User login
Microlearning during the pandemic
How to become a hospitalist
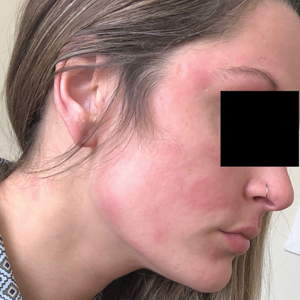
The vast amounts of information generated this past year related to the COVID-19 pandemic was a feat of wonder – recommendations and guidelines on the hospital level and on the national level came in a flurry, more often overwhelming and confusing than clarifying for the frontline provider. In addition, “routine” hospital care for non-infected patients and improvement processes had to continue as we all dealt with the whirlwind of increasing COVID cases, torrents of new guidelines, and educating our trainees.
Thus, the individual-level question: how does a clinician stay engaged and distill the relentless stream of new information?
In Spring 2020, when the first patients with COVID were admitted, our hospital medicine section was tasked to create a surge plan. This included organizing, orienting, and educating off-service providers on how to become hospitalists. Undoubtedly, the call to arms for our center was heard, and many responded. However, backgrounds were diverse in specialty – clinicians and trainees from psychiatry, general surgery, and various fellowships all answered. It was an exhausting and inefficient effort to produce the material, hold webinars, and schedule training, especially for those who were more removed from a hospital medicine experience. We knew we had to come up with an alternative plan moving forward.
Thus, the systems-level question: how does a health care system educate its clinicians, or any other health care providers, when reallocation of their talents and skills is both necessary, time-sensitive, and occuring during a period where new information is constantly being produced and changing?
To reach the most clinicians as possible, with the most succinct and distilled information, we had to come up with a method to do so. Ultimately, in considering the situation at hand, we had to understand who we were as the provider of the information, and who the recipient would be. We would like to share the initiatives and processes by which we constructed our solution to the two questions – microlearning through hospital podcasting.
Learning from our health care colleagues
With the initial webinars and training sessions for our staff, we assessed our learners’ motivations and background in managing in a hospital medicine capacity. Overall, we discovered that our trainees and clinicians have an innate drive to learn; all of them recognized the importance of keeping up with evidence-based information. However, the difficulty highlighted was the individual time available to dedicate to acquiring new information and awareness of new information being available to the health care sector during the chaotic times of the pandemic.
From our section’s perspective, we had a difficulty with coordinating among multiple professional development groups within our hospital, cost, and resources to execute training. These difficulties between providing knowledge and receiving knowledge have already been expertly analyzed.1
Parallel to this, the pedagogic paradigm shifts as we progress through our careers – the methods and skills we used in school contrast in many ways with those we use on a daily basis when it comes to learning. Instead of dedicating hours at a time to new challenges in our workflow or our interests, we watch videos, search retailers for product solutions, check our email correspondence, and peruse social media accounts several times a day. Information comes at us very quickly, but in small pieces.
One such innovation in pedagogy is the practice of microlearning. This refers to the use of small lesson modules and short-term activities intended to teach and reinforce concepts.2 It is the opposite of “macrolearning,” which is the principle of dedicating reading material, structured coursework, and traditional knowledge evaluation in the form of exams to reinforce learning. Certainly, microlearning has other names as well – “just-in-time,” “just-enough,” and “micro-courses” are a few synonyms seen in the current literature. Though a highly relevant concept for our situation, translating it to an endproduct for our trainees and clinicians required more thought.
From theory to application
Microlearning allows for faster delivery of information – fewer things to write means shorter course distribution times, allowing the learner to respond faster to changing educational goals and training demands. Microlearning is flexible – “micro-courses” can give a broad overview of a subject or cover complex topics broken down into simple parts. In addition, micro-learning promotes retention of key concepts – given the length of each lesson, repetition of the topic by the learner is possible at any point in time. The whole experience is similar to checking your favorite social media application on your smartphone.
Certainly, many examples of the application of microlearning are available in the health care sector – pharmaceutical and nursing training both have utilized the theory extensively.3-4 However, in many instances, individuals were still required to sit at a workstation to complete modules and lessons. We envisioned our application of microlearning to be “on-the-go,” without necessarily requiring a computer workstation or laptop to complete.
In thinking about how social media attracts and influences clinicians, many content creators on social media come to mind. In addition, most, if not all, have branched into various social media platforms – podcasting, blogging, YouTube, for example. In thinking about our colleagues and trainees, we wanted a platform that they could take on the go, without the need to focus their visual attention (such as while driving or running). Ultimately, we believe the podcast would be the best platform to disseminate our information.
Podcasting is not foreign to medicine. A variety of medical podcasts exist, whether produced by major medical journals or by various independent health care practitioners. Both, however, have their drawbacks – the podcasts created by major medical journals are typically a summary of the publication’s content and are less engaging. Alternatively, podcasts produced by independent creators are certainly engaging and entertaining, and have a wealth of information, but the line is often blurred between just that: education and entertainment. In both instances, there is no follow-up or feedback offered to the learner in the form of surveys, or other types of feedback, which is arguably an important piece in any form of pedagogy. Thus, we sought to strike a balance between the two forms for our purposes.
Process of two podcasts
Our section was aware of the two aims during the pandemic – (1) disseminate new information regarding COVID-19 to the rest of our staff members and trainees as quickly as possible, and (2) maintain and improve the current quality of care of our patients. Thus, we sought to apply the reach and efficiency of the podcasting medium to provide ongoing education and feedback with respect to these two aims.
“The Cure” podcast. We recognized the constant flow of new COVID-19 information and updates and we wanted to find a readily accessible platform to reach staff with timely updates. Our marketing & communications team later helped us realize that the content we wanted to share was relevant to our patients and the community, so we formatted the material to be practical and easily digestible- something that may help an individual make decisions at the bedside as well as have conversations at the dinner table. Most recently, we engaged with our human resources department to use our platform in orienting new hires with the goal of helping staff familiarize with the institutions policies, procedures, and job aids that keep staff and patients safe.
“Antibiotry” podcast. Prior to the COVID-19 pandemic, our antibiotic stewardship group noticed an increase in antibiotic use on our medical floors. This is monitored not only through internal metrics by our pharmacy department, but also via the SAAR (standardized antibiotic administration ratio). Both sources demonstrated an increase in antibiotic use, greater than expected. An initiative was formed between our hospital medicine and infectious disease sections, and our pharmacy department to raise awareness of this increase in use, provide education to our trainees, and to create systems solutions for clinicians.
Initially, we sought to hold in-person sessions once a month for our trainees. This was led by a senior resident at the time. Topics of discussion were geared towards clinical decision making regarding empiric antibiotic use on the hospital medicine service. At the same time, our team published empiric antibiotic use guidelines, accessible through our electronic medical record. In addition, the resident leader gave a voluntary survey at the end of the session to assess not only confidence of antibiotic use, but also baseline knowledge regarding antibiotics in various clinical scenarios. This survey was repeated at the end of the resident group’s month-long rotation. Altogether, each in-person session was no longer than 10 minutes.
Unfortunately, the initiative was just gaining momentum when the COVID-19 pandemic was declared. However, we sought to take this challenge and translate it into an opportunity.
We directed our focus towards stewardship during pandemic times. Initially, our resident leader sent out email primers, approximately 3-5 minute reads, as a substitute for the in-person sessions. Our primers’ uniqueness was in its incorporation of prescription pattern data that was developed by our resident leader and our initiative’s data analyst. In doing so, we provided professional feedback regarding our antibiotic use based on the clinical indication. This was a powerful tool to not only engage our learners and staff clinicians, but also as a benchmarking tool for continued quality improvement.
But email primers are not engaging, and despite the ubiquity of teleconferencing, it was difficult to ask our housestaff to break from their morning rounds for a 10 minute tele-meeting. Thus, we devised a podcast method of education – 5-10 minute audio clips with conversation regarding a topic of discussion. This way, our trainees and learners can access episodes of education on their own time throughout the pandemic without disrupting their workflow. Given the brevity of, but high-yield content in, each episode, it would not only be convenient for listeners to access and repeat, but also for the podcaster (our resident leader) to create, as recording of the audio portion takes anywhere between 10-20 minutes for each episode, with postprocessing similarly fast.
The interdisciplinary nature of continued medical education cannot be stressed enough. With the help of our professional development team and their educators, we were able to centralize our podcast and attach surveys and additional graphics for each episode, if appropriate. This additional detail allowed for feedback, engagement with our learners, and the chance to provide additional educational points, if the learner was interested. Given the integrated nature of this platform, quality metrics could easily be recorded in the form of “click” data and various other more conventional metrics, such as listener counts and the duration of each podcast played.
Future applications and initiatives
Thus far, we have had great success in the reception and use of both podcasts within our institution as an application of microlearning. “The Cure” has been widely listened to by all hospital staff from various services; it has caught the attention of state-wide radio programs, and plans to expand it into the community are being discussed.
As for “Antibiotry” podcast, the concept has been lauded by our medical educators. Given its centralization within our institution, we are able to publish institution-based data as a form of professional and educational feedback to our trainees and staff physicians. This is currently coupled with the development of a provider dashboard, visualizing antibiotic prescriptions and narrowing patterns of practice within our medicine department. We plan to expand “Antibiotry” to other services at the hospital.
For both podcasts, the steps it took to achieve the final product from the microlearning concept were possible through a combination of institutional need and a motivated team. We are fortunate to have highly energetic individuals, making the coordination and planning with our hospitalists, various sub-specialists, and professional development teams straightforward. As the team grows with more individuals interested in the initiatives, keen insight into interests, individual clinical expertise, presentation skills, and technical skills ought to be carefully weighed to sustain our podcasts most efficiently, and perhaps expand them through different social media platforms.
Our objective for sustainability is through the continued outreach to and recruitment of residents and medical students, who can play key roles in the development of future projects related to these educational innovations. Both microlearning podcasts were developed through the initial planning, trial and error, and execution by two resident leaders. Their initiative and motivation to educate our institution through these platforms were highly unique; their pathfinding set the foundation for sustainability and expansion to other services.
Of course, one of the key measures we would like to investigate is whether our microlearning platform translates to improved patient outcomes. Regarding “Antibiotry,” we hope to see a decrease in unnecessary broad-spectrum antibiotic use by drawing attention to clinician practice patterns. Quality and outcome metrics will continue to be developed and measured. In addition to patient care metrics, further investigation of pedagogical metrics will be conducted, especially in the evolving realm of graduate and continuing medical education.
Measuring educational quality is neither a new ethical nor philosophical debate – neither does it carry a definitive answer. Further help from education experts may be needed to assess the quality of the information provided and its impact on our learners.
Conclusion
Medicine is ever-changing – the guidelines and criteria for patient care and pathology that we learned in medical school have likely changed. There is no single “best” method of learning new information in medicine, simply due to the breadth and volume of such information generated on a daily basis. This poses both a challenge for present-day clinicians and trainees, and a stimulus for change in the methods of acquiring, absorbing, and applying new information to clinical decision making and practice.
We have found that podcasting is a well-received medium of information transfer that is convenient for both the learner and the content creator. Through the podcast format, we were able to distill non-engaging pieces of education and information and transform them into short-duration lessons that the learner can listen to at their own convenience. This proved to be especially handy during the chaos of the pandemic, not only for dissemination of information regarding the management of COVID-19, but also for sustaining quality improvement goals within our institution.
Further investigation on patient outcomes and information quality are the planned next steps. In addition, expansion of other microlearning media, such as group SMS texting, YouTube videos, and Twitter, ought to be considered. Though many publications discuss the theory, potential benefits, and predicted pitfalls of microlearning, few assess the real-world application of microlearning to the clinical setting for medical education.
So what did we learn? We should think of microlearning as moments when you turn to your smartphone or tablet in order to discover something, answer a question, or complete a task. These are moments when decisions are made and knowledge is reinforced. The goal is to capture these moments and fill them with essential pieces of information.
We offer these suggestions as a place to start. The microlearning platform allows for the collection of data on the interaction between user and course content. The data collected can be used for continuous quality improvement of the curriculum. Microlearning is a dynamic platform where creative ideas are encouraged and a multi-disciplinary approach is valuable to keeping an audience engaged. In the future, we hope to be able to correlate microlearning courses to provider performance and measurable patient outcomes.
Dr. Mercado is medical director at Alice Peck Day Memorial Hospital, and associate hospital epidemiologist, Dartmouth-Hitchcock Medical Center, both in Lebanon, N.H., and assistant professor at the Geisel School of Medicine at Dartmouth, Hanover, N.H. Dr. Feng is a Fellow in the Leadership/Preventive Medicine Program in the Department of Internal Medicine at Dartmouth-Hitchcock Medical Center.
References
1. Duggan F and Banwell L. Constructing a model of effective information dissemination in a crisis. Information Research. 2004;9(3). Paper 178 [Available at http://InformationR.net/ir/9-3/paper178.html].
2. Filipe HP, et al. Microlearning to improve CPD learning objectives. Clin Teach. 2020 Dec;17(6):695-699. doi: 10.1111/tct.13208.
3. Hegerius A, et al. E-Learning in Pharmacovigilance: An Evaluation of Microlearning-Based Modules Developed by Uppsala Monitoring Centre. Drug Saf. 2020 Nov;43(11):1171-1180. doi: 10.1007/s40264-020-00981-w.
4. Orwoll B, et al. Gamification and Microlearning for Engagement With Quality Improvement (GAMEQI): A Bundled Digital Intervention for the Prevention of Central Line-Associated Bloodstream Infection. Am J Med Qual. Jan/Feb 2018;33(1):21-29. doi: 10.1177/1062860617706542.
How to become a hospitalist
How to become a hospitalist
The vast amounts of information generated this past year related to the COVID-19 pandemic was a feat of wonder – recommendations and guidelines on the hospital level and on the national level came in a flurry, more often overwhelming and confusing than clarifying for the frontline provider. In addition, “routine” hospital care for non-infected patients and improvement processes had to continue as we all dealt with the whirlwind of increasing COVID cases, torrents of new guidelines, and educating our trainees.
Thus, the individual-level question: how does a clinician stay engaged and distill the relentless stream of new information?
In Spring 2020, when the first patients with COVID were admitted, our hospital medicine section was tasked to create a surge plan. This included organizing, orienting, and educating off-service providers on how to become hospitalists. Undoubtedly, the call to arms for our center was heard, and many responded. However, backgrounds were diverse in specialty – clinicians and trainees from psychiatry, general surgery, and various fellowships all answered. It was an exhausting and inefficient effort to produce the material, hold webinars, and schedule training, especially for those who were more removed from a hospital medicine experience. We knew we had to come up with an alternative plan moving forward.
Thus, the systems-level question: how does a health care system educate its clinicians, or any other health care providers, when reallocation of their talents and skills is both necessary, time-sensitive, and occuring during a period where new information is constantly being produced and changing?
To reach the most clinicians as possible, with the most succinct and distilled information, we had to come up with a method to do so. Ultimately, in considering the situation at hand, we had to understand who we were as the provider of the information, and who the recipient would be. We would like to share the initiatives and processes by which we constructed our solution to the two questions – microlearning through hospital podcasting.
Learning from our health care colleagues
With the initial webinars and training sessions for our staff, we assessed our learners’ motivations and background in managing in a hospital medicine capacity. Overall, we discovered that our trainees and clinicians have an innate drive to learn; all of them recognized the importance of keeping up with evidence-based information. However, the difficulty highlighted was the individual time available to dedicate to acquiring new information and awareness of new information being available to the health care sector during the chaotic times of the pandemic.
From our section’s perspective, we had a difficulty with coordinating among multiple professional development groups within our hospital, cost, and resources to execute training. These difficulties between providing knowledge and receiving knowledge have already been expertly analyzed.1
Parallel to this, the pedagogic paradigm shifts as we progress through our careers – the methods and skills we used in school contrast in many ways with those we use on a daily basis when it comes to learning. Instead of dedicating hours at a time to new challenges in our workflow or our interests, we watch videos, search retailers for product solutions, check our email correspondence, and peruse social media accounts several times a day. Information comes at us very quickly, but in small pieces.
One such innovation in pedagogy is the practice of microlearning. This refers to the use of small lesson modules and short-term activities intended to teach and reinforce concepts.2 It is the opposite of “macrolearning,” which is the principle of dedicating reading material, structured coursework, and traditional knowledge evaluation in the form of exams to reinforce learning. Certainly, microlearning has other names as well – “just-in-time,” “just-enough,” and “micro-courses” are a few synonyms seen in the current literature. Though a highly relevant concept for our situation, translating it to an endproduct for our trainees and clinicians required more thought.
From theory to application
Microlearning allows for faster delivery of information – fewer things to write means shorter course distribution times, allowing the learner to respond faster to changing educational goals and training demands. Microlearning is flexible – “micro-courses” can give a broad overview of a subject or cover complex topics broken down into simple parts. In addition, micro-learning promotes retention of key concepts – given the length of each lesson, repetition of the topic by the learner is possible at any point in time. The whole experience is similar to checking your favorite social media application on your smartphone.
Certainly, many examples of the application of microlearning are available in the health care sector – pharmaceutical and nursing training both have utilized the theory extensively.3-4 However, in many instances, individuals were still required to sit at a workstation to complete modules and lessons. We envisioned our application of microlearning to be “on-the-go,” without necessarily requiring a computer workstation or laptop to complete.
In thinking about how social media attracts and influences clinicians, many content creators on social media come to mind. In addition, most, if not all, have branched into various social media platforms – podcasting, blogging, YouTube, for example. In thinking about our colleagues and trainees, we wanted a platform that they could take on the go, without the need to focus their visual attention (such as while driving or running). Ultimately, we believe the podcast would be the best platform to disseminate our information.
Podcasting is not foreign to medicine. A variety of medical podcasts exist, whether produced by major medical journals or by various independent health care practitioners. Both, however, have their drawbacks – the podcasts created by major medical journals are typically a summary of the publication’s content and are less engaging. Alternatively, podcasts produced by independent creators are certainly engaging and entertaining, and have a wealth of information, but the line is often blurred between just that: education and entertainment. In both instances, there is no follow-up or feedback offered to the learner in the form of surveys, or other types of feedback, which is arguably an important piece in any form of pedagogy. Thus, we sought to strike a balance between the two forms for our purposes.
Process of two podcasts
Our section was aware of the two aims during the pandemic – (1) disseminate new information regarding COVID-19 to the rest of our staff members and trainees as quickly as possible, and (2) maintain and improve the current quality of care of our patients. Thus, we sought to apply the reach and efficiency of the podcasting medium to provide ongoing education and feedback with respect to these two aims.
“The Cure” podcast. We recognized the constant flow of new COVID-19 information and updates and we wanted to find a readily accessible platform to reach staff with timely updates. Our marketing & communications team later helped us realize that the content we wanted to share was relevant to our patients and the community, so we formatted the material to be practical and easily digestible- something that may help an individual make decisions at the bedside as well as have conversations at the dinner table. Most recently, we engaged with our human resources department to use our platform in orienting new hires with the goal of helping staff familiarize with the institutions policies, procedures, and job aids that keep staff and patients safe.
“Antibiotry” podcast. Prior to the COVID-19 pandemic, our antibiotic stewardship group noticed an increase in antibiotic use on our medical floors. This is monitored not only through internal metrics by our pharmacy department, but also via the SAAR (standardized antibiotic administration ratio). Both sources demonstrated an increase in antibiotic use, greater than expected. An initiative was formed between our hospital medicine and infectious disease sections, and our pharmacy department to raise awareness of this increase in use, provide education to our trainees, and to create systems solutions for clinicians.
Initially, we sought to hold in-person sessions once a month for our trainees. This was led by a senior resident at the time. Topics of discussion were geared towards clinical decision making regarding empiric antibiotic use on the hospital medicine service. At the same time, our team published empiric antibiotic use guidelines, accessible through our electronic medical record. In addition, the resident leader gave a voluntary survey at the end of the session to assess not only confidence of antibiotic use, but also baseline knowledge regarding antibiotics in various clinical scenarios. This survey was repeated at the end of the resident group’s month-long rotation. Altogether, each in-person session was no longer than 10 minutes.
Unfortunately, the initiative was just gaining momentum when the COVID-19 pandemic was declared. However, we sought to take this challenge and translate it into an opportunity.
We directed our focus towards stewardship during pandemic times. Initially, our resident leader sent out email primers, approximately 3-5 minute reads, as a substitute for the in-person sessions. Our primers’ uniqueness was in its incorporation of prescription pattern data that was developed by our resident leader and our initiative’s data analyst. In doing so, we provided professional feedback regarding our antibiotic use based on the clinical indication. This was a powerful tool to not only engage our learners and staff clinicians, but also as a benchmarking tool for continued quality improvement.
But email primers are not engaging, and despite the ubiquity of teleconferencing, it was difficult to ask our housestaff to break from their morning rounds for a 10 minute tele-meeting. Thus, we devised a podcast method of education – 5-10 minute audio clips with conversation regarding a topic of discussion. This way, our trainees and learners can access episodes of education on their own time throughout the pandemic without disrupting their workflow. Given the brevity of, but high-yield content in, each episode, it would not only be convenient for listeners to access and repeat, but also for the podcaster (our resident leader) to create, as recording of the audio portion takes anywhere between 10-20 minutes for each episode, with postprocessing similarly fast.
The interdisciplinary nature of continued medical education cannot be stressed enough. With the help of our professional development team and their educators, we were able to centralize our podcast and attach surveys and additional graphics for each episode, if appropriate. This additional detail allowed for feedback, engagement with our learners, and the chance to provide additional educational points, if the learner was interested. Given the integrated nature of this platform, quality metrics could easily be recorded in the form of “click” data and various other more conventional metrics, such as listener counts and the duration of each podcast played.
Future applications and initiatives
Thus far, we have had great success in the reception and use of both podcasts within our institution as an application of microlearning. “The Cure” has been widely listened to by all hospital staff from various services; it has caught the attention of state-wide radio programs, and plans to expand it into the community are being discussed.
As for “Antibiotry” podcast, the concept has been lauded by our medical educators. Given its centralization within our institution, we are able to publish institution-based data as a form of professional and educational feedback to our trainees and staff physicians. This is currently coupled with the development of a provider dashboard, visualizing antibiotic prescriptions and narrowing patterns of practice within our medicine department. We plan to expand “Antibiotry” to other services at the hospital.
For both podcasts, the steps it took to achieve the final product from the microlearning concept were possible through a combination of institutional need and a motivated team. We are fortunate to have highly energetic individuals, making the coordination and planning with our hospitalists, various sub-specialists, and professional development teams straightforward. As the team grows with more individuals interested in the initiatives, keen insight into interests, individual clinical expertise, presentation skills, and technical skills ought to be carefully weighed to sustain our podcasts most efficiently, and perhaps expand them through different social media platforms.
Our objective for sustainability is through the continued outreach to and recruitment of residents and medical students, who can play key roles in the development of future projects related to these educational innovations. Both microlearning podcasts were developed through the initial planning, trial and error, and execution by two resident leaders. Their initiative and motivation to educate our institution through these platforms were highly unique; their pathfinding set the foundation for sustainability and expansion to other services.
Of course, one of the key measures we would like to investigate is whether our microlearning platform translates to improved patient outcomes. Regarding “Antibiotry,” we hope to see a decrease in unnecessary broad-spectrum antibiotic use by drawing attention to clinician practice patterns. Quality and outcome metrics will continue to be developed and measured. In addition to patient care metrics, further investigation of pedagogical metrics will be conducted, especially in the evolving realm of graduate and continuing medical education.
Measuring educational quality is neither a new ethical nor philosophical debate – neither does it carry a definitive answer. Further help from education experts may be needed to assess the quality of the information provided and its impact on our learners.
Conclusion
Medicine is ever-changing – the guidelines and criteria for patient care and pathology that we learned in medical school have likely changed. There is no single “best” method of learning new information in medicine, simply due to the breadth and volume of such information generated on a daily basis. This poses both a challenge for present-day clinicians and trainees, and a stimulus for change in the methods of acquiring, absorbing, and applying new information to clinical decision making and practice.
We have found that podcasting is a well-received medium of information transfer that is convenient for both the learner and the content creator. Through the podcast format, we were able to distill non-engaging pieces of education and information and transform them into short-duration lessons that the learner can listen to at their own convenience. This proved to be especially handy during the chaos of the pandemic, not only for dissemination of information regarding the management of COVID-19, but also for sustaining quality improvement goals within our institution.
Further investigation on patient outcomes and information quality are the planned next steps. In addition, expansion of other microlearning media, such as group SMS texting, YouTube videos, and Twitter, ought to be considered. Though many publications discuss the theory, potential benefits, and predicted pitfalls of microlearning, few assess the real-world application of microlearning to the clinical setting for medical education.
So what did we learn? We should think of microlearning as moments when you turn to your smartphone or tablet in order to discover something, answer a question, or complete a task. These are moments when decisions are made and knowledge is reinforced. The goal is to capture these moments and fill them with essential pieces of information.
We offer these suggestions as a place to start. The microlearning platform allows for the collection of data on the interaction between user and course content. The data collected can be used for continuous quality improvement of the curriculum. Microlearning is a dynamic platform where creative ideas are encouraged and a multi-disciplinary approach is valuable to keeping an audience engaged. In the future, we hope to be able to correlate microlearning courses to provider performance and measurable patient outcomes.
Dr. Mercado is medical director at Alice Peck Day Memorial Hospital, and associate hospital epidemiologist, Dartmouth-Hitchcock Medical Center, both in Lebanon, N.H., and assistant professor at the Geisel School of Medicine at Dartmouth, Hanover, N.H. Dr. Feng is a Fellow in the Leadership/Preventive Medicine Program in the Department of Internal Medicine at Dartmouth-Hitchcock Medical Center.
References
1. Duggan F and Banwell L. Constructing a model of effective information dissemination in a crisis. Information Research. 2004;9(3). Paper 178 [Available at http://InformationR.net/ir/9-3/paper178.html].
2. Filipe HP, et al. Microlearning to improve CPD learning objectives. Clin Teach. 2020 Dec;17(6):695-699. doi: 10.1111/tct.13208.
3. Hegerius A, et al. E-Learning in Pharmacovigilance: An Evaluation of Microlearning-Based Modules Developed by Uppsala Monitoring Centre. Drug Saf. 2020 Nov;43(11):1171-1180. doi: 10.1007/s40264-020-00981-w.
4. Orwoll B, et al. Gamification and Microlearning for Engagement With Quality Improvement (GAMEQI): A Bundled Digital Intervention for the Prevention of Central Line-Associated Bloodstream Infection. Am J Med Qual. Jan/Feb 2018;33(1):21-29. doi: 10.1177/1062860617706542.
The vast amounts of information generated this past year related to the COVID-19 pandemic was a feat of wonder – recommendations and guidelines on the hospital level and on the national level came in a flurry, more often overwhelming and confusing than clarifying for the frontline provider. In addition, “routine” hospital care for non-infected patients and improvement processes had to continue as we all dealt with the whirlwind of increasing COVID cases, torrents of new guidelines, and educating our trainees.
Thus, the individual-level question: how does a clinician stay engaged and distill the relentless stream of new information?
In Spring 2020, when the first patients with COVID were admitted, our hospital medicine section was tasked to create a surge plan. This included organizing, orienting, and educating off-service providers on how to become hospitalists. Undoubtedly, the call to arms for our center was heard, and many responded. However, backgrounds were diverse in specialty – clinicians and trainees from psychiatry, general surgery, and various fellowships all answered. It was an exhausting and inefficient effort to produce the material, hold webinars, and schedule training, especially for those who were more removed from a hospital medicine experience. We knew we had to come up with an alternative plan moving forward.
Thus, the systems-level question: how does a health care system educate its clinicians, or any other health care providers, when reallocation of their talents and skills is both necessary, time-sensitive, and occuring during a period where new information is constantly being produced and changing?
To reach the most clinicians as possible, with the most succinct and distilled information, we had to come up with a method to do so. Ultimately, in considering the situation at hand, we had to understand who we were as the provider of the information, and who the recipient would be. We would like to share the initiatives and processes by which we constructed our solution to the two questions – microlearning through hospital podcasting.
Learning from our health care colleagues
With the initial webinars and training sessions for our staff, we assessed our learners’ motivations and background in managing in a hospital medicine capacity. Overall, we discovered that our trainees and clinicians have an innate drive to learn; all of them recognized the importance of keeping up with evidence-based information. However, the difficulty highlighted was the individual time available to dedicate to acquiring new information and awareness of new information being available to the health care sector during the chaotic times of the pandemic.
From our section’s perspective, we had a difficulty with coordinating among multiple professional development groups within our hospital, cost, and resources to execute training. These difficulties between providing knowledge and receiving knowledge have already been expertly analyzed.1
Parallel to this, the pedagogic paradigm shifts as we progress through our careers – the methods and skills we used in school contrast in many ways with those we use on a daily basis when it comes to learning. Instead of dedicating hours at a time to new challenges in our workflow or our interests, we watch videos, search retailers for product solutions, check our email correspondence, and peruse social media accounts several times a day. Information comes at us very quickly, but in small pieces.
One such innovation in pedagogy is the practice of microlearning. This refers to the use of small lesson modules and short-term activities intended to teach and reinforce concepts.2 It is the opposite of “macrolearning,” which is the principle of dedicating reading material, structured coursework, and traditional knowledge evaluation in the form of exams to reinforce learning. Certainly, microlearning has other names as well – “just-in-time,” “just-enough,” and “micro-courses” are a few synonyms seen in the current literature. Though a highly relevant concept for our situation, translating it to an endproduct for our trainees and clinicians required more thought.
From theory to application
Microlearning allows for faster delivery of information – fewer things to write means shorter course distribution times, allowing the learner to respond faster to changing educational goals and training demands. Microlearning is flexible – “micro-courses” can give a broad overview of a subject or cover complex topics broken down into simple parts. In addition, micro-learning promotes retention of key concepts – given the length of each lesson, repetition of the topic by the learner is possible at any point in time. The whole experience is similar to checking your favorite social media application on your smartphone.
Certainly, many examples of the application of microlearning are available in the health care sector – pharmaceutical and nursing training both have utilized the theory extensively.3-4 However, in many instances, individuals were still required to sit at a workstation to complete modules and lessons. We envisioned our application of microlearning to be “on-the-go,” without necessarily requiring a computer workstation or laptop to complete.
In thinking about how social media attracts and influences clinicians, many content creators on social media come to mind. In addition, most, if not all, have branched into various social media platforms – podcasting, blogging, YouTube, for example. In thinking about our colleagues and trainees, we wanted a platform that they could take on the go, without the need to focus their visual attention (such as while driving or running). Ultimately, we believe the podcast would be the best platform to disseminate our information.
Podcasting is not foreign to medicine. A variety of medical podcasts exist, whether produced by major medical journals or by various independent health care practitioners. Both, however, have their drawbacks – the podcasts created by major medical journals are typically a summary of the publication’s content and are less engaging. Alternatively, podcasts produced by independent creators are certainly engaging and entertaining, and have a wealth of information, but the line is often blurred between just that: education and entertainment. In both instances, there is no follow-up or feedback offered to the learner in the form of surveys, or other types of feedback, which is arguably an important piece in any form of pedagogy. Thus, we sought to strike a balance between the two forms for our purposes.
Process of two podcasts
Our section was aware of the two aims during the pandemic – (1) disseminate new information regarding COVID-19 to the rest of our staff members and trainees as quickly as possible, and (2) maintain and improve the current quality of care of our patients. Thus, we sought to apply the reach and efficiency of the podcasting medium to provide ongoing education and feedback with respect to these two aims.
“The Cure” podcast. We recognized the constant flow of new COVID-19 information and updates and we wanted to find a readily accessible platform to reach staff with timely updates. Our marketing & communications team later helped us realize that the content we wanted to share was relevant to our patients and the community, so we formatted the material to be practical and easily digestible- something that may help an individual make decisions at the bedside as well as have conversations at the dinner table. Most recently, we engaged with our human resources department to use our platform in orienting new hires with the goal of helping staff familiarize with the institutions policies, procedures, and job aids that keep staff and patients safe.
“Antibiotry” podcast. Prior to the COVID-19 pandemic, our antibiotic stewardship group noticed an increase in antibiotic use on our medical floors. This is monitored not only through internal metrics by our pharmacy department, but also via the SAAR (standardized antibiotic administration ratio). Both sources demonstrated an increase in antibiotic use, greater than expected. An initiative was formed between our hospital medicine and infectious disease sections, and our pharmacy department to raise awareness of this increase in use, provide education to our trainees, and to create systems solutions for clinicians.
Initially, we sought to hold in-person sessions once a month for our trainees. This was led by a senior resident at the time. Topics of discussion were geared towards clinical decision making regarding empiric antibiotic use on the hospital medicine service. At the same time, our team published empiric antibiotic use guidelines, accessible through our electronic medical record. In addition, the resident leader gave a voluntary survey at the end of the session to assess not only confidence of antibiotic use, but also baseline knowledge regarding antibiotics in various clinical scenarios. This survey was repeated at the end of the resident group’s month-long rotation. Altogether, each in-person session was no longer than 10 minutes.
Unfortunately, the initiative was just gaining momentum when the COVID-19 pandemic was declared. However, we sought to take this challenge and translate it into an opportunity.
We directed our focus towards stewardship during pandemic times. Initially, our resident leader sent out email primers, approximately 3-5 minute reads, as a substitute for the in-person sessions. Our primers’ uniqueness was in its incorporation of prescription pattern data that was developed by our resident leader and our initiative’s data analyst. In doing so, we provided professional feedback regarding our antibiotic use based on the clinical indication. This was a powerful tool to not only engage our learners and staff clinicians, but also as a benchmarking tool for continued quality improvement.
But email primers are not engaging, and despite the ubiquity of teleconferencing, it was difficult to ask our housestaff to break from their morning rounds for a 10 minute tele-meeting. Thus, we devised a podcast method of education – 5-10 minute audio clips with conversation regarding a topic of discussion. This way, our trainees and learners can access episodes of education on their own time throughout the pandemic without disrupting their workflow. Given the brevity of, but high-yield content in, each episode, it would not only be convenient for listeners to access and repeat, but also for the podcaster (our resident leader) to create, as recording of the audio portion takes anywhere between 10-20 minutes for each episode, with postprocessing similarly fast.
The interdisciplinary nature of continued medical education cannot be stressed enough. With the help of our professional development team and their educators, we were able to centralize our podcast and attach surveys and additional graphics for each episode, if appropriate. This additional detail allowed for feedback, engagement with our learners, and the chance to provide additional educational points, if the learner was interested. Given the integrated nature of this platform, quality metrics could easily be recorded in the form of “click” data and various other more conventional metrics, such as listener counts and the duration of each podcast played.
Future applications and initiatives
Thus far, we have had great success in the reception and use of both podcasts within our institution as an application of microlearning. “The Cure” has been widely listened to by all hospital staff from various services; it has caught the attention of state-wide radio programs, and plans to expand it into the community are being discussed.
As for “Antibiotry” podcast, the concept has been lauded by our medical educators. Given its centralization within our institution, we are able to publish institution-based data as a form of professional and educational feedback to our trainees and staff physicians. This is currently coupled with the development of a provider dashboard, visualizing antibiotic prescriptions and narrowing patterns of practice within our medicine department. We plan to expand “Antibiotry” to other services at the hospital.
For both podcasts, the steps it took to achieve the final product from the microlearning concept were possible through a combination of institutional need and a motivated team. We are fortunate to have highly energetic individuals, making the coordination and planning with our hospitalists, various sub-specialists, and professional development teams straightforward. As the team grows with more individuals interested in the initiatives, keen insight into interests, individual clinical expertise, presentation skills, and technical skills ought to be carefully weighed to sustain our podcasts most efficiently, and perhaps expand them through different social media platforms.
Our objective for sustainability is through the continued outreach to and recruitment of residents and medical students, who can play key roles in the development of future projects related to these educational innovations. Both microlearning podcasts were developed through the initial planning, trial and error, and execution by two resident leaders. Their initiative and motivation to educate our institution through these platforms were highly unique; their pathfinding set the foundation for sustainability and expansion to other services.
Of course, one of the key measures we would like to investigate is whether our microlearning platform translates to improved patient outcomes. Regarding “Antibiotry,” we hope to see a decrease in unnecessary broad-spectrum antibiotic use by drawing attention to clinician practice patterns. Quality and outcome metrics will continue to be developed and measured. In addition to patient care metrics, further investigation of pedagogical metrics will be conducted, especially in the evolving realm of graduate and continuing medical education.
Measuring educational quality is neither a new ethical nor philosophical debate – neither does it carry a definitive answer. Further help from education experts may be needed to assess the quality of the information provided and its impact on our learners.
Conclusion
Medicine is ever-changing – the guidelines and criteria for patient care and pathology that we learned in medical school have likely changed. There is no single “best” method of learning new information in medicine, simply due to the breadth and volume of such information generated on a daily basis. This poses both a challenge for present-day clinicians and trainees, and a stimulus for change in the methods of acquiring, absorbing, and applying new information to clinical decision making and practice.
We have found that podcasting is a well-received medium of information transfer that is convenient for both the learner and the content creator. Through the podcast format, we were able to distill non-engaging pieces of education and information and transform them into short-duration lessons that the learner can listen to at their own convenience. This proved to be especially handy during the chaos of the pandemic, not only for dissemination of information regarding the management of COVID-19, but also for sustaining quality improvement goals within our institution.
Further investigation on patient outcomes and information quality are the planned next steps. In addition, expansion of other microlearning media, such as group SMS texting, YouTube videos, and Twitter, ought to be considered. Though many publications discuss the theory, potential benefits, and predicted pitfalls of microlearning, few assess the real-world application of microlearning to the clinical setting for medical education.
So what did we learn? We should think of microlearning as moments when you turn to your smartphone or tablet in order to discover something, answer a question, or complete a task. These are moments when decisions are made and knowledge is reinforced. The goal is to capture these moments and fill them with essential pieces of information.
We offer these suggestions as a place to start. The microlearning platform allows for the collection of data on the interaction between user and course content. The data collected can be used for continuous quality improvement of the curriculum. Microlearning is a dynamic platform where creative ideas are encouraged and a multi-disciplinary approach is valuable to keeping an audience engaged. In the future, we hope to be able to correlate microlearning courses to provider performance and measurable patient outcomes.
Dr. Mercado is medical director at Alice Peck Day Memorial Hospital, and associate hospital epidemiologist, Dartmouth-Hitchcock Medical Center, both in Lebanon, N.H., and assistant professor at the Geisel School of Medicine at Dartmouth, Hanover, N.H. Dr. Feng is a Fellow in the Leadership/Preventive Medicine Program in the Department of Internal Medicine at Dartmouth-Hitchcock Medical Center.
References
1. Duggan F and Banwell L. Constructing a model of effective information dissemination in a crisis. Information Research. 2004;9(3). Paper 178 [Available at http://InformationR.net/ir/9-3/paper178.html].
2. Filipe HP, et al. Microlearning to improve CPD learning objectives. Clin Teach. 2020 Dec;17(6):695-699. doi: 10.1111/tct.13208.
3. Hegerius A, et al. E-Learning in Pharmacovigilance: An Evaluation of Microlearning-Based Modules Developed by Uppsala Monitoring Centre. Drug Saf. 2020 Nov;43(11):1171-1180. doi: 10.1007/s40264-020-00981-w.
4. Orwoll B, et al. Gamification and Microlearning for Engagement With Quality Improvement (GAMEQI): A Bundled Digital Intervention for the Prevention of Central Line-Associated Bloodstream Infection. Am J Med Qual. Jan/Feb 2018;33(1):21-29. doi: 10.1177/1062860617706542.
Internal mammary lymph node radiation safe over the long term
After a median follow-up 15.7 years among almost 4,000 women, for half of patients who received postoperative internal mammary and medial supraclavicular (IM-MS) lymph node irradiation, the “absolute rates and differences” of heart and lung complications “were very low, with no increased non–breast cancer–related mortality, even before introducing heart-sparing techniques,” said the investigators.
The findings come from the European Organization for Research and Treatment of Cancer (EORTC) trial. The investigators were led by Philip Poortmans, MD, PhD, a radiation oncologist at the University of Antwerp (Belgium).
The team had previously reported lower breast cancer mortality and breast cancer recurrence rates in the radiation group.
Women in the trial were treated from 1996 to 2004. “We expect that with contemporary volume-based radiation therapy outcomes will be even better, by improved coverage of target volumes, more homogeneous dose delivery and decreased doses to nontarget tissues,” the team wrote.
In the end, “our findings ... have important – reassuring – consequences for decision-making concerning elective lymph node treatment in breast cancer,” the researchers commented.
The study was published online on July 28, 2021, in the Journal of the National Cancer Institute.
Resolving the debate
There’s been debate for decades on whether the long-term risk associated with nodal irradiation, particularly collateral heart and lung damage from internal mammary irradiation, outweigh the benefits of better disease control, Julia White, MD, a radiation oncologist at the Ohio State University Breast Center, Columbus, noted in an accompanying editorial.
Concerns stem originally from trials conducted from the 1950s to the 1970s. In those trials, higher doses of radiation were delivered to the internal mammary node with far less precision than today. Subsequent studies have not laid the worry to rest, and protocols vary across institutions, Dr. White explained. Some treat IM nodes in high-risk patients, but others only treat the axilla and the medial supraclavicular lymph nodes.
Dr. White said the new EORTC trial “moves us one step closer to resolving the debate about the value of internal mammary nodal [IMN] radiation.”
She noted that, since 2014, advances in the field have led to an almost 50% reduction in cardiac radiation exposure during breast cancer treatment. Current guidelines recommend that internal mammary nodes “should generally be treated” as part of postmastectomy radiotherapy but that cardiopulmonary complications are still possible even with improved techniques, she wrote.
Mostly grade 1 morbidity
Women in the study had stage I-III breast cancer with axillary node involvement and/or medially located primary tumors. The median age at study entry was 54 years. The patients were treated at 46 centers in 13 countries.
The group that received IM-MS irradiation after surgery received 50 Gy in 25 fractions over 5 weeks.
The cumulative 15-year incidence of lung fibrosis was 5.7% among treated women versus 2.9% among control patients. The incidence of cardiac fibrosis was 1.9% with treatment, versus 1.1%.
The incidence of any cardiac disease was 11.1% in the radiation arm versus 9.4% in the control group.
Complications were mostly of grade 1. The only statistically significant difference in rates of events of grade 2 or higher was in the incidence of pulmonary morbidity, which was 0.8% with radiation versus 0.1% without. There were no differences in the incidence of second malignancies, contralateral breast cancer cases, or cardiovascular deaths with IMN irradiation.
The authors noted that their results conflict with a 2013 study that found a relative increase in major coronary events of 7.4% per Gy mean heart dose. The women in that trial were treated in Sweden and Denmark between 1958 and 2001.
Dr. Poortmans and colleagues noted, however, that this 2013 study and others found a proportional and not an absolute increase in risk. With a baseline risk of 10%, for instance, a 7% increase per 1 Gy translates to a total risk of 10.07%.
Also, no increased risk has been reported in more recently published trials, and a meta-analysis found no increase in non–breast cancer–related mortality with trials that began after 1988.
Still, “it seems logical to take the preexisting cardiac comorbidity of patients into consideration,” the investigators concluded. For patients with higher baseline cardiopulmonary risk factors, lower mean heart doses should be used, and such patients should undergo longer-term follow-up.
The study was funded by La Ligue Nationale Contre Le Cancer and the KWF Kanker Bestrijding from the Netherlands. The investigators and Dr. White disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After a median follow-up 15.7 years among almost 4,000 women, for half of patients who received postoperative internal mammary and medial supraclavicular (IM-MS) lymph node irradiation, the “absolute rates and differences” of heart and lung complications “were very low, with no increased non–breast cancer–related mortality, even before introducing heart-sparing techniques,” said the investigators.
The findings come from the European Organization for Research and Treatment of Cancer (EORTC) trial. The investigators were led by Philip Poortmans, MD, PhD, a radiation oncologist at the University of Antwerp (Belgium).
The team had previously reported lower breast cancer mortality and breast cancer recurrence rates in the radiation group.
Women in the trial were treated from 1996 to 2004. “We expect that with contemporary volume-based radiation therapy outcomes will be even better, by improved coverage of target volumes, more homogeneous dose delivery and decreased doses to nontarget tissues,” the team wrote.
In the end, “our findings ... have important – reassuring – consequences for decision-making concerning elective lymph node treatment in breast cancer,” the researchers commented.
The study was published online on July 28, 2021, in the Journal of the National Cancer Institute.
Resolving the debate
There’s been debate for decades on whether the long-term risk associated with nodal irradiation, particularly collateral heart and lung damage from internal mammary irradiation, outweigh the benefits of better disease control, Julia White, MD, a radiation oncologist at the Ohio State University Breast Center, Columbus, noted in an accompanying editorial.
Concerns stem originally from trials conducted from the 1950s to the 1970s. In those trials, higher doses of radiation were delivered to the internal mammary node with far less precision than today. Subsequent studies have not laid the worry to rest, and protocols vary across institutions, Dr. White explained. Some treat IM nodes in high-risk patients, but others only treat the axilla and the medial supraclavicular lymph nodes.
Dr. White said the new EORTC trial “moves us one step closer to resolving the debate about the value of internal mammary nodal [IMN] radiation.”
She noted that, since 2014, advances in the field have led to an almost 50% reduction in cardiac radiation exposure during breast cancer treatment. Current guidelines recommend that internal mammary nodes “should generally be treated” as part of postmastectomy radiotherapy but that cardiopulmonary complications are still possible even with improved techniques, she wrote.
Mostly grade 1 morbidity
Women in the study had stage I-III breast cancer with axillary node involvement and/or medially located primary tumors. The median age at study entry was 54 years. The patients were treated at 46 centers in 13 countries.
The group that received IM-MS irradiation after surgery received 50 Gy in 25 fractions over 5 weeks.
The cumulative 15-year incidence of lung fibrosis was 5.7% among treated women versus 2.9% among control patients. The incidence of cardiac fibrosis was 1.9% with treatment, versus 1.1%.
The incidence of any cardiac disease was 11.1% in the radiation arm versus 9.4% in the control group.
Complications were mostly of grade 1. The only statistically significant difference in rates of events of grade 2 or higher was in the incidence of pulmonary morbidity, which was 0.8% with radiation versus 0.1% without. There were no differences in the incidence of second malignancies, contralateral breast cancer cases, or cardiovascular deaths with IMN irradiation.
The authors noted that their results conflict with a 2013 study that found a relative increase in major coronary events of 7.4% per Gy mean heart dose. The women in that trial were treated in Sweden and Denmark between 1958 and 2001.
Dr. Poortmans and colleagues noted, however, that this 2013 study and others found a proportional and not an absolute increase in risk. With a baseline risk of 10%, for instance, a 7% increase per 1 Gy translates to a total risk of 10.07%.
Also, no increased risk has been reported in more recently published trials, and a meta-analysis found no increase in non–breast cancer–related mortality with trials that began after 1988.
Still, “it seems logical to take the preexisting cardiac comorbidity of patients into consideration,” the investigators concluded. For patients with higher baseline cardiopulmonary risk factors, lower mean heart doses should be used, and such patients should undergo longer-term follow-up.
The study was funded by La Ligue Nationale Contre Le Cancer and the KWF Kanker Bestrijding from the Netherlands. The investigators and Dr. White disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After a median follow-up 15.7 years among almost 4,000 women, for half of patients who received postoperative internal mammary and medial supraclavicular (IM-MS) lymph node irradiation, the “absolute rates and differences” of heart and lung complications “were very low, with no increased non–breast cancer–related mortality, even before introducing heart-sparing techniques,” said the investigators.
The findings come from the European Organization for Research and Treatment of Cancer (EORTC) trial. The investigators were led by Philip Poortmans, MD, PhD, a radiation oncologist at the University of Antwerp (Belgium).
The team had previously reported lower breast cancer mortality and breast cancer recurrence rates in the radiation group.
Women in the trial were treated from 1996 to 2004. “We expect that with contemporary volume-based radiation therapy outcomes will be even better, by improved coverage of target volumes, more homogeneous dose delivery and decreased doses to nontarget tissues,” the team wrote.
In the end, “our findings ... have important – reassuring – consequences for decision-making concerning elective lymph node treatment in breast cancer,” the researchers commented.
The study was published online on July 28, 2021, in the Journal of the National Cancer Institute.
Resolving the debate
There’s been debate for decades on whether the long-term risk associated with nodal irradiation, particularly collateral heart and lung damage from internal mammary irradiation, outweigh the benefits of better disease control, Julia White, MD, a radiation oncologist at the Ohio State University Breast Center, Columbus, noted in an accompanying editorial.
Concerns stem originally from trials conducted from the 1950s to the 1970s. In those trials, higher doses of radiation were delivered to the internal mammary node with far less precision than today. Subsequent studies have not laid the worry to rest, and protocols vary across institutions, Dr. White explained. Some treat IM nodes in high-risk patients, but others only treat the axilla and the medial supraclavicular lymph nodes.
Dr. White said the new EORTC trial “moves us one step closer to resolving the debate about the value of internal mammary nodal [IMN] radiation.”
She noted that, since 2014, advances in the field have led to an almost 50% reduction in cardiac radiation exposure during breast cancer treatment. Current guidelines recommend that internal mammary nodes “should generally be treated” as part of postmastectomy radiotherapy but that cardiopulmonary complications are still possible even with improved techniques, she wrote.
Mostly grade 1 morbidity
Women in the study had stage I-III breast cancer with axillary node involvement and/or medially located primary tumors. The median age at study entry was 54 years. The patients were treated at 46 centers in 13 countries.
The group that received IM-MS irradiation after surgery received 50 Gy in 25 fractions over 5 weeks.
The cumulative 15-year incidence of lung fibrosis was 5.7% among treated women versus 2.9% among control patients. The incidence of cardiac fibrosis was 1.9% with treatment, versus 1.1%.
The incidence of any cardiac disease was 11.1% in the radiation arm versus 9.4% in the control group.
Complications were mostly of grade 1. The only statistically significant difference in rates of events of grade 2 or higher was in the incidence of pulmonary morbidity, which was 0.8% with radiation versus 0.1% without. There were no differences in the incidence of second malignancies, contralateral breast cancer cases, or cardiovascular deaths with IMN irradiation.
The authors noted that their results conflict with a 2013 study that found a relative increase in major coronary events of 7.4% per Gy mean heart dose. The women in that trial were treated in Sweden and Denmark between 1958 and 2001.
Dr. Poortmans and colleagues noted, however, that this 2013 study and others found a proportional and not an absolute increase in risk. With a baseline risk of 10%, for instance, a 7% increase per 1 Gy translates to a total risk of 10.07%.
Also, no increased risk has been reported in more recently published trials, and a meta-analysis found no increase in non–breast cancer–related mortality with trials that began after 1988.
Still, “it seems logical to take the preexisting cardiac comorbidity of patients into consideration,” the investigators concluded. For patients with higher baseline cardiopulmonary risk factors, lower mean heart doses should be used, and such patients should undergo longer-term follow-up.
The study was funded by La Ligue Nationale Contre Le Cancer and the KWF Kanker Bestrijding from the Netherlands. The investigators and Dr. White disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HBV screening often incomplete or forgone when starting tocilizumab, tofacitinib
People beginning treatment with the immunosuppressive drugs tocilizumab (Actemra) or tofacitinib (Xeljanz) are infrequently screened for hepatitis B virus (HBV) infection, according to a new study of patients with rheumatic diseases who are starting one of the two treatments.
“Perhaps not unexpectedly, these screening patterns conform more with recommendations from the American College of Rheumatology, which do not explicitly stipulate universal HBV screening,” wrote lead author Amir M. Mohareb, MD, of Massachusetts General Hospital in Boston. The study was published in The Journal of Rheumatology.
To determine the frequency of HBV screening among this specific population, the researchers conducted a retrospective, cross-sectional study of patients 18 years or older within the Mass General Brigham health system in the Boston area who initiated either of the two drugs before Dec. 31, 2018. Tocilizumab was approved by the Food and Drug Administration on Jan. 11, 2010, and tofacitinib was approved on Nov. 6, 2012.
The final study population included 678 patients on tocilizumab and 391 patients on tofacitinib. The mean age of the patients in each group was 61 years for tocilizumab and 60 years for tofacitinib. A large majority were female (78% of the tocilizumab group, 88% of the tofacitinib group) and 84% of patients in both groups were white. Their primary diagnosis was rheumatoid arthritis (53% of the tocilizumab group, 77% of the tofacitinib group), and most of them – 57% of patients on tocilizumab and 72% of patients on tofacitinib – had a history of being on both conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs).
HBV screening patterns were classified into three categories: complete (all three of the HBV surface antigen [HBsAg], total core antibody [anti-HBcAb], and surface antibody [HBsAb] tests); partial (any one to two tests); and none. Of the 678 patients on tocilizumab, 194 (29%) underwent complete screening, 307 (45%) underwent partial screening, and 177 (26%) had no screening. Of the 391 patients on tofacitinib, 94 (24%) underwent complete screening, 195 (50%) underwent partial screening, and 102 (26%) had none.
Inappropriate testing – defined as either HBV e-antigen (HBeAg), anti-HBcAb IgM, or HBV DNA without a positive HBsAg or total anti-HBcAb – occurred in 22% of patients on tocilizumab and 23% of patients on tofacitinib. After multivariable analysis, the authors found that Whites were less likely to undergo complete screening (odds ratio, 0.74; 95% confidence interval, 0.57-0.95) compared to non-Whites. Previous use of immunosuppressive agents such as conventional synthetic DMARDs (OR, 1.05; 95% CI, 0.72-1.55) and biologic DMARDs with or without prior csDMARDs (OR, 0.73; 95% CI, 0.48-1.12) was not associated with a likelihood of complete appropriate screening.
“These data add to the evidence indicating that clinicians are not completing pretreatment screening for latent infections prior to patients starting high-risk immunosuppressant drugs,” Gabriela Schmajuk, MD, of the University of California, San Francisco, said in an interview. “It can be dangerous, since a fraction of these patients may reactivate latent infections with HBV that can result in liver failure or death.
“On the bright side,” she added, “we have antivirals that can be given as prophylaxis against reactivation of latent HBV if patients do test positive.”
Dr. Schmajuk was previously the senior author of a similar study from the 2019 American College of Rheumatology annual meeting that found only a small percentage of patients who were new users of biologics or new synthetic DMARDs were screened for HBV or hepatitis C virus.
When asked if anything in the study stood out, she acknowledged being “somewhat surprised that patients with prior immunosuppression did not have higher rates of screening. One might expect that since those patients had more opportunities for screening – since they started new medications more times – they would have higher rates, but this did not appear to be the case.”
As a message to rheumatologists who may be starting their patients on any biologic or new synthetic DMARD, she reinforced that “we need universal HBV screening for patients starting these medications. Many clinicians are used to ordering a hepatitis B surface antigen test, but one key message is that we also need to be ordering hepatitis B core antibody tests. Patients with a positive core antibody are still at risk for reactivation.”
The authors noted their study’s limitations, including the data being retrospectively collected and some of the subjects potentially being screened in laboratories outside of the Mass General Brigham health system. In addition, they stated that their findings “may not be generalizable to nonrheumatologic settings or other immunomodulators,” although they added that studies of other patient populations have also uncovered “similarly low HBV screening frequencies.”
Several of the authors reported being supported by institutes within the National Institutes of Health. Beyond that, they declared no potential conflicts of interest.
People beginning treatment with the immunosuppressive drugs tocilizumab (Actemra) or tofacitinib (Xeljanz) are infrequently screened for hepatitis B virus (HBV) infection, according to a new study of patients with rheumatic diseases who are starting one of the two treatments.
“Perhaps not unexpectedly, these screening patterns conform more with recommendations from the American College of Rheumatology, which do not explicitly stipulate universal HBV screening,” wrote lead author Amir M. Mohareb, MD, of Massachusetts General Hospital in Boston. The study was published in The Journal of Rheumatology.
To determine the frequency of HBV screening among this specific population, the researchers conducted a retrospective, cross-sectional study of patients 18 years or older within the Mass General Brigham health system in the Boston area who initiated either of the two drugs before Dec. 31, 2018. Tocilizumab was approved by the Food and Drug Administration on Jan. 11, 2010, and tofacitinib was approved on Nov. 6, 2012.
The final study population included 678 patients on tocilizumab and 391 patients on tofacitinib. The mean age of the patients in each group was 61 years for tocilizumab and 60 years for tofacitinib. A large majority were female (78% of the tocilizumab group, 88% of the tofacitinib group) and 84% of patients in both groups were white. Their primary diagnosis was rheumatoid arthritis (53% of the tocilizumab group, 77% of the tofacitinib group), and most of them – 57% of patients on tocilizumab and 72% of patients on tofacitinib – had a history of being on both conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs).
HBV screening patterns were classified into three categories: complete (all three of the HBV surface antigen [HBsAg], total core antibody [anti-HBcAb], and surface antibody [HBsAb] tests); partial (any one to two tests); and none. Of the 678 patients on tocilizumab, 194 (29%) underwent complete screening, 307 (45%) underwent partial screening, and 177 (26%) had no screening. Of the 391 patients on tofacitinib, 94 (24%) underwent complete screening, 195 (50%) underwent partial screening, and 102 (26%) had none.
Inappropriate testing – defined as either HBV e-antigen (HBeAg), anti-HBcAb IgM, or HBV DNA without a positive HBsAg or total anti-HBcAb – occurred in 22% of patients on tocilizumab and 23% of patients on tofacitinib. After multivariable analysis, the authors found that Whites were less likely to undergo complete screening (odds ratio, 0.74; 95% confidence interval, 0.57-0.95) compared to non-Whites. Previous use of immunosuppressive agents such as conventional synthetic DMARDs (OR, 1.05; 95% CI, 0.72-1.55) and biologic DMARDs with or without prior csDMARDs (OR, 0.73; 95% CI, 0.48-1.12) was not associated with a likelihood of complete appropriate screening.
“These data add to the evidence indicating that clinicians are not completing pretreatment screening for latent infections prior to patients starting high-risk immunosuppressant drugs,” Gabriela Schmajuk, MD, of the University of California, San Francisco, said in an interview. “It can be dangerous, since a fraction of these patients may reactivate latent infections with HBV that can result in liver failure or death.
“On the bright side,” she added, “we have antivirals that can be given as prophylaxis against reactivation of latent HBV if patients do test positive.”
Dr. Schmajuk was previously the senior author of a similar study from the 2019 American College of Rheumatology annual meeting that found only a small percentage of patients who were new users of biologics or new synthetic DMARDs were screened for HBV or hepatitis C virus.
When asked if anything in the study stood out, she acknowledged being “somewhat surprised that patients with prior immunosuppression did not have higher rates of screening. One might expect that since those patients had more opportunities for screening – since they started new medications more times – they would have higher rates, but this did not appear to be the case.”
As a message to rheumatologists who may be starting their patients on any biologic or new synthetic DMARD, she reinforced that “we need universal HBV screening for patients starting these medications. Many clinicians are used to ordering a hepatitis B surface antigen test, but one key message is that we also need to be ordering hepatitis B core antibody tests. Patients with a positive core antibody are still at risk for reactivation.”
The authors noted their study’s limitations, including the data being retrospectively collected and some of the subjects potentially being screened in laboratories outside of the Mass General Brigham health system. In addition, they stated that their findings “may not be generalizable to nonrheumatologic settings or other immunomodulators,” although they added that studies of other patient populations have also uncovered “similarly low HBV screening frequencies.”
Several of the authors reported being supported by institutes within the National Institutes of Health. Beyond that, they declared no potential conflicts of interest.
People beginning treatment with the immunosuppressive drugs tocilizumab (Actemra) or tofacitinib (Xeljanz) are infrequently screened for hepatitis B virus (HBV) infection, according to a new study of patients with rheumatic diseases who are starting one of the two treatments.
“Perhaps not unexpectedly, these screening patterns conform more with recommendations from the American College of Rheumatology, which do not explicitly stipulate universal HBV screening,” wrote lead author Amir M. Mohareb, MD, of Massachusetts General Hospital in Boston. The study was published in The Journal of Rheumatology.
To determine the frequency of HBV screening among this specific population, the researchers conducted a retrospective, cross-sectional study of patients 18 years or older within the Mass General Brigham health system in the Boston area who initiated either of the two drugs before Dec. 31, 2018. Tocilizumab was approved by the Food and Drug Administration on Jan. 11, 2010, and tofacitinib was approved on Nov. 6, 2012.
The final study population included 678 patients on tocilizumab and 391 patients on tofacitinib. The mean age of the patients in each group was 61 years for tocilizumab and 60 years for tofacitinib. A large majority were female (78% of the tocilizumab group, 88% of the tofacitinib group) and 84% of patients in both groups were white. Their primary diagnosis was rheumatoid arthritis (53% of the tocilizumab group, 77% of the tofacitinib group), and most of them – 57% of patients on tocilizumab and 72% of patients on tofacitinib – had a history of being on both conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs).
HBV screening patterns were classified into three categories: complete (all three of the HBV surface antigen [HBsAg], total core antibody [anti-HBcAb], and surface antibody [HBsAb] tests); partial (any one to two tests); and none. Of the 678 patients on tocilizumab, 194 (29%) underwent complete screening, 307 (45%) underwent partial screening, and 177 (26%) had no screening. Of the 391 patients on tofacitinib, 94 (24%) underwent complete screening, 195 (50%) underwent partial screening, and 102 (26%) had none.
Inappropriate testing – defined as either HBV e-antigen (HBeAg), anti-HBcAb IgM, or HBV DNA without a positive HBsAg or total anti-HBcAb – occurred in 22% of patients on tocilizumab and 23% of patients on tofacitinib. After multivariable analysis, the authors found that Whites were less likely to undergo complete screening (odds ratio, 0.74; 95% confidence interval, 0.57-0.95) compared to non-Whites. Previous use of immunosuppressive agents such as conventional synthetic DMARDs (OR, 1.05; 95% CI, 0.72-1.55) and biologic DMARDs with or without prior csDMARDs (OR, 0.73; 95% CI, 0.48-1.12) was not associated with a likelihood of complete appropriate screening.
“These data add to the evidence indicating that clinicians are not completing pretreatment screening for latent infections prior to patients starting high-risk immunosuppressant drugs,” Gabriela Schmajuk, MD, of the University of California, San Francisco, said in an interview. “It can be dangerous, since a fraction of these patients may reactivate latent infections with HBV that can result in liver failure or death.
“On the bright side,” she added, “we have antivirals that can be given as prophylaxis against reactivation of latent HBV if patients do test positive.”
Dr. Schmajuk was previously the senior author of a similar study from the 2019 American College of Rheumatology annual meeting that found only a small percentage of patients who were new users of biologics or new synthetic DMARDs were screened for HBV or hepatitis C virus.
When asked if anything in the study stood out, she acknowledged being “somewhat surprised that patients with prior immunosuppression did not have higher rates of screening. One might expect that since those patients had more opportunities for screening – since they started new medications more times – they would have higher rates, but this did not appear to be the case.”
As a message to rheumatologists who may be starting their patients on any biologic or new synthetic DMARD, she reinforced that “we need universal HBV screening for patients starting these medications. Many clinicians are used to ordering a hepatitis B surface antigen test, but one key message is that we also need to be ordering hepatitis B core antibody tests. Patients with a positive core antibody are still at risk for reactivation.”
The authors noted their study’s limitations, including the data being retrospectively collected and some of the subjects potentially being screened in laboratories outside of the Mass General Brigham health system. In addition, they stated that their findings “may not be generalizable to nonrheumatologic settings or other immunomodulators,” although they added that studies of other patient populations have also uncovered “similarly low HBV screening frequencies.”
Several of the authors reported being supported by institutes within the National Institutes of Health. Beyond that, they declared no potential conflicts of interest.
FROM THE JOURNAL OF RHEUMATOLOGY
Use of Complementary Alternative Medicine and Supplementation for Skin Disease
Complementary alternative medicine (CAM) has been described by the National Center for Complementary and Integrative Medicine as “health care approaches that are not typically part of conventional medical care or that may have origins outside of usual Western practice.”1 Although this definition is broad, CAM encompasses therapies such as traditional Chinese medicine, herbal therapies, dietary supplements, and mind/body interventions. The use of CAM has grown, and according to a 2012 National Center for Complementary and Integrative Health survey, more than 30% of US adults and 12% of US children use health care approaches that are considered outside of conventional medical practice. In a survey study of US adults, at least 17.7% of respondents said they had taken a dietary supplement other than a vitamin or mineral in the last year.1 Data from the 2007 National Health Interview Survey showed that the prevalence of adults with skin conditions using CAM was 84.5% compared to 38.3% in the general population.2 In addition, 8.15 million US patients with dermatologic conditions reported using CAM over a 5-year period.3 Complementary alternative medicine has emerged as an alternative or adjunct to standard treatments, making it important for dermatologists to understand the existing literature on these therapies. Herein, we review the current evidence-based literature that exists on CAM for the treatment of atopic dermatitis (AD), psoriasis, and alopecia areata (AA).
Atopic Dermatitis
Atopic dermatitis is a chronic, pruritic, inflammatory skin condition with considerable morbidity.4,5 The pathophysiology of AD is multifactorial and includes aspects of barrier dysfunction, IgE hypersensitivity, abnormal cell-mediated immune response, and environmental factors.6 Atopic dermatitis also is one of the most common inflammatory skin conditions in adults, affecting more than 7% of the US population and up to 20% of the total population in developed countries. Of those affected, 40% have moderate or severe symptoms that result in a substantial impact on quality of life.7 Despite advances in understanding disease pathology and treatment, a subset of patients opt to defer conventional treatments such as topical and systemic corticosteroids, antibiotics, nonsteroidal immunomodulators, and biologics. Patients may seek alternative therapies when typical treatments fail or when the perceived side effects outweigh the benefits.5,8 The use of CAM has been well described in patients with AD; however, the existing evidence supporting its use along with its safety profile have not been thoroughly explored. Herein, we will discuss some of the most well-studied supplements for treatment of AD, including evening primrose oil (EPO), fish oil, and probiotics.5
Oral supplementation with polyunsaturated fatty acids commonly is reported in patients with AD.5,8 The idea that a fatty acid deficiency could lead to atopic skin conditions has been around since 1937, when it was suggested that patients with AD had lower levels of blood unsaturated fatty acids.9 Conflicting evidence regarding oral fatty acid ingestion and AD disease severity has emerged.10,11 One unsaturated fatty acid, γ-linolenic acid (GLA), has demonstrated anti-inflammatory properties and involvement in barrier repair.12 It is converted to dihomo-GLA in the body, which acts on cyclooxygenase enzymes to produce the inflammatory mediator prostaglandin E1. The production of GLA is mediated by the enzyme delta-6 desaturase in the metabolization of linoleic acid.12 However, it has been reported that in a subset of patients with AD, a malfunction of delta-6 desaturase may play a role in disease progression and result in lower baseline levels of GLA.10,12 Evening primrose oil and borage oil contain high amounts of GLA (8%–10% and 23%, respectively); thus, supplementation with these oils has been studied in AD.13
EPO for AD
Studies investigating EPO (Oenothera biennis) and its association with AD severity have shown mixed results. A Cochrane review reported that oral borage oil and EPO were not effective treatments for AD,14 while another larger randomized controlled trial (RCT) found no statistically significant improvement in AD symptoms.15 However, multiple smaller studies have found that clinical symptoms of AD, such as erythema, xerosis, pruritus, and total body surface area involved, did improve with oral EPO supplementation when compared to placebo, and the results were statistically significant (P=.04).16,17 One study looked at different dosages of EPO and found that groups ingesting both 160 mg and 320 mg daily experienced reductions in eczema area and severity index score, with greater improvement noted with the higher dosage.17 Side effects associated with oral EPO include an anticoagulant effect and transient gastrointestinal tract upset.8,14 There currently is not enough evidence or safety data to recommend this supplement to AD patients.
Although topical use of fatty acids with high concentrations of GLA, such as EPO and borage oil, have demonstrated improvement in subjective symptom severity, most studies have not reached statistical significance.10,11 One study used a 10% EPO cream for 2 weeks compared to placebo and found statistically significant improvement in patient-reported AD symptoms (P=.045). However, this study only included 10 participants, and therefore larger studies are necessary to confirm this result.18 Some RCTs have shown that topical coconut oil, sunflower seed oil, and sandalwood album oil improve AD symptom severity, but again, large controlled trials are needed.5 Unfortunately, many essential oils, including EPO, can cause a secondary allergic contact dermatitis and potentially worsen AD.19
Fish Oil for AD
Fish oil is a commonly used supplement for AD due to its high content of the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Omega-3 fatty acids exert anti-inflammatory effects by displacing arachidonic acid, a proinflammatory omega-6 fatty acid thought to increase IgE, as well as helper T cell (TH2) cytokines and prostaglandin E2.8,20 A 2012 Cochrane review found that, while some studies revealed mild improvement in AD symptoms with oral fish oil supplementation, these RCTs were of poor methodological quality.21 Multiple smaller studies have shown a decrease in pruritus, severity, and physician-rated clinical scores with fish oil use.5,8,20,22 One study with 145 participants reported that 6 g of fish oil once daily compared to isoenergetic corn oil for 16 weeks identified no statistically significant differences between the treatment groups.20 No adverse events were identified in any of the reported trials. Further studies should be conducted to assess the utility and dosing of fish oil supplements in AD patients.
Probiotics for AD
Probiotics consist of live microorganisms that enhance the microflora of the gastrointestinal tract.8,20 They have been shown to influence food digestion and also have demonstrated potential influence on the skin-gut axis.23 The theory that intestinal dysbiosis plays a role in AD pathogenesis has been investigated in multiple studies.23-25 The central premise is that low-fiber and high-fat Western diets lead to fundamental changes in the gut microbiome, resulting in fewer anti-inflammatory metabolites, such as short-chain fatty acids (SCFAs).23-25 These SCFAs are produced by microbes during the fermentation of dietary fiber and are known for their effect on epithelial barrier integrity and anti-inflammatory properties mediated through G protein–coupled receptor 43.25 Multiple studies have shown that the gut microbiome in patients with AD have higher proportions of Clostridium difficile, Escherichia coli, and Staphylococcus aureus and lower levels of Bifidobacterium, Bacteroidetes, and Bacteroides species compared to healthy controls.26,27 Metagenomic analysis of fecal samples from patients with AD have shown a reduction of Faecalibacterium prausnitzii species when compared to controls, along with a decreased SCFA production, leading to the hypothesis that the gut microbiome may play a role in epithelial barrier disruption.28,29 Systematic reviews and smaller studies have found that oral probiotic use does lead to AD symptom improvement.8,30,31 A systematic review of 25 RCTs with 1599 participants found that supplementation with oral probiotics significantly decreased the SCORAD (SCORing Atopic Dermatitis) index in adults and children older than 1 year with AD but had no effect on infants younger than 1 year (P<.001). They also found that supplementation with diverse microbes or Lactobacillus species showed greater benefit than Bifidobacterium species alone.30 Another study analyzed the effect of oral Lactobacillus fermentum (1×109 CFU twice daily) in 53 children with AD vs placebo for 16 weeks. This study found a statically significant decrease in SCORAD index between oral probiotics and placebo, with 92% (n=24) of participants supplementing with probiotics having a lower SCORAD index than baseline compared to 63% (n=17) in the placebo group (P=.01).31 However, the use of probiotics for AD treatment has remained controversial. Two recent systematic reviews, including 39 RCTs of 2599 randomized patients, found that the use of currently available oral probiotics made little or no difference in patient-rated AD symptoms, investigator-rated AD symptoms, or quality of life.32,33 No adverse effects were observed in the included studies. Unfortunately, the individual RCTs included were heterogeneous, and future studies with standardized probiotic supplementation should be undertaken before probiotics can be routinely recommended.
The use of topical probiotics in AD also has recently emerged. Multiple studies have shown that patients with AD have higher levels of colonization with S aureus, which is associated with T-cell dysfunction, more severe allergic skin reactions, and disruptions in barrier function.34,35 Therefore, altering the skin microbiota through topical probiotics could theoretically reduce AD symptoms and flares. Multiple RCTs and smaller studies have shown that topical probiotics can alter the skin microbiota, improve erythema, and decrease scaling and pruritus in AD patients.35-38 One study used a heat-treated Lactobacillus johnsonii 0.3% lotion twice daily for 3 weeks vs placebo in patients with AD with positive S aureus skin cultures. The S aureus load decreased in patients using the topical probiotic lotion, which correlated with lower SCORAD index that was statistically significant compared to placebo (P=.012).36 More robust studies are needed to determine if topical probiotics should routinely be recommended in AD.
Psoriasis
Psoriasis vulgaris is a chronic inflammatory skin condition characterized by pruritic, hyperkeratotic, scaly plaques.39,40 Keratinocyte hyperproliferation is central to psoriasis pathogenesis and is thought to be a T-cell–driven reaction to antigens or trauma in genetically predisposed individuals. Standard treatments for psoriasis currently include topical corticosteroids and anti-inflammatories, oral immunomodulatory therapy, biologic agents, and phototherapy.40 The use of CAM is highly prevalent among patients with psoriasis, with one study reporting that 51% (n=162) of psoriatic patients interviewed had used CAM.41 The most common reasons for CAM use included dissatisfaction with current treatment, adverse side effects of standard therapy, and patient-reported attempts at “trying everything to heal disease.”42 Herein, we will discuss some of the most frequently used supplements for treatment of psoriatic disease.39
Fish Oil for Psoriasis
One of the most common supplements used by patients with psoriasis is fish oil due to its purported anti-inflammatory qualities.20,39 The consensus on fish oil supplementation for psoriasis is mixed.43-45 Multiple RCTs have reported reductions in psoriasis area and severity index (PASI) scores or symptomatic improvement with variable doses of fish oil.44,46 One RCT found that using EPA 1.8 g once daily and DHA 1.2 g once daily for 12 weeks resulted in significant improvement in pruritus, scaling, and erythema (P<.05).44 Another study reported a significant decrease in erythema (P=.02) and total body surface area affected (P=.0001) with EPA 3.6 g once daily and DHA 2.4 g once daily supplementation compared to olive oil supplementation for 15 weeks.46 Alternatively, multiple studies have failed to show statistically significant improvement in psoriatic symptoms with fish oil supplementation at variable doses and time frames (14–216 mg daily EPA, 9–80 mg daily DHA, from 2 weeks to 9 months).40,47,48 Fish oil may impart anticoagulant properties and should not be started without the guidance of a physician. Currently, there are no data to make specific recommendations on the use of fish oil as an adjunct psoriatic treatment.
Curcumin for Psoriasis
Another supplement routinely utilized in patients with psoriasis is curcumin,40,49,50 a yellow phytochemical that is a major component of the spice turmeric. Curcumin has been shown to inhibit certain proinflammatory cytokines including IL-17, IL-6, IFN-γ, and tumor necrosis factor α and has been regarded as having immune-modulating, anti-inflammatory, and antibacterial properties.40,50 Curcumin also has been reported to suppress phosphorylase kinase, an enzyme that has increased activity in psoriatic plaques that correlates with markers of psoriatic hyperproliferation.50,51 When applied topically, turmeric microgel 0.5% has been reported to decrease scaling, erythema, and psoriatic plaque thickness over the course of 9 weeks.50 In a nonrandomized trial with 10 participants, researchers found that phosphorylase kinase activity levels in psoriatic skin biopsies of patients applying topical curcumin 1% were lower than placebo and topical calcipotriol applied in combination. The lower phosphorylase kinase levels correlated with level of disease severity, and topical curcumin 1% showed a superior outcome when compared to topical calcipotriol.40,49 Although these preliminary results are interesting, there still are not enough data at this time to recommend topical curcumin as a treatment of psoriasis. No known adverse events have been reported with the use of topical curcumin to date.
Oral curcumin has poor oral bioavailability, and 40% to 90% of oral doses are excreted, making supplementation a challenge.40 In one RCT, oral curcumin 2 g daily (using a lecithin-based delivery system to increase bioavailability) was administered in combination with topical methylprednisolone aceponate 0.1%, resulting in significant improvement in psoriatic symptoms and lower IL-22 compared to placebo and topical methylprednisolone aceponate (P<.05).52 Other studies also have reported decreased PASI scores with oral curcumin supplementation.53,54 Adverse effects reported with oral curcumin included gastrointestinal tract upset and hot flashes.53 Although there is early evidence that may support the use of oral curcumin supplementation for psoriasis, more data are needed before recommending this therapy.
Indigo Naturalis for Psoriasis
Topical indigo naturalis (IN) also has been reported to improve psoriasis symptoms.39,53,55 The antipsoriatic effects are thought to occur through the active ingredient in IN (indirubin), which is responsible for inhibition of keratinocyte proliferation.40 One study reported that topical IN 1.4% containing indirubin 0.16% with a petroleum ointment vehicle applied to psoriatic plaques over 12 weeks resulted in a significant decrease in PASI scores from 18.9 at baseline to 6.3 after IN treatment (P<.001).56 Another study found that over 8 weeks, topical application of IN 2.83% containing indirubin 0.24% to psoriatic plaques vs petroleum jelly resulted in 56.3% (n=9) of the treatment group achieving PASI 75 compared to 0% in the placebo group (n=24).55 One deterrent in topical IN treatment is the dark blue pigment it contains; however, no other adverse outcomes were found with topical IN treatment.56 Larger clinical trials are necessary to further explore IN as a potential adjunct treatment in patients with mild psoriatic disease. When taken orally, IN has caused gastrointestinal tract disturbance and elevated liver enzyme levels.57
Herbal Toxicities
It is important to consider that oral supplements including curcumin and IN are widely available over-the-counter and online without oversight by the US Food and Drug Administration.40 Herbal supplements typically are compounded with other ingredients and have been associated with hepatotoxicity as well as drug-supplement interactions, including abnormal bleeding and clotting.58 There exists a lack of general surveillance data, making the true burden of herbal toxicities more difficult to accurately discern. Although some supplements have been associated with anti-inflammatory qualities and disease improvement, other herbal supplements have been shown to possess immunostimulatory characteristics. Herbal supplements such as spirulina, chlorella, Aphanizomenon flos-aquae, and echinacea have been shown to upregulate inflammatory pathways in a variety of autoimmune skin conditions.59
Probiotics for Psoriasis
Data on probiotic use in patients with psoriasis are limited.23 A distinct pattern of dysbiosis has been identified in psoriatic patients, as there is thought to be depletion of beneficial bacteria such as Bifidobacterium, lactobacilli, and F prausnitzii and increased colonization with pathogenic organisms such as Salmonella, E coli, Heliobacter, Campylobacter, and Alcaligenes in psoriasis patients.23,59,60 Early mouse studies have supported this hypothesis, as mice fed with Lactobacillus pentosus have developed milder forms of imiquimod-induced psoriasis compared to placebo,55 and mice receiving probiotic supplementation have lower levels of psoriasis-related proinflammatory markers such as TH17-associated cytokines.61 Another study in humans found that daily oral Bifidobacterium infantis supplementation for 8 weeks in psoriatic patients resulted in lower C-reactive protein and tumor necrosis factor α levels compared to placebo.62 Studies on the use of topical probiotics in psoriasis have been limited, and more research is needed to explore this relationship.38 At this time, no specific recommendations can be made on the use of probiotics in psoriatic patients.
Alopecia Areata
Alopecia areata is nonscarring hair loss that can affect the scalp, face, or body.63,64 The pathophysiology of AA involves the attack of the hair follicle matrix epithelium by inflammatory cells without hair follicle stem cell destruction. The precise events that precipitate these episodes are unknown, but triggers such as emotional or physical stress, vaccines, or viral infections have been reported.65 There is no cure for AA, and current treatments such as topical minoxidil and corticosteroids (topical, intralesional, or oral) vary widely in efficacy.64 Although Janus kinase inhibitors recently have shown promising results in the treatment of AA, the need for prolonged therapy may be frustrating to patients.66 Severity of AA also can vary, with 30% of patients experiencing extensive hair loss.67 The use of CAM has been widely reported in AA due to high levels of dissatisfaction with existing therapies.68 Herein, we discuss the most studied alternative treatments used in AA
Garlic and Onion for Alopecia
One alternative treatment that has shown promising initial results is application of topical garlic and onion extracts to affected areas.64,69,70 Both garlic and onion belong to the Allium genus and are high in sulfur and phenolic compounds.70 They have been reported to possess bactericidal and vasodilatory activity,71 and it has been hypothesized that onion and garlic extracts may induce therapeutic effects through induction of a mild contact dermatitis.70 One single-blinded, controlled trial using topical crude onion juice reported that 86.9% (n=20) of patients had full regrowth of hair compared to 13.3% (n=2) of patients treated with a tap water placebo at 8 weeks (P<.0001). This study also noted that patients using onion juice had a higher rate of erythema at application site; unfortunately, the study was small with only 38 patients.70 Another double-blind RCT using garlic gel 5% with betamethasone valerate cream 0.1% compared to betamethasone valerate cream alone found that after 3 months, patients in the garlic gel group had increased terminal hairs and smaller patch sizes compared to the betamethasone valerate cream group.69 More studies are needed to confirm these results.
Aromatherapy With Essential Oils for Alopecia
Another alternative treatment in AA that has demonstrated positive results is aromatherapy skin massage with essential oils to patches of alopecia.72 Although certain essential oils, such as tea tree oil, have been reported to have specific antibacterial or anti-inflammatory properties, essential oils have been reported to cause allergic contact dermatitis and should be used with caution.73,74 For example, tea tree oil is a well-known cause of allergic contact dermatitis, and positive patch testing has ranged from 0.1% to 3.5% in studies assessing topical tea tree oil 5% application.75 Overall, there have been nearly 80 essential oils implicated in contact dermatitis, with high-concentration products being one of the highest risk factors for an allergic contact reaction.76 One RCT compared daily scalp massage with essential oils (rosemary, lavender, thyme, and cedarwood in a carrier oil) to daily scalp massage with a placebo carrier oil in AA patients. The results showed that at 7 months of treatment, 44% (n=19) of the aromatherapy group showed improvement compared to 15% (n=6) in the control group.77 Another study used a similar group of essential oils (thyme, rosemary, atlas cedar, lavender, and EPO in a carrier oil) with daily scalp massage and reported similar improvement of AA symptoms compared to control; the investigators also reported irritation at application site in 1 patient.78 There currently are not enough data to recommend aromatherapy skin massage for the treatment of AA, and this practice may cause harm to the patient by induction of allergic contact dermatitis.
There have been a few studies to suggest that the use of total glucosides of peony with compound glycyrrhizin and oral Korean red ginseng may have beneficial effects on AA treatment, but efficacy and safety data are lacking, and these therapies should not be recommended without more information.64,79,80
Final Thoughts
Dermatologic patients frequently are opting for CAM,2 and although some therapies may show promising initial results, alternative medicines also can drive adverse events.19,30 The lack of oversight from the US Food and Drug Administration on the products leads to many unknowns for true health risks with over-the-counter CAM supplements.40 As the use of CAM becomes increasingly common among dermatologic patients, it is important for dermatologists to understand the benefits and risks, especially for commonly treated conditions. More data is needed before CAM can be routinely recommended.
- Complementary, alternative, or integrative health: what’s in a name? National Center for Complementary and Integrative Health website. Updated April 2021. Accessed April 25, 2021. https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name
- Fuhrmann T, Smith N, Tausk F. Use of complementary and alternative medicine among adults with skin disease: updated results from a national survey. J Am Acad Dermatol. 2010;63:1000-1005.
- Landis ET, Davis SA, Feldman SR, et al. Complementary and alternative medicine use in dermatology in the United States. J Altern Complement Med. 2014;20:392-398.
- Solman L, Lloyd‐Lavery A, Grindlay DJC, et al. What’s new in atopic eczema? an analysis of systematic reviews published in 2016. part 1: treatment and prevention. Clin Exp Dermatol. 2019;44:363-369.
- Vieira BL, Lim NR, Lohman ME, et al. Complementary and alternative medicine for atopic dermatitis: an evidence-based review. Am J Clin Dermatol. 2016;17:557-581.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. In: Fortson EA, Feldman SR, Strowd LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:21-37.
- Atopic dermatitis in America. Asthma and Allergy Foundation of America website. Accessed July 30, 2021. https://www.aafa.org/atopic-dermatitis-in-america
- Schlichte MJ, Vandersall A, Katta R. Diet and eczema: a review of dietary supplements for the treatment of atopic dermatitis. Dermatol Pract Concept. 2016;6:23-29.
- Brown WR, Hansen AE. Arachidonic and linolic acid of the serum in normal and eczematous human subjects. Proc Soc Exp Bio Med. 1937;36:113-117.
- Lee J, Bielory L. Complementary and alternative interventions in atopic dermatitis. Immunol Allergy Clin North Am. 2010;30:411-424.
- Ferreira MJ, Fiadeiro T, Silva M, et al. Topical γ-linolenic acid therapy in atopic dermatitis. Allergo J. 1998;7:213-216.
- Simon D, Eng PA, Borelli S, et al. Gamma-linolenic acid levels correlate with clinical efficacy of evening primrose oil in patients with atopic dermatitis. Adv Ther. 2014;31:180-188.
- Fan Y-Y, Chapkin RS. Importance of dietary γ-linolenic acid in human health and nutrition. J Nutr. 1998;128:1411-1414.
- Bamford JTM, Ray S, Musekiwa A, et al. Oral evening primrose oil and borage oil for eczema. Cochrane Database Syst Rev. 2013;4:CD004416.
- Williams H. Evening primrose oil for atopic dermatitis. BMJ. 2003;327:2.
- Schalin-Karrila M, Mattila L, Jansen CT, et al. Evening primrose oil in the treatment of atopic eczema: effect on clinical status, plasma phospholipid fatty acids and circulating blood prostaglandins. Br J Dermatol. 1987;117:11-19.
- Chung BY, Park SY, Jung MJ, et al. Effect of evening primrose oil on Korean patients with mild atopic dermatitis: a randomized, double-blinded, placebo-controlled clinical study. Ann Dermatol. 2018;30:409-416.
- Anstey A, Quigley M, Wilkinson JD. Topical evening primrose oil as treatment for atopic eczema. J Dermatolog Treat. 1990;1:199-201.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. Dermatitis. 2016;27:39-42.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review. Int J Dermatol. 2019;58:1371-1376.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema [published online February 15, 2012]. Cochrane Database Syst Rev. Accessed July 22, 2021. doi:10.1002/14651858.CD005205.pub3
- Balic´ A, Vlašic´ D, Žužul K, et al. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int J Mol Sci. 2020;21:741.
- Salem I, Ramser A, Isham N, et al. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459.
- Agrawal R, Wisniewski JA, Woodfolk JA. The role of regulatory T cells in atopic dermatitis. Pathogenesis Manage Atopic Dermatitis. 2011;41:112-124.
- Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282-1286.
- Lee E, Lee S-Y, Kang M-J, et al. Clostridia in the gut and onset of atopic dermatitis via eosinophilic inflammation. Ann Allergy Asthma Immunol. 2016;117:91-92.e1.
- Nylund L, Nermes M, Isolauri E, et al. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy. 2015;70:241-244.
- Kim H-J, Kim HY, Lee S-Y, et al. Clinical efficacy and mechanism of probiotics in allergic diseases. Korean J Pediatr. 2013;56:369-376.
- Song H, Yoo Y, Hwang J, et al. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. 2016;137:852-860.
- Kim S-O, Ah Y-M, Yu YM, et al. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2014;113:217-226.
- Weston S, Halbert A, Richmond P, et al. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005;90:892-897.
- Huang R, Ning H, Shen M, et al. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol. 2017;7:392.

- Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018;11:CD006135.
- Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020;29:15-21.
- Woo TE, Sibley CD. The emerging utility of the cutaneous microbiome in the treatment of acne and atopic dermatitis. J Am Acad Dermatol. 2020;82:222-228.
- Blanchet-Réthoré S, Bourdès V, Mercenier A, et al. Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin Cosmet Investig Dermatol. 2017;10:249-257.
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nature Medicine. 2021;27:700-709.
- França K. Topical probiotics in dermatological therapy and skincare: a concise review. Dermatol Ther (Heidelb). 2020;11:71-77.
- Talbott W, Duffy N. Complementary and alternative medicine for psoriasis: what the dermatologist needs to know. Am J Clin Dermatol. 2015;16:147-165.
- Gamret AC, Price A, Fertig RM, et al. Complementary and alternative medicine therapies for psoriasis: a systematic review. JAMA Dermatol. 2018;154:1330-1337.
- Fleischer AB, Feldman SR, Rapp SR, et al. Alternative therapies commonly used within a population of patients with psoriasis. Cutis. 1996;58:216-220.
- Ben-Arye E, Ziv M, Frenkel M, et al. Complementary medicine and psoriasis: linking the patient’s outlook with evidence-based medicine. Dermatology. 2003;207:302-307.
- Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis: part 3. role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
- Bittiner SB, Tucker WF, Cartwright I, et al. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1:378-380.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a Systematic review. JAMA Dermatol. 2018;154:934-950.
- Gupta AK, Ellis CN, Tellner DC, et al. Double-blind, placebo-controlled study to evaluate the efficacy of fish oil and low-dose UVB in the treatment of psoriasis. Br J Dermatol. 1989;120:801-807.
- Kristensen S, Schmidt EB, Schlemmer A, et al. Beneficial effect of n-3 polyunsaturated fatty acids on inflammation and analgesic use in psoriatic arthritis: a randomized, double blind, placebo-controlled trial. Scand J Rheumatol. 2018;47:27-36.
- Søyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Heng MCY, Song MK, Harker J, et al. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol. 2000;143:937-949.
- Sarafian G, Afshar M, Mansouri P, et al. Topical turmeric microemulgel in the management of plaque psoriasis; a clinical evaluation. Iran J Pharm Res. 2015;14:865-876.
- Reddy S, Aggarwal BB. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Letters. 1994;341:19-22.
- Antiga E, Bonciolini V, Volpi W, et al. Oral curcumin (meriva) is effective as an adjuvant treatment and is able to reduce IL-22 serum levels in patients with psoriasis vulgaris. Biomed Res Int. 2015;2015:283634.
- Kurd SK, Smith N, VanVoorhees A, et al. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: a prospective clinical trial. J Am Acad Dermatol. 2008;58:625-631.
- Carrion-Gutierrez M, Ramirez-Bosca A, Navarro-Lopez V, et al. Effects of Curcuma extract and visible light on adults with plaque psoriasis. Eur J Dermatol. 2015;25:240-246.
- Cheng H-M, Wu Y-C, Wang Q, et al. Clinical efficacy and IL-17 targeting mechanism of indigo naturalis as a topical agent in moderate psoriasis. BMC Complement Altern Med. 2017;17:439.
- Lin Y-K, Chang C-J, Chang Y-C, et al. Clinical assessment of patients with recalcitrant psoriasis in a randomized, observer-blind, vehicle-controlled trial using indigo naturalis. Arch Dermatol. 2008;144:1457-1464.
- Naganuma M, Sugimoto S, Suzuki H, et al. Adverse events in patients with ulcerative colitis treated with indigo naturalis: a Japanese nationwide survey. J Gastroenterol. 2019;54:891-896.
- Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Alimentary Pharmacol Ther. 2013;37:3-17.
- Bax CE, Chakka S, Concha JSS, et al. The effects of immunostimulatory herbal supplements on autoimmune skin diseases. J Am Acad Dermatol. 2021;84:1051-1058.
- Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes an altered gut microbiota in psoriatic arthritis and resembles dysbiosis of inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128-139.
- Chen Y-H, Wu C-S, Chao Y-H, et al. Lactobacillus pentosus GMNL-77 inhibits skin lesions in imiquimod-induced psoriasis-like mice. J Food Drug Anal. 2017;25:559-566.
- Groeger D, O’Mahony L, Murphy EF, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4:325-339.
- Hosking A-M, Juhasz M, Atanaskova Mesinkovska N. Complementary and alternative treatments for alopecia: a comprehensive review. Skin Appendage Disord. 2019;5:72-89.
- Tkachenko E, Okhovat J-P, Manjaly P, et al. Complementary & alternative medicine for alopecia areata: a systematic review [published online December 20, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.027
- Lepe K, Zito PM. Alopecia areata. In: StatPearls. StatPearls Publishing; 2021. Accessed July 22, 2021. https://pubmed.ncbi.nlm.nih.gov/30725685/
- Ismail FF, Sinclair R. JAK inhibition in the treatment of alopecia areata—a promising new dawn? Expert Rev Clin Pharmacol. 2020;13:43-51. doi:10.1080/17512433.2020.1702878
- van den Biggelaar FJHM, Smolders J, Jansen JFA. Complementary and alternative medicine in alopecia areata. AM J Clin Dermatol. 2010;11:11-20.
- Hussain ST, Mostaghimi A, Barr PJ, et al. Utilization of mental health resources and complementary and alternative therapies for alopecia areata: a U.S. survey. Int J Trichology. 2017;9:160-164.
- Hajheydari Z, Jamshidi M, Akbari J, et al. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73:29-32.
- Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343-346.
- Burian JP, Sacramento LVS, Carlos IZ. Fungal infection control by garlic extracts (Allium sativum L.) and modulation of peritoneal macrophages activity in murine model of sporotrichosis. Braz J Biol. 2017;77:848-855.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Lakshmi C, Srinivas CR. Allergic contact dermatitis following aromatherapy with valiya narayana thailam—an ayurvedic oil presenting as exfoliative dermatitis. Contact Dermatitis. 2009;61:297-298.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62.
- Groot AC de, Schmidt E. Tea tree oil: contact allergy and chemical composition. Contact Dermatitis. 2016;75:129-143.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. dermatitis. 2016;27:39-42.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Ozmen I, Caliskan E, Arca E, et al. Efficacy of aromatherapy in the treatment of localized alopecia areata: a double-blind placebo controlled study. Gulhane Med J. 2015;57:233.
- Oh GN, Son SW. Efficacy of Korean red ginseng in the treatment of alopecia areata. J Ginseng Res. 2012;36:391-395.
- Yang D-Q, You L-P, Song P-H, et al. A randomized controlled trial comparing total glucosides of paeony capsule and compound glycyrrhizin tablet for alopecia areata. Chin J Integr Med. 2012;18:621-625.
Complementary alternative medicine (CAM) has been described by the National Center for Complementary and Integrative Medicine as “health care approaches that are not typically part of conventional medical care or that may have origins outside of usual Western practice.”1 Although this definition is broad, CAM encompasses therapies such as traditional Chinese medicine, herbal therapies, dietary supplements, and mind/body interventions. The use of CAM has grown, and according to a 2012 National Center for Complementary and Integrative Health survey, more than 30% of US adults and 12% of US children use health care approaches that are considered outside of conventional medical practice. In a survey study of US adults, at least 17.7% of respondents said they had taken a dietary supplement other than a vitamin or mineral in the last year.1 Data from the 2007 National Health Interview Survey showed that the prevalence of adults with skin conditions using CAM was 84.5% compared to 38.3% in the general population.2 In addition, 8.15 million US patients with dermatologic conditions reported using CAM over a 5-year period.3 Complementary alternative medicine has emerged as an alternative or adjunct to standard treatments, making it important for dermatologists to understand the existing literature on these therapies. Herein, we review the current evidence-based literature that exists on CAM for the treatment of atopic dermatitis (AD), psoriasis, and alopecia areata (AA).
Atopic Dermatitis
Atopic dermatitis is a chronic, pruritic, inflammatory skin condition with considerable morbidity.4,5 The pathophysiology of AD is multifactorial and includes aspects of barrier dysfunction, IgE hypersensitivity, abnormal cell-mediated immune response, and environmental factors.6 Atopic dermatitis also is one of the most common inflammatory skin conditions in adults, affecting more than 7% of the US population and up to 20% of the total population in developed countries. Of those affected, 40% have moderate or severe symptoms that result in a substantial impact on quality of life.7 Despite advances in understanding disease pathology and treatment, a subset of patients opt to defer conventional treatments such as topical and systemic corticosteroids, antibiotics, nonsteroidal immunomodulators, and biologics. Patients may seek alternative therapies when typical treatments fail or when the perceived side effects outweigh the benefits.5,8 The use of CAM has been well described in patients with AD; however, the existing evidence supporting its use along with its safety profile have not been thoroughly explored. Herein, we will discuss some of the most well-studied supplements for treatment of AD, including evening primrose oil (EPO), fish oil, and probiotics.5
Oral supplementation with polyunsaturated fatty acids commonly is reported in patients with AD.5,8 The idea that a fatty acid deficiency could lead to atopic skin conditions has been around since 1937, when it was suggested that patients with AD had lower levels of blood unsaturated fatty acids.9 Conflicting evidence regarding oral fatty acid ingestion and AD disease severity has emerged.10,11 One unsaturated fatty acid, γ-linolenic acid (GLA), has demonstrated anti-inflammatory properties and involvement in barrier repair.12 It is converted to dihomo-GLA in the body, which acts on cyclooxygenase enzymes to produce the inflammatory mediator prostaglandin E1. The production of GLA is mediated by the enzyme delta-6 desaturase in the metabolization of linoleic acid.12 However, it has been reported that in a subset of patients with AD, a malfunction of delta-6 desaturase may play a role in disease progression and result in lower baseline levels of GLA.10,12 Evening primrose oil and borage oil contain high amounts of GLA (8%–10% and 23%, respectively); thus, supplementation with these oils has been studied in AD.13
EPO for AD
Studies investigating EPO (Oenothera biennis) and its association with AD severity have shown mixed results. A Cochrane review reported that oral borage oil and EPO were not effective treatments for AD,14 while another larger randomized controlled trial (RCT) found no statistically significant improvement in AD symptoms.15 However, multiple smaller studies have found that clinical symptoms of AD, such as erythema, xerosis, pruritus, and total body surface area involved, did improve with oral EPO supplementation when compared to placebo, and the results were statistically significant (P=.04).16,17 One study looked at different dosages of EPO and found that groups ingesting both 160 mg and 320 mg daily experienced reductions in eczema area and severity index score, with greater improvement noted with the higher dosage.17 Side effects associated with oral EPO include an anticoagulant effect and transient gastrointestinal tract upset.8,14 There currently is not enough evidence or safety data to recommend this supplement to AD patients.
Although topical use of fatty acids with high concentrations of GLA, such as EPO and borage oil, have demonstrated improvement in subjective symptom severity, most studies have not reached statistical significance.10,11 One study used a 10% EPO cream for 2 weeks compared to placebo and found statistically significant improvement in patient-reported AD symptoms (P=.045). However, this study only included 10 participants, and therefore larger studies are necessary to confirm this result.18 Some RCTs have shown that topical coconut oil, sunflower seed oil, and sandalwood album oil improve AD symptom severity, but again, large controlled trials are needed.5 Unfortunately, many essential oils, including EPO, can cause a secondary allergic contact dermatitis and potentially worsen AD.19
Fish Oil for AD
Fish oil is a commonly used supplement for AD due to its high content of the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Omega-3 fatty acids exert anti-inflammatory effects by displacing arachidonic acid, a proinflammatory omega-6 fatty acid thought to increase IgE, as well as helper T cell (TH2) cytokines and prostaglandin E2.8,20 A 2012 Cochrane review found that, while some studies revealed mild improvement in AD symptoms with oral fish oil supplementation, these RCTs were of poor methodological quality.21 Multiple smaller studies have shown a decrease in pruritus, severity, and physician-rated clinical scores with fish oil use.5,8,20,22 One study with 145 participants reported that 6 g of fish oil once daily compared to isoenergetic corn oil for 16 weeks identified no statistically significant differences between the treatment groups.20 No adverse events were identified in any of the reported trials. Further studies should be conducted to assess the utility and dosing of fish oil supplements in AD patients.
Probiotics for AD
Probiotics consist of live microorganisms that enhance the microflora of the gastrointestinal tract.8,20 They have been shown to influence food digestion and also have demonstrated potential influence on the skin-gut axis.23 The theory that intestinal dysbiosis plays a role in AD pathogenesis has been investigated in multiple studies.23-25 The central premise is that low-fiber and high-fat Western diets lead to fundamental changes in the gut microbiome, resulting in fewer anti-inflammatory metabolites, such as short-chain fatty acids (SCFAs).23-25 These SCFAs are produced by microbes during the fermentation of dietary fiber and are known for their effect on epithelial barrier integrity and anti-inflammatory properties mediated through G protein–coupled receptor 43.25 Multiple studies have shown that the gut microbiome in patients with AD have higher proportions of Clostridium difficile, Escherichia coli, and Staphylococcus aureus and lower levels of Bifidobacterium, Bacteroidetes, and Bacteroides species compared to healthy controls.26,27 Metagenomic analysis of fecal samples from patients with AD have shown a reduction of Faecalibacterium prausnitzii species when compared to controls, along with a decreased SCFA production, leading to the hypothesis that the gut microbiome may play a role in epithelial barrier disruption.28,29 Systematic reviews and smaller studies have found that oral probiotic use does lead to AD symptom improvement.8,30,31 A systematic review of 25 RCTs with 1599 participants found that supplementation with oral probiotics significantly decreased the SCORAD (SCORing Atopic Dermatitis) index in adults and children older than 1 year with AD but had no effect on infants younger than 1 year (P<.001). They also found that supplementation with diverse microbes or Lactobacillus species showed greater benefit than Bifidobacterium species alone.30 Another study analyzed the effect of oral Lactobacillus fermentum (1×109 CFU twice daily) in 53 children with AD vs placebo for 16 weeks. This study found a statically significant decrease in SCORAD index between oral probiotics and placebo, with 92% (n=24) of participants supplementing with probiotics having a lower SCORAD index than baseline compared to 63% (n=17) in the placebo group (P=.01).31 However, the use of probiotics for AD treatment has remained controversial. Two recent systematic reviews, including 39 RCTs of 2599 randomized patients, found that the use of currently available oral probiotics made little or no difference in patient-rated AD symptoms, investigator-rated AD symptoms, or quality of life.32,33 No adverse effects were observed in the included studies. Unfortunately, the individual RCTs included were heterogeneous, and future studies with standardized probiotic supplementation should be undertaken before probiotics can be routinely recommended.
The use of topical probiotics in AD also has recently emerged. Multiple studies have shown that patients with AD have higher levels of colonization with S aureus, which is associated with T-cell dysfunction, more severe allergic skin reactions, and disruptions in barrier function.34,35 Therefore, altering the skin microbiota through topical probiotics could theoretically reduce AD symptoms and flares. Multiple RCTs and smaller studies have shown that topical probiotics can alter the skin microbiota, improve erythema, and decrease scaling and pruritus in AD patients.35-38 One study used a heat-treated Lactobacillus johnsonii 0.3% lotion twice daily for 3 weeks vs placebo in patients with AD with positive S aureus skin cultures. The S aureus load decreased in patients using the topical probiotic lotion, which correlated with lower SCORAD index that was statistically significant compared to placebo (P=.012).36 More robust studies are needed to determine if topical probiotics should routinely be recommended in AD.
Psoriasis
Psoriasis vulgaris is a chronic inflammatory skin condition characterized by pruritic, hyperkeratotic, scaly plaques.39,40 Keratinocyte hyperproliferation is central to psoriasis pathogenesis and is thought to be a T-cell–driven reaction to antigens or trauma in genetically predisposed individuals. Standard treatments for psoriasis currently include topical corticosteroids and anti-inflammatories, oral immunomodulatory therapy, biologic agents, and phototherapy.40 The use of CAM is highly prevalent among patients with psoriasis, with one study reporting that 51% (n=162) of psoriatic patients interviewed had used CAM.41 The most common reasons for CAM use included dissatisfaction with current treatment, adverse side effects of standard therapy, and patient-reported attempts at “trying everything to heal disease.”42 Herein, we will discuss some of the most frequently used supplements for treatment of psoriatic disease.39
Fish Oil for Psoriasis
One of the most common supplements used by patients with psoriasis is fish oil due to its purported anti-inflammatory qualities.20,39 The consensus on fish oil supplementation for psoriasis is mixed.43-45 Multiple RCTs have reported reductions in psoriasis area and severity index (PASI) scores or symptomatic improvement with variable doses of fish oil.44,46 One RCT found that using EPA 1.8 g once daily and DHA 1.2 g once daily for 12 weeks resulted in significant improvement in pruritus, scaling, and erythema (P<.05).44 Another study reported a significant decrease in erythema (P=.02) and total body surface area affected (P=.0001) with EPA 3.6 g once daily and DHA 2.4 g once daily supplementation compared to olive oil supplementation for 15 weeks.46 Alternatively, multiple studies have failed to show statistically significant improvement in psoriatic symptoms with fish oil supplementation at variable doses and time frames (14–216 mg daily EPA, 9–80 mg daily DHA, from 2 weeks to 9 months).40,47,48 Fish oil may impart anticoagulant properties and should not be started without the guidance of a physician. Currently, there are no data to make specific recommendations on the use of fish oil as an adjunct psoriatic treatment.
Curcumin for Psoriasis
Another supplement routinely utilized in patients with psoriasis is curcumin,40,49,50 a yellow phytochemical that is a major component of the spice turmeric. Curcumin has been shown to inhibit certain proinflammatory cytokines including IL-17, IL-6, IFN-γ, and tumor necrosis factor α and has been regarded as having immune-modulating, anti-inflammatory, and antibacterial properties.40,50 Curcumin also has been reported to suppress phosphorylase kinase, an enzyme that has increased activity in psoriatic plaques that correlates with markers of psoriatic hyperproliferation.50,51 When applied topically, turmeric microgel 0.5% has been reported to decrease scaling, erythema, and psoriatic plaque thickness over the course of 9 weeks.50 In a nonrandomized trial with 10 participants, researchers found that phosphorylase kinase activity levels in psoriatic skin biopsies of patients applying topical curcumin 1% were lower than placebo and topical calcipotriol applied in combination. The lower phosphorylase kinase levels correlated with level of disease severity, and topical curcumin 1% showed a superior outcome when compared to topical calcipotriol.40,49 Although these preliminary results are interesting, there still are not enough data at this time to recommend topical curcumin as a treatment of psoriasis. No known adverse events have been reported with the use of topical curcumin to date.
Oral curcumin has poor oral bioavailability, and 40% to 90% of oral doses are excreted, making supplementation a challenge.40 In one RCT, oral curcumin 2 g daily (using a lecithin-based delivery system to increase bioavailability) was administered in combination with topical methylprednisolone aceponate 0.1%, resulting in significant improvement in psoriatic symptoms and lower IL-22 compared to placebo and topical methylprednisolone aceponate (P<.05).52 Other studies also have reported decreased PASI scores with oral curcumin supplementation.53,54 Adverse effects reported with oral curcumin included gastrointestinal tract upset and hot flashes.53 Although there is early evidence that may support the use of oral curcumin supplementation for psoriasis, more data are needed before recommending this therapy.
Indigo Naturalis for Psoriasis
Topical indigo naturalis (IN) also has been reported to improve psoriasis symptoms.39,53,55 The antipsoriatic effects are thought to occur through the active ingredient in IN (indirubin), which is responsible for inhibition of keratinocyte proliferation.40 One study reported that topical IN 1.4% containing indirubin 0.16% with a petroleum ointment vehicle applied to psoriatic plaques over 12 weeks resulted in a significant decrease in PASI scores from 18.9 at baseline to 6.3 after IN treatment (P<.001).56 Another study found that over 8 weeks, topical application of IN 2.83% containing indirubin 0.24% to psoriatic plaques vs petroleum jelly resulted in 56.3% (n=9) of the treatment group achieving PASI 75 compared to 0% in the placebo group (n=24).55 One deterrent in topical IN treatment is the dark blue pigment it contains; however, no other adverse outcomes were found with topical IN treatment.56 Larger clinical trials are necessary to further explore IN as a potential adjunct treatment in patients with mild psoriatic disease. When taken orally, IN has caused gastrointestinal tract disturbance and elevated liver enzyme levels.57
Herbal Toxicities
It is important to consider that oral supplements including curcumin and IN are widely available over-the-counter and online without oversight by the US Food and Drug Administration.40 Herbal supplements typically are compounded with other ingredients and have been associated with hepatotoxicity as well as drug-supplement interactions, including abnormal bleeding and clotting.58 There exists a lack of general surveillance data, making the true burden of herbal toxicities more difficult to accurately discern. Although some supplements have been associated with anti-inflammatory qualities and disease improvement, other herbal supplements have been shown to possess immunostimulatory characteristics. Herbal supplements such as spirulina, chlorella, Aphanizomenon flos-aquae, and echinacea have been shown to upregulate inflammatory pathways in a variety of autoimmune skin conditions.59
Probiotics for Psoriasis
Data on probiotic use in patients with psoriasis are limited.23 A distinct pattern of dysbiosis has been identified in psoriatic patients, as there is thought to be depletion of beneficial bacteria such as Bifidobacterium, lactobacilli, and F prausnitzii and increased colonization with pathogenic organisms such as Salmonella, E coli, Heliobacter, Campylobacter, and Alcaligenes in psoriasis patients.23,59,60 Early mouse studies have supported this hypothesis, as mice fed with Lactobacillus pentosus have developed milder forms of imiquimod-induced psoriasis compared to placebo,55 and mice receiving probiotic supplementation have lower levels of psoriasis-related proinflammatory markers such as TH17-associated cytokines.61 Another study in humans found that daily oral Bifidobacterium infantis supplementation for 8 weeks in psoriatic patients resulted in lower C-reactive protein and tumor necrosis factor α levels compared to placebo.62 Studies on the use of topical probiotics in psoriasis have been limited, and more research is needed to explore this relationship.38 At this time, no specific recommendations can be made on the use of probiotics in psoriatic patients.
Alopecia Areata
Alopecia areata is nonscarring hair loss that can affect the scalp, face, or body.63,64 The pathophysiology of AA involves the attack of the hair follicle matrix epithelium by inflammatory cells without hair follicle stem cell destruction. The precise events that precipitate these episodes are unknown, but triggers such as emotional or physical stress, vaccines, or viral infections have been reported.65 There is no cure for AA, and current treatments such as topical minoxidil and corticosteroids (topical, intralesional, or oral) vary widely in efficacy.64 Although Janus kinase inhibitors recently have shown promising results in the treatment of AA, the need for prolonged therapy may be frustrating to patients.66 Severity of AA also can vary, with 30% of patients experiencing extensive hair loss.67 The use of CAM has been widely reported in AA due to high levels of dissatisfaction with existing therapies.68 Herein, we discuss the most studied alternative treatments used in AA
Garlic and Onion for Alopecia
One alternative treatment that has shown promising initial results is application of topical garlic and onion extracts to affected areas.64,69,70 Both garlic and onion belong to the Allium genus and are high in sulfur and phenolic compounds.70 They have been reported to possess bactericidal and vasodilatory activity,71 and it has been hypothesized that onion and garlic extracts may induce therapeutic effects through induction of a mild contact dermatitis.70 One single-blinded, controlled trial using topical crude onion juice reported that 86.9% (n=20) of patients had full regrowth of hair compared to 13.3% (n=2) of patients treated with a tap water placebo at 8 weeks (P<.0001). This study also noted that patients using onion juice had a higher rate of erythema at application site; unfortunately, the study was small with only 38 patients.70 Another double-blind RCT using garlic gel 5% with betamethasone valerate cream 0.1% compared to betamethasone valerate cream alone found that after 3 months, patients in the garlic gel group had increased terminal hairs and smaller patch sizes compared to the betamethasone valerate cream group.69 More studies are needed to confirm these results.
Aromatherapy With Essential Oils for Alopecia
Another alternative treatment in AA that has demonstrated positive results is aromatherapy skin massage with essential oils to patches of alopecia.72 Although certain essential oils, such as tea tree oil, have been reported to have specific antibacterial or anti-inflammatory properties, essential oils have been reported to cause allergic contact dermatitis and should be used with caution.73,74 For example, tea tree oil is a well-known cause of allergic contact dermatitis, and positive patch testing has ranged from 0.1% to 3.5% in studies assessing topical tea tree oil 5% application.75 Overall, there have been nearly 80 essential oils implicated in contact dermatitis, with high-concentration products being one of the highest risk factors for an allergic contact reaction.76 One RCT compared daily scalp massage with essential oils (rosemary, lavender, thyme, and cedarwood in a carrier oil) to daily scalp massage with a placebo carrier oil in AA patients. The results showed that at 7 months of treatment, 44% (n=19) of the aromatherapy group showed improvement compared to 15% (n=6) in the control group.77 Another study used a similar group of essential oils (thyme, rosemary, atlas cedar, lavender, and EPO in a carrier oil) with daily scalp massage and reported similar improvement of AA symptoms compared to control; the investigators also reported irritation at application site in 1 patient.78 There currently are not enough data to recommend aromatherapy skin massage for the treatment of AA, and this practice may cause harm to the patient by induction of allergic contact dermatitis.
There have been a few studies to suggest that the use of total glucosides of peony with compound glycyrrhizin and oral Korean red ginseng may have beneficial effects on AA treatment, but efficacy and safety data are lacking, and these therapies should not be recommended without more information.64,79,80
Final Thoughts
Dermatologic patients frequently are opting for CAM,2 and although some therapies may show promising initial results, alternative medicines also can drive adverse events.19,30 The lack of oversight from the US Food and Drug Administration on the products leads to many unknowns for true health risks with over-the-counter CAM supplements.40 As the use of CAM becomes increasingly common among dermatologic patients, it is important for dermatologists to understand the benefits and risks, especially for commonly treated conditions. More data is needed before CAM can be routinely recommended.
Complementary alternative medicine (CAM) has been described by the National Center for Complementary and Integrative Medicine as “health care approaches that are not typically part of conventional medical care or that may have origins outside of usual Western practice.”1 Although this definition is broad, CAM encompasses therapies such as traditional Chinese medicine, herbal therapies, dietary supplements, and mind/body interventions. The use of CAM has grown, and according to a 2012 National Center for Complementary and Integrative Health survey, more than 30% of US adults and 12% of US children use health care approaches that are considered outside of conventional medical practice. In a survey study of US adults, at least 17.7% of respondents said they had taken a dietary supplement other than a vitamin or mineral in the last year.1 Data from the 2007 National Health Interview Survey showed that the prevalence of adults with skin conditions using CAM was 84.5% compared to 38.3% in the general population.2 In addition, 8.15 million US patients with dermatologic conditions reported using CAM over a 5-year period.3 Complementary alternative medicine has emerged as an alternative or adjunct to standard treatments, making it important for dermatologists to understand the existing literature on these therapies. Herein, we review the current evidence-based literature that exists on CAM for the treatment of atopic dermatitis (AD), psoriasis, and alopecia areata (AA).
Atopic Dermatitis
Atopic dermatitis is a chronic, pruritic, inflammatory skin condition with considerable morbidity.4,5 The pathophysiology of AD is multifactorial and includes aspects of barrier dysfunction, IgE hypersensitivity, abnormal cell-mediated immune response, and environmental factors.6 Atopic dermatitis also is one of the most common inflammatory skin conditions in adults, affecting more than 7% of the US population and up to 20% of the total population in developed countries. Of those affected, 40% have moderate or severe symptoms that result in a substantial impact on quality of life.7 Despite advances in understanding disease pathology and treatment, a subset of patients opt to defer conventional treatments such as topical and systemic corticosteroids, antibiotics, nonsteroidal immunomodulators, and biologics. Patients may seek alternative therapies when typical treatments fail or when the perceived side effects outweigh the benefits.5,8 The use of CAM has been well described in patients with AD; however, the existing evidence supporting its use along with its safety profile have not been thoroughly explored. Herein, we will discuss some of the most well-studied supplements for treatment of AD, including evening primrose oil (EPO), fish oil, and probiotics.5
Oral supplementation with polyunsaturated fatty acids commonly is reported in patients with AD.5,8 The idea that a fatty acid deficiency could lead to atopic skin conditions has been around since 1937, when it was suggested that patients with AD had lower levels of blood unsaturated fatty acids.9 Conflicting evidence regarding oral fatty acid ingestion and AD disease severity has emerged.10,11 One unsaturated fatty acid, γ-linolenic acid (GLA), has demonstrated anti-inflammatory properties and involvement in barrier repair.12 It is converted to dihomo-GLA in the body, which acts on cyclooxygenase enzymes to produce the inflammatory mediator prostaglandin E1. The production of GLA is mediated by the enzyme delta-6 desaturase in the metabolization of linoleic acid.12 However, it has been reported that in a subset of patients with AD, a malfunction of delta-6 desaturase may play a role in disease progression and result in lower baseline levels of GLA.10,12 Evening primrose oil and borage oil contain high amounts of GLA (8%–10% and 23%, respectively); thus, supplementation with these oils has been studied in AD.13
EPO for AD
Studies investigating EPO (Oenothera biennis) and its association with AD severity have shown mixed results. A Cochrane review reported that oral borage oil and EPO were not effective treatments for AD,14 while another larger randomized controlled trial (RCT) found no statistically significant improvement in AD symptoms.15 However, multiple smaller studies have found that clinical symptoms of AD, such as erythema, xerosis, pruritus, and total body surface area involved, did improve with oral EPO supplementation when compared to placebo, and the results were statistically significant (P=.04).16,17 One study looked at different dosages of EPO and found that groups ingesting both 160 mg and 320 mg daily experienced reductions in eczema area and severity index score, with greater improvement noted with the higher dosage.17 Side effects associated with oral EPO include an anticoagulant effect and transient gastrointestinal tract upset.8,14 There currently is not enough evidence or safety data to recommend this supplement to AD patients.
Although topical use of fatty acids with high concentrations of GLA, such as EPO and borage oil, have demonstrated improvement in subjective symptom severity, most studies have not reached statistical significance.10,11 One study used a 10% EPO cream for 2 weeks compared to placebo and found statistically significant improvement in patient-reported AD symptoms (P=.045). However, this study only included 10 participants, and therefore larger studies are necessary to confirm this result.18 Some RCTs have shown that topical coconut oil, sunflower seed oil, and sandalwood album oil improve AD symptom severity, but again, large controlled trials are needed.5 Unfortunately, many essential oils, including EPO, can cause a secondary allergic contact dermatitis and potentially worsen AD.19
Fish Oil for AD
Fish oil is a commonly used supplement for AD due to its high content of the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Omega-3 fatty acids exert anti-inflammatory effects by displacing arachidonic acid, a proinflammatory omega-6 fatty acid thought to increase IgE, as well as helper T cell (TH2) cytokines and prostaglandin E2.8,20 A 2012 Cochrane review found that, while some studies revealed mild improvement in AD symptoms with oral fish oil supplementation, these RCTs were of poor methodological quality.21 Multiple smaller studies have shown a decrease in pruritus, severity, and physician-rated clinical scores with fish oil use.5,8,20,22 One study with 145 participants reported that 6 g of fish oil once daily compared to isoenergetic corn oil for 16 weeks identified no statistically significant differences between the treatment groups.20 No adverse events were identified in any of the reported trials. Further studies should be conducted to assess the utility and dosing of fish oil supplements in AD patients.
Probiotics for AD
Probiotics consist of live microorganisms that enhance the microflora of the gastrointestinal tract.8,20 They have been shown to influence food digestion and also have demonstrated potential influence on the skin-gut axis.23 The theory that intestinal dysbiosis plays a role in AD pathogenesis has been investigated in multiple studies.23-25 The central premise is that low-fiber and high-fat Western diets lead to fundamental changes in the gut microbiome, resulting in fewer anti-inflammatory metabolites, such as short-chain fatty acids (SCFAs).23-25 These SCFAs are produced by microbes during the fermentation of dietary fiber and are known for their effect on epithelial barrier integrity and anti-inflammatory properties mediated through G protein–coupled receptor 43.25 Multiple studies have shown that the gut microbiome in patients with AD have higher proportions of Clostridium difficile, Escherichia coli, and Staphylococcus aureus and lower levels of Bifidobacterium, Bacteroidetes, and Bacteroides species compared to healthy controls.26,27 Metagenomic analysis of fecal samples from patients with AD have shown a reduction of Faecalibacterium prausnitzii species when compared to controls, along with a decreased SCFA production, leading to the hypothesis that the gut microbiome may play a role in epithelial barrier disruption.28,29 Systematic reviews and smaller studies have found that oral probiotic use does lead to AD symptom improvement.8,30,31 A systematic review of 25 RCTs with 1599 participants found that supplementation with oral probiotics significantly decreased the SCORAD (SCORing Atopic Dermatitis) index in adults and children older than 1 year with AD but had no effect on infants younger than 1 year (P<.001). They also found that supplementation with diverse microbes or Lactobacillus species showed greater benefit than Bifidobacterium species alone.30 Another study analyzed the effect of oral Lactobacillus fermentum (1×109 CFU twice daily) in 53 children with AD vs placebo for 16 weeks. This study found a statically significant decrease in SCORAD index between oral probiotics and placebo, with 92% (n=24) of participants supplementing with probiotics having a lower SCORAD index than baseline compared to 63% (n=17) in the placebo group (P=.01).31 However, the use of probiotics for AD treatment has remained controversial. Two recent systematic reviews, including 39 RCTs of 2599 randomized patients, found that the use of currently available oral probiotics made little or no difference in patient-rated AD symptoms, investigator-rated AD symptoms, or quality of life.32,33 No adverse effects were observed in the included studies. Unfortunately, the individual RCTs included were heterogeneous, and future studies with standardized probiotic supplementation should be undertaken before probiotics can be routinely recommended.
The use of topical probiotics in AD also has recently emerged. Multiple studies have shown that patients with AD have higher levels of colonization with S aureus, which is associated with T-cell dysfunction, more severe allergic skin reactions, and disruptions in barrier function.34,35 Therefore, altering the skin microbiota through topical probiotics could theoretically reduce AD symptoms and flares. Multiple RCTs and smaller studies have shown that topical probiotics can alter the skin microbiota, improve erythema, and decrease scaling and pruritus in AD patients.35-38 One study used a heat-treated Lactobacillus johnsonii 0.3% lotion twice daily for 3 weeks vs placebo in patients with AD with positive S aureus skin cultures. The S aureus load decreased in patients using the topical probiotic lotion, which correlated with lower SCORAD index that was statistically significant compared to placebo (P=.012).36 More robust studies are needed to determine if topical probiotics should routinely be recommended in AD.
Psoriasis
Psoriasis vulgaris is a chronic inflammatory skin condition characterized by pruritic, hyperkeratotic, scaly plaques.39,40 Keratinocyte hyperproliferation is central to psoriasis pathogenesis and is thought to be a T-cell–driven reaction to antigens or trauma in genetically predisposed individuals. Standard treatments for psoriasis currently include topical corticosteroids and anti-inflammatories, oral immunomodulatory therapy, biologic agents, and phototherapy.40 The use of CAM is highly prevalent among patients with psoriasis, with one study reporting that 51% (n=162) of psoriatic patients interviewed had used CAM.41 The most common reasons for CAM use included dissatisfaction with current treatment, adverse side effects of standard therapy, and patient-reported attempts at “trying everything to heal disease.”42 Herein, we will discuss some of the most frequently used supplements for treatment of psoriatic disease.39
Fish Oil for Psoriasis
One of the most common supplements used by patients with psoriasis is fish oil due to its purported anti-inflammatory qualities.20,39 The consensus on fish oil supplementation for psoriasis is mixed.43-45 Multiple RCTs have reported reductions in psoriasis area and severity index (PASI) scores or symptomatic improvement with variable doses of fish oil.44,46 One RCT found that using EPA 1.8 g once daily and DHA 1.2 g once daily for 12 weeks resulted in significant improvement in pruritus, scaling, and erythema (P<.05).44 Another study reported a significant decrease in erythema (P=.02) and total body surface area affected (P=.0001) with EPA 3.6 g once daily and DHA 2.4 g once daily supplementation compared to olive oil supplementation for 15 weeks.46 Alternatively, multiple studies have failed to show statistically significant improvement in psoriatic symptoms with fish oil supplementation at variable doses and time frames (14–216 mg daily EPA, 9–80 mg daily DHA, from 2 weeks to 9 months).40,47,48 Fish oil may impart anticoagulant properties and should not be started without the guidance of a physician. Currently, there are no data to make specific recommendations on the use of fish oil as an adjunct psoriatic treatment.
Curcumin for Psoriasis
Another supplement routinely utilized in patients with psoriasis is curcumin,40,49,50 a yellow phytochemical that is a major component of the spice turmeric. Curcumin has been shown to inhibit certain proinflammatory cytokines including IL-17, IL-6, IFN-γ, and tumor necrosis factor α and has been regarded as having immune-modulating, anti-inflammatory, and antibacterial properties.40,50 Curcumin also has been reported to suppress phosphorylase kinase, an enzyme that has increased activity in psoriatic plaques that correlates with markers of psoriatic hyperproliferation.50,51 When applied topically, turmeric microgel 0.5% has been reported to decrease scaling, erythema, and psoriatic plaque thickness over the course of 9 weeks.50 In a nonrandomized trial with 10 participants, researchers found that phosphorylase kinase activity levels in psoriatic skin biopsies of patients applying topical curcumin 1% were lower than placebo and topical calcipotriol applied in combination. The lower phosphorylase kinase levels correlated with level of disease severity, and topical curcumin 1% showed a superior outcome when compared to topical calcipotriol.40,49 Although these preliminary results are interesting, there still are not enough data at this time to recommend topical curcumin as a treatment of psoriasis. No known adverse events have been reported with the use of topical curcumin to date.
Oral curcumin has poor oral bioavailability, and 40% to 90% of oral doses are excreted, making supplementation a challenge.40 In one RCT, oral curcumin 2 g daily (using a lecithin-based delivery system to increase bioavailability) was administered in combination with topical methylprednisolone aceponate 0.1%, resulting in significant improvement in psoriatic symptoms and lower IL-22 compared to placebo and topical methylprednisolone aceponate (P<.05).52 Other studies also have reported decreased PASI scores with oral curcumin supplementation.53,54 Adverse effects reported with oral curcumin included gastrointestinal tract upset and hot flashes.53 Although there is early evidence that may support the use of oral curcumin supplementation for psoriasis, more data are needed before recommending this therapy.
Indigo Naturalis for Psoriasis
Topical indigo naturalis (IN) also has been reported to improve psoriasis symptoms.39,53,55 The antipsoriatic effects are thought to occur through the active ingredient in IN (indirubin), which is responsible for inhibition of keratinocyte proliferation.40 One study reported that topical IN 1.4% containing indirubin 0.16% with a petroleum ointment vehicle applied to psoriatic plaques over 12 weeks resulted in a significant decrease in PASI scores from 18.9 at baseline to 6.3 after IN treatment (P<.001).56 Another study found that over 8 weeks, topical application of IN 2.83% containing indirubin 0.24% to psoriatic plaques vs petroleum jelly resulted in 56.3% (n=9) of the treatment group achieving PASI 75 compared to 0% in the placebo group (n=24).55 One deterrent in topical IN treatment is the dark blue pigment it contains; however, no other adverse outcomes were found with topical IN treatment.56 Larger clinical trials are necessary to further explore IN as a potential adjunct treatment in patients with mild psoriatic disease. When taken orally, IN has caused gastrointestinal tract disturbance and elevated liver enzyme levels.57
Herbal Toxicities
It is important to consider that oral supplements including curcumin and IN are widely available over-the-counter and online without oversight by the US Food and Drug Administration.40 Herbal supplements typically are compounded with other ingredients and have been associated with hepatotoxicity as well as drug-supplement interactions, including abnormal bleeding and clotting.58 There exists a lack of general surveillance data, making the true burden of herbal toxicities more difficult to accurately discern. Although some supplements have been associated with anti-inflammatory qualities and disease improvement, other herbal supplements have been shown to possess immunostimulatory characteristics. Herbal supplements such as spirulina, chlorella, Aphanizomenon flos-aquae, and echinacea have been shown to upregulate inflammatory pathways in a variety of autoimmune skin conditions.59
Probiotics for Psoriasis
Data on probiotic use in patients with psoriasis are limited.23 A distinct pattern of dysbiosis has been identified in psoriatic patients, as there is thought to be depletion of beneficial bacteria such as Bifidobacterium, lactobacilli, and F prausnitzii and increased colonization with pathogenic organisms such as Salmonella, E coli, Heliobacter, Campylobacter, and Alcaligenes in psoriasis patients.23,59,60 Early mouse studies have supported this hypothesis, as mice fed with Lactobacillus pentosus have developed milder forms of imiquimod-induced psoriasis compared to placebo,55 and mice receiving probiotic supplementation have lower levels of psoriasis-related proinflammatory markers such as TH17-associated cytokines.61 Another study in humans found that daily oral Bifidobacterium infantis supplementation for 8 weeks in psoriatic patients resulted in lower C-reactive protein and tumor necrosis factor α levels compared to placebo.62 Studies on the use of topical probiotics in psoriasis have been limited, and more research is needed to explore this relationship.38 At this time, no specific recommendations can be made on the use of probiotics in psoriatic patients.
Alopecia Areata
Alopecia areata is nonscarring hair loss that can affect the scalp, face, or body.63,64 The pathophysiology of AA involves the attack of the hair follicle matrix epithelium by inflammatory cells without hair follicle stem cell destruction. The precise events that precipitate these episodes are unknown, but triggers such as emotional or physical stress, vaccines, or viral infections have been reported.65 There is no cure for AA, and current treatments such as topical minoxidil and corticosteroids (topical, intralesional, or oral) vary widely in efficacy.64 Although Janus kinase inhibitors recently have shown promising results in the treatment of AA, the need for prolonged therapy may be frustrating to patients.66 Severity of AA also can vary, with 30% of patients experiencing extensive hair loss.67 The use of CAM has been widely reported in AA due to high levels of dissatisfaction with existing therapies.68 Herein, we discuss the most studied alternative treatments used in AA
Garlic and Onion for Alopecia
One alternative treatment that has shown promising initial results is application of topical garlic and onion extracts to affected areas.64,69,70 Both garlic and onion belong to the Allium genus and are high in sulfur and phenolic compounds.70 They have been reported to possess bactericidal and vasodilatory activity,71 and it has been hypothesized that onion and garlic extracts may induce therapeutic effects through induction of a mild contact dermatitis.70 One single-blinded, controlled trial using topical crude onion juice reported that 86.9% (n=20) of patients had full regrowth of hair compared to 13.3% (n=2) of patients treated with a tap water placebo at 8 weeks (P<.0001). This study also noted that patients using onion juice had a higher rate of erythema at application site; unfortunately, the study was small with only 38 patients.70 Another double-blind RCT using garlic gel 5% with betamethasone valerate cream 0.1% compared to betamethasone valerate cream alone found that after 3 months, patients in the garlic gel group had increased terminal hairs and smaller patch sizes compared to the betamethasone valerate cream group.69 More studies are needed to confirm these results.
Aromatherapy With Essential Oils for Alopecia
Another alternative treatment in AA that has demonstrated positive results is aromatherapy skin massage with essential oils to patches of alopecia.72 Although certain essential oils, such as tea tree oil, have been reported to have specific antibacterial or anti-inflammatory properties, essential oils have been reported to cause allergic contact dermatitis and should be used with caution.73,74 For example, tea tree oil is a well-known cause of allergic contact dermatitis, and positive patch testing has ranged from 0.1% to 3.5% in studies assessing topical tea tree oil 5% application.75 Overall, there have been nearly 80 essential oils implicated in contact dermatitis, with high-concentration products being one of the highest risk factors for an allergic contact reaction.76 One RCT compared daily scalp massage with essential oils (rosemary, lavender, thyme, and cedarwood in a carrier oil) to daily scalp massage with a placebo carrier oil in AA patients. The results showed that at 7 months of treatment, 44% (n=19) of the aromatherapy group showed improvement compared to 15% (n=6) in the control group.77 Another study used a similar group of essential oils (thyme, rosemary, atlas cedar, lavender, and EPO in a carrier oil) with daily scalp massage and reported similar improvement of AA symptoms compared to control; the investigators also reported irritation at application site in 1 patient.78 There currently are not enough data to recommend aromatherapy skin massage for the treatment of AA, and this practice may cause harm to the patient by induction of allergic contact dermatitis.
There have been a few studies to suggest that the use of total glucosides of peony with compound glycyrrhizin and oral Korean red ginseng may have beneficial effects on AA treatment, but efficacy and safety data are lacking, and these therapies should not be recommended without more information.64,79,80
Final Thoughts
Dermatologic patients frequently are opting for CAM,2 and although some therapies may show promising initial results, alternative medicines also can drive adverse events.19,30 The lack of oversight from the US Food and Drug Administration on the products leads to many unknowns for true health risks with over-the-counter CAM supplements.40 As the use of CAM becomes increasingly common among dermatologic patients, it is important for dermatologists to understand the benefits and risks, especially for commonly treated conditions. More data is needed before CAM can be routinely recommended.
- Complementary, alternative, or integrative health: what’s in a name? National Center for Complementary and Integrative Health website. Updated April 2021. Accessed April 25, 2021. https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name
- Fuhrmann T, Smith N, Tausk F. Use of complementary and alternative medicine among adults with skin disease: updated results from a national survey. J Am Acad Dermatol. 2010;63:1000-1005.
- Landis ET, Davis SA, Feldman SR, et al. Complementary and alternative medicine use in dermatology in the United States. J Altern Complement Med. 2014;20:392-398.
- Solman L, Lloyd‐Lavery A, Grindlay DJC, et al. What’s new in atopic eczema? an analysis of systematic reviews published in 2016. part 1: treatment and prevention. Clin Exp Dermatol. 2019;44:363-369.
- Vieira BL, Lim NR, Lohman ME, et al. Complementary and alternative medicine for atopic dermatitis: an evidence-based review. Am J Clin Dermatol. 2016;17:557-581.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. In: Fortson EA, Feldman SR, Strowd LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:21-37.
- Atopic dermatitis in America. Asthma and Allergy Foundation of America website. Accessed July 30, 2021. https://www.aafa.org/atopic-dermatitis-in-america
- Schlichte MJ, Vandersall A, Katta R. Diet and eczema: a review of dietary supplements for the treatment of atopic dermatitis. Dermatol Pract Concept. 2016;6:23-29.
- Brown WR, Hansen AE. Arachidonic and linolic acid of the serum in normal and eczematous human subjects. Proc Soc Exp Bio Med. 1937;36:113-117.
- Lee J, Bielory L. Complementary and alternative interventions in atopic dermatitis. Immunol Allergy Clin North Am. 2010;30:411-424.
- Ferreira MJ, Fiadeiro T, Silva M, et al. Topical γ-linolenic acid therapy in atopic dermatitis. Allergo J. 1998;7:213-216.
- Simon D, Eng PA, Borelli S, et al. Gamma-linolenic acid levels correlate with clinical efficacy of evening primrose oil in patients with atopic dermatitis. Adv Ther. 2014;31:180-188.
- Fan Y-Y, Chapkin RS. Importance of dietary γ-linolenic acid in human health and nutrition. J Nutr. 1998;128:1411-1414.
- Bamford JTM, Ray S, Musekiwa A, et al. Oral evening primrose oil and borage oil for eczema. Cochrane Database Syst Rev. 2013;4:CD004416.
- Williams H. Evening primrose oil for atopic dermatitis. BMJ. 2003;327:2.
- Schalin-Karrila M, Mattila L, Jansen CT, et al. Evening primrose oil in the treatment of atopic eczema: effect on clinical status, plasma phospholipid fatty acids and circulating blood prostaglandins. Br J Dermatol. 1987;117:11-19.
- Chung BY, Park SY, Jung MJ, et al. Effect of evening primrose oil on Korean patients with mild atopic dermatitis: a randomized, double-blinded, placebo-controlled clinical study. Ann Dermatol. 2018;30:409-416.
- Anstey A, Quigley M, Wilkinson JD. Topical evening primrose oil as treatment for atopic eczema. J Dermatolog Treat. 1990;1:199-201.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. Dermatitis. 2016;27:39-42.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review. Int J Dermatol. 2019;58:1371-1376.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema [published online February 15, 2012]. Cochrane Database Syst Rev. Accessed July 22, 2021. doi:10.1002/14651858.CD005205.pub3
- Balic´ A, Vlašic´ D, Žužul K, et al. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int J Mol Sci. 2020;21:741.
- Salem I, Ramser A, Isham N, et al. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459.
- Agrawal R, Wisniewski JA, Woodfolk JA. The role of regulatory T cells in atopic dermatitis. Pathogenesis Manage Atopic Dermatitis. 2011;41:112-124.
- Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282-1286.
- Lee E, Lee S-Y, Kang M-J, et al. Clostridia in the gut and onset of atopic dermatitis via eosinophilic inflammation. Ann Allergy Asthma Immunol. 2016;117:91-92.e1.
- Nylund L, Nermes M, Isolauri E, et al. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy. 2015;70:241-244.
- Kim H-J, Kim HY, Lee S-Y, et al. Clinical efficacy and mechanism of probiotics in allergic diseases. Korean J Pediatr. 2013;56:369-376.
- Song H, Yoo Y, Hwang J, et al. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. 2016;137:852-860.
- Kim S-O, Ah Y-M, Yu YM, et al. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2014;113:217-226.
- Weston S, Halbert A, Richmond P, et al. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005;90:892-897.
- Huang R, Ning H, Shen M, et al. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol. 2017;7:392.

- Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018;11:CD006135.
- Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020;29:15-21.
- Woo TE, Sibley CD. The emerging utility of the cutaneous microbiome in the treatment of acne and atopic dermatitis. J Am Acad Dermatol. 2020;82:222-228.
- Blanchet-Réthoré S, Bourdès V, Mercenier A, et al. Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin Cosmet Investig Dermatol. 2017;10:249-257.
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nature Medicine. 2021;27:700-709.
- França K. Topical probiotics in dermatological therapy and skincare: a concise review. Dermatol Ther (Heidelb). 2020;11:71-77.
- Talbott W, Duffy N. Complementary and alternative medicine for psoriasis: what the dermatologist needs to know. Am J Clin Dermatol. 2015;16:147-165.
- Gamret AC, Price A, Fertig RM, et al. Complementary and alternative medicine therapies for psoriasis: a systematic review. JAMA Dermatol. 2018;154:1330-1337.
- Fleischer AB, Feldman SR, Rapp SR, et al. Alternative therapies commonly used within a population of patients with psoriasis. Cutis. 1996;58:216-220.
- Ben-Arye E, Ziv M, Frenkel M, et al. Complementary medicine and psoriasis: linking the patient’s outlook with evidence-based medicine. Dermatology. 2003;207:302-307.
- Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis: part 3. role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
- Bittiner SB, Tucker WF, Cartwright I, et al. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1:378-380.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a Systematic review. JAMA Dermatol. 2018;154:934-950.
- Gupta AK, Ellis CN, Tellner DC, et al. Double-blind, placebo-controlled study to evaluate the efficacy of fish oil and low-dose UVB in the treatment of psoriasis. Br J Dermatol. 1989;120:801-807.
- Kristensen S, Schmidt EB, Schlemmer A, et al. Beneficial effect of n-3 polyunsaturated fatty acids on inflammation and analgesic use in psoriatic arthritis: a randomized, double blind, placebo-controlled trial. Scand J Rheumatol. 2018;47:27-36.
- Søyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Heng MCY, Song MK, Harker J, et al. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol. 2000;143:937-949.
- Sarafian G, Afshar M, Mansouri P, et al. Topical turmeric microemulgel in the management of plaque psoriasis; a clinical evaluation. Iran J Pharm Res. 2015;14:865-876.
- Reddy S, Aggarwal BB. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Letters. 1994;341:19-22.
- Antiga E, Bonciolini V, Volpi W, et al. Oral curcumin (meriva) is effective as an adjuvant treatment and is able to reduce IL-22 serum levels in patients with psoriasis vulgaris. Biomed Res Int. 2015;2015:283634.
- Kurd SK, Smith N, VanVoorhees A, et al. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: a prospective clinical trial. J Am Acad Dermatol. 2008;58:625-631.
- Carrion-Gutierrez M, Ramirez-Bosca A, Navarro-Lopez V, et al. Effects of Curcuma extract and visible light on adults with plaque psoriasis. Eur J Dermatol. 2015;25:240-246.
- Cheng H-M, Wu Y-C, Wang Q, et al. Clinical efficacy and IL-17 targeting mechanism of indigo naturalis as a topical agent in moderate psoriasis. BMC Complement Altern Med. 2017;17:439.
- Lin Y-K, Chang C-J, Chang Y-C, et al. Clinical assessment of patients with recalcitrant psoriasis in a randomized, observer-blind, vehicle-controlled trial using indigo naturalis. Arch Dermatol. 2008;144:1457-1464.
- Naganuma M, Sugimoto S, Suzuki H, et al. Adverse events in patients with ulcerative colitis treated with indigo naturalis: a Japanese nationwide survey. J Gastroenterol. 2019;54:891-896.
- Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Alimentary Pharmacol Ther. 2013;37:3-17.
- Bax CE, Chakka S, Concha JSS, et al. The effects of immunostimulatory herbal supplements on autoimmune skin diseases. J Am Acad Dermatol. 2021;84:1051-1058.
- Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes an altered gut microbiota in psoriatic arthritis and resembles dysbiosis of inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128-139.
- Chen Y-H, Wu C-S, Chao Y-H, et al. Lactobacillus pentosus GMNL-77 inhibits skin lesions in imiquimod-induced psoriasis-like mice. J Food Drug Anal. 2017;25:559-566.
- Groeger D, O’Mahony L, Murphy EF, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4:325-339.
- Hosking A-M, Juhasz M, Atanaskova Mesinkovska N. Complementary and alternative treatments for alopecia: a comprehensive review. Skin Appendage Disord. 2019;5:72-89.
- Tkachenko E, Okhovat J-P, Manjaly P, et al. Complementary & alternative medicine for alopecia areata: a systematic review [published online December 20, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.027
- Lepe K, Zito PM. Alopecia areata. In: StatPearls. StatPearls Publishing; 2021. Accessed July 22, 2021. https://pubmed.ncbi.nlm.nih.gov/30725685/
- Ismail FF, Sinclair R. JAK inhibition in the treatment of alopecia areata—a promising new dawn? Expert Rev Clin Pharmacol. 2020;13:43-51. doi:10.1080/17512433.2020.1702878
- van den Biggelaar FJHM, Smolders J, Jansen JFA. Complementary and alternative medicine in alopecia areata. AM J Clin Dermatol. 2010;11:11-20.
- Hussain ST, Mostaghimi A, Barr PJ, et al. Utilization of mental health resources and complementary and alternative therapies for alopecia areata: a U.S. survey. Int J Trichology. 2017;9:160-164.
- Hajheydari Z, Jamshidi M, Akbari J, et al. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73:29-32.
- Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343-346.
- Burian JP, Sacramento LVS, Carlos IZ. Fungal infection control by garlic extracts (Allium sativum L.) and modulation of peritoneal macrophages activity in murine model of sporotrichosis. Braz J Biol. 2017;77:848-855.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Lakshmi C, Srinivas CR. Allergic contact dermatitis following aromatherapy with valiya narayana thailam—an ayurvedic oil presenting as exfoliative dermatitis. Contact Dermatitis. 2009;61:297-298.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62.
- Groot AC de, Schmidt E. Tea tree oil: contact allergy and chemical composition. Contact Dermatitis. 2016;75:129-143.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. dermatitis. 2016;27:39-42.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Ozmen I, Caliskan E, Arca E, et al. Efficacy of aromatherapy in the treatment of localized alopecia areata: a double-blind placebo controlled study. Gulhane Med J. 2015;57:233.
- Oh GN, Son SW. Efficacy of Korean red ginseng in the treatment of alopecia areata. J Ginseng Res. 2012;36:391-395.
- Yang D-Q, You L-P, Song P-H, et al. A randomized controlled trial comparing total glucosides of paeony capsule and compound glycyrrhizin tablet for alopecia areata. Chin J Integr Med. 2012;18:621-625.
- Complementary, alternative, or integrative health: what’s in a name? National Center for Complementary and Integrative Health website. Updated April 2021. Accessed April 25, 2021. https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name
- Fuhrmann T, Smith N, Tausk F. Use of complementary and alternative medicine among adults with skin disease: updated results from a national survey. J Am Acad Dermatol. 2010;63:1000-1005.
- Landis ET, Davis SA, Feldman SR, et al. Complementary and alternative medicine use in dermatology in the United States. J Altern Complement Med. 2014;20:392-398.
- Solman L, Lloyd‐Lavery A, Grindlay DJC, et al. What’s new in atopic eczema? an analysis of systematic reviews published in 2016. part 1: treatment and prevention. Clin Exp Dermatol. 2019;44:363-369.
- Vieira BL, Lim NR, Lohman ME, et al. Complementary and alternative medicine for atopic dermatitis: an evidence-based review. Am J Clin Dermatol. 2016;17:557-581.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. In: Fortson EA, Feldman SR, Strowd LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:21-37.
- Atopic dermatitis in America. Asthma and Allergy Foundation of America website. Accessed July 30, 2021. https://www.aafa.org/atopic-dermatitis-in-america
- Schlichte MJ, Vandersall A, Katta R. Diet and eczema: a review of dietary supplements for the treatment of atopic dermatitis. Dermatol Pract Concept. 2016;6:23-29.
- Brown WR, Hansen AE. Arachidonic and linolic acid of the serum in normal and eczematous human subjects. Proc Soc Exp Bio Med. 1937;36:113-117.
- Lee J, Bielory L. Complementary and alternative interventions in atopic dermatitis. Immunol Allergy Clin North Am. 2010;30:411-424.
- Ferreira MJ, Fiadeiro T, Silva M, et al. Topical γ-linolenic acid therapy in atopic dermatitis. Allergo J. 1998;7:213-216.
- Simon D, Eng PA, Borelli S, et al. Gamma-linolenic acid levels correlate with clinical efficacy of evening primrose oil in patients with atopic dermatitis. Adv Ther. 2014;31:180-188.
- Fan Y-Y, Chapkin RS. Importance of dietary γ-linolenic acid in human health and nutrition. J Nutr. 1998;128:1411-1414.
- Bamford JTM, Ray S, Musekiwa A, et al. Oral evening primrose oil and borage oil for eczema. Cochrane Database Syst Rev. 2013;4:CD004416.
- Williams H. Evening primrose oil for atopic dermatitis. BMJ. 2003;327:2.
- Schalin-Karrila M, Mattila L, Jansen CT, et al. Evening primrose oil in the treatment of atopic eczema: effect on clinical status, plasma phospholipid fatty acids and circulating blood prostaglandins. Br J Dermatol. 1987;117:11-19.
- Chung BY, Park SY, Jung MJ, et al. Effect of evening primrose oil on Korean patients with mild atopic dermatitis: a randomized, double-blinded, placebo-controlled clinical study. Ann Dermatol. 2018;30:409-416.
- Anstey A, Quigley M, Wilkinson JD. Topical evening primrose oil as treatment for atopic eczema. J Dermatolog Treat. 1990;1:199-201.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. Dermatitis. 2016;27:39-42.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review. Int J Dermatol. 2019;58:1371-1376.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema [published online February 15, 2012]. Cochrane Database Syst Rev. Accessed July 22, 2021. doi:10.1002/14651858.CD005205.pub3
- Balic´ A, Vlašic´ D, Žužul K, et al. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int J Mol Sci. 2020;21:741.
- Salem I, Ramser A, Isham N, et al. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459.
- Agrawal R, Wisniewski JA, Woodfolk JA. The role of regulatory T cells in atopic dermatitis. Pathogenesis Manage Atopic Dermatitis. 2011;41:112-124.
- Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282-1286.
- Lee E, Lee S-Y, Kang M-J, et al. Clostridia in the gut and onset of atopic dermatitis via eosinophilic inflammation. Ann Allergy Asthma Immunol. 2016;117:91-92.e1.
- Nylund L, Nermes M, Isolauri E, et al. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy. 2015;70:241-244.
- Kim H-J, Kim HY, Lee S-Y, et al. Clinical efficacy and mechanism of probiotics in allergic diseases. Korean J Pediatr. 2013;56:369-376.
- Song H, Yoo Y, Hwang J, et al. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. 2016;137:852-860.
- Kim S-O, Ah Y-M, Yu YM, et al. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2014;113:217-226.
- Weston S, Halbert A, Richmond P, et al. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005;90:892-897.
- Huang R, Ning H, Shen M, et al. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol. 2017;7:392.

- Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018;11:CD006135.
- Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020;29:15-21.
- Woo TE, Sibley CD. The emerging utility of the cutaneous microbiome in the treatment of acne and atopic dermatitis. J Am Acad Dermatol. 2020;82:222-228.
- Blanchet-Réthoré S, Bourdès V, Mercenier A, et al. Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin Cosmet Investig Dermatol. 2017;10:249-257.
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nature Medicine. 2021;27:700-709.
- França K. Topical probiotics in dermatological therapy and skincare: a concise review. Dermatol Ther (Heidelb). 2020;11:71-77.
- Talbott W, Duffy N. Complementary and alternative medicine for psoriasis: what the dermatologist needs to know. Am J Clin Dermatol. 2015;16:147-165.
- Gamret AC, Price A, Fertig RM, et al. Complementary and alternative medicine therapies for psoriasis: a systematic review. JAMA Dermatol. 2018;154:1330-1337.
- Fleischer AB, Feldman SR, Rapp SR, et al. Alternative therapies commonly used within a population of patients with psoriasis. Cutis. 1996;58:216-220.
- Ben-Arye E, Ziv M, Frenkel M, et al. Complementary medicine and psoriasis: linking the patient’s outlook with evidence-based medicine. Dermatology. 2003;207:302-307.
- Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis: part 3. role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
- Bittiner SB, Tucker WF, Cartwright I, et al. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1:378-380.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a Systematic review. JAMA Dermatol. 2018;154:934-950.
- Gupta AK, Ellis CN, Tellner DC, et al. Double-blind, placebo-controlled study to evaluate the efficacy of fish oil and low-dose UVB in the treatment of psoriasis. Br J Dermatol. 1989;120:801-807.
- Kristensen S, Schmidt EB, Schlemmer A, et al. Beneficial effect of n-3 polyunsaturated fatty acids on inflammation and analgesic use in psoriatic arthritis: a randomized, double blind, placebo-controlled trial. Scand J Rheumatol. 2018;47:27-36.
- Søyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Heng MCY, Song MK, Harker J, et al. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol. 2000;143:937-949.
- Sarafian G, Afshar M, Mansouri P, et al. Topical turmeric microemulgel in the management of plaque psoriasis; a clinical evaluation. Iran J Pharm Res. 2015;14:865-876.
- Reddy S, Aggarwal BB. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Letters. 1994;341:19-22.
- Antiga E, Bonciolini V, Volpi W, et al. Oral curcumin (meriva) is effective as an adjuvant treatment and is able to reduce IL-22 serum levels in patients with psoriasis vulgaris. Biomed Res Int. 2015;2015:283634.
- Kurd SK, Smith N, VanVoorhees A, et al. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: a prospective clinical trial. J Am Acad Dermatol. 2008;58:625-631.
- Carrion-Gutierrez M, Ramirez-Bosca A, Navarro-Lopez V, et al. Effects of Curcuma extract and visible light on adults with plaque psoriasis. Eur J Dermatol. 2015;25:240-246.
- Cheng H-M, Wu Y-C, Wang Q, et al. Clinical efficacy and IL-17 targeting mechanism of indigo naturalis as a topical agent in moderate psoriasis. BMC Complement Altern Med. 2017;17:439.
- Lin Y-K, Chang C-J, Chang Y-C, et al. Clinical assessment of patients with recalcitrant psoriasis in a randomized, observer-blind, vehicle-controlled trial using indigo naturalis. Arch Dermatol. 2008;144:1457-1464.
- Naganuma M, Sugimoto S, Suzuki H, et al. Adverse events in patients with ulcerative colitis treated with indigo naturalis: a Japanese nationwide survey. J Gastroenterol. 2019;54:891-896.
- Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Alimentary Pharmacol Ther. 2013;37:3-17.
- Bax CE, Chakka S, Concha JSS, et al. The effects of immunostimulatory herbal supplements on autoimmune skin diseases. J Am Acad Dermatol. 2021;84:1051-1058.
- Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes an altered gut microbiota in psoriatic arthritis and resembles dysbiosis of inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128-139.
- Chen Y-H, Wu C-S, Chao Y-H, et al. Lactobacillus pentosus GMNL-77 inhibits skin lesions in imiquimod-induced psoriasis-like mice. J Food Drug Anal. 2017;25:559-566.
- Groeger D, O’Mahony L, Murphy EF, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4:325-339.
- Hosking A-M, Juhasz M, Atanaskova Mesinkovska N. Complementary and alternative treatments for alopecia: a comprehensive review. Skin Appendage Disord. 2019;5:72-89.
- Tkachenko E, Okhovat J-P, Manjaly P, et al. Complementary & alternative medicine for alopecia areata: a systematic review [published online December 20, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.027
- Lepe K, Zito PM. Alopecia areata. In: StatPearls. StatPearls Publishing; 2021. Accessed July 22, 2021. https://pubmed.ncbi.nlm.nih.gov/30725685/
- Ismail FF, Sinclair R. JAK inhibition in the treatment of alopecia areata—a promising new dawn? Expert Rev Clin Pharmacol. 2020;13:43-51. doi:10.1080/17512433.2020.1702878
- van den Biggelaar FJHM, Smolders J, Jansen JFA. Complementary and alternative medicine in alopecia areata. AM J Clin Dermatol. 2010;11:11-20.
- Hussain ST, Mostaghimi A, Barr PJ, et al. Utilization of mental health resources and complementary and alternative therapies for alopecia areata: a U.S. survey. Int J Trichology. 2017;9:160-164.
- Hajheydari Z, Jamshidi M, Akbari J, et al. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73:29-32.
- Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343-346.
- Burian JP, Sacramento LVS, Carlos IZ. Fungal infection control by garlic extracts (Allium sativum L.) and modulation of peritoneal macrophages activity in murine model of sporotrichosis. Braz J Biol. 2017;77:848-855.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Lakshmi C, Srinivas CR. Allergic contact dermatitis following aromatherapy with valiya narayana thailam—an ayurvedic oil presenting as exfoliative dermatitis. Contact Dermatitis. 2009;61:297-298.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62.
- Groot AC de, Schmidt E. Tea tree oil: contact allergy and chemical composition. Contact Dermatitis. 2016;75:129-143.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. dermatitis. 2016;27:39-42.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Ozmen I, Caliskan E, Arca E, et al. Efficacy of aromatherapy in the treatment of localized alopecia areata: a double-blind placebo controlled study. Gulhane Med J. 2015;57:233.
- Oh GN, Son SW. Efficacy of Korean red ginseng in the treatment of alopecia areata. J Ginseng Res. 2012;36:391-395.
- Yang D-Q, You L-P, Song P-H, et al. A randomized controlled trial comparing total glucosides of paeony capsule and compound glycyrrhizin tablet for alopecia areata. Chin J Integr Med. 2012;18:621-625.
Practice Points
- Dermatologic patients are increasingly opting for alternative treatments in addition to or instead of standard therapies for many common skin conditions.
- Dermatologists should be aware of the emerging evidence regarding the risks and benefits of some of the most popular alternative treatments in common skin disorders.
- Counseling patients on the side effects that accompany many supplements and the lack of data to support others is a crucial component of patient care.
Rashes in Pregnancy
Rashes that develop during pregnancy often result in considerable anxiety or concern for patients and their families. Recognizing these pregnancy-specific dermatoses is important in identifying fetal risks as well as providing appropriate management and expert guidance for patients regarding future pregnancies. Managing cutaneous manifestations of pregnancy-related disorders is challenging and requires knowledge of potential side effects of therapy for both the mother and fetus. It also is important to appreciate the physiologic cutaneous changes of pregnancy along with their clinical significance and management.
In 2006, Ambrose-Rudolph et al1 proposed reclassification of pregnancy-specific dermatoses, which has since been widely accepted by the academic dermatology community. The 4 most prominent disorders include intrahepatic cholestasis of pregnancy (ICP); pemphigoid gestationis (PG); polymorphic eruption of pregnancy (PEP), also known as pruritic urticarial papules and plaques of pregnancy; and atopic eruption of pregnancy.2 It is important to recognize these pregnancy-specific disorders and to understand their clinical significance. The morphology of the eruption as well as the location and timing of the onset of the rash are important clues in making an accurate diagnosis.3
Clinical Presentation
Intrahepatic cholestasis of pregnancy presents with severe generalized pruritus, usually with involvement of the palms and soles, in the late second or third trimester. Pemphigoid gestationis presents with urticarial papules and/or bullae, often in the second or third trimester or postpartum. An important diagnostic clue for PG is involvement near the umbilicus. Polymorphic eruption of pregnancy presents with urticarial papules and plaques; onset occurs in the third trimester or postpartum and initially involves the striae while sparing the umbilicus, unlike in PG. Atopic eruption of pregnancy has an earlier onset than the other pregnancy-specific dermatoses, often in the first or second trimester, and presents with widespread eczematous lesions.3
Diagnosis
The pregnancy dermatoses with the greatest potential for fetal risks are ICP and PG; therefore, it is critical for health care providers to diagnose these dermatoses in a timely manner and initiate appropriate management. Intrahepatic cholestasis of pregnancy is confirmed by elevated serum bile acids (ie, >10 µmol/L), often during the third trimester. The risk of fetal morbidity is high in ICP with increased bile acids crossing the placenta causing placental anoxia and impaired cardiomyocyte function.4 Fetal risks, including preterm delivery, meconium-stained amniotic fluid, and stillbirth, correlate with the level of bile acids in the serum.5 Maternal prognosis is favorable, but there is an increased association with hepatitis C and hepatobiliary disease.6
Diagnosis of PG is confirmed by classic biopsy results and direct immunofluorescence revealing C3 with or without IgG in a linear band along the basement membrane zone. Additionally, complement indirect immunofluorescence reveals circulating IgG anti–basement membrane zone antibodies. Pemphigoid gestationis is associated with increased fetal risks of preterm labor and intrauterine growth retardation.7 Clinical findings of PG may present in the fetus upon delivery due to transmission of autoantibodies across the placenta. The symptoms usually are mild.8 An increased risk of Graves disease has been reported in mothers with PG.
In most cases, diagnosis of PEP is based on history and morphology, but if the presentation is not classic, skin biopsy must be used to differentiate it from PG as well as more common dermatologic conditions such as contact dermatitis, drug and viral eruptions, and urticaria.
Atopic eruption of pregnancy manifests as widespread eczematous excoriated papules and plaques. Lesions of prurigo nodularis are common.
Comorbidities
It is important to be aware of specific clinical associations related to pregnancy-specific dermatoses. Pemphigoid gestationis has been associated with gestational trophoblastic tumors including hydatiform mole and choriocarcinoma.4 An increased risk for Graves disease has been reported in patients with PG.9 Patients who develop ICP have a higher incidence of hepatitis C, postpartum cholecystitis, gallstones, and nonalcoholic cirrhosis.8 Polymorphic eruption of pregnancy is associated with a notably higher incidence in multiple gestation pregnancies.2
Treatment and Management
Management of ICP requires an accurate and timely diagnosis, and advanced neonatal-obstetric management is critical.3 Ursodeoxycholic acid is the treatment of choice and reduces pruritus, prolongs pregnancy, and reduces fetal risk.4 Most stillbirths cluster at the 38th week of pregnancy, and patients with ICP and highly elevated serum bile acids (>40 µmol/L) should be considered for delivery at 37 weeks or earlier.5
Management of the other cutaneous disorders of pregnancy can be challenging for health care providers based on safety concerns for the fetus. Although it is important to minimize risks to the fetus, it also is important to adequately treat the mother’s cutaneous disease, which requires a solid knowledge of drug safety during pregnancy. The former US Food and Drug Administration classification system using A, B, C, D, and X pregnancy categories was replaced by the Pregnancy Lactation Label Final Rule, which provides counseling on medication safety during pregnancy.10 In 2014, Murase et al11 published a review of dermatologic medication safety during pregnancy, which serves as an excellent guide.
Before instituting treatment, the therapeutic plan should be discussed with the physician managing the patient’s pregnancy. In general, topical steroids are considered safe during pregnancy, and low-potency to moderate-potency topical steroids are preferred. If possible, use of topical steroids should be limited to less than 300 g for the duration of the pregnancy. Fluticasone propionate should be avoided during pregnancy because it is not metabolized by the placenta. When systemic steroids are considered appropriate for management during pregnancy, nonhalogenated corticosteroids such as prednisone and prednisolone are preferred because they are enzymatically inactivated by the placenta, which results in a favorable maternal-fetal gradient.12 There has been concern expressed in the medical literature that systemic steroids during the first trimester may increase the risk of cleft lip and cleft palate.3,12 When managing pregnancy dermatoses, consideration should be given to keep prednisone exposure below 20 mg/d, and try to limit prolonged use to 7.5 mg/d. However, this may not be possible in PG.3 Vitamin D and calcium supplementation may be appropriate when patients are on prolonged systemic steroids to control disease.
Antihistamines can be used to control pruritus complicating pregnancy-associated dermatoses. First-generation antihistamines such as chlorpheniramine and diphenhydramine are preferred due to long-term safety data.3,11,12 Loratadine is the first choice and cetirizine is the second choice if a second-generation antihistamine is preferred.3 Loratadine is preferred during breastfeeding due to less sedation.12 High-dose antihistamines prior to delivery may cause concerns for potential side effects in the newborn, including tremulousness, irritability, and poor feeding.
Recurrence
Women with pregnancy dermatoses often are concerned about recurrence with future pregnancies. Pemphigoid gestationis may flare with subsequent pregnancies, subsequent menses, or with oral contraceptive use.3 Recurrence of PEP in subsequent pregnancies is rare and usually is less severe than the primary eruption.8 Often, the rare recurrent eruption of PEP is associated with multigestational pregnancies.2 Mothers can anticipate a recurrence of ICP in up to 60% to 70% of future pregnancies. Patients with AEP have an underlying atopic diathesis, and recurrence in future pregnancies is not uncommon.8
Final Thoughts
In summary, it is important for health care providers to recognize the specific cutaneous disorders of pregnancy and their potential fetal complications. The anatomical location of onset of the dermatosis and timing of onset during pregnancy can give important clues. Appropriate management, especially with ICP, can minimize fetal complications. A fundamental knowledge of medication safety and management during pregnancy is essential. Rashes during pregnancy can cause anxiety in the mother and family and require support, comfort, and guidance.
- Ambrose-Rudolph CM, Müllegger RR, Vaughn-Jones SA, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54:395-404.
- Bechtel M, Plotner A. Dermatoses of pregnancy. Clin Obstet Gynecol. 2015;58:104-111.
- Bechtel M. Pruritus in pregnancy and its management. Dermatol Clin. 2018;36:259-265.
- Ambrose-Rudolph CM. Dermatoses of pregnancy—clues to diagnosis, fetal risk, and therapy. Ann Dermatol. 2011;23:265-275.
- Geenes V, Chappell LC, Seed PT, et al. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-controlled study. Hepatology. 2014;59:1482-1491.
- Bergman H, Melamed N, Koven G. Pruritus in pregnancy: treatment of dermatoses unique to pregnancy. Can Fam Physician. 2013;59:1290-1294.
- Beard MP, Millington GW. Recent developments in the specific dermatoses of pregnancy. Clin Exp Dermatol. 2012;37:1-14.
- Shears S, Blaszczak A, Kaffenberger J. Pregnancy dermatosis. In: Tyler KH, ed. Cutaneous Disorders of Pregnancy. 1st ed. Springer Nature; 2020:13-39.
- Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2015;26:274-284.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Fed Registr. 2014;79:72064-72103. To be codified at 21 CFR § 201.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part 1. pregnancy. J Am Acad Dermatol. 2014;401:E1-E14.
- Friedman B, Bercovitch L. Atopic dermatitis in pregnancy. In: Tyler KH, ed. Cutaneous Disorders of Pregnancy. Springer Nature; 2020:59-74.
Rashes that develop during pregnancy often result in considerable anxiety or concern for patients and their families. Recognizing these pregnancy-specific dermatoses is important in identifying fetal risks as well as providing appropriate management and expert guidance for patients regarding future pregnancies. Managing cutaneous manifestations of pregnancy-related disorders is challenging and requires knowledge of potential side effects of therapy for both the mother and fetus. It also is important to appreciate the physiologic cutaneous changes of pregnancy along with their clinical significance and management.
In 2006, Ambrose-Rudolph et al1 proposed reclassification of pregnancy-specific dermatoses, which has since been widely accepted by the academic dermatology community. The 4 most prominent disorders include intrahepatic cholestasis of pregnancy (ICP); pemphigoid gestationis (PG); polymorphic eruption of pregnancy (PEP), also known as pruritic urticarial papules and plaques of pregnancy; and atopic eruption of pregnancy.2 It is important to recognize these pregnancy-specific disorders and to understand their clinical significance. The morphology of the eruption as well as the location and timing of the onset of the rash are important clues in making an accurate diagnosis.3
Clinical Presentation
Intrahepatic cholestasis of pregnancy presents with severe generalized pruritus, usually with involvement of the palms and soles, in the late second or third trimester. Pemphigoid gestationis presents with urticarial papules and/or bullae, often in the second or third trimester or postpartum. An important diagnostic clue for PG is involvement near the umbilicus. Polymorphic eruption of pregnancy presents with urticarial papules and plaques; onset occurs in the third trimester or postpartum and initially involves the striae while sparing the umbilicus, unlike in PG. Atopic eruption of pregnancy has an earlier onset than the other pregnancy-specific dermatoses, often in the first or second trimester, and presents with widespread eczematous lesions.3
Diagnosis
The pregnancy dermatoses with the greatest potential for fetal risks are ICP and PG; therefore, it is critical for health care providers to diagnose these dermatoses in a timely manner and initiate appropriate management. Intrahepatic cholestasis of pregnancy is confirmed by elevated serum bile acids (ie, >10 µmol/L), often during the third trimester. The risk of fetal morbidity is high in ICP with increased bile acids crossing the placenta causing placental anoxia and impaired cardiomyocyte function.4 Fetal risks, including preterm delivery, meconium-stained amniotic fluid, and stillbirth, correlate with the level of bile acids in the serum.5 Maternal prognosis is favorable, but there is an increased association with hepatitis C and hepatobiliary disease.6
Diagnosis of PG is confirmed by classic biopsy results and direct immunofluorescence revealing C3 with or without IgG in a linear band along the basement membrane zone. Additionally, complement indirect immunofluorescence reveals circulating IgG anti–basement membrane zone antibodies. Pemphigoid gestationis is associated with increased fetal risks of preterm labor and intrauterine growth retardation.7 Clinical findings of PG may present in the fetus upon delivery due to transmission of autoantibodies across the placenta. The symptoms usually are mild.8 An increased risk of Graves disease has been reported in mothers with PG.
In most cases, diagnosis of PEP is based on history and morphology, but if the presentation is not classic, skin biopsy must be used to differentiate it from PG as well as more common dermatologic conditions such as contact dermatitis, drug and viral eruptions, and urticaria.
Atopic eruption of pregnancy manifests as widespread eczematous excoriated papules and plaques. Lesions of prurigo nodularis are common.
Comorbidities
It is important to be aware of specific clinical associations related to pregnancy-specific dermatoses. Pemphigoid gestationis has been associated with gestational trophoblastic tumors including hydatiform mole and choriocarcinoma.4 An increased risk for Graves disease has been reported in patients with PG.9 Patients who develop ICP have a higher incidence of hepatitis C, postpartum cholecystitis, gallstones, and nonalcoholic cirrhosis.8 Polymorphic eruption of pregnancy is associated with a notably higher incidence in multiple gestation pregnancies.2
Treatment and Management
Management of ICP requires an accurate and timely diagnosis, and advanced neonatal-obstetric management is critical.3 Ursodeoxycholic acid is the treatment of choice and reduces pruritus, prolongs pregnancy, and reduces fetal risk.4 Most stillbirths cluster at the 38th week of pregnancy, and patients with ICP and highly elevated serum bile acids (>40 µmol/L) should be considered for delivery at 37 weeks or earlier.5
Management of the other cutaneous disorders of pregnancy can be challenging for health care providers based on safety concerns for the fetus. Although it is important to minimize risks to the fetus, it also is important to adequately treat the mother’s cutaneous disease, which requires a solid knowledge of drug safety during pregnancy. The former US Food and Drug Administration classification system using A, B, C, D, and X pregnancy categories was replaced by the Pregnancy Lactation Label Final Rule, which provides counseling on medication safety during pregnancy.10 In 2014, Murase et al11 published a review of dermatologic medication safety during pregnancy, which serves as an excellent guide.
Before instituting treatment, the therapeutic plan should be discussed with the physician managing the patient’s pregnancy. In general, topical steroids are considered safe during pregnancy, and low-potency to moderate-potency topical steroids are preferred. If possible, use of topical steroids should be limited to less than 300 g for the duration of the pregnancy. Fluticasone propionate should be avoided during pregnancy because it is not metabolized by the placenta. When systemic steroids are considered appropriate for management during pregnancy, nonhalogenated corticosteroids such as prednisone and prednisolone are preferred because they are enzymatically inactivated by the placenta, which results in a favorable maternal-fetal gradient.12 There has been concern expressed in the medical literature that systemic steroids during the first trimester may increase the risk of cleft lip and cleft palate.3,12 When managing pregnancy dermatoses, consideration should be given to keep prednisone exposure below 20 mg/d, and try to limit prolonged use to 7.5 mg/d. However, this may not be possible in PG.3 Vitamin D and calcium supplementation may be appropriate when patients are on prolonged systemic steroids to control disease.
Antihistamines can be used to control pruritus complicating pregnancy-associated dermatoses. First-generation antihistamines such as chlorpheniramine and diphenhydramine are preferred due to long-term safety data.3,11,12 Loratadine is the first choice and cetirizine is the second choice if a second-generation antihistamine is preferred.3 Loratadine is preferred during breastfeeding due to less sedation.12 High-dose antihistamines prior to delivery may cause concerns for potential side effects in the newborn, including tremulousness, irritability, and poor feeding.
Recurrence
Women with pregnancy dermatoses often are concerned about recurrence with future pregnancies. Pemphigoid gestationis may flare with subsequent pregnancies, subsequent menses, or with oral contraceptive use.3 Recurrence of PEP in subsequent pregnancies is rare and usually is less severe than the primary eruption.8 Often, the rare recurrent eruption of PEP is associated with multigestational pregnancies.2 Mothers can anticipate a recurrence of ICP in up to 60% to 70% of future pregnancies. Patients with AEP have an underlying atopic diathesis, and recurrence in future pregnancies is not uncommon.8
Final Thoughts
In summary, it is important for health care providers to recognize the specific cutaneous disorders of pregnancy and their potential fetal complications. The anatomical location of onset of the dermatosis and timing of onset during pregnancy can give important clues. Appropriate management, especially with ICP, can minimize fetal complications. A fundamental knowledge of medication safety and management during pregnancy is essential. Rashes during pregnancy can cause anxiety in the mother and family and require support, comfort, and guidance.
Rashes that develop during pregnancy often result in considerable anxiety or concern for patients and their families. Recognizing these pregnancy-specific dermatoses is important in identifying fetal risks as well as providing appropriate management and expert guidance for patients regarding future pregnancies. Managing cutaneous manifestations of pregnancy-related disorders is challenging and requires knowledge of potential side effects of therapy for both the mother and fetus. It also is important to appreciate the physiologic cutaneous changes of pregnancy along with their clinical significance and management.
In 2006, Ambrose-Rudolph et al1 proposed reclassification of pregnancy-specific dermatoses, which has since been widely accepted by the academic dermatology community. The 4 most prominent disorders include intrahepatic cholestasis of pregnancy (ICP); pemphigoid gestationis (PG); polymorphic eruption of pregnancy (PEP), also known as pruritic urticarial papules and plaques of pregnancy; and atopic eruption of pregnancy.2 It is important to recognize these pregnancy-specific disorders and to understand their clinical significance. The morphology of the eruption as well as the location and timing of the onset of the rash are important clues in making an accurate diagnosis.3
Clinical Presentation
Intrahepatic cholestasis of pregnancy presents with severe generalized pruritus, usually with involvement of the palms and soles, in the late second or third trimester. Pemphigoid gestationis presents with urticarial papules and/or bullae, often in the second or third trimester or postpartum. An important diagnostic clue for PG is involvement near the umbilicus. Polymorphic eruption of pregnancy presents with urticarial papules and plaques; onset occurs in the third trimester or postpartum and initially involves the striae while sparing the umbilicus, unlike in PG. Atopic eruption of pregnancy has an earlier onset than the other pregnancy-specific dermatoses, often in the first or second trimester, and presents with widespread eczematous lesions.3
Diagnosis
The pregnancy dermatoses with the greatest potential for fetal risks are ICP and PG; therefore, it is critical for health care providers to diagnose these dermatoses in a timely manner and initiate appropriate management. Intrahepatic cholestasis of pregnancy is confirmed by elevated serum bile acids (ie, >10 µmol/L), often during the third trimester. The risk of fetal morbidity is high in ICP with increased bile acids crossing the placenta causing placental anoxia and impaired cardiomyocyte function.4 Fetal risks, including preterm delivery, meconium-stained amniotic fluid, and stillbirth, correlate with the level of bile acids in the serum.5 Maternal prognosis is favorable, but there is an increased association with hepatitis C and hepatobiliary disease.6
Diagnosis of PG is confirmed by classic biopsy results and direct immunofluorescence revealing C3 with or without IgG in a linear band along the basement membrane zone. Additionally, complement indirect immunofluorescence reveals circulating IgG anti–basement membrane zone antibodies. Pemphigoid gestationis is associated with increased fetal risks of preterm labor and intrauterine growth retardation.7 Clinical findings of PG may present in the fetus upon delivery due to transmission of autoantibodies across the placenta. The symptoms usually are mild.8 An increased risk of Graves disease has been reported in mothers with PG.
In most cases, diagnosis of PEP is based on history and morphology, but if the presentation is not classic, skin biopsy must be used to differentiate it from PG as well as more common dermatologic conditions such as contact dermatitis, drug and viral eruptions, and urticaria.
Atopic eruption of pregnancy manifests as widespread eczematous excoriated papules and plaques. Lesions of prurigo nodularis are common.
Comorbidities
It is important to be aware of specific clinical associations related to pregnancy-specific dermatoses. Pemphigoid gestationis has been associated with gestational trophoblastic tumors including hydatiform mole and choriocarcinoma.4 An increased risk for Graves disease has been reported in patients with PG.9 Patients who develop ICP have a higher incidence of hepatitis C, postpartum cholecystitis, gallstones, and nonalcoholic cirrhosis.8 Polymorphic eruption of pregnancy is associated with a notably higher incidence in multiple gestation pregnancies.2
Treatment and Management
Management of ICP requires an accurate and timely diagnosis, and advanced neonatal-obstetric management is critical.3 Ursodeoxycholic acid is the treatment of choice and reduces pruritus, prolongs pregnancy, and reduces fetal risk.4 Most stillbirths cluster at the 38th week of pregnancy, and patients with ICP and highly elevated serum bile acids (>40 µmol/L) should be considered for delivery at 37 weeks or earlier.5
Management of the other cutaneous disorders of pregnancy can be challenging for health care providers based on safety concerns for the fetus. Although it is important to minimize risks to the fetus, it also is important to adequately treat the mother’s cutaneous disease, which requires a solid knowledge of drug safety during pregnancy. The former US Food and Drug Administration classification system using A, B, C, D, and X pregnancy categories was replaced by the Pregnancy Lactation Label Final Rule, which provides counseling on medication safety during pregnancy.10 In 2014, Murase et al11 published a review of dermatologic medication safety during pregnancy, which serves as an excellent guide.
Before instituting treatment, the therapeutic plan should be discussed with the physician managing the patient’s pregnancy. In general, topical steroids are considered safe during pregnancy, and low-potency to moderate-potency topical steroids are preferred. If possible, use of topical steroids should be limited to less than 300 g for the duration of the pregnancy. Fluticasone propionate should be avoided during pregnancy because it is not metabolized by the placenta. When systemic steroids are considered appropriate for management during pregnancy, nonhalogenated corticosteroids such as prednisone and prednisolone are preferred because they are enzymatically inactivated by the placenta, which results in a favorable maternal-fetal gradient.12 There has been concern expressed in the medical literature that systemic steroids during the first trimester may increase the risk of cleft lip and cleft palate.3,12 When managing pregnancy dermatoses, consideration should be given to keep prednisone exposure below 20 mg/d, and try to limit prolonged use to 7.5 mg/d. However, this may not be possible in PG.3 Vitamin D and calcium supplementation may be appropriate when patients are on prolonged systemic steroids to control disease.
Antihistamines can be used to control pruritus complicating pregnancy-associated dermatoses. First-generation antihistamines such as chlorpheniramine and diphenhydramine are preferred due to long-term safety data.3,11,12 Loratadine is the first choice and cetirizine is the second choice if a second-generation antihistamine is preferred.3 Loratadine is preferred during breastfeeding due to less sedation.12 High-dose antihistamines prior to delivery may cause concerns for potential side effects in the newborn, including tremulousness, irritability, and poor feeding.
Recurrence
Women with pregnancy dermatoses often are concerned about recurrence with future pregnancies. Pemphigoid gestationis may flare with subsequent pregnancies, subsequent menses, or with oral contraceptive use.3 Recurrence of PEP in subsequent pregnancies is rare and usually is less severe than the primary eruption.8 Often, the rare recurrent eruption of PEP is associated with multigestational pregnancies.2 Mothers can anticipate a recurrence of ICP in up to 60% to 70% of future pregnancies. Patients with AEP have an underlying atopic diathesis, and recurrence in future pregnancies is not uncommon.8
Final Thoughts
In summary, it is important for health care providers to recognize the specific cutaneous disorders of pregnancy and their potential fetal complications. The anatomical location of onset of the dermatosis and timing of onset during pregnancy can give important clues. Appropriate management, especially with ICP, can minimize fetal complications. A fundamental knowledge of medication safety and management during pregnancy is essential. Rashes during pregnancy can cause anxiety in the mother and family and require support, comfort, and guidance.
- Ambrose-Rudolph CM, Müllegger RR, Vaughn-Jones SA, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54:395-404.
- Bechtel M, Plotner A. Dermatoses of pregnancy. Clin Obstet Gynecol. 2015;58:104-111.
- Bechtel M. Pruritus in pregnancy and its management. Dermatol Clin. 2018;36:259-265.
- Ambrose-Rudolph CM. Dermatoses of pregnancy—clues to diagnosis, fetal risk, and therapy. Ann Dermatol. 2011;23:265-275.
- Geenes V, Chappell LC, Seed PT, et al. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-controlled study. Hepatology. 2014;59:1482-1491.
- Bergman H, Melamed N, Koven G. Pruritus in pregnancy: treatment of dermatoses unique to pregnancy. Can Fam Physician. 2013;59:1290-1294.
- Beard MP, Millington GW. Recent developments in the specific dermatoses of pregnancy. Clin Exp Dermatol. 2012;37:1-14.
- Shears S, Blaszczak A, Kaffenberger J. Pregnancy dermatosis. In: Tyler KH, ed. Cutaneous Disorders of Pregnancy. 1st ed. Springer Nature; 2020:13-39.
- Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2015;26:274-284.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Fed Registr. 2014;79:72064-72103. To be codified at 21 CFR § 201.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part 1. pregnancy. J Am Acad Dermatol. 2014;401:E1-E14.
- Friedman B, Bercovitch L. Atopic dermatitis in pregnancy. In: Tyler KH, ed. Cutaneous Disorders of Pregnancy. Springer Nature; 2020:59-74.
- Ambrose-Rudolph CM, Müllegger RR, Vaughn-Jones SA, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54:395-404.
- Bechtel M, Plotner A. Dermatoses of pregnancy. Clin Obstet Gynecol. 2015;58:104-111.
- Bechtel M. Pruritus in pregnancy and its management. Dermatol Clin. 2018;36:259-265.
- Ambrose-Rudolph CM. Dermatoses of pregnancy—clues to diagnosis, fetal risk, and therapy. Ann Dermatol. 2011;23:265-275.
- Geenes V, Chappell LC, Seed PT, et al. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-controlled study. Hepatology. 2014;59:1482-1491.
- Bergman H, Melamed N, Koven G. Pruritus in pregnancy: treatment of dermatoses unique to pregnancy. Can Fam Physician. 2013;59:1290-1294.
- Beard MP, Millington GW. Recent developments in the specific dermatoses of pregnancy. Clin Exp Dermatol. 2012;37:1-14.
- Shears S, Blaszczak A, Kaffenberger J. Pregnancy dermatosis. In: Tyler KH, ed. Cutaneous Disorders of Pregnancy. 1st ed. Springer Nature; 2020:13-39.
- Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2015;26:274-284.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Fed Registr. 2014;79:72064-72103. To be codified at 21 CFR § 201.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part 1. pregnancy. J Am Acad Dermatol. 2014;401:E1-E14.
- Friedman B, Bercovitch L. Atopic dermatitis in pregnancy. In: Tyler KH, ed. Cutaneous Disorders of Pregnancy. Springer Nature; 2020:59-74.
Dupilumab-Induced Facial Flushing After Alcohol Consumption
Dupilumab is a fully humanized monoclonal antibody to the α subunit of the IL-4 receptor that inhibits the action of helper T cell (TH2)–type cytokines IL-4 and IL-13. Dupilumab was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe atopic dermatitis (AD). We report 2 patients with AD who were treated with dupilumab and subsequently developed facial flushing after consuming alcohol.
Case Report
Patient 1
A 24-year-old woman presented to the dermatology clinic with a lifelong history of moderate to severe AD. She had a medical history of asthma and seasonal allergies, which were treated with fexofenadine and an inhaler, as needed. The patient had an affected body surface area of approximately 70% and had achieved only partial relief with topical corticosteroids and topical calcineurin inhibitors.
Because her disease was severe, the patient was started on dupilumab at FDA-approved dosing for AD: a 600-mg subcutaneous (SC) loading dose, followed by 300 mg SC every 2 weeks. She reported rapid skin clearance within 2 weeks of the start of treatment. Her course was complicated by mild head and neck dermatitis.
Seven months after starting treatment, the patient began to acutely experience erythema and warmth over the entire face that was triggered by drinking alcohol (Figure). Before starting dupilumab, she had consumed alcohol on multiple occasions without a flushing effect. This new finding was distinguishable from her facial dermatitis. Onset was within a few minutes after drinking alcohol; flushing self-resolved in 15 to 30 minutes. Although diffuse, erythema and warmth were concentrated around the jawline, eyebrows, and ears and occurred every time the patient drank alcohol. Moreover, she reported that consumption of hard (ie, distilled) liquor, specifically tequila, caused a more severe presentation. She denied other symptoms associated with dupilumab.
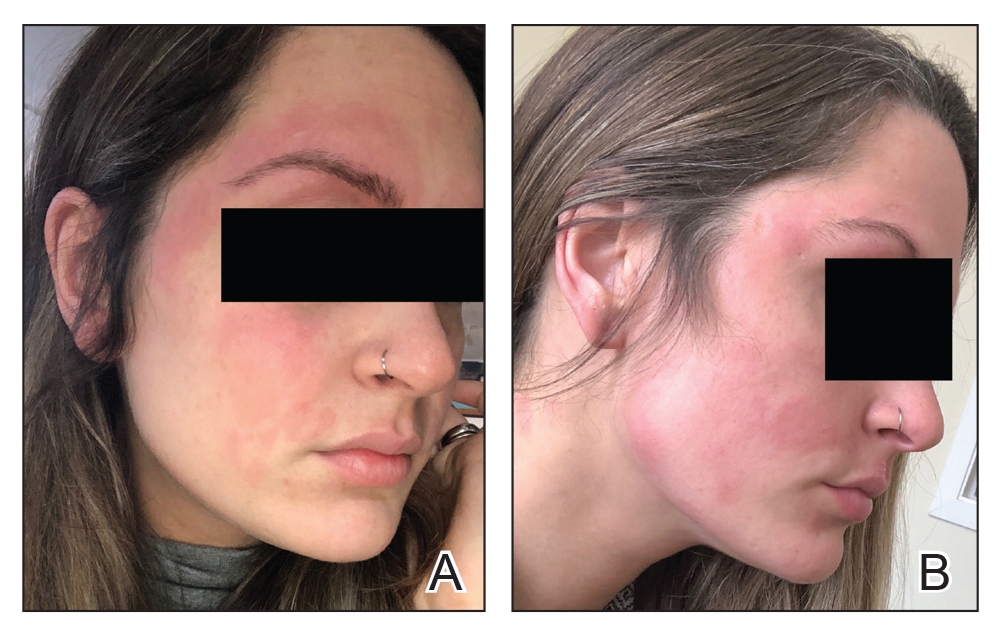
Patient 2
A 32-year-old man presented to the dermatology clinic with a 10-year history of moderate to severe AD. He had a medical history of asthma (treated with albuterol, montelukast, and fluticasone); allergic rhinitis; and severe environmental allergies, including sensitivity to dust mites, dogs, trees, and grass.
For AD, the patient had been treated with topical corticosteroids and the Goeckerman regimen (a combination of phototherapy and crude coal tar). He experienced only partial relief with topical corticosteroids; the Goeckerman regimen cleared his skin, but he had quick recurrence after approximately 1 month. Given his work schedule, the patient was unable to resume phototherapy.
Because of symptoms related to the patient’s severe allergies, his allergist prescribed dupilumab: a 600-mg SC loading dose, followed by 300 mg SC every 2 weeks. The patient reported near-complete resolution of AD symptoms approximately 2 months after initiating treatment. He reported a few episodes of mild conjunctivitis that self-resolved after the first month of treatment.
Three weeks after initiating dupilumab, the patient noticed new-onset facial flushing in response to consuming alcohol. He described flushing as sudden immediate redness and warmth concentrated around the forehead, eyes, and cheeks. He reported that flushing was worse with hard liquor than with beer. Flushing would slowly subside over approximately 30 minutes despite continued alcohol consumption.
Comment
Two other single-patient case reports have discussed similar findings of alcohol-induced flushing associated with dupilumab.1,2 Both of those patients—a 19-year-old woman and a 26-year-old woman—had not experienced flushing before beginning treatment with dupilumab for AD. Both experienced onset of facial flushing months after beginning dupilumab even though both had consumed alcohol before starting dupilumab, similar to the cases presented here. One patient had a history of asthma; the other had a history of seasonal and environmental allergies.
Possible Mechanism of Action
Acute alcohol ingestion causes dermal vasodilation of the skin (ie, flushing).3 A proposed mechanism is that flushing results from direct action on central vascular-control mechanisms. This theory results from observations that individuals with quadriplegia lack notable ethanol-induced vasodilation, suggesting that ethanol has a central neural site of action.Although some research has indicated that ethanol might induce these effects by altering the action of certain hormones (eg, angiotensin, vasopressin, and catecholamines), the precise mechanism by which ethanol alters vascular function in humans remains unexplained.3
Deficiencies in alcohol dehydrogenase (ADH), aldehyde dehydrogenase 2, and certain cytochrome P450 enzymes also might contribute to facial flushing. People of Asian, especially East Asian, descent often respond to an acute dose of ethanol with symptoms of facial flushing—predominantly the result of an elevated blood level of acetaldehyde caused by an inherited deficiency of aldehyde dehydrogenase 2,4 which is downstream from ADH in the metabolic pathway of alcohol. The major enzyme system responsible for metabolism of ethanol is ADH; however, the cytochrome P450–dependent ethanol-oxidizing system—including major CYP450 isoforms CYP3A, CYP2C19, CYP2C9, CYP1A2, and CYP2D6, as well as minor CYP450 isoforms, such as CYP2E1— also are involved, to a lesser extent.5
A Role for Dupilumab?
A recent pharmacokinetic study found that dupilumab appears to have little effect on the activity of the major CYP450 isoforms. However, the drug’s effect on ADH and minor CYP450 minor isoforms is unknown. Prior drug-drug interaction studies have shown that certain cytokines and cytokine modulators can markedly influence the expression, stability, and activity of specific CYP450 enzymes.6 For example, IL-6 causes a reduction in messenger RNA for CYP3A4 and, to a lesser extent, for other isoforms.7 Whether dupilumab influences enzymes involved in processing alcohol requires further study.
Conclusion
We describe 2 cases of dupilumab-induced facial flushing after alcohol consumption. The mechanism of this dupilumab-associated flushing is unknown and requires further research.
- Herz S, Petri M, Sondermann W. New alcohol flushing in a patient with atopic dermatitis under therapy with dupilumab. Dermatol Ther. 2019;32:e12762. doi:10.1111/dth.12762
- Igelman SJ, Na C, Simpson EL. Alcohol-induced facial flushing in a patient with atopic dermatitis treated with dupilumab. JAAD Case Rep. 2020;6:139-140. doi:10.1016/j.jdcr.2019.12.002
- Malpas SC, Robinson BJ, Maling TJ. Mechanism of ethanol-induced vasodilation. J Appl Physiol (1985). 1990;68:731-734. doi:10.1152/jappl.1990.68.2.731
- Brooks PJ, Enoch M-A, Goldman D, et al. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6:e50. doi:10.1371/journal.pmed.1000050
- Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16:667-685. doi:10.1016/j.cld.2012.08.002
- Davis JD, Bansal A, Hassman D, et al. Evaluation of potential disease-mediated drug-drug interaction in patients with moderate-to-severe atopic dermatitis receiving dupilumab. Clin Pharmacol Ther. 2018;104:1146-1154. doi:10.1002/cpt.1058
- Mimura H, Kobayashi K, Xu L, et al. Effects of cytokines on CYP3A4 expression and reversal of the effects by anti-cytokine agents in the three-dimensionally cultured human hepatoma cell line FLC-4. Drug Metab Pharmacokinet. 2015;30:105-110. doi:10.1016/j.dmpk.2014.09.004
Dupilumab is a fully humanized monoclonal antibody to the α subunit of the IL-4 receptor that inhibits the action of helper T cell (TH2)–type cytokines IL-4 and IL-13. Dupilumab was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe atopic dermatitis (AD). We report 2 patients with AD who were treated with dupilumab and subsequently developed facial flushing after consuming alcohol.
Case Report
Patient 1
A 24-year-old woman presented to the dermatology clinic with a lifelong history of moderate to severe AD. She had a medical history of asthma and seasonal allergies, which were treated with fexofenadine and an inhaler, as needed. The patient had an affected body surface area of approximately 70% and had achieved only partial relief with topical corticosteroids and topical calcineurin inhibitors.
Because her disease was severe, the patient was started on dupilumab at FDA-approved dosing for AD: a 600-mg subcutaneous (SC) loading dose, followed by 300 mg SC every 2 weeks. She reported rapid skin clearance within 2 weeks of the start of treatment. Her course was complicated by mild head and neck dermatitis.
Seven months after starting treatment, the patient began to acutely experience erythema and warmth over the entire face that was triggered by drinking alcohol (Figure). Before starting dupilumab, she had consumed alcohol on multiple occasions without a flushing effect. This new finding was distinguishable from her facial dermatitis. Onset was within a few minutes after drinking alcohol; flushing self-resolved in 15 to 30 minutes. Although diffuse, erythema and warmth were concentrated around the jawline, eyebrows, and ears and occurred every time the patient drank alcohol. Moreover, she reported that consumption of hard (ie, distilled) liquor, specifically tequila, caused a more severe presentation. She denied other symptoms associated with dupilumab.

Patient 2
A 32-year-old man presented to the dermatology clinic with a 10-year history of moderate to severe AD. He had a medical history of asthma (treated with albuterol, montelukast, and fluticasone); allergic rhinitis; and severe environmental allergies, including sensitivity to dust mites, dogs, trees, and grass.
For AD, the patient had been treated with topical corticosteroids and the Goeckerman regimen (a combination of phototherapy and crude coal tar). He experienced only partial relief with topical corticosteroids; the Goeckerman regimen cleared his skin, but he had quick recurrence after approximately 1 month. Given his work schedule, the patient was unable to resume phototherapy.
Because of symptoms related to the patient’s severe allergies, his allergist prescribed dupilumab: a 600-mg SC loading dose, followed by 300 mg SC every 2 weeks. The patient reported near-complete resolution of AD symptoms approximately 2 months after initiating treatment. He reported a few episodes of mild conjunctivitis that self-resolved after the first month of treatment.
Three weeks after initiating dupilumab, the patient noticed new-onset facial flushing in response to consuming alcohol. He described flushing as sudden immediate redness and warmth concentrated around the forehead, eyes, and cheeks. He reported that flushing was worse with hard liquor than with beer. Flushing would slowly subside over approximately 30 minutes despite continued alcohol consumption.
Comment
Two other single-patient case reports have discussed similar findings of alcohol-induced flushing associated with dupilumab.1,2 Both of those patients—a 19-year-old woman and a 26-year-old woman—had not experienced flushing before beginning treatment with dupilumab for AD. Both experienced onset of facial flushing months after beginning dupilumab even though both had consumed alcohol before starting dupilumab, similar to the cases presented here. One patient had a history of asthma; the other had a history of seasonal and environmental allergies.
Possible Mechanism of Action
Acute alcohol ingestion causes dermal vasodilation of the skin (ie, flushing).3 A proposed mechanism is that flushing results from direct action on central vascular-control mechanisms. This theory results from observations that individuals with quadriplegia lack notable ethanol-induced vasodilation, suggesting that ethanol has a central neural site of action.Although some research has indicated that ethanol might induce these effects by altering the action of certain hormones (eg, angiotensin, vasopressin, and catecholamines), the precise mechanism by which ethanol alters vascular function in humans remains unexplained.3
Deficiencies in alcohol dehydrogenase (ADH), aldehyde dehydrogenase 2, and certain cytochrome P450 enzymes also might contribute to facial flushing. People of Asian, especially East Asian, descent often respond to an acute dose of ethanol with symptoms of facial flushing—predominantly the result of an elevated blood level of acetaldehyde caused by an inherited deficiency of aldehyde dehydrogenase 2,4 which is downstream from ADH in the metabolic pathway of alcohol. The major enzyme system responsible for metabolism of ethanol is ADH; however, the cytochrome P450–dependent ethanol-oxidizing system—including major CYP450 isoforms CYP3A, CYP2C19, CYP2C9, CYP1A2, and CYP2D6, as well as minor CYP450 isoforms, such as CYP2E1— also are involved, to a lesser extent.5
A Role for Dupilumab?
A recent pharmacokinetic study found that dupilumab appears to have little effect on the activity of the major CYP450 isoforms. However, the drug’s effect on ADH and minor CYP450 minor isoforms is unknown. Prior drug-drug interaction studies have shown that certain cytokines and cytokine modulators can markedly influence the expression, stability, and activity of specific CYP450 enzymes.6 For example, IL-6 causes a reduction in messenger RNA for CYP3A4 and, to a lesser extent, for other isoforms.7 Whether dupilumab influences enzymes involved in processing alcohol requires further study.
Conclusion
We describe 2 cases of dupilumab-induced facial flushing after alcohol consumption. The mechanism of this dupilumab-associated flushing is unknown and requires further research.
Dupilumab is a fully humanized monoclonal antibody to the α subunit of the IL-4 receptor that inhibits the action of helper T cell (TH2)–type cytokines IL-4 and IL-13. Dupilumab was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe atopic dermatitis (AD). We report 2 patients with AD who were treated with dupilumab and subsequently developed facial flushing after consuming alcohol.
Case Report
Patient 1
A 24-year-old woman presented to the dermatology clinic with a lifelong history of moderate to severe AD. She had a medical history of asthma and seasonal allergies, which were treated with fexofenadine and an inhaler, as needed. The patient had an affected body surface area of approximately 70% and had achieved only partial relief with topical corticosteroids and topical calcineurin inhibitors.
Because her disease was severe, the patient was started on dupilumab at FDA-approved dosing for AD: a 600-mg subcutaneous (SC) loading dose, followed by 300 mg SC every 2 weeks. She reported rapid skin clearance within 2 weeks of the start of treatment. Her course was complicated by mild head and neck dermatitis.
Seven months after starting treatment, the patient began to acutely experience erythema and warmth over the entire face that was triggered by drinking alcohol (Figure). Before starting dupilumab, she had consumed alcohol on multiple occasions without a flushing effect. This new finding was distinguishable from her facial dermatitis. Onset was within a few minutes after drinking alcohol; flushing self-resolved in 15 to 30 minutes. Although diffuse, erythema and warmth were concentrated around the jawline, eyebrows, and ears and occurred every time the patient drank alcohol. Moreover, she reported that consumption of hard (ie, distilled) liquor, specifically tequila, caused a more severe presentation. She denied other symptoms associated with dupilumab.

Patient 2
A 32-year-old man presented to the dermatology clinic with a 10-year history of moderate to severe AD. He had a medical history of asthma (treated with albuterol, montelukast, and fluticasone); allergic rhinitis; and severe environmental allergies, including sensitivity to dust mites, dogs, trees, and grass.
For AD, the patient had been treated with topical corticosteroids and the Goeckerman regimen (a combination of phototherapy and crude coal tar). He experienced only partial relief with topical corticosteroids; the Goeckerman regimen cleared his skin, but he had quick recurrence after approximately 1 month. Given his work schedule, the patient was unable to resume phototherapy.
Because of symptoms related to the patient’s severe allergies, his allergist prescribed dupilumab: a 600-mg SC loading dose, followed by 300 mg SC every 2 weeks. The patient reported near-complete resolution of AD symptoms approximately 2 months after initiating treatment. He reported a few episodes of mild conjunctivitis that self-resolved after the first month of treatment.
Three weeks after initiating dupilumab, the patient noticed new-onset facial flushing in response to consuming alcohol. He described flushing as sudden immediate redness and warmth concentrated around the forehead, eyes, and cheeks. He reported that flushing was worse with hard liquor than with beer. Flushing would slowly subside over approximately 30 minutes despite continued alcohol consumption.
Comment
Two other single-patient case reports have discussed similar findings of alcohol-induced flushing associated with dupilumab.1,2 Both of those patients—a 19-year-old woman and a 26-year-old woman—had not experienced flushing before beginning treatment with dupilumab for AD. Both experienced onset of facial flushing months after beginning dupilumab even though both had consumed alcohol before starting dupilumab, similar to the cases presented here. One patient had a history of asthma; the other had a history of seasonal and environmental allergies.
Possible Mechanism of Action
Acute alcohol ingestion causes dermal vasodilation of the skin (ie, flushing).3 A proposed mechanism is that flushing results from direct action on central vascular-control mechanisms. This theory results from observations that individuals with quadriplegia lack notable ethanol-induced vasodilation, suggesting that ethanol has a central neural site of action.Although some research has indicated that ethanol might induce these effects by altering the action of certain hormones (eg, angiotensin, vasopressin, and catecholamines), the precise mechanism by which ethanol alters vascular function in humans remains unexplained.3
Deficiencies in alcohol dehydrogenase (ADH), aldehyde dehydrogenase 2, and certain cytochrome P450 enzymes also might contribute to facial flushing. People of Asian, especially East Asian, descent often respond to an acute dose of ethanol with symptoms of facial flushing—predominantly the result of an elevated blood level of acetaldehyde caused by an inherited deficiency of aldehyde dehydrogenase 2,4 which is downstream from ADH in the metabolic pathway of alcohol. The major enzyme system responsible for metabolism of ethanol is ADH; however, the cytochrome P450–dependent ethanol-oxidizing system—including major CYP450 isoforms CYP3A, CYP2C19, CYP2C9, CYP1A2, and CYP2D6, as well as minor CYP450 isoforms, such as CYP2E1— also are involved, to a lesser extent.5
A Role for Dupilumab?
A recent pharmacokinetic study found that dupilumab appears to have little effect on the activity of the major CYP450 isoforms. However, the drug’s effect on ADH and minor CYP450 minor isoforms is unknown. Prior drug-drug interaction studies have shown that certain cytokines and cytokine modulators can markedly influence the expression, stability, and activity of specific CYP450 enzymes.6 For example, IL-6 causes a reduction in messenger RNA for CYP3A4 and, to a lesser extent, for other isoforms.7 Whether dupilumab influences enzymes involved in processing alcohol requires further study.
Conclusion
We describe 2 cases of dupilumab-induced facial flushing after alcohol consumption. The mechanism of this dupilumab-associated flushing is unknown and requires further research.
- Herz S, Petri M, Sondermann W. New alcohol flushing in a patient with atopic dermatitis under therapy with dupilumab. Dermatol Ther. 2019;32:e12762. doi:10.1111/dth.12762
- Igelman SJ, Na C, Simpson EL. Alcohol-induced facial flushing in a patient with atopic dermatitis treated with dupilumab. JAAD Case Rep. 2020;6:139-140. doi:10.1016/j.jdcr.2019.12.002
- Malpas SC, Robinson BJ, Maling TJ. Mechanism of ethanol-induced vasodilation. J Appl Physiol (1985). 1990;68:731-734. doi:10.1152/jappl.1990.68.2.731
- Brooks PJ, Enoch M-A, Goldman D, et al. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6:e50. doi:10.1371/journal.pmed.1000050
- Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16:667-685. doi:10.1016/j.cld.2012.08.002
- Davis JD, Bansal A, Hassman D, et al. Evaluation of potential disease-mediated drug-drug interaction in patients with moderate-to-severe atopic dermatitis receiving dupilumab. Clin Pharmacol Ther. 2018;104:1146-1154. doi:10.1002/cpt.1058
- Mimura H, Kobayashi K, Xu L, et al. Effects of cytokines on CYP3A4 expression and reversal of the effects by anti-cytokine agents in the three-dimensionally cultured human hepatoma cell line FLC-4. Drug Metab Pharmacokinet. 2015;30:105-110. doi:10.1016/j.dmpk.2014.09.004
- Herz S, Petri M, Sondermann W. New alcohol flushing in a patient with atopic dermatitis under therapy with dupilumab. Dermatol Ther. 2019;32:e12762. doi:10.1111/dth.12762
- Igelman SJ, Na C, Simpson EL. Alcohol-induced facial flushing in a patient with atopic dermatitis treated with dupilumab. JAAD Case Rep. 2020;6:139-140. doi:10.1016/j.jdcr.2019.12.002
- Malpas SC, Robinson BJ, Maling TJ. Mechanism of ethanol-induced vasodilation. J Appl Physiol (1985). 1990;68:731-734. doi:10.1152/jappl.1990.68.2.731
- Brooks PJ, Enoch M-A, Goldman D, et al. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6:e50. doi:10.1371/journal.pmed.1000050
- Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16:667-685. doi:10.1016/j.cld.2012.08.002
- Davis JD, Bansal A, Hassman D, et al. Evaluation of potential disease-mediated drug-drug interaction in patients with moderate-to-severe atopic dermatitis receiving dupilumab. Clin Pharmacol Ther. 2018;104:1146-1154. doi:10.1002/cpt.1058
- Mimura H, Kobayashi K, Xu L, et al. Effects of cytokines on CYP3A4 expression and reversal of the effects by anti-cytokine agents in the three-dimensionally cultured human hepatoma cell line FLC-4. Drug Metab Pharmacokinet. 2015;30:105-110. doi:10.1016/j.dmpk.2014.09.004
Practice Points
- Dupilumab is a fully humanized monoclonal antibody that inhibits the action of IL-4 and IL-13. It was approved by the US Food and Drug Administration in 2017 for treatment of moderate to severe atopic dermatitis.
- Facial flushing after alcohol consumption may be an emerging side effect of dupilumab.
- Whether dupilumab influences enzymes involved in processing alcohol requires further study.
An Algorithm for Managing Spitting Sutures
Practice Gap
It is well established that surgical complications and a poor scar outcome can have a remarkable impact on patient satisfaction.1 A common complication following dermatologic surgery is suture spitting, in which a buried suture is extruded through the skin surface. When repairing a cutaneous defect following dermatologic surgery, absorbable or nonabsorbable sutures are placed under the skin surface to approximate wound edges, eliminate dead space, and reduce tension on the edges of the wound, improving the cosmetic outcomes.
Absorbable sutures constitute most buried sutures in cutaneous surgery and can be made of natural or synthetic fibers.2 Absorbable sutures made from synthetic fibers are degraded by hydrolysis, in which water breaks down polymer chains of the suture filament. Natural absorbable sutures are composed of mammalian collagen; they are broken down by the enzymatic process of proteolysis.
Tensile strength is lost long before a suture is fully absorbed. Although synthetic fibers have, in general, higher tensile strength and generate less tissue inflammation, they take much longer to absorb.2 During absorption, in some cases, a buried suture is pushed to the surface and extrudes along the wound edge or scar, which is known as spitting3 (Figure 1).
Suture spitting typically occurs in the 2-week to 3-month postoperative period. However, with the use of long-lasting absorbable or nonabsorbable sutures, spitting can occur several months or years postoperatively. Spitting sutures often are associated with surrounding erythema, edema, discharge, and a foreign-body sensation4—symptoms that can be highly distressing to the patient and can lead to postoperative infection or stitch abscess.3
Herein, we review techniques that can decrease the risk for suture spitting, and we present a stepwise approach to managing this common problem.
The Technique
Choice of suture material for buried sutures can influence the risk of spitting.
Factors Impacting Increased Spitting
The 3 most common absorbable sutures in dermatologic surgery include poliglecaprone 25, polyglactin 910, and polydioxanone; of them, polyglactin 910 has been found to have a higher rate of spitting than poliglecaprone 25 and polydioxanone.2 However, because complete absorption of polydioxanone can take as long as 8 months, this suture might “spit” much later than polyglactin 910 or poliglecaprone 25, which typically are fully hydrolyzed by 3 and 4 months, respectively.2 Placing sutures superficially in the dermis has been found to increase the rate of spitting.5 Throwing more knots per closure also has been found to increase the rate of spitting.5
How to Decrease Spitting
Careful choice of suture material and proper depth of suture placement might decrease the risk for spitting in dermatologic surgery. Furthermore, if polyglactin 910 or a long-lasting suture is to be used, sutures should be placed deeply.
What to Do If Sutures Spit
When a suture has begun to spit, the extruding foreign material needs to be removed and the surgical site assessed for infection or abscess. Exposed suture material typically can be removed with forceps without local anesthesia. In some cases, fine-tipped Bishop-Harmon tissue forceps or jewelers forceps might be required.
If the suture cannot be removed completely, it should be trimmed as short as possible. This can be accomplished by pulling on the exposed end of the suture, tenting the skin, and trimming it as close as possible to the surface. Once the foreign material is removed, assessment for signs of infection is paramount.
How to Manage Infection—Postoperative infection associated with a spitting suture can take the form of a periwound cellulitis or stitch abscess.3 A stitch abscess can reflect a sterile inflammatory response to the buried suture or a true infection4; the former is more common.3 In the event of an infected stitch abscess, provide warm compresses, obtain specimens for culture, and prescribe antibiotics after the spitting suture has been removed. Incision and drainage also might be required if notable fluctuance is present.
It is crucial for dermatologic surgeons to identify and manage these complications. Figure 2 illustrates an algorithmic approach to managing spitting sutures.
Practical Implications
Spitting sutures are a common occurrence following dermatologic surgery that can lead to remarkable patient distress. Fortunately, in the absence of superimposed infection, spitting sutures have not been shown to worsen outcomes of healing and scarring.5 Nevertheless, it is important to identify and appropriately treat this common complication. The simple algorithm we provide (Figure 2) aids in cutaneous surgery by providing a straightforward approach to managing spitting sutures and their complications.
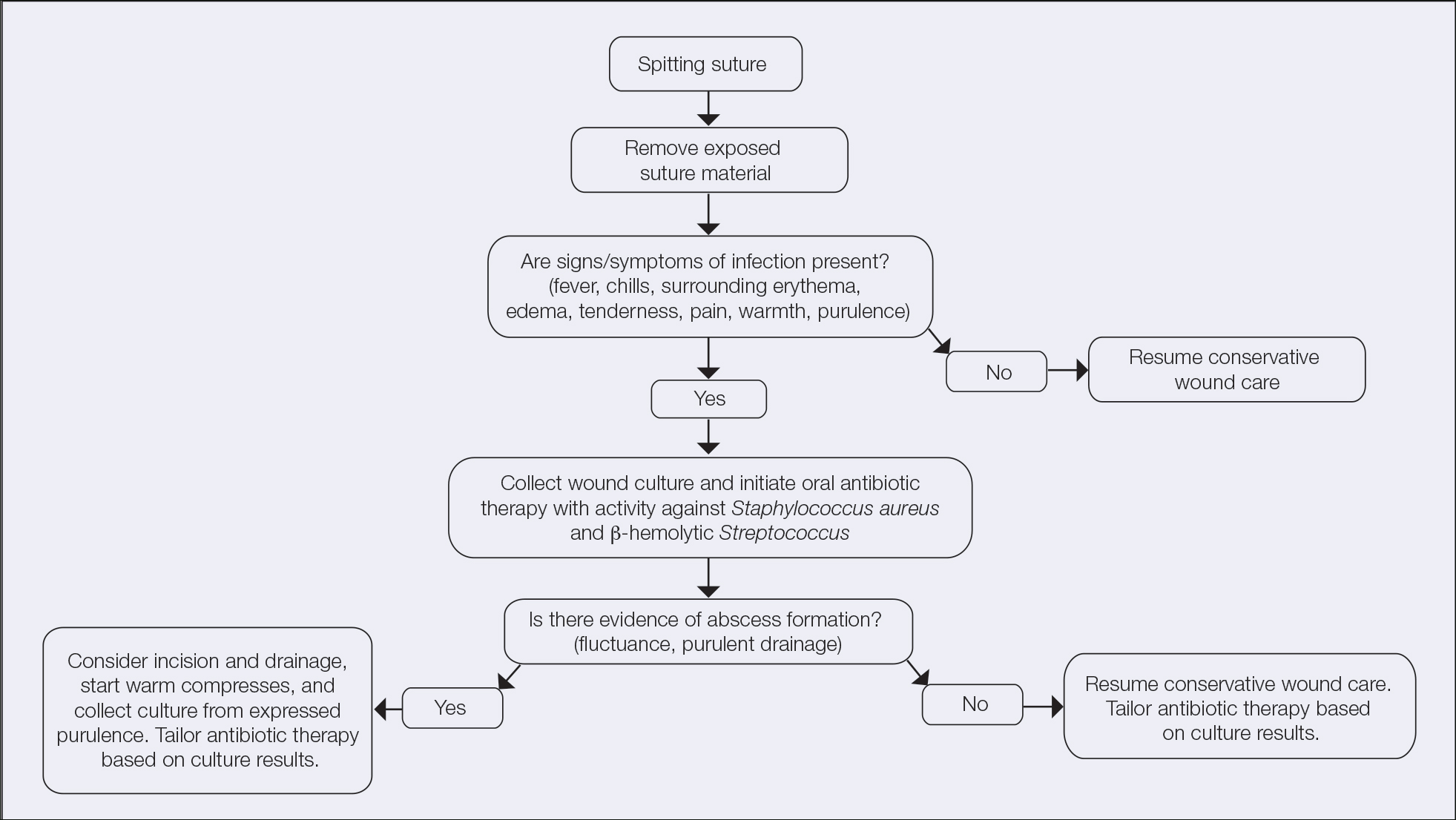
- Balaraman B, Geddes ER, Friedman PM. Best reconstructive techniques: improving the final scar. Dermatol Surg. 2015;41(suppl 10):S265-S275. doi:10.1097/DSS.0000000000000496
- Yag-Howard C. Sutures, needles, and tissue adhesives: a review for dermatologic surgery. Dermatol Surg. 2014;40(suppl 9):S3-S15. doi:10.1097/01.DSS.0000452738.23278.2d
- Gloster HM. Complications in Cutaneous Surgery. Springer; 2011.
- Slutsky JB, Fosko ST. Complications in Mohs surgery. In: Berlin A, ed. Mohs and Cutaneous Surgery: Maximizing Aesthetic Outcomes. CRC Press; 2015:55-89.
- Kim B, Sgarioto M, Hewitt D, et al. Scar outcomes in dermatological surgery. Australas J Dermatol. 2018;59:48-51. doi:10.1111/ajd.12570
Practice Gap
It is well established that surgical complications and a poor scar outcome can have a remarkable impact on patient satisfaction.1 A common complication following dermatologic surgery is suture spitting, in which a buried suture is extruded through the skin surface. When repairing a cutaneous defect following dermatologic surgery, absorbable or nonabsorbable sutures are placed under the skin surface to approximate wound edges, eliminate dead space, and reduce tension on the edges of the wound, improving the cosmetic outcomes.
Absorbable sutures constitute most buried sutures in cutaneous surgery and can be made of natural or synthetic fibers.2 Absorbable sutures made from synthetic fibers are degraded by hydrolysis, in which water breaks down polymer chains of the suture filament. Natural absorbable sutures are composed of mammalian collagen; they are broken down by the enzymatic process of proteolysis.
Tensile strength is lost long before a suture is fully absorbed. Although synthetic fibers have, in general, higher tensile strength and generate less tissue inflammation, they take much longer to absorb.2 During absorption, in some cases, a buried suture is pushed to the surface and extrudes along the wound edge or scar, which is known as spitting3 (Figure 1).

Suture spitting typically occurs in the 2-week to 3-month postoperative period. However, with the use of long-lasting absorbable or nonabsorbable sutures, spitting can occur several months or years postoperatively. Spitting sutures often are associated with surrounding erythema, edema, discharge, and a foreign-body sensation4—symptoms that can be highly distressing to the patient and can lead to postoperative infection or stitch abscess.3
Herein, we review techniques that can decrease the risk for suture spitting, and we present a stepwise approach to managing this common problem.
The Technique
Choice of suture material for buried sutures can influence the risk of spitting.
Factors Impacting Increased Spitting
The 3 most common absorbable sutures in dermatologic surgery include poliglecaprone 25, polyglactin 910, and polydioxanone; of them, polyglactin 910 has been found to have a higher rate of spitting than poliglecaprone 25 and polydioxanone.2 However, because complete absorption of polydioxanone can take as long as 8 months, this suture might “spit” much later than polyglactin 910 or poliglecaprone 25, which typically are fully hydrolyzed by 3 and 4 months, respectively.2 Placing sutures superficially in the dermis has been found to increase the rate of spitting.5 Throwing more knots per closure also has been found to increase the rate of spitting.5
How to Decrease Spitting
Careful choice of suture material and proper depth of suture placement might decrease the risk for spitting in dermatologic surgery. Furthermore, if polyglactin 910 or a long-lasting suture is to be used, sutures should be placed deeply.
What to Do If Sutures Spit
When a suture has begun to spit, the extruding foreign material needs to be removed and the surgical site assessed for infection or abscess. Exposed suture material typically can be removed with forceps without local anesthesia. In some cases, fine-tipped Bishop-Harmon tissue forceps or jewelers forceps might be required.
If the suture cannot be removed completely, it should be trimmed as short as possible. This can be accomplished by pulling on the exposed end of the suture, tenting the skin, and trimming it as close as possible to the surface. Once the foreign material is removed, assessment for signs of infection is paramount.
How to Manage Infection—Postoperative infection associated with a spitting suture can take the form of a periwound cellulitis or stitch abscess.3 A stitch abscess can reflect a sterile inflammatory response to the buried suture or a true infection4; the former is more common.3 In the event of an infected stitch abscess, provide warm compresses, obtain specimens for culture, and prescribe antibiotics after the spitting suture has been removed. Incision and drainage also might be required if notable fluctuance is present.
It is crucial for dermatologic surgeons to identify and manage these complications. Figure 2 illustrates an algorithmic approach to managing spitting sutures.
Practical Implications
Spitting sutures are a common occurrence following dermatologic surgery that can lead to remarkable patient distress. Fortunately, in the absence of superimposed infection, spitting sutures have not been shown to worsen outcomes of healing and scarring.5 Nevertheless, it is important to identify and appropriately treat this common complication. The simple algorithm we provide (Figure 2) aids in cutaneous surgery by providing a straightforward approach to managing spitting sutures and their complications.

Practice Gap
It is well established that surgical complications and a poor scar outcome can have a remarkable impact on patient satisfaction.1 A common complication following dermatologic surgery is suture spitting, in which a buried suture is extruded through the skin surface. When repairing a cutaneous defect following dermatologic surgery, absorbable or nonabsorbable sutures are placed under the skin surface to approximate wound edges, eliminate dead space, and reduce tension on the edges of the wound, improving the cosmetic outcomes.
Absorbable sutures constitute most buried sutures in cutaneous surgery and can be made of natural or synthetic fibers.2 Absorbable sutures made from synthetic fibers are degraded by hydrolysis, in which water breaks down polymer chains of the suture filament. Natural absorbable sutures are composed of mammalian collagen; they are broken down by the enzymatic process of proteolysis.
Tensile strength is lost long before a suture is fully absorbed. Although synthetic fibers have, in general, higher tensile strength and generate less tissue inflammation, they take much longer to absorb.2 During absorption, in some cases, a buried suture is pushed to the surface and extrudes along the wound edge or scar, which is known as spitting3 (Figure 1).

Suture spitting typically occurs in the 2-week to 3-month postoperative period. However, with the use of long-lasting absorbable or nonabsorbable sutures, spitting can occur several months or years postoperatively. Spitting sutures often are associated with surrounding erythema, edema, discharge, and a foreign-body sensation4—symptoms that can be highly distressing to the patient and can lead to postoperative infection or stitch abscess.3
Herein, we review techniques that can decrease the risk for suture spitting, and we present a stepwise approach to managing this common problem.
The Technique
Choice of suture material for buried sutures can influence the risk of spitting.
Factors Impacting Increased Spitting
The 3 most common absorbable sutures in dermatologic surgery include poliglecaprone 25, polyglactin 910, and polydioxanone; of them, polyglactin 910 has been found to have a higher rate of spitting than poliglecaprone 25 and polydioxanone.2 However, because complete absorption of polydioxanone can take as long as 8 months, this suture might “spit” much later than polyglactin 910 or poliglecaprone 25, which typically are fully hydrolyzed by 3 and 4 months, respectively.2 Placing sutures superficially in the dermis has been found to increase the rate of spitting.5 Throwing more knots per closure also has been found to increase the rate of spitting.5
How to Decrease Spitting
Careful choice of suture material and proper depth of suture placement might decrease the risk for spitting in dermatologic surgery. Furthermore, if polyglactin 910 or a long-lasting suture is to be used, sutures should be placed deeply.
What to Do If Sutures Spit
When a suture has begun to spit, the extruding foreign material needs to be removed and the surgical site assessed for infection or abscess. Exposed suture material typically can be removed with forceps without local anesthesia. In some cases, fine-tipped Bishop-Harmon tissue forceps or jewelers forceps might be required.
If the suture cannot be removed completely, it should be trimmed as short as possible. This can be accomplished by pulling on the exposed end of the suture, tenting the skin, and trimming it as close as possible to the surface. Once the foreign material is removed, assessment for signs of infection is paramount.
How to Manage Infection—Postoperative infection associated with a spitting suture can take the form of a periwound cellulitis or stitch abscess.3 A stitch abscess can reflect a sterile inflammatory response to the buried suture or a true infection4; the former is more common.3 In the event of an infected stitch abscess, provide warm compresses, obtain specimens for culture, and prescribe antibiotics after the spitting suture has been removed. Incision and drainage also might be required if notable fluctuance is present.
It is crucial for dermatologic surgeons to identify and manage these complications. Figure 2 illustrates an algorithmic approach to managing spitting sutures.
Practical Implications
Spitting sutures are a common occurrence following dermatologic surgery that can lead to remarkable patient distress. Fortunately, in the absence of superimposed infection, spitting sutures have not been shown to worsen outcomes of healing and scarring.5 Nevertheless, it is important to identify and appropriately treat this common complication. The simple algorithm we provide (Figure 2) aids in cutaneous surgery by providing a straightforward approach to managing spitting sutures and their complications.

- Balaraman B, Geddes ER, Friedman PM. Best reconstructive techniques: improving the final scar. Dermatol Surg. 2015;41(suppl 10):S265-S275. doi:10.1097/DSS.0000000000000496
- Yag-Howard C. Sutures, needles, and tissue adhesives: a review for dermatologic surgery. Dermatol Surg. 2014;40(suppl 9):S3-S15. doi:10.1097/01.DSS.0000452738.23278.2d
- Gloster HM. Complications in Cutaneous Surgery. Springer; 2011.
- Slutsky JB, Fosko ST. Complications in Mohs surgery. In: Berlin A, ed. Mohs and Cutaneous Surgery: Maximizing Aesthetic Outcomes. CRC Press; 2015:55-89.
- Kim B, Sgarioto M, Hewitt D, et al. Scar outcomes in dermatological surgery. Australas J Dermatol. 2018;59:48-51. doi:10.1111/ajd.12570
- Balaraman B, Geddes ER, Friedman PM. Best reconstructive techniques: improving the final scar. Dermatol Surg. 2015;41(suppl 10):S265-S275. doi:10.1097/DSS.0000000000000496
- Yag-Howard C. Sutures, needles, and tissue adhesives: a review for dermatologic surgery. Dermatol Surg. 2014;40(suppl 9):S3-S15. doi:10.1097/01.DSS.0000452738.23278.2d
- Gloster HM. Complications in Cutaneous Surgery. Springer; 2011.
- Slutsky JB, Fosko ST. Complications in Mohs surgery. In: Berlin A, ed. Mohs and Cutaneous Surgery: Maximizing Aesthetic Outcomes. CRC Press; 2015:55-89.
- Kim B, Sgarioto M, Hewitt D, et al. Scar outcomes in dermatological surgery. Australas J Dermatol. 2018;59:48-51. doi:10.1111/ajd.12570
Patch Test–Directed Dietary Avoidance in the Management of Irritable Bowel Syndrome
Irritable bowel syndrome (IBS) is one of the most common disorders managed by primary care physicians and gastroenterologists.1 Characterized by abdominal pain coinciding with altered stool form and/or frequency as defined by the Rome IV diagnostic criteria,2 symptoms range from mild to debilitating and may remarkably impair quality of life and work productivity.1
The cause of IBS is poorly understood. Proposed pathophysiologic factors include impaired mucosal function, microbial imbalance, visceral hypersensitivity, psychologic dysfunction, genetic factors, neurotransmitter imbalance, postinfectious gastroenteritis, inflammation, and food intolerance, any or all of which may lead to the development and maintenance of IBS symptoms.3 More recent observations of inflammation in the intestinal lining4,5 and proinflammatory peripherally circulating cytokines6 challenge its traditional classification as a functional disorder.
The cause of this inflammation is of intense interest, with speculation that the bacterial microbiota, bile acids, association with postinfectious gastroenteritis and inflammatory bowel disease cases, and/or foods may contribute. Although approximately 50% of individuals with IBS report that foods aggravate their symptoms,7 studies investigating type I antibody–mediated immediate hypersensitivity have largely failed to demonstrate a substantial link, prompting many authorities to regard these associations as food “intolerances” rather than true allergies. Based on this body of literature, a large 2010 consensus report on all aspects of food allergies advises against food allergy testing for IBS.8
In contrast, by utilizing type IV food allergen skin patch testing, 2 proof-of-concept studies9,10 investigated a different allergic mechanism in IBS, namely cell-mediated delayed-type hypersensitivity. Because many foods and food additives are known to cause allergic contact dermatitis,11 it was hypothesized that these foods may elicit a similar delayed-type hypersensitivity response in the intestinal lining in previously sensitized individuals. By following a patch test–guided food avoidance diet, a large subpopulation of patients with IBS experienced partial or complete IBS symptom relief.9,10 Our study further investigates a role for food-related delayed-type hypersensitivities in the pathogenesis of IBS.
Methods
Patient Selection
This study was conducted in a secondary care community-based setting. All patients were self-referred over an 18-month period ending in October 2019, had physician-diagnosed IBS, and/or met the Rome IV criteria for IBS and presented expressly for the food patch testing on a fee-for-service basis. Subtype of IBS was determined on presentation by the self-reported historically predominant symptom. Duration of IBS symptoms was self-reported and was rounded to the nearest year for purposes of data collection.
Exclusion criteria included pregnancy, known allergy to adhesive tape or any of the food allergens used in the study, severe skin rash, symptoms that had a known cause other than IBS, or active treatment with systemic immunosuppressive medications.
Patch Testing
Skin patch testing was initiated using an extensive panel of 117 type IV food allergens (eTable)11 identified in the literature,12 most of which utilized standard compounded formulations13 or were available from reputable patch test manufacturers (Brial Allergen GmbH; Chemotechnique Diagnostics). This panel was not approved by the US Food and Drug Administration. The freeze-dried vegetable formulations were taken from the 2018 report.9 Standard skin patch test procedure protocols12 were used, affixing the patches to the upper aspect of the back.
Following patch test application on day 1, two follow-up visits occurred on day 3 and either day 4 or day 5. On day 3, patches were removed, and the initial results were read by a board-certified dermatologist according to a standard grading system.14 Interpretation of patch tests included no reaction, questionable reaction consisting of macular erythema, weak reaction consisting of erythema and slight edema, or strong reaction consisting of erythema and marked edema. On day 4 or day 5, the final patch test reading was performed, and patients were informed of their results. Patients were advised to avoid ingestion of all foods that elicited a questionable or positive patch test response for at least 3 months, and information about the foods and their avoidance also was distributed and reviewed.
Food Avoidance Questionnaire
Patients with questionable or positive patch tests at 72 or 96 hours were advised of their eligibility to participate in an institutional review board–approved food avoidance questionnaire study investigating the utility of patch test–guided food avoidance on IBS symptoms. The questionnaire assessed the following: (1) baseline average abdominal pain prior to patch test–guided avoidance diet (0=no symptoms; 10=very severe); (2) average abdominal pain since initiation of patch test–guided avoidance diet (0=no symptoms; 10=very severe); (3) degree of improvement in overall IBS symptoms by the end of the food avoidance period (0=no improvement; 10=great improvement); (4) compliance with the avoidance diet for the duration of the avoidance period (completely, partially, not at all, or not sure).
Questionnaires and informed consent were mailed to patients via the US Postal Service 3 months after completing the patch testing. The questionnaire and consent were to be completed and returned after dietary avoidance of the identified allergens for at least 3 months. Patients were not compensated for participation in the study.
Statistical Analysis
Statistical analysis of data collected from study questionnaires was performed with Microsoft Excel. Mean abdominal pain and mean global improvement scores were reported along with 1 SD of the mean. For comparison of mean abdominal pain and improvement in global IBS symptoms from baseline to after 3 months of identified allergen avoidance, a Mann-Whitney U test was performed, with P<.05 being considered statistically significant.
Results
Thirty-seven consecutive patients underwent the testing and were eligible for the study. Nineteen patients were included in the study by virtue of completing and returning their posttest food avoidance questionnaire and informed consent. Eighteen patients were White and 1 was Asian. Subcategories of IBS were diarrhea predominant (9 [47.4%]), constipation predominant (3 [15.8%]), mixed type (5 [26.3%]), and undetermined type (2 [10.5%]). Questionnaire answers were reported after a mean (SD) duration of patch test–directed food avoidance of 4.5 (3.0) months (Table 1).
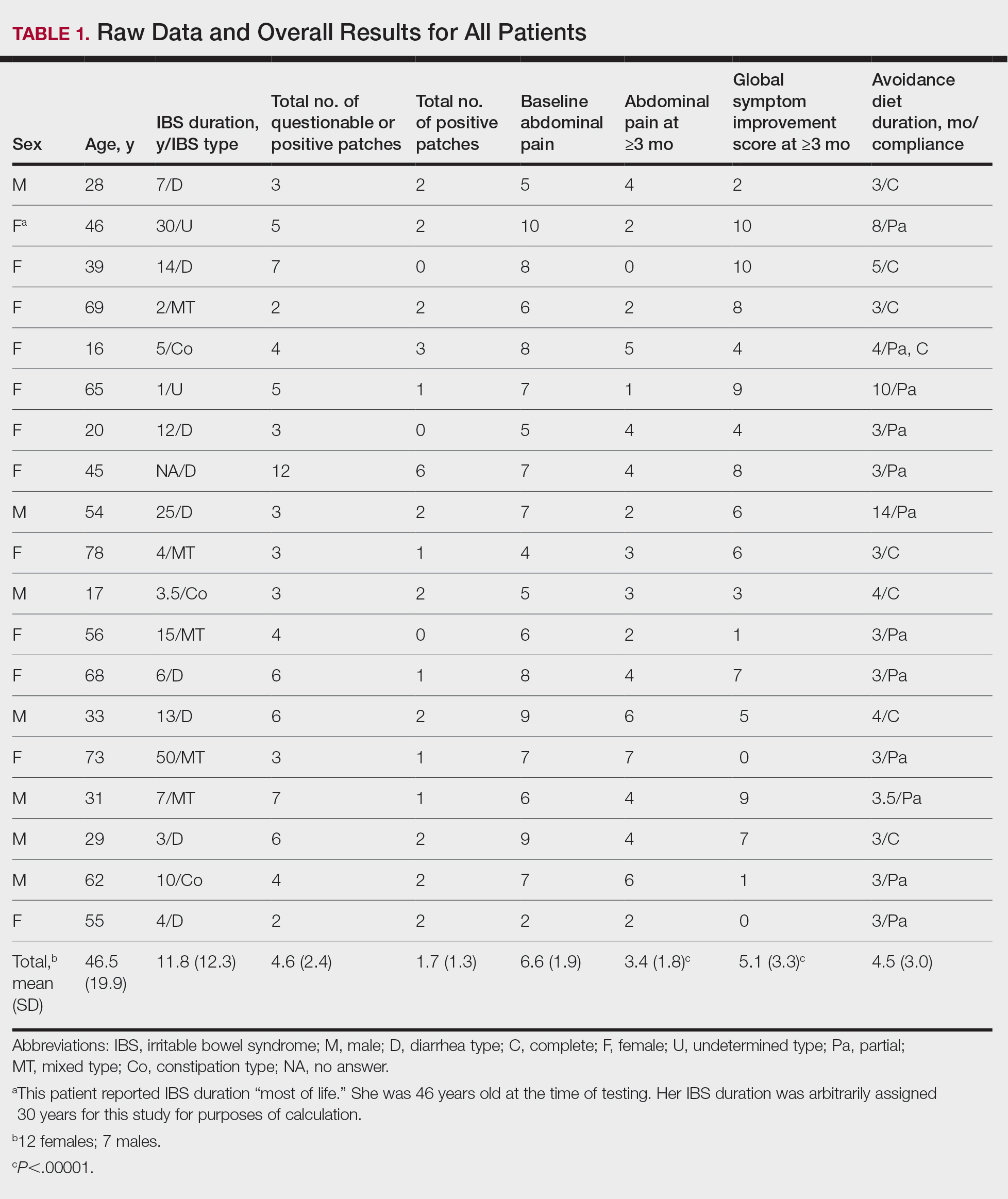
Overall Improvement
Fifteen (78.9%) patients reported at least slight to great improvement in their global IBS symptoms, and 4 (21.1%) reported no improvement (Table 2), with a mean (SD) improvement score of 5.1 (3.3)(P<.00001).
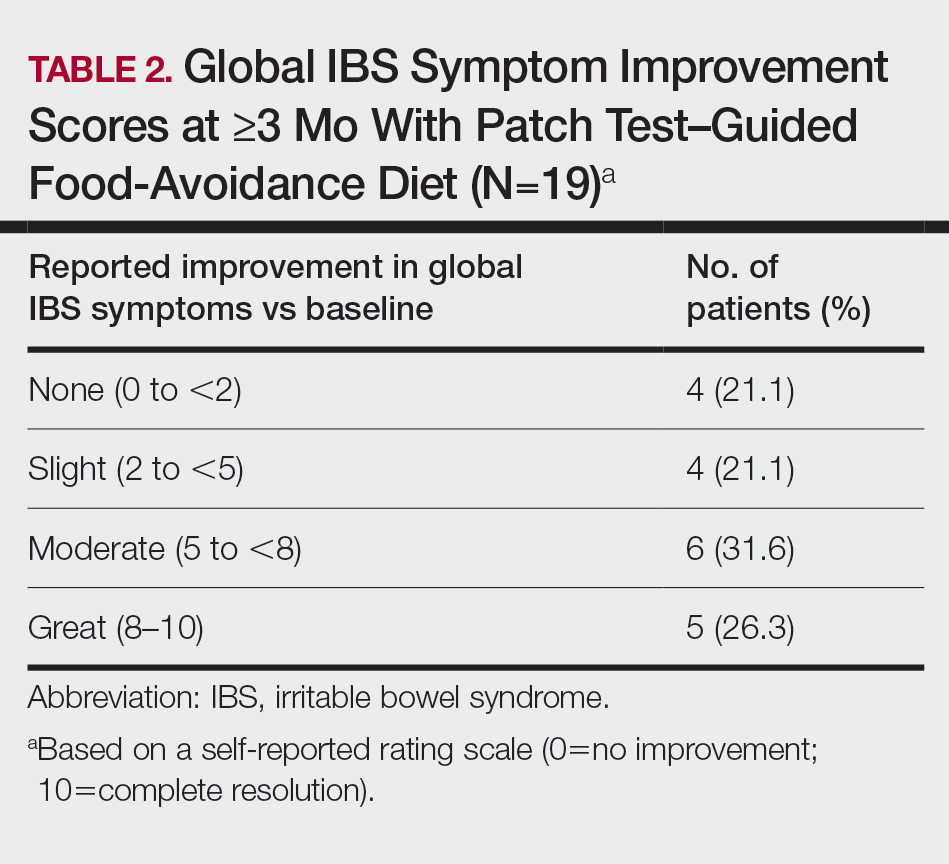
Abdominal Pain
All 19 patients reported mild to marked abdominal pain at baseline. The mean (SD) baseline pain score was 6.6 (1.9). The mean (SD) pain score was 3.4 (1.8)(P<.00001) after an average patch test–guided dietary avoidance of 4.5 (3.0) months (Table 3).
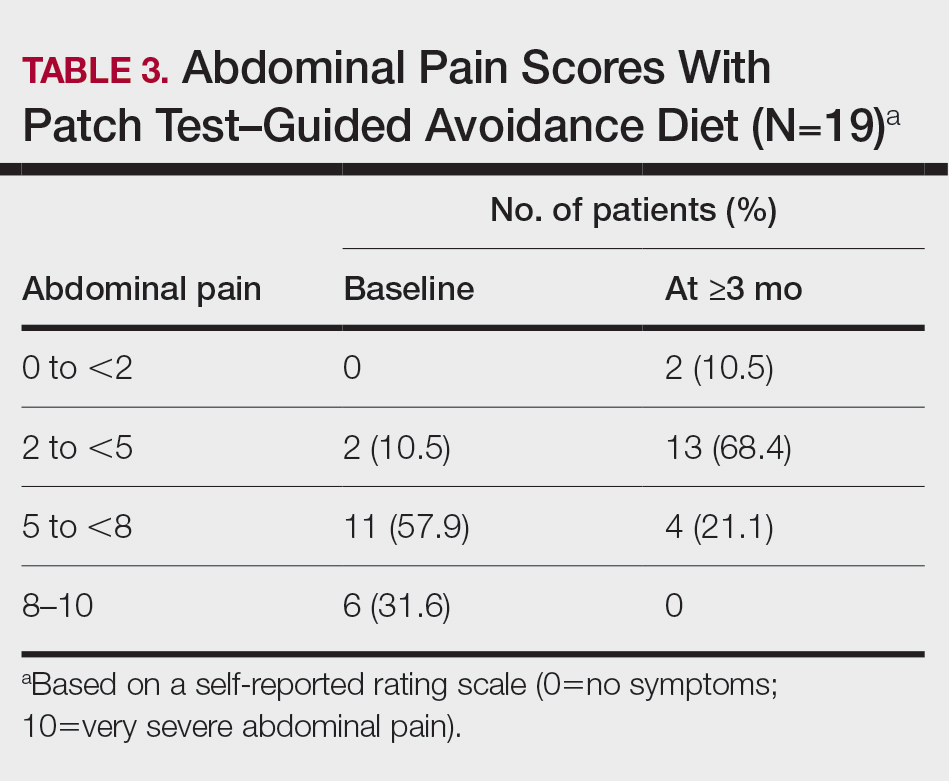
Comment
Despite intense research interest and a growing number of new medications for IBS approved by the US Food and Drug Administration, there remains a large void in the search for cost-effective and efficacious approaches for IBS evaluation and treatment. In addition to major disturbances in quality of life,14,15 the cost to society in direct medical expenses and indirect costs associated with loss of productivity and work absenteeism is considerable; estimates range from $21 billion or more annually.16
Food Hypersensitivities Triggering IBS
This study further evaluated a role for skin patch testing to identify delayed-type (type IV) food hypersensitivities that trigger IBS symptoms and differed from the prior investigations9,10 in that the symptoms used to define IBS were updated from the Rome III17 to the newer Rome IV2 criteria. The data presented here show moderate to great improvement in global IBS symptoms in 58% (11/19) of patients, which is in line with a 2018 report of 40 study participants for whom follow-up at 3 or more months was available,9 providing additional support for a role for type IV food allergies in causing the same gastrointestinal tract symptoms that define IBS. The distinction between food-related studies, including this one, that implicate food allergies9,10 and prior studies that did not support a role for food allergies in IBS pathogenesis8 can be accounted for by the type of allergy investigated. Conclusions that IBS flares after food ingestion were attributable to intolerance rather than true allergy were based on results investigating only the humoral arm and failed to consider the cell-mediated arm of the immune system. As such, foods that appear to trigger IBS symptoms on an allergic basis in our study are recognized in the literature12 as type IV allergens that elicit cell-mediated immunologic responses rather than more widely recognized type I allergens, such as peanuts and shellfish, that elicit immediate-type hypersensitivity responses. Although any type IV food allergen(s) could be responsible, a pattern emerged in this study and the study published in 2018.9 Namely, some foods stood out as more frequently inducing patch test reactions, with the 3 most common being carmine, cinnamon bark oil, and sodium bisulfite (eTable). The sample size is relatively small, but the results raise the question of whether these foods are the most likely to trigger IBS symptoms in the general population. If so, is it the result of a higher innate sensitizing potential and/or a higher frequency of exposure in commonly eaten foods? Larger randomized clinical trials are needed.
Immune Response and IBS
There is mounting evidence that the immune system may play a role in the pathophysiology of IBS.18 Both lymphocyte infiltration of the myenteric plexus and an increase in intestinal mucosal T lymphocytes have been observed, and it is generally accepted that the mucosal immune system seems to be activated, at least in a subset of patients with IBS.19 Irritable bowel syndrome associations with quiescent inflammatory bowel disease or postinfectious gastroenteritis provide 2 potential causes for the inflammation, but most IBS patients have had neither.20 The mucosal lining of the intestine and immune system have vast exposure to intraluminal allergens in transit, and it is hypothesized that the same delayed-type hypersensitivity response elicited in the skin by patch testing is elicited in the intestine, resulting in the inflammation that triggers IBS symptoms.10 The results here add to the growing body of evidence that ingestion of type IV food allergens by previously sensitized individuals could, in fact, be the primary source of the inflammation observed in a large subpopulation of individuals who carry a diagnosis of IBS.
Food Allergens in Patch Testing
Many of the food allergens used in this study are commonly found in various nonfood products that may contact the skin. For example, many flavorings are used as fragrances, and many preservatives, binders, thickeners, emulsifiers, and stabilizers serve the same role in moisturizers, cosmetics, and topical medications. Likewise, nickel sulfate hexahydrate, ubiquitous in foods that arise from the earth, often is found in metal in jewelry, clothing components, and cell phones. All are potential sensitizers. Thus, the question may arise whether the causal relationship between the food allergens identified by patch testing and IBS symptoms might be more of a systemic effect akin to systemic contact dermatitis as sometimes follows ingestion of an allergen to which an individual has been topically sensitized, rather than the proposed localized immunologic response in the intestinal lining. We were unaware of patient history of allergic contact dermatitis to any of the patch test allergens in this study, but the dermatologist author here (M.S.) has unpublished experience with 2 other patients with IBS who have benefited from low-nickel diets after having had positive patch tests to nickel sulfate hexahydrate and who, in retrospect, did report a history of earring dermatitis. Future investigations using pre– and post–food challenge histologic assessments of the intestinal mucosa in patients who benefit from patch test–guided food avoidance diets should help to better define the mechanism.
Because IBS has not been traditionally associated with structural or biochemical abnormalities detectable with current routine diagnostic tools, it has long been viewed as a functional disorder. The findings published more recently,9,10 in addition to this study’s results, would negate this functional classification in the subset of patients with IBS symptoms who experience sustained relief of their symptoms by patch test–directed food avoidance. The underlying delayed-type hypersensitivity pathogenesis of the IBS-like symptoms in these individuals would mandate an organic classification, aptly named allergic contact enteritis.10
Follow-up Data
The mean (SD) follow-up duration for this study and the 2018 report9 was 4.5 (3.0) months and 7.6 (3.9) months, respectively. The placebo effect is a concern for disorders such as IBS in which primarily subjective outcome measures are available,21 and in a retrospective analysis of 25 randomized, placebo-controlled IBS clinical trials, Spiller22 concluded the optimum length of such trials to be more than 3 months, which these studies exceed. Although not blinded or placebo controlled, the length of follow-up in the 2018 report9 and here enhances the validity of the results.
Limitation
The retrospective manner in which the self-assessments were reported in this study introduces the potential for recall bias, a variable that could affect results. The presence and direction of bias by any given individual cannot be known, making it difficult to determine any effect it may have had. Further investigation should include daily assessments and refine the primary study end points to include both abdominal pain and the defecation considerations that define IBS.
Conclusion
Food patch testing has the potential to offer a safe, cost-effective approach to the evaluation and management of IBS symptoms. Randomized clinical trials are needed to further investigate the validity of the proof-of-concept results to date. For patients who benefit from a patch test–guided avoidance diet, invasive and costly endoscopic, radiologic, and laboratory testing and pharmacologic management could be averted. Symptomatic relief could be attained simply by avoiding the implicated foods, essentially doing more by doing less.
- Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:1-24.
- Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. 2017;6:99.
- Barbara G, De Giorgio R, Stanghellini V, et al. New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(suppl 2):1-9
- Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002;122:1778-1783.
- Tornblom H, Lindberg G, Nyberg B, et al. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979.
- O’Mahony L, McCarthy J, Kelly
P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. - Ragnarsson G, Bodemar G. Pain is temporally related to eating but not to defecation in the irritable bowel syndrome (IBS): patients’ description of diarrhea, constipation and symptom variation during a prospective 6-week study. Eur J Gastroenterol Hepatol. 1998;10:415-421.
- Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
- Shin GH, Smith MS, Toro B, et al. Utility of food patch testing in the evaluation and management of irritable bowel syndrome. Skin. 2018;2:1-15.
- Stierstorfer MB, Sha CT. Food patch testing for irritable bowel syndrome. J Am Acad Dermatol. 2013;68:377-384.
- Marks JG, Belsito DV, DeLeo MD, et al. North American Contact Dermatitis Group patch test results for the detection of delayed-type hypersensitivity to topical allergens. J Am Acad Dermatol. 1998;38:911-918.
- Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. BC Decker; 2008.
- DeGroot AC. Patch Testing. acdegroot Publishing; 2008.
- Gralnek IM, Hays RD, Kilbourne A, et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654-660.
- Halder SL, Lock GR, Talley NJ, et al. Impact of functional gastrointestinal disorders on health-related quality of life: a population-based case–control study. Aliment Pharmacol Ther. 2004;19:233-242.
- International Foundation for Gastrointestinal Disorders. About IBS. statistics. Accessed July 20, 2021. https://www.aboutibs.org/facts-about-ibs/statistics.html
- Rome Foundation. Guidelines—Rome III diagnostic criteria for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:307-312.
- Collins SM. Is the irritable gut an inflamed gut? Scand J Gastroenterol. 1992;192(suppl):102-105.
- Park MI, Camilleri M. Is there a role of food allergy in irritable bowel syndrome and functional dyspepsia? a systemic review. Neurogastroenterol Motil. 2006;18:595-607.
- Grover M, Herfarth H, Drossman DA. The functional-organic dichotomy: postinfectious irritable bowel syndrome and inflammatory bowel disease–irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:48-53.
- Hrobiartsson A, Gotzsche PC. Is the placebo powerless? an analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594-1602.
- Spiller RC. Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am J Med. 1999;107:91S-97S.
Irritable bowel syndrome (IBS) is one of the most common disorders managed by primary care physicians and gastroenterologists.1 Characterized by abdominal pain coinciding with altered stool form and/or frequency as defined by the Rome IV diagnostic criteria,2 symptoms range from mild to debilitating and may remarkably impair quality of life and work productivity.1
The cause of IBS is poorly understood. Proposed pathophysiologic factors include impaired mucosal function, microbial imbalance, visceral hypersensitivity, psychologic dysfunction, genetic factors, neurotransmitter imbalance, postinfectious gastroenteritis, inflammation, and food intolerance, any or all of which may lead to the development and maintenance of IBS symptoms.3 More recent observations of inflammation in the intestinal lining4,5 and proinflammatory peripherally circulating cytokines6 challenge its traditional classification as a functional disorder.
The cause of this inflammation is of intense interest, with speculation that the bacterial microbiota, bile acids, association with postinfectious gastroenteritis and inflammatory bowel disease cases, and/or foods may contribute. Although approximately 50% of individuals with IBS report that foods aggravate their symptoms,7 studies investigating type I antibody–mediated immediate hypersensitivity have largely failed to demonstrate a substantial link, prompting many authorities to regard these associations as food “intolerances” rather than true allergies. Based on this body of literature, a large 2010 consensus report on all aspects of food allergies advises against food allergy testing for IBS.8
In contrast, by utilizing type IV food allergen skin patch testing, 2 proof-of-concept studies9,10 investigated a different allergic mechanism in IBS, namely cell-mediated delayed-type hypersensitivity. Because many foods and food additives are known to cause allergic contact dermatitis,11 it was hypothesized that these foods may elicit a similar delayed-type hypersensitivity response in the intestinal lining in previously sensitized individuals. By following a patch test–guided food avoidance diet, a large subpopulation of patients with IBS experienced partial or complete IBS symptom relief.9,10 Our study further investigates a role for food-related delayed-type hypersensitivities in the pathogenesis of IBS.
Methods
Patient Selection
This study was conducted in a secondary care community-based setting. All patients were self-referred over an 18-month period ending in October 2019, had physician-diagnosed IBS, and/or met the Rome IV criteria for IBS and presented expressly for the food patch testing on a fee-for-service basis. Subtype of IBS was determined on presentation by the self-reported historically predominant symptom. Duration of IBS symptoms was self-reported and was rounded to the nearest year for purposes of data collection.
Exclusion criteria included pregnancy, known allergy to adhesive tape or any of the food allergens used in the study, severe skin rash, symptoms that had a known cause other than IBS, or active treatment with systemic immunosuppressive medications.
Patch Testing
Skin patch testing was initiated using an extensive panel of 117 type IV food allergens (eTable)11 identified in the literature,12 most of which utilized standard compounded formulations13 or were available from reputable patch test manufacturers (Brial Allergen GmbH; Chemotechnique Diagnostics). This panel was not approved by the US Food and Drug Administration. The freeze-dried vegetable formulations were taken from the 2018 report.9 Standard skin patch test procedure protocols12 were used, affixing the patches to the upper aspect of the back.
Following patch test application on day 1, two follow-up visits occurred on day 3 and either day 4 or day 5. On day 3, patches were removed, and the initial results were read by a board-certified dermatologist according to a standard grading system.14 Interpretation of patch tests included no reaction, questionable reaction consisting of macular erythema, weak reaction consisting of erythema and slight edema, or strong reaction consisting of erythema and marked edema. On day 4 or day 5, the final patch test reading was performed, and patients were informed of their results. Patients were advised to avoid ingestion of all foods that elicited a questionable or positive patch test response for at least 3 months, and information about the foods and their avoidance also was distributed and reviewed.
Food Avoidance Questionnaire
Patients with questionable or positive patch tests at 72 or 96 hours were advised of their eligibility to participate in an institutional review board–approved food avoidance questionnaire study investigating the utility of patch test–guided food avoidance on IBS symptoms. The questionnaire assessed the following: (1) baseline average abdominal pain prior to patch test–guided avoidance diet (0=no symptoms; 10=very severe); (2) average abdominal pain since initiation of patch test–guided avoidance diet (0=no symptoms; 10=very severe); (3) degree of improvement in overall IBS symptoms by the end of the food avoidance period (0=no improvement; 10=great improvement); (4) compliance with the avoidance diet for the duration of the avoidance period (completely, partially, not at all, or not sure).
Questionnaires and informed consent were mailed to patients via the US Postal Service 3 months after completing the patch testing. The questionnaire and consent were to be completed and returned after dietary avoidance of the identified allergens for at least 3 months. Patients were not compensated for participation in the study.
Statistical Analysis
Statistical analysis of data collected from study questionnaires was performed with Microsoft Excel. Mean abdominal pain and mean global improvement scores were reported along with 1 SD of the mean. For comparison of mean abdominal pain and improvement in global IBS symptoms from baseline to after 3 months of identified allergen avoidance, a Mann-Whitney U test was performed, with P<.05 being considered statistically significant.
Results
Thirty-seven consecutive patients underwent the testing and were eligible for the study. Nineteen patients were included in the study by virtue of completing and returning their posttest food avoidance questionnaire and informed consent. Eighteen patients were White and 1 was Asian. Subcategories of IBS were diarrhea predominant (9 [47.4%]), constipation predominant (3 [15.8%]), mixed type (5 [26.3%]), and undetermined type (2 [10.5%]). Questionnaire answers were reported after a mean (SD) duration of patch test–directed food avoidance of 4.5 (3.0) months (Table 1).

Overall Improvement
Fifteen (78.9%) patients reported at least slight to great improvement in their global IBS symptoms, and 4 (21.1%) reported no improvement (Table 2), with a mean (SD) improvement score of 5.1 (3.3)(P<.00001).

Abdominal Pain
All 19 patients reported mild to marked abdominal pain at baseline. The mean (SD) baseline pain score was 6.6 (1.9). The mean (SD) pain score was 3.4 (1.8)(P<.00001) after an average patch test–guided dietary avoidance of 4.5 (3.0) months (Table 3).

Comment
Despite intense research interest and a growing number of new medications for IBS approved by the US Food and Drug Administration, there remains a large void in the search for cost-effective and efficacious approaches for IBS evaluation and treatment. In addition to major disturbances in quality of life,14,15 the cost to society in direct medical expenses and indirect costs associated with loss of productivity and work absenteeism is considerable; estimates range from $21 billion or more annually.16
Food Hypersensitivities Triggering IBS
This study further evaluated a role for skin patch testing to identify delayed-type (type IV) food hypersensitivities that trigger IBS symptoms and differed from the prior investigations9,10 in that the symptoms used to define IBS were updated from the Rome III17 to the newer Rome IV2 criteria. The data presented here show moderate to great improvement in global IBS symptoms in 58% (11/19) of patients, which is in line with a 2018 report of 40 study participants for whom follow-up at 3 or more months was available,9 providing additional support for a role for type IV food allergies in causing the same gastrointestinal tract symptoms that define IBS. The distinction between food-related studies, including this one, that implicate food allergies9,10 and prior studies that did not support a role for food allergies in IBS pathogenesis8 can be accounted for by the type of allergy investigated. Conclusions that IBS flares after food ingestion were attributable to intolerance rather than true allergy were based on results investigating only the humoral arm and failed to consider the cell-mediated arm of the immune system. As such, foods that appear to trigger IBS symptoms on an allergic basis in our study are recognized in the literature12 as type IV allergens that elicit cell-mediated immunologic responses rather than more widely recognized type I allergens, such as peanuts and shellfish, that elicit immediate-type hypersensitivity responses. Although any type IV food allergen(s) could be responsible, a pattern emerged in this study and the study published in 2018.9 Namely, some foods stood out as more frequently inducing patch test reactions, with the 3 most common being carmine, cinnamon bark oil, and sodium bisulfite (eTable). The sample size is relatively small, but the results raise the question of whether these foods are the most likely to trigger IBS symptoms in the general population. If so, is it the result of a higher innate sensitizing potential and/or a higher frequency of exposure in commonly eaten foods? Larger randomized clinical trials are needed.
Immune Response and IBS
There is mounting evidence that the immune system may play a role in the pathophysiology of IBS.18 Both lymphocyte infiltration of the myenteric plexus and an increase in intestinal mucosal T lymphocytes have been observed, and it is generally accepted that the mucosal immune system seems to be activated, at least in a subset of patients with IBS.19 Irritable bowel syndrome associations with quiescent inflammatory bowel disease or postinfectious gastroenteritis provide 2 potential causes for the inflammation, but most IBS patients have had neither.20 The mucosal lining of the intestine and immune system have vast exposure to intraluminal allergens in transit, and it is hypothesized that the same delayed-type hypersensitivity response elicited in the skin by patch testing is elicited in the intestine, resulting in the inflammation that triggers IBS symptoms.10 The results here add to the growing body of evidence that ingestion of type IV food allergens by previously sensitized individuals could, in fact, be the primary source of the inflammation observed in a large subpopulation of individuals who carry a diagnosis of IBS.
Food Allergens in Patch Testing
Many of the food allergens used in this study are commonly found in various nonfood products that may contact the skin. For example, many flavorings are used as fragrances, and many preservatives, binders, thickeners, emulsifiers, and stabilizers serve the same role in moisturizers, cosmetics, and topical medications. Likewise, nickel sulfate hexahydrate, ubiquitous in foods that arise from the earth, often is found in metal in jewelry, clothing components, and cell phones. All are potential sensitizers. Thus, the question may arise whether the causal relationship between the food allergens identified by patch testing and IBS symptoms might be more of a systemic effect akin to systemic contact dermatitis as sometimes follows ingestion of an allergen to which an individual has been topically sensitized, rather than the proposed localized immunologic response in the intestinal lining. We were unaware of patient history of allergic contact dermatitis to any of the patch test allergens in this study, but the dermatologist author here (M.S.) has unpublished experience with 2 other patients with IBS who have benefited from low-nickel diets after having had positive patch tests to nickel sulfate hexahydrate and who, in retrospect, did report a history of earring dermatitis. Future investigations using pre– and post–food challenge histologic assessments of the intestinal mucosa in patients who benefit from patch test–guided food avoidance diets should help to better define the mechanism.
Because IBS has not been traditionally associated with structural or biochemical abnormalities detectable with current routine diagnostic tools, it has long been viewed as a functional disorder. The findings published more recently,9,10 in addition to this study’s results, would negate this functional classification in the subset of patients with IBS symptoms who experience sustained relief of their symptoms by patch test–directed food avoidance. The underlying delayed-type hypersensitivity pathogenesis of the IBS-like symptoms in these individuals would mandate an organic classification, aptly named allergic contact enteritis.10
Follow-up Data
The mean (SD) follow-up duration for this study and the 2018 report9 was 4.5 (3.0) months and 7.6 (3.9) months, respectively. The placebo effect is a concern for disorders such as IBS in which primarily subjective outcome measures are available,21 and in a retrospective analysis of 25 randomized, placebo-controlled IBS clinical trials, Spiller22 concluded the optimum length of such trials to be more than 3 months, which these studies exceed. Although not blinded or placebo controlled, the length of follow-up in the 2018 report9 and here enhances the validity of the results.
Limitation
The retrospective manner in which the self-assessments were reported in this study introduces the potential for recall bias, a variable that could affect results. The presence and direction of bias by any given individual cannot be known, making it difficult to determine any effect it may have had. Further investigation should include daily assessments and refine the primary study end points to include both abdominal pain and the defecation considerations that define IBS.
Conclusion
Food patch testing has the potential to offer a safe, cost-effective approach to the evaluation and management of IBS symptoms. Randomized clinical trials are needed to further investigate the validity of the proof-of-concept results to date. For patients who benefit from a patch test–guided avoidance diet, invasive and costly endoscopic, radiologic, and laboratory testing and pharmacologic management could be averted. Symptomatic relief could be attained simply by avoiding the implicated foods, essentially doing more by doing less.
Irritable bowel syndrome (IBS) is one of the most common disorders managed by primary care physicians and gastroenterologists.1 Characterized by abdominal pain coinciding with altered stool form and/or frequency as defined by the Rome IV diagnostic criteria,2 symptoms range from mild to debilitating and may remarkably impair quality of life and work productivity.1
The cause of IBS is poorly understood. Proposed pathophysiologic factors include impaired mucosal function, microbial imbalance, visceral hypersensitivity, psychologic dysfunction, genetic factors, neurotransmitter imbalance, postinfectious gastroenteritis, inflammation, and food intolerance, any or all of which may lead to the development and maintenance of IBS symptoms.3 More recent observations of inflammation in the intestinal lining4,5 and proinflammatory peripherally circulating cytokines6 challenge its traditional classification as a functional disorder.
The cause of this inflammation is of intense interest, with speculation that the bacterial microbiota, bile acids, association with postinfectious gastroenteritis and inflammatory bowel disease cases, and/or foods may contribute. Although approximately 50% of individuals with IBS report that foods aggravate their symptoms,7 studies investigating type I antibody–mediated immediate hypersensitivity have largely failed to demonstrate a substantial link, prompting many authorities to regard these associations as food “intolerances” rather than true allergies. Based on this body of literature, a large 2010 consensus report on all aspects of food allergies advises against food allergy testing for IBS.8
In contrast, by utilizing type IV food allergen skin patch testing, 2 proof-of-concept studies9,10 investigated a different allergic mechanism in IBS, namely cell-mediated delayed-type hypersensitivity. Because many foods and food additives are known to cause allergic contact dermatitis,11 it was hypothesized that these foods may elicit a similar delayed-type hypersensitivity response in the intestinal lining in previously sensitized individuals. By following a patch test–guided food avoidance diet, a large subpopulation of patients with IBS experienced partial or complete IBS symptom relief.9,10 Our study further investigates a role for food-related delayed-type hypersensitivities in the pathogenesis of IBS.
Methods
Patient Selection
This study was conducted in a secondary care community-based setting. All patients were self-referred over an 18-month period ending in October 2019, had physician-diagnosed IBS, and/or met the Rome IV criteria for IBS and presented expressly for the food patch testing on a fee-for-service basis. Subtype of IBS was determined on presentation by the self-reported historically predominant symptom. Duration of IBS symptoms was self-reported and was rounded to the nearest year for purposes of data collection.
Exclusion criteria included pregnancy, known allergy to adhesive tape or any of the food allergens used in the study, severe skin rash, symptoms that had a known cause other than IBS, or active treatment with systemic immunosuppressive medications.
Patch Testing
Skin patch testing was initiated using an extensive panel of 117 type IV food allergens (eTable)11 identified in the literature,12 most of which utilized standard compounded formulations13 or were available from reputable patch test manufacturers (Brial Allergen GmbH; Chemotechnique Diagnostics). This panel was not approved by the US Food and Drug Administration. The freeze-dried vegetable formulations were taken from the 2018 report.9 Standard skin patch test procedure protocols12 were used, affixing the patches to the upper aspect of the back.
Following patch test application on day 1, two follow-up visits occurred on day 3 and either day 4 or day 5. On day 3, patches were removed, and the initial results were read by a board-certified dermatologist according to a standard grading system.14 Interpretation of patch tests included no reaction, questionable reaction consisting of macular erythema, weak reaction consisting of erythema and slight edema, or strong reaction consisting of erythema and marked edema. On day 4 or day 5, the final patch test reading was performed, and patients were informed of their results. Patients were advised to avoid ingestion of all foods that elicited a questionable or positive patch test response for at least 3 months, and information about the foods and their avoidance also was distributed and reviewed.
Food Avoidance Questionnaire
Patients with questionable or positive patch tests at 72 or 96 hours were advised of their eligibility to participate in an institutional review board–approved food avoidance questionnaire study investigating the utility of patch test–guided food avoidance on IBS symptoms. The questionnaire assessed the following: (1) baseline average abdominal pain prior to patch test–guided avoidance diet (0=no symptoms; 10=very severe); (2) average abdominal pain since initiation of patch test–guided avoidance diet (0=no symptoms; 10=very severe); (3) degree of improvement in overall IBS symptoms by the end of the food avoidance period (0=no improvement; 10=great improvement); (4) compliance with the avoidance diet for the duration of the avoidance period (completely, partially, not at all, or not sure).
Questionnaires and informed consent were mailed to patients via the US Postal Service 3 months after completing the patch testing. The questionnaire and consent were to be completed and returned after dietary avoidance of the identified allergens for at least 3 months. Patients were not compensated for participation in the study.
Statistical Analysis
Statistical analysis of data collected from study questionnaires was performed with Microsoft Excel. Mean abdominal pain and mean global improvement scores were reported along with 1 SD of the mean. For comparison of mean abdominal pain and improvement in global IBS symptoms from baseline to after 3 months of identified allergen avoidance, a Mann-Whitney U test was performed, with P<.05 being considered statistically significant.
Results
Thirty-seven consecutive patients underwent the testing and were eligible for the study. Nineteen patients were included in the study by virtue of completing and returning their posttest food avoidance questionnaire and informed consent. Eighteen patients were White and 1 was Asian. Subcategories of IBS were diarrhea predominant (9 [47.4%]), constipation predominant (3 [15.8%]), mixed type (5 [26.3%]), and undetermined type (2 [10.5%]). Questionnaire answers were reported after a mean (SD) duration of patch test–directed food avoidance of 4.5 (3.0) months (Table 1).

Overall Improvement
Fifteen (78.9%) patients reported at least slight to great improvement in their global IBS symptoms, and 4 (21.1%) reported no improvement (Table 2), with a mean (SD) improvement score of 5.1 (3.3)(P<.00001).

Abdominal Pain
All 19 patients reported mild to marked abdominal pain at baseline. The mean (SD) baseline pain score was 6.6 (1.9). The mean (SD) pain score was 3.4 (1.8)(P<.00001) after an average patch test–guided dietary avoidance of 4.5 (3.0) months (Table 3).

Comment
Despite intense research interest and a growing number of new medications for IBS approved by the US Food and Drug Administration, there remains a large void in the search for cost-effective and efficacious approaches for IBS evaluation and treatment. In addition to major disturbances in quality of life,14,15 the cost to society in direct medical expenses and indirect costs associated with loss of productivity and work absenteeism is considerable; estimates range from $21 billion or more annually.16
Food Hypersensitivities Triggering IBS
This study further evaluated a role for skin patch testing to identify delayed-type (type IV) food hypersensitivities that trigger IBS symptoms and differed from the prior investigations9,10 in that the symptoms used to define IBS were updated from the Rome III17 to the newer Rome IV2 criteria. The data presented here show moderate to great improvement in global IBS symptoms in 58% (11/19) of patients, which is in line with a 2018 report of 40 study participants for whom follow-up at 3 or more months was available,9 providing additional support for a role for type IV food allergies in causing the same gastrointestinal tract symptoms that define IBS. The distinction between food-related studies, including this one, that implicate food allergies9,10 and prior studies that did not support a role for food allergies in IBS pathogenesis8 can be accounted for by the type of allergy investigated. Conclusions that IBS flares after food ingestion were attributable to intolerance rather than true allergy were based on results investigating only the humoral arm and failed to consider the cell-mediated arm of the immune system. As such, foods that appear to trigger IBS symptoms on an allergic basis in our study are recognized in the literature12 as type IV allergens that elicit cell-mediated immunologic responses rather than more widely recognized type I allergens, such as peanuts and shellfish, that elicit immediate-type hypersensitivity responses. Although any type IV food allergen(s) could be responsible, a pattern emerged in this study and the study published in 2018.9 Namely, some foods stood out as more frequently inducing patch test reactions, with the 3 most common being carmine, cinnamon bark oil, and sodium bisulfite (eTable). The sample size is relatively small, but the results raise the question of whether these foods are the most likely to trigger IBS symptoms in the general population. If so, is it the result of a higher innate sensitizing potential and/or a higher frequency of exposure in commonly eaten foods? Larger randomized clinical trials are needed.
Immune Response and IBS
There is mounting evidence that the immune system may play a role in the pathophysiology of IBS.18 Both lymphocyte infiltration of the myenteric plexus and an increase in intestinal mucosal T lymphocytes have been observed, and it is generally accepted that the mucosal immune system seems to be activated, at least in a subset of patients with IBS.19 Irritable bowel syndrome associations with quiescent inflammatory bowel disease or postinfectious gastroenteritis provide 2 potential causes for the inflammation, but most IBS patients have had neither.20 The mucosal lining of the intestine and immune system have vast exposure to intraluminal allergens in transit, and it is hypothesized that the same delayed-type hypersensitivity response elicited in the skin by patch testing is elicited in the intestine, resulting in the inflammation that triggers IBS symptoms.10 The results here add to the growing body of evidence that ingestion of type IV food allergens by previously sensitized individuals could, in fact, be the primary source of the inflammation observed in a large subpopulation of individuals who carry a diagnosis of IBS.
Food Allergens in Patch Testing
Many of the food allergens used in this study are commonly found in various nonfood products that may contact the skin. For example, many flavorings are used as fragrances, and many preservatives, binders, thickeners, emulsifiers, and stabilizers serve the same role in moisturizers, cosmetics, and topical medications. Likewise, nickel sulfate hexahydrate, ubiquitous in foods that arise from the earth, often is found in metal in jewelry, clothing components, and cell phones. All are potential sensitizers. Thus, the question may arise whether the causal relationship between the food allergens identified by patch testing and IBS symptoms might be more of a systemic effect akin to systemic contact dermatitis as sometimes follows ingestion of an allergen to which an individual has been topically sensitized, rather than the proposed localized immunologic response in the intestinal lining. We were unaware of patient history of allergic contact dermatitis to any of the patch test allergens in this study, but the dermatologist author here (M.S.) has unpublished experience with 2 other patients with IBS who have benefited from low-nickel diets after having had positive patch tests to nickel sulfate hexahydrate and who, in retrospect, did report a history of earring dermatitis. Future investigations using pre– and post–food challenge histologic assessments of the intestinal mucosa in patients who benefit from patch test–guided food avoidance diets should help to better define the mechanism.
Because IBS has not been traditionally associated with structural or biochemical abnormalities detectable with current routine diagnostic tools, it has long been viewed as a functional disorder. The findings published more recently,9,10 in addition to this study’s results, would negate this functional classification in the subset of patients with IBS symptoms who experience sustained relief of their symptoms by patch test–directed food avoidance. The underlying delayed-type hypersensitivity pathogenesis of the IBS-like symptoms in these individuals would mandate an organic classification, aptly named allergic contact enteritis.10
Follow-up Data
The mean (SD) follow-up duration for this study and the 2018 report9 was 4.5 (3.0) months and 7.6 (3.9) months, respectively. The placebo effect is a concern for disorders such as IBS in which primarily subjective outcome measures are available,21 and in a retrospective analysis of 25 randomized, placebo-controlled IBS clinical trials, Spiller22 concluded the optimum length of such trials to be more than 3 months, which these studies exceed. Although not blinded or placebo controlled, the length of follow-up in the 2018 report9 and here enhances the validity of the results.
Limitation
The retrospective manner in which the self-assessments were reported in this study introduces the potential for recall bias, a variable that could affect results. The presence and direction of bias by any given individual cannot be known, making it difficult to determine any effect it may have had. Further investigation should include daily assessments and refine the primary study end points to include both abdominal pain and the defecation considerations that define IBS.
Conclusion
Food patch testing has the potential to offer a safe, cost-effective approach to the evaluation and management of IBS symptoms. Randomized clinical trials are needed to further investigate the validity of the proof-of-concept results to date. For patients who benefit from a patch test–guided avoidance diet, invasive and costly endoscopic, radiologic, and laboratory testing and pharmacologic management could be averted. Symptomatic relief could be attained simply by avoiding the implicated foods, essentially doing more by doing less.
- Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:1-24.
- Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. 2017;6:99.
- Barbara G, De Giorgio R, Stanghellini V, et al. New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(suppl 2):1-9
- Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002;122:1778-1783.
- Tornblom H, Lindberg G, Nyberg B, et al. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979.
- O’Mahony L, McCarthy J, Kelly
P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. - Ragnarsson G, Bodemar G. Pain is temporally related to eating but not to defecation in the irritable bowel syndrome (IBS): patients’ description of diarrhea, constipation and symptom variation during a prospective 6-week study. Eur J Gastroenterol Hepatol. 1998;10:415-421.
- Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
- Shin GH, Smith MS, Toro B, et al. Utility of food patch testing in the evaluation and management of irritable bowel syndrome. Skin. 2018;2:1-15.
- Stierstorfer MB, Sha CT. Food patch testing for irritable bowel syndrome. J Am Acad Dermatol. 2013;68:377-384.
- Marks JG, Belsito DV, DeLeo MD, et al. North American Contact Dermatitis Group patch test results for the detection of delayed-type hypersensitivity to topical allergens. J Am Acad Dermatol. 1998;38:911-918.
- Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. BC Decker; 2008.
- DeGroot AC. Patch Testing. acdegroot Publishing; 2008.
- Gralnek IM, Hays RD, Kilbourne A, et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654-660.
- Halder SL, Lock GR, Talley NJ, et al. Impact of functional gastrointestinal disorders on health-related quality of life: a population-based case–control study. Aliment Pharmacol Ther. 2004;19:233-242.
- International Foundation for Gastrointestinal Disorders. About IBS. statistics. Accessed July 20, 2021. https://www.aboutibs.org/facts-about-ibs/statistics.html
- Rome Foundation. Guidelines—Rome III diagnostic criteria for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:307-312.
- Collins SM. Is the irritable gut an inflamed gut? Scand J Gastroenterol. 1992;192(suppl):102-105.
- Park MI, Camilleri M. Is there a role of food allergy in irritable bowel syndrome and functional dyspepsia? a systemic review. Neurogastroenterol Motil. 2006;18:595-607.
- Grover M, Herfarth H, Drossman DA. The functional-organic dichotomy: postinfectious irritable bowel syndrome and inflammatory bowel disease–irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:48-53.
- Hrobiartsson A, Gotzsche PC. Is the placebo powerless? an analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594-1602.
- Spiller RC. Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am J Med. 1999;107:91S-97S.
- Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:1-24.
- Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. 2017;6:99.
- Barbara G, De Giorgio R, Stanghellini V, et al. New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(suppl 2):1-9
- Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002;122:1778-1783.
- Tornblom H, Lindberg G, Nyberg B, et al. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979.
- O’Mahony L, McCarthy J, Kelly
P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. - Ragnarsson G, Bodemar G. Pain is temporally related to eating but not to defecation in the irritable bowel syndrome (IBS): patients’ description of diarrhea, constipation and symptom variation during a prospective 6-week study. Eur J Gastroenterol Hepatol. 1998;10:415-421.
- Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
- Shin GH, Smith MS, Toro B, et al. Utility of food patch testing in the evaluation and management of irritable bowel syndrome. Skin. 2018;2:1-15.
- Stierstorfer MB, Sha CT. Food patch testing for irritable bowel syndrome. J Am Acad Dermatol. 2013;68:377-384.
- Marks JG, Belsito DV, DeLeo MD, et al. North American Contact Dermatitis Group patch test results for the detection of delayed-type hypersensitivity to topical allergens. J Am Acad Dermatol. 1998;38:911-918.
- Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. BC Decker; 2008.
- DeGroot AC. Patch Testing. acdegroot Publishing; 2008.
- Gralnek IM, Hays RD, Kilbourne A, et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654-660.
- Halder SL, Lock GR, Talley NJ, et al. Impact of functional gastrointestinal disorders on health-related quality of life: a population-based case–control study. Aliment Pharmacol Ther. 2004;19:233-242.
- International Foundation for Gastrointestinal Disorders. About IBS. statistics. Accessed July 20, 2021. https://www.aboutibs.org/facts-about-ibs/statistics.html
- Rome Foundation. Guidelines—Rome III diagnostic criteria for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:307-312.
- Collins SM. Is the irritable gut an inflamed gut? Scand J Gastroenterol. 1992;192(suppl):102-105.
- Park MI, Camilleri M. Is there a role of food allergy in irritable bowel syndrome and functional dyspepsia? a systemic review. Neurogastroenterol Motil. 2006;18:595-607.
- Grover M, Herfarth H, Drossman DA. The functional-organic dichotomy: postinfectious irritable bowel syndrome and inflammatory bowel disease–irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:48-53.
- Hrobiartsson A, Gotzsche PC. Is the placebo powerless? an analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594-1602.
- Spiller RC. Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am J Med. 1999;107:91S-97S.
Practice Points
- Recent observations of inflammation in irritable bowel syndrome (IBS) challenge its traditional classification as a functional disorder.
- Delayed-type food hypersensitivities, as detectable by skin patch testing, to type IV food allergens are one plausible cause for intestinal inflammation.
- Patch test–directed food avoidance improves IBS symptoms in some patients and offers a new approach to the evaluation and management of this condition.
- Dermatologists and other health care practitioners with expertise in patch testing are uniquely positioned to utilize these skills to help patients with IBS.
Aerobic exercise reduces BP in resistant hypertension
Aerobic exercise may help reduce blood pressure in patients whose hypertension responds poorly to medications, a new study suggests.
A randomized controlled clinical trial showed that patients with resistant hypertension assigned to a moderate-intensity aerobic exercise training program had lower blood pressure compared with patients who received usual care.
“Resistant hypertension persists as a big clinical challenge because the available treatment options to lower blood pressure in this clinical population, namely drugs and renal denervation, show limited success,” Fernando Ribeiro, PhD, University of Aveiro, Portugal, told this news organization. “Aerobic exercise was safe and associated with a significant and clinically relevant reduction in 24-hour, daytime ambulatory, and office blood pressure.”
The findings were published online August 4 in JAMA Cardiology.
The researchers enrolled 53 patients aged 40-75 years with a diagnosis of resistant hypertension in this prospective, single-blinded trial. Nearly half (24) were women.
Resistant hypertension was defined as having a “mean systolic BP of 130 mm Hg or greater on 24-hour ambulatory BP monitoring and/or 135 mm Hg or greater during daytime hours while taking maximally tolerated doses of at least 3 antihypertensive agents, including a diuretic, or to have a controlled BP while taking 4 or more antihypertensive agents.”
From March 2017 to December 2019 at two sites in Portugal, 26 patients were randomly assigned to a 12-week aerobic exercise training program involving three 40-minute supervised sessions per week in addition to usual care. Another 27 patients in the control group were allocated to receive usual care only.
24-hour ambulatory systolic blood pressure was reduced by 7.1 mm Hg (95% confidence interval, -12.8 to -1.4; P = .02) in patients in the exercise group compared with the control group. In the exercise group, there were additional reductions of:
- -5.1 mm Hg of 24-hour ambulatory diastolic blood pressure (95% CI, -7.9 to -2.3; P = .001)
- -8.4 mm Hg of daytime systolic blood pressure (95% CI, -14.3 to -2.5, P = .006)
- -5.7 mm Hg of daytime diastolic blood pressure (95% CI, -9.0 to -2.4; P = .001)
- -10.0 mm Hg of office systolic blood pressure (95% CI, -17.6 to -2.5; P = .01)
Additionally, a significant improvement in cardiorespiratory fitness (5.05 mL/kg per minute of oxygen consumption; 95% CI, 3.5-6.6; P < .001) was observed in the exercise group compared with the control group.
Although prior research has suggested that exercise may lower blood pressure, this study is particularly useful because it “outlines very specifically what types of exercise you can recommend,” said Daniel Lackland, DrPH, Medical University of South Carolina, Charleston.
Although important, exercise is “one part of the overall management of high blood pressure. If people are being prescribed medication, they should continue taking it and work on lifestyle changes like reducing salt intake and drinking in moderation,” added Dr. Lackland, who was not involved in the research.
Also commenting on the findings, Wanpen Vongpatanasin, MD, UT Southwestern Medical Center, Dallas, pointed out that there are many potential benefits from exercise training. “It might improve endothelial function, decrease vascular stiffness and nervous system reactivity to stress, and improve quality of life for patients,” she said.
The study has several limitations, including a small sample size and a patient population that mostly has “relatively mild hypertension,” Dr. Vongpatanasin said, adding, “We don’t know whether these findings will apply to patients with more severe hypertension.”
It would also have been helpful if investigators monitored patient adherence to prescribed medications through urine or blood samples rather than a questionnaire, and to measure nighttime blood pressure, which is a more important predictor of cardiovascular outcomes, said Dr. Vongpatanasin, who was not associated with the research.
Moving forward, it will be important to “investigate why some patients are nonresponders to the exercise intervention and why some are super-responders,” study author Dr. Ribeiro said.
Dr. Ribeiro, Dr. Lackland, and Dr. Vongpatanasin have disclosed no relevant financial relationships. This study was funded by the European Union through the European Regional Development Fund Operational Competitiveness Factors Program (COMPETE) and by the Portuguese government through the Foundation for Science and Technology. The funders had no role in the study.
A version of this article first appeared on Medscape.com.
Aerobic exercise may help reduce blood pressure in patients whose hypertension responds poorly to medications, a new study suggests.
A randomized controlled clinical trial showed that patients with resistant hypertension assigned to a moderate-intensity aerobic exercise training program had lower blood pressure compared with patients who received usual care.
“Resistant hypertension persists as a big clinical challenge because the available treatment options to lower blood pressure in this clinical population, namely drugs and renal denervation, show limited success,” Fernando Ribeiro, PhD, University of Aveiro, Portugal, told this news organization. “Aerobic exercise was safe and associated with a significant and clinically relevant reduction in 24-hour, daytime ambulatory, and office blood pressure.”
The findings were published online August 4 in JAMA Cardiology.
The researchers enrolled 53 patients aged 40-75 years with a diagnosis of resistant hypertension in this prospective, single-blinded trial. Nearly half (24) were women.
Resistant hypertension was defined as having a “mean systolic BP of 130 mm Hg or greater on 24-hour ambulatory BP monitoring and/or 135 mm Hg or greater during daytime hours while taking maximally tolerated doses of at least 3 antihypertensive agents, including a diuretic, or to have a controlled BP while taking 4 or more antihypertensive agents.”
From March 2017 to December 2019 at two sites in Portugal, 26 patients were randomly assigned to a 12-week aerobic exercise training program involving three 40-minute supervised sessions per week in addition to usual care. Another 27 patients in the control group were allocated to receive usual care only.
24-hour ambulatory systolic blood pressure was reduced by 7.1 mm Hg (95% confidence interval, -12.8 to -1.4; P = .02) in patients in the exercise group compared with the control group. In the exercise group, there were additional reductions of:
- -5.1 mm Hg of 24-hour ambulatory diastolic blood pressure (95% CI, -7.9 to -2.3; P = .001)
- -8.4 mm Hg of daytime systolic blood pressure (95% CI, -14.3 to -2.5, P = .006)
- -5.7 mm Hg of daytime diastolic blood pressure (95% CI, -9.0 to -2.4; P = .001)
- -10.0 mm Hg of office systolic blood pressure (95% CI, -17.6 to -2.5; P = .01)
Additionally, a significant improvement in cardiorespiratory fitness (5.05 mL/kg per minute of oxygen consumption; 95% CI, 3.5-6.6; P < .001) was observed in the exercise group compared with the control group.
Although prior research has suggested that exercise may lower blood pressure, this study is particularly useful because it “outlines very specifically what types of exercise you can recommend,” said Daniel Lackland, DrPH, Medical University of South Carolina, Charleston.
Although important, exercise is “one part of the overall management of high blood pressure. If people are being prescribed medication, they should continue taking it and work on lifestyle changes like reducing salt intake and drinking in moderation,” added Dr. Lackland, who was not involved in the research.
Also commenting on the findings, Wanpen Vongpatanasin, MD, UT Southwestern Medical Center, Dallas, pointed out that there are many potential benefits from exercise training. “It might improve endothelial function, decrease vascular stiffness and nervous system reactivity to stress, and improve quality of life for patients,” she said.
The study has several limitations, including a small sample size and a patient population that mostly has “relatively mild hypertension,” Dr. Vongpatanasin said, adding, “We don’t know whether these findings will apply to patients with more severe hypertension.”
It would also have been helpful if investigators monitored patient adherence to prescribed medications through urine or blood samples rather than a questionnaire, and to measure nighttime blood pressure, which is a more important predictor of cardiovascular outcomes, said Dr. Vongpatanasin, who was not associated with the research.
Moving forward, it will be important to “investigate why some patients are nonresponders to the exercise intervention and why some are super-responders,” study author Dr. Ribeiro said.
Dr. Ribeiro, Dr. Lackland, and Dr. Vongpatanasin have disclosed no relevant financial relationships. This study was funded by the European Union through the European Regional Development Fund Operational Competitiveness Factors Program (COMPETE) and by the Portuguese government through the Foundation for Science and Technology. The funders had no role in the study.
A version of this article first appeared on Medscape.com.
Aerobic exercise may help reduce blood pressure in patients whose hypertension responds poorly to medications, a new study suggests.
A randomized controlled clinical trial showed that patients with resistant hypertension assigned to a moderate-intensity aerobic exercise training program had lower blood pressure compared with patients who received usual care.
“Resistant hypertension persists as a big clinical challenge because the available treatment options to lower blood pressure in this clinical population, namely drugs and renal denervation, show limited success,” Fernando Ribeiro, PhD, University of Aveiro, Portugal, told this news organization. “Aerobic exercise was safe and associated with a significant and clinically relevant reduction in 24-hour, daytime ambulatory, and office blood pressure.”
The findings were published online August 4 in JAMA Cardiology.
The researchers enrolled 53 patients aged 40-75 years with a diagnosis of resistant hypertension in this prospective, single-blinded trial. Nearly half (24) were women.
Resistant hypertension was defined as having a “mean systolic BP of 130 mm Hg or greater on 24-hour ambulatory BP monitoring and/or 135 mm Hg or greater during daytime hours while taking maximally tolerated doses of at least 3 antihypertensive agents, including a diuretic, or to have a controlled BP while taking 4 or more antihypertensive agents.”
From March 2017 to December 2019 at two sites in Portugal, 26 patients were randomly assigned to a 12-week aerobic exercise training program involving three 40-minute supervised sessions per week in addition to usual care. Another 27 patients in the control group were allocated to receive usual care only.
24-hour ambulatory systolic blood pressure was reduced by 7.1 mm Hg (95% confidence interval, -12.8 to -1.4; P = .02) in patients in the exercise group compared with the control group. In the exercise group, there were additional reductions of:
- -5.1 mm Hg of 24-hour ambulatory diastolic blood pressure (95% CI, -7.9 to -2.3; P = .001)
- -8.4 mm Hg of daytime systolic blood pressure (95% CI, -14.3 to -2.5, P = .006)
- -5.7 mm Hg of daytime diastolic blood pressure (95% CI, -9.0 to -2.4; P = .001)
- -10.0 mm Hg of office systolic blood pressure (95% CI, -17.6 to -2.5; P = .01)
Additionally, a significant improvement in cardiorespiratory fitness (5.05 mL/kg per minute of oxygen consumption; 95% CI, 3.5-6.6; P < .001) was observed in the exercise group compared with the control group.
Although prior research has suggested that exercise may lower blood pressure, this study is particularly useful because it “outlines very specifically what types of exercise you can recommend,” said Daniel Lackland, DrPH, Medical University of South Carolina, Charleston.
Although important, exercise is “one part of the overall management of high blood pressure. If people are being prescribed medication, they should continue taking it and work on lifestyle changes like reducing salt intake and drinking in moderation,” added Dr. Lackland, who was not involved in the research.
Also commenting on the findings, Wanpen Vongpatanasin, MD, UT Southwestern Medical Center, Dallas, pointed out that there are many potential benefits from exercise training. “It might improve endothelial function, decrease vascular stiffness and nervous system reactivity to stress, and improve quality of life for patients,” she said.
The study has several limitations, including a small sample size and a patient population that mostly has “relatively mild hypertension,” Dr. Vongpatanasin said, adding, “We don’t know whether these findings will apply to patients with more severe hypertension.”
It would also have been helpful if investigators monitored patient adherence to prescribed medications through urine or blood samples rather than a questionnaire, and to measure nighttime blood pressure, which is a more important predictor of cardiovascular outcomes, said Dr. Vongpatanasin, who was not associated with the research.
Moving forward, it will be important to “investigate why some patients are nonresponders to the exercise intervention and why some are super-responders,” study author Dr. Ribeiro said.
Dr. Ribeiro, Dr. Lackland, and Dr. Vongpatanasin have disclosed no relevant financial relationships. This study was funded by the European Union through the European Regional Development Fund Operational Competitiveness Factors Program (COMPETE) and by the Portuguese government through the Foundation for Science and Technology. The funders had no role in the study.
A version of this article first appeared on Medscape.com.
CDC: Vaccination may cut risk of COVID reinfection in half
The Centers for Disease Control and Prevention has recommended that everyone get a COVID-19 vaccine, even if they’ve had the virus before. Yet many skeptics have held off getting the shots, believing that immunity generated by their previous infection will protect them if they should encounter the virus again.
A new study published in the CDC’s Morbidity and Mortality Weekly Report pokes holes in this notion. It shows people who have recovered from COVID-19 but haven’t been vaccinated have more than double the risk of testing positive for the virus again, compared with someone who was vaccinated after an initial infection.
The study looked at 738 Kentucky residents who had an initial bout of COVID-19 in 2020. About 250 of them tested positive for COVID-19 a second time between May and July of 2021, when the Delta variant became dominant in the United States.
The study matched each person who’d been reinfected with two people of the same sex and roughly the same age who had caught their initial COVID infection within the same week. The researchers then cross-matched those cases with data from Kentucky’s Immunization Registry.
They found that those who were unvaccinated had more than double the risk of being reinfected during the Delta wave. Partial vaccination appeared to have no significant impact on the risk of reinfection.
Among those who were reinfected, 20% were fully vaccinated, while 34% of those who did not get reinfected were fully vaccinated.
The study is observational, meaning it can’t show cause and effect; and the researchers had no information on the severity of the infections. Alyson Cavanaugh, PhD, a member of the CDC’s Epidemic Intelligence Service who led the study, said it is possible that some of the people who tested positive a second time had asymptomatic infections that were picked up through routine screening.
Still, the study backs up previous research and suggests that vaccination offers important additional protection.
“Our laboratory studies have shown that there’s an added benefit of vaccine for people who’ve had previous COVID-19. This is a real-world, epidemiologic study that found that among people who’d previously already had COVID-19, those who were vaccinated had lower odds of being reinfected,” Dr. Cavanaugh said.
“If you have had COVID-19 before, please still get vaccinated,” said CDC Director Rochelle Walensky, MD, in a written media statement. “This study shows you are twice as likely to get infected again if you are unvaccinated. Getting the vaccine is the best way to protect yourself and others around you, especially as the more contagious Delta variant spreads around the country.”
In a White House COVID-19 Response Team briefing in May, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease, explained why vaccines create stronger immunity than infection. He highlighted new research showing that two doses of an mRNA vaccine produce levels of neutralizing antibodies that are up to 10 times higher than the levels found in the blood of people who’ve recovered from COVID-19. Vaccines also enhance B cells and T cells in people who’ve recovered from COVID-19, which broadens the spectrum of protection and helps to fend off variants.
The study has some important limitations, which the authors acknowledged. The first is that second infections weren’t confirmed with genetic sequencing, so the researchers couldn’t definitively tell if a person tested positive a second time because they caught a new virus, or if they were somehow still shedding virus from their first infection. Given that the tests were at least 5 months apart, though, the researchers think reinfection is the most likely explanation.
Another bias in the study could have something to do with vaccination. Vaccinated people may have been less likely to be tested for COVID-19 after their vaccines, so the association or reinfection with a lack of vaccination may be overestimated.
Also, people who were vaccinated at federal sites or in another state were not logged in the state’s immunization registry, which may have skewed the data.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has recommended that everyone get a COVID-19 vaccine, even if they’ve had the virus before. Yet many skeptics have held off getting the shots, believing that immunity generated by their previous infection will protect them if they should encounter the virus again.
A new study published in the CDC’s Morbidity and Mortality Weekly Report pokes holes in this notion. It shows people who have recovered from COVID-19 but haven’t been vaccinated have more than double the risk of testing positive for the virus again, compared with someone who was vaccinated after an initial infection.
The study looked at 738 Kentucky residents who had an initial bout of COVID-19 in 2020. About 250 of them tested positive for COVID-19 a second time between May and July of 2021, when the Delta variant became dominant in the United States.
The study matched each person who’d been reinfected with two people of the same sex and roughly the same age who had caught their initial COVID infection within the same week. The researchers then cross-matched those cases with data from Kentucky’s Immunization Registry.
They found that those who were unvaccinated had more than double the risk of being reinfected during the Delta wave. Partial vaccination appeared to have no significant impact on the risk of reinfection.
Among those who were reinfected, 20% were fully vaccinated, while 34% of those who did not get reinfected were fully vaccinated.
The study is observational, meaning it can’t show cause and effect; and the researchers had no information on the severity of the infections. Alyson Cavanaugh, PhD, a member of the CDC’s Epidemic Intelligence Service who led the study, said it is possible that some of the people who tested positive a second time had asymptomatic infections that were picked up through routine screening.
Still, the study backs up previous research and suggests that vaccination offers important additional protection.
“Our laboratory studies have shown that there’s an added benefit of vaccine for people who’ve had previous COVID-19. This is a real-world, epidemiologic study that found that among people who’d previously already had COVID-19, those who were vaccinated had lower odds of being reinfected,” Dr. Cavanaugh said.
“If you have had COVID-19 before, please still get vaccinated,” said CDC Director Rochelle Walensky, MD, in a written media statement. “This study shows you are twice as likely to get infected again if you are unvaccinated. Getting the vaccine is the best way to protect yourself and others around you, especially as the more contagious Delta variant spreads around the country.”
In a White House COVID-19 Response Team briefing in May, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease, explained why vaccines create stronger immunity than infection. He highlighted new research showing that two doses of an mRNA vaccine produce levels of neutralizing antibodies that are up to 10 times higher than the levels found in the blood of people who’ve recovered from COVID-19. Vaccines also enhance B cells and T cells in people who’ve recovered from COVID-19, which broadens the spectrum of protection and helps to fend off variants.
The study has some important limitations, which the authors acknowledged. The first is that second infections weren’t confirmed with genetic sequencing, so the researchers couldn’t definitively tell if a person tested positive a second time because they caught a new virus, or if they were somehow still shedding virus from their first infection. Given that the tests were at least 5 months apart, though, the researchers think reinfection is the most likely explanation.
Another bias in the study could have something to do with vaccination. Vaccinated people may have been less likely to be tested for COVID-19 after their vaccines, so the association or reinfection with a lack of vaccination may be overestimated.
Also, people who were vaccinated at federal sites or in another state were not logged in the state’s immunization registry, which may have skewed the data.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has recommended that everyone get a COVID-19 vaccine, even if they’ve had the virus before. Yet many skeptics have held off getting the shots, believing that immunity generated by their previous infection will protect them if they should encounter the virus again.
A new study published in the CDC’s Morbidity and Mortality Weekly Report pokes holes in this notion. It shows people who have recovered from COVID-19 but haven’t been vaccinated have more than double the risk of testing positive for the virus again, compared with someone who was vaccinated after an initial infection.
The study looked at 738 Kentucky residents who had an initial bout of COVID-19 in 2020. About 250 of them tested positive for COVID-19 a second time between May and July of 2021, when the Delta variant became dominant in the United States.
The study matched each person who’d been reinfected with two people of the same sex and roughly the same age who had caught their initial COVID infection within the same week. The researchers then cross-matched those cases with data from Kentucky’s Immunization Registry.
They found that those who were unvaccinated had more than double the risk of being reinfected during the Delta wave. Partial vaccination appeared to have no significant impact on the risk of reinfection.
Among those who were reinfected, 20% were fully vaccinated, while 34% of those who did not get reinfected were fully vaccinated.
The study is observational, meaning it can’t show cause and effect; and the researchers had no information on the severity of the infections. Alyson Cavanaugh, PhD, a member of the CDC’s Epidemic Intelligence Service who led the study, said it is possible that some of the people who tested positive a second time had asymptomatic infections that were picked up through routine screening.
Still, the study backs up previous research and suggests that vaccination offers important additional protection.
“Our laboratory studies have shown that there’s an added benefit of vaccine for people who’ve had previous COVID-19. This is a real-world, epidemiologic study that found that among people who’d previously already had COVID-19, those who were vaccinated had lower odds of being reinfected,” Dr. Cavanaugh said.
“If you have had COVID-19 before, please still get vaccinated,” said CDC Director Rochelle Walensky, MD, in a written media statement. “This study shows you are twice as likely to get infected again if you are unvaccinated. Getting the vaccine is the best way to protect yourself and others around you, especially as the more contagious Delta variant spreads around the country.”
In a White House COVID-19 Response Team briefing in May, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease, explained why vaccines create stronger immunity than infection. He highlighted new research showing that two doses of an mRNA vaccine produce levels of neutralizing antibodies that are up to 10 times higher than the levels found in the blood of people who’ve recovered from COVID-19. Vaccines also enhance B cells and T cells in people who’ve recovered from COVID-19, which broadens the spectrum of protection and helps to fend off variants.
The study has some important limitations, which the authors acknowledged. The first is that second infections weren’t confirmed with genetic sequencing, so the researchers couldn’t definitively tell if a person tested positive a second time because they caught a new virus, or if they were somehow still shedding virus from their first infection. Given that the tests were at least 5 months apart, though, the researchers think reinfection is the most likely explanation.
Another bias in the study could have something to do with vaccination. Vaccinated people may have been less likely to be tested for COVID-19 after their vaccines, so the association or reinfection with a lack of vaccination may be overestimated.
Also, people who were vaccinated at federal sites or in another state were not logged in the state’s immunization registry, which may have skewed the data.
A version of this article first appeared on Medscape.com.