User login
ID doctors have the most paperwork, administrative demands
Infectious disease physicians are among the doctors carrying the largest burdens in the COVID-19 pandemic.
Perhaps not surprisingly, they were the specialists least likely to feel they were fairly compensated in the Medscape Infectious Diseases Physician Compensation Report 2021.
Only 44% said the pay was fair (down from 51% the prior year) compared with those at the high end – 79% in oncology, 69% in psychiatry, and 68% in plastic surgery who answered that way.
Income, which averaged $245,000, varied little from the previous year overall, according to the survey, but nearly one-third of ID physicians saw a decline in pay.
Again this year, ID physicians ranked near the bottom on the compensation spectrum. Pediatricians were lowest paid at $221,000. Plastic surgeons topped the chart at $526,000, followed by orthopedists at $511,000.
At the same time, the ID specialty is facing increasing shortages, a gap made even more visible in the pandemic. Medscape reported last year that nearly 80% of U.S. counties have no infectious disease specialists.
Thomas File Jr., MD, last year’s president of the Infectious Diseases Society of America, emphasized that COVID-19 is not the only threat that ID specialists have had to deal with or will have. He cited the threats that Zika and SARS posed in past years.
“COVID-19 illustrates the need for more trained ID specialists, because we know we’re going to be seeing more outbreaks in the future,” he said in an interview at the onset of the pandemic in March 2020.
Longer hours in pandemic
ID physicians’ hours generally increased during the pandemic, and they remain inflated by 8 hours per week (60 compared with 52 prepandemic) as the nation struggles to manage continuing COVID-19 infections. Physicians in critical care and public health and preventive medicine are seeing heavier workloads as well, by an average of 6-7 hours per week.
At the same time, ID physicians spent the most time of physicians in all specialties on paperwork and administrative tasks. Those tasks, which include electronic health record entry and clinical reading, took ID doctors 24.2 hours a week, more the twice the hours spent by those in anesthesiology (10.1), ophthalmology (10.3), and radiology (11.6).
The 24.2 hours was a substantial increase from the last report, when ID physicians said they spent 18.5 hours on the tasks.
The survey asked about the most challenging part of the job. ID physicians reported “long hours” as number one followed by “having so many rules and regulations.”
Only 4% said the danger or risk associated with treating COVID-19 patients was the most challenging part.
The top two aspects of their work they deemed most rewarding were “being very good at what I do” (chosen by 33%) and “knowing that I’m making the world a better place” (31%).
Patient volume up 17%
ID physicians reported seeing 78 patients per week in this report compared with 66 prepandemic, a 17% increase. Conversely, pediatricians saw an 18% drop in patient visits, followed by dermatologists, orthopedists and otolaryngologists (all down about 15%).
Despite the challenges and dissatisfaction with pay, the great majority of ID physicians said they would choose both medicine (83%) and their specialty (89%, up from 85% last year) again.
A version of this article first appeared on Medscape.com.
Infectious disease physicians are among the doctors carrying the largest burdens in the COVID-19 pandemic.
Perhaps not surprisingly, they were the specialists least likely to feel they were fairly compensated in the Medscape Infectious Diseases Physician Compensation Report 2021.
Only 44% said the pay was fair (down from 51% the prior year) compared with those at the high end – 79% in oncology, 69% in psychiatry, and 68% in plastic surgery who answered that way.
Income, which averaged $245,000, varied little from the previous year overall, according to the survey, but nearly one-third of ID physicians saw a decline in pay.
Again this year, ID physicians ranked near the bottom on the compensation spectrum. Pediatricians were lowest paid at $221,000. Plastic surgeons topped the chart at $526,000, followed by orthopedists at $511,000.
At the same time, the ID specialty is facing increasing shortages, a gap made even more visible in the pandemic. Medscape reported last year that nearly 80% of U.S. counties have no infectious disease specialists.
Thomas File Jr., MD, last year’s president of the Infectious Diseases Society of America, emphasized that COVID-19 is not the only threat that ID specialists have had to deal with or will have. He cited the threats that Zika and SARS posed in past years.
“COVID-19 illustrates the need for more trained ID specialists, because we know we’re going to be seeing more outbreaks in the future,” he said in an interview at the onset of the pandemic in March 2020.
Longer hours in pandemic
ID physicians’ hours generally increased during the pandemic, and they remain inflated by 8 hours per week (60 compared with 52 prepandemic) as the nation struggles to manage continuing COVID-19 infections. Physicians in critical care and public health and preventive medicine are seeing heavier workloads as well, by an average of 6-7 hours per week.
At the same time, ID physicians spent the most time of physicians in all specialties on paperwork and administrative tasks. Those tasks, which include electronic health record entry and clinical reading, took ID doctors 24.2 hours a week, more the twice the hours spent by those in anesthesiology (10.1), ophthalmology (10.3), and radiology (11.6).
The 24.2 hours was a substantial increase from the last report, when ID physicians said they spent 18.5 hours on the tasks.
The survey asked about the most challenging part of the job. ID physicians reported “long hours” as number one followed by “having so many rules and regulations.”
Only 4% said the danger or risk associated with treating COVID-19 patients was the most challenging part.
The top two aspects of their work they deemed most rewarding were “being very good at what I do” (chosen by 33%) and “knowing that I’m making the world a better place” (31%).
Patient volume up 17%
ID physicians reported seeing 78 patients per week in this report compared with 66 prepandemic, a 17% increase. Conversely, pediatricians saw an 18% drop in patient visits, followed by dermatologists, orthopedists and otolaryngologists (all down about 15%).
Despite the challenges and dissatisfaction with pay, the great majority of ID physicians said they would choose both medicine (83%) and their specialty (89%, up from 85% last year) again.
A version of this article first appeared on Medscape.com.
Infectious disease physicians are among the doctors carrying the largest burdens in the COVID-19 pandemic.
Perhaps not surprisingly, they were the specialists least likely to feel they were fairly compensated in the Medscape Infectious Diseases Physician Compensation Report 2021.
Only 44% said the pay was fair (down from 51% the prior year) compared with those at the high end – 79% in oncology, 69% in psychiatry, and 68% in plastic surgery who answered that way.
Income, which averaged $245,000, varied little from the previous year overall, according to the survey, but nearly one-third of ID physicians saw a decline in pay.
Again this year, ID physicians ranked near the bottom on the compensation spectrum. Pediatricians were lowest paid at $221,000. Plastic surgeons topped the chart at $526,000, followed by orthopedists at $511,000.
At the same time, the ID specialty is facing increasing shortages, a gap made even more visible in the pandemic. Medscape reported last year that nearly 80% of U.S. counties have no infectious disease specialists.
Thomas File Jr., MD, last year’s president of the Infectious Diseases Society of America, emphasized that COVID-19 is not the only threat that ID specialists have had to deal with or will have. He cited the threats that Zika and SARS posed in past years.
“COVID-19 illustrates the need for more trained ID specialists, because we know we’re going to be seeing more outbreaks in the future,” he said in an interview at the onset of the pandemic in March 2020.
Longer hours in pandemic
ID physicians’ hours generally increased during the pandemic, and they remain inflated by 8 hours per week (60 compared with 52 prepandemic) as the nation struggles to manage continuing COVID-19 infections. Physicians in critical care and public health and preventive medicine are seeing heavier workloads as well, by an average of 6-7 hours per week.
At the same time, ID physicians spent the most time of physicians in all specialties on paperwork and administrative tasks. Those tasks, which include electronic health record entry and clinical reading, took ID doctors 24.2 hours a week, more the twice the hours spent by those in anesthesiology (10.1), ophthalmology (10.3), and radiology (11.6).
The 24.2 hours was a substantial increase from the last report, when ID physicians said they spent 18.5 hours on the tasks.
The survey asked about the most challenging part of the job. ID physicians reported “long hours” as number one followed by “having so many rules and regulations.”
Only 4% said the danger or risk associated with treating COVID-19 patients was the most challenging part.
The top two aspects of their work they deemed most rewarding were “being very good at what I do” (chosen by 33%) and “knowing that I’m making the world a better place” (31%).
Patient volume up 17%
ID physicians reported seeing 78 patients per week in this report compared with 66 prepandemic, a 17% increase. Conversely, pediatricians saw an 18% drop in patient visits, followed by dermatologists, orthopedists and otolaryngologists (all down about 15%).
Despite the challenges and dissatisfaction with pay, the great majority of ID physicians said they would choose both medicine (83%) and their specialty (89%, up from 85% last year) again.
A version of this article first appeared on Medscape.com.
Admit or send home for GI bleeding? AI may help you decide
GI Genius recently became the first Food and Drug Administration–approved device to use artificial intelligence (AI) for endoscopy. Soon, similar technology may give gastroenterologists an edge before they even walk into the procedure room.
AI can provide highly accurate risk scores for patients with suspected upper GI bleeding, and make a recommendation for discharge or hospitalization, according to Dennis Shung, MD, MHS, a clinical instructor at Yale University, New Haven, Conn. And this could provide extensive benefit.
“Acute gastrointestinal bleeding is the most common gastrointestinal diagnosis requiring hospitalization. It costs around $19.2 billion per year,” Dr. Shung said, citing a study from Gastroenterology. He made these remarks during a virtual presentation at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Emergency department visits for upper GI bleeding increased 17% from 2006 to 2014, Dr. Shung added, suggesting a rising trend.
The trouble with using risk scores
A variety of conventional risk scores are presently available to help manage these patients. Generally, they use a composite outcome of hemostatic intervention, transfusion, or death to determine which patients should be hospitalized (high risk) and which patients can go home (low risk). Although these models can offer high sensitivity, they remain underutilized.
“[Clinical risk scores] are cumbersome, it’s difficult to calculate them, [and] you may not remember to do that in your busy workflow,” Dr. Shung said.
He pointed out that low implementation may also stem from poorly defined clinical responsibilities.
“[Observing] providers caring for patients with GI bleeding showed that there was a culture of not taking ownership,” he said. “Emergency department physicians thought that it was the gastroenterologists who needed to [perform risk scoring]. Gastroenterologists thought it was the ED [physicians’ responsibility].”
To overcome these pitfalls, Dr. Shung and colleagues are developing AI that automates risk analysis for upper GI bleeding by integrating the process into the clinical workflow. Like GI Genius, their strategy relies upon machine learning, which is a type of AI that can improve automatically without being explicitly programmed.
Their most recent study (Sci Rep. 2021 Apr 23;11[1]:8827) involved a machine learning model that could predict transfusion in patients admitted for acute GI bleeding. The model was developed and internally validated in a cohort of 2,524 patients, then shown to outperform conventional regression-based models when externally validated in 1,526 patients similarly admitted at large urban hospitals.
Google Maps for GI bleeding
“The future, as I envision it, is a Google Maps for GI bleeding,” Dr. Shung said, referring to how the popular web-mapping product analyzes real-time data, such as weather and traffic patterns, to provide the best route and an estimated time of arrival. “With the electronic health record, we have the ability to personalize care by basically using data obtained during the clinical encounter to generate risk assessment in real time.”
In other words, machine learning software reads a patient’s electronic health record, runs relevant data through an algorithm, and produces both a risk score and a clinical recommendation. In the case of suspected upper GI bleeding, the clinician is advised to either discharge for outpatient endoscopy or hospitalize for inpatient evaluation.
Because the quality and consistency of data in EHRs can vary, the most advanced form of machine learning – deep learning – is needed to make this a clinical reality. Deep learning converts simpler concepts into complex ones. In this scenario, that would mean deciding which clinical data are relevant and which are just noise. Taking this a step further, deep learning can actually “draw conclusions” from what’s missing.
“There are huge challenges in [irregular data] that need to be overcome,” Dr. Shung said in an interview. “But I see it as an opportunity. When you see things that are irregularly sampled, when you see things are missing – they mean something. They mean that a human has decided that that is not the way we should do things because this patient doesn’t need it. And I think there is a lot of value in learning how to model those things.”
The road to clinical implementation
With further research and validation, deep learning models for gastroenterology are likely to play a role in clinical decision-making, according to Dr. Shung. But to reach the clinic floor, developers will need to outsmart some more fundamental obstacles. “The main thing that’s really barring [AI risk modeling] from being used is the reimbursement issue,” he said, referring to uncertainty in how payers will cover associated costs.
In an interview, Sushovan Guha, MD, PhD, moderator of the virtual session and codirector of the center for interventional gastroenterology at UTHealth (iGUT) in Houston, pointed out another financial unknown: liability.
“What happens if there is an error?” he asked. “It’s done by the computers, but who is at fault?”
In addition to these challenges, some clinicians may need to be persuaded before they are willing to trust an algorithm with a patient’s life.
“We have to have community physicians convinced about the importance of using these tools to further improve their clinical practice,” Dr. Guha said. To this end, he added, “It’s time for us to accept and adapt, and make our decision-making process much more efficient.”
The investigators disclosed no relevant conflicts of interest.
GI Genius recently became the first Food and Drug Administration–approved device to use artificial intelligence (AI) for endoscopy. Soon, similar technology may give gastroenterologists an edge before they even walk into the procedure room.
AI can provide highly accurate risk scores for patients with suspected upper GI bleeding, and make a recommendation for discharge or hospitalization, according to Dennis Shung, MD, MHS, a clinical instructor at Yale University, New Haven, Conn. And this could provide extensive benefit.
“Acute gastrointestinal bleeding is the most common gastrointestinal diagnosis requiring hospitalization. It costs around $19.2 billion per year,” Dr. Shung said, citing a study from Gastroenterology. He made these remarks during a virtual presentation at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Emergency department visits for upper GI bleeding increased 17% from 2006 to 2014, Dr. Shung added, suggesting a rising trend.
The trouble with using risk scores
A variety of conventional risk scores are presently available to help manage these patients. Generally, they use a composite outcome of hemostatic intervention, transfusion, or death to determine which patients should be hospitalized (high risk) and which patients can go home (low risk). Although these models can offer high sensitivity, they remain underutilized.
“[Clinical risk scores] are cumbersome, it’s difficult to calculate them, [and] you may not remember to do that in your busy workflow,” Dr. Shung said.
He pointed out that low implementation may also stem from poorly defined clinical responsibilities.
“[Observing] providers caring for patients with GI bleeding showed that there was a culture of not taking ownership,” he said. “Emergency department physicians thought that it was the gastroenterologists who needed to [perform risk scoring]. Gastroenterologists thought it was the ED [physicians’ responsibility].”
To overcome these pitfalls, Dr. Shung and colleagues are developing AI that automates risk analysis for upper GI bleeding by integrating the process into the clinical workflow. Like GI Genius, their strategy relies upon machine learning, which is a type of AI that can improve automatically without being explicitly programmed.
Their most recent study (Sci Rep. 2021 Apr 23;11[1]:8827) involved a machine learning model that could predict transfusion in patients admitted for acute GI bleeding. The model was developed and internally validated in a cohort of 2,524 patients, then shown to outperform conventional regression-based models when externally validated in 1,526 patients similarly admitted at large urban hospitals.
Google Maps for GI bleeding
“The future, as I envision it, is a Google Maps for GI bleeding,” Dr. Shung said, referring to how the popular web-mapping product analyzes real-time data, such as weather and traffic patterns, to provide the best route and an estimated time of arrival. “With the electronic health record, we have the ability to personalize care by basically using data obtained during the clinical encounter to generate risk assessment in real time.”
In other words, machine learning software reads a patient’s electronic health record, runs relevant data through an algorithm, and produces both a risk score and a clinical recommendation. In the case of suspected upper GI bleeding, the clinician is advised to either discharge for outpatient endoscopy or hospitalize for inpatient evaluation.
Because the quality and consistency of data in EHRs can vary, the most advanced form of machine learning – deep learning – is needed to make this a clinical reality. Deep learning converts simpler concepts into complex ones. In this scenario, that would mean deciding which clinical data are relevant and which are just noise. Taking this a step further, deep learning can actually “draw conclusions” from what’s missing.
“There are huge challenges in [irregular data] that need to be overcome,” Dr. Shung said in an interview. “But I see it as an opportunity. When you see things that are irregularly sampled, when you see things are missing – they mean something. They mean that a human has decided that that is not the way we should do things because this patient doesn’t need it. And I think there is a lot of value in learning how to model those things.”
The road to clinical implementation
With further research and validation, deep learning models for gastroenterology are likely to play a role in clinical decision-making, according to Dr. Shung. But to reach the clinic floor, developers will need to outsmart some more fundamental obstacles. “The main thing that’s really barring [AI risk modeling] from being used is the reimbursement issue,” he said, referring to uncertainty in how payers will cover associated costs.
In an interview, Sushovan Guha, MD, PhD, moderator of the virtual session and codirector of the center for interventional gastroenterology at UTHealth (iGUT) in Houston, pointed out another financial unknown: liability.
“What happens if there is an error?” he asked. “It’s done by the computers, but who is at fault?”
In addition to these challenges, some clinicians may need to be persuaded before they are willing to trust an algorithm with a patient’s life.
“We have to have community physicians convinced about the importance of using these tools to further improve their clinical practice,” Dr. Guha said. To this end, he added, “It’s time for us to accept and adapt, and make our decision-making process much more efficient.”
The investigators disclosed no relevant conflicts of interest.
GI Genius recently became the first Food and Drug Administration–approved device to use artificial intelligence (AI) for endoscopy. Soon, similar technology may give gastroenterologists an edge before they even walk into the procedure room.
AI can provide highly accurate risk scores for patients with suspected upper GI bleeding, and make a recommendation for discharge or hospitalization, according to Dennis Shung, MD, MHS, a clinical instructor at Yale University, New Haven, Conn. And this could provide extensive benefit.
“Acute gastrointestinal bleeding is the most common gastrointestinal diagnosis requiring hospitalization. It costs around $19.2 billion per year,” Dr. Shung said, citing a study from Gastroenterology. He made these remarks during a virtual presentation at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Emergency department visits for upper GI bleeding increased 17% from 2006 to 2014, Dr. Shung added, suggesting a rising trend.
The trouble with using risk scores
A variety of conventional risk scores are presently available to help manage these patients. Generally, they use a composite outcome of hemostatic intervention, transfusion, or death to determine which patients should be hospitalized (high risk) and which patients can go home (low risk). Although these models can offer high sensitivity, they remain underutilized.
“[Clinical risk scores] are cumbersome, it’s difficult to calculate them, [and] you may not remember to do that in your busy workflow,” Dr. Shung said.
He pointed out that low implementation may also stem from poorly defined clinical responsibilities.
“[Observing] providers caring for patients with GI bleeding showed that there was a culture of not taking ownership,” he said. “Emergency department physicians thought that it was the gastroenterologists who needed to [perform risk scoring]. Gastroenterologists thought it was the ED [physicians’ responsibility].”
To overcome these pitfalls, Dr. Shung and colleagues are developing AI that automates risk analysis for upper GI bleeding by integrating the process into the clinical workflow. Like GI Genius, their strategy relies upon machine learning, which is a type of AI that can improve automatically without being explicitly programmed.
Their most recent study (Sci Rep. 2021 Apr 23;11[1]:8827) involved a machine learning model that could predict transfusion in patients admitted for acute GI bleeding. The model was developed and internally validated in a cohort of 2,524 patients, then shown to outperform conventional regression-based models when externally validated in 1,526 patients similarly admitted at large urban hospitals.
Google Maps for GI bleeding
“The future, as I envision it, is a Google Maps for GI bleeding,” Dr. Shung said, referring to how the popular web-mapping product analyzes real-time data, such as weather and traffic patterns, to provide the best route and an estimated time of arrival. “With the electronic health record, we have the ability to personalize care by basically using data obtained during the clinical encounter to generate risk assessment in real time.”
In other words, machine learning software reads a patient’s electronic health record, runs relevant data through an algorithm, and produces both a risk score and a clinical recommendation. In the case of suspected upper GI bleeding, the clinician is advised to either discharge for outpatient endoscopy or hospitalize for inpatient evaluation.
Because the quality and consistency of data in EHRs can vary, the most advanced form of machine learning – deep learning – is needed to make this a clinical reality. Deep learning converts simpler concepts into complex ones. In this scenario, that would mean deciding which clinical data are relevant and which are just noise. Taking this a step further, deep learning can actually “draw conclusions” from what’s missing.
“There are huge challenges in [irregular data] that need to be overcome,” Dr. Shung said in an interview. “But I see it as an opportunity. When you see things that are irregularly sampled, when you see things are missing – they mean something. They mean that a human has decided that that is not the way we should do things because this patient doesn’t need it. And I think there is a lot of value in learning how to model those things.”
The road to clinical implementation
With further research and validation, deep learning models for gastroenterology are likely to play a role in clinical decision-making, according to Dr. Shung. But to reach the clinic floor, developers will need to outsmart some more fundamental obstacles. “The main thing that’s really barring [AI risk modeling] from being used is the reimbursement issue,” he said, referring to uncertainty in how payers will cover associated costs.
In an interview, Sushovan Guha, MD, PhD, moderator of the virtual session and codirector of the center for interventional gastroenterology at UTHealth (iGUT) in Houston, pointed out another financial unknown: liability.
“What happens if there is an error?” he asked. “It’s done by the computers, but who is at fault?”
In addition to these challenges, some clinicians may need to be persuaded before they are willing to trust an algorithm with a patient’s life.
“We have to have community physicians convinced about the importance of using these tools to further improve their clinical practice,” Dr. Guha said. To this end, he added, “It’s time for us to accept and adapt, and make our decision-making process much more efficient.”
The investigators disclosed no relevant conflicts of interest.
FROM 2021 AGA TECH SUMMIT
COVID-19 fallout makes case for promoting the mental health czar
When the Biden administration announced who would serve on its COVID-19 task force, some asked why a mental health expert had not been included. I have a broader question: In light of the magnitude of the pandemic’s fallout, why doesn’t the administration create a mental health post parallel to the surgeon general?
I have been making the case for creation of a high-level mental health post for quite some time. In fact, in the late 1970s, toward the end of then-President Jimmy Carter’s term, I wrote and talked about the need for a special cabinet post of mental health. At the time I realized that, besides chronic mental disorders, the amount of mental distress people experienced from a myriad of life issues leading to anxiety, depression, even posttraumatic stress disorder (although not labeled as such then), needed focused and informed leadership.
Before the pandemic, the World Health Organization reported that depression was the leading cause of disability worldwide. In the prepandemic United States, mental and substance use disorders were the top cause of disability among younger people.
We’ve lost almost 600,000 people to COVID-19, and people have been unable to grieve properly. More than 2 million women have left the labor force to care for children and sick family members. As we continue to learn about the mental health–related devastation wrought by SARS-CoV-2 – particularly long-haul COVID-19 – it’s time to dust off my proposal, update it, and implement it.
Building on a good decision
Back in 2017, President Trump appointed Elinore F. McCance-Katz, MD, PhD, to a new post officially called “assistant secretary for mental health and substance use” and unofficially called the “mental health czar.” This was a groundbreaking step, because Dr. McCance-Katz, a psychiatrist, is known for developing innovative approaches to addressing the opioid crisis in her home state of Rhode Island. She resigned from her post on Jan. 7, 2021, citing her concerns about the Jan. 6 insurrection on the U.S. Capitol.
As of this writing, President Biden has nominated psychologist Miriam Delphin-Rittmon, PhD, who is commissioner of Connecticut Department of Mental Health and Addiction Services, as mental health czar. I’m glad to see that the new administration wants a new czar, but I would prefer to see a more expansive role for a mental health professional at the federal level. The reason is because
Processing the current crisis
Americans managed to recover emotionally from the ravages of death and dying from World War II; we lived through the “atomic age” of mutual destruction, sometimes calling it the age of anxiety. But nothing has come close to the overwhelming devastation that COVID-19 has brought to the world – and to this country.
A recent Government Accountability Office report shows 38% of U.S. adults reported symptoms of anxiety or depression from April 2020 through February 2021. That was up from 11% from January to June 2019, the report said, citing data from the Centers for Disease Control and Prevention. Meanwhile, the report cites data from the Substance Abuse and Mental Health Services Administration showing that opioid deaths were 25%-50% higher during the pandemic than a year earlier.
My sense is that people generally have opened up regarding their emotional problems in a freer manner, thus allowing us to speak about and accept mental health problems as part of our human reality – just as we accept physical disorders and search for treatment and care.
In terms of talk therapy, I still believe that the “thinking” therapies, that is, cognitive therapies that involved getting a new perspective on problems, are most effective in dealing with the myriad of emotional issues people experience as well as those that have arisen because of COVID-19, and the tremendous fear of severe illness and death that the virus can bring. Besides anxiety, depression, and fear, the psychological toll of a fractured lifestyle, coupled with social isolation, will lead many into a variety of PTSD-related conditions. Many of those conditions, including PTSD, might lift when COVID-19 is controlled, but the time frame for resolution is far from clear and will vary, depending on each person. National leadership, as well as therapists, need to be ready to work with the many mental health problems COVID-19 will leave in its wake.
Therapeutically, as we develop our cognitive approaches to the problems this pandemic has brought, whether affecting people with no past psychiatric history or those with a previous or ongoing problems, we are in a unique position ourselves to offer even more support based on our own experiences during the pandemic. Our patients have seen us wear masks and work remotely, and just as we know about their suffering, they know we have been affected as well. These shared experiences with patients can allow us to express even greater empathy and offer even greater support – which I believe enhances the cognitive process and adds more humanism to the therapeutic process.
The therapists I’ve talked with believe that sharing coping skills – even generally sharing anxieties – can be very therapeutic. They compared these exchanges to what is done in support or educational groups.
As a psychiatrist who has been treating patients using cognitive-behavioral therapy – the thinking therapy – for more than 40 years, I agree that sharing our experiences in this worldwide pandemic with those we are helping can be extremely beneficial. Using this approach would not distract from other cognitive work. CBT, after all, is a far cry from dynamic or psychoanalytic talking or listening.
Change is in the air. More and more Americans are getting vaccinated, and the CDC is constantly updating its guidance on COVID-19. That guidance should have a mental health component.
I urge the president to put mental health at the forefront by nominating an expert who could offer mental health solutions on a daily basis. This person should be on equal footing with the surgeon general. Taking this step would help destigmatize mental suffering and despair – and create greater awareness about how to address those conditions.
Dr. London has been a practicing psychiatrist for 4 decades and a newspaper columnist for almost as long. He has a private practice in New York and is author of “Find Freedom Fast: Short-Term Therapy That Works” (New York: Kettlehole Publishing, 2019). Dr. London has no conflicts of interest.
When the Biden administration announced who would serve on its COVID-19 task force, some asked why a mental health expert had not been included. I have a broader question: In light of the magnitude of the pandemic’s fallout, why doesn’t the administration create a mental health post parallel to the surgeon general?
I have been making the case for creation of a high-level mental health post for quite some time. In fact, in the late 1970s, toward the end of then-President Jimmy Carter’s term, I wrote and talked about the need for a special cabinet post of mental health. At the time I realized that, besides chronic mental disorders, the amount of mental distress people experienced from a myriad of life issues leading to anxiety, depression, even posttraumatic stress disorder (although not labeled as such then), needed focused and informed leadership.
Before the pandemic, the World Health Organization reported that depression was the leading cause of disability worldwide. In the prepandemic United States, mental and substance use disorders were the top cause of disability among younger people.
We’ve lost almost 600,000 people to COVID-19, and people have been unable to grieve properly. More than 2 million women have left the labor force to care for children and sick family members. As we continue to learn about the mental health–related devastation wrought by SARS-CoV-2 – particularly long-haul COVID-19 – it’s time to dust off my proposal, update it, and implement it.
Building on a good decision
Back in 2017, President Trump appointed Elinore F. McCance-Katz, MD, PhD, to a new post officially called “assistant secretary for mental health and substance use” and unofficially called the “mental health czar.” This was a groundbreaking step, because Dr. McCance-Katz, a psychiatrist, is known for developing innovative approaches to addressing the opioid crisis in her home state of Rhode Island. She resigned from her post on Jan. 7, 2021, citing her concerns about the Jan. 6 insurrection on the U.S. Capitol.
As of this writing, President Biden has nominated psychologist Miriam Delphin-Rittmon, PhD, who is commissioner of Connecticut Department of Mental Health and Addiction Services, as mental health czar. I’m glad to see that the new administration wants a new czar, but I would prefer to see a more expansive role for a mental health professional at the federal level. The reason is because
Processing the current crisis
Americans managed to recover emotionally from the ravages of death and dying from World War II; we lived through the “atomic age” of mutual destruction, sometimes calling it the age of anxiety. But nothing has come close to the overwhelming devastation that COVID-19 has brought to the world – and to this country.
A recent Government Accountability Office report shows 38% of U.S. adults reported symptoms of anxiety or depression from April 2020 through February 2021. That was up from 11% from January to June 2019, the report said, citing data from the Centers for Disease Control and Prevention. Meanwhile, the report cites data from the Substance Abuse and Mental Health Services Administration showing that opioid deaths were 25%-50% higher during the pandemic than a year earlier.
My sense is that people generally have opened up regarding their emotional problems in a freer manner, thus allowing us to speak about and accept mental health problems as part of our human reality – just as we accept physical disorders and search for treatment and care.
In terms of talk therapy, I still believe that the “thinking” therapies, that is, cognitive therapies that involved getting a new perspective on problems, are most effective in dealing with the myriad of emotional issues people experience as well as those that have arisen because of COVID-19, and the tremendous fear of severe illness and death that the virus can bring. Besides anxiety, depression, and fear, the psychological toll of a fractured lifestyle, coupled with social isolation, will lead many into a variety of PTSD-related conditions. Many of those conditions, including PTSD, might lift when COVID-19 is controlled, but the time frame for resolution is far from clear and will vary, depending on each person. National leadership, as well as therapists, need to be ready to work with the many mental health problems COVID-19 will leave in its wake.
Therapeutically, as we develop our cognitive approaches to the problems this pandemic has brought, whether affecting people with no past psychiatric history or those with a previous or ongoing problems, we are in a unique position ourselves to offer even more support based on our own experiences during the pandemic. Our patients have seen us wear masks and work remotely, and just as we know about their suffering, they know we have been affected as well. These shared experiences with patients can allow us to express even greater empathy and offer even greater support – which I believe enhances the cognitive process and adds more humanism to the therapeutic process.
The therapists I’ve talked with believe that sharing coping skills – even generally sharing anxieties – can be very therapeutic. They compared these exchanges to what is done in support or educational groups.
As a psychiatrist who has been treating patients using cognitive-behavioral therapy – the thinking therapy – for more than 40 years, I agree that sharing our experiences in this worldwide pandemic with those we are helping can be extremely beneficial. Using this approach would not distract from other cognitive work. CBT, after all, is a far cry from dynamic or psychoanalytic talking or listening.
Change is in the air. More and more Americans are getting vaccinated, and the CDC is constantly updating its guidance on COVID-19. That guidance should have a mental health component.
I urge the president to put mental health at the forefront by nominating an expert who could offer mental health solutions on a daily basis. This person should be on equal footing with the surgeon general. Taking this step would help destigmatize mental suffering and despair – and create greater awareness about how to address those conditions.
Dr. London has been a practicing psychiatrist for 4 decades and a newspaper columnist for almost as long. He has a private practice in New York and is author of “Find Freedom Fast: Short-Term Therapy That Works” (New York: Kettlehole Publishing, 2019). Dr. London has no conflicts of interest.
When the Biden administration announced who would serve on its COVID-19 task force, some asked why a mental health expert had not been included. I have a broader question: In light of the magnitude of the pandemic’s fallout, why doesn’t the administration create a mental health post parallel to the surgeon general?
I have been making the case for creation of a high-level mental health post for quite some time. In fact, in the late 1970s, toward the end of then-President Jimmy Carter’s term, I wrote and talked about the need for a special cabinet post of mental health. At the time I realized that, besides chronic mental disorders, the amount of mental distress people experienced from a myriad of life issues leading to anxiety, depression, even posttraumatic stress disorder (although not labeled as such then), needed focused and informed leadership.
Before the pandemic, the World Health Organization reported that depression was the leading cause of disability worldwide. In the prepandemic United States, mental and substance use disorders were the top cause of disability among younger people.
We’ve lost almost 600,000 people to COVID-19, and people have been unable to grieve properly. More than 2 million women have left the labor force to care for children and sick family members. As we continue to learn about the mental health–related devastation wrought by SARS-CoV-2 – particularly long-haul COVID-19 – it’s time to dust off my proposal, update it, and implement it.
Building on a good decision
Back in 2017, President Trump appointed Elinore F. McCance-Katz, MD, PhD, to a new post officially called “assistant secretary for mental health and substance use” and unofficially called the “mental health czar.” This was a groundbreaking step, because Dr. McCance-Katz, a psychiatrist, is known for developing innovative approaches to addressing the opioid crisis in her home state of Rhode Island. She resigned from her post on Jan. 7, 2021, citing her concerns about the Jan. 6 insurrection on the U.S. Capitol.
As of this writing, President Biden has nominated psychologist Miriam Delphin-Rittmon, PhD, who is commissioner of Connecticut Department of Mental Health and Addiction Services, as mental health czar. I’m glad to see that the new administration wants a new czar, but I would prefer to see a more expansive role for a mental health professional at the federal level. The reason is because
Processing the current crisis
Americans managed to recover emotionally from the ravages of death and dying from World War II; we lived through the “atomic age” of mutual destruction, sometimes calling it the age of anxiety. But nothing has come close to the overwhelming devastation that COVID-19 has brought to the world – and to this country.
A recent Government Accountability Office report shows 38% of U.S. adults reported symptoms of anxiety or depression from April 2020 through February 2021. That was up from 11% from January to June 2019, the report said, citing data from the Centers for Disease Control and Prevention. Meanwhile, the report cites data from the Substance Abuse and Mental Health Services Administration showing that opioid deaths were 25%-50% higher during the pandemic than a year earlier.
My sense is that people generally have opened up regarding their emotional problems in a freer manner, thus allowing us to speak about and accept mental health problems as part of our human reality – just as we accept physical disorders and search for treatment and care.
In terms of talk therapy, I still believe that the “thinking” therapies, that is, cognitive therapies that involved getting a new perspective on problems, are most effective in dealing with the myriad of emotional issues people experience as well as those that have arisen because of COVID-19, and the tremendous fear of severe illness and death that the virus can bring. Besides anxiety, depression, and fear, the psychological toll of a fractured lifestyle, coupled with social isolation, will lead many into a variety of PTSD-related conditions. Many of those conditions, including PTSD, might lift when COVID-19 is controlled, but the time frame for resolution is far from clear and will vary, depending on each person. National leadership, as well as therapists, need to be ready to work with the many mental health problems COVID-19 will leave in its wake.
Therapeutically, as we develop our cognitive approaches to the problems this pandemic has brought, whether affecting people with no past psychiatric history or those with a previous or ongoing problems, we are in a unique position ourselves to offer even more support based on our own experiences during the pandemic. Our patients have seen us wear masks and work remotely, and just as we know about their suffering, they know we have been affected as well. These shared experiences with patients can allow us to express even greater empathy and offer even greater support – which I believe enhances the cognitive process and adds more humanism to the therapeutic process.
The therapists I’ve talked with believe that sharing coping skills – even generally sharing anxieties – can be very therapeutic. They compared these exchanges to what is done in support or educational groups.
As a psychiatrist who has been treating patients using cognitive-behavioral therapy – the thinking therapy – for more than 40 years, I agree that sharing our experiences in this worldwide pandemic with those we are helping can be extremely beneficial. Using this approach would not distract from other cognitive work. CBT, after all, is a far cry from dynamic or psychoanalytic talking or listening.
Change is in the air. More and more Americans are getting vaccinated, and the CDC is constantly updating its guidance on COVID-19. That guidance should have a mental health component.
I urge the president to put mental health at the forefront by nominating an expert who could offer mental health solutions on a daily basis. This person should be on equal footing with the surgeon general. Taking this step would help destigmatize mental suffering and despair – and create greater awareness about how to address those conditions.
Dr. London has been a practicing psychiatrist for 4 decades and a newspaper columnist for almost as long. He has a private practice in New York and is author of “Find Freedom Fast: Short-Term Therapy That Works” (New York: Kettlehole Publishing, 2019). Dr. London has no conflicts of interest.
G-POEM: Is it the much-needed treatment for gastroparesis?
In refractory gastroparesis, gastric peroral endoscopic myotomy (G-POEM) led to improvements in some patients, but the benefits were modest overall, according to results from a multicenter prospective study.
The clinical success rate was 56% at 12 months, defined as a 1 unit or greater decrease in the Gastroparesis Cardinal Symptom Index (GCSI) score accompanied by a 25% or greater decrease in two subscales detailing specific symptoms. Though the results fell short of expectations, they represent progress. In a previous large, multicenter, prospective study of existing therapies, just 28% experienced an improvement of 1 or more in the GCSI after 48 weeks of standard of care treatment.
This recent study, led by Kia Vosoughi and senior author Mouen Khashab, MD, of Johns Hopkins Medicine, Baltimore, was published online March 19, 2021, in Gut.
Pylorospasm has been linked to the symptoms of gastroparesis, prompting pyloric-directed interventions such as botulinum toxin injection, transpyloric stent placement, and pneumatic dilation of the pylorus. However, none have proven to have long-term benefit. G-POEM was introduced in 2013 as a minimally invasive pyloric-directed procedure. Some small, retrospective studies showed encouraging results, but this was the first prospective study.
“There is a huge drive to find other ways of treating gastroparesis because our current methods are not very effective and potentially have side effects. Unfortunately, [G-POEM] was not as helpful as we expected, even though there was improvement,” said Gyanprakash A. Ketwaroo, MD, MSc, AGAF, who was asked to comment on the findings. Dr. Ketwaroo is an assistant professor of medicine at Baylor College of Medicine, Houston.
Dr. Ketwaroo still endorses G-POEM despite the results because of the limitations of existing treatments.
“Even though it’s disappointing, [G-POEM] may still be better than the other approaches for gastroparesis. The other potential benefit is that it may be more readily available to patients than such interventions as the neurostimulator,” said Dr. Ketwaroo.
He added that the results highlight the importance of patient selection, pointing out that the researchers classified patients by etiologies.
“It may not be as applicable to the wider variety of patients with gastroparesis as we had hoped. I think they initially tried to look for a group that may be more amenable to it. But I don’t think this study was able to pick up an outcome based on etiology,” said Dr. Ketwaroo.
He also noted that the study showed the procedure to be generally safe, despite being more invasive than some interventions for gastroparesis.
“The adverse events were very minimal,” Dr. Ketwaroo said.
The researchers analyzed data from 75 patients (mean age, 49.3 years) who underwent G-POEM and completed 12 months of follow-up. Out of this predominantly female (71.3%) group, 41.3% had idiopathic gastroparesis, 35% had postsurgical gastroparesis, and 23.8% had diabetic gastroparesis.
The clinical success was similar across the subgroups, in the 50%-60% range for all three (P = .913). On average, the GCSI score dropped from 2.8 at 1 month following the procedure, to 1.5 at 12 months (P < .001). All components of quality of life improved at 12 months, with the exception of physical functioning.
At 3 months, 66% of patients underwent a gastric emptying study, with gastric retention at 4 hours being compared with baseline values. From that group, 64.2% improved, and 47.1% achieved normalization. Those with improvement in gastric emptying at 3 months had a clinical success rate of 75.8% at the same time point, compared with 38.9% with no improvement (P = .015).
Five adverse events were reported (6.2%). All were mild and procedure related.
A multivariate analysis showed that predictors of clinical success at 12 months included a baseline GCSI score greater than 2.6 (odds ratio, 3.23; P = .04) and baseline gastric retention greater than 20% at 4 hours (OR, 3.65; P = .029).
“Our findings of mid-term clinical success and durability of G-POEM may help physicians to choose the best therapeutic strategy for patients with refractory gastroparesis. G-POEM may be considered in patients with more severe baseline symptoms and pre-G-POEM gastric retention,” the researchers concluded.
The authors disclosed no external funding. Dr. Ketwaroo has no relevant financial disclosures, although he is on the editorial advisory board for GI & Hepatology News.
In refractory gastroparesis, gastric peroral endoscopic myotomy (G-POEM) led to improvements in some patients, but the benefits were modest overall, according to results from a multicenter prospective study.
The clinical success rate was 56% at 12 months, defined as a 1 unit or greater decrease in the Gastroparesis Cardinal Symptom Index (GCSI) score accompanied by a 25% or greater decrease in two subscales detailing specific symptoms. Though the results fell short of expectations, they represent progress. In a previous large, multicenter, prospective study of existing therapies, just 28% experienced an improvement of 1 or more in the GCSI after 48 weeks of standard of care treatment.
This recent study, led by Kia Vosoughi and senior author Mouen Khashab, MD, of Johns Hopkins Medicine, Baltimore, was published online March 19, 2021, in Gut.
Pylorospasm has been linked to the symptoms of gastroparesis, prompting pyloric-directed interventions such as botulinum toxin injection, transpyloric stent placement, and pneumatic dilation of the pylorus. However, none have proven to have long-term benefit. G-POEM was introduced in 2013 as a minimally invasive pyloric-directed procedure. Some small, retrospective studies showed encouraging results, but this was the first prospective study.
“There is a huge drive to find other ways of treating gastroparesis because our current methods are not very effective and potentially have side effects. Unfortunately, [G-POEM] was not as helpful as we expected, even though there was improvement,” said Gyanprakash A. Ketwaroo, MD, MSc, AGAF, who was asked to comment on the findings. Dr. Ketwaroo is an assistant professor of medicine at Baylor College of Medicine, Houston.
Dr. Ketwaroo still endorses G-POEM despite the results because of the limitations of existing treatments.
“Even though it’s disappointing, [G-POEM] may still be better than the other approaches for gastroparesis. The other potential benefit is that it may be more readily available to patients than such interventions as the neurostimulator,” said Dr. Ketwaroo.
He added that the results highlight the importance of patient selection, pointing out that the researchers classified patients by etiologies.
“It may not be as applicable to the wider variety of patients with gastroparesis as we had hoped. I think they initially tried to look for a group that may be more amenable to it. But I don’t think this study was able to pick up an outcome based on etiology,” said Dr. Ketwaroo.
He also noted that the study showed the procedure to be generally safe, despite being more invasive than some interventions for gastroparesis.
“The adverse events were very minimal,” Dr. Ketwaroo said.
The researchers analyzed data from 75 patients (mean age, 49.3 years) who underwent G-POEM and completed 12 months of follow-up. Out of this predominantly female (71.3%) group, 41.3% had idiopathic gastroparesis, 35% had postsurgical gastroparesis, and 23.8% had diabetic gastroparesis.
The clinical success was similar across the subgroups, in the 50%-60% range for all three (P = .913). On average, the GCSI score dropped from 2.8 at 1 month following the procedure, to 1.5 at 12 months (P < .001). All components of quality of life improved at 12 months, with the exception of physical functioning.
At 3 months, 66% of patients underwent a gastric emptying study, with gastric retention at 4 hours being compared with baseline values. From that group, 64.2% improved, and 47.1% achieved normalization. Those with improvement in gastric emptying at 3 months had a clinical success rate of 75.8% at the same time point, compared with 38.9% with no improvement (P = .015).
Five adverse events were reported (6.2%). All were mild and procedure related.
A multivariate analysis showed that predictors of clinical success at 12 months included a baseline GCSI score greater than 2.6 (odds ratio, 3.23; P = .04) and baseline gastric retention greater than 20% at 4 hours (OR, 3.65; P = .029).
“Our findings of mid-term clinical success and durability of G-POEM may help physicians to choose the best therapeutic strategy for patients with refractory gastroparesis. G-POEM may be considered in patients with more severe baseline symptoms and pre-G-POEM gastric retention,” the researchers concluded.
The authors disclosed no external funding. Dr. Ketwaroo has no relevant financial disclosures, although he is on the editorial advisory board for GI & Hepatology News.
In refractory gastroparesis, gastric peroral endoscopic myotomy (G-POEM) led to improvements in some patients, but the benefits were modest overall, according to results from a multicenter prospective study.
The clinical success rate was 56% at 12 months, defined as a 1 unit or greater decrease in the Gastroparesis Cardinal Symptom Index (GCSI) score accompanied by a 25% or greater decrease in two subscales detailing specific symptoms. Though the results fell short of expectations, they represent progress. In a previous large, multicenter, prospective study of existing therapies, just 28% experienced an improvement of 1 or more in the GCSI after 48 weeks of standard of care treatment.
This recent study, led by Kia Vosoughi and senior author Mouen Khashab, MD, of Johns Hopkins Medicine, Baltimore, was published online March 19, 2021, in Gut.
Pylorospasm has been linked to the symptoms of gastroparesis, prompting pyloric-directed interventions such as botulinum toxin injection, transpyloric stent placement, and pneumatic dilation of the pylorus. However, none have proven to have long-term benefit. G-POEM was introduced in 2013 as a minimally invasive pyloric-directed procedure. Some small, retrospective studies showed encouraging results, but this was the first prospective study.
“There is a huge drive to find other ways of treating gastroparesis because our current methods are not very effective and potentially have side effects. Unfortunately, [G-POEM] was not as helpful as we expected, even though there was improvement,” said Gyanprakash A. Ketwaroo, MD, MSc, AGAF, who was asked to comment on the findings. Dr. Ketwaroo is an assistant professor of medicine at Baylor College of Medicine, Houston.
Dr. Ketwaroo still endorses G-POEM despite the results because of the limitations of existing treatments.
“Even though it’s disappointing, [G-POEM] may still be better than the other approaches for gastroparesis. The other potential benefit is that it may be more readily available to patients than such interventions as the neurostimulator,” said Dr. Ketwaroo.
He added that the results highlight the importance of patient selection, pointing out that the researchers classified patients by etiologies.
“It may not be as applicable to the wider variety of patients with gastroparesis as we had hoped. I think they initially tried to look for a group that may be more amenable to it. But I don’t think this study was able to pick up an outcome based on etiology,” said Dr. Ketwaroo.
He also noted that the study showed the procedure to be generally safe, despite being more invasive than some interventions for gastroparesis.
“The adverse events were very minimal,” Dr. Ketwaroo said.
The researchers analyzed data from 75 patients (mean age, 49.3 years) who underwent G-POEM and completed 12 months of follow-up. Out of this predominantly female (71.3%) group, 41.3% had idiopathic gastroparesis, 35% had postsurgical gastroparesis, and 23.8% had diabetic gastroparesis.
The clinical success was similar across the subgroups, in the 50%-60% range for all three (P = .913). On average, the GCSI score dropped from 2.8 at 1 month following the procedure, to 1.5 at 12 months (P < .001). All components of quality of life improved at 12 months, with the exception of physical functioning.
At 3 months, 66% of patients underwent a gastric emptying study, with gastric retention at 4 hours being compared with baseline values. From that group, 64.2% improved, and 47.1% achieved normalization. Those with improvement in gastric emptying at 3 months had a clinical success rate of 75.8% at the same time point, compared with 38.9% with no improvement (P = .015).
Five adverse events were reported (6.2%). All were mild and procedure related.
A multivariate analysis showed that predictors of clinical success at 12 months included a baseline GCSI score greater than 2.6 (odds ratio, 3.23; P = .04) and baseline gastric retention greater than 20% at 4 hours (OR, 3.65; P = .029).
“Our findings of mid-term clinical success and durability of G-POEM may help physicians to choose the best therapeutic strategy for patients with refractory gastroparesis. G-POEM may be considered in patients with more severe baseline symptoms and pre-G-POEM gastric retention,” the researchers concluded.
The authors disclosed no external funding. Dr. Ketwaroo has no relevant financial disclosures, although he is on the editorial advisory board for GI & Hepatology News.
FROM GUT
Morning Discharges and Patient Length of Stay in Inpatient General Internal Medicine
There is substantial interest in improving patient flow and reducing hospital length of stay (LOS).1-4 Impaired hospital flow may negatively impact both patient satisfaction and safety through, for example, emergency department (ED) overcrowding.5,6 Impaired hospital flow is associated with downstream effects on patient care, hospital costs, and availability of beds.7-9
A number of quality-improvement interventions aim to improve patient flow, including efforts to increase the number of discharges that occur before noon.10,11 Morning discharges have been hypothesized to free hospital beds earlier, thus reducing ED wait times for incoming patients and increasing beds for elective surgeries.11 Morning discharges may also be more predictable for staff and patients. However, it is unclear whether efforts to increase the number of morning discharges have a negative impact on inpatient LOS by incentivizing physicians to keep patients in the hospital for an extra night to facilitate discharge in the early morning rather than the late afternoon. Morning discharges have been associated with both increased12 and decreased LOS.10,11,13-15
The purpose of this study was to examine the associations between morning discharges and ED LOS and hospital LOS in general internal medicine (GIM) at seven hospitals. GIM patients represent nearly 40% of ED admissions to a hospital,16 and thus are an important determinant of patient flow through the ED and hospital. We hypothesized that patients who were admitted to GIM on days with more morning discharges would have shorter ED LOS and hospital LOS.
METHODS
Design, Setting, and Participants
This was a retrospective cohort study conducted using the General Medicine Inpatient Initiative (GEMINI) clinical dataset.16 The dataset includes all GIM admissions at seven large hospital sites in Toronto and Mississauga, Ontario, Canada. These include five academic hospitals and two community-based teaching hospitals. Each hospital is publicly funded and provides tertiary and/or quaternary care to diverse multiethnic populations. Research ethics board approval was obtained from all participating sites.
GIM care is delivered by several interdisciplinary clinical teams functioning in parallel. Attending physicians are predominantly internists who practice as hospitalists in discrete service blocks, typically lasting 2 weeks at a time. Although GIM patients are preferentially admitted to GIM wards, participating hospitals did not have strict policies regarding cohorting GIM patients to specific wards (ie, holding patients in ED until a specific bed becomes available) that would confound the association between morning discharge and ED wait times. Approximately 75% of GIM patients are cared for on dedicated GIM wards at participating hospitals, with the remainder cared for on other medical or surgical wards.
We included all hospitalized patients who were admitted to hospital and discharged from GIM between April 1, 2010, and October 31, 2017, from the seven GEMINI hospitals. We included only patients admitted through the ED. As such, we did not include elective admissions or interfacility transfers who would not experience ED wait times. We excluded patients who were discharged without a provincial health insurance number (N = 2,169; 1.1% of total sample) because they could not be linked across visits to measure readmissions.
Data Source
The GEMINI dataset has been rigorously validated and previously described in detail.16 GEMINI collects both administrative health data reported to the Canadian Institute for Health Information (including data about patient demographics, comorbidities, and discharge destination) as well as electronic clinical data extracted from hospital computer systems (including attending physicians, in-hospital patient room transfers, and laboratory test results). Data are collected for each individual hospital encounter, and the provincial health insurance number is used to link patients across encounters.
Exposures and Outcomes
The two primary outcomes were ED LOS and hospital LOS. ED LOS was calculated as the difference between the time from triage by nursing staff to a patient’s exit from the ED, measured in hours. We also examined 30-day readmission to GIM at any participating hospital as a balancing measure against premature discharges and inpatient mortality because it could modify hospital LOS.
Patient Characteristics
Baseline patient characteristics were measured, including age, sex, Charlson Comorbidity Index score,17 day of admission (categorized as weekend/holiday or weekday), time of admission to hospital (
Statistical Analysis
The study population and physician characteristics were summarized with descriptive statistics. The balance of baseline patient characteristics across morning discharge quartiles was assessed using standardized differences. A standardized difference of less than 0.1 reflects good balance.20
Unadjusted estimates of patient outcomes were reported across morning discharge quartiles. To model the overall association between morning discharge and outcomes, the number of morning GIM discharges on the day of admission was subtracted from the mean number of morning discharges at each hospital and considered as a continuous exposure. We used generalized linear mixed models to estimate the effect of morning discharges on patient outcomes. We fit negative binomial regression models with log link to examine the association between the number of morning discharges (centered by subtracting the hospital mean) and the two main outcomes, ED LOS and hospital LOS. Given the overdispersion of the study population due to the unequal mean and variance, a negative binomial model was preferred over a Poisson regression, as the mean and variance were not equal.21 For our secondary outcomes of binary measures (30-day readmission and morality), we fit logistic regression models. Adjustment for multiple comparisons was not performed.
Multivariable analysis was conducted to adjust for the baseline characteristics described above as well as the total number of GIM discharges on the day of admission and GIM census on the day of admission. Hospital and study month (to account for secular time trends) were included as fixed effects, and patients and admitting physicians were included as crossed random effects to account for the nested structure of admissions within patients and admissions within physicians within hospitals.
A sensitivity analysis was performed to assess for nonlinear associations between morning discharges and the four outcomes (hospital LOS, ED LOS, in-hospital mortality, and readmission) by inputting the term as a restricted cubic spline, with up to five knots
RESULTS
Study Population and Patient Characteristics
The study population consisted of 189,781 hospitalizations involving 115,630 unique patients. The median patient age was 73 years (interquartile range [IQR], 57-84), 50.3% were female, 43.8% had a high Charlson Comorbidity Index score, and 11.1% were admitted to GIM in the prior 30 days (Table 1). The median ED LOS was 14.5 hours (IQR, 10.0-23.1), and the mean was 18.1 hours (SD, 12.2). The median hospital LOS was 4.6 days (IQR, 2.4-9.0), and the mean was 8.6 days (SD, 18.7).
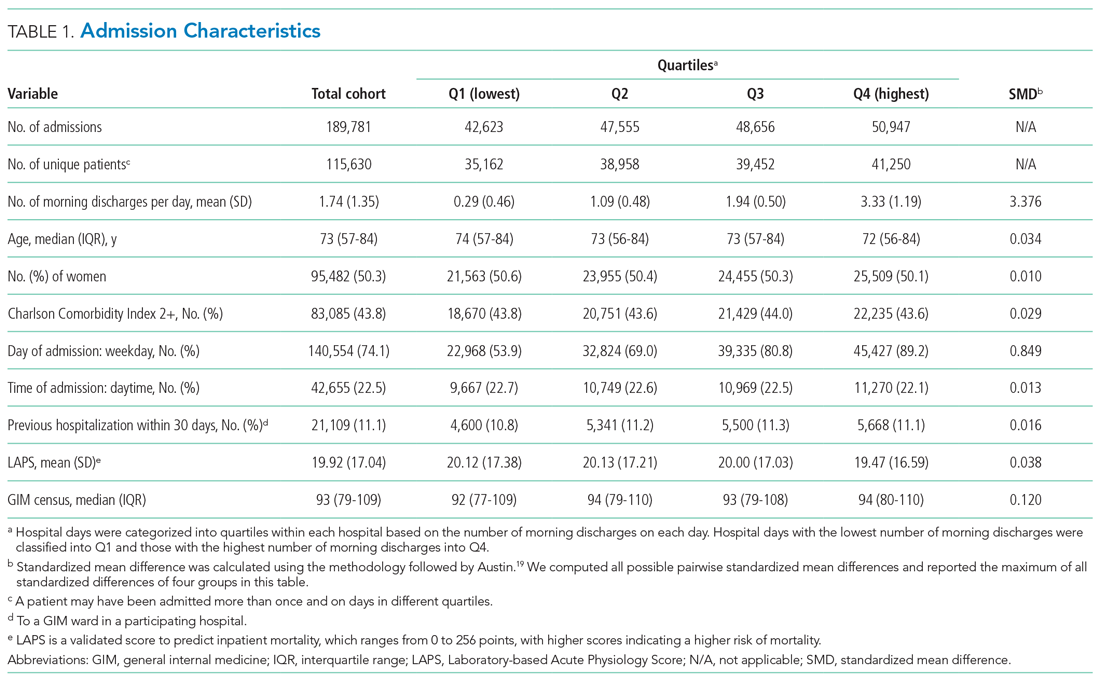
In total, 36,043 (19.0%) discharges occurred between 8:00
Outcomes
Unadjusted clinical outcomes by number of morning discharges are presented in Table 2. The median unadjusted ED LOS was 14.4 (SD, 14.1), 14.3 (SD, 13.2), 14.5 (SD, 13.0), and 14.8 (SD, 13.0) hours for the first to fourth quartiles (fewest to largest number of morning discharges), respectively. The median unadjusted hospital LOS was 4.6 (SD, 6.5), 4.6 (SD, 6.9), 4.7 (SD, 6.4), and 4.6 (SD, 6.4) days for the first to fourth quartiles, respectively.

Unadjusted inpatient mortality was 6.1%, 5.5%, 5.5%, and 5.2% across the first to fourth quartiles, respectively. Unadjusted 30-day readmission to GIM was 12.2%, 12.6%, 12.6%, and 12.5% across the first to fourth quartiles, respectively.
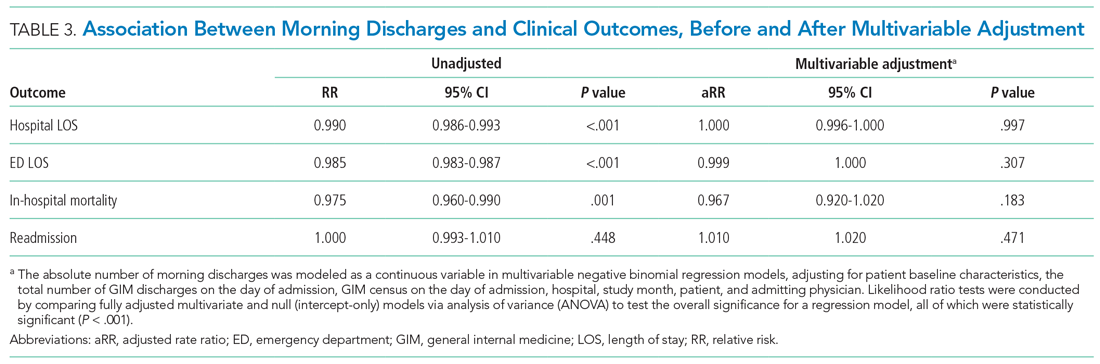
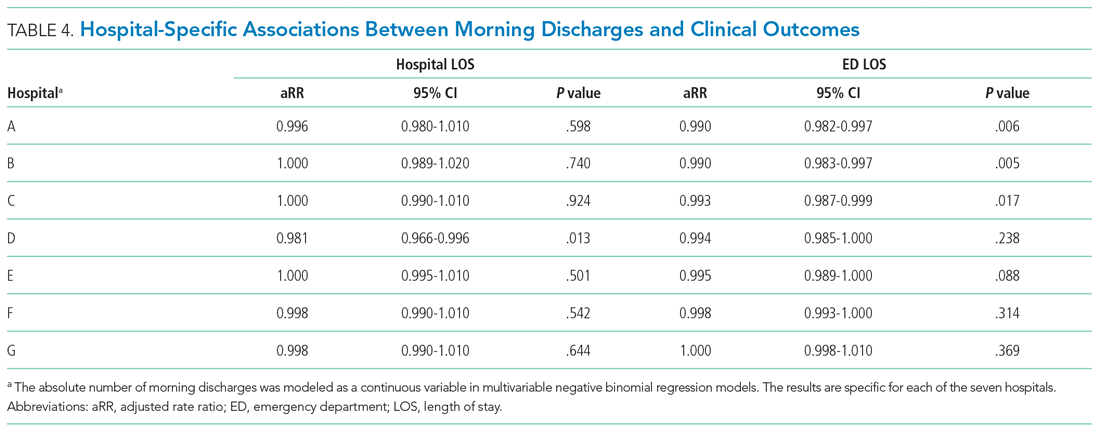
After multivariable adjustment, there was no significant association between morning discharge and hospital LOS (aRR, 1.000; 95% CI, 0.996-1.000; P = .997), ED LOS (aRR, 0.999; 95% CI, 0.997-1.000; P = .307), in-hospital mortality (aRR, 0.967; 95% CI, 0.920-1.020; P =.183), or 30-day readmission (aRR, 1.010; 95% CI, 0.991-1.020; P = .471) (Table 3, Appendix Table 2, Appendix Table 3, Appendix Table 4, Appendix Table 5). When examining each hospital separately, we found that morning discharge was significantly associated with hospital LOS at only one hospital (Hospital D; aRR, 0.981; 95% CI, 0.966-0.996; P = .013). Morning discharge was statistically significantly associated with ED LOS at three hospitals (A, B, and C), but the aRR was at least 0.99 in all three cases (Table 4).
In sensitivity analyses, we found no improvements in model fit when adding spline terms to the model, suggesting no significant nonlinear associations between morning discharges and the outcomes of interest.
DISCUSSION
This large multicenter cohort study found no significant overall association between the number of morning discharges and ED or hospital LOS in GIM. At one hospital, there was a 1.9% reduction in adjusted ED LOS for every additional morning discharge, but no difference in hospital LOS. We also did not observe differences in readmission or inpatient mortality associated with the number of morning discharges. Our observational findings suggest that there is unlikely to be a strong association between morning discharge and patient throughput in GIM. Given that there may be other downstream benefits of morning discharge, such as freeing beds for daytime surgeries,23 further research is needed to determine the effectiveness of specific interventions.
Several studies have posited morning discharge as a method of improving both patient care and hospital flow metrics.10,11,13-15,23 Quality improvement initiatives targeting morning discharges have included stakeholder meetings, incentives programs, discharge-centered breakfast programs, and creating deadlines for discharge orders.24-29 Although these initiatives have gained support, critics have suggested that their supporting evidence is not robust. Werthemier et al10 found a 9.0% reduction of observed to expected LOS associated with increasing the number of early discharges. However, a response article suggested that their findings were confounded by other hospital initiatives, such as allocation of medical and social services to weekends.30 Other observational studies have concluded that hospital LOS is not affected by the number of morning discharges, but this research has been limited by single-center analysis and relatively smaller sample sizes.12 Our study further calls into question the association between morning discharge and patient throughput.
An additional reason for the controversy is that physicians may actively work to discharge patients late in the day to avoid an additional night in hospital. A qualitative study by Minichiello et al31 evaluated staff perceptions regarding afternoon discharges. Physicians and medical students believed that afternoon discharges were a result of waiting for test results and procedures, with staff aiming to discharge patients immediately after obtaining results or finishing necessary procedures. As such, there are concerns that incentivizing morning discharge may lead physicians in the opposite direction, to consciously or unconsciously keep patients overnight in order to facilitate an early morning discharge.30
Our study’s greatest strength was the large sample size over 7 years at seven hospitals in two cities, including both academic and community hospitals with different models of care. To our knowledge, this is the first cohort study that has analyzed the association between early discharge and LOS using multiple centers. To avoid the confounding and reverse causality that may exist when examining the relationship between LOS and morning discharge at the patient level (eg, patients who stay in hospital longer may have more “planned” discharges and leave in the morning), we examined the association based on variation across different days within the GIM service of each hospital. Further, we included robust risk adjustment using clinical and laboratory data. Finally, since our study included a diverse patient population served by participating centers in a system with universal insurance for hospital care, our findings are likely generalizable to other urban and suburban hospitals.
There are several important limitations of our analysis. First, we could only include GIM patients, who represent nearly 40% of ED admissions to hospital at participating centers. A more holistic analysis across all hospital services could be justified; however, given that many quality improvement initiatives occur at the level of a single hospital service, we felt our approach would be informative for future research and improvement efforts. Approximately 75% of GIM patients at participating hospitals were cared for on a GIM ward, with 25% cared for on off-service units. We were unable to include the total hospital census in our models, and this could affect LOS and waiting times for GIM patients, particularly those admitted to off-service units. GIM census is likely highly correlated with hospital census, and we were able to adjust for this. Nevertheless, this remains an important potential source of unmeasured confounding. Second, we did not model the effects of morning discharges from GIM on patient-flow measures for non-GIM patients. Given the lack of effects for GIM patients, who would be more likely to be directly affected, it is unlikely that large effects would be seen for other hospital patients, but we did not measure effects on surgical delays or cancellations, for example.23 Third, we report 30-day readmission to GIM at participating hospitals only, rather than all readmissions. However, prior research in our region demonstrated that 82% of hospital readmissions occur to the same site.32 Thus, our measure, which includes admission to any participating hospital, likely captures more than 80% of all readmissions, and this was a secondary outcome in our analysis. Finally, qualitative metrics, such as patient or provider satisfaction, were not measured in our study. Earlier discharge may impact patient care in other ways by being more predictable for staff, improving bed allocation for daytime procedures, making medication pick-ups easier to arrange, or making consultations with allied health services more convenient.11,28,33 Conversely, if pressured to discharge before noon, providers may feel rushed to complete tasks and may face disruptions to typical workflow.24 As such, future research is needed to provide a more complete understanding of the impact of early-morning discharge beyond hospital flow.
CONCLUSION
The number of morning discharges was not significantly associated with shorter ED LOS or hospital LOS for GIM patients. Our observational findings suggest that increasing morning discharges alone may not substantially improve patient flow in GIM. Further research is needed to evaluate specific morning discharge interventions and assess hospital-wide effects.
1. Trzeciak S, Rivers EP. Emergency department overcrowding in the United States: an emerging threat to patient safety and public health. Emerg Med J. 2003;20(5):402-405. https://doi.org/10.1136/emj.20.5.402
2. McKenna P, Heslin SM, Viccellio P, Mallon WK, Hernandez C, Morley EJ. Emergency department and hospital crowding: causes, consequences, and cures. Clin Exp Emerg Med. 2019;6(3):189-195. https://doi.org/10.15441/ceem.18.022
3. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1-10. https://doi.org/10.1111/j.1553-2712.2008.00295.x
4. Derlet RW, Richards JR. Overcrowding in the nation’s emergency departments: complex causes and disturbing effects. Ann Emerg Med. 2000;35(1):63-68. https://doi.org/10.1016/s0196-0644(00)70105-3
5. Pines JM, Iyer S, Disbot M, Hollander JE, Shofer FS, Datner EM. The effect of emergency department crowding on patient satisfaction for admitted patients. Acad Emerg Med. 2008;15(9):825-831. https://doi.org/10.1111/j.1553-2712.2008.00200.x
6. Carter EJ, Pouch SM, Larson EL. The relationship between emergency department crowding and patient outcomes: a systematic review. J Nurs Scholarsh. 2014;46(2):106-115. https://doi.org/10.1111/jnu.1205
7. Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA. 2010;303(21):2141-2147. https://doi.org/10.1001/jama.2010.748
8. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. https://doi.org/ 10.1002/14651858.CD006632.pub2
9. Zodda D, Underwood J. Improving emergency department throughput: evidence-based strategies aimed at reducing boarding and overcrowding. Phys Leadership J. 2019;6(3):70-73.
10. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. https://doi.org/10.1002/jhm.2154
11. Kane M, Weinacker A, Arthofer R, et al. A multidisciplinary initiative to increase inpatient discharges before noon. J Nurs Adm. 2016;46(12):630-635. https://doi.org/10.1097/NNA.0000000000000418
12. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. https://doi.org/10.1002/jhm.2529
13. Patel H, Morduchowicz S, Mourad M. Using a systematic framework of interventions to improve early discharges. Jt Comm J Qual Patient Saf. 2017;43(4):189-196. https://doi.org/10.1016/j.jcjq.2016.12.003
14. El-Eid GR, Kaddoum R, Tamim H, Hitti EA. Improving hospital discharge time: a successful implementation of Six Sigma methodology. Medicine (Baltimore). 2015;94(12):e633. https://doi.org/10.1097/MD.0000000000000633
15. Mathews KS, Corso P, Bacon S, Jenq GY. Using the red/yellow/green discharge tool to improve the timeliness of hospital discharges. Jt Comm J Qual Patient Saf. 2014;40(6):243-252. https://doi.org/10.1016/s1553-7250(14)40033-3
16. Verma AA, Pasricha SV, Jung HY, et al. Assessing the quality of clinical and administrative data extracted from hospitals: the General Medicine Inpatient Initiative (GEMINI) experience. J Am Med Inform Assoc. 2021; 28(3):578-587. doi: 10.1093/jamia/ocaa225.
17. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(60:676-682. https://doi.org/10.1093/aje/kwq433
18. Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232-239. https://doi.org/10.1097/MLR.0b013e3181589bb6
19. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(60:1228-1234. https://doi.org/10.1080/03610910902859574
20. van Walraven C, Escobar GJ, Greene JD, Forster AJ. The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population. J Clin Epidemiol. 2010;63(7):798-803. https://doi.org/10.1016/j.jclinepi.2009.08.020
21. Hilbe JM. Negative binomial regression. In: Modeling Count Data. Cambridge University Press. 2014:126-160.
22. Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed. Springer; 2015.
23. Durvasula R, Kayihan A, Del Bene S, et al. A multidisciplinary care pathway significantly increases the number of early morning discharges in a large academic medical center. Qual Manag Health Care. 2015;24(1):45-51. https://doi.org/10.1097/QMH.0000000000000049
24. Goolsarran N, Olowo G, Ling Y, Abbasi S, Taub E, Teressa G. Outcomes of a resident-led early hospital discharge intervention. J Gen Intern Med. 2020;35(2):437-443. https://doi.org/10.1007/s11606-019-05563-w
25. Beck MJ, Okerblom D, Kumar A, Bandyopadhyay S, Scalzi LV. Lean intervention improves patient discharge times, improves emergency department throughput and reduces congestion. Hosp Pract (1995). 2016;44(5):252-259. https://doi.org/10.1080/21548331.2016.1254559
26. Karling A, Tang KW. Discharge before noon: a study in a medical emergency ward. 2015. Accessed February 11, 2021. http://publications.lib.chalmers.se/records/fulltext/231873/231873.pdf
27. Mathews K, Corso P, Bacon S, Jenq GY. Using the red/yellow/green discharge tool to improve the timeliness of hospital discharges. Jt Comm J Qual Patient Saf. 2014;40(6):243-252. https://doi.org/10.1016/s1553-7250(14)40033-3
28. Goodson AS, DeGuzman, PB, Honeycutt A, Summy C, Manly F. Total joint replacement discharge brunch: meeting patient education needs and a hospital initiative of discharge by noon. Orthop Nurs. 2014;33(3):159-162. https://doi.org/10.1097/NOR.0000000000000048
29. Kravet SJ, Levine RB, Rubin HR, Wright SM. Discharging patients earlier in the day: a concept worth evaluating. Health Care Manag (Frederick). 2007;26(2):142-146. https://doi.org/10.1097/01.HCM.0000268617.33491.60
30. Shine D. Discharge before noon: an urban legend. Am J Med. 2015;128(5):445-446. https://doi.org/10.1016/j.amjmed.2014.12.011
31. Minichiello TM, Auerbach AD, Wachter RM. Caregiver perceptions of the reasons for delayed hospital discharge. Eff Clin Pract. 2001;4(6):250-255.
32. Staples JA, Thiruchelvam D, Redelmeier DA. Site of hospital readmission and mortality: a population-based retrospective cohort study. CMAJ Open. 2014;2:E77-E85. https://doi.org/10.9778/cmajo.20130053
33. Bowles KH, Foust JB, Naylor MD. Hospital discharge referral decision making: a multidisciplinary perspective. Appl Nurs Res. 2003;16(3):134-143. https://doi.org/10.1016/s0897-1897(03)00048-x
There is substantial interest in improving patient flow and reducing hospital length of stay (LOS).1-4 Impaired hospital flow may negatively impact both patient satisfaction and safety through, for example, emergency department (ED) overcrowding.5,6 Impaired hospital flow is associated with downstream effects on patient care, hospital costs, and availability of beds.7-9
A number of quality-improvement interventions aim to improve patient flow, including efforts to increase the number of discharges that occur before noon.10,11 Morning discharges have been hypothesized to free hospital beds earlier, thus reducing ED wait times for incoming patients and increasing beds for elective surgeries.11 Morning discharges may also be more predictable for staff and patients. However, it is unclear whether efforts to increase the number of morning discharges have a negative impact on inpatient LOS by incentivizing physicians to keep patients in the hospital for an extra night to facilitate discharge in the early morning rather than the late afternoon. Morning discharges have been associated with both increased12 and decreased LOS.10,11,13-15
The purpose of this study was to examine the associations between morning discharges and ED LOS and hospital LOS in general internal medicine (GIM) at seven hospitals. GIM patients represent nearly 40% of ED admissions to a hospital,16 and thus are an important determinant of patient flow through the ED and hospital. We hypothesized that patients who were admitted to GIM on days with more morning discharges would have shorter ED LOS and hospital LOS.
METHODS
Design, Setting, and Participants
This was a retrospective cohort study conducted using the General Medicine Inpatient Initiative (GEMINI) clinical dataset.16 The dataset includes all GIM admissions at seven large hospital sites in Toronto and Mississauga, Ontario, Canada. These include five academic hospitals and two community-based teaching hospitals. Each hospital is publicly funded and provides tertiary and/or quaternary care to diverse multiethnic populations. Research ethics board approval was obtained from all participating sites.
GIM care is delivered by several interdisciplinary clinical teams functioning in parallel. Attending physicians are predominantly internists who practice as hospitalists in discrete service blocks, typically lasting 2 weeks at a time. Although GIM patients are preferentially admitted to GIM wards, participating hospitals did not have strict policies regarding cohorting GIM patients to specific wards (ie, holding patients in ED until a specific bed becomes available) that would confound the association between morning discharge and ED wait times. Approximately 75% of GIM patients are cared for on dedicated GIM wards at participating hospitals, with the remainder cared for on other medical or surgical wards.
We included all hospitalized patients who were admitted to hospital and discharged from GIM between April 1, 2010, and October 31, 2017, from the seven GEMINI hospitals. We included only patients admitted through the ED. As such, we did not include elective admissions or interfacility transfers who would not experience ED wait times. We excluded patients who were discharged without a provincial health insurance number (N = 2,169; 1.1% of total sample) because they could not be linked across visits to measure readmissions.
Data Source
The GEMINI dataset has been rigorously validated and previously described in detail.16 GEMINI collects both administrative health data reported to the Canadian Institute for Health Information (including data about patient demographics, comorbidities, and discharge destination) as well as electronic clinical data extracted from hospital computer systems (including attending physicians, in-hospital patient room transfers, and laboratory test results). Data are collected for each individual hospital encounter, and the provincial health insurance number is used to link patients across encounters.
Exposures and Outcomes
The two primary outcomes were ED LOS and hospital LOS. ED LOS was calculated as the difference between the time from triage by nursing staff to a patient’s exit from the ED, measured in hours. We also examined 30-day readmission to GIM at any participating hospital as a balancing measure against premature discharges and inpatient mortality because it could modify hospital LOS.
Patient Characteristics
Baseline patient characteristics were measured, including age, sex, Charlson Comorbidity Index score,17 day of admission (categorized as weekend/holiday or weekday), time of admission to hospital (
Statistical Analysis
The study population and physician characteristics were summarized with descriptive statistics. The balance of baseline patient characteristics across morning discharge quartiles was assessed using standardized differences. A standardized difference of less than 0.1 reflects good balance.20
Unadjusted estimates of patient outcomes were reported across morning discharge quartiles. To model the overall association between morning discharge and outcomes, the number of morning GIM discharges on the day of admission was subtracted from the mean number of morning discharges at each hospital and considered as a continuous exposure. We used generalized linear mixed models to estimate the effect of morning discharges on patient outcomes. We fit negative binomial regression models with log link to examine the association between the number of morning discharges (centered by subtracting the hospital mean) and the two main outcomes, ED LOS and hospital LOS. Given the overdispersion of the study population due to the unequal mean and variance, a negative binomial model was preferred over a Poisson regression, as the mean and variance were not equal.21 For our secondary outcomes of binary measures (30-day readmission and morality), we fit logistic regression models. Adjustment for multiple comparisons was not performed.
Multivariable analysis was conducted to adjust for the baseline characteristics described above as well as the total number of GIM discharges on the day of admission and GIM census on the day of admission. Hospital and study month (to account for secular time trends) were included as fixed effects, and patients and admitting physicians were included as crossed random effects to account for the nested structure of admissions within patients and admissions within physicians within hospitals.
A sensitivity analysis was performed to assess for nonlinear associations between morning discharges and the four outcomes (hospital LOS, ED LOS, in-hospital mortality, and readmission) by inputting the term as a restricted cubic spline, with up to five knots
RESULTS
Study Population and Patient Characteristics
The study population consisted of 189,781 hospitalizations involving 115,630 unique patients. The median patient age was 73 years (interquartile range [IQR], 57-84), 50.3% were female, 43.8% had a high Charlson Comorbidity Index score, and 11.1% were admitted to GIM in the prior 30 days (Table 1). The median ED LOS was 14.5 hours (IQR, 10.0-23.1), and the mean was 18.1 hours (SD, 12.2). The median hospital LOS was 4.6 days (IQR, 2.4-9.0), and the mean was 8.6 days (SD, 18.7).

In total, 36,043 (19.0%) discharges occurred between 8:00
Outcomes
Unadjusted clinical outcomes by number of morning discharges are presented in Table 2. The median unadjusted ED LOS was 14.4 (SD, 14.1), 14.3 (SD, 13.2), 14.5 (SD, 13.0), and 14.8 (SD, 13.0) hours for the first to fourth quartiles (fewest to largest number of morning discharges), respectively. The median unadjusted hospital LOS was 4.6 (SD, 6.5), 4.6 (SD, 6.9), 4.7 (SD, 6.4), and 4.6 (SD, 6.4) days for the first to fourth quartiles, respectively.

Unadjusted inpatient mortality was 6.1%, 5.5%, 5.5%, and 5.2% across the first to fourth quartiles, respectively. Unadjusted 30-day readmission to GIM was 12.2%, 12.6%, 12.6%, and 12.5% across the first to fourth quartiles, respectively.
After multivariable adjustment, there was no significant association between morning discharge and hospital LOS (aRR, 1.000; 95% CI, 0.996-1.000; P = .997), ED LOS (aRR, 0.999; 95% CI, 0.997-1.000; P = .307), in-hospital mortality (aRR, 0.967; 95% CI, 0.920-1.020; P =.183), or 30-day readmission (aRR, 1.010; 95% CI, 0.991-1.020; P = .471) (Table 3, Appendix Table 2, Appendix Table 3, Appendix Table 4, Appendix Table 5). When examining each hospital separately, we found that morning discharge was significantly associated with hospital LOS at only one hospital (Hospital D; aRR, 0.981; 95% CI, 0.966-0.996; P = .013). Morning discharge was statistically significantly associated with ED LOS at three hospitals (A, B, and C), but the aRR was at least 0.99 in all three cases (Table 4).

In sensitivity analyses, we found no improvements in model fit when adding spline terms to the model, suggesting no significant nonlinear associations between morning discharges and the outcomes of interest.

DISCUSSION
This large multicenter cohort study found no significant overall association between the number of morning discharges and ED or hospital LOS in GIM. At one hospital, there was a 1.9% reduction in adjusted ED LOS for every additional morning discharge, but no difference in hospital LOS. We also did not observe differences in readmission or inpatient mortality associated with the number of morning discharges. Our observational findings suggest that there is unlikely to be a strong association between morning discharge and patient throughput in GIM. Given that there may be other downstream benefits of morning discharge, such as freeing beds for daytime surgeries,23 further research is needed to determine the effectiveness of specific interventions.
Several studies have posited morning discharge as a method of improving both patient care and hospital flow metrics.10,11,13-15,23 Quality improvement initiatives targeting morning discharges have included stakeholder meetings, incentives programs, discharge-centered breakfast programs, and creating deadlines for discharge orders.24-29 Although these initiatives have gained support, critics have suggested that their supporting evidence is not robust. Werthemier et al10 found a 9.0% reduction of observed to expected LOS associated with increasing the number of early discharges. However, a response article suggested that their findings were confounded by other hospital initiatives, such as allocation of medical and social services to weekends.30 Other observational studies have concluded that hospital LOS is not affected by the number of morning discharges, but this research has been limited by single-center analysis and relatively smaller sample sizes.12 Our study further calls into question the association between morning discharge and patient throughput.
An additional reason for the controversy is that physicians may actively work to discharge patients late in the day to avoid an additional night in hospital. A qualitative study by Minichiello et al31 evaluated staff perceptions regarding afternoon discharges. Physicians and medical students believed that afternoon discharges were a result of waiting for test results and procedures, with staff aiming to discharge patients immediately after obtaining results or finishing necessary procedures. As such, there are concerns that incentivizing morning discharge may lead physicians in the opposite direction, to consciously or unconsciously keep patients overnight in order to facilitate an early morning discharge.30
Our study’s greatest strength was the large sample size over 7 years at seven hospitals in two cities, including both academic and community hospitals with different models of care. To our knowledge, this is the first cohort study that has analyzed the association between early discharge and LOS using multiple centers. To avoid the confounding and reverse causality that may exist when examining the relationship between LOS and morning discharge at the patient level (eg, patients who stay in hospital longer may have more “planned” discharges and leave in the morning), we examined the association based on variation across different days within the GIM service of each hospital. Further, we included robust risk adjustment using clinical and laboratory data. Finally, since our study included a diverse patient population served by participating centers in a system with universal insurance for hospital care, our findings are likely generalizable to other urban and suburban hospitals.
There are several important limitations of our analysis. First, we could only include GIM patients, who represent nearly 40% of ED admissions to hospital at participating centers. A more holistic analysis across all hospital services could be justified; however, given that many quality improvement initiatives occur at the level of a single hospital service, we felt our approach would be informative for future research and improvement efforts. Approximately 75% of GIM patients at participating hospitals were cared for on a GIM ward, with 25% cared for on off-service units. We were unable to include the total hospital census in our models, and this could affect LOS and waiting times for GIM patients, particularly those admitted to off-service units. GIM census is likely highly correlated with hospital census, and we were able to adjust for this. Nevertheless, this remains an important potential source of unmeasured confounding. Second, we did not model the effects of morning discharges from GIM on patient-flow measures for non-GIM patients. Given the lack of effects for GIM patients, who would be more likely to be directly affected, it is unlikely that large effects would be seen for other hospital patients, but we did not measure effects on surgical delays or cancellations, for example.23 Third, we report 30-day readmission to GIM at participating hospitals only, rather than all readmissions. However, prior research in our region demonstrated that 82% of hospital readmissions occur to the same site.32 Thus, our measure, which includes admission to any participating hospital, likely captures more than 80% of all readmissions, and this was a secondary outcome in our analysis. Finally, qualitative metrics, such as patient or provider satisfaction, were not measured in our study. Earlier discharge may impact patient care in other ways by being more predictable for staff, improving bed allocation for daytime procedures, making medication pick-ups easier to arrange, or making consultations with allied health services more convenient.11,28,33 Conversely, if pressured to discharge before noon, providers may feel rushed to complete tasks and may face disruptions to typical workflow.24 As such, future research is needed to provide a more complete understanding of the impact of early-morning discharge beyond hospital flow.
CONCLUSION
The number of morning discharges was not significantly associated with shorter ED LOS or hospital LOS for GIM patients. Our observational findings suggest that increasing morning discharges alone may not substantially improve patient flow in GIM. Further research is needed to evaluate specific morning discharge interventions and assess hospital-wide effects.
There is substantial interest in improving patient flow and reducing hospital length of stay (LOS).1-4 Impaired hospital flow may negatively impact both patient satisfaction and safety through, for example, emergency department (ED) overcrowding.5,6 Impaired hospital flow is associated with downstream effects on patient care, hospital costs, and availability of beds.7-9
A number of quality-improvement interventions aim to improve patient flow, including efforts to increase the number of discharges that occur before noon.10,11 Morning discharges have been hypothesized to free hospital beds earlier, thus reducing ED wait times for incoming patients and increasing beds for elective surgeries.11 Morning discharges may also be more predictable for staff and patients. However, it is unclear whether efforts to increase the number of morning discharges have a negative impact on inpatient LOS by incentivizing physicians to keep patients in the hospital for an extra night to facilitate discharge in the early morning rather than the late afternoon. Morning discharges have been associated with both increased12 and decreased LOS.10,11,13-15
The purpose of this study was to examine the associations between morning discharges and ED LOS and hospital LOS in general internal medicine (GIM) at seven hospitals. GIM patients represent nearly 40% of ED admissions to a hospital,16 and thus are an important determinant of patient flow through the ED and hospital. We hypothesized that patients who were admitted to GIM on days with more morning discharges would have shorter ED LOS and hospital LOS.
METHODS
Design, Setting, and Participants
This was a retrospective cohort study conducted using the General Medicine Inpatient Initiative (GEMINI) clinical dataset.16 The dataset includes all GIM admissions at seven large hospital sites in Toronto and Mississauga, Ontario, Canada. These include five academic hospitals and two community-based teaching hospitals. Each hospital is publicly funded and provides tertiary and/or quaternary care to diverse multiethnic populations. Research ethics board approval was obtained from all participating sites.
GIM care is delivered by several interdisciplinary clinical teams functioning in parallel. Attending physicians are predominantly internists who practice as hospitalists in discrete service blocks, typically lasting 2 weeks at a time. Although GIM patients are preferentially admitted to GIM wards, participating hospitals did not have strict policies regarding cohorting GIM patients to specific wards (ie, holding patients in ED until a specific bed becomes available) that would confound the association between morning discharge and ED wait times. Approximately 75% of GIM patients are cared for on dedicated GIM wards at participating hospitals, with the remainder cared for on other medical or surgical wards.
We included all hospitalized patients who were admitted to hospital and discharged from GIM between April 1, 2010, and October 31, 2017, from the seven GEMINI hospitals. We included only patients admitted through the ED. As such, we did not include elective admissions or interfacility transfers who would not experience ED wait times. We excluded patients who were discharged without a provincial health insurance number (N = 2,169; 1.1% of total sample) because they could not be linked across visits to measure readmissions.
Data Source
The GEMINI dataset has been rigorously validated and previously described in detail.16 GEMINI collects both administrative health data reported to the Canadian Institute for Health Information (including data about patient demographics, comorbidities, and discharge destination) as well as electronic clinical data extracted from hospital computer systems (including attending physicians, in-hospital patient room transfers, and laboratory test results). Data are collected for each individual hospital encounter, and the provincial health insurance number is used to link patients across encounters.
Exposures and Outcomes
The two primary outcomes were ED LOS and hospital LOS. ED LOS was calculated as the difference between the time from triage by nursing staff to a patient’s exit from the ED, measured in hours. We also examined 30-day readmission to GIM at any participating hospital as a balancing measure against premature discharges and inpatient mortality because it could modify hospital LOS.
Patient Characteristics
Baseline patient characteristics were measured, including age, sex, Charlson Comorbidity Index score,17 day of admission (categorized as weekend/holiday or weekday), time of admission to hospital (
Statistical Analysis
The study population and physician characteristics were summarized with descriptive statistics. The balance of baseline patient characteristics across morning discharge quartiles was assessed using standardized differences. A standardized difference of less than 0.1 reflects good balance.20
Unadjusted estimates of patient outcomes were reported across morning discharge quartiles. To model the overall association between morning discharge and outcomes, the number of morning GIM discharges on the day of admission was subtracted from the mean number of morning discharges at each hospital and considered as a continuous exposure. We used generalized linear mixed models to estimate the effect of morning discharges on patient outcomes. We fit negative binomial regression models with log link to examine the association between the number of morning discharges (centered by subtracting the hospital mean) and the two main outcomes, ED LOS and hospital LOS. Given the overdispersion of the study population due to the unequal mean and variance, a negative binomial model was preferred over a Poisson regression, as the mean and variance were not equal.21 For our secondary outcomes of binary measures (30-day readmission and morality), we fit logistic regression models. Adjustment for multiple comparisons was not performed.
Multivariable analysis was conducted to adjust for the baseline characteristics described above as well as the total number of GIM discharges on the day of admission and GIM census on the day of admission. Hospital and study month (to account for secular time trends) were included as fixed effects, and patients and admitting physicians were included as crossed random effects to account for the nested structure of admissions within patients and admissions within physicians within hospitals.
A sensitivity analysis was performed to assess for nonlinear associations between morning discharges and the four outcomes (hospital LOS, ED LOS, in-hospital mortality, and readmission) by inputting the term as a restricted cubic spline, with up to five knots
RESULTS
Study Population and Patient Characteristics
The study population consisted of 189,781 hospitalizations involving 115,630 unique patients. The median patient age was 73 years (interquartile range [IQR], 57-84), 50.3% were female, 43.8% had a high Charlson Comorbidity Index score, and 11.1% were admitted to GIM in the prior 30 days (Table 1). The median ED LOS was 14.5 hours (IQR, 10.0-23.1), and the mean was 18.1 hours (SD, 12.2). The median hospital LOS was 4.6 days (IQR, 2.4-9.0), and the mean was 8.6 days (SD, 18.7).

In total, 36,043 (19.0%) discharges occurred between 8:00
Outcomes
Unadjusted clinical outcomes by number of morning discharges are presented in Table 2. The median unadjusted ED LOS was 14.4 (SD, 14.1), 14.3 (SD, 13.2), 14.5 (SD, 13.0), and 14.8 (SD, 13.0) hours for the first to fourth quartiles (fewest to largest number of morning discharges), respectively. The median unadjusted hospital LOS was 4.6 (SD, 6.5), 4.6 (SD, 6.9), 4.7 (SD, 6.4), and 4.6 (SD, 6.4) days for the first to fourth quartiles, respectively.

Unadjusted inpatient mortality was 6.1%, 5.5%, 5.5%, and 5.2% across the first to fourth quartiles, respectively. Unadjusted 30-day readmission to GIM was 12.2%, 12.6%, 12.6%, and 12.5% across the first to fourth quartiles, respectively.
After multivariable adjustment, there was no significant association between morning discharge and hospital LOS (aRR, 1.000; 95% CI, 0.996-1.000; P = .997), ED LOS (aRR, 0.999; 95% CI, 0.997-1.000; P = .307), in-hospital mortality (aRR, 0.967; 95% CI, 0.920-1.020; P =.183), or 30-day readmission (aRR, 1.010; 95% CI, 0.991-1.020; P = .471) (Table 3, Appendix Table 2, Appendix Table 3, Appendix Table 4, Appendix Table 5). When examining each hospital separately, we found that morning discharge was significantly associated with hospital LOS at only one hospital (Hospital D; aRR, 0.981; 95% CI, 0.966-0.996; P = .013). Morning discharge was statistically significantly associated with ED LOS at three hospitals (A, B, and C), but the aRR was at least 0.99 in all three cases (Table 4).

In sensitivity analyses, we found no improvements in model fit when adding spline terms to the model, suggesting no significant nonlinear associations between morning discharges and the outcomes of interest.

DISCUSSION
This large multicenter cohort study found no significant overall association between the number of morning discharges and ED or hospital LOS in GIM. At one hospital, there was a 1.9% reduction in adjusted ED LOS for every additional morning discharge, but no difference in hospital LOS. We also did not observe differences in readmission or inpatient mortality associated with the number of morning discharges. Our observational findings suggest that there is unlikely to be a strong association between morning discharge and patient throughput in GIM. Given that there may be other downstream benefits of morning discharge, such as freeing beds for daytime surgeries,23 further research is needed to determine the effectiveness of specific interventions.
Several studies have posited morning discharge as a method of improving both patient care and hospital flow metrics.10,11,13-15,23 Quality improvement initiatives targeting morning discharges have included stakeholder meetings, incentives programs, discharge-centered breakfast programs, and creating deadlines for discharge orders.24-29 Although these initiatives have gained support, critics have suggested that their supporting evidence is not robust. Werthemier et al10 found a 9.0% reduction of observed to expected LOS associated with increasing the number of early discharges. However, a response article suggested that their findings were confounded by other hospital initiatives, such as allocation of medical and social services to weekends.30 Other observational studies have concluded that hospital LOS is not affected by the number of morning discharges, but this research has been limited by single-center analysis and relatively smaller sample sizes.12 Our study further calls into question the association between morning discharge and patient throughput.
An additional reason for the controversy is that physicians may actively work to discharge patients late in the day to avoid an additional night in hospital. A qualitative study by Minichiello et al31 evaluated staff perceptions regarding afternoon discharges. Physicians and medical students believed that afternoon discharges were a result of waiting for test results and procedures, with staff aiming to discharge patients immediately after obtaining results or finishing necessary procedures. As such, there are concerns that incentivizing morning discharge may lead physicians in the opposite direction, to consciously or unconsciously keep patients overnight in order to facilitate an early morning discharge.30
Our study’s greatest strength was the large sample size over 7 years at seven hospitals in two cities, including both academic and community hospitals with different models of care. To our knowledge, this is the first cohort study that has analyzed the association between early discharge and LOS using multiple centers. To avoid the confounding and reverse causality that may exist when examining the relationship between LOS and morning discharge at the patient level (eg, patients who stay in hospital longer may have more “planned” discharges and leave in the morning), we examined the association based on variation across different days within the GIM service of each hospital. Further, we included robust risk adjustment using clinical and laboratory data. Finally, since our study included a diverse patient population served by participating centers in a system with universal insurance for hospital care, our findings are likely generalizable to other urban and suburban hospitals.
There are several important limitations of our analysis. First, we could only include GIM patients, who represent nearly 40% of ED admissions to hospital at participating centers. A more holistic analysis across all hospital services could be justified; however, given that many quality improvement initiatives occur at the level of a single hospital service, we felt our approach would be informative for future research and improvement efforts. Approximately 75% of GIM patients at participating hospitals were cared for on a GIM ward, with 25% cared for on off-service units. We were unable to include the total hospital census in our models, and this could affect LOS and waiting times for GIM patients, particularly those admitted to off-service units. GIM census is likely highly correlated with hospital census, and we were able to adjust for this. Nevertheless, this remains an important potential source of unmeasured confounding. Second, we did not model the effects of morning discharges from GIM on patient-flow measures for non-GIM patients. Given the lack of effects for GIM patients, who would be more likely to be directly affected, it is unlikely that large effects would be seen for other hospital patients, but we did not measure effects on surgical delays or cancellations, for example.23 Third, we report 30-day readmission to GIM at participating hospitals only, rather than all readmissions. However, prior research in our region demonstrated that 82% of hospital readmissions occur to the same site.32 Thus, our measure, which includes admission to any participating hospital, likely captures more than 80% of all readmissions, and this was a secondary outcome in our analysis. Finally, qualitative metrics, such as patient or provider satisfaction, were not measured in our study. Earlier discharge may impact patient care in other ways by being more predictable for staff, improving bed allocation for daytime procedures, making medication pick-ups easier to arrange, or making consultations with allied health services more convenient.11,28,33 Conversely, if pressured to discharge before noon, providers may feel rushed to complete tasks and may face disruptions to typical workflow.24 As such, future research is needed to provide a more complete understanding of the impact of early-morning discharge beyond hospital flow.
CONCLUSION
The number of morning discharges was not significantly associated with shorter ED LOS or hospital LOS for GIM patients. Our observational findings suggest that increasing morning discharges alone may not substantially improve patient flow in GIM. Further research is needed to evaluate specific morning discharge interventions and assess hospital-wide effects.
1. Trzeciak S, Rivers EP. Emergency department overcrowding in the United States: an emerging threat to patient safety and public health. Emerg Med J. 2003;20(5):402-405. https://doi.org/10.1136/emj.20.5.402
2. McKenna P, Heslin SM, Viccellio P, Mallon WK, Hernandez C, Morley EJ. Emergency department and hospital crowding: causes, consequences, and cures. Clin Exp Emerg Med. 2019;6(3):189-195. https://doi.org/10.15441/ceem.18.022
3. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1-10. https://doi.org/10.1111/j.1553-2712.2008.00295.x
4. Derlet RW, Richards JR. Overcrowding in the nation’s emergency departments: complex causes and disturbing effects. Ann Emerg Med. 2000;35(1):63-68. https://doi.org/10.1016/s0196-0644(00)70105-3
5. Pines JM, Iyer S, Disbot M, Hollander JE, Shofer FS, Datner EM. The effect of emergency department crowding on patient satisfaction for admitted patients. Acad Emerg Med. 2008;15(9):825-831. https://doi.org/10.1111/j.1553-2712.2008.00200.x
6. Carter EJ, Pouch SM, Larson EL. The relationship between emergency department crowding and patient outcomes: a systematic review. J Nurs Scholarsh. 2014;46(2):106-115. https://doi.org/10.1111/jnu.1205
7. Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA. 2010;303(21):2141-2147. https://doi.org/10.1001/jama.2010.748
8. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. https://doi.org/ 10.1002/14651858.CD006632.pub2
9. Zodda D, Underwood J. Improving emergency department throughput: evidence-based strategies aimed at reducing boarding and overcrowding. Phys Leadership J. 2019;6(3):70-73.
10. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. https://doi.org/10.1002/jhm.2154
11. Kane M, Weinacker A, Arthofer R, et al. A multidisciplinary initiative to increase inpatient discharges before noon. J Nurs Adm. 2016;46(12):630-635. https://doi.org/10.1097/NNA.0000000000000418
12. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. https://doi.org/10.1002/jhm.2529
13. Patel H, Morduchowicz S, Mourad M. Using a systematic framework of interventions to improve early discharges. Jt Comm J Qual Patient Saf. 2017;43(4):189-196. https://doi.org/10.1016/j.jcjq.2016.12.003
14. El-Eid GR, Kaddoum R, Tamim H, Hitti EA. Improving hospital discharge time: a successful implementation of Six Sigma methodology. Medicine (Baltimore). 2015;94(12):e633. https://doi.org/10.1097/MD.0000000000000633
15. Mathews KS, Corso P, Bacon S, Jenq GY. Using the red/yellow/green discharge tool to improve the timeliness of hospital discharges. Jt Comm J Qual Patient Saf. 2014;40(6):243-252. https://doi.org/10.1016/s1553-7250(14)40033-3
16. Verma AA, Pasricha SV, Jung HY, et al. Assessing the quality of clinical and administrative data extracted from hospitals: the General Medicine Inpatient Initiative (GEMINI) experience. J Am Med Inform Assoc. 2021; 28(3):578-587. doi: 10.1093/jamia/ocaa225.
17. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(60:676-682. https://doi.org/10.1093/aje/kwq433
18. Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232-239. https://doi.org/10.1097/MLR.0b013e3181589bb6
19. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(60:1228-1234. https://doi.org/10.1080/03610910902859574
20. van Walraven C, Escobar GJ, Greene JD, Forster AJ. The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population. J Clin Epidemiol. 2010;63(7):798-803. https://doi.org/10.1016/j.jclinepi.2009.08.020
21. Hilbe JM. Negative binomial regression. In: Modeling Count Data. Cambridge University Press. 2014:126-160.
22. Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed. Springer; 2015.
23. Durvasula R, Kayihan A, Del Bene S, et al. A multidisciplinary care pathway significantly increases the number of early morning discharges in a large academic medical center. Qual Manag Health Care. 2015;24(1):45-51. https://doi.org/10.1097/QMH.0000000000000049
24. Goolsarran N, Olowo G, Ling Y, Abbasi S, Taub E, Teressa G. Outcomes of a resident-led early hospital discharge intervention. J Gen Intern Med. 2020;35(2):437-443. https://doi.org/10.1007/s11606-019-05563-w
25. Beck MJ, Okerblom D, Kumar A, Bandyopadhyay S, Scalzi LV. Lean intervention improves patient discharge times, improves emergency department throughput and reduces congestion. Hosp Pract (1995). 2016;44(5):252-259. https://doi.org/10.1080/21548331.2016.1254559
26. Karling A, Tang KW. Discharge before noon: a study in a medical emergency ward. 2015. Accessed February 11, 2021. http://publications.lib.chalmers.se/records/fulltext/231873/231873.pdf
27. Mathews K, Corso P, Bacon S, Jenq GY. Using the red/yellow/green discharge tool to improve the timeliness of hospital discharges. Jt Comm J Qual Patient Saf. 2014;40(6):243-252. https://doi.org/10.1016/s1553-7250(14)40033-3
28. Goodson AS, DeGuzman, PB, Honeycutt A, Summy C, Manly F. Total joint replacement discharge brunch: meeting patient education needs and a hospital initiative of discharge by noon. Orthop Nurs. 2014;33(3):159-162. https://doi.org/10.1097/NOR.0000000000000048
29. Kravet SJ, Levine RB, Rubin HR, Wright SM. Discharging patients earlier in the day: a concept worth evaluating. Health Care Manag (Frederick). 2007;26(2):142-146. https://doi.org/10.1097/01.HCM.0000268617.33491.60
30. Shine D. Discharge before noon: an urban legend. Am J Med. 2015;128(5):445-446. https://doi.org/10.1016/j.amjmed.2014.12.011
31. Minichiello TM, Auerbach AD, Wachter RM. Caregiver perceptions of the reasons for delayed hospital discharge. Eff Clin Pract. 2001;4(6):250-255.
32. Staples JA, Thiruchelvam D, Redelmeier DA. Site of hospital readmission and mortality: a population-based retrospective cohort study. CMAJ Open. 2014;2:E77-E85. https://doi.org/10.9778/cmajo.20130053
33. Bowles KH, Foust JB, Naylor MD. Hospital discharge referral decision making: a multidisciplinary perspective. Appl Nurs Res. 2003;16(3):134-143. https://doi.org/10.1016/s0897-1897(03)00048-x
1. Trzeciak S, Rivers EP. Emergency department overcrowding in the United States: an emerging threat to patient safety and public health. Emerg Med J. 2003;20(5):402-405. https://doi.org/10.1136/emj.20.5.402
2. McKenna P, Heslin SM, Viccellio P, Mallon WK, Hernandez C, Morley EJ. Emergency department and hospital crowding: causes, consequences, and cures. Clin Exp Emerg Med. 2019;6(3):189-195. https://doi.org/10.15441/ceem.18.022
3. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1-10. https://doi.org/10.1111/j.1553-2712.2008.00295.x
4. Derlet RW, Richards JR. Overcrowding in the nation’s emergency departments: complex causes and disturbing effects. Ann Emerg Med. 2000;35(1):63-68. https://doi.org/10.1016/s0196-0644(00)70105-3
5. Pines JM, Iyer S, Disbot M, Hollander JE, Shofer FS, Datner EM. The effect of emergency department crowding on patient satisfaction for admitted patients. Acad Emerg Med. 2008;15(9):825-831. https://doi.org/10.1111/j.1553-2712.2008.00200.x
6. Carter EJ, Pouch SM, Larson EL. The relationship between emergency department crowding and patient outcomes: a systematic review. J Nurs Scholarsh. 2014;46(2):106-115. https://doi.org/10.1111/jnu.1205
7. Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA. 2010;303(21):2141-2147. https://doi.org/10.1001/jama.2010.748
8. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. https://doi.org/ 10.1002/14651858.CD006632.pub2
9. Zodda D, Underwood J. Improving emergency department throughput: evidence-based strategies aimed at reducing boarding and overcrowding. Phys Leadership J. 2019;6(3):70-73.
10. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. https://doi.org/10.1002/jhm.2154
11. Kane M, Weinacker A, Arthofer R, et al. A multidisciplinary initiative to increase inpatient discharges before noon. J Nurs Adm. 2016;46(12):630-635. https://doi.org/10.1097/NNA.0000000000000418
12. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. https://doi.org/10.1002/jhm.2529
13. Patel H, Morduchowicz S, Mourad M. Using a systematic framework of interventions to improve early discharges. Jt Comm J Qual Patient Saf. 2017;43(4):189-196. https://doi.org/10.1016/j.jcjq.2016.12.003
14. El-Eid GR, Kaddoum R, Tamim H, Hitti EA. Improving hospital discharge time: a successful implementation of Six Sigma methodology. Medicine (Baltimore). 2015;94(12):e633. https://doi.org/10.1097/MD.0000000000000633
15. Mathews KS, Corso P, Bacon S, Jenq GY. Using the red/yellow/green discharge tool to improve the timeliness of hospital discharges. Jt Comm J Qual Patient Saf. 2014;40(6):243-252. https://doi.org/10.1016/s1553-7250(14)40033-3
16. Verma AA, Pasricha SV, Jung HY, et al. Assessing the quality of clinical and administrative data extracted from hospitals: the General Medicine Inpatient Initiative (GEMINI) experience. J Am Med Inform Assoc. 2021; 28(3):578-587. doi: 10.1093/jamia/ocaa225.
17. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(60:676-682. https://doi.org/10.1093/aje/kwq433
18. Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232-239. https://doi.org/10.1097/MLR.0b013e3181589bb6
19. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(60:1228-1234. https://doi.org/10.1080/03610910902859574
20. van Walraven C, Escobar GJ, Greene JD, Forster AJ. The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population. J Clin Epidemiol. 2010;63(7):798-803. https://doi.org/10.1016/j.jclinepi.2009.08.020
21. Hilbe JM. Negative binomial regression. In: Modeling Count Data. Cambridge University Press. 2014:126-160.
22. Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed. Springer; 2015.
23. Durvasula R, Kayihan A, Del Bene S, et al. A multidisciplinary care pathway significantly increases the number of early morning discharges in a large academic medical center. Qual Manag Health Care. 2015;24(1):45-51. https://doi.org/10.1097/QMH.0000000000000049
24. Goolsarran N, Olowo G, Ling Y, Abbasi S, Taub E, Teressa G. Outcomes of a resident-led early hospital discharge intervention. J Gen Intern Med. 2020;35(2):437-443. https://doi.org/10.1007/s11606-019-05563-w
25. Beck MJ, Okerblom D, Kumar A, Bandyopadhyay S, Scalzi LV. Lean intervention improves patient discharge times, improves emergency department throughput and reduces congestion. Hosp Pract (1995). 2016;44(5):252-259. https://doi.org/10.1080/21548331.2016.1254559
26. Karling A, Tang KW. Discharge before noon: a study in a medical emergency ward. 2015. Accessed February 11, 2021. http://publications.lib.chalmers.se/records/fulltext/231873/231873.pdf
27. Mathews K, Corso P, Bacon S, Jenq GY. Using the red/yellow/green discharge tool to improve the timeliness of hospital discharges. Jt Comm J Qual Patient Saf. 2014;40(6):243-252. https://doi.org/10.1016/s1553-7250(14)40033-3
28. Goodson AS, DeGuzman, PB, Honeycutt A, Summy C, Manly F. Total joint replacement discharge brunch: meeting patient education needs and a hospital initiative of discharge by noon. Orthop Nurs. 2014;33(3):159-162. https://doi.org/10.1097/NOR.0000000000000048
29. Kravet SJ, Levine RB, Rubin HR, Wright SM. Discharging patients earlier in the day: a concept worth evaluating. Health Care Manag (Frederick). 2007;26(2):142-146. https://doi.org/10.1097/01.HCM.0000268617.33491.60
30. Shine D. Discharge before noon: an urban legend. Am J Med. 2015;128(5):445-446. https://doi.org/10.1016/j.amjmed.2014.12.011
31. Minichiello TM, Auerbach AD, Wachter RM. Caregiver perceptions of the reasons for delayed hospital discharge. Eff Clin Pract. 2001;4(6):250-255.
32. Staples JA, Thiruchelvam D, Redelmeier DA. Site of hospital readmission and mortality: a population-based retrospective cohort study. CMAJ Open. 2014;2:E77-E85. https://doi.org/10.9778/cmajo.20130053
33. Bowles KH, Foust JB, Naylor MD. Hospital discharge referral decision making: a multidisciplinary perspective. Appl Nurs Res. 2003;16(3):134-143. https://doi.org/10.1016/s0897-1897(03)00048-x
© 2021 Society of Hospital Medicine
A Resident-Led Intervention to Increase Initiation of Buprenorphine Maintenance for Hospitalized Patients With Opioid Use Disorder
Nearly 48,000 Americans died from overdoses involving opioids in 2018, continuing a national crisis that has led to 446,000 deaths since 1999.1 Annually, opioids are responsible for more than 500,000 admissions, approximately 1% of all hospitalizations, costing the United States nearly $15 billion.2,3 Among hospitalized patients, chronic opioid use is associated with increased mortality, severe infectious complications, and higher rates of readmission.4 Opioid use disorder (OUD) is a chronic, relapsing medical condition with biopsychosocial origins and significant morbidity and mortality.5 Opioid agonist therapy (OAT) with buprenorphine or methadone maintenance, the evidence-based standard of treatment, reduces the mortality rate by half, decreases overdoses and hospital readmissions, and improves retention in care.6-10
OAT maintenance refers to using buprenorphine or methadone for long-term treatment of OUD rather than for acute treatment of opioid withdrawal. Despite evidence supporting OAT maintenance, clinicians start medications for only 11% to 15% of hospitalized patients with OUD, depending on practice contexts.11,12 Three significant barriers—stigma, insufficient clinician education, and restrictive regulations—prevent clinicians from starting OAT.13 Clinicians who do not have the Drug Enforcement Administration (DEA)–issued DATA-2000 waiver (X-waiver) for outpatient prescribing can order buprenorphine for admitted patients but cannot prescribe it at discharge.14 In hospitals where they exist, addiction medicine consult services offer primary teams guidance on pharmacotherapy, leading to reduced hospital readmissions and increased engagement in outpatient addiction treatment.15-17 However, in most hospitals around the country, such specialty services do not exist.18 In some hospitals without addiction medicine consult services, hospitalists with expertise in OUD have started assisting primary teams in starting OAT, but to our knowledge, no prior studies have described the impact of these interventions on patients or clinician experience with OAT.19
This quality improvement project aimed to increase the rate at which internal medicine resident teams at Johns Hopkins Hospital (JHH) in Baltimore, Maryland, started hospitalized patients with OUD on buprenorphine maintenance. We hypothesized that resident education and measures to increase the availability of X-waivered physicians would increase the rate of initiating buprenorphine maintenance. We additionally hypothesized that these interventions would increase knowledge about and comfort with buprenorphine across the residency. This represents the first study to examine the effects of clinician education and a team of X-waivered residents and hospitalists who assist in starting buprenorphine maintenance in a hospital without an addiction medicine consult service.
METHODS
Setting
This study took place from July 2018 to June 2019 at JHH, a large, academic, urban hospital in Baltimore. Prior to the intervention, internal medicine residents at JHH commonly used short courses of buprenorphine to treat withdrawal, but they did not have access to hospital-specific resources to assist with starting maintenance OAT. During the study period, JHH had a Substance Use Disorders team staffed by peer recovery specialists that could be consulted by hospitalists and residents to provide psychosocial support and link admitted patients to treatment after discharge. There were no providers on the team to guide pharmacotherapy or to write discharge buprenorphine prescriptions. The Osler Medical Residency Training Program at JHH has 140 internal medicine residents and 16 combined medicine-pediatrics residents. All residents receive 1 hour of formal education about opioid use disorder annually. In addition, 28 of those 156 residents, those in the Urban Health Primary Care track, spend 1 month on an Addiction Medicine rotation in which they complete the 8-hour training required to receive the X-waiver. Those residents are encouraged to apply for the X-waiver once they obtain a medical license subsidized by a Health Resources & Services Administration (HRSA) grant. Four internal medicine attending physicians on teaching services and one resident had X-waivers prior to the intervention.
Intervention
In November 2018, we administered a survey to residents to identify barriers to starting buprenorphine maintenance and to measure knowledge and confidence with using buprenorphine for OUD (Appendix Figure 1 and Figure 2). We focused on buprenorphine because providers at JHH were familiar with this medication and because Baltimore has widespread access to buprenorphine, with more than 490 local buprenorphine providers.20 Five residents piloted the survey and provided feedback. We then administered the survey to all internal medicine and medicine-pediatrics residents. Based on the results, we developed a targeted educational conference and also created the Buprenorphine Bridge Team (BBT).
In January 2019, we presented the educational conference for residents devoted to the use of buprenorphine for OUD and introduced the BBT. The conference started with a patient testimonial and included peer recovery specialists, pharmacists, nurses, and social workers. We summarized the evidence for buprenorphine and offered a practical guide to start treatment in a one-page protocol. This protocol included guidance on selecting patients, shared decision-making around OUD treatment, avoiding precipitated withdrawal, dosing buprenorphine, and establishing follow-up (Appendix Figure 3). We asked for input on this protocol from nursing leadership, social work teams, and peer recovery specialists. Dosing was adapted from the Guidelines from the American Society of Addiction Medicine, with expert input from physicians from the Addiction Medicine Consult service at Johns Hopkins Bayview Medical Center, also in Baltimore.5 We instructed residents to obtain discharge buprenorphine prescriptions from an X-waivered physician on their team or from the newly established BBT. We asked resident teams to set up a postdischarge appointment for patients with an X-waivered provider, either in a community practice or at the JHH After Care Clinic, a transitional care clinic for discharged patients.21
The BBT is a resident-led group of X-waivered JHH residents and hospitalists who volunteer to write discharge buprenorphine prescriptions for patients. The BBT serves to ensure primary teams have access to an X-waivered prescriber. It is not a consult service. We asked primary teams to contact the BBT after initiating buprenorphine and after securing a follow-up appointment. In response to each request, a member of the BBT reviews the patient chart, confirms the follow-up plan, writes a prescription for buprenorphine along with intranasal naloxone, and leaves a brief note. During the 6-month postintervention period, the team consisted of three residents and three hospitalist attendings. Each week, two members (residents or attendings) staffed the team Monday to Friday, 8
In May 2019, 5 months after the education session and implementation of the BBT, we administered a follow-up survey.
Outcomes
As a secondary outcome, we measured engagement in OUD treatment after discharge by calculating the proportion of patients started on buprenorphine who filled a buprenorphine prescription within 30 days after discharge. We chose 30 days based on the National Committee for Quality Assurance’s Healthcare Effectiveness Data and Information Set (HEDIS) measure for engagement of treatment for alcohol and other drugs.23 We obtained the data from the Chesapeake Regional Information System for our Patients (CRISP) Prescription Drug Monitoring Program, which monitors all prescriptions for controlled substances dispensed in Maryland and five neighboring states. As a balancing measure, we counted patients newly started on methadone maintenance for OUD before and after the intervention. Additional secondary process outcomes included frequency of BBT requests, the volume of buprenorphine prescriptions written by the team, and time required to complete a BBT request.
Clinician-level outcomes, measured with electronically administered pre- and postintervention surveys to residents, included knowledge about and comfort with buprenorphine. Of the 16 questions in the pre- and postimplementation surveys, we analyzed the 6 questions concerning knowledge and comfort that remained identical in the pre- and postintervention surveys and used 5-point Likert scale responses. As an incentive, we randomly distributed three $50 gift cards to survey completers.
Analysis
We used an interrupted time series analysis to evaluate the association between the intervention bundle and a change in the rate that medical teams started patients with OUD on buprenorphine maintenance. This approach allowed us to control for preintervention trends. To evaluate the impact of our interventions, our pre- and postintervention periods include the same residents during the 2018-2019 academic year. Both periods consisted of twelve 2-week intervals (preintervention: July 26, 2019, to January 9, 2019; postintervention: January 10, 2019, to June 26, 2019).
To evaluate for changes in engagement in OUD treatment after discharge, we used two-sample t tests. To evaluate for changes in resident-reported comfort and knowledge with initiating buprenorphine maintenance, we used Wilcoxon rank sum tests for survey data and Wilcoxon signed rank tests for paired data. All analyses employed two-sided P values with statistical significance evaluated at the .05 alpha level. We analyzed data using R version 3.6.3 (Foundation for Statistical Computing). The Institutional Review Board at JHH reviewed and approved the study protocol as a quality improvement project (IRB00193365).
Before the intervention, 13 of the 30 patients (40%) newly started on buprenorphine maintenance during their admission filled a follow-up buprenorphine prescription within 30 days of discharge. After the intervention, 31 of 64 patients (46%) filled a buprenorphine prescription within 30 days (P = .612). Two patients were started on methadone maintenance, one prior to and one after the intervention.
During the 6-month postintervention period, the BBT received 75 requests and wrote 70 prescriptions for buprenorphine. The median time required to complete a BBT request was 15 minutes (minimum, 5 minutes; maximum, 60 minutes).
Of 156 internal medicine and medicine-pediatrics residents, 89 residents (57%) completed the baseline survey and 66 residents (42%) completed the follow-up survey. Forty residents completed both surveys. After the intervention, residents were significantly more likely to feel comfortable dosing buprenorphine (P < .0001) and counseling patients about its use (P = .0237) and were more likely to report ease of establishing follow-up (P < .0001). Self-reported knowledge about preventing precipitated withdrawal increased significantly (P = .0191), as did knowledge about the effectiveness of buprenorphine (P = .0003) independent of formal drug counseling (P = .0066) (Table). Paired survey data also found statistically significant results for all questions except those about preventing precipitated withdrawal and efficacy. For the latter, respondents who completed both surveys were more knowledgeable before the intervention than the overall group that completed the baseline survey (Appendix Table).
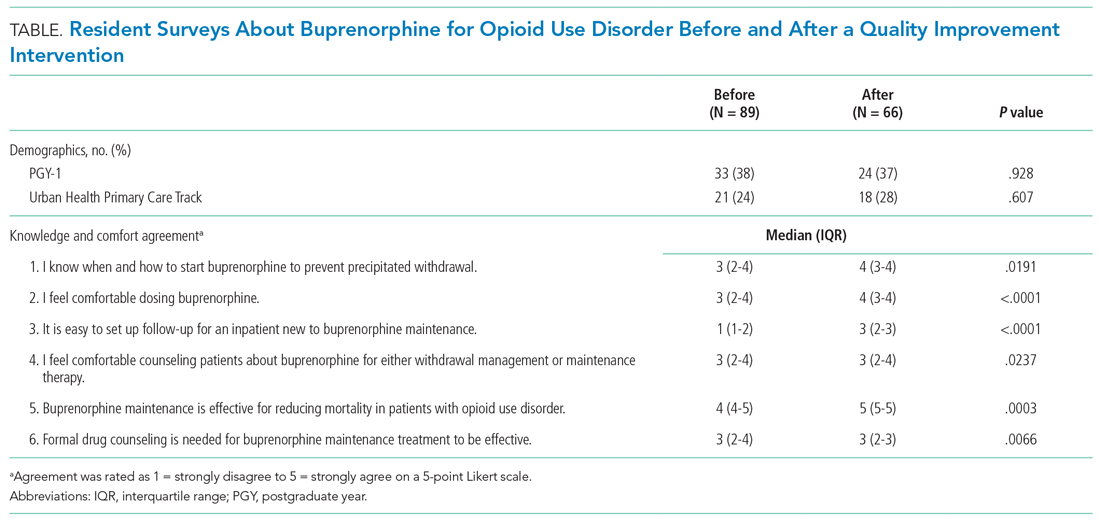
This study shows how a resident-led quality improvement project comprising clinician education and implementation of a novel BBT was associated with an increased rate of starting buprenorphine maintenance in hospitalized patients with OUD and improved resident knowledge about and comfort with buprenorphine. To our knowledge, this is the first study demonstrating how education and a team of X-waivered generalists can help primary teams initiate and discharge patients on buprenorphine maintenance in a hospital without an addiction medicine consult service.
Prior to the intervention, resident internal medicine teams at JHH started 10% of hospitalized patients with OUD on buprenorphine maintenance, consistent with prior studies showing rates of 11% to 15% for initiating OAT for hospitalized patients.11,12 After the intervention, the rate of initiating buprenorphine maintenance more than doubled, rising to 24% of eligible patients. Resident internal medicine teams at JHH started buprenorphine maintenance for 37 more patients over the 24-week postintervention period than would have been predicted prior to the intervention, or an additional three patients every 2 weeks.
Between 40% and 46% of hospitalized patients newly started on buprenorphine maintenance filled an outpatient buprenorphine prescription within 30 days of discharge. We are not aware of comparative data for 30-day follow-up for hospitalized patients newly started on buprenorphine maintenance. Data from other contexts show 5% to 10% of veterans were engaged in addiction treatment 30 days after initiation from inpatient or outpatient encounters. An analysis of an academic medical center in Oregon found engagement with an addiction medicine consult service increased after hospital engagement for patients with any substance use disorder from 23% to 39% using the 34-day HEDIS measure for engagement.17,24,25
The BBT required approximately 15 minutes per request and wrote an average of three prescriptions per week, demonstrating the feasibility of this approach and the high demand for this service. One strength of our approach is that residents gained experience starting buprenorphine independently using the aforementioned protocol instead of deferring to a full consult service. It is likely that this resident engagement in initiating longitudinal OUD care contributed to the success of this initiative, as did existing resident familiarity with using buprenorphine for opioid withdrawal.
This approach to resident education—promoting direct, first-person experience with medications in a clinical context—aligns with recommendations from a recent review about substance use disorder education for health professionals.26 Our interventions increased resident knowledge and comfort with buprenorphine, consistent with prior studies showing increased resident confidence in management of substance use disorders after curricular innovations.24,25
A few contextual features were essential for this project’s viability. Maryland allows American medical graduates to obtain a medical license after 1 year of postgraduate training. This allowed three residents to obtain X-waivers. These residents had access to HRSA funding to subsidize the expenses of applying for state licensure and DEA registration. BBT members volunteered their time while working on other services. Last, we were able to take advantage of buprenorphine-providing clinics in Baltimore, including the JHH After Care Clinic, to accept patients for follow-up appointments after discharge.
Limitations
The BBT required motivated clinicians willing to volunteer for additional clinical responsibilities during inpatient rotations and supportive faculty and residency leadership. Attending physicians, nurse practitioners, or physician assistants could staff a similar BBT in hospitals without residents or in hospitals where residents cannot obtain DEA registration. Crucially, other hospitals may not have access to practices with X-waivered physicians for outpatient follow-up. A recent study found X-waivered primary care physicians were less likely to be affiliated with hospital health systems. Other studies have shown limitations in access to buprenorphine at the county level based on geography and racial/ethnic segregation.27-29
Most patients hospitalized with OUD did not have ICD-10 codes associated with OUD. We addressed this by assuming patients had OUD if buprenorphine or methadone was ordered during their hospitalization, even if the medication was never administered. This may have overcounted patients prescribed these medications for indications other than OUD, and it may have undercounted patients with OUD for whom buprenorphine or methadone were never considered. The opioid withdrawal order set at JHH automatically offers an option to use buprenorphine to treat withdrawal. Patients with OUD for whom buprenorphine or methadone were never ordered likely did not experience withdrawal or were in withdrawal so mild that it escaped the attention of the team, which limits the generalizability of our intervention.
We identified several limitations to the internal validity of our study. First, we used a before-and-after study design without a control group. We could not ethically withhold access to evidence-based, mortality-reducing medications from patients. Without a control group, we cannot rule out the possibility that underlying temporal trends made residents more likely to start buprenorphine maintenance independent of our intervention. We attempted to control for unmeasured confounders by using an interrupted time series analysis to control for preintervention trends, comparing the same group of residents before and after our interventions, and selecting an intervention period during which residents were given only educational sessions and materials provided by our team. Our results may be biased by clustered data because certain residents may have been more likely to initiate buprenorphine, but these effects are likely marginal because resident schedules are balanced between outpatient and inpatient rotations during each 6-month period.
Finally, this project focused on buprenorphine, not on other medications for OUD, including methadone or naltrexone, or nonpharmacologic treatments for OUD.
Sustainability and Next Steps
Since the start of the BBT in January 2019, five additional PGY-2 residents obtained their medical licenses and X-waivers. These residents, with the support of two attending hospitalists, led the BBT and coordinated education sessions that were incorporated into the curriculum during the 2019-2020 academic year. These educational sessions will continue indefinitely. In 2020, JHH started an Addiction Medicine Consult Service staffed by physicians, NPs, and a pharmacist. The BBT continues to operate in conjunction with this service.
We found substantial variability in the rate of buprenorphine maintenance initiation despite our interventions. This is an area for future improvement. In a free-response prompt in our follow-up survey, residents requested additional education sessions and an order set to assist with initiation of buprenorphine. To address these gaps, three educational sessions were added, one of which included education on starting methadone maintenance therapy. We also added a new order set for starting buprenorphine maintenance. We hypothesize that these interventions will improve consistency.
In order for a similar program to be disseminated to other institutions, educational initiatives and a team of dedicated X-waivered prescribers are key. Materials to assist with this process are available in the Appendix.
CONCLUSION
This study shows how a resident-led intervention comprising clinician education and a team of X-waivered generalists was associated with improved treatment of OUD for hospitalized patients. We encourage residents and all clinicians at other hospitals without addiction medicine consult services to design, implement, and study similar interventions that directly increase the use of buprenorphine or methadone maintenance to treat OUD.
Preliminary results from this project were presented at the AMERSA National Conference on November 7, 2019.
1. Wilson N, Kariisa M, Seth P, Iv HS, Davis NL. Drug and opioid-involved overdose deaths – United States, 2017–2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. http://dx.doi.org/10.15585/mmwr.mm6911a4
2. Berk J, Rogers KM, Wilson DJ, Thakrar A, Feldman L. Missed opportunities for treatment of opioid use disorder in the hospital setting: updating an outdated policy. J Hosp Med. 2020;15(10):619-621. https://doi.org/10.12788/jhm.3352
3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002–12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424
4. Mosher HJ, Jiang L, Vaughan Sarrazin MS, Cram P, Kaboli PJ, Vander Weg MW. Prevalence and characteristics of hospitalized adults on chronic opioid therapy. J Hosp Med. 2014;9(2):82-87. https://doi.org/10.1002/jhm.2113
5. Crotty K, Freedman KI, Kampman KM. Executive summary of the focused update of the ASAM national practice guideline for the treatment of opioid use disorder. J Addict Med. 2020;14(2):99-112. https://doi.org/10.1097/adm.0000000000000635
6. Leshner AI, Mancher M, eds. Medications for Opioid Use Disorder Save Lives. The National Academies Press; 2019. https://www.nap.edu/catalog/25310
7. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357: j1550. https://doi.org/10.1136/bmj.j1550
8. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality. Ann Intern Med. 2018;169(3):137-145. https://dx.doi.org/10.7326%2FM17-3107
9. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368. https://doi.org/10.1056/nejmra1604339
10. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
11. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
12. Priest KC, Lovejoy TI, Englander H, Shull S, McCarty D. Opioid agonist therapy during hospitalization within the Veterans Health Administration: a pragmatic retrospective cohort analysis. J Gen Intern Med. 2020;35(8):2365-2374. https://doi.org/10.1007/s11606-020-05815-0
13. Madras BK, Ahmad NJ, Wen J, Sharfstein J; Prevention, Treatment, and Recovery Working Group of the Action Collaborative on Countering the U.S. Opioid Epidemic. Improving access to evidence-based medical treatment for opioid use disorder: strategies to address key barriers within the treatment system. NAM Perspectives. April 27, 2020. https://doi.org/10.31478/202004b
14. Fiscella K, Wakeman SE, Beletsky L. Buprenorphine deregulation and mainstreaming treatment for opioid use disorder: x the X Waiver. JAMA Psychiatry. 2019;76(3):229-230. https://doi.org/10.1001/jamapsychiatry.2018.3685
15. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/adm.0000000000000496
16. Weimer M, Morford K, Donroe J. Treatment of opioid use disorder in the acute hospital setting: a critical review of the literature (2014–2019). Curr Addict Rep. 2019;6(4):339-354.
17. Englander H, Dobbertin K, Lind BK, et al. Inpatient addiction medicine consultation and post-hospital substance use disorder treatment engagement: a propensity-matched analysis. J Gen Intern Med. 2019;34(12):2796-2803. https://doi.org/10.1007/s11606-019-05251-9
18. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2019;14(3):E1-E4. https://doi.org/10.12788/jhm.3311
19. Bottner R, Moriates C, Tirado C. The role of hospitalists in treating opioid use disorder. J Addict Med. 2020;14(2):178. https://doi.org/10.1097/adm.0000000000000545
20. Behavioral health treatment services locator. Substance Abuse and Mental Health Services Administration. Accessed May 14, 2020. https://findtreatment.samhsa.gov/
21. Groesbeck K, Whiteman LN, Stewart RW. Reducing readmission rates by improving transitional care. South Med J. 2015;108(12):758-760. https://doi.org/10.14423/smj.0000000000000376
22. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015 Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
23. Initiation and engagement of alcohol and other drug abuse or dependence treatment (IET). NCQA. Accessed April 20, 2020. https://www.ncqa.org/hedis/measures/initiation-and-engagement-of-alcohol-and-other-drug-abuse-or-dependence-treatment/
24. Wyse JJ, Robbins JL, McGinnis KA, et al. Predictors of timely opioid agonist treatment initiation among veterans with and without HIV. Drug Alcohol Depend. 2019;198:70-75. https://doi.org/10.1016/j.drugalcdep.2019.01.038
25. Harris AHS, Humphreys K, Finney JW. Veterans Affairs facility performance on Washington Circle indicators and casemix-adjusted effectiveness. J Subst Abuse Treat. 2007;33(4):333-339. https://doi.org/10.1016/j.jsat.2006.12.015
26. Muzyk A, Smothers ZPW, Andolsek KM, et al. Interprofessional substance use disorder education in health professions education programs: a scoping review. Acad Med. 2020;95(3):470-480. https://doi.org/10.1097/acm.0000000000003053
27. Saloner B, Lin L, Simon K. Geographic location of buprenorphine-waivered physicians and integration with health systems. J Subst Abuse Treat. 2020;115:108034. https://doi.org/10.1016/j.jsat.2020.108034
28. Jones CW, Christman Z, Smith CM, et al. Comparison between buprenorphine provider availability and opioid deaths among US counties. J Subst Abuse Treat. 2018;93:19-25. https://doi.org/10.1016/j.jsat.2018.07.008
29. Goedel WC, Shapiro A, Cerdá M, Tsai JW, Hadland SE, Marshall BDL. Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA Netw Open. 2020;3(4):e203711. https://doi.org/10.1001/jamanetworkopen.2020.3711
Nearly 48,000 Americans died from overdoses involving opioids in 2018, continuing a national crisis that has led to 446,000 deaths since 1999.1 Annually, opioids are responsible for more than 500,000 admissions, approximately 1% of all hospitalizations, costing the United States nearly $15 billion.2,3 Among hospitalized patients, chronic opioid use is associated with increased mortality, severe infectious complications, and higher rates of readmission.4 Opioid use disorder (OUD) is a chronic, relapsing medical condition with biopsychosocial origins and significant morbidity and mortality.5 Opioid agonist therapy (OAT) with buprenorphine or methadone maintenance, the evidence-based standard of treatment, reduces the mortality rate by half, decreases overdoses and hospital readmissions, and improves retention in care.6-10
OAT maintenance refers to using buprenorphine or methadone for long-term treatment of OUD rather than for acute treatment of opioid withdrawal. Despite evidence supporting OAT maintenance, clinicians start medications for only 11% to 15% of hospitalized patients with OUD, depending on practice contexts.11,12 Three significant barriers—stigma, insufficient clinician education, and restrictive regulations—prevent clinicians from starting OAT.13 Clinicians who do not have the Drug Enforcement Administration (DEA)–issued DATA-2000 waiver (X-waiver) for outpatient prescribing can order buprenorphine for admitted patients but cannot prescribe it at discharge.14 In hospitals where they exist, addiction medicine consult services offer primary teams guidance on pharmacotherapy, leading to reduced hospital readmissions and increased engagement in outpatient addiction treatment.15-17 However, in most hospitals around the country, such specialty services do not exist.18 In some hospitals without addiction medicine consult services, hospitalists with expertise in OUD have started assisting primary teams in starting OAT, but to our knowledge, no prior studies have described the impact of these interventions on patients or clinician experience with OAT.19
This quality improvement project aimed to increase the rate at which internal medicine resident teams at Johns Hopkins Hospital (JHH) in Baltimore, Maryland, started hospitalized patients with OUD on buprenorphine maintenance. We hypothesized that resident education and measures to increase the availability of X-waivered physicians would increase the rate of initiating buprenorphine maintenance. We additionally hypothesized that these interventions would increase knowledge about and comfort with buprenorphine across the residency. This represents the first study to examine the effects of clinician education and a team of X-waivered residents and hospitalists who assist in starting buprenorphine maintenance in a hospital without an addiction medicine consult service.
METHODS
Setting
This study took place from July 2018 to June 2019 at JHH, a large, academic, urban hospital in Baltimore. Prior to the intervention, internal medicine residents at JHH commonly used short courses of buprenorphine to treat withdrawal, but they did not have access to hospital-specific resources to assist with starting maintenance OAT. During the study period, JHH had a Substance Use Disorders team staffed by peer recovery specialists that could be consulted by hospitalists and residents to provide psychosocial support and link admitted patients to treatment after discharge. There were no providers on the team to guide pharmacotherapy or to write discharge buprenorphine prescriptions. The Osler Medical Residency Training Program at JHH has 140 internal medicine residents and 16 combined medicine-pediatrics residents. All residents receive 1 hour of formal education about opioid use disorder annually. In addition, 28 of those 156 residents, those in the Urban Health Primary Care track, spend 1 month on an Addiction Medicine rotation in which they complete the 8-hour training required to receive the X-waiver. Those residents are encouraged to apply for the X-waiver once they obtain a medical license subsidized by a Health Resources & Services Administration (HRSA) grant. Four internal medicine attending physicians on teaching services and one resident had X-waivers prior to the intervention.
Intervention
In November 2018, we administered a survey to residents to identify barriers to starting buprenorphine maintenance and to measure knowledge and confidence with using buprenorphine for OUD (Appendix Figure 1 and Figure 2). We focused on buprenorphine because providers at JHH were familiar with this medication and because Baltimore has widespread access to buprenorphine, with more than 490 local buprenorphine providers.20 Five residents piloted the survey and provided feedback. We then administered the survey to all internal medicine and medicine-pediatrics residents. Based on the results, we developed a targeted educational conference and also created the Buprenorphine Bridge Team (BBT).
In January 2019, we presented the educational conference for residents devoted to the use of buprenorphine for OUD and introduced the BBT. The conference started with a patient testimonial and included peer recovery specialists, pharmacists, nurses, and social workers. We summarized the evidence for buprenorphine and offered a practical guide to start treatment in a one-page protocol. This protocol included guidance on selecting patients, shared decision-making around OUD treatment, avoiding precipitated withdrawal, dosing buprenorphine, and establishing follow-up (Appendix Figure 3). We asked for input on this protocol from nursing leadership, social work teams, and peer recovery specialists. Dosing was adapted from the Guidelines from the American Society of Addiction Medicine, with expert input from physicians from the Addiction Medicine Consult service at Johns Hopkins Bayview Medical Center, also in Baltimore.5 We instructed residents to obtain discharge buprenorphine prescriptions from an X-waivered physician on their team or from the newly established BBT. We asked resident teams to set up a postdischarge appointment for patients with an X-waivered provider, either in a community practice or at the JHH After Care Clinic, a transitional care clinic for discharged patients.21
The BBT is a resident-led group of X-waivered JHH residents and hospitalists who volunteer to write discharge buprenorphine prescriptions for patients. The BBT serves to ensure primary teams have access to an X-waivered prescriber. It is not a consult service. We asked primary teams to contact the BBT after initiating buprenorphine and after securing a follow-up appointment. In response to each request, a member of the BBT reviews the patient chart, confirms the follow-up plan, writes a prescription for buprenorphine along with intranasal naloxone, and leaves a brief note. During the 6-month postintervention period, the team consisted of three residents and three hospitalist attendings. Each week, two members (residents or attendings) staffed the team Monday to Friday, 8
In May 2019, 5 months after the education session and implementation of the BBT, we administered a follow-up survey.
Outcomes
As a secondary outcome, we measured engagement in OUD treatment after discharge by calculating the proportion of patients started on buprenorphine who filled a buprenorphine prescription within 30 days after discharge. We chose 30 days based on the National Committee for Quality Assurance’s Healthcare Effectiveness Data and Information Set (HEDIS) measure for engagement of treatment for alcohol and other drugs.23 We obtained the data from the Chesapeake Regional Information System for our Patients (CRISP) Prescription Drug Monitoring Program, which monitors all prescriptions for controlled substances dispensed in Maryland and five neighboring states. As a balancing measure, we counted patients newly started on methadone maintenance for OUD before and after the intervention. Additional secondary process outcomes included frequency of BBT requests, the volume of buprenorphine prescriptions written by the team, and time required to complete a BBT request.
Clinician-level outcomes, measured with electronically administered pre- and postintervention surveys to residents, included knowledge about and comfort with buprenorphine. Of the 16 questions in the pre- and postimplementation surveys, we analyzed the 6 questions concerning knowledge and comfort that remained identical in the pre- and postintervention surveys and used 5-point Likert scale responses. As an incentive, we randomly distributed three $50 gift cards to survey completers.
Analysis
We used an interrupted time series analysis to evaluate the association between the intervention bundle and a change in the rate that medical teams started patients with OUD on buprenorphine maintenance. This approach allowed us to control for preintervention trends. To evaluate the impact of our interventions, our pre- and postintervention periods include the same residents during the 2018-2019 academic year. Both periods consisted of twelve 2-week intervals (preintervention: July 26, 2019, to January 9, 2019; postintervention: January 10, 2019, to June 26, 2019).
To evaluate for changes in engagement in OUD treatment after discharge, we used two-sample t tests. To evaluate for changes in resident-reported comfort and knowledge with initiating buprenorphine maintenance, we used Wilcoxon rank sum tests for survey data and Wilcoxon signed rank tests for paired data. All analyses employed two-sided P values with statistical significance evaluated at the .05 alpha level. We analyzed data using R version 3.6.3 (Foundation for Statistical Computing). The Institutional Review Board at JHH reviewed and approved the study protocol as a quality improvement project (IRB00193365).

Before the intervention, 13 of the 30 patients (40%) newly started on buprenorphine maintenance during their admission filled a follow-up buprenorphine prescription within 30 days of discharge. After the intervention, 31 of 64 patients (46%) filled a buprenorphine prescription within 30 days (P = .612). Two patients were started on methadone maintenance, one prior to and one after the intervention.
During the 6-month postintervention period, the BBT received 75 requests and wrote 70 prescriptions for buprenorphine. The median time required to complete a BBT request was 15 minutes (minimum, 5 minutes; maximum, 60 minutes).
Of 156 internal medicine and medicine-pediatrics residents, 89 residents (57%) completed the baseline survey and 66 residents (42%) completed the follow-up survey. Forty residents completed both surveys. After the intervention, residents were significantly more likely to feel comfortable dosing buprenorphine (P < .0001) and counseling patients about its use (P = .0237) and were more likely to report ease of establishing follow-up (P < .0001). Self-reported knowledge about preventing precipitated withdrawal increased significantly (P = .0191), as did knowledge about the effectiveness of buprenorphine (P = .0003) independent of formal drug counseling (P = .0066) (Table). Paired survey data also found statistically significant results for all questions except those about preventing precipitated withdrawal and efficacy. For the latter, respondents who completed both surveys were more knowledgeable before the intervention than the overall group that completed the baseline survey (Appendix Table).

This study shows how a resident-led quality improvement project comprising clinician education and implementation of a novel BBT was associated with an increased rate of starting buprenorphine maintenance in hospitalized patients with OUD and improved resident knowledge about and comfort with buprenorphine. To our knowledge, this is the first study demonstrating how education and a team of X-waivered generalists can help primary teams initiate and discharge patients on buprenorphine maintenance in a hospital without an addiction medicine consult service.
Prior to the intervention, resident internal medicine teams at JHH started 10% of hospitalized patients with OUD on buprenorphine maintenance, consistent with prior studies showing rates of 11% to 15% for initiating OAT for hospitalized patients.11,12 After the intervention, the rate of initiating buprenorphine maintenance more than doubled, rising to 24% of eligible patients. Resident internal medicine teams at JHH started buprenorphine maintenance for 37 more patients over the 24-week postintervention period than would have been predicted prior to the intervention, or an additional three patients every 2 weeks.
Between 40% and 46% of hospitalized patients newly started on buprenorphine maintenance filled an outpatient buprenorphine prescription within 30 days of discharge. We are not aware of comparative data for 30-day follow-up for hospitalized patients newly started on buprenorphine maintenance. Data from other contexts show 5% to 10% of veterans were engaged in addiction treatment 30 days after initiation from inpatient or outpatient encounters. An analysis of an academic medical center in Oregon found engagement with an addiction medicine consult service increased after hospital engagement for patients with any substance use disorder from 23% to 39% using the 34-day HEDIS measure for engagement.17,24,25
The BBT required approximately 15 minutes per request and wrote an average of three prescriptions per week, demonstrating the feasibility of this approach and the high demand for this service. One strength of our approach is that residents gained experience starting buprenorphine independently using the aforementioned protocol instead of deferring to a full consult service. It is likely that this resident engagement in initiating longitudinal OUD care contributed to the success of this initiative, as did existing resident familiarity with using buprenorphine for opioid withdrawal.
This approach to resident education—promoting direct, first-person experience with medications in a clinical context—aligns with recommendations from a recent review about substance use disorder education for health professionals.26 Our interventions increased resident knowledge and comfort with buprenorphine, consistent with prior studies showing increased resident confidence in management of substance use disorders after curricular innovations.24,25
A few contextual features were essential for this project’s viability. Maryland allows American medical graduates to obtain a medical license after 1 year of postgraduate training. This allowed three residents to obtain X-waivers. These residents had access to HRSA funding to subsidize the expenses of applying for state licensure and DEA registration. BBT members volunteered their time while working on other services. Last, we were able to take advantage of buprenorphine-providing clinics in Baltimore, including the JHH After Care Clinic, to accept patients for follow-up appointments after discharge.
Limitations
The BBT required motivated clinicians willing to volunteer for additional clinical responsibilities during inpatient rotations and supportive faculty and residency leadership. Attending physicians, nurse practitioners, or physician assistants could staff a similar BBT in hospitals without residents or in hospitals where residents cannot obtain DEA registration. Crucially, other hospitals may not have access to practices with X-waivered physicians for outpatient follow-up. A recent study found X-waivered primary care physicians were less likely to be affiliated with hospital health systems. Other studies have shown limitations in access to buprenorphine at the county level based on geography and racial/ethnic segregation.27-29
Most patients hospitalized with OUD did not have ICD-10 codes associated with OUD. We addressed this by assuming patients had OUD if buprenorphine or methadone was ordered during their hospitalization, even if the medication was never administered. This may have overcounted patients prescribed these medications for indications other than OUD, and it may have undercounted patients with OUD for whom buprenorphine or methadone were never considered. The opioid withdrawal order set at JHH automatically offers an option to use buprenorphine to treat withdrawal. Patients with OUD for whom buprenorphine or methadone were never ordered likely did not experience withdrawal or were in withdrawal so mild that it escaped the attention of the team, which limits the generalizability of our intervention.
We identified several limitations to the internal validity of our study. First, we used a before-and-after study design without a control group. We could not ethically withhold access to evidence-based, mortality-reducing medications from patients. Without a control group, we cannot rule out the possibility that underlying temporal trends made residents more likely to start buprenorphine maintenance independent of our intervention. We attempted to control for unmeasured confounders by using an interrupted time series analysis to control for preintervention trends, comparing the same group of residents before and after our interventions, and selecting an intervention period during which residents were given only educational sessions and materials provided by our team. Our results may be biased by clustered data because certain residents may have been more likely to initiate buprenorphine, but these effects are likely marginal because resident schedules are balanced between outpatient and inpatient rotations during each 6-month period.
Finally, this project focused on buprenorphine, not on other medications for OUD, including methadone or naltrexone, or nonpharmacologic treatments for OUD.
Sustainability and Next Steps
Since the start of the BBT in January 2019, five additional PGY-2 residents obtained their medical licenses and X-waivers. These residents, with the support of two attending hospitalists, led the BBT and coordinated education sessions that were incorporated into the curriculum during the 2019-2020 academic year. These educational sessions will continue indefinitely. In 2020, JHH started an Addiction Medicine Consult Service staffed by physicians, NPs, and a pharmacist. The BBT continues to operate in conjunction with this service.
We found substantial variability in the rate of buprenorphine maintenance initiation despite our interventions. This is an area for future improvement. In a free-response prompt in our follow-up survey, residents requested additional education sessions and an order set to assist with initiation of buprenorphine. To address these gaps, three educational sessions were added, one of which included education on starting methadone maintenance therapy. We also added a new order set for starting buprenorphine maintenance. We hypothesize that these interventions will improve consistency.
In order for a similar program to be disseminated to other institutions, educational initiatives and a team of dedicated X-waivered prescribers are key. Materials to assist with this process are available in the Appendix.
CONCLUSION
This study shows how a resident-led intervention comprising clinician education and a team of X-waivered generalists was associated with improved treatment of OUD for hospitalized patients. We encourage residents and all clinicians at other hospitals without addiction medicine consult services to design, implement, and study similar interventions that directly increase the use of buprenorphine or methadone maintenance to treat OUD.
Preliminary results from this project were presented at the AMERSA National Conference on November 7, 2019.
Nearly 48,000 Americans died from overdoses involving opioids in 2018, continuing a national crisis that has led to 446,000 deaths since 1999.1 Annually, opioids are responsible for more than 500,000 admissions, approximately 1% of all hospitalizations, costing the United States nearly $15 billion.2,3 Among hospitalized patients, chronic opioid use is associated with increased mortality, severe infectious complications, and higher rates of readmission.4 Opioid use disorder (OUD) is a chronic, relapsing medical condition with biopsychosocial origins and significant morbidity and mortality.5 Opioid agonist therapy (OAT) with buprenorphine or methadone maintenance, the evidence-based standard of treatment, reduces the mortality rate by half, decreases overdoses and hospital readmissions, and improves retention in care.6-10
OAT maintenance refers to using buprenorphine or methadone for long-term treatment of OUD rather than for acute treatment of opioid withdrawal. Despite evidence supporting OAT maintenance, clinicians start medications for only 11% to 15% of hospitalized patients with OUD, depending on practice contexts.11,12 Three significant barriers—stigma, insufficient clinician education, and restrictive regulations—prevent clinicians from starting OAT.13 Clinicians who do not have the Drug Enforcement Administration (DEA)–issued DATA-2000 waiver (X-waiver) for outpatient prescribing can order buprenorphine for admitted patients but cannot prescribe it at discharge.14 In hospitals where they exist, addiction medicine consult services offer primary teams guidance on pharmacotherapy, leading to reduced hospital readmissions and increased engagement in outpatient addiction treatment.15-17 However, in most hospitals around the country, such specialty services do not exist.18 In some hospitals without addiction medicine consult services, hospitalists with expertise in OUD have started assisting primary teams in starting OAT, but to our knowledge, no prior studies have described the impact of these interventions on patients or clinician experience with OAT.19
This quality improvement project aimed to increase the rate at which internal medicine resident teams at Johns Hopkins Hospital (JHH) in Baltimore, Maryland, started hospitalized patients with OUD on buprenorphine maintenance. We hypothesized that resident education and measures to increase the availability of X-waivered physicians would increase the rate of initiating buprenorphine maintenance. We additionally hypothesized that these interventions would increase knowledge about and comfort with buprenorphine across the residency. This represents the first study to examine the effects of clinician education and a team of X-waivered residents and hospitalists who assist in starting buprenorphine maintenance in a hospital without an addiction medicine consult service.
METHODS
Setting
This study took place from July 2018 to June 2019 at JHH, a large, academic, urban hospital in Baltimore. Prior to the intervention, internal medicine residents at JHH commonly used short courses of buprenorphine to treat withdrawal, but they did not have access to hospital-specific resources to assist with starting maintenance OAT. During the study period, JHH had a Substance Use Disorders team staffed by peer recovery specialists that could be consulted by hospitalists and residents to provide psychosocial support and link admitted patients to treatment after discharge. There were no providers on the team to guide pharmacotherapy or to write discharge buprenorphine prescriptions. The Osler Medical Residency Training Program at JHH has 140 internal medicine residents and 16 combined medicine-pediatrics residents. All residents receive 1 hour of formal education about opioid use disorder annually. In addition, 28 of those 156 residents, those in the Urban Health Primary Care track, spend 1 month on an Addiction Medicine rotation in which they complete the 8-hour training required to receive the X-waiver. Those residents are encouraged to apply for the X-waiver once they obtain a medical license subsidized by a Health Resources & Services Administration (HRSA) grant. Four internal medicine attending physicians on teaching services and one resident had X-waivers prior to the intervention.
Intervention
In November 2018, we administered a survey to residents to identify barriers to starting buprenorphine maintenance and to measure knowledge and confidence with using buprenorphine for OUD (Appendix Figure 1 and Figure 2). We focused on buprenorphine because providers at JHH were familiar with this medication and because Baltimore has widespread access to buprenorphine, with more than 490 local buprenorphine providers.20 Five residents piloted the survey and provided feedback. We then administered the survey to all internal medicine and medicine-pediatrics residents. Based on the results, we developed a targeted educational conference and also created the Buprenorphine Bridge Team (BBT).
In January 2019, we presented the educational conference for residents devoted to the use of buprenorphine for OUD and introduced the BBT. The conference started with a patient testimonial and included peer recovery specialists, pharmacists, nurses, and social workers. We summarized the evidence for buprenorphine and offered a practical guide to start treatment in a one-page protocol. This protocol included guidance on selecting patients, shared decision-making around OUD treatment, avoiding precipitated withdrawal, dosing buprenorphine, and establishing follow-up (Appendix Figure 3). We asked for input on this protocol from nursing leadership, social work teams, and peer recovery specialists. Dosing was adapted from the Guidelines from the American Society of Addiction Medicine, with expert input from physicians from the Addiction Medicine Consult service at Johns Hopkins Bayview Medical Center, also in Baltimore.5 We instructed residents to obtain discharge buprenorphine prescriptions from an X-waivered physician on their team or from the newly established BBT. We asked resident teams to set up a postdischarge appointment for patients with an X-waivered provider, either in a community practice or at the JHH After Care Clinic, a transitional care clinic for discharged patients.21
The BBT is a resident-led group of X-waivered JHH residents and hospitalists who volunteer to write discharge buprenorphine prescriptions for patients. The BBT serves to ensure primary teams have access to an X-waivered prescriber. It is not a consult service. We asked primary teams to contact the BBT after initiating buprenorphine and after securing a follow-up appointment. In response to each request, a member of the BBT reviews the patient chart, confirms the follow-up plan, writes a prescription for buprenorphine along with intranasal naloxone, and leaves a brief note. During the 6-month postintervention period, the team consisted of three residents and three hospitalist attendings. Each week, two members (residents or attendings) staffed the team Monday to Friday, 8
In May 2019, 5 months after the education session and implementation of the BBT, we administered a follow-up survey.
Outcomes
As a secondary outcome, we measured engagement in OUD treatment after discharge by calculating the proportion of patients started on buprenorphine who filled a buprenorphine prescription within 30 days after discharge. We chose 30 days based on the National Committee for Quality Assurance’s Healthcare Effectiveness Data and Information Set (HEDIS) measure for engagement of treatment for alcohol and other drugs.23 We obtained the data from the Chesapeake Regional Information System for our Patients (CRISP) Prescription Drug Monitoring Program, which monitors all prescriptions for controlled substances dispensed in Maryland and five neighboring states. As a balancing measure, we counted patients newly started on methadone maintenance for OUD before and after the intervention. Additional secondary process outcomes included frequency of BBT requests, the volume of buprenorphine prescriptions written by the team, and time required to complete a BBT request.
Clinician-level outcomes, measured with electronically administered pre- and postintervention surveys to residents, included knowledge about and comfort with buprenorphine. Of the 16 questions in the pre- and postimplementation surveys, we analyzed the 6 questions concerning knowledge and comfort that remained identical in the pre- and postintervention surveys and used 5-point Likert scale responses. As an incentive, we randomly distributed three $50 gift cards to survey completers.
Analysis
We used an interrupted time series analysis to evaluate the association between the intervention bundle and a change in the rate that medical teams started patients with OUD on buprenorphine maintenance. This approach allowed us to control for preintervention trends. To evaluate the impact of our interventions, our pre- and postintervention periods include the same residents during the 2018-2019 academic year. Both periods consisted of twelve 2-week intervals (preintervention: July 26, 2019, to January 9, 2019; postintervention: January 10, 2019, to June 26, 2019).
To evaluate for changes in engagement in OUD treatment after discharge, we used two-sample t tests. To evaluate for changes in resident-reported comfort and knowledge with initiating buprenorphine maintenance, we used Wilcoxon rank sum tests for survey data and Wilcoxon signed rank tests for paired data. All analyses employed two-sided P values with statistical significance evaluated at the .05 alpha level. We analyzed data using R version 3.6.3 (Foundation for Statistical Computing). The Institutional Review Board at JHH reviewed and approved the study protocol as a quality improvement project (IRB00193365).

Before the intervention, 13 of the 30 patients (40%) newly started on buprenorphine maintenance during their admission filled a follow-up buprenorphine prescription within 30 days of discharge. After the intervention, 31 of 64 patients (46%) filled a buprenorphine prescription within 30 days (P = .612). Two patients were started on methadone maintenance, one prior to and one after the intervention.
During the 6-month postintervention period, the BBT received 75 requests and wrote 70 prescriptions for buprenorphine. The median time required to complete a BBT request was 15 minutes (minimum, 5 minutes; maximum, 60 minutes).
Of 156 internal medicine and medicine-pediatrics residents, 89 residents (57%) completed the baseline survey and 66 residents (42%) completed the follow-up survey. Forty residents completed both surveys. After the intervention, residents were significantly more likely to feel comfortable dosing buprenorphine (P < .0001) and counseling patients about its use (P = .0237) and were more likely to report ease of establishing follow-up (P < .0001). Self-reported knowledge about preventing precipitated withdrawal increased significantly (P = .0191), as did knowledge about the effectiveness of buprenorphine (P = .0003) independent of formal drug counseling (P = .0066) (Table). Paired survey data also found statistically significant results for all questions except those about preventing precipitated withdrawal and efficacy. For the latter, respondents who completed both surveys were more knowledgeable before the intervention than the overall group that completed the baseline survey (Appendix Table).

This study shows how a resident-led quality improvement project comprising clinician education and implementation of a novel BBT was associated with an increased rate of starting buprenorphine maintenance in hospitalized patients with OUD and improved resident knowledge about and comfort with buprenorphine. To our knowledge, this is the first study demonstrating how education and a team of X-waivered generalists can help primary teams initiate and discharge patients on buprenorphine maintenance in a hospital without an addiction medicine consult service.
Prior to the intervention, resident internal medicine teams at JHH started 10% of hospitalized patients with OUD on buprenorphine maintenance, consistent with prior studies showing rates of 11% to 15% for initiating OAT for hospitalized patients.11,12 After the intervention, the rate of initiating buprenorphine maintenance more than doubled, rising to 24% of eligible patients. Resident internal medicine teams at JHH started buprenorphine maintenance for 37 more patients over the 24-week postintervention period than would have been predicted prior to the intervention, or an additional three patients every 2 weeks.
Between 40% and 46% of hospitalized patients newly started on buprenorphine maintenance filled an outpatient buprenorphine prescription within 30 days of discharge. We are not aware of comparative data for 30-day follow-up for hospitalized patients newly started on buprenorphine maintenance. Data from other contexts show 5% to 10% of veterans were engaged in addiction treatment 30 days after initiation from inpatient or outpatient encounters. An analysis of an academic medical center in Oregon found engagement with an addiction medicine consult service increased after hospital engagement for patients with any substance use disorder from 23% to 39% using the 34-day HEDIS measure for engagement.17,24,25
The BBT required approximately 15 minutes per request and wrote an average of three prescriptions per week, demonstrating the feasibility of this approach and the high demand for this service. One strength of our approach is that residents gained experience starting buprenorphine independently using the aforementioned protocol instead of deferring to a full consult service. It is likely that this resident engagement in initiating longitudinal OUD care contributed to the success of this initiative, as did existing resident familiarity with using buprenorphine for opioid withdrawal.
This approach to resident education—promoting direct, first-person experience with medications in a clinical context—aligns with recommendations from a recent review about substance use disorder education for health professionals.26 Our interventions increased resident knowledge and comfort with buprenorphine, consistent with prior studies showing increased resident confidence in management of substance use disorders after curricular innovations.24,25
A few contextual features were essential for this project’s viability. Maryland allows American medical graduates to obtain a medical license after 1 year of postgraduate training. This allowed three residents to obtain X-waivers. These residents had access to HRSA funding to subsidize the expenses of applying for state licensure and DEA registration. BBT members volunteered their time while working on other services. Last, we were able to take advantage of buprenorphine-providing clinics in Baltimore, including the JHH After Care Clinic, to accept patients for follow-up appointments after discharge.
Limitations
The BBT required motivated clinicians willing to volunteer for additional clinical responsibilities during inpatient rotations and supportive faculty and residency leadership. Attending physicians, nurse practitioners, or physician assistants could staff a similar BBT in hospitals without residents or in hospitals where residents cannot obtain DEA registration. Crucially, other hospitals may not have access to practices with X-waivered physicians for outpatient follow-up. A recent study found X-waivered primary care physicians were less likely to be affiliated with hospital health systems. Other studies have shown limitations in access to buprenorphine at the county level based on geography and racial/ethnic segregation.27-29
Most patients hospitalized with OUD did not have ICD-10 codes associated with OUD. We addressed this by assuming patients had OUD if buprenorphine or methadone was ordered during their hospitalization, even if the medication was never administered. This may have overcounted patients prescribed these medications for indications other than OUD, and it may have undercounted patients with OUD for whom buprenorphine or methadone were never considered. The opioid withdrawal order set at JHH automatically offers an option to use buprenorphine to treat withdrawal. Patients with OUD for whom buprenorphine or methadone were never ordered likely did not experience withdrawal or were in withdrawal so mild that it escaped the attention of the team, which limits the generalizability of our intervention.
We identified several limitations to the internal validity of our study. First, we used a before-and-after study design without a control group. We could not ethically withhold access to evidence-based, mortality-reducing medications from patients. Without a control group, we cannot rule out the possibility that underlying temporal trends made residents more likely to start buprenorphine maintenance independent of our intervention. We attempted to control for unmeasured confounders by using an interrupted time series analysis to control for preintervention trends, comparing the same group of residents before and after our interventions, and selecting an intervention period during which residents were given only educational sessions and materials provided by our team. Our results may be biased by clustered data because certain residents may have been more likely to initiate buprenorphine, but these effects are likely marginal because resident schedules are balanced between outpatient and inpatient rotations during each 6-month period.
Finally, this project focused on buprenorphine, not on other medications for OUD, including methadone or naltrexone, or nonpharmacologic treatments for OUD.
Sustainability and Next Steps
Since the start of the BBT in January 2019, five additional PGY-2 residents obtained their medical licenses and X-waivers. These residents, with the support of two attending hospitalists, led the BBT and coordinated education sessions that were incorporated into the curriculum during the 2019-2020 academic year. These educational sessions will continue indefinitely. In 2020, JHH started an Addiction Medicine Consult Service staffed by physicians, NPs, and a pharmacist. The BBT continues to operate in conjunction with this service.
We found substantial variability in the rate of buprenorphine maintenance initiation despite our interventions. This is an area for future improvement. In a free-response prompt in our follow-up survey, residents requested additional education sessions and an order set to assist with initiation of buprenorphine. To address these gaps, three educational sessions were added, one of which included education on starting methadone maintenance therapy. We also added a new order set for starting buprenorphine maintenance. We hypothesize that these interventions will improve consistency.
In order for a similar program to be disseminated to other institutions, educational initiatives and a team of dedicated X-waivered prescribers are key. Materials to assist with this process are available in the Appendix.
CONCLUSION
This study shows how a resident-led intervention comprising clinician education and a team of X-waivered generalists was associated with improved treatment of OUD for hospitalized patients. We encourage residents and all clinicians at other hospitals without addiction medicine consult services to design, implement, and study similar interventions that directly increase the use of buprenorphine or methadone maintenance to treat OUD.
Preliminary results from this project were presented at the AMERSA National Conference on November 7, 2019.
1. Wilson N, Kariisa M, Seth P, Iv HS, Davis NL. Drug and opioid-involved overdose deaths – United States, 2017–2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. http://dx.doi.org/10.15585/mmwr.mm6911a4
2. Berk J, Rogers KM, Wilson DJ, Thakrar A, Feldman L. Missed opportunities for treatment of opioid use disorder in the hospital setting: updating an outdated policy. J Hosp Med. 2020;15(10):619-621. https://doi.org/10.12788/jhm.3352
3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002–12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424
4. Mosher HJ, Jiang L, Vaughan Sarrazin MS, Cram P, Kaboli PJ, Vander Weg MW. Prevalence and characteristics of hospitalized adults on chronic opioid therapy. J Hosp Med. 2014;9(2):82-87. https://doi.org/10.1002/jhm.2113
5. Crotty K, Freedman KI, Kampman KM. Executive summary of the focused update of the ASAM national practice guideline for the treatment of opioid use disorder. J Addict Med. 2020;14(2):99-112. https://doi.org/10.1097/adm.0000000000000635
6. Leshner AI, Mancher M, eds. Medications for Opioid Use Disorder Save Lives. The National Academies Press; 2019. https://www.nap.edu/catalog/25310
7. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357: j1550. https://doi.org/10.1136/bmj.j1550
8. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality. Ann Intern Med. 2018;169(3):137-145. https://dx.doi.org/10.7326%2FM17-3107
9. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368. https://doi.org/10.1056/nejmra1604339
10. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
11. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
12. Priest KC, Lovejoy TI, Englander H, Shull S, McCarty D. Opioid agonist therapy during hospitalization within the Veterans Health Administration: a pragmatic retrospective cohort analysis. J Gen Intern Med. 2020;35(8):2365-2374. https://doi.org/10.1007/s11606-020-05815-0
13. Madras BK, Ahmad NJ, Wen J, Sharfstein J; Prevention, Treatment, and Recovery Working Group of the Action Collaborative on Countering the U.S. Opioid Epidemic. Improving access to evidence-based medical treatment for opioid use disorder: strategies to address key barriers within the treatment system. NAM Perspectives. April 27, 2020. https://doi.org/10.31478/202004b
14. Fiscella K, Wakeman SE, Beletsky L. Buprenorphine deregulation and mainstreaming treatment for opioid use disorder: x the X Waiver. JAMA Psychiatry. 2019;76(3):229-230. https://doi.org/10.1001/jamapsychiatry.2018.3685
15. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/adm.0000000000000496
16. Weimer M, Morford K, Donroe J. Treatment of opioid use disorder in the acute hospital setting: a critical review of the literature (2014–2019). Curr Addict Rep. 2019;6(4):339-354.
17. Englander H, Dobbertin K, Lind BK, et al. Inpatient addiction medicine consultation and post-hospital substance use disorder treatment engagement: a propensity-matched analysis. J Gen Intern Med. 2019;34(12):2796-2803. https://doi.org/10.1007/s11606-019-05251-9
18. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2019;14(3):E1-E4. https://doi.org/10.12788/jhm.3311
19. Bottner R, Moriates C, Tirado C. The role of hospitalists in treating opioid use disorder. J Addict Med. 2020;14(2):178. https://doi.org/10.1097/adm.0000000000000545
20. Behavioral health treatment services locator. Substance Abuse and Mental Health Services Administration. Accessed May 14, 2020. https://findtreatment.samhsa.gov/
21. Groesbeck K, Whiteman LN, Stewart RW. Reducing readmission rates by improving transitional care. South Med J. 2015;108(12):758-760. https://doi.org/10.14423/smj.0000000000000376
22. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015 Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
23. Initiation and engagement of alcohol and other drug abuse or dependence treatment (IET). NCQA. Accessed April 20, 2020. https://www.ncqa.org/hedis/measures/initiation-and-engagement-of-alcohol-and-other-drug-abuse-or-dependence-treatment/
24. Wyse JJ, Robbins JL, McGinnis KA, et al. Predictors of timely opioid agonist treatment initiation among veterans with and without HIV. Drug Alcohol Depend. 2019;198:70-75. https://doi.org/10.1016/j.drugalcdep.2019.01.038
25. Harris AHS, Humphreys K, Finney JW. Veterans Affairs facility performance on Washington Circle indicators and casemix-adjusted effectiveness. J Subst Abuse Treat. 2007;33(4):333-339. https://doi.org/10.1016/j.jsat.2006.12.015
26. Muzyk A, Smothers ZPW, Andolsek KM, et al. Interprofessional substance use disorder education in health professions education programs: a scoping review. Acad Med. 2020;95(3):470-480. https://doi.org/10.1097/acm.0000000000003053
27. Saloner B, Lin L, Simon K. Geographic location of buprenorphine-waivered physicians and integration with health systems. J Subst Abuse Treat. 2020;115:108034. https://doi.org/10.1016/j.jsat.2020.108034
28. Jones CW, Christman Z, Smith CM, et al. Comparison between buprenorphine provider availability and opioid deaths among US counties. J Subst Abuse Treat. 2018;93:19-25. https://doi.org/10.1016/j.jsat.2018.07.008
29. Goedel WC, Shapiro A, Cerdá M, Tsai JW, Hadland SE, Marshall BDL. Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA Netw Open. 2020;3(4):e203711. https://doi.org/10.1001/jamanetworkopen.2020.3711
1. Wilson N, Kariisa M, Seth P, Iv HS, Davis NL. Drug and opioid-involved overdose deaths – United States, 2017–2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. http://dx.doi.org/10.15585/mmwr.mm6911a4
2. Berk J, Rogers KM, Wilson DJ, Thakrar A, Feldman L. Missed opportunities for treatment of opioid use disorder in the hospital setting: updating an outdated policy. J Hosp Med. 2020;15(10):619-621. https://doi.org/10.12788/jhm.3352
3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002–12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424
4. Mosher HJ, Jiang L, Vaughan Sarrazin MS, Cram P, Kaboli PJ, Vander Weg MW. Prevalence and characteristics of hospitalized adults on chronic opioid therapy. J Hosp Med. 2014;9(2):82-87. https://doi.org/10.1002/jhm.2113
5. Crotty K, Freedman KI, Kampman KM. Executive summary of the focused update of the ASAM national practice guideline for the treatment of opioid use disorder. J Addict Med. 2020;14(2):99-112. https://doi.org/10.1097/adm.0000000000000635
6. Leshner AI, Mancher M, eds. Medications for Opioid Use Disorder Save Lives. The National Academies Press; 2019. https://www.nap.edu/catalog/25310
7. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357: j1550. https://doi.org/10.1136/bmj.j1550
8. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality. Ann Intern Med. 2018;169(3):137-145. https://dx.doi.org/10.7326%2FM17-3107
9. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368. https://doi.org/10.1056/nejmra1604339
10. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
11. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
12. Priest KC, Lovejoy TI, Englander H, Shull S, McCarty D. Opioid agonist therapy during hospitalization within the Veterans Health Administration: a pragmatic retrospective cohort analysis. J Gen Intern Med. 2020;35(8):2365-2374. https://doi.org/10.1007/s11606-020-05815-0
13. Madras BK, Ahmad NJ, Wen J, Sharfstein J; Prevention, Treatment, and Recovery Working Group of the Action Collaborative on Countering the U.S. Opioid Epidemic. Improving access to evidence-based medical treatment for opioid use disorder: strategies to address key barriers within the treatment system. NAM Perspectives. April 27, 2020. https://doi.org/10.31478/202004b
14. Fiscella K, Wakeman SE, Beletsky L. Buprenorphine deregulation and mainstreaming treatment for opioid use disorder: x the X Waiver. JAMA Psychiatry. 2019;76(3):229-230. https://doi.org/10.1001/jamapsychiatry.2018.3685
15. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/adm.0000000000000496
16. Weimer M, Morford K, Donroe J. Treatment of opioid use disorder in the acute hospital setting: a critical review of the literature (2014–2019). Curr Addict Rep. 2019;6(4):339-354.
17. Englander H, Dobbertin K, Lind BK, et al. Inpatient addiction medicine consultation and post-hospital substance use disorder treatment engagement: a propensity-matched analysis. J Gen Intern Med. 2019;34(12):2796-2803. https://doi.org/10.1007/s11606-019-05251-9
18. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2019;14(3):E1-E4. https://doi.org/10.12788/jhm.3311
19. Bottner R, Moriates C, Tirado C. The role of hospitalists in treating opioid use disorder. J Addict Med. 2020;14(2):178. https://doi.org/10.1097/adm.0000000000000545
20. Behavioral health treatment services locator. Substance Abuse and Mental Health Services Administration. Accessed May 14, 2020. https://findtreatment.samhsa.gov/
21. Groesbeck K, Whiteman LN, Stewart RW. Reducing readmission rates by improving transitional care. South Med J. 2015;108(12):758-760. https://doi.org/10.14423/smj.0000000000000376
22. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015 Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
23. Initiation and engagement of alcohol and other drug abuse or dependence treatment (IET). NCQA. Accessed April 20, 2020. https://www.ncqa.org/hedis/measures/initiation-and-engagement-of-alcohol-and-other-drug-abuse-or-dependence-treatment/
24. Wyse JJ, Robbins JL, McGinnis KA, et al. Predictors of timely opioid agonist treatment initiation among veterans with and without HIV. Drug Alcohol Depend. 2019;198:70-75. https://doi.org/10.1016/j.drugalcdep.2019.01.038
25. Harris AHS, Humphreys K, Finney JW. Veterans Affairs facility performance on Washington Circle indicators and casemix-adjusted effectiveness. J Subst Abuse Treat. 2007;33(4):333-339. https://doi.org/10.1016/j.jsat.2006.12.015
26. Muzyk A, Smothers ZPW, Andolsek KM, et al. Interprofessional substance use disorder education in health professions education programs: a scoping review. Acad Med. 2020;95(3):470-480. https://doi.org/10.1097/acm.0000000000003053
27. Saloner B, Lin L, Simon K. Geographic location of buprenorphine-waivered physicians and integration with health systems. J Subst Abuse Treat. 2020;115:108034. https://doi.org/10.1016/j.jsat.2020.108034
28. Jones CW, Christman Z, Smith CM, et al. Comparison between buprenorphine provider availability and opioid deaths among US counties. J Subst Abuse Treat. 2018;93:19-25. https://doi.org/10.1016/j.jsat.2018.07.008
29. Goedel WC, Shapiro A, Cerdá M, Tsai JW, Hadland SE, Marshall BDL. Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA Netw Open. 2020;3(4):e203711. https://doi.org/10.1001/jamanetworkopen.2020.3711
© 2021 Society of Hospital Medicine
Hospital Buprenorphine Program for Opioid Use Disorder Is Associated With Increased Inpatient and Outpatient Addiction Treatment
Hospitalizations related to opioid use disorder (OUD) have increased and now account for up to 6% of hospital admissions in certain areas of the United States.1 Patients with OUD who are started on buprenorphine during hospitalization are more likely to enter outpatient treatment, stay in treatment longer, and have more drug-free days compared with patients who only receive a referral for outpatient treatment.2,3 Therefore, a crucial comprehensive strategy for OUD care should include hospital-based programs that support initiation of treatment in the inpatient setting and strong bridges to outpatient care. One of the common barriers to initiating treatment in the inpatient setting, however, is a lack of access to addiction medicine specialists.4-6
In 2017, we created a hospitalist-led interprofessional team called the B-Team (Buprenorphine Team) to help primary care teams identify patients with OUD, initiate and maintain buprenorphine therapy during hospitalization, provide warm handoffs to outpatient treatment programs, and reduce institutional stigma related to people with substance use disorders.
METHODS
Program Description
The B-Team is led by a hospital medicine physician assistant and includes physicians from internal medicine, consult-liaison psychiatry, and palliative care; advanced practice and bedside nurses; a social worker; a pharmacist; a chaplain; a peer-recovery specialist; and medical trainees. The B-Team is notified of potential candidates for buprenorphine through a secure texting platform, one that is accessible to any healthcare provider at the hospital. Patients who are referred to the B-Team either self-identify or are identified by their primary team as having an underlying OUD. One of the B-Team providers assesses the patient to determine if they are eligible to receive inpatient therapy. Patients are considered eligible for the program if they meet Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria for OUD, have a desire to cease opioid use, and receive medical clearance to take buprenorphine.
For eligible patients, the B-Team provider orders a nurse-driven protocol to initiate buprenorphine for OUD. The chaplain offers psychospiritual counseling, and the social worker provides counseling and coordination of care. The B-Team partners with a nonhospital-affiliated, publicly-funded, office-based opioid treatment (OBOT) program that combines primary care with behavioral health programming. A follow-up outpatient appointment is secured prior to hospital discharge, and a member of the B-Team who has Drug Addiction Treatment Act of 2000 (DATA 2000) X-waiver certification prescribes buprenorphine as a bridge until the follow-up appointment. The medication is dispensed from the hospital’s retail pharmacy, and the patient leaves the hospital with the medication in-hand.
Patients who are not eligible for buprenorphine therapy are offered a harm-reduction intervention or referral to the psychiatry consult liaison service to assess for alternative diagnoses or treatment. These patients are also offered psychospiritual counseling and a prescription for naloxone.
Prior to the creation of the B-Team at our hospital, there was no structure in place to facilitate initiation of buprenorphine therapy during hospitalization and no linkage to outpatient treatment after discharge; furthermore, none of the hospitalists or other providers (including consulting psychiatrists) had an X-waiver to prescribe buprenorphine for OUD.
Program Evaluation
Study data were collected using Research Electronic Data Capture software. Inpatient and outpatient data were entered by a B-Team provider or a researcher via chart review. Patients were considered to be engaged in care if they attended at least one outpatient appointment for buprenorphine therapy during each of the following time periods: (1) 0 to 27 days (initial follow-up), 28-89 days (1- to 3-month follow-up), 90-179 days (3- to 6-month follow-up), and 180 days or more (>6-month follow-up). Only visits specifically for buprenorphine maintenance therapy were counted. If multiple encounters occurred within one time frame, the encounter closest to 0, 30, 90, or 180 days from discharge was used. If a patient did not attend any encounters during a specified time frame, they were considered to no longer be engaged in care and were no longer tracked for purposes of the evaluation. Data for the percentage of patients engaged in outpatient care are presented as the number of patients who attended at least one appointment during each of the follow-up periods (1 to 3 months, 3 to 6 months, or after 6 months, as noted above) divided by the number of patients who had been discharged with coordinated follow-up.
The number of patients admitted per month for whom there was an order to initiate inpatient buprenorphine therapy was analyzed using a statistical process control chart,
This program and study were considered quality improvement by The University of Texas Institutional Review Board and did not meet criteria for human subjects research.
RESULTS
During the first 2 years of the program (September 2018-September 2020), the B-Team received 260 patient referrals. Most of the patients were White (72%), male (62%), and between ages 25 and 44 years (53%) (Appendix Table). The team initiated buprenorphine therapy in 132 hospitalized patients. In the year prior to the creation of the B-Team program, the average number of hospitalized patients receiving buprenorphine for OUD per month was three; after the launch of the B-Team program, this number increased
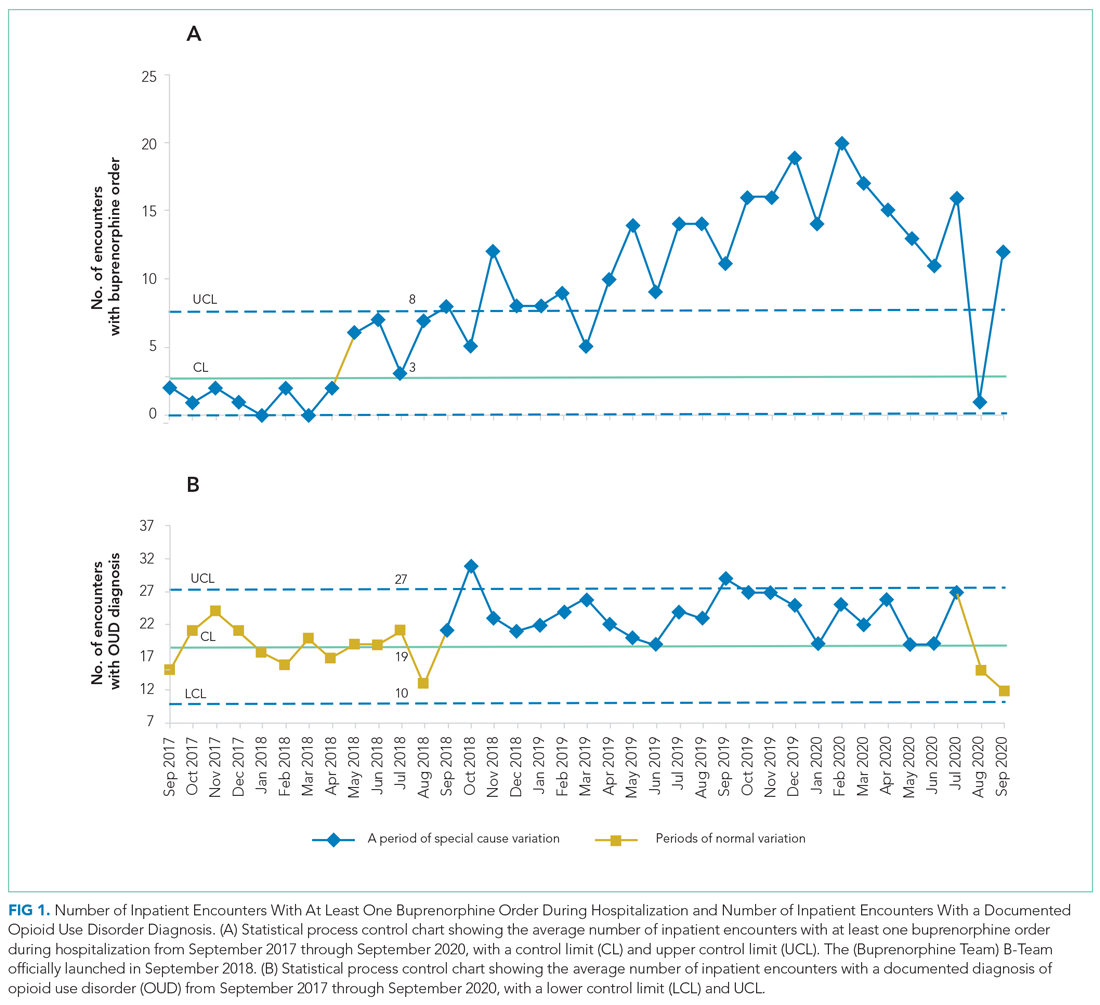
The B-Team saw a total of 132 eligible patients; members of the team provided counseling, support, and resources regarding buprenorphine therapy. In addition, the B-Team’s chaplain provided emotional support and spiritual connection (if desired) to 40 of these patients (30%). In the study, no cases of precipitated withdrawal were identified. Of the 132 patients seen, 110 (83%) were accepted to an outpatient OUD program upon discharge from the hospital; 98 (89%) of these patients were accepted at our partner OBOT clinic. The remaining patients were not interested in continuing OUD treatment (13%) or were denied acceptance to an outpatient program based on administrative and/or financial eligibility guidelines (4%). Patients who would not be attending an outpatient program were discontinued on buprenorphine therapy prior to discharge, counseled about naloxone, and provided printed resources.
Outpatient appointment attendance was used to measure ongoing treatment engagement of the 110 patients who were discharged with coordinated follow-up care. A total of 65 patients (59%) attended their first outpatient appointment; the average time between discharge and the first outpatient appointment was 5.9 days. Forty-two patients (38%) attended at least one appointment between 1 and 3 months; 29 (26%) between 3 and 6 months; and 24 (22%) after 6 months (Figure 2).
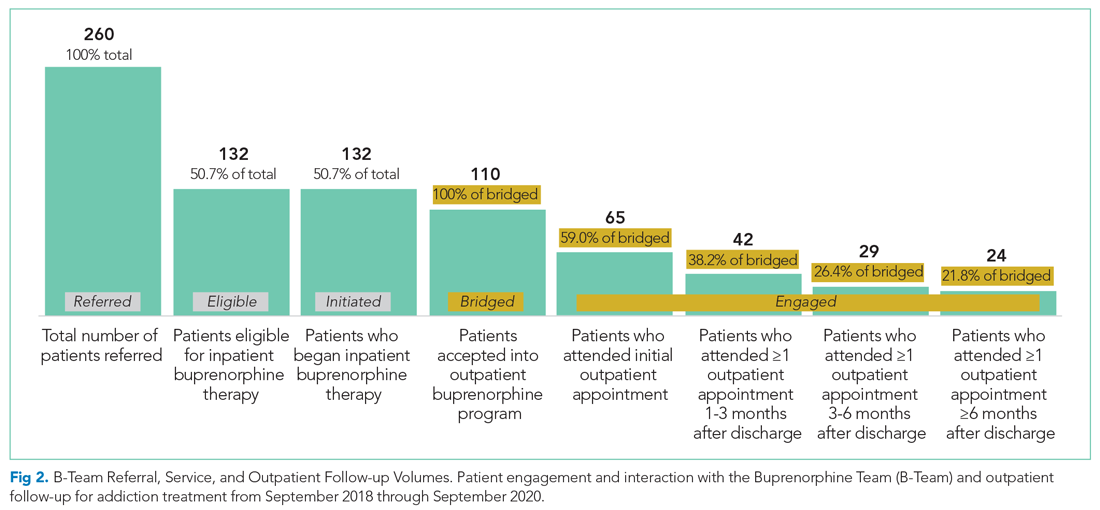
Of the 128 patients who were not administered buprenorphine therapy, 64 (50%) were not interested in starting treatment and/or were not ready to engage in treatment; 36 (28%) did not meet criteria for OUD treatment; 28 (22%) were already receiving treatment or preferred another type of OUD treatment; and 13 (10%) had severe comorbid addiction and/or illness requiring treatment that contraindicates the use of buprenorphine.
DISCUSSION
A volunteer hospitalist-led interprofessional team providing evidence-based care for hospitalized patients with OUD was associated with a substantial increase in patients receiving buprenorphine therapy—both during hospitalization and after discharge. In the program, 59% of patients attended initial follow-up appointments, and 22% of patients were still engaged at 6 months. These outpatient follow-up rates appear to be similar to, or higher than, other programs described in the literature. For example, a buprenorphine OUD-treatment initiative led by the psychiatry consult service at a Boston academic medical center resulted in less than half of patients receiving buprenorphine treatment within 2 months of discharge.7 In another study wherein an addiction medicine consult service administered buprenorphine to patients with OUD during hospitalization, 39%, 27%, and 18% of patients were retained in outpatient treatment at 30, 90, and 180 days, respectively.8
The B-Team model is likely generalizable to other hospital medicine groups that may not otherwise have access to inpatient care for substance use disorder. The B-Team is not an addiction medicine consultation service; rather, it is a hospitalist-led quality improvement initiative seeking to improve the standard of care for hospitalized patients with OUD.
A significant barrier is ensuring ongoing support for patients with OUD after discharge. In the B-Team program, a parallel OBOT program was created by a local nonaffiliated federally qualified health center. Although 89% of patients received treatment at this OBOT clinic, the inpatient team also has relationships with other local treatment centers, including programs that provide methadone. Another important barrier to high-quality outpatient care for OUD is the requirement of an X-waiver. To help overcome this barrier, our inpatient program partnered with a regional medical society to offer periodic X-waiver training to outpatient providers. In less than a year, more than 100 regional prescribers participated in this program.
Our study has several limitations. There was likely some degree of selection bias among the hospitalized patients who received initial buprenorphine treatment. To our knowledge, there is no specific validated screening tool for OUD in the inpatient acute care setting; moreover, we have been unable to implement standardized screening for OUD into the electronic health record. As such, we rely on the totality of the clinical circumstances approach to identify patients with OUD.
Furthermore, we had neither a comparison group nor a prospective plan to follow patients who did not remain engaged in care after discharge. In addition, our analysis of OUD admissions included F11 ICD-10 codes, which are limited by clinical documentation.9,10 Our program focuses exclusively on buprenorphine initiation due to insufficient immediate outpatient capacity for methadone initiated during hospitalization and lack of coverage for extended-release naltrexone. Limitations to outpatient data-sharing prevented the reporting of outpatient appointments external to the identified partner program; since these appointments were included in the analysis as “lost to follow-up,” actual engagement rates may be higher than those reported.
Moving forward, the B-Team is continuing to serve as a role model for appropriate, patient-centered, evidence-based care for hospitalized patients with OUD. Attending physicians and residents with an X-waiver are now encouraged to initiate buprenorphine treatment on their own. In June 2020, we added peer-recovery support services to the program, which has improved care for patients and increased adoption of hospital-initiated substance use disorder interventions.11 Lessons learned from inpatient implementation are being applied to our hospital’s emergency department and to an inpatient obstetrics unit at a partner hospital; they are also being employed to further empower hospitalists to diagnose and treat other substance use disorders, such as alcohol use disorder.
1. Owens PL, Weiss AJ, Barrett ML. Hospital Burden of Opioid-Related Inpatient Stays: Metropolitan and Rural Hospitals, 2016. HCUP Statistical Brief #258. Agency for Healthcare Research and Quality. May 2020. Accessed May 24, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559382/pdf/Bookshelf_NBK559382.pdf
2. Liebschutz J, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/jamainternmed.2014.2556
3. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
4. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736
5. Fanucchi L, Lofwall MR. Putting parity into practice — integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. https://doi.org/10.1056/nejmp1606157
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
7. Suzuki J, DeVido J, Kalra I, et al. Initiating buprenorphine treatment for hospitalized patients with opioid dependence: a case series. Am J Addict. 2015;24(1):10-14. https://doi.org/10.1111/ajad.12161
8. Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1-5. https://doi.org/10.1016/j.jsat.2017.05.007
9. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/adm.0000000000000454
10. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
11. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. 2019. https://doi.org/10.1007/s11606-019-05311-0
Hospitalizations related to opioid use disorder (OUD) have increased and now account for up to 6% of hospital admissions in certain areas of the United States.1 Patients with OUD who are started on buprenorphine during hospitalization are more likely to enter outpatient treatment, stay in treatment longer, and have more drug-free days compared with patients who only receive a referral for outpatient treatment.2,3 Therefore, a crucial comprehensive strategy for OUD care should include hospital-based programs that support initiation of treatment in the inpatient setting and strong bridges to outpatient care. One of the common barriers to initiating treatment in the inpatient setting, however, is a lack of access to addiction medicine specialists.4-6
In 2017, we created a hospitalist-led interprofessional team called the B-Team (Buprenorphine Team) to help primary care teams identify patients with OUD, initiate and maintain buprenorphine therapy during hospitalization, provide warm handoffs to outpatient treatment programs, and reduce institutional stigma related to people with substance use disorders.
METHODS
Program Description
The B-Team is led by a hospital medicine physician assistant and includes physicians from internal medicine, consult-liaison psychiatry, and palliative care; advanced practice and bedside nurses; a social worker; a pharmacist; a chaplain; a peer-recovery specialist; and medical trainees. The B-Team is notified of potential candidates for buprenorphine through a secure texting platform, one that is accessible to any healthcare provider at the hospital. Patients who are referred to the B-Team either self-identify or are identified by their primary team as having an underlying OUD. One of the B-Team providers assesses the patient to determine if they are eligible to receive inpatient therapy. Patients are considered eligible for the program if they meet Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria for OUD, have a desire to cease opioid use, and receive medical clearance to take buprenorphine.
For eligible patients, the B-Team provider orders a nurse-driven protocol to initiate buprenorphine for OUD. The chaplain offers psychospiritual counseling, and the social worker provides counseling and coordination of care. The B-Team partners with a nonhospital-affiliated, publicly-funded, office-based opioid treatment (OBOT) program that combines primary care with behavioral health programming. A follow-up outpatient appointment is secured prior to hospital discharge, and a member of the B-Team who has Drug Addiction Treatment Act of 2000 (DATA 2000) X-waiver certification prescribes buprenorphine as a bridge until the follow-up appointment. The medication is dispensed from the hospital’s retail pharmacy, and the patient leaves the hospital with the medication in-hand.
Patients who are not eligible for buprenorphine therapy are offered a harm-reduction intervention or referral to the psychiatry consult liaison service to assess for alternative diagnoses or treatment. These patients are also offered psychospiritual counseling and a prescription for naloxone.
Prior to the creation of the B-Team at our hospital, there was no structure in place to facilitate initiation of buprenorphine therapy during hospitalization and no linkage to outpatient treatment after discharge; furthermore, none of the hospitalists or other providers (including consulting psychiatrists) had an X-waiver to prescribe buprenorphine for OUD.
Program Evaluation
Study data were collected using Research Electronic Data Capture software. Inpatient and outpatient data were entered by a B-Team provider or a researcher via chart review. Patients were considered to be engaged in care if they attended at least one outpatient appointment for buprenorphine therapy during each of the following time periods: (1) 0 to 27 days (initial follow-up), 28-89 days (1- to 3-month follow-up), 90-179 days (3- to 6-month follow-up), and 180 days or more (>6-month follow-up). Only visits specifically for buprenorphine maintenance therapy were counted. If multiple encounters occurred within one time frame, the encounter closest to 0, 30, 90, or 180 days from discharge was used. If a patient did not attend any encounters during a specified time frame, they were considered to no longer be engaged in care and were no longer tracked for purposes of the evaluation. Data for the percentage of patients engaged in outpatient care are presented as the number of patients who attended at least one appointment during each of the follow-up periods (1 to 3 months, 3 to 6 months, or after 6 months, as noted above) divided by the number of patients who had been discharged with coordinated follow-up.
The number of patients admitted per month for whom there was an order to initiate inpatient buprenorphine therapy was analyzed using a statistical process control chart,
This program and study were considered quality improvement by The University of Texas Institutional Review Board and did not meet criteria for human subjects research.
RESULTS
During the first 2 years of the program (September 2018-September 2020), the B-Team received 260 patient referrals. Most of the patients were White (72%), male (62%), and between ages 25 and 44 years (53%) (Appendix Table). The team initiated buprenorphine therapy in 132 hospitalized patients. In the year prior to the creation of the B-Team program, the average number of hospitalized patients receiving buprenorphine for OUD per month was three; after the launch of the B-Team program, this number increased

The B-Team saw a total of 132 eligible patients; members of the team provided counseling, support, and resources regarding buprenorphine therapy. In addition, the B-Team’s chaplain provided emotional support and spiritual connection (if desired) to 40 of these patients (30%). In the study, no cases of precipitated withdrawal were identified. Of the 132 patients seen, 110 (83%) were accepted to an outpatient OUD program upon discharge from the hospital; 98 (89%) of these patients were accepted at our partner OBOT clinic. The remaining patients were not interested in continuing OUD treatment (13%) or were denied acceptance to an outpatient program based on administrative and/or financial eligibility guidelines (4%). Patients who would not be attending an outpatient program were discontinued on buprenorphine therapy prior to discharge, counseled about naloxone, and provided printed resources.
Outpatient appointment attendance was used to measure ongoing treatment engagement of the 110 patients who were discharged with coordinated follow-up care. A total of 65 patients (59%) attended their first outpatient appointment; the average time between discharge and the first outpatient appointment was 5.9 days. Forty-two patients (38%) attended at least one appointment between 1 and 3 months; 29 (26%) between 3 and 6 months; and 24 (22%) after 6 months (Figure 2).

Of the 128 patients who were not administered buprenorphine therapy, 64 (50%) were not interested in starting treatment and/or were not ready to engage in treatment; 36 (28%) did not meet criteria for OUD treatment; 28 (22%) were already receiving treatment or preferred another type of OUD treatment; and 13 (10%) had severe comorbid addiction and/or illness requiring treatment that contraindicates the use of buprenorphine.
DISCUSSION
A volunteer hospitalist-led interprofessional team providing evidence-based care for hospitalized patients with OUD was associated with a substantial increase in patients receiving buprenorphine therapy—both during hospitalization and after discharge. In the program, 59% of patients attended initial follow-up appointments, and 22% of patients were still engaged at 6 months. These outpatient follow-up rates appear to be similar to, or higher than, other programs described in the literature. For example, a buprenorphine OUD-treatment initiative led by the psychiatry consult service at a Boston academic medical center resulted in less than half of patients receiving buprenorphine treatment within 2 months of discharge.7 In another study wherein an addiction medicine consult service administered buprenorphine to patients with OUD during hospitalization, 39%, 27%, and 18% of patients were retained in outpatient treatment at 30, 90, and 180 days, respectively.8
The B-Team model is likely generalizable to other hospital medicine groups that may not otherwise have access to inpatient care for substance use disorder. The B-Team is not an addiction medicine consultation service; rather, it is a hospitalist-led quality improvement initiative seeking to improve the standard of care for hospitalized patients with OUD.
A significant barrier is ensuring ongoing support for patients with OUD after discharge. In the B-Team program, a parallel OBOT program was created by a local nonaffiliated federally qualified health center. Although 89% of patients received treatment at this OBOT clinic, the inpatient team also has relationships with other local treatment centers, including programs that provide methadone. Another important barrier to high-quality outpatient care for OUD is the requirement of an X-waiver. To help overcome this barrier, our inpatient program partnered with a regional medical society to offer periodic X-waiver training to outpatient providers. In less than a year, more than 100 regional prescribers participated in this program.
Our study has several limitations. There was likely some degree of selection bias among the hospitalized patients who received initial buprenorphine treatment. To our knowledge, there is no specific validated screening tool for OUD in the inpatient acute care setting; moreover, we have been unable to implement standardized screening for OUD into the electronic health record. As such, we rely on the totality of the clinical circumstances approach to identify patients with OUD.
Furthermore, we had neither a comparison group nor a prospective plan to follow patients who did not remain engaged in care after discharge. In addition, our analysis of OUD admissions included F11 ICD-10 codes, which are limited by clinical documentation.9,10 Our program focuses exclusively on buprenorphine initiation due to insufficient immediate outpatient capacity for methadone initiated during hospitalization and lack of coverage for extended-release naltrexone. Limitations to outpatient data-sharing prevented the reporting of outpatient appointments external to the identified partner program; since these appointments were included in the analysis as “lost to follow-up,” actual engagement rates may be higher than those reported.
Moving forward, the B-Team is continuing to serve as a role model for appropriate, patient-centered, evidence-based care for hospitalized patients with OUD. Attending physicians and residents with an X-waiver are now encouraged to initiate buprenorphine treatment on their own. In June 2020, we added peer-recovery support services to the program, which has improved care for patients and increased adoption of hospital-initiated substance use disorder interventions.11 Lessons learned from inpatient implementation are being applied to our hospital’s emergency department and to an inpatient obstetrics unit at a partner hospital; they are also being employed to further empower hospitalists to diagnose and treat other substance use disorders, such as alcohol use disorder.
Hospitalizations related to opioid use disorder (OUD) have increased and now account for up to 6% of hospital admissions in certain areas of the United States.1 Patients with OUD who are started on buprenorphine during hospitalization are more likely to enter outpatient treatment, stay in treatment longer, and have more drug-free days compared with patients who only receive a referral for outpatient treatment.2,3 Therefore, a crucial comprehensive strategy for OUD care should include hospital-based programs that support initiation of treatment in the inpatient setting and strong bridges to outpatient care. One of the common barriers to initiating treatment in the inpatient setting, however, is a lack of access to addiction medicine specialists.4-6
In 2017, we created a hospitalist-led interprofessional team called the B-Team (Buprenorphine Team) to help primary care teams identify patients with OUD, initiate and maintain buprenorphine therapy during hospitalization, provide warm handoffs to outpatient treatment programs, and reduce institutional stigma related to people with substance use disorders.
METHODS
Program Description
The B-Team is led by a hospital medicine physician assistant and includes physicians from internal medicine, consult-liaison psychiatry, and palliative care; advanced practice and bedside nurses; a social worker; a pharmacist; a chaplain; a peer-recovery specialist; and medical trainees. The B-Team is notified of potential candidates for buprenorphine through a secure texting platform, one that is accessible to any healthcare provider at the hospital. Patients who are referred to the B-Team either self-identify or are identified by their primary team as having an underlying OUD. One of the B-Team providers assesses the patient to determine if they are eligible to receive inpatient therapy. Patients are considered eligible for the program if they meet Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria for OUD, have a desire to cease opioid use, and receive medical clearance to take buprenorphine.
For eligible patients, the B-Team provider orders a nurse-driven protocol to initiate buprenorphine for OUD. The chaplain offers psychospiritual counseling, and the social worker provides counseling and coordination of care. The B-Team partners with a nonhospital-affiliated, publicly-funded, office-based opioid treatment (OBOT) program that combines primary care with behavioral health programming. A follow-up outpatient appointment is secured prior to hospital discharge, and a member of the B-Team who has Drug Addiction Treatment Act of 2000 (DATA 2000) X-waiver certification prescribes buprenorphine as a bridge until the follow-up appointment. The medication is dispensed from the hospital’s retail pharmacy, and the patient leaves the hospital with the medication in-hand.
Patients who are not eligible for buprenorphine therapy are offered a harm-reduction intervention or referral to the psychiatry consult liaison service to assess for alternative diagnoses or treatment. These patients are also offered psychospiritual counseling and a prescription for naloxone.
Prior to the creation of the B-Team at our hospital, there was no structure in place to facilitate initiation of buprenorphine therapy during hospitalization and no linkage to outpatient treatment after discharge; furthermore, none of the hospitalists or other providers (including consulting psychiatrists) had an X-waiver to prescribe buprenorphine for OUD.
Program Evaluation
Study data were collected using Research Electronic Data Capture software. Inpatient and outpatient data were entered by a B-Team provider or a researcher via chart review. Patients were considered to be engaged in care if they attended at least one outpatient appointment for buprenorphine therapy during each of the following time periods: (1) 0 to 27 days (initial follow-up), 28-89 days (1- to 3-month follow-up), 90-179 days (3- to 6-month follow-up), and 180 days or more (>6-month follow-up). Only visits specifically for buprenorphine maintenance therapy were counted. If multiple encounters occurred within one time frame, the encounter closest to 0, 30, 90, or 180 days from discharge was used. If a patient did not attend any encounters during a specified time frame, they were considered to no longer be engaged in care and were no longer tracked for purposes of the evaluation. Data for the percentage of patients engaged in outpatient care are presented as the number of patients who attended at least one appointment during each of the follow-up periods (1 to 3 months, 3 to 6 months, or after 6 months, as noted above) divided by the number of patients who had been discharged with coordinated follow-up.
The number of patients admitted per month for whom there was an order to initiate inpatient buprenorphine therapy was analyzed using a statistical process control chart,
This program and study were considered quality improvement by The University of Texas Institutional Review Board and did not meet criteria for human subjects research.
RESULTS
During the first 2 years of the program (September 2018-September 2020), the B-Team received 260 patient referrals. Most of the patients were White (72%), male (62%), and between ages 25 and 44 years (53%) (Appendix Table). The team initiated buprenorphine therapy in 132 hospitalized patients. In the year prior to the creation of the B-Team program, the average number of hospitalized patients receiving buprenorphine for OUD per month was three; after the launch of the B-Team program, this number increased

The B-Team saw a total of 132 eligible patients; members of the team provided counseling, support, and resources regarding buprenorphine therapy. In addition, the B-Team’s chaplain provided emotional support and spiritual connection (if desired) to 40 of these patients (30%). In the study, no cases of precipitated withdrawal were identified. Of the 132 patients seen, 110 (83%) were accepted to an outpatient OUD program upon discharge from the hospital; 98 (89%) of these patients were accepted at our partner OBOT clinic. The remaining patients were not interested in continuing OUD treatment (13%) or were denied acceptance to an outpatient program based on administrative and/or financial eligibility guidelines (4%). Patients who would not be attending an outpatient program were discontinued on buprenorphine therapy prior to discharge, counseled about naloxone, and provided printed resources.
Outpatient appointment attendance was used to measure ongoing treatment engagement of the 110 patients who were discharged with coordinated follow-up care. A total of 65 patients (59%) attended their first outpatient appointment; the average time between discharge and the first outpatient appointment was 5.9 days. Forty-two patients (38%) attended at least one appointment between 1 and 3 months; 29 (26%) between 3 and 6 months; and 24 (22%) after 6 months (Figure 2).

Of the 128 patients who were not administered buprenorphine therapy, 64 (50%) were not interested in starting treatment and/or were not ready to engage in treatment; 36 (28%) did not meet criteria for OUD treatment; 28 (22%) were already receiving treatment or preferred another type of OUD treatment; and 13 (10%) had severe comorbid addiction and/or illness requiring treatment that contraindicates the use of buprenorphine.
DISCUSSION
A volunteer hospitalist-led interprofessional team providing evidence-based care for hospitalized patients with OUD was associated with a substantial increase in patients receiving buprenorphine therapy—both during hospitalization and after discharge. In the program, 59% of patients attended initial follow-up appointments, and 22% of patients were still engaged at 6 months. These outpatient follow-up rates appear to be similar to, or higher than, other programs described in the literature. For example, a buprenorphine OUD-treatment initiative led by the psychiatry consult service at a Boston academic medical center resulted in less than half of patients receiving buprenorphine treatment within 2 months of discharge.7 In another study wherein an addiction medicine consult service administered buprenorphine to patients with OUD during hospitalization, 39%, 27%, and 18% of patients were retained in outpatient treatment at 30, 90, and 180 days, respectively.8
The B-Team model is likely generalizable to other hospital medicine groups that may not otherwise have access to inpatient care for substance use disorder. The B-Team is not an addiction medicine consultation service; rather, it is a hospitalist-led quality improvement initiative seeking to improve the standard of care for hospitalized patients with OUD.
A significant barrier is ensuring ongoing support for patients with OUD after discharge. In the B-Team program, a parallel OBOT program was created by a local nonaffiliated federally qualified health center. Although 89% of patients received treatment at this OBOT clinic, the inpatient team also has relationships with other local treatment centers, including programs that provide methadone. Another important barrier to high-quality outpatient care for OUD is the requirement of an X-waiver. To help overcome this barrier, our inpatient program partnered with a regional medical society to offer periodic X-waiver training to outpatient providers. In less than a year, more than 100 regional prescribers participated in this program.
Our study has several limitations. There was likely some degree of selection bias among the hospitalized patients who received initial buprenorphine treatment. To our knowledge, there is no specific validated screening tool for OUD in the inpatient acute care setting; moreover, we have been unable to implement standardized screening for OUD into the electronic health record. As such, we rely on the totality of the clinical circumstances approach to identify patients with OUD.
Furthermore, we had neither a comparison group nor a prospective plan to follow patients who did not remain engaged in care after discharge. In addition, our analysis of OUD admissions included F11 ICD-10 codes, which are limited by clinical documentation.9,10 Our program focuses exclusively on buprenorphine initiation due to insufficient immediate outpatient capacity for methadone initiated during hospitalization and lack of coverage for extended-release naltrexone. Limitations to outpatient data-sharing prevented the reporting of outpatient appointments external to the identified partner program; since these appointments were included in the analysis as “lost to follow-up,” actual engagement rates may be higher than those reported.
Moving forward, the B-Team is continuing to serve as a role model for appropriate, patient-centered, evidence-based care for hospitalized patients with OUD. Attending physicians and residents with an X-waiver are now encouraged to initiate buprenorphine treatment on their own. In June 2020, we added peer-recovery support services to the program, which has improved care for patients and increased adoption of hospital-initiated substance use disorder interventions.11 Lessons learned from inpatient implementation are being applied to our hospital’s emergency department and to an inpatient obstetrics unit at a partner hospital; they are also being employed to further empower hospitalists to diagnose and treat other substance use disorders, such as alcohol use disorder.
1. Owens PL, Weiss AJ, Barrett ML. Hospital Burden of Opioid-Related Inpatient Stays: Metropolitan and Rural Hospitals, 2016. HCUP Statistical Brief #258. Agency for Healthcare Research and Quality. May 2020. Accessed May 24, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559382/pdf/Bookshelf_NBK559382.pdf
2. Liebschutz J, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/jamainternmed.2014.2556
3. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
4. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736
5. Fanucchi L, Lofwall MR. Putting parity into practice — integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. https://doi.org/10.1056/nejmp1606157
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
7. Suzuki J, DeVido J, Kalra I, et al. Initiating buprenorphine treatment for hospitalized patients with opioid dependence: a case series. Am J Addict. 2015;24(1):10-14. https://doi.org/10.1111/ajad.12161
8. Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1-5. https://doi.org/10.1016/j.jsat.2017.05.007
9. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/adm.0000000000000454
10. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
11. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. 2019. https://doi.org/10.1007/s11606-019-05311-0
1. Owens PL, Weiss AJ, Barrett ML. Hospital Burden of Opioid-Related Inpatient Stays: Metropolitan and Rural Hospitals, 2016. HCUP Statistical Brief #258. Agency for Healthcare Research and Quality. May 2020. Accessed May 24, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559382/pdf/Bookshelf_NBK559382.pdf
2. Liebschutz J, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/jamainternmed.2014.2556
3. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
4. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736
5. Fanucchi L, Lofwall MR. Putting parity into practice — integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. https://doi.org/10.1056/nejmp1606157
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
7. Suzuki J, DeVido J, Kalra I, et al. Initiating buprenorphine treatment for hospitalized patients with opioid dependence: a case series. Am J Addict. 2015;24(1):10-14. https://doi.org/10.1111/ajad.12161
8. Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1-5. https://doi.org/10.1016/j.jsat.2017.05.007
9. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/adm.0000000000000454
10. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
11. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. 2019. https://doi.org/10.1007/s11606-019-05311-0
© 2021 Society of Hospital Medicine
Gender Differences in the Presentation and Outcomes of Hospitalized Patients With COVID-19
There is growing evidence that gender may be associated with COVID-19 infection, presentation, and prognosis
METHODS
Study methods and definitions are available in Appendix 1 and Appendix 2, respectively, and detailed in a previous paper5 and online on the web page of the study.6
Enrolled patients were divided into two groups according to their gender, then propensity score matching (PSM) analysis was performed (1:1 nearest neighbor matching, caliper = 0.01, without replacement and maximizing execution performance). Our
Statistical analysis methods are outlined in Appendix 1.
RESULTS
Of the 2,798 patients consecutively enrolled in the HOPE registry, 1,111 were women (39.7%) and 1,687 were men (60.3%). Of the 2,375 (84.9%) patients who had a nasopharyngeal swab positive for COVID-19, 962 were women and 1,413 were men.
Baseline Characteristics and Clinical Presentation
The baseline characteristics and clinical presentation of the overall population included in the study are summarized in Appendix Table 1. In the raw population, men had a significantly higher prevalence of conventional cardiovascular risk factors, such as diabetes, dyslipidemia, and smoking history, as well as a history of lung and cardiovascular diseases. On presentation, the most common symptoms for all patients were fever, cough, and dyspnea. Fever was more common in men, whereas vomiting, diarrhea, and upper airway symptoms (eg, sore throat, hyposmia/anosmia, dysgeusia) were more common in women.
Most patients had increased values of acute phase reactants. C-reactive protein (CRP) was elevated in 90.2% and D-dimer in 64.2% of patients, both significantly more often in men. Lymphocytopenia was present in 75.4% of patients, more commonly among men. Bilateral pneumonia occurred in 69.2% of the population, more frequently in men.
After PSM analysis (Appendix Table 2), a higher prevalence of hyposmia/anosmia and gastrointestinal symptoms in women was confirmed, as well as a higher prevalence of fever in men. Laboratory tests in men still presented alterations consistent with a more severe COVID-19 infection (significantly higher CRP, troponin, transaminases, lymphocytopenia, thrombocytopenia, and ferritin). There was no significant difference in the time between onset of symptoms and hospital admission by gender (6.2 ± 7.1 days in women vs 5.9 ± 7.6 days in men; P = .472).
The main findings after PSM analysis are summarized in Appendix Figure 1 and Appendix Figure 2.
In-Hospital Management and Outcomes
The supportive and pharmacologic treatments of study patients and their outcomes are summarized in Appendix Table 3. During the in-hospital stay, men required oxygen supplementation more frequently than women. Noninvasive mechanical ventilation, invasive mechanical ventilation, and pronation were more commonly used in men. Chloroquine/hydroxychloroquine, antivirals, and antibiotics were the medications most widely used in our population (84.5%, 65.8%, and 74.4% of patients, respectively), without significant differences between male and female patients, with the exception of antibiotics, which were used more often in men (76.6% vs 71.1%). Immunomodulators (corticosteroids, tocilizumab, and interferon) were used more often in male patients.
After PSM (Table), men more frequently received immunomodulators (corticosteroids and tocilizumab), antibiotics, and pronation. No differences in invasive and noninvasive mechanical ventilation were observed.
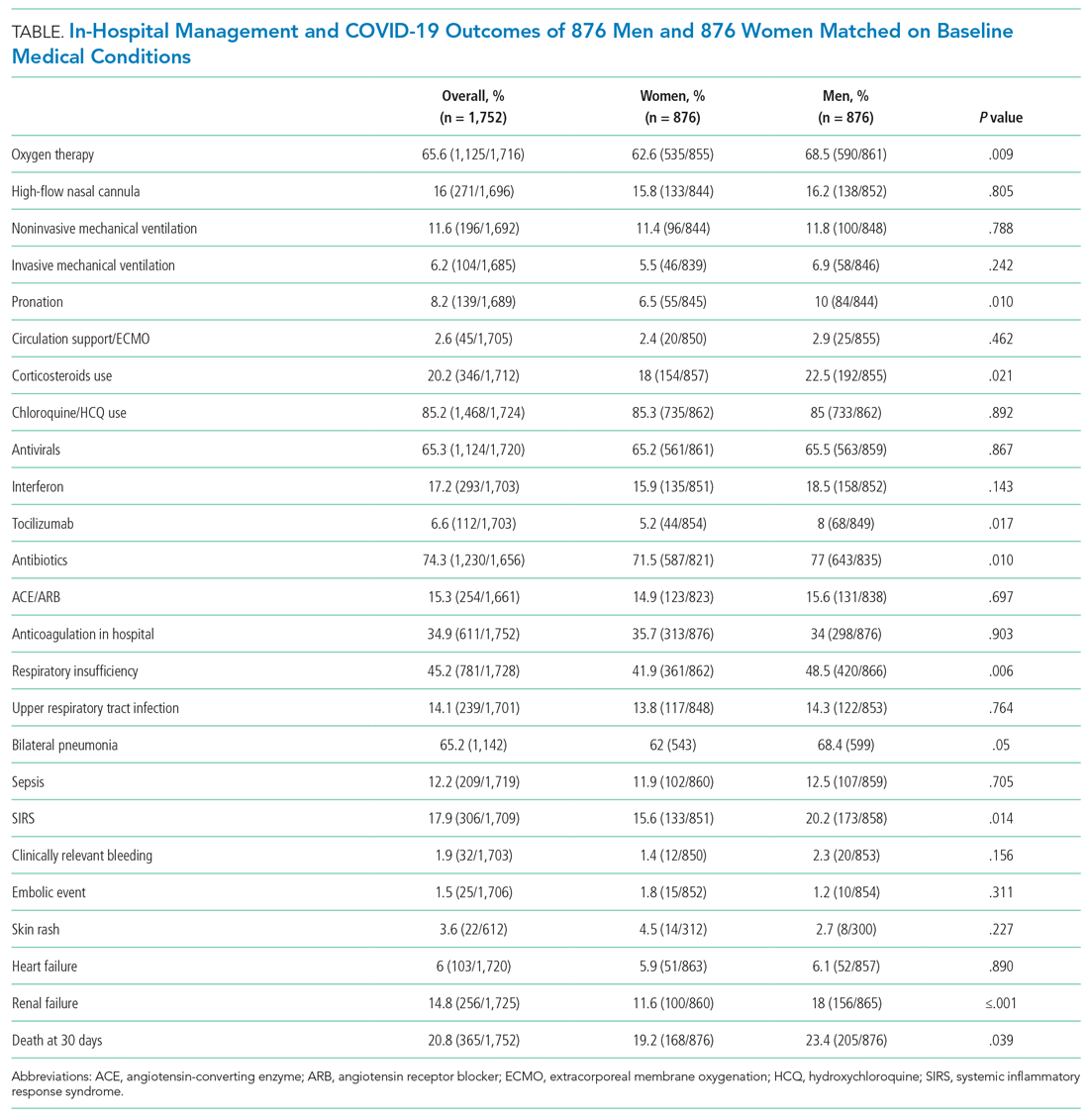
Thirty-day outcome data were available for all patients included in the analysis. During the in-hospital stay, 48% of patients developed respiratory insufficiency, 18.8%
The PSM analysis continued to show a higher 30-day mortality rate among men (Figure), as well as greater need for oxygen, pronation, and use of immunomodulators and antibiotics (Table).
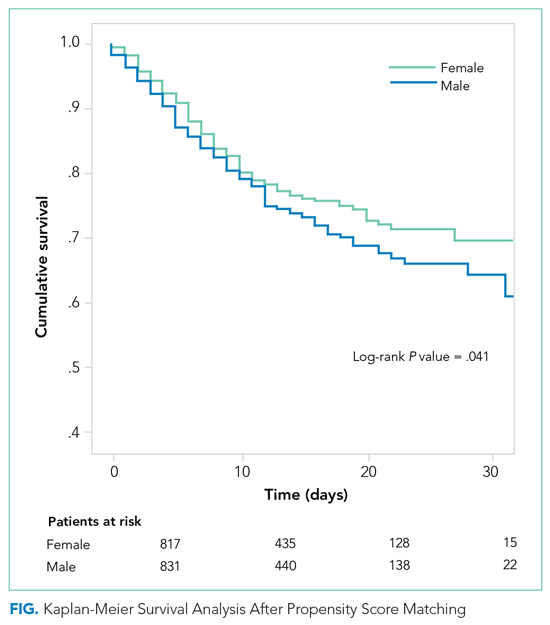
DISCUSSION
The results of our study confirm that among patients with COVID-19, men have a poorer prognosis than women. Because of the design of the study, it is not possible to determine if men are more prone to SARS-CoV-2 infection in our population; however, given the prevalence of men in our unselected, all-comers population, we can assume that men are either infected more often and/or more frequently symptomatic.
After PSM analysis, the 30-day all-cause mortality remained higher among men than women. The poorer prognosis of male patients is attributable not only to a higher burden of cardiovascular risk factors, but may also be related to unmodifiable biological factors, such as sex differences in angiotensin-converting enzyme 2 expression.7,8 The worse prognosis observed in our study confirms the higher incidence of death in male patients that was observed in previous studies.9 Liu et al questioned the role of gender as an independent prognostic factor in COVID-1910; however, that study included fewer patients, who were also younger and had less severe disease.
The clinical presentation of COVID-19 also differed by gender in our study. Gastrointestinal symptoms and hyposmia/anosmia were more common in women, whereas fever was more common in men. The prevalence of olfactory and gustatory dysfunction in women has already been described,11,12 and these symptoms have been linked with milder disease.13 It is possible that women presenting to the hospital had milder forms of COVID-19, or that there were systematic differences in how men and women sought medical care. The results of our study emphasize the need for a high level of suspicion for COVID-19 infection in women, even in the presence of mild mucosal or gastrointestinal symptoms and/or relatively minor laboratory abnormalities.
Laboratory values indicative of more severe COVID-19 infection in men could suggest a higher inflammatory response to the infection. Men also received more immunomodulators and antibiotics in this study. A recent paper from Scully et al14 pointed out the different immune response to viruses observed in men that could partially explain the higher level of inflammation markers and the more severe disease observed in men.
Limitations
Our study has several limitations. As an observational study of hospitalized patients, it may represent patients with more severe COVID-19. Men and women may have sought hospital care differently. Diagnosis, testing, and treatment were not standardized and may have been influenced by patient gender. Although we attempted to match patients on baseline medical conditions, we may not have completely controlled for differences in preexisting health. Finally, gender data were collected as binary and so did not capture other gender categories.
CONCLUSION
In our multicenter cohort of hospitalized COVID-19 patients, men had a higher burden of risk factors; different clinical presentations, with more fever and less olfactory and gastrointestinal symptoms; and a significantly poorer prognosis than women did at 30 days.
Acknowledgments
The authors thank Cardiovascular Excellence SL for their essential support regarding the database and HOPE web page as well as all HOPE researchers. The authors also thank Michael Andrews for his valuable contribution to the English revision.
1. Alkhouli M, Nanjundappa A, Annie F, Bates MC, Bhatt DL. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin Proc. 2020;95(8):1613-1620. https://doi.org/10.1016/j.mayocp.2020.05.014
2. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. https://doi.org/10.1186/s13293-020-00304-9
3. Gausman J, Langer A. Sex and gender disparities in the COVID-19 pandemic. J Womens Health (Larchmt). 2020;29(4):465-466. https://doi.org/10.1089/jwh.2020.8472
4. Walter LA, McGregor AJ. Sex- and gender-specific observations and implications for COVID-19. West J Emerg Med. 2020;21(3):507-509. https://doi.org/10.5811/westjem.2020.4.47536
5. Núñez-Gil IJ, Estrada V, Fernández-Pérez C, et al. Health outcome predictive evaluation for COVID 19 international registry (HOPE COVID-19), rationale and design. Contemp Clin Trials Commun. 2020;20:100654. https://doi.org/10.1016/j.conctc.2020.100654
6. International COVID-19 Clinical Evaluation Registry: HOPE-COVID 19. Accessed February 6, 2021. https://hopeprojectmd.com/en/
7. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37. https://doi.org/10.1038/s41420-020-0276-1
8. Ciaglia E, Vecchione C, Puca AA. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front Pediatr. 2020;8:206. https://doi.org/10.3389/fped.2020.00206
9. Peckham H, de Gruijter N, Raine C, et al. Sex-bias in COVID-19: a meta-analysis and review of sex differences in disease and immunity. Research Square. April 20, 2020. https://doi.org/10.21203/rs.3.rs-23651/v2
10. Liu J, Zhang L, Chen Y, et al. Association of sex with clinical outcomes in COVID-19 patients: a retrospective analysis of 1190 cases. Respir Med. 2020;173:106159. https://doi.org/10.1016/j.rmed.2020.106159
11. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms—a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722-728. https://doi.org/10.1177/0194599820934380
12. Costa KVTD, Carnaúba ATL, Rocha KW, Andrade KCLD, Ferreira SMS, Menezes PTL. Olfactory and taste disorders in COVID-19: a systematic review. Braz J Otorhinolaryngol. 2020;86(6):781-792. https://doi.org/10.1016/j.bjorl.2020.05.008
13. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251-2261. https://doi.org/10.1007/s00405-020-05965-1
14. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442-447. https://doi.org/10.1038/s41577-020-0348-8
There is growing evidence that gender may be associated with COVID-19 infection, presentation, and prognosis
METHODS
Study methods and definitions are available in Appendix 1 and Appendix 2, respectively, and detailed in a previous paper5 and online on the web page of the study.6
Enrolled patients were divided into two groups according to their gender, then propensity score matching (PSM) analysis was performed (1:1 nearest neighbor matching, caliper = 0.01, without replacement and maximizing execution performance). Our
Statistical analysis methods are outlined in Appendix 1.
RESULTS
Of the 2,798 patients consecutively enrolled in the HOPE registry, 1,111 were women (39.7%) and 1,687 were men (60.3%). Of the 2,375 (84.9%) patients who had a nasopharyngeal swab positive for COVID-19, 962 were women and 1,413 were men.
Baseline Characteristics and Clinical Presentation
The baseline characteristics and clinical presentation of the overall population included in the study are summarized in Appendix Table 1. In the raw population, men had a significantly higher prevalence of conventional cardiovascular risk factors, such as diabetes, dyslipidemia, and smoking history, as well as a history of lung and cardiovascular diseases. On presentation, the most common symptoms for all patients were fever, cough, and dyspnea. Fever was more common in men, whereas vomiting, diarrhea, and upper airway symptoms (eg, sore throat, hyposmia/anosmia, dysgeusia) were more common in women.
Most patients had increased values of acute phase reactants. C-reactive protein (CRP) was elevated in 90.2% and D-dimer in 64.2% of patients, both significantly more often in men. Lymphocytopenia was present in 75.4% of patients, more commonly among men. Bilateral pneumonia occurred in 69.2% of the population, more frequently in men.
After PSM analysis (Appendix Table 2), a higher prevalence of hyposmia/anosmia and gastrointestinal symptoms in women was confirmed, as well as a higher prevalence of fever in men. Laboratory tests in men still presented alterations consistent with a more severe COVID-19 infection (significantly higher CRP, troponin, transaminases, lymphocytopenia, thrombocytopenia, and ferritin). There was no significant difference in the time between onset of symptoms and hospital admission by gender (6.2 ± 7.1 days in women vs 5.9 ± 7.6 days in men; P = .472).
The main findings after PSM analysis are summarized in Appendix Figure 1 and Appendix Figure 2.
In-Hospital Management and Outcomes
The supportive and pharmacologic treatments of study patients and their outcomes are summarized in Appendix Table 3. During the in-hospital stay, men required oxygen supplementation more frequently than women. Noninvasive mechanical ventilation, invasive mechanical ventilation, and pronation were more commonly used in men. Chloroquine/hydroxychloroquine, antivirals, and antibiotics were the medications most widely used in our population (84.5%, 65.8%, and 74.4% of patients, respectively), without significant differences between male and female patients, with the exception of antibiotics, which were used more often in men (76.6% vs 71.1%). Immunomodulators (corticosteroids, tocilizumab, and interferon) were used more often in male patients.
After PSM (Table), men more frequently received immunomodulators (corticosteroids and tocilizumab), antibiotics, and pronation. No differences in invasive and noninvasive mechanical ventilation were observed.

Thirty-day outcome data were available for all patients included in the analysis. During the in-hospital stay, 48% of patients developed respiratory insufficiency, 18.8%
The PSM analysis continued to show a higher 30-day mortality rate among men (Figure), as well as greater need for oxygen, pronation, and use of immunomodulators and antibiotics (Table).

DISCUSSION
The results of our study confirm that among patients with COVID-19, men have a poorer prognosis than women. Because of the design of the study, it is not possible to determine if men are more prone to SARS-CoV-2 infection in our population; however, given the prevalence of men in our unselected, all-comers population, we can assume that men are either infected more often and/or more frequently symptomatic.
After PSM analysis, the 30-day all-cause mortality remained higher among men than women. The poorer prognosis of male patients is attributable not only to a higher burden of cardiovascular risk factors, but may also be related to unmodifiable biological factors, such as sex differences in angiotensin-converting enzyme 2 expression.7,8 The worse prognosis observed in our study confirms the higher incidence of death in male patients that was observed in previous studies.9 Liu et al questioned the role of gender as an independent prognostic factor in COVID-1910; however, that study included fewer patients, who were also younger and had less severe disease.
The clinical presentation of COVID-19 also differed by gender in our study. Gastrointestinal symptoms and hyposmia/anosmia were more common in women, whereas fever was more common in men. The prevalence of olfactory and gustatory dysfunction in women has already been described,11,12 and these symptoms have been linked with milder disease.13 It is possible that women presenting to the hospital had milder forms of COVID-19, or that there were systematic differences in how men and women sought medical care. The results of our study emphasize the need for a high level of suspicion for COVID-19 infection in women, even in the presence of mild mucosal or gastrointestinal symptoms and/or relatively minor laboratory abnormalities.
Laboratory values indicative of more severe COVID-19 infection in men could suggest a higher inflammatory response to the infection. Men also received more immunomodulators and antibiotics in this study. A recent paper from Scully et al14 pointed out the different immune response to viruses observed in men that could partially explain the higher level of inflammation markers and the more severe disease observed in men.
Limitations
Our study has several limitations. As an observational study of hospitalized patients, it may represent patients with more severe COVID-19. Men and women may have sought hospital care differently. Diagnosis, testing, and treatment were not standardized and may have been influenced by patient gender. Although we attempted to match patients on baseline medical conditions, we may not have completely controlled for differences in preexisting health. Finally, gender data were collected as binary and so did not capture other gender categories.
CONCLUSION
In our multicenter cohort of hospitalized COVID-19 patients, men had a higher burden of risk factors; different clinical presentations, with more fever and less olfactory and gastrointestinal symptoms; and a significantly poorer prognosis than women did at 30 days.
Acknowledgments
The authors thank Cardiovascular Excellence SL for their essential support regarding the database and HOPE web page as well as all HOPE researchers. The authors also thank Michael Andrews for his valuable contribution to the English revision.
There is growing evidence that gender may be associated with COVID-19 infection, presentation, and prognosis
METHODS
Study methods and definitions are available in Appendix 1 and Appendix 2, respectively, and detailed in a previous paper5 and online on the web page of the study.6
Enrolled patients were divided into two groups according to their gender, then propensity score matching (PSM) analysis was performed (1:1 nearest neighbor matching, caliper = 0.01, without replacement and maximizing execution performance). Our
Statistical analysis methods are outlined in Appendix 1.
RESULTS
Of the 2,798 patients consecutively enrolled in the HOPE registry, 1,111 were women (39.7%) and 1,687 were men (60.3%). Of the 2,375 (84.9%) patients who had a nasopharyngeal swab positive for COVID-19, 962 were women and 1,413 were men.
Baseline Characteristics and Clinical Presentation
The baseline characteristics and clinical presentation of the overall population included in the study are summarized in Appendix Table 1. In the raw population, men had a significantly higher prevalence of conventional cardiovascular risk factors, such as diabetes, dyslipidemia, and smoking history, as well as a history of lung and cardiovascular diseases. On presentation, the most common symptoms for all patients were fever, cough, and dyspnea. Fever was more common in men, whereas vomiting, diarrhea, and upper airway symptoms (eg, sore throat, hyposmia/anosmia, dysgeusia) were more common in women.
Most patients had increased values of acute phase reactants. C-reactive protein (CRP) was elevated in 90.2% and D-dimer in 64.2% of patients, both significantly more often in men. Lymphocytopenia was present in 75.4% of patients, more commonly among men. Bilateral pneumonia occurred in 69.2% of the population, more frequently in men.
After PSM analysis (Appendix Table 2), a higher prevalence of hyposmia/anosmia and gastrointestinal symptoms in women was confirmed, as well as a higher prevalence of fever in men. Laboratory tests in men still presented alterations consistent with a more severe COVID-19 infection (significantly higher CRP, troponin, transaminases, lymphocytopenia, thrombocytopenia, and ferritin). There was no significant difference in the time between onset of symptoms and hospital admission by gender (6.2 ± 7.1 days in women vs 5.9 ± 7.6 days in men; P = .472).
The main findings after PSM analysis are summarized in Appendix Figure 1 and Appendix Figure 2.
In-Hospital Management and Outcomes
The supportive and pharmacologic treatments of study patients and their outcomes are summarized in Appendix Table 3. During the in-hospital stay, men required oxygen supplementation more frequently than women. Noninvasive mechanical ventilation, invasive mechanical ventilation, and pronation were more commonly used in men. Chloroquine/hydroxychloroquine, antivirals, and antibiotics were the medications most widely used in our population (84.5%, 65.8%, and 74.4% of patients, respectively), without significant differences between male and female patients, with the exception of antibiotics, which were used more often in men (76.6% vs 71.1%). Immunomodulators (corticosteroids, tocilizumab, and interferon) were used more often in male patients.
After PSM (Table), men more frequently received immunomodulators (corticosteroids and tocilizumab), antibiotics, and pronation. No differences in invasive and noninvasive mechanical ventilation were observed.

Thirty-day outcome data were available for all patients included in the analysis. During the in-hospital stay, 48% of patients developed respiratory insufficiency, 18.8%
The PSM analysis continued to show a higher 30-day mortality rate among men (Figure), as well as greater need for oxygen, pronation, and use of immunomodulators and antibiotics (Table).

DISCUSSION
The results of our study confirm that among patients with COVID-19, men have a poorer prognosis than women. Because of the design of the study, it is not possible to determine if men are more prone to SARS-CoV-2 infection in our population; however, given the prevalence of men in our unselected, all-comers population, we can assume that men are either infected more often and/or more frequently symptomatic.
After PSM analysis, the 30-day all-cause mortality remained higher among men than women. The poorer prognosis of male patients is attributable not only to a higher burden of cardiovascular risk factors, but may also be related to unmodifiable biological factors, such as sex differences in angiotensin-converting enzyme 2 expression.7,8 The worse prognosis observed in our study confirms the higher incidence of death in male patients that was observed in previous studies.9 Liu et al questioned the role of gender as an independent prognostic factor in COVID-1910; however, that study included fewer patients, who were also younger and had less severe disease.
The clinical presentation of COVID-19 also differed by gender in our study. Gastrointestinal symptoms and hyposmia/anosmia were more common in women, whereas fever was more common in men. The prevalence of olfactory and gustatory dysfunction in women has already been described,11,12 and these symptoms have been linked with milder disease.13 It is possible that women presenting to the hospital had milder forms of COVID-19, or that there were systematic differences in how men and women sought medical care. The results of our study emphasize the need for a high level of suspicion for COVID-19 infection in women, even in the presence of mild mucosal or gastrointestinal symptoms and/or relatively minor laboratory abnormalities.
Laboratory values indicative of more severe COVID-19 infection in men could suggest a higher inflammatory response to the infection. Men also received more immunomodulators and antibiotics in this study. A recent paper from Scully et al14 pointed out the different immune response to viruses observed in men that could partially explain the higher level of inflammation markers and the more severe disease observed in men.
Limitations
Our study has several limitations. As an observational study of hospitalized patients, it may represent patients with more severe COVID-19. Men and women may have sought hospital care differently. Diagnosis, testing, and treatment were not standardized and may have been influenced by patient gender. Although we attempted to match patients on baseline medical conditions, we may not have completely controlled for differences in preexisting health. Finally, gender data were collected as binary and so did not capture other gender categories.
CONCLUSION
In our multicenter cohort of hospitalized COVID-19 patients, men had a higher burden of risk factors; different clinical presentations, with more fever and less olfactory and gastrointestinal symptoms; and a significantly poorer prognosis than women did at 30 days.
Acknowledgments
The authors thank Cardiovascular Excellence SL for their essential support regarding the database and HOPE web page as well as all HOPE researchers. The authors also thank Michael Andrews for his valuable contribution to the English revision.
1. Alkhouli M, Nanjundappa A, Annie F, Bates MC, Bhatt DL. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin Proc. 2020;95(8):1613-1620. https://doi.org/10.1016/j.mayocp.2020.05.014
2. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. https://doi.org/10.1186/s13293-020-00304-9
3. Gausman J, Langer A. Sex and gender disparities in the COVID-19 pandemic. J Womens Health (Larchmt). 2020;29(4):465-466. https://doi.org/10.1089/jwh.2020.8472
4. Walter LA, McGregor AJ. Sex- and gender-specific observations and implications for COVID-19. West J Emerg Med. 2020;21(3):507-509. https://doi.org/10.5811/westjem.2020.4.47536
5. Núñez-Gil IJ, Estrada V, Fernández-Pérez C, et al. Health outcome predictive evaluation for COVID 19 international registry (HOPE COVID-19), rationale and design. Contemp Clin Trials Commun. 2020;20:100654. https://doi.org/10.1016/j.conctc.2020.100654
6. International COVID-19 Clinical Evaluation Registry: HOPE-COVID 19. Accessed February 6, 2021. https://hopeprojectmd.com/en/
7. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37. https://doi.org/10.1038/s41420-020-0276-1
8. Ciaglia E, Vecchione C, Puca AA. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front Pediatr. 2020;8:206. https://doi.org/10.3389/fped.2020.00206
9. Peckham H, de Gruijter N, Raine C, et al. Sex-bias in COVID-19: a meta-analysis and review of sex differences in disease and immunity. Research Square. April 20, 2020. https://doi.org/10.21203/rs.3.rs-23651/v2
10. Liu J, Zhang L, Chen Y, et al. Association of sex with clinical outcomes in COVID-19 patients: a retrospective analysis of 1190 cases. Respir Med. 2020;173:106159. https://doi.org/10.1016/j.rmed.2020.106159
11. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms—a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722-728. https://doi.org/10.1177/0194599820934380
12. Costa KVTD, Carnaúba ATL, Rocha KW, Andrade KCLD, Ferreira SMS, Menezes PTL. Olfactory and taste disorders in COVID-19: a systematic review. Braz J Otorhinolaryngol. 2020;86(6):781-792. https://doi.org/10.1016/j.bjorl.2020.05.008
13. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251-2261. https://doi.org/10.1007/s00405-020-05965-1
14. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442-447. https://doi.org/10.1038/s41577-020-0348-8
1. Alkhouli M, Nanjundappa A, Annie F, Bates MC, Bhatt DL. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin Proc. 2020;95(8):1613-1620. https://doi.org/10.1016/j.mayocp.2020.05.014
2. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. https://doi.org/10.1186/s13293-020-00304-9
3. Gausman J, Langer A. Sex and gender disparities in the COVID-19 pandemic. J Womens Health (Larchmt). 2020;29(4):465-466. https://doi.org/10.1089/jwh.2020.8472
4. Walter LA, McGregor AJ. Sex- and gender-specific observations and implications for COVID-19. West J Emerg Med. 2020;21(3):507-509. https://doi.org/10.5811/westjem.2020.4.47536
5. Núñez-Gil IJ, Estrada V, Fernández-Pérez C, et al. Health outcome predictive evaluation for COVID 19 international registry (HOPE COVID-19), rationale and design. Contemp Clin Trials Commun. 2020;20:100654. https://doi.org/10.1016/j.conctc.2020.100654
6. International COVID-19 Clinical Evaluation Registry: HOPE-COVID 19. Accessed February 6, 2021. https://hopeprojectmd.com/en/
7. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37. https://doi.org/10.1038/s41420-020-0276-1
8. Ciaglia E, Vecchione C, Puca AA. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front Pediatr. 2020;8:206. https://doi.org/10.3389/fped.2020.00206
9. Peckham H, de Gruijter N, Raine C, et al. Sex-bias in COVID-19: a meta-analysis and review of sex differences in disease and immunity. Research Square. April 20, 2020. https://doi.org/10.21203/rs.3.rs-23651/v2
10. Liu J, Zhang L, Chen Y, et al. Association of sex with clinical outcomes in COVID-19 patients: a retrospective analysis of 1190 cases. Respir Med. 2020;173:106159. https://doi.org/10.1016/j.rmed.2020.106159
11. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms—a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722-728. https://doi.org/10.1177/0194599820934380
12. Costa KVTD, Carnaúba ATL, Rocha KW, Andrade KCLD, Ferreira SMS, Menezes PTL. Olfactory and taste disorders in COVID-19: a systematic review. Braz J Otorhinolaryngol. 2020;86(6):781-792. https://doi.org/10.1016/j.bjorl.2020.05.008
13. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251-2261. https://doi.org/10.1007/s00405-020-05965-1
14. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442-447. https://doi.org/10.1038/s41577-020-0348-8
© 2021 Society of Hospital Medicine
Mapping the Clinical Experience of a New York City Residency Program During the COVID-19 Pandemic
The COVID-19 pandemic has disrupted the educational experience of medical trainees around the world, and this has been especially true for those in New York City (NYC), the early epicenter of the global outbreak.1 The pandemic’s surge required redeployment of trainees away from scheduled rotations, focused didactics around emerging COVID-19 data, and seemingly narrowed trainees’ clinical exposure to a single respiratory infection.
While there is a small body of literature describing the programmatic responses2,3 and educational adaptations4-7 that have come about as a result of the pandemic’s disruptive force, a characterization of exactly how trainees’ clinical experiences have been affected is lacking. A detailed understanding of how trainees’ inpatient care activities evolved during the pandemic could provide valuable practice habits feedback, allow for comparisons across training sites, focus content selection for didactic learning and self-study, and potentially help forecast similar clinical changes in the event of a subsequent wave. Perhaps most important, as internal medicine (IM) trainees require broad exposure to diverse clinical conditions to mature toward independent practice, a characterization of exactly how the pandemic has narrowed the diversity of clinical exposure could inform changes in how trainees attain clinical competence.
Profiling IM residents’ clinical experiences in a meaningful way is particularly challenging given the extraordinary breadth of the field. We recently developed a strategy by which resident-attributed International Classification of Diseases, Tenth Revision (ICD-10) principal diagnosis codes are mapped to an educational taxonomy of medical content categories, yielding clinical exposure profiles.8 Here, we apply this mapping strategy to all four training hospitals of a large NYC IM residency program to catalogue the evolution of clinical diversity experienced by residents during the COVID-19 pandemic.
METHODS
Study Population
The NYU IM Residency Program comprises 225 resident physicians rotating at four inpatient training sites: NYU Langone Hospital–Brooklyn (NYU-BK), NYU Langone Hospitals–Manhattan (NYU-MN), Bellevue Hospital (BH), and VA–New York Harbor Healthcare (VA). The study period was defined as February 1, 2020, to May 31, 2020, to capture clinical exposure during baseline, surge, and immediate post-surge periods. The NYU IM residency program declared pandemic emergency status on March 23, 2020, after which all residents were assigned to inpatient acute care and intensive care rotations to augment the inpatient workforce.
Data Source
Clinical data at each training hospital are collected and stored, allowing for asynchronous querying. Given differences in data reporting, strategies for collecting principal ICD-10 codes of patients discharged during the study period differed slightly across sites. Principal ICD-10 codes from patients discharged from NYU-BK and NYU-MN were filtered by nursing unit, allowing selection for resident-staffed units. Principal ICD-10 codes from BH were curated by care team, allowing selection for resident-staffed teams. Principal ICD-10 codes from VA were filtered by both hospital unit and provider service to attribute to resident providers. Dates of each discharge were included, and mortalities were included as discharges. All methods yielded a dataset of principal ICD-10 discharge diagnosis codes attributed primarily to IM residents. Given the rapid changes in hospital staffing to care for increasing patient volumes, in rare circumstances residents and other providers (such as advanced practice providers) shared hospital units. While ICD-10 codes mined from each hospital are attributed primarily to residents, this attribution is not entirely exclusive. Data were analyzed both by training site and in aggregate across the four training sites. No individually identifiable data were analyzed, the primary goal of the project was to improve education, and the data were collected as part of a required aspect of training; as a result, this project met criteria for certification as a quality improvement, and not a human subject, research project.
The Crosswalk Tool
We previously developed a crosswalk tool containing 4,854 ICD-10 diagnoses uniquely mapped to 16 broad medical content areas as defined by the American Board of Internal Medicine (ABIM).8 Custom programs (MATLAB, MathWorks, Inc) captured and subsequently mapped resident-attributed ICD-10 discharge codes to content areas if the syntax of the ICD-10 code in question exactly matched or was nested within an ICD-10 code in the crosswalk. This tool allowed us to measure the daily discharge frequency of each content area across the sites.
Analysis
The sensitivity of the crosswalk tool was calculated as the number of ICD-10 codes captured divided by the total number of patients. Codes missed by the tool were excluded. The total number, as well as the 7-day running average of discharges per content area, across the sites during the study period were measured. To evaluate for differences in the distribution of content before and after pandemic emergency status, 2 × 16 χ2 contingency tables were constructed. To evaluate for changes in the mean relative proportions (%) of each content area, paired t tests were conducted. Confidence intervals were estimated from t distributions.
RESULTS
There were 6,613 patients discharged from all sites (NYU-BK, 2,062; NYU-MN, 2,188; BH, 1,711; VA, 652; Appendix Table). The crosswalk tool captured 6,384 principal discharge ICD-10 codes (96.5%). The five most common content areas during the study period were infectious diseases (ID; n = 2,892), cardiovascular disease (CVD; n = 1,199), gastroenterology (n = 406), pulmonary disease (n = 372), and nephrology and urology (n = 252). These were also the content areas most frequently encountered by residents at baseline (Figure and Table). The distribution of content prior to declaration of pandemic emergency status was significantly different than that after declaration (χ2 = 709; df, 15; P <.001). ID diagnoses, driven by COVID-19, rose steeply in the period following declaration, peaked in mid-April, and slowly waned in May (Figure). The mean relative percentage of ID discharges across the sites rose from 26.0% (16.5%-35.4%) at baseline to 58.3% (41.3%-75.3%) in the period after pandemic emergency status was declared (P = .005).
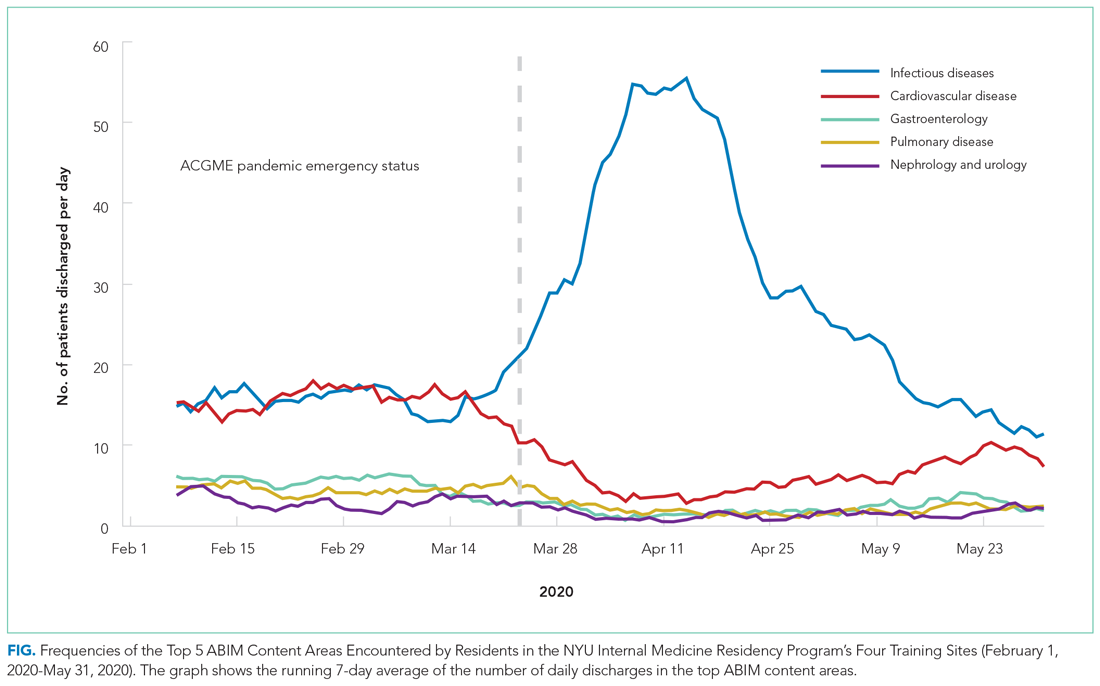
Frequencies of diagnoses mapping to other content areas decreased significantly, reflecting a marked tapering of clinical diversity (Figure and Table). Specifically, decreases were seen in CVD (27.6% [95% CI, 17.9%-37.2%] to 13.9% [95% CI, 5.5%-22.3%]; P = .013); gastroenterology (8.3% [95% CI, 6.2%-10.2%] to 4.6% [95% CI, 2.1%-6.9%]; P = .038); pulmonary disease (8.0% [95% CI, 5.6%-10.2%] to 4.6% [95% CI, 1.6%-7.4%]; P = .040); and nephrology and urology (4.8% [95% CI, 2.6%-6.9%] to 3.1% [95% CI, 1.9%-4.2%]; P = .047) (Table). In late April, diagnoses mapping to these content areas began to repopulate residents’ clinical experiences and by the end of the study period had nearly returned to baseline frequencies. These patterns were similar when discharge diagnoses from each training site were plotted individually (Appendix Figure).
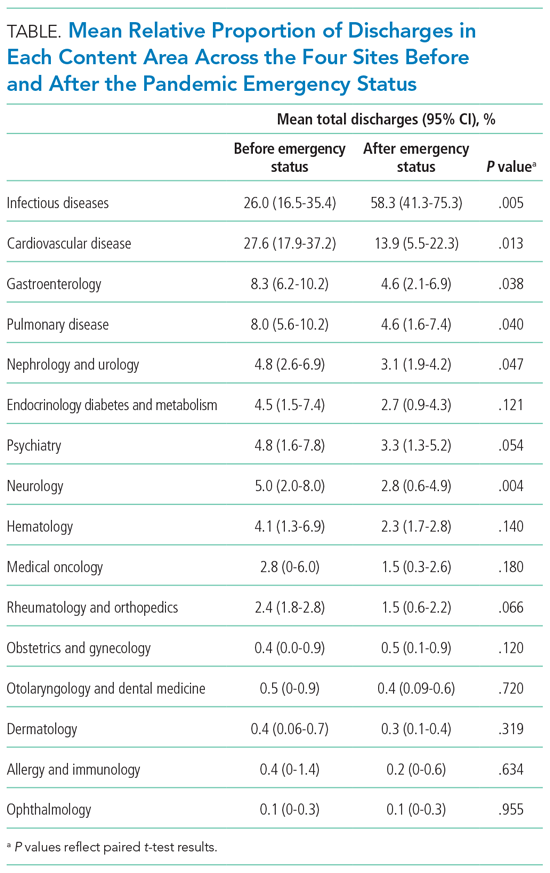
DISCUSSION
Here, we demonstrate how the clinical educational landscape changed for our residents during the COVID-19 pandemic. We uncover a dramatic deviation in the content to which residents were exposed through patient care activities that disproportionately favored ID at the expense of all other content. We demonstrate that this reduction in clinical diversity persisted for nearly 2 months and was similar at each of our training hospitals, and also provide a trajectory on which other content repopulated residents’ clinical experiences.
These data have served several valuable purposes and support ongoing efforts to map residents’ experiential curriculum at our program and others. Sharing this data with residents, as occurred routinely in town hall forums and noon conferences, has provided them with real-time practice feedback during a time of crisis. This has provided scope for their herculean efforts during the pandemic, served as a blueprint for underrepresented content most ripe for self-study, and offered reassurance of a return to normalcy given the trajectory of clinical content curves. As practice habits feedback is an Accreditation Council for Graduate Medical Education requirement, this strategy has also served as a robust and reproducible means of complying.
Our training program used this characterization of clinical content to help guide teaching in the pandemic era. For example, we preferentially structured case conferences and other didactics around reemerging content areas to capitalize on just-in-time education and harness residents’ eagerness for a respite from COVID-specific education. Residents required to quarantine at home were provided with learning plans centered on content underrepresented in clinical practice.
Given the critical importance of experiential learning in IM residents’ training, our findings quantifying significant changes in clinical exposure could form the basis for predicting poor outcomes in competency-based assessments for residents training in the COVID era, which continues to affect our trainees. For example, our characterization of clinical exposure may predict poor in-training exam or even ABIM certification exam performance in the content areas most drastically affected. Knowledge of this association of clinical exposure and clinical competence could allow training programs like ours to preempt poor performance in competency-based assessments by more aggressively shifting lectures, simulations, and other didactic programs toward content areas underrepresented in the pandemic’s wake.
Limitations of this study include the fact that availability of testing and ICD-10 coding for COVID-19 differed slightly across training sites, potentially contributing to site differences in mapping. Additionally, given our 1:1 mapping of ICD-10 codes to content categories, our strategy attributes COVID-19 to ID alone, and does not capture additional areas germane to this diagnosis, such as pulmonary disease.
CONCLUSION
We provide a detailed characterization of the evolution of a single IM program’s patient care experiences across four training hospitals during the COVID-19 pandemic. Such characterization can be leveraged to provide effective practice habits feedback and guide teaching efforts, and could form the basis to predict competency-based outcomes for trainees in the COVID era.
1. Accreditation Council for Graduate Medical Education. ACGME response to pandemic crisis. Accessed April 14, 2021. https://acgme.org/covid-19
2. Manson DK, Shen S, Lavelle MP, et al. Reorganizing a medicine residency program in response to the COVID-19 pandemic in New York. Acad Med. 2020;95(11):1670-1673. https://doi.org/10.1097/ACM.0000000000003548
3. Kee A, Archuleta S, Dan YY. Internal medicine residency training in the COVID-19 era—reflections from Singapore. J Grad Med Educ. 2020;12(4):406-408. https://doi.org/10.4300/JGME-D-20-00315.1
4. Kochis M, Goessling W. Learning during and from a crisis: the student-led development of a COVID-19 curriculum. Acad Med. 2021;96(3):399-401. https://doi.org/10.1097/ACM.0000000000003755
5 . Redinger JW, Cornia PB, Albert TJ. Teaching during a pandemic. J Grad Med Educ. 2020;12(4):403-405. https://doi.org/10.4300/JGME-D-20-00241.1
6. Liang ZC, Ooi SBS, Wang W. Pandemics and their impact on medical training: lessons from Singapore. Acad Med. 2020;95(9):1359-1361. https://doi.org/10.1097/ACM.0000000000003441
7. Tisdale R, Filsoof AR, Singhal S. Novel graduate medical education in the era of a novel virus. J Grad Med Educ. 2020;12(4):409-411. https://doi.org/10.4300/JGME-D-20-00225.1
8. Rhee DW, Chun JW, Stern DT, Sartori DJ. Experience and education in residency training: capturing the resident experience by mapping clinical data. Acad Med. Published online May 11, 2021. https://doi.org/10.1097/ACM.0000000000004162
The COVID-19 pandemic has disrupted the educational experience of medical trainees around the world, and this has been especially true for those in New York City (NYC), the early epicenter of the global outbreak.1 The pandemic’s surge required redeployment of trainees away from scheduled rotations, focused didactics around emerging COVID-19 data, and seemingly narrowed trainees’ clinical exposure to a single respiratory infection.
While there is a small body of literature describing the programmatic responses2,3 and educational adaptations4-7 that have come about as a result of the pandemic’s disruptive force, a characterization of exactly how trainees’ clinical experiences have been affected is lacking. A detailed understanding of how trainees’ inpatient care activities evolved during the pandemic could provide valuable practice habits feedback, allow for comparisons across training sites, focus content selection for didactic learning and self-study, and potentially help forecast similar clinical changes in the event of a subsequent wave. Perhaps most important, as internal medicine (IM) trainees require broad exposure to diverse clinical conditions to mature toward independent practice, a characterization of exactly how the pandemic has narrowed the diversity of clinical exposure could inform changes in how trainees attain clinical competence.
Profiling IM residents’ clinical experiences in a meaningful way is particularly challenging given the extraordinary breadth of the field. We recently developed a strategy by which resident-attributed International Classification of Diseases, Tenth Revision (ICD-10) principal diagnosis codes are mapped to an educational taxonomy of medical content categories, yielding clinical exposure profiles.8 Here, we apply this mapping strategy to all four training hospitals of a large NYC IM residency program to catalogue the evolution of clinical diversity experienced by residents during the COVID-19 pandemic.
METHODS
Study Population
The NYU IM Residency Program comprises 225 resident physicians rotating at four inpatient training sites: NYU Langone Hospital–Brooklyn (NYU-BK), NYU Langone Hospitals–Manhattan (NYU-MN), Bellevue Hospital (BH), and VA–New York Harbor Healthcare (VA). The study period was defined as February 1, 2020, to May 31, 2020, to capture clinical exposure during baseline, surge, and immediate post-surge periods. The NYU IM residency program declared pandemic emergency status on March 23, 2020, after which all residents were assigned to inpatient acute care and intensive care rotations to augment the inpatient workforce.
Data Source
Clinical data at each training hospital are collected and stored, allowing for asynchronous querying. Given differences in data reporting, strategies for collecting principal ICD-10 codes of patients discharged during the study period differed slightly across sites. Principal ICD-10 codes from patients discharged from NYU-BK and NYU-MN were filtered by nursing unit, allowing selection for resident-staffed units. Principal ICD-10 codes from BH were curated by care team, allowing selection for resident-staffed teams. Principal ICD-10 codes from VA were filtered by both hospital unit and provider service to attribute to resident providers. Dates of each discharge were included, and mortalities were included as discharges. All methods yielded a dataset of principal ICD-10 discharge diagnosis codes attributed primarily to IM residents. Given the rapid changes in hospital staffing to care for increasing patient volumes, in rare circumstances residents and other providers (such as advanced practice providers) shared hospital units. While ICD-10 codes mined from each hospital are attributed primarily to residents, this attribution is not entirely exclusive. Data were analyzed both by training site and in aggregate across the four training sites. No individually identifiable data were analyzed, the primary goal of the project was to improve education, and the data were collected as part of a required aspect of training; as a result, this project met criteria for certification as a quality improvement, and not a human subject, research project.
The Crosswalk Tool
We previously developed a crosswalk tool containing 4,854 ICD-10 diagnoses uniquely mapped to 16 broad medical content areas as defined by the American Board of Internal Medicine (ABIM).8 Custom programs (MATLAB, MathWorks, Inc) captured and subsequently mapped resident-attributed ICD-10 discharge codes to content areas if the syntax of the ICD-10 code in question exactly matched or was nested within an ICD-10 code in the crosswalk. This tool allowed us to measure the daily discharge frequency of each content area across the sites.
Analysis
The sensitivity of the crosswalk tool was calculated as the number of ICD-10 codes captured divided by the total number of patients. Codes missed by the tool were excluded. The total number, as well as the 7-day running average of discharges per content area, across the sites during the study period were measured. To evaluate for differences in the distribution of content before and after pandemic emergency status, 2 × 16 χ2 contingency tables were constructed. To evaluate for changes in the mean relative proportions (%) of each content area, paired t tests were conducted. Confidence intervals were estimated from t distributions.
RESULTS
There were 6,613 patients discharged from all sites (NYU-BK, 2,062; NYU-MN, 2,188; BH, 1,711; VA, 652; Appendix Table). The crosswalk tool captured 6,384 principal discharge ICD-10 codes (96.5%). The five most common content areas during the study period were infectious diseases (ID; n = 2,892), cardiovascular disease (CVD; n = 1,199), gastroenterology (n = 406), pulmonary disease (n = 372), and nephrology and urology (n = 252). These were also the content areas most frequently encountered by residents at baseline (Figure and Table). The distribution of content prior to declaration of pandemic emergency status was significantly different than that after declaration (χ2 = 709; df, 15; P <.001). ID diagnoses, driven by COVID-19, rose steeply in the period following declaration, peaked in mid-April, and slowly waned in May (Figure). The mean relative percentage of ID discharges across the sites rose from 26.0% (16.5%-35.4%) at baseline to 58.3% (41.3%-75.3%) in the period after pandemic emergency status was declared (P = .005).

Frequencies of diagnoses mapping to other content areas decreased significantly, reflecting a marked tapering of clinical diversity (Figure and Table). Specifically, decreases were seen in CVD (27.6% [95% CI, 17.9%-37.2%] to 13.9% [95% CI, 5.5%-22.3%]; P = .013); gastroenterology (8.3% [95% CI, 6.2%-10.2%] to 4.6% [95% CI, 2.1%-6.9%]; P = .038); pulmonary disease (8.0% [95% CI, 5.6%-10.2%] to 4.6% [95% CI, 1.6%-7.4%]; P = .040); and nephrology and urology (4.8% [95% CI, 2.6%-6.9%] to 3.1% [95% CI, 1.9%-4.2%]; P = .047) (Table). In late April, diagnoses mapping to these content areas began to repopulate residents’ clinical experiences and by the end of the study period had nearly returned to baseline frequencies. These patterns were similar when discharge diagnoses from each training site were plotted individually (Appendix Figure).

DISCUSSION
Here, we demonstrate how the clinical educational landscape changed for our residents during the COVID-19 pandemic. We uncover a dramatic deviation in the content to which residents were exposed through patient care activities that disproportionately favored ID at the expense of all other content. We demonstrate that this reduction in clinical diversity persisted for nearly 2 months and was similar at each of our training hospitals, and also provide a trajectory on which other content repopulated residents’ clinical experiences.
These data have served several valuable purposes and support ongoing efforts to map residents’ experiential curriculum at our program and others. Sharing this data with residents, as occurred routinely in town hall forums and noon conferences, has provided them with real-time practice feedback during a time of crisis. This has provided scope for their herculean efforts during the pandemic, served as a blueprint for underrepresented content most ripe for self-study, and offered reassurance of a return to normalcy given the trajectory of clinical content curves. As practice habits feedback is an Accreditation Council for Graduate Medical Education requirement, this strategy has also served as a robust and reproducible means of complying.
Our training program used this characterization of clinical content to help guide teaching in the pandemic era. For example, we preferentially structured case conferences and other didactics around reemerging content areas to capitalize on just-in-time education and harness residents’ eagerness for a respite from COVID-specific education. Residents required to quarantine at home were provided with learning plans centered on content underrepresented in clinical practice.
Given the critical importance of experiential learning in IM residents’ training, our findings quantifying significant changes in clinical exposure could form the basis for predicting poor outcomes in competency-based assessments for residents training in the COVID era, which continues to affect our trainees. For example, our characterization of clinical exposure may predict poor in-training exam or even ABIM certification exam performance in the content areas most drastically affected. Knowledge of this association of clinical exposure and clinical competence could allow training programs like ours to preempt poor performance in competency-based assessments by more aggressively shifting lectures, simulations, and other didactic programs toward content areas underrepresented in the pandemic’s wake.
Limitations of this study include the fact that availability of testing and ICD-10 coding for COVID-19 differed slightly across training sites, potentially contributing to site differences in mapping. Additionally, given our 1:1 mapping of ICD-10 codes to content categories, our strategy attributes COVID-19 to ID alone, and does not capture additional areas germane to this diagnosis, such as pulmonary disease.
CONCLUSION
We provide a detailed characterization of the evolution of a single IM program’s patient care experiences across four training hospitals during the COVID-19 pandemic. Such characterization can be leveraged to provide effective practice habits feedback and guide teaching efforts, and could form the basis to predict competency-based outcomes for trainees in the COVID era.
The COVID-19 pandemic has disrupted the educational experience of medical trainees around the world, and this has been especially true for those in New York City (NYC), the early epicenter of the global outbreak.1 The pandemic’s surge required redeployment of trainees away from scheduled rotations, focused didactics around emerging COVID-19 data, and seemingly narrowed trainees’ clinical exposure to a single respiratory infection.
While there is a small body of literature describing the programmatic responses2,3 and educational adaptations4-7 that have come about as a result of the pandemic’s disruptive force, a characterization of exactly how trainees’ clinical experiences have been affected is lacking. A detailed understanding of how trainees’ inpatient care activities evolved during the pandemic could provide valuable practice habits feedback, allow for comparisons across training sites, focus content selection for didactic learning and self-study, and potentially help forecast similar clinical changes in the event of a subsequent wave. Perhaps most important, as internal medicine (IM) trainees require broad exposure to diverse clinical conditions to mature toward independent practice, a characterization of exactly how the pandemic has narrowed the diversity of clinical exposure could inform changes in how trainees attain clinical competence.
Profiling IM residents’ clinical experiences in a meaningful way is particularly challenging given the extraordinary breadth of the field. We recently developed a strategy by which resident-attributed International Classification of Diseases, Tenth Revision (ICD-10) principal diagnosis codes are mapped to an educational taxonomy of medical content categories, yielding clinical exposure profiles.8 Here, we apply this mapping strategy to all four training hospitals of a large NYC IM residency program to catalogue the evolution of clinical diversity experienced by residents during the COVID-19 pandemic.
METHODS
Study Population
The NYU IM Residency Program comprises 225 resident physicians rotating at four inpatient training sites: NYU Langone Hospital–Brooklyn (NYU-BK), NYU Langone Hospitals–Manhattan (NYU-MN), Bellevue Hospital (BH), and VA–New York Harbor Healthcare (VA). The study period was defined as February 1, 2020, to May 31, 2020, to capture clinical exposure during baseline, surge, and immediate post-surge periods. The NYU IM residency program declared pandemic emergency status on March 23, 2020, after which all residents were assigned to inpatient acute care and intensive care rotations to augment the inpatient workforce.
Data Source
Clinical data at each training hospital are collected and stored, allowing for asynchronous querying. Given differences in data reporting, strategies for collecting principal ICD-10 codes of patients discharged during the study period differed slightly across sites. Principal ICD-10 codes from patients discharged from NYU-BK and NYU-MN were filtered by nursing unit, allowing selection for resident-staffed units. Principal ICD-10 codes from BH were curated by care team, allowing selection for resident-staffed teams. Principal ICD-10 codes from VA were filtered by both hospital unit and provider service to attribute to resident providers. Dates of each discharge were included, and mortalities were included as discharges. All methods yielded a dataset of principal ICD-10 discharge diagnosis codes attributed primarily to IM residents. Given the rapid changes in hospital staffing to care for increasing patient volumes, in rare circumstances residents and other providers (such as advanced practice providers) shared hospital units. While ICD-10 codes mined from each hospital are attributed primarily to residents, this attribution is not entirely exclusive. Data were analyzed both by training site and in aggregate across the four training sites. No individually identifiable data were analyzed, the primary goal of the project was to improve education, and the data were collected as part of a required aspect of training; as a result, this project met criteria for certification as a quality improvement, and not a human subject, research project.
The Crosswalk Tool
We previously developed a crosswalk tool containing 4,854 ICD-10 diagnoses uniquely mapped to 16 broad medical content areas as defined by the American Board of Internal Medicine (ABIM).8 Custom programs (MATLAB, MathWorks, Inc) captured and subsequently mapped resident-attributed ICD-10 discharge codes to content areas if the syntax of the ICD-10 code in question exactly matched or was nested within an ICD-10 code in the crosswalk. This tool allowed us to measure the daily discharge frequency of each content area across the sites.
Analysis
The sensitivity of the crosswalk tool was calculated as the number of ICD-10 codes captured divided by the total number of patients. Codes missed by the tool were excluded. The total number, as well as the 7-day running average of discharges per content area, across the sites during the study period were measured. To evaluate for differences in the distribution of content before and after pandemic emergency status, 2 × 16 χ2 contingency tables were constructed. To evaluate for changes in the mean relative proportions (%) of each content area, paired t tests were conducted. Confidence intervals were estimated from t distributions.
RESULTS
There were 6,613 patients discharged from all sites (NYU-BK, 2,062; NYU-MN, 2,188; BH, 1,711; VA, 652; Appendix Table). The crosswalk tool captured 6,384 principal discharge ICD-10 codes (96.5%). The five most common content areas during the study period were infectious diseases (ID; n = 2,892), cardiovascular disease (CVD; n = 1,199), gastroenterology (n = 406), pulmonary disease (n = 372), and nephrology and urology (n = 252). These were also the content areas most frequently encountered by residents at baseline (Figure and Table). The distribution of content prior to declaration of pandemic emergency status was significantly different than that after declaration (χ2 = 709; df, 15; P <.001). ID diagnoses, driven by COVID-19, rose steeply in the period following declaration, peaked in mid-April, and slowly waned in May (Figure). The mean relative percentage of ID discharges across the sites rose from 26.0% (16.5%-35.4%) at baseline to 58.3% (41.3%-75.3%) in the period after pandemic emergency status was declared (P = .005).

Frequencies of diagnoses mapping to other content areas decreased significantly, reflecting a marked tapering of clinical diversity (Figure and Table). Specifically, decreases were seen in CVD (27.6% [95% CI, 17.9%-37.2%] to 13.9% [95% CI, 5.5%-22.3%]; P = .013); gastroenterology (8.3% [95% CI, 6.2%-10.2%] to 4.6% [95% CI, 2.1%-6.9%]; P = .038); pulmonary disease (8.0% [95% CI, 5.6%-10.2%] to 4.6% [95% CI, 1.6%-7.4%]; P = .040); and nephrology and urology (4.8% [95% CI, 2.6%-6.9%] to 3.1% [95% CI, 1.9%-4.2%]; P = .047) (Table). In late April, diagnoses mapping to these content areas began to repopulate residents’ clinical experiences and by the end of the study period had nearly returned to baseline frequencies. These patterns were similar when discharge diagnoses from each training site were plotted individually (Appendix Figure).

DISCUSSION
Here, we demonstrate how the clinical educational landscape changed for our residents during the COVID-19 pandemic. We uncover a dramatic deviation in the content to which residents were exposed through patient care activities that disproportionately favored ID at the expense of all other content. We demonstrate that this reduction in clinical diversity persisted for nearly 2 months and was similar at each of our training hospitals, and also provide a trajectory on which other content repopulated residents’ clinical experiences.
These data have served several valuable purposes and support ongoing efforts to map residents’ experiential curriculum at our program and others. Sharing this data with residents, as occurred routinely in town hall forums and noon conferences, has provided them with real-time practice feedback during a time of crisis. This has provided scope for their herculean efforts during the pandemic, served as a blueprint for underrepresented content most ripe for self-study, and offered reassurance of a return to normalcy given the trajectory of clinical content curves. As practice habits feedback is an Accreditation Council for Graduate Medical Education requirement, this strategy has also served as a robust and reproducible means of complying.
Our training program used this characterization of clinical content to help guide teaching in the pandemic era. For example, we preferentially structured case conferences and other didactics around reemerging content areas to capitalize on just-in-time education and harness residents’ eagerness for a respite from COVID-specific education. Residents required to quarantine at home were provided with learning plans centered on content underrepresented in clinical practice.
Given the critical importance of experiential learning in IM residents’ training, our findings quantifying significant changes in clinical exposure could form the basis for predicting poor outcomes in competency-based assessments for residents training in the COVID era, which continues to affect our trainees. For example, our characterization of clinical exposure may predict poor in-training exam or even ABIM certification exam performance in the content areas most drastically affected. Knowledge of this association of clinical exposure and clinical competence could allow training programs like ours to preempt poor performance in competency-based assessments by more aggressively shifting lectures, simulations, and other didactic programs toward content areas underrepresented in the pandemic’s wake.
Limitations of this study include the fact that availability of testing and ICD-10 coding for COVID-19 differed slightly across training sites, potentially contributing to site differences in mapping. Additionally, given our 1:1 mapping of ICD-10 codes to content categories, our strategy attributes COVID-19 to ID alone, and does not capture additional areas germane to this diagnosis, such as pulmonary disease.
CONCLUSION
We provide a detailed characterization of the evolution of a single IM program’s patient care experiences across four training hospitals during the COVID-19 pandemic. Such characterization can be leveraged to provide effective practice habits feedback and guide teaching efforts, and could form the basis to predict competency-based outcomes for trainees in the COVID era.
1. Accreditation Council for Graduate Medical Education. ACGME response to pandemic crisis. Accessed April 14, 2021. https://acgme.org/covid-19
2. Manson DK, Shen S, Lavelle MP, et al. Reorganizing a medicine residency program in response to the COVID-19 pandemic in New York. Acad Med. 2020;95(11):1670-1673. https://doi.org/10.1097/ACM.0000000000003548
3. Kee A, Archuleta S, Dan YY. Internal medicine residency training in the COVID-19 era—reflections from Singapore. J Grad Med Educ. 2020;12(4):406-408. https://doi.org/10.4300/JGME-D-20-00315.1
4. Kochis M, Goessling W. Learning during and from a crisis: the student-led development of a COVID-19 curriculum. Acad Med. 2021;96(3):399-401. https://doi.org/10.1097/ACM.0000000000003755
5 . Redinger JW, Cornia PB, Albert TJ. Teaching during a pandemic. J Grad Med Educ. 2020;12(4):403-405. https://doi.org/10.4300/JGME-D-20-00241.1
6. Liang ZC, Ooi SBS, Wang W. Pandemics and their impact on medical training: lessons from Singapore. Acad Med. 2020;95(9):1359-1361. https://doi.org/10.1097/ACM.0000000000003441
7. Tisdale R, Filsoof AR, Singhal S. Novel graduate medical education in the era of a novel virus. J Grad Med Educ. 2020;12(4):409-411. https://doi.org/10.4300/JGME-D-20-00225.1
8. Rhee DW, Chun JW, Stern DT, Sartori DJ. Experience and education in residency training: capturing the resident experience by mapping clinical data. Acad Med. Published online May 11, 2021. https://doi.org/10.1097/ACM.0000000000004162
1. Accreditation Council for Graduate Medical Education. ACGME response to pandemic crisis. Accessed April 14, 2021. https://acgme.org/covid-19
2. Manson DK, Shen S, Lavelle MP, et al. Reorganizing a medicine residency program in response to the COVID-19 pandemic in New York. Acad Med. 2020;95(11):1670-1673. https://doi.org/10.1097/ACM.0000000000003548
3. Kee A, Archuleta S, Dan YY. Internal medicine residency training in the COVID-19 era—reflections from Singapore. J Grad Med Educ. 2020;12(4):406-408. https://doi.org/10.4300/JGME-D-20-00315.1
4. Kochis M, Goessling W. Learning during and from a crisis: the student-led development of a COVID-19 curriculum. Acad Med. 2021;96(3):399-401. https://doi.org/10.1097/ACM.0000000000003755
5 . Redinger JW, Cornia PB, Albert TJ. Teaching during a pandemic. J Grad Med Educ. 2020;12(4):403-405. https://doi.org/10.4300/JGME-D-20-00241.1
6. Liang ZC, Ooi SBS, Wang W. Pandemics and their impact on medical training: lessons from Singapore. Acad Med. 2020;95(9):1359-1361. https://doi.org/10.1097/ACM.0000000000003441
7. Tisdale R, Filsoof AR, Singhal S. Novel graduate medical education in the era of a novel virus. J Grad Med Educ. 2020;12(4):409-411. https://doi.org/10.4300/JGME-D-20-00225.1
8. Rhee DW, Chun JW, Stern DT, Sartori DJ. Experience and education in residency training: capturing the resident experience by mapping clinical data. Acad Med. Published online May 11, 2021. https://doi.org/10.1097/ACM.0000000000004162
© 2021 Society of Hospital Medicine
Impact of a Hospitalist-Run Procedure Service on Time to Paracentesis and Length of Stay
Peritoneal fluid examination is often recommended for hospitalized patients with ascites.1
Internal medicine residency programs are establishing procedure services to address concerns about resident training in procedures and patient safety.
METHODS
An inpatient hospitalist-run procedure service was introduced on July 1, 2016. The service was staffed by a hospitalist and second-year internal medicine residents. The service is available 7:00
Data on age, gender, race, ethnicity, date and time of hospital admission, and discharge date and time were retrieved. We also retrieved data on the absolute number of polymorphonuclear leukocytes (PMN) in the peritoneal fluid sample; a patient with a count higher than 250/uL was considered to have SBP. The timestamp for the peritoneal fluid results was used to approximate the A2P time. Paracenteses performed by or under direct supervision of procedure service hospitalists were identified through a procedure log maintained by procedure service hospitalists. We generated a binary variable to differentiate patients who were admitted during the day from those admitted during the night, when the procedure service was not available. For all patients, we calculated the model for end-stage liver disease and sodium (MELD-Na) score.13 Groups performing paracenteses were categorized into procedure service, residents, and radiology. Primary clinical services were categorized into general medicine, gastroenterology, surgery, and others.
Data were summarized as mean (SD) or median (interquartile range) for continuous variables and as percentages for categorical variables. Patients who had paracenteses by radiology or residents during the study period were considered controls. We used concurrent controls to address secular time trends (eg, measures to decrease LOS or changes in ordering tests in the electronic health record) in outcome measures. Patient characteristics were compared using the Wilcoxon rank-sum test or the χ2 test, as appropriate.
Two outcome variables were examined: LOS, and A2P time. Because both outcome variables were right skewed, we used generalized linear models with gamma distribution and log link. The advantage of a generalized linear model approach is that the transformed coefficients are better interpretable than when using the log transformation of the response variable.14 To account for time trends, we included time in months in the model. Models were adjusted for age, gender, race, whether the admission was during day or night, PMN in peritoneal fluid, MELD-Na score, platelet count on the day of procedure, presence or absence of cirrhosis, diagnosis-related groups weight, primary clinical service, and the group performing paracentesis. To address heterogeneity among patients included in our study and the fact that some patients had multiple paracenteses, we conducted sensitivity analyses by excluding all noncirrhotic patients and including only the first paracentesis. A P value less than .05 was considered significant. All statistical analyses were performed using Stata MP 16.0 for Windows (StataCorp LLC).
RESULTS
Of the 1,321 paracenteses included in our study, 509 (38.5%) were performed by the procedure service, 723 (54.7%) by residents, and 89 (6.7%) by radiology.
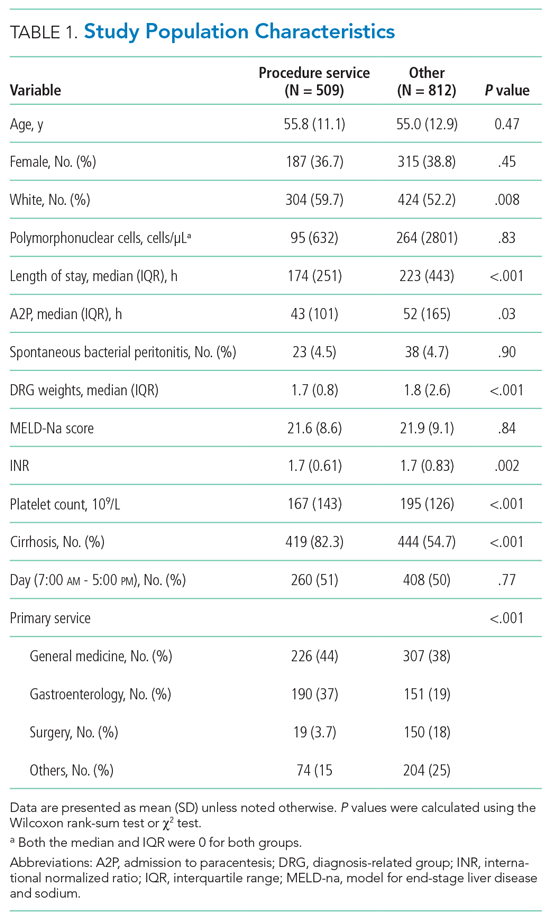
In unadjusted models but accounting for secular time trends, patients who had paracenteses performed by residents or by radiology had a 50% (95% CI, 22%-83%; P = .002) and 127% (95% CI, 65%-211%; P < .001) longer LOS, respectively, than when paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in LOS between radiology and the procedure service remained significant; patients who had a paracentesis performed by radiology had a 27% (95% CI, 2%-58%; P = .03) longer LOS than patients who had the procedure performed by the procedure service. This relative LOS translates into 88 (95% CI, 1-174 hours) additional hours in absolute LOS. There was no difference in LOS between the procedure service and residents in the adjusted analysis (Table 2).

Similarly, in unadjusted models for A2P time and accounting for secular time trends, patients who had a paracentesis performed by residents or by radiology had a 52% (95% CI, 23%-88%; P < .001) and 173% (95% CI, 109%-280%; P < .001) longer A2P time, respectively, than patients whose paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in A2P time between radiology and the procedure service remained significant. Patients who had paracentesis performed by radiology had a 40% (95% CI, 5%-87%; P = .02) longer A2P time than patients who had paracentesis performed by the procedure service. This relative increase translates into 52 (95% CI, 3.3-101 hours) additional hours in absolute A2P time. On the other hand, residents had a significantly shorter A2P time (–19%, 95% CI, –33% to 0.2%; P = .05) (Table 2).
In the sensitivity analysis, excluding noncirrhotic patients and including only the first paracentesis for patients who had multiple procedures performed during admission, the results remained unchanged. In adjusted analysis, patients who had paracentesis performed by radiology had a 47% (95% CI, 3.7%-108%; P = .03) longer LOS and 91% (95% CI, 19%-107%; P = .008) longer A2P time than when paracentesis was performed by the procedure service. There were no differences in LOS or A2P time between the procedure service and residents (Table 2).
DISCUSSION
In this study, we report that a hospitalist-run procedure service, when compared with a radiology service, is associated with decreased LOS and A2P time independent of studied potential confounders and secular time trends. We also showed that, compared with radiology, the A2P time for nonemergent procedures (those performed 6 hours after admission) was not adversely affected by the procedure service. Residents performing paracenteses independently had shorter A2P time than the procedure service.
Although several institutions have bedside procedure services, data are lacking on benefits. Previously, paracenteses performed by residents have been associated with decreased LOS and need for transfusions when compared with radiology.7 Our study extends these findings to show a shortened A2P time. Delays may occur when a patient is referred to radiology because of volume, triaging of higher-acuity procedures, and transportation. Procedure services provide consistent attending supervision, more procedures by upper-level residents, and a lower rate of unsuccessful procedures.12,15 Current study findings support the importance of continuing bedside procedure training for at least those residents who are interested in hospital medicine.7
Our study has several strengths and some potential limitations. The study examined outcomes that are important to patients as well as hospital administrators; it also had a large sample size, spanning 3 years.
CONCLUSION
We found that a hospitalist-run teaching procedure service is associated with shorter LOS and A2P time. Further research is needed to determine if the benefits of a procedure service extend to lowering morbidity and/or mortality, as well as to determine the cost-effectiveness of a procedure service and whether the significant investment by the institution in establishing a procedure service is mitigated by the gains from better patient outcomes and reduced LOS.
1. Runyon BA. AASLD guidelines. Management of adult patients with ascites due to cirrhosis: update 2012. April 2013. https://www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4.pdf
2. Rimola A, García-Tsao G, Navasa M, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32(1):142-153. https://doi.org/10.1016/S0168-8278(00)80201-9
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
4. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. https://doi.org/10.1111/jgh.13255
5. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
6. Chinnock B, Afarian H, Minnigan H, Butler J, Hendey GW. Physician clinical impression does not rule out spontaneous bacterial peritonitis in patients undergoing emergency department paracentesis. Ann Emerg Med. 2008;52(3):268-273. https://doi.org/10.1016/j.annemergmed.2008.02.016
7. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
8. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71.e17-24. https://doi.org/10.1016/j.amjmed.2005.08.007
9. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
10. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors—procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z
11. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018-1026. https://doi.org/10.1056/NEJMoa0801209
14. Lindsey JK, Jones B. Choosing among generalized linear models applied to medical data. Stat Med. 1998;17(1):59-68. https://doi.org/10.1002/(sici)1097-0258(19980115)17:1<59::aid-sim733>3.0.co;2-7
15. Miller R, Garber A, Smith H, Malik M, Kimberly C, Qayyum R. Volume and supervision of resident procedures logged after implementation of a procedure medicine curriculum. J Gen Intern Med. Published online March 17, 2020. https://doi.org/10.1007/s11606-020-05763-9
Peritoneal fluid examination is often recommended for hospitalized patients with ascites.1
Internal medicine residency programs are establishing procedure services to address concerns about resident training in procedures and patient safety.
METHODS
An inpatient hospitalist-run procedure service was introduced on July 1, 2016. The service was staffed by a hospitalist and second-year internal medicine residents. The service is available 7:00
Data on age, gender, race, ethnicity, date and time of hospital admission, and discharge date and time were retrieved. We also retrieved data on the absolute number of polymorphonuclear leukocytes (PMN) in the peritoneal fluid sample; a patient with a count higher than 250/uL was considered to have SBP. The timestamp for the peritoneal fluid results was used to approximate the A2P time. Paracenteses performed by or under direct supervision of procedure service hospitalists were identified through a procedure log maintained by procedure service hospitalists. We generated a binary variable to differentiate patients who were admitted during the day from those admitted during the night, when the procedure service was not available. For all patients, we calculated the model for end-stage liver disease and sodium (MELD-Na) score.13 Groups performing paracenteses were categorized into procedure service, residents, and radiology. Primary clinical services were categorized into general medicine, gastroenterology, surgery, and others.
Data were summarized as mean (SD) or median (interquartile range) for continuous variables and as percentages for categorical variables. Patients who had paracenteses by radiology or residents during the study period were considered controls. We used concurrent controls to address secular time trends (eg, measures to decrease LOS or changes in ordering tests in the electronic health record) in outcome measures. Patient characteristics were compared using the Wilcoxon rank-sum test or the χ2 test, as appropriate.
Two outcome variables were examined: LOS, and A2P time. Because both outcome variables were right skewed, we used generalized linear models with gamma distribution and log link. The advantage of a generalized linear model approach is that the transformed coefficients are better interpretable than when using the log transformation of the response variable.14 To account for time trends, we included time in months in the model. Models were adjusted for age, gender, race, whether the admission was during day or night, PMN in peritoneal fluid, MELD-Na score, platelet count on the day of procedure, presence or absence of cirrhosis, diagnosis-related groups weight, primary clinical service, and the group performing paracentesis. To address heterogeneity among patients included in our study and the fact that some patients had multiple paracenteses, we conducted sensitivity analyses by excluding all noncirrhotic patients and including only the first paracentesis. A P value less than .05 was considered significant. All statistical analyses were performed using Stata MP 16.0 for Windows (StataCorp LLC).
RESULTS
Of the 1,321 paracenteses included in our study, 509 (38.5%) were performed by the procedure service, 723 (54.7%) by residents, and 89 (6.7%) by radiology.

In unadjusted models but accounting for secular time trends, patients who had paracenteses performed by residents or by radiology had a 50% (95% CI, 22%-83%; P = .002) and 127% (95% CI, 65%-211%; P < .001) longer LOS, respectively, than when paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in LOS between radiology and the procedure service remained significant; patients who had a paracentesis performed by radiology had a 27% (95% CI, 2%-58%; P = .03) longer LOS than patients who had the procedure performed by the procedure service. This relative LOS translates into 88 (95% CI, 1-174 hours) additional hours in absolute LOS. There was no difference in LOS between the procedure service and residents in the adjusted analysis (Table 2).

Similarly, in unadjusted models for A2P time and accounting for secular time trends, patients who had a paracentesis performed by residents or by radiology had a 52% (95% CI, 23%-88%; P < .001) and 173% (95% CI, 109%-280%; P < .001) longer A2P time, respectively, than patients whose paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in A2P time between radiology and the procedure service remained significant. Patients who had paracentesis performed by radiology had a 40% (95% CI, 5%-87%; P = .02) longer A2P time than patients who had paracentesis performed by the procedure service. This relative increase translates into 52 (95% CI, 3.3-101 hours) additional hours in absolute A2P time. On the other hand, residents had a significantly shorter A2P time (–19%, 95% CI, –33% to 0.2%; P = .05) (Table 2).
In the sensitivity analysis, excluding noncirrhotic patients and including only the first paracentesis for patients who had multiple procedures performed during admission, the results remained unchanged. In adjusted analysis, patients who had paracentesis performed by radiology had a 47% (95% CI, 3.7%-108%; P = .03) longer LOS and 91% (95% CI, 19%-107%; P = .008) longer A2P time than when paracentesis was performed by the procedure service. There were no differences in LOS or A2P time between the procedure service and residents (Table 2).
DISCUSSION
In this study, we report that a hospitalist-run procedure service, when compared with a radiology service, is associated with decreased LOS and A2P time independent of studied potential confounders and secular time trends. We also showed that, compared with radiology, the A2P time for nonemergent procedures (those performed 6 hours after admission) was not adversely affected by the procedure service. Residents performing paracenteses independently had shorter A2P time than the procedure service.
Although several institutions have bedside procedure services, data are lacking on benefits. Previously, paracenteses performed by residents have been associated with decreased LOS and need for transfusions when compared with radiology.7 Our study extends these findings to show a shortened A2P time. Delays may occur when a patient is referred to radiology because of volume, triaging of higher-acuity procedures, and transportation. Procedure services provide consistent attending supervision, more procedures by upper-level residents, and a lower rate of unsuccessful procedures.12,15 Current study findings support the importance of continuing bedside procedure training for at least those residents who are interested in hospital medicine.7
Our study has several strengths and some potential limitations. The study examined outcomes that are important to patients as well as hospital administrators; it also had a large sample size, spanning 3 years.
CONCLUSION
We found that a hospitalist-run teaching procedure service is associated with shorter LOS and A2P time. Further research is needed to determine if the benefits of a procedure service extend to lowering morbidity and/or mortality, as well as to determine the cost-effectiveness of a procedure service and whether the significant investment by the institution in establishing a procedure service is mitigated by the gains from better patient outcomes and reduced LOS.
Peritoneal fluid examination is often recommended for hospitalized patients with ascites.1
Internal medicine residency programs are establishing procedure services to address concerns about resident training in procedures and patient safety.
METHODS
An inpatient hospitalist-run procedure service was introduced on July 1, 2016. The service was staffed by a hospitalist and second-year internal medicine residents. The service is available 7:00
Data on age, gender, race, ethnicity, date and time of hospital admission, and discharge date and time were retrieved. We also retrieved data on the absolute number of polymorphonuclear leukocytes (PMN) in the peritoneal fluid sample; a patient with a count higher than 250/uL was considered to have SBP. The timestamp for the peritoneal fluid results was used to approximate the A2P time. Paracenteses performed by or under direct supervision of procedure service hospitalists were identified through a procedure log maintained by procedure service hospitalists. We generated a binary variable to differentiate patients who were admitted during the day from those admitted during the night, when the procedure service was not available. For all patients, we calculated the model for end-stage liver disease and sodium (MELD-Na) score.13 Groups performing paracenteses were categorized into procedure service, residents, and radiology. Primary clinical services were categorized into general medicine, gastroenterology, surgery, and others.
Data were summarized as mean (SD) or median (interquartile range) for continuous variables and as percentages for categorical variables. Patients who had paracenteses by radiology or residents during the study period were considered controls. We used concurrent controls to address secular time trends (eg, measures to decrease LOS or changes in ordering tests in the electronic health record) in outcome measures. Patient characteristics were compared using the Wilcoxon rank-sum test or the χ2 test, as appropriate.
Two outcome variables were examined: LOS, and A2P time. Because both outcome variables were right skewed, we used generalized linear models with gamma distribution and log link. The advantage of a generalized linear model approach is that the transformed coefficients are better interpretable than when using the log transformation of the response variable.14 To account for time trends, we included time in months in the model. Models were adjusted for age, gender, race, whether the admission was during day or night, PMN in peritoneal fluid, MELD-Na score, platelet count on the day of procedure, presence or absence of cirrhosis, diagnosis-related groups weight, primary clinical service, and the group performing paracentesis. To address heterogeneity among patients included in our study and the fact that some patients had multiple paracenteses, we conducted sensitivity analyses by excluding all noncirrhotic patients and including only the first paracentesis. A P value less than .05 was considered significant. All statistical analyses were performed using Stata MP 16.0 for Windows (StataCorp LLC).
RESULTS
Of the 1,321 paracenteses included in our study, 509 (38.5%) were performed by the procedure service, 723 (54.7%) by residents, and 89 (6.7%) by radiology.

In unadjusted models but accounting for secular time trends, patients who had paracenteses performed by residents or by radiology had a 50% (95% CI, 22%-83%; P = .002) and 127% (95% CI, 65%-211%; P < .001) longer LOS, respectively, than when paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in LOS between radiology and the procedure service remained significant; patients who had a paracentesis performed by radiology had a 27% (95% CI, 2%-58%; P = .03) longer LOS than patients who had the procedure performed by the procedure service. This relative LOS translates into 88 (95% CI, 1-174 hours) additional hours in absolute LOS. There was no difference in LOS between the procedure service and residents in the adjusted analysis (Table 2).

Similarly, in unadjusted models for A2P time and accounting for secular time trends, patients who had a paracentesis performed by residents or by radiology had a 52% (95% CI, 23%-88%; P < .001) and 173% (95% CI, 109%-280%; P < .001) longer A2P time, respectively, than patients whose paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in A2P time between radiology and the procedure service remained significant. Patients who had paracentesis performed by radiology had a 40% (95% CI, 5%-87%; P = .02) longer A2P time than patients who had paracentesis performed by the procedure service. This relative increase translates into 52 (95% CI, 3.3-101 hours) additional hours in absolute A2P time. On the other hand, residents had a significantly shorter A2P time (–19%, 95% CI, –33% to 0.2%; P = .05) (Table 2).
In the sensitivity analysis, excluding noncirrhotic patients and including only the first paracentesis for patients who had multiple procedures performed during admission, the results remained unchanged. In adjusted analysis, patients who had paracentesis performed by radiology had a 47% (95% CI, 3.7%-108%; P = .03) longer LOS and 91% (95% CI, 19%-107%; P = .008) longer A2P time than when paracentesis was performed by the procedure service. There were no differences in LOS or A2P time between the procedure service and residents (Table 2).
DISCUSSION
In this study, we report that a hospitalist-run procedure service, when compared with a radiology service, is associated with decreased LOS and A2P time independent of studied potential confounders and secular time trends. We also showed that, compared with radiology, the A2P time for nonemergent procedures (those performed 6 hours after admission) was not adversely affected by the procedure service. Residents performing paracenteses independently had shorter A2P time than the procedure service.
Although several institutions have bedside procedure services, data are lacking on benefits. Previously, paracenteses performed by residents have been associated with decreased LOS and need for transfusions when compared with radiology.7 Our study extends these findings to show a shortened A2P time. Delays may occur when a patient is referred to radiology because of volume, triaging of higher-acuity procedures, and transportation. Procedure services provide consistent attending supervision, more procedures by upper-level residents, and a lower rate of unsuccessful procedures.12,15 Current study findings support the importance of continuing bedside procedure training for at least those residents who are interested in hospital medicine.7
Our study has several strengths and some potential limitations. The study examined outcomes that are important to patients as well as hospital administrators; it also had a large sample size, spanning 3 years.
CONCLUSION
We found that a hospitalist-run teaching procedure service is associated with shorter LOS and A2P time. Further research is needed to determine if the benefits of a procedure service extend to lowering morbidity and/or mortality, as well as to determine the cost-effectiveness of a procedure service and whether the significant investment by the institution in establishing a procedure service is mitigated by the gains from better patient outcomes and reduced LOS.
1. Runyon BA. AASLD guidelines. Management of adult patients with ascites due to cirrhosis: update 2012. April 2013. https://www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4.pdf
2. Rimola A, García-Tsao G, Navasa M, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32(1):142-153. https://doi.org/10.1016/S0168-8278(00)80201-9
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
4. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. https://doi.org/10.1111/jgh.13255
5. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
6. Chinnock B, Afarian H, Minnigan H, Butler J, Hendey GW. Physician clinical impression does not rule out spontaneous bacterial peritonitis in patients undergoing emergency department paracentesis. Ann Emerg Med. 2008;52(3):268-273. https://doi.org/10.1016/j.annemergmed.2008.02.016
7. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
8. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71.e17-24. https://doi.org/10.1016/j.amjmed.2005.08.007
9. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
10. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors—procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z
11. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018-1026. https://doi.org/10.1056/NEJMoa0801209
14. Lindsey JK, Jones B. Choosing among generalized linear models applied to medical data. Stat Med. 1998;17(1):59-68. https://doi.org/10.1002/(sici)1097-0258(19980115)17:1<59::aid-sim733>3.0.co;2-7
15. Miller R, Garber A, Smith H, Malik M, Kimberly C, Qayyum R. Volume and supervision of resident procedures logged after implementation of a procedure medicine curriculum. J Gen Intern Med. Published online March 17, 2020. https://doi.org/10.1007/s11606-020-05763-9
1. Runyon BA. AASLD guidelines. Management of adult patients with ascites due to cirrhosis: update 2012. April 2013. https://www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4.pdf
2. Rimola A, García-Tsao G, Navasa M, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32(1):142-153. https://doi.org/10.1016/S0168-8278(00)80201-9
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
4. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. https://doi.org/10.1111/jgh.13255
5. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
6. Chinnock B, Afarian H, Minnigan H, Butler J, Hendey GW. Physician clinical impression does not rule out spontaneous bacterial peritonitis in patients undergoing emergency department paracentesis. Ann Emerg Med. 2008;52(3):268-273. https://doi.org/10.1016/j.annemergmed.2008.02.016
7. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
8. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71.e17-24. https://doi.org/10.1016/j.amjmed.2005.08.007
9. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
10. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors—procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z
11. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018-1026. https://doi.org/10.1056/NEJMoa0801209
14. Lindsey JK, Jones B. Choosing among generalized linear models applied to medical data. Stat Med. 1998;17(1):59-68. https://doi.org/10.1002/(sici)1097-0258(19980115)17:1<59::aid-sim733>3.0.co;2-7
15. Miller R, Garber A, Smith H, Malik M, Kimberly C, Qayyum R. Volume and supervision of resident procedures logged after implementation of a procedure medicine curriculum. J Gen Intern Med. Published online March 17, 2020. https://doi.org/10.1007/s11606-020-05763-9
© Society of Hospital Medicine






