User login
High-flow nasal cannula improves dyspnea in palliative care patients with respiratory failure
Background: For patients receiving palliative care who develop respiratory distress, conventional oxygen therapy may not adequately relieve symptoms of dyspnea, and noninvasive ventilation may not promote comfort. Few randomized controlled trials have investigated the use of high-flow nasal cannula (HFNC) for treatment of palliative care patients who present to the hospital with respiratory distress.
Study design: Randomized crossover study.
Setting: Emergency department of a single institution.
Synopsis: Forty-eight palliative care patients who presented to the ED with acute dyspnea were enrolled and randomized to receive HFNC for 1 hour, followed by conventional oxygen therapy for 1 hour, or vice versa. The authors found that patients using HFNC reported significantly less dyspnea on a breathlessness severity scale, compared with patients using conventional oxygen therapy. Additionally, patients using HFNC had significantly lower respiratory rates, and HFNC use was associated with significantly lower need for morphine in a 1-hour period. The study was limited because of its single institution and small sample size, and therefore the results may not be generalizable to other patient populations.
Bottom line: Treatment with a high-flow nasal cannula may improve symptoms of acute dysp-nea in palliative patients when compared with conventional oxygen therapy.
Citation: Ruangsomboon O et al. High-flow nasal cannula versus conventional oxygen therapy in relieving dyspnea in emergency palliative patients with do-not-intubate status: A randomized crossover study. Ann Emerg Med. 2019 Dec 18. doi: 10.1016/j.annemergmed.2019.09.009.
Dr. Halford is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
Background: For patients receiving palliative care who develop respiratory distress, conventional oxygen therapy may not adequately relieve symptoms of dyspnea, and noninvasive ventilation may not promote comfort. Few randomized controlled trials have investigated the use of high-flow nasal cannula (HFNC) for treatment of palliative care patients who present to the hospital with respiratory distress.
Study design: Randomized crossover study.
Setting: Emergency department of a single institution.
Synopsis: Forty-eight palliative care patients who presented to the ED with acute dyspnea were enrolled and randomized to receive HFNC for 1 hour, followed by conventional oxygen therapy for 1 hour, or vice versa. The authors found that patients using HFNC reported significantly less dyspnea on a breathlessness severity scale, compared with patients using conventional oxygen therapy. Additionally, patients using HFNC had significantly lower respiratory rates, and HFNC use was associated with significantly lower need for morphine in a 1-hour period. The study was limited because of its single institution and small sample size, and therefore the results may not be generalizable to other patient populations.
Bottom line: Treatment with a high-flow nasal cannula may improve symptoms of acute dysp-nea in palliative patients when compared with conventional oxygen therapy.
Citation: Ruangsomboon O et al. High-flow nasal cannula versus conventional oxygen therapy in relieving dyspnea in emergency palliative patients with do-not-intubate status: A randomized crossover study. Ann Emerg Med. 2019 Dec 18. doi: 10.1016/j.annemergmed.2019.09.009.
Dr. Halford is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
Background: For patients receiving palliative care who develop respiratory distress, conventional oxygen therapy may not adequately relieve symptoms of dyspnea, and noninvasive ventilation may not promote comfort. Few randomized controlled trials have investigated the use of high-flow nasal cannula (HFNC) for treatment of palliative care patients who present to the hospital with respiratory distress.
Study design: Randomized crossover study.
Setting: Emergency department of a single institution.
Synopsis: Forty-eight palliative care patients who presented to the ED with acute dyspnea were enrolled and randomized to receive HFNC for 1 hour, followed by conventional oxygen therapy for 1 hour, or vice versa. The authors found that patients using HFNC reported significantly less dyspnea on a breathlessness severity scale, compared with patients using conventional oxygen therapy. Additionally, patients using HFNC had significantly lower respiratory rates, and HFNC use was associated with significantly lower need for morphine in a 1-hour period. The study was limited because of its single institution and small sample size, and therefore the results may not be generalizable to other patient populations.
Bottom line: Treatment with a high-flow nasal cannula may improve symptoms of acute dysp-nea in palliative patients when compared with conventional oxygen therapy.
Citation: Ruangsomboon O et al. High-flow nasal cannula versus conventional oxygen therapy in relieving dyspnea in emergency palliative patients with do-not-intubate status: A randomized crossover study. Ann Emerg Med. 2019 Dec 18. doi: 10.1016/j.annemergmed.2019.09.009.
Dr. Halford is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
Pericardial fat an independent risk factor for heart failure
Pericardial fat is associated with a heightened risk for heart failure, particularly in women, new research suggests.
In a prospective cohort study of nearly 7,000 individuals, excess pericardial fat was linked to a higher risk for heart failure, even after adjustment for established risk factors for heart failure.
Women with high pericardial fat volume (PFV), defined as more than 70 cm3 or 2.4 fluid ounces, had double the risk of developing heart failure. For men, high PFV, defined as more than 120 cm3 or 4.0 fluid ounces, was associated with a 50% increase in the risk for heart failure.
The findings were published in the Journal of the American College of Cardiology.
“People will ask why should they measure fat around the heart. Why can’t they just take the waist circumference or body mass index as a measure for increased risk?” lead author Satish Kenchaiah, MD, MPH, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Yet, when we adjusted for waist circumference, hip circumference, waist to hip ratio, and other known variables, pericardial fat was still associated with an increased risk of heart failure. This tells me that it is not just overall fat in the body but something about its location around the heart that is playing a role,” Dr. Kenchaiah said.
“Now that we have found an association between any amount of fat around the pericardium and heart failure, it gives us an impetus to build future research on identifying how exactly these fat deposits influence the development of cardiomyopathy,” he said.
Dr. Kenchaiah and colleagues investigated the association of pericardial fat with incident heart failure by examining chest CT scans from 6,785 participants (3,584 women and 3,201 men aged 45-84 years) in the Multi-Ethnic Study of Atherosclerosis.
The participants were from four different ethnic groups: 38% were White; 28% were Black, 22% were Hispanic, and 12% were Chinese American. They were recruited between July 17, 2000, and Aug. 31, 2002, from six communities in the United States: Baltimore and Baltimore County; Chicago; Forsyth County, N.C.; Los Angeles County northern Manhattan and the Bronx, New York; and St. Paul, Minn.
All participants were free of cardiovascular disease at baseline.
The researchers followed participants for more than 17 years. During this time, 385 (5.7%; 164 women and 221 men) developed newly diagnosed heart failure.
In women, the hazard ratio for every 42 cm3 increase in PFV was 1.44 (95% confidence interval, 1.21-1.71; P < .001). In men, the HR was 1.13 (95% CI, 1.01-1.27; P = .03).
High PVF conferred a twofold greater risk for heart failure in women (HR, 2.06; 95% CI, 1.48-2.87; P < .001) and a 53% higher risk in men (HR, 1.53; 95% CI, 1.13-2.07; P = .006).
These associations remained significant after further adjustment for circulating markers of systemic inflammation (that is, C-reactive protein and interleukin-6), and abdominal subcutaneous or visceral fat.
They also found that the heightened risk persisted, even after adjustment for established risk factors for heart failure, such as age, cigarette smoking, alcohol consumption, sedentary lifestyle, high blood pressure, high blood sugar, high cholesterol, and myocardial infarction.
Results were similar among all of the ethnic groups studied.
A surprise finding
“The most surprising part of this study was that the risk for heart failure with increased pericardial fat does not seem to be explained by obesity and systemic inflammation alone,” Andreas P. Kalogeropoulos, MD, MPH, PhD, Stony Brook (N.Y.) University, said in an interview.
“If pericardial fat was merely a proxy for increased visceral fat, one would expect the association of pericardial fat with heart failure risk to go away after factoring in abdominal CT findings, which was not the case here. Also, accounting for inflammatory markers did not change things dramatically. However, we need to be careful here, as abdominal CT scans have not been done simultaneously with the pericardial fat scans in the study,” said Dr. Kalogeropoulos, who coauthored an accompanying editorial with Michael E. Hall, MD, University of Mississippi Medical Center, Jackson.
The other striking finding, although not entirely surprising, was the stronger association of pericardial fat with heart failure risk in women, he noted.
“Although several clues have been reported pointing to women being more sensitive to the adverse cardiac effects of pericardial fat, this is the first large prospective study to connect the dots and show much higher risk in women in a convincing way. For the record, this is the first prospective study to show the connection between pericardial fat and heart failure risk altogether,” Dr. Kalogeropoulos said.
“Obviously, we need to do more work to see how we can use the important findings of Kenchaiah and colleagues to reduce risk for heart failure among patients with increased pericardial fat, especially women. For starters, we would need a way to identify these patients,” he said. “In this aspect, it is encouraging that pericardial fat can be measured in low-radiation CT scans, similar to those used for coronary calcium, and that automation technology to speed up pericardial fat measurements is already in the pipeline.
“The next step would be to see what kind of interventions would reduce risk for heart failure in these patients,” he added. “Weight loss would be an obvious thing, but novel agents with favorable cardiometabolic effects, like newer antidiabetic medications, are intriguing options, too.”
The study was supported by the National Heart, Lung, and Blood Institute and the National Institutes of Health. Dr. Kenchaiah and Dr. Kalogeropoulos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pericardial fat is associated with a heightened risk for heart failure, particularly in women, new research suggests.
In a prospective cohort study of nearly 7,000 individuals, excess pericardial fat was linked to a higher risk for heart failure, even after adjustment for established risk factors for heart failure.
Women with high pericardial fat volume (PFV), defined as more than 70 cm3 or 2.4 fluid ounces, had double the risk of developing heart failure. For men, high PFV, defined as more than 120 cm3 or 4.0 fluid ounces, was associated with a 50% increase in the risk for heart failure.
The findings were published in the Journal of the American College of Cardiology.
“People will ask why should they measure fat around the heart. Why can’t they just take the waist circumference or body mass index as a measure for increased risk?” lead author Satish Kenchaiah, MD, MPH, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Yet, when we adjusted for waist circumference, hip circumference, waist to hip ratio, and other known variables, pericardial fat was still associated with an increased risk of heart failure. This tells me that it is not just overall fat in the body but something about its location around the heart that is playing a role,” Dr. Kenchaiah said.
“Now that we have found an association between any amount of fat around the pericardium and heart failure, it gives us an impetus to build future research on identifying how exactly these fat deposits influence the development of cardiomyopathy,” he said.
Dr. Kenchaiah and colleagues investigated the association of pericardial fat with incident heart failure by examining chest CT scans from 6,785 participants (3,584 women and 3,201 men aged 45-84 years) in the Multi-Ethnic Study of Atherosclerosis.
The participants were from four different ethnic groups: 38% were White; 28% were Black, 22% were Hispanic, and 12% were Chinese American. They were recruited between July 17, 2000, and Aug. 31, 2002, from six communities in the United States: Baltimore and Baltimore County; Chicago; Forsyth County, N.C.; Los Angeles County northern Manhattan and the Bronx, New York; and St. Paul, Minn.
All participants were free of cardiovascular disease at baseline.
The researchers followed participants for more than 17 years. During this time, 385 (5.7%; 164 women and 221 men) developed newly diagnosed heart failure.
In women, the hazard ratio for every 42 cm3 increase in PFV was 1.44 (95% confidence interval, 1.21-1.71; P < .001). In men, the HR was 1.13 (95% CI, 1.01-1.27; P = .03).
High PVF conferred a twofold greater risk for heart failure in women (HR, 2.06; 95% CI, 1.48-2.87; P < .001) and a 53% higher risk in men (HR, 1.53; 95% CI, 1.13-2.07; P = .006).
These associations remained significant after further adjustment for circulating markers of systemic inflammation (that is, C-reactive protein and interleukin-6), and abdominal subcutaneous or visceral fat.
They also found that the heightened risk persisted, even after adjustment for established risk factors for heart failure, such as age, cigarette smoking, alcohol consumption, sedentary lifestyle, high blood pressure, high blood sugar, high cholesterol, and myocardial infarction.
Results were similar among all of the ethnic groups studied.
A surprise finding
“The most surprising part of this study was that the risk for heart failure with increased pericardial fat does not seem to be explained by obesity and systemic inflammation alone,” Andreas P. Kalogeropoulos, MD, MPH, PhD, Stony Brook (N.Y.) University, said in an interview.
“If pericardial fat was merely a proxy for increased visceral fat, one would expect the association of pericardial fat with heart failure risk to go away after factoring in abdominal CT findings, which was not the case here. Also, accounting for inflammatory markers did not change things dramatically. However, we need to be careful here, as abdominal CT scans have not been done simultaneously with the pericardial fat scans in the study,” said Dr. Kalogeropoulos, who coauthored an accompanying editorial with Michael E. Hall, MD, University of Mississippi Medical Center, Jackson.
The other striking finding, although not entirely surprising, was the stronger association of pericardial fat with heart failure risk in women, he noted.
“Although several clues have been reported pointing to women being more sensitive to the adverse cardiac effects of pericardial fat, this is the first large prospective study to connect the dots and show much higher risk in women in a convincing way. For the record, this is the first prospective study to show the connection between pericardial fat and heart failure risk altogether,” Dr. Kalogeropoulos said.
“Obviously, we need to do more work to see how we can use the important findings of Kenchaiah and colleagues to reduce risk for heart failure among patients with increased pericardial fat, especially women. For starters, we would need a way to identify these patients,” he said. “In this aspect, it is encouraging that pericardial fat can be measured in low-radiation CT scans, similar to those used for coronary calcium, and that automation technology to speed up pericardial fat measurements is already in the pipeline.
“The next step would be to see what kind of interventions would reduce risk for heart failure in these patients,” he added. “Weight loss would be an obvious thing, but novel agents with favorable cardiometabolic effects, like newer antidiabetic medications, are intriguing options, too.”
The study was supported by the National Heart, Lung, and Blood Institute and the National Institutes of Health. Dr. Kenchaiah and Dr. Kalogeropoulos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pericardial fat is associated with a heightened risk for heart failure, particularly in women, new research suggests.
In a prospective cohort study of nearly 7,000 individuals, excess pericardial fat was linked to a higher risk for heart failure, even after adjustment for established risk factors for heart failure.
Women with high pericardial fat volume (PFV), defined as more than 70 cm3 or 2.4 fluid ounces, had double the risk of developing heart failure. For men, high PFV, defined as more than 120 cm3 or 4.0 fluid ounces, was associated with a 50% increase in the risk for heart failure.
The findings were published in the Journal of the American College of Cardiology.
“People will ask why should they measure fat around the heart. Why can’t they just take the waist circumference or body mass index as a measure for increased risk?” lead author Satish Kenchaiah, MD, MPH, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Yet, when we adjusted for waist circumference, hip circumference, waist to hip ratio, and other known variables, pericardial fat was still associated with an increased risk of heart failure. This tells me that it is not just overall fat in the body but something about its location around the heart that is playing a role,” Dr. Kenchaiah said.
“Now that we have found an association between any amount of fat around the pericardium and heart failure, it gives us an impetus to build future research on identifying how exactly these fat deposits influence the development of cardiomyopathy,” he said.
Dr. Kenchaiah and colleagues investigated the association of pericardial fat with incident heart failure by examining chest CT scans from 6,785 participants (3,584 women and 3,201 men aged 45-84 years) in the Multi-Ethnic Study of Atherosclerosis.
The participants were from four different ethnic groups: 38% were White; 28% were Black, 22% were Hispanic, and 12% were Chinese American. They were recruited between July 17, 2000, and Aug. 31, 2002, from six communities in the United States: Baltimore and Baltimore County; Chicago; Forsyth County, N.C.; Los Angeles County northern Manhattan and the Bronx, New York; and St. Paul, Minn.
All participants were free of cardiovascular disease at baseline.
The researchers followed participants for more than 17 years. During this time, 385 (5.7%; 164 women and 221 men) developed newly diagnosed heart failure.
In women, the hazard ratio for every 42 cm3 increase in PFV was 1.44 (95% confidence interval, 1.21-1.71; P < .001). In men, the HR was 1.13 (95% CI, 1.01-1.27; P = .03).
High PVF conferred a twofold greater risk for heart failure in women (HR, 2.06; 95% CI, 1.48-2.87; P < .001) and a 53% higher risk in men (HR, 1.53; 95% CI, 1.13-2.07; P = .006).
These associations remained significant after further adjustment for circulating markers of systemic inflammation (that is, C-reactive protein and interleukin-6), and abdominal subcutaneous or visceral fat.
They also found that the heightened risk persisted, even after adjustment for established risk factors for heart failure, such as age, cigarette smoking, alcohol consumption, sedentary lifestyle, high blood pressure, high blood sugar, high cholesterol, and myocardial infarction.
Results were similar among all of the ethnic groups studied.
A surprise finding
“The most surprising part of this study was that the risk for heart failure with increased pericardial fat does not seem to be explained by obesity and systemic inflammation alone,” Andreas P. Kalogeropoulos, MD, MPH, PhD, Stony Brook (N.Y.) University, said in an interview.
“If pericardial fat was merely a proxy for increased visceral fat, one would expect the association of pericardial fat with heart failure risk to go away after factoring in abdominal CT findings, which was not the case here. Also, accounting for inflammatory markers did not change things dramatically. However, we need to be careful here, as abdominal CT scans have not been done simultaneously with the pericardial fat scans in the study,” said Dr. Kalogeropoulos, who coauthored an accompanying editorial with Michael E. Hall, MD, University of Mississippi Medical Center, Jackson.
The other striking finding, although not entirely surprising, was the stronger association of pericardial fat with heart failure risk in women, he noted.
“Although several clues have been reported pointing to women being more sensitive to the adverse cardiac effects of pericardial fat, this is the first large prospective study to connect the dots and show much higher risk in women in a convincing way. For the record, this is the first prospective study to show the connection between pericardial fat and heart failure risk altogether,” Dr. Kalogeropoulos said.
“Obviously, we need to do more work to see how we can use the important findings of Kenchaiah and colleagues to reduce risk for heart failure among patients with increased pericardial fat, especially women. For starters, we would need a way to identify these patients,” he said. “In this aspect, it is encouraging that pericardial fat can be measured in low-radiation CT scans, similar to those used for coronary calcium, and that automation technology to speed up pericardial fat measurements is already in the pipeline.
“The next step would be to see what kind of interventions would reduce risk for heart failure in these patients,” he added. “Weight loss would be an obvious thing, but novel agents with favorable cardiometabolic effects, like newer antidiabetic medications, are intriguing options, too.”
The study was supported by the National Heart, Lung, and Blood Institute and the National Institutes of Health. Dr. Kenchaiah and Dr. Kalogeropoulos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘Smart toilet’ with AI automatically scans stool for blood and consistency
A “smart toilet” in development uses artificial intelligence (AI) to scan stool for consistency and presence of blood – and early evidence suggests it is more accurate than patient self-reporting, a study reveals.
The remote, automated, real-time analysis and reporting increase the likelihood of physicians detecting gastrointestinal issues earlier, investigators reported.
In a proof-of-concept study, the smart toilet was 85% accurate in categorizing stool consistency as loose, normal, or constipated. The findings were presented at the annual Digestive Disease Week® (DDW).
“This study highlights a very innovative and practical tool that could have major implications for patients and clinicians alike,” Andrea Shin, MD, who was not affiliated with the research, said in an interview.
“Stool form or consistency and signs of bleeding are some of the most important pieces of clinical history when it comes to GI or bowel symptoms,” added Dr. Shin, assistant professor of medicine in the department of gastroenterology and hepatology at Indiana University, Indianapolis.
Image analysis
The researchers tested their AI algorithm on 3,328 images. They assessed photos from the Internet and some submitted anonymously by participants in the study.
Two gastroenterologists also rated a subset of 552 images. The physicians showed “satisfactory agreement” on interrater reliability (the extent to which two or more “raters” [for example, observers, examiners] agree), the investigators noted.
The smart toilet also was 76% accurate for gross blood detection.
“It’s objective and more accurate,” study author Sonia Grego, PhD, said in an interview. In contrast to asking patients to keep a bowel movement diary or recall the frequency and consistency of their stool over time, “the system does it for you,” she added.
“Our technology – by automating the image acquisition – removes the burden of having to track your pattern for weeks or months,” added Dr. Grego, founding director of the Duke Smart Toilet Lab at Duke University in Durham, N.C.
Information provided by patients “can have a big impact on decision-making,” Dr. Shin said. “For example, if I am talking to an individual who suffers from irritable bowel syndrome [IBS], I commonly ask them about how loose or watery and hard or formed their stool is, because this information gives me clues as to the underlying problems that may be driving their symptoms.”
Dr. Shin agreed it can be challenging for people to know what is important to report to their doctor. “This tool has the potential to relieve patient burden and facilitate communication between a patient and their clinician. It’s a great example of how technology can be leveraged to enhance care.”
Working behind the scenes
Together with gastroenterologist Deborah Anne Fisher, MD, an associate professor of medicine at Duke, Dr. Grego and colleagues devised a prototype that positions the image analyzer in the pipes behind the toilet. So the analysis is done post flush.
“We are experts of toilets and toilet technology,” Dr. Grego said. “We have learned that people really don’t like to see anything weird around the toilet bowl.”
The smart toilet system is designed for multiple users in a residential or commercial setting. The technology could be used in hospitals or long-term care facilities, for example. A fingerprint scanner on the flush mechanism tracks each individual user.
Mixed reactions
Dr. Grego gets a range of reactions when she tells people she is developing smart toilet technology.
“Friends and family laugh about the concept of the smart toilet,” she said, “so all the possible jokes that have been done on poops, we know.”
In fact, the researchers also are collecting the jokes they hear. “We’re being very systematic.”
In contrast, gastroenterologists who learn of the technology in development are more enthusiastic, Dr. Grego said. “There is such a need for removing the uncertainty of the patient recall about bowel movement frequency and appearance.
“We are seeking to expand through collaboration with additional GI doctors. We want to develop a more advanced prototype and do further validation studies,” Dr. Grego said.
Digital health tool
There is an aversion among patients to handling stool “or even talking about it,” Dr. Grego said. Colleagues tell her that people are more willing to provide a blood sample, which requires a needle, than a stool sample.
“But a lot of health data is there [in the stool],” she added. “We think this will empower a lot of research as well as consumer data gathering.”
For example, Dr. Grego envisions pharmaceutical companies using the technology to detect or monitor any changes in stool or gut health based on a treatment in development during clinical trials.
Furthermore, the technology might empower health-conscious consumers who want to track their own gut health. “This technology will be a whole new entry in the digital health toolkit,” Dr. Grego said.
Although not included in the research presented at this year’s DDW, the developers plan to add a sampling capability. Biochemic analysis of stool samples could provide “metabolically relevant information,” including stool biomarkers and microbiome composition.
“We have demonstrated it in the laboratory. It will be part of the technology when developed into a product,” Dr. Grego said.
This proof-of-concept study “is the first step in a path we are aggressively pursuing,” Dr. Grego said. She estimated it will take about 12-18 months to develop a prototype for use with patients. “We hope to move to a product soon after that.”
“I’m looking forward to seeing future iterations of this tool,” Dr. Shin said. “It could have a role in monitoring important GI diseases and disorders, including IBS and inflammatory bowel disease, or even for the detection of ‘alarm symptoms’ that shouldn’t be ignored.
“I could even see it having a role in preventative health in the future,” Dr. Shin added.
The technology has been licensed to the spin-off company Coprata to develop the product further.
“We hope to have an impact on people’s health very soon,” Dr. Grego said.
Duke University funded the study. Dr. Grego holds a management position at Coprata. Dr. Shin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A “smart toilet” in development uses artificial intelligence (AI) to scan stool for consistency and presence of blood – and early evidence suggests it is more accurate than patient self-reporting, a study reveals.
The remote, automated, real-time analysis and reporting increase the likelihood of physicians detecting gastrointestinal issues earlier, investigators reported.
In a proof-of-concept study, the smart toilet was 85% accurate in categorizing stool consistency as loose, normal, or constipated. The findings were presented at the annual Digestive Disease Week® (DDW).
“This study highlights a very innovative and practical tool that could have major implications for patients and clinicians alike,” Andrea Shin, MD, who was not affiliated with the research, said in an interview.
“Stool form or consistency and signs of bleeding are some of the most important pieces of clinical history when it comes to GI or bowel symptoms,” added Dr. Shin, assistant professor of medicine in the department of gastroenterology and hepatology at Indiana University, Indianapolis.
Image analysis
The researchers tested their AI algorithm on 3,328 images. They assessed photos from the Internet and some submitted anonymously by participants in the study.
Two gastroenterologists also rated a subset of 552 images. The physicians showed “satisfactory agreement” on interrater reliability (the extent to which two or more “raters” [for example, observers, examiners] agree), the investigators noted.
The smart toilet also was 76% accurate for gross blood detection.
“It’s objective and more accurate,” study author Sonia Grego, PhD, said in an interview. In contrast to asking patients to keep a bowel movement diary or recall the frequency and consistency of their stool over time, “the system does it for you,” she added.
“Our technology – by automating the image acquisition – removes the burden of having to track your pattern for weeks or months,” added Dr. Grego, founding director of the Duke Smart Toilet Lab at Duke University in Durham, N.C.
Information provided by patients “can have a big impact on decision-making,” Dr. Shin said. “For example, if I am talking to an individual who suffers from irritable bowel syndrome [IBS], I commonly ask them about how loose or watery and hard or formed their stool is, because this information gives me clues as to the underlying problems that may be driving their symptoms.”
Dr. Shin agreed it can be challenging for people to know what is important to report to their doctor. “This tool has the potential to relieve patient burden and facilitate communication between a patient and their clinician. It’s a great example of how technology can be leveraged to enhance care.”
Working behind the scenes
Together with gastroenterologist Deborah Anne Fisher, MD, an associate professor of medicine at Duke, Dr. Grego and colleagues devised a prototype that positions the image analyzer in the pipes behind the toilet. So the analysis is done post flush.
“We are experts of toilets and toilet technology,” Dr. Grego said. “We have learned that people really don’t like to see anything weird around the toilet bowl.”
The smart toilet system is designed for multiple users in a residential or commercial setting. The technology could be used in hospitals or long-term care facilities, for example. A fingerprint scanner on the flush mechanism tracks each individual user.
Mixed reactions
Dr. Grego gets a range of reactions when she tells people she is developing smart toilet technology.
“Friends and family laugh about the concept of the smart toilet,” she said, “so all the possible jokes that have been done on poops, we know.”
In fact, the researchers also are collecting the jokes they hear. “We’re being very systematic.”
In contrast, gastroenterologists who learn of the technology in development are more enthusiastic, Dr. Grego said. “There is such a need for removing the uncertainty of the patient recall about bowel movement frequency and appearance.
“We are seeking to expand through collaboration with additional GI doctors. We want to develop a more advanced prototype and do further validation studies,” Dr. Grego said.
Digital health tool
There is an aversion among patients to handling stool “or even talking about it,” Dr. Grego said. Colleagues tell her that people are more willing to provide a blood sample, which requires a needle, than a stool sample.
“But a lot of health data is there [in the stool],” she added. “We think this will empower a lot of research as well as consumer data gathering.”
For example, Dr. Grego envisions pharmaceutical companies using the technology to detect or monitor any changes in stool or gut health based on a treatment in development during clinical trials.
Furthermore, the technology might empower health-conscious consumers who want to track their own gut health. “This technology will be a whole new entry in the digital health toolkit,” Dr. Grego said.
Although not included in the research presented at this year’s DDW, the developers plan to add a sampling capability. Biochemic analysis of stool samples could provide “metabolically relevant information,” including stool biomarkers and microbiome composition.
“We have demonstrated it in the laboratory. It will be part of the technology when developed into a product,” Dr. Grego said.
This proof-of-concept study “is the first step in a path we are aggressively pursuing,” Dr. Grego said. She estimated it will take about 12-18 months to develop a prototype for use with patients. “We hope to move to a product soon after that.”
“I’m looking forward to seeing future iterations of this tool,” Dr. Shin said. “It could have a role in monitoring important GI diseases and disorders, including IBS and inflammatory bowel disease, or even for the detection of ‘alarm symptoms’ that shouldn’t be ignored.
“I could even see it having a role in preventative health in the future,” Dr. Shin added.
The technology has been licensed to the spin-off company Coprata to develop the product further.
“We hope to have an impact on people’s health very soon,” Dr. Grego said.
Duke University funded the study. Dr. Grego holds a management position at Coprata. Dr. Shin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A “smart toilet” in development uses artificial intelligence (AI) to scan stool for consistency and presence of blood – and early evidence suggests it is more accurate than patient self-reporting, a study reveals.
The remote, automated, real-time analysis and reporting increase the likelihood of physicians detecting gastrointestinal issues earlier, investigators reported.
In a proof-of-concept study, the smart toilet was 85% accurate in categorizing stool consistency as loose, normal, or constipated. The findings were presented at the annual Digestive Disease Week® (DDW).
“This study highlights a very innovative and practical tool that could have major implications for patients and clinicians alike,” Andrea Shin, MD, who was not affiliated with the research, said in an interview.
“Stool form or consistency and signs of bleeding are some of the most important pieces of clinical history when it comes to GI or bowel symptoms,” added Dr. Shin, assistant professor of medicine in the department of gastroenterology and hepatology at Indiana University, Indianapolis.
Image analysis
The researchers tested their AI algorithm on 3,328 images. They assessed photos from the Internet and some submitted anonymously by participants in the study.
Two gastroenterologists also rated a subset of 552 images. The physicians showed “satisfactory agreement” on interrater reliability (the extent to which two or more “raters” [for example, observers, examiners] agree), the investigators noted.
The smart toilet also was 76% accurate for gross blood detection.
“It’s objective and more accurate,” study author Sonia Grego, PhD, said in an interview. In contrast to asking patients to keep a bowel movement diary or recall the frequency and consistency of their stool over time, “the system does it for you,” she added.
“Our technology – by automating the image acquisition – removes the burden of having to track your pattern for weeks or months,” added Dr. Grego, founding director of the Duke Smart Toilet Lab at Duke University in Durham, N.C.
Information provided by patients “can have a big impact on decision-making,” Dr. Shin said. “For example, if I am talking to an individual who suffers from irritable bowel syndrome [IBS], I commonly ask them about how loose or watery and hard or formed their stool is, because this information gives me clues as to the underlying problems that may be driving their symptoms.”
Dr. Shin agreed it can be challenging for people to know what is important to report to their doctor. “This tool has the potential to relieve patient burden and facilitate communication between a patient and their clinician. It’s a great example of how technology can be leveraged to enhance care.”
Working behind the scenes
Together with gastroenterologist Deborah Anne Fisher, MD, an associate professor of medicine at Duke, Dr. Grego and colleagues devised a prototype that positions the image analyzer in the pipes behind the toilet. So the analysis is done post flush.
“We are experts of toilets and toilet technology,” Dr. Grego said. “We have learned that people really don’t like to see anything weird around the toilet bowl.”
The smart toilet system is designed for multiple users in a residential or commercial setting. The technology could be used in hospitals or long-term care facilities, for example. A fingerprint scanner on the flush mechanism tracks each individual user.
Mixed reactions
Dr. Grego gets a range of reactions when she tells people she is developing smart toilet technology.
“Friends and family laugh about the concept of the smart toilet,” she said, “so all the possible jokes that have been done on poops, we know.”
In fact, the researchers also are collecting the jokes they hear. “We’re being very systematic.”
In contrast, gastroenterologists who learn of the technology in development are more enthusiastic, Dr. Grego said. “There is such a need for removing the uncertainty of the patient recall about bowel movement frequency and appearance.
“We are seeking to expand through collaboration with additional GI doctors. We want to develop a more advanced prototype and do further validation studies,” Dr. Grego said.
Digital health tool
There is an aversion among patients to handling stool “or even talking about it,” Dr. Grego said. Colleagues tell her that people are more willing to provide a blood sample, which requires a needle, than a stool sample.
“But a lot of health data is there [in the stool],” she added. “We think this will empower a lot of research as well as consumer data gathering.”
For example, Dr. Grego envisions pharmaceutical companies using the technology to detect or monitor any changes in stool or gut health based on a treatment in development during clinical trials.
Furthermore, the technology might empower health-conscious consumers who want to track their own gut health. “This technology will be a whole new entry in the digital health toolkit,” Dr. Grego said.
Although not included in the research presented at this year’s DDW, the developers plan to add a sampling capability. Biochemic analysis of stool samples could provide “metabolically relevant information,” including stool biomarkers and microbiome composition.
“We have demonstrated it in the laboratory. It will be part of the technology when developed into a product,” Dr. Grego said.
This proof-of-concept study “is the first step in a path we are aggressively pursuing,” Dr. Grego said. She estimated it will take about 12-18 months to develop a prototype for use with patients. “We hope to move to a product soon after that.”
“I’m looking forward to seeing future iterations of this tool,” Dr. Shin said. “It could have a role in monitoring important GI diseases and disorders, including IBS and inflammatory bowel disease, or even for the detection of ‘alarm symptoms’ that shouldn’t be ignored.
“I could even see it having a role in preventative health in the future,” Dr. Shin added.
The technology has been licensed to the spin-off company Coprata to develop the product further.
“We hope to have an impact on people’s health very soon,” Dr. Grego said.
Duke University funded the study. Dr. Grego holds a management position at Coprata. Dr. Shin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Avoiding excess oxygen in mechanically ventilated patients ‘seems sensible’
The respiratory therapists at Mount Sinai Beth Israel, New York, know when Lina Miyakawa, MD, starts a week in the ICU, because she turns down the fraction of inspired oxygen (FiO2) levels if patients tolerate it.
“Hyperoxia in mechanical ventilation is a topic that’s near and dear to my heart,” Dr. Miyakawa, a pulmonary and critical care medicine specialist at Mount Sinai Beth Israel, said during SHM Converge, the annual conference of the Society of Hospital Medicine. “You can always find ‘wean down FiO2’ in my consult notes.”
While it is believed that humans have built up evolutionary defenses against hypoxia but not against hyperoxia, medical literature on the topic of hyperoxia with supplemental oxygen is fairly young. “In medical school we were taught to give oxygen for anybody with chest pain and concern about acute coronary syndrome,” she said. “This was until recent data suggested harm from liberal oxygen use.”
In a single-center trial of 434 critical care patients with an ICU length of stay of 72 hours or longer, Italian researchers examined the effects of a conservative protocol for oxygen therapy versus conventional therapy on ICU mortality (JAMA. 2016;316[15]:1583-9). The trial was stopped because the patients who were assigned to receive conservative therapy had a significantly lower mortality than the ones who received usual care (P = .01). “The study was not perfect, and the premature stoppage likely exaggerated the effect size,” said Dr. Miyakawa, who was not affiliated with the trial. “However, subsequent retrospective studies continue to support a benefit with conservative oxygen use, especially in different groups of patients. One of note is hyperoxia following cardiac arrest. There’s something called a two-hit model that speaks to worsening ischemia with reperfusion injury after the initial hypoxic event from the cardiac arrest itself” (See Intensive Care Med. 2015;41:534-6).
In a multicenter cohort study that drew from the Project IMPACT critical care database of ICUs at 120 U.S. hospitals between 2001 and 2005, researchers led by J. Hope Kilgannon, MD, tested the hypothesis that post-resuscitation hyperoxia is associated with increased in-hospital mortality (JAMA. 2010;303[21]:2165-71). The study population consisted of 6,326 patients who were divided into three groups: the hypoxic group (a PaO2 of less than 60 mm Hg); the normoxic group (a PaO2 of 60-299 mm Hg), and the hyperoxic group (a PaO2 of over 300 mm Hg). The mortality for the hyperoxic group was 63%, the hypoxic group at 57%, and the normoxic group at 45%.
More recently, the ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group evaluated conservative versus liberal approaches in providing oxygen to 965 patients who were mechanically ventilated between 2015 and 2018 at 21 ICUs (N Eng J Med. 2020;382:989-98). Of the 965 patients, 484 were randomly assigned to the conservative oxygen group (defined as an SpO2 of 97% or lower) and 481 were assigned to the usual oxygen group (defined as having no specific measures limiting FiO2 or the SpO2). The primary outcome was the number of ventilator-free days from randomization until day 28, while the secondary outcome was mortality at 180 days. The researchers also performed a subgroup analysis of patients at risk for hypoxic-ischemic encephalopathy.
No significant differences were observed in the number of ventilator days between the two group (a median of 21 days in the conservative oxygen group versus 22 days in the usual oxygen group, respectively; P = .80) nor in mortality at 180 days (35.7% vs. 34.5%). However, in the subgroup analysis, patients with hypoxic-ischemic encephalopathy were noted to have more ventilator-free days (21 vs. 0 days), improved 180-day mortality (43% vs. 59%), and less functional impairment (55% vs. 68%) in the conservative-oxygen group.
“The results of this study suggest that conservative oxygen therapy has no additional advantage over standard oxygen therapy, but there may be benefits in those vulnerable to hyperoxia, which warrants further investigation,” Dr. Miyakawa said. “There are a few points to note on this topic. First, many of the previous studies had more liberal oxygen strategies than the ones used in this study, which could be the reason why we are seeing these results. In addition, O2 titration relies on imperfect approximations. PaO2 cannot be measured continuously; we really depend on the SpO2 on a minute-by-minute basis. Critically ill patients can also undergo episodes of hypoperfusion and shock state minute-by-minute. That’s when they’re at risk for hypoxemia. This would not be captured continuously with just O2 saturations.”
Dr. Miyakawa also highlighted the Liberal Oxygenation versus Conservative Oxygenation in Acute Respiratory Distress Syndrome trial (LOCO2) a prospective, multicenter, randomized, open-label trial involving patients with ARDS. It was carried out at 13 ICUs in France between June 2016 and September 2018 in an effort determine whether conservative oxygenation would reduce mortality at 28 days compared with the usual liberal-oxygen strategy (N Eng J Med. 2020;382:999-1008). The researchers detected a signal of increased mortality in the conservative oxygen group (34% vs. 27%), which led to a premature stoppage of the trial. “I’d like to postulate that the higher incidence of proning in the liberal oxygenation group compared to the conservative oxygen group (51% to 34%) may be the reason for the difference in mortality,” said Dr. Miyakawa, who was not affiliated with LOCO2. “This is supported from the 2013 PROSEVA Study Group, which reported that prone positioning in ARDS significantly decreases 28- and 90-day mortality” (see N Engl J Med. 2013; 368:2159-68).
She said that future trials on this topic “will have to address how a particular [oxygenation] target is both set and achieved in each group of patients, particularly those with specific organ injuries. In the meantime, in my opinion, avoiding excess oxygen seems sensible.”
Dr. Miyakawa reported having no financial disclosures.
The respiratory therapists at Mount Sinai Beth Israel, New York, know when Lina Miyakawa, MD, starts a week in the ICU, because she turns down the fraction of inspired oxygen (FiO2) levels if patients tolerate it.
“Hyperoxia in mechanical ventilation is a topic that’s near and dear to my heart,” Dr. Miyakawa, a pulmonary and critical care medicine specialist at Mount Sinai Beth Israel, said during SHM Converge, the annual conference of the Society of Hospital Medicine. “You can always find ‘wean down FiO2’ in my consult notes.”
While it is believed that humans have built up evolutionary defenses against hypoxia but not against hyperoxia, medical literature on the topic of hyperoxia with supplemental oxygen is fairly young. “In medical school we were taught to give oxygen for anybody with chest pain and concern about acute coronary syndrome,” she said. “This was until recent data suggested harm from liberal oxygen use.”
In a single-center trial of 434 critical care patients with an ICU length of stay of 72 hours or longer, Italian researchers examined the effects of a conservative protocol for oxygen therapy versus conventional therapy on ICU mortality (JAMA. 2016;316[15]:1583-9). The trial was stopped because the patients who were assigned to receive conservative therapy had a significantly lower mortality than the ones who received usual care (P = .01). “The study was not perfect, and the premature stoppage likely exaggerated the effect size,” said Dr. Miyakawa, who was not affiliated with the trial. “However, subsequent retrospective studies continue to support a benefit with conservative oxygen use, especially in different groups of patients. One of note is hyperoxia following cardiac arrest. There’s something called a two-hit model that speaks to worsening ischemia with reperfusion injury after the initial hypoxic event from the cardiac arrest itself” (See Intensive Care Med. 2015;41:534-6).
In a multicenter cohort study that drew from the Project IMPACT critical care database of ICUs at 120 U.S. hospitals between 2001 and 2005, researchers led by J. Hope Kilgannon, MD, tested the hypothesis that post-resuscitation hyperoxia is associated with increased in-hospital mortality (JAMA. 2010;303[21]:2165-71). The study population consisted of 6,326 patients who were divided into three groups: the hypoxic group (a PaO2 of less than 60 mm Hg); the normoxic group (a PaO2 of 60-299 mm Hg), and the hyperoxic group (a PaO2 of over 300 mm Hg). The mortality for the hyperoxic group was 63%, the hypoxic group at 57%, and the normoxic group at 45%.
More recently, the ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group evaluated conservative versus liberal approaches in providing oxygen to 965 patients who were mechanically ventilated between 2015 and 2018 at 21 ICUs (N Eng J Med. 2020;382:989-98). Of the 965 patients, 484 were randomly assigned to the conservative oxygen group (defined as an SpO2 of 97% or lower) and 481 were assigned to the usual oxygen group (defined as having no specific measures limiting FiO2 or the SpO2). The primary outcome was the number of ventilator-free days from randomization until day 28, while the secondary outcome was mortality at 180 days. The researchers also performed a subgroup analysis of patients at risk for hypoxic-ischemic encephalopathy.
No significant differences were observed in the number of ventilator days between the two group (a median of 21 days in the conservative oxygen group versus 22 days in the usual oxygen group, respectively; P = .80) nor in mortality at 180 days (35.7% vs. 34.5%). However, in the subgroup analysis, patients with hypoxic-ischemic encephalopathy were noted to have more ventilator-free days (21 vs. 0 days), improved 180-day mortality (43% vs. 59%), and less functional impairment (55% vs. 68%) in the conservative-oxygen group.
“The results of this study suggest that conservative oxygen therapy has no additional advantage over standard oxygen therapy, but there may be benefits in those vulnerable to hyperoxia, which warrants further investigation,” Dr. Miyakawa said. “There are a few points to note on this topic. First, many of the previous studies had more liberal oxygen strategies than the ones used in this study, which could be the reason why we are seeing these results. In addition, O2 titration relies on imperfect approximations. PaO2 cannot be measured continuously; we really depend on the SpO2 on a minute-by-minute basis. Critically ill patients can also undergo episodes of hypoperfusion and shock state minute-by-minute. That’s when they’re at risk for hypoxemia. This would not be captured continuously with just O2 saturations.”
Dr. Miyakawa also highlighted the Liberal Oxygenation versus Conservative Oxygenation in Acute Respiratory Distress Syndrome trial (LOCO2) a prospective, multicenter, randomized, open-label trial involving patients with ARDS. It was carried out at 13 ICUs in France between June 2016 and September 2018 in an effort determine whether conservative oxygenation would reduce mortality at 28 days compared with the usual liberal-oxygen strategy (N Eng J Med. 2020;382:999-1008). The researchers detected a signal of increased mortality in the conservative oxygen group (34% vs. 27%), which led to a premature stoppage of the trial. “I’d like to postulate that the higher incidence of proning in the liberal oxygenation group compared to the conservative oxygen group (51% to 34%) may be the reason for the difference in mortality,” said Dr. Miyakawa, who was not affiliated with LOCO2. “This is supported from the 2013 PROSEVA Study Group, which reported that prone positioning in ARDS significantly decreases 28- and 90-day mortality” (see N Engl J Med. 2013; 368:2159-68).
She said that future trials on this topic “will have to address how a particular [oxygenation] target is both set and achieved in each group of patients, particularly those with specific organ injuries. In the meantime, in my opinion, avoiding excess oxygen seems sensible.”
Dr. Miyakawa reported having no financial disclosures.
The respiratory therapists at Mount Sinai Beth Israel, New York, know when Lina Miyakawa, MD, starts a week in the ICU, because she turns down the fraction of inspired oxygen (FiO2) levels if patients tolerate it.
“Hyperoxia in mechanical ventilation is a topic that’s near and dear to my heart,” Dr. Miyakawa, a pulmonary and critical care medicine specialist at Mount Sinai Beth Israel, said during SHM Converge, the annual conference of the Society of Hospital Medicine. “You can always find ‘wean down FiO2’ in my consult notes.”
While it is believed that humans have built up evolutionary defenses against hypoxia but not against hyperoxia, medical literature on the topic of hyperoxia with supplemental oxygen is fairly young. “In medical school we were taught to give oxygen for anybody with chest pain and concern about acute coronary syndrome,” she said. “This was until recent data suggested harm from liberal oxygen use.”
In a single-center trial of 434 critical care patients with an ICU length of stay of 72 hours or longer, Italian researchers examined the effects of a conservative protocol for oxygen therapy versus conventional therapy on ICU mortality (JAMA. 2016;316[15]:1583-9). The trial was stopped because the patients who were assigned to receive conservative therapy had a significantly lower mortality than the ones who received usual care (P = .01). “The study was not perfect, and the premature stoppage likely exaggerated the effect size,” said Dr. Miyakawa, who was not affiliated with the trial. “However, subsequent retrospective studies continue to support a benefit with conservative oxygen use, especially in different groups of patients. One of note is hyperoxia following cardiac arrest. There’s something called a two-hit model that speaks to worsening ischemia with reperfusion injury after the initial hypoxic event from the cardiac arrest itself” (See Intensive Care Med. 2015;41:534-6).
In a multicenter cohort study that drew from the Project IMPACT critical care database of ICUs at 120 U.S. hospitals between 2001 and 2005, researchers led by J. Hope Kilgannon, MD, tested the hypothesis that post-resuscitation hyperoxia is associated with increased in-hospital mortality (JAMA. 2010;303[21]:2165-71). The study population consisted of 6,326 patients who were divided into three groups: the hypoxic group (a PaO2 of less than 60 mm Hg); the normoxic group (a PaO2 of 60-299 mm Hg), and the hyperoxic group (a PaO2 of over 300 mm Hg). The mortality for the hyperoxic group was 63%, the hypoxic group at 57%, and the normoxic group at 45%.
More recently, the ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group evaluated conservative versus liberal approaches in providing oxygen to 965 patients who were mechanically ventilated between 2015 and 2018 at 21 ICUs (N Eng J Med. 2020;382:989-98). Of the 965 patients, 484 were randomly assigned to the conservative oxygen group (defined as an SpO2 of 97% or lower) and 481 were assigned to the usual oxygen group (defined as having no specific measures limiting FiO2 or the SpO2). The primary outcome was the number of ventilator-free days from randomization until day 28, while the secondary outcome was mortality at 180 days. The researchers also performed a subgroup analysis of patients at risk for hypoxic-ischemic encephalopathy.
No significant differences were observed in the number of ventilator days between the two group (a median of 21 days in the conservative oxygen group versus 22 days in the usual oxygen group, respectively; P = .80) nor in mortality at 180 days (35.7% vs. 34.5%). However, in the subgroup analysis, patients with hypoxic-ischemic encephalopathy were noted to have more ventilator-free days (21 vs. 0 days), improved 180-day mortality (43% vs. 59%), and less functional impairment (55% vs. 68%) in the conservative-oxygen group.
“The results of this study suggest that conservative oxygen therapy has no additional advantage over standard oxygen therapy, but there may be benefits in those vulnerable to hyperoxia, which warrants further investigation,” Dr. Miyakawa said. “There are a few points to note on this topic. First, many of the previous studies had more liberal oxygen strategies than the ones used in this study, which could be the reason why we are seeing these results. In addition, O2 titration relies on imperfect approximations. PaO2 cannot be measured continuously; we really depend on the SpO2 on a minute-by-minute basis. Critically ill patients can also undergo episodes of hypoperfusion and shock state minute-by-minute. That’s when they’re at risk for hypoxemia. This would not be captured continuously with just O2 saturations.”
Dr. Miyakawa also highlighted the Liberal Oxygenation versus Conservative Oxygenation in Acute Respiratory Distress Syndrome trial (LOCO2) a prospective, multicenter, randomized, open-label trial involving patients with ARDS. It was carried out at 13 ICUs in France between June 2016 and September 2018 in an effort determine whether conservative oxygenation would reduce mortality at 28 days compared with the usual liberal-oxygen strategy (N Eng J Med. 2020;382:999-1008). The researchers detected a signal of increased mortality in the conservative oxygen group (34% vs. 27%), which led to a premature stoppage of the trial. “I’d like to postulate that the higher incidence of proning in the liberal oxygenation group compared to the conservative oxygen group (51% to 34%) may be the reason for the difference in mortality,” said Dr. Miyakawa, who was not affiliated with LOCO2. “This is supported from the 2013 PROSEVA Study Group, which reported that prone positioning in ARDS significantly decreases 28- and 90-day mortality” (see N Engl J Med. 2013; 368:2159-68).
She said that future trials on this topic “will have to address how a particular [oxygenation] target is both set and achieved in each group of patients, particularly those with specific organ injuries. In the meantime, in my opinion, avoiding excess oxygen seems sensible.”
Dr. Miyakawa reported having no financial disclosures.
FROM SHM CONVERGE 2021
PASCAL mitral valve repair shines at 2 years in CLASP
Transcatheter mitral valve repair with the PASCAL device showed high rates of survival and freedom from heart failure rehospitalization at 2 years in the single-arm, safety and efficacy CLASP study.
The early reductions in mitral regurgitation (MR) were sustained with 97% of patients having MR grades of 2+ or less and 78% having MR grades of 1+ or less at 2 years.
There was also evidence of left ventricular (LV) reverse remodeling and significant improvements in functional status, Molly Szerlip, MD, Baylor Scott & White Health, Plano, Texas, reported as lead author. The results were published online May 18 in JACC: Cardiovascular Interventions.
“The PASCAL transcatheter valve repair system is a favorable option for treating patients with MR,” she said in a simultaneous virtual presentation at the 2021 Congress of European Association of Percutaneous Cardiovascular Interventions (EuroPCR 2021).
The PASCAL system is not approved in the United States, but Dr. Szerlip observed that the investigators are eagerly awaiting results from the ongoing, pivotal CLASP IID/IIF trial comparing the edge-to-edge repair system with another such device, MitraClip, in 1,275 patients with functional or degenerative MR. The primary completion date is set for December 2023.
Abbott’s MitraClip has been available in the United States since 2013 and in Europe since 2008; Edwards Lifesciences received a CE mark for the PASCAL system in 2019.
“The results of the CLASP study are remarkable and indicate an additional differentiated tool ready for clinical routine,” Georg Goliasch, MD, PhD, and Philipp Bartko, MD, both from the Medical University of Vienna, write in an accompanying editorial.
As both systems target similar lesions, there might be “significant overlap in this particular patient population,” Dr. Goliasch told this news organization. From a technical perspective, the separate leaflet grasping was initially one of the advantages of the PASCAL, but this has also been recently introduced for the MitraClip.
That said, the “PASCAL device may offer a leaflet repair with decreased mechanical leaflet traction – specifically appealing to treat ventricular secondary MR – because mechanical forces applied to leaflets remain low, and the [central] spacer augments the leaflet surface in a way that reduces restrictive diastolic opening,” he added. “However, this remains highly speculative.”
The CLASP study enrolled 124 patients (56% male) with symptomatic MR grade of at least 3+ who were receiving optimal medical therapy at 14 sites in five countries. Their mean age was 75 years, 69% had functional MR (FMR), 31% had degenerative MR (DMR), and 60% were NYHA functional class III to IVa.
The primary endpoints of procedural and clinical success and adverse events at 30 days and 1-year outcomes were published last year. Echocardiographic data were available for 36 patients at 2 years with follow-up ongoing.
Composite major adverse event rates were 8.1% at 30 days, 18.5% at 1 year, and 16.9% at 2 years, driven mostly by severe bleeding at 7.3%, 11.3%, and 7.3%, respectively, Dr. Szerlip said.
Kaplan-Meier estimates showed 80.3% survival at 2 years (72.3% FMR, 94.3% DMR) and 84.3% freedom from heart failure rehospitalization (77.5% FMR, 97.3% DMR). The annualized HF rehospitalization rate fell to 85% at 2 years.
These results, the authors noted, hinged on minimizing residual MR. In the FMR group, 100% and 95% of patients achieved MR of 2+ or less at 1 year and 2 years, respectively, compared with 95% and 99% treated with the MitraClip in the COAPT study.
In the DMR group, 100% of patients achieved MR of 2+ or less at both 1 and 2 years, which “compares favorably to 94% from the EXPAND study at 1 year” with the MitraClip NTR and XTR systems, they write.
In CLASP, the LV end-diastolic volume decreased by 11 mL at 30 days and continued to decrease at 1 year (25 mL) and 2 years (33 mL; P < .001).
LV end-diastolic diameter (LVEDD) fell by 2.7 mm at 30 days, 3.9 mm at 1 year, and by 2.7 mm at 2 years (P = .002). At 2 years, 93% of patients were in NYHA class I or II (P < .001).
“The authors of the trial observed significant LV reverse remodeling with a decrease in LVEDD. These findings are indeed of particular interest and warrant further investigation by future studies as this has not been shown to such an extent in previous E2E [edge-to-edge] repair studies,” Dr. Goliasch said in an interview.
He raised an eyebrow, however, at the cross-trial comparisons, adding, “We should be very careful to draw any hasty conclusions considering the high proportion of missing echocardiographic data. Nevertheless, all these aspects might make the design of future studies for direct comparisons between E2E devices in the various structural aspects of mitral valve disease attractive to tailor treatment and optimize patient care.”
Dr. Szerlip and colleagues cited several study limitations including the absence of a control arm that may have contributed to a Hawthorne effect; not all patients had reached 2-year follow-up at the time of the analysis; and adjudication of events and assessment of the 6-minute walk test and quality-of-life measures were limited to 1 year based on the protocol.
The study was sponsored by Edwards Lifesciences. Dr. Szerlip reported serving as a proctor/speaker for Edwards; a national principal investigator for EFS; a speaker for Boston Scientific, and serving on steering committees for Medtronic and Abbott. Dr. Goliasch and Dr. Bartko have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Transcatheter mitral valve repair with the PASCAL device showed high rates of survival and freedom from heart failure rehospitalization at 2 years in the single-arm, safety and efficacy CLASP study.
The early reductions in mitral regurgitation (MR) were sustained with 97% of patients having MR grades of 2+ or less and 78% having MR grades of 1+ or less at 2 years.
There was also evidence of left ventricular (LV) reverse remodeling and significant improvements in functional status, Molly Szerlip, MD, Baylor Scott & White Health, Plano, Texas, reported as lead author. The results were published online May 18 in JACC: Cardiovascular Interventions.
“The PASCAL transcatheter valve repair system is a favorable option for treating patients with MR,” she said in a simultaneous virtual presentation at the 2021 Congress of European Association of Percutaneous Cardiovascular Interventions (EuroPCR 2021).
The PASCAL system is not approved in the United States, but Dr. Szerlip observed that the investigators are eagerly awaiting results from the ongoing, pivotal CLASP IID/IIF trial comparing the edge-to-edge repair system with another such device, MitraClip, in 1,275 patients with functional or degenerative MR. The primary completion date is set for December 2023.
Abbott’s MitraClip has been available in the United States since 2013 and in Europe since 2008; Edwards Lifesciences received a CE mark for the PASCAL system in 2019.
“The results of the CLASP study are remarkable and indicate an additional differentiated tool ready for clinical routine,” Georg Goliasch, MD, PhD, and Philipp Bartko, MD, both from the Medical University of Vienna, write in an accompanying editorial.
As both systems target similar lesions, there might be “significant overlap in this particular patient population,” Dr. Goliasch told this news organization. From a technical perspective, the separate leaflet grasping was initially one of the advantages of the PASCAL, but this has also been recently introduced for the MitraClip.
That said, the “PASCAL device may offer a leaflet repair with decreased mechanical leaflet traction – specifically appealing to treat ventricular secondary MR – because mechanical forces applied to leaflets remain low, and the [central] spacer augments the leaflet surface in a way that reduces restrictive diastolic opening,” he added. “However, this remains highly speculative.”
The CLASP study enrolled 124 patients (56% male) with symptomatic MR grade of at least 3+ who were receiving optimal medical therapy at 14 sites in five countries. Their mean age was 75 years, 69% had functional MR (FMR), 31% had degenerative MR (DMR), and 60% were NYHA functional class III to IVa.
The primary endpoints of procedural and clinical success and adverse events at 30 days and 1-year outcomes were published last year. Echocardiographic data were available for 36 patients at 2 years with follow-up ongoing.
Composite major adverse event rates were 8.1% at 30 days, 18.5% at 1 year, and 16.9% at 2 years, driven mostly by severe bleeding at 7.3%, 11.3%, and 7.3%, respectively, Dr. Szerlip said.
Kaplan-Meier estimates showed 80.3% survival at 2 years (72.3% FMR, 94.3% DMR) and 84.3% freedom from heart failure rehospitalization (77.5% FMR, 97.3% DMR). The annualized HF rehospitalization rate fell to 85% at 2 years.
These results, the authors noted, hinged on minimizing residual MR. In the FMR group, 100% and 95% of patients achieved MR of 2+ or less at 1 year and 2 years, respectively, compared with 95% and 99% treated with the MitraClip in the COAPT study.
In the DMR group, 100% of patients achieved MR of 2+ or less at both 1 and 2 years, which “compares favorably to 94% from the EXPAND study at 1 year” with the MitraClip NTR and XTR systems, they write.
In CLASP, the LV end-diastolic volume decreased by 11 mL at 30 days and continued to decrease at 1 year (25 mL) and 2 years (33 mL; P < .001).
LV end-diastolic diameter (LVEDD) fell by 2.7 mm at 30 days, 3.9 mm at 1 year, and by 2.7 mm at 2 years (P = .002). At 2 years, 93% of patients were in NYHA class I or II (P < .001).
“The authors of the trial observed significant LV reverse remodeling with a decrease in LVEDD. These findings are indeed of particular interest and warrant further investigation by future studies as this has not been shown to such an extent in previous E2E [edge-to-edge] repair studies,” Dr. Goliasch said in an interview.
He raised an eyebrow, however, at the cross-trial comparisons, adding, “We should be very careful to draw any hasty conclusions considering the high proportion of missing echocardiographic data. Nevertheless, all these aspects might make the design of future studies for direct comparisons between E2E devices in the various structural aspects of mitral valve disease attractive to tailor treatment and optimize patient care.”
Dr. Szerlip and colleagues cited several study limitations including the absence of a control arm that may have contributed to a Hawthorne effect; not all patients had reached 2-year follow-up at the time of the analysis; and adjudication of events and assessment of the 6-minute walk test and quality-of-life measures were limited to 1 year based on the protocol.
The study was sponsored by Edwards Lifesciences. Dr. Szerlip reported serving as a proctor/speaker for Edwards; a national principal investigator for EFS; a speaker for Boston Scientific, and serving on steering committees for Medtronic and Abbott. Dr. Goliasch and Dr. Bartko have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Transcatheter mitral valve repair with the PASCAL device showed high rates of survival and freedom from heart failure rehospitalization at 2 years in the single-arm, safety and efficacy CLASP study.
The early reductions in mitral regurgitation (MR) were sustained with 97% of patients having MR grades of 2+ or less and 78% having MR grades of 1+ or less at 2 years.
There was also evidence of left ventricular (LV) reverse remodeling and significant improvements in functional status, Molly Szerlip, MD, Baylor Scott & White Health, Plano, Texas, reported as lead author. The results were published online May 18 in JACC: Cardiovascular Interventions.
“The PASCAL transcatheter valve repair system is a favorable option for treating patients with MR,” she said in a simultaneous virtual presentation at the 2021 Congress of European Association of Percutaneous Cardiovascular Interventions (EuroPCR 2021).
The PASCAL system is not approved in the United States, but Dr. Szerlip observed that the investigators are eagerly awaiting results from the ongoing, pivotal CLASP IID/IIF trial comparing the edge-to-edge repair system with another such device, MitraClip, in 1,275 patients with functional or degenerative MR. The primary completion date is set for December 2023.
Abbott’s MitraClip has been available in the United States since 2013 and in Europe since 2008; Edwards Lifesciences received a CE mark for the PASCAL system in 2019.
“The results of the CLASP study are remarkable and indicate an additional differentiated tool ready for clinical routine,” Georg Goliasch, MD, PhD, and Philipp Bartko, MD, both from the Medical University of Vienna, write in an accompanying editorial.
As both systems target similar lesions, there might be “significant overlap in this particular patient population,” Dr. Goliasch told this news organization. From a technical perspective, the separate leaflet grasping was initially one of the advantages of the PASCAL, but this has also been recently introduced for the MitraClip.
That said, the “PASCAL device may offer a leaflet repair with decreased mechanical leaflet traction – specifically appealing to treat ventricular secondary MR – because mechanical forces applied to leaflets remain low, and the [central] spacer augments the leaflet surface in a way that reduces restrictive diastolic opening,” he added. “However, this remains highly speculative.”
The CLASP study enrolled 124 patients (56% male) with symptomatic MR grade of at least 3+ who were receiving optimal medical therapy at 14 sites in five countries. Their mean age was 75 years, 69% had functional MR (FMR), 31% had degenerative MR (DMR), and 60% were NYHA functional class III to IVa.
The primary endpoints of procedural and clinical success and adverse events at 30 days and 1-year outcomes were published last year. Echocardiographic data were available for 36 patients at 2 years with follow-up ongoing.
Composite major adverse event rates were 8.1% at 30 days, 18.5% at 1 year, and 16.9% at 2 years, driven mostly by severe bleeding at 7.3%, 11.3%, and 7.3%, respectively, Dr. Szerlip said.
Kaplan-Meier estimates showed 80.3% survival at 2 years (72.3% FMR, 94.3% DMR) and 84.3% freedom from heart failure rehospitalization (77.5% FMR, 97.3% DMR). The annualized HF rehospitalization rate fell to 85% at 2 years.
These results, the authors noted, hinged on minimizing residual MR. In the FMR group, 100% and 95% of patients achieved MR of 2+ or less at 1 year and 2 years, respectively, compared with 95% and 99% treated with the MitraClip in the COAPT study.
In the DMR group, 100% of patients achieved MR of 2+ or less at both 1 and 2 years, which “compares favorably to 94% from the EXPAND study at 1 year” with the MitraClip NTR and XTR systems, they write.
In CLASP, the LV end-diastolic volume decreased by 11 mL at 30 days and continued to decrease at 1 year (25 mL) and 2 years (33 mL; P < .001).
LV end-diastolic diameter (LVEDD) fell by 2.7 mm at 30 days, 3.9 mm at 1 year, and by 2.7 mm at 2 years (P = .002). At 2 years, 93% of patients were in NYHA class I or II (P < .001).
“The authors of the trial observed significant LV reverse remodeling with a decrease in LVEDD. These findings are indeed of particular interest and warrant further investigation by future studies as this has not been shown to such an extent in previous E2E [edge-to-edge] repair studies,” Dr. Goliasch said in an interview.
He raised an eyebrow, however, at the cross-trial comparisons, adding, “We should be very careful to draw any hasty conclusions considering the high proportion of missing echocardiographic data. Nevertheless, all these aspects might make the design of future studies for direct comparisons between E2E devices in the various structural aspects of mitral valve disease attractive to tailor treatment and optimize patient care.”
Dr. Szerlip and colleagues cited several study limitations including the absence of a control arm that may have contributed to a Hawthorne effect; not all patients had reached 2-year follow-up at the time of the analysis; and adjudication of events and assessment of the 6-minute walk test and quality-of-life measures were limited to 1 year based on the protocol.
The study was sponsored by Edwards Lifesciences. Dr. Szerlip reported serving as a proctor/speaker for Edwards; a national principal investigator for EFS; a speaker for Boston Scientific, and serving on steering committees for Medtronic and Abbott. Dr. Goliasch and Dr. Bartko have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
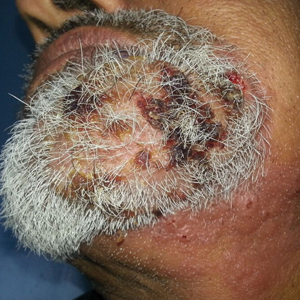
Multiple Crusted Swellings on the Chin
The Diagnosis: Cutaneous Cryptococcosis
Histologic examination revealed infiltration of the dermis and subcutaneous tissue with rounded basophilic cells on low magnification (Figure 1A). On higher magnification, encapsulated yeast cells (cryptococci) of varying size accompanied by chronic granulomatous inflammatory infiltration with occasional giant cells were seen (Figure 1B). Alcian blue stain showed mucinous capsular material (Figure 1C). There was no history of diabetes mellitus, tuberculosis, steroid therapy, or immunosuppression. Moreover, systemic involvement or systemic focus of infection was ruled out after computed tomography of the head, chest, and abdomen. Therefore, the diagnosis of primary cutaneous cryptococcosis (PCC) was established. The patient was started on oral itraconazole 100 mg twice daily along with 5 drops of a saturated solution of potassium iodide 3 times daily that later was increased to 20 drops 3 times daily at a weekly interval. The lesions started improving after 1 month and healed completely after 9 months of treatment (Figure 2).
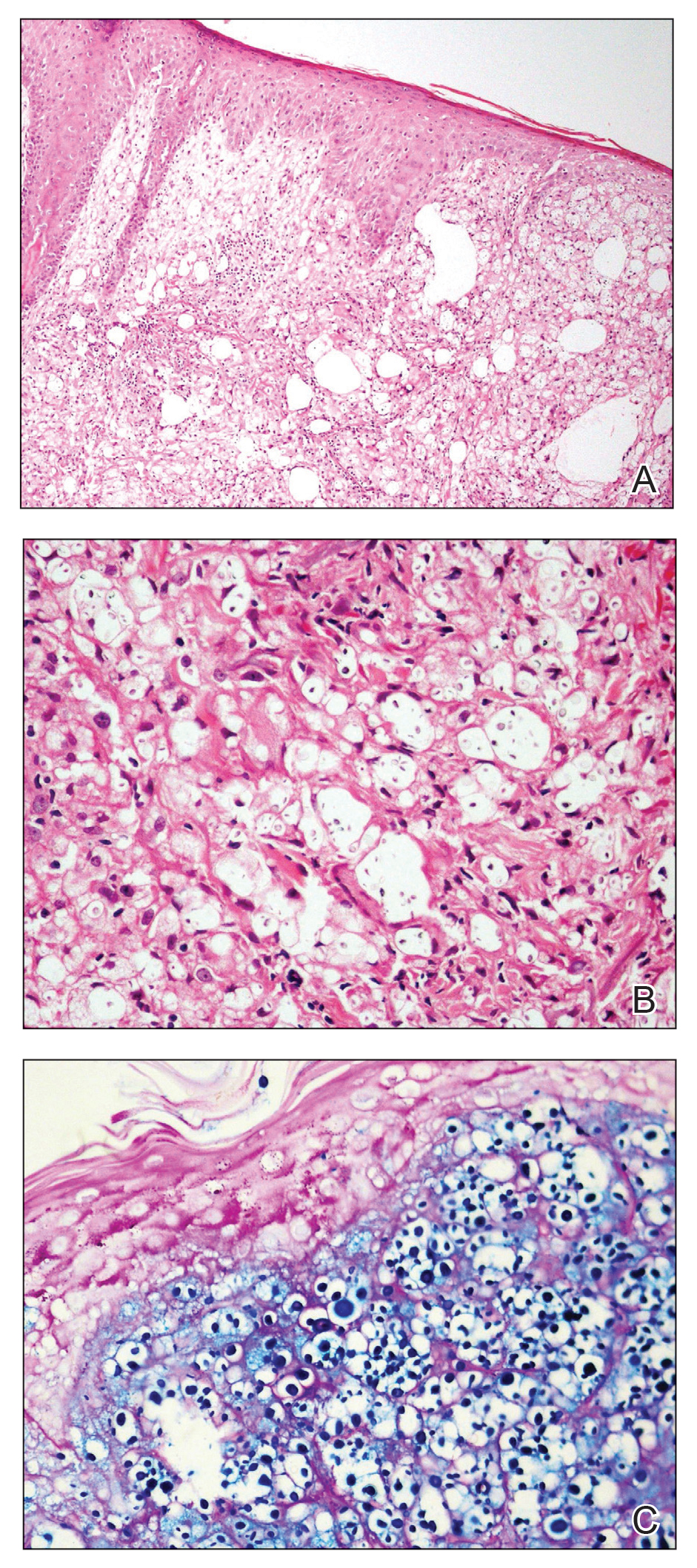
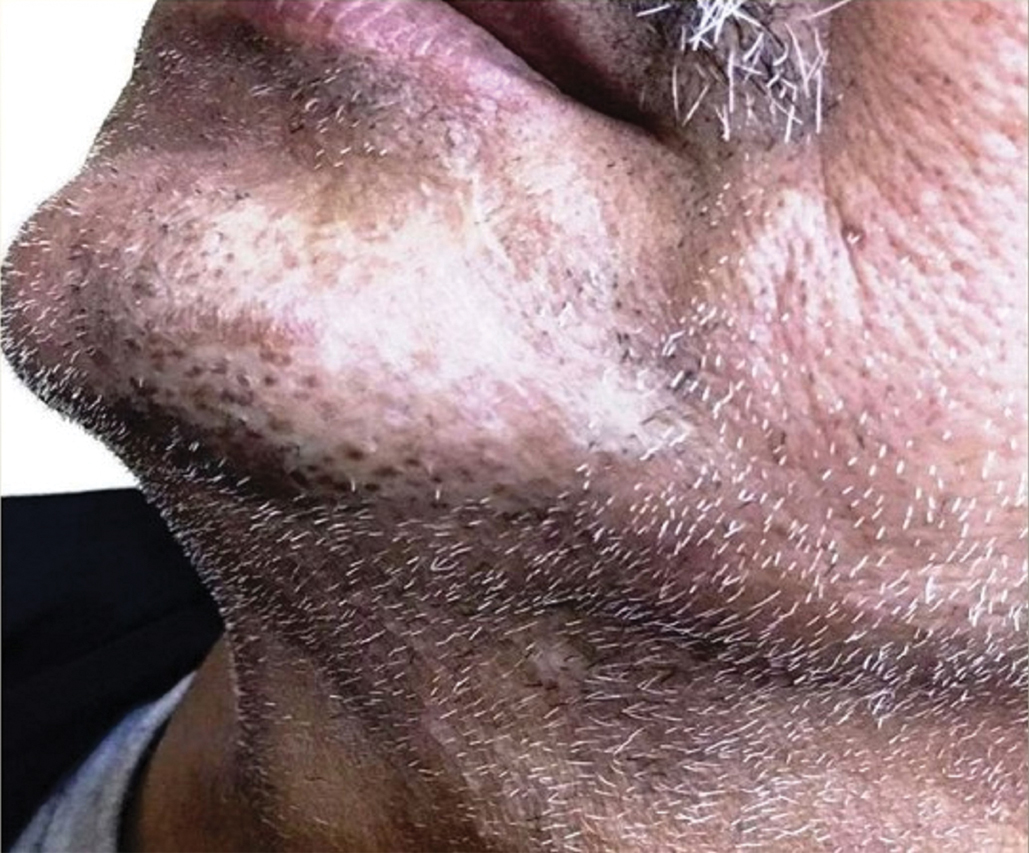
Primary cutaneous cryptococcosis is the identification of Cryptococcus neoformans in a skin lesion without evidence of simultaneous disseminated disease. Neuville et al1 observed that skin lesions resemble cellulitis, ulcerations, or whitlows and were located on unclothed areas. In contrast, lesions from disseminated disease presented as scattered umbilicated papules resembling molluscum contagiosum. Diagnosis of PCC is based on the observation of encapsulated yeasts by direct microscopic examination, isolation of C neoformans or Cryptococcus gattii in culture, and by the demonstration of capsular antigen in various fluids, including serum and cerebrospinal fluid by latex particle agglutination or enzyme-linked immunosorbent assay. Histologically, Cryptococcus species produce a proliferative inflammatory reaction in immunocompetent hosts with the formation of compact epithelioid granulomas, with giant cells and a peripheral layer of lymphocytes. Treatment options for PCC infection range from antifungal medications and surgical debridement to observation.
The differential diagnosis may include cutaneous leishmaniasis, cutaneous tuberculosis, cutaneous histoplasmosis, and basal cell carcinoma. These entities may have similar presentations and can only be confidently differentiated on direct microscopy and histopathologic examination. The characteristic Leishmania donovani bodies on microscopy in cutaneous leishmaniasis and tubercular granuloma with central necrosis on histology in cutaneous tuberculosis can differentiate these conditions from cryptococcosis. In some patients with cryptococcosis, the yeast may produce a less characteristic polysaccharide capsule and thus may be confused with histoplasmosis. Fontana-Masson staining may show melanin-producing yeast, which is characteristic of cryptococci.2 Ulcerated basal cell carcinoma may present similar clinically; however, histopathology will rule it out.
Cutaneous cryptococcal infection should be presumed to be disseminated until proven otherwise, and a search for other sites of involvement must immediately be undertaken. Cutaneous signs may be the first indication of infection, preceding the diagnosis of disseminated disease by 2 to 8 months, making its recognition crucial to early treatment. It is not possible to diagnose PCC on a specific clinical manifestation because a diverse range of skin lesions may be present. Therefore, culture and histology are the gold standards for diagnosis of cryptococcosis.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347.
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280.
The Diagnosis: Cutaneous Cryptococcosis
Histologic examination revealed infiltration of the dermis and subcutaneous tissue with rounded basophilic cells on low magnification (Figure 1A). On higher magnification, encapsulated yeast cells (cryptococci) of varying size accompanied by chronic granulomatous inflammatory infiltration with occasional giant cells were seen (Figure 1B). Alcian blue stain showed mucinous capsular material (Figure 1C). There was no history of diabetes mellitus, tuberculosis, steroid therapy, or immunosuppression. Moreover, systemic involvement or systemic focus of infection was ruled out after computed tomography of the head, chest, and abdomen. Therefore, the diagnosis of primary cutaneous cryptococcosis (PCC) was established. The patient was started on oral itraconazole 100 mg twice daily along with 5 drops of a saturated solution of potassium iodide 3 times daily that later was increased to 20 drops 3 times daily at a weekly interval. The lesions started improving after 1 month and healed completely after 9 months of treatment (Figure 2).


Primary cutaneous cryptococcosis is the identification of Cryptococcus neoformans in a skin lesion without evidence of simultaneous disseminated disease. Neuville et al1 observed that skin lesions resemble cellulitis, ulcerations, or whitlows and were located on unclothed areas. In contrast, lesions from disseminated disease presented as scattered umbilicated papules resembling molluscum contagiosum. Diagnosis of PCC is based on the observation of encapsulated yeasts by direct microscopic examination, isolation of C neoformans or Cryptococcus gattii in culture, and by the demonstration of capsular antigen in various fluids, including serum and cerebrospinal fluid by latex particle agglutination or enzyme-linked immunosorbent assay. Histologically, Cryptococcus species produce a proliferative inflammatory reaction in immunocompetent hosts with the formation of compact epithelioid granulomas, with giant cells and a peripheral layer of lymphocytes. Treatment options for PCC infection range from antifungal medications and surgical debridement to observation.
The differential diagnosis may include cutaneous leishmaniasis, cutaneous tuberculosis, cutaneous histoplasmosis, and basal cell carcinoma. These entities may have similar presentations and can only be confidently differentiated on direct microscopy and histopathologic examination. The characteristic Leishmania donovani bodies on microscopy in cutaneous leishmaniasis and tubercular granuloma with central necrosis on histology in cutaneous tuberculosis can differentiate these conditions from cryptococcosis. In some patients with cryptococcosis, the yeast may produce a less characteristic polysaccharide capsule and thus may be confused with histoplasmosis. Fontana-Masson staining may show melanin-producing yeast, which is characteristic of cryptococci.2 Ulcerated basal cell carcinoma may present similar clinically; however, histopathology will rule it out.
Cutaneous cryptococcal infection should be presumed to be disseminated until proven otherwise, and a search for other sites of involvement must immediately be undertaken. Cutaneous signs may be the first indication of infection, preceding the diagnosis of disseminated disease by 2 to 8 months, making its recognition crucial to early treatment. It is not possible to diagnose PCC on a specific clinical manifestation because a diverse range of skin lesions may be present. Therefore, culture and histology are the gold standards for diagnosis of cryptococcosis.
The Diagnosis: Cutaneous Cryptococcosis
Histologic examination revealed infiltration of the dermis and subcutaneous tissue with rounded basophilic cells on low magnification (Figure 1A). On higher magnification, encapsulated yeast cells (cryptococci) of varying size accompanied by chronic granulomatous inflammatory infiltration with occasional giant cells were seen (Figure 1B). Alcian blue stain showed mucinous capsular material (Figure 1C). There was no history of diabetes mellitus, tuberculosis, steroid therapy, or immunosuppression. Moreover, systemic involvement or systemic focus of infection was ruled out after computed tomography of the head, chest, and abdomen. Therefore, the diagnosis of primary cutaneous cryptococcosis (PCC) was established. The patient was started on oral itraconazole 100 mg twice daily along with 5 drops of a saturated solution of potassium iodide 3 times daily that later was increased to 20 drops 3 times daily at a weekly interval. The lesions started improving after 1 month and healed completely after 9 months of treatment (Figure 2).


Primary cutaneous cryptococcosis is the identification of Cryptococcus neoformans in a skin lesion without evidence of simultaneous disseminated disease. Neuville et al1 observed that skin lesions resemble cellulitis, ulcerations, or whitlows and were located on unclothed areas. In contrast, lesions from disseminated disease presented as scattered umbilicated papules resembling molluscum contagiosum. Diagnosis of PCC is based on the observation of encapsulated yeasts by direct microscopic examination, isolation of C neoformans or Cryptococcus gattii in culture, and by the demonstration of capsular antigen in various fluids, including serum and cerebrospinal fluid by latex particle agglutination or enzyme-linked immunosorbent assay. Histologically, Cryptococcus species produce a proliferative inflammatory reaction in immunocompetent hosts with the formation of compact epithelioid granulomas, with giant cells and a peripheral layer of lymphocytes. Treatment options for PCC infection range from antifungal medications and surgical debridement to observation.
The differential diagnosis may include cutaneous leishmaniasis, cutaneous tuberculosis, cutaneous histoplasmosis, and basal cell carcinoma. These entities may have similar presentations and can only be confidently differentiated on direct microscopy and histopathologic examination. The characteristic Leishmania donovani bodies on microscopy in cutaneous leishmaniasis and tubercular granuloma with central necrosis on histology in cutaneous tuberculosis can differentiate these conditions from cryptococcosis. In some patients with cryptococcosis, the yeast may produce a less characteristic polysaccharide capsule and thus may be confused with histoplasmosis. Fontana-Masson staining may show melanin-producing yeast, which is characteristic of cryptococci.2 Ulcerated basal cell carcinoma may present similar clinically; however, histopathology will rule it out.
Cutaneous cryptococcal infection should be presumed to be disseminated until proven otherwise, and a search for other sites of involvement must immediately be undertaken. Cutaneous signs may be the first indication of infection, preceding the diagnosis of disseminated disease by 2 to 8 months, making its recognition crucial to early treatment. It is not possible to diagnose PCC on a specific clinical manifestation because a diverse range of skin lesions may be present. Therefore, culture and histology are the gold standards for diagnosis of cryptococcosis.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347.
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347.
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280.
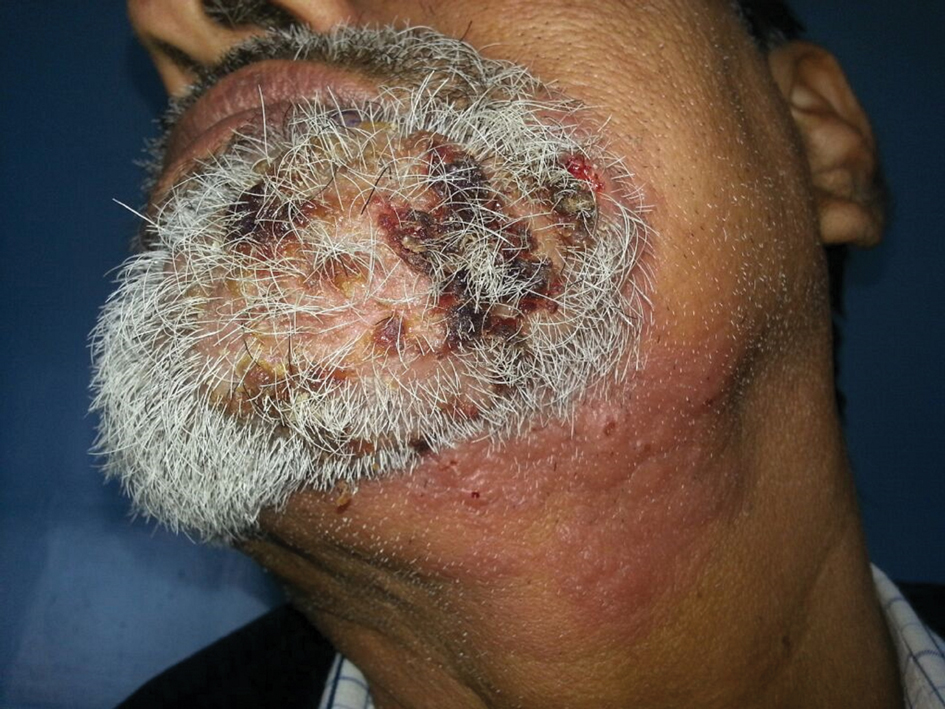
A 54-year-old man with no comorbidities presented with multiple painless swellings on the left side of the chin of 1 month’s duration that progressively were increasing, both in size and number. He denied any discharge of pus or grains from the lesion, facial trauma, insect bites, or dental procedures. The patient was treated with oral antibiotics for 15 days with no relief at an outside hospital. All routine blood and serologic investigations including viral markers and chest radiography were normal. Bacterial and fungal cultures as well as an acid-fast bacilli culture were negative. Systemic examination was normal, and vitals were within reference range. Mucocutaneous examination revealed multiple nontender small nodules and plaques with yellow-brown to dark brown hemorrhagic crusts with mild perilesional erythema on the left side of the chin extending to the adjacent neck. All mucosal sites were normal, and a biopsy was performed.
DOJ charges 14 with COVID-19–related fraud nearing $150M
The U.S. Department of Justice (DOJ) on May 26 announced charges against 14 defendants across the country who allegedly engaged in health care fraud schemes that exploited the COVID-19 pandemic and resulted in over $143 million in false billings to Medicare.
Among the defendants, a DOJ news release said, were a telemedicine company executive, a physician, marketers, and medical business owners.
In addition, the Centers for Medicare and Medicaid Services separately announced that it had taken “adverse administrative actions” against more than 50 providers for their involvement in fraud schemes related to COVID-19 or the abuse of CMS programs that were designed to encourage access to medical care during the pandemic.
Several of the defendants allegedly offered COVID-19 tests to Medicare beneficiaries in senior living facilities, drive-through COVID-19 testing sites, and medical offices to induce the beneficiaries to provide their personal identifying information and a saliva or a blood sample.
The DOJ charges claim the defendants then misused the information and the samples to submit claims to Medicare for unrelated, medically unnecessary, and far more expensive lab tests, including cancer genetic testing, allergy testing, and respiratory pathogen panel tests.
In some cases, it’s alleged, the lab results were not provided to the individuals in a timely fashion or were not reliable.
Other defendants are charged with exploiting temporary changes in CMS telehealth regulations that were designed to increase access to health care during the pandemic. In these cases, which the DOJ said were the first charges related to the expansion of telehealth under the COVID-19 emergency declaration, the defendants allegedly submitted false and fraudulent claims to Medicare for sham telemedicine encounters that did not occur.
“As part of these cases, medical professionals are alleged to have [been] offered and paid bribes in exchange for the medical professionals’ referral of unnecessary testing,” the DOJ news release said. However, no physicians were identified by the department.
Commenting on this aspect of the law enforcement action, FBI Director Christopher Wray said in the release: “Medical providers have been the unsung heroes for the American public throughout the pandemic. It’s disheartening that some have abused their authorities and committed COVID-19–related fraud against trusting citizens. The FBI, along with our federal law enforcement and private sector partners, are committed to continuing to combat health care fraud and protect the American people.”
The law enforcement action includes the third set of criminal charges related to the misuse of Provider Relief Fund monies, according to the release.
More than 340 individuals were charged in September 2020 with submitting $6 billion in fraudulent claims to federal health care programs and private insurers for telehealth consultations and substance abuse treatment. About $4.5 billion of that was related to telehealth, as reported by this news organization.
The new criminal charges were brought in federal district courts in Arkansas, California, Louisiana, Florida, New Jersey, and New York.
Case summaries
The DOJ provided several case summaries. One defendant, lab owner Billy Joe Taylor of Lavaca, Ark., was charged with participating in a scheme to defraud the government of over $42 million by filing false claims that were billed in combination with COVID-19 testing claims. He also allegedly billed for tests that were not performed.
Petros Hannesyan of Burbank, Calif., the owner of a home health agency, was charged with obtaining over $229,000 from COVID-19 relief programs under false pretenses. His firm allegedly misappropriated funds from the CARES Act Provider Relief Fund and submitted false loan applications and a false loan agreement to the Economic Injury Disaster Loan Program.
Michael Stein and Leonel Palatnik of Palm Beach County, Fla., were charged in a connection with an alleged $73 million conspiracy to defraud the government and to pay and receive health care kickbacks during the pandemic.
Mr. Stein, who owned a “purported” consulting company, and Mr. Palatnik, who owned testing labs in Texas, allegedly exploited Medicare’s waiver of telehealth restrictions “by offering telehealth providers access to Medicare beneficiaries for whom they could bill consultations. In exchange, these providers agreed to refer beneficiaries to [Mr. Palatnik’s] laboratories for expensive and medically unnecessary cancer and cardiovascular genetic testing.”
A version of this article first appeared on Medscape.com.
The U.S. Department of Justice (DOJ) on May 26 announced charges against 14 defendants across the country who allegedly engaged in health care fraud schemes that exploited the COVID-19 pandemic and resulted in over $143 million in false billings to Medicare.
Among the defendants, a DOJ news release said, were a telemedicine company executive, a physician, marketers, and medical business owners.
In addition, the Centers for Medicare and Medicaid Services separately announced that it had taken “adverse administrative actions” against more than 50 providers for their involvement in fraud schemes related to COVID-19 or the abuse of CMS programs that were designed to encourage access to medical care during the pandemic.
Several of the defendants allegedly offered COVID-19 tests to Medicare beneficiaries in senior living facilities, drive-through COVID-19 testing sites, and medical offices to induce the beneficiaries to provide their personal identifying information and a saliva or a blood sample.
The DOJ charges claim the defendants then misused the information and the samples to submit claims to Medicare for unrelated, medically unnecessary, and far more expensive lab tests, including cancer genetic testing, allergy testing, and respiratory pathogen panel tests.
In some cases, it’s alleged, the lab results were not provided to the individuals in a timely fashion or were not reliable.
Other defendants are charged with exploiting temporary changes in CMS telehealth regulations that were designed to increase access to health care during the pandemic. In these cases, which the DOJ said were the first charges related to the expansion of telehealth under the COVID-19 emergency declaration, the defendants allegedly submitted false and fraudulent claims to Medicare for sham telemedicine encounters that did not occur.
“As part of these cases, medical professionals are alleged to have [been] offered and paid bribes in exchange for the medical professionals’ referral of unnecessary testing,” the DOJ news release said. However, no physicians were identified by the department.
Commenting on this aspect of the law enforcement action, FBI Director Christopher Wray said in the release: “Medical providers have been the unsung heroes for the American public throughout the pandemic. It’s disheartening that some have abused their authorities and committed COVID-19–related fraud against trusting citizens. The FBI, along with our federal law enforcement and private sector partners, are committed to continuing to combat health care fraud and protect the American people.”
The law enforcement action includes the third set of criminal charges related to the misuse of Provider Relief Fund monies, according to the release.
More than 340 individuals were charged in September 2020 with submitting $6 billion in fraudulent claims to federal health care programs and private insurers for telehealth consultations and substance abuse treatment. About $4.5 billion of that was related to telehealth, as reported by this news organization.
The new criminal charges were brought in federal district courts in Arkansas, California, Louisiana, Florida, New Jersey, and New York.
Case summaries
The DOJ provided several case summaries. One defendant, lab owner Billy Joe Taylor of Lavaca, Ark., was charged with participating in a scheme to defraud the government of over $42 million by filing false claims that were billed in combination with COVID-19 testing claims. He also allegedly billed for tests that were not performed.
Petros Hannesyan of Burbank, Calif., the owner of a home health agency, was charged with obtaining over $229,000 from COVID-19 relief programs under false pretenses. His firm allegedly misappropriated funds from the CARES Act Provider Relief Fund and submitted false loan applications and a false loan agreement to the Economic Injury Disaster Loan Program.
Michael Stein and Leonel Palatnik of Palm Beach County, Fla., were charged in a connection with an alleged $73 million conspiracy to defraud the government and to pay and receive health care kickbacks during the pandemic.
Mr. Stein, who owned a “purported” consulting company, and Mr. Palatnik, who owned testing labs in Texas, allegedly exploited Medicare’s waiver of telehealth restrictions “by offering telehealth providers access to Medicare beneficiaries for whom they could bill consultations. In exchange, these providers agreed to refer beneficiaries to [Mr. Palatnik’s] laboratories for expensive and medically unnecessary cancer and cardiovascular genetic testing.”
A version of this article first appeared on Medscape.com.
The U.S. Department of Justice (DOJ) on May 26 announced charges against 14 defendants across the country who allegedly engaged in health care fraud schemes that exploited the COVID-19 pandemic and resulted in over $143 million in false billings to Medicare.
Among the defendants, a DOJ news release said, were a telemedicine company executive, a physician, marketers, and medical business owners.
In addition, the Centers for Medicare and Medicaid Services separately announced that it had taken “adverse administrative actions” against more than 50 providers for their involvement in fraud schemes related to COVID-19 or the abuse of CMS programs that were designed to encourage access to medical care during the pandemic.
Several of the defendants allegedly offered COVID-19 tests to Medicare beneficiaries in senior living facilities, drive-through COVID-19 testing sites, and medical offices to induce the beneficiaries to provide their personal identifying information and a saliva or a blood sample.
The DOJ charges claim the defendants then misused the information and the samples to submit claims to Medicare for unrelated, medically unnecessary, and far more expensive lab tests, including cancer genetic testing, allergy testing, and respiratory pathogen panel tests.
In some cases, it’s alleged, the lab results were not provided to the individuals in a timely fashion or were not reliable.
Other defendants are charged with exploiting temporary changes in CMS telehealth regulations that were designed to increase access to health care during the pandemic. In these cases, which the DOJ said were the first charges related to the expansion of telehealth under the COVID-19 emergency declaration, the defendants allegedly submitted false and fraudulent claims to Medicare for sham telemedicine encounters that did not occur.
“As part of these cases, medical professionals are alleged to have [been] offered and paid bribes in exchange for the medical professionals’ referral of unnecessary testing,” the DOJ news release said. However, no physicians were identified by the department.
Commenting on this aspect of the law enforcement action, FBI Director Christopher Wray said in the release: “Medical providers have been the unsung heroes for the American public throughout the pandemic. It’s disheartening that some have abused their authorities and committed COVID-19–related fraud against trusting citizens. The FBI, along with our federal law enforcement and private sector partners, are committed to continuing to combat health care fraud and protect the American people.”
The law enforcement action includes the third set of criminal charges related to the misuse of Provider Relief Fund monies, according to the release.
More than 340 individuals were charged in September 2020 with submitting $6 billion in fraudulent claims to federal health care programs and private insurers for telehealth consultations and substance abuse treatment. About $4.5 billion of that was related to telehealth, as reported by this news organization.
The new criminal charges were brought in federal district courts in Arkansas, California, Louisiana, Florida, New Jersey, and New York.
Case summaries
The DOJ provided several case summaries. One defendant, lab owner Billy Joe Taylor of Lavaca, Ark., was charged with participating in a scheme to defraud the government of over $42 million by filing false claims that were billed in combination with COVID-19 testing claims. He also allegedly billed for tests that were not performed.
Petros Hannesyan of Burbank, Calif., the owner of a home health agency, was charged with obtaining over $229,000 from COVID-19 relief programs under false pretenses. His firm allegedly misappropriated funds from the CARES Act Provider Relief Fund and submitted false loan applications and a false loan agreement to the Economic Injury Disaster Loan Program.
Michael Stein and Leonel Palatnik of Palm Beach County, Fla., were charged in a connection with an alleged $73 million conspiracy to defraud the government and to pay and receive health care kickbacks during the pandemic.
Mr. Stein, who owned a “purported” consulting company, and Mr. Palatnik, who owned testing labs in Texas, allegedly exploited Medicare’s waiver of telehealth restrictions “by offering telehealth providers access to Medicare beneficiaries for whom they could bill consultations. In exchange, these providers agreed to refer beneficiaries to [Mr. Palatnik’s] laboratories for expensive and medically unnecessary cancer and cardiovascular genetic testing.”
A version of this article first appeared on Medscape.com.
Elevated factor VIII troughs can lead to a higher proportion of zero bleeds in hemophilia
Rurioctocog alfa pegol prophylaxis was linked to fewer bleeding episodes in people with hemophilia A when it targeted higher levels of factor VIII (FVIII) troughs, according to a report published in Blood (2021;137[13]:1818-27).
Earlier studies demonstrated that the treatment effectively prevented bleeds with an acceptable safety profile in people with hemophilia A. The current prospective, randomized, open label PROPEL trial compared safety and efficacy of two target FVIII troughs in this population. Targeting 1%-3% and 8%-12% FVIII troughs was efficacious, with fewer bleeds in the latter arm and acceptable safety across both, according to Robert Klamroth, MD, of Vivantes Klinikum Friedrichshain, Berlin, and colleagues.
The PROPEL trial (NCT02585960) population comprised 155 patients with hepatitis A, aged 12-65 years, with severe disease and an annualized bleeding rate of at least 2 during the 12 months before enrollment in the study. All had previous FVIII treatment. Patients were randomized to 12 months’ pharmacokinetic rurioctocog alfa pegol prophylaxis targeting FVIII troughs of 1%-3% (reference arm) or 8%-12%.
The primary endpoint was absence of bleeds during the second 6-month period. A total of 95 patients completed the protocol.
Promising results
In the 1%-3% and 8%-12% arms, the proportions of patients who completed the protocol and had no bleeds were 40% and 67% respectively (P = .015). Serious adverse events occurred in 7 of 115 (6%) patients, including one treatment-related event in the 8%-12% arm. There were no deaths, serious thrombotic events, or adverse event-related discontinuations.
“Targeting 8% to 12% FVIII troughs resulted in a higher proportion of [patients] with no bleeds than prophylaxis that targeted 1% to 3% FVIII troughs. These results support the hypothesis that an elevated FVIII trough can benefit [patients]without changing the safety profile,” the researchers reported. Personalized treatment in this patient population should be considered, they added.
Problems remain
In an invited commentary, Christine L. Kempton, MD, of Emory University, Atlanta, pointed out that the study did not answer the question of what trough level is best, and that the target trough level may be up to a patient’s individual clinician to decide. “Many participants (42%) treated with the target trough level of 1% to 3% had no bleeding events during the study period, but some (38%) continued to have bleeding events despite higher target trough levels,” Dr. Kempton wrote. She added that, beyond this concern, the presence of subclinical bleeding is difficult to study and quantify, but its presence is supported in the literature by magnetic resonance imaging that demonstrated joint damage despite a lack of clinically evident bleeding.
“Thus, targeting zero clinical bleeding events does not mean that all joint disease, dysfunction, and pain will be eliminated. This reality underscores the need for better, not just more convenient, therapies,” she concluded.
The authors reported numerous relationships with a variety of pharmaceutical companies including grants, honoraria, and participation in speakers bureaus. Dr. Kempton reported honoraria from Takeda, Spark, Octapharma, and Pfizer, and research grants from Novo Nordisk.
Rurioctocog alfa pegol prophylaxis was linked to fewer bleeding episodes in people with hemophilia A when it targeted higher levels of factor VIII (FVIII) troughs, according to a report published in Blood (2021;137[13]:1818-27).
Earlier studies demonstrated that the treatment effectively prevented bleeds with an acceptable safety profile in people with hemophilia A. The current prospective, randomized, open label PROPEL trial compared safety and efficacy of two target FVIII troughs in this population. Targeting 1%-3% and 8%-12% FVIII troughs was efficacious, with fewer bleeds in the latter arm and acceptable safety across both, according to Robert Klamroth, MD, of Vivantes Klinikum Friedrichshain, Berlin, and colleagues.
The PROPEL trial (NCT02585960) population comprised 155 patients with hepatitis A, aged 12-65 years, with severe disease and an annualized bleeding rate of at least 2 during the 12 months before enrollment in the study. All had previous FVIII treatment. Patients were randomized to 12 months’ pharmacokinetic rurioctocog alfa pegol prophylaxis targeting FVIII troughs of 1%-3% (reference arm) or 8%-12%.
The primary endpoint was absence of bleeds during the second 6-month period. A total of 95 patients completed the protocol.
Promising results
In the 1%-3% and 8%-12% arms, the proportions of patients who completed the protocol and had no bleeds were 40% and 67% respectively (P = .015). Serious adverse events occurred in 7 of 115 (6%) patients, including one treatment-related event in the 8%-12% arm. There were no deaths, serious thrombotic events, or adverse event-related discontinuations.
“Targeting 8% to 12% FVIII troughs resulted in a higher proportion of [patients] with no bleeds than prophylaxis that targeted 1% to 3% FVIII troughs. These results support the hypothesis that an elevated FVIII trough can benefit [patients]without changing the safety profile,” the researchers reported. Personalized treatment in this patient population should be considered, they added.
Problems remain
In an invited commentary, Christine L. Kempton, MD, of Emory University, Atlanta, pointed out that the study did not answer the question of what trough level is best, and that the target trough level may be up to a patient’s individual clinician to decide. “Many participants (42%) treated with the target trough level of 1% to 3% had no bleeding events during the study period, but some (38%) continued to have bleeding events despite higher target trough levels,” Dr. Kempton wrote. She added that, beyond this concern, the presence of subclinical bleeding is difficult to study and quantify, but its presence is supported in the literature by magnetic resonance imaging that demonstrated joint damage despite a lack of clinically evident bleeding.
“Thus, targeting zero clinical bleeding events does not mean that all joint disease, dysfunction, and pain will be eliminated. This reality underscores the need for better, not just more convenient, therapies,” she concluded.
The authors reported numerous relationships with a variety of pharmaceutical companies including grants, honoraria, and participation in speakers bureaus. Dr. Kempton reported honoraria from Takeda, Spark, Octapharma, and Pfizer, and research grants from Novo Nordisk.
Rurioctocog alfa pegol prophylaxis was linked to fewer bleeding episodes in people with hemophilia A when it targeted higher levels of factor VIII (FVIII) troughs, according to a report published in Blood (2021;137[13]:1818-27).
Earlier studies demonstrated that the treatment effectively prevented bleeds with an acceptable safety profile in people with hemophilia A. The current prospective, randomized, open label PROPEL trial compared safety and efficacy of two target FVIII troughs in this population. Targeting 1%-3% and 8%-12% FVIII troughs was efficacious, with fewer bleeds in the latter arm and acceptable safety across both, according to Robert Klamroth, MD, of Vivantes Klinikum Friedrichshain, Berlin, and colleagues.
The PROPEL trial (NCT02585960) population comprised 155 patients with hepatitis A, aged 12-65 years, with severe disease and an annualized bleeding rate of at least 2 during the 12 months before enrollment in the study. All had previous FVIII treatment. Patients were randomized to 12 months’ pharmacokinetic rurioctocog alfa pegol prophylaxis targeting FVIII troughs of 1%-3% (reference arm) or 8%-12%.
The primary endpoint was absence of bleeds during the second 6-month period. A total of 95 patients completed the protocol.
Promising results
In the 1%-3% and 8%-12% arms, the proportions of patients who completed the protocol and had no bleeds were 40% and 67% respectively (P = .015). Serious adverse events occurred in 7 of 115 (6%) patients, including one treatment-related event in the 8%-12% arm. There were no deaths, serious thrombotic events, or adverse event-related discontinuations.
“Targeting 8% to 12% FVIII troughs resulted in a higher proportion of [patients] with no bleeds than prophylaxis that targeted 1% to 3% FVIII troughs. These results support the hypothesis that an elevated FVIII trough can benefit [patients]without changing the safety profile,” the researchers reported. Personalized treatment in this patient population should be considered, they added.
Problems remain
In an invited commentary, Christine L. Kempton, MD, of Emory University, Atlanta, pointed out that the study did not answer the question of what trough level is best, and that the target trough level may be up to a patient’s individual clinician to decide. “Many participants (42%) treated with the target trough level of 1% to 3% had no bleeding events during the study period, but some (38%) continued to have bleeding events despite higher target trough levels,” Dr. Kempton wrote. She added that, beyond this concern, the presence of subclinical bleeding is difficult to study and quantify, but its presence is supported in the literature by magnetic resonance imaging that demonstrated joint damage despite a lack of clinically evident bleeding.
“Thus, targeting zero clinical bleeding events does not mean that all joint disease, dysfunction, and pain will be eliminated. This reality underscores the need for better, not just more convenient, therapies,” she concluded.
The authors reported numerous relationships with a variety of pharmaceutical companies including grants, honoraria, and participation in speakers bureaus. Dr. Kempton reported honoraria from Takeda, Spark, Octapharma, and Pfizer, and research grants from Novo Nordisk.
FROM BLOOD
Visa worries intensify pandemic stress for immigrant hospitalist moms
The COVID-19 pandemic has been difficult for all hospitalists, especially those who are parents of young children. For hospitalist moms who are also immigrants working on temporary H1-B visas, this stress is exacerbated. Though each story is unique, the underlying themes are the same: Worries over visa renewals, the immigration process, family members back home, and the risk of illness, job loss, and deportation.
Supporting the family
Like all health care workers, Prasanna Palabindela, MD, a hospitalist at Jennie Stuart Health in Hopkinsville, Ky., has been worried about bringing COVID-19 home to her family, especially in the beginning. Her in-laws had just arrived from India for a visit in March 2020 when the pandemic began, everything was shut down, and her in-laws were forced to settle in for an unexpected months-long stay.
Along with her elderly in-laws, who also have chronic conditions, Dr. Palabindela had two small children to worry about – a then-5-month-old daughter and a 5-year-old son. “I was more worried about them than me,” she said. “I used to take showers before coming home and just do all precautions as much as I can. I’m glad that I did not bring COVID, so far, to the family.”
Once she could safely send her in-laws back to India, Dr. Palabindela began searching for a nanny. Daycare was out of the question because she didn’t want her children to be exposed to illness. After a long search, she found a nanny who could also help her son with virtual school. “It’s expensive, but still, my family and my family’s health is my priority,” she said.
Working on visas has caused multiple issues for Dr. Palabindela and her husband. After living in different states because of their jobs, her husband joined her in West Virginia for her residency and found a job there. When Dr. Palabindela took her current position, her husband had to quit his job in West Virginia and move with her to Kentucky for them to stay together. Unfortunately, he couldn’t find a good fit for work in Kentucky, so the couple decided to put him on her visa so they wouldn’t have to live apart.
Now Dr. Palabindela is the family’s sole breadwinner. “That means if something happens to me, I always worry what’s going to happen with my family because legally, my husband cannot work. Technically, everyone is deported back to home,” she said. Not being able to work is hard for her husband too. “It’s just so much stress in the family because he worked for 11 years,” said Dr. Palabindela.
Through all the upheavals, Dr. Palabindela has had support from all sides. Her husband has been the biggest source. “He’s my backbone. Every time, he supported me in each and every aspect,” she said. Her parents and her brothers check in on her constantly to make sure she’s staying safe. Even the chief at her hospital has played a significant role, going to bat for his physicians to ensure their safety.
Dr. Palabindela credits everyone who works with COVID-19 patients as heroes. “The nurses, the physicians, the housekeeping, respiratory therapist, speech therapist, physical therapy ... everybody has a role. Everybody is a hero,” she said. “Whoever is wearing a mask is a hero, too, because they are contributing to this community.”
Advocating for immigration reform
A lack of transparency and information in the beginning of the pandemic significantly contributed to anxiety, said Anuradha Amara, MD, MBBS, a hospitalist in Wilmington, Del. She felt that what was on the news and what was actually going on in the hospitals were quite different. Colleagues were getting sick, there wasn’t enough personal protective equipment, and planning went out the window. “It’s like a meteor hitting a place and then we start dealing with the aftermath, but we weren’t ready before,” Dr. Amara said. “We didn’t have a plan for a pandemic.”
Then there was the concern of either her or her husband, a cardiologist, getting sick and potentially losing their jobs and immigration status. “How am I going to go back to my country if I had to? What will happen to my family if I die? If I go on the ventilator? Those are the insecurities we found additional to the pandemic challenges we had,” Dr. Amara said.
Not being able to go see their family in India or have them come visit was difficult – “it was pretty bad up there,” said Dr. Amara. Fortunately, her family members in India remained safe, but there’s a very real uneasiness about returning should an emergency arise. “Should I go back and then take the risk of losing my job and losing my position and my kids are here, they’re going to school here. How do you decide that?” she asked.
One of the worst effects of her visa restrictions was not being able to help in New York when hospitals were so short-staffed, and the morgues were overflowing. “New York is 3 hours away from where I live, but I was in chains. I couldn’t help them because of these visa restrictions,” Dr. Amara said. During the emergency, the state allowed physicians from other states to practice without being licensed in New York, but immigrant physicians were not included. “Even if we wanted to, we couldn’t volunteer,” said Dr. Amara. “I have family in New York, and I was really worried. Out of compassion I wanted to help, but I couldn’t do anything.”
Before the pandemic, Dr. Amara joined in advocacy efforts for immigrant physicians through Physicians for American Healthcare Access (PAHA). “In uncertain times, like COVID, it gets worse that you’re challenged with everything on top of your health, your family, and you have to be worried about deportation,” she said. “We need to strengthen legislation. Nobody should suffer with immigration processes during an active pandemic or otherwise.”
In the United States, 28% of physicians are immigrants. Dr. Amara pointed out that these physicians go through years of expensive training with extensive background checks at every level, yet they’re classified as second preference (EB-2) workers. She believes that physicians as a group should be excluded from this category and allowed to automatically become citizens after 5 years of living in the United States and working in an underserved area.
There have been an estimated 15,000 unused green cards since 2005. And if Congress went back to 1992, there could be more than 220,000 previously unused green cards recaptured. These unused green cards are the basis behind bills H.R.2255 and S.1024, the Healthcare Workforce Resiliency Act, which has been championed by SHM and PAHA. “It will allow the frontline physicians, 15,000 of them, and 25,000 nurses, to obtain their permanent residency,” said Dr. Amara. “These are people who already applied for their permanent residencies and they’re still waiting.”
SHM has consistently advocated for the Act since it was first introduced, written multiple letters on the issue, and supported it both on and off Capitol Hill. The society says the legislation would be an “important first step toward addressing a critical shortage” in the U.S. health care system by “recognizing the vital role immigrant physicians and nurses are playing in the fight against COVID-19.”
Currently, SHM has a live action alert open for the reintroduced bill, and encourages members to contact their legislators and urge them to support the reintroduction of the Act by cosponsoring and working to pass the legislation
Dr. Amara encourages physicians to start engaging in advocacy efforts early. Though she didn’t begin participating until late in her career, she said being aware of and part of policies that affect medicine is important. If more physicians get involved, “there are so many things we can take care of,” said Dr. Amara. “The medical profession doesn’t have to be so difficult and so busy. There are ways we can make it better and I believe that. And obviously I’ll continue to work and advocate for the entire medical profession, their problems, their health and well-being, to prevent burnout.”
Making time for positivity and self-care
Sandhya Tagaram, MD, a hospitalist at UMass Memorial Medical Center in Worcester, Mass., and her husband, also a hospitalist physician, had only ever read about pandemics in books. They certainly never expected to be in the middle of one. “That was a totally different level of anxiety to work as frontline physicians with two kids under 5 years and families away back home in India,” she said.
Dr. Tagaram and her husband work opposite shifts so that one of them is always home with their two young children. “Our schedules became more challenging when the pandemic started. Between both of our schedules and with minimal childcare facilities, we managed to strike a decent work-family balance, although we experience less vacation time together. We are fortunate to have an understanding work group,” said Dr. Tagaram.
Even before COVID-19, Dr. Tagaram found working on the temporary work visa challenging. “I think the pandemic has exposed the layer of uncertainty associated with it,” she said. “It’s incredibly stressful to imagine any minor turbulence that could alter our family and work lives. As a frontline physician mom, I take pride in raising my kids and taking care of my patients. We want to serve our communities and at the same time secure our families.”
Not being able to visit family back home and travel is exceedingly difficult. Dr. Tagaram said it would be helpful if there was a separate permanent residence pathway for physicians because they play a critical role in public health and they have been an integral part of the COVID-19 pandemic response team. A separate pathway could help keep their families secure and enable them to give their best to their communities.
Amid all the anxiety, Dr. Tagaram said she and her husband realized they could not keep living with so much pressure. As parents and as physicians, they did not want their stress to leak out and affect their ability and commitment to care for their children or their patients. They decided they needed to figure out how to be positive and constructive.
“We try some daily fun activities with the kids after returning home from work,” said Dr. Tagaram. They also formed a bubble group with two other physician families so the children could interact safely. She said that it’s critical that physicians take time for themselves. “We have to cultivate a serious hobby that helps to rejuvenate and calm our busy minds,” said Dr. Tagaram.
She makes time every day to exercise and to read at least a few pages from a good book. She is also learning Carnatic music along with one of her daughters. And every month since March 2020, she has journaled about her work and what she learned so her daughters can read it someday. “These things keep me jazzed up,” she said.
The pandemic has highlighted the fact that we are all part of one global community. “Although we hail from different backgrounds, we learned that we do have some common goals of being kind and supportive to each other and to give back to our communities. Hopefully we will continue this spirit,” said Dr. Tagaram. As a physician mother, “I feel it’s a privilege and honor to take care of my family and my community.”
Soldiering on in the COVID-19 war
The uncertainty everyone felt at the beginning of the pandemic was “very, very scary,” said Mamtha Balla, MD, MPH, a hospitalist and clinical assistant professor in northwest Ohio. “Initially, I was so involved in it and I felt like it was like a war, a COVID-19 war, and we are soldiers in that and trying to protect and do whatever we can.”
She and her husband, a geriatrician also working on an H-1B visa, have worked hard not to bring the virus home to their 2-year-old daughter. Going into 2021, the past 2 years have been “the most hectic and emotionally draining – and physically exhausting – years of my life,” said Dr. Balla.
The COVID-19 vaccine has helped reduce some pressure, but Dr. Balla is still concerned about the high risk to health care workers and the new COVID-19 strains coming out. “We are really not sure what we are dealing with and how the COVID will calm,” she said. “It is pretty challenging being a health care worker because not only are you responsible for your patients at the end of the day, but you are also responsible for your families.”
Initially in the United States from India on a student visa in 2008, Dr. Balla was placed on an H-1B visa when she started her residency. It was during this time that her mother was diagnosed with cancer and went through surgeries and chemotherapy. “She was pretty ill,” recalled Dr. Balla.
Despite the situation, Dr. Balla was afraid to go stay with her mother in case her visa application was rejected, and she couldn’t complete her third year of education. “I opted not to go to India at that time because I did not want to take a chance,” Dr. Balla said. “I have tears in my eyes because those are not easy moments, to withhold from seeing your parents, or to be in any other emergency where you cannot travel. That especially puts us at a higher risk emotionally and physically.”
She has not seen her parents in 2½ years. Between the very real possibility of not being able to get her visa stamp and the unpredictability of how other countries are dealing with COVID-19, Dr. Balla feels it is impossible to even think of going to visit. “Even if I go, what if something happens where my visa gets stuck, or the visa office is not open?” she said. If she could not get back to the United States as planned, she would have patients left behind here.
Recently, Dr. Balla did travel to India and her passport stamp did not come on time, so her husband had to come back to the United States by himself. She had to wait for her stamp for a couple more weeks before she could leave and, in the meantime, had to make arrangements at her hospital. “It is so much trauma,” she said.
There’s also the worry she has about getting sick or disabled and not being able to work anymore, resulting in deportation. “Is that what we are doing for people who are working like soldiers? Are we really treating them the correct way?” Dr. Balla asked.
Dr. Balla considers all health care workers to be soldiers in the COVID-19 war. As such, she believes the government should step up to make sure they are supporting and helping these immigrant physician-soldiers who are so necessary. She applauds France’s recent decision to grant citizenship to its frontline immigrant health care workers and feels that the same should be done in the United States. She filed her green card application in 2012, but she is nowhere close to getting it. (The backlog for employment-based green cards is more than 900,000 now.)
As people putting their own and their family’s lives at risk to care for patients with COVID-19, Dr. Balla and her husband have talked about moving to another country or even back to India. “I am a taxpayer; I am a good human being working for the community and for the job. This is my 13th year here. If I am not eligible [for citizenship] still, then I am not sure what else I have to do to prove myself,” she said. “I am owning United States citizens as my people, so please own us and help us out in this difficult scenario.”
The COVID-19 pandemic has been difficult for all hospitalists, especially those who are parents of young children. For hospitalist moms who are also immigrants working on temporary H1-B visas, this stress is exacerbated. Though each story is unique, the underlying themes are the same: Worries over visa renewals, the immigration process, family members back home, and the risk of illness, job loss, and deportation.
Supporting the family
Like all health care workers, Prasanna Palabindela, MD, a hospitalist at Jennie Stuart Health in Hopkinsville, Ky., has been worried about bringing COVID-19 home to her family, especially in the beginning. Her in-laws had just arrived from India for a visit in March 2020 when the pandemic began, everything was shut down, and her in-laws were forced to settle in for an unexpected months-long stay.
Along with her elderly in-laws, who also have chronic conditions, Dr. Palabindela had two small children to worry about – a then-5-month-old daughter and a 5-year-old son. “I was more worried about them than me,” she said. “I used to take showers before coming home and just do all precautions as much as I can. I’m glad that I did not bring COVID, so far, to the family.”
Once she could safely send her in-laws back to India, Dr. Palabindela began searching for a nanny. Daycare was out of the question because she didn’t want her children to be exposed to illness. After a long search, she found a nanny who could also help her son with virtual school. “It’s expensive, but still, my family and my family’s health is my priority,” she said.
Working on visas has caused multiple issues for Dr. Palabindela and her husband. After living in different states because of their jobs, her husband joined her in West Virginia for her residency and found a job there. When Dr. Palabindela took her current position, her husband had to quit his job in West Virginia and move with her to Kentucky for them to stay together. Unfortunately, he couldn’t find a good fit for work in Kentucky, so the couple decided to put him on her visa so they wouldn’t have to live apart.
Now Dr. Palabindela is the family’s sole breadwinner. “That means if something happens to me, I always worry what’s going to happen with my family because legally, my husband cannot work. Technically, everyone is deported back to home,” she said. Not being able to work is hard for her husband too. “It’s just so much stress in the family because he worked for 11 years,” said Dr. Palabindela.
Through all the upheavals, Dr. Palabindela has had support from all sides. Her husband has been the biggest source. “He’s my backbone. Every time, he supported me in each and every aspect,” she said. Her parents and her brothers check in on her constantly to make sure she’s staying safe. Even the chief at her hospital has played a significant role, going to bat for his physicians to ensure their safety.
Dr. Palabindela credits everyone who works with COVID-19 patients as heroes. “The nurses, the physicians, the housekeeping, respiratory therapist, speech therapist, physical therapy ... everybody has a role. Everybody is a hero,” she said. “Whoever is wearing a mask is a hero, too, because they are contributing to this community.”
Advocating for immigration reform
A lack of transparency and information in the beginning of the pandemic significantly contributed to anxiety, said Anuradha Amara, MD, MBBS, a hospitalist in Wilmington, Del. She felt that what was on the news and what was actually going on in the hospitals were quite different. Colleagues were getting sick, there wasn’t enough personal protective equipment, and planning went out the window. “It’s like a meteor hitting a place and then we start dealing with the aftermath, but we weren’t ready before,” Dr. Amara said. “We didn’t have a plan for a pandemic.”
Then there was the concern of either her or her husband, a cardiologist, getting sick and potentially losing their jobs and immigration status. “How am I going to go back to my country if I had to? What will happen to my family if I die? If I go on the ventilator? Those are the insecurities we found additional to the pandemic challenges we had,” Dr. Amara said.
Not being able to go see their family in India or have them come visit was difficult – “it was pretty bad up there,” said Dr. Amara. Fortunately, her family members in India remained safe, but there’s a very real uneasiness about returning should an emergency arise. “Should I go back and then take the risk of losing my job and losing my position and my kids are here, they’re going to school here. How do you decide that?” she asked.
One of the worst effects of her visa restrictions was not being able to help in New York when hospitals were so short-staffed, and the morgues were overflowing. “New York is 3 hours away from where I live, but I was in chains. I couldn’t help them because of these visa restrictions,” Dr. Amara said. During the emergency, the state allowed physicians from other states to practice without being licensed in New York, but immigrant physicians were not included. “Even if we wanted to, we couldn’t volunteer,” said Dr. Amara. “I have family in New York, and I was really worried. Out of compassion I wanted to help, but I couldn’t do anything.”
Before the pandemic, Dr. Amara joined in advocacy efforts for immigrant physicians through Physicians for American Healthcare Access (PAHA). “In uncertain times, like COVID, it gets worse that you’re challenged with everything on top of your health, your family, and you have to be worried about deportation,” she said. “We need to strengthen legislation. Nobody should suffer with immigration processes during an active pandemic or otherwise.”
In the United States, 28% of physicians are immigrants. Dr. Amara pointed out that these physicians go through years of expensive training with extensive background checks at every level, yet they’re classified as second preference (EB-2) workers. She believes that physicians as a group should be excluded from this category and allowed to automatically become citizens after 5 years of living in the United States and working in an underserved area.
There have been an estimated 15,000 unused green cards since 2005. And if Congress went back to 1992, there could be more than 220,000 previously unused green cards recaptured. These unused green cards are the basis behind bills H.R.2255 and S.1024, the Healthcare Workforce Resiliency Act, which has been championed by SHM and PAHA. “It will allow the frontline physicians, 15,000 of them, and 25,000 nurses, to obtain their permanent residency,” said Dr. Amara. “These are people who already applied for their permanent residencies and they’re still waiting.”
SHM has consistently advocated for the Act since it was first introduced, written multiple letters on the issue, and supported it both on and off Capitol Hill. The society says the legislation would be an “important first step toward addressing a critical shortage” in the U.S. health care system by “recognizing the vital role immigrant physicians and nurses are playing in the fight against COVID-19.”
Currently, SHM has a live action alert open for the reintroduced bill, and encourages members to contact their legislators and urge them to support the reintroduction of the Act by cosponsoring and working to pass the legislation
Dr. Amara encourages physicians to start engaging in advocacy efforts early. Though she didn’t begin participating until late in her career, she said being aware of and part of policies that affect medicine is important. If more physicians get involved, “there are so many things we can take care of,” said Dr. Amara. “The medical profession doesn’t have to be so difficult and so busy. There are ways we can make it better and I believe that. And obviously I’ll continue to work and advocate for the entire medical profession, their problems, their health and well-being, to prevent burnout.”
Making time for positivity and self-care
Sandhya Tagaram, MD, a hospitalist at UMass Memorial Medical Center in Worcester, Mass., and her husband, also a hospitalist physician, had only ever read about pandemics in books. They certainly never expected to be in the middle of one. “That was a totally different level of anxiety to work as frontline physicians with two kids under 5 years and families away back home in India,” she said.
Dr. Tagaram and her husband work opposite shifts so that one of them is always home with their two young children. “Our schedules became more challenging when the pandemic started. Between both of our schedules and with minimal childcare facilities, we managed to strike a decent work-family balance, although we experience less vacation time together. We are fortunate to have an understanding work group,” said Dr. Tagaram.
Even before COVID-19, Dr. Tagaram found working on the temporary work visa challenging. “I think the pandemic has exposed the layer of uncertainty associated with it,” she said. “It’s incredibly stressful to imagine any minor turbulence that could alter our family and work lives. As a frontline physician mom, I take pride in raising my kids and taking care of my patients. We want to serve our communities and at the same time secure our families.”
Not being able to visit family back home and travel is exceedingly difficult. Dr. Tagaram said it would be helpful if there was a separate permanent residence pathway for physicians because they play a critical role in public health and they have been an integral part of the COVID-19 pandemic response team. A separate pathway could help keep their families secure and enable them to give their best to their communities.
Amid all the anxiety, Dr. Tagaram said she and her husband realized they could not keep living with so much pressure. As parents and as physicians, they did not want their stress to leak out and affect their ability and commitment to care for their children or their patients. They decided they needed to figure out how to be positive and constructive.
“We try some daily fun activities with the kids after returning home from work,” said Dr. Tagaram. They also formed a bubble group with two other physician families so the children could interact safely. She said that it’s critical that physicians take time for themselves. “We have to cultivate a serious hobby that helps to rejuvenate and calm our busy minds,” said Dr. Tagaram.
She makes time every day to exercise and to read at least a few pages from a good book. She is also learning Carnatic music along with one of her daughters. And every month since March 2020, she has journaled about her work and what she learned so her daughters can read it someday. “These things keep me jazzed up,” she said.
The pandemic has highlighted the fact that we are all part of one global community. “Although we hail from different backgrounds, we learned that we do have some common goals of being kind and supportive to each other and to give back to our communities. Hopefully we will continue this spirit,” said Dr. Tagaram. As a physician mother, “I feel it’s a privilege and honor to take care of my family and my community.”
Soldiering on in the COVID-19 war
The uncertainty everyone felt at the beginning of the pandemic was “very, very scary,” said Mamtha Balla, MD, MPH, a hospitalist and clinical assistant professor in northwest Ohio. “Initially, I was so involved in it and I felt like it was like a war, a COVID-19 war, and we are soldiers in that and trying to protect and do whatever we can.”
She and her husband, a geriatrician also working on an H-1B visa, have worked hard not to bring the virus home to their 2-year-old daughter. Going into 2021, the past 2 years have been “the most hectic and emotionally draining – and physically exhausting – years of my life,” said Dr. Balla.
The COVID-19 vaccine has helped reduce some pressure, but Dr. Balla is still concerned about the high risk to health care workers and the new COVID-19 strains coming out. “We are really not sure what we are dealing with and how the COVID will calm,” she said. “It is pretty challenging being a health care worker because not only are you responsible for your patients at the end of the day, but you are also responsible for your families.”
Initially in the United States from India on a student visa in 2008, Dr. Balla was placed on an H-1B visa when she started her residency. It was during this time that her mother was diagnosed with cancer and went through surgeries and chemotherapy. “She was pretty ill,” recalled Dr. Balla.
Despite the situation, Dr. Balla was afraid to go stay with her mother in case her visa application was rejected, and she couldn’t complete her third year of education. “I opted not to go to India at that time because I did not want to take a chance,” Dr. Balla said. “I have tears in my eyes because those are not easy moments, to withhold from seeing your parents, or to be in any other emergency where you cannot travel. That especially puts us at a higher risk emotionally and physically.”
She has not seen her parents in 2½ years. Between the very real possibility of not being able to get her visa stamp and the unpredictability of how other countries are dealing with COVID-19, Dr. Balla feels it is impossible to even think of going to visit. “Even if I go, what if something happens where my visa gets stuck, or the visa office is not open?” she said. If she could not get back to the United States as planned, she would have patients left behind here.
Recently, Dr. Balla did travel to India and her passport stamp did not come on time, so her husband had to come back to the United States by himself. She had to wait for her stamp for a couple more weeks before she could leave and, in the meantime, had to make arrangements at her hospital. “It is so much trauma,” she said.
There’s also the worry she has about getting sick or disabled and not being able to work anymore, resulting in deportation. “Is that what we are doing for people who are working like soldiers? Are we really treating them the correct way?” Dr. Balla asked.
Dr. Balla considers all health care workers to be soldiers in the COVID-19 war. As such, she believes the government should step up to make sure they are supporting and helping these immigrant physician-soldiers who are so necessary. She applauds France’s recent decision to grant citizenship to its frontline immigrant health care workers and feels that the same should be done in the United States. She filed her green card application in 2012, but she is nowhere close to getting it. (The backlog for employment-based green cards is more than 900,000 now.)
As people putting their own and their family’s lives at risk to care for patients with COVID-19, Dr. Balla and her husband have talked about moving to another country or even back to India. “I am a taxpayer; I am a good human being working for the community and for the job. This is my 13th year here. If I am not eligible [for citizenship] still, then I am not sure what else I have to do to prove myself,” she said. “I am owning United States citizens as my people, so please own us and help us out in this difficult scenario.”
The COVID-19 pandemic has been difficult for all hospitalists, especially those who are parents of young children. For hospitalist moms who are also immigrants working on temporary H1-B visas, this stress is exacerbated. Though each story is unique, the underlying themes are the same: Worries over visa renewals, the immigration process, family members back home, and the risk of illness, job loss, and deportation.
Supporting the family
Like all health care workers, Prasanna Palabindela, MD, a hospitalist at Jennie Stuart Health in Hopkinsville, Ky., has been worried about bringing COVID-19 home to her family, especially in the beginning. Her in-laws had just arrived from India for a visit in March 2020 when the pandemic began, everything was shut down, and her in-laws were forced to settle in for an unexpected months-long stay.
Along with her elderly in-laws, who also have chronic conditions, Dr. Palabindela had two small children to worry about – a then-5-month-old daughter and a 5-year-old son. “I was more worried about them than me,” she said. “I used to take showers before coming home and just do all precautions as much as I can. I’m glad that I did not bring COVID, so far, to the family.”
Once she could safely send her in-laws back to India, Dr. Palabindela began searching for a nanny. Daycare was out of the question because she didn’t want her children to be exposed to illness. After a long search, she found a nanny who could also help her son with virtual school. “It’s expensive, but still, my family and my family’s health is my priority,” she said.
Working on visas has caused multiple issues for Dr. Palabindela and her husband. After living in different states because of their jobs, her husband joined her in West Virginia for her residency and found a job there. When Dr. Palabindela took her current position, her husband had to quit his job in West Virginia and move with her to Kentucky for them to stay together. Unfortunately, he couldn’t find a good fit for work in Kentucky, so the couple decided to put him on her visa so they wouldn’t have to live apart.
Now Dr. Palabindela is the family’s sole breadwinner. “That means if something happens to me, I always worry what’s going to happen with my family because legally, my husband cannot work. Technically, everyone is deported back to home,” she said. Not being able to work is hard for her husband too. “It’s just so much stress in the family because he worked for 11 years,” said Dr. Palabindela.
Through all the upheavals, Dr. Palabindela has had support from all sides. Her husband has been the biggest source. “He’s my backbone. Every time, he supported me in each and every aspect,” she said. Her parents and her brothers check in on her constantly to make sure she’s staying safe. Even the chief at her hospital has played a significant role, going to bat for his physicians to ensure their safety.
Dr. Palabindela credits everyone who works with COVID-19 patients as heroes. “The nurses, the physicians, the housekeeping, respiratory therapist, speech therapist, physical therapy ... everybody has a role. Everybody is a hero,” she said. “Whoever is wearing a mask is a hero, too, because they are contributing to this community.”
Advocating for immigration reform
A lack of transparency and information in the beginning of the pandemic significantly contributed to anxiety, said Anuradha Amara, MD, MBBS, a hospitalist in Wilmington, Del. She felt that what was on the news and what was actually going on in the hospitals were quite different. Colleagues were getting sick, there wasn’t enough personal protective equipment, and planning went out the window. “It’s like a meteor hitting a place and then we start dealing with the aftermath, but we weren’t ready before,” Dr. Amara said. “We didn’t have a plan for a pandemic.”
Then there was the concern of either her or her husband, a cardiologist, getting sick and potentially losing their jobs and immigration status. “How am I going to go back to my country if I had to? What will happen to my family if I die? If I go on the ventilator? Those are the insecurities we found additional to the pandemic challenges we had,” Dr. Amara said.
Not being able to go see their family in India or have them come visit was difficult – “it was pretty bad up there,” said Dr. Amara. Fortunately, her family members in India remained safe, but there’s a very real uneasiness about returning should an emergency arise. “Should I go back and then take the risk of losing my job and losing my position and my kids are here, they’re going to school here. How do you decide that?” she asked.
One of the worst effects of her visa restrictions was not being able to help in New York when hospitals were so short-staffed, and the morgues were overflowing. “New York is 3 hours away from where I live, but I was in chains. I couldn’t help them because of these visa restrictions,” Dr. Amara said. During the emergency, the state allowed physicians from other states to practice without being licensed in New York, but immigrant physicians were not included. “Even if we wanted to, we couldn’t volunteer,” said Dr. Amara. “I have family in New York, and I was really worried. Out of compassion I wanted to help, but I couldn’t do anything.”
Before the pandemic, Dr. Amara joined in advocacy efforts for immigrant physicians through Physicians for American Healthcare Access (PAHA). “In uncertain times, like COVID, it gets worse that you’re challenged with everything on top of your health, your family, and you have to be worried about deportation,” she said. “We need to strengthen legislation. Nobody should suffer with immigration processes during an active pandemic or otherwise.”
In the United States, 28% of physicians are immigrants. Dr. Amara pointed out that these physicians go through years of expensive training with extensive background checks at every level, yet they’re classified as second preference (EB-2) workers. She believes that physicians as a group should be excluded from this category and allowed to automatically become citizens after 5 years of living in the United States and working in an underserved area.
There have been an estimated 15,000 unused green cards since 2005. And if Congress went back to 1992, there could be more than 220,000 previously unused green cards recaptured. These unused green cards are the basis behind bills H.R.2255 and S.1024, the Healthcare Workforce Resiliency Act, which has been championed by SHM and PAHA. “It will allow the frontline physicians, 15,000 of them, and 25,000 nurses, to obtain their permanent residency,” said Dr. Amara. “These are people who already applied for their permanent residencies and they’re still waiting.”
SHM has consistently advocated for the Act since it was first introduced, written multiple letters on the issue, and supported it both on and off Capitol Hill. The society says the legislation would be an “important first step toward addressing a critical shortage” in the U.S. health care system by “recognizing the vital role immigrant physicians and nurses are playing in the fight against COVID-19.”
Currently, SHM has a live action alert open for the reintroduced bill, and encourages members to contact their legislators and urge them to support the reintroduction of the Act by cosponsoring and working to pass the legislation
Dr. Amara encourages physicians to start engaging in advocacy efforts early. Though she didn’t begin participating until late in her career, she said being aware of and part of policies that affect medicine is important. If more physicians get involved, “there are so many things we can take care of,” said Dr. Amara. “The medical profession doesn’t have to be so difficult and so busy. There are ways we can make it better and I believe that. And obviously I’ll continue to work and advocate for the entire medical profession, their problems, their health and well-being, to prevent burnout.”
Making time for positivity and self-care
Sandhya Tagaram, MD, a hospitalist at UMass Memorial Medical Center in Worcester, Mass., and her husband, also a hospitalist physician, had only ever read about pandemics in books. They certainly never expected to be in the middle of one. “That was a totally different level of anxiety to work as frontline physicians with two kids under 5 years and families away back home in India,” she said.
Dr. Tagaram and her husband work opposite shifts so that one of them is always home with their two young children. “Our schedules became more challenging when the pandemic started. Between both of our schedules and with minimal childcare facilities, we managed to strike a decent work-family balance, although we experience less vacation time together. We are fortunate to have an understanding work group,” said Dr. Tagaram.
Even before COVID-19, Dr. Tagaram found working on the temporary work visa challenging. “I think the pandemic has exposed the layer of uncertainty associated with it,” she said. “It’s incredibly stressful to imagine any minor turbulence that could alter our family and work lives. As a frontline physician mom, I take pride in raising my kids and taking care of my patients. We want to serve our communities and at the same time secure our families.”
Not being able to visit family back home and travel is exceedingly difficult. Dr. Tagaram said it would be helpful if there was a separate permanent residence pathway for physicians because they play a critical role in public health and they have been an integral part of the COVID-19 pandemic response team. A separate pathway could help keep their families secure and enable them to give their best to their communities.
Amid all the anxiety, Dr. Tagaram said she and her husband realized they could not keep living with so much pressure. As parents and as physicians, they did not want their stress to leak out and affect their ability and commitment to care for their children or their patients. They decided they needed to figure out how to be positive and constructive.
“We try some daily fun activities with the kids after returning home from work,” said Dr. Tagaram. They also formed a bubble group with two other physician families so the children could interact safely. She said that it’s critical that physicians take time for themselves. “We have to cultivate a serious hobby that helps to rejuvenate and calm our busy minds,” said Dr. Tagaram.
She makes time every day to exercise and to read at least a few pages from a good book. She is also learning Carnatic music along with one of her daughters. And every month since March 2020, she has journaled about her work and what she learned so her daughters can read it someday. “These things keep me jazzed up,” she said.
The pandemic has highlighted the fact that we are all part of one global community. “Although we hail from different backgrounds, we learned that we do have some common goals of being kind and supportive to each other and to give back to our communities. Hopefully we will continue this spirit,” said Dr. Tagaram. As a physician mother, “I feel it’s a privilege and honor to take care of my family and my community.”
Soldiering on in the COVID-19 war
The uncertainty everyone felt at the beginning of the pandemic was “very, very scary,” said Mamtha Balla, MD, MPH, a hospitalist and clinical assistant professor in northwest Ohio. “Initially, I was so involved in it and I felt like it was like a war, a COVID-19 war, and we are soldiers in that and trying to protect and do whatever we can.”
She and her husband, a geriatrician also working on an H-1B visa, have worked hard not to bring the virus home to their 2-year-old daughter. Going into 2021, the past 2 years have been “the most hectic and emotionally draining – and physically exhausting – years of my life,” said Dr. Balla.
The COVID-19 vaccine has helped reduce some pressure, but Dr. Balla is still concerned about the high risk to health care workers and the new COVID-19 strains coming out. “We are really not sure what we are dealing with and how the COVID will calm,” she said. “It is pretty challenging being a health care worker because not only are you responsible for your patients at the end of the day, but you are also responsible for your families.”
Initially in the United States from India on a student visa in 2008, Dr. Balla was placed on an H-1B visa when she started her residency. It was during this time that her mother was diagnosed with cancer and went through surgeries and chemotherapy. “She was pretty ill,” recalled Dr. Balla.
Despite the situation, Dr. Balla was afraid to go stay with her mother in case her visa application was rejected, and she couldn’t complete her third year of education. “I opted not to go to India at that time because I did not want to take a chance,” Dr. Balla said. “I have tears in my eyes because those are not easy moments, to withhold from seeing your parents, or to be in any other emergency where you cannot travel. That especially puts us at a higher risk emotionally and physically.”
She has not seen her parents in 2½ years. Between the very real possibility of not being able to get her visa stamp and the unpredictability of how other countries are dealing with COVID-19, Dr. Balla feels it is impossible to even think of going to visit. “Even if I go, what if something happens where my visa gets stuck, or the visa office is not open?” she said. If she could not get back to the United States as planned, she would have patients left behind here.
Recently, Dr. Balla did travel to India and her passport stamp did not come on time, so her husband had to come back to the United States by himself. She had to wait for her stamp for a couple more weeks before she could leave and, in the meantime, had to make arrangements at her hospital. “It is so much trauma,” she said.
There’s also the worry she has about getting sick or disabled and not being able to work anymore, resulting in deportation. “Is that what we are doing for people who are working like soldiers? Are we really treating them the correct way?” Dr. Balla asked.
Dr. Balla considers all health care workers to be soldiers in the COVID-19 war. As such, she believes the government should step up to make sure they are supporting and helping these immigrant physician-soldiers who are so necessary. She applauds France’s recent decision to grant citizenship to its frontline immigrant health care workers and feels that the same should be done in the United States. She filed her green card application in 2012, but she is nowhere close to getting it. (The backlog for employment-based green cards is more than 900,000 now.)
As people putting their own and their family’s lives at risk to care for patients with COVID-19, Dr. Balla and her husband have talked about moving to another country or even back to India. “I am a taxpayer; I am a good human being working for the community and for the job. This is my 13th year here. If I am not eligible [for citizenship] still, then I am not sure what else I have to do to prove myself,” she said. “I am owning United States citizens as my people, so please own us and help us out in this difficult scenario.”
‘A better picture’: First AACE guidelines on diabetes technology
The American Association of Clinical Endocrinology (AACE) has issued its first-ever official guidelines addressing the use of advanced technologies in the management of people with diabetes.
The guidelines cover use of continuous glucose monitoring (CGM), insulin pumps, connected pens, automated insulin delivery systems, telemedicine technologies, and smartphone apps. They also address safety considerations, special situations such as hospitalization, and implementation in clinical practice.
They were presented on May 28 at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists and simultaneously published in Endocrine Practice.
Previous AACE guidance on the clinical use of insulin pumps and CGM over the past decade has been published in the form of consensus or position statements rather than official evidence-based guidelines, task force cochair George Grunberger, MD, of the Grunberger Diabetes Institute, Bloomfield Hills, Mich., explained.
“There’s never really been, until now, hardcore evidence, [with] peer-reviewed, quality trials published in the literature to go after the evidence that is required for guidelines. ... This is not an opinion piece or position statement.”
The problem with that strict approach to “guidelines” is how quickly the diabetes technology field is evolving, he acknowledged. “It’s frustrating because we know what’s [coming up], but we can’t put it in a guideline because it hasn’t been published yet.”
In an AACE podcast, Dr. Grunberger said the guidelines will likely become a “living” document, along the lines of the American Diabetes Association’s annual Standards of Care, as “any cutoff date is arbitrary. More and more papers will be published on these technologies. ... This is certainly not a static field.”
In the meantime, task force cochair and author Jennifer Sherr, MD, PhD, a pediatric endocrinologist, said she hopes the guidelines will help to reduce insurance company barriers to use of the currently available technologies.
“I am very hopeful that these guidelines will also encourage payers to change their stance. And I think that we as a community can continue to advocate and inform them of these guidelines so they can appropriately change their coverage practices,” added Dr. Sherr, of Yale University, New Haven, Conn.
Recommendations address CGM, pumps, and connected systems
In the guidelines, CGM is “strongly recommended for all persons with diabetes treated with intensive insulin therapy, defined as three or more injections of insulin per day or the use of an insulin pump.” For those with diabetes who use CGM, “priority metrics” include a “time in range” of greater than 70% from 14 days of active use. Targets for mean glucose should be individualized, with glycemic variability 36% or lower.
Further specific CGM target metrics are given for people with type 1 diabetes, older/high risk individuals, and for pregnant women. The recommendations align with those issued in a 2019 joint consensus statement on CGM time-in-range endorsed by several organizations, including AACE.
In response to an audience question about whether AACE is advising that time-in-range replace A1c for glycemia assessment, Dr. Sherr responded: “I think currently we’re not in a position where we can completely replace A1c with time in range. However, I’m hopeful that in future years we’ll see further data gathered ... to allow for that recommendation to occur.”
For now, she said, “What we really want to hone in on in the guidelines is that time-in-range and use of CGM truly allow clinicians to better understand how to optimize care for their persons with diabetes. It gives us a better picture. It’s not just a number of whether we’re hitting target. It tells us whether we need to attack time above range or time below range. So we really think it’s critical for clinical care.”
The document also provides specifics about real-time versus intermittently scanned CGM and use of diagnostic/professional CGM.
The “insulin delivery technologies” section covers use of connected pens, insulin pumps without CGM, insulin pumps with separate CGM, and the more advanced combined insulin pump-CGM systems including those with low-glucose suspend, predictive low-glucose suspend, and hybrid closed-loops (sometimes called the artificial pancreas).
In general, these automated insulin delivery systems (artificial pancreas), “are strongly recommended for all persons with [type 1 diabetes], since their use has been shown to increase time in range, especially in the overnight period, without causing an increased risk of hypoglycemia,” Dr. Sherr observed.
Other tech topics: Apps, telemedicine, and safety
The new guidelines say that “clinically validated” smartphone apps should be recommended to help teach or reinforce diabetes self-management skills and provide support and encouragement for healthy behaviors around food and exercise.
Dr. Grunberger pointed out: “As we know, there are tons of apps out there, and patients are using them. The problem is that very few of them have actually been validated in clinical trials in published peer-reviewed [journals].”
He recommended a joint statement on diabetes apps from the American Diabetes Association and the European Association for the Study of Diabetes that was initially discussed at the 2019 EASD meeting, as reported by this news organization, and subsequently published in January 2020 in Diabetes Care and Diabetologia.
“Telemedicine, including periodic phone calls, smartphone-web interactions ... by health care professionals ... is strongly recommended to treat persons with diabetes, provide diabetes education, remotely monitor glucose and/or insulin data to indicate the need for therapy adjustments, and improve diabetes-related outcomes/control with better engagement,” the document says.
Safety concerns addressed include the issue of certain medications interfering with CGM [readings] ... including acetaminophen, high-dose vitamin C, and hydroxyurea, as well as cautions about what to do in the event of device malfunction and assessing that the patient is sufficiently trained in proper device use. Criteria for insulin pump discontinuation are also given.
Implementation: Who will be prescribing? ‘This is not for amateurs’
A final section on implementation recommends that “initiation and use of diabetes technology should be implemented by health care professionals who are trained, committed, and experienced to prescribe and direct the use of these tools. Clinicians should have the infrastructure to support the needs of persons with diabetes using the technology.”
Dr. Grunberger commented: “I think the key is going to be who should be doing this? What is the role of a clinical endocrinologist in the future? What is our responsibility, [since] we don’t have the manpower and womanpower to take care of all these people as these technologies advance? It’s our responsibility to provide these hopefully valued recommendations as a resource for those who want to know more about it.”
However, he noted, “This is not for amateurs. If you want to actually use this in your practice, you need the infrastructure, the expertise, the training, the dedication, and the energy to be there for the patients all the time ... This clinical practice guideline is a foundation.”
Dr. Sherr added: “To me, it’s really thinking about ... changing our mindset from who is an appropriate candidate to who can benefit and how vast a group that entails ... I’m hopeful that we will see more technology use through continued conversations with our patients with diabetes, and hopefully through more clinicians being excited to be part of this revolution.”
Dr. Grunberger has reported being on speakers bureaus for Eli Lilly, Novo Nordisk, and Abbott. Dr. Sherr has reported being a consultant and speaker for Lilly and Medtronic Diabetes, a consultant for Insulet and Sanofi, and on advisory boards for Bigfoot Biomedical, Cecelia Health, Insulet, JDRF T1D fund, and Medtronic.
A version of this article first appeared on Medscape.com.
The American Association of Clinical Endocrinology (AACE) has issued its first-ever official guidelines addressing the use of advanced technologies in the management of people with diabetes.
The guidelines cover use of continuous glucose monitoring (CGM), insulin pumps, connected pens, automated insulin delivery systems, telemedicine technologies, and smartphone apps. They also address safety considerations, special situations such as hospitalization, and implementation in clinical practice.
They were presented on May 28 at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists and simultaneously published in Endocrine Practice.
Previous AACE guidance on the clinical use of insulin pumps and CGM over the past decade has been published in the form of consensus or position statements rather than official evidence-based guidelines, task force cochair George Grunberger, MD, of the Grunberger Diabetes Institute, Bloomfield Hills, Mich., explained.
“There’s never really been, until now, hardcore evidence, [with] peer-reviewed, quality trials published in the literature to go after the evidence that is required for guidelines. ... This is not an opinion piece or position statement.”
The problem with that strict approach to “guidelines” is how quickly the diabetes technology field is evolving, he acknowledged. “It’s frustrating because we know what’s [coming up], but we can’t put it in a guideline because it hasn’t been published yet.”
In an AACE podcast, Dr. Grunberger said the guidelines will likely become a “living” document, along the lines of the American Diabetes Association’s annual Standards of Care, as “any cutoff date is arbitrary. More and more papers will be published on these technologies. ... This is certainly not a static field.”
In the meantime, task force cochair and author Jennifer Sherr, MD, PhD, a pediatric endocrinologist, said she hopes the guidelines will help to reduce insurance company barriers to use of the currently available technologies.
“I am very hopeful that these guidelines will also encourage payers to change their stance. And I think that we as a community can continue to advocate and inform them of these guidelines so they can appropriately change their coverage practices,” added Dr. Sherr, of Yale University, New Haven, Conn.
Recommendations address CGM, pumps, and connected systems
In the guidelines, CGM is “strongly recommended for all persons with diabetes treated with intensive insulin therapy, defined as three or more injections of insulin per day or the use of an insulin pump.” For those with diabetes who use CGM, “priority metrics” include a “time in range” of greater than 70% from 14 days of active use. Targets for mean glucose should be individualized, with glycemic variability 36% or lower.
Further specific CGM target metrics are given for people with type 1 diabetes, older/high risk individuals, and for pregnant women. The recommendations align with those issued in a 2019 joint consensus statement on CGM time-in-range endorsed by several organizations, including AACE.
In response to an audience question about whether AACE is advising that time-in-range replace A1c for glycemia assessment, Dr. Sherr responded: “I think currently we’re not in a position where we can completely replace A1c with time in range. However, I’m hopeful that in future years we’ll see further data gathered ... to allow for that recommendation to occur.”
For now, she said, “What we really want to hone in on in the guidelines is that time-in-range and use of CGM truly allow clinicians to better understand how to optimize care for their persons with diabetes. It gives us a better picture. It’s not just a number of whether we’re hitting target. It tells us whether we need to attack time above range or time below range. So we really think it’s critical for clinical care.”
The document also provides specifics about real-time versus intermittently scanned CGM and use of diagnostic/professional CGM.
The “insulin delivery technologies” section covers use of connected pens, insulin pumps without CGM, insulin pumps with separate CGM, and the more advanced combined insulin pump-CGM systems including those with low-glucose suspend, predictive low-glucose suspend, and hybrid closed-loops (sometimes called the artificial pancreas).
In general, these automated insulin delivery systems (artificial pancreas), “are strongly recommended for all persons with [type 1 diabetes], since their use has been shown to increase time in range, especially in the overnight period, without causing an increased risk of hypoglycemia,” Dr. Sherr observed.
Other tech topics: Apps, telemedicine, and safety
The new guidelines say that “clinically validated” smartphone apps should be recommended to help teach or reinforce diabetes self-management skills and provide support and encouragement for healthy behaviors around food and exercise.
Dr. Grunberger pointed out: “As we know, there are tons of apps out there, and patients are using them. The problem is that very few of them have actually been validated in clinical trials in published peer-reviewed [journals].”
He recommended a joint statement on diabetes apps from the American Diabetes Association and the European Association for the Study of Diabetes that was initially discussed at the 2019 EASD meeting, as reported by this news organization, and subsequently published in January 2020 in Diabetes Care and Diabetologia.
“Telemedicine, including periodic phone calls, smartphone-web interactions ... by health care professionals ... is strongly recommended to treat persons with diabetes, provide diabetes education, remotely monitor glucose and/or insulin data to indicate the need for therapy adjustments, and improve diabetes-related outcomes/control with better engagement,” the document says.
Safety concerns addressed include the issue of certain medications interfering with CGM [readings] ... including acetaminophen, high-dose vitamin C, and hydroxyurea, as well as cautions about what to do in the event of device malfunction and assessing that the patient is sufficiently trained in proper device use. Criteria for insulin pump discontinuation are also given.
Implementation: Who will be prescribing? ‘This is not for amateurs’
A final section on implementation recommends that “initiation and use of diabetes technology should be implemented by health care professionals who are trained, committed, and experienced to prescribe and direct the use of these tools. Clinicians should have the infrastructure to support the needs of persons with diabetes using the technology.”
Dr. Grunberger commented: “I think the key is going to be who should be doing this? What is the role of a clinical endocrinologist in the future? What is our responsibility, [since] we don’t have the manpower and womanpower to take care of all these people as these technologies advance? It’s our responsibility to provide these hopefully valued recommendations as a resource for those who want to know more about it.”
However, he noted, “This is not for amateurs. If you want to actually use this in your practice, you need the infrastructure, the expertise, the training, the dedication, and the energy to be there for the patients all the time ... This clinical practice guideline is a foundation.”
Dr. Sherr added: “To me, it’s really thinking about ... changing our mindset from who is an appropriate candidate to who can benefit and how vast a group that entails ... I’m hopeful that we will see more technology use through continued conversations with our patients with diabetes, and hopefully through more clinicians being excited to be part of this revolution.”
Dr. Grunberger has reported being on speakers bureaus for Eli Lilly, Novo Nordisk, and Abbott. Dr. Sherr has reported being a consultant and speaker for Lilly and Medtronic Diabetes, a consultant for Insulet and Sanofi, and on advisory boards for Bigfoot Biomedical, Cecelia Health, Insulet, JDRF T1D fund, and Medtronic.
A version of this article first appeared on Medscape.com.
The American Association of Clinical Endocrinology (AACE) has issued its first-ever official guidelines addressing the use of advanced technologies in the management of people with diabetes.
The guidelines cover use of continuous glucose monitoring (CGM), insulin pumps, connected pens, automated insulin delivery systems, telemedicine technologies, and smartphone apps. They also address safety considerations, special situations such as hospitalization, and implementation in clinical practice.
They were presented on May 28 at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists and simultaneously published in Endocrine Practice.
Previous AACE guidance on the clinical use of insulin pumps and CGM over the past decade has been published in the form of consensus or position statements rather than official evidence-based guidelines, task force cochair George Grunberger, MD, of the Grunberger Diabetes Institute, Bloomfield Hills, Mich., explained.
“There’s never really been, until now, hardcore evidence, [with] peer-reviewed, quality trials published in the literature to go after the evidence that is required for guidelines. ... This is not an opinion piece or position statement.”
The problem with that strict approach to “guidelines” is how quickly the diabetes technology field is evolving, he acknowledged. “It’s frustrating because we know what’s [coming up], but we can’t put it in a guideline because it hasn’t been published yet.”
In an AACE podcast, Dr. Grunberger said the guidelines will likely become a “living” document, along the lines of the American Diabetes Association’s annual Standards of Care, as “any cutoff date is arbitrary. More and more papers will be published on these technologies. ... This is certainly not a static field.”
In the meantime, task force cochair and author Jennifer Sherr, MD, PhD, a pediatric endocrinologist, said she hopes the guidelines will help to reduce insurance company barriers to use of the currently available technologies.
“I am very hopeful that these guidelines will also encourage payers to change their stance. And I think that we as a community can continue to advocate and inform them of these guidelines so they can appropriately change their coverage practices,” added Dr. Sherr, of Yale University, New Haven, Conn.
Recommendations address CGM, pumps, and connected systems
In the guidelines, CGM is “strongly recommended for all persons with diabetes treated with intensive insulin therapy, defined as three or more injections of insulin per day or the use of an insulin pump.” For those with diabetes who use CGM, “priority metrics” include a “time in range” of greater than 70% from 14 days of active use. Targets for mean glucose should be individualized, with glycemic variability 36% or lower.
Further specific CGM target metrics are given for people with type 1 diabetes, older/high risk individuals, and for pregnant women. The recommendations align with those issued in a 2019 joint consensus statement on CGM time-in-range endorsed by several organizations, including AACE.
In response to an audience question about whether AACE is advising that time-in-range replace A1c for glycemia assessment, Dr. Sherr responded: “I think currently we’re not in a position where we can completely replace A1c with time in range. However, I’m hopeful that in future years we’ll see further data gathered ... to allow for that recommendation to occur.”
For now, she said, “What we really want to hone in on in the guidelines is that time-in-range and use of CGM truly allow clinicians to better understand how to optimize care for their persons with diabetes. It gives us a better picture. It’s not just a number of whether we’re hitting target. It tells us whether we need to attack time above range or time below range. So we really think it’s critical for clinical care.”
The document also provides specifics about real-time versus intermittently scanned CGM and use of diagnostic/professional CGM.
The “insulin delivery technologies” section covers use of connected pens, insulin pumps without CGM, insulin pumps with separate CGM, and the more advanced combined insulin pump-CGM systems including those with low-glucose suspend, predictive low-glucose suspend, and hybrid closed-loops (sometimes called the artificial pancreas).
In general, these automated insulin delivery systems (artificial pancreas), “are strongly recommended for all persons with [type 1 diabetes], since their use has been shown to increase time in range, especially in the overnight period, without causing an increased risk of hypoglycemia,” Dr. Sherr observed.
Other tech topics: Apps, telemedicine, and safety
The new guidelines say that “clinically validated” smartphone apps should be recommended to help teach or reinforce diabetes self-management skills and provide support and encouragement for healthy behaviors around food and exercise.
Dr. Grunberger pointed out: “As we know, there are tons of apps out there, and patients are using them. The problem is that very few of them have actually been validated in clinical trials in published peer-reviewed [journals].”
He recommended a joint statement on diabetes apps from the American Diabetes Association and the European Association for the Study of Diabetes that was initially discussed at the 2019 EASD meeting, as reported by this news organization, and subsequently published in January 2020 in Diabetes Care and Diabetologia.
“Telemedicine, including periodic phone calls, smartphone-web interactions ... by health care professionals ... is strongly recommended to treat persons with diabetes, provide diabetes education, remotely monitor glucose and/or insulin data to indicate the need for therapy adjustments, and improve diabetes-related outcomes/control with better engagement,” the document says.
Safety concerns addressed include the issue of certain medications interfering with CGM [readings] ... including acetaminophen, high-dose vitamin C, and hydroxyurea, as well as cautions about what to do in the event of device malfunction and assessing that the patient is sufficiently trained in proper device use. Criteria for insulin pump discontinuation are also given.
Implementation: Who will be prescribing? ‘This is not for amateurs’
A final section on implementation recommends that “initiation and use of diabetes technology should be implemented by health care professionals who are trained, committed, and experienced to prescribe and direct the use of these tools. Clinicians should have the infrastructure to support the needs of persons with diabetes using the technology.”
Dr. Grunberger commented: “I think the key is going to be who should be doing this? What is the role of a clinical endocrinologist in the future? What is our responsibility, [since] we don’t have the manpower and womanpower to take care of all these people as these technologies advance? It’s our responsibility to provide these hopefully valued recommendations as a resource for those who want to know more about it.”
However, he noted, “This is not for amateurs. If you want to actually use this in your practice, you need the infrastructure, the expertise, the training, the dedication, and the energy to be there for the patients all the time ... This clinical practice guideline is a foundation.”
Dr. Sherr added: “To me, it’s really thinking about ... changing our mindset from who is an appropriate candidate to who can benefit and how vast a group that entails ... I’m hopeful that we will see more technology use through continued conversations with our patients with diabetes, and hopefully through more clinicians being excited to be part of this revolution.”
Dr. Grunberger has reported being on speakers bureaus for Eli Lilly, Novo Nordisk, and Abbott. Dr. Sherr has reported being a consultant and speaker for Lilly and Medtronic Diabetes, a consultant for Insulet and Sanofi, and on advisory boards for Bigfoot Biomedical, Cecelia Health, Insulet, JDRF T1D fund, and Medtronic.
A version of this article first appeared on Medscape.com.