User login
Planning for SHM Converge 2022 now underway
A hospitalist for 18 years and Annual Conference Committee (ACC) member for the last 4 years, I have always felt immense pride in this meeting. This year, we experienced constant evolution and adapted in ways unimaginable; frameshifts, detours, course corrections, wearing out words like “pivot” and “unprecedented,” whilst contending with virus lulls and surges at hospitals across the country. And SHM Converge 2021 was a landmark success despite it all.
Our SHM community successfully connected through the marvels of modern technology and enjoyed a snappy new logo and name to mark the occasion. Our unflappable course director Dan Steinberg, MD, SFHM, led an intrepid and creative team through uncertainty and produced an extraordinary educational event truly worthy of the term “unprecedented.” ACC members, talented in so many ways, each brought a unique perspective to the planning table to craft a balanced, relevant, and cutting-edge program. The only thing harder than planning a conference for thousands of hospitalists is planning TWO CONFERENCES – one in person, then one virtually.
For their facilitation of virtual adaptation of everything from clinical talks to hot dog sales, our SHM administrative staff deserve a medal. Industry sponsors likewise performed pretzel maneuvers for the virtual interface, and we thank them for their creativity and support. Freshly minted SHM CEO Eric Howell, MD, MHM, kicked off Converge by adeptly filling some very large shoes with aplomb, humor, and humility – telegraphing that our society is in good hands indeed (and that 2020 was NOT the ‘final frontier’). And, finally, each of you, in the suspended reality of a conference hall, tapped into session after session from the comfort of your hometown chairs, indefatigably learning and networking during a pandemic year.
So, beyond adaptability, what did we learn? We renewed our commitment to resilience and wellness in medicine, and reemphasized how critical diversity, equity, and inclusion are in both the workplace and in clinical practice. These topics were complemented by the usual standing-room-only clinical updates and rapid-fire sessions – where everyone could enjoy a front row seat. We talked about parenting in the pandemic, compared clinical approaches in friendly debates – for patients big and small – and deeply dived into leadership strategies for a sustainable workforce.
Here are some SHM Converge 2021 nuggets (Apologies for so few ... there were thousands!):
Plenaries
Eric Howell, MD, MHM
- Make the world a better place, be transparent and act with integrity, invest in others, do what you love.
- SHM has been leading the pack in providing e-learning options, promoting clinician self-care, and intensifying diversity, equity, and inclusion efforts before and throughout the pandemic.
- SHM has 18,000 members, 68 chapters, 26 special interest groups, 15 committees, 12 board of directors, 50 staff – growing and getting stronger every day.
- Rainbows need both rain and sunshine to form.
Gen. Mark Hertling
- Our COVID experience as hospitalists shared many features with active combat, including post-COVID combat fog.
- Use your ears, eyes, and mouth in that order: Listen more, see more, speak less.
Vineet Arora, MD, MHM
- Don’t pass up your “career gates.”
- Find “zero-gravity thinkers” – not innovation killers.
- Keep track of your state of mind using the “Bob Wachter scale.”
U.S. Surgeon Gen. Vivek Murthy, MD, and Danielle Scheurer, MD, SFHM
- Mental health and well-being of clinicians is imperative; “heal thyself” doesn’t work. Culture must support policies to truly craft a more sustaining and rewarding environment.
- We are a nation hyperfocused on episodic and salvage care (and are good at it) but must move the needle toward continuity and prevention. Sadly, nobody celebrates the heart attack that was prevented.
- What can hospitalists do about social determinants of health? Advocate for policies individually or through SHM – if you don’t know how, receive training – this is invaluable. More lobbying as a profession may yield legislation and funding aimed at such determinants and improve healthcare.
Larry Wellikson, MD, MHM
- New models hospitalists may soon inhabit: Hospital at Home, ED+, Micro-Hospitals.
- More than 50% of revenue comes from “vertical” services (outside the hospital) rather than horizontal services (in hospital) – trend to increase efforts in population health initiatives.
- Emphasis on value must go from looking at episodes of care to outcomes.
- Hospitalists Complexologists? Be relevant, add value – survive, thrive, and prosper.
Other sessions
Stroke
- Mobile stroke units are a thing!
- Neurologists are not great at predictions after stroke – but scoring tools are!
- Focus on patient-centered outcomes (100% disability free vs. able to walk vs. happy to be alive).
Drug allergies
- Penicillin allergy: 2% cross-reactivity for cephalosporins – not 10%.
Navigating work/life balance
- Have two phones for work/home – church and state – keep them separate!
Becoming an expert
- Avoid “analysis paralysis”: “Better a good decision quickly than the best decision too late” – H. Geneen
Misc. revelations
- It’s pretty cool to know the Surgeon General is a hospitalist!
- Our SHM community rocks!
- Eric Howell is an avid Star Trek and overalls enthusiast!
- It’s exceedingly difficult to become a MHM – 35 total, 3 this year.
- Danielle Scheurer is a warm and natural interviewer, sensational leader, and closet REM-rapper.
- No matter how hard I try, I’ll always be a social media Luddite: “Am I hashtagging?”
Convenience notwithstanding, this year’s conference-from-home luxury is one we hope to dispense with for SHM Converge 2022, in exchange for wandering of halls, jockeying to be closer to the front of the room, collecting freebies in exhibit halls, and seeing 50 old friends on the way to the session for which you’re already late.
Nashville, Tenn., aka Music City, will be the site of our first in-person meeting in 3 years in April 2022. I will be there with my guitar for SHM’s open mic and I hope you too bring your diverse talents from across the country to spend a week learning and energizing with us, making hospital medicine music in “Honky Tonk Hall,” “Elvis Lives Lounge,” or the “Grand Ol’ Opry-ation Suite.” The band is getting back together! Be a part of the excitement. Bring your voice, bring your talent, and let’s do Nashville in numbers!
Planning is now underway ... and we need your ideas and suggestions! Share thoughts on topics and speakers through the OPEN CALL site through June 1st ... and don’t forget to watch on-demand talks you missed from SHM Converge 2021 – a veritable treasure trove of learning.
Dr. Nye is a hospitalist and professor of medicine at the University of California, San Francisco. She is the course director of SHM Converge 2022.
A hospitalist for 18 years and Annual Conference Committee (ACC) member for the last 4 years, I have always felt immense pride in this meeting. This year, we experienced constant evolution and adapted in ways unimaginable; frameshifts, detours, course corrections, wearing out words like “pivot” and “unprecedented,” whilst contending with virus lulls and surges at hospitals across the country. And SHM Converge 2021 was a landmark success despite it all.
Our SHM community successfully connected through the marvels of modern technology and enjoyed a snappy new logo and name to mark the occasion. Our unflappable course director Dan Steinberg, MD, SFHM, led an intrepid and creative team through uncertainty and produced an extraordinary educational event truly worthy of the term “unprecedented.” ACC members, talented in so many ways, each brought a unique perspective to the planning table to craft a balanced, relevant, and cutting-edge program. The only thing harder than planning a conference for thousands of hospitalists is planning TWO CONFERENCES – one in person, then one virtually.
For their facilitation of virtual adaptation of everything from clinical talks to hot dog sales, our SHM administrative staff deserve a medal. Industry sponsors likewise performed pretzel maneuvers for the virtual interface, and we thank them for their creativity and support. Freshly minted SHM CEO Eric Howell, MD, MHM, kicked off Converge by adeptly filling some very large shoes with aplomb, humor, and humility – telegraphing that our society is in good hands indeed (and that 2020 was NOT the ‘final frontier’). And, finally, each of you, in the suspended reality of a conference hall, tapped into session after session from the comfort of your hometown chairs, indefatigably learning and networking during a pandemic year.
So, beyond adaptability, what did we learn? We renewed our commitment to resilience and wellness in medicine, and reemphasized how critical diversity, equity, and inclusion are in both the workplace and in clinical practice. These topics were complemented by the usual standing-room-only clinical updates and rapid-fire sessions – where everyone could enjoy a front row seat. We talked about parenting in the pandemic, compared clinical approaches in friendly debates – for patients big and small – and deeply dived into leadership strategies for a sustainable workforce.
Here are some SHM Converge 2021 nuggets (Apologies for so few ... there were thousands!):
Plenaries
Eric Howell, MD, MHM
- Make the world a better place, be transparent and act with integrity, invest in others, do what you love.
- SHM has been leading the pack in providing e-learning options, promoting clinician self-care, and intensifying diversity, equity, and inclusion efforts before and throughout the pandemic.
- SHM has 18,000 members, 68 chapters, 26 special interest groups, 15 committees, 12 board of directors, 50 staff – growing and getting stronger every day.
- Rainbows need both rain and sunshine to form.
Gen. Mark Hertling
- Our COVID experience as hospitalists shared many features with active combat, including post-COVID combat fog.
- Use your ears, eyes, and mouth in that order: Listen more, see more, speak less.
Vineet Arora, MD, MHM
- Don’t pass up your “career gates.”
- Find “zero-gravity thinkers” – not innovation killers.
- Keep track of your state of mind using the “Bob Wachter scale.”
U.S. Surgeon Gen. Vivek Murthy, MD, and Danielle Scheurer, MD, SFHM
- Mental health and well-being of clinicians is imperative; “heal thyself” doesn’t work. Culture must support policies to truly craft a more sustaining and rewarding environment.
- We are a nation hyperfocused on episodic and salvage care (and are good at it) but must move the needle toward continuity and prevention. Sadly, nobody celebrates the heart attack that was prevented.
- What can hospitalists do about social determinants of health? Advocate for policies individually or through SHM – if you don’t know how, receive training – this is invaluable. More lobbying as a profession may yield legislation and funding aimed at such determinants and improve healthcare.
Larry Wellikson, MD, MHM
- New models hospitalists may soon inhabit: Hospital at Home, ED+, Micro-Hospitals.
- More than 50% of revenue comes from “vertical” services (outside the hospital) rather than horizontal services (in hospital) – trend to increase efforts in population health initiatives.
- Emphasis on value must go from looking at episodes of care to outcomes.
- Hospitalists Complexologists? Be relevant, add value – survive, thrive, and prosper.
Other sessions
Stroke
- Mobile stroke units are a thing!
- Neurologists are not great at predictions after stroke – but scoring tools are!
- Focus on patient-centered outcomes (100% disability free vs. able to walk vs. happy to be alive).
Drug allergies
- Penicillin allergy: 2% cross-reactivity for cephalosporins – not 10%.
Navigating work/life balance
- Have two phones for work/home – church and state – keep them separate!
Becoming an expert
- Avoid “analysis paralysis”: “Better a good decision quickly than the best decision too late” – H. Geneen
Misc. revelations
- It’s pretty cool to know the Surgeon General is a hospitalist!
- Our SHM community rocks!
- Eric Howell is an avid Star Trek and overalls enthusiast!
- It’s exceedingly difficult to become a MHM – 35 total, 3 this year.
- Danielle Scheurer is a warm and natural interviewer, sensational leader, and closet REM-rapper.
- No matter how hard I try, I’ll always be a social media Luddite: “Am I hashtagging?”
Convenience notwithstanding, this year’s conference-from-home luxury is one we hope to dispense with for SHM Converge 2022, in exchange for wandering of halls, jockeying to be closer to the front of the room, collecting freebies in exhibit halls, and seeing 50 old friends on the way to the session for which you’re already late.
Nashville, Tenn., aka Music City, will be the site of our first in-person meeting in 3 years in April 2022. I will be there with my guitar for SHM’s open mic and I hope you too bring your diverse talents from across the country to spend a week learning and energizing with us, making hospital medicine music in “Honky Tonk Hall,” “Elvis Lives Lounge,” or the “Grand Ol’ Opry-ation Suite.” The band is getting back together! Be a part of the excitement. Bring your voice, bring your talent, and let’s do Nashville in numbers!
Planning is now underway ... and we need your ideas and suggestions! Share thoughts on topics and speakers through the OPEN CALL site through June 1st ... and don’t forget to watch on-demand talks you missed from SHM Converge 2021 – a veritable treasure trove of learning.
Dr. Nye is a hospitalist and professor of medicine at the University of California, San Francisco. She is the course director of SHM Converge 2022.
A hospitalist for 18 years and Annual Conference Committee (ACC) member for the last 4 years, I have always felt immense pride in this meeting. This year, we experienced constant evolution and adapted in ways unimaginable; frameshifts, detours, course corrections, wearing out words like “pivot” and “unprecedented,” whilst contending with virus lulls and surges at hospitals across the country. And SHM Converge 2021 was a landmark success despite it all.
Our SHM community successfully connected through the marvels of modern technology and enjoyed a snappy new logo and name to mark the occasion. Our unflappable course director Dan Steinberg, MD, SFHM, led an intrepid and creative team through uncertainty and produced an extraordinary educational event truly worthy of the term “unprecedented.” ACC members, talented in so many ways, each brought a unique perspective to the planning table to craft a balanced, relevant, and cutting-edge program. The only thing harder than planning a conference for thousands of hospitalists is planning TWO CONFERENCES – one in person, then one virtually.
For their facilitation of virtual adaptation of everything from clinical talks to hot dog sales, our SHM administrative staff deserve a medal. Industry sponsors likewise performed pretzel maneuvers for the virtual interface, and we thank them for their creativity and support. Freshly minted SHM CEO Eric Howell, MD, MHM, kicked off Converge by adeptly filling some very large shoes with aplomb, humor, and humility – telegraphing that our society is in good hands indeed (and that 2020 was NOT the ‘final frontier’). And, finally, each of you, in the suspended reality of a conference hall, tapped into session after session from the comfort of your hometown chairs, indefatigably learning and networking during a pandemic year.
So, beyond adaptability, what did we learn? We renewed our commitment to resilience and wellness in medicine, and reemphasized how critical diversity, equity, and inclusion are in both the workplace and in clinical practice. These topics were complemented by the usual standing-room-only clinical updates and rapid-fire sessions – where everyone could enjoy a front row seat. We talked about parenting in the pandemic, compared clinical approaches in friendly debates – for patients big and small – and deeply dived into leadership strategies for a sustainable workforce.
Here are some SHM Converge 2021 nuggets (Apologies for so few ... there were thousands!):
Plenaries
Eric Howell, MD, MHM
- Make the world a better place, be transparent and act with integrity, invest in others, do what you love.
- SHM has been leading the pack in providing e-learning options, promoting clinician self-care, and intensifying diversity, equity, and inclusion efforts before and throughout the pandemic.
- SHM has 18,000 members, 68 chapters, 26 special interest groups, 15 committees, 12 board of directors, 50 staff – growing and getting stronger every day.
- Rainbows need both rain and sunshine to form.
Gen. Mark Hertling
- Our COVID experience as hospitalists shared many features with active combat, including post-COVID combat fog.
- Use your ears, eyes, and mouth in that order: Listen more, see more, speak less.
Vineet Arora, MD, MHM
- Don’t pass up your “career gates.”
- Find “zero-gravity thinkers” – not innovation killers.
- Keep track of your state of mind using the “Bob Wachter scale.”
U.S. Surgeon Gen. Vivek Murthy, MD, and Danielle Scheurer, MD, SFHM
- Mental health and well-being of clinicians is imperative; “heal thyself” doesn’t work. Culture must support policies to truly craft a more sustaining and rewarding environment.
- We are a nation hyperfocused on episodic and salvage care (and are good at it) but must move the needle toward continuity and prevention. Sadly, nobody celebrates the heart attack that was prevented.
- What can hospitalists do about social determinants of health? Advocate for policies individually or through SHM – if you don’t know how, receive training – this is invaluable. More lobbying as a profession may yield legislation and funding aimed at such determinants and improve healthcare.
Larry Wellikson, MD, MHM
- New models hospitalists may soon inhabit: Hospital at Home, ED+, Micro-Hospitals.
- More than 50% of revenue comes from “vertical” services (outside the hospital) rather than horizontal services (in hospital) – trend to increase efforts in population health initiatives.
- Emphasis on value must go from looking at episodes of care to outcomes.
- Hospitalists Complexologists? Be relevant, add value – survive, thrive, and prosper.
Other sessions
Stroke
- Mobile stroke units are a thing!
- Neurologists are not great at predictions after stroke – but scoring tools are!
- Focus on patient-centered outcomes (100% disability free vs. able to walk vs. happy to be alive).
Drug allergies
- Penicillin allergy: 2% cross-reactivity for cephalosporins – not 10%.
Navigating work/life balance
- Have two phones for work/home – church and state – keep them separate!
Becoming an expert
- Avoid “analysis paralysis”: “Better a good decision quickly than the best decision too late” – H. Geneen
Misc. revelations
- It’s pretty cool to know the Surgeon General is a hospitalist!
- Our SHM community rocks!
- Eric Howell is an avid Star Trek and overalls enthusiast!
- It’s exceedingly difficult to become a MHM – 35 total, 3 this year.
- Danielle Scheurer is a warm and natural interviewer, sensational leader, and closet REM-rapper.
- No matter how hard I try, I’ll always be a social media Luddite: “Am I hashtagging?”
Convenience notwithstanding, this year’s conference-from-home luxury is one we hope to dispense with for SHM Converge 2022, in exchange for wandering of halls, jockeying to be closer to the front of the room, collecting freebies in exhibit halls, and seeing 50 old friends on the way to the session for which you’re already late.
Nashville, Tenn., aka Music City, will be the site of our first in-person meeting in 3 years in April 2022. I will be there with my guitar for SHM’s open mic and I hope you too bring your diverse talents from across the country to spend a week learning and energizing with us, making hospital medicine music in “Honky Tonk Hall,” “Elvis Lives Lounge,” or the “Grand Ol’ Opry-ation Suite.” The band is getting back together! Be a part of the excitement. Bring your voice, bring your talent, and let’s do Nashville in numbers!
Planning is now underway ... and we need your ideas and suggestions! Share thoughts on topics and speakers through the OPEN CALL site through June 1st ... and don’t forget to watch on-demand talks you missed from SHM Converge 2021 – a veritable treasure trove of learning.
Dr. Nye is a hospitalist and professor of medicine at the University of California, San Francisco. She is the course director of SHM Converge 2022.
Less ambulatory care occurred than expected in pandemic, according to study
according to an analysis of national claims data from Jan. 1, 2019, to Oct. 31, 2020.
“The COVID-19 pandemic has seriously disrupted access to U.S. ambulatory care, endangering population health,” said John N. Mafi, MD, of the University of California, Los Angeles, in a presentation at the annual meeting of the Society of General Internal Medicine.
Dr. Mafi and colleagues conducted the analysis, which included 20 monthly cohorts, and measured outpatient visit rates per 100 members across all 20 study months. The researchers used a “difference-in-differences study design” and compared changes in rates of ambulatory care visits in January-February 2019 through September-October 2019 with the same periods in 2020.
They found that overall utilization fell to 68.9% of expected rates. This number increased to 82.6% of expected rates by May-June 2020 and to 87.7% of expected rates by July-August 2020.
To examine the impact of COVID-19 on U.S. ambulatory care patterns, the researchers identified 10.4 million individuals aged 18 years and older using the MedInsight research claims database. This database included Medicaid, commercial, dual eligible (receiving both Medicare and Medicaid benefits), Medicare Advantage (MA), and Medicare fee-for-service (FFS) patients. The average age of the individuals studied was 52 years, and 55% of the population were women. The researchers measured outpatient visit rates per 100 beneficiaries for several types of ambulatory care visits: emergency, urgent care, office, physical exams, preventive, alcohol/drug, and psychiatric care.
The researchers verified parallel trends in visits between 2018 and 2019 to establish a historical benchmark and divided the patient population into three groups based on insurance enrollment (continuously enrolled, not continuously enrolled, and fully enrolled) to account for new members adding insurance and disrupted coverage caused by job losses or other factors. The trends in ambulatory care utilization were similar between cohorts across the groups.
The rebound seen by the summer of 2020 showed variation when broken out by insurance type: 94.0% for Medicare FFS; 88.9% for commercial insurance; 86.3% for Medicare Advantage; 83.6% for dual eligible; and 78.0% for Medicaid.
“The big picture is that utilization looks similar across the three groups and has not attained prepandemic levels,” Dr. Mafi said.
When the results were divided by service type, utilization rates remained below expected rates while needs remain similar for U.S. Preventive Services Task Force–recommended preventive screening services, Dr. Mafi noted. The demand for psychiatric and substance use services has increased, but use rates are below expected rates. In addition, both avoidable and nonavoidable ED utilization both remained below expected rates.
In-person visits are down across insurance groups, but virtual visits are skyrocketing, across all insurance groups, Dr. Mafi added. However, virtual care visits have not completely compensated for declines in in-person visits, notably among dual-eligible and Medicaid insurance members.
Takeaways for policy makers include the fact that, while some reductions in unnecessary care, such as avoidable ED visits, may be beneficial, the “reduced USPSTF-recommended cancer and other evidence-based disease prevention may worsen health outcomes, particularly for Medicaid beneficiaries,” he said.
Outreach and outcomes
The study is important because “understanding ambulatory care patterns during the pandemic can highlight vulnerabilities and opportunities in our health care system,” Dr. Mafi said in an interview.
“While the COVID-19 pandemic has seriously disrupted access to U.S. ambulatory care, most studies have focused on the early months of the pandemic,” he noted.
Dr. Mafi said he was not surprised that ambulatory care utilization has not rebounded among Medicaid beneficiaries relative to other insurance groups.
“Medicaid beneficiaries are underresourced individuals who are disproportionately racial/ethnic minorities, and they historically have had difficulties accessing care. Our data suggest that the COVID-19 pandemic may be widening these preexisting inequities in access to ambulatory care,” he observed.
The study findings were limited by the use of the MedInsight research dataset, which is a convenience sample; and, therefore, the results might not be generalizable nationally, Dr. Mafi said. “However, it does include beneficiaries from all major insurance types across all 50 U.S. states. Additionally, our analysis was completed at the population level rather than the patient level, and so we were unable to account for patient-level characteristics such as clinical complexity,” he explained.
“The take-home message for clinicians is that our patients with Medicaid insurance may need additional efforts to overcome barriers to accessing ambulatory care, such as creating robust telemedicine outreach programs,” said Dr. Mafi. “Policy makers should also consider providing additional support and resources to safety net health systems who disproportionately care for Medicaid beneficiaries, such as higher reimbursements for both in-person and telemedicine visits.”
More research is needed, he emphasized. “We urgently need further inquiry into the impact of this persistently deferred ambulatory care utilization on important health outcomes such as preventable death/disability and quality of care.”
COVID consequences challenge ambulatory care
“These study findings mirror what we are seeing in primary care settings,” Maureen Lyons, MD, of Washington University. St. Louis, said in an interview. “With the pandemic, there are many additional barriers for patients accessing care, and these barriers have disproportionately impacted those who are already disadvantaged.
“From clinical experience, there are barriers directly related to COVID-19, such as the risk of infection or discomfort being in a clinic setting with other people. However, there also are barriers related to change in financial situation or insurance related to changes or loss of employment,” she said.
“Additionally, many patients have needed to take on increased responsibilities in other areas of their lives, such as caring for an ill family member or being responsible for children’s virtual school,” she said. These new responsibilities can lead people to skip or postpone ambulatory care visits.
“Loss of ambulatory care is likely to lead to increases in preventable illnesses with long-lasting effects,” Dr. Lyons noted. “Studying this in a robust fashion, as Dr. Mafi and colleagues have done, is a critical step in understanding and addressing this urgent need.”
Dr. Mafi noted that the data he presented is preliminary, and that he and his team hope to publish finalized estimates of ambulatory utilization rates in a forthcoming scientific paper.
The study was a collaboration between UCLA and Millman MedInsight, an actuarial health analytics company. Several coauthors are Millman employees. Dr. Mafi and the other researchers had no other relevant financial conflicts to disclose. Dr. Lyons had no financial conflicts to disclose.
according to an analysis of national claims data from Jan. 1, 2019, to Oct. 31, 2020.
“The COVID-19 pandemic has seriously disrupted access to U.S. ambulatory care, endangering population health,” said John N. Mafi, MD, of the University of California, Los Angeles, in a presentation at the annual meeting of the Society of General Internal Medicine.
Dr. Mafi and colleagues conducted the analysis, which included 20 monthly cohorts, and measured outpatient visit rates per 100 members across all 20 study months. The researchers used a “difference-in-differences study design” and compared changes in rates of ambulatory care visits in January-February 2019 through September-October 2019 with the same periods in 2020.
They found that overall utilization fell to 68.9% of expected rates. This number increased to 82.6% of expected rates by May-June 2020 and to 87.7% of expected rates by July-August 2020.
To examine the impact of COVID-19 on U.S. ambulatory care patterns, the researchers identified 10.4 million individuals aged 18 years and older using the MedInsight research claims database. This database included Medicaid, commercial, dual eligible (receiving both Medicare and Medicaid benefits), Medicare Advantage (MA), and Medicare fee-for-service (FFS) patients. The average age of the individuals studied was 52 years, and 55% of the population were women. The researchers measured outpatient visit rates per 100 beneficiaries for several types of ambulatory care visits: emergency, urgent care, office, physical exams, preventive, alcohol/drug, and psychiatric care.
The researchers verified parallel trends in visits between 2018 and 2019 to establish a historical benchmark and divided the patient population into three groups based on insurance enrollment (continuously enrolled, not continuously enrolled, and fully enrolled) to account for new members adding insurance and disrupted coverage caused by job losses or other factors. The trends in ambulatory care utilization were similar between cohorts across the groups.
The rebound seen by the summer of 2020 showed variation when broken out by insurance type: 94.0% for Medicare FFS; 88.9% for commercial insurance; 86.3% for Medicare Advantage; 83.6% for dual eligible; and 78.0% for Medicaid.
“The big picture is that utilization looks similar across the three groups and has not attained prepandemic levels,” Dr. Mafi said.
When the results were divided by service type, utilization rates remained below expected rates while needs remain similar for U.S. Preventive Services Task Force–recommended preventive screening services, Dr. Mafi noted. The demand for psychiatric and substance use services has increased, but use rates are below expected rates. In addition, both avoidable and nonavoidable ED utilization both remained below expected rates.
In-person visits are down across insurance groups, but virtual visits are skyrocketing, across all insurance groups, Dr. Mafi added. However, virtual care visits have not completely compensated for declines in in-person visits, notably among dual-eligible and Medicaid insurance members.
Takeaways for policy makers include the fact that, while some reductions in unnecessary care, such as avoidable ED visits, may be beneficial, the “reduced USPSTF-recommended cancer and other evidence-based disease prevention may worsen health outcomes, particularly for Medicaid beneficiaries,” he said.
Outreach and outcomes
The study is important because “understanding ambulatory care patterns during the pandemic can highlight vulnerabilities and opportunities in our health care system,” Dr. Mafi said in an interview.
“While the COVID-19 pandemic has seriously disrupted access to U.S. ambulatory care, most studies have focused on the early months of the pandemic,” he noted.
Dr. Mafi said he was not surprised that ambulatory care utilization has not rebounded among Medicaid beneficiaries relative to other insurance groups.
“Medicaid beneficiaries are underresourced individuals who are disproportionately racial/ethnic minorities, and they historically have had difficulties accessing care. Our data suggest that the COVID-19 pandemic may be widening these preexisting inequities in access to ambulatory care,” he observed.
The study findings were limited by the use of the MedInsight research dataset, which is a convenience sample; and, therefore, the results might not be generalizable nationally, Dr. Mafi said. “However, it does include beneficiaries from all major insurance types across all 50 U.S. states. Additionally, our analysis was completed at the population level rather than the patient level, and so we were unable to account for patient-level characteristics such as clinical complexity,” he explained.
“The take-home message for clinicians is that our patients with Medicaid insurance may need additional efforts to overcome barriers to accessing ambulatory care, such as creating robust telemedicine outreach programs,” said Dr. Mafi. “Policy makers should also consider providing additional support and resources to safety net health systems who disproportionately care for Medicaid beneficiaries, such as higher reimbursements for both in-person and telemedicine visits.”
More research is needed, he emphasized. “We urgently need further inquiry into the impact of this persistently deferred ambulatory care utilization on important health outcomes such as preventable death/disability and quality of care.”
COVID consequences challenge ambulatory care
“These study findings mirror what we are seeing in primary care settings,” Maureen Lyons, MD, of Washington University. St. Louis, said in an interview. “With the pandemic, there are many additional barriers for patients accessing care, and these barriers have disproportionately impacted those who are already disadvantaged.
“From clinical experience, there are barriers directly related to COVID-19, such as the risk of infection or discomfort being in a clinic setting with other people. However, there also are barriers related to change in financial situation or insurance related to changes or loss of employment,” she said.
“Additionally, many patients have needed to take on increased responsibilities in other areas of their lives, such as caring for an ill family member or being responsible for children’s virtual school,” she said. These new responsibilities can lead people to skip or postpone ambulatory care visits.
“Loss of ambulatory care is likely to lead to increases in preventable illnesses with long-lasting effects,” Dr. Lyons noted. “Studying this in a robust fashion, as Dr. Mafi and colleagues have done, is a critical step in understanding and addressing this urgent need.”
Dr. Mafi noted that the data he presented is preliminary, and that he and his team hope to publish finalized estimates of ambulatory utilization rates in a forthcoming scientific paper.
The study was a collaboration between UCLA and Millman MedInsight, an actuarial health analytics company. Several coauthors are Millman employees. Dr. Mafi and the other researchers had no other relevant financial conflicts to disclose. Dr. Lyons had no financial conflicts to disclose.
according to an analysis of national claims data from Jan. 1, 2019, to Oct. 31, 2020.
“The COVID-19 pandemic has seriously disrupted access to U.S. ambulatory care, endangering population health,” said John N. Mafi, MD, of the University of California, Los Angeles, in a presentation at the annual meeting of the Society of General Internal Medicine.
Dr. Mafi and colleagues conducted the analysis, which included 20 monthly cohorts, and measured outpatient visit rates per 100 members across all 20 study months. The researchers used a “difference-in-differences study design” and compared changes in rates of ambulatory care visits in January-February 2019 through September-October 2019 with the same periods in 2020.
They found that overall utilization fell to 68.9% of expected rates. This number increased to 82.6% of expected rates by May-June 2020 and to 87.7% of expected rates by July-August 2020.
To examine the impact of COVID-19 on U.S. ambulatory care patterns, the researchers identified 10.4 million individuals aged 18 years and older using the MedInsight research claims database. This database included Medicaid, commercial, dual eligible (receiving both Medicare and Medicaid benefits), Medicare Advantage (MA), and Medicare fee-for-service (FFS) patients. The average age of the individuals studied was 52 years, and 55% of the population were women. The researchers measured outpatient visit rates per 100 beneficiaries for several types of ambulatory care visits: emergency, urgent care, office, physical exams, preventive, alcohol/drug, and psychiatric care.
The researchers verified parallel trends in visits between 2018 and 2019 to establish a historical benchmark and divided the patient population into three groups based on insurance enrollment (continuously enrolled, not continuously enrolled, and fully enrolled) to account for new members adding insurance and disrupted coverage caused by job losses or other factors. The trends in ambulatory care utilization were similar between cohorts across the groups.
The rebound seen by the summer of 2020 showed variation when broken out by insurance type: 94.0% for Medicare FFS; 88.9% for commercial insurance; 86.3% for Medicare Advantage; 83.6% for dual eligible; and 78.0% for Medicaid.
“The big picture is that utilization looks similar across the three groups and has not attained prepandemic levels,” Dr. Mafi said.
When the results were divided by service type, utilization rates remained below expected rates while needs remain similar for U.S. Preventive Services Task Force–recommended preventive screening services, Dr. Mafi noted. The demand for psychiatric and substance use services has increased, but use rates are below expected rates. In addition, both avoidable and nonavoidable ED utilization both remained below expected rates.
In-person visits are down across insurance groups, but virtual visits are skyrocketing, across all insurance groups, Dr. Mafi added. However, virtual care visits have not completely compensated for declines in in-person visits, notably among dual-eligible and Medicaid insurance members.
Takeaways for policy makers include the fact that, while some reductions in unnecessary care, such as avoidable ED visits, may be beneficial, the “reduced USPSTF-recommended cancer and other evidence-based disease prevention may worsen health outcomes, particularly for Medicaid beneficiaries,” he said.
Outreach and outcomes
The study is important because “understanding ambulatory care patterns during the pandemic can highlight vulnerabilities and opportunities in our health care system,” Dr. Mafi said in an interview.
“While the COVID-19 pandemic has seriously disrupted access to U.S. ambulatory care, most studies have focused on the early months of the pandemic,” he noted.
Dr. Mafi said he was not surprised that ambulatory care utilization has not rebounded among Medicaid beneficiaries relative to other insurance groups.
“Medicaid beneficiaries are underresourced individuals who are disproportionately racial/ethnic minorities, and they historically have had difficulties accessing care. Our data suggest that the COVID-19 pandemic may be widening these preexisting inequities in access to ambulatory care,” he observed.
The study findings were limited by the use of the MedInsight research dataset, which is a convenience sample; and, therefore, the results might not be generalizable nationally, Dr. Mafi said. “However, it does include beneficiaries from all major insurance types across all 50 U.S. states. Additionally, our analysis was completed at the population level rather than the patient level, and so we were unable to account for patient-level characteristics such as clinical complexity,” he explained.
“The take-home message for clinicians is that our patients with Medicaid insurance may need additional efforts to overcome barriers to accessing ambulatory care, such as creating robust telemedicine outreach programs,” said Dr. Mafi. “Policy makers should also consider providing additional support and resources to safety net health systems who disproportionately care for Medicaid beneficiaries, such as higher reimbursements for both in-person and telemedicine visits.”
More research is needed, he emphasized. “We urgently need further inquiry into the impact of this persistently deferred ambulatory care utilization on important health outcomes such as preventable death/disability and quality of care.”
COVID consequences challenge ambulatory care
“These study findings mirror what we are seeing in primary care settings,” Maureen Lyons, MD, of Washington University. St. Louis, said in an interview. “With the pandemic, there are many additional barriers for patients accessing care, and these barriers have disproportionately impacted those who are already disadvantaged.
“From clinical experience, there are barriers directly related to COVID-19, such as the risk of infection or discomfort being in a clinic setting with other people. However, there also are barriers related to change in financial situation or insurance related to changes or loss of employment,” she said.
“Additionally, many patients have needed to take on increased responsibilities in other areas of their lives, such as caring for an ill family member or being responsible for children’s virtual school,” she said. These new responsibilities can lead people to skip or postpone ambulatory care visits.
“Loss of ambulatory care is likely to lead to increases in preventable illnesses with long-lasting effects,” Dr. Lyons noted. “Studying this in a robust fashion, as Dr. Mafi and colleagues have done, is a critical step in understanding and addressing this urgent need.”
Dr. Mafi noted that the data he presented is preliminary, and that he and his team hope to publish finalized estimates of ambulatory utilization rates in a forthcoming scientific paper.
The study was a collaboration between UCLA and Millman MedInsight, an actuarial health analytics company. Several coauthors are Millman employees. Dr. Mafi and the other researchers had no other relevant financial conflicts to disclose. Dr. Lyons had no financial conflicts to disclose.
FROM SGIM 2021
Novel rehab program fights frailty, boosts capacity in advanced HF
A novel physical rehabilitation program for patients with advanced heart failure that aimed to improve their ability to exercise before focusing on endurance was successful in a randomized trial in ways that seem to have eluded some earlier exercise-training studies in the setting of HF.
The often-frail patients following the training regimen, initiated before discharge from hospitalization for acute decompensation, worked on capabilities such as mobility, balance, and strength deemed necessary if exercises meant to build exercise capacity were to succeed.
A huge percentage stayed with the 12-week program, which featured personalized, one-on-one training from a physical therapist. The patients benefited, with improvements in balance, walking ability, and strength, which were followed by significant gains in 6-minute walk distance (6MWD) and measures of physical functioning, frailty, and quality of life. The patients then continued elements of the program at home out to 6 months.
At that time, death and rehospitalizations did not differ between those assigned to the regimen and similar patients who had not participated in the program, although the trial wasn’t powered for clinical events.
The rehab strategy seemed to work across a wide range of patient subgroups. In particular, there was evidence that the benefits were more pronounced in patients with HF and preserved ejection fraction (HFpEF) than in those with HF and reduced ejection fraction (HFrEF), observed Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C.
Dr. Kitzman presented results from the REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial at the annual scientific sessions of the American College of Cardiology and is lead author on its same-day publication in the New England Journal of Medicine.
An earlier pilot program unexpectedly showed that such patients recently hospitalized with HF “have significant impairments in mobility and balance,” he explained. If so, “it would be hazardous to subject them to traditional endurance training, such as walking-based treadmill or even bicycle.”
The unusual program, said Dr. Kitzman, looks to those issues before engaging the patients in endurance exercise by addressing mobility, balance, and basic strength – enough to repeatedly stand up from a sitting position, for example. “If you’re not able to stand with confidence, then you’re not able to walk on a treadmill.”
This model of exercise rehab “is used in geriatrics research, and enables them to safely increase endurance. It’s well known from geriatric studies that if you go directly to endurance in these, frail, older patients, you have little improvement and often have injuries and falls,” he added.
Guidance from telemedicine?
The functional outcomes examined in REHAB-HF “are the ones that matter to patients the most,” observed Eileen M. Handberg, PhD, of Shands Hospital at the University of Florida, Gainesville, at a presentation on the trial for the media.
“This is about being able to get out of a chair without assistance, not falling, walking farther, and feeling better as opposed to the more traditional outcome measure that has been used in cardiac rehab trials, which has been the exercise treadmill test – which most patients don’t have the capacity to do very well anyway,” said Dr. Handberg, who is not a part of REHAB-HF.
“This opens up rehab, potentially, to the more sick, who also need a better quality of life,” she said.
However, many patients invited to participate in the trial could not because they lived too far from the program, Dr. Handberg observed. “It would be nice to see if the lessons from COVID-19 might apply to this population” by making participation possible remotely, “perhaps using family members as rehab assistance,” she said.
“I was really very impressed that you had 83% adherence to a home exercise 6 months down the road, which far eclipses what we had in HF-ACTION,” said Vera Bittner, MD, University of Alabama at Birmingham, as the invited discussant following Dr. Kitzman’s formal presentation of the trial. “And it certainly eclipses what we see in the typical cardiac rehab program.”
Both Dr. Bittner and Dr. Kitzman participated in HF-ACTION, a randomized exercise-training trial for patients with chronic, stable HFrEF who were all-around less sick than those in REHAB-HF.
Four functional domains
Historically, HF exercise or rehab trials have excluded patients hospitalized with acute decompensation, and third-party reimbursement often has not covered such programs because of a lack of supporting evidence and a supposed potential for harm, Dr. Kitzman said.
Entry to REHAB-HF required the patients to be fit enough to walk 4 meters, with or without a walker or other assistant device, and to have been in the hospital for at least 24 hours with a primary diagnosis of acute decompensated HF.
The intervention relied on exercises aimed at improving the four functional domains of strength, balance, mobility, and – when those three were sufficiently developed – endurance, Dr. Kitzman and associates wrote in their published report.
“The intervention was initiated in the hospital when feasible and was subsequently transitioned to an outpatient facility as soon as possible after discharge,” they wrote. Afterward, “a key goal of the intervention during the first 3 months [the outpatient phase] was to prepare the patient to transition to the independent maintenance phase (months 4-6).”
The study’s control patients “received frequent calls from study staff to try to approximate the increased attention received by the intervention group,” Dr. Kitzman said in an interview. “They were allowed to receive all usual care as ordered by their treating physicians. This included, if ordered, standard physical therapy or cardiac rehabilitation” in 43% of the control cohort. Of the trial’s 349 patients, those assigned to the intervention scored significantly higher on the three-component Short Physical Performance Battery (SPPB) at 12 weeks than those assigned to a usual care approach that included, for some, more conventional cardiac rehabilitation (8.3 vs. 6.9; P < .001).
The SPPB, validated in trials as a proxy for clinical outcomes includes tests of balance while standing, gait speed during a 4-minute walk, and strength. The latter is the test that measures time needed to rise from a chair five times.
They also showed consistent gains in other measures of physical functioning and quality of life by 12 weeks months.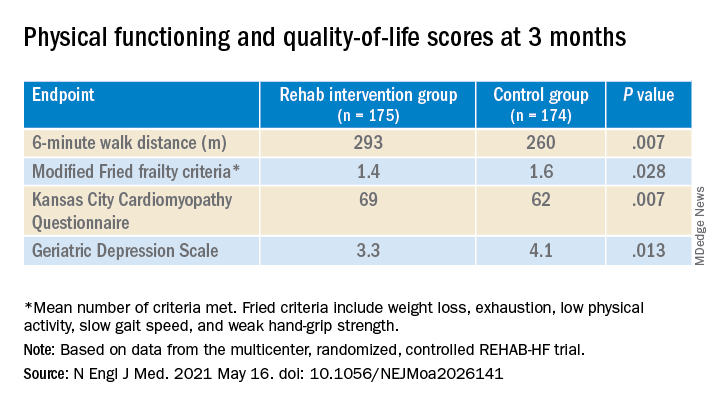
The observed SPPB treatment effect is “impressive” and “compares very favorably with previously reported estimates,” observed an accompanying editorial from Stefan D. Anker, MD, PhD, of the German Center for Cardiovascular Research and Charité Universitätsmedizin, Berlin, and Andrew J.S. Coats, DM, of the University of Warwick, Coventry, England.
“Similarly, the between-group differences seen in 6-minute walk distance (34 m) and gait speed (0.12 m/s) are clinically meaningful and sizable.”
They propose that some of the substantial quality-of-life benefit in the intervention group “may be due to better physical performance, and that part may be due to improvements in psychosocial factors and mood. It appears that exercise also resulted in patients becoming happier, or at least less depressed, as evidenced by the positive results on the Geriatric Depression Scale.”
Similar results across most subgroups
In subgroup analyses, the intervention was successful against the standard-care approach in both men and women at all ages and regardless of ejection fraction; symptom status; and whether the patient had diabetes, ischemic heart disease, or atrial fibrillation, or was obese.
Clinical outcomes were not significantly different at 6 months. The rate of death from any cause was 13% for the intervention group and 10% for the control group. There were 194 and 213 hospitalizations from any cause, respectively.
Not included in the trial’s current publication but soon to be published, Dr. Kitzman said when interviewed, is a comparison of outcomes in patients with HFpEF and HFrEF. “We found at baseline that those with HFpEF had worse impairment in physical function, quality of life, and frailty. After the intervention, there appeared to be consistently larger improvements in all outcomes, including SPPB, 6-minute walk, qualify of life, and frailty, in HFpEF versus HFrEF.”
The signals of potential benefit in HFpEF extended to clinical endpoints, he said. In contrast to similar rates of all-cause rehospitalization in HFrEF, “in patients with HFpEF, rehospitalizations were 17% lower in the intervention group, compared to the control group.” Still, he noted, the interaction P value wasn’t significant.
However, Dr. Kitzman added, mortality in the intervention group, compared with the control group, was reduced by 35% among patients with HFpEF, “but was 250% higher in HFrEF,” with a significant interaction P value.
He was careful to note that, as a phase 2 trial, REHAB-HF was underpowered for clinical events, “and even the results in the HFpEF group should not be seen as adequate evidence to change clinical care.” They were from an exploratory analysis that included relatively few events.
“Because definitive demonstration of improvement in clinical events is critical for altering clinical care guidelines and for third-party payer reimbursement decisions, we believe that a subsequent phase 3 trial is needed and are currently planning toward that,” Dr. Kitzman said.
The study was supported by research grants from the National Institutes of Health, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine, and the Oristano Family Fund at Wake Forest. Dr. Kitzman disclosed receiving consulting fees or honoraria from AbbVie, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, CinRx, Corviamedical, GlaxoSmithKline, and Merck; and having an unspecified relationship with Gilead. Dr. Handberg disclosed receiving grants from Aastom Biosciences, Abbott Laboratories, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Everyfit, Gilead, Ionis, Medtronic, Merck, Mesoblast, Relypsa, and Sanofi-Aventis. Dr. Bittner discloses receiving consulting fees or honoraria from Pfizer and Sanofi; receiving research grants from Amgen and The Medicines Company; and having unspecified relationships with AstraZeneca, DalCor, Esperion, and Sanofi-Aventis. Dr. Anker reported receiving grants and personal fees from Abbott Vascular and Vifor; personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Cardiac Dimensions, Thermo Fisher Scientific, AstraZeneca, Occlutech, Actimed, and Respicardia. Dr. Coats disclosed receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia.
A version of this article first appeared on Medscape.com.
A novel physical rehabilitation program for patients with advanced heart failure that aimed to improve their ability to exercise before focusing on endurance was successful in a randomized trial in ways that seem to have eluded some earlier exercise-training studies in the setting of HF.
The often-frail patients following the training regimen, initiated before discharge from hospitalization for acute decompensation, worked on capabilities such as mobility, balance, and strength deemed necessary if exercises meant to build exercise capacity were to succeed.
A huge percentage stayed with the 12-week program, which featured personalized, one-on-one training from a physical therapist. The patients benefited, with improvements in balance, walking ability, and strength, which were followed by significant gains in 6-minute walk distance (6MWD) and measures of physical functioning, frailty, and quality of life. The patients then continued elements of the program at home out to 6 months.
At that time, death and rehospitalizations did not differ between those assigned to the regimen and similar patients who had not participated in the program, although the trial wasn’t powered for clinical events.
The rehab strategy seemed to work across a wide range of patient subgroups. In particular, there was evidence that the benefits were more pronounced in patients with HF and preserved ejection fraction (HFpEF) than in those with HF and reduced ejection fraction (HFrEF), observed Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C.
Dr. Kitzman presented results from the REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial at the annual scientific sessions of the American College of Cardiology and is lead author on its same-day publication in the New England Journal of Medicine.
An earlier pilot program unexpectedly showed that such patients recently hospitalized with HF “have significant impairments in mobility and balance,” he explained. If so, “it would be hazardous to subject them to traditional endurance training, such as walking-based treadmill or even bicycle.”
The unusual program, said Dr. Kitzman, looks to those issues before engaging the patients in endurance exercise by addressing mobility, balance, and basic strength – enough to repeatedly stand up from a sitting position, for example. “If you’re not able to stand with confidence, then you’re not able to walk on a treadmill.”
This model of exercise rehab “is used in geriatrics research, and enables them to safely increase endurance. It’s well known from geriatric studies that if you go directly to endurance in these, frail, older patients, you have little improvement and often have injuries and falls,” he added.
Guidance from telemedicine?
The functional outcomes examined in REHAB-HF “are the ones that matter to patients the most,” observed Eileen M. Handberg, PhD, of Shands Hospital at the University of Florida, Gainesville, at a presentation on the trial for the media.
“This is about being able to get out of a chair without assistance, not falling, walking farther, and feeling better as opposed to the more traditional outcome measure that has been used in cardiac rehab trials, which has been the exercise treadmill test – which most patients don’t have the capacity to do very well anyway,” said Dr. Handberg, who is not a part of REHAB-HF.
“This opens up rehab, potentially, to the more sick, who also need a better quality of life,” she said.
However, many patients invited to participate in the trial could not because they lived too far from the program, Dr. Handberg observed. “It would be nice to see if the lessons from COVID-19 might apply to this population” by making participation possible remotely, “perhaps using family members as rehab assistance,” she said.
“I was really very impressed that you had 83% adherence to a home exercise 6 months down the road, which far eclipses what we had in HF-ACTION,” said Vera Bittner, MD, University of Alabama at Birmingham, as the invited discussant following Dr. Kitzman’s formal presentation of the trial. “And it certainly eclipses what we see in the typical cardiac rehab program.”
Both Dr. Bittner and Dr. Kitzman participated in HF-ACTION, a randomized exercise-training trial for patients with chronic, stable HFrEF who were all-around less sick than those in REHAB-HF.
Four functional domains
Historically, HF exercise or rehab trials have excluded patients hospitalized with acute decompensation, and third-party reimbursement often has not covered such programs because of a lack of supporting evidence and a supposed potential for harm, Dr. Kitzman said.
Entry to REHAB-HF required the patients to be fit enough to walk 4 meters, with or without a walker or other assistant device, and to have been in the hospital for at least 24 hours with a primary diagnosis of acute decompensated HF.
The intervention relied on exercises aimed at improving the four functional domains of strength, balance, mobility, and – when those three were sufficiently developed – endurance, Dr. Kitzman and associates wrote in their published report.
“The intervention was initiated in the hospital when feasible and was subsequently transitioned to an outpatient facility as soon as possible after discharge,” they wrote. Afterward, “a key goal of the intervention during the first 3 months [the outpatient phase] was to prepare the patient to transition to the independent maintenance phase (months 4-6).”
The study’s control patients “received frequent calls from study staff to try to approximate the increased attention received by the intervention group,” Dr. Kitzman said in an interview. “They were allowed to receive all usual care as ordered by their treating physicians. This included, if ordered, standard physical therapy or cardiac rehabilitation” in 43% of the control cohort. Of the trial’s 349 patients, those assigned to the intervention scored significantly higher on the three-component Short Physical Performance Battery (SPPB) at 12 weeks than those assigned to a usual care approach that included, for some, more conventional cardiac rehabilitation (8.3 vs. 6.9; P < .001).
The SPPB, validated in trials as a proxy for clinical outcomes includes tests of balance while standing, gait speed during a 4-minute walk, and strength. The latter is the test that measures time needed to rise from a chair five times.
They also showed consistent gains in other measures of physical functioning and quality of life by 12 weeks months.
The observed SPPB treatment effect is “impressive” and “compares very favorably with previously reported estimates,” observed an accompanying editorial from Stefan D. Anker, MD, PhD, of the German Center for Cardiovascular Research and Charité Universitätsmedizin, Berlin, and Andrew J.S. Coats, DM, of the University of Warwick, Coventry, England.
“Similarly, the between-group differences seen in 6-minute walk distance (34 m) and gait speed (0.12 m/s) are clinically meaningful and sizable.”
They propose that some of the substantial quality-of-life benefit in the intervention group “may be due to better physical performance, and that part may be due to improvements in psychosocial factors and mood. It appears that exercise also resulted in patients becoming happier, or at least less depressed, as evidenced by the positive results on the Geriatric Depression Scale.”
Similar results across most subgroups
In subgroup analyses, the intervention was successful against the standard-care approach in both men and women at all ages and regardless of ejection fraction; symptom status; and whether the patient had diabetes, ischemic heart disease, or atrial fibrillation, or was obese.
Clinical outcomes were not significantly different at 6 months. The rate of death from any cause was 13% for the intervention group and 10% for the control group. There were 194 and 213 hospitalizations from any cause, respectively.
Not included in the trial’s current publication but soon to be published, Dr. Kitzman said when interviewed, is a comparison of outcomes in patients with HFpEF and HFrEF. “We found at baseline that those with HFpEF had worse impairment in physical function, quality of life, and frailty. After the intervention, there appeared to be consistently larger improvements in all outcomes, including SPPB, 6-minute walk, qualify of life, and frailty, in HFpEF versus HFrEF.”
The signals of potential benefit in HFpEF extended to clinical endpoints, he said. In contrast to similar rates of all-cause rehospitalization in HFrEF, “in patients with HFpEF, rehospitalizations were 17% lower in the intervention group, compared to the control group.” Still, he noted, the interaction P value wasn’t significant.
However, Dr. Kitzman added, mortality in the intervention group, compared with the control group, was reduced by 35% among patients with HFpEF, “but was 250% higher in HFrEF,” with a significant interaction P value.
He was careful to note that, as a phase 2 trial, REHAB-HF was underpowered for clinical events, “and even the results in the HFpEF group should not be seen as adequate evidence to change clinical care.” They were from an exploratory analysis that included relatively few events.
“Because definitive demonstration of improvement in clinical events is critical for altering clinical care guidelines and for third-party payer reimbursement decisions, we believe that a subsequent phase 3 trial is needed and are currently planning toward that,” Dr. Kitzman said.
The study was supported by research grants from the National Institutes of Health, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine, and the Oristano Family Fund at Wake Forest. Dr. Kitzman disclosed receiving consulting fees or honoraria from AbbVie, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, CinRx, Corviamedical, GlaxoSmithKline, and Merck; and having an unspecified relationship with Gilead. Dr. Handberg disclosed receiving grants from Aastom Biosciences, Abbott Laboratories, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Everyfit, Gilead, Ionis, Medtronic, Merck, Mesoblast, Relypsa, and Sanofi-Aventis. Dr. Bittner discloses receiving consulting fees or honoraria from Pfizer and Sanofi; receiving research grants from Amgen and The Medicines Company; and having unspecified relationships with AstraZeneca, DalCor, Esperion, and Sanofi-Aventis. Dr. Anker reported receiving grants and personal fees from Abbott Vascular and Vifor; personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Cardiac Dimensions, Thermo Fisher Scientific, AstraZeneca, Occlutech, Actimed, and Respicardia. Dr. Coats disclosed receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia.
A version of this article first appeared on Medscape.com.
A novel physical rehabilitation program for patients with advanced heart failure that aimed to improve their ability to exercise before focusing on endurance was successful in a randomized trial in ways that seem to have eluded some earlier exercise-training studies in the setting of HF.
The often-frail patients following the training regimen, initiated before discharge from hospitalization for acute decompensation, worked on capabilities such as mobility, balance, and strength deemed necessary if exercises meant to build exercise capacity were to succeed.
A huge percentage stayed with the 12-week program, which featured personalized, one-on-one training from a physical therapist. The patients benefited, with improvements in balance, walking ability, and strength, which were followed by significant gains in 6-minute walk distance (6MWD) and measures of physical functioning, frailty, and quality of life. The patients then continued elements of the program at home out to 6 months.
At that time, death and rehospitalizations did not differ between those assigned to the regimen and similar patients who had not participated in the program, although the trial wasn’t powered for clinical events.
The rehab strategy seemed to work across a wide range of patient subgroups. In particular, there was evidence that the benefits were more pronounced in patients with HF and preserved ejection fraction (HFpEF) than in those with HF and reduced ejection fraction (HFrEF), observed Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C.
Dr. Kitzman presented results from the REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial at the annual scientific sessions of the American College of Cardiology and is lead author on its same-day publication in the New England Journal of Medicine.
An earlier pilot program unexpectedly showed that such patients recently hospitalized with HF “have significant impairments in mobility and balance,” he explained. If so, “it would be hazardous to subject them to traditional endurance training, such as walking-based treadmill or even bicycle.”
The unusual program, said Dr. Kitzman, looks to those issues before engaging the patients in endurance exercise by addressing mobility, balance, and basic strength – enough to repeatedly stand up from a sitting position, for example. “If you’re not able to stand with confidence, then you’re not able to walk on a treadmill.”
This model of exercise rehab “is used in geriatrics research, and enables them to safely increase endurance. It’s well known from geriatric studies that if you go directly to endurance in these, frail, older patients, you have little improvement and often have injuries and falls,” he added.
Guidance from telemedicine?
The functional outcomes examined in REHAB-HF “are the ones that matter to patients the most,” observed Eileen M. Handberg, PhD, of Shands Hospital at the University of Florida, Gainesville, at a presentation on the trial for the media.
“This is about being able to get out of a chair without assistance, not falling, walking farther, and feeling better as opposed to the more traditional outcome measure that has been used in cardiac rehab trials, which has been the exercise treadmill test – which most patients don’t have the capacity to do very well anyway,” said Dr. Handberg, who is not a part of REHAB-HF.
“This opens up rehab, potentially, to the more sick, who also need a better quality of life,” she said.
However, many patients invited to participate in the trial could not because they lived too far from the program, Dr. Handberg observed. “It would be nice to see if the lessons from COVID-19 might apply to this population” by making participation possible remotely, “perhaps using family members as rehab assistance,” she said.
“I was really very impressed that you had 83% adherence to a home exercise 6 months down the road, which far eclipses what we had in HF-ACTION,” said Vera Bittner, MD, University of Alabama at Birmingham, as the invited discussant following Dr. Kitzman’s formal presentation of the trial. “And it certainly eclipses what we see in the typical cardiac rehab program.”
Both Dr. Bittner and Dr. Kitzman participated in HF-ACTION, a randomized exercise-training trial for patients with chronic, stable HFrEF who were all-around less sick than those in REHAB-HF.
Four functional domains
Historically, HF exercise or rehab trials have excluded patients hospitalized with acute decompensation, and third-party reimbursement often has not covered such programs because of a lack of supporting evidence and a supposed potential for harm, Dr. Kitzman said.
Entry to REHAB-HF required the patients to be fit enough to walk 4 meters, with or without a walker or other assistant device, and to have been in the hospital for at least 24 hours with a primary diagnosis of acute decompensated HF.
The intervention relied on exercises aimed at improving the four functional domains of strength, balance, mobility, and – when those three were sufficiently developed – endurance, Dr. Kitzman and associates wrote in their published report.
“The intervention was initiated in the hospital when feasible and was subsequently transitioned to an outpatient facility as soon as possible after discharge,” they wrote. Afterward, “a key goal of the intervention during the first 3 months [the outpatient phase] was to prepare the patient to transition to the independent maintenance phase (months 4-6).”
The study’s control patients “received frequent calls from study staff to try to approximate the increased attention received by the intervention group,” Dr. Kitzman said in an interview. “They were allowed to receive all usual care as ordered by their treating physicians. This included, if ordered, standard physical therapy or cardiac rehabilitation” in 43% of the control cohort. Of the trial’s 349 patients, those assigned to the intervention scored significantly higher on the three-component Short Physical Performance Battery (SPPB) at 12 weeks than those assigned to a usual care approach that included, for some, more conventional cardiac rehabilitation (8.3 vs. 6.9; P < .001).
The SPPB, validated in trials as a proxy for clinical outcomes includes tests of balance while standing, gait speed during a 4-minute walk, and strength. The latter is the test that measures time needed to rise from a chair five times.
They also showed consistent gains in other measures of physical functioning and quality of life by 12 weeks months.
The observed SPPB treatment effect is “impressive” and “compares very favorably with previously reported estimates,” observed an accompanying editorial from Stefan D. Anker, MD, PhD, of the German Center for Cardiovascular Research and Charité Universitätsmedizin, Berlin, and Andrew J.S. Coats, DM, of the University of Warwick, Coventry, England.
“Similarly, the between-group differences seen in 6-minute walk distance (34 m) and gait speed (0.12 m/s) are clinically meaningful and sizable.”
They propose that some of the substantial quality-of-life benefit in the intervention group “may be due to better physical performance, and that part may be due to improvements in psychosocial factors and mood. It appears that exercise also resulted in patients becoming happier, or at least less depressed, as evidenced by the positive results on the Geriatric Depression Scale.”
Similar results across most subgroups
In subgroup analyses, the intervention was successful against the standard-care approach in both men and women at all ages and regardless of ejection fraction; symptom status; and whether the patient had diabetes, ischemic heart disease, or atrial fibrillation, or was obese.
Clinical outcomes were not significantly different at 6 months. The rate of death from any cause was 13% for the intervention group and 10% for the control group. There were 194 and 213 hospitalizations from any cause, respectively.
Not included in the trial’s current publication but soon to be published, Dr. Kitzman said when interviewed, is a comparison of outcomes in patients with HFpEF and HFrEF. “We found at baseline that those with HFpEF had worse impairment in physical function, quality of life, and frailty. After the intervention, there appeared to be consistently larger improvements in all outcomes, including SPPB, 6-minute walk, qualify of life, and frailty, in HFpEF versus HFrEF.”
The signals of potential benefit in HFpEF extended to clinical endpoints, he said. In contrast to similar rates of all-cause rehospitalization in HFrEF, “in patients with HFpEF, rehospitalizations were 17% lower in the intervention group, compared to the control group.” Still, he noted, the interaction P value wasn’t significant.
However, Dr. Kitzman added, mortality in the intervention group, compared with the control group, was reduced by 35% among patients with HFpEF, “but was 250% higher in HFrEF,” with a significant interaction P value.
He was careful to note that, as a phase 2 trial, REHAB-HF was underpowered for clinical events, “and even the results in the HFpEF group should not be seen as adequate evidence to change clinical care.” They were from an exploratory analysis that included relatively few events.
“Because definitive demonstration of improvement in clinical events is critical for altering clinical care guidelines and for third-party payer reimbursement decisions, we believe that a subsequent phase 3 trial is needed and are currently planning toward that,” Dr. Kitzman said.
The study was supported by research grants from the National Institutes of Health, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine, and the Oristano Family Fund at Wake Forest. Dr. Kitzman disclosed receiving consulting fees or honoraria from AbbVie, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, CinRx, Corviamedical, GlaxoSmithKline, and Merck; and having an unspecified relationship with Gilead. Dr. Handberg disclosed receiving grants from Aastom Biosciences, Abbott Laboratories, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Everyfit, Gilead, Ionis, Medtronic, Merck, Mesoblast, Relypsa, and Sanofi-Aventis. Dr. Bittner discloses receiving consulting fees or honoraria from Pfizer and Sanofi; receiving research grants from Amgen and The Medicines Company; and having unspecified relationships with AstraZeneca, DalCor, Esperion, and Sanofi-Aventis. Dr. Anker reported receiving grants and personal fees from Abbott Vascular and Vifor; personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Cardiac Dimensions, Thermo Fisher Scientific, AstraZeneca, Occlutech, Actimed, and Respicardia. Dr. Coats disclosed receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia.
A version of this article first appeared on Medscape.com.
Omics analysis links blood type to COVID-19
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Checkpoint inhibitors earn spot in new ESMO SCLC guidelines
In new small cell lung cancer guidelines, the European Society of Medical Oncology calls for upfront atezolizumab or durvalumab in combination with four to six cycles of etoposide and a platinum for stage 4 disease.
The strong recommendation is based on two phase 3 trials that showed improved overall survival when the checkpoint inhibitors were added to standard chemotherapy. “With very similar results, and in the context of a severe unmet need, both trials justify the need for immunotherapy in the frontline setting” and established “new standards of care” for stage 4 disease, the group said. “Atezolizumab or durvalumab in combination with a platinum plus etoposide should be offered to all eligible chemotherapy-naive patients” with a performance status of 0-1, said the group led by Anne-Marie Dingemans, MD, PhD, a pulmonology professor at Maastricht (the Netherlands) University Medical Center.
Alessio Cortellini, MD, a consulting oncologist and visiting researcher at Imperial College London, said he strongly endorses the recommendation when asked for comment.
“The addition of a PD-L1 inhibitor to a platinum/etoposide backbone is the first strategy that has led to a significant benefit in terms of overall survival. After decades of disappointing results, the bar has” been raised, said Dr. Cortellini.
New inhibitor
EMSO also incorporated the new RNA polymerase II inhibitor lurbinectedin into their guidelines as an option for patients progressing on or after first-line platinum-based chemotherapy.
The agent was approved by the U.S. Food and Drug Administration in June 2020 for metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
The recommendations – more than 50 in all – are based on a literature review and expert opinion, and cover SCLC diagnosis, staging, treatment, and follow-up, with flowcharts outlining treatment pathways.
Atezolizumab earned the endorsement following the IMpower133 trial, which showed a median overall survival of 12.3 months for atezolizumab in combination with carboplatin and etoposide, versus 10.3 months on chemotherapy alone; 34% of the atezolizumab group was alive at 18 months versus 21% in the placebo arm.
The durvalumab recommendation is based on the CASPIAN trial, in which the addition of durvalumab to platinum plus etoposide improved median overall survival from 10.5 to 12.9 months; 32% of durvalumab patients were alive at 18 months versus 24.8% in the chemotherapy-alone arm.
ESMO said “it is important to stress that, in both trials,” patients were in good clinical condition with a median age in the early 60s, so relatively young for SCLC. Also, the modest benefits “clearly emphasize the need for” biomarkers that predict response in order to better select patients.
Immunotherapy has improved cancer treatment across many malignancies and continues to be actively investigated in SCLC, but so far only atezolizumab and durvalumab have phase 3 evidence of benefit.
Makers of the blockbuster checkpoint inhibitors nivolumab and pembrolizumab recently withdrew their FDA approval for stage 4 SCLC that’s progressed after platinum-based chemotherapy and at least one other line of therapy; phase 3 trials failed to confirm the modest survival benefit found in early studies.
Lurbinectedin earned its place in the guidelines based on a single-arm study with 105 relapsed patients that showed an overall response rate of 22.2% in platinum-resistant and 45% in platinum-sensitive patients, with a median overall survival of 9.3 months.
The jury is still out, however. A phase 3 trial of lurbinectedin plus doxorubicin versus topotecan or CAV [cyclophosphamide, adriamycin, and vincristine] for advanced recurrent disease failed to meet its endpoint of superior overall survival, according to a recent press release from the its maker.
“It might be a bit early to discuss” routine use of lurbinectedin, although having it available is good “since literally nothing works in the second line setting,” Dr. Cortellini said.
There was no external funding for the work. The authors had numerous ties to pharmaceutical companies, including Dr. Dingemans who reported adviser and speakers fees and/or research funding from Roche, Lilly, Bristol-Myers Squib, and others. Dr. Costellini reported speakers fees from Novartis, Astrazeneca, and Astellas and consultant payments from Bristol-Myers Squibb, Roche, MSD, and AstraZeneca.
In new small cell lung cancer guidelines, the European Society of Medical Oncology calls for upfront atezolizumab or durvalumab in combination with four to six cycles of etoposide and a platinum for stage 4 disease.
The strong recommendation is based on two phase 3 trials that showed improved overall survival when the checkpoint inhibitors were added to standard chemotherapy. “With very similar results, and in the context of a severe unmet need, both trials justify the need for immunotherapy in the frontline setting” and established “new standards of care” for stage 4 disease, the group said. “Atezolizumab or durvalumab in combination with a platinum plus etoposide should be offered to all eligible chemotherapy-naive patients” with a performance status of 0-1, said the group led by Anne-Marie Dingemans, MD, PhD, a pulmonology professor at Maastricht (the Netherlands) University Medical Center.
Alessio Cortellini, MD, a consulting oncologist and visiting researcher at Imperial College London, said he strongly endorses the recommendation when asked for comment.
“The addition of a PD-L1 inhibitor to a platinum/etoposide backbone is the first strategy that has led to a significant benefit in terms of overall survival. After decades of disappointing results, the bar has” been raised, said Dr. Cortellini.
New inhibitor
EMSO also incorporated the new RNA polymerase II inhibitor lurbinectedin into their guidelines as an option for patients progressing on or after first-line platinum-based chemotherapy.
The agent was approved by the U.S. Food and Drug Administration in June 2020 for metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
The recommendations – more than 50 in all – are based on a literature review and expert opinion, and cover SCLC diagnosis, staging, treatment, and follow-up, with flowcharts outlining treatment pathways.
Atezolizumab earned the endorsement following the IMpower133 trial, which showed a median overall survival of 12.3 months for atezolizumab in combination with carboplatin and etoposide, versus 10.3 months on chemotherapy alone; 34% of the atezolizumab group was alive at 18 months versus 21% in the placebo arm.
The durvalumab recommendation is based on the CASPIAN trial, in which the addition of durvalumab to platinum plus etoposide improved median overall survival from 10.5 to 12.9 months; 32% of durvalumab patients were alive at 18 months versus 24.8% in the chemotherapy-alone arm.
ESMO said “it is important to stress that, in both trials,” patients were in good clinical condition with a median age in the early 60s, so relatively young for SCLC. Also, the modest benefits “clearly emphasize the need for” biomarkers that predict response in order to better select patients.
Immunotherapy has improved cancer treatment across many malignancies and continues to be actively investigated in SCLC, but so far only atezolizumab and durvalumab have phase 3 evidence of benefit.
Makers of the blockbuster checkpoint inhibitors nivolumab and pembrolizumab recently withdrew their FDA approval for stage 4 SCLC that’s progressed after platinum-based chemotherapy and at least one other line of therapy; phase 3 trials failed to confirm the modest survival benefit found in early studies.
Lurbinectedin earned its place in the guidelines based on a single-arm study with 105 relapsed patients that showed an overall response rate of 22.2% in platinum-resistant and 45% in platinum-sensitive patients, with a median overall survival of 9.3 months.
The jury is still out, however. A phase 3 trial of lurbinectedin plus doxorubicin versus topotecan or CAV [cyclophosphamide, adriamycin, and vincristine] for advanced recurrent disease failed to meet its endpoint of superior overall survival, according to a recent press release from the its maker.
“It might be a bit early to discuss” routine use of lurbinectedin, although having it available is good “since literally nothing works in the second line setting,” Dr. Cortellini said.
There was no external funding for the work. The authors had numerous ties to pharmaceutical companies, including Dr. Dingemans who reported adviser and speakers fees and/or research funding from Roche, Lilly, Bristol-Myers Squib, and others. Dr. Costellini reported speakers fees from Novartis, Astrazeneca, and Astellas and consultant payments from Bristol-Myers Squibb, Roche, MSD, and AstraZeneca.
In new small cell lung cancer guidelines, the European Society of Medical Oncology calls for upfront atezolizumab or durvalumab in combination with four to six cycles of etoposide and a platinum for stage 4 disease.
The strong recommendation is based on two phase 3 trials that showed improved overall survival when the checkpoint inhibitors were added to standard chemotherapy. “With very similar results, and in the context of a severe unmet need, both trials justify the need for immunotherapy in the frontline setting” and established “new standards of care” for stage 4 disease, the group said. “Atezolizumab or durvalumab in combination with a platinum plus etoposide should be offered to all eligible chemotherapy-naive patients” with a performance status of 0-1, said the group led by Anne-Marie Dingemans, MD, PhD, a pulmonology professor at Maastricht (the Netherlands) University Medical Center.
Alessio Cortellini, MD, a consulting oncologist and visiting researcher at Imperial College London, said he strongly endorses the recommendation when asked for comment.
“The addition of a PD-L1 inhibitor to a platinum/etoposide backbone is the first strategy that has led to a significant benefit in terms of overall survival. After decades of disappointing results, the bar has” been raised, said Dr. Cortellini.
New inhibitor
EMSO also incorporated the new RNA polymerase II inhibitor lurbinectedin into their guidelines as an option for patients progressing on or after first-line platinum-based chemotherapy.
The agent was approved by the U.S. Food and Drug Administration in June 2020 for metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
The recommendations – more than 50 in all – are based on a literature review and expert opinion, and cover SCLC diagnosis, staging, treatment, and follow-up, with flowcharts outlining treatment pathways.
Atezolizumab earned the endorsement following the IMpower133 trial, which showed a median overall survival of 12.3 months for atezolizumab in combination with carboplatin and etoposide, versus 10.3 months on chemotherapy alone; 34% of the atezolizumab group was alive at 18 months versus 21% in the placebo arm.
The durvalumab recommendation is based on the CASPIAN trial, in which the addition of durvalumab to platinum plus etoposide improved median overall survival from 10.5 to 12.9 months; 32% of durvalumab patients were alive at 18 months versus 24.8% in the chemotherapy-alone arm.
ESMO said “it is important to stress that, in both trials,” patients were in good clinical condition with a median age in the early 60s, so relatively young for SCLC. Also, the modest benefits “clearly emphasize the need for” biomarkers that predict response in order to better select patients.
Immunotherapy has improved cancer treatment across many malignancies and continues to be actively investigated in SCLC, but so far only atezolizumab and durvalumab have phase 3 evidence of benefit.
Makers of the blockbuster checkpoint inhibitors nivolumab and pembrolizumab recently withdrew their FDA approval for stage 4 SCLC that’s progressed after platinum-based chemotherapy and at least one other line of therapy; phase 3 trials failed to confirm the modest survival benefit found in early studies.
Lurbinectedin earned its place in the guidelines based on a single-arm study with 105 relapsed patients that showed an overall response rate of 22.2% in platinum-resistant and 45% in platinum-sensitive patients, with a median overall survival of 9.3 months.
The jury is still out, however. A phase 3 trial of lurbinectedin plus doxorubicin versus topotecan or CAV [cyclophosphamide, adriamycin, and vincristine] for advanced recurrent disease failed to meet its endpoint of superior overall survival, according to a recent press release from the its maker.
“It might be a bit early to discuss” routine use of lurbinectedin, although having it available is good “since literally nothing works in the second line setting,” Dr. Cortellini said.
There was no external funding for the work. The authors had numerous ties to pharmaceutical companies, including Dr. Dingemans who reported adviser and speakers fees and/or research funding from Roche, Lilly, Bristol-Myers Squib, and others. Dr. Costellini reported speakers fees from Novartis, Astrazeneca, and Astellas and consultant payments from Bristol-Myers Squibb, Roche, MSD, and AstraZeneca.
FROM ANNALS OF ONCOLOGY
Opioid addiction meds may curb growing problem of kratom dependence
Medications typically used to treat opioid use disorder (OUD) may* also be effective for the growing public health problem of kratom addiction, new research shows.
Results of a comprehensive literature review and an expert survey suggest buprenorphine, naltrexone, and methadone may be effective for patients seeking help for kratom addiction, and if further research confirms these findings, the indication for OUD medications could potentially be expanded to include moderate-to-severe kratom addiction, study investigator Saeed Ahmed, MD, medical director of West Ridge Center at Rutland Regional Medical Center, Rutland, Vermont, said in an interview.
Dr. Ahmed, who practices general psychiatry and addiction psychiatry, presented the findings at the virtual American Psychiatric Association 2021 Annual Meeting.
Emerging public health problem
Kratom can be ingested in pill or capsule form or as an extract. Its leaves can be chewed or dried and powdered to make a tea. It can also be incorporated into topical creams, balms, or tinctures.
Products containing the substance are “readily available and legal for sale in many states and cities in the U.S.,” said Dr. Ahmed, adding that it can be purchased online or at local smoke shops and is increasingly used by individuals to self-treat a variety of conditions including pain, anxiety, and mood conditions and as an opioid substitute.
As reported by this news organization, a 2018 analysis conducted by the U.S. Food and Drug Administration showed kratom is, in fact, an opioid, a finding that garnered significant push-back from the American Kratom Association.
Kratom addiction is an “emerging public health problem,” said Dr. Ahmed, adding that in recent years the number of calls to poison control centers across the country has increased 52-fold – from one per month to two per day. He believes misinformation through social media has helped fuel its use.
Kratom use, the investigators note, can lead to muscle pain, weight loss, insomnia, hallucinations and, in some cases (particularly when combined with synthetic opioids or benzodiazepines), it can lead to respiratory depression, seizures, coma, and death.
In addition,
To investigate, the researchers conducted a systematic literature search for cases pertaining to maintenance treatment for kratom dependence. They also tapped into case reports and scientific posters from reliable online sources and conference proceedings. In addition, they conducted a survey of members from the American Society of Addiction Medicine (ASAM).
The researchers found 14 reports of long-term management of kratom addiction, half of which did not involve an OUD. It’s important to exclude OUDs to avoid possible confounding.
In most cases, buprenorphine was used, but in a few cases naltrexone or methadone were prescribed. All cases had a favorable outcome. Dr. Ahmed noted that buprenorphine maintenance doses appear to be lower than those required to effectively treat OUD.
With a response rate of 11.5% (82 respondents) the ASAM survey results showed 82.6% of respondents (n = 57) had experience managing KUD, including 27.5% (n = 19) who had kratom addiction only. Of these, 89.5% (n = 17-19), used buprenorphine to manage KUD and of these, 6 combined it with talk therapy.
Dr. Ahmed cautioned that the included cases varied significantly in terms of relevant data, including kratom dose and route of administration, toxicology screening used to monitor abstinence, and duration of maintenance follow-up.
Despite these limitations, the review and survey underscore the importance of including moderate to severe kratom dependence as an indication for current OUD medications, the researchers note.
Including kratom addiction as an indication for these medications is important, especially for patients who are heavily addicted, to meet DSM-5 diagnostic criteria for moderate or severe SUD, they add.
In addition, the researchers recommend that clinicians consider referring patients with moderate to severe kratom dependence for counseling or enrollment in 12-step addiction treatment programs.
A separate diagnosis?
Dr. Ahmed said he would like to see kratom dependence included in the DSM-5 as a separate entity because it is a botanical with properties similar to, but different from, traditional opioids.
“This will not only help to better inform clinicians about a diagnostic criteria encompassing problematic use and facilitate screening, but it will also pave the way for treatments to be explored for this diagnosable condition,” he said. Dr. Ahmed pointed to a review published in the Wisconsin Medical Journal earlier this year that explored potential treatments for kratom dependence.
Commenting on the study for an interview, Petros Levounis, MD, professor and chair, department of psychiatry, and associate dean for professional development, Rutgers New Jersey Medical School, Newark, said the authors “have done a great job reviewing the literature and asking experts” about kratom addiction treatment.
“The punchline of their study is that kratom behaves very much like an opioid and is treated like an opioid.”
Dr. Levounis noted that kratom dependence is so new that experts don’t know much about it. However, he added, emerging evidence suggests that kratom “should be considered an opioid more than anything else,” but specified that he does not believe it warrants its own diagnosis.
He noted that individual opioids don’t have their own diagnostic category and that opioid use disorder is an umbrella term that covers all of these drugs.
Dr. Ahmed and Dr. Levounis have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*Updated 5/18/2021
Medications typically used to treat opioid use disorder (OUD) may* also be effective for the growing public health problem of kratom addiction, new research shows.
Results of a comprehensive literature review and an expert survey suggest buprenorphine, naltrexone, and methadone may be effective for patients seeking help for kratom addiction, and if further research confirms these findings, the indication for OUD medications could potentially be expanded to include moderate-to-severe kratom addiction, study investigator Saeed Ahmed, MD, medical director of West Ridge Center at Rutland Regional Medical Center, Rutland, Vermont, said in an interview.
Dr. Ahmed, who practices general psychiatry and addiction psychiatry, presented the findings at the virtual American Psychiatric Association 2021 Annual Meeting.
Emerging public health problem
Kratom can be ingested in pill or capsule form or as an extract. Its leaves can be chewed or dried and powdered to make a tea. It can also be incorporated into topical creams, balms, or tinctures.
Products containing the substance are “readily available and legal for sale in many states and cities in the U.S.,” said Dr. Ahmed, adding that it can be purchased online or at local smoke shops and is increasingly used by individuals to self-treat a variety of conditions including pain, anxiety, and mood conditions and as an opioid substitute.
As reported by this news organization, a 2018 analysis conducted by the U.S. Food and Drug Administration showed kratom is, in fact, an opioid, a finding that garnered significant push-back from the American Kratom Association.
Kratom addiction is an “emerging public health problem,” said Dr. Ahmed, adding that in recent years the number of calls to poison control centers across the country has increased 52-fold – from one per month to two per day. He believes misinformation through social media has helped fuel its use.
Kratom use, the investigators note, can lead to muscle pain, weight loss, insomnia, hallucinations and, in some cases (particularly when combined with synthetic opioids or benzodiazepines), it can lead to respiratory depression, seizures, coma, and death.
In addition,
To investigate, the researchers conducted a systematic literature search for cases pertaining to maintenance treatment for kratom dependence. They also tapped into case reports and scientific posters from reliable online sources and conference proceedings. In addition, they conducted a survey of members from the American Society of Addiction Medicine (ASAM).
The researchers found 14 reports of long-term management of kratom addiction, half of which did not involve an OUD. It’s important to exclude OUDs to avoid possible confounding.
In most cases, buprenorphine was used, but in a few cases naltrexone or methadone were prescribed. All cases had a favorable outcome. Dr. Ahmed noted that buprenorphine maintenance doses appear to be lower than those required to effectively treat OUD.
With a response rate of 11.5% (82 respondents) the ASAM survey results showed 82.6% of respondents (n = 57) had experience managing KUD, including 27.5% (n = 19) who had kratom addiction only. Of these, 89.5% (n = 17-19), used buprenorphine to manage KUD and of these, 6 combined it with talk therapy.
Dr. Ahmed cautioned that the included cases varied significantly in terms of relevant data, including kratom dose and route of administration, toxicology screening used to monitor abstinence, and duration of maintenance follow-up.
Despite these limitations, the review and survey underscore the importance of including moderate to severe kratom dependence as an indication for current OUD medications, the researchers note.
Including kratom addiction as an indication for these medications is important, especially for patients who are heavily addicted, to meet DSM-5 diagnostic criteria for moderate or severe SUD, they add.
In addition, the researchers recommend that clinicians consider referring patients with moderate to severe kratom dependence for counseling or enrollment in 12-step addiction treatment programs.
A separate diagnosis?
Dr. Ahmed said he would like to see kratom dependence included in the DSM-5 as a separate entity because it is a botanical with properties similar to, but different from, traditional opioids.
“This will not only help to better inform clinicians about a diagnostic criteria encompassing problematic use and facilitate screening, but it will also pave the way for treatments to be explored for this diagnosable condition,” he said. Dr. Ahmed pointed to a review published in the Wisconsin Medical Journal earlier this year that explored potential treatments for kratom dependence.
Commenting on the study for an interview, Petros Levounis, MD, professor and chair, department of psychiatry, and associate dean for professional development, Rutgers New Jersey Medical School, Newark, said the authors “have done a great job reviewing the literature and asking experts” about kratom addiction treatment.
“The punchline of their study is that kratom behaves very much like an opioid and is treated like an opioid.”
Dr. Levounis noted that kratom dependence is so new that experts don’t know much about it. However, he added, emerging evidence suggests that kratom “should be considered an opioid more than anything else,” but specified that he does not believe it warrants its own diagnosis.
He noted that individual opioids don’t have their own diagnostic category and that opioid use disorder is an umbrella term that covers all of these drugs.
Dr. Ahmed and Dr. Levounis have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*Updated 5/18/2021
Medications typically used to treat opioid use disorder (OUD) may* also be effective for the growing public health problem of kratom addiction, new research shows.
Results of a comprehensive literature review and an expert survey suggest buprenorphine, naltrexone, and methadone may be effective for patients seeking help for kratom addiction, and if further research confirms these findings, the indication for OUD medications could potentially be expanded to include moderate-to-severe kratom addiction, study investigator Saeed Ahmed, MD, medical director of West Ridge Center at Rutland Regional Medical Center, Rutland, Vermont, said in an interview.
Dr. Ahmed, who practices general psychiatry and addiction psychiatry, presented the findings at the virtual American Psychiatric Association 2021 Annual Meeting.
Emerging public health problem
Kratom can be ingested in pill or capsule form or as an extract. Its leaves can be chewed or dried and powdered to make a tea. It can also be incorporated into topical creams, balms, or tinctures.
Products containing the substance are “readily available and legal for sale in many states and cities in the U.S.,” said Dr. Ahmed, adding that it can be purchased online or at local smoke shops and is increasingly used by individuals to self-treat a variety of conditions including pain, anxiety, and mood conditions and as an opioid substitute.
As reported by this news organization, a 2018 analysis conducted by the U.S. Food and Drug Administration showed kratom is, in fact, an opioid, a finding that garnered significant push-back from the American Kratom Association.
Kratom addiction is an “emerging public health problem,” said Dr. Ahmed, adding that in recent years the number of calls to poison control centers across the country has increased 52-fold – from one per month to two per day. He believes misinformation through social media has helped fuel its use.
Kratom use, the investigators note, can lead to muscle pain, weight loss, insomnia, hallucinations and, in some cases (particularly when combined with synthetic opioids or benzodiazepines), it can lead to respiratory depression, seizures, coma, and death.
In addition,
To investigate, the researchers conducted a systematic literature search for cases pertaining to maintenance treatment for kratom dependence. They also tapped into case reports and scientific posters from reliable online sources and conference proceedings. In addition, they conducted a survey of members from the American Society of Addiction Medicine (ASAM).
The researchers found 14 reports of long-term management of kratom addiction, half of which did not involve an OUD. It’s important to exclude OUDs to avoid possible confounding.
In most cases, buprenorphine was used, but in a few cases naltrexone or methadone were prescribed. All cases had a favorable outcome. Dr. Ahmed noted that buprenorphine maintenance doses appear to be lower than those required to effectively treat OUD.
With a response rate of 11.5% (82 respondents) the ASAM survey results showed 82.6% of respondents (n = 57) had experience managing KUD, including 27.5% (n = 19) who had kratom addiction only. Of these, 89.5% (n = 17-19), used buprenorphine to manage KUD and of these, 6 combined it with talk therapy.
Dr. Ahmed cautioned that the included cases varied significantly in terms of relevant data, including kratom dose and route of administration, toxicology screening used to monitor abstinence, and duration of maintenance follow-up.
Despite these limitations, the review and survey underscore the importance of including moderate to severe kratom dependence as an indication for current OUD medications, the researchers note.
Including kratom addiction as an indication for these medications is important, especially for patients who are heavily addicted, to meet DSM-5 diagnostic criteria for moderate or severe SUD, they add.
In addition, the researchers recommend that clinicians consider referring patients with moderate to severe kratom dependence for counseling or enrollment in 12-step addiction treatment programs.
A separate diagnosis?
Dr. Ahmed said he would like to see kratom dependence included in the DSM-5 as a separate entity because it is a botanical with properties similar to, but different from, traditional opioids.
“This will not only help to better inform clinicians about a diagnostic criteria encompassing problematic use and facilitate screening, but it will also pave the way for treatments to be explored for this diagnosable condition,” he said. Dr. Ahmed pointed to a review published in the Wisconsin Medical Journal earlier this year that explored potential treatments for kratom dependence.
Commenting on the study for an interview, Petros Levounis, MD, professor and chair, department of psychiatry, and associate dean for professional development, Rutgers New Jersey Medical School, Newark, said the authors “have done a great job reviewing the literature and asking experts” about kratom addiction treatment.
“The punchline of their study is that kratom behaves very much like an opioid and is treated like an opioid.”
Dr. Levounis noted that kratom dependence is so new that experts don’t know much about it. However, he added, emerging evidence suggests that kratom “should be considered an opioid more than anything else,” but specified that he does not believe it warrants its own diagnosis.
He noted that individual opioids don’t have their own diagnostic category and that opioid use disorder is an umbrella term that covers all of these drugs.
Dr. Ahmed and Dr. Levounis have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*Updated 5/18/2021
FDA preparing an environmental impact statement for 2 sunscreen ingredients
The Food and Drug Administration is launching a process to prepare an environmental impact statement (EIS) regarding the use oxybenzone and octinoxate in over-the-counter sunscreen products.
According to the “Intent to Prepare an Environmental Impact Statement for Certain Sunscreen Drug Products for Over-The-Counter Use,” which was published in the Federal Register on May 13, 2021, the FDA will prepare an EIS “when data or information in an environmental assessment or otherwise available to the Agency leads to a finding that the proposed agency action may significantly affect the quality of the human environment.” The first step in this effort involves a “public scoping process” to evaluate any potential environmental impacts associated with the use of oxybenzone and octinoxate in sunscreens so that an EIS, if required, “can be completed prior to issuance of a final sunscreen order addressing sunscreens containing these ingredients.”
The American Academy of Dermatology Association weighed in on the FDA’s announcement, noting that it “appreciates the efforts of the agency to thoroughly examine all relevant science before issuing a final sunscreen order on these ingredients,” according to a statement released by the AADA on May 13, 2021.
The statement added: “Skin cancer is the most common cancer in the U.S., and unprotected exposure to the sun’s harmful ultraviolet rays is a major risk factor. The AADA continues to focus on encouraging members of the public to protect themselves by seeking shade, wearing protective clothing – including a lightweight and long-sleeved shirt, pants, a wide-brimmed hat and sunglasses – and applying a broad-spectrum sunscreen with an SPF of 30 or higher to all exposed skin.”
According to the FDA document, a series of developments regarding oxybenzone and octinoxate prompted the agency to take this step, including comments the agency received in response to the 2019 proposed rule titled “Sunscreen Drug Products for Over-The-Counter Human Use,” which raised concern about the potential effects of the two ingredients on coral and/or coral reefs, as well as research efforts by the National Oceanic and Atmospheric Administration Coral Reef Conservation Programs on the potential impacts of sunscreen products that include oxybenzone and octinoxate on coral reefs and other aquatic systems. Hawaii’s 2018 state law prohibiting the sale, offer of sale, and distribution of sunscreens that contain oxybenzone and/or octinoxate also influenced the agency’s decision to further evaluate the topic.
“The purpose of the public scoping process is to determine relevant issues that will influence the scope of the environmental analysis, including potential alternatives and the extent to which those issues and impacts will be analyzed,” the FDA document states. “At this initial stage of the scoping process, we have identified the following four alternatives: FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA [new drug application] is impermissible; FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA is permissible; FDA will conclude that inclusion of oxybenzone in sunscreens marketed without an NDA is permissible but that the inclusion of octinoxate in sunscreens marketed without an NDA is impermissible; or FDA will conclude that inclusion of octinoxate in sunscreens marketed without an NDA is permissible but that the inclusion of oxybenzone in sunscreens marketed without an NDA is impermissible.”
Until June 14, the FDA is accepting comments from the public electronically via the Federal eRulemaking Portal at www.regulations.gov (search for Docket No. FDA-2021-N-0352) or by mail to: Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, Md., 20852. Refer to Docket No. FDA-2021-N-0352.
The Food and Drug Administration is launching a process to prepare an environmental impact statement (EIS) regarding the use oxybenzone and octinoxate in over-the-counter sunscreen products.
According to the “Intent to Prepare an Environmental Impact Statement for Certain Sunscreen Drug Products for Over-The-Counter Use,” which was published in the Federal Register on May 13, 2021, the FDA will prepare an EIS “when data or information in an environmental assessment or otherwise available to the Agency leads to a finding that the proposed agency action may significantly affect the quality of the human environment.” The first step in this effort involves a “public scoping process” to evaluate any potential environmental impacts associated with the use of oxybenzone and octinoxate in sunscreens so that an EIS, if required, “can be completed prior to issuance of a final sunscreen order addressing sunscreens containing these ingredients.”
The American Academy of Dermatology Association weighed in on the FDA’s announcement, noting that it “appreciates the efforts of the agency to thoroughly examine all relevant science before issuing a final sunscreen order on these ingredients,” according to a statement released by the AADA on May 13, 2021.
The statement added: “Skin cancer is the most common cancer in the U.S., and unprotected exposure to the sun’s harmful ultraviolet rays is a major risk factor. The AADA continues to focus on encouraging members of the public to protect themselves by seeking shade, wearing protective clothing – including a lightweight and long-sleeved shirt, pants, a wide-brimmed hat and sunglasses – and applying a broad-spectrum sunscreen with an SPF of 30 or higher to all exposed skin.”
According to the FDA document, a series of developments regarding oxybenzone and octinoxate prompted the agency to take this step, including comments the agency received in response to the 2019 proposed rule titled “Sunscreen Drug Products for Over-The-Counter Human Use,” which raised concern about the potential effects of the two ingredients on coral and/or coral reefs, as well as research efforts by the National Oceanic and Atmospheric Administration Coral Reef Conservation Programs on the potential impacts of sunscreen products that include oxybenzone and octinoxate on coral reefs and other aquatic systems. Hawaii’s 2018 state law prohibiting the sale, offer of sale, and distribution of sunscreens that contain oxybenzone and/or octinoxate also influenced the agency’s decision to further evaluate the topic.
“The purpose of the public scoping process is to determine relevant issues that will influence the scope of the environmental analysis, including potential alternatives and the extent to which those issues and impacts will be analyzed,” the FDA document states. “At this initial stage of the scoping process, we have identified the following four alternatives: FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA [new drug application] is impermissible; FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA is permissible; FDA will conclude that inclusion of oxybenzone in sunscreens marketed without an NDA is permissible but that the inclusion of octinoxate in sunscreens marketed without an NDA is impermissible; or FDA will conclude that inclusion of octinoxate in sunscreens marketed without an NDA is permissible but that the inclusion of oxybenzone in sunscreens marketed without an NDA is impermissible.”
Until June 14, the FDA is accepting comments from the public electronically via the Federal eRulemaking Portal at www.regulations.gov (search for Docket No. FDA-2021-N-0352) or by mail to: Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, Md., 20852. Refer to Docket No. FDA-2021-N-0352.
The Food and Drug Administration is launching a process to prepare an environmental impact statement (EIS) regarding the use oxybenzone and octinoxate in over-the-counter sunscreen products.
According to the “Intent to Prepare an Environmental Impact Statement for Certain Sunscreen Drug Products for Over-The-Counter Use,” which was published in the Federal Register on May 13, 2021, the FDA will prepare an EIS “when data or information in an environmental assessment or otherwise available to the Agency leads to a finding that the proposed agency action may significantly affect the quality of the human environment.” The first step in this effort involves a “public scoping process” to evaluate any potential environmental impacts associated with the use of oxybenzone and octinoxate in sunscreens so that an EIS, if required, “can be completed prior to issuance of a final sunscreen order addressing sunscreens containing these ingredients.”
The American Academy of Dermatology Association weighed in on the FDA’s announcement, noting that it “appreciates the efforts of the agency to thoroughly examine all relevant science before issuing a final sunscreen order on these ingredients,” according to a statement released by the AADA on May 13, 2021.
The statement added: “Skin cancer is the most common cancer in the U.S., and unprotected exposure to the sun’s harmful ultraviolet rays is a major risk factor. The AADA continues to focus on encouraging members of the public to protect themselves by seeking shade, wearing protective clothing – including a lightweight and long-sleeved shirt, pants, a wide-brimmed hat and sunglasses – and applying a broad-spectrum sunscreen with an SPF of 30 or higher to all exposed skin.”
According to the FDA document, a series of developments regarding oxybenzone and octinoxate prompted the agency to take this step, including comments the agency received in response to the 2019 proposed rule titled “Sunscreen Drug Products for Over-The-Counter Human Use,” which raised concern about the potential effects of the two ingredients on coral and/or coral reefs, as well as research efforts by the National Oceanic and Atmospheric Administration Coral Reef Conservation Programs on the potential impacts of sunscreen products that include oxybenzone and octinoxate on coral reefs and other aquatic systems. Hawaii’s 2018 state law prohibiting the sale, offer of sale, and distribution of sunscreens that contain oxybenzone and/or octinoxate also influenced the agency’s decision to further evaluate the topic.
“The purpose of the public scoping process is to determine relevant issues that will influence the scope of the environmental analysis, including potential alternatives and the extent to which those issues and impacts will be analyzed,” the FDA document states. “At this initial stage of the scoping process, we have identified the following four alternatives: FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA [new drug application] is impermissible; FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA is permissible; FDA will conclude that inclusion of oxybenzone in sunscreens marketed without an NDA is permissible but that the inclusion of octinoxate in sunscreens marketed without an NDA is impermissible; or FDA will conclude that inclusion of octinoxate in sunscreens marketed without an NDA is permissible but that the inclusion of oxybenzone in sunscreens marketed without an NDA is impermissible.”
Until June 14, the FDA is accepting comments from the public electronically via the Federal eRulemaking Portal at www.regulations.gov (search for Docket No. FDA-2021-N-0352) or by mail to: Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, Md., 20852. Refer to Docket No. FDA-2021-N-0352.
Telemedicine is popular among Mohs surgeons – for now
A majority of
A variety of factors combine to make it “very difficult for surgeons to make long-term plans for implementing telemedicine in their practices,” said Mario Maruthur, MD, who presented the findings at the annual meeting of the American College of Mohs Surgery. “Telemedicine likely has a role in Mohs practices, particularly with postop follow-up visits. However, postpandemic reimbursement and regulatory issues need to be formally laid out before Mohs surgeons are able to incorporate it into their permanent work flow.”
Dr. Maruthur, a Mohs surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York, and colleagues sent a survey to ACMS members in September and October 2020. “We saw first-hand in our surgical practice that telemedicine quickly became an important tool when the pandemic surged in the spring of 2020,” he said. Considering that surgical practices are highly dependent on in-person visits, the impetus for this study was to assess to what degree Mohs practices from across the spectrum, including academic and private practices, embraced telemedicine during the pandemic, and “what these surgical practices used telemedicine for, how it was received by their patients, which telemedicine platforms were most often utilized, and lastly, what are their plans if any for incorporating telemedicine into their surgical practices after the pandemic subsides.”
The researchers received responses from 115 surgeons representing all regions of the country (40% Northeast, 21% South, 21% Midwest, and 18% West). Half practiced in urban areas (37%) and large cities (13%), and 40% were in an academic setting versus 36% in a single-specialty private practice.
More than 70% of the respondents said their case load fell by at least 75% during the initial surge of the pandemic; 80% turned to telemedicine, compared with just 23% who relied on the technology prior to the pandemic. The most commonly used telemedicine technologies were FaceTime, Zoom, Doximity, and Epic.
Mohs surgeons reported most commonly using telemedicine for postsurgery management (77% of the total 115 responses). “Telemedicine is a great fit for this category of visits as they allow the surgeon to view the surgical site and answer any questions they patient may have,” Dr. Maruthur said. “If the surgeon does suspect a postop infection or other concern based on a patient’s signs or symptoms, they can easily schedule the patient for an in-person assessment. We suspect that postop follow-up visits may be the best candidate for long-term use of telemedicine in Mohs surgery practices.”
Surgeons also reported using telemedicine for “spot checks” (61%) and surgical consultations (59%).
However, Dr. Maruther noted that preoperative assessments and spot checks can be difficult to perform using telemedicine. “The quality of the video image is not always great, patients can have a difficult time pointing the camera at the right spot and at the right distance. Even appreciating the actual size of the lesion are all difficult over a video encounter. And there is a lot of information gleaned from in-person physical examination, such as whether the lesion is fixed to a deeper structure and whether there are any nearby scars or other suspicious lesions.”
Nearly three-quarters of the surgeons using the technology said most or all patients were receptive to telemedicine.
However, the surgeons reported multiple barriers to the use of telemedicine: Limitations when compared with physical exams (88%), fitting it into the work flow (58%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
In an interview, Sumaira Z. Aasi, MD, director of Mohs and dermatologic surgery, Stanford University, agreed that there are many obstacles to routine use of telemedicine by Mohs surgeons. “As surgeons, we rely on the physical and tactile exam to get a sense of the size and extent of the cancer and characteristics such as the laxity of the surrounding tissue whether the tumor is fixed,” she said. “It is very difficult to access this on a telemedicine visit.”
In addition, she said, “many of our patients are in the elderly population, and some may not be comfortable using this technology. Also, it’s not a work flow that we are comfortable or familiar with. And I think that the technology has to improve to allow for better resolution of images as we ‘examine’ patients through a telemedicine visit.”
She added that “another con is there is a reliance on having the patient point out lesions of concern. Many cancers are picked by a careful in-person examination by a qualified physician/dermatologist/Mohs surgeon when the lesion is quite small or subtle and not even noticed by the patient themselves. This approach invariably leads to earlier biopsies and earlier treatments that can prevent morbidity and save health care money.”
On the other hand, she said, telemedicine “may save patients some time and money in terms of the effort and cost of transportation to come in for simpler postoperative medical visits that are often short in their very nature, such as postop check-ups.”
Most of the surgeons surveyed (69%) said telemedicine probably or definitely deserves a place in the practice Mohs surgery, but only 50% said they’d like to or would definitely pursue giving telemedicine a role in their practices once the pandemic is over.
“At the start of the pandemic, many regulations in areas such as HIPAA were eased, and reimbursements were increased, which allowed telemedicine to be quickly adopted,” Dr. Maruther said. “The government and payers have yet to decide which regulations and reimbursements will be in place after the pandemic. That makes it very difficult for surgeons to make long-term plans for implementing telemedicine in their practices.”
Dr. Aasi predicted that telemedicine will become more appealing to patients and physicians as it its technology and usability improves. More familiarity with its use will also be helpful, she said, and surgeons will be more receptive as it’s incorporated into efficient daily work flow.
The study was funded in part by the National Institutes of Health.
A majority of
A variety of factors combine to make it “very difficult for surgeons to make long-term plans for implementing telemedicine in their practices,” said Mario Maruthur, MD, who presented the findings at the annual meeting of the American College of Mohs Surgery. “Telemedicine likely has a role in Mohs practices, particularly with postop follow-up visits. However, postpandemic reimbursement and regulatory issues need to be formally laid out before Mohs surgeons are able to incorporate it into their permanent work flow.”
Dr. Maruthur, a Mohs surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York, and colleagues sent a survey to ACMS members in September and October 2020. “We saw first-hand in our surgical practice that telemedicine quickly became an important tool when the pandemic surged in the spring of 2020,” he said. Considering that surgical practices are highly dependent on in-person visits, the impetus for this study was to assess to what degree Mohs practices from across the spectrum, including academic and private practices, embraced telemedicine during the pandemic, and “what these surgical practices used telemedicine for, how it was received by their patients, which telemedicine platforms were most often utilized, and lastly, what are their plans if any for incorporating telemedicine into their surgical practices after the pandemic subsides.”
The researchers received responses from 115 surgeons representing all regions of the country (40% Northeast, 21% South, 21% Midwest, and 18% West). Half practiced in urban areas (37%) and large cities (13%), and 40% were in an academic setting versus 36% in a single-specialty private practice.
More than 70% of the respondents said their case load fell by at least 75% during the initial surge of the pandemic; 80% turned to telemedicine, compared with just 23% who relied on the technology prior to the pandemic. The most commonly used telemedicine technologies were FaceTime, Zoom, Doximity, and Epic.
Mohs surgeons reported most commonly using telemedicine for postsurgery management (77% of the total 115 responses). “Telemedicine is a great fit for this category of visits as they allow the surgeon to view the surgical site and answer any questions they patient may have,” Dr. Maruthur said. “If the surgeon does suspect a postop infection or other concern based on a patient’s signs or symptoms, they can easily schedule the patient for an in-person assessment. We suspect that postop follow-up visits may be the best candidate for long-term use of telemedicine in Mohs surgery practices.”
Surgeons also reported using telemedicine for “spot checks” (61%) and surgical consultations (59%).
However, Dr. Maruther noted that preoperative assessments and spot checks can be difficult to perform using telemedicine. “The quality of the video image is not always great, patients can have a difficult time pointing the camera at the right spot and at the right distance. Even appreciating the actual size of the lesion are all difficult over a video encounter. And there is a lot of information gleaned from in-person physical examination, such as whether the lesion is fixed to a deeper structure and whether there are any nearby scars or other suspicious lesions.”
Nearly three-quarters of the surgeons using the technology said most or all patients were receptive to telemedicine.
However, the surgeons reported multiple barriers to the use of telemedicine: Limitations when compared with physical exams (88%), fitting it into the work flow (58%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
In an interview, Sumaira Z. Aasi, MD, director of Mohs and dermatologic surgery, Stanford University, agreed that there are many obstacles to routine use of telemedicine by Mohs surgeons. “As surgeons, we rely on the physical and tactile exam to get a sense of the size and extent of the cancer and characteristics such as the laxity of the surrounding tissue whether the tumor is fixed,” she said. “It is very difficult to access this on a telemedicine visit.”
In addition, she said, “many of our patients are in the elderly population, and some may not be comfortable using this technology. Also, it’s not a work flow that we are comfortable or familiar with. And I think that the technology has to improve to allow for better resolution of images as we ‘examine’ patients through a telemedicine visit.”
She added that “another con is there is a reliance on having the patient point out lesions of concern. Many cancers are picked by a careful in-person examination by a qualified physician/dermatologist/Mohs surgeon when the lesion is quite small or subtle and not even noticed by the patient themselves. This approach invariably leads to earlier biopsies and earlier treatments that can prevent morbidity and save health care money.”
On the other hand, she said, telemedicine “may save patients some time and money in terms of the effort and cost of transportation to come in for simpler postoperative medical visits that are often short in their very nature, such as postop check-ups.”
Most of the surgeons surveyed (69%) said telemedicine probably or definitely deserves a place in the practice Mohs surgery, but only 50% said they’d like to or would definitely pursue giving telemedicine a role in their practices once the pandemic is over.
“At the start of the pandemic, many regulations in areas such as HIPAA were eased, and reimbursements were increased, which allowed telemedicine to be quickly adopted,” Dr. Maruther said. “The government and payers have yet to decide which regulations and reimbursements will be in place after the pandemic. That makes it very difficult for surgeons to make long-term plans for implementing telemedicine in their practices.”
Dr. Aasi predicted that telemedicine will become more appealing to patients and physicians as it its technology and usability improves. More familiarity with its use will also be helpful, she said, and surgeons will be more receptive as it’s incorporated into efficient daily work flow.
The study was funded in part by the National Institutes of Health.
A majority of
A variety of factors combine to make it “very difficult for surgeons to make long-term plans for implementing telemedicine in their practices,” said Mario Maruthur, MD, who presented the findings at the annual meeting of the American College of Mohs Surgery. “Telemedicine likely has a role in Mohs practices, particularly with postop follow-up visits. However, postpandemic reimbursement and regulatory issues need to be formally laid out before Mohs surgeons are able to incorporate it into their permanent work flow.”
Dr. Maruthur, a Mohs surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York, and colleagues sent a survey to ACMS members in September and October 2020. “We saw first-hand in our surgical practice that telemedicine quickly became an important tool when the pandemic surged in the spring of 2020,” he said. Considering that surgical practices are highly dependent on in-person visits, the impetus for this study was to assess to what degree Mohs practices from across the spectrum, including academic and private practices, embraced telemedicine during the pandemic, and “what these surgical practices used telemedicine for, how it was received by their patients, which telemedicine platforms were most often utilized, and lastly, what are their plans if any for incorporating telemedicine into their surgical practices after the pandemic subsides.”
The researchers received responses from 115 surgeons representing all regions of the country (40% Northeast, 21% South, 21% Midwest, and 18% West). Half practiced in urban areas (37%) and large cities (13%), and 40% were in an academic setting versus 36% in a single-specialty private practice.
More than 70% of the respondents said their case load fell by at least 75% during the initial surge of the pandemic; 80% turned to telemedicine, compared with just 23% who relied on the technology prior to the pandemic. The most commonly used telemedicine technologies were FaceTime, Zoom, Doximity, and Epic.
Mohs surgeons reported most commonly using telemedicine for postsurgery management (77% of the total 115 responses). “Telemedicine is a great fit for this category of visits as they allow the surgeon to view the surgical site and answer any questions they patient may have,” Dr. Maruthur said. “If the surgeon does suspect a postop infection or other concern based on a patient’s signs or symptoms, they can easily schedule the patient for an in-person assessment. We suspect that postop follow-up visits may be the best candidate for long-term use of telemedicine in Mohs surgery practices.”
Surgeons also reported using telemedicine for “spot checks” (61%) and surgical consultations (59%).
However, Dr. Maruther noted that preoperative assessments and spot checks can be difficult to perform using telemedicine. “The quality of the video image is not always great, patients can have a difficult time pointing the camera at the right spot and at the right distance. Even appreciating the actual size of the lesion are all difficult over a video encounter. And there is a lot of information gleaned from in-person physical examination, such as whether the lesion is fixed to a deeper structure and whether there are any nearby scars or other suspicious lesions.”
Nearly three-quarters of the surgeons using the technology said most or all patients were receptive to telemedicine.
However, the surgeons reported multiple barriers to the use of telemedicine: Limitations when compared with physical exams (88%), fitting it into the work flow (58%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
In an interview, Sumaira Z. Aasi, MD, director of Mohs and dermatologic surgery, Stanford University, agreed that there are many obstacles to routine use of telemedicine by Mohs surgeons. “As surgeons, we rely on the physical and tactile exam to get a sense of the size and extent of the cancer and characteristics such as the laxity of the surrounding tissue whether the tumor is fixed,” she said. “It is very difficult to access this on a telemedicine visit.”
In addition, she said, “many of our patients are in the elderly population, and some may not be comfortable using this technology. Also, it’s not a work flow that we are comfortable or familiar with. And I think that the technology has to improve to allow for better resolution of images as we ‘examine’ patients through a telemedicine visit.”
She added that “another con is there is a reliance on having the patient point out lesions of concern. Many cancers are picked by a careful in-person examination by a qualified physician/dermatologist/Mohs surgeon when the lesion is quite small or subtle and not even noticed by the patient themselves. This approach invariably leads to earlier biopsies and earlier treatments that can prevent morbidity and save health care money.”
On the other hand, she said, telemedicine “may save patients some time and money in terms of the effort and cost of transportation to come in for simpler postoperative medical visits that are often short in their very nature, such as postop check-ups.”
Most of the surgeons surveyed (69%) said telemedicine probably or definitely deserves a place in the practice Mohs surgery, but only 50% said they’d like to or would definitely pursue giving telemedicine a role in their practices once the pandemic is over.
“At the start of the pandemic, many regulations in areas such as HIPAA were eased, and reimbursements were increased, which allowed telemedicine to be quickly adopted,” Dr. Maruther said. “The government and payers have yet to decide which regulations and reimbursements will be in place after the pandemic. That makes it very difficult for surgeons to make long-term plans for implementing telemedicine in their practices.”
Dr. Aasi predicted that telemedicine will become more appealing to patients and physicians as it its technology and usability improves. More familiarity with its use will also be helpful, she said, and surgeons will be more receptive as it’s incorporated into efficient daily work flow.
The study was funded in part by the National Institutes of Health.
FROM THE ACMS ANNUAL MEETING
Pediatricians see drop in income during the pandemic
The average income for pediatricians declined slightly from 2019 to 2020, according to the Medscape Pediatrician Compensation Report 2021.

The report, which was conducted between October 2020 and February 2021, found that the average pediatrician income was down $11,000 – from $232,000 in 2019 to $221,000 in 2020, with 48% of pediatricians reporting at least some decline in compensation.
The specialty also earned the least amount of money in 2020, compared with all of the other specialties, which isn’t surprising since pediatricians have been among the lowest-paid physician specialties since 2013. The highest-earning specialty was plastic surgery with an average income of $526,000 annually.
Most pediatricians who saw a drop in income cited pandemic-related issues such as job loss, fewer hours, and fewer patients.
Jesse Hackell, MD, vice president and chief operating officer of Ponoma Pediatrics in New York, said in an interview the reduced wages pediatricians saw in 2020 didn’t surprise him because many pediatric offices saw a huge drop in visits that were not urgent.
“[The report] shows that procedural specialties tended to do a lot better than the nonprocedural specialties,” Dr. Hackell said. “That’s because, during the shutdown, if you broke your leg, you still needed the orthopedist. And even though the hospitals weren’t doing elective surgeries, they were certainly doing the emergency stuff.”
Meanwhile, in pediatrician offices, where Dr. Hackell said office visits dropped 70%-80% at the beginning of the pandemic, “parents weren’t going to bring a healthy kid out for routine visits and they weren’t going to bring a kid out for minor illnesses and expose them to possibly communicable diseases in the office.”
About 52% of pediatricians who lost income because of the pandemic believe their income levels will return to normal in 2-3 years. Meanwhile 30% of pediatricians expect their income to return to normal within a year, and 8% believe it will take 4 years for them to bounce back.
Physician work hours generally declined for some time during the pandemic, according to the report. However, most pediatricians are working about the same number of hours as they did before the pandemic, which is 47 hours per week.
Despite working the same number of hours per week that they did prepandemic, they are seeing fewer patients. They are currently seeing on average 64 patients per week, compared with the 78 patients they used to see weekly before the pandemic.
Dr. Hackell said that might be because pediatric offices are trying to make up the loss of revenue during the beginning of the pandemic, from the reduced number of well visits and immunizations, in the second half of the year with outreach.
“Since about June 2020, we’ve been making concerted efforts to remind parents that preventing other infectious diseases is critically important,” Dr. Hackell explained. “And so actually, for the second half of the year, many of us saw more well visits and immunization volume than in 2019 as we sought to make up the gap. It wasn’t that we were seeing more overall, but we’re trying to make up the gap that happened from March, April, May, [and] June.”
Most pediatricians find their work rewarding. One-third say the most rewarding part of their job is gratitude from and relationships with their patients. Meanwhile, 31% of pediatricians said knowing they are making the world a better place was a rewarding part of their job. Only 8% of them said making money was a rewarding part of their job.
Dr. Hackell said he did not go into pediatrics to make money, it was because he found it stimulating and has “no complaints.”
“I’ve been a pediatrician for 40 years and I wouldn’t do anything else,” Dr. Hackell said. “I don’t know that there’s anything that I would find as rewarding as the relationships that I’ve had over 40 years with my patients. You know, getting invited to weddings of kids who I saw when they were newborns is pretty impressive. It’s the gratification of having ongoing relationships with families.”
Furthermore, the report revealed that 77% of pediatricians said they would pick medicine again if they had a choice, and 82% said they would choose the same specialty.
The experts disclosed no relevant financial interests.
*This story was updated on 5/18/2021.
The average income for pediatricians declined slightly from 2019 to 2020, according to the Medscape Pediatrician Compensation Report 2021.

The report, which was conducted between October 2020 and February 2021, found that the average pediatrician income was down $11,000 – from $232,000 in 2019 to $221,000 in 2020, with 48% of pediatricians reporting at least some decline in compensation.
The specialty also earned the least amount of money in 2020, compared with all of the other specialties, which isn’t surprising since pediatricians have been among the lowest-paid physician specialties since 2013. The highest-earning specialty was plastic surgery with an average income of $526,000 annually.
Most pediatricians who saw a drop in income cited pandemic-related issues such as job loss, fewer hours, and fewer patients.
Jesse Hackell, MD, vice president and chief operating officer of Ponoma Pediatrics in New York, said in an interview the reduced wages pediatricians saw in 2020 didn’t surprise him because many pediatric offices saw a huge drop in visits that were not urgent.
“[The report] shows that procedural specialties tended to do a lot better than the nonprocedural specialties,” Dr. Hackell said. “That’s because, during the shutdown, if you broke your leg, you still needed the orthopedist. And even though the hospitals weren’t doing elective surgeries, they were certainly doing the emergency stuff.”
Meanwhile, in pediatrician offices, where Dr. Hackell said office visits dropped 70%-80% at the beginning of the pandemic, “parents weren’t going to bring a healthy kid out for routine visits and they weren’t going to bring a kid out for minor illnesses and expose them to possibly communicable diseases in the office.”
About 52% of pediatricians who lost income because of the pandemic believe their income levels will return to normal in 2-3 years. Meanwhile 30% of pediatricians expect their income to return to normal within a year, and 8% believe it will take 4 years for them to bounce back.
Physician work hours generally declined for some time during the pandemic, according to the report. However, most pediatricians are working about the same number of hours as they did before the pandemic, which is 47 hours per week.
Despite working the same number of hours per week that they did prepandemic, they are seeing fewer patients. They are currently seeing on average 64 patients per week, compared with the 78 patients they used to see weekly before the pandemic.
Dr. Hackell said that might be because pediatric offices are trying to make up the loss of revenue during the beginning of the pandemic, from the reduced number of well visits and immunizations, in the second half of the year with outreach.
“Since about June 2020, we’ve been making concerted efforts to remind parents that preventing other infectious diseases is critically important,” Dr. Hackell explained. “And so actually, for the second half of the year, many of us saw more well visits and immunization volume than in 2019 as we sought to make up the gap. It wasn’t that we were seeing more overall, but we’re trying to make up the gap that happened from March, April, May, [and] June.”
Most pediatricians find their work rewarding. One-third say the most rewarding part of their job is gratitude from and relationships with their patients. Meanwhile, 31% of pediatricians said knowing they are making the world a better place was a rewarding part of their job. Only 8% of them said making money was a rewarding part of their job.
Dr. Hackell said he did not go into pediatrics to make money, it was because he found it stimulating and has “no complaints.”
“I’ve been a pediatrician for 40 years and I wouldn’t do anything else,” Dr. Hackell said. “I don’t know that there’s anything that I would find as rewarding as the relationships that I’ve had over 40 years with my patients. You know, getting invited to weddings of kids who I saw when they were newborns is pretty impressive. It’s the gratification of having ongoing relationships with families.”
Furthermore, the report revealed that 77% of pediatricians said they would pick medicine again if they had a choice, and 82% said they would choose the same specialty.
The experts disclosed no relevant financial interests.
*This story was updated on 5/18/2021.
The average income for pediatricians declined slightly from 2019 to 2020, according to the Medscape Pediatrician Compensation Report 2021.

The report, which was conducted between October 2020 and February 2021, found that the average pediatrician income was down $11,000 – from $232,000 in 2019 to $221,000 in 2020, with 48% of pediatricians reporting at least some decline in compensation.
The specialty also earned the least amount of money in 2020, compared with all of the other specialties, which isn’t surprising since pediatricians have been among the lowest-paid physician specialties since 2013. The highest-earning specialty was plastic surgery with an average income of $526,000 annually.
Most pediatricians who saw a drop in income cited pandemic-related issues such as job loss, fewer hours, and fewer patients.
Jesse Hackell, MD, vice president and chief operating officer of Ponoma Pediatrics in New York, said in an interview the reduced wages pediatricians saw in 2020 didn’t surprise him because many pediatric offices saw a huge drop in visits that were not urgent.
“[The report] shows that procedural specialties tended to do a lot better than the nonprocedural specialties,” Dr. Hackell said. “That’s because, during the shutdown, if you broke your leg, you still needed the orthopedist. And even though the hospitals weren’t doing elective surgeries, they were certainly doing the emergency stuff.”
Meanwhile, in pediatrician offices, where Dr. Hackell said office visits dropped 70%-80% at the beginning of the pandemic, “parents weren’t going to bring a healthy kid out for routine visits and they weren’t going to bring a kid out for minor illnesses and expose them to possibly communicable diseases in the office.”
About 52% of pediatricians who lost income because of the pandemic believe their income levels will return to normal in 2-3 years. Meanwhile 30% of pediatricians expect their income to return to normal within a year, and 8% believe it will take 4 years for them to bounce back.
Physician work hours generally declined for some time during the pandemic, according to the report. However, most pediatricians are working about the same number of hours as they did before the pandemic, which is 47 hours per week.
Despite working the same number of hours per week that they did prepandemic, they are seeing fewer patients. They are currently seeing on average 64 patients per week, compared with the 78 patients they used to see weekly before the pandemic.
Dr. Hackell said that might be because pediatric offices are trying to make up the loss of revenue during the beginning of the pandemic, from the reduced number of well visits and immunizations, in the second half of the year with outreach.
“Since about June 2020, we’ve been making concerted efforts to remind parents that preventing other infectious diseases is critically important,” Dr. Hackell explained. “And so actually, for the second half of the year, many of us saw more well visits and immunization volume than in 2019 as we sought to make up the gap. It wasn’t that we were seeing more overall, but we’re trying to make up the gap that happened from March, April, May, [and] June.”
Most pediatricians find their work rewarding. One-third say the most rewarding part of their job is gratitude from and relationships with their patients. Meanwhile, 31% of pediatricians said knowing they are making the world a better place was a rewarding part of their job. Only 8% of them said making money was a rewarding part of their job.
Dr. Hackell said he did not go into pediatrics to make money, it was because he found it stimulating and has “no complaints.”
“I’ve been a pediatrician for 40 years and I wouldn’t do anything else,” Dr. Hackell said. “I don’t know that there’s anything that I would find as rewarding as the relationships that I’ve had over 40 years with my patients. You know, getting invited to weddings of kids who I saw when they were newborns is pretty impressive. It’s the gratification of having ongoing relationships with families.”
Furthermore, the report revealed that 77% of pediatricians said they would pick medicine again if they had a choice, and 82% said they would choose the same specialty.
The experts disclosed no relevant financial interests.
*This story was updated on 5/18/2021.
Are more naturopaths trying to compete with docs?
Jon Hislop, MD, PhD, hadn’t been in practice very long before patients began coming to him with requests to order tests that their naturopaths had recommended.
The family physician in North Vancouver, British Columbia, knew little about naturopathy but began researching it.
“I was finding that some of what the naturopaths were telling them was a little odd. Some of the tests they were asking for were unnecessary,” Dr. Hislop said.
The more he learned about naturopathy, the more appalled he became. He eventually took to Twitter, where he wages a campaign against naturopathy and alternative medicine.
“There is no alternative medicine,” he said. “There’s medicine and there’s other stuff. We need to stick to medicine and stay away from the other stuff.”
Dr. Hislop is not alone in his criticism of naturopathic medicine. Professional medical societies almost universally oppose naturopathy, but that has not stopped its spread or prevented it from becoming part of some health care systems.
Americans spent $30.2 billion on out-of-pocket complementary health care, according to a 2016 report from the National Institutes of Health. That includes everything from herbal supplements and massage therapy to chiropractic care.
What is naturopathic medicine?
Naturopathy came to the United States from Germany in the 1800s, but some of its practices are thousands of years old. Naturopathic treatments include homeopathy, IV vitamin infusions, acupuncture, Reiki, and herbal supplements.
Naturopathy is based on the belief that the body has an innate ability to heal itself. It discourages drugs and surgery in favor of supplements, herbs, and other so-called natural treatments. Much of it centers around addressing lifestyle issues and counseling patients to improve their diets, quit smoking, exercise more, lose weight, etc., in order to address the root causes of some health problems.
Practitioners are critical of Western medicine for what they regard as an over-reliance on drugs and technology and for treating symptoms rather than the causes of disease.
“We get a lot of people who are at the end of their ropes, people with hard-to-diagnose diseases who know they are sick but whose labs are normal,” said Jaquel Patterson, ND, former president of the American Association of Naturopathic Physicians (AANP) and medical director of a naturopathic practice in Connecticut.
Separate training and licensing
There are major differences among naturopaths.
At one extreme are unlicensed, self-taught “healers,” who can embrace everything from homeopathy to aromatherapy.
At the other end are naturopathic doctors (NDs), who are more likely to become part of health care systems. These caregivers are trained and licensed, though not by the same institutions as traditional physicians.
To be licensed, NDs must graduate from one of seven accredited naturopathic medical schools in the United States and Canada. In addition to a standard medical curriculum, schools require graduates to complete 4 years of training in clinical nutrition, acupuncture, homeopathic medicine, botanical medicine, physical medicine, and counseling. Medical students intern in clinical settings for 2 years.
NDs are eager to distinguish themselves from their uncredentialed counterparts.
“Some people go to a weekend class and call themselves naturopaths. That’s very concerning. I don’t want those people to be licensed either,” said Hallie Armstrong, ND, who practices in Michigan.
In the United States, there are 6,000 practicing NDs and an unknown number of unlicensed naturopathic healers.
Can naturopaths call themselves ‘physicians’?
Twenty-two states, the District of Columbia, Puerto Rico, and the U.S. Virgin Islands have licensing or registration laws for naturopathic doctors. Three states – South Carolina, Tennessee, and Florida – prohibit practicing naturopathic medicine without a license, according to the AANP.
States that license NDs differ in what they permit them to do.
Nine states allow licensed NDs to use the term “physician,” although this is prohibited in seven states. Most licensed states allow naturopathic practitioners some prescribing authority, including the prescribing of many controlled substances, although only a few states permit full prescribing rights. Most states that license NDs allow them to prescribe and administer nonprescription therapeutic substances, drugs, and therapies.
Twelve states and the District of Columbia allow licensed naturopathic doctors to perform some minor procedures, such as stitching up wounds. Additionally, 13 states allow NDs to order diagnostic tests.
Although the AANP lobbies to get licensure in more states and to expand the activities that NDs can perform, the medical establishment in those states nearly always opposes the legislation, as do national organizations, such as the American Academy of Family Physicians and the American College of Physicians.
“They absolutely will not stop until they get licenses. They’ve done a really good job of selling themselves as legitimate health care professionals to state legislatures,” said David Gorski, MD, PhD, FACS, a surgical oncologist and managing editor of Science-Based Medicine, a blog that attacks unproven medical claims and defends traditional medicine. Naturopathy is a favorite target.
Are naturopaths gaining ground anyway?
Despite the opposition of the medical establishment and many individual health care professionals, a growing number of health care systems are adopting alternative medicine.
In 2018, the AANP stated that 28 prominent health systems, hospitals, and cancer treatment centers had one or more licensed NDs on staff. Among them were Cancer Treatment Centers of America, Cedars-Sinai, Columbia University’s Herbert Irving Comprehensive Cancer Center, and the Fred Hutchinson Cancer Research Center.
Other health care systems may not have NDs on staff but provide naturopathic treatments, usually under the heading of “complementary medicine” or “integrative medicine.” For example, the Cleveland Clinic’s Center for Integrative and Lifestyle Medicine offers acupuncture, Chinese herbal medicine, Reiki, yoga, and culinary medicine.
Critics find this appalling.
“I think it’s a mistake to integrate that kind of practice into a science-based health care setting. If we learned anything over the past year, it’s that medicine based on magical thinking is dangerous,” said Timothy Caulfield, LLM, FCAHS, research director at the Health Law Institute of the University of Alberta, Edmonton.
Dr. Gorski added: “I’m not exactly sure why doctors who should know better have become more accepting of practices that aren’t science-based or are outright quackery.”
Becoming part of the system
Beaumont Health, Michigan’s largest health care system, added integrative medicine in 2006 and hired its first naturopathic practitioners a year later.
The integrative practitioners began in oncology, offering such things as massage therapy, acupuncture, guided imagery, and Reiki. “Very quickly, people outside oncology began saying, ‘I’ve got a cardiology patient who would really benefit from this ... I’ve got a GI patient who could benefit from this...,’” said Maureen Anderson, MD, medical director of Beaumont Integrative Medicine.
Beaumont now offers integrative medicine at three locations. They average 20,000 visits a year and work with 50 to 60 practitioners, many of whom work part-time.
Because Michigan does not license NDs, their scope of practice at Beaumont is limited. They take patient histories, provide advice on nutrition, diet, and exercise, and prescribe herbs and supplements. Beaumont operates its own herbal and supplement pharmacy.
NDs work under the medical supervision of Dr. Anderson, an emergency medicine physician who became interested in naturopathy because she thought traditional medicine doesn’t do a good job of providing care for chronic conditions. Any initial skepticism on the part of the medical staff has been overcome by seeing the benefits naturopathy provides, Dr. Anderson said. The claim is echoed by Mr. Armstrong, an ND who works in the system part-time: “As soon as [doctors] understand our schooling and where we’re coming from and understand that we want to do the same things, then they’re very accepting.”
The University of California, Irvine, health care system has one of the largest naturopathic medicine programs in the country, the result of a $200 million donation in 2017 from a couple who champion alternative medicine. The Susan Samueli Integrative Health Institute includes 28 health care professionals, including MDs, NDs, RNs, acupuncturists, dietitians, yoga instructors, and others. It includes a research arm, which is focused primarily on acupuncture.
The alternative medicine offerings benefit the system, said Kim Hecht, DO, medical director of inpatient and ambulatory services at the Samueli Institute.
“I’m not against traditional medicine, because I think everything has a time and a place,” Dr. Hecht said. However, she rejects the idea that MDs can offer the same holistic approach as NDs.
“Medical science likes to say we’re interested in treating the whole person, but if you look at medical school courses, that’s not what’s being taught,” she said.
The chance to work within a traditional health care system was attractive to Arvin Jenab, ND, medical director of naturopathic medicine at the institute.
“It offers the opportunity to refine our medicine and trim the things that aren’t necessary or are controversial and concentrate on the things at the core of what we do,” he said.
UCI Health practices a conservative model of naturopathy that supports traditional practitioners, Mr. Jenab said.
Is there any harm?
Some patients clearly want what naturopathy offers. So what’s the harm?
Health care systems that integrate alternative medicine legitimize it and lower the overall standard of care, Mr. Caulfield said. Most naturopathy claims are not backed by evidence, and making it available to patients amounts to deceiving them, he said.
“If there’s good science behind it, it’s not going to be alternative medicine; it’s going to be medicine,” Mr. Caulfield said.
Family physician Dr. Hislop said that refusing to order naturopath-recommended tests interferes with his relationships with patients and often requires lengthy conversations to explain the problems with naturopathy.
Naturopathic medicine can deter patients from seeking proven conventional treatments, which can put their health at risk, Dr. Gorski said.
Some naturopaths could potentially be harmful.
In 2017, a California woman died after receiving an IV preparation of curcumin, a chemical constituent in the Indian spice turmeric featured in alternative medicine. The U.S. Food and Drug Administration found that the treating ND mixed the curcumin emulsion product with ungraded castor oil that had a warning label stating: “CAUTION: For manufacturing or laboratory use only.”
Because naturopathic care is generally not covered by insurance, it can also be expensive for patients who pay out of pocket.
Ironically, the mainstream health care system helps create the environment in which naturopathic medicine thrives.
It offers patients a more relaxed and personal alternative to rushed visits with harried doctors scrambling to see the required number of patients in a day. By contrast, an initial visit with an ND might last a leisurely 60 minutes, with 30-minute follow-up appointments.
Mr. Caulfield acknowledged that the relaxed naturopathic approach can be more attractive to patients but said the answer is to reform the current system: “You don’t fix a broken arm by acupuncture.”
A version of this article first appeared on Medscape.com.
Jon Hislop, MD, PhD, hadn’t been in practice very long before patients began coming to him with requests to order tests that their naturopaths had recommended.
The family physician in North Vancouver, British Columbia, knew little about naturopathy but began researching it.
“I was finding that some of what the naturopaths were telling them was a little odd. Some of the tests they were asking for were unnecessary,” Dr. Hislop said.
The more he learned about naturopathy, the more appalled he became. He eventually took to Twitter, where he wages a campaign against naturopathy and alternative medicine.
“There is no alternative medicine,” he said. “There’s medicine and there’s other stuff. We need to stick to medicine and stay away from the other stuff.”
Dr. Hislop is not alone in his criticism of naturopathic medicine. Professional medical societies almost universally oppose naturopathy, but that has not stopped its spread or prevented it from becoming part of some health care systems.
Americans spent $30.2 billion on out-of-pocket complementary health care, according to a 2016 report from the National Institutes of Health. That includes everything from herbal supplements and massage therapy to chiropractic care.
What is naturopathic medicine?
Naturopathy came to the United States from Germany in the 1800s, but some of its practices are thousands of years old. Naturopathic treatments include homeopathy, IV vitamin infusions, acupuncture, Reiki, and herbal supplements.
Naturopathy is based on the belief that the body has an innate ability to heal itself. It discourages drugs and surgery in favor of supplements, herbs, and other so-called natural treatments. Much of it centers around addressing lifestyle issues and counseling patients to improve their diets, quit smoking, exercise more, lose weight, etc., in order to address the root causes of some health problems.
Practitioners are critical of Western medicine for what they regard as an over-reliance on drugs and technology and for treating symptoms rather than the causes of disease.
“We get a lot of people who are at the end of their ropes, people with hard-to-diagnose diseases who know they are sick but whose labs are normal,” said Jaquel Patterson, ND, former president of the American Association of Naturopathic Physicians (AANP) and medical director of a naturopathic practice in Connecticut.
Separate training and licensing
There are major differences among naturopaths.
At one extreme are unlicensed, self-taught “healers,” who can embrace everything from homeopathy to aromatherapy.
At the other end are naturopathic doctors (NDs), who are more likely to become part of health care systems. These caregivers are trained and licensed, though not by the same institutions as traditional physicians.
To be licensed, NDs must graduate from one of seven accredited naturopathic medical schools in the United States and Canada. In addition to a standard medical curriculum, schools require graduates to complete 4 years of training in clinical nutrition, acupuncture, homeopathic medicine, botanical medicine, physical medicine, and counseling. Medical students intern in clinical settings for 2 years.
NDs are eager to distinguish themselves from their uncredentialed counterparts.
“Some people go to a weekend class and call themselves naturopaths. That’s very concerning. I don’t want those people to be licensed either,” said Hallie Armstrong, ND, who practices in Michigan.
In the United States, there are 6,000 practicing NDs and an unknown number of unlicensed naturopathic healers.
Can naturopaths call themselves ‘physicians’?
Twenty-two states, the District of Columbia, Puerto Rico, and the U.S. Virgin Islands have licensing or registration laws for naturopathic doctors. Three states – South Carolina, Tennessee, and Florida – prohibit practicing naturopathic medicine without a license, according to the AANP.
States that license NDs differ in what they permit them to do.
Nine states allow licensed NDs to use the term “physician,” although this is prohibited in seven states. Most licensed states allow naturopathic practitioners some prescribing authority, including the prescribing of many controlled substances, although only a few states permit full prescribing rights. Most states that license NDs allow them to prescribe and administer nonprescription therapeutic substances, drugs, and therapies.
Twelve states and the District of Columbia allow licensed naturopathic doctors to perform some minor procedures, such as stitching up wounds. Additionally, 13 states allow NDs to order diagnostic tests.
Although the AANP lobbies to get licensure in more states and to expand the activities that NDs can perform, the medical establishment in those states nearly always opposes the legislation, as do national organizations, such as the American Academy of Family Physicians and the American College of Physicians.
“They absolutely will not stop until they get licenses. They’ve done a really good job of selling themselves as legitimate health care professionals to state legislatures,” said David Gorski, MD, PhD, FACS, a surgical oncologist and managing editor of Science-Based Medicine, a blog that attacks unproven medical claims and defends traditional medicine. Naturopathy is a favorite target.
Are naturopaths gaining ground anyway?
Despite the opposition of the medical establishment and many individual health care professionals, a growing number of health care systems are adopting alternative medicine.
In 2018, the AANP stated that 28 prominent health systems, hospitals, and cancer treatment centers had one or more licensed NDs on staff. Among them were Cancer Treatment Centers of America, Cedars-Sinai, Columbia University’s Herbert Irving Comprehensive Cancer Center, and the Fred Hutchinson Cancer Research Center.
Other health care systems may not have NDs on staff but provide naturopathic treatments, usually under the heading of “complementary medicine” or “integrative medicine.” For example, the Cleveland Clinic’s Center for Integrative and Lifestyle Medicine offers acupuncture, Chinese herbal medicine, Reiki, yoga, and culinary medicine.
Critics find this appalling.
“I think it’s a mistake to integrate that kind of practice into a science-based health care setting. If we learned anything over the past year, it’s that medicine based on magical thinking is dangerous,” said Timothy Caulfield, LLM, FCAHS, research director at the Health Law Institute of the University of Alberta, Edmonton.
Dr. Gorski added: “I’m not exactly sure why doctors who should know better have become more accepting of practices that aren’t science-based or are outright quackery.”
Becoming part of the system
Beaumont Health, Michigan’s largest health care system, added integrative medicine in 2006 and hired its first naturopathic practitioners a year later.
The integrative practitioners began in oncology, offering such things as massage therapy, acupuncture, guided imagery, and Reiki. “Very quickly, people outside oncology began saying, ‘I’ve got a cardiology patient who would really benefit from this ... I’ve got a GI patient who could benefit from this...,’” said Maureen Anderson, MD, medical director of Beaumont Integrative Medicine.
Beaumont now offers integrative medicine at three locations. They average 20,000 visits a year and work with 50 to 60 practitioners, many of whom work part-time.
Because Michigan does not license NDs, their scope of practice at Beaumont is limited. They take patient histories, provide advice on nutrition, diet, and exercise, and prescribe herbs and supplements. Beaumont operates its own herbal and supplement pharmacy.
NDs work under the medical supervision of Dr. Anderson, an emergency medicine physician who became interested in naturopathy because she thought traditional medicine doesn’t do a good job of providing care for chronic conditions. Any initial skepticism on the part of the medical staff has been overcome by seeing the benefits naturopathy provides, Dr. Anderson said. The claim is echoed by Mr. Armstrong, an ND who works in the system part-time: “As soon as [doctors] understand our schooling and where we’re coming from and understand that we want to do the same things, then they’re very accepting.”
The University of California, Irvine, health care system has one of the largest naturopathic medicine programs in the country, the result of a $200 million donation in 2017 from a couple who champion alternative medicine. The Susan Samueli Integrative Health Institute includes 28 health care professionals, including MDs, NDs, RNs, acupuncturists, dietitians, yoga instructors, and others. It includes a research arm, which is focused primarily on acupuncture.
The alternative medicine offerings benefit the system, said Kim Hecht, DO, medical director of inpatient and ambulatory services at the Samueli Institute.
“I’m not against traditional medicine, because I think everything has a time and a place,” Dr. Hecht said. However, she rejects the idea that MDs can offer the same holistic approach as NDs.
“Medical science likes to say we’re interested in treating the whole person, but if you look at medical school courses, that’s not what’s being taught,” she said.
The chance to work within a traditional health care system was attractive to Arvin Jenab, ND, medical director of naturopathic medicine at the institute.
“It offers the opportunity to refine our medicine and trim the things that aren’t necessary or are controversial and concentrate on the things at the core of what we do,” he said.
UCI Health practices a conservative model of naturopathy that supports traditional practitioners, Mr. Jenab said.
Is there any harm?
Some patients clearly want what naturopathy offers. So what’s the harm?
Health care systems that integrate alternative medicine legitimize it and lower the overall standard of care, Mr. Caulfield said. Most naturopathy claims are not backed by evidence, and making it available to patients amounts to deceiving them, he said.
“If there’s good science behind it, it’s not going to be alternative medicine; it’s going to be medicine,” Mr. Caulfield said.
Family physician Dr. Hislop said that refusing to order naturopath-recommended tests interferes with his relationships with patients and often requires lengthy conversations to explain the problems with naturopathy.
Naturopathic medicine can deter patients from seeking proven conventional treatments, which can put their health at risk, Dr. Gorski said.
Some naturopaths could potentially be harmful.
In 2017, a California woman died after receiving an IV preparation of curcumin, a chemical constituent in the Indian spice turmeric featured in alternative medicine. The U.S. Food and Drug Administration found that the treating ND mixed the curcumin emulsion product with ungraded castor oil that had a warning label stating: “CAUTION: For manufacturing or laboratory use only.”
Because naturopathic care is generally not covered by insurance, it can also be expensive for patients who pay out of pocket.
Ironically, the mainstream health care system helps create the environment in which naturopathic medicine thrives.
It offers patients a more relaxed and personal alternative to rushed visits with harried doctors scrambling to see the required number of patients in a day. By contrast, an initial visit with an ND might last a leisurely 60 minutes, with 30-minute follow-up appointments.
Mr. Caulfield acknowledged that the relaxed naturopathic approach can be more attractive to patients but said the answer is to reform the current system: “You don’t fix a broken arm by acupuncture.”
A version of this article first appeared on Medscape.com.
Jon Hislop, MD, PhD, hadn’t been in practice very long before patients began coming to him with requests to order tests that their naturopaths had recommended.
The family physician in North Vancouver, British Columbia, knew little about naturopathy but began researching it.
“I was finding that some of what the naturopaths were telling them was a little odd. Some of the tests they were asking for were unnecessary,” Dr. Hislop said.
The more he learned about naturopathy, the more appalled he became. He eventually took to Twitter, where he wages a campaign against naturopathy and alternative medicine.
“There is no alternative medicine,” he said. “There’s medicine and there’s other stuff. We need to stick to medicine and stay away from the other stuff.”
Dr. Hislop is not alone in his criticism of naturopathic medicine. Professional medical societies almost universally oppose naturopathy, but that has not stopped its spread or prevented it from becoming part of some health care systems.
Americans spent $30.2 billion on out-of-pocket complementary health care, according to a 2016 report from the National Institutes of Health. That includes everything from herbal supplements and massage therapy to chiropractic care.
What is naturopathic medicine?
Naturopathy came to the United States from Germany in the 1800s, but some of its practices are thousands of years old. Naturopathic treatments include homeopathy, IV vitamin infusions, acupuncture, Reiki, and herbal supplements.
Naturopathy is based on the belief that the body has an innate ability to heal itself. It discourages drugs and surgery in favor of supplements, herbs, and other so-called natural treatments. Much of it centers around addressing lifestyle issues and counseling patients to improve their diets, quit smoking, exercise more, lose weight, etc., in order to address the root causes of some health problems.
Practitioners are critical of Western medicine for what they regard as an over-reliance on drugs and technology and for treating symptoms rather than the causes of disease.
“We get a lot of people who are at the end of their ropes, people with hard-to-diagnose diseases who know they are sick but whose labs are normal,” said Jaquel Patterson, ND, former president of the American Association of Naturopathic Physicians (AANP) and medical director of a naturopathic practice in Connecticut.
Separate training and licensing
There are major differences among naturopaths.
At one extreme are unlicensed, self-taught “healers,” who can embrace everything from homeopathy to aromatherapy.
At the other end are naturopathic doctors (NDs), who are more likely to become part of health care systems. These caregivers are trained and licensed, though not by the same institutions as traditional physicians.
To be licensed, NDs must graduate from one of seven accredited naturopathic medical schools in the United States and Canada. In addition to a standard medical curriculum, schools require graduates to complete 4 years of training in clinical nutrition, acupuncture, homeopathic medicine, botanical medicine, physical medicine, and counseling. Medical students intern in clinical settings for 2 years.
NDs are eager to distinguish themselves from their uncredentialed counterparts.
“Some people go to a weekend class and call themselves naturopaths. That’s very concerning. I don’t want those people to be licensed either,” said Hallie Armstrong, ND, who practices in Michigan.
In the United States, there are 6,000 practicing NDs and an unknown number of unlicensed naturopathic healers.
Can naturopaths call themselves ‘physicians’?
Twenty-two states, the District of Columbia, Puerto Rico, and the U.S. Virgin Islands have licensing or registration laws for naturopathic doctors. Three states – South Carolina, Tennessee, and Florida – prohibit practicing naturopathic medicine without a license, according to the AANP.
States that license NDs differ in what they permit them to do.
Nine states allow licensed NDs to use the term “physician,” although this is prohibited in seven states. Most licensed states allow naturopathic practitioners some prescribing authority, including the prescribing of many controlled substances, although only a few states permit full prescribing rights. Most states that license NDs allow them to prescribe and administer nonprescription therapeutic substances, drugs, and therapies.
Twelve states and the District of Columbia allow licensed naturopathic doctors to perform some minor procedures, such as stitching up wounds. Additionally, 13 states allow NDs to order diagnostic tests.
Although the AANP lobbies to get licensure in more states and to expand the activities that NDs can perform, the medical establishment in those states nearly always opposes the legislation, as do national organizations, such as the American Academy of Family Physicians and the American College of Physicians.
“They absolutely will not stop until they get licenses. They’ve done a really good job of selling themselves as legitimate health care professionals to state legislatures,” said David Gorski, MD, PhD, FACS, a surgical oncologist and managing editor of Science-Based Medicine, a blog that attacks unproven medical claims and defends traditional medicine. Naturopathy is a favorite target.
Are naturopaths gaining ground anyway?
Despite the opposition of the medical establishment and many individual health care professionals, a growing number of health care systems are adopting alternative medicine.
In 2018, the AANP stated that 28 prominent health systems, hospitals, and cancer treatment centers had one or more licensed NDs on staff. Among them were Cancer Treatment Centers of America, Cedars-Sinai, Columbia University’s Herbert Irving Comprehensive Cancer Center, and the Fred Hutchinson Cancer Research Center.
Other health care systems may not have NDs on staff but provide naturopathic treatments, usually under the heading of “complementary medicine” or “integrative medicine.” For example, the Cleveland Clinic’s Center for Integrative and Lifestyle Medicine offers acupuncture, Chinese herbal medicine, Reiki, yoga, and culinary medicine.
Critics find this appalling.
“I think it’s a mistake to integrate that kind of practice into a science-based health care setting. If we learned anything over the past year, it’s that medicine based on magical thinking is dangerous,” said Timothy Caulfield, LLM, FCAHS, research director at the Health Law Institute of the University of Alberta, Edmonton.
Dr. Gorski added: “I’m not exactly sure why doctors who should know better have become more accepting of practices that aren’t science-based or are outright quackery.”
Becoming part of the system
Beaumont Health, Michigan’s largest health care system, added integrative medicine in 2006 and hired its first naturopathic practitioners a year later.
The integrative practitioners began in oncology, offering such things as massage therapy, acupuncture, guided imagery, and Reiki. “Very quickly, people outside oncology began saying, ‘I’ve got a cardiology patient who would really benefit from this ... I’ve got a GI patient who could benefit from this...,’” said Maureen Anderson, MD, medical director of Beaumont Integrative Medicine.
Beaumont now offers integrative medicine at three locations. They average 20,000 visits a year and work with 50 to 60 practitioners, many of whom work part-time.
Because Michigan does not license NDs, their scope of practice at Beaumont is limited. They take patient histories, provide advice on nutrition, diet, and exercise, and prescribe herbs and supplements. Beaumont operates its own herbal and supplement pharmacy.
NDs work under the medical supervision of Dr. Anderson, an emergency medicine physician who became interested in naturopathy because she thought traditional medicine doesn’t do a good job of providing care for chronic conditions. Any initial skepticism on the part of the medical staff has been overcome by seeing the benefits naturopathy provides, Dr. Anderson said. The claim is echoed by Mr. Armstrong, an ND who works in the system part-time: “As soon as [doctors] understand our schooling and where we’re coming from and understand that we want to do the same things, then they’re very accepting.”
The University of California, Irvine, health care system has one of the largest naturopathic medicine programs in the country, the result of a $200 million donation in 2017 from a couple who champion alternative medicine. The Susan Samueli Integrative Health Institute includes 28 health care professionals, including MDs, NDs, RNs, acupuncturists, dietitians, yoga instructors, and others. It includes a research arm, which is focused primarily on acupuncture.
The alternative medicine offerings benefit the system, said Kim Hecht, DO, medical director of inpatient and ambulatory services at the Samueli Institute.
“I’m not against traditional medicine, because I think everything has a time and a place,” Dr. Hecht said. However, she rejects the idea that MDs can offer the same holistic approach as NDs.
“Medical science likes to say we’re interested in treating the whole person, but if you look at medical school courses, that’s not what’s being taught,” she said.
The chance to work within a traditional health care system was attractive to Arvin Jenab, ND, medical director of naturopathic medicine at the institute.
“It offers the opportunity to refine our medicine and trim the things that aren’t necessary or are controversial and concentrate on the things at the core of what we do,” he said.
UCI Health practices a conservative model of naturopathy that supports traditional practitioners, Mr. Jenab said.
Is there any harm?
Some patients clearly want what naturopathy offers. So what’s the harm?
Health care systems that integrate alternative medicine legitimize it and lower the overall standard of care, Mr. Caulfield said. Most naturopathy claims are not backed by evidence, and making it available to patients amounts to deceiving them, he said.
“If there’s good science behind it, it’s not going to be alternative medicine; it’s going to be medicine,” Mr. Caulfield said.
Family physician Dr. Hislop said that refusing to order naturopath-recommended tests interferes with his relationships with patients and often requires lengthy conversations to explain the problems with naturopathy.
Naturopathic medicine can deter patients from seeking proven conventional treatments, which can put their health at risk, Dr. Gorski said.
Some naturopaths could potentially be harmful.
In 2017, a California woman died after receiving an IV preparation of curcumin, a chemical constituent in the Indian spice turmeric featured in alternative medicine. The U.S. Food and Drug Administration found that the treating ND mixed the curcumin emulsion product with ungraded castor oil that had a warning label stating: “CAUTION: For manufacturing or laboratory use only.”
Because naturopathic care is generally not covered by insurance, it can also be expensive for patients who pay out of pocket.
Ironically, the mainstream health care system helps create the environment in which naturopathic medicine thrives.
It offers patients a more relaxed and personal alternative to rushed visits with harried doctors scrambling to see the required number of patients in a day. By contrast, an initial visit with an ND might last a leisurely 60 minutes, with 30-minute follow-up appointments.
Mr. Caulfield acknowledged that the relaxed naturopathic approach can be more attractive to patients but said the answer is to reform the current system: “You don’t fix a broken arm by acupuncture.”
A version of this article first appeared on Medscape.com.











