User login
Stopping Empagliflozin Unmasks Heart Failure
SGLT2 inhibitors have been shown to have a role in the management of heart failure in patients with type 2 diabetes mellitus, but there is a risk of exacerbation when discontinued.
About 40% of patients with heart failure (HF) also have type 2 diabetes mellitus (T2DM).1 Certain sodium-glucose cotransporter-2 (SGLT2) inhibitors have benefited patients with HF.2 We report a case of a patient with T2DM who had signs and symptoms of hypervolemia after discontinuing the SGLT2 inhibitor empagliflozin. The patient was found to have previously undiagnosed HF.
Case Presentation
A 58-year-old male presented for care at Malcolm Randall Veterans Affairs Medical Center in Gainseville, Florida, diabetes clinic. The patient was diagnosed with T2DM at age 32 years. At 36 years, he was started on subcutaneous insulin injections, and was switched to insulin pump therapy in his early 40s. At the time of evaluation, the T2DM was managed using an insulin pump, metformin, and acarbose. He had been prescribed empagliflozin 10 mg several months before presentation, but the medication ran out about 1 month prior to evaluation, and additional refills were unavailable.
The patient reported a 1-month history of worsening exertional shortness of breath, decreased exercise tolerance, and lower extremity swelling. Vitals signs, including respiratory rate and oxygen saturation were within normal limits. Bibasilar crackles and bilateral 2+ pitting pedal edema were noted. The remaining examination was unrevealing. His most recent glycated hemoglobin A1c level from 1 month prior to the presentation was 6.4%.
Given the patient’s shortness of breath and evidence of fluid overload on examination, brain natriuretic peptide was obtained and was significantly elevated at 5,895 pg/mL. A transthoracic echocardiogram revealed left ventricular ejection fraction < 20%. The patient was started on furosemide 40 mg, pending receipt of empagliflozin. A cardiology evaluation also was recommended.
Cardiac catheterization identified significant obstructions to the left anterior descending and left circumflex arteries. The patient underwent successful percutaneous coronary intervention to these areas. Following initiation of medications and coronary revascularization, the patient reported significant symptom improvement. At the follow-up evaluation 8 weeks later, he was symptom free, and his physical examination was consistent with euvolemia.
Discussion
T2DM has been associated with adverse cardiovascular outcomes, including atherosclerotic heart disease and HF. There are several theories about the relationship between T2DM and HF, though the exact pathophysiology of this relationship is unknown.3,4 One theory suggests diabetic cardiomyopathy as the cause. In patients with diabetic cardiomyopathy, there is early development of diastolic dysfunction, which eventually progresses to ventricular dysfunction. There is continued stimulation of the renin-angiotensin-aldosterone system that leads to death of cardiomyocytes, fibrosis, and remodeling, which worsens pump failure.5
SGLT2 inhibitors decrease hyperglycemia and hyperinsulinemia, potentially reducing HF risk. SGLT2 inhibitors decrease blood glucose levels by inhibiting SGLT2 in the proximal tubule, leading to a decrease of glucose reabsorption and an increase in excretion.6,7 The EMPA-REG OUTCOME trial looked at cardiovascular outcomes in patients with T2DM at high risk for adverse cardiac events. There was a significant risk reduction in deaths and hospitalizations for HF in patients treated with empagliflozin.8
The EMPRISE study specifically examined empagliflozin and its effects on hospitalization for HF.2 When compared with patients treated with sitagliptin, there was a statistically significant decrease in hospitalization for HF in patients with T2DM, both with and without preexisting cardiovascular disease.
This case highlights the relationship between T2DM and HF. We also show how the use of empagliflozin may have helped manage the patient’s undiagnosed HF and how its discontinuation luckily unmasked it. Routine evaluation for HF in patients with T2DM is not done, but likely there are patients who would benefit, especially given the strong, albeit less known, association between these 2 conditions.
Further studies are needed to determine the type of patients who would benefit most from HF screening. For now, the best practice is to obtain a complete medical history that includes current and recently discontinued medications as well a thorough physical examination for signs of fluid overload and cardiovascular compromise. Patients who may have signs concerning for HF can have appropriate testing and intervention.
Conclusions
SGLT2 inhibitors have been shown to have a role in the management of HF in patients with T2DM. There is a risk of exacerbation or unmasking of HF when discontinuing SGLT2 inhibitors. To our knowledge, this is the first paper describing the discovery of HF following interruption of SGLT2 inhibitor treatment. The clinician and patient should monitor for signs and symptoms of fluid overload when stopping therapy. Further research into the benefits of a more comprehensive evaluation is needed.
1. Thomas MC. Type 2 diabetes and heart failure: challenges and solutions. Curr Cardiol Rev. 2016;12(3):249-255. doi:10.2174/1573403X12666160606120254
2. Patorno E, Pawar A, Franklin J, et al. Empagliflozin and the risk of heart failure hospitalization in routine clinical care: a first analysis from the EMPRISE study. Circulation. 2019;139(25):2822-2830. doi:10.1161/CIRCULATIONAHA.118.039177
3. Packer M. Heart failure: the most important, preventable, and treatable cardiovascular complication of type 2 diabetes. Diabetes Care. 2018;41(1):11-13. doi:10.2337/dci17-0052
4. Thrainsdottir I, Aspelund T, Thorheirsson G, et al. The association between glucose abnormalities and heart failure in the population-based Reykjavík study. Diabetes Care. 2005;28(3):612-616. doi:10.2337/diacare.28.3.612
5. Bell D, Goncalves E. Heart failure in the patient with diabetes: epidemiology, aetiology, prognosis, therapy and the effect of glucose-lowering medications. Diabetes Obes Metab. 2019;21(6):1277-1290. doi:10.1111/dom.13652
6. Nair S, Wilding JPH. Sodium glucose cotransporter 2 Inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab. 2010;95(1):34-42. doi:10.1210/jc.2009-0473
7. Ali A, Bain S, Hicks D, et al; Improving Diabetes Steering Committee. SGLT2 inhibitors: cardiovascular benefits beyond HbA1c- translating evidence into practice. Diabetes Ther. 2019;10(5):1595-1622. doi:10.1007/s13300-019-0657-8
8. Zinman B, Wanner C, Lachin J, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/NEJMoa1504720
SGLT2 inhibitors have been shown to have a role in the management of heart failure in patients with type 2 diabetes mellitus, but there is a risk of exacerbation when discontinued.
SGLT2 inhibitors have been shown to have a role in the management of heart failure in patients with type 2 diabetes mellitus, but there is a risk of exacerbation when discontinued.
About 40% of patients with heart failure (HF) also have type 2 diabetes mellitus (T2DM).1 Certain sodium-glucose cotransporter-2 (SGLT2) inhibitors have benefited patients with HF.2 We report a case of a patient with T2DM who had signs and symptoms of hypervolemia after discontinuing the SGLT2 inhibitor empagliflozin. The patient was found to have previously undiagnosed HF.
Case Presentation
A 58-year-old male presented for care at Malcolm Randall Veterans Affairs Medical Center in Gainseville, Florida, diabetes clinic. The patient was diagnosed with T2DM at age 32 years. At 36 years, he was started on subcutaneous insulin injections, and was switched to insulin pump therapy in his early 40s. At the time of evaluation, the T2DM was managed using an insulin pump, metformin, and acarbose. He had been prescribed empagliflozin 10 mg several months before presentation, but the medication ran out about 1 month prior to evaluation, and additional refills were unavailable.
The patient reported a 1-month history of worsening exertional shortness of breath, decreased exercise tolerance, and lower extremity swelling. Vitals signs, including respiratory rate and oxygen saturation were within normal limits. Bibasilar crackles and bilateral 2+ pitting pedal edema were noted. The remaining examination was unrevealing. His most recent glycated hemoglobin A1c level from 1 month prior to the presentation was 6.4%.
Given the patient’s shortness of breath and evidence of fluid overload on examination, brain natriuretic peptide was obtained and was significantly elevated at 5,895 pg/mL. A transthoracic echocardiogram revealed left ventricular ejection fraction < 20%. The patient was started on furosemide 40 mg, pending receipt of empagliflozin. A cardiology evaluation also was recommended.
Cardiac catheterization identified significant obstructions to the left anterior descending and left circumflex arteries. The patient underwent successful percutaneous coronary intervention to these areas. Following initiation of medications and coronary revascularization, the patient reported significant symptom improvement. At the follow-up evaluation 8 weeks later, he was symptom free, and his physical examination was consistent with euvolemia.
Discussion
T2DM has been associated with adverse cardiovascular outcomes, including atherosclerotic heart disease and HF. There are several theories about the relationship between T2DM and HF, though the exact pathophysiology of this relationship is unknown.3,4 One theory suggests diabetic cardiomyopathy as the cause. In patients with diabetic cardiomyopathy, there is early development of diastolic dysfunction, which eventually progresses to ventricular dysfunction. There is continued stimulation of the renin-angiotensin-aldosterone system that leads to death of cardiomyocytes, fibrosis, and remodeling, which worsens pump failure.5
SGLT2 inhibitors decrease hyperglycemia and hyperinsulinemia, potentially reducing HF risk. SGLT2 inhibitors decrease blood glucose levels by inhibiting SGLT2 in the proximal tubule, leading to a decrease of glucose reabsorption and an increase in excretion.6,7 The EMPA-REG OUTCOME trial looked at cardiovascular outcomes in patients with T2DM at high risk for adverse cardiac events. There was a significant risk reduction in deaths and hospitalizations for HF in patients treated with empagliflozin.8
The EMPRISE study specifically examined empagliflozin and its effects on hospitalization for HF.2 When compared with patients treated with sitagliptin, there was a statistically significant decrease in hospitalization for HF in patients with T2DM, both with and without preexisting cardiovascular disease.
This case highlights the relationship between T2DM and HF. We also show how the use of empagliflozin may have helped manage the patient’s undiagnosed HF and how its discontinuation luckily unmasked it. Routine evaluation for HF in patients with T2DM is not done, but likely there are patients who would benefit, especially given the strong, albeit less known, association between these 2 conditions.
Further studies are needed to determine the type of patients who would benefit most from HF screening. For now, the best practice is to obtain a complete medical history that includes current and recently discontinued medications as well a thorough physical examination for signs of fluid overload and cardiovascular compromise. Patients who may have signs concerning for HF can have appropriate testing and intervention.
Conclusions
SGLT2 inhibitors have been shown to have a role in the management of HF in patients with T2DM. There is a risk of exacerbation or unmasking of HF when discontinuing SGLT2 inhibitors. To our knowledge, this is the first paper describing the discovery of HF following interruption of SGLT2 inhibitor treatment. The clinician and patient should monitor for signs and symptoms of fluid overload when stopping therapy. Further research into the benefits of a more comprehensive evaluation is needed.
About 40% of patients with heart failure (HF) also have type 2 diabetes mellitus (T2DM).1 Certain sodium-glucose cotransporter-2 (SGLT2) inhibitors have benefited patients with HF.2 We report a case of a patient with T2DM who had signs and symptoms of hypervolemia after discontinuing the SGLT2 inhibitor empagliflozin. The patient was found to have previously undiagnosed HF.
Case Presentation
A 58-year-old male presented for care at Malcolm Randall Veterans Affairs Medical Center in Gainseville, Florida, diabetes clinic. The patient was diagnosed with T2DM at age 32 years. At 36 years, he was started on subcutaneous insulin injections, and was switched to insulin pump therapy in his early 40s. At the time of evaluation, the T2DM was managed using an insulin pump, metformin, and acarbose. He had been prescribed empagliflozin 10 mg several months before presentation, but the medication ran out about 1 month prior to evaluation, and additional refills were unavailable.
The patient reported a 1-month history of worsening exertional shortness of breath, decreased exercise tolerance, and lower extremity swelling. Vitals signs, including respiratory rate and oxygen saturation were within normal limits. Bibasilar crackles and bilateral 2+ pitting pedal edema were noted. The remaining examination was unrevealing. His most recent glycated hemoglobin A1c level from 1 month prior to the presentation was 6.4%.
Given the patient’s shortness of breath and evidence of fluid overload on examination, brain natriuretic peptide was obtained and was significantly elevated at 5,895 pg/mL. A transthoracic echocardiogram revealed left ventricular ejection fraction < 20%. The patient was started on furosemide 40 mg, pending receipt of empagliflozin. A cardiology evaluation also was recommended.
Cardiac catheterization identified significant obstructions to the left anterior descending and left circumflex arteries. The patient underwent successful percutaneous coronary intervention to these areas. Following initiation of medications and coronary revascularization, the patient reported significant symptom improvement. At the follow-up evaluation 8 weeks later, he was symptom free, and his physical examination was consistent with euvolemia.
Discussion
T2DM has been associated with adverse cardiovascular outcomes, including atherosclerotic heart disease and HF. There are several theories about the relationship between T2DM and HF, though the exact pathophysiology of this relationship is unknown.3,4 One theory suggests diabetic cardiomyopathy as the cause. In patients with diabetic cardiomyopathy, there is early development of diastolic dysfunction, which eventually progresses to ventricular dysfunction. There is continued stimulation of the renin-angiotensin-aldosterone system that leads to death of cardiomyocytes, fibrosis, and remodeling, which worsens pump failure.5
SGLT2 inhibitors decrease hyperglycemia and hyperinsulinemia, potentially reducing HF risk. SGLT2 inhibitors decrease blood glucose levels by inhibiting SGLT2 in the proximal tubule, leading to a decrease of glucose reabsorption and an increase in excretion.6,7 The EMPA-REG OUTCOME trial looked at cardiovascular outcomes in patients with T2DM at high risk for adverse cardiac events. There was a significant risk reduction in deaths and hospitalizations for HF in patients treated with empagliflozin.8
The EMPRISE study specifically examined empagliflozin and its effects on hospitalization for HF.2 When compared with patients treated with sitagliptin, there was a statistically significant decrease in hospitalization for HF in patients with T2DM, both with and without preexisting cardiovascular disease.
This case highlights the relationship between T2DM and HF. We also show how the use of empagliflozin may have helped manage the patient’s undiagnosed HF and how its discontinuation luckily unmasked it. Routine evaluation for HF in patients with T2DM is not done, but likely there are patients who would benefit, especially given the strong, albeit less known, association between these 2 conditions.
Further studies are needed to determine the type of patients who would benefit most from HF screening. For now, the best practice is to obtain a complete medical history that includes current and recently discontinued medications as well a thorough physical examination for signs of fluid overload and cardiovascular compromise. Patients who may have signs concerning for HF can have appropriate testing and intervention.
Conclusions
SGLT2 inhibitors have been shown to have a role in the management of HF in patients with T2DM. There is a risk of exacerbation or unmasking of HF when discontinuing SGLT2 inhibitors. To our knowledge, this is the first paper describing the discovery of HF following interruption of SGLT2 inhibitor treatment. The clinician and patient should monitor for signs and symptoms of fluid overload when stopping therapy. Further research into the benefits of a more comprehensive evaluation is needed.
1. Thomas MC. Type 2 diabetes and heart failure: challenges and solutions. Curr Cardiol Rev. 2016;12(3):249-255. doi:10.2174/1573403X12666160606120254
2. Patorno E, Pawar A, Franklin J, et al. Empagliflozin and the risk of heart failure hospitalization in routine clinical care: a first analysis from the EMPRISE study. Circulation. 2019;139(25):2822-2830. doi:10.1161/CIRCULATIONAHA.118.039177
3. Packer M. Heart failure: the most important, preventable, and treatable cardiovascular complication of type 2 diabetes. Diabetes Care. 2018;41(1):11-13. doi:10.2337/dci17-0052
4. Thrainsdottir I, Aspelund T, Thorheirsson G, et al. The association between glucose abnormalities and heart failure in the population-based Reykjavík study. Diabetes Care. 2005;28(3):612-616. doi:10.2337/diacare.28.3.612
5. Bell D, Goncalves E. Heart failure in the patient with diabetes: epidemiology, aetiology, prognosis, therapy and the effect of glucose-lowering medications. Diabetes Obes Metab. 2019;21(6):1277-1290. doi:10.1111/dom.13652
6. Nair S, Wilding JPH. Sodium glucose cotransporter 2 Inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab. 2010;95(1):34-42. doi:10.1210/jc.2009-0473
7. Ali A, Bain S, Hicks D, et al; Improving Diabetes Steering Committee. SGLT2 inhibitors: cardiovascular benefits beyond HbA1c- translating evidence into practice. Diabetes Ther. 2019;10(5):1595-1622. doi:10.1007/s13300-019-0657-8
8. Zinman B, Wanner C, Lachin J, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/NEJMoa1504720
1. Thomas MC. Type 2 diabetes and heart failure: challenges and solutions. Curr Cardiol Rev. 2016;12(3):249-255. doi:10.2174/1573403X12666160606120254
2. Patorno E, Pawar A, Franklin J, et al. Empagliflozin and the risk of heart failure hospitalization in routine clinical care: a first analysis from the EMPRISE study. Circulation. 2019;139(25):2822-2830. doi:10.1161/CIRCULATIONAHA.118.039177
3. Packer M. Heart failure: the most important, preventable, and treatable cardiovascular complication of type 2 diabetes. Diabetes Care. 2018;41(1):11-13. doi:10.2337/dci17-0052
4. Thrainsdottir I, Aspelund T, Thorheirsson G, et al. The association between glucose abnormalities and heart failure in the population-based Reykjavík study. Diabetes Care. 2005;28(3):612-616. doi:10.2337/diacare.28.3.612
5. Bell D, Goncalves E. Heart failure in the patient with diabetes: epidemiology, aetiology, prognosis, therapy and the effect of glucose-lowering medications. Diabetes Obes Metab. 2019;21(6):1277-1290. doi:10.1111/dom.13652
6. Nair S, Wilding JPH. Sodium glucose cotransporter 2 Inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab. 2010;95(1):34-42. doi:10.1210/jc.2009-0473
7. Ali A, Bain S, Hicks D, et al; Improving Diabetes Steering Committee. SGLT2 inhibitors: cardiovascular benefits beyond HbA1c- translating evidence into practice. Diabetes Ther. 2019;10(5):1595-1622. doi:10.1007/s13300-019-0657-8
8. Zinman B, Wanner C, Lachin J, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/NEJMoa1504720
Cortical surface changes tied to risk for movement disorders in schizophrenia
Schizophrenia patients with parkinsonism show distinctive patterns of cortical surface markers, compared with schizophrenia patients without parkinsonism and healthy controls, results of a multimodal magnetic resonance imaging study suggest.
Sensorimotor abnormalities are common in schizophrenia patients, however, “the neurobiological mechanisms underlying parkinsonism in [schizophrenia], which in treated samples represents the unity of interplay between spontaneous and antipsychotic drug-exacerbated movement disorder, are poorly understood,” wrote Robert Christian Wolf, MD, of Heidelberg (Germany) University, and colleagues.
In a study published in Schizophrenia Research (2021 May;231:54-60), the investigators examined brain imaging findings from 20 healthy controls, 38 schizophrenia patients with parkinsonism (SZ-P), and 35 schizophrenia patients without parkinsonism (SZ-nonP). Dr. Wolf and colleagues examined three cortical surface markers: cortical thickness, complexity of cortical folding, and sulcus depth.
Compared with SZ-nonP patients, the SZ-P patients showed significantly increased complexity of cortical folding in the left supplementary motor cortex (SMC) and significantly decreased left postcentral sulcus (PCS) depth. In addition, left SMC activity was higher in both SZ-P and SZ-nonP patient groups, compared with controls.
In a regression analysis, the researchers examined relationships between parkinsonism severity and brain structure. They found that parkinsonism severity was negatively associated with left middle frontal complexity of cortical folding and left anterior cingulate cortex cortical thickness.
“Overall, the data support the notion that cortical features of distinct neurodevelopmental origin, particularly cortical folding indices such as [complexity of cortical folding] and sulcus depth, contribute to the pathogenesis of parkinsonism in SZ,” the researchers wrote.
The study findings were limited by several factors, including the cross-sectional design, the potential limitations of the Simpson-Angus Scale in characterizing parkinsonism, the inability to record lifetime antibiotics exposure in the patient population, and the inability to identify changes in brain stem nuclei, the researchers noted. However, the results were strengthened by the well-matched study groups and use of multimodal MRI, they said.
Consequently, “,” and suggest a link between abnormal neurodevelopmental processes and an increased risk for movement disorders in schizophrenia, they concluded.
The study was funded by the German Research Foundation and the German Federal Ministry of Education and Research. Dr. Wolf and colleagues disclosed no conflicts.
Schizophrenia patients with parkinsonism show distinctive patterns of cortical surface markers, compared with schizophrenia patients without parkinsonism and healthy controls, results of a multimodal magnetic resonance imaging study suggest.
Sensorimotor abnormalities are common in schizophrenia patients, however, “the neurobiological mechanisms underlying parkinsonism in [schizophrenia], which in treated samples represents the unity of interplay between spontaneous and antipsychotic drug-exacerbated movement disorder, are poorly understood,” wrote Robert Christian Wolf, MD, of Heidelberg (Germany) University, and colleagues.
In a study published in Schizophrenia Research (2021 May;231:54-60), the investigators examined brain imaging findings from 20 healthy controls, 38 schizophrenia patients with parkinsonism (SZ-P), and 35 schizophrenia patients without parkinsonism (SZ-nonP). Dr. Wolf and colleagues examined three cortical surface markers: cortical thickness, complexity of cortical folding, and sulcus depth.
Compared with SZ-nonP patients, the SZ-P patients showed significantly increased complexity of cortical folding in the left supplementary motor cortex (SMC) and significantly decreased left postcentral sulcus (PCS) depth. In addition, left SMC activity was higher in both SZ-P and SZ-nonP patient groups, compared with controls.
In a regression analysis, the researchers examined relationships between parkinsonism severity and brain structure. They found that parkinsonism severity was negatively associated with left middle frontal complexity of cortical folding and left anterior cingulate cortex cortical thickness.
“Overall, the data support the notion that cortical features of distinct neurodevelopmental origin, particularly cortical folding indices such as [complexity of cortical folding] and sulcus depth, contribute to the pathogenesis of parkinsonism in SZ,” the researchers wrote.
The study findings were limited by several factors, including the cross-sectional design, the potential limitations of the Simpson-Angus Scale in characterizing parkinsonism, the inability to record lifetime antibiotics exposure in the patient population, and the inability to identify changes in brain stem nuclei, the researchers noted. However, the results were strengthened by the well-matched study groups and use of multimodal MRI, they said.
Consequently, “,” and suggest a link between abnormal neurodevelopmental processes and an increased risk for movement disorders in schizophrenia, they concluded.
The study was funded by the German Research Foundation and the German Federal Ministry of Education and Research. Dr. Wolf and colleagues disclosed no conflicts.
Schizophrenia patients with parkinsonism show distinctive patterns of cortical surface markers, compared with schizophrenia patients without parkinsonism and healthy controls, results of a multimodal magnetic resonance imaging study suggest.
Sensorimotor abnormalities are common in schizophrenia patients, however, “the neurobiological mechanisms underlying parkinsonism in [schizophrenia], which in treated samples represents the unity of interplay between spontaneous and antipsychotic drug-exacerbated movement disorder, are poorly understood,” wrote Robert Christian Wolf, MD, of Heidelberg (Germany) University, and colleagues.
In a study published in Schizophrenia Research (2021 May;231:54-60), the investigators examined brain imaging findings from 20 healthy controls, 38 schizophrenia patients with parkinsonism (SZ-P), and 35 schizophrenia patients without parkinsonism (SZ-nonP). Dr. Wolf and colleagues examined three cortical surface markers: cortical thickness, complexity of cortical folding, and sulcus depth.
Compared with SZ-nonP patients, the SZ-P patients showed significantly increased complexity of cortical folding in the left supplementary motor cortex (SMC) and significantly decreased left postcentral sulcus (PCS) depth. In addition, left SMC activity was higher in both SZ-P and SZ-nonP patient groups, compared with controls.
In a regression analysis, the researchers examined relationships between parkinsonism severity and brain structure. They found that parkinsonism severity was negatively associated with left middle frontal complexity of cortical folding and left anterior cingulate cortex cortical thickness.
“Overall, the data support the notion that cortical features of distinct neurodevelopmental origin, particularly cortical folding indices such as [complexity of cortical folding] and sulcus depth, contribute to the pathogenesis of parkinsonism in SZ,” the researchers wrote.
The study findings were limited by several factors, including the cross-sectional design, the potential limitations of the Simpson-Angus Scale in characterizing parkinsonism, the inability to record lifetime antibiotics exposure in the patient population, and the inability to identify changes in brain stem nuclei, the researchers noted. However, the results were strengthened by the well-matched study groups and use of multimodal MRI, they said.
Consequently, “,” and suggest a link between abnormal neurodevelopmental processes and an increased risk for movement disorders in schizophrenia, they concluded.
The study was funded by the German Research Foundation and the German Federal Ministry of Education and Research. Dr. Wolf and colleagues disclosed no conflicts.
FROM SCHIZOPHRENIA RESEARCH
Reflections on 10 years of hospitalist productivity
Successful programs will recruit lifelong learners
The workload of individual hospitalists has long been a hot-button issue. In a 2013 survey of hospitalists, 40% felt workloads were unsafe on a monthly basis, and 22% reported ordering unnecessary testing or procedures because of time pressure.1 In a 2014 analysis of over 20,000 admissions to an academic hospital medicine service, increasing workload led to increased length of stay and cost per case.2 Although these studies suggest a “sweet spot” for hospitalist workload, many groups face constant pressure to increase revenue.
Over the past decade there has been a significant change in how hospital medicine programs are financed. In the 2010 State of Hospital Medicine (SoHM), the median financial support per physician hospitalist in adult hospital medicine groups (HMGs) was $98,253. By the 2020 SoHM, the financial support was $198,750, an increase of $100,497 in just 10 years. When this is combined with the explosive growth in the number of hospitalists, there is one inescapable conclusion – hospital medicine is expensive.
Over this same 10 years, net collections per hospitalist grew from $194,440 in 2010 to $216,779 in 2020, an increase of $22,339. The increase was caused by higher collections per encounter, not more encounters. Additionally, median compensation for adult/internal medicine hospitalists increased over the same period from $215,000 to $307,336, an increase of $92,336, or 43%. That is an increase of 3.7% per year, more than twice the rate of inflation or wage growth in the general economy over the same period. About 75% of this increase was funded by hospital support. It is clear – health care systems continue to find value in investing in hospitalists and hospital medicine programs.
With mounting costs for hospitals, there is pressure for the hospitalist model of care to change or for yearly billable encounters per hospitalist full-time equivalent to increase. Yet, the productivity of hospitalists, as measured by median billable encounters per year has remained flat. The 2010 SoHM listed median number of billable encounters per year for an internal medicine hospitalist as 2,230. In 2020, the number is 2,246 – a trivial 0.7% increase per decade, what amounts to a rounding error. There has been wiggle up and down over the years, but I suspect these are not trends but noise.
So the question is why. I think it is partly because hospital medicine leaders together with the leaders of their health care systems seem to be reaching an equilibrium. Productivity will always remain an expectation. This expectation will vary based on local circumstances. But for many HMGs, the days when productivity is pushed as the primary objective seem to be disappearing. Most hospital leaders seem to now understand that high productivity can be detrimental to other program goals.
But if productivity is flat, do 40% of hospitalists still feel they are providing unsafe care on a monthly basis? Without another study we don’t know, but here are some reasons why I’m hopeful. First, the hospitalist workforce is more experienced than 10 years ago and may be more efficient. Second, hospital medicine groups are larger and are therefore enabled to schedule more flexibly or enact jeopardy systems to level out workload on busy days. And lastly, hospitalists who feel they are providing unsafe care find greener pastures. The 2010 SoHM reported adult hospital medicine programs had a median 14.3% turnover rate. The 2020 SoHM turnover was 10.9%. While this is up from 2018 (7.4%) and 2016 (6.9%), the general trend is down.
Additionally, we all need to consider the possibility that there will be a disruptive innovation that will allow greater productivity for individual hospitalists while maintaining value. It is apparent the EHR is not yet that breakthrough. We all need to keep our eyes open, stay flexible, and be prepared to meet evolving demands on our programs.
We will see constant demands on hospitalists. But I’m hopeful that going forward expectations will increasingly shift away from simply working harder and seeing more patients, toward goals related to improving performance. Training programs generally produce excellent clinicians, but they often do not equip physicians to be excellent hospitalists. Successful hospital medicine programs will recruit lifelong learners and career hospitalists who are flexible and willing to innovate and adapt. The best programs will have structures in place to help excellent clinicians mature into the role of excellent hospitalists, and leaders that create and foster an environment of excellence.
Discover more 2020 SoHM Report data at www.hospitalmedicine.org/sohm.
Dr. Frederickson is medical director, hospital medicine and palliative care, at CHI Health, Omaha, Neb., and assistant professor at Creighton University, Omaha.
References
1. Michtalik HJ et al. Impact of Attending Physician Workload on Patient Care: A Survey of Hospitalists. JAMA Intern Med. 2013;173(5):375-7. doi: 10.1001/jamainternmed.2013.1864.
2. Elliott DJ et al. Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786-93. doi: 10.1001/jamainternmed.2014.300.
Successful programs will recruit lifelong learners
Successful programs will recruit lifelong learners
The workload of individual hospitalists has long been a hot-button issue. In a 2013 survey of hospitalists, 40% felt workloads were unsafe on a monthly basis, and 22% reported ordering unnecessary testing or procedures because of time pressure.1 In a 2014 analysis of over 20,000 admissions to an academic hospital medicine service, increasing workload led to increased length of stay and cost per case.2 Although these studies suggest a “sweet spot” for hospitalist workload, many groups face constant pressure to increase revenue.
Over the past decade there has been a significant change in how hospital medicine programs are financed. In the 2010 State of Hospital Medicine (SoHM), the median financial support per physician hospitalist in adult hospital medicine groups (HMGs) was $98,253. By the 2020 SoHM, the financial support was $198,750, an increase of $100,497 in just 10 years. When this is combined with the explosive growth in the number of hospitalists, there is one inescapable conclusion – hospital medicine is expensive.
Over this same 10 years, net collections per hospitalist grew from $194,440 in 2010 to $216,779 in 2020, an increase of $22,339. The increase was caused by higher collections per encounter, not more encounters. Additionally, median compensation for adult/internal medicine hospitalists increased over the same period from $215,000 to $307,336, an increase of $92,336, or 43%. That is an increase of 3.7% per year, more than twice the rate of inflation or wage growth in the general economy over the same period. About 75% of this increase was funded by hospital support. It is clear – health care systems continue to find value in investing in hospitalists and hospital medicine programs.
With mounting costs for hospitals, there is pressure for the hospitalist model of care to change or for yearly billable encounters per hospitalist full-time equivalent to increase. Yet, the productivity of hospitalists, as measured by median billable encounters per year has remained flat. The 2010 SoHM listed median number of billable encounters per year for an internal medicine hospitalist as 2,230. In 2020, the number is 2,246 – a trivial 0.7% increase per decade, what amounts to a rounding error. There has been wiggle up and down over the years, but I suspect these are not trends but noise.
So the question is why. I think it is partly because hospital medicine leaders together with the leaders of their health care systems seem to be reaching an equilibrium. Productivity will always remain an expectation. This expectation will vary based on local circumstances. But for many HMGs, the days when productivity is pushed as the primary objective seem to be disappearing. Most hospital leaders seem to now understand that high productivity can be detrimental to other program goals.
But if productivity is flat, do 40% of hospitalists still feel they are providing unsafe care on a monthly basis? Without another study we don’t know, but here are some reasons why I’m hopeful. First, the hospitalist workforce is more experienced than 10 years ago and may be more efficient. Second, hospital medicine groups are larger and are therefore enabled to schedule more flexibly or enact jeopardy systems to level out workload on busy days. And lastly, hospitalists who feel they are providing unsafe care find greener pastures. The 2010 SoHM reported adult hospital medicine programs had a median 14.3% turnover rate. The 2020 SoHM turnover was 10.9%. While this is up from 2018 (7.4%) and 2016 (6.9%), the general trend is down.
Additionally, we all need to consider the possibility that there will be a disruptive innovation that will allow greater productivity for individual hospitalists while maintaining value. It is apparent the EHR is not yet that breakthrough. We all need to keep our eyes open, stay flexible, and be prepared to meet evolving demands on our programs.
We will see constant demands on hospitalists. But I’m hopeful that going forward expectations will increasingly shift away from simply working harder and seeing more patients, toward goals related to improving performance. Training programs generally produce excellent clinicians, but they often do not equip physicians to be excellent hospitalists. Successful hospital medicine programs will recruit lifelong learners and career hospitalists who are flexible and willing to innovate and adapt. The best programs will have structures in place to help excellent clinicians mature into the role of excellent hospitalists, and leaders that create and foster an environment of excellence.
Discover more 2020 SoHM Report data at www.hospitalmedicine.org/sohm.
Dr. Frederickson is medical director, hospital medicine and palliative care, at CHI Health, Omaha, Neb., and assistant professor at Creighton University, Omaha.
References
1. Michtalik HJ et al. Impact of Attending Physician Workload on Patient Care: A Survey of Hospitalists. JAMA Intern Med. 2013;173(5):375-7. doi: 10.1001/jamainternmed.2013.1864.
2. Elliott DJ et al. Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786-93. doi: 10.1001/jamainternmed.2014.300.
The workload of individual hospitalists has long been a hot-button issue. In a 2013 survey of hospitalists, 40% felt workloads were unsafe on a monthly basis, and 22% reported ordering unnecessary testing or procedures because of time pressure.1 In a 2014 analysis of over 20,000 admissions to an academic hospital medicine service, increasing workload led to increased length of stay and cost per case.2 Although these studies suggest a “sweet spot” for hospitalist workload, many groups face constant pressure to increase revenue.
Over the past decade there has been a significant change in how hospital medicine programs are financed. In the 2010 State of Hospital Medicine (SoHM), the median financial support per physician hospitalist in adult hospital medicine groups (HMGs) was $98,253. By the 2020 SoHM, the financial support was $198,750, an increase of $100,497 in just 10 years. When this is combined with the explosive growth in the number of hospitalists, there is one inescapable conclusion – hospital medicine is expensive.
Over this same 10 years, net collections per hospitalist grew from $194,440 in 2010 to $216,779 in 2020, an increase of $22,339. The increase was caused by higher collections per encounter, not more encounters. Additionally, median compensation for adult/internal medicine hospitalists increased over the same period from $215,000 to $307,336, an increase of $92,336, or 43%. That is an increase of 3.7% per year, more than twice the rate of inflation or wage growth in the general economy over the same period. About 75% of this increase was funded by hospital support. It is clear – health care systems continue to find value in investing in hospitalists and hospital medicine programs.
With mounting costs for hospitals, there is pressure for the hospitalist model of care to change or for yearly billable encounters per hospitalist full-time equivalent to increase. Yet, the productivity of hospitalists, as measured by median billable encounters per year has remained flat. The 2010 SoHM listed median number of billable encounters per year for an internal medicine hospitalist as 2,230. In 2020, the number is 2,246 – a trivial 0.7% increase per decade, what amounts to a rounding error. There has been wiggle up and down over the years, but I suspect these are not trends but noise.
So the question is why. I think it is partly because hospital medicine leaders together with the leaders of their health care systems seem to be reaching an equilibrium. Productivity will always remain an expectation. This expectation will vary based on local circumstances. But for many HMGs, the days when productivity is pushed as the primary objective seem to be disappearing. Most hospital leaders seem to now understand that high productivity can be detrimental to other program goals.
But if productivity is flat, do 40% of hospitalists still feel they are providing unsafe care on a monthly basis? Without another study we don’t know, but here are some reasons why I’m hopeful. First, the hospitalist workforce is more experienced than 10 years ago and may be more efficient. Second, hospital medicine groups are larger and are therefore enabled to schedule more flexibly or enact jeopardy systems to level out workload on busy days. And lastly, hospitalists who feel they are providing unsafe care find greener pastures. The 2010 SoHM reported adult hospital medicine programs had a median 14.3% turnover rate. The 2020 SoHM turnover was 10.9%. While this is up from 2018 (7.4%) and 2016 (6.9%), the general trend is down.
Additionally, we all need to consider the possibility that there will be a disruptive innovation that will allow greater productivity for individual hospitalists while maintaining value. It is apparent the EHR is not yet that breakthrough. We all need to keep our eyes open, stay flexible, and be prepared to meet evolving demands on our programs.
We will see constant demands on hospitalists. But I’m hopeful that going forward expectations will increasingly shift away from simply working harder and seeing more patients, toward goals related to improving performance. Training programs generally produce excellent clinicians, but they often do not equip physicians to be excellent hospitalists. Successful hospital medicine programs will recruit lifelong learners and career hospitalists who are flexible and willing to innovate and adapt. The best programs will have structures in place to help excellent clinicians mature into the role of excellent hospitalists, and leaders that create and foster an environment of excellence.
Discover more 2020 SoHM Report data at www.hospitalmedicine.org/sohm.
Dr. Frederickson is medical director, hospital medicine and palliative care, at CHI Health, Omaha, Neb., and assistant professor at Creighton University, Omaha.
References
1. Michtalik HJ et al. Impact of Attending Physician Workload on Patient Care: A Survey of Hospitalists. JAMA Intern Med. 2013;173(5):375-7. doi: 10.1001/jamainternmed.2013.1864.
2. Elliott DJ et al. Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786-93. doi: 10.1001/jamainternmed.2014.300.
Why getting a COVID-19 vaccine to children could take time
Testing COVID-19 vaccines in young children is going to be tricky. Deciding how to approve them and who should get them may be even more difficult.
So far, the vaccines available to Americans ages 12 and up have sailed through the U.S. Food and Drug Administration’s regulatory checks, taking advantage of an accelerated clearance process called an Emergency Use Authorization (EUA).
EUAs set a lower bar for effectiveness, saying the vaccines may be safe and effective based on just a few months of data.
But with COVID cases plummeting in the United States and children historically seeing far less serious disease than adults, a panel of expert advisors to the FDA was asked to deliberate on Thursday whether the agency could consider vaccines for this age group under the same standard.
Stated another way: Is COVID an emergency for kids?
There’s another wrinkle in the mix, too – heart inflammation, which appears to be a very rare emerging adverse event tied to vaccination. It seems to happen more often in teens and young adults. To date, cases of myocarditis and pericarditis appear to be happening in 16 to 30 people for every 1 million doses given.
But if it is conclusively linked to the shots, some wonder whether it might tip the balance between benefits and risks for kids.
That left some of the experts who sit on the FDA’s advisory committee for vaccines and related biological products urging the FDA to take its time and more thoroughly study the shots before they’re given to millions of children.
Vaccine studies different in children?
Clinical studies of the vaccines in teens and adults have thus far relied on some straightforward math. You take two groups of similar people. You give half the vaccine and half a placebo. Then you wait and see which group has more symptomatic infections. To date, the vaccines have dramatically cut the risk of getting severely ill with COVID for every age group tested.
But COVID infections are falling rapidly in the U.S., and that may make it more difficult for researchers to conduct a similar kind of experiment in children.
The FDA is considering different approaches to figure out whether a vaccine would be effective in kids, including something called an “immunobridging trial.”
In bridging trials, researchers don’t look for infections; rather, they look for proven signs that someone has developed immunity, like antibody levels. Those biomarkers are then compared to the immune responses of younger adults who have demonstrated good protection against infection.
The main advantage of bridging studies is speed. It’s possible to get a snapshot of how the immune system responds to a vaccine within weeks of the final dose.
The drawback is that researchers don’t know exactly what to look for to judge how well the shots are generating protection.
That’s made even more difficult because kids’ immune systems are still developing, so it may be tough to draw direct parallels to adults.
“We don’t know what the serologic correlate of immunity is now. We don’t know how much antibody you have to get in order to be protected. We don’t know what the role of T cells will be,” said H. Cody Meissner, MD, chief of the division of pediatric infectious disease at Tufts Medical Center, Boston.
“I have so much sympathy for the FDA because these are enormous problems, and you have to make a decision,” said Dr. Meissner, who is a member of the FDA’s vaccines and related biological products advisory committee.
Speed vaccines to market, or gather more data?
The plummeting rate of infections in the United States also means that it may be more difficult for the FDA to justify allowing a vaccine on the market for emergency use for children under age 12.
In its recent advisory committee meeting, the agency asked the panel whether it should consider COVID vaccines for children under an EUA or a biologics license application (BLA), aka full approval.
A BLA typically means the agency considers a year or two of data on a new product, rather than just 2 months’ worth. Emergency use also allows products on the market under a looser standard – they “may be” safe and effective, instead of has been proven to be safe and effective.
Several committee members said they didn’t feel the United States was still in an emergency with COVID and couldn’t see the FDA allowing a vaccine to be used in kids that wasn’t given the agency’s highest level of scrutiny, particularly with reports of adverse events like myocarditis coming to light.
“I just want to be sure the price we pay for vaccinating millions of children justifies the side effects, and I don’t think we know that yet,” Dr. Meissner said.
Others acknowledged that there was little risk to kids now with infections on the decline but said that picture could change as variants spread, schools reopen, and colder temperatures force people indoors.
The FDA must decide whether to act based on where we are now or where we could be in a few months.
“I think it’s the million-dollar question right now,” said Hannah Kirking, MD, a medical epidemiologist with the Centers for Disease Control and Prevention who presented new and unpublished data on COVID’s impact in children to the FDA’s advisory committee.
She said prospective studies tracking the way COVID moves through a household with weekly testing from New York City and Utah had found that children catch and transmit COVID almost as readily as adults. But they don’t usually get as sick as adults do, so their cases are easy to miss.
She also presented the results of blood tests from samples around the country looking for evidence of past infection. In these seroprevalence studies, about 27% of children under age 17 had antibodies to COVID – the most of any age group. So more than 1 in 4 kids already has some natural immunity.
That means the main benefit of vaccinating children might be the protection of others, while they still bear the risks – however tiny.
Some experts felt that wasn’t enough reason to justify mass distribution of the vaccines to kids, and from a regulatory standpoint, it might not be permissible.
“FDA can only approve a medical product in a population if the benefits outweigh the risks in that population,” said Peter Doshi, PhD, assistant professor of pharmaceutical health services research in the University of Maryland’s school of pharmacy, Baltimore.
“If benefits don’t outweigh risks in children, it can’t be indicated for children. Full stop,” said Dr. Doshi, who is also an editor at the BMJ.
He said there’s another way to give children access to vaccines, through an expanded access or compassionate use program. Because most COVID deaths have been in children with underlying health conditions, Dr. Doshi and others said it might make sense to allow expanded access – which would get vaccines to children at high risk for complications – without turning them loose on millions before they are more thoroughly studied.
“It’s not a particularly attractive option for industry, because there’s no money to be made. Your medicine can’t be commercialized under expanded access. The most you can reap is manufacturing cost, which is not a lot,” he said.
Art Caplan, a professor of bioethics at New York University’s Langone medical center, said the argument for vaccinating children for flu falls along the same lines. The benefit-to-risk ratio is finely balanced in children. The main value of protecting them is to protect others.
“Flu rarely kills young folks. But you’re really trying to protect old folks and that’s the classic example,” he said.
What’s more, he said the idea that children would take on some risk with a vaccine for little personal benefit is oversimplified.
“Yes, you might get vaccinated to prevent harm to others, but those others are providing benefits to you. It’s not a one-way street. I think that’s a little morally distorted,” Mr. Caplan said. “Being able to keep society open benefits kids and adults alike.”
Other committee members felt like it was too early to sound the all-clear on COVID and said the FDA should authorize vaccines for children as quickly as it had for other age groups.
“We are still, I believe, in an emergency situation. I think that when this virus goes into our children, which is what it’s going to do, that will give it an incubator to change,” said Oveta Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan, Ann Arbor.
Fuller said that for the good of the world, Americans needed to vaccinate children to prevent the virus from mutating and creating new and potentially more dangerous variants.
Weighing risk over safety
Beth Thielen, MD, PhD, pediatric infectious disease specialist and virologist at the University of Minnesota, Minneapolis, said she had not followed the committee’s discussions, but about once a month she treats kids who are very sick because of the virus – either because of a COVID infection or because of multisystem inflammatory syndrome (MIS-C), an inflammatory reaction that strikes after infection.
She’s worried about how the virus has already changed. She said the kind of disease she’s seeing in kids now is different than what she saw in the early months of the pandemic.
“In the last couple of months, I’ve actually seen a few cases of severe pulmonary disease, more similar to adult disease in children,” Dr. Thielen said. “I see on the horizon that we could start seeing more significant disease in young people, and then the risks of being unvaccinated go up substantially.”
But she also knows nobody has a crystal ball, and right now, everything seems to be trending in the right direction with COVID. That makes the risk-to-benefit consideration murkier.
“The question in my mind is, what is the risk of side effects from the vaccine?” she said. “I think we really need to know what the safety profile of vaccine looks like in children because we do have a decent understanding now what risk from disease looks like, because it’s small, but we are seeing it.”
Dr. Thielen said she’ll be keeping an eye on the next meeting of the CDC’s Advisory Committee on Immunization Practices for more answers.
A version of this article first appeared on Medscape.com.
Testing COVID-19 vaccines in young children is going to be tricky. Deciding how to approve them and who should get them may be even more difficult.
So far, the vaccines available to Americans ages 12 and up have sailed through the U.S. Food and Drug Administration’s regulatory checks, taking advantage of an accelerated clearance process called an Emergency Use Authorization (EUA).
EUAs set a lower bar for effectiveness, saying the vaccines may be safe and effective based on just a few months of data.
But with COVID cases plummeting in the United States and children historically seeing far less serious disease than adults, a panel of expert advisors to the FDA was asked to deliberate on Thursday whether the agency could consider vaccines for this age group under the same standard.
Stated another way: Is COVID an emergency for kids?
There’s another wrinkle in the mix, too – heart inflammation, which appears to be a very rare emerging adverse event tied to vaccination. It seems to happen more often in teens and young adults. To date, cases of myocarditis and pericarditis appear to be happening in 16 to 30 people for every 1 million doses given.
But if it is conclusively linked to the shots, some wonder whether it might tip the balance between benefits and risks for kids.
That left some of the experts who sit on the FDA’s advisory committee for vaccines and related biological products urging the FDA to take its time and more thoroughly study the shots before they’re given to millions of children.
Vaccine studies different in children?
Clinical studies of the vaccines in teens and adults have thus far relied on some straightforward math. You take two groups of similar people. You give half the vaccine and half a placebo. Then you wait and see which group has more symptomatic infections. To date, the vaccines have dramatically cut the risk of getting severely ill with COVID for every age group tested.
But COVID infections are falling rapidly in the U.S., and that may make it more difficult for researchers to conduct a similar kind of experiment in children.
The FDA is considering different approaches to figure out whether a vaccine would be effective in kids, including something called an “immunobridging trial.”
In bridging trials, researchers don’t look for infections; rather, they look for proven signs that someone has developed immunity, like antibody levels. Those biomarkers are then compared to the immune responses of younger adults who have demonstrated good protection against infection.
The main advantage of bridging studies is speed. It’s possible to get a snapshot of how the immune system responds to a vaccine within weeks of the final dose.
The drawback is that researchers don’t know exactly what to look for to judge how well the shots are generating protection.
That’s made even more difficult because kids’ immune systems are still developing, so it may be tough to draw direct parallels to adults.
“We don’t know what the serologic correlate of immunity is now. We don’t know how much antibody you have to get in order to be protected. We don’t know what the role of T cells will be,” said H. Cody Meissner, MD, chief of the division of pediatric infectious disease at Tufts Medical Center, Boston.
“I have so much sympathy for the FDA because these are enormous problems, and you have to make a decision,” said Dr. Meissner, who is a member of the FDA’s vaccines and related biological products advisory committee.
Speed vaccines to market, or gather more data?
The plummeting rate of infections in the United States also means that it may be more difficult for the FDA to justify allowing a vaccine on the market for emergency use for children under age 12.
In its recent advisory committee meeting, the agency asked the panel whether it should consider COVID vaccines for children under an EUA or a biologics license application (BLA), aka full approval.
A BLA typically means the agency considers a year or two of data on a new product, rather than just 2 months’ worth. Emergency use also allows products on the market under a looser standard – they “may be” safe and effective, instead of has been proven to be safe and effective.
Several committee members said they didn’t feel the United States was still in an emergency with COVID and couldn’t see the FDA allowing a vaccine to be used in kids that wasn’t given the agency’s highest level of scrutiny, particularly with reports of adverse events like myocarditis coming to light.
“I just want to be sure the price we pay for vaccinating millions of children justifies the side effects, and I don’t think we know that yet,” Dr. Meissner said.
Others acknowledged that there was little risk to kids now with infections on the decline but said that picture could change as variants spread, schools reopen, and colder temperatures force people indoors.
The FDA must decide whether to act based on where we are now or where we could be in a few months.
“I think it’s the million-dollar question right now,” said Hannah Kirking, MD, a medical epidemiologist with the Centers for Disease Control and Prevention who presented new and unpublished data on COVID’s impact in children to the FDA’s advisory committee.
She said prospective studies tracking the way COVID moves through a household with weekly testing from New York City and Utah had found that children catch and transmit COVID almost as readily as adults. But they don’t usually get as sick as adults do, so their cases are easy to miss.
She also presented the results of blood tests from samples around the country looking for evidence of past infection. In these seroprevalence studies, about 27% of children under age 17 had antibodies to COVID – the most of any age group. So more than 1 in 4 kids already has some natural immunity.
That means the main benefit of vaccinating children might be the protection of others, while they still bear the risks – however tiny.
Some experts felt that wasn’t enough reason to justify mass distribution of the vaccines to kids, and from a regulatory standpoint, it might not be permissible.
“FDA can only approve a medical product in a population if the benefits outweigh the risks in that population,” said Peter Doshi, PhD, assistant professor of pharmaceutical health services research in the University of Maryland’s school of pharmacy, Baltimore.
“If benefits don’t outweigh risks in children, it can’t be indicated for children. Full stop,” said Dr. Doshi, who is also an editor at the BMJ.
He said there’s another way to give children access to vaccines, through an expanded access or compassionate use program. Because most COVID deaths have been in children with underlying health conditions, Dr. Doshi and others said it might make sense to allow expanded access – which would get vaccines to children at high risk for complications – without turning them loose on millions before they are more thoroughly studied.
“It’s not a particularly attractive option for industry, because there’s no money to be made. Your medicine can’t be commercialized under expanded access. The most you can reap is manufacturing cost, which is not a lot,” he said.
Art Caplan, a professor of bioethics at New York University’s Langone medical center, said the argument for vaccinating children for flu falls along the same lines. The benefit-to-risk ratio is finely balanced in children. The main value of protecting them is to protect others.
“Flu rarely kills young folks. But you’re really trying to protect old folks and that’s the classic example,” he said.
What’s more, he said the idea that children would take on some risk with a vaccine for little personal benefit is oversimplified.
“Yes, you might get vaccinated to prevent harm to others, but those others are providing benefits to you. It’s not a one-way street. I think that’s a little morally distorted,” Mr. Caplan said. “Being able to keep society open benefits kids and adults alike.”
Other committee members felt like it was too early to sound the all-clear on COVID and said the FDA should authorize vaccines for children as quickly as it had for other age groups.
“We are still, I believe, in an emergency situation. I think that when this virus goes into our children, which is what it’s going to do, that will give it an incubator to change,” said Oveta Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan, Ann Arbor.
Fuller said that for the good of the world, Americans needed to vaccinate children to prevent the virus from mutating and creating new and potentially more dangerous variants.
Weighing risk over safety
Beth Thielen, MD, PhD, pediatric infectious disease specialist and virologist at the University of Minnesota, Minneapolis, said she had not followed the committee’s discussions, but about once a month she treats kids who are very sick because of the virus – either because of a COVID infection or because of multisystem inflammatory syndrome (MIS-C), an inflammatory reaction that strikes after infection.
She’s worried about how the virus has already changed. She said the kind of disease she’s seeing in kids now is different than what she saw in the early months of the pandemic.
“In the last couple of months, I’ve actually seen a few cases of severe pulmonary disease, more similar to adult disease in children,” Dr. Thielen said. “I see on the horizon that we could start seeing more significant disease in young people, and then the risks of being unvaccinated go up substantially.”
But she also knows nobody has a crystal ball, and right now, everything seems to be trending in the right direction with COVID. That makes the risk-to-benefit consideration murkier.
“The question in my mind is, what is the risk of side effects from the vaccine?” she said. “I think we really need to know what the safety profile of vaccine looks like in children because we do have a decent understanding now what risk from disease looks like, because it’s small, but we are seeing it.”
Dr. Thielen said she’ll be keeping an eye on the next meeting of the CDC’s Advisory Committee on Immunization Practices for more answers.
A version of this article first appeared on Medscape.com.
Testing COVID-19 vaccines in young children is going to be tricky. Deciding how to approve them and who should get them may be even more difficult.
So far, the vaccines available to Americans ages 12 and up have sailed through the U.S. Food and Drug Administration’s regulatory checks, taking advantage of an accelerated clearance process called an Emergency Use Authorization (EUA).
EUAs set a lower bar for effectiveness, saying the vaccines may be safe and effective based on just a few months of data.
But with COVID cases plummeting in the United States and children historically seeing far less serious disease than adults, a panel of expert advisors to the FDA was asked to deliberate on Thursday whether the agency could consider vaccines for this age group under the same standard.
Stated another way: Is COVID an emergency for kids?
There’s another wrinkle in the mix, too – heart inflammation, which appears to be a very rare emerging adverse event tied to vaccination. It seems to happen more often in teens and young adults. To date, cases of myocarditis and pericarditis appear to be happening in 16 to 30 people for every 1 million doses given.
But if it is conclusively linked to the shots, some wonder whether it might tip the balance between benefits and risks for kids.
That left some of the experts who sit on the FDA’s advisory committee for vaccines and related biological products urging the FDA to take its time and more thoroughly study the shots before they’re given to millions of children.
Vaccine studies different in children?
Clinical studies of the vaccines in teens and adults have thus far relied on some straightforward math. You take two groups of similar people. You give half the vaccine and half a placebo. Then you wait and see which group has more symptomatic infections. To date, the vaccines have dramatically cut the risk of getting severely ill with COVID for every age group tested.
But COVID infections are falling rapidly in the U.S., and that may make it more difficult for researchers to conduct a similar kind of experiment in children.
The FDA is considering different approaches to figure out whether a vaccine would be effective in kids, including something called an “immunobridging trial.”
In bridging trials, researchers don’t look for infections; rather, they look for proven signs that someone has developed immunity, like antibody levels. Those biomarkers are then compared to the immune responses of younger adults who have demonstrated good protection against infection.
The main advantage of bridging studies is speed. It’s possible to get a snapshot of how the immune system responds to a vaccine within weeks of the final dose.
The drawback is that researchers don’t know exactly what to look for to judge how well the shots are generating protection.
That’s made even more difficult because kids’ immune systems are still developing, so it may be tough to draw direct parallels to adults.
“We don’t know what the serologic correlate of immunity is now. We don’t know how much antibody you have to get in order to be protected. We don’t know what the role of T cells will be,” said H. Cody Meissner, MD, chief of the division of pediatric infectious disease at Tufts Medical Center, Boston.
“I have so much sympathy for the FDA because these are enormous problems, and you have to make a decision,” said Dr. Meissner, who is a member of the FDA’s vaccines and related biological products advisory committee.
Speed vaccines to market, or gather more data?
The plummeting rate of infections in the United States also means that it may be more difficult for the FDA to justify allowing a vaccine on the market for emergency use for children under age 12.
In its recent advisory committee meeting, the agency asked the panel whether it should consider COVID vaccines for children under an EUA or a biologics license application (BLA), aka full approval.
A BLA typically means the agency considers a year or two of data on a new product, rather than just 2 months’ worth. Emergency use also allows products on the market under a looser standard – they “may be” safe and effective, instead of has been proven to be safe and effective.
Several committee members said they didn’t feel the United States was still in an emergency with COVID and couldn’t see the FDA allowing a vaccine to be used in kids that wasn’t given the agency’s highest level of scrutiny, particularly with reports of adverse events like myocarditis coming to light.
“I just want to be sure the price we pay for vaccinating millions of children justifies the side effects, and I don’t think we know that yet,” Dr. Meissner said.
Others acknowledged that there was little risk to kids now with infections on the decline but said that picture could change as variants spread, schools reopen, and colder temperatures force people indoors.
The FDA must decide whether to act based on where we are now or where we could be in a few months.
“I think it’s the million-dollar question right now,” said Hannah Kirking, MD, a medical epidemiologist with the Centers for Disease Control and Prevention who presented new and unpublished data on COVID’s impact in children to the FDA’s advisory committee.
She said prospective studies tracking the way COVID moves through a household with weekly testing from New York City and Utah had found that children catch and transmit COVID almost as readily as adults. But they don’t usually get as sick as adults do, so their cases are easy to miss.
She also presented the results of blood tests from samples around the country looking for evidence of past infection. In these seroprevalence studies, about 27% of children under age 17 had antibodies to COVID – the most of any age group. So more than 1 in 4 kids already has some natural immunity.
That means the main benefit of vaccinating children might be the protection of others, while they still bear the risks – however tiny.
Some experts felt that wasn’t enough reason to justify mass distribution of the vaccines to kids, and from a regulatory standpoint, it might not be permissible.
“FDA can only approve a medical product in a population if the benefits outweigh the risks in that population,” said Peter Doshi, PhD, assistant professor of pharmaceutical health services research in the University of Maryland’s school of pharmacy, Baltimore.
“If benefits don’t outweigh risks in children, it can’t be indicated for children. Full stop,” said Dr. Doshi, who is also an editor at the BMJ.
He said there’s another way to give children access to vaccines, through an expanded access or compassionate use program. Because most COVID deaths have been in children with underlying health conditions, Dr. Doshi and others said it might make sense to allow expanded access – which would get vaccines to children at high risk for complications – without turning them loose on millions before they are more thoroughly studied.
“It’s not a particularly attractive option for industry, because there’s no money to be made. Your medicine can’t be commercialized under expanded access. The most you can reap is manufacturing cost, which is not a lot,” he said.
Art Caplan, a professor of bioethics at New York University’s Langone medical center, said the argument for vaccinating children for flu falls along the same lines. The benefit-to-risk ratio is finely balanced in children. The main value of protecting them is to protect others.
“Flu rarely kills young folks. But you’re really trying to protect old folks and that’s the classic example,” he said.
What’s more, he said the idea that children would take on some risk with a vaccine for little personal benefit is oversimplified.
“Yes, you might get vaccinated to prevent harm to others, but those others are providing benefits to you. It’s not a one-way street. I think that’s a little morally distorted,” Mr. Caplan said. “Being able to keep society open benefits kids and adults alike.”
Other committee members felt like it was too early to sound the all-clear on COVID and said the FDA should authorize vaccines for children as quickly as it had for other age groups.
“We are still, I believe, in an emergency situation. I think that when this virus goes into our children, which is what it’s going to do, that will give it an incubator to change,” said Oveta Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan, Ann Arbor.
Fuller said that for the good of the world, Americans needed to vaccinate children to prevent the virus from mutating and creating new and potentially more dangerous variants.
Weighing risk over safety
Beth Thielen, MD, PhD, pediatric infectious disease specialist and virologist at the University of Minnesota, Minneapolis, said she had not followed the committee’s discussions, but about once a month she treats kids who are very sick because of the virus – either because of a COVID infection or because of multisystem inflammatory syndrome (MIS-C), an inflammatory reaction that strikes after infection.
She’s worried about how the virus has already changed. She said the kind of disease she’s seeing in kids now is different than what she saw in the early months of the pandemic.
“In the last couple of months, I’ve actually seen a few cases of severe pulmonary disease, more similar to adult disease in children,” Dr. Thielen said. “I see on the horizon that we could start seeing more significant disease in young people, and then the risks of being unvaccinated go up substantially.”
But she also knows nobody has a crystal ball, and right now, everything seems to be trending in the right direction with COVID. That makes the risk-to-benefit consideration murkier.
“The question in my mind is, what is the risk of side effects from the vaccine?” she said. “I think we really need to know what the safety profile of vaccine looks like in children because we do have a decent understanding now what risk from disease looks like, because it’s small, but we are seeing it.”
Dr. Thielen said she’ll be keeping an eye on the next meeting of the CDC’s Advisory Committee on Immunization Practices for more answers.
A version of this article first appeared on Medscape.com.
Is trouble falling asleep a modifiable risk factor for dementia?
, new research suggests.
Trouble falling asleep “may be a modifiable risk factor for later-life cognitive impairment and dementia,” said lead author Afsara Zaheed, a PhD candidate in clinical science, department of psychology, University of Michigan, Ann Arbor.
“Patients should also be aware of the importance of insomnia on cognitive functioning so that they can bring up these concerns with their providers early,” she said.
The findings were presented at Virtual SLEEP 2021, the 35th Annual Meeting of the Associated Professional Sleep Societies.
Poor sleep common with age
As many as one-half of older adults report having poor sleep quality and insomnia, and growing evidence suggests that insomnia may be a unique risk factor for cognitive decline in later life, Ms. Zaheed explained.
To investigate further, the researchers analyzed data on 2,496 adults aged 51 years and older who were participants in the Health and Retirement Study, a longitudinal study of aging in a nationally representative population of older adults.
In 2002, participants were asked how often they had trouble falling asleep, woke up during the night, woke up too early, and were not able to fall asleep again and how often they felt really rested when they woke up in the morning.
In 2016, participants’ cognition was assessed using a battery of neuropsychological tests that gauged episodic memory, executive function, language, visuospatial/construction, and processing speed.
Analyses controlled for sociodemographics, baseline global cognitive performance, and the influence of depressive symptoms and vascular disease.
Compared with other insomnia symptoms, having difficulty falling asleep in 2002 was the main insomnia symptom that was predictive of cognitive impairment 14 years later, in 2016.
More frequent trouble falling asleep was predictive of poorer episodic memory, executive function, language, processing speed, and visuospatial performance.
The associations between sleep initiation and later cognitive impairment were partially explained by depressive symptoms and vascular disease burden for all domains except episodic memory, which was only partially explained by depressive symptoms.
Unclear mechanism
Ms. Zaheed said research is needed to uncover neurophysiologic mechanisms underlying the observed associations. “It may be that chronic difficulty with falling asleep is associated with inflammatory or metabolic processes that negatively affect brain structure and function over time,” she said.
“Insomnia has also been linked with higher accumulation of protein aggregates in the brain that disrupt cell communication and are characteristic of late-life disorders such as Alzheimer’s disease,” she added.
“While our project did not directly investigate these potential causal pathways between insomnia and cognition, our results suggest that investigating these potential mechanisms is an important area for future research,” Ms. Zaheed said.
“While additional intervention research is needed to determine whether targeting insomnia in older patients can have lasting cognitive benefits, results from this study suggest that discussing insomnia symptoms at the primary care level may be beneficial for both doctors and patients,” she added.
“By targeting insomnia – for example, through an evidence-based cognitive–behavioral therapy approach – individuals may improve various mental and physical health outcomes in addition to improving their sleep quality,” Ms. Zaheed said.
Reached for comment, Shaheen E. Lakhan, MD, PhD, neurologist in Newton, Massachusetts, said, “There is a strong link between chronic sleep disturbances and cognitive impairment, including dementia.”
“This study further supports this link and specifically calls out initiating sleep (as opposed to staying asleep) as the culprit. It also raises the hypothesis that the link is primarily mediated by depression and vascular disease; however, the verdict is still out,” said Dr. Lakhan.
The study was funded by the National Institute on Aging. Ms. Zaheed and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Trouble falling asleep “may be a modifiable risk factor for later-life cognitive impairment and dementia,” said lead author Afsara Zaheed, a PhD candidate in clinical science, department of psychology, University of Michigan, Ann Arbor.
“Patients should also be aware of the importance of insomnia on cognitive functioning so that they can bring up these concerns with their providers early,” she said.
The findings were presented at Virtual SLEEP 2021, the 35th Annual Meeting of the Associated Professional Sleep Societies.
Poor sleep common with age
As many as one-half of older adults report having poor sleep quality and insomnia, and growing evidence suggests that insomnia may be a unique risk factor for cognitive decline in later life, Ms. Zaheed explained.
To investigate further, the researchers analyzed data on 2,496 adults aged 51 years and older who were participants in the Health and Retirement Study, a longitudinal study of aging in a nationally representative population of older adults.
In 2002, participants were asked how often they had trouble falling asleep, woke up during the night, woke up too early, and were not able to fall asleep again and how often they felt really rested when they woke up in the morning.
In 2016, participants’ cognition was assessed using a battery of neuropsychological tests that gauged episodic memory, executive function, language, visuospatial/construction, and processing speed.
Analyses controlled for sociodemographics, baseline global cognitive performance, and the influence of depressive symptoms and vascular disease.
Compared with other insomnia symptoms, having difficulty falling asleep in 2002 was the main insomnia symptom that was predictive of cognitive impairment 14 years later, in 2016.
More frequent trouble falling asleep was predictive of poorer episodic memory, executive function, language, processing speed, and visuospatial performance.
The associations between sleep initiation and later cognitive impairment were partially explained by depressive symptoms and vascular disease burden for all domains except episodic memory, which was only partially explained by depressive symptoms.
Unclear mechanism
Ms. Zaheed said research is needed to uncover neurophysiologic mechanisms underlying the observed associations. “It may be that chronic difficulty with falling asleep is associated with inflammatory or metabolic processes that negatively affect brain structure and function over time,” she said.
“Insomnia has also been linked with higher accumulation of protein aggregates in the brain that disrupt cell communication and are characteristic of late-life disorders such as Alzheimer’s disease,” she added.
“While our project did not directly investigate these potential causal pathways between insomnia and cognition, our results suggest that investigating these potential mechanisms is an important area for future research,” Ms. Zaheed said.
“While additional intervention research is needed to determine whether targeting insomnia in older patients can have lasting cognitive benefits, results from this study suggest that discussing insomnia symptoms at the primary care level may be beneficial for both doctors and patients,” she added.
“By targeting insomnia – for example, through an evidence-based cognitive–behavioral therapy approach – individuals may improve various mental and physical health outcomes in addition to improving their sleep quality,” Ms. Zaheed said.
Reached for comment, Shaheen E. Lakhan, MD, PhD, neurologist in Newton, Massachusetts, said, “There is a strong link between chronic sleep disturbances and cognitive impairment, including dementia.”
“This study further supports this link and specifically calls out initiating sleep (as opposed to staying asleep) as the culprit. It also raises the hypothesis that the link is primarily mediated by depression and vascular disease; however, the verdict is still out,” said Dr. Lakhan.
The study was funded by the National Institute on Aging. Ms. Zaheed and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Trouble falling asleep “may be a modifiable risk factor for later-life cognitive impairment and dementia,” said lead author Afsara Zaheed, a PhD candidate in clinical science, department of psychology, University of Michigan, Ann Arbor.
“Patients should also be aware of the importance of insomnia on cognitive functioning so that they can bring up these concerns with their providers early,” she said.
The findings were presented at Virtual SLEEP 2021, the 35th Annual Meeting of the Associated Professional Sleep Societies.
Poor sleep common with age
As many as one-half of older adults report having poor sleep quality and insomnia, and growing evidence suggests that insomnia may be a unique risk factor for cognitive decline in later life, Ms. Zaheed explained.
To investigate further, the researchers analyzed data on 2,496 adults aged 51 years and older who were participants in the Health and Retirement Study, a longitudinal study of aging in a nationally representative population of older adults.
In 2002, participants were asked how often they had trouble falling asleep, woke up during the night, woke up too early, and were not able to fall asleep again and how often they felt really rested when they woke up in the morning.
In 2016, participants’ cognition was assessed using a battery of neuropsychological tests that gauged episodic memory, executive function, language, visuospatial/construction, and processing speed.
Analyses controlled for sociodemographics, baseline global cognitive performance, and the influence of depressive symptoms and vascular disease.
Compared with other insomnia symptoms, having difficulty falling asleep in 2002 was the main insomnia symptom that was predictive of cognitive impairment 14 years later, in 2016.
More frequent trouble falling asleep was predictive of poorer episodic memory, executive function, language, processing speed, and visuospatial performance.
The associations between sleep initiation and later cognitive impairment were partially explained by depressive symptoms and vascular disease burden for all domains except episodic memory, which was only partially explained by depressive symptoms.
Unclear mechanism
Ms. Zaheed said research is needed to uncover neurophysiologic mechanisms underlying the observed associations. “It may be that chronic difficulty with falling asleep is associated with inflammatory or metabolic processes that negatively affect brain structure and function over time,” she said.
“Insomnia has also been linked with higher accumulation of protein aggregates in the brain that disrupt cell communication and are characteristic of late-life disorders such as Alzheimer’s disease,” she added.
“While our project did not directly investigate these potential causal pathways between insomnia and cognition, our results suggest that investigating these potential mechanisms is an important area for future research,” Ms. Zaheed said.
“While additional intervention research is needed to determine whether targeting insomnia in older patients can have lasting cognitive benefits, results from this study suggest that discussing insomnia symptoms at the primary care level may be beneficial for both doctors and patients,” she added.
“By targeting insomnia – for example, through an evidence-based cognitive–behavioral therapy approach – individuals may improve various mental and physical health outcomes in addition to improving their sleep quality,” Ms. Zaheed said.
Reached for comment, Shaheen E. Lakhan, MD, PhD, neurologist in Newton, Massachusetts, said, “There is a strong link between chronic sleep disturbances and cognitive impairment, including dementia.”
“This study further supports this link and specifically calls out initiating sleep (as opposed to staying asleep) as the culprit. It also raises the hypothesis that the link is primarily mediated by depression and vascular disease; however, the verdict is still out,” said Dr. Lakhan.
The study was funded by the National Institute on Aging. Ms. Zaheed and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Insomnia in children tied to mood and anxiety disorders in adulthood
, new research indicates. However, insomnia symptoms in childhood that remit in the transition to adolescence do not confer increased risk of mood or anxiety disorders later on, the study found.
“As insomnia symptoms may precipitate or maintain internalizing disorders, our findings further reinforce the need for early sleep interventions to prevent future mental health disorders,” said lead investigator Julio Fernandez-Mendoza, PhD, associate professor at Penn State University, Hershey.
He presented his research at Virtual SLEEP 2021, the 35th annual meeting of the Associated Professional Sleep Societies.
Results ‘very clear’
The findings are based on data from the Penn State Child Cohort, a longitudinal, population-based sample of 700 children with a median age of 9 years, including 421 who were followed up 8 years later as adolescents (median age, 16 years) and 502 who were followed up 15 years later as young adults (median age, 24 years).
The data are “very clear that the risk of having internalizing disorders in young adulthood associated with having persistent insomnia symptoms, since childhood through adolescence into young adulthood,” Dr. Fernandez-Mendoza said in his presentation.
A persistent developmental trajectory was associated with a threefold increased risk of adult internalizing disorder (hazard ratio, 3.19).
The risk of having an internalizing disorder in young adulthood associated with newly developing (incident) insomnia symptoms is about twofold higher (HR, 1.94), whereas the risk associated with the waxing and waning pattern of insomnia is 1.5-fold (HR, 1.53) higher and only marginally significant, he reported.
An equally important finding, said Dr. Fernandez-Mendoza, is that those who had remitted insomnia symptoms in the transition to adolescence and throughout young adulthood were not at increased risk of having an internalizing disorder in young adulthood.
“Insomnia symptoms in a persistent manner associated with long-term adverse mental health outcomes, but remission of those insomnia symptoms associated with a good prognosis,” he said.
It’s also important to note, he said, that about 40% of children do not outgrow their insomnia symptoms in the transition to adolescence and are at risk of developing mental health disorders later on during early adulthood.
Reached for comment, Nitun Verma, MD, a spokesperson for the American Academy of Sleep Medicine, said: “There is a connection with mood and anxiety disorders with sleep, especially insomnia. This is a good reminder that reviewing someone’s sleep habits should always be a part of assessing someone’s mental health.”
A version of this article first appeared on Medscape.com.
, new research indicates. However, insomnia symptoms in childhood that remit in the transition to adolescence do not confer increased risk of mood or anxiety disorders later on, the study found.
“As insomnia symptoms may precipitate or maintain internalizing disorders, our findings further reinforce the need for early sleep interventions to prevent future mental health disorders,” said lead investigator Julio Fernandez-Mendoza, PhD, associate professor at Penn State University, Hershey.
He presented his research at Virtual SLEEP 2021, the 35th annual meeting of the Associated Professional Sleep Societies.
Results ‘very clear’
The findings are based on data from the Penn State Child Cohort, a longitudinal, population-based sample of 700 children with a median age of 9 years, including 421 who were followed up 8 years later as adolescents (median age, 16 years) and 502 who were followed up 15 years later as young adults (median age, 24 years).
The data are “very clear that the risk of having internalizing disorders in young adulthood associated with having persistent insomnia symptoms, since childhood through adolescence into young adulthood,” Dr. Fernandez-Mendoza said in his presentation.
A persistent developmental trajectory was associated with a threefold increased risk of adult internalizing disorder (hazard ratio, 3.19).
The risk of having an internalizing disorder in young adulthood associated with newly developing (incident) insomnia symptoms is about twofold higher (HR, 1.94), whereas the risk associated with the waxing and waning pattern of insomnia is 1.5-fold (HR, 1.53) higher and only marginally significant, he reported.
An equally important finding, said Dr. Fernandez-Mendoza, is that those who had remitted insomnia symptoms in the transition to adolescence and throughout young adulthood were not at increased risk of having an internalizing disorder in young adulthood.
“Insomnia symptoms in a persistent manner associated with long-term adverse mental health outcomes, but remission of those insomnia symptoms associated with a good prognosis,” he said.
It’s also important to note, he said, that about 40% of children do not outgrow their insomnia symptoms in the transition to adolescence and are at risk of developing mental health disorders later on during early adulthood.
Reached for comment, Nitun Verma, MD, a spokesperson for the American Academy of Sleep Medicine, said: “There is a connection with mood and anxiety disorders with sleep, especially insomnia. This is a good reminder that reviewing someone’s sleep habits should always be a part of assessing someone’s mental health.”
A version of this article first appeared on Medscape.com.
, new research indicates. However, insomnia symptoms in childhood that remit in the transition to adolescence do not confer increased risk of mood or anxiety disorders later on, the study found.
“As insomnia symptoms may precipitate or maintain internalizing disorders, our findings further reinforce the need for early sleep interventions to prevent future mental health disorders,” said lead investigator Julio Fernandez-Mendoza, PhD, associate professor at Penn State University, Hershey.
He presented his research at Virtual SLEEP 2021, the 35th annual meeting of the Associated Professional Sleep Societies.
Results ‘very clear’
The findings are based on data from the Penn State Child Cohort, a longitudinal, population-based sample of 700 children with a median age of 9 years, including 421 who were followed up 8 years later as adolescents (median age, 16 years) and 502 who were followed up 15 years later as young adults (median age, 24 years).
The data are “very clear that the risk of having internalizing disorders in young adulthood associated with having persistent insomnia symptoms, since childhood through adolescence into young adulthood,” Dr. Fernandez-Mendoza said in his presentation.
A persistent developmental trajectory was associated with a threefold increased risk of adult internalizing disorder (hazard ratio, 3.19).
The risk of having an internalizing disorder in young adulthood associated with newly developing (incident) insomnia symptoms is about twofold higher (HR, 1.94), whereas the risk associated with the waxing and waning pattern of insomnia is 1.5-fold (HR, 1.53) higher and only marginally significant, he reported.
An equally important finding, said Dr. Fernandez-Mendoza, is that those who had remitted insomnia symptoms in the transition to adolescence and throughout young adulthood were not at increased risk of having an internalizing disorder in young adulthood.
“Insomnia symptoms in a persistent manner associated with long-term adverse mental health outcomes, but remission of those insomnia symptoms associated with a good prognosis,” he said.
It’s also important to note, he said, that about 40% of children do not outgrow their insomnia symptoms in the transition to adolescence and are at risk of developing mental health disorders later on during early adulthood.
Reached for comment, Nitun Verma, MD, a spokesperson for the American Academy of Sleep Medicine, said: “There is a connection with mood and anxiety disorders with sleep, especially insomnia. This is a good reminder that reviewing someone’s sleep habits should always be a part of assessing someone’s mental health.”
A version of this article first appeared on Medscape.com.
BBB integrity linked to cortical spreading depression
CSD has been linked to migraine aura, but a connection to pain symptoms is uncertain. “There’s just a lack of knowledge. We don’t understand migraine pathophysiology,” said Michael G. Harrington, MBChB, who was asked to comment on the study.
“The evidence for altered transport across the barrier in cortical spreading depression and the associated aura of migraine is pretty strong. The evidence for regular migraine, not so. In fact, there’s really no strong evidence for leakage in those people, and so it is still unresolved whether this initial cortical spreading depression that occurs in aura then triggers migraine afterwards, because it’s occurring during the aura. And in people who do not have the aura, is there a silent cortical spreading depression phenomenon with some leakage that triggers the migraine? That question is definitely not answered,” said Dr. Harrington, a research professor of neurology at the University of Southern California, Los Angeles.
Leakage of the BBB might allow passage of nociceptive compounds that could trigger migraine. Loss of BBB integrity has also been seen in other central nervous system pain disorders, suggesting that alterations to BBB functioning could have broader implications.
“In this model that we’re using, we’re seeing loss of overall barrier integrity, which lends itself to a whole cascade of further pathological possibilities,” Jared Wahl, a PhD candidate at the University of Arizona, Tucson, said in an interview. He presented the research at the American Headache Society’s 2021 annual meeting.
A leaky BBB could allow infiltration of a range of substances, but the potential for such a mechanism in migraine pathology is not well understood.
The researchers specifically investigated the potential role of claudin-5 in the tight junction (TJ) region of the BBB. The decision was made in part because the proteins involved in the BBB are difficult to study, and there is some familiarity with claudin-5, according to Mr. Wahl. ”Of all the proteins that are out there, for claudin-5 (there are) somewhat better techniques and products available to work with, and there’s been some previous research done to show that it’s implicated in blood brain barrier pathology. So it seemed like a good candidate to start with investigating this whole possible pathophysiological link between barrier disruption and migration of pronociceptive substances into the CNS during migraine attacks,” he said. The claudin proteins are also the major components of the tight junctions that seal off gaps between endothelial cells along the BBB.
Dynamic changes seen in the in vitro model
To simulate a CSD event, the researchers pulsed cultured cells for 5 minutes with astrocyte-conditioned media, artificial cerebrospinal fluid, KCl, glutamate, altered pH, or adenosine triphosphate (ATP). They used trans endothelial electrical resistance (TEER) to quickly and qualitatively screen for loss of barrier integrity, which is characterized by loss of electrical resistance. To quantify the magnitude of a breach, the researchers applied carbon-14 (C14)–labeled sucrose to one side of the barrier, and determined the amount of labeled sucrose transmitted to the other side of the barrier.
ATP and pH pulses that were outside normal physiological limits led to permeability. The team then used immunocytochemistry assays to visualize the condition of the model BBB, and found discontinuity of the tight junction membranes. Imaging of claudin-5 showed organizational changes within the tight junction, but there was no change in expression level, suggesting that the alterations were due to dynamic reorganization, according to Mr. Wahl.
Transient openings could allow passage of molecules such as bradykinin, calcitonin gene-related peptide (CGRP), and substance P, which could go on to affect the trigeminal nerve complex and trigger a migraine. “That’s sort of the crux of a lot of this migraine research, is gluing this physiological (mechanism) to how it is actually activating the CNS. And this is sort of where we’re going with it at the moment,” said Mr. Wahl.
Next steps
The researchers next plan to generate a cell line with claudin-5 linked to green fluorescent protein, then use confocal microscopy to image claudin-5 in real time as the BBB model responds to a simulated CSD.
Another important step will be to link physiological findings like those presented by Mr. Wahl to migraine-specific mechanisms. The results from this model will need to be expanded to include more than endothelial cells, especially astrocytes, pericytes, and neurons, as well as organoids, brain slices, or in vivo animal models, according to Dr. Harrington. “I think you could try and block the changes in occludin [another protein in the tight junction] or claudin-5 to see if, under the same provocation, that prevented the changes in a migraine model. That would be a direct way of connecting from CSD to migraine,” said Dr. Harrington.
If BBB disruption is confirmed to play an important role in migraine, and claudin-5 or other specific proteins are confirmed to be the cause, it could have clinical implications. A drug that could prevent those changes in the proteins and prevent a leak in the BBB could be a migraine preventative. “That could help prevent things like nociceptive substances migrating into the CNS, and could possibly be a well-tolerated drug target that doesn’t have the side effects or the overuse problems that a lot of stuff on the market has today,” said Mr. Wahl.
CSD has been linked to migraine aura, but a connection to pain symptoms is uncertain. “There’s just a lack of knowledge. We don’t understand migraine pathophysiology,” said Michael G. Harrington, MBChB, who was asked to comment on the study.
“The evidence for altered transport across the barrier in cortical spreading depression and the associated aura of migraine is pretty strong. The evidence for regular migraine, not so. In fact, there’s really no strong evidence for leakage in those people, and so it is still unresolved whether this initial cortical spreading depression that occurs in aura then triggers migraine afterwards, because it’s occurring during the aura. And in people who do not have the aura, is there a silent cortical spreading depression phenomenon with some leakage that triggers the migraine? That question is definitely not answered,” said Dr. Harrington, a research professor of neurology at the University of Southern California, Los Angeles.
Leakage of the BBB might allow passage of nociceptive compounds that could trigger migraine. Loss of BBB integrity has also been seen in other central nervous system pain disorders, suggesting that alterations to BBB functioning could have broader implications.
“In this model that we’re using, we’re seeing loss of overall barrier integrity, which lends itself to a whole cascade of further pathological possibilities,” Jared Wahl, a PhD candidate at the University of Arizona, Tucson, said in an interview. He presented the research at the American Headache Society’s 2021 annual meeting.
A leaky BBB could allow infiltration of a range of substances, but the potential for such a mechanism in migraine pathology is not well understood.
The researchers specifically investigated the potential role of claudin-5 in the tight junction (TJ) region of the BBB. The decision was made in part because the proteins involved in the BBB are difficult to study, and there is some familiarity with claudin-5, according to Mr. Wahl. ”Of all the proteins that are out there, for claudin-5 (there are) somewhat better techniques and products available to work with, and there’s been some previous research done to show that it’s implicated in blood brain barrier pathology. So it seemed like a good candidate to start with investigating this whole possible pathophysiological link between barrier disruption and migration of pronociceptive substances into the CNS during migraine attacks,” he said. The claudin proteins are also the major components of the tight junctions that seal off gaps between endothelial cells along the BBB.
Dynamic changes seen in the in vitro model
To simulate a CSD event, the researchers pulsed cultured cells for 5 minutes with astrocyte-conditioned media, artificial cerebrospinal fluid, KCl, glutamate, altered pH, or adenosine triphosphate (ATP). They used trans endothelial electrical resistance (TEER) to quickly and qualitatively screen for loss of barrier integrity, which is characterized by loss of electrical resistance. To quantify the magnitude of a breach, the researchers applied carbon-14 (C14)–labeled sucrose to one side of the barrier, and determined the amount of labeled sucrose transmitted to the other side of the barrier.
ATP and pH pulses that were outside normal physiological limits led to permeability. The team then used immunocytochemistry assays to visualize the condition of the model BBB, and found discontinuity of the tight junction membranes. Imaging of claudin-5 showed organizational changes within the tight junction, but there was no change in expression level, suggesting that the alterations were due to dynamic reorganization, according to Mr. Wahl.
Transient openings could allow passage of molecules such as bradykinin, calcitonin gene-related peptide (CGRP), and substance P, which could go on to affect the trigeminal nerve complex and trigger a migraine. “That’s sort of the crux of a lot of this migraine research, is gluing this physiological (mechanism) to how it is actually activating the CNS. And this is sort of where we’re going with it at the moment,” said Mr. Wahl.
Next steps
The researchers next plan to generate a cell line with claudin-5 linked to green fluorescent protein, then use confocal microscopy to image claudin-5 in real time as the BBB model responds to a simulated CSD.
Another important step will be to link physiological findings like those presented by Mr. Wahl to migraine-specific mechanisms. The results from this model will need to be expanded to include more than endothelial cells, especially astrocytes, pericytes, and neurons, as well as organoids, brain slices, or in vivo animal models, according to Dr. Harrington. “I think you could try and block the changes in occludin [another protein in the tight junction] or claudin-5 to see if, under the same provocation, that prevented the changes in a migraine model. That would be a direct way of connecting from CSD to migraine,” said Dr. Harrington.
If BBB disruption is confirmed to play an important role in migraine, and claudin-5 or other specific proteins are confirmed to be the cause, it could have clinical implications. A drug that could prevent those changes in the proteins and prevent a leak in the BBB could be a migraine preventative. “That could help prevent things like nociceptive substances migrating into the CNS, and could possibly be a well-tolerated drug target that doesn’t have the side effects or the overuse problems that a lot of stuff on the market has today,” said Mr. Wahl.
CSD has been linked to migraine aura, but a connection to pain symptoms is uncertain. “There’s just a lack of knowledge. We don’t understand migraine pathophysiology,” said Michael G. Harrington, MBChB, who was asked to comment on the study.
“The evidence for altered transport across the barrier in cortical spreading depression and the associated aura of migraine is pretty strong. The evidence for regular migraine, not so. In fact, there’s really no strong evidence for leakage in those people, and so it is still unresolved whether this initial cortical spreading depression that occurs in aura then triggers migraine afterwards, because it’s occurring during the aura. And in people who do not have the aura, is there a silent cortical spreading depression phenomenon with some leakage that triggers the migraine? That question is definitely not answered,” said Dr. Harrington, a research professor of neurology at the University of Southern California, Los Angeles.
Leakage of the BBB might allow passage of nociceptive compounds that could trigger migraine. Loss of BBB integrity has also been seen in other central nervous system pain disorders, suggesting that alterations to BBB functioning could have broader implications.
“In this model that we’re using, we’re seeing loss of overall barrier integrity, which lends itself to a whole cascade of further pathological possibilities,” Jared Wahl, a PhD candidate at the University of Arizona, Tucson, said in an interview. He presented the research at the American Headache Society’s 2021 annual meeting.
A leaky BBB could allow infiltration of a range of substances, but the potential for such a mechanism in migraine pathology is not well understood.
The researchers specifically investigated the potential role of claudin-5 in the tight junction (TJ) region of the BBB. The decision was made in part because the proteins involved in the BBB are difficult to study, and there is some familiarity with claudin-5, according to Mr. Wahl. ”Of all the proteins that are out there, for claudin-5 (there are) somewhat better techniques and products available to work with, and there’s been some previous research done to show that it’s implicated in blood brain barrier pathology. So it seemed like a good candidate to start with investigating this whole possible pathophysiological link between barrier disruption and migration of pronociceptive substances into the CNS during migraine attacks,” he said. The claudin proteins are also the major components of the tight junctions that seal off gaps between endothelial cells along the BBB.
Dynamic changes seen in the in vitro model
To simulate a CSD event, the researchers pulsed cultured cells for 5 minutes with astrocyte-conditioned media, artificial cerebrospinal fluid, KCl, glutamate, altered pH, or adenosine triphosphate (ATP). They used trans endothelial electrical resistance (TEER) to quickly and qualitatively screen for loss of barrier integrity, which is characterized by loss of electrical resistance. To quantify the magnitude of a breach, the researchers applied carbon-14 (C14)–labeled sucrose to one side of the barrier, and determined the amount of labeled sucrose transmitted to the other side of the barrier.
ATP and pH pulses that were outside normal physiological limits led to permeability. The team then used immunocytochemistry assays to visualize the condition of the model BBB, and found discontinuity of the tight junction membranes. Imaging of claudin-5 showed organizational changes within the tight junction, but there was no change in expression level, suggesting that the alterations were due to dynamic reorganization, according to Mr. Wahl.
Transient openings could allow passage of molecules such as bradykinin, calcitonin gene-related peptide (CGRP), and substance P, which could go on to affect the trigeminal nerve complex and trigger a migraine. “That’s sort of the crux of a lot of this migraine research, is gluing this physiological (mechanism) to how it is actually activating the CNS. And this is sort of where we’re going with it at the moment,” said Mr. Wahl.
Next steps
The researchers next plan to generate a cell line with claudin-5 linked to green fluorescent protein, then use confocal microscopy to image claudin-5 in real time as the BBB model responds to a simulated CSD.
Another important step will be to link physiological findings like those presented by Mr. Wahl to migraine-specific mechanisms. The results from this model will need to be expanded to include more than endothelial cells, especially astrocytes, pericytes, and neurons, as well as organoids, brain slices, or in vivo animal models, according to Dr. Harrington. “I think you could try and block the changes in occludin [another protein in the tight junction] or claudin-5 to see if, under the same provocation, that prevented the changes in a migraine model. That would be a direct way of connecting from CSD to migraine,” said Dr. Harrington.
If BBB disruption is confirmed to play an important role in migraine, and claudin-5 or other specific proteins are confirmed to be the cause, it could have clinical implications. A drug that could prevent those changes in the proteins and prevent a leak in the BBB could be a migraine preventative. “That could help prevent things like nociceptive substances migrating into the CNS, and could possibly be a well-tolerated drug target that doesn’t have the side effects or the overuse problems that a lot of stuff on the market has today,” said Mr. Wahl.
FROM AHS 2021
No increased risk of hypertension with erenumab?
, easing earlier concerns that this may be one of the drug’s adverse events, an analysis of postmarketing data shows. Nevertheless, investigators noted that more research is needed to confirm that this is the case.
While randomized clinical trials have shown no increased risk of hypertension related to the drug, it has been reported in postmarketing data. However, many of these events occurred in patients with previously documented hypertension or risk factors for the disorder, the investigators noted.
The rate of hypertension adverse events in postmarketing data was 0.144 per 100 person-years. Most such reports described only one instance of elevated blood pressure. In April 2020, the prescribing information for the drug was updated to include a mention of the risk of hypertension.
“Given the limitations of postmarketing reports, including incomplete information, lack of a control arm, and others, additional data are certainly needed to fully characterize the nature, the timing, and the extent to which hypertension is a risk associated with erenumab, and indeed other [calcitonin-gene-related peptide (CGRP)] pathway antagonists,” said study investigator David W. Dodick, MD, professor of neurology at the Mayo Clinic College of Medicine, Phoenix.
The findings were presented at the American Headache Society’s 2021 annual meeting.
No increased risk over time
A monoclonal antibody and CGRP antagonist, erenumab is approved in the United States for migraine prevention in adults. CGRP medications are vasodilators, and, therefore, migraine treatments that target this pathway could theoretically have hypertensive effects.
To assess the risk of hypertension in migraine patients treated with erenumab, investigators examined clinical trial and postmarketing data. The analysis included cases with limited information and patients with a different etiology for the development of hypertension.
Using Amgen Clinical Trial data, the researchers performed a pooled safety analysis of four placebo-controlled, double-blind phase 2 or 3 studies of the drug. Participants had episodic or chronic migraine and were between ages 18 and 60 years or age 65 years. The doses studied were 70 mg and 140 mg.
In these studies, blood pressure data for each patient were based on an average of at least two measurements taken after patients were in rested state for at least 5 minutes. The position used for blood pressure measurement for each patient was consistent throughout the study.
The investigators also analyzed postmarketing reports of hypertension from May 17, 2018, to Jan. 31, 2020, identified in Amgen Global Safety data.
The pooled studies included 1,043 participants receiving placebo, 893 receiving 70 mg of erenumab, and 507 receiving 140 mg of the drug. During the treatment phase, the incidence of hypertension was 0.9% among controls, 0.8% in the 70-mg group, and 0.2% in the 140-mg group. The proportion of patients who started a new antihypertensive medication was 1.2% in controls, 0.8% in the 70-mg group, and 0.2% in the 140-mg group.
In a long-term, open-label study, patients with episodic migraine received erenumab treatment for up to 5 years. The incidence of hypertension did not increase with time in this population.
The postmarketing data encompassed 245,682 person-years of erenumab exposure. The researchers identified 362 hypertension events (355 cases). The rate of these events was 0.144 per 100 person-years. The exposure-adjusted incidence of hypertension was 1.9 per 100 patient-years for erenumab.
Of the 362 hypertensive events, 158 (43.6%) were in patients with a medical history of hypertension or risk factors for hypertension. Information about the time to onset of hypertension was available for 121 (33.4%) adverse events.
Of this group, 56 (46.2%) occurred within 1 week of erenumab initiation, including 43 (35.5%) that occurred within 1 day. This rapid time to hypertension onset “is inconsistent with the pharmacokinetic profile of once-monthly erenumab 70 mg or 140 mg, which has a peak serum concentration in approximately 6 days,” said Dr. Dodick.
Hypertension ‘not a barrier’ to treatment
Commenting on the findings, Richard B. Lipton, MD, Edwin S. Lowe professor and vice chair of neurology at Albert Einstein College of Medicine, New York, noted that in theory blocking CGRP could increase blood pressure. However, he noted that the data and clinical experience do not suggest erenumab poses a serious risk.
“I have rarely seen new-onset hypertension with erenumab in the patients treated at our medical center. The few cases I have seen were in older adults with pre-existing hypertension,” he said.
The investigators’ review of clinical trial data together with global safety databases was a strength of their study, said Dr. Lipton. In clinical trials, patients are monitored carefully, and a placebo control group aids in the determination of background rates.
“In the trials, the incidence of new-onset hypertension or hypertension exacerbation was 0.9%, while the placebo rate was 0.2%,” said Dr. Lipton. “The absolute increase in the risk of hypertension was 0.6% or six cases per thousand: A low rate.” But clinical trials enroll carefully selected patients who do not represent the broad group of people treated with erenumab in clinical practice, he added.
The global safety data are more representative of patients who receive erenumab in real-world settings. The weaknesses of these data, though, are the lack of a control group and the incomplete ascertainment of data associated with spontaneous reporting.
Yet both types of studies, with their complementary strengths and weaknesses, indicated low rates of hypertension.
“The low incidence of hypertension, in my view, is not a barrier to the use of erenumab, though checking blood pressure after starting any new migraine therapy is prudent,” Dr. Lipton concluded.
The study was funded by Amgen. Dr. Dodick had no relevant disclosures. Dr. Lipton has consulted for and conducted studies funded by Amgen and by other companies that manufacture CGRP drugs. He has stock options in Biohaven.
A version of this article first appeared on Medscape.com.
, easing earlier concerns that this may be one of the drug’s adverse events, an analysis of postmarketing data shows. Nevertheless, investigators noted that more research is needed to confirm that this is the case.
While randomized clinical trials have shown no increased risk of hypertension related to the drug, it has been reported in postmarketing data. However, many of these events occurred in patients with previously documented hypertension or risk factors for the disorder, the investigators noted.
The rate of hypertension adverse events in postmarketing data was 0.144 per 100 person-years. Most such reports described only one instance of elevated blood pressure. In April 2020, the prescribing information for the drug was updated to include a mention of the risk of hypertension.
“Given the limitations of postmarketing reports, including incomplete information, lack of a control arm, and others, additional data are certainly needed to fully characterize the nature, the timing, and the extent to which hypertension is a risk associated with erenumab, and indeed other [calcitonin-gene-related peptide (CGRP)] pathway antagonists,” said study investigator David W. Dodick, MD, professor of neurology at the Mayo Clinic College of Medicine, Phoenix.
The findings were presented at the American Headache Society’s 2021 annual meeting.
No increased risk over time
A monoclonal antibody and CGRP antagonist, erenumab is approved in the United States for migraine prevention in adults. CGRP medications are vasodilators, and, therefore, migraine treatments that target this pathway could theoretically have hypertensive effects.
To assess the risk of hypertension in migraine patients treated with erenumab, investigators examined clinical trial and postmarketing data. The analysis included cases with limited information and patients with a different etiology for the development of hypertension.
Using Amgen Clinical Trial data, the researchers performed a pooled safety analysis of four placebo-controlled, double-blind phase 2 or 3 studies of the drug. Participants had episodic or chronic migraine and were between ages 18 and 60 years or age 65 years. The doses studied were 70 mg and 140 mg.
In these studies, blood pressure data for each patient were based on an average of at least two measurements taken after patients were in rested state for at least 5 minutes. The position used for blood pressure measurement for each patient was consistent throughout the study.
The investigators also analyzed postmarketing reports of hypertension from May 17, 2018, to Jan. 31, 2020, identified in Amgen Global Safety data.
The pooled studies included 1,043 participants receiving placebo, 893 receiving 70 mg of erenumab, and 507 receiving 140 mg of the drug. During the treatment phase, the incidence of hypertension was 0.9% among controls, 0.8% in the 70-mg group, and 0.2% in the 140-mg group. The proportion of patients who started a new antihypertensive medication was 1.2% in controls, 0.8% in the 70-mg group, and 0.2% in the 140-mg group.
In a long-term, open-label study, patients with episodic migraine received erenumab treatment for up to 5 years. The incidence of hypertension did not increase with time in this population.
The postmarketing data encompassed 245,682 person-years of erenumab exposure. The researchers identified 362 hypertension events (355 cases). The rate of these events was 0.144 per 100 person-years. The exposure-adjusted incidence of hypertension was 1.9 per 100 patient-years for erenumab.
Of the 362 hypertensive events, 158 (43.6%) were in patients with a medical history of hypertension or risk factors for hypertension. Information about the time to onset of hypertension was available for 121 (33.4%) adverse events.
Of this group, 56 (46.2%) occurred within 1 week of erenumab initiation, including 43 (35.5%) that occurred within 1 day. This rapid time to hypertension onset “is inconsistent with the pharmacokinetic profile of once-monthly erenumab 70 mg or 140 mg, which has a peak serum concentration in approximately 6 days,” said Dr. Dodick.
Hypertension ‘not a barrier’ to treatment
Commenting on the findings, Richard B. Lipton, MD, Edwin S. Lowe professor and vice chair of neurology at Albert Einstein College of Medicine, New York, noted that in theory blocking CGRP could increase blood pressure. However, he noted that the data and clinical experience do not suggest erenumab poses a serious risk.
“I have rarely seen new-onset hypertension with erenumab in the patients treated at our medical center. The few cases I have seen were in older adults with pre-existing hypertension,” he said.
The investigators’ review of clinical trial data together with global safety databases was a strength of their study, said Dr. Lipton. In clinical trials, patients are monitored carefully, and a placebo control group aids in the determination of background rates.
“In the trials, the incidence of new-onset hypertension or hypertension exacerbation was 0.9%, while the placebo rate was 0.2%,” said Dr. Lipton. “The absolute increase in the risk of hypertension was 0.6% or six cases per thousand: A low rate.” But clinical trials enroll carefully selected patients who do not represent the broad group of people treated with erenumab in clinical practice, he added.
The global safety data are more representative of patients who receive erenumab in real-world settings. The weaknesses of these data, though, are the lack of a control group and the incomplete ascertainment of data associated with spontaneous reporting.
Yet both types of studies, with their complementary strengths and weaknesses, indicated low rates of hypertension.
“The low incidence of hypertension, in my view, is not a barrier to the use of erenumab, though checking blood pressure after starting any new migraine therapy is prudent,” Dr. Lipton concluded.
The study was funded by Amgen. Dr. Dodick had no relevant disclosures. Dr. Lipton has consulted for and conducted studies funded by Amgen and by other companies that manufacture CGRP drugs. He has stock options in Biohaven.
A version of this article first appeared on Medscape.com.
, easing earlier concerns that this may be one of the drug’s adverse events, an analysis of postmarketing data shows. Nevertheless, investigators noted that more research is needed to confirm that this is the case.
While randomized clinical trials have shown no increased risk of hypertension related to the drug, it has been reported in postmarketing data. However, many of these events occurred in patients with previously documented hypertension or risk factors for the disorder, the investigators noted.
The rate of hypertension adverse events in postmarketing data was 0.144 per 100 person-years. Most such reports described only one instance of elevated blood pressure. In April 2020, the prescribing information for the drug was updated to include a mention of the risk of hypertension.
“Given the limitations of postmarketing reports, including incomplete information, lack of a control arm, and others, additional data are certainly needed to fully characterize the nature, the timing, and the extent to which hypertension is a risk associated with erenumab, and indeed other [calcitonin-gene-related peptide (CGRP)] pathway antagonists,” said study investigator David W. Dodick, MD, professor of neurology at the Mayo Clinic College of Medicine, Phoenix.
The findings were presented at the American Headache Society’s 2021 annual meeting.
No increased risk over time
A monoclonal antibody and CGRP antagonist, erenumab is approved in the United States for migraine prevention in adults. CGRP medications are vasodilators, and, therefore, migraine treatments that target this pathway could theoretically have hypertensive effects.
To assess the risk of hypertension in migraine patients treated with erenumab, investigators examined clinical trial and postmarketing data. The analysis included cases with limited information and patients with a different etiology for the development of hypertension.
Using Amgen Clinical Trial data, the researchers performed a pooled safety analysis of four placebo-controlled, double-blind phase 2 or 3 studies of the drug. Participants had episodic or chronic migraine and were between ages 18 and 60 years or age 65 years. The doses studied were 70 mg and 140 mg.
In these studies, blood pressure data for each patient were based on an average of at least two measurements taken after patients were in rested state for at least 5 minutes. The position used for blood pressure measurement for each patient was consistent throughout the study.
The investigators also analyzed postmarketing reports of hypertension from May 17, 2018, to Jan. 31, 2020, identified in Amgen Global Safety data.
The pooled studies included 1,043 participants receiving placebo, 893 receiving 70 mg of erenumab, and 507 receiving 140 mg of the drug. During the treatment phase, the incidence of hypertension was 0.9% among controls, 0.8% in the 70-mg group, and 0.2% in the 140-mg group. The proportion of patients who started a new antihypertensive medication was 1.2% in controls, 0.8% in the 70-mg group, and 0.2% in the 140-mg group.
In a long-term, open-label study, patients with episodic migraine received erenumab treatment for up to 5 years. The incidence of hypertension did not increase with time in this population.
The postmarketing data encompassed 245,682 person-years of erenumab exposure. The researchers identified 362 hypertension events (355 cases). The rate of these events was 0.144 per 100 person-years. The exposure-adjusted incidence of hypertension was 1.9 per 100 patient-years for erenumab.
Of the 362 hypertensive events, 158 (43.6%) were in patients with a medical history of hypertension or risk factors for hypertension. Information about the time to onset of hypertension was available for 121 (33.4%) adverse events.
Of this group, 56 (46.2%) occurred within 1 week of erenumab initiation, including 43 (35.5%) that occurred within 1 day. This rapid time to hypertension onset “is inconsistent with the pharmacokinetic profile of once-monthly erenumab 70 mg or 140 mg, which has a peak serum concentration in approximately 6 days,” said Dr. Dodick.
Hypertension ‘not a barrier’ to treatment
Commenting on the findings, Richard B. Lipton, MD, Edwin S. Lowe professor and vice chair of neurology at Albert Einstein College of Medicine, New York, noted that in theory blocking CGRP could increase blood pressure. However, he noted that the data and clinical experience do not suggest erenumab poses a serious risk.
“I have rarely seen new-onset hypertension with erenumab in the patients treated at our medical center. The few cases I have seen were in older adults with pre-existing hypertension,” he said.
The investigators’ review of clinical trial data together with global safety databases was a strength of their study, said Dr. Lipton. In clinical trials, patients are monitored carefully, and a placebo control group aids in the determination of background rates.
“In the trials, the incidence of new-onset hypertension or hypertension exacerbation was 0.9%, while the placebo rate was 0.2%,” said Dr. Lipton. “The absolute increase in the risk of hypertension was 0.6% or six cases per thousand: A low rate.” But clinical trials enroll carefully selected patients who do not represent the broad group of people treated with erenumab in clinical practice, he added.
The global safety data are more representative of patients who receive erenumab in real-world settings. The weaknesses of these data, though, are the lack of a control group and the incomplete ascertainment of data associated with spontaneous reporting.
Yet both types of studies, with their complementary strengths and weaknesses, indicated low rates of hypertension.
“The low incidence of hypertension, in my view, is not a barrier to the use of erenumab, though checking blood pressure after starting any new migraine therapy is prudent,” Dr. Lipton concluded.
The study was funded by Amgen. Dr. Dodick had no relevant disclosures. Dr. Lipton has consulted for and conducted studies funded by Amgen and by other companies that manufacture CGRP drugs. He has stock options in Biohaven.
A version of this article first appeared on Medscape.com.
FROM AHS 2021
Can laparoscopic lavage beat resection for acute perforated diverticulitis?
Severe complications at 5 years were no different for patients with perforated purulent diverticulitis who underwent laparoscopic peritoneal lavage or colon resection, according to data from 199 individuals treated at 21 hospitals in Norway and Sweden. But it may yet prove appropriate in the right patient.
Acute perforated diverticulitis with peritonitis remains a challenging complication with high morbidity and mortality among patients with diverticular disease, and bowel resection remains the standard of treatment, Najia Azhar, MD, of Skåne University Hospital, Malmö, Sweden, and colleagues wrote.
Short-term data suggest that laparoscopic lavage with drainage and antibiotics might be a viable alternative, but long-term data are lacking, they said.
In the Scandinavian Diverticulitis (SCANDIV) trial, published in JAMA Surgery, researchers randomized 101 patients to laparoscopic peritoneal lavage and 98 to colon resection. With 3 patients lost to follow-up, the final analysis included 73 patients who underwent laparoscopic lavage and 69 who underwent resection. The mean age of the lavage patients was 66.4 years, and 39 were men. The mean age of the resection patients was 63.5 years, and 36 were men. The primary outcome was severe complications – excluding stoma reversals and elective sigmoid resections because of recurrence – at an average of 5 years’ follow-up. Secondary outcomes included stoma prevalence, diverticulitis recurrence, and secondary sigmoid resection.
Severe complications were similar for the lavage and resection groups (36% and 35%, respectively), as were the overall mortality rates (32% and 25%, respectively).
The prevalence of stoma was significantly lower in the lavage group, compared with the resection group (8% vs. 33%, P = .002). However, secondary operations (including reversal of stoma) were similar between the lavage and resection groups, performed in 26 lavage patients (36%) versus 24 resection patients (35%).
Diverticulitis recurrence was significantly more common in the lavage, compared with the resection group (21% vs. 4%, P = .004), the researchers noted.
In the laparoscopic lavage group, 30% (n = 21) underwent a sigmoid resection; all but one of these occurred within a year of the index procedure, the researchers wrote. In addition, overall length of hospital stay was similar for both groups.
No significant differences in quality of life were noted between the groups, based on the EuroQoL-5D questionnaire or Cleveland Global Quality of Life scores.
Balance secondary pros and cons
Laparoscopic lavage is not common practice today in the United States, the researchers noted. In clinical practice guidelines issued in 2020, the American Society of Colon and Rectal Surgeons strongly recommend colectomy over laparoscopic lavage for the treatment of left-sided colonic diverticulitis. However, the European Society of Coloproctology’s guidelines state that laparoscopic lavage is feasible for patients with peritonitis at Hinchey stage III.
The findings of the current study were limited primarily by the exclusion of 50% of eligible patients because of challenges associated with conducting randomized trials in emergency settings, the researchers noted. However, the number of excluded patients and their baseline characteristics after exclusion were very similar in the two groups, and the study represents the largest randomized trial to date to examine long-term outcomes in patients with perforated diverticulitis.
“Laparoscopic lavage is faster and cost-effective but leads to a higher reoperation rate and recurrence rate, often requiring secondary sigmoid resection,” the researchers emphasized. Consequently, patients undergoing lavage should have consented for resection surgery.
The similar rates of severe complications and quality of life scores support laparoscopic lavage as an option for perforated purulent diverticulitis, but shared decision-making will be essential for better optimal patient management, the researchers concluded.
Similar outcomes, but unanswered questions
Even though the primary outcome of disease-related morbidity was similar for both groups, “the issue still remains regarding when and how, if ever, this therapeutic approach should be considered for purulent peritonitis,” Kellie E. Cunningham, MD, and Brian S. Zuckerbraun, MD, both of the University of Pittsburgh, wrote in an accompanying editorial.
Although laparoscopic lavage has the obvious advantages of avoiding a laparotomy and stoma, previous studies have shown a higher rate of early reoperations and recurrent diverticulitis, despite lower stoma prevalence and equal mortality rates, they said. In addition, “patients who are immunosuppressed or would be expected to have a higher mortality rate with failure to achieve definitive source control should likely not be offered this therapy.”
A “philosophical” argument could be made in favor of laparoscopic lavage based on the potential consequences of early treatment failure, they wrote.
“Although one may consider the need for early reoperation a complication, some would argue it affects the minority of patients, thus avoiding the more morbid procedure with creation of a stoma at the index operation in the majority of patients,” they noted. “Additionally, patients who underwent lavage that subsequently proceed to colectomy would have otherwise been offered this therapy initially at the time of the index operation.”
More research is needed to answer questions such as which, if any, operative findings are associated with failure. In addition, an analysis of long-term cost benefits between the two options should be explored, the authors wrote.
Based on current evidence, shared decision-making is necessary, with individualized care and short and long-term trade-offs taken into account, they wrote.
Gastroenterologist perspective: Study fills gap in follow-up data
In an interview, David A. Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia School of Medicine, Norfolk, said the study is important because data have been lacking on outcomes of a laparoscopic lavage without a resection.
The findings represent “a major shift” in the growing consensus among surgeons that laparoscopic lavage is a viable option in appropriate patients, he said.
A key issue is the high rate of morbidity in patients who undergo traditional diverticulitis surgery. Complications can include wound infection and poor quality of life associated with stoma, Dr. Johnson said. Consequently, “a nonoperative approach from a patient perspective is certainly refreshing.”
Dr. Johnson said he was surprised by how well the patients fared after lavage given the severity of the diverticulitis in the patient population. However, this may be in part because of the relatively small numbers of patients at highest risk for complications, such as those with diabetes or immunocompromising conditions.
Dr. Johnson also said he was struck by the fact that the adenocarcinomas in the lavage group were diagnosed within the first year after the procedure. “The cancer diagnosis shouldn’t reflect on the lavage group,” but emphasizes the importance of having an earlier colonoscopy, he noted.
Next steps for research might include identifying a standardized endpoint for lavage, and determining how expanded use of the procedure might impact community practice, Dr. Johnson said. In addition, more research is needed to more clearly define patients most likely to benefit from laparoscopic lavage.
The study was supported in part by the department of surgery at Skåne University Hospital, Akershus University Hospital, and a fellowship to one of the study coauthors from the Southeastern Norway Regional Health Authority. Lead author Dr. Azhar disclosed grants from the department of surgery of Skåne University Hospital. Dr. Cunningham and Dr. Zuckerbraun had no financial conflicts to disclose. Dr. Johnson had no relevant financial disclosures.
Severe complications at 5 years were no different for patients with perforated purulent diverticulitis who underwent laparoscopic peritoneal lavage or colon resection, according to data from 199 individuals treated at 21 hospitals in Norway and Sweden. But it may yet prove appropriate in the right patient.
Acute perforated diverticulitis with peritonitis remains a challenging complication with high morbidity and mortality among patients with diverticular disease, and bowel resection remains the standard of treatment, Najia Azhar, MD, of Skåne University Hospital, Malmö, Sweden, and colleagues wrote.
Short-term data suggest that laparoscopic lavage with drainage and antibiotics might be a viable alternative, but long-term data are lacking, they said.
In the Scandinavian Diverticulitis (SCANDIV) trial, published in JAMA Surgery, researchers randomized 101 patients to laparoscopic peritoneal lavage and 98 to colon resection. With 3 patients lost to follow-up, the final analysis included 73 patients who underwent laparoscopic lavage and 69 who underwent resection. The mean age of the lavage patients was 66.4 years, and 39 were men. The mean age of the resection patients was 63.5 years, and 36 were men. The primary outcome was severe complications – excluding stoma reversals and elective sigmoid resections because of recurrence – at an average of 5 years’ follow-up. Secondary outcomes included stoma prevalence, diverticulitis recurrence, and secondary sigmoid resection.
Severe complications were similar for the lavage and resection groups (36% and 35%, respectively), as were the overall mortality rates (32% and 25%, respectively).
The prevalence of stoma was significantly lower in the lavage group, compared with the resection group (8% vs. 33%, P = .002). However, secondary operations (including reversal of stoma) were similar between the lavage and resection groups, performed in 26 lavage patients (36%) versus 24 resection patients (35%).
Diverticulitis recurrence was significantly more common in the lavage, compared with the resection group (21% vs. 4%, P = .004), the researchers noted.
In the laparoscopic lavage group, 30% (n = 21) underwent a sigmoid resection; all but one of these occurred within a year of the index procedure, the researchers wrote. In addition, overall length of hospital stay was similar for both groups.
No significant differences in quality of life were noted between the groups, based on the EuroQoL-5D questionnaire or Cleveland Global Quality of Life scores.
Balance secondary pros and cons
Laparoscopic lavage is not common practice today in the United States, the researchers noted. In clinical practice guidelines issued in 2020, the American Society of Colon and Rectal Surgeons strongly recommend colectomy over laparoscopic lavage for the treatment of left-sided colonic diverticulitis. However, the European Society of Coloproctology’s guidelines state that laparoscopic lavage is feasible for patients with peritonitis at Hinchey stage III.
The findings of the current study were limited primarily by the exclusion of 50% of eligible patients because of challenges associated with conducting randomized trials in emergency settings, the researchers noted. However, the number of excluded patients and their baseline characteristics after exclusion were very similar in the two groups, and the study represents the largest randomized trial to date to examine long-term outcomes in patients with perforated diverticulitis.
“Laparoscopic lavage is faster and cost-effective but leads to a higher reoperation rate and recurrence rate, often requiring secondary sigmoid resection,” the researchers emphasized. Consequently, patients undergoing lavage should have consented for resection surgery.
The similar rates of severe complications and quality of life scores support laparoscopic lavage as an option for perforated purulent diverticulitis, but shared decision-making will be essential for better optimal patient management, the researchers concluded.
Similar outcomes, but unanswered questions
Even though the primary outcome of disease-related morbidity was similar for both groups, “the issue still remains regarding when and how, if ever, this therapeutic approach should be considered for purulent peritonitis,” Kellie E. Cunningham, MD, and Brian S. Zuckerbraun, MD, both of the University of Pittsburgh, wrote in an accompanying editorial.
Although laparoscopic lavage has the obvious advantages of avoiding a laparotomy and stoma, previous studies have shown a higher rate of early reoperations and recurrent diverticulitis, despite lower stoma prevalence and equal mortality rates, they said. In addition, “patients who are immunosuppressed or would be expected to have a higher mortality rate with failure to achieve definitive source control should likely not be offered this therapy.”
A “philosophical” argument could be made in favor of laparoscopic lavage based on the potential consequences of early treatment failure, they wrote.
“Although one may consider the need for early reoperation a complication, some would argue it affects the minority of patients, thus avoiding the more morbid procedure with creation of a stoma at the index operation in the majority of patients,” they noted. “Additionally, patients who underwent lavage that subsequently proceed to colectomy would have otherwise been offered this therapy initially at the time of the index operation.”
More research is needed to answer questions such as which, if any, operative findings are associated with failure. In addition, an analysis of long-term cost benefits between the two options should be explored, the authors wrote.
Based on current evidence, shared decision-making is necessary, with individualized care and short and long-term trade-offs taken into account, they wrote.
Gastroenterologist perspective: Study fills gap in follow-up data
In an interview, David A. Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia School of Medicine, Norfolk, said the study is important because data have been lacking on outcomes of a laparoscopic lavage without a resection.
The findings represent “a major shift” in the growing consensus among surgeons that laparoscopic lavage is a viable option in appropriate patients, he said.
A key issue is the high rate of morbidity in patients who undergo traditional diverticulitis surgery. Complications can include wound infection and poor quality of life associated with stoma, Dr. Johnson said. Consequently, “a nonoperative approach from a patient perspective is certainly refreshing.”
Dr. Johnson said he was surprised by how well the patients fared after lavage given the severity of the diverticulitis in the patient population. However, this may be in part because of the relatively small numbers of patients at highest risk for complications, such as those with diabetes or immunocompromising conditions.
Dr. Johnson also said he was struck by the fact that the adenocarcinomas in the lavage group were diagnosed within the first year after the procedure. “The cancer diagnosis shouldn’t reflect on the lavage group,” but emphasizes the importance of having an earlier colonoscopy, he noted.
Next steps for research might include identifying a standardized endpoint for lavage, and determining how expanded use of the procedure might impact community practice, Dr. Johnson said. In addition, more research is needed to more clearly define patients most likely to benefit from laparoscopic lavage.
The study was supported in part by the department of surgery at Skåne University Hospital, Akershus University Hospital, and a fellowship to one of the study coauthors from the Southeastern Norway Regional Health Authority. Lead author Dr. Azhar disclosed grants from the department of surgery of Skåne University Hospital. Dr. Cunningham and Dr. Zuckerbraun had no financial conflicts to disclose. Dr. Johnson had no relevant financial disclosures.
Severe complications at 5 years were no different for patients with perforated purulent diverticulitis who underwent laparoscopic peritoneal lavage or colon resection, according to data from 199 individuals treated at 21 hospitals in Norway and Sweden. But it may yet prove appropriate in the right patient.
Acute perforated diverticulitis with peritonitis remains a challenging complication with high morbidity and mortality among patients with diverticular disease, and bowel resection remains the standard of treatment, Najia Azhar, MD, of Skåne University Hospital, Malmö, Sweden, and colleagues wrote.
Short-term data suggest that laparoscopic lavage with drainage and antibiotics might be a viable alternative, but long-term data are lacking, they said.
In the Scandinavian Diverticulitis (SCANDIV) trial, published in JAMA Surgery, researchers randomized 101 patients to laparoscopic peritoneal lavage and 98 to colon resection. With 3 patients lost to follow-up, the final analysis included 73 patients who underwent laparoscopic lavage and 69 who underwent resection. The mean age of the lavage patients was 66.4 years, and 39 were men. The mean age of the resection patients was 63.5 years, and 36 were men. The primary outcome was severe complications – excluding stoma reversals and elective sigmoid resections because of recurrence – at an average of 5 years’ follow-up. Secondary outcomes included stoma prevalence, diverticulitis recurrence, and secondary sigmoid resection.
Severe complications were similar for the lavage and resection groups (36% and 35%, respectively), as were the overall mortality rates (32% and 25%, respectively).
The prevalence of stoma was significantly lower in the lavage group, compared with the resection group (8% vs. 33%, P = .002). However, secondary operations (including reversal of stoma) were similar between the lavage and resection groups, performed in 26 lavage patients (36%) versus 24 resection patients (35%).
Diverticulitis recurrence was significantly more common in the lavage, compared with the resection group (21% vs. 4%, P = .004), the researchers noted.
In the laparoscopic lavage group, 30% (n = 21) underwent a sigmoid resection; all but one of these occurred within a year of the index procedure, the researchers wrote. In addition, overall length of hospital stay was similar for both groups.
No significant differences in quality of life were noted between the groups, based on the EuroQoL-5D questionnaire or Cleveland Global Quality of Life scores.
Balance secondary pros and cons
Laparoscopic lavage is not common practice today in the United States, the researchers noted. In clinical practice guidelines issued in 2020, the American Society of Colon and Rectal Surgeons strongly recommend colectomy over laparoscopic lavage for the treatment of left-sided colonic diverticulitis. However, the European Society of Coloproctology’s guidelines state that laparoscopic lavage is feasible for patients with peritonitis at Hinchey stage III.
The findings of the current study were limited primarily by the exclusion of 50% of eligible patients because of challenges associated with conducting randomized trials in emergency settings, the researchers noted. However, the number of excluded patients and their baseline characteristics after exclusion were very similar in the two groups, and the study represents the largest randomized trial to date to examine long-term outcomes in patients with perforated diverticulitis.
“Laparoscopic lavage is faster and cost-effective but leads to a higher reoperation rate and recurrence rate, often requiring secondary sigmoid resection,” the researchers emphasized. Consequently, patients undergoing lavage should have consented for resection surgery.
The similar rates of severe complications and quality of life scores support laparoscopic lavage as an option for perforated purulent diverticulitis, but shared decision-making will be essential for better optimal patient management, the researchers concluded.
Similar outcomes, but unanswered questions
Even though the primary outcome of disease-related morbidity was similar for both groups, “the issue still remains regarding when and how, if ever, this therapeutic approach should be considered for purulent peritonitis,” Kellie E. Cunningham, MD, and Brian S. Zuckerbraun, MD, both of the University of Pittsburgh, wrote in an accompanying editorial.
Although laparoscopic lavage has the obvious advantages of avoiding a laparotomy and stoma, previous studies have shown a higher rate of early reoperations and recurrent diverticulitis, despite lower stoma prevalence and equal mortality rates, they said. In addition, “patients who are immunosuppressed or would be expected to have a higher mortality rate with failure to achieve definitive source control should likely not be offered this therapy.”
A “philosophical” argument could be made in favor of laparoscopic lavage based on the potential consequences of early treatment failure, they wrote.
“Although one may consider the need for early reoperation a complication, some would argue it affects the minority of patients, thus avoiding the more morbid procedure with creation of a stoma at the index operation in the majority of patients,” they noted. “Additionally, patients who underwent lavage that subsequently proceed to colectomy would have otherwise been offered this therapy initially at the time of the index operation.”
More research is needed to answer questions such as which, if any, operative findings are associated with failure. In addition, an analysis of long-term cost benefits between the two options should be explored, the authors wrote.
Based on current evidence, shared decision-making is necessary, with individualized care and short and long-term trade-offs taken into account, they wrote.
Gastroenterologist perspective: Study fills gap in follow-up data
In an interview, David A. Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia School of Medicine, Norfolk, said the study is important because data have been lacking on outcomes of a laparoscopic lavage without a resection.
The findings represent “a major shift” in the growing consensus among surgeons that laparoscopic lavage is a viable option in appropriate patients, he said.
A key issue is the high rate of morbidity in patients who undergo traditional diverticulitis surgery. Complications can include wound infection and poor quality of life associated with stoma, Dr. Johnson said. Consequently, “a nonoperative approach from a patient perspective is certainly refreshing.”
Dr. Johnson said he was surprised by how well the patients fared after lavage given the severity of the diverticulitis in the patient population. However, this may be in part because of the relatively small numbers of patients at highest risk for complications, such as those with diabetes or immunocompromising conditions.
Dr. Johnson also said he was struck by the fact that the adenocarcinomas in the lavage group were diagnosed within the first year after the procedure. “The cancer diagnosis shouldn’t reflect on the lavage group,” but emphasizes the importance of having an earlier colonoscopy, he noted.
Next steps for research might include identifying a standardized endpoint for lavage, and determining how expanded use of the procedure might impact community practice, Dr. Johnson said. In addition, more research is needed to more clearly define patients most likely to benefit from laparoscopic lavage.
The study was supported in part by the department of surgery at Skåne University Hospital, Akershus University Hospital, and a fellowship to one of the study coauthors from the Southeastern Norway Regional Health Authority. Lead author Dr. Azhar disclosed grants from the department of surgery of Skåne University Hospital. Dr. Cunningham and Dr. Zuckerbraun had no financial conflicts to disclose. Dr. Johnson had no relevant financial disclosures.
FROM JAMA SURGERY
Osteoporosis management: Use a goal-oriented, individualized approach
Recommendations for care are evolving, with increasingly sophisticated screening and diagnostic tools and a broadening array of treatment options.
As the population of older adults rises, primary osteoporosis has become a problem of public health significance, resulting in more than 2 million fractures and $19 billion in related costs annually in the United States.1 Despite the availability of effective primary and secondary preventive measures, many older adults do not receive adequate information on bone health from their primary care provider.2 Initiation of osteoporosis treatment is low even among patients who have had an osteoporotic fracture: Fewer than one-quarter of older adults with hip fracture have begun taking osteoporosis medication within 12 months of hospital discharge.3
In this overview of osteoporosis care, we provide information on how to evaluate and manage older adults in primary care settings who are at risk of, or have been given a diagnosis of, primary osteoporosis. The guidance that we offer reflects the most recent updates and recommendations by relevant professional societies.1,4-7
The nature and scope of an urgent problem
Osteoporosis is a skeletal disorder characterized by low bone mass and deterioration of bone structure that causes bone fragility and increases the risk of fracture.8 Operationally, it is defined by the World Health Organization as a bone mineral density (BMD) score below 2.5 SD from the mean value for a young White woman (ie, T-score ≤ –2.5).9 Primary osteoporosis is age related and occurs mostly in postmenopausal women and older men, affecting 25% of women and 5% of men ≥ 65 years.10
An osteoporotic fracture is particularly devastating in an older adult because it can cause pain, reduced mobility, depression, and social isolation and can increase the risk of related mortality.1 The National Osteoporosis Foundation estimates that 20% of older adults who sustain a hip fracture die within 1 year due to complications of the fracture itself or surgical repair.1 Therefore, it is of paramount importance to identify patients who are at increased risk of fracture and intervene early.
Clinical manifestations
Osteoporosis does not have a primary presentation; rather, disease manifests clinically when a patient develops complications. Often, a fragility fracture is the first sign in an older person.11
A fracture is the most important complication of osteoporosis and can result from low-trauma injury or a fall from standing height—thus, the term “fragility fracture.” Osteoporotic fractures commonly involve the vertebra, hip, and wrist. Hip and extremity fractures can result in limited or lost mobility and depression. Vertebral fractures can be asymptomatic or result in kyphosis and loss of height. Fractures can give rise to pain.
Age and female sexare risk factors
TABLE 11,6,10 lists risk factors associated with osteoporosis. Age is the most important; prevalence of osteoporosis increases with age. Other nonmodifiable risk factors include female sex (the disease appears earlier in women who enter menopause prematurely), family history of osteoporosis, and race and ethnicity. Twenty percent of Asian and non-Hispanic White women > 50 years have osteoporosis.1 A study showed that Mexican Americans are at higher risk of osteoporosis than non-Hispanic Whites; non-Hispanic Blacks are least affected.10
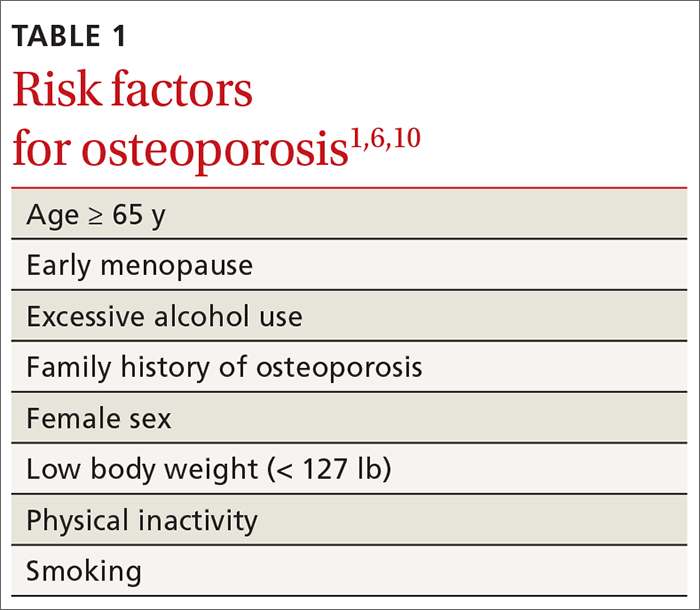
Other risk factors include low body weight (< 127 lb) and a history of fractures after age 50. Behavioral risk factors include smoking, excessive alcohol intake (> 3 drinks/d), poor nutrition, and a sedentary lifestyle.1,6
Continue to: Who should be screened?...
Who should be screened?
Screening is generally performed with a clinical evaluation and a dual-energy x-ray absorptiometry (DXA) scan of BMD. Measurement of BMD is generally recommended for screening all women ≥ 65 years and those < 65 years whose 10-year risk of fracture is equivalent to that of a 65-year-old White woman (see “Assessment of fracture risk” later in the article). For men, the US Preventive Services Task Force recommends screening those with a prior fracture or a secondary risk factor for disease.5 However, the National Osteoporosis Foundation recommends screening all men ≥ 70 years and those 50 to 69 years whose risk profile shows heightened risk.1,4
DXA of the spine and hip is preferred; the distal one-third of the radius (termed “33% radius”) of the nondominant arm can be used when spine and hip BMD cannot be interpreted because of bone changes from the disease process or artifacts, or in certain diseases in which the wrist region shows the earliest change (eg, primary hyperparathyroidism).6,7
Clinical evaluation includes a detailed history, physical examination, laboratory screening, and assessment for risk of fracture.
❚ History. Explore the presence of risk factors, including fractures in adulthood, falls, medication use, alcohol and tobacco use, family history of osteoporosis, and chronic disease.6,7
❚ Physical exam. Assess height, including any loss (> 1.5 in) since the patient’s second or third decade of life; kyphosis; frailty; and balance and mobility problems.4,6,7
❚ Laboratory and imaging studies. Perform basic laboratory testing when DXA is abnormal, including thyroid function, serum calcium, and renal function.6,12 Radiography of the lateral spine might be necessary, especially when there is kyphosis or loss of height. Assess for vertebral fracture, using lateral spine radiography, when vertebral involvement is suspected.6,7
❚ Assessment of fracture risk. Fracture risk can be assessed with any of a number of tools, including:
- Simplified Calculated Osteoporosis Risk Estimation (SCORE): www.medicalalgorithms.com/simplified-calculated-osteoporosis-risk-estimation-tool
- Osteoporosis Risk Assessment Instrument (ORAI): www.physio-pedia.com/The_Osteoporosis_Risk_Assessment_Instrument_(ORAI)
- Osteoporosis Index of Risk (OSIRIS): https://www.tandfonline.com/doi/abs/10.1080/gye.16.3.245.250?journalCode=igye20
- Osteoporosis Self-Assessment Tool (OST): www.ncbi.nlm.nih.gov/books/NBK45516/figure/ch10.f2/
- FRAX tool5: www.sheffield.ac.uk/FRAX.
The FRAX tool is widely used. It assesses a patient’s 10-year risk of fracture.
Diagnosis is based on these criteria
Diagnosis of osteoporosis is based on any 1 or more of the following criteria6:
- a history of fragility fracture not explained by metabolic bone disease
- T-score ≤ –2.5 (lumbar, hip, femoral neck, or 33% radius)
- a nation-specific FRAX score (in the absence of access to DXA).
❚ Secondary disease. Patients in whom secondary osteoporosis is suspected should undergo laboratory investigation to ascertain the cause; treatment of the underlying pathology might then be required. Evaluation for a secondary cause might include a complete blood count, comprehensive metabolic panel, protein electrophoresis and urinary protein electrophoresis (to rule out myeloproliferative and hematologic diseases), and tests of serum 25-hydroxyvitamin D, parathyroid hormone, serum calcium, alkaline phosphatase, 24-hour urinary calcium, sodium, and creatinine.6,7 Specialized testing for biochemical markers of bone turnover—so-called bone-turnover markers—can be considered as part of the initial evaluation and follow-up, although the tests are not recommended by the US Preventive Services Task Force (see “Monitoring the efficacy of treatment,” later in the article, for more information about these markers).6
Although BMD by DXA remains the gold standard in screening for and diagnosing osteoporosis, a high rate of fracture is seen in patients with certain diseases, such as type 2 diabetes and ankylosing spondylitis, who have a nonosteoporotic low T-score. This raises concerns about the usefulness of BMD for diagnosing osteoporosis in patients who have one of these diseases.13-16
❚
❚ Trabecular bone score (TBS), a surrogate bone-quality measure that is calculated based on the spine DXA image, has recently been introduced in clinical practice, and can be used to predict fracture risk in conjunction with BMD assessment by DXA and the FRAX score.17 TBS provides an indirect index of the trabecular microarchitecture using pixel gray-level variation in lumbar spine DXA images.18 Three categories of TBS (≤ 1.200, degraded microarchitecture; 1.200-1.350, partially degraded microarchitecture; and > 1.350, normal microarchitecture) have been reported to correspond with a T-score of, respectively, ≤ −2.5; −2.5 to −1.0; and > −1.0.18 TBS can be used only in patients with a body mass index of 15 to 37.5.19,20
There is no recommendation for monitoring bone quality using TBS after osteoporosis treatment. Such monitoring is at the clinician’s discretion for appropriate patients who might not show a risk of fracture, based on BMD measurement.
Continue to: Putting preventive measures into practice...
Putting preventive measures into practice
Measures to prevent osteoporosis and preserve bone health (TABLE 21,6) are best started in childhood but can be initiated at any age and maintained through the lifespan. Encourage older adults to adopt dietary and behavioral strategies to improve their bone health and prevent fracture. We recommend the following strategies; take each patient’s individual situation into consideration when electing to adopt any of these measures.

❚ Vitamin D. Consider checking the serum 25-hydroxyvitamin D level and providing supplementation (800-1000 IU daily, the National Osteoporosis Foundation recommends1) as necessary to maintain the level at 30-50 ng/mL.6
❚ Calcium. Encourage a daily dietary calcium intake of 1000-1200 mg. Supplement calcium if you determine that diet does not provide an adequate amount.
❚ Alcohol. Advise patients to limit consumption to < 3 drinks a day.
❚ Tobacco. Advise smoking cessation.
❚ Activity. Encourage an active lifestyle, including regular weight-bearing and balance exercises and resistance exercises such as Pilates, weightlifting, and tai chi. The regimen should be tailored to the patient’s individual situation.
❚ Medical therapy for concomitant illness. When possible, prescribe medications for chronic comorbidities that can also benefit bone health. For example, long-term use of angiotensin-converting enzyme (ACE) inhibitors and thiazide diuretics for hypertension are associated with a slower decline in BMD in some populations.21-23
Tailor treatment to patient’s circumstances
TABLE 34,6,24 describes indications for pharmacotherapy in osteoporosis. Pharmacotherapy is recommended in all cases of osteoporosis and osteopenia when fracture risk is high.24

Generally, you should undertake a discussion with the patient of the relative risks and benefits of treatment, taking into account their values and preferences, to come to a shared decision. Tailoring treatment, based on the patient’s distinctive circumstances, through shared decision-making is key to compliance.25
Pharmacotherapy is not indicated in patients whose risk of fracture is low; however, you should reassess such patients every 2 to 4 years.26 Women with a very high BMD might not need to be retested with DXA any sooner than every 10 to 15 years.
There are 3 main classes of first-line pharmacotherapeutic agents for osteoporosis in older adults (TABLE 44,6,7,26-41): antiresorptives (bisphosphonates and denosumab), anabolics (teriparatide and abaloparatide), and a monoclonal sclerostin antibody (romosozumab). (TABLE 44,6,7,26-41 and the discussion in this section also remark on the selective estrogen-receptor modulator raloxifene, which is used in special clinical circumstances but has been removed from the first line of osteoporosis pharmacotherapy.)

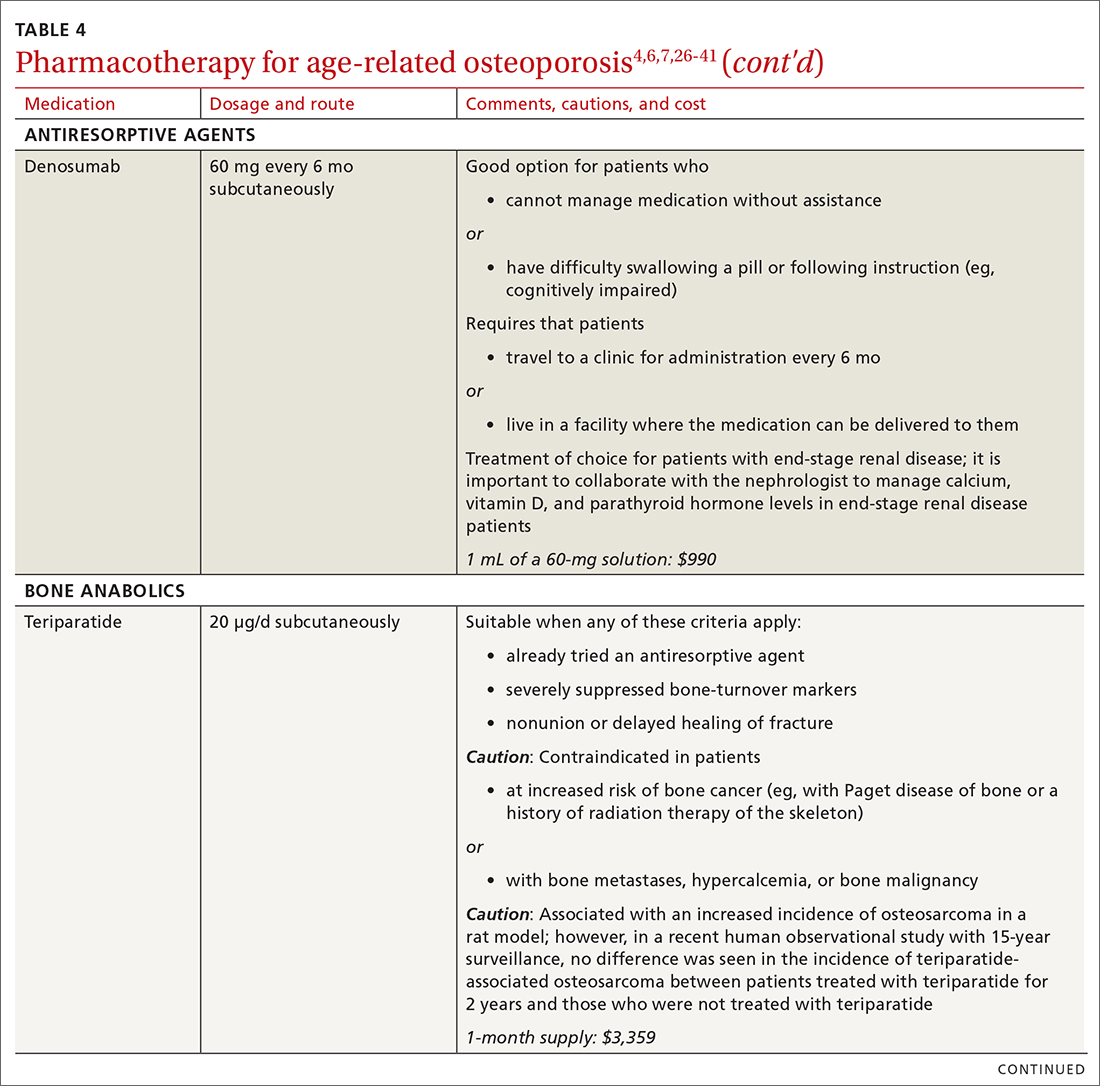
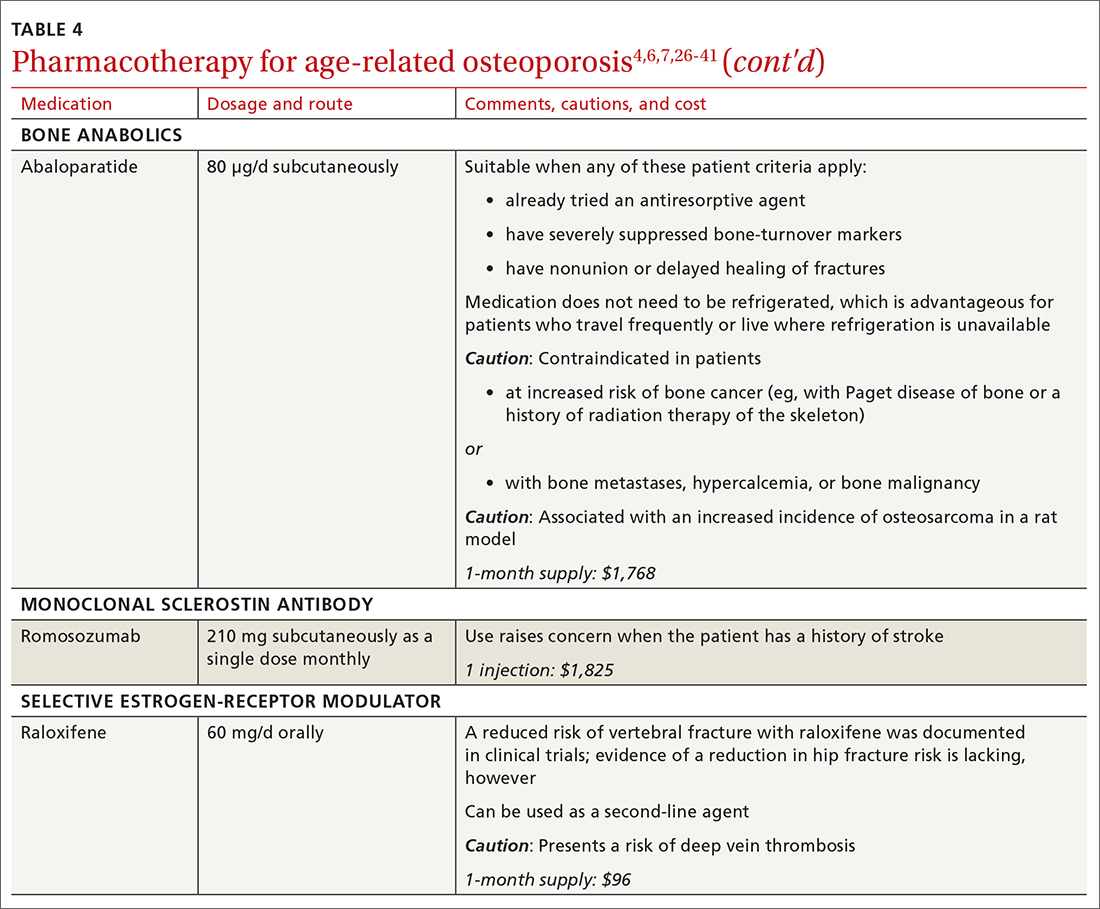
❚ Bisphosphonates. Oral bisphosphonates (alendronate, ibandronate, risedronate) can be used as initial treatment in patients with a high risk of fracture.35 Bisphosphonates have been shown to reduce fracture risk and improve BMD. When an oral bisphosphonate cannot be tolerated, intravenous zoledronate or ibandronate can be used.41
Patients treated with a bisphosphonate should be assessed for their fracture risk after 3 to 5 years of treatment26; when intravenous zoledronate is given as initial therapy, patients should be assessed after 3 years. After assessment, patients who remain at high risk should continue treatment; those whose fracture risk has decreased to low or moderate should have treatment temporarily suspended (bisphosphonate holiday) for as long as 5 years.26 Patients on bisphosphonate holiday should have their fracture risk assessed at 2- to 4-year intervals.26 Restart treatment if there is an increase in fracture risk (eg, a decrease in BMD) or if a fracture occurs. Bisphosphonates have a prolonged effect on BMD—for many years after treatment is discontinued.27,28
Oral bisphosphonates are associated with gastroesophageal reflux disease, difficulty swallowing, and gastritis. Rare adverse effects include osteonecrosis of the jaw and atypical femur fracture.29
❚ Denosumab, a recombinant human antibody, is a relatively newer antiresorptive for initial treatment. Denosumab, 60 mg, is given subcutaneously every 6 months. The drug can be used when bisphosphonates are contraindicated, the patient finds the bisphosphonate dosing regimen difficult to follow, or the patient is unresponsive to bisphosphonates.
Patients taking denosumab are reassessed every 5 to 10 years to determine whether to continue therapy or change to a new drug. Abrupt discontinuation of therapy can lead to rebound bone loss and increased risk of fracture.30-32 As with bisphosphonates, long-term use can be associated with osteonecrosis of the jaw and atypical femur fracture.33
There is no recommendation for a drug holiday for denosumab. An increase in, or no loss of, bone density and no new fractures while being treated are signs of effective treatment. There is no guideline for stopping denosumab, unless the patient develops adverse effects.
❚ Bone anabolics. Patients with a very high risk of fracture (eg, who have sustained multiple vertebral fractures), can begin treatment with teriparatide (20 μg/d subcutaneously) or abaloparatide (80 μg/d subcutaneously) for as long as 2 years, followed by treatment with an antiresorptive, such as a bisphosphonate.4,6 Teriparatide can be used in patients who have not responded to an antiresorptive as first-line treatment.
Both abaloparatide and teriparatide might be associated with a risk of osteosarcoma and are contraindicated in patients who are at increased risk of osteosarcoma.36,39,40
❚ Romosozumab, a monoclonal sclerostin antibody, can be used in patients with very high risk of fracture or with multiple vertebral fractures. Romosozumab increases bone formation and reduces bone resorption. It is given monthly, 210 mg subcutaneously, for 1 year. The recommendation is that patients who have completed a course of romosozumab continue with antiresorptive treatment.26
Romosozumab is associated with an increase in the risk of cardiovascular disease, including stroke and myocardial infarction.26
❚ Raloxifene, a selective estrogen-receptor modulator, is no longer a first-line agent for osteoporosis in older adults34 because of its association with an increased risk of deep-vein thrombosis, pulmonary embolism, and lethal stroke. However, raloxifene can be used, at 60 mg/d, when bisphosphonates or denosumab are unsuitable. In addition, raloxifene is particularly useful in women with a high risk of breast cancer and in men who are taking a long-acting gonadotropin-releasing hormone agonist for prostate cancer.37,38
Continue to: Influence of chronic...
Influence of chronic diseaseon bone health
Chronic diseases—hypertension, type 2 diabetes, hyperthyroidism, rheumatoid arthritis, ankylosing spondylitis, and gastroenterologic disorders such as celiac disease and ulcerative colitis—are known to affect bone loss that can hasten osteoporosis.16,18,21 Furthermore, medications used to treat chronic diseases are known to affect bone health: Some, such as statins, ACE inhibitors, and hydrochlorothiazide, are bone protective; others, such as steroids, pioglitazone, and selective serotonin reuptake inhibitors, accelerate bone loss.1,14,42,43 It is important to be aware of the effect of a patient’s chronic diseases, and treatments for those diseases, on bone health, to help develop an individualized osteoporosis prevention plan.
Monitoring the efficacy of treatment
Treatment of osteoporosis should not be initiated without baseline measurement of BMD of the spine and hip. Subsequent to establishing that baseline, serial measurement of BMD can be used to (1) determine when treatment needs to be initiated for an untreated patient and (2) assess response in a treated patient. There is no consensus on the interval at which DXA should be repeated for the purpose of monitoring treatment response; frequency depends on the individual’s circumstances and the medication used. Notably, many physicians repeat DXA after 2 years of treatment8; however, the American College of Physicians recommends against repeating DXA within the first 5 years of pharmacotherapy in women.24
Patients with suspected vertebral fracture or those with loss of height > 1.5 inches require lateral radiographs of the thoracic and lumbar spine to assess the status of fractures.4,6
❚ Bone-turnover markers measured in serum can be used to assess treatment efficacy and patient adherence. The formation marker procollagen type I N-terminal propeptide (P1NP) and the resorption marker beta C-terminal cross-linking telopeptide of type 1 collagen (bCTX) are preferred for evaluating bone turnover in the clinical setting. Assessing P1NP and bCTX at baseline and after 3 months of treatment might be effective in monitoring adherence, particularly in patients taking a bisphosphonate.44
Be sure to address fall prevention
It is important to address falls, and how to prevent them, in patients with osteoporosis. Falls can precipitate fracture in older adults with reduced BMD, and fractures are the most common and debilitating manifestation of osteoporosis. Your discussion of falls with patients should include45:
- consequences of falls
- cautions about medications that can cloud mental alertness
- use of appropriate footwear
- home safety, such as adequate lighting, removal of floor clutter, and installation of handrails in the bathroom and stairwells and on outside steps.
- having an annual comprehensive eye exam.
Osteoporosis is avoidable and treatable
Earlier research reported various expressions of number needed to treat for medical management of osteoporosis—making it difficult to follow a single number as a reference for gauging the effectiveness of pharmacotherapy.46,47 However, for older adults of different ethnic and racial backgrounds with multiple comorbidities and polypharmacy, it might be more pragmatic in primary care to establish a model of goal-oriented, individualized care. By focusing on prevention of bone loss, and being mindful that the risk of fracture almost doubles with a decrease of 1 SD in BMD, you can translate numbers to goals of care.48
In the United States, approximately one-half of osteoporosis cases in adults ≥ 50 years are managed by primary care providers. As a chronic disease, osteoporosis requires that you, first, provide regular monitoring and assessment, because risk can vary with comorbidities,49 and, second, discuss and initiate screening and treatment as appropriate, which can be done annually during a well-care visit.
CORRESPONDENCE
Nahid Rianon, MD, DrPH, Department of Family and Community Medicine, UTHealth McGovern Medical School, 6431 Fannin Street #JJL 324C, Houston, TX, 77030; [email protected]
- What is osteoporosis and what causes it? National Osteoporosis Foundation Website. 2020. Accessed April 28, 2021. www.nof.org/patients/what-is-osteoporosis/
- des Bordes J, Prasad S, Pratt G, et al. Knowledge, beliefs, and concerns about bone health from a systematic review and metasynthesis of qualitative studies. PLoS One. 2020;15:e0227765. doi: 10.1371/journal.pone.0227765
- Solomon DH, Johnston SS, Boytsov NN, et al. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929-1937. doi: 10.1002/jbmr.2202
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381. doi: 10.1007/s00198-014-2794-2
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531. doi: 10.1001/jama.2018.7498
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL
- Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline.J Clin Endocrinol Metab. 2012;97:1802-1822. doi: 10.1210/jc.2011-3045
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. Accessed April 28, 2021. www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
- Looker AC, Frenk SM. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005--2010. Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health and Nutrition Examination Surveys. August 2015. Accessed April 28, 2021. www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf
- Kerschan-Schindl K. Prevention and rehabilitation of osteoporosis. Wien Med Wochenschr. 2016;166:22-27. doi: 10.1007/s10354-015-0417-y
- Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(suppl 1):3-36. doi: 10.1007/s10195-017-0474-7
- Martineau P, Leslie WD, Johansson H, et al. In which patients does lumbar spine trabecular bone score (TBS) have the largest effect? Bone. 2018;113:161-168. doi: 10.1016/j.bone.2018.05.026
- Rianon NJ, Smith SM, Lee M, et al. Glycemic control and bone turnover in older Mexican Americans with type 2 diabetes. J Osteoporos. 2018;2018:7153021. doi: 10.1155/2018/7153021
- Richards C, Hans D, Leslie WD. Trabecular bone score (TBS) predicts fracture in ankylosing spondylitis: The Manitoba BMD Registry. J Clin Densitom. 2020;23:543-548. doi: 10.1016/j.jocd.2020.01.003
- Xue Y, Baker AL, Nader S, et al. Lumbar spine trabecular bone score (TBS) reflects diminished bone quality in patients with diabetes mellitus and oral glucocorticoid therapy. J Clin Densitom. 2018;21:185-192. doi: 10.1016/j.jocd.2017.09.003
- Silva BC, Broy SB, Boutroy S, et al. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18:309-330. doi: 10.1016/j.jocd.2015.06.008
- Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518-530. doi: 10.1002/jbmr.2176
- Leslie WD, Aubry-Rozier B, Lamy O, et al; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602-609.
- Looker AC, Sarafrazi Isfahani N, Fan B, et al. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27:2467-2475. doi: 10.1007/s00198-016-3550-6
- Rianon N, Ambrose CG, Pervin H, et al. Long-term use of angiotensin-converting enzyme inhibitors protects against bone loss in African-American elderly men. Arch Osteoporos. 2017;12:94. doi: 10.1007/s11657-017-0387-3
- Morton DJ, Barrett-Connor EL, Edelstein SL. Thiazides and bone mineral density in elderly men and women. Am J Epidemiol. 1994;139:1107-1115. doi: 10.1093/oxfordjournals.aje.a116954
- Sigurdsson G, Franzson L. Increased bone mineral density in a population-based group of 70-year-old women on thiazide diuretics, independent of parathyroid hormone levels. J Intern Med. 2001;250:51-56. doi: 10.1046/j.1365-2796.2001.00850.x
- Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839. doi: 10.7326/M15-1361
- des Bordes JKA, Suarez-Almazor ME, Volk RJ, et al. Online educational tool to promote bone health in cancer survivors. J Health Commun. 2017;22:808-817. doi: 10.1080/10810730.2017.1360415
- Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:587-594. doi: 10.1210/clinem/dgaa048
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. doi: 10.1001/jama.296.24.2927
- Bone HG, Hosking D, Devogelaer J-P, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. doi: 10.1056/NEJMoa030897
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491. doi: 10.1359/jbmr.0707onj
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972-980. doi: 10.1210/jc.2010-1502
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198. doi: 10.1002/jbmr.3337
- Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018;190:E485-E486. doi: 10.1503/cmaj.180115
- Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46:1515-1525. doi: 10.1016/j.jcms.2018.05.046
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137. doi: 10.1056/NEJMoa062462
- Chesnut CH 3rd, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249. doi: 10.1359/JBMR.040325
- Gilsenban A, Midkiff K, Kellier-Steele N, et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res. 2021;36:244-252. doi: 10.1002/jbmr.4188
- Cuzick J, Sestak I, Bonanni B, et al; SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827-1834. doi: 10.1016/S0140-6736(13)60140-3
- Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841-3846. doi: 10.1210/jc.2003-032058
- TYMLOS. Prescribing information. Radius Health, Inc.; April 2017. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf
- FORTEO. Prescribing information. Eli Lilly and Co.; April 2020. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf
- Wooltorton E. Patients receiving intravenous bisphosphonates should avoid invasive dental procedures. Can Med Assoc J. 2003;172:1684. doi: https://doi.org/10.1503/cmaj.050640
- Chiadika SM, Shobayo FO, Naqvi SH, et al. Lower femoral neck bone mineral density (BMD) in elderly women not on statins. Women Health. 2019;59:845-853. doi: 10.1080/03630242.2019.1567646
- Saraykar S, John V, Cao B, et al. Association of selective serotonin reuptake inhibitors and bone mineral density in elderly women. J Clin Densitom. 2018;21:193-199. doi: 10.1016/j.jocd.2017.05.016
- Lorentzon M, Branco J, Brandi ML, et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 2019;36:2811-2824. doi: 10.1007/s12325-019-01063-9
- STEADI--older adult fall prevention. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2019. Accessed April 28, 2021. www.cdc.gov/steadi/patient.html
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
- Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7:2113-2122.
- Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15:183-187. doi: 10.1359/jbmr.2000.15.2.183
- Rianon N, Anand D, Rasu R. Changing trends in osteoporosis care from specialty to primary care physicians. Curr Med Res Opin. 2013;29:881-888. doi: 10.1185/03007995.2013.809335
Recommendations for care are evolving, with increasingly sophisticated screening and diagnostic tools and a broadening array of treatment options.
Recommendations for care are evolving, with increasingly sophisticated screening and diagnostic tools and a broadening array of treatment options.
As the population of older adults rises, primary osteoporosis has become a problem of public health significance, resulting in more than 2 million fractures and $19 billion in related costs annually in the United States.1 Despite the availability of effective primary and secondary preventive measures, many older adults do not receive adequate information on bone health from their primary care provider.2 Initiation of osteoporosis treatment is low even among patients who have had an osteoporotic fracture: Fewer than one-quarter of older adults with hip fracture have begun taking osteoporosis medication within 12 months of hospital discharge.3
In this overview of osteoporosis care, we provide information on how to evaluate and manage older adults in primary care settings who are at risk of, or have been given a diagnosis of, primary osteoporosis. The guidance that we offer reflects the most recent updates and recommendations by relevant professional societies.1,4-7
The nature and scope of an urgent problem
Osteoporosis is a skeletal disorder characterized by low bone mass and deterioration of bone structure that causes bone fragility and increases the risk of fracture.8 Operationally, it is defined by the World Health Organization as a bone mineral density (BMD) score below 2.5 SD from the mean value for a young White woman (ie, T-score ≤ –2.5).9 Primary osteoporosis is age related and occurs mostly in postmenopausal women and older men, affecting 25% of women and 5% of men ≥ 65 years.10
An osteoporotic fracture is particularly devastating in an older adult because it can cause pain, reduced mobility, depression, and social isolation and can increase the risk of related mortality.1 The National Osteoporosis Foundation estimates that 20% of older adults who sustain a hip fracture die within 1 year due to complications of the fracture itself or surgical repair.1 Therefore, it is of paramount importance to identify patients who are at increased risk of fracture and intervene early.
Clinical manifestations
Osteoporosis does not have a primary presentation; rather, disease manifests clinically when a patient develops complications. Often, a fragility fracture is the first sign in an older person.11
A fracture is the most important complication of osteoporosis and can result from low-trauma injury or a fall from standing height—thus, the term “fragility fracture.” Osteoporotic fractures commonly involve the vertebra, hip, and wrist. Hip and extremity fractures can result in limited or lost mobility and depression. Vertebral fractures can be asymptomatic or result in kyphosis and loss of height. Fractures can give rise to pain.
Age and female sexare risk factors
TABLE 11,6,10 lists risk factors associated with osteoporosis. Age is the most important; prevalence of osteoporosis increases with age. Other nonmodifiable risk factors include female sex (the disease appears earlier in women who enter menopause prematurely), family history of osteoporosis, and race and ethnicity. Twenty percent of Asian and non-Hispanic White women > 50 years have osteoporosis.1 A study showed that Mexican Americans are at higher risk of osteoporosis than non-Hispanic Whites; non-Hispanic Blacks are least affected.10

Other risk factors include low body weight (< 127 lb) and a history of fractures after age 50. Behavioral risk factors include smoking, excessive alcohol intake (> 3 drinks/d), poor nutrition, and a sedentary lifestyle.1,6
Continue to: Who should be screened?...
Who should be screened?
Screening is generally performed with a clinical evaluation and a dual-energy x-ray absorptiometry (DXA) scan of BMD. Measurement of BMD is generally recommended for screening all women ≥ 65 years and those < 65 years whose 10-year risk of fracture is equivalent to that of a 65-year-old White woman (see “Assessment of fracture risk” later in the article). For men, the US Preventive Services Task Force recommends screening those with a prior fracture or a secondary risk factor for disease.5 However, the National Osteoporosis Foundation recommends screening all men ≥ 70 years and those 50 to 69 years whose risk profile shows heightened risk.1,4
DXA of the spine and hip is preferred; the distal one-third of the radius (termed “33% radius”) of the nondominant arm can be used when spine and hip BMD cannot be interpreted because of bone changes from the disease process or artifacts, or in certain diseases in which the wrist region shows the earliest change (eg, primary hyperparathyroidism).6,7
Clinical evaluation includes a detailed history, physical examination, laboratory screening, and assessment for risk of fracture.
❚ History. Explore the presence of risk factors, including fractures in adulthood, falls, medication use, alcohol and tobacco use, family history of osteoporosis, and chronic disease.6,7
❚ Physical exam. Assess height, including any loss (> 1.5 in) since the patient’s second or third decade of life; kyphosis; frailty; and balance and mobility problems.4,6,7
❚ Laboratory and imaging studies. Perform basic laboratory testing when DXA is abnormal, including thyroid function, serum calcium, and renal function.6,12 Radiography of the lateral spine might be necessary, especially when there is kyphosis or loss of height. Assess for vertebral fracture, using lateral spine radiography, when vertebral involvement is suspected.6,7
❚ Assessment of fracture risk. Fracture risk can be assessed with any of a number of tools, including:
- Simplified Calculated Osteoporosis Risk Estimation (SCORE): www.medicalalgorithms.com/simplified-calculated-osteoporosis-risk-estimation-tool
- Osteoporosis Risk Assessment Instrument (ORAI): www.physio-pedia.com/The_Osteoporosis_Risk_Assessment_Instrument_(ORAI)
- Osteoporosis Index of Risk (OSIRIS): https://www.tandfonline.com/doi/abs/10.1080/gye.16.3.245.250?journalCode=igye20
- Osteoporosis Self-Assessment Tool (OST): www.ncbi.nlm.nih.gov/books/NBK45516/figure/ch10.f2/
- FRAX tool5: www.sheffield.ac.uk/FRAX.
The FRAX tool is widely used. It assesses a patient’s 10-year risk of fracture.
Diagnosis is based on these criteria
Diagnosis of osteoporosis is based on any 1 or more of the following criteria6:
- a history of fragility fracture not explained by metabolic bone disease
- T-score ≤ –2.5 (lumbar, hip, femoral neck, or 33% radius)
- a nation-specific FRAX score (in the absence of access to DXA).
❚ Secondary disease. Patients in whom secondary osteoporosis is suspected should undergo laboratory investigation to ascertain the cause; treatment of the underlying pathology might then be required. Evaluation for a secondary cause might include a complete blood count, comprehensive metabolic panel, protein electrophoresis and urinary protein electrophoresis (to rule out myeloproliferative and hematologic diseases), and tests of serum 25-hydroxyvitamin D, parathyroid hormone, serum calcium, alkaline phosphatase, 24-hour urinary calcium, sodium, and creatinine.6,7 Specialized testing for biochemical markers of bone turnover—so-called bone-turnover markers—can be considered as part of the initial evaluation and follow-up, although the tests are not recommended by the US Preventive Services Task Force (see “Monitoring the efficacy of treatment,” later in the article, for more information about these markers).6
Although BMD by DXA remains the gold standard in screening for and diagnosing osteoporosis, a high rate of fracture is seen in patients with certain diseases, such as type 2 diabetes and ankylosing spondylitis, who have a nonosteoporotic low T-score. This raises concerns about the usefulness of BMD for diagnosing osteoporosis in patients who have one of these diseases.13-16
❚
❚ Trabecular bone score (TBS), a surrogate bone-quality measure that is calculated based on the spine DXA image, has recently been introduced in clinical practice, and can be used to predict fracture risk in conjunction with BMD assessment by DXA and the FRAX score.17 TBS provides an indirect index of the trabecular microarchitecture using pixel gray-level variation in lumbar spine DXA images.18 Three categories of TBS (≤ 1.200, degraded microarchitecture; 1.200-1.350, partially degraded microarchitecture; and > 1.350, normal microarchitecture) have been reported to correspond with a T-score of, respectively, ≤ −2.5; −2.5 to −1.0; and > −1.0.18 TBS can be used only in patients with a body mass index of 15 to 37.5.19,20
There is no recommendation for monitoring bone quality using TBS after osteoporosis treatment. Such monitoring is at the clinician’s discretion for appropriate patients who might not show a risk of fracture, based on BMD measurement.
Continue to: Putting preventive measures into practice...
Putting preventive measures into practice
Measures to prevent osteoporosis and preserve bone health (TABLE 21,6) are best started in childhood but can be initiated at any age and maintained through the lifespan. Encourage older adults to adopt dietary and behavioral strategies to improve their bone health and prevent fracture. We recommend the following strategies; take each patient’s individual situation into consideration when electing to adopt any of these measures.

❚ Vitamin D. Consider checking the serum 25-hydroxyvitamin D level and providing supplementation (800-1000 IU daily, the National Osteoporosis Foundation recommends1) as necessary to maintain the level at 30-50 ng/mL.6
❚ Calcium. Encourage a daily dietary calcium intake of 1000-1200 mg. Supplement calcium if you determine that diet does not provide an adequate amount.
❚ Alcohol. Advise patients to limit consumption to < 3 drinks a day.
❚ Tobacco. Advise smoking cessation.
❚ Activity. Encourage an active lifestyle, including regular weight-bearing and balance exercises and resistance exercises such as Pilates, weightlifting, and tai chi. The regimen should be tailored to the patient’s individual situation.
❚ Medical therapy for concomitant illness. When possible, prescribe medications for chronic comorbidities that can also benefit bone health. For example, long-term use of angiotensin-converting enzyme (ACE) inhibitors and thiazide diuretics for hypertension are associated with a slower decline in BMD in some populations.21-23
Tailor treatment to patient’s circumstances
TABLE 34,6,24 describes indications for pharmacotherapy in osteoporosis. Pharmacotherapy is recommended in all cases of osteoporosis and osteopenia when fracture risk is high.24

Generally, you should undertake a discussion with the patient of the relative risks and benefits of treatment, taking into account their values and preferences, to come to a shared decision. Tailoring treatment, based on the patient’s distinctive circumstances, through shared decision-making is key to compliance.25
Pharmacotherapy is not indicated in patients whose risk of fracture is low; however, you should reassess such patients every 2 to 4 years.26 Women with a very high BMD might not need to be retested with DXA any sooner than every 10 to 15 years.
There are 3 main classes of first-line pharmacotherapeutic agents for osteoporosis in older adults (TABLE 44,6,7,26-41): antiresorptives (bisphosphonates and denosumab), anabolics (teriparatide and abaloparatide), and a monoclonal sclerostin antibody (romosozumab). (TABLE 44,6,7,26-41 and the discussion in this section also remark on the selective estrogen-receptor modulator raloxifene, which is used in special clinical circumstances but has been removed from the first line of osteoporosis pharmacotherapy.)



❚ Bisphosphonates. Oral bisphosphonates (alendronate, ibandronate, risedronate) can be used as initial treatment in patients with a high risk of fracture.35 Bisphosphonates have been shown to reduce fracture risk and improve BMD. When an oral bisphosphonate cannot be tolerated, intravenous zoledronate or ibandronate can be used.41
Patients treated with a bisphosphonate should be assessed for their fracture risk after 3 to 5 years of treatment26; when intravenous zoledronate is given as initial therapy, patients should be assessed after 3 years. After assessment, patients who remain at high risk should continue treatment; those whose fracture risk has decreased to low or moderate should have treatment temporarily suspended (bisphosphonate holiday) for as long as 5 years.26 Patients on bisphosphonate holiday should have their fracture risk assessed at 2- to 4-year intervals.26 Restart treatment if there is an increase in fracture risk (eg, a decrease in BMD) or if a fracture occurs. Bisphosphonates have a prolonged effect on BMD—for many years after treatment is discontinued.27,28
Oral bisphosphonates are associated with gastroesophageal reflux disease, difficulty swallowing, and gastritis. Rare adverse effects include osteonecrosis of the jaw and atypical femur fracture.29
❚ Denosumab, a recombinant human antibody, is a relatively newer antiresorptive for initial treatment. Denosumab, 60 mg, is given subcutaneously every 6 months. The drug can be used when bisphosphonates are contraindicated, the patient finds the bisphosphonate dosing regimen difficult to follow, or the patient is unresponsive to bisphosphonates.
Patients taking denosumab are reassessed every 5 to 10 years to determine whether to continue therapy or change to a new drug. Abrupt discontinuation of therapy can lead to rebound bone loss and increased risk of fracture.30-32 As with bisphosphonates, long-term use can be associated with osteonecrosis of the jaw and atypical femur fracture.33
There is no recommendation for a drug holiday for denosumab. An increase in, or no loss of, bone density and no new fractures while being treated are signs of effective treatment. There is no guideline for stopping denosumab, unless the patient develops adverse effects.
❚ Bone anabolics. Patients with a very high risk of fracture (eg, who have sustained multiple vertebral fractures), can begin treatment with teriparatide (20 μg/d subcutaneously) or abaloparatide (80 μg/d subcutaneously) for as long as 2 years, followed by treatment with an antiresorptive, such as a bisphosphonate.4,6 Teriparatide can be used in patients who have not responded to an antiresorptive as first-line treatment.
Both abaloparatide and teriparatide might be associated with a risk of osteosarcoma and are contraindicated in patients who are at increased risk of osteosarcoma.36,39,40
❚ Romosozumab, a monoclonal sclerostin antibody, can be used in patients with very high risk of fracture or with multiple vertebral fractures. Romosozumab increases bone formation and reduces bone resorption. It is given monthly, 210 mg subcutaneously, for 1 year. The recommendation is that patients who have completed a course of romosozumab continue with antiresorptive treatment.26
Romosozumab is associated with an increase in the risk of cardiovascular disease, including stroke and myocardial infarction.26
❚ Raloxifene, a selective estrogen-receptor modulator, is no longer a first-line agent for osteoporosis in older adults34 because of its association with an increased risk of deep-vein thrombosis, pulmonary embolism, and lethal stroke. However, raloxifene can be used, at 60 mg/d, when bisphosphonates or denosumab are unsuitable. In addition, raloxifene is particularly useful in women with a high risk of breast cancer and in men who are taking a long-acting gonadotropin-releasing hormone agonist for prostate cancer.37,38
Continue to: Influence of chronic...
Influence of chronic diseaseon bone health
Chronic diseases—hypertension, type 2 diabetes, hyperthyroidism, rheumatoid arthritis, ankylosing spondylitis, and gastroenterologic disorders such as celiac disease and ulcerative colitis—are known to affect bone loss that can hasten osteoporosis.16,18,21 Furthermore, medications used to treat chronic diseases are known to affect bone health: Some, such as statins, ACE inhibitors, and hydrochlorothiazide, are bone protective; others, such as steroids, pioglitazone, and selective serotonin reuptake inhibitors, accelerate bone loss.1,14,42,43 It is important to be aware of the effect of a patient’s chronic diseases, and treatments for those diseases, on bone health, to help develop an individualized osteoporosis prevention plan.
Monitoring the efficacy of treatment
Treatment of osteoporosis should not be initiated without baseline measurement of BMD of the spine and hip. Subsequent to establishing that baseline, serial measurement of BMD can be used to (1) determine when treatment needs to be initiated for an untreated patient and (2) assess response in a treated patient. There is no consensus on the interval at which DXA should be repeated for the purpose of monitoring treatment response; frequency depends on the individual’s circumstances and the medication used. Notably, many physicians repeat DXA after 2 years of treatment8; however, the American College of Physicians recommends against repeating DXA within the first 5 years of pharmacotherapy in women.24
Patients with suspected vertebral fracture or those with loss of height > 1.5 inches require lateral radiographs of the thoracic and lumbar spine to assess the status of fractures.4,6
❚ Bone-turnover markers measured in serum can be used to assess treatment efficacy and patient adherence. The formation marker procollagen type I N-terminal propeptide (P1NP) and the resorption marker beta C-terminal cross-linking telopeptide of type 1 collagen (bCTX) are preferred for evaluating bone turnover in the clinical setting. Assessing P1NP and bCTX at baseline and after 3 months of treatment might be effective in monitoring adherence, particularly in patients taking a bisphosphonate.44
Be sure to address fall prevention
It is important to address falls, and how to prevent them, in patients with osteoporosis. Falls can precipitate fracture in older adults with reduced BMD, and fractures are the most common and debilitating manifestation of osteoporosis. Your discussion of falls with patients should include45:
- consequences of falls
- cautions about medications that can cloud mental alertness
- use of appropriate footwear
- home safety, such as adequate lighting, removal of floor clutter, and installation of handrails in the bathroom and stairwells and on outside steps.
- having an annual comprehensive eye exam.
Osteoporosis is avoidable and treatable
Earlier research reported various expressions of number needed to treat for medical management of osteoporosis—making it difficult to follow a single number as a reference for gauging the effectiveness of pharmacotherapy.46,47 However, for older adults of different ethnic and racial backgrounds with multiple comorbidities and polypharmacy, it might be more pragmatic in primary care to establish a model of goal-oriented, individualized care. By focusing on prevention of bone loss, and being mindful that the risk of fracture almost doubles with a decrease of 1 SD in BMD, you can translate numbers to goals of care.48
In the United States, approximately one-half of osteoporosis cases in adults ≥ 50 years are managed by primary care providers. As a chronic disease, osteoporosis requires that you, first, provide regular monitoring and assessment, because risk can vary with comorbidities,49 and, second, discuss and initiate screening and treatment as appropriate, which can be done annually during a well-care visit.
CORRESPONDENCE
Nahid Rianon, MD, DrPH, Department of Family and Community Medicine, UTHealth McGovern Medical School, 6431 Fannin Street #JJL 324C, Houston, TX, 77030; [email protected]
As the population of older adults rises, primary osteoporosis has become a problem of public health significance, resulting in more than 2 million fractures and $19 billion in related costs annually in the United States.1 Despite the availability of effective primary and secondary preventive measures, many older adults do not receive adequate information on bone health from their primary care provider.2 Initiation of osteoporosis treatment is low even among patients who have had an osteoporotic fracture: Fewer than one-quarter of older adults with hip fracture have begun taking osteoporosis medication within 12 months of hospital discharge.3
In this overview of osteoporosis care, we provide information on how to evaluate and manage older adults in primary care settings who are at risk of, or have been given a diagnosis of, primary osteoporosis. The guidance that we offer reflects the most recent updates and recommendations by relevant professional societies.1,4-7
The nature and scope of an urgent problem
Osteoporosis is a skeletal disorder characterized by low bone mass and deterioration of bone structure that causes bone fragility and increases the risk of fracture.8 Operationally, it is defined by the World Health Organization as a bone mineral density (BMD) score below 2.5 SD from the mean value for a young White woman (ie, T-score ≤ –2.5).9 Primary osteoporosis is age related and occurs mostly in postmenopausal women and older men, affecting 25% of women and 5% of men ≥ 65 years.10
An osteoporotic fracture is particularly devastating in an older adult because it can cause pain, reduced mobility, depression, and social isolation and can increase the risk of related mortality.1 The National Osteoporosis Foundation estimates that 20% of older adults who sustain a hip fracture die within 1 year due to complications of the fracture itself or surgical repair.1 Therefore, it is of paramount importance to identify patients who are at increased risk of fracture and intervene early.
Clinical manifestations
Osteoporosis does not have a primary presentation; rather, disease manifests clinically when a patient develops complications. Often, a fragility fracture is the first sign in an older person.11
A fracture is the most important complication of osteoporosis and can result from low-trauma injury or a fall from standing height—thus, the term “fragility fracture.” Osteoporotic fractures commonly involve the vertebra, hip, and wrist. Hip and extremity fractures can result in limited or lost mobility and depression. Vertebral fractures can be asymptomatic or result in kyphosis and loss of height. Fractures can give rise to pain.
Age and female sexare risk factors
TABLE 11,6,10 lists risk factors associated with osteoporosis. Age is the most important; prevalence of osteoporosis increases with age. Other nonmodifiable risk factors include female sex (the disease appears earlier in women who enter menopause prematurely), family history of osteoporosis, and race and ethnicity. Twenty percent of Asian and non-Hispanic White women > 50 years have osteoporosis.1 A study showed that Mexican Americans are at higher risk of osteoporosis than non-Hispanic Whites; non-Hispanic Blacks are least affected.10

Other risk factors include low body weight (< 127 lb) and a history of fractures after age 50. Behavioral risk factors include smoking, excessive alcohol intake (> 3 drinks/d), poor nutrition, and a sedentary lifestyle.1,6
Continue to: Who should be screened?...
Who should be screened?
Screening is generally performed with a clinical evaluation and a dual-energy x-ray absorptiometry (DXA) scan of BMD. Measurement of BMD is generally recommended for screening all women ≥ 65 years and those < 65 years whose 10-year risk of fracture is equivalent to that of a 65-year-old White woman (see “Assessment of fracture risk” later in the article). For men, the US Preventive Services Task Force recommends screening those with a prior fracture or a secondary risk factor for disease.5 However, the National Osteoporosis Foundation recommends screening all men ≥ 70 years and those 50 to 69 years whose risk profile shows heightened risk.1,4
DXA of the spine and hip is preferred; the distal one-third of the radius (termed “33% radius”) of the nondominant arm can be used when spine and hip BMD cannot be interpreted because of bone changes from the disease process or artifacts, or in certain diseases in which the wrist region shows the earliest change (eg, primary hyperparathyroidism).6,7
Clinical evaluation includes a detailed history, physical examination, laboratory screening, and assessment for risk of fracture.
❚ History. Explore the presence of risk factors, including fractures in adulthood, falls, medication use, alcohol and tobacco use, family history of osteoporosis, and chronic disease.6,7
❚ Physical exam. Assess height, including any loss (> 1.5 in) since the patient’s second or third decade of life; kyphosis; frailty; and balance and mobility problems.4,6,7
❚ Laboratory and imaging studies. Perform basic laboratory testing when DXA is abnormal, including thyroid function, serum calcium, and renal function.6,12 Radiography of the lateral spine might be necessary, especially when there is kyphosis or loss of height. Assess for vertebral fracture, using lateral spine radiography, when vertebral involvement is suspected.6,7
❚ Assessment of fracture risk. Fracture risk can be assessed with any of a number of tools, including:
- Simplified Calculated Osteoporosis Risk Estimation (SCORE): www.medicalalgorithms.com/simplified-calculated-osteoporosis-risk-estimation-tool
- Osteoporosis Risk Assessment Instrument (ORAI): www.physio-pedia.com/The_Osteoporosis_Risk_Assessment_Instrument_(ORAI)
- Osteoporosis Index of Risk (OSIRIS): https://www.tandfonline.com/doi/abs/10.1080/gye.16.3.245.250?journalCode=igye20
- Osteoporosis Self-Assessment Tool (OST): www.ncbi.nlm.nih.gov/books/NBK45516/figure/ch10.f2/
- FRAX tool5: www.sheffield.ac.uk/FRAX.
The FRAX tool is widely used. It assesses a patient’s 10-year risk of fracture.
Diagnosis is based on these criteria
Diagnosis of osteoporosis is based on any 1 or more of the following criteria6:
- a history of fragility fracture not explained by metabolic bone disease
- T-score ≤ –2.5 (lumbar, hip, femoral neck, or 33% radius)
- a nation-specific FRAX score (in the absence of access to DXA).
❚ Secondary disease. Patients in whom secondary osteoporosis is suspected should undergo laboratory investigation to ascertain the cause; treatment of the underlying pathology might then be required. Evaluation for a secondary cause might include a complete blood count, comprehensive metabolic panel, protein electrophoresis and urinary protein electrophoresis (to rule out myeloproliferative and hematologic diseases), and tests of serum 25-hydroxyvitamin D, parathyroid hormone, serum calcium, alkaline phosphatase, 24-hour urinary calcium, sodium, and creatinine.6,7 Specialized testing for biochemical markers of bone turnover—so-called bone-turnover markers—can be considered as part of the initial evaluation and follow-up, although the tests are not recommended by the US Preventive Services Task Force (see “Monitoring the efficacy of treatment,” later in the article, for more information about these markers).6
Although BMD by DXA remains the gold standard in screening for and diagnosing osteoporosis, a high rate of fracture is seen in patients with certain diseases, such as type 2 diabetes and ankylosing spondylitis, who have a nonosteoporotic low T-score. This raises concerns about the usefulness of BMD for diagnosing osteoporosis in patients who have one of these diseases.13-16
❚
❚ Trabecular bone score (TBS), a surrogate bone-quality measure that is calculated based on the spine DXA image, has recently been introduced in clinical practice, and can be used to predict fracture risk in conjunction with BMD assessment by DXA and the FRAX score.17 TBS provides an indirect index of the trabecular microarchitecture using pixel gray-level variation in lumbar spine DXA images.18 Three categories of TBS (≤ 1.200, degraded microarchitecture; 1.200-1.350, partially degraded microarchitecture; and > 1.350, normal microarchitecture) have been reported to correspond with a T-score of, respectively, ≤ −2.5; −2.5 to −1.0; and > −1.0.18 TBS can be used only in patients with a body mass index of 15 to 37.5.19,20
There is no recommendation for monitoring bone quality using TBS after osteoporosis treatment. Such monitoring is at the clinician’s discretion for appropriate patients who might not show a risk of fracture, based on BMD measurement.
Continue to: Putting preventive measures into practice...
Putting preventive measures into practice
Measures to prevent osteoporosis and preserve bone health (TABLE 21,6) are best started in childhood but can be initiated at any age and maintained through the lifespan. Encourage older adults to adopt dietary and behavioral strategies to improve their bone health and prevent fracture. We recommend the following strategies; take each patient’s individual situation into consideration when electing to adopt any of these measures.

❚ Vitamin D. Consider checking the serum 25-hydroxyvitamin D level and providing supplementation (800-1000 IU daily, the National Osteoporosis Foundation recommends1) as necessary to maintain the level at 30-50 ng/mL.6
❚ Calcium. Encourage a daily dietary calcium intake of 1000-1200 mg. Supplement calcium if you determine that diet does not provide an adequate amount.
❚ Alcohol. Advise patients to limit consumption to < 3 drinks a day.
❚ Tobacco. Advise smoking cessation.
❚ Activity. Encourage an active lifestyle, including regular weight-bearing and balance exercises and resistance exercises such as Pilates, weightlifting, and tai chi. The regimen should be tailored to the patient’s individual situation.
❚ Medical therapy for concomitant illness. When possible, prescribe medications for chronic comorbidities that can also benefit bone health. For example, long-term use of angiotensin-converting enzyme (ACE) inhibitors and thiazide diuretics for hypertension are associated with a slower decline in BMD in some populations.21-23
Tailor treatment to patient’s circumstances
TABLE 34,6,24 describes indications for pharmacotherapy in osteoporosis. Pharmacotherapy is recommended in all cases of osteoporosis and osteopenia when fracture risk is high.24

Generally, you should undertake a discussion with the patient of the relative risks and benefits of treatment, taking into account their values and preferences, to come to a shared decision. Tailoring treatment, based on the patient’s distinctive circumstances, through shared decision-making is key to compliance.25
Pharmacotherapy is not indicated in patients whose risk of fracture is low; however, you should reassess such patients every 2 to 4 years.26 Women with a very high BMD might not need to be retested with DXA any sooner than every 10 to 15 years.
There are 3 main classes of first-line pharmacotherapeutic agents for osteoporosis in older adults (TABLE 44,6,7,26-41): antiresorptives (bisphosphonates and denosumab), anabolics (teriparatide and abaloparatide), and a monoclonal sclerostin antibody (romosozumab). (TABLE 44,6,7,26-41 and the discussion in this section also remark on the selective estrogen-receptor modulator raloxifene, which is used in special clinical circumstances but has been removed from the first line of osteoporosis pharmacotherapy.)



❚ Bisphosphonates. Oral bisphosphonates (alendronate, ibandronate, risedronate) can be used as initial treatment in patients with a high risk of fracture.35 Bisphosphonates have been shown to reduce fracture risk and improve BMD. When an oral bisphosphonate cannot be tolerated, intravenous zoledronate or ibandronate can be used.41
Patients treated with a bisphosphonate should be assessed for their fracture risk after 3 to 5 years of treatment26; when intravenous zoledronate is given as initial therapy, patients should be assessed after 3 years. After assessment, patients who remain at high risk should continue treatment; those whose fracture risk has decreased to low or moderate should have treatment temporarily suspended (bisphosphonate holiday) for as long as 5 years.26 Patients on bisphosphonate holiday should have their fracture risk assessed at 2- to 4-year intervals.26 Restart treatment if there is an increase in fracture risk (eg, a decrease in BMD) or if a fracture occurs. Bisphosphonates have a prolonged effect on BMD—for many years after treatment is discontinued.27,28
Oral bisphosphonates are associated with gastroesophageal reflux disease, difficulty swallowing, and gastritis. Rare adverse effects include osteonecrosis of the jaw and atypical femur fracture.29
❚ Denosumab, a recombinant human antibody, is a relatively newer antiresorptive for initial treatment. Denosumab, 60 mg, is given subcutaneously every 6 months. The drug can be used when bisphosphonates are contraindicated, the patient finds the bisphosphonate dosing regimen difficult to follow, or the patient is unresponsive to bisphosphonates.
Patients taking denosumab are reassessed every 5 to 10 years to determine whether to continue therapy or change to a new drug. Abrupt discontinuation of therapy can lead to rebound bone loss and increased risk of fracture.30-32 As with bisphosphonates, long-term use can be associated with osteonecrosis of the jaw and atypical femur fracture.33
There is no recommendation for a drug holiday for denosumab. An increase in, or no loss of, bone density and no new fractures while being treated are signs of effective treatment. There is no guideline for stopping denosumab, unless the patient develops adverse effects.
❚ Bone anabolics. Patients with a very high risk of fracture (eg, who have sustained multiple vertebral fractures), can begin treatment with teriparatide (20 μg/d subcutaneously) or abaloparatide (80 μg/d subcutaneously) for as long as 2 years, followed by treatment with an antiresorptive, such as a bisphosphonate.4,6 Teriparatide can be used in patients who have not responded to an antiresorptive as first-line treatment.
Both abaloparatide and teriparatide might be associated with a risk of osteosarcoma and are contraindicated in patients who are at increased risk of osteosarcoma.36,39,40
❚ Romosozumab, a monoclonal sclerostin antibody, can be used in patients with very high risk of fracture or with multiple vertebral fractures. Romosozumab increases bone formation and reduces bone resorption. It is given monthly, 210 mg subcutaneously, for 1 year. The recommendation is that patients who have completed a course of romosozumab continue with antiresorptive treatment.26
Romosozumab is associated with an increase in the risk of cardiovascular disease, including stroke and myocardial infarction.26
❚ Raloxifene, a selective estrogen-receptor modulator, is no longer a first-line agent for osteoporosis in older adults34 because of its association with an increased risk of deep-vein thrombosis, pulmonary embolism, and lethal stroke. However, raloxifene can be used, at 60 mg/d, when bisphosphonates or denosumab are unsuitable. In addition, raloxifene is particularly useful in women with a high risk of breast cancer and in men who are taking a long-acting gonadotropin-releasing hormone agonist for prostate cancer.37,38
Continue to: Influence of chronic...
Influence of chronic diseaseon bone health
Chronic diseases—hypertension, type 2 diabetes, hyperthyroidism, rheumatoid arthritis, ankylosing spondylitis, and gastroenterologic disorders such as celiac disease and ulcerative colitis—are known to affect bone loss that can hasten osteoporosis.16,18,21 Furthermore, medications used to treat chronic diseases are known to affect bone health: Some, such as statins, ACE inhibitors, and hydrochlorothiazide, are bone protective; others, such as steroids, pioglitazone, and selective serotonin reuptake inhibitors, accelerate bone loss.1,14,42,43 It is important to be aware of the effect of a patient’s chronic diseases, and treatments for those diseases, on bone health, to help develop an individualized osteoporosis prevention plan.
Monitoring the efficacy of treatment
Treatment of osteoporosis should not be initiated without baseline measurement of BMD of the spine and hip. Subsequent to establishing that baseline, serial measurement of BMD can be used to (1) determine when treatment needs to be initiated for an untreated patient and (2) assess response in a treated patient. There is no consensus on the interval at which DXA should be repeated for the purpose of monitoring treatment response; frequency depends on the individual’s circumstances and the medication used. Notably, many physicians repeat DXA after 2 years of treatment8; however, the American College of Physicians recommends against repeating DXA within the first 5 years of pharmacotherapy in women.24
Patients with suspected vertebral fracture or those with loss of height > 1.5 inches require lateral radiographs of the thoracic and lumbar spine to assess the status of fractures.4,6
❚ Bone-turnover markers measured in serum can be used to assess treatment efficacy and patient adherence. The formation marker procollagen type I N-terminal propeptide (P1NP) and the resorption marker beta C-terminal cross-linking telopeptide of type 1 collagen (bCTX) are preferred for evaluating bone turnover in the clinical setting. Assessing P1NP and bCTX at baseline and after 3 months of treatment might be effective in monitoring adherence, particularly in patients taking a bisphosphonate.44
Be sure to address fall prevention
It is important to address falls, and how to prevent them, in patients with osteoporosis. Falls can precipitate fracture in older adults with reduced BMD, and fractures are the most common and debilitating manifestation of osteoporosis. Your discussion of falls with patients should include45:
- consequences of falls
- cautions about medications that can cloud mental alertness
- use of appropriate footwear
- home safety, such as adequate lighting, removal of floor clutter, and installation of handrails in the bathroom and stairwells and on outside steps.
- having an annual comprehensive eye exam.
Osteoporosis is avoidable and treatable
Earlier research reported various expressions of number needed to treat for medical management of osteoporosis—making it difficult to follow a single number as a reference for gauging the effectiveness of pharmacotherapy.46,47 However, for older adults of different ethnic and racial backgrounds with multiple comorbidities and polypharmacy, it might be more pragmatic in primary care to establish a model of goal-oriented, individualized care. By focusing on prevention of bone loss, and being mindful that the risk of fracture almost doubles with a decrease of 1 SD in BMD, you can translate numbers to goals of care.48
In the United States, approximately one-half of osteoporosis cases in adults ≥ 50 years are managed by primary care providers. As a chronic disease, osteoporosis requires that you, first, provide regular monitoring and assessment, because risk can vary with comorbidities,49 and, second, discuss and initiate screening and treatment as appropriate, which can be done annually during a well-care visit.
CORRESPONDENCE
Nahid Rianon, MD, DrPH, Department of Family and Community Medicine, UTHealth McGovern Medical School, 6431 Fannin Street #JJL 324C, Houston, TX, 77030; [email protected]
- What is osteoporosis and what causes it? National Osteoporosis Foundation Website. 2020. Accessed April 28, 2021. www.nof.org/patients/what-is-osteoporosis/
- des Bordes J, Prasad S, Pratt G, et al. Knowledge, beliefs, and concerns about bone health from a systematic review and metasynthesis of qualitative studies. PLoS One. 2020;15:e0227765. doi: 10.1371/journal.pone.0227765
- Solomon DH, Johnston SS, Boytsov NN, et al. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929-1937. doi: 10.1002/jbmr.2202
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381. doi: 10.1007/s00198-014-2794-2
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531. doi: 10.1001/jama.2018.7498
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL
- Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline.J Clin Endocrinol Metab. 2012;97:1802-1822. doi: 10.1210/jc.2011-3045
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. Accessed April 28, 2021. www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
- Looker AC, Frenk SM. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005--2010. Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health and Nutrition Examination Surveys. August 2015. Accessed April 28, 2021. www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf
- Kerschan-Schindl K. Prevention and rehabilitation of osteoporosis. Wien Med Wochenschr. 2016;166:22-27. doi: 10.1007/s10354-015-0417-y
- Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(suppl 1):3-36. doi: 10.1007/s10195-017-0474-7
- Martineau P, Leslie WD, Johansson H, et al. In which patients does lumbar spine trabecular bone score (TBS) have the largest effect? Bone. 2018;113:161-168. doi: 10.1016/j.bone.2018.05.026
- Rianon NJ, Smith SM, Lee M, et al. Glycemic control and bone turnover in older Mexican Americans with type 2 diabetes. J Osteoporos. 2018;2018:7153021. doi: 10.1155/2018/7153021
- Richards C, Hans D, Leslie WD. Trabecular bone score (TBS) predicts fracture in ankylosing spondylitis: The Manitoba BMD Registry. J Clin Densitom. 2020;23:543-548. doi: 10.1016/j.jocd.2020.01.003
- Xue Y, Baker AL, Nader S, et al. Lumbar spine trabecular bone score (TBS) reflects diminished bone quality in patients with diabetes mellitus and oral glucocorticoid therapy. J Clin Densitom. 2018;21:185-192. doi: 10.1016/j.jocd.2017.09.003
- Silva BC, Broy SB, Boutroy S, et al. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18:309-330. doi: 10.1016/j.jocd.2015.06.008
- Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518-530. doi: 10.1002/jbmr.2176
- Leslie WD, Aubry-Rozier B, Lamy O, et al; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602-609.
- Looker AC, Sarafrazi Isfahani N, Fan B, et al. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27:2467-2475. doi: 10.1007/s00198-016-3550-6
- Rianon N, Ambrose CG, Pervin H, et al. Long-term use of angiotensin-converting enzyme inhibitors protects against bone loss in African-American elderly men. Arch Osteoporos. 2017;12:94. doi: 10.1007/s11657-017-0387-3
- Morton DJ, Barrett-Connor EL, Edelstein SL. Thiazides and bone mineral density in elderly men and women. Am J Epidemiol. 1994;139:1107-1115. doi: 10.1093/oxfordjournals.aje.a116954
- Sigurdsson G, Franzson L. Increased bone mineral density in a population-based group of 70-year-old women on thiazide diuretics, independent of parathyroid hormone levels. J Intern Med. 2001;250:51-56. doi: 10.1046/j.1365-2796.2001.00850.x
- Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839. doi: 10.7326/M15-1361
- des Bordes JKA, Suarez-Almazor ME, Volk RJ, et al. Online educational tool to promote bone health in cancer survivors. J Health Commun. 2017;22:808-817. doi: 10.1080/10810730.2017.1360415
- Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:587-594. doi: 10.1210/clinem/dgaa048
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. doi: 10.1001/jama.296.24.2927
- Bone HG, Hosking D, Devogelaer J-P, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. doi: 10.1056/NEJMoa030897
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491. doi: 10.1359/jbmr.0707onj
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972-980. doi: 10.1210/jc.2010-1502
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198. doi: 10.1002/jbmr.3337
- Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018;190:E485-E486. doi: 10.1503/cmaj.180115
- Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46:1515-1525. doi: 10.1016/j.jcms.2018.05.046
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137. doi: 10.1056/NEJMoa062462
- Chesnut CH 3rd, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249. doi: 10.1359/JBMR.040325
- Gilsenban A, Midkiff K, Kellier-Steele N, et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res. 2021;36:244-252. doi: 10.1002/jbmr.4188
- Cuzick J, Sestak I, Bonanni B, et al; SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827-1834. doi: 10.1016/S0140-6736(13)60140-3
- Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841-3846. doi: 10.1210/jc.2003-032058
- TYMLOS. Prescribing information. Radius Health, Inc.; April 2017. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf
- FORTEO. Prescribing information. Eli Lilly and Co.; April 2020. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf
- Wooltorton E. Patients receiving intravenous bisphosphonates should avoid invasive dental procedures. Can Med Assoc J. 2003;172:1684. doi: https://doi.org/10.1503/cmaj.050640
- Chiadika SM, Shobayo FO, Naqvi SH, et al. Lower femoral neck bone mineral density (BMD) in elderly women not on statins. Women Health. 2019;59:845-853. doi: 10.1080/03630242.2019.1567646
- Saraykar S, John V, Cao B, et al. Association of selective serotonin reuptake inhibitors and bone mineral density in elderly women. J Clin Densitom. 2018;21:193-199. doi: 10.1016/j.jocd.2017.05.016
- Lorentzon M, Branco J, Brandi ML, et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 2019;36:2811-2824. doi: 10.1007/s12325-019-01063-9
- STEADI--older adult fall prevention. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2019. Accessed April 28, 2021. www.cdc.gov/steadi/patient.html
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
- Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7:2113-2122.
- Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15:183-187. doi: 10.1359/jbmr.2000.15.2.183
- Rianon N, Anand D, Rasu R. Changing trends in osteoporosis care from specialty to primary care physicians. Curr Med Res Opin. 2013;29:881-888. doi: 10.1185/03007995.2013.809335
- What is osteoporosis and what causes it? National Osteoporosis Foundation Website. 2020. Accessed April 28, 2021. www.nof.org/patients/what-is-osteoporosis/
- des Bordes J, Prasad S, Pratt G, et al. Knowledge, beliefs, and concerns about bone health from a systematic review and metasynthesis of qualitative studies. PLoS One. 2020;15:e0227765. doi: 10.1371/journal.pone.0227765
- Solomon DH, Johnston SS, Boytsov NN, et al. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929-1937. doi: 10.1002/jbmr.2202
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381. doi: 10.1007/s00198-014-2794-2
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531. doi: 10.1001/jama.2018.7498
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL
- Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline.J Clin Endocrinol Metab. 2012;97:1802-1822. doi: 10.1210/jc.2011-3045
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. Accessed April 28, 2021. www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
- Looker AC, Frenk SM. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005--2010. Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health and Nutrition Examination Surveys. August 2015. Accessed April 28, 2021. www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf
- Kerschan-Schindl K. Prevention and rehabilitation of osteoporosis. Wien Med Wochenschr. 2016;166:22-27. doi: 10.1007/s10354-015-0417-y
- Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(suppl 1):3-36. doi: 10.1007/s10195-017-0474-7
- Martineau P, Leslie WD, Johansson H, et al. In which patients does lumbar spine trabecular bone score (TBS) have the largest effect? Bone. 2018;113:161-168. doi: 10.1016/j.bone.2018.05.026
- Rianon NJ, Smith SM, Lee M, et al. Glycemic control and bone turnover in older Mexican Americans with type 2 diabetes. J Osteoporos. 2018;2018:7153021. doi: 10.1155/2018/7153021
- Richards C, Hans D, Leslie WD. Trabecular bone score (TBS) predicts fracture in ankylosing spondylitis: The Manitoba BMD Registry. J Clin Densitom. 2020;23:543-548. doi: 10.1016/j.jocd.2020.01.003
- Xue Y, Baker AL, Nader S, et al. Lumbar spine trabecular bone score (TBS) reflects diminished bone quality in patients with diabetes mellitus and oral glucocorticoid therapy. J Clin Densitom. 2018;21:185-192. doi: 10.1016/j.jocd.2017.09.003
- Silva BC, Broy SB, Boutroy S, et al. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18:309-330. doi: 10.1016/j.jocd.2015.06.008
- Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518-530. doi: 10.1002/jbmr.2176
- Leslie WD, Aubry-Rozier B, Lamy O, et al; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602-609.
- Looker AC, Sarafrazi Isfahani N, Fan B, et al. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27:2467-2475. doi: 10.1007/s00198-016-3550-6
- Rianon N, Ambrose CG, Pervin H, et al. Long-term use of angiotensin-converting enzyme inhibitors protects against bone loss in African-American elderly men. Arch Osteoporos. 2017;12:94. doi: 10.1007/s11657-017-0387-3
- Morton DJ, Barrett-Connor EL, Edelstein SL. Thiazides and bone mineral density in elderly men and women. Am J Epidemiol. 1994;139:1107-1115. doi: 10.1093/oxfordjournals.aje.a116954
- Sigurdsson G, Franzson L. Increased bone mineral density in a population-based group of 70-year-old women on thiazide diuretics, independent of parathyroid hormone levels. J Intern Med. 2001;250:51-56. doi: 10.1046/j.1365-2796.2001.00850.x
- Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839. doi: 10.7326/M15-1361
- des Bordes JKA, Suarez-Almazor ME, Volk RJ, et al. Online educational tool to promote bone health in cancer survivors. J Health Commun. 2017;22:808-817. doi: 10.1080/10810730.2017.1360415
- Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:587-594. doi: 10.1210/clinem/dgaa048
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938. doi: 10.1001/jama.296.24.2927
- Bone HG, Hosking D, Devogelaer J-P, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. doi: 10.1056/NEJMoa030897
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491. doi: 10.1359/jbmr.0707onj
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972-980. doi: 10.1210/jc.2010-1502
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198. doi: 10.1002/jbmr.3337
- Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018;190:E485-E486. doi: 10.1503/cmaj.180115
- Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46:1515-1525. doi: 10.1016/j.jcms.2018.05.046
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137. doi: 10.1056/NEJMoa062462
- Chesnut CH 3rd, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249. doi: 10.1359/JBMR.040325
- Gilsenban A, Midkiff K, Kellier-Steele N, et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res. 2021;36:244-252. doi: 10.1002/jbmr.4188
- Cuzick J, Sestak I, Bonanni B, et al; SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827-1834. doi: 10.1016/S0140-6736(13)60140-3
- Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841-3846. doi: 10.1210/jc.2003-032058
- TYMLOS. Prescribing information. Radius Health, Inc.; April 2017. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf
- FORTEO. Prescribing information. Eli Lilly and Co.; April 2020. Accessed May 20, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf
- Wooltorton E. Patients receiving intravenous bisphosphonates should avoid invasive dental procedures. Can Med Assoc J. 2003;172:1684. doi: https://doi.org/10.1503/cmaj.050640
- Chiadika SM, Shobayo FO, Naqvi SH, et al. Lower femoral neck bone mineral density (BMD) in elderly women not on statins. Women Health. 2019;59:845-853. doi: 10.1080/03630242.2019.1567646
- Saraykar S, John V, Cao B, et al. Association of selective serotonin reuptake inhibitors and bone mineral density in elderly women. J Clin Densitom. 2018;21:193-199. doi: 10.1016/j.jocd.2017.05.016
- Lorentzon M, Branco J, Brandi ML, et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 2019;36:2811-2824. doi: 10.1007/s12325-019-01063-9
- STEADI--older adult fall prevention. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2019. Accessed April 28, 2021. www.cdc.gov/steadi/patient.html
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
- Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7:2113-2122.
- Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15:183-187. doi: 10.1359/jbmr.2000.15.2.183
- Rianon N, Anand D, Rasu R. Changing trends in osteoporosis care from specialty to primary care physicians. Curr Med Res Opin. 2013;29:881-888. doi: 10.1185/03007995.2013.809335
PRACTICE RECOMMENDATIONS
❯ Consider screening for osteoporosis, using bone mineral density (BMD) by dual-energy X-ray absorptiometry (DXA), in all postmenopausal women ≥ 65 years and in women < 65 years at high risk of osteoporosis.
❯ Consider screening in men ≥ 70 years and in younger men at high risk of fracture.
❯ Use the trabecular bone score with DXA BMD to screen patients at high risk of fracture who have a normal BMD—eg, patients with type 2 diabetes or ankylosing spondylitis.
❯ Offer individualized pharmacotherapy to older patients with a diagnosis of osteoporosis and to those at high risk of fracture.