User login
By the numbers: Children and COVID-19 prevention
Over 6.3 million doses of COVID-19 vaccine have been administered to children aged 12-17 years as of June 7, according to data from the Centers for Disease Control and Prevention.
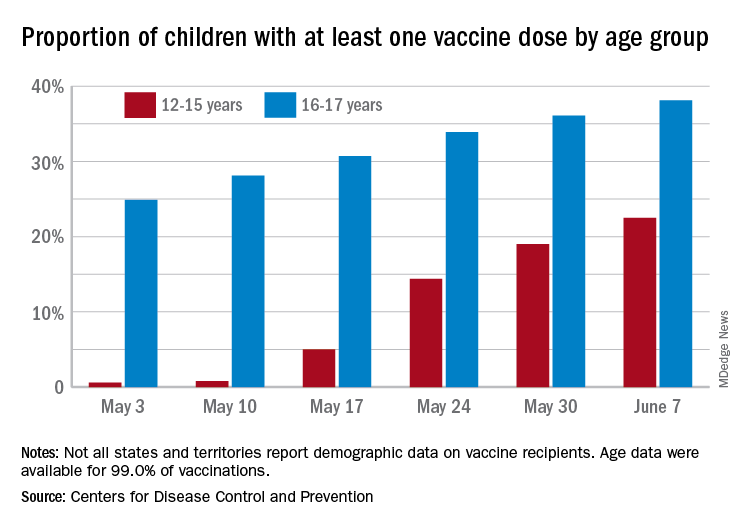
The latest results from the CDC’s COVID Data Tracker show that , with the corresponding figures for vaccine completion coming in at 4.1% and 26.4%. Compared with a week earlier, those numbers are up by 15.4% (one dose) and 486% (completion) for the younger group and by 4.7% and 8.6%, respectively, for the older children.
Children aged 12-15 represented 17.9% of all persons who initiated vaccination in the last 14 days up to June 7, while children aged 16-17 made up 4.8% of vaccine initiation over that period. The 25- to 39-year-olds, at 23.7% of all vaccine initiators, were the only group ahead of those aged 12-15, and the 50- to 64-year-olds were just behind at 17.7%, the CDC data show.
Both groups of children were on the low side, however, when it came to vaccine completion in the last 14 days, with those aged 12-15 at 6.7% of the total and those aged 16-17 years at 4.3%. The only age groups lower than that were ≥75 at 3.5% and <12 at 0.2%, and the highest share of vaccine completion was 26.0% for those aged 25-39, which also happens to be the group with the largest share of the U.S. population (20.5%), the CDC said.
People considered fully vaccinated are those who have received the second dose of a two-dose series or one dose of a single-shot vaccine, but children under age 18 years are eligible only for the Pfizer-BioNTech version, the CDC noted.
Meanwhile, back on the incidence side of the COVID-19 pandemic, the number of new cases in U.S. children for the week ending June 3 was at its lowest point (16,281) since mid-June of 2020, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Cases among children now total 3.99 million, which represents 14.1% of cases among all ages, a proportion that hasn’t increased since mid-May, which hasn’t happened since the two groups started keeping track in mid-April of 2020 in the 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam that report such data by age.
Less encouraging was the CDC’s report that “COVID-19-associated hospitalization rates among adolescents ages 12-17 years increased during March and April, following declines in January and February 2021.”
Children have been experiencing much lower rates of severe disease than those of adults throughout the pandemic, the CDC pointed out, but “recent increases in COVID-19-associated hospitalization rates and the potential for severe disease in adolescents reinforce the importance of continued prevention strategies, including vaccination and the correct and consistent use of masks in those who are not yet fully vaccinated.”
Over 6.3 million doses of COVID-19 vaccine have been administered to children aged 12-17 years as of June 7, according to data from the Centers for Disease Control and Prevention.

The latest results from the CDC’s COVID Data Tracker show that , with the corresponding figures for vaccine completion coming in at 4.1% and 26.4%. Compared with a week earlier, those numbers are up by 15.4% (one dose) and 486% (completion) for the younger group and by 4.7% and 8.6%, respectively, for the older children.
Children aged 12-15 represented 17.9% of all persons who initiated vaccination in the last 14 days up to June 7, while children aged 16-17 made up 4.8% of vaccine initiation over that period. The 25- to 39-year-olds, at 23.7% of all vaccine initiators, were the only group ahead of those aged 12-15, and the 50- to 64-year-olds were just behind at 17.7%, the CDC data show.
Both groups of children were on the low side, however, when it came to vaccine completion in the last 14 days, with those aged 12-15 at 6.7% of the total and those aged 16-17 years at 4.3%. The only age groups lower than that were ≥75 at 3.5% and <12 at 0.2%, and the highest share of vaccine completion was 26.0% for those aged 25-39, which also happens to be the group with the largest share of the U.S. population (20.5%), the CDC said.
People considered fully vaccinated are those who have received the second dose of a two-dose series or one dose of a single-shot vaccine, but children under age 18 years are eligible only for the Pfizer-BioNTech version, the CDC noted.
Meanwhile, back on the incidence side of the COVID-19 pandemic, the number of new cases in U.S. children for the week ending June 3 was at its lowest point (16,281) since mid-June of 2020, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Cases among children now total 3.99 million, which represents 14.1% of cases among all ages, a proportion that hasn’t increased since mid-May, which hasn’t happened since the two groups started keeping track in mid-April of 2020 in the 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam that report such data by age.
Less encouraging was the CDC’s report that “COVID-19-associated hospitalization rates among adolescents ages 12-17 years increased during March and April, following declines in January and February 2021.”
Children have been experiencing much lower rates of severe disease than those of adults throughout the pandemic, the CDC pointed out, but “recent increases in COVID-19-associated hospitalization rates and the potential for severe disease in adolescents reinforce the importance of continued prevention strategies, including vaccination and the correct and consistent use of masks in those who are not yet fully vaccinated.”
Over 6.3 million doses of COVID-19 vaccine have been administered to children aged 12-17 years as of June 7, according to data from the Centers for Disease Control and Prevention.

The latest results from the CDC’s COVID Data Tracker show that , with the corresponding figures for vaccine completion coming in at 4.1% and 26.4%. Compared with a week earlier, those numbers are up by 15.4% (one dose) and 486% (completion) for the younger group and by 4.7% and 8.6%, respectively, for the older children.
Children aged 12-15 represented 17.9% of all persons who initiated vaccination in the last 14 days up to June 7, while children aged 16-17 made up 4.8% of vaccine initiation over that period. The 25- to 39-year-olds, at 23.7% of all vaccine initiators, were the only group ahead of those aged 12-15, and the 50- to 64-year-olds were just behind at 17.7%, the CDC data show.
Both groups of children were on the low side, however, when it came to vaccine completion in the last 14 days, with those aged 12-15 at 6.7% of the total and those aged 16-17 years at 4.3%. The only age groups lower than that were ≥75 at 3.5% and <12 at 0.2%, and the highest share of vaccine completion was 26.0% for those aged 25-39, which also happens to be the group with the largest share of the U.S. population (20.5%), the CDC said.
People considered fully vaccinated are those who have received the second dose of a two-dose series or one dose of a single-shot vaccine, but children under age 18 years are eligible only for the Pfizer-BioNTech version, the CDC noted.
Meanwhile, back on the incidence side of the COVID-19 pandemic, the number of new cases in U.S. children for the week ending June 3 was at its lowest point (16,281) since mid-June of 2020, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Cases among children now total 3.99 million, which represents 14.1% of cases among all ages, a proportion that hasn’t increased since mid-May, which hasn’t happened since the two groups started keeping track in mid-April of 2020 in the 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam that report such data by age.
Less encouraging was the CDC’s report that “COVID-19-associated hospitalization rates among adolescents ages 12-17 years increased during March and April, following declines in January and February 2021.”
Children have been experiencing much lower rates of severe disease than those of adults throughout the pandemic, the CDC pointed out, but “recent increases in COVID-19-associated hospitalization rates and the potential for severe disease in adolescents reinforce the importance of continued prevention strategies, including vaccination and the correct and consistent use of masks in those who are not yet fully vaccinated.”
Air pollution linked to increased fibroid risk in Black women
Black women exposed to ozone air pollution have an increased risk of developing fibroids, according to new research published in Human Production.
Uterine fibroids are a common type of pelvic growth, affecting up to 80% of women by the time they reach age 50, according to the U.S. Department of Health and Human Services. Black women are hit hardest by fibroids; they are diagnosed two to three times the rate of White women and tend to have more severe symptoms.
Researchers are unclear on why exposure to ozone air pollution increases the risk of developing fibroids. However, they believe that when it comes to identifying causes of fibroids and explanations for racial disparities in fibroids, more research that focuses on environmental and neighborhood-level risk factors could help inform policy and interventions to improve gynecologic health.
“A large body of literature from the environmental justice field has documented that people of color, and Black people specifically, are inequitably exposed to air pollution,” study author Amelia K. Wesselink, PhD, assistant professor at Boston University School of Public Health, said in an interview. “And there is growing evidence that air pollution can influence gynecologic health and therefore may contribute to racial disparities in gynecologic outcomes.”
Dr. Wesselink and colleagues wanted to know the extent to which three air pollutants – particulate matter (PM2.5), nitrogen dioxide (NO2) and ozone (03) – were linked to the development of fibroids. To figure this out, they analyzed data on nearly 22,000 premenopausal Black women who lived in 56 metropolitan areas in the United States between 2007 and 2011. They assigned air pollution exposures to participants’ residential addresses collected at baseline and over follow-up and tried to capture long-term exposure to air pollutants.
During the study, nearly 30% of participants reported that they were diagnosed with fibroids. Researchers observed that the exposure to PM2.5 and NO2 was not associated with an increased risk of developing these fibroids.
Dr. Wesselink said the findings may have underestimated fibroid incidence, so they “need to be replicated in a prospective, ultrasound-based study that can identify all fibroid cases.”
“There has not been a lot of research on how air pollution influences fibroid risk, but the two studies that are out there show some evidence of an association,” said Dr. Wesselink. “The fact that our results were consistent with this is interesting. The surprising part of our findings was that we observed an association for ozone, but not for PM2.5 or NO2.”
Nathaniel DeNicola, MD, MSHP, FACOG, a Washington-based obstetrics and gynecology physician affiliated with John Hopkins Health System, applauded the methodology of the study and said the findings prove that patients and doctors should be talking about the environment and exposures to air pollutants.
“[Air pollution] has numerous components to it. And we should try to figure out exactly what components are most dangerous to human health and what doses and what times of life,” said Dr. DeNicola, an environmental health expert.
The increased risk of developing fibroids is a “historical observation” and air pollution may be part of a multifactorial cause of that, Dr. DeNicola said. He said he wouldn’t be surprised if future studies show that “higher exposure [to air pollution] – due to how city planning works, often communities of color are in the areas with the most dense air pollution – exacerbates some other mechanism already in place.
Although it’s unclear how ozone exposure increases fibroid risk, Dr. Wesselink said it may be through a mechanism that is unique to ozone.
“In other words, it might be that there is a factor related to ozone that we did not account for that explains our findings. Vitamin D is a factor that we were not able to account for in this study,” Dr. Wesselink said. “Future work on this topic should consider the role of vitamin D [exposure or deficiency].”
Dr. DeNicola said ozone’s impact may also be tied to its “known association” with hypertension. A 2017 study by Drew B. Day, PhD, of Duke University, Durham, N.C., and colleagues, found that ozone exposure has been linked to hypertension. Meanwhile, a 2015 study has found an association between hypertension and fibroids.
“[This study] does raise an important message. It shines a light where more research needs to be done,” Dr. DeNicola said. “The ozone connection to hypertension was probably most compelling as a true risk factor for uterine fibroids.”
Dr. Wesselink said future work on fibroid etiology should focus on environmental and neighborhood-level exposures to pollutants.
Black women exposed to ozone air pollution have an increased risk of developing fibroids, according to new research published in Human Production.
Uterine fibroids are a common type of pelvic growth, affecting up to 80% of women by the time they reach age 50, according to the U.S. Department of Health and Human Services. Black women are hit hardest by fibroids; they are diagnosed two to three times the rate of White women and tend to have more severe symptoms.
Researchers are unclear on why exposure to ozone air pollution increases the risk of developing fibroids. However, they believe that when it comes to identifying causes of fibroids and explanations for racial disparities in fibroids, more research that focuses on environmental and neighborhood-level risk factors could help inform policy and interventions to improve gynecologic health.
“A large body of literature from the environmental justice field has documented that people of color, and Black people specifically, are inequitably exposed to air pollution,” study author Amelia K. Wesselink, PhD, assistant professor at Boston University School of Public Health, said in an interview. “And there is growing evidence that air pollution can influence gynecologic health and therefore may contribute to racial disparities in gynecologic outcomes.”
Dr. Wesselink and colleagues wanted to know the extent to which three air pollutants – particulate matter (PM2.5), nitrogen dioxide (NO2) and ozone (03) – were linked to the development of fibroids. To figure this out, they analyzed data on nearly 22,000 premenopausal Black women who lived in 56 metropolitan areas in the United States between 2007 and 2011. They assigned air pollution exposures to participants’ residential addresses collected at baseline and over follow-up and tried to capture long-term exposure to air pollutants.
During the study, nearly 30% of participants reported that they were diagnosed with fibroids. Researchers observed that the exposure to PM2.5 and NO2 was not associated with an increased risk of developing these fibroids.
Dr. Wesselink said the findings may have underestimated fibroid incidence, so they “need to be replicated in a prospective, ultrasound-based study that can identify all fibroid cases.”
“There has not been a lot of research on how air pollution influences fibroid risk, but the two studies that are out there show some evidence of an association,” said Dr. Wesselink. “The fact that our results were consistent with this is interesting. The surprising part of our findings was that we observed an association for ozone, but not for PM2.5 or NO2.”
Nathaniel DeNicola, MD, MSHP, FACOG, a Washington-based obstetrics and gynecology physician affiliated with John Hopkins Health System, applauded the methodology of the study and said the findings prove that patients and doctors should be talking about the environment and exposures to air pollutants.
“[Air pollution] has numerous components to it. And we should try to figure out exactly what components are most dangerous to human health and what doses and what times of life,” said Dr. DeNicola, an environmental health expert.
The increased risk of developing fibroids is a “historical observation” and air pollution may be part of a multifactorial cause of that, Dr. DeNicola said. He said he wouldn’t be surprised if future studies show that “higher exposure [to air pollution] – due to how city planning works, often communities of color are in the areas with the most dense air pollution – exacerbates some other mechanism already in place.
Although it’s unclear how ozone exposure increases fibroid risk, Dr. Wesselink said it may be through a mechanism that is unique to ozone.
“In other words, it might be that there is a factor related to ozone that we did not account for that explains our findings. Vitamin D is a factor that we were not able to account for in this study,” Dr. Wesselink said. “Future work on this topic should consider the role of vitamin D [exposure or deficiency].”
Dr. DeNicola said ozone’s impact may also be tied to its “known association” with hypertension. A 2017 study by Drew B. Day, PhD, of Duke University, Durham, N.C., and colleagues, found that ozone exposure has been linked to hypertension. Meanwhile, a 2015 study has found an association between hypertension and fibroids.
“[This study] does raise an important message. It shines a light where more research needs to be done,” Dr. DeNicola said. “The ozone connection to hypertension was probably most compelling as a true risk factor for uterine fibroids.”
Dr. Wesselink said future work on fibroid etiology should focus on environmental and neighborhood-level exposures to pollutants.
Black women exposed to ozone air pollution have an increased risk of developing fibroids, according to new research published in Human Production.
Uterine fibroids are a common type of pelvic growth, affecting up to 80% of women by the time they reach age 50, according to the U.S. Department of Health and Human Services. Black women are hit hardest by fibroids; they are diagnosed two to three times the rate of White women and tend to have more severe symptoms.
Researchers are unclear on why exposure to ozone air pollution increases the risk of developing fibroids. However, they believe that when it comes to identifying causes of fibroids and explanations for racial disparities in fibroids, more research that focuses on environmental and neighborhood-level risk factors could help inform policy and interventions to improve gynecologic health.
“A large body of literature from the environmental justice field has documented that people of color, and Black people specifically, are inequitably exposed to air pollution,” study author Amelia K. Wesselink, PhD, assistant professor at Boston University School of Public Health, said in an interview. “And there is growing evidence that air pollution can influence gynecologic health and therefore may contribute to racial disparities in gynecologic outcomes.”
Dr. Wesselink and colleagues wanted to know the extent to which three air pollutants – particulate matter (PM2.5), nitrogen dioxide (NO2) and ozone (03) – were linked to the development of fibroids. To figure this out, they analyzed data on nearly 22,000 premenopausal Black women who lived in 56 metropolitan areas in the United States between 2007 and 2011. They assigned air pollution exposures to participants’ residential addresses collected at baseline and over follow-up and tried to capture long-term exposure to air pollutants.
During the study, nearly 30% of participants reported that they were diagnosed with fibroids. Researchers observed that the exposure to PM2.5 and NO2 was not associated with an increased risk of developing these fibroids.
Dr. Wesselink said the findings may have underestimated fibroid incidence, so they “need to be replicated in a prospective, ultrasound-based study that can identify all fibroid cases.”
“There has not been a lot of research on how air pollution influences fibroid risk, but the two studies that are out there show some evidence of an association,” said Dr. Wesselink. “The fact that our results were consistent with this is interesting. The surprising part of our findings was that we observed an association for ozone, but not for PM2.5 or NO2.”
Nathaniel DeNicola, MD, MSHP, FACOG, a Washington-based obstetrics and gynecology physician affiliated with John Hopkins Health System, applauded the methodology of the study and said the findings prove that patients and doctors should be talking about the environment and exposures to air pollutants.
“[Air pollution] has numerous components to it. And we should try to figure out exactly what components are most dangerous to human health and what doses and what times of life,” said Dr. DeNicola, an environmental health expert.
The increased risk of developing fibroids is a “historical observation” and air pollution may be part of a multifactorial cause of that, Dr. DeNicola said. He said he wouldn’t be surprised if future studies show that “higher exposure [to air pollution] – due to how city planning works, often communities of color are in the areas with the most dense air pollution – exacerbates some other mechanism already in place.
Although it’s unclear how ozone exposure increases fibroid risk, Dr. Wesselink said it may be through a mechanism that is unique to ozone.
“In other words, it might be that there is a factor related to ozone that we did not account for that explains our findings. Vitamin D is a factor that we were not able to account for in this study,” Dr. Wesselink said. “Future work on this topic should consider the role of vitamin D [exposure or deficiency].”
Dr. DeNicola said ozone’s impact may also be tied to its “known association” with hypertension. A 2017 study by Drew B. Day, PhD, of Duke University, Durham, N.C., and colleagues, found that ozone exposure has been linked to hypertension. Meanwhile, a 2015 study has found an association between hypertension and fibroids.
“[This study] does raise an important message. It shines a light where more research needs to be done,” Dr. DeNicola said. “The ozone connection to hypertension was probably most compelling as a true risk factor for uterine fibroids.”
Dr. Wesselink said future work on fibroid etiology should focus on environmental and neighborhood-level exposures to pollutants.
NIAID advances universal flu vaccine candidate into phase 1 trial
Last month, U.S. government researchers began a test of an experimental influenza vaccine that they hope will provide long-lasting immunity against multiple strains of the virus. Their project adds to the many approaches that have been tried in the decades-long quest for a universal flu shot.
For the first time, the National Institute of Allergy and Infectious Diseases (NIAID) is testing an investigational flu vaccine, known as FluMos-v1, on people. Researchers in recent years have targeted the stalk or stem of an influenza surface protein called hemagglutinin (HA) in trying to develop better flu vaccines. NIAID said FluMos-v1 is designed to spark production of antibodies against the HA protein from different virus strains, which could make it superior to vaccines now available, NIAID said.
“It could be longer lasting than the traditional flu vaccine and give us what we call super seasonal protection that might go beyond just one flu season to next year’s or the year after, or offer additional protection in a pandemic setting,” Alicia T. Widge, MD, of NIAID’s Vaccine Research Center, who is the principal investigator of the trial, said in an interview.
The phase 1 study (NCT04896086) aims to enroll 35 participants, 15 of whom will receive a single intramuscular injection of a comparator treatment, Flucelvax, which has already been approved by the U.S. Food and Drug Administration. The FluMos-v1 group will start with five participants who will receive one 20-μg dose. If no safety problems emerge at that dosage, another 15 volunteers will receive one 60-μg dose of the investigational vaccine.
The incorporation of a comparator group in the phase 1 study may help investigators get an early idea of how well FluMos-v1 compares to a marketed product, Dr. Widge said. The test will be carried out through the National Institutes of Health Clinical Center.
‘Renaissance’ of flu-vaccine research?
Currently, flu vaccines are reformulated each year in an attempt to match the dominant strain for the upcoming season, an effort that often falls notably short. The estimated vaccine effectiveness rate in the United States has ranged from a low of 19% to a high of 60% in recent years, according to the Centers for Disease Control and Prevention.
Scientists have been working for decades on a universal flu vaccine that would offer better results but haven’t yet identified the right strategy to outwit mutations in the virus. Recent setbacks include BiondVax Pharmaceuticals’ October 2020 announcement of a failed phase 3 trial of its experimental M-001 universal flu vaccine candidate.
But advances in understanding the immune system may set the stage for a “renaissance” in efforts to develop a universal flu vaccine, Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said in an interview.
The COVID-19 pandemic has spurred greater interest in the need to develop a universal flu vaccine, he said. Dr. Osterholm said he is “more optimistic now than ever” about the chances for developing vaccines that can fend off multiple strains over longer periods, although the goal of a shot that can ward off influenza in all cases may remain elusive.
“How good can we make them? Will they ever be really universal? Will they have long periods of protection? I don’t think any of us know that yet,” Dr. Osterholm said. “But this is not the influenza vaccine world of 5 or 7 years ago.”
The mRNA technology used to develop the world’s first approved COVID-19 vaccines, for example, may be applied against influenza, Dr. Osterholm said.
In January 2021, Moderna announced plans to test three development candidates for a seasonal influenza vaccine and aims to start a phase 1 study this year. In an April interview on CNBC’s Squawk Box program, Moderna’s chief executive, Stephané Bancel, spoke about the company’s plans to eventually create a combination vaccine for SARS-Cov-2 and flu viruses.
SARS-CoV-2 “is not going away.” Like flu, this virus will persist and change forms, Ms. Bancel said. Creating a flu shot that outperforms the existing ones would boost confidence in influenza vaccines, which many people now skip, Ms. Bancel said. People might someday be able to get a combination of this more effective flu shot with a COVID-19 vaccine booster in their local pharmacies.
“You can take one dose and then have a nice winter,” Ms. Bancel said of Moderna’s goal for a combination vaccine.
A version of this article first appeared on Medscape.com.
Last month, U.S. government researchers began a test of an experimental influenza vaccine that they hope will provide long-lasting immunity against multiple strains of the virus. Their project adds to the many approaches that have been tried in the decades-long quest for a universal flu shot.
For the first time, the National Institute of Allergy and Infectious Diseases (NIAID) is testing an investigational flu vaccine, known as FluMos-v1, on people. Researchers in recent years have targeted the stalk or stem of an influenza surface protein called hemagglutinin (HA) in trying to develop better flu vaccines. NIAID said FluMos-v1 is designed to spark production of antibodies against the HA protein from different virus strains, which could make it superior to vaccines now available, NIAID said.
“It could be longer lasting than the traditional flu vaccine and give us what we call super seasonal protection that might go beyond just one flu season to next year’s or the year after, or offer additional protection in a pandemic setting,” Alicia T. Widge, MD, of NIAID’s Vaccine Research Center, who is the principal investigator of the trial, said in an interview.
The phase 1 study (NCT04896086) aims to enroll 35 participants, 15 of whom will receive a single intramuscular injection of a comparator treatment, Flucelvax, which has already been approved by the U.S. Food and Drug Administration. The FluMos-v1 group will start with five participants who will receive one 20-μg dose. If no safety problems emerge at that dosage, another 15 volunteers will receive one 60-μg dose of the investigational vaccine.
The incorporation of a comparator group in the phase 1 study may help investigators get an early idea of how well FluMos-v1 compares to a marketed product, Dr. Widge said. The test will be carried out through the National Institutes of Health Clinical Center.
‘Renaissance’ of flu-vaccine research?
Currently, flu vaccines are reformulated each year in an attempt to match the dominant strain for the upcoming season, an effort that often falls notably short. The estimated vaccine effectiveness rate in the United States has ranged from a low of 19% to a high of 60% in recent years, according to the Centers for Disease Control and Prevention.
Scientists have been working for decades on a universal flu vaccine that would offer better results but haven’t yet identified the right strategy to outwit mutations in the virus. Recent setbacks include BiondVax Pharmaceuticals’ October 2020 announcement of a failed phase 3 trial of its experimental M-001 universal flu vaccine candidate.
But advances in understanding the immune system may set the stage for a “renaissance” in efforts to develop a universal flu vaccine, Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said in an interview.
The COVID-19 pandemic has spurred greater interest in the need to develop a universal flu vaccine, he said. Dr. Osterholm said he is “more optimistic now than ever” about the chances for developing vaccines that can fend off multiple strains over longer periods, although the goal of a shot that can ward off influenza in all cases may remain elusive.
“How good can we make them? Will they ever be really universal? Will they have long periods of protection? I don’t think any of us know that yet,” Dr. Osterholm said. “But this is not the influenza vaccine world of 5 or 7 years ago.”
The mRNA technology used to develop the world’s first approved COVID-19 vaccines, for example, may be applied against influenza, Dr. Osterholm said.
In January 2021, Moderna announced plans to test three development candidates for a seasonal influenza vaccine and aims to start a phase 1 study this year. In an April interview on CNBC’s Squawk Box program, Moderna’s chief executive, Stephané Bancel, spoke about the company’s plans to eventually create a combination vaccine for SARS-Cov-2 and flu viruses.
SARS-CoV-2 “is not going away.” Like flu, this virus will persist and change forms, Ms. Bancel said. Creating a flu shot that outperforms the existing ones would boost confidence in influenza vaccines, which many people now skip, Ms. Bancel said. People might someday be able to get a combination of this more effective flu shot with a COVID-19 vaccine booster in their local pharmacies.
“You can take one dose and then have a nice winter,” Ms. Bancel said of Moderna’s goal for a combination vaccine.
A version of this article first appeared on Medscape.com.
Last month, U.S. government researchers began a test of an experimental influenza vaccine that they hope will provide long-lasting immunity against multiple strains of the virus. Their project adds to the many approaches that have been tried in the decades-long quest for a universal flu shot.
For the first time, the National Institute of Allergy and Infectious Diseases (NIAID) is testing an investigational flu vaccine, known as FluMos-v1, on people. Researchers in recent years have targeted the stalk or stem of an influenza surface protein called hemagglutinin (HA) in trying to develop better flu vaccines. NIAID said FluMos-v1 is designed to spark production of antibodies against the HA protein from different virus strains, which could make it superior to vaccines now available, NIAID said.
“It could be longer lasting than the traditional flu vaccine and give us what we call super seasonal protection that might go beyond just one flu season to next year’s or the year after, or offer additional protection in a pandemic setting,” Alicia T. Widge, MD, of NIAID’s Vaccine Research Center, who is the principal investigator of the trial, said in an interview.
The phase 1 study (NCT04896086) aims to enroll 35 participants, 15 of whom will receive a single intramuscular injection of a comparator treatment, Flucelvax, which has already been approved by the U.S. Food and Drug Administration. The FluMos-v1 group will start with five participants who will receive one 20-μg dose. If no safety problems emerge at that dosage, another 15 volunteers will receive one 60-μg dose of the investigational vaccine.
The incorporation of a comparator group in the phase 1 study may help investigators get an early idea of how well FluMos-v1 compares to a marketed product, Dr. Widge said. The test will be carried out through the National Institutes of Health Clinical Center.
‘Renaissance’ of flu-vaccine research?
Currently, flu vaccines are reformulated each year in an attempt to match the dominant strain for the upcoming season, an effort that often falls notably short. The estimated vaccine effectiveness rate in the United States has ranged from a low of 19% to a high of 60% in recent years, according to the Centers for Disease Control and Prevention.
Scientists have been working for decades on a universal flu vaccine that would offer better results but haven’t yet identified the right strategy to outwit mutations in the virus. Recent setbacks include BiondVax Pharmaceuticals’ October 2020 announcement of a failed phase 3 trial of its experimental M-001 universal flu vaccine candidate.
But advances in understanding the immune system may set the stage for a “renaissance” in efforts to develop a universal flu vaccine, Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said in an interview.
The COVID-19 pandemic has spurred greater interest in the need to develop a universal flu vaccine, he said. Dr. Osterholm said he is “more optimistic now than ever” about the chances for developing vaccines that can fend off multiple strains over longer periods, although the goal of a shot that can ward off influenza in all cases may remain elusive.
“How good can we make them? Will they ever be really universal? Will they have long periods of protection? I don’t think any of us know that yet,” Dr. Osterholm said. “But this is not the influenza vaccine world of 5 or 7 years ago.”
The mRNA technology used to develop the world’s first approved COVID-19 vaccines, for example, may be applied against influenza, Dr. Osterholm said.
In January 2021, Moderna announced plans to test three development candidates for a seasonal influenza vaccine and aims to start a phase 1 study this year. In an April interview on CNBC’s Squawk Box program, Moderna’s chief executive, Stephané Bancel, spoke about the company’s plans to eventually create a combination vaccine for SARS-Cov-2 and flu viruses.
SARS-CoV-2 “is not going away.” Like flu, this virus will persist and change forms, Ms. Bancel said. Creating a flu shot that outperforms the existing ones would boost confidence in influenza vaccines, which many people now skip, Ms. Bancel said. People might someday be able to get a combination of this more effective flu shot with a COVID-19 vaccine booster in their local pharmacies.
“You can take one dose and then have a nice winter,” Ms. Bancel said of Moderna’s goal for a combination vaccine.
A version of this article first appeared on Medscape.com.
First year of life sees initial bleeding episodes in children with von Willebrand disease
To remedy a lack of data on infants and toddlers with von Willebrand disease (VWD), researchers examined data on patients collected from the U.S. Hemophilia Treatment Center Network. They examined birth characteristics, bleeding episodes, and complications experienced by 105 patients with VWD who were younger than 2 years of age.
For these patients, the mean age of diagnosis was 7 months, with little variation by sex. Patients with type 2 VWD were diagnosed earlier than those with types 1 or 3 (P = .04), and those with a family history of VWD were diagnosed approximately 4 months earlier than those with none (P < .001), according to the report by Brandi Dupervil, DHSC, of the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, and colleagues.
Approximately 14% of the patients were born preterm and 13% had low birth weight, proportions that were higher than national preterm birth rates (approximately 12% and 8%, respectively). There was no way of knowing from the data whether this was due to the presence of VWD or other factors, according to the report (Blood Adv. 2021;5[8]:2079-86).
Specialized care
The study found that initial bleeding episodes were most commonly oropharyngeal, related to circumcision, or intracranial or extracranial, and that most initial bleeding episodes occurred within the first year of life, according to the researchers.
Overall, there were 274 bleeding episodes among 73 children, including oral/nasal episodes (38 patients experienced 166 episodes), soft tissue hematomas (15 patients experienced 57 episodes), and head injuries, including skull fractures (13 patients experienced 19 episodes), according to the report.
In terms of treatment, among the two-thirds of the patients who had intervention to prevent or treat bleeding, most received either plasma-derived VW factor/factor VIII concentrates or antifibrinolytics.
Overall, the researchers advocated a team approach to treating these children “including genetic counselors throughout the prepartum period who work to increase expectant mothers’ understanding of the risks associated with having a child with VWD.”
They also recommended the input of “adult and pediatric hematologists, obstetrician gynecologists, genetic counselors, nurses, and social workers throughout the pre- and postpartum period who seek to optimize outcomes and disease management.”
The authors reported that they had no competing interests.
To remedy a lack of data on infants and toddlers with von Willebrand disease (VWD), researchers examined data on patients collected from the U.S. Hemophilia Treatment Center Network. They examined birth characteristics, bleeding episodes, and complications experienced by 105 patients with VWD who were younger than 2 years of age.
For these patients, the mean age of diagnosis was 7 months, with little variation by sex. Patients with type 2 VWD were diagnosed earlier than those with types 1 or 3 (P = .04), and those with a family history of VWD were diagnosed approximately 4 months earlier than those with none (P < .001), according to the report by Brandi Dupervil, DHSC, of the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, and colleagues.
Approximately 14% of the patients were born preterm and 13% had low birth weight, proportions that were higher than national preterm birth rates (approximately 12% and 8%, respectively). There was no way of knowing from the data whether this was due to the presence of VWD or other factors, according to the report (Blood Adv. 2021;5[8]:2079-86).
Specialized care
The study found that initial bleeding episodes were most commonly oropharyngeal, related to circumcision, or intracranial or extracranial, and that most initial bleeding episodes occurred within the first year of life, according to the researchers.
Overall, there were 274 bleeding episodes among 73 children, including oral/nasal episodes (38 patients experienced 166 episodes), soft tissue hematomas (15 patients experienced 57 episodes), and head injuries, including skull fractures (13 patients experienced 19 episodes), according to the report.
In terms of treatment, among the two-thirds of the patients who had intervention to prevent or treat bleeding, most received either plasma-derived VW factor/factor VIII concentrates or antifibrinolytics.
Overall, the researchers advocated a team approach to treating these children “including genetic counselors throughout the prepartum period who work to increase expectant mothers’ understanding of the risks associated with having a child with VWD.”
They also recommended the input of “adult and pediatric hematologists, obstetrician gynecologists, genetic counselors, nurses, and social workers throughout the pre- and postpartum period who seek to optimize outcomes and disease management.”
The authors reported that they had no competing interests.
To remedy a lack of data on infants and toddlers with von Willebrand disease (VWD), researchers examined data on patients collected from the U.S. Hemophilia Treatment Center Network. They examined birth characteristics, bleeding episodes, and complications experienced by 105 patients with VWD who were younger than 2 years of age.
For these patients, the mean age of diagnosis was 7 months, with little variation by sex. Patients with type 2 VWD were diagnosed earlier than those with types 1 or 3 (P = .04), and those with a family history of VWD were diagnosed approximately 4 months earlier than those with none (P < .001), according to the report by Brandi Dupervil, DHSC, of the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, and colleagues.
Approximately 14% of the patients were born preterm and 13% had low birth weight, proportions that were higher than national preterm birth rates (approximately 12% and 8%, respectively). There was no way of knowing from the data whether this was due to the presence of VWD or other factors, according to the report (Blood Adv. 2021;5[8]:2079-86).
Specialized care
The study found that initial bleeding episodes were most commonly oropharyngeal, related to circumcision, or intracranial or extracranial, and that most initial bleeding episodes occurred within the first year of life, according to the researchers.
Overall, there were 274 bleeding episodes among 73 children, including oral/nasal episodes (38 patients experienced 166 episodes), soft tissue hematomas (15 patients experienced 57 episodes), and head injuries, including skull fractures (13 patients experienced 19 episodes), according to the report.
In terms of treatment, among the two-thirds of the patients who had intervention to prevent or treat bleeding, most received either plasma-derived VW factor/factor VIII concentrates or antifibrinolytics.
Overall, the researchers advocated a team approach to treating these children “including genetic counselors throughout the prepartum period who work to increase expectant mothers’ understanding of the risks associated with having a child with VWD.”
They also recommended the input of “adult and pediatric hematologists, obstetrician gynecologists, genetic counselors, nurses, and social workers throughout the pre- and postpartum period who seek to optimize outcomes and disease management.”
The authors reported that they had no competing interests.
FROM BLOOD ADVANCES
Improving emergency care for children living outside of urban areas
A new physician workforce study documents that almost all clinically active pediatric emergency physicians in the United States – 99% of them – work in urban areas, and that those who do practice in rural areas are significantly older and closer to retirement age.
The portrait of approximately 2,400 self-identified pediatric emergency medicine (EM) physicians may be unsurprising given the overall propensity of physicians – including board-certified general emergency physicians – to practice in urban areas. Even so, it underscores a decades-long concern that many children do not have access to optimal pediatric emergency care.
And the findings highlight the need, the authors say, to keep pressing to improve emergency care for a population of children with “a mortality rate that is already higher than that of its suburban and urban peers (JAMA Network Open 2021;4[5]:e2110084).”
Emergent care of pediatric patients is well within the scope of practice for physicians with training and board certification in general EM, but children and adolescents have different clinical needs and “there are high-stakes scenarios [in children] that we [as emergency physicians] don’t get exposed to as often because we’re not in a children’s hospital or we just don’t have that additional level of training,” said Christopher L. Bennett, MD, MA, of the department of emergency medicine at Stanford University and lead author of the study.
Researchers have documented that some emergency physicians have some discomfort in caring for very ill pediatric patients, he and his coauthors wrote.
Children account for more than 20% of annual ED visits, and most children who seek emergency care in the United States – upwards of 80% – present to general emergency departments. Yet the vast majority of these EDs care for fewer than 14-15 children a day.
With such low pediatric volume, “there will never be pediatric emergency medicine physicians in the rural hospitals in [our] health care system,” said Kathleen M. Brown, MD, medical director for quality and safety of the Emergency Medicine and Trauma Center at Children’s National Medical Center in Washington.
Redistribution “is not a practical solution, and we’ve known that for a long time,” said Dr. Brown, past chairperson of the American College of Emergency Physicians’ pediatric emergency medicine committee. “That’s why national efforts have focused on better preparing the general emergency department and making sure the hospital workforce is ready to take care of children ... to manage and stabilize [them] and recognize when they need more definitive care.”
Continuing efforts to increase “pediatric readiness” in general EDs is one of the recommendations issued by the American Academy of Pediatrics, ACEP, and Emergency Nurses Association in its most recent joint policy statement on emergency care for children, published in May (Pediatrics 2021;147[5]:e2021050787). A 2018 joint policy statement detailed the resources – medications, equipment, policies, and education – necessary for EDs to provide effective pediatric care (Pediatrics 2018;142[5]:e20182459).
There is some evidence that pediatric readiness has improved and that EDs with higher readiness scores may have better pediatric outcomes and lower mortality, said Dr. Brown, a coauthor of both policy statements. (One study cited in the 2018 policy statement, for example, found that children with extremity immobilization and a pain score of 5 or greater had faster management of their pain and decreased exposure to radiation when they were treated in a better-prepared facility than in a facility with less readiness.)
Yet many hospitals still do not have designated pediatric emergency care coordinators (PECCs) – roles that are widely believed to be central to pediatric readiness. PECCs (physicians and nurses) were recommended in 2006 by the then-Institute of Medicine and have been advocated by the AAP, ACEP, and other organizations.
According to 2013 data from the National Pediatric Readiness Project (NPRP), launched that year by the AAP, ACEP, ENA, and the federal Emergency Medical Services for Children program of the Health Resources and Services Administration, at least 15% of EDs lacked at least 1 piece of recommended equipment, and 81% reported barriers to pediatric emergency care guidelines. The NPRP is currently conducting an updated assessment, Dr. Brown said.
Some experts have proposed a different kind of solution – one in which American Board of Pediatrics–certified pediatric EM physicians would care for selective adult patients with common disease patterns who present to rural EDs, in addition to children. They might provide direct patient care across several hospitals in a region, while also addressing quality improvement and assisting EPs and other providers in the region on pediatric care issues.
The proposal, published in May 2020, comes from the 13-member special subcommittee of the ACEP committee on PEM that was tasked with exploring strategies to improve access to emergency pediatric expertise and disaster preparedness in all settings. The proposal was endorsed by the ACEP board of directors, said Jim Homme, MD, a coauthor of the paper (JACEP Open 2020;1:1520-6.)
“We’re saying, look at the ped-trained pediatric emergency provider more broadly. They can actually successfully care for a broader patient population and make it financially feasible ... [for that physician] to be a part of the system,” said Dr. Homme, program director of the emergency medicine residency at the Mayo Clinic College of Medicine and Science in Rochester, Minn.
“The benefit would be not only having the expertise to see children, but to train up other individuals in the institution, and be advocates for the care of children,” he said.
“We’re not saying we want a pediatrics-trained EM physician in every site so that every child would be seen by one – that’s not the goal,” Dr. Homme said. “The goal is to distribute them more broadly than they currently are, and in doing so, make available the other benefits besides direct patient care.”
Most of the physicians in the United States who identify as pediatric EM physicians have completed either a pediatrics or EM residency, followed by a pediatric EM fellowship. It is much more common to have primary training in pediatrics than in EM, said Dr. Homme and Dr. Bennett. A small number of physicians, like Dr. Homme, are dually trained in pediatrics and EM through the completion of two residencies. Dr. Bennett’s workforce study used the American Medical Association Physician Masterfile database and identified 2,403 clinically active pediatric EPs – 5% of all clinically active emergency physicians. Those practicing in rural areas had a median age of 59, compared with a median age of 46 in urban areas. More than half of the pediatric EPs – 68% – reported having pediatric EM board certification.
Three states – Montana, South Dakota, and Wyoming – had no pediatric EMs at all, Dr. Bennett noted.
Readiness in rural Oregon, New England
Torree McGowan, MD, an emergency physician with the St. Charles Health System in Oregon, works in small critical access hospitals in the rural towns of Madras and Prineville, each several hours by ground to the nearest pediatric hospital. She said she feels well equipped to care for children through her training (a rotation in a pediatric ICU and several months working in pediatric EDs) and through her ongoing work with pediatric patients. Children and adolescents comprise about 20%-30% of her volume.
She sees more pediatric illness – children with respiratory syncytial virus who need respiratory support, for instance, and children with severe asthma or diabetic ketoacidosis – than pediatric trauma. When she faces questions, uncertainties, or wants confirmation of a decision, she consults by phone with pediatric subspecialists.
“I don’t take care of kids on vasopressor drips on a regular basis [for instance],” said Dr. McGowan, who sits on ACEP’s disaster preparedness committee and is an Air Force veteran. “But I know the basics and can phone a colleague to be sure I’m doing it correctly. The ability to outreach is there.”
Telemedicine is valuable, she said, but there may also be value in working alongside a pediatric EM physician. One of her EP colleagues is fellowship-trained in ultrasonography and “leads us in training and quality control,” Dr. McGowan said. “And if she’s on shift with you she’ll teach you all about ultrasound. There’s probably utility in having a pediatric EP who does that as well. But incentivizing that and taking them away from the pediatric hospital is a paradigm shift.”
Either way, she said, “being able to bring that expertise out of urban centers, whether it’s to a hospital group like ours or whether it’s by telemedicine, is really, really helpful.”
Her group does not have official PECCs, but the joint policy statements by AAP/ACEP/ENA on pediatric readiness and the “whole pediatric readiness effort’ have been valuable in “driving conversations” with administrators about needs such as purchasing pediatric-sized video laryngoscope blades and other equipment needed for pediatric emergencies, however infrequently they may occur, Dr. McGowan said.
In New England, researchers leading a grassroots regional intervention to establish a PECC in every ED in the region have reported an increased prevalence of “pediatric champions” from less than 30% 5 years ago to greater than 90% in 2019, investigators have reported (Pediatr Emerg Care. 2021. doi: 10.1097/PEC.0000000000002456).
The initiative involved individual outreach to leaders of each ED – largely through phone and e-mail appeals – and collaboration among the State Emergency Medical Services for Children agencies and ACEP and ENA state chapters. The researchers are currently investigating the direct impact of PECCs on patient outcomes.
More on regionalization of ped-trained EPs
Dr. Bennett sees telemedicine as a primary part of improving pediatric emergency care. “I think that’s where things are going to go in pediatric emergency medicine,” he said, especially in the wake of COVID-19: “I don’t see how it’s not going to become much more common.”
Dr. Homme maintains that a broader integration of ABP-certified pediatric EM physicians into underserved regions would advance ED preparedness in a way that telemedicine, or even the appointment of PECCs, does not, said Dr. Homme.
Institutions would need to acknowledge that many of the current restrictions on pediatric EM physicians’ scope of practice are based on arbitrary age cut-offs, and their leaders would need to expand hospital-defined privileges to better align with training and capabilities, he said. Local credentialing provisions and other policies would also need to be adjusted.
Pediatric EM physicians spend at least 4 months of their graduate EM training in an adult ED, and there is significant overlap in the core competencies and the procedures considered essential for practice between pediatric EM fellowship programs and EM programs, Dr. Homme and his coauthors wrote in their proposal. “The pandemic really reinforced this concept,” Dr. Homme said. “As the number of patients in pediatric EDs plummeted, many of the ped-trained providers had to pivot and help care for adults. ... It worked great.”
The broader integration of pediatrics-trained pediatric EM physicians fits well, he believes, with current workforce dynamics. “There aren’t enough individuals coming out of an EM background and doing that subspecialty training to have any hope that they’d be able to cover these underserved areas,” he said. “And the academic pediatric workforce is getting kind of saturated. So having additional employment opportunities would be great.”
Dr. Homme pursued an EM residency after pediatrics training (rather than a pediatric EM fellowship) because he did not want to be limited geographically and because, while he wanted to focus on children, he also “wanted to be available to a larger population.”
He believes that some pediatrics-trained pediatric EM physicians would choose rural practice options, and hopes that the proposal will gain traction. Some EPs will be opposed, he said, and some pediatrics-trained EPs will not interested, “but if we can find people open to the idea on both sides, I think we can really move the needle in the direction we’re trying to, which is to disseminate an area of expertise into areas that just don’t have it.”
A new physician workforce study documents that almost all clinically active pediatric emergency physicians in the United States – 99% of them – work in urban areas, and that those who do practice in rural areas are significantly older and closer to retirement age.
The portrait of approximately 2,400 self-identified pediatric emergency medicine (EM) physicians may be unsurprising given the overall propensity of physicians – including board-certified general emergency physicians – to practice in urban areas. Even so, it underscores a decades-long concern that many children do not have access to optimal pediatric emergency care.
And the findings highlight the need, the authors say, to keep pressing to improve emergency care for a population of children with “a mortality rate that is already higher than that of its suburban and urban peers (JAMA Network Open 2021;4[5]:e2110084).”
Emergent care of pediatric patients is well within the scope of practice for physicians with training and board certification in general EM, but children and adolescents have different clinical needs and “there are high-stakes scenarios [in children] that we [as emergency physicians] don’t get exposed to as often because we’re not in a children’s hospital or we just don’t have that additional level of training,” said Christopher L. Bennett, MD, MA, of the department of emergency medicine at Stanford University and lead author of the study.
Researchers have documented that some emergency physicians have some discomfort in caring for very ill pediatric patients, he and his coauthors wrote.
Children account for more than 20% of annual ED visits, and most children who seek emergency care in the United States – upwards of 80% – present to general emergency departments. Yet the vast majority of these EDs care for fewer than 14-15 children a day.
With such low pediatric volume, “there will never be pediatric emergency medicine physicians in the rural hospitals in [our] health care system,” said Kathleen M. Brown, MD, medical director for quality and safety of the Emergency Medicine and Trauma Center at Children’s National Medical Center in Washington.
Redistribution “is not a practical solution, and we’ve known that for a long time,” said Dr. Brown, past chairperson of the American College of Emergency Physicians’ pediatric emergency medicine committee. “That’s why national efforts have focused on better preparing the general emergency department and making sure the hospital workforce is ready to take care of children ... to manage and stabilize [them] and recognize when they need more definitive care.”
Continuing efforts to increase “pediatric readiness” in general EDs is one of the recommendations issued by the American Academy of Pediatrics, ACEP, and Emergency Nurses Association in its most recent joint policy statement on emergency care for children, published in May (Pediatrics 2021;147[5]:e2021050787). A 2018 joint policy statement detailed the resources – medications, equipment, policies, and education – necessary for EDs to provide effective pediatric care (Pediatrics 2018;142[5]:e20182459).
There is some evidence that pediatric readiness has improved and that EDs with higher readiness scores may have better pediatric outcomes and lower mortality, said Dr. Brown, a coauthor of both policy statements. (One study cited in the 2018 policy statement, for example, found that children with extremity immobilization and a pain score of 5 or greater had faster management of their pain and decreased exposure to radiation when they were treated in a better-prepared facility than in a facility with less readiness.)
Yet many hospitals still do not have designated pediatric emergency care coordinators (PECCs) – roles that are widely believed to be central to pediatric readiness. PECCs (physicians and nurses) were recommended in 2006 by the then-Institute of Medicine and have been advocated by the AAP, ACEP, and other organizations.
According to 2013 data from the National Pediatric Readiness Project (NPRP), launched that year by the AAP, ACEP, ENA, and the federal Emergency Medical Services for Children program of the Health Resources and Services Administration, at least 15% of EDs lacked at least 1 piece of recommended equipment, and 81% reported barriers to pediatric emergency care guidelines. The NPRP is currently conducting an updated assessment, Dr. Brown said.
Some experts have proposed a different kind of solution – one in which American Board of Pediatrics–certified pediatric EM physicians would care for selective adult patients with common disease patterns who present to rural EDs, in addition to children. They might provide direct patient care across several hospitals in a region, while also addressing quality improvement and assisting EPs and other providers in the region on pediatric care issues.
The proposal, published in May 2020, comes from the 13-member special subcommittee of the ACEP committee on PEM that was tasked with exploring strategies to improve access to emergency pediatric expertise and disaster preparedness in all settings. The proposal was endorsed by the ACEP board of directors, said Jim Homme, MD, a coauthor of the paper (JACEP Open 2020;1:1520-6.)
“We’re saying, look at the ped-trained pediatric emergency provider more broadly. They can actually successfully care for a broader patient population and make it financially feasible ... [for that physician] to be a part of the system,” said Dr. Homme, program director of the emergency medicine residency at the Mayo Clinic College of Medicine and Science in Rochester, Minn.
“The benefit would be not only having the expertise to see children, but to train up other individuals in the institution, and be advocates for the care of children,” he said.
“We’re not saying we want a pediatrics-trained EM physician in every site so that every child would be seen by one – that’s not the goal,” Dr. Homme said. “The goal is to distribute them more broadly than they currently are, and in doing so, make available the other benefits besides direct patient care.”
Most of the physicians in the United States who identify as pediatric EM physicians have completed either a pediatrics or EM residency, followed by a pediatric EM fellowship. It is much more common to have primary training in pediatrics than in EM, said Dr. Homme and Dr. Bennett. A small number of physicians, like Dr. Homme, are dually trained in pediatrics and EM through the completion of two residencies. Dr. Bennett’s workforce study used the American Medical Association Physician Masterfile database and identified 2,403 clinically active pediatric EPs – 5% of all clinically active emergency physicians. Those practicing in rural areas had a median age of 59, compared with a median age of 46 in urban areas. More than half of the pediatric EPs – 68% – reported having pediatric EM board certification.
Three states – Montana, South Dakota, and Wyoming – had no pediatric EMs at all, Dr. Bennett noted.
Readiness in rural Oregon, New England
Torree McGowan, MD, an emergency physician with the St. Charles Health System in Oregon, works in small critical access hospitals in the rural towns of Madras and Prineville, each several hours by ground to the nearest pediatric hospital. She said she feels well equipped to care for children through her training (a rotation in a pediatric ICU and several months working in pediatric EDs) and through her ongoing work with pediatric patients. Children and adolescents comprise about 20%-30% of her volume.
She sees more pediatric illness – children with respiratory syncytial virus who need respiratory support, for instance, and children with severe asthma or diabetic ketoacidosis – than pediatric trauma. When she faces questions, uncertainties, or wants confirmation of a decision, she consults by phone with pediatric subspecialists.
“I don’t take care of kids on vasopressor drips on a regular basis [for instance],” said Dr. McGowan, who sits on ACEP’s disaster preparedness committee and is an Air Force veteran. “But I know the basics and can phone a colleague to be sure I’m doing it correctly. The ability to outreach is there.”
Telemedicine is valuable, she said, but there may also be value in working alongside a pediatric EM physician. One of her EP colleagues is fellowship-trained in ultrasonography and “leads us in training and quality control,” Dr. McGowan said. “And if she’s on shift with you she’ll teach you all about ultrasound. There’s probably utility in having a pediatric EP who does that as well. But incentivizing that and taking them away from the pediatric hospital is a paradigm shift.”
Either way, she said, “being able to bring that expertise out of urban centers, whether it’s to a hospital group like ours or whether it’s by telemedicine, is really, really helpful.”
Her group does not have official PECCs, but the joint policy statements by AAP/ACEP/ENA on pediatric readiness and the “whole pediatric readiness effort’ have been valuable in “driving conversations” with administrators about needs such as purchasing pediatric-sized video laryngoscope blades and other equipment needed for pediatric emergencies, however infrequently they may occur, Dr. McGowan said.
In New England, researchers leading a grassroots regional intervention to establish a PECC in every ED in the region have reported an increased prevalence of “pediatric champions” from less than 30% 5 years ago to greater than 90% in 2019, investigators have reported (Pediatr Emerg Care. 2021. doi: 10.1097/PEC.0000000000002456).
The initiative involved individual outreach to leaders of each ED – largely through phone and e-mail appeals – and collaboration among the State Emergency Medical Services for Children agencies and ACEP and ENA state chapters. The researchers are currently investigating the direct impact of PECCs on patient outcomes.
More on regionalization of ped-trained EPs
Dr. Bennett sees telemedicine as a primary part of improving pediatric emergency care. “I think that’s where things are going to go in pediatric emergency medicine,” he said, especially in the wake of COVID-19: “I don’t see how it’s not going to become much more common.”
Dr. Homme maintains that a broader integration of ABP-certified pediatric EM physicians into underserved regions would advance ED preparedness in a way that telemedicine, or even the appointment of PECCs, does not, said Dr. Homme.
Institutions would need to acknowledge that many of the current restrictions on pediatric EM physicians’ scope of practice are based on arbitrary age cut-offs, and their leaders would need to expand hospital-defined privileges to better align with training and capabilities, he said. Local credentialing provisions and other policies would also need to be adjusted.
Pediatric EM physicians spend at least 4 months of their graduate EM training in an adult ED, and there is significant overlap in the core competencies and the procedures considered essential for practice between pediatric EM fellowship programs and EM programs, Dr. Homme and his coauthors wrote in their proposal. “The pandemic really reinforced this concept,” Dr. Homme said. “As the number of patients in pediatric EDs plummeted, many of the ped-trained providers had to pivot and help care for adults. ... It worked great.”
The broader integration of pediatrics-trained pediatric EM physicians fits well, he believes, with current workforce dynamics. “There aren’t enough individuals coming out of an EM background and doing that subspecialty training to have any hope that they’d be able to cover these underserved areas,” he said. “And the academic pediatric workforce is getting kind of saturated. So having additional employment opportunities would be great.”
Dr. Homme pursued an EM residency after pediatrics training (rather than a pediatric EM fellowship) because he did not want to be limited geographically and because, while he wanted to focus on children, he also “wanted to be available to a larger population.”
He believes that some pediatrics-trained pediatric EM physicians would choose rural practice options, and hopes that the proposal will gain traction. Some EPs will be opposed, he said, and some pediatrics-trained EPs will not interested, “but if we can find people open to the idea on both sides, I think we can really move the needle in the direction we’re trying to, which is to disseminate an area of expertise into areas that just don’t have it.”
A new physician workforce study documents that almost all clinically active pediatric emergency physicians in the United States – 99% of them – work in urban areas, and that those who do practice in rural areas are significantly older and closer to retirement age.
The portrait of approximately 2,400 self-identified pediatric emergency medicine (EM) physicians may be unsurprising given the overall propensity of physicians – including board-certified general emergency physicians – to practice in urban areas. Even so, it underscores a decades-long concern that many children do not have access to optimal pediatric emergency care.
And the findings highlight the need, the authors say, to keep pressing to improve emergency care for a population of children with “a mortality rate that is already higher than that of its suburban and urban peers (JAMA Network Open 2021;4[5]:e2110084).”
Emergent care of pediatric patients is well within the scope of practice for physicians with training and board certification in general EM, but children and adolescents have different clinical needs and “there are high-stakes scenarios [in children] that we [as emergency physicians] don’t get exposed to as often because we’re not in a children’s hospital or we just don’t have that additional level of training,” said Christopher L. Bennett, MD, MA, of the department of emergency medicine at Stanford University and lead author of the study.
Researchers have documented that some emergency physicians have some discomfort in caring for very ill pediatric patients, he and his coauthors wrote.
Children account for more than 20% of annual ED visits, and most children who seek emergency care in the United States – upwards of 80% – present to general emergency departments. Yet the vast majority of these EDs care for fewer than 14-15 children a day.
With such low pediatric volume, “there will never be pediatric emergency medicine physicians in the rural hospitals in [our] health care system,” said Kathleen M. Brown, MD, medical director for quality and safety of the Emergency Medicine and Trauma Center at Children’s National Medical Center in Washington.
Redistribution “is not a practical solution, and we’ve known that for a long time,” said Dr. Brown, past chairperson of the American College of Emergency Physicians’ pediatric emergency medicine committee. “That’s why national efforts have focused on better preparing the general emergency department and making sure the hospital workforce is ready to take care of children ... to manage and stabilize [them] and recognize when they need more definitive care.”
Continuing efforts to increase “pediatric readiness” in general EDs is one of the recommendations issued by the American Academy of Pediatrics, ACEP, and Emergency Nurses Association in its most recent joint policy statement on emergency care for children, published in May (Pediatrics 2021;147[5]:e2021050787). A 2018 joint policy statement detailed the resources – medications, equipment, policies, and education – necessary for EDs to provide effective pediatric care (Pediatrics 2018;142[5]:e20182459).
There is some evidence that pediatric readiness has improved and that EDs with higher readiness scores may have better pediatric outcomes and lower mortality, said Dr. Brown, a coauthor of both policy statements. (One study cited in the 2018 policy statement, for example, found that children with extremity immobilization and a pain score of 5 or greater had faster management of their pain and decreased exposure to radiation when they were treated in a better-prepared facility than in a facility with less readiness.)
Yet many hospitals still do not have designated pediatric emergency care coordinators (PECCs) – roles that are widely believed to be central to pediatric readiness. PECCs (physicians and nurses) were recommended in 2006 by the then-Institute of Medicine and have been advocated by the AAP, ACEP, and other organizations.
According to 2013 data from the National Pediatric Readiness Project (NPRP), launched that year by the AAP, ACEP, ENA, and the federal Emergency Medical Services for Children program of the Health Resources and Services Administration, at least 15% of EDs lacked at least 1 piece of recommended equipment, and 81% reported barriers to pediatric emergency care guidelines. The NPRP is currently conducting an updated assessment, Dr. Brown said.
Some experts have proposed a different kind of solution – one in which American Board of Pediatrics–certified pediatric EM physicians would care for selective adult patients with common disease patterns who present to rural EDs, in addition to children. They might provide direct patient care across several hospitals in a region, while also addressing quality improvement and assisting EPs and other providers in the region on pediatric care issues.
The proposal, published in May 2020, comes from the 13-member special subcommittee of the ACEP committee on PEM that was tasked with exploring strategies to improve access to emergency pediatric expertise and disaster preparedness in all settings. The proposal was endorsed by the ACEP board of directors, said Jim Homme, MD, a coauthor of the paper (JACEP Open 2020;1:1520-6.)
“We’re saying, look at the ped-trained pediatric emergency provider more broadly. They can actually successfully care for a broader patient population and make it financially feasible ... [for that physician] to be a part of the system,” said Dr. Homme, program director of the emergency medicine residency at the Mayo Clinic College of Medicine and Science in Rochester, Minn.
“The benefit would be not only having the expertise to see children, but to train up other individuals in the institution, and be advocates for the care of children,” he said.
“We’re not saying we want a pediatrics-trained EM physician in every site so that every child would be seen by one – that’s not the goal,” Dr. Homme said. “The goal is to distribute them more broadly than they currently are, and in doing so, make available the other benefits besides direct patient care.”
Most of the physicians in the United States who identify as pediatric EM physicians have completed either a pediatrics or EM residency, followed by a pediatric EM fellowship. It is much more common to have primary training in pediatrics than in EM, said Dr. Homme and Dr. Bennett. A small number of physicians, like Dr. Homme, are dually trained in pediatrics and EM through the completion of two residencies. Dr. Bennett’s workforce study used the American Medical Association Physician Masterfile database and identified 2,403 clinically active pediatric EPs – 5% of all clinically active emergency physicians. Those practicing in rural areas had a median age of 59, compared with a median age of 46 in urban areas. More than half of the pediatric EPs – 68% – reported having pediatric EM board certification.
Three states – Montana, South Dakota, and Wyoming – had no pediatric EMs at all, Dr. Bennett noted.
Readiness in rural Oregon, New England
Torree McGowan, MD, an emergency physician with the St. Charles Health System in Oregon, works in small critical access hospitals in the rural towns of Madras and Prineville, each several hours by ground to the nearest pediatric hospital. She said she feels well equipped to care for children through her training (a rotation in a pediatric ICU and several months working in pediatric EDs) and through her ongoing work with pediatric patients. Children and adolescents comprise about 20%-30% of her volume.
She sees more pediatric illness – children with respiratory syncytial virus who need respiratory support, for instance, and children with severe asthma or diabetic ketoacidosis – than pediatric trauma. When she faces questions, uncertainties, or wants confirmation of a decision, she consults by phone with pediatric subspecialists.
“I don’t take care of kids on vasopressor drips on a regular basis [for instance],” said Dr. McGowan, who sits on ACEP’s disaster preparedness committee and is an Air Force veteran. “But I know the basics and can phone a colleague to be sure I’m doing it correctly. The ability to outreach is there.”
Telemedicine is valuable, she said, but there may also be value in working alongside a pediatric EM physician. One of her EP colleagues is fellowship-trained in ultrasonography and “leads us in training and quality control,” Dr. McGowan said. “And if she’s on shift with you she’ll teach you all about ultrasound. There’s probably utility in having a pediatric EP who does that as well. But incentivizing that and taking them away from the pediatric hospital is a paradigm shift.”
Either way, she said, “being able to bring that expertise out of urban centers, whether it’s to a hospital group like ours or whether it’s by telemedicine, is really, really helpful.”
Her group does not have official PECCs, but the joint policy statements by AAP/ACEP/ENA on pediatric readiness and the “whole pediatric readiness effort’ have been valuable in “driving conversations” with administrators about needs such as purchasing pediatric-sized video laryngoscope blades and other equipment needed for pediatric emergencies, however infrequently they may occur, Dr. McGowan said.
In New England, researchers leading a grassroots regional intervention to establish a PECC in every ED in the region have reported an increased prevalence of “pediatric champions” from less than 30% 5 years ago to greater than 90% in 2019, investigators have reported (Pediatr Emerg Care. 2021. doi: 10.1097/PEC.0000000000002456).
The initiative involved individual outreach to leaders of each ED – largely through phone and e-mail appeals – and collaboration among the State Emergency Medical Services for Children agencies and ACEP and ENA state chapters. The researchers are currently investigating the direct impact of PECCs on patient outcomes.
More on regionalization of ped-trained EPs
Dr. Bennett sees telemedicine as a primary part of improving pediatric emergency care. “I think that’s where things are going to go in pediatric emergency medicine,” he said, especially in the wake of COVID-19: “I don’t see how it’s not going to become much more common.”
Dr. Homme maintains that a broader integration of ABP-certified pediatric EM physicians into underserved regions would advance ED preparedness in a way that telemedicine, or even the appointment of PECCs, does not, said Dr. Homme.
Institutions would need to acknowledge that many of the current restrictions on pediatric EM physicians’ scope of practice are based on arbitrary age cut-offs, and their leaders would need to expand hospital-defined privileges to better align with training and capabilities, he said. Local credentialing provisions and other policies would also need to be adjusted.
Pediatric EM physicians spend at least 4 months of their graduate EM training in an adult ED, and there is significant overlap in the core competencies and the procedures considered essential for practice between pediatric EM fellowship programs and EM programs, Dr. Homme and his coauthors wrote in their proposal. “The pandemic really reinforced this concept,” Dr. Homme said. “As the number of patients in pediatric EDs plummeted, many of the ped-trained providers had to pivot and help care for adults. ... It worked great.”
The broader integration of pediatrics-trained pediatric EM physicians fits well, he believes, with current workforce dynamics. “There aren’t enough individuals coming out of an EM background and doing that subspecialty training to have any hope that they’d be able to cover these underserved areas,” he said. “And the academic pediatric workforce is getting kind of saturated. So having additional employment opportunities would be great.”
Dr. Homme pursued an EM residency after pediatrics training (rather than a pediatric EM fellowship) because he did not want to be limited geographically and because, while he wanted to focus on children, he also “wanted to be available to a larger population.”
He believes that some pediatrics-trained pediatric EM physicians would choose rural practice options, and hopes that the proposal will gain traction. Some EPs will be opposed, he said, and some pediatrics-trained EPs will not interested, “but if we can find people open to the idea on both sides, I think we can really move the needle in the direction we’re trying to, which is to disseminate an area of expertise into areas that just don’t have it.”
Cortical thinning in adolescence ‘definitively’ tied to subsequent psychosis
Subtle differences in brain morphometric features present in adolescence were associated with the subsequent development of psychosis in what is believed to be the largest neuroimaging investigation to date involving people at clinical high risk (CHR).
Investigators found widespread lower cortical thickness (CT) in individuals at CHR, consistent with previously reported CT differences in individuals with an established psychotic disorder.
“This is the first study to definitively show that there are subtle, widespread structural brain differences in high-risk youth before they develop psychosis,” study investigator Maria Jalbrzikowski, PhD, assistant professor of psychiatry, University of Pittsburgh, said in an interview.
The findings also suggest that there are developmental periods during which certain brain abnormalities may be more apparent, “highlighting the need to consider developmental period when developing objective, biological risk factors for early intervention in psychosis,” Dr. Jalbrzikowski said.
The study was published online May 5 in JAMA Psychiatry.
‘Sobering’ results
The findings are based on pooled structural MRI scans from 3,169 individuals recruited at 31 international sites participating in the Enhancing Neuro Imaging Genetics Through Meta-Analysis (ENIGMA) Clinical High Risk for Psychosis Working Group.
Forty-five percent of the participants were female; the mean age was 21 years (range, 9.5 to 39.9 years).
The cohort included 1,792 individuals at CHR for psychosis and 1,377 healthy control persons. Using longitudinal clinical information, the researchers identified 253 individuals at CHR who went on to develop a psychotic disorder (CHR-PS+) and 1,234 at CHR who did not develop a psychotic disorder (CHR-PS-). For the remaining 305 individuals at CHR, follow-up data were unavailable.
Compared with healthy control persons, individuals at CHR had widespread lower CT measures but not lower surface area or subcortical volume. Lower CT measures in the fusiform, superior temporal, and paracentral regions were associated with conversion to psychosis.
The pattern of differences in cortical thickness in those in the CHR-PS+ group mirrored patterns seen in people with schizophrenia and in people with 22q11.2 microdeletion syndrome who developed a psychotic disorder.
The researchers note that although all individuals experience cortical thinning as they move into adulthood, in their study, cortical thinning was already present in participants aged 12 to 16 years who developed psychosis.
“We don’t yet know exactly what this means, but adolescence is a critical time in a child’s life – it’s a time of opportunity to take risks and explore, but also a period of vulnerability,” Dr. Jalbrzikowski said in a news release.
“We could be seeing the result of something that happened even earlier in brain development but only begins to influence behavior during this developmental stage,” she noted.
This analysis represents the largest-ever pooling of brain scans in children and young adults who were determined by psychiatric assessment to be at high risk of developing psychosis.
“These results were, in a sense, sobering. On the one hand, our dataset includes 600% more high-risk youth who developed psychosis than any existing study, allowing us to see statistically significant results in brain structure.”
“But the variance between whether or not a high-risk youth develops psychosis is so small that it would be impossible to see a difference at the individual level,” Dr. Jalbrzikowski said.
More work is needed in order for the findings to be translated into clinical care, she added.
Definitive findings
Commenting on the findings for an interview, Russell Margolis, MD, clinical director, Johns Hopkins Schizophrenia Center, said that “it’s not so much that the findings are novel but rather that they’re fairly definitive in that this is by far the largest study of its kind looking at this particular question, and that gives it power. The problem with imaging studies has often been inconsistent results from study to study because of small sample size.”
“In order to see these differences in a robust way, you need a large sample size, meaning that for any one individual, this kind of structural imaging is not going to add much to the prediction of whether someone will eventually develop a schizophrenia-like illness,” said Dr. Margolis, professor of psychiatry and behavioral sciences, Johns Hopkins University, Baltimore.
From a clinical standpoint,
“The predominant hypothesis in the field is that early developmental abnormalities are the root cause of schizophrenia and related disorders, and this study is consistent with that, particularly the age-related differences, which are suggestive of neurodevelopmental abnormalities preceding the development of overt symptoms, which many other findings have also suggested,” Dr. Margolis said.
“Abnormalities in cortical thickness could be from a number of different neurobiological processes, and research into those processes are worth investigating,” he added.
The researchers received support from numerous funders, all of which are listed in the original article, along with author disclosures for ENIGMA working group members. Dr. Margolis has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Subtle differences in brain morphometric features present in adolescence were associated with the subsequent development of psychosis in what is believed to be the largest neuroimaging investigation to date involving people at clinical high risk (CHR).
Investigators found widespread lower cortical thickness (CT) in individuals at CHR, consistent with previously reported CT differences in individuals with an established psychotic disorder.
“This is the first study to definitively show that there are subtle, widespread structural brain differences in high-risk youth before they develop psychosis,” study investigator Maria Jalbrzikowski, PhD, assistant professor of psychiatry, University of Pittsburgh, said in an interview.
The findings also suggest that there are developmental periods during which certain brain abnormalities may be more apparent, “highlighting the need to consider developmental period when developing objective, biological risk factors for early intervention in psychosis,” Dr. Jalbrzikowski said.
The study was published online May 5 in JAMA Psychiatry.
‘Sobering’ results
The findings are based on pooled structural MRI scans from 3,169 individuals recruited at 31 international sites participating in the Enhancing Neuro Imaging Genetics Through Meta-Analysis (ENIGMA) Clinical High Risk for Psychosis Working Group.
Forty-five percent of the participants were female; the mean age was 21 years (range, 9.5 to 39.9 years).
The cohort included 1,792 individuals at CHR for psychosis and 1,377 healthy control persons. Using longitudinal clinical information, the researchers identified 253 individuals at CHR who went on to develop a psychotic disorder (CHR-PS+) and 1,234 at CHR who did not develop a psychotic disorder (CHR-PS-). For the remaining 305 individuals at CHR, follow-up data were unavailable.
Compared with healthy control persons, individuals at CHR had widespread lower CT measures but not lower surface area or subcortical volume. Lower CT measures in the fusiform, superior temporal, and paracentral regions were associated with conversion to psychosis.
The pattern of differences in cortical thickness in those in the CHR-PS+ group mirrored patterns seen in people with schizophrenia and in people with 22q11.2 microdeletion syndrome who developed a psychotic disorder.
The researchers note that although all individuals experience cortical thinning as they move into adulthood, in their study, cortical thinning was already present in participants aged 12 to 16 years who developed psychosis.
“We don’t yet know exactly what this means, but adolescence is a critical time in a child’s life – it’s a time of opportunity to take risks and explore, but also a period of vulnerability,” Dr. Jalbrzikowski said in a news release.
“We could be seeing the result of something that happened even earlier in brain development but only begins to influence behavior during this developmental stage,” she noted.
This analysis represents the largest-ever pooling of brain scans in children and young adults who were determined by psychiatric assessment to be at high risk of developing psychosis.
“These results were, in a sense, sobering. On the one hand, our dataset includes 600% more high-risk youth who developed psychosis than any existing study, allowing us to see statistically significant results in brain structure.”
“But the variance between whether or not a high-risk youth develops psychosis is so small that it would be impossible to see a difference at the individual level,” Dr. Jalbrzikowski said.
More work is needed in order for the findings to be translated into clinical care, she added.
Definitive findings
Commenting on the findings for an interview, Russell Margolis, MD, clinical director, Johns Hopkins Schizophrenia Center, said that “it’s not so much that the findings are novel but rather that they’re fairly definitive in that this is by far the largest study of its kind looking at this particular question, and that gives it power. The problem with imaging studies has often been inconsistent results from study to study because of small sample size.”
“In order to see these differences in a robust way, you need a large sample size, meaning that for any one individual, this kind of structural imaging is not going to add much to the prediction of whether someone will eventually develop a schizophrenia-like illness,” said Dr. Margolis, professor of psychiatry and behavioral sciences, Johns Hopkins University, Baltimore.
From a clinical standpoint,
“The predominant hypothesis in the field is that early developmental abnormalities are the root cause of schizophrenia and related disorders, and this study is consistent with that, particularly the age-related differences, which are suggestive of neurodevelopmental abnormalities preceding the development of overt symptoms, which many other findings have also suggested,” Dr. Margolis said.
“Abnormalities in cortical thickness could be from a number of different neurobiological processes, and research into those processes are worth investigating,” he added.
The researchers received support from numerous funders, all of which are listed in the original article, along with author disclosures for ENIGMA working group members. Dr. Margolis has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Subtle differences in brain morphometric features present in adolescence were associated with the subsequent development of psychosis in what is believed to be the largest neuroimaging investigation to date involving people at clinical high risk (CHR).
Investigators found widespread lower cortical thickness (CT) in individuals at CHR, consistent with previously reported CT differences in individuals with an established psychotic disorder.
“This is the first study to definitively show that there are subtle, widespread structural brain differences in high-risk youth before they develop psychosis,” study investigator Maria Jalbrzikowski, PhD, assistant professor of psychiatry, University of Pittsburgh, said in an interview.
The findings also suggest that there are developmental periods during which certain brain abnormalities may be more apparent, “highlighting the need to consider developmental period when developing objective, biological risk factors for early intervention in psychosis,” Dr. Jalbrzikowski said.
The study was published online May 5 in JAMA Psychiatry.
‘Sobering’ results
The findings are based on pooled structural MRI scans from 3,169 individuals recruited at 31 international sites participating in the Enhancing Neuro Imaging Genetics Through Meta-Analysis (ENIGMA) Clinical High Risk for Psychosis Working Group.
Forty-five percent of the participants were female; the mean age was 21 years (range, 9.5 to 39.9 years).
The cohort included 1,792 individuals at CHR for psychosis and 1,377 healthy control persons. Using longitudinal clinical information, the researchers identified 253 individuals at CHR who went on to develop a psychotic disorder (CHR-PS+) and 1,234 at CHR who did not develop a psychotic disorder (CHR-PS-). For the remaining 305 individuals at CHR, follow-up data were unavailable.
Compared with healthy control persons, individuals at CHR had widespread lower CT measures but not lower surface area or subcortical volume. Lower CT measures in the fusiform, superior temporal, and paracentral regions were associated with conversion to psychosis.
The pattern of differences in cortical thickness in those in the CHR-PS+ group mirrored patterns seen in people with schizophrenia and in people with 22q11.2 microdeletion syndrome who developed a psychotic disorder.
The researchers note that although all individuals experience cortical thinning as they move into adulthood, in their study, cortical thinning was already present in participants aged 12 to 16 years who developed psychosis.
“We don’t yet know exactly what this means, but adolescence is a critical time in a child’s life – it’s a time of opportunity to take risks and explore, but also a period of vulnerability,” Dr. Jalbrzikowski said in a news release.
“We could be seeing the result of something that happened even earlier in brain development but only begins to influence behavior during this developmental stage,” she noted.
This analysis represents the largest-ever pooling of brain scans in children and young adults who were determined by psychiatric assessment to be at high risk of developing psychosis.
“These results were, in a sense, sobering. On the one hand, our dataset includes 600% more high-risk youth who developed psychosis than any existing study, allowing us to see statistically significant results in brain structure.”
“But the variance between whether or not a high-risk youth develops psychosis is so small that it would be impossible to see a difference at the individual level,” Dr. Jalbrzikowski said.
More work is needed in order for the findings to be translated into clinical care, she added.
Definitive findings
Commenting on the findings for an interview, Russell Margolis, MD, clinical director, Johns Hopkins Schizophrenia Center, said that “it’s not so much that the findings are novel but rather that they’re fairly definitive in that this is by far the largest study of its kind looking at this particular question, and that gives it power. The problem with imaging studies has often been inconsistent results from study to study because of small sample size.”
“In order to see these differences in a robust way, you need a large sample size, meaning that for any one individual, this kind of structural imaging is not going to add much to the prediction of whether someone will eventually develop a schizophrenia-like illness,” said Dr. Margolis, professor of psychiatry and behavioral sciences, Johns Hopkins University, Baltimore.
From a clinical standpoint,
“The predominant hypothesis in the field is that early developmental abnormalities are the root cause of schizophrenia and related disorders, and this study is consistent with that, particularly the age-related differences, which are suggestive of neurodevelopmental abnormalities preceding the development of overt symptoms, which many other findings have also suggested,” Dr. Margolis said.
“Abnormalities in cortical thickness could be from a number of different neurobiological processes, and research into those processes are worth investigating,” he added.
The researchers received support from numerous funders, all of which are listed in the original article, along with author disclosures for ENIGMA working group members. Dr. Margolis has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Novel study links air pollution to increased risk of rheumatoid arthritis flares
Pollution appears to trigger inflammation
In patients with rheumatoid arthritis, exposure to air pollution is associated with both elevated levels of C-reactive protein (CRP) and increased risk of arthritis flares, according to a novel longitudinal study presented at the annual European Congress of Rheumatology.
The data revealed “a striking association between air pollution and increased CRP levels and risk of an arthritis flare,” reported first author Giovanni Adami, MD, DSc, of the rheumatology unit at the University of Verona (Italy).
The excess risk of elevated CRP and flares began “at very low levels of exposure, even those below commonly used thresholds for risk to human health,” he added.
Study details
Researchers collected data on 888 patients with RA from numerous patient visits in the context of more than 13,000 air pollution records. The CRP levels and RA flares were evaluated in the context of air pollution monitoring that is performed on a daily basis at several sites in the city of Verona where the study was conducted. Verona is an industrial city in northern Italy that has high but variable levels of air pollution based on factory activity and weather conditions.
Patients with RA who provided clinical data for this study were matched by their proximity to specific air pollution monitoring sites. By linking CRP levels and disease activity to air pollution levels over multiple follow-up visits, the design allowed the RA study participants “to serve as their own controls,” Dr. Adami explained.
At each patient visit during the study, CRP levels were measured and disease activity assessed. Patients were considered to have elevated CRP when levels were 5 mg/L or higher. The presence of an RA flare was defined by a 1.2-point increase or more in 28-joint Disease Activity Score using CRP (DAS28-CRP).
Both the CRP level and the presence or absence of a flare were evaluated in relationship to the patient’s specific local air pollution levels in the prior 60 days.
Increased levels of CRP, a surrogate for inflammatory activity, and increased disease activity, were both associated with elevated exposure to air pollutants prior to an office visit. These associations remained statistically significant when evaluated by specific air pollutants such as carbon monoxide (CO), nitrogen oxides (NO2, NO), small particulate matter (PM10; particles ≤ 10 mcm), and ozone (O3).
The relationship between increased exposure to air pollution contaminants and elevated CRP was supported by a dose effect. In the case of PM10, for example, the odds ratio of having elevated CRP was increased by only about 25% (OR, 1.25) when mean levels were 30 mcg/m3 or lower in the period prior to the office visit. This rose incrementally for higher mean levels of PM10, reaching 70% (OR, 1.70) for levels > 50 mcg/m3.
The researchers detected statistically significant differences in mean and area-under-the curve (AUC) values of most air pollutants in the 60 days prior to office visits when patients had a flare versus when disease activity was low. For example, the difference in mean and AUC levels in the period prior to a flare relative to a period with low disease activity was significant for CO (P = .001 for both) and NO and NO2 (P = .003 for both), and O3 (P = .002 and P = .001, respectively). For PM10, P values were .011 and .005, respectively.
“Remarkably, we found that the cumulative exposure to NO2 in the 60 days preceding a flare was approximately 500 mcg/m3 higher than the low disease activity visit, an exposure that equates to approximately 200 passively smoked cigarettes,” Dr. Adami reported.
Trying to confirm causality of association
Dr. Adami’s study is not the first study to link air pollution to risk of RA. Several have suggested that air pollution is a risk factor for developing joint disease, but a recently published study conducted in Kuwait associated greater disease activity with NO2 and another air pollutant, sulfur dioxide (SO2), although not CO, PM10, or O3.
A coauthor of that study, which evaluated pollution in regard to disease activity on DAS score, Adeeba Al-Herz, MD, a rheumatology consultant at Al-Amiri Hospital, Kuwait City, said in an interview, “We proved the correlation between them but not the causality.”
However, she believes that this is an important area of inquiry.
“We are working now on another paper in which we studied a causal relationship between the two, meaning that we are evaluating whether SO2 and NO2 trigger RA activity,” Dr. Al-Herz said. That study is now complete, and the manuscript is being written.
The magnitude of the association in these two studies suggest that there might be a clinical message if causality can be confirmed, according to Dr. Adami. Although there are many reasons to seek to reduce and avoid air pollution, these data suggest risk of a proinflammatory state might be one of them.
Dr. Adami believes that the evidence of an adverse effect on patients with RA is strong.
“In order to reduce the burden of RA, public and environmental health policy makers should aim to diminish gaseous and particulate matter emissions to a larger extent than currently recommended,” he said.
In an interview after his presentation, Dr. Adami suggested that the risk of an inflammatory response and increases in arthritis flares from air pollution is not surprising. Previous studies have linked cigarette smoking to both.
“The mechanisms underlying the development of inflammation are very similar. Indeed, the toxic components contained in cigarette smoking are largely shared with diesel exhaust and fossil fuel combustion,” he said.
Although causality between air pollution and arthritis flares cannot be confirmed in these data, a basis for suspecting a causal relationship is supported by “plenty of in vitro and animal studies,” according to Dr. Adami.
On the basis of these studies, several mechanisms have been postulated.
“As an example, exposure to air pollution can promote the activation of the bronchus-associated lymphoid tissue (BALT), which can trigger the activation of the transcription factor nuclear factor-kappaB,” he said. This, in turn, can “lead to the secretion of proinflammatory cytokines, such as tumor necrosis factor–alpha and interleukin-1.”
Another theory is that posttranslational modification of proteins in the lung, a process called citrullination, “can lead to production of autoantibodies known to have a pathogenic role in RA,” he added.
Proving a causal relationship, however, is difficult.
“We certainly cannot conduct a randomized clinical trial on that and voluntarily expose some patients to pollution. Thus, we need to rely on observational data,” Dr. Adami said.
Of strategies being considered to generate evidence of a causal relationship between pollution and the exacerbation of RA, “we certainly will try to study those patients that move from a highly polluted area to a greener zone and vice versa,” he said. This will allow us “to explore what happens when the exposure to pollution changes dramatically in a short period of time.”
In the meantime, “given what is known to date, I would certainly advise my RA patients to avoid exposure to air pollution,” Dr. Adami said. He acknowledged there is no proof that this will help patients to reduce the risk of flares, but there are already many good reasons to minimize exposure to air pollution.
Dr. Adami and Dr. Al-Herz report no potential conflicts of interest.
Pollution appears to trigger inflammation
Pollution appears to trigger inflammation
In patients with rheumatoid arthritis, exposure to air pollution is associated with both elevated levels of C-reactive protein (CRP) and increased risk of arthritis flares, according to a novel longitudinal study presented at the annual European Congress of Rheumatology.
The data revealed “a striking association between air pollution and increased CRP levels and risk of an arthritis flare,” reported first author Giovanni Adami, MD, DSc, of the rheumatology unit at the University of Verona (Italy).
The excess risk of elevated CRP and flares began “at very low levels of exposure, even those below commonly used thresholds for risk to human health,” he added.
Study details
Researchers collected data on 888 patients with RA from numerous patient visits in the context of more than 13,000 air pollution records. The CRP levels and RA flares were evaluated in the context of air pollution monitoring that is performed on a daily basis at several sites in the city of Verona where the study was conducted. Verona is an industrial city in northern Italy that has high but variable levels of air pollution based on factory activity and weather conditions.
Patients with RA who provided clinical data for this study were matched by their proximity to specific air pollution monitoring sites. By linking CRP levels and disease activity to air pollution levels over multiple follow-up visits, the design allowed the RA study participants “to serve as their own controls,” Dr. Adami explained.
At each patient visit during the study, CRP levels were measured and disease activity assessed. Patients were considered to have elevated CRP when levels were 5 mg/L or higher. The presence of an RA flare was defined by a 1.2-point increase or more in 28-joint Disease Activity Score using CRP (DAS28-CRP).
Both the CRP level and the presence or absence of a flare were evaluated in relationship to the patient’s specific local air pollution levels in the prior 60 days.
Increased levels of CRP, a surrogate for inflammatory activity, and increased disease activity, were both associated with elevated exposure to air pollutants prior to an office visit. These associations remained statistically significant when evaluated by specific air pollutants such as carbon monoxide (CO), nitrogen oxides (NO2, NO), small particulate matter (PM10; particles ≤ 10 mcm), and ozone (O3).
The relationship between increased exposure to air pollution contaminants and elevated CRP was supported by a dose effect. In the case of PM10, for example, the odds ratio of having elevated CRP was increased by only about 25% (OR, 1.25) when mean levels were 30 mcg/m3 or lower in the period prior to the office visit. This rose incrementally for higher mean levels of PM10, reaching 70% (OR, 1.70) for levels > 50 mcg/m3.
The researchers detected statistically significant differences in mean and area-under-the curve (AUC) values of most air pollutants in the 60 days prior to office visits when patients had a flare versus when disease activity was low. For example, the difference in mean and AUC levels in the period prior to a flare relative to a period with low disease activity was significant for CO (P = .001 for both) and NO and NO2 (P = .003 for both), and O3 (P = .002 and P = .001, respectively). For PM10, P values were .011 and .005, respectively.
“Remarkably, we found that the cumulative exposure to NO2 in the 60 days preceding a flare was approximately 500 mcg/m3 higher than the low disease activity visit, an exposure that equates to approximately 200 passively smoked cigarettes,” Dr. Adami reported.
Trying to confirm causality of association
Dr. Adami’s study is not the first study to link air pollution to risk of RA. Several have suggested that air pollution is a risk factor for developing joint disease, but a recently published study conducted in Kuwait associated greater disease activity with NO2 and another air pollutant, sulfur dioxide (SO2), although not CO, PM10, or O3.
A coauthor of that study, which evaluated pollution in regard to disease activity on DAS score, Adeeba Al-Herz, MD, a rheumatology consultant at Al-Amiri Hospital, Kuwait City, said in an interview, “We proved the correlation between them but not the causality.”
However, she believes that this is an important area of inquiry.
“We are working now on another paper in which we studied a causal relationship between the two, meaning that we are evaluating whether SO2 and NO2 trigger RA activity,” Dr. Al-Herz said. That study is now complete, and the manuscript is being written.
The magnitude of the association in these two studies suggest that there might be a clinical message if causality can be confirmed, according to Dr. Adami. Although there are many reasons to seek to reduce and avoid air pollution, these data suggest risk of a proinflammatory state might be one of them.
Dr. Adami believes that the evidence of an adverse effect on patients with RA is strong.
“In order to reduce the burden of RA, public and environmental health policy makers should aim to diminish gaseous and particulate matter emissions to a larger extent than currently recommended,” he said.
In an interview after his presentation, Dr. Adami suggested that the risk of an inflammatory response and increases in arthritis flares from air pollution is not surprising. Previous studies have linked cigarette smoking to both.
“The mechanisms underlying the development of inflammation are very similar. Indeed, the toxic components contained in cigarette smoking are largely shared with diesel exhaust and fossil fuel combustion,” he said.
Although causality between air pollution and arthritis flares cannot be confirmed in these data, a basis for suspecting a causal relationship is supported by “plenty of in vitro and animal studies,” according to Dr. Adami.
On the basis of these studies, several mechanisms have been postulated.
“As an example, exposure to air pollution can promote the activation of the bronchus-associated lymphoid tissue (BALT), which can trigger the activation of the transcription factor nuclear factor-kappaB,” he said. This, in turn, can “lead to the secretion of proinflammatory cytokines, such as tumor necrosis factor–alpha and interleukin-1.”
Another theory is that posttranslational modification of proteins in the lung, a process called citrullination, “can lead to production of autoantibodies known to have a pathogenic role in RA,” he added.
Proving a causal relationship, however, is difficult.
“We certainly cannot conduct a randomized clinical trial on that and voluntarily expose some patients to pollution. Thus, we need to rely on observational data,” Dr. Adami said.
Of strategies being considered to generate evidence of a causal relationship between pollution and the exacerbation of RA, “we certainly will try to study those patients that move from a highly polluted area to a greener zone and vice versa,” he said. This will allow us “to explore what happens when the exposure to pollution changes dramatically in a short period of time.”
In the meantime, “given what is known to date, I would certainly advise my RA patients to avoid exposure to air pollution,” Dr. Adami said. He acknowledged there is no proof that this will help patients to reduce the risk of flares, but there are already many good reasons to minimize exposure to air pollution.
Dr. Adami and Dr. Al-Herz report no potential conflicts of interest.
In patients with rheumatoid arthritis, exposure to air pollution is associated with both elevated levels of C-reactive protein (CRP) and increased risk of arthritis flares, according to a novel longitudinal study presented at the annual European Congress of Rheumatology.
The data revealed “a striking association between air pollution and increased CRP levels and risk of an arthritis flare,” reported first author Giovanni Adami, MD, DSc, of the rheumatology unit at the University of Verona (Italy).
The excess risk of elevated CRP and flares began “at very low levels of exposure, even those below commonly used thresholds for risk to human health,” he added.
Study details
Researchers collected data on 888 patients with RA from numerous patient visits in the context of more than 13,000 air pollution records. The CRP levels and RA flares were evaluated in the context of air pollution monitoring that is performed on a daily basis at several sites in the city of Verona where the study was conducted. Verona is an industrial city in northern Italy that has high but variable levels of air pollution based on factory activity and weather conditions.
Patients with RA who provided clinical data for this study were matched by their proximity to specific air pollution monitoring sites. By linking CRP levels and disease activity to air pollution levels over multiple follow-up visits, the design allowed the RA study participants “to serve as their own controls,” Dr. Adami explained.
At each patient visit during the study, CRP levels were measured and disease activity assessed. Patients were considered to have elevated CRP when levels were 5 mg/L or higher. The presence of an RA flare was defined by a 1.2-point increase or more in 28-joint Disease Activity Score using CRP (DAS28-CRP).
Both the CRP level and the presence or absence of a flare were evaluated in relationship to the patient’s specific local air pollution levels in the prior 60 days.
Increased levels of CRP, a surrogate for inflammatory activity, and increased disease activity, were both associated with elevated exposure to air pollutants prior to an office visit. These associations remained statistically significant when evaluated by specific air pollutants such as carbon monoxide (CO), nitrogen oxides (NO2, NO), small particulate matter (PM10; particles ≤ 10 mcm), and ozone (O3).
The relationship between increased exposure to air pollution contaminants and elevated CRP was supported by a dose effect. In the case of PM10, for example, the odds ratio of having elevated CRP was increased by only about 25% (OR, 1.25) when mean levels were 30 mcg/m3 or lower in the period prior to the office visit. This rose incrementally for higher mean levels of PM10, reaching 70% (OR, 1.70) for levels > 50 mcg/m3.
The researchers detected statistically significant differences in mean and area-under-the curve (AUC) values of most air pollutants in the 60 days prior to office visits when patients had a flare versus when disease activity was low. For example, the difference in mean and AUC levels in the period prior to a flare relative to a period with low disease activity was significant for CO (P = .001 for both) and NO and NO2 (P = .003 for both), and O3 (P = .002 and P = .001, respectively). For PM10, P values were .011 and .005, respectively.
“Remarkably, we found that the cumulative exposure to NO2 in the 60 days preceding a flare was approximately 500 mcg/m3 higher than the low disease activity visit, an exposure that equates to approximately 200 passively smoked cigarettes,” Dr. Adami reported.
Trying to confirm causality of association
Dr. Adami’s study is not the first study to link air pollution to risk of RA. Several have suggested that air pollution is a risk factor for developing joint disease, but a recently published study conducted in Kuwait associated greater disease activity with NO2 and another air pollutant, sulfur dioxide (SO2), although not CO, PM10, or O3.
A coauthor of that study, which evaluated pollution in regard to disease activity on DAS score, Adeeba Al-Herz, MD, a rheumatology consultant at Al-Amiri Hospital, Kuwait City, said in an interview, “We proved the correlation between them but not the causality.”
However, she believes that this is an important area of inquiry.
“We are working now on another paper in which we studied a causal relationship between the two, meaning that we are evaluating whether SO2 and NO2 trigger RA activity,” Dr. Al-Herz said. That study is now complete, and the manuscript is being written.
The magnitude of the association in these two studies suggest that there might be a clinical message if causality can be confirmed, according to Dr. Adami. Although there are many reasons to seek to reduce and avoid air pollution, these data suggest risk of a proinflammatory state might be one of them.
Dr. Adami believes that the evidence of an adverse effect on patients with RA is strong.
“In order to reduce the burden of RA, public and environmental health policy makers should aim to diminish gaseous and particulate matter emissions to a larger extent than currently recommended,” he said.
In an interview after his presentation, Dr. Adami suggested that the risk of an inflammatory response and increases in arthritis flares from air pollution is not surprising. Previous studies have linked cigarette smoking to both.
“The mechanisms underlying the development of inflammation are very similar. Indeed, the toxic components contained in cigarette smoking are largely shared with diesel exhaust and fossil fuel combustion,” he said.
Although causality between air pollution and arthritis flares cannot be confirmed in these data, a basis for suspecting a causal relationship is supported by “plenty of in vitro and animal studies,” according to Dr. Adami.
On the basis of these studies, several mechanisms have been postulated.
“As an example, exposure to air pollution can promote the activation of the bronchus-associated lymphoid tissue (BALT), which can trigger the activation of the transcription factor nuclear factor-kappaB,” he said. This, in turn, can “lead to the secretion of proinflammatory cytokines, such as tumor necrosis factor–alpha and interleukin-1.”
Another theory is that posttranslational modification of proteins in the lung, a process called citrullination, “can lead to production of autoantibodies known to have a pathogenic role in RA,” he added.
Proving a causal relationship, however, is difficult.
“We certainly cannot conduct a randomized clinical trial on that and voluntarily expose some patients to pollution. Thus, we need to rely on observational data,” Dr. Adami said.
Of strategies being considered to generate evidence of a causal relationship between pollution and the exacerbation of RA, “we certainly will try to study those patients that move from a highly polluted area to a greener zone and vice versa,” he said. This will allow us “to explore what happens when the exposure to pollution changes dramatically in a short period of time.”
In the meantime, “given what is known to date, I would certainly advise my RA patients to avoid exposure to air pollution,” Dr. Adami said. He acknowledged there is no proof that this will help patients to reduce the risk of flares, but there are already many good reasons to minimize exposure to air pollution.
Dr. Adami and Dr. Al-Herz report no potential conflicts of interest.
FROM THE EULAR 2021 CONGRESS
FDA clears next-generation DBS system for movement disorders
The SenSight Directional Lead System for DBS therapy combines two recent advancements: sensing capability that allows real-time monitoring of brain signals to optimize settings for stimulation, and a “directional lead” that enables steering of electric current for more precise targeting of stimulation through the electrode.
“Until now, sensing capability and directional leads have not been available in the same DBS system, so we have had to choose one technology or the other, based on the predicted needs of each patient,” neurosurgeon Kelly Foote, MD, who performed the first implant of the SenSight System at University of Florida (UF) Health, said in a news release.
“Now, by coupling this new directional lead with a pulse generator capable of brain sensing, we are excited to be able to offer our patients the synergistic benefits of both technologies,” added Dr. Foote, codirector of the Norman Fixel Institute for Neurological Diseases at UF Health.
Dr. Foote said DBS systems capable of adjusting therapeutic stimulation in response to continuously recorded brain signals may lead to better DBS outcomes with fewer adverse effects.
“Adding a directional lead to such a system will improve our ability to localize abnormal signals and enable us to steer current more effectively to areas in the brain where it is most beneficial,” Dr. Foote said.
“We are excited to see the clinical benefits that the new SenSight directional lead system will provide to patients and physicians in the U.S.,” added Mike Daly, vice president and general manager of brain modulation at Medtronic.
Medtronic’s SenSight directional lead DBS system received CE Mark approval in Europe in March.
A version of this article first appeared on Medscape.com.
The SenSight Directional Lead System for DBS therapy combines two recent advancements: sensing capability that allows real-time monitoring of brain signals to optimize settings for stimulation, and a “directional lead” that enables steering of electric current for more precise targeting of stimulation through the electrode.
“Until now, sensing capability and directional leads have not been available in the same DBS system, so we have had to choose one technology or the other, based on the predicted needs of each patient,” neurosurgeon Kelly Foote, MD, who performed the first implant of the SenSight System at University of Florida (UF) Health, said in a news release.
“Now, by coupling this new directional lead with a pulse generator capable of brain sensing, we are excited to be able to offer our patients the synergistic benefits of both technologies,” added Dr. Foote, codirector of the Norman Fixel Institute for Neurological Diseases at UF Health.
Dr. Foote said DBS systems capable of adjusting therapeutic stimulation in response to continuously recorded brain signals may lead to better DBS outcomes with fewer adverse effects.
“Adding a directional lead to such a system will improve our ability to localize abnormal signals and enable us to steer current more effectively to areas in the brain where it is most beneficial,” Dr. Foote said.
“We are excited to see the clinical benefits that the new SenSight directional lead system will provide to patients and physicians in the U.S.,” added Mike Daly, vice president and general manager of brain modulation at Medtronic.
Medtronic’s SenSight directional lead DBS system received CE Mark approval in Europe in March.
A version of this article first appeared on Medscape.com.
The SenSight Directional Lead System for DBS therapy combines two recent advancements: sensing capability that allows real-time monitoring of brain signals to optimize settings for stimulation, and a “directional lead” that enables steering of electric current for more precise targeting of stimulation through the electrode.
“Until now, sensing capability and directional leads have not been available in the same DBS system, so we have had to choose one technology or the other, based on the predicted needs of each patient,” neurosurgeon Kelly Foote, MD, who performed the first implant of the SenSight System at University of Florida (UF) Health, said in a news release.
“Now, by coupling this new directional lead with a pulse generator capable of brain sensing, we are excited to be able to offer our patients the synergistic benefits of both technologies,” added Dr. Foote, codirector of the Norman Fixel Institute for Neurological Diseases at UF Health.
Dr. Foote said DBS systems capable of adjusting therapeutic stimulation in response to continuously recorded brain signals may lead to better DBS outcomes with fewer adverse effects.
“Adding a directional lead to such a system will improve our ability to localize abnormal signals and enable us to steer current more effectively to areas in the brain where it is most beneficial,” Dr. Foote said.
“We are excited to see the clinical benefits that the new SenSight directional lead system will provide to patients and physicians in the U.S.,” added Mike Daly, vice president and general manager of brain modulation at Medtronic.
Medtronic’s SenSight directional lead DBS system received CE Mark approval in Europe in March.
A version of this article first appeared on Medscape.com.
Waist circumference a marker for NAFL in type 1 diabetes
It follows that, as the prevalence of obesity among people with type 1 diabetes mellitus (T1DM) increases, so would the incidence of nonalcoholic fatty liver (NAFL), as it does in type 2 diabetes.
However, researchers in Finland report that the incidence of NAFL in T1DM is much lower, and that the use of the waist-to-height ratio to calculate midsection girth could be a low-cost alternative to MRI and computed tomography to more precisely diagnose NAFL in T1DM.
In a cross-sectional analysis of 121 adults with T1DM in the Finnish Diabetic Nephropathy study, known as FinnDiane, researchers from the University of Helsinki report in Diabetes Care that a waist-to-height ratio of 0.5 showed a relatively high rate of accuracy for identifying NAFL that was statistically significant (P = .04).
Lead author Erika B. Parente, MD, PhD, a researcher at the Folkhälsän Research Center in Helsinki, noted that the findings do not identify any causality between what the researchers called visceral adiposity and NAFL. “As long as they have accumulation of fat in the center of body and they can develop this low-grade inflammation that also goes to insulin-load sensitivity, people with T1DM can accumulate fat in the liver as do people with T2DM and the general population,” she said in an interview.
These findings build on her group’s previous work published in Scientific Reports showing a strong relationship between waist-to-height ratio and visceral fat percentage in adults with T1DM. The most recent FinnDiane analysis found no similar relationship between NAFL and fat tissue in the hips, arms and legs, and total adipose tissue.
Better than BMI as a measure
“We also found that waist-to-height ratio is better than body mass index to identify those individuals at higher risk of having NAFL,” Dr. Parente said. However, it’s not possible to predict which patients referred to imaging evaluation after being screened by waist-to-height ratio of 0.5 will surely have NAFL, she added.
That answer, she said, would require a longitudinal and cost-effectiveness study with larger population.
The waist-to-height ratio cutoff of 0.5 showed an 86% sensitivity and 55% specificity for NAFL, whereas BMI of 26.6 kg/m2 showed an 79% sensitivity and 57% specificity.
“The most important message from our research is that health care professionals should be aware that individuals with T1DM can have NAFL, and waist-to-height ratio may help to identify those at higher risk,” she said.
The prevalence of NAFL among the adults with T1DM in the study was 11.6%, which is lower than the prevalence other studies reported in T2DM – 76% in a U.S. study – and in the general population – ranging from 19% to 46%. This underscores, Dr. Parente noted, the importance of using waist-to-height ratio in T1DM patients to determine the status of NAFL.
She said that few studies have investigated the consequences of NAFL in T1DM, pointing to two that linked NAFL with chronic kidney disease and cardiovascular disease in T1DM (Diabetes Care. 2014;37:1729-36; J Hepatol. 2010;53:713-8). “Most of the studies about the consequences of NAFL included people with T2DM,” she said. “From our research, we cannot conclude about the impact of NAFL in cardiovascular or kidney complications in our population because this is a cross-sectional study.”
That question may be answered by a future follow-up study of the ongoing FinnDiane study, she said.
The study is a “good reminder” that people with central adiposity and metabolic syndrome can develop NAFL disease, said Jeanne Marie Clark, MD, MPH, of Johns Hopkins University, Baltimore. “Even patients we may not think of having insulin resistance, such as those with T1DM.”
However, Dr. Clark added, “I do not think we can really determine which measure of central adiposity is best.” She noted that the study was “pretty small” with only 14 patients who had NAFL disease. “Waist-to-height ratio is certainly a reasonable option,” she added. “Waist circumference alone is known to be a strong predictor. I would say some measure is better than none, and it should be more routine in clinical practice.”
Dr. Parente disclosed financial relationships with Eli Lilly, Abbott, AstraZeneca, Sanofi, and Boehringer Ingelheim. Two of eight coauthors disclosed financial relationships with AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Elo Water, Fresenius, GE Healthcare, Medscape, Merck Sharpe and Dohme, Mundipharma, Novo Nordisk, Peer-Voice, Sanofi, and Sciarc. The remaining coauthors had no disclosures.
Dr. Clark had no disclosures.
It follows that, as the prevalence of obesity among people with type 1 diabetes mellitus (T1DM) increases, so would the incidence of nonalcoholic fatty liver (NAFL), as it does in type 2 diabetes.
However, researchers in Finland report that the incidence of NAFL in T1DM is much lower, and that the use of the waist-to-height ratio to calculate midsection girth could be a low-cost alternative to MRI and computed tomography to more precisely diagnose NAFL in T1DM.
In a cross-sectional analysis of 121 adults with T1DM in the Finnish Diabetic Nephropathy study, known as FinnDiane, researchers from the University of Helsinki report in Diabetes Care that a waist-to-height ratio of 0.5 showed a relatively high rate of accuracy for identifying NAFL that was statistically significant (P = .04).
Lead author Erika B. Parente, MD, PhD, a researcher at the Folkhälsän Research Center in Helsinki, noted that the findings do not identify any causality between what the researchers called visceral adiposity and NAFL. “As long as they have accumulation of fat in the center of body and they can develop this low-grade inflammation that also goes to insulin-load sensitivity, people with T1DM can accumulate fat in the liver as do people with T2DM and the general population,” she said in an interview.
These findings build on her group’s previous work published in Scientific Reports showing a strong relationship between waist-to-height ratio and visceral fat percentage in adults with T1DM. The most recent FinnDiane analysis found no similar relationship between NAFL and fat tissue in the hips, arms and legs, and total adipose tissue.
Better than BMI as a measure
“We also found that waist-to-height ratio is better than body mass index to identify those individuals at higher risk of having NAFL,” Dr. Parente said. However, it’s not possible to predict which patients referred to imaging evaluation after being screened by waist-to-height ratio of 0.5 will surely have NAFL, she added.
That answer, she said, would require a longitudinal and cost-effectiveness study with larger population.
The waist-to-height ratio cutoff of 0.5 showed an 86% sensitivity and 55% specificity for NAFL, whereas BMI of 26.6 kg/m2 showed an 79% sensitivity and 57% specificity.
“The most important message from our research is that health care professionals should be aware that individuals with T1DM can have NAFL, and waist-to-height ratio may help to identify those at higher risk,” she said.
The prevalence of NAFL among the adults with T1DM in the study was 11.6%, which is lower than the prevalence other studies reported in T2DM – 76% in a U.S. study – and in the general population – ranging from 19% to 46%. This underscores, Dr. Parente noted, the importance of using waist-to-height ratio in T1DM patients to determine the status of NAFL.
She said that few studies have investigated the consequences of NAFL in T1DM, pointing to two that linked NAFL with chronic kidney disease and cardiovascular disease in T1DM (Diabetes Care. 2014;37:1729-36; J Hepatol. 2010;53:713-8). “Most of the studies about the consequences of NAFL included people with T2DM,” she said. “From our research, we cannot conclude about the impact of NAFL in cardiovascular or kidney complications in our population because this is a cross-sectional study.”
That question may be answered by a future follow-up study of the ongoing FinnDiane study, she said.
The study is a “good reminder” that people with central adiposity and metabolic syndrome can develop NAFL disease, said Jeanne Marie Clark, MD, MPH, of Johns Hopkins University, Baltimore. “Even patients we may not think of having insulin resistance, such as those with T1DM.”
However, Dr. Clark added, “I do not think we can really determine which measure of central adiposity is best.” She noted that the study was “pretty small” with only 14 patients who had NAFL disease. “Waist-to-height ratio is certainly a reasonable option,” she added. “Waist circumference alone is known to be a strong predictor. I would say some measure is better than none, and it should be more routine in clinical practice.”
Dr. Parente disclosed financial relationships with Eli Lilly, Abbott, AstraZeneca, Sanofi, and Boehringer Ingelheim. Two of eight coauthors disclosed financial relationships with AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Elo Water, Fresenius, GE Healthcare, Medscape, Merck Sharpe and Dohme, Mundipharma, Novo Nordisk, Peer-Voice, Sanofi, and Sciarc. The remaining coauthors had no disclosures.
Dr. Clark had no disclosures.
It follows that, as the prevalence of obesity among people with type 1 diabetes mellitus (T1DM) increases, so would the incidence of nonalcoholic fatty liver (NAFL), as it does in type 2 diabetes.
However, researchers in Finland report that the incidence of NAFL in T1DM is much lower, and that the use of the waist-to-height ratio to calculate midsection girth could be a low-cost alternative to MRI and computed tomography to more precisely diagnose NAFL in T1DM.
In a cross-sectional analysis of 121 adults with T1DM in the Finnish Diabetic Nephropathy study, known as FinnDiane, researchers from the University of Helsinki report in Diabetes Care that a waist-to-height ratio of 0.5 showed a relatively high rate of accuracy for identifying NAFL that was statistically significant (P = .04).
Lead author Erika B. Parente, MD, PhD, a researcher at the Folkhälsän Research Center in Helsinki, noted that the findings do not identify any causality between what the researchers called visceral adiposity and NAFL. “As long as they have accumulation of fat in the center of body and they can develop this low-grade inflammation that also goes to insulin-load sensitivity, people with T1DM can accumulate fat in the liver as do people with T2DM and the general population,” she said in an interview.
These findings build on her group’s previous work published in Scientific Reports showing a strong relationship between waist-to-height ratio and visceral fat percentage in adults with T1DM. The most recent FinnDiane analysis found no similar relationship between NAFL and fat tissue in the hips, arms and legs, and total adipose tissue.
Better than BMI as a measure
“We also found that waist-to-height ratio is better than body mass index to identify those individuals at higher risk of having NAFL,” Dr. Parente said. However, it’s not possible to predict which patients referred to imaging evaluation after being screened by waist-to-height ratio of 0.5 will surely have NAFL, she added.
That answer, she said, would require a longitudinal and cost-effectiveness study with larger population.
The waist-to-height ratio cutoff of 0.5 showed an 86% sensitivity and 55% specificity for NAFL, whereas BMI of 26.6 kg/m2 showed an 79% sensitivity and 57% specificity.
“The most important message from our research is that health care professionals should be aware that individuals with T1DM can have NAFL, and waist-to-height ratio may help to identify those at higher risk,” she said.
The prevalence of NAFL among the adults with T1DM in the study was 11.6%, which is lower than the prevalence other studies reported in T2DM – 76% in a U.S. study – and in the general population – ranging from 19% to 46%. This underscores, Dr. Parente noted, the importance of using waist-to-height ratio in T1DM patients to determine the status of NAFL.
She said that few studies have investigated the consequences of NAFL in T1DM, pointing to two that linked NAFL with chronic kidney disease and cardiovascular disease in T1DM (Diabetes Care. 2014;37:1729-36; J Hepatol. 2010;53:713-8). “Most of the studies about the consequences of NAFL included people with T2DM,” she said. “From our research, we cannot conclude about the impact of NAFL in cardiovascular or kidney complications in our population because this is a cross-sectional study.”
That question may be answered by a future follow-up study of the ongoing FinnDiane study, she said.
The study is a “good reminder” that people with central adiposity and metabolic syndrome can develop NAFL disease, said Jeanne Marie Clark, MD, MPH, of Johns Hopkins University, Baltimore. “Even patients we may not think of having insulin resistance, such as those with T1DM.”
However, Dr. Clark added, “I do not think we can really determine which measure of central adiposity is best.” She noted that the study was “pretty small” with only 14 patients who had NAFL disease. “Waist-to-height ratio is certainly a reasonable option,” she added. “Waist circumference alone is known to be a strong predictor. I would say some measure is better than none, and it should be more routine in clinical practice.”
Dr. Parente disclosed financial relationships with Eli Lilly, Abbott, AstraZeneca, Sanofi, and Boehringer Ingelheim. Two of eight coauthors disclosed financial relationships with AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Elo Water, Fresenius, GE Healthcare, Medscape, Merck Sharpe and Dohme, Mundipharma, Novo Nordisk, Peer-Voice, Sanofi, and Sciarc. The remaining coauthors had no disclosures.
Dr. Clark had no disclosures.
FROM DIABETES CARE
CDC director cites rise in hospitalizations in urging teen vaccinations
“I am deeply concerned by the numbers of hospitalized adolescents and saddened to see the number of adolescents who required treatment in intensive care units or mechanical ventilation,” CDC Director Rochelle Walensky, MD, said in a statement.
While urging teenagers to wear masks and take precautions around others, she asked “parents, relatives, and close friends to join me and talk with teens about the importance of these prevention strategies and to encourage them to get vaccinated.”
Dr. Walensky referred to the CDC’s Morbidity and Mortality Weekly Report that showed adolescent hospitalizations peaked at 2.1 per 100,000 in early January 2021, then dropped to 0.6 per 100,000 in mid-March.
Alarmingly, hospitalizations rose to 1.3 per 100,000 in April, and a number of teens required serious interventions.
“Among hospitalized adolescents, nearly one-third required intensive care unit admission, and 5% required invasive mechanical ventilation,” the report said. No deaths occurred.
The study looked at 376 adolescents aged 12-17 who were hospitalized and tested positive for coronavirus. Of that group, 204 were hospitalized for COVID-19 and the other 172 were hospitalized for reasons not directly related to COVID-19.
Of the 204 hospitalized for COVID-19, 70.6% had an underlying medical condition such as obesity or chronic lung disease.
The study noted that children and teenagers have lower hospitalization rates and generally show less severe symptoms than do older people.
Possible causes for the rise in adolescent COVID-19 hospitalizations include the arrival of variants, the growing number of children returning to in-person education, and the changes in mask-wearing and other safety precautions, the study said.
The American Academy of Pediatrics said that as of May 27, 4 million children have tested positive for COVID-19 since the pandemic began, with about 34,500 new child cases reported for the week ending May 27.
The AAP said children have represented 14.1% of total cases since the pandemic began, but for the week ending May 27, children represented 24.3% of new reported weekly COVID-19 cases.
On May 10, the FDA granted emergency use authorization for the Pfizer coronavirus vaccine to be given to children aged 12-15 years. Previously, the FDA had authorized the Pfizer vaccine for people aged 16 years and up, whereas the Moderna and Johnson & Johnson vaccines are authorized for people aged 18 years and up.
“Vaccination is our way out of this pandemic,” Dr. Walensky said in her statement. “I continue to see promising signs in CDC data that we are nearing the end of this pandemic in this country; however, we all have to do our part and get vaccinated to cross the finish line.”
A version of this article was first published on WebMD.com.
“I am deeply concerned by the numbers of hospitalized adolescents and saddened to see the number of adolescents who required treatment in intensive care units or mechanical ventilation,” CDC Director Rochelle Walensky, MD, said in a statement.
While urging teenagers to wear masks and take precautions around others, she asked “parents, relatives, and close friends to join me and talk with teens about the importance of these prevention strategies and to encourage them to get vaccinated.”
Dr. Walensky referred to the CDC’s Morbidity and Mortality Weekly Report that showed adolescent hospitalizations peaked at 2.1 per 100,000 in early January 2021, then dropped to 0.6 per 100,000 in mid-March.
Alarmingly, hospitalizations rose to 1.3 per 100,000 in April, and a number of teens required serious interventions.
“Among hospitalized adolescents, nearly one-third required intensive care unit admission, and 5% required invasive mechanical ventilation,” the report said. No deaths occurred.
The study looked at 376 adolescents aged 12-17 who were hospitalized and tested positive for coronavirus. Of that group, 204 were hospitalized for COVID-19 and the other 172 were hospitalized for reasons not directly related to COVID-19.
Of the 204 hospitalized for COVID-19, 70.6% had an underlying medical condition such as obesity or chronic lung disease.
The study noted that children and teenagers have lower hospitalization rates and generally show less severe symptoms than do older people.
Possible causes for the rise in adolescent COVID-19 hospitalizations include the arrival of variants, the growing number of children returning to in-person education, and the changes in mask-wearing and other safety precautions, the study said.
The American Academy of Pediatrics said that as of May 27, 4 million children have tested positive for COVID-19 since the pandemic began, with about 34,500 new child cases reported for the week ending May 27.
The AAP said children have represented 14.1% of total cases since the pandemic began, but for the week ending May 27, children represented 24.3% of new reported weekly COVID-19 cases.
On May 10, the FDA granted emergency use authorization for the Pfizer coronavirus vaccine to be given to children aged 12-15 years. Previously, the FDA had authorized the Pfizer vaccine for people aged 16 years and up, whereas the Moderna and Johnson & Johnson vaccines are authorized for people aged 18 years and up.
“Vaccination is our way out of this pandemic,” Dr. Walensky said in her statement. “I continue to see promising signs in CDC data that we are nearing the end of this pandemic in this country; however, we all have to do our part and get vaccinated to cross the finish line.”
A version of this article was first published on WebMD.com.
“I am deeply concerned by the numbers of hospitalized adolescents and saddened to see the number of adolescents who required treatment in intensive care units or mechanical ventilation,” CDC Director Rochelle Walensky, MD, said in a statement.
While urging teenagers to wear masks and take precautions around others, she asked “parents, relatives, and close friends to join me and talk with teens about the importance of these prevention strategies and to encourage them to get vaccinated.”
Dr. Walensky referred to the CDC’s Morbidity and Mortality Weekly Report that showed adolescent hospitalizations peaked at 2.1 per 100,000 in early January 2021, then dropped to 0.6 per 100,000 in mid-March.
Alarmingly, hospitalizations rose to 1.3 per 100,000 in April, and a number of teens required serious interventions.
“Among hospitalized adolescents, nearly one-third required intensive care unit admission, and 5% required invasive mechanical ventilation,” the report said. No deaths occurred.
The study looked at 376 adolescents aged 12-17 who were hospitalized and tested positive for coronavirus. Of that group, 204 were hospitalized for COVID-19 and the other 172 were hospitalized for reasons not directly related to COVID-19.
Of the 204 hospitalized for COVID-19, 70.6% had an underlying medical condition such as obesity or chronic lung disease.
The study noted that children and teenagers have lower hospitalization rates and generally show less severe symptoms than do older people.
Possible causes for the rise in adolescent COVID-19 hospitalizations include the arrival of variants, the growing number of children returning to in-person education, and the changes in mask-wearing and other safety precautions, the study said.
The American Academy of Pediatrics said that as of May 27, 4 million children have tested positive for COVID-19 since the pandemic began, with about 34,500 new child cases reported for the week ending May 27.
The AAP said children have represented 14.1% of total cases since the pandemic began, but for the week ending May 27, children represented 24.3% of new reported weekly COVID-19 cases.
On May 10, the FDA granted emergency use authorization for the Pfizer coronavirus vaccine to be given to children aged 12-15 years. Previously, the FDA had authorized the Pfizer vaccine for people aged 16 years and up, whereas the Moderna and Johnson & Johnson vaccines are authorized for people aged 18 years and up.
“Vaccination is our way out of this pandemic,” Dr. Walensky said in her statement. “I continue to see promising signs in CDC data that we are nearing the end of this pandemic in this country; however, we all have to do our part and get vaccinated to cross the finish line.”
A version of this article was first published on WebMD.com.




