User login
Increased cumulative exposure to melphalan in multiple myeloma patients increases MDS risk
Key clinical point: Increased cumulative exposure to the alkylating agent melphalan increases the subsequent risk for developing acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) in patients with multiple myeloma (MM).
Major finding: Cumulative exposure to melphalan was significantly higher (odds ratio, 2.8; P less than .001) among patients with MM and AML/MDS (median, 988 mg) than control participants (median, 578 mg). The median time to development of AML/MDS was 3.8 years.
Study details: The study included 26,627 patients diagnosed with MM between 1985 and 2011, of which 124 (0.5%) patients developed subsequent AML/MDS. Each patient with MM and AML/MDS diagnosis was matched with a control MM patient without AML/MDS.
Disclosures: The study was supported by grants from the Asrun Einarsdottir Foundation in Iceland, University of Iceland Research Fund, Icelandic Centre for Research, Landspitali University Hospital Research Fund, Thorman’s foundation, and Sylvester Comprehensive Cancer Center NCI Core Grant. O Landgren and M Björkholm reported ties with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Jonsdottir G et al. Eur J Haematol. 2021 May 9. doi: 10.1111/ejh.13650.
Key clinical point: Increased cumulative exposure to the alkylating agent melphalan increases the subsequent risk for developing acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) in patients with multiple myeloma (MM).
Major finding: Cumulative exposure to melphalan was significantly higher (odds ratio, 2.8; P less than .001) among patients with MM and AML/MDS (median, 988 mg) than control participants (median, 578 mg). The median time to development of AML/MDS was 3.8 years.
Study details: The study included 26,627 patients diagnosed with MM between 1985 and 2011, of which 124 (0.5%) patients developed subsequent AML/MDS. Each patient with MM and AML/MDS diagnosis was matched with a control MM patient without AML/MDS.
Disclosures: The study was supported by grants from the Asrun Einarsdottir Foundation in Iceland, University of Iceland Research Fund, Icelandic Centre for Research, Landspitali University Hospital Research Fund, Thorman’s foundation, and Sylvester Comprehensive Cancer Center NCI Core Grant. O Landgren and M Björkholm reported ties with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Jonsdottir G et al. Eur J Haematol. 2021 May 9. doi: 10.1111/ejh.13650.
Key clinical point: Increased cumulative exposure to the alkylating agent melphalan increases the subsequent risk for developing acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) in patients with multiple myeloma (MM).
Major finding: Cumulative exposure to melphalan was significantly higher (odds ratio, 2.8; P less than .001) among patients with MM and AML/MDS (median, 988 mg) than control participants (median, 578 mg). The median time to development of AML/MDS was 3.8 years.
Study details: The study included 26,627 patients diagnosed with MM between 1985 and 2011, of which 124 (0.5%) patients developed subsequent AML/MDS. Each patient with MM and AML/MDS diagnosis was matched with a control MM patient without AML/MDS.
Disclosures: The study was supported by grants from the Asrun Einarsdottir Foundation in Iceland, University of Iceland Research Fund, Icelandic Centre for Research, Landspitali University Hospital Research Fund, Thorman’s foundation, and Sylvester Comprehensive Cancer Center NCI Core Grant. O Landgren and M Björkholm reported ties with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Jonsdottir G et al. Eur J Haematol. 2021 May 9. doi: 10.1111/ejh.13650.
COVID-19 Vaccine Reactions in Dermatology: “Filling” in the Gaps
As we marked the 1-year anniversary of the COVID-19 pandemic, nearly 100 million Americans had received their first dose of the COVID-19 vaccine, heralding some sense of relief and enabling us to envision a return to something resembling life before lockdown.1 Amid these breakthroughs and vaccination campaigns forging ahead worldwide, we saw new questions and problems arise. Vaccine hesitancy was already an issue in many segments of society where misinformation and mistrust of the medical establishment have served as barriers to the progress of public health. Once reports of adverse reactions following COVID-19 vaccination—such as those linked to use of facial fillers—made news headlines, many in the dermatology community began facing inquiries from patients questioning if they should wait to receive the vaccine or skip it entirely. As dermatologists, we must be informed and prepared to address these situations, to manage adverse reactions when they arise, and to encourage and promote vaccination during this critical time for public health in our society.
Cutaneous Vaccine Reactions and Facial Fillers
As public COVID-19 vaccinations move forward, dermatologic side effects, which were first noted during clinical trials, have received amplified attention, despite the fact that these cutaneous reactions—including localized injection-site redness and swelling, generalized urticarial and morbilliform eruptions, and even facial filler reactions—have been reported as relatively minor and self-limited.2 The excipient polyethylene glycol has been suspected as a possible etiology of vaccine-related allergic and hypersensitivity reactions, suggesting care be taken in those who are patch-test positive or have a history of allergy to polyethylene glycol–containing products (eg, penicillin, laxatives, makeup, certain dermal fillers).2,3 Although rare, facial and lip swelling reactions in those with a prior history of facial fillers in COVID-19 vaccine trials have drawn particular public concern and potential vaccine hesitancy given that more than 2.7 million Americans seek treatment with dermal fillers annually. There has been continued demand for these treatments during the pandemic, particularly due to aesthetic sensitivity surrounding video conferencing.4
Release of trial data from the Moderna COVID-19 vaccine prompted a discourse around safety and recommended protocols for filler procedures in the community of aesthetic medicine, as 3 participants in the experimental arm—all of whom had a history of treatment with facial filler injections—were reported to have facial or lip swelling shortly following vaccination. Two of these cases were considered to be serious adverse events due to extensive facial swelling, with the participants having received filler injections 6 months and 2 weeks prior to vaccination, respectively.5 A third participant experienced lip swelling only, which according to the US Food and Drug Administration briefing document was considered “medically significant” but not a serious adverse event, with unknown timing of the most recent filler injection. In all cases, symptom onset began 1 or 2 days following vaccination, and all resolved with either no or minimal intervention.6 The US Food and Drug Administration briefing document does not detail which type of fillers each participant had received, but subsequent reports indicated hyaluronic acid (HA) fillers. Of note, one patient in the placebo arm of the trial also developed progressive periorbital and facial edema in the setting of known filler injections performed 5 weeks prior, requiring treatment with corticosteroids and barring her from receiving a second injection in the trial.7
After public vaccination started, additional reports have emerged of facial edema occurring following administration of both the Pfizer and Moderna COVID-19 vaccines.2,8,9 In one series, 4 cases of facial swelling were reported in patients who had HA filler placed more than 1 year prior to vaccination.9 The first patient, who had a history of HA fillers in the temples and cheeks, developed moderate periorbital swelling 2 days following her second dose of the Pfizer vaccine. Another patient who had received a series of filler injections over the last 3 years experienced facial swelling 24 hours after her second dose of the Moderna vaccine and also reported a similar reaction in the past following an upper respiratory tract infection. The third patient developed perioral and infraorbital edema 18 hours after her first dose of the Moderna vaccine. The fourth patient developed inflammation in filler-treated areas 10 days after the first dose of the Pfizer vaccine and notably had a history of filler reaction to an unknown trigger in 2019 that was treated with hyaluronidase, intralesional steroids, and 5-fluorouracil. All cases of facial edema reportedly resolved.9
The observed adverse events have been proposed as delayed-type hypersensitivity reactions (DTRs) to facial fillers and are suspected to be triggered by the COVID-19 spike protein and subsequent immunogenic response. This reaction is not unique to the COVID-19 vaccines; in fact, many inflammatory stimuli such as sinus infections, flulike illnesses, facial injury, dental procedures, and exposure to certain medications and chemotherapeutics have triggered DTRs in filler patients, especially in those with genetic or immunologic risk factors including certain human leukocyte antigen subtypes or autoimmune disorders.3
Counseling Patients and Reducing Risks
As reports of DTRs to facial fillers after COVID-19 vaccination continue to emerge, it is not surprising that patients may become confused by potential side effects and postpone vaccination as a result. This evolving situation has called upon aesthetic physicians to adapt our practice and prepare our patients. Most importantly, we must continue to follow the data and integrate evidence-based COVID-19 vaccine–related counseling into our office visits. It is paramount to encourage vaccination and inform patients that these rare adverse events are both temporary and treatable. Given the currently available data, patients with a history of treatment with dermal fillers should not be discouraged from receiving the vaccine; however, we may provide suggestions to lessen the likelihood of adverse reactions and ease patient concerns. For example, it may be helpful to consider a time frame between vaccination and filler procedures that is longer than 2 weeks, just as would be advised for those having dental procedures or with recent infections, and potentially longer windows for those with risk factors such as prior sensitivity to dermal fillers, autoimmune disorders, or those on immunomodulatory medications. Dilution of fillers with saline or lidocaine or use of non-HA fillers also may be suggested around the time of vaccination to mitigate the risk of DTRs.3
Managing Vaccine Reactions
If facial swelling does occur despite these precautions and lasts longer than 48 hours, treatment with antihistamines, steroids, and/or hyaluronidase has been successful in vaccine trial and posttrial patients, both alone or in combination, and are likely to resolve edema promptly without altering the effectiveness of the vaccine.3,5,9 Angiotensin-converting enzyme inhibitors such as lisinopril more recently have been recommended for treatment of facial edema following COVID-19 vaccination,9 but questions remain regarding the true efficacy in this scenario given that the majority of swelling reactions resolve without this treatment. Additionally, there were no controls to indicate treatment with the angiotensin-converting enzyme inhibitor demonstrated an actual impact. Dermatologists generally are wary of adding medications of questionable utility that are associated with potential side effects and drug reactions, given that we often are tasked with managing the consequences of such mistakes. Thus, to avoid additional harm in the setting of insufficient evidence, as was seen following widespread use of hydroxychloroquine at the outset of the COVID-19 pandemic, well-structured studies are required before such interventions can be recommended.
If symptoms arise following the first vaccine injection, they can be managed if needed while patients are reassured and advised to obtain their second dose, with pretreatment considerations including antihistamines and instruction to present to the emergency department if a more severe reaction is suspected.2 In a larger sense, we also can contribute to the collective knowledge, growth, and preparedness of the medical community by reporting cases of adverse events to vaccine reporting systems and registries, such as the US Department of Health and Human Services’ Vaccine Adverse Event Reporting System, the Centers for Disease Control and Prevention’s V-Safe After Vaccination Health Checker, and the American Academy of Dermatology’s COVID-19 Dermatology Registry.
Final Thoughts
As dermatologists, we now find ourselves in the familiar role of balancing the aesthetic goals of our patients with our primary mission of public health and safety at a time when their health and well-being is particularly vulnerable. Adverse reactions will continue to occur as larger segments of the world’s population become vaccinated. Meanwhile, we must continue to manage symptoms, dispel myths, emphasize that any dermatologic risk posed by the COVID-19 vaccines is far outweighed by the benefits of immunization, and promote health and education, looking ahead to life beyond the pandemic.
- Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus (COVID-19) vaccinations. Our World in Data website. Accessed May 10, 2021. https://ourworldindata.org/covid-vaccinations
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases [published online April 7, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.092
- Rice SM, Ferree SD, Mesinkovska NA, et al. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. Int J Womens Dermatol. 2021;7:209-212. doi:10.1016/j.ijwd.2021.01.007
- Rice SM, Siegel JA, Libby T, et al. Zooming into cosmetic procedures during the COVID-19 pandemic: the provider’s perspective. Int J Womens Dermatol. 2021;7:213-216.
- FDA Briefing Document: Moderna COVID-19 Vaccine. US Department of Health and Human Services; 2020. Accessed May 11, 2021. https://www.fda.gov/media/144434/download
- Moderna’s COVID-19 vaccine may cause swelling, inflammation in those with facial fillers. American Society of Plastic Surgeons website. Published December 27, 2020. Accessed May 11, 2021. http://www.plasticsurgery.org/for-medical-professionals/publications/psn-extra/news/modernas-covid19-vaccine-may-cause-swelling-inflammation-in-those-with-facial-fillers
- Munavalli GG, Guthridge R, Knutsen-Larson S, et al. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment [published online February 9, 2021]. Arch Dermatol Res. doi:10.1007/s00403-021-02190-6
- Schlessinger J. Update on COVID-19 vaccines and dermal fillers. Practical Dermatol. February 2021:46-47. Accessed May 10, 2021. https://practicaldermatology.com/articles/2021-feb/update-on-covid-19-vaccines-and-dermal-fillers/pdf
- Munavalli GG, Knutsen-Larson S, Lupo MP, et al. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination—a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021;10:63-68. doi:10.1016/j.jdcr.2021.02.018
As we marked the 1-year anniversary of the COVID-19 pandemic, nearly 100 million Americans had received their first dose of the COVID-19 vaccine, heralding some sense of relief and enabling us to envision a return to something resembling life before lockdown.1 Amid these breakthroughs and vaccination campaigns forging ahead worldwide, we saw new questions and problems arise. Vaccine hesitancy was already an issue in many segments of society where misinformation and mistrust of the medical establishment have served as barriers to the progress of public health. Once reports of adverse reactions following COVID-19 vaccination—such as those linked to use of facial fillers—made news headlines, many in the dermatology community began facing inquiries from patients questioning if they should wait to receive the vaccine or skip it entirely. As dermatologists, we must be informed and prepared to address these situations, to manage adverse reactions when they arise, and to encourage and promote vaccination during this critical time for public health in our society.
Cutaneous Vaccine Reactions and Facial Fillers
As public COVID-19 vaccinations move forward, dermatologic side effects, which were first noted during clinical trials, have received amplified attention, despite the fact that these cutaneous reactions—including localized injection-site redness and swelling, generalized urticarial and morbilliform eruptions, and even facial filler reactions—have been reported as relatively minor and self-limited.2 The excipient polyethylene glycol has been suspected as a possible etiology of vaccine-related allergic and hypersensitivity reactions, suggesting care be taken in those who are patch-test positive or have a history of allergy to polyethylene glycol–containing products (eg, penicillin, laxatives, makeup, certain dermal fillers).2,3 Although rare, facial and lip swelling reactions in those with a prior history of facial fillers in COVID-19 vaccine trials have drawn particular public concern and potential vaccine hesitancy given that more than 2.7 million Americans seek treatment with dermal fillers annually. There has been continued demand for these treatments during the pandemic, particularly due to aesthetic sensitivity surrounding video conferencing.4
Release of trial data from the Moderna COVID-19 vaccine prompted a discourse around safety and recommended protocols for filler procedures in the community of aesthetic medicine, as 3 participants in the experimental arm—all of whom had a history of treatment with facial filler injections—were reported to have facial or lip swelling shortly following vaccination. Two of these cases were considered to be serious adverse events due to extensive facial swelling, with the participants having received filler injections 6 months and 2 weeks prior to vaccination, respectively.5 A third participant experienced lip swelling only, which according to the US Food and Drug Administration briefing document was considered “medically significant” but not a serious adverse event, with unknown timing of the most recent filler injection. In all cases, symptom onset began 1 or 2 days following vaccination, and all resolved with either no or minimal intervention.6 The US Food and Drug Administration briefing document does not detail which type of fillers each participant had received, but subsequent reports indicated hyaluronic acid (HA) fillers. Of note, one patient in the placebo arm of the trial also developed progressive periorbital and facial edema in the setting of known filler injections performed 5 weeks prior, requiring treatment with corticosteroids and barring her from receiving a second injection in the trial.7
After public vaccination started, additional reports have emerged of facial edema occurring following administration of both the Pfizer and Moderna COVID-19 vaccines.2,8,9 In one series, 4 cases of facial swelling were reported in patients who had HA filler placed more than 1 year prior to vaccination.9 The first patient, who had a history of HA fillers in the temples and cheeks, developed moderate periorbital swelling 2 days following her second dose of the Pfizer vaccine. Another patient who had received a series of filler injections over the last 3 years experienced facial swelling 24 hours after her second dose of the Moderna vaccine and also reported a similar reaction in the past following an upper respiratory tract infection. The third patient developed perioral and infraorbital edema 18 hours after her first dose of the Moderna vaccine. The fourth patient developed inflammation in filler-treated areas 10 days after the first dose of the Pfizer vaccine and notably had a history of filler reaction to an unknown trigger in 2019 that was treated with hyaluronidase, intralesional steroids, and 5-fluorouracil. All cases of facial edema reportedly resolved.9
The observed adverse events have been proposed as delayed-type hypersensitivity reactions (DTRs) to facial fillers and are suspected to be triggered by the COVID-19 spike protein and subsequent immunogenic response. This reaction is not unique to the COVID-19 vaccines; in fact, many inflammatory stimuli such as sinus infections, flulike illnesses, facial injury, dental procedures, and exposure to certain medications and chemotherapeutics have triggered DTRs in filler patients, especially in those with genetic or immunologic risk factors including certain human leukocyte antigen subtypes or autoimmune disorders.3
Counseling Patients and Reducing Risks
As reports of DTRs to facial fillers after COVID-19 vaccination continue to emerge, it is not surprising that patients may become confused by potential side effects and postpone vaccination as a result. This evolving situation has called upon aesthetic physicians to adapt our practice and prepare our patients. Most importantly, we must continue to follow the data and integrate evidence-based COVID-19 vaccine–related counseling into our office visits. It is paramount to encourage vaccination and inform patients that these rare adverse events are both temporary and treatable. Given the currently available data, patients with a history of treatment with dermal fillers should not be discouraged from receiving the vaccine; however, we may provide suggestions to lessen the likelihood of adverse reactions and ease patient concerns. For example, it may be helpful to consider a time frame between vaccination and filler procedures that is longer than 2 weeks, just as would be advised for those having dental procedures or with recent infections, and potentially longer windows for those with risk factors such as prior sensitivity to dermal fillers, autoimmune disorders, or those on immunomodulatory medications. Dilution of fillers with saline or lidocaine or use of non-HA fillers also may be suggested around the time of vaccination to mitigate the risk of DTRs.3
Managing Vaccine Reactions
If facial swelling does occur despite these precautions and lasts longer than 48 hours, treatment with antihistamines, steroids, and/or hyaluronidase has been successful in vaccine trial and posttrial patients, both alone or in combination, and are likely to resolve edema promptly without altering the effectiveness of the vaccine.3,5,9 Angiotensin-converting enzyme inhibitors such as lisinopril more recently have been recommended for treatment of facial edema following COVID-19 vaccination,9 but questions remain regarding the true efficacy in this scenario given that the majority of swelling reactions resolve without this treatment. Additionally, there were no controls to indicate treatment with the angiotensin-converting enzyme inhibitor demonstrated an actual impact. Dermatologists generally are wary of adding medications of questionable utility that are associated with potential side effects and drug reactions, given that we often are tasked with managing the consequences of such mistakes. Thus, to avoid additional harm in the setting of insufficient evidence, as was seen following widespread use of hydroxychloroquine at the outset of the COVID-19 pandemic, well-structured studies are required before such interventions can be recommended.
If symptoms arise following the first vaccine injection, they can be managed if needed while patients are reassured and advised to obtain their second dose, with pretreatment considerations including antihistamines and instruction to present to the emergency department if a more severe reaction is suspected.2 In a larger sense, we also can contribute to the collective knowledge, growth, and preparedness of the medical community by reporting cases of adverse events to vaccine reporting systems and registries, such as the US Department of Health and Human Services’ Vaccine Adverse Event Reporting System, the Centers for Disease Control and Prevention’s V-Safe After Vaccination Health Checker, and the American Academy of Dermatology’s COVID-19 Dermatology Registry.
Final Thoughts
As dermatologists, we now find ourselves in the familiar role of balancing the aesthetic goals of our patients with our primary mission of public health and safety at a time when their health and well-being is particularly vulnerable. Adverse reactions will continue to occur as larger segments of the world’s population become vaccinated. Meanwhile, we must continue to manage symptoms, dispel myths, emphasize that any dermatologic risk posed by the COVID-19 vaccines is far outweighed by the benefits of immunization, and promote health and education, looking ahead to life beyond the pandemic.
As we marked the 1-year anniversary of the COVID-19 pandemic, nearly 100 million Americans had received their first dose of the COVID-19 vaccine, heralding some sense of relief and enabling us to envision a return to something resembling life before lockdown.1 Amid these breakthroughs and vaccination campaigns forging ahead worldwide, we saw new questions and problems arise. Vaccine hesitancy was already an issue in many segments of society where misinformation and mistrust of the medical establishment have served as barriers to the progress of public health. Once reports of adverse reactions following COVID-19 vaccination—such as those linked to use of facial fillers—made news headlines, many in the dermatology community began facing inquiries from patients questioning if they should wait to receive the vaccine or skip it entirely. As dermatologists, we must be informed and prepared to address these situations, to manage adverse reactions when they arise, and to encourage and promote vaccination during this critical time for public health in our society.
Cutaneous Vaccine Reactions and Facial Fillers
As public COVID-19 vaccinations move forward, dermatologic side effects, which were first noted during clinical trials, have received amplified attention, despite the fact that these cutaneous reactions—including localized injection-site redness and swelling, generalized urticarial and morbilliform eruptions, and even facial filler reactions—have been reported as relatively minor and self-limited.2 The excipient polyethylene glycol has been suspected as a possible etiology of vaccine-related allergic and hypersensitivity reactions, suggesting care be taken in those who are patch-test positive or have a history of allergy to polyethylene glycol–containing products (eg, penicillin, laxatives, makeup, certain dermal fillers).2,3 Although rare, facial and lip swelling reactions in those with a prior history of facial fillers in COVID-19 vaccine trials have drawn particular public concern and potential vaccine hesitancy given that more than 2.7 million Americans seek treatment with dermal fillers annually. There has been continued demand for these treatments during the pandemic, particularly due to aesthetic sensitivity surrounding video conferencing.4
Release of trial data from the Moderna COVID-19 vaccine prompted a discourse around safety and recommended protocols for filler procedures in the community of aesthetic medicine, as 3 participants in the experimental arm—all of whom had a history of treatment with facial filler injections—were reported to have facial or lip swelling shortly following vaccination. Two of these cases were considered to be serious adverse events due to extensive facial swelling, with the participants having received filler injections 6 months and 2 weeks prior to vaccination, respectively.5 A third participant experienced lip swelling only, which according to the US Food and Drug Administration briefing document was considered “medically significant” but not a serious adverse event, with unknown timing of the most recent filler injection. In all cases, symptom onset began 1 or 2 days following vaccination, and all resolved with either no or minimal intervention.6 The US Food and Drug Administration briefing document does not detail which type of fillers each participant had received, but subsequent reports indicated hyaluronic acid (HA) fillers. Of note, one patient in the placebo arm of the trial also developed progressive periorbital and facial edema in the setting of known filler injections performed 5 weeks prior, requiring treatment with corticosteroids and barring her from receiving a second injection in the trial.7
After public vaccination started, additional reports have emerged of facial edema occurring following administration of both the Pfizer and Moderna COVID-19 vaccines.2,8,9 In one series, 4 cases of facial swelling were reported in patients who had HA filler placed more than 1 year prior to vaccination.9 The first patient, who had a history of HA fillers in the temples and cheeks, developed moderate periorbital swelling 2 days following her second dose of the Pfizer vaccine. Another patient who had received a series of filler injections over the last 3 years experienced facial swelling 24 hours after her second dose of the Moderna vaccine and also reported a similar reaction in the past following an upper respiratory tract infection. The third patient developed perioral and infraorbital edema 18 hours after her first dose of the Moderna vaccine. The fourth patient developed inflammation in filler-treated areas 10 days after the first dose of the Pfizer vaccine and notably had a history of filler reaction to an unknown trigger in 2019 that was treated with hyaluronidase, intralesional steroids, and 5-fluorouracil. All cases of facial edema reportedly resolved.9
The observed adverse events have been proposed as delayed-type hypersensitivity reactions (DTRs) to facial fillers and are suspected to be triggered by the COVID-19 spike protein and subsequent immunogenic response. This reaction is not unique to the COVID-19 vaccines; in fact, many inflammatory stimuli such as sinus infections, flulike illnesses, facial injury, dental procedures, and exposure to certain medications and chemotherapeutics have triggered DTRs in filler patients, especially in those with genetic or immunologic risk factors including certain human leukocyte antigen subtypes or autoimmune disorders.3
Counseling Patients and Reducing Risks
As reports of DTRs to facial fillers after COVID-19 vaccination continue to emerge, it is not surprising that patients may become confused by potential side effects and postpone vaccination as a result. This evolving situation has called upon aesthetic physicians to adapt our practice and prepare our patients. Most importantly, we must continue to follow the data and integrate evidence-based COVID-19 vaccine–related counseling into our office visits. It is paramount to encourage vaccination and inform patients that these rare adverse events are both temporary and treatable. Given the currently available data, patients with a history of treatment with dermal fillers should not be discouraged from receiving the vaccine; however, we may provide suggestions to lessen the likelihood of adverse reactions and ease patient concerns. For example, it may be helpful to consider a time frame between vaccination and filler procedures that is longer than 2 weeks, just as would be advised for those having dental procedures or with recent infections, and potentially longer windows for those with risk factors such as prior sensitivity to dermal fillers, autoimmune disorders, or those on immunomodulatory medications. Dilution of fillers with saline or lidocaine or use of non-HA fillers also may be suggested around the time of vaccination to mitigate the risk of DTRs.3
Managing Vaccine Reactions
If facial swelling does occur despite these precautions and lasts longer than 48 hours, treatment with antihistamines, steroids, and/or hyaluronidase has been successful in vaccine trial and posttrial patients, both alone or in combination, and are likely to resolve edema promptly without altering the effectiveness of the vaccine.3,5,9 Angiotensin-converting enzyme inhibitors such as lisinopril more recently have been recommended for treatment of facial edema following COVID-19 vaccination,9 but questions remain regarding the true efficacy in this scenario given that the majority of swelling reactions resolve without this treatment. Additionally, there were no controls to indicate treatment with the angiotensin-converting enzyme inhibitor demonstrated an actual impact. Dermatologists generally are wary of adding medications of questionable utility that are associated with potential side effects and drug reactions, given that we often are tasked with managing the consequences of such mistakes. Thus, to avoid additional harm in the setting of insufficient evidence, as was seen following widespread use of hydroxychloroquine at the outset of the COVID-19 pandemic, well-structured studies are required before such interventions can be recommended.
If symptoms arise following the first vaccine injection, they can be managed if needed while patients are reassured and advised to obtain their second dose, with pretreatment considerations including antihistamines and instruction to present to the emergency department if a more severe reaction is suspected.2 In a larger sense, we also can contribute to the collective knowledge, growth, and preparedness of the medical community by reporting cases of adverse events to vaccine reporting systems and registries, such as the US Department of Health and Human Services’ Vaccine Adverse Event Reporting System, the Centers for Disease Control and Prevention’s V-Safe After Vaccination Health Checker, and the American Academy of Dermatology’s COVID-19 Dermatology Registry.
Final Thoughts
As dermatologists, we now find ourselves in the familiar role of balancing the aesthetic goals of our patients with our primary mission of public health and safety at a time when their health and well-being is particularly vulnerable. Adverse reactions will continue to occur as larger segments of the world’s population become vaccinated. Meanwhile, we must continue to manage symptoms, dispel myths, emphasize that any dermatologic risk posed by the COVID-19 vaccines is far outweighed by the benefits of immunization, and promote health and education, looking ahead to life beyond the pandemic.
- Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus (COVID-19) vaccinations. Our World in Data website. Accessed May 10, 2021. https://ourworldindata.org/covid-vaccinations
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases [published online April 7, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.092
- Rice SM, Ferree SD, Mesinkovska NA, et al. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. Int J Womens Dermatol. 2021;7:209-212. doi:10.1016/j.ijwd.2021.01.007
- Rice SM, Siegel JA, Libby T, et al. Zooming into cosmetic procedures during the COVID-19 pandemic: the provider’s perspective. Int J Womens Dermatol. 2021;7:213-216.
- FDA Briefing Document: Moderna COVID-19 Vaccine. US Department of Health and Human Services; 2020. Accessed May 11, 2021. https://www.fda.gov/media/144434/download
- Moderna’s COVID-19 vaccine may cause swelling, inflammation in those with facial fillers. American Society of Plastic Surgeons website. Published December 27, 2020. Accessed May 11, 2021. http://www.plasticsurgery.org/for-medical-professionals/publications/psn-extra/news/modernas-covid19-vaccine-may-cause-swelling-inflammation-in-those-with-facial-fillers
- Munavalli GG, Guthridge R, Knutsen-Larson S, et al. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment [published online February 9, 2021]. Arch Dermatol Res. doi:10.1007/s00403-021-02190-6
- Schlessinger J. Update on COVID-19 vaccines and dermal fillers. Practical Dermatol. February 2021:46-47. Accessed May 10, 2021. https://practicaldermatology.com/articles/2021-feb/update-on-covid-19-vaccines-and-dermal-fillers/pdf
- Munavalli GG, Knutsen-Larson S, Lupo MP, et al. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination—a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021;10:63-68. doi:10.1016/j.jdcr.2021.02.018
- Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus (COVID-19) vaccinations. Our World in Data website. Accessed May 10, 2021. https://ourworldindata.org/covid-vaccinations
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases [published online April 7, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.092
- Rice SM, Ferree SD, Mesinkovska NA, et al. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. Int J Womens Dermatol. 2021;7:209-212. doi:10.1016/j.ijwd.2021.01.007
- Rice SM, Siegel JA, Libby T, et al. Zooming into cosmetic procedures during the COVID-19 pandemic: the provider’s perspective. Int J Womens Dermatol. 2021;7:213-216.
- FDA Briefing Document: Moderna COVID-19 Vaccine. US Department of Health and Human Services; 2020. Accessed May 11, 2021. https://www.fda.gov/media/144434/download
- Moderna’s COVID-19 vaccine may cause swelling, inflammation in those with facial fillers. American Society of Plastic Surgeons website. Published December 27, 2020. Accessed May 11, 2021. http://www.plasticsurgery.org/for-medical-professionals/publications/psn-extra/news/modernas-covid19-vaccine-may-cause-swelling-inflammation-in-those-with-facial-fillers
- Munavalli GG, Guthridge R, Knutsen-Larson S, et al. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment [published online February 9, 2021]. Arch Dermatol Res. doi:10.1007/s00403-021-02190-6
- Schlessinger J. Update on COVID-19 vaccines and dermal fillers. Practical Dermatol. February 2021:46-47. Accessed May 10, 2021. https://practicaldermatology.com/articles/2021-feb/update-on-covid-19-vaccines-and-dermal-fillers/pdf
- Munavalli GG, Knutsen-Larson S, Lupo MP, et al. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination—a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021;10:63-68. doi:10.1016/j.jdcr.2021.02.018
Physician convicted in buprenorphine scheme faces up to 20 years in prison
A West Virginia physician faces up to 20 years in prison in the wake of his conviction by a federal jury for illegally distributing buprenorphine.
The jury convicted Sriramloo Kesari, MD, 78, of Charleston, for distributing buprenorphine outside the scope of medical practice, according to a U.S. Department of Justice statement.
Investigators from the Drug Enforcement Administration presented evidence at the trial that Dr. Kesari, a general practitioner, operated a cash-only business selling buprenorphine prescriptions.
Federal prosecutors said that the physician signed prescriptions, which were then distributed by an employee in exchange for cash. Dr. Kesari was often absent, at times physically located in California, according to the federal government.
Prosecutors indicted the West Virginia physician in September 2019 as part of an “opioid strikeforce takedown” in Ohio, Virginia, and West Virginia that resulted in charges against 13 individuals, including 11 physicians.
Dr. Kesari’s attorneys filed motions during the course of the lengthy case showing that psychiatric and neurological exams indicated that the physician was cognitively impaired.
Based on that evidence and the federal indictment, the West Virginia Board of Medicine suspended Dr. Kesari’s license in February 2020, stating that he is not “mentally and/or physically fit to practice medicine and surgery with reasonable skill and safety.”
Dr. Kesari was first licensed in West Virginia in 1979. In 1987, the Board of Medicine placed Dr. Kesari on a 3-year probation because of his failure to keep records for patients for whom he was prescribing controlled substances.
However, within a few months, the Board changed the probation order to allow Dr. Kesari to write prescriptions for schedule II and III substances in the Boone Hospital emergency room where he continued to work.
The physician had no other disciplinary actions until his license suspension, but the Board lists settlement of four malpractice cases and the dismissal of a fifth between 1986 and 2001.
A version of this article first appeared on Medscape.com.
A West Virginia physician faces up to 20 years in prison in the wake of his conviction by a federal jury for illegally distributing buprenorphine.
The jury convicted Sriramloo Kesari, MD, 78, of Charleston, for distributing buprenorphine outside the scope of medical practice, according to a U.S. Department of Justice statement.
Investigators from the Drug Enforcement Administration presented evidence at the trial that Dr. Kesari, a general practitioner, operated a cash-only business selling buprenorphine prescriptions.
Federal prosecutors said that the physician signed prescriptions, which were then distributed by an employee in exchange for cash. Dr. Kesari was often absent, at times physically located in California, according to the federal government.
Prosecutors indicted the West Virginia physician in September 2019 as part of an “opioid strikeforce takedown” in Ohio, Virginia, and West Virginia that resulted in charges against 13 individuals, including 11 physicians.
Dr. Kesari’s attorneys filed motions during the course of the lengthy case showing that psychiatric and neurological exams indicated that the physician was cognitively impaired.
Based on that evidence and the federal indictment, the West Virginia Board of Medicine suspended Dr. Kesari’s license in February 2020, stating that he is not “mentally and/or physically fit to practice medicine and surgery with reasonable skill and safety.”
Dr. Kesari was first licensed in West Virginia in 1979. In 1987, the Board of Medicine placed Dr. Kesari on a 3-year probation because of his failure to keep records for patients for whom he was prescribing controlled substances.
However, within a few months, the Board changed the probation order to allow Dr. Kesari to write prescriptions for schedule II and III substances in the Boone Hospital emergency room where he continued to work.
The physician had no other disciplinary actions until his license suspension, but the Board lists settlement of four malpractice cases and the dismissal of a fifth between 1986 and 2001.
A version of this article first appeared on Medscape.com.
A West Virginia physician faces up to 20 years in prison in the wake of his conviction by a federal jury for illegally distributing buprenorphine.
The jury convicted Sriramloo Kesari, MD, 78, of Charleston, for distributing buprenorphine outside the scope of medical practice, according to a U.S. Department of Justice statement.
Investigators from the Drug Enforcement Administration presented evidence at the trial that Dr. Kesari, a general practitioner, operated a cash-only business selling buprenorphine prescriptions.
Federal prosecutors said that the physician signed prescriptions, which were then distributed by an employee in exchange for cash. Dr. Kesari was often absent, at times physically located in California, according to the federal government.
Prosecutors indicted the West Virginia physician in September 2019 as part of an “opioid strikeforce takedown” in Ohio, Virginia, and West Virginia that resulted in charges against 13 individuals, including 11 physicians.
Dr. Kesari’s attorneys filed motions during the course of the lengthy case showing that psychiatric and neurological exams indicated that the physician was cognitively impaired.
Based on that evidence and the federal indictment, the West Virginia Board of Medicine suspended Dr. Kesari’s license in February 2020, stating that he is not “mentally and/or physically fit to practice medicine and surgery with reasonable skill and safety.”
Dr. Kesari was first licensed in West Virginia in 1979. In 1987, the Board of Medicine placed Dr. Kesari on a 3-year probation because of his failure to keep records for patients for whom he was prescribing controlled substances.
However, within a few months, the Board changed the probation order to allow Dr. Kesari to write prescriptions for schedule II and III substances in the Boone Hospital emergency room where he continued to work.
The physician had no other disciplinary actions until his license suspension, but the Board lists settlement of four malpractice cases and the dismissal of a fifth between 1986 and 2001.
A version of this article first appeared on Medscape.com.
MDS: Adolescents and young adults have a favorable survival with allo-HSCT
Key clinical point: Adolescent and young adult (AYA) patients with myelodysplastic syndrome (MDS) receiving allogeneic hematopoietic stem cell transplantation (allo-HSCT) generally exhibit better survival than their non-AYA counterparts.
Major finding: The 3-year overall survival (OS) in AYA patients was 71.2% (95% confidence interval, 67.4%-74.6%). Predictors of poor 3-year OS were active disease status (adjusted hazard ratio [aHR], 1.54; P = .016), poor cytogenetic risk (aHR, 1.62; P = .011), poor performance status (aHR, 2.01; P = .016), human leukocyte antigen (HLA)-matched unrelated donors (aHR, 2.23; P less than .001), HLA-mismatched unrelated donors (aHR, 2.16; P = .027), and cord blood transplantation (aHR, 2.44; P = 0.001).
Study details: The study included 645 AYA patients with MDS, aged 16-39 years, who received first allo-HSCT.
Disclosures: No funding source was identified. The authors declared no conflicts of interest.
Source: Shimomura Y et al. Bone Marrow Transplant. 2021 May 15. doi: 10.1038/s41409-021-01324-8.
Key clinical point: Adolescent and young adult (AYA) patients with myelodysplastic syndrome (MDS) receiving allogeneic hematopoietic stem cell transplantation (allo-HSCT) generally exhibit better survival than their non-AYA counterparts.
Major finding: The 3-year overall survival (OS) in AYA patients was 71.2% (95% confidence interval, 67.4%-74.6%). Predictors of poor 3-year OS were active disease status (adjusted hazard ratio [aHR], 1.54; P = .016), poor cytogenetic risk (aHR, 1.62; P = .011), poor performance status (aHR, 2.01; P = .016), human leukocyte antigen (HLA)-matched unrelated donors (aHR, 2.23; P less than .001), HLA-mismatched unrelated donors (aHR, 2.16; P = .027), and cord blood transplantation (aHR, 2.44; P = 0.001).
Study details: The study included 645 AYA patients with MDS, aged 16-39 years, who received first allo-HSCT.
Disclosures: No funding source was identified. The authors declared no conflicts of interest.
Source: Shimomura Y et al. Bone Marrow Transplant. 2021 May 15. doi: 10.1038/s41409-021-01324-8.
Key clinical point: Adolescent and young adult (AYA) patients with myelodysplastic syndrome (MDS) receiving allogeneic hematopoietic stem cell transplantation (allo-HSCT) generally exhibit better survival than their non-AYA counterparts.
Major finding: The 3-year overall survival (OS) in AYA patients was 71.2% (95% confidence interval, 67.4%-74.6%). Predictors of poor 3-year OS were active disease status (adjusted hazard ratio [aHR], 1.54; P = .016), poor cytogenetic risk (aHR, 1.62; P = .011), poor performance status (aHR, 2.01; P = .016), human leukocyte antigen (HLA)-matched unrelated donors (aHR, 2.23; P less than .001), HLA-mismatched unrelated donors (aHR, 2.16; P = .027), and cord blood transplantation (aHR, 2.44; P = 0.001).
Study details: The study included 645 AYA patients with MDS, aged 16-39 years, who received first allo-HSCT.
Disclosures: No funding source was identified. The authors declared no conflicts of interest.
Source: Shimomura Y et al. Bone Marrow Transplant. 2021 May 15. doi: 10.1038/s41409-021-01324-8.
MDS del5q: Could DNA methylation patterns predict response to lenalidomide?
Key clinical point: Lenalidomide had no relevant effect on DNA methylation status in patients with myelodysplastic syndrome with isolated deletion of chromosome 5q (MDS del5q).
Major finding: Lenalidomide treatment did not have a relevant impact on genome-wide DNA methylation in patients with MDS del5q. However, methylation analysis before treatment could identify a distinct subgroup of patients (27%) with a trend toward inferior overall survival but not inferior progression-free survival.
Study details: DNA methylation analysis was performed on 51 MDS del5q patients treated with lenalidomide. Direct effects of lenalidomide on DNA methylation were studied using 17 paired samples pre- and posttreatment.
Disclosures: Open access funding by Projekt DEAL. This study was supported by funds from the Deutsche Forschungsgemeinschaft, “Deutsche Krebshilfe,” Gutermuth Foundation, H.W. & J. Hector fund, Baden Wuerttemberg, and Dr Rolf M Schwiete Fund. Mannheim DN is an endowed Professor of the German José-Carreras-Stiftung. The study was partly funded by a research grant from Celgene Inc.
Source: Hecht A et al. Ann Hematol. 2021 April 27. doi: 10.1007/s00277-021-04492-1.
Key clinical point: Lenalidomide had no relevant effect on DNA methylation status in patients with myelodysplastic syndrome with isolated deletion of chromosome 5q (MDS del5q).
Major finding: Lenalidomide treatment did not have a relevant impact on genome-wide DNA methylation in patients with MDS del5q. However, methylation analysis before treatment could identify a distinct subgroup of patients (27%) with a trend toward inferior overall survival but not inferior progression-free survival.
Study details: DNA methylation analysis was performed on 51 MDS del5q patients treated with lenalidomide. Direct effects of lenalidomide on DNA methylation were studied using 17 paired samples pre- and posttreatment.
Disclosures: Open access funding by Projekt DEAL. This study was supported by funds from the Deutsche Forschungsgemeinschaft, “Deutsche Krebshilfe,” Gutermuth Foundation, H.W. & J. Hector fund, Baden Wuerttemberg, and Dr Rolf M Schwiete Fund. Mannheim DN is an endowed Professor of the German José-Carreras-Stiftung. The study was partly funded by a research grant from Celgene Inc.
Source: Hecht A et al. Ann Hematol. 2021 April 27. doi: 10.1007/s00277-021-04492-1.
Key clinical point: Lenalidomide had no relevant effect on DNA methylation status in patients with myelodysplastic syndrome with isolated deletion of chromosome 5q (MDS del5q).
Major finding: Lenalidomide treatment did not have a relevant impact on genome-wide DNA methylation in patients with MDS del5q. However, methylation analysis before treatment could identify a distinct subgroup of patients (27%) with a trend toward inferior overall survival but not inferior progression-free survival.
Study details: DNA methylation analysis was performed on 51 MDS del5q patients treated with lenalidomide. Direct effects of lenalidomide on DNA methylation were studied using 17 paired samples pre- and posttreatment.
Disclosures: Open access funding by Projekt DEAL. This study was supported by funds from the Deutsche Forschungsgemeinschaft, “Deutsche Krebshilfe,” Gutermuth Foundation, H.W. & J. Hector fund, Baden Wuerttemberg, and Dr Rolf M Schwiete Fund. Mannheim DN is an endowed Professor of the German José-Carreras-Stiftung. The study was partly funded by a research grant from Celgene Inc.
Source: Hecht A et al. Ann Hematol. 2021 April 27. doi: 10.1007/s00277-021-04492-1.
Upfront allo-HSCT preferable for MDS
Key clinical point: Pretransplant cytoreductive therapy is not associated with improved outcomes in patients with myelodysplastic syndromes (MDS) who have undergone allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Major finding: Five-year overall survival after diagnosis was 73.6% (95% confidence interval [CI], 70.3%-76.9%), 43.4% (95% CI, 39.2%-47.6%), and 46.9% (95% CI, 44.7%-49.1%) in the upfront transplantation (upfront), induction chemotherapy (CT), and hypomethylating agents alone (HMA) groups, respectively (P = .033). Treatment-related mortality was 13.0% (95% CI, 11.4%-15.1%), 32.4% (95% CI, 30.1%-35.3%), and 28.4% (95% CI, 26.2%-30.3%) in the 3 groups, respectively (P = .028).
Study details: A total of 157 MDS patients were categorized into 3 groups based on the pretransplantation therapy: upfront (n=54), CT (n=66), and HMA (n=37). In addition, 124 patients underwent allo-HSCT.
Disclosures: This study was supported by the National Natural Science Foundation of China, Research and Development Program in Key Areas of Guangdong Province, Science and Technology Program of Guangzhou, and Clinical Research Start-up Project of Southern Medical University. The authors declared no conflicts of interest.
Source: Chen Y et al. Int J Cancer. 2021 Apr 25. doi: 10.1002/ijc.33608.
Key clinical point: Pretransplant cytoreductive therapy is not associated with improved outcomes in patients with myelodysplastic syndromes (MDS) who have undergone allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Major finding: Five-year overall survival after diagnosis was 73.6% (95% confidence interval [CI], 70.3%-76.9%), 43.4% (95% CI, 39.2%-47.6%), and 46.9% (95% CI, 44.7%-49.1%) in the upfront transplantation (upfront), induction chemotherapy (CT), and hypomethylating agents alone (HMA) groups, respectively (P = .033). Treatment-related mortality was 13.0% (95% CI, 11.4%-15.1%), 32.4% (95% CI, 30.1%-35.3%), and 28.4% (95% CI, 26.2%-30.3%) in the 3 groups, respectively (P = .028).
Study details: A total of 157 MDS patients were categorized into 3 groups based on the pretransplantation therapy: upfront (n=54), CT (n=66), and HMA (n=37). In addition, 124 patients underwent allo-HSCT.
Disclosures: This study was supported by the National Natural Science Foundation of China, Research and Development Program in Key Areas of Guangdong Province, Science and Technology Program of Guangzhou, and Clinical Research Start-up Project of Southern Medical University. The authors declared no conflicts of interest.
Source: Chen Y et al. Int J Cancer. 2021 Apr 25. doi: 10.1002/ijc.33608.
Key clinical point: Pretransplant cytoreductive therapy is not associated with improved outcomes in patients with myelodysplastic syndromes (MDS) who have undergone allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Major finding: Five-year overall survival after diagnosis was 73.6% (95% confidence interval [CI], 70.3%-76.9%), 43.4% (95% CI, 39.2%-47.6%), and 46.9% (95% CI, 44.7%-49.1%) in the upfront transplantation (upfront), induction chemotherapy (CT), and hypomethylating agents alone (HMA) groups, respectively (P = .033). Treatment-related mortality was 13.0% (95% CI, 11.4%-15.1%), 32.4% (95% CI, 30.1%-35.3%), and 28.4% (95% CI, 26.2%-30.3%) in the 3 groups, respectively (P = .028).
Study details: A total of 157 MDS patients were categorized into 3 groups based on the pretransplantation therapy: upfront (n=54), CT (n=66), and HMA (n=37). In addition, 124 patients underwent allo-HSCT.
Disclosures: This study was supported by the National Natural Science Foundation of China, Research and Development Program in Key Areas of Guangdong Province, Science and Technology Program of Guangzhou, and Clinical Research Start-up Project of Southern Medical University. The authors declared no conflicts of interest.
Source: Chen Y et al. Int J Cancer. 2021 Apr 25. doi: 10.1002/ijc.33608.
How to Save a Limb: Identification of Pyoderma Gangrenosum
Case Report
A 67-year-old woman presented with a painful expanding ulcer on the left leg and a new nearby ulcer of 2 months’ duration. She initially was seen 2 months prior for a wound on the left knee due to a fall as well as cellulitis, which was treated with intravenous vancomycin and ceftriaxone. Wound cultures were negative for bacteria, and she was discharged without antibiotics. She presented to the emergency department 1 month later for malodorous discharge of the first ulcer with zero systemic inflammatory response syndrome criteria; no fever; and no abnormal heart rate, respiratory rate, or leukocyte count. She was discharged with wound care. After 3 weeks, she returned with a second ulcer and worsening drainage but zero systemic inflammatory response syndrome criteria. She had a medical history of Crohn disease with 9-year remission, atrial fibrillation, pacemaker, mitral valve replacement, chronic obstructive pulmonary disease, and a 51 pack-year smoking history.
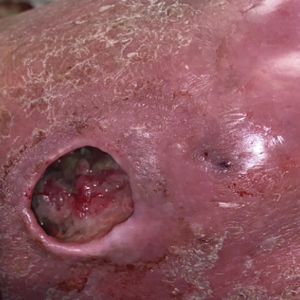
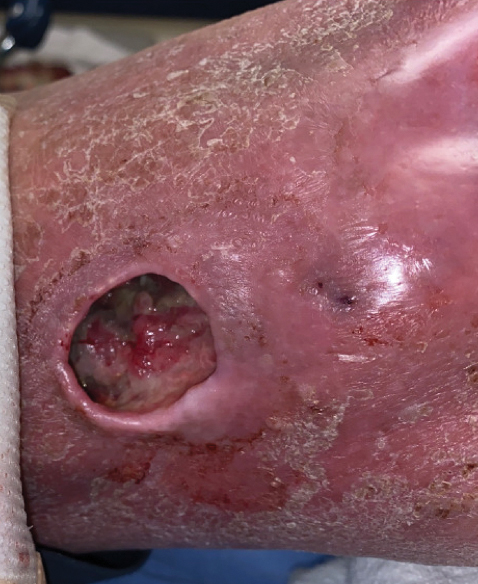
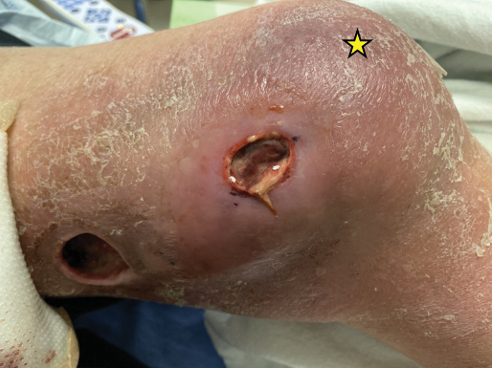
Physical examination of the left leg revealed a 3×3-cm deep lesion (ulcer A) on the distal left thigh located superomedial to the knee (Figure 1) as well as a 2×1-cm deep lesion (ulcer B) on the anteromedial knee with undermining and tunneling (Figure 2). A large amount of malodorous tan bloody discharge was present on both ulcers. There were no signs of induration or crepitus.Due to concerns of skin and soft tissue infection (SSTI) or osteomyelitis, a bone scan and wound and blood cultures were ordered. The patient was started on vancomycin and piperacillin-tazobactam in the emergency department, which later was augmented with cefepime. Trauma surgery scheduled debridement for the following morning with suspicion of necrotizing fasciitis. Additional consultations were requested, including infectious disease, wound care, and dermatology. Dermatology evaluated the wound, performed a punch biopsy, and canceled debridement due to unclear diagnosis. The clinical differential at that time included pyoderma gangrenosum (PG), atypical vasculitis, or infection. Additional workup revealed positive antineutrophil cytoplasmic antibodies but negative proteinase 3 and myeloperoxidase, disfavoring vasculitis. Wound cultures grew Staphylococcus aureus and Pseudomonas aeruginosa.
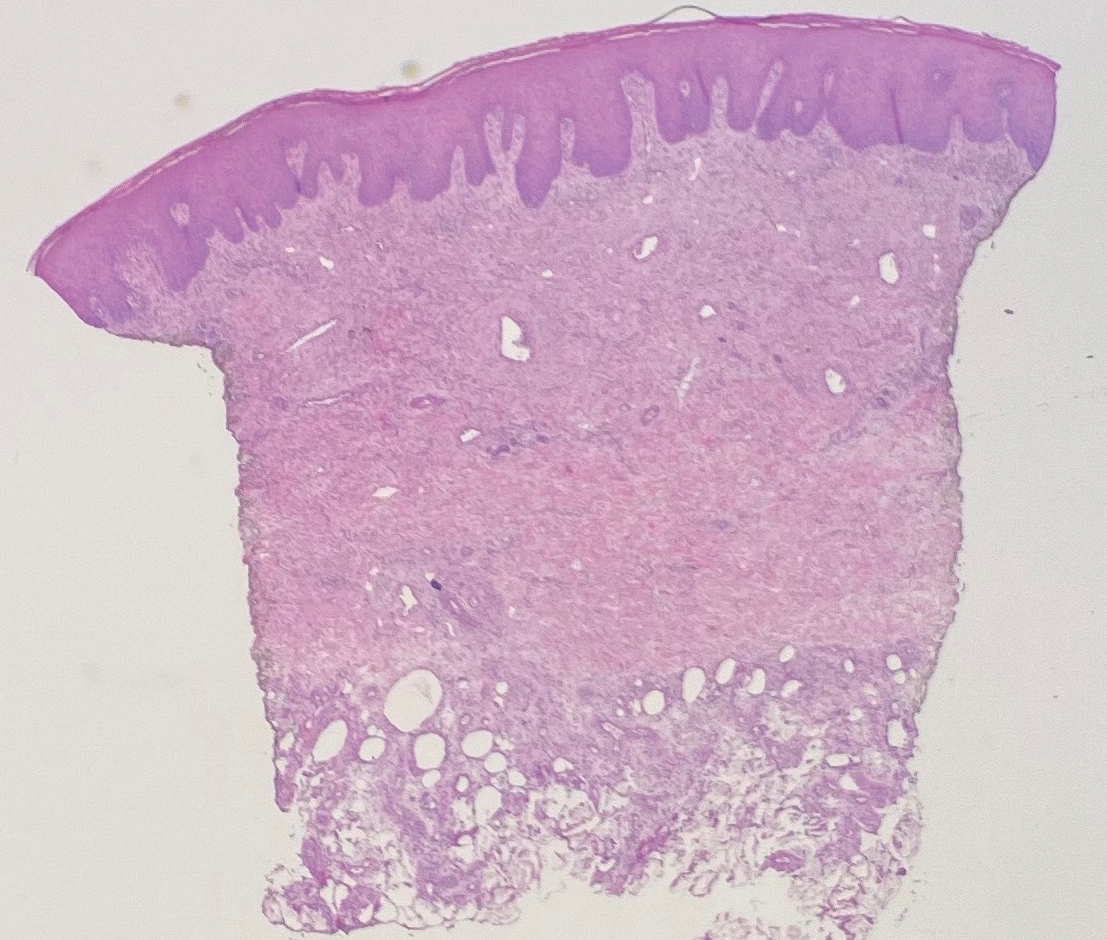
Histologic evaluation revealed deep dermal necrosis with a mixed inflammatory infiltrate (Figure 3) and no organisms or vasculitis. Antibiotics were discontinued, and she was discharged on a 14-day course of prednisone 60 mg daily for empirical treatment of PG with dermatology follow-up. Medical management included a 6-month course of dapsone that was extended to 7 months because of an intensive care unit stay for a cerebrovascular accident. Daily dosing was as follows: 100 mg for 5 months, 50 mg for 1 month, and 25 mg for 1 month, then stopped. She was followed with serial complete blood cell count every 1 to 2 months and home-health wound care. One month after dapsone initiation, the ulcers decreased in size. Ulcer B was fully healed after 4 months, and ulcer A was nearly closed at 6 months without any new flares.
Comment
Pyoderma gangrenosum is a rare inflammatory skin condition that classically presents as tender papules or pustules evolving into painful ulcers, most commonly on the lower extremities. Pyoderma gangrenosum has a propensity to exhibit pathergy, the hyperreactivity of the skin in response to minor trauma. This phenomenon in PG manifests as the rapid evolution from pustule to ulceration with violaceous undermining borders.
Diagnosis of PG
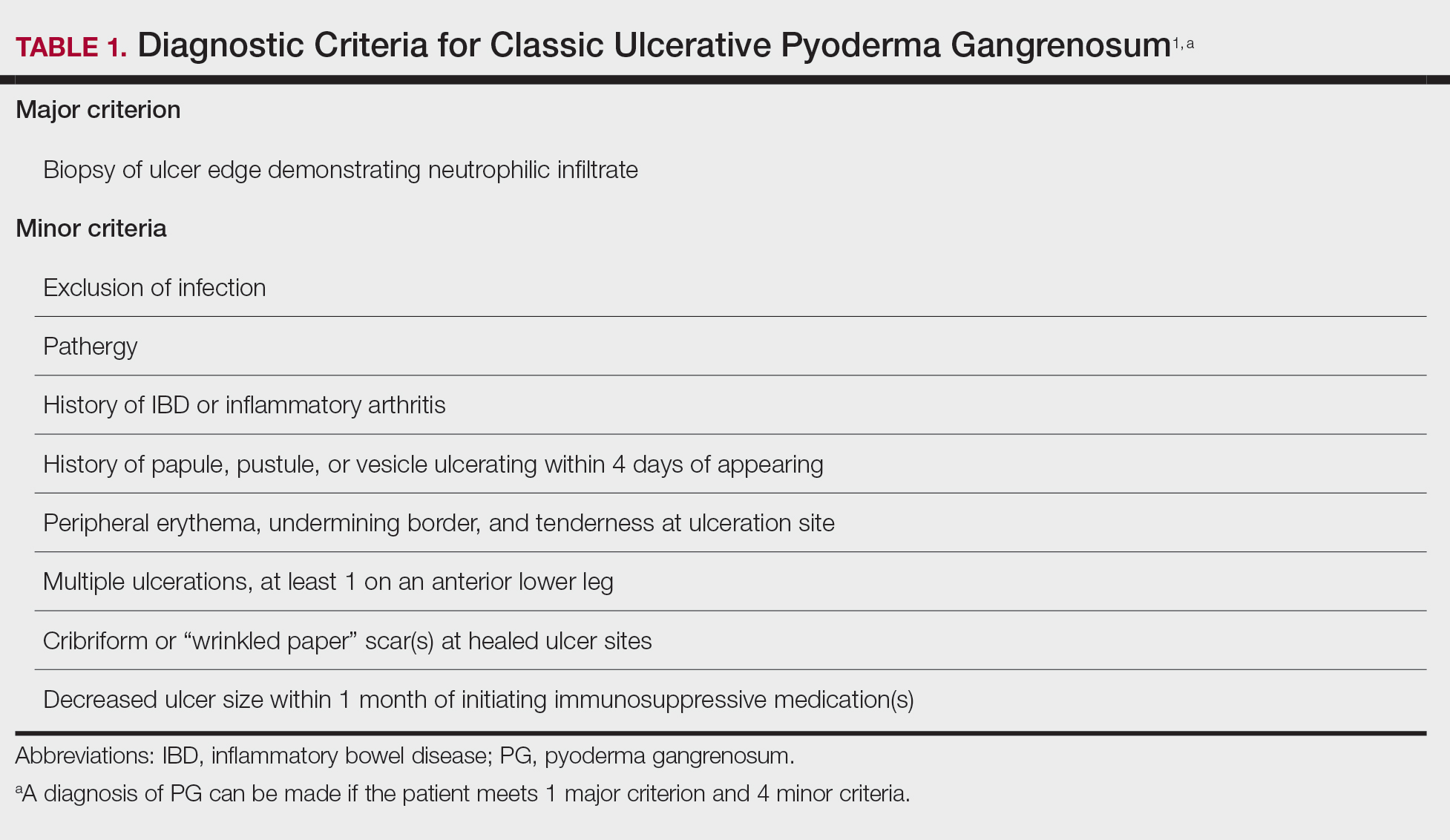
Pyoderma gangrenosum has been described as a diagnosis of exclusion, as its findings frequently mimic SSTIs. Important findings to obtain are histology, history, ulcer morphology, and response to treatment.
In 2018, Maverakis et al1 proposed diagnostic criteria for classic ulcerative PG (Table 1). A diagnosis of PG can be made if the patient meets 1 major criterion and 4 minor criteria. Our case met 0 major criteria and 5 minor criteria: history of inflammatory bowel disease (IBD); history of pustule ulcerating within 4 days of appearing; peripheral erythema, undermining border, and tenderness at ulceration site; multiple ulcerations, with at least 1 on an anterior lower leg; and decreased ulcer size within 1 month of initiating immunosuppressive medication(s). Although our patient’s biopsy demonstrated a mixed infiltrate, PG was not excluded due to spontaneous resolution at the time of biopsy, emphasizing the need to biopsy subsequent new lesions if neutrophils are not initially seen.1 Pyoderma gangrenosum frequently is associated with IBD, most often Crohn disease, as seen in our patient.2-4 Although IBD classically is associated with smoking, studies have yet to conclude if smoking is a predictive factor of PG.5 Our patient presented with an initial ulcer that evolved into 2 ulcers, similar to a case of bilateral ulcers.6
Differential Diagnosis of PG
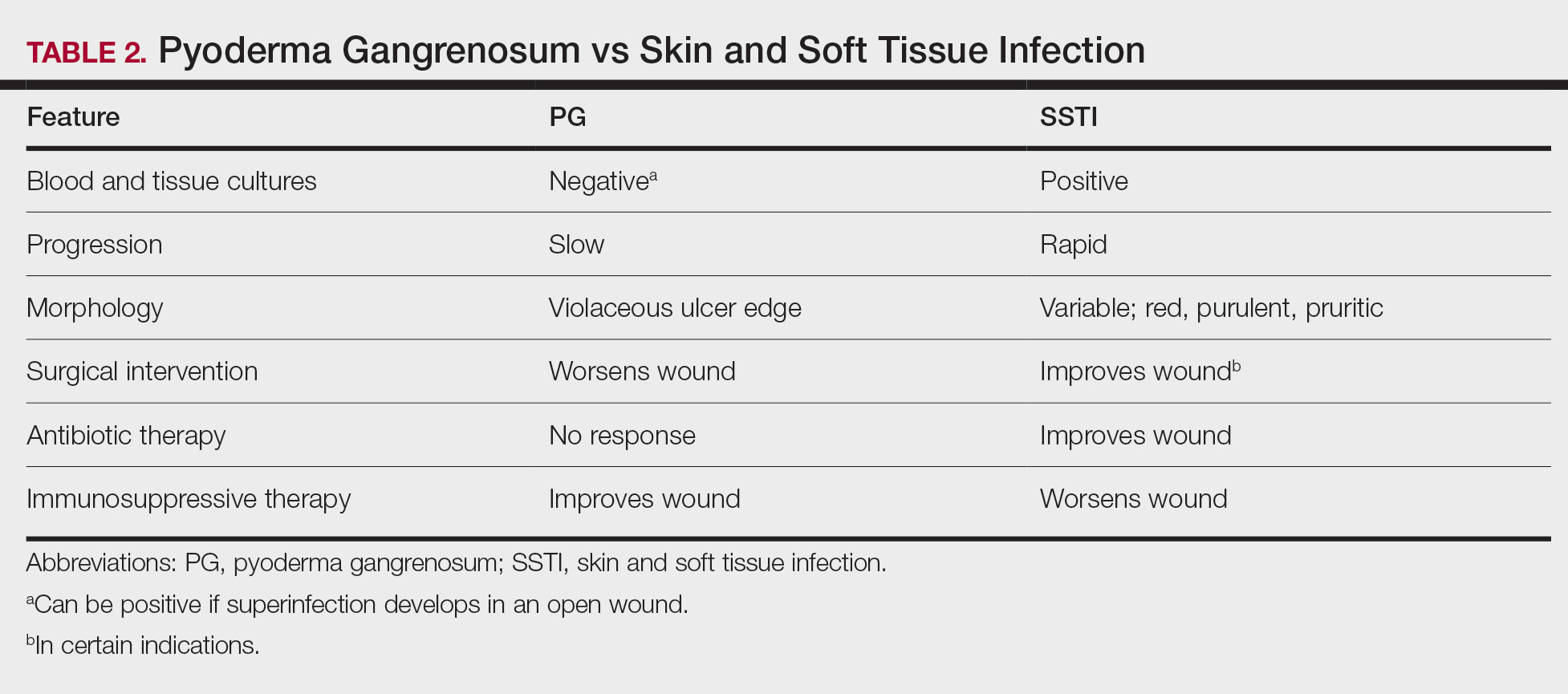
Other possible diagnoses to consider are SSTI and vasculitis, the latter being disfavored by no evidence of vasculitis on biopsy and negative titers for proteinase 3 and myeloperoxidase antibodies. However, the presence of either, similar to a mixed infiltrate, does not exclude a diagnosis of PG, as they can occur simultaneously. Consequently, superinfection of a chronically open wound can occur due to underlying PG.7 The differences between PG and SSTI are listed in Table 2.
Although we know PG involves neutrophilic dysfunction, the pathophysiology remains poorly understood, contributing to the lack of clinical guidelines.8 Therefore, the diagnosis of PG often is delayed and is associated with severe consequences such as necrotizing fasciitis, osteomyelitis, cosmetic morbidity, and limb amputation.9,10 Dermatologic consultation can aid in early diagnosis and avoid amputation.7,10 Amputation has been used as a last resort to preserve optimal outcomes in patients with severe PG.11
Management of PG
A gold standard of treatment of PG does not exist, but the goal is to promote wound healing. Patients with limited disease typically can be managed with wound care and topical steroids or calcineurin inhibitors, though data on efficacy are limited. However, our patient had more extensive disease and needed to be treated with systemic therapy. First-line therapy for extensive disease includes oral prednisone or cyclosporine for patients who cannot tolerate systemic corticosteroids.12 Second-line and adjunctive therapy options include dapsone, minocycline, methotrexate, and infliximab. Our patient was prescribed a 7-month course of dapsone with outpatient dermatology and demonstrated resolution of both ulcers. Dapsone was tapered from a daily dose of 100 mg to 50 mg to 25 mg to none over the course of 2 to 3 months. Close monitoring with wound care is recommended, and petroleum jelly can be used for dry skin around the lesion for comfort.
Conclusion
The diagnosis of PG is challenging because it relies heavily on clinical signs and often mimics SSTI. Gathering a detailed medical history is critical to make the diagnosis of PG. In a patient with associated features of PG, dermatologic consultation and biopsy of skin lesions should be considered. Physicians should evaluate for suspected PG prior to proceeding with surgical intervention to avoid unnecessary amputation. The diagnostic criteria for classic ulcerative PG are gaining wider acceptance and are a useful tool for clinicians.
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466.
- Bisarya K, Azzopardi S, Lye G, et al. Necrotizing fasciitis versus pyoderma gangrenosum: securing the correct diagnosis! a case report and literature review. Eplasty. 2011;11:E24.
- Perricone G, Vangeli M. Pyoderma gangrenosum in ulcerative colitis. N Engl J Med. 2018;379:E7.
- Ashchyan HJ, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Ampuero J, Rojas-Feria M, Castro-Fernández M, et al. Predictive factors for erythema nodosum and pyoderma gangrenosum in inflammatory bowel disease. J Gastroenterol Hepatol. 2014;29:291-295.
- Ebner DW, Hu M, Poterucha TH. 29-year-old woman with fever and bilateral lower extremity lesions. Mayo Clin Proc. 2018;93:1659-1663.
- Marzak H, Von Hunolstein JJ, Lipsker D, et al. Management of a superinfected pyoderma gangrenosum after pacemaker implant. HeartRhythm Case Rep. 2018;5:63-65.
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691-698.
- Saffie MG, Shroff A. A case of pyoderma gangrenosum misdiagnosed as necrotizing infection: a potential diagnostic catastrophe. Case Rep Infect Dis. 2018;2018:8907542.
- Haag CK, Nutan F, Cyrus JW, et al. Pyoderma gangrenosum misdiagnosis resulting in amputation: a review. J Trauma Acute Care Surg. 2019;86:307-313.
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol. 2019;155:79-84.
- Alavi A, French LE, Davis MD, et al. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2017;18:355-372.
Case Report
A 67-year-old woman presented with a painful expanding ulcer on the left leg and a new nearby ulcer of 2 months’ duration. She initially was seen 2 months prior for a wound on the left knee due to a fall as well as cellulitis, which was treated with intravenous vancomycin and ceftriaxone. Wound cultures were negative for bacteria, and she was discharged without antibiotics. She presented to the emergency department 1 month later for malodorous discharge of the first ulcer with zero systemic inflammatory response syndrome criteria; no fever; and no abnormal heart rate, respiratory rate, or leukocyte count. She was discharged with wound care. After 3 weeks, she returned with a second ulcer and worsening drainage but zero systemic inflammatory response syndrome criteria. She had a medical history of Crohn disease with 9-year remission, atrial fibrillation, pacemaker, mitral valve replacement, chronic obstructive pulmonary disease, and a 51 pack-year smoking history.
Physical examination of the left leg revealed a 3×3-cm deep lesion (ulcer A) on the distal left thigh located superomedial to the knee (Figure 1) as well as a 2×1-cm deep lesion (ulcer B) on the anteromedial knee with undermining and tunneling (Figure 2). A large amount of malodorous tan bloody discharge was present on both ulcers. There were no signs of induration or crepitus.Due to concerns of skin and soft tissue infection (SSTI) or osteomyelitis, a bone scan and wound and blood cultures were ordered. The patient was started on vancomycin and piperacillin-tazobactam in the emergency department, which later was augmented with cefepime. Trauma surgery scheduled debridement for the following morning with suspicion of necrotizing fasciitis. Additional consultations were requested, including infectious disease, wound care, and dermatology. Dermatology evaluated the wound, performed a punch biopsy, and canceled debridement due to unclear diagnosis. The clinical differential at that time included pyoderma gangrenosum (PG), atypical vasculitis, or infection. Additional workup revealed positive antineutrophil cytoplasmic antibodies but negative proteinase 3 and myeloperoxidase, disfavoring vasculitis. Wound cultures grew Staphylococcus aureus and Pseudomonas aeruginosa.


Histologic evaluation revealed deep dermal necrosis with a mixed inflammatory infiltrate (Figure 3) and no organisms or vasculitis. Antibiotics were discontinued, and she was discharged on a 14-day course of prednisone 60 mg daily for empirical treatment of PG with dermatology follow-up. Medical management included a 6-month course of dapsone that was extended to 7 months because of an intensive care unit stay for a cerebrovascular accident. Daily dosing was as follows: 100 mg for 5 months, 50 mg for 1 month, and 25 mg for 1 month, then stopped. She was followed with serial complete blood cell count every 1 to 2 months and home-health wound care. One month after dapsone initiation, the ulcers decreased in size. Ulcer B was fully healed after 4 months, and ulcer A was nearly closed at 6 months without any new flares.

Comment
Pyoderma gangrenosum is a rare inflammatory skin condition that classically presents as tender papules or pustules evolving into painful ulcers, most commonly on the lower extremities. Pyoderma gangrenosum has a propensity to exhibit pathergy, the hyperreactivity of the skin in response to minor trauma. This phenomenon in PG manifests as the rapid evolution from pustule to ulceration with violaceous undermining borders.
Diagnosis of PG
Pyoderma gangrenosum has been described as a diagnosis of exclusion, as its findings frequently mimic SSTIs. Important findings to obtain are histology, history, ulcer morphology, and response to treatment.
In 2018, Maverakis et al1 proposed diagnostic criteria for classic ulcerative PG (Table 1). A diagnosis of PG can be made if the patient meets 1 major criterion and 4 minor criteria. Our case met 0 major criteria and 5 minor criteria: history of inflammatory bowel disease (IBD); history of pustule ulcerating within 4 days of appearing; peripheral erythema, undermining border, and tenderness at ulceration site; multiple ulcerations, with at least 1 on an anterior lower leg; and decreased ulcer size within 1 month of initiating immunosuppressive medication(s). Although our patient’s biopsy demonstrated a mixed infiltrate, PG was not excluded due to spontaneous resolution at the time of biopsy, emphasizing the need to biopsy subsequent new lesions if neutrophils are not initially seen.1 Pyoderma gangrenosum frequently is associated with IBD, most often Crohn disease, as seen in our patient.2-4 Although IBD classically is associated with smoking, studies have yet to conclude if smoking is a predictive factor of PG.5 Our patient presented with an initial ulcer that evolved into 2 ulcers, similar to a case of bilateral ulcers.6

Differential Diagnosis of PG
Other possible diagnoses to consider are SSTI and vasculitis, the latter being disfavored by no evidence of vasculitis on biopsy and negative titers for proteinase 3 and myeloperoxidase antibodies. However, the presence of either, similar to a mixed infiltrate, does not exclude a diagnosis of PG, as they can occur simultaneously. Consequently, superinfection of a chronically open wound can occur due to underlying PG.7 The differences between PG and SSTI are listed in Table 2.

Although we know PG involves neutrophilic dysfunction, the pathophysiology remains poorly understood, contributing to the lack of clinical guidelines.8 Therefore, the diagnosis of PG often is delayed and is associated with severe consequences such as necrotizing fasciitis, osteomyelitis, cosmetic morbidity, and limb amputation.9,10 Dermatologic consultation can aid in early diagnosis and avoid amputation.7,10 Amputation has been used as a last resort to preserve optimal outcomes in patients with severe PG.11
Management of PG
A gold standard of treatment of PG does not exist, but the goal is to promote wound healing. Patients with limited disease typically can be managed with wound care and topical steroids or calcineurin inhibitors, though data on efficacy are limited. However, our patient had more extensive disease and needed to be treated with systemic therapy. First-line therapy for extensive disease includes oral prednisone or cyclosporine for patients who cannot tolerate systemic corticosteroids.12 Second-line and adjunctive therapy options include dapsone, minocycline, methotrexate, and infliximab. Our patient was prescribed a 7-month course of dapsone with outpatient dermatology and demonstrated resolution of both ulcers. Dapsone was tapered from a daily dose of 100 mg to 50 mg to 25 mg to none over the course of 2 to 3 months. Close monitoring with wound care is recommended, and petroleum jelly can be used for dry skin around the lesion for comfort.
Conclusion
The diagnosis of PG is challenging because it relies heavily on clinical signs and often mimics SSTI. Gathering a detailed medical history is critical to make the diagnosis of PG. In a patient with associated features of PG, dermatologic consultation and biopsy of skin lesions should be considered. Physicians should evaluate for suspected PG prior to proceeding with surgical intervention to avoid unnecessary amputation. The diagnostic criteria for classic ulcerative PG are gaining wider acceptance and are a useful tool for clinicians.
Case Report
A 67-year-old woman presented with a painful expanding ulcer on the left leg and a new nearby ulcer of 2 months’ duration. She initially was seen 2 months prior for a wound on the left knee due to a fall as well as cellulitis, which was treated with intravenous vancomycin and ceftriaxone. Wound cultures were negative for bacteria, and she was discharged without antibiotics. She presented to the emergency department 1 month later for malodorous discharge of the first ulcer with zero systemic inflammatory response syndrome criteria; no fever; and no abnormal heart rate, respiratory rate, or leukocyte count. She was discharged with wound care. After 3 weeks, she returned with a second ulcer and worsening drainage but zero systemic inflammatory response syndrome criteria. She had a medical history of Crohn disease with 9-year remission, atrial fibrillation, pacemaker, mitral valve replacement, chronic obstructive pulmonary disease, and a 51 pack-year smoking history.
Physical examination of the left leg revealed a 3×3-cm deep lesion (ulcer A) on the distal left thigh located superomedial to the knee (Figure 1) as well as a 2×1-cm deep lesion (ulcer B) on the anteromedial knee with undermining and tunneling (Figure 2). A large amount of malodorous tan bloody discharge was present on both ulcers. There were no signs of induration or crepitus.Due to concerns of skin and soft tissue infection (SSTI) or osteomyelitis, a bone scan and wound and blood cultures were ordered. The patient was started on vancomycin and piperacillin-tazobactam in the emergency department, which later was augmented with cefepime. Trauma surgery scheduled debridement for the following morning with suspicion of necrotizing fasciitis. Additional consultations were requested, including infectious disease, wound care, and dermatology. Dermatology evaluated the wound, performed a punch biopsy, and canceled debridement due to unclear diagnosis. The clinical differential at that time included pyoderma gangrenosum (PG), atypical vasculitis, or infection. Additional workup revealed positive antineutrophil cytoplasmic antibodies but negative proteinase 3 and myeloperoxidase, disfavoring vasculitis. Wound cultures grew Staphylococcus aureus and Pseudomonas aeruginosa.


Histologic evaluation revealed deep dermal necrosis with a mixed inflammatory infiltrate (Figure 3) and no organisms or vasculitis. Antibiotics were discontinued, and she was discharged on a 14-day course of prednisone 60 mg daily for empirical treatment of PG with dermatology follow-up. Medical management included a 6-month course of dapsone that was extended to 7 months because of an intensive care unit stay for a cerebrovascular accident. Daily dosing was as follows: 100 mg for 5 months, 50 mg for 1 month, and 25 mg for 1 month, then stopped. She was followed with serial complete blood cell count every 1 to 2 months and home-health wound care. One month after dapsone initiation, the ulcers decreased in size. Ulcer B was fully healed after 4 months, and ulcer A was nearly closed at 6 months without any new flares.

Comment
Pyoderma gangrenosum is a rare inflammatory skin condition that classically presents as tender papules or pustules evolving into painful ulcers, most commonly on the lower extremities. Pyoderma gangrenosum has a propensity to exhibit pathergy, the hyperreactivity of the skin in response to minor trauma. This phenomenon in PG manifests as the rapid evolution from pustule to ulceration with violaceous undermining borders.
Diagnosis of PG
Pyoderma gangrenosum has been described as a diagnosis of exclusion, as its findings frequently mimic SSTIs. Important findings to obtain are histology, history, ulcer morphology, and response to treatment.
In 2018, Maverakis et al1 proposed diagnostic criteria for classic ulcerative PG (Table 1). A diagnosis of PG can be made if the patient meets 1 major criterion and 4 minor criteria. Our case met 0 major criteria and 5 minor criteria: history of inflammatory bowel disease (IBD); history of pustule ulcerating within 4 days of appearing; peripheral erythema, undermining border, and tenderness at ulceration site; multiple ulcerations, with at least 1 on an anterior lower leg; and decreased ulcer size within 1 month of initiating immunosuppressive medication(s). Although our patient’s biopsy demonstrated a mixed infiltrate, PG was not excluded due to spontaneous resolution at the time of biopsy, emphasizing the need to biopsy subsequent new lesions if neutrophils are not initially seen.1 Pyoderma gangrenosum frequently is associated with IBD, most often Crohn disease, as seen in our patient.2-4 Although IBD classically is associated with smoking, studies have yet to conclude if smoking is a predictive factor of PG.5 Our patient presented with an initial ulcer that evolved into 2 ulcers, similar to a case of bilateral ulcers.6

Differential Diagnosis of PG
Other possible diagnoses to consider are SSTI and vasculitis, the latter being disfavored by no evidence of vasculitis on biopsy and negative titers for proteinase 3 and myeloperoxidase antibodies. However, the presence of either, similar to a mixed infiltrate, does not exclude a diagnosis of PG, as they can occur simultaneously. Consequently, superinfection of a chronically open wound can occur due to underlying PG.7 The differences between PG and SSTI are listed in Table 2.

Although we know PG involves neutrophilic dysfunction, the pathophysiology remains poorly understood, contributing to the lack of clinical guidelines.8 Therefore, the diagnosis of PG often is delayed and is associated with severe consequences such as necrotizing fasciitis, osteomyelitis, cosmetic morbidity, and limb amputation.9,10 Dermatologic consultation can aid in early diagnosis and avoid amputation.7,10 Amputation has been used as a last resort to preserve optimal outcomes in patients with severe PG.11
Management of PG
A gold standard of treatment of PG does not exist, but the goal is to promote wound healing. Patients with limited disease typically can be managed with wound care and topical steroids or calcineurin inhibitors, though data on efficacy are limited. However, our patient had more extensive disease and needed to be treated with systemic therapy. First-line therapy for extensive disease includes oral prednisone or cyclosporine for patients who cannot tolerate systemic corticosteroids.12 Second-line and adjunctive therapy options include dapsone, minocycline, methotrexate, and infliximab. Our patient was prescribed a 7-month course of dapsone with outpatient dermatology and demonstrated resolution of both ulcers. Dapsone was tapered from a daily dose of 100 mg to 50 mg to 25 mg to none over the course of 2 to 3 months. Close monitoring with wound care is recommended, and petroleum jelly can be used for dry skin around the lesion for comfort.
Conclusion
The diagnosis of PG is challenging because it relies heavily on clinical signs and often mimics SSTI. Gathering a detailed medical history is critical to make the diagnosis of PG. In a patient with associated features of PG, dermatologic consultation and biopsy of skin lesions should be considered. Physicians should evaluate for suspected PG prior to proceeding with surgical intervention to avoid unnecessary amputation. The diagnostic criteria for classic ulcerative PG are gaining wider acceptance and are a useful tool for clinicians.
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466.
- Bisarya K, Azzopardi S, Lye G, et al. Necrotizing fasciitis versus pyoderma gangrenosum: securing the correct diagnosis! a case report and literature review. Eplasty. 2011;11:E24.
- Perricone G, Vangeli M. Pyoderma gangrenosum in ulcerative colitis. N Engl J Med. 2018;379:E7.
- Ashchyan HJ, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Ampuero J, Rojas-Feria M, Castro-Fernández M, et al. Predictive factors for erythema nodosum and pyoderma gangrenosum in inflammatory bowel disease. J Gastroenterol Hepatol. 2014;29:291-295.
- Ebner DW, Hu M, Poterucha TH. 29-year-old woman with fever and bilateral lower extremity lesions. Mayo Clin Proc. 2018;93:1659-1663.
- Marzak H, Von Hunolstein JJ, Lipsker D, et al. Management of a superinfected pyoderma gangrenosum after pacemaker implant. HeartRhythm Case Rep. 2018;5:63-65.
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691-698.
- Saffie MG, Shroff A. A case of pyoderma gangrenosum misdiagnosed as necrotizing infection: a potential diagnostic catastrophe. Case Rep Infect Dis. 2018;2018:8907542.
- Haag CK, Nutan F, Cyrus JW, et al. Pyoderma gangrenosum misdiagnosis resulting in amputation: a review. J Trauma Acute Care Surg. 2019;86:307-313.
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol. 2019;155:79-84.
- Alavi A, French LE, Davis MD, et al. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2017;18:355-372.
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466.
- Bisarya K, Azzopardi S, Lye G, et al. Necrotizing fasciitis versus pyoderma gangrenosum: securing the correct diagnosis! a case report and literature review. Eplasty. 2011;11:E24.
- Perricone G, Vangeli M. Pyoderma gangrenosum in ulcerative colitis. N Engl J Med. 2018;379:E7.
- Ashchyan HJ, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Ampuero J, Rojas-Feria M, Castro-Fernández M, et al. Predictive factors for erythema nodosum and pyoderma gangrenosum in inflammatory bowel disease. J Gastroenterol Hepatol. 2014;29:291-295.
- Ebner DW, Hu M, Poterucha TH. 29-year-old woman with fever and bilateral lower extremity lesions. Mayo Clin Proc. 2018;93:1659-1663.
- Marzak H, Von Hunolstein JJ, Lipsker D, et al. Management of a superinfected pyoderma gangrenosum after pacemaker implant. HeartRhythm Case Rep. 2018;5:63-65.
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691-698.
- Saffie MG, Shroff A. A case of pyoderma gangrenosum misdiagnosed as necrotizing infection: a potential diagnostic catastrophe. Case Rep Infect Dis. 2018;2018:8907542.
- Haag CK, Nutan F, Cyrus JW, et al. Pyoderma gangrenosum misdiagnosis resulting in amputation: a review. J Trauma Acute Care Surg. 2019;86:307-313.
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol. 2019;155:79-84.
- Alavi A, French LE, Davis MD, et al. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2017;18:355-372.
Practice Points
- Pyoderma gangrenosum (PG) frequently is misdiagnosed due to its similar presentation to other skin and soft tissue infections (SSTIs). Patients with known risk factors for PG should be evaluated with a high index of suspicion to ensure early diagnosis and avoid serious complications. Common associations include inflammatory bowel disease (IBD), hematologic malignancies, and rheumatologic disorders.
- Response to treatment may be used to guide management when the diagnosis of SSTIs vs PG cannot be distinguished with clinical and histologic findings alone. In a worsening ulcer that has failed antibiotic therapy, clinicians should consider the diagnosis of PG and the risk of pathergy prior to surgical intervention such as debridement.
- Although typically a diagnosis of exclusion, clinicians can consider the use of diagnostic criteria for PG in patients of high clinical suspicion. A trial of immunosuppressants can be considered after infection has been ruled out.
TP53-mutated MDS: Eprenetapopt plus azacitidine is safe and favorable
Key clinical point: In patients with very high-risk TP53-mutated myelodysplastic syndromes (MDS), addition of eprenetapopt to azacitidine (AZA) was safe and led to better clinical outcomes than AZA alone.
Major finding: The overall response rate in MDS patients was 62% including 47% complete remission. The median duration of response in MDS patients was 10.4 months. At a median follow-up of 9.7 months, the median overall survival in MDS patients was 12.1 months. Eprenetapopt plus AZA was generally well tolerated.
Study details: Phase 2 study of eprenetapopt plus AZA vs. AZA alone in 34 very high-risk TP53-mutated MDS patients.
Disclosures: The study was supported by Groupe Francophone des Myelodysplasies. The authors reported relationships with various pharmaceutical companies.
Source: Cluzeau T et al. J Clin Oncol. 2021 Feb 18. doi: 10.1200/JCO.20.02342.
Key clinical point: In patients with very high-risk TP53-mutated myelodysplastic syndromes (MDS), addition of eprenetapopt to azacitidine (AZA) was safe and led to better clinical outcomes than AZA alone.
Major finding: The overall response rate in MDS patients was 62% including 47% complete remission. The median duration of response in MDS patients was 10.4 months. At a median follow-up of 9.7 months, the median overall survival in MDS patients was 12.1 months. Eprenetapopt plus AZA was generally well tolerated.
Study details: Phase 2 study of eprenetapopt plus AZA vs. AZA alone in 34 very high-risk TP53-mutated MDS patients.
Disclosures: The study was supported by Groupe Francophone des Myelodysplasies. The authors reported relationships with various pharmaceutical companies.
Source: Cluzeau T et al. J Clin Oncol. 2021 Feb 18. doi: 10.1200/JCO.20.02342.
Key clinical point: In patients with very high-risk TP53-mutated myelodysplastic syndromes (MDS), addition of eprenetapopt to azacitidine (AZA) was safe and led to better clinical outcomes than AZA alone.
Major finding: The overall response rate in MDS patients was 62% including 47% complete remission. The median duration of response in MDS patients was 10.4 months. At a median follow-up of 9.7 months, the median overall survival in MDS patients was 12.1 months. Eprenetapopt plus AZA was generally well tolerated.
Study details: Phase 2 study of eprenetapopt plus AZA vs. AZA alone in 34 very high-risk TP53-mutated MDS patients.
Disclosures: The study was supported by Groupe Francophone des Myelodysplasies. The authors reported relationships with various pharmaceutical companies.
Source: Cluzeau T et al. J Clin Oncol. 2021 Feb 18. doi: 10.1200/JCO.20.02342.
Clinical Edge Journal Scan Commentary: MDS June 2021
Allogeneic stem cell transplantation (SCT) remains the only potential curable option for patients with myelodysplastic syndromes (MDS). While MDS mainly occurs in older patients, MDS does occur in younger patients and prior studies have suggested better overall survival (OS) for younger patients undergoing allogeneic SCT. Shimomura et al (BMT 2021) analyzed the outcome of 645 patients aged 16-39 years old who received allogeneic SCT in Japan. In this multicenter retrospective analysis, 3 year OS was 71%. In the cohort, 37% of patients had either intermediate-2 or high risk by IPSS, 41% had active disease prior to SCT, and 10% had secondary MDS. In a multivariate analysis, active disease status, poor cytogenetic risk, and transplant other than from related donor were associated with poor 3 year OS. Cumulative 3 year relapse was 11% and 3 year non-relapse mortality was 19%, which is lower than those of older patients with MDS which ranges from 30-40%. Limitations from this analysis are lack of genetic mutation data, as well as lack of incorporation of prior MDS directed therapy with outcome analysis, however this analysis from a large retrospective cohort provides valuable information regarding outcome in young patients with MDS.
Use of melphalan in multiple myeloma is associated with increased risk of developing MDS and acute myeloid leukemia (AML). Jonsdottir et al (European Journal of Hematology) reported results of a large population-based study using the Swedish cancer registry to determine relationship of exposure to melphalan treatment for multiple myeloma and development of subsequent MDS and AML. Out of 26,627 patients with multiple myeloma in the Swedish cancer registry, 0.5% (124) patients developed subsequent MDS or AML. The median time from multiple myeloma diagnosis to MDS or AML diagnosis was 3.8 years. There was about threefold higher cumulative exposure to melphalan among those who developed MDS or AML compared to those who did not develop MDS or AML (OR=2.8, 95% CI 1.7-5.2, P < 0.001). There was no difference among the groups in exposure and dosing of radiation therapy, cumulative dose of cyclophosphamide, or doxorubicine. This large population-based study confirms the association between melphalan therapy and development of MDS/AML.
Febrile neutropenia is a common complication for neutropenic patients undergoing therapy. Patients who develop febrile neutropenia are treated with empiric broad-spectrum antibiotics, however there is conflicting recommended duration of antimicrobial treatment in absence of evidence of bacterial infection. The Infectious Disease Society of America recommends antibiotic treatment until marrow recovery, while the European Conference of Infections in Leukemia guidelines suggest consideration of discontinuation after 72 hours if the patient is hemodynamically stable and afebrile for 48 hours. Schauwvlieghe et al (EClinical Medicine 2021) compared the outcome of MDS and AML patients with febrile neutropenia during induction chemotherapy receiving a shortened 3 day empiric antibiotic therapy in absence of documented infection to those receiving antibiotics until neutrophil recovery in a retrospective comparative cohort study in two hospitals in the HOVON network. The shorted antibiotic group received a median of 9 days of antibiotics, compared to 19 days. The primary endpoint was the rate of serious medical complications; there was no statistical difference between the two groups (12.5% for shortened antibiotics vs 8.9% for prolonged antibiotics, P = 0.17). There was no difference in serious medical complications when adjusting for age, AML risk, non-pulmonary HCT-CI score, and year of admission. There was also no significant difference in infection-related 30-day mortality among the two groups. This study suggests that for patients who develop febrile neutropenia while receiving induction chemotherapy, it may be safe to consider stopping antibiotics after 3 days in absence of infection. A prospective trial should be considered to further evaluate the safety of shortened antibiotic course.
Allogeneic stem cell transplantation (SCT) remains the only potential curable option for patients with myelodysplastic syndromes (MDS). While MDS mainly occurs in older patients, MDS does occur in younger patients and prior studies have suggested better overall survival (OS) for younger patients undergoing allogeneic SCT. Shimomura et al (BMT 2021) analyzed the outcome of 645 patients aged 16-39 years old who received allogeneic SCT in Japan. In this multicenter retrospective analysis, 3 year OS was 71%. In the cohort, 37% of patients had either intermediate-2 or high risk by IPSS, 41% had active disease prior to SCT, and 10% had secondary MDS. In a multivariate analysis, active disease status, poor cytogenetic risk, and transplant other than from related donor were associated with poor 3 year OS. Cumulative 3 year relapse was 11% and 3 year non-relapse mortality was 19%, which is lower than those of older patients with MDS which ranges from 30-40%. Limitations from this analysis are lack of genetic mutation data, as well as lack of incorporation of prior MDS directed therapy with outcome analysis, however this analysis from a large retrospective cohort provides valuable information regarding outcome in young patients with MDS.
Use of melphalan in multiple myeloma is associated with increased risk of developing MDS and acute myeloid leukemia (AML). Jonsdottir et al (European Journal of Hematology) reported results of a large population-based study using the Swedish cancer registry to determine relationship of exposure to melphalan treatment for multiple myeloma and development of subsequent MDS and AML. Out of 26,627 patients with multiple myeloma in the Swedish cancer registry, 0.5% (124) patients developed subsequent MDS or AML. The median time from multiple myeloma diagnosis to MDS or AML diagnosis was 3.8 years. There was about threefold higher cumulative exposure to melphalan among those who developed MDS or AML compared to those who did not develop MDS or AML (OR=2.8, 95% CI 1.7-5.2, P < 0.001). There was no difference among the groups in exposure and dosing of radiation therapy, cumulative dose of cyclophosphamide, or doxorubicine. This large population-based study confirms the association between melphalan therapy and development of MDS/AML.
Febrile neutropenia is a common complication for neutropenic patients undergoing therapy. Patients who develop febrile neutropenia are treated with empiric broad-spectrum antibiotics, however there is conflicting recommended duration of antimicrobial treatment in absence of evidence of bacterial infection. The Infectious Disease Society of America recommends antibiotic treatment until marrow recovery, while the European Conference of Infections in Leukemia guidelines suggest consideration of discontinuation after 72 hours if the patient is hemodynamically stable and afebrile for 48 hours. Schauwvlieghe et al (EClinical Medicine 2021) compared the outcome of MDS and AML patients with febrile neutropenia during induction chemotherapy receiving a shortened 3 day empiric antibiotic therapy in absence of documented infection to those receiving antibiotics until neutrophil recovery in a retrospective comparative cohort study in two hospitals in the HOVON network. The shorted antibiotic group received a median of 9 days of antibiotics, compared to 19 days. The primary endpoint was the rate of serious medical complications; there was no statistical difference between the two groups (12.5% for shortened antibiotics vs 8.9% for prolonged antibiotics, P = 0.17). There was no difference in serious medical complications when adjusting for age, AML risk, non-pulmonary HCT-CI score, and year of admission. There was also no significant difference in infection-related 30-day mortality among the two groups. This study suggests that for patients who develop febrile neutropenia while receiving induction chemotherapy, it may be safe to consider stopping antibiotics after 3 days in absence of infection. A prospective trial should be considered to further evaluate the safety of shortened antibiotic course.
Allogeneic stem cell transplantation (SCT) remains the only potential curable option for patients with myelodysplastic syndromes (MDS). While MDS mainly occurs in older patients, MDS does occur in younger patients and prior studies have suggested better overall survival (OS) for younger patients undergoing allogeneic SCT. Shimomura et al (BMT 2021) analyzed the outcome of 645 patients aged 16-39 years old who received allogeneic SCT in Japan. In this multicenter retrospective analysis, 3 year OS was 71%. In the cohort, 37% of patients had either intermediate-2 or high risk by IPSS, 41% had active disease prior to SCT, and 10% had secondary MDS. In a multivariate analysis, active disease status, poor cytogenetic risk, and transplant other than from related donor were associated with poor 3 year OS. Cumulative 3 year relapse was 11% and 3 year non-relapse mortality was 19%, which is lower than those of older patients with MDS which ranges from 30-40%. Limitations from this analysis are lack of genetic mutation data, as well as lack of incorporation of prior MDS directed therapy with outcome analysis, however this analysis from a large retrospective cohort provides valuable information regarding outcome in young patients with MDS.
Use of melphalan in multiple myeloma is associated with increased risk of developing MDS and acute myeloid leukemia (AML). Jonsdottir et al (European Journal of Hematology) reported results of a large population-based study using the Swedish cancer registry to determine relationship of exposure to melphalan treatment for multiple myeloma and development of subsequent MDS and AML. Out of 26,627 patients with multiple myeloma in the Swedish cancer registry, 0.5% (124) patients developed subsequent MDS or AML. The median time from multiple myeloma diagnosis to MDS or AML diagnosis was 3.8 years. There was about threefold higher cumulative exposure to melphalan among those who developed MDS or AML compared to those who did not develop MDS or AML (OR=2.8, 95% CI 1.7-5.2, P < 0.001). There was no difference among the groups in exposure and dosing of radiation therapy, cumulative dose of cyclophosphamide, or doxorubicine. This large population-based study confirms the association between melphalan therapy and development of MDS/AML.
Febrile neutropenia is a common complication for neutropenic patients undergoing therapy. Patients who develop febrile neutropenia are treated with empiric broad-spectrum antibiotics, however there is conflicting recommended duration of antimicrobial treatment in absence of evidence of bacterial infection. The Infectious Disease Society of America recommends antibiotic treatment until marrow recovery, while the European Conference of Infections in Leukemia guidelines suggest consideration of discontinuation after 72 hours if the patient is hemodynamically stable and afebrile for 48 hours. Schauwvlieghe et al (EClinical Medicine 2021) compared the outcome of MDS and AML patients with febrile neutropenia during induction chemotherapy receiving a shortened 3 day empiric antibiotic therapy in absence of documented infection to those receiving antibiotics until neutrophil recovery in a retrospective comparative cohort study in two hospitals in the HOVON network. The shorted antibiotic group received a median of 9 days of antibiotics, compared to 19 days. The primary endpoint was the rate of serious medical complications; there was no statistical difference between the two groups (12.5% for shortened antibiotics vs 8.9% for prolonged antibiotics, P = 0.17). There was no difference in serious medical complications when adjusting for age, AML risk, non-pulmonary HCT-CI score, and year of admission. There was also no significant difference in infection-related 30-day mortality among the two groups. This study suggests that for patients who develop febrile neutropenia while receiving induction chemotherapy, it may be safe to consider stopping antibiotics after 3 days in absence of infection. A prospective trial should be considered to further evaluate the safety of shortened antibiotic course.
Patients with RA on rituximab at risk for worse COVID-19 outcomes
Patients with rheumatoid arthritis who were using rituximab at the time of COVID-19 onset had a fourfold higher risk of being hospitalized, needing mechanical ventilation, or dying, compared with patients taking a tumor necrosis factor inhibitor (TNFi), according to a report given at the annual European Congress of Rheumatology.
The use of Janus kinase inhibitors (JAKi) also was associated with a twofold higher risk for these COVID-19 outcomes, said Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, in presenting the analysis from the COVID-19 Global Rheumatology Alliance (GRA) Physician Registry.
“The strong association of rituximab and JAK inhibitor use with poor COVID-19 outcomes highlights the prioritization of risk mitigation strategies for these patients,” Dr. Sparks said at the meeting.
The full findings have now been published in Annals of the Rheumatic Diseases.
JAKi association questioned
These findings provide “an important understanding for the risk of our patients in times before vaccination,” said Hendrik Schulze-Koops, MD, of Ludwig Maximilian University of Munich, who chaired the session in which the study was presented.
However, “recently, baricitinib was licensed to prevent particular aspects of severe COVID. What’s the explanation for this discrepancy?” he asked.
“Certainly, the JAK inhibitor finding deserves further study,” Dr. Sparks acknowledged, adding that the data were analyzed by class rather than for individual drugs.
“One possible explanation could be when JAK inhibitors are used,” he suggested. “It might be different for patients who [have been] just infected – that might have different biologic effects – as opposed to choosing to treat patients right when there’s a hyperinflammatory cascade, or there’s oxygen need.”
Regarding the JAK inhibitor finding, Ronald van Vollenhoven, MD, PhD, of the University of Amsterdam, pointed out during the online Q&A that “JAKi have a very short half-life compared to biologics.”
Dr. van Vollenhoven asked: “Could the practice of stopping these drugs upon COVID infection have a negative impact on the course?” To which Dr. Sparks responded: “The different half-life of drugs would be a promising avenue to look at, to see whether increases in disease activity might have imparted some of the effects we saw.”
Performing the analysis
As of April 12, 2021, the GRA Physician Registry contained the records of more than 15,000 patients. Dr. Sparks, collaborator Zachary Wallace, MD, of Massachusetts General Hospital, Boston, and associates limited their analysis to 2,869 patients with RA who had been treated with either a biologic or targeted synthetic disease-modifying antirheumatic drug (b/tsDMARD) at the time they were diagnosed with COVID-19.
“We wanted to limit it to a single disease and also limit it to drugs that are considered for that disease,” Dr. Sparks explained in an interview.
“Because patients with rheumatoid arthritis are often treated sequentially, we wanted to further limit the analysis to patients who were on advanced therapies so that they were at a similar disease state, and also had the opportunity to receive advanced therapies.”
This approach hopefully minimizes the possibility of confounding by indication, Dr. Sparks said.
Most of the patients included in the analysis had received a TNFi (n = 1,388), and they were used as the control arm of the analysis. Outcomes associated with treatment with the other b/tsDMARDs, which included abatacept (n = 237), rituximab (n = 364), interleukin-6 inhibitors (IL-6i; n = 317), and JAKi (n = 563), were then compared with TNFi.
Baseline characteristics of patients were broadly similar across the groups. The mean age was 56.7 years and 80.8% of the study population was female. There were a few expected differences among users of rituximab versus TNFi, notably a higher percentage of patients with interstitial lung disease (11% vs. 1.4% of TNFi users) or cancer (7.4% vs. 0.9%) among patients treated with rituximab since it is commonly used in these patients, Dr. Sparks said.
“We did perform a sensitivity analysis where we restricted the population to not having ILD or cancer and we actually found really similar findings,” he added.
Four COVID-19 outcomes assessed
The researchers used a four-point ordinal scale modeled after one set by the World Health Organization to assess four COVID-19 outcomes: not hospitalized, hospitalized without oxygenation, hospitalized with oxygenation or ventilation, and death.
Odds ratios (ORs) comparing rituximab to TNFi for these four COVID-19 outcomes were a respective 4.53, 2.87, 4.05, and 4.57. The ORs for JAKi versus TNFi were a respective 2.4, 1.55, 2.03, and 2.04.
“We found no consistent associations of abatacept or interleukin-6 inhibitors with COVID-19 severity, compared to TNF inhibitors,” which is reassuring, Dr. Sparks said.
ORs for the four COVID-19 outcomes with abatacept were a respective 1.18, 1.12, 1.41, and 1.46, and for IL-6i were 0.84, 0.72, 0.75, and 1.13.
Rituximab use in patients with RA who develop COVID-19
So, should rituximab be stopped in patients with RA if they develop COVID-19? “This is an important question and one that would be decided on a case-by-case basis,” Dr. Sparks said. “Of course, the drug has a very long half-life, so risk mitigation strategies are still of utmost importance,” he added.
“I think everyone’s a bit reticent to want to start rituximab in this environment, but it might also make me pause about starting a JAK inhibitor,” Dr. Sparks added. “Given that this is a first finding, I’m not sure I would necessarily change patients who are doing well on these medications. I think what it really makes me want to do is to try to obviously vaccinate the patients on JAK inhibitors as they do have a short half-life.”
More observational studies would be helpful, Dr. Sparks said, adding that “the most pressing need is to try to figure out how to protect our patients with rituximab.”
The COVID-19 Global Rheumatology Alliance Physician Registry is supported by the American College of Rheumatology and the European Alliance of Associations for Rheumatology. Dr. Sparks disclosed serving as a consultant for Bristol Myers Squibb, Gilead, Inova, Optum, and Pfizer for work unrelated to this study. Dr. Wallace disclosed receiving grant support from Bristol Myers Squibb and Principia/Sanofi and serving as a consultant for Viela Bio and Medpace for work unrelated to this study.
Patients with rheumatoid arthritis who were using rituximab at the time of COVID-19 onset had a fourfold higher risk of being hospitalized, needing mechanical ventilation, or dying, compared with patients taking a tumor necrosis factor inhibitor (TNFi), according to a report given at the annual European Congress of Rheumatology.
The use of Janus kinase inhibitors (JAKi) also was associated with a twofold higher risk for these COVID-19 outcomes, said Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, in presenting the analysis from the COVID-19 Global Rheumatology Alliance (GRA) Physician Registry.
“The strong association of rituximab and JAK inhibitor use with poor COVID-19 outcomes highlights the prioritization of risk mitigation strategies for these patients,” Dr. Sparks said at the meeting.
The full findings have now been published in Annals of the Rheumatic Diseases.
JAKi association questioned
These findings provide “an important understanding for the risk of our patients in times before vaccination,” said Hendrik Schulze-Koops, MD, of Ludwig Maximilian University of Munich, who chaired the session in which the study was presented.
However, “recently, baricitinib was licensed to prevent particular aspects of severe COVID. What’s the explanation for this discrepancy?” he asked.
“Certainly, the JAK inhibitor finding deserves further study,” Dr. Sparks acknowledged, adding that the data were analyzed by class rather than for individual drugs.
“One possible explanation could be when JAK inhibitors are used,” he suggested. “It might be different for patients who [have been] just infected – that might have different biologic effects – as opposed to choosing to treat patients right when there’s a hyperinflammatory cascade, or there’s oxygen need.”
Regarding the JAK inhibitor finding, Ronald van Vollenhoven, MD, PhD, of the University of Amsterdam, pointed out during the online Q&A that “JAKi have a very short half-life compared to biologics.”
Dr. van Vollenhoven asked: “Could the practice of stopping these drugs upon COVID infection have a negative impact on the course?” To which Dr. Sparks responded: “The different half-life of drugs would be a promising avenue to look at, to see whether increases in disease activity might have imparted some of the effects we saw.”
Performing the analysis
As of April 12, 2021, the GRA Physician Registry contained the records of more than 15,000 patients. Dr. Sparks, collaborator Zachary Wallace, MD, of Massachusetts General Hospital, Boston, and associates limited their analysis to 2,869 patients with RA who had been treated with either a biologic or targeted synthetic disease-modifying antirheumatic drug (b/tsDMARD) at the time they were diagnosed with COVID-19.
“We wanted to limit it to a single disease and also limit it to drugs that are considered for that disease,” Dr. Sparks explained in an interview.
“Because patients with rheumatoid arthritis are often treated sequentially, we wanted to further limit the analysis to patients who were on advanced therapies so that they were at a similar disease state, and also had the opportunity to receive advanced therapies.”
This approach hopefully minimizes the possibility of confounding by indication, Dr. Sparks said.
Most of the patients included in the analysis had received a TNFi (n = 1,388), and they were used as the control arm of the analysis. Outcomes associated with treatment with the other b/tsDMARDs, which included abatacept (n = 237), rituximab (n = 364), interleukin-6 inhibitors (IL-6i; n = 317), and JAKi (n = 563), were then compared with TNFi.
Baseline characteristics of patients were broadly similar across the groups. The mean age was 56.7 years and 80.8% of the study population was female. There were a few expected differences among users of rituximab versus TNFi, notably a higher percentage of patients with interstitial lung disease (11% vs. 1.4% of TNFi users) or cancer (7.4% vs. 0.9%) among patients treated with rituximab since it is commonly used in these patients, Dr. Sparks said.
“We did perform a sensitivity analysis where we restricted the population to not having ILD or cancer and we actually found really similar findings,” he added.
Four COVID-19 outcomes assessed
The researchers used a four-point ordinal scale modeled after one set by the World Health Organization to assess four COVID-19 outcomes: not hospitalized, hospitalized without oxygenation, hospitalized with oxygenation or ventilation, and death.
Odds ratios (ORs) comparing rituximab to TNFi for these four COVID-19 outcomes were a respective 4.53, 2.87, 4.05, and 4.57. The ORs for JAKi versus TNFi were a respective 2.4, 1.55, 2.03, and 2.04.
“We found no consistent associations of abatacept or interleukin-6 inhibitors with COVID-19 severity, compared to TNF inhibitors,” which is reassuring, Dr. Sparks said.
ORs for the four COVID-19 outcomes with abatacept were a respective 1.18, 1.12, 1.41, and 1.46, and for IL-6i were 0.84, 0.72, 0.75, and 1.13.
Rituximab use in patients with RA who develop COVID-19
So, should rituximab be stopped in patients with RA if they develop COVID-19? “This is an important question and one that would be decided on a case-by-case basis,” Dr. Sparks said. “Of course, the drug has a very long half-life, so risk mitigation strategies are still of utmost importance,” he added.
“I think everyone’s a bit reticent to want to start rituximab in this environment, but it might also make me pause about starting a JAK inhibitor,” Dr. Sparks added. “Given that this is a first finding, I’m not sure I would necessarily change patients who are doing well on these medications. I think what it really makes me want to do is to try to obviously vaccinate the patients on JAK inhibitors as they do have a short half-life.”
More observational studies would be helpful, Dr. Sparks said, adding that “the most pressing need is to try to figure out how to protect our patients with rituximab.”
The COVID-19 Global Rheumatology Alliance Physician Registry is supported by the American College of Rheumatology and the European Alliance of Associations for Rheumatology. Dr. Sparks disclosed serving as a consultant for Bristol Myers Squibb, Gilead, Inova, Optum, and Pfizer for work unrelated to this study. Dr. Wallace disclosed receiving grant support from Bristol Myers Squibb and Principia/Sanofi and serving as a consultant for Viela Bio and Medpace for work unrelated to this study.
Patients with rheumatoid arthritis who were using rituximab at the time of COVID-19 onset had a fourfold higher risk of being hospitalized, needing mechanical ventilation, or dying, compared with patients taking a tumor necrosis factor inhibitor (TNFi), according to a report given at the annual European Congress of Rheumatology.
The use of Janus kinase inhibitors (JAKi) also was associated with a twofold higher risk for these COVID-19 outcomes, said Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, in presenting the analysis from the COVID-19 Global Rheumatology Alliance (GRA) Physician Registry.
“The strong association of rituximab and JAK inhibitor use with poor COVID-19 outcomes highlights the prioritization of risk mitigation strategies for these patients,” Dr. Sparks said at the meeting.
The full findings have now been published in Annals of the Rheumatic Diseases.
JAKi association questioned
These findings provide “an important understanding for the risk of our patients in times before vaccination,” said Hendrik Schulze-Koops, MD, of Ludwig Maximilian University of Munich, who chaired the session in which the study was presented.
However, “recently, baricitinib was licensed to prevent particular aspects of severe COVID. What’s the explanation for this discrepancy?” he asked.
“Certainly, the JAK inhibitor finding deserves further study,” Dr. Sparks acknowledged, adding that the data were analyzed by class rather than for individual drugs.
“One possible explanation could be when JAK inhibitors are used,” he suggested. “It might be different for patients who [have been] just infected – that might have different biologic effects – as opposed to choosing to treat patients right when there’s a hyperinflammatory cascade, or there’s oxygen need.”
Regarding the JAK inhibitor finding, Ronald van Vollenhoven, MD, PhD, of the University of Amsterdam, pointed out during the online Q&A that “JAKi have a very short half-life compared to biologics.”
Dr. van Vollenhoven asked: “Could the practice of stopping these drugs upon COVID infection have a negative impact on the course?” To which Dr. Sparks responded: “The different half-life of drugs would be a promising avenue to look at, to see whether increases in disease activity might have imparted some of the effects we saw.”
Performing the analysis
As of April 12, 2021, the GRA Physician Registry contained the records of more than 15,000 patients. Dr. Sparks, collaborator Zachary Wallace, MD, of Massachusetts General Hospital, Boston, and associates limited their analysis to 2,869 patients with RA who had been treated with either a biologic or targeted synthetic disease-modifying antirheumatic drug (b/tsDMARD) at the time they were diagnosed with COVID-19.
“We wanted to limit it to a single disease and also limit it to drugs that are considered for that disease,” Dr. Sparks explained in an interview.
“Because patients with rheumatoid arthritis are often treated sequentially, we wanted to further limit the analysis to patients who were on advanced therapies so that they were at a similar disease state, and also had the opportunity to receive advanced therapies.”
This approach hopefully minimizes the possibility of confounding by indication, Dr. Sparks said.
Most of the patients included in the analysis had received a TNFi (n = 1,388), and they were used as the control arm of the analysis. Outcomes associated with treatment with the other b/tsDMARDs, which included abatacept (n = 237), rituximab (n = 364), interleukin-6 inhibitors (IL-6i; n = 317), and JAKi (n = 563), were then compared with TNFi.
Baseline characteristics of patients were broadly similar across the groups. The mean age was 56.7 years and 80.8% of the study population was female. There were a few expected differences among users of rituximab versus TNFi, notably a higher percentage of patients with interstitial lung disease (11% vs. 1.4% of TNFi users) or cancer (7.4% vs. 0.9%) among patients treated with rituximab since it is commonly used in these patients, Dr. Sparks said.
“We did perform a sensitivity analysis where we restricted the population to not having ILD or cancer and we actually found really similar findings,” he added.
Four COVID-19 outcomes assessed
The researchers used a four-point ordinal scale modeled after one set by the World Health Organization to assess four COVID-19 outcomes: not hospitalized, hospitalized without oxygenation, hospitalized with oxygenation or ventilation, and death.
Odds ratios (ORs) comparing rituximab to TNFi for these four COVID-19 outcomes were a respective 4.53, 2.87, 4.05, and 4.57. The ORs for JAKi versus TNFi were a respective 2.4, 1.55, 2.03, and 2.04.
“We found no consistent associations of abatacept or interleukin-6 inhibitors with COVID-19 severity, compared to TNF inhibitors,” which is reassuring, Dr. Sparks said.
ORs for the four COVID-19 outcomes with abatacept were a respective 1.18, 1.12, 1.41, and 1.46, and for IL-6i were 0.84, 0.72, 0.75, and 1.13.
Rituximab use in patients with RA who develop COVID-19
So, should rituximab be stopped in patients with RA if they develop COVID-19? “This is an important question and one that would be decided on a case-by-case basis,” Dr. Sparks said. “Of course, the drug has a very long half-life, so risk mitigation strategies are still of utmost importance,” he added.
“I think everyone’s a bit reticent to want to start rituximab in this environment, but it might also make me pause about starting a JAK inhibitor,” Dr. Sparks added. “Given that this is a first finding, I’m not sure I would necessarily change patients who are doing well on these medications. I think what it really makes me want to do is to try to obviously vaccinate the patients on JAK inhibitors as they do have a short half-life.”
More observational studies would be helpful, Dr. Sparks said, adding that “the most pressing need is to try to figure out how to protect our patients with rituximab.”
The COVID-19 Global Rheumatology Alliance Physician Registry is supported by the American College of Rheumatology and the European Alliance of Associations for Rheumatology. Dr. Sparks disclosed serving as a consultant for Bristol Myers Squibb, Gilead, Inova, Optum, and Pfizer for work unrelated to this study. Dr. Wallace disclosed receiving grant support from Bristol Myers Squibb and Principia/Sanofi and serving as a consultant for Viela Bio and Medpace for work unrelated to this study.
FROM THE EULAR 2021 CONGRESS