User login
Reassuring rates of ADHD after assisted reproductive techniques
according to a study published in Pediatrics.
The findings, based on an analysis of data from more than 1.5 million children in Sweden, provide “additional reassurance concerning offspring neurodevelopment after use of ART,” study author Chen Wang, MPH, and colleagues said. The results show the importance of accounting for underlying infertility when studying ART safety, they added. Mr. Wang is a researcher in the department of medical epidemiology and biostatistics at Karolinska Institutet in Stockholm.
Prior research has not shown major differences during early childhood between children conceived with ART and those conceived spontaneously. To examine long-term neurodevelopmental outcomes, including ADHD and school performance, the investigators analyzed data in Swedish population registers from children born between 1986 and 2012.
Infertility and the use of ART became increasingly common during the study period, the researchers noted. Between 1986 and 2001, 7% of births were to couples with known infertility, and 13% of these births were achieved with ART. Between 1996 and 2012, 11% of births were to couples with infertility, and 26% of these births were achieved with ART.
“Couples with infertility were more likely older and married or cohabiting, compared with couples with no known infertility,” Mr. Wang and colleagues reported. “Among infertile couples, those that conceived with ART had, on average, higher age and education, and the women were less likely to smoke.”
The investigators estimated that the cumulative incidence of ADHD by age 15 years was 6.2% in children conceived with ART, 7.3% among children of couples with infertility who did not use ART, and 7.1% in children born to couples with no known infertility.
Overall, children conceived with ART were at lower risk of ADHD (hazard ratio, 0.83). But after adjusting for parental characteristics and health factors, the researchers found a “slightly elevated risk of ADHD with ART,” with adjusted HRs of 1.05-1.07.
When the researchers focused on children born to couples with infertility, ART was associated with a lower risk of ADHD (adjusted HR, 0.80), compared with spontaneous conception. Accounting for parental characteristics and health history, however, “attenuated the association toward the null,” the researchers reported.
The researchers also compared ART methods, including intracytoplasmic sperm injection versus standard in vitro fertilization (IVF), and fresh embryo transfer versus frozen embryo transfer. The various procedures were not associated with substantially different risks.
Patterns for school performance were generally similar to those for ADHD.
“In this large follow-up of nationwide birth cohorts, we observed lower risk of ADHD and slightly better overall school performance in children conceived with ART, compared with all other children. Differences in parental characteristics appeared to completely explain and even slightly reverse the associations,” the study authors said. “When the comparison was restricted to children of couples with known infertility, no differences were seen.”
The study was well designed and “spans more than 25 years of ART during which treatments have changed dramatically,” commented Barbara Luke, ScD, MPH, professor of obstetrics, gynecology, and reproductive biology at Michigan State University, East Lansing.
Dr. Luke and colleagues have studied academic achievement in children conceived with IVF in Texas. The results of the Swedish study “are in line with U.S. studies, and are generally reassuring,” Dr. Luke said.
The U.S. studies also showed that parental factors may play a role in understanding academic performance.
“In our studies of third-grade and sixth-grade academic outcomes, we found differences by racial/Hispanic origin groups, gender, and maternal age,” she said.
The study by Mr. Wang and coauthors was funded by grants from a Swedish government agency and the National Institutes of Health. The researchers and Dr. Luke had no relevant financial disclosures.
according to a study published in Pediatrics.
The findings, based on an analysis of data from more than 1.5 million children in Sweden, provide “additional reassurance concerning offspring neurodevelopment after use of ART,” study author Chen Wang, MPH, and colleagues said. The results show the importance of accounting for underlying infertility when studying ART safety, they added. Mr. Wang is a researcher in the department of medical epidemiology and biostatistics at Karolinska Institutet in Stockholm.
Prior research has not shown major differences during early childhood between children conceived with ART and those conceived spontaneously. To examine long-term neurodevelopmental outcomes, including ADHD and school performance, the investigators analyzed data in Swedish population registers from children born between 1986 and 2012.
Infertility and the use of ART became increasingly common during the study period, the researchers noted. Between 1986 and 2001, 7% of births were to couples with known infertility, and 13% of these births were achieved with ART. Between 1996 and 2012, 11% of births were to couples with infertility, and 26% of these births were achieved with ART.
“Couples with infertility were more likely older and married or cohabiting, compared with couples with no known infertility,” Mr. Wang and colleagues reported. “Among infertile couples, those that conceived with ART had, on average, higher age and education, and the women were less likely to smoke.”
The investigators estimated that the cumulative incidence of ADHD by age 15 years was 6.2% in children conceived with ART, 7.3% among children of couples with infertility who did not use ART, and 7.1% in children born to couples with no known infertility.
Overall, children conceived with ART were at lower risk of ADHD (hazard ratio, 0.83). But after adjusting for parental characteristics and health factors, the researchers found a “slightly elevated risk of ADHD with ART,” with adjusted HRs of 1.05-1.07.
When the researchers focused on children born to couples with infertility, ART was associated with a lower risk of ADHD (adjusted HR, 0.80), compared with spontaneous conception. Accounting for parental characteristics and health history, however, “attenuated the association toward the null,” the researchers reported.
The researchers also compared ART methods, including intracytoplasmic sperm injection versus standard in vitro fertilization (IVF), and fresh embryo transfer versus frozen embryo transfer. The various procedures were not associated with substantially different risks.
Patterns for school performance were generally similar to those for ADHD.
“In this large follow-up of nationwide birth cohorts, we observed lower risk of ADHD and slightly better overall school performance in children conceived with ART, compared with all other children. Differences in parental characteristics appeared to completely explain and even slightly reverse the associations,” the study authors said. “When the comparison was restricted to children of couples with known infertility, no differences were seen.”
The study was well designed and “spans more than 25 years of ART during which treatments have changed dramatically,” commented Barbara Luke, ScD, MPH, professor of obstetrics, gynecology, and reproductive biology at Michigan State University, East Lansing.
Dr. Luke and colleagues have studied academic achievement in children conceived with IVF in Texas. The results of the Swedish study “are in line with U.S. studies, and are generally reassuring,” Dr. Luke said.
The U.S. studies also showed that parental factors may play a role in understanding academic performance.
“In our studies of third-grade and sixth-grade academic outcomes, we found differences by racial/Hispanic origin groups, gender, and maternal age,” she said.
The study by Mr. Wang and coauthors was funded by grants from a Swedish government agency and the National Institutes of Health. The researchers and Dr. Luke had no relevant financial disclosures.
according to a study published in Pediatrics.
The findings, based on an analysis of data from more than 1.5 million children in Sweden, provide “additional reassurance concerning offspring neurodevelopment after use of ART,” study author Chen Wang, MPH, and colleagues said. The results show the importance of accounting for underlying infertility when studying ART safety, they added. Mr. Wang is a researcher in the department of medical epidemiology and biostatistics at Karolinska Institutet in Stockholm.
Prior research has not shown major differences during early childhood between children conceived with ART and those conceived spontaneously. To examine long-term neurodevelopmental outcomes, including ADHD and school performance, the investigators analyzed data in Swedish population registers from children born between 1986 and 2012.
Infertility and the use of ART became increasingly common during the study period, the researchers noted. Between 1986 and 2001, 7% of births were to couples with known infertility, and 13% of these births were achieved with ART. Between 1996 and 2012, 11% of births were to couples with infertility, and 26% of these births were achieved with ART.
“Couples with infertility were more likely older and married or cohabiting, compared with couples with no known infertility,” Mr. Wang and colleagues reported. “Among infertile couples, those that conceived with ART had, on average, higher age and education, and the women were less likely to smoke.”
The investigators estimated that the cumulative incidence of ADHD by age 15 years was 6.2% in children conceived with ART, 7.3% among children of couples with infertility who did not use ART, and 7.1% in children born to couples with no known infertility.
Overall, children conceived with ART were at lower risk of ADHD (hazard ratio, 0.83). But after adjusting for parental characteristics and health factors, the researchers found a “slightly elevated risk of ADHD with ART,” with adjusted HRs of 1.05-1.07.
When the researchers focused on children born to couples with infertility, ART was associated with a lower risk of ADHD (adjusted HR, 0.80), compared with spontaneous conception. Accounting for parental characteristics and health history, however, “attenuated the association toward the null,” the researchers reported.
The researchers also compared ART methods, including intracytoplasmic sperm injection versus standard in vitro fertilization (IVF), and fresh embryo transfer versus frozen embryo transfer. The various procedures were not associated with substantially different risks.
Patterns for school performance were generally similar to those for ADHD.
“In this large follow-up of nationwide birth cohorts, we observed lower risk of ADHD and slightly better overall school performance in children conceived with ART, compared with all other children. Differences in parental characteristics appeared to completely explain and even slightly reverse the associations,” the study authors said. “When the comparison was restricted to children of couples with known infertility, no differences were seen.”
The study was well designed and “spans more than 25 years of ART during which treatments have changed dramatically,” commented Barbara Luke, ScD, MPH, professor of obstetrics, gynecology, and reproductive biology at Michigan State University, East Lansing.
Dr. Luke and colleagues have studied academic achievement in children conceived with IVF in Texas. The results of the Swedish study “are in line with U.S. studies, and are generally reassuring,” Dr. Luke said.
The U.S. studies also showed that parental factors may play a role in understanding academic performance.
“In our studies of third-grade and sixth-grade academic outcomes, we found differences by racial/Hispanic origin groups, gender, and maternal age,” she said.
The study by Mr. Wang and coauthors was funded by grants from a Swedish government agency and the National Institutes of Health. The researchers and Dr. Luke had no relevant financial disclosures.
FROM PEDIATRICS
Delta becomes dominant coronavirus variant in U.S.
The contagious Delta variant has become the dominant form of the coronavirus in the United States, now accounting for more than 51% of COVID-19 cases in the country, according to new CDC data to updated on July 6.
The variant, also known as B.1.617.2 and first detected in India, makes up more than 80% of new cases in some Midwestern states, including Iowa, Kansas, and Missouri. Delta also accounts for 74% of cases in Western states such as Colorado and Utah and 59% of cases in Southern states such as Louisiana and Texas.
Communities with low vaccination rates are bearing the brunt of new Delta cases. Public health experts are urging those who are unvaccinated to get a shot to protect themselves and their communities against future surges.
“Right now we have two Americas: the vaccinated and the unvaccinated,” Paul Offit, MD, an infectious disease specialist at Children’s Hospital of Philadelphia, told NPR.
“We’re feeling pretty good right now because it’s the summer,” he said. “But come winter, if we still have a significant percentage of the population that is unvaccinated, we’re going to see this virus surge again.”
So far, COVID-19 vaccines appear to protect people against the Delta variant. But health officials are watching other variants that could evade vaccine protection and lead to major outbreaks this year.
For instance, certain mutations in the Epsilon variant may allow it to evade the immunity from past infections and current COVID-19 vaccines, according to a new study published July 1 in the Science. The variant, also known as B.1.427/B.1.429 and first identified in California, has now been reported in 34 countries and could become widespread in the United States.
Researchers from the University of Washington and clinics in Switzerland tested the variant in blood samples from vaccinated people, as well as those who were previously infected with COVID-19. They found that the neutralizing power was reduced by about 2 to 3½ times.
The research team also visualized the variant and found that three mutations on Epsilon’s spike protein allow the virus to escape certain antibodies and lower the strength of vaccines.
Epsilon “relies on an indirect and unusual neutralization-escape strategy,” they wrote, saying that understanding these escape routes could help scientists track new variants, curb the pandemic, and create booster shots.
In Australia, for instance, public health officials have detected the Lambda variant, which could be more infectious than the Delta variant and resistant to vaccines, according to Sky News.
A hotel quarantine program in New South Wales identified the variant in someone who had returned from travel, the news outlet reported. Also known as C.37, Lambda was named a “variant of interest” by the World Health Organization in June.
Lambda was first identified in Peru in December and now accounts for more than 80% of the country’s cases, according to the Financial Times. It has since been found in 27 countries, including the U.S., U.K., and Germany.
The variant has seven mutations on the spike protein that allow the virus to infect human cells, the news outlet reported. One mutation is like another mutation on the Delta variant, which could make it more contagious.
In a preprint study published July 1, researchers at the University of Chile at Santiago found that Lambda is better able to escape antibodies created by the CoronaVac vaccine made by Sinovac in China. In the paper, which hasn’t yet been peer-reviewed, researchers tested blood samples from local health care workers in Santiago who had received two doses of the vaccine.
“Our data revealed that the spike protein ... carries mutations conferring increased infectivity and the ability to escape from neutralizing antibodies,” they wrote.
The research team urged countries to continue testing for contagious variants, even in areas with high vaccination rates, so scientists can identify mutations quickly and analyze whether new variants can escape vaccines.
“The world has to get its act together,” Saad Omer, PhD, director of the Yale Institute for Global Health, told NPR. “Otherwise yet another, potentially more dangerous, variant could emerge.”
A version of this article first appeared on WebMD.com.
The contagious Delta variant has become the dominant form of the coronavirus in the United States, now accounting for more than 51% of COVID-19 cases in the country, according to new CDC data to updated on July 6.
The variant, also known as B.1.617.2 and first detected in India, makes up more than 80% of new cases in some Midwestern states, including Iowa, Kansas, and Missouri. Delta also accounts for 74% of cases in Western states such as Colorado and Utah and 59% of cases in Southern states such as Louisiana and Texas.
Communities with low vaccination rates are bearing the brunt of new Delta cases. Public health experts are urging those who are unvaccinated to get a shot to protect themselves and their communities against future surges.
“Right now we have two Americas: the vaccinated and the unvaccinated,” Paul Offit, MD, an infectious disease specialist at Children’s Hospital of Philadelphia, told NPR.
“We’re feeling pretty good right now because it’s the summer,” he said. “But come winter, if we still have a significant percentage of the population that is unvaccinated, we’re going to see this virus surge again.”
So far, COVID-19 vaccines appear to protect people against the Delta variant. But health officials are watching other variants that could evade vaccine protection and lead to major outbreaks this year.
For instance, certain mutations in the Epsilon variant may allow it to evade the immunity from past infections and current COVID-19 vaccines, according to a new study published July 1 in the Science. The variant, also known as B.1.427/B.1.429 and first identified in California, has now been reported in 34 countries and could become widespread in the United States.
Researchers from the University of Washington and clinics in Switzerland tested the variant in blood samples from vaccinated people, as well as those who were previously infected with COVID-19. They found that the neutralizing power was reduced by about 2 to 3½ times.
The research team also visualized the variant and found that three mutations on Epsilon’s spike protein allow the virus to escape certain antibodies and lower the strength of vaccines.
Epsilon “relies on an indirect and unusual neutralization-escape strategy,” they wrote, saying that understanding these escape routes could help scientists track new variants, curb the pandemic, and create booster shots.
In Australia, for instance, public health officials have detected the Lambda variant, which could be more infectious than the Delta variant and resistant to vaccines, according to Sky News.
A hotel quarantine program in New South Wales identified the variant in someone who had returned from travel, the news outlet reported. Also known as C.37, Lambda was named a “variant of interest” by the World Health Organization in June.
Lambda was first identified in Peru in December and now accounts for more than 80% of the country’s cases, according to the Financial Times. It has since been found in 27 countries, including the U.S., U.K., and Germany.
The variant has seven mutations on the spike protein that allow the virus to infect human cells, the news outlet reported. One mutation is like another mutation on the Delta variant, which could make it more contagious.
In a preprint study published July 1, researchers at the University of Chile at Santiago found that Lambda is better able to escape antibodies created by the CoronaVac vaccine made by Sinovac in China. In the paper, which hasn’t yet been peer-reviewed, researchers tested blood samples from local health care workers in Santiago who had received two doses of the vaccine.
“Our data revealed that the spike protein ... carries mutations conferring increased infectivity and the ability to escape from neutralizing antibodies,” they wrote.
The research team urged countries to continue testing for contagious variants, even in areas with high vaccination rates, so scientists can identify mutations quickly and analyze whether new variants can escape vaccines.
“The world has to get its act together,” Saad Omer, PhD, director of the Yale Institute for Global Health, told NPR. “Otherwise yet another, potentially more dangerous, variant could emerge.”
A version of this article first appeared on WebMD.com.
The contagious Delta variant has become the dominant form of the coronavirus in the United States, now accounting for more than 51% of COVID-19 cases in the country, according to new CDC data to updated on July 6.
The variant, also known as B.1.617.2 and first detected in India, makes up more than 80% of new cases in some Midwestern states, including Iowa, Kansas, and Missouri. Delta also accounts for 74% of cases in Western states such as Colorado and Utah and 59% of cases in Southern states such as Louisiana and Texas.
Communities with low vaccination rates are bearing the brunt of new Delta cases. Public health experts are urging those who are unvaccinated to get a shot to protect themselves and their communities against future surges.
“Right now we have two Americas: the vaccinated and the unvaccinated,” Paul Offit, MD, an infectious disease specialist at Children’s Hospital of Philadelphia, told NPR.
“We’re feeling pretty good right now because it’s the summer,” he said. “But come winter, if we still have a significant percentage of the population that is unvaccinated, we’re going to see this virus surge again.”
So far, COVID-19 vaccines appear to protect people against the Delta variant. But health officials are watching other variants that could evade vaccine protection and lead to major outbreaks this year.
For instance, certain mutations in the Epsilon variant may allow it to evade the immunity from past infections and current COVID-19 vaccines, according to a new study published July 1 in the Science. The variant, also known as B.1.427/B.1.429 and first identified in California, has now been reported in 34 countries and could become widespread in the United States.
Researchers from the University of Washington and clinics in Switzerland tested the variant in blood samples from vaccinated people, as well as those who were previously infected with COVID-19. They found that the neutralizing power was reduced by about 2 to 3½ times.
The research team also visualized the variant and found that three mutations on Epsilon’s spike protein allow the virus to escape certain antibodies and lower the strength of vaccines.
Epsilon “relies on an indirect and unusual neutralization-escape strategy,” they wrote, saying that understanding these escape routes could help scientists track new variants, curb the pandemic, and create booster shots.
In Australia, for instance, public health officials have detected the Lambda variant, which could be more infectious than the Delta variant and resistant to vaccines, according to Sky News.
A hotel quarantine program in New South Wales identified the variant in someone who had returned from travel, the news outlet reported. Also known as C.37, Lambda was named a “variant of interest” by the World Health Organization in June.
Lambda was first identified in Peru in December and now accounts for more than 80% of the country’s cases, according to the Financial Times. It has since been found in 27 countries, including the U.S., U.K., and Germany.
The variant has seven mutations on the spike protein that allow the virus to infect human cells, the news outlet reported. One mutation is like another mutation on the Delta variant, which could make it more contagious.
In a preprint study published July 1, researchers at the University of Chile at Santiago found that Lambda is better able to escape antibodies created by the CoronaVac vaccine made by Sinovac in China. In the paper, which hasn’t yet been peer-reviewed, researchers tested blood samples from local health care workers in Santiago who had received two doses of the vaccine.
“Our data revealed that the spike protein ... carries mutations conferring increased infectivity and the ability to escape from neutralizing antibodies,” they wrote.
The research team urged countries to continue testing for contagious variants, even in areas with high vaccination rates, so scientists can identify mutations quickly and analyze whether new variants can escape vaccines.
“The world has to get its act together,” Saad Omer, PhD, director of the Yale Institute for Global Health, told NPR. “Otherwise yet another, potentially more dangerous, variant could emerge.”
A version of this article first appeared on WebMD.com.
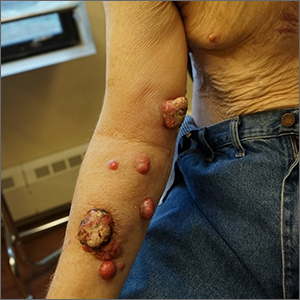
Nodules on the arm
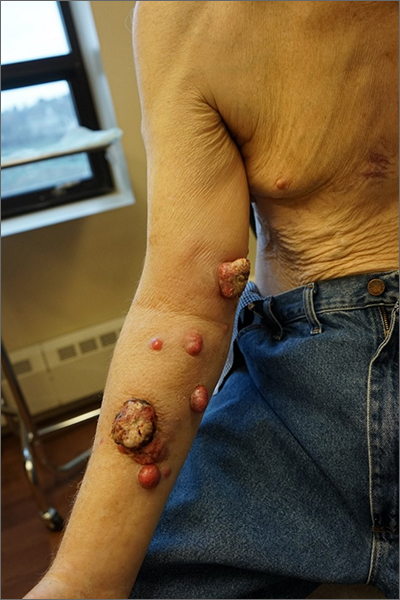
Excisional biopsy was performed on a small tumor and immune staining suggested lung cancer as the primary source.
Metastatic cancer of an unknown primary source accounts for 3% to 5% of invasive cancers.1 Poorly differentiated SCC, as in this case, may arise from skin, lung, or head and neck SCC. Risks of metastatic disease in cutaneous SCC include size greater than 2 cm, depth greater than 2 mm, and location on the ear, hand, lip, or within a burn.2 Recurrent tumors, poorly differentiated tumors, and tumors demonstrating perineural invasion also increase the risk of metastasis.
Having failed both previous treatments, further tissue characterization offered some hope of effective immunotherapy. Specifically, SCC from lung cancer as a primary source may express programmed-death ligand 1 (PD-L1), a transmembrane protein that suppresses adaptive immune responses. Pembrolizumab inhibits this molecule leading to a more aggressive immune response to tumor cells. Additionally, tumor profiling of specific oncogenes can highlight potentially beneficial therapies.
In this case, gene profiling identified several active tumor oncogenes, but PD-L1 was not strongly expressed. Despite the effort, this process did not uncover practical or novel treatment options. The patient was offered an empiric trial of immunotherapy but opted to pursue palliative therapy alone and passed away 2 months later.
This case highlights the role of multidisciplinary care of metastatic disease to the skin and the potential role, and limitations, of skin biopsy in tumor profiling and treatment guidance. Even though the most likely primary site was lung, cutaneous metastases can be equally devastating.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet. 2012;379:1428-1435. doi: 10.1016/S0140-6736(11)61178-1
2. Brougham NDLS, Dennett ER, Cameron R, et al. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J Surg Oncol. 2012;106:811-815. doi: 10.1002/jso.23155

Excisional biopsy was performed on a small tumor and immune staining suggested lung cancer as the primary source.
Metastatic cancer of an unknown primary source accounts for 3% to 5% of invasive cancers.1 Poorly differentiated SCC, as in this case, may arise from skin, lung, or head and neck SCC. Risks of metastatic disease in cutaneous SCC include size greater than 2 cm, depth greater than 2 mm, and location on the ear, hand, lip, or within a burn.2 Recurrent tumors, poorly differentiated tumors, and tumors demonstrating perineural invasion also increase the risk of metastasis.
Having failed both previous treatments, further tissue characterization offered some hope of effective immunotherapy. Specifically, SCC from lung cancer as a primary source may express programmed-death ligand 1 (PD-L1), a transmembrane protein that suppresses adaptive immune responses. Pembrolizumab inhibits this molecule leading to a more aggressive immune response to tumor cells. Additionally, tumor profiling of specific oncogenes can highlight potentially beneficial therapies.
In this case, gene profiling identified several active tumor oncogenes, but PD-L1 was not strongly expressed. Despite the effort, this process did not uncover practical or novel treatment options. The patient was offered an empiric trial of immunotherapy but opted to pursue palliative therapy alone and passed away 2 months later.
This case highlights the role of multidisciplinary care of metastatic disease to the skin and the potential role, and limitations, of skin biopsy in tumor profiling and treatment guidance. Even though the most likely primary site was lung, cutaneous metastases can be equally devastating.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Excisional biopsy was performed on a small tumor and immune staining suggested lung cancer as the primary source.
Metastatic cancer of an unknown primary source accounts for 3% to 5% of invasive cancers.1 Poorly differentiated SCC, as in this case, may arise from skin, lung, or head and neck SCC. Risks of metastatic disease in cutaneous SCC include size greater than 2 cm, depth greater than 2 mm, and location on the ear, hand, lip, or within a burn.2 Recurrent tumors, poorly differentiated tumors, and tumors demonstrating perineural invasion also increase the risk of metastasis.
Having failed both previous treatments, further tissue characterization offered some hope of effective immunotherapy. Specifically, SCC from lung cancer as a primary source may express programmed-death ligand 1 (PD-L1), a transmembrane protein that suppresses adaptive immune responses. Pembrolizumab inhibits this molecule leading to a more aggressive immune response to tumor cells. Additionally, tumor profiling of specific oncogenes can highlight potentially beneficial therapies.
In this case, gene profiling identified several active tumor oncogenes, but PD-L1 was not strongly expressed. Despite the effort, this process did not uncover practical or novel treatment options. The patient was offered an empiric trial of immunotherapy but opted to pursue palliative therapy alone and passed away 2 months later.
This case highlights the role of multidisciplinary care of metastatic disease to the skin and the potential role, and limitations, of skin biopsy in tumor profiling and treatment guidance. Even though the most likely primary site was lung, cutaneous metastases can be equally devastating.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet. 2012;379:1428-1435. doi: 10.1016/S0140-6736(11)61178-1
2. Brougham NDLS, Dennett ER, Cameron R, et al. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J Surg Oncol. 2012;106:811-815. doi: 10.1002/jso.23155
1. Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet. 2012;379:1428-1435. doi: 10.1016/S0140-6736(11)61178-1
2. Brougham NDLS, Dennett ER, Cameron R, et al. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J Surg Oncol. 2012;106:811-815. doi: 10.1002/jso.23155
FDA okays 1-month dual antiplatelet therapy for Abbott’s Xience stents
The U.S. Food and Drug Administration, Abbott announced on June 30.
Patients who receive stents are typically on DAPT regimens such as aspirin and P2Y12 inhibitors for 6 to 12 months to prevent blood clots, but high-bleeding risk patients can experience bleeding during prolonged DAPT.
“The new FDA approval for DAPT for the XIENCE family of stents provides interventional cardiologists confidence they are delivering the best care to patients with high bleeding risk. A short DAPT duration minimizes risks for high bleeding risk patients and allows them to return to daily life sooner and with more assurance,” Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York and the global principal investigator for Abbott’s Short DAPT program (XIENCE 28 and XIENCE 90), said in a news release.
The new labeling comes on the heels of European CE Mark approval for the Xience stents with DAPT as short as 28 days, “giving Xience stents the shortest DAPT indication in the world,” the company noted.
Results of the XIENCE 28 trial were used to support the new CE Mark DAPT indication. The trial showed no increase in death of myocardial infarction between 1 and 6 months and a significantly lower risk for severe bleeding with the Xience stent and 1-month DAPT, compared with 6-month DAPT in more than 1,600 high-bleeding risk patients.
The XIENCE 90 trial involving more than 2,000 high-bleeding risk patients reported no difference in death or MI between 3 and 12 months with Xience and 3-month DAPT versus 12-month DAPT.
Abbott scored a second win, also announcing FDA and CE Mark approval of its next-generation Xience Skypoint stent in high-bleeding risk patients with 1-month DAPT.
“XIENCE Skypoint is easier to place and allows physicians to treat larger blood vessels through improved stent expansion that can open clogged vessels more effectively,” the company said.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration, Abbott announced on June 30.
Patients who receive stents are typically on DAPT regimens such as aspirin and P2Y12 inhibitors for 6 to 12 months to prevent blood clots, but high-bleeding risk patients can experience bleeding during prolonged DAPT.
“The new FDA approval for DAPT for the XIENCE family of stents provides interventional cardiologists confidence they are delivering the best care to patients with high bleeding risk. A short DAPT duration minimizes risks for high bleeding risk patients and allows them to return to daily life sooner and with more assurance,” Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York and the global principal investigator for Abbott’s Short DAPT program (XIENCE 28 and XIENCE 90), said in a news release.
The new labeling comes on the heels of European CE Mark approval for the Xience stents with DAPT as short as 28 days, “giving Xience stents the shortest DAPT indication in the world,” the company noted.
Results of the XIENCE 28 trial were used to support the new CE Mark DAPT indication. The trial showed no increase in death of myocardial infarction between 1 and 6 months and a significantly lower risk for severe bleeding with the Xience stent and 1-month DAPT, compared with 6-month DAPT in more than 1,600 high-bleeding risk patients.
The XIENCE 90 trial involving more than 2,000 high-bleeding risk patients reported no difference in death or MI between 3 and 12 months with Xience and 3-month DAPT versus 12-month DAPT.
Abbott scored a second win, also announcing FDA and CE Mark approval of its next-generation Xience Skypoint stent in high-bleeding risk patients with 1-month DAPT.
“XIENCE Skypoint is easier to place and allows physicians to treat larger blood vessels through improved stent expansion that can open clogged vessels more effectively,” the company said.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration, Abbott announced on June 30.
Patients who receive stents are typically on DAPT regimens such as aspirin and P2Y12 inhibitors for 6 to 12 months to prevent blood clots, but high-bleeding risk patients can experience bleeding during prolonged DAPT.
“The new FDA approval for DAPT for the XIENCE family of stents provides interventional cardiologists confidence they are delivering the best care to patients with high bleeding risk. A short DAPT duration minimizes risks for high bleeding risk patients and allows them to return to daily life sooner and with more assurance,” Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York and the global principal investigator for Abbott’s Short DAPT program (XIENCE 28 and XIENCE 90), said in a news release.
The new labeling comes on the heels of European CE Mark approval for the Xience stents with DAPT as short as 28 days, “giving Xience stents the shortest DAPT indication in the world,” the company noted.
Results of the XIENCE 28 trial were used to support the new CE Mark DAPT indication. The trial showed no increase in death of myocardial infarction between 1 and 6 months and a significantly lower risk for severe bleeding with the Xience stent and 1-month DAPT, compared with 6-month DAPT in more than 1,600 high-bleeding risk patients.
The XIENCE 90 trial involving more than 2,000 high-bleeding risk patients reported no difference in death or MI between 3 and 12 months with Xience and 3-month DAPT versus 12-month DAPT.
Abbott scored a second win, also announcing FDA and CE Mark approval of its next-generation Xience Skypoint stent in high-bleeding risk patients with 1-month DAPT.
“XIENCE Skypoint is easier to place and allows physicians to treat larger blood vessels through improved stent expansion that can open clogged vessels more effectively,” the company said.
A version of this article first appeared on Medscape.com.
Ending the ED ‘boarding’ of youth with mental health needs
All over the country, high numbers of youth experiencing a mental health crisis are presenting to emergency departments, where they are assessed to need an inpatient psychiatric hospitalization but then wait for days and sometimes weeks with nowhere to go. In Colorado, one of the largest children’s hospitals in the state declared their own state of emergency to call attention to the problem after facing a 72% increase in volume for mental health emergency visits.1 This problem is hardly new, but the COVID pandemic has appeared to take the problem to new heights. In Massachusetts, the “boarding” of youth awaiting psychiatric hospitalization has more than doubled since the pandemic, according to a recent report from National Public Radio.2 Like many public health problems, there is evidence that the burden falls disproportionately on groups that have faced health inequities in the past.3
What is causing this? The proximal cause is fairly simple: Acute mental health problems in youth are rising while the supply of intensive services is dropping. The number of available inpatient psychiatric beds has steadily been falling over the years even prior to the COVID pandemic, which then took more capacity offline because of staffing shortages and requirements for additional distance between patients (such as eliminating double-occupancy rooms). Meanwhile, levels of anxiety, depression, and suicidality have been rising in youth for reasons still not adequately understood.
The stories of these youth and their families waiting for stabilization and treatment are heartbreaking, and nobody disagrees with the idea that a child being confined to a small ED room for days is not good care. What is debated, however, is how best to fix this problem both in the short and long term. In the eyes of many, the ultimate solution is clear: more inpatient beds. This may indeed be required for some areas, but a closer look at how an entire mental health system operates often reveals both more complex problems and some alternative potential solutions. For example, hospital staff will often acknowledge that they have patients ready for discharge but who need more intensive step-down services like a residential treatment or partial hospital program to be able to do so safely. You can’t have hospital admissions if you don’t have hospital discharges, so without good step-down options patients back up and the regular flow is disrupted. Upstream of the crisis that sends many youth to EDs is another opportunity area, as these tipping points are often seen coming by others, including their pediatricians, but referrals to clinicians or programs that might bring improvement and prevent the need for an ED evaluation are also in short supply.
In the short term, efforts are being directed by some EDs to make the physical space more therapeutic for individuals experiencing mental health problems and to offer more actual treatment when people are there. This can take the form of having a secure space in which to move around, or being offered some supportive psychotherapy sessions and possible medication changes while in the ED. It can also involve simple things like the availability of books, video games, and toys to help pass the time. Such efforts are greatly needed, and many feel that the notion of mental health emergencies somehow being outside the “lane” of emergency medicine training and practice should have been retired long ago.
Medium-term solutions can involve the standing up of more intensive mental health programs that are below the level of inpatient hospitalizations, such as intensive outpatient or partial hospitalization programs, or improved mobile response services that go beyond triage and actually bring supports and techniques directly to families in need. As mentioned, these levels of services can provide both a step-down option that facilitates a hospital discharge and a measure that can prevent the need for some hospitalizations in the first place.
Looking over the long term, health care systems and governments need to evaluate the degree to which more hospital or residential beds may still be needed, despite our best efforts to improve flow and prevent mental health crises from originating. This can often be a contentious topic, however, and securing public dollars to support more beds is often quite difficult even where there seems to be a clear need.
Hovering over nearly all potential solutions, of course, is the challenge of finding the mental health workforce to implement any new programs and initiatives without stealing from services already in place. This dilemma speaks to ongoing issues of parity between resources devoted to mental health versus physical health care. Some mental health care organizations are currently trying to recruit new workers with bonuses or new incentives, but longer-term fixes are likely to require a hard look at the degree to which our actual commitment to mental health care matches the political rhetoric.
Discussions of how to solve the problem of ED boarding can easily deteriorate into a lot of finger pointing of what somebody else should be doing. The truth is, however, that there are many actions that can be taken by those in very different roles.
While many of these steps require efforts from mental health organizations, emergency departments, government agencies, and hospitals, there are things that can be done within the purview of the primary care clinician. First, look for opportunities to increase your collaboration with mental health professionals through initiatives such as integrated care programs. The Health Resources and Services Administration is now using funds from the American Rescue Plan Act to strengthen integrated care programs across the country and new opportunities may well be available soon to get additional mental health supports to primary care offices. Second, get involved and advocate for the mental health of your patients by communicating with other groups to make other potential solutions a reality.
Children and adolescents waiting for days to get the mental health care they need and deserve is an unacceptable situation that we can and must overcome. Quick fixes will be hard to find, but with some collaborative effort, forward thinking, and, yes, financial investments, we can find solutions that reflect the principle of mental health being a foundation for all health.
Dr. Rettew is a child and adolescent psychiatrist and associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Follow him on Twitter @PediPsych. His latest book is “Parenting Made Complicated: What Science Really Knows About the Greatest Debates of Early Childhood.”
References
1. Tabachnik S. Colorado health leaders declare youth mental health state of emergency: “Our kids have run out of resilience.” Denver Post. 2021 May 25.
2. Bebinger M. Kids in mental health crisis can languish for days inside ERs. National Public Radio. 2021 Jun 23.
3. Nash KA et al. Pediatrics. 2021:147:5. e2020030692.
All over the country, high numbers of youth experiencing a mental health crisis are presenting to emergency departments, where they are assessed to need an inpatient psychiatric hospitalization but then wait for days and sometimes weeks with nowhere to go. In Colorado, one of the largest children’s hospitals in the state declared their own state of emergency to call attention to the problem after facing a 72% increase in volume for mental health emergency visits.1 This problem is hardly new, but the COVID pandemic has appeared to take the problem to new heights. In Massachusetts, the “boarding” of youth awaiting psychiatric hospitalization has more than doubled since the pandemic, according to a recent report from National Public Radio.2 Like many public health problems, there is evidence that the burden falls disproportionately on groups that have faced health inequities in the past.3
What is causing this? The proximal cause is fairly simple: Acute mental health problems in youth are rising while the supply of intensive services is dropping. The number of available inpatient psychiatric beds has steadily been falling over the years even prior to the COVID pandemic, which then took more capacity offline because of staffing shortages and requirements for additional distance between patients (such as eliminating double-occupancy rooms). Meanwhile, levels of anxiety, depression, and suicidality have been rising in youth for reasons still not adequately understood.
The stories of these youth and their families waiting for stabilization and treatment are heartbreaking, and nobody disagrees with the idea that a child being confined to a small ED room for days is not good care. What is debated, however, is how best to fix this problem both in the short and long term. In the eyes of many, the ultimate solution is clear: more inpatient beds. This may indeed be required for some areas, but a closer look at how an entire mental health system operates often reveals both more complex problems and some alternative potential solutions. For example, hospital staff will often acknowledge that they have patients ready for discharge but who need more intensive step-down services like a residential treatment or partial hospital program to be able to do so safely. You can’t have hospital admissions if you don’t have hospital discharges, so without good step-down options patients back up and the regular flow is disrupted. Upstream of the crisis that sends many youth to EDs is another opportunity area, as these tipping points are often seen coming by others, including their pediatricians, but referrals to clinicians or programs that might bring improvement and prevent the need for an ED evaluation are also in short supply.
In the short term, efforts are being directed by some EDs to make the physical space more therapeutic for individuals experiencing mental health problems and to offer more actual treatment when people are there. This can take the form of having a secure space in which to move around, or being offered some supportive psychotherapy sessions and possible medication changes while in the ED. It can also involve simple things like the availability of books, video games, and toys to help pass the time. Such efforts are greatly needed, and many feel that the notion of mental health emergencies somehow being outside the “lane” of emergency medicine training and practice should have been retired long ago.
Medium-term solutions can involve the standing up of more intensive mental health programs that are below the level of inpatient hospitalizations, such as intensive outpatient or partial hospitalization programs, or improved mobile response services that go beyond triage and actually bring supports and techniques directly to families in need. As mentioned, these levels of services can provide both a step-down option that facilitates a hospital discharge and a measure that can prevent the need for some hospitalizations in the first place.
Looking over the long term, health care systems and governments need to evaluate the degree to which more hospital or residential beds may still be needed, despite our best efforts to improve flow and prevent mental health crises from originating. This can often be a contentious topic, however, and securing public dollars to support more beds is often quite difficult even where there seems to be a clear need.
Hovering over nearly all potential solutions, of course, is the challenge of finding the mental health workforce to implement any new programs and initiatives without stealing from services already in place. This dilemma speaks to ongoing issues of parity between resources devoted to mental health versus physical health care. Some mental health care organizations are currently trying to recruit new workers with bonuses or new incentives, but longer-term fixes are likely to require a hard look at the degree to which our actual commitment to mental health care matches the political rhetoric.
Discussions of how to solve the problem of ED boarding can easily deteriorate into a lot of finger pointing of what somebody else should be doing. The truth is, however, that there are many actions that can be taken by those in very different roles.
While many of these steps require efforts from mental health organizations, emergency departments, government agencies, and hospitals, there are things that can be done within the purview of the primary care clinician. First, look for opportunities to increase your collaboration with mental health professionals through initiatives such as integrated care programs. The Health Resources and Services Administration is now using funds from the American Rescue Plan Act to strengthen integrated care programs across the country and new opportunities may well be available soon to get additional mental health supports to primary care offices. Second, get involved and advocate for the mental health of your patients by communicating with other groups to make other potential solutions a reality.
Children and adolescents waiting for days to get the mental health care they need and deserve is an unacceptable situation that we can and must overcome. Quick fixes will be hard to find, but with some collaborative effort, forward thinking, and, yes, financial investments, we can find solutions that reflect the principle of mental health being a foundation for all health.
Dr. Rettew is a child and adolescent psychiatrist and associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Follow him on Twitter @PediPsych. His latest book is “Parenting Made Complicated: What Science Really Knows About the Greatest Debates of Early Childhood.”
References
1. Tabachnik S. Colorado health leaders declare youth mental health state of emergency: “Our kids have run out of resilience.” Denver Post. 2021 May 25.
2. Bebinger M. Kids in mental health crisis can languish for days inside ERs. National Public Radio. 2021 Jun 23.
3. Nash KA et al. Pediatrics. 2021:147:5. e2020030692.
All over the country, high numbers of youth experiencing a mental health crisis are presenting to emergency departments, where they are assessed to need an inpatient psychiatric hospitalization but then wait for days and sometimes weeks with nowhere to go. In Colorado, one of the largest children’s hospitals in the state declared their own state of emergency to call attention to the problem after facing a 72% increase in volume for mental health emergency visits.1 This problem is hardly new, but the COVID pandemic has appeared to take the problem to new heights. In Massachusetts, the “boarding” of youth awaiting psychiatric hospitalization has more than doubled since the pandemic, according to a recent report from National Public Radio.2 Like many public health problems, there is evidence that the burden falls disproportionately on groups that have faced health inequities in the past.3
What is causing this? The proximal cause is fairly simple: Acute mental health problems in youth are rising while the supply of intensive services is dropping. The number of available inpatient psychiatric beds has steadily been falling over the years even prior to the COVID pandemic, which then took more capacity offline because of staffing shortages and requirements for additional distance between patients (such as eliminating double-occupancy rooms). Meanwhile, levels of anxiety, depression, and suicidality have been rising in youth for reasons still not adequately understood.
The stories of these youth and their families waiting for stabilization and treatment are heartbreaking, and nobody disagrees with the idea that a child being confined to a small ED room for days is not good care. What is debated, however, is how best to fix this problem both in the short and long term. In the eyes of many, the ultimate solution is clear: more inpatient beds. This may indeed be required for some areas, but a closer look at how an entire mental health system operates often reveals both more complex problems and some alternative potential solutions. For example, hospital staff will often acknowledge that they have patients ready for discharge but who need more intensive step-down services like a residential treatment or partial hospital program to be able to do so safely. You can’t have hospital admissions if you don’t have hospital discharges, so without good step-down options patients back up and the regular flow is disrupted. Upstream of the crisis that sends many youth to EDs is another opportunity area, as these tipping points are often seen coming by others, including their pediatricians, but referrals to clinicians or programs that might bring improvement and prevent the need for an ED evaluation are also in short supply.
In the short term, efforts are being directed by some EDs to make the physical space more therapeutic for individuals experiencing mental health problems and to offer more actual treatment when people are there. This can take the form of having a secure space in which to move around, or being offered some supportive psychotherapy sessions and possible medication changes while in the ED. It can also involve simple things like the availability of books, video games, and toys to help pass the time. Such efforts are greatly needed, and many feel that the notion of mental health emergencies somehow being outside the “lane” of emergency medicine training and practice should have been retired long ago.
Medium-term solutions can involve the standing up of more intensive mental health programs that are below the level of inpatient hospitalizations, such as intensive outpatient or partial hospitalization programs, or improved mobile response services that go beyond triage and actually bring supports and techniques directly to families in need. As mentioned, these levels of services can provide both a step-down option that facilitates a hospital discharge and a measure that can prevent the need for some hospitalizations in the first place.
Looking over the long term, health care systems and governments need to evaluate the degree to which more hospital or residential beds may still be needed, despite our best efforts to improve flow and prevent mental health crises from originating. This can often be a contentious topic, however, and securing public dollars to support more beds is often quite difficult even where there seems to be a clear need.
Hovering over nearly all potential solutions, of course, is the challenge of finding the mental health workforce to implement any new programs and initiatives without stealing from services already in place. This dilemma speaks to ongoing issues of parity between resources devoted to mental health versus physical health care. Some mental health care organizations are currently trying to recruit new workers with bonuses or new incentives, but longer-term fixes are likely to require a hard look at the degree to which our actual commitment to mental health care matches the political rhetoric.
Discussions of how to solve the problem of ED boarding can easily deteriorate into a lot of finger pointing of what somebody else should be doing. The truth is, however, that there are many actions that can be taken by those in very different roles.
While many of these steps require efforts from mental health organizations, emergency departments, government agencies, and hospitals, there are things that can be done within the purview of the primary care clinician. First, look for opportunities to increase your collaboration with mental health professionals through initiatives such as integrated care programs. The Health Resources and Services Administration is now using funds from the American Rescue Plan Act to strengthen integrated care programs across the country and new opportunities may well be available soon to get additional mental health supports to primary care offices. Second, get involved and advocate for the mental health of your patients by communicating with other groups to make other potential solutions a reality.
Children and adolescents waiting for days to get the mental health care they need and deserve is an unacceptable situation that we can and must overcome. Quick fixes will be hard to find, but with some collaborative effort, forward thinking, and, yes, financial investments, we can find solutions that reflect the principle of mental health being a foundation for all health.
Dr. Rettew is a child and adolescent psychiatrist and associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Follow him on Twitter @PediPsych. His latest book is “Parenting Made Complicated: What Science Really Knows About the Greatest Debates of Early Childhood.”
References
1. Tabachnik S. Colorado health leaders declare youth mental health state of emergency: “Our kids have run out of resilience.” Denver Post. 2021 May 25.
2. Bebinger M. Kids in mental health crisis can languish for days inside ERs. National Public Radio. 2021 Jun 23.
3. Nash KA et al. Pediatrics. 2021:147:5. e2020030692.
Chronic stress and genetics can raise the risk of Alzheimer’s disease
The researchers also proposed a mechanism to account for how genetic factors may affect HPA axis reactivity and lead to inflammation, which is a core component of neurodegeneration.
“Chronic stress can impact the way immune cells in the brain function and increase inflammation. Genetic variants within that stress response can further affect the function of immune cells,” lead author Ayeisha Milligan Armstrong, a PhD candidate at Curtin Health Innovation Research Institute in Perth, Australia, said in an interview.
The findings were published online June 22 in Biological Reviews).
Research has found that long-term stress during early and mid-life is increasingly associated with cognitive decline and neurodegeneration. There is already evidence to suggest that chronic stress is a risk factor for the “sporadic” or late-onset subtype of Alzheimer’s disease.
A cascade of events
Stress activates the HPA, which in turn regulates bodily levels of cortisol, a glucocorticoid stress hormone. Increased levels of cortisol are frequently observed in patients with Alzheimer’s disease and “make a major contribution to the disease process,” the authors wrote. For example, the hippocampus – a part of the brain involved in processing and forming memories – has numerous glucocorticoid receptors and is “therefore particularly sensitive to the effects of glucocorticoids.” However, the molecular mechanisms involved remain poorly understood.
“There is an intimate interplay between exposure to chronic stress and pathways influencing the body’s reaction to such stress,” senior author David Groth, PhD, said in a statement. Dr. Groth is an associate professor at Curtin University in Perth, Australia.
There is variation between individuals with regard to how sensitive they are to stress and glucocorticoid responses. Environmental factors such as stress are thought to be at least partly responsible, as are genetic factors such as genetic polymorphisms and epigenetics. “Genetic variations within these pathways can influence the way the brain’s immune system behaves, leading to a dysfunctional response. In the brain, this leads to a chronic disruption of normal brain processes, increasing the risk of subsequent neurodegeneration and ultimately dementia,” Dr. Groth said.
The researchers suggested that these variations may prime the immune cells of the brain, the microglia, to cause inflammation in the brain. Normally, microglia are involved in monitoring the brain tissue for and responding to damage and infections to keep the brain healthy. However, in an inflammatory state, the microglia instead contribute to a “more neurotoxic environment through the production of proinflammatory cytokines, altered synaptic pruning, and the reduced production of protective neurotrophic factors,” the authors wrote. Microglia may also promote the accumulation of amyloid beta and tau protein, which damage the brain tissue and can cause neurodegeneration. There are different groups of microglia in the brain, each of which may respond differently to genetic and environmental stressors.
“Genome-wide association studies have found that of the genes identified as being associated with Alzheimer’s disease, 60.5% are expressed in microglia,” the authors noted.
To connect the roles of chronic stress and brain inflammation in Alzheimer’s disease, the researchers proposed a “two-hit” hypothesis: Early or mid-life exposure to stress primes the microglia to enter an inflammatory state in response to a secondary stimulus later in life.
Pay attention to stress
For clinicians, this paper highlights the importance of managing stress in patients and their families.
“Clinicians need to be attuned to the effects of stress on patients and their caregivers, and how that [stress] can affect their morbidity and mortality,” Cynthia Munro, PhD, associate professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, said in an interview. She added that attention must be paid to modifiable risk factors such as poor sleep and diet.
Although managing stress is important, that doesn’t mean that everyone who’s experienced chronic stress will develop Alzheimer’s disease. “Chronic stress can alter the HPA axis but it doesn’t necessarily do so in everyone. A cascade of events needs to occur,” said Dr. Munro. “People should always try to reduce the effects of stress to the extent that they can. Stress can lead to a whole host of negative health outcomes, not just Alzheimer’s disease.”
Next steps
Moving forward, the researchers plan to further investigate the molecular mechanisms responsible for the role of stress in Alzheimer’s disease and how genetic variants affect neurodegeneration, Ms. Armstrong said. Ultimately, understanding how stress and genetics contribute to Alzheimer’s disease may lead to the identification of possible therapeutic targets.
Ms. Armstrong and Dr. Munro declared no relevant financial relationships. The study was independently funded.
The researchers also proposed a mechanism to account for how genetic factors may affect HPA axis reactivity and lead to inflammation, which is a core component of neurodegeneration.
“Chronic stress can impact the way immune cells in the brain function and increase inflammation. Genetic variants within that stress response can further affect the function of immune cells,” lead author Ayeisha Milligan Armstrong, a PhD candidate at Curtin Health Innovation Research Institute in Perth, Australia, said in an interview.
The findings were published online June 22 in Biological Reviews).
Research has found that long-term stress during early and mid-life is increasingly associated with cognitive decline and neurodegeneration. There is already evidence to suggest that chronic stress is a risk factor for the “sporadic” or late-onset subtype of Alzheimer’s disease.
A cascade of events
Stress activates the HPA, which in turn regulates bodily levels of cortisol, a glucocorticoid stress hormone. Increased levels of cortisol are frequently observed in patients with Alzheimer’s disease and “make a major contribution to the disease process,” the authors wrote. For example, the hippocampus – a part of the brain involved in processing and forming memories – has numerous glucocorticoid receptors and is “therefore particularly sensitive to the effects of glucocorticoids.” However, the molecular mechanisms involved remain poorly understood.
“There is an intimate interplay between exposure to chronic stress and pathways influencing the body’s reaction to such stress,” senior author David Groth, PhD, said in a statement. Dr. Groth is an associate professor at Curtin University in Perth, Australia.
There is variation between individuals with regard to how sensitive they are to stress and glucocorticoid responses. Environmental factors such as stress are thought to be at least partly responsible, as are genetic factors such as genetic polymorphisms and epigenetics. “Genetic variations within these pathways can influence the way the brain’s immune system behaves, leading to a dysfunctional response. In the brain, this leads to a chronic disruption of normal brain processes, increasing the risk of subsequent neurodegeneration and ultimately dementia,” Dr. Groth said.
The researchers suggested that these variations may prime the immune cells of the brain, the microglia, to cause inflammation in the brain. Normally, microglia are involved in monitoring the brain tissue for and responding to damage and infections to keep the brain healthy. However, in an inflammatory state, the microglia instead contribute to a “more neurotoxic environment through the production of proinflammatory cytokines, altered synaptic pruning, and the reduced production of protective neurotrophic factors,” the authors wrote. Microglia may also promote the accumulation of amyloid beta and tau protein, which damage the brain tissue and can cause neurodegeneration. There are different groups of microglia in the brain, each of which may respond differently to genetic and environmental stressors.
“Genome-wide association studies have found that of the genes identified as being associated with Alzheimer’s disease, 60.5% are expressed in microglia,” the authors noted.
To connect the roles of chronic stress and brain inflammation in Alzheimer’s disease, the researchers proposed a “two-hit” hypothesis: Early or mid-life exposure to stress primes the microglia to enter an inflammatory state in response to a secondary stimulus later in life.
Pay attention to stress
For clinicians, this paper highlights the importance of managing stress in patients and their families.
“Clinicians need to be attuned to the effects of stress on patients and their caregivers, and how that [stress] can affect their morbidity and mortality,” Cynthia Munro, PhD, associate professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, said in an interview. She added that attention must be paid to modifiable risk factors such as poor sleep and diet.
Although managing stress is important, that doesn’t mean that everyone who’s experienced chronic stress will develop Alzheimer’s disease. “Chronic stress can alter the HPA axis but it doesn’t necessarily do so in everyone. A cascade of events needs to occur,” said Dr. Munro. “People should always try to reduce the effects of stress to the extent that they can. Stress can lead to a whole host of negative health outcomes, not just Alzheimer’s disease.”
Next steps
Moving forward, the researchers plan to further investigate the molecular mechanisms responsible for the role of stress in Alzheimer’s disease and how genetic variants affect neurodegeneration, Ms. Armstrong said. Ultimately, understanding how stress and genetics contribute to Alzheimer’s disease may lead to the identification of possible therapeutic targets.
Ms. Armstrong and Dr. Munro declared no relevant financial relationships. The study was independently funded.
The researchers also proposed a mechanism to account for how genetic factors may affect HPA axis reactivity and lead to inflammation, which is a core component of neurodegeneration.
“Chronic stress can impact the way immune cells in the brain function and increase inflammation. Genetic variants within that stress response can further affect the function of immune cells,” lead author Ayeisha Milligan Armstrong, a PhD candidate at Curtin Health Innovation Research Institute in Perth, Australia, said in an interview.
The findings were published online June 22 in Biological Reviews).
Research has found that long-term stress during early and mid-life is increasingly associated with cognitive decline and neurodegeneration. There is already evidence to suggest that chronic stress is a risk factor for the “sporadic” or late-onset subtype of Alzheimer’s disease.
A cascade of events
Stress activates the HPA, which in turn regulates bodily levels of cortisol, a glucocorticoid stress hormone. Increased levels of cortisol are frequently observed in patients with Alzheimer’s disease and “make a major contribution to the disease process,” the authors wrote. For example, the hippocampus – a part of the brain involved in processing and forming memories – has numerous glucocorticoid receptors and is “therefore particularly sensitive to the effects of glucocorticoids.” However, the molecular mechanisms involved remain poorly understood.
“There is an intimate interplay between exposure to chronic stress and pathways influencing the body’s reaction to such stress,” senior author David Groth, PhD, said in a statement. Dr. Groth is an associate professor at Curtin University in Perth, Australia.
There is variation between individuals with regard to how sensitive they are to stress and glucocorticoid responses. Environmental factors such as stress are thought to be at least partly responsible, as are genetic factors such as genetic polymorphisms and epigenetics. “Genetic variations within these pathways can influence the way the brain’s immune system behaves, leading to a dysfunctional response. In the brain, this leads to a chronic disruption of normal brain processes, increasing the risk of subsequent neurodegeneration and ultimately dementia,” Dr. Groth said.
The researchers suggested that these variations may prime the immune cells of the brain, the microglia, to cause inflammation in the brain. Normally, microglia are involved in monitoring the brain tissue for and responding to damage and infections to keep the brain healthy. However, in an inflammatory state, the microglia instead contribute to a “more neurotoxic environment through the production of proinflammatory cytokines, altered synaptic pruning, and the reduced production of protective neurotrophic factors,” the authors wrote. Microglia may also promote the accumulation of amyloid beta and tau protein, which damage the brain tissue and can cause neurodegeneration. There are different groups of microglia in the brain, each of which may respond differently to genetic and environmental stressors.
“Genome-wide association studies have found that of the genes identified as being associated with Alzheimer’s disease, 60.5% are expressed in microglia,” the authors noted.
To connect the roles of chronic stress and brain inflammation in Alzheimer’s disease, the researchers proposed a “two-hit” hypothesis: Early or mid-life exposure to stress primes the microglia to enter an inflammatory state in response to a secondary stimulus later in life.
Pay attention to stress
For clinicians, this paper highlights the importance of managing stress in patients and their families.
“Clinicians need to be attuned to the effects of stress on patients and their caregivers, and how that [stress] can affect their morbidity and mortality,” Cynthia Munro, PhD, associate professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, said in an interview. She added that attention must be paid to modifiable risk factors such as poor sleep and diet.
Although managing stress is important, that doesn’t mean that everyone who’s experienced chronic stress will develop Alzheimer’s disease. “Chronic stress can alter the HPA axis but it doesn’t necessarily do so in everyone. A cascade of events needs to occur,” said Dr. Munro. “People should always try to reduce the effects of stress to the extent that they can. Stress can lead to a whole host of negative health outcomes, not just Alzheimer’s disease.”
Next steps
Moving forward, the researchers plan to further investigate the molecular mechanisms responsible for the role of stress in Alzheimer’s disease and how genetic variants affect neurodegeneration, Ms. Armstrong said. Ultimately, understanding how stress and genetics contribute to Alzheimer’s disease may lead to the identification of possible therapeutic targets.
Ms. Armstrong and Dr. Munro declared no relevant financial relationships. The study was independently funded.
FROM BIOLOGICAL REVIEWS
Pandemic upped telemedicine use 100-fold in type 2 diabetes
The COVID-19 pandemic jump-started a significant role for telemedicine in the routine follow-up of U.S. patients with type 2 diabetes, based on insurance claims records for more than 2.7 million American adults during 2019 and 2020.
During 2019, 0.3% of 1,357,029 adults with type 2 diabetes in a U.S. claims database, OptumLabs Data Warehouse, had one or more telemedicine visits. During 2020, this jumped to 29% of a similar group of U.S. adults once the pandemic kicked in, a nearly 100-fold increase, Sadiq Y. Patel, PhD, and coauthors wrote in a research letter published July 6, 2021, in JAMA Internal Medicine.
The data show that telemedicine visits didn’t seem to negatively impact care, with hemoglobin A1c levels and medication fills remaining constant across the year.
But Robert A. Gabbay, MD, PhD, chief science and medical officer for the American Diabetes Association, said these results, while reassuring, seem “quite surprising” relative to anecdotal reports from colleagues around the United States.
It’s possible they may only apply to the specific patients included in this study – which was limited to those with either commercial or Medicare Advantage health insurance – he noted in an interview.
Diabetes well-suited to telemedicine
Dr. Patel, of the department of health care policy at Harvard Medical School, Boston, and coauthors said the information from their study showed “no evidence of a negative association with medication fills or glycemic control” among these patients during the pandemic in 2020, compared with the prepandemic year 2019.
During the first 48 weeks in 2020, A1c levels averaged 7.16% among patients with type 2 diabetes, compared with an average of 7.14% for patients with type 2 diabetes during the first 48 weeks of 2019. Fill rates for prescription medications were 64% during 2020 and 62% during 2019.
A1c levels and medication fill rates “are important markers of the quality of diabetes care, but obviously not the only important things,” said Ateev Mehrotra, MD, corresponding author for the study and a researcher in the same department as Dr. Patel.
“Limited to the metrics we looked at and in this population we did not see any substantial negative impact of the pandemic on the care for patients with type 2 diabetes,” Dr. Mehrotra said in an interview.
“The pandemic catalyzed a tremendous shift to telemedicine among patients with diabetes. Because it is a chronic illness that requires frequent check-ins, diabetes is particularly well suited to using telemedicine,” he added.
Telemedicine not a complete replacement for in-patient visits
Dr. Gabbay agreed that “providers and patients have found telemedicine to be a helpful tool for managing patients with diabetes.”
But “most people do not think of this as a complete replacement for in-person visits, and most [U.S.] institutions have started to have more in-person visits. It’s probably about 50/50 at this point,” he said in an interview.
“It represents an impressive effort by the health care community to pivot toward telehealth to ensure that patients with diabetes continue to get care.”
Nevertheless, Dr. Gabbay added that “despite the success of telemedicine many patients still prefer to see their providers in person. I have a number of patients who were overjoyed to come in and be seen in person even when I offered telemedicine as an alternative. There is a relationship and trust piece that is more profound in person.”
And he cautioned that, although A1c “is a helpful measure, it may not fully demonstrate the percentage of patients at high risk.”
The data in the study by Dr. Patel and coauthors showing a steady level of medication refills during the pandemic “is encouraging,” he said, speculating that “people may have had more time [during the pandemic] to focus on medication adherence.”
More evidence of telemedicine’s leap
Other U.S. sites that follow patients with type 2 diabetes have recently reported similar findings, albeit on a much more localized level.
At Vanderbilt University Medical Center in Nashville, Tenn., telemedicine consultations for patients with diabetes or other endocrinology disorders spurted from essentially none prior to March 2020 to a peak of nearly 700 visits/week in early May 2020, and then maintained a rate of roughly 500 telemedicine consultations weekly through the end of 2020, said Michelle L. Griffth, MD, during a talk at the 2021 annual ADA scientific sessions.
“We’ve made telehealth a permanent part of our practice,” said Dr. Griffith, medical director of telehealth ambulatory services at Vanderbilt. “We can use this boom in telehealth as a catalyst for diabetes-practice evolution,” she suggested.
It was a similar story at Scripps Health in southern California. During March and April 2020, video telemedicine consultations jumped from a prior rate of about 60/month to about 13,000/week, and then settled back to a monthly rate of about 25,000-30,000 during the balance of 2020, said Athena Philis-Tsimikas, MD, an endocrinologist and vice president of the Scripps Whittier Diabetes Institute in La Jolla, Calif. (These numbers include all telehealth consultations for patients at Scripps, not just patients with diabetes.)
“COVID sped up the process of integrating digital technology into health care,” concluded Dr. Philis-Tsimikas. A big factor driving this transition was the decision of many insurers to reimburse for telemedicine visits, something not done prepandemic.
The study received no commercial support. Dr. Patel, Dr. Mehrotra, Dr. Griffith, Dr. Philis-Tsimikas, and Dr. Gabbay reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The COVID-19 pandemic jump-started a significant role for telemedicine in the routine follow-up of U.S. patients with type 2 diabetes, based on insurance claims records for more than 2.7 million American adults during 2019 and 2020.
During 2019, 0.3% of 1,357,029 adults with type 2 diabetes in a U.S. claims database, OptumLabs Data Warehouse, had one or more telemedicine visits. During 2020, this jumped to 29% of a similar group of U.S. adults once the pandemic kicked in, a nearly 100-fold increase, Sadiq Y. Patel, PhD, and coauthors wrote in a research letter published July 6, 2021, in JAMA Internal Medicine.
The data show that telemedicine visits didn’t seem to negatively impact care, with hemoglobin A1c levels and medication fills remaining constant across the year.
But Robert A. Gabbay, MD, PhD, chief science and medical officer for the American Diabetes Association, said these results, while reassuring, seem “quite surprising” relative to anecdotal reports from colleagues around the United States.
It’s possible they may only apply to the specific patients included in this study – which was limited to those with either commercial or Medicare Advantage health insurance – he noted in an interview.
Diabetes well-suited to telemedicine
Dr. Patel, of the department of health care policy at Harvard Medical School, Boston, and coauthors said the information from their study showed “no evidence of a negative association with medication fills or glycemic control” among these patients during the pandemic in 2020, compared with the prepandemic year 2019.
During the first 48 weeks in 2020, A1c levels averaged 7.16% among patients with type 2 diabetes, compared with an average of 7.14% for patients with type 2 diabetes during the first 48 weeks of 2019. Fill rates for prescription medications were 64% during 2020 and 62% during 2019.
A1c levels and medication fill rates “are important markers of the quality of diabetes care, but obviously not the only important things,” said Ateev Mehrotra, MD, corresponding author for the study and a researcher in the same department as Dr. Patel.
“Limited to the metrics we looked at and in this population we did not see any substantial negative impact of the pandemic on the care for patients with type 2 diabetes,” Dr. Mehrotra said in an interview.
“The pandemic catalyzed a tremendous shift to telemedicine among patients with diabetes. Because it is a chronic illness that requires frequent check-ins, diabetes is particularly well suited to using telemedicine,” he added.
Telemedicine not a complete replacement for in-patient visits
Dr. Gabbay agreed that “providers and patients have found telemedicine to be a helpful tool for managing patients with diabetes.”
But “most people do not think of this as a complete replacement for in-person visits, and most [U.S.] institutions have started to have more in-person visits. It’s probably about 50/50 at this point,” he said in an interview.
“It represents an impressive effort by the health care community to pivot toward telehealth to ensure that patients with diabetes continue to get care.”
Nevertheless, Dr. Gabbay added that “despite the success of telemedicine many patients still prefer to see their providers in person. I have a number of patients who were overjoyed to come in and be seen in person even when I offered telemedicine as an alternative. There is a relationship and trust piece that is more profound in person.”
And he cautioned that, although A1c “is a helpful measure, it may not fully demonstrate the percentage of patients at high risk.”
The data in the study by Dr. Patel and coauthors showing a steady level of medication refills during the pandemic “is encouraging,” he said, speculating that “people may have had more time [during the pandemic] to focus on medication adherence.”
More evidence of telemedicine’s leap
Other U.S. sites that follow patients with type 2 diabetes have recently reported similar findings, albeit on a much more localized level.
At Vanderbilt University Medical Center in Nashville, Tenn., telemedicine consultations for patients with diabetes or other endocrinology disorders spurted from essentially none prior to March 2020 to a peak of nearly 700 visits/week in early May 2020, and then maintained a rate of roughly 500 telemedicine consultations weekly through the end of 2020, said Michelle L. Griffth, MD, during a talk at the 2021 annual ADA scientific sessions.
“We’ve made telehealth a permanent part of our practice,” said Dr. Griffith, medical director of telehealth ambulatory services at Vanderbilt. “We can use this boom in telehealth as a catalyst for diabetes-practice evolution,” she suggested.
It was a similar story at Scripps Health in southern California. During March and April 2020, video telemedicine consultations jumped from a prior rate of about 60/month to about 13,000/week, and then settled back to a monthly rate of about 25,000-30,000 during the balance of 2020, said Athena Philis-Tsimikas, MD, an endocrinologist and vice president of the Scripps Whittier Diabetes Institute in La Jolla, Calif. (These numbers include all telehealth consultations for patients at Scripps, not just patients with diabetes.)
“COVID sped up the process of integrating digital technology into health care,” concluded Dr. Philis-Tsimikas. A big factor driving this transition was the decision of many insurers to reimburse for telemedicine visits, something not done prepandemic.
The study received no commercial support. Dr. Patel, Dr. Mehrotra, Dr. Griffith, Dr. Philis-Tsimikas, and Dr. Gabbay reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The COVID-19 pandemic jump-started a significant role for telemedicine in the routine follow-up of U.S. patients with type 2 diabetes, based on insurance claims records for more than 2.7 million American adults during 2019 and 2020.
During 2019, 0.3% of 1,357,029 adults with type 2 diabetes in a U.S. claims database, OptumLabs Data Warehouse, had one or more telemedicine visits. During 2020, this jumped to 29% of a similar group of U.S. adults once the pandemic kicked in, a nearly 100-fold increase, Sadiq Y. Patel, PhD, and coauthors wrote in a research letter published July 6, 2021, in JAMA Internal Medicine.
The data show that telemedicine visits didn’t seem to negatively impact care, with hemoglobin A1c levels and medication fills remaining constant across the year.
But Robert A. Gabbay, MD, PhD, chief science and medical officer for the American Diabetes Association, said these results, while reassuring, seem “quite surprising” relative to anecdotal reports from colleagues around the United States.
It’s possible they may only apply to the specific patients included in this study – which was limited to those with either commercial or Medicare Advantage health insurance – he noted in an interview.
Diabetes well-suited to telemedicine
Dr. Patel, of the department of health care policy at Harvard Medical School, Boston, and coauthors said the information from their study showed “no evidence of a negative association with medication fills or glycemic control” among these patients during the pandemic in 2020, compared with the prepandemic year 2019.
During the first 48 weeks in 2020, A1c levels averaged 7.16% among patients with type 2 diabetes, compared with an average of 7.14% for patients with type 2 diabetes during the first 48 weeks of 2019. Fill rates for prescription medications were 64% during 2020 and 62% during 2019.
A1c levels and medication fill rates “are important markers of the quality of diabetes care, but obviously not the only important things,” said Ateev Mehrotra, MD, corresponding author for the study and a researcher in the same department as Dr. Patel.
“Limited to the metrics we looked at and in this population we did not see any substantial negative impact of the pandemic on the care for patients with type 2 diabetes,” Dr. Mehrotra said in an interview.
“The pandemic catalyzed a tremendous shift to telemedicine among patients with diabetes. Because it is a chronic illness that requires frequent check-ins, diabetes is particularly well suited to using telemedicine,” he added.
Telemedicine not a complete replacement for in-patient visits
Dr. Gabbay agreed that “providers and patients have found telemedicine to be a helpful tool for managing patients with diabetes.”
But “most people do not think of this as a complete replacement for in-person visits, and most [U.S.] institutions have started to have more in-person visits. It’s probably about 50/50 at this point,” he said in an interview.
“It represents an impressive effort by the health care community to pivot toward telehealth to ensure that patients with diabetes continue to get care.”
Nevertheless, Dr. Gabbay added that “despite the success of telemedicine many patients still prefer to see their providers in person. I have a number of patients who were overjoyed to come in and be seen in person even when I offered telemedicine as an alternative. There is a relationship and trust piece that is more profound in person.”
And he cautioned that, although A1c “is a helpful measure, it may not fully demonstrate the percentage of patients at high risk.”
The data in the study by Dr. Patel and coauthors showing a steady level of medication refills during the pandemic “is encouraging,” he said, speculating that “people may have had more time [during the pandemic] to focus on medication adherence.”
More evidence of telemedicine’s leap
Other U.S. sites that follow patients with type 2 diabetes have recently reported similar findings, albeit on a much more localized level.
At Vanderbilt University Medical Center in Nashville, Tenn., telemedicine consultations for patients with diabetes or other endocrinology disorders spurted from essentially none prior to March 2020 to a peak of nearly 700 visits/week in early May 2020, and then maintained a rate of roughly 500 telemedicine consultations weekly through the end of 2020, said Michelle L. Griffth, MD, during a talk at the 2021 annual ADA scientific sessions.
“We’ve made telehealth a permanent part of our practice,” said Dr. Griffith, medical director of telehealth ambulatory services at Vanderbilt. “We can use this boom in telehealth as a catalyst for diabetes-practice evolution,” she suggested.
It was a similar story at Scripps Health in southern California. During March and April 2020, video telemedicine consultations jumped from a prior rate of about 60/month to about 13,000/week, and then settled back to a monthly rate of about 25,000-30,000 during the balance of 2020, said Athena Philis-Tsimikas, MD, an endocrinologist and vice president of the Scripps Whittier Diabetes Institute in La Jolla, Calif. (These numbers include all telehealth consultations for patients at Scripps, not just patients with diabetes.)
“COVID sped up the process of integrating digital technology into health care,” concluded Dr. Philis-Tsimikas. A big factor driving this transition was the decision of many insurers to reimburse for telemedicine visits, something not done prepandemic.
The study received no commercial support. Dr. Patel, Dr. Mehrotra, Dr. Griffith, Dr. Philis-Tsimikas, and Dr. Gabbay reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Postpartum depression affects dads, too
Michael W., a 38-year-old New Jersey–based attorney, and his wife had been excitedly planning for the birth of their baby and were overjoyed when she was born.
But after that, “I found that parenting a newborn was shockingly exhausting. I felt unprepared for the task, overwhelmed by the burden of the 24-hour-schedule and lack of sleep, and I struggled with feelings of inadequacy,” he said in an interview.
Michael never thought he had postpartum depression (PPD), perhaps because the condition is more commonly associated with women. But a study published in the American Journal of Men’s Health suggests that PPD also affects men.
A team of Danish investigators led by researcher Sarah Pedersen, of the department of public health, Aarhus University, extensively interviewed eight fathers with PPD and found their primary experiences involved feelings of being overwhelmed and powerless or inadequate, which sometimes turned into anger and frustration.
“I think one of the most important take-home messages is that practicing clinicians working with new parents should invite fathers to your consultations and engage the fathers as much as possible,” Ms. Pedersen said in an interview.
The findings also contained a message for parents, she says.
“I hope you will support each other and talk about your feelings and how you experience the transition to parenthood – know that it will take time to adjust to your new role,” she said.
Not enough attention
There’s been too little focus on fathers when it comes to PPD, according to Ms. Pedersen.
“During the last decade, several studies have examined the prevalence of PPD in men, and there is rising evidence that paternal PPD is associated with increased risk of long-term adverse behavioral and emotional outcomes in children,” she said.
Nevertheless, only three studies have been based on interviews with fathers who had personal experience with PPD.
“The purpose of our study was, first of all, to explore the lived experience of fathers who had PPD and, secondly, to gain deeper understanding of their help-seeking behavior – barriers to seeking help and facilitators of help-seeking,” Ms. Pedersen said.
The study was based on “semistructured” interviews with eight Danish fathers (ages 29-38 years) who had had PPD, none of whom had a previous history of depression.
All of them had received a formal diagnosis of PPD by a general practitioner or psychologist, and all had sought or received mental health care and considered themselves recovered from depression at the time of the interview.
The researchers used a technique called interpretative phenomenological analysis to analyze the interviews.
This method “aims to produce in-depth examinations of certain phenomena by examining how individuals make meaning of their own life experiences,” the authors wrote.
A ‘radical change’
Of the fathers, five described the period of pregnancy as a “time of happiness, full of positive expectations about fatherhood.”
But “the fathers’ great expectations were later replaced by a very different reality of fatherhood,” the authors wrote, noting that the transition to fatherhood was, in the words of one participant, a “radical change that you just can’t imagine.”
Most fathers expressed a feeling of being overwhelmed, and three felt unready for the task, which added to their depression.
“The participants wanted to be emotionally and physically present in their child’s life, but during the time of their depression, these kind-hearted intentions changed into feelings of guilt and inadequacy, as the participants did not feel they had enough energy and mental strength to become the kind of fathers they wanted to be,” the authors wrote.
The fathers mentioned stressors they believed contributed to their PPD, including complications during their partners’ pregnancies, unplanned cesarean birth (three fathers), the partners’ difficulties with breastfeeding (five fathers), and employment-related concerns. Five reported that their partners had postpartum emotional distress.
‘Masculine norms’
A second focus of the research was to examine fathers’ help-seeking behaviors, Ms. Pedersen said.
Ultimately, all the men sought formal help, either from their general practitioners or from a health visitor, with two seeking help right after birth.
Although the men were able to recognize changes in mood and behavior in retrospect, many did not regard them as signs of depression before their diagnosis.
Most had heard of PPD, but primarily as it affects women. Three sought information online about paternal PPD but couldn’t find any.
Four participants described experiencing PPD as “taboo,” based on a “combination of false beliefs, stigma, and masculine norms,” the authors stated, since men “are supposed to be big and strong and take care of everything, and suddenly you can’t.”
The authors reported that seven participants were screened for PPD or depression by a health care professional.
“The screening was an important part of the help-seeking process, as this was the first time two of the fathers were introduced to PPD,” the authors noted.
Although the screening “had the potential to spark conversation” about PPD, it was geared toward women, and some participants did not feel it was relevant to them.
“Future research should focus on identification of educational needs about paternal PPD among both parents, health care professionals, and other professionals taking care of new families,” Ms. Pedersen said.
Michael W. says it would have been helpful if someone had prepared him and his wife for what to expect, or if there had been some type of screening. Also, he advises expectant parents to “get some real-life experience by spending time around a newborn to see what’s involved.”
Different symptoms
“We often talk about mothers suffering from PPD, so it is more normalized for mothers to bring it up or for loved ones to ask mothers about how they are doing physically and psychologically after the birth,” Craig Garfield, MD, an attending physician and founder/director of Family and Child Health innovations at Ann and Robert H. Lurie Children’s Hospital, Chicago, said in an interview.
For fathers, “it is not discussed as commonly, so friends and families don’t often ask dads, and dads don’t know where to turn,” said Dr. Garfield, professor of pediatrics and medical social sciences at Northwestern University, Chicago. He was not involved with the study.
He noted that symptoms in fathers might differ from those of mothers.
“I have seen fathers who are anxious or more moody than they had been prior, or more angry, and I have seen fathers who throw themselves into work or begin drinking more – all related to changes in mood and depressive symptoms in the postnatal period,” he said.
Symptoms in men may last longer than in women. Dr. Garfield’s group published a study in which they surveyed 400 mothers and fathers of premature infants in the neonatal intensive care unit (NICU) about depressive symptoms around the time of NICU admission, at discharge home, and then after 30 days at home.
Roughly one-third of mothers screened positive for depressive symptoms around NICU admission, as did 17% of fathers. But the mothers’ depression scores improved by discharge and 30 days after being home, while the fathers’ remained “essentially unchanged,” he said.
“Further, we found that if doctors were to screen mothers and fathers during the NICU stay – at admission or even at discharge – that would greatly improve their ability to predict who would still have depressive symptoms 1 month after going home.”
Ms. Pedersen agrees that clinicians should incorporate screening for PPD into their practices and be proactive in encouraging fathers to get help.
“Keep pushing,” she advised, as “men rarely seek help, compared to women, in matters of mental health.”
A version of this article first appeared on WebMD.com.
Michael W., a 38-year-old New Jersey–based attorney, and his wife had been excitedly planning for the birth of their baby and were overjoyed when she was born.
But after that, “I found that parenting a newborn was shockingly exhausting. I felt unprepared for the task, overwhelmed by the burden of the 24-hour-schedule and lack of sleep, and I struggled with feelings of inadequacy,” he said in an interview.
Michael never thought he had postpartum depression (PPD), perhaps because the condition is more commonly associated with women. But a study published in the American Journal of Men’s Health suggests that PPD also affects men.
A team of Danish investigators led by researcher Sarah Pedersen, of the department of public health, Aarhus University, extensively interviewed eight fathers with PPD and found their primary experiences involved feelings of being overwhelmed and powerless or inadequate, which sometimes turned into anger and frustration.
“I think one of the most important take-home messages is that practicing clinicians working with new parents should invite fathers to your consultations and engage the fathers as much as possible,” Ms. Pedersen said in an interview.
The findings also contained a message for parents, she says.
“I hope you will support each other and talk about your feelings and how you experience the transition to parenthood – know that it will take time to adjust to your new role,” she said.
Not enough attention
There’s been too little focus on fathers when it comes to PPD, according to Ms. Pedersen.
“During the last decade, several studies have examined the prevalence of PPD in men, and there is rising evidence that paternal PPD is associated with increased risk of long-term adverse behavioral and emotional outcomes in children,” she said.
Nevertheless, only three studies have been based on interviews with fathers who had personal experience with PPD.
“The purpose of our study was, first of all, to explore the lived experience of fathers who had PPD and, secondly, to gain deeper understanding of their help-seeking behavior – barriers to seeking help and facilitators of help-seeking,” Ms. Pedersen said.
The study was based on “semistructured” interviews with eight Danish fathers (ages 29-38 years) who had had PPD, none of whom had a previous history of depression.
All of them had received a formal diagnosis of PPD by a general practitioner or psychologist, and all had sought or received mental health care and considered themselves recovered from depression at the time of the interview.
The researchers used a technique called interpretative phenomenological analysis to analyze the interviews.
This method “aims to produce in-depth examinations of certain phenomena by examining how individuals make meaning of their own life experiences,” the authors wrote.
A ‘radical change’
Of the fathers, five described the period of pregnancy as a “time of happiness, full of positive expectations about fatherhood.”
But “the fathers’ great expectations were later replaced by a very different reality of fatherhood,” the authors wrote, noting that the transition to fatherhood was, in the words of one participant, a “radical change that you just can’t imagine.”
Most fathers expressed a feeling of being overwhelmed, and three felt unready for the task, which added to their depression.
“The participants wanted to be emotionally and physically present in their child’s life, but during the time of their depression, these kind-hearted intentions changed into feelings of guilt and inadequacy, as the participants did not feel they had enough energy and mental strength to become the kind of fathers they wanted to be,” the authors wrote.
The fathers mentioned stressors they believed contributed to their PPD, including complications during their partners’ pregnancies, unplanned cesarean birth (three fathers), the partners’ difficulties with breastfeeding (five fathers), and employment-related concerns. Five reported that their partners had postpartum emotional distress.
‘Masculine norms’
A second focus of the research was to examine fathers’ help-seeking behaviors, Ms. Pedersen said.
Ultimately, all the men sought formal help, either from their general practitioners or from a health visitor, with two seeking help right after birth.
Although the men were able to recognize changes in mood and behavior in retrospect, many did not regard them as signs of depression before their diagnosis.
Most had heard of PPD, but primarily as it affects women. Three sought information online about paternal PPD but couldn’t find any.
Four participants described experiencing PPD as “taboo,” based on a “combination of false beliefs, stigma, and masculine norms,” the authors stated, since men “are supposed to be big and strong and take care of everything, and suddenly you can’t.”
The authors reported that seven participants were screened for PPD or depression by a health care professional.
“The screening was an important part of the help-seeking process, as this was the first time two of the fathers were introduced to PPD,” the authors noted.
Although the screening “had the potential to spark conversation” about PPD, it was geared toward women, and some participants did not feel it was relevant to them.
“Future research should focus on identification of educational needs about paternal PPD among both parents, health care professionals, and other professionals taking care of new families,” Ms. Pedersen said.
Michael W. says it would have been helpful if someone had prepared him and his wife for what to expect, or if there had been some type of screening. Also, he advises expectant parents to “get some real-life experience by spending time around a newborn to see what’s involved.”
Different symptoms
“We often talk about mothers suffering from PPD, so it is more normalized for mothers to bring it up or for loved ones to ask mothers about how they are doing physically and psychologically after the birth,” Craig Garfield, MD, an attending physician and founder/director of Family and Child Health innovations at Ann and Robert H. Lurie Children’s Hospital, Chicago, said in an interview.
For fathers, “it is not discussed as commonly, so friends and families don’t often ask dads, and dads don’t know where to turn,” said Dr. Garfield, professor of pediatrics and medical social sciences at Northwestern University, Chicago. He was not involved with the study.
He noted that symptoms in fathers might differ from those of mothers.
“I have seen fathers who are anxious or more moody than they had been prior, or more angry, and I have seen fathers who throw themselves into work or begin drinking more – all related to changes in mood and depressive symptoms in the postnatal period,” he said.
Symptoms in men may last longer than in women. Dr. Garfield’s group published a study in which they surveyed 400 mothers and fathers of premature infants in the neonatal intensive care unit (NICU) about depressive symptoms around the time of NICU admission, at discharge home, and then after 30 days at home.
Roughly one-third of mothers screened positive for depressive symptoms around NICU admission, as did 17% of fathers. But the mothers’ depression scores improved by discharge and 30 days after being home, while the fathers’ remained “essentially unchanged,” he said.
“Further, we found that if doctors were to screen mothers and fathers during the NICU stay – at admission or even at discharge – that would greatly improve their ability to predict who would still have depressive symptoms 1 month after going home.”
Ms. Pedersen agrees that clinicians should incorporate screening for PPD into their practices and be proactive in encouraging fathers to get help.
“Keep pushing,” she advised, as “men rarely seek help, compared to women, in matters of mental health.”
A version of this article first appeared on WebMD.com.
Michael W., a 38-year-old New Jersey–based attorney, and his wife had been excitedly planning for the birth of their baby and were overjoyed when she was born.
But after that, “I found that parenting a newborn was shockingly exhausting. I felt unprepared for the task, overwhelmed by the burden of the 24-hour-schedule and lack of sleep, and I struggled with feelings of inadequacy,” he said in an interview.
Michael never thought he had postpartum depression (PPD), perhaps because the condition is more commonly associated with women. But a study published in the American Journal of Men’s Health suggests that PPD also affects men.
A team of Danish investigators led by researcher Sarah Pedersen, of the department of public health, Aarhus University, extensively interviewed eight fathers with PPD and found their primary experiences involved feelings of being overwhelmed and powerless or inadequate, which sometimes turned into anger and frustration.
“I think one of the most important take-home messages is that practicing clinicians working with new parents should invite fathers to your consultations and engage the fathers as much as possible,” Ms. Pedersen said in an interview.
The findings also contained a message for parents, she says.
“I hope you will support each other and talk about your feelings and how you experience the transition to parenthood – know that it will take time to adjust to your new role,” she said.
Not enough attention
There’s been too little focus on fathers when it comes to PPD, according to Ms. Pedersen.
“During the last decade, several studies have examined the prevalence of PPD in men, and there is rising evidence that paternal PPD is associated with increased risk of long-term adverse behavioral and emotional outcomes in children,” she said.
Nevertheless, only three studies have been based on interviews with fathers who had personal experience with PPD.
“The purpose of our study was, first of all, to explore the lived experience of fathers who had PPD and, secondly, to gain deeper understanding of their help-seeking behavior – barriers to seeking help and facilitators of help-seeking,” Ms. Pedersen said.
The study was based on “semistructured” interviews with eight Danish fathers (ages 29-38 years) who had had PPD, none of whom had a previous history of depression.
All of them had received a formal diagnosis of PPD by a general practitioner or psychologist, and all had sought or received mental health care and considered themselves recovered from depression at the time of the interview.
The researchers used a technique called interpretative phenomenological analysis to analyze the interviews.
This method “aims to produce in-depth examinations of certain phenomena by examining how individuals make meaning of their own life experiences,” the authors wrote.
A ‘radical change’
Of the fathers, five described the period of pregnancy as a “time of happiness, full of positive expectations about fatherhood.”
But “the fathers’ great expectations were later replaced by a very different reality of fatherhood,” the authors wrote, noting that the transition to fatherhood was, in the words of one participant, a “radical change that you just can’t imagine.”
Most fathers expressed a feeling of being overwhelmed, and three felt unready for the task, which added to their depression.
“The participants wanted to be emotionally and physically present in their child’s life, but during the time of their depression, these kind-hearted intentions changed into feelings of guilt and inadequacy, as the participants did not feel they had enough energy and mental strength to become the kind of fathers they wanted to be,” the authors wrote.
The fathers mentioned stressors they believed contributed to their PPD, including complications during their partners’ pregnancies, unplanned cesarean birth (three fathers), the partners’ difficulties with breastfeeding (five fathers), and employment-related concerns. Five reported that their partners had postpartum emotional distress.
‘Masculine norms’
A second focus of the research was to examine fathers’ help-seeking behaviors, Ms. Pedersen said.
Ultimately, all the men sought formal help, either from their general practitioners or from a health visitor, with two seeking help right after birth.
Although the men were able to recognize changes in mood and behavior in retrospect, many did not regard them as signs of depression before their diagnosis.
Most had heard of PPD, but primarily as it affects women. Three sought information online about paternal PPD but couldn’t find any.
Four participants described experiencing PPD as “taboo,” based on a “combination of false beliefs, stigma, and masculine norms,” the authors stated, since men “are supposed to be big and strong and take care of everything, and suddenly you can’t.”
The authors reported that seven participants were screened for PPD or depression by a health care professional.
“The screening was an important part of the help-seeking process, as this was the first time two of the fathers were introduced to PPD,” the authors noted.
Although the screening “had the potential to spark conversation” about PPD, it was geared toward women, and some participants did not feel it was relevant to them.
“Future research should focus on identification of educational needs about paternal PPD among both parents, health care professionals, and other professionals taking care of new families,” Ms. Pedersen said.
Michael W. says it would have been helpful if someone had prepared him and his wife for what to expect, or if there had been some type of screening. Also, he advises expectant parents to “get some real-life experience by spending time around a newborn to see what’s involved.”
Different symptoms
“We often talk about mothers suffering from PPD, so it is more normalized for mothers to bring it up or for loved ones to ask mothers about how they are doing physically and psychologically after the birth,” Craig Garfield, MD, an attending physician and founder/director of Family and Child Health innovations at Ann and Robert H. Lurie Children’s Hospital, Chicago, said in an interview.
For fathers, “it is not discussed as commonly, so friends and families don’t often ask dads, and dads don’t know where to turn,” said Dr. Garfield, professor of pediatrics and medical social sciences at Northwestern University, Chicago. He was not involved with the study.
He noted that symptoms in fathers might differ from those of mothers.
“I have seen fathers who are anxious or more moody than they had been prior, or more angry, and I have seen fathers who throw themselves into work or begin drinking more – all related to changes in mood and depressive symptoms in the postnatal period,” he said.
Symptoms in men may last longer than in women. Dr. Garfield’s group published a study in which they surveyed 400 mothers and fathers of premature infants in the neonatal intensive care unit (NICU) about depressive symptoms around the time of NICU admission, at discharge home, and then after 30 days at home.
Roughly one-third of mothers screened positive for depressive symptoms around NICU admission, as did 17% of fathers. But the mothers’ depression scores improved by discharge and 30 days after being home, while the fathers’ remained “essentially unchanged,” he said.
“Further, we found that if doctors were to screen mothers and fathers during the NICU stay – at admission or even at discharge – that would greatly improve their ability to predict who would still have depressive symptoms 1 month after going home.”
Ms. Pedersen agrees that clinicians should incorporate screening for PPD into their practices and be proactive in encouraging fathers to get help.
“Keep pushing,” she advised, as “men rarely seek help, compared to women, in matters of mental health.”
A version of this article first appeared on WebMD.com.
Fibrosis severity in ALD predicts survival
Moderate fibrosis in patients with alcoholic liver disease is not a benign condition, say investigators who studied the natural history of ALD according to biopsy-proven fibrosis stage.
In a study of 422 patients who were followed for a median of 43 months, 5.8% of patients with no or minimal fibrosis had a liver decompensation event. This compares with 21% (hazard ratio, 3.8; 95% confidence interval, 1.9-7.5; P < .01) of patients with significant fibrosis and 45% (HR, 9.6; 95% CI, 5.2-18.0; P < .01) of patients with advanced fibrosis, said Ditlev Nytoft Rasmussen, PhD, in an oral presentation at the meeting sponsored by the European Association for the Study of the Liver.
“The most obvious implication of this study is that the stage of fibrosis is a very strong predictor for the prognosis in early asymptomatic alcohol-related liver disease,” he said.
The findings suggest that gastroenterologists do not need to follow patients with no or only minor fibrosis, but patients with significant (F2) fibrosis, or greater, should be closely followed, said Dr. Rasmussen, who is with the FLASH Center for Liver Research at Odense (Denmark) University.
A gastroenterologist who was not involved in the study commented on the excess mortality the investigators saw.
“The really striking finding here to me is the excess mortality in the significant fibrosis group. They are not healthy, and some of that excess mortality does appear to be liver related, but some of it may not be liver related,“ said Esperance A. Schaefer, MD, MPH, of Massachusetts General Hospital in Boston.
“I think it will be important to be vigilant about the causes of death that are not liver related in patients with significant and advanced fibrosis, and the healthy group,” she said in an interview.
Ewan H. Forrest, MD, from the University of Glasgow, who was not involved in the study, said: “These are a challenging group of patients to identify with early disease. You quite rightly raise the question of how we should follow-up with these patients.”
Managing asymptomatic patients
Although noninvasive tests can identify ALD in the early fibrotic stage, it’s unclear how patients with early asymptomatic disease should be managed, Dr. Rasmussen said.
This uncertainty prompted FLASH investigators to study patients with ALD to determine how fibrosis affected outcomes such as decompensation, hospitalization, and death.
They looked at a prospective cohort of patients diagnosed with ALD by biopsy and transient elastography (FibroScan) from 2013 to 2018. Follow-up data for the patients were collected retrospectively from electronic health records or charts.
The patients, who all had alcohol overuse and no history of decompensation or other etiologies at baseline, were classified into three groups: 225 liver-healthy patients with minimal to no fibrosis (F1 or transient elastography <6 kPa kPa); 104 patients with significant fibrosis (F2); and, 93 patients with advanced fibrosis (F3 or F4).
The median patient age was 57 years, and 75% of the patients were men. The patients were followed for 1,149 patient-years.
Findings and outcomes
During follow-up, 53 patients died, and 51 had a decompensation event: overt hepatoencephalopathy, ascites, variceal bleeding, hepatorenal syndrome, or jaundice. Of the 51 patients, 27 died.
There was a protocol change during the study, with the new protocol stating that patients with transient elastography below 6 kPa could not undergo biopsy. Based on the 106 patients who had both a FibroScan and biopsy, of whom 20% had F2 fibrosis, the investigators calculated that 14 patients who did not undergo biopsy may have been incorrectly classified as having healthy livers, Dr. Rasmussen said.
Patients with healthy livers had a significantly better decompensation-free survival rates, with only 5.8% of those with healthy livers having a decompensation event out to 4.5 years, compared with 21% of patients with significant fibrosis or advanced fibrosis.
Within the first year of follow-up, 98% of patients with healthy livers were alive and free of decompensations, compared with 94% with significant fibrosis and 84% with advanced fibrosis. The respective rates at 3 years were 95%, 82%, and 55%.
The percentage of hospital admissions that were liver related was 5% in the healthy-liver group, 24% in the significant group, and 47% in the advanced group.
Ongoing alcohol use during follow-up was also a significant predictor of worse decompensation-free survival (HR, 1.6; P = .04), with two in three decompensation events occurring in patients with alcohol overuse. The effect of alcohol was less strong than fibrosis stage, however, Dr. Rasmussen noted.
Dr. Schaefer said that “we see individuals who with heavy alcohol use progress much more rapidly than you’d anticipate with the rule of thumb that you see in other diseases of one fibrosis stage every 5-7 years.”
Among the strengths of this study included the use of biopsy and the inclusion of all stages of fibrosis. The investigators acknowledged as potential limitations the retrospective collection of follow-up data, reliance on medical charts to estimate alcohol consumption, and the single-center design.
The study was supported by grants from the European Union’s Horizon 2020 research and innovation program and the Novo Nordisk Foundation Challenge program. Dr. Rasmussen, Dr. Schaefer, and Dr. Forrest reported no conflicts of interest to disclose.
Moderate fibrosis in patients with alcoholic liver disease is not a benign condition, say investigators who studied the natural history of ALD according to biopsy-proven fibrosis stage.
In a study of 422 patients who were followed for a median of 43 months, 5.8% of patients with no or minimal fibrosis had a liver decompensation event. This compares with 21% (hazard ratio, 3.8; 95% confidence interval, 1.9-7.5; P < .01) of patients with significant fibrosis and 45% (HR, 9.6; 95% CI, 5.2-18.0; P < .01) of patients with advanced fibrosis, said Ditlev Nytoft Rasmussen, PhD, in an oral presentation at the meeting sponsored by the European Association for the Study of the Liver.
“The most obvious implication of this study is that the stage of fibrosis is a very strong predictor for the prognosis in early asymptomatic alcohol-related liver disease,” he said.
The findings suggest that gastroenterologists do not need to follow patients with no or only minor fibrosis, but patients with significant (F2) fibrosis, or greater, should be closely followed, said Dr. Rasmussen, who is with the FLASH Center for Liver Research at Odense (Denmark) University.
A gastroenterologist who was not involved in the study commented on the excess mortality the investigators saw.
“The really striking finding here to me is the excess mortality in the significant fibrosis group. They are not healthy, and some of that excess mortality does appear to be liver related, but some of it may not be liver related,“ said Esperance A. Schaefer, MD, MPH, of Massachusetts General Hospital in Boston.
“I think it will be important to be vigilant about the causes of death that are not liver related in patients with significant and advanced fibrosis, and the healthy group,” she said in an interview.
Ewan H. Forrest, MD, from the University of Glasgow, who was not involved in the study, said: “These are a challenging group of patients to identify with early disease. You quite rightly raise the question of how we should follow-up with these patients.”
Managing asymptomatic patients
Although noninvasive tests can identify ALD in the early fibrotic stage, it’s unclear how patients with early asymptomatic disease should be managed, Dr. Rasmussen said.
This uncertainty prompted FLASH investigators to study patients with ALD to determine how fibrosis affected outcomes such as decompensation, hospitalization, and death.
They looked at a prospective cohort of patients diagnosed with ALD by biopsy and transient elastography (FibroScan) from 2013 to 2018. Follow-up data for the patients were collected retrospectively from electronic health records or charts.
The patients, who all had alcohol overuse and no history of decompensation or other etiologies at baseline, were classified into three groups: 225 liver-healthy patients with minimal to no fibrosis (F1 or transient elastography <6 kPa kPa); 104 patients with significant fibrosis (F2); and, 93 patients with advanced fibrosis (F3 or F4).
The median patient age was 57 years, and 75% of the patients were men. The patients were followed for 1,149 patient-years.
Findings and outcomes
During follow-up, 53 patients died, and 51 had a decompensation event: overt hepatoencephalopathy, ascites, variceal bleeding, hepatorenal syndrome, or jaundice. Of the 51 patients, 27 died.
There was a protocol change during the study, with the new protocol stating that patients with transient elastography below 6 kPa could not undergo biopsy. Based on the 106 patients who had both a FibroScan and biopsy, of whom 20% had F2 fibrosis, the investigators calculated that 14 patients who did not undergo biopsy may have been incorrectly classified as having healthy livers, Dr. Rasmussen said.
Patients with healthy livers had a significantly better decompensation-free survival rates, with only 5.8% of those with healthy livers having a decompensation event out to 4.5 years, compared with 21% of patients with significant fibrosis or advanced fibrosis.
Within the first year of follow-up, 98% of patients with healthy livers were alive and free of decompensations, compared with 94% with significant fibrosis and 84% with advanced fibrosis. The respective rates at 3 years were 95%, 82%, and 55%.
The percentage of hospital admissions that were liver related was 5% in the healthy-liver group, 24% in the significant group, and 47% in the advanced group.
Ongoing alcohol use during follow-up was also a significant predictor of worse decompensation-free survival (HR, 1.6; P = .04), with two in three decompensation events occurring in patients with alcohol overuse. The effect of alcohol was less strong than fibrosis stage, however, Dr. Rasmussen noted.
Dr. Schaefer said that “we see individuals who with heavy alcohol use progress much more rapidly than you’d anticipate with the rule of thumb that you see in other diseases of one fibrosis stage every 5-7 years.”
Among the strengths of this study included the use of biopsy and the inclusion of all stages of fibrosis. The investigators acknowledged as potential limitations the retrospective collection of follow-up data, reliance on medical charts to estimate alcohol consumption, and the single-center design.
The study was supported by grants from the European Union’s Horizon 2020 research and innovation program and the Novo Nordisk Foundation Challenge program. Dr. Rasmussen, Dr. Schaefer, and Dr. Forrest reported no conflicts of interest to disclose.
Moderate fibrosis in patients with alcoholic liver disease is not a benign condition, say investigators who studied the natural history of ALD according to biopsy-proven fibrosis stage.
In a study of 422 patients who were followed for a median of 43 months, 5.8% of patients with no or minimal fibrosis had a liver decompensation event. This compares with 21% (hazard ratio, 3.8; 95% confidence interval, 1.9-7.5; P < .01) of patients with significant fibrosis and 45% (HR, 9.6; 95% CI, 5.2-18.0; P < .01) of patients with advanced fibrosis, said Ditlev Nytoft Rasmussen, PhD, in an oral presentation at the meeting sponsored by the European Association for the Study of the Liver.
“The most obvious implication of this study is that the stage of fibrosis is a very strong predictor for the prognosis in early asymptomatic alcohol-related liver disease,” he said.
The findings suggest that gastroenterologists do not need to follow patients with no or only minor fibrosis, but patients with significant (F2) fibrosis, or greater, should be closely followed, said Dr. Rasmussen, who is with the FLASH Center for Liver Research at Odense (Denmark) University.
A gastroenterologist who was not involved in the study commented on the excess mortality the investigators saw.
“The really striking finding here to me is the excess mortality in the significant fibrosis group. They are not healthy, and some of that excess mortality does appear to be liver related, but some of it may not be liver related,“ said Esperance A. Schaefer, MD, MPH, of Massachusetts General Hospital in Boston.
“I think it will be important to be vigilant about the causes of death that are not liver related in patients with significant and advanced fibrosis, and the healthy group,” she said in an interview.
Ewan H. Forrest, MD, from the University of Glasgow, who was not involved in the study, said: “These are a challenging group of patients to identify with early disease. You quite rightly raise the question of how we should follow-up with these patients.”
Managing asymptomatic patients
Although noninvasive tests can identify ALD in the early fibrotic stage, it’s unclear how patients with early asymptomatic disease should be managed, Dr. Rasmussen said.
This uncertainty prompted FLASH investigators to study patients with ALD to determine how fibrosis affected outcomes such as decompensation, hospitalization, and death.
They looked at a prospective cohort of patients diagnosed with ALD by biopsy and transient elastography (FibroScan) from 2013 to 2018. Follow-up data for the patients were collected retrospectively from electronic health records or charts.
The patients, who all had alcohol overuse and no history of decompensation or other etiologies at baseline, were classified into three groups: 225 liver-healthy patients with minimal to no fibrosis (F1 or transient elastography <6 kPa kPa); 104 patients with significant fibrosis (F2); and, 93 patients with advanced fibrosis (F3 or F4).
The median patient age was 57 years, and 75% of the patients were men. The patients were followed for 1,149 patient-years.
Findings and outcomes
During follow-up, 53 patients died, and 51 had a decompensation event: overt hepatoencephalopathy, ascites, variceal bleeding, hepatorenal syndrome, or jaundice. Of the 51 patients, 27 died.
There was a protocol change during the study, with the new protocol stating that patients with transient elastography below 6 kPa could not undergo biopsy. Based on the 106 patients who had both a FibroScan and biopsy, of whom 20% had F2 fibrosis, the investigators calculated that 14 patients who did not undergo biopsy may have been incorrectly classified as having healthy livers, Dr. Rasmussen said.
Patients with healthy livers had a significantly better decompensation-free survival rates, with only 5.8% of those with healthy livers having a decompensation event out to 4.5 years, compared with 21% of patients with significant fibrosis or advanced fibrosis.
Within the first year of follow-up, 98% of patients with healthy livers were alive and free of decompensations, compared with 94% with significant fibrosis and 84% with advanced fibrosis. The respective rates at 3 years were 95%, 82%, and 55%.
The percentage of hospital admissions that were liver related was 5% in the healthy-liver group, 24% in the significant group, and 47% in the advanced group.
Ongoing alcohol use during follow-up was also a significant predictor of worse decompensation-free survival (HR, 1.6; P = .04), with two in three decompensation events occurring in patients with alcohol overuse. The effect of alcohol was less strong than fibrosis stage, however, Dr. Rasmussen noted.
Dr. Schaefer said that “we see individuals who with heavy alcohol use progress much more rapidly than you’d anticipate with the rule of thumb that you see in other diseases of one fibrosis stage every 5-7 years.”
Among the strengths of this study included the use of biopsy and the inclusion of all stages of fibrosis. The investigators acknowledged as potential limitations the retrospective collection of follow-up data, reliance on medical charts to estimate alcohol consumption, and the single-center design.
The study was supported by grants from the European Union’s Horizon 2020 research and innovation program and the Novo Nordisk Foundation Challenge program. Dr. Rasmussen, Dr. Schaefer, and Dr. Forrest reported no conflicts of interest to disclose.
FROM ILC 2021
2021 Update on abnormal uterine bleeding
Abnormal uterine bleeding (AUB) continues to be a top-10 reason why women present for gynecologic care, which makes keeping up with clinical therapies important. Over the past year, we have learned a tremendous amount about elagolix with hormonal add-back therapy for the treatment of bleeding associated with uterine fibroids. In this Update, we provide an overview from 3 randomized clinical trials on the recent US Food and Drug Administration (FDA)-approved drug, elagolix with hormonal add-back therapy (approved May 29, 2020). In addition, we review the data on the Cerene cryotherapy device (Channel Medsystems), as one might rightly ask, do we need another endometrial ablation device? We will address that question, as this device has some unique features that gynecologists should be aware of. Last, we review a study on the importance of considering quality of life in patients with uterine fibroids, which provides sobering information on the psychosocial aspects of uterine fibroids that all clinicians who care for such patients should be aware of.
Endometrial ablation with a new cryotherapy device: Is less more?
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
The phrase “less is more,” in the world of architecture and design, is often associated with Ludwig Mies van der Rohe (1886–1969). One could argue that this principle is one key advantage with the addition of yet another non-resectoscopic endometrial ablation device. The Cerene cryotherapy device, FDA approved in 2019, is presented as a simple, disposable device for in-office use that takes advantage of natural cryoanesthesia and results in less tissue destruction than many other ablation methods.
Device reduces bleeding and permits greater ability for future evaluation
Recently, Curlin and colleagues conducted a prospective, multicenter clinical trial to evaluate the safety and efficacy of the Cerene device in reducing menstrual blood loss.1 They followed 230 patients over 12 months and found that 81% (77% with intention-to-treat analysis) met the primary end point of a pictorial blood loss assessment chart (PBLAC) score of 75 or lower. Clinically, this translated to 44% of patients experiencing light bleeding; 27%, eumenorrhea; and 10%, amenorrhea. This is clearly “less” in terms of the rate of amenorrhea in most endometrial ablation studies. However, this also may translate into “more” ability to evaluate the endometrial cavity in the future, as 97% of the patients were able to undergo hysteroscopy at the 12-month mark and, of those, 93% were able to have the entire endometrial cavity assessed.
Further, of 97 patients who had a tubal sterilization, none had symptoms or evidence of postablation tubal sterilization syndrome. Three patients were unable to undergo hysteroscopy due to pain intolerance (2) or cervical stenosis (1). This is important because some gynecologists have expressed concern over intrauterine synechiae, which may result in scarring and associated future difficulty in assessing the endometrium for possible cancer.
Details about the device
The Cerene device is a single use, disposable device that uses cryothermal energy from nitrous oxide that results in a liquid-to-gas phase change within a polyurethane balloon (resulting in a temperature of -86°C) and delivered through a 6-mm sheath. It may be used in uterine cavities that measure between 2.5 and 6.5 cm in length, corresponding to approximately 10 cm in a uterine sound measurement. Treatment time is 2.5 minutes of nitrous oxide flow.
As mentioned, another benefit claimed is that the Cerene device’s cryoanalgesia properties enable the procedure to be more tolerated in the office setting. Of the 230 patients studied in the Curlin trial, no procedures were performed under general anesthesia.1 Medications used included paracervical block (PCB) only (8%), PCB plus nonsteroidal anti-inflammatory drugs (19.8%), PCB plus oral narcotics/anxiolytics (69%), and PCB plus intravenous sedation (2.9%), showing that this device is ideally suited for in-office use.
The rate of serious adverse events was 2.5% (7 total events in 6 patients within 12 months). All serious adverse events were reviewed by a Clinical Events Committee and none were deemed to be device-related events.
Long-term outcomes remain to be seen
For physicians and patients who worry about the ability to access the endometrial cavity in the future, less may be more. It will be interesting to see what the long-term outcomes show with use of the Cerene cryotherapy device, and whether a lower amenorrhea rate will translate into a higher repeat intervention rate or not. Of course, not all are minimalists. As the architect Robert Venturi (1925–2018) was quoted as saying, “Less is a bore.”
The new Cerene cryotherapy endometrial ablation method meets the FDA’s target for reduction of menstrual blood loss, but it has a slightly lower amenorrhea rate than other devices. Its most significant features are the potential for improved analgesia for in-office use and the possibility that there may be less scarring of the endometrial cavity for future assessment if needed.
Continue to: QoL assessment in women with fibroids is useful in evaluating treatment success...
QoL assessment in women with fibroids is useful in evaluating treatment success
Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674- 708.e8.
In many studies that assess AUB, the primary emphasis generally is placed on quantitation of menstrual bleeding by using PBLAC and alkaline hematin scores. In a systematic review, Go and colleagues argue the case for the importance of measuring the psychosocial impact of abnormal bleeding, emphasizing the concerning finding that many women with fibroids report lower vitality and lower social function scores than women with breast cancer.2
Fibroids associated with inconvenience—and anxiety
The authors analyzed and reviewed 18 randomized trials and 39 observational studies after screening 3,625 records from electronic database searches, with the goal to include only studies with validated quality of life (QoL) questionnaires that were administered both before and after treatment. A highlighted aspect of the reviewed studies was that “control” and “concern” subscales were most affected by fibroids, noting the inconvenience and anxiety that are related to the unpredictable onset and intensity of menses and the feeling of loss of control over one’s health and future.
This systematic review is important because although previous research has shown that fibroids significantly affect QoL, the psychosocial burden of fibroid symptoms had not been compared across different QoL instruments for both disease-specific and general validated health subscales.
Disability levels with fibroids are similar to those with other chronic diseases
Go and colleagues further reported that uterine fibroids have considerable psychosocial impact and lead to poor overall QoL physically and emotionally, with diminished sexual function and increased urinary or defecatory issues. Women with fibroids experienced a level of disability that was similar to that seen in other chronic diseases, and their vitality scores were lower than those associated with heart disease, diabetes, and as mentioned, breast cancer.
The authors concluded that “although objective clinical measures are important to establish a comprehensive understanding of health status, patient reported QoL outcomes play a critical role in evaluating success of a therapy.” They suggested that a larger emphasis on patient-centered care may help to mitigate the psychosocial effects of fibroids.
The study by Go and colleagues highlights the significant psychosocial aspects of the heavy menstrual bleeding associated with fibroids, and the authors found that many women with fibroids score in the range of those with other significant diseases, such as breast cancer and diabetes.
We have noted the trend of including QoL in research, and Go and colleagues make an excellent and compelling argument for this trend using quantitative analysis. It is important to consider this not only in our design of future research but also, and perhaps more importantly, in our clinical care of women as we try to better understand what they are experiencing.
Continue to: What have we learned over the past year about elagolix for uterine fibroids?...
What have we learned over the past year about elagolix for uterine fibroids?
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
Data from the Elaris UF-1 and UF-2 6-month, phase 3 trials3 and the results of the Elaris UF-EXTEND trial with a 6-month extension (totaling 12 months of use)4 were published in 2020, and the 12-month results were discussed in OBG Management (2020;32[7]:35, 39-40). An additional data analysis from the same researchers assessed the effect of elagolix with hormonal add-back therapy in a number of patient subgroups.5 These 3 publications have added to our knowledge of this therapy, and it is worth reviewing them in this context
Design of the elagolix plus hormonal add-back therapy trials
The initial UF-1 and UF-2 trials were 2 identical, double-blind, randomized, placebo-controlled, 6-month, phase 3 trials designed to evaluate the safety and efficacy of elagolix and hormonal add-back therapy.3 UF-1 was conducted at 76 sites in the United States from December 2015 through December 2018, whereas UF-2 was conducted at 77 sites in the United States and Canada from February 2016 through January 2019; the trials were registered separately. Both trials had a 2:1:1 randomization of elagolix (300 mg twice daily) with hormonal add-back therapy (estradiol 1 mg and norethindrone acetate 0.5 mg daily), elagolix alone (300 mg twice daily), or placebo.
In the 6-month studies, the primary end point was both menstrual blood loss of less than 80 mL and at least a 50% reduction of menstrual blood loss as measured by the alkaline hematin method.3 Among several secondary end points was the assessment of QoL using the Uterine Fibroid Symptom QoL questionnaire (UFS-QoL).
Trial results. In UF-1, 68.5% of 206 women, and in UF-2, 76.5% of 189 women, respectively, taking elagolix with add-back therapy met the primary objective. Among women taking elagolix alone, in UF-1, 84.1% of 104 women, and in UF-2, 77% of 95 women, respectively, met criteria. There was improvement in UFS-QoL scores in women receiving elagolix plus add-back therapy with a reduction of symptom severity of -33.2 in UF-1 and -41.4 in UF-2, as compared with the placebo-treated groups (-10.3 and -7.7, respectively).
Adverse effects. Elagolix was associated with a low incidence of serious adverse effects, and the addition of hormonal add-back therapy attenuated the decreases in bone mineral density observed with elagolix alone. In both UF-1 and UF-2 trials, bone mineral density did not differ significantly in the groups of women who received elagolix with hormonal addback therapy versus placebo.
The extension trial results
Of note, in the 12-month study (6-month extension), the authors reported that 87.9% of the women taking elagolix with hormonal add-back therapy met the primary objective.4 Among the women taking elagolix alone, 89.4% met the primary objective.
In a review of the AbbVie-funded extension study, the editorial comments in the Obstetrical and Gynecological Survey expressed concern over the high proportion of data loss, comparing the number of patients joining the extended trial, patients who completed an additional 6 months of treatment, and patients who completed the posttreatment follow-up period of “up to 12 months.”6 Approximately one-third of patients were lost between initial enrollment to the subset who completed follow-up. There was concern that “losses of that magnitude pose a serious threat to validity.”6
Effectiveness in subgroups
Al-Hendy and colleagues analyzed data from the Elaris UF-1 and UF-2 trials to see if the outcomes for elagolix with hormonal addback therapy demonstrated safety and efficacy in subgroups of patients of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume.5
Results. In all subgroups, they found a statistically significant reduction in blood loss in mean menstrual blood loss volume for those treated with elagolix plus hormonal addback therapy compared with those treated with placebo. As well, in terms of QoL, among all subgroups, the mean change in symptom severity score as well as health-related QoL total score from baseline to month 6 was statistically significantly greater than the mean change in the placebo group.
The bottom line
Elagolix with hormonal add-back therapy appears to be a safe and effective method to reduce menstrual blood loss associated with uterine fibroids. It also has a favorable effect on QoL and appears to have benefits in subgroups of women of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume. ●
Elagolix plus hormonal add-back therapy provides several advantages to fibroid care, including a pill form that, as a gonadotropin-releasing hormone (GnRH) antagonist, provides much quicker action than GnRH agonists. The hormonal add-back feature seems to improve QoL measures and has a favorable reported bleeding reduction rate. It also appears to be reasonably safe. Although the studies reviewed here may have some weaknesses, it helps to have another therapy to offer to women who have blood loss associated with fibroids. Deciding on the drug’s optimal clinical use has not been fully explored, as it may be a short-term solution to a long-term problem and may not be ideal for all patients with fibroids. Elagolix and hormonal add-back therapy may be advantageous for patients who need to stop bleeding quickly and are trying to decide about their reproductive plans, for patients close to menopause who need a therapy to bridge this gap, and for patients trying to obtain relief between pregnancies.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
- Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674-708.e8.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
- Obstetrical & Gynecological Survey. 2020;75:545-547.
Abnormal uterine bleeding (AUB) continues to be a top-10 reason why women present for gynecologic care, which makes keeping up with clinical therapies important. Over the past year, we have learned a tremendous amount about elagolix with hormonal add-back therapy for the treatment of bleeding associated with uterine fibroids. In this Update, we provide an overview from 3 randomized clinical trials on the recent US Food and Drug Administration (FDA)-approved drug, elagolix with hormonal add-back therapy (approved May 29, 2020). In addition, we review the data on the Cerene cryotherapy device (Channel Medsystems), as one might rightly ask, do we need another endometrial ablation device? We will address that question, as this device has some unique features that gynecologists should be aware of. Last, we review a study on the importance of considering quality of life in patients with uterine fibroids, which provides sobering information on the psychosocial aspects of uterine fibroids that all clinicians who care for such patients should be aware of.
Endometrial ablation with a new cryotherapy device: Is less more?
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
The phrase “less is more,” in the world of architecture and design, is often associated with Ludwig Mies van der Rohe (1886–1969). One could argue that this principle is one key advantage with the addition of yet another non-resectoscopic endometrial ablation device. The Cerene cryotherapy device, FDA approved in 2019, is presented as a simple, disposable device for in-office use that takes advantage of natural cryoanesthesia and results in less tissue destruction than many other ablation methods.
Device reduces bleeding and permits greater ability for future evaluation
Recently, Curlin and colleagues conducted a prospective, multicenter clinical trial to evaluate the safety and efficacy of the Cerene device in reducing menstrual blood loss.1 They followed 230 patients over 12 months and found that 81% (77% with intention-to-treat analysis) met the primary end point of a pictorial blood loss assessment chart (PBLAC) score of 75 or lower. Clinically, this translated to 44% of patients experiencing light bleeding; 27%, eumenorrhea; and 10%, amenorrhea. This is clearly “less” in terms of the rate of amenorrhea in most endometrial ablation studies. However, this also may translate into “more” ability to evaluate the endometrial cavity in the future, as 97% of the patients were able to undergo hysteroscopy at the 12-month mark and, of those, 93% were able to have the entire endometrial cavity assessed.
Further, of 97 patients who had a tubal sterilization, none had symptoms or evidence of postablation tubal sterilization syndrome. Three patients were unable to undergo hysteroscopy due to pain intolerance (2) or cervical stenosis (1). This is important because some gynecologists have expressed concern over intrauterine synechiae, which may result in scarring and associated future difficulty in assessing the endometrium for possible cancer.
Details about the device
The Cerene device is a single use, disposable device that uses cryothermal energy from nitrous oxide that results in a liquid-to-gas phase change within a polyurethane balloon (resulting in a temperature of -86°C) and delivered through a 6-mm sheath. It may be used in uterine cavities that measure between 2.5 and 6.5 cm in length, corresponding to approximately 10 cm in a uterine sound measurement. Treatment time is 2.5 minutes of nitrous oxide flow.
As mentioned, another benefit claimed is that the Cerene device’s cryoanalgesia properties enable the procedure to be more tolerated in the office setting. Of the 230 patients studied in the Curlin trial, no procedures were performed under general anesthesia.1 Medications used included paracervical block (PCB) only (8%), PCB plus nonsteroidal anti-inflammatory drugs (19.8%), PCB plus oral narcotics/anxiolytics (69%), and PCB plus intravenous sedation (2.9%), showing that this device is ideally suited for in-office use.
The rate of serious adverse events was 2.5% (7 total events in 6 patients within 12 months). All serious adverse events were reviewed by a Clinical Events Committee and none were deemed to be device-related events.
Long-term outcomes remain to be seen
For physicians and patients who worry about the ability to access the endometrial cavity in the future, less may be more. It will be interesting to see what the long-term outcomes show with use of the Cerene cryotherapy device, and whether a lower amenorrhea rate will translate into a higher repeat intervention rate or not. Of course, not all are minimalists. As the architect Robert Venturi (1925–2018) was quoted as saying, “Less is a bore.”
The new Cerene cryotherapy endometrial ablation method meets the FDA’s target for reduction of menstrual blood loss, but it has a slightly lower amenorrhea rate than other devices. Its most significant features are the potential for improved analgesia for in-office use and the possibility that there may be less scarring of the endometrial cavity for future assessment if needed.
Continue to: QoL assessment in women with fibroids is useful in evaluating treatment success...
QoL assessment in women with fibroids is useful in evaluating treatment success
Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674- 708.e8.
In many studies that assess AUB, the primary emphasis generally is placed on quantitation of menstrual bleeding by using PBLAC and alkaline hematin scores. In a systematic review, Go and colleagues argue the case for the importance of measuring the psychosocial impact of abnormal bleeding, emphasizing the concerning finding that many women with fibroids report lower vitality and lower social function scores than women with breast cancer.2
Fibroids associated with inconvenience—and anxiety
The authors analyzed and reviewed 18 randomized trials and 39 observational studies after screening 3,625 records from electronic database searches, with the goal to include only studies with validated quality of life (QoL) questionnaires that were administered both before and after treatment. A highlighted aspect of the reviewed studies was that “control” and “concern” subscales were most affected by fibroids, noting the inconvenience and anxiety that are related to the unpredictable onset and intensity of menses and the feeling of loss of control over one’s health and future.
This systematic review is important because although previous research has shown that fibroids significantly affect QoL, the psychosocial burden of fibroid symptoms had not been compared across different QoL instruments for both disease-specific and general validated health subscales.
Disability levels with fibroids are similar to those with other chronic diseases
Go and colleagues further reported that uterine fibroids have considerable psychosocial impact and lead to poor overall QoL physically and emotionally, with diminished sexual function and increased urinary or defecatory issues. Women with fibroids experienced a level of disability that was similar to that seen in other chronic diseases, and their vitality scores were lower than those associated with heart disease, diabetes, and as mentioned, breast cancer.
The authors concluded that “although objective clinical measures are important to establish a comprehensive understanding of health status, patient reported QoL outcomes play a critical role in evaluating success of a therapy.” They suggested that a larger emphasis on patient-centered care may help to mitigate the psychosocial effects of fibroids.
The study by Go and colleagues highlights the significant psychosocial aspects of the heavy menstrual bleeding associated with fibroids, and the authors found that many women with fibroids score in the range of those with other significant diseases, such as breast cancer and diabetes.
We have noted the trend of including QoL in research, and Go and colleagues make an excellent and compelling argument for this trend using quantitative analysis. It is important to consider this not only in our design of future research but also, and perhaps more importantly, in our clinical care of women as we try to better understand what they are experiencing.
Continue to: What have we learned over the past year about elagolix for uterine fibroids?...
What have we learned over the past year about elagolix for uterine fibroids?
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
Data from the Elaris UF-1 and UF-2 6-month, phase 3 trials3 and the results of the Elaris UF-EXTEND trial with a 6-month extension (totaling 12 months of use)4 were published in 2020, and the 12-month results were discussed in OBG Management (2020;32[7]:35, 39-40). An additional data analysis from the same researchers assessed the effect of elagolix with hormonal add-back therapy in a number of patient subgroups.5 These 3 publications have added to our knowledge of this therapy, and it is worth reviewing them in this context
Design of the elagolix plus hormonal add-back therapy trials
The initial UF-1 and UF-2 trials were 2 identical, double-blind, randomized, placebo-controlled, 6-month, phase 3 trials designed to evaluate the safety and efficacy of elagolix and hormonal add-back therapy.3 UF-1 was conducted at 76 sites in the United States from December 2015 through December 2018, whereas UF-2 was conducted at 77 sites in the United States and Canada from February 2016 through January 2019; the trials were registered separately. Both trials had a 2:1:1 randomization of elagolix (300 mg twice daily) with hormonal add-back therapy (estradiol 1 mg and norethindrone acetate 0.5 mg daily), elagolix alone (300 mg twice daily), or placebo.
In the 6-month studies, the primary end point was both menstrual blood loss of less than 80 mL and at least a 50% reduction of menstrual blood loss as measured by the alkaline hematin method.3 Among several secondary end points was the assessment of QoL using the Uterine Fibroid Symptom QoL questionnaire (UFS-QoL).
Trial results. In UF-1, 68.5% of 206 women, and in UF-2, 76.5% of 189 women, respectively, taking elagolix with add-back therapy met the primary objective. Among women taking elagolix alone, in UF-1, 84.1% of 104 women, and in UF-2, 77% of 95 women, respectively, met criteria. There was improvement in UFS-QoL scores in women receiving elagolix plus add-back therapy with a reduction of symptom severity of -33.2 in UF-1 and -41.4 in UF-2, as compared with the placebo-treated groups (-10.3 and -7.7, respectively).
Adverse effects. Elagolix was associated with a low incidence of serious adverse effects, and the addition of hormonal add-back therapy attenuated the decreases in bone mineral density observed with elagolix alone. In both UF-1 and UF-2 trials, bone mineral density did not differ significantly in the groups of women who received elagolix with hormonal addback therapy versus placebo.
The extension trial results
Of note, in the 12-month study (6-month extension), the authors reported that 87.9% of the women taking elagolix with hormonal add-back therapy met the primary objective.4 Among the women taking elagolix alone, 89.4% met the primary objective.
In a review of the AbbVie-funded extension study, the editorial comments in the Obstetrical and Gynecological Survey expressed concern over the high proportion of data loss, comparing the number of patients joining the extended trial, patients who completed an additional 6 months of treatment, and patients who completed the posttreatment follow-up period of “up to 12 months.”6 Approximately one-third of patients were lost between initial enrollment to the subset who completed follow-up. There was concern that “losses of that magnitude pose a serious threat to validity.”6
Effectiveness in subgroups
Al-Hendy and colleagues analyzed data from the Elaris UF-1 and UF-2 trials to see if the outcomes for elagolix with hormonal addback therapy demonstrated safety and efficacy in subgroups of patients of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume.5
Results. In all subgroups, they found a statistically significant reduction in blood loss in mean menstrual blood loss volume for those treated with elagolix plus hormonal addback therapy compared with those treated with placebo. As well, in terms of QoL, among all subgroups, the mean change in symptom severity score as well as health-related QoL total score from baseline to month 6 was statistically significantly greater than the mean change in the placebo group.
The bottom line
Elagolix with hormonal add-back therapy appears to be a safe and effective method to reduce menstrual blood loss associated with uterine fibroids. It also has a favorable effect on QoL and appears to have benefits in subgroups of women of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume. ●
Elagolix plus hormonal add-back therapy provides several advantages to fibroid care, including a pill form that, as a gonadotropin-releasing hormone (GnRH) antagonist, provides much quicker action than GnRH agonists. The hormonal add-back feature seems to improve QoL measures and has a favorable reported bleeding reduction rate. It also appears to be reasonably safe. Although the studies reviewed here may have some weaknesses, it helps to have another therapy to offer to women who have blood loss associated with fibroids. Deciding on the drug’s optimal clinical use has not been fully explored, as it may be a short-term solution to a long-term problem and may not be ideal for all patients with fibroids. Elagolix and hormonal add-back therapy may be advantageous for patients who need to stop bleeding quickly and are trying to decide about their reproductive plans, for patients close to menopause who need a therapy to bridge this gap, and for patients trying to obtain relief between pregnancies.
Abnormal uterine bleeding (AUB) continues to be a top-10 reason why women present for gynecologic care, which makes keeping up with clinical therapies important. Over the past year, we have learned a tremendous amount about elagolix with hormonal add-back therapy for the treatment of bleeding associated with uterine fibroids. In this Update, we provide an overview from 3 randomized clinical trials on the recent US Food and Drug Administration (FDA)-approved drug, elagolix with hormonal add-back therapy (approved May 29, 2020). In addition, we review the data on the Cerene cryotherapy device (Channel Medsystems), as one might rightly ask, do we need another endometrial ablation device? We will address that question, as this device has some unique features that gynecologists should be aware of. Last, we review a study on the importance of considering quality of life in patients with uterine fibroids, which provides sobering information on the psychosocial aspects of uterine fibroids that all clinicians who care for such patients should be aware of.
Endometrial ablation with a new cryotherapy device: Is less more?
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
The phrase “less is more,” in the world of architecture and design, is often associated with Ludwig Mies van der Rohe (1886–1969). One could argue that this principle is one key advantage with the addition of yet another non-resectoscopic endometrial ablation device. The Cerene cryotherapy device, FDA approved in 2019, is presented as a simple, disposable device for in-office use that takes advantage of natural cryoanesthesia and results in less tissue destruction than many other ablation methods.
Device reduces bleeding and permits greater ability for future evaluation
Recently, Curlin and colleagues conducted a prospective, multicenter clinical trial to evaluate the safety and efficacy of the Cerene device in reducing menstrual blood loss.1 They followed 230 patients over 12 months and found that 81% (77% with intention-to-treat analysis) met the primary end point of a pictorial blood loss assessment chart (PBLAC) score of 75 or lower. Clinically, this translated to 44% of patients experiencing light bleeding; 27%, eumenorrhea; and 10%, amenorrhea. This is clearly “less” in terms of the rate of amenorrhea in most endometrial ablation studies. However, this also may translate into “more” ability to evaluate the endometrial cavity in the future, as 97% of the patients were able to undergo hysteroscopy at the 12-month mark and, of those, 93% were able to have the entire endometrial cavity assessed.
Further, of 97 patients who had a tubal sterilization, none had symptoms or evidence of postablation tubal sterilization syndrome. Three patients were unable to undergo hysteroscopy due to pain intolerance (2) or cervical stenosis (1). This is important because some gynecologists have expressed concern over intrauterine synechiae, which may result in scarring and associated future difficulty in assessing the endometrium for possible cancer.
Details about the device
The Cerene device is a single use, disposable device that uses cryothermal energy from nitrous oxide that results in a liquid-to-gas phase change within a polyurethane balloon (resulting in a temperature of -86°C) and delivered through a 6-mm sheath. It may be used in uterine cavities that measure between 2.5 and 6.5 cm in length, corresponding to approximately 10 cm in a uterine sound measurement. Treatment time is 2.5 minutes of nitrous oxide flow.
As mentioned, another benefit claimed is that the Cerene device’s cryoanalgesia properties enable the procedure to be more tolerated in the office setting. Of the 230 patients studied in the Curlin trial, no procedures were performed under general anesthesia.1 Medications used included paracervical block (PCB) only (8%), PCB plus nonsteroidal anti-inflammatory drugs (19.8%), PCB plus oral narcotics/anxiolytics (69%), and PCB plus intravenous sedation (2.9%), showing that this device is ideally suited for in-office use.
The rate of serious adverse events was 2.5% (7 total events in 6 patients within 12 months). All serious adverse events were reviewed by a Clinical Events Committee and none were deemed to be device-related events.
Long-term outcomes remain to be seen
For physicians and patients who worry about the ability to access the endometrial cavity in the future, less may be more. It will be interesting to see what the long-term outcomes show with use of the Cerene cryotherapy device, and whether a lower amenorrhea rate will translate into a higher repeat intervention rate or not. Of course, not all are minimalists. As the architect Robert Venturi (1925–2018) was quoted as saying, “Less is a bore.”
The new Cerene cryotherapy endometrial ablation method meets the FDA’s target for reduction of menstrual blood loss, but it has a slightly lower amenorrhea rate than other devices. Its most significant features are the potential for improved analgesia for in-office use and the possibility that there may be less scarring of the endometrial cavity for future assessment if needed.
Continue to: QoL assessment in women with fibroids is useful in evaluating treatment success...
QoL assessment in women with fibroids is useful in evaluating treatment success
Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674- 708.e8.
In many studies that assess AUB, the primary emphasis generally is placed on quantitation of menstrual bleeding by using PBLAC and alkaline hematin scores. In a systematic review, Go and colleagues argue the case for the importance of measuring the psychosocial impact of abnormal bleeding, emphasizing the concerning finding that many women with fibroids report lower vitality and lower social function scores than women with breast cancer.2
Fibroids associated with inconvenience—and anxiety
The authors analyzed and reviewed 18 randomized trials and 39 observational studies after screening 3,625 records from electronic database searches, with the goal to include only studies with validated quality of life (QoL) questionnaires that were administered both before and after treatment. A highlighted aspect of the reviewed studies was that “control” and “concern” subscales were most affected by fibroids, noting the inconvenience and anxiety that are related to the unpredictable onset and intensity of menses and the feeling of loss of control over one’s health and future.
This systematic review is important because although previous research has shown that fibroids significantly affect QoL, the psychosocial burden of fibroid symptoms had not been compared across different QoL instruments for both disease-specific and general validated health subscales.
Disability levels with fibroids are similar to those with other chronic diseases
Go and colleagues further reported that uterine fibroids have considerable psychosocial impact and lead to poor overall QoL physically and emotionally, with diminished sexual function and increased urinary or defecatory issues. Women with fibroids experienced a level of disability that was similar to that seen in other chronic diseases, and their vitality scores were lower than those associated with heart disease, diabetes, and as mentioned, breast cancer.
The authors concluded that “although objective clinical measures are important to establish a comprehensive understanding of health status, patient reported QoL outcomes play a critical role in evaluating success of a therapy.” They suggested that a larger emphasis on patient-centered care may help to mitigate the psychosocial effects of fibroids.
The study by Go and colleagues highlights the significant psychosocial aspects of the heavy menstrual bleeding associated with fibroids, and the authors found that many women with fibroids score in the range of those with other significant diseases, such as breast cancer and diabetes.
We have noted the trend of including QoL in research, and Go and colleagues make an excellent and compelling argument for this trend using quantitative analysis. It is important to consider this not only in our design of future research but also, and perhaps more importantly, in our clinical care of women as we try to better understand what they are experiencing.
Continue to: What have we learned over the past year about elagolix for uterine fibroids?...
What have we learned over the past year about elagolix for uterine fibroids?
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
Data from the Elaris UF-1 and UF-2 6-month, phase 3 trials3 and the results of the Elaris UF-EXTEND trial with a 6-month extension (totaling 12 months of use)4 were published in 2020, and the 12-month results were discussed in OBG Management (2020;32[7]:35, 39-40). An additional data analysis from the same researchers assessed the effect of elagolix with hormonal add-back therapy in a number of patient subgroups.5 These 3 publications have added to our knowledge of this therapy, and it is worth reviewing them in this context
Design of the elagolix plus hormonal add-back therapy trials
The initial UF-1 and UF-2 trials were 2 identical, double-blind, randomized, placebo-controlled, 6-month, phase 3 trials designed to evaluate the safety and efficacy of elagolix and hormonal add-back therapy.3 UF-1 was conducted at 76 sites in the United States from December 2015 through December 2018, whereas UF-2 was conducted at 77 sites in the United States and Canada from February 2016 through January 2019; the trials were registered separately. Both trials had a 2:1:1 randomization of elagolix (300 mg twice daily) with hormonal add-back therapy (estradiol 1 mg and norethindrone acetate 0.5 mg daily), elagolix alone (300 mg twice daily), or placebo.
In the 6-month studies, the primary end point was both menstrual blood loss of less than 80 mL and at least a 50% reduction of menstrual blood loss as measured by the alkaline hematin method.3 Among several secondary end points was the assessment of QoL using the Uterine Fibroid Symptom QoL questionnaire (UFS-QoL).
Trial results. In UF-1, 68.5% of 206 women, and in UF-2, 76.5% of 189 women, respectively, taking elagolix with add-back therapy met the primary objective. Among women taking elagolix alone, in UF-1, 84.1% of 104 women, and in UF-2, 77% of 95 women, respectively, met criteria. There was improvement in UFS-QoL scores in women receiving elagolix plus add-back therapy with a reduction of symptom severity of -33.2 in UF-1 and -41.4 in UF-2, as compared with the placebo-treated groups (-10.3 and -7.7, respectively).
Adverse effects. Elagolix was associated with a low incidence of serious adverse effects, and the addition of hormonal add-back therapy attenuated the decreases in bone mineral density observed with elagolix alone. In both UF-1 and UF-2 trials, bone mineral density did not differ significantly in the groups of women who received elagolix with hormonal addback therapy versus placebo.
The extension trial results
Of note, in the 12-month study (6-month extension), the authors reported that 87.9% of the women taking elagolix with hormonal add-back therapy met the primary objective.4 Among the women taking elagolix alone, 89.4% met the primary objective.
In a review of the AbbVie-funded extension study, the editorial comments in the Obstetrical and Gynecological Survey expressed concern over the high proportion of data loss, comparing the number of patients joining the extended trial, patients who completed an additional 6 months of treatment, and patients who completed the posttreatment follow-up period of “up to 12 months.”6 Approximately one-third of patients were lost between initial enrollment to the subset who completed follow-up. There was concern that “losses of that magnitude pose a serious threat to validity.”6
Effectiveness in subgroups
Al-Hendy and colleagues analyzed data from the Elaris UF-1 and UF-2 trials to see if the outcomes for elagolix with hormonal addback therapy demonstrated safety and efficacy in subgroups of patients of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume.5
Results. In all subgroups, they found a statistically significant reduction in blood loss in mean menstrual blood loss volume for those treated with elagolix plus hormonal addback therapy compared with those treated with placebo. As well, in terms of QoL, among all subgroups, the mean change in symptom severity score as well as health-related QoL total score from baseline to month 6 was statistically significantly greater than the mean change in the placebo group.
The bottom line
Elagolix with hormonal add-back therapy appears to be a safe and effective method to reduce menstrual blood loss associated with uterine fibroids. It also has a favorable effect on QoL and appears to have benefits in subgroups of women of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume. ●
Elagolix plus hormonal add-back therapy provides several advantages to fibroid care, including a pill form that, as a gonadotropin-releasing hormone (GnRH) antagonist, provides much quicker action than GnRH agonists. The hormonal add-back feature seems to improve QoL measures and has a favorable reported bleeding reduction rate. It also appears to be reasonably safe. Although the studies reviewed here may have some weaknesses, it helps to have another therapy to offer to women who have blood loss associated with fibroids. Deciding on the drug’s optimal clinical use has not been fully explored, as it may be a short-term solution to a long-term problem and may not be ideal for all patients with fibroids. Elagolix and hormonal add-back therapy may be advantageous for patients who need to stop bleeding quickly and are trying to decide about their reproductive plans, for patients close to menopause who need a therapy to bridge this gap, and for patients trying to obtain relief between pregnancies.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
- Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674-708.e8.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
- Obstetrical & Gynecological Survey. 2020;75:545-547.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
- Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674-708.e8.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
- Obstetrical & Gynecological Survey. 2020;75:545-547.