User login
Hospital acquisition had no significant change in the rate of readmission or mortality
Background: Prior studies have examined the impact of hospital system mergers on health care costs, but few studies have previously examined impact on quality and patient experience.
Study design: Retrospective, difference-in-difference analysis.
Setting: 2,232 U.S. hospitals during 2007-2016.
Synopsis: The authors identified 2,232 hospitals, including 246 hospitals that were acquired between 2009 and 2013 and 1,986 control hospitals that were not acquired during this period. They used a difference-in-difference analysis to compare hospital performance on quality and patient experience measures from before and after an acquisition to concurrent changes in control hospitals. Hospital acquisition was associated with a significant decline in measured patient experience. There was no significant differential change in 30-day readmission or mortality. Although there was an association between acquisition and significant improvement in clinical process metrics, the authors found that this improvement occurred almost entirely prior to acquisition.
Bottom line: Hospital acquisition was associated with worse experience for patients and had no significant impact on readmission or mortality rates.
Citation: Beaulieu ND et al. Changes in quality of care after hospital mergers and acquisitions. N Engl J Med. 2020 Jan 2;382:51-9.
Dr. Midha is a hospitalist at Beth Israel Deaconess Medical Center, instructor of medicine, Boston University, and part-time instructor in medicine, Harvard Medical School, all in Boston.
Background: Prior studies have examined the impact of hospital system mergers on health care costs, but few studies have previously examined impact on quality and patient experience.
Study design: Retrospective, difference-in-difference analysis.
Setting: 2,232 U.S. hospitals during 2007-2016.
Synopsis: The authors identified 2,232 hospitals, including 246 hospitals that were acquired between 2009 and 2013 and 1,986 control hospitals that were not acquired during this period. They used a difference-in-difference analysis to compare hospital performance on quality and patient experience measures from before and after an acquisition to concurrent changes in control hospitals. Hospital acquisition was associated with a significant decline in measured patient experience. There was no significant differential change in 30-day readmission or mortality. Although there was an association between acquisition and significant improvement in clinical process metrics, the authors found that this improvement occurred almost entirely prior to acquisition.
Bottom line: Hospital acquisition was associated with worse experience for patients and had no significant impact on readmission or mortality rates.
Citation: Beaulieu ND et al. Changes in quality of care after hospital mergers and acquisitions. N Engl J Med. 2020 Jan 2;382:51-9.
Dr. Midha is a hospitalist at Beth Israel Deaconess Medical Center, instructor of medicine, Boston University, and part-time instructor in medicine, Harvard Medical School, all in Boston.
Background: Prior studies have examined the impact of hospital system mergers on health care costs, but few studies have previously examined impact on quality and patient experience.
Study design: Retrospective, difference-in-difference analysis.
Setting: 2,232 U.S. hospitals during 2007-2016.
Synopsis: The authors identified 2,232 hospitals, including 246 hospitals that were acquired between 2009 and 2013 and 1,986 control hospitals that were not acquired during this period. They used a difference-in-difference analysis to compare hospital performance on quality and patient experience measures from before and after an acquisition to concurrent changes in control hospitals. Hospital acquisition was associated with a significant decline in measured patient experience. There was no significant differential change in 30-day readmission or mortality. Although there was an association between acquisition and significant improvement in clinical process metrics, the authors found that this improvement occurred almost entirely prior to acquisition.
Bottom line: Hospital acquisition was associated with worse experience for patients and had no significant impact on readmission or mortality rates.
Citation: Beaulieu ND et al. Changes in quality of care after hospital mergers and acquisitions. N Engl J Med. 2020 Jan 2;382:51-9.
Dr. Midha is a hospitalist at Beth Israel Deaconess Medical Center, instructor of medicine, Boston University, and part-time instructor in medicine, Harvard Medical School, all in Boston.
Revised dispatch system boosts bystander CPR in those with limited English
The improved Los Angeles medical dispatch system prompted more callers with limited English proficiency to initiate telecommunicator-assisted cardiopulmonary resuscitation (T-CPR), compared with the previous system, a new study shows.
The Los Angeles Tiered Dispatch System (LA-TDS), adopted in late 2014, used simplified questions aimed at identifying cardiac arrest, compared with the city’s earlier Medical Priority Dispatch System (MPDS).
The result was substantially decreased call processing times, decreased “undertriage” of out-of-hospital cardiac arrest (OHCA), and improved overall T-CPR rates (Resuscitation. 2020 Oct;155:74-81).
But now, a secondary analysis of the data shows there was a much higher jump in T-CPR rates among a small subset of callers with limited English proficiency, compared with those proficient in English (JAMA Network Open. 2021;4[6]:e216827).
“This was an unanticipated, significant, and disproportionate change, but fortunately a very good change,” lead author Stephen Sanko, MD, said in an interview.
While the T-CPR rate among English-proficient callers increased from 55% with the MPDS to 67% with the LA-TDS (odds ratio, 1.66; P = .007), it rose from 28% to 69% (OR, 5.66; P = .003) among callers with limited English proficiency. In the adjusted analysis, the new LA-TDS was associated with a 69% higher prevalence of T-CPR among English-proficient callers, compared with a 350% greater prevalence among callers with limited English proficiency.
“The emergency communication process between a caller and 911 telecommunicator is more complex than we thought, and likely constitutes a unique subsubspecialty that interacts with fields as diverse as medicine, health equity, linguistics, sociology, consumer behavior and others,” said Dr. Sanko, who is from the division of emergency medical services at the University of Southern California in Los Angeles.
“Yet in spite of this complexity, we’re starting to be able to reproducibly classify elements of the emergency conversation that we believe are tied to outcomes we all care about. ... Modulators of health disparities are present as early as the dispatch conversation, and, importantly, they can be intervened upon to promote improved outcomes,” he continued.
The retrospective cohort study was a predefined secondary analysis of a previously published study comparing telecommunicator management of out-of-hospital cardiac arrest over 3 months with the MPDS versus 3 months with the LA-TDS. The primary outcome was the number of patients who received telecommunicator-assisted chest compressions from callers with limited English proficiency.
Of the 597 emergency calls that met the inclusion criteria, 289 (48%) were in the MPDS cohort and 308 (52%) were in the LA-TDS cohort. In the MPDS cohort, 263 callers had English proficiency and 26 had limited proficiency; in the latter cohort, those figures were 273 and 35, respectively.
There were no significant differences between cohorts in the use of real-time translation services, which were employed 27%-31% of the time.
The reason for the overall T-CPR improvement is likely that the LA-TDS was tailored to the community needs, said Dr. Sanko. “Most people, including doctors, think of 911 dispatch as something simple and straightforward, like ordering a pizza or calling a ride share. [But] LA-TDS is a ‘home grown’ dispatch system whose structure, questions, and emergency instructions were all developed by EMS medical directors and telecommunicators with extensive experience in our community.”
That being said, the researchers acknowledge that the reason behind the bigger T-CPR boost in LEP callers remains unclear. Although the link between language and system was statistically significant, they noted “it was not an a priori hypothesis and appeared to be largely attributable to the low T-CPR rates for callers with limited English proficiency using MPDS.” Additionally, such callers were “remarkably under-represented” in the sample, “which included approximately 600 calls over two quarters in a large city,” said Dr Sanko.
“We hypothesize that a more direct structure, earlier commitment to treating patients with abnormal life status indicators as being suspected cardiac arrest cases, and earlier reassurance may have improved caller confidence that telecommunicators knew what they were doing. This in turn may have translated into an increased likelihood of bystander caller willingness to perform immediate life-saving maneuvers.”
Despite a number of limitations, “the study is important and highlights instructive topics for discussion that suggest potential next-step opportunities,” noted Richard Chocron, MD, PhD, Miranda Lewis, MD, and Thomas Rea, MD, MPH, in an invited commentary that accompanied the publication. Dr. Chocron is from the Paris University, Paris Research Cardiovascular Center, INSERM; Dr. Lewis is from the Georges Pompidou European Hospital in Paris; and Dr. Rea is from the Division of Emergency Medical Services, Public Health–Seattle & King County. Both Dr. Lewis and Dr. Rea are also at the University of Washington, Seattle.
“Sanko et al. found that approximately 10% of all emergency calls were classified as limited English proficiency calls in a community in which 19% of the population was considered to have limited English proficiency,” they added. “This finding suggests the possibility that populations with limited English proficiency are less likely to activate 911 for incidence of cardiac arrest. If true, this finding would compound the health disparity observed among those with limited English proficiency. This topic is important in that it transcends the role of EMS personnel and engages a broad spectrum of societal stakeholders. We must listen, learn, and ultimately deliver public safety resources to groups who have not been well served by conventional approaches.”
None of the authors or editorialists reported any conflicts of interest.
The improved Los Angeles medical dispatch system prompted more callers with limited English proficiency to initiate telecommunicator-assisted cardiopulmonary resuscitation (T-CPR), compared with the previous system, a new study shows.
The Los Angeles Tiered Dispatch System (LA-TDS), adopted in late 2014, used simplified questions aimed at identifying cardiac arrest, compared with the city’s earlier Medical Priority Dispatch System (MPDS).
The result was substantially decreased call processing times, decreased “undertriage” of out-of-hospital cardiac arrest (OHCA), and improved overall T-CPR rates (Resuscitation. 2020 Oct;155:74-81).
But now, a secondary analysis of the data shows there was a much higher jump in T-CPR rates among a small subset of callers with limited English proficiency, compared with those proficient in English (JAMA Network Open. 2021;4[6]:e216827).
“This was an unanticipated, significant, and disproportionate change, but fortunately a very good change,” lead author Stephen Sanko, MD, said in an interview.
While the T-CPR rate among English-proficient callers increased from 55% with the MPDS to 67% with the LA-TDS (odds ratio, 1.66; P = .007), it rose from 28% to 69% (OR, 5.66; P = .003) among callers with limited English proficiency. In the adjusted analysis, the new LA-TDS was associated with a 69% higher prevalence of T-CPR among English-proficient callers, compared with a 350% greater prevalence among callers with limited English proficiency.
“The emergency communication process between a caller and 911 telecommunicator is more complex than we thought, and likely constitutes a unique subsubspecialty that interacts with fields as diverse as medicine, health equity, linguistics, sociology, consumer behavior and others,” said Dr. Sanko, who is from the division of emergency medical services at the University of Southern California in Los Angeles.
“Yet in spite of this complexity, we’re starting to be able to reproducibly classify elements of the emergency conversation that we believe are tied to outcomes we all care about. ... Modulators of health disparities are present as early as the dispatch conversation, and, importantly, they can be intervened upon to promote improved outcomes,” he continued.
The retrospective cohort study was a predefined secondary analysis of a previously published study comparing telecommunicator management of out-of-hospital cardiac arrest over 3 months with the MPDS versus 3 months with the LA-TDS. The primary outcome was the number of patients who received telecommunicator-assisted chest compressions from callers with limited English proficiency.
Of the 597 emergency calls that met the inclusion criteria, 289 (48%) were in the MPDS cohort and 308 (52%) were in the LA-TDS cohort. In the MPDS cohort, 263 callers had English proficiency and 26 had limited proficiency; in the latter cohort, those figures were 273 and 35, respectively.
There were no significant differences between cohorts in the use of real-time translation services, which were employed 27%-31% of the time.
The reason for the overall T-CPR improvement is likely that the LA-TDS was tailored to the community needs, said Dr. Sanko. “Most people, including doctors, think of 911 dispatch as something simple and straightforward, like ordering a pizza or calling a ride share. [But] LA-TDS is a ‘home grown’ dispatch system whose structure, questions, and emergency instructions were all developed by EMS medical directors and telecommunicators with extensive experience in our community.”
That being said, the researchers acknowledge that the reason behind the bigger T-CPR boost in LEP callers remains unclear. Although the link between language and system was statistically significant, they noted “it was not an a priori hypothesis and appeared to be largely attributable to the low T-CPR rates for callers with limited English proficiency using MPDS.” Additionally, such callers were “remarkably under-represented” in the sample, “which included approximately 600 calls over two quarters in a large city,” said Dr Sanko.
“We hypothesize that a more direct structure, earlier commitment to treating patients with abnormal life status indicators as being suspected cardiac arrest cases, and earlier reassurance may have improved caller confidence that telecommunicators knew what they were doing. This in turn may have translated into an increased likelihood of bystander caller willingness to perform immediate life-saving maneuvers.”
Despite a number of limitations, “the study is important and highlights instructive topics for discussion that suggest potential next-step opportunities,” noted Richard Chocron, MD, PhD, Miranda Lewis, MD, and Thomas Rea, MD, MPH, in an invited commentary that accompanied the publication. Dr. Chocron is from the Paris University, Paris Research Cardiovascular Center, INSERM; Dr. Lewis is from the Georges Pompidou European Hospital in Paris; and Dr. Rea is from the Division of Emergency Medical Services, Public Health–Seattle & King County. Both Dr. Lewis and Dr. Rea are also at the University of Washington, Seattle.
“Sanko et al. found that approximately 10% of all emergency calls were classified as limited English proficiency calls in a community in which 19% of the population was considered to have limited English proficiency,” they added. “This finding suggests the possibility that populations with limited English proficiency are less likely to activate 911 for incidence of cardiac arrest. If true, this finding would compound the health disparity observed among those with limited English proficiency. This topic is important in that it transcends the role of EMS personnel and engages a broad spectrum of societal stakeholders. We must listen, learn, and ultimately deliver public safety resources to groups who have not been well served by conventional approaches.”
None of the authors or editorialists reported any conflicts of interest.
The improved Los Angeles medical dispatch system prompted more callers with limited English proficiency to initiate telecommunicator-assisted cardiopulmonary resuscitation (T-CPR), compared with the previous system, a new study shows.
The Los Angeles Tiered Dispatch System (LA-TDS), adopted in late 2014, used simplified questions aimed at identifying cardiac arrest, compared with the city’s earlier Medical Priority Dispatch System (MPDS).
The result was substantially decreased call processing times, decreased “undertriage” of out-of-hospital cardiac arrest (OHCA), and improved overall T-CPR rates (Resuscitation. 2020 Oct;155:74-81).
But now, a secondary analysis of the data shows there was a much higher jump in T-CPR rates among a small subset of callers with limited English proficiency, compared with those proficient in English (JAMA Network Open. 2021;4[6]:e216827).
“This was an unanticipated, significant, and disproportionate change, but fortunately a very good change,” lead author Stephen Sanko, MD, said in an interview.
While the T-CPR rate among English-proficient callers increased from 55% with the MPDS to 67% with the LA-TDS (odds ratio, 1.66; P = .007), it rose from 28% to 69% (OR, 5.66; P = .003) among callers with limited English proficiency. In the adjusted analysis, the new LA-TDS was associated with a 69% higher prevalence of T-CPR among English-proficient callers, compared with a 350% greater prevalence among callers with limited English proficiency.
“The emergency communication process between a caller and 911 telecommunicator is more complex than we thought, and likely constitutes a unique subsubspecialty that interacts with fields as diverse as medicine, health equity, linguistics, sociology, consumer behavior and others,” said Dr. Sanko, who is from the division of emergency medical services at the University of Southern California in Los Angeles.
“Yet in spite of this complexity, we’re starting to be able to reproducibly classify elements of the emergency conversation that we believe are tied to outcomes we all care about. ... Modulators of health disparities are present as early as the dispatch conversation, and, importantly, they can be intervened upon to promote improved outcomes,” he continued.
The retrospective cohort study was a predefined secondary analysis of a previously published study comparing telecommunicator management of out-of-hospital cardiac arrest over 3 months with the MPDS versus 3 months with the LA-TDS. The primary outcome was the number of patients who received telecommunicator-assisted chest compressions from callers with limited English proficiency.
Of the 597 emergency calls that met the inclusion criteria, 289 (48%) were in the MPDS cohort and 308 (52%) were in the LA-TDS cohort. In the MPDS cohort, 263 callers had English proficiency and 26 had limited proficiency; in the latter cohort, those figures were 273 and 35, respectively.
There were no significant differences between cohorts in the use of real-time translation services, which were employed 27%-31% of the time.
The reason for the overall T-CPR improvement is likely that the LA-TDS was tailored to the community needs, said Dr. Sanko. “Most people, including doctors, think of 911 dispatch as something simple and straightforward, like ordering a pizza or calling a ride share. [But] LA-TDS is a ‘home grown’ dispatch system whose structure, questions, and emergency instructions were all developed by EMS medical directors and telecommunicators with extensive experience in our community.”
That being said, the researchers acknowledge that the reason behind the bigger T-CPR boost in LEP callers remains unclear. Although the link between language and system was statistically significant, they noted “it was not an a priori hypothesis and appeared to be largely attributable to the low T-CPR rates for callers with limited English proficiency using MPDS.” Additionally, such callers were “remarkably under-represented” in the sample, “which included approximately 600 calls over two quarters in a large city,” said Dr Sanko.
“We hypothesize that a more direct structure, earlier commitment to treating patients with abnormal life status indicators as being suspected cardiac arrest cases, and earlier reassurance may have improved caller confidence that telecommunicators knew what they were doing. This in turn may have translated into an increased likelihood of bystander caller willingness to perform immediate life-saving maneuvers.”
Despite a number of limitations, “the study is important and highlights instructive topics for discussion that suggest potential next-step opportunities,” noted Richard Chocron, MD, PhD, Miranda Lewis, MD, and Thomas Rea, MD, MPH, in an invited commentary that accompanied the publication. Dr. Chocron is from the Paris University, Paris Research Cardiovascular Center, INSERM; Dr. Lewis is from the Georges Pompidou European Hospital in Paris; and Dr. Rea is from the Division of Emergency Medical Services, Public Health–Seattle & King County. Both Dr. Lewis and Dr. Rea are also at the University of Washington, Seattle.
“Sanko et al. found that approximately 10% of all emergency calls were classified as limited English proficiency calls in a community in which 19% of the population was considered to have limited English proficiency,” they added. “This finding suggests the possibility that populations with limited English proficiency are less likely to activate 911 for incidence of cardiac arrest. If true, this finding would compound the health disparity observed among those with limited English proficiency. This topic is important in that it transcends the role of EMS personnel and engages a broad spectrum of societal stakeholders. We must listen, learn, and ultimately deliver public safety resources to groups who have not been well served by conventional approaches.”
None of the authors or editorialists reported any conflicts of interest.
FROM JAMA NETWORK OPEN
CRC screening guidelines: 45 is the new 50, and 85 is the new 75
Build a better mousetrap, and the world will beat a path to your door. Find an accurate, completely noninvasive method for colorectal cancer screening and you’ll probably win the Nobel Prize for Medicine or Physiology.
But until then, we’ll have to make do with colonoscopy, fecal immunochemical testing (FIT), and other messy but necessary means for preventing full-blown CRC and reducing the risk of CRC morbidity and mortality. And start turning to them earlier in patients’ lives.
The U.S. Preventive Services Task Force (USPSTF) has issued an update of its 2016 recommendations for CRC screening, for the first time advising that screening for all average-risk adults begin at age 45. This new recommendation is in line with the guidelines issued by the American Cancer Society, which were updated in 2018, to reflect the inescapable truth that CRC is increasingly being diagnosed at a younger age.
Not to be left out, the U.S. Multi-Society Task Force (MSTF) – which represents the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy – issued a statement supporting lowering the age of initial screening in normal-risk adults to 45, and promised that an update of the 2017 guidelines would include the new recommendation.
Recommendations influence reimbursement
Guidelines are often honored as much in the breach as in the observance, but those issued by the USPSTF have unique sway, according to Sonia S. Kupfer, MD, of the section of gastroenterology, hepatology, and nutrition at the University of Chicago, and colleagues.
“While other guidelines have recommended this younger age, the USPSTF guidelines directly inform insurance coverage and waiving of cost sharing as part of federal law,” they wrote in an editorial accompanying the USPSTF guideline statement in the Journal of the American Medical Association.
Although the USPSTF rated its recommendation on starting at age 45 a ‘B’ level – indicating a moderate certainty of moderate benefit – it’s an important step, Dr. Kupfer said in an interview.
“The big advantage here is that we may be able to make a dent in this early-onset colorectal cancer, which, having seen many of these patients, is very alarming, and they don’t always seem to have classic risk factors,” she said. “So, getting them when we can potentially prevent cancer by taking out polyps, or even getting them in an earlier stage, certainly will be beneficial.”
The MSTF also considered recommending 45 as the starting age for normal-risk patients in its 2017 guidelines, noted Douglas Rex, MD, who was chair of the committee that drew up those guidelines, as well as director of endoscopy at Indiana University Hospital in Indianapolis.
“Since that time there has been more evidence, and there’s also some empiric evidence, about the yield of screening in the 45- to 49-year-old age group,” he said in an interview.
The one that gets done
Although the various guidelines differ in specifics, all are in agreement on the general proposition that colonoscopy is the gold standard for screening and detecting the presence of polyps, adenomas, and CRC.
But as USPSTF member Martha Kubik, PhD, RN, director of the George Mason University School of Nursing in Fairfax, Va., said in a statement: “The right test is the one that gets done.”
Gastroenterologists acknowledge that despite its efficacy, colonoscopy is an invasive procedure involving meticulous and unpleasant and/or uncomfortable bowel prep, sedation, and significant time requirements.
In the theory that something is better than nothing, with clinical evidence of varying degrees of quality, the USPSTF recommends the following procedures or tests for average-risk adults:
- Colonoscopy screening every 10 years.
- every 10 years plus annual FIT.
- CT colonography every 5 years.
- High-sensitivity guaiac fecal occult blood test (gFOBT; Hemoccult II) or FIT every year.
- Stool DNA-FIT (Cologuard) every 1 to 3 years.
The Food and Drug Administration also recently approved an artificial intelligence device designed for use with an endoscope, which its manufacturer says can help clinicians detect gastrointestinal lesions they might otherwise miss. This is not a new screening method, but rather an enhancement of existing ones. It neither diagnoses lesions nor recommends treatments, and is not intended to take the place of laboratory sampling.
“I think artificial intelligence is poised to make colonoscopy more effective,” Dr. Rex said. “In the first five trials that we’ve seen, the average increase in the adenoma detection rate has been 11%, and for each 1% gain in the adenoma detection rate, patients have about a 3% decline in their risk of getting cancer after a colonoscopy and about a 5% decline in their risk for fatal cancer. Those are the largest gains that we’ve seen from a technology.”
Different evidence, varied outcomes
Despite the recommendations, a quick dive into the morass of evidence from multiple studies featured in the updated USPSTF guidelines shows that not all screening methods are created equal.
A single colonoscopy, for example, has been shown in large cohort studies to be associated with a 68% reduction in CRC mortality vs. no screening, compared with a 26% reduction with flexible sigmoidoscopy performed every 3-5 years, 22% reduction with Hemoccult II, and 10% with FIT every 2 years.
The USPSTF investigators did not find any studies evaluating the effectiveness of CT colonography, high-sensitivity gFOBT, stool DNA with or without FIT, or serum tests on CRC incidence, CRC mortality, or both.
The two visualization methods for which studies were available, colonoscopy and CT colonography, were generally comparable in sensitivity and specificity for detecting and correctly identifying adenomas 6 mm and larger, although colonography had higher sensitivity for CRC than colonoscopy.
When performed in two to nine annual or biennial rounds, gFOBT was associated with a reduction of CRC-specific mortality of 9% after 19.5 years and 22% at 30 years, compared with no screening.
In observational studies, screening colonoscopy and FIT were both associated with lower risk of CRC incidence or mortality, compared with no screening.
When to stop?
The major guidelines are all in agreement that once an individual reaches age 75, the decision about whether to continue screening should be made on a case-by-case basis, depending on the patient’s overall health, relative risks, and life expectancy.
But if a study published 2 days after the release of the USPSTF guidelines is any indication, just as 45 is the new 50 for starting screening, 85 may be the new 75 for stopping it.
As researchers from Mass General Cancer Center in Boston reported in JAMA Oncology, screening endoscopy for persons older than 75 in otherwise good health can reduce the risk for CRC incidence and CRC-related death by approximately 40%.
The researchers also found, however, that screening did not provide a significant survival benefit for individuals older than 75 with cardiovascular disease, diabetes, or three or more other health conditions.
“Until now, there really weren’t clear data to help us decide whether patients should be screened after age 75,” coinvestigator Andrew T. Chan. MD, MPH, a gastroenterologist and chief of the clinical and translational epidemiology unit at Mass General, said in a statement. “Current guidance was largely based on modeling and extrapolation of studies conducted in other age groups, and not on solid data to show whether screening was actually helpful in an older population.”
In an interview, Dr. Chan said that while the recommendation to screen older adults has to be tailored to individual risk factors, “it should help to provide more confidence for clinicians and patients.”
“I think this is particularly important, because we know that the population as a whole is aging, so more and more people are in this category of over the age of 75, and it’s not an infrequent issue in the clinic as to what to continue with respect to preventative interventions,” he said.
Dr. Kupfer said that the findings by Dr. Chan and colleagues are largely in keeping with guideline recommendations.
“We factor in a lot of different things, including comorbidities, in making the decision to continue screening up to age 85. Certainly, physiological age and chronological age aren’t always the same, so not every 75-year-old is going to be in the same boat,” she said.
“The risk goes up as people get older, but there starts to be competing mortality at some point, and if you have to do a colonoscopy there are obviously issues related to sedation that, as someone gets older, we have to take into consideration,” she added.
Patients frequently confuse screening with surveillance, Dr. Rex said, and he has had patients tell him: “I hear you don’t do these anymore on people over the age of 75.”
“But that’s not true,” Dr. Rex emphasized.
“Screening is generally considered appropriate even up to the age of 85, but between 75 and 85 it should be considered on an individual basis, and there are several considerations there,” Dr. Rex said. “One is whether a patient has ever been screened before. The second is how they were screened. Third is their life expectancy and how many comorbidities they have. And fourth is their personal feelings about it and interest in it.”
He pointed out that the false-positive rate of stool DNA-FIT tests increases with age, and that for older patients who were previously screened, a standard FIT test may be a more appropriate.
So doc, what should I do?
Multiple guidelines, levels of evidence, different screening methods with varying efficacy, individual risk factors – how can clinicians make sense of all these data at the practice level?
“Any modality can be used for screening. Colorectal cancer screening can be done in a number of different ways, and I think that sometimes gets lost in the shuffle, and the thought becomes that everybody has to get a colonoscopy at 45, but there are certainly other tests,” Dr. Kupfer said.
“This just reminds us that we should be thinking about ways we can be doing screening on a population basis, so that we make sure there is equity,” she said.
It’s also important to remember that patients with familial CRC syndromes should begin screening at an even earlier age than average-risk adults, she emphasized.
“To really make a dent in early-onset colorectal cancer, we have to continue to take an active case-finding approach,” she said.
Dr. Rex noted that despite minor differences, the major guidelines are all similar in their initial statements that screening works.
“We’ve still got 50,000 people a year dying from colorectal cancer, lots more than that of new cases,” he said. “If you look at a single factor contributing to that the most, it’s that a lot of the American public is not getting screened at all – it can be up to 40% of the population, depending on what state you’re in.”
Although there are a variety of screening methods available, there are few studies directly comparing them, leaving clinicians at the practice level with the task of presenting all or some of them to patients.
“What the Multi-Society Task Force says that is different, and I think that they get right, is that we don’t have any data [indicating] that offering five, six, or seven options increases the chance of screening – there’s really no evidence that going past two does,” Dr. Rex said.
“The list of options also includes things that nobody actually does,” he added. “For example, flexible sigmoidoscopy has dropped off the map, and FIT has largely replaced guaiac-based testing, even high-sensitivity guaiac. Nobody is really doing CT colonography. The three tests that are being used are colonoscopy, FIT, and [stool DNA-FIT].”
Dr. Rex said that he favors sequential offers, presenting colonoscopy first, emphasizing the benefits for higher-risk patients, and if the patients refuse, offering a fecal-based test.
“Minimizing the number of options makes the conversation feasible, and it’s still very responsible,” he said.
Dr. Kupfer has performed collaborative research with Myriad Genetic Laboratories. She is an editorial advisory board member for GI & Hepatology News from MDedge, part of the Medscape Professional Network. Dr. Rex serves or served as a consultant for Olympus Corporation; Boston Scientific; Medtronic; and Aries; and received research support from Endo-Aid; Olympus Corporation; and Medivators. He has ownership in ai4gi. He is an editorial board member for Medscape Gastroenterology. Dr. Chan has served as a consultant to Pfizer, Bayer AG, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
This article was updated June 8, 2021.
Build a better mousetrap, and the world will beat a path to your door. Find an accurate, completely noninvasive method for colorectal cancer screening and you’ll probably win the Nobel Prize for Medicine or Physiology.
But until then, we’ll have to make do with colonoscopy, fecal immunochemical testing (FIT), and other messy but necessary means for preventing full-blown CRC and reducing the risk of CRC morbidity and mortality. And start turning to them earlier in patients’ lives.
The U.S. Preventive Services Task Force (USPSTF) has issued an update of its 2016 recommendations for CRC screening, for the first time advising that screening for all average-risk adults begin at age 45. This new recommendation is in line with the guidelines issued by the American Cancer Society, which were updated in 2018, to reflect the inescapable truth that CRC is increasingly being diagnosed at a younger age.
Not to be left out, the U.S. Multi-Society Task Force (MSTF) – which represents the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy – issued a statement supporting lowering the age of initial screening in normal-risk adults to 45, and promised that an update of the 2017 guidelines would include the new recommendation.
Recommendations influence reimbursement
Guidelines are often honored as much in the breach as in the observance, but those issued by the USPSTF have unique sway, according to Sonia S. Kupfer, MD, of the section of gastroenterology, hepatology, and nutrition at the University of Chicago, and colleagues.
“While other guidelines have recommended this younger age, the USPSTF guidelines directly inform insurance coverage and waiving of cost sharing as part of federal law,” they wrote in an editorial accompanying the USPSTF guideline statement in the Journal of the American Medical Association.
Although the USPSTF rated its recommendation on starting at age 45 a ‘B’ level – indicating a moderate certainty of moderate benefit – it’s an important step, Dr. Kupfer said in an interview.
“The big advantage here is that we may be able to make a dent in this early-onset colorectal cancer, which, having seen many of these patients, is very alarming, and they don’t always seem to have classic risk factors,” she said. “So, getting them when we can potentially prevent cancer by taking out polyps, or even getting them in an earlier stage, certainly will be beneficial.”
The MSTF also considered recommending 45 as the starting age for normal-risk patients in its 2017 guidelines, noted Douglas Rex, MD, who was chair of the committee that drew up those guidelines, as well as director of endoscopy at Indiana University Hospital in Indianapolis.
“Since that time there has been more evidence, and there’s also some empiric evidence, about the yield of screening in the 45- to 49-year-old age group,” he said in an interview.
The one that gets done
Although the various guidelines differ in specifics, all are in agreement on the general proposition that colonoscopy is the gold standard for screening and detecting the presence of polyps, adenomas, and CRC.
But as USPSTF member Martha Kubik, PhD, RN, director of the George Mason University School of Nursing in Fairfax, Va., said in a statement: “The right test is the one that gets done.”
Gastroenterologists acknowledge that despite its efficacy, colonoscopy is an invasive procedure involving meticulous and unpleasant and/or uncomfortable bowel prep, sedation, and significant time requirements.
In the theory that something is better than nothing, with clinical evidence of varying degrees of quality, the USPSTF recommends the following procedures or tests for average-risk adults:
- Colonoscopy screening every 10 years.
- every 10 years plus annual FIT.
- CT colonography every 5 years.
- High-sensitivity guaiac fecal occult blood test (gFOBT; Hemoccult II) or FIT every year.
- Stool DNA-FIT (Cologuard) every 1 to 3 years.
The Food and Drug Administration also recently approved an artificial intelligence device designed for use with an endoscope, which its manufacturer says can help clinicians detect gastrointestinal lesions they might otherwise miss. This is not a new screening method, but rather an enhancement of existing ones. It neither diagnoses lesions nor recommends treatments, and is not intended to take the place of laboratory sampling.
“I think artificial intelligence is poised to make colonoscopy more effective,” Dr. Rex said. “In the first five trials that we’ve seen, the average increase in the adenoma detection rate has been 11%, and for each 1% gain in the adenoma detection rate, patients have about a 3% decline in their risk of getting cancer after a colonoscopy and about a 5% decline in their risk for fatal cancer. Those are the largest gains that we’ve seen from a technology.”
Different evidence, varied outcomes
Despite the recommendations, a quick dive into the morass of evidence from multiple studies featured in the updated USPSTF guidelines shows that not all screening methods are created equal.
A single colonoscopy, for example, has been shown in large cohort studies to be associated with a 68% reduction in CRC mortality vs. no screening, compared with a 26% reduction with flexible sigmoidoscopy performed every 3-5 years, 22% reduction with Hemoccult II, and 10% with FIT every 2 years.
The USPSTF investigators did not find any studies evaluating the effectiveness of CT colonography, high-sensitivity gFOBT, stool DNA with or without FIT, or serum tests on CRC incidence, CRC mortality, or both.
The two visualization methods for which studies were available, colonoscopy and CT colonography, were generally comparable in sensitivity and specificity for detecting and correctly identifying adenomas 6 mm and larger, although colonography had higher sensitivity for CRC than colonoscopy.
When performed in two to nine annual or biennial rounds, gFOBT was associated with a reduction of CRC-specific mortality of 9% after 19.5 years and 22% at 30 years, compared with no screening.
In observational studies, screening colonoscopy and FIT were both associated with lower risk of CRC incidence or mortality, compared with no screening.
When to stop?
The major guidelines are all in agreement that once an individual reaches age 75, the decision about whether to continue screening should be made on a case-by-case basis, depending on the patient’s overall health, relative risks, and life expectancy.
But if a study published 2 days after the release of the USPSTF guidelines is any indication, just as 45 is the new 50 for starting screening, 85 may be the new 75 for stopping it.
As researchers from Mass General Cancer Center in Boston reported in JAMA Oncology, screening endoscopy for persons older than 75 in otherwise good health can reduce the risk for CRC incidence and CRC-related death by approximately 40%.
The researchers also found, however, that screening did not provide a significant survival benefit for individuals older than 75 with cardiovascular disease, diabetes, or three or more other health conditions.
“Until now, there really weren’t clear data to help us decide whether patients should be screened after age 75,” coinvestigator Andrew T. Chan. MD, MPH, a gastroenterologist and chief of the clinical and translational epidemiology unit at Mass General, said in a statement. “Current guidance was largely based on modeling and extrapolation of studies conducted in other age groups, and not on solid data to show whether screening was actually helpful in an older population.”
In an interview, Dr. Chan said that while the recommendation to screen older adults has to be tailored to individual risk factors, “it should help to provide more confidence for clinicians and patients.”
“I think this is particularly important, because we know that the population as a whole is aging, so more and more people are in this category of over the age of 75, and it’s not an infrequent issue in the clinic as to what to continue with respect to preventative interventions,” he said.
Dr. Kupfer said that the findings by Dr. Chan and colleagues are largely in keeping with guideline recommendations.
“We factor in a lot of different things, including comorbidities, in making the decision to continue screening up to age 85. Certainly, physiological age and chronological age aren’t always the same, so not every 75-year-old is going to be in the same boat,” she said.
“The risk goes up as people get older, but there starts to be competing mortality at some point, and if you have to do a colonoscopy there are obviously issues related to sedation that, as someone gets older, we have to take into consideration,” she added.
Patients frequently confuse screening with surveillance, Dr. Rex said, and he has had patients tell him: “I hear you don’t do these anymore on people over the age of 75.”
“But that’s not true,” Dr. Rex emphasized.
“Screening is generally considered appropriate even up to the age of 85, but between 75 and 85 it should be considered on an individual basis, and there are several considerations there,” Dr. Rex said. “One is whether a patient has ever been screened before. The second is how they were screened. Third is their life expectancy and how many comorbidities they have. And fourth is their personal feelings about it and interest in it.”
He pointed out that the false-positive rate of stool DNA-FIT tests increases with age, and that for older patients who were previously screened, a standard FIT test may be a more appropriate.
So doc, what should I do?
Multiple guidelines, levels of evidence, different screening methods with varying efficacy, individual risk factors – how can clinicians make sense of all these data at the practice level?
“Any modality can be used for screening. Colorectal cancer screening can be done in a number of different ways, and I think that sometimes gets lost in the shuffle, and the thought becomes that everybody has to get a colonoscopy at 45, but there are certainly other tests,” Dr. Kupfer said.
“This just reminds us that we should be thinking about ways we can be doing screening on a population basis, so that we make sure there is equity,” she said.
It’s also important to remember that patients with familial CRC syndromes should begin screening at an even earlier age than average-risk adults, she emphasized.
“To really make a dent in early-onset colorectal cancer, we have to continue to take an active case-finding approach,” she said.
Dr. Rex noted that despite minor differences, the major guidelines are all similar in their initial statements that screening works.
“We’ve still got 50,000 people a year dying from colorectal cancer, lots more than that of new cases,” he said. “If you look at a single factor contributing to that the most, it’s that a lot of the American public is not getting screened at all – it can be up to 40% of the population, depending on what state you’re in.”
Although there are a variety of screening methods available, there are few studies directly comparing them, leaving clinicians at the practice level with the task of presenting all or some of them to patients.
“What the Multi-Society Task Force says that is different, and I think that they get right, is that we don’t have any data [indicating] that offering five, six, or seven options increases the chance of screening – there’s really no evidence that going past two does,” Dr. Rex said.
“The list of options also includes things that nobody actually does,” he added. “For example, flexible sigmoidoscopy has dropped off the map, and FIT has largely replaced guaiac-based testing, even high-sensitivity guaiac. Nobody is really doing CT colonography. The three tests that are being used are colonoscopy, FIT, and [stool DNA-FIT].”
Dr. Rex said that he favors sequential offers, presenting colonoscopy first, emphasizing the benefits for higher-risk patients, and if the patients refuse, offering a fecal-based test.
“Minimizing the number of options makes the conversation feasible, and it’s still very responsible,” he said.
Dr. Kupfer has performed collaborative research with Myriad Genetic Laboratories. She is an editorial advisory board member for GI & Hepatology News from MDedge, part of the Medscape Professional Network. Dr. Rex serves or served as a consultant for Olympus Corporation; Boston Scientific; Medtronic; and Aries; and received research support from Endo-Aid; Olympus Corporation; and Medivators. He has ownership in ai4gi. He is an editorial board member for Medscape Gastroenterology. Dr. Chan has served as a consultant to Pfizer, Bayer AG, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
This article was updated June 8, 2021.
Build a better mousetrap, and the world will beat a path to your door. Find an accurate, completely noninvasive method for colorectal cancer screening and you’ll probably win the Nobel Prize for Medicine or Physiology.
But until then, we’ll have to make do with colonoscopy, fecal immunochemical testing (FIT), and other messy but necessary means for preventing full-blown CRC and reducing the risk of CRC morbidity and mortality. And start turning to them earlier in patients’ lives.
The U.S. Preventive Services Task Force (USPSTF) has issued an update of its 2016 recommendations for CRC screening, for the first time advising that screening for all average-risk adults begin at age 45. This new recommendation is in line with the guidelines issued by the American Cancer Society, which were updated in 2018, to reflect the inescapable truth that CRC is increasingly being diagnosed at a younger age.
Not to be left out, the U.S. Multi-Society Task Force (MSTF) – which represents the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy – issued a statement supporting lowering the age of initial screening in normal-risk adults to 45, and promised that an update of the 2017 guidelines would include the new recommendation.
Recommendations influence reimbursement
Guidelines are often honored as much in the breach as in the observance, but those issued by the USPSTF have unique sway, according to Sonia S. Kupfer, MD, of the section of gastroenterology, hepatology, and nutrition at the University of Chicago, and colleagues.
“While other guidelines have recommended this younger age, the USPSTF guidelines directly inform insurance coverage and waiving of cost sharing as part of federal law,” they wrote in an editorial accompanying the USPSTF guideline statement in the Journal of the American Medical Association.
Although the USPSTF rated its recommendation on starting at age 45 a ‘B’ level – indicating a moderate certainty of moderate benefit – it’s an important step, Dr. Kupfer said in an interview.
“The big advantage here is that we may be able to make a dent in this early-onset colorectal cancer, which, having seen many of these patients, is very alarming, and they don’t always seem to have classic risk factors,” she said. “So, getting them when we can potentially prevent cancer by taking out polyps, or even getting them in an earlier stage, certainly will be beneficial.”
The MSTF also considered recommending 45 as the starting age for normal-risk patients in its 2017 guidelines, noted Douglas Rex, MD, who was chair of the committee that drew up those guidelines, as well as director of endoscopy at Indiana University Hospital in Indianapolis.
“Since that time there has been more evidence, and there’s also some empiric evidence, about the yield of screening in the 45- to 49-year-old age group,” he said in an interview.
The one that gets done
Although the various guidelines differ in specifics, all are in agreement on the general proposition that colonoscopy is the gold standard for screening and detecting the presence of polyps, adenomas, and CRC.
But as USPSTF member Martha Kubik, PhD, RN, director of the George Mason University School of Nursing in Fairfax, Va., said in a statement: “The right test is the one that gets done.”
Gastroenterologists acknowledge that despite its efficacy, colonoscopy is an invasive procedure involving meticulous and unpleasant and/or uncomfortable bowel prep, sedation, and significant time requirements.
In the theory that something is better than nothing, with clinical evidence of varying degrees of quality, the USPSTF recommends the following procedures or tests for average-risk adults:
- Colonoscopy screening every 10 years.
- every 10 years plus annual FIT.
- CT colonography every 5 years.
- High-sensitivity guaiac fecal occult blood test (gFOBT; Hemoccult II) or FIT every year.
- Stool DNA-FIT (Cologuard) every 1 to 3 years.
The Food and Drug Administration also recently approved an artificial intelligence device designed for use with an endoscope, which its manufacturer says can help clinicians detect gastrointestinal lesions they might otherwise miss. This is not a new screening method, but rather an enhancement of existing ones. It neither diagnoses lesions nor recommends treatments, and is not intended to take the place of laboratory sampling.
“I think artificial intelligence is poised to make colonoscopy more effective,” Dr. Rex said. “In the first five trials that we’ve seen, the average increase in the adenoma detection rate has been 11%, and for each 1% gain in the adenoma detection rate, patients have about a 3% decline in their risk of getting cancer after a colonoscopy and about a 5% decline in their risk for fatal cancer. Those are the largest gains that we’ve seen from a technology.”
Different evidence, varied outcomes
Despite the recommendations, a quick dive into the morass of evidence from multiple studies featured in the updated USPSTF guidelines shows that not all screening methods are created equal.
A single colonoscopy, for example, has been shown in large cohort studies to be associated with a 68% reduction in CRC mortality vs. no screening, compared with a 26% reduction with flexible sigmoidoscopy performed every 3-5 years, 22% reduction with Hemoccult II, and 10% with FIT every 2 years.
The USPSTF investigators did not find any studies evaluating the effectiveness of CT colonography, high-sensitivity gFOBT, stool DNA with or without FIT, or serum tests on CRC incidence, CRC mortality, or both.
The two visualization methods for which studies were available, colonoscopy and CT colonography, were generally comparable in sensitivity and specificity for detecting and correctly identifying adenomas 6 mm and larger, although colonography had higher sensitivity for CRC than colonoscopy.
When performed in two to nine annual or biennial rounds, gFOBT was associated with a reduction of CRC-specific mortality of 9% after 19.5 years and 22% at 30 years, compared with no screening.
In observational studies, screening colonoscopy and FIT were both associated with lower risk of CRC incidence or mortality, compared with no screening.
When to stop?
The major guidelines are all in agreement that once an individual reaches age 75, the decision about whether to continue screening should be made on a case-by-case basis, depending on the patient’s overall health, relative risks, and life expectancy.
But if a study published 2 days after the release of the USPSTF guidelines is any indication, just as 45 is the new 50 for starting screening, 85 may be the new 75 for stopping it.
As researchers from Mass General Cancer Center in Boston reported in JAMA Oncology, screening endoscopy for persons older than 75 in otherwise good health can reduce the risk for CRC incidence and CRC-related death by approximately 40%.
The researchers also found, however, that screening did not provide a significant survival benefit for individuals older than 75 with cardiovascular disease, diabetes, or three or more other health conditions.
“Until now, there really weren’t clear data to help us decide whether patients should be screened after age 75,” coinvestigator Andrew T. Chan. MD, MPH, a gastroenterologist and chief of the clinical and translational epidemiology unit at Mass General, said in a statement. “Current guidance was largely based on modeling and extrapolation of studies conducted in other age groups, and not on solid data to show whether screening was actually helpful in an older population.”
In an interview, Dr. Chan said that while the recommendation to screen older adults has to be tailored to individual risk factors, “it should help to provide more confidence for clinicians and patients.”
“I think this is particularly important, because we know that the population as a whole is aging, so more and more people are in this category of over the age of 75, and it’s not an infrequent issue in the clinic as to what to continue with respect to preventative interventions,” he said.
Dr. Kupfer said that the findings by Dr. Chan and colleagues are largely in keeping with guideline recommendations.
“We factor in a lot of different things, including comorbidities, in making the decision to continue screening up to age 85. Certainly, physiological age and chronological age aren’t always the same, so not every 75-year-old is going to be in the same boat,” she said.
“The risk goes up as people get older, but there starts to be competing mortality at some point, and if you have to do a colonoscopy there are obviously issues related to sedation that, as someone gets older, we have to take into consideration,” she added.
Patients frequently confuse screening with surveillance, Dr. Rex said, and he has had patients tell him: “I hear you don’t do these anymore on people over the age of 75.”
“But that’s not true,” Dr. Rex emphasized.
“Screening is generally considered appropriate even up to the age of 85, but between 75 and 85 it should be considered on an individual basis, and there are several considerations there,” Dr. Rex said. “One is whether a patient has ever been screened before. The second is how they were screened. Third is their life expectancy and how many comorbidities they have. And fourth is their personal feelings about it and interest in it.”
He pointed out that the false-positive rate of stool DNA-FIT tests increases with age, and that for older patients who were previously screened, a standard FIT test may be a more appropriate.
So doc, what should I do?
Multiple guidelines, levels of evidence, different screening methods with varying efficacy, individual risk factors – how can clinicians make sense of all these data at the practice level?
“Any modality can be used for screening. Colorectal cancer screening can be done in a number of different ways, and I think that sometimes gets lost in the shuffle, and the thought becomes that everybody has to get a colonoscopy at 45, but there are certainly other tests,” Dr. Kupfer said.
“This just reminds us that we should be thinking about ways we can be doing screening on a population basis, so that we make sure there is equity,” she said.
It’s also important to remember that patients with familial CRC syndromes should begin screening at an even earlier age than average-risk adults, she emphasized.
“To really make a dent in early-onset colorectal cancer, we have to continue to take an active case-finding approach,” she said.
Dr. Rex noted that despite minor differences, the major guidelines are all similar in their initial statements that screening works.
“We’ve still got 50,000 people a year dying from colorectal cancer, lots more than that of new cases,” he said. “If you look at a single factor contributing to that the most, it’s that a lot of the American public is not getting screened at all – it can be up to 40% of the population, depending on what state you’re in.”
Although there are a variety of screening methods available, there are few studies directly comparing them, leaving clinicians at the practice level with the task of presenting all or some of them to patients.
“What the Multi-Society Task Force says that is different, and I think that they get right, is that we don’t have any data [indicating] that offering five, six, or seven options increases the chance of screening – there’s really no evidence that going past two does,” Dr. Rex said.
“The list of options also includes things that nobody actually does,” he added. “For example, flexible sigmoidoscopy has dropped off the map, and FIT has largely replaced guaiac-based testing, even high-sensitivity guaiac. Nobody is really doing CT colonography. The three tests that are being used are colonoscopy, FIT, and [stool DNA-FIT].”
Dr. Rex said that he favors sequential offers, presenting colonoscopy first, emphasizing the benefits for higher-risk patients, and if the patients refuse, offering a fecal-based test.
“Minimizing the number of options makes the conversation feasible, and it’s still very responsible,” he said.
Dr. Kupfer has performed collaborative research with Myriad Genetic Laboratories. She is an editorial advisory board member for GI & Hepatology News from MDedge, part of the Medscape Professional Network. Dr. Rex serves or served as a consultant for Olympus Corporation; Boston Scientific; Medtronic; and Aries; and received research support from Endo-Aid; Olympus Corporation; and Medivators. He has ownership in ai4gi. He is an editorial board member for Medscape Gastroenterology. Dr. Chan has served as a consultant to Pfizer, Bayer AG, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
This article was updated June 8, 2021.
Reexamining the Role of Diet in Dermatology
Within the last decade, almost 3000 articles have been published on the role of diet in the prevention and management of dermatologic conditions. Patients are increasingly interested in—and employing—dietary modifications that may influence skin appearance and aid in the treatment of cutaneous disease.1 It is essential that dermatologists are familiar with existing evidence on the role of diet in dermatology to counsel patients appropriately. Herein, we discuss the compositions of several popular diets and their proposed utility for dermatologic purposes. We highlight the limited literature that exists surrounding this topic and emphasize the need for future, well-designed clinical trials that study the impact of diet on skin disease.
Ketogenic Diet
The ketogenic diet has a macronutrient profile composed of high fat, low to moderate protein, and very low carbohydrates. Nutritional ketosis occurs as the body begins to use free fatty acids (via beta oxidation) as the primary metabolite driving cellular metabolism. It has been suggested that the ketogenic diet may impart beneficial effects on skin disease; however, limited literature exists on the role of nutritional ketosis in the treatment of dermatologic conditions.
Mechanistically, the ketogenic diet decreases the secretion of insulin and insulinlike growth factor 1, resulting in a reduction of circulating androgens and increased activity of the retinoid X receptor.2 In acne vulgaris, it has been suggested that the ketogenic diet may be beneficial in decreasing androgen-induced sebum production and the overproliferation of keratinocytes.2-7 The ketogenic diet is one of the most rapidly effective dietary strategies for normalizing both insulin and androgens, thus it may theoretically be useful for other metabolic and hormone-dependent skin diseases, such as hidradenitis suppurativa.8,9
The cutaneous manifestations associated with chronic hyperinsulinemia and hyperglycemia are numerous and include acanthosis nigricans, acrochordons, diabetic dermopathy, scleredema diabeticorum, bullosis diabeticorum, keratosis pilaris, and generalized granuloma annulare. There also is an increased risk for bacterial and fungal skin infections associated with hyperglycemic states.10 The ketogenic diet is an effective nonpharmacologic tool for normalizing serum insulin and glucose levels in most patients and may have utility in the aforementioned conditions.11,12 In addition to improving insulin sensitivity, it has been used as a dietary strategy for weight loss.11-15 Because obesity and metabolic syndrome are highly correlated with common skin conditions such as psoriasis, hidradenitis suppurativa, and androgenetic alopecia, there may be a role for employing the ketogenic diet in these patient populations.16,17
Although robust clinical studies on ketogenic diets in skin disease are lacking, a recent single-arm, open-label clinical trial observed benefit in all 37 drug-naïve, overweight patients with chronic plaque psoriasis who underwent a ketogenic weight loss protocol. Significant reductions in psoriasis area and severity index (PASI) score and dermatology life quality index score were reported (P<.001).18 Another study of 30 patients with psoriasis found that a 4-week, low-calorie, ketogenic diet resulted in 50% improvement of PASI scores, 10% weight loss, and a reduction in the proinflammatory cytokines IL-1β and IL-2.19 Despite these results, it is a challenge to tease out if the specific dietary intervention or its associated weight loss was the main driver in these reported improvements in skin disease.
There is mixed evidence on the anti-inflammatory nature of the ketogenic diet, likely due to wide variation in the composition of foods included in individual diets. In many instances, the ketogenic diet is thought to possess considerable antioxidant and anti-inflammatory capabilities. Ketones are known activators of the nuclear factor erythroid 2–related factor 2 pathway, which upregulates the production of glutathione, a major endogenous intracellular antioxidant.20 Additionally, dietary compounds from foods that are encouraged while on the ketogenic diet, such as sulforaphane from broccoli, also are independent activators of nuclear factor erythroid 2–related factor 2.21 Ketones are efficiently utilized by mitochondria, which also may result in the decreased production of reactive oxygen species and lower oxidative stress.22 Moreover, the ketone body β-hydroxybutyrate has demonstrated the ability to reduce proinflammatory IL-1β levels via suppression of nucleotide-binding domain-like receptor protein 3 inflammasome activity.23,24 The activity of IL-1β is known to be elevated in many dermatologic conditions, including juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, hidradenitis suppurativa, Behçet disease, and other autoinflammatory syndromes.25 Ketones also have been shown to inhibit the nuclear factor–κB proinflammatory signaling pathway.22,26,27 Overexpression of IL-1β and aberrant activation of nuclear factor–κB are implicated in a variety of inflammatory, autoimmune, and oncologic cutaneous pathologies. The ketogenic diet may prove to be an effective adjunctive treatment for dermatologists to consider in select patient populations.23,24,28-30
For patients with keratinocyte carcinomas, the ketogenic diet may offer the aforementioned anti-inflammatory and antioxidant effects, as well as suppression of the mechanistic target of rapamycin, a major regulator of cell metabolism and proliferation.31,32 Inhibition of mechanistic target of rapamycin activity has been shown to slow tumor growth and reduce the development of squamous cell carcinoma.25,33,34 The ketogenic diet also may exploit the preferential utilization of glucose exhibited by many types of cancer cells, thereby “starving” the tumor of its primary fuel source.35,36 In vitro and animal studies in a variety of cancer types have demonstrated that a ketogenic metabolic state—achieved through the ketogenic diet or fasting—can sensitize tumor cells to chemotherapy and radiation while conferring a protective effect to normal cells.37-40 This recently described phenomenon is known as differential stress resistance, but it has not been studied in keratinocyte malignancies or melanoma to date. Importantly, some basal cell carcinomas and BRAF V600E–mutated melanomas have worsened while on the ketogenic diet, suggesting more data is needed before it can be recommended for all cancer patients.41,42 Furthermore, other skin conditions such as prurigo pigmentosa have been associated with initiation of the ketogenic diet.43
Low FODMAP Diet
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) are short-chain carbohydrates that are poorly absorbed, osmotically active, and rapidly fermented by intestinal bacteria.44 The low FODMAP diet has been shown to be efficacious for treatment of irritable bowel syndrome, small intestinal bacterial overgrowth (SIBO), and some cases of inflammatory bowel disease (IBD).44-49 A low FODMAP diet may have potential implications for several dermatologic conditions.
Rosacea has been associated with various gastrointestinal tract disorders including irritable bowel syndrome, SIBO, and IBD.50-54 A single study found that patients with rosacea had a 13-fold increased risk for SIBO.55,56 Treatment of 40 patients with SIBO using rifaximin resulted in complete resolution of rosacea in all patients, with no relapse after a 3-year follow-up period.55 Psoriasis also has been associated with SIBO and IBD.57,58 One small study found that eradication of SIBO in psoriatic patients resulted in improved PASI scores and colorimetric values.59
Although the long-term health consequences of the low FODMAP diet are unknown, further research on such dietary interventions for inflammatory skin conditions is warranted given the mounting evidence of a gut-skin connection and the role of the intestinal microbiome in skin health.50,51
Gluten-Free Diet
Gluten is a protein found in a variety of grains. Although the role of gluten in the pathogenesis of celiac disease and dermatitis herpetiformis is indisputable, the deleterious effects of gluten outside of the context of these diseases remain controversial. There may be a compelling case for eliminating gluten in psoriasis patients with seropositivity for celiac disease. A recent systematic review found a 2.2-fold increased risk for celiac disease in psoriasis patients.60 Antigliadin antibody titers also were found to be positively correlated with psoriatic disease severity.61 In addition, one open-label study found a reduction in PASI scores in 73% of patients with antigliadin antibodies after 3 months on a gluten-free diet compared to those without antibodies; however, the study only included 22 patients.62 Several other small studies have yielded similar results63,64; however, antigliadin antibodies are neither the most sensitive nor specific markers of celiac disease, and additional testing should be completed in any patient who may carry this diagnosis. A survey study by the National Psoriasis Foundation found that the dietary change associated with the greatest skin improvement was removal of gluten and nightshade vegetables in approximately 50% of the 1200 psoriasis patients that responded.65 Case reports of various dermatologic conditions including sarcoidosis, vitiligo, alopecia areata, lichen planus, dermatomyositis, pyoderma gangrenosum, erythema nodosum, leukocytoclastic vasculitis, linear IgA bullous dermatosis, and aphthous ulcerations have reportedly improved with a gluten-free diet; however, this should not be used as primary therapy in patients without celiac disease.66-71 Because gluten-free diets can be expensive and challenging to follow, a formal assessment for celiac disease should be considered before recommendation of this dietary intervention.
Low Histamine Diet
Histamine is a biogenic amine produced by the decarboxylation of the amino acid histidine.72 It is found in several foods in varying amounts. Because bacteria can convert histidine into histamine, many fermented and aged foods such as kimchi, sauerkraut, cheese, and red wine contain high levels of histamine. Individuals who have decreased activity of diamine oxidase (DAO), an enzyme that degrades histamine, may be more susceptible to histamine intolerance.72 The symptoms of histamine intolerance are numerous and include gastrointestinal tract distress, rhinorrhea and nasal congestion, headache, urticaria, flushing, and pruritus. Histamine intolerance can mimic an IgE-mediated food allergy; however, allergy testing is negative in these patients. Unfortunately, there is no laboratory test for histamine intolerance; a double-blind, placebo-controlled food challenge is considered the gold-standard test.72
As it pertains to dermatology, a low histamine diet may play a role in the treatment of certain patients with atopic dermatitis and chronic spontaneous urticaria. One study reported that 17 of 54 (31.5%) atopic patients had higher basal levels of serum histamine compared to controls.73 Another study found that a histamine-free diet led to improvement in both histamine intolerance symptoms and atopic dermatitis disease severity (SCORing atopic dermatitis) in patients with low DAO activity.74 In chronic spontaneous urticaria, a recent systematic review found that in 223 patients placed on a low histamine diet for 3 to 4 weeks, 12% and 44% achieved complete and partial remission, respectively.75 Although treatment response based on a patient’s DAO activity level has not been correlated, a diet low in histamine may prove useful for patients with persistent atopic dermatitis and chronic spontaneous urticaria who have negative food allergy tests and report exacerbation of symptoms after ingestion of histamine-rich foods.76,77
Mediterranean Diet
The Mediterranean diet has been touted as one of the healthiest diets to date, and large randomized clinical trials have demonstrated its effectiveness in weight loss, improving insulin sensitivity, and reducing inflammatory cytokine profiles.78,79 A major criticism of the Mediterranean diet is that it has considerable ambiguity and lacks a precise definition due to the variability of what is consumed in different Mediterranean regions. Generally, the diet emphasizes high consumption of colorful fruits and vegetables, aromatic herbs and spices, olive oil, nuts, and seafood, as well as modest amounts of dairy, eggs, and red meat.80 The anti-inflammatory effects of this diet largely have been attributed to its abundance of polyphenols, carotenoids, monounsaturated fatty acids, and omega-3 polyunsaturated fatty acids (PUFAs).80,81 Examples of polyphenols include resveratrol in red grapes, quercetin in apples and red onions, and curcumin in turmeric, while examples of carotenoids include lycopene in tomatoes and zeaxanthin in dark leafy greens. Oleic acid is a monounsaturated fatty acid present in high concentrations in olive oil, while eicosapentaenoic acid and docosahexaenoic acid are omega-3 PUFAs predominantly found in fish.82
Unfortunately, rigorous clinical trials regarding the Mediterranean diet as it pertains to dermatology have not been undertaken. Numerous observational studies in patients with psoriasis have suggested that close adherence to the Mediterranean diet was associated with improvement in PASI scores.83-86 The National Psoriasis Foundation now recommends a trial of the Mediterranean diet in some patients with psoriasis, emphasizing increased dietary intake of olive oil, fish, and vegetables.87 Adherence to a Mediterranean diet also has been inversely correlated to the severity of acne vulgaris and hidradenitis suppurativa88,89; however, these studies failed to account for the multifactorial risk factors associated with these conditions. Mediterranean diets also may impart a chemopreventive effect, supported by a number of in vivo and in vitro studies demonstrating the inhibition and/or reversal of cutaneous DNA damage induced by UV radiation through supplementation with various phytonutrients and omega-3 PUFAs.81,90-92 Although small case-control studies have found a decreased risk of basal cell carcinoma in those who closely adhered to a Mediterranean diet, more rigorous clinical research is needed.93
Whole-Food, Plant-Based Diet
A whole-food, plant-based (WFPB) diet is another popular dietary approach that consists of eating fruits, vegetables, legumes, nuts, seeds, and grains in their whole natural form.94 This diet discourages all animal products, including red meat, seafood, dairy, and eggs. It is similar to a vegan diet except that it eliminates all highly refined carbohydrates, vegetable oils, and other processed foods.94 Randomized clinical studies have demonstrated the WFPB diet to be effective in the treatment of obesity and metabolic syndrome.95,96
A WFPB diet has been shown to increase the antioxidant capacity of cells, lengthen telomeres, and reduce formation of advanced glycation end products.94,97,98 These benefits may help combat accelerated skin aging, including increased skin permeability, reduced elasticity and hydration, decreased angiogenesis, impaired immune function, and decreased vitamin D synthesis. Accelerated skin aging can result in delayed wound healing and susceptibility to skin tears and ecchymoses and also may promote the development of cutaneous malignancies.99 There remains a lack of clinical data studying a properly formulated WFPB diet in the dermatologic setting.
Paleolithic Diet
The paleolithic (Paleo) diet is an increasingly popular way of eating that attempts to mirror what our ancestors may have consumed between 10,000 and 2.5 million years ago.100 It is similar to the Mediterranean diet but excludes grains, dairy, legumes, and nightshade vegetables. It also calls for elimination of highly processed sugars and oils as well as chemical food additives and preservatives. There is a strict variation of the diet for individuals with autoimmune disease that also excludes eggs, nuts, and seeds, as these can be inflammatory or immunogenic in some patients.100-106 Other variations of the diet exist, including the ketogenic Paleo diet, pegan (Paleo vegan) diet, and lacto-Paleo diet.100 An often cited criticism of the Paleo diet is the low intake of calcium and risk for osteoporosis; however, consumption of calcium-rich foods or a calcium supplement can address this concern.107
Although small clinical studies have found the Paleo diet to be beneficial for various autoimmune diseases, clinical data evaluating the utility of the diet for cutaneous disease is lacking.108,109 Numerous randomized trials have demonstrated the Paleo diet to be effective for weight loss and improving insulin sensitivity and lipid levels.110-116 Thus, the Paleo diet may theoretically serve as a viable adjunct dietary approach to the treatment of cutaneous diseases associated with obesity and metabolic derangement.117
Carnivore Diet
Arguably the most controversial and radical diet is the carnivore diet. As the name implies, the carnivore diet is based on consuming solely animal products. A properly structured carnivore diet emphasizes a “nose-to-tail” eating approach where all parts of the animal including the muscle meats, organs, and fat are consumed. Proponents of the diet cite anthropologic evidence from fossil-stable carbon-13/carbon-12 isotope analyses, craniodental features, and numerous other adaptations that indicate increased consumption of meat during human evolution.118-122 Notably, many early humans ate a carnivore diet, but life span was very short at this time, suggesting the diet may not be as beneficial as has been suggested.
Despite the abundance of anecdotal evidence supporting its use for a variety of chronic conditions, including cutaneous autoimmune disease, there is a virtual absence of high-quality research on the carnivore diet.123-125
The purported benefits of the carnivore diet may be attributed to the consumption of organ meats that contain highly bioavailable essential vitamins and minerals, such as iron, zinc, copper, selenium, thiamine, niacin, folate, vitamin B6, vitamin B12, vitamin A, vitamin D, vitamin K, and choline.126-128 Other dietary compounds that have demonstrated benefit for skin health and are predominantly found in animal foods include carnosine, carnitine, creatine, taurine, coenzyme Q10, and collagen.129-134 Nevertheless, there is no data to recommend the elimination of antioxidant- and micronutrient-dense plant-based foods. Rigorous clinical research evaluating the efficacy and safety of the carnivore diet in dermatologic patients is needed. A carnivore diet should not be undertaken without the assistance of a dietician who can ensure adequate micronutrient and macronutrient support.
Final Thoughts
The adjunctive role of diet in the treatment of skin disease is expanding and becoming more widely accepted among dermatologists. Unfortunately, there remains a lack of randomized controlled trials confirming the efficacy of various dietary interventions in the dermatologic setting. Although evidence-based dietary recommendations currently are limited, it is important for dermatologists to be aware of the varied and nuanced dietary interventions employed by patients.
Ultimately, dietary recommendations must be personalized, considering a patient’s comorbidities, personal beliefs and preferences, and nutrigenetics. The emerging field of dermatonutrigenomics—the study of how dietary compounds interact with one’s genes to influence skin health—may allow for precise dietary recommendations to be made in dermatologic practice. Direct-to-consumer genetic tests targeted toward dermatology patients are already on the market, but their clinical utility awaits validation.1 Because nutritional science is a constantly evolving field, becoming familiar with these popular diets will serve both dermatologists and their patients well.
- Jaros J, Katta R, Shi VY. Dermatonutrigenomics: past, present, and future. Dermatology. 2019;235:164-166.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Smith R, Mann N, Mäkeläinen H, et al. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52:718-726.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a "male" therapy for a predominantly "female" disease. J Clin Aesthet Dermatol. 2016;9:44.
- Nikolakis G, Karagiannidis I, Vaiopoulos AG, et al. Endocrinological mechanisms in the pathophysiology of hidradenitis suppurativa [in German]. Hautarzt. 2020;71:762-771.
- Karadag AS, Ozlu E, Lavery MJ. Cutaneous manifestations of diabetes mellitus and the metabolic syndrome. Clin Dermatology. 2018;36:89-93.
- Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969-977.
- Anton SD, Hida A, Heekin K, et al. Effects of popular diets without specific calorie targets on weight loss outcomes: systematic review of findings from clinical trials. Nutrients. 2017;9:822.
- Castellana M, Conte E, Cignarelli A, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:5-16.
- Paoli A, Mancin L, Giacona MC, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18:104.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Lian N, Chen M. Metabolic syndrome and skin disease: potential connection and risk. Int J Dermatol Venereol. 2019;2:89-93.
- Hu Y, Zhu Y, Lian N, et al. Metabolic syndrome and skin diseases. Front Endocrinol (Lausanne). 2019;10:788.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Milder J, Liang L-P, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238-244.
- Kubo E, Chhunchha B, Singh P, et al. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep. 2017;7:14130.
- Pinto A, Bonucci A, Maggi E, et al. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer's disease. Antioxidants (Basel). 2018;7:63.
- Youm Y-H, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Rahman M, Muhammad S, Khan MA, et al. The β-hydroxybutyrate receptor HCA 2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:1-11.
- Lu Y, Yang YY, Zhou MW, et al. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett. 2018;683:13-18.
- Hamarsheh S, Zeiser R. NLRP3 inflammasome activation in cancer: a double-edged sword. Front Immunol. 2020;11:1444.
- Bell S, Degitz K, Quirling M, et al. Involvement of NF-κB signalling in skin physiology and disease. Cell Signal. 2003;15:1-7.
- Goldminz AM, Au SC, Kim N, et al. NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69:89-94.
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589.
- McDaniel S, Rensing N, Yamada K, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:E7-E11.
- Alter M, Satzger I, Schrem H, et al. Non-melanoma skin cancer is reduced after switch of immunosuppression to mTOR-inhibitors in organ transplant recipients. J Dtsch Dermatol Ges. 2014;12:480-488.
- Feldmeyer L, Hofbauer GF, Böni T, et al. Mammalian target of rapamycin (mTOR) inhibitors slow skin carcinogenesis, but impair wound healing. Br J Dermatol. 2012;166:422-424.
- Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211-218.
- Li W. "Warburg effect" and mitochondrial metabolism in skin cancer.J Carcinogene Mutagene. 2012:S4.
- Naveed S, Aslam M, Ahmad A. Starvation based differential chemotherapy: a novel approach for cancer treatment. Oman Med J. 2014;29:391-398.
- Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215-8220.
- Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29:271-280.
- de Groot S, Pijl H, van der Hoeven JJM, et al. Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res. 2019;38:209.
- Hosseini M, Kasraian Z, Rezvani HR. Energy metabolism in skin cancers: a therapeutic perspective. Biochim Biophys Acta Bioenerg. 2017;1858:712-722.
- Feichtinger RG, Lang R, Geilberger R, et al. Melanoma tumors exhibit a variable but distinct metabolic signature. Exp Dermatol. 2018;27:204-207.
- Alshaya MA, Turkmani MG, Alissa AM. Prurigo pigmentosa following ketogenic diet and bariatric surgery: a growing association. JAAD Case Rep. 2019;5:504-507.
- Bellini M, Tonarelli S, Nagy AG, et al. Low FODMAP diet: evidence, doubts, and hopes. Nutrients. 2020;12:148.
- Kwiatkowski L, Rice E, Langland J. Integrative treatment of chronic abdominal bloating and pain associated with overgrowth of small intestinal bacteria: a case report. Altern Ther Health Med. 2017;23:56-61.
- Hubkova T. No more pain in the gut: lifestyle medicine approach to irritable bowel syndrome. Am J Lifestyle Med. 2017;11:223-226.
- Schumann D, Klose P, Lauche R, et al. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition. 2018;45:24-31.
- Cox SR, Prince AC, Myers CE, et al. Fermentable carbohydrates [FODMAPs] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: a randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J Crohns Colitis. 2017;11:1420-1429.
- Damas OM, Garces L, Abreu MT. Diet as adjunctive treatment for inflammatory bowel disease: review and update of the latest literature. Curr Treat Options Gastroenterol. 2019;17:313-325.
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424.
- Daou H, Paradiso M, Hennessy K, et al. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Weinstock LB, Steinhoff M. Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin. J Am Acad Dermatol. 2013;68:875-876.
- Wu CY, Chang YT, Juan CK, et al. Risk of inflammatory bowel disease in patients with rosacea: results from a nationwide cohort study in Taiwan. J Am Acad Dermatol. 2017;76:911-917.
- Egeberg A, Weinstock LB, Thyssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Drago F, De Col E, Agnoletti AF, et al. The role of small intestinal bacterial overgrowth in rosacea: a 3-year follow-up. J Am Acad Dermatol. 2016;75:E113-E115.
- Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759-764.
- Ojetti V, De Simone C, Aguilar Sanchez J, et al. Malabsorption in psoriatic patients: cause or consequence? Scand J Gastroenterol. 2006;41:1267-1271.
- Kim M, Choi KH, Hwang SW, et al. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017;76:40-48.
- Drago F, Ciccarese G, Indemini E, et al. Psoriasis and small intestine bacterial overgrowth. Int J Dermatol. 2018;57:112-113.
- Acharya P, Mathur M. Association between psoriasis and celiac disease: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1376-1385.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2018;11:13-19.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Loche F, Bazex J. Celiac disease associated with cutaneous sarcoidosic granuloma [in French]. Rev Med Interne. 1997;18:975-978.
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210.
- Wijarnpreecha K, Panjawatanan P, Corral JE, et al. Celiac disease and risk of sarcoidosis: a systematic review and meta-analysis. J Evid Based Med. 2019;12:194-199.
- Rodrigo L, Beteta-Gorriti V, Alvarez N, et al. Cutaneous and mucosal manifestations associated with celiac disease. Nutrients. 2018;10:800.
- Song MS, Farber D, Bitton A, et al. Dermatomyositis associated with celiac disease: response to a gluten-free diet. Can J Gastroenterol. 2006;20:433-435.
- Egan CA, Smith EP, Taylor TB, et al. Linear IgA bullous dermatosis responsive to a gluten-free diet. Am J Gastroenterol. 2001;96:1927-1929.
- Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, et al. Histamine intolerance: the current state of the art. Biomolecules. 2020;10:1181.
- Ring J. Plasma histamine concentrations in atopic eczema. Clin Allergy. 1983;13:545-552.
- Maintz L, Benfadal S, Allam JP, et al. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J Allergy Clin Immunol. 2006;117:1106-1112.
- Cornillier H, Giraudeau B, Samimi M, et al. Effect of diet in chronic spontaneous urticaria: a systematic review. Acta Derm Venereol. 2019;99:127-132.
- Son JH, Chung BY, Kim HO, et al. A histamine-free diet is helpful for treatment of adult patients with chronic spontaneous urticaria. Ann Dermatol. 2018;30:164-172.
- Wagner N, Dirk D, Peveling-Oberhag A, et al. A popular myth - low-histamine diet improves chronic spontaneous urticaria - fact or fiction? J Eur Acad Dermatol Venereol. 2017;31:650-655.
- Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
- Steffen LM, Van Horn L, Daviglus ML, et al. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (coronary artery risk development in young adults) study. Br J Nutr. 2014;112:1654-1661.
- Bower A, Marquez S, de Mejia EG. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit Rev Food Sci Nutr. 2016;56:2728-2746.
- Bosch R, Philips N, Suárez-Pérez JA, et al. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants (Basel). 2015;4:248-268.
- Katsimbri P, Korakas E, Kountouri A, et al. The effect of antioxidant and anti-inflammatory capacity of diet on psoriasis and psoriatic arthritis phenotype: nutrition as therapeutic tool? Antioxidants. 2021;10:157.
- Molina-Leyva A, Cuenca-Barrales C, Vega-Castillo JJ, et al. Adherence to Mediterranean diet in Spanish patients with psoriasis: cardiovascular benefits? Dermatol Ther. 2019;32:E12810.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:1-10.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Korovesi A, Dalamaga M, Kotopouli M, et al. Adherence to the Mediterranean diet is independently associated with psoriasis risk, severity, and quality of life: a cross-sectional observational study. Int J Dermatol. 2019;58:E164-E165.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934-950.
- Skroza N, Tolino E, Semyonov L, et al. Mediterranean diet and familial dysmetabolism as factors influencing the development of acne. Scand J Public Health. 2012;40:466-474.
- Barrea L, Fabbrocini G, Annunziata G, et al. Role of nutrition and adherence to the Mediterranean diet in the multidisciplinary approach of hidradenitis suppurativa: evaluation of nutritional status and its association with severity of disease. Nutrients. 2018;11:57.
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71-83.
- Huang T-H, Wang P-W, Yang S-C, et al. Cosmetic and therapeutic applications of fish oil's fatty acids on the skin. Mar Drugs. 2018;16:256.
- Rizwan M, Rodriguez-Blanco I, Harbottle A, et al. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: a randomized controlled trial. Br J Dermatol. 2011;164:154-162.
- Leone A, Martínez-González M, Martin-Gorgojo A, et al. Mediterranean diet, dietary approaches to stop hypertension, and pro-vegetarian dietary pattern in relation to the risk of basal cell carcinoma: a nested case-control study within the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2020;112:364-372.
- Solway J, McBride M, Haq F, et al. Diet and dermatology: the role of a whole-food, plant-based diet in preventing and reversing skin aging--a review. J Clin Aesthet Dermatol. 2020;13:38-43.
- Greger M. A whole food plant-based diet is effective for weight loss: the evidence. Am J Lifestyle Med. 2020;14:500-510.
- Wright N, Wilson L, Smith M, et al. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7:E256.
- Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14:1112-1120.
- Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048-1057.
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3-14.
- Gupta L, Khandelwal D, Lal PR, et al. Palaeolithic diet in diabesity and endocrinopathies--a vegan's perspective. Eur Endocrinol. 2019;15:77-82.
- Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66:1414-1427.
- Thorburn Alison N, Macia L, Mackay Charles R. Diet, metabolites, and "Western lifestyle" inflammatory diseases. Immunity. 2014;40:833-842.
- Katta R, Schlichte M. Diet and dermatitis: food triggers. J Clin Aesthet Dermatol. 2014;7:30-36.
- Dhar S, Srinivas SM. Food allergy in atopic dermatitis. Indian J Dermatol. 2016;61:645-648.
- Birmingham N, Thanesvorakul S, Gangur V. Relative immunogenicity of commonly allergenic foods versus rarely allergenic and nonallergenic foods in mice. J Food Prot. 2002;65:1988-1991.
- Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16:751-765.
- Kowalski LM, Bujko J. Evaluation of biological and clinical potential of paleolithic diet [in Polish]. Rocz Panstw Zakl Hig. 2012;63:9-15.
- Lee JE, Titcomb TJ, Bisht B, et al. A modified MCT-based ketogenic diet increases plasma β-hydroxybutyrate but has less effect on fatigue and quality of life in people with multiple sclerosis compared to a modified paleolithic diet: a waitlist-controlled, randomized pilot study. J Am Coll Nutr. 2021;40:13-25.
- Abbott RD, Sadowski A, Alt AG. Efficacy of the autoimmune protocol diet as part of a multi-disciplinary, supported lifestyle intervention for Hashimoto's thyroiditis. Cureus. 2019;11:E4556.
- Lindeberg S, Jönsson T, Granfeldt Y, et al. A palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia. 2007;50:1795-1807.
- Jönsson T, Granfeldt Y, Ahrén B, et al. Beneficial effects of a paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35.
- Boers I, Muskiet FAJ, Berkelaar E, et al. Favourable effects of consuming a palaeolithic-type diet on characteristics of the metabolic syndrome: a randomized controlled pilot-study. Lipids Health Dis. 2014;13:160.
- Ghaedi E, Mohammadi M, Mohammadi H, et al. Effects of a paleolithic diet on cardiovascular disease risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10:634-646.
- Mellberg C, Sandberg S, Ryberg M, et al. Long-term effects of a palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68:350-357.
- Pastore RL, Brooks JT, Carbone JW. Paleolithic nutrition improves plasma lipid concentrations of hypercholesterolemic adults to a greater extent than traditional heart-healthy dietary recommendations. Nutr Res. 2015;35:474-479.
- Otten J, Stomby A, Waling M, et al. Benefits of a paleolithic diet with and without supervised exercise on fat mass, insulin sensitivity, and glycemic control: a randomized controlled trial in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2017;33:E2828.
- Stefanadi EC, Dimitrakakis G, Antoniou C-K, et al. Metabolic syndrome and the skin: a more than superficial association. reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol Metab Syndr. 2018;10:9.
- Mann N. Meat in the human diet: an anthropological perspective. Nutr Dietetics. 2007;64(suppl 4):S102-S107.
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345-352.
- Kuhn JE. Throwing, the shoulder, and human evolution. Am J Orthop (Belle Mead NJ). 2016;45:110-114.
- Kobayashi H, Kohshima S. Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J Hum Evol. 2001;40:419-435.
- Cordain L, Eaton SB, Miller JB, et al. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002;56(suppl 1):S42-S52.
- McClellan WS, Du Bois EF. Clinical calorimetry: XLV. prolonged meat diets with a study of kidney function and ketosis. J Biol Chem. 1930;87:651-668.
- O'Hearn A. Can a carnivore diet provide all essential nutrients? Curr Opin Endocrinol Diabetes Obes. 2020;27:312-316.
- O'Hearn LA. A survey of improvements experienced on a carnivore diet compared to only carbohydrate restriction. Open Science Forum website. Published February 12, 2019. Accessed May 17, 2021. doi:10.17605/OSF.IO/5FU4D
- Williams P. Nutritional composition of red meat. Nutrition & Dietetics. 2007;64(suppl 4):S113-S119.
- Biel W, Czerniawska-Piątkowska E, Kowalczyk A. Offal chemical composition from veal, beef, and lamb maintained in organic production systems. Animals (Basel). 2019;9:489.
- Elmadfa I, Meyer AL. The role of the status of selected micronutrients in shaping the immune function. Endocr Metab Immune Disord Drug Targets. 2019;19:1100-1115.
- Babizhayev M. Treatment of skin aging and photoaging with innovative oral dosage forms of nonhydrolized carnosine and carcinine. Int J Clin Derm Res. 2017;5:116-143.
- Danby FW. Nutrition and aging skin: sugar and glycation. Clin Dermatol. 2010;28:409-411.
- Siefken W, Carstensen S, Springmann G, et al. Role of taurine accumulation in keratinocyte hydration. J Invest Dermatol. 2003;121:354-361.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19:3059.
- Fischer F, Achterberg V, März A, et al. Folic acid and creatineimprove the firmness of human skin in vivo. J Cosmet Dermatol. 2011;10:15-23.
- Blatt T, Lenz H, Weber T. Topical application of creatine is multibeneficial for human skin. J Am Acad Dermatol. 2005;52:P32.
Within the last decade, almost 3000 articles have been published on the role of diet in the prevention and management of dermatologic conditions. Patients are increasingly interested in—and employing—dietary modifications that may influence skin appearance and aid in the treatment of cutaneous disease.1 It is essential that dermatologists are familiar with existing evidence on the role of diet in dermatology to counsel patients appropriately. Herein, we discuss the compositions of several popular diets and their proposed utility for dermatologic purposes. We highlight the limited literature that exists surrounding this topic and emphasize the need for future, well-designed clinical trials that study the impact of diet on skin disease.
Ketogenic Diet
The ketogenic diet has a macronutrient profile composed of high fat, low to moderate protein, and very low carbohydrates. Nutritional ketosis occurs as the body begins to use free fatty acids (via beta oxidation) as the primary metabolite driving cellular metabolism. It has been suggested that the ketogenic diet may impart beneficial effects on skin disease; however, limited literature exists on the role of nutritional ketosis in the treatment of dermatologic conditions.
Mechanistically, the ketogenic diet decreases the secretion of insulin and insulinlike growth factor 1, resulting in a reduction of circulating androgens and increased activity of the retinoid X receptor.2 In acne vulgaris, it has been suggested that the ketogenic diet may be beneficial in decreasing androgen-induced sebum production and the overproliferation of keratinocytes.2-7 The ketogenic diet is one of the most rapidly effective dietary strategies for normalizing both insulin and androgens, thus it may theoretically be useful for other metabolic and hormone-dependent skin diseases, such as hidradenitis suppurativa.8,9
The cutaneous manifestations associated with chronic hyperinsulinemia and hyperglycemia are numerous and include acanthosis nigricans, acrochordons, diabetic dermopathy, scleredema diabeticorum, bullosis diabeticorum, keratosis pilaris, and generalized granuloma annulare. There also is an increased risk for bacterial and fungal skin infections associated with hyperglycemic states.10 The ketogenic diet is an effective nonpharmacologic tool for normalizing serum insulin and glucose levels in most patients and may have utility in the aforementioned conditions.11,12 In addition to improving insulin sensitivity, it has been used as a dietary strategy for weight loss.11-15 Because obesity and metabolic syndrome are highly correlated with common skin conditions such as psoriasis, hidradenitis suppurativa, and androgenetic alopecia, there may be a role for employing the ketogenic diet in these patient populations.16,17
Although robust clinical studies on ketogenic diets in skin disease are lacking, a recent single-arm, open-label clinical trial observed benefit in all 37 drug-naïve, overweight patients with chronic plaque psoriasis who underwent a ketogenic weight loss protocol. Significant reductions in psoriasis area and severity index (PASI) score and dermatology life quality index score were reported (P<.001).18 Another study of 30 patients with psoriasis found that a 4-week, low-calorie, ketogenic diet resulted in 50% improvement of PASI scores, 10% weight loss, and a reduction in the proinflammatory cytokines IL-1β and IL-2.19 Despite these results, it is a challenge to tease out if the specific dietary intervention or its associated weight loss was the main driver in these reported improvements in skin disease.
There is mixed evidence on the anti-inflammatory nature of the ketogenic diet, likely due to wide variation in the composition of foods included in individual diets. In many instances, the ketogenic diet is thought to possess considerable antioxidant and anti-inflammatory capabilities. Ketones are known activators of the nuclear factor erythroid 2–related factor 2 pathway, which upregulates the production of glutathione, a major endogenous intracellular antioxidant.20 Additionally, dietary compounds from foods that are encouraged while on the ketogenic diet, such as sulforaphane from broccoli, also are independent activators of nuclear factor erythroid 2–related factor 2.21 Ketones are efficiently utilized by mitochondria, which also may result in the decreased production of reactive oxygen species and lower oxidative stress.22 Moreover, the ketone body β-hydroxybutyrate has demonstrated the ability to reduce proinflammatory IL-1β levels via suppression of nucleotide-binding domain-like receptor protein 3 inflammasome activity.23,24 The activity of IL-1β is known to be elevated in many dermatologic conditions, including juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, hidradenitis suppurativa, Behçet disease, and other autoinflammatory syndromes.25 Ketones also have been shown to inhibit the nuclear factor–κB proinflammatory signaling pathway.22,26,27 Overexpression of IL-1β and aberrant activation of nuclear factor–κB are implicated in a variety of inflammatory, autoimmune, and oncologic cutaneous pathologies. The ketogenic diet may prove to be an effective adjunctive treatment for dermatologists to consider in select patient populations.23,24,28-30
For patients with keratinocyte carcinomas, the ketogenic diet may offer the aforementioned anti-inflammatory and antioxidant effects, as well as suppression of the mechanistic target of rapamycin, a major regulator of cell metabolism and proliferation.31,32 Inhibition of mechanistic target of rapamycin activity has been shown to slow tumor growth and reduce the development of squamous cell carcinoma.25,33,34 The ketogenic diet also may exploit the preferential utilization of glucose exhibited by many types of cancer cells, thereby “starving” the tumor of its primary fuel source.35,36 In vitro and animal studies in a variety of cancer types have demonstrated that a ketogenic metabolic state—achieved through the ketogenic diet or fasting—can sensitize tumor cells to chemotherapy and radiation while conferring a protective effect to normal cells.37-40 This recently described phenomenon is known as differential stress resistance, but it has not been studied in keratinocyte malignancies or melanoma to date. Importantly, some basal cell carcinomas and BRAF V600E–mutated melanomas have worsened while on the ketogenic diet, suggesting more data is needed before it can be recommended for all cancer patients.41,42 Furthermore, other skin conditions such as prurigo pigmentosa have been associated with initiation of the ketogenic diet.43
Low FODMAP Diet
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) are short-chain carbohydrates that are poorly absorbed, osmotically active, and rapidly fermented by intestinal bacteria.44 The low FODMAP diet has been shown to be efficacious for treatment of irritable bowel syndrome, small intestinal bacterial overgrowth (SIBO), and some cases of inflammatory bowel disease (IBD).44-49 A low FODMAP diet may have potential implications for several dermatologic conditions.
Rosacea has been associated with various gastrointestinal tract disorders including irritable bowel syndrome, SIBO, and IBD.50-54 A single study found that patients with rosacea had a 13-fold increased risk for SIBO.55,56 Treatment of 40 patients with SIBO using rifaximin resulted in complete resolution of rosacea in all patients, with no relapse after a 3-year follow-up period.55 Psoriasis also has been associated with SIBO and IBD.57,58 One small study found that eradication of SIBO in psoriatic patients resulted in improved PASI scores and colorimetric values.59
Although the long-term health consequences of the low FODMAP diet are unknown, further research on such dietary interventions for inflammatory skin conditions is warranted given the mounting evidence of a gut-skin connection and the role of the intestinal microbiome in skin health.50,51
Gluten-Free Diet
Gluten is a protein found in a variety of grains. Although the role of gluten in the pathogenesis of celiac disease and dermatitis herpetiformis is indisputable, the deleterious effects of gluten outside of the context of these diseases remain controversial. There may be a compelling case for eliminating gluten in psoriasis patients with seropositivity for celiac disease. A recent systematic review found a 2.2-fold increased risk for celiac disease in psoriasis patients.60 Antigliadin antibody titers also were found to be positively correlated with psoriatic disease severity.61 In addition, one open-label study found a reduction in PASI scores in 73% of patients with antigliadin antibodies after 3 months on a gluten-free diet compared to those without antibodies; however, the study only included 22 patients.62 Several other small studies have yielded similar results63,64; however, antigliadin antibodies are neither the most sensitive nor specific markers of celiac disease, and additional testing should be completed in any patient who may carry this diagnosis. A survey study by the National Psoriasis Foundation found that the dietary change associated with the greatest skin improvement was removal of gluten and nightshade vegetables in approximately 50% of the 1200 psoriasis patients that responded.65 Case reports of various dermatologic conditions including sarcoidosis, vitiligo, alopecia areata, lichen planus, dermatomyositis, pyoderma gangrenosum, erythema nodosum, leukocytoclastic vasculitis, linear IgA bullous dermatosis, and aphthous ulcerations have reportedly improved with a gluten-free diet; however, this should not be used as primary therapy in patients without celiac disease.66-71 Because gluten-free diets can be expensive and challenging to follow, a formal assessment for celiac disease should be considered before recommendation of this dietary intervention.
Low Histamine Diet
Histamine is a biogenic amine produced by the decarboxylation of the amino acid histidine.72 It is found in several foods in varying amounts. Because bacteria can convert histidine into histamine, many fermented and aged foods such as kimchi, sauerkraut, cheese, and red wine contain high levels of histamine. Individuals who have decreased activity of diamine oxidase (DAO), an enzyme that degrades histamine, may be more susceptible to histamine intolerance.72 The symptoms of histamine intolerance are numerous and include gastrointestinal tract distress, rhinorrhea and nasal congestion, headache, urticaria, flushing, and pruritus. Histamine intolerance can mimic an IgE-mediated food allergy; however, allergy testing is negative in these patients. Unfortunately, there is no laboratory test for histamine intolerance; a double-blind, placebo-controlled food challenge is considered the gold-standard test.72
As it pertains to dermatology, a low histamine diet may play a role in the treatment of certain patients with atopic dermatitis and chronic spontaneous urticaria. One study reported that 17 of 54 (31.5%) atopic patients had higher basal levels of serum histamine compared to controls.73 Another study found that a histamine-free diet led to improvement in both histamine intolerance symptoms and atopic dermatitis disease severity (SCORing atopic dermatitis) in patients with low DAO activity.74 In chronic spontaneous urticaria, a recent systematic review found that in 223 patients placed on a low histamine diet for 3 to 4 weeks, 12% and 44% achieved complete and partial remission, respectively.75 Although treatment response based on a patient’s DAO activity level has not been correlated, a diet low in histamine may prove useful for patients with persistent atopic dermatitis and chronic spontaneous urticaria who have negative food allergy tests and report exacerbation of symptoms after ingestion of histamine-rich foods.76,77
Mediterranean Diet
The Mediterranean diet has been touted as one of the healthiest diets to date, and large randomized clinical trials have demonstrated its effectiveness in weight loss, improving insulin sensitivity, and reducing inflammatory cytokine profiles.78,79 A major criticism of the Mediterranean diet is that it has considerable ambiguity and lacks a precise definition due to the variability of what is consumed in different Mediterranean regions. Generally, the diet emphasizes high consumption of colorful fruits and vegetables, aromatic herbs and spices, olive oil, nuts, and seafood, as well as modest amounts of dairy, eggs, and red meat.80 The anti-inflammatory effects of this diet largely have been attributed to its abundance of polyphenols, carotenoids, monounsaturated fatty acids, and omega-3 polyunsaturated fatty acids (PUFAs).80,81 Examples of polyphenols include resveratrol in red grapes, quercetin in apples and red onions, and curcumin in turmeric, while examples of carotenoids include lycopene in tomatoes and zeaxanthin in dark leafy greens. Oleic acid is a monounsaturated fatty acid present in high concentrations in olive oil, while eicosapentaenoic acid and docosahexaenoic acid are omega-3 PUFAs predominantly found in fish.82
Unfortunately, rigorous clinical trials regarding the Mediterranean diet as it pertains to dermatology have not been undertaken. Numerous observational studies in patients with psoriasis have suggested that close adherence to the Mediterranean diet was associated with improvement in PASI scores.83-86 The National Psoriasis Foundation now recommends a trial of the Mediterranean diet in some patients with psoriasis, emphasizing increased dietary intake of olive oil, fish, and vegetables.87 Adherence to a Mediterranean diet also has been inversely correlated to the severity of acne vulgaris and hidradenitis suppurativa88,89; however, these studies failed to account for the multifactorial risk factors associated with these conditions. Mediterranean diets also may impart a chemopreventive effect, supported by a number of in vivo and in vitro studies demonstrating the inhibition and/or reversal of cutaneous DNA damage induced by UV radiation through supplementation with various phytonutrients and omega-3 PUFAs.81,90-92 Although small case-control studies have found a decreased risk of basal cell carcinoma in those who closely adhered to a Mediterranean diet, more rigorous clinical research is needed.93
Whole-Food, Plant-Based Diet
A whole-food, plant-based (WFPB) diet is another popular dietary approach that consists of eating fruits, vegetables, legumes, nuts, seeds, and grains in their whole natural form.94 This diet discourages all animal products, including red meat, seafood, dairy, and eggs. It is similar to a vegan diet except that it eliminates all highly refined carbohydrates, vegetable oils, and other processed foods.94 Randomized clinical studies have demonstrated the WFPB diet to be effective in the treatment of obesity and metabolic syndrome.95,96
A WFPB diet has been shown to increase the antioxidant capacity of cells, lengthen telomeres, and reduce formation of advanced glycation end products.94,97,98 These benefits may help combat accelerated skin aging, including increased skin permeability, reduced elasticity and hydration, decreased angiogenesis, impaired immune function, and decreased vitamin D synthesis. Accelerated skin aging can result in delayed wound healing and susceptibility to skin tears and ecchymoses and also may promote the development of cutaneous malignancies.99 There remains a lack of clinical data studying a properly formulated WFPB diet in the dermatologic setting.
Paleolithic Diet
The paleolithic (Paleo) diet is an increasingly popular way of eating that attempts to mirror what our ancestors may have consumed between 10,000 and 2.5 million years ago.100 It is similar to the Mediterranean diet but excludes grains, dairy, legumes, and nightshade vegetables. It also calls for elimination of highly processed sugars and oils as well as chemical food additives and preservatives. There is a strict variation of the diet for individuals with autoimmune disease that also excludes eggs, nuts, and seeds, as these can be inflammatory or immunogenic in some patients.100-106 Other variations of the diet exist, including the ketogenic Paleo diet, pegan (Paleo vegan) diet, and lacto-Paleo diet.100 An often cited criticism of the Paleo diet is the low intake of calcium and risk for osteoporosis; however, consumption of calcium-rich foods or a calcium supplement can address this concern.107
Although small clinical studies have found the Paleo diet to be beneficial for various autoimmune diseases, clinical data evaluating the utility of the diet for cutaneous disease is lacking.108,109 Numerous randomized trials have demonstrated the Paleo diet to be effective for weight loss and improving insulin sensitivity and lipid levels.110-116 Thus, the Paleo diet may theoretically serve as a viable adjunct dietary approach to the treatment of cutaneous diseases associated with obesity and metabolic derangement.117
Carnivore Diet
Arguably the most controversial and radical diet is the carnivore diet. As the name implies, the carnivore diet is based on consuming solely animal products. A properly structured carnivore diet emphasizes a “nose-to-tail” eating approach where all parts of the animal including the muscle meats, organs, and fat are consumed. Proponents of the diet cite anthropologic evidence from fossil-stable carbon-13/carbon-12 isotope analyses, craniodental features, and numerous other adaptations that indicate increased consumption of meat during human evolution.118-122 Notably, many early humans ate a carnivore diet, but life span was very short at this time, suggesting the diet may not be as beneficial as has been suggested.
Despite the abundance of anecdotal evidence supporting its use for a variety of chronic conditions, including cutaneous autoimmune disease, there is a virtual absence of high-quality research on the carnivore diet.123-125
The purported benefits of the carnivore diet may be attributed to the consumption of organ meats that contain highly bioavailable essential vitamins and minerals, such as iron, zinc, copper, selenium, thiamine, niacin, folate, vitamin B6, vitamin B12, vitamin A, vitamin D, vitamin K, and choline.126-128 Other dietary compounds that have demonstrated benefit for skin health and are predominantly found in animal foods include carnosine, carnitine, creatine, taurine, coenzyme Q10, and collagen.129-134 Nevertheless, there is no data to recommend the elimination of antioxidant- and micronutrient-dense plant-based foods. Rigorous clinical research evaluating the efficacy and safety of the carnivore diet in dermatologic patients is needed. A carnivore diet should not be undertaken without the assistance of a dietician who can ensure adequate micronutrient and macronutrient support.
Final Thoughts
The adjunctive role of diet in the treatment of skin disease is expanding and becoming more widely accepted among dermatologists. Unfortunately, there remains a lack of randomized controlled trials confirming the efficacy of various dietary interventions in the dermatologic setting. Although evidence-based dietary recommendations currently are limited, it is important for dermatologists to be aware of the varied and nuanced dietary interventions employed by patients.
Ultimately, dietary recommendations must be personalized, considering a patient’s comorbidities, personal beliefs and preferences, and nutrigenetics. The emerging field of dermatonutrigenomics—the study of how dietary compounds interact with one’s genes to influence skin health—may allow for precise dietary recommendations to be made in dermatologic practice. Direct-to-consumer genetic tests targeted toward dermatology patients are already on the market, but their clinical utility awaits validation.1 Because nutritional science is a constantly evolving field, becoming familiar with these popular diets will serve both dermatologists and their patients well.
Within the last decade, almost 3000 articles have been published on the role of diet in the prevention and management of dermatologic conditions. Patients are increasingly interested in—and employing—dietary modifications that may influence skin appearance and aid in the treatment of cutaneous disease.1 It is essential that dermatologists are familiar with existing evidence on the role of diet in dermatology to counsel patients appropriately. Herein, we discuss the compositions of several popular diets and their proposed utility for dermatologic purposes. We highlight the limited literature that exists surrounding this topic and emphasize the need for future, well-designed clinical trials that study the impact of diet on skin disease.
Ketogenic Diet
The ketogenic diet has a macronutrient profile composed of high fat, low to moderate protein, and very low carbohydrates. Nutritional ketosis occurs as the body begins to use free fatty acids (via beta oxidation) as the primary metabolite driving cellular metabolism. It has been suggested that the ketogenic diet may impart beneficial effects on skin disease; however, limited literature exists on the role of nutritional ketosis in the treatment of dermatologic conditions.
Mechanistically, the ketogenic diet decreases the secretion of insulin and insulinlike growth factor 1, resulting in a reduction of circulating androgens and increased activity of the retinoid X receptor.2 In acne vulgaris, it has been suggested that the ketogenic diet may be beneficial in decreasing androgen-induced sebum production and the overproliferation of keratinocytes.2-7 The ketogenic diet is one of the most rapidly effective dietary strategies for normalizing both insulin and androgens, thus it may theoretically be useful for other metabolic and hormone-dependent skin diseases, such as hidradenitis suppurativa.8,9
The cutaneous manifestations associated with chronic hyperinsulinemia and hyperglycemia are numerous and include acanthosis nigricans, acrochordons, diabetic dermopathy, scleredema diabeticorum, bullosis diabeticorum, keratosis pilaris, and generalized granuloma annulare. There also is an increased risk for bacterial and fungal skin infections associated with hyperglycemic states.10 The ketogenic diet is an effective nonpharmacologic tool for normalizing serum insulin and glucose levels in most patients and may have utility in the aforementioned conditions.11,12 In addition to improving insulin sensitivity, it has been used as a dietary strategy for weight loss.11-15 Because obesity and metabolic syndrome are highly correlated with common skin conditions such as psoriasis, hidradenitis suppurativa, and androgenetic alopecia, there may be a role for employing the ketogenic diet in these patient populations.16,17
Although robust clinical studies on ketogenic diets in skin disease are lacking, a recent single-arm, open-label clinical trial observed benefit in all 37 drug-naïve, overweight patients with chronic plaque psoriasis who underwent a ketogenic weight loss protocol. Significant reductions in psoriasis area and severity index (PASI) score and dermatology life quality index score were reported (P<.001).18 Another study of 30 patients with psoriasis found that a 4-week, low-calorie, ketogenic diet resulted in 50% improvement of PASI scores, 10% weight loss, and a reduction in the proinflammatory cytokines IL-1β and IL-2.19 Despite these results, it is a challenge to tease out if the specific dietary intervention or its associated weight loss was the main driver in these reported improvements in skin disease.
There is mixed evidence on the anti-inflammatory nature of the ketogenic diet, likely due to wide variation in the composition of foods included in individual diets. In many instances, the ketogenic diet is thought to possess considerable antioxidant and anti-inflammatory capabilities. Ketones are known activators of the nuclear factor erythroid 2–related factor 2 pathway, which upregulates the production of glutathione, a major endogenous intracellular antioxidant.20 Additionally, dietary compounds from foods that are encouraged while on the ketogenic diet, such as sulforaphane from broccoli, also are independent activators of nuclear factor erythroid 2–related factor 2.21 Ketones are efficiently utilized by mitochondria, which also may result in the decreased production of reactive oxygen species and lower oxidative stress.22 Moreover, the ketone body β-hydroxybutyrate has demonstrated the ability to reduce proinflammatory IL-1β levels via suppression of nucleotide-binding domain-like receptor protein 3 inflammasome activity.23,24 The activity of IL-1β is known to be elevated in many dermatologic conditions, including juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, hidradenitis suppurativa, Behçet disease, and other autoinflammatory syndromes.25 Ketones also have been shown to inhibit the nuclear factor–κB proinflammatory signaling pathway.22,26,27 Overexpression of IL-1β and aberrant activation of nuclear factor–κB are implicated in a variety of inflammatory, autoimmune, and oncologic cutaneous pathologies. The ketogenic diet may prove to be an effective adjunctive treatment for dermatologists to consider in select patient populations.23,24,28-30
For patients with keratinocyte carcinomas, the ketogenic diet may offer the aforementioned anti-inflammatory and antioxidant effects, as well as suppression of the mechanistic target of rapamycin, a major regulator of cell metabolism and proliferation.31,32 Inhibition of mechanistic target of rapamycin activity has been shown to slow tumor growth and reduce the development of squamous cell carcinoma.25,33,34 The ketogenic diet also may exploit the preferential utilization of glucose exhibited by many types of cancer cells, thereby “starving” the tumor of its primary fuel source.35,36 In vitro and animal studies in a variety of cancer types have demonstrated that a ketogenic metabolic state—achieved through the ketogenic diet or fasting—can sensitize tumor cells to chemotherapy and radiation while conferring a protective effect to normal cells.37-40 This recently described phenomenon is known as differential stress resistance, but it has not been studied in keratinocyte malignancies or melanoma to date. Importantly, some basal cell carcinomas and BRAF V600E–mutated melanomas have worsened while on the ketogenic diet, suggesting more data is needed before it can be recommended for all cancer patients.41,42 Furthermore, other skin conditions such as prurigo pigmentosa have been associated with initiation of the ketogenic diet.43
Low FODMAP Diet
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) are short-chain carbohydrates that are poorly absorbed, osmotically active, and rapidly fermented by intestinal bacteria.44 The low FODMAP diet has been shown to be efficacious for treatment of irritable bowel syndrome, small intestinal bacterial overgrowth (SIBO), and some cases of inflammatory bowel disease (IBD).44-49 A low FODMAP diet may have potential implications for several dermatologic conditions.
Rosacea has been associated with various gastrointestinal tract disorders including irritable bowel syndrome, SIBO, and IBD.50-54 A single study found that patients with rosacea had a 13-fold increased risk for SIBO.55,56 Treatment of 40 patients with SIBO using rifaximin resulted in complete resolution of rosacea in all patients, with no relapse after a 3-year follow-up period.55 Psoriasis also has been associated with SIBO and IBD.57,58 One small study found that eradication of SIBO in psoriatic patients resulted in improved PASI scores and colorimetric values.59
Although the long-term health consequences of the low FODMAP diet are unknown, further research on such dietary interventions for inflammatory skin conditions is warranted given the mounting evidence of a gut-skin connection and the role of the intestinal microbiome in skin health.50,51
Gluten-Free Diet
Gluten is a protein found in a variety of grains. Although the role of gluten in the pathogenesis of celiac disease and dermatitis herpetiformis is indisputable, the deleterious effects of gluten outside of the context of these diseases remain controversial. There may be a compelling case for eliminating gluten in psoriasis patients with seropositivity for celiac disease. A recent systematic review found a 2.2-fold increased risk for celiac disease in psoriasis patients.60 Antigliadin antibody titers also were found to be positively correlated with psoriatic disease severity.61 In addition, one open-label study found a reduction in PASI scores in 73% of patients with antigliadin antibodies after 3 months on a gluten-free diet compared to those without antibodies; however, the study only included 22 patients.62 Several other small studies have yielded similar results63,64; however, antigliadin antibodies are neither the most sensitive nor specific markers of celiac disease, and additional testing should be completed in any patient who may carry this diagnosis. A survey study by the National Psoriasis Foundation found that the dietary change associated with the greatest skin improvement was removal of gluten and nightshade vegetables in approximately 50% of the 1200 psoriasis patients that responded.65 Case reports of various dermatologic conditions including sarcoidosis, vitiligo, alopecia areata, lichen planus, dermatomyositis, pyoderma gangrenosum, erythema nodosum, leukocytoclastic vasculitis, linear IgA bullous dermatosis, and aphthous ulcerations have reportedly improved with a gluten-free diet; however, this should not be used as primary therapy in patients without celiac disease.66-71 Because gluten-free diets can be expensive and challenging to follow, a formal assessment for celiac disease should be considered before recommendation of this dietary intervention.
Low Histamine Diet
Histamine is a biogenic amine produced by the decarboxylation of the amino acid histidine.72 It is found in several foods in varying amounts. Because bacteria can convert histidine into histamine, many fermented and aged foods such as kimchi, sauerkraut, cheese, and red wine contain high levels of histamine. Individuals who have decreased activity of diamine oxidase (DAO), an enzyme that degrades histamine, may be more susceptible to histamine intolerance.72 The symptoms of histamine intolerance are numerous and include gastrointestinal tract distress, rhinorrhea and nasal congestion, headache, urticaria, flushing, and pruritus. Histamine intolerance can mimic an IgE-mediated food allergy; however, allergy testing is negative in these patients. Unfortunately, there is no laboratory test for histamine intolerance; a double-blind, placebo-controlled food challenge is considered the gold-standard test.72
As it pertains to dermatology, a low histamine diet may play a role in the treatment of certain patients with atopic dermatitis and chronic spontaneous urticaria. One study reported that 17 of 54 (31.5%) atopic patients had higher basal levels of serum histamine compared to controls.73 Another study found that a histamine-free diet led to improvement in both histamine intolerance symptoms and atopic dermatitis disease severity (SCORing atopic dermatitis) in patients with low DAO activity.74 In chronic spontaneous urticaria, a recent systematic review found that in 223 patients placed on a low histamine diet for 3 to 4 weeks, 12% and 44% achieved complete and partial remission, respectively.75 Although treatment response based on a patient’s DAO activity level has not been correlated, a diet low in histamine may prove useful for patients with persistent atopic dermatitis and chronic spontaneous urticaria who have negative food allergy tests and report exacerbation of symptoms after ingestion of histamine-rich foods.76,77
Mediterranean Diet
The Mediterranean diet has been touted as one of the healthiest diets to date, and large randomized clinical trials have demonstrated its effectiveness in weight loss, improving insulin sensitivity, and reducing inflammatory cytokine profiles.78,79 A major criticism of the Mediterranean diet is that it has considerable ambiguity and lacks a precise definition due to the variability of what is consumed in different Mediterranean regions. Generally, the diet emphasizes high consumption of colorful fruits and vegetables, aromatic herbs and spices, olive oil, nuts, and seafood, as well as modest amounts of dairy, eggs, and red meat.80 The anti-inflammatory effects of this diet largely have been attributed to its abundance of polyphenols, carotenoids, monounsaturated fatty acids, and omega-3 polyunsaturated fatty acids (PUFAs).80,81 Examples of polyphenols include resveratrol in red grapes, quercetin in apples and red onions, and curcumin in turmeric, while examples of carotenoids include lycopene in tomatoes and zeaxanthin in dark leafy greens. Oleic acid is a monounsaturated fatty acid present in high concentrations in olive oil, while eicosapentaenoic acid and docosahexaenoic acid are omega-3 PUFAs predominantly found in fish.82
Unfortunately, rigorous clinical trials regarding the Mediterranean diet as it pertains to dermatology have not been undertaken. Numerous observational studies in patients with psoriasis have suggested that close adherence to the Mediterranean diet was associated with improvement in PASI scores.83-86 The National Psoriasis Foundation now recommends a trial of the Mediterranean diet in some patients with psoriasis, emphasizing increased dietary intake of olive oil, fish, and vegetables.87 Adherence to a Mediterranean diet also has been inversely correlated to the severity of acne vulgaris and hidradenitis suppurativa88,89; however, these studies failed to account for the multifactorial risk factors associated with these conditions. Mediterranean diets also may impart a chemopreventive effect, supported by a number of in vivo and in vitro studies demonstrating the inhibition and/or reversal of cutaneous DNA damage induced by UV radiation through supplementation with various phytonutrients and omega-3 PUFAs.81,90-92 Although small case-control studies have found a decreased risk of basal cell carcinoma in those who closely adhered to a Mediterranean diet, more rigorous clinical research is needed.93
Whole-Food, Plant-Based Diet
A whole-food, plant-based (WFPB) diet is another popular dietary approach that consists of eating fruits, vegetables, legumes, nuts, seeds, and grains in their whole natural form.94 This diet discourages all animal products, including red meat, seafood, dairy, and eggs. It is similar to a vegan diet except that it eliminates all highly refined carbohydrates, vegetable oils, and other processed foods.94 Randomized clinical studies have demonstrated the WFPB diet to be effective in the treatment of obesity and metabolic syndrome.95,96
A WFPB diet has been shown to increase the antioxidant capacity of cells, lengthen telomeres, and reduce formation of advanced glycation end products.94,97,98 These benefits may help combat accelerated skin aging, including increased skin permeability, reduced elasticity and hydration, decreased angiogenesis, impaired immune function, and decreased vitamin D synthesis. Accelerated skin aging can result in delayed wound healing and susceptibility to skin tears and ecchymoses and also may promote the development of cutaneous malignancies.99 There remains a lack of clinical data studying a properly formulated WFPB diet in the dermatologic setting.
Paleolithic Diet
The paleolithic (Paleo) diet is an increasingly popular way of eating that attempts to mirror what our ancestors may have consumed between 10,000 and 2.5 million years ago.100 It is similar to the Mediterranean diet but excludes grains, dairy, legumes, and nightshade vegetables. It also calls for elimination of highly processed sugars and oils as well as chemical food additives and preservatives. There is a strict variation of the diet for individuals with autoimmune disease that also excludes eggs, nuts, and seeds, as these can be inflammatory or immunogenic in some patients.100-106 Other variations of the diet exist, including the ketogenic Paleo diet, pegan (Paleo vegan) diet, and lacto-Paleo diet.100 An often cited criticism of the Paleo diet is the low intake of calcium and risk for osteoporosis; however, consumption of calcium-rich foods or a calcium supplement can address this concern.107
Although small clinical studies have found the Paleo diet to be beneficial for various autoimmune diseases, clinical data evaluating the utility of the diet for cutaneous disease is lacking.108,109 Numerous randomized trials have demonstrated the Paleo diet to be effective for weight loss and improving insulin sensitivity and lipid levels.110-116 Thus, the Paleo diet may theoretically serve as a viable adjunct dietary approach to the treatment of cutaneous diseases associated with obesity and metabolic derangement.117
Carnivore Diet
Arguably the most controversial and radical diet is the carnivore diet. As the name implies, the carnivore diet is based on consuming solely animal products. A properly structured carnivore diet emphasizes a “nose-to-tail” eating approach where all parts of the animal including the muscle meats, organs, and fat are consumed. Proponents of the diet cite anthropologic evidence from fossil-stable carbon-13/carbon-12 isotope analyses, craniodental features, and numerous other adaptations that indicate increased consumption of meat during human evolution.118-122 Notably, many early humans ate a carnivore diet, but life span was very short at this time, suggesting the diet may not be as beneficial as has been suggested.
Despite the abundance of anecdotal evidence supporting its use for a variety of chronic conditions, including cutaneous autoimmune disease, there is a virtual absence of high-quality research on the carnivore diet.123-125
The purported benefits of the carnivore diet may be attributed to the consumption of organ meats that contain highly bioavailable essential vitamins and minerals, such as iron, zinc, copper, selenium, thiamine, niacin, folate, vitamin B6, vitamin B12, vitamin A, vitamin D, vitamin K, and choline.126-128 Other dietary compounds that have demonstrated benefit for skin health and are predominantly found in animal foods include carnosine, carnitine, creatine, taurine, coenzyme Q10, and collagen.129-134 Nevertheless, there is no data to recommend the elimination of antioxidant- and micronutrient-dense plant-based foods. Rigorous clinical research evaluating the efficacy and safety of the carnivore diet in dermatologic patients is needed. A carnivore diet should not be undertaken without the assistance of a dietician who can ensure adequate micronutrient and macronutrient support.
Final Thoughts
The adjunctive role of diet in the treatment of skin disease is expanding and becoming more widely accepted among dermatologists. Unfortunately, there remains a lack of randomized controlled trials confirming the efficacy of various dietary interventions in the dermatologic setting. Although evidence-based dietary recommendations currently are limited, it is important for dermatologists to be aware of the varied and nuanced dietary interventions employed by patients.
Ultimately, dietary recommendations must be personalized, considering a patient’s comorbidities, personal beliefs and preferences, and nutrigenetics. The emerging field of dermatonutrigenomics—the study of how dietary compounds interact with one’s genes to influence skin health—may allow for precise dietary recommendations to be made in dermatologic practice. Direct-to-consumer genetic tests targeted toward dermatology patients are already on the market, but their clinical utility awaits validation.1 Because nutritional science is a constantly evolving field, becoming familiar with these popular diets will serve both dermatologists and their patients well.
- Jaros J, Katta R, Shi VY. Dermatonutrigenomics: past, present, and future. Dermatology. 2019;235:164-166.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Smith R, Mann N, Mäkeläinen H, et al. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52:718-726.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a "male" therapy for a predominantly "female" disease. J Clin Aesthet Dermatol. 2016;9:44.
- Nikolakis G, Karagiannidis I, Vaiopoulos AG, et al. Endocrinological mechanisms in the pathophysiology of hidradenitis suppurativa [in German]. Hautarzt. 2020;71:762-771.
- Karadag AS, Ozlu E, Lavery MJ. Cutaneous manifestations of diabetes mellitus and the metabolic syndrome. Clin Dermatology. 2018;36:89-93.
- Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969-977.
- Anton SD, Hida A, Heekin K, et al. Effects of popular diets without specific calorie targets on weight loss outcomes: systematic review of findings from clinical trials. Nutrients. 2017;9:822.
- Castellana M, Conte E, Cignarelli A, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:5-16.
- Paoli A, Mancin L, Giacona MC, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18:104.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Lian N, Chen M. Metabolic syndrome and skin disease: potential connection and risk. Int J Dermatol Venereol. 2019;2:89-93.
- Hu Y, Zhu Y, Lian N, et al. Metabolic syndrome and skin diseases. Front Endocrinol (Lausanne). 2019;10:788.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Milder J, Liang L-P, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238-244.
- Kubo E, Chhunchha B, Singh P, et al. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep. 2017;7:14130.
- Pinto A, Bonucci A, Maggi E, et al. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer's disease. Antioxidants (Basel). 2018;7:63.
- Youm Y-H, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Rahman M, Muhammad S, Khan MA, et al. The β-hydroxybutyrate receptor HCA 2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:1-11.
- Lu Y, Yang YY, Zhou MW, et al. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett. 2018;683:13-18.
- Hamarsheh S, Zeiser R. NLRP3 inflammasome activation in cancer: a double-edged sword. Front Immunol. 2020;11:1444.
- Bell S, Degitz K, Quirling M, et al. Involvement of NF-κB signalling in skin physiology and disease. Cell Signal. 2003;15:1-7.
- Goldminz AM, Au SC, Kim N, et al. NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69:89-94.
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589.
- McDaniel S, Rensing N, Yamada K, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:E7-E11.
- Alter M, Satzger I, Schrem H, et al. Non-melanoma skin cancer is reduced after switch of immunosuppression to mTOR-inhibitors in organ transplant recipients. J Dtsch Dermatol Ges. 2014;12:480-488.
- Feldmeyer L, Hofbauer GF, Böni T, et al. Mammalian target of rapamycin (mTOR) inhibitors slow skin carcinogenesis, but impair wound healing. Br J Dermatol. 2012;166:422-424.
- Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211-218.
- Li W. "Warburg effect" and mitochondrial metabolism in skin cancer.J Carcinogene Mutagene. 2012:S4.
- Naveed S, Aslam M, Ahmad A. Starvation based differential chemotherapy: a novel approach for cancer treatment. Oman Med J. 2014;29:391-398.
- Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215-8220.
- Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29:271-280.
- de Groot S, Pijl H, van der Hoeven JJM, et al. Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res. 2019;38:209.
- Hosseini M, Kasraian Z, Rezvani HR. Energy metabolism in skin cancers: a therapeutic perspective. Biochim Biophys Acta Bioenerg. 2017;1858:712-722.
- Feichtinger RG, Lang R, Geilberger R, et al. Melanoma tumors exhibit a variable but distinct metabolic signature. Exp Dermatol. 2018;27:204-207.
- Alshaya MA, Turkmani MG, Alissa AM. Prurigo pigmentosa following ketogenic diet and bariatric surgery: a growing association. JAAD Case Rep. 2019;5:504-507.
- Bellini M, Tonarelli S, Nagy AG, et al. Low FODMAP diet: evidence, doubts, and hopes. Nutrients. 2020;12:148.
- Kwiatkowski L, Rice E, Langland J. Integrative treatment of chronic abdominal bloating and pain associated with overgrowth of small intestinal bacteria: a case report. Altern Ther Health Med. 2017;23:56-61.
- Hubkova T. No more pain in the gut: lifestyle medicine approach to irritable bowel syndrome. Am J Lifestyle Med. 2017;11:223-226.
- Schumann D, Klose P, Lauche R, et al. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition. 2018;45:24-31.
- Cox SR, Prince AC, Myers CE, et al. Fermentable carbohydrates [FODMAPs] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: a randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J Crohns Colitis. 2017;11:1420-1429.
- Damas OM, Garces L, Abreu MT. Diet as adjunctive treatment for inflammatory bowel disease: review and update of the latest literature. Curr Treat Options Gastroenterol. 2019;17:313-325.
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424.
- Daou H, Paradiso M, Hennessy K, et al. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Weinstock LB, Steinhoff M. Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin. J Am Acad Dermatol. 2013;68:875-876.
- Wu CY, Chang YT, Juan CK, et al. Risk of inflammatory bowel disease in patients with rosacea: results from a nationwide cohort study in Taiwan. J Am Acad Dermatol. 2017;76:911-917.
- Egeberg A, Weinstock LB, Thyssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Drago F, De Col E, Agnoletti AF, et al. The role of small intestinal bacterial overgrowth in rosacea: a 3-year follow-up. J Am Acad Dermatol. 2016;75:E113-E115.
- Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759-764.
- Ojetti V, De Simone C, Aguilar Sanchez J, et al. Malabsorption in psoriatic patients: cause or consequence? Scand J Gastroenterol. 2006;41:1267-1271.
- Kim M, Choi KH, Hwang SW, et al. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017;76:40-48.
- Drago F, Ciccarese G, Indemini E, et al. Psoriasis and small intestine bacterial overgrowth. Int J Dermatol. 2018;57:112-113.
- Acharya P, Mathur M. Association between psoriasis and celiac disease: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1376-1385.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2018;11:13-19.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Loche F, Bazex J. Celiac disease associated with cutaneous sarcoidosic granuloma [in French]. Rev Med Interne. 1997;18:975-978.
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210.
- Wijarnpreecha K, Panjawatanan P, Corral JE, et al. Celiac disease and risk of sarcoidosis: a systematic review and meta-analysis. J Evid Based Med. 2019;12:194-199.
- Rodrigo L, Beteta-Gorriti V, Alvarez N, et al. Cutaneous and mucosal manifestations associated with celiac disease. Nutrients. 2018;10:800.
- Song MS, Farber D, Bitton A, et al. Dermatomyositis associated with celiac disease: response to a gluten-free diet. Can J Gastroenterol. 2006;20:433-435.
- Egan CA, Smith EP, Taylor TB, et al. Linear IgA bullous dermatosis responsive to a gluten-free diet. Am J Gastroenterol. 2001;96:1927-1929.
- Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, et al. Histamine intolerance: the current state of the art. Biomolecules. 2020;10:1181.
- Ring J. Plasma histamine concentrations in atopic eczema. Clin Allergy. 1983;13:545-552.
- Maintz L, Benfadal S, Allam JP, et al. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J Allergy Clin Immunol. 2006;117:1106-1112.
- Cornillier H, Giraudeau B, Samimi M, et al. Effect of diet in chronic spontaneous urticaria: a systematic review. Acta Derm Venereol. 2019;99:127-132.
- Son JH, Chung BY, Kim HO, et al. A histamine-free diet is helpful for treatment of adult patients with chronic spontaneous urticaria. Ann Dermatol. 2018;30:164-172.
- Wagner N, Dirk D, Peveling-Oberhag A, et al. A popular myth - low-histamine diet improves chronic spontaneous urticaria - fact or fiction? J Eur Acad Dermatol Venereol. 2017;31:650-655.
- Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
- Steffen LM, Van Horn L, Daviglus ML, et al. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (coronary artery risk development in young adults) study. Br J Nutr. 2014;112:1654-1661.
- Bower A, Marquez S, de Mejia EG. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit Rev Food Sci Nutr. 2016;56:2728-2746.
- Bosch R, Philips N, Suárez-Pérez JA, et al. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants (Basel). 2015;4:248-268.
- Katsimbri P, Korakas E, Kountouri A, et al. The effect of antioxidant and anti-inflammatory capacity of diet on psoriasis and psoriatic arthritis phenotype: nutrition as therapeutic tool? Antioxidants. 2021;10:157.
- Molina-Leyva A, Cuenca-Barrales C, Vega-Castillo JJ, et al. Adherence to Mediterranean diet in Spanish patients with psoriasis: cardiovascular benefits? Dermatol Ther. 2019;32:E12810.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:1-10.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Korovesi A, Dalamaga M, Kotopouli M, et al. Adherence to the Mediterranean diet is independently associated with psoriasis risk, severity, and quality of life: a cross-sectional observational study. Int J Dermatol. 2019;58:E164-E165.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934-950.
- Skroza N, Tolino E, Semyonov L, et al. Mediterranean diet and familial dysmetabolism as factors influencing the development of acne. Scand J Public Health. 2012;40:466-474.
- Barrea L, Fabbrocini G, Annunziata G, et al. Role of nutrition and adherence to the Mediterranean diet in the multidisciplinary approach of hidradenitis suppurativa: evaluation of nutritional status and its association with severity of disease. Nutrients. 2018;11:57.
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71-83.
- Huang T-H, Wang P-W, Yang S-C, et al. Cosmetic and therapeutic applications of fish oil's fatty acids on the skin. Mar Drugs. 2018;16:256.
- Rizwan M, Rodriguez-Blanco I, Harbottle A, et al. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: a randomized controlled trial. Br J Dermatol. 2011;164:154-162.
- Leone A, Martínez-González M, Martin-Gorgojo A, et al. Mediterranean diet, dietary approaches to stop hypertension, and pro-vegetarian dietary pattern in relation to the risk of basal cell carcinoma: a nested case-control study within the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2020;112:364-372.
- Solway J, McBride M, Haq F, et al. Diet and dermatology: the role of a whole-food, plant-based diet in preventing and reversing skin aging--a review. J Clin Aesthet Dermatol. 2020;13:38-43.
- Greger M. A whole food plant-based diet is effective for weight loss: the evidence. Am J Lifestyle Med. 2020;14:500-510.
- Wright N, Wilson L, Smith M, et al. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7:E256.
- Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14:1112-1120.
- Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048-1057.
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3-14.
- Gupta L, Khandelwal D, Lal PR, et al. Palaeolithic diet in diabesity and endocrinopathies--a vegan's perspective. Eur Endocrinol. 2019;15:77-82.
- Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66:1414-1427.
- Thorburn Alison N, Macia L, Mackay Charles R. Diet, metabolites, and "Western lifestyle" inflammatory diseases. Immunity. 2014;40:833-842.
- Katta R, Schlichte M. Diet and dermatitis: food triggers. J Clin Aesthet Dermatol. 2014;7:30-36.
- Dhar S, Srinivas SM. Food allergy in atopic dermatitis. Indian J Dermatol. 2016;61:645-648.
- Birmingham N, Thanesvorakul S, Gangur V. Relative immunogenicity of commonly allergenic foods versus rarely allergenic and nonallergenic foods in mice. J Food Prot. 2002;65:1988-1991.
- Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16:751-765.
- Kowalski LM, Bujko J. Evaluation of biological and clinical potential of paleolithic diet [in Polish]. Rocz Panstw Zakl Hig. 2012;63:9-15.
- Lee JE, Titcomb TJ, Bisht B, et al. A modified MCT-based ketogenic diet increases plasma β-hydroxybutyrate but has less effect on fatigue and quality of life in people with multiple sclerosis compared to a modified paleolithic diet: a waitlist-controlled, randomized pilot study. J Am Coll Nutr. 2021;40:13-25.
- Abbott RD, Sadowski A, Alt AG. Efficacy of the autoimmune protocol diet as part of a multi-disciplinary, supported lifestyle intervention for Hashimoto's thyroiditis. Cureus. 2019;11:E4556.
- Lindeberg S, Jönsson T, Granfeldt Y, et al. A palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia. 2007;50:1795-1807.
- Jönsson T, Granfeldt Y, Ahrén B, et al. Beneficial effects of a paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35.
- Boers I, Muskiet FAJ, Berkelaar E, et al. Favourable effects of consuming a palaeolithic-type diet on characteristics of the metabolic syndrome: a randomized controlled pilot-study. Lipids Health Dis. 2014;13:160.
- Ghaedi E, Mohammadi M, Mohammadi H, et al. Effects of a paleolithic diet on cardiovascular disease risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10:634-646.
- Mellberg C, Sandberg S, Ryberg M, et al. Long-term effects of a palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68:350-357.
- Pastore RL, Brooks JT, Carbone JW. Paleolithic nutrition improves plasma lipid concentrations of hypercholesterolemic adults to a greater extent than traditional heart-healthy dietary recommendations. Nutr Res. 2015;35:474-479.
- Otten J, Stomby A, Waling M, et al. Benefits of a paleolithic diet with and without supervised exercise on fat mass, insulin sensitivity, and glycemic control: a randomized controlled trial in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2017;33:E2828.
- Stefanadi EC, Dimitrakakis G, Antoniou C-K, et al. Metabolic syndrome and the skin: a more than superficial association. reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol Metab Syndr. 2018;10:9.
- Mann N. Meat in the human diet: an anthropological perspective. Nutr Dietetics. 2007;64(suppl 4):S102-S107.
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345-352.
- Kuhn JE. Throwing, the shoulder, and human evolution. Am J Orthop (Belle Mead NJ). 2016;45:110-114.
- Kobayashi H, Kohshima S. Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J Hum Evol. 2001;40:419-435.
- Cordain L, Eaton SB, Miller JB, et al. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002;56(suppl 1):S42-S52.
- McClellan WS, Du Bois EF. Clinical calorimetry: XLV. prolonged meat diets with a study of kidney function and ketosis. J Biol Chem. 1930;87:651-668.
- O'Hearn A. Can a carnivore diet provide all essential nutrients? Curr Opin Endocrinol Diabetes Obes. 2020;27:312-316.
- O'Hearn LA. A survey of improvements experienced on a carnivore diet compared to only carbohydrate restriction. Open Science Forum website. Published February 12, 2019. Accessed May 17, 2021. doi:10.17605/OSF.IO/5FU4D
- Williams P. Nutritional composition of red meat. Nutrition & Dietetics. 2007;64(suppl 4):S113-S119.
- Biel W, Czerniawska-Piątkowska E, Kowalczyk A. Offal chemical composition from veal, beef, and lamb maintained in organic production systems. Animals (Basel). 2019;9:489.
- Elmadfa I, Meyer AL. The role of the status of selected micronutrients in shaping the immune function. Endocr Metab Immune Disord Drug Targets. 2019;19:1100-1115.
- Babizhayev M. Treatment of skin aging and photoaging with innovative oral dosage forms of nonhydrolized carnosine and carcinine. Int J Clin Derm Res. 2017;5:116-143.
- Danby FW. Nutrition and aging skin: sugar and glycation. Clin Dermatol. 2010;28:409-411.
- Siefken W, Carstensen S, Springmann G, et al. Role of taurine accumulation in keratinocyte hydration. J Invest Dermatol. 2003;121:354-361.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19:3059.
- Fischer F, Achterberg V, März A, et al. Folic acid and creatineimprove the firmness of human skin in vivo. J Cosmet Dermatol. 2011;10:15-23.
- Blatt T, Lenz H, Weber T. Topical application of creatine is multibeneficial for human skin. J Am Acad Dermatol. 2005;52:P32.
- Jaros J, Katta R, Shi VY. Dermatonutrigenomics: past, present, and future. Dermatology. 2019;235:164-166.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Smith R, Mann N, Mäkeläinen H, et al. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52:718-726.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a "male" therapy for a predominantly "female" disease. J Clin Aesthet Dermatol. 2016;9:44.
- Nikolakis G, Karagiannidis I, Vaiopoulos AG, et al. Endocrinological mechanisms in the pathophysiology of hidradenitis suppurativa [in German]. Hautarzt. 2020;71:762-771.
- Karadag AS, Ozlu E, Lavery MJ. Cutaneous manifestations of diabetes mellitus and the metabolic syndrome. Clin Dermatology. 2018;36:89-93.
- Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969-977.
- Anton SD, Hida A, Heekin K, et al. Effects of popular diets without specific calorie targets on weight loss outcomes: systematic review of findings from clinical trials. Nutrients. 2017;9:822.
- Castellana M, Conte E, Cignarelli A, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:5-16.
- Paoli A, Mancin L, Giacona MC, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18:104.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Lian N, Chen M. Metabolic syndrome and skin disease: potential connection and risk. Int J Dermatol Venereol. 2019;2:89-93.
- Hu Y, Zhu Y, Lian N, et al. Metabolic syndrome and skin diseases. Front Endocrinol (Lausanne). 2019;10:788.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Milder J, Liang L-P, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238-244.
- Kubo E, Chhunchha B, Singh P, et al. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep. 2017;7:14130.
- Pinto A, Bonucci A, Maggi E, et al. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer's disease. Antioxidants (Basel). 2018;7:63.
- Youm Y-H, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Rahman M, Muhammad S, Khan MA, et al. The β-hydroxybutyrate receptor HCA 2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:1-11.
- Lu Y, Yang YY, Zhou MW, et al. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett. 2018;683:13-18.
- Hamarsheh S, Zeiser R. NLRP3 inflammasome activation in cancer: a double-edged sword. Front Immunol. 2020;11:1444.
- Bell S, Degitz K, Quirling M, et al. Involvement of NF-κB signalling in skin physiology and disease. Cell Signal. 2003;15:1-7.
- Goldminz AM, Au SC, Kim N, et al. NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69:89-94.
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589.
- McDaniel S, Rensing N, Yamada K, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:E7-E11.
- Alter M, Satzger I, Schrem H, et al. Non-melanoma skin cancer is reduced after switch of immunosuppression to mTOR-inhibitors in organ transplant recipients. J Dtsch Dermatol Ges. 2014;12:480-488.
- Feldmeyer L, Hofbauer GF, Böni T, et al. Mammalian target of rapamycin (mTOR) inhibitors slow skin carcinogenesis, but impair wound healing. Br J Dermatol. 2012;166:422-424.
- Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211-218.
- Li W. "Warburg effect" and mitochondrial metabolism in skin cancer.J Carcinogene Mutagene. 2012:S4.
- Naveed S, Aslam M, Ahmad A. Starvation based differential chemotherapy: a novel approach for cancer treatment. Oman Med J. 2014;29:391-398.
- Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215-8220.
- Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29:271-280.
- de Groot S, Pijl H, van der Hoeven JJM, et al. Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res. 2019;38:209.
- Hosseini M, Kasraian Z, Rezvani HR. Energy metabolism in skin cancers: a therapeutic perspective. Biochim Biophys Acta Bioenerg. 2017;1858:712-722.
- Feichtinger RG, Lang R, Geilberger R, et al. Melanoma tumors exhibit a variable but distinct metabolic signature. Exp Dermatol. 2018;27:204-207.
- Alshaya MA, Turkmani MG, Alissa AM. Prurigo pigmentosa following ketogenic diet and bariatric surgery: a growing association. JAAD Case Rep. 2019;5:504-507.
- Bellini M, Tonarelli S, Nagy AG, et al. Low FODMAP diet: evidence, doubts, and hopes. Nutrients. 2020;12:148.
- Kwiatkowski L, Rice E, Langland J. Integrative treatment of chronic abdominal bloating and pain associated with overgrowth of small intestinal bacteria: a case report. Altern Ther Health Med. 2017;23:56-61.
- Hubkova T. No more pain in the gut: lifestyle medicine approach to irritable bowel syndrome. Am J Lifestyle Med. 2017;11:223-226.
- Schumann D, Klose P, Lauche R, et al. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition. 2018;45:24-31.
- Cox SR, Prince AC, Myers CE, et al. Fermentable carbohydrates [FODMAPs] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: a randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J Crohns Colitis. 2017;11:1420-1429.
- Damas OM, Garces L, Abreu MT. Diet as adjunctive treatment for inflammatory bowel disease: review and update of the latest literature. Curr Treat Options Gastroenterol. 2019;17:313-325.
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424.
- Daou H, Paradiso M, Hennessy K, et al. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Weinstock LB, Steinhoff M. Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin. J Am Acad Dermatol. 2013;68:875-876.
- Wu CY, Chang YT, Juan CK, et al. Risk of inflammatory bowel disease in patients with rosacea: results from a nationwide cohort study in Taiwan. J Am Acad Dermatol. 2017;76:911-917.
- Egeberg A, Weinstock LB, Thyssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Drago F, De Col E, Agnoletti AF, et al. The role of small intestinal bacterial overgrowth in rosacea: a 3-year follow-up. J Am Acad Dermatol. 2016;75:E113-E115.
- Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759-764.
- Ojetti V, De Simone C, Aguilar Sanchez J, et al. Malabsorption in psoriatic patients: cause or consequence? Scand J Gastroenterol. 2006;41:1267-1271.
- Kim M, Choi KH, Hwang SW, et al. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017;76:40-48.
- Drago F, Ciccarese G, Indemini E, et al. Psoriasis and small intestine bacterial overgrowth. Int J Dermatol. 2018;57:112-113.
- Acharya P, Mathur M. Association between psoriasis and celiac disease: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1376-1385.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2018;11:13-19.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Loche F, Bazex J. Celiac disease associated with cutaneous sarcoidosic granuloma [in French]. Rev Med Interne. 1997;18:975-978.
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210.
- Wijarnpreecha K, Panjawatanan P, Corral JE, et al. Celiac disease and risk of sarcoidosis: a systematic review and meta-analysis. J Evid Based Med. 2019;12:194-199.
- Rodrigo L, Beteta-Gorriti V, Alvarez N, et al. Cutaneous and mucosal manifestations associated with celiac disease. Nutrients. 2018;10:800.
- Song MS, Farber D, Bitton A, et al. Dermatomyositis associated with celiac disease: response to a gluten-free diet. Can J Gastroenterol. 2006;20:433-435.
- Egan CA, Smith EP, Taylor TB, et al. Linear IgA bullous dermatosis responsive to a gluten-free diet. Am J Gastroenterol. 2001;96:1927-1929.
- Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, et al. Histamine intolerance: the current state of the art. Biomolecules. 2020;10:1181.
- Ring J. Plasma histamine concentrations in atopic eczema. Clin Allergy. 1983;13:545-552.
- Maintz L, Benfadal S, Allam JP, et al. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J Allergy Clin Immunol. 2006;117:1106-1112.
- Cornillier H, Giraudeau B, Samimi M, et al. Effect of diet in chronic spontaneous urticaria: a systematic review. Acta Derm Venereol. 2019;99:127-132.
- Son JH, Chung BY, Kim HO, et al. A histamine-free diet is helpful for treatment of adult patients with chronic spontaneous urticaria. Ann Dermatol. 2018;30:164-172.
- Wagner N, Dirk D, Peveling-Oberhag A, et al. A popular myth - low-histamine diet improves chronic spontaneous urticaria - fact or fiction? J Eur Acad Dermatol Venereol. 2017;31:650-655.
- Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
- Steffen LM, Van Horn L, Daviglus ML, et al. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (coronary artery risk development in young adults) study. Br J Nutr. 2014;112:1654-1661.
- Bower A, Marquez S, de Mejia EG. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit Rev Food Sci Nutr. 2016;56:2728-2746.
- Bosch R, Philips N, Suárez-Pérez JA, et al. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants (Basel). 2015;4:248-268.
- Katsimbri P, Korakas E, Kountouri A, et al. The effect of antioxidant and anti-inflammatory capacity of diet on psoriasis and psoriatic arthritis phenotype: nutrition as therapeutic tool? Antioxidants. 2021;10:157.
- Molina-Leyva A, Cuenca-Barrales C, Vega-Castillo JJ, et al. Adherence to Mediterranean diet in Spanish patients with psoriasis: cardiovascular benefits? Dermatol Ther. 2019;32:E12810.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:1-10.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Korovesi A, Dalamaga M, Kotopouli M, et al. Adherence to the Mediterranean diet is independently associated with psoriasis risk, severity, and quality of life: a cross-sectional observational study. Int J Dermatol. 2019;58:E164-E165.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934-950.
- Skroza N, Tolino E, Semyonov L, et al. Mediterranean diet and familial dysmetabolism as factors influencing the development of acne. Scand J Public Health. 2012;40:466-474.
- Barrea L, Fabbrocini G, Annunziata G, et al. Role of nutrition and adherence to the Mediterranean diet in the multidisciplinary approach of hidradenitis suppurativa: evaluation of nutritional status and its association with severity of disease. Nutrients. 2018;11:57.
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71-83.
- Huang T-H, Wang P-W, Yang S-C, et al. Cosmetic and therapeutic applications of fish oil's fatty acids on the skin. Mar Drugs. 2018;16:256.
- Rizwan M, Rodriguez-Blanco I, Harbottle A, et al. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: a randomized controlled trial. Br J Dermatol. 2011;164:154-162.
- Leone A, Martínez-González M, Martin-Gorgojo A, et al. Mediterranean diet, dietary approaches to stop hypertension, and pro-vegetarian dietary pattern in relation to the risk of basal cell carcinoma: a nested case-control study within the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2020;112:364-372.
- Solway J, McBride M, Haq F, et al. Diet and dermatology: the role of a whole-food, plant-based diet in preventing and reversing skin aging--a review. J Clin Aesthet Dermatol. 2020;13:38-43.
- Greger M. A whole food plant-based diet is effective for weight loss: the evidence. Am J Lifestyle Med. 2020;14:500-510.
- Wright N, Wilson L, Smith M, et al. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7:E256.
- Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14:1112-1120.
- Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048-1057.
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3-14.
- Gupta L, Khandelwal D, Lal PR, et al. Palaeolithic diet in diabesity and endocrinopathies--a vegan's perspective. Eur Endocrinol. 2019;15:77-82.
- Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66:1414-1427.
- Thorburn Alison N, Macia L, Mackay Charles R. Diet, metabolites, and "Western lifestyle" inflammatory diseases. Immunity. 2014;40:833-842.
- Katta R, Schlichte M. Diet and dermatitis: food triggers. J Clin Aesthet Dermatol. 2014;7:30-36.
- Dhar S, Srinivas SM. Food allergy in atopic dermatitis. Indian J Dermatol. 2016;61:645-648.
- Birmingham N, Thanesvorakul S, Gangur V. Relative immunogenicity of commonly allergenic foods versus rarely allergenic and nonallergenic foods in mice. J Food Prot. 2002;65:1988-1991.
- Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16:751-765.
- Kowalski LM, Bujko J. Evaluation of biological and clinical potential of paleolithic diet [in Polish]. Rocz Panstw Zakl Hig. 2012;63:9-15.
- Lee JE, Titcomb TJ, Bisht B, et al. A modified MCT-based ketogenic diet increases plasma β-hydroxybutyrate but has less effect on fatigue and quality of life in people with multiple sclerosis compared to a modified paleolithic diet: a waitlist-controlled, randomized pilot study. J Am Coll Nutr. 2021;40:13-25.
- Abbott RD, Sadowski A, Alt AG. Efficacy of the autoimmune protocol diet as part of a multi-disciplinary, supported lifestyle intervention for Hashimoto's thyroiditis. Cureus. 2019;11:E4556.
- Lindeberg S, Jönsson T, Granfeldt Y, et al. A palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia. 2007;50:1795-1807.
- Jönsson T, Granfeldt Y, Ahrén B, et al. Beneficial effects of a paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35.
- Boers I, Muskiet FAJ, Berkelaar E, et al. Favourable effects of consuming a palaeolithic-type diet on characteristics of the metabolic syndrome: a randomized controlled pilot-study. Lipids Health Dis. 2014;13:160.
- Ghaedi E, Mohammadi M, Mohammadi H, et al. Effects of a paleolithic diet on cardiovascular disease risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10:634-646.
- Mellberg C, Sandberg S, Ryberg M, et al. Long-term effects of a palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68:350-357.
- Pastore RL, Brooks JT, Carbone JW. Paleolithic nutrition improves plasma lipid concentrations of hypercholesterolemic adults to a greater extent than traditional heart-healthy dietary recommendations. Nutr Res. 2015;35:474-479.
- Otten J, Stomby A, Waling M, et al. Benefits of a paleolithic diet with and without supervised exercise on fat mass, insulin sensitivity, and glycemic control: a randomized controlled trial in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2017;33:E2828.
- Stefanadi EC, Dimitrakakis G, Antoniou C-K, et al. Metabolic syndrome and the skin: a more than superficial association. reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol Metab Syndr. 2018;10:9.
- Mann N. Meat in the human diet: an anthropological perspective. Nutr Dietetics. 2007;64(suppl 4):S102-S107.
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345-352.
- Kuhn JE. Throwing, the shoulder, and human evolution. Am J Orthop (Belle Mead NJ). 2016;45:110-114.
- Kobayashi H, Kohshima S. Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J Hum Evol. 2001;40:419-435.
- Cordain L, Eaton SB, Miller JB, et al. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002;56(suppl 1):S42-S52.
- McClellan WS, Du Bois EF. Clinical calorimetry: XLV. prolonged meat diets with a study of kidney function and ketosis. J Biol Chem. 1930;87:651-668.
- O'Hearn A. Can a carnivore diet provide all essential nutrients? Curr Opin Endocrinol Diabetes Obes. 2020;27:312-316.
- O'Hearn LA. A survey of improvements experienced on a carnivore diet compared to only carbohydrate restriction. Open Science Forum website. Published February 12, 2019. Accessed May 17, 2021. doi:10.17605/OSF.IO/5FU4D
- Williams P. Nutritional composition of red meat. Nutrition & Dietetics. 2007;64(suppl 4):S113-S119.
- Biel W, Czerniawska-Piątkowska E, Kowalczyk A. Offal chemical composition from veal, beef, and lamb maintained in organic production systems. Animals (Basel). 2019;9:489.
- Elmadfa I, Meyer AL. The role of the status of selected micronutrients in shaping the immune function. Endocr Metab Immune Disord Drug Targets. 2019;19:1100-1115.
- Babizhayev M. Treatment of skin aging and photoaging with innovative oral dosage forms of nonhydrolized carnosine and carcinine. Int J Clin Derm Res. 2017;5:116-143.
- Danby FW. Nutrition and aging skin: sugar and glycation. Clin Dermatol. 2010;28:409-411.
- Siefken W, Carstensen S, Springmann G, et al. Role of taurine accumulation in keratinocyte hydration. J Invest Dermatol. 2003;121:354-361.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19:3059.
- Fischer F, Achterberg V, März A, et al. Folic acid and creatineimprove the firmness of human skin in vivo. J Cosmet Dermatol. 2011;10:15-23.
- Blatt T, Lenz H, Weber T. Topical application of creatine is multibeneficial for human skin. J Am Acad Dermatol. 2005;52:P32.
Practice Points
- Patients are increasingly interested in dietary modifications that may influence skin appearance and aid in the treatment of cutaneous disease.
- Although evidence-based dietary recommendations currently are limited, it is important for dermatologists to be aware of the varied and nuanced dietary interventions employed by patients.
- There remains a lack of randomized controlled trials assessing the efficacy of various dietary interventions in the dermatologic setting.
E/M Coding in 2021: The Times (and More) Are A-Changin’
Effective on January 1, 2021, the outpatient evaluation and management (E/M) codes underwent substantial changes, which were the culmination of multiple years of revision and surveying via the American Medical Association (AMA) Relative Value Scale Update Committee and Current Procedural Terminology (RUC-CPT) process to streamline definitions and promote consistency as well as to decrease the administrative burden for all specialties within the house of medicine.1 These updates represent a notable change from the previous documentation requirements for this oft used family of codes. Herein, we break down some of the highlights of the changes and how they may be applied for some commonly used dermatologic diagnoses.
Time Is Time Is Time
Prior to the 2021 revisions, a physician generally could only code for an E/M level by time for a face-to-face encounter dominated by counseling and/or care coordination. With the new updates, any encounter can be coded by total time spent by the physician with the patient1; however, clinical staff time is not included. There also are now clear guidelines of the time ranges corresponding to the level of E/M,1 as noted in Table 1.
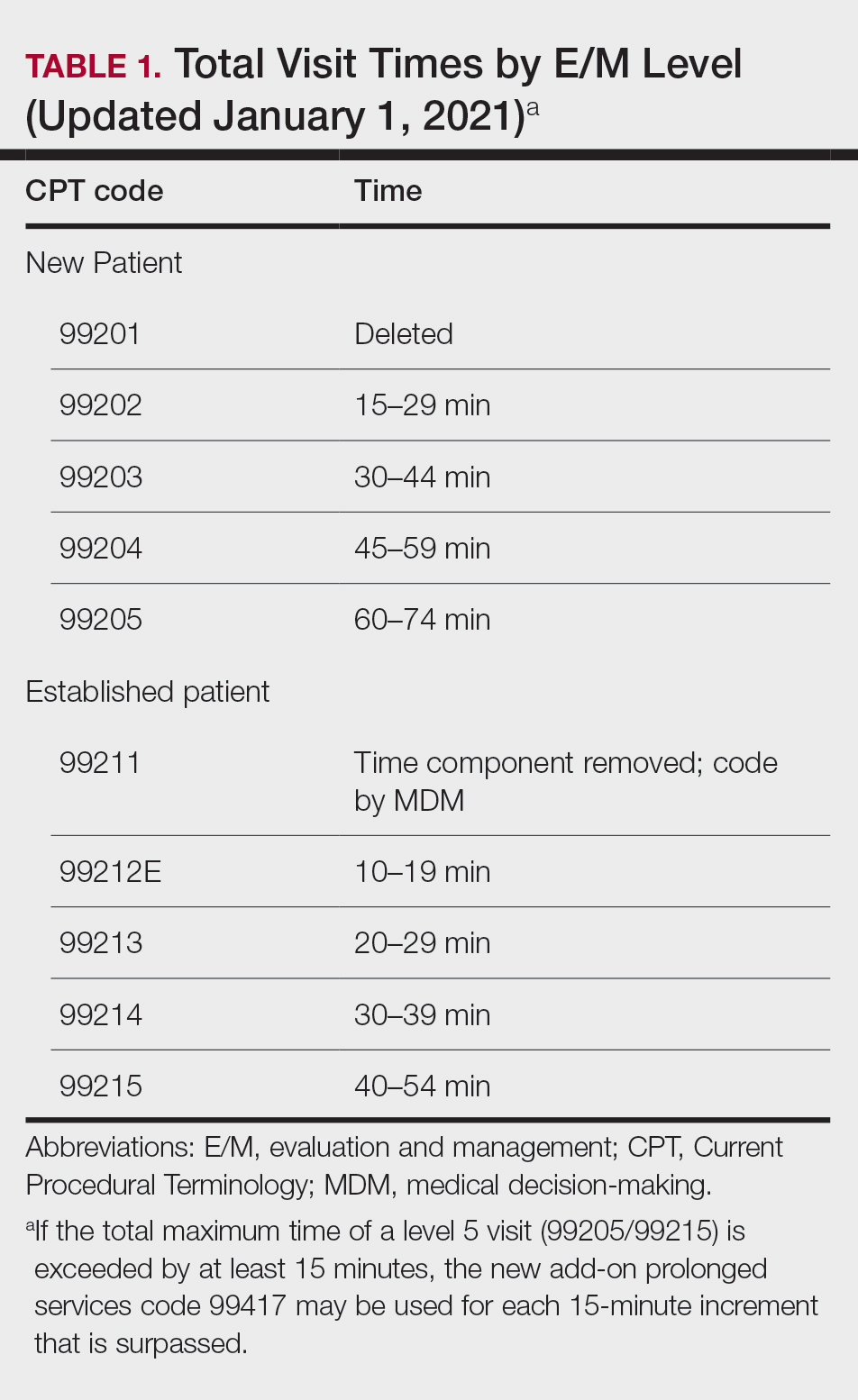
Importantly, time now includes not just face-to-face time with the patient but also any time on the date of the encounter that the physician is involved in the care of the patient when not reported with a separate code. This can include reviewing notes or data before or after the examination, care coordination, ordering laboratory tests, and providing any documentation related to the encounter. Importantly, this applies only when these activities are done on the date of the encounter.
If you work with a nurse practitioner or physician assistant (PA) who assists you and you are the one reporting the service, you cannot double-dip. For example, if your PA spends 10 minutes alone with a patient, you are in the room together for 5 minutes, the PA spends another 10 minutes alone with the patient afterward, and you do chart work for 10 minutes at the end of the day, the total time spent is 35 minutes, not 40 minutes, as you cannot count the time you and the PA spent together twice.
Decisions, Decisions
Evaluation and management coding also can be determined via the level of medical decision-making (MDM). Per the 2021 guidelines, MDM is comprised of 3 categories: (1) number and complexity of problems addressed at the encounter, (2) amount and/or complexity of data to be reviewed or analyzed, and (3) risk of complications and/or morbidity or mortality of patient management.1 To reach a certain overall E/M level, 2 of 3 categories must be met or exceeded. Let’s dive into each of these in a little more detail.
Number and Complexity of Problems Addressed at the Encounter
First, it is important to understand the definition of a problem addressed. Per AMA guidelines, this includes a disease, condition, illness, injury, symptom, sign, finding, complaint, or other matter addressed at the encounter that is evaluated or treated at the encounter by the physician. If the problem is referred to another provider without evaluation or consideration of treatment, it is not considered to be a problem addressed and cannot count toward this first category. An example could be a patient with a lump on the abdomen that you refer to plastic or general surgery for evaluation and treatment.
Once you have determined that you are addressing a problem, you will need to determine the level of complexity of the problem, as outlined in Table 2. Keep in mind that some entities and disease states in dermatology may fit the requirements of more than 1 level of complexity depending on the clinical situation, while there are many entities in dermatology that may not be perfectly captured by any of the levels described. In these situations, clinical judgement is required to determine where the problem would best fit. Importantly, whatever you decide, your documentation should support that decision.
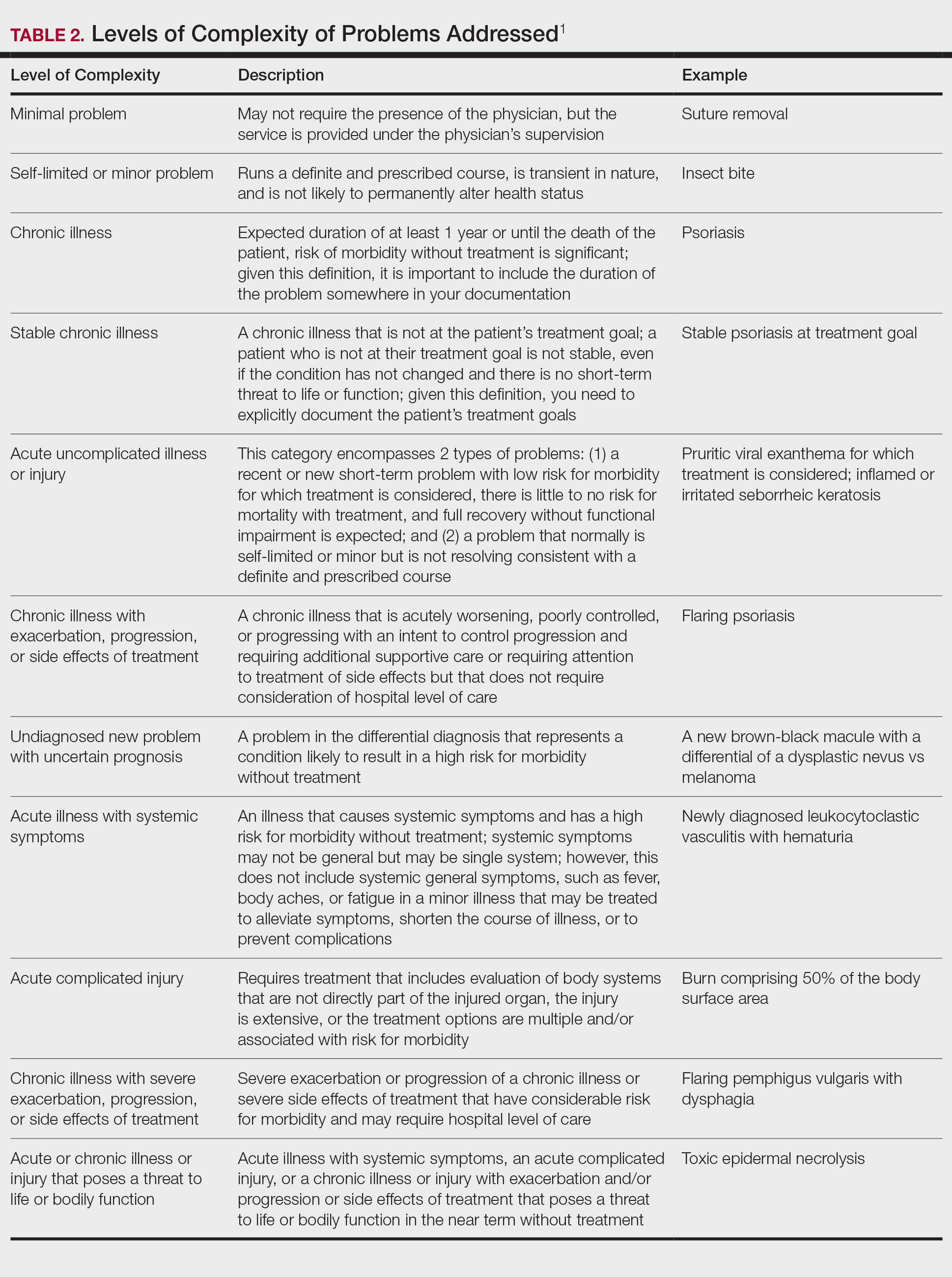
Amount and/or Complexity of Data to Be Reviewed and Analyzed
This category encompasses any external notes reviewed, unique laboratory tests or imaging ordered or reviewed, the need for an independent historian or discussion with external health care providers or appropriate sources, or independent interpretation of tests. Some high-yield definitions in this category are outlined in Table 3.
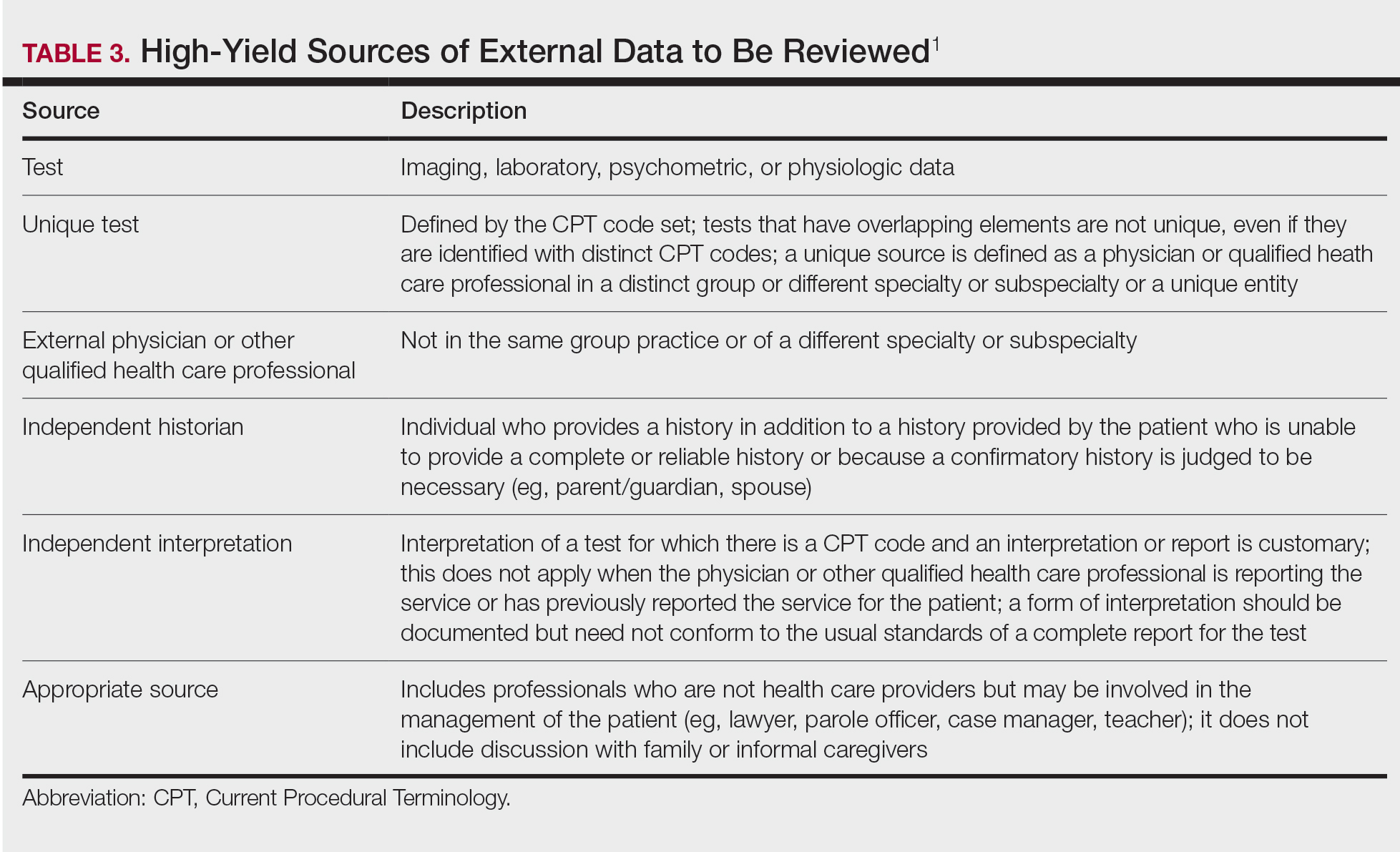
Risk of Complications and/or Morbidity or Mortality of Patient Management
In this category, risk relates to both the patient’s diagnosis and treatment(s). Importantly, for treatment and diagnostic options, these include both the options selected and those considered but not selected. Risk is defined as the probability and/or consequences of an event and is based on the usual behavior and thought processes of a physician in the same specialty. In other words, think of the risk as compared to risk in the setting of other dermatologists diagnosing and/or treating the same condition.
Social determinants of health also play a part in this category and are defined as economic and social conditions that influence the health of individuals and communities. Social determinants of health can be indicated by the specific corresponding International Statistical Classification of Diseases, Tenth Revision code and may need to be included in your billing according to specific institutional or carrier guidelines if they are a factor in your level of MDM.
For the purposes of MDM, risk is stratified into minimal, low, moderate, and high. Some examples for each level are outlined in Table 4.

Putting It All Together
Once you have determined each of the above 3 categories, you can put them together into the MDM chart to ascertain the overall level of MDM. (The official AMA medical decision-making grid is available online [https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf]). Keep in mind that 2 of 3 columns in the table must be obtained in that level to reach an overall E/M level; for example, a visit that addresses 2 self-limited or minor problems (level 3) in which no data is reviewed (level 2) and involves prescribing a new medication (level 4), would be an overall level 3 visit.
Final Thoughts
The outpatient E/M guidelines have undergone substantial revisions; therefore, it is crucial to understand the updated definitions to ensure proper billing and documentation. History and physical examination documentation must be medically appropriate but are no longer used to determine overall E/M level; time and MDM are the sole options that can be used. Importantly, try to code as accurately as possible, documenting which problems were both noted and addressed. If you are unsure of a definition within the updated changes and MDM table, referencing the appropriate sources for guidance is recommended.
Although representing a considerable shift, the revaluation of this family of codes and the intended decrease in documentation burden has the ability to be a positive gain for dermatologists. Expect other code families to mirror these changes in the next few years.
- American Medical Association. CPT® Evaluation and management (E/M) office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Accessed May 14, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
Effective on January 1, 2021, the outpatient evaluation and management (E/M) codes underwent substantial changes, which were the culmination of multiple years of revision and surveying via the American Medical Association (AMA) Relative Value Scale Update Committee and Current Procedural Terminology (RUC-CPT) process to streamline definitions and promote consistency as well as to decrease the administrative burden for all specialties within the house of medicine.1 These updates represent a notable change from the previous documentation requirements for this oft used family of codes. Herein, we break down some of the highlights of the changes and how they may be applied for some commonly used dermatologic diagnoses.
Time Is Time Is Time
Prior to the 2021 revisions, a physician generally could only code for an E/M level by time for a face-to-face encounter dominated by counseling and/or care coordination. With the new updates, any encounter can be coded by total time spent by the physician with the patient1; however, clinical staff time is not included. There also are now clear guidelines of the time ranges corresponding to the level of E/M,1 as noted in Table 1.

Importantly, time now includes not just face-to-face time with the patient but also any time on the date of the encounter that the physician is involved in the care of the patient when not reported with a separate code. This can include reviewing notes or data before or after the examination, care coordination, ordering laboratory tests, and providing any documentation related to the encounter. Importantly, this applies only when these activities are done on the date of the encounter.
If you work with a nurse practitioner or physician assistant (PA) who assists you and you are the one reporting the service, you cannot double-dip. For example, if your PA spends 10 minutes alone with a patient, you are in the room together for 5 minutes, the PA spends another 10 minutes alone with the patient afterward, and you do chart work for 10 minutes at the end of the day, the total time spent is 35 minutes, not 40 minutes, as you cannot count the time you and the PA spent together twice.
Decisions, Decisions
Evaluation and management coding also can be determined via the level of medical decision-making (MDM). Per the 2021 guidelines, MDM is comprised of 3 categories: (1) number and complexity of problems addressed at the encounter, (2) amount and/or complexity of data to be reviewed or analyzed, and (3) risk of complications and/or morbidity or mortality of patient management.1 To reach a certain overall E/M level, 2 of 3 categories must be met or exceeded. Let’s dive into each of these in a little more detail.
Number and Complexity of Problems Addressed at the Encounter
First, it is important to understand the definition of a problem addressed. Per AMA guidelines, this includes a disease, condition, illness, injury, symptom, sign, finding, complaint, or other matter addressed at the encounter that is evaluated or treated at the encounter by the physician. If the problem is referred to another provider without evaluation or consideration of treatment, it is not considered to be a problem addressed and cannot count toward this first category. An example could be a patient with a lump on the abdomen that you refer to plastic or general surgery for evaluation and treatment.
Once you have determined that you are addressing a problem, you will need to determine the level of complexity of the problem, as outlined in Table 2. Keep in mind that some entities and disease states in dermatology may fit the requirements of more than 1 level of complexity depending on the clinical situation, while there are many entities in dermatology that may not be perfectly captured by any of the levels described. In these situations, clinical judgement is required to determine where the problem would best fit. Importantly, whatever you decide, your documentation should support that decision.

Amount and/or Complexity of Data to Be Reviewed and Analyzed
This category encompasses any external notes reviewed, unique laboratory tests or imaging ordered or reviewed, the need for an independent historian or discussion with external health care providers or appropriate sources, or independent interpretation of tests. Some high-yield definitions in this category are outlined in Table 3.

Risk of Complications and/or Morbidity or Mortality of Patient Management
In this category, risk relates to both the patient’s diagnosis and treatment(s). Importantly, for treatment and diagnostic options, these include both the options selected and those considered but not selected. Risk is defined as the probability and/or consequences of an event and is based on the usual behavior and thought processes of a physician in the same specialty. In other words, think of the risk as compared to risk in the setting of other dermatologists diagnosing and/or treating the same condition.
Social determinants of health also play a part in this category and are defined as economic and social conditions that influence the health of individuals and communities. Social determinants of health can be indicated by the specific corresponding International Statistical Classification of Diseases, Tenth Revision code and may need to be included in your billing according to specific institutional or carrier guidelines if they are a factor in your level of MDM.
For the purposes of MDM, risk is stratified into minimal, low, moderate, and high. Some examples for each level are outlined in Table 4.

Putting It All Together
Once you have determined each of the above 3 categories, you can put them together into the MDM chart to ascertain the overall level of MDM. (The official AMA medical decision-making grid is available online [https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf]). Keep in mind that 2 of 3 columns in the table must be obtained in that level to reach an overall E/M level; for example, a visit that addresses 2 self-limited or minor problems (level 3) in which no data is reviewed (level 2) and involves prescribing a new medication (level 4), would be an overall level 3 visit.
Final Thoughts
The outpatient E/M guidelines have undergone substantial revisions; therefore, it is crucial to understand the updated definitions to ensure proper billing and documentation. History and physical examination documentation must be medically appropriate but are no longer used to determine overall E/M level; time and MDM are the sole options that can be used. Importantly, try to code as accurately as possible, documenting which problems were both noted and addressed. If you are unsure of a definition within the updated changes and MDM table, referencing the appropriate sources for guidance is recommended.
Although representing a considerable shift, the revaluation of this family of codes and the intended decrease in documentation burden has the ability to be a positive gain for dermatologists. Expect other code families to mirror these changes in the next few years.
Effective on January 1, 2021, the outpatient evaluation and management (E/M) codes underwent substantial changes, which were the culmination of multiple years of revision and surveying via the American Medical Association (AMA) Relative Value Scale Update Committee and Current Procedural Terminology (RUC-CPT) process to streamline definitions and promote consistency as well as to decrease the administrative burden for all specialties within the house of medicine.1 These updates represent a notable change from the previous documentation requirements for this oft used family of codes. Herein, we break down some of the highlights of the changes and how they may be applied for some commonly used dermatologic diagnoses.
Time Is Time Is Time
Prior to the 2021 revisions, a physician generally could only code for an E/M level by time for a face-to-face encounter dominated by counseling and/or care coordination. With the new updates, any encounter can be coded by total time spent by the physician with the patient1; however, clinical staff time is not included. There also are now clear guidelines of the time ranges corresponding to the level of E/M,1 as noted in Table 1.

Importantly, time now includes not just face-to-face time with the patient but also any time on the date of the encounter that the physician is involved in the care of the patient when not reported with a separate code. This can include reviewing notes or data before or after the examination, care coordination, ordering laboratory tests, and providing any documentation related to the encounter. Importantly, this applies only when these activities are done on the date of the encounter.
If you work with a nurse practitioner or physician assistant (PA) who assists you and you are the one reporting the service, you cannot double-dip. For example, if your PA spends 10 minutes alone with a patient, you are in the room together for 5 minutes, the PA spends another 10 minutes alone with the patient afterward, and you do chart work for 10 minutes at the end of the day, the total time spent is 35 minutes, not 40 minutes, as you cannot count the time you and the PA spent together twice.
Decisions, Decisions
Evaluation and management coding also can be determined via the level of medical decision-making (MDM). Per the 2021 guidelines, MDM is comprised of 3 categories: (1) number and complexity of problems addressed at the encounter, (2) amount and/or complexity of data to be reviewed or analyzed, and (3) risk of complications and/or morbidity or mortality of patient management.1 To reach a certain overall E/M level, 2 of 3 categories must be met or exceeded. Let’s dive into each of these in a little more detail.
Number and Complexity of Problems Addressed at the Encounter
First, it is important to understand the definition of a problem addressed. Per AMA guidelines, this includes a disease, condition, illness, injury, symptom, sign, finding, complaint, or other matter addressed at the encounter that is evaluated or treated at the encounter by the physician. If the problem is referred to another provider without evaluation or consideration of treatment, it is not considered to be a problem addressed and cannot count toward this first category. An example could be a patient with a lump on the abdomen that you refer to plastic or general surgery for evaluation and treatment.
Once you have determined that you are addressing a problem, you will need to determine the level of complexity of the problem, as outlined in Table 2. Keep in mind that some entities and disease states in dermatology may fit the requirements of more than 1 level of complexity depending on the clinical situation, while there are many entities in dermatology that may not be perfectly captured by any of the levels described. In these situations, clinical judgement is required to determine where the problem would best fit. Importantly, whatever you decide, your documentation should support that decision.

Amount and/or Complexity of Data to Be Reviewed and Analyzed
This category encompasses any external notes reviewed, unique laboratory tests or imaging ordered or reviewed, the need for an independent historian or discussion with external health care providers or appropriate sources, or independent interpretation of tests. Some high-yield definitions in this category are outlined in Table 3.

Risk of Complications and/or Morbidity or Mortality of Patient Management
In this category, risk relates to both the patient’s diagnosis and treatment(s). Importantly, for treatment and diagnostic options, these include both the options selected and those considered but not selected. Risk is defined as the probability and/or consequences of an event and is based on the usual behavior and thought processes of a physician in the same specialty. In other words, think of the risk as compared to risk in the setting of other dermatologists diagnosing and/or treating the same condition.
Social determinants of health also play a part in this category and are defined as economic and social conditions that influence the health of individuals and communities. Social determinants of health can be indicated by the specific corresponding International Statistical Classification of Diseases, Tenth Revision code and may need to be included in your billing according to specific institutional or carrier guidelines if they are a factor in your level of MDM.
For the purposes of MDM, risk is stratified into minimal, low, moderate, and high. Some examples for each level are outlined in Table 4.

Putting It All Together
Once you have determined each of the above 3 categories, you can put them together into the MDM chart to ascertain the overall level of MDM. (The official AMA medical decision-making grid is available online [https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf]). Keep in mind that 2 of 3 columns in the table must be obtained in that level to reach an overall E/M level; for example, a visit that addresses 2 self-limited or minor problems (level 3) in which no data is reviewed (level 2) and involves prescribing a new medication (level 4), would be an overall level 3 visit.
Final Thoughts
The outpatient E/M guidelines have undergone substantial revisions; therefore, it is crucial to understand the updated definitions to ensure proper billing and documentation. History and physical examination documentation must be medically appropriate but are no longer used to determine overall E/M level; time and MDM are the sole options that can be used. Importantly, try to code as accurately as possible, documenting which problems were both noted and addressed. If you are unsure of a definition within the updated changes and MDM table, referencing the appropriate sources for guidance is recommended.
Although representing a considerable shift, the revaluation of this family of codes and the intended decrease in documentation burden has the ability to be a positive gain for dermatologists. Expect other code families to mirror these changes in the next few years.
- American Medical Association. CPT® Evaluation and management (E/M) office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Accessed May 14, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
- American Medical Association. CPT® Evaluation and management (E/M) office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Accessed May 14, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
Practice Points
- The outpatient evaluation and management (E/M) codes have undergone substantial changes that took effect January 1, 2021.
- Outpatient E/M visits are now coded based on time or level of medical decision-making (MDM).
- Time now includes all preservice, intraservice, and postservice time the physician spends with the patient on the date of the encounter.
- Many of the key definitions used in order to determine level of MDM have been streamlined and updated.
MDS: Elevated mature monocytes in bone marrow can supplement IPSS-R as a prognostic indicator
Key clinical point: Increased percentage of mature monocyte in bone marrow (PMMBM) may assist the Revised International Prognostic Scoring System (IPSS-R) to predict poor prognosis in patients with myelodysplastic syndromes (MDS).
Major finding: Elevated (>6%) vs. normal PMMBM was associated with shorter overall survival (24 months vs. 37 months; P = .026) along with higher risk distribution in terms of IPSS-R (P = .025) and higher frequency of IDH2 mutation (P = .007).
Study details: The data come from a retrospective analysis of 216 MDS patients, categorized into elevated and normal PMMBM groups.
Disclosures: The study was supported by the Zhejiang Provincial Natural Science Foundation of China, Medical and Health Science and Technology Projects of Zhejiang Province, National Science Foundation of Ningbo, and Chinese Medicine Science and Technology Plan Project of Zhejiang Province. The authors declared no conflicts of interest.
Source: Wu A et al. BMC Cancer. 2021 May 13. doi: 10.1186/s12885-021-08303-8.
Key clinical point: Increased percentage of mature monocyte in bone marrow (PMMBM) may assist the Revised International Prognostic Scoring System (IPSS-R) to predict poor prognosis in patients with myelodysplastic syndromes (MDS).
Major finding: Elevated (>6%) vs. normal PMMBM was associated with shorter overall survival (24 months vs. 37 months; P = .026) along with higher risk distribution in terms of IPSS-R (P = .025) and higher frequency of IDH2 mutation (P = .007).
Study details: The data come from a retrospective analysis of 216 MDS patients, categorized into elevated and normal PMMBM groups.
Disclosures: The study was supported by the Zhejiang Provincial Natural Science Foundation of China, Medical and Health Science and Technology Projects of Zhejiang Province, National Science Foundation of Ningbo, and Chinese Medicine Science and Technology Plan Project of Zhejiang Province. The authors declared no conflicts of interest.
Source: Wu A et al. BMC Cancer. 2021 May 13. doi: 10.1186/s12885-021-08303-8.
Key clinical point: Increased percentage of mature monocyte in bone marrow (PMMBM) may assist the Revised International Prognostic Scoring System (IPSS-R) to predict poor prognosis in patients with myelodysplastic syndromes (MDS).
Major finding: Elevated (>6%) vs. normal PMMBM was associated with shorter overall survival (24 months vs. 37 months; P = .026) along with higher risk distribution in terms of IPSS-R (P = .025) and higher frequency of IDH2 mutation (P = .007).
Study details: The data come from a retrospective analysis of 216 MDS patients, categorized into elevated and normal PMMBM groups.
Disclosures: The study was supported by the Zhejiang Provincial Natural Science Foundation of China, Medical and Health Science and Technology Projects of Zhejiang Province, National Science Foundation of Ningbo, and Chinese Medicine Science and Technology Plan Project of Zhejiang Province. The authors declared no conflicts of interest.
Source: Wu A et al. BMC Cancer. 2021 May 13. doi: 10.1186/s12885-021-08303-8.
MDS: Antibiotics can be stopped after 3 days in patients with febrile neutropenia after chemotherapy
Key clinical point: During remission induction chemotherapy in patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), antibiotics can be safely stopped after 3 days of febrile neutropenia in the absence of infection.
Major finding: Serious medical complication (SMC) was seen in 12.5% of patients receiving the 3-day empirical broad-spectrum antibiotic therapy (EBAT) vs. 8.9% of patients receiving the prolonged regimen (P = .17). After adjustment for confounders, there was no significant difference between both strategies in the number of SMCs (hazard ratio, 1.357; P = .297).
Study details: AML or MDS patients who received chemotherapy were treated with either 3-day EBAT or a prolonged antibiotic regimen (until neutrophil recovery).
Disclosures: The study did not receive any specific funding. A Schauwvlieghe, J Maertens, and T Mercier reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Schauwvlieghe A et al. EClinicalMedicine. 2021 Apr 25. doi: 10.1016/j.eclinm.2021.100855.
Key clinical point: During remission induction chemotherapy in patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), antibiotics can be safely stopped after 3 days of febrile neutropenia in the absence of infection.
Major finding: Serious medical complication (SMC) was seen in 12.5% of patients receiving the 3-day empirical broad-spectrum antibiotic therapy (EBAT) vs. 8.9% of patients receiving the prolonged regimen (P = .17). After adjustment for confounders, there was no significant difference between both strategies in the number of SMCs (hazard ratio, 1.357; P = .297).
Study details: AML or MDS patients who received chemotherapy were treated with either 3-day EBAT or a prolonged antibiotic regimen (until neutrophil recovery).
Disclosures: The study did not receive any specific funding. A Schauwvlieghe, J Maertens, and T Mercier reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Schauwvlieghe A et al. EClinicalMedicine. 2021 Apr 25. doi: 10.1016/j.eclinm.2021.100855.
Key clinical point: During remission induction chemotherapy in patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), antibiotics can be safely stopped after 3 days of febrile neutropenia in the absence of infection.
Major finding: Serious medical complication (SMC) was seen in 12.5% of patients receiving the 3-day empirical broad-spectrum antibiotic therapy (EBAT) vs. 8.9% of patients receiving the prolonged regimen (P = .17). After adjustment for confounders, there was no significant difference between both strategies in the number of SMCs (hazard ratio, 1.357; P = .297).
Study details: AML or MDS patients who received chemotherapy were treated with either 3-day EBAT or a prolonged antibiotic regimen (until neutrophil recovery).
Disclosures: The study did not receive any specific funding. A Schauwvlieghe, J Maertens, and T Mercier reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Schauwvlieghe A et al. EClinicalMedicine. 2021 Apr 25. doi: 10.1016/j.eclinm.2021.100855.
De novo MDS: Pretransplant RBC and platelet transfusion burden tied to poor survival outcomes
Key clinical point: Higher pretransplant red blood cell (RBC) and platelet transfusion burden has an independent association with higher overall and relapse-related mortality following allogeneic hematopoietic stem cell transplantation (allo-HSCT) for de novo myelodysplastic syndrome (MDS).
Major finding: A higher pretransplant RBC transfusion burden was significantly associated with lower overall survival (OS; P less than .001) and higher relapse-related mortality (P less than .001). Similarly, a higher pretransplant platelet transfusion burden was associated with lower OS (P less than .001) and higher relapse-related mortality (P = .001).
Study details: A retrospective study examined the effects of pretransplant RBC and platelet transfusion burden on outcomes after allo-HSCT in 1,007 adults with de novo MDS.
Disclosures: This study was supported in part by the Practical Research Project for Allergic Diseases and Immunology (Research Technology of Medical Transplantation) from the Japan Agency for Medical Research and Development. The authors declared no conflicts of interest.
Source: Konuma T et al. Transplant Cell Ther. 2021 May 12. doi: 10.1016/j.jtct.2021.05.003.
Key clinical point: Higher pretransplant red blood cell (RBC) and platelet transfusion burden has an independent association with higher overall and relapse-related mortality following allogeneic hematopoietic stem cell transplantation (allo-HSCT) for de novo myelodysplastic syndrome (MDS).
Major finding: A higher pretransplant RBC transfusion burden was significantly associated with lower overall survival (OS; P less than .001) and higher relapse-related mortality (P less than .001). Similarly, a higher pretransplant platelet transfusion burden was associated with lower OS (P less than .001) and higher relapse-related mortality (P = .001).
Study details: A retrospective study examined the effects of pretransplant RBC and platelet transfusion burden on outcomes after allo-HSCT in 1,007 adults with de novo MDS.
Disclosures: This study was supported in part by the Practical Research Project for Allergic Diseases and Immunology (Research Technology of Medical Transplantation) from the Japan Agency for Medical Research and Development. The authors declared no conflicts of interest.
Source: Konuma T et al. Transplant Cell Ther. 2021 May 12. doi: 10.1016/j.jtct.2021.05.003.
Key clinical point: Higher pretransplant red blood cell (RBC) and platelet transfusion burden has an independent association with higher overall and relapse-related mortality following allogeneic hematopoietic stem cell transplantation (allo-HSCT) for de novo myelodysplastic syndrome (MDS).
Major finding: A higher pretransplant RBC transfusion burden was significantly associated with lower overall survival (OS; P less than .001) and higher relapse-related mortality (P less than .001). Similarly, a higher pretransplant platelet transfusion burden was associated with lower OS (P less than .001) and higher relapse-related mortality (P = .001).
Study details: A retrospective study examined the effects of pretransplant RBC and platelet transfusion burden on outcomes after allo-HSCT in 1,007 adults with de novo MDS.
Disclosures: This study was supported in part by the Practical Research Project for Allergic Diseases and Immunology (Research Technology of Medical Transplantation) from the Japan Agency for Medical Research and Development. The authors declared no conflicts of interest.
Source: Konuma T et al. Transplant Cell Ther. 2021 May 12. doi: 10.1016/j.jtct.2021.05.003.
Less restrictive enrollment criteria warranted for MDS clinical trials
Key clinical point: Less restrictive inclusion and exclusion criteria are warranted to improve the participation of patients with myelodysplastic syndrome (MDS) in clinical trials.
Major finding: Each trial was suitable for ~18% of patients in the cohort, whereas 34% of the patients were eligible for at least 1 of the 9 trials. Pharma-initiated trials excluded more than twice the fraction of patients vs. investigator-initiated trials (inclusion, 10% vs. 21%). Key reasons for exclusion included karyotype (average exclusion rate, 58%), comorbidities (40%), and previous therapies (55%)
Study details: A simulation exercise was performed to estimate the average proportion of MDS patients eligible for participation in a clinical trial. A total of 1,809 patients were included in the cohort.
Disclosures: This study did not receive any funding. K Nachtkamp, T Schroeder, E Schuler, J Kaivers, A Giagounidis, C Rautenberg, N Gattermann, and U Germing reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Nachtkamp K et al. Leuk Res. 2021 May 11. doi: 10.1016/j.leukres.2021.106611.
Key clinical point: Less restrictive inclusion and exclusion criteria are warranted to improve the participation of patients with myelodysplastic syndrome (MDS) in clinical trials.
Major finding: Each trial was suitable for ~18% of patients in the cohort, whereas 34% of the patients were eligible for at least 1 of the 9 trials. Pharma-initiated trials excluded more than twice the fraction of patients vs. investigator-initiated trials (inclusion, 10% vs. 21%). Key reasons for exclusion included karyotype (average exclusion rate, 58%), comorbidities (40%), and previous therapies (55%)
Study details: A simulation exercise was performed to estimate the average proportion of MDS patients eligible for participation in a clinical trial. A total of 1,809 patients were included in the cohort.
Disclosures: This study did not receive any funding. K Nachtkamp, T Schroeder, E Schuler, J Kaivers, A Giagounidis, C Rautenberg, N Gattermann, and U Germing reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Nachtkamp K et al. Leuk Res. 2021 May 11. doi: 10.1016/j.leukres.2021.106611.
Key clinical point: Less restrictive inclusion and exclusion criteria are warranted to improve the participation of patients with myelodysplastic syndrome (MDS) in clinical trials.
Major finding: Each trial was suitable for ~18% of patients in the cohort, whereas 34% of the patients were eligible for at least 1 of the 9 trials. Pharma-initiated trials excluded more than twice the fraction of patients vs. investigator-initiated trials (inclusion, 10% vs. 21%). Key reasons for exclusion included karyotype (average exclusion rate, 58%), comorbidities (40%), and previous therapies (55%)
Study details: A simulation exercise was performed to estimate the average proportion of MDS patients eligible for participation in a clinical trial. A total of 1,809 patients were included in the cohort.
Disclosures: This study did not receive any funding. K Nachtkamp, T Schroeder, E Schuler, J Kaivers, A Giagounidis, C Rautenberg, N Gattermann, and U Germing reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Nachtkamp K et al. Leuk Res. 2021 May 11. doi: 10.1016/j.leukres.2021.106611.
T-cell inhibition by PD-L1-expressing stem cells may play a role in MDS development
Key clinical point: Inhibition of T cells by programmed death-ligand 1 (PD-L1)-expressing hematopoietic stem cells could be an underlying mechanism in the development of myelodysplastic syndrome (MDS). The findings support the potential use of immune checkpoint inhibitors in the treatment of suitable MDS patients.
Major finding: Significantly increased proportions of PD-L1+CD34+ stem cells were seen in MDS patients compared with hematopoietic stem cell transplantation (HSCT) recipients in remission for both the CD38− subset (P = .0127) and CD38+ subset (P = .0336).
Study details: The study included 7 MDS and 9 acute myeloid leukemia samples. Six HSCT recipients who remained in remission for more than 6 months were considered controls.
Disclosures: The study was supported by the Düsseldorf School of Oncology (funded by the Comprehensive Cancer Center Düsseldorf/Deutsche Krebshilfe and the Medical Faculty HHU Düsseldorf). The authors declared no conflicts of interest.
Source: Moskorz W et al. Br J Haematol. 2021 May 6. doi: 10.1111/bjh.17461.
Key clinical point: Inhibition of T cells by programmed death-ligand 1 (PD-L1)-expressing hematopoietic stem cells could be an underlying mechanism in the development of myelodysplastic syndrome (MDS). The findings support the potential use of immune checkpoint inhibitors in the treatment of suitable MDS patients.
Major finding: Significantly increased proportions of PD-L1+CD34+ stem cells were seen in MDS patients compared with hematopoietic stem cell transplantation (HSCT) recipients in remission for both the CD38− subset (P = .0127) and CD38+ subset (P = .0336).
Study details: The study included 7 MDS and 9 acute myeloid leukemia samples. Six HSCT recipients who remained in remission for more than 6 months were considered controls.
Disclosures: The study was supported by the Düsseldorf School of Oncology (funded by the Comprehensive Cancer Center Düsseldorf/Deutsche Krebshilfe and the Medical Faculty HHU Düsseldorf). The authors declared no conflicts of interest.
Source: Moskorz W et al. Br J Haematol. 2021 May 6. doi: 10.1111/bjh.17461.
Key clinical point: Inhibition of T cells by programmed death-ligand 1 (PD-L1)-expressing hematopoietic stem cells could be an underlying mechanism in the development of myelodysplastic syndrome (MDS). The findings support the potential use of immune checkpoint inhibitors in the treatment of suitable MDS patients.
Major finding: Significantly increased proportions of PD-L1+CD34+ stem cells were seen in MDS patients compared with hematopoietic stem cell transplantation (HSCT) recipients in remission for both the CD38− subset (P = .0127) and CD38+ subset (P = .0336).
Study details: The study included 7 MDS and 9 acute myeloid leukemia samples. Six HSCT recipients who remained in remission for more than 6 months were considered controls.
Disclosures: The study was supported by the Düsseldorf School of Oncology (funded by the Comprehensive Cancer Center Düsseldorf/Deutsche Krebshilfe and the Medical Faculty HHU Düsseldorf). The authors declared no conflicts of interest.
Source: Moskorz W et al. Br J Haematol. 2021 May 6. doi: 10.1111/bjh.17461.

