User login
How well do JAK inhibitors work for atopic dermatitis?
largely because of the heterogeneous nature of the disease.
“Atopic dermatitis patients have different complaints,” Jacob P. Thyssen, MD, PhD, said during the Revolutionizing Atopic Dermatitis symposium. “Some of them have repeated infections. Some have psychiatric symptoms. Others have widespread eczema. When you talk about how well they work, it really depends on what aspects of AD, what subgroups of AD, and how well they work with comorbidities of AD.”
Baricitinib, a JAK1/JAK2 inhibitor in 2-mg and 4-mg tablets, is available in the European Union, and is under Food and Drug Administration review for AD in the United States. Two JAK1 inhibitors continue to be evaluated in AD clinical trials and are also under FDA review for AD: abrocitinib (100 mg and 200 mg) and upadacitinib (15 mg and 30 mg). None of these agents have been tested in head-to-head trials and only one (abrocitinib) has been compared with the interleukin-4 receptor–alpha antagonist dupilumab, which makes meaningful direct comparisons impossible. (Baricitinib and upadacitinib are approved for treating RA in the United States.)
In his informal assessment from clinical trial data of how these three JAK inhibitors compare with the biologic agents dupilumab and tralokinumab, with potency as an indication, Dr. Thyssen, professor of dermatology at the University of Copenhagen, observed that abrocitinib and dupilumab “are somewhere in the middle,” tralokinumab and baricitinib are “slightly weaker,” while upadacitinib is “very potent.” (Dupilumab is approved by the FDA for treating AD ages 6 and older, and tralokinumab, a fully human monoclonal antibody that binds to IL-13, is under FDA review for AD.)
However, he cautioned that making direct comparisons of these drugs is limited by differences in clinical trial designs, trial length, severity of disease at baseline, and demographics. “Placebo effects also differ between trials, and the speed of onset is different between JAK inhibitors and biologic agents. Because of this, efficacy can be difficult to assess over 12-16 weeks. That’s why long-term studies are necessary.”
It’s also tricky to compare safety signals with baricitinib, abrocitinib, and upadacitinib, “because some of them are JAK1 inhibitors; others are JAK1/JAK2 inhibitors,” he continued. “Even the molecules that inhibit JAK1 are different, so making a comparison between abrocitinib and upadacitinib requires studies that do this is in the best way and over a long period of time.”
Safety signals
Common safety signals in this drug class include nasopharyngitis, nausea, and headache. “Many of these are short lasting, meaning that patients will perhaps have a headache for a day or two and then it will be over,” said Dr. Thyssen, who is also a consultant dermatologist at Bispebjerg Hospital in Copenhagen. “This means that even though we see high proportions of safety signals, this is probably not going to limit the use of JAK inhibitors in most of our patients. Then we have an acne signal in higher proportions for abrocitinib and upadacitinib than for baricitinib, so perhaps this is related to the potency.”
There is also an increased risk for infections, including herpes zoster. “Is this a class effect?” he asked. “We see quite a bit for baricitinib, particularly when it’s used for rheumatoid arthritis. We also see it in AD patients, but we don’t know to what degree yet. We need the real-world evidence before we can make any conclusions.” Routine blood monitoring tests are also required in patients taking JAK inhibitors, because of the risk for leukopenia and effects on liver enzymes.
Then there’s the risk of deep vein thrombosis/pulmonary embolism. “This is mostly linked to baricitinib use, but is this a class effect or is it specific to baricitinib?” he asked. “We’ll have to wait and see, but I think overall, this is not something I have great fear of because we see that AD patients are young, usually with a normal [body mass index], at least in Europe. But we have to study this closely.”
From a clinical standpoint, JAK1/2 inhibitors work well on every measurable aspect of AD, he said, including eczema severity, itch, skin pain, sleep, and quality of life. “Based on conference abstracts and publications, they seem to work equally well independent of race, BMI, atopy status, age, and whether their AD is extrinsic or intrinsic,” Dr. Thyssen added. “One thing we haven’t learned from the companies is, what patients have the highest likelihood of getting a good treatment response? We don’t have good biomarkers yet, but anything the companies can do to help us identify the patients with the greatest chance of success would be so welcome.”
The best available data suggest that JAK inhibitors benefit AD patients with certain comorbidities, including inflammatory bowel disease (with upadacitinib), RA (with both baricitinib and upadacitinib), and alopecia areata (with baricitinib). “These drugs also have been shown to work well for the psychiatric symptoms of disease,” he said.
“As for patients with type 2 inflammation in the airways such as asthma and rhinitis, dupilumab works, but do the JAK inhibitors work? It’s possible from a mode of action standpoint, but we don’t know.” It also remains unclear how JAK inhibitors will fare in the treatment of chronic hand eczema and ocular surface disease, like allergic conjunctivitis, he said.
Despite the unknowns, Dr. Thyssen emphasized the promise that JAK inhibitors hold for AD patients. “We know they provide good AD control,” he said. “For some, like baricitinib, you may need to instruct the patient to use topical corticosteroids as well, but this does not seem to be necessary for upadacitinib and abrocitinib. You really have a single bullet here that will take away most of the problems for many patients, with very fast onset of action, which is important for our patients.”
Dr. Thyssen disclosed that he is a speaker, advisory board member, and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
largely because of the heterogeneous nature of the disease.
“Atopic dermatitis patients have different complaints,” Jacob P. Thyssen, MD, PhD, said during the Revolutionizing Atopic Dermatitis symposium. “Some of them have repeated infections. Some have psychiatric symptoms. Others have widespread eczema. When you talk about how well they work, it really depends on what aspects of AD, what subgroups of AD, and how well they work with comorbidities of AD.”
Baricitinib, a JAK1/JAK2 inhibitor in 2-mg and 4-mg tablets, is available in the European Union, and is under Food and Drug Administration review for AD in the United States. Two JAK1 inhibitors continue to be evaluated in AD clinical trials and are also under FDA review for AD: abrocitinib (100 mg and 200 mg) and upadacitinib (15 mg and 30 mg). None of these agents have been tested in head-to-head trials and only one (abrocitinib) has been compared with the interleukin-4 receptor–alpha antagonist dupilumab, which makes meaningful direct comparisons impossible. (Baricitinib and upadacitinib are approved for treating RA in the United States.)
In his informal assessment from clinical trial data of how these three JAK inhibitors compare with the biologic agents dupilumab and tralokinumab, with potency as an indication, Dr. Thyssen, professor of dermatology at the University of Copenhagen, observed that abrocitinib and dupilumab “are somewhere in the middle,” tralokinumab and baricitinib are “slightly weaker,” while upadacitinib is “very potent.” (Dupilumab is approved by the FDA for treating AD ages 6 and older, and tralokinumab, a fully human monoclonal antibody that binds to IL-13, is under FDA review for AD.)
However, he cautioned that making direct comparisons of these drugs is limited by differences in clinical trial designs, trial length, severity of disease at baseline, and demographics. “Placebo effects also differ between trials, and the speed of onset is different between JAK inhibitors and biologic agents. Because of this, efficacy can be difficult to assess over 12-16 weeks. That’s why long-term studies are necessary.”
It’s also tricky to compare safety signals with baricitinib, abrocitinib, and upadacitinib, “because some of them are JAK1 inhibitors; others are JAK1/JAK2 inhibitors,” he continued. “Even the molecules that inhibit JAK1 are different, so making a comparison between abrocitinib and upadacitinib requires studies that do this is in the best way and over a long period of time.”
Safety signals
Common safety signals in this drug class include nasopharyngitis, nausea, and headache. “Many of these are short lasting, meaning that patients will perhaps have a headache for a day or two and then it will be over,” said Dr. Thyssen, who is also a consultant dermatologist at Bispebjerg Hospital in Copenhagen. “This means that even though we see high proportions of safety signals, this is probably not going to limit the use of JAK inhibitors in most of our patients. Then we have an acne signal in higher proportions for abrocitinib and upadacitinib than for baricitinib, so perhaps this is related to the potency.”
There is also an increased risk for infections, including herpes zoster. “Is this a class effect?” he asked. “We see quite a bit for baricitinib, particularly when it’s used for rheumatoid arthritis. We also see it in AD patients, but we don’t know to what degree yet. We need the real-world evidence before we can make any conclusions.” Routine blood monitoring tests are also required in patients taking JAK inhibitors, because of the risk for leukopenia and effects on liver enzymes.
Then there’s the risk of deep vein thrombosis/pulmonary embolism. “This is mostly linked to baricitinib use, but is this a class effect or is it specific to baricitinib?” he asked. “We’ll have to wait and see, but I think overall, this is not something I have great fear of because we see that AD patients are young, usually with a normal [body mass index], at least in Europe. But we have to study this closely.”
From a clinical standpoint, JAK1/2 inhibitors work well on every measurable aspect of AD, he said, including eczema severity, itch, skin pain, sleep, and quality of life. “Based on conference abstracts and publications, they seem to work equally well independent of race, BMI, atopy status, age, and whether their AD is extrinsic or intrinsic,” Dr. Thyssen added. “One thing we haven’t learned from the companies is, what patients have the highest likelihood of getting a good treatment response? We don’t have good biomarkers yet, but anything the companies can do to help us identify the patients with the greatest chance of success would be so welcome.”
The best available data suggest that JAK inhibitors benefit AD patients with certain comorbidities, including inflammatory bowel disease (with upadacitinib), RA (with both baricitinib and upadacitinib), and alopecia areata (with baricitinib). “These drugs also have been shown to work well for the psychiatric symptoms of disease,” he said.
“As for patients with type 2 inflammation in the airways such as asthma and rhinitis, dupilumab works, but do the JAK inhibitors work? It’s possible from a mode of action standpoint, but we don’t know.” It also remains unclear how JAK inhibitors will fare in the treatment of chronic hand eczema and ocular surface disease, like allergic conjunctivitis, he said.
Despite the unknowns, Dr. Thyssen emphasized the promise that JAK inhibitors hold for AD patients. “We know they provide good AD control,” he said. “For some, like baricitinib, you may need to instruct the patient to use topical corticosteroids as well, but this does not seem to be necessary for upadacitinib and abrocitinib. You really have a single bullet here that will take away most of the problems for many patients, with very fast onset of action, which is important for our patients.”
Dr. Thyssen disclosed that he is a speaker, advisory board member, and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
largely because of the heterogeneous nature of the disease.
“Atopic dermatitis patients have different complaints,” Jacob P. Thyssen, MD, PhD, said during the Revolutionizing Atopic Dermatitis symposium. “Some of them have repeated infections. Some have psychiatric symptoms. Others have widespread eczema. When you talk about how well they work, it really depends on what aspects of AD, what subgroups of AD, and how well they work with comorbidities of AD.”
Baricitinib, a JAK1/JAK2 inhibitor in 2-mg and 4-mg tablets, is available in the European Union, and is under Food and Drug Administration review for AD in the United States. Two JAK1 inhibitors continue to be evaluated in AD clinical trials and are also under FDA review for AD: abrocitinib (100 mg and 200 mg) and upadacitinib (15 mg and 30 mg). None of these agents have been tested in head-to-head trials and only one (abrocitinib) has been compared with the interleukin-4 receptor–alpha antagonist dupilumab, which makes meaningful direct comparisons impossible. (Baricitinib and upadacitinib are approved for treating RA in the United States.)
In his informal assessment from clinical trial data of how these three JAK inhibitors compare with the biologic agents dupilumab and tralokinumab, with potency as an indication, Dr. Thyssen, professor of dermatology at the University of Copenhagen, observed that abrocitinib and dupilumab “are somewhere in the middle,” tralokinumab and baricitinib are “slightly weaker,” while upadacitinib is “very potent.” (Dupilumab is approved by the FDA for treating AD ages 6 and older, and tralokinumab, a fully human monoclonal antibody that binds to IL-13, is under FDA review for AD.)
However, he cautioned that making direct comparisons of these drugs is limited by differences in clinical trial designs, trial length, severity of disease at baseline, and demographics. “Placebo effects also differ between trials, and the speed of onset is different between JAK inhibitors and biologic agents. Because of this, efficacy can be difficult to assess over 12-16 weeks. That’s why long-term studies are necessary.”
It’s also tricky to compare safety signals with baricitinib, abrocitinib, and upadacitinib, “because some of them are JAK1 inhibitors; others are JAK1/JAK2 inhibitors,” he continued. “Even the molecules that inhibit JAK1 are different, so making a comparison between abrocitinib and upadacitinib requires studies that do this is in the best way and over a long period of time.”
Safety signals
Common safety signals in this drug class include nasopharyngitis, nausea, and headache. “Many of these are short lasting, meaning that patients will perhaps have a headache for a day or two and then it will be over,” said Dr. Thyssen, who is also a consultant dermatologist at Bispebjerg Hospital in Copenhagen. “This means that even though we see high proportions of safety signals, this is probably not going to limit the use of JAK inhibitors in most of our patients. Then we have an acne signal in higher proportions for abrocitinib and upadacitinib than for baricitinib, so perhaps this is related to the potency.”
There is also an increased risk for infections, including herpes zoster. “Is this a class effect?” he asked. “We see quite a bit for baricitinib, particularly when it’s used for rheumatoid arthritis. We also see it in AD patients, but we don’t know to what degree yet. We need the real-world evidence before we can make any conclusions.” Routine blood monitoring tests are also required in patients taking JAK inhibitors, because of the risk for leukopenia and effects on liver enzymes.
Then there’s the risk of deep vein thrombosis/pulmonary embolism. “This is mostly linked to baricitinib use, but is this a class effect or is it specific to baricitinib?” he asked. “We’ll have to wait and see, but I think overall, this is not something I have great fear of because we see that AD patients are young, usually with a normal [body mass index], at least in Europe. But we have to study this closely.”
From a clinical standpoint, JAK1/2 inhibitors work well on every measurable aspect of AD, he said, including eczema severity, itch, skin pain, sleep, and quality of life. “Based on conference abstracts and publications, they seem to work equally well independent of race, BMI, atopy status, age, and whether their AD is extrinsic or intrinsic,” Dr. Thyssen added. “One thing we haven’t learned from the companies is, what patients have the highest likelihood of getting a good treatment response? We don’t have good biomarkers yet, but anything the companies can do to help us identify the patients with the greatest chance of success would be so welcome.”
The best available data suggest that JAK inhibitors benefit AD patients with certain comorbidities, including inflammatory bowel disease (with upadacitinib), RA (with both baricitinib and upadacitinib), and alopecia areata (with baricitinib). “These drugs also have been shown to work well for the psychiatric symptoms of disease,” he said.
“As for patients with type 2 inflammation in the airways such as asthma and rhinitis, dupilumab works, but do the JAK inhibitors work? It’s possible from a mode of action standpoint, but we don’t know.” It also remains unclear how JAK inhibitors will fare in the treatment of chronic hand eczema and ocular surface disease, like allergic conjunctivitis, he said.
Despite the unknowns, Dr. Thyssen emphasized the promise that JAK inhibitors hold for AD patients. “We know they provide good AD control,” he said. “For some, like baricitinib, you may need to instruct the patient to use topical corticosteroids as well, but this does not seem to be necessary for upadacitinib and abrocitinib. You really have a single bullet here that will take away most of the problems for many patients, with very fast onset of action, which is important for our patients.”
Dr. Thyssen disclosed that he is a speaker, advisory board member, and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
FROM REVOLUTIONIZING AD 2021
Clinical Edge Commentary: RA July 2021
Several recent studies have evaluated risks of therapy in rheumatoid arthritis (RA). One question regarding treatment of early RA is whether different initial treatment strategies confer different risks. In a systematic review with network meta-analysis, Adas et al reviewed differences between methotrexate, biologic disease-modifying antirheumatic drug (bDMARD), and steroid use in early RA. Overall, risk of serious adverse events was higher with bDMARD monotherapy than methotrexate monotherapy. Of note, while generally long-term steroid use is disfavored due to adverse effects, serious adverse events were not increased in patients treated with methotrexate and steroids together. The size of differences in risk was small and study heterogeneity, including the class of bDMARDs, limits generalizability of this information; thus, variations in the studies themselves may account for these differences.
Pazmino et al also looked at treatment strategies in early RA in a post hoc analysis of participants at “low-risk” for poor prognosis in the CareRA trial, in which patients were randomized to step-up methotrexate without glucocorticoids or step-down with glucocorticoids. While pain scores and disease activity scores were similar among the two groups, analgesic use (including non-steroidal anti-inflammatory drugs [NSAIDs] and opioids) was significantly lower among those randomized to the glucocorticoid-bridging arm. Though this information is reassuring as to the utility of glucocorticoids, it is not clear that this correlation is broadly applicable, for example, among the “higher-risk” patients who might otherwise be more likely to receive glucocorticoids.
A recent analysis of the COVID-19 global rheumatology alliance physician registry by Sparks et al of cases of COVID-19 in patients with rheumatic disease looked more specifically at COVID-19 outcomes in patients with RA on biologic therapy. These are of interest due both to the risk of immunosuppression overall as well as the use of immunosuppressive medications in COVID-19-associated hyperinflammation. The study evaluated outcomes of hospitalization (including respiratory support and mortality). While hospitalization is difficult to evaluate as an outcome without knowing the background rate of COVID in the different areas, of the hospitalized patients, patients who used Janus kinase inhibitors (JAKi) and rituximab received oxygen or ventilator support and had higher mortality than those who were on abatacept, IL-6 inhibitors, or TNF inhibitors. Wider interpretation is difficult due to lack of knowledge of when medications were given (including rituximab dosing), but the results suggest that concern is warranted in improving outcomes for patients with RA on these therapies.
Finally, regarding the well-known cardiovascular risk associated with RA, several observational studies have suggested that methotrexate is associated with reduction in risk of cardiovascular events. This cohort study of the Veterans Affairs RA registry followed over 2000 patients for a mean of about 5 years; a reduction in incidence of cardiovascular events was associated with methotrexate use, independent of age, body mass index (BMI), cardiovascular risk factors, RA disease activity, and other RA therapies. It may be that methotrexate use is associated with an unknown mediator of cardiovascular disease not evaluated in this study, such as reduced glucocorticoid or NSAID use, but this area deserves further investigation.
Several recent studies have evaluated risks of therapy in rheumatoid arthritis (RA). One question regarding treatment of early RA is whether different initial treatment strategies confer different risks. In a systematic review with network meta-analysis, Adas et al reviewed differences between methotrexate, biologic disease-modifying antirheumatic drug (bDMARD), and steroid use in early RA. Overall, risk of serious adverse events was higher with bDMARD monotherapy than methotrexate monotherapy. Of note, while generally long-term steroid use is disfavored due to adverse effects, serious adverse events were not increased in patients treated with methotrexate and steroids together. The size of differences in risk was small and study heterogeneity, including the class of bDMARDs, limits generalizability of this information; thus, variations in the studies themselves may account for these differences.
Pazmino et al also looked at treatment strategies in early RA in a post hoc analysis of participants at “low-risk” for poor prognosis in the CareRA trial, in which patients were randomized to step-up methotrexate without glucocorticoids or step-down with glucocorticoids. While pain scores and disease activity scores were similar among the two groups, analgesic use (including non-steroidal anti-inflammatory drugs [NSAIDs] and opioids) was significantly lower among those randomized to the glucocorticoid-bridging arm. Though this information is reassuring as to the utility of glucocorticoids, it is not clear that this correlation is broadly applicable, for example, among the “higher-risk” patients who might otherwise be more likely to receive glucocorticoids.
A recent analysis of the COVID-19 global rheumatology alliance physician registry by Sparks et al of cases of COVID-19 in patients with rheumatic disease looked more specifically at COVID-19 outcomes in patients with RA on biologic therapy. These are of interest due both to the risk of immunosuppression overall as well as the use of immunosuppressive medications in COVID-19-associated hyperinflammation. The study evaluated outcomes of hospitalization (including respiratory support and mortality). While hospitalization is difficult to evaluate as an outcome without knowing the background rate of COVID in the different areas, of the hospitalized patients, patients who used Janus kinase inhibitors (JAKi) and rituximab received oxygen or ventilator support and had higher mortality than those who were on abatacept, IL-6 inhibitors, or TNF inhibitors. Wider interpretation is difficult due to lack of knowledge of when medications were given (including rituximab dosing), but the results suggest that concern is warranted in improving outcomes for patients with RA on these therapies.
Finally, regarding the well-known cardiovascular risk associated with RA, several observational studies have suggested that methotrexate is associated with reduction in risk of cardiovascular events. This cohort study of the Veterans Affairs RA registry followed over 2000 patients for a mean of about 5 years; a reduction in incidence of cardiovascular events was associated with methotrexate use, independent of age, body mass index (BMI), cardiovascular risk factors, RA disease activity, and other RA therapies. It may be that methotrexate use is associated with an unknown mediator of cardiovascular disease not evaluated in this study, such as reduced glucocorticoid or NSAID use, but this area deserves further investigation.
Several recent studies have evaluated risks of therapy in rheumatoid arthritis (RA). One question regarding treatment of early RA is whether different initial treatment strategies confer different risks. In a systematic review with network meta-analysis, Adas et al reviewed differences between methotrexate, biologic disease-modifying antirheumatic drug (bDMARD), and steroid use in early RA. Overall, risk of serious adverse events was higher with bDMARD monotherapy than methotrexate monotherapy. Of note, while generally long-term steroid use is disfavored due to adverse effects, serious adverse events were not increased in patients treated with methotrexate and steroids together. The size of differences in risk was small and study heterogeneity, including the class of bDMARDs, limits generalizability of this information; thus, variations in the studies themselves may account for these differences.
Pazmino et al also looked at treatment strategies in early RA in a post hoc analysis of participants at “low-risk” for poor prognosis in the CareRA trial, in which patients were randomized to step-up methotrexate without glucocorticoids or step-down with glucocorticoids. While pain scores and disease activity scores were similar among the two groups, analgesic use (including non-steroidal anti-inflammatory drugs [NSAIDs] and opioids) was significantly lower among those randomized to the glucocorticoid-bridging arm. Though this information is reassuring as to the utility of glucocorticoids, it is not clear that this correlation is broadly applicable, for example, among the “higher-risk” patients who might otherwise be more likely to receive glucocorticoids.
A recent analysis of the COVID-19 global rheumatology alliance physician registry by Sparks et al of cases of COVID-19 in patients with rheumatic disease looked more specifically at COVID-19 outcomes in patients with RA on biologic therapy. These are of interest due both to the risk of immunosuppression overall as well as the use of immunosuppressive medications in COVID-19-associated hyperinflammation. The study evaluated outcomes of hospitalization (including respiratory support and mortality). While hospitalization is difficult to evaluate as an outcome without knowing the background rate of COVID in the different areas, of the hospitalized patients, patients who used Janus kinase inhibitors (JAKi) and rituximab received oxygen or ventilator support and had higher mortality than those who were on abatacept, IL-6 inhibitors, or TNF inhibitors. Wider interpretation is difficult due to lack of knowledge of when medications were given (including rituximab dosing), but the results suggest that concern is warranted in improving outcomes for patients with RA on these therapies.
Finally, regarding the well-known cardiovascular risk associated with RA, several observational studies have suggested that methotrexate is associated with reduction in risk of cardiovascular events. This cohort study of the Veterans Affairs RA registry followed over 2000 patients for a mean of about 5 years; a reduction in incidence of cardiovascular events was associated with methotrexate use, independent of age, body mass index (BMI), cardiovascular risk factors, RA disease activity, and other RA therapies. It may be that methotrexate use is associated with an unknown mediator of cardiovascular disease not evaluated in this study, such as reduced glucocorticoid or NSAID use, but this area deserves further investigation.
Indoor tanning ICD-10 codes may be underused, study finds
according to a study presented at the annual meeting of the Society for Investigative Dermatology.
“Since indoor tanning ICD-10 codes were only recently universally implemented in 2015, and providers may still be using other codes that cover similar services, we think our data likely underestimate the number of encounters and sequelae associated with indoor tanning,” Alexandria M. Brown, BSA, of Baylor College of Medicine, Houston, said in her presentation. “We think increased usage of these indoor tanning exposure codes in coming years will strengthen this body of indoor tanning literature and data.”
Using insurance claims data on about 43 million patients from Truven Health MarketScan, Ms. Brown and colleagues analyzed patient encounters with ICD-10 indoor tanning codes W89.1, W89.1XXA, W89.1XXD, and W89.1XXS between 2016 and 2018 for about 43 million patients. Overall, there were 4,550 patient encounters where these codes had been recorded, with most (99%) occurring in an outpatient setting. The majority of providers at these encounters were dermatologists (72%). Patients were mostly women (85%); and most were ages 25-34 years (19.4%), 35-44 years (20.6%), 45-54 years (22.7%), and 55-64 years (19%). Almost 5% were 65 and over, 11.7% were ages 18-24, and 1.6% were under age 18.
The use of indoor tanning codes were most common in the Midwest (55 per 100,000 encounters with dermatologists), compared with 16 per 100,000 in the Northeast, 21 per 100,000 in the West, and 28 per 100,000 in the South. CPT codes for “destruction of a premalignant lesion” and “biopsy” were the most frequently used codes entered at visits where indoor tanning codes were also entered, and were present in 15.1% of encounters and 18.4% of encounters, respectively.
“This suggests that many of these encounters may have been for skin cancer surveillance and that indoor tanning exposure may have been coded as part of a patient’s skin cancer risk profile,” Ms. Brown noted.
The study shows how these codes are being used and could help determine health care use patterns for these patients as well as their comorbidities, behaviors, and risk factors, according to the authors, who believe this is the first study to look at the use of ICD-10 indoor tanning codes.
“Any effort to reduce indoor tanning requires knowledge of the population at risk. It has been shown that the ability to recognize and provide counseling to at-risk patients can improve sun protective behaviors and reduce indoor tanning,” Ms. Brown said. Claims databases can be a “valuable tool to better understand patients who have been exposed to indoor tanning and their associated risk factors, comorbidities, behaviors, and health care utilization.”
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said the study was interesting and “provides some guidance with respect to who, when, and where in the U.S. to target educational initiatives on the harms of tanning beds.”
Dr. Friedman, who was not involved with the research, agreed with the authors’ assertion that their study was underestimating the use of indoor tanning beds. “Using a large database provides the means to better generalize one’s dataset; however in this case, it relies on proper coding by the practitioner,” or even using the code for tanning bed use at all.
“There also could be some inherent bias given most of the cases for which the code was used was for skin cancer surveillance, and therefore tanning bed use was top of mind,” he said.
While he believes this study may not be most efficient way of determining demographics of at-risk individuals using tanning beds, Dr. Friedman said the results “should serve as the impetus to develop public health campaigns around this information, following which research can be conducted to evaluate if the intervention had an impact.”
Ms. Brown and Dr. Friedman reported no relevant financial disclosures.
according to a study presented at the annual meeting of the Society for Investigative Dermatology.
“Since indoor tanning ICD-10 codes were only recently universally implemented in 2015, and providers may still be using other codes that cover similar services, we think our data likely underestimate the number of encounters and sequelae associated with indoor tanning,” Alexandria M. Brown, BSA, of Baylor College of Medicine, Houston, said in her presentation. “We think increased usage of these indoor tanning exposure codes in coming years will strengthen this body of indoor tanning literature and data.”
Using insurance claims data on about 43 million patients from Truven Health MarketScan, Ms. Brown and colleagues analyzed patient encounters with ICD-10 indoor tanning codes W89.1, W89.1XXA, W89.1XXD, and W89.1XXS between 2016 and 2018 for about 43 million patients. Overall, there were 4,550 patient encounters where these codes had been recorded, with most (99%) occurring in an outpatient setting. The majority of providers at these encounters were dermatologists (72%). Patients were mostly women (85%); and most were ages 25-34 years (19.4%), 35-44 years (20.6%), 45-54 years (22.7%), and 55-64 years (19%). Almost 5% were 65 and over, 11.7% were ages 18-24, and 1.6% were under age 18.
The use of indoor tanning codes were most common in the Midwest (55 per 100,000 encounters with dermatologists), compared with 16 per 100,000 in the Northeast, 21 per 100,000 in the West, and 28 per 100,000 in the South. CPT codes for “destruction of a premalignant lesion” and “biopsy” were the most frequently used codes entered at visits where indoor tanning codes were also entered, and were present in 15.1% of encounters and 18.4% of encounters, respectively.
“This suggests that many of these encounters may have been for skin cancer surveillance and that indoor tanning exposure may have been coded as part of a patient’s skin cancer risk profile,” Ms. Brown noted.
The study shows how these codes are being used and could help determine health care use patterns for these patients as well as their comorbidities, behaviors, and risk factors, according to the authors, who believe this is the first study to look at the use of ICD-10 indoor tanning codes.
“Any effort to reduce indoor tanning requires knowledge of the population at risk. It has been shown that the ability to recognize and provide counseling to at-risk patients can improve sun protective behaviors and reduce indoor tanning,” Ms. Brown said. Claims databases can be a “valuable tool to better understand patients who have been exposed to indoor tanning and their associated risk factors, comorbidities, behaviors, and health care utilization.”
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said the study was interesting and “provides some guidance with respect to who, when, and where in the U.S. to target educational initiatives on the harms of tanning beds.”
Dr. Friedman, who was not involved with the research, agreed with the authors’ assertion that their study was underestimating the use of indoor tanning beds. “Using a large database provides the means to better generalize one’s dataset; however in this case, it relies on proper coding by the practitioner,” or even using the code for tanning bed use at all.
“There also could be some inherent bias given most of the cases for which the code was used was for skin cancer surveillance, and therefore tanning bed use was top of mind,” he said.
While he believes this study may not be most efficient way of determining demographics of at-risk individuals using tanning beds, Dr. Friedman said the results “should serve as the impetus to develop public health campaigns around this information, following which research can be conducted to evaluate if the intervention had an impact.”
Ms. Brown and Dr. Friedman reported no relevant financial disclosures.
according to a study presented at the annual meeting of the Society for Investigative Dermatology.
“Since indoor tanning ICD-10 codes were only recently universally implemented in 2015, and providers may still be using other codes that cover similar services, we think our data likely underestimate the number of encounters and sequelae associated with indoor tanning,” Alexandria M. Brown, BSA, of Baylor College of Medicine, Houston, said in her presentation. “We think increased usage of these indoor tanning exposure codes in coming years will strengthen this body of indoor tanning literature and data.”
Using insurance claims data on about 43 million patients from Truven Health MarketScan, Ms. Brown and colleagues analyzed patient encounters with ICD-10 indoor tanning codes W89.1, W89.1XXA, W89.1XXD, and W89.1XXS between 2016 and 2018 for about 43 million patients. Overall, there were 4,550 patient encounters where these codes had been recorded, with most (99%) occurring in an outpatient setting. The majority of providers at these encounters were dermatologists (72%). Patients were mostly women (85%); and most were ages 25-34 years (19.4%), 35-44 years (20.6%), 45-54 years (22.7%), and 55-64 years (19%). Almost 5% were 65 and over, 11.7% were ages 18-24, and 1.6% were under age 18.
The use of indoor tanning codes were most common in the Midwest (55 per 100,000 encounters with dermatologists), compared with 16 per 100,000 in the Northeast, 21 per 100,000 in the West, and 28 per 100,000 in the South. CPT codes for “destruction of a premalignant lesion” and “biopsy” were the most frequently used codes entered at visits where indoor tanning codes were also entered, and were present in 15.1% of encounters and 18.4% of encounters, respectively.
“This suggests that many of these encounters may have been for skin cancer surveillance and that indoor tanning exposure may have been coded as part of a patient’s skin cancer risk profile,” Ms. Brown noted.
The study shows how these codes are being used and could help determine health care use patterns for these patients as well as their comorbidities, behaviors, and risk factors, according to the authors, who believe this is the first study to look at the use of ICD-10 indoor tanning codes.
“Any effort to reduce indoor tanning requires knowledge of the population at risk. It has been shown that the ability to recognize and provide counseling to at-risk patients can improve sun protective behaviors and reduce indoor tanning,” Ms. Brown said. Claims databases can be a “valuable tool to better understand patients who have been exposed to indoor tanning and their associated risk factors, comorbidities, behaviors, and health care utilization.”
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said the study was interesting and “provides some guidance with respect to who, when, and where in the U.S. to target educational initiatives on the harms of tanning beds.”
Dr. Friedman, who was not involved with the research, agreed with the authors’ assertion that their study was underestimating the use of indoor tanning beds. “Using a large database provides the means to better generalize one’s dataset; however in this case, it relies on proper coding by the practitioner,” or even using the code for tanning bed use at all.
“There also could be some inherent bias given most of the cases for which the code was used was for skin cancer surveillance, and therefore tanning bed use was top of mind,” he said.
While he believes this study may not be most efficient way of determining demographics of at-risk individuals using tanning beds, Dr. Friedman said the results “should serve as the impetus to develop public health campaigns around this information, following which research can be conducted to evaluate if the intervention had an impact.”
Ms. Brown and Dr. Friedman reported no relevant financial disclosures.
FROM SID 2021
Female doctors of color say they feel pressure to change their look
It started when a Latina doctor tweeted that she lost points on a practical exam in medical school because of her hoop earrings, with the evaluator writing “earrings, unprofessional.”
That led other female doctors to cite their own experiences, reported The Lily, a Washington Post publication aimed at millennial women. Many women posted photos of themselves wearing hoops, which have long been associated with Latina and African American women, the outlet said.
“There’s a big movement to police women of color and how they present themselves in medical spaces,” said Briana Christophers, an MD-PhD student at the Tri-Institutional MD-PhD Program in New York. “I think in part it’s a way of trying to make people who don’t usually fit the mold, fit the mold.”
Ms. Christophers, who identifies as Latina, said she was urged to wear a black or navy suit when interviewing for doctorate programs. She wore a black suit with a lavender blouse and received comments about that – some positive, some not, she said.
“Sometimes you don’t know how to interpret those sorts of comments,” Ms. Christophers said. “Do you remember because you like the shirt, or because you don’t think I should have done that?”
Doctors of color still stand out in American medicine. The Lily cited the Association of American Medical Colleges as saying that in 2018, Hispanics made up 5.8% of active American doctors and African Americans made up 5%.
Studies show that medical professionals of color often don’t receive the same respect as their White counterparts, with some people questioning whether they’re actually doctors.
“At work, wearing my white coat that has my name pretty big on it with a badge that says doctor on it, I still get asked if I’m the environmental services staff,” Alexandra Sims, MD, a pediatrician in Cincinnati, told The Lily. “I think it just demonstrates how deeply ingrained bias, racism, and sexism are in society and that we have a lot of work to do to disrupt that.”
Dr. Sims said the tweet about hoop earrings led her to wonder about daily decisions she makes about dress.
“Am I too much? Is this too much? Is this earring too big? Is this nail polish color too loud? And how will that be received at work?” she said, noting that she may opt not to wear hoops in certain situations, such as when she’s dealing with a grabby baby.
Monica Verduzco-Gutierrez, MD, professor and chair of the department of rehabilitation medicine at University of Texas Health, San Antonio, said doctors should be judged on the care they provide, not their appearance.
“Judging someone based on their earrings or their jumpsuit or whatever else that they’re noticing about the student is not an appropriate way to judge the student’s ability to take care of a patient,” Dr. Verduzco-Gutierrez said, noting that she was not speaking on behalf of the school.
A version of this article was first published on WebMD.com .
It started when a Latina doctor tweeted that she lost points on a practical exam in medical school because of her hoop earrings, with the evaluator writing “earrings, unprofessional.”
That led other female doctors to cite their own experiences, reported The Lily, a Washington Post publication aimed at millennial women. Many women posted photos of themselves wearing hoops, which have long been associated with Latina and African American women, the outlet said.
“There’s a big movement to police women of color and how they present themselves in medical spaces,” said Briana Christophers, an MD-PhD student at the Tri-Institutional MD-PhD Program in New York. “I think in part it’s a way of trying to make people who don’t usually fit the mold, fit the mold.”
Ms. Christophers, who identifies as Latina, said she was urged to wear a black or navy suit when interviewing for doctorate programs. She wore a black suit with a lavender blouse and received comments about that – some positive, some not, she said.
“Sometimes you don’t know how to interpret those sorts of comments,” Ms. Christophers said. “Do you remember because you like the shirt, or because you don’t think I should have done that?”
Doctors of color still stand out in American medicine. The Lily cited the Association of American Medical Colleges as saying that in 2018, Hispanics made up 5.8% of active American doctors and African Americans made up 5%.
Studies show that medical professionals of color often don’t receive the same respect as their White counterparts, with some people questioning whether they’re actually doctors.
“At work, wearing my white coat that has my name pretty big on it with a badge that says doctor on it, I still get asked if I’m the environmental services staff,” Alexandra Sims, MD, a pediatrician in Cincinnati, told The Lily. “I think it just demonstrates how deeply ingrained bias, racism, and sexism are in society and that we have a lot of work to do to disrupt that.”
Dr. Sims said the tweet about hoop earrings led her to wonder about daily decisions she makes about dress.
“Am I too much? Is this too much? Is this earring too big? Is this nail polish color too loud? And how will that be received at work?” she said, noting that she may opt not to wear hoops in certain situations, such as when she’s dealing with a grabby baby.
Monica Verduzco-Gutierrez, MD, professor and chair of the department of rehabilitation medicine at University of Texas Health, San Antonio, said doctors should be judged on the care they provide, not their appearance.
“Judging someone based on their earrings or their jumpsuit or whatever else that they’re noticing about the student is not an appropriate way to judge the student’s ability to take care of a patient,” Dr. Verduzco-Gutierrez said, noting that she was not speaking on behalf of the school.
A version of this article was first published on WebMD.com .
It started when a Latina doctor tweeted that she lost points on a practical exam in medical school because of her hoop earrings, with the evaluator writing “earrings, unprofessional.”
That led other female doctors to cite their own experiences, reported The Lily, a Washington Post publication aimed at millennial women. Many women posted photos of themselves wearing hoops, which have long been associated with Latina and African American women, the outlet said.
“There’s a big movement to police women of color and how they present themselves in medical spaces,” said Briana Christophers, an MD-PhD student at the Tri-Institutional MD-PhD Program in New York. “I think in part it’s a way of trying to make people who don’t usually fit the mold, fit the mold.”
Ms. Christophers, who identifies as Latina, said she was urged to wear a black or navy suit when interviewing for doctorate programs. She wore a black suit with a lavender blouse and received comments about that – some positive, some not, she said.
“Sometimes you don’t know how to interpret those sorts of comments,” Ms. Christophers said. “Do you remember because you like the shirt, or because you don’t think I should have done that?”
Doctors of color still stand out in American medicine. The Lily cited the Association of American Medical Colleges as saying that in 2018, Hispanics made up 5.8% of active American doctors and African Americans made up 5%.
Studies show that medical professionals of color often don’t receive the same respect as their White counterparts, with some people questioning whether they’re actually doctors.
“At work, wearing my white coat that has my name pretty big on it with a badge that says doctor on it, I still get asked if I’m the environmental services staff,” Alexandra Sims, MD, a pediatrician in Cincinnati, told The Lily. “I think it just demonstrates how deeply ingrained bias, racism, and sexism are in society and that we have a lot of work to do to disrupt that.”
Dr. Sims said the tweet about hoop earrings led her to wonder about daily decisions she makes about dress.
“Am I too much? Is this too much? Is this earring too big? Is this nail polish color too loud? And how will that be received at work?” she said, noting that she may opt not to wear hoops in certain situations, such as when she’s dealing with a grabby baby.
Monica Verduzco-Gutierrez, MD, professor and chair of the department of rehabilitation medicine at University of Texas Health, San Antonio, said doctors should be judged on the care they provide, not their appearance.
“Judging someone based on their earrings or their jumpsuit or whatever else that they’re noticing about the student is not an appropriate way to judge the student’s ability to take care of a patient,” Dr. Verduzco-Gutierrez said, noting that she was not speaking on behalf of the school.
A version of this article was first published on WebMD.com .
CDC notes sharp declines in breast and cervical cancer screening
The new data come from the National Breast and Cervical Cancer Early Detection Program (NBCCEDP), a program that provides cancer screening services to women with low income and inadequate health insurance.
The data show that the total number of screenings funded by the NBCCEDP declined by 87% for breast cancer screening and by 84% for cervical cancer screening in April 2020 in comparison with the previous 5-year averages for that month.
The declines in breast cancer screening varied from 84% among Hispanic women to 98% among American Indian/Alaskan Native women. The declines in cervical cancer screening varied from 82% among Black women to 92% among Asian Pacific Islander women.
In April 2020, breast cancer screening declined by 86% in metro areas, 88% in urban areas, and 89% in rural areas in comparison with respective 5-year averages. For cervical cancer screenings, the corresponding declines were 85%, 77%, and 82%.
The findings are consistent with those from studies conducted in insured populations, note the authors, led by the Amy DeGroff, PhD, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
“Prolonged delays in screening related to the COVID-19 pandemic may lead to delayed diagnoses, poor health consequences, and an increase in cancer disparities among women already experiencing health inequities,” the CDC states in a press release.
Women from racial and ethnic minority groups already face a disproportionate burden of cervical and breast cancers in the United States: Black women and Hispanic women have the highest rates of cervical cancer incidence (8.3 and 8.9 per 100,000 women, respectively, vs. 7.3 per 100,000 among White women) and the highest rates of cervical cancer deaths. Black women have the highest rate of breast cancer death (26.9 per 100,000 women, vs. 19.4 per 100,000 among White women), the study authors explain.
Although the volume of screening began to recover in May 2020 – test volumes for breast and cervical cancer were 39% and 40% below the 5-year average by June 2020 – breast cancer screening in rural areas remained 52% below the 5-year average, they report.
The findings were published online June 30 in Preventive Medicine.
“This study highlights a decline in cancer screening among women of racial and ethnic minority groups with low incomes when their access to medical services decreased at the beginning of the pandemic,” Dr. DeGroff comments in the CDC press release.
The findings “reinforce the need to safely maintain routine health care services during the pandemic, especially when the health care environment meets COVID-19 safety guidelines,” she adds.
The investigators used NBCCEDP administrative and program data reported to the CDC by awardees – organizations that receive funding to implement the NBCCEDP – to assess the impact of COVID-19 on the number of breast and cervical cancer screening tests administered through the program and the effects of COVID-19 on the availability of screening services and NBCCEDP awardees’ capacity to support partner clinics.
A total of 630,264 breast and 594,566 cervical cancer screening tests were conducted during the review period of January-June 2015-2020.
Despite COVID-related challenges, “a large number of awardees reported flexibility and creative efforts to reach women and support clinics’ resumption of clinical care, including screening, during the COVID-19 pandemic,” the authors write.
“[The] CDC encourages health care professionals to help minimize delays in testing by continuing routine cancer screening for women having symptoms or at high risk for breast or cervical cancer,” Dr. DeGroff commented. “The Early Detection Program can help women overcome barriers to health equity by educating them about the importance of routine screening, addressing their concerns about COVID-19 transmission, and helping them to safely access screening through interventions like patient navigation.”
Future studies will examine the effect of the pandemic on screening during the second half of 2020, when surges of COVID-19 and their timing varied geographically, they note.
A version of this article first appeared on Medscape.com.
The new data come from the National Breast and Cervical Cancer Early Detection Program (NBCCEDP), a program that provides cancer screening services to women with low income and inadequate health insurance.
The data show that the total number of screenings funded by the NBCCEDP declined by 87% for breast cancer screening and by 84% for cervical cancer screening in April 2020 in comparison with the previous 5-year averages for that month.
The declines in breast cancer screening varied from 84% among Hispanic women to 98% among American Indian/Alaskan Native women. The declines in cervical cancer screening varied from 82% among Black women to 92% among Asian Pacific Islander women.
In April 2020, breast cancer screening declined by 86% in metro areas, 88% in urban areas, and 89% in rural areas in comparison with respective 5-year averages. For cervical cancer screenings, the corresponding declines were 85%, 77%, and 82%.
The findings are consistent with those from studies conducted in insured populations, note the authors, led by the Amy DeGroff, PhD, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
“Prolonged delays in screening related to the COVID-19 pandemic may lead to delayed diagnoses, poor health consequences, and an increase in cancer disparities among women already experiencing health inequities,” the CDC states in a press release.
Women from racial and ethnic minority groups already face a disproportionate burden of cervical and breast cancers in the United States: Black women and Hispanic women have the highest rates of cervical cancer incidence (8.3 and 8.9 per 100,000 women, respectively, vs. 7.3 per 100,000 among White women) and the highest rates of cervical cancer deaths. Black women have the highest rate of breast cancer death (26.9 per 100,000 women, vs. 19.4 per 100,000 among White women), the study authors explain.
Although the volume of screening began to recover in May 2020 – test volumes for breast and cervical cancer were 39% and 40% below the 5-year average by June 2020 – breast cancer screening in rural areas remained 52% below the 5-year average, they report.
The findings were published online June 30 in Preventive Medicine.
“This study highlights a decline in cancer screening among women of racial and ethnic minority groups with low incomes when their access to medical services decreased at the beginning of the pandemic,” Dr. DeGroff comments in the CDC press release.
The findings “reinforce the need to safely maintain routine health care services during the pandemic, especially when the health care environment meets COVID-19 safety guidelines,” she adds.
The investigators used NBCCEDP administrative and program data reported to the CDC by awardees – organizations that receive funding to implement the NBCCEDP – to assess the impact of COVID-19 on the number of breast and cervical cancer screening tests administered through the program and the effects of COVID-19 on the availability of screening services and NBCCEDP awardees’ capacity to support partner clinics.
A total of 630,264 breast and 594,566 cervical cancer screening tests were conducted during the review period of January-June 2015-2020.
Despite COVID-related challenges, “a large number of awardees reported flexibility and creative efforts to reach women and support clinics’ resumption of clinical care, including screening, during the COVID-19 pandemic,” the authors write.
“[The] CDC encourages health care professionals to help minimize delays in testing by continuing routine cancer screening for women having symptoms or at high risk for breast or cervical cancer,” Dr. DeGroff commented. “The Early Detection Program can help women overcome barriers to health equity by educating them about the importance of routine screening, addressing their concerns about COVID-19 transmission, and helping them to safely access screening through interventions like patient navigation.”
Future studies will examine the effect of the pandemic on screening during the second half of 2020, when surges of COVID-19 and their timing varied geographically, they note.
A version of this article first appeared on Medscape.com.
The new data come from the National Breast and Cervical Cancer Early Detection Program (NBCCEDP), a program that provides cancer screening services to women with low income and inadequate health insurance.
The data show that the total number of screenings funded by the NBCCEDP declined by 87% for breast cancer screening and by 84% for cervical cancer screening in April 2020 in comparison with the previous 5-year averages for that month.
The declines in breast cancer screening varied from 84% among Hispanic women to 98% among American Indian/Alaskan Native women. The declines in cervical cancer screening varied from 82% among Black women to 92% among Asian Pacific Islander women.
In April 2020, breast cancer screening declined by 86% in metro areas, 88% in urban areas, and 89% in rural areas in comparison with respective 5-year averages. For cervical cancer screenings, the corresponding declines were 85%, 77%, and 82%.
The findings are consistent with those from studies conducted in insured populations, note the authors, led by the Amy DeGroff, PhD, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
“Prolonged delays in screening related to the COVID-19 pandemic may lead to delayed diagnoses, poor health consequences, and an increase in cancer disparities among women already experiencing health inequities,” the CDC states in a press release.
Women from racial and ethnic minority groups already face a disproportionate burden of cervical and breast cancers in the United States: Black women and Hispanic women have the highest rates of cervical cancer incidence (8.3 and 8.9 per 100,000 women, respectively, vs. 7.3 per 100,000 among White women) and the highest rates of cervical cancer deaths. Black women have the highest rate of breast cancer death (26.9 per 100,000 women, vs. 19.4 per 100,000 among White women), the study authors explain.
Although the volume of screening began to recover in May 2020 – test volumes for breast and cervical cancer were 39% and 40% below the 5-year average by June 2020 – breast cancer screening in rural areas remained 52% below the 5-year average, they report.
The findings were published online June 30 in Preventive Medicine.
“This study highlights a decline in cancer screening among women of racial and ethnic minority groups with low incomes when their access to medical services decreased at the beginning of the pandemic,” Dr. DeGroff comments in the CDC press release.
The findings “reinforce the need to safely maintain routine health care services during the pandemic, especially when the health care environment meets COVID-19 safety guidelines,” she adds.
The investigators used NBCCEDP administrative and program data reported to the CDC by awardees – organizations that receive funding to implement the NBCCEDP – to assess the impact of COVID-19 on the number of breast and cervical cancer screening tests administered through the program and the effects of COVID-19 on the availability of screening services and NBCCEDP awardees’ capacity to support partner clinics.
A total of 630,264 breast and 594,566 cervical cancer screening tests were conducted during the review period of January-June 2015-2020.
Despite COVID-related challenges, “a large number of awardees reported flexibility and creative efforts to reach women and support clinics’ resumption of clinical care, including screening, during the COVID-19 pandemic,” the authors write.
“[The] CDC encourages health care professionals to help minimize delays in testing by continuing routine cancer screening for women having symptoms or at high risk for breast or cervical cancer,” Dr. DeGroff commented. “The Early Detection Program can help women overcome barriers to health equity by educating them about the importance of routine screening, addressing their concerns about COVID-19 transmission, and helping them to safely access screening through interventions like patient navigation.”
Future studies will examine the effect of the pandemic on screening during the second half of 2020, when surges of COVID-19 and their timing varied geographically, they note.
A version of this article first appeared on Medscape.com.
Secnidazole gets FDA nod for trichomoniasis
The Food and Drug Administration has expanded the approval of secnidazole to include treatment of trichomoniasis in adults, according to a statement from manufacturer Lupin Pharmaceuticals.
Trichomoniasis vaginalis is a common, nonviral, curable sexually transmitted disease that affects approximately 3 million to 5 million adults in the United States each year; the infection can linger for months or years if left untreated, and may have a negative impact on reproductive health. The drug was approved for the treatment of bacterial vaginosis in 2017.
The availability of a single-dose oral treatment for both trichomoniasis and bacterial vaginosis may help improve adherence and reduce risk factors associated with these conditions, including pelvic inflammatory disease and other sexually transmitted infections, according to the statement.
The approval for the new indication was based primarily on data from a phase 3 clinical trial in which women with a confirmed trichomoniasis diagnosis were randomized to a single dose of 2 g oral secnidazole or a placebo. Secnidazole showed a 92.2% cure rate for patients with trichomoniasis, compared with placebo, based on cultures collected 6-12 days after dosing. Cure rates in subsets of patients with HIV and bacterial vaginosis were 100% and 95%, respectively.
The most common treatment-related adverse events were vulvovaginal candidiasis and nausea, each reported in 2.7% of study participants. The study findings were published in March 2021 in Clinical Infections Diseases.
Secnidazole also is approved for treatment of trichomoniasis in men, based on data from four open-label studies, one with men only and three including both men and women, according to the statement.
Full prescribing information for secnidazole is available here.
The Food and Drug Administration has expanded the approval of secnidazole to include treatment of trichomoniasis in adults, according to a statement from manufacturer Lupin Pharmaceuticals.
Trichomoniasis vaginalis is a common, nonviral, curable sexually transmitted disease that affects approximately 3 million to 5 million adults in the United States each year; the infection can linger for months or years if left untreated, and may have a negative impact on reproductive health. The drug was approved for the treatment of bacterial vaginosis in 2017.
The availability of a single-dose oral treatment for both trichomoniasis and bacterial vaginosis may help improve adherence and reduce risk factors associated with these conditions, including pelvic inflammatory disease and other sexually transmitted infections, according to the statement.
The approval for the new indication was based primarily on data from a phase 3 clinical trial in which women with a confirmed trichomoniasis diagnosis were randomized to a single dose of 2 g oral secnidazole or a placebo. Secnidazole showed a 92.2% cure rate for patients with trichomoniasis, compared with placebo, based on cultures collected 6-12 days after dosing. Cure rates in subsets of patients with HIV and bacterial vaginosis were 100% and 95%, respectively.
The most common treatment-related adverse events were vulvovaginal candidiasis and nausea, each reported in 2.7% of study participants. The study findings were published in March 2021 in Clinical Infections Diseases.
Secnidazole also is approved for treatment of trichomoniasis in men, based on data from four open-label studies, one with men only and three including both men and women, according to the statement.
Full prescribing information for secnidazole is available here.
The Food and Drug Administration has expanded the approval of secnidazole to include treatment of trichomoniasis in adults, according to a statement from manufacturer Lupin Pharmaceuticals.
Trichomoniasis vaginalis is a common, nonviral, curable sexually transmitted disease that affects approximately 3 million to 5 million adults in the United States each year; the infection can linger for months or years if left untreated, and may have a negative impact on reproductive health. The drug was approved for the treatment of bacterial vaginosis in 2017.
The availability of a single-dose oral treatment for both trichomoniasis and bacterial vaginosis may help improve adherence and reduce risk factors associated with these conditions, including pelvic inflammatory disease and other sexually transmitted infections, according to the statement.
The approval for the new indication was based primarily on data from a phase 3 clinical trial in which women with a confirmed trichomoniasis diagnosis were randomized to a single dose of 2 g oral secnidazole or a placebo. Secnidazole showed a 92.2% cure rate for patients with trichomoniasis, compared with placebo, based on cultures collected 6-12 days after dosing. Cure rates in subsets of patients with HIV and bacterial vaginosis were 100% and 95%, respectively.
The most common treatment-related adverse events were vulvovaginal candidiasis and nausea, each reported in 2.7% of study participants. The study findings were published in March 2021 in Clinical Infections Diseases.
Secnidazole also is approved for treatment of trichomoniasis in men, based on data from four open-label studies, one with men only and three including both men and women, according to the statement.
Full prescribing information for secnidazole is available here.
New analysis puts U.S. psoriasis prevalence at 3%
, according to an analysis of national survey data from 2011 to 2014.
“The adult prevalence rate of 3.0% continues to place psoriasis as one of the most common immune-mediated diseases affecting adults” in the United States, April W. Armstrong, MD, MPH, and associates said in a report published in JAMA Dermatology. At that rate, approximately 7,560,000 Americans aged 20 years or older have psoriasis.
That overall rate among adults aged 20 years and older, based on data from the 2011-2012 and 2013-2014 cycles of the National Health and Nutrition Examination Survey (NHANES), did not change significantly when compared with the 2003-2004 NHANES, when it was 3.15% among those aged 20-59, said Dr. Armstrong, professor of dermatology, University of Southern California, Los Angeles, and associates.
For the 2011-2014 period, psoriasis prevalence was similar between women (3.2%) and men (2.8%) but was significantly associated with older age and White/non-White status. Those aged 50-59 years had the highest prevalence of any age group at 4.3% and those aged 70 and older had a rate of 3.9%, while those aged 20-29 were the lowest at 1.6%, the investigators reported.
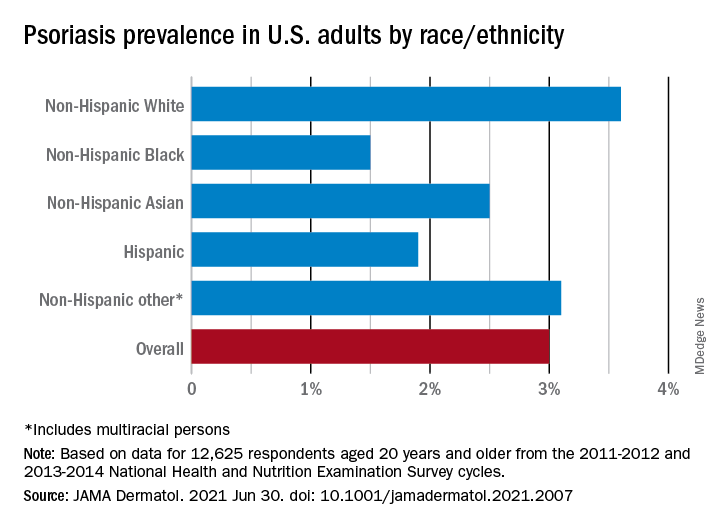
The prevalence in non-Hispanic Whites in the United States was 3.6% over the study period, and their odds ratio for having psoriasis was 1.92, compared with non-White individuals. Asian respondents had a prevalence of 2.5%, with the Hispanic population at 1.9%, non-Hispanic Black respondents at 1.5%, and those identifying as other (including multiracial persons) at 3.1%, they said.
The NHANES sample consisted of 12,638 people who had participated in the question that asked if they had ever been diagnosed with psoriasis by a physician or other health care professional, of whom 12,625 gave a definitive yes or no answer, the investigators noted.
A much smaller number, 329, also answered a question about the severity of their disease: Fifty-six percent had little or no psoriasis, almost 22% reported 1-2 palms of involvement, 16% had 3-10 palms of involvement, and 5.5% said the coverage was more than 10 palms. Since the survey did not distinguish between treated and untreated patients, however, some “of those reporting low body surface area involvement may be receiving treatments that are controlling their otherwise more extensive disease,” they wrote.
Dr. Armstrong and another investigator said that they have received grants, personal fees, and honoraria from a number of pharmaceutical companies; two other investigators are employees of the National Psoriasis Foundation.
, according to an analysis of national survey data from 2011 to 2014.

“The adult prevalence rate of 3.0% continues to place psoriasis as one of the most common immune-mediated diseases affecting adults” in the United States, April W. Armstrong, MD, MPH, and associates said in a report published in JAMA Dermatology. At that rate, approximately 7,560,000 Americans aged 20 years or older have psoriasis.
That overall rate among adults aged 20 years and older, based on data from the 2011-2012 and 2013-2014 cycles of the National Health and Nutrition Examination Survey (NHANES), did not change significantly when compared with the 2003-2004 NHANES, when it was 3.15% among those aged 20-59, said Dr. Armstrong, professor of dermatology, University of Southern California, Los Angeles, and associates.
For the 2011-2014 period, psoriasis prevalence was similar between women (3.2%) and men (2.8%) but was significantly associated with older age and White/non-White status. Those aged 50-59 years had the highest prevalence of any age group at 4.3% and those aged 70 and older had a rate of 3.9%, while those aged 20-29 were the lowest at 1.6%, the investigators reported.
The prevalence in non-Hispanic Whites in the United States was 3.6% over the study period, and their odds ratio for having psoriasis was 1.92, compared with non-White individuals. Asian respondents had a prevalence of 2.5%, with the Hispanic population at 1.9%, non-Hispanic Black respondents at 1.5%, and those identifying as other (including multiracial persons) at 3.1%, they said.
The NHANES sample consisted of 12,638 people who had participated in the question that asked if they had ever been diagnosed with psoriasis by a physician or other health care professional, of whom 12,625 gave a definitive yes or no answer, the investigators noted.
A much smaller number, 329, also answered a question about the severity of their disease: Fifty-six percent had little or no psoriasis, almost 22% reported 1-2 palms of involvement, 16% had 3-10 palms of involvement, and 5.5% said the coverage was more than 10 palms. Since the survey did not distinguish between treated and untreated patients, however, some “of those reporting low body surface area involvement may be receiving treatments that are controlling their otherwise more extensive disease,” they wrote.
Dr. Armstrong and another investigator said that they have received grants, personal fees, and honoraria from a number of pharmaceutical companies; two other investigators are employees of the National Psoriasis Foundation.
, according to an analysis of national survey data from 2011 to 2014.

“The adult prevalence rate of 3.0% continues to place psoriasis as one of the most common immune-mediated diseases affecting adults” in the United States, April W. Armstrong, MD, MPH, and associates said in a report published in JAMA Dermatology. At that rate, approximately 7,560,000 Americans aged 20 years or older have psoriasis.
That overall rate among adults aged 20 years and older, based on data from the 2011-2012 and 2013-2014 cycles of the National Health and Nutrition Examination Survey (NHANES), did not change significantly when compared with the 2003-2004 NHANES, when it was 3.15% among those aged 20-59, said Dr. Armstrong, professor of dermatology, University of Southern California, Los Angeles, and associates.
For the 2011-2014 period, psoriasis prevalence was similar between women (3.2%) and men (2.8%) but was significantly associated with older age and White/non-White status. Those aged 50-59 years had the highest prevalence of any age group at 4.3% and those aged 70 and older had a rate of 3.9%, while those aged 20-29 were the lowest at 1.6%, the investigators reported.
The prevalence in non-Hispanic Whites in the United States was 3.6% over the study period, and their odds ratio for having psoriasis was 1.92, compared with non-White individuals. Asian respondents had a prevalence of 2.5%, with the Hispanic population at 1.9%, non-Hispanic Black respondents at 1.5%, and those identifying as other (including multiracial persons) at 3.1%, they said.
The NHANES sample consisted of 12,638 people who had participated in the question that asked if they had ever been diagnosed with psoriasis by a physician or other health care professional, of whom 12,625 gave a definitive yes or no answer, the investigators noted.
A much smaller number, 329, also answered a question about the severity of their disease: Fifty-six percent had little or no psoriasis, almost 22% reported 1-2 palms of involvement, 16% had 3-10 palms of involvement, and 5.5% said the coverage was more than 10 palms. Since the survey did not distinguish between treated and untreated patients, however, some “of those reporting low body surface area involvement may be receiving treatments that are controlling their otherwise more extensive disease,” they wrote.
Dr. Armstrong and another investigator said that they have received grants, personal fees, and honoraria from a number of pharmaceutical companies; two other investigators are employees of the National Psoriasis Foundation.
FROM JAMA DERMATOLOGY
Clinician practices to connect with patients
Background: As technology and medical advances improve patient care, physicians and patients have become more dissatisfied with their interactions and relationships. Practices are needed to improve the connection between physician and patient.
Study design: Mixed-methods.
Setting: Three diverse primary care settings (academic medical center, Veterans Affairs facility, federally qualified health center).
Synopsis: Initial evidence- and narrative-based practices were identified from a systematic literature review, clinical observations of primary care encounters, and qualitative discussions with physicians, patients, and nonmedical professionals. A three-round modified Delphi process was performed with experts representing different aspects of the patient-physician relationship.
Five recommended clinical practices were recognized to foster presence and meaningful connections with patients: 1. Prepare with intention (becoming familiar with the patient before you meet them); 2. Listen intently and completely (sit down, lean forward, and don’t interrupt, but listen); 3. Agree on what matters most (discover your patient’s goals and fit them into the visit); 4. Connect with the patient’s story (take notice of efforts by the patient and successes); 5. Explore emotional cues (be aware of your patient’s emotions). Limitations of this study include the use of convenience sampling for the qualitative research, lack of international diversity of the expert panelists, and the lack of validation of the five practices as a whole.
Bottom line: The five practices of prepare with intention, listen intently and completely, agree on what matters most, connect with the patient’s story, and explore emotional cues may improve the patient-physician connection.
Citation: Zulman DM et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323(1):70-81.
Dr. Trammell-Velasquez is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: As technology and medical advances improve patient care, physicians and patients have become more dissatisfied with their interactions and relationships. Practices are needed to improve the connection between physician and patient.
Study design: Mixed-methods.
Setting: Three diverse primary care settings (academic medical center, Veterans Affairs facility, federally qualified health center).
Synopsis: Initial evidence- and narrative-based practices were identified from a systematic literature review, clinical observations of primary care encounters, and qualitative discussions with physicians, patients, and nonmedical professionals. A three-round modified Delphi process was performed with experts representing different aspects of the patient-physician relationship.
Five recommended clinical practices were recognized to foster presence and meaningful connections with patients: 1. Prepare with intention (becoming familiar with the patient before you meet them); 2. Listen intently and completely (sit down, lean forward, and don’t interrupt, but listen); 3. Agree on what matters most (discover your patient’s goals and fit them into the visit); 4. Connect with the patient’s story (take notice of efforts by the patient and successes); 5. Explore emotional cues (be aware of your patient’s emotions). Limitations of this study include the use of convenience sampling for the qualitative research, lack of international diversity of the expert panelists, and the lack of validation of the five practices as a whole.
Bottom line: The five practices of prepare with intention, listen intently and completely, agree on what matters most, connect with the patient’s story, and explore emotional cues may improve the patient-physician connection.
Citation: Zulman DM et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323(1):70-81.
Dr. Trammell-Velasquez is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: As technology and medical advances improve patient care, physicians and patients have become more dissatisfied with their interactions and relationships. Practices are needed to improve the connection between physician and patient.
Study design: Mixed-methods.
Setting: Three diverse primary care settings (academic medical center, Veterans Affairs facility, federally qualified health center).
Synopsis: Initial evidence- and narrative-based practices were identified from a systematic literature review, clinical observations of primary care encounters, and qualitative discussions with physicians, patients, and nonmedical professionals. A three-round modified Delphi process was performed with experts representing different aspects of the patient-physician relationship.
Five recommended clinical practices were recognized to foster presence and meaningful connections with patients: 1. Prepare with intention (becoming familiar with the patient before you meet them); 2. Listen intently and completely (sit down, lean forward, and don’t interrupt, but listen); 3. Agree on what matters most (discover your patient’s goals and fit them into the visit); 4. Connect with the patient’s story (take notice of efforts by the patient and successes); 5. Explore emotional cues (be aware of your patient’s emotions). Limitations of this study include the use of convenience sampling for the qualitative research, lack of international diversity of the expert panelists, and the lack of validation of the five practices as a whole.
Bottom line: The five practices of prepare with intention, listen intently and completely, agree on what matters most, connect with the patient’s story, and explore emotional cues may improve the patient-physician connection.
Citation: Zulman DM et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323(1):70-81.
Dr. Trammell-Velasquez is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Hearing loss tied to decline in physical functioning
published online in JAMA Network Open.
Hearing loss is associated with slower gait and, in particular, worse balance, the data suggest.
“Because hearing impairment is amenable to prevention and management, it potentially serves as a target for interventions to slow physical decline with aging,” the researchers said.
To examine how hearing impairment relates to physical function in older adults, Pablo Martinez-Amezcua, MD, PhD, MHS, a researcher in the department of epidemiology at Johns Hopkins University, Baltimore, and colleagues analyzed data from the ongoing Atherosclerosis Risk in Communities (ARIC) study.
ARIC initially enrolled more than 15,000 adults in Maryland, Minnesota, Mississippi, and North Carolina between 1987 and 1989. In the present study, the researchers focused on data from 2,956 participants who attended a study visit between 2016 and 2017, during which researchers assessed their hearing using pure tone audiometry.
Hearing-study participants had an average age of 79 years, about 58% were women, and 80% were White. Approximately 33% of the participants had normal hearing, 40% had mild hearing impairment, 23% had moderate hearing impairment, and 4% had severe hearing impairment.
Participants had also undergone assessment of physical functioning at study visits between 2011 and 2019, including a fast-paced 2-minute walk test to measure their walking endurance. Another assessment, the Short Physical Performance Battery (SPPB), tests balance, gait speed, and chair stands (seated participants stand up and sit back down five times as quickly as possible while their arms are crossed).
Dr. Martinez-Amezcua and colleagues found that severe hearing impairment was associated with a lower average SPPB score compared with normal hearing in a regression analysis. Specifically, compared with those with normal hearing, participants with severe hearing impairment were more likely to have low scores on the SPPB (odds ratio, 2.72), balance (OR, 2.72), and gait speed (OR, 2.16).
However, hearing impairment was not significantly associated with the chair stand test results. The researchers note that chair stands may rely more on strength, whereas balance and gait speed may rely more on coordination and movement.
The team also found that people with worse hearing tended to walk a shorter distance during the 2-minute walk test. Compared with participants with normal hearing, participants with moderate hearing impairment walked 2.81 meters less and those with severe hearing impairment walked 5.31 meters less on average, after adjustment for variables including age, sex, and health conditions.
Participants with hearing impairment also tended to have faster declines in physical function over time.
Various mechanisms could explain associations between hearing and physical function, the authors said. For example, an underlying condition such as cardiovascular disease might affect both hearing and physical function. Damage to the inner ear could affect vestibular and auditory systems at the same time. In addition, hearing impairment may relate to cognition, depression, or social isolation, which could influence physical activity.
“Age-related hearing loss is traditionally seen as a barrier for communication,” Dr. Martinez-Amezcua told this news organization. “In the past decade, research on the consequences of hearing loss has identified it as a risk factor for cognitive decline and dementia. Our findings contribute to our understanding of other negative outcomes associated with hearing loss.”
Randomized clinical trials are the best way to assess whether addressing hearing loss might improve physical function, Dr. Martinez-Amezcua said. “Currently there is one clinical trial (ACHIEVE) that will, among other outcomes, study the impact of hearing aids on cognitive and physical function,” he said.
Although interventions may not reverse hearing loss, hearing rehabilitation strategies, including hearing aids and cochlear implants, may help, he added. Educating caregivers and changing a person’s environment can also reduce the effects hearing loss has on daily life, Dr. Martinez-Amezcua said.
“We rely so much in our sense of vision for activities of daily living that we tend to underestimate how important hearing is, and the consequences of hearing loss go beyond having trouble communicating with someone,” he said.
This study and prior research “raise the intriguing idea that hearing may provide essential information to the neural circuits underpinning movement in our environment and that correction for hearing loss may help promote physical well-being,” Willa D. Brenowitz, PhD, MPH, and Margaret I. Wallhagen, PhD, GNP-BC, both at the University of California, San Francisco, wrote in an accompanying commentary. “While this hypothesis is appealing and warrants further investigation, there are multiple other potential explanations of such an association, including potential sources of bias that may affect observational studies such as this one.”
Beyond treating hearing loss, interventions such as physical therapy or tai chi may benefit patients, they suggested.
Because many changes occur during older age, it can be difficult to understand which factor is influencing another, Dr. Brenowitz said in an interview. There are potentially relevant mechanisms through which hearing could affect cognition and physical functioning. Still another explanation could be that some people are “aging in a faster way” than others, Dr. Brenowitz said.
Dr. Martinez-Amezcua and a coauthor disclosed receiving sponsorship from the Cochlear Center for Hearing and Public Health. Another author, Frank R. Lin, MD, PhD, directs the research center, which is partly funded by a philanthropic gift from Cochlear to the Johns Hopkins Bloomberg School of Public Health. Dr. Lin also disclosed personal fees from Frequency Therapeutics and Caption Call. One author serves on a scientific advisory board for Shoebox and Good Machine Studio.
Dr. Wallhagen has served on the board of trustees of the Hearing Loss Association of America and is a member of the board of the Hearing Loss Association of America–California. Dr. Wallhagen also received funding for a pilot project on the impact of hearing loss on communication in the context of chronic serious illness from the National Palliative Care Research Center outside the submitted work.
A version of this article first appeared on Medscape.com.
published online in JAMA Network Open.
Hearing loss is associated with slower gait and, in particular, worse balance, the data suggest.
“Because hearing impairment is amenable to prevention and management, it potentially serves as a target for interventions to slow physical decline with aging,” the researchers said.
To examine how hearing impairment relates to physical function in older adults, Pablo Martinez-Amezcua, MD, PhD, MHS, a researcher in the department of epidemiology at Johns Hopkins University, Baltimore, and colleagues analyzed data from the ongoing Atherosclerosis Risk in Communities (ARIC) study.
ARIC initially enrolled more than 15,000 adults in Maryland, Minnesota, Mississippi, and North Carolina between 1987 and 1989. In the present study, the researchers focused on data from 2,956 participants who attended a study visit between 2016 and 2017, during which researchers assessed their hearing using pure tone audiometry.
Hearing-study participants had an average age of 79 years, about 58% were women, and 80% were White. Approximately 33% of the participants had normal hearing, 40% had mild hearing impairment, 23% had moderate hearing impairment, and 4% had severe hearing impairment.
Participants had also undergone assessment of physical functioning at study visits between 2011 and 2019, including a fast-paced 2-minute walk test to measure their walking endurance. Another assessment, the Short Physical Performance Battery (SPPB), tests balance, gait speed, and chair stands (seated participants stand up and sit back down five times as quickly as possible while their arms are crossed).
Dr. Martinez-Amezcua and colleagues found that severe hearing impairment was associated with a lower average SPPB score compared with normal hearing in a regression analysis. Specifically, compared with those with normal hearing, participants with severe hearing impairment were more likely to have low scores on the SPPB (odds ratio, 2.72), balance (OR, 2.72), and gait speed (OR, 2.16).
However, hearing impairment was not significantly associated with the chair stand test results. The researchers note that chair stands may rely more on strength, whereas balance and gait speed may rely more on coordination and movement.
The team also found that people with worse hearing tended to walk a shorter distance during the 2-minute walk test. Compared with participants with normal hearing, participants with moderate hearing impairment walked 2.81 meters less and those with severe hearing impairment walked 5.31 meters less on average, after adjustment for variables including age, sex, and health conditions.
Participants with hearing impairment also tended to have faster declines in physical function over time.
Various mechanisms could explain associations between hearing and physical function, the authors said. For example, an underlying condition such as cardiovascular disease might affect both hearing and physical function. Damage to the inner ear could affect vestibular and auditory systems at the same time. In addition, hearing impairment may relate to cognition, depression, or social isolation, which could influence physical activity.
“Age-related hearing loss is traditionally seen as a barrier for communication,” Dr. Martinez-Amezcua told this news organization. “In the past decade, research on the consequences of hearing loss has identified it as a risk factor for cognitive decline and dementia. Our findings contribute to our understanding of other negative outcomes associated with hearing loss.”
Randomized clinical trials are the best way to assess whether addressing hearing loss might improve physical function, Dr. Martinez-Amezcua said. “Currently there is one clinical trial (ACHIEVE) that will, among other outcomes, study the impact of hearing aids on cognitive and physical function,” he said.
Although interventions may not reverse hearing loss, hearing rehabilitation strategies, including hearing aids and cochlear implants, may help, he added. Educating caregivers and changing a person’s environment can also reduce the effects hearing loss has on daily life, Dr. Martinez-Amezcua said.
“We rely so much in our sense of vision for activities of daily living that we tend to underestimate how important hearing is, and the consequences of hearing loss go beyond having trouble communicating with someone,” he said.
This study and prior research “raise the intriguing idea that hearing may provide essential information to the neural circuits underpinning movement in our environment and that correction for hearing loss may help promote physical well-being,” Willa D. Brenowitz, PhD, MPH, and Margaret I. Wallhagen, PhD, GNP-BC, both at the University of California, San Francisco, wrote in an accompanying commentary. “While this hypothesis is appealing and warrants further investigation, there are multiple other potential explanations of such an association, including potential sources of bias that may affect observational studies such as this one.”
Beyond treating hearing loss, interventions such as physical therapy or tai chi may benefit patients, they suggested.
Because many changes occur during older age, it can be difficult to understand which factor is influencing another, Dr. Brenowitz said in an interview. There are potentially relevant mechanisms through which hearing could affect cognition and physical functioning. Still another explanation could be that some people are “aging in a faster way” than others, Dr. Brenowitz said.
Dr. Martinez-Amezcua and a coauthor disclosed receiving sponsorship from the Cochlear Center for Hearing and Public Health. Another author, Frank R. Lin, MD, PhD, directs the research center, which is partly funded by a philanthropic gift from Cochlear to the Johns Hopkins Bloomberg School of Public Health. Dr. Lin also disclosed personal fees from Frequency Therapeutics and Caption Call. One author serves on a scientific advisory board for Shoebox and Good Machine Studio.
Dr. Wallhagen has served on the board of trustees of the Hearing Loss Association of America and is a member of the board of the Hearing Loss Association of America–California. Dr. Wallhagen also received funding for a pilot project on the impact of hearing loss on communication in the context of chronic serious illness from the National Palliative Care Research Center outside the submitted work.
A version of this article first appeared on Medscape.com.
published online in JAMA Network Open.
Hearing loss is associated with slower gait and, in particular, worse balance, the data suggest.
“Because hearing impairment is amenable to prevention and management, it potentially serves as a target for interventions to slow physical decline with aging,” the researchers said.
To examine how hearing impairment relates to physical function in older adults, Pablo Martinez-Amezcua, MD, PhD, MHS, a researcher in the department of epidemiology at Johns Hopkins University, Baltimore, and colleagues analyzed data from the ongoing Atherosclerosis Risk in Communities (ARIC) study.
ARIC initially enrolled more than 15,000 adults in Maryland, Minnesota, Mississippi, and North Carolina between 1987 and 1989. In the present study, the researchers focused on data from 2,956 participants who attended a study visit between 2016 and 2017, during which researchers assessed their hearing using pure tone audiometry.
Hearing-study participants had an average age of 79 years, about 58% were women, and 80% were White. Approximately 33% of the participants had normal hearing, 40% had mild hearing impairment, 23% had moderate hearing impairment, and 4% had severe hearing impairment.
Participants had also undergone assessment of physical functioning at study visits between 2011 and 2019, including a fast-paced 2-minute walk test to measure their walking endurance. Another assessment, the Short Physical Performance Battery (SPPB), tests balance, gait speed, and chair stands (seated participants stand up and sit back down five times as quickly as possible while their arms are crossed).
Dr. Martinez-Amezcua and colleagues found that severe hearing impairment was associated with a lower average SPPB score compared with normal hearing in a regression analysis. Specifically, compared with those with normal hearing, participants with severe hearing impairment were more likely to have low scores on the SPPB (odds ratio, 2.72), balance (OR, 2.72), and gait speed (OR, 2.16).
However, hearing impairment was not significantly associated with the chair stand test results. The researchers note that chair stands may rely more on strength, whereas balance and gait speed may rely more on coordination and movement.
The team also found that people with worse hearing tended to walk a shorter distance during the 2-minute walk test. Compared with participants with normal hearing, participants with moderate hearing impairment walked 2.81 meters less and those with severe hearing impairment walked 5.31 meters less on average, after adjustment for variables including age, sex, and health conditions.
Participants with hearing impairment also tended to have faster declines in physical function over time.
Various mechanisms could explain associations between hearing and physical function, the authors said. For example, an underlying condition such as cardiovascular disease might affect both hearing and physical function. Damage to the inner ear could affect vestibular and auditory systems at the same time. In addition, hearing impairment may relate to cognition, depression, or social isolation, which could influence physical activity.
“Age-related hearing loss is traditionally seen as a barrier for communication,” Dr. Martinez-Amezcua told this news organization. “In the past decade, research on the consequences of hearing loss has identified it as a risk factor for cognitive decline and dementia. Our findings contribute to our understanding of other negative outcomes associated with hearing loss.”
Randomized clinical trials are the best way to assess whether addressing hearing loss might improve physical function, Dr. Martinez-Amezcua said. “Currently there is one clinical trial (ACHIEVE) that will, among other outcomes, study the impact of hearing aids on cognitive and physical function,” he said.
Although interventions may not reverse hearing loss, hearing rehabilitation strategies, including hearing aids and cochlear implants, may help, he added. Educating caregivers and changing a person’s environment can also reduce the effects hearing loss has on daily life, Dr. Martinez-Amezcua said.
“We rely so much in our sense of vision for activities of daily living that we tend to underestimate how important hearing is, and the consequences of hearing loss go beyond having trouble communicating with someone,” he said.
This study and prior research “raise the intriguing idea that hearing may provide essential information to the neural circuits underpinning movement in our environment and that correction for hearing loss may help promote physical well-being,” Willa D. Brenowitz, PhD, MPH, and Margaret I. Wallhagen, PhD, GNP-BC, both at the University of California, San Francisco, wrote in an accompanying commentary. “While this hypothesis is appealing and warrants further investigation, there are multiple other potential explanations of such an association, including potential sources of bias that may affect observational studies such as this one.”
Beyond treating hearing loss, interventions such as physical therapy or tai chi may benefit patients, they suggested.
Because many changes occur during older age, it can be difficult to understand which factor is influencing another, Dr. Brenowitz said in an interview. There are potentially relevant mechanisms through which hearing could affect cognition and physical functioning. Still another explanation could be that some people are “aging in a faster way” than others, Dr. Brenowitz said.
Dr. Martinez-Amezcua and a coauthor disclosed receiving sponsorship from the Cochlear Center for Hearing and Public Health. Another author, Frank R. Lin, MD, PhD, directs the research center, which is partly funded by a philanthropic gift from Cochlear to the Johns Hopkins Bloomberg School of Public Health. Dr. Lin also disclosed personal fees from Frequency Therapeutics and Caption Call. One author serves on a scientific advisory board for Shoebox and Good Machine Studio.
Dr. Wallhagen has served on the board of trustees of the Hearing Loss Association of America and is a member of the board of the Hearing Loss Association of America–California. Dr. Wallhagen also received funding for a pilot project on the impact of hearing loss on communication in the context of chronic serious illness from the National Palliative Care Research Center outside the submitted work.
A version of this article first appeared on Medscape.com.
New details of myocarditis linked to COVID vaccines
Further details from multiple cases of myocarditis linked to the Pfizer and Moderna mRNA COVID vaccines have been described in recent papers in the medical literature.
The cases appear to occur almost exclusively in males and most often in younger age groups. While symptoms and signs of myocarditis mostly resolved with a few days of supportive care, long-term effects are unknown at present.
The authors of all the reports and of two accompanying editorials in JAMA Cardiology are unanimous in their opinion that the benefits of vaccination still outweigh the risks.
The Centers for Disease Control and Prevention’s but committee members delivered a strong endorsement for continuing to vaccinate young people with the mRNA vaccines.
The current case reports are published in two papers in JAMA Cardiology and in three in Circulation.
U.S. military reports 23 cases
In one report in JAMA Cardiology, authors led by Jay Montgomery, MD, from Walter Reed National Military Medical Center in Bethesda, Md., described 23 cases from the U.S. Military Health System of individuals with acute myocarditis who presented within 4 days after mRNA-based COVID-19 vaccination (7 Pfizer and 16 Moderna).
All patients were male, 22 of 23 were on active duty, and the median age was 25 years (range, 20-51); 20 of the 23 cases occurred after receipt of a second dose of an mRNA COVID-19 vaccine.
The patients all presented with acute onset of marked chest pain. All patients had significantly elevated cardiac troponin levels. Among eight patients who underwent cardiac MRI (cMRI), all had findings consistent with the clinical diagnosis of myocarditis.
Additional testing did not identify other possible causes of myocarditis. All patients received brief supportive care and were recovered or recovering.
The authors reported that the military administered more than 2.8 million doses of mRNA COVID-19 vaccine in this period, and while the observed number of myocarditis cases was small, the number was “substantially higher” than expected among male military members after a second vaccine dose.
They noted that, based on historical data, among the 544,000 second doses to military members there may have been 0-10 expected myocarditis cases, but they observed 19 cases.
“All patients in this series reflect substantial similarities in demographic characteristics, proximate vaccine dose, onset interval, and character of vaccine-associated myocarditis. The consistent pattern of clinical presentation, rapid recovery, and absence of evidence of other causes support the diagnosis of hypersensitivity myocarditis,” they stated.
They added that presentation after a second vaccine dose or, in three patients, when vaccination followed SARS-CoV-2 infection, suggests that prior exposure was relevant in the hypersensitivity response.
“The spectrum of clinical presentation and reliance on patients seeking health care and on health care professionals recognizing a rare vaccine-associated adverse event limits determination of the true incidence of this condition,” the authors wrote.
They stressed that recognition of vaccine-associated myocarditis is clinically important because diagnosis impacts management, recommendations for exercise, and monitoring for cardiomyopathy.
But the authors also acknowledged that it is important to frame concerns about potential vaccine-associated myocarditis within the context of the current pandemic.
“Infection with SARS-CoV-2 is a clear cause of serious cardiac injury in many patients. ... Prevalence of cardiac injury may be as high as 60% in seriously ill patients. Notably, nearly 1% of highly fit athletes with mild COVID-19 infection have evidence of myocarditis on cMRI,” they wrote.
“Given that COVID-19 vaccines are remarkably effective at preventing infection, any risk of rare adverse events following immunization must be carefully weighed against the very substantial benefit of vaccination,” they concluded.
Four cases at Duke
In the second paper in JAMA Cardiology, a group led by Han W. Kim, MD, reported four patients with acute myocarditis occurring within days of mRNA COVID-19 vaccination (two Pfizer and two Moderna) in patients treated at Duke University Medical Center, Durham, N.C. The hospital courses of the four patients with myocarditis following COVID-19 vaccination were uneventful, and they were discharged within 2-4 days.
The authors said that, although a causal relationship cannot be established, none of the patients had a viral prodrome or had coincident testing that revealed an alternative explanation.
They stated that these four patients represent the majority of patients with acute myocarditis identified in the past 3 months at their institution, and this led to the highest total number of patients with acute myocarditis, compared with the same 3-month period for the past 5 years.
“Additionally, we identified only those patients with severe unremitting chest pain who sought medical attention. Those with mild or moderate chest pain might not seek medical attention, and it is possible that subclinical myocarditis may occur and could be detected by active surveillance, as has been described with smallpox vaccination,” they wrote.
Further case reports
In one of the papers in Circulation, a group led by Kathryn F. Larson, MD, from the Mayo Clinic in Rochester, Minn., described eight patients hospitalized with chest pain who were diagnosed with myocarditis within 2-4 days of receiving either the Pfizer or Moderna vaccine.
Two of the patients had previously been infected by SARS-CoV-2 without need for hospitalization. All individuals were otherwise healthy males between the ages of 21 and 56 years. All but one patient developed symptoms after their second dose, and the one patient who developed myocarditis after the first vaccine dose had previously been infected with SARS-CoV-2.
Systemic symptoms began within 24 hours after vaccine administration in five of eight patients, with chest pain presenting between 48 and 96 hours later. Troponin values were elevated in all individuals and appeared to peak the day after admission, whereas none had eosinophilia.
Cardiac MRI revealed findings consistent with myocarditis in all patients. All patients had resolution of their chest pain and were discharged from the hospital in stable condition.
“The patients presented here demonstrated typical signs, symptoms, and diagnostic features of acute myocarditis. The temporal association between receiving an mRNA-based COVID-19 vaccine and the development of myocarditis is notable,” the authors said.
They added that they would consider the use of corticosteroids in these patients but cautioned that this could reduce the specific immune response against SARS-COV-2 triggered by the vaccine. “Thus, the duration of corticosteroid administration should be limited to the resolution of the symptoms or ventricular arrhythmias or the recovery of the left ventricular ejection fraction.”
Pending publication of long-term outcome data after SARS-CoV-2 vaccine–related myocarditis, they suggest adherence to the current consensus recommendation to abstain from competitive sports for a period of 3-6 months with reevaluation prior to sports participation.
In another of the Circulation papers, a group led by Carolyn M. Rosner, MSN, presented a case series of seven patients hospitalized for acute myocarditis-like illness following COVID-19 vaccination, from two U.S. medical centers, in Falls Church, Va., and Dallas. All patients were males below the age of 40 years and of White or Hispanic race/ethnicity. Only one patient reported prior history of COVID-19 infection. Six patients received mRNA (Moderna or Pfizer) and one received the adenovirus (Johnson & Johnson) vaccine. All patients presented 3-7 days post vaccination with acute onset chest pain and biochemical evidence of myocardial injury.
Hospital length of stay was 3 days, and all patients’ symptoms resolved by hospital discharge.
And finally, the third paper in Circulation reported a detailed description of one patient – a 52-year-old, previously healthy male who presented with acute myocarditis 3 days after the administration of the second dose of Moderna’s COVID-19 vaccine. The symptoms resolved, and there was a gradual improvement in cMRI findings. Ischemic injury and other potential causes of acute myocardial injury were excluded, as were other potential infectious causes of myocarditis, and there was no evidence of systemic autoimmune disease.
“Clinicians should be aware that myocarditis may be present in patients exhibiting cardiac signs and symptoms 2-4 days after COVID-19 vaccination,” the authors said.
They added that additional surveillance of such adverse events post–COVID-19 vaccination will help identify subgroups at higher risk for this vaccine-related effect, and whether additional precautions are necessary.
‘Benefits outweigh risk’
In an accompanying editorial in JAMA Cardiology, three doctors from the CDC cite several other reports of myocarditis after mRNA COVID vaccination. These include a case report published in Pediatrics of seven male adolescents aged 14-19 years who presented with myocarditis or myopericarditis within 4 days after receipt of a second dose of the Pfizer vaccine.
But the editorialists noted that the most comprehensive data about the risk for myocarditis following immunization with mRNA vaccines comes from Israel.
The Israeli Ministry of Health recently posted data describing 121 myocarditis cases occurring within 30 days of a second dose of mRNA vaccine among 5,049,424 persons, suggesting a crude incidence rate of approximately 24 cases per million.
On the current case reports, the CDC doctors wrote: “The striking clinical similarities in the presentations of these patients, their recent vaccination with an mRNA-based COVID-19 vaccine, and the lack of any alternative etiologies for acute myocarditis suggest an association with immunization.”
They said that acute onset of chest pain 3-5 days after vaccine administration, usually after a second dose, is a typical feature of reported cases and suggests an immune-mediated mechanism.
But SARS-CoV-2 infection also causes cardiac injury which may result in severe outcomes, and based on currently available data, myocarditis following immunization with current mRNA-based vaccines is rare.
“At present, the benefits of immunization in preventing severe morbidity favors continued COVID-19 vaccination, particularly considering the increasing COVID-19 hospitalization rates among adolescents reported during spring 2021,” the editorialists stated.
But they added that many questions remain. These include whether modifications are needed to the vaccine schedule among persons with a history of possible or confirmed myocarditis after COVID vaccine, how should postvaccine myocarditis be managed, how often should follow-up assessments be performed, how might follow-up assessments affect recommendations to avoid vigorous physical activity following the diagnosis of myocarditis, and do all likely cases of acute myocarditis that appear to be uncomplicated require cardiac MRI for more definitive diagnosis?
“While the data needed to answer such questions are being collected, there is an opportunity for researchers with expertise in myocarditis to develop a comprehensive, national assessment of the natural history, pathogenesis, and treatment of acute myocarditis associated with receipt of mRNA-based COVID-19 vaccines,” they concluded.
In a second editorial in JAMA Cardiology, a group of editors from the journal acknowledged that publication of the current case reports may contribute to additional public concern regarding immunization. But they added that clinicians discussing immunization with patients should recognize that these case series suggest that the symptomatic events consistent with myocarditis are still very rare and appear to be self-limiting.
“Given the risks of COVID-19, including the risk of myocarditis from COVID-19 infection, the editors do not believe these case reports are sufficient to interrupt the march toward maximal vaccination against SARS-CoV-2 as expeditiously as possible,” they said.
A version of this article first appeared on Medscape.com.
Further details from multiple cases of myocarditis linked to the Pfizer and Moderna mRNA COVID vaccines have been described in recent papers in the medical literature.
The cases appear to occur almost exclusively in males and most often in younger age groups. While symptoms and signs of myocarditis mostly resolved with a few days of supportive care, long-term effects are unknown at present.
The authors of all the reports and of two accompanying editorials in JAMA Cardiology are unanimous in their opinion that the benefits of vaccination still outweigh the risks.
The Centers for Disease Control and Prevention’s but committee members delivered a strong endorsement for continuing to vaccinate young people with the mRNA vaccines.
The current case reports are published in two papers in JAMA Cardiology and in three in Circulation.
U.S. military reports 23 cases
In one report in JAMA Cardiology, authors led by Jay Montgomery, MD, from Walter Reed National Military Medical Center in Bethesda, Md., described 23 cases from the U.S. Military Health System of individuals with acute myocarditis who presented within 4 days after mRNA-based COVID-19 vaccination (7 Pfizer and 16 Moderna).
All patients were male, 22 of 23 were on active duty, and the median age was 25 years (range, 20-51); 20 of the 23 cases occurred after receipt of a second dose of an mRNA COVID-19 vaccine.
The patients all presented with acute onset of marked chest pain. All patients had significantly elevated cardiac troponin levels. Among eight patients who underwent cardiac MRI (cMRI), all had findings consistent with the clinical diagnosis of myocarditis.
Additional testing did not identify other possible causes of myocarditis. All patients received brief supportive care and were recovered or recovering.
The authors reported that the military administered more than 2.8 million doses of mRNA COVID-19 vaccine in this period, and while the observed number of myocarditis cases was small, the number was “substantially higher” than expected among male military members after a second vaccine dose.
They noted that, based on historical data, among the 544,000 second doses to military members there may have been 0-10 expected myocarditis cases, but they observed 19 cases.
“All patients in this series reflect substantial similarities in demographic characteristics, proximate vaccine dose, onset interval, and character of vaccine-associated myocarditis. The consistent pattern of clinical presentation, rapid recovery, and absence of evidence of other causes support the diagnosis of hypersensitivity myocarditis,” they stated.
They added that presentation after a second vaccine dose or, in three patients, when vaccination followed SARS-CoV-2 infection, suggests that prior exposure was relevant in the hypersensitivity response.
“The spectrum of clinical presentation and reliance on patients seeking health care and on health care professionals recognizing a rare vaccine-associated adverse event limits determination of the true incidence of this condition,” the authors wrote.
They stressed that recognition of vaccine-associated myocarditis is clinically important because diagnosis impacts management, recommendations for exercise, and monitoring for cardiomyopathy.
But the authors also acknowledged that it is important to frame concerns about potential vaccine-associated myocarditis within the context of the current pandemic.
“Infection with SARS-CoV-2 is a clear cause of serious cardiac injury in many patients. ... Prevalence of cardiac injury may be as high as 60% in seriously ill patients. Notably, nearly 1% of highly fit athletes with mild COVID-19 infection have evidence of myocarditis on cMRI,” they wrote.
“Given that COVID-19 vaccines are remarkably effective at preventing infection, any risk of rare adverse events following immunization must be carefully weighed against the very substantial benefit of vaccination,” they concluded.
Four cases at Duke
In the second paper in JAMA Cardiology, a group led by Han W. Kim, MD, reported four patients with acute myocarditis occurring within days of mRNA COVID-19 vaccination (two Pfizer and two Moderna) in patients treated at Duke University Medical Center, Durham, N.C. The hospital courses of the four patients with myocarditis following COVID-19 vaccination were uneventful, and they were discharged within 2-4 days.
The authors said that, although a causal relationship cannot be established, none of the patients had a viral prodrome or had coincident testing that revealed an alternative explanation.
They stated that these four patients represent the majority of patients with acute myocarditis identified in the past 3 months at their institution, and this led to the highest total number of patients with acute myocarditis, compared with the same 3-month period for the past 5 years.
“Additionally, we identified only those patients with severe unremitting chest pain who sought medical attention. Those with mild or moderate chest pain might not seek medical attention, and it is possible that subclinical myocarditis may occur and could be detected by active surveillance, as has been described with smallpox vaccination,” they wrote.
Further case reports
In one of the papers in Circulation, a group led by Kathryn F. Larson, MD, from the Mayo Clinic in Rochester, Minn., described eight patients hospitalized with chest pain who were diagnosed with myocarditis within 2-4 days of receiving either the Pfizer or Moderna vaccine.
Two of the patients had previously been infected by SARS-CoV-2 without need for hospitalization. All individuals were otherwise healthy males between the ages of 21 and 56 years. All but one patient developed symptoms after their second dose, and the one patient who developed myocarditis after the first vaccine dose had previously been infected with SARS-CoV-2.
Systemic symptoms began within 24 hours after vaccine administration in five of eight patients, with chest pain presenting between 48 and 96 hours later. Troponin values were elevated in all individuals and appeared to peak the day after admission, whereas none had eosinophilia.
Cardiac MRI revealed findings consistent with myocarditis in all patients. All patients had resolution of their chest pain and were discharged from the hospital in stable condition.
“The patients presented here demonstrated typical signs, symptoms, and diagnostic features of acute myocarditis. The temporal association between receiving an mRNA-based COVID-19 vaccine and the development of myocarditis is notable,” the authors said.
They added that they would consider the use of corticosteroids in these patients but cautioned that this could reduce the specific immune response against SARS-COV-2 triggered by the vaccine. “Thus, the duration of corticosteroid administration should be limited to the resolution of the symptoms or ventricular arrhythmias or the recovery of the left ventricular ejection fraction.”
Pending publication of long-term outcome data after SARS-CoV-2 vaccine–related myocarditis, they suggest adherence to the current consensus recommendation to abstain from competitive sports for a period of 3-6 months with reevaluation prior to sports participation.
In another of the Circulation papers, a group led by Carolyn M. Rosner, MSN, presented a case series of seven patients hospitalized for acute myocarditis-like illness following COVID-19 vaccination, from two U.S. medical centers, in Falls Church, Va., and Dallas. All patients were males below the age of 40 years and of White or Hispanic race/ethnicity. Only one patient reported prior history of COVID-19 infection. Six patients received mRNA (Moderna or Pfizer) and one received the adenovirus (Johnson & Johnson) vaccine. All patients presented 3-7 days post vaccination with acute onset chest pain and biochemical evidence of myocardial injury.
Hospital length of stay was 3 days, and all patients’ symptoms resolved by hospital discharge.
And finally, the third paper in Circulation reported a detailed description of one patient – a 52-year-old, previously healthy male who presented with acute myocarditis 3 days after the administration of the second dose of Moderna’s COVID-19 vaccine. The symptoms resolved, and there was a gradual improvement in cMRI findings. Ischemic injury and other potential causes of acute myocardial injury were excluded, as were other potential infectious causes of myocarditis, and there was no evidence of systemic autoimmune disease.
“Clinicians should be aware that myocarditis may be present in patients exhibiting cardiac signs and symptoms 2-4 days after COVID-19 vaccination,” the authors said.
They added that additional surveillance of such adverse events post–COVID-19 vaccination will help identify subgroups at higher risk for this vaccine-related effect, and whether additional precautions are necessary.
‘Benefits outweigh risk’
In an accompanying editorial in JAMA Cardiology, three doctors from the CDC cite several other reports of myocarditis after mRNA COVID vaccination. These include a case report published in Pediatrics of seven male adolescents aged 14-19 years who presented with myocarditis or myopericarditis within 4 days after receipt of a second dose of the Pfizer vaccine.
But the editorialists noted that the most comprehensive data about the risk for myocarditis following immunization with mRNA vaccines comes from Israel.
The Israeli Ministry of Health recently posted data describing 121 myocarditis cases occurring within 30 days of a second dose of mRNA vaccine among 5,049,424 persons, suggesting a crude incidence rate of approximately 24 cases per million.
On the current case reports, the CDC doctors wrote: “The striking clinical similarities in the presentations of these patients, their recent vaccination with an mRNA-based COVID-19 vaccine, and the lack of any alternative etiologies for acute myocarditis suggest an association with immunization.”
They said that acute onset of chest pain 3-5 days after vaccine administration, usually after a second dose, is a typical feature of reported cases and suggests an immune-mediated mechanism.
But SARS-CoV-2 infection also causes cardiac injury which may result in severe outcomes, and based on currently available data, myocarditis following immunization with current mRNA-based vaccines is rare.
“At present, the benefits of immunization in preventing severe morbidity favors continued COVID-19 vaccination, particularly considering the increasing COVID-19 hospitalization rates among adolescents reported during spring 2021,” the editorialists stated.
But they added that many questions remain. These include whether modifications are needed to the vaccine schedule among persons with a history of possible or confirmed myocarditis after COVID vaccine, how should postvaccine myocarditis be managed, how often should follow-up assessments be performed, how might follow-up assessments affect recommendations to avoid vigorous physical activity following the diagnosis of myocarditis, and do all likely cases of acute myocarditis that appear to be uncomplicated require cardiac MRI for more definitive diagnosis?
“While the data needed to answer such questions are being collected, there is an opportunity for researchers with expertise in myocarditis to develop a comprehensive, national assessment of the natural history, pathogenesis, and treatment of acute myocarditis associated with receipt of mRNA-based COVID-19 vaccines,” they concluded.
In a second editorial in JAMA Cardiology, a group of editors from the journal acknowledged that publication of the current case reports may contribute to additional public concern regarding immunization. But they added that clinicians discussing immunization with patients should recognize that these case series suggest that the symptomatic events consistent with myocarditis are still very rare and appear to be self-limiting.
“Given the risks of COVID-19, including the risk of myocarditis from COVID-19 infection, the editors do not believe these case reports are sufficient to interrupt the march toward maximal vaccination against SARS-CoV-2 as expeditiously as possible,” they said.
A version of this article first appeared on Medscape.com.
Further details from multiple cases of myocarditis linked to the Pfizer and Moderna mRNA COVID vaccines have been described in recent papers in the medical literature.
The cases appear to occur almost exclusively in males and most often in younger age groups. While symptoms and signs of myocarditis mostly resolved with a few days of supportive care, long-term effects are unknown at present.
The authors of all the reports and of two accompanying editorials in JAMA Cardiology are unanimous in their opinion that the benefits of vaccination still outweigh the risks.
The Centers for Disease Control and Prevention’s but committee members delivered a strong endorsement for continuing to vaccinate young people with the mRNA vaccines.
The current case reports are published in two papers in JAMA Cardiology and in three in Circulation.
U.S. military reports 23 cases
In one report in JAMA Cardiology, authors led by Jay Montgomery, MD, from Walter Reed National Military Medical Center in Bethesda, Md., described 23 cases from the U.S. Military Health System of individuals with acute myocarditis who presented within 4 days after mRNA-based COVID-19 vaccination (7 Pfizer and 16 Moderna).
All patients were male, 22 of 23 were on active duty, and the median age was 25 years (range, 20-51); 20 of the 23 cases occurred after receipt of a second dose of an mRNA COVID-19 vaccine.
The patients all presented with acute onset of marked chest pain. All patients had significantly elevated cardiac troponin levels. Among eight patients who underwent cardiac MRI (cMRI), all had findings consistent with the clinical diagnosis of myocarditis.
Additional testing did not identify other possible causes of myocarditis. All patients received brief supportive care and were recovered or recovering.
The authors reported that the military administered more than 2.8 million doses of mRNA COVID-19 vaccine in this period, and while the observed number of myocarditis cases was small, the number was “substantially higher” than expected among male military members after a second vaccine dose.
They noted that, based on historical data, among the 544,000 second doses to military members there may have been 0-10 expected myocarditis cases, but they observed 19 cases.
“All patients in this series reflect substantial similarities in demographic characteristics, proximate vaccine dose, onset interval, and character of vaccine-associated myocarditis. The consistent pattern of clinical presentation, rapid recovery, and absence of evidence of other causes support the diagnosis of hypersensitivity myocarditis,” they stated.
They added that presentation after a second vaccine dose or, in three patients, when vaccination followed SARS-CoV-2 infection, suggests that prior exposure was relevant in the hypersensitivity response.
“The spectrum of clinical presentation and reliance on patients seeking health care and on health care professionals recognizing a rare vaccine-associated adverse event limits determination of the true incidence of this condition,” the authors wrote.
They stressed that recognition of vaccine-associated myocarditis is clinically important because diagnosis impacts management, recommendations for exercise, and monitoring for cardiomyopathy.
But the authors also acknowledged that it is important to frame concerns about potential vaccine-associated myocarditis within the context of the current pandemic.
“Infection with SARS-CoV-2 is a clear cause of serious cardiac injury in many patients. ... Prevalence of cardiac injury may be as high as 60% in seriously ill patients. Notably, nearly 1% of highly fit athletes with mild COVID-19 infection have evidence of myocarditis on cMRI,” they wrote.
“Given that COVID-19 vaccines are remarkably effective at preventing infection, any risk of rare adverse events following immunization must be carefully weighed against the very substantial benefit of vaccination,” they concluded.
Four cases at Duke
In the second paper in JAMA Cardiology, a group led by Han W. Kim, MD, reported four patients with acute myocarditis occurring within days of mRNA COVID-19 vaccination (two Pfizer and two Moderna) in patients treated at Duke University Medical Center, Durham, N.C. The hospital courses of the four patients with myocarditis following COVID-19 vaccination were uneventful, and they were discharged within 2-4 days.
The authors said that, although a causal relationship cannot be established, none of the patients had a viral prodrome or had coincident testing that revealed an alternative explanation.
They stated that these four patients represent the majority of patients with acute myocarditis identified in the past 3 months at their institution, and this led to the highest total number of patients with acute myocarditis, compared with the same 3-month period for the past 5 years.
“Additionally, we identified only those patients with severe unremitting chest pain who sought medical attention. Those with mild or moderate chest pain might not seek medical attention, and it is possible that subclinical myocarditis may occur and could be detected by active surveillance, as has been described with smallpox vaccination,” they wrote.
Further case reports
In one of the papers in Circulation, a group led by Kathryn F. Larson, MD, from the Mayo Clinic in Rochester, Minn., described eight patients hospitalized with chest pain who were diagnosed with myocarditis within 2-4 days of receiving either the Pfizer or Moderna vaccine.
Two of the patients had previously been infected by SARS-CoV-2 without need for hospitalization. All individuals were otherwise healthy males between the ages of 21 and 56 years. All but one patient developed symptoms after their second dose, and the one patient who developed myocarditis after the first vaccine dose had previously been infected with SARS-CoV-2.
Systemic symptoms began within 24 hours after vaccine administration in five of eight patients, with chest pain presenting between 48 and 96 hours later. Troponin values were elevated in all individuals and appeared to peak the day after admission, whereas none had eosinophilia.
Cardiac MRI revealed findings consistent with myocarditis in all patients. All patients had resolution of their chest pain and were discharged from the hospital in stable condition.
“The patients presented here demonstrated typical signs, symptoms, and diagnostic features of acute myocarditis. The temporal association between receiving an mRNA-based COVID-19 vaccine and the development of myocarditis is notable,” the authors said.
They added that they would consider the use of corticosteroids in these patients but cautioned that this could reduce the specific immune response against SARS-COV-2 triggered by the vaccine. “Thus, the duration of corticosteroid administration should be limited to the resolution of the symptoms or ventricular arrhythmias or the recovery of the left ventricular ejection fraction.”
Pending publication of long-term outcome data after SARS-CoV-2 vaccine–related myocarditis, they suggest adherence to the current consensus recommendation to abstain from competitive sports for a period of 3-6 months with reevaluation prior to sports participation.
In another of the Circulation papers, a group led by Carolyn M. Rosner, MSN, presented a case series of seven patients hospitalized for acute myocarditis-like illness following COVID-19 vaccination, from two U.S. medical centers, in Falls Church, Va., and Dallas. All patients were males below the age of 40 years and of White or Hispanic race/ethnicity. Only one patient reported prior history of COVID-19 infection. Six patients received mRNA (Moderna or Pfizer) and one received the adenovirus (Johnson & Johnson) vaccine. All patients presented 3-7 days post vaccination with acute onset chest pain and biochemical evidence of myocardial injury.
Hospital length of stay was 3 days, and all patients’ symptoms resolved by hospital discharge.
And finally, the third paper in Circulation reported a detailed description of one patient – a 52-year-old, previously healthy male who presented with acute myocarditis 3 days after the administration of the second dose of Moderna’s COVID-19 vaccine. The symptoms resolved, and there was a gradual improvement in cMRI findings. Ischemic injury and other potential causes of acute myocardial injury were excluded, as were other potential infectious causes of myocarditis, and there was no evidence of systemic autoimmune disease.
“Clinicians should be aware that myocarditis may be present in patients exhibiting cardiac signs and symptoms 2-4 days after COVID-19 vaccination,” the authors said.
They added that additional surveillance of such adverse events post–COVID-19 vaccination will help identify subgroups at higher risk for this vaccine-related effect, and whether additional precautions are necessary.
‘Benefits outweigh risk’
In an accompanying editorial in JAMA Cardiology, three doctors from the CDC cite several other reports of myocarditis after mRNA COVID vaccination. These include a case report published in Pediatrics of seven male adolescents aged 14-19 years who presented with myocarditis or myopericarditis within 4 days after receipt of a second dose of the Pfizer vaccine.
But the editorialists noted that the most comprehensive data about the risk for myocarditis following immunization with mRNA vaccines comes from Israel.
The Israeli Ministry of Health recently posted data describing 121 myocarditis cases occurring within 30 days of a second dose of mRNA vaccine among 5,049,424 persons, suggesting a crude incidence rate of approximately 24 cases per million.
On the current case reports, the CDC doctors wrote: “The striking clinical similarities in the presentations of these patients, their recent vaccination with an mRNA-based COVID-19 vaccine, and the lack of any alternative etiologies for acute myocarditis suggest an association with immunization.”
They said that acute onset of chest pain 3-5 days after vaccine administration, usually after a second dose, is a typical feature of reported cases and suggests an immune-mediated mechanism.
But SARS-CoV-2 infection also causes cardiac injury which may result in severe outcomes, and based on currently available data, myocarditis following immunization with current mRNA-based vaccines is rare.
“At present, the benefits of immunization in preventing severe morbidity favors continued COVID-19 vaccination, particularly considering the increasing COVID-19 hospitalization rates among adolescents reported during spring 2021,” the editorialists stated.
But they added that many questions remain. These include whether modifications are needed to the vaccine schedule among persons with a history of possible or confirmed myocarditis after COVID vaccine, how should postvaccine myocarditis be managed, how often should follow-up assessments be performed, how might follow-up assessments affect recommendations to avoid vigorous physical activity following the diagnosis of myocarditis, and do all likely cases of acute myocarditis that appear to be uncomplicated require cardiac MRI for more definitive diagnosis?
“While the data needed to answer such questions are being collected, there is an opportunity for researchers with expertise in myocarditis to develop a comprehensive, national assessment of the natural history, pathogenesis, and treatment of acute myocarditis associated with receipt of mRNA-based COVID-19 vaccines,” they concluded.
In a second editorial in JAMA Cardiology, a group of editors from the journal acknowledged that publication of the current case reports may contribute to additional public concern regarding immunization. But they added that clinicians discussing immunization with patients should recognize that these case series suggest that the symptomatic events consistent with myocarditis are still very rare and appear to be self-limiting.
“Given the risks of COVID-19, including the risk of myocarditis from COVID-19 infection, the editors do not believe these case reports are sufficient to interrupt the march toward maximal vaccination against SARS-CoV-2 as expeditiously as possible,” they said.
A version of this article first appeared on Medscape.com.