User login
CAG Clinical Practice Guideline: Vaccination in patients with IBD
The Canadian Association of Gastroenterology (CAG) has published a two-part clinical practice guideline for immunizing patients with inflammatory bowel disease (IBD) that covers both live and inactivated vaccines across pediatric and adult patients.
The guideline, which has been endorsed by the American Gastroenterological Association, is composed of recommendations drawn from a broader body of data than prior publications on the same topic, according to Eric I. Benchimol, MD, PhD, of the University of Ottawa and the University of Toronto, and colleagues.
“Previous guidelines on immunizations of patients with IBD considered only the limited available evidence of vaccine safety and effectiveness in IBD populations, and failed to consider the ample evidence available in the general population or in other immune-mediated inflammatory diseases when assessing the certainty of evidence or developing their recommendations,” they wrote in Gastroenterology.
Part 1: Live vaccine recommendations
The first part of the guideline includes seven recommendations for use of live vaccines in patients with IBD.
In this area, decision-making is largely dependent upon use of immunosuppressive therapy, which the investigators defined as “corticosteroids, thiopurines, biologics, small molecules such as JAK [Janus kinase] inhibitors, and combinations thereof,” with the caveat that “there is no standard definition of immunosuppression,” and “the degree to which immunosuppressive therapy causes clinically significant immunosuppression generally is dose related and varies by drug.”
Before offering specific recommendations, Dr. Benchimol and colleagues provided three general principles to abide by: 1. Clinicians should review each patient’s history of immunization and vaccine-preventable diseases at diagnosis and on a routine basis; 2. Appropriate vaccinations should ideally be given prior to starting immunosuppressive therapy; and 3. Immunosuppressive therapy (when urgently needed) should not be delayed so that immunizations can be given in advance.
“[Delaying therapy] could lead to more anticipated harms than benefits, due to the risk of progression of the inflammatory activity and resulting complications,” the investigators wrote.
Specific recommendations in the guideline address measles, mumps, and rubella (MMR); and varicella. Both vaccines are recommended for susceptible pediatric and adult patients not taking immunosuppressive therapy. In contrast, neither vaccine is recommended for immunosuppressed patients of any age. Certainty of evidence ranged from very low to moderate.
Concerning vaccination within the first 6 months of life for infants born of mothers taking biologics, the expert panel did not reach a consensus.
“[T]he group was unable to recommend for or against their routine use because the desirable and undesirable effects were closely balanced and the evidence on safety outcomes was insufficient to justify a recommendation,” wrote Dr. Benchimol and colleagues. “Health care providers should be cautious with the administration of live vaccines in the first year of life in the infants of mothers using biologics. These infants should be evaluated by clinicians with expertise in the impact of exposure to monoclonal antibody biologics in utero.”
Part 2: Inactivated vaccine recommendations
The second part of the guideline, by lead author Jennifer L. Jones, MD, of Dalhousie University, Queen Elizabeth II Health Sciences Center, Halifax, N.S., and colleagues, provides 15 recommendations for giving inactivated vaccines to patients with IBD.
The panel considered eight vaccines: Haemophilus influenzae type B (Hib); herpes zoster (HZ); hepatitis B; influenza; Streptococcus pneumoniae (pneumococcal vaccine); Neisseria meningitidis (meningococcal vaccine); human papillomavirus (HPV); and diphtheria, tetanus, and pertussis.
Generally, the above vaccines are recommended on an age-appropriate basis, regardless of immunosuppression status, albeit with varying levels of confidence. For example, the Hib vaccine is strongly recommended for pediatric patients 5 years and younger, whereas the same recommendation for older children and adults is conditional.
For several patient populations and vaccines, the guideline panel did not reach a consensus, including use of double-dose hepatitis B vaccine for immunosuppressed adults, timing seasonal flu shots with dosing of biologics, use of pneumococcal vaccines in nonimmunosuppressed patents without a risk factor for pneumococcal disease, use of meningococcal vaccines in adults not at risk for invasive meningococcal disease, and use of HPV vaccine in patients aged 27-45 years.
While immunosuppressive therapy is not a contraindication for giving inactivated vaccines, Dr. Jones and colleagues noted that immunosuppression may hinder vaccine responses.
“Given that patients with IBD on immunosuppressive therapy may have lower immune response to vaccine, further research will be needed to assess the safety and effectiveness of high-dose vs. standard-dose vaccination strategy,” they wrote, also noting that more work is needed to determine if accelerated vaccinations strategies may be feasible prior to initiation of immunosuppressive therapy.
Because of a lack of evidence, the guideline panel did not issue IBD-specific recommendations for vaccines against SARS-CoV-2; however, Dr. Jones and colleagues suggested that clinicians reference a CAG publication on the subject published earlier this year.
The guideline was supported by grants to the Canadian Association of Gastroenterology from the Canadian Institutes of Health Research’s Institute of Nutrition, Metabolism and Diabetes; and CANImmunize. Dr. Benchimol disclosed additional relationships with the Canadian Institutes of Health Research, Crohn’s and Colitis Canada; and the Canadian Child Health Clinician Scientist Program.
The Canadian Association of Gastroenterology (CAG) has published a two-part clinical practice guideline for immunizing patients with inflammatory bowel disease (IBD) that covers both live and inactivated vaccines across pediatric and adult patients.
The guideline, which has been endorsed by the American Gastroenterological Association, is composed of recommendations drawn from a broader body of data than prior publications on the same topic, according to Eric I. Benchimol, MD, PhD, of the University of Ottawa and the University of Toronto, and colleagues.
“Previous guidelines on immunizations of patients with IBD considered only the limited available evidence of vaccine safety and effectiveness in IBD populations, and failed to consider the ample evidence available in the general population or in other immune-mediated inflammatory diseases when assessing the certainty of evidence or developing their recommendations,” they wrote in Gastroenterology.
Part 1: Live vaccine recommendations
The first part of the guideline includes seven recommendations for use of live vaccines in patients with IBD.
In this area, decision-making is largely dependent upon use of immunosuppressive therapy, which the investigators defined as “corticosteroids, thiopurines, biologics, small molecules such as JAK [Janus kinase] inhibitors, and combinations thereof,” with the caveat that “there is no standard definition of immunosuppression,” and “the degree to which immunosuppressive therapy causes clinically significant immunosuppression generally is dose related and varies by drug.”
Before offering specific recommendations, Dr. Benchimol and colleagues provided three general principles to abide by: 1. Clinicians should review each patient’s history of immunization and vaccine-preventable diseases at diagnosis and on a routine basis; 2. Appropriate vaccinations should ideally be given prior to starting immunosuppressive therapy; and 3. Immunosuppressive therapy (when urgently needed) should not be delayed so that immunizations can be given in advance.
“[Delaying therapy] could lead to more anticipated harms than benefits, due to the risk of progression of the inflammatory activity and resulting complications,” the investigators wrote.
Specific recommendations in the guideline address measles, mumps, and rubella (MMR); and varicella. Both vaccines are recommended for susceptible pediatric and adult patients not taking immunosuppressive therapy. In contrast, neither vaccine is recommended for immunosuppressed patients of any age. Certainty of evidence ranged from very low to moderate.
Concerning vaccination within the first 6 months of life for infants born of mothers taking biologics, the expert panel did not reach a consensus.
“[T]he group was unable to recommend for or against their routine use because the desirable and undesirable effects were closely balanced and the evidence on safety outcomes was insufficient to justify a recommendation,” wrote Dr. Benchimol and colleagues. “Health care providers should be cautious with the administration of live vaccines in the first year of life in the infants of mothers using biologics. These infants should be evaluated by clinicians with expertise in the impact of exposure to monoclonal antibody biologics in utero.”
Part 2: Inactivated vaccine recommendations
The second part of the guideline, by lead author Jennifer L. Jones, MD, of Dalhousie University, Queen Elizabeth II Health Sciences Center, Halifax, N.S., and colleagues, provides 15 recommendations for giving inactivated vaccines to patients with IBD.
The panel considered eight vaccines: Haemophilus influenzae type B (Hib); herpes zoster (HZ); hepatitis B; influenza; Streptococcus pneumoniae (pneumococcal vaccine); Neisseria meningitidis (meningococcal vaccine); human papillomavirus (HPV); and diphtheria, tetanus, and pertussis.
Generally, the above vaccines are recommended on an age-appropriate basis, regardless of immunosuppression status, albeit with varying levels of confidence. For example, the Hib vaccine is strongly recommended for pediatric patients 5 years and younger, whereas the same recommendation for older children and adults is conditional.
For several patient populations and vaccines, the guideline panel did not reach a consensus, including use of double-dose hepatitis B vaccine for immunosuppressed adults, timing seasonal flu shots with dosing of biologics, use of pneumococcal vaccines in nonimmunosuppressed patents without a risk factor for pneumococcal disease, use of meningococcal vaccines in adults not at risk for invasive meningococcal disease, and use of HPV vaccine in patients aged 27-45 years.
While immunosuppressive therapy is not a contraindication for giving inactivated vaccines, Dr. Jones and colleagues noted that immunosuppression may hinder vaccine responses.
“Given that patients with IBD on immunosuppressive therapy may have lower immune response to vaccine, further research will be needed to assess the safety and effectiveness of high-dose vs. standard-dose vaccination strategy,” they wrote, also noting that more work is needed to determine if accelerated vaccinations strategies may be feasible prior to initiation of immunosuppressive therapy.
Because of a lack of evidence, the guideline panel did not issue IBD-specific recommendations for vaccines against SARS-CoV-2; however, Dr. Jones and colleagues suggested that clinicians reference a CAG publication on the subject published earlier this year.
The guideline was supported by grants to the Canadian Association of Gastroenterology from the Canadian Institutes of Health Research’s Institute of Nutrition, Metabolism and Diabetes; and CANImmunize. Dr. Benchimol disclosed additional relationships with the Canadian Institutes of Health Research, Crohn’s and Colitis Canada; and the Canadian Child Health Clinician Scientist Program.
The Canadian Association of Gastroenterology (CAG) has published a two-part clinical practice guideline for immunizing patients with inflammatory bowel disease (IBD) that covers both live and inactivated vaccines across pediatric and adult patients.
The guideline, which has been endorsed by the American Gastroenterological Association, is composed of recommendations drawn from a broader body of data than prior publications on the same topic, according to Eric I. Benchimol, MD, PhD, of the University of Ottawa and the University of Toronto, and colleagues.
“Previous guidelines on immunizations of patients with IBD considered only the limited available evidence of vaccine safety and effectiveness in IBD populations, and failed to consider the ample evidence available in the general population or in other immune-mediated inflammatory diseases when assessing the certainty of evidence or developing their recommendations,” they wrote in Gastroenterology.
Part 1: Live vaccine recommendations
The first part of the guideline includes seven recommendations for use of live vaccines in patients with IBD.
In this area, decision-making is largely dependent upon use of immunosuppressive therapy, which the investigators defined as “corticosteroids, thiopurines, biologics, small molecules such as JAK [Janus kinase] inhibitors, and combinations thereof,” with the caveat that “there is no standard definition of immunosuppression,” and “the degree to which immunosuppressive therapy causes clinically significant immunosuppression generally is dose related and varies by drug.”
Before offering specific recommendations, Dr. Benchimol and colleagues provided three general principles to abide by: 1. Clinicians should review each patient’s history of immunization and vaccine-preventable diseases at diagnosis and on a routine basis; 2. Appropriate vaccinations should ideally be given prior to starting immunosuppressive therapy; and 3. Immunosuppressive therapy (when urgently needed) should not be delayed so that immunizations can be given in advance.
“[Delaying therapy] could lead to more anticipated harms than benefits, due to the risk of progression of the inflammatory activity and resulting complications,” the investigators wrote.
Specific recommendations in the guideline address measles, mumps, and rubella (MMR); and varicella. Both vaccines are recommended for susceptible pediatric and adult patients not taking immunosuppressive therapy. In contrast, neither vaccine is recommended for immunosuppressed patients of any age. Certainty of evidence ranged from very low to moderate.
Concerning vaccination within the first 6 months of life for infants born of mothers taking biologics, the expert panel did not reach a consensus.
“[T]he group was unable to recommend for or against their routine use because the desirable and undesirable effects were closely balanced and the evidence on safety outcomes was insufficient to justify a recommendation,” wrote Dr. Benchimol and colleagues. “Health care providers should be cautious with the administration of live vaccines in the first year of life in the infants of mothers using biologics. These infants should be evaluated by clinicians with expertise in the impact of exposure to monoclonal antibody biologics in utero.”
Part 2: Inactivated vaccine recommendations
The second part of the guideline, by lead author Jennifer L. Jones, MD, of Dalhousie University, Queen Elizabeth II Health Sciences Center, Halifax, N.S., and colleagues, provides 15 recommendations for giving inactivated vaccines to patients with IBD.
The panel considered eight vaccines: Haemophilus influenzae type B (Hib); herpes zoster (HZ); hepatitis B; influenza; Streptococcus pneumoniae (pneumococcal vaccine); Neisseria meningitidis (meningococcal vaccine); human papillomavirus (HPV); and diphtheria, tetanus, and pertussis.
Generally, the above vaccines are recommended on an age-appropriate basis, regardless of immunosuppression status, albeit with varying levels of confidence. For example, the Hib vaccine is strongly recommended for pediatric patients 5 years and younger, whereas the same recommendation for older children and adults is conditional.
For several patient populations and vaccines, the guideline panel did not reach a consensus, including use of double-dose hepatitis B vaccine for immunosuppressed adults, timing seasonal flu shots with dosing of biologics, use of pneumococcal vaccines in nonimmunosuppressed patents without a risk factor for pneumococcal disease, use of meningococcal vaccines in adults not at risk for invasive meningococcal disease, and use of HPV vaccine in patients aged 27-45 years.
While immunosuppressive therapy is not a contraindication for giving inactivated vaccines, Dr. Jones and colleagues noted that immunosuppression may hinder vaccine responses.
“Given that patients with IBD on immunosuppressive therapy may have lower immune response to vaccine, further research will be needed to assess the safety and effectiveness of high-dose vs. standard-dose vaccination strategy,” they wrote, also noting that more work is needed to determine if accelerated vaccinations strategies may be feasible prior to initiation of immunosuppressive therapy.
Because of a lack of evidence, the guideline panel did not issue IBD-specific recommendations for vaccines against SARS-CoV-2; however, Dr. Jones and colleagues suggested that clinicians reference a CAG publication on the subject published earlier this year.
The guideline was supported by grants to the Canadian Association of Gastroenterology from the Canadian Institutes of Health Research’s Institute of Nutrition, Metabolism and Diabetes; and CANImmunize. Dr. Benchimol disclosed additional relationships with the Canadian Institutes of Health Research, Crohn’s and Colitis Canada; and the Canadian Child Health Clinician Scientist Program.
FROM GASTROENTEROLOGY
Short-term approach is best for seizure prevention after intracerebral hemorrhage
(sICH), new research shows.
Investigators created a model that simulated common clinical scenarios to compare four antiseizure drug strategies – conservative, moderate, aggressive, and risk-guided. They used the 2HELPS2B score as a risk stratification tool to guide clinical decisions.
The investigators found that the short-term, early-seizure prophylaxis strategies “dominated” long-term therapy under most clinical scenarios, underscoring the importance of early discontinuation of antiseizure drug therapy.
“The main message here was that strategies that involved long-term antiseizure drug prescription (moderate and aggressive) fail to provide better outcomes in most clinical scenarios, when compared with strategies using short-term prophylaxis (conservative and risk-guided),” senior investigator Lidia M.V.R. Moura, MD, MPH, assistant professor of neurology, Harvard Medical School, Boston, said in an interview.
The study was published online July 26 in JAMA Neurology.
Common complication
“Acute asymptomatic seizures [early seizures ≤7 days after stroke] are a common complication of sICH,” the authors noted.
Potential safety concerns have prompted recommendations against the use of antiseizure medications for primary prophylaxis. However, approximately 40% of U.S. patients with sICH do receive prophylactic levetiracetam before seizure development. For these patients, the duration of prophylaxis varies widely.
“Because seizure risk is a key determinant of which patient groups might benefit most from different prophylaxis strategies, validated tools for predicting early ... and late ... seizure risks could aid physicians in treatment decisions. However, no clinical trials or prospective studies have evaluated the net benefit of various strategies after sICH,” the investigators noted.
“Our patients who were survivors of an intracerebral hemorrhage motivated us to conduct the study,” said Dr. Moura, who is also director of the MGH NeuroValue Laboratory. “Some would come to the clinic with a long list of medications; some of them were taking antiseizure drugs for many years, but they never had a documented seizure.” These patients did not know why they had been taking an antiseizure drug for so long.
“In these conversations, we noted so much variability in indications and variability in patient access to specialty care to make treatment decisions. We noted that the evidence behind our current guidelines on seizure management was limited,” she added.
Dr. Moura and colleagues were “committed to improve outcome for people with neurological conditions by leveraging research methods that can help guide providers and systems, especially when data from clinical trials is lacking,” so they “decided to compare different strategies head to head using available data and generate evidence that could be used in situations with many trade-offs in risks and benefits.”
To investigate, the researchers used a simulation model and decision analysis to compare four treatment strategies on the basis of type of therapy (primary vs. secondary prophylaxis), timing of event (early vs. late seizures), and duration of therapy (1-week [short-term] versus indefinite [long-term] therapy).
These four strategies were as follows:
- Conservative: short-term (7-day) secondary early-seizure prophylaxis with long-term therapy after late seizure
- Moderate: long-term secondary early-seizure prophylaxis or late-seizure therapy
- Aggressive: long-term primary prophylaxis
- Risk-guided: short-term secondary early-seizure prophylaxis among low-risk patients (2HELPS2b score, 0), short-term primary prophylaxis among patients at higher risk (2HELPS2B score ≥1), and long-term secondary therapy for late seizure
The decision tree’s outcome measure was the number of expected quality-adjusted life-years.
Primary prophylaxis was defined as “treatment initiated immediately on hospital admission.” Secondary prophylaxis was defined as “treatment started after a seizure” and was subdivided into secondary early-seizure prophylaxis, defined as treatment started after a seizure occurring in the first 7 days after the stroke, or secondary late-seizure therapy, defined as treatment started or restarted after a seizure occurring after the first poststroke week.
Incorporate early-risk stratification tool
The researchers created four common clinical scenarios and then applied the decision-making model to each. They found that the preferred strategies differed, depending on the particular scenario.
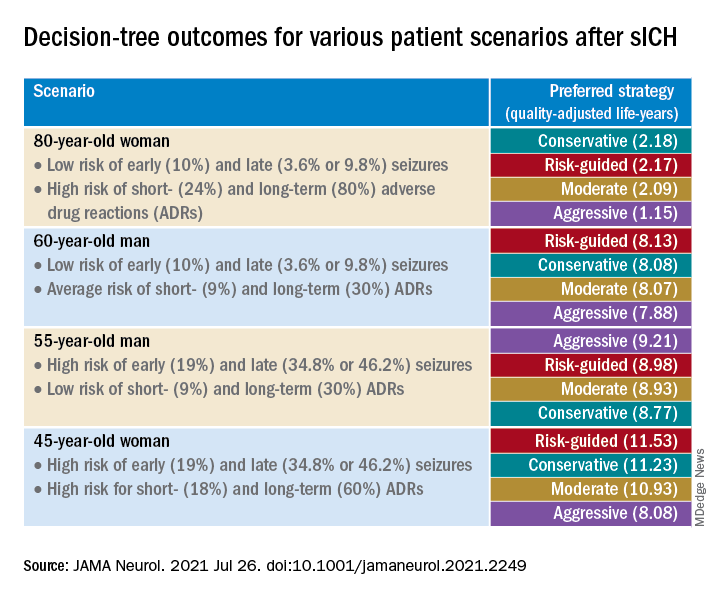
Sensitivity analyses revealed that short-term strategies, including the conservative and risk-guided approaches, were preferable in most cases, with the risk-guided strategy performing comparably or even better than alternative strategies in most cases.
“Our findings suggest that a strategy that incorporates an early-seizure risk stratification tool [2HELPS2B] is favored over alternative strategies in most settings,” Dr. Moura commented.
“Current services with rapidly available EEG may consider using a 1-hour screening with EEG upon admission for all patients presenting with sICH to risk-stratify those patients, using the 2HELPS2B tool,” she continued. “If EEG is unavailable for early-seizure risk stratification, the conservative strategy seems most reasonable.”
‘Potential fallacies’
Commenting on the study, José Biller, MD, professor and chairman, department of neurology, Loyola University Chicago, Maywood, Ill., called it a “well-written and intriguing contribution [to the field], with potential fallacies.”
The bottom line, he said, is that only a randomized, long-term, prospective, multicenter, high-quality study with larger cohorts can prove or disprove the investigators’ assumption.
The authors acknowledged that a limitation of the study was the use of published literature to obtain data to estimate model parameters and that they did not account for other possible factors that might modify some parameter estimates.
Nevertheless, Dr. Moura said the findings have important practical implications because they “highlight the importance of discontinuing antiseizure medications that were started during a hospitalization for sICH in patients that only had an early seizure.”
It is “of great importance for all providers to reassess the indication of antiseizure medications. Those drugs are not free of risks and can impact the patient’s health and quality of life,” she added.
The study was supported by grants from the National Institutes of Health. Dr. Moura reported receiving funding from the Centers for Disease Control and Prevention, the NIH, and the Epilepsy Foundation of America (Epilepsy Learning Healthcare System) as the director of the data coordinating center. Dr. Biller is the editor-in-chief of the Journal of Stroke and Cerebrovascular Diseases and a section editor of UpToDate.
A version of this article first appeared on Medscape.com.
(sICH), new research shows.
Investigators created a model that simulated common clinical scenarios to compare four antiseizure drug strategies – conservative, moderate, aggressive, and risk-guided. They used the 2HELPS2B score as a risk stratification tool to guide clinical decisions.
The investigators found that the short-term, early-seizure prophylaxis strategies “dominated” long-term therapy under most clinical scenarios, underscoring the importance of early discontinuation of antiseizure drug therapy.
“The main message here was that strategies that involved long-term antiseizure drug prescription (moderate and aggressive) fail to provide better outcomes in most clinical scenarios, when compared with strategies using short-term prophylaxis (conservative and risk-guided),” senior investigator Lidia M.V.R. Moura, MD, MPH, assistant professor of neurology, Harvard Medical School, Boston, said in an interview.
The study was published online July 26 in JAMA Neurology.
Common complication
“Acute asymptomatic seizures [early seizures ≤7 days after stroke] are a common complication of sICH,” the authors noted.
Potential safety concerns have prompted recommendations against the use of antiseizure medications for primary prophylaxis. However, approximately 40% of U.S. patients with sICH do receive prophylactic levetiracetam before seizure development. For these patients, the duration of prophylaxis varies widely.
“Because seizure risk is a key determinant of which patient groups might benefit most from different prophylaxis strategies, validated tools for predicting early ... and late ... seizure risks could aid physicians in treatment decisions. However, no clinical trials or prospective studies have evaluated the net benefit of various strategies after sICH,” the investigators noted.
“Our patients who were survivors of an intracerebral hemorrhage motivated us to conduct the study,” said Dr. Moura, who is also director of the MGH NeuroValue Laboratory. “Some would come to the clinic with a long list of medications; some of them were taking antiseizure drugs for many years, but they never had a documented seizure.” These patients did not know why they had been taking an antiseizure drug for so long.
“In these conversations, we noted so much variability in indications and variability in patient access to specialty care to make treatment decisions. We noted that the evidence behind our current guidelines on seizure management was limited,” she added.
Dr. Moura and colleagues were “committed to improve outcome for people with neurological conditions by leveraging research methods that can help guide providers and systems, especially when data from clinical trials is lacking,” so they “decided to compare different strategies head to head using available data and generate evidence that could be used in situations with many trade-offs in risks and benefits.”
To investigate, the researchers used a simulation model and decision analysis to compare four treatment strategies on the basis of type of therapy (primary vs. secondary prophylaxis), timing of event (early vs. late seizures), and duration of therapy (1-week [short-term] versus indefinite [long-term] therapy).
These four strategies were as follows:
- Conservative: short-term (7-day) secondary early-seizure prophylaxis with long-term therapy after late seizure
- Moderate: long-term secondary early-seizure prophylaxis or late-seizure therapy
- Aggressive: long-term primary prophylaxis
- Risk-guided: short-term secondary early-seizure prophylaxis among low-risk patients (2HELPS2b score, 0), short-term primary prophylaxis among patients at higher risk (2HELPS2B score ≥1), and long-term secondary therapy for late seizure
The decision tree’s outcome measure was the number of expected quality-adjusted life-years.
Primary prophylaxis was defined as “treatment initiated immediately on hospital admission.” Secondary prophylaxis was defined as “treatment started after a seizure” and was subdivided into secondary early-seizure prophylaxis, defined as treatment started after a seizure occurring in the first 7 days after the stroke, or secondary late-seizure therapy, defined as treatment started or restarted after a seizure occurring after the first poststroke week.
Incorporate early-risk stratification tool
The researchers created four common clinical scenarios and then applied the decision-making model to each. They found that the preferred strategies differed, depending on the particular scenario.

Sensitivity analyses revealed that short-term strategies, including the conservative and risk-guided approaches, were preferable in most cases, with the risk-guided strategy performing comparably or even better than alternative strategies in most cases.
“Our findings suggest that a strategy that incorporates an early-seizure risk stratification tool [2HELPS2B] is favored over alternative strategies in most settings,” Dr. Moura commented.
“Current services with rapidly available EEG may consider using a 1-hour screening with EEG upon admission for all patients presenting with sICH to risk-stratify those patients, using the 2HELPS2B tool,” she continued. “If EEG is unavailable for early-seizure risk stratification, the conservative strategy seems most reasonable.”
‘Potential fallacies’
Commenting on the study, José Biller, MD, professor and chairman, department of neurology, Loyola University Chicago, Maywood, Ill., called it a “well-written and intriguing contribution [to the field], with potential fallacies.”
The bottom line, he said, is that only a randomized, long-term, prospective, multicenter, high-quality study with larger cohorts can prove or disprove the investigators’ assumption.
The authors acknowledged that a limitation of the study was the use of published literature to obtain data to estimate model parameters and that they did not account for other possible factors that might modify some parameter estimates.
Nevertheless, Dr. Moura said the findings have important practical implications because they “highlight the importance of discontinuing antiseizure medications that were started during a hospitalization for sICH in patients that only had an early seizure.”
It is “of great importance for all providers to reassess the indication of antiseizure medications. Those drugs are not free of risks and can impact the patient’s health and quality of life,” she added.
The study was supported by grants from the National Institutes of Health. Dr. Moura reported receiving funding from the Centers for Disease Control and Prevention, the NIH, and the Epilepsy Foundation of America (Epilepsy Learning Healthcare System) as the director of the data coordinating center. Dr. Biller is the editor-in-chief of the Journal of Stroke and Cerebrovascular Diseases and a section editor of UpToDate.
A version of this article first appeared on Medscape.com.
(sICH), new research shows.
Investigators created a model that simulated common clinical scenarios to compare four antiseizure drug strategies – conservative, moderate, aggressive, and risk-guided. They used the 2HELPS2B score as a risk stratification tool to guide clinical decisions.
The investigators found that the short-term, early-seizure prophylaxis strategies “dominated” long-term therapy under most clinical scenarios, underscoring the importance of early discontinuation of antiseizure drug therapy.
“The main message here was that strategies that involved long-term antiseizure drug prescription (moderate and aggressive) fail to provide better outcomes in most clinical scenarios, when compared with strategies using short-term prophylaxis (conservative and risk-guided),” senior investigator Lidia M.V.R. Moura, MD, MPH, assistant professor of neurology, Harvard Medical School, Boston, said in an interview.
The study was published online July 26 in JAMA Neurology.
Common complication
“Acute asymptomatic seizures [early seizures ≤7 days after stroke] are a common complication of sICH,” the authors noted.
Potential safety concerns have prompted recommendations against the use of antiseizure medications for primary prophylaxis. However, approximately 40% of U.S. patients with sICH do receive prophylactic levetiracetam before seizure development. For these patients, the duration of prophylaxis varies widely.
“Because seizure risk is a key determinant of which patient groups might benefit most from different prophylaxis strategies, validated tools for predicting early ... and late ... seizure risks could aid physicians in treatment decisions. However, no clinical trials or prospective studies have evaluated the net benefit of various strategies after sICH,” the investigators noted.
“Our patients who were survivors of an intracerebral hemorrhage motivated us to conduct the study,” said Dr. Moura, who is also director of the MGH NeuroValue Laboratory. “Some would come to the clinic with a long list of medications; some of them were taking antiseizure drugs for many years, but they never had a documented seizure.” These patients did not know why they had been taking an antiseizure drug for so long.
“In these conversations, we noted so much variability in indications and variability in patient access to specialty care to make treatment decisions. We noted that the evidence behind our current guidelines on seizure management was limited,” she added.
Dr. Moura and colleagues were “committed to improve outcome for people with neurological conditions by leveraging research methods that can help guide providers and systems, especially when data from clinical trials is lacking,” so they “decided to compare different strategies head to head using available data and generate evidence that could be used in situations with many trade-offs in risks and benefits.”
To investigate, the researchers used a simulation model and decision analysis to compare four treatment strategies on the basis of type of therapy (primary vs. secondary prophylaxis), timing of event (early vs. late seizures), and duration of therapy (1-week [short-term] versus indefinite [long-term] therapy).
These four strategies were as follows:
- Conservative: short-term (7-day) secondary early-seizure prophylaxis with long-term therapy after late seizure
- Moderate: long-term secondary early-seizure prophylaxis or late-seizure therapy
- Aggressive: long-term primary prophylaxis
- Risk-guided: short-term secondary early-seizure prophylaxis among low-risk patients (2HELPS2b score, 0), short-term primary prophylaxis among patients at higher risk (2HELPS2B score ≥1), and long-term secondary therapy for late seizure
The decision tree’s outcome measure was the number of expected quality-adjusted life-years.
Primary prophylaxis was defined as “treatment initiated immediately on hospital admission.” Secondary prophylaxis was defined as “treatment started after a seizure” and was subdivided into secondary early-seizure prophylaxis, defined as treatment started after a seizure occurring in the first 7 days after the stroke, or secondary late-seizure therapy, defined as treatment started or restarted after a seizure occurring after the first poststroke week.
Incorporate early-risk stratification tool
The researchers created four common clinical scenarios and then applied the decision-making model to each. They found that the preferred strategies differed, depending on the particular scenario.

Sensitivity analyses revealed that short-term strategies, including the conservative and risk-guided approaches, were preferable in most cases, with the risk-guided strategy performing comparably or even better than alternative strategies in most cases.
“Our findings suggest that a strategy that incorporates an early-seizure risk stratification tool [2HELPS2B] is favored over alternative strategies in most settings,” Dr. Moura commented.
“Current services with rapidly available EEG may consider using a 1-hour screening with EEG upon admission for all patients presenting with sICH to risk-stratify those patients, using the 2HELPS2B tool,” she continued. “If EEG is unavailable for early-seizure risk stratification, the conservative strategy seems most reasonable.”
‘Potential fallacies’
Commenting on the study, José Biller, MD, professor and chairman, department of neurology, Loyola University Chicago, Maywood, Ill., called it a “well-written and intriguing contribution [to the field], with potential fallacies.”
The bottom line, he said, is that only a randomized, long-term, prospective, multicenter, high-quality study with larger cohorts can prove or disprove the investigators’ assumption.
The authors acknowledged that a limitation of the study was the use of published literature to obtain data to estimate model parameters and that they did not account for other possible factors that might modify some parameter estimates.
Nevertheless, Dr. Moura said the findings have important practical implications because they “highlight the importance of discontinuing antiseizure medications that were started during a hospitalization for sICH in patients that only had an early seizure.”
It is “of great importance for all providers to reassess the indication of antiseizure medications. Those drugs are not free of risks and can impact the patient’s health and quality of life,” she added.
The study was supported by grants from the National Institutes of Health. Dr. Moura reported receiving funding from the Centers for Disease Control and Prevention, the NIH, and the Epilepsy Foundation of America (Epilepsy Learning Healthcare System) as the director of the data coordinating center. Dr. Biller is the editor-in-chief of the Journal of Stroke and Cerebrovascular Diseases and a section editor of UpToDate.
A version of this article first appeared on Medscape.com.
Major musculoskeletal surgery in children with medically complex conditions
A review of the International Committee’s guide
The International Committee on Perioperative Care for Children with Medical Complexity developed an online guide, “Deciding on and Preparing for Major Musculoskeletal Surgery in Children with Cerebral Palsy, Neurodevelopmental Disorders, and Other Medically Complex Conditions,” published on Dec. 20, 2020, detailing how to prepare pediatric patients with medical complexity prior to musculoskeletal surgery. The guide was developed from a dearth of information regarding optimal care practices for these patients.
The multidisciplinary committee included members from orthopedic surgery, general pediatrics, pediatric hospital medicine, anesthesiology, critical care medicine, pain medicine, physiotherapy, developmental and behavioral pediatrics, and families of children with cerebral palsy. Mirna Giordano, MD, FAAP, FHM, associate professor of pediatrics at Columbia University, New York, and International Committee member, helped develop these recommendations to “improve quality of care in the perioperative period for children with medical complexities and neurodisabilities all over the world.”
The guide meticulously details the steps required to successfully prepare for an operation and postoperative recovery. It includes an algorithm and comprehensive assessment plan that can be implemented to assess and optimize the child’s health and wellbeing prior to surgery. It encourages shared decision making and highlights the need for ongoing, open communication between providers, patients, and families to set goals and expectations, discuss potential complications, and describe outcomes and the recovery process.
The module elaborates on several key factors that must be evaluated and addressed long before surgery to ensure success. Baseline nutrition is critical and must be evaluated with body composition and anthropometric measurements. Respiratory health must be assessed with consideration of pulmonology consultation, specific testing, and ventilator or assistive-device optimization. Moreover, children with innate muscular weakness or restrictive lung disease should have baseline physiology evaluated in anticipation of potential postoperative complications, including atelectasis, hypoventilation, and pneumonia. Coexisting chronic medical conditions must also be optimized in anticipation of expected deviations from baseline.
In anticipation of peri- and postoperative care, the medical team should also be aware of details surrounding patients’ indwelling medical devices, such as cardiac implantable devices and tracheostomies. Particular attention should be paid to baclofen pumps, as malfunction or mistitration can lead to periprocedural hypotension or withdrawal.
Of paramount importance is understanding how the child appears and responds when in pain or discomfort, especially for a child with limited verbal communication. The module provides pain assessment tools, tailored to verbal and nonverbal patients in both the inpatient and outpatient settings. The module also shares guidance on establishing communication and goals with the family and within the care team on how the child appears when in distress and how he/she/they respond to pain medications. The pain plan should encompass both pharmacologic and nonpharmacologic therapeutics. Furthermore, as pain and discomfort may present from multiple sources, not limited to the regions involved in the procedure, understanding how the child responds to urinary retention, constipation, dyspnea, and uncomfortable positions is important to care. Postoperative immobilization must also be addressed as it may lead to pressure injury, manifesting as behavioral changes.
The module also presents laboratory testing as part of the preoperative health assessment. It details the utility or lack thereof of several common practices and provides recommendations on components that should be part of each patient’s assessment. It also contains videos showcased from the Courage Parents Network on family and provider perceptions of spinal fusion.
Family and social assessments must not be neglected prior to surgery, as these areas may also affect surgical outcomes. The module shares several screening tools that care team members can use to screen for family and social issues. Challenges to discharge planning are also discussed, including how to approach transportation, medical equipment, and school transitions needs.
The module is available for review in OPEN Pediatrics (www.openpediatrics.org), an online community for pediatric health professionals who share peer-reviewed best practices. “Our aim is to disseminate the recommendations as widely as possible to bring about the maximum good to the most,” Dr. Giordano said. The International Committee on Perioperative Care for Children with Medical Complexity is planning further guides regarding perioperative care, particularly for intraoperative and postoperative considerations.
Dr. Tantoco is a med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago, and instructor of medicine (hospital medicine) and pediatrics in Northwestern University, in Chicago. She is also a member of the SHM Pediatrics Special Interest Group Executive Committee. Dr. Bhasin is a med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital, and assistant professor of medicine (hospital medicine) and pediatrics in Northwestern University.
A review of the International Committee’s guide
A review of the International Committee’s guide
The International Committee on Perioperative Care for Children with Medical Complexity developed an online guide, “Deciding on and Preparing for Major Musculoskeletal Surgery in Children with Cerebral Palsy, Neurodevelopmental Disorders, and Other Medically Complex Conditions,” published on Dec. 20, 2020, detailing how to prepare pediatric patients with medical complexity prior to musculoskeletal surgery. The guide was developed from a dearth of information regarding optimal care practices for these patients.
The multidisciplinary committee included members from orthopedic surgery, general pediatrics, pediatric hospital medicine, anesthesiology, critical care medicine, pain medicine, physiotherapy, developmental and behavioral pediatrics, and families of children with cerebral palsy. Mirna Giordano, MD, FAAP, FHM, associate professor of pediatrics at Columbia University, New York, and International Committee member, helped develop these recommendations to “improve quality of care in the perioperative period for children with medical complexities and neurodisabilities all over the world.”
The guide meticulously details the steps required to successfully prepare for an operation and postoperative recovery. It includes an algorithm and comprehensive assessment plan that can be implemented to assess and optimize the child’s health and wellbeing prior to surgery. It encourages shared decision making and highlights the need for ongoing, open communication between providers, patients, and families to set goals and expectations, discuss potential complications, and describe outcomes and the recovery process.
The module elaborates on several key factors that must be evaluated and addressed long before surgery to ensure success. Baseline nutrition is critical and must be evaluated with body composition and anthropometric measurements. Respiratory health must be assessed with consideration of pulmonology consultation, specific testing, and ventilator or assistive-device optimization. Moreover, children with innate muscular weakness or restrictive lung disease should have baseline physiology evaluated in anticipation of potential postoperative complications, including atelectasis, hypoventilation, and pneumonia. Coexisting chronic medical conditions must also be optimized in anticipation of expected deviations from baseline.
In anticipation of peri- and postoperative care, the medical team should also be aware of details surrounding patients’ indwelling medical devices, such as cardiac implantable devices and tracheostomies. Particular attention should be paid to baclofen pumps, as malfunction or mistitration can lead to periprocedural hypotension or withdrawal.
Of paramount importance is understanding how the child appears and responds when in pain or discomfort, especially for a child with limited verbal communication. The module provides pain assessment tools, tailored to verbal and nonverbal patients in both the inpatient and outpatient settings. The module also shares guidance on establishing communication and goals with the family and within the care team on how the child appears when in distress and how he/she/they respond to pain medications. The pain plan should encompass both pharmacologic and nonpharmacologic therapeutics. Furthermore, as pain and discomfort may present from multiple sources, not limited to the regions involved in the procedure, understanding how the child responds to urinary retention, constipation, dyspnea, and uncomfortable positions is important to care. Postoperative immobilization must also be addressed as it may lead to pressure injury, manifesting as behavioral changes.
The module also presents laboratory testing as part of the preoperative health assessment. It details the utility or lack thereof of several common practices and provides recommendations on components that should be part of each patient’s assessment. It also contains videos showcased from the Courage Parents Network on family and provider perceptions of spinal fusion.
Family and social assessments must not be neglected prior to surgery, as these areas may also affect surgical outcomes. The module shares several screening tools that care team members can use to screen for family and social issues. Challenges to discharge planning are also discussed, including how to approach transportation, medical equipment, and school transitions needs.
The module is available for review in OPEN Pediatrics (www.openpediatrics.org), an online community for pediatric health professionals who share peer-reviewed best practices. “Our aim is to disseminate the recommendations as widely as possible to bring about the maximum good to the most,” Dr. Giordano said. The International Committee on Perioperative Care for Children with Medical Complexity is planning further guides regarding perioperative care, particularly for intraoperative and postoperative considerations.
Dr. Tantoco is a med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago, and instructor of medicine (hospital medicine) and pediatrics in Northwestern University, in Chicago. She is also a member of the SHM Pediatrics Special Interest Group Executive Committee. Dr. Bhasin is a med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital, and assistant professor of medicine (hospital medicine) and pediatrics in Northwestern University.
The International Committee on Perioperative Care for Children with Medical Complexity developed an online guide, “Deciding on and Preparing for Major Musculoskeletal Surgery in Children with Cerebral Palsy, Neurodevelopmental Disorders, and Other Medically Complex Conditions,” published on Dec. 20, 2020, detailing how to prepare pediatric patients with medical complexity prior to musculoskeletal surgery. The guide was developed from a dearth of information regarding optimal care practices for these patients.
The multidisciplinary committee included members from orthopedic surgery, general pediatrics, pediatric hospital medicine, anesthesiology, critical care medicine, pain medicine, physiotherapy, developmental and behavioral pediatrics, and families of children with cerebral palsy. Mirna Giordano, MD, FAAP, FHM, associate professor of pediatrics at Columbia University, New York, and International Committee member, helped develop these recommendations to “improve quality of care in the perioperative period for children with medical complexities and neurodisabilities all over the world.”
The guide meticulously details the steps required to successfully prepare for an operation and postoperative recovery. It includes an algorithm and comprehensive assessment plan that can be implemented to assess and optimize the child’s health and wellbeing prior to surgery. It encourages shared decision making and highlights the need for ongoing, open communication between providers, patients, and families to set goals and expectations, discuss potential complications, and describe outcomes and the recovery process.
The module elaborates on several key factors that must be evaluated and addressed long before surgery to ensure success. Baseline nutrition is critical and must be evaluated with body composition and anthropometric measurements. Respiratory health must be assessed with consideration of pulmonology consultation, specific testing, and ventilator or assistive-device optimization. Moreover, children with innate muscular weakness or restrictive lung disease should have baseline physiology evaluated in anticipation of potential postoperative complications, including atelectasis, hypoventilation, and pneumonia. Coexisting chronic medical conditions must also be optimized in anticipation of expected deviations from baseline.
In anticipation of peri- and postoperative care, the medical team should also be aware of details surrounding patients’ indwelling medical devices, such as cardiac implantable devices and tracheostomies. Particular attention should be paid to baclofen pumps, as malfunction or mistitration can lead to periprocedural hypotension or withdrawal.
Of paramount importance is understanding how the child appears and responds when in pain or discomfort, especially for a child with limited verbal communication. The module provides pain assessment tools, tailored to verbal and nonverbal patients in both the inpatient and outpatient settings. The module also shares guidance on establishing communication and goals with the family and within the care team on how the child appears when in distress and how he/she/they respond to pain medications. The pain plan should encompass both pharmacologic and nonpharmacologic therapeutics. Furthermore, as pain and discomfort may present from multiple sources, not limited to the regions involved in the procedure, understanding how the child responds to urinary retention, constipation, dyspnea, and uncomfortable positions is important to care. Postoperative immobilization must also be addressed as it may lead to pressure injury, manifesting as behavioral changes.
The module also presents laboratory testing as part of the preoperative health assessment. It details the utility or lack thereof of several common practices and provides recommendations on components that should be part of each patient’s assessment. It also contains videos showcased from the Courage Parents Network on family and provider perceptions of spinal fusion.
Family and social assessments must not be neglected prior to surgery, as these areas may also affect surgical outcomes. The module shares several screening tools that care team members can use to screen for family and social issues. Challenges to discharge planning are also discussed, including how to approach transportation, medical equipment, and school transitions needs.
The module is available for review in OPEN Pediatrics (www.openpediatrics.org), an online community for pediatric health professionals who share peer-reviewed best practices. “Our aim is to disseminate the recommendations as widely as possible to bring about the maximum good to the most,” Dr. Giordano said. The International Committee on Perioperative Care for Children with Medical Complexity is planning further guides regarding perioperative care, particularly for intraoperative and postoperative considerations.
Dr. Tantoco is a med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago, and instructor of medicine (hospital medicine) and pediatrics in Northwestern University, in Chicago. She is also a member of the SHM Pediatrics Special Interest Group Executive Committee. Dr. Bhasin is a med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital, and assistant professor of medicine (hospital medicine) and pediatrics in Northwestern University.
Ultraprocessed foods comprise most of the calories for youths
In the 2 decades from 1999 to 2018, ultraprocessed foods consistently accounted for the majority of energy intake by American young people, a large cross-sectional study of National Health and Nutrition Examination Survey (NHANES) data shows.
In young people aged 2-19 years, the estimated percentage of total energy from consumption of ultraprocessed foods increased from 61.4% to 67.0%, for a difference of 5.6% (95% confidence interval [CI] 3.5-7.7, P < .001 for trend), according to Lu Wang, PhD, MPH, a postdoctoral fellow at the Friedman School of Nutrition Science and Policy at Tufts University in Boston, and colleagues.
In contrast, total energy from non- or minimally processed foods decreased from 28.8% to 23.5% (difference −5.3%, 95% CI, −7.5 to −3.2, P < .001 for trend).
“The estimated percentage of energy consumed from ultraprocessed foods increased from 1999 to 2018, with an increasing trend in ready-to-heat and -eat mixed dishes and a decreasing trend in sugar-sweetened beverages,” the authors wrote. The report was published online Aug. 10 in JAMA.
The findings held regardless of the educational and socioeconomic status of the children’s parents.
Significant disparities by race and ethnicity emerged, however, with the ultraprocessed food phenomenon more marked in non-Hispanic Black youths and Mexican-American youths than in their non-Hispanic White counterparts. “Targeted marketing of junk foods toward racial/ethnic minority youths may partly contribute to such differences,” the authors wrote. “However, persistently lower consumption of ultraprocessed foods among Mexican-American youths may reflect more home cooking among Hispanic families.”
Among non-Hispanic Black youths consumption rose from 62.2% to 72.5% (difference 10.3%, 95% CI, 6.8-13.8) and among Mexican-American youths from 55.8% to 63.5% (difference 7.6%, 95% CI, 4.4-10.9). In non-Hispanic White youths intake rose from 63.4% to 68.6% (difference 5.2%, 95% CI, 2.1-8.3, P = .04 for trends).
In addition, a higher consumption of ultraprocessed foods among school-aged youths than among preschool children aged 2-5 years may reflect increased marketing, availability, and selection of ultraprocessed foods for older youths, the authors noted.
Food processing, with its potential adverse effects, may need to be considered as a food dimension in addition to nutrients and food groups in future dietary recommendations and food policies, they added.
“An increasing number of studies are showing a link between ultraprocessed food consumption and adverse health outcomes in children,” corresponding author Fang Fang Zhang, MD, PhD, Neely Family Professor and associate professor at Tufts’ Friedman School of Nutrition Science and Policy, said in an interview. “Health care providers can play a larger role in encouraging patients – and their parents – to replace unhealthy ultraprocessed foods such as ultraprocessed sweet bakery products with healthy unprocessed or minimally processed foods in their diet such as less processed whole grains. “
In Dr. Zhang’s view, teachers also have a part to play in promoting nutrition literacy. “Schools can play an important role in empowering children with knowledge and skills to make healthy food choices,” she said. “Nutrition literacy should be an integral part of the health education curriculum in all K-12 schools.”
Commenting on the study but not involved in it, Michelle Katzow, MD, a pediatrician/obesity medicine specialist and assistant professor at the Feinstein Institutes for Medical Research in Manhasset, N.Y., said the work highlights an often overlooked aspect of the modern American diet that may well be contributing to poor health outcomes in young people.
“It suggests that even as the science advances and we learn more about the adverse health effects of ultraprocessed foods, public health efforts to improve nutrition and food quality in children have not been successful,” she said in an interview. “This is because it is so hard for public health advocates to compete with the food industry, which stands to really benefit financially from hooking kids on processed foods that are not good for their health.”
Dr. Katzow added that the observed racial/ethnic disparities are not surprising in light of a growing body of evidence that racism exists in food marketing. “We need to put forward policies that regulate the food industry, particularly in relation to its most susceptible targets, our kids.”
Study details
The serial cross-sectional analysis used 24-hour dietary recall data from a nationally representative sample from 10 NHANES cycles for the range of 1999-2000 to 2017-2018. The weighted mean age of the cohort was 10.7 years and 49.1% were girls.
Among the subgroups of ultraprocessed foods, the estimated percentage of energy from ready-to-heat and ready-to-eat mixed dishes increased from 2.2% to 11.2% (difference 8.9%; 95%, CI, 7.7-10.2).
Energy from sweets and sweet snacks increased from 10.7% to 12.9% (difference 2.3%; 95% CI, 1.0-3.6), but the estimated percentage of energy decreased for sugar-sweetened beverages from 10.8% to 5.3% (difference −5.5%; 95% CI, −6.5 to −4.5).
In other categories, estimated energy intake from processed fats and oils, condiments, and sauces fell from 7.1% to 4.0% (difference −3.1%; 95% CI, −3.7 to −2.6, all P < .05 for trend).
Not surprisingly, ultraprocessed foods had an overall poorer nutrient profile than that of nonultraprocessed, although they often contained less saturated fat, and they also contained more carbohydrates, mostly from low-quality sources with added sugars and low levels of dietary fiber and protein.
And despite a higher total folate content in ultraprocessed foods because of fortification, higher-level consumers took in less total folate owing to their lower consumption of whole foods.
The authors cautioned that in addition to poor nutrient profiles, processing itself may harm health by changing the physical structure and chemical composition of food, which could lead to elevated glycemic response and reduced satiety. Furthermore, recent research has linked food additives such as emulsifiers, stabilizers, and artificial sweeteners to adverse metabolomic effects and obesity risk. Pointing to the recent success of efforts to reduce consumption of sugary beverages, Dr. Zhang said, “We need to mobilize the same energy and level of commitment when it comes to other unhealthy ultraprocessed foods such as cakes, cookies, doughnuts, and brownies.”
The trends identified by the Tufts study “are concerning and potentially have major public health significance,” according to an accompanying JAMA editorial.
“Better dietary assessment methods are needed to document trends and understand the unique role of ultraprocessed foods to inform future evidence-based policy and dietary recommendations,” wrote Katie A. Meyer, ScD, and Lindsey Smith Taillie, PhD, of the Gillings School of Global Public Health at the University of North Carolina in Chapel Hill.
The editorialists share the authors’ view that “a conceptual advancement would be to consider the level and characteristics of processing as just one of multiple dimensions (including nutrients and food groups) used to classify foods as healthy or unhealthy.” They pointed out that the Pan American Health Organization already recommends targeting products that are ultraprocessed and high in concerning add-in nutrients.
They cautioned, however, that the classification of ultraprocessed foods will not be easy because it requires data on a full list of ingredients, and the effects of processing generally cannot be separated from the composite nutrients of ultraprocessed foods.
This presents a challenge for national food consumption research “given that most large epidemiological studies rely on food frequency questionnaires that lack the information necessary to classify processing levels,” they wrote.
This study was supported by the National Institutes of Health and the São Paulo Research Foundation. Coauthor Dariush Mozaffarian, MD, a cardiologist at Tufts University, disclosed support from the Bill & Melinda Gates Foundation, the National Institutes of Health, and the Rockefeller Foundation as well as personal fees from several commercial companies. He has served on several scientific advisory boards and received royalties from UpToDate, all outside of the submitted work. Dr. Meyer reported a grant from choline manufacturer Balchem. Dr. Taillie reported funding from Bloomberg Philanthropies. Dr. Zhang had no disclosures. Dr. Katzow disclosed no competing interests.
In the 2 decades from 1999 to 2018, ultraprocessed foods consistently accounted for the majority of energy intake by American young people, a large cross-sectional study of National Health and Nutrition Examination Survey (NHANES) data shows.
In young people aged 2-19 years, the estimated percentage of total energy from consumption of ultraprocessed foods increased from 61.4% to 67.0%, for a difference of 5.6% (95% confidence interval [CI] 3.5-7.7, P < .001 for trend), according to Lu Wang, PhD, MPH, a postdoctoral fellow at the Friedman School of Nutrition Science and Policy at Tufts University in Boston, and colleagues.
In contrast, total energy from non- or minimally processed foods decreased from 28.8% to 23.5% (difference −5.3%, 95% CI, −7.5 to −3.2, P < .001 for trend).
“The estimated percentage of energy consumed from ultraprocessed foods increased from 1999 to 2018, with an increasing trend in ready-to-heat and -eat mixed dishes and a decreasing trend in sugar-sweetened beverages,” the authors wrote. The report was published online Aug. 10 in JAMA.
The findings held regardless of the educational and socioeconomic status of the children’s parents.
Significant disparities by race and ethnicity emerged, however, with the ultraprocessed food phenomenon more marked in non-Hispanic Black youths and Mexican-American youths than in their non-Hispanic White counterparts. “Targeted marketing of junk foods toward racial/ethnic minority youths may partly contribute to such differences,” the authors wrote. “However, persistently lower consumption of ultraprocessed foods among Mexican-American youths may reflect more home cooking among Hispanic families.”
Among non-Hispanic Black youths consumption rose from 62.2% to 72.5% (difference 10.3%, 95% CI, 6.8-13.8) and among Mexican-American youths from 55.8% to 63.5% (difference 7.6%, 95% CI, 4.4-10.9). In non-Hispanic White youths intake rose from 63.4% to 68.6% (difference 5.2%, 95% CI, 2.1-8.3, P = .04 for trends).
In addition, a higher consumption of ultraprocessed foods among school-aged youths than among preschool children aged 2-5 years may reflect increased marketing, availability, and selection of ultraprocessed foods for older youths, the authors noted.
Food processing, with its potential adverse effects, may need to be considered as a food dimension in addition to nutrients and food groups in future dietary recommendations and food policies, they added.
“An increasing number of studies are showing a link between ultraprocessed food consumption and adverse health outcomes in children,” corresponding author Fang Fang Zhang, MD, PhD, Neely Family Professor and associate professor at Tufts’ Friedman School of Nutrition Science and Policy, said in an interview. “Health care providers can play a larger role in encouraging patients – and their parents – to replace unhealthy ultraprocessed foods such as ultraprocessed sweet bakery products with healthy unprocessed or minimally processed foods in their diet such as less processed whole grains. “
In Dr. Zhang’s view, teachers also have a part to play in promoting nutrition literacy. “Schools can play an important role in empowering children with knowledge and skills to make healthy food choices,” she said. “Nutrition literacy should be an integral part of the health education curriculum in all K-12 schools.”
Commenting on the study but not involved in it, Michelle Katzow, MD, a pediatrician/obesity medicine specialist and assistant professor at the Feinstein Institutes for Medical Research in Manhasset, N.Y., said the work highlights an often overlooked aspect of the modern American diet that may well be contributing to poor health outcomes in young people.
“It suggests that even as the science advances and we learn more about the adverse health effects of ultraprocessed foods, public health efforts to improve nutrition and food quality in children have not been successful,” she said in an interview. “This is because it is so hard for public health advocates to compete with the food industry, which stands to really benefit financially from hooking kids on processed foods that are not good for their health.”
Dr. Katzow added that the observed racial/ethnic disparities are not surprising in light of a growing body of evidence that racism exists in food marketing. “We need to put forward policies that regulate the food industry, particularly in relation to its most susceptible targets, our kids.”
Study details
The serial cross-sectional analysis used 24-hour dietary recall data from a nationally representative sample from 10 NHANES cycles for the range of 1999-2000 to 2017-2018. The weighted mean age of the cohort was 10.7 years and 49.1% were girls.
Among the subgroups of ultraprocessed foods, the estimated percentage of energy from ready-to-heat and ready-to-eat mixed dishes increased from 2.2% to 11.2% (difference 8.9%; 95%, CI, 7.7-10.2).
Energy from sweets and sweet snacks increased from 10.7% to 12.9% (difference 2.3%; 95% CI, 1.0-3.6), but the estimated percentage of energy decreased for sugar-sweetened beverages from 10.8% to 5.3% (difference −5.5%; 95% CI, −6.5 to −4.5).
In other categories, estimated energy intake from processed fats and oils, condiments, and sauces fell from 7.1% to 4.0% (difference −3.1%; 95% CI, −3.7 to −2.6, all P < .05 for trend).
Not surprisingly, ultraprocessed foods had an overall poorer nutrient profile than that of nonultraprocessed, although they often contained less saturated fat, and they also contained more carbohydrates, mostly from low-quality sources with added sugars and low levels of dietary fiber and protein.
And despite a higher total folate content in ultraprocessed foods because of fortification, higher-level consumers took in less total folate owing to their lower consumption of whole foods.
The authors cautioned that in addition to poor nutrient profiles, processing itself may harm health by changing the physical structure and chemical composition of food, which could lead to elevated glycemic response and reduced satiety. Furthermore, recent research has linked food additives such as emulsifiers, stabilizers, and artificial sweeteners to adverse metabolomic effects and obesity risk. Pointing to the recent success of efforts to reduce consumption of sugary beverages, Dr. Zhang said, “We need to mobilize the same energy and level of commitment when it comes to other unhealthy ultraprocessed foods such as cakes, cookies, doughnuts, and brownies.”
The trends identified by the Tufts study “are concerning and potentially have major public health significance,” according to an accompanying JAMA editorial.
“Better dietary assessment methods are needed to document trends and understand the unique role of ultraprocessed foods to inform future evidence-based policy and dietary recommendations,” wrote Katie A. Meyer, ScD, and Lindsey Smith Taillie, PhD, of the Gillings School of Global Public Health at the University of North Carolina in Chapel Hill.
The editorialists share the authors’ view that “a conceptual advancement would be to consider the level and characteristics of processing as just one of multiple dimensions (including nutrients and food groups) used to classify foods as healthy or unhealthy.” They pointed out that the Pan American Health Organization already recommends targeting products that are ultraprocessed and high in concerning add-in nutrients.
They cautioned, however, that the classification of ultraprocessed foods will not be easy because it requires data on a full list of ingredients, and the effects of processing generally cannot be separated from the composite nutrients of ultraprocessed foods.
This presents a challenge for national food consumption research “given that most large epidemiological studies rely on food frequency questionnaires that lack the information necessary to classify processing levels,” they wrote.
This study was supported by the National Institutes of Health and the São Paulo Research Foundation. Coauthor Dariush Mozaffarian, MD, a cardiologist at Tufts University, disclosed support from the Bill & Melinda Gates Foundation, the National Institutes of Health, and the Rockefeller Foundation as well as personal fees from several commercial companies. He has served on several scientific advisory boards and received royalties from UpToDate, all outside of the submitted work. Dr. Meyer reported a grant from choline manufacturer Balchem. Dr. Taillie reported funding from Bloomberg Philanthropies. Dr. Zhang had no disclosures. Dr. Katzow disclosed no competing interests.
In the 2 decades from 1999 to 2018, ultraprocessed foods consistently accounted for the majority of energy intake by American young people, a large cross-sectional study of National Health and Nutrition Examination Survey (NHANES) data shows.
In young people aged 2-19 years, the estimated percentage of total energy from consumption of ultraprocessed foods increased from 61.4% to 67.0%, for a difference of 5.6% (95% confidence interval [CI] 3.5-7.7, P < .001 for trend), according to Lu Wang, PhD, MPH, a postdoctoral fellow at the Friedman School of Nutrition Science and Policy at Tufts University in Boston, and colleagues.
In contrast, total energy from non- or minimally processed foods decreased from 28.8% to 23.5% (difference −5.3%, 95% CI, −7.5 to −3.2, P < .001 for trend).
“The estimated percentage of energy consumed from ultraprocessed foods increased from 1999 to 2018, with an increasing trend in ready-to-heat and -eat mixed dishes and a decreasing trend in sugar-sweetened beverages,” the authors wrote. The report was published online Aug. 10 in JAMA.
The findings held regardless of the educational and socioeconomic status of the children’s parents.
Significant disparities by race and ethnicity emerged, however, with the ultraprocessed food phenomenon more marked in non-Hispanic Black youths and Mexican-American youths than in their non-Hispanic White counterparts. “Targeted marketing of junk foods toward racial/ethnic minority youths may partly contribute to such differences,” the authors wrote. “However, persistently lower consumption of ultraprocessed foods among Mexican-American youths may reflect more home cooking among Hispanic families.”
Among non-Hispanic Black youths consumption rose from 62.2% to 72.5% (difference 10.3%, 95% CI, 6.8-13.8) and among Mexican-American youths from 55.8% to 63.5% (difference 7.6%, 95% CI, 4.4-10.9). In non-Hispanic White youths intake rose from 63.4% to 68.6% (difference 5.2%, 95% CI, 2.1-8.3, P = .04 for trends).
In addition, a higher consumption of ultraprocessed foods among school-aged youths than among preschool children aged 2-5 years may reflect increased marketing, availability, and selection of ultraprocessed foods for older youths, the authors noted.
Food processing, with its potential adverse effects, may need to be considered as a food dimension in addition to nutrients and food groups in future dietary recommendations and food policies, they added.
“An increasing number of studies are showing a link between ultraprocessed food consumption and adverse health outcomes in children,” corresponding author Fang Fang Zhang, MD, PhD, Neely Family Professor and associate professor at Tufts’ Friedman School of Nutrition Science and Policy, said in an interview. “Health care providers can play a larger role in encouraging patients – and their parents – to replace unhealthy ultraprocessed foods such as ultraprocessed sweet bakery products with healthy unprocessed or minimally processed foods in their diet such as less processed whole grains. “
In Dr. Zhang’s view, teachers also have a part to play in promoting nutrition literacy. “Schools can play an important role in empowering children with knowledge and skills to make healthy food choices,” she said. “Nutrition literacy should be an integral part of the health education curriculum in all K-12 schools.”
Commenting on the study but not involved in it, Michelle Katzow, MD, a pediatrician/obesity medicine specialist and assistant professor at the Feinstein Institutes for Medical Research in Manhasset, N.Y., said the work highlights an often overlooked aspect of the modern American diet that may well be contributing to poor health outcomes in young people.
“It suggests that even as the science advances and we learn more about the adverse health effects of ultraprocessed foods, public health efforts to improve nutrition and food quality in children have not been successful,” she said in an interview. “This is because it is so hard for public health advocates to compete with the food industry, which stands to really benefit financially from hooking kids on processed foods that are not good for their health.”
Dr. Katzow added that the observed racial/ethnic disparities are not surprising in light of a growing body of evidence that racism exists in food marketing. “We need to put forward policies that regulate the food industry, particularly in relation to its most susceptible targets, our kids.”
Study details
The serial cross-sectional analysis used 24-hour dietary recall data from a nationally representative sample from 10 NHANES cycles for the range of 1999-2000 to 2017-2018. The weighted mean age of the cohort was 10.7 years and 49.1% were girls.
Among the subgroups of ultraprocessed foods, the estimated percentage of energy from ready-to-heat and ready-to-eat mixed dishes increased from 2.2% to 11.2% (difference 8.9%; 95%, CI, 7.7-10.2).
Energy from sweets and sweet snacks increased from 10.7% to 12.9% (difference 2.3%; 95% CI, 1.0-3.6), but the estimated percentage of energy decreased for sugar-sweetened beverages from 10.8% to 5.3% (difference −5.5%; 95% CI, −6.5 to −4.5).
In other categories, estimated energy intake from processed fats and oils, condiments, and sauces fell from 7.1% to 4.0% (difference −3.1%; 95% CI, −3.7 to −2.6, all P < .05 for trend).
Not surprisingly, ultraprocessed foods had an overall poorer nutrient profile than that of nonultraprocessed, although they often contained less saturated fat, and they also contained more carbohydrates, mostly from low-quality sources with added sugars and low levels of dietary fiber and protein.
And despite a higher total folate content in ultraprocessed foods because of fortification, higher-level consumers took in less total folate owing to their lower consumption of whole foods.
The authors cautioned that in addition to poor nutrient profiles, processing itself may harm health by changing the physical structure and chemical composition of food, which could lead to elevated glycemic response and reduced satiety. Furthermore, recent research has linked food additives such as emulsifiers, stabilizers, and artificial sweeteners to adverse metabolomic effects and obesity risk. Pointing to the recent success of efforts to reduce consumption of sugary beverages, Dr. Zhang said, “We need to mobilize the same energy and level of commitment when it comes to other unhealthy ultraprocessed foods such as cakes, cookies, doughnuts, and brownies.”
The trends identified by the Tufts study “are concerning and potentially have major public health significance,” according to an accompanying JAMA editorial.
“Better dietary assessment methods are needed to document trends and understand the unique role of ultraprocessed foods to inform future evidence-based policy and dietary recommendations,” wrote Katie A. Meyer, ScD, and Lindsey Smith Taillie, PhD, of the Gillings School of Global Public Health at the University of North Carolina in Chapel Hill.
The editorialists share the authors’ view that “a conceptual advancement would be to consider the level and characteristics of processing as just one of multiple dimensions (including nutrients and food groups) used to classify foods as healthy or unhealthy.” They pointed out that the Pan American Health Organization already recommends targeting products that are ultraprocessed and high in concerning add-in nutrients.
They cautioned, however, that the classification of ultraprocessed foods will not be easy because it requires data on a full list of ingredients, and the effects of processing generally cannot be separated from the composite nutrients of ultraprocessed foods.
This presents a challenge for national food consumption research “given that most large epidemiological studies rely on food frequency questionnaires that lack the information necessary to classify processing levels,” they wrote.
This study was supported by the National Institutes of Health and the São Paulo Research Foundation. Coauthor Dariush Mozaffarian, MD, a cardiologist at Tufts University, disclosed support from the Bill & Melinda Gates Foundation, the National Institutes of Health, and the Rockefeller Foundation as well as personal fees from several commercial companies. He has served on several scientific advisory boards and received royalties from UpToDate, all outside of the submitted work. Dr. Meyer reported a grant from choline manufacturer Balchem. Dr. Taillie reported funding from Bloomberg Philanthropies. Dr. Zhang had no disclosures. Dr. Katzow disclosed no competing interests.
FROM JAMA
Virtual roller-coaster may explain the ups and downs of migraine
and may explain the mechanisms underlying common symptoms and increased activity in certain brain regions in migraine patients.
In a new study, the prevalence of dizziness was 65% among patients with migraine who underwent a virtual roller-coaster ride versus 30% among those without migraine. In addition, imaging showed greater neuronal activity after the simulation in those with migraine.
“Migraine patients reported more dizziness and motion sickness, as well as longer symptom duration and intensity, in a virtual roller-coaster ride,” even though the videos and timing were identical for both groups, said study investigator Arne May, MD, PhD, professor of neurology at the University of Hamburg (Germany).
“We found differences not just in behavioral results but also in specific activations of areas within the cerebellum and the frontal gyrus. Migraine patients process such visual input differently from controls and activate a specific brain network to do so,” he added.
The findings were published online July 21, 2021, in Neurology.
The brain’s response
Nausea, which is among the diagnostic criteria for migraine, is the main symptom of motion sickness. Vestibular symptoms such as dizziness are also components of migraine.
Previous research has examined how the brain processes visual and motion stimuli in migraine, but the reasons patients with migraine are susceptible to motion sickness and dizziness remain unclear.
The researchers used a simulated roller-coaster ride to study the clinical and brain responses to motion among participants with and participants without migraine. They enrolled 20 consecutive patients with migraine who presented to a tertiary headache clinic between January and March 2020 and enrolled 20 healthy participants from a university hospital and the community. The average age of the study population was 30 years, and more than 80% were women.
In response to a questionnaire, participants provided information about demographics and headache features, including onset, frequency, and intensity. They also provided information about their status within the migraine phase and about vestibular symptoms experienced in daily life.
While undergoing functional MRI (fMRI), all participants watched two short videos that provided a first-person perspective of a roller-coaster ride. During the videos, they wore ear buds that conveyed the sound of a car riding over the rails.
The first video included more horizontal perspectives, and the second had more vertical perspectives. Each video was shown three times in random order.
During fMRI, participants reported intensity of nausea and vestibular symptoms using an 11-point Likert scale. After the experiment, they responded to a questionnaire that evaluated intensity and duration of nausea, dizziness, and vertigo experienced during the videos.
Participants also were given the Simulator Sickness Questionnaire (SSQ), which assessed motion sickness. A 100-point visual analog scale (VAS) was used to rate how realistic the roller-coaster experience had been.
There were no differences in sex or age between the migraine group and the healthy control group. Half of the patients with migraine reported aura. The mean number of migraine attacks within the previous month was 3.7. The mean Migraine Disability Assessment score was 21.5, which indicates severe disability.
Nausea, dizziness often neglected
Baseline prevalence of vestibular symptoms was 75% in the migraine group and 5% in the control group (P < .0001). These symptoms included dizziness (60% and 5%, respectively; P < .0001) and postural symptoms (40% and 0%, respectively; P = .003).
At baseline, vestibular symptoms were more frequent (P = .001), more intense (P < .0001), and were associated with greater disability (P = .001) in patients with migraine, compared with participants without migraine. The patients with migraine were also more susceptible to motion sickness (P = .02) and had higher depression scores (P = .001).
During the roller-coaster simulation, dizziness was more prevalent among patients with migraine than among those without migraine (65% vs. 30%; P = .03). Patients with migraine also reported more motion sickness (SSQ score, 47.3 vs. 24.3; P = .004), longer symptom duration (1:19 minutes vs. 00:27 minutes; P = .03), and symptoms of greater intensity (VAS, 22.0 vs. 9.9; P = .03).
Brain activity also differed between groups. Among patients with migraine, neuronal activity was greater in clusters within the right superior and left inferior occipital gyrus, the left pontine nuclei, and the left cerebellar lobules V and VI.
There was a moderately negative correlation of activation of the inferior occipital gyrus with migraine disability (r = –0.46; P = .04). Activation within the pontine nuclei correlated positively with motion sickness scores (r = 0.32; P = .04).
In addition, among patients with migraine, activity in the cerebellar lobule VIIb and in the left middle frontal gyrus was decreased in comparison with persons without migraine. Also among patients with migraine, there was enhanced connectivity between the pontine nuclei, cerebellar areas V and VI, and the interior and superior occipital gyrus and numerous cortical areas.
Clinicians often neglect to treat dizziness and nausea in patients with migraine, said Dr. May. However, these symptoms are part of migraine, even when attacks are not occurring.
“I have learned that if we can explain such symptoms, they are better accepted,” said Dr. May. “We need more and better basic research because we need to understand before we treat.”
Toward faster, more effective treatment
Commenting on the study, Erik Viirre, MD, PhD, professor in the department of neurosciences, University of California, San Diego, said, “we can be excited and celebrate that these researchers are using these news tools to investigate the operation of the migraine brain.
“That will combine with the new therapies and the genomics to give us a powerful approach to this particular condition,” said Dr. Viirre, who was not involved with the research.
The findings provide significant detail about the interconnections between the various brain regions affected by migraine, he noted. These regions include not just the sensory centers but also areas involved in higher executive function and emotional responses.
By identifying these regions, the findings show “some of the underlying mechanisms of these clinically relevant features,” said Dr. Viirre, who is also director of UCSD’s Arthur C. Clarke Center for Human Imagination.
The investigators set up the motion simulation well and used sound fMRI methodology, he added. However, imaging studies of the brain’s response to motion pose several challenges.
“The biggest challenge in any of these circumstances is that you can’t put an actual fMRI scanner on a roller-coaster,” said Dr. Viirre. “The actual acceleration and gravitational sensations delivered by a roller-coaster and gravity, of course, do not occur when you’re lying still in an MRI scanner.” Nevertheless, the pseudoacceleration produced by a visual stimulus is a reasonable proxy.
The findings also suggest that researchers in the future could examine whether any new therapeutic interventions for migraine modulate the brain functions differently for individuals with migraine than for those without migraine, he noted.
“That’s going to lead us to a faster, more effective, more reliable suite of migraine therapies,” said Dr. Viirre.
The study also reminds clinicians to take a broader approach to patients with migraine, and it underscores the value of strategies such as self-calming techniques, which can reduce the number and intensity of headaches, he said.
“Literally demonstrating these functional differences in the migraine brain is a hugely important message of advocacy for people with migraine,” Dr. Viirre concluded.
The study was funded by the German Research Foundation. Drs. May and Viirre have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and may explain the mechanisms underlying common symptoms and increased activity in certain brain regions in migraine patients.
In a new study, the prevalence of dizziness was 65% among patients with migraine who underwent a virtual roller-coaster ride versus 30% among those without migraine. In addition, imaging showed greater neuronal activity after the simulation in those with migraine.
“Migraine patients reported more dizziness and motion sickness, as well as longer symptom duration and intensity, in a virtual roller-coaster ride,” even though the videos and timing were identical for both groups, said study investigator Arne May, MD, PhD, professor of neurology at the University of Hamburg (Germany).
“We found differences not just in behavioral results but also in specific activations of areas within the cerebellum and the frontal gyrus. Migraine patients process such visual input differently from controls and activate a specific brain network to do so,” he added.
The findings were published online July 21, 2021, in Neurology.
The brain’s response
Nausea, which is among the diagnostic criteria for migraine, is the main symptom of motion sickness. Vestibular symptoms such as dizziness are also components of migraine.
Previous research has examined how the brain processes visual and motion stimuli in migraine, but the reasons patients with migraine are susceptible to motion sickness and dizziness remain unclear.
The researchers used a simulated roller-coaster ride to study the clinical and brain responses to motion among participants with and participants without migraine. They enrolled 20 consecutive patients with migraine who presented to a tertiary headache clinic between January and March 2020 and enrolled 20 healthy participants from a university hospital and the community. The average age of the study population was 30 years, and more than 80% were women.
In response to a questionnaire, participants provided information about demographics and headache features, including onset, frequency, and intensity. They also provided information about their status within the migraine phase and about vestibular symptoms experienced in daily life.
While undergoing functional MRI (fMRI), all participants watched two short videos that provided a first-person perspective of a roller-coaster ride. During the videos, they wore ear buds that conveyed the sound of a car riding over the rails.
The first video included more horizontal perspectives, and the second had more vertical perspectives. Each video was shown three times in random order.
During fMRI, participants reported intensity of nausea and vestibular symptoms using an 11-point Likert scale. After the experiment, they responded to a questionnaire that evaluated intensity and duration of nausea, dizziness, and vertigo experienced during the videos.
Participants also were given the Simulator Sickness Questionnaire (SSQ), which assessed motion sickness. A 100-point visual analog scale (VAS) was used to rate how realistic the roller-coaster experience had been.
There were no differences in sex or age between the migraine group and the healthy control group. Half of the patients with migraine reported aura. The mean number of migraine attacks within the previous month was 3.7. The mean Migraine Disability Assessment score was 21.5, which indicates severe disability.
Nausea, dizziness often neglected
Baseline prevalence of vestibular symptoms was 75% in the migraine group and 5% in the control group (P < .0001). These symptoms included dizziness (60% and 5%, respectively; P < .0001) and postural symptoms (40% and 0%, respectively; P = .003).
At baseline, vestibular symptoms were more frequent (P = .001), more intense (P < .0001), and were associated with greater disability (P = .001) in patients with migraine, compared with participants without migraine. The patients with migraine were also more susceptible to motion sickness (P = .02) and had higher depression scores (P = .001).
During the roller-coaster simulation, dizziness was more prevalent among patients with migraine than among those without migraine (65% vs. 30%; P = .03). Patients with migraine also reported more motion sickness (SSQ score, 47.3 vs. 24.3; P = .004), longer symptom duration (1:19 minutes vs. 00:27 minutes; P = .03), and symptoms of greater intensity (VAS, 22.0 vs. 9.9; P = .03).
Brain activity also differed between groups. Among patients with migraine, neuronal activity was greater in clusters within the right superior and left inferior occipital gyrus, the left pontine nuclei, and the left cerebellar lobules V and VI.
There was a moderately negative correlation of activation of the inferior occipital gyrus with migraine disability (r = –0.46; P = .04). Activation within the pontine nuclei correlated positively with motion sickness scores (r = 0.32; P = .04).
In addition, among patients with migraine, activity in the cerebellar lobule VIIb and in the left middle frontal gyrus was decreased in comparison with persons without migraine. Also among patients with migraine, there was enhanced connectivity between the pontine nuclei, cerebellar areas V and VI, and the interior and superior occipital gyrus and numerous cortical areas.
Clinicians often neglect to treat dizziness and nausea in patients with migraine, said Dr. May. However, these symptoms are part of migraine, even when attacks are not occurring.
“I have learned that if we can explain such symptoms, they are better accepted,” said Dr. May. “We need more and better basic research because we need to understand before we treat.”
Toward faster, more effective treatment
Commenting on the study, Erik Viirre, MD, PhD, professor in the department of neurosciences, University of California, San Diego, said, “we can be excited and celebrate that these researchers are using these news tools to investigate the operation of the migraine brain.
“That will combine with the new therapies and the genomics to give us a powerful approach to this particular condition,” said Dr. Viirre, who was not involved with the research.
The findings provide significant detail about the interconnections between the various brain regions affected by migraine, he noted. These regions include not just the sensory centers but also areas involved in higher executive function and emotional responses.
By identifying these regions, the findings show “some of the underlying mechanisms of these clinically relevant features,” said Dr. Viirre, who is also director of UCSD’s Arthur C. Clarke Center for Human Imagination.
The investigators set up the motion simulation well and used sound fMRI methodology, he added. However, imaging studies of the brain’s response to motion pose several challenges.
“The biggest challenge in any of these circumstances is that you can’t put an actual fMRI scanner on a roller-coaster,” said Dr. Viirre. “The actual acceleration and gravitational sensations delivered by a roller-coaster and gravity, of course, do not occur when you’re lying still in an MRI scanner.” Nevertheless, the pseudoacceleration produced by a visual stimulus is a reasonable proxy.
The findings also suggest that researchers in the future could examine whether any new therapeutic interventions for migraine modulate the brain functions differently for individuals with migraine than for those without migraine, he noted.
“That’s going to lead us to a faster, more effective, more reliable suite of migraine therapies,” said Dr. Viirre.
The study also reminds clinicians to take a broader approach to patients with migraine, and it underscores the value of strategies such as self-calming techniques, which can reduce the number and intensity of headaches, he said.
“Literally demonstrating these functional differences in the migraine brain is a hugely important message of advocacy for people with migraine,” Dr. Viirre concluded.
The study was funded by the German Research Foundation. Drs. May and Viirre have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and may explain the mechanisms underlying common symptoms and increased activity in certain brain regions in migraine patients.
In a new study, the prevalence of dizziness was 65% among patients with migraine who underwent a virtual roller-coaster ride versus 30% among those without migraine. In addition, imaging showed greater neuronal activity after the simulation in those with migraine.
“Migraine patients reported more dizziness and motion sickness, as well as longer symptom duration and intensity, in a virtual roller-coaster ride,” even though the videos and timing were identical for both groups, said study investigator Arne May, MD, PhD, professor of neurology at the University of Hamburg (Germany).
“We found differences not just in behavioral results but also in specific activations of areas within the cerebellum and the frontal gyrus. Migraine patients process such visual input differently from controls and activate a specific brain network to do so,” he added.
The findings were published online July 21, 2021, in Neurology.
The brain’s response
Nausea, which is among the diagnostic criteria for migraine, is the main symptom of motion sickness. Vestibular symptoms such as dizziness are also components of migraine.
Previous research has examined how the brain processes visual and motion stimuli in migraine, but the reasons patients with migraine are susceptible to motion sickness and dizziness remain unclear.
The researchers used a simulated roller-coaster ride to study the clinical and brain responses to motion among participants with and participants without migraine. They enrolled 20 consecutive patients with migraine who presented to a tertiary headache clinic between January and March 2020 and enrolled 20 healthy participants from a university hospital and the community. The average age of the study population was 30 years, and more than 80% were women.
In response to a questionnaire, participants provided information about demographics and headache features, including onset, frequency, and intensity. They also provided information about their status within the migraine phase and about vestibular symptoms experienced in daily life.
While undergoing functional MRI (fMRI), all participants watched two short videos that provided a first-person perspective of a roller-coaster ride. During the videos, they wore ear buds that conveyed the sound of a car riding over the rails.
The first video included more horizontal perspectives, and the second had more vertical perspectives. Each video was shown three times in random order.
During fMRI, participants reported intensity of nausea and vestibular symptoms using an 11-point Likert scale. After the experiment, they responded to a questionnaire that evaluated intensity and duration of nausea, dizziness, and vertigo experienced during the videos.
Participants also were given the Simulator Sickness Questionnaire (SSQ), which assessed motion sickness. A 100-point visual analog scale (VAS) was used to rate how realistic the roller-coaster experience had been.
There were no differences in sex or age between the migraine group and the healthy control group. Half of the patients with migraine reported aura. The mean number of migraine attacks within the previous month was 3.7. The mean Migraine Disability Assessment score was 21.5, which indicates severe disability.
Nausea, dizziness often neglected
Baseline prevalence of vestibular symptoms was 75% in the migraine group and 5% in the control group (P < .0001). These symptoms included dizziness (60% and 5%, respectively; P < .0001) and postural symptoms (40% and 0%, respectively; P = .003).
At baseline, vestibular symptoms were more frequent (P = .001), more intense (P < .0001), and were associated with greater disability (P = .001) in patients with migraine, compared with participants without migraine. The patients with migraine were also more susceptible to motion sickness (P = .02) and had higher depression scores (P = .001).
During the roller-coaster simulation, dizziness was more prevalent among patients with migraine than among those without migraine (65% vs. 30%; P = .03). Patients with migraine also reported more motion sickness (SSQ score, 47.3 vs. 24.3; P = .004), longer symptom duration (1:19 minutes vs. 00:27 minutes; P = .03), and symptoms of greater intensity (VAS, 22.0 vs. 9.9; P = .03).
Brain activity also differed between groups. Among patients with migraine, neuronal activity was greater in clusters within the right superior and left inferior occipital gyrus, the left pontine nuclei, and the left cerebellar lobules V and VI.
There was a moderately negative correlation of activation of the inferior occipital gyrus with migraine disability (r = –0.46; P = .04). Activation within the pontine nuclei correlated positively with motion sickness scores (r = 0.32; P = .04).
In addition, among patients with migraine, activity in the cerebellar lobule VIIb and in the left middle frontal gyrus was decreased in comparison with persons without migraine. Also among patients with migraine, there was enhanced connectivity between the pontine nuclei, cerebellar areas V and VI, and the interior and superior occipital gyrus and numerous cortical areas.
Clinicians often neglect to treat dizziness and nausea in patients with migraine, said Dr. May. However, these symptoms are part of migraine, even when attacks are not occurring.
“I have learned that if we can explain such symptoms, they are better accepted,” said Dr. May. “We need more and better basic research because we need to understand before we treat.”
Toward faster, more effective treatment
Commenting on the study, Erik Viirre, MD, PhD, professor in the department of neurosciences, University of California, San Diego, said, “we can be excited and celebrate that these researchers are using these news tools to investigate the operation of the migraine brain.
“That will combine with the new therapies and the genomics to give us a powerful approach to this particular condition,” said Dr. Viirre, who was not involved with the research.
The findings provide significant detail about the interconnections between the various brain regions affected by migraine, he noted. These regions include not just the sensory centers but also areas involved in higher executive function and emotional responses.
By identifying these regions, the findings show “some of the underlying mechanisms of these clinically relevant features,” said Dr. Viirre, who is also director of UCSD’s Arthur C. Clarke Center for Human Imagination.
The investigators set up the motion simulation well and used sound fMRI methodology, he added. However, imaging studies of the brain’s response to motion pose several challenges.
“The biggest challenge in any of these circumstances is that you can’t put an actual fMRI scanner on a roller-coaster,” said Dr. Viirre. “The actual acceleration and gravitational sensations delivered by a roller-coaster and gravity, of course, do not occur when you’re lying still in an MRI scanner.” Nevertheless, the pseudoacceleration produced by a visual stimulus is a reasonable proxy.
The findings also suggest that researchers in the future could examine whether any new therapeutic interventions for migraine modulate the brain functions differently for individuals with migraine than for those without migraine, he noted.
“That’s going to lead us to a faster, more effective, more reliable suite of migraine therapies,” said Dr. Viirre.
The study also reminds clinicians to take a broader approach to patients with migraine, and it underscores the value of strategies such as self-calming techniques, which can reduce the number and intensity of headaches, he said.
“Literally demonstrating these functional differences in the migraine brain is a hugely important message of advocacy for people with migraine,” Dr. Viirre concluded.
The study was funded by the German Research Foundation. Drs. May and Viirre have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Surge of new child COVID cases continues for 6th consecutive week
The current COVID-19 surge has brought new cases in children to their highest level since February, according to a new report.
New pediatric cases rose for the 6th straight week, with almost 94,000 reported for the week ending Aug. 5.
That weekly total was up by 31% over the previous week and by over 1,000% since late June, when the new-case figure was at its lowest point (8,447) since early in the pandemic, the American Academy of Pediatrics and the Children’s Hospital Association said. COVID-related deaths – 13 for the week – were also higher than at any time since March 2021.
Almost 4.3 million children have been infected with SARS-CoV-2, which is 14.3% of all cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. Children represented 15.0% of the new cases reported in those jurisdictions during the week ending Aug. 5, the AAP and CHA said in their weekly report.
Another measure that has been trending upward recently is vaccine initiation among 12- to 15-year-olds, although the latest weekly total is still well below the high of 1.4 million seen in May. First-time vaccinations reached almost 411,000 for the week of Aug. 3-9, marking the fourth consecutive increase in that age group, the Centers for Disease Control and Prevention said on its COVID Data Tracker. Vaccinations also increased, although more modestly, for 16- and 17-year-olds in the most recent week.
Cumulative figures for children aged 12-17 show that almost 10.4 million have received at least one dose and that 7.7 million are fully vaccinated as of Aug. 9. By age group, 42.2% of those aged 12-15 have received at least one dose, and 30.4% have completed the vaccine regimen. Among those aged 16-17 years, 52.2% have gotten their first dose, and 41.4% are fully vaccinated, according to the COVID Data Tracker.
Looking at vaccination rates on the state level shows that only 20% of children aged 12-17 in Wyoming and 21% in Mississippi have gotten at least one dose as of Aug. 4, while Massachusetts is up to 68% and Vermont reports 70%. Rates for full vaccination range from 11% in Mississippi and Alabama to 61% in Vermont, based on an AAP analysis of CDC data, which is not available for Idaho.
The current COVID-19 surge has brought new cases in children to their highest level since February, according to a new report.

New pediatric cases rose for the 6th straight week, with almost 94,000 reported for the week ending Aug. 5.
That weekly total was up by 31% over the previous week and by over 1,000% since late June, when the new-case figure was at its lowest point (8,447) since early in the pandemic, the American Academy of Pediatrics and the Children’s Hospital Association said. COVID-related deaths – 13 for the week – were also higher than at any time since March 2021.
Almost 4.3 million children have been infected with SARS-CoV-2, which is 14.3% of all cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. Children represented 15.0% of the new cases reported in those jurisdictions during the week ending Aug. 5, the AAP and CHA said in their weekly report.
Another measure that has been trending upward recently is vaccine initiation among 12- to 15-year-olds, although the latest weekly total is still well below the high of 1.4 million seen in May. First-time vaccinations reached almost 411,000 for the week of Aug. 3-9, marking the fourth consecutive increase in that age group, the Centers for Disease Control and Prevention said on its COVID Data Tracker. Vaccinations also increased, although more modestly, for 16- and 17-year-olds in the most recent week.
Cumulative figures for children aged 12-17 show that almost 10.4 million have received at least one dose and that 7.7 million are fully vaccinated as of Aug. 9. By age group, 42.2% of those aged 12-15 have received at least one dose, and 30.4% have completed the vaccine regimen. Among those aged 16-17 years, 52.2% have gotten their first dose, and 41.4% are fully vaccinated, according to the COVID Data Tracker.
Looking at vaccination rates on the state level shows that only 20% of children aged 12-17 in Wyoming and 21% in Mississippi have gotten at least one dose as of Aug. 4, while Massachusetts is up to 68% and Vermont reports 70%. Rates for full vaccination range from 11% in Mississippi and Alabama to 61% in Vermont, based on an AAP analysis of CDC data, which is not available for Idaho.
The current COVID-19 surge has brought new cases in children to their highest level since February, according to a new report.

New pediatric cases rose for the 6th straight week, with almost 94,000 reported for the week ending Aug. 5.
That weekly total was up by 31% over the previous week and by over 1,000% since late June, when the new-case figure was at its lowest point (8,447) since early in the pandemic, the American Academy of Pediatrics and the Children’s Hospital Association said. COVID-related deaths – 13 for the week – were also higher than at any time since March 2021.
Almost 4.3 million children have been infected with SARS-CoV-2, which is 14.3% of all cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. Children represented 15.0% of the new cases reported in those jurisdictions during the week ending Aug. 5, the AAP and CHA said in their weekly report.
Another measure that has been trending upward recently is vaccine initiation among 12- to 15-year-olds, although the latest weekly total is still well below the high of 1.4 million seen in May. First-time vaccinations reached almost 411,000 for the week of Aug. 3-9, marking the fourth consecutive increase in that age group, the Centers for Disease Control and Prevention said on its COVID Data Tracker. Vaccinations also increased, although more modestly, for 16- and 17-year-olds in the most recent week.
Cumulative figures for children aged 12-17 show that almost 10.4 million have received at least one dose and that 7.7 million are fully vaccinated as of Aug. 9. By age group, 42.2% of those aged 12-15 have received at least one dose, and 30.4% have completed the vaccine regimen. Among those aged 16-17 years, 52.2% have gotten their first dose, and 41.4% are fully vaccinated, according to the COVID Data Tracker.
Looking at vaccination rates on the state level shows that only 20% of children aged 12-17 in Wyoming and 21% in Mississippi have gotten at least one dose as of Aug. 4, while Massachusetts is up to 68% and Vermont reports 70%. Rates for full vaccination range from 11% in Mississippi and Alabama to 61% in Vermont, based on an AAP analysis of CDC data, which is not available for Idaho.
Physician ‘predator’ sentenced for opioid-related patient death; more
Doctor’s illegal opioid distribution results in patient death
Thomas K. Ballard III, MD, of Jackson, Tenn., pleaded guilty to causing the death of one of his patients in 2015 by illegally prescribing hydrocodone. He faces a minimum of 20 years in prison for one count of illegal drug distribution resulting in death. He will be sentenced in September.
Dr. Ballard, 63, owned and operated the Ballard Clinic, from which he prescribed dangerous and addictive drugs without legitimate medical purpose. according to the U.S. Department of Justice.
Dr. Ballard’s treatment records show that he believed a patient had psychiatric problems and was abusing her medication, evidenced by positive drug screens and prescriptions obtained elsewhere for suboxone, a drug used to treat opioid dependency. However, Dr. Ballard continued to prescribe hydrocodone to the patient, including on May 28, 2015, when the patient fatally overdosed on the drug.
“Ballard has proven himself to be nothing more than a predator in a white lab coat, and he should expect to be punished accordingly,” said Special Agent in Charge J. Todd Scott of the Drug Enforcement Administration’s Louisville Division. “Doctors take an oath to first do no harm, and instead, Ballard chose to put his own licentious interests above his patients’ well-being.”
Clinical researchers falsify drug trial data
Eduardo Navarro, of Miami, and Nayade Varona, of Port St. Lucie, Fla., pleaded guilty to conspiring to falsify clinical trial data. Mr. Navarro, 52, was sentenced to 46 months in prison and Ms. Varona, 50, received 30 months in prison.
The court also ordered the defendants to pay $2,134,503 in restitution.
Mr. Navarro and Ms. Varona both worked for Tellus Clinical Research, where Mr. Navarro was a subinvestigator and nurse practitioner, and Ms. Varona was an assistant study coordinator. They admitted to agreeing with one another and others to falsify data in medical records for two clinical trials that were evaluating a treatment for irritable bowel syndrome. Mr. Navarro and Ms. Varona falsified data to make it appear as though patients were participating in the trials, which never occurred.
Doctor faces decade in prison for $6 million health care fraud
Keyvan Amirikhorheh, MD, a family physician in Seal Beach, Calif., pleaded guilty to conspiring to commit health care fraud. He faces a maximum of 10 years in prison.
While working at Los Angeles Community Clinic, Dr. Amirikhorheh submitted fraudulent claims for family planning services, diagnostic testing, and prescriptions for nonexistent patients, defrauding the Family Planning, Access, Care and Treatment (Family PACT) program administered by Medi-Cal, the California Medicaid program.
Between March 2016 and April 2019, Los Angeles Community Clinic and associated laboratories and pharmacies submitted approximately $8,406,204 in claims to Medi-Cal and were paid approximately $6,660,028. Dr. Amirikhorheh, 61, is the final defendant of five to plead guilty in the case, according to the DOJ.
Dentist office sued for HIV discrimination
Night and Day Dental, of North Carolina, settled with the DOJ to resolve a claim that it discriminated against a woman with HIV in violation of the Americans With Disabilities Act.
Title III of the ADA prohibits health care professionals from discriminating against people with disabilities, including those with HIV. The DOJ found that Night and Day Dental refused to accept a woman as a new patient because of her HIV-positive status. The patient was seeking routine dental care, including a cleaning and check-up. Night and Day Dental additionally requires certain blood work results from patients with HIV before deciding whether to provide dental care, when requiring such results is not medically necessary.
They will pay $30,000 to the victim of the discrimination, train their staff on the ADA, develop an antidiscrimination policy, and report to the DOJ every time they refuse to treat a person with HIV or stop providing treatment after learning of a patient’s HIV-positive status. “Turning away patients with HIV or requiring them to provide information that is not medically recommended creates unfair barriers to health care for people with HIV,” said Kristen Clarke, assistant attorney general in the DOJ’s Civil Rights Division.
A version of this article first appeared on Medscape.com.
This article was updated 8/12/21.
Doctor’s illegal opioid distribution results in patient death
Thomas K. Ballard III, MD, of Jackson, Tenn., pleaded guilty to causing the death of one of his patients in 2015 by illegally prescribing hydrocodone. He faces a minimum of 20 years in prison for one count of illegal drug distribution resulting in death. He will be sentenced in September.
Dr. Ballard, 63, owned and operated the Ballard Clinic, from which he prescribed dangerous and addictive drugs without legitimate medical purpose. according to the U.S. Department of Justice.
Dr. Ballard’s treatment records show that he believed a patient had psychiatric problems and was abusing her medication, evidenced by positive drug screens and prescriptions obtained elsewhere for suboxone, a drug used to treat opioid dependency. However, Dr. Ballard continued to prescribe hydrocodone to the patient, including on May 28, 2015, when the patient fatally overdosed on the drug.
“Ballard has proven himself to be nothing more than a predator in a white lab coat, and he should expect to be punished accordingly,” said Special Agent in Charge J. Todd Scott of the Drug Enforcement Administration’s Louisville Division. “Doctors take an oath to first do no harm, and instead, Ballard chose to put his own licentious interests above his patients’ well-being.”
Clinical researchers falsify drug trial data
Eduardo Navarro, of Miami, and Nayade Varona, of Port St. Lucie, Fla., pleaded guilty to conspiring to falsify clinical trial data. Mr. Navarro, 52, was sentenced to 46 months in prison and Ms. Varona, 50, received 30 months in prison.
The court also ordered the defendants to pay $2,134,503 in restitution.
Mr. Navarro and Ms. Varona both worked for Tellus Clinical Research, where Mr. Navarro was a subinvestigator and nurse practitioner, and Ms. Varona was an assistant study coordinator. They admitted to agreeing with one another and others to falsify data in medical records for two clinical trials that were evaluating a treatment for irritable bowel syndrome. Mr. Navarro and Ms. Varona falsified data to make it appear as though patients were participating in the trials, which never occurred.
Doctor faces decade in prison for $6 million health care fraud
Keyvan Amirikhorheh, MD, a family physician in Seal Beach, Calif., pleaded guilty to conspiring to commit health care fraud. He faces a maximum of 10 years in prison.
While working at Los Angeles Community Clinic, Dr. Amirikhorheh submitted fraudulent claims for family planning services, diagnostic testing, and prescriptions for nonexistent patients, defrauding the Family Planning, Access, Care and Treatment (Family PACT) program administered by Medi-Cal, the California Medicaid program.
Between March 2016 and April 2019, Los Angeles Community Clinic and associated laboratories and pharmacies submitted approximately $8,406,204 in claims to Medi-Cal and were paid approximately $6,660,028. Dr. Amirikhorheh, 61, is the final defendant of five to plead guilty in the case, according to the DOJ.
Dentist office sued for HIV discrimination
Night and Day Dental, of North Carolina, settled with the DOJ to resolve a claim that it discriminated against a woman with HIV in violation of the Americans With Disabilities Act.
Title III of the ADA prohibits health care professionals from discriminating against people with disabilities, including those with HIV. The DOJ found that Night and Day Dental refused to accept a woman as a new patient because of her HIV-positive status. The patient was seeking routine dental care, including a cleaning and check-up. Night and Day Dental additionally requires certain blood work results from patients with HIV before deciding whether to provide dental care, when requiring such results is not medically necessary.
They will pay $30,000 to the victim of the discrimination, train their staff on the ADA, develop an antidiscrimination policy, and report to the DOJ every time they refuse to treat a person with HIV or stop providing treatment after learning of a patient’s HIV-positive status. “Turning away patients with HIV or requiring them to provide information that is not medically recommended creates unfair barriers to health care for people with HIV,” said Kristen Clarke, assistant attorney general in the DOJ’s Civil Rights Division.
A version of this article first appeared on Medscape.com.
This article was updated 8/12/21.
Doctor’s illegal opioid distribution results in patient death
Thomas K. Ballard III, MD, of Jackson, Tenn., pleaded guilty to causing the death of one of his patients in 2015 by illegally prescribing hydrocodone. He faces a minimum of 20 years in prison for one count of illegal drug distribution resulting in death. He will be sentenced in September.
Dr. Ballard, 63, owned and operated the Ballard Clinic, from which he prescribed dangerous and addictive drugs without legitimate medical purpose. according to the U.S. Department of Justice.
Dr. Ballard’s treatment records show that he believed a patient had psychiatric problems and was abusing her medication, evidenced by positive drug screens and prescriptions obtained elsewhere for suboxone, a drug used to treat opioid dependency. However, Dr. Ballard continued to prescribe hydrocodone to the patient, including on May 28, 2015, when the patient fatally overdosed on the drug.
“Ballard has proven himself to be nothing more than a predator in a white lab coat, and he should expect to be punished accordingly,” said Special Agent in Charge J. Todd Scott of the Drug Enforcement Administration’s Louisville Division. “Doctors take an oath to first do no harm, and instead, Ballard chose to put his own licentious interests above his patients’ well-being.”
Clinical researchers falsify drug trial data
Eduardo Navarro, of Miami, and Nayade Varona, of Port St. Lucie, Fla., pleaded guilty to conspiring to falsify clinical trial data. Mr. Navarro, 52, was sentenced to 46 months in prison and Ms. Varona, 50, received 30 months in prison.
The court also ordered the defendants to pay $2,134,503 in restitution.
Mr. Navarro and Ms. Varona both worked for Tellus Clinical Research, where Mr. Navarro was a subinvestigator and nurse practitioner, and Ms. Varona was an assistant study coordinator. They admitted to agreeing with one another and others to falsify data in medical records for two clinical trials that were evaluating a treatment for irritable bowel syndrome. Mr. Navarro and Ms. Varona falsified data to make it appear as though patients were participating in the trials, which never occurred.
Doctor faces decade in prison for $6 million health care fraud
Keyvan Amirikhorheh, MD, a family physician in Seal Beach, Calif., pleaded guilty to conspiring to commit health care fraud. He faces a maximum of 10 years in prison.
While working at Los Angeles Community Clinic, Dr. Amirikhorheh submitted fraudulent claims for family planning services, diagnostic testing, and prescriptions for nonexistent patients, defrauding the Family Planning, Access, Care and Treatment (Family PACT) program administered by Medi-Cal, the California Medicaid program.
Between March 2016 and April 2019, Los Angeles Community Clinic and associated laboratories and pharmacies submitted approximately $8,406,204 in claims to Medi-Cal and were paid approximately $6,660,028. Dr. Amirikhorheh, 61, is the final defendant of five to plead guilty in the case, according to the DOJ.
Dentist office sued for HIV discrimination
Night and Day Dental, of North Carolina, settled with the DOJ to resolve a claim that it discriminated against a woman with HIV in violation of the Americans With Disabilities Act.
Title III of the ADA prohibits health care professionals from discriminating against people with disabilities, including those with HIV. The DOJ found that Night and Day Dental refused to accept a woman as a new patient because of her HIV-positive status. The patient was seeking routine dental care, including a cleaning and check-up. Night and Day Dental additionally requires certain blood work results from patients with HIV before deciding whether to provide dental care, when requiring such results is not medically necessary.
They will pay $30,000 to the victim of the discrimination, train their staff on the ADA, develop an antidiscrimination policy, and report to the DOJ every time they refuse to treat a person with HIV or stop providing treatment after learning of a patient’s HIV-positive status. “Turning away patients with HIV or requiring them to provide information that is not medically recommended creates unfair barriers to health care for people with HIV,” said Kristen Clarke, assistant attorney general in the DOJ’s Civil Rights Division.
A version of this article first appeared on Medscape.com.
This article was updated 8/12/21.
MS CONFERENCE NEWS
- Common MS treatment wears off more quickly in Black patients
- A less expensive, more convenient treatment option for MS?
- Investigational drug reduces brain lesions in highly active MS
- Evobrutinib may lower nerve damage biomarker levels
- Disease progression and therapy response vary in MS by ethnicity
- Some MS treatments may heighten COVID risk
- Natalizumab postinfusion reactions
- Common MS treatment wears off more quickly in Black patients
- A less expensive, more convenient treatment option for MS?
- Investigational drug reduces brain lesions in highly active MS
- Evobrutinib may lower nerve damage biomarker levels
- Disease progression and therapy response vary in MS by ethnicity
- Some MS treatments may heighten COVID risk
- Natalizumab postinfusion reactions
- Common MS treatment wears off more quickly in Black patients
- A less expensive, more convenient treatment option for MS?
- Investigational drug reduces brain lesions in highly active MS
- Evobrutinib may lower nerve damage biomarker levels
- Disease progression and therapy response vary in MS by ethnicity
- Some MS treatments may heighten COVID risk
- Natalizumab postinfusion reactions
MS CONFERENCE NEWS
- Common MS treatment wears off more quickly in Black patients
- A less expensive, more convenient treatment option for MS?
- Investigational drug reduces brain lesions in highly active MS
- Evobrutinib may lower nerve damage biomarker levels
- Disease progression and therapy response vary in MS by ethnicity
- Some MS treatments may heighten COVID risk
- Natalizumab postinfusion reactions
- Common MS treatment wears off more quickly in Black patients
- A less expensive, more convenient treatment option for MS?
- Investigational drug reduces brain lesions in highly active MS
- Evobrutinib may lower nerve damage biomarker levels
- Disease progression and therapy response vary in MS by ethnicity
- Some MS treatments may heighten COVID risk
- Natalizumab postinfusion reactions
- Common MS treatment wears off more quickly in Black patients
- A less expensive, more convenient treatment option for MS?
- Investigational drug reduces brain lesions in highly active MS
- Evobrutinib may lower nerve damage biomarker levels
- Disease progression and therapy response vary in MS by ethnicity
- Some MS treatments may heighten COVID risk
- Natalizumab postinfusion reactions
Clinical pearls for Muslim patients with suicide risk
The United States of America is home to a rapidly growing population of more than 3.5 million Muslims. The American Muslim population is a microcosm of global Islamic culture and religious practice, with heterogeneity across age, sex, ethnic origin, immigration status, socioeconomic background, and religiosity. Muslims in America face stressors, including challenges with migration, language barriers, and acculturation.
Some Muslim subgroups (for example, Black Muslims) face additional, intersectional struggles, such as racial discrimination and multigenerational trauma. These challenges may lead to the onset or exacerbation of psychopathology. Nevertheless, the mental health needs of this segment of the American population remain unmet.
Among mental health problems, suicide is inadequately researched among American Muslims. Global studies from both Muslim majority and non-Muslim majority countries consistently indicate that Muslims have among the lowest rates of suicide in comparison with other religious and nonreligious groups. Overall, this body of literature alludes to suicide resiliency in Muslim populations.
However, these studies may not depict the reality for American Muslims. A new research letter, published by two of us (R.A. and B.Z.) and other colleagues at Stanford (Calif.) University’s Muslim Mental Health and Islamic Psychology Lab, highlights the possibility of risk rather than resilience among American Muslims.
In a widely sampled population-based poll, we found that across religious groups in America, Muslims were up to twice as likely to endorse a lifetime history of suicide attempt than other religious or nonreligious groups.
Because of the paucity of suicide research, further inquiry is needed to explain American Muslim evident suicide risk. Nevertheless, our research shows that discrimination and marginalization, both religious and racial, are prominent suicide risk factors in the American Muslim narrative. From 2016 to 2020, almost two-thirds of American Muslims reported facing religious discrimination every year. In 2020, Muslim children in public K-12 systems were twice as likely to face bullying, a third of whom indicated that their bully was a school staff member. While the suicide literature has yet to explore Islamophobia in depth, marginalization and discrimination are demonstrably linked to suicide.
Here are a few clinical pearls that we think will help clinicians meet the needs of these patients:
1. Emphasize the basics. Muslims may be hesitant to engage with mental health practitioners and are often unfamiliar with confidentiality standards. Some may have experience with paternalistic health care cultures where patient privacy is violated. Consequently, some Muslim patients may have concerns that medical professionals can share personal medical history with family members or allied health professionals without obtaining consent. They may worry that private matters will be spread in their community, resulting in stigmatization or discrimination.
Providers should clearly communicate the terms of confidentiality and emphasize patient autonomy over information disclosed outside of the therapeutic partnership.
2. Develop a therapeutic alliance with cultural humility. Since Muslim patients have likely witnessed discrimination, either directly or indirectly, clinicians must adopt a nonjudgmental stance when discussing cultural, religious, or moral values different from their own. Muslim patients may find defending their faith and cultural norms stigmatizing, when faced with clinicians’ assumptions.
Providers should be transparent about their knowledge limitations, ask humbly for a partnership of shared learning, and allow the patient to lead where appropriate. Clinicians should develop a working understanding of Islamic values and cultural norms. See below for Muslim Mental Health resources.
3. Assess suicide risk and ask follow-up questions. Some clinicians may not deem suicide assessments valuable for Muslim patients, believing that strong religious values may preclude them from suicide risk. New findings that suicide risk is prominent among American Muslims highlights the necessity for assessment.
Practitioners should conduct thorough suicide risk assessments, including: past and present ideation, plan, intent, means, relevant risk, and resilience factors. Muslims may be culturally inclined to deny ideation, especially when accompanied by family members. Providers should be on alert for incongruent cues in patient affect and behavior.
4. Accommodate inpatient religious practice. Muslims navigate daily religious choices, from prayers at prescribed times to observing Islamic dietary guidelines. During psychiatric admissions, many of these norms are suspended temporarily. Treatments that do not include the flexibility to address these concerns may mirror patients’ experiences with Islamophobia. For example, being asked to remove the hijab, even with good cause (that is, self-harm precautions), may trigger familiar discriminatory threats to safety and belonging.
Religious accommodations should be addressed in rounds so that all interacting staff maintain collective accountability for religious needs. Accommodations may require adaptive solutions, such as one-piece pull-on–style hijabs as safer alternatives to rectangular wraps. To prevent pathologizing religious observance, providers should consider meeting with Muslim chaplains and patient advocates, including family members or religious care providers, where appropriate.
Addressing the mental health needs of Muslim patients not only requires cultural humility but knowledge about unique challenges facing this diverse community.
To help further advance understanding of these issues, consider taking the American Psychiatric Association’s Muslim Mental Health CME course, which will be taught by Dr. Awaad. In addition, we have included a list of resources below.
Further reading
Moffic S et al. Islamophobia and Psychiatry: Recognition, Prevention and Treatment. New York: Springer, 2019.
Keshavarzi H et al. Applying Islamic Principles to Clinical Mental Health Care: Introducing Traditional Islamically Integrated Psychotherapy. New York: Routledge, 2020.
Ahmed S and MM Amer. Counseling Muslims: Handbook of Mental Health Issues and Interventions. New York: Routledge/Taylor & Francis Group, 2012.
American Psychiatric Association. Stress & Trauma Toolkit for Treating Muslims in a Changing Political and Social Environment, 2019.
American Psychiatric Association. Mental Health Disparities: Muslim Americans, 2019.
Awaad R et al. JAMA Psychiatry. 2021 Jul 21. doi: 10.1001/jamapsychiatry.2021.1813.
Baca-Garcia E et al. J Affect Disord. 2011;134(1-3):327-32.
Institute for Muslim Mental Health: https://muslimmentalhealth.com/
Institute for Social Policy and Understanding. “Religious Discrimination in Multiple Forms Impacts Muslims of All Ages: American Muslim Poll, 2020.
Silverman JJ et al. Am J Psychiatry. 2015 Aug 1;172(8):798-802.
Resources
Stanford Muslim Mental Health and Islamic Psychology Lab: http://med.stanford.edu/psychiatry/research/MuslimMHLab.html
Maristan: https://maristan.org/
Naseeha mental health hotline: https://naseeha.org/
Dr. Awaad is a clinical associate professor in the department of psychiatry and behavioral sciences at Stanford University. She also serves as associate division chief of public mental health and population sciences, and diversity section chief in the psychiatry department. In addition, Dr. Awaad is executive director of Maristan, an organization focused on using authentic traditions to meet the mental health needs of the Islamic community, and is affiliated with the department of psychiatry and behavioral sciences at Stanford. Dr. Awaad is coeditor of “Islamophobia and Psychiatry: Recognition, Prevention and Treatment” (New York: Springer, 2019), and “Applying Islamic Principles to Clinical Mental Health Care: Introducing Traditional Islamically Integrated Psychotherapy” (New York: Routledge/Taylor & Francis Group, 2020).
Dr. Husain completed her medical degree from St. George’s University in True Blue, Grenada; she is currently a graduate student in the department of public health concentrating on mental health parity in the United States. She also works as a researcher at the Stanford Muslim Mental Health & Islamic Psychology Lab and as an organizer for Team Liyna, a national effort aimed at diversifying the stem cell registry responsible for more than 10,000 new registrants since 2019.
Mr. Zia, who has been affiliated with the department of psychiatry and behavioral sciences at Stanford, is a PhD candidate and Canada-Vanier scholar in the department of clinical psychology at the University of Manitoba, Winnipeg. Mr. Zia is also a psychological associate at the New Leaf Psychology Centre in Milton, Ont. He has no relevant financial relationships.
The United States of America is home to a rapidly growing population of more than 3.5 million Muslims. The American Muslim population is a microcosm of global Islamic culture and religious practice, with heterogeneity across age, sex, ethnic origin, immigration status, socioeconomic background, and religiosity. Muslims in America face stressors, including challenges with migration, language barriers, and acculturation.
Some Muslim subgroups (for example, Black Muslims) face additional, intersectional struggles, such as racial discrimination and multigenerational trauma. These challenges may lead to the onset or exacerbation of psychopathology. Nevertheless, the mental health needs of this segment of the American population remain unmet.
Among mental health problems, suicide is inadequately researched among American Muslims. Global studies from both Muslim majority and non-Muslim majority countries consistently indicate that Muslims have among the lowest rates of suicide in comparison with other religious and nonreligious groups. Overall, this body of literature alludes to suicide resiliency in Muslim populations.
However, these studies may not depict the reality for American Muslims. A new research letter, published by two of us (R.A. and B.Z.) and other colleagues at Stanford (Calif.) University’s Muslim Mental Health and Islamic Psychology Lab, highlights the possibility of risk rather than resilience among American Muslims.
In a widely sampled population-based poll, we found that across religious groups in America, Muslims were up to twice as likely to endorse a lifetime history of suicide attempt than other religious or nonreligious groups.
Because of the paucity of suicide research, further inquiry is needed to explain American Muslim evident suicide risk. Nevertheless, our research shows that discrimination and marginalization, both religious and racial, are prominent suicide risk factors in the American Muslim narrative. From 2016 to 2020, almost two-thirds of American Muslims reported facing religious discrimination every year. In 2020, Muslim children in public K-12 systems were twice as likely to face bullying, a third of whom indicated that their bully was a school staff member. While the suicide literature has yet to explore Islamophobia in depth, marginalization and discrimination are demonstrably linked to suicide.
Here are a few clinical pearls that we think will help clinicians meet the needs of these patients:
1. Emphasize the basics. Muslims may be hesitant to engage with mental health practitioners and are often unfamiliar with confidentiality standards. Some may have experience with paternalistic health care cultures where patient privacy is violated. Consequently, some Muslim patients may have concerns that medical professionals can share personal medical history with family members or allied health professionals without obtaining consent. They may worry that private matters will be spread in their community, resulting in stigmatization or discrimination.
Providers should clearly communicate the terms of confidentiality and emphasize patient autonomy over information disclosed outside of the therapeutic partnership.
2. Develop a therapeutic alliance with cultural humility. Since Muslim patients have likely witnessed discrimination, either directly or indirectly, clinicians must adopt a nonjudgmental stance when discussing cultural, religious, or moral values different from their own. Muslim patients may find defending their faith and cultural norms stigmatizing, when faced with clinicians’ assumptions.
Providers should be transparent about their knowledge limitations, ask humbly for a partnership of shared learning, and allow the patient to lead where appropriate. Clinicians should develop a working understanding of Islamic values and cultural norms. See below for Muslim Mental Health resources.
3. Assess suicide risk and ask follow-up questions. Some clinicians may not deem suicide assessments valuable for Muslim patients, believing that strong religious values may preclude them from suicide risk. New findings that suicide risk is prominent among American Muslims highlights the necessity for assessment.
Practitioners should conduct thorough suicide risk assessments, including: past and present ideation, plan, intent, means, relevant risk, and resilience factors. Muslims may be culturally inclined to deny ideation, especially when accompanied by family members. Providers should be on alert for incongruent cues in patient affect and behavior.
4. Accommodate inpatient religious practice. Muslims navigate daily religious choices, from prayers at prescribed times to observing Islamic dietary guidelines. During psychiatric admissions, many of these norms are suspended temporarily. Treatments that do not include the flexibility to address these concerns may mirror patients’ experiences with Islamophobia. For example, being asked to remove the hijab, even with good cause (that is, self-harm precautions), may trigger familiar discriminatory threats to safety and belonging.
Religious accommodations should be addressed in rounds so that all interacting staff maintain collective accountability for religious needs. Accommodations may require adaptive solutions, such as one-piece pull-on–style hijabs as safer alternatives to rectangular wraps. To prevent pathologizing religious observance, providers should consider meeting with Muslim chaplains and patient advocates, including family members or religious care providers, where appropriate.
Addressing the mental health needs of Muslim patients not only requires cultural humility but knowledge about unique challenges facing this diverse community.
To help further advance understanding of these issues, consider taking the American Psychiatric Association’s Muslim Mental Health CME course, which will be taught by Dr. Awaad. In addition, we have included a list of resources below.
Further reading
Moffic S et al. Islamophobia and Psychiatry: Recognition, Prevention and Treatment. New York: Springer, 2019.
Keshavarzi H et al. Applying Islamic Principles to Clinical Mental Health Care: Introducing Traditional Islamically Integrated Psychotherapy. New York: Routledge, 2020.
Ahmed S and MM Amer. Counseling Muslims: Handbook of Mental Health Issues and Interventions. New York: Routledge/Taylor & Francis Group, 2012.
American Psychiatric Association. Stress & Trauma Toolkit for Treating Muslims in a Changing Political and Social Environment, 2019.
American Psychiatric Association. Mental Health Disparities: Muslim Americans, 2019.
Awaad R et al. JAMA Psychiatry. 2021 Jul 21. doi: 10.1001/jamapsychiatry.2021.1813.
Baca-Garcia E et al. J Affect Disord. 2011;134(1-3):327-32.
Institute for Muslim Mental Health: https://muslimmentalhealth.com/
Institute for Social Policy and Understanding. “Religious Discrimination in Multiple Forms Impacts Muslims of All Ages: American Muslim Poll, 2020.
Silverman JJ et al. Am J Psychiatry. 2015 Aug 1;172(8):798-802.
Resources
Stanford Muslim Mental Health and Islamic Psychology Lab: http://med.stanford.edu/psychiatry/research/MuslimMHLab.html
Maristan: https://maristan.org/
Naseeha mental health hotline: https://naseeha.org/
Dr. Awaad is a clinical associate professor in the department of psychiatry and behavioral sciences at Stanford University. She also serves as associate division chief of public mental health and population sciences, and diversity section chief in the psychiatry department. In addition, Dr. Awaad is executive director of Maristan, an organization focused on using authentic traditions to meet the mental health needs of the Islamic community, and is affiliated with the department of psychiatry and behavioral sciences at Stanford. Dr. Awaad is coeditor of “Islamophobia and Psychiatry: Recognition, Prevention and Treatment” (New York: Springer, 2019), and “Applying Islamic Principles to Clinical Mental Health Care: Introducing Traditional Islamically Integrated Psychotherapy” (New York: Routledge/Taylor & Francis Group, 2020).
Dr. Husain completed her medical degree from St. George’s University in True Blue, Grenada; she is currently a graduate student in the department of public health concentrating on mental health parity in the United States. She also works as a researcher at the Stanford Muslim Mental Health & Islamic Psychology Lab and as an organizer for Team Liyna, a national effort aimed at diversifying the stem cell registry responsible for more than 10,000 new registrants since 2019.
Mr. Zia, who has been affiliated with the department of psychiatry and behavioral sciences at Stanford, is a PhD candidate and Canada-Vanier scholar in the department of clinical psychology at the University of Manitoba, Winnipeg. Mr. Zia is also a psychological associate at the New Leaf Psychology Centre in Milton, Ont. He has no relevant financial relationships.
The United States of America is home to a rapidly growing population of more than 3.5 million Muslims. The American Muslim population is a microcosm of global Islamic culture and religious practice, with heterogeneity across age, sex, ethnic origin, immigration status, socioeconomic background, and religiosity. Muslims in America face stressors, including challenges with migration, language barriers, and acculturation.
Some Muslim subgroups (for example, Black Muslims) face additional, intersectional struggles, such as racial discrimination and multigenerational trauma. These challenges may lead to the onset or exacerbation of psychopathology. Nevertheless, the mental health needs of this segment of the American population remain unmet.
Among mental health problems, suicide is inadequately researched among American Muslims. Global studies from both Muslim majority and non-Muslim majority countries consistently indicate that Muslims have among the lowest rates of suicide in comparison with other religious and nonreligious groups. Overall, this body of literature alludes to suicide resiliency in Muslim populations.
However, these studies may not depict the reality for American Muslims. A new research letter, published by two of us (R.A. and B.Z.) and other colleagues at Stanford (Calif.) University’s Muslim Mental Health and Islamic Psychology Lab, highlights the possibility of risk rather than resilience among American Muslims.
In a widely sampled population-based poll, we found that across religious groups in America, Muslims were up to twice as likely to endorse a lifetime history of suicide attempt than other religious or nonreligious groups.
Because of the paucity of suicide research, further inquiry is needed to explain American Muslim evident suicide risk. Nevertheless, our research shows that discrimination and marginalization, both religious and racial, are prominent suicide risk factors in the American Muslim narrative. From 2016 to 2020, almost two-thirds of American Muslims reported facing religious discrimination every year. In 2020, Muslim children in public K-12 systems were twice as likely to face bullying, a third of whom indicated that their bully was a school staff member. While the suicide literature has yet to explore Islamophobia in depth, marginalization and discrimination are demonstrably linked to suicide.
Here are a few clinical pearls that we think will help clinicians meet the needs of these patients:
1. Emphasize the basics. Muslims may be hesitant to engage with mental health practitioners and are often unfamiliar with confidentiality standards. Some may have experience with paternalistic health care cultures where patient privacy is violated. Consequently, some Muslim patients may have concerns that medical professionals can share personal medical history with family members or allied health professionals without obtaining consent. They may worry that private matters will be spread in their community, resulting in stigmatization or discrimination.
Providers should clearly communicate the terms of confidentiality and emphasize patient autonomy over information disclosed outside of the therapeutic partnership.
2. Develop a therapeutic alliance with cultural humility. Since Muslim patients have likely witnessed discrimination, either directly or indirectly, clinicians must adopt a nonjudgmental stance when discussing cultural, religious, or moral values different from their own. Muslim patients may find defending their faith and cultural norms stigmatizing, when faced with clinicians’ assumptions.
Providers should be transparent about their knowledge limitations, ask humbly for a partnership of shared learning, and allow the patient to lead where appropriate. Clinicians should develop a working understanding of Islamic values and cultural norms. See below for Muslim Mental Health resources.
3. Assess suicide risk and ask follow-up questions. Some clinicians may not deem suicide assessments valuable for Muslim patients, believing that strong religious values may preclude them from suicide risk. New findings that suicide risk is prominent among American Muslims highlights the necessity for assessment.
Practitioners should conduct thorough suicide risk assessments, including: past and present ideation, plan, intent, means, relevant risk, and resilience factors. Muslims may be culturally inclined to deny ideation, especially when accompanied by family members. Providers should be on alert for incongruent cues in patient affect and behavior.
4. Accommodate inpatient religious practice. Muslims navigate daily religious choices, from prayers at prescribed times to observing Islamic dietary guidelines. During psychiatric admissions, many of these norms are suspended temporarily. Treatments that do not include the flexibility to address these concerns may mirror patients’ experiences with Islamophobia. For example, being asked to remove the hijab, even with good cause (that is, self-harm precautions), may trigger familiar discriminatory threats to safety and belonging.
Religious accommodations should be addressed in rounds so that all interacting staff maintain collective accountability for religious needs. Accommodations may require adaptive solutions, such as one-piece pull-on–style hijabs as safer alternatives to rectangular wraps. To prevent pathologizing religious observance, providers should consider meeting with Muslim chaplains and patient advocates, including family members or religious care providers, where appropriate.
Addressing the mental health needs of Muslim patients not only requires cultural humility but knowledge about unique challenges facing this diverse community.
To help further advance understanding of these issues, consider taking the American Psychiatric Association’s Muslim Mental Health CME course, which will be taught by Dr. Awaad. In addition, we have included a list of resources below.
Further reading
Moffic S et al. Islamophobia and Psychiatry: Recognition, Prevention and Treatment. New York: Springer, 2019.
Keshavarzi H et al. Applying Islamic Principles to Clinical Mental Health Care: Introducing Traditional Islamically Integrated Psychotherapy. New York: Routledge, 2020.
Ahmed S and MM Amer. Counseling Muslims: Handbook of Mental Health Issues and Interventions. New York: Routledge/Taylor & Francis Group, 2012.
American Psychiatric Association. Stress & Trauma Toolkit for Treating Muslims in a Changing Political and Social Environment, 2019.
American Psychiatric Association. Mental Health Disparities: Muslim Americans, 2019.
Awaad R et al. JAMA Psychiatry. 2021 Jul 21. doi: 10.1001/jamapsychiatry.2021.1813.
Baca-Garcia E et al. J Affect Disord. 2011;134(1-3):327-32.
Institute for Muslim Mental Health: https://muslimmentalhealth.com/
Institute for Social Policy and Understanding. “Religious Discrimination in Multiple Forms Impacts Muslims of All Ages: American Muslim Poll, 2020.
Silverman JJ et al. Am J Psychiatry. 2015 Aug 1;172(8):798-802.
Resources
Stanford Muslim Mental Health and Islamic Psychology Lab: http://med.stanford.edu/psychiatry/research/MuslimMHLab.html
Maristan: https://maristan.org/
Naseeha mental health hotline: https://naseeha.org/
Dr. Awaad is a clinical associate professor in the department of psychiatry and behavioral sciences at Stanford University. She also serves as associate division chief of public mental health and population sciences, and diversity section chief in the psychiatry department. In addition, Dr. Awaad is executive director of Maristan, an organization focused on using authentic traditions to meet the mental health needs of the Islamic community, and is affiliated with the department of psychiatry and behavioral sciences at Stanford. Dr. Awaad is coeditor of “Islamophobia and Psychiatry: Recognition, Prevention and Treatment” (New York: Springer, 2019), and “Applying Islamic Principles to Clinical Mental Health Care: Introducing Traditional Islamically Integrated Psychotherapy” (New York: Routledge/Taylor & Francis Group, 2020).
Dr. Husain completed her medical degree from St. George’s University in True Blue, Grenada; she is currently a graduate student in the department of public health concentrating on mental health parity in the United States. She also works as a researcher at the Stanford Muslim Mental Health & Islamic Psychology Lab and as an organizer for Team Liyna, a national effort aimed at diversifying the stem cell registry responsible for more than 10,000 new registrants since 2019.
Mr. Zia, who has been affiliated with the department of psychiatry and behavioral sciences at Stanford, is a PhD candidate and Canada-Vanier scholar in the department of clinical psychology at the University of Manitoba, Winnipeg. Mr. Zia is also a psychological associate at the New Leaf Psychology Centre in Milton, Ont. He has no relevant financial relationships.











