User login
SHM Converge Daily News -- Day 1
Click here for the Wednesday issue of the SHM Converge Daily News newsletter.
Click here for the Wednesday issue of the SHM Converge Daily News newsletter.
Click here for the Wednesday issue of the SHM Converge Daily News newsletter.
CML-CP: Bosutinib outscores imatinib as a frontline treatment option in Asian subpopulation
Key clinical point: Bosutinib was more effective than imatinib as first-line treatment of newly diagnosed chronic-phase chronic myeloid leukemia (CML-CP) with manageable toxicity, particularly in the Asian subpopulation of BFORE trial.
Major finding: Rates of major molecular response favored bosutinib vs. imatinib, particularly more in Asian (odds ratio [OR], 2.28; 95% confidence interval [CI], 0.87-5.97) than non-Asian (OR, 1.43; 95% CI, 0.99-2.07) subpopulation. Grade 3/4 treatment-emergent adverse events were more frequent with bosutinib vs. imatinib (72.7% vs. 36.4%) but were manageable.
Study details: This study evaluated 536 patients (Asian, n=67; non-Asian, n=469) with newly diagnosed CML-CP from the phase 3 BFORE trial randomly allocated to either bosutinib or imatinib.
Disclosures: This study was sponsored by Pfizer. The lead author and some of his coinvestigators reported advisory roles, speaker fees, owning stock in, being an employee of, receiving support from, and/or consulting for various pharmaceutical companies, including Pfizer.
Source: Chuah C et al. Int J Hematol. 2021 Apr 13. doi: 10.1007/s12185-021-03144-4.
Key clinical point: Bosutinib was more effective than imatinib as first-line treatment of newly diagnosed chronic-phase chronic myeloid leukemia (CML-CP) with manageable toxicity, particularly in the Asian subpopulation of BFORE trial.
Major finding: Rates of major molecular response favored bosutinib vs. imatinib, particularly more in Asian (odds ratio [OR], 2.28; 95% confidence interval [CI], 0.87-5.97) than non-Asian (OR, 1.43; 95% CI, 0.99-2.07) subpopulation. Grade 3/4 treatment-emergent adverse events were more frequent with bosutinib vs. imatinib (72.7% vs. 36.4%) but were manageable.
Study details: This study evaluated 536 patients (Asian, n=67; non-Asian, n=469) with newly diagnosed CML-CP from the phase 3 BFORE trial randomly allocated to either bosutinib or imatinib.
Disclosures: This study was sponsored by Pfizer. The lead author and some of his coinvestigators reported advisory roles, speaker fees, owning stock in, being an employee of, receiving support from, and/or consulting for various pharmaceutical companies, including Pfizer.
Source: Chuah C et al. Int J Hematol. 2021 Apr 13. doi: 10.1007/s12185-021-03144-4.
Key clinical point: Bosutinib was more effective than imatinib as first-line treatment of newly diagnosed chronic-phase chronic myeloid leukemia (CML-CP) with manageable toxicity, particularly in the Asian subpopulation of BFORE trial.
Major finding: Rates of major molecular response favored bosutinib vs. imatinib, particularly more in Asian (odds ratio [OR], 2.28; 95% confidence interval [CI], 0.87-5.97) than non-Asian (OR, 1.43; 95% CI, 0.99-2.07) subpopulation. Grade 3/4 treatment-emergent adverse events were more frequent with bosutinib vs. imatinib (72.7% vs. 36.4%) but were manageable.
Study details: This study evaluated 536 patients (Asian, n=67; non-Asian, n=469) with newly diagnosed CML-CP from the phase 3 BFORE trial randomly allocated to either bosutinib or imatinib.
Disclosures: This study was sponsored by Pfizer. The lead author and some of his coinvestigators reported advisory roles, speaker fees, owning stock in, being an employee of, receiving support from, and/or consulting for various pharmaceutical companies, including Pfizer.
Source: Chuah C et al. Int J Hematol. 2021 Apr 13. doi: 10.1007/s12185-021-03144-4.
Fatal Case of Levamisole-Induced Vasculopathy in a Cocaine User
To the Editor:
Levamisole is a veterinary anthelmintic drug with immunomodulating properties that was once approved by the US Food and Drug Administration for the treatment of various conditions, including autoimmune diseases, cancer, pediatric kidney disease, and chronic infections.1-4 Levamisole was banned in 2000 after reports of associated agranulocytosis and a characteristic painful purpuric vasculitis.4,5 Despite the ban, its use persists due to its increasing incorporation as an adulterant in cocaine, presumably for its dopaminergic properties that potentiate psychotropic effects.6 In 2009, the Drug Enforcement Administration reported that 69% of seized cocaine in the United States contains this chemical, with an average concentration of 10%.5 Levamisole-induced vasculopathy (LIV) typically resolves following the cessation of cocaine without further treatment necessary. We present a fatal case of LIV to emphasize that early recognition and discontinuation of the offending agent could be lifesaving.
A 40-year-old woman with a history of cocaine abuse was admitted with tender, reticular, purpuric, and erythematous patches and plaques on the lower extremities with areas of necrosis (Figure 1). The lesions had been present intermittently for 6 months. She tried topical mupirocin and oral amoxicillin clavulanate without improvement. She also described polyarthralgia in the hands, but the remainder of the review of symptoms and physical examination was negative.
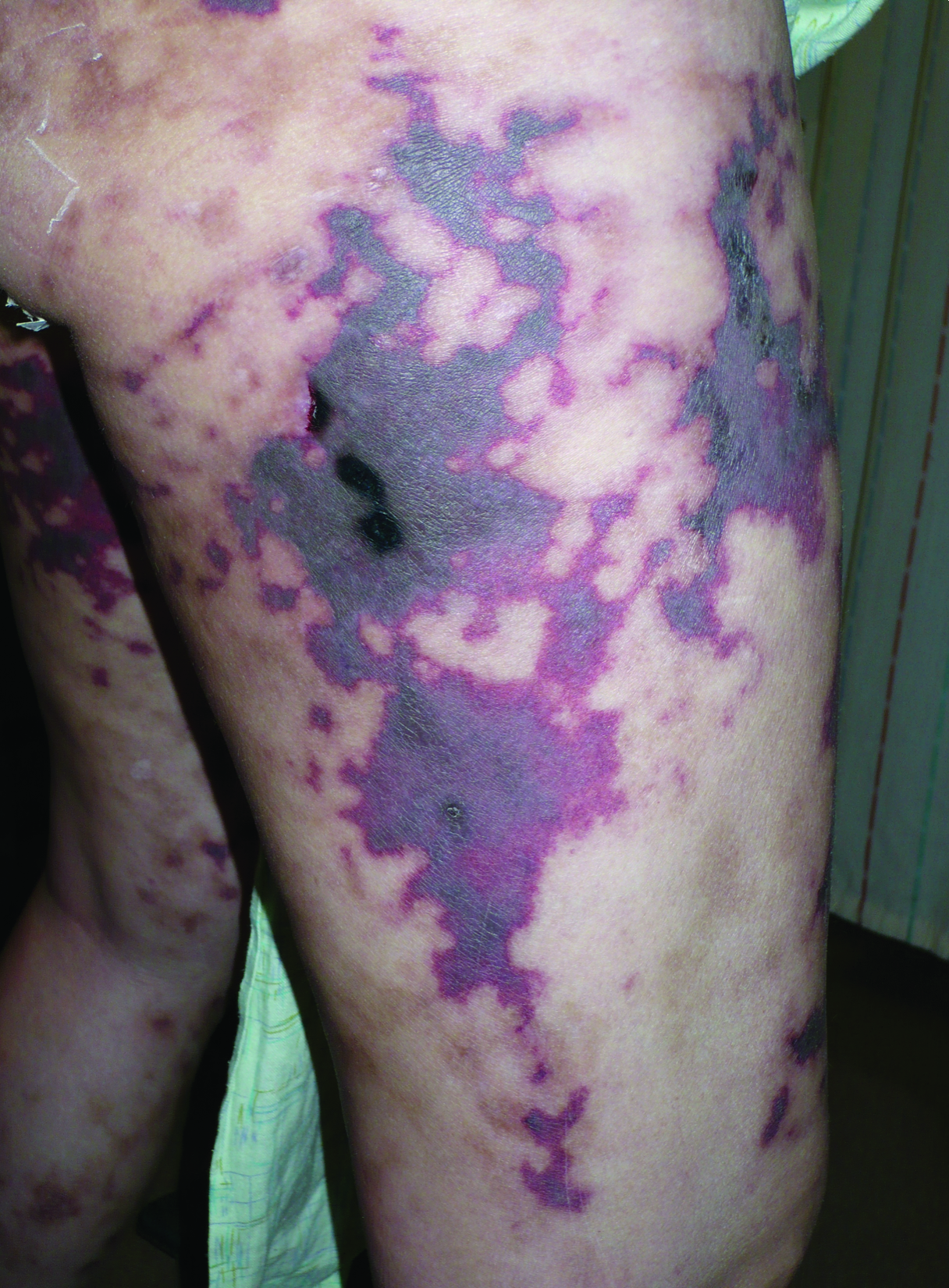
Coagulation studies and white blood cell counts were within reference range. A urine toxicology screen was positive for cocaine; however, urine testing for levamisole was not performed given the short half-life of levamisole in vivo. A biopsy of one of the skin lesions on the right thigh showed pauci-inflammatory superficial and deep vein thrombosis with recanalization (Figure 2). A rheumatology workup revealed an elevated C-reactive protein level, low C3, positive antinuclear antibody, positive anti–double-stranded DNA, positive anticardiolipin antibody, positive lupus anticoagulant, and positive perinuclear antineutrophil cytoplasmic antibody (ANCA). Tests for HIV, hepatitis B and C, cryoglobulinemia, and cytomegalovirus were negative. Given the clinical picture and laboratory findings, levamisole-induced vasculitis was deemed likely. The patient was treated with appropriate skin and wound care. She was discharged with a prednisone taper and oral cephalexin and was counseled on cocaine cessation.

Five months later, the patient was readmitted for lower extremity edema and worsening painful lesions that had progressed to involve the legs, thighs, buttocks, flanks, and the tip of her nose. A deep vein thrombosis workup was negative. She admitted to ongoing cocaine use that was confirmed with urine toxicology. Coagulation studies and white blood cell counts remained within reference range. Repeat skin biopsy was consistent with prior findings, demonstrating thrombosis of superficial and deep vessels with recanalization. In addition, it showed focal epidermal necrosis and a perivascular infiltrate of lymphocytes, histiocytes, and rare neutrophils. She was placed on high-dose methylprednisolone. Over the course of the next month, her urine continued to test positive for cocaine, and she developed necrotizing fasciitis necessitating lower extremity amputation, abdominal washout, and debridement. She quickly deteriorated, developing multiorgan failure with sepsis, leading to death. Of note, the patient was never found to have neutropenia or agranulocytosis throughout the disease course.
Because levamisole is no longer in clinical use, reports of its adverse effects come exclusively from users of cocaine, whether via smoking or snorting. Levamisole-induced vasculopathy typically is painful and purpuric, with or without necrosis, in a retiform or stellate pattern and commonly involves the extremities, trunk, face, and external ears.7 The average age of presentation is 43 years and it more commonly is seen in women.8
Levamisole-induced vasculopathy remains a diagnosis of exclusion, so it is important to rule out other treatable causes. The differential diagnosis for purpura associated with vasculitis also includes other antineutrophilic cytoplasmic–associated vasculitides (eg, granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis), infectious purpura fulminans, antiphospholipid syndrome, cryoglobulinemia, and disseminated intravascular coagulation.9 In LIV patients, perinuclear ANCAs are present in up to 90% of cases, and cytoplasmic ANCAs in 19% to 59% of cases.10,11 Although leukopenia and neutropenia complicate approximately 60% of LIV cases, they are not required to make the diagnosis.11,12 Elevated erythrocyte sedimentation rate, normal coagulation studies, and positive antineutrophil antibodies and lupus anticoagulant further aid in the diagnosis.8 Urine should be tested for cocaine in suspected patients. Urine also can be tested for levamisole, which is challenging because of the short half-life of 5.6 hours. Only 2% to 5% of levamisole is excreted unchanged in the urine, and testing requires gas chromatography and mass spectrometry that was not readily available to perform on our patient.7 In addition to laboratory and urine studies, hair strand testing,10 skin biopsy, and histologic findings also can be used to support the diagnosis.
The pathogenesis of LIV is not completely understood, but it is thought to be an immune complex–mediated process based on immunofluorescence studies in the skin.13,14 Classic pathologic findings include multiple fibrin thrombi within small vessels in the superficial and deep dermis, leukocytoclastic vasculitis of small vessels consisting of fibrinoid necrosis of the vessel wall, extravasated erythrocytes, karyorrhectic debris, and angiocentric inflammation.14 Direct immunofluorescence is not routinely performed but most commonly demonstrates deposition of IgA, IgM, and C3.14,15
Levamisole-induced vasculopathy usually resolves upon cessation of cocaine use without long-term sequelae. Steroids have been used as treatment of prominent vasculitis with variable success; however, immunosuppressive effects should be closely monitored, especially with inpatients with concurrent granulocytopenia. Broad-spectrum antibiotics have been used in cases with fever and agranulocytosis. Cutaneous lesions typically disappear within 2 to 3 weeks, and serologic markers resolve within 2 to 10 months. Recurrent use of cocaine generally results in recurrent neutropenia and skin eruptions, supporting the causal role. Our patient’s recurrent prolonged cocaine use with vasculopathy was assumed to be the source of the necrotizing fasciitis that led to a cascade of sepsis, rapidly progressing multiorgan failure, and ultimate demise.
Presentation of a purpuric vasculopathy, with or without associated neutropenia and positive autoantibodies, should prompt the consideration of levamisole-contaminated cocaine use in the clinician’s differential. Although the patient may initially deny cocaine use, it is important to keep this diagnosis in mind when the clinical picture fits, and urine toxicology screen should be ordered when there is question. Physicians and patients should be wary of potential complications, even death. Early recognition and discontinuation of the offending agent could be lifesaving.
- Menni S, Pistritto G, Gianotti R, et al. Ear lobe necrosis by levamisole-induced occlusive vasculitis in a pediatric patient. Pediatr Dermatol. 1997;14:477-479.
- Symoens J, Veys E, Mielants M, et al. Adverse reactions to levamisole. Cancer Treat Rep. 1978;62:1721-1730.
- Vogel CL, Silverman MA, Mansell PW, et al. Mechanism of levamisole-induced granulocytopenia in breast cancer patients. Am J Hematol. 1980;9:171-183.
- Rongioletti F, Ghio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol. 2011;30:1385-1392.
- Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2011;35:545-550.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Pearson T, Bremmer M, Cohen J, et al. Vasculopathy related to cocaine adulterated with levamisole: a review of the literature. Dermatol Online J. 2012;18:1.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Chai PR, Bastan W, Machan J, et al. Levamisole exposure and hematologic indices in cocaine users. Acad Emerg Med. 2011;18:1141-1147.
- Lazareth H, Peytavin G, Polivka L, et al. The hairy-print for levamisole-induced vasculitis. BMJ Case Rep. 2012;2012:bcr2012006602.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
- Jenkins J, Babu K, Hsu-Hung E, et al. ANCA-positive necrotizing vasculitis and thrombotic vasculopathy induced by levamisole-adulterated cocaine: a distinctive clinicopathologic presentation. J Am Acad Dermatol. 2011;65:E14-E16.
To the Editor:
Levamisole is a veterinary anthelmintic drug with immunomodulating properties that was once approved by the US Food and Drug Administration for the treatment of various conditions, including autoimmune diseases, cancer, pediatric kidney disease, and chronic infections.1-4 Levamisole was banned in 2000 after reports of associated agranulocytosis and a characteristic painful purpuric vasculitis.4,5 Despite the ban, its use persists due to its increasing incorporation as an adulterant in cocaine, presumably for its dopaminergic properties that potentiate psychotropic effects.6 In 2009, the Drug Enforcement Administration reported that 69% of seized cocaine in the United States contains this chemical, with an average concentration of 10%.5 Levamisole-induced vasculopathy (LIV) typically resolves following the cessation of cocaine without further treatment necessary. We present a fatal case of LIV to emphasize that early recognition and discontinuation of the offending agent could be lifesaving.
A 40-year-old woman with a history of cocaine abuse was admitted with tender, reticular, purpuric, and erythematous patches and plaques on the lower extremities with areas of necrosis (Figure 1). The lesions had been present intermittently for 6 months. She tried topical mupirocin and oral amoxicillin clavulanate without improvement. She also described polyarthralgia in the hands, but the remainder of the review of symptoms and physical examination was negative.

Coagulation studies and white blood cell counts were within reference range. A urine toxicology screen was positive for cocaine; however, urine testing for levamisole was not performed given the short half-life of levamisole in vivo. A biopsy of one of the skin lesions on the right thigh showed pauci-inflammatory superficial and deep vein thrombosis with recanalization (Figure 2). A rheumatology workup revealed an elevated C-reactive protein level, low C3, positive antinuclear antibody, positive anti–double-stranded DNA, positive anticardiolipin antibody, positive lupus anticoagulant, and positive perinuclear antineutrophil cytoplasmic antibody (ANCA). Tests for HIV, hepatitis B and C, cryoglobulinemia, and cytomegalovirus were negative. Given the clinical picture and laboratory findings, levamisole-induced vasculitis was deemed likely. The patient was treated with appropriate skin and wound care. She was discharged with a prednisone taper and oral cephalexin and was counseled on cocaine cessation.

Five months later, the patient was readmitted for lower extremity edema and worsening painful lesions that had progressed to involve the legs, thighs, buttocks, flanks, and the tip of her nose. A deep vein thrombosis workup was negative. She admitted to ongoing cocaine use that was confirmed with urine toxicology. Coagulation studies and white blood cell counts remained within reference range. Repeat skin biopsy was consistent with prior findings, demonstrating thrombosis of superficial and deep vessels with recanalization. In addition, it showed focal epidermal necrosis and a perivascular infiltrate of lymphocytes, histiocytes, and rare neutrophils. She was placed on high-dose methylprednisolone. Over the course of the next month, her urine continued to test positive for cocaine, and she developed necrotizing fasciitis necessitating lower extremity amputation, abdominal washout, and debridement. She quickly deteriorated, developing multiorgan failure with sepsis, leading to death. Of note, the patient was never found to have neutropenia or agranulocytosis throughout the disease course.
Because levamisole is no longer in clinical use, reports of its adverse effects come exclusively from users of cocaine, whether via smoking or snorting. Levamisole-induced vasculopathy typically is painful and purpuric, with or without necrosis, in a retiform or stellate pattern and commonly involves the extremities, trunk, face, and external ears.7 The average age of presentation is 43 years and it more commonly is seen in women.8
Levamisole-induced vasculopathy remains a diagnosis of exclusion, so it is important to rule out other treatable causes. The differential diagnosis for purpura associated with vasculitis also includes other antineutrophilic cytoplasmic–associated vasculitides (eg, granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis), infectious purpura fulminans, antiphospholipid syndrome, cryoglobulinemia, and disseminated intravascular coagulation.9 In LIV patients, perinuclear ANCAs are present in up to 90% of cases, and cytoplasmic ANCAs in 19% to 59% of cases.10,11 Although leukopenia and neutropenia complicate approximately 60% of LIV cases, they are not required to make the diagnosis.11,12 Elevated erythrocyte sedimentation rate, normal coagulation studies, and positive antineutrophil antibodies and lupus anticoagulant further aid in the diagnosis.8 Urine should be tested for cocaine in suspected patients. Urine also can be tested for levamisole, which is challenging because of the short half-life of 5.6 hours. Only 2% to 5% of levamisole is excreted unchanged in the urine, and testing requires gas chromatography and mass spectrometry that was not readily available to perform on our patient.7 In addition to laboratory and urine studies, hair strand testing,10 skin biopsy, and histologic findings also can be used to support the diagnosis.
The pathogenesis of LIV is not completely understood, but it is thought to be an immune complex–mediated process based on immunofluorescence studies in the skin.13,14 Classic pathologic findings include multiple fibrin thrombi within small vessels in the superficial and deep dermis, leukocytoclastic vasculitis of small vessels consisting of fibrinoid necrosis of the vessel wall, extravasated erythrocytes, karyorrhectic debris, and angiocentric inflammation.14 Direct immunofluorescence is not routinely performed but most commonly demonstrates deposition of IgA, IgM, and C3.14,15
Levamisole-induced vasculopathy usually resolves upon cessation of cocaine use without long-term sequelae. Steroids have been used as treatment of prominent vasculitis with variable success; however, immunosuppressive effects should be closely monitored, especially with inpatients with concurrent granulocytopenia. Broad-spectrum antibiotics have been used in cases with fever and agranulocytosis. Cutaneous lesions typically disappear within 2 to 3 weeks, and serologic markers resolve within 2 to 10 months. Recurrent use of cocaine generally results in recurrent neutropenia and skin eruptions, supporting the causal role. Our patient’s recurrent prolonged cocaine use with vasculopathy was assumed to be the source of the necrotizing fasciitis that led to a cascade of sepsis, rapidly progressing multiorgan failure, and ultimate demise.
Presentation of a purpuric vasculopathy, with or without associated neutropenia and positive autoantibodies, should prompt the consideration of levamisole-contaminated cocaine use in the clinician’s differential. Although the patient may initially deny cocaine use, it is important to keep this diagnosis in mind when the clinical picture fits, and urine toxicology screen should be ordered when there is question. Physicians and patients should be wary of potential complications, even death. Early recognition and discontinuation of the offending agent could be lifesaving.
To the Editor:
Levamisole is a veterinary anthelmintic drug with immunomodulating properties that was once approved by the US Food and Drug Administration for the treatment of various conditions, including autoimmune diseases, cancer, pediatric kidney disease, and chronic infections.1-4 Levamisole was banned in 2000 after reports of associated agranulocytosis and a characteristic painful purpuric vasculitis.4,5 Despite the ban, its use persists due to its increasing incorporation as an adulterant in cocaine, presumably for its dopaminergic properties that potentiate psychotropic effects.6 In 2009, the Drug Enforcement Administration reported that 69% of seized cocaine in the United States contains this chemical, with an average concentration of 10%.5 Levamisole-induced vasculopathy (LIV) typically resolves following the cessation of cocaine without further treatment necessary. We present a fatal case of LIV to emphasize that early recognition and discontinuation of the offending agent could be lifesaving.
A 40-year-old woman with a history of cocaine abuse was admitted with tender, reticular, purpuric, and erythematous patches and plaques on the lower extremities with areas of necrosis (Figure 1). The lesions had been present intermittently for 6 months. She tried topical mupirocin and oral amoxicillin clavulanate without improvement. She also described polyarthralgia in the hands, but the remainder of the review of symptoms and physical examination was negative.

Coagulation studies and white blood cell counts were within reference range. A urine toxicology screen was positive for cocaine; however, urine testing for levamisole was not performed given the short half-life of levamisole in vivo. A biopsy of one of the skin lesions on the right thigh showed pauci-inflammatory superficial and deep vein thrombosis with recanalization (Figure 2). A rheumatology workup revealed an elevated C-reactive protein level, low C3, positive antinuclear antibody, positive anti–double-stranded DNA, positive anticardiolipin antibody, positive lupus anticoagulant, and positive perinuclear antineutrophil cytoplasmic antibody (ANCA). Tests for HIV, hepatitis B and C, cryoglobulinemia, and cytomegalovirus were negative. Given the clinical picture and laboratory findings, levamisole-induced vasculitis was deemed likely. The patient was treated with appropriate skin and wound care. She was discharged with a prednisone taper and oral cephalexin and was counseled on cocaine cessation.

Five months later, the patient was readmitted for lower extremity edema and worsening painful lesions that had progressed to involve the legs, thighs, buttocks, flanks, and the tip of her nose. A deep vein thrombosis workup was negative. She admitted to ongoing cocaine use that was confirmed with urine toxicology. Coagulation studies and white blood cell counts remained within reference range. Repeat skin biopsy was consistent with prior findings, demonstrating thrombosis of superficial and deep vessels with recanalization. In addition, it showed focal epidermal necrosis and a perivascular infiltrate of lymphocytes, histiocytes, and rare neutrophils. She was placed on high-dose methylprednisolone. Over the course of the next month, her urine continued to test positive for cocaine, and she developed necrotizing fasciitis necessitating lower extremity amputation, abdominal washout, and debridement. She quickly deteriorated, developing multiorgan failure with sepsis, leading to death. Of note, the patient was never found to have neutropenia or agranulocytosis throughout the disease course.
Because levamisole is no longer in clinical use, reports of its adverse effects come exclusively from users of cocaine, whether via smoking or snorting. Levamisole-induced vasculopathy typically is painful and purpuric, with or without necrosis, in a retiform or stellate pattern and commonly involves the extremities, trunk, face, and external ears.7 The average age of presentation is 43 years and it more commonly is seen in women.8
Levamisole-induced vasculopathy remains a diagnosis of exclusion, so it is important to rule out other treatable causes. The differential diagnosis for purpura associated with vasculitis also includes other antineutrophilic cytoplasmic–associated vasculitides (eg, granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis), infectious purpura fulminans, antiphospholipid syndrome, cryoglobulinemia, and disseminated intravascular coagulation.9 In LIV patients, perinuclear ANCAs are present in up to 90% of cases, and cytoplasmic ANCAs in 19% to 59% of cases.10,11 Although leukopenia and neutropenia complicate approximately 60% of LIV cases, they are not required to make the diagnosis.11,12 Elevated erythrocyte sedimentation rate, normal coagulation studies, and positive antineutrophil antibodies and lupus anticoagulant further aid in the diagnosis.8 Urine should be tested for cocaine in suspected patients. Urine also can be tested for levamisole, which is challenging because of the short half-life of 5.6 hours. Only 2% to 5% of levamisole is excreted unchanged in the urine, and testing requires gas chromatography and mass spectrometry that was not readily available to perform on our patient.7 In addition to laboratory and urine studies, hair strand testing,10 skin biopsy, and histologic findings also can be used to support the diagnosis.
The pathogenesis of LIV is not completely understood, but it is thought to be an immune complex–mediated process based on immunofluorescence studies in the skin.13,14 Classic pathologic findings include multiple fibrin thrombi within small vessels in the superficial and deep dermis, leukocytoclastic vasculitis of small vessels consisting of fibrinoid necrosis of the vessel wall, extravasated erythrocytes, karyorrhectic debris, and angiocentric inflammation.14 Direct immunofluorescence is not routinely performed but most commonly demonstrates deposition of IgA, IgM, and C3.14,15
Levamisole-induced vasculopathy usually resolves upon cessation of cocaine use without long-term sequelae. Steroids have been used as treatment of prominent vasculitis with variable success; however, immunosuppressive effects should be closely monitored, especially with inpatients with concurrent granulocytopenia. Broad-spectrum antibiotics have been used in cases with fever and agranulocytosis. Cutaneous lesions typically disappear within 2 to 3 weeks, and serologic markers resolve within 2 to 10 months. Recurrent use of cocaine generally results in recurrent neutropenia and skin eruptions, supporting the causal role. Our patient’s recurrent prolonged cocaine use with vasculopathy was assumed to be the source of the necrotizing fasciitis that led to a cascade of sepsis, rapidly progressing multiorgan failure, and ultimate demise.
Presentation of a purpuric vasculopathy, with or without associated neutropenia and positive autoantibodies, should prompt the consideration of levamisole-contaminated cocaine use in the clinician’s differential. Although the patient may initially deny cocaine use, it is important to keep this diagnosis in mind when the clinical picture fits, and urine toxicology screen should be ordered when there is question. Physicians and patients should be wary of potential complications, even death. Early recognition and discontinuation of the offending agent could be lifesaving.
- Menni S, Pistritto G, Gianotti R, et al. Ear lobe necrosis by levamisole-induced occlusive vasculitis in a pediatric patient. Pediatr Dermatol. 1997;14:477-479.
- Symoens J, Veys E, Mielants M, et al. Adverse reactions to levamisole. Cancer Treat Rep. 1978;62:1721-1730.
- Vogel CL, Silverman MA, Mansell PW, et al. Mechanism of levamisole-induced granulocytopenia in breast cancer patients. Am J Hematol. 1980;9:171-183.
- Rongioletti F, Ghio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol. 2011;30:1385-1392.
- Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2011;35:545-550.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Pearson T, Bremmer M, Cohen J, et al. Vasculopathy related to cocaine adulterated with levamisole: a review of the literature. Dermatol Online J. 2012;18:1.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Chai PR, Bastan W, Machan J, et al. Levamisole exposure and hematologic indices in cocaine users. Acad Emerg Med. 2011;18:1141-1147.
- Lazareth H, Peytavin G, Polivka L, et al. The hairy-print for levamisole-induced vasculitis. BMJ Case Rep. 2012;2012:bcr2012006602.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
- Jenkins J, Babu K, Hsu-Hung E, et al. ANCA-positive necrotizing vasculitis and thrombotic vasculopathy induced by levamisole-adulterated cocaine: a distinctive clinicopathologic presentation. J Am Acad Dermatol. 2011;65:E14-E16.
- Menni S, Pistritto G, Gianotti R, et al. Ear lobe necrosis by levamisole-induced occlusive vasculitis in a pediatric patient. Pediatr Dermatol. 1997;14:477-479.
- Symoens J, Veys E, Mielants M, et al. Adverse reactions to levamisole. Cancer Treat Rep. 1978;62:1721-1730.
- Vogel CL, Silverman MA, Mansell PW, et al. Mechanism of levamisole-induced granulocytopenia in breast cancer patients. Am J Hematol. 1980;9:171-183.
- Rongioletti F, Ghio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol. 2011;30:1385-1392.
- Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2011;35:545-550.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Pearson T, Bremmer M, Cohen J, et al. Vasculopathy related to cocaine adulterated with levamisole: a review of the literature. Dermatol Online J. 2012;18:1.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Chai PR, Bastan W, Machan J, et al. Levamisole exposure and hematologic indices in cocaine users. Acad Emerg Med. 2011;18:1141-1147.
- Lazareth H, Peytavin G, Polivka L, et al. The hairy-print for levamisole-induced vasculitis. BMJ Case Rep. 2012;2012:bcr2012006602.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
- Jenkins J, Babu K, Hsu-Hung E, et al. ANCA-positive necrotizing vasculitis and thrombotic vasculopathy induced by levamisole-adulterated cocaine: a distinctive clinicopathologic presentation. J Am Acad Dermatol. 2011;65:E14-E16.
Practice Points
- Levamisole-induced vasculopathy usually resolves upon cessation of cocaine use without long-term sequelae.
- Presentation of a purpuric vasculitis, with or without associated neutropenia and positive autoantibodies, should prompt the consideration of levamisole-contaminated cocaine use in the clinician’s differential. Early recognition and discontinuation of the offending agent could be lifesaving.
To fight anti-Asian hate, we must talk about it
Words matter. So, hear me when I say: I am Asian. I am American. I am a woman. I am not COVID-19. I did not create this virus. I did not place it in my pocket and bring it to the world, sprinkling it like pixie dust, along each path I’ve crossed.
My words create a story, and not just those of one psychiatrist reaching out to others. It’s the story of how the powerful use of words throughout my life inflicted racism upon me, even when unacknowledged by my conscious self. I share my story to let you know you are not alone in your journey of unwinding the cumulative systemic racist words and actions that might have affected your self-identification and self-love. I hope you channel that renewed sense of discovery to empower you to use your own words to create a positive impact for yourself and others – whether it is for your patients, friends, or community.
Currently, I serve as a physician and an advocate. I lead telehealth and developed software that screens for suicide risk (with support of a digital health grant). I also joined friends to develop a by-clinician, for-clinician telemental health platform.
Outside of my Hippocratic Oath, my mission, at its core, was to destigmatize mental illness through cultivating thoughtful conversations. I am a daughter, a sister, an aunt, a friend, and an American. I am working hard to create a life I love – the embodiment of the American Dream. If you meet me face to face, no curriculum vitae, no email, I’m Vietnamese. However, I am not the color of my skin or the shape of my eyes. I am no more defined by my lingering Vietnamese accent than I am by its Texan counterpart. Yet, throughout my life, my Vietnamese ethnicity has been a marker that others have used to define and objectify me.
Trauma emerges on national stage
I never thought it would happen to me, but as a resident physician, one of my most traumatic experiences of abusive power was when Donald Trump was running for president in 2016. He was using words and rhetoric that objectified women by classifying and quantifying their “attractiveness.” This culminated in a scandal surrounding a recording in which he said he would grab women by the $%&#@ ... and had been allowed to do so because he was a celebrity. That episode affected me profoundly, maybe more than most. As a child and adolescent psychiatrist, I knew the impact those words could have on future generations, and, as a woman and an aunt, I was appalled. But then, the effect turned to assault. Words matter.
I was living in New York City and as I made my nightly walk home on the Upper East Side, a man followed me. When I walked up the stairs to my building, he actually grabbed me ... by the $%&#@. He did this with the same casual manner that one might greet a coworker with a high-five. He then turned and walked away, laughing. I was overcome with shock; shocked that I could be so violated and yet thankful that he hadn’t taken any more aggressive liberties. He didn’t run away. He walked out as calmly as he had walked in, despite violating a most private piece of my femininity. And he laughed. As much as it jilted me, angered me, and made me feel demeaned and less-than, I know it’s a blessing that the story ended there; so often attacks against women end so much worse.
I questioned: “Why?” Why would this man do this to me? To anyone? I don’t know the answer, but I do know this: The things we normalize through the words we hear in the world, on the news, and at our dinner tables become action. It happened. This man didn’t skulk off into the alleyway. He didn’t hide. He laughed because he felt entitled. That’s because words matter.
My journey is paved with words that mattered. I was born in Vietnam; my family legally immigrated to the United States when I was 5 years old. Throughout grade school, I began to realize the power of spoken words, especially when I was frequently told to go back to where I came from. Questions flew at me like bullets, and whether innocent or borne of curiosity, were hurtful reminders that, through no choice of my own, I was an unwelcome foreigner. “Where are you from?” ... “No, where are you really, really from?” I felt eyes peering through me when my mother packed for me our culture’s traditional foods for lunch. “Ew, what’s that?” ... “That’s gross it smells.” How I longed for the cloak of a peanut butter and jelly sandwich and blonde hair.
As I approached high school, college, and postgraduate work, the “where are you from?” questions didn’t stop but took on a new connotation, as if I were some exotic pet that men had seen walking down the street. “Ooh, what is that?” While history is riddled with the objectification of women, rarely would any woman expect to have a stranger approach her and objectify her with a statement such as: “I only date girls with breast implants.” For Asian women, however, experiencing verbal objectification has become the norm. Each approach I faced was followed by a story about Asian girlfriends of their past and a request for my phone number that felt more like a demand.
What these men probably meant as flirtation, I internalized as inescapable concerns of whether or not they had true desire to get to know me as a person. I became used to unsolicited words and attention from men who objectified me as an exotic fetish. I tried to pretend it was okay, but why? Objectifying Asian women is racism. Their words remind me, and I still hear them, that America has a long history of hypersexualizing Asian women. These words – at their core – dehumanize Asian women, and as we have seen, lead to violence.
Over the past few weeks, there’s been discourse about the mass shooting in Atlanta. We need to pause and remember that the victims, like us, were human. These women killed in Atlanta had husbands, children, siblings, parents, and communities that they were taken away from, senselessly, based solely on their outward appearance. Whether or not this act was perpetrated by someone with a sexual addiction doesn’t matter. What happened is rooted in the systemic racism that has stereotyped Asian women as sexual objects. The perpetrator targeted a group of people because of the systemic racism ingrained in him, plain and simple.
Everybody, no matter how evolved one’s thinking, is influenced by words. You don’t have to have mental illness or malicious intent to fall for propaganda – that’s what makes it so scary, it works so well. Even among my own friends and family, some of the most compassionate people I know, I’ve heard disparaging remarks against Chinese people, from other Asians, repeating the same rhetoric they’ve seen in American newspapers and Asian media outlets, echoing the former president’s coronavirus references to the “Chinese virus.”
But what makes something systemic? What feeds this virus of hate and gives these practices their longevity? Pointing out problems doesn’t make them go away; we have to cultivate conversation based around solutions. And that’s our next step. What can we do to make a positive impact?
Words have affected my life, and my words have given me power. I encourage others to engage in activities where they too can feel empowered. Since the beginning of the pandemic, I’ve leveraged my leadership position with the American Psychiatric Association’s Caucus of Asian American Psychiatrists and used my words to promote advocacy. I’ve also used my voice to raise national attention to the anti-Asian hate activities. Motivated by my own desire to seek a supportive space with others to reflect on our racial identities, I’ve also launched various free support groups for Asian American and Pacific Islanders (AAPI) professionals and health care providers. I want to feel a sense of connection with others who share my experiences, as I never underestimate the phenomenal force of comforting words from a healing community.
Clinicians need their own space for processing, too. It is vitally important for us to take care of ourselves, because our patients’ words can affect our own mental health. My colleagues are shocked by the amount of AAPI patients who are reaching out to them for care. Most of them have not worked with AAPI patients before, because so many people of AAPI descent do not often seek treatment. Many of our patients are dealing with anxiety surrounding their own health and wellness, coupled with financial uncertainties and social unrest. In particular, AAPI clinicians may start to experience bystander trauma, because, for the first time, they are thinking: “It could have been me.” AAPI clinicians are in a unique situation where they have the extra burden of providing a safe space for processing clients’ trauma while also processing their own. We may have experiences of discrimination or racially motivated assaults and can reexperience this trauma through our work. Before we can help others, we have to do a self-check and reflect on how we are doing and seek our own support.
If you are able to take care of yourself and feel empowered to make a difference, there are many ways to help fight against anti-Asian sentiment, both on a personal and more global scale.
We have to check our biases and those of our family, friends, and colleagues. Everyone, even mental health professionals, has biases and is affected by disinformation. We have to dig deep into our own unconscious biases, reflect on them, and commit to changing the biases around us. Do we, or our families, have unconscious biases against a particular minority group? If so, discuss it. No one is to blame. This is systemic, and no one is at fault. White men are not to be vilified. Conservative Republicans are not our enemy. Each of us is human, with our own flaws that can influence our own conscious and unconscious thoughts and actions. Let’s discuss racial issues with our family and friends. Whenever someone says something hateful or discriminatory toward another ethnic group or racial background, we have to call it out, and help them realize their biases and change them.
If you are able, use your words to write to your elected representatives. Send them a short email, no need to be fancy. For example, you can send a note of support for legislation that is similar to the COVID-19 Hate Crimes Act, which passed the Senate on Thursday, April 22, with 94:1 bipartisan support. This kind of legislation is a step in the right direction, but there is still more we must do to stop anti-Asian biases and hate. There is empowerment and healing through making your own voice heard. I hope that these tragic incidents will lead to impactful policy changes.
The next step in this journey of empowerment is speaking about your lived experiences publicly and promoting the voices of others. I dedicated a section of my social media platforms to amplifying Asian voices, sharing news, and updating my hashtags to support the #StopAsianHate movement. I made it a point to form relationships with other advocates, AAPI mental health professionals and those personally affected by anti-Asian hate. Speaking up and speaking out didn’t take away my worries, but it did remind me that I’m powerful and that I am not alone. I can take action and demand action. I do not have to hide in the shadows but can stand in the light, using my voice like a megaphone to call out injustice and intolerance.
I hope that, for AAPI clinicians who may be affected by these current events, this validates your experiences. You are not alone. This is a reminder to treat yourself with empathy as you would your patients. For others, I hope this helps you to learn the plight of many AAPI community members in this country. Together, we can use words to create better neighborhoods, a better country, and safe spaces for all communities, especially the marginalized. As we know, words matter.
Dr. Vo is a board-certified psychiatrist and is the medical director of telehealth for the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia. She is also a faculty member at the University of Pennsylvania, also in Philadelphia. Dr. Vo conducts digital health research focused on using automation and artificial intelligence for suicide risk screening and connecting patients to mental health care services. She disclosed serving as cofounder of telemental health software, Orchid, that eliminates burdensome administrative tasks so that clinicians can focus on their patients and have time for their loved ones.
Words matter. So, hear me when I say: I am Asian. I am American. I am a woman. I am not COVID-19. I did not create this virus. I did not place it in my pocket and bring it to the world, sprinkling it like pixie dust, along each path I’ve crossed.
My words create a story, and not just those of one psychiatrist reaching out to others. It’s the story of how the powerful use of words throughout my life inflicted racism upon me, even when unacknowledged by my conscious self. I share my story to let you know you are not alone in your journey of unwinding the cumulative systemic racist words and actions that might have affected your self-identification and self-love. I hope you channel that renewed sense of discovery to empower you to use your own words to create a positive impact for yourself and others – whether it is for your patients, friends, or community.
Currently, I serve as a physician and an advocate. I lead telehealth and developed software that screens for suicide risk (with support of a digital health grant). I also joined friends to develop a by-clinician, for-clinician telemental health platform.
Outside of my Hippocratic Oath, my mission, at its core, was to destigmatize mental illness through cultivating thoughtful conversations. I am a daughter, a sister, an aunt, a friend, and an American. I am working hard to create a life I love – the embodiment of the American Dream. If you meet me face to face, no curriculum vitae, no email, I’m Vietnamese. However, I am not the color of my skin or the shape of my eyes. I am no more defined by my lingering Vietnamese accent than I am by its Texan counterpart. Yet, throughout my life, my Vietnamese ethnicity has been a marker that others have used to define and objectify me.
Trauma emerges on national stage
I never thought it would happen to me, but as a resident physician, one of my most traumatic experiences of abusive power was when Donald Trump was running for president in 2016. He was using words and rhetoric that objectified women by classifying and quantifying their “attractiveness.” This culminated in a scandal surrounding a recording in which he said he would grab women by the $%&#@ ... and had been allowed to do so because he was a celebrity. That episode affected me profoundly, maybe more than most. As a child and adolescent psychiatrist, I knew the impact those words could have on future generations, and, as a woman and an aunt, I was appalled. But then, the effect turned to assault. Words matter.
I was living in New York City and as I made my nightly walk home on the Upper East Side, a man followed me. When I walked up the stairs to my building, he actually grabbed me ... by the $%&#@. He did this with the same casual manner that one might greet a coworker with a high-five. He then turned and walked away, laughing. I was overcome with shock; shocked that I could be so violated and yet thankful that he hadn’t taken any more aggressive liberties. He didn’t run away. He walked out as calmly as he had walked in, despite violating a most private piece of my femininity. And he laughed. As much as it jilted me, angered me, and made me feel demeaned and less-than, I know it’s a blessing that the story ended there; so often attacks against women end so much worse.
I questioned: “Why?” Why would this man do this to me? To anyone? I don’t know the answer, but I do know this: The things we normalize through the words we hear in the world, on the news, and at our dinner tables become action. It happened. This man didn’t skulk off into the alleyway. He didn’t hide. He laughed because he felt entitled. That’s because words matter.
My journey is paved with words that mattered. I was born in Vietnam; my family legally immigrated to the United States when I was 5 years old. Throughout grade school, I began to realize the power of spoken words, especially when I was frequently told to go back to where I came from. Questions flew at me like bullets, and whether innocent or borne of curiosity, were hurtful reminders that, through no choice of my own, I was an unwelcome foreigner. “Where are you from?” ... “No, where are you really, really from?” I felt eyes peering through me when my mother packed for me our culture’s traditional foods for lunch. “Ew, what’s that?” ... “That’s gross it smells.” How I longed for the cloak of a peanut butter and jelly sandwich and blonde hair.
As I approached high school, college, and postgraduate work, the “where are you from?” questions didn’t stop but took on a new connotation, as if I were some exotic pet that men had seen walking down the street. “Ooh, what is that?” While history is riddled with the objectification of women, rarely would any woman expect to have a stranger approach her and objectify her with a statement such as: “I only date girls with breast implants.” For Asian women, however, experiencing verbal objectification has become the norm. Each approach I faced was followed by a story about Asian girlfriends of their past and a request for my phone number that felt more like a demand.
What these men probably meant as flirtation, I internalized as inescapable concerns of whether or not they had true desire to get to know me as a person. I became used to unsolicited words and attention from men who objectified me as an exotic fetish. I tried to pretend it was okay, but why? Objectifying Asian women is racism. Their words remind me, and I still hear them, that America has a long history of hypersexualizing Asian women. These words – at their core – dehumanize Asian women, and as we have seen, lead to violence.
Over the past few weeks, there’s been discourse about the mass shooting in Atlanta. We need to pause and remember that the victims, like us, were human. These women killed in Atlanta had husbands, children, siblings, parents, and communities that they were taken away from, senselessly, based solely on their outward appearance. Whether or not this act was perpetrated by someone with a sexual addiction doesn’t matter. What happened is rooted in the systemic racism that has stereotyped Asian women as sexual objects. The perpetrator targeted a group of people because of the systemic racism ingrained in him, plain and simple.
Everybody, no matter how evolved one’s thinking, is influenced by words. You don’t have to have mental illness or malicious intent to fall for propaganda – that’s what makes it so scary, it works so well. Even among my own friends and family, some of the most compassionate people I know, I’ve heard disparaging remarks against Chinese people, from other Asians, repeating the same rhetoric they’ve seen in American newspapers and Asian media outlets, echoing the former president’s coronavirus references to the “Chinese virus.”
But what makes something systemic? What feeds this virus of hate and gives these practices their longevity? Pointing out problems doesn’t make them go away; we have to cultivate conversation based around solutions. And that’s our next step. What can we do to make a positive impact?
Words have affected my life, and my words have given me power. I encourage others to engage in activities where they too can feel empowered. Since the beginning of the pandemic, I’ve leveraged my leadership position with the American Psychiatric Association’s Caucus of Asian American Psychiatrists and used my words to promote advocacy. I’ve also used my voice to raise national attention to the anti-Asian hate activities. Motivated by my own desire to seek a supportive space with others to reflect on our racial identities, I’ve also launched various free support groups for Asian American and Pacific Islanders (AAPI) professionals and health care providers. I want to feel a sense of connection with others who share my experiences, as I never underestimate the phenomenal force of comforting words from a healing community.
Clinicians need their own space for processing, too. It is vitally important for us to take care of ourselves, because our patients’ words can affect our own mental health. My colleagues are shocked by the amount of AAPI patients who are reaching out to them for care. Most of them have not worked with AAPI patients before, because so many people of AAPI descent do not often seek treatment. Many of our patients are dealing with anxiety surrounding their own health and wellness, coupled with financial uncertainties and social unrest. In particular, AAPI clinicians may start to experience bystander trauma, because, for the first time, they are thinking: “It could have been me.” AAPI clinicians are in a unique situation where they have the extra burden of providing a safe space for processing clients’ trauma while also processing their own. We may have experiences of discrimination or racially motivated assaults and can reexperience this trauma through our work. Before we can help others, we have to do a self-check and reflect on how we are doing and seek our own support.
If you are able to take care of yourself and feel empowered to make a difference, there are many ways to help fight against anti-Asian sentiment, both on a personal and more global scale.
We have to check our biases and those of our family, friends, and colleagues. Everyone, even mental health professionals, has biases and is affected by disinformation. We have to dig deep into our own unconscious biases, reflect on them, and commit to changing the biases around us. Do we, or our families, have unconscious biases against a particular minority group? If so, discuss it. No one is to blame. This is systemic, and no one is at fault. White men are not to be vilified. Conservative Republicans are not our enemy. Each of us is human, with our own flaws that can influence our own conscious and unconscious thoughts and actions. Let’s discuss racial issues with our family and friends. Whenever someone says something hateful or discriminatory toward another ethnic group or racial background, we have to call it out, and help them realize their biases and change them.
If you are able, use your words to write to your elected representatives. Send them a short email, no need to be fancy. For example, you can send a note of support for legislation that is similar to the COVID-19 Hate Crimes Act, which passed the Senate on Thursday, April 22, with 94:1 bipartisan support. This kind of legislation is a step in the right direction, but there is still more we must do to stop anti-Asian biases and hate. There is empowerment and healing through making your own voice heard. I hope that these tragic incidents will lead to impactful policy changes.
The next step in this journey of empowerment is speaking about your lived experiences publicly and promoting the voices of others. I dedicated a section of my social media platforms to amplifying Asian voices, sharing news, and updating my hashtags to support the #StopAsianHate movement. I made it a point to form relationships with other advocates, AAPI mental health professionals and those personally affected by anti-Asian hate. Speaking up and speaking out didn’t take away my worries, but it did remind me that I’m powerful and that I am not alone. I can take action and demand action. I do not have to hide in the shadows but can stand in the light, using my voice like a megaphone to call out injustice and intolerance.
I hope that, for AAPI clinicians who may be affected by these current events, this validates your experiences. You are not alone. This is a reminder to treat yourself with empathy as you would your patients. For others, I hope this helps you to learn the plight of many AAPI community members in this country. Together, we can use words to create better neighborhoods, a better country, and safe spaces for all communities, especially the marginalized. As we know, words matter.
Dr. Vo is a board-certified psychiatrist and is the medical director of telehealth for the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia. She is also a faculty member at the University of Pennsylvania, also in Philadelphia. Dr. Vo conducts digital health research focused on using automation and artificial intelligence for suicide risk screening and connecting patients to mental health care services. She disclosed serving as cofounder of telemental health software, Orchid, that eliminates burdensome administrative tasks so that clinicians can focus on their patients and have time for their loved ones.
Words matter. So, hear me when I say: I am Asian. I am American. I am a woman. I am not COVID-19. I did not create this virus. I did not place it in my pocket and bring it to the world, sprinkling it like pixie dust, along each path I’ve crossed.
My words create a story, and not just those of one psychiatrist reaching out to others. It’s the story of how the powerful use of words throughout my life inflicted racism upon me, even when unacknowledged by my conscious self. I share my story to let you know you are not alone in your journey of unwinding the cumulative systemic racist words and actions that might have affected your self-identification and self-love. I hope you channel that renewed sense of discovery to empower you to use your own words to create a positive impact for yourself and others – whether it is for your patients, friends, or community.
Currently, I serve as a physician and an advocate. I lead telehealth and developed software that screens for suicide risk (with support of a digital health grant). I also joined friends to develop a by-clinician, for-clinician telemental health platform.
Outside of my Hippocratic Oath, my mission, at its core, was to destigmatize mental illness through cultivating thoughtful conversations. I am a daughter, a sister, an aunt, a friend, and an American. I am working hard to create a life I love – the embodiment of the American Dream. If you meet me face to face, no curriculum vitae, no email, I’m Vietnamese. However, I am not the color of my skin or the shape of my eyes. I am no more defined by my lingering Vietnamese accent than I am by its Texan counterpart. Yet, throughout my life, my Vietnamese ethnicity has been a marker that others have used to define and objectify me.
Trauma emerges on national stage
I never thought it would happen to me, but as a resident physician, one of my most traumatic experiences of abusive power was when Donald Trump was running for president in 2016. He was using words and rhetoric that objectified women by classifying and quantifying their “attractiveness.” This culminated in a scandal surrounding a recording in which he said he would grab women by the $%&#@ ... and had been allowed to do so because he was a celebrity. That episode affected me profoundly, maybe more than most. As a child and adolescent psychiatrist, I knew the impact those words could have on future generations, and, as a woman and an aunt, I was appalled. But then, the effect turned to assault. Words matter.
I was living in New York City and as I made my nightly walk home on the Upper East Side, a man followed me. When I walked up the stairs to my building, he actually grabbed me ... by the $%&#@. He did this with the same casual manner that one might greet a coworker with a high-five. He then turned and walked away, laughing. I was overcome with shock; shocked that I could be so violated and yet thankful that he hadn’t taken any more aggressive liberties. He didn’t run away. He walked out as calmly as he had walked in, despite violating a most private piece of my femininity. And he laughed. As much as it jilted me, angered me, and made me feel demeaned and less-than, I know it’s a blessing that the story ended there; so often attacks against women end so much worse.
I questioned: “Why?” Why would this man do this to me? To anyone? I don’t know the answer, but I do know this: The things we normalize through the words we hear in the world, on the news, and at our dinner tables become action. It happened. This man didn’t skulk off into the alleyway. He didn’t hide. He laughed because he felt entitled. That’s because words matter.
My journey is paved with words that mattered. I was born in Vietnam; my family legally immigrated to the United States when I was 5 years old. Throughout grade school, I began to realize the power of spoken words, especially when I was frequently told to go back to where I came from. Questions flew at me like bullets, and whether innocent or borne of curiosity, were hurtful reminders that, through no choice of my own, I was an unwelcome foreigner. “Where are you from?” ... “No, where are you really, really from?” I felt eyes peering through me when my mother packed for me our culture’s traditional foods for lunch. “Ew, what’s that?” ... “That’s gross it smells.” How I longed for the cloak of a peanut butter and jelly sandwich and blonde hair.
As I approached high school, college, and postgraduate work, the “where are you from?” questions didn’t stop but took on a new connotation, as if I were some exotic pet that men had seen walking down the street. “Ooh, what is that?” While history is riddled with the objectification of women, rarely would any woman expect to have a stranger approach her and objectify her with a statement such as: “I only date girls with breast implants.” For Asian women, however, experiencing verbal objectification has become the norm. Each approach I faced was followed by a story about Asian girlfriends of their past and a request for my phone number that felt more like a demand.
What these men probably meant as flirtation, I internalized as inescapable concerns of whether or not they had true desire to get to know me as a person. I became used to unsolicited words and attention from men who objectified me as an exotic fetish. I tried to pretend it was okay, but why? Objectifying Asian women is racism. Their words remind me, and I still hear them, that America has a long history of hypersexualizing Asian women. These words – at their core – dehumanize Asian women, and as we have seen, lead to violence.
Over the past few weeks, there’s been discourse about the mass shooting in Atlanta. We need to pause and remember that the victims, like us, were human. These women killed in Atlanta had husbands, children, siblings, parents, and communities that they were taken away from, senselessly, based solely on their outward appearance. Whether or not this act was perpetrated by someone with a sexual addiction doesn’t matter. What happened is rooted in the systemic racism that has stereotyped Asian women as sexual objects. The perpetrator targeted a group of people because of the systemic racism ingrained in him, plain and simple.
Everybody, no matter how evolved one’s thinking, is influenced by words. You don’t have to have mental illness or malicious intent to fall for propaganda – that’s what makes it so scary, it works so well. Even among my own friends and family, some of the most compassionate people I know, I’ve heard disparaging remarks against Chinese people, from other Asians, repeating the same rhetoric they’ve seen in American newspapers and Asian media outlets, echoing the former president’s coronavirus references to the “Chinese virus.”
But what makes something systemic? What feeds this virus of hate and gives these practices their longevity? Pointing out problems doesn’t make them go away; we have to cultivate conversation based around solutions. And that’s our next step. What can we do to make a positive impact?
Words have affected my life, and my words have given me power. I encourage others to engage in activities where they too can feel empowered. Since the beginning of the pandemic, I’ve leveraged my leadership position with the American Psychiatric Association’s Caucus of Asian American Psychiatrists and used my words to promote advocacy. I’ve also used my voice to raise national attention to the anti-Asian hate activities. Motivated by my own desire to seek a supportive space with others to reflect on our racial identities, I’ve also launched various free support groups for Asian American and Pacific Islanders (AAPI) professionals and health care providers. I want to feel a sense of connection with others who share my experiences, as I never underestimate the phenomenal force of comforting words from a healing community.
Clinicians need their own space for processing, too. It is vitally important for us to take care of ourselves, because our patients’ words can affect our own mental health. My colleagues are shocked by the amount of AAPI patients who are reaching out to them for care. Most of them have not worked with AAPI patients before, because so many people of AAPI descent do not often seek treatment. Many of our patients are dealing with anxiety surrounding their own health and wellness, coupled with financial uncertainties and social unrest. In particular, AAPI clinicians may start to experience bystander trauma, because, for the first time, they are thinking: “It could have been me.” AAPI clinicians are in a unique situation where they have the extra burden of providing a safe space for processing clients’ trauma while also processing their own. We may have experiences of discrimination or racially motivated assaults and can reexperience this trauma through our work. Before we can help others, we have to do a self-check and reflect on how we are doing and seek our own support.
If you are able to take care of yourself and feel empowered to make a difference, there are many ways to help fight against anti-Asian sentiment, both on a personal and more global scale.
We have to check our biases and those of our family, friends, and colleagues. Everyone, even mental health professionals, has biases and is affected by disinformation. We have to dig deep into our own unconscious biases, reflect on them, and commit to changing the biases around us. Do we, or our families, have unconscious biases against a particular minority group? If so, discuss it. No one is to blame. This is systemic, and no one is at fault. White men are not to be vilified. Conservative Republicans are not our enemy. Each of us is human, with our own flaws that can influence our own conscious and unconscious thoughts and actions. Let’s discuss racial issues with our family and friends. Whenever someone says something hateful or discriminatory toward another ethnic group or racial background, we have to call it out, and help them realize their biases and change them.
If you are able, use your words to write to your elected representatives. Send them a short email, no need to be fancy. For example, you can send a note of support for legislation that is similar to the COVID-19 Hate Crimes Act, which passed the Senate on Thursday, April 22, with 94:1 bipartisan support. This kind of legislation is a step in the right direction, but there is still more we must do to stop anti-Asian biases and hate. There is empowerment and healing through making your own voice heard. I hope that these tragic incidents will lead to impactful policy changes.
The next step in this journey of empowerment is speaking about your lived experiences publicly and promoting the voices of others. I dedicated a section of my social media platforms to amplifying Asian voices, sharing news, and updating my hashtags to support the #StopAsianHate movement. I made it a point to form relationships with other advocates, AAPI mental health professionals and those personally affected by anti-Asian hate. Speaking up and speaking out didn’t take away my worries, but it did remind me that I’m powerful and that I am not alone. I can take action and demand action. I do not have to hide in the shadows but can stand in the light, using my voice like a megaphone to call out injustice and intolerance.
I hope that, for AAPI clinicians who may be affected by these current events, this validates your experiences. You are not alone. This is a reminder to treat yourself with empathy as you would your patients. For others, I hope this helps you to learn the plight of many AAPI community members in this country. Together, we can use words to create better neighborhoods, a better country, and safe spaces for all communities, especially the marginalized. As we know, words matter.
Dr. Vo is a board-certified psychiatrist and is the medical director of telehealth for the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia. She is also a faculty member at the University of Pennsylvania, also in Philadelphia. Dr. Vo conducts digital health research focused on using automation and artificial intelligence for suicide risk screening and connecting patients to mental health care services. She disclosed serving as cofounder of telemental health software, Orchid, that eliminates burdensome administrative tasks so that clinicians can focus on their patients and have time for their loved ones.
Genital Primary Herpetic Infection With Concurrent Hepatitis in an Infant
To the Editor:
Cutaneous herpes simplex virus (HSV) infection generally involves mucocutaneous junctions, but virtually any area of the skin can be affected.1 When the genital area of adult patients is affected, the disease usually is sexually transmitted and mainly caused by HSV-2. In infants, genital primary herpetic infection is rare and more commonly is caused by HSV-1 than by HSV-2. We report a rare case of genital primary herpetic infection with concurrent hepatitis in an infant.
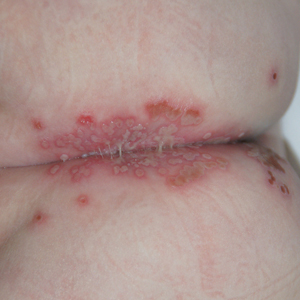
An 8-month-old infant with no underlying medical problems, including atopic dermatitis, was referred for erythematous grouped vesicles with erosions on the perianal area of 4 days’ duration (Figure). The skin color appeared normal, not icterus. She also had a fever (temperature, 37.9 °C), and her urination pattern had changed from normal to frequent leakage, possibly owing to pain related to the eroded lesions. Physical examination did not reveal palpable inguinal lymph nodes. The oral mucosa was not involved. The patient’s father had a history of recurrent herpetic infection on both the perioral and perianal areas.
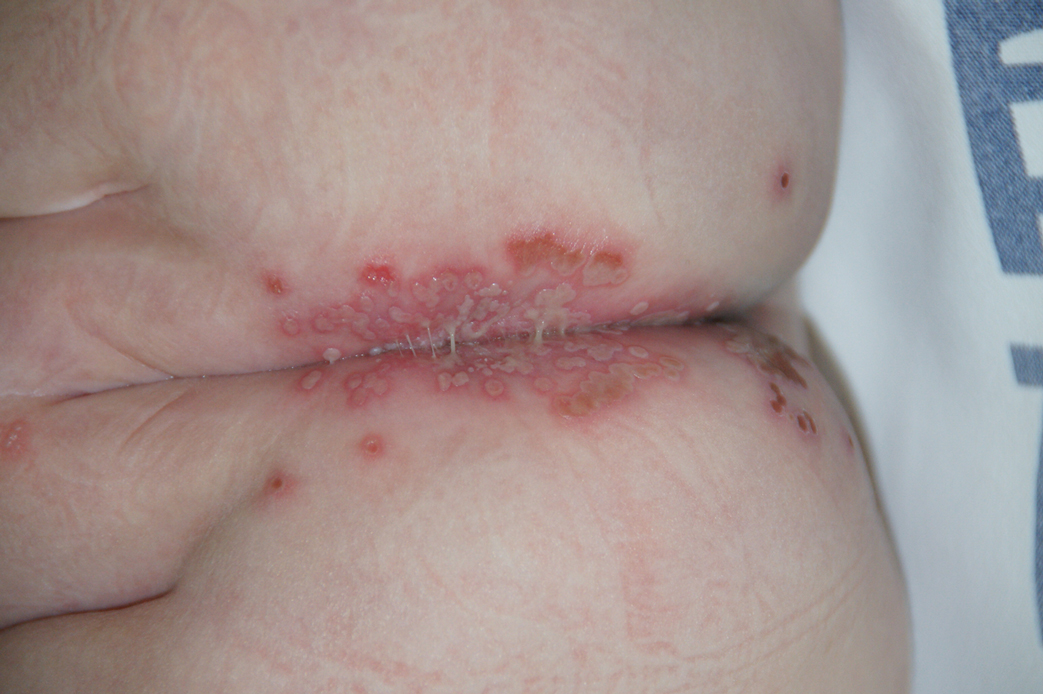
A Tzanck smear revealed giant multinucleated cells with multiple inflammatory cells. Laboratory tests revealed marked leukocytosis, elevated liver enzymes (aspartate aminotransferase, 141 IU/L [reference range, 15 IU/L–60 IU/L]; alanine aminotransferase, 422 IU/L [reference range, 13 IU/L–45 IU/L]), and was positive for herpes simplex viral IgM but negative for herpes simplex viral IgG. A viral culture also demonstrated the growth of HSV. An abdominal ultrasound was normal. Based on the cutaneous and laboratory findings, genital primary herpetic infection with concurrent hepatitis was diagnosed. Intravenous acyclovir 50 mg was administered 3 times daily for 7 days, and a wet dressing with topical mupirocin was employed daily until the skin lesions healed. The fever subsided soon after starting treatment. The liver enzyme counts decreased gradually in serial follow-up (aspartate aminotransferase, 75 IU/L; alanine aminotransferase, 70 IU/L).
Primary herpetic infection usually is asymptomatic, but when symptoms do occur, it is characterized by the sudden onset of painful vesicle clusters over erythematous edematous skin. Lesions can be associated with fever and malaise and may involve the perineum. Urinary symptoms may occur. The average age of onset ranges from 6 months to 4 years. The virus commonly is transmitted by asymptomatic carriers. Autoinoculation from concomitant oral primary herpetic infection or individuals with active herpetic infection is one possible route of transmission. In our patient, we assumed that she acquired the virus from her father during close contact. A diagnosis can be made clinically using direct methods including culture, Tzanck smear, or polymerase chain reaction, or indirect methods such as serologic tests.2
Hepatitis secondary to HSV infection is rare, especially in immunocompetent patients. It occurs during primary infection and rarely during recurrent infection with or without concomitant skin lesions.3 Symptoms include fever, anorexia, nausea, vomiting, abdominal pain, leukopenia, coagulopathy, and marked elevation of serum transaminase levels without jaundice. Based on our patient’s elevated liver enzyme levels and virological evidence of acute primary HSV infection, a lack of evidence of other hepatic viral infections, and the presence of herpes simplex viremia, we concluded that this infant had viral hepatitis as a part of the clinical presentation of primary HSV infection. We did not perform a direct liver biopsy considering her age and accompanying risks.4
Primary herpetic infection usually has a benign course and a short duration. In children, the prognosis depends on underlying immunologic status, not a particular type of HSV. In children with atopic dermatitis, primary herpetic infection tends to occur earlier and is more severe. Early treatment with acyclovir is effective; intravenous treatment is not required unless local complications or systemic involvement are present. Long-term follow-up is recommended because of the possibility of recurrence.
Although the possibility of systemic involvement including hepatitis due to HSV infection is low, awareness among dermatologists about primary herpetic infection and its possible complications would be helpful in the diagnosis and treatment, especially for atypical or extensive cases.
- Jenson HB, Shapiro ED. Primary herpes simplex virus infection of a diaper rash. Pediatr Infect Dis J. 1987;6:1136-1138.
- Batalla A, Flórez A, Dávila P, et al. Genital primary herpes simplexinfection in a 5-month-old infant. Dermatol Online J. 2011;17:8.
- Norvell JP, Blei AT, Jovanovic BD, et al. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428-1434.
- Chen CK, Wu SH, Huang YC. Herpetic gingivostomatitis with severe hepatitis in a previously healthy child. J Microbiol Immunol Infect. 2012;45:324-325.
To the Editor:
Cutaneous herpes simplex virus (HSV) infection generally involves mucocutaneous junctions, but virtually any area of the skin can be affected.1 When the genital area of adult patients is affected, the disease usually is sexually transmitted and mainly caused by HSV-2. In infants, genital primary herpetic infection is rare and more commonly is caused by HSV-1 than by HSV-2. We report a rare case of genital primary herpetic infection with concurrent hepatitis in an infant.
An 8-month-old infant with no underlying medical problems, including atopic dermatitis, was referred for erythematous grouped vesicles with erosions on the perianal area of 4 days’ duration (Figure). The skin color appeared normal, not icterus. She also had a fever (temperature, 37.9 °C), and her urination pattern had changed from normal to frequent leakage, possibly owing to pain related to the eroded lesions. Physical examination did not reveal palpable inguinal lymph nodes. The oral mucosa was not involved. The patient’s father had a history of recurrent herpetic infection on both the perioral and perianal areas.

A Tzanck smear revealed giant multinucleated cells with multiple inflammatory cells. Laboratory tests revealed marked leukocytosis, elevated liver enzymes (aspartate aminotransferase, 141 IU/L [reference range, 15 IU/L–60 IU/L]; alanine aminotransferase, 422 IU/L [reference range, 13 IU/L–45 IU/L]), and was positive for herpes simplex viral IgM but negative for herpes simplex viral IgG. A viral culture also demonstrated the growth of HSV. An abdominal ultrasound was normal. Based on the cutaneous and laboratory findings, genital primary herpetic infection with concurrent hepatitis was diagnosed. Intravenous acyclovir 50 mg was administered 3 times daily for 7 days, and a wet dressing with topical mupirocin was employed daily until the skin lesions healed. The fever subsided soon after starting treatment. The liver enzyme counts decreased gradually in serial follow-up (aspartate aminotransferase, 75 IU/L; alanine aminotransferase, 70 IU/L).
Primary herpetic infection usually is asymptomatic, but when symptoms do occur, it is characterized by the sudden onset of painful vesicle clusters over erythematous edematous skin. Lesions can be associated with fever and malaise and may involve the perineum. Urinary symptoms may occur. The average age of onset ranges from 6 months to 4 years. The virus commonly is transmitted by asymptomatic carriers. Autoinoculation from concomitant oral primary herpetic infection or individuals with active herpetic infection is one possible route of transmission. In our patient, we assumed that she acquired the virus from her father during close contact. A diagnosis can be made clinically using direct methods including culture, Tzanck smear, or polymerase chain reaction, or indirect methods such as serologic tests.2
Hepatitis secondary to HSV infection is rare, especially in immunocompetent patients. It occurs during primary infection and rarely during recurrent infection with or without concomitant skin lesions.3 Symptoms include fever, anorexia, nausea, vomiting, abdominal pain, leukopenia, coagulopathy, and marked elevation of serum transaminase levels without jaundice. Based on our patient’s elevated liver enzyme levels and virological evidence of acute primary HSV infection, a lack of evidence of other hepatic viral infections, and the presence of herpes simplex viremia, we concluded that this infant had viral hepatitis as a part of the clinical presentation of primary HSV infection. We did not perform a direct liver biopsy considering her age and accompanying risks.4
Primary herpetic infection usually has a benign course and a short duration. In children, the prognosis depends on underlying immunologic status, not a particular type of HSV. In children with atopic dermatitis, primary herpetic infection tends to occur earlier and is more severe. Early treatment with acyclovir is effective; intravenous treatment is not required unless local complications or systemic involvement are present. Long-term follow-up is recommended because of the possibility of recurrence.
Although the possibility of systemic involvement including hepatitis due to HSV infection is low, awareness among dermatologists about primary herpetic infection and its possible complications would be helpful in the diagnosis and treatment, especially for atypical or extensive cases.
To the Editor:
Cutaneous herpes simplex virus (HSV) infection generally involves mucocutaneous junctions, but virtually any area of the skin can be affected.1 When the genital area of adult patients is affected, the disease usually is sexually transmitted and mainly caused by HSV-2. In infants, genital primary herpetic infection is rare and more commonly is caused by HSV-1 than by HSV-2. We report a rare case of genital primary herpetic infection with concurrent hepatitis in an infant.
An 8-month-old infant with no underlying medical problems, including atopic dermatitis, was referred for erythematous grouped vesicles with erosions on the perianal area of 4 days’ duration (Figure). The skin color appeared normal, not icterus. She also had a fever (temperature, 37.9 °C), and her urination pattern had changed from normal to frequent leakage, possibly owing to pain related to the eroded lesions. Physical examination did not reveal palpable inguinal lymph nodes. The oral mucosa was not involved. The patient’s father had a history of recurrent herpetic infection on both the perioral and perianal areas.

A Tzanck smear revealed giant multinucleated cells with multiple inflammatory cells. Laboratory tests revealed marked leukocytosis, elevated liver enzymes (aspartate aminotransferase, 141 IU/L [reference range, 15 IU/L–60 IU/L]; alanine aminotransferase, 422 IU/L [reference range, 13 IU/L–45 IU/L]), and was positive for herpes simplex viral IgM but negative for herpes simplex viral IgG. A viral culture also demonstrated the growth of HSV. An abdominal ultrasound was normal. Based on the cutaneous and laboratory findings, genital primary herpetic infection with concurrent hepatitis was diagnosed. Intravenous acyclovir 50 mg was administered 3 times daily for 7 days, and a wet dressing with topical mupirocin was employed daily until the skin lesions healed. The fever subsided soon after starting treatment. The liver enzyme counts decreased gradually in serial follow-up (aspartate aminotransferase, 75 IU/L; alanine aminotransferase, 70 IU/L).
Primary herpetic infection usually is asymptomatic, but when symptoms do occur, it is characterized by the sudden onset of painful vesicle clusters over erythematous edematous skin. Lesions can be associated with fever and malaise and may involve the perineum. Urinary symptoms may occur. The average age of onset ranges from 6 months to 4 years. The virus commonly is transmitted by asymptomatic carriers. Autoinoculation from concomitant oral primary herpetic infection or individuals with active herpetic infection is one possible route of transmission. In our patient, we assumed that she acquired the virus from her father during close contact. A diagnosis can be made clinically using direct methods including culture, Tzanck smear, or polymerase chain reaction, or indirect methods such as serologic tests.2
Hepatitis secondary to HSV infection is rare, especially in immunocompetent patients. It occurs during primary infection and rarely during recurrent infection with or without concomitant skin lesions.3 Symptoms include fever, anorexia, nausea, vomiting, abdominal pain, leukopenia, coagulopathy, and marked elevation of serum transaminase levels without jaundice. Based on our patient’s elevated liver enzyme levels and virological evidence of acute primary HSV infection, a lack of evidence of other hepatic viral infections, and the presence of herpes simplex viremia, we concluded that this infant had viral hepatitis as a part of the clinical presentation of primary HSV infection. We did not perform a direct liver biopsy considering her age and accompanying risks.4
Primary herpetic infection usually has a benign course and a short duration. In children, the prognosis depends on underlying immunologic status, not a particular type of HSV. In children with atopic dermatitis, primary herpetic infection tends to occur earlier and is more severe. Early treatment with acyclovir is effective; intravenous treatment is not required unless local complications or systemic involvement are present. Long-term follow-up is recommended because of the possibility of recurrence.
Although the possibility of systemic involvement including hepatitis due to HSV infection is low, awareness among dermatologists about primary herpetic infection and its possible complications would be helpful in the diagnosis and treatment, especially for atypical or extensive cases.
- Jenson HB, Shapiro ED. Primary herpes simplex virus infection of a diaper rash. Pediatr Infect Dis J. 1987;6:1136-1138.
- Batalla A, Flórez A, Dávila P, et al. Genital primary herpes simplexinfection in a 5-month-old infant. Dermatol Online J. 2011;17:8.
- Norvell JP, Blei AT, Jovanovic BD, et al. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428-1434.
- Chen CK, Wu SH, Huang YC. Herpetic gingivostomatitis with severe hepatitis in a previously healthy child. J Microbiol Immunol Infect. 2012;45:324-325.
- Jenson HB, Shapiro ED. Primary herpes simplex virus infection of a diaper rash. Pediatr Infect Dis J. 1987;6:1136-1138.
- Batalla A, Flórez A, Dávila P, et al. Genital primary herpes simplexinfection in a 5-month-old infant. Dermatol Online J. 2011;17:8.
- Norvell JP, Blei AT, Jovanovic BD, et al. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428-1434.
- Chen CK, Wu SH, Huang YC. Herpetic gingivostomatitis with severe hepatitis in a previously healthy child. J Microbiol Immunol Infect. 2012;45:324-325.
Practice Points
- Parents with a history of herpes simplex virus (HSV) need to be educated before the baby is born to be careful about direct skin contact with the child to prevent the spread of HSV infection.
- Although systemic involvement is not typical, additional tests to rule out internal organ involvement may be required, especially in children.
Pruritic and Pustular Eruption on the Face
The Diagnosis: Demodicosis
A clinical diagnosis of facial demodicosis triggered by topical corticosteroid therapy was suspected in our patient. A 3-mm punch biopsy of the right forehead demonstrated a follicular infundibulum rich in Demodex folliculorum with a discrete mononuclear infiltrate in the dermis (Figure 1), confirming the diagnosis. The patient was prescribed metronidazole gel 2% twice daily. Complete clearance of the facial rash was observed at 1-week follow-up (Figure 2).
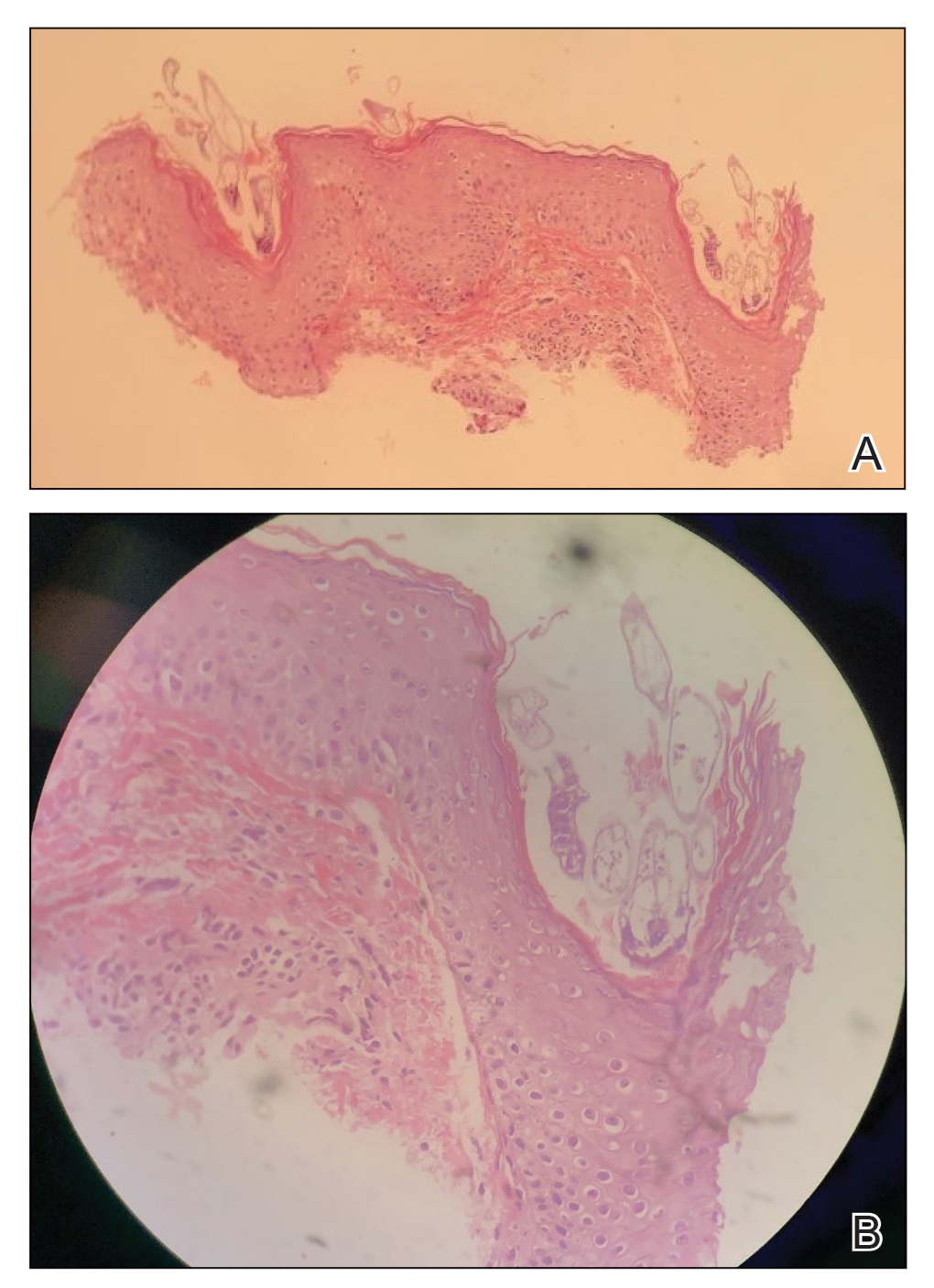
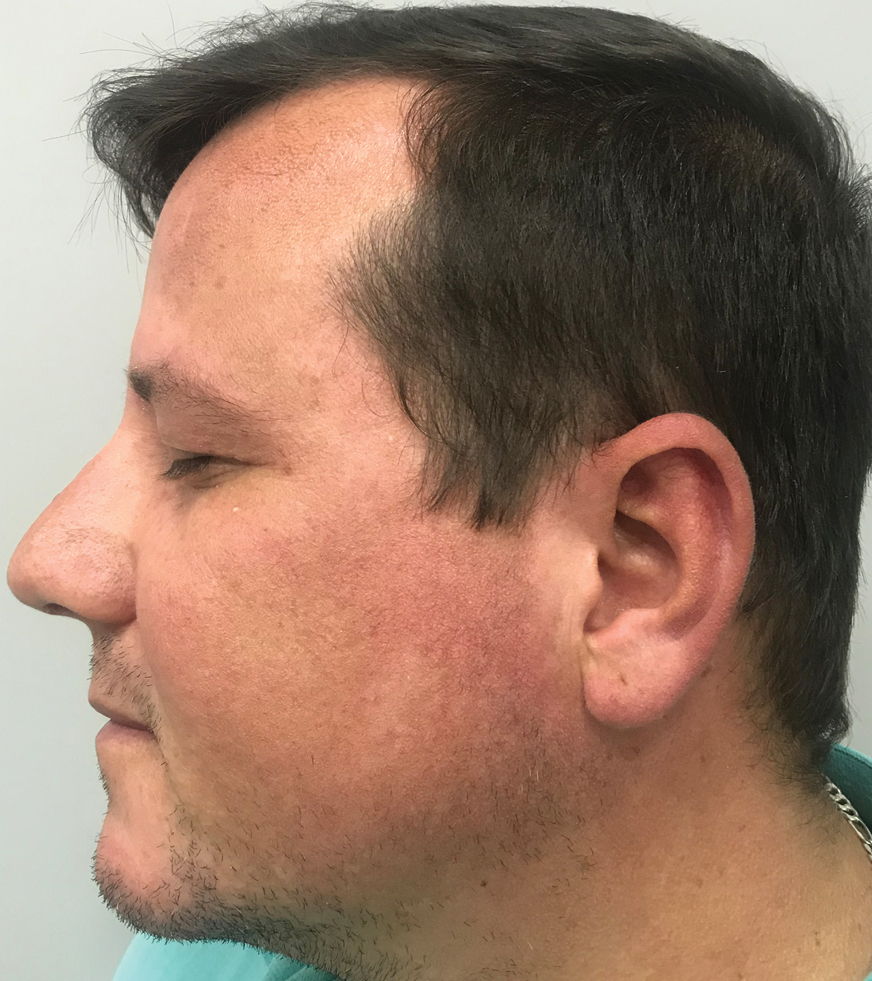
Demodex folliculorum is a mite that parasitizes the pilosebaceous follicles of human skin, becoming pathogenic with excessive colonization.1-3 Facial demodicosis presents as pruritic papules and pustules in a pilosebaceous distribution on the face.2,3 The diagnosis of facial demodicosis can be difficult due to similarities with many other common facial rashes such as acne, rosacea, contact dermatitis, and folliculitis.
Similar to facial demodicosis, rosacea can present with erythema, papules, and pustules on the face. Patients with rosacea also have a higher prevalence and degree of Demodex mite infestation.4 However, these facial rashes can be differentiated when symptom acuity and personal and family histories are considered. In patients with a long-standing personal and/or family history of erythema, papules, and pustules, the chronicity of disease implies a diagnosis of rosacea. The acute onset of symptomatology and absence of personal or family history of rosacea in our patient favored a diagnosis of demodicosis.
Pityrosporum folliculitis is an eruption caused by Malassezia furfur, the organism implicated in tinea versicolor. It can present similarly to Demodex folliculitis with follicular papules and pustules on the forehead and back. Features that help to differentiate Pityrosporum folliculitis from Demodex folliculitis include involvement of the upper trunk and onset after antibiotic usage.5 Diagnosis of Pityrosporum folliculitis can be confirmed by potassium hydroxide preparation, which demonstrates spaghetti-and-meatball-like hyphae and spores.
Eosinophilic folliculitis is a chronic sterile folliculitis that usually presents as intensely pruritic papules and pustules on the face with an eruptive onset. There are 3 variants of disease: the classic form (also known as Ofuji disease), immunosuppression-associated disease, and infancy-associated disease.6 Eosinophilic folliculitis also may present with annular plaques and peripheral blood eosinophilia, and it is more prevalent in patients of Japanese descent. Differentiation from Demodex folliculitis can be done by histologic examination, which demonstrates spongiosis with exocytosis of eosinophils into the epithelium and a clear deficiency of infiltrating mites.6
Miliaria, also known as sweat rash, is a common condition that occurs due to occlusion of eccrine sweat glands.7 Clinically, miliaria is characterized by erythematous papules ranging from 2 to 4 mm in size that may be vesicular or pustular. Miliaria and demodicosis may have similar clinical presentations; however, several characteristic differences can be noted. Based on the pathophysiology, miliaria will not be folliculocentric, which differs from demodicosis. Additionally, miliaria most commonly occurs in areas of occlusion, such as in skin folds or on the trunk under tight clothing. Miliaria rarely can appear confluent and sunburnlike.8 Furthermore, miliaria is less common on the face, while demodicosis almost exclusively is found on the face.
We present the case of an otherwise healthy patient with acute onset of pruritic papules and pustules involving the superior face following topical corticosteroid use. Similar cases frequently are encountered by dermatologists and require broad differential diagnoses.
- Aylesworth R, Vance JC. Demodex folliculorum and Demodex brevis in cutaneous biopsies. J Am Acad Dermatol. 1982;7:583-589.
- Kligman AM, Christensen MS. Demodex folliculorum: requirements for understanding its role in human skin disease. J Invest Dermatol. 2011;131:8-10.
- Zomorodian K, Geramishoar M, Saadat F, et al. Facial demodicosis. Eur J Dermatol. 2004;14:121-122.
- Chang YS, Huang YC. Role of Demodex mite infestation in rosacea: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:441-447.
- Prindaville B, Belazarian L, Levin NA, et al. Pityrosporum folliculitis: a retrospective review of 110 cases. J Am Acad Dermatol. 2018;78:511-514.
- Lankerani L, Thompson R. Eosinophilic pustular folliculitis: case report and review of the literature. Cutis. 2010;86:190-194.
- Hölzle ER, Kligman AM. The pathogenesis of miliaria rubra. role of the resident microflora. Br J Dermatol. 1978;99:117-137.
- Al-Hilo MM, Al-Saedy SJ, Alwan AI. Atypical presentation of miliaria in Iraqi patients attending Al-Kindy Teaching Hospital in Baghdad: a clinical descriptive study. Am J Dermatol Venereol. 2012;1:41-46.
The Diagnosis: Demodicosis
A clinical diagnosis of facial demodicosis triggered by topical corticosteroid therapy was suspected in our patient. A 3-mm punch biopsy of the right forehead demonstrated a follicular infundibulum rich in Demodex folliculorum with a discrete mononuclear infiltrate in the dermis (Figure 1), confirming the diagnosis. The patient was prescribed metronidazole gel 2% twice daily. Complete clearance of the facial rash was observed at 1-week follow-up (Figure 2).


Demodex folliculorum is a mite that parasitizes the pilosebaceous follicles of human skin, becoming pathogenic with excessive colonization.1-3 Facial demodicosis presents as pruritic papules and pustules in a pilosebaceous distribution on the face.2,3 The diagnosis of facial demodicosis can be difficult due to similarities with many other common facial rashes such as acne, rosacea, contact dermatitis, and folliculitis.
Similar to facial demodicosis, rosacea can present with erythema, papules, and pustules on the face. Patients with rosacea also have a higher prevalence and degree of Demodex mite infestation.4 However, these facial rashes can be differentiated when symptom acuity and personal and family histories are considered. In patients with a long-standing personal and/or family history of erythema, papules, and pustules, the chronicity of disease implies a diagnosis of rosacea. The acute onset of symptomatology and absence of personal or family history of rosacea in our patient favored a diagnosis of demodicosis.
Pityrosporum folliculitis is an eruption caused by Malassezia furfur, the organism implicated in tinea versicolor. It can present similarly to Demodex folliculitis with follicular papules and pustules on the forehead and back. Features that help to differentiate Pityrosporum folliculitis from Demodex folliculitis include involvement of the upper trunk and onset after antibiotic usage.5 Diagnosis of Pityrosporum folliculitis can be confirmed by potassium hydroxide preparation, which demonstrates spaghetti-and-meatball-like hyphae and spores.
Eosinophilic folliculitis is a chronic sterile folliculitis that usually presents as intensely pruritic papules and pustules on the face with an eruptive onset. There are 3 variants of disease: the classic form (also known as Ofuji disease), immunosuppression-associated disease, and infancy-associated disease.6 Eosinophilic folliculitis also may present with annular plaques and peripheral blood eosinophilia, and it is more prevalent in patients of Japanese descent. Differentiation from Demodex folliculitis can be done by histologic examination, which demonstrates spongiosis with exocytosis of eosinophils into the epithelium and a clear deficiency of infiltrating mites.6
Miliaria, also known as sweat rash, is a common condition that occurs due to occlusion of eccrine sweat glands.7 Clinically, miliaria is characterized by erythematous papules ranging from 2 to 4 mm in size that may be vesicular or pustular. Miliaria and demodicosis may have similar clinical presentations; however, several characteristic differences can be noted. Based on the pathophysiology, miliaria will not be folliculocentric, which differs from demodicosis. Additionally, miliaria most commonly occurs in areas of occlusion, such as in skin folds or on the trunk under tight clothing. Miliaria rarely can appear confluent and sunburnlike.8 Furthermore, miliaria is less common on the face, while demodicosis almost exclusively is found on the face.
We present the case of an otherwise healthy patient with acute onset of pruritic papules and pustules involving the superior face following topical corticosteroid use. Similar cases frequently are encountered by dermatologists and require broad differential diagnoses.
The Diagnosis: Demodicosis
A clinical diagnosis of facial demodicosis triggered by topical corticosteroid therapy was suspected in our patient. A 3-mm punch biopsy of the right forehead demonstrated a follicular infundibulum rich in Demodex folliculorum with a discrete mononuclear infiltrate in the dermis (Figure 1), confirming the diagnosis. The patient was prescribed metronidazole gel 2% twice daily. Complete clearance of the facial rash was observed at 1-week follow-up (Figure 2).


Demodex folliculorum is a mite that parasitizes the pilosebaceous follicles of human skin, becoming pathogenic with excessive colonization.1-3 Facial demodicosis presents as pruritic papules and pustules in a pilosebaceous distribution on the face.2,3 The diagnosis of facial demodicosis can be difficult due to similarities with many other common facial rashes such as acne, rosacea, contact dermatitis, and folliculitis.
Similar to facial demodicosis, rosacea can present with erythema, papules, and pustules on the face. Patients with rosacea also have a higher prevalence and degree of Demodex mite infestation.4 However, these facial rashes can be differentiated when symptom acuity and personal and family histories are considered. In patients with a long-standing personal and/or family history of erythema, papules, and pustules, the chronicity of disease implies a diagnosis of rosacea. The acute onset of symptomatology and absence of personal or family history of rosacea in our patient favored a diagnosis of demodicosis.
Pityrosporum folliculitis is an eruption caused by Malassezia furfur, the organism implicated in tinea versicolor. It can present similarly to Demodex folliculitis with follicular papules and pustules on the forehead and back. Features that help to differentiate Pityrosporum folliculitis from Demodex folliculitis include involvement of the upper trunk and onset after antibiotic usage.5 Diagnosis of Pityrosporum folliculitis can be confirmed by potassium hydroxide preparation, which demonstrates spaghetti-and-meatball-like hyphae and spores.
Eosinophilic folliculitis is a chronic sterile folliculitis that usually presents as intensely pruritic papules and pustules on the face with an eruptive onset. There are 3 variants of disease: the classic form (also known as Ofuji disease), immunosuppression-associated disease, and infancy-associated disease.6 Eosinophilic folliculitis also may present with annular plaques and peripheral blood eosinophilia, and it is more prevalent in patients of Japanese descent. Differentiation from Demodex folliculitis can be done by histologic examination, which demonstrates spongiosis with exocytosis of eosinophils into the epithelium and a clear deficiency of infiltrating mites.6
Miliaria, also known as sweat rash, is a common condition that occurs due to occlusion of eccrine sweat glands.7 Clinically, miliaria is characterized by erythematous papules ranging from 2 to 4 mm in size that may be vesicular or pustular. Miliaria and demodicosis may have similar clinical presentations; however, several characteristic differences can be noted. Based on the pathophysiology, miliaria will not be folliculocentric, which differs from demodicosis. Additionally, miliaria most commonly occurs in areas of occlusion, such as in skin folds or on the trunk under tight clothing. Miliaria rarely can appear confluent and sunburnlike.8 Furthermore, miliaria is less common on the face, while demodicosis almost exclusively is found on the face.
We present the case of an otherwise healthy patient with acute onset of pruritic papules and pustules involving the superior face following topical corticosteroid use. Similar cases frequently are encountered by dermatologists and require broad differential diagnoses.
- Aylesworth R, Vance JC. Demodex folliculorum and Demodex brevis in cutaneous biopsies. J Am Acad Dermatol. 1982;7:583-589.
- Kligman AM, Christensen MS. Demodex folliculorum: requirements for understanding its role in human skin disease. J Invest Dermatol. 2011;131:8-10.
- Zomorodian K, Geramishoar M, Saadat F, et al. Facial demodicosis. Eur J Dermatol. 2004;14:121-122.
- Chang YS, Huang YC. Role of Demodex mite infestation in rosacea: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:441-447.
- Prindaville B, Belazarian L, Levin NA, et al. Pityrosporum folliculitis: a retrospective review of 110 cases. J Am Acad Dermatol. 2018;78:511-514.
- Lankerani L, Thompson R. Eosinophilic pustular folliculitis: case report and review of the literature. Cutis. 2010;86:190-194.
- Hölzle ER, Kligman AM. The pathogenesis of miliaria rubra. role of the resident microflora. Br J Dermatol. 1978;99:117-137.
- Al-Hilo MM, Al-Saedy SJ, Alwan AI. Atypical presentation of miliaria in Iraqi patients attending Al-Kindy Teaching Hospital in Baghdad: a clinical descriptive study. Am J Dermatol Venereol. 2012;1:41-46.
- Aylesworth R, Vance JC. Demodex folliculorum and Demodex brevis in cutaneous biopsies. J Am Acad Dermatol. 1982;7:583-589.
- Kligman AM, Christensen MS. Demodex folliculorum: requirements for understanding its role in human skin disease. J Invest Dermatol. 2011;131:8-10.
- Zomorodian K, Geramishoar M, Saadat F, et al. Facial demodicosis. Eur J Dermatol. 2004;14:121-122.
- Chang YS, Huang YC. Role of Demodex mite infestation in rosacea: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:441-447.
- Prindaville B, Belazarian L, Levin NA, et al. Pityrosporum folliculitis: a retrospective review of 110 cases. J Am Acad Dermatol. 2018;78:511-514.
- Lankerani L, Thompson R. Eosinophilic pustular folliculitis: case report and review of the literature. Cutis. 2010;86:190-194.
- Hölzle ER, Kligman AM. The pathogenesis of miliaria rubra. role of the resident microflora. Br J Dermatol. 1978;99:117-137.
- Al-Hilo MM, Al-Saedy SJ, Alwan AI. Atypical presentation of miliaria in Iraqi patients attending Al-Kindy Teaching Hospital in Baghdad: a clinical descriptive study. Am J Dermatol Venereol. 2012;1:41-46.
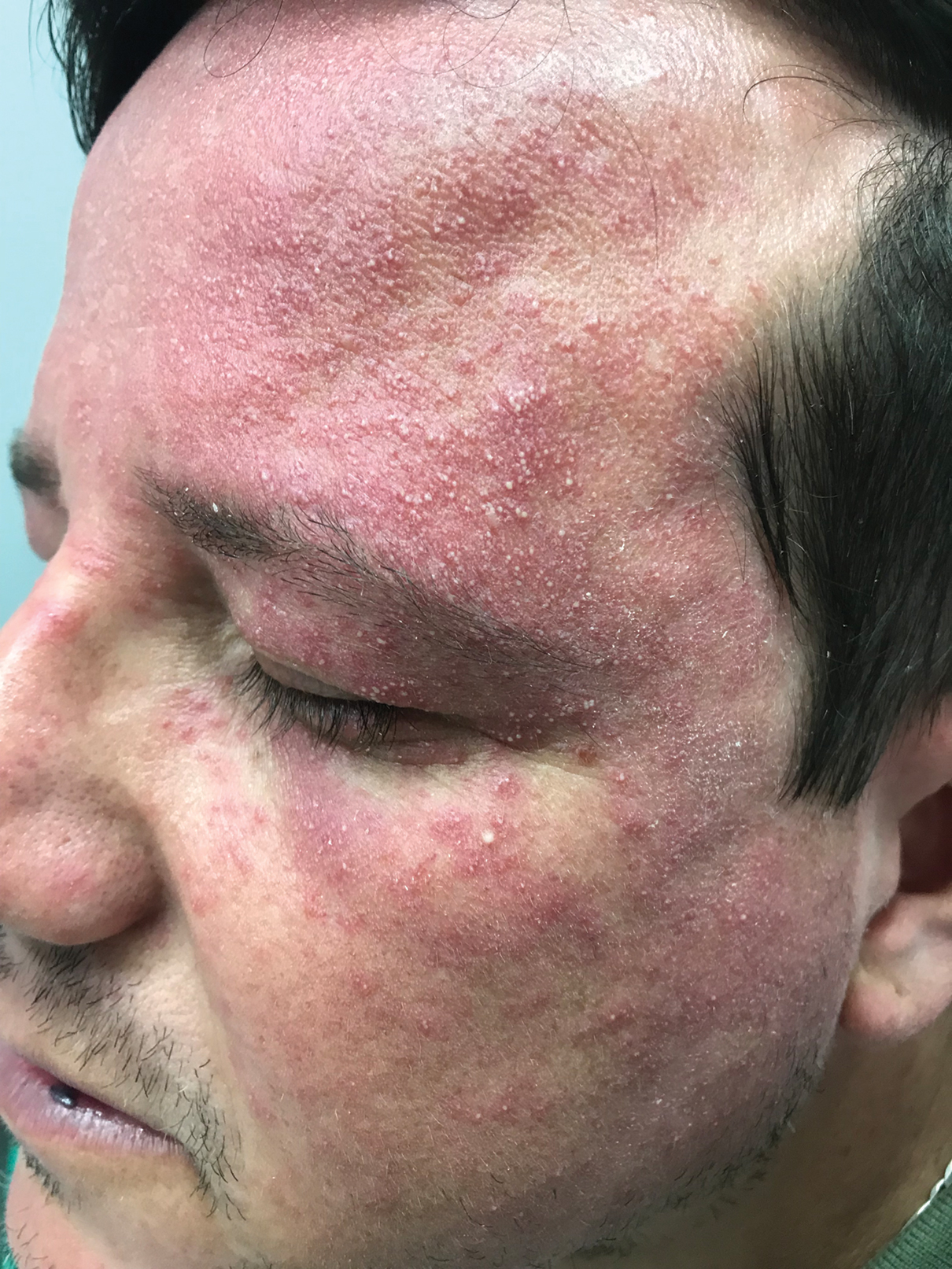
A 37-year-old man presented with a progressively pruritic and pustular eruption on the face of 2 weeks’ duration. Twenty days prior to the rash onset, he began treatment for scalp psoriasis with a mixture of salicylic acid 20 mg/mL and betamethasone dipropionate 0.5 mg/mL, which inadvertently extended to the facial area. One week after rash onset, he presented to the emergency department at a local hospital, where he was given intravenous hydrocortisone 100 mg with no improvement of the rash. He had no history of skin cancer, and his family history was negative for dermatologic disease. At the time of examination, he had an erythematous eruption of follicular micropustules on the forehead, upper and lower eyelids, temples, cheeks, and mandible.
Improving health disparities starts with acknowledging structural racism
Earlier this spring, Kimberly D. Manning, MD, FACP, FAAP, was caring for an elderly Black man with multiple comorbidities at Grady Memorial Hospital in Atlanta, assembling an order for medications and a discharge plan.
“It was very challenging,” Dr. Manning, professor of medicine and associate vice chair of diversity, equity, and inclusion at Emory University, Atlanta, recalled during a May 4 session at SHM Converge, the annual conference of the Society of Hospital Medicine.
At one point, the patient glanced at her, shrugged, and said: “You know, Doc, we get in where we fit in.”
“He was talking about the idea that people who come from historically disadvantaged backgrounds just have to try to figure it out, have to try to make a dollar out of 15 cents,” Dr. Manning said. “This, to me, really underscores what we mean when we say health disparities, this idea that there are people who are working hard and doing the best that they can but who still are forced to ‘get in where they fit in.’”
The Centers for Disease Control and Prevention defines health disparities as preventable differences in the burden of disease, injury, violence, or opportunities to achieve optimal health that are experienced by socially disadvantaged populations. “When we think about health disparities we often think about many diagnoses,” Dr. Manning continued. “We think about HIV and the disparate care and outcomes we’ve seen in populations of individuals who come from minority backgrounds. We see disparities in obesity, cancer, cardiovascular disease, infant mortality and maternal death, hospital readmissions, and COVID-19. We know that people who do not have access to health care or to healthy neighborhoods and environments or who are economically disadvantaged have poorer outcomes. It plays out with all of these diagnoses.”
In her opinion, health disparities in hospital medicine fall into in one of three buckets: diagnosis and triage, hospital stay and treatment, and sticking the landing – “that is, after a patient leaves the hospital,” Dr. Manning explained. “The hospital stay is the turn on the balance beam. You can do everything perfectly, but then you must dismount. To score a ‘10’ you have to stick the landing. That means being able to get your medications, being able to get to and from clinic appointments, being able to understand the directions you’ve been given. All of these things are intertwined, the inpatient and outpatient care.”
The roots of health disparities in hospitalized patients stem from centuries ago, she said, when America’s health care system was built to benefit white male landowners and their families. Health care for Blacks, on the other hand, “was focused on function, almost like veterinary care, or experimentation,” Dr. Manning said. “After slavery ended, many historically Black institutions of higher learning opened, including medical schools. In 1909, there were seven historically Black medical schools. Acknowledging the history that preceded disparities is essential.”
In her view, the path to improving health care disparities starts with conceding that structural racism exists in the practice of medicine. “This means that health disparities are connected to systemic and individual issues, including our biases,” Dr. Manning said. “Our system was built on this idea that there is greater value of one group of people above others. Access to care, physician workforce, and biases are impacted by system design. Health equity and health equality are not the same.”
She also underscored the importance of the social determinants of health, or “those things we need to be healthy,” including economic stability, neighborhood and physical environment, educational opportunities, access to good food, community and social context, and the idea of health care as a human right and understanding our health care system. “This is what is necessary,” she declared. “Without all of these together, we can’t have the health outcomes that we desire.”
As hospital leaders work to build a more diverse physician workforce, Dr. Manning emphasized the importance of forming antiracism policies by addressing questions such as what will we not stand for? How will we protect and create psychologically safe environments? What is our commitment to diversity in leadership and in trainees? What is our commitment to implicit bias training and bystander training?
“We have to get uncomfortable enough to advocate with urgency because all of these are necessary factors to mitigate health disparities,” she said. “Though the systemic issues are the most urgent, on an individual level, we must continue to disrupt the negative ideology and stereotypes that threaten our environment every day. When we see those negative things, we have to call them out. We need to continue to listen, to humanize those things that are happening around us, and to understand historical context.”
Dr. Manning reported having no financial disclosures.
Earlier this spring, Kimberly D. Manning, MD, FACP, FAAP, was caring for an elderly Black man with multiple comorbidities at Grady Memorial Hospital in Atlanta, assembling an order for medications and a discharge plan.
“It was very challenging,” Dr. Manning, professor of medicine and associate vice chair of diversity, equity, and inclusion at Emory University, Atlanta, recalled during a May 4 session at SHM Converge, the annual conference of the Society of Hospital Medicine.
At one point, the patient glanced at her, shrugged, and said: “You know, Doc, we get in where we fit in.”
“He was talking about the idea that people who come from historically disadvantaged backgrounds just have to try to figure it out, have to try to make a dollar out of 15 cents,” Dr. Manning said. “This, to me, really underscores what we mean when we say health disparities, this idea that there are people who are working hard and doing the best that they can but who still are forced to ‘get in where they fit in.’”
The Centers for Disease Control and Prevention defines health disparities as preventable differences in the burden of disease, injury, violence, or opportunities to achieve optimal health that are experienced by socially disadvantaged populations. “When we think about health disparities we often think about many diagnoses,” Dr. Manning continued. “We think about HIV and the disparate care and outcomes we’ve seen in populations of individuals who come from minority backgrounds. We see disparities in obesity, cancer, cardiovascular disease, infant mortality and maternal death, hospital readmissions, and COVID-19. We know that people who do not have access to health care or to healthy neighborhoods and environments or who are economically disadvantaged have poorer outcomes. It plays out with all of these diagnoses.”
In her opinion, health disparities in hospital medicine fall into in one of three buckets: diagnosis and triage, hospital stay and treatment, and sticking the landing – “that is, after a patient leaves the hospital,” Dr. Manning explained. “The hospital stay is the turn on the balance beam. You can do everything perfectly, but then you must dismount. To score a ‘10’ you have to stick the landing. That means being able to get your medications, being able to get to and from clinic appointments, being able to understand the directions you’ve been given. All of these things are intertwined, the inpatient and outpatient care.”
The roots of health disparities in hospitalized patients stem from centuries ago, she said, when America’s health care system was built to benefit white male landowners and their families. Health care for Blacks, on the other hand, “was focused on function, almost like veterinary care, or experimentation,” Dr. Manning said. “After slavery ended, many historically Black institutions of higher learning opened, including medical schools. In 1909, there were seven historically Black medical schools. Acknowledging the history that preceded disparities is essential.”
In her view, the path to improving health care disparities starts with conceding that structural racism exists in the practice of medicine. “This means that health disparities are connected to systemic and individual issues, including our biases,” Dr. Manning said. “Our system was built on this idea that there is greater value of one group of people above others. Access to care, physician workforce, and biases are impacted by system design. Health equity and health equality are not the same.”
She also underscored the importance of the social determinants of health, or “those things we need to be healthy,” including economic stability, neighborhood and physical environment, educational opportunities, access to good food, community and social context, and the idea of health care as a human right and understanding our health care system. “This is what is necessary,” she declared. “Without all of these together, we can’t have the health outcomes that we desire.”
As hospital leaders work to build a more diverse physician workforce, Dr. Manning emphasized the importance of forming antiracism policies by addressing questions such as what will we not stand for? How will we protect and create psychologically safe environments? What is our commitment to diversity in leadership and in trainees? What is our commitment to implicit bias training and bystander training?
“We have to get uncomfortable enough to advocate with urgency because all of these are necessary factors to mitigate health disparities,” she said. “Though the systemic issues are the most urgent, on an individual level, we must continue to disrupt the negative ideology and stereotypes that threaten our environment every day. When we see those negative things, we have to call them out. We need to continue to listen, to humanize those things that are happening around us, and to understand historical context.”
Dr. Manning reported having no financial disclosures.
Earlier this spring, Kimberly D. Manning, MD, FACP, FAAP, was caring for an elderly Black man with multiple comorbidities at Grady Memorial Hospital in Atlanta, assembling an order for medications and a discharge plan.
“It was very challenging,” Dr. Manning, professor of medicine and associate vice chair of diversity, equity, and inclusion at Emory University, Atlanta, recalled during a May 4 session at SHM Converge, the annual conference of the Society of Hospital Medicine.
At one point, the patient glanced at her, shrugged, and said: “You know, Doc, we get in where we fit in.”
“He was talking about the idea that people who come from historically disadvantaged backgrounds just have to try to figure it out, have to try to make a dollar out of 15 cents,” Dr. Manning said. “This, to me, really underscores what we mean when we say health disparities, this idea that there are people who are working hard and doing the best that they can but who still are forced to ‘get in where they fit in.’”
The Centers for Disease Control and Prevention defines health disparities as preventable differences in the burden of disease, injury, violence, or opportunities to achieve optimal health that are experienced by socially disadvantaged populations. “When we think about health disparities we often think about many diagnoses,” Dr. Manning continued. “We think about HIV and the disparate care and outcomes we’ve seen in populations of individuals who come from minority backgrounds. We see disparities in obesity, cancer, cardiovascular disease, infant mortality and maternal death, hospital readmissions, and COVID-19. We know that people who do not have access to health care or to healthy neighborhoods and environments or who are economically disadvantaged have poorer outcomes. It plays out with all of these diagnoses.”
In her opinion, health disparities in hospital medicine fall into in one of three buckets: diagnosis and triage, hospital stay and treatment, and sticking the landing – “that is, after a patient leaves the hospital,” Dr. Manning explained. “The hospital stay is the turn on the balance beam. You can do everything perfectly, but then you must dismount. To score a ‘10’ you have to stick the landing. That means being able to get your medications, being able to get to and from clinic appointments, being able to understand the directions you’ve been given. All of these things are intertwined, the inpatient and outpatient care.”
The roots of health disparities in hospitalized patients stem from centuries ago, she said, when America’s health care system was built to benefit white male landowners and their families. Health care for Blacks, on the other hand, “was focused on function, almost like veterinary care, or experimentation,” Dr. Manning said. “After slavery ended, many historically Black institutions of higher learning opened, including medical schools. In 1909, there were seven historically Black medical schools. Acknowledging the history that preceded disparities is essential.”
In her view, the path to improving health care disparities starts with conceding that structural racism exists in the practice of medicine. “This means that health disparities are connected to systemic and individual issues, including our biases,” Dr. Manning said. “Our system was built on this idea that there is greater value of one group of people above others. Access to care, physician workforce, and biases are impacted by system design. Health equity and health equality are not the same.”
She also underscored the importance of the social determinants of health, or “those things we need to be healthy,” including economic stability, neighborhood and physical environment, educational opportunities, access to good food, community and social context, and the idea of health care as a human right and understanding our health care system. “This is what is necessary,” she declared. “Without all of these together, we can’t have the health outcomes that we desire.”
As hospital leaders work to build a more diverse physician workforce, Dr. Manning emphasized the importance of forming antiracism policies by addressing questions such as what will we not stand for? How will we protect and create psychologically safe environments? What is our commitment to diversity in leadership and in trainees? What is our commitment to implicit bias training and bystander training?
“We have to get uncomfortable enough to advocate with urgency because all of these are necessary factors to mitigate health disparities,” she said. “Though the systemic issues are the most urgent, on an individual level, we must continue to disrupt the negative ideology and stereotypes that threaten our environment every day. When we see those negative things, we have to call them out. We need to continue to listen, to humanize those things that are happening around us, and to understand historical context.”
Dr. Manning reported having no financial disclosures.
FROM SHM CONVERGE 2021
Preventing endoscopist injuries starts with ergonomics
Endoscopists are at high risk of musculoskeletal issues, and a multifaceted strategy is needed to reduce rates of injury, including better body posture and endoscopic suite layout, according to leading experts.
Latha Alaparthi, MD, director of committee operations at Gastroenterology Center of Connecticut, Hamden, and assistant clinical professor at Yale University, New Haven, Conn., noted that female gastroenterologists are at particular risk because they often work with outsize equipment and suboptimal room setup.
“I think it’s something for us to recognize, and [we need to] find ways to protect ourselves,” Dr. Alaparthi said during a virtual presentation at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Prevalence of musculoskeletal injuries in gastroenterology
Gastroenterologists spend 43% of their time performing procedures, Dr. Alaparthi said, and all those hours take a toll on the body. Up to 89% of gastroenterologists report musculoskeletal symptoms – most often back pain, followed by neck pain and hand pain.
Even newcomers to the field are at risk, she added, noting that 47% of gastroenterology fellows report injury in their first year of training. And with one out of three fellows now female, the issue may be a growing concern.
“As female gastroenterologists, we are even more at risk,” Dr. Alaparthi said. This is partly due to differences in equipment and room design, which “take into consideration 5% of female average measurements and 95% of that of males.”
The resultant injuries may be enough to drive female doctors from the field. Dr. Alaparthi recounted her colleague’s experience in leaving gastroenterology for the pharmaceutical industry after experiencing ongoing neck pain.
“She called me and said 1 week after she stopped doing endoscopies, her neck pain was gone.”
For gastroenterologists of any gender, musculoskeletal injuries can cause pain and suffering, reduced quality of life, lost or reduced work output, short-term or permanent disability, lost wages, and impediment to career advancement. Yet physicians aren’t the only stakeholders affected by these injuries. Employers stand to lose financially from decreased productivity and increased compensation costs.
“[Injuries have] implications not just to the individual but to the company and to patient care,” Dr. Alaparthi said.
She went on to suggest that an effective solution to the problem will require efforts from both gastroenterologists and institutions, including greater self-awareness of body positioning, access to anthropometrically suitable equipment, better room design, and a work culture that supports breaks during procedures, if needed.
“We definitely need programs to provide comprehensive work force injury prevention and protection specific to GI endoscopy – not just for gastroenterologists, but for the whole team involved.”
Ergonomics in endoscopy training
Presenting after Dr. Alaparthi, Katherine Garman, MD, associate professor of medicine and vice chief of research, gastroenterology, at Duke University, Durham, N.C., offered ways to incorporate ergonomics into an endoscopy training curriculum.
“Ergonomics evaluates how a job can best fit to an individual, instead of forcing an individual to fit into a job,” Dr. Garman said. “[This] is a really important concept when we think about training,”
Yet this concept may run counter to most fellows’ natural instinct to fit in and avoid being obtrusive, she noted.
“We need to think about empowering [fellows] from the very beginning to be proactive about how [they] interact with the equipment and the space,” Dr. Garman said. “[They should know] it is perfectly acceptable to adjust the monitor height, move the bed height to an appropriate level, and make the space comfortable ... at the beginning of what should be a long, productive career.”
Dr. Garman offered several more key points to include in a training program, including increased postural awareness, microbreaks during procedures, and early intervention for prior injuries that may increase risk.
“We’ve had fellows who’ve come in who’ve had fractures, wrist [injuries], shoulder injuries,” she said. “We advise early consultation with a physical therapist for those fellows.”
In a recently published study, Dr. Garman and colleagues invited a physical therapist into the endoscopy suite, allowing for real-time assessment of ergonomic positioning and posturing, as well as wellness planning. Out of eight participating endoscopists, all said that the posture education and procedure suite recommendations were helpful, 87.5% said that the pictures of their posture and movement analysis were helpful, 50% said that the pain education was helpful, and 25% found the personalized exercise plans helpful.
“Endoscopists are not always excited about doing exercises at home,” Dr. Garman said.
The ergonomically optimized endoscopy suite
In the next presentation, Mehnaz Shafi, MD, professor of medicine and ad interim chair of the department of gastroenterology, hepatology, and nutrition at MD Anderson Cancer Center, Houston, described how clinicians and institutions can create ergonomically optimized endoscopy suites.
She began by reviewing specific causes of injury, including repetitive motion, high pinch force, and awkward posture, the latter of which can lead to microtrauma, inflammation, and connective tissue injury.
According to Dr. Shafi, endoscopists should stand in a neutral position with back straight and knees slightly bent. The patient should be positioned at the edge of the bed, which should be 85-120 cm off the floor. Monitors should be 93-162 cm off the floor and 15-25 degrees below eye level. When interacting with multiple monitors, endoscopists should rotate their entire bodies to maintain a neutral position. Hands and elbows also should be kept neutral, with less than 10 degrees of angulation from the height of the bed. To ensure safer hand grip, Dr. Shafi suggested removing any cord loops that may increase tension and using a towel to more evenly distribute gripping force.
Finally, Dr. Shafi encouraged awareness of other room hazards, such as slippery floors and exposed wires and tubing.
The presenters reported having no conflicts of interest.
This article was updated May 5, 2021.
Endoscopists are at high risk of musculoskeletal issues, and a multifaceted strategy is needed to reduce rates of injury, including better body posture and endoscopic suite layout, according to leading experts.
Latha Alaparthi, MD, director of committee operations at Gastroenterology Center of Connecticut, Hamden, and assistant clinical professor at Yale University, New Haven, Conn., noted that female gastroenterologists are at particular risk because they often work with outsize equipment and suboptimal room setup.
“I think it’s something for us to recognize, and [we need to] find ways to protect ourselves,” Dr. Alaparthi said during a virtual presentation at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Prevalence of musculoskeletal injuries in gastroenterology
Gastroenterologists spend 43% of their time performing procedures, Dr. Alaparthi said, and all those hours take a toll on the body. Up to 89% of gastroenterologists report musculoskeletal symptoms – most often back pain, followed by neck pain and hand pain.
Even newcomers to the field are at risk, she added, noting that 47% of gastroenterology fellows report injury in their first year of training. And with one out of three fellows now female, the issue may be a growing concern.
“As female gastroenterologists, we are even more at risk,” Dr. Alaparthi said. This is partly due to differences in equipment and room design, which “take into consideration 5% of female average measurements and 95% of that of males.”
The resultant injuries may be enough to drive female doctors from the field. Dr. Alaparthi recounted her colleague’s experience in leaving gastroenterology for the pharmaceutical industry after experiencing ongoing neck pain.
“She called me and said 1 week after she stopped doing endoscopies, her neck pain was gone.”
For gastroenterologists of any gender, musculoskeletal injuries can cause pain and suffering, reduced quality of life, lost or reduced work output, short-term or permanent disability, lost wages, and impediment to career advancement. Yet physicians aren’t the only stakeholders affected by these injuries. Employers stand to lose financially from decreased productivity and increased compensation costs.
“[Injuries have] implications not just to the individual but to the company and to patient care,” Dr. Alaparthi said.
She went on to suggest that an effective solution to the problem will require efforts from both gastroenterologists and institutions, including greater self-awareness of body positioning, access to anthropometrically suitable equipment, better room design, and a work culture that supports breaks during procedures, if needed.
“We definitely need programs to provide comprehensive work force injury prevention and protection specific to GI endoscopy – not just for gastroenterologists, but for the whole team involved.”
Ergonomics in endoscopy training
Presenting after Dr. Alaparthi, Katherine Garman, MD, associate professor of medicine and vice chief of research, gastroenterology, at Duke University, Durham, N.C., offered ways to incorporate ergonomics into an endoscopy training curriculum.
“Ergonomics evaluates how a job can best fit to an individual, instead of forcing an individual to fit into a job,” Dr. Garman said. “[This] is a really important concept when we think about training,”
Yet this concept may run counter to most fellows’ natural instinct to fit in and avoid being obtrusive, she noted.
“We need to think about empowering [fellows] from the very beginning to be proactive about how [they] interact with the equipment and the space,” Dr. Garman said. “[They should know] it is perfectly acceptable to adjust the monitor height, move the bed height to an appropriate level, and make the space comfortable ... at the beginning of what should be a long, productive career.”
Dr. Garman offered several more key points to include in a training program, including increased postural awareness, microbreaks during procedures, and early intervention for prior injuries that may increase risk.
“We’ve had fellows who’ve come in who’ve had fractures, wrist [injuries], shoulder injuries,” she said. “We advise early consultation with a physical therapist for those fellows.”
In a recently published study, Dr. Garman and colleagues invited a physical therapist into the endoscopy suite, allowing for real-time assessment of ergonomic positioning and posturing, as well as wellness planning. Out of eight participating endoscopists, all said that the posture education and procedure suite recommendations were helpful, 87.5% said that the pictures of their posture and movement analysis were helpful, 50% said that the pain education was helpful, and 25% found the personalized exercise plans helpful.
“Endoscopists are not always excited about doing exercises at home,” Dr. Garman said.
The ergonomically optimized endoscopy suite
In the next presentation, Mehnaz Shafi, MD, professor of medicine and ad interim chair of the department of gastroenterology, hepatology, and nutrition at MD Anderson Cancer Center, Houston, described how clinicians and institutions can create ergonomically optimized endoscopy suites.
She began by reviewing specific causes of injury, including repetitive motion, high pinch force, and awkward posture, the latter of which can lead to microtrauma, inflammation, and connective tissue injury.
According to Dr. Shafi, endoscopists should stand in a neutral position with back straight and knees slightly bent. The patient should be positioned at the edge of the bed, which should be 85-120 cm off the floor. Monitors should be 93-162 cm off the floor and 15-25 degrees below eye level. When interacting with multiple monitors, endoscopists should rotate their entire bodies to maintain a neutral position. Hands and elbows also should be kept neutral, with less than 10 degrees of angulation from the height of the bed. To ensure safer hand grip, Dr. Shafi suggested removing any cord loops that may increase tension and using a towel to more evenly distribute gripping force.
Finally, Dr. Shafi encouraged awareness of other room hazards, such as slippery floors and exposed wires and tubing.
The presenters reported having no conflicts of interest.
This article was updated May 5, 2021.
Endoscopists are at high risk of musculoskeletal issues, and a multifaceted strategy is needed to reduce rates of injury, including better body posture and endoscopic suite layout, according to leading experts.
Latha Alaparthi, MD, director of committee operations at Gastroenterology Center of Connecticut, Hamden, and assistant clinical professor at Yale University, New Haven, Conn., noted that female gastroenterologists are at particular risk because they often work with outsize equipment and suboptimal room setup.
“I think it’s something for us to recognize, and [we need to] find ways to protect ourselves,” Dr. Alaparthi said during a virtual presentation at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Prevalence of musculoskeletal injuries in gastroenterology
Gastroenterologists spend 43% of their time performing procedures, Dr. Alaparthi said, and all those hours take a toll on the body. Up to 89% of gastroenterologists report musculoskeletal symptoms – most often back pain, followed by neck pain and hand pain.
Even newcomers to the field are at risk, she added, noting that 47% of gastroenterology fellows report injury in their first year of training. And with one out of three fellows now female, the issue may be a growing concern.
“As female gastroenterologists, we are even more at risk,” Dr. Alaparthi said. This is partly due to differences in equipment and room design, which “take into consideration 5% of female average measurements and 95% of that of males.”
The resultant injuries may be enough to drive female doctors from the field. Dr. Alaparthi recounted her colleague’s experience in leaving gastroenterology for the pharmaceutical industry after experiencing ongoing neck pain.
“She called me and said 1 week after she stopped doing endoscopies, her neck pain was gone.”
For gastroenterologists of any gender, musculoskeletal injuries can cause pain and suffering, reduced quality of life, lost or reduced work output, short-term or permanent disability, lost wages, and impediment to career advancement. Yet physicians aren’t the only stakeholders affected by these injuries. Employers stand to lose financially from decreased productivity and increased compensation costs.
“[Injuries have] implications not just to the individual but to the company and to patient care,” Dr. Alaparthi said.
She went on to suggest that an effective solution to the problem will require efforts from both gastroenterologists and institutions, including greater self-awareness of body positioning, access to anthropometrically suitable equipment, better room design, and a work culture that supports breaks during procedures, if needed.
“We definitely need programs to provide comprehensive work force injury prevention and protection specific to GI endoscopy – not just for gastroenterologists, but for the whole team involved.”
Ergonomics in endoscopy training
Presenting after Dr. Alaparthi, Katherine Garman, MD, associate professor of medicine and vice chief of research, gastroenterology, at Duke University, Durham, N.C., offered ways to incorporate ergonomics into an endoscopy training curriculum.
“Ergonomics evaluates how a job can best fit to an individual, instead of forcing an individual to fit into a job,” Dr. Garman said. “[This] is a really important concept when we think about training,”
Yet this concept may run counter to most fellows’ natural instinct to fit in and avoid being obtrusive, she noted.
“We need to think about empowering [fellows] from the very beginning to be proactive about how [they] interact with the equipment and the space,” Dr. Garman said. “[They should know] it is perfectly acceptable to adjust the monitor height, move the bed height to an appropriate level, and make the space comfortable ... at the beginning of what should be a long, productive career.”
Dr. Garman offered several more key points to include in a training program, including increased postural awareness, microbreaks during procedures, and early intervention for prior injuries that may increase risk.
“We’ve had fellows who’ve come in who’ve had fractures, wrist [injuries], shoulder injuries,” she said. “We advise early consultation with a physical therapist for those fellows.”
In a recently published study, Dr. Garman and colleagues invited a physical therapist into the endoscopy suite, allowing for real-time assessment of ergonomic positioning and posturing, as well as wellness planning. Out of eight participating endoscopists, all said that the posture education and procedure suite recommendations were helpful, 87.5% said that the pictures of their posture and movement analysis were helpful, 50% said that the pain education was helpful, and 25% found the personalized exercise plans helpful.
“Endoscopists are not always excited about doing exercises at home,” Dr. Garman said.
The ergonomically optimized endoscopy suite
In the next presentation, Mehnaz Shafi, MD, professor of medicine and ad interim chair of the department of gastroenterology, hepatology, and nutrition at MD Anderson Cancer Center, Houston, described how clinicians and institutions can create ergonomically optimized endoscopy suites.
She began by reviewing specific causes of injury, including repetitive motion, high pinch force, and awkward posture, the latter of which can lead to microtrauma, inflammation, and connective tissue injury.
According to Dr. Shafi, endoscopists should stand in a neutral position with back straight and knees slightly bent. The patient should be positioned at the edge of the bed, which should be 85-120 cm off the floor. Monitors should be 93-162 cm off the floor and 15-25 degrees below eye level. When interacting with multiple monitors, endoscopists should rotate their entire bodies to maintain a neutral position. Hands and elbows also should be kept neutral, with less than 10 degrees of angulation from the height of the bed. To ensure safer hand grip, Dr. Shafi suggested removing any cord loops that may increase tension and using a towel to more evenly distribute gripping force.
Finally, Dr. Shafi encouraged awareness of other room hazards, such as slippery floors and exposed wires and tubing.
The presenters reported having no conflicts of interest.
This article was updated May 5, 2021.
FROM 2021 AGA TECH SUMMIT MEETING
Formal geriatric assessment should be routine
a geriatric oncologist said during a presentation at the American College of Physicians annual Internal Medicine meeting.
A 2020 ASCO survey, which the speaker, Grant R. Williams, MD, coauthored, found that 9 out of 10 community oncologists assessed at least some older patients differently than younger patients. But only 1 out of 3 did so in a formal manner, Dr. Williams, director of the cancer and aging program at the University of Alabama at Birmingham, said during presentation at virtual meeting.
In most cases, informal geriatric assessment considers only the tip of the ‘geriatric oncology iceberg,’ including chronological age, performance status, tumor characteristics, and organ function, Dr. Williams noted.
In contrast, formal geriatric assessment dives deeper, measuring a series of additional outcome-associated factors: polypharmacy, comorbidities, falls, psychosocial dysfunction, social support, sarcopenia, nutritional deficits, cognitive impairment, and functional issues.
“All these other factors under the surface are critically important to developing a personalized and individualized cancer treatment plan for older adults,” Dr. Williams said.
He went on to explain that elderly cancer patients can be sorted into three broad categories: fit, vulnerable, and frail. Fit and frail patients are relatively easy to identify, but most elderly patients fall into the vulnerable category, Dr. Williams noted.
“It’s really more challenging to identify those individuals across the spectrum than those at the extremes,” Dr. Williams said, noting that formal geriatric assessment can detect problems not found routinely.
Formal geriatric assessment’s value
Geriatric assessment can be used for risk modeling and making life-expectancy calculations. It can also be used as an interventional tool, guiding cancer treatment selection, he said. Furthermore, it can open doors to general health interventions, such as occupational therapy, to reduce fall risk.
Beneficial interventions identified by geriatric assessment have been shown to improve function, reduce chemotherapy toxicities, improve quality of life, and extend survival, Dr. Williams noted.
Formal geriatric assessment may be particularly useful for primary care providers considering referral to an oncologist, he said.
“I think performing a geriatric assessment [prior to referral] would be a great idea. And that’s twofold: Even before you send them to the oncologist, it gives you an idea of how they may tolerate treatment, and frankly, it may give you an idea that they don’t need a referral to the oncologist if they’re particularly frail,” noted Dr. Williams.
Alternatives to formal assessments
When asked how providers can incorporate formal assessments into a busy day at the clinic, Dr. Williams encouraged the use of abbreviated formal assessments, then adding further testing if needed.
“Given known time and support staff restraints, modified geriatric assessment tools have been developed that are either mostly or completely patient-reported,” he said in an interview, referring to the Cancer and Aging Research Group (CARG) Geriatric Assessment and the Cancer and Aging Resilience Evaluation (CARE), respectively.
“[These assessments] can easily be completed before clinical visits or while in the waiting room,” Dr. Williams noted. “The additional objective tests, such as Timed Up and Go, and Mental Status Exam, can be completed if deemed necessary based on these initial assessments.”
Martine Extermann, MD, PhD, provided her suggestions in an interview for what physicians can do to get better outcomes for this patient group.
“The secret of successful anti-cancer treatment in an older person is to be proactive with supportive care,” said Dr. Extermann, leader of the senior adult oncology program at H. Lee Moffitt Cancer Center & Research Institute, Tampa, Fla. “You have to really plan ahead, identify the support gaps, identify the potential problems, and prevent them thoroughly. The upfront work of good patient evaluation will save you a lot of trouble down the line,” she added.
Ms. Extermann also mentioned the challenges to providing care to geriatric patients with cancer, including a lack of financial incentive for physicians to specialize in geriatrics.
Gerontology remains a practice gap
Oncologists who don’t perform geriatric assessments are probably missing more than they think, Dr. Extermann said in an interview.
“Many oncologists don’t fully realize the importance of [geriatric assessment] yet,” Dr. Extermann said. “They kind of think that their internal medicine training will carry through, and they’ll be able to identify everything; actually, we know very well we miss half of what is found by geriatric assessment clinically.”
Gerontology remains a practice gap, Dr. Extermann said, not only within oncology, but across specialties.
“One of the big problems with the U.S. health care system is we don’t have enough geriatricians, and the reason we don’t have enough geriatricians is because we don’t pay them,” she said.
“Geriatrics is the only specialty where you do more training to be paid less, because Medicare doesn’t reimburse geriatric assessment, [and] it doesn’t reimburse geriatric consultation. [This] doesn’t motivate universities to create geriatric clinics and geriatric programs because they will lose money, basically, doing that. If we want to really solve the problem, we have to solve the reimbursement problem up front,” she explained.
Dr. Williams disclosed financial relationships with Carevive Health Systems, Cardinal Health, the National Cancer Institute, and the American Cancer Society. Dr. Extermann reported no conflicts of interest.
a geriatric oncologist said during a presentation at the American College of Physicians annual Internal Medicine meeting.
A 2020 ASCO survey, which the speaker, Grant R. Williams, MD, coauthored, found that 9 out of 10 community oncologists assessed at least some older patients differently than younger patients. But only 1 out of 3 did so in a formal manner, Dr. Williams, director of the cancer and aging program at the University of Alabama at Birmingham, said during presentation at virtual meeting.
In most cases, informal geriatric assessment considers only the tip of the ‘geriatric oncology iceberg,’ including chronological age, performance status, tumor characteristics, and organ function, Dr. Williams noted.
In contrast, formal geriatric assessment dives deeper, measuring a series of additional outcome-associated factors: polypharmacy, comorbidities, falls, psychosocial dysfunction, social support, sarcopenia, nutritional deficits, cognitive impairment, and functional issues.
“All these other factors under the surface are critically important to developing a personalized and individualized cancer treatment plan for older adults,” Dr. Williams said.
He went on to explain that elderly cancer patients can be sorted into three broad categories: fit, vulnerable, and frail. Fit and frail patients are relatively easy to identify, but most elderly patients fall into the vulnerable category, Dr. Williams noted.
“It’s really more challenging to identify those individuals across the spectrum than those at the extremes,” Dr. Williams said, noting that formal geriatric assessment can detect problems not found routinely.
Formal geriatric assessment’s value
Geriatric assessment can be used for risk modeling and making life-expectancy calculations. It can also be used as an interventional tool, guiding cancer treatment selection, he said. Furthermore, it can open doors to general health interventions, such as occupational therapy, to reduce fall risk.
Beneficial interventions identified by geriatric assessment have been shown to improve function, reduce chemotherapy toxicities, improve quality of life, and extend survival, Dr. Williams noted.
Formal geriatric assessment may be particularly useful for primary care providers considering referral to an oncologist, he said.
“I think performing a geriatric assessment [prior to referral] would be a great idea. And that’s twofold: Even before you send them to the oncologist, it gives you an idea of how they may tolerate treatment, and frankly, it may give you an idea that they don’t need a referral to the oncologist if they’re particularly frail,” noted Dr. Williams.
Alternatives to formal assessments
When asked how providers can incorporate formal assessments into a busy day at the clinic, Dr. Williams encouraged the use of abbreviated formal assessments, then adding further testing if needed.
“Given known time and support staff restraints, modified geriatric assessment tools have been developed that are either mostly or completely patient-reported,” he said in an interview, referring to the Cancer and Aging Research Group (CARG) Geriatric Assessment and the Cancer and Aging Resilience Evaluation (CARE), respectively.
“[These assessments] can easily be completed before clinical visits or while in the waiting room,” Dr. Williams noted. “The additional objective tests, such as Timed Up and Go, and Mental Status Exam, can be completed if deemed necessary based on these initial assessments.”
Martine Extermann, MD, PhD, provided her suggestions in an interview for what physicians can do to get better outcomes for this patient group.
“The secret of successful anti-cancer treatment in an older person is to be proactive with supportive care,” said Dr. Extermann, leader of the senior adult oncology program at H. Lee Moffitt Cancer Center & Research Institute, Tampa, Fla. “You have to really plan ahead, identify the support gaps, identify the potential problems, and prevent them thoroughly. The upfront work of good patient evaluation will save you a lot of trouble down the line,” she added.
Ms. Extermann also mentioned the challenges to providing care to geriatric patients with cancer, including a lack of financial incentive for physicians to specialize in geriatrics.
Gerontology remains a practice gap
Oncologists who don’t perform geriatric assessments are probably missing more than they think, Dr. Extermann said in an interview.
“Many oncologists don’t fully realize the importance of [geriatric assessment] yet,” Dr. Extermann said. “They kind of think that their internal medicine training will carry through, and they’ll be able to identify everything; actually, we know very well we miss half of what is found by geriatric assessment clinically.”
Gerontology remains a practice gap, Dr. Extermann said, not only within oncology, but across specialties.
“One of the big problems with the U.S. health care system is we don’t have enough geriatricians, and the reason we don’t have enough geriatricians is because we don’t pay them,” she said.
“Geriatrics is the only specialty where you do more training to be paid less, because Medicare doesn’t reimburse geriatric assessment, [and] it doesn’t reimburse geriatric consultation. [This] doesn’t motivate universities to create geriatric clinics and geriatric programs because they will lose money, basically, doing that. If we want to really solve the problem, we have to solve the reimbursement problem up front,” she explained.
Dr. Williams disclosed financial relationships with Carevive Health Systems, Cardinal Health, the National Cancer Institute, and the American Cancer Society. Dr. Extermann reported no conflicts of interest.
a geriatric oncologist said during a presentation at the American College of Physicians annual Internal Medicine meeting.
A 2020 ASCO survey, which the speaker, Grant R. Williams, MD, coauthored, found that 9 out of 10 community oncologists assessed at least some older patients differently than younger patients. But only 1 out of 3 did so in a formal manner, Dr. Williams, director of the cancer and aging program at the University of Alabama at Birmingham, said during presentation at virtual meeting.
In most cases, informal geriatric assessment considers only the tip of the ‘geriatric oncology iceberg,’ including chronological age, performance status, tumor characteristics, and organ function, Dr. Williams noted.
In contrast, formal geriatric assessment dives deeper, measuring a series of additional outcome-associated factors: polypharmacy, comorbidities, falls, psychosocial dysfunction, social support, sarcopenia, nutritional deficits, cognitive impairment, and functional issues.
“All these other factors under the surface are critically important to developing a personalized and individualized cancer treatment plan for older adults,” Dr. Williams said.
He went on to explain that elderly cancer patients can be sorted into three broad categories: fit, vulnerable, and frail. Fit and frail patients are relatively easy to identify, but most elderly patients fall into the vulnerable category, Dr. Williams noted.
“It’s really more challenging to identify those individuals across the spectrum than those at the extremes,” Dr. Williams said, noting that formal geriatric assessment can detect problems not found routinely.
Formal geriatric assessment’s value
Geriatric assessment can be used for risk modeling and making life-expectancy calculations. It can also be used as an interventional tool, guiding cancer treatment selection, he said. Furthermore, it can open doors to general health interventions, such as occupational therapy, to reduce fall risk.
Beneficial interventions identified by geriatric assessment have been shown to improve function, reduce chemotherapy toxicities, improve quality of life, and extend survival, Dr. Williams noted.
Formal geriatric assessment may be particularly useful for primary care providers considering referral to an oncologist, he said.
“I think performing a geriatric assessment [prior to referral] would be a great idea. And that’s twofold: Even before you send them to the oncologist, it gives you an idea of how they may tolerate treatment, and frankly, it may give you an idea that they don’t need a referral to the oncologist if they’re particularly frail,” noted Dr. Williams.
Alternatives to formal assessments
When asked how providers can incorporate formal assessments into a busy day at the clinic, Dr. Williams encouraged the use of abbreviated formal assessments, then adding further testing if needed.
“Given known time and support staff restraints, modified geriatric assessment tools have been developed that are either mostly or completely patient-reported,” he said in an interview, referring to the Cancer and Aging Research Group (CARG) Geriatric Assessment and the Cancer and Aging Resilience Evaluation (CARE), respectively.
“[These assessments] can easily be completed before clinical visits or while in the waiting room,” Dr. Williams noted. “The additional objective tests, such as Timed Up and Go, and Mental Status Exam, can be completed if deemed necessary based on these initial assessments.”
Martine Extermann, MD, PhD, provided her suggestions in an interview for what physicians can do to get better outcomes for this patient group.
“The secret of successful anti-cancer treatment in an older person is to be proactive with supportive care,” said Dr. Extermann, leader of the senior adult oncology program at H. Lee Moffitt Cancer Center & Research Institute, Tampa, Fla. “You have to really plan ahead, identify the support gaps, identify the potential problems, and prevent them thoroughly. The upfront work of good patient evaluation will save you a lot of trouble down the line,” she added.
Ms. Extermann also mentioned the challenges to providing care to geriatric patients with cancer, including a lack of financial incentive for physicians to specialize in geriatrics.
Gerontology remains a practice gap
Oncologists who don’t perform geriatric assessments are probably missing more than they think, Dr. Extermann said in an interview.
“Many oncologists don’t fully realize the importance of [geriatric assessment] yet,” Dr. Extermann said. “They kind of think that their internal medicine training will carry through, and they’ll be able to identify everything; actually, we know very well we miss half of what is found by geriatric assessment clinically.”
Gerontology remains a practice gap, Dr. Extermann said, not only within oncology, but across specialties.
“One of the big problems with the U.S. health care system is we don’t have enough geriatricians, and the reason we don’t have enough geriatricians is because we don’t pay them,” she said.
“Geriatrics is the only specialty where you do more training to be paid less, because Medicare doesn’t reimburse geriatric assessment, [and] it doesn’t reimburse geriatric consultation. [This] doesn’t motivate universities to create geriatric clinics and geriatric programs because they will lose money, basically, doing that. If we want to really solve the problem, we have to solve the reimbursement problem up front,” she explained.
Dr. Williams disclosed financial relationships with Carevive Health Systems, Cardinal Health, the National Cancer Institute, and the American Cancer Society. Dr. Extermann reported no conflicts of interest.
FROM INTERNAL MEDICINE 2021
Jack Remington, MD, noted toxoplasmosis researcher, dies at 90
Jack. S. Remington, MD, the Stanford (Calif.) University clinical scientist who developed a test to identify babies at risk for dangerous toxoplasmosis, died on April 8 at the age of 90.
Dr. Remington was professor emeritus of infectious diseases at Stanford Medicine. A legendary researcher, Dr. Remington was described by colleagues and trainees as a dogged clinician. Known as “Stat Jack” for his sense of urgency, he retired in 2005.
He died after a fall; it was the last of many. When he wasn’t treating patients or conducting research, Dr. Remington was often rock climbing. Friends said he had broken many bones but was always a passionate climber.
Dr. Remington was retired when Upinder Singh, MD, arrived at Stanford. Now she is chief of infectious diseases and geographic medicine at Stanford Medicine. Dr. Singh said in an interview that Dr. Remington was a bright, forward-thinking scientist.
Dr. Remington conducted research at the Palo Alto Medical Foundation (PAMF), part of the Sutter Health network. He ran a toxoplasmosis serology lab, and it was his baby, Dr. Singh said. In 2019, it was renamed for him: The Dr Jack S. Remington Laboratory for Specialty Diagnostics.
While he conducted research at PAMF, he treated patients at Stanford, where he could see his research benefit them.
“What he held closest to his heart was that scientific endeavors should help patients,” Dr. Singh said.
Born in Chicago in 1931, Dr. Remington did his undergraduate work at Loyola University in Chicago and the University of Illinois, where he graduated from medical school in 1956, according to a statement from Stanford. He spent 2 years as a senior assistant surgeon for the United States Public Health Service and as a researcher at the National Institute of Allergy and Infectious Diseases.
There, he conducted key research on Toxoplasma gondii, a usually dormant parasite that poses a serious risk to anyone with a compromised immune system – a group that includes babies, transplant recipients, and people with HIV. T gondii is the reason pregnant women are told not to clean out litter boxes, because it can be spread through cat feces. Humans also contract toxoplasmosis by eating contaminated meat. The Centers for Disease Control and Prevention estimates that 300 to 4,000 babies are exposed each year and develop toxoplasmosis. Often symptom-free for a period, the children can go on to develop vision problems or developmental delays.
Dr. Remington developed a blood test that measures a baby’s exposure and, therefore, risk for toxoplasmosis. According to the Stanford announcement, “The test distinguished between antibodies that a newborn has passively acquired from its mother through the placental barrier and antibodies that indicate a newborn has actually been infected in the womb by pathogens, notably T. gondii, that had been residing in the mother’s tissues. The latter case meant a baby needed immediate treatment to stave off active toxoplasmosis.”
Dr. Remington also led clinical trials and developed drugs to treat the condition. Stanford reports that he authored or coauthored more than 600 articles and held 11 patents.
He also coauthored the most authoritative textbook in the field. Remington and Klein’s Infectious Diseases of the Fetus and Newborn Infant is now in its eighth edition.
Dr. Remington was elected a fellow of the American College of Physicians in 1966, the London-based Royal College of Physicians in 1999, the American Association for the Advancement of Science in 2000, and the American Academy of Microbiology in 2000. He was a past president of the Western Society for Clinical Research, the Infectious Diseases Society of America, and the International Immunocompromised Host Society.
Friends and colleagues remember him as a dedicated mentor, evidenced by the many trainees who traveled to his 70th birthday party, said Philip Pizzo, MD, professor of pediatrics and immunology at Stanford Medicine. Dr. Pizzo, the former dean of the School of Medicine, met Dr. Remington in 1977 after presenting a research paper on the subject of the immunocompromised host at a New York meeting of the Infectious Diseases Society of America. They became lifelong colleagues and friends.
Dr. Remington had his own kind of confidence and self-assurance, Dr. Pizzo said: “He climbed the most challenging rock faces in the world. It takes a certain kind of personality to do that.”
A version of this article first appeared on Medscape.com.
Jack. S. Remington, MD, the Stanford (Calif.) University clinical scientist who developed a test to identify babies at risk for dangerous toxoplasmosis, died on April 8 at the age of 90.
Dr. Remington was professor emeritus of infectious diseases at Stanford Medicine. A legendary researcher, Dr. Remington was described by colleagues and trainees as a dogged clinician. Known as “Stat Jack” for his sense of urgency, he retired in 2005.
He died after a fall; it was the last of many. When he wasn’t treating patients or conducting research, Dr. Remington was often rock climbing. Friends said he had broken many bones but was always a passionate climber.
Dr. Remington was retired when Upinder Singh, MD, arrived at Stanford. Now she is chief of infectious diseases and geographic medicine at Stanford Medicine. Dr. Singh said in an interview that Dr. Remington was a bright, forward-thinking scientist.
Dr. Remington conducted research at the Palo Alto Medical Foundation (PAMF), part of the Sutter Health network. He ran a toxoplasmosis serology lab, and it was his baby, Dr. Singh said. In 2019, it was renamed for him: The Dr Jack S. Remington Laboratory for Specialty Diagnostics.
While he conducted research at PAMF, he treated patients at Stanford, where he could see his research benefit them.
“What he held closest to his heart was that scientific endeavors should help patients,” Dr. Singh said.
Born in Chicago in 1931, Dr. Remington did his undergraduate work at Loyola University in Chicago and the University of Illinois, where he graduated from medical school in 1956, according to a statement from Stanford. He spent 2 years as a senior assistant surgeon for the United States Public Health Service and as a researcher at the National Institute of Allergy and Infectious Diseases.
There, he conducted key research on Toxoplasma gondii, a usually dormant parasite that poses a serious risk to anyone with a compromised immune system – a group that includes babies, transplant recipients, and people with HIV. T gondii is the reason pregnant women are told not to clean out litter boxes, because it can be spread through cat feces. Humans also contract toxoplasmosis by eating contaminated meat. The Centers for Disease Control and Prevention estimates that 300 to 4,000 babies are exposed each year and develop toxoplasmosis. Often symptom-free for a period, the children can go on to develop vision problems or developmental delays.
Dr. Remington developed a blood test that measures a baby’s exposure and, therefore, risk for toxoplasmosis. According to the Stanford announcement, “The test distinguished between antibodies that a newborn has passively acquired from its mother through the placental barrier and antibodies that indicate a newborn has actually been infected in the womb by pathogens, notably T. gondii, that had been residing in the mother’s tissues. The latter case meant a baby needed immediate treatment to stave off active toxoplasmosis.”
Dr. Remington also led clinical trials and developed drugs to treat the condition. Stanford reports that he authored or coauthored more than 600 articles and held 11 patents.
He also coauthored the most authoritative textbook in the field. Remington and Klein’s Infectious Diseases of the Fetus and Newborn Infant is now in its eighth edition.
Dr. Remington was elected a fellow of the American College of Physicians in 1966, the London-based Royal College of Physicians in 1999, the American Association for the Advancement of Science in 2000, and the American Academy of Microbiology in 2000. He was a past president of the Western Society for Clinical Research, the Infectious Diseases Society of America, and the International Immunocompromised Host Society.
Friends and colleagues remember him as a dedicated mentor, evidenced by the many trainees who traveled to his 70th birthday party, said Philip Pizzo, MD, professor of pediatrics and immunology at Stanford Medicine. Dr. Pizzo, the former dean of the School of Medicine, met Dr. Remington in 1977 after presenting a research paper on the subject of the immunocompromised host at a New York meeting of the Infectious Diseases Society of America. They became lifelong colleagues and friends.
Dr. Remington had his own kind of confidence and self-assurance, Dr. Pizzo said: “He climbed the most challenging rock faces in the world. It takes a certain kind of personality to do that.”
A version of this article first appeared on Medscape.com.
Jack. S. Remington, MD, the Stanford (Calif.) University clinical scientist who developed a test to identify babies at risk for dangerous toxoplasmosis, died on April 8 at the age of 90.
Dr. Remington was professor emeritus of infectious diseases at Stanford Medicine. A legendary researcher, Dr. Remington was described by colleagues and trainees as a dogged clinician. Known as “Stat Jack” for his sense of urgency, he retired in 2005.
He died after a fall; it was the last of many. When he wasn’t treating patients or conducting research, Dr. Remington was often rock climbing. Friends said he had broken many bones but was always a passionate climber.
Dr. Remington was retired when Upinder Singh, MD, arrived at Stanford. Now she is chief of infectious diseases and geographic medicine at Stanford Medicine. Dr. Singh said in an interview that Dr. Remington was a bright, forward-thinking scientist.
Dr. Remington conducted research at the Palo Alto Medical Foundation (PAMF), part of the Sutter Health network. He ran a toxoplasmosis serology lab, and it was his baby, Dr. Singh said. In 2019, it was renamed for him: The Dr Jack S. Remington Laboratory for Specialty Diagnostics.
While he conducted research at PAMF, he treated patients at Stanford, where he could see his research benefit them.
“What he held closest to his heart was that scientific endeavors should help patients,” Dr. Singh said.
Born in Chicago in 1931, Dr. Remington did his undergraduate work at Loyola University in Chicago and the University of Illinois, where he graduated from medical school in 1956, according to a statement from Stanford. He spent 2 years as a senior assistant surgeon for the United States Public Health Service and as a researcher at the National Institute of Allergy and Infectious Diseases.
There, he conducted key research on Toxoplasma gondii, a usually dormant parasite that poses a serious risk to anyone with a compromised immune system – a group that includes babies, transplant recipients, and people with HIV. T gondii is the reason pregnant women are told not to clean out litter boxes, because it can be spread through cat feces. Humans also contract toxoplasmosis by eating contaminated meat. The Centers for Disease Control and Prevention estimates that 300 to 4,000 babies are exposed each year and develop toxoplasmosis. Often symptom-free for a period, the children can go on to develop vision problems or developmental delays.
Dr. Remington developed a blood test that measures a baby’s exposure and, therefore, risk for toxoplasmosis. According to the Stanford announcement, “The test distinguished between antibodies that a newborn has passively acquired from its mother through the placental barrier and antibodies that indicate a newborn has actually been infected in the womb by pathogens, notably T. gondii, that had been residing in the mother’s tissues. The latter case meant a baby needed immediate treatment to stave off active toxoplasmosis.”
Dr. Remington also led clinical trials and developed drugs to treat the condition. Stanford reports that he authored or coauthored more than 600 articles and held 11 patents.
He also coauthored the most authoritative textbook in the field. Remington and Klein’s Infectious Diseases of the Fetus and Newborn Infant is now in its eighth edition.
Dr. Remington was elected a fellow of the American College of Physicians in 1966, the London-based Royal College of Physicians in 1999, the American Association for the Advancement of Science in 2000, and the American Academy of Microbiology in 2000. He was a past president of the Western Society for Clinical Research, the Infectious Diseases Society of America, and the International Immunocompromised Host Society.
Friends and colleagues remember him as a dedicated mentor, evidenced by the many trainees who traveled to his 70th birthday party, said Philip Pizzo, MD, professor of pediatrics and immunology at Stanford Medicine. Dr. Pizzo, the former dean of the School of Medicine, met Dr. Remington in 1977 after presenting a research paper on the subject of the immunocompromised host at a New York meeting of the Infectious Diseases Society of America. They became lifelong colleagues and friends.
Dr. Remington had his own kind of confidence and self-assurance, Dr. Pizzo said: “He climbed the most challenging rock faces in the world. It takes a certain kind of personality to do that.”
A version of this article first appeared on Medscape.com.