User login
Commentary: COVID-19 Treatment and Disease-Modifying Therapies in MS, June 2022
One of the invisible treatment concerns is the effect of disease-modifying therapies (DMT) on vaccination, vaccination hesitancy, recurring COVID-19 variants and their ability to elude detection, and the protection of PWMS. This includes our ability to treat vaccinated PWMS if breakthrough recurrent infection occurs and identify how best to mitigate risk for recurrent infection. Prior comments have explored the impact of varied DMT on B-cell–related antibody response. With little surprise, a decreased SARS-CoV-2 antibody level is the major contributor to breakthrough SARS-CoV-2 infection in vaccinated PWMS taking various DMT, with a third vaccine dose significantly reducing the risk for infection. A prospective study (N = 1705) by Sormani and colleagues examined PWMS taking various DMT who received two doses of the BNT162b2 (BioNTech-Pfizer) (n = 1391) or mRNA-1273 (Moderna, aka CX-024414) (n = 314) SARS-CoV-2 vaccine, with most receiving a third dose. After the second dose, the only significant factor associated with risk for breakthrough infection was low antibody level (hazard ratio [HR] 0.51; P < .001), with the third dose reducing the risk for infection by 56% (HR 0.44; P = .025) during the Omicron COVID-19 wave.
In another recent prospective study, Cabeza and colleagues noted that ocrelizumab-treated PWMS who received a third SARS-CoV-2 vaccine dose had a boosted T-cell response, but there was no additive effect on the maximal T-cell response. The study included PWMS taking DMT (ocrelizumab, n = 24; fingolimod, n = 12; or no DMT, n = 10) and healthy controls (n = 12), all of whom received three SARS-CoV-2 vaccine doses (BioNTech-Pfizer or Moderna). The SARS-CoV-2–specific T-cell response in patients treated with ocrelizumab was comparable to that in PWMS who were not treated with DMT and to that in healthy controls after the second SARS-CoV-2 vaccination. However, the third SARS-CoV-2 vaccination had no additive effect on T-cell response, but it did induce a booster response (P < .05).
The relationship and interplay of both T-cell and B-cell responses to viral infection is important to understand and appreciate. However, for PWMS who have had, do have, or will experience breakthrough infection, early use of anti-SARS-CoV-2 monoclonal antibodies (mAb) was effective and safe in treating acute COVID-19 in PWMS treated with fingolimod or ocrelizumab. Manzano and colleagues reported on an observational study including 23 PWMS, most of whom had completed the initial COVID-19 vaccine series before infection and were either untreated or treated with fingolimod+ ocrelizumab and then received anti–SARS-CoV2 mAbs (bamlanivimab + etesevimab, casirivimab + imdevimab, sotrovimab, or an undocumented formulation) for treatment of active COVID-19. In this study, 74% of PWMS were able to be managed as outpatients (median duration to mAb receipt, 4 days), and 48% of PWMS recovered from COVID-19 within 7 days after mAb receipt, with no clinical MS relapses documented during or shortly after COVID-19 (median follow-up, 18 days). No adverse events or deaths were reported in this series.
Pivotal trials and package insert information affect DMT choice and dosing, the timing of ongoing treatment, and the awareness of efficacy and potential adverse reactions. Foley and colleagues demonstrated that switching to once-every-6-weeks (QW6) dosing of natalizumab from a stable dosing of once every 4 weeks (QW4) was safe, without any clinically meaningful loss of efficacy in most patients with relapsing-remitting MS (RRMS). In the phase 3b NOVA trial (N = 499), patients with RRMS receiving stable intravenous natalizumab QW4 dosing were randomly assigned to continue QW4 (n = 248) or switch to QW6 (n = 251) natalizumab dosing. The mean number of new or newly enlarging T2 hyperintense lesions at 72 weeks was 0.20 (95% CI 0.07-0.63) with natalizumab QW6 vs 0.05 (95% CI 0.01-0.22) with natalizumab QW4, with only two of the PWMS developing 25 or more lesions; this contributed to most of the excess lesions in the QW6 dosing regimen. The safety profile was similar for both the regimens.
Both DMT choice and vaccine-related antibody production matter. Various DMT have different and problematic impact on antibody production and response, and unrecognized immune deficiency or poor antibody response are problematic as variant COVID-19 strains continue to evolve. Protection against both MS disease activity and infections from variants remain a complex issue. Establishing and maintaining protection are important. Identifying PWMS who are at high risk for poor or sustained antibody response is important in addition to the ongoing effective treatment of MS. The landscape of available DMT choice, treatment paradigms, and COVID-19 variants and COVID-19 family protection continues to evolve.
One of the invisible treatment concerns is the effect of disease-modifying therapies (DMT) on vaccination, vaccination hesitancy, recurring COVID-19 variants and their ability to elude detection, and the protection of PWMS. This includes our ability to treat vaccinated PWMS if breakthrough recurrent infection occurs and identify how best to mitigate risk for recurrent infection. Prior comments have explored the impact of varied DMT on B-cell–related antibody response. With little surprise, a decreased SARS-CoV-2 antibody level is the major contributor to breakthrough SARS-CoV-2 infection in vaccinated PWMS taking various DMT, with a third vaccine dose significantly reducing the risk for infection. A prospective study (N = 1705) by Sormani and colleagues examined PWMS taking various DMT who received two doses of the BNT162b2 (BioNTech-Pfizer) (n = 1391) or mRNA-1273 (Moderna, aka CX-024414) (n = 314) SARS-CoV-2 vaccine, with most receiving a third dose. After the second dose, the only significant factor associated with risk for breakthrough infection was low antibody level (hazard ratio [HR] 0.51; P < .001), with the third dose reducing the risk for infection by 56% (HR 0.44; P = .025) during the Omicron COVID-19 wave.
In another recent prospective study, Cabeza and colleagues noted that ocrelizumab-treated PWMS who received a third SARS-CoV-2 vaccine dose had a boosted T-cell response, but there was no additive effect on the maximal T-cell response. The study included PWMS taking DMT (ocrelizumab, n = 24; fingolimod, n = 12; or no DMT, n = 10) and healthy controls (n = 12), all of whom received three SARS-CoV-2 vaccine doses (BioNTech-Pfizer or Moderna). The SARS-CoV-2–specific T-cell response in patients treated with ocrelizumab was comparable to that in PWMS who were not treated with DMT and to that in healthy controls after the second SARS-CoV-2 vaccination. However, the third SARS-CoV-2 vaccination had no additive effect on T-cell response, but it did induce a booster response (P < .05).
The relationship and interplay of both T-cell and B-cell responses to viral infection is important to understand and appreciate. However, for PWMS who have had, do have, or will experience breakthrough infection, early use of anti-SARS-CoV-2 monoclonal antibodies (mAb) was effective and safe in treating acute COVID-19 in PWMS treated with fingolimod or ocrelizumab. Manzano and colleagues reported on an observational study including 23 PWMS, most of whom had completed the initial COVID-19 vaccine series before infection and were either untreated or treated with fingolimod+ ocrelizumab and then received anti–SARS-CoV2 mAbs (bamlanivimab + etesevimab, casirivimab + imdevimab, sotrovimab, or an undocumented formulation) for treatment of active COVID-19. In this study, 74% of PWMS were able to be managed as outpatients (median duration to mAb receipt, 4 days), and 48% of PWMS recovered from COVID-19 within 7 days after mAb receipt, with no clinical MS relapses documented during or shortly after COVID-19 (median follow-up, 18 days). No adverse events or deaths were reported in this series.
Pivotal trials and package insert information affect DMT choice and dosing, the timing of ongoing treatment, and the awareness of efficacy and potential adverse reactions. Foley and colleagues demonstrated that switching to once-every-6-weeks (QW6) dosing of natalizumab from a stable dosing of once every 4 weeks (QW4) was safe, without any clinically meaningful loss of efficacy in most patients with relapsing-remitting MS (RRMS). In the phase 3b NOVA trial (N = 499), patients with RRMS receiving stable intravenous natalizumab QW4 dosing were randomly assigned to continue QW4 (n = 248) or switch to QW6 (n = 251) natalizumab dosing. The mean number of new or newly enlarging T2 hyperintense lesions at 72 weeks was 0.20 (95% CI 0.07-0.63) with natalizumab QW6 vs 0.05 (95% CI 0.01-0.22) with natalizumab QW4, with only two of the PWMS developing 25 or more lesions; this contributed to most of the excess lesions in the QW6 dosing regimen. The safety profile was similar for both the regimens.
Both DMT choice and vaccine-related antibody production matter. Various DMT have different and problematic impact on antibody production and response, and unrecognized immune deficiency or poor antibody response are problematic as variant COVID-19 strains continue to evolve. Protection against both MS disease activity and infections from variants remain a complex issue. Establishing and maintaining protection are important. Identifying PWMS who are at high risk for poor or sustained antibody response is important in addition to the ongoing effective treatment of MS. The landscape of available DMT choice, treatment paradigms, and COVID-19 variants and COVID-19 family protection continues to evolve.
One of the invisible treatment concerns is the effect of disease-modifying therapies (DMT) on vaccination, vaccination hesitancy, recurring COVID-19 variants and their ability to elude detection, and the protection of PWMS. This includes our ability to treat vaccinated PWMS if breakthrough recurrent infection occurs and identify how best to mitigate risk for recurrent infection. Prior comments have explored the impact of varied DMT on B-cell–related antibody response. With little surprise, a decreased SARS-CoV-2 antibody level is the major contributor to breakthrough SARS-CoV-2 infection in vaccinated PWMS taking various DMT, with a third vaccine dose significantly reducing the risk for infection. A prospective study (N = 1705) by Sormani and colleagues examined PWMS taking various DMT who received two doses of the BNT162b2 (BioNTech-Pfizer) (n = 1391) or mRNA-1273 (Moderna, aka CX-024414) (n = 314) SARS-CoV-2 vaccine, with most receiving a third dose. After the second dose, the only significant factor associated with risk for breakthrough infection was low antibody level (hazard ratio [HR] 0.51; P < .001), with the third dose reducing the risk for infection by 56% (HR 0.44; P = .025) during the Omicron COVID-19 wave.
In another recent prospective study, Cabeza and colleagues noted that ocrelizumab-treated PWMS who received a third SARS-CoV-2 vaccine dose had a boosted T-cell response, but there was no additive effect on the maximal T-cell response. The study included PWMS taking DMT (ocrelizumab, n = 24; fingolimod, n = 12; or no DMT, n = 10) and healthy controls (n = 12), all of whom received three SARS-CoV-2 vaccine doses (BioNTech-Pfizer or Moderna). The SARS-CoV-2–specific T-cell response in patients treated with ocrelizumab was comparable to that in PWMS who were not treated with DMT and to that in healthy controls after the second SARS-CoV-2 vaccination. However, the third SARS-CoV-2 vaccination had no additive effect on T-cell response, but it did induce a booster response (P < .05).
The relationship and interplay of both T-cell and B-cell responses to viral infection is important to understand and appreciate. However, for PWMS who have had, do have, or will experience breakthrough infection, early use of anti-SARS-CoV-2 monoclonal antibodies (mAb) was effective and safe in treating acute COVID-19 in PWMS treated with fingolimod or ocrelizumab. Manzano and colleagues reported on an observational study including 23 PWMS, most of whom had completed the initial COVID-19 vaccine series before infection and were either untreated or treated with fingolimod+ ocrelizumab and then received anti–SARS-CoV2 mAbs (bamlanivimab + etesevimab, casirivimab + imdevimab, sotrovimab, or an undocumented formulation) for treatment of active COVID-19. In this study, 74% of PWMS were able to be managed as outpatients (median duration to mAb receipt, 4 days), and 48% of PWMS recovered from COVID-19 within 7 days after mAb receipt, with no clinical MS relapses documented during or shortly after COVID-19 (median follow-up, 18 days). No adverse events or deaths were reported in this series.
Pivotal trials and package insert information affect DMT choice and dosing, the timing of ongoing treatment, and the awareness of efficacy and potential adverse reactions. Foley and colleagues demonstrated that switching to once-every-6-weeks (QW6) dosing of natalizumab from a stable dosing of once every 4 weeks (QW4) was safe, without any clinically meaningful loss of efficacy in most patients with relapsing-remitting MS (RRMS). In the phase 3b NOVA trial (N = 499), patients with RRMS receiving stable intravenous natalizumab QW4 dosing were randomly assigned to continue QW4 (n = 248) or switch to QW6 (n = 251) natalizumab dosing. The mean number of new or newly enlarging T2 hyperintense lesions at 72 weeks was 0.20 (95% CI 0.07-0.63) with natalizumab QW6 vs 0.05 (95% CI 0.01-0.22) with natalizumab QW4, with only two of the PWMS developing 25 or more lesions; this contributed to most of the excess lesions in the QW6 dosing regimen. The safety profile was similar for both the regimens.
Both DMT choice and vaccine-related antibody production matter. Various DMT have different and problematic impact on antibody production and response, and unrecognized immune deficiency or poor antibody response are problematic as variant COVID-19 strains continue to evolve. Protection against both MS disease activity and infections from variants remain a complex issue. Establishing and maintaining protection are important. Identifying PWMS who are at high risk for poor or sustained antibody response is important in addition to the ongoing effective treatment of MS. The landscape of available DMT choice, treatment paradigms, and COVID-19 variants and COVID-19 family protection continues to evolve.
Abortion debate may affect Rx decisions for pregnant women
Obstetrician Beverly Gray, MD, is already seeing the effects of the Roe v. Wade abortion debate in her North Carolina practice.
The state allows abortion but requires that women get counseling with a qualified health professional 72 hours before the procedure. “Aside from that, we still have patients asking for more efficacious contraceptive methods just in case,” said Dr. Gray, residency director and division director for women’s community and population health and associate professor for obstetrics and gynecology at Duke University, Durham, N.C.
Patients and staff in her clinic have also been approaching her about tubal ligation. “They’re asking about additional birth control methods because they’re concerned about what’s going to happen” with the challenge to the historic Roe v. Wade decision in the Supreme Court and subsequent actions in the states to restrict or ban abortion, she said.
This has implications not just for abortion but for medications known to affect pregnancy. “What I’m really worried about is physicians will be withholding medicine because they’re concerned about teratogenic effects,” said Dr. Gray.
With more states issuing restrictions on abortion, doctors are worried that patients needing certain drugs to maintain their lupus flares, cancer, or other diseases may decide not to take them in the event they accidentally become pregnant. If the drug is known to affect the fetus, the fear is a patient who lives in a state with abortion restrictions will no longer have the option to terminate a pregnancy.
Instead, a scenario may arise in which the patient – and their physician – may opt not to treat at all with an otherwise lifesaving medication, experts told this news organization.
The U.S. landscape on abortion restrictions
A leaked draft of a U.S. Supreme Court opinion on Mississippi’s 15-week abortion ban has sent the medical community into a tailspin. The case, Dobbs v. Jackson Women’s Health Organization, challenges the 1973 Roe v. Wade decision that affirms the constitutional right to abortion. It’s anticipated the high court will decide on the case in June.
Although the upcoming decision is subject to change, the draft indicated the high court would uphold the Mississippi ban. This would essentially overturn the 1973 ruling. An earlier Supreme Court decision allowing a Texas law banning abortion at 6 weeks suggests the court may already be heading in this direction. At the state level, legislatures have been moving on divergent paths – some taking steps to preserve abortion rights, others initiating restrictions.
More than 100 abortion restrictions in 19 states took effect in 2021, according to the Guttmacher Institute, which tracks such metrics. In 2022, “two key themes are anti-abortion policymakers’ continued pursuit of various types of abortion bans and restrictions on medication abortion,” the institute reported.
Forty-six states and the District of Columbia have introduced 2,025 restrictions or proactive measures on sexual and reproductive health and rights so far this year. The latest tally from Guttmacher, updated in late May, revealed that 11 states so far have enacted 42 abortion restrictions. A total of 6 states (Arizona, Florida, Idaho, Kentucky, Oklahoma, and Wyoming) have issued nine bans on abortion.
Comparatively, 11 states have enacted 19 protective abortion measures.
Twenty-two states have introduced 117 restrictions on medication abortions, which account for 54% of U.S. abortions. This includes seven measures that would ban medication abortion outright, according to Guttmacher. Kentucky and South Dakota collectively have enacted 14 restrictions on medication abortion, as well as provisions that ban mailing of abortion pills.
Chilling effect on prescribing
Some physicians anticipate that drugs such as the “morning-after” pill (levonorgestrel) will become less available as restrictions go into effect, since these are medications designed to prevent pregnancy.*
However, the ongoing effort to put a lid on abortion measures has prompted concerns about a trickle-down effect on other medications that are otherwise life-changing or lifesaving to patients but pose a risk to the fetus.
Several drugs are well documented to affect fetal growth and development of the fetus, ranging from mild, transitory effects to severe, permanent birth defects, said Ronald G. Grifka, MD, chief medical officer of University of Michigan Health-West and clinical professor of pediatrics at the University of Michigan Medical School, Ann Arbor. “As new medications are developed, we will need heightened attention to make sure they are safe for the fetus,” he added.
Certain teratogenic medications are associated with a high risk of abortion even though this isn’t their primary use, noted Christina Chambers, PhD, MPH, co-director of the Center for Better Beginnings and associate director with the Altman Clinical & Translational Research Institute at the University of California, San Diego.
“I don’t think anyone would intentionally take these drugs to induce spontaneous abortion. But if the drugs pose a risk for it, I can see how the laws might be stretched” to include them, said Dr. Chambers.
Methotrexate, a medication for autoimmune disorders, has a high risk of spontaneous abortion. So do acne medications such as isotretinoin.
Patients are usually told they’re not supposed to get pregnant on these drugs because there’s a high risk of pregnancy loss and risk of malformations and potential learning problems in the fetus. But many pregnancies aren’t planned, said Dr. Chambers. “Patients may forget about the side effects or think their birth control will protect them. And the next time they refill the medication, they may not hear about the warnings again.”
With a restrictive abortion law or ban in effect, a woman might think: “I won’t take this drug because if there’s any potential that I might get pregnant, I won’t have the option to abort an at-risk pregnancy.” Women and their doctors, for that matter, don’t want to put themselves in this position, said Dr. Chambers.
Rheumatologist Megan Clowse, MD, who prescribes several medications that potentially cause major birth defects and pregnancy loss, worries about the ramifications of these accumulating bans.
“Methotrexate has been a leading drug for us for decades for rheumatoid arthritis. Mycophenolate is a vital drug for lupus,” said Dr. Clowse, associate professor of medicine at Duke University’s division of rheumatology and immunology.
Both methotrexate and mycophenolate pose about a 40% risk of pregnancy loss and significantly increase the risk for birth defects. “I’m definitely concerned that there might be doctors or women who elect not to use those medications in women of reproductive age because of the potential risk for pregnancy and absence of abortion rights,” said Dr. Clowse.
These situations might force women to use contraceptives they don’t want to use, such as hormonal implants or intrauterine devices, she added. Another side effect is that women and their partners may decide to abstain from sex.
The iPLEDGE factor
Some rheumatology drugs like lenalidomide (Revlimid) require a valid negative pregnancy test in a lab every month. Similarly, the iPLEDGE Risk Evaluation and Mitigation Strategy seeks to reduce the teratogenicity of isotretinoin by requiring two types of birth control and regular pregnancy tests by users.
For isotretinoin specifically, abortion restrictions “could lead to increased adherence to pregnancy prevention measures which are already stringent in iPLEDGE. But on the other hand, it could lead to reduced willingness of physicians to prescribe or patients to take the medication,” said Dr. Chambers.
With programs like iPLEDGE in effect, the rate of pregnancies and abortions that occur in dermatology are relatively low, said Jenny Murase, MD, associate clinical professor of dermatology at the University of California, San Francisco.
Nevertheless, as a physician who regularly prescribes medications like isotretinoin in women of childbearing age, “it’s terrifying to me that a woman wouldn’t have the option to terminate the pregnancy if a teratogenic effect from the medication caused a severe birth defect,” said Dr. Murase.
Dermatologists use other teratogenic medications such as thalidomide, mycophenolate mofetil, and methotrexate for chronic dermatologic disease like psoriasis and atopic dermatitis.
The situation is especially tricky for dermatologists since most patients – about 80% – never discuss their pregnancy with their specialist prior to pregnancy initiation. Dr. Murase recalls when a patient with chronic plaque psoriasis on methotrexate in her late 40s became pregnant and had an abortion even before Dr. Murase became aware of the pregnancy.
Because dermatologists routinely prescribe long-term medications for chronic diseases like acne, psoriasis, and atopic dermatitis, it is important to have a conversation regarding the risks and benefits of long-term medication should a pregnancy occur in any woman of childbearing age, she said.
Fewer women in clinical trials?
Abortion restrictions could possibly discourage women of reproductive age to participate in a clinical trial for a new medication, said Dr. Chambers.
A female patient with a chronic disease who’s randomized to receive a new medication may be required to use certain types of birth control because of unknown potential adverse effects the drug may have on the fetus. But in some cases, accidental pregnancies happen.
The participant in the trial may say, “I don’t know enough about the safety of this drug in pregnancy, and I’ve already taken it. I want to terminate the pregnancy,” said Dr. Chambers. Thinking ahead, a woman may decide not to do the trial to avoid the risk of getting pregnant and not having the option to terminate the pregnancy.
This could apply to new drugs such as antiviral treatments, or medications for severe chronic disease that typically have no clinical trial data in pregnancy prior to initial release into the market.
Women may start taking the drug without thinking about getting pregnant, then realize there are no safety data and become concerned about its effects on a future pregnancy.
The question is: Will abortion restrictions have a chilling effect on these new drugs as well? Patients and their doctors may decide not to try it until more data are available. “I can see where abortion restrictions would change the risk or benefit calculation in thinking about what you do or don’t prescribe or take during reproductive age,” said Dr. Chambers.
The upside of restrictions?
If there’s a positive side to these developments with abortion bans, it may encourage women taking new medications or joining clinical trials to think even more carefully about adherence to effective contraception, said Dr. Chambers.
Some methods are more effective than others, she emphasized. “When you have an unplanned pregnancy, it could mean that the method you used wasn’t optimal or you weren’t using it as recommended.” A goal moving forward is to encourage more thoughtful use of highly effective contraceptives, thus reducing the number of unplanned pregnancies, she added.
If patients are taking methotrexate, “the time to think about pregnancy is before getting pregnant so you can switch to a drug that’s compatible with pregnancy,” she said.
This whole thought process regarding pregnancy planning could work toward useful health goals, said Dr. Chambers. “Nobody thinks termination is the preferred method, but planning ahead should involve a discussion of what works best for the patient.”
Patients do have other choices, said Dr. Grifka. “Fortunately, there are many commonly prescribed medications which cross the placenta and have no ill effects on the fetus.”
Talking to patients about choices
Dr. Clowse, who spends a lot of time training rheumatologists, encourages them to have conversations with patients about pregnancy planning. It’s a lot to manage, getting the right drug to a female patient with chronic illness, especially in this current climate of abortion upheaval, she noted.
Her approach is to have an open and honest conversation with patients about their concerns and fears, what the realities are, and what the potential future options are for certain rheumatology drugs in the United States.
Some women who see what’s happening across the country may become so risk averse that they may choose to die rather than take a lifesaving drug that poses certain risks under new restrictions.
“I think that’s tragic,” said Dr. Clowse.
To help their patients, Dr. Gray believes physicians across specialties should better educate themselves about physiology in pregnancy and how to counsel patients on the impact of not taking medications in pregnancy.
In her view, it’s almost coercive to say to a patient, “You really need to have effective contraception if I’m going to give you this lifesaving or quality-of-life-improving medication.”
When confronting such scenarios, Dr. Gray doesn’t think physicians need to change how they counsel patients about contraception. “I don’t think we should be putting pressure on patients to consider other permanent methods just because there’s a lack of abortion options.”
Patients will eventually make those decisions for themselves, she said. “They’re going to want a more efficacious method because they’re worried about not having access to abortion if they get pregnant.”
Dr. Gray reports being a site principal investigator for a phase 3 trial for VeraCept IUD, funded by Sebela Pharmaceuticals. Dr. Clowse reports receiving research funding and doing consulting for GlaxoSmithKline.
*Correction, 6/2/2022: A previous version of this article misstated the intended use of drugs such as the “morning-after” pill (levonorgestrel). They are taken to prevent unintended pregnancy.
A version of this article first appeared on Medscape.com .
Obstetrician Beverly Gray, MD, is already seeing the effects of the Roe v. Wade abortion debate in her North Carolina practice.
The state allows abortion but requires that women get counseling with a qualified health professional 72 hours before the procedure. “Aside from that, we still have patients asking for more efficacious contraceptive methods just in case,” said Dr. Gray, residency director and division director for women’s community and population health and associate professor for obstetrics and gynecology at Duke University, Durham, N.C.
Patients and staff in her clinic have also been approaching her about tubal ligation. “They’re asking about additional birth control methods because they’re concerned about what’s going to happen” with the challenge to the historic Roe v. Wade decision in the Supreme Court and subsequent actions in the states to restrict or ban abortion, she said.
This has implications not just for abortion but for medications known to affect pregnancy. “What I’m really worried about is physicians will be withholding medicine because they’re concerned about teratogenic effects,” said Dr. Gray.
With more states issuing restrictions on abortion, doctors are worried that patients needing certain drugs to maintain their lupus flares, cancer, or other diseases may decide not to take them in the event they accidentally become pregnant. If the drug is known to affect the fetus, the fear is a patient who lives in a state with abortion restrictions will no longer have the option to terminate a pregnancy.
Instead, a scenario may arise in which the patient – and their physician – may opt not to treat at all with an otherwise lifesaving medication, experts told this news organization.
The U.S. landscape on abortion restrictions
A leaked draft of a U.S. Supreme Court opinion on Mississippi’s 15-week abortion ban has sent the medical community into a tailspin. The case, Dobbs v. Jackson Women’s Health Organization, challenges the 1973 Roe v. Wade decision that affirms the constitutional right to abortion. It’s anticipated the high court will decide on the case in June.
Although the upcoming decision is subject to change, the draft indicated the high court would uphold the Mississippi ban. This would essentially overturn the 1973 ruling. An earlier Supreme Court decision allowing a Texas law banning abortion at 6 weeks suggests the court may already be heading in this direction. At the state level, legislatures have been moving on divergent paths – some taking steps to preserve abortion rights, others initiating restrictions.
More than 100 abortion restrictions in 19 states took effect in 2021, according to the Guttmacher Institute, which tracks such metrics. In 2022, “two key themes are anti-abortion policymakers’ continued pursuit of various types of abortion bans and restrictions on medication abortion,” the institute reported.
Forty-six states and the District of Columbia have introduced 2,025 restrictions or proactive measures on sexual and reproductive health and rights so far this year. The latest tally from Guttmacher, updated in late May, revealed that 11 states so far have enacted 42 abortion restrictions. A total of 6 states (Arizona, Florida, Idaho, Kentucky, Oklahoma, and Wyoming) have issued nine bans on abortion.
Comparatively, 11 states have enacted 19 protective abortion measures.
Twenty-two states have introduced 117 restrictions on medication abortions, which account for 54% of U.S. abortions. This includes seven measures that would ban medication abortion outright, according to Guttmacher. Kentucky and South Dakota collectively have enacted 14 restrictions on medication abortion, as well as provisions that ban mailing of abortion pills.
Chilling effect on prescribing
Some physicians anticipate that drugs such as the “morning-after” pill (levonorgestrel) will become less available as restrictions go into effect, since these are medications designed to prevent pregnancy.*
However, the ongoing effort to put a lid on abortion measures has prompted concerns about a trickle-down effect on other medications that are otherwise life-changing or lifesaving to patients but pose a risk to the fetus.
Several drugs are well documented to affect fetal growth and development of the fetus, ranging from mild, transitory effects to severe, permanent birth defects, said Ronald G. Grifka, MD, chief medical officer of University of Michigan Health-West and clinical professor of pediatrics at the University of Michigan Medical School, Ann Arbor. “As new medications are developed, we will need heightened attention to make sure they are safe for the fetus,” he added.
Certain teratogenic medications are associated with a high risk of abortion even though this isn’t their primary use, noted Christina Chambers, PhD, MPH, co-director of the Center for Better Beginnings and associate director with the Altman Clinical & Translational Research Institute at the University of California, San Diego.
“I don’t think anyone would intentionally take these drugs to induce spontaneous abortion. But if the drugs pose a risk for it, I can see how the laws might be stretched” to include them, said Dr. Chambers.
Methotrexate, a medication for autoimmune disorders, has a high risk of spontaneous abortion. So do acne medications such as isotretinoin.
Patients are usually told they’re not supposed to get pregnant on these drugs because there’s a high risk of pregnancy loss and risk of malformations and potential learning problems in the fetus. But many pregnancies aren’t planned, said Dr. Chambers. “Patients may forget about the side effects or think their birth control will protect them. And the next time they refill the medication, they may not hear about the warnings again.”
With a restrictive abortion law or ban in effect, a woman might think: “I won’t take this drug because if there’s any potential that I might get pregnant, I won’t have the option to abort an at-risk pregnancy.” Women and their doctors, for that matter, don’t want to put themselves in this position, said Dr. Chambers.
Rheumatologist Megan Clowse, MD, who prescribes several medications that potentially cause major birth defects and pregnancy loss, worries about the ramifications of these accumulating bans.
“Methotrexate has been a leading drug for us for decades for rheumatoid arthritis. Mycophenolate is a vital drug for lupus,” said Dr. Clowse, associate professor of medicine at Duke University’s division of rheumatology and immunology.
Both methotrexate and mycophenolate pose about a 40% risk of pregnancy loss and significantly increase the risk for birth defects. “I’m definitely concerned that there might be doctors or women who elect not to use those medications in women of reproductive age because of the potential risk for pregnancy and absence of abortion rights,” said Dr. Clowse.
These situations might force women to use contraceptives they don’t want to use, such as hormonal implants or intrauterine devices, she added. Another side effect is that women and their partners may decide to abstain from sex.
The iPLEDGE factor
Some rheumatology drugs like lenalidomide (Revlimid) require a valid negative pregnancy test in a lab every month. Similarly, the iPLEDGE Risk Evaluation and Mitigation Strategy seeks to reduce the teratogenicity of isotretinoin by requiring two types of birth control and regular pregnancy tests by users.
For isotretinoin specifically, abortion restrictions “could lead to increased adherence to pregnancy prevention measures which are already stringent in iPLEDGE. But on the other hand, it could lead to reduced willingness of physicians to prescribe or patients to take the medication,” said Dr. Chambers.
With programs like iPLEDGE in effect, the rate of pregnancies and abortions that occur in dermatology are relatively low, said Jenny Murase, MD, associate clinical professor of dermatology at the University of California, San Francisco.
Nevertheless, as a physician who regularly prescribes medications like isotretinoin in women of childbearing age, “it’s terrifying to me that a woman wouldn’t have the option to terminate the pregnancy if a teratogenic effect from the medication caused a severe birth defect,” said Dr. Murase.
Dermatologists use other teratogenic medications such as thalidomide, mycophenolate mofetil, and methotrexate for chronic dermatologic disease like psoriasis and atopic dermatitis.
The situation is especially tricky for dermatologists since most patients – about 80% – never discuss their pregnancy with their specialist prior to pregnancy initiation. Dr. Murase recalls when a patient with chronic plaque psoriasis on methotrexate in her late 40s became pregnant and had an abortion even before Dr. Murase became aware of the pregnancy.
Because dermatologists routinely prescribe long-term medications for chronic diseases like acne, psoriasis, and atopic dermatitis, it is important to have a conversation regarding the risks and benefits of long-term medication should a pregnancy occur in any woman of childbearing age, she said.
Fewer women in clinical trials?
Abortion restrictions could possibly discourage women of reproductive age to participate in a clinical trial for a new medication, said Dr. Chambers.
A female patient with a chronic disease who’s randomized to receive a new medication may be required to use certain types of birth control because of unknown potential adverse effects the drug may have on the fetus. But in some cases, accidental pregnancies happen.
The participant in the trial may say, “I don’t know enough about the safety of this drug in pregnancy, and I’ve already taken it. I want to terminate the pregnancy,” said Dr. Chambers. Thinking ahead, a woman may decide not to do the trial to avoid the risk of getting pregnant and not having the option to terminate the pregnancy.
This could apply to new drugs such as antiviral treatments, or medications for severe chronic disease that typically have no clinical trial data in pregnancy prior to initial release into the market.
Women may start taking the drug without thinking about getting pregnant, then realize there are no safety data and become concerned about its effects on a future pregnancy.
The question is: Will abortion restrictions have a chilling effect on these new drugs as well? Patients and their doctors may decide not to try it until more data are available. “I can see where abortion restrictions would change the risk or benefit calculation in thinking about what you do or don’t prescribe or take during reproductive age,” said Dr. Chambers.
The upside of restrictions?
If there’s a positive side to these developments with abortion bans, it may encourage women taking new medications or joining clinical trials to think even more carefully about adherence to effective contraception, said Dr. Chambers.
Some methods are more effective than others, she emphasized. “When you have an unplanned pregnancy, it could mean that the method you used wasn’t optimal or you weren’t using it as recommended.” A goal moving forward is to encourage more thoughtful use of highly effective contraceptives, thus reducing the number of unplanned pregnancies, she added.
If patients are taking methotrexate, “the time to think about pregnancy is before getting pregnant so you can switch to a drug that’s compatible with pregnancy,” she said.
This whole thought process regarding pregnancy planning could work toward useful health goals, said Dr. Chambers. “Nobody thinks termination is the preferred method, but planning ahead should involve a discussion of what works best for the patient.”
Patients do have other choices, said Dr. Grifka. “Fortunately, there are many commonly prescribed medications which cross the placenta and have no ill effects on the fetus.”
Talking to patients about choices
Dr. Clowse, who spends a lot of time training rheumatologists, encourages them to have conversations with patients about pregnancy planning. It’s a lot to manage, getting the right drug to a female patient with chronic illness, especially in this current climate of abortion upheaval, she noted.
Her approach is to have an open and honest conversation with patients about their concerns and fears, what the realities are, and what the potential future options are for certain rheumatology drugs in the United States.
Some women who see what’s happening across the country may become so risk averse that they may choose to die rather than take a lifesaving drug that poses certain risks under new restrictions.
“I think that’s tragic,” said Dr. Clowse.
To help their patients, Dr. Gray believes physicians across specialties should better educate themselves about physiology in pregnancy and how to counsel patients on the impact of not taking medications in pregnancy.
In her view, it’s almost coercive to say to a patient, “You really need to have effective contraception if I’m going to give you this lifesaving or quality-of-life-improving medication.”
When confronting such scenarios, Dr. Gray doesn’t think physicians need to change how they counsel patients about contraception. “I don’t think we should be putting pressure on patients to consider other permanent methods just because there’s a lack of abortion options.”
Patients will eventually make those decisions for themselves, she said. “They’re going to want a more efficacious method because they’re worried about not having access to abortion if they get pregnant.”
Dr. Gray reports being a site principal investigator for a phase 3 trial for VeraCept IUD, funded by Sebela Pharmaceuticals. Dr. Clowse reports receiving research funding and doing consulting for GlaxoSmithKline.
*Correction, 6/2/2022: A previous version of this article misstated the intended use of drugs such as the “morning-after” pill (levonorgestrel). They are taken to prevent unintended pregnancy.
A version of this article first appeared on Medscape.com .
Obstetrician Beverly Gray, MD, is already seeing the effects of the Roe v. Wade abortion debate in her North Carolina practice.
The state allows abortion but requires that women get counseling with a qualified health professional 72 hours before the procedure. “Aside from that, we still have patients asking for more efficacious contraceptive methods just in case,” said Dr. Gray, residency director and division director for women’s community and population health and associate professor for obstetrics and gynecology at Duke University, Durham, N.C.
Patients and staff in her clinic have also been approaching her about tubal ligation. “They’re asking about additional birth control methods because they’re concerned about what’s going to happen” with the challenge to the historic Roe v. Wade decision in the Supreme Court and subsequent actions in the states to restrict or ban abortion, she said.
This has implications not just for abortion but for medications known to affect pregnancy. “What I’m really worried about is physicians will be withholding medicine because they’re concerned about teratogenic effects,” said Dr. Gray.
With more states issuing restrictions on abortion, doctors are worried that patients needing certain drugs to maintain their lupus flares, cancer, or other diseases may decide not to take them in the event they accidentally become pregnant. If the drug is known to affect the fetus, the fear is a patient who lives in a state with abortion restrictions will no longer have the option to terminate a pregnancy.
Instead, a scenario may arise in which the patient – and their physician – may opt not to treat at all with an otherwise lifesaving medication, experts told this news organization.
The U.S. landscape on abortion restrictions
A leaked draft of a U.S. Supreme Court opinion on Mississippi’s 15-week abortion ban has sent the medical community into a tailspin. The case, Dobbs v. Jackson Women’s Health Organization, challenges the 1973 Roe v. Wade decision that affirms the constitutional right to abortion. It’s anticipated the high court will decide on the case in June.
Although the upcoming decision is subject to change, the draft indicated the high court would uphold the Mississippi ban. This would essentially overturn the 1973 ruling. An earlier Supreme Court decision allowing a Texas law banning abortion at 6 weeks suggests the court may already be heading in this direction. At the state level, legislatures have been moving on divergent paths – some taking steps to preserve abortion rights, others initiating restrictions.
More than 100 abortion restrictions in 19 states took effect in 2021, according to the Guttmacher Institute, which tracks such metrics. In 2022, “two key themes are anti-abortion policymakers’ continued pursuit of various types of abortion bans and restrictions on medication abortion,” the institute reported.
Forty-six states and the District of Columbia have introduced 2,025 restrictions or proactive measures on sexual and reproductive health and rights so far this year. The latest tally from Guttmacher, updated in late May, revealed that 11 states so far have enacted 42 abortion restrictions. A total of 6 states (Arizona, Florida, Idaho, Kentucky, Oklahoma, and Wyoming) have issued nine bans on abortion.
Comparatively, 11 states have enacted 19 protective abortion measures.
Twenty-two states have introduced 117 restrictions on medication abortions, which account for 54% of U.S. abortions. This includes seven measures that would ban medication abortion outright, according to Guttmacher. Kentucky and South Dakota collectively have enacted 14 restrictions on medication abortion, as well as provisions that ban mailing of abortion pills.
Chilling effect on prescribing
Some physicians anticipate that drugs such as the “morning-after” pill (levonorgestrel) will become less available as restrictions go into effect, since these are medications designed to prevent pregnancy.*
However, the ongoing effort to put a lid on abortion measures has prompted concerns about a trickle-down effect on other medications that are otherwise life-changing or lifesaving to patients but pose a risk to the fetus.
Several drugs are well documented to affect fetal growth and development of the fetus, ranging from mild, transitory effects to severe, permanent birth defects, said Ronald G. Grifka, MD, chief medical officer of University of Michigan Health-West and clinical professor of pediatrics at the University of Michigan Medical School, Ann Arbor. “As new medications are developed, we will need heightened attention to make sure they are safe for the fetus,” he added.
Certain teratogenic medications are associated with a high risk of abortion even though this isn’t their primary use, noted Christina Chambers, PhD, MPH, co-director of the Center for Better Beginnings and associate director with the Altman Clinical & Translational Research Institute at the University of California, San Diego.
“I don’t think anyone would intentionally take these drugs to induce spontaneous abortion. But if the drugs pose a risk for it, I can see how the laws might be stretched” to include them, said Dr. Chambers.
Methotrexate, a medication for autoimmune disorders, has a high risk of spontaneous abortion. So do acne medications such as isotretinoin.
Patients are usually told they’re not supposed to get pregnant on these drugs because there’s a high risk of pregnancy loss and risk of malformations and potential learning problems in the fetus. But many pregnancies aren’t planned, said Dr. Chambers. “Patients may forget about the side effects or think their birth control will protect them. And the next time they refill the medication, they may not hear about the warnings again.”
With a restrictive abortion law or ban in effect, a woman might think: “I won’t take this drug because if there’s any potential that I might get pregnant, I won’t have the option to abort an at-risk pregnancy.” Women and their doctors, for that matter, don’t want to put themselves in this position, said Dr. Chambers.
Rheumatologist Megan Clowse, MD, who prescribes several medications that potentially cause major birth defects and pregnancy loss, worries about the ramifications of these accumulating bans.
“Methotrexate has been a leading drug for us for decades for rheumatoid arthritis. Mycophenolate is a vital drug for lupus,” said Dr. Clowse, associate professor of medicine at Duke University’s division of rheumatology and immunology.
Both methotrexate and mycophenolate pose about a 40% risk of pregnancy loss and significantly increase the risk for birth defects. “I’m definitely concerned that there might be doctors or women who elect not to use those medications in women of reproductive age because of the potential risk for pregnancy and absence of abortion rights,” said Dr. Clowse.
These situations might force women to use contraceptives they don’t want to use, such as hormonal implants or intrauterine devices, she added. Another side effect is that women and their partners may decide to abstain from sex.
The iPLEDGE factor
Some rheumatology drugs like lenalidomide (Revlimid) require a valid negative pregnancy test in a lab every month. Similarly, the iPLEDGE Risk Evaluation and Mitigation Strategy seeks to reduce the teratogenicity of isotretinoin by requiring two types of birth control and regular pregnancy tests by users.
For isotretinoin specifically, abortion restrictions “could lead to increased adherence to pregnancy prevention measures which are already stringent in iPLEDGE. But on the other hand, it could lead to reduced willingness of physicians to prescribe or patients to take the medication,” said Dr. Chambers.
With programs like iPLEDGE in effect, the rate of pregnancies and abortions that occur in dermatology are relatively low, said Jenny Murase, MD, associate clinical professor of dermatology at the University of California, San Francisco.
Nevertheless, as a physician who regularly prescribes medications like isotretinoin in women of childbearing age, “it’s terrifying to me that a woman wouldn’t have the option to terminate the pregnancy if a teratogenic effect from the medication caused a severe birth defect,” said Dr. Murase.
Dermatologists use other teratogenic medications such as thalidomide, mycophenolate mofetil, and methotrexate for chronic dermatologic disease like psoriasis and atopic dermatitis.
The situation is especially tricky for dermatologists since most patients – about 80% – never discuss their pregnancy with their specialist prior to pregnancy initiation. Dr. Murase recalls when a patient with chronic plaque psoriasis on methotrexate in her late 40s became pregnant and had an abortion even before Dr. Murase became aware of the pregnancy.
Because dermatologists routinely prescribe long-term medications for chronic diseases like acne, psoriasis, and atopic dermatitis, it is important to have a conversation regarding the risks and benefits of long-term medication should a pregnancy occur in any woman of childbearing age, she said.
Fewer women in clinical trials?
Abortion restrictions could possibly discourage women of reproductive age to participate in a clinical trial for a new medication, said Dr. Chambers.
A female patient with a chronic disease who’s randomized to receive a new medication may be required to use certain types of birth control because of unknown potential adverse effects the drug may have on the fetus. But in some cases, accidental pregnancies happen.
The participant in the trial may say, “I don’t know enough about the safety of this drug in pregnancy, and I’ve already taken it. I want to terminate the pregnancy,” said Dr. Chambers. Thinking ahead, a woman may decide not to do the trial to avoid the risk of getting pregnant and not having the option to terminate the pregnancy.
This could apply to new drugs such as antiviral treatments, or medications for severe chronic disease that typically have no clinical trial data in pregnancy prior to initial release into the market.
Women may start taking the drug without thinking about getting pregnant, then realize there are no safety data and become concerned about its effects on a future pregnancy.
The question is: Will abortion restrictions have a chilling effect on these new drugs as well? Patients and their doctors may decide not to try it until more data are available. “I can see where abortion restrictions would change the risk or benefit calculation in thinking about what you do or don’t prescribe or take during reproductive age,” said Dr. Chambers.
The upside of restrictions?
If there’s a positive side to these developments with abortion bans, it may encourage women taking new medications or joining clinical trials to think even more carefully about adherence to effective contraception, said Dr. Chambers.
Some methods are more effective than others, she emphasized. “When you have an unplanned pregnancy, it could mean that the method you used wasn’t optimal or you weren’t using it as recommended.” A goal moving forward is to encourage more thoughtful use of highly effective contraceptives, thus reducing the number of unplanned pregnancies, she added.
If patients are taking methotrexate, “the time to think about pregnancy is before getting pregnant so you can switch to a drug that’s compatible with pregnancy,” she said.
This whole thought process regarding pregnancy planning could work toward useful health goals, said Dr. Chambers. “Nobody thinks termination is the preferred method, but planning ahead should involve a discussion of what works best for the patient.”
Patients do have other choices, said Dr. Grifka. “Fortunately, there are many commonly prescribed medications which cross the placenta and have no ill effects on the fetus.”
Talking to patients about choices
Dr. Clowse, who spends a lot of time training rheumatologists, encourages them to have conversations with patients about pregnancy planning. It’s a lot to manage, getting the right drug to a female patient with chronic illness, especially in this current climate of abortion upheaval, she noted.
Her approach is to have an open and honest conversation with patients about their concerns and fears, what the realities are, and what the potential future options are for certain rheumatology drugs in the United States.
Some women who see what’s happening across the country may become so risk averse that they may choose to die rather than take a lifesaving drug that poses certain risks under new restrictions.
“I think that’s tragic,” said Dr. Clowse.
To help their patients, Dr. Gray believes physicians across specialties should better educate themselves about physiology in pregnancy and how to counsel patients on the impact of not taking medications in pregnancy.
In her view, it’s almost coercive to say to a patient, “You really need to have effective contraception if I’m going to give you this lifesaving or quality-of-life-improving medication.”
When confronting such scenarios, Dr. Gray doesn’t think physicians need to change how they counsel patients about contraception. “I don’t think we should be putting pressure on patients to consider other permanent methods just because there’s a lack of abortion options.”
Patients will eventually make those decisions for themselves, she said. “They’re going to want a more efficacious method because they’re worried about not having access to abortion if they get pregnant.”
Dr. Gray reports being a site principal investigator for a phase 3 trial for VeraCept IUD, funded by Sebela Pharmaceuticals. Dr. Clowse reports receiving research funding and doing consulting for GlaxoSmithKline.
*Correction, 6/2/2022: A previous version of this article misstated the intended use of drugs such as the “morning-after” pill (levonorgestrel). They are taken to prevent unintended pregnancy.
A version of this article first appeared on Medscape.com .
Psychological intervention looks promising in Crohn’s disease
SAN DIEGO – A combination of cognitive-behavioral therapy and mindfulness meditation could reduce pain and fatigue from Crohn’s disease, researchers say.
Patients who followed the program not only felt better but were also more often able to show up for work and leisure activities, compared with a control group assigned to a wait list, said Shmuel Odes, MD, a professor of internal medicine at Ben-Gurion University of the Negev in Beersheba, Israel. He presented the finding at Digestive Diseases Week® (DDW) 2022.
Psychological and social factors affect the gut and vice versa, Dr. Odes said. Yet many inflammatory bowel disease clinics overlook psychological interventions.
To address these issues, Dr. Odes and colleagues developed cognitive-behavioral– and mindfulness-based stress reduction (COBMINDEX) training, which can be taught by clinical social workers over the Internet. “The patient learns to relax,” Dr. Odes told MDedge News. “He learns not to fight his condition.”
In a previous paper, published in the journal Inflammatory Bowel Diseases, Dr. Odes and colleagues reported that patients who learned the technique showed improvement on a variety of psychological and quality-of-life measures, accompanied by changes in inflammatory cytokines and cortisol.
In a follow-up analysis presented here, the researchers looked at measures of pain and fatigue and then examined whether these were associated with productivity at work and other daily activities.
The study investigators randomly assigned 72 patients to an intervention group who got COBMINDEX training right away, and another 70 to a control group assigned to a wait list of 12 weeks before they could get the training. At baseline, the two groups were not significantly different in any demographic or clinical variable the researchers could find.
Social workers provided COBMINDEX training for the patients in seven 60-minute session over 12 weeks. Five of the sessions were devoted to cognitive-behavioral therapy and two to mindfulness-based stress reduction. The social workers asked the patients to do exercises at least once a day and report outcomes through an app.
Twelve patients dropped out of the COBMINDEX group and four dropped from the wait-list group because of lack of interest, time constraints, pregnancy, or illness.
The researchers created a composite score with a 0-15 scale (with higher scores indicating greater pain) from three pain items from the Harvey-Bradshaw Index for Crohn’s Disease, the Short Inflammatory Bowel Disease Questionnaire, and the 12-Item Short Form Survey.
To measure fatigue, they used the Functional Assessment of Chronic Illness Therapy-Fatigue, which has a 0-52 scale, with lower scores indicating greater fatigue.
To measure impairment while working and other daily activities, they used the Work Productivity and Activity Impairment Questionnaire: Crohn’s Disease. Scores on this measure are expressed as a percentage, with higher values indicating greater impairment.
Both the COBMINDEX and the wait-list groups improved on all these scales, but the improvements were significantly greater for the COBMINDEX group.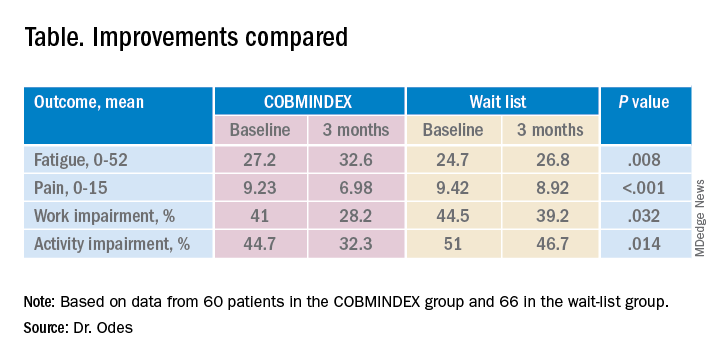
Through statistical analysis, the researchers found that the improvements in pain and fatigue indirectly caused the improvements in work and activity impairment, and that pain and fatigue improvements made independent contributions of similar magnitudes. COBMINDEX did not directly improve work or activity.
Psychological interventions are too often overlooked in Crohn’s disease, said the session comoderator Paul Moayyedi, MD, a professor of gastroenterology at McMaster University in Hamilton, Ont. “We need to realize how important this is to patients and urgently make this available,” he told MDedge.
A variety of interventions are being researched, and this study makes an important contribution, he said. However, he questioned whether people on a wait list can serve as an adequate control. “If you have to wait for something, you tend to have more pain, and you could have less productivity just because of waiting,” he said. “Ideally they should do a randomized trial with a sham intervention, not a wait list.”
Dr. Odes responded that it is very difficult to recruit people to a trial if they only have a 50% chance of getting a real treatment. And he noted that the people on the wait list in this trial did not show any signs of increased symptoms.
Physicians wanting to provide psychological help to their Crohn’s disease patients can refer them to social workers or psychotherapists, Dr. Odes said, but these professionals may lack training for applying cognitive-behavioral therapy and mindfulness-based stress reduction to patients with Crohn’s disease. His team hopes to make an app publicly available soon.
Neither Dr. Odes nor Dr. Moayyedi reported any relevant financial interests. The study was supported by a grant from the Leona M. and Harry B. Helmsley Charitable Trust.
SAN DIEGO – A combination of cognitive-behavioral therapy and mindfulness meditation could reduce pain and fatigue from Crohn’s disease, researchers say.
Patients who followed the program not only felt better but were also more often able to show up for work and leisure activities, compared with a control group assigned to a wait list, said Shmuel Odes, MD, a professor of internal medicine at Ben-Gurion University of the Negev in Beersheba, Israel. He presented the finding at Digestive Diseases Week® (DDW) 2022.
Psychological and social factors affect the gut and vice versa, Dr. Odes said. Yet many inflammatory bowel disease clinics overlook psychological interventions.
To address these issues, Dr. Odes and colleagues developed cognitive-behavioral– and mindfulness-based stress reduction (COBMINDEX) training, which can be taught by clinical social workers over the Internet. “The patient learns to relax,” Dr. Odes told MDedge News. “He learns not to fight his condition.”
In a previous paper, published in the journal Inflammatory Bowel Diseases, Dr. Odes and colleagues reported that patients who learned the technique showed improvement on a variety of psychological and quality-of-life measures, accompanied by changes in inflammatory cytokines and cortisol.
In a follow-up analysis presented here, the researchers looked at measures of pain and fatigue and then examined whether these were associated with productivity at work and other daily activities.
The study investigators randomly assigned 72 patients to an intervention group who got COBMINDEX training right away, and another 70 to a control group assigned to a wait list of 12 weeks before they could get the training. At baseline, the two groups were not significantly different in any demographic or clinical variable the researchers could find.
Social workers provided COBMINDEX training for the patients in seven 60-minute session over 12 weeks. Five of the sessions were devoted to cognitive-behavioral therapy and two to mindfulness-based stress reduction. The social workers asked the patients to do exercises at least once a day and report outcomes through an app.
Twelve patients dropped out of the COBMINDEX group and four dropped from the wait-list group because of lack of interest, time constraints, pregnancy, or illness.
The researchers created a composite score with a 0-15 scale (with higher scores indicating greater pain) from three pain items from the Harvey-Bradshaw Index for Crohn’s Disease, the Short Inflammatory Bowel Disease Questionnaire, and the 12-Item Short Form Survey.
To measure fatigue, they used the Functional Assessment of Chronic Illness Therapy-Fatigue, which has a 0-52 scale, with lower scores indicating greater fatigue.
To measure impairment while working and other daily activities, they used the Work Productivity and Activity Impairment Questionnaire: Crohn’s Disease. Scores on this measure are expressed as a percentage, with higher values indicating greater impairment.
Both the COBMINDEX and the wait-list groups improved on all these scales, but the improvements were significantly greater for the COBMINDEX group.
Through statistical analysis, the researchers found that the improvements in pain and fatigue indirectly caused the improvements in work and activity impairment, and that pain and fatigue improvements made independent contributions of similar magnitudes. COBMINDEX did not directly improve work or activity.
Psychological interventions are too often overlooked in Crohn’s disease, said the session comoderator Paul Moayyedi, MD, a professor of gastroenterology at McMaster University in Hamilton, Ont. “We need to realize how important this is to patients and urgently make this available,” he told MDedge.
A variety of interventions are being researched, and this study makes an important contribution, he said. However, he questioned whether people on a wait list can serve as an adequate control. “If you have to wait for something, you tend to have more pain, and you could have less productivity just because of waiting,” he said. “Ideally they should do a randomized trial with a sham intervention, not a wait list.”
Dr. Odes responded that it is very difficult to recruit people to a trial if they only have a 50% chance of getting a real treatment. And he noted that the people on the wait list in this trial did not show any signs of increased symptoms.
Physicians wanting to provide psychological help to their Crohn’s disease patients can refer them to social workers or psychotherapists, Dr. Odes said, but these professionals may lack training for applying cognitive-behavioral therapy and mindfulness-based stress reduction to patients with Crohn’s disease. His team hopes to make an app publicly available soon.
Neither Dr. Odes nor Dr. Moayyedi reported any relevant financial interests. The study was supported by a grant from the Leona M. and Harry B. Helmsley Charitable Trust.
SAN DIEGO – A combination of cognitive-behavioral therapy and mindfulness meditation could reduce pain and fatigue from Crohn’s disease, researchers say.
Patients who followed the program not only felt better but were also more often able to show up for work and leisure activities, compared with a control group assigned to a wait list, said Shmuel Odes, MD, a professor of internal medicine at Ben-Gurion University of the Negev in Beersheba, Israel. He presented the finding at Digestive Diseases Week® (DDW) 2022.
Psychological and social factors affect the gut and vice versa, Dr. Odes said. Yet many inflammatory bowel disease clinics overlook psychological interventions.
To address these issues, Dr. Odes and colleagues developed cognitive-behavioral– and mindfulness-based stress reduction (COBMINDEX) training, which can be taught by clinical social workers over the Internet. “The patient learns to relax,” Dr. Odes told MDedge News. “He learns not to fight his condition.”
In a previous paper, published in the journal Inflammatory Bowel Diseases, Dr. Odes and colleagues reported that patients who learned the technique showed improvement on a variety of psychological and quality-of-life measures, accompanied by changes in inflammatory cytokines and cortisol.
In a follow-up analysis presented here, the researchers looked at measures of pain and fatigue and then examined whether these were associated with productivity at work and other daily activities.
The study investigators randomly assigned 72 patients to an intervention group who got COBMINDEX training right away, and another 70 to a control group assigned to a wait list of 12 weeks before they could get the training. At baseline, the two groups were not significantly different in any demographic or clinical variable the researchers could find.
Social workers provided COBMINDEX training for the patients in seven 60-minute session over 12 weeks. Five of the sessions were devoted to cognitive-behavioral therapy and two to mindfulness-based stress reduction. The social workers asked the patients to do exercises at least once a day and report outcomes through an app.
Twelve patients dropped out of the COBMINDEX group and four dropped from the wait-list group because of lack of interest, time constraints, pregnancy, or illness.
The researchers created a composite score with a 0-15 scale (with higher scores indicating greater pain) from three pain items from the Harvey-Bradshaw Index for Crohn’s Disease, the Short Inflammatory Bowel Disease Questionnaire, and the 12-Item Short Form Survey.
To measure fatigue, they used the Functional Assessment of Chronic Illness Therapy-Fatigue, which has a 0-52 scale, with lower scores indicating greater fatigue.
To measure impairment while working and other daily activities, they used the Work Productivity and Activity Impairment Questionnaire: Crohn’s Disease. Scores on this measure are expressed as a percentage, with higher values indicating greater impairment.
Both the COBMINDEX and the wait-list groups improved on all these scales, but the improvements were significantly greater for the COBMINDEX group.
Through statistical analysis, the researchers found that the improvements in pain and fatigue indirectly caused the improvements in work and activity impairment, and that pain and fatigue improvements made independent contributions of similar magnitudes. COBMINDEX did not directly improve work or activity.
Psychological interventions are too often overlooked in Crohn’s disease, said the session comoderator Paul Moayyedi, MD, a professor of gastroenterology at McMaster University in Hamilton, Ont. “We need to realize how important this is to patients and urgently make this available,” he told MDedge.
A variety of interventions are being researched, and this study makes an important contribution, he said. However, he questioned whether people on a wait list can serve as an adequate control. “If you have to wait for something, you tend to have more pain, and you could have less productivity just because of waiting,” he said. “Ideally they should do a randomized trial with a sham intervention, not a wait list.”
Dr. Odes responded that it is very difficult to recruit people to a trial if they only have a 50% chance of getting a real treatment. And he noted that the people on the wait list in this trial did not show any signs of increased symptoms.
Physicians wanting to provide psychological help to their Crohn’s disease patients can refer them to social workers or psychotherapists, Dr. Odes said, but these professionals may lack training for applying cognitive-behavioral therapy and mindfulness-based stress reduction to patients with Crohn’s disease. His team hopes to make an app publicly available soon.
Neither Dr. Odes nor Dr. Moayyedi reported any relevant financial interests. The study was supported by a grant from the Leona M. and Harry B. Helmsley Charitable Trust.
AT DDW 2022
Medical trauma an under-recognized trigger for PTSD
NEW ORLEANS – Recent studies have confirmed that posttraumatic stress disorder can be triggered by health-related stress such as stints in the ICU and life-threatening medical emergencies, but most psychiatrists may not be aware of the latest research, according to an expert in mental trauma.
“This is true among children as well as adults, but it is not generally appreciated by psychiatrists and not at all by non-physicians,” said Charles B. Nemeroff, MD, PhD, professor and chair of the department of psychiatry and behavioral sciences at the University of Texas at Austin’s Dell Medical School, in a presentation at the annual meeting of the American Psychiatric Association. “It’s something that we all need to educate our colleagues about.”
As Dr. Nemeroff noted in a wide-ranging discussion about the latest trends in PTSD diagnosis and treatment, the DSM-5 doesn’t yet mention medical trauma in its definition of PTSD but refers more vaguely to triggering events that involve “actual or threatened death, serious injury, or sexual violence.”
However, multiple recent studies have linked medical trauma to PTSD. A 2019 study in Intensive Care Medicine found that 25% of 99 patients who were treated for emergency respiratory or cardiovascular crises showed PTSD symptoms at 6 months, and the percentage of childhood cancer survivors with PTSD was estimated at as high as 22%, according to research published in Frontiers in Psychology.In 2013, a meta-analysis suggested that 23% of stroke survivors have PTSD symptoms within 1 year, and 11% after 1 year.
PTSD is unique
Dr. Nemeroff noted that PTSD is the only diagnosis in the DSM-5 that’s directly linked to an environmental event. Specifically, he said, PTSD is caused by “very unexpected traumatic events that occur outside the normal repertoire of human behavior.”
In response, “most people that have an acute stress disorder response will fundamentally extinguish it and end up returning to the baseline level of functioning,” he said. But those with PTSD do not recover.
Dr. Nemeroff recommends the use of the 20-question self-report tool known as PCL-5. “It’s your friend,” he said. “It takes a few minutes for the patients to fill out while in the front office, and it doesn’t cost anything. Most patients who have PTSD will have a score of 50-55, maybe 60. You’re going to try to get them down to below 30, and you’re going to give this to them every time they come to your office to follow their progress. It works like a charm.”
As for treatment, psychotherapy and medications remain standard, he said, although “PTSD is a tough disorder to treat.”
According to him, brief cognitive behavioral therapy (CBT) – 4-5 sessions – has shown the greatest benefit and highest level of evidence in support when initiated within 4-30 days of trauma. Group therapy may be helpful, while it’s not clear if spiritual support and “psychological first aid” are useful during this time period.
There’s no evidence that medications such as SSRIs and atypical antipsychotics will prevent PTSD from developing; typical antipsychotics are not recommended. Individual or group “debriefing” is highly not recommended, Dr. Nemeroff said, because the experience can re-traumatize patients, as researchers learned after 9/11 when encouraging people to relive their experiences triggered PTSD and heartbreak.
Also not recommended: Benzodiazepines and formal psychotherapy in people without symptoms.
Exposure-based CBT has been proven to be successful, Dr. Nemeroff said, but it must be provided by a trained professional. “Going for a weekend course isn’t sufficient,” he said, and research suggests that group CBT is not as helpfulas individual CBT.
As for medication over the longer term, research supports SNRIs and SSRIs such as sertaline (Zoloft) and paroxetine (Paxil). Dr. Nemeroff is a fan of venlafaxine (Effexor): “It has a wide dose range. I can go from 75 to 150 milligrams at the low end and 450 and even 600 milligrams at the high end. I’ve had some amazing successes.”
In addition, atypical antipsychotics can be helpful in non-responders or psychotic PTSD patients, he said.
Dr. Nemeroff said he’s skeptical of ketamine as a treatment for PTSD, but he’s most hopeful about MDMA-assisted therapy due to “impressive data” regarding PTSD that was released last year. A bid for FDA approval is in the works, he said.
He added that data is promising from trials examining transcranial magnetic stimulationand (in work by his own team) electroconvulsive therapy. Both therapies are worth considering, he said.
Dr. Nemeroff reported multiple disclosures including research/grant support, stock holdings, scientific advisory board service, consulting relationships, board of director service, and patents.
NEW ORLEANS – Recent studies have confirmed that posttraumatic stress disorder can be triggered by health-related stress such as stints in the ICU and life-threatening medical emergencies, but most psychiatrists may not be aware of the latest research, according to an expert in mental trauma.
“This is true among children as well as adults, but it is not generally appreciated by psychiatrists and not at all by non-physicians,” said Charles B. Nemeroff, MD, PhD, professor and chair of the department of psychiatry and behavioral sciences at the University of Texas at Austin’s Dell Medical School, in a presentation at the annual meeting of the American Psychiatric Association. “It’s something that we all need to educate our colleagues about.”
As Dr. Nemeroff noted in a wide-ranging discussion about the latest trends in PTSD diagnosis and treatment, the DSM-5 doesn’t yet mention medical trauma in its definition of PTSD but refers more vaguely to triggering events that involve “actual or threatened death, serious injury, or sexual violence.”
However, multiple recent studies have linked medical trauma to PTSD. A 2019 study in Intensive Care Medicine found that 25% of 99 patients who were treated for emergency respiratory or cardiovascular crises showed PTSD symptoms at 6 months, and the percentage of childhood cancer survivors with PTSD was estimated at as high as 22%, according to research published in Frontiers in Psychology.In 2013, a meta-analysis suggested that 23% of stroke survivors have PTSD symptoms within 1 year, and 11% after 1 year.
PTSD is unique
Dr. Nemeroff noted that PTSD is the only diagnosis in the DSM-5 that’s directly linked to an environmental event. Specifically, he said, PTSD is caused by “very unexpected traumatic events that occur outside the normal repertoire of human behavior.”
In response, “most people that have an acute stress disorder response will fundamentally extinguish it and end up returning to the baseline level of functioning,” he said. But those with PTSD do not recover.
Dr. Nemeroff recommends the use of the 20-question self-report tool known as PCL-5. “It’s your friend,” he said. “It takes a few minutes for the patients to fill out while in the front office, and it doesn’t cost anything. Most patients who have PTSD will have a score of 50-55, maybe 60. You’re going to try to get them down to below 30, and you’re going to give this to them every time they come to your office to follow their progress. It works like a charm.”
As for treatment, psychotherapy and medications remain standard, he said, although “PTSD is a tough disorder to treat.”
According to him, brief cognitive behavioral therapy (CBT) – 4-5 sessions – has shown the greatest benefit and highest level of evidence in support when initiated within 4-30 days of trauma. Group therapy may be helpful, while it’s not clear if spiritual support and “psychological first aid” are useful during this time period.
There’s no evidence that medications such as SSRIs and atypical antipsychotics will prevent PTSD from developing; typical antipsychotics are not recommended. Individual or group “debriefing” is highly not recommended, Dr. Nemeroff said, because the experience can re-traumatize patients, as researchers learned after 9/11 when encouraging people to relive their experiences triggered PTSD and heartbreak.
Also not recommended: Benzodiazepines and formal psychotherapy in people without symptoms.
Exposure-based CBT has been proven to be successful, Dr. Nemeroff said, but it must be provided by a trained professional. “Going for a weekend course isn’t sufficient,” he said, and research suggests that group CBT is not as helpfulas individual CBT.
As for medication over the longer term, research supports SNRIs and SSRIs such as sertaline (Zoloft) and paroxetine (Paxil). Dr. Nemeroff is a fan of venlafaxine (Effexor): “It has a wide dose range. I can go from 75 to 150 milligrams at the low end and 450 and even 600 milligrams at the high end. I’ve had some amazing successes.”
In addition, atypical antipsychotics can be helpful in non-responders or psychotic PTSD patients, he said.
Dr. Nemeroff said he’s skeptical of ketamine as a treatment for PTSD, but he’s most hopeful about MDMA-assisted therapy due to “impressive data” regarding PTSD that was released last year. A bid for FDA approval is in the works, he said.
He added that data is promising from trials examining transcranial magnetic stimulationand (in work by his own team) electroconvulsive therapy. Both therapies are worth considering, he said.
Dr. Nemeroff reported multiple disclosures including research/grant support, stock holdings, scientific advisory board service, consulting relationships, board of director service, and patents.
NEW ORLEANS – Recent studies have confirmed that posttraumatic stress disorder can be triggered by health-related stress such as stints in the ICU and life-threatening medical emergencies, but most psychiatrists may not be aware of the latest research, according to an expert in mental trauma.
“This is true among children as well as adults, but it is not generally appreciated by psychiatrists and not at all by non-physicians,” said Charles B. Nemeroff, MD, PhD, professor and chair of the department of psychiatry and behavioral sciences at the University of Texas at Austin’s Dell Medical School, in a presentation at the annual meeting of the American Psychiatric Association. “It’s something that we all need to educate our colleagues about.”
As Dr. Nemeroff noted in a wide-ranging discussion about the latest trends in PTSD diagnosis and treatment, the DSM-5 doesn’t yet mention medical trauma in its definition of PTSD but refers more vaguely to triggering events that involve “actual or threatened death, serious injury, or sexual violence.”
However, multiple recent studies have linked medical trauma to PTSD. A 2019 study in Intensive Care Medicine found that 25% of 99 patients who were treated for emergency respiratory or cardiovascular crises showed PTSD symptoms at 6 months, and the percentage of childhood cancer survivors with PTSD was estimated at as high as 22%, according to research published in Frontiers in Psychology.In 2013, a meta-analysis suggested that 23% of stroke survivors have PTSD symptoms within 1 year, and 11% after 1 year.
PTSD is unique
Dr. Nemeroff noted that PTSD is the only diagnosis in the DSM-5 that’s directly linked to an environmental event. Specifically, he said, PTSD is caused by “very unexpected traumatic events that occur outside the normal repertoire of human behavior.”
In response, “most people that have an acute stress disorder response will fundamentally extinguish it and end up returning to the baseline level of functioning,” he said. But those with PTSD do not recover.
Dr. Nemeroff recommends the use of the 20-question self-report tool known as PCL-5. “It’s your friend,” he said. “It takes a few minutes for the patients to fill out while in the front office, and it doesn’t cost anything. Most patients who have PTSD will have a score of 50-55, maybe 60. You’re going to try to get them down to below 30, and you’re going to give this to them every time they come to your office to follow their progress. It works like a charm.”
As for treatment, psychotherapy and medications remain standard, he said, although “PTSD is a tough disorder to treat.”
According to him, brief cognitive behavioral therapy (CBT) – 4-5 sessions – has shown the greatest benefit and highest level of evidence in support when initiated within 4-30 days of trauma. Group therapy may be helpful, while it’s not clear if spiritual support and “psychological first aid” are useful during this time period.
There’s no evidence that medications such as SSRIs and atypical antipsychotics will prevent PTSD from developing; typical antipsychotics are not recommended. Individual or group “debriefing” is highly not recommended, Dr. Nemeroff said, because the experience can re-traumatize patients, as researchers learned after 9/11 when encouraging people to relive their experiences triggered PTSD and heartbreak.
Also not recommended: Benzodiazepines and formal psychotherapy in people without symptoms.
Exposure-based CBT has been proven to be successful, Dr. Nemeroff said, but it must be provided by a trained professional. “Going for a weekend course isn’t sufficient,” he said, and research suggests that group CBT is not as helpfulas individual CBT.
As for medication over the longer term, research supports SNRIs and SSRIs such as sertaline (Zoloft) and paroxetine (Paxil). Dr. Nemeroff is a fan of venlafaxine (Effexor): “It has a wide dose range. I can go from 75 to 150 milligrams at the low end and 450 and even 600 milligrams at the high end. I’ve had some amazing successes.”
In addition, atypical antipsychotics can be helpful in non-responders or psychotic PTSD patients, he said.
Dr. Nemeroff said he’s skeptical of ketamine as a treatment for PTSD, but he’s most hopeful about MDMA-assisted therapy due to “impressive data” regarding PTSD that was released last year. A bid for FDA approval is in the works, he said.
He added that data is promising from trials examining transcranial magnetic stimulationand (in work by his own team) electroconvulsive therapy. Both therapies are worth considering, he said.
Dr. Nemeroff reported multiple disclosures including research/grant support, stock holdings, scientific advisory board service, consulting relationships, board of director service, and patents.
at APA 2022
Telepsychiatry helped maintain standard of schizophrenia care during COVID
, new survey data show.
“Mental health centers rose to the challenge and did what they needed to do for their patients,” study investigator Dawn Velligan, PhD, University of Texas Health Science Center at San Antonio, told this news organization.
“Some decided to put patients on longer-acting injectable formulations. Some centers gave injections outside to make people feel safer,” Dr. Velligan said.
She added that other patients who might not have had transportation, or were too afraid to come in, were switched to oral medications. However, “switching to orals isn’t something that should be done lightly. I would only want patients to switch to orals as a last resort, but you do what you have to do,” Dr. Velligan said.
The findings were presented at the annual meeting of the American Psychiatric Association.
No going back?
When COVID hit, many mental health clinics closed for in-person visits. “This was unprecedented and we wanted to understand how clinics adapted their services and clinical management of patients with schizophrenia” on LAIs, Dr. Velligan said.
She and her colleagues surveyed 35 mental health clinics, with one respondent at each clinic, between October and November 2020.
All 35 clinics reported using telepsychiatry; 15 had been using telepsychiatry before the pandemic, while 20 (57%) began using it after COVID hit.
Across outpatient visit types, telepsychiatry use for noninjection visits rose from 12%-15% before the pandemic to 45%-69% after the pandemic.
In addition, patients were more apt to keep their telehealth visit. The frequency of appointment “no shows” and/or cancellations for telepsychiatry visits decreased by roughly one-third after the pandemic, compared with before the pandemic.
For patients with schizophrenia treated with LAIs, the frequency of telepsychiatry visits increased in 46% of the clinics during the pandemic.
For these patients, management options included switching patients from LAIs to oral antipsychotics in 34% of clinics and switching patients to LAIs with longer injection intervals in 31% of clinics.
Chief barriers to telepsychiatry visits were low reimbursement rate and lack of access to technology/reliable Internet.
Nearly all respondents reported being satisfied with the use of telepsychiatry to support patients with schizophrenia, whether treated with LAIs (94%) or with oral antipsychotics (97%).
Sixty percent of respondents reported no change in medication adherence for patients treated with LAIs since the start of the pandemic, while less than half (43%) reported no change in adherence to oral antipsychotics.
Most respondents (69%) felt that telepsychiatry visits would very likely continue to be used in combination with in-person office visits after the pandemic.
“Telemedicine is here to stay,” Dr. Velligan said.
Moving to a ‘hybrid universe’
Hector Colon-Rivera, MD, University of Pittsburgh Medical Center and president of the APA’s Hispanic Caucus, agrees.
Commenting on the findings, he noted that, because of shifts in care brought on by COVID, psychiatrists had to adopt telemedicine practices. As a result, many “now feel more comfortable” with telehealth visits for medication management and psychotherapy, said Dr. Colon-Rivera, who was not involved with the research.
He added this study is important because it shows that even patients with severe mental illness can be successfully managed with telepsychiatry, and with good adherence.
“Especially for patients with schizophrenia who have access issues, telepsychiatry is really helpful,” Dr. Colon-Rivera said.
“Telepsychiatry is becoming standard. Most clinics are moving to the hybrid universe now by having a telemedicine component and also seeing patients in person. Even places like emergency rooms and psychiatrists who do consults on medical floors are using telepsychiatry as an option,” he added.
Study funding was provided by Alkermes. Dr. Velligan has reported financial relationships with Alkermes, Otsuka, Janssen, and Lyndra. Dr. Colon-Rivera has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new survey data show.
“Mental health centers rose to the challenge and did what they needed to do for their patients,” study investigator Dawn Velligan, PhD, University of Texas Health Science Center at San Antonio, told this news organization.
“Some decided to put patients on longer-acting injectable formulations. Some centers gave injections outside to make people feel safer,” Dr. Velligan said.
She added that other patients who might not have had transportation, or were too afraid to come in, were switched to oral medications. However, “switching to orals isn’t something that should be done lightly. I would only want patients to switch to orals as a last resort, but you do what you have to do,” Dr. Velligan said.
The findings were presented at the annual meeting of the American Psychiatric Association.
No going back?
When COVID hit, many mental health clinics closed for in-person visits. “This was unprecedented and we wanted to understand how clinics adapted their services and clinical management of patients with schizophrenia” on LAIs, Dr. Velligan said.
She and her colleagues surveyed 35 mental health clinics, with one respondent at each clinic, between October and November 2020.
All 35 clinics reported using telepsychiatry; 15 had been using telepsychiatry before the pandemic, while 20 (57%) began using it after COVID hit.
Across outpatient visit types, telepsychiatry use for noninjection visits rose from 12%-15% before the pandemic to 45%-69% after the pandemic.
In addition, patients were more apt to keep their telehealth visit. The frequency of appointment “no shows” and/or cancellations for telepsychiatry visits decreased by roughly one-third after the pandemic, compared with before the pandemic.
For patients with schizophrenia treated with LAIs, the frequency of telepsychiatry visits increased in 46% of the clinics during the pandemic.
For these patients, management options included switching patients from LAIs to oral antipsychotics in 34% of clinics and switching patients to LAIs with longer injection intervals in 31% of clinics.
Chief barriers to telepsychiatry visits were low reimbursement rate and lack of access to technology/reliable Internet.
Nearly all respondents reported being satisfied with the use of telepsychiatry to support patients with schizophrenia, whether treated with LAIs (94%) or with oral antipsychotics (97%).
Sixty percent of respondents reported no change in medication adherence for patients treated with LAIs since the start of the pandemic, while less than half (43%) reported no change in adherence to oral antipsychotics.
Most respondents (69%) felt that telepsychiatry visits would very likely continue to be used in combination with in-person office visits after the pandemic.
“Telemedicine is here to stay,” Dr. Velligan said.
Moving to a ‘hybrid universe’
Hector Colon-Rivera, MD, University of Pittsburgh Medical Center and president of the APA’s Hispanic Caucus, agrees.
Commenting on the findings, he noted that, because of shifts in care brought on by COVID, psychiatrists had to adopt telemedicine practices. As a result, many “now feel more comfortable” with telehealth visits for medication management and psychotherapy, said Dr. Colon-Rivera, who was not involved with the research.
He added this study is important because it shows that even patients with severe mental illness can be successfully managed with telepsychiatry, and with good adherence.
“Especially for patients with schizophrenia who have access issues, telepsychiatry is really helpful,” Dr. Colon-Rivera said.
“Telepsychiatry is becoming standard. Most clinics are moving to the hybrid universe now by having a telemedicine component and also seeing patients in person. Even places like emergency rooms and psychiatrists who do consults on medical floors are using telepsychiatry as an option,” he added.
Study funding was provided by Alkermes. Dr. Velligan has reported financial relationships with Alkermes, Otsuka, Janssen, and Lyndra. Dr. Colon-Rivera has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new survey data show.
“Mental health centers rose to the challenge and did what they needed to do for their patients,” study investigator Dawn Velligan, PhD, University of Texas Health Science Center at San Antonio, told this news organization.
“Some decided to put patients on longer-acting injectable formulations. Some centers gave injections outside to make people feel safer,” Dr. Velligan said.
She added that other patients who might not have had transportation, or were too afraid to come in, were switched to oral medications. However, “switching to orals isn’t something that should be done lightly. I would only want patients to switch to orals as a last resort, but you do what you have to do,” Dr. Velligan said.
The findings were presented at the annual meeting of the American Psychiatric Association.
No going back?
When COVID hit, many mental health clinics closed for in-person visits. “This was unprecedented and we wanted to understand how clinics adapted their services and clinical management of patients with schizophrenia” on LAIs, Dr. Velligan said.
She and her colleagues surveyed 35 mental health clinics, with one respondent at each clinic, between October and November 2020.
All 35 clinics reported using telepsychiatry; 15 had been using telepsychiatry before the pandemic, while 20 (57%) began using it after COVID hit.
Across outpatient visit types, telepsychiatry use for noninjection visits rose from 12%-15% before the pandemic to 45%-69% after the pandemic.
In addition, patients were more apt to keep their telehealth visit. The frequency of appointment “no shows” and/or cancellations for telepsychiatry visits decreased by roughly one-third after the pandemic, compared with before the pandemic.
For patients with schizophrenia treated with LAIs, the frequency of telepsychiatry visits increased in 46% of the clinics during the pandemic.
For these patients, management options included switching patients from LAIs to oral antipsychotics in 34% of clinics and switching patients to LAIs with longer injection intervals in 31% of clinics.
Chief barriers to telepsychiatry visits were low reimbursement rate and lack of access to technology/reliable Internet.
Nearly all respondents reported being satisfied with the use of telepsychiatry to support patients with schizophrenia, whether treated with LAIs (94%) or with oral antipsychotics (97%).
Sixty percent of respondents reported no change in medication adherence for patients treated with LAIs since the start of the pandemic, while less than half (43%) reported no change in adherence to oral antipsychotics.
Most respondents (69%) felt that telepsychiatry visits would very likely continue to be used in combination with in-person office visits after the pandemic.
“Telemedicine is here to stay,” Dr. Velligan said.
Moving to a ‘hybrid universe’
Hector Colon-Rivera, MD, University of Pittsburgh Medical Center and president of the APA’s Hispanic Caucus, agrees.
Commenting on the findings, he noted that, because of shifts in care brought on by COVID, psychiatrists had to adopt telemedicine practices. As a result, many “now feel more comfortable” with telehealth visits for medication management and psychotherapy, said Dr. Colon-Rivera, who was not involved with the research.
He added this study is important because it shows that even patients with severe mental illness can be successfully managed with telepsychiatry, and with good adherence.
“Especially for patients with schizophrenia who have access issues, telepsychiatry is really helpful,” Dr. Colon-Rivera said.
“Telepsychiatry is becoming standard. Most clinics are moving to the hybrid universe now by having a telemedicine component and also seeing patients in person. Even places like emergency rooms and psychiatrists who do consults on medical floors are using telepsychiatry as an option,” he added.
Study funding was provided by Alkermes. Dr. Velligan has reported financial relationships with Alkermes, Otsuka, Janssen, and Lyndra. Dr. Colon-Rivera has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM APA 2022
Are docs getting fed up with hearing about burnout?
There is a feeling of exhaustion, being unable to shake a lingering cold, suffering from frequent headaches and gastrointestinal disturbances, sleeplessness and shortness of breath ...
That was how burnout was described by clinical psychologist Herbert Freudenberger, PhD, who first used the phrase in a paper back in 1974, after observing the emotional depletion and accompanying psychosomatic symptoms among volunteer staff of a free clinic in New York City. He called it “burnout,” a term borrowed from the slang of substance abusers.
It has now been established beyond a shadow of a doubt that burnout is a serious issue facing physicians across specialties, albeit some more intensely than others. But with the constant barrage of stories published on an almost daily basis, along with studies and surveys, it begs the question:
Some have suggested that the focus should be more on tackling burnout and instituting viable solutions rather than rehashing the problem.
There haven’t been studies or surveys on this question, but several experts have offered their opinion.
Jonathan Fisher, MD, a cardiologist and organizational well-being and resiliency leader at Novant Health, Charlotte, N.C., cautioned that he hesitates to speak about what physicians in general believe. “We are a diverse group of nearly 1 million in the United States alone,” he said.
But he noted that there is a specific phenomenon among burned-out health care providers who are “burned out on burnout.”
“Essentially, the underlying thought is ‘talk is cheap and we want action,’” said Dr. Fisher, who is chair and co-founder of the Ending Physician Burnout Global Summit that was held in 2021. “This reaction is often a reflection of disheartened physicians’ sense of hopelessness and cynicism that systemic change to improve working conditions will happen in our lifetime.”
Dr. Fisher explained that “typically, anyone suffering – physicians or nonphysicians – cares more about ending the suffering as soon as possible than learning its causes, but to alleviate suffering at its core – including the emotional suffering of burnout – we must understand the many causes.”
“To address both the organizational and individual drivers of burnout requires a keen awareness of the thoughts, fears, and dreams of physicians, health care executives, and all other stakeholders in health care,” he added.
Burnout, of course, is a very real problem. The 2022 Medscape Physician Burnout & Depression Report found that nearly half of all respondents (47%) said they are burned out, which was higher than the prior year. Perhaps not surprisingly, burnout among emergency physicians took the biggest leap, jumping from 43% in 2021 to 60% this year. More than half of critical care physicians (56%) also reported that they were burned out.
The World Health Organization’s International Classification of Diseases (ICD-11) – the official compendium of diseases – has categorized burnout as a “syndrome” that results from “chronic workplace stress that has not been successfully managed.” It is considered to be an occupational phenomenon and is not classified as a medical condition.
But whether or not physicians are burned out on hearing about burnout remains unclear. “I am not sure if physicians are tired of hearing about ‘burnout,’ but I do think that they want to hear about solutions that go beyond just telling them to take better care of themselves,” said Anne Thorndike, MD, MPH, an internal medicine physician at Massachusetts General Hospital and associate professor of medicine at Harvard Medical School, Boston. “There are major systematic factors that contribute to physicians burning out.”
Why talk about negative outcomes?
Jonathan Ripp, MD, MPH, however, is familiar with this sentiment. “‘Why do we keep identifying a problem without solutions’ is certainly a sentiment that is being expressed,” he said. “It’s a negative outcome, so why do we keep talking about negative outcomes?”
Dr. Ripp, who is a professor of medicine, medical education, and geriatrics and palliative medicine; the senior associate dean for well-being and resilience; and chief wellness officer at Icahn School of Medicine at Mount Sinai, New York, is also a well-known expert and researcher in burnout and physician well-being.
He noted that burnout was one of the first “tools” used as a metric to measure well-being, but it is a negative measurement. “It’s been around a long time, so it has a lot of evidence,” said Dr. Ripp. “But that said, there are other ways of measuring well-being without a negative association, and ways of measuring meaning in work – fulfillment and satisfaction, and so on. It should be balanced.”
But for the average physician not familiar with the long legacy of research, they may be frustrated by this situation. “Then they ask, ‘Why are you just showing me more of this instead of doing something about it?’ but we are actually doing something about it,” said Dr. Ripp.
There are many efforts underway, he explained, but it’s a challenging and complex issue. “There are numerous drivers impacting the well-being of any given segment within the health care workforce,” he said. “It will also vary by discipline and location, and there are also a host of individual factors that may have very little to do with the work environment. There are some very well-established efforts for an organizational approach, but it remains to be seen which is the most effective.”
But in broad strokes, he continued, it’s about tackling the system and not about making an individual more resilient. “Individuals that do engage in activities that improve resilience do better, but that’s not what this is about – it’s not going to solve the problem,” said Dr. Ripp. “Those of us like myself, who are working in this space, are trying to promote a culture of well-being – at the system level.”
The question is how to enable the workforce to do their best work in an efficient way so that the balance of their activities are not the meaningless aspects. “And instead, shoot that balance to the meaningful aspects of work,” he added. “There are enormous challenges, but even though we are working on solutions, I can see how the individual may not see that – they may say, ‘Stop telling me to be resilient, stop telling me there’s a problem,’ but we’re working on it.”
Moving medicine forward
James Jerzak, MD, a family physician in Green Bay, Wisc., and physician lead at Bellin Health, noted that “it seems to me that doctors aren’t burned out talking about burnout, but they are burned out hearing that the solution to burnout is simply for them to become more resilient,” he said. “In actuality, the path to dealing with this huge problem is to make meaningful systemic changes in how medicine is practiced.”
He reiterated that medical care has become increasingly complex, with the aging of the population; the increasing incidence of chronic diseases, such as diabetes; the challenges with the increasing cost of care, higher copays, and lack of health insurance for a large portion of the country; and general incivility toward health care workers that was exacerbated by the pandemic.
“This has all led to significantly increased stress levels for medical workers,” he said. “Couple all of that with the increased work involved in meeting the demands of the electronic health record, and it is clear that the current situation is unsustainable.”
In his own health care system, moving medicine forward has meant advancing team-based care, which translates to expanding teams to include adequate support for physicians. This strategy addressed problems in health care delivery, part of which is burnout.
“In many systems practicing advanced team-based care, the ancillary staff – medical assistants, LPNs, and RNs – play an enhanced role in the patient visit and perform functions such as quality care gap closure, medication review and refill pending, pending orders, and helping with documentation,” he said. “Although the current health care workforce shortages has created challenges, there are a lot of innovative approaches being tried [that are] aimed at providing solutions.”
The second key factor is for systems is to develop robust support for their providers with a broad range of team members, such as case managers, clinical pharmacists, diabetic educators, care coordinators, and others. “The day has passed where individual physicians can effectivity manage all of the complexities of care, especially since there are so many nonclinical factors affecting care,” said Dr. Jerzak.
“The recent focus on the social determinants of health and health equity underlies the fact that it truly takes a team of health care professionals working together to provide optimal care for patients,” he said.
Dr. Thorndike, who mentors premedical and medical trainees, has pointed out that burnout begins way before an individual enters the workplace as a doctor. Burnout begins in the earliest stages of medical practice, with the application process to medical school. The admissions process extends over a 12-month period, causing a great deal of “toxic stress.”
One study found that, compared with non-premedical students, premedical students had greater depression severity and emotional exhaustion.
“The current system of medical school admissions ignores the toll that the lengthy and emotionally exhausting process takes on aspiring physicians,” she said. “This is just one example of many in training and health care that requires physicians to set aside their own lives to achieve their goals and to provide the best possible care to others.”
A version of this article first appeared on Medscape.com.
There is a feeling of exhaustion, being unable to shake a lingering cold, suffering from frequent headaches and gastrointestinal disturbances, sleeplessness and shortness of breath ...
That was how burnout was described by clinical psychologist Herbert Freudenberger, PhD, who first used the phrase in a paper back in 1974, after observing the emotional depletion and accompanying psychosomatic symptoms among volunteer staff of a free clinic in New York City. He called it “burnout,” a term borrowed from the slang of substance abusers.
It has now been established beyond a shadow of a doubt that burnout is a serious issue facing physicians across specialties, albeit some more intensely than others. But with the constant barrage of stories published on an almost daily basis, along with studies and surveys, it begs the question:
Some have suggested that the focus should be more on tackling burnout and instituting viable solutions rather than rehashing the problem.
There haven’t been studies or surveys on this question, but several experts have offered their opinion.
Jonathan Fisher, MD, a cardiologist and organizational well-being and resiliency leader at Novant Health, Charlotte, N.C., cautioned that he hesitates to speak about what physicians in general believe. “We are a diverse group of nearly 1 million in the United States alone,” he said.
But he noted that there is a specific phenomenon among burned-out health care providers who are “burned out on burnout.”
“Essentially, the underlying thought is ‘talk is cheap and we want action,’” said Dr. Fisher, who is chair and co-founder of the Ending Physician Burnout Global Summit that was held in 2021. “This reaction is often a reflection of disheartened physicians’ sense of hopelessness and cynicism that systemic change to improve working conditions will happen in our lifetime.”
Dr. Fisher explained that “typically, anyone suffering – physicians or nonphysicians – cares more about ending the suffering as soon as possible than learning its causes, but to alleviate suffering at its core – including the emotional suffering of burnout – we must understand the many causes.”
“To address both the organizational and individual drivers of burnout requires a keen awareness of the thoughts, fears, and dreams of physicians, health care executives, and all other stakeholders in health care,” he added.
Burnout, of course, is a very real problem. The 2022 Medscape Physician Burnout & Depression Report found that nearly half of all respondents (47%) said they are burned out, which was higher than the prior year. Perhaps not surprisingly, burnout among emergency physicians took the biggest leap, jumping from 43% in 2021 to 60% this year. More than half of critical care physicians (56%) also reported that they were burned out.
The World Health Organization’s International Classification of Diseases (ICD-11) – the official compendium of diseases – has categorized burnout as a “syndrome” that results from “chronic workplace stress that has not been successfully managed.” It is considered to be an occupational phenomenon and is not classified as a medical condition.
But whether or not physicians are burned out on hearing about burnout remains unclear. “I am not sure if physicians are tired of hearing about ‘burnout,’ but I do think that they want to hear about solutions that go beyond just telling them to take better care of themselves,” said Anne Thorndike, MD, MPH, an internal medicine physician at Massachusetts General Hospital and associate professor of medicine at Harvard Medical School, Boston. “There are major systematic factors that contribute to physicians burning out.”
Why talk about negative outcomes?
Jonathan Ripp, MD, MPH, however, is familiar with this sentiment. “‘Why do we keep identifying a problem without solutions’ is certainly a sentiment that is being expressed,” he said. “It’s a negative outcome, so why do we keep talking about negative outcomes?”
Dr. Ripp, who is a professor of medicine, medical education, and geriatrics and palliative medicine; the senior associate dean for well-being and resilience; and chief wellness officer at Icahn School of Medicine at Mount Sinai, New York, is also a well-known expert and researcher in burnout and physician well-being.
He noted that burnout was one of the first “tools” used as a metric to measure well-being, but it is a negative measurement. “It’s been around a long time, so it has a lot of evidence,” said Dr. Ripp. “But that said, there are other ways of measuring well-being without a negative association, and ways of measuring meaning in work – fulfillment and satisfaction, and so on. It should be balanced.”
But for the average physician not familiar with the long legacy of research, they may be frustrated by this situation. “Then they ask, ‘Why are you just showing me more of this instead of doing something about it?’ but we are actually doing something about it,” said Dr. Ripp.
There are many efforts underway, he explained, but it’s a challenging and complex issue. “There are numerous drivers impacting the well-being of any given segment within the health care workforce,” he said. “It will also vary by discipline and location, and there are also a host of individual factors that may have very little to do with the work environment. There are some very well-established efforts for an organizational approach, but it remains to be seen which is the most effective.”
But in broad strokes, he continued, it’s about tackling the system and not about making an individual more resilient. “Individuals that do engage in activities that improve resilience do better, but that’s not what this is about – it’s not going to solve the problem,” said Dr. Ripp. “Those of us like myself, who are working in this space, are trying to promote a culture of well-being – at the system level.”
The question is how to enable the workforce to do their best work in an efficient way so that the balance of their activities are not the meaningless aspects. “And instead, shoot that balance to the meaningful aspects of work,” he added. “There are enormous challenges, but even though we are working on solutions, I can see how the individual may not see that – they may say, ‘Stop telling me to be resilient, stop telling me there’s a problem,’ but we’re working on it.”
Moving medicine forward
James Jerzak, MD, a family physician in Green Bay, Wisc., and physician lead at Bellin Health, noted that “it seems to me that doctors aren’t burned out talking about burnout, but they are burned out hearing that the solution to burnout is simply for them to become more resilient,” he said. “In actuality, the path to dealing with this huge problem is to make meaningful systemic changes in how medicine is practiced.”
He reiterated that medical care has become increasingly complex, with the aging of the population; the increasing incidence of chronic diseases, such as diabetes; the challenges with the increasing cost of care, higher copays, and lack of health insurance for a large portion of the country; and general incivility toward health care workers that was exacerbated by the pandemic.
“This has all led to significantly increased stress levels for medical workers,” he said. “Couple all of that with the increased work involved in meeting the demands of the electronic health record, and it is clear that the current situation is unsustainable.”
In his own health care system, moving medicine forward has meant advancing team-based care, which translates to expanding teams to include adequate support for physicians. This strategy addressed problems in health care delivery, part of which is burnout.
“In many systems practicing advanced team-based care, the ancillary staff – medical assistants, LPNs, and RNs – play an enhanced role in the patient visit and perform functions such as quality care gap closure, medication review and refill pending, pending orders, and helping with documentation,” he said. “Although the current health care workforce shortages has created challenges, there are a lot of innovative approaches being tried [that are] aimed at providing solutions.”
The second key factor is for systems is to develop robust support for their providers with a broad range of team members, such as case managers, clinical pharmacists, diabetic educators, care coordinators, and others. “The day has passed where individual physicians can effectivity manage all of the complexities of care, especially since there are so many nonclinical factors affecting care,” said Dr. Jerzak.
“The recent focus on the social determinants of health and health equity underlies the fact that it truly takes a team of health care professionals working together to provide optimal care for patients,” he said.
Dr. Thorndike, who mentors premedical and medical trainees, has pointed out that burnout begins way before an individual enters the workplace as a doctor. Burnout begins in the earliest stages of medical practice, with the application process to medical school. The admissions process extends over a 12-month period, causing a great deal of “toxic stress.”
One study found that, compared with non-premedical students, premedical students had greater depression severity and emotional exhaustion.
“The current system of medical school admissions ignores the toll that the lengthy and emotionally exhausting process takes on aspiring physicians,” she said. “This is just one example of many in training and health care that requires physicians to set aside their own lives to achieve their goals and to provide the best possible care to others.”
A version of this article first appeared on Medscape.com.
There is a feeling of exhaustion, being unable to shake a lingering cold, suffering from frequent headaches and gastrointestinal disturbances, sleeplessness and shortness of breath ...
That was how burnout was described by clinical psychologist Herbert Freudenberger, PhD, who first used the phrase in a paper back in 1974, after observing the emotional depletion and accompanying psychosomatic symptoms among volunteer staff of a free clinic in New York City. He called it “burnout,” a term borrowed from the slang of substance abusers.
It has now been established beyond a shadow of a doubt that burnout is a serious issue facing physicians across specialties, albeit some more intensely than others. But with the constant barrage of stories published on an almost daily basis, along with studies and surveys, it begs the question:
Some have suggested that the focus should be more on tackling burnout and instituting viable solutions rather than rehashing the problem.
There haven’t been studies or surveys on this question, but several experts have offered their opinion.
Jonathan Fisher, MD, a cardiologist and organizational well-being and resiliency leader at Novant Health, Charlotte, N.C., cautioned that he hesitates to speak about what physicians in general believe. “We are a diverse group of nearly 1 million in the United States alone,” he said.
But he noted that there is a specific phenomenon among burned-out health care providers who are “burned out on burnout.”
“Essentially, the underlying thought is ‘talk is cheap and we want action,’” said Dr. Fisher, who is chair and co-founder of the Ending Physician Burnout Global Summit that was held in 2021. “This reaction is often a reflection of disheartened physicians’ sense of hopelessness and cynicism that systemic change to improve working conditions will happen in our lifetime.”
Dr. Fisher explained that “typically, anyone suffering – physicians or nonphysicians – cares more about ending the suffering as soon as possible than learning its causes, but to alleviate suffering at its core – including the emotional suffering of burnout – we must understand the many causes.”
“To address both the organizational and individual drivers of burnout requires a keen awareness of the thoughts, fears, and dreams of physicians, health care executives, and all other stakeholders in health care,” he added.
Burnout, of course, is a very real problem. The 2022 Medscape Physician Burnout & Depression Report found that nearly half of all respondents (47%) said they are burned out, which was higher than the prior year. Perhaps not surprisingly, burnout among emergency physicians took the biggest leap, jumping from 43% in 2021 to 60% this year. More than half of critical care physicians (56%) also reported that they were burned out.
The World Health Organization’s International Classification of Diseases (ICD-11) – the official compendium of diseases – has categorized burnout as a “syndrome” that results from “chronic workplace stress that has not been successfully managed.” It is considered to be an occupational phenomenon and is not classified as a medical condition.
But whether or not physicians are burned out on hearing about burnout remains unclear. “I am not sure if physicians are tired of hearing about ‘burnout,’ but I do think that they want to hear about solutions that go beyond just telling them to take better care of themselves,” said Anne Thorndike, MD, MPH, an internal medicine physician at Massachusetts General Hospital and associate professor of medicine at Harvard Medical School, Boston. “There are major systematic factors that contribute to physicians burning out.”
Why talk about negative outcomes?
Jonathan Ripp, MD, MPH, however, is familiar with this sentiment. “‘Why do we keep identifying a problem without solutions’ is certainly a sentiment that is being expressed,” he said. “It’s a negative outcome, so why do we keep talking about negative outcomes?”
Dr. Ripp, who is a professor of medicine, medical education, and geriatrics and palliative medicine; the senior associate dean for well-being and resilience; and chief wellness officer at Icahn School of Medicine at Mount Sinai, New York, is also a well-known expert and researcher in burnout and physician well-being.
He noted that burnout was one of the first “tools” used as a metric to measure well-being, but it is a negative measurement. “It’s been around a long time, so it has a lot of evidence,” said Dr. Ripp. “But that said, there are other ways of measuring well-being without a negative association, and ways of measuring meaning in work – fulfillment and satisfaction, and so on. It should be balanced.”
But for the average physician not familiar with the long legacy of research, they may be frustrated by this situation. “Then they ask, ‘Why are you just showing me more of this instead of doing something about it?’ but we are actually doing something about it,” said Dr. Ripp.
There are many efforts underway, he explained, but it’s a challenging and complex issue. “There are numerous drivers impacting the well-being of any given segment within the health care workforce,” he said. “It will also vary by discipline and location, and there are also a host of individual factors that may have very little to do with the work environment. There are some very well-established efforts for an organizational approach, but it remains to be seen which is the most effective.”
But in broad strokes, he continued, it’s about tackling the system and not about making an individual more resilient. “Individuals that do engage in activities that improve resilience do better, but that’s not what this is about – it’s not going to solve the problem,” said Dr. Ripp. “Those of us like myself, who are working in this space, are trying to promote a culture of well-being – at the system level.”
The question is how to enable the workforce to do their best work in an efficient way so that the balance of their activities are not the meaningless aspects. “And instead, shoot that balance to the meaningful aspects of work,” he added. “There are enormous challenges, but even though we are working on solutions, I can see how the individual may not see that – they may say, ‘Stop telling me to be resilient, stop telling me there’s a problem,’ but we’re working on it.”
Moving medicine forward
James Jerzak, MD, a family physician in Green Bay, Wisc., and physician lead at Bellin Health, noted that “it seems to me that doctors aren’t burned out talking about burnout, but they are burned out hearing that the solution to burnout is simply for them to become more resilient,” he said. “In actuality, the path to dealing with this huge problem is to make meaningful systemic changes in how medicine is practiced.”
He reiterated that medical care has become increasingly complex, with the aging of the population; the increasing incidence of chronic diseases, such as diabetes; the challenges with the increasing cost of care, higher copays, and lack of health insurance for a large portion of the country; and general incivility toward health care workers that was exacerbated by the pandemic.
“This has all led to significantly increased stress levels for medical workers,” he said. “Couple all of that with the increased work involved in meeting the demands of the electronic health record, and it is clear that the current situation is unsustainable.”
In his own health care system, moving medicine forward has meant advancing team-based care, which translates to expanding teams to include adequate support for physicians. This strategy addressed problems in health care delivery, part of which is burnout.
“In many systems practicing advanced team-based care, the ancillary staff – medical assistants, LPNs, and RNs – play an enhanced role in the patient visit and perform functions such as quality care gap closure, medication review and refill pending, pending orders, and helping with documentation,” he said. “Although the current health care workforce shortages has created challenges, there are a lot of innovative approaches being tried [that are] aimed at providing solutions.”
The second key factor is for systems is to develop robust support for their providers with a broad range of team members, such as case managers, clinical pharmacists, diabetic educators, care coordinators, and others. “The day has passed where individual physicians can effectivity manage all of the complexities of care, especially since there are so many nonclinical factors affecting care,” said Dr. Jerzak.
“The recent focus on the social determinants of health and health equity underlies the fact that it truly takes a team of health care professionals working together to provide optimal care for patients,” he said.
Dr. Thorndike, who mentors premedical and medical trainees, has pointed out that burnout begins way before an individual enters the workplace as a doctor. Burnout begins in the earliest stages of medical practice, with the application process to medical school. The admissions process extends over a 12-month period, causing a great deal of “toxic stress.”
One study found that, compared with non-premedical students, premedical students had greater depression severity and emotional exhaustion.
“The current system of medical school admissions ignores the toll that the lengthy and emotionally exhausting process takes on aspiring physicians,” she said. “This is just one example of many in training and health care that requires physicians to set aside their own lives to achieve their goals and to provide the best possible care to others.”
A version of this article first appeared on Medscape.com.
Very high HDL-C: Too much of a good thing?
A new study suggests that .
Investigators studied close to 10,000 patients with CAD in two separate cohorts. After adjusting for an array of covariates, they found that individuals with HDL-C levels greater than 80 mg/dL had a 96% higher risk for all-cause mortality and a 71% higher risk for cardiovascular mortality than those with HDL-C levels between 40 and 60 mg/dL.
A U-shaped association was found, with higher risk for all-cause and cardiovascular mortality in patients with both very low and very high, compared with midrange, HDL-C values.
“Very high HDL levels are associated with increased risk of adverse outcomes, not lower risk, as previously thought. This is true not only in the general population, but also in people with known coronary artery disease,” senior author Arshed A. Quyyumi, MD, professor of medicine, division of cardiology, Emory University, Atlanta, told this news organization.
“Physicians have to be cognizant of the fact that, at levels of HDL-C above 80 mg/dL, they [should be] more aggressive with risk reduction and not believe that the patient is at ‘low risk’ because of high levels of ‘good’ cholesterol,” said Dr. Quyyumi, director of the Emory Clinical Cardiovascular Research Institute.
The study was published online in JAMA Cardiology.
Inverse association?
HDL-C levels have “historically been inversely associated with increased cardiovascular disease (CVD) risk; however, recent studies have questioned the efficacy of therapies designed to increase HDL-C levels,” the authors wrote. Moreover, genetic variants associated with HDL-C have not been found to be linked to CVD risk.
Whether “very high HDL-C levels in patients with coronary artery disease (CAD) are associated with mortality risk remains unknown,” they wrote. In this study, the researchers investigated not only the potential risk of elevated HDL-C levels in these patients, but also the association of known HDL-C genetic variants with this risk.
To do so, they analyzed data from a subset of patients with CAD in two independent study groups: the UK Biobank (UKB; n = 14,478; mean [standard deviation] age, 61.2 [5.8] years; 76.2% male; 93.8% White) and the Emory Cardiovascular Biobank (EmCAB; n = 5,467; mean age, 63.8 [12.3] years; 66.4% male; 73.2% White). Participants were followed prospectively for a median of 8.9 (interquartile range, 8.0-9.7) years and 6.7 (IQR, 4.0-10.8) years, respectively.
Additional data collected included medical and medication history and demographic characteristics, which were used as covariates, as well as genomic information.
Of the UKB cohort, 12.4% and 7.9% sustained all-cause or cardiovascular death, respectively, during the follow-up period, and 1.8% of participants had an HDL-C level above 80 mg/dL.
Among these participants with very high HDL-C levels, 16.9% and 8.6% had all-cause or cardiovascular death, respectively. Compared with the reference category (HDL-C level of 40-60 mg/dL), those with low HDL-C levels (≤ 30 mg/dL) had an expected higher risk for both all-cause and cardiovascular mortality, even after adjustment for covariates (hazard ratio, 1.33; 95% confidence interval, 1.07-1.64 and HR, 1.42; 95% CI, 1.09-1.85, respectively; P = .009).
“Importantly,” the authors stated, “compared with the reference category, individuals with very high HDL-C levels (>80 mg/dL) also had a higher risk of all-cause death (HR, 1.58 [1.16-2.14], P = .004).”
Although cardiovascular death rates were not significantly greater in unadjusted analyses, after adjustment, the highest HDL-C group had an increased risk for all-cause and cardiovascular death (HR, 1.96; 95% CI, 1.42-2.71; P < .001 and HR, 1.71; 95% CI, 1.09-2.68, respectively; P = .02).
Compared with females, males with HDL-C levels above 80 mg/dL had a higher risk for all-cause and cardiovascular death.
Similar findings were obtained in the EmCAB patients, 1.6% of whom had HDL-C levels above 80 mg/dL. During the follow-up period, 26.9% and 13.8% of participants sustained all-cause and cardiovascular death, respectively. Of those with HDL-C levels above 80 mg/dL, 30.0% and 16.7% experienced all-cause and cardiovascular death, respectively.
Compared with those with HDL-C levels of 40-60 mg/dL, those in the lowest (≤30 mg/dL) and highest (>80 mg/dL) groups had a “significant or near-significant greater risk for all-cause death in both unadjusted and fully adjusted models.
“Using adjusted HR curves, a U-shaped association between HDL-C and adverse events was evident with higher mortality at both very high and low HDL-C levels,” the authors noted.
Compared with patients without diabetes, those with diabetes and an HDL-C level above 80 mg/dL had a higher risk for all-cause and cardiovascular death, and patients younger than 65 years had a higher risk for cardiovascular death than patients 65 years and older.
The researchers found a “positive linear association” between the HDL-C genetic risk score (GRS) and HDL levels, wherein a 1-SD higher HDL-C GRS was associated with a 3.03 mg/dL higher HDL-C level (2.83-3.22; P < .001; R 2 = 0.06).
The HDL-C GRS was not associated with the risk for all-cause or cardiovascular death in unadjusted models, and after the HDL-C GRS was added to the fully adjusted models, the association with HDL-C level above 80 mg/dL was not attenuated, “indicating that HDL-C genetic variations in the GRS do not contribute substantially to the risk.”
“Potential mechanisms through which very high HDL-C might cause adverse cardiovascular outcomes in patients with CAD need to be studied,” Dr. Quyyumi said. “Whether the functional capacity of the HDL particle is altered when the level is very high remains unknown. Whether it is more able to oxidize and thus shift from being protective to harmful also needs to be investigated.”
Red flag
Commenting for this news organization, Sadiya Sana Khan, MD, MSc, assistant professor of medicine (cardiology) and preventive medicine (epidemiology), Northwestern University, Chicago, said: “I think the most important point [of the study] is to identify people with very high HDL-C. This can serve as a reminder to discuss heart-healthy lifestyles and discussion of statin therapy if needed, based on LDL-C.”
In an accompanying editorial coauthored with Gregg Fonarow, MD, Ahmanson-UCLA Cardiomyopathy Center, University of California, Los Angeles, the pair wrote: “Although the present findings may be related to residual confounding, high HDL-C levels should not automatically be assumed to be protective.”
They advised clinicians to “use HDL-C levels as a surrogate marker, with very low and very high levels as a red flag to target for more intensive primary and secondary prevention, as the maxim for HDL-C as ‘good’ cholesterol only holds for HDL-C levels of 80 mg/dL or less.”
This study was supported in part by grants from the National Institutes of Health, the American Heart Association, and the Abraham J. & Phyllis Katz Foundation. Dr. Quyyumi and coauthors report no relevant financial relationships. Dr. Khan reports receiving grants from the American Heart Association and the National Institutes of Health outside the submitted work. Dr. Fonarow reports receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
A new study suggests that .
Investigators studied close to 10,000 patients with CAD in two separate cohorts. After adjusting for an array of covariates, they found that individuals with HDL-C levels greater than 80 mg/dL had a 96% higher risk for all-cause mortality and a 71% higher risk for cardiovascular mortality than those with HDL-C levels between 40 and 60 mg/dL.
A U-shaped association was found, with higher risk for all-cause and cardiovascular mortality in patients with both very low and very high, compared with midrange, HDL-C values.
“Very high HDL levels are associated with increased risk of adverse outcomes, not lower risk, as previously thought. This is true not only in the general population, but also in people with known coronary artery disease,” senior author Arshed A. Quyyumi, MD, professor of medicine, division of cardiology, Emory University, Atlanta, told this news organization.
“Physicians have to be cognizant of the fact that, at levels of HDL-C above 80 mg/dL, they [should be] more aggressive with risk reduction and not believe that the patient is at ‘low risk’ because of high levels of ‘good’ cholesterol,” said Dr. Quyyumi, director of the Emory Clinical Cardiovascular Research Institute.
The study was published online in JAMA Cardiology.
Inverse association?
HDL-C levels have “historically been inversely associated with increased cardiovascular disease (CVD) risk; however, recent studies have questioned the efficacy of therapies designed to increase HDL-C levels,” the authors wrote. Moreover, genetic variants associated with HDL-C have not been found to be linked to CVD risk.
Whether “very high HDL-C levels in patients with coronary artery disease (CAD) are associated with mortality risk remains unknown,” they wrote. In this study, the researchers investigated not only the potential risk of elevated HDL-C levels in these patients, but also the association of known HDL-C genetic variants with this risk.
To do so, they analyzed data from a subset of patients with CAD in two independent study groups: the UK Biobank (UKB; n = 14,478; mean [standard deviation] age, 61.2 [5.8] years; 76.2% male; 93.8% White) and the Emory Cardiovascular Biobank (EmCAB; n = 5,467; mean age, 63.8 [12.3] years; 66.4% male; 73.2% White). Participants were followed prospectively for a median of 8.9 (interquartile range, 8.0-9.7) years and 6.7 (IQR, 4.0-10.8) years, respectively.
Additional data collected included medical and medication history and demographic characteristics, which were used as covariates, as well as genomic information.
Of the UKB cohort, 12.4% and 7.9% sustained all-cause or cardiovascular death, respectively, during the follow-up period, and 1.8% of participants had an HDL-C level above 80 mg/dL.
Among these participants with very high HDL-C levels, 16.9% and 8.6% had all-cause or cardiovascular death, respectively. Compared with the reference category (HDL-C level of 40-60 mg/dL), those with low HDL-C levels (≤ 30 mg/dL) had an expected higher risk for both all-cause and cardiovascular mortality, even after adjustment for covariates (hazard ratio, 1.33; 95% confidence interval, 1.07-1.64 and HR, 1.42; 95% CI, 1.09-1.85, respectively; P = .009).
“Importantly,” the authors stated, “compared with the reference category, individuals with very high HDL-C levels (>80 mg/dL) also had a higher risk of all-cause death (HR, 1.58 [1.16-2.14], P = .004).”
Although cardiovascular death rates were not significantly greater in unadjusted analyses, after adjustment, the highest HDL-C group had an increased risk for all-cause and cardiovascular death (HR, 1.96; 95% CI, 1.42-2.71; P < .001 and HR, 1.71; 95% CI, 1.09-2.68, respectively; P = .02).
Compared with females, males with HDL-C levels above 80 mg/dL had a higher risk for all-cause and cardiovascular death.
Similar findings were obtained in the EmCAB patients, 1.6% of whom had HDL-C levels above 80 mg/dL. During the follow-up period, 26.9% and 13.8% of participants sustained all-cause and cardiovascular death, respectively. Of those with HDL-C levels above 80 mg/dL, 30.0% and 16.7% experienced all-cause and cardiovascular death, respectively.
Compared with those with HDL-C levels of 40-60 mg/dL, those in the lowest (≤30 mg/dL) and highest (>80 mg/dL) groups had a “significant or near-significant greater risk for all-cause death in both unadjusted and fully adjusted models.
“Using adjusted HR curves, a U-shaped association between HDL-C and adverse events was evident with higher mortality at both very high and low HDL-C levels,” the authors noted.
Compared with patients without diabetes, those with diabetes and an HDL-C level above 80 mg/dL had a higher risk for all-cause and cardiovascular death, and patients younger than 65 years had a higher risk for cardiovascular death than patients 65 years and older.
The researchers found a “positive linear association” between the HDL-C genetic risk score (GRS) and HDL levels, wherein a 1-SD higher HDL-C GRS was associated with a 3.03 mg/dL higher HDL-C level (2.83-3.22; P < .001; R 2 = 0.06).
The HDL-C GRS was not associated with the risk for all-cause or cardiovascular death in unadjusted models, and after the HDL-C GRS was added to the fully adjusted models, the association with HDL-C level above 80 mg/dL was not attenuated, “indicating that HDL-C genetic variations in the GRS do not contribute substantially to the risk.”
“Potential mechanisms through which very high HDL-C might cause adverse cardiovascular outcomes in patients with CAD need to be studied,” Dr. Quyyumi said. “Whether the functional capacity of the HDL particle is altered when the level is very high remains unknown. Whether it is more able to oxidize and thus shift from being protective to harmful also needs to be investigated.”
Red flag
Commenting for this news organization, Sadiya Sana Khan, MD, MSc, assistant professor of medicine (cardiology) and preventive medicine (epidemiology), Northwestern University, Chicago, said: “I think the most important point [of the study] is to identify people with very high HDL-C. This can serve as a reminder to discuss heart-healthy lifestyles and discussion of statin therapy if needed, based on LDL-C.”
In an accompanying editorial coauthored with Gregg Fonarow, MD, Ahmanson-UCLA Cardiomyopathy Center, University of California, Los Angeles, the pair wrote: “Although the present findings may be related to residual confounding, high HDL-C levels should not automatically be assumed to be protective.”
They advised clinicians to “use HDL-C levels as a surrogate marker, with very low and very high levels as a red flag to target for more intensive primary and secondary prevention, as the maxim for HDL-C as ‘good’ cholesterol only holds for HDL-C levels of 80 mg/dL or less.”
This study was supported in part by grants from the National Institutes of Health, the American Heart Association, and the Abraham J. & Phyllis Katz Foundation. Dr. Quyyumi and coauthors report no relevant financial relationships. Dr. Khan reports receiving grants from the American Heart Association and the National Institutes of Health outside the submitted work. Dr. Fonarow reports receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
A new study suggests that .
Investigators studied close to 10,000 patients with CAD in two separate cohorts. After adjusting for an array of covariates, they found that individuals with HDL-C levels greater than 80 mg/dL had a 96% higher risk for all-cause mortality and a 71% higher risk for cardiovascular mortality than those with HDL-C levels between 40 and 60 mg/dL.
A U-shaped association was found, with higher risk for all-cause and cardiovascular mortality in patients with both very low and very high, compared with midrange, HDL-C values.
“Very high HDL levels are associated with increased risk of adverse outcomes, not lower risk, as previously thought. This is true not only in the general population, but also in people with known coronary artery disease,” senior author Arshed A. Quyyumi, MD, professor of medicine, division of cardiology, Emory University, Atlanta, told this news organization.
“Physicians have to be cognizant of the fact that, at levels of HDL-C above 80 mg/dL, they [should be] more aggressive with risk reduction and not believe that the patient is at ‘low risk’ because of high levels of ‘good’ cholesterol,” said Dr. Quyyumi, director of the Emory Clinical Cardiovascular Research Institute.
The study was published online in JAMA Cardiology.
Inverse association?
HDL-C levels have “historically been inversely associated with increased cardiovascular disease (CVD) risk; however, recent studies have questioned the efficacy of therapies designed to increase HDL-C levels,” the authors wrote. Moreover, genetic variants associated with HDL-C have not been found to be linked to CVD risk.
Whether “very high HDL-C levels in patients with coronary artery disease (CAD) are associated with mortality risk remains unknown,” they wrote. In this study, the researchers investigated not only the potential risk of elevated HDL-C levels in these patients, but also the association of known HDL-C genetic variants with this risk.
To do so, they analyzed data from a subset of patients with CAD in two independent study groups: the UK Biobank (UKB; n = 14,478; mean [standard deviation] age, 61.2 [5.8] years; 76.2% male; 93.8% White) and the Emory Cardiovascular Biobank (EmCAB; n = 5,467; mean age, 63.8 [12.3] years; 66.4% male; 73.2% White). Participants were followed prospectively for a median of 8.9 (interquartile range, 8.0-9.7) years and 6.7 (IQR, 4.0-10.8) years, respectively.
Additional data collected included medical and medication history and demographic characteristics, which were used as covariates, as well as genomic information.
Of the UKB cohort, 12.4% and 7.9% sustained all-cause or cardiovascular death, respectively, during the follow-up period, and 1.8% of participants had an HDL-C level above 80 mg/dL.
Among these participants with very high HDL-C levels, 16.9% and 8.6% had all-cause or cardiovascular death, respectively. Compared with the reference category (HDL-C level of 40-60 mg/dL), those with low HDL-C levels (≤ 30 mg/dL) had an expected higher risk for both all-cause and cardiovascular mortality, even after adjustment for covariates (hazard ratio, 1.33; 95% confidence interval, 1.07-1.64 and HR, 1.42; 95% CI, 1.09-1.85, respectively; P = .009).
“Importantly,” the authors stated, “compared with the reference category, individuals with very high HDL-C levels (>80 mg/dL) also had a higher risk of all-cause death (HR, 1.58 [1.16-2.14], P = .004).”
Although cardiovascular death rates were not significantly greater in unadjusted analyses, after adjustment, the highest HDL-C group had an increased risk for all-cause and cardiovascular death (HR, 1.96; 95% CI, 1.42-2.71; P < .001 and HR, 1.71; 95% CI, 1.09-2.68, respectively; P = .02).
Compared with females, males with HDL-C levels above 80 mg/dL had a higher risk for all-cause and cardiovascular death.
Similar findings were obtained in the EmCAB patients, 1.6% of whom had HDL-C levels above 80 mg/dL. During the follow-up period, 26.9% and 13.8% of participants sustained all-cause and cardiovascular death, respectively. Of those with HDL-C levels above 80 mg/dL, 30.0% and 16.7% experienced all-cause and cardiovascular death, respectively.
Compared with those with HDL-C levels of 40-60 mg/dL, those in the lowest (≤30 mg/dL) and highest (>80 mg/dL) groups had a “significant or near-significant greater risk for all-cause death in both unadjusted and fully adjusted models.
“Using adjusted HR curves, a U-shaped association between HDL-C and adverse events was evident with higher mortality at both very high and low HDL-C levels,” the authors noted.
Compared with patients without diabetes, those with diabetes and an HDL-C level above 80 mg/dL had a higher risk for all-cause and cardiovascular death, and patients younger than 65 years had a higher risk for cardiovascular death than patients 65 years and older.
The researchers found a “positive linear association” between the HDL-C genetic risk score (GRS) and HDL levels, wherein a 1-SD higher HDL-C GRS was associated with a 3.03 mg/dL higher HDL-C level (2.83-3.22; P < .001; R 2 = 0.06).
The HDL-C GRS was not associated with the risk for all-cause or cardiovascular death in unadjusted models, and after the HDL-C GRS was added to the fully adjusted models, the association with HDL-C level above 80 mg/dL was not attenuated, “indicating that HDL-C genetic variations in the GRS do not contribute substantially to the risk.”
“Potential mechanisms through which very high HDL-C might cause adverse cardiovascular outcomes in patients with CAD need to be studied,” Dr. Quyyumi said. “Whether the functional capacity of the HDL particle is altered when the level is very high remains unknown. Whether it is more able to oxidize and thus shift from being protective to harmful also needs to be investigated.”
Red flag
Commenting for this news organization, Sadiya Sana Khan, MD, MSc, assistant professor of medicine (cardiology) and preventive medicine (epidemiology), Northwestern University, Chicago, said: “I think the most important point [of the study] is to identify people with very high HDL-C. This can serve as a reminder to discuss heart-healthy lifestyles and discussion of statin therapy if needed, based on LDL-C.”
In an accompanying editorial coauthored with Gregg Fonarow, MD, Ahmanson-UCLA Cardiomyopathy Center, University of California, Los Angeles, the pair wrote: “Although the present findings may be related to residual confounding, high HDL-C levels should not automatically be assumed to be protective.”
They advised clinicians to “use HDL-C levels as a surrogate marker, with very low and very high levels as a red flag to target for more intensive primary and secondary prevention, as the maxim for HDL-C as ‘good’ cholesterol only holds for HDL-C levels of 80 mg/dL or less.”
This study was supported in part by grants from the National Institutes of Health, the American Heart Association, and the Abraham J. & Phyllis Katz Foundation. Dr. Quyyumi and coauthors report no relevant financial relationships. Dr. Khan reports receiving grants from the American Heart Association and the National Institutes of Health outside the submitted work. Dr. Fonarow reports receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
Race-, ethnicity-based clinical guidelines miss the mark: Study
SAN DIEGO – Race-based recommendations and clinical algorithms may be doing more harm than good, according to a systematic review of databases and guidelines.
The study found examples of screening recommendations based on race or ethnicity that were likely misleading since these are social constructs that don’t reflect a patient’s individual risk, said Shazia Siddique, MD, who presented the study at the annual Digestive Disease Week® (DDW). “Historically, we’ve made so many clinical decisions based on somebody’s race and ethnicity. We walk into a room, we don’t even ask people which racial or ethnic category they identify with. We just look at them and we say, ‘Their skin color looks black, and therefore we’re going to apply a different equation to them.’ ”
However, a patient’s risks and unique health circumstances are much more complicated than that. They may be related to genetics, or environmental exposures, or level of access to quality health care. Race can often be inappropriately used as a stand-in for these and other factors, she explained.
“These [racial] categories are truly a social construct. It’s becoming very problematic that people are literally making decisions based on somebody’s skin color. That’s just not what the science supports. If there are specific genes or environmental factors, or differences in access to health care that then impact outcomes for certain racial or ethnic groups, we need to figure out what those are,” said Dr. Siddique, who is an assistant professor of medicine at the University of Pennsylvania, Philadelphia.
Those messages are still entrenched in medical education. “I graduated medical school in 2012, and it was taught to me to use race and ethnicity in clinical decision-making. We need to start in medical education to shift the way that we’re thinking. On the research side, we really need to think about how we can replace or remove race and ethnicity and understand the consequences of that, so that over time we can make a shift,” said Dr. Siddique.
For example, Dr. Siddique discussed recommendations that suggest Asian heritage as a risk factor for hepatitis B screening, but that’s not a good factor to consider: “People were saying that Asians should be screened at an earlier age, but it’s really people that were born and raised in Asian countries where it’s endemic or they may have gotten it from their mothers at birth. It’s a marker for how long you have had the disease and how much virus is in your bloodstream. It’s not because you’re Asian. If you’re born and raised in the United States, and you don’t have any of those risk factors, you shouldn’t be treated differently based on your identified racial and ethnic group,” said Dr. Siddique.
These questions have become even more important in recent years because of patients with multiracial identifies and other considerations. “Now the proxy for which race was being used is even messier,” said Dr. Siddique.
So, how should physicians think about assessing a patient’s personalized risks? The key, said Dr. Siddique, is to look at each patient’s individual factors, such as health care access, environmental exposures from jobs or living conditions, or the country they emigrated from if they weren’t born in the United States. “Disease prevalences are different in different areas, and that changes your index of suspicion,” she said.
And when considering current guidelines that incorporate race or ethnicity, she recommends viewing them skeptically: “If there is a current algorithm in your health system or in a guideline that you’re reading that says you should be making a change based on race and ethnicity, you should look at that with a close eye and say, “What do I think it’s being used as a proxy for, and how can I elicit that from my patient?’ ”
The issues raised by Dr. Siddique’s study are important, but there also could be concerns in taking them too far, according to Gary Falk, MD, a professor of medicine at the University of Pennsylvania who comoderated the session where Dr. Siddique presented. He was not involved in the study, but was listed on Dr. Siddique’s acknowledgement slide.
Dr. Falk coauthored Barrett’s esophagus guidelines in 2016 that incorporated White race as a risk factor.
“There are certain clear ethnic factors or country of origin factors that impact one’s risk for cancer, and there are certain diseases that are more common in certain ethnic groups. I think that if we homogenize everybody, we may potentially hurt some people in the effort to be inclusive. That’s my only concern. I think it’s totally correct that we have to get out of our comfort zone, but I hate to see us reach too far on the other end, and homogenize things to the point that people who have increased risk are not being recognized for that reason,” said Dr. Falk.
He acknowledged that White race as a risk for Barrett’s is not easy to define given the uncertainty of the genetic risk, for example, in patients with mixed heritage. “This is all very provocative. We have to think about it carefully,” said Dr. Falk.
Dr. Siddique and Dr. Falk have no relevant financial disclosures.
SAN DIEGO – Race-based recommendations and clinical algorithms may be doing more harm than good, according to a systematic review of databases and guidelines.
The study found examples of screening recommendations based on race or ethnicity that were likely misleading since these are social constructs that don’t reflect a patient’s individual risk, said Shazia Siddique, MD, who presented the study at the annual Digestive Disease Week® (DDW). “Historically, we’ve made so many clinical decisions based on somebody’s race and ethnicity. We walk into a room, we don’t even ask people which racial or ethnic category they identify with. We just look at them and we say, ‘Their skin color looks black, and therefore we’re going to apply a different equation to them.’ ”
However, a patient’s risks and unique health circumstances are much more complicated than that. They may be related to genetics, or environmental exposures, or level of access to quality health care. Race can often be inappropriately used as a stand-in for these and other factors, she explained.
“These [racial] categories are truly a social construct. It’s becoming very problematic that people are literally making decisions based on somebody’s skin color. That’s just not what the science supports. If there are specific genes or environmental factors, or differences in access to health care that then impact outcomes for certain racial or ethnic groups, we need to figure out what those are,” said Dr. Siddique, who is an assistant professor of medicine at the University of Pennsylvania, Philadelphia.
Those messages are still entrenched in medical education. “I graduated medical school in 2012, and it was taught to me to use race and ethnicity in clinical decision-making. We need to start in medical education to shift the way that we’re thinking. On the research side, we really need to think about how we can replace or remove race and ethnicity and understand the consequences of that, so that over time we can make a shift,” said Dr. Siddique.
For example, Dr. Siddique discussed recommendations that suggest Asian heritage as a risk factor for hepatitis B screening, but that’s not a good factor to consider: “People were saying that Asians should be screened at an earlier age, but it’s really people that were born and raised in Asian countries where it’s endemic or they may have gotten it from their mothers at birth. It’s a marker for how long you have had the disease and how much virus is in your bloodstream. It’s not because you’re Asian. If you’re born and raised in the United States, and you don’t have any of those risk factors, you shouldn’t be treated differently based on your identified racial and ethnic group,” said Dr. Siddique.
These questions have become even more important in recent years because of patients with multiracial identifies and other considerations. “Now the proxy for which race was being used is even messier,” said Dr. Siddique.
So, how should physicians think about assessing a patient’s personalized risks? The key, said Dr. Siddique, is to look at each patient’s individual factors, such as health care access, environmental exposures from jobs or living conditions, or the country they emigrated from if they weren’t born in the United States. “Disease prevalences are different in different areas, and that changes your index of suspicion,” she said.
And when considering current guidelines that incorporate race or ethnicity, she recommends viewing them skeptically: “If there is a current algorithm in your health system or in a guideline that you’re reading that says you should be making a change based on race and ethnicity, you should look at that with a close eye and say, “What do I think it’s being used as a proxy for, and how can I elicit that from my patient?’ ”
The issues raised by Dr. Siddique’s study are important, but there also could be concerns in taking them too far, according to Gary Falk, MD, a professor of medicine at the University of Pennsylvania who comoderated the session where Dr. Siddique presented. He was not involved in the study, but was listed on Dr. Siddique’s acknowledgement slide.
Dr. Falk coauthored Barrett’s esophagus guidelines in 2016 that incorporated White race as a risk factor.
“There are certain clear ethnic factors or country of origin factors that impact one’s risk for cancer, and there are certain diseases that are more common in certain ethnic groups. I think that if we homogenize everybody, we may potentially hurt some people in the effort to be inclusive. That’s my only concern. I think it’s totally correct that we have to get out of our comfort zone, but I hate to see us reach too far on the other end, and homogenize things to the point that people who have increased risk are not being recognized for that reason,” said Dr. Falk.
He acknowledged that White race as a risk for Barrett’s is not easy to define given the uncertainty of the genetic risk, for example, in patients with mixed heritage. “This is all very provocative. We have to think about it carefully,” said Dr. Falk.
Dr. Siddique and Dr. Falk have no relevant financial disclosures.
SAN DIEGO – Race-based recommendations and clinical algorithms may be doing more harm than good, according to a systematic review of databases and guidelines.
The study found examples of screening recommendations based on race or ethnicity that were likely misleading since these are social constructs that don’t reflect a patient’s individual risk, said Shazia Siddique, MD, who presented the study at the annual Digestive Disease Week® (DDW). “Historically, we’ve made so many clinical decisions based on somebody’s race and ethnicity. We walk into a room, we don’t even ask people which racial or ethnic category they identify with. We just look at them and we say, ‘Their skin color looks black, and therefore we’re going to apply a different equation to them.’ ”
However, a patient’s risks and unique health circumstances are much more complicated than that. They may be related to genetics, or environmental exposures, or level of access to quality health care. Race can often be inappropriately used as a stand-in for these and other factors, she explained.
“These [racial] categories are truly a social construct. It’s becoming very problematic that people are literally making decisions based on somebody’s skin color. That’s just not what the science supports. If there are specific genes or environmental factors, or differences in access to health care that then impact outcomes for certain racial or ethnic groups, we need to figure out what those are,” said Dr. Siddique, who is an assistant professor of medicine at the University of Pennsylvania, Philadelphia.
Those messages are still entrenched in medical education. “I graduated medical school in 2012, and it was taught to me to use race and ethnicity in clinical decision-making. We need to start in medical education to shift the way that we’re thinking. On the research side, we really need to think about how we can replace or remove race and ethnicity and understand the consequences of that, so that over time we can make a shift,” said Dr. Siddique.
For example, Dr. Siddique discussed recommendations that suggest Asian heritage as a risk factor for hepatitis B screening, but that’s not a good factor to consider: “People were saying that Asians should be screened at an earlier age, but it’s really people that were born and raised in Asian countries where it’s endemic or they may have gotten it from their mothers at birth. It’s a marker for how long you have had the disease and how much virus is in your bloodstream. It’s not because you’re Asian. If you’re born and raised in the United States, and you don’t have any of those risk factors, you shouldn’t be treated differently based on your identified racial and ethnic group,” said Dr. Siddique.
These questions have become even more important in recent years because of patients with multiracial identifies and other considerations. “Now the proxy for which race was being used is even messier,” said Dr. Siddique.
So, how should physicians think about assessing a patient’s personalized risks? The key, said Dr. Siddique, is to look at each patient’s individual factors, such as health care access, environmental exposures from jobs or living conditions, or the country they emigrated from if they weren’t born in the United States. “Disease prevalences are different in different areas, and that changes your index of suspicion,” she said.
And when considering current guidelines that incorporate race or ethnicity, she recommends viewing them skeptically: “If there is a current algorithm in your health system or in a guideline that you’re reading that says you should be making a change based on race and ethnicity, you should look at that with a close eye and say, “What do I think it’s being used as a proxy for, and how can I elicit that from my patient?’ ”
The issues raised by Dr. Siddique’s study are important, but there also could be concerns in taking them too far, according to Gary Falk, MD, a professor of medicine at the University of Pennsylvania who comoderated the session where Dr. Siddique presented. He was not involved in the study, but was listed on Dr. Siddique’s acknowledgement slide.
Dr. Falk coauthored Barrett’s esophagus guidelines in 2016 that incorporated White race as a risk factor.
“There are certain clear ethnic factors or country of origin factors that impact one’s risk for cancer, and there are certain diseases that are more common in certain ethnic groups. I think that if we homogenize everybody, we may potentially hurt some people in the effort to be inclusive. That’s my only concern. I think it’s totally correct that we have to get out of our comfort zone, but I hate to see us reach too far on the other end, and homogenize things to the point that people who have increased risk are not being recognized for that reason,” said Dr. Falk.
He acknowledged that White race as a risk for Barrett’s is not easy to define given the uncertainty of the genetic risk, for example, in patients with mixed heritage. “This is all very provocative. We have to think about it carefully,” said Dr. Falk.
Dr. Siddique and Dr. Falk have no relevant financial disclosures.
AT DDW 2022
C. diff.: How did a community hospital cut infections by 77%?
Teamwork by a wide range of professional staff, coupled with support from leadership, enabled one academic community hospital to cut its rate of hospital-onset Clostridioides difficile infections (HO-CDIs) by almost two-thirds in 1 year and by over three-quarters in 3 years, a study published in the American Journal of Infection Control reports.
C. diff. is a major health threat. According to the U.S. Centers for Disease Control and Prevention, CDIs, mainly linked with hospitals, caused an estimated 223,900 cases in hospitalized patients and 12,800 deaths in the United States in 2017.
“The interventions and outcomes of the project improved patient care by ensuring early testing, diagnosis, treatment if warranted, and proper isolation, which helped reduce C. diff. transmission to staff and other patients,” lead study author Cherith Walter, MSN, RN, a clinical nurse specialist at Emory Saint Joseph’s Hospital, Atlanta, told this news organization. “Had we not worked together as a team, we would not have had the ability to carry out such a robust project,” she added in an email.
Each HO-CDI case costs a health care system an estimated $12,313, and high rates of HO-CDIs incur fines from the Hospital-Acquired Condition Reduction Program of the Centers for Medicare & Medicaid Services (CMS), the authors write.
A diverse staff team collaborated
Emory Saint Joseph’s, a 410-bed hospital in Atlanta, had a history of being above the national CMS benchmark for HO-CDIs. To reduce these infections, comply with CMS requirements, and avoid fines, Ms. Walter and colleagues launched a quality improvement project between 2015 and 2020.
With the approval of the chief nursing officer, chief quality officer, and hospital board, researchers mobilized a diverse team of professionals: a clinical nurse specialist, a physician champion, unit nurse champions, a hospital epidemiologist, an infection preventionist, a clinical microbiologist, an antimicrobial stewardship pharmacist, and an environmental services representative.
The team investigated what caused their hospital’s HO-CDIs from 2014 through 2016 and developed appropriate, evidence-based infection prevention interventions. The integrated approach involved:
- Diagnostic stewardship, including a diarrhea decision-tree algorithm that enabled nurses to order tests of any loose or unformed stool for C. diff. during the first 3 days of admission.
- Enhanced environmental cleaning, which involved switching from sporicidal disinfectant only in isolation rooms to using a more effective Environmental Protection Agency–approved sporicidal disinfectant containing hydrogen peroxide and peracetic acid in all patient rooms for daily cleaning and after discharge. Every day, high-touch surfaces in C. diff. isolation rooms were cleaned and shared equipment was disinfected with bleach wipes. After patient discharge, staff cleaned mattresses on all sides, wiped walls with disinfectant, and used ultraviolet light.
- Antimicrobial stewardship. Formulary fluoroquinolones were removed as standalone orders and made available only through order sets with built-in clinical decision support.
- Education of staff on best practices, through emails, flyers, meetings, and training sessions. Two nurses needed to approve the appropriateness of testing specific specimens for CDI. All HO-CDIs were reviewed and findings presented at CDI team meetings.
- Accountability. Staff on the team and units received emailed notices about compliance issues and held meetings to discuss how to improve compliance.
After 1 year, HO-CDI incidence dropped 63% from baseline, from above 12 cases per 10,000 patient-days to 4.72 per 10,000 patient-days. And after 3 years, infections dropped 77% to 2.80 per 10,000 patient-days.
The hospital’s HO-CDI standardized infection ratio – the total number of infections divided by the National Healthcare Safety Network’s risk-adjusted predicted number of infections – dropped below the national benchmark, from 1.11 in 2015 to 0.43 in 2020.
The hospital also increased testing of appropriate patients for CDI within the first 3 days of admission, from 54% in 2014 to 81% in late 2019.
“By testing patients within 3 days of admission, we discovered that many had acquired C. diff. before admission,” Ms. Walter said. “I don’t think we realized how prevalent C. diff. was in the community.”
Benjamin D. Galvan, MLS(ASCP), CIC, an infection preventionist at Tampa General Hospital and a member of the Association for Professionals in Infection Control and Epidemiology, welcomed the study’s results.
“Effective collaboration within the health care setting is a highly effective way to implement and sustain evidence-based practices related to infection reduction. When buy-in is obtained from the top, and pertinent stakeholders are engaged for their expertise, we can see sustainable change and improved patient outcomes,” Mr. Galvan, who was not involved in the study, said in an email.
“The researchers did a fantastic job,” he added. “I am grateful to see this important work addressed in the literature, as it will only improve buy-in for improvement efforts aimed at reducing infections moving forward across the health care continuum.”
Douglas S. Paauw, MD, a professor of medicine and chair for patient-centered clinical education at the University of Washington School of Medicine, Seattle, said that the team’s most important interventions were changing the environmental cleaning protocol and using agents that kill C. diff. spores.
“We know that as many as 10%-20% of hospitalized patients carry C. diff. Cleaning only the rooms where you know you have C. diff. (isolation rooms) will miss most of it,” said Dr. Paauw, who was also not involved in the study. “Cleaning every room with cleaners that actually work is very important but costs money.”
Handwashing with soap and water works, alcohol hand gels do not
“We know that handwashing with soap and water is the most important way to prevent hospital C. diff. transmission,” Dr. Paauw noted. “Handwashing protocols implemented prior to the study were probably a big part of the team’s success.”
Handwashing with soap and water works but alcohol hand gels do not, he cautioned.
“C. diff. rates in hospitals went up years ago when we started putting alcohol gels outside patients’ rooms,” Dr. Paauw explained. “Now, instead of washing their hands, staff quickly pump gel before they see patients. Applying gel is easy, but gel does not eliminate C. diff. spores. Handwashing is such a simple way to fix the C. diff. problem, but doctors don’t take the time.”
“We need to take the C. diff. problem seriously. We have enough information, and we know the right things to do. We need to wash our hands. We need to clean the rooms. We need to stop cutting corners if we want to give good care,” he said.
The authors plan to conduct further related research.
The study was not funded. All study authors, as well as Mr. Galvan and Dr. Paauw, have reported no relevant financial interests.
A version of this article first appeared on Medscape.com.
Teamwork by a wide range of professional staff, coupled with support from leadership, enabled one academic community hospital to cut its rate of hospital-onset Clostridioides difficile infections (HO-CDIs) by almost two-thirds in 1 year and by over three-quarters in 3 years, a study published in the American Journal of Infection Control reports.
C. diff. is a major health threat. According to the U.S. Centers for Disease Control and Prevention, CDIs, mainly linked with hospitals, caused an estimated 223,900 cases in hospitalized patients and 12,800 deaths in the United States in 2017.
“The interventions and outcomes of the project improved patient care by ensuring early testing, diagnosis, treatment if warranted, and proper isolation, which helped reduce C. diff. transmission to staff and other patients,” lead study author Cherith Walter, MSN, RN, a clinical nurse specialist at Emory Saint Joseph’s Hospital, Atlanta, told this news organization. “Had we not worked together as a team, we would not have had the ability to carry out such a robust project,” she added in an email.
Each HO-CDI case costs a health care system an estimated $12,313, and high rates of HO-CDIs incur fines from the Hospital-Acquired Condition Reduction Program of the Centers for Medicare & Medicaid Services (CMS), the authors write.
A diverse staff team collaborated
Emory Saint Joseph’s, a 410-bed hospital in Atlanta, had a history of being above the national CMS benchmark for HO-CDIs. To reduce these infections, comply with CMS requirements, and avoid fines, Ms. Walter and colleagues launched a quality improvement project between 2015 and 2020.
With the approval of the chief nursing officer, chief quality officer, and hospital board, researchers mobilized a diverse team of professionals: a clinical nurse specialist, a physician champion, unit nurse champions, a hospital epidemiologist, an infection preventionist, a clinical microbiologist, an antimicrobial stewardship pharmacist, and an environmental services representative.
The team investigated what caused their hospital’s HO-CDIs from 2014 through 2016 and developed appropriate, evidence-based infection prevention interventions. The integrated approach involved:
- Diagnostic stewardship, including a diarrhea decision-tree algorithm that enabled nurses to order tests of any loose or unformed stool for C. diff. during the first 3 days of admission.
- Enhanced environmental cleaning, which involved switching from sporicidal disinfectant only in isolation rooms to using a more effective Environmental Protection Agency–approved sporicidal disinfectant containing hydrogen peroxide and peracetic acid in all patient rooms for daily cleaning and after discharge. Every day, high-touch surfaces in C. diff. isolation rooms were cleaned and shared equipment was disinfected with bleach wipes. After patient discharge, staff cleaned mattresses on all sides, wiped walls with disinfectant, and used ultraviolet light.
- Antimicrobial stewardship. Formulary fluoroquinolones were removed as standalone orders and made available only through order sets with built-in clinical decision support.
- Education of staff on best practices, through emails, flyers, meetings, and training sessions. Two nurses needed to approve the appropriateness of testing specific specimens for CDI. All HO-CDIs were reviewed and findings presented at CDI team meetings.
- Accountability. Staff on the team and units received emailed notices about compliance issues and held meetings to discuss how to improve compliance.
After 1 year, HO-CDI incidence dropped 63% from baseline, from above 12 cases per 10,000 patient-days to 4.72 per 10,000 patient-days. And after 3 years, infections dropped 77% to 2.80 per 10,000 patient-days.
The hospital’s HO-CDI standardized infection ratio – the total number of infections divided by the National Healthcare Safety Network’s risk-adjusted predicted number of infections – dropped below the national benchmark, from 1.11 in 2015 to 0.43 in 2020.
The hospital also increased testing of appropriate patients for CDI within the first 3 days of admission, from 54% in 2014 to 81% in late 2019.
“By testing patients within 3 days of admission, we discovered that many had acquired C. diff. before admission,” Ms. Walter said. “I don’t think we realized how prevalent C. diff. was in the community.”
Benjamin D. Galvan, MLS(ASCP), CIC, an infection preventionist at Tampa General Hospital and a member of the Association for Professionals in Infection Control and Epidemiology, welcomed the study’s results.
“Effective collaboration within the health care setting is a highly effective way to implement and sustain evidence-based practices related to infection reduction. When buy-in is obtained from the top, and pertinent stakeholders are engaged for their expertise, we can see sustainable change and improved patient outcomes,” Mr. Galvan, who was not involved in the study, said in an email.
“The researchers did a fantastic job,” he added. “I am grateful to see this important work addressed in the literature, as it will only improve buy-in for improvement efforts aimed at reducing infections moving forward across the health care continuum.”
Douglas S. Paauw, MD, a professor of medicine and chair for patient-centered clinical education at the University of Washington School of Medicine, Seattle, said that the team’s most important interventions were changing the environmental cleaning protocol and using agents that kill C. diff. spores.
“We know that as many as 10%-20% of hospitalized patients carry C. diff. Cleaning only the rooms where you know you have C. diff. (isolation rooms) will miss most of it,” said Dr. Paauw, who was also not involved in the study. “Cleaning every room with cleaners that actually work is very important but costs money.”
Handwashing with soap and water works, alcohol hand gels do not
“We know that handwashing with soap and water is the most important way to prevent hospital C. diff. transmission,” Dr. Paauw noted. “Handwashing protocols implemented prior to the study were probably a big part of the team’s success.”
Handwashing with soap and water works but alcohol hand gels do not, he cautioned.
“C. diff. rates in hospitals went up years ago when we started putting alcohol gels outside patients’ rooms,” Dr. Paauw explained. “Now, instead of washing their hands, staff quickly pump gel before they see patients. Applying gel is easy, but gel does not eliminate C. diff. spores. Handwashing is such a simple way to fix the C. diff. problem, but doctors don’t take the time.”
“We need to take the C. diff. problem seriously. We have enough information, and we know the right things to do. We need to wash our hands. We need to clean the rooms. We need to stop cutting corners if we want to give good care,” he said.
The authors plan to conduct further related research.
The study was not funded. All study authors, as well as Mr. Galvan and Dr. Paauw, have reported no relevant financial interests.
A version of this article first appeared on Medscape.com.
Teamwork by a wide range of professional staff, coupled with support from leadership, enabled one academic community hospital to cut its rate of hospital-onset Clostridioides difficile infections (HO-CDIs) by almost two-thirds in 1 year and by over three-quarters in 3 years, a study published in the American Journal of Infection Control reports.
C. diff. is a major health threat. According to the U.S. Centers for Disease Control and Prevention, CDIs, mainly linked with hospitals, caused an estimated 223,900 cases in hospitalized patients and 12,800 deaths in the United States in 2017.
“The interventions and outcomes of the project improved patient care by ensuring early testing, diagnosis, treatment if warranted, and proper isolation, which helped reduce C. diff. transmission to staff and other patients,” lead study author Cherith Walter, MSN, RN, a clinical nurse specialist at Emory Saint Joseph’s Hospital, Atlanta, told this news organization. “Had we not worked together as a team, we would not have had the ability to carry out such a robust project,” she added in an email.
Each HO-CDI case costs a health care system an estimated $12,313, and high rates of HO-CDIs incur fines from the Hospital-Acquired Condition Reduction Program of the Centers for Medicare & Medicaid Services (CMS), the authors write.
A diverse staff team collaborated
Emory Saint Joseph’s, a 410-bed hospital in Atlanta, had a history of being above the national CMS benchmark for HO-CDIs. To reduce these infections, comply with CMS requirements, and avoid fines, Ms. Walter and colleagues launched a quality improvement project between 2015 and 2020.
With the approval of the chief nursing officer, chief quality officer, and hospital board, researchers mobilized a diverse team of professionals: a clinical nurse specialist, a physician champion, unit nurse champions, a hospital epidemiologist, an infection preventionist, a clinical microbiologist, an antimicrobial stewardship pharmacist, and an environmental services representative.
The team investigated what caused their hospital’s HO-CDIs from 2014 through 2016 and developed appropriate, evidence-based infection prevention interventions. The integrated approach involved:
- Diagnostic stewardship, including a diarrhea decision-tree algorithm that enabled nurses to order tests of any loose or unformed stool for C. diff. during the first 3 days of admission.
- Enhanced environmental cleaning, which involved switching from sporicidal disinfectant only in isolation rooms to using a more effective Environmental Protection Agency–approved sporicidal disinfectant containing hydrogen peroxide and peracetic acid in all patient rooms for daily cleaning and after discharge. Every day, high-touch surfaces in C. diff. isolation rooms were cleaned and shared equipment was disinfected with bleach wipes. After patient discharge, staff cleaned mattresses on all sides, wiped walls with disinfectant, and used ultraviolet light.
- Antimicrobial stewardship. Formulary fluoroquinolones were removed as standalone orders and made available only through order sets with built-in clinical decision support.
- Education of staff on best practices, through emails, flyers, meetings, and training sessions. Two nurses needed to approve the appropriateness of testing specific specimens for CDI. All HO-CDIs were reviewed and findings presented at CDI team meetings.
- Accountability. Staff on the team and units received emailed notices about compliance issues and held meetings to discuss how to improve compliance.
After 1 year, HO-CDI incidence dropped 63% from baseline, from above 12 cases per 10,000 patient-days to 4.72 per 10,000 patient-days. And after 3 years, infections dropped 77% to 2.80 per 10,000 patient-days.
The hospital’s HO-CDI standardized infection ratio – the total number of infections divided by the National Healthcare Safety Network’s risk-adjusted predicted number of infections – dropped below the national benchmark, from 1.11 in 2015 to 0.43 in 2020.
The hospital also increased testing of appropriate patients for CDI within the first 3 days of admission, from 54% in 2014 to 81% in late 2019.
“By testing patients within 3 days of admission, we discovered that many had acquired C. diff. before admission,” Ms. Walter said. “I don’t think we realized how prevalent C. diff. was in the community.”
Benjamin D. Galvan, MLS(ASCP), CIC, an infection preventionist at Tampa General Hospital and a member of the Association for Professionals in Infection Control and Epidemiology, welcomed the study’s results.
“Effective collaboration within the health care setting is a highly effective way to implement and sustain evidence-based practices related to infection reduction. When buy-in is obtained from the top, and pertinent stakeholders are engaged for their expertise, we can see sustainable change and improved patient outcomes,” Mr. Galvan, who was not involved in the study, said in an email.
“The researchers did a fantastic job,” he added. “I am grateful to see this important work addressed in the literature, as it will only improve buy-in for improvement efforts aimed at reducing infections moving forward across the health care continuum.”
Douglas S. Paauw, MD, a professor of medicine and chair for patient-centered clinical education at the University of Washington School of Medicine, Seattle, said that the team’s most important interventions were changing the environmental cleaning protocol and using agents that kill C. diff. spores.
“We know that as many as 10%-20% of hospitalized patients carry C. diff. Cleaning only the rooms where you know you have C. diff. (isolation rooms) will miss most of it,” said Dr. Paauw, who was also not involved in the study. “Cleaning every room with cleaners that actually work is very important but costs money.”
Handwashing with soap and water works, alcohol hand gels do not
“We know that handwashing with soap and water is the most important way to prevent hospital C. diff. transmission,” Dr. Paauw noted. “Handwashing protocols implemented prior to the study were probably a big part of the team’s success.”
Handwashing with soap and water works but alcohol hand gels do not, he cautioned.
“C. diff. rates in hospitals went up years ago when we started putting alcohol gels outside patients’ rooms,” Dr. Paauw explained. “Now, instead of washing their hands, staff quickly pump gel before they see patients. Applying gel is easy, but gel does not eliminate C. diff. spores. Handwashing is such a simple way to fix the C. diff. problem, but doctors don’t take the time.”
“We need to take the C. diff. problem seriously. We have enough information, and we know the right things to do. We need to wash our hands. We need to clean the rooms. We need to stop cutting corners if we want to give good care,” he said.
The authors plan to conduct further related research.
The study was not funded. All study authors, as well as Mr. Galvan and Dr. Paauw, have reported no relevant financial interests.
A version of this article first appeared on Medscape.com.
Focus on antivirals, vaccines as monkeypox continues
Since the first case of monkeypox on May 6, reports of outbreaks have come from multiple countries, with the United Kingdom, Spain, and Portugal in the lead, followed by Canada, Israel, and Australia, among others. The United States has reported cases in Boston and New York, and presumed cases have occurred in Utah and Florida. As of May 25, close to 350 cases, either suspected (83) or confirmed (265), have been reported globally.
Monkeypox outbreaks have previously been confined to Central and West Africa, except for an impressively large outbreak in the United States in 2003, during which 47 people were infected across six states. The epidemic was traced to a Gambian rat, rope squirrels, and dormice that had been imported from Ghana as pets and that had infected prairie dogs at a large wholesale pet store.
“It’s amazing how many of these viruses – COVID, now monkeypox and others – [exist]. They’re out there in the wild in the animal reservoir,” said Dennis Hruby, PhD, executive VP/chief scientific officer and scientific founder of SIGA Technologies.
“When it comes to the human population, they sometimes behave in ways we’re not expecting. That and a few mutations change those strains and pathogenicity and can be pandemic,” he told this news organization.
Now that the virus is pandemic, there is an urgent interest in medicines and vaccines that might halt its spread.
Smallpox drug tecovirimat
SIGA’s drug is tecovirimat, initially known as ST-246 and now branded as TPOXX. The U.S. Food and Drug Administration approved an oral formulation to treat smallpox in 2018. While smallpox was eradicated by 1980, there have been ongoing concerns about its potential use in a bioterrorism attack.
Tecovirimat is also approved for smallpox in Canada. In Europe, the approval includes treatment of monkeypox, cowpox, and complications from immunization with vaccinia. On May 19, the FDA approved an IV formulation of tecovirimat for those unable to tolerate oral medications.
In a press release, SIGA notes that tecovirimat was “developed through funding and collaboration with the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health & Human Services, as well as early-stage development supported by the National Institutes of Health, US Centers for Disease Control and Prevention, and the Department of Defense. Tecovirimat is stockpiled by the U.S. Government to mitigate the impact of a potential outbreak or bioterror attack.”
SIGA adds that, under Project Bioshield, “the United States maintains a stockpile of 1.7 million courses in the Strategic National Stockpile.” The drug is only available through the government’s stockpile.
Tecovirimat works by preventing the viruses from reproducing by interfering with a protein, VP37. The virus cannot escape the cell and so cannot infect other cells, Dr. Hruby explained.
Tecovirimat was developed under the FDA’s so-called Animal Rule, which allows approval on the basis of animal studies when human efficacy studies are unethical or impractical.
In a placebo-controlled human pharmacokinetic and safety study, only 2% of the 359 who received TPOXX had to have treatment stopped because of adverse reactions, a rate similar to placebo. The most common reactions (≥2%) were headache, nausea, and abdominal pain. Significant drug interactions were found with the coadministration of repaglinide and midazolam.
Of note is that tecovirimat’s efficacy may be reduced in immunocompromised patients. The smallpox vaccine is contraindicated for those who are immunocompromised. Those people should be offered vaccinia immune globulin.
With monkeypox, “the earlier the disease is recognized and you start treating, [the] more effective,” said Dr. Hruby. “In a monkey model which, much like humans, if we treat early on as the first lesions emerged or even several days after the lesions emerged, we see close to 100% protection.”
The other alternative drug for smallpox and (likely) monkeypox is Chimerix’s brincidofovir (BCV, Tembexa), a lipid conjugate of cidofovir, a drug for cytomegalovirus. Brincidofovir has a better safety profile than cidofovir and was also approved under the Animal Rule.
UpToDate suggests that tecovirimat is the drug of choice for monkeypox. They note that for severely infected patients, it can be combined with brincidofovir after consultation with the CDC or state health department officials.
Two vaccines available
Two vaccines are currently available. The oldest is ACAM2000, a replication-competent vaccine that replaced Dryvax, whose use was stopped in 1977, the last year in which naturally occurring cases of smallpox occurred. ACAM2000 is used to immunize military recruits. It was produced by Sanofi and is now produced by Emergent Biosolutions. Being a live vaccinia vaccine, it is contraindicated for people who are immunocompromised or pregnant, as well as for children and those with eczema, because serious and occasionally fatal reactions have occurred. Because of unexpected cardiac complications in first responders who received Dryvax, having a history of cardiac disease or significant risk factors is considered a contraindication to replication-competent (live) vaccination except in the setting of a bioterrorism event.
ACAM2000 is not FDA approved for monkeypox, but it is readily available. The United States stockpile has more than 100 million doses, according to the CDC.
“ACAM is not very different from Dryvax in terms of safety profile,” Melvin Sanicas, MD, a vaccinologist and health educator, told this news organization.
The newest option is a replication-deficient modified vaccinia Ankara vaccine called Jynneos in the United States (Imvanex in Europe; Imvamune in Canada). The vaccine is made by Denmark-based Bavarian Nordic. The FDA approved Jynneos in 2019. It, too, is available through BARDA’s stockpiles; 1,000 doses are available now and more are on order.
In the current monkeypox outbreak, Jynneos has been offered to higher-risk contacts in the United Kingdom. The CDC is planning to provide it to high-risk contacts of infected persons in the United States. This strategy is called “ring vaccination,” through which only close contacts are immunized initially. The rings are then enlarged to include more people as needed. Ring vaccination works well for easily identified diseases such as monkeypox and in situations in which there are few cases. It has been used very effectively for smallpox and Ebola.
Jynneos is not associated with the same risks as the live vaccine. In solicited reactions, injection-site reactions were common. Other reported systemic symptoms were muscle pain (42.8%), headache (34.8%), fatigue (30.4%), nausea (17.3%), and chills (10.4%).
Other vaccines are expected to be developed. Moderna has just thrown its hat into the ring, announcing it is beginning preclinical trials for monkeypox.
Prolonged close contact
Monkeypox is spread by large droplets or contact with infected lesions or body fluids. It’s thought to require prolonged close contact. In an email interview, Dr. Sanicas told this news organization that the “contact can be with (1) skin lesions of an infected person, (2) respiratory droplets in prolonged face-to-face contact, (3) fomites. The cases in the United Kingdom are in men having sex with men, but it does not mean the disease is now sexually transmitted. People do not need to have sex to be infected, but of course, sexual contact means there is prolonged contact.” The household transmission rate is less than 10%.
Dr. Sanicas confirmed that, as with smallpox, monkeypox could be transmitted by contact with clothing or bedding that has been contaminated through contact with the infected lesions, as smallpox was transmitted to Native Americans by colonizers. Airborne transmission is a theoretical possibility but is not considered likely. Being a DNA virus, monkeypox is less likely to mutate than COVID. “If it were as infectious as flu or coronavirus, there would be more infections and outbreaks in countries where MPX [monkeypox] is endemic in Western Africa or Congo Basin,” said Dr. Sanicas.
Fortunately, this clade of monkeypox, which appears to have originated in West Africa, is estimated to have a mortality rate of about 1%. In contrast, the Congo Basin clade has a death rate of up to 10%.
Dr. Sanicas concluded, “Be cautious, but there’s no need for further fear and panic on top of what we have for COVID-19. Monkeypox is not COVID and will not cause the same devastation/death/lockdowns as COVID-19.”
Dr. Hruby is an employee and stockholder of SIGA. Dr. Sanicas reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Since the first case of monkeypox on May 6, reports of outbreaks have come from multiple countries, with the United Kingdom, Spain, and Portugal in the lead, followed by Canada, Israel, and Australia, among others. The United States has reported cases in Boston and New York, and presumed cases have occurred in Utah and Florida. As of May 25, close to 350 cases, either suspected (83) or confirmed (265), have been reported globally.
Monkeypox outbreaks have previously been confined to Central and West Africa, except for an impressively large outbreak in the United States in 2003, during which 47 people were infected across six states. The epidemic was traced to a Gambian rat, rope squirrels, and dormice that had been imported from Ghana as pets and that had infected prairie dogs at a large wholesale pet store.
“It’s amazing how many of these viruses – COVID, now monkeypox and others – [exist]. They’re out there in the wild in the animal reservoir,” said Dennis Hruby, PhD, executive VP/chief scientific officer and scientific founder of SIGA Technologies.
“When it comes to the human population, they sometimes behave in ways we’re not expecting. That and a few mutations change those strains and pathogenicity and can be pandemic,” he told this news organization.
Now that the virus is pandemic, there is an urgent interest in medicines and vaccines that might halt its spread.
Smallpox drug tecovirimat
SIGA’s drug is tecovirimat, initially known as ST-246 and now branded as TPOXX. The U.S. Food and Drug Administration approved an oral formulation to treat smallpox in 2018. While smallpox was eradicated by 1980, there have been ongoing concerns about its potential use in a bioterrorism attack.
Tecovirimat is also approved for smallpox in Canada. In Europe, the approval includes treatment of monkeypox, cowpox, and complications from immunization with vaccinia. On May 19, the FDA approved an IV formulation of tecovirimat for those unable to tolerate oral medications.
In a press release, SIGA notes that tecovirimat was “developed through funding and collaboration with the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health & Human Services, as well as early-stage development supported by the National Institutes of Health, US Centers for Disease Control and Prevention, and the Department of Defense. Tecovirimat is stockpiled by the U.S. Government to mitigate the impact of a potential outbreak or bioterror attack.”
SIGA adds that, under Project Bioshield, “the United States maintains a stockpile of 1.7 million courses in the Strategic National Stockpile.” The drug is only available through the government’s stockpile.
Tecovirimat works by preventing the viruses from reproducing by interfering with a protein, VP37. The virus cannot escape the cell and so cannot infect other cells, Dr. Hruby explained.
Tecovirimat was developed under the FDA’s so-called Animal Rule, which allows approval on the basis of animal studies when human efficacy studies are unethical or impractical.
In a placebo-controlled human pharmacokinetic and safety study, only 2% of the 359 who received TPOXX had to have treatment stopped because of adverse reactions, a rate similar to placebo. The most common reactions (≥2%) were headache, nausea, and abdominal pain. Significant drug interactions were found with the coadministration of repaglinide and midazolam.
Of note is that tecovirimat’s efficacy may be reduced in immunocompromised patients. The smallpox vaccine is contraindicated for those who are immunocompromised. Those people should be offered vaccinia immune globulin.
With monkeypox, “the earlier the disease is recognized and you start treating, [the] more effective,” said Dr. Hruby. “In a monkey model which, much like humans, if we treat early on as the first lesions emerged or even several days after the lesions emerged, we see close to 100% protection.”
The other alternative drug for smallpox and (likely) monkeypox is Chimerix’s brincidofovir (BCV, Tembexa), a lipid conjugate of cidofovir, a drug for cytomegalovirus. Brincidofovir has a better safety profile than cidofovir and was also approved under the Animal Rule.
UpToDate suggests that tecovirimat is the drug of choice for monkeypox. They note that for severely infected patients, it can be combined with brincidofovir after consultation with the CDC or state health department officials.
Two vaccines available
Two vaccines are currently available. The oldest is ACAM2000, a replication-competent vaccine that replaced Dryvax, whose use was stopped in 1977, the last year in which naturally occurring cases of smallpox occurred. ACAM2000 is used to immunize military recruits. It was produced by Sanofi and is now produced by Emergent Biosolutions. Being a live vaccinia vaccine, it is contraindicated for people who are immunocompromised or pregnant, as well as for children and those with eczema, because serious and occasionally fatal reactions have occurred. Because of unexpected cardiac complications in first responders who received Dryvax, having a history of cardiac disease or significant risk factors is considered a contraindication to replication-competent (live) vaccination except in the setting of a bioterrorism event.
ACAM2000 is not FDA approved for monkeypox, but it is readily available. The United States stockpile has more than 100 million doses, according to the CDC.
“ACAM is not very different from Dryvax in terms of safety profile,” Melvin Sanicas, MD, a vaccinologist and health educator, told this news organization.
The newest option is a replication-deficient modified vaccinia Ankara vaccine called Jynneos in the United States (Imvanex in Europe; Imvamune in Canada). The vaccine is made by Denmark-based Bavarian Nordic. The FDA approved Jynneos in 2019. It, too, is available through BARDA’s stockpiles; 1,000 doses are available now and more are on order.
In the current monkeypox outbreak, Jynneos has been offered to higher-risk contacts in the United Kingdom. The CDC is planning to provide it to high-risk contacts of infected persons in the United States. This strategy is called “ring vaccination,” through which only close contacts are immunized initially. The rings are then enlarged to include more people as needed. Ring vaccination works well for easily identified diseases such as monkeypox and in situations in which there are few cases. It has been used very effectively for smallpox and Ebola.
Jynneos is not associated with the same risks as the live vaccine. In solicited reactions, injection-site reactions were common. Other reported systemic symptoms were muscle pain (42.8%), headache (34.8%), fatigue (30.4%), nausea (17.3%), and chills (10.4%).
Other vaccines are expected to be developed. Moderna has just thrown its hat into the ring, announcing it is beginning preclinical trials for monkeypox.
Prolonged close contact
Monkeypox is spread by large droplets or contact with infected lesions or body fluids. It’s thought to require prolonged close contact. In an email interview, Dr. Sanicas told this news organization that the “contact can be with (1) skin lesions of an infected person, (2) respiratory droplets in prolonged face-to-face contact, (3) fomites. The cases in the United Kingdom are in men having sex with men, but it does not mean the disease is now sexually transmitted. People do not need to have sex to be infected, but of course, sexual contact means there is prolonged contact.” The household transmission rate is less than 10%.
Dr. Sanicas confirmed that, as with smallpox, monkeypox could be transmitted by contact with clothing or bedding that has been contaminated through contact with the infected lesions, as smallpox was transmitted to Native Americans by colonizers. Airborne transmission is a theoretical possibility but is not considered likely. Being a DNA virus, monkeypox is less likely to mutate than COVID. “If it were as infectious as flu or coronavirus, there would be more infections and outbreaks in countries where MPX [monkeypox] is endemic in Western Africa or Congo Basin,” said Dr. Sanicas.
Fortunately, this clade of monkeypox, which appears to have originated in West Africa, is estimated to have a mortality rate of about 1%. In contrast, the Congo Basin clade has a death rate of up to 10%.
Dr. Sanicas concluded, “Be cautious, but there’s no need for further fear and panic on top of what we have for COVID-19. Monkeypox is not COVID and will not cause the same devastation/death/lockdowns as COVID-19.”
Dr. Hruby is an employee and stockholder of SIGA. Dr. Sanicas reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Since the first case of monkeypox on May 6, reports of outbreaks have come from multiple countries, with the United Kingdom, Spain, and Portugal in the lead, followed by Canada, Israel, and Australia, among others. The United States has reported cases in Boston and New York, and presumed cases have occurred in Utah and Florida. As of May 25, close to 350 cases, either suspected (83) or confirmed (265), have been reported globally.
Monkeypox outbreaks have previously been confined to Central and West Africa, except for an impressively large outbreak in the United States in 2003, during which 47 people were infected across six states. The epidemic was traced to a Gambian rat, rope squirrels, and dormice that had been imported from Ghana as pets and that had infected prairie dogs at a large wholesale pet store.
“It’s amazing how many of these viruses – COVID, now monkeypox and others – [exist]. They’re out there in the wild in the animal reservoir,” said Dennis Hruby, PhD, executive VP/chief scientific officer and scientific founder of SIGA Technologies.
“When it comes to the human population, they sometimes behave in ways we’re not expecting. That and a few mutations change those strains and pathogenicity and can be pandemic,” he told this news organization.
Now that the virus is pandemic, there is an urgent interest in medicines and vaccines that might halt its spread.
Smallpox drug tecovirimat
SIGA’s drug is tecovirimat, initially known as ST-246 and now branded as TPOXX. The U.S. Food and Drug Administration approved an oral formulation to treat smallpox in 2018. While smallpox was eradicated by 1980, there have been ongoing concerns about its potential use in a bioterrorism attack.
Tecovirimat is also approved for smallpox in Canada. In Europe, the approval includes treatment of monkeypox, cowpox, and complications from immunization with vaccinia. On May 19, the FDA approved an IV formulation of tecovirimat for those unable to tolerate oral medications.
In a press release, SIGA notes that tecovirimat was “developed through funding and collaboration with the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health & Human Services, as well as early-stage development supported by the National Institutes of Health, US Centers for Disease Control and Prevention, and the Department of Defense. Tecovirimat is stockpiled by the U.S. Government to mitigate the impact of a potential outbreak or bioterror attack.”
SIGA adds that, under Project Bioshield, “the United States maintains a stockpile of 1.7 million courses in the Strategic National Stockpile.” The drug is only available through the government’s stockpile.
Tecovirimat works by preventing the viruses from reproducing by interfering with a protein, VP37. The virus cannot escape the cell and so cannot infect other cells, Dr. Hruby explained.
Tecovirimat was developed under the FDA’s so-called Animal Rule, which allows approval on the basis of animal studies when human efficacy studies are unethical or impractical.
In a placebo-controlled human pharmacokinetic and safety study, only 2% of the 359 who received TPOXX had to have treatment stopped because of adverse reactions, a rate similar to placebo. The most common reactions (≥2%) were headache, nausea, and abdominal pain. Significant drug interactions were found with the coadministration of repaglinide and midazolam.
Of note is that tecovirimat’s efficacy may be reduced in immunocompromised patients. The smallpox vaccine is contraindicated for those who are immunocompromised. Those people should be offered vaccinia immune globulin.
With monkeypox, “the earlier the disease is recognized and you start treating, [the] more effective,” said Dr. Hruby. “In a monkey model which, much like humans, if we treat early on as the first lesions emerged or even several days after the lesions emerged, we see close to 100% protection.”
The other alternative drug for smallpox and (likely) monkeypox is Chimerix’s brincidofovir (BCV, Tembexa), a lipid conjugate of cidofovir, a drug for cytomegalovirus. Brincidofovir has a better safety profile than cidofovir and was also approved under the Animal Rule.
UpToDate suggests that tecovirimat is the drug of choice for monkeypox. They note that for severely infected patients, it can be combined with brincidofovir after consultation with the CDC or state health department officials.
Two vaccines available
Two vaccines are currently available. The oldest is ACAM2000, a replication-competent vaccine that replaced Dryvax, whose use was stopped in 1977, the last year in which naturally occurring cases of smallpox occurred. ACAM2000 is used to immunize military recruits. It was produced by Sanofi and is now produced by Emergent Biosolutions. Being a live vaccinia vaccine, it is contraindicated for people who are immunocompromised or pregnant, as well as for children and those with eczema, because serious and occasionally fatal reactions have occurred. Because of unexpected cardiac complications in first responders who received Dryvax, having a history of cardiac disease or significant risk factors is considered a contraindication to replication-competent (live) vaccination except in the setting of a bioterrorism event.
ACAM2000 is not FDA approved for monkeypox, but it is readily available. The United States stockpile has more than 100 million doses, according to the CDC.
“ACAM is not very different from Dryvax in terms of safety profile,” Melvin Sanicas, MD, a vaccinologist and health educator, told this news organization.
The newest option is a replication-deficient modified vaccinia Ankara vaccine called Jynneos in the United States (Imvanex in Europe; Imvamune in Canada). The vaccine is made by Denmark-based Bavarian Nordic. The FDA approved Jynneos in 2019. It, too, is available through BARDA’s stockpiles; 1,000 doses are available now and more are on order.
In the current monkeypox outbreak, Jynneos has been offered to higher-risk contacts in the United Kingdom. The CDC is planning to provide it to high-risk contacts of infected persons in the United States. This strategy is called “ring vaccination,” through which only close contacts are immunized initially. The rings are then enlarged to include more people as needed. Ring vaccination works well for easily identified diseases such as monkeypox and in situations in which there are few cases. It has been used very effectively for smallpox and Ebola.
Jynneos is not associated with the same risks as the live vaccine. In solicited reactions, injection-site reactions were common. Other reported systemic symptoms were muscle pain (42.8%), headache (34.8%), fatigue (30.4%), nausea (17.3%), and chills (10.4%).
Other vaccines are expected to be developed. Moderna has just thrown its hat into the ring, announcing it is beginning preclinical trials for monkeypox.
Prolonged close contact
Monkeypox is spread by large droplets or contact with infected lesions or body fluids. It’s thought to require prolonged close contact. In an email interview, Dr. Sanicas told this news organization that the “contact can be with (1) skin lesions of an infected person, (2) respiratory droplets in prolonged face-to-face contact, (3) fomites. The cases in the United Kingdom are in men having sex with men, but it does not mean the disease is now sexually transmitted. People do not need to have sex to be infected, but of course, sexual contact means there is prolonged contact.” The household transmission rate is less than 10%.
Dr. Sanicas confirmed that, as with smallpox, monkeypox could be transmitted by contact with clothing or bedding that has been contaminated through contact with the infected lesions, as smallpox was transmitted to Native Americans by colonizers. Airborne transmission is a theoretical possibility but is not considered likely. Being a DNA virus, monkeypox is less likely to mutate than COVID. “If it were as infectious as flu or coronavirus, there would be more infections and outbreaks in countries where MPX [monkeypox] is endemic in Western Africa or Congo Basin,” said Dr. Sanicas.
Fortunately, this clade of monkeypox, which appears to have originated in West Africa, is estimated to have a mortality rate of about 1%. In contrast, the Congo Basin clade has a death rate of up to 10%.
Dr. Sanicas concluded, “Be cautious, but there’s no need for further fear and panic on top of what we have for COVID-19. Monkeypox is not COVID and will not cause the same devastation/death/lockdowns as COVID-19.”
Dr. Hruby is an employee and stockholder of SIGA. Dr. Sanicas reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.













