User login
Osteoporosis linked to increased risk of hearing loss
Women with osteoporosis, low bone density, or a previous vertebral fracture show significant increases in the risk of hearing loss compared to those without osteoporosis, according to a new study with more than 3 decades of follow-up.
The use of bisphosphonate therapy did not alter the risk, the researchers found.
“To the best of our knowledge, this is the first large longitudinal study to evaluate the relations of bone density, bisphosphonate use, fractures, and risk of hearing loss,” reported Sharon Curhan, MD, and colleagues in research published online in the Journal of the American Geriatric Society.
“In this large nationwide longitudinal study of nearly 144,000 women with up to 34 years of follow-up, we found that osteoporosis or low bone density was independently associated with higher risk of incident moderate or worse hearing loss,” the authors wrote.
“The magnitude of the elevated risk was similar among women who did and did not use bisphosphonates,” they added.
Participants were from the nurses’ health study and NHS II
With recent research suggesting a potential link between bisphosphonate use and prevention of noise-induced hearing loss in mice, Dr. Curhan, of the Channing Division of Network Medicine at Brigham and Women’s Hospital, Boston, and colleagues turned to the large longitudinal cohorts of the Nurses’ Health Study (NHS), conducted from 1982 to 2016, and the Nurses’ Health Study II (NHS II), from 1995 to 2017.
In total, the primary analysis included 60,821 women in the NHS and 83,078 in the NHS II.
Women in the NHS were aged 36-61 years at baseline and 70-95 years at the end of follow-up, while in the NHS II, women were aged 31-48 years at baseline and 53-70 years at the end of follow-up.
After multivariate adjustment for key factors including age, race/ethnicity, oral hormone use, and a variety of other factors, women in the NHS with osteoporosis had an increased risk of moderate or worse hearing loss, as self-reported every 2 years, compared to those without osteoporosis (relative risk, 1.14; 95% confidence interval, 1.09-1.19).
And in the NHS II, which also included data on low bone density, the risk of self-reported hearing loss was higher among those with osteoporosis or low bone density (RR, 1.30; 95% CI, 1.21-1.40).
No significant differences were observed in hearing loss risk based on whether women were treated with bisphosphonates, with the mean duration of use of the medication being 5.8 years in the NHS and 3.4 years in the NHS II.
Those who sustained a vertebral fracture also had a higher risk of hearing loss in both studies (NHS: RR, 1.31; NHS II: RR, 1.39).
However, the increased risk of hearing loss was not observed with hip fracture.
“Our findings of up to a 40% higher risk among women with vertebral fracture, but not hip fracture, were intriguing and merit further study,” the authors noted.
“The discordant findings between these skeletal sites may reflect differences in composition and metabolism of bones in the spine and hip and could provide insight into the pathophysiological changes in the ear that may lead to hearing loss,” they added.
Audiometric subanalysis
In an analysis of a subcohort of 3,749 women looking at audiometric thresholds for a more precise measure of hearing loss, women with osteoporosis or low bone density continued to show significantly worse hearing loss when treated with bisphosphonates compared to those without osteoporosis or low bone density.
However, there were no significant hearing loss differences among those with osteoporosis who did not take bisphosphonates versus those without osteoporosis.
The authors speculate that the use of bisphosphonates could have been indicative of more severe osteoporosis, hence the poorer audiometric thresholds.
In an interview, Dr. Curhan said the details of bisphosphonate use, such as type and duration, and their role in hearing loss should be further evaluated.
“Possibly, a potential influence of bisphosphonates on the relation of osteoporosis and hearing loss in humans may depend on the type, dose, and timing of bisphosphonate administration,” she observed. “This is an important question for further study.”
Mechanisms: Bone loss may extend to ear structures
In terms of the mechanisms linking osteoporosis itself to hearing loss, the authors noted that bone loss, in addition to compromising more prominent skeletal sites, could logically extend to bone-related structures in the ear.
“Bone mass at peripheral sites is correlated with bone mass at central sites, such as hip and spine, with correlation coefficients between 0.6 and 0.7,” they explained. “Plausibly, systemic bone demineralization could involve the temporal bone, the otic capsule, and the middle ear ossicles.”
They noted that hearing loss has been linked to other pathologic bone disorders, including otosclerosis and Paget disease.
Furthermore, imbalances in bone formation and resorption in osteoporosis may lead to alterations in ionic metabolism, which can lead to hearing loss.
Looking ahead, Dr. Curhan and colleagues plan to further examine whether calcium and vitamin D, which are associated with the prevention of osteoporosis, have a role in preventing hearing loss.
In the meantime, the findings underscore that clinicians treating patients with osteoporosis should routinely check patients’ hearing, Dr. Curhan said.
“Undetected and untreated hearing loss can adversely impact social interactions, physical and mental well-being, and daily life,” she said.
“Early detection of hearing loss offers greater opportunity for successful management and to learn strategies for rehabilitation and prevention of further progression.”
The study received support from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Women with osteoporosis, low bone density, or a previous vertebral fracture show significant increases in the risk of hearing loss compared to those without osteoporosis, according to a new study with more than 3 decades of follow-up.
The use of bisphosphonate therapy did not alter the risk, the researchers found.
“To the best of our knowledge, this is the first large longitudinal study to evaluate the relations of bone density, bisphosphonate use, fractures, and risk of hearing loss,” reported Sharon Curhan, MD, and colleagues in research published online in the Journal of the American Geriatric Society.
“In this large nationwide longitudinal study of nearly 144,000 women with up to 34 years of follow-up, we found that osteoporosis or low bone density was independently associated with higher risk of incident moderate or worse hearing loss,” the authors wrote.
“The magnitude of the elevated risk was similar among women who did and did not use bisphosphonates,” they added.
Participants were from the nurses’ health study and NHS II
With recent research suggesting a potential link between bisphosphonate use and prevention of noise-induced hearing loss in mice, Dr. Curhan, of the Channing Division of Network Medicine at Brigham and Women’s Hospital, Boston, and colleagues turned to the large longitudinal cohorts of the Nurses’ Health Study (NHS), conducted from 1982 to 2016, and the Nurses’ Health Study II (NHS II), from 1995 to 2017.
In total, the primary analysis included 60,821 women in the NHS and 83,078 in the NHS II.
Women in the NHS were aged 36-61 years at baseline and 70-95 years at the end of follow-up, while in the NHS II, women were aged 31-48 years at baseline and 53-70 years at the end of follow-up.
After multivariate adjustment for key factors including age, race/ethnicity, oral hormone use, and a variety of other factors, women in the NHS with osteoporosis had an increased risk of moderate or worse hearing loss, as self-reported every 2 years, compared to those without osteoporosis (relative risk, 1.14; 95% confidence interval, 1.09-1.19).
And in the NHS II, which also included data on low bone density, the risk of self-reported hearing loss was higher among those with osteoporosis or low bone density (RR, 1.30; 95% CI, 1.21-1.40).
No significant differences were observed in hearing loss risk based on whether women were treated with bisphosphonates, with the mean duration of use of the medication being 5.8 years in the NHS and 3.4 years in the NHS II.
Those who sustained a vertebral fracture also had a higher risk of hearing loss in both studies (NHS: RR, 1.31; NHS II: RR, 1.39).
However, the increased risk of hearing loss was not observed with hip fracture.
“Our findings of up to a 40% higher risk among women with vertebral fracture, but not hip fracture, were intriguing and merit further study,” the authors noted.
“The discordant findings between these skeletal sites may reflect differences in composition and metabolism of bones in the spine and hip and could provide insight into the pathophysiological changes in the ear that may lead to hearing loss,” they added.
Audiometric subanalysis
In an analysis of a subcohort of 3,749 women looking at audiometric thresholds for a more precise measure of hearing loss, women with osteoporosis or low bone density continued to show significantly worse hearing loss when treated with bisphosphonates compared to those without osteoporosis or low bone density.
However, there were no significant hearing loss differences among those with osteoporosis who did not take bisphosphonates versus those without osteoporosis.
The authors speculate that the use of bisphosphonates could have been indicative of more severe osteoporosis, hence the poorer audiometric thresholds.
In an interview, Dr. Curhan said the details of bisphosphonate use, such as type and duration, and their role in hearing loss should be further evaluated.
“Possibly, a potential influence of bisphosphonates on the relation of osteoporosis and hearing loss in humans may depend on the type, dose, and timing of bisphosphonate administration,” she observed. “This is an important question for further study.”
Mechanisms: Bone loss may extend to ear structures
In terms of the mechanisms linking osteoporosis itself to hearing loss, the authors noted that bone loss, in addition to compromising more prominent skeletal sites, could logically extend to bone-related structures in the ear.
“Bone mass at peripheral sites is correlated with bone mass at central sites, such as hip and spine, with correlation coefficients between 0.6 and 0.7,” they explained. “Plausibly, systemic bone demineralization could involve the temporal bone, the otic capsule, and the middle ear ossicles.”
They noted that hearing loss has been linked to other pathologic bone disorders, including otosclerosis and Paget disease.
Furthermore, imbalances in bone formation and resorption in osteoporosis may lead to alterations in ionic metabolism, which can lead to hearing loss.
Looking ahead, Dr. Curhan and colleagues plan to further examine whether calcium and vitamin D, which are associated with the prevention of osteoporosis, have a role in preventing hearing loss.
In the meantime, the findings underscore that clinicians treating patients with osteoporosis should routinely check patients’ hearing, Dr. Curhan said.
“Undetected and untreated hearing loss can adversely impact social interactions, physical and mental well-being, and daily life,” she said.
“Early detection of hearing loss offers greater opportunity for successful management and to learn strategies for rehabilitation and prevention of further progression.”
The study received support from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Women with osteoporosis, low bone density, or a previous vertebral fracture show significant increases in the risk of hearing loss compared to those without osteoporosis, according to a new study with more than 3 decades of follow-up.
The use of bisphosphonate therapy did not alter the risk, the researchers found.
“To the best of our knowledge, this is the first large longitudinal study to evaluate the relations of bone density, bisphosphonate use, fractures, and risk of hearing loss,” reported Sharon Curhan, MD, and colleagues in research published online in the Journal of the American Geriatric Society.
“In this large nationwide longitudinal study of nearly 144,000 women with up to 34 years of follow-up, we found that osteoporosis or low bone density was independently associated with higher risk of incident moderate or worse hearing loss,” the authors wrote.
“The magnitude of the elevated risk was similar among women who did and did not use bisphosphonates,” they added.
Participants were from the nurses’ health study and NHS II
With recent research suggesting a potential link between bisphosphonate use and prevention of noise-induced hearing loss in mice, Dr. Curhan, of the Channing Division of Network Medicine at Brigham and Women’s Hospital, Boston, and colleagues turned to the large longitudinal cohorts of the Nurses’ Health Study (NHS), conducted from 1982 to 2016, and the Nurses’ Health Study II (NHS II), from 1995 to 2017.
In total, the primary analysis included 60,821 women in the NHS and 83,078 in the NHS II.
Women in the NHS were aged 36-61 years at baseline and 70-95 years at the end of follow-up, while in the NHS II, women were aged 31-48 years at baseline and 53-70 years at the end of follow-up.
After multivariate adjustment for key factors including age, race/ethnicity, oral hormone use, and a variety of other factors, women in the NHS with osteoporosis had an increased risk of moderate or worse hearing loss, as self-reported every 2 years, compared to those without osteoporosis (relative risk, 1.14; 95% confidence interval, 1.09-1.19).
And in the NHS II, which also included data on low bone density, the risk of self-reported hearing loss was higher among those with osteoporosis or low bone density (RR, 1.30; 95% CI, 1.21-1.40).
No significant differences were observed in hearing loss risk based on whether women were treated with bisphosphonates, with the mean duration of use of the medication being 5.8 years in the NHS and 3.4 years in the NHS II.
Those who sustained a vertebral fracture also had a higher risk of hearing loss in both studies (NHS: RR, 1.31; NHS II: RR, 1.39).
However, the increased risk of hearing loss was not observed with hip fracture.
“Our findings of up to a 40% higher risk among women with vertebral fracture, but not hip fracture, were intriguing and merit further study,” the authors noted.
“The discordant findings between these skeletal sites may reflect differences in composition and metabolism of bones in the spine and hip and could provide insight into the pathophysiological changes in the ear that may lead to hearing loss,” they added.
Audiometric subanalysis
In an analysis of a subcohort of 3,749 women looking at audiometric thresholds for a more precise measure of hearing loss, women with osteoporosis or low bone density continued to show significantly worse hearing loss when treated with bisphosphonates compared to those without osteoporosis or low bone density.
However, there were no significant hearing loss differences among those with osteoporosis who did not take bisphosphonates versus those without osteoporosis.
The authors speculate that the use of bisphosphonates could have been indicative of more severe osteoporosis, hence the poorer audiometric thresholds.
In an interview, Dr. Curhan said the details of bisphosphonate use, such as type and duration, and their role in hearing loss should be further evaluated.
“Possibly, a potential influence of bisphosphonates on the relation of osteoporosis and hearing loss in humans may depend on the type, dose, and timing of bisphosphonate administration,” she observed. “This is an important question for further study.”
Mechanisms: Bone loss may extend to ear structures
In terms of the mechanisms linking osteoporosis itself to hearing loss, the authors noted that bone loss, in addition to compromising more prominent skeletal sites, could logically extend to bone-related structures in the ear.
“Bone mass at peripheral sites is correlated with bone mass at central sites, such as hip and spine, with correlation coefficients between 0.6 and 0.7,” they explained. “Plausibly, systemic bone demineralization could involve the temporal bone, the otic capsule, and the middle ear ossicles.”
They noted that hearing loss has been linked to other pathologic bone disorders, including otosclerosis and Paget disease.
Furthermore, imbalances in bone formation and resorption in osteoporosis may lead to alterations in ionic metabolism, which can lead to hearing loss.
Looking ahead, Dr. Curhan and colleagues plan to further examine whether calcium and vitamin D, which are associated with the prevention of osteoporosis, have a role in preventing hearing loss.
In the meantime, the findings underscore that clinicians treating patients with osteoporosis should routinely check patients’ hearing, Dr. Curhan said.
“Undetected and untreated hearing loss can adversely impact social interactions, physical and mental well-being, and daily life,” she said.
“Early detection of hearing loss offers greater opportunity for successful management and to learn strategies for rehabilitation and prevention of further progression.”
The study received support from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Combination therapy may benefit patients with migraine
, according to a large retrospective analysis. The results lend hope that the combination may be synergistic, according to Andrew Blumenfeld, MD, director of the Headache Center of Southern California in Carlsbad. Dr. Blumenfeld presented at the American Headache Society’s 2021 annual meeting. The study was published online April 21 in Pain Therapy.
The retrospective analysis showed a 4-day reduction in headache days per month. In contrast, in the pivotal study for erenumab, the most commonly used anti-CGRP antibody among subjects in the study, showed a 2-day benefit in a subanalysis of patients who had failed at least two oral preventives.
There is mechanistic evidence to suggest the two therapies could be synergistic. OnabotulinumtoxinA is believed to inhibit the release of CGRP, and antibodies reduce CGRP levels. OnabotulinumtoxinA prevents activation of C-fibers in the trigeminal sensory afferents, but does not affect A-delta fibers.
On the other hand, most data indicate that the anti-CGRP antibody fremanezumab prevents activation of A-delta but not C-fibers, and a recent review argues that CGRP antibody nonresponders may have migraines driven by C-fibers or other pathways. “Thus, concomitant use of medications blocking the activation of meningeal C-fibers may provide a synergistic effect on the trigeminal nociceptive pathway,” the authors wrote.
Study finding match clinical practice
The results of the new study strengthen the case that the combination is effective, though proof would require prospective, randomized trials. “I think that it really gives credibility to what we are seeing in practice, which is that combined therapy often is much better than therapy with onabotulinumtoxinA alone, said Deborah Friedman, MD, MPH, who was asked to comment on the findings. Dr. Friedman is professor of neurology and ophthalmology at the University of Texas, Dallas.
The extra 4 migraine-free days per month is a significant benefit, said Stewart Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H. “It’s an extra month and a half of no disability per year, and that’s on top of what onabotulinumtoxinA does. So it’s really a very important clinical finding,” Dr. Tepper said in an interview.
Many insurance companies refuse to pay for the combination therapy, despite the fact that relatively few migraine patients would likely seek it out, according to Dr. Friedman. “It’s just kind of a shame,” she said.
Insurance companies often object that the combination therapy is experimental, despite the widespread use of combination therapies in migraine. “It’s no more experimental in my opinion than any other combination of medications that we use. For people that have severe migraine, we use combination therapy all the time,” said Dr. Friedman.
Improvements with combination therapy
The study was a chart review of 257 patients who started on onabotulinumtoxinA and later initiated anti-CGRP antibody therapy. A total of 104 completed four visits after initiation of anti-CGRP antibody therapy (completers). Before starting any therapy, patients reported an average of 21 headache days per month in the overall group, and 22 among completers. That frequency dropped to 12 in both groups after onabotulinumtoxinA therapy (overall group difference, –9 days; 95% confidence interval, –8 to –11 days; completers group difference, –10; 95% CI, –7 to –12 days).
A total of 77.8% of subjects in the overall cohort took erenumab, 16.3% took galcanezumab, and 5.8% took fremanezumab. In the completers cohort, the percentages were 84.5%, 10.7%, and 4.9%, respectively.
Compared with baseline, both completers and noncompleters had clinically significant improvements in disability, as measured by at least a 5-point improvement in Migraine Disability Assessment (MIDAS) score at the 3-month visit (–5.8 for completers and –6.3 for the overall cohort group), the 6-month visit (–6.6 and –11.1), the 9-month visit (–8.3 and –6.1), and 1 year (–12.7 and –8.4).
At the first visit, 33.0% of completers had at least a 5-point reduction in MIDAS, as did 36.0% of the overall cohort group, and the trend continued at 6 months (39.8% and 45.1%), 9 months (43.7% and 43.7%), and at 1 year (45.3% and 44.8%).
The study was funded by Allergan. Dr. Blumenfeld has served on advisory boards for Aeon, AbbVie, Amgen, Alder, Biohaven, Teva, Supernus, Promius, Eaglet, and Lilly, and has received funding for speaking from AbbVie, Amgen, Pernix, Supernus, Depomed, Avanir, Promius, Teva, Eli Lilly, Lundbeck, Novartis, and Theranica. Dr. Tepper has consulted for Teva. Dr. Friedman has been on the advisory board for Allergan, Amgen, Lundbeck, Eli Lilly, and Teva Pharmaceuticals, and has received grant support from Allergan and Eli Lilly.
, according to a large retrospective analysis. The results lend hope that the combination may be synergistic, according to Andrew Blumenfeld, MD, director of the Headache Center of Southern California in Carlsbad. Dr. Blumenfeld presented at the American Headache Society’s 2021 annual meeting. The study was published online April 21 in Pain Therapy.
The retrospective analysis showed a 4-day reduction in headache days per month. In contrast, in the pivotal study for erenumab, the most commonly used anti-CGRP antibody among subjects in the study, showed a 2-day benefit in a subanalysis of patients who had failed at least two oral preventives.
There is mechanistic evidence to suggest the two therapies could be synergistic. OnabotulinumtoxinA is believed to inhibit the release of CGRP, and antibodies reduce CGRP levels. OnabotulinumtoxinA prevents activation of C-fibers in the trigeminal sensory afferents, but does not affect A-delta fibers.
On the other hand, most data indicate that the anti-CGRP antibody fremanezumab prevents activation of A-delta but not C-fibers, and a recent review argues that CGRP antibody nonresponders may have migraines driven by C-fibers or other pathways. “Thus, concomitant use of medications blocking the activation of meningeal C-fibers may provide a synergistic effect on the trigeminal nociceptive pathway,” the authors wrote.
Study finding match clinical practice
The results of the new study strengthen the case that the combination is effective, though proof would require prospective, randomized trials. “I think that it really gives credibility to what we are seeing in practice, which is that combined therapy often is much better than therapy with onabotulinumtoxinA alone, said Deborah Friedman, MD, MPH, who was asked to comment on the findings. Dr. Friedman is professor of neurology and ophthalmology at the University of Texas, Dallas.
The extra 4 migraine-free days per month is a significant benefit, said Stewart Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H. “It’s an extra month and a half of no disability per year, and that’s on top of what onabotulinumtoxinA does. So it’s really a very important clinical finding,” Dr. Tepper said in an interview.
Many insurance companies refuse to pay for the combination therapy, despite the fact that relatively few migraine patients would likely seek it out, according to Dr. Friedman. “It’s just kind of a shame,” she said.
Insurance companies often object that the combination therapy is experimental, despite the widespread use of combination therapies in migraine. “It’s no more experimental in my opinion than any other combination of medications that we use. For people that have severe migraine, we use combination therapy all the time,” said Dr. Friedman.
Improvements with combination therapy
The study was a chart review of 257 patients who started on onabotulinumtoxinA and later initiated anti-CGRP antibody therapy. A total of 104 completed four visits after initiation of anti-CGRP antibody therapy (completers). Before starting any therapy, patients reported an average of 21 headache days per month in the overall group, and 22 among completers. That frequency dropped to 12 in both groups after onabotulinumtoxinA therapy (overall group difference, –9 days; 95% confidence interval, –8 to –11 days; completers group difference, –10; 95% CI, –7 to –12 days).
A total of 77.8% of subjects in the overall cohort took erenumab, 16.3% took galcanezumab, and 5.8% took fremanezumab. In the completers cohort, the percentages were 84.5%, 10.7%, and 4.9%, respectively.
Compared with baseline, both completers and noncompleters had clinically significant improvements in disability, as measured by at least a 5-point improvement in Migraine Disability Assessment (MIDAS) score at the 3-month visit (–5.8 for completers and –6.3 for the overall cohort group), the 6-month visit (–6.6 and –11.1), the 9-month visit (–8.3 and –6.1), and 1 year (–12.7 and –8.4).
At the first visit, 33.0% of completers had at least a 5-point reduction in MIDAS, as did 36.0% of the overall cohort group, and the trend continued at 6 months (39.8% and 45.1%), 9 months (43.7% and 43.7%), and at 1 year (45.3% and 44.8%).
The study was funded by Allergan. Dr. Blumenfeld has served on advisory boards for Aeon, AbbVie, Amgen, Alder, Biohaven, Teva, Supernus, Promius, Eaglet, and Lilly, and has received funding for speaking from AbbVie, Amgen, Pernix, Supernus, Depomed, Avanir, Promius, Teva, Eli Lilly, Lundbeck, Novartis, and Theranica. Dr. Tepper has consulted for Teva. Dr. Friedman has been on the advisory board for Allergan, Amgen, Lundbeck, Eli Lilly, and Teva Pharmaceuticals, and has received grant support from Allergan and Eli Lilly.
, according to a large retrospective analysis. The results lend hope that the combination may be synergistic, according to Andrew Blumenfeld, MD, director of the Headache Center of Southern California in Carlsbad. Dr. Blumenfeld presented at the American Headache Society’s 2021 annual meeting. The study was published online April 21 in Pain Therapy.
The retrospective analysis showed a 4-day reduction in headache days per month. In contrast, in the pivotal study for erenumab, the most commonly used anti-CGRP antibody among subjects in the study, showed a 2-day benefit in a subanalysis of patients who had failed at least two oral preventives.
There is mechanistic evidence to suggest the two therapies could be synergistic. OnabotulinumtoxinA is believed to inhibit the release of CGRP, and antibodies reduce CGRP levels. OnabotulinumtoxinA prevents activation of C-fibers in the trigeminal sensory afferents, but does not affect A-delta fibers.
On the other hand, most data indicate that the anti-CGRP antibody fremanezumab prevents activation of A-delta but not C-fibers, and a recent review argues that CGRP antibody nonresponders may have migraines driven by C-fibers or other pathways. “Thus, concomitant use of medications blocking the activation of meningeal C-fibers may provide a synergistic effect on the trigeminal nociceptive pathway,” the authors wrote.
Study finding match clinical practice
The results of the new study strengthen the case that the combination is effective, though proof would require prospective, randomized trials. “I think that it really gives credibility to what we are seeing in practice, which is that combined therapy often is much better than therapy with onabotulinumtoxinA alone, said Deborah Friedman, MD, MPH, who was asked to comment on the findings. Dr. Friedman is professor of neurology and ophthalmology at the University of Texas, Dallas.
The extra 4 migraine-free days per month is a significant benefit, said Stewart Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H. “It’s an extra month and a half of no disability per year, and that’s on top of what onabotulinumtoxinA does. So it’s really a very important clinical finding,” Dr. Tepper said in an interview.
Many insurance companies refuse to pay for the combination therapy, despite the fact that relatively few migraine patients would likely seek it out, according to Dr. Friedman. “It’s just kind of a shame,” she said.
Insurance companies often object that the combination therapy is experimental, despite the widespread use of combination therapies in migraine. “It’s no more experimental in my opinion than any other combination of medications that we use. For people that have severe migraine, we use combination therapy all the time,” said Dr. Friedman.
Improvements with combination therapy
The study was a chart review of 257 patients who started on onabotulinumtoxinA and later initiated anti-CGRP antibody therapy. A total of 104 completed four visits after initiation of anti-CGRP antibody therapy (completers). Before starting any therapy, patients reported an average of 21 headache days per month in the overall group, and 22 among completers. That frequency dropped to 12 in both groups after onabotulinumtoxinA therapy (overall group difference, –9 days; 95% confidence interval, –8 to –11 days; completers group difference, –10; 95% CI, –7 to –12 days).
A total of 77.8% of subjects in the overall cohort took erenumab, 16.3% took galcanezumab, and 5.8% took fremanezumab. In the completers cohort, the percentages were 84.5%, 10.7%, and 4.9%, respectively.
Compared with baseline, both completers and noncompleters had clinically significant improvements in disability, as measured by at least a 5-point improvement in Migraine Disability Assessment (MIDAS) score at the 3-month visit (–5.8 for completers and –6.3 for the overall cohort group), the 6-month visit (–6.6 and –11.1), the 9-month visit (–8.3 and –6.1), and 1 year (–12.7 and –8.4).
At the first visit, 33.0% of completers had at least a 5-point reduction in MIDAS, as did 36.0% of the overall cohort group, and the trend continued at 6 months (39.8% and 45.1%), 9 months (43.7% and 43.7%), and at 1 year (45.3% and 44.8%).
The study was funded by Allergan. Dr. Blumenfeld has served on advisory boards for Aeon, AbbVie, Amgen, Alder, Biohaven, Teva, Supernus, Promius, Eaglet, and Lilly, and has received funding for speaking from AbbVie, Amgen, Pernix, Supernus, Depomed, Avanir, Promius, Teva, Eli Lilly, Lundbeck, Novartis, and Theranica. Dr. Tepper has consulted for Teva. Dr. Friedman has been on the advisory board for Allergan, Amgen, Lundbeck, Eli Lilly, and Teva Pharmaceuticals, and has received grant support from Allergan and Eli Lilly.
FROM AHS 2021
Improving racial and gender equity in pediatric HM programs
Converge 2021 session
Racial and Gender Equity in Your PHM Program
Presenters
Jorge Ganem, MD, FAAP, and Vanessa N. Durand, DO, FAAP
Session summary
Dr. Ganem, associate professor of pediatrics at the University of Texas at Austin and director of pediatric hospital medicine at Dell Children’s Medical Center, and Dr. Durand, assistant professor of pediatrics at Drexel University and pediatric hospitalist at St. Christopher’s Hospital for Children, Philadelphia, presented an engaging session regarding gender equity in the workplace during SHM Converge 2021.
Dr. Ganem and Dr. Durand first presented data to illustrate the gender equity problem. They touched on the mental burden underrepresented minorities face professionally. Dr. Ganem and Dr. Durand discussed cognitive biases, defined allyship, sponsorship, and mentorship and shared how to distinguish between the three. They concluded their session with concrete ways to narrow gaps in equity in hospital medicine programs.
The highlights of this session included evidence-based “best-practices” that pediatric hospital medicine divisions can adopt. One important theme was regarding metrics. Dr. Ganem and Dr. Durand shared how important it is to evaluate divisions for pay and diversity gaps. Armed with these data, programs can be more effective in developing solutions. Some solutions provided by the presenters included “blind” interviews where traditional “cognitive metrics” (i.e., board scores) are not shared with interviewers to minimize anchoring and confirmation biases. Instead, interviewers should focus on the experiences and attributes of the job that the applicant can hopefully embody. This could be accomplished using a holistic review tool from the Association of American Medical Colleges.
One of the most powerful ideas shared in this session was a quote from a Harvard student shown in a video regarding bias and racism where he said, “Nothing in all the world is more dangerous than sincere ignorance and conscious stupidity.” Changes will only happen if we make them happen.
Key takeaways
- Racial and gender equity are problems that are undeniable, even in pediatrics.
- Be wary of conscious biases and the mental burden placed unfairly on underrepresented minorities in your institution.
- Becoming an amplifier, a sponsor, or a champion are ways to make a small individual difference.
- Measure your program’s data and commit to making change using evidence-based actions and assessments aimed at decreasing bias and increasing equity.
References
Association of American Medical Colleges. Holistic Review. 2021. www.aamc.org/services/member-capacity-building/holistic-review.
Dr. Singh is a board-certified pediatric hospitalist at Stanford University and Lucile Packard Children’s Hospital Stanford, both in Palo Alto, Calif. He is a native Texan living in the San Francisco Bay area with his wife and two young boys. His nonclinical passions include bedside communication and inpatient health care information technology.
Converge 2021 session
Racial and Gender Equity in Your PHM Program
Presenters
Jorge Ganem, MD, FAAP, and Vanessa N. Durand, DO, FAAP
Session summary
Dr. Ganem, associate professor of pediatrics at the University of Texas at Austin and director of pediatric hospital medicine at Dell Children’s Medical Center, and Dr. Durand, assistant professor of pediatrics at Drexel University and pediatric hospitalist at St. Christopher’s Hospital for Children, Philadelphia, presented an engaging session regarding gender equity in the workplace during SHM Converge 2021.
Dr. Ganem and Dr. Durand first presented data to illustrate the gender equity problem. They touched on the mental burden underrepresented minorities face professionally. Dr. Ganem and Dr. Durand discussed cognitive biases, defined allyship, sponsorship, and mentorship and shared how to distinguish between the three. They concluded their session with concrete ways to narrow gaps in equity in hospital medicine programs.
The highlights of this session included evidence-based “best-practices” that pediatric hospital medicine divisions can adopt. One important theme was regarding metrics. Dr. Ganem and Dr. Durand shared how important it is to evaluate divisions for pay and diversity gaps. Armed with these data, programs can be more effective in developing solutions. Some solutions provided by the presenters included “blind” interviews where traditional “cognitive metrics” (i.e., board scores) are not shared with interviewers to minimize anchoring and confirmation biases. Instead, interviewers should focus on the experiences and attributes of the job that the applicant can hopefully embody. This could be accomplished using a holistic review tool from the Association of American Medical Colleges.
One of the most powerful ideas shared in this session was a quote from a Harvard student shown in a video regarding bias and racism where he said, “Nothing in all the world is more dangerous than sincere ignorance and conscious stupidity.” Changes will only happen if we make them happen.
Key takeaways
- Racial and gender equity are problems that are undeniable, even in pediatrics.
- Be wary of conscious biases and the mental burden placed unfairly on underrepresented minorities in your institution.
- Becoming an amplifier, a sponsor, or a champion are ways to make a small individual difference.
- Measure your program’s data and commit to making change using evidence-based actions and assessments aimed at decreasing bias and increasing equity.
References
Association of American Medical Colleges. Holistic Review. 2021. www.aamc.org/services/member-capacity-building/holistic-review.
Dr. Singh is a board-certified pediatric hospitalist at Stanford University and Lucile Packard Children’s Hospital Stanford, both in Palo Alto, Calif. He is a native Texan living in the San Francisco Bay area with his wife and two young boys. His nonclinical passions include bedside communication and inpatient health care information technology.
Converge 2021 session
Racial and Gender Equity in Your PHM Program
Presenters
Jorge Ganem, MD, FAAP, and Vanessa N. Durand, DO, FAAP
Session summary
Dr. Ganem, associate professor of pediatrics at the University of Texas at Austin and director of pediatric hospital medicine at Dell Children’s Medical Center, and Dr. Durand, assistant professor of pediatrics at Drexel University and pediatric hospitalist at St. Christopher’s Hospital for Children, Philadelphia, presented an engaging session regarding gender equity in the workplace during SHM Converge 2021.
Dr. Ganem and Dr. Durand first presented data to illustrate the gender equity problem. They touched on the mental burden underrepresented minorities face professionally. Dr. Ganem and Dr. Durand discussed cognitive biases, defined allyship, sponsorship, and mentorship and shared how to distinguish between the three. They concluded their session with concrete ways to narrow gaps in equity in hospital medicine programs.
The highlights of this session included evidence-based “best-practices” that pediatric hospital medicine divisions can adopt. One important theme was regarding metrics. Dr. Ganem and Dr. Durand shared how important it is to evaluate divisions for pay and diversity gaps. Armed with these data, programs can be more effective in developing solutions. Some solutions provided by the presenters included “blind” interviews where traditional “cognitive metrics” (i.e., board scores) are not shared with interviewers to minimize anchoring and confirmation biases. Instead, interviewers should focus on the experiences and attributes of the job that the applicant can hopefully embody. This could be accomplished using a holistic review tool from the Association of American Medical Colleges.
One of the most powerful ideas shared in this session was a quote from a Harvard student shown in a video regarding bias and racism where he said, “Nothing in all the world is more dangerous than sincere ignorance and conscious stupidity.” Changes will only happen if we make them happen.
Key takeaways
- Racial and gender equity are problems that are undeniable, even in pediatrics.
- Be wary of conscious biases and the mental burden placed unfairly on underrepresented minorities in your institution.
- Becoming an amplifier, a sponsor, or a champion are ways to make a small individual difference.
- Measure your program’s data and commit to making change using evidence-based actions and assessments aimed at decreasing bias and increasing equity.
References
Association of American Medical Colleges. Holistic Review. 2021. www.aamc.org/services/member-capacity-building/holistic-review.
Dr. Singh is a board-certified pediatric hospitalist at Stanford University and Lucile Packard Children’s Hospital Stanford, both in Palo Alto, Calif. He is a native Texan living in the San Francisco Bay area with his wife and two young boys. His nonclinical passions include bedside communication and inpatient health care information technology.
FROM SHM CONVERGE 2021
Schizophrenia meds a key contributor to cognitive impairment
Anticholinergic medication burden from antipsychotics, antidepressants, and other psychotropics has a cumulative effect of worsening cognitive function in patients with schizophrenia, new research indicates.
“The link between long-term use of anticholinergic medications and cognitive impairment is well-known and growing,” lead researcher Yash Joshi, MD, department of psychiatry, University of California, San Diego, said in an interview.
“While this association is relevant for everyone, it is particularly important for those living with schizophrenia, who often struggle with cognitive difficulties conferred by the illness itself,” said Dr. Joshi.
“Brain health in schizophrenia is a game of inches, and even small negative effects on cognitive functioning through anticholinergic medication burden may have large impacts on patients’ lives,” he added.
The study was published online May 14 in the American Journal of Psychiatry.
‘Striking’ results
Dr. Joshi and colleagues set out to comprehensively characterize how the cumulative anticholinergic burden from different classes of medications affect cognition in patients with schizophrenia.
They assessed medical records, including all prescribed medications, for 1,120 adults with a diagnosis of schizophrenia or schizoaffective disorder.
For each participant, prescribed medications were rated and summed using a modified anticholinergic cognitive burden (ACB) scale. Cognitive functioning was assessed by performance on domains of the Penn Computerized Neurocognitive Battery (PCNB).
The investigators found that 63% of participants had an ACB score of at least 3, which is “striking,” said Dr. Joshi, given that previous studies have shown that an ACB score of 3 in a healthy, older adult is associated with cognitive dysfunction and a 50% increased risk of developing dementia.
About one-quarter of participants had an ACB score of 6 or higher.
Yet, these high ACB scores are not hard to achieve in routine psychiatric care, the researchers note.
For example, a patient taking olanzapine daily to ease symptoms of psychosis would have an ACB score of 3; if hydroxyzine was added for anxiety or insomnia, the patient’s ACB score would rise to 6, they point out.
Lightening the load
Antipsychotics contributed more than half of the anticholinergic burden, while traditional anticholinergics, antidepressants, mood stabilizers, and benzodiazepines accounted for the remainder.
“It is easy even for well-meaning clinicians to inadvertently contribute to anticholinergic medication burden through routine and appropriate care. The unique finding here is that this burden comes from medications we don’t usually think of as typical anticholinergic agents,” senior author Gregory Light, PhD, with University of California, San Diego, said in a news release.
Anticholinergic medication burden was significantly associated with generalized impairments in cognitive functioning across all cognitive domains on the PCNB with comparable magnitude and after controlling for multiple proxies of functioning or disease severity.
Higher anticholinergic medication burden was associated with worse cognitive performance. The PCNB global cognitive averages for none, low, average, high, and very high anticholinergic burdens were, respectively (in z values), -0.51, -0.70, -0.85, -0.96, and -1.15.
The results suggest “total cumulative anticholinergic burden – rather than anticholinergic burden attributable to a specific antipsychotic or psychotropic medication class – is a key contributor to cognitive impairment in schizophrenia,” the researchers write.
“The results imply that if it is clinically safe and practical,” said Dr. Joshi.
“This may be accomplished by reducing overall polypharmacy or transitioning to equivalent medications with lower overall anticholinergic burden. While ‘traditional’ anticholinergic medications should always be scrutinized, all medications should be carefully evaluated to understand whether they contribute to cumulative anticholinergic medication burden,” he added.
Confirmatory findings
Commenting on the study for this news organization, Jessica Gannon, MD, assistant professor of psychiatry, University of Pittsburgh, said the author’s findings “aren’t surprising, but the work that they did was pretty comprehensive [and] further fleshed out some of our concerns about the impact of anticholinergics on cognitive function in patients with schizophrenia.”
“We certainly have to use some of these medications for patients, like antipsychotics that do have some anticholinergic burden associated with them. We don’t really have other options,” Dr. Gannon said.
“But certainly I think this calls us to be better stewards of medication in general. And when we prescribe for comorbid conditions, like depression and anxiety, we should be careful in our prescribing practices, try not to prescribe an anticholinergic medication, and, if they have been prescribed, to deprescribe them,” Dr. Gannon added.
The study was supported by grants from the National Institute of Mental Health; the Sidney R. Baer, Jr. Foundation; the Brain and Behavior Research Foundation; the VISN-22 Mental Illness Research, Education, and Clinical Center; and the Department of Veterans Affairs. Dr. Joshi and Dr. Gannon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Anticholinergic medication burden from antipsychotics, antidepressants, and other psychotropics has a cumulative effect of worsening cognitive function in patients with schizophrenia, new research indicates.
“The link between long-term use of anticholinergic medications and cognitive impairment is well-known and growing,” lead researcher Yash Joshi, MD, department of psychiatry, University of California, San Diego, said in an interview.
“While this association is relevant for everyone, it is particularly important for those living with schizophrenia, who often struggle with cognitive difficulties conferred by the illness itself,” said Dr. Joshi.
“Brain health in schizophrenia is a game of inches, and even small negative effects on cognitive functioning through anticholinergic medication burden may have large impacts on patients’ lives,” he added.
The study was published online May 14 in the American Journal of Psychiatry.
‘Striking’ results
Dr. Joshi and colleagues set out to comprehensively characterize how the cumulative anticholinergic burden from different classes of medications affect cognition in patients with schizophrenia.
They assessed medical records, including all prescribed medications, for 1,120 adults with a diagnosis of schizophrenia or schizoaffective disorder.
For each participant, prescribed medications were rated and summed using a modified anticholinergic cognitive burden (ACB) scale. Cognitive functioning was assessed by performance on domains of the Penn Computerized Neurocognitive Battery (PCNB).
The investigators found that 63% of participants had an ACB score of at least 3, which is “striking,” said Dr. Joshi, given that previous studies have shown that an ACB score of 3 in a healthy, older adult is associated with cognitive dysfunction and a 50% increased risk of developing dementia.
About one-quarter of participants had an ACB score of 6 or higher.
Yet, these high ACB scores are not hard to achieve in routine psychiatric care, the researchers note.
For example, a patient taking olanzapine daily to ease symptoms of psychosis would have an ACB score of 3; if hydroxyzine was added for anxiety or insomnia, the patient’s ACB score would rise to 6, they point out.
Lightening the load
Antipsychotics contributed more than half of the anticholinergic burden, while traditional anticholinergics, antidepressants, mood stabilizers, and benzodiazepines accounted for the remainder.
“It is easy even for well-meaning clinicians to inadvertently contribute to anticholinergic medication burden through routine and appropriate care. The unique finding here is that this burden comes from medications we don’t usually think of as typical anticholinergic agents,” senior author Gregory Light, PhD, with University of California, San Diego, said in a news release.
Anticholinergic medication burden was significantly associated with generalized impairments in cognitive functioning across all cognitive domains on the PCNB with comparable magnitude and after controlling for multiple proxies of functioning or disease severity.
Higher anticholinergic medication burden was associated with worse cognitive performance. The PCNB global cognitive averages for none, low, average, high, and very high anticholinergic burdens were, respectively (in z values), -0.51, -0.70, -0.85, -0.96, and -1.15.
The results suggest “total cumulative anticholinergic burden – rather than anticholinergic burden attributable to a specific antipsychotic or psychotropic medication class – is a key contributor to cognitive impairment in schizophrenia,” the researchers write.
“The results imply that if it is clinically safe and practical,” said Dr. Joshi.
“This may be accomplished by reducing overall polypharmacy or transitioning to equivalent medications with lower overall anticholinergic burden. While ‘traditional’ anticholinergic medications should always be scrutinized, all medications should be carefully evaluated to understand whether they contribute to cumulative anticholinergic medication burden,” he added.
Confirmatory findings
Commenting on the study for this news organization, Jessica Gannon, MD, assistant professor of psychiatry, University of Pittsburgh, said the author’s findings “aren’t surprising, but the work that they did was pretty comprehensive [and] further fleshed out some of our concerns about the impact of anticholinergics on cognitive function in patients with schizophrenia.”
“We certainly have to use some of these medications for patients, like antipsychotics that do have some anticholinergic burden associated with them. We don’t really have other options,” Dr. Gannon said.
“But certainly I think this calls us to be better stewards of medication in general. And when we prescribe for comorbid conditions, like depression and anxiety, we should be careful in our prescribing practices, try not to prescribe an anticholinergic medication, and, if they have been prescribed, to deprescribe them,” Dr. Gannon added.
The study was supported by grants from the National Institute of Mental Health; the Sidney R. Baer, Jr. Foundation; the Brain and Behavior Research Foundation; the VISN-22 Mental Illness Research, Education, and Clinical Center; and the Department of Veterans Affairs. Dr. Joshi and Dr. Gannon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Anticholinergic medication burden from antipsychotics, antidepressants, and other psychotropics has a cumulative effect of worsening cognitive function in patients with schizophrenia, new research indicates.
“The link between long-term use of anticholinergic medications and cognitive impairment is well-known and growing,” lead researcher Yash Joshi, MD, department of psychiatry, University of California, San Diego, said in an interview.
“While this association is relevant for everyone, it is particularly important for those living with schizophrenia, who often struggle with cognitive difficulties conferred by the illness itself,” said Dr. Joshi.
“Brain health in schizophrenia is a game of inches, and even small negative effects on cognitive functioning through anticholinergic medication burden may have large impacts on patients’ lives,” he added.
The study was published online May 14 in the American Journal of Psychiatry.
‘Striking’ results
Dr. Joshi and colleagues set out to comprehensively characterize how the cumulative anticholinergic burden from different classes of medications affect cognition in patients with schizophrenia.
They assessed medical records, including all prescribed medications, for 1,120 adults with a diagnosis of schizophrenia or schizoaffective disorder.
For each participant, prescribed medications were rated and summed using a modified anticholinergic cognitive burden (ACB) scale. Cognitive functioning was assessed by performance on domains of the Penn Computerized Neurocognitive Battery (PCNB).
The investigators found that 63% of participants had an ACB score of at least 3, which is “striking,” said Dr. Joshi, given that previous studies have shown that an ACB score of 3 in a healthy, older adult is associated with cognitive dysfunction and a 50% increased risk of developing dementia.
About one-quarter of participants had an ACB score of 6 or higher.
Yet, these high ACB scores are not hard to achieve in routine psychiatric care, the researchers note.
For example, a patient taking olanzapine daily to ease symptoms of psychosis would have an ACB score of 3; if hydroxyzine was added for anxiety or insomnia, the patient’s ACB score would rise to 6, they point out.
Lightening the load
Antipsychotics contributed more than half of the anticholinergic burden, while traditional anticholinergics, antidepressants, mood stabilizers, and benzodiazepines accounted for the remainder.
“It is easy even for well-meaning clinicians to inadvertently contribute to anticholinergic medication burden through routine and appropriate care. The unique finding here is that this burden comes from medications we don’t usually think of as typical anticholinergic agents,” senior author Gregory Light, PhD, with University of California, San Diego, said in a news release.
Anticholinergic medication burden was significantly associated with generalized impairments in cognitive functioning across all cognitive domains on the PCNB with comparable magnitude and after controlling for multiple proxies of functioning or disease severity.
Higher anticholinergic medication burden was associated with worse cognitive performance. The PCNB global cognitive averages for none, low, average, high, and very high anticholinergic burdens were, respectively (in z values), -0.51, -0.70, -0.85, -0.96, and -1.15.
The results suggest “total cumulative anticholinergic burden – rather than anticholinergic burden attributable to a specific antipsychotic or psychotropic medication class – is a key contributor to cognitive impairment in schizophrenia,” the researchers write.
“The results imply that if it is clinically safe and practical,” said Dr. Joshi.
“This may be accomplished by reducing overall polypharmacy or transitioning to equivalent medications with lower overall anticholinergic burden. While ‘traditional’ anticholinergic medications should always be scrutinized, all medications should be carefully evaluated to understand whether they contribute to cumulative anticholinergic medication burden,” he added.
Confirmatory findings
Commenting on the study for this news organization, Jessica Gannon, MD, assistant professor of psychiatry, University of Pittsburgh, said the author’s findings “aren’t surprising, but the work that they did was pretty comprehensive [and] further fleshed out some of our concerns about the impact of anticholinergics on cognitive function in patients with schizophrenia.”
“We certainly have to use some of these medications for patients, like antipsychotics that do have some anticholinergic burden associated with them. We don’t really have other options,” Dr. Gannon said.
“But certainly I think this calls us to be better stewards of medication in general. And when we prescribe for comorbid conditions, like depression and anxiety, we should be careful in our prescribing practices, try not to prescribe an anticholinergic medication, and, if they have been prescribed, to deprescribe them,” Dr. Gannon added.
The study was supported by grants from the National Institute of Mental Health; the Sidney R. Baer, Jr. Foundation; the Brain and Behavior Research Foundation; the VISN-22 Mental Illness Research, Education, and Clinical Center; and the Department of Veterans Affairs. Dr. Joshi and Dr. Gannon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Choosing the right R-CHOP dosage for elderly patients with DLBCL
Physicians often face the choice of whether to treat elderly patients with diffuse large B-cell lymphoma (DLBCL) with a full or reduced dose intensity (DI) of R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone + rituximab), according to Edward J. Bataillard of the Imperial College Healthcare National Health Service Trust, London, and colleagues.
To address this issue, the researchers conducted a systematic review assessing the impact of R-CHOP DI on DLBCL survival outcomes, according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Protocols (PRISMA-P) guidelines. They found that greater than 80 years of age is an important cutoff for treating patients with a reduced R-CHOP dosage, according to their results, published in Blood Advances (2021;5[9]:2426-37).
Cutoff at 80 years of age
Their final review comprised 13 studies including 5,188 patients. Overall, the lower DI (intended or relative) was associated with inferior survival in seven of nine studies reporting crude survival analyses. In addition, most studies and those larger studies of higher quality showed poorer outcomes associated with reduced R-CHOP DI.
However, in subgroups of patients aged 80 years or more, survival was not consistently affected by the use of lower dosage R-CHOP, according to the researchers.
“We found evidence of improved survival with higher RDIs (up to R-CHOP-21) in those aged < 80 years, but the literature to date does not support full-dose intensity in those 80 years [or older],” they stated.
However, the researchers concluded that: “In the absence of improved options beyond R-CHOP in DLBCL over the past 20 years, prospective studies of DI are warranted, despite the recognized challenges involved.”
Two of the authors reported being previously employed by Roche. A third served as a consultant and adviser and received honoraria from Roche and other pharmaceutical companies. Several authors reported disclosures related to multiple other pharmaceutical companies.
Physicians often face the choice of whether to treat elderly patients with diffuse large B-cell lymphoma (DLBCL) with a full or reduced dose intensity (DI) of R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone + rituximab), according to Edward J. Bataillard of the Imperial College Healthcare National Health Service Trust, London, and colleagues.
To address this issue, the researchers conducted a systematic review assessing the impact of R-CHOP DI on DLBCL survival outcomes, according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Protocols (PRISMA-P) guidelines. They found that greater than 80 years of age is an important cutoff for treating patients with a reduced R-CHOP dosage, according to their results, published in Blood Advances (2021;5[9]:2426-37).
Cutoff at 80 years of age
Their final review comprised 13 studies including 5,188 patients. Overall, the lower DI (intended or relative) was associated with inferior survival in seven of nine studies reporting crude survival analyses. In addition, most studies and those larger studies of higher quality showed poorer outcomes associated with reduced R-CHOP DI.
However, in subgroups of patients aged 80 years or more, survival was not consistently affected by the use of lower dosage R-CHOP, according to the researchers.
“We found evidence of improved survival with higher RDIs (up to R-CHOP-21) in those aged < 80 years, but the literature to date does not support full-dose intensity in those 80 years [or older],” they stated.
However, the researchers concluded that: “In the absence of improved options beyond R-CHOP in DLBCL over the past 20 years, prospective studies of DI are warranted, despite the recognized challenges involved.”
Two of the authors reported being previously employed by Roche. A third served as a consultant and adviser and received honoraria from Roche and other pharmaceutical companies. Several authors reported disclosures related to multiple other pharmaceutical companies.
Physicians often face the choice of whether to treat elderly patients with diffuse large B-cell lymphoma (DLBCL) with a full or reduced dose intensity (DI) of R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone + rituximab), according to Edward J. Bataillard of the Imperial College Healthcare National Health Service Trust, London, and colleagues.
To address this issue, the researchers conducted a systematic review assessing the impact of R-CHOP DI on DLBCL survival outcomes, according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Protocols (PRISMA-P) guidelines. They found that greater than 80 years of age is an important cutoff for treating patients with a reduced R-CHOP dosage, according to their results, published in Blood Advances (2021;5[9]:2426-37).
Cutoff at 80 years of age
Their final review comprised 13 studies including 5,188 patients. Overall, the lower DI (intended or relative) was associated with inferior survival in seven of nine studies reporting crude survival analyses. In addition, most studies and those larger studies of higher quality showed poorer outcomes associated with reduced R-CHOP DI.
However, in subgroups of patients aged 80 years or more, survival was not consistently affected by the use of lower dosage R-CHOP, according to the researchers.
“We found evidence of improved survival with higher RDIs (up to R-CHOP-21) in those aged < 80 years, but the literature to date does not support full-dose intensity in those 80 years [or older],” they stated.
However, the researchers concluded that: “In the absence of improved options beyond R-CHOP in DLBCL over the past 20 years, prospective studies of DI are warranted, despite the recognized challenges involved.”
Two of the authors reported being previously employed by Roche. A third served as a consultant and adviser and received honoraria from Roche and other pharmaceutical companies. Several authors reported disclosures related to multiple other pharmaceutical companies.
FROM BLOOD ADVANCES
Outcomes Following Implementation of a Hospital-Wide, Multicomponent Delirium Care Pathway
Delirium is an acute disturbance in mental status characterized by fluctuations in cognition and attention that affects more than 2.6 million hospitalized older adults in the United States annually, a rate that is expected to increase as the population ages.1-4 Hospital-acquired delirium is associated with poor outcomes, including prolonged hospital length of stay (LOS), loss of independence, cognitive impairment, and even death.5-10 Individuals who develop delirium do poorly after hospital discharge and are more likely to be readmitted within 30 days.11 Approximately 30% to 40% of hospital-acquired delirium cases are preventable.10,12 However, programs designed to prevent delirium and associated complications, such as increased LOS, have demonstrated variable success.12-14 Many studies are limited by small sample sizes, lack of generalizability to different hospitalized patient populations, poor adherence, or reliance on outside funding.12,13,15-18
Delirium prevention programs face several challenges because delirium could be caused by a variety of risk factors and precipitants.19,20 Some risk factors that occur frequently among hospitalized patients can be mitigated, such as sensory impairment, immobility from physical restraints or urinary catheters, and polypharmacy.20,21 Effective delirium care pathways targeting these risk factors must be multifaceted, interdisciplinary, and interprofessional. Accurate risk assessment is critical to allocate resources to high-risk patients. Delirium affects patients in all medical and surgical disciplines, and often is underdiagnosed.19,22 Comprehensive screening is necessary to identify cases early and track outcomes, and educational efforts must reach all providers in the hospital. These challenges require a systematic, pragmatic approach to change.
The purpose of this study was to evaluate the association between a delirium care pathway and clinical outcomes for hospitalized patients. We hypothesized that this program would be associated with reduced hospital LOS, with secondary benefits to hospitalization costs, odds of 30-day readmission, and delirium rates.
METHODS
Study Design
In this retrospective cohort study, we compared clinical outcomes the year before and after implementation of a delirium care pathway across seven hospital units. The study period spanned October 1, 2015, through February 28, 2019. The study was approved by the University of California, San Francisco Institutional Review Board (#13-12500).
Multicomponent Delirium Care Pathway
The delirium care pathway was developed collaboratively among geriatrics, hospital medicine, neurology, anesthesiology, surgery, and psychiatry services, with an interprofessional team of physicians, nurses, pharmacists, and physical and occupational therapists. This pathway was implemented in units consecutively, approximately every 4 months in the following order: neurosciences, medicine, cardiology, general surgery, specialty surgery, hematology-oncology, and transplant. The same implementation education protocols were performed in each unit. The pathway consisted of several components targeting delirium prevention and management (Appendix Figure 1 and Appendix Figure 2). Systematic screening for delirium was introduced as part of the multicomponent intervention. Nursing staff assessed each patient’s risk of developing delirium at admission using the AWOL score, a validated delirium prediction tool.23 AWOL consists of: patient Age, spelling “World” backwards correctly, Orientation, and assessment of iLlness severity by the nurse. For patients who spoke a language other than English, spelling of “world” backwards was translated to his or her primary language, or if this was not possible, the task was modified to serial 7s (subtracting 7 from 100 in a serial fashion). This modification has been validated for use in other languages.24 Patients at high risk for delirium based on an AWOL score ≥2 received a multidisciplinary intervention with four components: (1) notifying the primary team by pager and electronic medical record (EMR), (2) a nurse-led, evidence-based, nonpharmacologic multicomponent intervention,25 (3) placement of a delirium order set by the physician, and (4) review of medications by the unit pharmacist who adjusted administration timing to occur during waking hours and placed a note in the EMR notifying the primary team of potentially deliriogenic medications. The delirium order set reinforced the nonpharmacologic multicomponent intervention through a nursing order, placed an automatic consult to occupational therapy, and included options to order physical therapy, order speech/language therapy, obtain vital signs three times daily with minimal night interruptions, remove an indwelling bladder catheter, and prescribe melatonin as a sleep aid.
The bedside nurse screened all patients for active delirium every 12-hour shift using the Nursing Delirium Screening Scale (NuDESC) and entered the results into the EMR.23,26 Capturing NuDESC results in the EMR allowed communication across medical providers as well as monitoring of screening adherence. Each nurse received two in-person trainings in staff meetings and one-to-one instruction during the first week of implementation. All nurses were required to complete a 15-minute training module and had the option of completing an additional 1-hour continuing medical education module. If a patient was transferred to the intensive care unit (ICU), delirium was identified through use of the ICU-specific Confusion Assessment Method (CAM-ICU) assessments, which the bedside nurse performed each shift throughout the intervention period.27 Nurses were instructed to call the primary team physician after every positive screen. Before each unit’s implementation start date, physicians with patients on that unit received education through a combination of grand rounds, resident lectures and seminars, and a pocket card on delirium evaluation and management.
Participants and Eligibility Criteria
We included all patients aged ≥50 years hospitalized for >1 day on each hospital unit (Figure). We included adults aged ≥50 years to maximize the number of participants for this study while also capturing a population at risk for delirium. Because the delirium care pathway was unit-based and the pathway was rolled out sequentially across units, only patients who were admitted to and discharged from the same unit were included to better isolate the effect of the pathway. Patients who were transferred to the ICU were only included if they were discharged from the original unit of admission. Only the first hospitalization was included for patients with multiple hospitalizations during the study period.
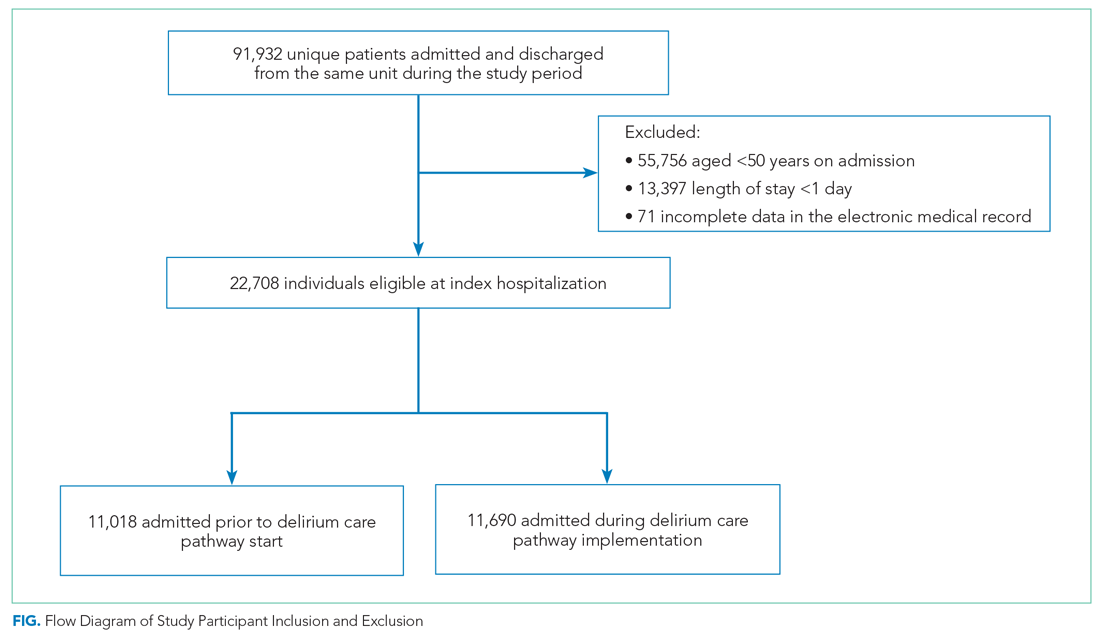
Patient Characteristics
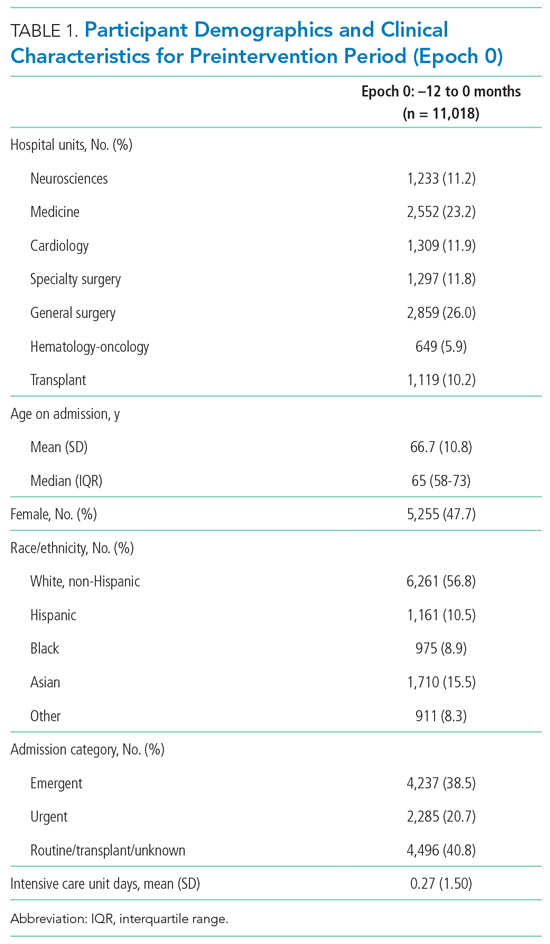
Patient demographics and clinical data were collected after discharge through Clarity and Vizient electronic databases (Table 1 and Table 2). All Elixhauser comorbidities were included except for the following International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10) codes that overlapped with a delirium diagnosis: G31.2, G93.89, G93.9, G94, R41.0, and R41.82 (Appendix Table 1). Severity of illness was obtained from Vizient, which calculates illness severity based on clinical and claims data (Appendix Table 1).
Delirium Metrics
Delirium screening was introduced as part of the multicomponent intervention, and therefore delirium rates before the intervention could not be determined. Trends in delirium prevalence and incidence after the intervention are reported. Prevalent delirium was defined as a single score of ≥2 on the nurse-administered NuDESC or a positive CAM-ICU at any point during the hospital stay. Incident delirium was identified if the first NuDESC score was negative and any subsequent NuDESC or CAM-ICU score was positive.

Outcomes
The primary study outcome was hospital LOS across all participants. Secondary outcomes included total direct cost and odds of 30-day hospital readmission. Readmissions tracked as part of hospital quality reporting were obtained from Vizient and were not captured if they occurred at another hospital. We also examined rates of safety attendant and restraint use during the study period, defined as the number of safety attendant days or restraint days per 1,000 patient days.
Because previous studies have demonstrated the effectiveness of multicomponent delirium interventions among elderly general medical patients,12 we also investigated these same outcomes in the medicine unit alone.
Statistical Analysis
The date of intervention implementation was determined for each hospital unit, which was defined as time(0) [t(0)]. The 12-month postintervention period was divided into four 3-month epochs to assess for trends. Data were aggregated across the seven units using t(0) as the start date, agnostic to the calendar month. Demographic and clinical characteristics were collected for the 12-months before t(0) and the four 3-month epochs after t(0). Univariate analysis of outcome variables comparing trends across the same epochs were conducted in the same manner, except for the rate of delirium, which was measured after t(0) and therefore could not be compared with the preintervention period.
Multivariable models were adjusted for age, sex, race/ethnicity, admission category, Elixhauser comorbidities, severity of illness quartile, and number days spent in the ICU. Admission category referred to whether the admission was emergent, urgent, or elective/unknown. Because it took 3 months after t(0) for each unit to reach a delirium screening compliance rate of 90%, the intervention was only considered fully implemented after this period. A ramp-up variable was set to 0 for admissions occurring prior to the intervention to t(0), 1/3 for admissions occurring 1 month post intervention, 2/3 for 2 months post intervention, and 1 for admissions occurring 3 to 12 months post intervention. In this way, the coefficient for the ramp-up variable estimated the postintervention versus preintervention effect. Numerical outcomes (LOS, cost) were log transformed to reduce skewness and analyzed using linear models. Coefficients were back-transformed to provide interpretations as proportional change in the median outcomes.
For LOS and readmission, we assessed secular trends by including admission date and admission date squared, in case the trend was nonlinear, as possible predictors; admission date was the specific date—not time from t(0)—to account for secular trends and allow contemporaneous controls in the analysis. To be conservative, we retained secular terms (first considering the quadratic and then the linear) if P <.10. The categorical outcome (30-day readmission) was analyzed using a logistic model. Count variables (delirium, safety attendants, restraints) were analyzed using Poisson regression models with a log link, and coefficients were back-transformed to provide rate ratio interpretations. Because delirium was not measured before t(0), and because the intervention was considered to take 3 months to become fully effective, baseline delirium rates were defined as those in the first 3 months adjusted by the ramp-up variable. For each outcome we included hospital unit, a ramp-up variable (measuring the pre- vs postintervention effect), and their interaction. If there was no statistically significant interaction, we presented the outcome for all units combined. If the interaction was statistically significant, we looked for consistency across units and reported results for all units combined when consistent, along with site-specific results. If the results were not consistent across the units, we provided site-specific results only. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
RESULTS
Participant Demographics and Clinical Characteristics
A total of 22,708 individuals were included in this study, with 11,018 in the preintervention period (Table 1 and Table 2). Most patients were cared for on the general surgery unit (n = 5,899), followed by the medicine unit (n = 4,923). The smallest number of patients were cared for on the hematology-oncology unit (n = 1,709). Across the five epochs, patients were of similar age and sex, and spent a similar number of days in the ICU. The population was diverse with regard to race and ethnicity; there were minor differences in admission category. There were also minor differences in severity of illness and some comorbidities between timepoints (Appendix Table 1).
Delirium Metrics
Delirium prevalence was 13.0% during the first epoch post intervention, followed by 12.0%, 11.7%, and 13.0% in the subsequent epochs (P = .91). Incident delirium occurred in 6.1% of patients during the first epoch post intervention, followed by 5.3%, 5.3%, and 5.8% in the subsequent epochs (P = .63).
Primary Outcome
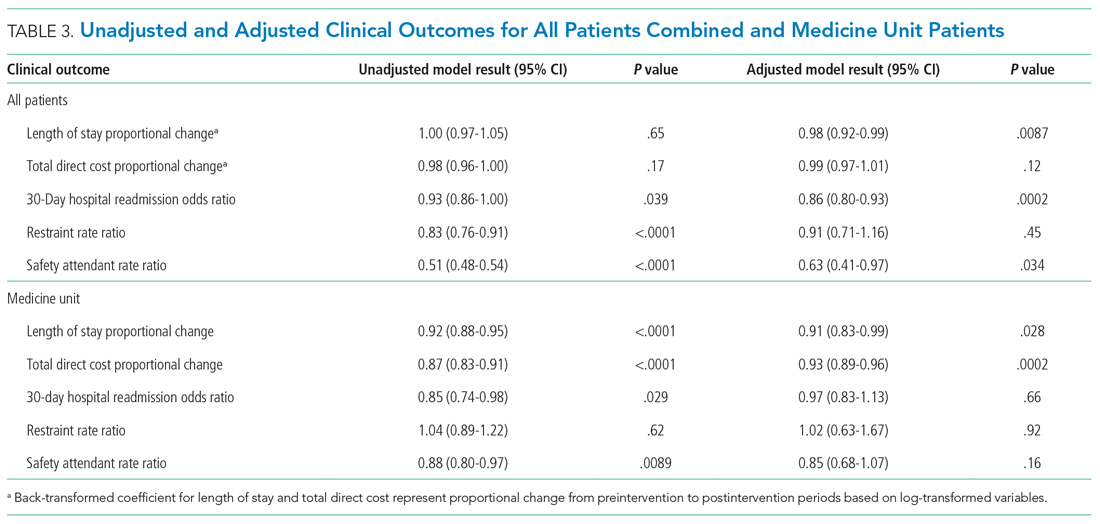
Epoch-level data for LOS before and after the intervention is shown in Appendix Table 2. The mean unadjusted LOS for all units combined did not decrease after the intervention, but in the adjusted model, the mean LOS decreased by 2% after the intervention (P = .0087; Table 3).
Secondary Outcomes
The odds of 30-day readmission decreased by 14% (P = .0002) in the adjusted models for all units combined (Table 3). There was no statistically significant reduction in adjusted total direct hospitalization cost or rate of restraint use. The safety attendant results showed strong effect modification across sites; the site-specific estimates are provided in Appendix Table 3. However, the estimated values all showed reductions, and a number were large and statistically significant.
Medicine Unit Outcomes
On the medicine unit alone, we observed a statistically significant reduction in LOS of 9% after implementation of the delirium care pathway (P = .028) in the adjusted model (Table 3). There was an associated 7% proportional decrease in total direct cost (P = .0002). Reductions in 30-day readmission and safety attendant use did not remain statistically significant in the adjusted models.
DISCUSSION
Implementation of a hospital-wide multicomponent delirium care pathway was associated with reduced hospital LOS and 30-day hospital readmission in a study of 22,708 hospitalized adults at a tertiary care, university hospital in Northern California, encompassing both medical and surgical acute care patients. When evaluating general medicine patients alone, pathway implementation was associated with reductions in LOS and total direct cost. The cost savings of 7% among medical patients translates to median savings of $1,237 per hospitalization. This study—one of the largest to date examining implementation of a hospital-wide delirium care pathway—supports use of a multicomponent delirium care pathway for older adults hospitalized for a range of conditions.
Multicomponent pathways for delirium prevention and management are increasingly being used in hospital settings. The United Kingdom National Institute for Health and Care Excellence guidelines recommend delirium assessment and intervention by a multidisciplinary team within 24 hours of hospital admission for those at risk.25 These guidelines are based on evidence accumulated in clinical studies over the past 30 years suggesting that multicomponent interventions reduce incident delirium by 30% to 40% among medical and surgical patients.12,13,25,28
Although multicomponent delirium care pathways are associated with improved patient outcomes, the specific clinical benefits might vary across patient populations. Here, we found larger reductions in LOS and total direct cost among medicine patients. Medical patients might respond more robustly to nonpharmacologic multicomponent delirium interventions because of differing delirium etiologies (eg, constipation and sleep deprivation in a medical patient vs seizures or encephalitis in a neurosciences patient). Another explanation for the difference observed in total direct cost might be the inclusion of surgical units in the total study population. For example, not all hospital days are equivalent in cost for patients on a surgical unit.29 For patients requiring surgical care, most of the hospitalization cost might be incurred during the initial days of hospitalization, when there are perioperative costs; therefore, reduced LOS might have a lower economic impact.29 Multicomponent, nonpharmacologic delirium interventions encourage discontinuing restraints. As a result, one might expect a need for more frequent safety attendant use and an associated cost increase. However, we found that the estimated unit-specific values for safety attendant use showed reductions, which were large and highly statistically significant. For all units combined and the medicine unit alone, we found that the rate of restraint use decreased, although the change was not statistically significant. It is possible that some of the interventions taught to nurses and physicians as part of care pathway implementation, such as the use of family support for at-risk and delirious patients, led to a reduction in both safety attendants and restraints.
Our study had several strengths. This is one of the largest hospital-based delirium interventions studied, both in terms of its scope across seven diverse medical and surgical hospital units and the number of hospitalized patients studied. This intervention did not require additional staff or creating a specialized ward. Adherence to the pathway, as measured by risk assessment and delirium screening, was high (>90%) 3 months after implementation. This allowed for robust outcome ascertainment. The patient population’s characteristics and rates of delirium were stable over time. Because different hospital units incorporated the multicomponent delirium care pathway at different times, limiting enrollment to patients admitted and discharged from the same unit isolated the analysis to patients exposed to the pathway on each unit. This design also limited potential influence of other hospital quality improvement projects that might have occurred at the same time.
The primary limitation of this study is that screening for delirium was introduced as part of the multicomponent intervention. This decision was made to maximize buy-in from bedside nurses performing delirium screening because this addition to their workflow was explicitly linked to delirium prevention and management measures. Delirium could not be ascertained preintervention from the EMR because it is a clinical diagnosis and is coded inadequately.30 We could only measure the change in delirium metrics after implementation of the delirium care pathway. Because baseline delirium rates before the intervention were not measured systematically, conclusions about the intervention’s association with delirium metrics are limited. All other outcomes were measured before and after the intervention.
Although the comprehensive delirium screening program and high rate of adherence are a methodologic strength of this study, a second limitation is the use of the NuDESC. Our previous research demonstrated that the NuDESC has low sensitivity but high specificity and positive predictive value,26 which might underestimate delirium rates in this study. However, any underestimation should be stable over time and temporal trends should remain meaningful. This could allow more widespread study of delirium among hospitalized individuals. Because this care pathway was hospital-wide, it was important to ensure both consistency of screening and longevity of the initiative, and it was necessary to select a delirium assessment tool that was efficient and validated for nursing implementation. For these reasons, the NuDESC was an appropriate choice.
It is possible that our results could be influenced by unmeasured confounders. For example, although we incorporated Elixhauser medical comorbidities and illness severity into our model, we were unable to adjust for baseline functional status or frailty. Baseline functional status and frailty were not reliably recorded in the EMR, although these are potential confounders when investigating clinical outcomes including hospital readmission.
CONCLUSION
Implementation of a systematic, hospital-wide multicomponent delirium care pathway is associated with reductions in hospital LOS and 30-day readmission. In general medicine units, the reduction in LOS and associated cost savings were robust. These results demonstrate the feasibility and effectiveness of implementing an interprofessional, multidisciplinary multicomponent delirium care pathway through medical center funding to benefit patients and the hospital system.
Acknowledgments
The authors thank the many hospital staff members, especially the nurses, pharmacists, therapists, and patient care assistants, who helped implement the multicomponent delirium care pathway. All persons who have contributed significantly to this work are listed as authors of this work.
1. Bidwell J. Interventions for preventing delirium in hospitalized non-ICU patients: A Cochrane review summary. Int J Nurs Stud. 2017;70:142-143. https://doi.org/ 10.1016/j.ijnurstu.2016.11.010
2. Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis and treatment. Crit Care Clin. 2008;24(4):657-722, vii. https://doi.org/10.1016/j.ccc.2008.05.008
3. Field RR, Wall MH. Delirium: past, present, and future. Semin Cardiothorac Vasc Anesth. 2013;17(3):170-179. https://doi.org/10.1177/1089253213476957
4. Oh ST, Park JY. Postoperative delirium. Korean J Anesthesiol. 2019;72(1):4-12. https://doi.org/10.4097/kja.d.18.00073.1
5. Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263(8):1097-1101.
6. Salluh JI, Soares M, Teles JM, et al. Delirium epidemiology in critical care (DECCA): an international study. Crit Care. 2010;14(6):R210. https://doi.org/10.1186/cc9333
7. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. https://doi.org/
8. McCusker J, Cole MG, Dendukuri N, Belzile E. Does delirium increase hospital stay? J Am Geriatr Soc. 2003;51(11):1539-1546. https://doi.org/10.1001/jama.291.14.1753
9. Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13(4):234-242. https://doi.org/10.1046/j.1525-1497.1998.00073.x
10. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364. https://doi.org/10.1093/ageing/afl005
11. LaHue SC, Douglas VC, Kuo T, et al. Association between inpatient delirium and hospital readmission in patients >/= 65 years of age: a retrospective cohort study. J Hosp Med. 2019;14(4):201-206. https://doi.org/10.12788/jhm.3130
12. Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. https://doi.org/10.1001/jamainternmed.2014.7779
13. Inouye SK, Bogardus ST, Jr., Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901
14. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. https://doi.org/
15. Alhaidari AA, Allen-Narker RA. An evolving approach to delirium: A mixed-methods process evaluation of a hospital-wide delirium program in New Zealand. Australas J Ageing. 2017. https://doi.org/10.1046/j.1532-5415.2001.49108.x
16. Holroyd-Leduc JM, Khandwala F, Sink KM. How can delirium best be prevented and managed in older patients in hospital? CMAJ. 2010;182(5):465-470. https://doi.org/10.1503/cmaj.080519
17. Siddiqi N, Stockdale R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalised patients. Cochrane Database Syst Rev. 2007(2):CD005563. https://doi.org/ 10.1002/14651858.CD005563.pub2
18. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. https://doi.org/10.1002/14651858.CD005563.pub3
19. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. https://doi.org/10.1016/S0140-6736(13)60688-1
20. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857.
21. LaHue SC, Liu VX. Loud and clear: sensory impairment, delirium, and functional recovery in critical illness. Am J Respir Crit Care Med. 2016;194(3):252-253. https://doi.org/10.1164/rccm.201602-0372ED
22. Ritter SRF, Cardoso AF, Lins MMP, Zoccoli TLV, Freitas MPD, Camargos EF. Underdiagnosis of delirium in the elderly in acute care hospital settings: lessons not learned. Psychogeriatrics. 2018;18(4):268-275. https://doi.org/10.1111/psyg.12324
23. Douglas VC, Hessler CS, Dhaliwal G, et al. The AWOL tool: derivation and validation of a delirium prediction rule. J Hosp Med. 2013;8(9):493-499. https://doi.org/10.1002/jhm.2062
24. Tombaugh TN, McDowell I, Kristjansson B, Hubley AM. Mini-Mental State Examination (MMSE) and the modified MMSE (3MS): A psychometric comparison and normative data. Psychol Assessment. 1996;8(1):48-59. https://doi.org/10.1037/1040-3590.8.1.48
25. Young J, Murthy L, Westby M, Akunne A, O’Mahony R, Guideline Development Group. Diagnosis, prevention, and management of delirium: summary of NICE guidance. BMJ. 2010;341:c3704. https://doi.org/10.1136/bmj.c3704
26. Hargrave A, Bastiaens J, Bourgeois JA, et al. Validation of a nurse-based delirium-screening tool for hospitalized patients. Psychosomatics. 2017;58(6):594-603. https://doi.org/10.1016/j.psym.2017.05.005
27. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710. https://doi.org/10.1001/jama.286.21.2703
28. Strijbos MJ, Steunenberg B, van der Mast RC, Inouye SK, Schuurmans MJ. Design and methods of the Hospital Elder Life Program (HELP), a multicomponent targeted intervention to prevent delirium in hospitalized older patients: efficacy and cost-effectiveness in Dutch health care. BMC Geriatr. 2013;13:78. https://doi.org/10.1186/1471-2318-13-78
29. Taheri PA, Butz DA, Greenfield LJ. Length of stay has minimal impact on the cost of hospital admission. J Am Coll Surg. 2000;191(2):123-130. https://doi.org/10.1016/s1072-7515(00)00352-5
30. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. https://doi.org/10.1038/nrneurol.2009.24
Delirium is an acute disturbance in mental status characterized by fluctuations in cognition and attention that affects more than 2.6 million hospitalized older adults in the United States annually, a rate that is expected to increase as the population ages.1-4 Hospital-acquired delirium is associated with poor outcomes, including prolonged hospital length of stay (LOS), loss of independence, cognitive impairment, and even death.5-10 Individuals who develop delirium do poorly after hospital discharge and are more likely to be readmitted within 30 days.11 Approximately 30% to 40% of hospital-acquired delirium cases are preventable.10,12 However, programs designed to prevent delirium and associated complications, such as increased LOS, have demonstrated variable success.12-14 Many studies are limited by small sample sizes, lack of generalizability to different hospitalized patient populations, poor adherence, or reliance on outside funding.12,13,15-18
Delirium prevention programs face several challenges because delirium could be caused by a variety of risk factors and precipitants.19,20 Some risk factors that occur frequently among hospitalized patients can be mitigated, such as sensory impairment, immobility from physical restraints or urinary catheters, and polypharmacy.20,21 Effective delirium care pathways targeting these risk factors must be multifaceted, interdisciplinary, and interprofessional. Accurate risk assessment is critical to allocate resources to high-risk patients. Delirium affects patients in all medical and surgical disciplines, and often is underdiagnosed.19,22 Comprehensive screening is necessary to identify cases early and track outcomes, and educational efforts must reach all providers in the hospital. These challenges require a systematic, pragmatic approach to change.
The purpose of this study was to evaluate the association between a delirium care pathway and clinical outcomes for hospitalized patients. We hypothesized that this program would be associated with reduced hospital LOS, with secondary benefits to hospitalization costs, odds of 30-day readmission, and delirium rates.
METHODS
Study Design
In this retrospective cohort study, we compared clinical outcomes the year before and after implementation of a delirium care pathway across seven hospital units. The study period spanned October 1, 2015, through February 28, 2019. The study was approved by the University of California, San Francisco Institutional Review Board (#13-12500).
Multicomponent Delirium Care Pathway
The delirium care pathway was developed collaboratively among geriatrics, hospital medicine, neurology, anesthesiology, surgery, and psychiatry services, with an interprofessional team of physicians, nurses, pharmacists, and physical and occupational therapists. This pathway was implemented in units consecutively, approximately every 4 months in the following order: neurosciences, medicine, cardiology, general surgery, specialty surgery, hematology-oncology, and transplant. The same implementation education protocols were performed in each unit. The pathway consisted of several components targeting delirium prevention and management (Appendix Figure 1 and Appendix Figure 2). Systematic screening for delirium was introduced as part of the multicomponent intervention. Nursing staff assessed each patient’s risk of developing delirium at admission using the AWOL score, a validated delirium prediction tool.23 AWOL consists of: patient Age, spelling “World” backwards correctly, Orientation, and assessment of iLlness severity by the nurse. For patients who spoke a language other than English, spelling of “world” backwards was translated to his or her primary language, or if this was not possible, the task was modified to serial 7s (subtracting 7 from 100 in a serial fashion). This modification has been validated for use in other languages.24 Patients at high risk for delirium based on an AWOL score ≥2 received a multidisciplinary intervention with four components: (1) notifying the primary team by pager and electronic medical record (EMR), (2) a nurse-led, evidence-based, nonpharmacologic multicomponent intervention,25 (3) placement of a delirium order set by the physician, and (4) review of medications by the unit pharmacist who adjusted administration timing to occur during waking hours and placed a note in the EMR notifying the primary team of potentially deliriogenic medications. The delirium order set reinforced the nonpharmacologic multicomponent intervention through a nursing order, placed an automatic consult to occupational therapy, and included options to order physical therapy, order speech/language therapy, obtain vital signs three times daily with minimal night interruptions, remove an indwelling bladder catheter, and prescribe melatonin as a sleep aid.
The bedside nurse screened all patients for active delirium every 12-hour shift using the Nursing Delirium Screening Scale (NuDESC) and entered the results into the EMR.23,26 Capturing NuDESC results in the EMR allowed communication across medical providers as well as monitoring of screening adherence. Each nurse received two in-person trainings in staff meetings and one-to-one instruction during the first week of implementation. All nurses were required to complete a 15-minute training module and had the option of completing an additional 1-hour continuing medical education module. If a patient was transferred to the intensive care unit (ICU), delirium was identified through use of the ICU-specific Confusion Assessment Method (CAM-ICU) assessments, which the bedside nurse performed each shift throughout the intervention period.27 Nurses were instructed to call the primary team physician after every positive screen. Before each unit’s implementation start date, physicians with patients on that unit received education through a combination of grand rounds, resident lectures and seminars, and a pocket card on delirium evaluation and management.
Participants and Eligibility Criteria
We included all patients aged ≥50 years hospitalized for >1 day on each hospital unit (Figure). We included adults aged ≥50 years to maximize the number of participants for this study while also capturing a population at risk for delirium. Because the delirium care pathway was unit-based and the pathway was rolled out sequentially across units, only patients who were admitted to and discharged from the same unit were included to better isolate the effect of the pathway. Patients who were transferred to the ICU were only included if they were discharged from the original unit of admission. Only the first hospitalization was included for patients with multiple hospitalizations during the study period.

Patient Characteristics
Patient demographics and clinical data were collected after discharge through Clarity and Vizient electronic databases (Table 1 and Table 2). All Elixhauser comorbidities were included except for the following International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10) codes that overlapped with a delirium diagnosis: G31.2, G93.89, G93.9, G94, R41.0, and R41.82 (Appendix Table 1). Severity of illness was obtained from Vizient, which calculates illness severity based on clinical and claims data (Appendix Table 1).

Delirium Metrics
Delirium screening was introduced as part of the multicomponent intervention, and therefore delirium rates before the intervention could not be determined. Trends in delirium prevalence and incidence after the intervention are reported. Prevalent delirium was defined as a single score of ≥2 on the nurse-administered NuDESC or a positive CAM-ICU at any point during the hospital stay. Incident delirium was identified if the first NuDESC score was negative and any subsequent NuDESC or CAM-ICU score was positive.

Outcomes
The primary study outcome was hospital LOS across all participants. Secondary outcomes included total direct cost and odds of 30-day hospital readmission. Readmissions tracked as part of hospital quality reporting were obtained from Vizient and were not captured if they occurred at another hospital. We also examined rates of safety attendant and restraint use during the study period, defined as the number of safety attendant days or restraint days per 1,000 patient days.
Because previous studies have demonstrated the effectiveness of multicomponent delirium interventions among elderly general medical patients,12 we also investigated these same outcomes in the medicine unit alone.
Statistical Analysis
The date of intervention implementation was determined for each hospital unit, which was defined as time(0) [t(0)]. The 12-month postintervention period was divided into four 3-month epochs to assess for trends. Data were aggregated across the seven units using t(0) as the start date, agnostic to the calendar month. Demographic and clinical characteristics were collected for the 12-months before t(0) and the four 3-month epochs after t(0). Univariate analysis of outcome variables comparing trends across the same epochs were conducted in the same manner, except for the rate of delirium, which was measured after t(0) and therefore could not be compared with the preintervention period.
Multivariable models were adjusted for age, sex, race/ethnicity, admission category, Elixhauser comorbidities, severity of illness quartile, and number days spent in the ICU. Admission category referred to whether the admission was emergent, urgent, or elective/unknown. Because it took 3 months after t(0) for each unit to reach a delirium screening compliance rate of 90%, the intervention was only considered fully implemented after this period. A ramp-up variable was set to 0 for admissions occurring prior to the intervention to t(0), 1/3 for admissions occurring 1 month post intervention, 2/3 for 2 months post intervention, and 1 for admissions occurring 3 to 12 months post intervention. In this way, the coefficient for the ramp-up variable estimated the postintervention versus preintervention effect. Numerical outcomes (LOS, cost) were log transformed to reduce skewness and analyzed using linear models. Coefficients were back-transformed to provide interpretations as proportional change in the median outcomes.
For LOS and readmission, we assessed secular trends by including admission date and admission date squared, in case the trend was nonlinear, as possible predictors; admission date was the specific date—not time from t(0)—to account for secular trends and allow contemporaneous controls in the analysis. To be conservative, we retained secular terms (first considering the quadratic and then the linear) if P <.10. The categorical outcome (30-day readmission) was analyzed using a logistic model. Count variables (delirium, safety attendants, restraints) were analyzed using Poisson regression models with a log link, and coefficients were back-transformed to provide rate ratio interpretations. Because delirium was not measured before t(0), and because the intervention was considered to take 3 months to become fully effective, baseline delirium rates were defined as those in the first 3 months adjusted by the ramp-up variable. For each outcome we included hospital unit, a ramp-up variable (measuring the pre- vs postintervention effect), and their interaction. If there was no statistically significant interaction, we presented the outcome for all units combined. If the interaction was statistically significant, we looked for consistency across units and reported results for all units combined when consistent, along with site-specific results. If the results were not consistent across the units, we provided site-specific results only. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
RESULTS
Participant Demographics and Clinical Characteristics
A total of 22,708 individuals were included in this study, with 11,018 in the preintervention period (Table 1 and Table 2). Most patients were cared for on the general surgery unit (n = 5,899), followed by the medicine unit (n = 4,923). The smallest number of patients were cared for on the hematology-oncology unit (n = 1,709). Across the five epochs, patients were of similar age and sex, and spent a similar number of days in the ICU. The population was diverse with regard to race and ethnicity; there were minor differences in admission category. There were also minor differences in severity of illness and some comorbidities between timepoints (Appendix Table 1).
Delirium Metrics
Delirium prevalence was 13.0% during the first epoch post intervention, followed by 12.0%, 11.7%, and 13.0% in the subsequent epochs (P = .91). Incident delirium occurred in 6.1% of patients during the first epoch post intervention, followed by 5.3%, 5.3%, and 5.8% in the subsequent epochs (P = .63).
Primary Outcome
Epoch-level data for LOS before and after the intervention is shown in Appendix Table 2. The mean unadjusted LOS for all units combined did not decrease after the intervention, but in the adjusted model, the mean LOS decreased by 2% after the intervention (P = .0087; Table 3).

Secondary Outcomes
The odds of 30-day readmission decreased by 14% (P = .0002) in the adjusted models for all units combined (Table 3). There was no statistically significant reduction in adjusted total direct hospitalization cost or rate of restraint use. The safety attendant results showed strong effect modification across sites; the site-specific estimates are provided in Appendix Table 3. However, the estimated values all showed reductions, and a number were large and statistically significant.
Medicine Unit Outcomes
On the medicine unit alone, we observed a statistically significant reduction in LOS of 9% after implementation of the delirium care pathway (P = .028) in the adjusted model (Table 3). There was an associated 7% proportional decrease in total direct cost (P = .0002). Reductions in 30-day readmission and safety attendant use did not remain statistically significant in the adjusted models.
DISCUSSION
Implementation of a hospital-wide multicomponent delirium care pathway was associated with reduced hospital LOS and 30-day hospital readmission in a study of 22,708 hospitalized adults at a tertiary care, university hospital in Northern California, encompassing both medical and surgical acute care patients. When evaluating general medicine patients alone, pathway implementation was associated with reductions in LOS and total direct cost. The cost savings of 7% among medical patients translates to median savings of $1,237 per hospitalization. This study—one of the largest to date examining implementation of a hospital-wide delirium care pathway—supports use of a multicomponent delirium care pathway for older adults hospitalized for a range of conditions.
Multicomponent pathways for delirium prevention and management are increasingly being used in hospital settings. The United Kingdom National Institute for Health and Care Excellence guidelines recommend delirium assessment and intervention by a multidisciplinary team within 24 hours of hospital admission for those at risk.25 These guidelines are based on evidence accumulated in clinical studies over the past 30 years suggesting that multicomponent interventions reduce incident delirium by 30% to 40% among medical and surgical patients.12,13,25,28
Although multicomponent delirium care pathways are associated with improved patient outcomes, the specific clinical benefits might vary across patient populations. Here, we found larger reductions in LOS and total direct cost among medicine patients. Medical patients might respond more robustly to nonpharmacologic multicomponent delirium interventions because of differing delirium etiologies (eg, constipation and sleep deprivation in a medical patient vs seizures or encephalitis in a neurosciences patient). Another explanation for the difference observed in total direct cost might be the inclusion of surgical units in the total study population. For example, not all hospital days are equivalent in cost for patients on a surgical unit.29 For patients requiring surgical care, most of the hospitalization cost might be incurred during the initial days of hospitalization, when there are perioperative costs; therefore, reduced LOS might have a lower economic impact.29 Multicomponent, nonpharmacologic delirium interventions encourage discontinuing restraints. As a result, one might expect a need for more frequent safety attendant use and an associated cost increase. However, we found that the estimated unit-specific values for safety attendant use showed reductions, which were large and highly statistically significant. For all units combined and the medicine unit alone, we found that the rate of restraint use decreased, although the change was not statistically significant. It is possible that some of the interventions taught to nurses and physicians as part of care pathway implementation, such as the use of family support for at-risk and delirious patients, led to a reduction in both safety attendants and restraints.
Our study had several strengths. This is one of the largest hospital-based delirium interventions studied, both in terms of its scope across seven diverse medical and surgical hospital units and the number of hospitalized patients studied. This intervention did not require additional staff or creating a specialized ward. Adherence to the pathway, as measured by risk assessment and delirium screening, was high (>90%) 3 months after implementation. This allowed for robust outcome ascertainment. The patient population’s characteristics and rates of delirium were stable over time. Because different hospital units incorporated the multicomponent delirium care pathway at different times, limiting enrollment to patients admitted and discharged from the same unit isolated the analysis to patients exposed to the pathway on each unit. This design also limited potential influence of other hospital quality improvement projects that might have occurred at the same time.
The primary limitation of this study is that screening for delirium was introduced as part of the multicomponent intervention. This decision was made to maximize buy-in from bedside nurses performing delirium screening because this addition to their workflow was explicitly linked to delirium prevention and management measures. Delirium could not be ascertained preintervention from the EMR because it is a clinical diagnosis and is coded inadequately.30 We could only measure the change in delirium metrics after implementation of the delirium care pathway. Because baseline delirium rates before the intervention were not measured systematically, conclusions about the intervention’s association with delirium metrics are limited. All other outcomes were measured before and after the intervention.
Although the comprehensive delirium screening program and high rate of adherence are a methodologic strength of this study, a second limitation is the use of the NuDESC. Our previous research demonstrated that the NuDESC has low sensitivity but high specificity and positive predictive value,26 which might underestimate delirium rates in this study. However, any underestimation should be stable over time and temporal trends should remain meaningful. This could allow more widespread study of delirium among hospitalized individuals. Because this care pathway was hospital-wide, it was important to ensure both consistency of screening and longevity of the initiative, and it was necessary to select a delirium assessment tool that was efficient and validated for nursing implementation. For these reasons, the NuDESC was an appropriate choice.
It is possible that our results could be influenced by unmeasured confounders. For example, although we incorporated Elixhauser medical comorbidities and illness severity into our model, we were unable to adjust for baseline functional status or frailty. Baseline functional status and frailty were not reliably recorded in the EMR, although these are potential confounders when investigating clinical outcomes including hospital readmission.
CONCLUSION
Implementation of a systematic, hospital-wide multicomponent delirium care pathway is associated with reductions in hospital LOS and 30-day readmission. In general medicine units, the reduction in LOS and associated cost savings were robust. These results demonstrate the feasibility and effectiveness of implementing an interprofessional, multidisciplinary multicomponent delirium care pathway through medical center funding to benefit patients and the hospital system.
Acknowledgments
The authors thank the many hospital staff members, especially the nurses, pharmacists, therapists, and patient care assistants, who helped implement the multicomponent delirium care pathway. All persons who have contributed significantly to this work are listed as authors of this work.
Delirium is an acute disturbance in mental status characterized by fluctuations in cognition and attention that affects more than 2.6 million hospitalized older adults in the United States annually, a rate that is expected to increase as the population ages.1-4 Hospital-acquired delirium is associated with poor outcomes, including prolonged hospital length of stay (LOS), loss of independence, cognitive impairment, and even death.5-10 Individuals who develop delirium do poorly after hospital discharge and are more likely to be readmitted within 30 days.11 Approximately 30% to 40% of hospital-acquired delirium cases are preventable.10,12 However, programs designed to prevent delirium and associated complications, such as increased LOS, have demonstrated variable success.12-14 Many studies are limited by small sample sizes, lack of generalizability to different hospitalized patient populations, poor adherence, or reliance on outside funding.12,13,15-18
Delirium prevention programs face several challenges because delirium could be caused by a variety of risk factors and precipitants.19,20 Some risk factors that occur frequently among hospitalized patients can be mitigated, such as sensory impairment, immobility from physical restraints or urinary catheters, and polypharmacy.20,21 Effective delirium care pathways targeting these risk factors must be multifaceted, interdisciplinary, and interprofessional. Accurate risk assessment is critical to allocate resources to high-risk patients. Delirium affects patients in all medical and surgical disciplines, and often is underdiagnosed.19,22 Comprehensive screening is necessary to identify cases early and track outcomes, and educational efforts must reach all providers in the hospital. These challenges require a systematic, pragmatic approach to change.
The purpose of this study was to evaluate the association between a delirium care pathway and clinical outcomes for hospitalized patients. We hypothesized that this program would be associated with reduced hospital LOS, with secondary benefits to hospitalization costs, odds of 30-day readmission, and delirium rates.
METHODS
Study Design
In this retrospective cohort study, we compared clinical outcomes the year before and after implementation of a delirium care pathway across seven hospital units. The study period spanned October 1, 2015, through February 28, 2019. The study was approved by the University of California, San Francisco Institutional Review Board (#13-12500).
Multicomponent Delirium Care Pathway
The delirium care pathway was developed collaboratively among geriatrics, hospital medicine, neurology, anesthesiology, surgery, and psychiatry services, with an interprofessional team of physicians, nurses, pharmacists, and physical and occupational therapists. This pathway was implemented in units consecutively, approximately every 4 months in the following order: neurosciences, medicine, cardiology, general surgery, specialty surgery, hematology-oncology, and transplant. The same implementation education protocols were performed in each unit. The pathway consisted of several components targeting delirium prevention and management (Appendix Figure 1 and Appendix Figure 2). Systematic screening for delirium was introduced as part of the multicomponent intervention. Nursing staff assessed each patient’s risk of developing delirium at admission using the AWOL score, a validated delirium prediction tool.23 AWOL consists of: patient Age, spelling “World” backwards correctly, Orientation, and assessment of iLlness severity by the nurse. For patients who spoke a language other than English, spelling of “world” backwards was translated to his or her primary language, or if this was not possible, the task was modified to serial 7s (subtracting 7 from 100 in a serial fashion). This modification has been validated for use in other languages.24 Patients at high risk for delirium based on an AWOL score ≥2 received a multidisciplinary intervention with four components: (1) notifying the primary team by pager and electronic medical record (EMR), (2) a nurse-led, evidence-based, nonpharmacologic multicomponent intervention,25 (3) placement of a delirium order set by the physician, and (4) review of medications by the unit pharmacist who adjusted administration timing to occur during waking hours and placed a note in the EMR notifying the primary team of potentially deliriogenic medications. The delirium order set reinforced the nonpharmacologic multicomponent intervention through a nursing order, placed an automatic consult to occupational therapy, and included options to order physical therapy, order speech/language therapy, obtain vital signs three times daily with minimal night interruptions, remove an indwelling bladder catheter, and prescribe melatonin as a sleep aid.
The bedside nurse screened all patients for active delirium every 12-hour shift using the Nursing Delirium Screening Scale (NuDESC) and entered the results into the EMR.23,26 Capturing NuDESC results in the EMR allowed communication across medical providers as well as monitoring of screening adherence. Each nurse received two in-person trainings in staff meetings and one-to-one instruction during the first week of implementation. All nurses were required to complete a 15-minute training module and had the option of completing an additional 1-hour continuing medical education module. If a patient was transferred to the intensive care unit (ICU), delirium was identified through use of the ICU-specific Confusion Assessment Method (CAM-ICU) assessments, which the bedside nurse performed each shift throughout the intervention period.27 Nurses were instructed to call the primary team physician after every positive screen. Before each unit’s implementation start date, physicians with patients on that unit received education through a combination of grand rounds, resident lectures and seminars, and a pocket card on delirium evaluation and management.
Participants and Eligibility Criteria
We included all patients aged ≥50 years hospitalized for >1 day on each hospital unit (Figure). We included adults aged ≥50 years to maximize the number of participants for this study while also capturing a population at risk for delirium. Because the delirium care pathway was unit-based and the pathway was rolled out sequentially across units, only patients who were admitted to and discharged from the same unit were included to better isolate the effect of the pathway. Patients who were transferred to the ICU were only included if they were discharged from the original unit of admission. Only the first hospitalization was included for patients with multiple hospitalizations during the study period.

Patient Characteristics
Patient demographics and clinical data were collected after discharge through Clarity and Vizient electronic databases (Table 1 and Table 2). All Elixhauser comorbidities were included except for the following International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10) codes that overlapped with a delirium diagnosis: G31.2, G93.89, G93.9, G94, R41.0, and R41.82 (Appendix Table 1). Severity of illness was obtained from Vizient, which calculates illness severity based on clinical and claims data (Appendix Table 1).

Delirium Metrics
Delirium screening was introduced as part of the multicomponent intervention, and therefore delirium rates before the intervention could not be determined. Trends in delirium prevalence and incidence after the intervention are reported. Prevalent delirium was defined as a single score of ≥2 on the nurse-administered NuDESC or a positive CAM-ICU at any point during the hospital stay. Incident delirium was identified if the first NuDESC score was negative and any subsequent NuDESC or CAM-ICU score was positive.

Outcomes
The primary study outcome was hospital LOS across all participants. Secondary outcomes included total direct cost and odds of 30-day hospital readmission. Readmissions tracked as part of hospital quality reporting were obtained from Vizient and were not captured if they occurred at another hospital. We also examined rates of safety attendant and restraint use during the study period, defined as the number of safety attendant days or restraint days per 1,000 patient days.
Because previous studies have demonstrated the effectiveness of multicomponent delirium interventions among elderly general medical patients,12 we also investigated these same outcomes in the medicine unit alone.
Statistical Analysis
The date of intervention implementation was determined for each hospital unit, which was defined as time(0) [t(0)]. The 12-month postintervention period was divided into four 3-month epochs to assess for trends. Data were aggregated across the seven units using t(0) as the start date, agnostic to the calendar month. Demographic and clinical characteristics were collected for the 12-months before t(0) and the four 3-month epochs after t(0). Univariate analysis of outcome variables comparing trends across the same epochs were conducted in the same manner, except for the rate of delirium, which was measured after t(0) and therefore could not be compared with the preintervention period.
Multivariable models were adjusted for age, sex, race/ethnicity, admission category, Elixhauser comorbidities, severity of illness quartile, and number days spent in the ICU. Admission category referred to whether the admission was emergent, urgent, or elective/unknown. Because it took 3 months after t(0) for each unit to reach a delirium screening compliance rate of 90%, the intervention was only considered fully implemented after this period. A ramp-up variable was set to 0 for admissions occurring prior to the intervention to t(0), 1/3 for admissions occurring 1 month post intervention, 2/3 for 2 months post intervention, and 1 for admissions occurring 3 to 12 months post intervention. In this way, the coefficient for the ramp-up variable estimated the postintervention versus preintervention effect. Numerical outcomes (LOS, cost) were log transformed to reduce skewness and analyzed using linear models. Coefficients were back-transformed to provide interpretations as proportional change in the median outcomes.
For LOS and readmission, we assessed secular trends by including admission date and admission date squared, in case the trend was nonlinear, as possible predictors; admission date was the specific date—not time from t(0)—to account for secular trends and allow contemporaneous controls in the analysis. To be conservative, we retained secular terms (first considering the quadratic and then the linear) if P <.10. The categorical outcome (30-day readmission) was analyzed using a logistic model. Count variables (delirium, safety attendants, restraints) were analyzed using Poisson regression models with a log link, and coefficients were back-transformed to provide rate ratio interpretations. Because delirium was not measured before t(0), and because the intervention was considered to take 3 months to become fully effective, baseline delirium rates were defined as those in the first 3 months adjusted by the ramp-up variable. For each outcome we included hospital unit, a ramp-up variable (measuring the pre- vs postintervention effect), and their interaction. If there was no statistically significant interaction, we presented the outcome for all units combined. If the interaction was statistically significant, we looked for consistency across units and reported results for all units combined when consistent, along with site-specific results. If the results were not consistent across the units, we provided site-specific results only. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
RESULTS
Participant Demographics and Clinical Characteristics
A total of 22,708 individuals were included in this study, with 11,018 in the preintervention period (Table 1 and Table 2). Most patients were cared for on the general surgery unit (n = 5,899), followed by the medicine unit (n = 4,923). The smallest number of patients were cared for on the hematology-oncology unit (n = 1,709). Across the five epochs, patients were of similar age and sex, and spent a similar number of days in the ICU. The population was diverse with regard to race and ethnicity; there were minor differences in admission category. There were also minor differences in severity of illness and some comorbidities between timepoints (Appendix Table 1).
Delirium Metrics
Delirium prevalence was 13.0% during the first epoch post intervention, followed by 12.0%, 11.7%, and 13.0% in the subsequent epochs (P = .91). Incident delirium occurred in 6.1% of patients during the first epoch post intervention, followed by 5.3%, 5.3%, and 5.8% in the subsequent epochs (P = .63).
Primary Outcome
Epoch-level data for LOS before and after the intervention is shown in Appendix Table 2. The mean unadjusted LOS for all units combined did not decrease after the intervention, but in the adjusted model, the mean LOS decreased by 2% after the intervention (P = .0087; Table 3).

Secondary Outcomes
The odds of 30-day readmission decreased by 14% (P = .0002) in the adjusted models for all units combined (Table 3). There was no statistically significant reduction in adjusted total direct hospitalization cost or rate of restraint use. The safety attendant results showed strong effect modification across sites; the site-specific estimates are provided in Appendix Table 3. However, the estimated values all showed reductions, and a number were large and statistically significant.
Medicine Unit Outcomes
On the medicine unit alone, we observed a statistically significant reduction in LOS of 9% after implementation of the delirium care pathway (P = .028) in the adjusted model (Table 3). There was an associated 7% proportional decrease in total direct cost (P = .0002). Reductions in 30-day readmission and safety attendant use did not remain statistically significant in the adjusted models.
DISCUSSION
Implementation of a hospital-wide multicomponent delirium care pathway was associated with reduced hospital LOS and 30-day hospital readmission in a study of 22,708 hospitalized adults at a tertiary care, university hospital in Northern California, encompassing both medical and surgical acute care patients. When evaluating general medicine patients alone, pathway implementation was associated with reductions in LOS and total direct cost. The cost savings of 7% among medical patients translates to median savings of $1,237 per hospitalization. This study—one of the largest to date examining implementation of a hospital-wide delirium care pathway—supports use of a multicomponent delirium care pathway for older adults hospitalized for a range of conditions.
Multicomponent pathways for delirium prevention and management are increasingly being used in hospital settings. The United Kingdom National Institute for Health and Care Excellence guidelines recommend delirium assessment and intervention by a multidisciplinary team within 24 hours of hospital admission for those at risk.25 These guidelines are based on evidence accumulated in clinical studies over the past 30 years suggesting that multicomponent interventions reduce incident delirium by 30% to 40% among medical and surgical patients.12,13,25,28
Although multicomponent delirium care pathways are associated with improved patient outcomes, the specific clinical benefits might vary across patient populations. Here, we found larger reductions in LOS and total direct cost among medicine patients. Medical patients might respond more robustly to nonpharmacologic multicomponent delirium interventions because of differing delirium etiologies (eg, constipation and sleep deprivation in a medical patient vs seizures or encephalitis in a neurosciences patient). Another explanation for the difference observed in total direct cost might be the inclusion of surgical units in the total study population. For example, not all hospital days are equivalent in cost for patients on a surgical unit.29 For patients requiring surgical care, most of the hospitalization cost might be incurred during the initial days of hospitalization, when there are perioperative costs; therefore, reduced LOS might have a lower economic impact.29 Multicomponent, nonpharmacologic delirium interventions encourage discontinuing restraints. As a result, one might expect a need for more frequent safety attendant use and an associated cost increase. However, we found that the estimated unit-specific values for safety attendant use showed reductions, which were large and highly statistically significant. For all units combined and the medicine unit alone, we found that the rate of restraint use decreased, although the change was not statistically significant. It is possible that some of the interventions taught to nurses and physicians as part of care pathway implementation, such as the use of family support for at-risk and delirious patients, led to a reduction in both safety attendants and restraints.
Our study had several strengths. This is one of the largest hospital-based delirium interventions studied, both in terms of its scope across seven diverse medical and surgical hospital units and the number of hospitalized patients studied. This intervention did not require additional staff or creating a specialized ward. Adherence to the pathway, as measured by risk assessment and delirium screening, was high (>90%) 3 months after implementation. This allowed for robust outcome ascertainment. The patient population’s characteristics and rates of delirium were stable over time. Because different hospital units incorporated the multicomponent delirium care pathway at different times, limiting enrollment to patients admitted and discharged from the same unit isolated the analysis to patients exposed to the pathway on each unit. This design also limited potential influence of other hospital quality improvement projects that might have occurred at the same time.
The primary limitation of this study is that screening for delirium was introduced as part of the multicomponent intervention. This decision was made to maximize buy-in from bedside nurses performing delirium screening because this addition to their workflow was explicitly linked to delirium prevention and management measures. Delirium could not be ascertained preintervention from the EMR because it is a clinical diagnosis and is coded inadequately.30 We could only measure the change in delirium metrics after implementation of the delirium care pathway. Because baseline delirium rates before the intervention were not measured systematically, conclusions about the intervention’s association with delirium metrics are limited. All other outcomes were measured before and after the intervention.
Although the comprehensive delirium screening program and high rate of adherence are a methodologic strength of this study, a second limitation is the use of the NuDESC. Our previous research demonstrated that the NuDESC has low sensitivity but high specificity and positive predictive value,26 which might underestimate delirium rates in this study. However, any underestimation should be stable over time and temporal trends should remain meaningful. This could allow more widespread study of delirium among hospitalized individuals. Because this care pathway was hospital-wide, it was important to ensure both consistency of screening and longevity of the initiative, and it was necessary to select a delirium assessment tool that was efficient and validated for nursing implementation. For these reasons, the NuDESC was an appropriate choice.
It is possible that our results could be influenced by unmeasured confounders. For example, although we incorporated Elixhauser medical comorbidities and illness severity into our model, we were unable to adjust for baseline functional status or frailty. Baseline functional status and frailty were not reliably recorded in the EMR, although these are potential confounders when investigating clinical outcomes including hospital readmission.
CONCLUSION
Implementation of a systematic, hospital-wide multicomponent delirium care pathway is associated with reductions in hospital LOS and 30-day readmission. In general medicine units, the reduction in LOS and associated cost savings were robust. These results demonstrate the feasibility and effectiveness of implementing an interprofessional, multidisciplinary multicomponent delirium care pathway through medical center funding to benefit patients and the hospital system.
Acknowledgments
The authors thank the many hospital staff members, especially the nurses, pharmacists, therapists, and patient care assistants, who helped implement the multicomponent delirium care pathway. All persons who have contributed significantly to this work are listed as authors of this work.
1. Bidwell J. Interventions for preventing delirium in hospitalized non-ICU patients: A Cochrane review summary. Int J Nurs Stud. 2017;70:142-143. https://doi.org/ 10.1016/j.ijnurstu.2016.11.010
2. Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis and treatment. Crit Care Clin. 2008;24(4):657-722, vii. https://doi.org/10.1016/j.ccc.2008.05.008
3. Field RR, Wall MH. Delirium: past, present, and future. Semin Cardiothorac Vasc Anesth. 2013;17(3):170-179. https://doi.org/10.1177/1089253213476957
4. Oh ST, Park JY. Postoperative delirium. Korean J Anesthesiol. 2019;72(1):4-12. https://doi.org/10.4097/kja.d.18.00073.1
5. Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263(8):1097-1101.
6. Salluh JI, Soares M, Teles JM, et al. Delirium epidemiology in critical care (DECCA): an international study. Crit Care. 2010;14(6):R210. https://doi.org/10.1186/cc9333
7. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. https://doi.org/
8. McCusker J, Cole MG, Dendukuri N, Belzile E. Does delirium increase hospital stay? J Am Geriatr Soc. 2003;51(11):1539-1546. https://doi.org/10.1001/jama.291.14.1753
9. Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13(4):234-242. https://doi.org/10.1046/j.1525-1497.1998.00073.x
10. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364. https://doi.org/10.1093/ageing/afl005
11. LaHue SC, Douglas VC, Kuo T, et al. Association between inpatient delirium and hospital readmission in patients >/= 65 years of age: a retrospective cohort study. J Hosp Med. 2019;14(4):201-206. https://doi.org/10.12788/jhm.3130
12. Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. https://doi.org/10.1001/jamainternmed.2014.7779
13. Inouye SK, Bogardus ST, Jr., Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901
14. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. https://doi.org/
15. Alhaidari AA, Allen-Narker RA. An evolving approach to delirium: A mixed-methods process evaluation of a hospital-wide delirium program in New Zealand. Australas J Ageing. 2017. https://doi.org/10.1046/j.1532-5415.2001.49108.x
16. Holroyd-Leduc JM, Khandwala F, Sink KM. How can delirium best be prevented and managed in older patients in hospital? CMAJ. 2010;182(5):465-470. https://doi.org/10.1503/cmaj.080519
17. Siddiqi N, Stockdale R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalised patients. Cochrane Database Syst Rev. 2007(2):CD005563. https://doi.org/ 10.1002/14651858.CD005563.pub2
18. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. https://doi.org/10.1002/14651858.CD005563.pub3
19. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. https://doi.org/10.1016/S0140-6736(13)60688-1
20. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857.
21. LaHue SC, Liu VX. Loud and clear: sensory impairment, delirium, and functional recovery in critical illness. Am J Respir Crit Care Med. 2016;194(3):252-253. https://doi.org/10.1164/rccm.201602-0372ED
22. Ritter SRF, Cardoso AF, Lins MMP, Zoccoli TLV, Freitas MPD, Camargos EF. Underdiagnosis of delirium in the elderly in acute care hospital settings: lessons not learned. Psychogeriatrics. 2018;18(4):268-275. https://doi.org/10.1111/psyg.12324
23. Douglas VC, Hessler CS, Dhaliwal G, et al. The AWOL tool: derivation and validation of a delirium prediction rule. J Hosp Med. 2013;8(9):493-499. https://doi.org/10.1002/jhm.2062
24. Tombaugh TN, McDowell I, Kristjansson B, Hubley AM. Mini-Mental State Examination (MMSE) and the modified MMSE (3MS): A psychometric comparison and normative data. Psychol Assessment. 1996;8(1):48-59. https://doi.org/10.1037/1040-3590.8.1.48
25. Young J, Murthy L, Westby M, Akunne A, O’Mahony R, Guideline Development Group. Diagnosis, prevention, and management of delirium: summary of NICE guidance. BMJ. 2010;341:c3704. https://doi.org/10.1136/bmj.c3704
26. Hargrave A, Bastiaens J, Bourgeois JA, et al. Validation of a nurse-based delirium-screening tool for hospitalized patients. Psychosomatics. 2017;58(6):594-603. https://doi.org/10.1016/j.psym.2017.05.005
27. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710. https://doi.org/10.1001/jama.286.21.2703
28. Strijbos MJ, Steunenberg B, van der Mast RC, Inouye SK, Schuurmans MJ. Design and methods of the Hospital Elder Life Program (HELP), a multicomponent targeted intervention to prevent delirium in hospitalized older patients: efficacy and cost-effectiveness in Dutch health care. BMC Geriatr. 2013;13:78. https://doi.org/10.1186/1471-2318-13-78
29. Taheri PA, Butz DA, Greenfield LJ. Length of stay has minimal impact on the cost of hospital admission. J Am Coll Surg. 2000;191(2):123-130. https://doi.org/10.1016/s1072-7515(00)00352-5
30. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. https://doi.org/10.1038/nrneurol.2009.24
1. Bidwell J. Interventions for preventing delirium in hospitalized non-ICU patients: A Cochrane review summary. Int J Nurs Stud. 2017;70:142-143. https://doi.org/ 10.1016/j.ijnurstu.2016.11.010
2. Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis and treatment. Crit Care Clin. 2008;24(4):657-722, vii. https://doi.org/10.1016/j.ccc.2008.05.008
3. Field RR, Wall MH. Delirium: past, present, and future. Semin Cardiothorac Vasc Anesth. 2013;17(3):170-179. https://doi.org/10.1177/1089253213476957
4. Oh ST, Park JY. Postoperative delirium. Korean J Anesthesiol. 2019;72(1):4-12. https://doi.org/10.4097/kja.d.18.00073.1
5. Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263(8):1097-1101.
6. Salluh JI, Soares M, Teles JM, et al. Delirium epidemiology in critical care (DECCA): an international study. Crit Care. 2010;14(6):R210. https://doi.org/10.1186/cc9333
7. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. https://doi.org/
8. McCusker J, Cole MG, Dendukuri N, Belzile E. Does delirium increase hospital stay? J Am Geriatr Soc. 2003;51(11):1539-1546. https://doi.org/10.1001/jama.291.14.1753
9. Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13(4):234-242. https://doi.org/10.1046/j.1525-1497.1998.00073.x
10. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364. https://doi.org/10.1093/ageing/afl005
11. LaHue SC, Douglas VC, Kuo T, et al. Association between inpatient delirium and hospital readmission in patients >/= 65 years of age: a retrospective cohort study. J Hosp Med. 2019;14(4):201-206. https://doi.org/10.12788/jhm.3130
12. Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. https://doi.org/10.1001/jamainternmed.2014.7779
13. Inouye SK, Bogardus ST, Jr., Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901
14. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. https://doi.org/
15. Alhaidari AA, Allen-Narker RA. An evolving approach to delirium: A mixed-methods process evaluation of a hospital-wide delirium program in New Zealand. Australas J Ageing. 2017. https://doi.org/10.1046/j.1532-5415.2001.49108.x
16. Holroyd-Leduc JM, Khandwala F, Sink KM. How can delirium best be prevented and managed in older patients in hospital? CMAJ. 2010;182(5):465-470. https://doi.org/10.1503/cmaj.080519
17. Siddiqi N, Stockdale R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalised patients. Cochrane Database Syst Rev. 2007(2):CD005563. https://doi.org/ 10.1002/14651858.CD005563.pub2
18. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. https://doi.org/10.1002/14651858.CD005563.pub3
19. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. https://doi.org/10.1016/S0140-6736(13)60688-1
20. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857.
21. LaHue SC, Liu VX. Loud and clear: sensory impairment, delirium, and functional recovery in critical illness. Am J Respir Crit Care Med. 2016;194(3):252-253. https://doi.org/10.1164/rccm.201602-0372ED
22. Ritter SRF, Cardoso AF, Lins MMP, Zoccoli TLV, Freitas MPD, Camargos EF. Underdiagnosis of delirium in the elderly in acute care hospital settings: lessons not learned. Psychogeriatrics. 2018;18(4):268-275. https://doi.org/10.1111/psyg.12324
23. Douglas VC, Hessler CS, Dhaliwal G, et al. The AWOL tool: derivation and validation of a delirium prediction rule. J Hosp Med. 2013;8(9):493-499. https://doi.org/10.1002/jhm.2062
24. Tombaugh TN, McDowell I, Kristjansson B, Hubley AM. Mini-Mental State Examination (MMSE) and the modified MMSE (3MS): A psychometric comparison and normative data. Psychol Assessment. 1996;8(1):48-59. https://doi.org/10.1037/1040-3590.8.1.48
25. Young J, Murthy L, Westby M, Akunne A, O’Mahony R, Guideline Development Group. Diagnosis, prevention, and management of delirium: summary of NICE guidance. BMJ. 2010;341:c3704. https://doi.org/10.1136/bmj.c3704
26. Hargrave A, Bastiaens J, Bourgeois JA, et al. Validation of a nurse-based delirium-screening tool for hospitalized patients. Psychosomatics. 2017;58(6):594-603. https://doi.org/10.1016/j.psym.2017.05.005
27. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710. https://doi.org/10.1001/jama.286.21.2703
28. Strijbos MJ, Steunenberg B, van der Mast RC, Inouye SK, Schuurmans MJ. Design and methods of the Hospital Elder Life Program (HELP), a multicomponent targeted intervention to prevent delirium in hospitalized older patients: efficacy and cost-effectiveness in Dutch health care. BMC Geriatr. 2013;13:78. https://doi.org/10.1186/1471-2318-13-78
29. Taheri PA, Butz DA, Greenfield LJ. Length of stay has minimal impact on the cost of hospital admission. J Am Coll Surg. 2000;191(2):123-130. https://doi.org/10.1016/s1072-7515(00)00352-5
30. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. https://doi.org/10.1038/nrneurol.2009.24
© 2021 Society of Hospital Medicine
Sleep Medicine A Special Report
Sleep Medicine A Special Report
- MOBILE PHONE RELIANCE
How it’s linked to young adults’ sleep problems in two studies - OBSTRUCTIVE SLEEP APNEA
New research validates phenotypes in Latinos - DEMENTIA
Papers show associations between disease and sleep therapies
Sleep Medicine A Special Report
- MOBILE PHONE RELIANCE
How it’s linked to young adults’ sleep problems in two studies - OBSTRUCTIVE SLEEP APNEA
New research validates phenotypes in Latinos - DEMENTIA
Papers show associations between disease and sleep therapies
Sleep Medicine A Special Report
- MOBILE PHONE RELIANCE
How it’s linked to young adults’ sleep problems in two studies - OBSTRUCTIVE SLEEP APNEA
New research validates phenotypes in Latinos - DEMENTIA
Papers show associations between disease and sleep therapies
VENUS: Ustekinumab appears superior to vedolizumab for refractory Crohn’s disease
Ustekinumab (Stelara, Janssen) appears superior to vedolizumab (Entyvio, Takeda) on multiple measures of response and remission among patients with Crohn’s disease who failed at least one anti–tumor necrosis factor (TNF) therapy, in a retrospective analysis.
Of patients taking ustekinumab, a higher proportion (51%) met the primary endpoint of corticosteroid-free clinical remission at week 54. In the vedolizumab group, only 41% achieved the same outcome.
“Failure to anti-TNF therapy is a major concern in Crohn’s disease,” Anthony Buisson, MD, PhD, head of the Inflammatory Bowel Disease Unit, University Hospital Estaing, Clermont-Ferrand, France, said during the Digestive Disease Week 2021 virtual meeting.
Dr. Buisson estimated that 15% of patients with Crohn’s disease experience primary failure from an anti-TNF agent. Also, only slightly more than one-third (37%) remain in clinical remission at 1 year.
With that in mind, Dr. Buisson and his colleagues conducted the VENUS study to evaluate outcomes between ustekinumab and vedolizumab. These two biologic agents are indicated for Crohn’s disease and feature different mechanisms of action, compared with anti-TNF agents.
They assessed 312 adults with Crohn’s disease from two patient cohorts in France. All participants failed prior treatment with at least one anti-TNF agent, including approximately 20% who experienced a primary nonresponse.
The retrospective analysis included 224 patients treated with ustekinumab and another 88 with vedolizumab between July 2014 and May 2020. The two groups were comparable based on a propensity analysis. Other medications were allowed at the physician’s discretion.
Nonresponders and other outcomes
“Vedolizumab patients were more likely to be primary nonresponders than ustekinumab patients,” Dr. Buisson said. This group included 6% of patients taking ustekinumab versus 14% of those taking vedolizumab.
In contrast, regarding secondary loss of response, “we did not observe any [significant] difference between two groups,” he added.
The investigators defined corticosteroid-free remission as a Crohn’s Disease Activity Index less than 150 at week 54. They also assessed “deep remission” at 14 weeks, which was defined as meeting corticosteroid-free remission and a fecal calprotectin of less than 100 mcg/g.
They found that 26% of patients who received ustekinumab met the deep remission criteria versus 4% of those who received vedolizumab.
Dr. Buisson and colleagues also looked at time to drug escalation. A Kaplan Meier curve revealed that patients taking vedolizumab were more likely to be escalated, compared with those taking ustekinumab (hazard ratio, 1.35).
Furthermore, those treated with vedolizumab also featured a higher risk for drug discontinuation because of therapeutic failure (HR, 1.53).
“This is an interesting study comparing ustekinumab with vedolizumab in Crohn’s disease patients who have failed prior anti-TNF therapy,” Farah Monzur, MD, who was not affiliated with the study, told this news organization. “Although the researchers assessed corticosteroid-free remission and ‘deep remission,’ ” she said, endoscopic and histologic remission were not studied, “which have become the new targets to achieve.”
“Even so, this study adds to the literature, aiming to position these newer biologics in the treatment algorithm,” added Dr. Monzur, assistant professor of medicine and medical director of ambulatory GI at Stony Brook (N.Y.) Medicine.
Superior in subgroup analyses as well
“No subgroups were identified where vedolizumab was more effective than ustekinumab,” Dr. Buisson said. “In contrast, ustekinumab was more effective in five subgroups.”
The subgroups favoring ustekinumab included those patients not taking steroids at baseline, with no prior bowel resection, with a noncomplicated phenotype, with upper gastrointestinal involvement, and older than 35 years of age.
The retrospective analysis design was a limitation. The long follow-up and large sample size were strengths.
The authors concluded that ustekinumab was more effective to achieve early and long-term efficacy than vedolizumab in patients with Crohn’s disease who previously failed to anti-TNF agents.
“However, these data should be confirmed in a head-to-head randomized controlled trial,” Dr. Buisson said.
Dr. Buisson disclosed that he is a consultant for Janssen and Takeda. Dr. Monzur has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com..
Ustekinumab (Stelara, Janssen) appears superior to vedolizumab (Entyvio, Takeda) on multiple measures of response and remission among patients with Crohn’s disease who failed at least one anti–tumor necrosis factor (TNF) therapy, in a retrospective analysis.
Of patients taking ustekinumab, a higher proportion (51%) met the primary endpoint of corticosteroid-free clinical remission at week 54. In the vedolizumab group, only 41% achieved the same outcome.
“Failure to anti-TNF therapy is a major concern in Crohn’s disease,” Anthony Buisson, MD, PhD, head of the Inflammatory Bowel Disease Unit, University Hospital Estaing, Clermont-Ferrand, France, said during the Digestive Disease Week 2021 virtual meeting.
Dr. Buisson estimated that 15% of patients with Crohn’s disease experience primary failure from an anti-TNF agent. Also, only slightly more than one-third (37%) remain in clinical remission at 1 year.
With that in mind, Dr. Buisson and his colleagues conducted the VENUS study to evaluate outcomes between ustekinumab and vedolizumab. These two biologic agents are indicated for Crohn’s disease and feature different mechanisms of action, compared with anti-TNF agents.
They assessed 312 adults with Crohn’s disease from two patient cohorts in France. All participants failed prior treatment with at least one anti-TNF agent, including approximately 20% who experienced a primary nonresponse.
The retrospective analysis included 224 patients treated with ustekinumab and another 88 with vedolizumab between July 2014 and May 2020. The two groups were comparable based on a propensity analysis. Other medications were allowed at the physician’s discretion.
Nonresponders and other outcomes
“Vedolizumab patients were more likely to be primary nonresponders than ustekinumab patients,” Dr. Buisson said. This group included 6% of patients taking ustekinumab versus 14% of those taking vedolizumab.
In contrast, regarding secondary loss of response, “we did not observe any [significant] difference between two groups,” he added.
The investigators defined corticosteroid-free remission as a Crohn’s Disease Activity Index less than 150 at week 54. They also assessed “deep remission” at 14 weeks, which was defined as meeting corticosteroid-free remission and a fecal calprotectin of less than 100 mcg/g.
They found that 26% of patients who received ustekinumab met the deep remission criteria versus 4% of those who received vedolizumab.
Dr. Buisson and colleagues also looked at time to drug escalation. A Kaplan Meier curve revealed that patients taking vedolizumab were more likely to be escalated, compared with those taking ustekinumab (hazard ratio, 1.35).
Furthermore, those treated with vedolizumab also featured a higher risk for drug discontinuation because of therapeutic failure (HR, 1.53).
“This is an interesting study comparing ustekinumab with vedolizumab in Crohn’s disease patients who have failed prior anti-TNF therapy,” Farah Monzur, MD, who was not affiliated with the study, told this news organization. “Although the researchers assessed corticosteroid-free remission and ‘deep remission,’ ” she said, endoscopic and histologic remission were not studied, “which have become the new targets to achieve.”
“Even so, this study adds to the literature, aiming to position these newer biologics in the treatment algorithm,” added Dr. Monzur, assistant professor of medicine and medical director of ambulatory GI at Stony Brook (N.Y.) Medicine.
Superior in subgroup analyses as well
“No subgroups were identified where vedolizumab was more effective than ustekinumab,” Dr. Buisson said. “In contrast, ustekinumab was more effective in five subgroups.”
The subgroups favoring ustekinumab included those patients not taking steroids at baseline, with no prior bowel resection, with a noncomplicated phenotype, with upper gastrointestinal involvement, and older than 35 years of age.
The retrospective analysis design was a limitation. The long follow-up and large sample size were strengths.
The authors concluded that ustekinumab was more effective to achieve early and long-term efficacy than vedolizumab in patients with Crohn’s disease who previously failed to anti-TNF agents.
“However, these data should be confirmed in a head-to-head randomized controlled trial,” Dr. Buisson said.
Dr. Buisson disclosed that he is a consultant for Janssen and Takeda. Dr. Monzur has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com..
Ustekinumab (Stelara, Janssen) appears superior to vedolizumab (Entyvio, Takeda) on multiple measures of response and remission among patients with Crohn’s disease who failed at least one anti–tumor necrosis factor (TNF) therapy, in a retrospective analysis.
Of patients taking ustekinumab, a higher proportion (51%) met the primary endpoint of corticosteroid-free clinical remission at week 54. In the vedolizumab group, only 41% achieved the same outcome.
“Failure to anti-TNF therapy is a major concern in Crohn’s disease,” Anthony Buisson, MD, PhD, head of the Inflammatory Bowel Disease Unit, University Hospital Estaing, Clermont-Ferrand, France, said during the Digestive Disease Week 2021 virtual meeting.
Dr. Buisson estimated that 15% of patients with Crohn’s disease experience primary failure from an anti-TNF agent. Also, only slightly more than one-third (37%) remain in clinical remission at 1 year.
With that in mind, Dr. Buisson and his colleagues conducted the VENUS study to evaluate outcomes between ustekinumab and vedolizumab. These two biologic agents are indicated for Crohn’s disease and feature different mechanisms of action, compared with anti-TNF agents.
They assessed 312 adults with Crohn’s disease from two patient cohorts in France. All participants failed prior treatment with at least one anti-TNF agent, including approximately 20% who experienced a primary nonresponse.
The retrospective analysis included 224 patients treated with ustekinumab and another 88 with vedolizumab between July 2014 and May 2020. The two groups were comparable based on a propensity analysis. Other medications were allowed at the physician’s discretion.
Nonresponders and other outcomes
“Vedolizumab patients were more likely to be primary nonresponders than ustekinumab patients,” Dr. Buisson said. This group included 6% of patients taking ustekinumab versus 14% of those taking vedolizumab.
In contrast, regarding secondary loss of response, “we did not observe any [significant] difference between two groups,” he added.
The investigators defined corticosteroid-free remission as a Crohn’s Disease Activity Index less than 150 at week 54. They also assessed “deep remission” at 14 weeks, which was defined as meeting corticosteroid-free remission and a fecal calprotectin of less than 100 mcg/g.
They found that 26% of patients who received ustekinumab met the deep remission criteria versus 4% of those who received vedolizumab.
Dr. Buisson and colleagues also looked at time to drug escalation. A Kaplan Meier curve revealed that patients taking vedolizumab were more likely to be escalated, compared with those taking ustekinumab (hazard ratio, 1.35).
Furthermore, those treated with vedolizumab also featured a higher risk for drug discontinuation because of therapeutic failure (HR, 1.53).
“This is an interesting study comparing ustekinumab with vedolizumab in Crohn’s disease patients who have failed prior anti-TNF therapy,” Farah Monzur, MD, who was not affiliated with the study, told this news organization. “Although the researchers assessed corticosteroid-free remission and ‘deep remission,’ ” she said, endoscopic and histologic remission were not studied, “which have become the new targets to achieve.”
“Even so, this study adds to the literature, aiming to position these newer biologics in the treatment algorithm,” added Dr. Monzur, assistant professor of medicine and medical director of ambulatory GI at Stony Brook (N.Y.) Medicine.
Superior in subgroup analyses as well
“No subgroups were identified where vedolizumab was more effective than ustekinumab,” Dr. Buisson said. “In contrast, ustekinumab was more effective in five subgroups.”
The subgroups favoring ustekinumab included those patients not taking steroids at baseline, with no prior bowel resection, with a noncomplicated phenotype, with upper gastrointestinal involvement, and older than 35 years of age.
The retrospective analysis design was a limitation. The long follow-up and large sample size were strengths.
The authors concluded that ustekinumab was more effective to achieve early and long-term efficacy than vedolizumab in patients with Crohn’s disease who previously failed to anti-TNF agents.
“However, these data should be confirmed in a head-to-head randomized controlled trial,” Dr. Buisson said.
Dr. Buisson disclosed that he is a consultant for Janssen and Takeda. Dr. Monzur has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com..
New obesity target? Dopamine circuit in brainstem affects satiety
Researchers have discovered a new dopaminergic neural circuit leading to the hindbrain that is involved in satiety (feeling full and eating cessation) in mice, which may eventually lead to new ways to treat obesity.
Moreover, when mice were given methylphenidate (Ritalin, Concerta) – a stimulant approved to treat attention deficit hyperactivity disorder (ADHD) with a well-known side effect of decreasing appetite – signals in this dopaminergic pathway were enhanced and the mice ate less.
The study by Yong Han, PhD, a postdoctoral associate at Baylor College of Medicine, Houston, and colleagues was published online May 27 in Science Advances.
“We identified a new dopamine neural circuit from the midbrain to the hindbrain (brainstem) that regulates feeding behavior through an enhanced satiation response,” senior author Qi Wu, PhD, assistant professor in pediatrics-nutrition at Baylor College of Medicine, summarized in an interview.
The findings suggest that “people with obesity have a compromised dopaminergic neural pathway, presumably in ways that delay the satiation response, which makes them eat more, have a larger meal,” he explained.
Newly identified brain circuit plays a key role in satiety response
The study is about a circuit in the brain that helps precisely regulate the size of food portion consumed, Dr. Wu emphasized in a statement from the university, adding that the satiation response is as important as appetite.
Importantly, the results also provide clues about how methylphenidate can lead to weight loss.
Regulators have deemed that methylphenidate, a controlled substance with other side effects such as anxiety and a fast heart rate, is safe and effective for ADHD, Dr. Wu noted.
He speculated that, “If researchers want to do clinical trials of methylphenidate for obesity, it ultimately could evolve to be an anti-obesity drug, alone or combined with other drugs, or possibly derivatives of methylphenidate could be tested.”
The brain circuit “we discovered is the first to be fully described to regulate portion size via dopamine signaling,” Dr. Han stressed in the statement.
“Our new study shows that a circuit connecting neurons that produce dopamine, a chemical messenger previously known for the regulation of motivation and pleasure, has a new [critical] role in the control of feeding through dynamically regulating the satiety response,” he explained.
Brain signals that control portion size
Earlier studies that investigated how the dopaminergic system may regulate food intake, appetite, and body weight, have produced conflicting results, Dr. Wu said.
The researchers performed several experiments in mice that included the use of cell-specific circuitry mapping, optogenetics, and real-time recordings of brain activity.
They identified a new dopaminergic neural circuit comprised of dopaminergic neurons in the caudal ventral tegmental area (DA-VTA neurons) in the midbrain that directly innervate dopamine receptor D1-expressing neurons within the lateral parabrachial nucleus (DRD1-LPBN neurons) in the hindbrain.
There were four main findings:
- DA-VTA neurons were activated immediately before the cessation of each feeding bout.
- Actively inhibiting DA-VTA neurons before the end of each feeding bout prolonged the feeding.
- Activating DRD1-LPBN neurons inhibited feeding.
- Mice that lacked the DRD1 gene ate much more and gained weight.
“Our study illuminates a hindbrain dopaminergic circuit that controls feeding through dynamic regulation in satiety response and meal structure,” the researchers reiterate.
The study was supported by grants from the National Institutes of Health, NIH Digestive Diseases Center, Pew Charitable Trust, American Diabetes Association, Baylor Collaborative Faculty Research Investment Program, USDA/CRIS, USDA/ARS, American Heart Association, and NIH Centers of Biomedical Research Excellence, and by Pew and Kavli scholarships. The researchers have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Researchers have discovered a new dopaminergic neural circuit leading to the hindbrain that is involved in satiety (feeling full and eating cessation) in mice, which may eventually lead to new ways to treat obesity.
Moreover, when mice were given methylphenidate (Ritalin, Concerta) – a stimulant approved to treat attention deficit hyperactivity disorder (ADHD) with a well-known side effect of decreasing appetite – signals in this dopaminergic pathway were enhanced and the mice ate less.
The study by Yong Han, PhD, a postdoctoral associate at Baylor College of Medicine, Houston, and colleagues was published online May 27 in Science Advances.
“We identified a new dopamine neural circuit from the midbrain to the hindbrain (brainstem) that regulates feeding behavior through an enhanced satiation response,” senior author Qi Wu, PhD, assistant professor in pediatrics-nutrition at Baylor College of Medicine, summarized in an interview.
The findings suggest that “people with obesity have a compromised dopaminergic neural pathway, presumably in ways that delay the satiation response, which makes them eat more, have a larger meal,” he explained.
Newly identified brain circuit plays a key role in satiety response
The study is about a circuit in the brain that helps precisely regulate the size of food portion consumed, Dr. Wu emphasized in a statement from the university, adding that the satiation response is as important as appetite.
Importantly, the results also provide clues about how methylphenidate can lead to weight loss.
Regulators have deemed that methylphenidate, a controlled substance with other side effects such as anxiety and a fast heart rate, is safe and effective for ADHD, Dr. Wu noted.
He speculated that, “If researchers want to do clinical trials of methylphenidate for obesity, it ultimately could evolve to be an anti-obesity drug, alone or combined with other drugs, or possibly derivatives of methylphenidate could be tested.”
The brain circuit “we discovered is the first to be fully described to regulate portion size via dopamine signaling,” Dr. Han stressed in the statement.
“Our new study shows that a circuit connecting neurons that produce dopamine, a chemical messenger previously known for the regulation of motivation and pleasure, has a new [critical] role in the control of feeding through dynamically regulating the satiety response,” he explained.
Brain signals that control portion size
Earlier studies that investigated how the dopaminergic system may regulate food intake, appetite, and body weight, have produced conflicting results, Dr. Wu said.
The researchers performed several experiments in mice that included the use of cell-specific circuitry mapping, optogenetics, and real-time recordings of brain activity.
They identified a new dopaminergic neural circuit comprised of dopaminergic neurons in the caudal ventral tegmental area (DA-VTA neurons) in the midbrain that directly innervate dopamine receptor D1-expressing neurons within the lateral parabrachial nucleus (DRD1-LPBN neurons) in the hindbrain.
There were four main findings:
- DA-VTA neurons were activated immediately before the cessation of each feeding bout.
- Actively inhibiting DA-VTA neurons before the end of each feeding bout prolonged the feeding.
- Activating DRD1-LPBN neurons inhibited feeding.
- Mice that lacked the DRD1 gene ate much more and gained weight.
“Our study illuminates a hindbrain dopaminergic circuit that controls feeding through dynamic regulation in satiety response and meal structure,” the researchers reiterate.
The study was supported by grants from the National Institutes of Health, NIH Digestive Diseases Center, Pew Charitable Trust, American Diabetes Association, Baylor Collaborative Faculty Research Investment Program, USDA/CRIS, USDA/ARS, American Heart Association, and NIH Centers of Biomedical Research Excellence, and by Pew and Kavli scholarships. The researchers have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Researchers have discovered a new dopaminergic neural circuit leading to the hindbrain that is involved in satiety (feeling full and eating cessation) in mice, which may eventually lead to new ways to treat obesity.
Moreover, when mice were given methylphenidate (Ritalin, Concerta) – a stimulant approved to treat attention deficit hyperactivity disorder (ADHD) with a well-known side effect of decreasing appetite – signals in this dopaminergic pathway were enhanced and the mice ate less.
The study by Yong Han, PhD, a postdoctoral associate at Baylor College of Medicine, Houston, and colleagues was published online May 27 in Science Advances.
“We identified a new dopamine neural circuit from the midbrain to the hindbrain (brainstem) that regulates feeding behavior through an enhanced satiation response,” senior author Qi Wu, PhD, assistant professor in pediatrics-nutrition at Baylor College of Medicine, summarized in an interview.
The findings suggest that “people with obesity have a compromised dopaminergic neural pathway, presumably in ways that delay the satiation response, which makes them eat more, have a larger meal,” he explained.
Newly identified brain circuit plays a key role in satiety response
The study is about a circuit in the brain that helps precisely regulate the size of food portion consumed, Dr. Wu emphasized in a statement from the university, adding that the satiation response is as important as appetite.
Importantly, the results also provide clues about how methylphenidate can lead to weight loss.
Regulators have deemed that methylphenidate, a controlled substance with other side effects such as anxiety and a fast heart rate, is safe and effective for ADHD, Dr. Wu noted.
He speculated that, “If researchers want to do clinical trials of methylphenidate for obesity, it ultimately could evolve to be an anti-obesity drug, alone or combined with other drugs, or possibly derivatives of methylphenidate could be tested.”
The brain circuit “we discovered is the first to be fully described to regulate portion size via dopamine signaling,” Dr. Han stressed in the statement.
“Our new study shows that a circuit connecting neurons that produce dopamine, a chemical messenger previously known for the regulation of motivation and pleasure, has a new [critical] role in the control of feeding through dynamically regulating the satiety response,” he explained.
Brain signals that control portion size
Earlier studies that investigated how the dopaminergic system may regulate food intake, appetite, and body weight, have produced conflicting results, Dr. Wu said.
The researchers performed several experiments in mice that included the use of cell-specific circuitry mapping, optogenetics, and real-time recordings of brain activity.
They identified a new dopaminergic neural circuit comprised of dopaminergic neurons in the caudal ventral tegmental area (DA-VTA neurons) in the midbrain that directly innervate dopamine receptor D1-expressing neurons within the lateral parabrachial nucleus (DRD1-LPBN neurons) in the hindbrain.
There were four main findings:
- DA-VTA neurons were activated immediately before the cessation of each feeding bout.
- Actively inhibiting DA-VTA neurons before the end of each feeding bout prolonged the feeding.
- Activating DRD1-LPBN neurons inhibited feeding.
- Mice that lacked the DRD1 gene ate much more and gained weight.
“Our study illuminates a hindbrain dopaminergic circuit that controls feeding through dynamic regulation in satiety response and meal structure,” the researchers reiterate.
The study was supported by grants from the National Institutes of Health, NIH Digestive Diseases Center, Pew Charitable Trust, American Diabetes Association, Baylor Collaborative Faculty Research Investment Program, USDA/CRIS, USDA/ARS, American Heart Association, and NIH Centers of Biomedical Research Excellence, and by Pew and Kavli scholarships. The researchers have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
A primer on COVID-19 in hospitalized children
Converge 2021 session
COVID-19 in Children
Presenter
Philip Zachariah, MD, MPH
Session summary
Children have been less severely affected by COVID-19 than adults (hospitalization rates around 5%). However, once hospitalized, ICU admission rates in children have been similar to adults, around 30%. Mortality has been 1%-2%. Risk factors for more severe acute SARS CoV-2 infections include age extremes, minorities, obesity, medical complexity, immunocompromised pediatric patients, and asthma.
Multisystem-inflammatory-syndrome-in-children (MIS-C) continues to present among persistently febrile children with multisystem findings and the history of acute COVID-19 infection in prior 3-6 weeks. There seems to be a link between the immunological defects in type I and II interferon production, as autoantibodies to type I interferon may predispose to severe disease. Dr. Zachariah of Columbia University Medical Center in New York, discussed the recent study exploring intravenous immunoglobulin (IVIG) alone versus IVIG and steroids as treatment options for MIS-C. So far, the failure rates in IVIG-alone group were higher (51%) versus IVIG and steroids (9%).
Besides MIS-C, many neurological manifestations of COVID-19 have been seen among children including GBS, seizures, encephalitis, cranial neuropathies, and demyelination cases. Diabetic ketoacidosis (DKA), secondary hemophagocytic lymphohistiocytosis (HLH), and pseudo-appendicitis have all been described in the literature, however, larger case control studied are needed.
In children, clinical vascular thrombotic events (VTEs) are rare. Anticoagulant thromboprophylaxis is suggested for hospitalized patients with COVID-19–related illness, whose D-dimer is >5 times upper limit of normal values and who have one or more non–COVID-19 related clinical risk factors for hospital acquired VTEs.
Key takeaways
- Once hospitalized, the ICU admission rates for children have been similar to those in adults, ~30%.
- MIS-C is showing lower failure rates if treated with IVIG and steroids, and most reliable laboratory findings should be elevated C-reactive protein, lymphopenia, and elevated brain natriuretic peptide.
- In hospitalized children with COVID-19, clinical VTEs are rare.
Dr. Giordano is an associate professor of pediatrics at Columbia University Medical Center in New York. She is a pediatric hospitalist with expertise in pediatric surgical comanagement
Converge 2021 session
COVID-19 in Children
Presenter
Philip Zachariah, MD, MPH
Session summary
Children have been less severely affected by COVID-19 than adults (hospitalization rates around 5%). However, once hospitalized, ICU admission rates in children have been similar to adults, around 30%. Mortality has been 1%-2%. Risk factors for more severe acute SARS CoV-2 infections include age extremes, minorities, obesity, medical complexity, immunocompromised pediatric patients, and asthma.
Multisystem-inflammatory-syndrome-in-children (MIS-C) continues to present among persistently febrile children with multisystem findings and the history of acute COVID-19 infection in prior 3-6 weeks. There seems to be a link between the immunological defects in type I and II interferon production, as autoantibodies to type I interferon may predispose to severe disease. Dr. Zachariah of Columbia University Medical Center in New York, discussed the recent study exploring intravenous immunoglobulin (IVIG) alone versus IVIG and steroids as treatment options for MIS-C. So far, the failure rates in IVIG-alone group were higher (51%) versus IVIG and steroids (9%).
Besides MIS-C, many neurological manifestations of COVID-19 have been seen among children including GBS, seizures, encephalitis, cranial neuropathies, and demyelination cases. Diabetic ketoacidosis (DKA), secondary hemophagocytic lymphohistiocytosis (HLH), and pseudo-appendicitis have all been described in the literature, however, larger case control studied are needed.
In children, clinical vascular thrombotic events (VTEs) are rare. Anticoagulant thromboprophylaxis is suggested for hospitalized patients with COVID-19–related illness, whose D-dimer is >5 times upper limit of normal values and who have one or more non–COVID-19 related clinical risk factors for hospital acquired VTEs.
Key takeaways
- Once hospitalized, the ICU admission rates for children have been similar to those in adults, ~30%.
- MIS-C is showing lower failure rates if treated with IVIG and steroids, and most reliable laboratory findings should be elevated C-reactive protein, lymphopenia, and elevated brain natriuretic peptide.
- In hospitalized children with COVID-19, clinical VTEs are rare.
Dr. Giordano is an associate professor of pediatrics at Columbia University Medical Center in New York. She is a pediatric hospitalist with expertise in pediatric surgical comanagement
Converge 2021 session
COVID-19 in Children
Presenter
Philip Zachariah, MD, MPH
Session summary
Children have been less severely affected by COVID-19 than adults (hospitalization rates around 5%). However, once hospitalized, ICU admission rates in children have been similar to adults, around 30%. Mortality has been 1%-2%. Risk factors for more severe acute SARS CoV-2 infections include age extremes, minorities, obesity, medical complexity, immunocompromised pediatric patients, and asthma.
Multisystem-inflammatory-syndrome-in-children (MIS-C) continues to present among persistently febrile children with multisystem findings and the history of acute COVID-19 infection in prior 3-6 weeks. There seems to be a link between the immunological defects in type I and II interferon production, as autoantibodies to type I interferon may predispose to severe disease. Dr. Zachariah of Columbia University Medical Center in New York, discussed the recent study exploring intravenous immunoglobulin (IVIG) alone versus IVIG and steroids as treatment options for MIS-C. So far, the failure rates in IVIG-alone group were higher (51%) versus IVIG and steroids (9%).
Besides MIS-C, many neurological manifestations of COVID-19 have been seen among children including GBS, seizures, encephalitis, cranial neuropathies, and demyelination cases. Diabetic ketoacidosis (DKA), secondary hemophagocytic lymphohistiocytosis (HLH), and pseudo-appendicitis have all been described in the literature, however, larger case control studied are needed.
In children, clinical vascular thrombotic events (VTEs) are rare. Anticoagulant thromboprophylaxis is suggested for hospitalized patients with COVID-19–related illness, whose D-dimer is >5 times upper limit of normal values and who have one or more non–COVID-19 related clinical risk factors for hospital acquired VTEs.
Key takeaways
- Once hospitalized, the ICU admission rates for children have been similar to those in adults, ~30%.
- MIS-C is showing lower failure rates if treated with IVIG and steroids, and most reliable laboratory findings should be elevated C-reactive protein, lymphopenia, and elevated brain natriuretic peptide.
- In hospitalized children with COVID-19, clinical VTEs are rare.
Dr. Giordano is an associate professor of pediatrics at Columbia University Medical Center in New York. She is a pediatric hospitalist with expertise in pediatric surgical comanagement
FROM SHM CONVERGE 2021











