User login
Pediatric cancer survivors at risk for opioid misuse
Survivors of childhood cancers are at increased risk for prescription opioid misuse compared with their peers, a review of a claims database revealed.
Among more than 8,000 patients age 21 or younger who had completed treatment for hematologic, central nervous system, bone, or gonadal cancers, survivors were significantly more likely than were their peers to have an opioid prescription, longer duration of prescription, and higher daily doses of opioids, and to have opioid prescriptions overlapping for a week or more, reported Xu Ji, PhD, of Emory University in Atlanta.
Teenage and young adult patients were at higher risk than were patients younger than 12, and the risk was highest among patients who had been treated for bone malignancies, as well as those who had undergone any hematopoietic stem cell transplant.
“These findings suggest that health care providers who regularly see survivors should explore nonopioid options to help prevent opioid misuse, and screen for potential misuse in those who actually receive opioids,” she said in an oral abstract presented during the annual meeting of the American Society of Pediatric Hematology/Oncology.
“This is a really important topic, and something that’s probably been underinvestigated and underexplored in our patient population,” said session comoderator Sheri Spunt, MD, Endowed Professor of Pediatric Cancer at Stanford (Calif.) University.
Database review
Dr. Ji and colleagues used the IBM MarketScan Commercial Claims and Encounters database from 2009 to 2018 to examine prescription opioid use, potential misuse, and substance use disorders in pediatric cancer survivors in the first year after completion of therapy, and to identify factors associated with risk for misuse or substance use disorders. Specifically, the period of interest was the first year after completion of all treatments, including surgery, chemotherapy, radiation, and stem cell transplant (Abstract 2015).
They looked at deidentified records on any opioid prescription and for treatment of any opioid use or substance use disorder (alcohol, psychotherapeutic drugs, marijuana, or illicit drug use disorders).
They defined indicators of potential misuse as either prescriptions for long-acting or extended-release opioids for acute pain conditions; opioid and benzodiazepine prescriptions overlapping by a week or more; opioid prescriptions overlapping by a week or more; high daily opioid dosage (prescribed daily dose of 100 or greater morphine milligram equivalent [MME]; and/or opioid dose escalation (an increase of at least 50% in mean MMEs per month twice consecutively within 1 year).
They compared outcomes between a total of 8,635 survivors and 44,175 controls, matched on a 1:5 basis with survivors by age, sex, and region, and continuous enrollment during the 1-year posttherapy period.
In each of three age categories – 0 to 11 years, 12 to 17 years, and 18 years and older – survivors were significantly more likely to have received an opioid prescription, at 15% for the youngest survivors vs. 2% of controls, 25% vs. 8% for 12- to 17-year-olds, and 28% vs. 12% for those 18 and older (P < .01 for all three comparisons).
Survivors were also significantly more likely to have any indicator of potential misuse (1.6% vs. 0.1%, 4.6% vs. 0.5%, and 7.4% vs. 1.2%, respectively, P < .001 for all) and both the youngest and oldest groups (but not 12- to 17-year-olds) were significantly more like to have opioid or substance use disorder (0.4% vs. 0% for 0-11 years, 5.76% vs. 4.2% for 18 years and older, P < .001 for both).
Among patients with any opioid prescription, survivors were significantly more likely than were controls of any age to have indicators for potential misuse. For example, 13% of survivors aged 18 years and older had prescriptions for high opioid doses, compared with 5% of controls, and 12% had prescription overlap, vs. 2%.
Compared with patients with leukemia, patients treated for bone malignancies had a 6% greater risk for having any indicator of misuse, while patients with other malignancies were at slightly lower risk for misuse than those who completed leukemia therapy.
Patients who received any stem cell transplant had an 8.4% greater risk for misuse compared with patients who had surgery only.
Opioids pre- and posttreatment?
“Being someone who takes care of a lot of bone cancer patients, I do see patients with these issues,” Dr. Spunt said.
Audience member Jack H. Staddon, MD, PhD, of the Billings (Montana) Clinic, noted the possibility that opioid use during treatment may have been carried on into the posttreatment period, and asked whether use of narcotics during treatment was an independent risk factor for posttreatment narcotic use or misuse.
The researchers plan to investigate this question in future studies, Dr. Ji replied.
They did not report a study funding source. Dr. Ji and coauthors and Dr. Staddon reported no relevant disclosures.
Survivors of childhood cancers are at increased risk for prescription opioid misuse compared with their peers, a review of a claims database revealed.
Among more than 8,000 patients age 21 or younger who had completed treatment for hematologic, central nervous system, bone, or gonadal cancers, survivors were significantly more likely than were their peers to have an opioid prescription, longer duration of prescription, and higher daily doses of opioids, and to have opioid prescriptions overlapping for a week or more, reported Xu Ji, PhD, of Emory University in Atlanta.
Teenage and young adult patients were at higher risk than were patients younger than 12, and the risk was highest among patients who had been treated for bone malignancies, as well as those who had undergone any hematopoietic stem cell transplant.
“These findings suggest that health care providers who regularly see survivors should explore nonopioid options to help prevent opioid misuse, and screen for potential misuse in those who actually receive opioids,” she said in an oral abstract presented during the annual meeting of the American Society of Pediatric Hematology/Oncology.
“This is a really important topic, and something that’s probably been underinvestigated and underexplored in our patient population,” said session comoderator Sheri Spunt, MD, Endowed Professor of Pediatric Cancer at Stanford (Calif.) University.
Database review
Dr. Ji and colleagues used the IBM MarketScan Commercial Claims and Encounters database from 2009 to 2018 to examine prescription opioid use, potential misuse, and substance use disorders in pediatric cancer survivors in the first year after completion of therapy, and to identify factors associated with risk for misuse or substance use disorders. Specifically, the period of interest was the first year after completion of all treatments, including surgery, chemotherapy, radiation, and stem cell transplant (Abstract 2015).
They looked at deidentified records on any opioid prescription and for treatment of any opioid use or substance use disorder (alcohol, psychotherapeutic drugs, marijuana, or illicit drug use disorders).
They defined indicators of potential misuse as either prescriptions for long-acting or extended-release opioids for acute pain conditions; opioid and benzodiazepine prescriptions overlapping by a week or more; opioid prescriptions overlapping by a week or more; high daily opioid dosage (prescribed daily dose of 100 or greater morphine milligram equivalent [MME]; and/or opioid dose escalation (an increase of at least 50% in mean MMEs per month twice consecutively within 1 year).
They compared outcomes between a total of 8,635 survivors and 44,175 controls, matched on a 1:5 basis with survivors by age, sex, and region, and continuous enrollment during the 1-year posttherapy period.
In each of three age categories – 0 to 11 years, 12 to 17 years, and 18 years and older – survivors were significantly more likely to have received an opioid prescription, at 15% for the youngest survivors vs. 2% of controls, 25% vs. 8% for 12- to 17-year-olds, and 28% vs. 12% for those 18 and older (P < .01 for all three comparisons).
Survivors were also significantly more likely to have any indicator of potential misuse (1.6% vs. 0.1%, 4.6% vs. 0.5%, and 7.4% vs. 1.2%, respectively, P < .001 for all) and both the youngest and oldest groups (but not 12- to 17-year-olds) were significantly more like to have opioid or substance use disorder (0.4% vs. 0% for 0-11 years, 5.76% vs. 4.2% for 18 years and older, P < .001 for both).
Among patients with any opioid prescription, survivors were significantly more likely than were controls of any age to have indicators for potential misuse. For example, 13% of survivors aged 18 years and older had prescriptions for high opioid doses, compared with 5% of controls, and 12% had prescription overlap, vs. 2%.
Compared with patients with leukemia, patients treated for bone malignancies had a 6% greater risk for having any indicator of misuse, while patients with other malignancies were at slightly lower risk for misuse than those who completed leukemia therapy.
Patients who received any stem cell transplant had an 8.4% greater risk for misuse compared with patients who had surgery only.
Opioids pre- and posttreatment?
“Being someone who takes care of a lot of bone cancer patients, I do see patients with these issues,” Dr. Spunt said.
Audience member Jack H. Staddon, MD, PhD, of the Billings (Montana) Clinic, noted the possibility that opioid use during treatment may have been carried on into the posttreatment period, and asked whether use of narcotics during treatment was an independent risk factor for posttreatment narcotic use or misuse.
The researchers plan to investigate this question in future studies, Dr. Ji replied.
They did not report a study funding source. Dr. Ji and coauthors and Dr. Staddon reported no relevant disclosures.
Survivors of childhood cancers are at increased risk for prescription opioid misuse compared with their peers, a review of a claims database revealed.
Among more than 8,000 patients age 21 or younger who had completed treatment for hematologic, central nervous system, bone, or gonadal cancers, survivors were significantly more likely than were their peers to have an opioid prescription, longer duration of prescription, and higher daily doses of opioids, and to have opioid prescriptions overlapping for a week or more, reported Xu Ji, PhD, of Emory University in Atlanta.
Teenage and young adult patients were at higher risk than were patients younger than 12, and the risk was highest among patients who had been treated for bone malignancies, as well as those who had undergone any hematopoietic stem cell transplant.
“These findings suggest that health care providers who regularly see survivors should explore nonopioid options to help prevent opioid misuse, and screen for potential misuse in those who actually receive opioids,” she said in an oral abstract presented during the annual meeting of the American Society of Pediatric Hematology/Oncology.
“This is a really important topic, and something that’s probably been underinvestigated and underexplored in our patient population,” said session comoderator Sheri Spunt, MD, Endowed Professor of Pediatric Cancer at Stanford (Calif.) University.
Database review
Dr. Ji and colleagues used the IBM MarketScan Commercial Claims and Encounters database from 2009 to 2018 to examine prescription opioid use, potential misuse, and substance use disorders in pediatric cancer survivors in the first year after completion of therapy, and to identify factors associated with risk for misuse or substance use disorders. Specifically, the period of interest was the first year after completion of all treatments, including surgery, chemotherapy, radiation, and stem cell transplant (Abstract 2015).
They looked at deidentified records on any opioid prescription and for treatment of any opioid use or substance use disorder (alcohol, psychotherapeutic drugs, marijuana, or illicit drug use disorders).
They defined indicators of potential misuse as either prescriptions for long-acting or extended-release opioids for acute pain conditions; opioid and benzodiazepine prescriptions overlapping by a week or more; opioid prescriptions overlapping by a week or more; high daily opioid dosage (prescribed daily dose of 100 or greater morphine milligram equivalent [MME]; and/or opioid dose escalation (an increase of at least 50% in mean MMEs per month twice consecutively within 1 year).
They compared outcomes between a total of 8,635 survivors and 44,175 controls, matched on a 1:5 basis with survivors by age, sex, and region, and continuous enrollment during the 1-year posttherapy period.
In each of three age categories – 0 to 11 years, 12 to 17 years, and 18 years and older – survivors were significantly more likely to have received an opioid prescription, at 15% for the youngest survivors vs. 2% of controls, 25% vs. 8% for 12- to 17-year-olds, and 28% vs. 12% for those 18 and older (P < .01 for all three comparisons).
Survivors were also significantly more likely to have any indicator of potential misuse (1.6% vs. 0.1%, 4.6% vs. 0.5%, and 7.4% vs. 1.2%, respectively, P < .001 for all) and both the youngest and oldest groups (but not 12- to 17-year-olds) were significantly more like to have opioid or substance use disorder (0.4% vs. 0% for 0-11 years, 5.76% vs. 4.2% for 18 years and older, P < .001 for both).
Among patients with any opioid prescription, survivors were significantly more likely than were controls of any age to have indicators for potential misuse. For example, 13% of survivors aged 18 years and older had prescriptions for high opioid doses, compared with 5% of controls, and 12% had prescription overlap, vs. 2%.
Compared with patients with leukemia, patients treated for bone malignancies had a 6% greater risk for having any indicator of misuse, while patients with other malignancies were at slightly lower risk for misuse than those who completed leukemia therapy.
Patients who received any stem cell transplant had an 8.4% greater risk for misuse compared with patients who had surgery only.
Opioids pre- and posttreatment?
“Being someone who takes care of a lot of bone cancer patients, I do see patients with these issues,” Dr. Spunt said.
Audience member Jack H. Staddon, MD, PhD, of the Billings (Montana) Clinic, noted the possibility that opioid use during treatment may have been carried on into the posttreatment period, and asked whether use of narcotics during treatment was an independent risk factor for posttreatment narcotic use or misuse.
The researchers plan to investigate this question in future studies, Dr. Ji replied.
They did not report a study funding source. Dr. Ji and coauthors and Dr. Staddon reported no relevant disclosures.
FROM 2021 ASPHO CONFERENCE
COVID-19 coaching program provides ‘psychological PPE’ for HCPs
A novel program that coaches healthcare workers effectively bolsters wellness and resilience in the face of the ongoing COVID-19 pandemic.
Investigators found the program they developed successfully reduced the severity of mental health threats in healthcare workers.
The pandemic has been “an enormous threat to the resilience of healthcare workers,” said program leader Benjamin Rosen, MD, assistant professor, department of psychiatry, University of Toronto, and staff psychiatrist at Sinai Health in Toronto.
“Working at a hospital this year, you’re not only worried about battling COVID, but you’re also enduring uncertainty and fear and moral distress, which has contributed to unprecedented levels of burnout,” Dr. Rosen added.
The findings were presented at the annual meeting of the American Psychiatric Association, held virtually this year.
‘Psychological PPE’
Building on previous experience supporting colleagues through the 2003 severe acute respiratory syndrome (SARS) outbreak in Toronto, Dr. Rosen’s team designed and implemented an initiative to support colleagues’ wellness and resilience early in the COVID-19 pandemic.
The Resilience Coaching for Healthcare Workers program is designed to support psychological well-being during times of chronic stress and help healthcare workers “keep their heads in the game so that they can sustain the focus and the rigor that they need to do their work,” Dr. Rosen said during a press briefing.
Participating coaches are mental health clinicians with training in psychological first aid, resilience, and psychotherapy to provide peer support to units and teams working on the front line. The program provides a kind of “psychological PPE” to complement other protective measures, Dr. Rosen explained.
There are currently 15 coaches working with 17 units and clinical teams at Sinai Health, which encompasses Mount Sinai Hospital and Bridgepoint Active Health, both in Toronto. Most coaches provide support to groups of up to 15 people either virtually or in person. More than 5,300 staff members have received coaching support since the program’s launch in April 2020.
Mary Preisman, MD, consultation liaison psychiatrist at Mount Sinai Hospital, who is involved with the program, said it’s important to note that coaches are not in clinical relationships with healthcare providers, but rather are applying diverse psychotherapeutic tools to deliver collegial support. When clinical support is requested, coaches facilitate connection with other psychiatrists.
‘An excellent model’
Preliminary analysis of qualitative data, which includes interviews with coaches and providers, suggests that coaching is successful in mitigating the severity of mental health threats that healthcare workers face.
“The feedback so far is that coaching has really helped to strengthen team cohesiveness and resilience, which has been really encouraging for us,” Dr. Rosen said.
For example, some participants said the coaching improved relationships with their colleagues, decreased loneliness, and increased the sense of support from their employer.
Others commented on the value of regularly scheduled coaching “huddles” that are embedded within the work environment.
Dr. Rosen said the program is funded by academic grants through the end of next year, which is key given that Toronto is currently in the middle of a third wave of the pandemic.
“ There have been studies that show, even years after a pandemic or an epidemic has ended, the psychological consequences of anxiety and distress persist,” Dr. Rosen said.
Briefing moderator Jeffrey Borenstein, MD, president and CEO, Brain & Behavior Research Foundation and editor-in-chief, Psychiatric News, said the Toronto team has developed “an excellent model that could be used around the world to support the well-being of healthcare workers who are on the front lines of a pandemic.”
This research had no commercial funding. Dr. Rosen, Dr. Preisman, and Dr. Borenstein have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A novel program that coaches healthcare workers effectively bolsters wellness and resilience in the face of the ongoing COVID-19 pandemic.
Investigators found the program they developed successfully reduced the severity of mental health threats in healthcare workers.
The pandemic has been “an enormous threat to the resilience of healthcare workers,” said program leader Benjamin Rosen, MD, assistant professor, department of psychiatry, University of Toronto, and staff psychiatrist at Sinai Health in Toronto.
“Working at a hospital this year, you’re not only worried about battling COVID, but you’re also enduring uncertainty and fear and moral distress, which has contributed to unprecedented levels of burnout,” Dr. Rosen added.
The findings were presented at the annual meeting of the American Psychiatric Association, held virtually this year.
‘Psychological PPE’
Building on previous experience supporting colleagues through the 2003 severe acute respiratory syndrome (SARS) outbreak in Toronto, Dr. Rosen’s team designed and implemented an initiative to support colleagues’ wellness and resilience early in the COVID-19 pandemic.
The Resilience Coaching for Healthcare Workers program is designed to support psychological well-being during times of chronic stress and help healthcare workers “keep their heads in the game so that they can sustain the focus and the rigor that they need to do their work,” Dr. Rosen said during a press briefing.
Participating coaches are mental health clinicians with training in psychological first aid, resilience, and psychotherapy to provide peer support to units and teams working on the front line. The program provides a kind of “psychological PPE” to complement other protective measures, Dr. Rosen explained.
There are currently 15 coaches working with 17 units and clinical teams at Sinai Health, which encompasses Mount Sinai Hospital and Bridgepoint Active Health, both in Toronto. Most coaches provide support to groups of up to 15 people either virtually or in person. More than 5,300 staff members have received coaching support since the program’s launch in April 2020.
Mary Preisman, MD, consultation liaison psychiatrist at Mount Sinai Hospital, who is involved with the program, said it’s important to note that coaches are not in clinical relationships with healthcare providers, but rather are applying diverse psychotherapeutic tools to deliver collegial support. When clinical support is requested, coaches facilitate connection with other psychiatrists.
‘An excellent model’
Preliminary analysis of qualitative data, which includes interviews with coaches and providers, suggests that coaching is successful in mitigating the severity of mental health threats that healthcare workers face.
“The feedback so far is that coaching has really helped to strengthen team cohesiveness and resilience, which has been really encouraging for us,” Dr. Rosen said.
For example, some participants said the coaching improved relationships with their colleagues, decreased loneliness, and increased the sense of support from their employer.
Others commented on the value of regularly scheduled coaching “huddles” that are embedded within the work environment.
Dr. Rosen said the program is funded by academic grants through the end of next year, which is key given that Toronto is currently in the middle of a third wave of the pandemic.
“ There have been studies that show, even years after a pandemic or an epidemic has ended, the psychological consequences of anxiety and distress persist,” Dr. Rosen said.
Briefing moderator Jeffrey Borenstein, MD, president and CEO, Brain & Behavior Research Foundation and editor-in-chief, Psychiatric News, said the Toronto team has developed “an excellent model that could be used around the world to support the well-being of healthcare workers who are on the front lines of a pandemic.”
This research had no commercial funding. Dr. Rosen, Dr. Preisman, and Dr. Borenstein have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A novel program that coaches healthcare workers effectively bolsters wellness and resilience in the face of the ongoing COVID-19 pandemic.
Investigators found the program they developed successfully reduced the severity of mental health threats in healthcare workers.
The pandemic has been “an enormous threat to the resilience of healthcare workers,” said program leader Benjamin Rosen, MD, assistant professor, department of psychiatry, University of Toronto, and staff psychiatrist at Sinai Health in Toronto.
“Working at a hospital this year, you’re not only worried about battling COVID, but you’re also enduring uncertainty and fear and moral distress, which has contributed to unprecedented levels of burnout,” Dr. Rosen added.
The findings were presented at the annual meeting of the American Psychiatric Association, held virtually this year.
‘Psychological PPE’
Building on previous experience supporting colleagues through the 2003 severe acute respiratory syndrome (SARS) outbreak in Toronto, Dr. Rosen’s team designed and implemented an initiative to support colleagues’ wellness and resilience early in the COVID-19 pandemic.
The Resilience Coaching for Healthcare Workers program is designed to support psychological well-being during times of chronic stress and help healthcare workers “keep their heads in the game so that they can sustain the focus and the rigor that they need to do their work,” Dr. Rosen said during a press briefing.
Participating coaches are mental health clinicians with training in psychological first aid, resilience, and psychotherapy to provide peer support to units and teams working on the front line. The program provides a kind of “psychological PPE” to complement other protective measures, Dr. Rosen explained.
There are currently 15 coaches working with 17 units and clinical teams at Sinai Health, which encompasses Mount Sinai Hospital and Bridgepoint Active Health, both in Toronto. Most coaches provide support to groups of up to 15 people either virtually or in person. More than 5,300 staff members have received coaching support since the program’s launch in April 2020.
Mary Preisman, MD, consultation liaison psychiatrist at Mount Sinai Hospital, who is involved with the program, said it’s important to note that coaches are not in clinical relationships with healthcare providers, but rather are applying diverse psychotherapeutic tools to deliver collegial support. When clinical support is requested, coaches facilitate connection with other psychiatrists.
‘An excellent model’
Preliminary analysis of qualitative data, which includes interviews with coaches and providers, suggests that coaching is successful in mitigating the severity of mental health threats that healthcare workers face.
“The feedback so far is that coaching has really helped to strengthen team cohesiveness and resilience, which has been really encouraging for us,” Dr. Rosen said.
For example, some participants said the coaching improved relationships with their colleagues, decreased loneliness, and increased the sense of support from their employer.
Others commented on the value of regularly scheduled coaching “huddles” that are embedded within the work environment.
Dr. Rosen said the program is funded by academic grants through the end of next year, which is key given that Toronto is currently in the middle of a third wave of the pandemic.
“ There have been studies that show, even years after a pandemic or an epidemic has ended, the psychological consequences of anxiety and distress persist,” Dr. Rosen said.
Briefing moderator Jeffrey Borenstein, MD, president and CEO, Brain & Behavior Research Foundation and editor-in-chief, Psychiatric News, said the Toronto team has developed “an excellent model that could be used around the world to support the well-being of healthcare workers who are on the front lines of a pandemic.”
This research had no commercial funding. Dr. Rosen, Dr. Preisman, and Dr. Borenstein have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Strategies to turn the tide on racial and gender inequity
Working to mitigate racial and gender inequity in hospital medicine may seem like a daunting task, but every physician can play a role in turning the tide toward equity, according to Jorge Ganem, MD, FAAP.
“Talking about bias, racism, sexism, gender inequity, and health disparities is difficult,” Dr. Ganem, associate professor of pediatrics at the University of Texas at Austin and director of pediatric hospital medicine at Dell Children’s Medical Center in Austin, said May 5 at SHM Converge, the annual conference of the Society of Hospital Medicine. “There certainly comes a heavy weight and responsibility that we all feel. But I believe that we should approach gender inequities and racial disparities through a quality and patient safety lens, and looking through that lens.”
Dr. Ganem – along with Vanessa Durand, DO, FAAP, of St. Christopher’s Hospital for Children in Philadelphia, and Yemisi O. Jones, MD, FAAP, FHM, of Cincinnati Children’s Hospital – devised the concept of “functional allyship” as one way to improve representation in hospital medicine. The approach consists of three categories: listeners, amplifiers, and champions. Listeners are “those who take the time to listen and give space to the voices who are oppressed and disadvantaged,” Dr. Ganem said. “Action may not always be possible, but the space gives those who are marginalized validation to the feelings that the oppression produces.”
He described amplifiers as those who use their position of privilege to spread the message by educating their colleagues and other peers. “This includes elevating those from marginalized communities to speak on their own behalf and giving them the spotlight, given their expertise,” he said.
Champions are those who actively work to dismantle the oppression within systems. Dr. Ganem cited organizations such as ADVANCE PHM, FEMinEM and HeForShe as examples of national and global efforts, “but this also includes those working in committees that are addressing diversity and inclusion in their workplace and coming up with policies and procedures to increase equity,” he said.
Finding opportunities to practice mentorship and sponsorship are also important. “Positive mentorship relationships are key in avoiding burnout and decreasing attrition,” he said. “The development of successful mentorship programs are a must in order to retain women physicians and ‘underrepresented in medicine’ physicians in your organization.” He described a “sponsor” as someone who is in a position of influence and power who actively supports the career of a “protégé” whom they have identified as having high potential. “The sponsor may advance a protégé’s career by nominating them for leadership opportunities and introducing them into career networks,” he said.
Dr. Durand discussed additional ways to improve disparities of gender, race, and ethnicity. “It can all start with measuring the data,” said Dr. Durand, also an assistant professor of pediatrics at Drexel University, Philadelphia. “This means looking at gender and race and ethnicity data by unit or section at your institution, as well as leadership positions.” In 2017, authors led by Hilary Sanfey, MBBBCh, MHPE, FACS, published an article addressing strategies to identify and close the gender salary gap in surgery (J Am Coll Surg. 2017;225[2]:333-8). Their recommendations included changing policies, transparency, oversight of metrics, promoting women to senior leadership positions, and evaluating the organizational culture. “It goes back to culture, because it leads to accountability,” Dr. Durand said. “Behavior change comes with accountability.”
Part of holding people accountable within a culture change includes addressing microaggressions, or indirect expressions of prejudice. In 2016, authors led by Floyd Cheung, PhD., established a framework using the acronym A.C.T.I.O.N., which identifies a microaggression without being aggressive or evoking defensiveness towards the person communicating the microaggression. A.C.T.I.O.N. stands for Ask clarifying questions; Come from curiosity, not judgment; Tell what you observed in a factual manner; Impact exploration – discuss what the impact was; Own your own thoughts and feelings around the situation; and discuss Next steps.
“Granted, this might take a little time, but when we state microaggressions, most of us don’t realize that those statements could be hurtful or uncomfortable for the person receiving them,” Dr. Durand said.
Another strategy to address disparities involves partially blinding the interview process for trainees. “You can do this by not giving any ‘cognitive information’ to your interviewers – such as United States Medical Licensing Examination Step scores – that may anchor their position prior to the interview taking place,” she explained. “You can also standardize one or two questions that all interviewees have to answer, to have a more objective way to compare answers horizontally rather than vertically.”
This complements the notion of the Association of American Medical Colleges’ “holistic review,” a principle that it describes as allowing admissions committees “to consider the ‘whole’ applicant, rather than disproportionately focusing on any one factor.”
“The overall concept is to evaluate what are criteria of the position you are hiring for,” Dr. Durand said. “Different criteria will have different levels of importance. You would take into consideration the values of the group or the institution and make sure those criteria are most important for selection, at the forefront.”
Dr. Ganem and Dr. Durand reported having no financial disclosures.
Working to mitigate racial and gender inequity in hospital medicine may seem like a daunting task, but every physician can play a role in turning the tide toward equity, according to Jorge Ganem, MD, FAAP.
“Talking about bias, racism, sexism, gender inequity, and health disparities is difficult,” Dr. Ganem, associate professor of pediatrics at the University of Texas at Austin and director of pediatric hospital medicine at Dell Children’s Medical Center in Austin, said May 5 at SHM Converge, the annual conference of the Society of Hospital Medicine. “There certainly comes a heavy weight and responsibility that we all feel. But I believe that we should approach gender inequities and racial disparities through a quality and patient safety lens, and looking through that lens.”
Dr. Ganem – along with Vanessa Durand, DO, FAAP, of St. Christopher’s Hospital for Children in Philadelphia, and Yemisi O. Jones, MD, FAAP, FHM, of Cincinnati Children’s Hospital – devised the concept of “functional allyship” as one way to improve representation in hospital medicine. The approach consists of three categories: listeners, amplifiers, and champions. Listeners are “those who take the time to listen and give space to the voices who are oppressed and disadvantaged,” Dr. Ganem said. “Action may not always be possible, but the space gives those who are marginalized validation to the feelings that the oppression produces.”
He described amplifiers as those who use their position of privilege to spread the message by educating their colleagues and other peers. “This includes elevating those from marginalized communities to speak on their own behalf and giving them the spotlight, given their expertise,” he said.
Champions are those who actively work to dismantle the oppression within systems. Dr. Ganem cited organizations such as ADVANCE PHM, FEMinEM and HeForShe as examples of national and global efforts, “but this also includes those working in committees that are addressing diversity and inclusion in their workplace and coming up with policies and procedures to increase equity,” he said.
Finding opportunities to practice mentorship and sponsorship are also important. “Positive mentorship relationships are key in avoiding burnout and decreasing attrition,” he said. “The development of successful mentorship programs are a must in order to retain women physicians and ‘underrepresented in medicine’ physicians in your organization.” He described a “sponsor” as someone who is in a position of influence and power who actively supports the career of a “protégé” whom they have identified as having high potential. “The sponsor may advance a protégé’s career by nominating them for leadership opportunities and introducing them into career networks,” he said.
Dr. Durand discussed additional ways to improve disparities of gender, race, and ethnicity. “It can all start with measuring the data,” said Dr. Durand, also an assistant professor of pediatrics at Drexel University, Philadelphia. “This means looking at gender and race and ethnicity data by unit or section at your institution, as well as leadership positions.” In 2017, authors led by Hilary Sanfey, MBBBCh, MHPE, FACS, published an article addressing strategies to identify and close the gender salary gap in surgery (J Am Coll Surg. 2017;225[2]:333-8). Their recommendations included changing policies, transparency, oversight of metrics, promoting women to senior leadership positions, and evaluating the organizational culture. “It goes back to culture, because it leads to accountability,” Dr. Durand said. “Behavior change comes with accountability.”
Part of holding people accountable within a culture change includes addressing microaggressions, or indirect expressions of prejudice. In 2016, authors led by Floyd Cheung, PhD., established a framework using the acronym A.C.T.I.O.N., which identifies a microaggression without being aggressive or evoking defensiveness towards the person communicating the microaggression. A.C.T.I.O.N. stands for Ask clarifying questions; Come from curiosity, not judgment; Tell what you observed in a factual manner; Impact exploration – discuss what the impact was; Own your own thoughts and feelings around the situation; and discuss Next steps.
“Granted, this might take a little time, but when we state microaggressions, most of us don’t realize that those statements could be hurtful or uncomfortable for the person receiving them,” Dr. Durand said.
Another strategy to address disparities involves partially blinding the interview process for trainees. “You can do this by not giving any ‘cognitive information’ to your interviewers – such as United States Medical Licensing Examination Step scores – that may anchor their position prior to the interview taking place,” she explained. “You can also standardize one or two questions that all interviewees have to answer, to have a more objective way to compare answers horizontally rather than vertically.”
This complements the notion of the Association of American Medical Colleges’ “holistic review,” a principle that it describes as allowing admissions committees “to consider the ‘whole’ applicant, rather than disproportionately focusing on any one factor.”
“The overall concept is to evaluate what are criteria of the position you are hiring for,” Dr. Durand said. “Different criteria will have different levels of importance. You would take into consideration the values of the group or the institution and make sure those criteria are most important for selection, at the forefront.”
Dr. Ganem and Dr. Durand reported having no financial disclosures.
Working to mitigate racial and gender inequity in hospital medicine may seem like a daunting task, but every physician can play a role in turning the tide toward equity, according to Jorge Ganem, MD, FAAP.
“Talking about bias, racism, sexism, gender inequity, and health disparities is difficult,” Dr. Ganem, associate professor of pediatrics at the University of Texas at Austin and director of pediatric hospital medicine at Dell Children’s Medical Center in Austin, said May 5 at SHM Converge, the annual conference of the Society of Hospital Medicine. “There certainly comes a heavy weight and responsibility that we all feel. But I believe that we should approach gender inequities and racial disparities through a quality and patient safety lens, and looking through that lens.”
Dr. Ganem – along with Vanessa Durand, DO, FAAP, of St. Christopher’s Hospital for Children in Philadelphia, and Yemisi O. Jones, MD, FAAP, FHM, of Cincinnati Children’s Hospital – devised the concept of “functional allyship” as one way to improve representation in hospital medicine. The approach consists of three categories: listeners, amplifiers, and champions. Listeners are “those who take the time to listen and give space to the voices who are oppressed and disadvantaged,” Dr. Ganem said. “Action may not always be possible, but the space gives those who are marginalized validation to the feelings that the oppression produces.”
He described amplifiers as those who use their position of privilege to spread the message by educating their colleagues and other peers. “This includes elevating those from marginalized communities to speak on their own behalf and giving them the spotlight, given their expertise,” he said.
Champions are those who actively work to dismantle the oppression within systems. Dr. Ganem cited organizations such as ADVANCE PHM, FEMinEM and HeForShe as examples of national and global efforts, “but this also includes those working in committees that are addressing diversity and inclusion in their workplace and coming up with policies and procedures to increase equity,” he said.
Finding opportunities to practice mentorship and sponsorship are also important. “Positive mentorship relationships are key in avoiding burnout and decreasing attrition,” he said. “The development of successful mentorship programs are a must in order to retain women physicians and ‘underrepresented in medicine’ physicians in your organization.” He described a “sponsor” as someone who is in a position of influence and power who actively supports the career of a “protégé” whom they have identified as having high potential. “The sponsor may advance a protégé’s career by nominating them for leadership opportunities and introducing them into career networks,” he said.
Dr. Durand discussed additional ways to improve disparities of gender, race, and ethnicity. “It can all start with measuring the data,” said Dr. Durand, also an assistant professor of pediatrics at Drexel University, Philadelphia. “This means looking at gender and race and ethnicity data by unit or section at your institution, as well as leadership positions.” In 2017, authors led by Hilary Sanfey, MBBBCh, MHPE, FACS, published an article addressing strategies to identify and close the gender salary gap in surgery (J Am Coll Surg. 2017;225[2]:333-8). Their recommendations included changing policies, transparency, oversight of metrics, promoting women to senior leadership positions, and evaluating the organizational culture. “It goes back to culture, because it leads to accountability,” Dr. Durand said. “Behavior change comes with accountability.”
Part of holding people accountable within a culture change includes addressing microaggressions, or indirect expressions of prejudice. In 2016, authors led by Floyd Cheung, PhD., established a framework using the acronym A.C.T.I.O.N., which identifies a microaggression without being aggressive or evoking defensiveness towards the person communicating the microaggression. A.C.T.I.O.N. stands for Ask clarifying questions; Come from curiosity, not judgment; Tell what you observed in a factual manner; Impact exploration – discuss what the impact was; Own your own thoughts and feelings around the situation; and discuss Next steps.
“Granted, this might take a little time, but when we state microaggressions, most of us don’t realize that those statements could be hurtful or uncomfortable for the person receiving them,” Dr. Durand said.
Another strategy to address disparities involves partially blinding the interview process for trainees. “You can do this by not giving any ‘cognitive information’ to your interviewers – such as United States Medical Licensing Examination Step scores – that may anchor their position prior to the interview taking place,” she explained. “You can also standardize one or two questions that all interviewees have to answer, to have a more objective way to compare answers horizontally rather than vertically.”
This complements the notion of the Association of American Medical Colleges’ “holistic review,” a principle that it describes as allowing admissions committees “to consider the ‘whole’ applicant, rather than disproportionately focusing on any one factor.”
“The overall concept is to evaluate what are criteria of the position you are hiring for,” Dr. Durand said. “Different criteria will have different levels of importance. You would take into consideration the values of the group or the institution and make sure those criteria are most important for selection, at the forefront.”
Dr. Ganem and Dr. Durand reported having no financial disclosures.
FROM SHM CONVERGE 2021
Torsemide over furosemide as first-line loop diuretic for HF
When starting a new loop diuretic for a patient with heart failure, strongly consider torsemide over furosemide, Anthony C. Breu, MD, advised at SHM Converge, the annual conference of the Society of Hospital Medicine.
“Whether or not you take a patient who’s already on furosemide and you make the switch to torsemide is a little bit tougher for me to advocate, though that has happened in clinical trials,” said Dr. Breu, assistant professor of medicine at Harvard Medical School, Boston, who spoke May 5 at the Converge session “Things We Do for No Reason.” He co-presented the session with Leonard Feldman, MD, SFHM, director of the Osler Medical Residency Urban Health Track and associate professor at Johns Hopkins Medicine, Baltimore.
“If you consider doing this it would make sense to do so concert with the outpatient primary doctor and the outpatient cardiologist,” Dr. Breu said. “But in my review of the literature, it’s at least worth having these discussions, particularly for a patient who has multiple readmissions for heart failure. That may be a time to pause and ask: ‘Could torsemide be of benefit here?’ ”
In Dr. Breu’s opinion, there are at least three reasons why consider torsemide should be considered a first-line treatment for heart failure. For one thing, the current evidence says so. In a trial published in 2001, researchers randomized 234 patients with heart failure to receive torsemide or furosemide for 1 year. The percentage of patients who had one or more hospital readmissions was lower among those who received torsemide, compared with those who received furosemide in the torsemide group for heart failure (17% vs. 32%, respectively; P < .01) and for other cardiovascular causes (44% vs. 59%; P = .03). In addition, the number of total admissions was numerically lower for patients in the torsemide group, compared with the furosemide group for heart failure (23 vs. 61; P < .01) and for cardiovascular causes (78 vs. 130; P = .02).
In a separate study, researchers conducted an open-label trial of 237 patients with New York Heart Association (NYHA) class II-IV heart failure who were randomized to torsemide or furosemide. They found that a significantly higher percentage of patients in the torsemide group improved by one or more NYHA heart failure class, compared with those in the furosemide group (40%; P = .001 vs. 31%; P = .3). Moreover, patients treated with furosemide had more restrictions of daily life at 9 months, compared with those treated with torsemide (P < .001).
A separate, open-label, nonrandomized, postmarketing surveillance trial also found benefits of torsemide over furosemide or other agents used for patients with NYHA class III and IV heart failure. Patients treated with torsemide had a lower total mortality, compared with those treated with furosemide or other agents (2.2% vs. 4.5%, respectively; P < .05) as well as a lower cardiac mortality (1.4% vs. 3.5%; P < .05). They were also more likely to improve by one or more heart failure class (46% vs. 37%; P < .01) and less likely to have potassium levels less than 3.5 mEq/L or greater than 5.0 mEq/L (13% vs. 18%; P = .01).
According to Dr. Breu, meta-analyses of this topic consistently show that the NYHA class improved more with torsemide than with furosemide. “Some meta-analyses find a mortality benefit, while others find a readmissions benefit,” he said. “None of them show a benefit of furosemide over torsemide.”
A second reason to use torsemide as a first-line treatment for heart failure is that it has superior pharmacokinetics/dynamics, compared with furosemide. “We’ve all heard that furosemide has variable bioavailability,” said Dr. Breu, who also deputy editor of the Journal of Hospital Medicine’s “Things We Do For No Reason” article series. “Torsemide and bumetanide are much more reliably absorbed, partially because they are not affected by food, whereas furosemide is. That could be potentially problematic for patients who take their diuretic with meals. The fact that torsemide has less renal clearance is a benefit, because patients with heart failure have changing renal function.” In addition, the half-life of torsemide is 3-4 hours and the duration of action is 12 hours, “which are both longer than those for furosemide or bumetanide,” he added.
He also pointed out that torsemide has been shown to block the aldosterone receptor in vitro and in rat models – an effect that has not been observed with other loop diuretics. A randomized trial of patients with chronic heart failure found that levels of renin and aldosterone increased more with torsemide, compared with furosemide, supporting the hypothesis of aldosterone receptor blockade.
A third main reason to use torsemide as your go-to for heart failure has to do with its purported antifibrotic effects, “so that it could be more than a diuretic,” Dr. Breu said. “In heart failure, myocardial fibrosis occurs from increased collagen synthesis and turnover. Aldosterone has been shown to play a role in this myocardial fibrosis. Spironolactone has been shown to mitigate this to some extent. If torsemide acts a little like spironolactone, maybe that could explain some of the long-term effects that we see in these studies.”
A study supporting this notion found that torsemide but not furosemide reduced levels of serum carboxyl-terminal peptide of procollagen type I, which is associated with exaggerated myocardial deposition of collagen type I fibers in cardiac diseases.
Going forward, a study known as TRANSFORM-HF, which is currently recruiting about 6,000 patients, should bring more clarity to the topic. The primary objective is to compare the treatment strategy of torsemide versus furosemide on clinical outcomes over 12 months in patients with heart failure who are hospitalized. The estimated completion is mid-2022.
Dr. Breu and Dr. Feldman reported having no relevant financial disclosures.
When starting a new loop diuretic for a patient with heart failure, strongly consider torsemide over furosemide, Anthony C. Breu, MD, advised at SHM Converge, the annual conference of the Society of Hospital Medicine.
“Whether or not you take a patient who’s already on furosemide and you make the switch to torsemide is a little bit tougher for me to advocate, though that has happened in clinical trials,” said Dr. Breu, assistant professor of medicine at Harvard Medical School, Boston, who spoke May 5 at the Converge session “Things We Do for No Reason.” He co-presented the session with Leonard Feldman, MD, SFHM, director of the Osler Medical Residency Urban Health Track and associate professor at Johns Hopkins Medicine, Baltimore.
“If you consider doing this it would make sense to do so concert with the outpatient primary doctor and the outpatient cardiologist,” Dr. Breu said. “But in my review of the literature, it’s at least worth having these discussions, particularly for a patient who has multiple readmissions for heart failure. That may be a time to pause and ask: ‘Could torsemide be of benefit here?’ ”
In Dr. Breu’s opinion, there are at least three reasons why consider torsemide should be considered a first-line treatment for heart failure. For one thing, the current evidence says so. In a trial published in 2001, researchers randomized 234 patients with heart failure to receive torsemide or furosemide for 1 year. The percentage of patients who had one or more hospital readmissions was lower among those who received torsemide, compared with those who received furosemide in the torsemide group for heart failure (17% vs. 32%, respectively; P < .01) and for other cardiovascular causes (44% vs. 59%; P = .03). In addition, the number of total admissions was numerically lower for patients in the torsemide group, compared with the furosemide group for heart failure (23 vs. 61; P < .01) and for cardiovascular causes (78 vs. 130; P = .02).
In a separate study, researchers conducted an open-label trial of 237 patients with New York Heart Association (NYHA) class II-IV heart failure who were randomized to torsemide or furosemide. They found that a significantly higher percentage of patients in the torsemide group improved by one or more NYHA heart failure class, compared with those in the furosemide group (40%; P = .001 vs. 31%; P = .3). Moreover, patients treated with furosemide had more restrictions of daily life at 9 months, compared with those treated with torsemide (P < .001).
A separate, open-label, nonrandomized, postmarketing surveillance trial also found benefits of torsemide over furosemide or other agents used for patients with NYHA class III and IV heart failure. Patients treated with torsemide had a lower total mortality, compared with those treated with furosemide or other agents (2.2% vs. 4.5%, respectively; P < .05) as well as a lower cardiac mortality (1.4% vs. 3.5%; P < .05). They were also more likely to improve by one or more heart failure class (46% vs. 37%; P < .01) and less likely to have potassium levels less than 3.5 mEq/L or greater than 5.0 mEq/L (13% vs. 18%; P = .01).
According to Dr. Breu, meta-analyses of this topic consistently show that the NYHA class improved more with torsemide than with furosemide. “Some meta-analyses find a mortality benefit, while others find a readmissions benefit,” he said. “None of them show a benefit of furosemide over torsemide.”
A second reason to use torsemide as a first-line treatment for heart failure is that it has superior pharmacokinetics/dynamics, compared with furosemide. “We’ve all heard that furosemide has variable bioavailability,” said Dr. Breu, who also deputy editor of the Journal of Hospital Medicine’s “Things We Do For No Reason” article series. “Torsemide and bumetanide are much more reliably absorbed, partially because they are not affected by food, whereas furosemide is. That could be potentially problematic for patients who take their diuretic with meals. The fact that torsemide has less renal clearance is a benefit, because patients with heart failure have changing renal function.” In addition, the half-life of torsemide is 3-4 hours and the duration of action is 12 hours, “which are both longer than those for furosemide or bumetanide,” he added.
He also pointed out that torsemide has been shown to block the aldosterone receptor in vitro and in rat models – an effect that has not been observed with other loop diuretics. A randomized trial of patients with chronic heart failure found that levels of renin and aldosterone increased more with torsemide, compared with furosemide, supporting the hypothesis of aldosterone receptor blockade.
A third main reason to use torsemide as your go-to for heart failure has to do with its purported antifibrotic effects, “so that it could be more than a diuretic,” Dr. Breu said. “In heart failure, myocardial fibrosis occurs from increased collagen synthesis and turnover. Aldosterone has been shown to play a role in this myocardial fibrosis. Spironolactone has been shown to mitigate this to some extent. If torsemide acts a little like spironolactone, maybe that could explain some of the long-term effects that we see in these studies.”
A study supporting this notion found that torsemide but not furosemide reduced levels of serum carboxyl-terminal peptide of procollagen type I, which is associated with exaggerated myocardial deposition of collagen type I fibers in cardiac diseases.
Going forward, a study known as TRANSFORM-HF, which is currently recruiting about 6,000 patients, should bring more clarity to the topic. The primary objective is to compare the treatment strategy of torsemide versus furosemide on clinical outcomes over 12 months in patients with heart failure who are hospitalized. The estimated completion is mid-2022.
Dr. Breu and Dr. Feldman reported having no relevant financial disclosures.
When starting a new loop diuretic for a patient with heart failure, strongly consider torsemide over furosemide, Anthony C. Breu, MD, advised at SHM Converge, the annual conference of the Society of Hospital Medicine.
“Whether or not you take a patient who’s already on furosemide and you make the switch to torsemide is a little bit tougher for me to advocate, though that has happened in clinical trials,” said Dr. Breu, assistant professor of medicine at Harvard Medical School, Boston, who spoke May 5 at the Converge session “Things We Do for No Reason.” He co-presented the session with Leonard Feldman, MD, SFHM, director of the Osler Medical Residency Urban Health Track and associate professor at Johns Hopkins Medicine, Baltimore.
“If you consider doing this it would make sense to do so concert with the outpatient primary doctor and the outpatient cardiologist,” Dr. Breu said. “But in my review of the literature, it’s at least worth having these discussions, particularly for a patient who has multiple readmissions for heart failure. That may be a time to pause and ask: ‘Could torsemide be of benefit here?’ ”
In Dr. Breu’s opinion, there are at least three reasons why consider torsemide should be considered a first-line treatment for heart failure. For one thing, the current evidence says so. In a trial published in 2001, researchers randomized 234 patients with heart failure to receive torsemide or furosemide for 1 year. The percentage of patients who had one or more hospital readmissions was lower among those who received torsemide, compared with those who received furosemide in the torsemide group for heart failure (17% vs. 32%, respectively; P < .01) and for other cardiovascular causes (44% vs. 59%; P = .03). In addition, the number of total admissions was numerically lower for patients in the torsemide group, compared with the furosemide group for heart failure (23 vs. 61; P < .01) and for cardiovascular causes (78 vs. 130; P = .02).
In a separate study, researchers conducted an open-label trial of 237 patients with New York Heart Association (NYHA) class II-IV heart failure who were randomized to torsemide or furosemide. They found that a significantly higher percentage of patients in the torsemide group improved by one or more NYHA heart failure class, compared with those in the furosemide group (40%; P = .001 vs. 31%; P = .3). Moreover, patients treated with furosemide had more restrictions of daily life at 9 months, compared with those treated with torsemide (P < .001).
A separate, open-label, nonrandomized, postmarketing surveillance trial also found benefits of torsemide over furosemide or other agents used for patients with NYHA class III and IV heart failure. Patients treated with torsemide had a lower total mortality, compared with those treated with furosemide or other agents (2.2% vs. 4.5%, respectively; P < .05) as well as a lower cardiac mortality (1.4% vs. 3.5%; P < .05). They were also more likely to improve by one or more heart failure class (46% vs. 37%; P < .01) and less likely to have potassium levels less than 3.5 mEq/L or greater than 5.0 mEq/L (13% vs. 18%; P = .01).
According to Dr. Breu, meta-analyses of this topic consistently show that the NYHA class improved more with torsemide than with furosemide. “Some meta-analyses find a mortality benefit, while others find a readmissions benefit,” he said. “None of them show a benefit of furosemide over torsemide.”
A second reason to use torsemide as a first-line treatment for heart failure is that it has superior pharmacokinetics/dynamics, compared with furosemide. “We’ve all heard that furosemide has variable bioavailability,” said Dr. Breu, who also deputy editor of the Journal of Hospital Medicine’s “Things We Do For No Reason” article series. “Torsemide and bumetanide are much more reliably absorbed, partially because they are not affected by food, whereas furosemide is. That could be potentially problematic for patients who take their diuretic with meals. The fact that torsemide has less renal clearance is a benefit, because patients with heart failure have changing renal function.” In addition, the half-life of torsemide is 3-4 hours and the duration of action is 12 hours, “which are both longer than those for furosemide or bumetanide,” he added.
He also pointed out that torsemide has been shown to block the aldosterone receptor in vitro and in rat models – an effect that has not been observed with other loop diuretics. A randomized trial of patients with chronic heart failure found that levels of renin and aldosterone increased more with torsemide, compared with furosemide, supporting the hypothesis of aldosterone receptor blockade.
A third main reason to use torsemide as your go-to for heart failure has to do with its purported antifibrotic effects, “so that it could be more than a diuretic,” Dr. Breu said. “In heart failure, myocardial fibrosis occurs from increased collagen synthesis and turnover. Aldosterone has been shown to play a role in this myocardial fibrosis. Spironolactone has been shown to mitigate this to some extent. If torsemide acts a little like spironolactone, maybe that could explain some of the long-term effects that we see in these studies.”
A study supporting this notion found that torsemide but not furosemide reduced levels of serum carboxyl-terminal peptide of procollagen type I, which is associated with exaggerated myocardial deposition of collagen type I fibers in cardiac diseases.
Going forward, a study known as TRANSFORM-HF, which is currently recruiting about 6,000 patients, should bring more clarity to the topic. The primary objective is to compare the treatment strategy of torsemide versus furosemide on clinical outcomes over 12 months in patients with heart failure who are hospitalized. The estimated completion is mid-2022.
Dr. Breu and Dr. Feldman reported having no relevant financial disclosures.
FROM SHM CONVERGE 2021
SHM names new Masters in Hospital Medicine at Converge 2021
This year, the Society of Hospital Medicine will induct three new Masters in Hospital Medicine (MHM), the society’s highest professional honor.
SHM first introduced the MHM designation in 2010. The honor is reserved for hospitalists who have uniquely distinguished themselves in the specialty through the excellence and significance of their contributions to hospital medicine specifically and healthcare as a whole. SHM members are nominated for MHM consideration, and the SHM Board of Directors rigorously reviews qualifications and selects each year’s MHM class.
The three hospitalists receiving the MHM designation at SHM Converge 2021 are Dr. Nasim Afsar, Dr. Shaun D. Frost, and Dr. Jeffrey L. Schnipper.
Nasim Afsar, MD, MBA, MHM
Dr. Nasim Afsar has been elected a Master in Hospital Medicine, honoring her unwavering dedication to hospital medicine and the Society as an accomplished medical leader.
She is known for her accomplishments in establishing and optimizing complex systems of care in the ambulatory and inpatient settings. Her contributions to hospital medicine can be seen through her extensive leadership experience in health care operations, quality, finance, and management.
Dr. Afsar received her MD from the UC Davis School of Medicine, and went on to complete her residency and internship at UCSD and UCLA, internal medicine programs. She currently serves as the chief operating officer for ambulatory care for UCI Health, with the vision of delivering flawless care for the population of patients in Orange County, Calif.
Over her career, Dr. Afsar has led the development and successful implementation of forward-thinking and ambitious healthcare quality strategies across multiple organizations. Her work in patient safety and quality improvement has earned her numerous accolades and awards, including the 2011 John M. Eisenberg Award.
She served on SHM’s Board of Directors from April 2012 through April 2020, including as president and treasurer. During her time on the board, she was instrumental in defining SHM’s role in population health.
Dr. Afsar has held a variety of positions within the Society, including as chair of SHM’s Hospital Quality & Patient Safety Committee, founder and past copresident of SHM’s Los Angeles Chapter, and as faculty at numerous annual conferences. She was an esteemed mentor within SHM’s Project BOOST, a program within SHM’s Center for Quality Improvement focused on care transitions, and served as an associate editor of the Journal of Hospital Medicine for nearly 13 years.
Dr. Afsar is a hospital medicine leader and true champion for our Society and for our specialty. She epitomizes what it means to be a passionate, driven, and accomplished hospital medicine pioneer.
Shaun D. Frost, MD, MHM
Dr. Shaun D. Frost has been elected a Master in Hospital Medicine, celebrating his enduring commitment to hospital medicine and to the Society for more than 20 years.
After completing his internal medicine residency at the University of Texas, Southwestern Medical School in 1998, he launched his career at the Cleveland Clinic Foundation, where he was a clinical assistant professor of internal medicine at Penn State’s College of Medicine.
His contributions to hospital medicine can be seen through his comprehensive leadership background, commitment to medical education, innovation in hospitalist program operations, and various publications. He is also well known for his mentorship of young hospitalist leaders.
Dr. Frost currently serves as the associate medical director of care delivery systems at HealthPartners Health Insurance Plan. He is a practicing hospitalist at Regions Hospital in St. Paul, Minn., and is assistant professor at the University of Minnesota’s Medical School. Prior to this role, he worked at the Cleveland Clinic as the director of Nonteaching Inpatient Services for 6 years, and also served as the Northeast Region chief medical officer of Cogent Healthcare from 2006 to 2012 where he standardized program operations through structured leadership training according to phased priorities and critical functions.
Dr. Frost is well known for his expansive contributions to the Society of Hospital Medicine, and was recognized by SHM in 2005 with the National Award for Clinical Excellence.
Dr. Frost joined the Society in 1999, and soon thereafter founded and led the Northeast Ohio Chapter. His influence, leadership, and guidance helped to shape the creation of SHM’s Chapter Program, which is an integral part of the Society, connecting hospitalists at the local level.
He served on SHM’s Board of Directors for 6 years, including as president and treasurer. He has spoken at many of SHM’s annual conferences, participated on annual meeting planning committees, and served as course director for the annual meeting’s Perioperative Medicine Precourse. He also has served as a facilitator at SHM’s Leadership Academies.
Dr. Frost has led and actively participated in numerous SHM committees, councils, and workgroups, including service as the chair of SHM’s Membership Committee for 5 years. In fact, the SHM fellow designations, including this very distinction, the Master in Hospital Medicine, originated within this committee under his leadership.
During his tenure as SHM president in 2012-2013, he helped to focus the organization’s work to define hospital medicine’s strengths and benefits to healthcare, culminating in the publication of the “Key Principles and Characteristics of an Effective Hospital Medicine Group.”
Dr. Frost is a specialty vanguard, innovator, and SHM leader. He demonstrates what it means to be a passionate and dedicated hospital medicine professional in his community and within the field at-large.
Jeffrey L. Schnipper, MD, MPH, MHM
Dr. Jeffrey L. Schnipper has been elected a Master in Hospital Medicine, honoring his commitment to hospital medicine as an accomplished hospitalist, researcher, and quality improvement enthusiast.
He graduated from Harvard Medical School in 1996 and went on to receive his master’s degree in public health from the Harvard School of Public Health in 2001. Dr. Schnipper also completed his residency, along with a General Medicine Fellowship, at Massachusetts General Hospital in 2001.
Dr. Schnipper currently serves as the director of clinical research in Brigham and Women’s Hospital’s Hospital Medicine Unit. He also serves as the research director of its General Internal Medicine and Primary Care Division and the fellowship director of the Harvard-Brigham Research Fellowship in Hospital Medicine. He is an associate physician at Brigham and Women’s Hospital and is professor of medicine at Harvard Medical School.
His contributions to hospital medicine are demonstrated through his research efforts focused on improving the quality of healthcare delivery for general medical patients, including inpatient diabetes management, care transitions, medication reconciliation, and hospital at home care. His medication reconciliation research project (known as MARQUIS) was funded through AHRQ and led to a 5+ year partnership with the SHM Center for Quality Improvement. When Dr. Schnipper obtained a second AHRQ grant for the MARQUIS2 study, he also partnered with SHM’s Center for Quality Improvement.
Dr. Schnipper joined the Society in 2005 and remains an engaged member of the Boston Association of Academic Hospital Medicine Chapter. He has been a member of SHM’s Annual Conference Committee and serves on the editorial team of the Journal of Hospital Medicine as an associate editor. He has been invited to speak at numerous SHM annual conferences. His research efforts and impact on the medical field can be found in over 150 peer-reviewed publications including JHM, JAMA Internal Medicine, Annals of Internal Medicine, and the Journal of the American Medical Informatics Association.
Dr. Schnipper has received numerous awards and honors throughout his career, including SHM’s Excellence in Research Award in 2013. He is an established researcher, educator, and physician with a broad spectrum of clinical interests that embody what it means to be a hospitalist leader and a top-notch patient care provider.
This year, the Society of Hospital Medicine will induct three new Masters in Hospital Medicine (MHM), the society’s highest professional honor.
SHM first introduced the MHM designation in 2010. The honor is reserved for hospitalists who have uniquely distinguished themselves in the specialty through the excellence and significance of their contributions to hospital medicine specifically and healthcare as a whole. SHM members are nominated for MHM consideration, and the SHM Board of Directors rigorously reviews qualifications and selects each year’s MHM class.
The three hospitalists receiving the MHM designation at SHM Converge 2021 are Dr. Nasim Afsar, Dr. Shaun D. Frost, and Dr. Jeffrey L. Schnipper.
Nasim Afsar, MD, MBA, MHM
Dr. Nasim Afsar has been elected a Master in Hospital Medicine, honoring her unwavering dedication to hospital medicine and the Society as an accomplished medical leader.
She is known for her accomplishments in establishing and optimizing complex systems of care in the ambulatory and inpatient settings. Her contributions to hospital medicine can be seen through her extensive leadership experience in health care operations, quality, finance, and management.
Dr. Afsar received her MD from the UC Davis School of Medicine, and went on to complete her residency and internship at UCSD and UCLA, internal medicine programs. She currently serves as the chief operating officer for ambulatory care for UCI Health, with the vision of delivering flawless care for the population of patients in Orange County, Calif.
Over her career, Dr. Afsar has led the development and successful implementation of forward-thinking and ambitious healthcare quality strategies across multiple organizations. Her work in patient safety and quality improvement has earned her numerous accolades and awards, including the 2011 John M. Eisenberg Award.
She served on SHM’s Board of Directors from April 2012 through April 2020, including as president and treasurer. During her time on the board, she was instrumental in defining SHM’s role in population health.
Dr. Afsar has held a variety of positions within the Society, including as chair of SHM’s Hospital Quality & Patient Safety Committee, founder and past copresident of SHM’s Los Angeles Chapter, and as faculty at numerous annual conferences. She was an esteemed mentor within SHM’s Project BOOST, a program within SHM’s Center for Quality Improvement focused on care transitions, and served as an associate editor of the Journal of Hospital Medicine for nearly 13 years.
Dr. Afsar is a hospital medicine leader and true champion for our Society and for our specialty. She epitomizes what it means to be a passionate, driven, and accomplished hospital medicine pioneer.
Shaun D. Frost, MD, MHM
Dr. Shaun D. Frost has been elected a Master in Hospital Medicine, celebrating his enduring commitment to hospital medicine and to the Society for more than 20 years.
After completing his internal medicine residency at the University of Texas, Southwestern Medical School in 1998, he launched his career at the Cleveland Clinic Foundation, where he was a clinical assistant professor of internal medicine at Penn State’s College of Medicine.
His contributions to hospital medicine can be seen through his comprehensive leadership background, commitment to medical education, innovation in hospitalist program operations, and various publications. He is also well known for his mentorship of young hospitalist leaders.
Dr. Frost currently serves as the associate medical director of care delivery systems at HealthPartners Health Insurance Plan. He is a practicing hospitalist at Regions Hospital in St. Paul, Minn., and is assistant professor at the University of Minnesota’s Medical School. Prior to this role, he worked at the Cleveland Clinic as the director of Nonteaching Inpatient Services for 6 years, and also served as the Northeast Region chief medical officer of Cogent Healthcare from 2006 to 2012 where he standardized program operations through structured leadership training according to phased priorities and critical functions.
Dr. Frost is well known for his expansive contributions to the Society of Hospital Medicine, and was recognized by SHM in 2005 with the National Award for Clinical Excellence.
Dr. Frost joined the Society in 1999, and soon thereafter founded and led the Northeast Ohio Chapter. His influence, leadership, and guidance helped to shape the creation of SHM’s Chapter Program, which is an integral part of the Society, connecting hospitalists at the local level.
He served on SHM’s Board of Directors for 6 years, including as president and treasurer. He has spoken at many of SHM’s annual conferences, participated on annual meeting planning committees, and served as course director for the annual meeting’s Perioperative Medicine Precourse. He also has served as a facilitator at SHM’s Leadership Academies.
Dr. Frost has led and actively participated in numerous SHM committees, councils, and workgroups, including service as the chair of SHM’s Membership Committee for 5 years. In fact, the SHM fellow designations, including this very distinction, the Master in Hospital Medicine, originated within this committee under his leadership.
During his tenure as SHM president in 2012-2013, he helped to focus the organization’s work to define hospital medicine’s strengths and benefits to healthcare, culminating in the publication of the “Key Principles and Characteristics of an Effective Hospital Medicine Group.”
Dr. Frost is a specialty vanguard, innovator, and SHM leader. He demonstrates what it means to be a passionate and dedicated hospital medicine professional in his community and within the field at-large.
Jeffrey L. Schnipper, MD, MPH, MHM
Dr. Jeffrey L. Schnipper has been elected a Master in Hospital Medicine, honoring his commitment to hospital medicine as an accomplished hospitalist, researcher, and quality improvement enthusiast.
He graduated from Harvard Medical School in 1996 and went on to receive his master’s degree in public health from the Harvard School of Public Health in 2001. Dr. Schnipper also completed his residency, along with a General Medicine Fellowship, at Massachusetts General Hospital in 2001.
Dr. Schnipper currently serves as the director of clinical research in Brigham and Women’s Hospital’s Hospital Medicine Unit. He also serves as the research director of its General Internal Medicine and Primary Care Division and the fellowship director of the Harvard-Brigham Research Fellowship in Hospital Medicine. He is an associate physician at Brigham and Women’s Hospital and is professor of medicine at Harvard Medical School.
His contributions to hospital medicine are demonstrated through his research efforts focused on improving the quality of healthcare delivery for general medical patients, including inpatient diabetes management, care transitions, medication reconciliation, and hospital at home care. His medication reconciliation research project (known as MARQUIS) was funded through AHRQ and led to a 5+ year partnership with the SHM Center for Quality Improvement. When Dr. Schnipper obtained a second AHRQ grant for the MARQUIS2 study, he also partnered with SHM’s Center for Quality Improvement.
Dr. Schnipper joined the Society in 2005 and remains an engaged member of the Boston Association of Academic Hospital Medicine Chapter. He has been a member of SHM’s Annual Conference Committee and serves on the editorial team of the Journal of Hospital Medicine as an associate editor. He has been invited to speak at numerous SHM annual conferences. His research efforts and impact on the medical field can be found in over 150 peer-reviewed publications including JHM, JAMA Internal Medicine, Annals of Internal Medicine, and the Journal of the American Medical Informatics Association.
Dr. Schnipper has received numerous awards and honors throughout his career, including SHM’s Excellence in Research Award in 2013. He is an established researcher, educator, and physician with a broad spectrum of clinical interests that embody what it means to be a hospitalist leader and a top-notch patient care provider.
This year, the Society of Hospital Medicine will induct three new Masters in Hospital Medicine (MHM), the society’s highest professional honor.
SHM first introduced the MHM designation in 2010. The honor is reserved for hospitalists who have uniquely distinguished themselves in the specialty through the excellence and significance of their contributions to hospital medicine specifically and healthcare as a whole. SHM members are nominated for MHM consideration, and the SHM Board of Directors rigorously reviews qualifications and selects each year’s MHM class.
The three hospitalists receiving the MHM designation at SHM Converge 2021 are Dr. Nasim Afsar, Dr. Shaun D. Frost, and Dr. Jeffrey L. Schnipper.
Nasim Afsar, MD, MBA, MHM
Dr. Nasim Afsar has been elected a Master in Hospital Medicine, honoring her unwavering dedication to hospital medicine and the Society as an accomplished medical leader.
She is known for her accomplishments in establishing and optimizing complex systems of care in the ambulatory and inpatient settings. Her contributions to hospital medicine can be seen through her extensive leadership experience in health care operations, quality, finance, and management.
Dr. Afsar received her MD from the UC Davis School of Medicine, and went on to complete her residency and internship at UCSD and UCLA, internal medicine programs. She currently serves as the chief operating officer for ambulatory care for UCI Health, with the vision of delivering flawless care for the population of patients in Orange County, Calif.
Over her career, Dr. Afsar has led the development and successful implementation of forward-thinking and ambitious healthcare quality strategies across multiple organizations. Her work in patient safety and quality improvement has earned her numerous accolades and awards, including the 2011 John M. Eisenberg Award.
She served on SHM’s Board of Directors from April 2012 through April 2020, including as president and treasurer. During her time on the board, she was instrumental in defining SHM’s role in population health.
Dr. Afsar has held a variety of positions within the Society, including as chair of SHM’s Hospital Quality & Patient Safety Committee, founder and past copresident of SHM’s Los Angeles Chapter, and as faculty at numerous annual conferences. She was an esteemed mentor within SHM’s Project BOOST, a program within SHM’s Center for Quality Improvement focused on care transitions, and served as an associate editor of the Journal of Hospital Medicine for nearly 13 years.
Dr. Afsar is a hospital medicine leader and true champion for our Society and for our specialty. She epitomizes what it means to be a passionate, driven, and accomplished hospital medicine pioneer.
Shaun D. Frost, MD, MHM
Dr. Shaun D. Frost has been elected a Master in Hospital Medicine, celebrating his enduring commitment to hospital medicine and to the Society for more than 20 years.
After completing his internal medicine residency at the University of Texas, Southwestern Medical School in 1998, he launched his career at the Cleveland Clinic Foundation, where he was a clinical assistant professor of internal medicine at Penn State’s College of Medicine.
His contributions to hospital medicine can be seen through his comprehensive leadership background, commitment to medical education, innovation in hospitalist program operations, and various publications. He is also well known for his mentorship of young hospitalist leaders.
Dr. Frost currently serves as the associate medical director of care delivery systems at HealthPartners Health Insurance Plan. He is a practicing hospitalist at Regions Hospital in St. Paul, Minn., and is assistant professor at the University of Minnesota’s Medical School. Prior to this role, he worked at the Cleveland Clinic as the director of Nonteaching Inpatient Services for 6 years, and also served as the Northeast Region chief medical officer of Cogent Healthcare from 2006 to 2012 where he standardized program operations through structured leadership training according to phased priorities and critical functions.
Dr. Frost is well known for his expansive contributions to the Society of Hospital Medicine, and was recognized by SHM in 2005 with the National Award for Clinical Excellence.
Dr. Frost joined the Society in 1999, and soon thereafter founded and led the Northeast Ohio Chapter. His influence, leadership, and guidance helped to shape the creation of SHM’s Chapter Program, which is an integral part of the Society, connecting hospitalists at the local level.
He served on SHM’s Board of Directors for 6 years, including as president and treasurer. He has spoken at many of SHM’s annual conferences, participated on annual meeting planning committees, and served as course director for the annual meeting’s Perioperative Medicine Precourse. He also has served as a facilitator at SHM’s Leadership Academies.
Dr. Frost has led and actively participated in numerous SHM committees, councils, and workgroups, including service as the chair of SHM’s Membership Committee for 5 years. In fact, the SHM fellow designations, including this very distinction, the Master in Hospital Medicine, originated within this committee under his leadership.
During his tenure as SHM president in 2012-2013, he helped to focus the organization’s work to define hospital medicine’s strengths and benefits to healthcare, culminating in the publication of the “Key Principles and Characteristics of an Effective Hospital Medicine Group.”
Dr. Frost is a specialty vanguard, innovator, and SHM leader. He demonstrates what it means to be a passionate and dedicated hospital medicine professional in his community and within the field at-large.
Jeffrey L. Schnipper, MD, MPH, MHM
Dr. Jeffrey L. Schnipper has been elected a Master in Hospital Medicine, honoring his commitment to hospital medicine as an accomplished hospitalist, researcher, and quality improvement enthusiast.
He graduated from Harvard Medical School in 1996 and went on to receive his master’s degree in public health from the Harvard School of Public Health in 2001. Dr. Schnipper also completed his residency, along with a General Medicine Fellowship, at Massachusetts General Hospital in 2001.
Dr. Schnipper currently serves as the director of clinical research in Brigham and Women’s Hospital’s Hospital Medicine Unit. He also serves as the research director of its General Internal Medicine and Primary Care Division and the fellowship director of the Harvard-Brigham Research Fellowship in Hospital Medicine. He is an associate physician at Brigham and Women’s Hospital and is professor of medicine at Harvard Medical School.
His contributions to hospital medicine are demonstrated through his research efforts focused on improving the quality of healthcare delivery for general medical patients, including inpatient diabetes management, care transitions, medication reconciliation, and hospital at home care. His medication reconciliation research project (known as MARQUIS) was funded through AHRQ and led to a 5+ year partnership with the SHM Center for Quality Improvement. When Dr. Schnipper obtained a second AHRQ grant for the MARQUIS2 study, he also partnered with SHM’s Center for Quality Improvement.
Dr. Schnipper joined the Society in 2005 and remains an engaged member of the Boston Association of Academic Hospital Medicine Chapter. He has been a member of SHM’s Annual Conference Committee and serves on the editorial team of the Journal of Hospital Medicine as an associate editor. He has been invited to speak at numerous SHM annual conferences. His research efforts and impact on the medical field can be found in over 150 peer-reviewed publications including JHM, JAMA Internal Medicine, Annals of Internal Medicine, and the Journal of the American Medical Informatics Association.
Dr. Schnipper has received numerous awards and honors throughout his career, including SHM’s Excellence in Research Award in 2013. He is an established researcher, educator, and physician with a broad spectrum of clinical interests that embody what it means to be a hospitalist leader and a top-notch patient care provider.
FROM SHM CONVERGE 2021
Parental attitudes to kids’ sexual orientation: Unexpected findings
For gay and lesbian individuals, consistency in parents’ attitudes toward their child’s sexual orientation, even when they are negative, is an important factor in positive mental health outcomes, new research shows.
Study investigator Matthew Verdun, MS, a licensed marriage and family therapist and doctoral student at the Chicago School of Professional Psychology at Los Angeles, California, found that gays and lesbians whose parents were not supportive of their sexual orientation could still have good outcomes.
The findings were presented at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
High rates of mental illness
Research shows that members of the gay and lesbian community experience higher rates of mental illness and substance use disorders and that psychological well-being declines during periods close to when sexual orientation is disclosed.
Mr. Verdun referred to a theory in the literature of homosexual identity formation that describes how individuals go through six stages: confusion, comparison, tolerance, acceptance, pride, and synthesis.
Research shows a U-shaped relationship between subjective reports of well-being at these six stages. The lowest rates occur during the identity comparison and identity tolerance stages.
“Those stages roughly correspond with the time when people would disclose their sexual orientation to parents and family members. The time when a person discloses is probably one of the most anxious times in their life; it’s also where their rate of well-being is the lowest,” said Mr. Verdun.
Mr. Verdun said he “wanted to know what happens when a parent is supportive or rejecting at that moment, but also what happens over time.”
To determine whether parental support affects depression, anxiety, or substance abuse in members of the gay and lesbian community, Mr. Verdun studied 175 individuals who self-identified as gay or lesbian (77 males and 98 females) and were recruited via social media. Most (70.3%) were of White race or ethnicity.
Participants completed surveys asking about their parents’ initial and current level of support regarding their sexual orientation. They also completed the nine-item Patient Health Questionnaire (PHQ-9), the seven-item General Anxiety Disorder (GAD-7), and the 20-item Drug Abuse Screening Tool (DAST-20).
The investigators categorized participants into one of three groups on the basis of parental support:
- Consistently positive.
- Negative to positive.
- Consistently negative.
A fourth group, positive to negative, was excluded from the analysis because it was too small.
Mr. Verdun was unable analyze results for substance abuse. “The DAST-20 results violated the assumption of homogeneity of variances, which meant the analysis could result in error,” he explained.
Analyses for the PHQ-9 and GAD-7 showed that the consistently positive group had the lowest symptom scores.
“People whose parents were accepting had the lowest scores for anxiety and depression,” said Mr. Verdun.
For both the PHQ and GAD, the findings were significant (P < .05) for the consistently positive and the consistently negative groups in comparison with the negative to positive group.
The difference between the consistently positive and the consistently negative groups was not statistically significant.
Surprise finding
Previous research has shown that current levels of parental support relate to better mental health, so Mr. Verdun initially thought children whose parents were consistently supportive or those whose parents became supportive over time would have the best mental health outcomes.
“But, interestingly, what I found was that people whose parents vacillated between being accepting and rejecting over time actually had significantly more mental health symptoms at the time of the assessment than people whose parents were consistently accepting or consistently rejecting,” he said.
Although the study provided evidence of better outcomes for those with consistently unsupportive parents, Mr. Verdun believes some hypotheses are worthy of further research.
One is that people with unsupportive parents receive support elsewhere and could, for example, turn to peers, teachers, or other community members, including faith leaders, and that symptoms of mental illness may improve with such support, said Mr. Verdun.
These individuals may also develop ways to “buffer their mental health symptoms,” possibly by cultivating meaningful relationships “where they’re seen as a complete and total person, not just in terms of their sexual orientation,” he said.
Gay and lesbian individuals may also benefit from “healing activities,” which might include engagement and involvement in their community, such as performing volunteer work and learning about the history of their community, said Mr. Verdun.
Mental health providers can play a role in creating a positive environment by referring patients to support groups, to centers that cater to gays and lesbians, to faith communities, or by encouraging recreational activities, said Mr. Verdun.
Clinicians can also help gay and lesbian patients determine how and when to safely disclose their sexual orientation, he said.
The study did not include bisexual or transsexual individuals because processes of identifying sexual orientation differ for those persons, said Mr. Verdun.
“I would like to conduct future research that includes bisexual, trans people, and intersectional groups within the LGBTQIA [lesbian, gay, bisexual, transgender, queer, intersex, asexual] community,” he said.
Important research
Commenting on the study, Jeffrey Borenstein, MD, president and CEO of the Brain and Behavior Research Foundation and editor-in-chief of Psychiatric News, said the work is “extremely important and that it has the potential to lead to clinical guidance.”
The finding that levels of depression and anxiety were lower in children whose parents were accepting of their sexual orientation is not surprising, said Dr. Borenstein. “It’s common sense, but it’s always good to have such a finding demonstrate it,” he said.
Parents who understand this relationship may be better able to help their child who is depressed or anxious, he added.
Dr. Borenstein agreed that further research is needed regarding the finding of benefits from consistent parenting, even when that parenting involves rejection.
Such research might uncover “what types of other supports these individuals have that allow for lower levels of depression and anxiety,” he said.
“For this population, the risk of mental health issues is higher, and the risk of suicide is higher, so anything we can do to provide support and improved treatment is extremely important,” he said.
A version of this article first appeared on Medscape.com.
For gay and lesbian individuals, consistency in parents’ attitudes toward their child’s sexual orientation, even when they are negative, is an important factor in positive mental health outcomes, new research shows.
Study investigator Matthew Verdun, MS, a licensed marriage and family therapist and doctoral student at the Chicago School of Professional Psychology at Los Angeles, California, found that gays and lesbians whose parents were not supportive of their sexual orientation could still have good outcomes.
The findings were presented at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
High rates of mental illness
Research shows that members of the gay and lesbian community experience higher rates of mental illness and substance use disorders and that psychological well-being declines during periods close to when sexual orientation is disclosed.
Mr. Verdun referred to a theory in the literature of homosexual identity formation that describes how individuals go through six stages: confusion, comparison, tolerance, acceptance, pride, and synthesis.
Research shows a U-shaped relationship between subjective reports of well-being at these six stages. The lowest rates occur during the identity comparison and identity tolerance stages.
“Those stages roughly correspond with the time when people would disclose their sexual orientation to parents and family members. The time when a person discloses is probably one of the most anxious times in their life; it’s also where their rate of well-being is the lowest,” said Mr. Verdun.
Mr. Verdun said he “wanted to know what happens when a parent is supportive or rejecting at that moment, but also what happens over time.”
To determine whether parental support affects depression, anxiety, or substance abuse in members of the gay and lesbian community, Mr. Verdun studied 175 individuals who self-identified as gay or lesbian (77 males and 98 females) and were recruited via social media. Most (70.3%) were of White race or ethnicity.
Participants completed surveys asking about their parents’ initial and current level of support regarding their sexual orientation. They also completed the nine-item Patient Health Questionnaire (PHQ-9), the seven-item General Anxiety Disorder (GAD-7), and the 20-item Drug Abuse Screening Tool (DAST-20).
The investigators categorized participants into one of three groups on the basis of parental support:
- Consistently positive.
- Negative to positive.
- Consistently negative.
A fourth group, positive to negative, was excluded from the analysis because it was too small.
Mr. Verdun was unable analyze results for substance abuse. “The DAST-20 results violated the assumption of homogeneity of variances, which meant the analysis could result in error,” he explained.
Analyses for the PHQ-9 and GAD-7 showed that the consistently positive group had the lowest symptom scores.
“People whose parents were accepting had the lowest scores for anxiety and depression,” said Mr. Verdun.
For both the PHQ and GAD, the findings were significant (P < .05) for the consistently positive and the consistently negative groups in comparison with the negative to positive group.
The difference between the consistently positive and the consistently negative groups was not statistically significant.
Surprise finding
Previous research has shown that current levels of parental support relate to better mental health, so Mr. Verdun initially thought children whose parents were consistently supportive or those whose parents became supportive over time would have the best mental health outcomes.
“But, interestingly, what I found was that people whose parents vacillated between being accepting and rejecting over time actually had significantly more mental health symptoms at the time of the assessment than people whose parents were consistently accepting or consistently rejecting,” he said.
Although the study provided evidence of better outcomes for those with consistently unsupportive parents, Mr. Verdun believes some hypotheses are worthy of further research.
One is that people with unsupportive parents receive support elsewhere and could, for example, turn to peers, teachers, or other community members, including faith leaders, and that symptoms of mental illness may improve with such support, said Mr. Verdun.
These individuals may also develop ways to “buffer their mental health symptoms,” possibly by cultivating meaningful relationships “where they’re seen as a complete and total person, not just in terms of their sexual orientation,” he said.
Gay and lesbian individuals may also benefit from “healing activities,” which might include engagement and involvement in their community, such as performing volunteer work and learning about the history of their community, said Mr. Verdun.
Mental health providers can play a role in creating a positive environment by referring patients to support groups, to centers that cater to gays and lesbians, to faith communities, or by encouraging recreational activities, said Mr. Verdun.
Clinicians can also help gay and lesbian patients determine how and when to safely disclose their sexual orientation, he said.
The study did not include bisexual or transsexual individuals because processes of identifying sexual orientation differ for those persons, said Mr. Verdun.
“I would like to conduct future research that includes bisexual, trans people, and intersectional groups within the LGBTQIA [lesbian, gay, bisexual, transgender, queer, intersex, asexual] community,” he said.
Important research
Commenting on the study, Jeffrey Borenstein, MD, president and CEO of the Brain and Behavior Research Foundation and editor-in-chief of Psychiatric News, said the work is “extremely important and that it has the potential to lead to clinical guidance.”
The finding that levels of depression and anxiety were lower in children whose parents were accepting of their sexual orientation is not surprising, said Dr. Borenstein. “It’s common sense, but it’s always good to have such a finding demonstrate it,” he said.
Parents who understand this relationship may be better able to help their child who is depressed or anxious, he added.
Dr. Borenstein agreed that further research is needed regarding the finding of benefits from consistent parenting, even when that parenting involves rejection.
Such research might uncover “what types of other supports these individuals have that allow for lower levels of depression and anxiety,” he said.
“For this population, the risk of mental health issues is higher, and the risk of suicide is higher, so anything we can do to provide support and improved treatment is extremely important,” he said.
A version of this article first appeared on Medscape.com.
For gay and lesbian individuals, consistency in parents’ attitudes toward their child’s sexual orientation, even when they are negative, is an important factor in positive mental health outcomes, new research shows.
Study investigator Matthew Verdun, MS, a licensed marriage and family therapist and doctoral student at the Chicago School of Professional Psychology at Los Angeles, California, found that gays and lesbians whose parents were not supportive of their sexual orientation could still have good outcomes.
The findings were presented at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
High rates of mental illness
Research shows that members of the gay and lesbian community experience higher rates of mental illness and substance use disorders and that psychological well-being declines during periods close to when sexual orientation is disclosed.
Mr. Verdun referred to a theory in the literature of homosexual identity formation that describes how individuals go through six stages: confusion, comparison, tolerance, acceptance, pride, and synthesis.
Research shows a U-shaped relationship between subjective reports of well-being at these six stages. The lowest rates occur during the identity comparison and identity tolerance stages.
“Those stages roughly correspond with the time when people would disclose their sexual orientation to parents and family members. The time when a person discloses is probably one of the most anxious times in their life; it’s also where their rate of well-being is the lowest,” said Mr. Verdun.
Mr. Verdun said he “wanted to know what happens when a parent is supportive or rejecting at that moment, but also what happens over time.”
To determine whether parental support affects depression, anxiety, or substance abuse in members of the gay and lesbian community, Mr. Verdun studied 175 individuals who self-identified as gay or lesbian (77 males and 98 females) and were recruited via social media. Most (70.3%) were of White race or ethnicity.
Participants completed surveys asking about their parents’ initial and current level of support regarding their sexual orientation. They also completed the nine-item Patient Health Questionnaire (PHQ-9), the seven-item General Anxiety Disorder (GAD-7), and the 20-item Drug Abuse Screening Tool (DAST-20).
The investigators categorized participants into one of three groups on the basis of parental support:
- Consistently positive.
- Negative to positive.
- Consistently negative.
A fourth group, positive to negative, was excluded from the analysis because it was too small.
Mr. Verdun was unable analyze results for substance abuse. “The DAST-20 results violated the assumption of homogeneity of variances, which meant the analysis could result in error,” he explained.
Analyses for the PHQ-9 and GAD-7 showed that the consistently positive group had the lowest symptom scores.
“People whose parents were accepting had the lowest scores for anxiety and depression,” said Mr. Verdun.
For both the PHQ and GAD, the findings were significant (P < .05) for the consistently positive and the consistently negative groups in comparison with the negative to positive group.
The difference between the consistently positive and the consistently negative groups was not statistically significant.
Surprise finding
Previous research has shown that current levels of parental support relate to better mental health, so Mr. Verdun initially thought children whose parents were consistently supportive or those whose parents became supportive over time would have the best mental health outcomes.
“But, interestingly, what I found was that people whose parents vacillated between being accepting and rejecting over time actually had significantly more mental health symptoms at the time of the assessment than people whose parents were consistently accepting or consistently rejecting,” he said.
Although the study provided evidence of better outcomes for those with consistently unsupportive parents, Mr. Verdun believes some hypotheses are worthy of further research.
One is that people with unsupportive parents receive support elsewhere and could, for example, turn to peers, teachers, or other community members, including faith leaders, and that symptoms of mental illness may improve with such support, said Mr. Verdun.
These individuals may also develop ways to “buffer their mental health symptoms,” possibly by cultivating meaningful relationships “where they’re seen as a complete and total person, not just in terms of their sexual orientation,” he said.
Gay and lesbian individuals may also benefit from “healing activities,” which might include engagement and involvement in their community, such as performing volunteer work and learning about the history of their community, said Mr. Verdun.
Mental health providers can play a role in creating a positive environment by referring patients to support groups, to centers that cater to gays and lesbians, to faith communities, or by encouraging recreational activities, said Mr. Verdun.
Clinicians can also help gay and lesbian patients determine how and when to safely disclose their sexual orientation, he said.
The study did not include bisexual or transsexual individuals because processes of identifying sexual orientation differ for those persons, said Mr. Verdun.
“I would like to conduct future research that includes bisexual, trans people, and intersectional groups within the LGBTQIA [lesbian, gay, bisexual, transgender, queer, intersex, asexual] community,” he said.
Important research
Commenting on the study, Jeffrey Borenstein, MD, president and CEO of the Brain and Behavior Research Foundation and editor-in-chief of Psychiatric News, said the work is “extremely important and that it has the potential to lead to clinical guidance.”
The finding that levels of depression and anxiety were lower in children whose parents were accepting of their sexual orientation is not surprising, said Dr. Borenstein. “It’s common sense, but it’s always good to have such a finding demonstrate it,” he said.
Parents who understand this relationship may be better able to help their child who is depressed or anxious, he added.
Dr. Borenstein agreed that further research is needed regarding the finding of benefits from consistent parenting, even when that parenting involves rejection.
Such research might uncover “what types of other supports these individuals have that allow for lower levels of depression and anxiety,” he said.
“For this population, the risk of mental health issues is higher, and the risk of suicide is higher, so anything we can do to provide support and improved treatment is extremely important,” he said.
A version of this article first appeared on Medscape.com.
Hospitalist leader offers a post–COVID-19 approach to career advancement
After navigating a pandemic that turned the world – including the world of hospital medicine – upside down for so long, the very idea of returning to a “normal” career and way of life can seem strange.
Vineet Arora, MD, MAPP, MHM, assistant dean for scholarship and discovery and associate chief medical officer for clinical learning environment at the University of Chicago, offered guidance to hospitalists on the transition from pandemic life to postpandemic life on May 5 at SHM Converge, the annual conference of the Society of Hospital Medicine.
The pandemic, Dr. Arora said, showed how important it is to develop trust. When resources were scarce as dire COVID-19 cases flooded hospitals, a culture of trust was essential to getting through the crisis.
“My team expects me to speak up on their behalf – it’s how we do things. It’s so germane to safety,” Dr. Arora said. “This is what you’re looking for in your organization – a place of psychological safety and trust.”
Surveys show that patients do trust their physicians, and healthcare providers “got a big bump” in trust during the pandemic, she said, which offers a unique opportunity.
“Doctors are trusted messengers for the COVID vaccine,” she said. “It really does matter.” But clinicians should also advocate for social justice, she said. “We must speak up even louder to fight everyday racism.”
As hospitalists move into the postpandemic medical world, Dr. Arora encouraged them to “get rid of delusions of grandeur,” expecting incredible accomplishments around every corner.
“Amazing things do happen, but oftentimes they happen because we sustain the things we start,” Dr. Arora said. For instance, physicians should consider small changes in workflow, but then sustain those changes. Maintaining pushes for change is not necessarily the norm, she said, adding that all hospitalists are probably familiar with quality improvement projects that generate only 3 months of data, because of lost focus.
Hospitalists should also “seek out information brokers” in the postpandemic medical world, or those interacting with a variety of groups who are often good sources of ideas. Hospitalists, she said, are “natural information brokers,” communicating routinely with a wide variety of specialists and healthcare professionals.
“You’ve got to know what’s important to your organization and to your patients and to everybody else,” Dr. Arora said.
She suggested that hospitalists find “zero-gravity thinkers,” and even to be this type of thinker themselves – one who stays open to new ideas and has diverse interests and experiences.
It is easy to settle into the same ways we’ve always done things, Dr. Arora said.
“The truth is there are ways that it can be better,” she said. “But we sometimes have to seek out new ideas and maintain an open mind – and sometimes we need someone to do it for us.”
Often, those closest to us are the least valuable in this regard, she said, referring to them as “innovation killers.”
“They’re not going to give you the next breakthrough idea,” she said. “You have to get outside of your network to understand where the good ideas are coming from.”
With the trauma that hospitalists have experienced for more than a year, well-being might never have been a more vital topic than it is now, Dr. Arora said.
“We’re done with online wellness modules,” she said. “Fix the system and not the person because we all know the system is not working for us. As hospitalists, we actually are experts at fixing systems.”
Dr. Arora said that one way to think of how to improve hospitalist well-being is by emphasizing “the Four Ts” – teamwork (such as the use of scribes and good communication), time (consider new work schedule models), transitions (refining workflows) and tech (technology that works for clinicians rather than creating a burden).
As hospitalists attempt to move ahead in their post–COVID-19 careers, the key is finding new challenges and never stopping the learning process, Dr. Arora said. Referring to a concept described by career coach May Busch, she said physicians can consider successful careers as a “series of S curves” – at the beginning, there is a lot of work without much advancement, followed by a rapid rise, and then arrival at the destination, which brings you to a new plateau higher up the ladder. At the higher plateau, hospitalists should “jump to a new S curve,” learning a new skill and embarking on a new endeavor, which will lift them even higher.
“Success,” Dr. Arora said, “is defined by continuous growth and learning.”
Dr. Arora reported having no financial disclosures.
After navigating a pandemic that turned the world – including the world of hospital medicine – upside down for so long, the very idea of returning to a “normal” career and way of life can seem strange.
Vineet Arora, MD, MAPP, MHM, assistant dean for scholarship and discovery and associate chief medical officer for clinical learning environment at the University of Chicago, offered guidance to hospitalists on the transition from pandemic life to postpandemic life on May 5 at SHM Converge, the annual conference of the Society of Hospital Medicine.
The pandemic, Dr. Arora said, showed how important it is to develop trust. When resources were scarce as dire COVID-19 cases flooded hospitals, a culture of trust was essential to getting through the crisis.
“My team expects me to speak up on their behalf – it’s how we do things. It’s so germane to safety,” Dr. Arora said. “This is what you’re looking for in your organization – a place of psychological safety and trust.”
Surveys show that patients do trust their physicians, and healthcare providers “got a big bump” in trust during the pandemic, she said, which offers a unique opportunity.
“Doctors are trusted messengers for the COVID vaccine,” she said. “It really does matter.” But clinicians should also advocate for social justice, she said. “We must speak up even louder to fight everyday racism.”
As hospitalists move into the postpandemic medical world, Dr. Arora encouraged them to “get rid of delusions of grandeur,” expecting incredible accomplishments around every corner.
“Amazing things do happen, but oftentimes they happen because we sustain the things we start,” Dr. Arora said. For instance, physicians should consider small changes in workflow, but then sustain those changes. Maintaining pushes for change is not necessarily the norm, she said, adding that all hospitalists are probably familiar with quality improvement projects that generate only 3 months of data, because of lost focus.
Hospitalists should also “seek out information brokers” in the postpandemic medical world, or those interacting with a variety of groups who are often good sources of ideas. Hospitalists, she said, are “natural information brokers,” communicating routinely with a wide variety of specialists and healthcare professionals.
“You’ve got to know what’s important to your organization and to your patients and to everybody else,” Dr. Arora said.
She suggested that hospitalists find “zero-gravity thinkers,” and even to be this type of thinker themselves – one who stays open to new ideas and has diverse interests and experiences.
It is easy to settle into the same ways we’ve always done things, Dr. Arora said.
“The truth is there are ways that it can be better,” she said. “But we sometimes have to seek out new ideas and maintain an open mind – and sometimes we need someone to do it for us.”
Often, those closest to us are the least valuable in this regard, she said, referring to them as “innovation killers.”
“They’re not going to give you the next breakthrough idea,” she said. “You have to get outside of your network to understand where the good ideas are coming from.”
With the trauma that hospitalists have experienced for more than a year, well-being might never have been a more vital topic than it is now, Dr. Arora said.
“We’re done with online wellness modules,” she said. “Fix the system and not the person because we all know the system is not working for us. As hospitalists, we actually are experts at fixing systems.”
Dr. Arora said that one way to think of how to improve hospitalist well-being is by emphasizing “the Four Ts” – teamwork (such as the use of scribes and good communication), time (consider new work schedule models), transitions (refining workflows) and tech (technology that works for clinicians rather than creating a burden).
As hospitalists attempt to move ahead in their post–COVID-19 careers, the key is finding new challenges and never stopping the learning process, Dr. Arora said. Referring to a concept described by career coach May Busch, she said physicians can consider successful careers as a “series of S curves” – at the beginning, there is a lot of work without much advancement, followed by a rapid rise, and then arrival at the destination, which brings you to a new plateau higher up the ladder. At the higher plateau, hospitalists should “jump to a new S curve,” learning a new skill and embarking on a new endeavor, which will lift them even higher.
“Success,” Dr. Arora said, “is defined by continuous growth and learning.”
Dr. Arora reported having no financial disclosures.
After navigating a pandemic that turned the world – including the world of hospital medicine – upside down for so long, the very idea of returning to a “normal” career and way of life can seem strange.
Vineet Arora, MD, MAPP, MHM, assistant dean for scholarship and discovery and associate chief medical officer for clinical learning environment at the University of Chicago, offered guidance to hospitalists on the transition from pandemic life to postpandemic life on May 5 at SHM Converge, the annual conference of the Society of Hospital Medicine.
The pandemic, Dr. Arora said, showed how important it is to develop trust. When resources were scarce as dire COVID-19 cases flooded hospitals, a culture of trust was essential to getting through the crisis.
“My team expects me to speak up on their behalf – it’s how we do things. It’s so germane to safety,” Dr. Arora said. “This is what you’re looking for in your organization – a place of psychological safety and trust.”
Surveys show that patients do trust their physicians, and healthcare providers “got a big bump” in trust during the pandemic, she said, which offers a unique opportunity.
“Doctors are trusted messengers for the COVID vaccine,” she said. “It really does matter.” But clinicians should also advocate for social justice, she said. “We must speak up even louder to fight everyday racism.”
As hospitalists move into the postpandemic medical world, Dr. Arora encouraged them to “get rid of delusions of grandeur,” expecting incredible accomplishments around every corner.
“Amazing things do happen, but oftentimes they happen because we sustain the things we start,” Dr. Arora said. For instance, physicians should consider small changes in workflow, but then sustain those changes. Maintaining pushes for change is not necessarily the norm, she said, adding that all hospitalists are probably familiar with quality improvement projects that generate only 3 months of data, because of lost focus.
Hospitalists should also “seek out information brokers” in the postpandemic medical world, or those interacting with a variety of groups who are often good sources of ideas. Hospitalists, she said, are “natural information brokers,” communicating routinely with a wide variety of specialists and healthcare professionals.
“You’ve got to know what’s important to your organization and to your patients and to everybody else,” Dr. Arora said.
She suggested that hospitalists find “zero-gravity thinkers,” and even to be this type of thinker themselves – one who stays open to new ideas and has diverse interests and experiences.
It is easy to settle into the same ways we’ve always done things, Dr. Arora said.
“The truth is there are ways that it can be better,” she said. “But we sometimes have to seek out new ideas and maintain an open mind – and sometimes we need someone to do it for us.”
Often, those closest to us are the least valuable in this regard, she said, referring to them as “innovation killers.”
“They’re not going to give you the next breakthrough idea,” she said. “You have to get outside of your network to understand where the good ideas are coming from.”
With the trauma that hospitalists have experienced for more than a year, well-being might never have been a more vital topic than it is now, Dr. Arora said.
“We’re done with online wellness modules,” she said. “Fix the system and not the person because we all know the system is not working for us. As hospitalists, we actually are experts at fixing systems.”
Dr. Arora said that one way to think of how to improve hospitalist well-being is by emphasizing “the Four Ts” – teamwork (such as the use of scribes and good communication), time (consider new work schedule models), transitions (refining workflows) and tech (technology that works for clinicians rather than creating a burden).
As hospitalists attempt to move ahead in their post–COVID-19 careers, the key is finding new challenges and never stopping the learning process, Dr. Arora said. Referring to a concept described by career coach May Busch, she said physicians can consider successful careers as a “series of S curves” – at the beginning, there is a lot of work without much advancement, followed by a rapid rise, and then arrival at the destination, which brings you to a new plateau higher up the ladder. At the higher plateau, hospitalists should “jump to a new S curve,” learning a new skill and embarking on a new endeavor, which will lift them even higher.
“Success,” Dr. Arora said, “is defined by continuous growth and learning.”
Dr. Arora reported having no financial disclosures.
FROM SHM Converge 2021
No SPARKLE with ibrutinib plus chemo in r/r pediatric B-NHL
Adding ibrutinib to chemotherapy did not improve outcomes for children and young adults with relapsed or refractory mature B-cell non-Hodgkin lymphoma (B-NHL), an interim analysis of the SPARKLE trial showed.
Among 51 patients aged 1-30 years with mature B-NHL that had been diagnosed before age 18, there was no significant difference in the primary endpoint of event-free survival (EFS) between patients assigned on a 2:1 basis to receive either ibrutinib (Imbruvica) plus one of two chemotherapy regimens or to chemotherapy alone. In fact, EFS was shorter among patients assigned to ibrutinib, although a larger proportion of these patients had previously received rituximab, a known factor for poor prognosis, reported Amos Burke, MD, from Cambridge (England) University.
The trial was stopped for futility in May 2020, after a median follow-up of 17.97 months.
“Further studies are required to determine the optimal therapy for patients with relapsed, mature B-NHL, especially those who have received prior rituximab,” he said in an audio walk-through of a scientific poster presented during the annual meeting of the American Society of Pediatric Hematology/Oncology.
“This is a very challenging patient population because they historically have had a very poor survival rate,” commented Paul J. Galardy, MD, a pediatric hematologist/oncologist at the Mayo Clinic in Rochester, Minn., who was not involved in the study.
“The field has struggled to improve outcomes for these patients in part because there are relatively few patients per year with relapsed refractory mature B-cell lymphoma due to the very effective nature of the up-front therapy. This makes new clinical trials difficult to perform,” he said.
Poor prognosis
Ibrutinib, an inhibitor of Bruton tyrosine kinase, is approved in the United States for treatment of marginal zone lymphoma, mantle cell lymphoma, and chronic lymphocytic leukemia/small lymphocytic lymphoma, as well as other indications, all in adults only. It has also been shown to have activity against B-NHL in preclinical and early human trials, Dr. Burke said.
Given the poor prognosis of children and young adults with relapsed/refractory mature B-NHL – a 2-year overall survival (OS) of 30% or less with chemoimmunotherapy – the investigators tested whether adding ibrutinib to the standard of care could improve outcomes.
They enrolled patients with relapsed/refractory B-NHL in first relapse or primarily refractory to conventional therapy, with measurable disease (greater than 1 cm) by CT, bone marrow involvement, or cerebrospinal fluid with blasts. The patients were required to have Karnofsky-Lansky performance scores of 50 or greater.
The histologies included Burkitt lymphoma, diffuse large B-cell lymphoma (DLBCL), Burkitt-like lymphoma, Burkitt leukemia, primary mediastinal B-cell lymphoma, and other unspecified types.
Dr. Burke reported results on 48 patients included in the May 2020 analysis, plus 3 additional patients who were enrolled between the data cutoff for the first analysis and the meeting of the independent data monitoring committee where the decision was made to stop the trial.
A total of 35 patients were randomized to receive ibrutinib with either the RICE (rituximab plus ifosfamide, carboplatin, and etoposide) or RVICI (rituximab plus vincristine, ifosfamide, carboplatin, idarubicin, and dexamethasone) regimen. All of these patients received treatment on study.
Of the 18 patients randomized to receive either RICE or RVICI alone, 1 did not receive any cycles of chemoimmunotherapy.
At the data cutoff for the updated analysis in November 2020, 14 patients assigned to ibrutinib and 4 assigned to chemoimmunotherapy alone remained on study; no patients in either arm were still receiving therapy.
A total of 17 patients assigned to the combination arm died and 4 withdrew consent. In the chemoimmunotherapy-alone arm, 10 died and 2 withdrew consent.
In both arms, patients were treated until either completing three cycles of therapy, start of conditioning treatment prior to stem cell transplantation, disease progression, or unacceptable toxicity.
In the ibrutinib arm, the median EFS was 5.36 months, compared with 6.97 months with chemoimmunotherapy alone, translating into a hazard ratio for EFS with ibrutinib of 1.078 (nonsignificant).
The respective median overall survival was 13.44 versus 11.07 months,
Subgroup analysis showed that EFS and OS did not differ significantly by age, histology, background regimen, or central nervous system or bone marrow involvement.
Overall response rates were 68.6% in the ibrutinib arm, and 81.3% in the chemoimmunotherapy arm. The respective complete response rates were 8.6% and 18.8%, and partial response rates were 60% and 62.5%.
The overall treatment-emergent adverse event (TEAE) profile was similar between the treatment arms, although six patients in the ibrutinib arm versus one in the chemoimmunotherapy arm experienced a major hemorrhage. One patients in the ibrutinib arm died from pulmonary hemorrhage.
Dr. Burke noted that, although the numbers were small, the failure to see a difference in efficacy between study arms may have been caused in part by a greater number of patients assigned to ibrutinib who had received prior treatment with rituximab (85.7% vs. 56.3%).
Not the right partner?
“The results of this study would suggest that ibrutinib is not the right agent. This is not altogether unexpected,” Dr. Galardy said. “The benefit of ibrutinib in adults with mature B-cell lymphoma is primarily based on biological characteristics of lymphomas that develop in older individuals.”
He noted that mature B-cell lymphoma in older adults is often of the activated B-cell subtype, which frequently has mutations that make it sensitive to ibrutinib. In contrast, children, adolescents, and young adults more commonly have the germinal center B-cell subtype that doesn’t have similarly targetable mutations.
He added that, although the reasons for poor prognosis in patients with prior rituximab exposure are unclear, “it is likely that patients who have recurrent or refractory disease after therapy that included rituximab may have developed resistance to this drug. Since both arms of this study included rituximab as a component of the therapy, the patients with prior exposure to this drug may have had reduced benefit of the additional rituximab, compared with those who had not received the drug before.”
The SPARKLE trial was funded by Janssen Research & Development. Dr. Burke disclosed consultancy fees from Janssen and others. Dr. Galardy is an equity holder in Abbott and AbbVie.
Adding ibrutinib to chemotherapy did not improve outcomes for children and young adults with relapsed or refractory mature B-cell non-Hodgkin lymphoma (B-NHL), an interim analysis of the SPARKLE trial showed.
Among 51 patients aged 1-30 years with mature B-NHL that had been diagnosed before age 18, there was no significant difference in the primary endpoint of event-free survival (EFS) between patients assigned on a 2:1 basis to receive either ibrutinib (Imbruvica) plus one of two chemotherapy regimens or to chemotherapy alone. In fact, EFS was shorter among patients assigned to ibrutinib, although a larger proportion of these patients had previously received rituximab, a known factor for poor prognosis, reported Amos Burke, MD, from Cambridge (England) University.
The trial was stopped for futility in May 2020, after a median follow-up of 17.97 months.
“Further studies are required to determine the optimal therapy for patients with relapsed, mature B-NHL, especially those who have received prior rituximab,” he said in an audio walk-through of a scientific poster presented during the annual meeting of the American Society of Pediatric Hematology/Oncology.
“This is a very challenging patient population because they historically have had a very poor survival rate,” commented Paul J. Galardy, MD, a pediatric hematologist/oncologist at the Mayo Clinic in Rochester, Minn., who was not involved in the study.
“The field has struggled to improve outcomes for these patients in part because there are relatively few patients per year with relapsed refractory mature B-cell lymphoma due to the very effective nature of the up-front therapy. This makes new clinical trials difficult to perform,” he said.
Poor prognosis
Ibrutinib, an inhibitor of Bruton tyrosine kinase, is approved in the United States for treatment of marginal zone lymphoma, mantle cell lymphoma, and chronic lymphocytic leukemia/small lymphocytic lymphoma, as well as other indications, all in adults only. It has also been shown to have activity against B-NHL in preclinical and early human trials, Dr. Burke said.
Given the poor prognosis of children and young adults with relapsed/refractory mature B-NHL – a 2-year overall survival (OS) of 30% or less with chemoimmunotherapy – the investigators tested whether adding ibrutinib to the standard of care could improve outcomes.
They enrolled patients with relapsed/refractory B-NHL in first relapse or primarily refractory to conventional therapy, with measurable disease (greater than 1 cm) by CT, bone marrow involvement, or cerebrospinal fluid with blasts. The patients were required to have Karnofsky-Lansky performance scores of 50 or greater.
The histologies included Burkitt lymphoma, diffuse large B-cell lymphoma (DLBCL), Burkitt-like lymphoma, Burkitt leukemia, primary mediastinal B-cell lymphoma, and other unspecified types.
Dr. Burke reported results on 48 patients included in the May 2020 analysis, plus 3 additional patients who were enrolled between the data cutoff for the first analysis and the meeting of the independent data monitoring committee where the decision was made to stop the trial.
A total of 35 patients were randomized to receive ibrutinib with either the RICE (rituximab plus ifosfamide, carboplatin, and etoposide) or RVICI (rituximab plus vincristine, ifosfamide, carboplatin, idarubicin, and dexamethasone) regimen. All of these patients received treatment on study.
Of the 18 patients randomized to receive either RICE or RVICI alone, 1 did not receive any cycles of chemoimmunotherapy.
At the data cutoff for the updated analysis in November 2020, 14 patients assigned to ibrutinib and 4 assigned to chemoimmunotherapy alone remained on study; no patients in either arm were still receiving therapy.
A total of 17 patients assigned to the combination arm died and 4 withdrew consent. In the chemoimmunotherapy-alone arm, 10 died and 2 withdrew consent.
In both arms, patients were treated until either completing three cycles of therapy, start of conditioning treatment prior to stem cell transplantation, disease progression, or unacceptable toxicity.
In the ibrutinib arm, the median EFS was 5.36 months, compared with 6.97 months with chemoimmunotherapy alone, translating into a hazard ratio for EFS with ibrutinib of 1.078 (nonsignificant).
The respective median overall survival was 13.44 versus 11.07 months,
Subgroup analysis showed that EFS and OS did not differ significantly by age, histology, background regimen, or central nervous system or bone marrow involvement.
Overall response rates were 68.6% in the ibrutinib arm, and 81.3% in the chemoimmunotherapy arm. The respective complete response rates were 8.6% and 18.8%, and partial response rates were 60% and 62.5%.
The overall treatment-emergent adverse event (TEAE) profile was similar between the treatment arms, although six patients in the ibrutinib arm versus one in the chemoimmunotherapy arm experienced a major hemorrhage. One patients in the ibrutinib arm died from pulmonary hemorrhage.
Dr. Burke noted that, although the numbers were small, the failure to see a difference in efficacy between study arms may have been caused in part by a greater number of patients assigned to ibrutinib who had received prior treatment with rituximab (85.7% vs. 56.3%).
Not the right partner?
“The results of this study would suggest that ibrutinib is not the right agent. This is not altogether unexpected,” Dr. Galardy said. “The benefit of ibrutinib in adults with mature B-cell lymphoma is primarily based on biological characteristics of lymphomas that develop in older individuals.”
He noted that mature B-cell lymphoma in older adults is often of the activated B-cell subtype, which frequently has mutations that make it sensitive to ibrutinib. In contrast, children, adolescents, and young adults more commonly have the germinal center B-cell subtype that doesn’t have similarly targetable mutations.
He added that, although the reasons for poor prognosis in patients with prior rituximab exposure are unclear, “it is likely that patients who have recurrent or refractory disease after therapy that included rituximab may have developed resistance to this drug. Since both arms of this study included rituximab as a component of the therapy, the patients with prior exposure to this drug may have had reduced benefit of the additional rituximab, compared with those who had not received the drug before.”
The SPARKLE trial was funded by Janssen Research & Development. Dr. Burke disclosed consultancy fees from Janssen and others. Dr. Galardy is an equity holder in Abbott and AbbVie.
Adding ibrutinib to chemotherapy did not improve outcomes for children and young adults with relapsed or refractory mature B-cell non-Hodgkin lymphoma (B-NHL), an interim analysis of the SPARKLE trial showed.
Among 51 patients aged 1-30 years with mature B-NHL that had been diagnosed before age 18, there was no significant difference in the primary endpoint of event-free survival (EFS) between patients assigned on a 2:1 basis to receive either ibrutinib (Imbruvica) plus one of two chemotherapy regimens or to chemotherapy alone. In fact, EFS was shorter among patients assigned to ibrutinib, although a larger proportion of these patients had previously received rituximab, a known factor for poor prognosis, reported Amos Burke, MD, from Cambridge (England) University.
The trial was stopped for futility in May 2020, after a median follow-up of 17.97 months.
“Further studies are required to determine the optimal therapy for patients with relapsed, mature B-NHL, especially those who have received prior rituximab,” he said in an audio walk-through of a scientific poster presented during the annual meeting of the American Society of Pediatric Hematology/Oncology.
“This is a very challenging patient population because they historically have had a very poor survival rate,” commented Paul J. Galardy, MD, a pediatric hematologist/oncologist at the Mayo Clinic in Rochester, Minn., who was not involved in the study.
“The field has struggled to improve outcomes for these patients in part because there are relatively few patients per year with relapsed refractory mature B-cell lymphoma due to the very effective nature of the up-front therapy. This makes new clinical trials difficult to perform,” he said.
Poor prognosis
Ibrutinib, an inhibitor of Bruton tyrosine kinase, is approved in the United States for treatment of marginal zone lymphoma, mantle cell lymphoma, and chronic lymphocytic leukemia/small lymphocytic lymphoma, as well as other indications, all in adults only. It has also been shown to have activity against B-NHL in preclinical and early human trials, Dr. Burke said.
Given the poor prognosis of children and young adults with relapsed/refractory mature B-NHL – a 2-year overall survival (OS) of 30% or less with chemoimmunotherapy – the investigators tested whether adding ibrutinib to the standard of care could improve outcomes.
They enrolled patients with relapsed/refractory B-NHL in first relapse or primarily refractory to conventional therapy, with measurable disease (greater than 1 cm) by CT, bone marrow involvement, or cerebrospinal fluid with blasts. The patients were required to have Karnofsky-Lansky performance scores of 50 or greater.
The histologies included Burkitt lymphoma, diffuse large B-cell lymphoma (DLBCL), Burkitt-like lymphoma, Burkitt leukemia, primary mediastinal B-cell lymphoma, and other unspecified types.
Dr. Burke reported results on 48 patients included in the May 2020 analysis, plus 3 additional patients who were enrolled between the data cutoff for the first analysis and the meeting of the independent data monitoring committee where the decision was made to stop the trial.
A total of 35 patients were randomized to receive ibrutinib with either the RICE (rituximab plus ifosfamide, carboplatin, and etoposide) or RVICI (rituximab plus vincristine, ifosfamide, carboplatin, idarubicin, and dexamethasone) regimen. All of these patients received treatment on study.
Of the 18 patients randomized to receive either RICE or RVICI alone, 1 did not receive any cycles of chemoimmunotherapy.
At the data cutoff for the updated analysis in November 2020, 14 patients assigned to ibrutinib and 4 assigned to chemoimmunotherapy alone remained on study; no patients in either arm were still receiving therapy.
A total of 17 patients assigned to the combination arm died and 4 withdrew consent. In the chemoimmunotherapy-alone arm, 10 died and 2 withdrew consent.
In both arms, patients were treated until either completing three cycles of therapy, start of conditioning treatment prior to stem cell transplantation, disease progression, or unacceptable toxicity.
In the ibrutinib arm, the median EFS was 5.36 months, compared with 6.97 months with chemoimmunotherapy alone, translating into a hazard ratio for EFS with ibrutinib of 1.078 (nonsignificant).
The respective median overall survival was 13.44 versus 11.07 months,
Subgroup analysis showed that EFS and OS did not differ significantly by age, histology, background regimen, or central nervous system or bone marrow involvement.
Overall response rates were 68.6% in the ibrutinib arm, and 81.3% in the chemoimmunotherapy arm. The respective complete response rates were 8.6% and 18.8%, and partial response rates were 60% and 62.5%.
The overall treatment-emergent adverse event (TEAE) profile was similar between the treatment arms, although six patients in the ibrutinib arm versus one in the chemoimmunotherapy arm experienced a major hemorrhage. One patients in the ibrutinib arm died from pulmonary hemorrhage.
Dr. Burke noted that, although the numbers were small, the failure to see a difference in efficacy between study arms may have been caused in part by a greater number of patients assigned to ibrutinib who had received prior treatment with rituximab (85.7% vs. 56.3%).
Not the right partner?
“The results of this study would suggest that ibrutinib is not the right agent. This is not altogether unexpected,” Dr. Galardy said. “The benefit of ibrutinib in adults with mature B-cell lymphoma is primarily based on biological characteristics of lymphomas that develop in older individuals.”
He noted that mature B-cell lymphoma in older adults is often of the activated B-cell subtype, which frequently has mutations that make it sensitive to ibrutinib. In contrast, children, adolescents, and young adults more commonly have the germinal center B-cell subtype that doesn’t have similarly targetable mutations.
He added that, although the reasons for poor prognosis in patients with prior rituximab exposure are unclear, “it is likely that patients who have recurrent or refractory disease after therapy that included rituximab may have developed resistance to this drug. Since both arms of this study included rituximab as a component of the therapy, the patients with prior exposure to this drug may have had reduced benefit of the additional rituximab, compared with those who had not received the drug before.”
The SPARKLE trial was funded by Janssen Research & Development. Dr. Burke disclosed consultancy fees from Janssen and others. Dr. Galardy is an equity holder in Abbott and AbbVie.
FROM ASPHO 2021
Treatment Delay in Melanoma: A Risk Factor Analysis of an Impending Crisis
Melanoma is the most lethal skin cancer and is the second most common cancer in adolescents and young adults.1 It is the fifth most common cancer in the United States based on incidence, which has steadily risen for the last 2 decades.2,3 For melanoma management, delayed initial diagnosis has been associated with more advanced lesions at presentation and poorer outcomes.4 However, the prognostic implications of delaying melanoma management after diagnosis merits further scrutiny.
This study investigates the associations between melanoma treatment delay (MTD) and patient and tumor characteristics. Although most cases undergo surgical treatment first, more advanced stages may require initiating chemotherapy, radiation therapy, or immunotherapy. In addition, patients who are poor surgical candidates may opt for topical field therapy, such as imiquimod for superficial lesions, prior to more definitive treatment.5 In the Medicaid population, patients who are older than 85 years, married, and previously diagnosed with another melanoma and who also have an increased comorbidity burden have a higher likelihood of MTD.6 For nonmelanoma skin cancers, patient denial is the most common patient-specific factor accounting for treatment delay.7 For this study, our aim was to further evaluate the independent risk factors associated with MTD.
Methods
Case Selection
The National Cancer Database (NCDB) was queried for all cutaneous melanoma cases from 2004 to 2015 (N=525,271). The NCDB is an oncology database sourced from more than 1500 accredited cancer facilities in the United States and Puerto Rico. It receives cases from academic hospitals, Veterans Health Administration hospitals, and community centers.8 Annually, the database collects approximately 70% of cancer diagnoses and 48% of melanoma diagnoses in the United States.9,10 Per institutional guidelines, this analysis was determined to be exempt from institutional review board approval due to the deidentified nature of the dataset.
The selection scheme is illustrated in Table 1. International Statistical Classification of Diseases and Related Health Problems histology codes 8720/3 through 8780/3 combined with the site and morphology primary codes C44.0 through C44.9 identified all patients with a diagnosis of cutaneous melanoma. Primary site was established with the histology codes in the following manner: C44.0 through C44.4 for head/neck primary, C44.5 for trunk primary, C44.6 through C44.7 for extremity primary, and C44.8 through C44.9 for not otherwise specified. Because the NCDB does not specify cause of death, any cases in which the melanoma diagnosis was not the patient’s primary (or first) cancer diagnosis were excluded because of potential ambiguity. Cases lacking histologic confirmation of the diagnosis after primary site biopsy or cases diagnosed from autopsy reports also were excluded. Reports missing staging data or undergoing palliative management were removed. In total, 104,118 cases met the inclusion criteria.

Variables of Interest
The NCDB database codes for a variable “Treatment Started, Days from Dx” are defined as the number of days between the date of diagnosis and the date on which treatment—surgery, radiation, systemic, or other therapy—of the patient began at any facility.11 Treatment delays were classified as more than 45 days or more than 90 days. These thresholds were chosen based on previous studies citing a 45-day recommendation as the timeframe in which primary site excision of melanoma should occur for improved outcomes.1,6,12 Additionally, the postponement cutoffs were aligned with prior studies on surgical delay in melanoma for the Medicaid population.6 Delays of 45 days were labeled as moderate MTD (mMTD), whereas postponements more than 90 days were designated as severe MTD (sMTD).
Patient and tumor characteristics were analyzed for associations with MTD (Table 2). Covariates included age, sex, race (white vs nonwhite), Hispanic ethnicity, insurance status (private; Medicare, Medicaid or other government insurance; and no insurance), median annual income of the patient’s residential zip code (based on 2008-2012 census data), percentage of the population of the patient’s residential zip code without a high school degree (based on 2008-2012 census data), Charlson-Deyo (CD) comorbidity score (a weighted score derived from the sum scores for comorbid conditions), geographic location (rural, urban, and metropolitan), and treatment facility (academic vs nonacademic). Tumor characteristics included primary site (head/neck, trunk, and extremities), stage, and Breslow depth of invasion. Tumor stage was determined using the American Joint Committee on Cancer 6th and 7th editions, depending on the patient’s year of diagnosis.
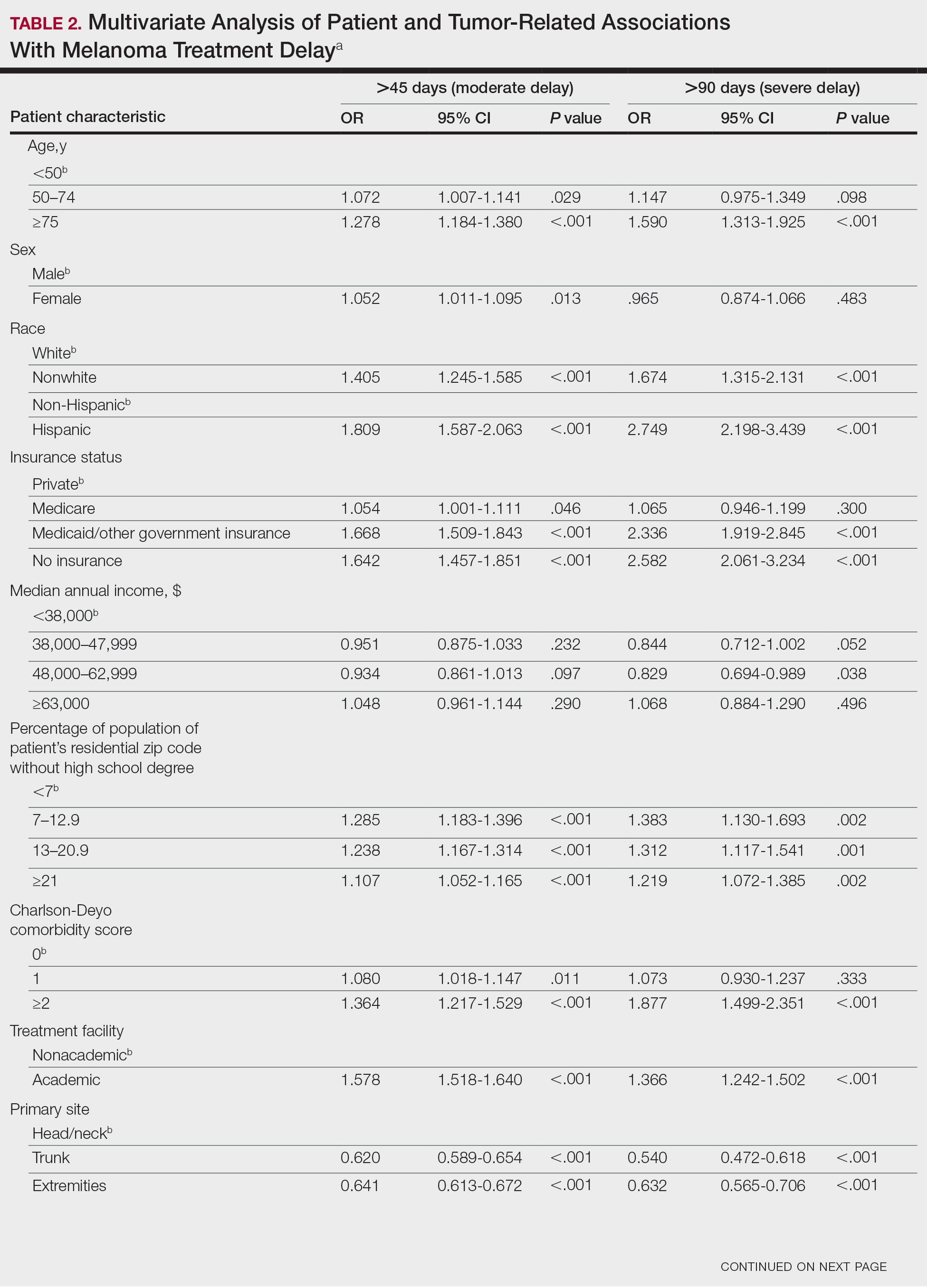

Statistical Methods
χ2 and Fisher exact tests were used to analyze categorical variables involving patient demographics and tumor characteristics by bivariate analysis (Tables 3 and 4). Multivariate analysis determined the relative impact on MTD by including variables that significantly differed on bivariate χ2 analysis (Table 2). Multivariate modeling determined odds ratio (OR) and corresponding 95% CI for the risk-adjusted associations of the variables with MTD. All statistical analyses were performed using SPSS Statistics version 23 (IBM). P<.05 was considered statistically significant, and all statistical tests were 2-tailed. Line graph figures by year of diagnosis were modeled by SPSS using the mean days of delay per year. Independent sample t tests assessed for differences in mean values.
Results
The final study population included 104,118 patients, most of whom were male (56.4%), white (96.6%), and aged 50 to 74 years (54.4%). Most patients were privately insured (52.6%), had no CD comorbidities (87.5%), and lived in metropolitan cities (80.4%)(Table 3). A large majority (95,473 [91.7%]) of patients received surgery as the first means of treatment, with a smaller portion (863 [0.8%]) having unspecified systemic therapy first. The remaining cases were first treated with chemotherapy (1738 [1.7%]), immunotherapy (382 [0.4%]), or radiation (490 [0.5%]), and the rest did not specify treatment sequence. The tumors were most commonly located on the extremities (40.7%), were stage I (41.2%), and had a Breslow depth of less than 1 mm (41.6%).
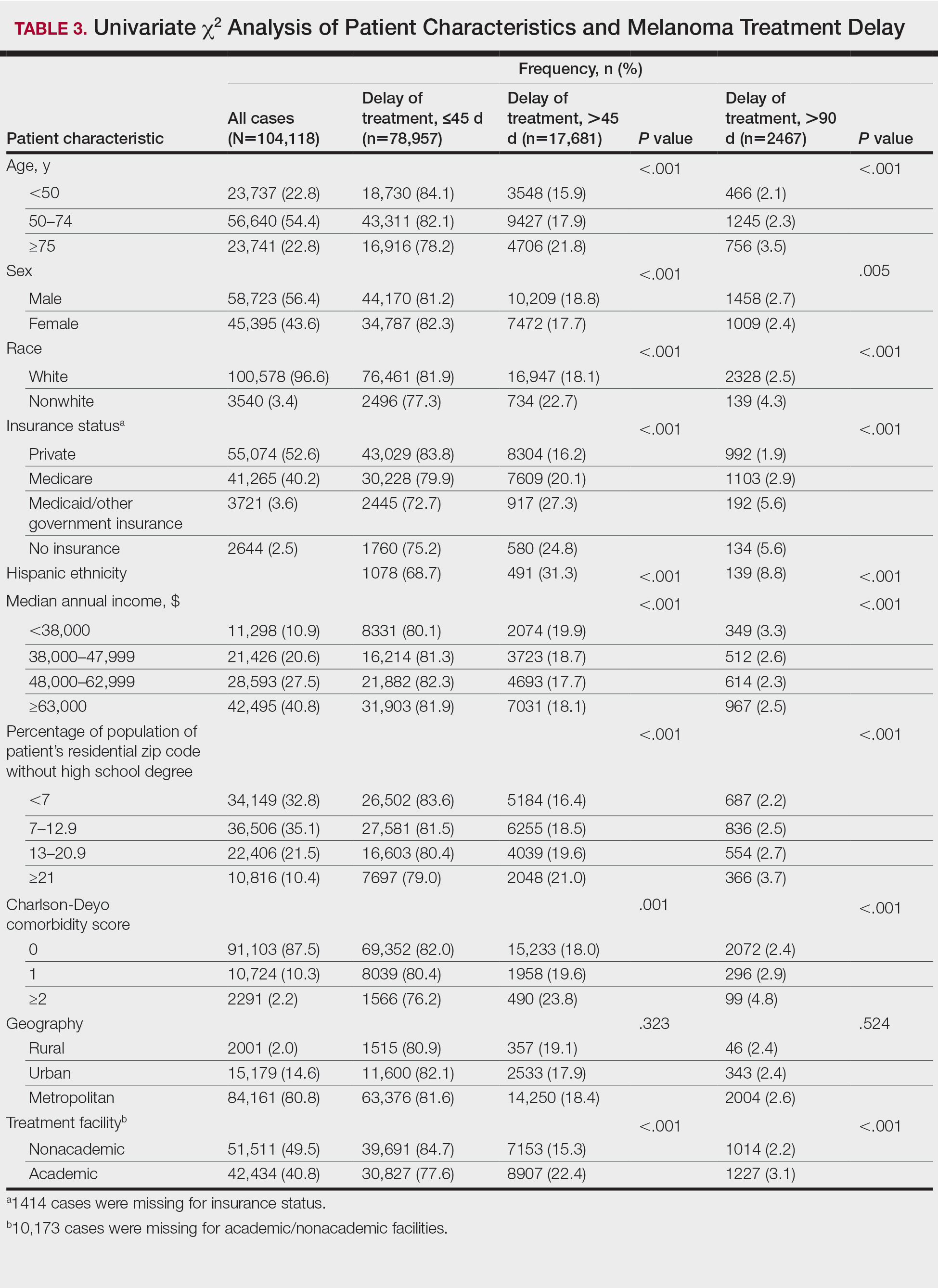
Treatment delay averaged 31.55 days, with a median of 27 days. Overall mean MTD increased significantly from 29.74 days in 2004 to 32.55 days in 2015 (2-tailed t test; P<.001)(Figure). A total of 78,957 cases (75.8%) received treatment within 45 days, whereas 2467 cases (2.5%) were postponed past 90 days. On bivariate analysis, age, sex, race, insurance status, Hispanic ethnicity, median annual income of residential zip code, percentage of the population of the patient’s residential zip code with high school degrees, CD score, and academic treatment facility held significant associations with mMTD and sMTD (P<.05)(Table 3). Analyzing bivariate associations with pertinent tumor characteristics—primary site, stage, and Breslow depth—also held significant associations with mMTD and sMTD (P<.001)(Table 4).
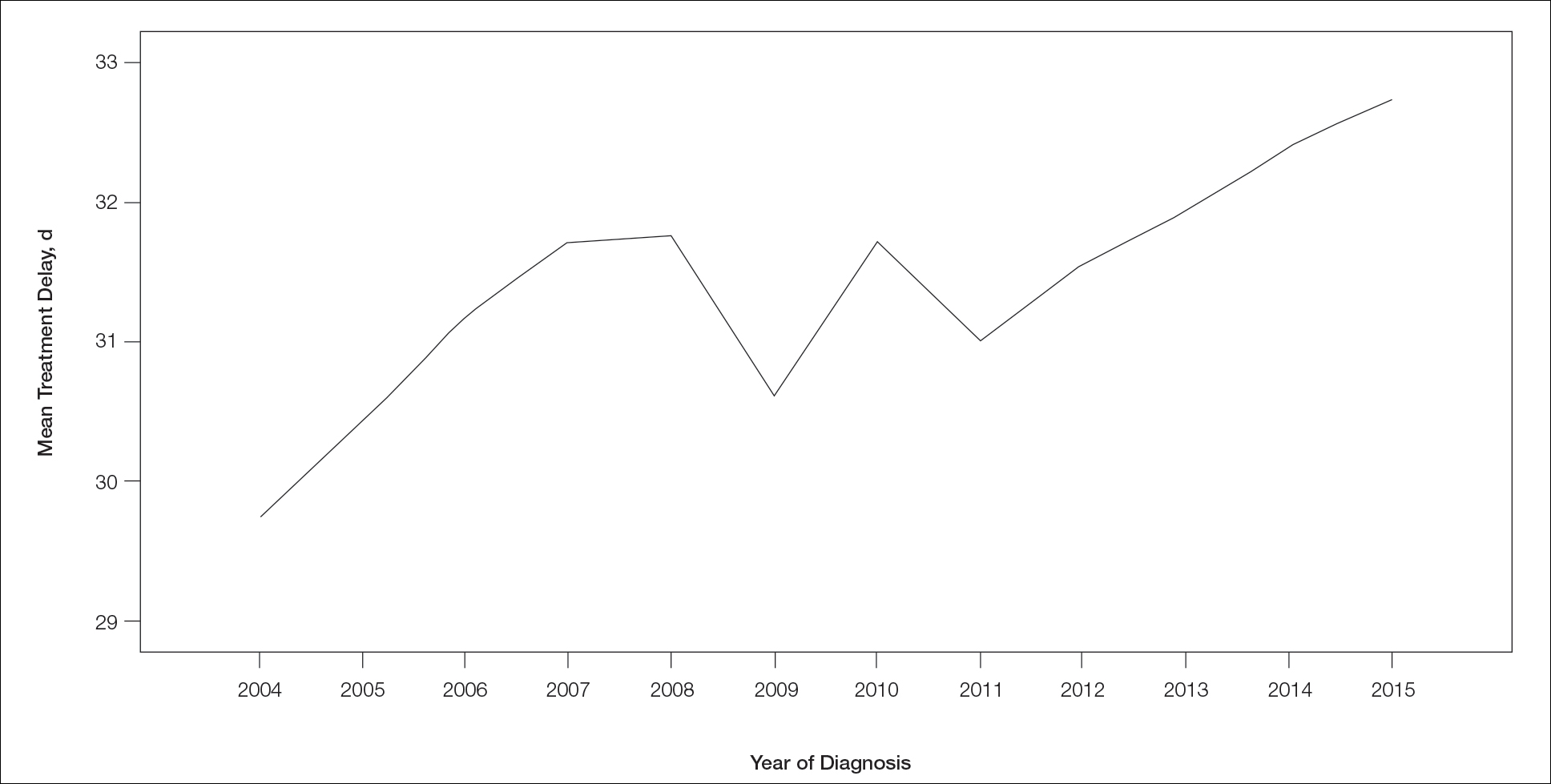
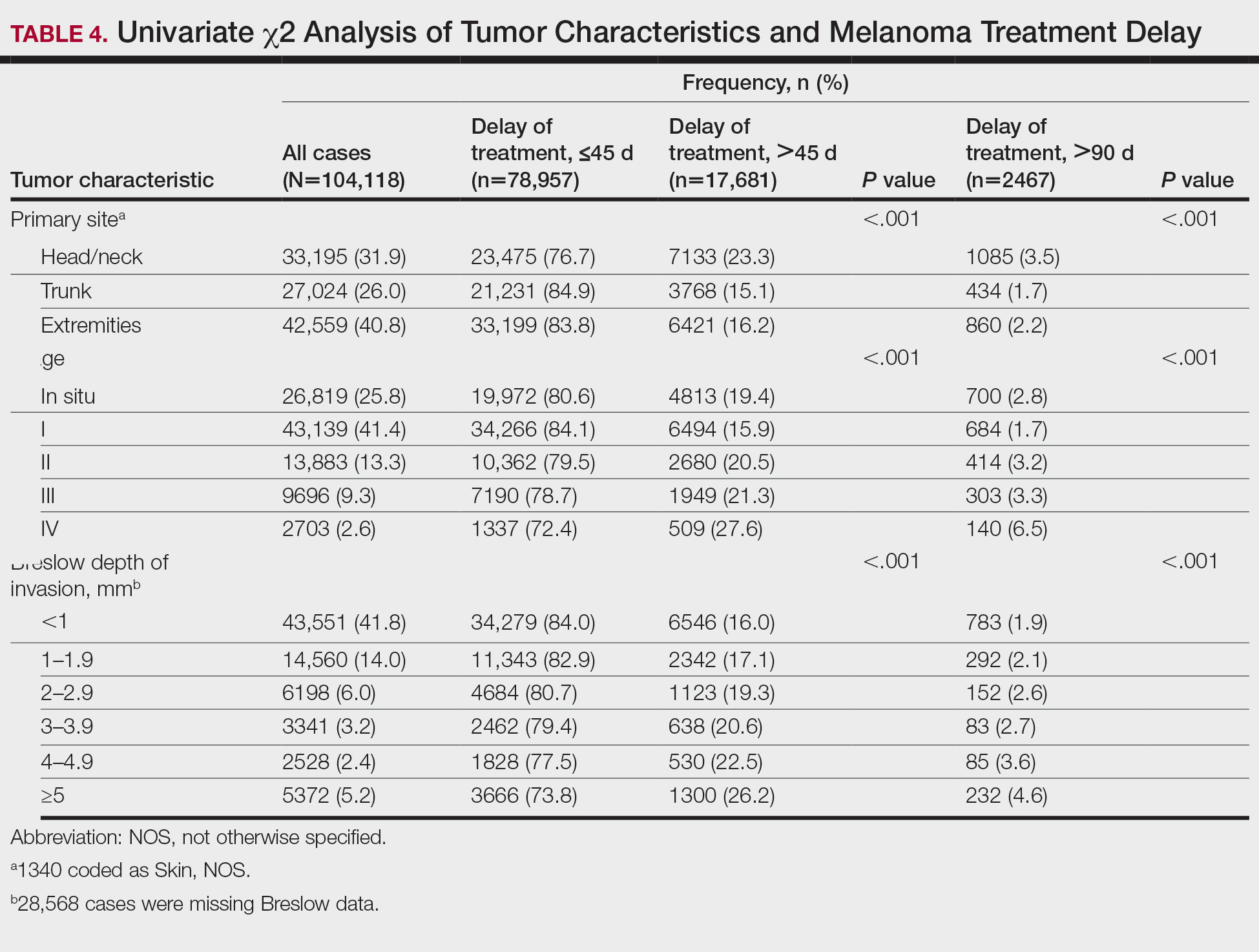
On multivariate analysis, controlling for the variables significant on bivariate analysis, multiple factors showed independent associations with MTD (Table 2). Patients aged 50 to 74 years were more likely to have mMTD (reference: <50 years; P=.029; OR=1.072). Patients 75 years and older showed greater rates of mMTD (reference: <50 years; P<.001; OR=1.278) and sMTD (P<.001; OR=1.590). Women had more mMTD (P=.013; OR=1.052). Nonwhite patients had greater rates of both mMTD (reference: white; P<.001; OR=1.405) and sMTD (P<.001; OR=1.674). Hispanic patients also had greater mMTD (reference: non-Hispanic: P<.001; OR=1.809) and sMTD (P<.001; OR=2.749). Compared to patients with private insurance, those with Medicare were more likely to have mMTD (P=.046; OR=1.054). Patients with no insurance or Medicaid/other government insurance showed more mMTD (no insurance: P<.001, OR=1.642; Medicaid/other: P<.001, OR=1.668) and sMTD (no insurance: P<.001, OR=2.582; Medicaid/other: P<.001, OR=2.336).
With respect to the median annual income of the patient’s residential zip code, patients residing in areas with a median income of $48,000 to $62,999 were less likely to have an sMTD (reference: <$38,000; P=.038; OR=0.829). Compared with patients residing in zip codes where a high percentage of the population had high school degrees, areas with higher nongraduate rates had greater overall rates of MTD (P<.001). Patients with more CD comorbidities also held an association with mMTD (CD1 with reference: CD0; P=.011; OR=1.080)(CD2 with reference: CD0; P<.001; OR=1.364) and sMTD (CD2 with reference: CD0; P<.001; OR=1.877). Academic facilities had greater rates of mMTD (reference: nonacademic facilities; P<.001; OR=1.578) and sMTD (P<.001; OR=1.366). In reference to head/neck primaries, primary sites on the trunk and extremities showed fewer mMTD (trunk: P<.001, OR=0.620; extremities: P<.001, OR=0.641) and sMTD (trunk: P<.001, OR=0.540; extremities: P<.001, OR=0.632). Compared with in situ disease, stage I melanomas were less likely to have treatment delay (mMTD: P<.001, OR=0.902; sMTD: P<.001, OR=0.690), whereas stages II (mMTD: P<.001, OR=1.130), III (mMTD: P<.001, OR=1.196; sMTD: P=.023, OR=1.204), and IV (mMTD: P<.001, OR=1.690; sMTD: P<.001, OR=2.240) were more highly associated with treatments delays.
Comment
The path to successful melanoma management involves 2 timeframes. One is time to diagnosis and the other is time to treatment. With 24.2% of patients receiving treatment later than 45 days after diagnosis, MTD is common and, according to our results, has increased on average from 2004 to 2015. This delay may be partially explained by a shortage of dermatologists, leading to longer wait times and follow-up.13,14 Melanoma treatment delay also varied based on insurance status. Unsurprisingly, those with private insurance showed the lowest rates of MTD. Those with no insurance, Medicare, or Medicaid/other government insurance likely faced greater socioeconomic barriers to health care, such as coverage issues.15 Transportation, low health literacy, and limited work schedule flexibility have been described as additional hurdles to health care that could contribute to this finding.16,17 Similarly, nonwhite patients, Hispanic patients, and those from zip codes with low high school graduation rates had more MTD. Although these findings may be explained by socioeconomic barriers and heightened distrust of the health care system, it also is important to consider physician accessibility.18,19
Considering the 2011 Affordable Care Act along with the 2014 Medicaid expansion, our study holds implications on the impact of these legislations on melanoma treatment. Studies have supported expected rises in Medicaid coverage.20,21 The overall uninsured rate in the United States declined from 16% in 2010 to 9.1% in 2015.22 In our study, the uninsured population showed the highest average MTD rates, though those with Medicaid also had significant MTD. Another treacherous hurdle for patients is the coordination of care among dermatologists, oncologists, general surgeons, plastic surgeons, and Mohs surgeons as a multidisciplinary team. Lott et al6 found that patients who received both biopsy and excision from a dermatologist had the shortest treatment delays, whereas those who had a dermatologist biopsy the site and a different surgeon—including Mohs surgeons—excise it experienced significantly greater MTDs (probablility of MTD >45 days was 31% [95% CI, 24%-37%]. This discordant care and referrals could explain the surprising finding that treatment at an academic facility was independently associated with more MTD, possibly due to the care transitions and referrals that disproportionately affect academic centers and multidisciplinary teams, as mentioned above, regarding the transition of care to other physicians (eg, plastic surgeon). A total of 70.1% of our cases treated at academic facilities reported a prior diagnosis at another facility. These results should not dissuade the pursuit of multidisciplinary treatment teams but should raise caution to untimely referrals.
Age, sex, and race were all associated with more MTD. Patients older than 50 years likely face more complex decisions regarding treatment burden, quality of life, and functional outcomes of more aggressive treatments. High rates of surgical refusal for a number of malignancies have been documented in the elderly population,23-25 which is of particular concern for the high surgery burden of head and neck melanomas,26 as further supported by the findings of more MTD for head and neck primaries. As with elderly patients, patients with higher comorbidity scores and more advanced tumors face similar family–patient care discussions to guide treatment. Additionally, women were more likely to experience MTD, which may be connected to a greater concern for cosmesis27 and necessitate more complex management options, such as Mohs micrographic surgery (a procedure that has gained some support for melanoma excision with the help of immunostaining).28
There are several limitations to this study. Accurate data rely on precise record keeping, reporting, and coding by the contributing institutions. The NCDB case diagnosis is derived from data entry without a centralized review process by experienced dermatopathologists. We could not assess the effects of tumor diameter, as these data were inadequately recorded within the dataset. The NCDB also does not provide details on specific immunotherapy or chemotherapy agents. The NCDB also is a facility-based data source, potentially biasing the melanoma data toward thicker advanced tumors more readily managed at such institutions. Lastly, it is impossible to distinguish between patient-related (ie, difficult decision-making) and health care–related (ie, health care accessibility) delays. Nonetheless, we maintain that minimizing MTD is important for survival outcomes and for limiting the progression of melanomas, regardless of the underlying rationale. We believe that our study expands on conclusions previously limited to a Medicare population.
Conclusion
According to the NCDB, mean MTD has increased significantly from 2004 to 2015. Our results suggest that MTD is relatively common in the United States, thereby increasing the risk for metastases. Higher MTD rates are independently associated with being older than 50 years, female, nonwhite, not privately insured, Hispanic, and treated at an academic facility; having a positive comorbidity history and stage II to IV tumors; and residing in a zip code with a low high school graduation rate. Stage I tumors, primaries not located on the head or neck, and residing in a zip code with a higher median income are associated with lower MTD rates. Policymakers, patients, and dermatologists should better recognize these risk factors to facilitate patient guidance and health equity.
- Huff LS, Chang CA, Thomas JF, et al. Defining an acceptable period of time from melanoma biopsy to excision. Dermatol Reports. 2012;4:E2.
- Matthews NH, Li WQ, Qureshi AA, et al. Epidemiology of Melanoma. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Nelson BR, Hamlet KR, Gillard M, et al. Sebaceous carcinoma. J Am Acad Dermatol. 1995;33:1-15.
- Fan Q, Cohen S, John B, et al. Melanoma in situ treated with topical imiquimod for management of persistently positive margins: a review of treatment methods. Ochsner J. 2015;15:443-447.
- Lott JP, Narayan D, Soulos PR, et al. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151:731-741.
- Renzi C, Mastroeni S, Mannooranparampil TJ, et al. Delay in diagnosis and treatment of squamous cell carcinoma of the skin. Acta Derm Venereol. 2010;90:595-601.
- Winchester DP, Stewart AK, Phillips JL, et al. The National Cancer Database: past, present, and future. Ann Surg Oncol. 2010;17:4-7.
- Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488-490.
- Turkeltaub AE, Pezzi TA, Pezzi CM, et al. Characteristics, treatment, and survival of invasive malignant melanoma (MM) in giant pigmented nevi (GPN) in adults: 976 cases from the National Cancer Data Base (NCDB). J Am Acad Dermatol. 2016;74:1128-1134.
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3:1722-1728.
- Riker AI, Glass F, Perez I, et al. Cutaneous melanoma: methods of biopsy and definitive surgical excision. Dermatol Ther. 2005;18:387-393.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Glazer AM, Farberg AS, Winkelmann RR, et al. Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153:322-325.
- Okoro CA, Zhao G, Dhingra SS, et al. Peer reviewed: lack of health insurance among adults aged 18 to 64 years: findings from the 2013 Behavioral Risk Factor Surveillance System. Prev Chronic Dis. 2015;12:E231.
- Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health. 2013;38:976-993.
- Valerio M, Cabana MD, White DF, et al. Understanding of asthma management: Medicaid parents’ perspectives. Chest. 2006;129:594-601.
- Kaplan CP, Nápoles A, Davis S, et al. Latinos and cancer information: perspectives of patients, health professionals and telephone cancer information specialists. J Health Dispar Res Pract. 2016;9:154-167.
- Armstrong K, Ravenell KL, McMurphy S, et al. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97:1283-1289.
- Moss HA, Havrilesky LJ, Chino J. Insurance coverage among women diagnosed with a gynecologic malignancy before and after implementation of the Affordable Care Act. Gynecol Oncol. 2017;146:457-464.
- Moss HA, Havrilesky LJ, Zafar SY, et al. Trends in insurance status among patients diagnosed with cancer before and after implementation of the Affordable Care Act. J Oncol Pract. 2018;14:E92-E102.
- Obama B. United States health care reform: progress to date and next steps. JAMA. 2016;316:525-532.
- Crippen MM, Brady JS, Mozeika AM, et al. Impact of body mass index on operative outcomes in head and neck free flap surgery. Otolaryngol Head Neck Surg. 2018;159:817-823.
- Verkooijen HM, Fioretta GM, Rapiti E, et al. Patients’ refusal of surgery strongly impairs breast cancer survival. Ann Surg. 2005;242:276-280.
- Wang J, Wang FW. Refusal of cancer-directed surgery strongly impairs survival of patients with localized hepatocellular carcinoma. Int J Surg Oncol. 2010;2010:381795.
- Zito PM, Scharf R. Cancer, melanoma, head and neck. StatPearls. StatPearls Publishing; 2018.
- Al-Dujaili Z, Henry M, Dorizas A, et al. Skin cancer concerns particular to women. Int J Womens Dermatol. 2017;3:S49-S51.
- Etzkorn JR, Jew OS, Shin TM, et al. Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining for atypical intraepidermal melanocytic proliferation. J Am Acad Dermatol. 2018;79:1109-1116.e1
Melanoma is the most lethal skin cancer and is the second most common cancer in adolescents and young adults.1 It is the fifth most common cancer in the United States based on incidence, which has steadily risen for the last 2 decades.2,3 For melanoma management, delayed initial diagnosis has been associated with more advanced lesions at presentation and poorer outcomes.4 However, the prognostic implications of delaying melanoma management after diagnosis merits further scrutiny.
This study investigates the associations between melanoma treatment delay (MTD) and patient and tumor characteristics. Although most cases undergo surgical treatment first, more advanced stages may require initiating chemotherapy, radiation therapy, or immunotherapy. In addition, patients who are poor surgical candidates may opt for topical field therapy, such as imiquimod for superficial lesions, prior to more definitive treatment.5 In the Medicaid population, patients who are older than 85 years, married, and previously diagnosed with another melanoma and who also have an increased comorbidity burden have a higher likelihood of MTD.6 For nonmelanoma skin cancers, patient denial is the most common patient-specific factor accounting for treatment delay.7 For this study, our aim was to further evaluate the independent risk factors associated with MTD.
Methods
Case Selection
The National Cancer Database (NCDB) was queried for all cutaneous melanoma cases from 2004 to 2015 (N=525,271). The NCDB is an oncology database sourced from more than 1500 accredited cancer facilities in the United States and Puerto Rico. It receives cases from academic hospitals, Veterans Health Administration hospitals, and community centers.8 Annually, the database collects approximately 70% of cancer diagnoses and 48% of melanoma diagnoses in the United States.9,10 Per institutional guidelines, this analysis was determined to be exempt from institutional review board approval due to the deidentified nature of the dataset.
The selection scheme is illustrated in Table 1. International Statistical Classification of Diseases and Related Health Problems histology codes 8720/3 through 8780/3 combined with the site and morphology primary codes C44.0 through C44.9 identified all patients with a diagnosis of cutaneous melanoma. Primary site was established with the histology codes in the following manner: C44.0 through C44.4 for head/neck primary, C44.5 for trunk primary, C44.6 through C44.7 for extremity primary, and C44.8 through C44.9 for not otherwise specified. Because the NCDB does not specify cause of death, any cases in which the melanoma diagnosis was not the patient’s primary (or first) cancer diagnosis were excluded because of potential ambiguity. Cases lacking histologic confirmation of the diagnosis after primary site biopsy or cases diagnosed from autopsy reports also were excluded. Reports missing staging data or undergoing palliative management were removed. In total, 104,118 cases met the inclusion criteria.

Variables of Interest
The NCDB database codes for a variable “Treatment Started, Days from Dx” are defined as the number of days between the date of diagnosis and the date on which treatment—surgery, radiation, systemic, or other therapy—of the patient began at any facility.11 Treatment delays were classified as more than 45 days or more than 90 days. These thresholds were chosen based on previous studies citing a 45-day recommendation as the timeframe in which primary site excision of melanoma should occur for improved outcomes.1,6,12 Additionally, the postponement cutoffs were aligned with prior studies on surgical delay in melanoma for the Medicaid population.6 Delays of 45 days were labeled as moderate MTD (mMTD), whereas postponements more than 90 days were designated as severe MTD (sMTD).
Patient and tumor characteristics were analyzed for associations with MTD (Table 2). Covariates included age, sex, race (white vs nonwhite), Hispanic ethnicity, insurance status (private; Medicare, Medicaid or other government insurance; and no insurance), median annual income of the patient’s residential zip code (based on 2008-2012 census data), percentage of the population of the patient’s residential zip code without a high school degree (based on 2008-2012 census data), Charlson-Deyo (CD) comorbidity score (a weighted score derived from the sum scores for comorbid conditions), geographic location (rural, urban, and metropolitan), and treatment facility (academic vs nonacademic). Tumor characteristics included primary site (head/neck, trunk, and extremities), stage, and Breslow depth of invasion. Tumor stage was determined using the American Joint Committee on Cancer 6th and 7th editions, depending on the patient’s year of diagnosis.


Statistical Methods
χ2 and Fisher exact tests were used to analyze categorical variables involving patient demographics and tumor characteristics by bivariate analysis (Tables 3 and 4). Multivariate analysis determined the relative impact on MTD by including variables that significantly differed on bivariate χ2 analysis (Table 2). Multivariate modeling determined odds ratio (OR) and corresponding 95% CI for the risk-adjusted associations of the variables with MTD. All statistical analyses were performed using SPSS Statistics version 23 (IBM). P<.05 was considered statistically significant, and all statistical tests were 2-tailed. Line graph figures by year of diagnosis were modeled by SPSS using the mean days of delay per year. Independent sample t tests assessed for differences in mean values.
Results
The final study population included 104,118 patients, most of whom were male (56.4%), white (96.6%), and aged 50 to 74 years (54.4%). Most patients were privately insured (52.6%), had no CD comorbidities (87.5%), and lived in metropolitan cities (80.4%)(Table 3). A large majority (95,473 [91.7%]) of patients received surgery as the first means of treatment, with a smaller portion (863 [0.8%]) having unspecified systemic therapy first. The remaining cases were first treated with chemotherapy (1738 [1.7%]), immunotherapy (382 [0.4%]), or radiation (490 [0.5%]), and the rest did not specify treatment sequence. The tumors were most commonly located on the extremities (40.7%), were stage I (41.2%), and had a Breslow depth of less than 1 mm (41.6%).

Treatment delay averaged 31.55 days, with a median of 27 days. Overall mean MTD increased significantly from 29.74 days in 2004 to 32.55 days in 2015 (2-tailed t test; P<.001)(Figure). A total of 78,957 cases (75.8%) received treatment within 45 days, whereas 2467 cases (2.5%) were postponed past 90 days. On bivariate analysis, age, sex, race, insurance status, Hispanic ethnicity, median annual income of residential zip code, percentage of the population of the patient’s residential zip code with high school degrees, CD score, and academic treatment facility held significant associations with mMTD and sMTD (P<.05)(Table 3). Analyzing bivariate associations with pertinent tumor characteristics—primary site, stage, and Breslow depth—also held significant associations with mMTD and sMTD (P<.001)(Table 4).


On multivariate analysis, controlling for the variables significant on bivariate analysis, multiple factors showed independent associations with MTD (Table 2). Patients aged 50 to 74 years were more likely to have mMTD (reference: <50 years; P=.029; OR=1.072). Patients 75 years and older showed greater rates of mMTD (reference: <50 years; P<.001; OR=1.278) and sMTD (P<.001; OR=1.590). Women had more mMTD (P=.013; OR=1.052). Nonwhite patients had greater rates of both mMTD (reference: white; P<.001; OR=1.405) and sMTD (P<.001; OR=1.674). Hispanic patients also had greater mMTD (reference: non-Hispanic: P<.001; OR=1.809) and sMTD (P<.001; OR=2.749). Compared to patients with private insurance, those with Medicare were more likely to have mMTD (P=.046; OR=1.054). Patients with no insurance or Medicaid/other government insurance showed more mMTD (no insurance: P<.001, OR=1.642; Medicaid/other: P<.001, OR=1.668) and sMTD (no insurance: P<.001, OR=2.582; Medicaid/other: P<.001, OR=2.336).
With respect to the median annual income of the patient’s residential zip code, patients residing in areas with a median income of $48,000 to $62,999 were less likely to have an sMTD (reference: <$38,000; P=.038; OR=0.829). Compared with patients residing in zip codes where a high percentage of the population had high school degrees, areas with higher nongraduate rates had greater overall rates of MTD (P<.001). Patients with more CD comorbidities also held an association with mMTD (CD1 with reference: CD0; P=.011; OR=1.080)(CD2 with reference: CD0; P<.001; OR=1.364) and sMTD (CD2 with reference: CD0; P<.001; OR=1.877). Academic facilities had greater rates of mMTD (reference: nonacademic facilities; P<.001; OR=1.578) and sMTD (P<.001; OR=1.366). In reference to head/neck primaries, primary sites on the trunk and extremities showed fewer mMTD (trunk: P<.001, OR=0.620; extremities: P<.001, OR=0.641) and sMTD (trunk: P<.001, OR=0.540; extremities: P<.001, OR=0.632). Compared with in situ disease, stage I melanomas were less likely to have treatment delay (mMTD: P<.001, OR=0.902; sMTD: P<.001, OR=0.690), whereas stages II (mMTD: P<.001, OR=1.130), III (mMTD: P<.001, OR=1.196; sMTD: P=.023, OR=1.204), and IV (mMTD: P<.001, OR=1.690; sMTD: P<.001, OR=2.240) were more highly associated with treatments delays.
Comment
The path to successful melanoma management involves 2 timeframes. One is time to diagnosis and the other is time to treatment. With 24.2% of patients receiving treatment later than 45 days after diagnosis, MTD is common and, according to our results, has increased on average from 2004 to 2015. This delay may be partially explained by a shortage of dermatologists, leading to longer wait times and follow-up.13,14 Melanoma treatment delay also varied based on insurance status. Unsurprisingly, those with private insurance showed the lowest rates of MTD. Those with no insurance, Medicare, or Medicaid/other government insurance likely faced greater socioeconomic barriers to health care, such as coverage issues.15 Transportation, low health literacy, and limited work schedule flexibility have been described as additional hurdles to health care that could contribute to this finding.16,17 Similarly, nonwhite patients, Hispanic patients, and those from zip codes with low high school graduation rates had more MTD. Although these findings may be explained by socioeconomic barriers and heightened distrust of the health care system, it also is important to consider physician accessibility.18,19
Considering the 2011 Affordable Care Act along with the 2014 Medicaid expansion, our study holds implications on the impact of these legislations on melanoma treatment. Studies have supported expected rises in Medicaid coverage.20,21 The overall uninsured rate in the United States declined from 16% in 2010 to 9.1% in 2015.22 In our study, the uninsured population showed the highest average MTD rates, though those with Medicaid also had significant MTD. Another treacherous hurdle for patients is the coordination of care among dermatologists, oncologists, general surgeons, plastic surgeons, and Mohs surgeons as a multidisciplinary team. Lott et al6 found that patients who received both biopsy and excision from a dermatologist had the shortest treatment delays, whereas those who had a dermatologist biopsy the site and a different surgeon—including Mohs surgeons—excise it experienced significantly greater MTDs (probablility of MTD >45 days was 31% [95% CI, 24%-37%]. This discordant care and referrals could explain the surprising finding that treatment at an academic facility was independently associated with more MTD, possibly due to the care transitions and referrals that disproportionately affect academic centers and multidisciplinary teams, as mentioned above, regarding the transition of care to other physicians (eg, plastic surgeon). A total of 70.1% of our cases treated at academic facilities reported a prior diagnosis at another facility. These results should not dissuade the pursuit of multidisciplinary treatment teams but should raise caution to untimely referrals.
Age, sex, and race were all associated with more MTD. Patients older than 50 years likely face more complex decisions regarding treatment burden, quality of life, and functional outcomes of more aggressive treatments. High rates of surgical refusal for a number of malignancies have been documented in the elderly population,23-25 which is of particular concern for the high surgery burden of head and neck melanomas,26 as further supported by the findings of more MTD for head and neck primaries. As with elderly patients, patients with higher comorbidity scores and more advanced tumors face similar family–patient care discussions to guide treatment. Additionally, women were more likely to experience MTD, which may be connected to a greater concern for cosmesis27 and necessitate more complex management options, such as Mohs micrographic surgery (a procedure that has gained some support for melanoma excision with the help of immunostaining).28
There are several limitations to this study. Accurate data rely on precise record keeping, reporting, and coding by the contributing institutions. The NCDB case diagnosis is derived from data entry without a centralized review process by experienced dermatopathologists. We could not assess the effects of tumor diameter, as these data were inadequately recorded within the dataset. The NCDB also does not provide details on specific immunotherapy or chemotherapy agents. The NCDB also is a facility-based data source, potentially biasing the melanoma data toward thicker advanced tumors more readily managed at such institutions. Lastly, it is impossible to distinguish between patient-related (ie, difficult decision-making) and health care–related (ie, health care accessibility) delays. Nonetheless, we maintain that minimizing MTD is important for survival outcomes and for limiting the progression of melanomas, regardless of the underlying rationale. We believe that our study expands on conclusions previously limited to a Medicare population.
Conclusion
According to the NCDB, mean MTD has increased significantly from 2004 to 2015. Our results suggest that MTD is relatively common in the United States, thereby increasing the risk for metastases. Higher MTD rates are independently associated with being older than 50 years, female, nonwhite, not privately insured, Hispanic, and treated at an academic facility; having a positive comorbidity history and stage II to IV tumors; and residing in a zip code with a low high school graduation rate. Stage I tumors, primaries not located on the head or neck, and residing in a zip code with a higher median income are associated with lower MTD rates. Policymakers, patients, and dermatologists should better recognize these risk factors to facilitate patient guidance and health equity.
Melanoma is the most lethal skin cancer and is the second most common cancer in adolescents and young adults.1 It is the fifth most common cancer in the United States based on incidence, which has steadily risen for the last 2 decades.2,3 For melanoma management, delayed initial diagnosis has been associated with more advanced lesions at presentation and poorer outcomes.4 However, the prognostic implications of delaying melanoma management after diagnosis merits further scrutiny.
This study investigates the associations between melanoma treatment delay (MTD) and patient and tumor characteristics. Although most cases undergo surgical treatment first, more advanced stages may require initiating chemotherapy, radiation therapy, or immunotherapy. In addition, patients who are poor surgical candidates may opt for topical field therapy, such as imiquimod for superficial lesions, prior to more definitive treatment.5 In the Medicaid population, patients who are older than 85 years, married, and previously diagnosed with another melanoma and who also have an increased comorbidity burden have a higher likelihood of MTD.6 For nonmelanoma skin cancers, patient denial is the most common patient-specific factor accounting for treatment delay.7 For this study, our aim was to further evaluate the independent risk factors associated with MTD.
Methods
Case Selection
The National Cancer Database (NCDB) was queried for all cutaneous melanoma cases from 2004 to 2015 (N=525,271). The NCDB is an oncology database sourced from more than 1500 accredited cancer facilities in the United States and Puerto Rico. It receives cases from academic hospitals, Veterans Health Administration hospitals, and community centers.8 Annually, the database collects approximately 70% of cancer diagnoses and 48% of melanoma diagnoses in the United States.9,10 Per institutional guidelines, this analysis was determined to be exempt from institutional review board approval due to the deidentified nature of the dataset.
The selection scheme is illustrated in Table 1. International Statistical Classification of Diseases and Related Health Problems histology codes 8720/3 through 8780/3 combined with the site and morphology primary codes C44.0 through C44.9 identified all patients with a diagnosis of cutaneous melanoma. Primary site was established with the histology codes in the following manner: C44.0 through C44.4 for head/neck primary, C44.5 for trunk primary, C44.6 through C44.7 for extremity primary, and C44.8 through C44.9 for not otherwise specified. Because the NCDB does not specify cause of death, any cases in which the melanoma diagnosis was not the patient’s primary (or first) cancer diagnosis were excluded because of potential ambiguity. Cases lacking histologic confirmation of the diagnosis after primary site biopsy or cases diagnosed from autopsy reports also were excluded. Reports missing staging data or undergoing palliative management were removed. In total, 104,118 cases met the inclusion criteria.

Variables of Interest
The NCDB database codes for a variable “Treatment Started, Days from Dx” are defined as the number of days between the date of diagnosis and the date on which treatment—surgery, radiation, systemic, or other therapy—of the patient began at any facility.11 Treatment delays were classified as more than 45 days or more than 90 days. These thresholds were chosen based on previous studies citing a 45-day recommendation as the timeframe in which primary site excision of melanoma should occur for improved outcomes.1,6,12 Additionally, the postponement cutoffs were aligned with prior studies on surgical delay in melanoma for the Medicaid population.6 Delays of 45 days were labeled as moderate MTD (mMTD), whereas postponements more than 90 days were designated as severe MTD (sMTD).
Patient and tumor characteristics were analyzed for associations with MTD (Table 2). Covariates included age, sex, race (white vs nonwhite), Hispanic ethnicity, insurance status (private; Medicare, Medicaid or other government insurance; and no insurance), median annual income of the patient’s residential zip code (based on 2008-2012 census data), percentage of the population of the patient’s residential zip code without a high school degree (based on 2008-2012 census data), Charlson-Deyo (CD) comorbidity score (a weighted score derived from the sum scores for comorbid conditions), geographic location (rural, urban, and metropolitan), and treatment facility (academic vs nonacademic). Tumor characteristics included primary site (head/neck, trunk, and extremities), stage, and Breslow depth of invasion. Tumor stage was determined using the American Joint Committee on Cancer 6th and 7th editions, depending on the patient’s year of diagnosis.


Statistical Methods
χ2 and Fisher exact tests were used to analyze categorical variables involving patient demographics and tumor characteristics by bivariate analysis (Tables 3 and 4). Multivariate analysis determined the relative impact on MTD by including variables that significantly differed on bivariate χ2 analysis (Table 2). Multivariate modeling determined odds ratio (OR) and corresponding 95% CI for the risk-adjusted associations of the variables with MTD. All statistical analyses were performed using SPSS Statistics version 23 (IBM). P<.05 was considered statistically significant, and all statistical tests were 2-tailed. Line graph figures by year of diagnosis were modeled by SPSS using the mean days of delay per year. Independent sample t tests assessed for differences in mean values.
Results
The final study population included 104,118 patients, most of whom were male (56.4%), white (96.6%), and aged 50 to 74 years (54.4%). Most patients were privately insured (52.6%), had no CD comorbidities (87.5%), and lived in metropolitan cities (80.4%)(Table 3). A large majority (95,473 [91.7%]) of patients received surgery as the first means of treatment, with a smaller portion (863 [0.8%]) having unspecified systemic therapy first. The remaining cases were first treated with chemotherapy (1738 [1.7%]), immunotherapy (382 [0.4%]), or radiation (490 [0.5%]), and the rest did not specify treatment sequence. The tumors were most commonly located on the extremities (40.7%), were stage I (41.2%), and had a Breslow depth of less than 1 mm (41.6%).

Treatment delay averaged 31.55 days, with a median of 27 days. Overall mean MTD increased significantly from 29.74 days in 2004 to 32.55 days in 2015 (2-tailed t test; P<.001)(Figure). A total of 78,957 cases (75.8%) received treatment within 45 days, whereas 2467 cases (2.5%) were postponed past 90 days. On bivariate analysis, age, sex, race, insurance status, Hispanic ethnicity, median annual income of residential zip code, percentage of the population of the patient’s residential zip code with high school degrees, CD score, and academic treatment facility held significant associations with mMTD and sMTD (P<.05)(Table 3). Analyzing bivariate associations with pertinent tumor characteristics—primary site, stage, and Breslow depth—also held significant associations with mMTD and sMTD (P<.001)(Table 4).


On multivariate analysis, controlling for the variables significant on bivariate analysis, multiple factors showed independent associations with MTD (Table 2). Patients aged 50 to 74 years were more likely to have mMTD (reference: <50 years; P=.029; OR=1.072). Patients 75 years and older showed greater rates of mMTD (reference: <50 years; P<.001; OR=1.278) and sMTD (P<.001; OR=1.590). Women had more mMTD (P=.013; OR=1.052). Nonwhite patients had greater rates of both mMTD (reference: white; P<.001; OR=1.405) and sMTD (P<.001; OR=1.674). Hispanic patients also had greater mMTD (reference: non-Hispanic: P<.001; OR=1.809) and sMTD (P<.001; OR=2.749). Compared to patients with private insurance, those with Medicare were more likely to have mMTD (P=.046; OR=1.054). Patients with no insurance or Medicaid/other government insurance showed more mMTD (no insurance: P<.001, OR=1.642; Medicaid/other: P<.001, OR=1.668) and sMTD (no insurance: P<.001, OR=2.582; Medicaid/other: P<.001, OR=2.336).
With respect to the median annual income of the patient’s residential zip code, patients residing in areas with a median income of $48,000 to $62,999 were less likely to have an sMTD (reference: <$38,000; P=.038; OR=0.829). Compared with patients residing in zip codes where a high percentage of the population had high school degrees, areas with higher nongraduate rates had greater overall rates of MTD (P<.001). Patients with more CD comorbidities also held an association with mMTD (CD1 with reference: CD0; P=.011; OR=1.080)(CD2 with reference: CD0; P<.001; OR=1.364) and sMTD (CD2 with reference: CD0; P<.001; OR=1.877). Academic facilities had greater rates of mMTD (reference: nonacademic facilities; P<.001; OR=1.578) and sMTD (P<.001; OR=1.366). In reference to head/neck primaries, primary sites on the trunk and extremities showed fewer mMTD (trunk: P<.001, OR=0.620; extremities: P<.001, OR=0.641) and sMTD (trunk: P<.001, OR=0.540; extremities: P<.001, OR=0.632). Compared with in situ disease, stage I melanomas were less likely to have treatment delay (mMTD: P<.001, OR=0.902; sMTD: P<.001, OR=0.690), whereas stages II (mMTD: P<.001, OR=1.130), III (mMTD: P<.001, OR=1.196; sMTD: P=.023, OR=1.204), and IV (mMTD: P<.001, OR=1.690; sMTD: P<.001, OR=2.240) were more highly associated with treatments delays.
Comment
The path to successful melanoma management involves 2 timeframes. One is time to diagnosis and the other is time to treatment. With 24.2% of patients receiving treatment later than 45 days after diagnosis, MTD is common and, according to our results, has increased on average from 2004 to 2015. This delay may be partially explained by a shortage of dermatologists, leading to longer wait times and follow-up.13,14 Melanoma treatment delay also varied based on insurance status. Unsurprisingly, those with private insurance showed the lowest rates of MTD. Those with no insurance, Medicare, or Medicaid/other government insurance likely faced greater socioeconomic barriers to health care, such as coverage issues.15 Transportation, low health literacy, and limited work schedule flexibility have been described as additional hurdles to health care that could contribute to this finding.16,17 Similarly, nonwhite patients, Hispanic patients, and those from zip codes with low high school graduation rates had more MTD. Although these findings may be explained by socioeconomic barriers and heightened distrust of the health care system, it also is important to consider physician accessibility.18,19
Considering the 2011 Affordable Care Act along with the 2014 Medicaid expansion, our study holds implications on the impact of these legislations on melanoma treatment. Studies have supported expected rises in Medicaid coverage.20,21 The overall uninsured rate in the United States declined from 16% in 2010 to 9.1% in 2015.22 In our study, the uninsured population showed the highest average MTD rates, though those with Medicaid also had significant MTD. Another treacherous hurdle for patients is the coordination of care among dermatologists, oncologists, general surgeons, plastic surgeons, and Mohs surgeons as a multidisciplinary team. Lott et al6 found that patients who received both biopsy and excision from a dermatologist had the shortest treatment delays, whereas those who had a dermatologist biopsy the site and a different surgeon—including Mohs surgeons—excise it experienced significantly greater MTDs (probablility of MTD >45 days was 31% [95% CI, 24%-37%]. This discordant care and referrals could explain the surprising finding that treatment at an academic facility was independently associated with more MTD, possibly due to the care transitions and referrals that disproportionately affect academic centers and multidisciplinary teams, as mentioned above, regarding the transition of care to other physicians (eg, plastic surgeon). A total of 70.1% of our cases treated at academic facilities reported a prior diagnosis at another facility. These results should not dissuade the pursuit of multidisciplinary treatment teams but should raise caution to untimely referrals.
Age, sex, and race were all associated with more MTD. Patients older than 50 years likely face more complex decisions regarding treatment burden, quality of life, and functional outcomes of more aggressive treatments. High rates of surgical refusal for a number of malignancies have been documented in the elderly population,23-25 which is of particular concern for the high surgery burden of head and neck melanomas,26 as further supported by the findings of more MTD for head and neck primaries. As with elderly patients, patients with higher comorbidity scores and more advanced tumors face similar family–patient care discussions to guide treatment. Additionally, women were more likely to experience MTD, which may be connected to a greater concern for cosmesis27 and necessitate more complex management options, such as Mohs micrographic surgery (a procedure that has gained some support for melanoma excision with the help of immunostaining).28
There are several limitations to this study. Accurate data rely on precise record keeping, reporting, and coding by the contributing institutions. The NCDB case diagnosis is derived from data entry without a centralized review process by experienced dermatopathologists. We could not assess the effects of tumor diameter, as these data were inadequately recorded within the dataset. The NCDB also does not provide details on specific immunotherapy or chemotherapy agents. The NCDB also is a facility-based data source, potentially biasing the melanoma data toward thicker advanced tumors more readily managed at such institutions. Lastly, it is impossible to distinguish between patient-related (ie, difficult decision-making) and health care–related (ie, health care accessibility) delays. Nonetheless, we maintain that minimizing MTD is important for survival outcomes and for limiting the progression of melanomas, regardless of the underlying rationale. We believe that our study expands on conclusions previously limited to a Medicare population.
Conclusion
According to the NCDB, mean MTD has increased significantly from 2004 to 2015. Our results suggest that MTD is relatively common in the United States, thereby increasing the risk for metastases. Higher MTD rates are independently associated with being older than 50 years, female, nonwhite, not privately insured, Hispanic, and treated at an academic facility; having a positive comorbidity history and stage II to IV tumors; and residing in a zip code with a low high school graduation rate. Stage I tumors, primaries not located on the head or neck, and residing in a zip code with a higher median income are associated with lower MTD rates. Policymakers, patients, and dermatologists should better recognize these risk factors to facilitate patient guidance and health equity.
- Huff LS, Chang CA, Thomas JF, et al. Defining an acceptable period of time from melanoma biopsy to excision. Dermatol Reports. 2012;4:E2.
- Matthews NH, Li WQ, Qureshi AA, et al. Epidemiology of Melanoma. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Nelson BR, Hamlet KR, Gillard M, et al. Sebaceous carcinoma. J Am Acad Dermatol. 1995;33:1-15.
- Fan Q, Cohen S, John B, et al. Melanoma in situ treated with topical imiquimod for management of persistently positive margins: a review of treatment methods. Ochsner J. 2015;15:443-447.
- Lott JP, Narayan D, Soulos PR, et al. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151:731-741.
- Renzi C, Mastroeni S, Mannooranparampil TJ, et al. Delay in diagnosis and treatment of squamous cell carcinoma of the skin. Acta Derm Venereol. 2010;90:595-601.
- Winchester DP, Stewart AK, Phillips JL, et al. The National Cancer Database: past, present, and future. Ann Surg Oncol. 2010;17:4-7.
- Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488-490.
- Turkeltaub AE, Pezzi TA, Pezzi CM, et al. Characteristics, treatment, and survival of invasive malignant melanoma (MM) in giant pigmented nevi (GPN) in adults: 976 cases from the National Cancer Data Base (NCDB). J Am Acad Dermatol. 2016;74:1128-1134.
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3:1722-1728.
- Riker AI, Glass F, Perez I, et al. Cutaneous melanoma: methods of biopsy and definitive surgical excision. Dermatol Ther. 2005;18:387-393.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Glazer AM, Farberg AS, Winkelmann RR, et al. Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153:322-325.
- Okoro CA, Zhao G, Dhingra SS, et al. Peer reviewed: lack of health insurance among adults aged 18 to 64 years: findings from the 2013 Behavioral Risk Factor Surveillance System. Prev Chronic Dis. 2015;12:E231.
- Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health. 2013;38:976-993.
- Valerio M, Cabana MD, White DF, et al. Understanding of asthma management: Medicaid parents’ perspectives. Chest. 2006;129:594-601.
- Kaplan CP, Nápoles A, Davis S, et al. Latinos and cancer information: perspectives of patients, health professionals and telephone cancer information specialists. J Health Dispar Res Pract. 2016;9:154-167.
- Armstrong K, Ravenell KL, McMurphy S, et al. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97:1283-1289.
- Moss HA, Havrilesky LJ, Chino J. Insurance coverage among women diagnosed with a gynecologic malignancy before and after implementation of the Affordable Care Act. Gynecol Oncol. 2017;146:457-464.
- Moss HA, Havrilesky LJ, Zafar SY, et al. Trends in insurance status among patients diagnosed with cancer before and after implementation of the Affordable Care Act. J Oncol Pract. 2018;14:E92-E102.
- Obama B. United States health care reform: progress to date and next steps. JAMA. 2016;316:525-532.
- Crippen MM, Brady JS, Mozeika AM, et al. Impact of body mass index on operative outcomes in head and neck free flap surgery. Otolaryngol Head Neck Surg. 2018;159:817-823.
- Verkooijen HM, Fioretta GM, Rapiti E, et al. Patients’ refusal of surgery strongly impairs breast cancer survival. Ann Surg. 2005;242:276-280.
- Wang J, Wang FW. Refusal of cancer-directed surgery strongly impairs survival of patients with localized hepatocellular carcinoma. Int J Surg Oncol. 2010;2010:381795.
- Zito PM, Scharf R. Cancer, melanoma, head and neck. StatPearls. StatPearls Publishing; 2018.
- Al-Dujaili Z, Henry M, Dorizas A, et al. Skin cancer concerns particular to women. Int J Womens Dermatol. 2017;3:S49-S51.
- Etzkorn JR, Jew OS, Shin TM, et al. Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining for atypical intraepidermal melanocytic proliferation. J Am Acad Dermatol. 2018;79:1109-1116.e1
- Huff LS, Chang CA, Thomas JF, et al. Defining an acceptable period of time from melanoma biopsy to excision. Dermatol Reports. 2012;4:E2.
- Matthews NH, Li WQ, Qureshi AA, et al. Epidemiology of Melanoma. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Nelson BR, Hamlet KR, Gillard M, et al. Sebaceous carcinoma. J Am Acad Dermatol. 1995;33:1-15.
- Fan Q, Cohen S, John B, et al. Melanoma in situ treated with topical imiquimod for management of persistently positive margins: a review of treatment methods. Ochsner J. 2015;15:443-447.
- Lott JP, Narayan D, Soulos PR, et al. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151:731-741.
- Renzi C, Mastroeni S, Mannooranparampil TJ, et al. Delay in diagnosis and treatment of squamous cell carcinoma of the skin. Acta Derm Venereol. 2010;90:595-601.
- Winchester DP, Stewart AK, Phillips JL, et al. The National Cancer Database: past, present, and future. Ann Surg Oncol. 2010;17:4-7.
- Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488-490.
- Turkeltaub AE, Pezzi TA, Pezzi CM, et al. Characteristics, treatment, and survival of invasive malignant melanoma (MM) in giant pigmented nevi (GPN) in adults: 976 cases from the National Cancer Data Base (NCDB). J Am Acad Dermatol. 2016;74:1128-1134.
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3:1722-1728.
- Riker AI, Glass F, Perez I, et al. Cutaneous melanoma: methods of biopsy and definitive surgical excision. Dermatol Ther. 2005;18:387-393.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Glazer AM, Farberg AS, Winkelmann RR, et al. Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153:322-325.
- Okoro CA, Zhao G, Dhingra SS, et al. Peer reviewed: lack of health insurance among adults aged 18 to 64 years: findings from the 2013 Behavioral Risk Factor Surveillance System. Prev Chronic Dis. 2015;12:E231.
- Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health. 2013;38:976-993.
- Valerio M, Cabana MD, White DF, et al. Understanding of asthma management: Medicaid parents’ perspectives. Chest. 2006;129:594-601.
- Kaplan CP, Nápoles A, Davis S, et al. Latinos and cancer information: perspectives of patients, health professionals and telephone cancer information specialists. J Health Dispar Res Pract. 2016;9:154-167.
- Armstrong K, Ravenell KL, McMurphy S, et al. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97:1283-1289.
- Moss HA, Havrilesky LJ, Chino J. Insurance coverage among women diagnosed with a gynecologic malignancy before and after implementation of the Affordable Care Act. Gynecol Oncol. 2017;146:457-464.
- Moss HA, Havrilesky LJ, Zafar SY, et al. Trends in insurance status among patients diagnosed with cancer before and after implementation of the Affordable Care Act. J Oncol Pract. 2018;14:E92-E102.
- Obama B. United States health care reform: progress to date and next steps. JAMA. 2016;316:525-532.
- Crippen MM, Brady JS, Mozeika AM, et al. Impact of body mass index on operative outcomes in head and neck free flap surgery. Otolaryngol Head Neck Surg. 2018;159:817-823.
- Verkooijen HM, Fioretta GM, Rapiti E, et al. Patients’ refusal of surgery strongly impairs breast cancer survival. Ann Surg. 2005;242:276-280.
- Wang J, Wang FW. Refusal of cancer-directed surgery strongly impairs survival of patients with localized hepatocellular carcinoma. Int J Surg Oncol. 2010;2010:381795.
- Zito PM, Scharf R. Cancer, melanoma, head and neck. StatPearls. StatPearls Publishing; 2018.
- Al-Dujaili Z, Henry M, Dorizas A, et al. Skin cancer concerns particular to women. Int J Womens Dermatol. 2017;3:S49-S51.
- Etzkorn JR, Jew OS, Shin TM, et al. Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining for atypical intraepidermal melanocytic proliferation. J Am Acad Dermatol. 2018;79:1109-1116.e1
Practice Points
- Melanoma treatment delays (MTDs) have been linked to poor outcomes.
- Based on the National Cancer Database, the mean MTD has increased significantly from 2004 to 2015 (P11<.001).
- More delays are seen in patients who are older than 50 years, female, nonwhite, not privately insured, and treated at an academic facility and who have more advanced tumor stage and head/neck primaries.
Clinical Edge Journal Scan Commentary: CML May 2021
One of the most important goals in the treatment of patients with CP-CML is to avoid the progression to advanced phases, such as accelerated and blast phase, where the treatments are limited and the outcomes inferior. The long term outcomes of patients with lymphoid blast crisis treated with HyperCVAD plus dasatinib was recently reported by Morita et al. The authors reviewed 85 patients (23 with CML- LBP and 62 with newly diagnosed Ph- positive ALL) who received hyper- CVAD plus dasatinib. In the CML- LBP cohort, 19 had prior chronic myeloid leukemia as chronic phase (n = 17; 74%), accelerated phase (n = 1; 4%), or myeloid blastic phase (n = 1; 4%); 4 (17%) presented with de novo CML- LBP. Patients with CML- LBP were less likely to achieve deep molecular remission than patients with Ph- positive ALL. The major molecular response (MMR) rates were 70% and 95%, respectively (P = .007), and the complete molecular response (CMR) rates were 55% and 74%, respectively (P = .16). However, the survival outcomes were similar for CML- LBP and Ph- positive ALL: The 5- year overall survival (OS) rates were 59% and 48%, respectively (P = .97). Allogeneic stem cell transplantation was associated with a better outcome in CML- LBP (5- year OS rate, 88% vs 57%; P = .04), while in Ph- positive ALL, the outcome was driven by deeper molecular remission: the 5- year OS rates were 63% and 25% with CMR and MMR, respectively (P = .002). Although the outcome of CML- LBP has improved with hyper- CVAD plus dasatinib therapy with survival comparable to that of Ph- positive ALL, data with third generation TKI may even improve these outcomes in the near future.
Allogeneic BMT is the ultimate therapy for resistant or intolerant to TKI patients with CP-CML as well as for advances phases of this disease. Since the introduction of TKI the rates of allo BMT had overall decreased, so Yassine and colleagues performed a systematic review/meta-analysis of the available literature to assess the evidence regarding allo-HCT efficacy in CP-CML patients. Data from eligible studies were extracted in relation to benefits (overall survival [OS], progression-free survival, disease-free survival [DFS], complete remission [CR], and molecular response [MR]) and harms (nonrelapse mortality [NRM], relapse, and acute and chronic graft-versus-host disease) and stratified by age into adult and pediatric groups. Overall for adult allo-HCT recipients, the pooled OS, DFS, CR and, MR were 84%, 66%, 56%, and 88%, respectively. Pooled NRM and relapse were 20% and 19%, respectively. As a conclusion, these results suggest that allo-HCT still is an effective treatment for TKI-resistant or TKI-intolerant CP-CML and the risk-befit ratio is favorable based on the lack of other alternatives.
One of the most important goals in the treatment of patients with CP-CML is to avoid the progression to advanced phases, such as accelerated and blast phase, where the treatments are limited and the outcomes inferior. The long term outcomes of patients with lymphoid blast crisis treated with HyperCVAD plus dasatinib was recently reported by Morita et al. The authors reviewed 85 patients (23 with CML- LBP and 62 with newly diagnosed Ph- positive ALL) who received hyper- CVAD plus dasatinib. In the CML- LBP cohort, 19 had prior chronic myeloid leukemia as chronic phase (n = 17; 74%), accelerated phase (n = 1; 4%), or myeloid blastic phase (n = 1; 4%); 4 (17%) presented with de novo CML- LBP. Patients with CML- LBP were less likely to achieve deep molecular remission than patients with Ph- positive ALL. The major molecular response (MMR) rates were 70% and 95%, respectively (P = .007), and the complete molecular response (CMR) rates were 55% and 74%, respectively (P = .16). However, the survival outcomes were similar for CML- LBP and Ph- positive ALL: The 5- year overall survival (OS) rates were 59% and 48%, respectively (P = .97). Allogeneic stem cell transplantation was associated with a better outcome in CML- LBP (5- year OS rate, 88% vs 57%; P = .04), while in Ph- positive ALL, the outcome was driven by deeper molecular remission: the 5- year OS rates were 63% and 25% with CMR and MMR, respectively (P = .002). Although the outcome of CML- LBP has improved with hyper- CVAD plus dasatinib therapy with survival comparable to that of Ph- positive ALL, data with third generation TKI may even improve these outcomes in the near future.
Allogeneic BMT is the ultimate therapy for resistant or intolerant to TKI patients with CP-CML as well as for advances phases of this disease. Since the introduction of TKI the rates of allo BMT had overall decreased, so Yassine and colleagues performed a systematic review/meta-analysis of the available literature to assess the evidence regarding allo-HCT efficacy in CP-CML patients. Data from eligible studies were extracted in relation to benefits (overall survival [OS], progression-free survival, disease-free survival [DFS], complete remission [CR], and molecular response [MR]) and harms (nonrelapse mortality [NRM], relapse, and acute and chronic graft-versus-host disease) and stratified by age into adult and pediatric groups. Overall for adult allo-HCT recipients, the pooled OS, DFS, CR and, MR were 84%, 66%, 56%, and 88%, respectively. Pooled NRM and relapse were 20% and 19%, respectively. As a conclusion, these results suggest that allo-HCT still is an effective treatment for TKI-resistant or TKI-intolerant CP-CML and the risk-befit ratio is favorable based on the lack of other alternatives.
One of the most important goals in the treatment of patients with CP-CML is to avoid the progression to advanced phases, such as accelerated and blast phase, where the treatments are limited and the outcomes inferior. The long term outcomes of patients with lymphoid blast crisis treated with HyperCVAD plus dasatinib was recently reported by Morita et al. The authors reviewed 85 patients (23 with CML- LBP and 62 with newly diagnosed Ph- positive ALL) who received hyper- CVAD plus dasatinib. In the CML- LBP cohort, 19 had prior chronic myeloid leukemia as chronic phase (n = 17; 74%), accelerated phase (n = 1; 4%), or myeloid blastic phase (n = 1; 4%); 4 (17%) presented with de novo CML- LBP. Patients with CML- LBP were less likely to achieve deep molecular remission than patients with Ph- positive ALL. The major molecular response (MMR) rates were 70% and 95%, respectively (P = .007), and the complete molecular response (CMR) rates were 55% and 74%, respectively (P = .16). However, the survival outcomes were similar for CML- LBP and Ph- positive ALL: The 5- year overall survival (OS) rates were 59% and 48%, respectively (P = .97). Allogeneic stem cell transplantation was associated with a better outcome in CML- LBP (5- year OS rate, 88% vs 57%; P = .04), while in Ph- positive ALL, the outcome was driven by deeper molecular remission: the 5- year OS rates were 63% and 25% with CMR and MMR, respectively (P = .002). Although the outcome of CML- LBP has improved with hyper- CVAD plus dasatinib therapy with survival comparable to that of Ph- positive ALL, data with third generation TKI may even improve these outcomes in the near future.
Allogeneic BMT is the ultimate therapy for resistant or intolerant to TKI patients with CP-CML as well as for advances phases of this disease. Since the introduction of TKI the rates of allo BMT had overall decreased, so Yassine and colleagues performed a systematic review/meta-analysis of the available literature to assess the evidence regarding allo-HCT efficacy in CP-CML patients. Data from eligible studies were extracted in relation to benefits (overall survival [OS], progression-free survival, disease-free survival [DFS], complete remission [CR], and molecular response [MR]) and harms (nonrelapse mortality [NRM], relapse, and acute and chronic graft-versus-host disease) and stratified by age into adult and pediatric groups. Overall for adult allo-HCT recipients, the pooled OS, DFS, CR and, MR were 84%, 66%, 56%, and 88%, respectively. Pooled NRM and relapse were 20% and 19%, respectively. As a conclusion, these results suggest that allo-HCT still is an effective treatment for TKI-resistant or TKI-intolerant CP-CML and the risk-befit ratio is favorable based on the lack of other alternatives.













