User login
DIY nerve stimulation effective in episodic migraine
results from a phase 3 study show.
This is great news for headache patients who want to explore nondrug treatment options, said study investigator Deena E. Kuruvilla, MD, neurologist and headache specialist at the Westport Headache Institute, Connecticut.
She added that such devices “aren’t always part of the conversation when we’re discussing preventive and acute treatments with our patients. Making this a regular part of the conversation might be helpful to patients.”
The findings were presented at ANA 2021: 146th Annual Meeting of the American Neurological Association (ANA), which was held online.
A key therapeutic target
The randomized, double-blind trial compared E-TNS with sham stimulation for the acute treatment of migraine.
The E-TNS device (Verum Cefaly Abortive Program) stimulates the supraorbital nerve in the forehead. “This nerve is a branch of the trigeminal nerve, which is thought to be the key player in migraine pathophysiology,” Dr. Kuruvilla noted.
The device has been cleared by the U.S. Food and Drug Administration for acute and preventive treatment of migraine.
During a run-in period before randomization, patients were asked to keep a detailed headache diary and to become comfortable using the trial device to treat an acute migraine attack at home.
The study enrolled 538 adult patients at 10 centers. The patients were aged 18 to 65 years, and they had been having episodic migraines, with or without aura, for at least a year. The participants had to have received a migraine diagnosis before age 50, and they had to be experiencing an attack of migraine 2 to 8 days per month.
The patients used the device only for a migraine of at least moderate intensity that was accompanied by at least one migraine-associated symptom, such as photophobia, phonophobia, or nausea. They were asked not to take rescue medication prior to or during a therapy session.
Study participants applied either neurostimulation or sham stimulation for a continuous 2-hour period within 4 hours of a migraine attack over the 2-month study period.
The two primary endpoints were pain freedom and freedom from the most bothersome migraine-associated symptoms at 2 hours.
Compared to sham treatment, active stimulation was more effective in achieving pain freedom (P = .043) and freedom from the most bothersome migraine-associated symptom (P = .001) at 2 hours.
“So the study did meet both primary endpoints with statistical significance,” said Dr. Kuruvilla.
The five secondary endpoints included pain relief at 2 hours; absence of all migraine-associated symptoms at 2 hours; use of rescue medication within 24 hours; sustained pain freedom at 24 hours; and sustained pain relief at 24 hours.
All but one of these endpoints reached statistical significance, showing superiority for the active intervention. The only exception was in regard to use of rescue medication.
The most common adverse event (AE) was forehead paresthesia, discomfort, or burning, which was more common in the active-treatment group than in the sham-treatment group (P = .009). There were four cases of nausea or vomiting in the active-treatment group and none in the sham-treatment group. There were no serious AEs.
Available over the counter
Both moderators of the headache poster tour that featured this study – Justin C. McArthur, MBBS, from Johns Hopkins University, Baltimore, and Steven Galetta, MD, from NYU Grossman School of Medicine – praised the presentation.
Dr. Galetta questioned whether patients were receiving preventive therapies. Dr. Kuruvilla said that the patients were allowed to enter the trial while taking preventive therapies, including antiepileptic treatments, blood pressure medications, and antidepressants, but that they had to be receiving stable doses.
The investigators didn’t distinguish between participants who were taking preventive therapies and those who weren’t, she said. “The aim was really to look at acute treatment for migraine,” and patients taking such medication “had been stable on their regimen for a pretty prolonged period of time.”
Dr. McArthur asked about the origin of the nausea some patients experienced.
It was difficult to determine whether the nausea was an aspect of an individual patient’s migraine attack or was an effect of the stimulation, said Dr. Kuruvilla. She noted that some patients found the vibrating sensation from the device uncomfortable and that nausea could be associated with pain at the site.
The device costs $300 to $400 (U.S.) and is available over the counter.
Dr. Kuruvilla is a consultant for Cefaly, Neurolief, Theranica, Now What Media, and Kx Advisors. She is on the speakers bureau for AbbVie/Allergan, Amgen/Novartis, Lilly, the American Headache Society, Biohaven, and CME meeting, and she is on an advisory board at AbbVie/Allergan, Lilly, Theranica, and Amgen/Novartis. She is editor and associate editor of Healthline and is an author for WebMD/Medscape, Healthline.
A version of this article first appeared on Medscape.com.
results from a phase 3 study show.
This is great news for headache patients who want to explore nondrug treatment options, said study investigator Deena E. Kuruvilla, MD, neurologist and headache specialist at the Westport Headache Institute, Connecticut.
She added that such devices “aren’t always part of the conversation when we’re discussing preventive and acute treatments with our patients. Making this a regular part of the conversation might be helpful to patients.”
The findings were presented at ANA 2021: 146th Annual Meeting of the American Neurological Association (ANA), which was held online.
A key therapeutic target
The randomized, double-blind trial compared E-TNS with sham stimulation for the acute treatment of migraine.
The E-TNS device (Verum Cefaly Abortive Program) stimulates the supraorbital nerve in the forehead. “This nerve is a branch of the trigeminal nerve, which is thought to be the key player in migraine pathophysiology,” Dr. Kuruvilla noted.
The device has been cleared by the U.S. Food and Drug Administration for acute and preventive treatment of migraine.
During a run-in period before randomization, patients were asked to keep a detailed headache diary and to become comfortable using the trial device to treat an acute migraine attack at home.
The study enrolled 538 adult patients at 10 centers. The patients were aged 18 to 65 years, and they had been having episodic migraines, with or without aura, for at least a year. The participants had to have received a migraine diagnosis before age 50, and they had to be experiencing an attack of migraine 2 to 8 days per month.
The patients used the device only for a migraine of at least moderate intensity that was accompanied by at least one migraine-associated symptom, such as photophobia, phonophobia, or nausea. They were asked not to take rescue medication prior to or during a therapy session.
Study participants applied either neurostimulation or sham stimulation for a continuous 2-hour period within 4 hours of a migraine attack over the 2-month study period.
The two primary endpoints were pain freedom and freedom from the most bothersome migraine-associated symptoms at 2 hours.
Compared to sham treatment, active stimulation was more effective in achieving pain freedom (P = .043) and freedom from the most bothersome migraine-associated symptom (P = .001) at 2 hours.
“So the study did meet both primary endpoints with statistical significance,” said Dr. Kuruvilla.
The five secondary endpoints included pain relief at 2 hours; absence of all migraine-associated symptoms at 2 hours; use of rescue medication within 24 hours; sustained pain freedom at 24 hours; and sustained pain relief at 24 hours.
All but one of these endpoints reached statistical significance, showing superiority for the active intervention. The only exception was in regard to use of rescue medication.
The most common adverse event (AE) was forehead paresthesia, discomfort, or burning, which was more common in the active-treatment group than in the sham-treatment group (P = .009). There were four cases of nausea or vomiting in the active-treatment group and none in the sham-treatment group. There were no serious AEs.
Available over the counter
Both moderators of the headache poster tour that featured this study – Justin C. McArthur, MBBS, from Johns Hopkins University, Baltimore, and Steven Galetta, MD, from NYU Grossman School of Medicine – praised the presentation.
Dr. Galetta questioned whether patients were receiving preventive therapies. Dr. Kuruvilla said that the patients were allowed to enter the trial while taking preventive therapies, including antiepileptic treatments, blood pressure medications, and antidepressants, but that they had to be receiving stable doses.
The investigators didn’t distinguish between participants who were taking preventive therapies and those who weren’t, she said. “The aim was really to look at acute treatment for migraine,” and patients taking such medication “had been stable on their regimen for a pretty prolonged period of time.”
Dr. McArthur asked about the origin of the nausea some patients experienced.
It was difficult to determine whether the nausea was an aspect of an individual patient’s migraine attack or was an effect of the stimulation, said Dr. Kuruvilla. She noted that some patients found the vibrating sensation from the device uncomfortable and that nausea could be associated with pain at the site.
The device costs $300 to $400 (U.S.) and is available over the counter.
Dr. Kuruvilla is a consultant for Cefaly, Neurolief, Theranica, Now What Media, and Kx Advisors. She is on the speakers bureau for AbbVie/Allergan, Amgen/Novartis, Lilly, the American Headache Society, Biohaven, and CME meeting, and she is on an advisory board at AbbVie/Allergan, Lilly, Theranica, and Amgen/Novartis. She is editor and associate editor of Healthline and is an author for WebMD/Medscape, Healthline.
A version of this article first appeared on Medscape.com.
results from a phase 3 study show.
This is great news for headache patients who want to explore nondrug treatment options, said study investigator Deena E. Kuruvilla, MD, neurologist and headache specialist at the Westport Headache Institute, Connecticut.
She added that such devices “aren’t always part of the conversation when we’re discussing preventive and acute treatments with our patients. Making this a regular part of the conversation might be helpful to patients.”
The findings were presented at ANA 2021: 146th Annual Meeting of the American Neurological Association (ANA), which was held online.
A key therapeutic target
The randomized, double-blind trial compared E-TNS with sham stimulation for the acute treatment of migraine.
The E-TNS device (Verum Cefaly Abortive Program) stimulates the supraorbital nerve in the forehead. “This nerve is a branch of the trigeminal nerve, which is thought to be the key player in migraine pathophysiology,” Dr. Kuruvilla noted.
The device has been cleared by the U.S. Food and Drug Administration for acute and preventive treatment of migraine.
During a run-in period before randomization, patients were asked to keep a detailed headache diary and to become comfortable using the trial device to treat an acute migraine attack at home.
The study enrolled 538 adult patients at 10 centers. The patients were aged 18 to 65 years, and they had been having episodic migraines, with or without aura, for at least a year. The participants had to have received a migraine diagnosis before age 50, and they had to be experiencing an attack of migraine 2 to 8 days per month.
The patients used the device only for a migraine of at least moderate intensity that was accompanied by at least one migraine-associated symptom, such as photophobia, phonophobia, or nausea. They were asked not to take rescue medication prior to or during a therapy session.
Study participants applied either neurostimulation or sham stimulation for a continuous 2-hour period within 4 hours of a migraine attack over the 2-month study period.
The two primary endpoints were pain freedom and freedom from the most bothersome migraine-associated symptoms at 2 hours.
Compared to sham treatment, active stimulation was more effective in achieving pain freedom (P = .043) and freedom from the most bothersome migraine-associated symptom (P = .001) at 2 hours.
“So the study did meet both primary endpoints with statistical significance,” said Dr. Kuruvilla.
The five secondary endpoints included pain relief at 2 hours; absence of all migraine-associated symptoms at 2 hours; use of rescue medication within 24 hours; sustained pain freedom at 24 hours; and sustained pain relief at 24 hours.
All but one of these endpoints reached statistical significance, showing superiority for the active intervention. The only exception was in regard to use of rescue medication.
The most common adverse event (AE) was forehead paresthesia, discomfort, or burning, which was more common in the active-treatment group than in the sham-treatment group (P = .009). There were four cases of nausea or vomiting in the active-treatment group and none in the sham-treatment group. There were no serious AEs.
Available over the counter
Both moderators of the headache poster tour that featured this study – Justin C. McArthur, MBBS, from Johns Hopkins University, Baltimore, and Steven Galetta, MD, from NYU Grossman School of Medicine – praised the presentation.
Dr. Galetta questioned whether patients were receiving preventive therapies. Dr. Kuruvilla said that the patients were allowed to enter the trial while taking preventive therapies, including antiepileptic treatments, blood pressure medications, and antidepressants, but that they had to be receiving stable doses.
The investigators didn’t distinguish between participants who were taking preventive therapies and those who weren’t, she said. “The aim was really to look at acute treatment for migraine,” and patients taking such medication “had been stable on their regimen for a pretty prolonged period of time.”
Dr. McArthur asked about the origin of the nausea some patients experienced.
It was difficult to determine whether the nausea was an aspect of an individual patient’s migraine attack or was an effect of the stimulation, said Dr. Kuruvilla. She noted that some patients found the vibrating sensation from the device uncomfortable and that nausea could be associated with pain at the site.
The device costs $300 to $400 (U.S.) and is available over the counter.
Dr. Kuruvilla is a consultant for Cefaly, Neurolief, Theranica, Now What Media, and Kx Advisors. She is on the speakers bureau for AbbVie/Allergan, Amgen/Novartis, Lilly, the American Headache Society, Biohaven, and CME meeting, and she is on an advisory board at AbbVie/Allergan, Lilly, Theranica, and Amgen/Novartis. She is editor and associate editor of Healthline and is an author for WebMD/Medscape, Healthline.
A version of this article first appeared on Medscape.com.
FROM ANA
Strategies for safe dissection of cervical fibroids during hysterectomy
Geographic cohorting increased direct care time and interruptions
Background: Geographic cohorting localizes hospitalist teams to a single unit. It has previously been shown to improve outcomes.
Design: Prospective time and motion study.
Setting: 11 geographically cohorted services and 4 noncohorted teams at Indiana University Health, a large academic medical center.
Synopsis: Geotracking was used to monitor time spent inside and outside of patient rooms for 17 hospitalists over at least 6 weeks. Eight hospitalists were also directly observed. Both groups spent roughly three times more time outside patient rooms than inside. Geographic cohorting was associated with longer patient visits (ranging from 69.6 to 101.7 minutes per day depending on team structure) and a higher percentage of time in patient rooms. Interruptions were more common with geographic cohorting. These hospitalists were interrupted every 14 minutes in the morning and every 8 minutes in the afternoon. Of these interruptions, 62% were face-to-face, 25% were electronic, and 13% were both simultaneously.
An important limitation of this study is that the investigators did not evaluate clinical outcomes or provider satisfaction. This may give some pause to the widespread push toward geographic cohorting.
Bottom line: More frequent interruptions may partially offset potential increases in patient-hospitalist interactions achieved through geographic cohorting.
Citation: Kara A et al. A time motion study evaluating the impact of geographic cohorting of hospitalists. J Hosp Med. 2020;15:338-44.
Dr. Sweigart is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: Geographic cohorting localizes hospitalist teams to a single unit. It has previously been shown to improve outcomes.
Design: Prospective time and motion study.
Setting: 11 geographically cohorted services and 4 noncohorted teams at Indiana University Health, a large academic medical center.
Synopsis: Geotracking was used to monitor time spent inside and outside of patient rooms for 17 hospitalists over at least 6 weeks. Eight hospitalists were also directly observed. Both groups spent roughly three times more time outside patient rooms than inside. Geographic cohorting was associated with longer patient visits (ranging from 69.6 to 101.7 minutes per day depending on team structure) and a higher percentage of time in patient rooms. Interruptions were more common with geographic cohorting. These hospitalists were interrupted every 14 minutes in the morning and every 8 minutes in the afternoon. Of these interruptions, 62% were face-to-face, 25% were electronic, and 13% were both simultaneously.
An important limitation of this study is that the investigators did not evaluate clinical outcomes or provider satisfaction. This may give some pause to the widespread push toward geographic cohorting.
Bottom line: More frequent interruptions may partially offset potential increases in patient-hospitalist interactions achieved through geographic cohorting.
Citation: Kara A et al. A time motion study evaluating the impact of geographic cohorting of hospitalists. J Hosp Med. 2020;15:338-44.
Dr. Sweigart is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: Geographic cohorting localizes hospitalist teams to a single unit. It has previously been shown to improve outcomes.
Design: Prospective time and motion study.
Setting: 11 geographically cohorted services and 4 noncohorted teams at Indiana University Health, a large academic medical center.
Synopsis: Geotracking was used to monitor time spent inside and outside of patient rooms for 17 hospitalists over at least 6 weeks. Eight hospitalists were also directly observed. Both groups spent roughly three times more time outside patient rooms than inside. Geographic cohorting was associated with longer patient visits (ranging from 69.6 to 101.7 minutes per day depending on team structure) and a higher percentage of time in patient rooms. Interruptions were more common with geographic cohorting. These hospitalists were interrupted every 14 minutes in the morning and every 8 minutes in the afternoon. Of these interruptions, 62% were face-to-face, 25% were electronic, and 13% were both simultaneously.
An important limitation of this study is that the investigators did not evaluate clinical outcomes or provider satisfaction. This may give some pause to the widespread push toward geographic cohorting.
Bottom line: More frequent interruptions may partially offset potential increases in patient-hospitalist interactions achieved through geographic cohorting.
Citation: Kara A et al. A time motion study evaluating the impact of geographic cohorting of hospitalists. J Hosp Med. 2020;15:338-44.
Dr. Sweigart is a hospitalist at the Lexington (Ky.) VA Health Care System.
Survey spotlights double-edged sword for minority cardiologists
Survey results paint a stark picture of discrimination among racial minorities in the cardiology workforce but also a strong sense of belonging.
Among respondents to the 2015 American College of Cardiology (ACC) Professional Life Survey, which is the most recent survey, over half (52.3%) of underrepresented racial and ethnic minorities (URMs) and 45.5% of Asian or Pacific Islanders reported experiencing discrimination compared with 36.4% of Whites (both P < .01).
Nevertheless, 91.2% of URMs reported being satisfied with their career, as did 90% of Asians or Pacific Islanders and 89.1% of Whites.
Satisfaction with financial compensation also did not differ between groups, and most cardiologists believed their opportunities for advancement were similar to those of their peers.
One possible explanation is that the respondents may simply be people who’ve had better experiences, lead author Kevin L. Thomas, MD, Duke Clinical Research Institute, Durham, N.C., and colleagues told this news organization. A second hypothesis looks more to sheer determination, or grit.
“Perhaps along the sometimes circuitous pathway to being a cardiologist – which is a lot of training, a lot of standardized testing, a lot of applications – that maybe you sub-select a group of individuals who are simply more resilient based on their life experiences and things that they’ve overcome to get where they are,” he said.
Interestingly, rates of burnout were lower among URMs (22.4%) and Asians/Pacific Islanders (20.1%) than Whites (30.3%; P = .02 and P < .01, respectively). The finding is unexpected but in line with a recent report of more than 4,400 U.S. physicians finding lower odds of burnout among Asian, Hispanic/Latinx, and Black physicians.
The new study, published October 18 in the Journal of the American College of Cardiology, however, affirms that women of all racial and ethnic groups face significant headwinds in the White, male-dominated cardiology workforce.
Just 13.9% of White men reported experiencing discrimination, compared with 44.6% of URM men and 36.2% of Asians/Pacific Islander men. In comparison, 69.2% of White women reported discrimination, as did 62.7% of URM women and 57% of Asian/Pacific Islander women (both P <.01).
“When you look specifically at White men versus White women, there is a large discrepancy there, and it just shows us, I think, for a lot of different groups, we still have a long way to go in terms of trying to achieve equity and to try to be inclusive in the workplace,” Dr. Thomas said.
Men were more likely to experience race- and religion-based discrimination in the workplace, whereas nearly all women reported sex discrimination, with parenting an important second. Approximately 85% of cardiologists reported being satisfied with their family lives, although unpublished data suggest URMs were less likely to be married and to have fewer children, Dr. Thomas said.
During job negotiations, URM cardiologists were less likely to prioritize salary, benefits, and work hours for their first job (13.6%, 10.9% 19.3%) than White cardiologists (20.6%, 23.3%, 31.3%; P < .02 for all).
In subsequent negotiations, URMs placed more emphasis on salary, benefits, and work hours than Whites, whereas both URMs and Asians/Pacific Islanders placed a greater importance on travel benefits, diversity, mentoring, workspace, time to promotion, academic rank, and roles with community, institutional, or national recognition, which the authors say, “might indicate a greater need to overcome systemic barriers.”
Three-fourths of all cardiologist respondents had a mentor during training, which can take many shapes, Dr. Thomas noted. “Within my own section as an electrophysiologist, which is a very subspecialized category, we have four Black electrophysiologists, and I think it was because many of us mentored each other as we came along, and it inspired us.”
URMs are more likely to experience the so-called “minority tax” of being tapped for added responsibilities in the name of inclusivity efforts, he said, and called on individuals from the dominant culture to mentor or sponsor cardiologists from other racial groups and to carve out leadership pathways for women and minorities so they “can use their gifts to benefit the profession at large,” leading clinical trials or steering committees and serving in high-profile roles.
Although the events of 2020 sharpened attention on the issue of diversity in America, Dr. Thomas and colleagues say that more work needs to be done defining the problem and that professional organizations and health systems also should systematically collect sex, racial, and ethnic identifies of members using classifications similar to the 2020 U.S. Census.
The study was based on 2,245 respondents to the 2015 Professional Life Survey, which was not specifically designed to assess racial/ethnic diversity topics and had a response rate of 21%, which limited representatives of each group.
In all, 197 were from URMs (80 Blacks, 113 Hispanics, 4 Native Americans), 564 were Asians/Pacific Islanders, 1,447 were Whites, and 37 listed multiracial/other. More than half (58%) were men, and most were adult cardiologists (83% to 85%), followed by pediatric cardiology (6% to 10%) and cardiovascular surgery (1% to 2%).
“Further research is needed to understand these findings and their significance, because ongoing efforts within ACC and other organizations to increase diversity will fail unless this is successfully addressed,” the authors conclude.
To that end, Dr. Thomas said they are looking to develop a new survey that taps other groups like the Association of Black Cardiologists and members of the LGBTQ community.
“I’m really excited about the opportunity to develop a survey that specifically has the objective of trying to understand the experiences of systematically disadvantaged, historically marginalized groups to see if we can see the same information, but maybe through a clear lens, and then be able to develop strategies to mitigate some of the challenges that we see” he said. “So we can increase the numbers and also have a workforce that is reflective of the populations that we take care of and the nation as a whole.”
The study was funded by the American College of Cardiology. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Survey results paint a stark picture of discrimination among racial minorities in the cardiology workforce but also a strong sense of belonging.
Among respondents to the 2015 American College of Cardiology (ACC) Professional Life Survey, which is the most recent survey, over half (52.3%) of underrepresented racial and ethnic minorities (URMs) and 45.5% of Asian or Pacific Islanders reported experiencing discrimination compared with 36.4% of Whites (both P < .01).
Nevertheless, 91.2% of URMs reported being satisfied with their career, as did 90% of Asians or Pacific Islanders and 89.1% of Whites.
Satisfaction with financial compensation also did not differ between groups, and most cardiologists believed their opportunities for advancement were similar to those of their peers.
One possible explanation is that the respondents may simply be people who’ve had better experiences, lead author Kevin L. Thomas, MD, Duke Clinical Research Institute, Durham, N.C., and colleagues told this news organization. A second hypothesis looks more to sheer determination, or grit.
“Perhaps along the sometimes circuitous pathway to being a cardiologist – which is a lot of training, a lot of standardized testing, a lot of applications – that maybe you sub-select a group of individuals who are simply more resilient based on their life experiences and things that they’ve overcome to get where they are,” he said.
Interestingly, rates of burnout were lower among URMs (22.4%) and Asians/Pacific Islanders (20.1%) than Whites (30.3%; P = .02 and P < .01, respectively). The finding is unexpected but in line with a recent report of more than 4,400 U.S. physicians finding lower odds of burnout among Asian, Hispanic/Latinx, and Black physicians.
The new study, published October 18 in the Journal of the American College of Cardiology, however, affirms that women of all racial and ethnic groups face significant headwinds in the White, male-dominated cardiology workforce.
Just 13.9% of White men reported experiencing discrimination, compared with 44.6% of URM men and 36.2% of Asians/Pacific Islander men. In comparison, 69.2% of White women reported discrimination, as did 62.7% of URM women and 57% of Asian/Pacific Islander women (both P <.01).
“When you look specifically at White men versus White women, there is a large discrepancy there, and it just shows us, I think, for a lot of different groups, we still have a long way to go in terms of trying to achieve equity and to try to be inclusive in the workplace,” Dr. Thomas said.
Men were more likely to experience race- and religion-based discrimination in the workplace, whereas nearly all women reported sex discrimination, with parenting an important second. Approximately 85% of cardiologists reported being satisfied with their family lives, although unpublished data suggest URMs were less likely to be married and to have fewer children, Dr. Thomas said.
During job negotiations, URM cardiologists were less likely to prioritize salary, benefits, and work hours for their first job (13.6%, 10.9% 19.3%) than White cardiologists (20.6%, 23.3%, 31.3%; P < .02 for all).
In subsequent negotiations, URMs placed more emphasis on salary, benefits, and work hours than Whites, whereas both URMs and Asians/Pacific Islanders placed a greater importance on travel benefits, diversity, mentoring, workspace, time to promotion, academic rank, and roles with community, institutional, or national recognition, which the authors say, “might indicate a greater need to overcome systemic barriers.”
Three-fourths of all cardiologist respondents had a mentor during training, which can take many shapes, Dr. Thomas noted. “Within my own section as an electrophysiologist, which is a very subspecialized category, we have four Black electrophysiologists, and I think it was because many of us mentored each other as we came along, and it inspired us.”
URMs are more likely to experience the so-called “minority tax” of being tapped for added responsibilities in the name of inclusivity efforts, he said, and called on individuals from the dominant culture to mentor or sponsor cardiologists from other racial groups and to carve out leadership pathways for women and minorities so they “can use their gifts to benefit the profession at large,” leading clinical trials or steering committees and serving in high-profile roles.
Although the events of 2020 sharpened attention on the issue of diversity in America, Dr. Thomas and colleagues say that more work needs to be done defining the problem and that professional organizations and health systems also should systematically collect sex, racial, and ethnic identifies of members using classifications similar to the 2020 U.S. Census.
The study was based on 2,245 respondents to the 2015 Professional Life Survey, which was not specifically designed to assess racial/ethnic diversity topics and had a response rate of 21%, which limited representatives of each group.
In all, 197 were from URMs (80 Blacks, 113 Hispanics, 4 Native Americans), 564 were Asians/Pacific Islanders, 1,447 were Whites, and 37 listed multiracial/other. More than half (58%) were men, and most were adult cardiologists (83% to 85%), followed by pediatric cardiology (6% to 10%) and cardiovascular surgery (1% to 2%).
“Further research is needed to understand these findings and their significance, because ongoing efforts within ACC and other organizations to increase diversity will fail unless this is successfully addressed,” the authors conclude.
To that end, Dr. Thomas said they are looking to develop a new survey that taps other groups like the Association of Black Cardiologists and members of the LGBTQ community.
“I’m really excited about the opportunity to develop a survey that specifically has the objective of trying to understand the experiences of systematically disadvantaged, historically marginalized groups to see if we can see the same information, but maybe through a clear lens, and then be able to develop strategies to mitigate some of the challenges that we see” he said. “So we can increase the numbers and also have a workforce that is reflective of the populations that we take care of and the nation as a whole.”
The study was funded by the American College of Cardiology. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Survey results paint a stark picture of discrimination among racial minorities in the cardiology workforce but also a strong sense of belonging.
Among respondents to the 2015 American College of Cardiology (ACC) Professional Life Survey, which is the most recent survey, over half (52.3%) of underrepresented racial and ethnic minorities (URMs) and 45.5% of Asian or Pacific Islanders reported experiencing discrimination compared with 36.4% of Whites (both P < .01).
Nevertheless, 91.2% of URMs reported being satisfied with their career, as did 90% of Asians or Pacific Islanders and 89.1% of Whites.
Satisfaction with financial compensation also did not differ between groups, and most cardiologists believed their opportunities for advancement were similar to those of their peers.
One possible explanation is that the respondents may simply be people who’ve had better experiences, lead author Kevin L. Thomas, MD, Duke Clinical Research Institute, Durham, N.C., and colleagues told this news organization. A second hypothesis looks more to sheer determination, or grit.
“Perhaps along the sometimes circuitous pathway to being a cardiologist – which is a lot of training, a lot of standardized testing, a lot of applications – that maybe you sub-select a group of individuals who are simply more resilient based on their life experiences and things that they’ve overcome to get where they are,” he said.
Interestingly, rates of burnout were lower among URMs (22.4%) and Asians/Pacific Islanders (20.1%) than Whites (30.3%; P = .02 and P < .01, respectively). The finding is unexpected but in line with a recent report of more than 4,400 U.S. physicians finding lower odds of burnout among Asian, Hispanic/Latinx, and Black physicians.
The new study, published October 18 in the Journal of the American College of Cardiology, however, affirms that women of all racial and ethnic groups face significant headwinds in the White, male-dominated cardiology workforce.
Just 13.9% of White men reported experiencing discrimination, compared with 44.6% of URM men and 36.2% of Asians/Pacific Islander men. In comparison, 69.2% of White women reported discrimination, as did 62.7% of URM women and 57% of Asian/Pacific Islander women (both P <.01).
“When you look specifically at White men versus White women, there is a large discrepancy there, and it just shows us, I think, for a lot of different groups, we still have a long way to go in terms of trying to achieve equity and to try to be inclusive in the workplace,” Dr. Thomas said.
Men were more likely to experience race- and religion-based discrimination in the workplace, whereas nearly all women reported sex discrimination, with parenting an important second. Approximately 85% of cardiologists reported being satisfied with their family lives, although unpublished data suggest URMs were less likely to be married and to have fewer children, Dr. Thomas said.
During job negotiations, URM cardiologists were less likely to prioritize salary, benefits, and work hours for their first job (13.6%, 10.9% 19.3%) than White cardiologists (20.6%, 23.3%, 31.3%; P < .02 for all).
In subsequent negotiations, URMs placed more emphasis on salary, benefits, and work hours than Whites, whereas both URMs and Asians/Pacific Islanders placed a greater importance on travel benefits, diversity, mentoring, workspace, time to promotion, academic rank, and roles with community, institutional, or national recognition, which the authors say, “might indicate a greater need to overcome systemic barriers.”
Three-fourths of all cardiologist respondents had a mentor during training, which can take many shapes, Dr. Thomas noted. “Within my own section as an electrophysiologist, which is a very subspecialized category, we have four Black electrophysiologists, and I think it was because many of us mentored each other as we came along, and it inspired us.”
URMs are more likely to experience the so-called “minority tax” of being tapped for added responsibilities in the name of inclusivity efforts, he said, and called on individuals from the dominant culture to mentor or sponsor cardiologists from other racial groups and to carve out leadership pathways for women and minorities so they “can use their gifts to benefit the profession at large,” leading clinical trials or steering committees and serving in high-profile roles.
Although the events of 2020 sharpened attention on the issue of diversity in America, Dr. Thomas and colleagues say that more work needs to be done defining the problem and that professional organizations and health systems also should systematically collect sex, racial, and ethnic identifies of members using classifications similar to the 2020 U.S. Census.
The study was based on 2,245 respondents to the 2015 Professional Life Survey, which was not specifically designed to assess racial/ethnic diversity topics and had a response rate of 21%, which limited representatives of each group.
In all, 197 were from URMs (80 Blacks, 113 Hispanics, 4 Native Americans), 564 were Asians/Pacific Islanders, 1,447 were Whites, and 37 listed multiracial/other. More than half (58%) were men, and most were adult cardiologists (83% to 85%), followed by pediatric cardiology (6% to 10%) and cardiovascular surgery (1% to 2%).
“Further research is needed to understand these findings and their significance, because ongoing efforts within ACC and other organizations to increase diversity will fail unless this is successfully addressed,” the authors conclude.
To that end, Dr. Thomas said they are looking to develop a new survey that taps other groups like the Association of Black Cardiologists and members of the LGBTQ community.
“I’m really excited about the opportunity to develop a survey that specifically has the objective of trying to understand the experiences of systematically disadvantaged, historically marginalized groups to see if we can see the same information, but maybe through a clear lens, and then be able to develop strategies to mitigate some of the challenges that we see” he said. “So we can increase the numbers and also have a workforce that is reflective of the populations that we take care of and the nation as a whole.”
The study was funded by the American College of Cardiology. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Guidelines for managing hypo- and hyperparathyroidism
A large international team of experts has developed two comprehensive guidelines for diagnosing, evaluating, and managing hypoparathyroidism and hyperparathyroidism, which replace guidelines issued 5 and 7 years ago.
Aliya A. Khan, MD, presented an overview of the hypoparathyroidism guidelines and John P. Bilezikian, MD, presented key aspects of the hyperparathyroidism guidelines at the American Society of Bone and Mineral Research (ASBMR) 2021 Annual Meeting.
The guidelines will be published as 17 articles in two issues of the society’s Journal of Bone and Mineral Research in 2022 – one on hypoparathyroidism and the other on hyperparathyroidism.
The work represents an “unprecedented effort” by more than 100 experts from 16 countries (United States, Canada, Australia, Brazil, China, Denmark, France, Germany, India, Italy, Israel, Lebanon, Singapore, Spain, Sweden, and the United Kingdom), Dr. Bilezikian told this news organization in an interview.
More than 100 international and national endocrine and osteoporosis organizations, societies, and patient advocacy groups from more than 50 countries have expressed interest in endorsing the guidelines.
Management of hypoparathyroidism
The new guidelines on hypoparathyroidism replace the guidelines issued in 2016 that were developed at the First International Conference on the Management of Hypoparathyroidism, Dr. Khan, from McMaster University, Hamilton, Ont., said in an email.
There was a need for new hypoparathyroidism guidelines, she explained, because of the better understanding of associated complications, how to predict who will develop hypoparathyroidism postoperatively (and how to prevent this), how and when to investigate a genetic cause further, when to consider parathyroid hormone (PTH) replacement therapy (and the benefits of the various molecules available today as well as those being evaluated in clinical research), and how to diagnose and manage hypoparathyroidism during pregnancy and lactation.
The experts in hypoparathyroidism were divided into four task forces that covered epidemiology and financial burden, etiology and pathophysiology, genetics and diagnosis, and patient evaluation and management.
The guidelines, developed over the past 18 months, provide detailed evidence-based graded (strong to weak) as well as ungraded (current practice) recommendations.
Summarizing a few key takeaways, Dr. Khan noted the guidelines recommend that clinicians treating patients with hypoparathyroidism should:
- Diagnose hypoparathyroidism if serum calcium corrected for albumin is low in the presence of a low or inappropriately normal PTH confirmed on two occasions 2 weeks apart (which may be supported by other specified abnormalities).
- Determine the cause for the hypoparathyroidism (which includes postsurgery, genetic variant, autoimmune, radiation, or idiopathic causes).
- Evaluate target organ damage.
- Try to achieve treatment goals and minimize risks for long-term complications.
- Consider PTH replacement therapy if patients have inadequate control, with symptoms of hypocalcemia or hypercalcemia, high phosphate, kidney disease, or high urine calcium, or poor quality of life.
The guideline strongly recommends using PTH measurements after total thyroidectomy to try to predict which patients will develop permanent postsurgical hypoparathyroidism.
It provides a clinical approach for establishing the genetic etiology of hypoparathyroidism.
A meta-analysis of 81 studies identified that the most common symptoms/complications of chronic hypoparathyroidism were, in descending order, cataract (24%), infection (18%), nephrolithiasis, renal insufficiency, seizures, depression, ischemic heart disease, and arrhythmias.
Based on the best available evidence, the guideline advises that “clinicians need to carefully determine why a patient has hypoparathyroidism and develop an individualized treatment plan with conventional therapy consisting of calcium, active vitamin D, hydrochlorothiazide, and plain vitamin D,” Dr. Khan continued.
“If a patient has poorly controlled hypoparathyroidism with many symptoms or is not doing well, then clinicians must consider PTH replacement therapy, since this will replace the missing hormone, lower the urine calcium losses, bring the serum calcium back up to the normal reference range, and lower phosphate (which appears to be associated with kidney calcification and may also contribute to basal ganglia calcification and calcium deposits in the eye),” she noted.
The guideline also discusses the optimal way to monitor and treat patients during pregnancy, delivery, and breastfeeding to optimize outcomes for mother and baby. The key points are closer patient monitoring with normalization of calcium, urine calcium, phosphate, and vitamin D.
Management of primary hyperparathyroidism
There was a need to update the previous 2014 guidelines developed at the Fourth International Workshop on the Management of Primary Hyperparathyroidism because, among other things, recent studies have provided new evidence about the different clinical phenotypes of primary hyperparathyroidism and ways the disease affects the skeleton and kidneys, Dr. Bilezikian, from the College of Physicians and Surgeons, Columbia University, New York, explained.
The experts in hyperparathyroidism were divided into four task forces that covered epidemiology, pathophysiology and genetics; classical and nonclassical disease manifestations; surgical aspects; and patient evaluation and management.
As part of these topics, the experts reviewed biochemical, skeletal, and renal findings, nonclassical features (such as neurocognitive complaints), nutritional and pharmacologic approaches, and disease course with or without surgical or medical intervention.
They made recommendations for diagnosis of hypercalcemic and normocalcemic phenotypes, differential diagnosis, evaluation of the skeleton and the kidney, indications for surgery, role of parathyroid imaging, indications for pharmacologic intervention, and monitoring.
“Consider the way this disease has appeared to change in the last 50 years,” said Dr. Bilezikian. In the 1940s, 50s, and 60s, patients with hyperparathyroidism were really sick and had severe bone disease and kidney disease. Then in the 70s, 80s, and 90s, the disease was more often discovered because of a screening test; high serum calcium was a hallmark of finding asymptomatic hyperparathyroidism.
In recent years, hyperparathyroidism is often discovered incidentally, when examining the skeleton or kidneys, he continued.
Primary hyperparathyroidism can now be subdivided into three types: patients who have target organ (kidney, bone) involvement, patients who don’t have this, and patients who have normocalcemic primary hyperparathyroidism.
The guideline discusses new medications that have become available for hyperparathyroidism, as well as surgery (the only cure), including how preoperative imaging can identify the overactive parathyroid gland, and the guidelines go into detail about how to monitor a patient and why a clinician would or would not recommend surgery, Dr. Bilezikian explained.
In the end, treatment is tailored to the individual.
Last, the guideline identifies eight areas where more research is needed.
The guidelines were funded by unrestricted educational grants from Amolyt, Ascendis, Calcilytix, and Takeda. Dr. Khan has reported participating on advisory boards for Alexion, Amgen, Amolyt, and Takeda, being a consultant for Amgen, receiving grants from Alexion, Amgen, Takeda, and Ascendis, being an investigator for Alexion, Amgen, Takeda, Ascendis, and Chugai, and being a speaker for Alexion, Amgen, Takeda, and Ultragenyx. Dr. Bilezikian has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large international team of experts has developed two comprehensive guidelines for diagnosing, evaluating, and managing hypoparathyroidism and hyperparathyroidism, which replace guidelines issued 5 and 7 years ago.
Aliya A. Khan, MD, presented an overview of the hypoparathyroidism guidelines and John P. Bilezikian, MD, presented key aspects of the hyperparathyroidism guidelines at the American Society of Bone and Mineral Research (ASBMR) 2021 Annual Meeting.
The guidelines will be published as 17 articles in two issues of the society’s Journal of Bone and Mineral Research in 2022 – one on hypoparathyroidism and the other on hyperparathyroidism.
The work represents an “unprecedented effort” by more than 100 experts from 16 countries (United States, Canada, Australia, Brazil, China, Denmark, France, Germany, India, Italy, Israel, Lebanon, Singapore, Spain, Sweden, and the United Kingdom), Dr. Bilezikian told this news organization in an interview.
More than 100 international and national endocrine and osteoporosis organizations, societies, and patient advocacy groups from more than 50 countries have expressed interest in endorsing the guidelines.
Management of hypoparathyroidism
The new guidelines on hypoparathyroidism replace the guidelines issued in 2016 that were developed at the First International Conference on the Management of Hypoparathyroidism, Dr. Khan, from McMaster University, Hamilton, Ont., said in an email.
There was a need for new hypoparathyroidism guidelines, she explained, because of the better understanding of associated complications, how to predict who will develop hypoparathyroidism postoperatively (and how to prevent this), how and when to investigate a genetic cause further, when to consider parathyroid hormone (PTH) replacement therapy (and the benefits of the various molecules available today as well as those being evaluated in clinical research), and how to diagnose and manage hypoparathyroidism during pregnancy and lactation.
The experts in hypoparathyroidism were divided into four task forces that covered epidemiology and financial burden, etiology and pathophysiology, genetics and diagnosis, and patient evaluation and management.
The guidelines, developed over the past 18 months, provide detailed evidence-based graded (strong to weak) as well as ungraded (current practice) recommendations.
Summarizing a few key takeaways, Dr. Khan noted the guidelines recommend that clinicians treating patients with hypoparathyroidism should:
- Diagnose hypoparathyroidism if serum calcium corrected for albumin is low in the presence of a low or inappropriately normal PTH confirmed on two occasions 2 weeks apart (which may be supported by other specified abnormalities).
- Determine the cause for the hypoparathyroidism (which includes postsurgery, genetic variant, autoimmune, radiation, or idiopathic causes).
- Evaluate target organ damage.
- Try to achieve treatment goals and minimize risks for long-term complications.
- Consider PTH replacement therapy if patients have inadequate control, with symptoms of hypocalcemia or hypercalcemia, high phosphate, kidney disease, or high urine calcium, or poor quality of life.
The guideline strongly recommends using PTH measurements after total thyroidectomy to try to predict which patients will develop permanent postsurgical hypoparathyroidism.
It provides a clinical approach for establishing the genetic etiology of hypoparathyroidism.
A meta-analysis of 81 studies identified that the most common symptoms/complications of chronic hypoparathyroidism were, in descending order, cataract (24%), infection (18%), nephrolithiasis, renal insufficiency, seizures, depression, ischemic heart disease, and arrhythmias.
Based on the best available evidence, the guideline advises that “clinicians need to carefully determine why a patient has hypoparathyroidism and develop an individualized treatment plan with conventional therapy consisting of calcium, active vitamin D, hydrochlorothiazide, and plain vitamin D,” Dr. Khan continued.
“If a patient has poorly controlled hypoparathyroidism with many symptoms or is not doing well, then clinicians must consider PTH replacement therapy, since this will replace the missing hormone, lower the urine calcium losses, bring the serum calcium back up to the normal reference range, and lower phosphate (which appears to be associated with kidney calcification and may also contribute to basal ganglia calcification and calcium deposits in the eye),” she noted.
The guideline also discusses the optimal way to monitor and treat patients during pregnancy, delivery, and breastfeeding to optimize outcomes for mother and baby. The key points are closer patient monitoring with normalization of calcium, urine calcium, phosphate, and vitamin D.
Management of primary hyperparathyroidism
There was a need to update the previous 2014 guidelines developed at the Fourth International Workshop on the Management of Primary Hyperparathyroidism because, among other things, recent studies have provided new evidence about the different clinical phenotypes of primary hyperparathyroidism and ways the disease affects the skeleton and kidneys, Dr. Bilezikian, from the College of Physicians and Surgeons, Columbia University, New York, explained.
The experts in hyperparathyroidism were divided into four task forces that covered epidemiology, pathophysiology and genetics; classical and nonclassical disease manifestations; surgical aspects; and patient evaluation and management.
As part of these topics, the experts reviewed biochemical, skeletal, and renal findings, nonclassical features (such as neurocognitive complaints), nutritional and pharmacologic approaches, and disease course with or without surgical or medical intervention.
They made recommendations for diagnosis of hypercalcemic and normocalcemic phenotypes, differential diagnosis, evaluation of the skeleton and the kidney, indications for surgery, role of parathyroid imaging, indications for pharmacologic intervention, and monitoring.
“Consider the way this disease has appeared to change in the last 50 years,” said Dr. Bilezikian. In the 1940s, 50s, and 60s, patients with hyperparathyroidism were really sick and had severe bone disease and kidney disease. Then in the 70s, 80s, and 90s, the disease was more often discovered because of a screening test; high serum calcium was a hallmark of finding asymptomatic hyperparathyroidism.
In recent years, hyperparathyroidism is often discovered incidentally, when examining the skeleton or kidneys, he continued.
Primary hyperparathyroidism can now be subdivided into three types: patients who have target organ (kidney, bone) involvement, patients who don’t have this, and patients who have normocalcemic primary hyperparathyroidism.
The guideline discusses new medications that have become available for hyperparathyroidism, as well as surgery (the only cure), including how preoperative imaging can identify the overactive parathyroid gland, and the guidelines go into detail about how to monitor a patient and why a clinician would or would not recommend surgery, Dr. Bilezikian explained.
In the end, treatment is tailored to the individual.
Last, the guideline identifies eight areas where more research is needed.
The guidelines were funded by unrestricted educational grants from Amolyt, Ascendis, Calcilytix, and Takeda. Dr. Khan has reported participating on advisory boards for Alexion, Amgen, Amolyt, and Takeda, being a consultant for Amgen, receiving grants from Alexion, Amgen, Takeda, and Ascendis, being an investigator for Alexion, Amgen, Takeda, Ascendis, and Chugai, and being a speaker for Alexion, Amgen, Takeda, and Ultragenyx. Dr. Bilezikian has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large international team of experts has developed two comprehensive guidelines for diagnosing, evaluating, and managing hypoparathyroidism and hyperparathyroidism, which replace guidelines issued 5 and 7 years ago.
Aliya A. Khan, MD, presented an overview of the hypoparathyroidism guidelines and John P. Bilezikian, MD, presented key aspects of the hyperparathyroidism guidelines at the American Society of Bone and Mineral Research (ASBMR) 2021 Annual Meeting.
The guidelines will be published as 17 articles in two issues of the society’s Journal of Bone and Mineral Research in 2022 – one on hypoparathyroidism and the other on hyperparathyroidism.
The work represents an “unprecedented effort” by more than 100 experts from 16 countries (United States, Canada, Australia, Brazil, China, Denmark, France, Germany, India, Italy, Israel, Lebanon, Singapore, Spain, Sweden, and the United Kingdom), Dr. Bilezikian told this news organization in an interview.
More than 100 international and national endocrine and osteoporosis organizations, societies, and patient advocacy groups from more than 50 countries have expressed interest in endorsing the guidelines.
Management of hypoparathyroidism
The new guidelines on hypoparathyroidism replace the guidelines issued in 2016 that were developed at the First International Conference on the Management of Hypoparathyroidism, Dr. Khan, from McMaster University, Hamilton, Ont., said in an email.
There was a need for new hypoparathyroidism guidelines, she explained, because of the better understanding of associated complications, how to predict who will develop hypoparathyroidism postoperatively (and how to prevent this), how and when to investigate a genetic cause further, when to consider parathyroid hormone (PTH) replacement therapy (and the benefits of the various molecules available today as well as those being evaluated in clinical research), and how to diagnose and manage hypoparathyroidism during pregnancy and lactation.
The experts in hypoparathyroidism were divided into four task forces that covered epidemiology and financial burden, etiology and pathophysiology, genetics and diagnosis, and patient evaluation and management.
The guidelines, developed over the past 18 months, provide detailed evidence-based graded (strong to weak) as well as ungraded (current practice) recommendations.
Summarizing a few key takeaways, Dr. Khan noted the guidelines recommend that clinicians treating patients with hypoparathyroidism should:
- Diagnose hypoparathyroidism if serum calcium corrected for albumin is low in the presence of a low or inappropriately normal PTH confirmed on two occasions 2 weeks apart (which may be supported by other specified abnormalities).
- Determine the cause for the hypoparathyroidism (which includes postsurgery, genetic variant, autoimmune, radiation, or idiopathic causes).
- Evaluate target organ damage.
- Try to achieve treatment goals and minimize risks for long-term complications.
- Consider PTH replacement therapy if patients have inadequate control, with symptoms of hypocalcemia or hypercalcemia, high phosphate, kidney disease, or high urine calcium, or poor quality of life.
The guideline strongly recommends using PTH measurements after total thyroidectomy to try to predict which patients will develop permanent postsurgical hypoparathyroidism.
It provides a clinical approach for establishing the genetic etiology of hypoparathyroidism.
A meta-analysis of 81 studies identified that the most common symptoms/complications of chronic hypoparathyroidism were, in descending order, cataract (24%), infection (18%), nephrolithiasis, renal insufficiency, seizures, depression, ischemic heart disease, and arrhythmias.
Based on the best available evidence, the guideline advises that “clinicians need to carefully determine why a patient has hypoparathyroidism and develop an individualized treatment plan with conventional therapy consisting of calcium, active vitamin D, hydrochlorothiazide, and plain vitamin D,” Dr. Khan continued.
“If a patient has poorly controlled hypoparathyroidism with many symptoms or is not doing well, then clinicians must consider PTH replacement therapy, since this will replace the missing hormone, lower the urine calcium losses, bring the serum calcium back up to the normal reference range, and lower phosphate (which appears to be associated with kidney calcification and may also contribute to basal ganglia calcification and calcium deposits in the eye),” she noted.
The guideline also discusses the optimal way to monitor and treat patients during pregnancy, delivery, and breastfeeding to optimize outcomes for mother and baby. The key points are closer patient monitoring with normalization of calcium, urine calcium, phosphate, and vitamin D.
Management of primary hyperparathyroidism
There was a need to update the previous 2014 guidelines developed at the Fourth International Workshop on the Management of Primary Hyperparathyroidism because, among other things, recent studies have provided new evidence about the different clinical phenotypes of primary hyperparathyroidism and ways the disease affects the skeleton and kidneys, Dr. Bilezikian, from the College of Physicians and Surgeons, Columbia University, New York, explained.
The experts in hyperparathyroidism were divided into four task forces that covered epidemiology, pathophysiology and genetics; classical and nonclassical disease manifestations; surgical aspects; and patient evaluation and management.
As part of these topics, the experts reviewed biochemical, skeletal, and renal findings, nonclassical features (such as neurocognitive complaints), nutritional and pharmacologic approaches, and disease course with or without surgical or medical intervention.
They made recommendations for diagnosis of hypercalcemic and normocalcemic phenotypes, differential diagnosis, evaluation of the skeleton and the kidney, indications for surgery, role of parathyroid imaging, indications for pharmacologic intervention, and monitoring.
“Consider the way this disease has appeared to change in the last 50 years,” said Dr. Bilezikian. In the 1940s, 50s, and 60s, patients with hyperparathyroidism were really sick and had severe bone disease and kidney disease. Then in the 70s, 80s, and 90s, the disease was more often discovered because of a screening test; high serum calcium was a hallmark of finding asymptomatic hyperparathyroidism.
In recent years, hyperparathyroidism is often discovered incidentally, when examining the skeleton or kidneys, he continued.
Primary hyperparathyroidism can now be subdivided into three types: patients who have target organ (kidney, bone) involvement, patients who don’t have this, and patients who have normocalcemic primary hyperparathyroidism.
The guideline discusses new medications that have become available for hyperparathyroidism, as well as surgery (the only cure), including how preoperative imaging can identify the overactive parathyroid gland, and the guidelines go into detail about how to monitor a patient and why a clinician would or would not recommend surgery, Dr. Bilezikian explained.
In the end, treatment is tailored to the individual.
Last, the guideline identifies eight areas where more research is needed.
The guidelines were funded by unrestricted educational grants from Amolyt, Ascendis, Calcilytix, and Takeda. Dr. Khan has reported participating on advisory boards for Alexion, Amgen, Amolyt, and Takeda, being a consultant for Amgen, receiving grants from Alexion, Amgen, Takeda, and Ascendis, being an investigator for Alexion, Amgen, Takeda, Ascendis, and Chugai, and being a speaker for Alexion, Amgen, Takeda, and Ultragenyx. Dr. Bilezikian has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Severe Asthma: Allergic Asthma
On improving DLBCL outcomes, single-agent regimens fall short
, a review of the relevant literature suggests.
“In addition ... single-agent regimens are most likely not efficient enough to substantially improve the outcome of patients with DLBCL,” Wendan Xu and colleagues at University Hospital Munster, Germany, concluded, based on their review.
Indeed, novel combinations that include B-cell receptor (BCR) signaling and phosphatidylinositol 3-kinase (PI3K) inhibitors are needed for DLBCL treatment, and treatment should also include conventional chemoimmunotherapeutic regimens as well as other targeted agents and novel immunologic approaches, they wrote. Such novel combinations could overcome mechanisms of resistance and increase cure rates in individuals with DLBCL, they contended.
The authors’ observations are based on a search of the available data, from which they summarized the “current understanding of BCR signaling with a special focus on the PI3K pathway and its role in the pathogenesis of DLBCL.”
The addition of the anti-CD20 antibody rituximab to the CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone) significantly improved outcomes for patients with DLBCL, but about a third of patients are not cured by the rituximab-CHOP (R-CHOP) regimen and subsequent therapies, they said, explaining their rationale for the review.
“A better understanding of the molecular pathogenesis is warranted to use novel targeted agents in an optimal manner,” they said.
The authors also addressed clinical implications of the findings, and mechanisms of resistance to PI3k inhibitors. For example, they noted that:
–Bruton’s tyrosine kinase (BTK) inhibitors may be beneficial when added to R-CHOP.
In the randomized phase 3 PHOENIX trial, ibrutinib plus R-CHOP versus R-CHOP alone in patients with non–germinal center B-cell (non-GCB) DLBCL showed a survival benefit in patients over 60 years of age, which suggests a possible role for “an intensified R-CHOP regimen that includes a BTK inhibitor” in these patients, they said. They added that confirmatory trials are under way, including the ESCALADE trial looking at the second-generation BTK inhibitor acalabrutinib combined with R-CHOP versus R-CHOP alone in patients with untreated DLBCL.
–Results have been mixed with PI3K inhibitors.
Various PI3K inhibitors have been evaluated for the treatment of patients with DLBCL.
Idelalisib, a first-in-class PI3K-specific inhibitor approved for treatment of relapsed/refractory (r/r) follicular lymphoma, small lymphocytic lymphoma, and chronic lymphocytic leukemia (CLL), showed only modest activity in preclinical DLBCL models, and no responses were detectable in a small trial of patients with r/r DLBCL, the authors said. “Severe toxic side effects and treatment-related deaths occurred in several clinical trials that tested idelalisib in combination with antibodies alone or with antibodies and chemotherapy, leading to the premature discontinuation of some of these studies,” they noted.
Other studies investigating idelalisib plus lenalidomide and rituximab or the spleen tyrosine kinase (SYK) inhibitor entospletinib in patients with r/r CLL or lymphoma were also halted because of “overwhelming, immune-mediated pulmonary and/or hepatic toxicities.”
Copanlisib, an intravenous pan-class I PI3K inhibitor with preferential inhibition of PI3Ka and PI3Kd, showed some promise as monotherapy in a phase 2 trial of patients with r/r DLBCL. The overall response rate was about 20%, and response was “numerically higher” in activated B-cell like (ABC) DLBCL, compared with GCB DLBCL (32% vs. 13%), confirming preclinical data that showed PI3Ka/d inhibition effectiveness mainly in ABC DLBCL.
“Compared with idelalisib, copanlisib appears to have a more favorable toxicity profile, with a lower incidence of severe complications,” they said, adding that a phase 2 trial of copanlisib plus R-CHOP as first-line therapy for patients with DLBCL is under way.
Further, monotherapy with buparlisib, a pan-class I PI3K inhibitor, was associated with a low response rate of 11.5% in a DLBCL subcohort in a phase 2 study, whereas parsaclisib, a next-generation inhibitor with specificity to the PI3Kd isoform, showed efficacy as a monotherapy in patients with r/r DLBCL in a phase 2 study (overall response rate, 25.5%), they said, adding that other PI3K inhibitors with additional inhibitory effects are under clinical development.
–Various molecular mechanisms of resistance to PI3K inhibitors have been described preclinically and clinically.
In an unbiased exploratory analysis of samples from patients treated with copanlisib, a 16-gene mutation signature that separated responders from nonresponders was identified, the authors said.
The finding suggests that genetic aberrations dictate response to PI3K inhibitors, they noted.
“This 16-gene signature included TNFAIP3, CREBBP, and PRDM1, which are known to be important in the molecular pathogenesis of DLBCL,” they wrote. A composite score was developed to reflect the numerical presence or absence of mutations in the gene set, they explained, adding that patients with a high composite score had a significantly higher overall response rate and longer progression-free survival than did patients with a lower score.
In addition, idelalisib treatment resulted in a feedback activation of PI3Ka in ABC DLBCL cells.
“This rebound of PI3K activity was overcome by subsequent PI3Ka inhibition in preclinical DLBCL models, further underscoring the necessity of inhibiting both PI3Ka and PI3Kd to achieve responses in ABC DLBCL,” they wrote, adding that “[i]n ABC DLBCL models treated with the PI3Ka/PI3Kd inhibitor AZD8835, activated CARD11 mutations were identified as a mechanism of resistance.”
Investigations looking at various treatment combinations to overcome resistance to PI3K inhibition and improve the efficacy of targeted approaches are under way, they said.
For example, copanlisib plus the BCL-2 inhibitor venetoclax showed “synergistic activity in BCR-dependent DLBCLs, with genetic bases for BCL-2 dysregulation in vitro and in vivo,” and combination treatment with umbralisib and the proteasome inhibitor carfilzomib showed synergistic cytotoxicity in B-cell lymphoma, they said, noting that the latter combination is currently being evaluated in patients with DLBCL.
This work was supported by a research grant from the Deutsche Krebshilfe. Dr. Xu reported having no financial disclosures.
, a review of the relevant literature suggests.
“In addition ... single-agent regimens are most likely not efficient enough to substantially improve the outcome of patients with DLBCL,” Wendan Xu and colleagues at University Hospital Munster, Germany, concluded, based on their review.
Indeed, novel combinations that include B-cell receptor (BCR) signaling and phosphatidylinositol 3-kinase (PI3K) inhibitors are needed for DLBCL treatment, and treatment should also include conventional chemoimmunotherapeutic regimens as well as other targeted agents and novel immunologic approaches, they wrote. Such novel combinations could overcome mechanisms of resistance and increase cure rates in individuals with DLBCL, they contended.
The authors’ observations are based on a search of the available data, from which they summarized the “current understanding of BCR signaling with a special focus on the PI3K pathway and its role in the pathogenesis of DLBCL.”
The addition of the anti-CD20 antibody rituximab to the CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone) significantly improved outcomes for patients with DLBCL, but about a third of patients are not cured by the rituximab-CHOP (R-CHOP) regimen and subsequent therapies, they said, explaining their rationale for the review.
“A better understanding of the molecular pathogenesis is warranted to use novel targeted agents in an optimal manner,” they said.
The authors also addressed clinical implications of the findings, and mechanisms of resistance to PI3k inhibitors. For example, they noted that:
–Bruton’s tyrosine kinase (BTK) inhibitors may be beneficial when added to R-CHOP.
In the randomized phase 3 PHOENIX trial, ibrutinib plus R-CHOP versus R-CHOP alone in patients with non–germinal center B-cell (non-GCB) DLBCL showed a survival benefit in patients over 60 years of age, which suggests a possible role for “an intensified R-CHOP regimen that includes a BTK inhibitor” in these patients, they said. They added that confirmatory trials are under way, including the ESCALADE trial looking at the second-generation BTK inhibitor acalabrutinib combined with R-CHOP versus R-CHOP alone in patients with untreated DLBCL.
–Results have been mixed with PI3K inhibitors.
Various PI3K inhibitors have been evaluated for the treatment of patients with DLBCL.
Idelalisib, a first-in-class PI3K-specific inhibitor approved for treatment of relapsed/refractory (r/r) follicular lymphoma, small lymphocytic lymphoma, and chronic lymphocytic leukemia (CLL), showed only modest activity in preclinical DLBCL models, and no responses were detectable in a small trial of patients with r/r DLBCL, the authors said. “Severe toxic side effects and treatment-related deaths occurred in several clinical trials that tested idelalisib in combination with antibodies alone or with antibodies and chemotherapy, leading to the premature discontinuation of some of these studies,” they noted.
Other studies investigating idelalisib plus lenalidomide and rituximab or the spleen tyrosine kinase (SYK) inhibitor entospletinib in patients with r/r CLL or lymphoma were also halted because of “overwhelming, immune-mediated pulmonary and/or hepatic toxicities.”
Copanlisib, an intravenous pan-class I PI3K inhibitor with preferential inhibition of PI3Ka and PI3Kd, showed some promise as monotherapy in a phase 2 trial of patients with r/r DLBCL. The overall response rate was about 20%, and response was “numerically higher” in activated B-cell like (ABC) DLBCL, compared with GCB DLBCL (32% vs. 13%), confirming preclinical data that showed PI3Ka/d inhibition effectiveness mainly in ABC DLBCL.
“Compared with idelalisib, copanlisib appears to have a more favorable toxicity profile, with a lower incidence of severe complications,” they said, adding that a phase 2 trial of copanlisib plus R-CHOP as first-line therapy for patients with DLBCL is under way.
Further, monotherapy with buparlisib, a pan-class I PI3K inhibitor, was associated with a low response rate of 11.5% in a DLBCL subcohort in a phase 2 study, whereas parsaclisib, a next-generation inhibitor with specificity to the PI3Kd isoform, showed efficacy as a monotherapy in patients with r/r DLBCL in a phase 2 study (overall response rate, 25.5%), they said, adding that other PI3K inhibitors with additional inhibitory effects are under clinical development.
–Various molecular mechanisms of resistance to PI3K inhibitors have been described preclinically and clinically.
In an unbiased exploratory analysis of samples from patients treated with copanlisib, a 16-gene mutation signature that separated responders from nonresponders was identified, the authors said.
The finding suggests that genetic aberrations dictate response to PI3K inhibitors, they noted.
“This 16-gene signature included TNFAIP3, CREBBP, and PRDM1, which are known to be important in the molecular pathogenesis of DLBCL,” they wrote. A composite score was developed to reflect the numerical presence or absence of mutations in the gene set, they explained, adding that patients with a high composite score had a significantly higher overall response rate and longer progression-free survival than did patients with a lower score.
In addition, idelalisib treatment resulted in a feedback activation of PI3Ka in ABC DLBCL cells.
“This rebound of PI3K activity was overcome by subsequent PI3Ka inhibition in preclinical DLBCL models, further underscoring the necessity of inhibiting both PI3Ka and PI3Kd to achieve responses in ABC DLBCL,” they wrote, adding that “[i]n ABC DLBCL models treated with the PI3Ka/PI3Kd inhibitor AZD8835, activated CARD11 mutations were identified as a mechanism of resistance.”
Investigations looking at various treatment combinations to overcome resistance to PI3K inhibition and improve the efficacy of targeted approaches are under way, they said.
For example, copanlisib plus the BCL-2 inhibitor venetoclax showed “synergistic activity in BCR-dependent DLBCLs, with genetic bases for BCL-2 dysregulation in vitro and in vivo,” and combination treatment with umbralisib and the proteasome inhibitor carfilzomib showed synergistic cytotoxicity in B-cell lymphoma, they said, noting that the latter combination is currently being evaluated in patients with DLBCL.
This work was supported by a research grant from the Deutsche Krebshilfe. Dr. Xu reported having no financial disclosures.
, a review of the relevant literature suggests.
“In addition ... single-agent regimens are most likely not efficient enough to substantially improve the outcome of patients with DLBCL,” Wendan Xu and colleagues at University Hospital Munster, Germany, concluded, based on their review.
Indeed, novel combinations that include B-cell receptor (BCR) signaling and phosphatidylinositol 3-kinase (PI3K) inhibitors are needed for DLBCL treatment, and treatment should also include conventional chemoimmunotherapeutic regimens as well as other targeted agents and novel immunologic approaches, they wrote. Such novel combinations could overcome mechanisms of resistance and increase cure rates in individuals with DLBCL, they contended.
The authors’ observations are based on a search of the available data, from which they summarized the “current understanding of BCR signaling with a special focus on the PI3K pathway and its role in the pathogenesis of DLBCL.”
The addition of the anti-CD20 antibody rituximab to the CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone) significantly improved outcomes for patients with DLBCL, but about a third of patients are not cured by the rituximab-CHOP (R-CHOP) regimen and subsequent therapies, they said, explaining their rationale for the review.
“A better understanding of the molecular pathogenesis is warranted to use novel targeted agents in an optimal manner,” they said.
The authors also addressed clinical implications of the findings, and mechanisms of resistance to PI3k inhibitors. For example, they noted that:
–Bruton’s tyrosine kinase (BTK) inhibitors may be beneficial when added to R-CHOP.
In the randomized phase 3 PHOENIX trial, ibrutinib plus R-CHOP versus R-CHOP alone in patients with non–germinal center B-cell (non-GCB) DLBCL showed a survival benefit in patients over 60 years of age, which suggests a possible role for “an intensified R-CHOP regimen that includes a BTK inhibitor” in these patients, they said. They added that confirmatory trials are under way, including the ESCALADE trial looking at the second-generation BTK inhibitor acalabrutinib combined with R-CHOP versus R-CHOP alone in patients with untreated DLBCL.
–Results have been mixed with PI3K inhibitors.
Various PI3K inhibitors have been evaluated for the treatment of patients with DLBCL.
Idelalisib, a first-in-class PI3K-specific inhibitor approved for treatment of relapsed/refractory (r/r) follicular lymphoma, small lymphocytic lymphoma, and chronic lymphocytic leukemia (CLL), showed only modest activity in preclinical DLBCL models, and no responses were detectable in a small trial of patients with r/r DLBCL, the authors said. “Severe toxic side effects and treatment-related deaths occurred in several clinical trials that tested idelalisib in combination with antibodies alone or with antibodies and chemotherapy, leading to the premature discontinuation of some of these studies,” they noted.
Other studies investigating idelalisib plus lenalidomide and rituximab or the spleen tyrosine kinase (SYK) inhibitor entospletinib in patients with r/r CLL or lymphoma were also halted because of “overwhelming, immune-mediated pulmonary and/or hepatic toxicities.”
Copanlisib, an intravenous pan-class I PI3K inhibitor with preferential inhibition of PI3Ka and PI3Kd, showed some promise as monotherapy in a phase 2 trial of patients with r/r DLBCL. The overall response rate was about 20%, and response was “numerically higher” in activated B-cell like (ABC) DLBCL, compared with GCB DLBCL (32% vs. 13%), confirming preclinical data that showed PI3Ka/d inhibition effectiveness mainly in ABC DLBCL.
“Compared with idelalisib, copanlisib appears to have a more favorable toxicity profile, with a lower incidence of severe complications,” they said, adding that a phase 2 trial of copanlisib plus R-CHOP as first-line therapy for patients with DLBCL is under way.
Further, monotherapy with buparlisib, a pan-class I PI3K inhibitor, was associated with a low response rate of 11.5% in a DLBCL subcohort in a phase 2 study, whereas parsaclisib, a next-generation inhibitor with specificity to the PI3Kd isoform, showed efficacy as a monotherapy in patients with r/r DLBCL in a phase 2 study (overall response rate, 25.5%), they said, adding that other PI3K inhibitors with additional inhibitory effects are under clinical development.
–Various molecular mechanisms of resistance to PI3K inhibitors have been described preclinically and clinically.
In an unbiased exploratory analysis of samples from patients treated with copanlisib, a 16-gene mutation signature that separated responders from nonresponders was identified, the authors said.
The finding suggests that genetic aberrations dictate response to PI3K inhibitors, they noted.
“This 16-gene signature included TNFAIP3, CREBBP, and PRDM1, which are known to be important in the molecular pathogenesis of DLBCL,” they wrote. A composite score was developed to reflect the numerical presence or absence of mutations in the gene set, they explained, adding that patients with a high composite score had a significantly higher overall response rate and longer progression-free survival than did patients with a lower score.
In addition, idelalisib treatment resulted in a feedback activation of PI3Ka in ABC DLBCL cells.
“This rebound of PI3K activity was overcome by subsequent PI3Ka inhibition in preclinical DLBCL models, further underscoring the necessity of inhibiting both PI3Ka and PI3Kd to achieve responses in ABC DLBCL,” they wrote, adding that “[i]n ABC DLBCL models treated with the PI3Ka/PI3Kd inhibitor AZD8835, activated CARD11 mutations were identified as a mechanism of resistance.”
Investigations looking at various treatment combinations to overcome resistance to PI3K inhibition and improve the efficacy of targeted approaches are under way, they said.
For example, copanlisib plus the BCL-2 inhibitor venetoclax showed “synergistic activity in BCR-dependent DLBCLs, with genetic bases for BCL-2 dysregulation in vitro and in vivo,” and combination treatment with umbralisib and the proteasome inhibitor carfilzomib showed synergistic cytotoxicity in B-cell lymphoma, they said, noting that the latter combination is currently being evaluated in patients with DLBCL.
This work was supported by a research grant from the Deutsche Krebshilfe. Dr. Xu reported having no financial disclosures.
FROM BLOOD
Painful facial abscess
A 35-year-old woman presented to our clinic with a purple-red cyst on her right cheek that had been present for about 4 years but had worsened over the prior 2 weeks (FIGURE 1). She said she was experiencing excruciating pain and that the cyst had purulent drainage. She denied any history of diabetes, dental problems, recent trauma, or an inciting event.
On physical examination, there was no cervical lymphadenopathy, and her vital signs were normal. An incision and drainage procedure was performed. About 2 mL of purulent fluid was extracted and sent for aerobic and anaerobic cultures.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cervicofacial actinomycosis
Direct Gram stain showed gram-positive cocci, so the patient was started on a 7-day course of cephalexin 500 mg tid. Five days later, the anaerobic culture grew Actinomyces neuii, revealing the diagnosis as cervicofacial actinomycosis; the patient stopped taking cephalexin. The patient was then switched to a 3-month course of amoxicillin 875 mg bid.
Actinomyces are natural inhabitants of the human oropharynx and gastrointestinal and genitourinary tracts.1-4 They are filamentous, gram-positive rods with characteristic sulfur granules (although these are not always present).1-4 It is believed that actinomycosis is endogenously acquired from deep tissue either through dental trauma, penetrating wounds, or compound fractures.2,4
The most common presentations of actinomycosis include cervicofacial (sometimes referred to as “lumpy jaw syndrome”), followed by abdominopelvic and thoracic/pulmonary, manifestations.2-4 Primary cutaneous actinomycosis is rare.5-9 Actinomycosis infection often manifests with indolent constitutional symptoms such as fatigue and anorexia.1 Most cases occur in men ages 20 to 60 years, although cases in women are increasingly being reported.2-4
Risk factors include poor dental hygiene or dental procedures, alcoholism, intrauterine device use, immunosuppression, appendicitis, and diverticulitis.2-4 The exact cause of this patient’s actinomycosis was unknown, as she did not have any known risk factors.
Furunculosis and sporotrichosis are part of the differential
Actinomycosis is often called a “great mimicker” due to its ability to masquerade as infection, malignancy, or fungus.1 The differential diagnosis for this patient’s presentation included bacterial soft-tissue infection (eg, furunculosis), infected epidermoid cyst, cutaneous tuberculosis, sporotrichosis, deep fungal infection, and nocardiosis.
Continue to: Furunculosis was initially suspected
Furunculosis was initially suspected, but the original wound culture demonstrated actinomycoses instead of traditional gram-positive bacteria.
A clinical diagnosis
The diagnosis of actinomycosis is usually made clinically, but definitive confirmation requires culture, which can be challenging with a slow-growing facultative or strict anaerobe that may take up to 14 days to appear.2-4 A Gram stain can aid in the diagnosis, but overall, there is a high false-negative rate in identifying actinomycosis.1,3,4
Treatment time can be lengthy, but prognosis is favorable
Unfortunately, there are no randomized controlled studies for treatment of actinomycosis. The majority of evidence for treatment comes from in vitro and clinical case studies.2-4,10 In general, prognosis of actinomycosis is favorable with low mortality, but chronic infection without complete resolution of symptoms can occur.1-4,7,8,10
First-line therapy for actinomycosis is a beta-lactam antibiotic, typically penicillin G or amoxicillin.2-4,10 High doses of prolonged intravenous (IV) and oral antibiotic therapy (2 to 12 months) based on location and complexity are standard.3,11 However, if there is minimal bone involvement and the patient shows rapid improvement, treatment could be shortened to a 4 to 6–week oral regimen.1,11 Surgical intervention can also shorten the required length of antibiotic duration.1,10
Cutaneous actinomycosis Tx. Amoxicillin/clavulanic acid has been shown to be an effective treatment for cutaneous actinomycosis, especially if polymicrobial infection is suspected.5,6 Individualized regimens for cutaneous actinomycosis—based on severity, location, and treatment response—are acceptable with close monitoring.1,2,11
Continue to: A lengthy recovery for our patient
A lengthy recovery for our patient
Seven weeks after the initial visit, the patient reported that she had taken only 20 days’ worth of the recommended 3-month course of amoxicillin. Fortunately, the lesion appeared to be healing well with no apparent fluid collection (FIGURE 2).
The patient was then prescribed, and completed, a 3-month course of amoxicillin/clavulanic acid
Nineteen months after initial treatment, the lesion reappeared as a painless cyst in a similar location (FIGURE 3). Plastic Surgery incised and drained the lesion and Infectious Diseases continued her on 3 months of amoxicillin/clavulanic acid 875 mg/125 mg bid, which she did complete.
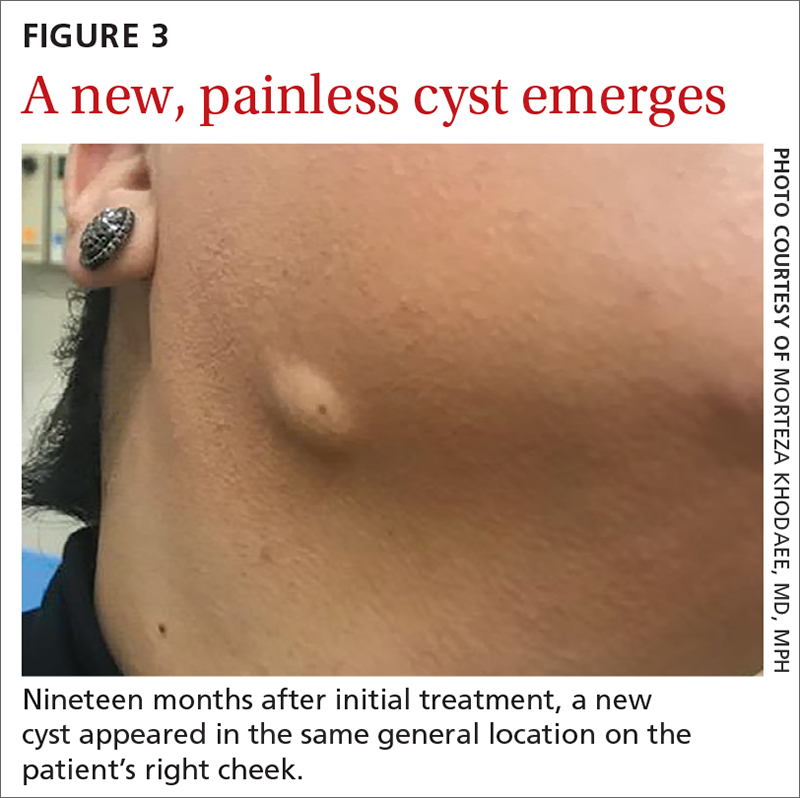
Due to the continued presence of the lesion, a computed tomography scan of the face was ordered 2 years after the initial visit and demonstrated a superficial skin lesion with no mandibular involvement (FIGURE 4). She was then treated with 3 more months of amoxicillin/clavulanic acid 875 mg/125 mg bid, with the possibility of deep debridement if not improved. However, debridement was unnecessary as the cyst did not recur.
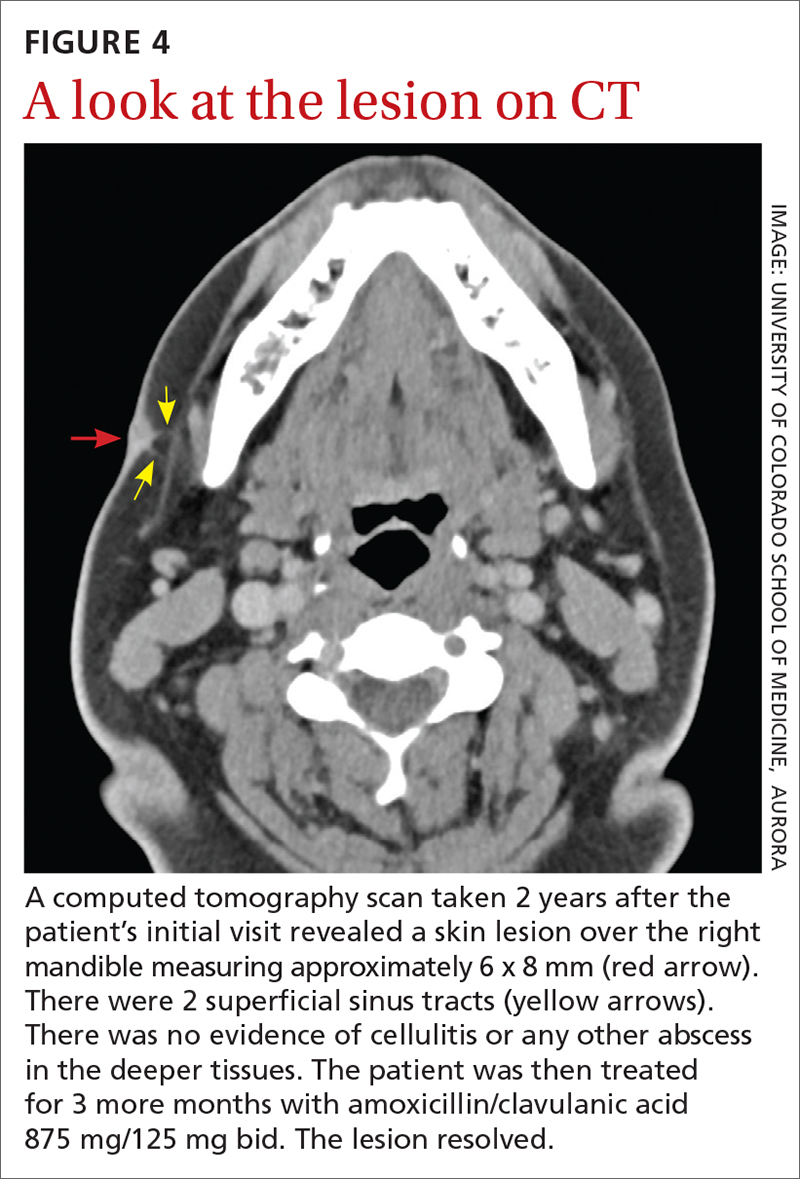
We believe that the course of this patient’s treatment was protracted because she never took oral antibiotics for more than 3 months at a time, and thus, her infection never completely resolved. In retrospect, we would have treated her more aggressively from the outset.
1. Najmi AH, Najmi IH, Tawhari MMH, et al. Cutaneous actinomycosis and long-term management through using oral and topical antibiotics: a case report. Clin Pract. 2018;8:1102. doi: 10.4081/ cp.2018.1102
2. Sharma S, Hashmi MF, Valentino ID. Actinomycosis. StatPearls Publishing; 2021.
3. Valour F, Sénécha A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-97. doi: 10.2147/IDR.S39601
4. Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011;343:d6099. doi: 10.1136/bmj.d6099
5. Akhtar M, Zade MP, Shahane PL, et al. Scalp actinomycosis presenting as soft tissue tumour: a case report with literature review. Int J Surg Case Rep. 2015;16:99-101. doi: 10.1016/ j.ijscr.2015.09.030
6. Bose M, Ghosh R, Mukherjee K, et al. Primary cutaneous actinomycosis:a case report. J Clin Diagn Res. 2014;8:YD03-5. doi: 10.7860/JCDR/2014/8286.4591
7. Cataño JC, Gómez Villegas SI. Images in clinical medicine. Cutaneous actinomycosis. N Engl J Med. 2016;374:1773. doi: 10.1056/ NEJMicm1511213
8. Mehta V, Balachandran C. Primary cutaneous actinomycosis on the chest wall. Dermatol Online J. 2008;14:13.
9. Piggott SA, Khodaee M. A bump in the groin: cutaneous actinomycosis. J Family Community Med. 2017;24:203. doi: 10.4103/jfcm.JFCM_79_17
10. Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Treatment of cutaneous actinomycosis with amoxicillin/clavulanic acid. J Dermatolog Treat. 2017;28:59-64. doi: 10.1080/09546634.2016.1178373
11. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;;7:183-197. doi: 10.2147/IDR.S39601
A 35-year-old woman presented to our clinic with a purple-red cyst on her right cheek that had been present for about 4 years but had worsened over the prior 2 weeks (FIGURE 1). She said she was experiencing excruciating pain and that the cyst had purulent drainage. She denied any history of diabetes, dental problems, recent trauma, or an inciting event.
On physical examination, there was no cervical lymphadenopathy, and her vital signs were normal. An incision and drainage procedure was performed. About 2 mL of purulent fluid was extracted and sent for aerobic and anaerobic cultures.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cervicofacial actinomycosis
Direct Gram stain showed gram-positive cocci, so the patient was started on a 7-day course of cephalexin 500 mg tid. Five days later, the anaerobic culture grew Actinomyces neuii, revealing the diagnosis as cervicofacial actinomycosis; the patient stopped taking cephalexin. The patient was then switched to a 3-month course of amoxicillin 875 mg bid.
Actinomyces are natural inhabitants of the human oropharynx and gastrointestinal and genitourinary tracts.1-4 They are filamentous, gram-positive rods with characteristic sulfur granules (although these are not always present).1-4 It is believed that actinomycosis is endogenously acquired from deep tissue either through dental trauma, penetrating wounds, or compound fractures.2,4
The most common presentations of actinomycosis include cervicofacial (sometimes referred to as “lumpy jaw syndrome”), followed by abdominopelvic and thoracic/pulmonary, manifestations.2-4 Primary cutaneous actinomycosis is rare.5-9 Actinomycosis infection often manifests with indolent constitutional symptoms such as fatigue and anorexia.1 Most cases occur in men ages 20 to 60 years, although cases in women are increasingly being reported.2-4
Risk factors include poor dental hygiene or dental procedures, alcoholism, intrauterine device use, immunosuppression, appendicitis, and diverticulitis.2-4 The exact cause of this patient’s actinomycosis was unknown, as she did not have any known risk factors.
Furunculosis and sporotrichosis are part of the differential
Actinomycosis is often called a “great mimicker” due to its ability to masquerade as infection, malignancy, or fungus.1 The differential diagnosis for this patient’s presentation included bacterial soft-tissue infection (eg, furunculosis), infected epidermoid cyst, cutaneous tuberculosis, sporotrichosis, deep fungal infection, and nocardiosis.
Continue to: Furunculosis was initially suspected
Furunculosis was initially suspected, but the original wound culture demonstrated actinomycoses instead of traditional gram-positive bacteria.
A clinical diagnosis
The diagnosis of actinomycosis is usually made clinically, but definitive confirmation requires culture, which can be challenging with a slow-growing facultative or strict anaerobe that may take up to 14 days to appear.2-4 A Gram stain can aid in the diagnosis, but overall, there is a high false-negative rate in identifying actinomycosis.1,3,4
Treatment time can be lengthy, but prognosis is favorable
Unfortunately, there are no randomized controlled studies for treatment of actinomycosis. The majority of evidence for treatment comes from in vitro and clinical case studies.2-4,10 In general, prognosis of actinomycosis is favorable with low mortality, but chronic infection without complete resolution of symptoms can occur.1-4,7,8,10
First-line therapy for actinomycosis is a beta-lactam antibiotic, typically penicillin G or amoxicillin.2-4,10 High doses of prolonged intravenous (IV) and oral antibiotic therapy (2 to 12 months) based on location and complexity are standard.3,11 However, if there is minimal bone involvement and the patient shows rapid improvement, treatment could be shortened to a 4 to 6–week oral regimen.1,11 Surgical intervention can also shorten the required length of antibiotic duration.1,10
Cutaneous actinomycosis Tx. Amoxicillin/clavulanic acid has been shown to be an effective treatment for cutaneous actinomycosis, especially if polymicrobial infection is suspected.5,6 Individualized regimens for cutaneous actinomycosis—based on severity, location, and treatment response—are acceptable with close monitoring.1,2,11
Continue to: A lengthy recovery for our patient
A lengthy recovery for our patient
Seven weeks after the initial visit, the patient reported that she had taken only 20 days’ worth of the recommended 3-month course of amoxicillin. Fortunately, the lesion appeared to be healing well with no apparent fluid collection (FIGURE 2).

The patient was then prescribed, and completed, a 3-month course of amoxicillin/clavulanic acid
Nineteen months after initial treatment, the lesion reappeared as a painless cyst in a similar location (FIGURE 3). Plastic Surgery incised and drained the lesion and Infectious Diseases continued her on 3 months of amoxicillin/clavulanic acid 875 mg/125 mg bid, which she did complete.

Due to the continued presence of the lesion, a computed tomography scan of the face was ordered 2 years after the initial visit and demonstrated a superficial skin lesion with no mandibular involvement (FIGURE 4). She was then treated with 3 more months of amoxicillin/clavulanic acid 875 mg/125 mg bid, with the possibility of deep debridement if not improved. However, debridement was unnecessary as the cyst did not recur.

We believe that the course of this patient’s treatment was protracted because she never took oral antibiotics for more than 3 months at a time, and thus, her infection never completely resolved. In retrospect, we would have treated her more aggressively from the outset.
A 35-year-old woman presented to our clinic with a purple-red cyst on her right cheek that had been present for about 4 years but had worsened over the prior 2 weeks (FIGURE 1). She said she was experiencing excruciating pain and that the cyst had purulent drainage. She denied any history of diabetes, dental problems, recent trauma, or an inciting event.
On physical examination, there was no cervical lymphadenopathy, and her vital signs were normal. An incision and drainage procedure was performed. About 2 mL of purulent fluid was extracted and sent for aerobic and anaerobic cultures.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cervicofacial actinomycosis
Direct Gram stain showed gram-positive cocci, so the patient was started on a 7-day course of cephalexin 500 mg tid. Five days later, the anaerobic culture grew Actinomyces neuii, revealing the diagnosis as cervicofacial actinomycosis; the patient stopped taking cephalexin. The patient was then switched to a 3-month course of amoxicillin 875 mg bid.
Actinomyces are natural inhabitants of the human oropharynx and gastrointestinal and genitourinary tracts.1-4 They are filamentous, gram-positive rods with characteristic sulfur granules (although these are not always present).1-4 It is believed that actinomycosis is endogenously acquired from deep tissue either through dental trauma, penetrating wounds, or compound fractures.2,4
The most common presentations of actinomycosis include cervicofacial (sometimes referred to as “lumpy jaw syndrome”), followed by abdominopelvic and thoracic/pulmonary, manifestations.2-4 Primary cutaneous actinomycosis is rare.5-9 Actinomycosis infection often manifests with indolent constitutional symptoms such as fatigue and anorexia.1 Most cases occur in men ages 20 to 60 years, although cases in women are increasingly being reported.2-4
Risk factors include poor dental hygiene or dental procedures, alcoholism, intrauterine device use, immunosuppression, appendicitis, and diverticulitis.2-4 The exact cause of this patient’s actinomycosis was unknown, as she did not have any known risk factors.
Furunculosis and sporotrichosis are part of the differential
Actinomycosis is often called a “great mimicker” due to its ability to masquerade as infection, malignancy, or fungus.1 The differential diagnosis for this patient’s presentation included bacterial soft-tissue infection (eg, furunculosis), infected epidermoid cyst, cutaneous tuberculosis, sporotrichosis, deep fungal infection, and nocardiosis.
Continue to: Furunculosis was initially suspected
Furunculosis was initially suspected, but the original wound culture demonstrated actinomycoses instead of traditional gram-positive bacteria.
A clinical diagnosis
The diagnosis of actinomycosis is usually made clinically, but definitive confirmation requires culture, which can be challenging with a slow-growing facultative or strict anaerobe that may take up to 14 days to appear.2-4 A Gram stain can aid in the diagnosis, but overall, there is a high false-negative rate in identifying actinomycosis.1,3,4
Treatment time can be lengthy, but prognosis is favorable
Unfortunately, there are no randomized controlled studies for treatment of actinomycosis. The majority of evidence for treatment comes from in vitro and clinical case studies.2-4,10 In general, prognosis of actinomycosis is favorable with low mortality, but chronic infection without complete resolution of symptoms can occur.1-4,7,8,10
First-line therapy for actinomycosis is a beta-lactam antibiotic, typically penicillin G or amoxicillin.2-4,10 High doses of prolonged intravenous (IV) and oral antibiotic therapy (2 to 12 months) based on location and complexity are standard.3,11 However, if there is minimal bone involvement and the patient shows rapid improvement, treatment could be shortened to a 4 to 6–week oral regimen.1,11 Surgical intervention can also shorten the required length of antibiotic duration.1,10
Cutaneous actinomycosis Tx. Amoxicillin/clavulanic acid has been shown to be an effective treatment for cutaneous actinomycosis, especially if polymicrobial infection is suspected.5,6 Individualized regimens for cutaneous actinomycosis—based on severity, location, and treatment response—are acceptable with close monitoring.1,2,11
Continue to: A lengthy recovery for our patient
A lengthy recovery for our patient
Seven weeks after the initial visit, the patient reported that she had taken only 20 days’ worth of the recommended 3-month course of amoxicillin. Fortunately, the lesion appeared to be healing well with no apparent fluid collection (FIGURE 2).

The patient was then prescribed, and completed, a 3-month course of amoxicillin/clavulanic acid
Nineteen months after initial treatment, the lesion reappeared as a painless cyst in a similar location (FIGURE 3). Plastic Surgery incised and drained the lesion and Infectious Diseases continued her on 3 months of amoxicillin/clavulanic acid 875 mg/125 mg bid, which she did complete.

Due to the continued presence of the lesion, a computed tomography scan of the face was ordered 2 years after the initial visit and demonstrated a superficial skin lesion with no mandibular involvement (FIGURE 4). She was then treated with 3 more months of amoxicillin/clavulanic acid 875 mg/125 mg bid, with the possibility of deep debridement if not improved. However, debridement was unnecessary as the cyst did not recur.

We believe that the course of this patient’s treatment was protracted because she never took oral antibiotics for more than 3 months at a time, and thus, her infection never completely resolved. In retrospect, we would have treated her more aggressively from the outset.
1. Najmi AH, Najmi IH, Tawhari MMH, et al. Cutaneous actinomycosis and long-term management through using oral and topical antibiotics: a case report. Clin Pract. 2018;8:1102. doi: 10.4081/ cp.2018.1102
2. Sharma S, Hashmi MF, Valentino ID. Actinomycosis. StatPearls Publishing; 2021.
3. Valour F, Sénécha A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-97. doi: 10.2147/IDR.S39601
4. Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011;343:d6099. doi: 10.1136/bmj.d6099
5. Akhtar M, Zade MP, Shahane PL, et al. Scalp actinomycosis presenting as soft tissue tumour: a case report with literature review. Int J Surg Case Rep. 2015;16:99-101. doi: 10.1016/ j.ijscr.2015.09.030
6. Bose M, Ghosh R, Mukherjee K, et al. Primary cutaneous actinomycosis:a case report. J Clin Diagn Res. 2014;8:YD03-5. doi: 10.7860/JCDR/2014/8286.4591
7. Cataño JC, Gómez Villegas SI. Images in clinical medicine. Cutaneous actinomycosis. N Engl J Med. 2016;374:1773. doi: 10.1056/ NEJMicm1511213
8. Mehta V, Balachandran C. Primary cutaneous actinomycosis on the chest wall. Dermatol Online J. 2008;14:13.
9. Piggott SA, Khodaee M. A bump in the groin: cutaneous actinomycosis. J Family Community Med. 2017;24:203. doi: 10.4103/jfcm.JFCM_79_17
10. Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Treatment of cutaneous actinomycosis with amoxicillin/clavulanic acid. J Dermatolog Treat. 2017;28:59-64. doi: 10.1080/09546634.2016.1178373
11. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;;7:183-197. doi: 10.2147/IDR.S39601
1. Najmi AH, Najmi IH, Tawhari MMH, et al. Cutaneous actinomycosis and long-term management through using oral and topical antibiotics: a case report. Clin Pract. 2018;8:1102. doi: 10.4081/ cp.2018.1102
2. Sharma S, Hashmi MF, Valentino ID. Actinomycosis. StatPearls Publishing; 2021.
3. Valour F, Sénécha A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-97. doi: 10.2147/IDR.S39601
4. Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011;343:d6099. doi: 10.1136/bmj.d6099
5. Akhtar M, Zade MP, Shahane PL, et al. Scalp actinomycosis presenting as soft tissue tumour: a case report with literature review. Int J Surg Case Rep. 2015;16:99-101. doi: 10.1016/ j.ijscr.2015.09.030
6. Bose M, Ghosh R, Mukherjee K, et al. Primary cutaneous actinomycosis:a case report. J Clin Diagn Res. 2014;8:YD03-5. doi: 10.7860/JCDR/2014/8286.4591
7. Cataño JC, Gómez Villegas SI. Images in clinical medicine. Cutaneous actinomycosis. N Engl J Med. 2016;374:1773. doi: 10.1056/ NEJMicm1511213
8. Mehta V, Balachandran C. Primary cutaneous actinomycosis on the chest wall. Dermatol Online J. 2008;14:13.
9. Piggott SA, Khodaee M. A bump in the groin: cutaneous actinomycosis. J Family Community Med. 2017;24:203. doi: 10.4103/jfcm.JFCM_79_17
10. Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Treatment of cutaneous actinomycosis with amoxicillin/clavulanic acid. J Dermatolog Treat. 2017;28:59-64. doi: 10.1080/09546634.2016.1178373
11. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;;7:183-197. doi: 10.2147/IDR.S39601
Painful lumps in the axilla
A 30-year-old man presented to the clinic with a complaint of small painful lumps in his armpit. He stated that he initially experienced some itching and discomfort, but after a while he noticed some red, tender, swollen areas. He also mentioned an odorous yellow fluid that would sometimes drain from the lumps. Since first noticing them 2 years earlier, he reported that the nodules had disappeared and reappeared on their own several times.
On physical exam, several small red subcutaneous nodules were present in the axilla and tender to palpation (FIGURE 1A). The patient also had comedonal acne on his back (FIGURE 1B). The patient’s body mass index was 31, and he was a nonsmoker.
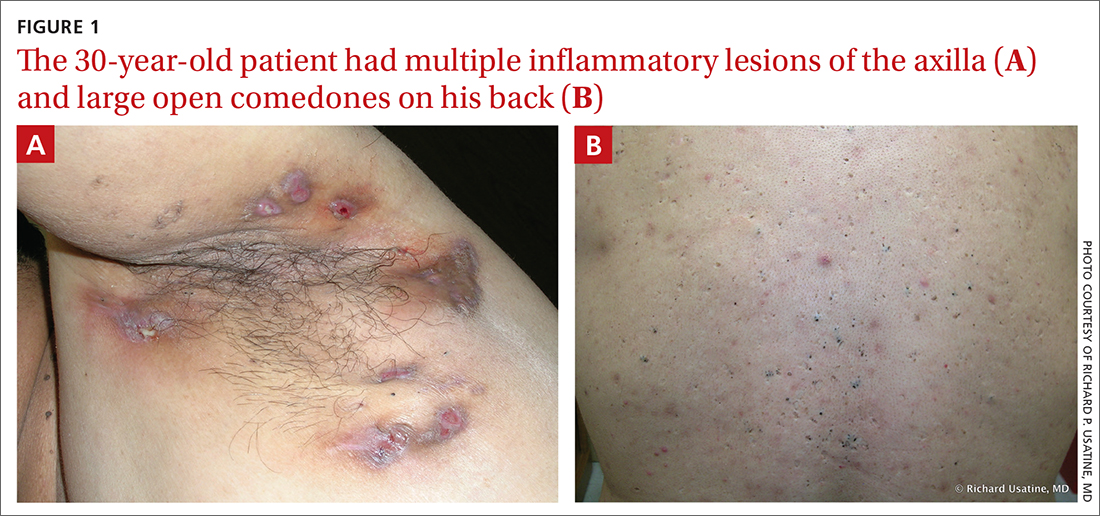
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Hidradenitis suppurativa
The characteristic location and morphology of the lesions, along with the chronicity and odor, were critical in arriving at a diagnosis of hidradenitis suppurativa (HS).
HS is a chronic, inflammatory skin condition that normally manifests in areas of apocrine sweat glands, including the axilla, groin, and perianal, perineal, and inframammary locations.1 It begins when an abnormal hair follicle gets occluded and ruptures, spilling keratin and bacteria into the dermis. An inflammatory response can ensue with surrounding neutrophils and lymphocytes, which leads to abscess formation and destruction of the pilosebaceous unit. Sinus tracts form between the lesions, and a cycle of scarring, fistulas, and contractures can occur.
In this case, the comedones from acne conglobata on the patient’s back indicated a more global follicular occlusion disorder. The characteristic triad is hidradenitis suppurativa, acne conglobata, and dissecting cellulitis of the scalp—of which the patient had 2.
Other potential causes of the pathology include abnormal secretion of apocrine glands, abnormal antimicrobial peptides, deficient numbers of sebaceous glands, and abnormal invaginations of the epidermis.2 Increased levels of tumor necrosis factor alpha and other cytokines have been detected in HS lesions and are a potential target for therapy.
The prevalence of HS in the United States is approximately 0.1%.3 The condition typically begins between the ages of 18 and 39 years. The ratio of women to men affected by the condition is 3:1.2 There is no evident racial or ethnic predilection. There is an association with diabetes and Crohn disease.3 Obesity and smoking are risk factors.1
Continue to: The differential includes an array of common skin conditions
The differential includes an array of common skin conditions
The differential diagnosis in this case included carbuncles, cysts, acne, and abscesses.
A furuncle or carbuncle can result from an infection of hair follicles that can manifest as individual (furuncle) or clusters of (carbuncle) red, painful boils. They form on parts of the skin where hair grows, including the face, neck, armpits, shoulders, and buttocks. They respond well to treatment with antibiotics and incision and drainage. They can be recurrent but usually don’t cluster together in apocrine-rich areas, as seen with HS.
Epidermal inclusion cysts are keratin-filled inclusion cysts with epithelial-lined cyst walls. The cysts are subcutaneous and occasionally more superficial. They can occur almost anywhere but are most often found on the back, scalp, neck, face, and chest. They are usually solitary; however, when there are multiple cysts, they are not linked by sinus tracts as found in HS.
Inflammatory acne lesions tend to form on the face, neck, back, chest, and shoulders, while HS lesions appear most often in apocrine-rich intertriginous areas.
Skin abscesses are local deep infections of the skin caused by bacterial pathogens. The most common agent is Staphylococcus aureus (frequently methicillin resistant). Injection drug use and immunosuppression are risk factors. Although bacteria do not cause HS lesions, bacteria can exacerbate HS through colonization.
Continue to: No lab test needed to diagnosis hidradenitis suppurativa
No lab test needed to diagnose hidradenitis suppurativa
Diagnosis of HS is largely clinical and based on a patient’s history and physical exam findings.2 No specific laboratory test is needed.
Although the patient in this case did have comedonal acne on his back, the lesions that prompted his visit were in an apocrine-rich area, were recurrent, and broke open on their own to release foul-smelling contents—all typical characteristics of HS.
Treatment depends on the severity of the condition
There are 3 stages of HS: Hurley stage I involves abscess formation without tracts or scars. Hurley stage II involves recurrent abscesses with sinus tracts and scarring. Hurley stage III has diffuse involvement with multiple interconnected sinus tracts and abscesses across an entire area.2 Our patient fits into Hurley stage III.
Evidence-based treatment of mild disease (Hurley stage I) includes topical clindamycin 1% solution/gel bid or doxycycline 100 mg bid for widespread disease (Hurley stage II or resistant stage I).2 Chlorhexidine and benzoyl peroxide washes are also often recommended.3 If a patient does not respond to this treatment or the condition is moderate to severe, then clindamycin 300 po bid (with or without rifampin 600/d po) for 10 weeks should be considered.4,5 In a randomized placebo-controlled trial that compared the efficacy of oral clindamycin vs clindamycin plus rifampin in patients with HS, both therapeutic options were statistically equivalent.5 One small, randomized controlled study of patients with mild-to-moderate HS showed that tetracycline 500 mg bid for 3 months resulted in fewer abscesses and nodules but was not superior to topical clindamycin.3
If the patient doesn’t show improvement (Hurley stage III), then adalimumab is an option, as follows: 160 mg subcutaneously at Week 0, 80 mg at Week 2, and then 40 mg weekly, if needed.4 Adalimumab is currently the only FDA-approved treatment for HS. Infliximab by IV infusion can be effective in improving pain, disease severity, and quality of life in patients with moderate-to-severe HS.3 This patient was also a candidate for treatment with systemic retinoids (isotretinoin or acitretin), which could have helped both the HS and the acne conglobata.
Continue to: Intralesional steroid injectiosn with triamcinolone
Intralesional steroid injections with triamcinolone 10 mg/mL can reduce local pain and inflammation rapidly. Pain management is also critical, as HS is painful. First-line therapy includes nonsteroidal anti-inflammatory drugs, acetaminophen, atypical anticonvulsants, and serotonin and norepinephrine reuptake inhibitors.2 Opiate analgesics may be needed for breakthrough pain in patients with severe disease. Avoiding tight clothing, harsh products, and adhesive dressings, as well as using clear petroleum jelly, can prevent skin trauma and help with healing. Weight loss and smoking cessation are also associated with better outcomes.6,7
If medical management fails …
If there is no improvement with medical management, it may be time to consider local procedures such as unroofing/deroofing, punch debridement, skin-tissue-sparing excision with electrosurgical peeling, and laser excision. Incision and drainage may be necessary for acutely inflamed, painful abscesses but should not be routinely performed because lesions can recur.3
Referral to a plastic surgeon is necessary when patients are considering wide excisions of largely affected areas. Even when surgical excisions are performed, medical treatment is needed to prevent new lesions and recurrences.
Our patient was treated initially with oral doxycycline 100 mg bid and intralesional triamcinolone (10 mg/mL) in the most tender lesions. He was also provided with a prescription for ibuprofen 800 mg tid to be taken with meals. The family physician encouraged the patient to lose weight. The patient derived some benefit from treatment but continued to experience new painful lesions.
The physician prescribed oral clindamycin 300 mg bid at a follow-up visit to replace the oral doxycycline. When this failed, the patient was sent for labs to determine if he would be a candidate for adalimumab. When the screening labs were normal, a prescription for adalimumab was provided: 160 mg subcutaneously at Week 0, 80 mg at Week 2, and then 40 mg weekly.4
1. Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi: 10.2147/CCID.S111019
2. Ballard K, Shuman VL. Hidradenitis Suppurativa. StatPearls Publishing; 2021.
3. Wipperman J, Bragg DA, Litzner B. Hidradenitis suppurativa: rapid evidence review. Am Fam Physician. 2019;100:562-569.
4. Alikhan A, Lymch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561. doi:10.10126/j.jaad.2008.11.911
5. Caro RDC, Cannizzaro MV, Botti E, et al. Clindamycin versus clindamycin plus rifampicin in hidradenitis suppurativa treatment: clinical and ultrasound observations. J Am Acad Dermatol. 2019;80:1314-1321. doi: 10.1016/j.jaad.2018.11.035
6. Hendricks AJ, Hirt PA, Sekhon S, et al. Non-pharmacologic approaches for hidradenitis suppurativa - a systematic review. J Dermatolog Treat. 2021;32:11-18. doi: 10.1080/09546634.2019.1621981
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi: 10.1016/j.jaad.2019.02.067
A 30-year-old man presented to the clinic with a complaint of small painful lumps in his armpit. He stated that he initially experienced some itching and discomfort, but after a while he noticed some red, tender, swollen areas. He also mentioned an odorous yellow fluid that would sometimes drain from the lumps. Since first noticing them 2 years earlier, he reported that the nodules had disappeared and reappeared on their own several times.
On physical exam, several small red subcutaneous nodules were present in the axilla and tender to palpation (FIGURE 1A). The patient also had comedonal acne on his back (FIGURE 1B). The patient’s body mass index was 31, and he was a nonsmoker.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Hidradenitis suppurativa
The characteristic location and morphology of the lesions, along with the chronicity and odor, were critical in arriving at a diagnosis of hidradenitis suppurativa (HS).
HS is a chronic, inflammatory skin condition that normally manifests in areas of apocrine sweat glands, including the axilla, groin, and perianal, perineal, and inframammary locations.1 It begins when an abnormal hair follicle gets occluded and ruptures, spilling keratin and bacteria into the dermis. An inflammatory response can ensue with surrounding neutrophils and lymphocytes, which leads to abscess formation and destruction of the pilosebaceous unit. Sinus tracts form between the lesions, and a cycle of scarring, fistulas, and contractures can occur.
In this case, the comedones from acne conglobata on the patient’s back indicated a more global follicular occlusion disorder. The characteristic triad is hidradenitis suppurativa, acne conglobata, and dissecting cellulitis of the scalp—of which the patient had 2.
Other potential causes of the pathology include abnormal secretion of apocrine glands, abnormal antimicrobial peptides, deficient numbers of sebaceous glands, and abnormal invaginations of the epidermis.2 Increased levels of tumor necrosis factor alpha and other cytokines have been detected in HS lesions and are a potential target for therapy.
The prevalence of HS in the United States is approximately 0.1%.3 The condition typically begins between the ages of 18 and 39 years. The ratio of women to men affected by the condition is 3:1.2 There is no evident racial or ethnic predilection. There is an association with diabetes and Crohn disease.3 Obesity and smoking are risk factors.1
Continue to: The differential includes an array of common skin conditions
The differential includes an array of common skin conditions
The differential diagnosis in this case included carbuncles, cysts, acne, and abscesses.
A furuncle or carbuncle can result from an infection of hair follicles that can manifest as individual (furuncle) or clusters of (carbuncle) red, painful boils. They form on parts of the skin where hair grows, including the face, neck, armpits, shoulders, and buttocks. They respond well to treatment with antibiotics and incision and drainage. They can be recurrent but usually don’t cluster together in apocrine-rich areas, as seen with HS.
Epidermal inclusion cysts are keratin-filled inclusion cysts with epithelial-lined cyst walls. The cysts are subcutaneous and occasionally more superficial. They can occur almost anywhere but are most often found on the back, scalp, neck, face, and chest. They are usually solitary; however, when there are multiple cysts, they are not linked by sinus tracts as found in HS.
Inflammatory acne lesions tend to form on the face, neck, back, chest, and shoulders, while HS lesions appear most often in apocrine-rich intertriginous areas.
Skin abscesses are local deep infections of the skin caused by bacterial pathogens. The most common agent is Staphylococcus aureus (frequently methicillin resistant). Injection drug use and immunosuppression are risk factors. Although bacteria do not cause HS lesions, bacteria can exacerbate HS through colonization.
Continue to: No lab test needed to diagnosis hidradenitis suppurativa
No lab test needed to diagnose hidradenitis suppurativa
Diagnosis of HS is largely clinical and based on a patient’s history and physical exam findings.2 No specific laboratory test is needed.
Although the patient in this case did have comedonal acne on his back, the lesions that prompted his visit were in an apocrine-rich area, were recurrent, and broke open on their own to release foul-smelling contents—all typical characteristics of HS.
Treatment depends on the severity of the condition
There are 3 stages of HS: Hurley stage I involves abscess formation without tracts or scars. Hurley stage II involves recurrent abscesses with sinus tracts and scarring. Hurley stage III has diffuse involvement with multiple interconnected sinus tracts and abscesses across an entire area.2 Our patient fits into Hurley stage III.
Evidence-based treatment of mild disease (Hurley stage I) includes topical clindamycin 1% solution/gel bid or doxycycline 100 mg bid for widespread disease (Hurley stage II or resistant stage I).2 Chlorhexidine and benzoyl peroxide washes are also often recommended.3 If a patient does not respond to this treatment or the condition is moderate to severe, then clindamycin 300 po bid (with or without rifampin 600/d po) for 10 weeks should be considered.4,5 In a randomized placebo-controlled trial that compared the efficacy of oral clindamycin vs clindamycin plus rifampin in patients with HS, both therapeutic options were statistically equivalent.5 One small, randomized controlled study of patients with mild-to-moderate HS showed that tetracycline 500 mg bid for 3 months resulted in fewer abscesses and nodules but was not superior to topical clindamycin.3
If the patient doesn’t show improvement (Hurley stage III), then adalimumab is an option, as follows: 160 mg subcutaneously at Week 0, 80 mg at Week 2, and then 40 mg weekly, if needed.4 Adalimumab is currently the only FDA-approved treatment for HS. Infliximab by IV infusion can be effective in improving pain, disease severity, and quality of life in patients with moderate-to-severe HS.3 This patient was also a candidate for treatment with systemic retinoids (isotretinoin or acitretin), which could have helped both the HS and the acne conglobata.
Continue to: Intralesional steroid injectiosn with triamcinolone
Intralesional steroid injections with triamcinolone 10 mg/mL can reduce local pain and inflammation rapidly. Pain management is also critical, as HS is painful. First-line therapy includes nonsteroidal anti-inflammatory drugs, acetaminophen, atypical anticonvulsants, and serotonin and norepinephrine reuptake inhibitors.2 Opiate analgesics may be needed for breakthrough pain in patients with severe disease. Avoiding tight clothing, harsh products, and adhesive dressings, as well as using clear petroleum jelly, can prevent skin trauma and help with healing. Weight loss and smoking cessation are also associated with better outcomes.6,7
If medical management fails …
If there is no improvement with medical management, it may be time to consider local procedures such as unroofing/deroofing, punch debridement, skin-tissue-sparing excision with electrosurgical peeling, and laser excision. Incision and drainage may be necessary for acutely inflamed, painful abscesses but should not be routinely performed because lesions can recur.3
Referral to a plastic surgeon is necessary when patients are considering wide excisions of largely affected areas. Even when surgical excisions are performed, medical treatment is needed to prevent new lesions and recurrences.
Our patient was treated initially with oral doxycycline 100 mg bid and intralesional triamcinolone (10 mg/mL) in the most tender lesions. He was also provided with a prescription for ibuprofen 800 mg tid to be taken with meals. The family physician encouraged the patient to lose weight. The patient derived some benefit from treatment but continued to experience new painful lesions.
The physician prescribed oral clindamycin 300 mg bid at a follow-up visit to replace the oral doxycycline. When this failed, the patient was sent for labs to determine if he would be a candidate for adalimumab. When the screening labs were normal, a prescription for adalimumab was provided: 160 mg subcutaneously at Week 0, 80 mg at Week 2, and then 40 mg weekly.4
A 30-year-old man presented to the clinic with a complaint of small painful lumps in his armpit. He stated that he initially experienced some itching and discomfort, but after a while he noticed some red, tender, swollen areas. He also mentioned an odorous yellow fluid that would sometimes drain from the lumps. Since first noticing them 2 years earlier, he reported that the nodules had disappeared and reappeared on their own several times.
On physical exam, several small red subcutaneous nodules were present in the axilla and tender to palpation (FIGURE 1A). The patient also had comedonal acne on his back (FIGURE 1B). The patient’s body mass index was 31, and he was a nonsmoker.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Hidradenitis suppurativa
The characteristic location and morphology of the lesions, along with the chronicity and odor, were critical in arriving at a diagnosis of hidradenitis suppurativa (HS).
HS is a chronic, inflammatory skin condition that normally manifests in areas of apocrine sweat glands, including the axilla, groin, and perianal, perineal, and inframammary locations.1 It begins when an abnormal hair follicle gets occluded and ruptures, spilling keratin and bacteria into the dermis. An inflammatory response can ensue with surrounding neutrophils and lymphocytes, which leads to abscess formation and destruction of the pilosebaceous unit. Sinus tracts form between the lesions, and a cycle of scarring, fistulas, and contractures can occur.
In this case, the comedones from acne conglobata on the patient’s back indicated a more global follicular occlusion disorder. The characteristic triad is hidradenitis suppurativa, acne conglobata, and dissecting cellulitis of the scalp—of which the patient had 2.
Other potential causes of the pathology include abnormal secretion of apocrine glands, abnormal antimicrobial peptides, deficient numbers of sebaceous glands, and abnormal invaginations of the epidermis.2 Increased levels of tumor necrosis factor alpha and other cytokines have been detected in HS lesions and are a potential target for therapy.
The prevalence of HS in the United States is approximately 0.1%.3 The condition typically begins between the ages of 18 and 39 years. The ratio of women to men affected by the condition is 3:1.2 There is no evident racial or ethnic predilection. There is an association with diabetes and Crohn disease.3 Obesity and smoking are risk factors.1
Continue to: The differential includes an array of common skin conditions
The differential includes an array of common skin conditions
The differential diagnosis in this case included carbuncles, cysts, acne, and abscesses.
A furuncle or carbuncle can result from an infection of hair follicles that can manifest as individual (furuncle) or clusters of (carbuncle) red, painful boils. They form on parts of the skin where hair grows, including the face, neck, armpits, shoulders, and buttocks. They respond well to treatment with antibiotics and incision and drainage. They can be recurrent but usually don’t cluster together in apocrine-rich areas, as seen with HS.
Epidermal inclusion cysts are keratin-filled inclusion cysts with epithelial-lined cyst walls. The cysts are subcutaneous and occasionally more superficial. They can occur almost anywhere but are most often found on the back, scalp, neck, face, and chest. They are usually solitary; however, when there are multiple cysts, they are not linked by sinus tracts as found in HS.
Inflammatory acne lesions tend to form on the face, neck, back, chest, and shoulders, while HS lesions appear most often in apocrine-rich intertriginous areas.
Skin abscesses are local deep infections of the skin caused by bacterial pathogens. The most common agent is Staphylococcus aureus (frequently methicillin resistant). Injection drug use and immunosuppression are risk factors. Although bacteria do not cause HS lesions, bacteria can exacerbate HS through colonization.
Continue to: No lab test needed to diagnosis hidradenitis suppurativa
No lab test needed to diagnose hidradenitis suppurativa
Diagnosis of HS is largely clinical and based on a patient’s history and physical exam findings.2 No specific laboratory test is needed.
Although the patient in this case did have comedonal acne on his back, the lesions that prompted his visit were in an apocrine-rich area, were recurrent, and broke open on their own to release foul-smelling contents—all typical characteristics of HS.
Treatment depends on the severity of the condition
There are 3 stages of HS: Hurley stage I involves abscess formation without tracts or scars. Hurley stage II involves recurrent abscesses with sinus tracts and scarring. Hurley stage III has diffuse involvement with multiple interconnected sinus tracts and abscesses across an entire area.2 Our patient fits into Hurley stage III.
Evidence-based treatment of mild disease (Hurley stage I) includes topical clindamycin 1% solution/gel bid or doxycycline 100 mg bid for widespread disease (Hurley stage II or resistant stage I).2 Chlorhexidine and benzoyl peroxide washes are also often recommended.3 If a patient does not respond to this treatment or the condition is moderate to severe, then clindamycin 300 po bid (with or without rifampin 600/d po) for 10 weeks should be considered.4,5 In a randomized placebo-controlled trial that compared the efficacy of oral clindamycin vs clindamycin plus rifampin in patients with HS, both therapeutic options were statistically equivalent.5 One small, randomized controlled study of patients with mild-to-moderate HS showed that tetracycline 500 mg bid for 3 months resulted in fewer abscesses and nodules but was not superior to topical clindamycin.3
If the patient doesn’t show improvement (Hurley stage III), then adalimumab is an option, as follows: 160 mg subcutaneously at Week 0, 80 mg at Week 2, and then 40 mg weekly, if needed.4 Adalimumab is currently the only FDA-approved treatment for HS. Infliximab by IV infusion can be effective in improving pain, disease severity, and quality of life in patients with moderate-to-severe HS.3 This patient was also a candidate for treatment with systemic retinoids (isotretinoin or acitretin), which could have helped both the HS and the acne conglobata.
Continue to: Intralesional steroid injectiosn with triamcinolone
Intralesional steroid injections with triamcinolone 10 mg/mL can reduce local pain and inflammation rapidly. Pain management is also critical, as HS is painful. First-line therapy includes nonsteroidal anti-inflammatory drugs, acetaminophen, atypical anticonvulsants, and serotonin and norepinephrine reuptake inhibitors.2 Opiate analgesics may be needed for breakthrough pain in patients with severe disease. Avoiding tight clothing, harsh products, and adhesive dressings, as well as using clear petroleum jelly, can prevent skin trauma and help with healing. Weight loss and smoking cessation are also associated with better outcomes.6,7
If medical management fails …
If there is no improvement with medical management, it may be time to consider local procedures such as unroofing/deroofing, punch debridement, skin-tissue-sparing excision with electrosurgical peeling, and laser excision. Incision and drainage may be necessary for acutely inflamed, painful abscesses but should not be routinely performed because lesions can recur.3
Referral to a plastic surgeon is necessary when patients are considering wide excisions of largely affected areas. Even when surgical excisions are performed, medical treatment is needed to prevent new lesions and recurrences.
Our patient was treated initially with oral doxycycline 100 mg bid and intralesional triamcinolone (10 mg/mL) in the most tender lesions. He was also provided with a prescription for ibuprofen 800 mg tid to be taken with meals. The family physician encouraged the patient to lose weight. The patient derived some benefit from treatment but continued to experience new painful lesions.
The physician prescribed oral clindamycin 300 mg bid at a follow-up visit to replace the oral doxycycline. When this failed, the patient was sent for labs to determine if he would be a candidate for adalimumab. When the screening labs were normal, a prescription for adalimumab was provided: 160 mg subcutaneously at Week 0, 80 mg at Week 2, and then 40 mg weekly.4
1. Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi: 10.2147/CCID.S111019
2. Ballard K, Shuman VL. Hidradenitis Suppurativa. StatPearls Publishing; 2021.
3. Wipperman J, Bragg DA, Litzner B. Hidradenitis suppurativa: rapid evidence review. Am Fam Physician. 2019;100:562-569.
4. Alikhan A, Lymch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561. doi:10.10126/j.jaad.2008.11.911
5. Caro RDC, Cannizzaro MV, Botti E, et al. Clindamycin versus clindamycin plus rifampicin in hidradenitis suppurativa treatment: clinical and ultrasound observations. J Am Acad Dermatol. 2019;80:1314-1321. doi: 10.1016/j.jaad.2018.11.035
6. Hendricks AJ, Hirt PA, Sekhon S, et al. Non-pharmacologic approaches for hidradenitis suppurativa - a systematic review. J Dermatolog Treat. 2021;32:11-18. doi: 10.1080/09546634.2019.1621981
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi: 10.1016/j.jaad.2019.02.067
1. Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi: 10.2147/CCID.S111019
2. Ballard K, Shuman VL. Hidradenitis Suppurativa. StatPearls Publishing; 2021.
3. Wipperman J, Bragg DA, Litzner B. Hidradenitis suppurativa: rapid evidence review. Am Fam Physician. 2019;100:562-569.
4. Alikhan A, Lymch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561. doi:10.10126/j.jaad.2008.11.911
5. Caro RDC, Cannizzaro MV, Botti E, et al. Clindamycin versus clindamycin plus rifampicin in hidradenitis suppurativa treatment: clinical and ultrasound observations. J Am Acad Dermatol. 2019;80:1314-1321. doi: 10.1016/j.jaad.2018.11.035
6. Hendricks AJ, Hirt PA, Sekhon S, et al. Non-pharmacologic approaches for hidradenitis suppurativa - a systematic review. J Dermatolog Treat. 2021;32:11-18. doi: 10.1080/09546634.2019.1621981
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi: 10.1016/j.jaad.2019.02.067