User login
Assessing perinatal anxiety: What to ask
Emerging data demonstrate that untreated perinatal anxiety is associated with negative outcomes, including an increased risk for suicide.1 A 2017 systematic review and meta-analysis that included 102 studies with a total of 221,974 women from 34 countries found that the prevalence of self-reported anxiety symptoms and any anxiety disorder was 22.9% and 15.2%, respectively, across the 3 trimesters.1 During pregnancy, anxiety disorders (eg, generalized anxiety disorder) and anxiety-related disorders (eg, obsessive-compulsive disorder [OCD] and posttraumatic stress disorder [PTSD]) can present as new illnesses or as a reoccurrence of an existing illness. Patients with pre-existing OCD may notice that the nature of their obsessions is changing. Women with pre-existing PTSD may have their symptoms triggered by pregnancy or delivery or may develop PTSD as a result of a traumatic delivery. Anxiety is frequently comorbid with depression, and high anxiety during pregnancy is one of the strongest risk factors for depression.1,2
In light of this data, awareness and recognition of perinatal anxiety is critical. In this article, we describe how to accurately assess perinatal anxiety by avoiding assumptions and asking key questions during the clinical interview.
Avoid these common assumptions
Assessment begins with avoiding assumptions typically associated with maternal mental health. One common assumption is that pregnancy is a joyous occasion for all women. Pregnancy can be a stressful time that has its own unique difficulties, including the potential to develop or have a relapse of a mental illness. Another assumption is that the only concern is “postpartum depression.” In actuality, a significant percentage of women will experience depression during their pregnancy (not just in the postpartum period), and many other psychiatric illnesses are common during the perinatal period, including anxiety disorders.
Conduct a focused interview
Risk factors associated with antenatal anxiety include2:
- previous history of mental illness (particularly a history of anxiety and depression and a history of psychiatric treatment)
- lack of partner or social support
- history of abuse or domestic violence
- unplanned or unwanted pregnancy
- adverse events in life and high perceived stress
- present/past pregnancy complications
- pregnancy loss.
Symptoms of anxiety. The presence of anxiety or worrying does not necessarily mean a mother has an anxiety disorder. Using the DSM-5 as a guide, we should use the questions outlined in the following sections to inquire about all of the symptoms related to a particular illness, the pervasiveness of these symptoms, and to what extent these symptoms impair a woman’s ability to function and carry out her usual activities.3
Past psychiatric history. Ask your patient the following: Have you previously experienced anxiety and/or depressive symptoms? Were those symptoms limited only to times when you were pregnant or postpartum? Were your symptoms severe enough to disrupt your life (job, school, relationships, ability to complete daily tasks)? What treatments were effective for your symptoms? What treatments were ineffective?3
Social factors. Learn more about your patient’s support systems by asking: Who do you consider to be part of your social support? How is your relationship with your social support? Are there challenges in your relationship with your friends, family, or partner? If yes, what are those challenges? Are there other children in the home, and do you have support for them? Is your home environment safe? Do you feel that you have what you need for the baby? What stressors are you currently experiencing? Do you attend support groups for expectant mothers? Are you engaged in perinatal care?3
Continue to: Given the high prevalence...
Given the high prevalence of interpersonal violence in women of reproductive age, all patients should be screened for this. The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women recommends screening for interpersonal violence at the first visit during the perinatal period, during each trimester, and at the postpartum visit (at minimum).4 Potential screening questions include (but are not limited to): Have you and/or your children ever been threatened by or felt afraid of your partner? When you argue with your partner, do either of you get physical? Has your partner ever physically hurt you (eg, hit, choked)? Do you feel safe at home? Do you have a safe place to go with resources you and your children will need in case of an emergency?4-6
Feelings toward pregnancy, past/current pregnancy complications, and pregnancy loss. Ask your patient: Was this pregnancy planned? How do you feel about your pregnancy? How do you see yourself as a mother? Do you currently have pregnancy complications and/or have had them in the past, and, if so, what are/were they? Have you lost a pregnancy? If so, what was that like? Do you have fears related to childbirth, and, if so, what are they?3
Intrusive thoughts about harming the baby. Intrusive thoughts are common in postpartum women with anxiety disorders, including OCD.7 Merely asking patients if they’ve had thoughts of harming their baby is incomplete and insufficient to assess for intrusive thoughts. This question does not distinguish between intrusive thoughts and homicidal ideation; this distinction is absolutely necessary given the difference in potential risk to the infant.
Intrusive thoughts are generally associated with a low risk of mothers acting on their thoughts. These thoughts are typically ego dystonic and, in the most severe form, can be distressing to an extent that they cause behavioral changes, such as avoiding bathing the infant, avoiding diaper changes, avoiding knives, or separating themselves from the infant.7 On the contrary, having homicidal ideation carries a higher risk for harm to the infant. Homicidal ideation may be seen in patients with co-occurring psychosis, poor reality testing, and delusions.5,7
Questions such as “Do you worry about harm coming to your baby?” “Do you worry about you causing harm to your baby?” and “Have you had an upsetting thought about harming your baby?” are more likely to reveal intrusive thoughts and prompt further exploration. Statements such as “Some people tell me that they have distressing thoughts about harm coming to their baby” can gently open the door to a having a dialogue about such thoughts. This dialogue is significantly important in making informed assessments as we develop comprehensive treatment plans.
1. Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. B J Psychiatry. 2017;210(5):315-323.
2. Biaggi A, Conroy S, Pawlby S, et al. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord. 2016;191:62-77.
3. Kirby N, Kilsby A, Walker R. Assessing low mood during pregnancy. BMJ. 2019;366:I4584. doi: 10.1136/bmj.I4584
4. American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee opinion: Intimate partner violence. Number 518. February 2012. Accessed March 23, 2020. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2012/02/intimate-partner-violence
5. Massachusetts Child Psychiatry Access Program for Moms Provider Toolkit. Accessed March 18, 2020. https://www.mcpapformoms.org/Docs/AdultProviderToolkit12.09.2019.pdf
6. Ashur ML. Asking about domestic violence: SAFE questions. JAMA. 1993;269(18):2367.
7. Brandes M, Soares CN, Cohen LS. Postpartum onset obsessive-compulsive disorder: diagnosis and management. Arch Womens Ment Health. 2004;7(2):99-110.
Emerging data demonstrate that untreated perinatal anxiety is associated with negative outcomes, including an increased risk for suicide.1 A 2017 systematic review and meta-analysis that included 102 studies with a total of 221,974 women from 34 countries found that the prevalence of self-reported anxiety symptoms and any anxiety disorder was 22.9% and 15.2%, respectively, across the 3 trimesters.1 During pregnancy, anxiety disorders (eg, generalized anxiety disorder) and anxiety-related disorders (eg, obsessive-compulsive disorder [OCD] and posttraumatic stress disorder [PTSD]) can present as new illnesses or as a reoccurrence of an existing illness. Patients with pre-existing OCD may notice that the nature of their obsessions is changing. Women with pre-existing PTSD may have their symptoms triggered by pregnancy or delivery or may develop PTSD as a result of a traumatic delivery. Anxiety is frequently comorbid with depression, and high anxiety during pregnancy is one of the strongest risk factors for depression.1,2
In light of this data, awareness and recognition of perinatal anxiety is critical. In this article, we describe how to accurately assess perinatal anxiety by avoiding assumptions and asking key questions during the clinical interview.
Avoid these common assumptions
Assessment begins with avoiding assumptions typically associated with maternal mental health. One common assumption is that pregnancy is a joyous occasion for all women. Pregnancy can be a stressful time that has its own unique difficulties, including the potential to develop or have a relapse of a mental illness. Another assumption is that the only concern is “postpartum depression.” In actuality, a significant percentage of women will experience depression during their pregnancy (not just in the postpartum period), and many other psychiatric illnesses are common during the perinatal period, including anxiety disorders.
Conduct a focused interview
Risk factors associated with antenatal anxiety include2:
- previous history of mental illness (particularly a history of anxiety and depression and a history of psychiatric treatment)
- lack of partner or social support
- history of abuse or domestic violence
- unplanned or unwanted pregnancy
- adverse events in life and high perceived stress
- present/past pregnancy complications
- pregnancy loss.
Symptoms of anxiety. The presence of anxiety or worrying does not necessarily mean a mother has an anxiety disorder. Using the DSM-5 as a guide, we should use the questions outlined in the following sections to inquire about all of the symptoms related to a particular illness, the pervasiveness of these symptoms, and to what extent these symptoms impair a woman’s ability to function and carry out her usual activities.3
Past psychiatric history. Ask your patient the following: Have you previously experienced anxiety and/or depressive symptoms? Were those symptoms limited only to times when you were pregnant or postpartum? Were your symptoms severe enough to disrupt your life (job, school, relationships, ability to complete daily tasks)? What treatments were effective for your symptoms? What treatments were ineffective?3
Social factors. Learn more about your patient’s support systems by asking: Who do you consider to be part of your social support? How is your relationship with your social support? Are there challenges in your relationship with your friends, family, or partner? If yes, what are those challenges? Are there other children in the home, and do you have support for them? Is your home environment safe? Do you feel that you have what you need for the baby? What stressors are you currently experiencing? Do you attend support groups for expectant mothers? Are you engaged in perinatal care?3
Continue to: Given the high prevalence...
Given the high prevalence of interpersonal violence in women of reproductive age, all patients should be screened for this. The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women recommends screening for interpersonal violence at the first visit during the perinatal period, during each trimester, and at the postpartum visit (at minimum).4 Potential screening questions include (but are not limited to): Have you and/or your children ever been threatened by or felt afraid of your partner? When you argue with your partner, do either of you get physical? Has your partner ever physically hurt you (eg, hit, choked)? Do you feel safe at home? Do you have a safe place to go with resources you and your children will need in case of an emergency?4-6
Feelings toward pregnancy, past/current pregnancy complications, and pregnancy loss. Ask your patient: Was this pregnancy planned? How do you feel about your pregnancy? How do you see yourself as a mother? Do you currently have pregnancy complications and/or have had them in the past, and, if so, what are/were they? Have you lost a pregnancy? If so, what was that like? Do you have fears related to childbirth, and, if so, what are they?3
Intrusive thoughts about harming the baby. Intrusive thoughts are common in postpartum women with anxiety disorders, including OCD.7 Merely asking patients if they’ve had thoughts of harming their baby is incomplete and insufficient to assess for intrusive thoughts. This question does not distinguish between intrusive thoughts and homicidal ideation; this distinction is absolutely necessary given the difference in potential risk to the infant.
Intrusive thoughts are generally associated with a low risk of mothers acting on their thoughts. These thoughts are typically ego dystonic and, in the most severe form, can be distressing to an extent that they cause behavioral changes, such as avoiding bathing the infant, avoiding diaper changes, avoiding knives, or separating themselves from the infant.7 On the contrary, having homicidal ideation carries a higher risk for harm to the infant. Homicidal ideation may be seen in patients with co-occurring psychosis, poor reality testing, and delusions.5,7
Questions such as “Do you worry about harm coming to your baby?” “Do you worry about you causing harm to your baby?” and “Have you had an upsetting thought about harming your baby?” are more likely to reveal intrusive thoughts and prompt further exploration. Statements such as “Some people tell me that they have distressing thoughts about harm coming to their baby” can gently open the door to a having a dialogue about such thoughts. This dialogue is significantly important in making informed assessments as we develop comprehensive treatment plans.
Emerging data demonstrate that untreated perinatal anxiety is associated with negative outcomes, including an increased risk for suicide.1 A 2017 systematic review and meta-analysis that included 102 studies with a total of 221,974 women from 34 countries found that the prevalence of self-reported anxiety symptoms and any anxiety disorder was 22.9% and 15.2%, respectively, across the 3 trimesters.1 During pregnancy, anxiety disorders (eg, generalized anxiety disorder) and anxiety-related disorders (eg, obsessive-compulsive disorder [OCD] and posttraumatic stress disorder [PTSD]) can present as new illnesses or as a reoccurrence of an existing illness. Patients with pre-existing OCD may notice that the nature of their obsessions is changing. Women with pre-existing PTSD may have their symptoms triggered by pregnancy or delivery or may develop PTSD as a result of a traumatic delivery. Anxiety is frequently comorbid with depression, and high anxiety during pregnancy is one of the strongest risk factors for depression.1,2
In light of this data, awareness and recognition of perinatal anxiety is critical. In this article, we describe how to accurately assess perinatal anxiety by avoiding assumptions and asking key questions during the clinical interview.
Avoid these common assumptions
Assessment begins with avoiding assumptions typically associated with maternal mental health. One common assumption is that pregnancy is a joyous occasion for all women. Pregnancy can be a stressful time that has its own unique difficulties, including the potential to develop or have a relapse of a mental illness. Another assumption is that the only concern is “postpartum depression.” In actuality, a significant percentage of women will experience depression during their pregnancy (not just in the postpartum period), and many other psychiatric illnesses are common during the perinatal period, including anxiety disorders.
Conduct a focused interview
Risk factors associated with antenatal anxiety include2:
- previous history of mental illness (particularly a history of anxiety and depression and a history of psychiatric treatment)
- lack of partner or social support
- history of abuse or domestic violence
- unplanned or unwanted pregnancy
- adverse events in life and high perceived stress
- present/past pregnancy complications
- pregnancy loss.
Symptoms of anxiety. The presence of anxiety or worrying does not necessarily mean a mother has an anxiety disorder. Using the DSM-5 as a guide, we should use the questions outlined in the following sections to inquire about all of the symptoms related to a particular illness, the pervasiveness of these symptoms, and to what extent these symptoms impair a woman’s ability to function and carry out her usual activities.3
Past psychiatric history. Ask your patient the following: Have you previously experienced anxiety and/or depressive symptoms? Were those symptoms limited only to times when you were pregnant or postpartum? Were your symptoms severe enough to disrupt your life (job, school, relationships, ability to complete daily tasks)? What treatments were effective for your symptoms? What treatments were ineffective?3
Social factors. Learn more about your patient’s support systems by asking: Who do you consider to be part of your social support? How is your relationship with your social support? Are there challenges in your relationship with your friends, family, or partner? If yes, what are those challenges? Are there other children in the home, and do you have support for them? Is your home environment safe? Do you feel that you have what you need for the baby? What stressors are you currently experiencing? Do you attend support groups for expectant mothers? Are you engaged in perinatal care?3
Continue to: Given the high prevalence...
Given the high prevalence of interpersonal violence in women of reproductive age, all patients should be screened for this. The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women recommends screening for interpersonal violence at the first visit during the perinatal period, during each trimester, and at the postpartum visit (at minimum).4 Potential screening questions include (but are not limited to): Have you and/or your children ever been threatened by or felt afraid of your partner? When you argue with your partner, do either of you get physical? Has your partner ever physically hurt you (eg, hit, choked)? Do you feel safe at home? Do you have a safe place to go with resources you and your children will need in case of an emergency?4-6
Feelings toward pregnancy, past/current pregnancy complications, and pregnancy loss. Ask your patient: Was this pregnancy planned? How do you feel about your pregnancy? How do you see yourself as a mother? Do you currently have pregnancy complications and/or have had them in the past, and, if so, what are/were they? Have you lost a pregnancy? If so, what was that like? Do you have fears related to childbirth, and, if so, what are they?3
Intrusive thoughts about harming the baby. Intrusive thoughts are common in postpartum women with anxiety disorders, including OCD.7 Merely asking patients if they’ve had thoughts of harming their baby is incomplete and insufficient to assess for intrusive thoughts. This question does not distinguish between intrusive thoughts and homicidal ideation; this distinction is absolutely necessary given the difference in potential risk to the infant.
Intrusive thoughts are generally associated with a low risk of mothers acting on their thoughts. These thoughts are typically ego dystonic and, in the most severe form, can be distressing to an extent that they cause behavioral changes, such as avoiding bathing the infant, avoiding diaper changes, avoiding knives, or separating themselves from the infant.7 On the contrary, having homicidal ideation carries a higher risk for harm to the infant. Homicidal ideation may be seen in patients with co-occurring psychosis, poor reality testing, and delusions.5,7
Questions such as “Do you worry about harm coming to your baby?” “Do you worry about you causing harm to your baby?” and “Have you had an upsetting thought about harming your baby?” are more likely to reveal intrusive thoughts and prompt further exploration. Statements such as “Some people tell me that they have distressing thoughts about harm coming to their baby” can gently open the door to a having a dialogue about such thoughts. This dialogue is significantly important in making informed assessments as we develop comprehensive treatment plans.
1. Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. B J Psychiatry. 2017;210(5):315-323.
2. Biaggi A, Conroy S, Pawlby S, et al. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord. 2016;191:62-77.
3. Kirby N, Kilsby A, Walker R. Assessing low mood during pregnancy. BMJ. 2019;366:I4584. doi: 10.1136/bmj.I4584
4. American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee opinion: Intimate partner violence. Number 518. February 2012. Accessed March 23, 2020. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2012/02/intimate-partner-violence
5. Massachusetts Child Psychiatry Access Program for Moms Provider Toolkit. Accessed March 18, 2020. https://www.mcpapformoms.org/Docs/AdultProviderToolkit12.09.2019.pdf
6. Ashur ML. Asking about domestic violence: SAFE questions. JAMA. 1993;269(18):2367.
7. Brandes M, Soares CN, Cohen LS. Postpartum onset obsessive-compulsive disorder: diagnosis and management. Arch Womens Ment Health. 2004;7(2):99-110.
1. Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. B J Psychiatry. 2017;210(5):315-323.
2. Biaggi A, Conroy S, Pawlby S, et al. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord. 2016;191:62-77.
3. Kirby N, Kilsby A, Walker R. Assessing low mood during pregnancy. BMJ. 2019;366:I4584. doi: 10.1136/bmj.I4584
4. American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee opinion: Intimate partner violence. Number 518. February 2012. Accessed March 23, 2020. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2012/02/intimate-partner-violence
5. Massachusetts Child Psychiatry Access Program for Moms Provider Toolkit. Accessed March 18, 2020. https://www.mcpapformoms.org/Docs/AdultProviderToolkit12.09.2019.pdf
6. Ashur ML. Asking about domestic violence: SAFE questions. JAMA. 1993;269(18):2367.
7. Brandes M, Soares CN, Cohen LS. Postpartum onset obsessive-compulsive disorder: diagnosis and management. Arch Womens Ment Health. 2004;7(2):99-110.
Systemic trauma in the Black community: My perspective as an Asian American
Being a physician gives me great privilege. However, this privilege did not start the moment I donned the white coat, but when I was born Asian American, to parents who hold advanced education degrees. It grew when our family moved to a White neighborhood and I was accepted into an elite college. For medical school and residency, I chose an academic program embedded in an urban setting that serves underprivileged minority communities. I entered psychiatry to facilitate healing. Yet as I read the headlines about people of color who had died at the hands of law enforcement, I found myself feeling overwhelmingly hopeless and numb.
In these individuals, I saw people who looked and lived just like the patients I chose to serve. But during this time, I did not see myself as the healer, but part of the system that brought pain and distress. As an Asian American, I identified with Tou Thao—the Asian American police officer involved in George Floyd’s death. In the medical community with which I identified, I found that ever-rising cases of COVID-19 were disproportionately affecting lower-income minority communities. In a polarizing world, I felt my Asian American identity prevented me from experiencing the pain and suffering Black communities faced. This was not my fight, and if it was, I was more immersed in the side that brought trauma to my patients. From a purely rational perspective, I had no right to feel sad. Intellectually, I felt unqualified to share in their pain, yet here I was, crying in my room.
An evolving transformation
As much as I wanted to take a break, training did not stop. A transformation occurred from an emerging awareness of the unique environment within which I was training and the intersection of who I knew myself to be. Serving in an urban program, I was given the opportunity for candid conversations with health professionals of color. I was humbled when Black colleagues proactively reached out to educate me about the historical context of these events and help me process them. I asked hard questions of my fellow residents who were Black, and listened to their answers and personal stories, which was difficult.
With my patients, I began to listen more intently and think about the systemic issues I had previously written off. One patient missed their appointment because public transportation was closed due to COVID-19. Another patient who was homeless was helped immensely by assistance with housing when he could no longer sleep at his place of residence. Really listening to him revealed that his street had become a common route for protests. With my therapy patient who experienced panic attacks listening to the news, I simply sat and grieved with them. I chose these interactions not because I was uniquely qualified, intelligent, or had any ability to change the trajectory of our country, but because they grew from me simply working in the context I chose and seeking the relationships I naturally sought.
How I define myself
As doctors, we accept the burden of caring for society’s ailments with the ultimate hope of celebrating triumph over the adversity of psychiatric illness. However, superseding our profession is the social system in which we live. I am part of a system that has historically caused trauma to some while benefitting others. Thus, between the calling of my practice and the country I practice in, I found a divergence. Once I accepted the truth of this system and the very personal way it affects me, my colleagues, and patients I serve, I was able to internally reconcile and rediscover hope. While I cannot change my experiences, advantages, or privilege, these facts do not change the reality that I am a citizen of the globe and human first. This realization is the silver lining of these perilous times; training among people of color who graciously included me in their experiences, and my willingness to listen and self-reflect. I now choose to define myself by what makes me similar to my patients instead of what isolates me from them. The tangible results of this deliberate step toward authenticity are renewed inspiration and joy.
For those of you who may have found yourself with no “ethnic home team” (or a desire for a new one), I leave you with this simple charge: Let your emotional reactions guide you to truth, and challenge yourself to process them with someone who doesn’t look like you. Leave your title at the door and embrace humility. You might be pleasantly surprised at the human you find when you look in the mirror.
Being a physician gives me great privilege. However, this privilege did not start the moment I donned the white coat, but when I was born Asian American, to parents who hold advanced education degrees. It grew when our family moved to a White neighborhood and I was accepted into an elite college. For medical school and residency, I chose an academic program embedded in an urban setting that serves underprivileged minority communities. I entered psychiatry to facilitate healing. Yet as I read the headlines about people of color who had died at the hands of law enforcement, I found myself feeling overwhelmingly hopeless and numb.
In these individuals, I saw people who looked and lived just like the patients I chose to serve. But during this time, I did not see myself as the healer, but part of the system that brought pain and distress. As an Asian American, I identified with Tou Thao—the Asian American police officer involved in George Floyd’s death. In the medical community with which I identified, I found that ever-rising cases of COVID-19 were disproportionately affecting lower-income minority communities. In a polarizing world, I felt my Asian American identity prevented me from experiencing the pain and suffering Black communities faced. This was not my fight, and if it was, I was more immersed in the side that brought trauma to my patients. From a purely rational perspective, I had no right to feel sad. Intellectually, I felt unqualified to share in their pain, yet here I was, crying in my room.
An evolving transformation
As much as I wanted to take a break, training did not stop. A transformation occurred from an emerging awareness of the unique environment within which I was training and the intersection of who I knew myself to be. Serving in an urban program, I was given the opportunity for candid conversations with health professionals of color. I was humbled when Black colleagues proactively reached out to educate me about the historical context of these events and help me process them. I asked hard questions of my fellow residents who were Black, and listened to their answers and personal stories, which was difficult.
With my patients, I began to listen more intently and think about the systemic issues I had previously written off. One patient missed their appointment because public transportation was closed due to COVID-19. Another patient who was homeless was helped immensely by assistance with housing when he could no longer sleep at his place of residence. Really listening to him revealed that his street had become a common route for protests. With my therapy patient who experienced panic attacks listening to the news, I simply sat and grieved with them. I chose these interactions not because I was uniquely qualified, intelligent, or had any ability to change the trajectory of our country, but because they grew from me simply working in the context I chose and seeking the relationships I naturally sought.
How I define myself
As doctors, we accept the burden of caring for society’s ailments with the ultimate hope of celebrating triumph over the adversity of psychiatric illness. However, superseding our profession is the social system in which we live. I am part of a system that has historically caused trauma to some while benefitting others. Thus, between the calling of my practice and the country I practice in, I found a divergence. Once I accepted the truth of this system and the very personal way it affects me, my colleagues, and patients I serve, I was able to internally reconcile and rediscover hope. While I cannot change my experiences, advantages, or privilege, these facts do not change the reality that I am a citizen of the globe and human first. This realization is the silver lining of these perilous times; training among people of color who graciously included me in their experiences, and my willingness to listen and self-reflect. I now choose to define myself by what makes me similar to my patients instead of what isolates me from them. The tangible results of this deliberate step toward authenticity are renewed inspiration and joy.
For those of you who may have found yourself with no “ethnic home team” (or a desire for a new one), I leave you with this simple charge: Let your emotional reactions guide you to truth, and challenge yourself to process them with someone who doesn’t look like you. Leave your title at the door and embrace humility. You might be pleasantly surprised at the human you find when you look in the mirror.
Being a physician gives me great privilege. However, this privilege did not start the moment I donned the white coat, but when I was born Asian American, to parents who hold advanced education degrees. It grew when our family moved to a White neighborhood and I was accepted into an elite college. For medical school and residency, I chose an academic program embedded in an urban setting that serves underprivileged minority communities. I entered psychiatry to facilitate healing. Yet as I read the headlines about people of color who had died at the hands of law enforcement, I found myself feeling overwhelmingly hopeless and numb.
In these individuals, I saw people who looked and lived just like the patients I chose to serve. But during this time, I did not see myself as the healer, but part of the system that brought pain and distress. As an Asian American, I identified with Tou Thao—the Asian American police officer involved in George Floyd’s death. In the medical community with which I identified, I found that ever-rising cases of COVID-19 were disproportionately affecting lower-income minority communities. In a polarizing world, I felt my Asian American identity prevented me from experiencing the pain and suffering Black communities faced. This was not my fight, and if it was, I was more immersed in the side that brought trauma to my patients. From a purely rational perspective, I had no right to feel sad. Intellectually, I felt unqualified to share in their pain, yet here I was, crying in my room.
An evolving transformation
As much as I wanted to take a break, training did not stop. A transformation occurred from an emerging awareness of the unique environment within which I was training and the intersection of who I knew myself to be. Serving in an urban program, I was given the opportunity for candid conversations with health professionals of color. I was humbled when Black colleagues proactively reached out to educate me about the historical context of these events and help me process them. I asked hard questions of my fellow residents who were Black, and listened to their answers and personal stories, which was difficult.
With my patients, I began to listen more intently and think about the systemic issues I had previously written off. One patient missed their appointment because public transportation was closed due to COVID-19. Another patient who was homeless was helped immensely by assistance with housing when he could no longer sleep at his place of residence. Really listening to him revealed that his street had become a common route for protests. With my therapy patient who experienced panic attacks listening to the news, I simply sat and grieved with them. I chose these interactions not because I was uniquely qualified, intelligent, or had any ability to change the trajectory of our country, but because they grew from me simply working in the context I chose and seeking the relationships I naturally sought.
How I define myself
As doctors, we accept the burden of caring for society’s ailments with the ultimate hope of celebrating triumph over the adversity of psychiatric illness. However, superseding our profession is the social system in which we live. I am part of a system that has historically caused trauma to some while benefitting others. Thus, between the calling of my practice and the country I practice in, I found a divergence. Once I accepted the truth of this system and the very personal way it affects me, my colleagues, and patients I serve, I was able to internally reconcile and rediscover hope. While I cannot change my experiences, advantages, or privilege, these facts do not change the reality that I am a citizen of the globe and human first. This realization is the silver lining of these perilous times; training among people of color who graciously included me in their experiences, and my willingness to listen and self-reflect. I now choose to define myself by what makes me similar to my patients instead of what isolates me from them. The tangible results of this deliberate step toward authenticity are renewed inspiration and joy.
For those of you who may have found yourself with no “ethnic home team” (or a desire for a new one), I leave you with this simple charge: Let your emotional reactions guide you to truth, and challenge yourself to process them with someone who doesn’t look like you. Leave your title at the door and embrace humility. You might be pleasantly surprised at the human you find when you look in the mirror.
Evidence-based medicine: It’s not a cookbook!
The term evidence-based medicine (EBM) has been derided by some as “cookbook medicine.” To others, EBM conjures up the efforts of describing interventions in terms of comparative effectiveness, drowning us in a deluge of “evidence-based” publications. The moniker has also been hijacked by companies to name their Health Economics and Outcomes research divisions. The spirit behind EBM is getting lost. EBM is not just about the evidence; it is about how we use it.1
In this commentary, we describe the concept of EBM and discuss teaching EBM to medical students and residents, its role in continuing medical education, and how it may be applied to practice, using a case scenario as a guide.
What is evidence-based medicine?
Sackett et al2 summed it best in an editorial published in the BMJ in 1996, where he emphasized decision-making in the care of individual patients. When making clinical decisions, using the best evidence available makes sense, but so does integrating individual clinical expertise and considering the individual patient’s preferences. Sackett et al2 warns about practice becoming tyrannized by evidence: “even excellent external evidence may be inapplicable to or inappropriate for an individual patient.” Clearly, EBM is not cookbook medicine.
Figure 13 illustrates EBM as the confluence of clinical judgment, relevant scientific evidence, and patients’ values and preferences. The results from a clinical trial are only one part of the equation. As practitioners, we have the advantage of detailed knowledge about the patient, and our decisions are not “one size fits all.” Prior information about the patient dictates how we apply the evidence that supports potential interventions.
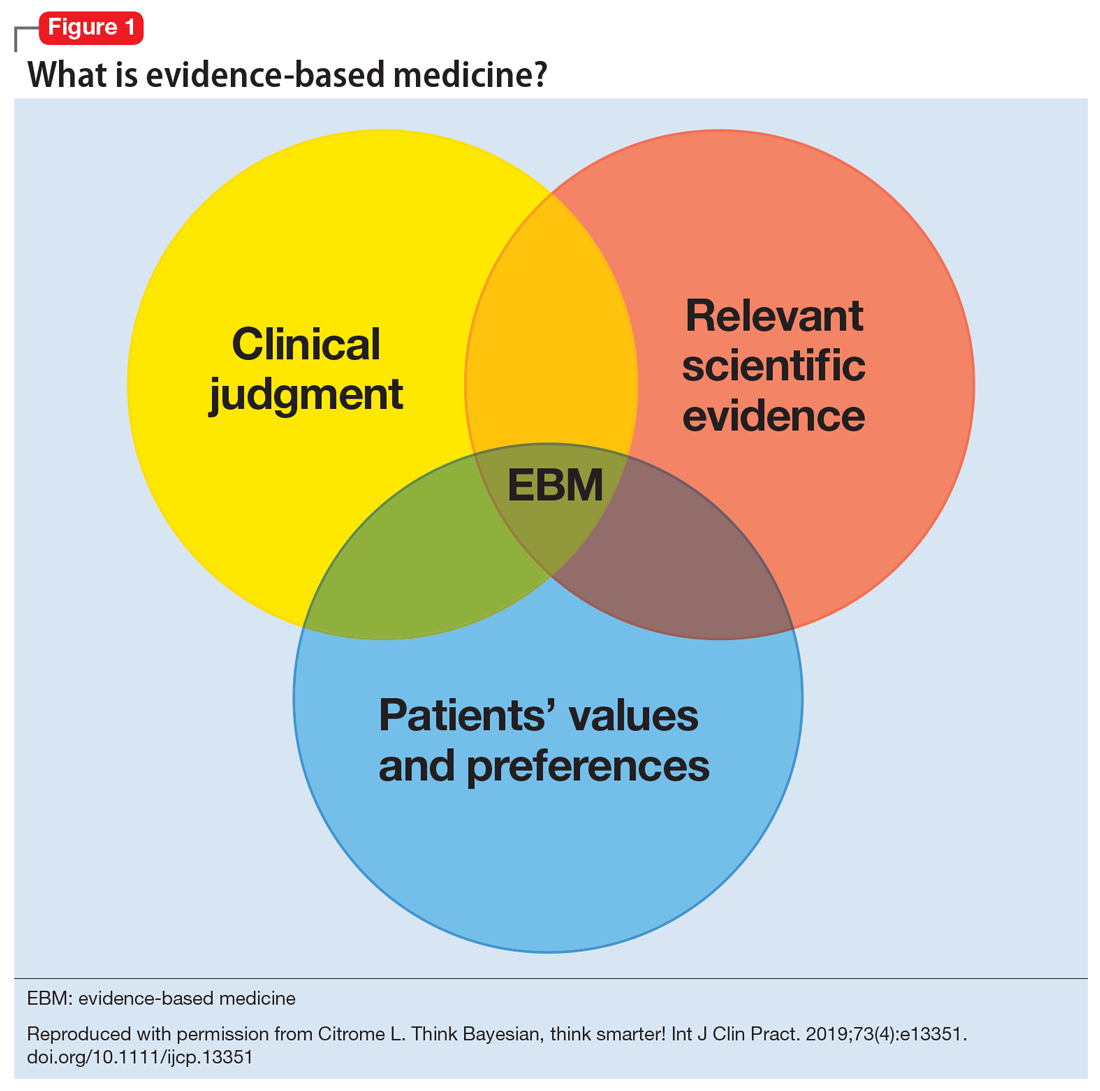
The concept of EBM was born out of necessity to bring scientific principles into the heart of medicine. As outlined by Sackett,4 the practice of EBM is a process of lifelong, self-directed learning in which caring for our own patients creates the need for clinically important information about diagnosis, prognosis, therapy, and other clinical and health care issues. Through EBM, we:
- convert these information needs into answerable questions
- track down, with maximum efficiency, the best evidence with which to answer questions (whether from clinical examination, diagnostic laboratory results, research evidence, or other sources)
- critically appraise that evidence for its validity (closeness to the truth) and usefulness (clinical applicability)
- integrate this appraisal with our clinical expertise and apply it in practice
- evaluate our performance.
Over the years, the original aim of EBM as a self-directed method for clinicians to practice high-quality medicine was morphed by some into a tool of enforced standardization and a boilerplate approach to managing costs across systems of care. As a result, the term EBM has been criticized because of:
- its reliance on empiricism
- a narrow definition of evidence
- a lack of evidence of efficacy
- its limited usefulness for individual patients
- threats to the autonomy of the doctor-patient relationship.
These 5 categories are associated with severe drawbacks when used for individual patient care.5 In addition to problems with applying standardized population research to a specific patient with a specific set of symptoms, medications, genetic variations, and unique environment, it can take years for clinicians to change their practices to incorporate new information.6
Continue to: Evidence that is too narrow...
Evidence that is too narrow in scope may not be useful. Single-molecule pharmaceutical clinical trials have erroneously become a synonym of EBM. Such studies do not reflect complex, real-life situations. Based on such studies, FDA product labeling can be inadequate in its guidance, particularly when faced with complex comorbidities. The standard comparison of active treatment to placebo is also seen as EBM, narrowing its scope and deflecting from clinical medicine when physicians measure one treatment’s success against another vs measuring real treatments against shams. Real-life treatment choice is frequently based on considering adverse effects as important to consider as therapeutic efficacy; however, this concept is outside of the common (mis)understanding of EBM.
Conflicting and ever-changing data and the push to replace clinical thinking with general dogmas trivializes medical practice and endangers treatment outcomes. This would not happen to the extent we see now if EBM was again seen as a guide and general direction rather than a blanket, distorted requirement to follow rigid recommendations for specific patients.
Insurance companies have driven a change in the understanding of EBM by using the FDA label as an excuse to deny, delay, and/or refuse to pay for treatments that are not explicitly and narrowly on-label. Dependence on on-label treatments is even more challenging in specialty medicine because primary care clinicians generally have tried the conventional approaches before referring patients to a specialist. However, insurance denials rarely differentiate between practice settings.
Medicolegal issues have cemented the present situation when clinically valid “off-label” treatments may be a reasonable consideration for patients but can place health care practitioners in jeopardy. The distorted EBM doctrine has become a justification for legal actions against clinicians who practice individualized medicine.
Concision bias (selectively focusing on information, losing nuance) and selection bias (patients in clinical trials who do not reflect real-life patients) have become an impediment to progress and EBM as originally intended.
Continue to: Training medical students and residents
Training medical students and residents
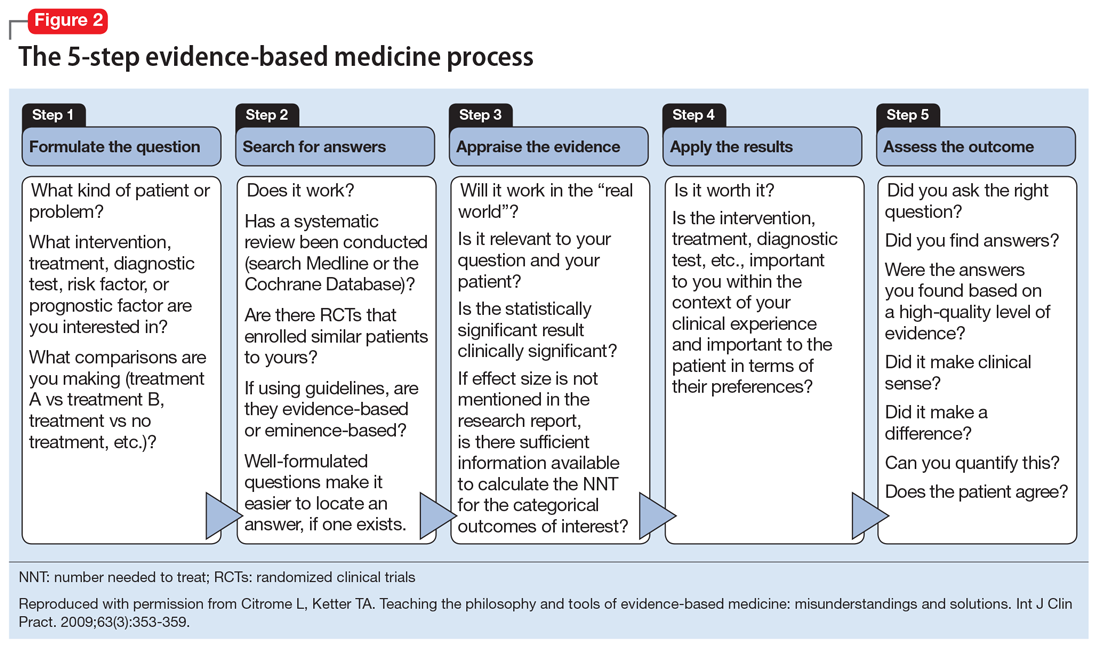
Although there is some variation in how EBM is taught to medical students and residents,7,8 the expectation is that such education occurs. The Accreditation Council for Graduate Medical Education requirements for a residency program state that “the program must advance residents’ knowledge and practice of the scholarly approach to evidence-based patient care.”9 The topic has been part of the American Society of Clinical Psychopharmacology Model Psychopharmacology Curriculum, but only in an optional lecture.10 The formal teaching of EBM includes how to find relevant biomedical publications for the clinical issues at hand, understand the different hierarchies of evidence, interpret results in terms of effect size, and apply this knowledge in the care of patients. This 5-step process is illustrated in Figure 28. See Related Resources for 3 books that provide a scholarly yet clinically relevant approach to EBM.
Continuing medical education
Most
Practical applications
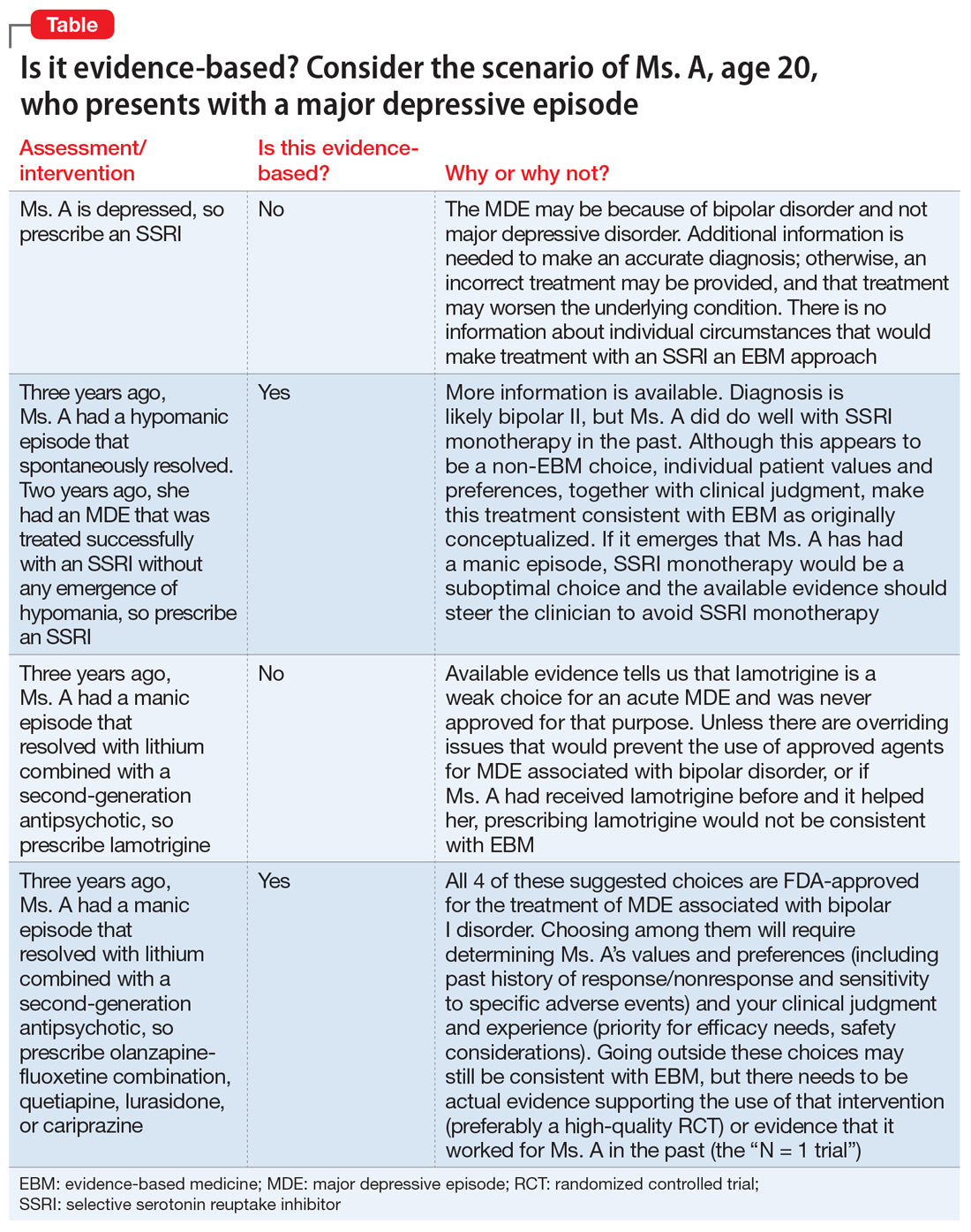
There are common clinical scenarios where evidence is ignored, or where it is overvalued. For example, the treatment of bipolar depression can be made worse with the use of antidepressants.14 Does this mean that antidepressants should never be used? What about patient history and preference? What if the approved agents fail to relieve symptoms or are not well tolerated? Available FDA-approved choices may not always be suitable.15 The Table illustrates some of these scenarios.
1. Citrome L. Evidence-based medicine: it’s not just about the evidence. Int J Clin Pract. 2011;65(6):634-635.
2. Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71.
3. Citrome L. Think Bayesian, think smarter! Int J Clin Pract. 2019;73(4):e13351. doi.org/10.1111/ijcp.13351
4. Sackett DL. Evidence-based medicine. Semin Perinatol. 1997;21(1):3-5.
5. Cohen AM, Stavri PZ, Hersh WR. A categorization and analysis of the criticisms of evidence-based medicine. Int J Med Inform. 2004;73(1):35-43.
6. Dutton DB. Worse than the disease: pitfalls of medical progress. Cambridge University Press; 1988.
7. Maggio LA. Educating physicians in evidence based medicine: current practices and curricular strategies. Perspect Med Educ. 2016;5(6):358-361.
8. Citrome L, Ketter TA. Teaching the philosophy and tools of evidence-based medicine: misunderstandings and solutions. Int J Clin Pract. 2009;63(3):353-359.
9. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Residency). Revised February 3, 2020. Accessed March 30, 2021. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRResidency2020.pdf
10. Citrome L, Ellison JM. Show me the evidence! Understanding the philosophy of evidence-based medicine and interpreting clinical trials. In: Glick ID, Macaluso M (Chair, Co-chair). ASCP model psychopharmacology curriculum for training directors and teachers of psychopharmacology in psychiatric residency programs, 10th ed. American Society of Clinical Psychopharmacology; 2019.
11. Citrome L. Interpreting and applying the CATIE results: with CATIE, context is key, when sorting out Phases 1, 1A, 1B, 2E, and 2T. Psychiatry (Edgmont). 2007;4(10):23-29.
12. Citrome L, Stroup TS. Schizophrenia, clinical antipsychotic trials of intervention effectiveness (CATIE) and number needed to treat: how can CATIE inform clinicians? Int J Clin Pract. 2006;60(8):933-940. doi: 10.1111/j.1742-1241.2006.01044.x
13. Citrome L. Dissecting clinical trials with ‘number needed to treat’. Current Psychiatry. 2007;6(3):66-71.
14. Goldberg JF, Freeman MP, Balon R, et al. The American Society of Clinical Psychopharmacology survey of psychopharmacologists’ practice patterns for the treatment of mood disorders. Depress Anxiety. 2015;32(8):605-613.
15. Citrome L. Food and Drug Administration-approved treatments for acute bipolar depression: what we have and what we need. J Clin Psychopharmacol. 2020;40(4):334-338.
The term evidence-based medicine (EBM) has been derided by some as “cookbook medicine.” To others, EBM conjures up the efforts of describing interventions in terms of comparative effectiveness, drowning us in a deluge of “evidence-based” publications. The moniker has also been hijacked by companies to name their Health Economics and Outcomes research divisions. The spirit behind EBM is getting lost. EBM is not just about the evidence; it is about how we use it.1
In this commentary, we describe the concept of EBM and discuss teaching EBM to medical students and residents, its role in continuing medical education, and how it may be applied to practice, using a case scenario as a guide.
What is evidence-based medicine?
Sackett et al2 summed it best in an editorial published in the BMJ in 1996, where he emphasized decision-making in the care of individual patients. When making clinical decisions, using the best evidence available makes sense, but so does integrating individual clinical expertise and considering the individual patient’s preferences. Sackett et al2 warns about practice becoming tyrannized by evidence: “even excellent external evidence may be inapplicable to or inappropriate for an individual patient.” Clearly, EBM is not cookbook medicine.
Figure 13 illustrates EBM as the confluence of clinical judgment, relevant scientific evidence, and patients’ values and preferences. The results from a clinical trial are only one part of the equation. As practitioners, we have the advantage of detailed knowledge about the patient, and our decisions are not “one size fits all.” Prior information about the patient dictates how we apply the evidence that supports potential interventions.

The concept of EBM was born out of necessity to bring scientific principles into the heart of medicine. As outlined by Sackett,4 the practice of EBM is a process of lifelong, self-directed learning in which caring for our own patients creates the need for clinically important information about diagnosis, prognosis, therapy, and other clinical and health care issues. Through EBM, we:
- convert these information needs into answerable questions
- track down, with maximum efficiency, the best evidence with which to answer questions (whether from clinical examination, diagnostic laboratory results, research evidence, or other sources)
- critically appraise that evidence for its validity (closeness to the truth) and usefulness (clinical applicability)
- integrate this appraisal with our clinical expertise and apply it in practice
- evaluate our performance.
Over the years, the original aim of EBM as a self-directed method for clinicians to practice high-quality medicine was morphed by some into a tool of enforced standardization and a boilerplate approach to managing costs across systems of care. As a result, the term EBM has been criticized because of:
- its reliance on empiricism
- a narrow definition of evidence
- a lack of evidence of efficacy
- its limited usefulness for individual patients
- threats to the autonomy of the doctor-patient relationship.
These 5 categories are associated with severe drawbacks when used for individual patient care.5 In addition to problems with applying standardized population research to a specific patient with a specific set of symptoms, medications, genetic variations, and unique environment, it can take years for clinicians to change their practices to incorporate new information.6
Continue to: Evidence that is too narrow...
Evidence that is too narrow in scope may not be useful. Single-molecule pharmaceutical clinical trials have erroneously become a synonym of EBM. Such studies do not reflect complex, real-life situations. Based on such studies, FDA product labeling can be inadequate in its guidance, particularly when faced with complex comorbidities. The standard comparison of active treatment to placebo is also seen as EBM, narrowing its scope and deflecting from clinical medicine when physicians measure one treatment’s success against another vs measuring real treatments against shams. Real-life treatment choice is frequently based on considering adverse effects as important to consider as therapeutic efficacy; however, this concept is outside of the common (mis)understanding of EBM.
Conflicting and ever-changing data and the push to replace clinical thinking with general dogmas trivializes medical practice and endangers treatment outcomes. This would not happen to the extent we see now if EBM was again seen as a guide and general direction rather than a blanket, distorted requirement to follow rigid recommendations for specific patients.
Insurance companies have driven a change in the understanding of EBM by using the FDA label as an excuse to deny, delay, and/or refuse to pay for treatments that are not explicitly and narrowly on-label. Dependence on on-label treatments is even more challenging in specialty medicine because primary care clinicians generally have tried the conventional approaches before referring patients to a specialist. However, insurance denials rarely differentiate between practice settings.
Medicolegal issues have cemented the present situation when clinically valid “off-label” treatments may be a reasonable consideration for patients but can place health care practitioners in jeopardy. The distorted EBM doctrine has become a justification for legal actions against clinicians who practice individualized medicine.
Concision bias (selectively focusing on information, losing nuance) and selection bias (patients in clinical trials who do not reflect real-life patients) have become an impediment to progress and EBM as originally intended.
Continue to: Training medical students and residents
Training medical students and residents
Although there is some variation in how EBM is taught to medical students and residents,7,8 the expectation is that such education occurs. The Accreditation Council for Graduate Medical Education requirements for a residency program state that “the program must advance residents’ knowledge and practice of the scholarly approach to evidence-based patient care.”9 The topic has been part of the American Society of Clinical Psychopharmacology Model Psychopharmacology Curriculum, but only in an optional lecture.10 The formal teaching of EBM includes how to find relevant biomedical publications for the clinical issues at hand, understand the different hierarchies of evidence, interpret results in terms of effect size, and apply this knowledge in the care of patients. This 5-step process is illustrated in Figure 28. See Related Resources for 3 books that provide a scholarly yet clinically relevant approach to EBM.

Continuing medical education
Most
Practical applications
There are common clinical scenarios where evidence is ignored, or where it is overvalued. For example, the treatment of bipolar depression can be made worse with the use of antidepressants.14 Does this mean that antidepressants should never be used? What about patient history and preference? What if the approved agents fail to relieve symptoms or are not well tolerated? Available FDA-approved choices may not always be suitable.15 The Table illustrates some of these scenarios.

The term evidence-based medicine (EBM) has been derided by some as “cookbook medicine.” To others, EBM conjures up the efforts of describing interventions in terms of comparative effectiveness, drowning us in a deluge of “evidence-based” publications. The moniker has also been hijacked by companies to name their Health Economics and Outcomes research divisions. The spirit behind EBM is getting lost. EBM is not just about the evidence; it is about how we use it.1
In this commentary, we describe the concept of EBM and discuss teaching EBM to medical students and residents, its role in continuing medical education, and how it may be applied to practice, using a case scenario as a guide.
What is evidence-based medicine?
Sackett et al2 summed it best in an editorial published in the BMJ in 1996, where he emphasized decision-making in the care of individual patients. When making clinical decisions, using the best evidence available makes sense, but so does integrating individual clinical expertise and considering the individual patient’s preferences. Sackett et al2 warns about practice becoming tyrannized by evidence: “even excellent external evidence may be inapplicable to or inappropriate for an individual patient.” Clearly, EBM is not cookbook medicine.
Figure 13 illustrates EBM as the confluence of clinical judgment, relevant scientific evidence, and patients’ values and preferences. The results from a clinical trial are only one part of the equation. As practitioners, we have the advantage of detailed knowledge about the patient, and our decisions are not “one size fits all.” Prior information about the patient dictates how we apply the evidence that supports potential interventions.

The concept of EBM was born out of necessity to bring scientific principles into the heart of medicine. As outlined by Sackett,4 the practice of EBM is a process of lifelong, self-directed learning in which caring for our own patients creates the need for clinically important information about diagnosis, prognosis, therapy, and other clinical and health care issues. Through EBM, we:
- convert these information needs into answerable questions
- track down, with maximum efficiency, the best evidence with which to answer questions (whether from clinical examination, diagnostic laboratory results, research evidence, or other sources)
- critically appraise that evidence for its validity (closeness to the truth) and usefulness (clinical applicability)
- integrate this appraisal with our clinical expertise and apply it in practice
- evaluate our performance.
Over the years, the original aim of EBM as a self-directed method for clinicians to practice high-quality medicine was morphed by some into a tool of enforced standardization and a boilerplate approach to managing costs across systems of care. As a result, the term EBM has been criticized because of:
- its reliance on empiricism
- a narrow definition of evidence
- a lack of evidence of efficacy
- its limited usefulness for individual patients
- threats to the autonomy of the doctor-patient relationship.
These 5 categories are associated with severe drawbacks when used for individual patient care.5 In addition to problems with applying standardized population research to a specific patient with a specific set of symptoms, medications, genetic variations, and unique environment, it can take years for clinicians to change their practices to incorporate new information.6
Continue to: Evidence that is too narrow...
Evidence that is too narrow in scope may not be useful. Single-molecule pharmaceutical clinical trials have erroneously become a synonym of EBM. Such studies do not reflect complex, real-life situations. Based on such studies, FDA product labeling can be inadequate in its guidance, particularly when faced with complex comorbidities. The standard comparison of active treatment to placebo is also seen as EBM, narrowing its scope and deflecting from clinical medicine when physicians measure one treatment’s success against another vs measuring real treatments against shams. Real-life treatment choice is frequently based on considering adverse effects as important to consider as therapeutic efficacy; however, this concept is outside of the common (mis)understanding of EBM.
Conflicting and ever-changing data and the push to replace clinical thinking with general dogmas trivializes medical practice and endangers treatment outcomes. This would not happen to the extent we see now if EBM was again seen as a guide and general direction rather than a blanket, distorted requirement to follow rigid recommendations for specific patients.
Insurance companies have driven a change in the understanding of EBM by using the FDA label as an excuse to deny, delay, and/or refuse to pay for treatments that are not explicitly and narrowly on-label. Dependence on on-label treatments is even more challenging in specialty medicine because primary care clinicians generally have tried the conventional approaches before referring patients to a specialist. However, insurance denials rarely differentiate between practice settings.
Medicolegal issues have cemented the present situation when clinically valid “off-label” treatments may be a reasonable consideration for patients but can place health care practitioners in jeopardy. The distorted EBM doctrine has become a justification for legal actions against clinicians who practice individualized medicine.
Concision bias (selectively focusing on information, losing nuance) and selection bias (patients in clinical trials who do not reflect real-life patients) have become an impediment to progress and EBM as originally intended.
Continue to: Training medical students and residents
Training medical students and residents
Although there is some variation in how EBM is taught to medical students and residents,7,8 the expectation is that such education occurs. The Accreditation Council for Graduate Medical Education requirements for a residency program state that “the program must advance residents’ knowledge and practice of the scholarly approach to evidence-based patient care.”9 The topic has been part of the American Society of Clinical Psychopharmacology Model Psychopharmacology Curriculum, but only in an optional lecture.10 The formal teaching of EBM includes how to find relevant biomedical publications for the clinical issues at hand, understand the different hierarchies of evidence, interpret results in terms of effect size, and apply this knowledge in the care of patients. This 5-step process is illustrated in Figure 28. See Related Resources for 3 books that provide a scholarly yet clinically relevant approach to EBM.

Continuing medical education
Most
Practical applications
There are common clinical scenarios where evidence is ignored, or where it is overvalued. For example, the treatment of bipolar depression can be made worse with the use of antidepressants.14 Does this mean that antidepressants should never be used? What about patient history and preference? What if the approved agents fail to relieve symptoms or are not well tolerated? Available FDA-approved choices may not always be suitable.15 The Table illustrates some of these scenarios.

1. Citrome L. Evidence-based medicine: it’s not just about the evidence. Int J Clin Pract. 2011;65(6):634-635.
2. Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71.
3. Citrome L. Think Bayesian, think smarter! Int J Clin Pract. 2019;73(4):e13351. doi.org/10.1111/ijcp.13351
4. Sackett DL. Evidence-based medicine. Semin Perinatol. 1997;21(1):3-5.
5. Cohen AM, Stavri PZ, Hersh WR. A categorization and analysis of the criticisms of evidence-based medicine. Int J Med Inform. 2004;73(1):35-43.
6. Dutton DB. Worse than the disease: pitfalls of medical progress. Cambridge University Press; 1988.
7. Maggio LA. Educating physicians in evidence based medicine: current practices and curricular strategies. Perspect Med Educ. 2016;5(6):358-361.
8. Citrome L, Ketter TA. Teaching the philosophy and tools of evidence-based medicine: misunderstandings and solutions. Int J Clin Pract. 2009;63(3):353-359.
9. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Residency). Revised February 3, 2020. Accessed March 30, 2021. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRResidency2020.pdf
10. Citrome L, Ellison JM. Show me the evidence! Understanding the philosophy of evidence-based medicine and interpreting clinical trials. In: Glick ID, Macaluso M (Chair, Co-chair). ASCP model psychopharmacology curriculum for training directors and teachers of psychopharmacology in psychiatric residency programs, 10th ed. American Society of Clinical Psychopharmacology; 2019.
11. Citrome L. Interpreting and applying the CATIE results: with CATIE, context is key, when sorting out Phases 1, 1A, 1B, 2E, and 2T. Psychiatry (Edgmont). 2007;4(10):23-29.
12. Citrome L, Stroup TS. Schizophrenia, clinical antipsychotic trials of intervention effectiveness (CATIE) and number needed to treat: how can CATIE inform clinicians? Int J Clin Pract. 2006;60(8):933-940. doi: 10.1111/j.1742-1241.2006.01044.x
13. Citrome L. Dissecting clinical trials with ‘number needed to treat’. Current Psychiatry. 2007;6(3):66-71.
14. Goldberg JF, Freeman MP, Balon R, et al. The American Society of Clinical Psychopharmacology survey of psychopharmacologists’ practice patterns for the treatment of mood disorders. Depress Anxiety. 2015;32(8):605-613.
15. Citrome L. Food and Drug Administration-approved treatments for acute bipolar depression: what we have and what we need. J Clin Psychopharmacol. 2020;40(4):334-338.
1. Citrome L. Evidence-based medicine: it’s not just about the evidence. Int J Clin Pract. 2011;65(6):634-635.
2. Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71.
3. Citrome L. Think Bayesian, think smarter! Int J Clin Pract. 2019;73(4):e13351. doi.org/10.1111/ijcp.13351
4. Sackett DL. Evidence-based medicine. Semin Perinatol. 1997;21(1):3-5.
5. Cohen AM, Stavri PZ, Hersh WR. A categorization and analysis of the criticisms of evidence-based medicine. Int J Med Inform. 2004;73(1):35-43.
6. Dutton DB. Worse than the disease: pitfalls of medical progress. Cambridge University Press; 1988.
7. Maggio LA. Educating physicians in evidence based medicine: current practices and curricular strategies. Perspect Med Educ. 2016;5(6):358-361.
8. Citrome L, Ketter TA. Teaching the philosophy and tools of evidence-based medicine: misunderstandings and solutions. Int J Clin Pract. 2009;63(3):353-359.
9. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Residency). Revised February 3, 2020. Accessed March 30, 2021. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRResidency2020.pdf
10. Citrome L, Ellison JM. Show me the evidence! Understanding the philosophy of evidence-based medicine and interpreting clinical trials. In: Glick ID, Macaluso M (Chair, Co-chair). ASCP model psychopharmacology curriculum for training directors and teachers of psychopharmacology in psychiatric residency programs, 10th ed. American Society of Clinical Psychopharmacology; 2019.
11. Citrome L. Interpreting and applying the CATIE results: with CATIE, context is key, when sorting out Phases 1, 1A, 1B, 2E, and 2T. Psychiatry (Edgmont). 2007;4(10):23-29.
12. Citrome L, Stroup TS. Schizophrenia, clinical antipsychotic trials of intervention effectiveness (CATIE) and number needed to treat: how can CATIE inform clinicians? Int J Clin Pract. 2006;60(8):933-940. doi: 10.1111/j.1742-1241.2006.01044.x
13. Citrome L. Dissecting clinical trials with ‘number needed to treat’. Current Psychiatry. 2007;6(3):66-71.
14. Goldberg JF, Freeman MP, Balon R, et al. The American Society of Clinical Psychopharmacology survey of psychopharmacologists’ practice patterns for the treatment of mood disorders. Depress Anxiety. 2015;32(8):605-613.
15. Citrome L. Food and Drug Administration-approved treatments for acute bipolar depression: what we have and what we need. J Clin Psychopharmacol. 2020;40(4):334-338.
10 devastating consequences of psychotic relapses
It breaks my heart every time young patients with functional disability and a history of several psychotic episodes are referred to me. It makes me wonder why they weren’t protected from a lifetime of disability with the use of one of the FDA-approved long-acting injectable (LAI) antipsychotics right after discharge from their initial hospitalization for first-episode psychosis (FEP).
Two decades ago, psychiatric research discovered that psychotic episodes are neurotoxic and neurodegenerative, with grave consequences for the brain if they recur. Although many clinicians are aware of the high rate of nonadherence in patients with schizophrenia—which inevitably leads to a psychotic relapse—the vast majority (>99%, in my estimate) never prescribe an LAI after the FEP to guarantee full adherence and protect the patient’s brain from further atrophy due to relapses. The overall rate of LAI antipsychotic use is astonishingly low (approximately 10%), despite the neurologic malignancy of psychotic episodes. Further, LAIs are most often used after a patient has experienced multiple psychotic episodes, at which point the patient has already lost a significant amount of brain tissue and has already descended into a life of permanent disability.
Oral antipsychotics have the same efficacy as their LAI counterparts, and certainly should be used initially in the hospital during FEP to ascertain the absence of an allergic reaction after initial exposure, and to establish tolerability. Inpatient nurses are experts at making sure a reluctant patient actually swallows the pills and does not cheek them to spit them out later. So patients who have had FEP do improve with oral medications in the hospital, but all bets are off that those patients will regularly ingest tablets every day after discharge. Studies show patients have a high rate of nonadherence within days or weeks after leaving the hospital for FEP.1 This leads to repetitive psychotic relapses and rehospitalizations, with dire consequences for young patients with schizophrenia—a very serious brain disorder that had been labeled “the worst disease of mankind”2 in the era before studies showed LAI second-generation antipsychotics for FEP had remarkable rates of relapse prevention and recovery.3,4
Psychiatrists should approach FEP the same way oncologists approach cancer when it is diagnosed as Stage 1. Oncologists immediately take action to prevent the recurrence of the patient’s cancer with chemotherapy and/or radiation therapy, and do not wait for the cancer to advance to Stage 4, with widespread metastasis, before administering these potentially life-saving therapies (despite their toxic adverse effects). In schizophrenia, functional disability is the equivalent of Stage 4 cancer and should be aggressively prevented by using LAIs at the time of initial diagnosis, which is Stage 1 schizophrenia. Knowing the grave consequences of psychotic relapses, there is no logical reason whatsoever not to switch patients who have had FEP to an LAI before they are discharged from the hospital. A well-known study by a UCLA research group that compared patients who had FEP and were assigned to oral vs LAI antipsychotics at the time of discharge reported a stunning difference at the end of 1 year: a 650% higher relapse rate among the oral medication group compared with the LAI group!5 In light of such a massive difference, wouldn’t psychiatrists want to treat their sons or daughters with an LAI antipsychotic right after FEP? I certainly would, and I have always believed in treating every patient like a family member.
Catastrophic consequences
This lack of early intervention with LAI antipsychotics following FEP is the main reason schizophrenia is associated with poor clinical and functional outcomes. Patients are prescribed pills that they often take erratically or not at all, and end up relapsing repeatedly, with multiple catastrophic consequences, such as:
1. Brain tissue loss. Until recently, psychiatry did not know that psychosis destroys gray and white matter in the brain and causes progressive brain atrophy with every psychotic relapse.6,7 The neurotoxicity of psychosis is attributed to 2 destructive processes: neuroinflammation8,9 and free radicals.10 Approximately 11 cc of brain tissue is lost during FEP and with every subsequent relapse.6 Simple math shows that after 3 to 5 relapses, patients’ brains will shrink by 35 cc to 60 cc. No wonder recurrent psychoses lead to a life of permanent disability. As I have said in a past editorial,11 just as cardiologists do everything they can to prevent a second myocardial infarction (“heart attack”), psychiatrists must do the same to prevent a second psychotic episode (“brain attack”).
2. Treatment resistance. With each psychotic episode, the low antipsychotic dose that worked well in FEP is no longer enough and must be increased. The neurodegenerative effects of psychosis implies that the brain structure changes with each episode. Higher and higher doses become necessary with every psychotic recurrence, and studies show that approximately 1 in 8 patients may stop responding altogether after a psychotic relapse.12
Continue to: Disability
3. Disability. Functional disability, both vocational and social, usually begins after the second psychotic episode, which is why it is so important to prevent the second episode.13 Patients usually must drop out of high school or college or quit the job they held before FEP. Most patients with multiple psychotic episodes will never be able to work, get married, have children, live independently, or develop a circle of friends. Disability in schizophrenia is essentially a functional death sentence.14
4. Incarceration and criminalization. So many of our patients with schizophrenia get arrested when they become psychotic and behave erratically due to delusions, hallucinations, or both. They typically are taken to jail instead of a hospital because almost all the state hospitals around the country have been closed. It is outrageous that a medical condition of the brain leads to criminalization of patients with schizophrenia.15 The only solution for this ongoing crisis of incarceration of our patients with schizophrenia is to prevent them from relapsing into psychosis. The so-called deinstitutionalization movement has mutated into trans-institutionalization, moving patients who are medically ill from state hospitals to more restrictive state prisons. Patients with schizophrenia should be surrounded by a mental health team, not by armed prison guards. The rate of recidivism among these individuals is extremely high because patients who are released often stop taking their medications and get re-arrested when their behavior deteriorates.
5. Suicide. The rate of suicide in the first year after FEP is astronomical. A recent study reported an unimaginably high suicide rate: 17,000% higher than that of the general population.16 Many patients with FEP commit suicide after they stop taking their antipsychotic medication, and often no antipsychotic medication is detected in their postmortem blood samples.
6. Homelessness. A disproportionate number of patients with schizophrenia become homeless.17 It started in the 1980s, when the shuttering of state hospitals began and patients with chronic illnesses were released into the community to fend for themselves. Many perished. Others became homeless, living on the streets of urban areas.
7. Early mortality. Schizophrenia has repeatedly been shown to be associated with early mortality, with a loss of approximately 25 potential years of life.17 This is attributed to lifestyle risk factors (eg, sedentary living, poor diet) and multiple medical comorbidities (eg, obesity, diabetes, hypertension). To make things worse, patients with schizophrenia do not receive basic medical care to protect them from cardiovascular morbidity, an appalling disparity of care.18 Interestingly, a recent 7-year follow-up study of patients with schizophrenia found that the lowest rate of mortality from all causes was among patients receiving a second-generation LAI.19 Relapse prevention with LAIs can reduce mortality! According to that study, the worst mortality rate was observed in patients with schizophrenia who were not receiving any antipsychotic medication.
Continue to: Posttraumatic stress disorder
8. Posttraumatic stress disorder (PTSD). Many studies report that psychosis triggers PTSD symptoms20 because delusions and hallucinations can represent a life-threatening experience. The symptoms of PTSD get embedded within the positive and negative symptoms of schizophrenia, and every psychotic relapse serves as a “booster shot” for PTSD, leading to depression, anxiety, personality changes, aggressive behavior, and suicide.
9. Hopelessness, depression, and demoralization. The stigma of a severe psychiatric brain disorder such as schizophrenia, with multiple episodes, disability, incarceration, and homelessness, extends to the patients themselves, who become hopeless and demoralized by a chronic illness that marginalizes them into desperately ill individuals.21 The more psychotic episodes, the more intense the demoralization, hopelessness, and depression.
10. Family burden. The repercussions of psychotic relapses after FEP leads to significant financial and emotional stress on patients’ families.22 The heavy burden of caregiving among family members can be highly distressing, leading to depression and medical illness due to compromised immune functions.
Preventing relapse: It is not rocket science
It is obvious that the single most important therapeutic action for patients with schizophrenia is to prevent psychotic relapses. Even partial nonadherence must be prevented, because a drop of 25% in a patient’s serum antipsychotic level has been reported to lead to a psychotic relapse.23 Preventing relapse after FEP is not rocket science: Switch the patient to an LAI before discharge from the hospital,24 and provide the clinically necessary psychosocial treatments at every monthly follow-up visit (supportive psychotherapy, social skill training, vocational rehabilitation, and cognitive remediation). I have witnessed firsthand how stable and functional a patient who has had FEP can become when started on a second-generation LAI very soon after the onset of the illness.
I will finish with a simple question to my clinician readers: given the many devastating consequences of psychotic relapses, what would you do for your young patient with FEP? I hope you will treat them like a family member, and protect them from brain atrophy, disability, incarceration, homelessness, and suicide by starting them on an LAI antipsychotic before they leave the hospital. We must do no less for this highly vulnerable, young patient population.
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449-468.
2. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
3. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
4. Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021:S2215-0366(21)00039-0. doi: 10.1016/S2215-0366(21)00039-0
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
7. Lei W, Kirkpatrick B, Wang Q, et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res Neuroimaging. 2019;288:12-20.
8. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
9. Köhler-Forsberg O, Müller N, Lennox BR. Editorial: The role of inflammation in the etiology and treatment of schizophrenia. Front Psychiatry. 2020;11:603296. doi: 10.3389/fpsyt.2020.603296
10. Noto C, Ota VK, Gadelha A, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210-216.
11. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
12. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
13. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
14. Weye N, Santomauro DF, Agerbo E, et al. Register-based metrics of years lived with disability associated with mental and substance use disorders: a register-based cohort study in Denmark. Lancet Psychiatry. 2021;8(4):310-319.
15. Kirchebner J, Günther MP, Lau S. Identifying influential factors distinguishing recidivists among offender patients with a diagnosis of schizophrenia via machine learning algorithms. Forensic Sci Int. 2020;315:110435. doi: 10.1016/j.forsciint.2020.110435
16. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
17. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
18. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Seedat S, Stein MB, Oosthuizen PP, et al. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J Nerv Ment Dis. 2003;191(10):675-681.
21. Berardelli I, Sarubbi S, Rogante E, et al. The role of demoralization and hopelessness in suicide risk in schizophrenia: A review of the literature. Medicina (Kaunas). 2019;55(5):200.
22. Khalil SA, Elbatrawy AN, Saleh NM, et al. The burden of care and burn out syndrome in caregivers of an Egyptian sample of schizophrenia patients. Int J Soc Psychiatry. 2021;10. doi: 10.1177/0020764021993155
23. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
24. Garner KN, Nasrallah HA. Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33-45.
It breaks my heart every time young patients with functional disability and a history of several psychotic episodes are referred to me. It makes me wonder why they weren’t protected from a lifetime of disability with the use of one of the FDA-approved long-acting injectable (LAI) antipsychotics right after discharge from their initial hospitalization for first-episode psychosis (FEP).
Two decades ago, psychiatric research discovered that psychotic episodes are neurotoxic and neurodegenerative, with grave consequences for the brain if they recur. Although many clinicians are aware of the high rate of nonadherence in patients with schizophrenia—which inevitably leads to a psychotic relapse—the vast majority (>99%, in my estimate) never prescribe an LAI after the FEP to guarantee full adherence and protect the patient’s brain from further atrophy due to relapses. The overall rate of LAI antipsychotic use is astonishingly low (approximately 10%), despite the neurologic malignancy of psychotic episodes. Further, LAIs are most often used after a patient has experienced multiple psychotic episodes, at which point the patient has already lost a significant amount of brain tissue and has already descended into a life of permanent disability.
Oral antipsychotics have the same efficacy as their LAI counterparts, and certainly should be used initially in the hospital during FEP to ascertain the absence of an allergic reaction after initial exposure, and to establish tolerability. Inpatient nurses are experts at making sure a reluctant patient actually swallows the pills and does not cheek them to spit them out later. So patients who have had FEP do improve with oral medications in the hospital, but all bets are off that those patients will regularly ingest tablets every day after discharge. Studies show patients have a high rate of nonadherence within days or weeks after leaving the hospital for FEP.1 This leads to repetitive psychotic relapses and rehospitalizations, with dire consequences for young patients with schizophrenia—a very serious brain disorder that had been labeled “the worst disease of mankind”2 in the era before studies showed LAI second-generation antipsychotics for FEP had remarkable rates of relapse prevention and recovery.3,4
Psychiatrists should approach FEP the same way oncologists approach cancer when it is diagnosed as Stage 1. Oncologists immediately take action to prevent the recurrence of the patient’s cancer with chemotherapy and/or radiation therapy, and do not wait for the cancer to advance to Stage 4, with widespread metastasis, before administering these potentially life-saving therapies (despite their toxic adverse effects). In schizophrenia, functional disability is the equivalent of Stage 4 cancer and should be aggressively prevented by using LAIs at the time of initial diagnosis, which is Stage 1 schizophrenia. Knowing the grave consequences of psychotic relapses, there is no logical reason whatsoever not to switch patients who have had FEP to an LAI before they are discharged from the hospital. A well-known study by a UCLA research group that compared patients who had FEP and were assigned to oral vs LAI antipsychotics at the time of discharge reported a stunning difference at the end of 1 year: a 650% higher relapse rate among the oral medication group compared with the LAI group!5 In light of such a massive difference, wouldn’t psychiatrists want to treat their sons or daughters with an LAI antipsychotic right after FEP? I certainly would, and I have always believed in treating every patient like a family member.
Catastrophic consequences
This lack of early intervention with LAI antipsychotics following FEP is the main reason schizophrenia is associated with poor clinical and functional outcomes. Patients are prescribed pills that they often take erratically or not at all, and end up relapsing repeatedly, with multiple catastrophic consequences, such as:
1. Brain tissue loss. Until recently, psychiatry did not know that psychosis destroys gray and white matter in the brain and causes progressive brain atrophy with every psychotic relapse.6,7 The neurotoxicity of psychosis is attributed to 2 destructive processes: neuroinflammation8,9 and free radicals.10 Approximately 11 cc of brain tissue is lost during FEP and with every subsequent relapse.6 Simple math shows that after 3 to 5 relapses, patients’ brains will shrink by 35 cc to 60 cc. No wonder recurrent psychoses lead to a life of permanent disability. As I have said in a past editorial,11 just as cardiologists do everything they can to prevent a second myocardial infarction (“heart attack”), psychiatrists must do the same to prevent a second psychotic episode (“brain attack”).
2. Treatment resistance. With each psychotic episode, the low antipsychotic dose that worked well in FEP is no longer enough and must be increased. The neurodegenerative effects of psychosis implies that the brain structure changes with each episode. Higher and higher doses become necessary with every psychotic recurrence, and studies show that approximately 1 in 8 patients may stop responding altogether after a psychotic relapse.12
Continue to: Disability
3. Disability. Functional disability, both vocational and social, usually begins after the second psychotic episode, which is why it is so important to prevent the second episode.13 Patients usually must drop out of high school or college or quit the job they held before FEP. Most patients with multiple psychotic episodes will never be able to work, get married, have children, live independently, or develop a circle of friends. Disability in schizophrenia is essentially a functional death sentence.14
4. Incarceration and criminalization. So many of our patients with schizophrenia get arrested when they become psychotic and behave erratically due to delusions, hallucinations, or both. They typically are taken to jail instead of a hospital because almost all the state hospitals around the country have been closed. It is outrageous that a medical condition of the brain leads to criminalization of patients with schizophrenia.15 The only solution for this ongoing crisis of incarceration of our patients with schizophrenia is to prevent them from relapsing into psychosis. The so-called deinstitutionalization movement has mutated into trans-institutionalization, moving patients who are medically ill from state hospitals to more restrictive state prisons. Patients with schizophrenia should be surrounded by a mental health team, not by armed prison guards. The rate of recidivism among these individuals is extremely high because patients who are released often stop taking their medications and get re-arrested when their behavior deteriorates.
5. Suicide. The rate of suicide in the first year after FEP is astronomical. A recent study reported an unimaginably high suicide rate: 17,000% higher than that of the general population.16 Many patients with FEP commit suicide after they stop taking their antipsychotic medication, and often no antipsychotic medication is detected in their postmortem blood samples.
6. Homelessness. A disproportionate number of patients with schizophrenia become homeless.17 It started in the 1980s, when the shuttering of state hospitals began and patients with chronic illnesses were released into the community to fend for themselves. Many perished. Others became homeless, living on the streets of urban areas.
7. Early mortality. Schizophrenia has repeatedly been shown to be associated with early mortality, with a loss of approximately 25 potential years of life.17 This is attributed to lifestyle risk factors (eg, sedentary living, poor diet) and multiple medical comorbidities (eg, obesity, diabetes, hypertension). To make things worse, patients with schizophrenia do not receive basic medical care to protect them from cardiovascular morbidity, an appalling disparity of care.18 Interestingly, a recent 7-year follow-up study of patients with schizophrenia found that the lowest rate of mortality from all causes was among patients receiving a second-generation LAI.19 Relapse prevention with LAIs can reduce mortality! According to that study, the worst mortality rate was observed in patients with schizophrenia who were not receiving any antipsychotic medication.
Continue to: Posttraumatic stress disorder
8. Posttraumatic stress disorder (PTSD). Many studies report that psychosis triggers PTSD symptoms20 because delusions and hallucinations can represent a life-threatening experience. The symptoms of PTSD get embedded within the positive and negative symptoms of schizophrenia, and every psychotic relapse serves as a “booster shot” for PTSD, leading to depression, anxiety, personality changes, aggressive behavior, and suicide.
9. Hopelessness, depression, and demoralization. The stigma of a severe psychiatric brain disorder such as schizophrenia, with multiple episodes, disability, incarceration, and homelessness, extends to the patients themselves, who become hopeless and demoralized by a chronic illness that marginalizes them into desperately ill individuals.21 The more psychotic episodes, the more intense the demoralization, hopelessness, and depression.
10. Family burden. The repercussions of psychotic relapses after FEP leads to significant financial and emotional stress on patients’ families.22 The heavy burden of caregiving among family members can be highly distressing, leading to depression and medical illness due to compromised immune functions.
Preventing relapse: It is not rocket science
It is obvious that the single most important therapeutic action for patients with schizophrenia is to prevent psychotic relapses. Even partial nonadherence must be prevented, because a drop of 25% in a patient’s serum antipsychotic level has been reported to lead to a psychotic relapse.23 Preventing relapse after FEP is not rocket science: Switch the patient to an LAI before discharge from the hospital,24 and provide the clinically necessary psychosocial treatments at every monthly follow-up visit (supportive psychotherapy, social skill training, vocational rehabilitation, and cognitive remediation). I have witnessed firsthand how stable and functional a patient who has had FEP can become when started on a second-generation LAI very soon after the onset of the illness.
I will finish with a simple question to my clinician readers: given the many devastating consequences of psychotic relapses, what would you do for your young patient with FEP? I hope you will treat them like a family member, and protect them from brain atrophy, disability, incarceration, homelessness, and suicide by starting them on an LAI antipsychotic before they leave the hospital. We must do no less for this highly vulnerable, young patient population.
It breaks my heart every time young patients with functional disability and a history of several psychotic episodes are referred to me. It makes me wonder why they weren’t protected from a lifetime of disability with the use of one of the FDA-approved long-acting injectable (LAI) antipsychotics right after discharge from their initial hospitalization for first-episode psychosis (FEP).
Two decades ago, psychiatric research discovered that psychotic episodes are neurotoxic and neurodegenerative, with grave consequences for the brain if they recur. Although many clinicians are aware of the high rate of nonadherence in patients with schizophrenia—which inevitably leads to a psychotic relapse—the vast majority (>99%, in my estimate) never prescribe an LAI after the FEP to guarantee full adherence and protect the patient’s brain from further atrophy due to relapses. The overall rate of LAI antipsychotic use is astonishingly low (approximately 10%), despite the neurologic malignancy of psychotic episodes. Further, LAIs are most often used after a patient has experienced multiple psychotic episodes, at which point the patient has already lost a significant amount of brain tissue and has already descended into a life of permanent disability.
Oral antipsychotics have the same efficacy as their LAI counterparts, and certainly should be used initially in the hospital during FEP to ascertain the absence of an allergic reaction after initial exposure, and to establish tolerability. Inpatient nurses are experts at making sure a reluctant patient actually swallows the pills and does not cheek them to spit them out later. So patients who have had FEP do improve with oral medications in the hospital, but all bets are off that those patients will regularly ingest tablets every day after discharge. Studies show patients have a high rate of nonadherence within days or weeks after leaving the hospital for FEP.1 This leads to repetitive psychotic relapses and rehospitalizations, with dire consequences for young patients with schizophrenia—a very serious brain disorder that had been labeled “the worst disease of mankind”2 in the era before studies showed LAI second-generation antipsychotics for FEP had remarkable rates of relapse prevention and recovery.3,4
Psychiatrists should approach FEP the same way oncologists approach cancer when it is diagnosed as Stage 1. Oncologists immediately take action to prevent the recurrence of the patient’s cancer with chemotherapy and/or radiation therapy, and do not wait for the cancer to advance to Stage 4, with widespread metastasis, before administering these potentially life-saving therapies (despite their toxic adverse effects). In schizophrenia, functional disability is the equivalent of Stage 4 cancer and should be aggressively prevented by using LAIs at the time of initial diagnosis, which is Stage 1 schizophrenia. Knowing the grave consequences of psychotic relapses, there is no logical reason whatsoever not to switch patients who have had FEP to an LAI before they are discharged from the hospital. A well-known study by a UCLA research group that compared patients who had FEP and were assigned to oral vs LAI antipsychotics at the time of discharge reported a stunning difference at the end of 1 year: a 650% higher relapse rate among the oral medication group compared with the LAI group!5 In light of such a massive difference, wouldn’t psychiatrists want to treat their sons or daughters with an LAI antipsychotic right after FEP? I certainly would, and I have always believed in treating every patient like a family member.
Catastrophic consequences
This lack of early intervention with LAI antipsychotics following FEP is the main reason schizophrenia is associated with poor clinical and functional outcomes. Patients are prescribed pills that they often take erratically or not at all, and end up relapsing repeatedly, with multiple catastrophic consequences, such as:
1. Brain tissue loss. Until recently, psychiatry did not know that psychosis destroys gray and white matter in the brain and causes progressive brain atrophy with every psychotic relapse.6,7 The neurotoxicity of psychosis is attributed to 2 destructive processes: neuroinflammation8,9 and free radicals.10 Approximately 11 cc of brain tissue is lost during FEP and with every subsequent relapse.6 Simple math shows that after 3 to 5 relapses, patients’ brains will shrink by 35 cc to 60 cc. No wonder recurrent psychoses lead to a life of permanent disability. As I have said in a past editorial,11 just as cardiologists do everything they can to prevent a second myocardial infarction (“heart attack”), psychiatrists must do the same to prevent a second psychotic episode (“brain attack”).
2. Treatment resistance. With each psychotic episode, the low antipsychotic dose that worked well in FEP is no longer enough and must be increased. The neurodegenerative effects of psychosis implies that the brain structure changes with each episode. Higher and higher doses become necessary with every psychotic recurrence, and studies show that approximately 1 in 8 patients may stop responding altogether after a psychotic relapse.12
Continue to: Disability
3. Disability. Functional disability, both vocational and social, usually begins after the second psychotic episode, which is why it is so important to prevent the second episode.13 Patients usually must drop out of high school or college or quit the job they held before FEP. Most patients with multiple psychotic episodes will never be able to work, get married, have children, live independently, or develop a circle of friends. Disability in schizophrenia is essentially a functional death sentence.14
4. Incarceration and criminalization. So many of our patients with schizophrenia get arrested when they become psychotic and behave erratically due to delusions, hallucinations, or both. They typically are taken to jail instead of a hospital because almost all the state hospitals around the country have been closed. It is outrageous that a medical condition of the brain leads to criminalization of patients with schizophrenia.15 The only solution for this ongoing crisis of incarceration of our patients with schizophrenia is to prevent them from relapsing into psychosis. The so-called deinstitutionalization movement has mutated into trans-institutionalization, moving patients who are medically ill from state hospitals to more restrictive state prisons. Patients with schizophrenia should be surrounded by a mental health team, not by armed prison guards. The rate of recidivism among these individuals is extremely high because patients who are released often stop taking their medications and get re-arrested when their behavior deteriorates.
5. Suicide. The rate of suicide in the first year after FEP is astronomical. A recent study reported an unimaginably high suicide rate: 17,000% higher than that of the general population.16 Many patients with FEP commit suicide after they stop taking their antipsychotic medication, and often no antipsychotic medication is detected in their postmortem blood samples.
6. Homelessness. A disproportionate number of patients with schizophrenia become homeless.17 It started in the 1980s, when the shuttering of state hospitals began and patients with chronic illnesses were released into the community to fend for themselves. Many perished. Others became homeless, living on the streets of urban areas.
7. Early mortality. Schizophrenia has repeatedly been shown to be associated with early mortality, with a loss of approximately 25 potential years of life.17 This is attributed to lifestyle risk factors (eg, sedentary living, poor diet) and multiple medical comorbidities (eg, obesity, diabetes, hypertension). To make things worse, patients with schizophrenia do not receive basic medical care to protect them from cardiovascular morbidity, an appalling disparity of care.18 Interestingly, a recent 7-year follow-up study of patients with schizophrenia found that the lowest rate of mortality from all causes was among patients receiving a second-generation LAI.19 Relapse prevention with LAIs can reduce mortality! According to that study, the worst mortality rate was observed in patients with schizophrenia who were not receiving any antipsychotic medication.
Continue to: Posttraumatic stress disorder
8. Posttraumatic stress disorder (PTSD). Many studies report that psychosis triggers PTSD symptoms20 because delusions and hallucinations can represent a life-threatening experience. The symptoms of PTSD get embedded within the positive and negative symptoms of schizophrenia, and every psychotic relapse serves as a “booster shot” for PTSD, leading to depression, anxiety, personality changes, aggressive behavior, and suicide.
9. Hopelessness, depression, and demoralization. The stigma of a severe psychiatric brain disorder such as schizophrenia, with multiple episodes, disability, incarceration, and homelessness, extends to the patients themselves, who become hopeless and demoralized by a chronic illness that marginalizes them into desperately ill individuals.21 The more psychotic episodes, the more intense the demoralization, hopelessness, and depression.
10. Family burden. The repercussions of psychotic relapses after FEP leads to significant financial and emotional stress on patients’ families.22 The heavy burden of caregiving among family members can be highly distressing, leading to depression and medical illness due to compromised immune functions.
Preventing relapse: It is not rocket science
It is obvious that the single most important therapeutic action for patients with schizophrenia is to prevent psychotic relapses. Even partial nonadherence must be prevented, because a drop of 25% in a patient’s serum antipsychotic level has been reported to lead to a psychotic relapse.23 Preventing relapse after FEP is not rocket science: Switch the patient to an LAI before discharge from the hospital,24 and provide the clinically necessary psychosocial treatments at every monthly follow-up visit (supportive psychotherapy, social skill training, vocational rehabilitation, and cognitive remediation). I have witnessed firsthand how stable and functional a patient who has had FEP can become when started on a second-generation LAI very soon after the onset of the illness.
I will finish with a simple question to my clinician readers: given the many devastating consequences of psychotic relapses, what would you do for your young patient with FEP? I hope you will treat them like a family member, and protect them from brain atrophy, disability, incarceration, homelessness, and suicide by starting them on an LAI antipsychotic before they leave the hospital. We must do no less for this highly vulnerable, young patient population.
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449-468.
2. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
3. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
4. Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021:S2215-0366(21)00039-0. doi: 10.1016/S2215-0366(21)00039-0
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
7. Lei W, Kirkpatrick B, Wang Q, et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res Neuroimaging. 2019;288:12-20.
8. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
9. Köhler-Forsberg O, Müller N, Lennox BR. Editorial: The role of inflammation in the etiology and treatment of schizophrenia. Front Psychiatry. 2020;11:603296. doi: 10.3389/fpsyt.2020.603296
10. Noto C, Ota VK, Gadelha A, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210-216.
11. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
12. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
13. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
14. Weye N, Santomauro DF, Agerbo E, et al. Register-based metrics of years lived with disability associated with mental and substance use disorders: a register-based cohort study in Denmark. Lancet Psychiatry. 2021;8(4):310-319.
15. Kirchebner J, Günther MP, Lau S. Identifying influential factors distinguishing recidivists among offender patients with a diagnosis of schizophrenia via machine learning algorithms. Forensic Sci Int. 2020;315:110435. doi: 10.1016/j.forsciint.2020.110435
16. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
17. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
18. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Seedat S, Stein MB, Oosthuizen PP, et al. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J Nerv Ment Dis. 2003;191(10):675-681.
21. Berardelli I, Sarubbi S, Rogante E, et al. The role of demoralization and hopelessness in suicide risk in schizophrenia: A review of the literature. Medicina (Kaunas). 2019;55(5):200.
22. Khalil SA, Elbatrawy AN, Saleh NM, et al. The burden of care and burn out syndrome in caregivers of an Egyptian sample of schizophrenia patients. Int J Soc Psychiatry. 2021;10. doi: 10.1177/0020764021993155
23. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
24. Garner KN, Nasrallah HA. Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33-45.
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449-468.
2. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
3. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
4. Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021:S2215-0366(21)00039-0. doi: 10.1016/S2215-0366(21)00039-0
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
7. Lei W, Kirkpatrick B, Wang Q, et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res Neuroimaging. 2019;288:12-20.
8. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
9. Köhler-Forsberg O, Müller N, Lennox BR. Editorial: The role of inflammation in the etiology and treatment of schizophrenia. Front Psychiatry. 2020;11:603296. doi: 10.3389/fpsyt.2020.603296
10. Noto C, Ota VK, Gadelha A, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210-216.
11. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
12. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
13. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
14. Weye N, Santomauro DF, Agerbo E, et al. Register-based metrics of years lived with disability associated with mental and substance use disorders: a register-based cohort study in Denmark. Lancet Psychiatry. 2021;8(4):310-319.
15. Kirchebner J, Günther MP, Lau S. Identifying influential factors distinguishing recidivists among offender patients with a diagnosis of schizophrenia via machine learning algorithms. Forensic Sci Int. 2020;315:110435. doi: 10.1016/j.forsciint.2020.110435
16. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
17. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
18. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Seedat S, Stein MB, Oosthuizen PP, et al. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J Nerv Ment Dis. 2003;191(10):675-681.
21. Berardelli I, Sarubbi S, Rogante E, et al. The role of demoralization and hopelessness in suicide risk in schizophrenia: A review of the literature. Medicina (Kaunas). 2019;55(5):200.
22. Khalil SA, Elbatrawy AN, Saleh NM, et al. The burden of care and burn out syndrome in caregivers of an Egyptian sample of schizophrenia patients. Int J Soc Psychiatry. 2021;10. doi: 10.1177/0020764021993155
23. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
24. Garner KN, Nasrallah HA. Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33-45.
‘Canceling’ obsolete terms
I wanted to thank Dr. Nasrallah for his most important editorial, “Let’s ‘cancel’ these obsolete terms in DSM” (From the Editor,
Robert Barris, MD
Nassau University Medical Center
East Meadow, New York
How sad! This is my reaction to reading Dr. Nasrallah’s January 2021 editorial. Although biological psychiatry is synonymous with brain neurotransmitters and psychopharmacology, absent from this perspective is the visible biology of the human organism, specifically Sigmund Freud’s discovery of the psychosexual development of the infant and child and Wilhelm Reich’s discovery of characterological and muscular armor. Medicine, a natural science, is founded and grounded in observation. Psychiatry, having ignored and eliminated (“canceled”) recognition of these readily observable phenomena essential to understanding psychiatric disorders, including neurosis and schizophrenia, allows Dr. Nasrallah to suggest we “cancel” what should be at the heart of psychiatric diagnosis and treatment. Sadly, this heart has been lost for decades.
Howard Chavis, MD
New York, New York
Dr. Nasrallah responds
Psychiatry, like all medical and scientific disciplines, must go through an ongoing renewal, including the update of its terminology, with or without a change in its concepts or principles. Anxiety is a more accurate description of clinical symptoms than neurosis, and psychosis spectrum is more accurate than schizophrenia. Besides the accuracy issue, “neurotic” and “schizophrenic” have unfortunately devolved into pejorative and stigmatizing terms. The lexicon of psychiatry has gone through seismic changes over the past several decades, as I described in a previous editorial.1 Psychiatry is a vibrant, constantly evolving biopsychosocial/clinical neuroscience, not a static descriptive discipline.
Reference
1. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
I found myself having difficulty with Dr. Nasrallah’s editorial about canceling “obsolete” terms. I agree that making a diagnosis of borderline or narcissistic personality disorder can be pejorative if the clinician is using it to manage their own unprocessed countertransference. While all behavior is brain-mediated, human behavior is influenced by psychological events great and small. I am concerned that you seem to be reducing personality trait disturbances to biological abnormality, pure and simple. Losing psychological understanding of patients while overexplaining behavior as pathological brain dysfunction risks losing why patients see us in the first place.
Michael Friedman, DO
Cherry Hill, New Jersey
Dr. Nasrallah responds
The renaming I suggest goes beyond countertransference. It has to do with scientific validity of the diagnostic construct. And yes, personality traits are heavily genetic, but with some modulation by environmental factors. I suggest reading the seminal works of Thomas J. Bouchard Jr., PhD, and Kenneth S. Kendler, MD, on identical twins reared together or apart for more details about the genetics of personality traits.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
I wanted to thank Dr. Nasrallah for his most important editorial, “Let’s ‘cancel’ these obsolete terms in DSM” (From the Editor,
Robert Barris, MD
Nassau University Medical Center
East Meadow, New York
How sad! This is my reaction to reading Dr. Nasrallah’s January 2021 editorial. Although biological psychiatry is synonymous with brain neurotransmitters and psychopharmacology, absent from this perspective is the visible biology of the human organism, specifically Sigmund Freud’s discovery of the psychosexual development of the infant and child and Wilhelm Reich’s discovery of characterological and muscular armor. Medicine, a natural science, is founded and grounded in observation. Psychiatry, having ignored and eliminated (“canceled”) recognition of these readily observable phenomena essential to understanding psychiatric disorders, including neurosis and schizophrenia, allows Dr. Nasrallah to suggest we “cancel” what should be at the heart of psychiatric diagnosis and treatment. Sadly, this heart has been lost for decades.
Howard Chavis, MD
New York, New York
Dr. Nasrallah responds
Psychiatry, like all medical and scientific disciplines, must go through an ongoing renewal, including the update of its terminology, with or without a change in its concepts or principles. Anxiety is a more accurate description of clinical symptoms than neurosis, and psychosis spectrum is more accurate than schizophrenia. Besides the accuracy issue, “neurotic” and “schizophrenic” have unfortunately devolved into pejorative and stigmatizing terms. The lexicon of psychiatry has gone through seismic changes over the past several decades, as I described in a previous editorial.1 Psychiatry is a vibrant, constantly evolving biopsychosocial/clinical neuroscience, not a static descriptive discipline.
Reference
1. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
I found myself having difficulty with Dr. Nasrallah’s editorial about canceling “obsolete” terms. I agree that making a diagnosis of borderline or narcissistic personality disorder can be pejorative if the clinician is using it to manage their own unprocessed countertransference. While all behavior is brain-mediated, human behavior is influenced by psychological events great and small. I am concerned that you seem to be reducing personality trait disturbances to biological abnormality, pure and simple. Losing psychological understanding of patients while overexplaining behavior as pathological brain dysfunction risks losing why patients see us in the first place.
Michael Friedman, DO
Cherry Hill, New Jersey
Dr. Nasrallah responds
The renaming I suggest goes beyond countertransference. It has to do with scientific validity of the diagnostic construct. And yes, personality traits are heavily genetic, but with some modulation by environmental factors. I suggest reading the seminal works of Thomas J. Bouchard Jr., PhD, and Kenneth S. Kendler, MD, on identical twins reared together or apart for more details about the genetics of personality traits.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
I wanted to thank Dr. Nasrallah for his most important editorial, “Let’s ‘cancel’ these obsolete terms in DSM” (From the Editor,
Robert Barris, MD
Nassau University Medical Center
East Meadow, New York
How sad! This is my reaction to reading Dr. Nasrallah’s January 2021 editorial. Although biological psychiatry is synonymous with brain neurotransmitters and psychopharmacology, absent from this perspective is the visible biology of the human organism, specifically Sigmund Freud’s discovery of the psychosexual development of the infant and child and Wilhelm Reich’s discovery of characterological and muscular armor. Medicine, a natural science, is founded and grounded in observation. Psychiatry, having ignored and eliminated (“canceled”) recognition of these readily observable phenomena essential to understanding psychiatric disorders, including neurosis and schizophrenia, allows Dr. Nasrallah to suggest we “cancel” what should be at the heart of psychiatric diagnosis and treatment. Sadly, this heart has been lost for decades.
Howard Chavis, MD
New York, New York
Dr. Nasrallah responds
Psychiatry, like all medical and scientific disciplines, must go through an ongoing renewal, including the update of its terminology, with or without a change in its concepts or principles. Anxiety is a more accurate description of clinical symptoms than neurosis, and psychosis spectrum is more accurate than schizophrenia. Besides the accuracy issue, “neurotic” and “schizophrenic” have unfortunately devolved into pejorative and stigmatizing terms. The lexicon of psychiatry has gone through seismic changes over the past several decades, as I described in a previous editorial.1 Psychiatry is a vibrant, constantly evolving biopsychosocial/clinical neuroscience, not a static descriptive discipline.
Reference
1. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
I found myself having difficulty with Dr. Nasrallah’s editorial about canceling “obsolete” terms. I agree that making a diagnosis of borderline or narcissistic personality disorder can be pejorative if the clinician is using it to manage their own unprocessed countertransference. While all behavior is brain-mediated, human behavior is influenced by psychological events great and small. I am concerned that you seem to be reducing personality trait disturbances to biological abnormality, pure and simple. Losing psychological understanding of patients while overexplaining behavior as pathological brain dysfunction risks losing why patients see us in the first place.
Michael Friedman, DO
Cherry Hill, New Jersey
Dr. Nasrallah responds
The renaming I suggest goes beyond countertransference. It has to do with scientific validity of the diagnostic construct. And yes, personality traits are heavily genetic, but with some modulation by environmental factors. I suggest reading the seminal works of Thomas J. Bouchard Jr., PhD, and Kenneth S. Kendler, MD, on identical twins reared together or apart for more details about the genetics of personality traits.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
Bright light therapy for bipolar depression: A review of 6 studies
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).
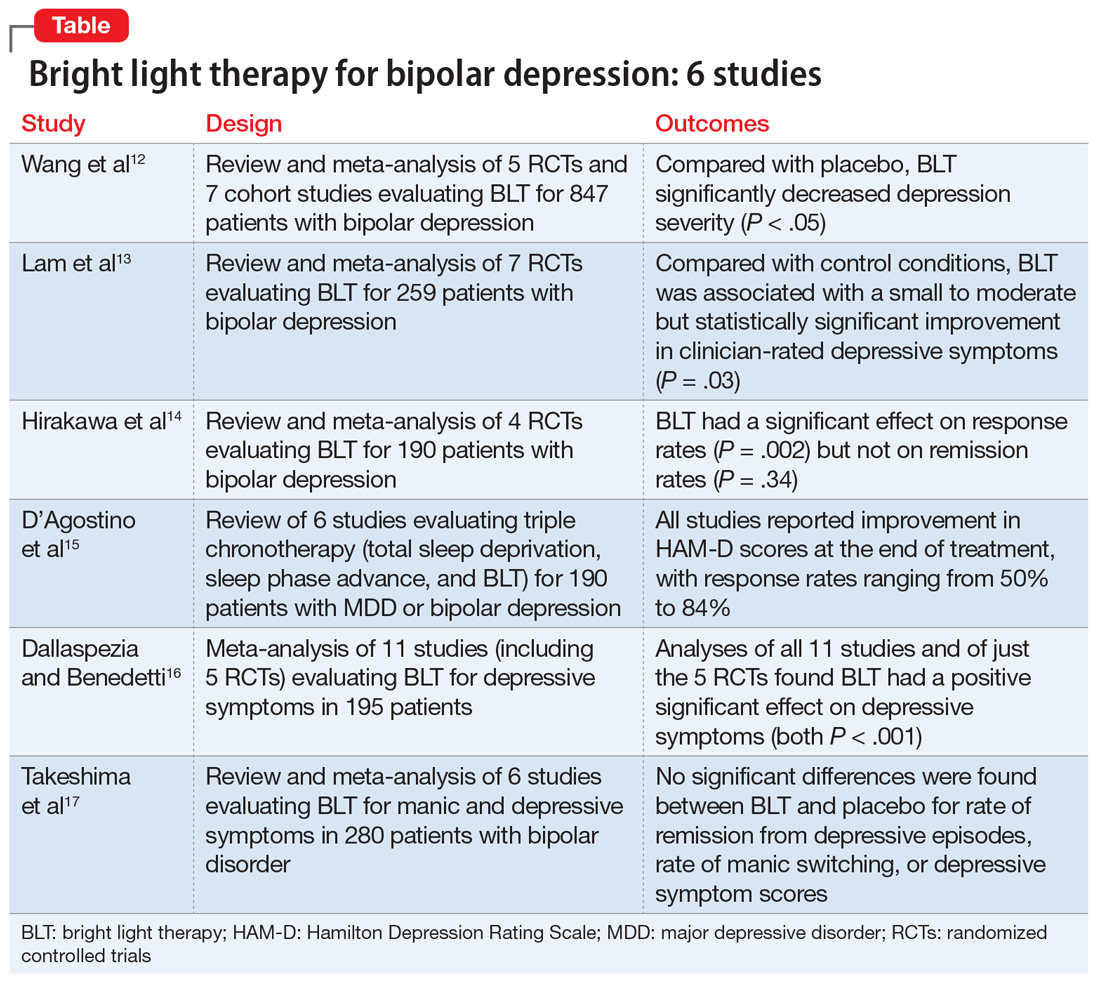
1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).

1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).

1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
ARISE to supportive psychotherapy
Supportive psychotherapy is a common type of therapy that often is used in combination with other modalities. By focusing on improving symptoms and accepting the patient’s limitations, it is particularly helpful for individuals who might have difficulty engaging in insight-oriented psychotherapies, such as those struggling with external stressors, including exposure to trauma, bereavement, physical disabilities, or socioeconomic challenges. Personal limitations, including severe personality disorder or intellectual disabilities, might also limit a patient’s ability to self-reflect on subconscious issues, which can lead to choosing a supportive modality.
While being supportive in the vernacular sense can be helpful, formal supportive psychotherapy employs well-defined goals and techniques.1 A therapist can facilitate progress by explicitly referring to these goals and techniques. The acronym ARISE can help therapists and other clinicians to use and appraise therapeutic progress toward these goals.
Alliance-building. The therapeutic alliance is an important predictor of the success of psychotherapy.2 Warmly encourage positive transference toward the therapist. The patient’s appreciation of the therapist’s empathic interactions can further the alliance. Paraphrasing the patient’s words can demonstrate and enhance empathy. Doing so allows clarification of the patient’s thoughts and helps the patient feel understood. Formulate and partner around shared therapeutic goals. Monitor the strength of the alliance and intervene if it is threatened. For example, if you misunderstand your patient and inadvertently offend them, apologizing may be helpful. In the face of disagreement between the patient and therapist, reorienting back to shared goals reinforces common ground.
Reduce anxiety and negative affect. In contrast to the caricature of the stiff psychoanalyst, the supportive therapist adopts an engaged conversational style to help the patient feel relaxed and to diminish the power differential between therapist and patient. If the patient appears uncomfortable with silence, maintaining the flow of conversation may reduce discomfort.1 Minimize your patient’s discomfort by approaching uncomfortable topics in manageable portions. Seek permission before introducing a subject that induces anxiety. Explain the reasoning behind approaching such topics.3 Reassurance and encouragement can further reduce anxiety.4 When not incongruous to the discussion, appropriate use of warm affect (eg, a smile) or even humor can elicit positive affect.
Increase awareness. Use psychoeducation and psychological interpretation (whether cognitive-behavioral or psychodynamic) to expand your patient’s awareness and help them understand their social contacts’ point of view. Clarification, gentle confrontation, and interpretation can make patients aware of biopsychosocial precipitants of distress.4
Strengthen coping mechanisms. Reinforce adaptive defense mechanisms, such as mature humor or suppression. Educating patients on practical organizational skills, problem-solving, relaxation techniques, and other relevant skills, can help them cope more effectively. Give advice only in limited circumstances, and when doing so, back up your advice with a rationale derived from your professional expertise. Because it is important for patients to realize that their life choices are their own, usually it is best to help the patient understand how they might come to their own decisions rather than to prescribe life choices in the form of advice.
Enhance self-esteem. Many patients in distress suffer from low self-esteem.5,6 Active encouragement and honest praise can nurture your patient’s ability to correct a distorted self-image and challenge self-reproach. Praise should not be false but reality-based. Praise can address preexisting strengths, highlight the patient’s willingness to express challenging material, or provide reinforcement on progress made toward treatment goals.
1. Rothe, EM. Supportive psychotherapy in everyday clinical practice: it’s like riding a bicycle. Psychiatric Times. Published May 24, 2017. Accessed April 12, 2021. https://www.psychiatrictimes.com/view/supportive-psychotherapy-everyday-clinical-practice-its-riding-bicycle
2. Flückiger C, Del Re AC, Wampold BE, et al. The alliance in adult psychotherapy: a meta-analytic synthesis. Psychotherapy (Chic). 2018;55(4):316-340.
3. Pine F. The interpretive moment. Variations on classical themes. Bull Menninger Clin. 1984;48(1), 54-71.
4. Grover S, Avasthi A, Jagiwala M. Clinical practice guidelines for practice of supportive psychotherapy. Indian J Psychiatry. 2020;62(Suppl 2):S173-S182.
5. Leary MR, Schreindorfer LS, Haupt AL. The role of low self-esteem in emotional and behavioral problems: why is low self-esteem dysfunctional? J Soc Clin Psychol. 1995;14(3):297-314.
6. Zahn R, Lythe KE, Gethin JA, et al. The role of self-blame and worthlessness in the psychopathology of major depressive disorder. J Affect Disord. 2015;186:337-341.
Supportive psychotherapy is a common type of therapy that often is used in combination with other modalities. By focusing on improving symptoms and accepting the patient’s limitations, it is particularly helpful for individuals who might have difficulty engaging in insight-oriented psychotherapies, such as those struggling with external stressors, including exposure to trauma, bereavement, physical disabilities, or socioeconomic challenges. Personal limitations, including severe personality disorder or intellectual disabilities, might also limit a patient’s ability to self-reflect on subconscious issues, which can lead to choosing a supportive modality.
While being supportive in the vernacular sense can be helpful, formal supportive psychotherapy employs well-defined goals and techniques.1 A therapist can facilitate progress by explicitly referring to these goals and techniques. The acronym ARISE can help therapists and other clinicians to use and appraise therapeutic progress toward these goals.
Alliance-building. The therapeutic alliance is an important predictor of the success of psychotherapy.2 Warmly encourage positive transference toward the therapist. The patient’s appreciation of the therapist’s empathic interactions can further the alliance. Paraphrasing the patient’s words can demonstrate and enhance empathy. Doing so allows clarification of the patient’s thoughts and helps the patient feel understood. Formulate and partner around shared therapeutic goals. Monitor the strength of the alliance and intervene if it is threatened. For example, if you misunderstand your patient and inadvertently offend them, apologizing may be helpful. In the face of disagreement between the patient and therapist, reorienting back to shared goals reinforces common ground.
Reduce anxiety and negative affect. In contrast to the caricature of the stiff psychoanalyst, the supportive therapist adopts an engaged conversational style to help the patient feel relaxed and to diminish the power differential between therapist and patient. If the patient appears uncomfortable with silence, maintaining the flow of conversation may reduce discomfort.1 Minimize your patient’s discomfort by approaching uncomfortable topics in manageable portions. Seek permission before introducing a subject that induces anxiety. Explain the reasoning behind approaching such topics.3 Reassurance and encouragement can further reduce anxiety.4 When not incongruous to the discussion, appropriate use of warm affect (eg, a smile) or even humor can elicit positive affect.
Increase awareness. Use psychoeducation and psychological interpretation (whether cognitive-behavioral or psychodynamic) to expand your patient’s awareness and help them understand their social contacts’ point of view. Clarification, gentle confrontation, and interpretation can make patients aware of biopsychosocial precipitants of distress.4
Strengthen coping mechanisms. Reinforce adaptive defense mechanisms, such as mature humor or suppression. Educating patients on practical organizational skills, problem-solving, relaxation techniques, and other relevant skills, can help them cope more effectively. Give advice only in limited circumstances, and when doing so, back up your advice with a rationale derived from your professional expertise. Because it is important for patients to realize that their life choices are their own, usually it is best to help the patient understand how they might come to their own decisions rather than to prescribe life choices in the form of advice.
Enhance self-esteem. Many patients in distress suffer from low self-esteem.5,6 Active encouragement and honest praise can nurture your patient’s ability to correct a distorted self-image and challenge self-reproach. Praise should not be false but reality-based. Praise can address preexisting strengths, highlight the patient’s willingness to express challenging material, or provide reinforcement on progress made toward treatment goals.
Supportive psychotherapy is a common type of therapy that often is used in combination with other modalities. By focusing on improving symptoms and accepting the patient’s limitations, it is particularly helpful for individuals who might have difficulty engaging in insight-oriented psychotherapies, such as those struggling with external stressors, including exposure to trauma, bereavement, physical disabilities, or socioeconomic challenges. Personal limitations, including severe personality disorder or intellectual disabilities, might also limit a patient’s ability to self-reflect on subconscious issues, which can lead to choosing a supportive modality.
While being supportive in the vernacular sense can be helpful, formal supportive psychotherapy employs well-defined goals and techniques.1 A therapist can facilitate progress by explicitly referring to these goals and techniques. The acronym ARISE can help therapists and other clinicians to use and appraise therapeutic progress toward these goals.
Alliance-building. The therapeutic alliance is an important predictor of the success of psychotherapy.2 Warmly encourage positive transference toward the therapist. The patient’s appreciation of the therapist’s empathic interactions can further the alliance. Paraphrasing the patient’s words can demonstrate and enhance empathy. Doing so allows clarification of the patient’s thoughts and helps the patient feel understood. Formulate and partner around shared therapeutic goals. Monitor the strength of the alliance and intervene if it is threatened. For example, if you misunderstand your patient and inadvertently offend them, apologizing may be helpful. In the face of disagreement between the patient and therapist, reorienting back to shared goals reinforces common ground.
Reduce anxiety and negative affect. In contrast to the caricature of the stiff psychoanalyst, the supportive therapist adopts an engaged conversational style to help the patient feel relaxed and to diminish the power differential between therapist and patient. If the patient appears uncomfortable with silence, maintaining the flow of conversation may reduce discomfort.1 Minimize your patient’s discomfort by approaching uncomfortable topics in manageable portions. Seek permission before introducing a subject that induces anxiety. Explain the reasoning behind approaching such topics.3 Reassurance and encouragement can further reduce anxiety.4 When not incongruous to the discussion, appropriate use of warm affect (eg, a smile) or even humor can elicit positive affect.
Increase awareness. Use psychoeducation and psychological interpretation (whether cognitive-behavioral or psychodynamic) to expand your patient’s awareness and help them understand their social contacts’ point of view. Clarification, gentle confrontation, and interpretation can make patients aware of biopsychosocial precipitants of distress.4
Strengthen coping mechanisms. Reinforce adaptive defense mechanisms, such as mature humor or suppression. Educating patients on practical organizational skills, problem-solving, relaxation techniques, and other relevant skills, can help them cope more effectively. Give advice only in limited circumstances, and when doing so, back up your advice with a rationale derived from your professional expertise. Because it is important for patients to realize that their life choices are their own, usually it is best to help the patient understand how they might come to their own decisions rather than to prescribe life choices in the form of advice.
Enhance self-esteem. Many patients in distress suffer from low self-esteem.5,6 Active encouragement and honest praise can nurture your patient’s ability to correct a distorted self-image and challenge self-reproach. Praise should not be false but reality-based. Praise can address preexisting strengths, highlight the patient’s willingness to express challenging material, or provide reinforcement on progress made toward treatment goals.
1. Rothe, EM. Supportive psychotherapy in everyday clinical practice: it’s like riding a bicycle. Psychiatric Times. Published May 24, 2017. Accessed April 12, 2021. https://www.psychiatrictimes.com/view/supportive-psychotherapy-everyday-clinical-practice-its-riding-bicycle
2. Flückiger C, Del Re AC, Wampold BE, et al. The alliance in adult psychotherapy: a meta-analytic synthesis. Psychotherapy (Chic). 2018;55(4):316-340.
3. Pine F. The interpretive moment. Variations on classical themes. Bull Menninger Clin. 1984;48(1), 54-71.
4. Grover S, Avasthi A, Jagiwala M. Clinical practice guidelines for practice of supportive psychotherapy. Indian J Psychiatry. 2020;62(Suppl 2):S173-S182.
5. Leary MR, Schreindorfer LS, Haupt AL. The role of low self-esteem in emotional and behavioral problems: why is low self-esteem dysfunctional? J Soc Clin Psychol. 1995;14(3):297-314.
6. Zahn R, Lythe KE, Gethin JA, et al. The role of self-blame and worthlessness in the psychopathology of major depressive disorder. J Affect Disord. 2015;186:337-341.
1. Rothe, EM. Supportive psychotherapy in everyday clinical practice: it’s like riding a bicycle. Psychiatric Times. Published May 24, 2017. Accessed April 12, 2021. https://www.psychiatrictimes.com/view/supportive-psychotherapy-everyday-clinical-practice-its-riding-bicycle
2. Flückiger C, Del Re AC, Wampold BE, et al. The alliance in adult psychotherapy: a meta-analytic synthesis. Psychotherapy (Chic). 2018;55(4):316-340.
3. Pine F. The interpretive moment. Variations on classical themes. Bull Menninger Clin. 1984;48(1), 54-71.
4. Grover S, Avasthi A, Jagiwala M. Clinical practice guidelines for practice of supportive psychotherapy. Indian J Psychiatry. 2020;62(Suppl 2):S173-S182.
5. Leary MR, Schreindorfer LS, Haupt AL. The role of low self-esteem in emotional and behavioral problems: why is low self-esteem dysfunctional? J Soc Clin Psychol. 1995;14(3):297-314.
6. Zahn R, Lythe KE, Gethin JA, et al. The role of self-blame and worthlessness in the psychopathology of major depressive disorder. J Affect Disord. 2015;186:337-341.
Management of patients with HCV who fail first line DAA regimens
Joseph K. Lim, MD, is the Director of Clinical Hepatology and Professor of Medicine in the Department of Medicine, Section of Digestive Diseases, Yale Liver Center, at the Yale University School of Medicine in New Haven, Connecticut. Dr. Lim's primary clinical and research interests are focused on viral hepatitis and non-alcoholic steatohepatitis.
What are some of the reasons for first line direct-acting antiviral (DAA) regimen failures in hepatitis C virus (HCV)?
Dr. Lim: In clinical practice, approximately 5% to 10% of patients will fail to achieve sustained virologic response (SVR). The most common reason is incompletion of treatment as a result of non-adherence, intolerance, adverse effects, or other medical/logistical factors that interfere with treatment. Another reason is reinfection, in which an individual who achieves viral eradication is re-exposed to HCV and develops a new infection. Each scenario warrants a careful evaluation to help identify which factors contributed to treatment failure.
Regarding incomplete treatment, it is important to identify other issues that require attention before considering retreatment. If there are other potential medications with potential drug-drug interaction (DDI) which influence the absorption of the DAA medications, that is important to identify (e.g. proton pump inhibitors).
Finally, if there are other non-medical reasons—psychosocial reasons, substance use reasons, or psychiatric reasons—that may prevent a patient from completing a full treatment course, it is important to identify and manage these before reconsidering DAA therapy.
What is the current standard of care for patients who failed a first line DAA regimen?
Dr. Lim: Fewer than 5% to 10% of patients treated with a first-line regimen fail to achieve a sustained virologic response (SVR). A significant proportion may develop resistance-associated substitutions (RASs) which may affect susceptibility to other DAA regimens, but fortunately, multiple studies have confirmed that retreatment of these patients with a contemporary DAA treatment regimen is associated with similarly high rates of SVR exceeding 90%.
The current guidelines by the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) suggest retreating these patients with a triple combination regimen of sofosbuvir, velpatasvir, plus voxilaprevir (also known as SOF/VEL/VOX) or glecaprevir plus pibrentasvir (GP).
Both are viable options for those who fail first line regimens, but with important nuances: 1) for patients who are genotype 1, either of these options is considered a valid regimen strategy; 2) for patients with genotype 2 through 6, the SOF/VEL/VOX combination is recommended; 3) for patients who have signature mutations for either the protease or the nonstructural protein 5A (NS5A), an individualized approach is needed to determine which strategy will be most efficacious; 4) for patients who have cirrhosis, and particularly those with decompensated cirrhosis, protease inhibitor-based combinations are contraindicated because of the risk of liver toxicity and/or hepatic decompensation, which is associated with an FDA black box warning.
Treatment of the very small number of patients who fail the second-line regimen with either GP or SOF/VEL/VOXis an area of significant controversy, and for which the evidence-based guidance in 2021 is quite limited. However, the AASLD and the IDSA recommend 2 potential regimens, including SOF/VEL/VOX plus weight-based ribavirin for 24 weeks, or sofosbuvir plus GP plus weight-based ribavirin for 16 weeks.
Why can it be difficult to differentiate true virological failure from relapse caused by non-adherence or from reinfection?
Dr. Lim: When I speak with patients who have failed DAAs, the majority state that they took every single pill. And although that leaves us in a conundrum where we cannot chalk it up to possible non-adherence, we do wish to determine whether the timing of when they took the medications was consistent.
We ask about whether there are other medications that may interact with these medications. Before we start treatment, we work with a pharmacist and online drug interaction websites to make sure that we do our best to avoid other medications that may interact with DAAs and impact the chance of achieving sustained virologic response (SVR). Despite those efforts, on occasion, patients report that they forgot they were taking a drug or supplement that could interfere with DAA absorption.
Lastly, in patients who offered no history of taking other medications and a report of 100% adherence, we make a presumption that this is probably true virological failure with development of virologic resistance. In 2021, AASLD/IDSA guidelines do not routinely recommend resistance testing for all patients who fail a first-line regimen because it frequently does not influence our decision on retreatment and may not always impact susceptibility to SOF/VEL/VOX or GP. However, in patients who have a history of multiple lines of treatment failure, some of whom have failed 4 or more previous treatment regimens, I do perform resistance testing on an individualized basis to inform our retreatment strategy.
With regards to the question about reinfection, there is no routine way this is assessed in clinical practice. From a public health perspective, we can perform phylogenetic testing, which can help distinguish the very specific viral strains to connect individuals in terms of the root of the infection. But in clinical practice, we do not use phylogenetic testing because if a patient’s initial genotype was genotype 1 and then they get reinfected, and their genotype was genotype 3, you do not need any special testing. At that point you know it is a different virus than what you initially had, and so that is the easiest way to make a distinction. If they have a different genotype of HCV the second time around, we generally will conclude that it represents reinfection rather than virologic relapse, although conversely, the discovery of the same genotype does not exclude reinfection.
Ninety-five percent of individuals treated with DAA agents are cured of HCV infection. Of the 5% who failed first line DAA regimens, what are their options and chances for being cured?
Dr. Lim: Again, retreatment with either SOF/VEL/VOX or GP is associated with a very high rate of SVR. Specifically, for SOF/VEL/VOX, there are two phase 3 clinical trials. One is POLARIS-1 for genotypes 1 through 6 and the other is POLARIS-4 for those with genotype 1 through 4. In both of those clinical trials, they reported a 96% to 97% chance of achieving SVR.1, 2 For GP, the MAGELLAN-1 protocol looked at patients, specifically genotype 1, and found that GP was associated with a 96% chance of SVR.3 As such, there is robust prospective RCT evidence validating the safety and efficacy of both SOF/VEL/VOX and GP for patients who fail first line DAA regimens.
How do people with HIV and persons who use drugs factor into DAA failures, and how does it affect them? How can their unique needs be met, and what special considerations can be made for them going forward?
Dr. Lim: Historically, HIV was viewed within our community as a “special population.” And the reason why was that with some older regimens, including those requiring pegylated interferon, the chance of achieving SVR was about 20% lower than those who had HCV monoinfection. But with our contemporary DAA regimens, that differential has washed away. The expected sustained virologic response rates of those with HCV alone or with coinfection of HIV is identical, around 95%. However, from a reinfection perspective, the available data suggests no difference in rates of virologic relapse or virologic failure or treatment failure or the risk of reinfection in HIV coinfected individuals. However, patients with HIV are taking antiretroviral therapy (ART) regimens that are associated with potential DDIs, which require special attention. On occasion, modification of the ART is needed to permit safe administration of HCV DAAs. In contrast, persons who inject drugs (PWID) remain a special population because of unique challenges and considerations required in the decision of who to treat, when to treat, and how to treat.
In terms of who to treat, there has been a paradigm shift. In the past, we would want to delay treatment until patients were drug-free for 6 months or greater. Many of our current insurance policies still mandate that patients be confirmed to be drug-free for a required amount of time before they will authorize the drug's release from the pharmacy. But at this time, that paradigm has shifted to where we, as a liver community, view HCV treatment as prevention. The concept is that within injection drug communities, if we can treat and eradicate HCV in super users of injection drugs, not only does it benefit that individual patient, but it may also benefit their community of injection drug users and prevent spread to others.
It is a high priority in 2021 that clinicians within the GI, liver, and infectious disease communities are willing to treat patients who are actively injecting drugs or in the process of going through relapse prevention, and/or rehabilitation. We must accept that persons who inject drugs may experience relapse to substance use which may be associated with HCV reinfection rates as high as 10% to 15% of cases. While these numbers are significant in my view, from a public health perspective and a clinical perspective, this should not dissuade clinicians from considering an individualized approach to offering DAA therapy to all patients, including those who are PWID.
- Safety and Efficacy of Sofosbuvir/Velpatasvir/Voxilaprevir in Adults with Chronic HCV Infection who have Previously Received Treatment with Direct-Acting Antiviral Therapy (POLARIS-1). Accessed- https://clinicaltrials.gov/ct2/show/NCT02607735
- Safety and Efficacy of SOF/VEL/VOX FDC for 12 Weeks and SOF/VEL for 12 Weeks in DAA-Experienced Adults with Chronic HCV Infection who have not Received an NS5A Inhibitor (POLARIS-4). Accessed- https://clinicaltrials.gov/ct2/show/NCT02639247
- Glecaprevir-Pibrentasvir (Mavyret). Accessed- https://www.hepatitisc.uw.edu/page/treatment/drugs/glecaprevir-pibrentasvir/clinical-trials
Joseph K. Lim, MD, is the Director of Clinical Hepatology and Professor of Medicine in the Department of Medicine, Section of Digestive Diseases, Yale Liver Center, at the Yale University School of Medicine in New Haven, Connecticut. Dr. Lim's primary clinical and research interests are focused on viral hepatitis and non-alcoholic steatohepatitis.
What are some of the reasons for first line direct-acting antiviral (DAA) regimen failures in hepatitis C virus (HCV)?
Dr. Lim: In clinical practice, approximately 5% to 10% of patients will fail to achieve sustained virologic response (SVR). The most common reason is incompletion of treatment as a result of non-adherence, intolerance, adverse effects, or other medical/logistical factors that interfere with treatment. Another reason is reinfection, in which an individual who achieves viral eradication is re-exposed to HCV and develops a new infection. Each scenario warrants a careful evaluation to help identify which factors contributed to treatment failure.
Regarding incomplete treatment, it is important to identify other issues that require attention before considering retreatment. If there are other potential medications with potential drug-drug interaction (DDI) which influence the absorption of the DAA medications, that is important to identify (e.g. proton pump inhibitors).
Finally, if there are other non-medical reasons—psychosocial reasons, substance use reasons, or psychiatric reasons—that may prevent a patient from completing a full treatment course, it is important to identify and manage these before reconsidering DAA therapy.
What is the current standard of care for patients who failed a first line DAA regimen?
Dr. Lim: Fewer than 5% to 10% of patients treated with a first-line regimen fail to achieve a sustained virologic response (SVR). A significant proportion may develop resistance-associated substitutions (RASs) which may affect susceptibility to other DAA regimens, but fortunately, multiple studies have confirmed that retreatment of these patients with a contemporary DAA treatment regimen is associated with similarly high rates of SVR exceeding 90%.
The current guidelines by the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) suggest retreating these patients with a triple combination regimen of sofosbuvir, velpatasvir, plus voxilaprevir (also known as SOF/VEL/VOX) or glecaprevir plus pibrentasvir (GP).
Both are viable options for those who fail first line regimens, but with important nuances: 1) for patients who are genotype 1, either of these options is considered a valid regimen strategy; 2) for patients with genotype 2 through 6, the SOF/VEL/VOX combination is recommended; 3) for patients who have signature mutations for either the protease or the nonstructural protein 5A (NS5A), an individualized approach is needed to determine which strategy will be most efficacious; 4) for patients who have cirrhosis, and particularly those with decompensated cirrhosis, protease inhibitor-based combinations are contraindicated because of the risk of liver toxicity and/or hepatic decompensation, which is associated with an FDA black box warning.
Treatment of the very small number of patients who fail the second-line regimen with either GP or SOF/VEL/VOXis an area of significant controversy, and for which the evidence-based guidance in 2021 is quite limited. However, the AASLD and the IDSA recommend 2 potential regimens, including SOF/VEL/VOX plus weight-based ribavirin for 24 weeks, or sofosbuvir plus GP plus weight-based ribavirin for 16 weeks.
Why can it be difficult to differentiate true virological failure from relapse caused by non-adherence or from reinfection?
Dr. Lim: When I speak with patients who have failed DAAs, the majority state that they took every single pill. And although that leaves us in a conundrum where we cannot chalk it up to possible non-adherence, we do wish to determine whether the timing of when they took the medications was consistent.
We ask about whether there are other medications that may interact with these medications. Before we start treatment, we work with a pharmacist and online drug interaction websites to make sure that we do our best to avoid other medications that may interact with DAAs and impact the chance of achieving sustained virologic response (SVR). Despite those efforts, on occasion, patients report that they forgot they were taking a drug or supplement that could interfere with DAA absorption.
Lastly, in patients who offered no history of taking other medications and a report of 100% adherence, we make a presumption that this is probably true virological failure with development of virologic resistance. In 2021, AASLD/IDSA guidelines do not routinely recommend resistance testing for all patients who fail a first-line regimen because it frequently does not influence our decision on retreatment and may not always impact susceptibility to SOF/VEL/VOX or GP. However, in patients who have a history of multiple lines of treatment failure, some of whom have failed 4 or more previous treatment regimens, I do perform resistance testing on an individualized basis to inform our retreatment strategy.
With regards to the question about reinfection, there is no routine way this is assessed in clinical practice. From a public health perspective, we can perform phylogenetic testing, which can help distinguish the very specific viral strains to connect individuals in terms of the root of the infection. But in clinical practice, we do not use phylogenetic testing because if a patient’s initial genotype was genotype 1 and then they get reinfected, and their genotype was genotype 3, you do not need any special testing. At that point you know it is a different virus than what you initially had, and so that is the easiest way to make a distinction. If they have a different genotype of HCV the second time around, we generally will conclude that it represents reinfection rather than virologic relapse, although conversely, the discovery of the same genotype does not exclude reinfection.
Ninety-five percent of individuals treated with DAA agents are cured of HCV infection. Of the 5% who failed first line DAA regimens, what are their options and chances for being cured?
Dr. Lim: Again, retreatment with either SOF/VEL/VOX or GP is associated with a very high rate of SVR. Specifically, for SOF/VEL/VOX, there are two phase 3 clinical trials. One is POLARIS-1 for genotypes 1 through 6 and the other is POLARIS-4 for those with genotype 1 through 4. In both of those clinical trials, they reported a 96% to 97% chance of achieving SVR.1, 2 For GP, the MAGELLAN-1 protocol looked at patients, specifically genotype 1, and found that GP was associated with a 96% chance of SVR.3 As such, there is robust prospective RCT evidence validating the safety and efficacy of both SOF/VEL/VOX and GP for patients who fail first line DAA regimens.
How do people with HIV and persons who use drugs factor into DAA failures, and how does it affect them? How can their unique needs be met, and what special considerations can be made for them going forward?
Dr. Lim: Historically, HIV was viewed within our community as a “special population.” And the reason why was that with some older regimens, including those requiring pegylated interferon, the chance of achieving SVR was about 20% lower than those who had HCV monoinfection. But with our contemporary DAA regimens, that differential has washed away. The expected sustained virologic response rates of those with HCV alone or with coinfection of HIV is identical, around 95%. However, from a reinfection perspective, the available data suggests no difference in rates of virologic relapse or virologic failure or treatment failure or the risk of reinfection in HIV coinfected individuals. However, patients with HIV are taking antiretroviral therapy (ART) regimens that are associated with potential DDIs, which require special attention. On occasion, modification of the ART is needed to permit safe administration of HCV DAAs. In contrast, persons who inject drugs (PWID) remain a special population because of unique challenges and considerations required in the decision of who to treat, when to treat, and how to treat.
In terms of who to treat, there has been a paradigm shift. In the past, we would want to delay treatment until patients were drug-free for 6 months or greater. Many of our current insurance policies still mandate that patients be confirmed to be drug-free for a required amount of time before they will authorize the drug's release from the pharmacy. But at this time, that paradigm has shifted to where we, as a liver community, view HCV treatment as prevention. The concept is that within injection drug communities, if we can treat and eradicate HCV in super users of injection drugs, not only does it benefit that individual patient, but it may also benefit their community of injection drug users and prevent spread to others.
It is a high priority in 2021 that clinicians within the GI, liver, and infectious disease communities are willing to treat patients who are actively injecting drugs or in the process of going through relapse prevention, and/or rehabilitation. We must accept that persons who inject drugs may experience relapse to substance use which may be associated with HCV reinfection rates as high as 10% to 15% of cases. While these numbers are significant in my view, from a public health perspective and a clinical perspective, this should not dissuade clinicians from considering an individualized approach to offering DAA therapy to all patients, including those who are PWID.
Joseph K. Lim, MD, is the Director of Clinical Hepatology and Professor of Medicine in the Department of Medicine, Section of Digestive Diseases, Yale Liver Center, at the Yale University School of Medicine in New Haven, Connecticut. Dr. Lim's primary clinical and research interests are focused on viral hepatitis and non-alcoholic steatohepatitis.
What are some of the reasons for first line direct-acting antiviral (DAA) regimen failures in hepatitis C virus (HCV)?
Dr. Lim: In clinical practice, approximately 5% to 10% of patients will fail to achieve sustained virologic response (SVR). The most common reason is incompletion of treatment as a result of non-adherence, intolerance, adverse effects, or other medical/logistical factors that interfere with treatment. Another reason is reinfection, in which an individual who achieves viral eradication is re-exposed to HCV and develops a new infection. Each scenario warrants a careful evaluation to help identify which factors contributed to treatment failure.
Regarding incomplete treatment, it is important to identify other issues that require attention before considering retreatment. If there are other potential medications with potential drug-drug interaction (DDI) which influence the absorption of the DAA medications, that is important to identify (e.g. proton pump inhibitors).
Finally, if there are other non-medical reasons—psychosocial reasons, substance use reasons, or psychiatric reasons—that may prevent a patient from completing a full treatment course, it is important to identify and manage these before reconsidering DAA therapy.
What is the current standard of care for patients who failed a first line DAA regimen?
Dr. Lim: Fewer than 5% to 10% of patients treated with a first-line regimen fail to achieve a sustained virologic response (SVR). A significant proportion may develop resistance-associated substitutions (RASs) which may affect susceptibility to other DAA regimens, but fortunately, multiple studies have confirmed that retreatment of these patients with a contemporary DAA treatment regimen is associated with similarly high rates of SVR exceeding 90%.
The current guidelines by the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) suggest retreating these patients with a triple combination regimen of sofosbuvir, velpatasvir, plus voxilaprevir (also known as SOF/VEL/VOX) or glecaprevir plus pibrentasvir (GP).
Both are viable options for those who fail first line regimens, but with important nuances: 1) for patients who are genotype 1, either of these options is considered a valid regimen strategy; 2) for patients with genotype 2 through 6, the SOF/VEL/VOX combination is recommended; 3) for patients who have signature mutations for either the protease or the nonstructural protein 5A (NS5A), an individualized approach is needed to determine which strategy will be most efficacious; 4) for patients who have cirrhosis, and particularly those with decompensated cirrhosis, protease inhibitor-based combinations are contraindicated because of the risk of liver toxicity and/or hepatic decompensation, which is associated with an FDA black box warning.
Treatment of the very small number of patients who fail the second-line regimen with either GP or SOF/VEL/VOXis an area of significant controversy, and for which the evidence-based guidance in 2021 is quite limited. However, the AASLD and the IDSA recommend 2 potential regimens, including SOF/VEL/VOX plus weight-based ribavirin for 24 weeks, or sofosbuvir plus GP plus weight-based ribavirin for 16 weeks.
Why can it be difficult to differentiate true virological failure from relapse caused by non-adherence or from reinfection?
Dr. Lim: When I speak with patients who have failed DAAs, the majority state that they took every single pill. And although that leaves us in a conundrum where we cannot chalk it up to possible non-adherence, we do wish to determine whether the timing of when they took the medications was consistent.
We ask about whether there are other medications that may interact with these medications. Before we start treatment, we work with a pharmacist and online drug interaction websites to make sure that we do our best to avoid other medications that may interact with DAAs and impact the chance of achieving sustained virologic response (SVR). Despite those efforts, on occasion, patients report that they forgot they were taking a drug or supplement that could interfere with DAA absorption.
Lastly, in patients who offered no history of taking other medications and a report of 100% adherence, we make a presumption that this is probably true virological failure with development of virologic resistance. In 2021, AASLD/IDSA guidelines do not routinely recommend resistance testing for all patients who fail a first-line regimen because it frequently does not influence our decision on retreatment and may not always impact susceptibility to SOF/VEL/VOX or GP. However, in patients who have a history of multiple lines of treatment failure, some of whom have failed 4 or more previous treatment regimens, I do perform resistance testing on an individualized basis to inform our retreatment strategy.
With regards to the question about reinfection, there is no routine way this is assessed in clinical practice. From a public health perspective, we can perform phylogenetic testing, which can help distinguish the very specific viral strains to connect individuals in terms of the root of the infection. But in clinical practice, we do not use phylogenetic testing because if a patient’s initial genotype was genotype 1 and then they get reinfected, and their genotype was genotype 3, you do not need any special testing. At that point you know it is a different virus than what you initially had, and so that is the easiest way to make a distinction. If they have a different genotype of HCV the second time around, we generally will conclude that it represents reinfection rather than virologic relapse, although conversely, the discovery of the same genotype does not exclude reinfection.
Ninety-five percent of individuals treated with DAA agents are cured of HCV infection. Of the 5% who failed first line DAA regimens, what are their options and chances for being cured?
Dr. Lim: Again, retreatment with either SOF/VEL/VOX or GP is associated with a very high rate of SVR. Specifically, for SOF/VEL/VOX, there are two phase 3 clinical trials. One is POLARIS-1 for genotypes 1 through 6 and the other is POLARIS-4 for those with genotype 1 through 4. In both of those clinical trials, they reported a 96% to 97% chance of achieving SVR.1, 2 For GP, the MAGELLAN-1 protocol looked at patients, specifically genotype 1, and found that GP was associated with a 96% chance of SVR.3 As such, there is robust prospective RCT evidence validating the safety and efficacy of both SOF/VEL/VOX and GP for patients who fail first line DAA regimens.
How do people with HIV and persons who use drugs factor into DAA failures, and how does it affect them? How can their unique needs be met, and what special considerations can be made for them going forward?
Dr. Lim: Historically, HIV was viewed within our community as a “special population.” And the reason why was that with some older regimens, including those requiring pegylated interferon, the chance of achieving SVR was about 20% lower than those who had HCV monoinfection. But with our contemporary DAA regimens, that differential has washed away. The expected sustained virologic response rates of those with HCV alone or with coinfection of HIV is identical, around 95%. However, from a reinfection perspective, the available data suggests no difference in rates of virologic relapse or virologic failure or treatment failure or the risk of reinfection in HIV coinfected individuals. However, patients with HIV are taking antiretroviral therapy (ART) regimens that are associated with potential DDIs, which require special attention. On occasion, modification of the ART is needed to permit safe administration of HCV DAAs. In contrast, persons who inject drugs (PWID) remain a special population because of unique challenges and considerations required in the decision of who to treat, when to treat, and how to treat.
In terms of who to treat, there has been a paradigm shift. In the past, we would want to delay treatment until patients were drug-free for 6 months or greater. Many of our current insurance policies still mandate that patients be confirmed to be drug-free for a required amount of time before they will authorize the drug's release from the pharmacy. But at this time, that paradigm has shifted to where we, as a liver community, view HCV treatment as prevention. The concept is that within injection drug communities, if we can treat and eradicate HCV in super users of injection drugs, not only does it benefit that individual patient, but it may also benefit their community of injection drug users and prevent spread to others.
It is a high priority in 2021 that clinicians within the GI, liver, and infectious disease communities are willing to treat patients who are actively injecting drugs or in the process of going through relapse prevention, and/or rehabilitation. We must accept that persons who inject drugs may experience relapse to substance use which may be associated with HCV reinfection rates as high as 10% to 15% of cases. While these numbers are significant in my view, from a public health perspective and a clinical perspective, this should not dissuade clinicians from considering an individualized approach to offering DAA therapy to all patients, including those who are PWID.
- Safety and Efficacy of Sofosbuvir/Velpatasvir/Voxilaprevir in Adults with Chronic HCV Infection who have Previously Received Treatment with Direct-Acting Antiviral Therapy (POLARIS-1). Accessed- https://clinicaltrials.gov/ct2/show/NCT02607735
- Safety and Efficacy of SOF/VEL/VOX FDC for 12 Weeks and SOF/VEL for 12 Weeks in DAA-Experienced Adults with Chronic HCV Infection who have not Received an NS5A Inhibitor (POLARIS-4). Accessed- https://clinicaltrials.gov/ct2/show/NCT02639247
- Glecaprevir-Pibrentasvir (Mavyret). Accessed- https://www.hepatitisc.uw.edu/page/treatment/drugs/glecaprevir-pibrentasvir/clinical-trials
- Safety and Efficacy of Sofosbuvir/Velpatasvir/Voxilaprevir in Adults with Chronic HCV Infection who have Previously Received Treatment with Direct-Acting Antiviral Therapy (POLARIS-1). Accessed- https://clinicaltrials.gov/ct2/show/NCT02607735
- Safety and Efficacy of SOF/VEL/VOX FDC for 12 Weeks and SOF/VEL for 12 Weeks in DAA-Experienced Adults with Chronic HCV Infection who have not Received an NS5A Inhibitor (POLARIS-4). Accessed- https://clinicaltrials.gov/ct2/show/NCT02639247
- Glecaprevir-Pibrentasvir (Mavyret). Accessed- https://www.hepatitisc.uw.edu/page/treatment/drugs/glecaprevir-pibrentasvir/clinical-trials
Applying a Text-Search Algorithm to Radiology Reports Can Find More Patients With Pulmonary Nodules Than Radiology Coding Alone (FULL)
Rapid advances in imaging technology have led to better spatial resolution with lower radiation doses to patients. These advances have helped to increase the use of diagnostic chest imaging, particularly in emergency departments and oncology centers, and in screening for coronary artery disease. As a result, there has been an explosion of incidental findings on chest imaging—including indeterminate lung nodules.1,2
Lung nodules are rounded and well-circumscribed lung opacities (≤ 3 cm in diameter) that may present as solitary or multiple lesions in usually asymptomatic patients. Most lung nodules are benign, the result of an infectious or inflammatory process. Nodules that are ≤ 8 mm in diameter, unless they show increase in size over time, often can be safely followed with imaging surveillance. In contrast, lung nodules > 8 mm could represent an early-stage lung cancer, especially among patients with high-risk for developing lung cancer (ie, those with advanced age, heavy tobacco abuse, or emphysema) and should be further assessed with close imaging surveillance, either chest computed tomography (CT) alone or positron-emission tomography (PET)/CT, or tissue biopsy, based on the underlying likelihood of malignancy.
Patients who receive an early-stage lung cancer diagnosis can be offered curative treatments leading to improved 5-year survival rates.3,4 Consequently, health care systems need to be able to identify these nodules accurately, in order to categorize and manage them accordingly to the Fleischner radiographic and American College of Chest Physicians clinical guidelines.5,6 Unfortunately, many hospitals struggle to identify patients with incidental lung nodules found during diagnostic chest and abdominal imaging, due in part to poor adherence to Fleischner guidelines among radiologists for categorizing pulmonary nodules.7,8
The Veterans Health Administration (VHA) system is interested in effectively detecting patients with incidental lung nodules. Veterans have a higher risk of developing lung cancer when compared with the entire US population, mainly due to a higher incidence of tobacco use.6 The prevalence of lung nodules among veterans with significant risk factors for lung cancer is about 60% nationwide, and up to 85% in the Midwest, due to the high prevalence of histoplasmosis.7 However, only a small percentage of these nodules represent an early stage primary lung cancer.
Several Veterans Integrated Service Networks (VISNs) in the VHA use a radiology diagnostic code to systematically identify imaging studies with presence of lung nodules. In VISN 23, which includes Minnesota, North Dakota, South Dakota, Iowa, and portions of neighboring states, the code used to identify these radiology studies is 44. However, there is high variability in the reporting and coding of imaging studies among radiologists, which could lead to misclassifying patients with lung nodules.8
Some studies suggest that using an automated text search algorithm within radiology reports can be a highly effective strategy to identify patients with lung nodules.9,10 In this study, we compared the diagnostic performance of a newly developed text search algorithm applied to radiology reports with the current standard practice of using a radiology diagnostic code for identifying patients with lung nodules at the Iowa City US Department of Veterans Affairs (VA) Health Care System (ICVAHCS) hospital in Iowa.
Methods
Since 2014, The ICVAHCS has used a radiology diagnostic code to identify any imaging studies with lung nodules. The radiologist enters “44” at the end of the reading process using the Nuance Powerscribe 360 radiation reporting system. The code is uploaded into the VHA Corporate Data Warehouse (CDW), and it is located within the radiology exam domain. This strategy was created and implemented by the Minneapolis VA Health Care System in Minnesota for all the VA hospitals in VISN 23. A lung nodule registry nurse was provided with a list of radiology studies flagged with this radiology diagnostic code every 2 weeks. A chart review was then performed for all these studies to determine the presence of a lung nodule. When detected, the ordering health care provider was alerted and given recommendations for managing the nodule.
We initially searched for the radiology studies with a presumptive lung nodule using the radiology code 44 within the CDW. Separately, we applied the text search strategy only to radiology reports from chest and abdomen studies (ie, X-rays, CT, magnetic resonance imaging [MRI], and PET) that contained any of the keyword phrases. The text search strategy was modeled based on a natural language processing (NLP) algorithm developed by the Puget Sound VA Healthcare System in Seattle, Washington to identify lung nodules on radiology reports.9 Our algorithm included a series of text searches using Microsoft SQL. After several simulations using a random group of radiology reports, we chose the keywords: “lung AND nodul”; “pulm AND nodul”; “pulm AND mass”; “lung AND mass”; and “ground glass”. We selected only chest and abdomen studies because on several simulations using a random group of radiology reports, the vast majority of lung nodules were identified on chest and abdomen imaging studies. Also, it would not have been feasible to chart review the approximately 30,000 total radiology reports that were generated during the study period.
From January 1, 2016 through November 30, 2016, we applied both search strategies independently: radiology diagnostic code for lung nodules to all imaging studies, and text search to all radiology reports of chest and abdomen imaging studies in the CDW (Figure). We also collected demographic (eg, age, sex, race, rurality) and clinical (eg, medical comorbidities, tobacco use) information that were uploaded to the database automatically from CDW using International Statistical Classification of Diseases, Tenth Edition and demographic codes. The VHA uses the Rural-Urban Commuting Areas (RUCA) system to define rurality, which takes into account population density and how closely a community is linked socioeconomically to larger urban centers.11 The protocol was reviewed and approved by the institutional review board of ICVAHCS and the University of Iowa.
The presence of a lung nodule was established by having the lung nodule registry nurse manually review the charts of every patient with a radiology report identified by either code 44 or the text search algorithm. The goal was to ensure that our text search strategy identified all reports with a code 44 to be compliant with VISN expectations. Cases in which a lung nodule was described in the radiology report were considered true positives, and those without a lung nodule description were considered false positives.
We compared the sociodemographic and clinical characteristics of patients with lung nodules between those identified with both code 44 and the text search and those identified with the text search alone. We used χ2 tests for categorical variables (eg, age, gender, RUCA, chronic obstructive pulmonary disease (COPD), smoking status) and t tests for continuous variables (eg, Charlson comorbidity score). A P value ≤ .05 was considered statistically significant. To assess the yield of each search strategy, we determined the number of patients with lung nodules detected by the text search and the radiology diagnostic code. We also calculated the positive predictive value (PPV) and 95% CI of each search strategy.
Results
We identified 12,983 radiology studies that required manual review during the study period. We confirmed that 8,516 imaging studies had lung nodules, representing 2,912 patients. Subjects with lung nodules were predominantly male (96%), aged between 60 and 79 years (71%), and lived in a rural area (72%). More than 50% of these patients had COPD and over a third were current smokers (Table 1). The text search algorithm identified all of the patients identified by the radiology diagnostic code (n = 1,251). It also identified an additional 1,661 patients with lung nodules that otherwise would have been missed by the radiology code. Compared with those identified only by the text search, those identified by both the radiology coding and text search were older, had lower Charlson comorbidity scores, and were more likely to be a current smoker.
The text search algorithm identified more than twice as many patients with potential lung nodules compared with the radiology diagnostic code (4,071 vs 1,363) (Table 2). However, the text search algorithm was associated with a much higher number of false positives than was the diagnostic code (1,159 vs 112) and a lower PPV (72% [95% CI, 70.6-73.4] vs 92% [95% CI, 90.6-93.4], respectively). The text search algorithm identified 130 patients with lung nodules of moderate to high risk for malignancy (> 8 mm diameter) that were not identified by the radiology code. When the PPV of each search strategy was calculated based on imaging studies with nodules (most patients had > 1 imaging study), the results remained similar (98% for radiology code and 66% for text search). A larger proportion of the lung nodules detected by code 44 vs the text search algorithm were from CT chest studies.
Discussion
In a population of predominantly older male veterans with significant risk factors for lung cancer and high incidence of incidental lung nodules, applying a text search algorithm on radiology reports identified a substantial number of patients with lung nodules, including some with nodules > 8 mm, that were missed by the radiologist-generated code.9,10 Improving the yield of detection for lung nodules in a population with high risk for lung cancer would increase the likelihood of detecting patients with potentially curable early-stage lung cancers, decreasing lung cancer mortality.
The reasons for the high number of patients with lung nodules missed by the radiology code are unclear. Potential explanations may include the lack of standardization of imaging reports by the radiologists (ie, only 21% of chest CTs used a standardized template describing a lung nodule in our study), a problem well recognized both within and outside VHA.8,12
The text search algorithm identified more patients with lung nodules but had a higher rate of false positives when compared with the diagnostic code. The high rate of false positives resulted in more charts to review and an increased workload for the lung nodule registry team. The challenges presented by an increased workload should be balanced against the potential harms of missing nodules that develop into advanced cancer.
Text Search Adjustments
Refining the text search criteria algorithm and the chart review process may decrease the rate of false positives significantly without affecting detection of lung nodules. In subsequent simulations, we found that by adding an exclusion criteria to text search algorithm to remove reports with specific keywords we could substantially reduce the number of false positive reports without affecting the detection rate of the lung nodules. These exclusion criteria would exclude any reports that: (1) contain “nodul” within the next 8 words after mentioning “no”; (2) contain “clear” within the next 8 words after mentioning “lung” in the text (eg, “lungs appear to be clear”); (3) contain “clear” within the next 4 words after mentioning “otherwise” in the text (eg, “otherwise appear to be clear”). Based on our study results, we further refined the text search strategy by limiting the search to only chest imaging studies. When we applied the revised algorithm to a random sample of imaging reports, we found all the code 44 radiology reports were still captured, but we were able to reduce the number of radiology reports needing review by about 80%.
Although classification approaches are being refined to improve radiology performance in multiple categories of nodules, this study suggests that alternative approaches based on text algorithms can improve the capture of pulmonary nodules that require surveillance. These algorithms also can be used to augment radiologist reporting systems. This represents an investment in resources to build a team that should include a bioinformatics specialist, lung nodule registry personnel (review charts of the detected imaging studies with lung nodules, populating the lung nodule database, and determining and tracking the need of imaging follow up), a lung nodule clinic nurse coordinator, and a dedicated lung nodule clinic pulmonologist.
Radiology departments could employ this text search approach to identify missed nodules and use an audit and feedback system to train radiologists to code lung nodules consistently at the time of the initial reading to avoid delays in identifying patients with nodules. Alternatively, the more widespread use of a standardized CT chest radiology reports using Fleischner or the American College of Radiology Lung Imaging Reporting and Data System (Lung RADS) templates might improve the detection of patients with lung nodules.5,13,14
The VHA system should have an effective strategy for identifying incidental lung nodules during routine radiology examinations. Relying only on radiologists to identify and code pulmonary nodules can lead to missing a significant number of patients with lung nodules and some patients with early stage lung cancer who could receive curative therapy.12,14-16 The use of a standardized algorithm, like a text search strategy, might decrease the risk of variation in the execution and result in a more sensitive detection of patients with lung nodules. The text search strategy might be easily implemented and shared with other hospitals both within and outside the VHA.
Limitations
This study was performed in a single VHA hospital and the findings may not be generalizable to other settings of care. Second, our study design is susceptible to work-up bias because the results of a diagnostic test (eg, chest or abdomen imaging) affected whether the chart review was used to verify the test result. It was not feasible to review the patient records of all radiology studies done at the facility during the study period, consequently complete 2 × 2 tables could not be created to calculate sensitivity, specificity, and negative predictive value.
Conclusion
A text search algorithm of radiology reports increased the detection of patients with lung nodules when compared with radiology diagnostic coding alone. However, the improved detection was associated with a higher rate of false positives, which requires manually reviewing a larger number of patient’s chart reports. Future research and quality improvement should focus on standardizing the radiology reporting process and improving the efficiency and reliability of follow up and tracking of incidental lung nodules.
Acknowledgments
The work reported here was supported by a grant from the Office of Rural Health (N32-FY16Q1-S1-P01577), US Department of Veterans Affairs, Veterans Health Administration. We also had the support from the Veterans Rural Health Resource Center-Iowa City, and the Health Services Research and Development (HSR&D) Service through the Comprehensive Access and Delivery Research and Evaluation (CADRE) Center (REA 09-220).
1. Jacobs PC, Mali WP, Grobbee DE, van der Graaf Y. Prevalence of incidental findings in computed tomographic screening of the chest: a systematic review. Journal of computer assisted tomography. 2008;32(2):214-221.
2. Frank L, Quint LE. Chest CT incidentalomas: thyroid lesions, enlarged mediastinal lymph nodes, and lung nodules. Cancer Imaging. 2012;12(1):41-48.
3. National Institutes of Health, National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed April 8, 2020.
4. Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e1S-e29S.
5. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
6. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
7. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406.
8. Iqbal MN, Stott E, Huml AM, et al. What’s in a name? Factors associated with documentation and evaluation of incidental pulmonary nodules. Ann Am Thorac Soc. 2016;13(10):1704-1711.
9. Farjah F, Halgrim S, Buist DS, et al. An automated method for identifying individuals with a lung nodule can be feasibly implemented across health systems. Egems (Wash DC). 2016;4(1):1254.
10. Danforth KN, Early MI, Ngan S, Kosco AE, Zheng C, Gould MK. Automated identification of patients with pulmonary nodules in an integrated health system using administrative health plan data, radiology reports, and natural language processing. J Thorac Oncol. 2012;7(8):1257-1262.
11. US Department of Veterans Affairs, Office of Rural Health. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp. Updated January 28, 2020. Accessed April 8, 2020.
12. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2016;13(2 suppl):R18-R24.
13. Eisenberg RL, Fleischner S. Ways to improve radiologists’ adherence to Fleischner Society guidelines for management of pulmonary nodules. J Am Coll Radiol. 2013;10(6):439-441.
14. Aberle DR. Implementing lung cancer screening: the US experience. Clin Radiol. 2017;72(5):401-406.
15. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S-e120S.
16. Callister ME, Baldwin DR. How should pulmonary nodules be optimally investigated and managed? Lung Cancer. 2016;91:48-55.
Rapid advances in imaging technology have led to better spatial resolution with lower radiation doses to patients. These advances have helped to increase the use of diagnostic chest imaging, particularly in emergency departments and oncology centers, and in screening for coronary artery disease. As a result, there has been an explosion of incidental findings on chest imaging—including indeterminate lung nodules.1,2
Lung nodules are rounded and well-circumscribed lung opacities (≤ 3 cm in diameter) that may present as solitary or multiple lesions in usually asymptomatic patients. Most lung nodules are benign, the result of an infectious or inflammatory process. Nodules that are ≤ 8 mm in diameter, unless they show increase in size over time, often can be safely followed with imaging surveillance. In contrast, lung nodules > 8 mm could represent an early-stage lung cancer, especially among patients with high-risk for developing lung cancer (ie, those with advanced age, heavy tobacco abuse, or emphysema) and should be further assessed with close imaging surveillance, either chest computed tomography (CT) alone or positron-emission tomography (PET)/CT, or tissue biopsy, based on the underlying likelihood of malignancy.
Patients who receive an early-stage lung cancer diagnosis can be offered curative treatments leading to improved 5-year survival rates.3,4 Consequently, health care systems need to be able to identify these nodules accurately, in order to categorize and manage them accordingly to the Fleischner radiographic and American College of Chest Physicians clinical guidelines.5,6 Unfortunately, many hospitals struggle to identify patients with incidental lung nodules found during diagnostic chest and abdominal imaging, due in part to poor adherence to Fleischner guidelines among radiologists for categorizing pulmonary nodules.7,8
The Veterans Health Administration (VHA) system is interested in effectively detecting patients with incidental lung nodules. Veterans have a higher risk of developing lung cancer when compared with the entire US population, mainly due to a higher incidence of tobacco use.6 The prevalence of lung nodules among veterans with significant risk factors for lung cancer is about 60% nationwide, and up to 85% in the Midwest, due to the high prevalence of histoplasmosis.7 However, only a small percentage of these nodules represent an early stage primary lung cancer.
Several Veterans Integrated Service Networks (VISNs) in the VHA use a radiology diagnostic code to systematically identify imaging studies with presence of lung nodules. In VISN 23, which includes Minnesota, North Dakota, South Dakota, Iowa, and portions of neighboring states, the code used to identify these radiology studies is 44. However, there is high variability in the reporting and coding of imaging studies among radiologists, which could lead to misclassifying patients with lung nodules.8
Some studies suggest that using an automated text search algorithm within radiology reports can be a highly effective strategy to identify patients with lung nodules.9,10 In this study, we compared the diagnostic performance of a newly developed text search algorithm applied to radiology reports with the current standard practice of using a radiology diagnostic code for identifying patients with lung nodules at the Iowa City US Department of Veterans Affairs (VA) Health Care System (ICVAHCS) hospital in Iowa.
Methods
Since 2014, The ICVAHCS has used a radiology diagnostic code to identify any imaging studies with lung nodules. The radiologist enters “44” at the end of the reading process using the Nuance Powerscribe 360 radiation reporting system. The code is uploaded into the VHA Corporate Data Warehouse (CDW), and it is located within the radiology exam domain. This strategy was created and implemented by the Minneapolis VA Health Care System in Minnesota for all the VA hospitals in VISN 23. A lung nodule registry nurse was provided with a list of radiology studies flagged with this radiology diagnostic code every 2 weeks. A chart review was then performed for all these studies to determine the presence of a lung nodule. When detected, the ordering health care provider was alerted and given recommendations for managing the nodule.
We initially searched for the radiology studies with a presumptive lung nodule using the radiology code 44 within the CDW. Separately, we applied the text search strategy only to radiology reports from chest and abdomen studies (ie, X-rays, CT, magnetic resonance imaging [MRI], and PET) that contained any of the keyword phrases. The text search strategy was modeled based on a natural language processing (NLP) algorithm developed by the Puget Sound VA Healthcare System in Seattle, Washington to identify lung nodules on radiology reports.9 Our algorithm included a series of text searches using Microsoft SQL. After several simulations using a random group of radiology reports, we chose the keywords: “lung AND nodul”; “pulm AND nodul”; “pulm AND mass”; “lung AND mass”; and “ground glass”. We selected only chest and abdomen studies because on several simulations using a random group of radiology reports, the vast majority of lung nodules were identified on chest and abdomen imaging studies. Also, it would not have been feasible to chart review the approximately 30,000 total radiology reports that were generated during the study period.
From January 1, 2016 through November 30, 2016, we applied both search strategies independently: radiology diagnostic code for lung nodules to all imaging studies, and text search to all radiology reports of chest and abdomen imaging studies in the CDW (Figure). We also collected demographic (eg, age, sex, race, rurality) and clinical (eg, medical comorbidities, tobacco use) information that were uploaded to the database automatically from CDW using International Statistical Classification of Diseases, Tenth Edition and demographic codes. The VHA uses the Rural-Urban Commuting Areas (RUCA) system to define rurality, which takes into account population density and how closely a community is linked socioeconomically to larger urban centers.11 The protocol was reviewed and approved by the institutional review board of ICVAHCS and the University of Iowa.
The presence of a lung nodule was established by having the lung nodule registry nurse manually review the charts of every patient with a radiology report identified by either code 44 or the text search algorithm. The goal was to ensure that our text search strategy identified all reports with a code 44 to be compliant with VISN expectations. Cases in which a lung nodule was described in the radiology report were considered true positives, and those without a lung nodule description were considered false positives.
We compared the sociodemographic and clinical characteristics of patients with lung nodules between those identified with both code 44 and the text search and those identified with the text search alone. We used χ2 tests for categorical variables (eg, age, gender, RUCA, chronic obstructive pulmonary disease (COPD), smoking status) and t tests for continuous variables (eg, Charlson comorbidity score). A P value ≤ .05 was considered statistically significant. To assess the yield of each search strategy, we determined the number of patients with lung nodules detected by the text search and the radiology diagnostic code. We also calculated the positive predictive value (PPV) and 95% CI of each search strategy.
Results
We identified 12,983 radiology studies that required manual review during the study period. We confirmed that 8,516 imaging studies had lung nodules, representing 2,912 patients. Subjects with lung nodules were predominantly male (96%), aged between 60 and 79 years (71%), and lived in a rural area (72%). More than 50% of these patients had COPD and over a third were current smokers (Table 1). The text search algorithm identified all of the patients identified by the radiology diagnostic code (n = 1,251). It also identified an additional 1,661 patients with lung nodules that otherwise would have been missed by the radiology code. Compared with those identified only by the text search, those identified by both the radiology coding and text search were older, had lower Charlson comorbidity scores, and were more likely to be a current smoker.
The text search algorithm identified more than twice as many patients with potential lung nodules compared with the radiology diagnostic code (4,071 vs 1,363) (Table 2). However, the text search algorithm was associated with a much higher number of false positives than was the diagnostic code (1,159 vs 112) and a lower PPV (72% [95% CI, 70.6-73.4] vs 92% [95% CI, 90.6-93.4], respectively). The text search algorithm identified 130 patients with lung nodules of moderate to high risk for malignancy (> 8 mm diameter) that were not identified by the radiology code. When the PPV of each search strategy was calculated based on imaging studies with nodules (most patients had > 1 imaging study), the results remained similar (98% for radiology code and 66% for text search). A larger proportion of the lung nodules detected by code 44 vs the text search algorithm were from CT chest studies.
Discussion
In a population of predominantly older male veterans with significant risk factors for lung cancer and high incidence of incidental lung nodules, applying a text search algorithm on radiology reports identified a substantial number of patients with lung nodules, including some with nodules > 8 mm, that were missed by the radiologist-generated code.9,10 Improving the yield of detection for lung nodules in a population with high risk for lung cancer would increase the likelihood of detecting patients with potentially curable early-stage lung cancers, decreasing lung cancer mortality.
The reasons for the high number of patients with lung nodules missed by the radiology code are unclear. Potential explanations may include the lack of standardization of imaging reports by the radiologists (ie, only 21% of chest CTs used a standardized template describing a lung nodule in our study), a problem well recognized both within and outside VHA.8,12
The text search algorithm identified more patients with lung nodules but had a higher rate of false positives when compared with the diagnostic code. The high rate of false positives resulted in more charts to review and an increased workload for the lung nodule registry team. The challenges presented by an increased workload should be balanced against the potential harms of missing nodules that develop into advanced cancer.
Text Search Adjustments
Refining the text search criteria algorithm and the chart review process may decrease the rate of false positives significantly without affecting detection of lung nodules. In subsequent simulations, we found that by adding an exclusion criteria to text search algorithm to remove reports with specific keywords we could substantially reduce the number of false positive reports without affecting the detection rate of the lung nodules. These exclusion criteria would exclude any reports that: (1) contain “nodul” within the next 8 words after mentioning “no”; (2) contain “clear” within the next 8 words after mentioning “lung” in the text (eg, “lungs appear to be clear”); (3) contain “clear” within the next 4 words after mentioning “otherwise” in the text (eg, “otherwise appear to be clear”). Based on our study results, we further refined the text search strategy by limiting the search to only chest imaging studies. When we applied the revised algorithm to a random sample of imaging reports, we found all the code 44 radiology reports were still captured, but we were able to reduce the number of radiology reports needing review by about 80%.
Although classification approaches are being refined to improve radiology performance in multiple categories of nodules, this study suggests that alternative approaches based on text algorithms can improve the capture of pulmonary nodules that require surveillance. These algorithms also can be used to augment radiologist reporting systems. This represents an investment in resources to build a team that should include a bioinformatics specialist, lung nodule registry personnel (review charts of the detected imaging studies with lung nodules, populating the lung nodule database, and determining and tracking the need of imaging follow up), a lung nodule clinic nurse coordinator, and a dedicated lung nodule clinic pulmonologist.
Radiology departments could employ this text search approach to identify missed nodules and use an audit and feedback system to train radiologists to code lung nodules consistently at the time of the initial reading to avoid delays in identifying patients with nodules. Alternatively, the more widespread use of a standardized CT chest radiology reports using Fleischner or the American College of Radiology Lung Imaging Reporting and Data System (Lung RADS) templates might improve the detection of patients with lung nodules.5,13,14
The VHA system should have an effective strategy for identifying incidental lung nodules during routine radiology examinations. Relying only on radiologists to identify and code pulmonary nodules can lead to missing a significant number of patients with lung nodules and some patients with early stage lung cancer who could receive curative therapy.12,14-16 The use of a standardized algorithm, like a text search strategy, might decrease the risk of variation in the execution and result in a more sensitive detection of patients with lung nodules. The text search strategy might be easily implemented and shared with other hospitals both within and outside the VHA.
Limitations
This study was performed in a single VHA hospital and the findings may not be generalizable to other settings of care. Second, our study design is susceptible to work-up bias because the results of a diagnostic test (eg, chest or abdomen imaging) affected whether the chart review was used to verify the test result. It was not feasible to review the patient records of all radiology studies done at the facility during the study period, consequently complete 2 × 2 tables could not be created to calculate sensitivity, specificity, and negative predictive value.
Conclusion
A text search algorithm of radiology reports increased the detection of patients with lung nodules when compared with radiology diagnostic coding alone. However, the improved detection was associated with a higher rate of false positives, which requires manually reviewing a larger number of patient’s chart reports. Future research and quality improvement should focus on standardizing the radiology reporting process and improving the efficiency and reliability of follow up and tracking of incidental lung nodules.
Acknowledgments
The work reported here was supported by a grant from the Office of Rural Health (N32-FY16Q1-S1-P01577), US Department of Veterans Affairs, Veterans Health Administration. We also had the support from the Veterans Rural Health Resource Center-Iowa City, and the Health Services Research and Development (HSR&D) Service through the Comprehensive Access and Delivery Research and Evaluation (CADRE) Center (REA 09-220).
Rapid advances in imaging technology have led to better spatial resolution with lower radiation doses to patients. These advances have helped to increase the use of diagnostic chest imaging, particularly in emergency departments and oncology centers, and in screening for coronary artery disease. As a result, there has been an explosion of incidental findings on chest imaging—including indeterminate lung nodules.1,2
Lung nodules are rounded and well-circumscribed lung opacities (≤ 3 cm in diameter) that may present as solitary or multiple lesions in usually asymptomatic patients. Most lung nodules are benign, the result of an infectious or inflammatory process. Nodules that are ≤ 8 mm in diameter, unless they show increase in size over time, often can be safely followed with imaging surveillance. In contrast, lung nodules > 8 mm could represent an early-stage lung cancer, especially among patients with high-risk for developing lung cancer (ie, those with advanced age, heavy tobacco abuse, or emphysema) and should be further assessed with close imaging surveillance, either chest computed tomography (CT) alone or positron-emission tomography (PET)/CT, or tissue biopsy, based on the underlying likelihood of malignancy.
Patients who receive an early-stage lung cancer diagnosis can be offered curative treatments leading to improved 5-year survival rates.3,4 Consequently, health care systems need to be able to identify these nodules accurately, in order to categorize and manage them accordingly to the Fleischner radiographic and American College of Chest Physicians clinical guidelines.5,6 Unfortunately, many hospitals struggle to identify patients with incidental lung nodules found during diagnostic chest and abdominal imaging, due in part to poor adherence to Fleischner guidelines among radiologists for categorizing pulmonary nodules.7,8
The Veterans Health Administration (VHA) system is interested in effectively detecting patients with incidental lung nodules. Veterans have a higher risk of developing lung cancer when compared with the entire US population, mainly due to a higher incidence of tobacco use.6 The prevalence of lung nodules among veterans with significant risk factors for lung cancer is about 60% nationwide, and up to 85% in the Midwest, due to the high prevalence of histoplasmosis.7 However, only a small percentage of these nodules represent an early stage primary lung cancer.
Several Veterans Integrated Service Networks (VISNs) in the VHA use a radiology diagnostic code to systematically identify imaging studies with presence of lung nodules. In VISN 23, which includes Minnesota, North Dakota, South Dakota, Iowa, and portions of neighboring states, the code used to identify these radiology studies is 44. However, there is high variability in the reporting and coding of imaging studies among radiologists, which could lead to misclassifying patients with lung nodules.8
Some studies suggest that using an automated text search algorithm within radiology reports can be a highly effective strategy to identify patients with lung nodules.9,10 In this study, we compared the diagnostic performance of a newly developed text search algorithm applied to radiology reports with the current standard practice of using a radiology diagnostic code for identifying patients with lung nodules at the Iowa City US Department of Veterans Affairs (VA) Health Care System (ICVAHCS) hospital in Iowa.
Methods
Since 2014, The ICVAHCS has used a radiology diagnostic code to identify any imaging studies with lung nodules. The radiologist enters “44” at the end of the reading process using the Nuance Powerscribe 360 radiation reporting system. The code is uploaded into the VHA Corporate Data Warehouse (CDW), and it is located within the radiology exam domain. This strategy was created and implemented by the Minneapolis VA Health Care System in Minnesota for all the VA hospitals in VISN 23. A lung nodule registry nurse was provided with a list of radiology studies flagged with this radiology diagnostic code every 2 weeks. A chart review was then performed for all these studies to determine the presence of a lung nodule. When detected, the ordering health care provider was alerted and given recommendations for managing the nodule.
We initially searched for the radiology studies with a presumptive lung nodule using the radiology code 44 within the CDW. Separately, we applied the text search strategy only to radiology reports from chest and abdomen studies (ie, X-rays, CT, magnetic resonance imaging [MRI], and PET) that contained any of the keyword phrases. The text search strategy was modeled based on a natural language processing (NLP) algorithm developed by the Puget Sound VA Healthcare System in Seattle, Washington to identify lung nodules on radiology reports.9 Our algorithm included a series of text searches using Microsoft SQL. After several simulations using a random group of radiology reports, we chose the keywords: “lung AND nodul”; “pulm AND nodul”; “pulm AND mass”; “lung AND mass”; and “ground glass”. We selected only chest and abdomen studies because on several simulations using a random group of radiology reports, the vast majority of lung nodules were identified on chest and abdomen imaging studies. Also, it would not have been feasible to chart review the approximately 30,000 total radiology reports that were generated during the study period.
From January 1, 2016 through November 30, 2016, we applied both search strategies independently: radiology diagnostic code for lung nodules to all imaging studies, and text search to all radiology reports of chest and abdomen imaging studies in the CDW (Figure). We also collected demographic (eg, age, sex, race, rurality) and clinical (eg, medical comorbidities, tobacco use) information that were uploaded to the database automatically from CDW using International Statistical Classification of Diseases, Tenth Edition and demographic codes. The VHA uses the Rural-Urban Commuting Areas (RUCA) system to define rurality, which takes into account population density and how closely a community is linked socioeconomically to larger urban centers.11 The protocol was reviewed and approved by the institutional review board of ICVAHCS and the University of Iowa.
The presence of a lung nodule was established by having the lung nodule registry nurse manually review the charts of every patient with a radiology report identified by either code 44 or the text search algorithm. The goal was to ensure that our text search strategy identified all reports with a code 44 to be compliant with VISN expectations. Cases in which a lung nodule was described in the radiology report were considered true positives, and those without a lung nodule description were considered false positives.
We compared the sociodemographic and clinical characteristics of patients with lung nodules between those identified with both code 44 and the text search and those identified with the text search alone. We used χ2 tests for categorical variables (eg, age, gender, RUCA, chronic obstructive pulmonary disease (COPD), smoking status) and t tests for continuous variables (eg, Charlson comorbidity score). A P value ≤ .05 was considered statistically significant. To assess the yield of each search strategy, we determined the number of patients with lung nodules detected by the text search and the radiology diagnostic code. We also calculated the positive predictive value (PPV) and 95% CI of each search strategy.
Results
We identified 12,983 radiology studies that required manual review during the study period. We confirmed that 8,516 imaging studies had lung nodules, representing 2,912 patients. Subjects with lung nodules were predominantly male (96%), aged between 60 and 79 years (71%), and lived in a rural area (72%). More than 50% of these patients had COPD and over a third were current smokers (Table 1). The text search algorithm identified all of the patients identified by the radiology diagnostic code (n = 1,251). It also identified an additional 1,661 patients with lung nodules that otherwise would have been missed by the radiology code. Compared with those identified only by the text search, those identified by both the radiology coding and text search were older, had lower Charlson comorbidity scores, and were more likely to be a current smoker.
The text search algorithm identified more than twice as many patients with potential lung nodules compared with the radiology diagnostic code (4,071 vs 1,363) (Table 2). However, the text search algorithm was associated with a much higher number of false positives than was the diagnostic code (1,159 vs 112) and a lower PPV (72% [95% CI, 70.6-73.4] vs 92% [95% CI, 90.6-93.4], respectively). The text search algorithm identified 130 patients with lung nodules of moderate to high risk for malignancy (> 8 mm diameter) that were not identified by the radiology code. When the PPV of each search strategy was calculated based on imaging studies with nodules (most patients had > 1 imaging study), the results remained similar (98% for radiology code and 66% for text search). A larger proportion of the lung nodules detected by code 44 vs the text search algorithm were from CT chest studies.
Discussion
In a population of predominantly older male veterans with significant risk factors for lung cancer and high incidence of incidental lung nodules, applying a text search algorithm on radiology reports identified a substantial number of patients with lung nodules, including some with nodules > 8 mm, that were missed by the radiologist-generated code.9,10 Improving the yield of detection for lung nodules in a population with high risk for lung cancer would increase the likelihood of detecting patients with potentially curable early-stage lung cancers, decreasing lung cancer mortality.
The reasons for the high number of patients with lung nodules missed by the radiology code are unclear. Potential explanations may include the lack of standardization of imaging reports by the radiologists (ie, only 21% of chest CTs used a standardized template describing a lung nodule in our study), a problem well recognized both within and outside VHA.8,12
The text search algorithm identified more patients with lung nodules but had a higher rate of false positives when compared with the diagnostic code. The high rate of false positives resulted in more charts to review and an increased workload for the lung nodule registry team. The challenges presented by an increased workload should be balanced against the potential harms of missing nodules that develop into advanced cancer.
Text Search Adjustments
Refining the text search criteria algorithm and the chart review process may decrease the rate of false positives significantly without affecting detection of lung nodules. In subsequent simulations, we found that by adding an exclusion criteria to text search algorithm to remove reports with specific keywords we could substantially reduce the number of false positive reports without affecting the detection rate of the lung nodules. These exclusion criteria would exclude any reports that: (1) contain “nodul” within the next 8 words after mentioning “no”; (2) contain “clear” within the next 8 words after mentioning “lung” in the text (eg, “lungs appear to be clear”); (3) contain “clear” within the next 4 words after mentioning “otherwise” in the text (eg, “otherwise appear to be clear”). Based on our study results, we further refined the text search strategy by limiting the search to only chest imaging studies. When we applied the revised algorithm to a random sample of imaging reports, we found all the code 44 radiology reports were still captured, but we were able to reduce the number of radiology reports needing review by about 80%.
Although classification approaches are being refined to improve radiology performance in multiple categories of nodules, this study suggests that alternative approaches based on text algorithms can improve the capture of pulmonary nodules that require surveillance. These algorithms also can be used to augment radiologist reporting systems. This represents an investment in resources to build a team that should include a bioinformatics specialist, lung nodule registry personnel (review charts of the detected imaging studies with lung nodules, populating the lung nodule database, and determining and tracking the need of imaging follow up), a lung nodule clinic nurse coordinator, and a dedicated lung nodule clinic pulmonologist.
Radiology departments could employ this text search approach to identify missed nodules and use an audit and feedback system to train radiologists to code lung nodules consistently at the time of the initial reading to avoid delays in identifying patients with nodules. Alternatively, the more widespread use of a standardized CT chest radiology reports using Fleischner or the American College of Radiology Lung Imaging Reporting and Data System (Lung RADS) templates might improve the detection of patients with lung nodules.5,13,14
The VHA system should have an effective strategy for identifying incidental lung nodules during routine radiology examinations. Relying only on radiologists to identify and code pulmonary nodules can lead to missing a significant number of patients with lung nodules and some patients with early stage lung cancer who could receive curative therapy.12,14-16 The use of a standardized algorithm, like a text search strategy, might decrease the risk of variation in the execution and result in a more sensitive detection of patients with lung nodules. The text search strategy might be easily implemented and shared with other hospitals both within and outside the VHA.
Limitations
This study was performed in a single VHA hospital and the findings may not be generalizable to other settings of care. Second, our study design is susceptible to work-up bias because the results of a diagnostic test (eg, chest or abdomen imaging) affected whether the chart review was used to verify the test result. It was not feasible to review the patient records of all radiology studies done at the facility during the study period, consequently complete 2 × 2 tables could not be created to calculate sensitivity, specificity, and negative predictive value.
Conclusion
A text search algorithm of radiology reports increased the detection of patients with lung nodules when compared with radiology diagnostic coding alone. However, the improved detection was associated with a higher rate of false positives, which requires manually reviewing a larger number of patient’s chart reports. Future research and quality improvement should focus on standardizing the radiology reporting process and improving the efficiency and reliability of follow up and tracking of incidental lung nodules.
Acknowledgments
The work reported here was supported by a grant from the Office of Rural Health (N32-FY16Q1-S1-P01577), US Department of Veterans Affairs, Veterans Health Administration. We also had the support from the Veterans Rural Health Resource Center-Iowa City, and the Health Services Research and Development (HSR&D) Service through the Comprehensive Access and Delivery Research and Evaluation (CADRE) Center (REA 09-220).
1. Jacobs PC, Mali WP, Grobbee DE, van der Graaf Y. Prevalence of incidental findings in computed tomographic screening of the chest: a systematic review. Journal of computer assisted tomography. 2008;32(2):214-221.
2. Frank L, Quint LE. Chest CT incidentalomas: thyroid lesions, enlarged mediastinal lymph nodes, and lung nodules. Cancer Imaging. 2012;12(1):41-48.
3. National Institutes of Health, National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed April 8, 2020.
4. Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e1S-e29S.
5. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
6. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
7. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406.
8. Iqbal MN, Stott E, Huml AM, et al. What’s in a name? Factors associated with documentation and evaluation of incidental pulmonary nodules. Ann Am Thorac Soc. 2016;13(10):1704-1711.
9. Farjah F, Halgrim S, Buist DS, et al. An automated method for identifying individuals with a lung nodule can be feasibly implemented across health systems. Egems (Wash DC). 2016;4(1):1254.
10. Danforth KN, Early MI, Ngan S, Kosco AE, Zheng C, Gould MK. Automated identification of patients with pulmonary nodules in an integrated health system using administrative health plan data, radiology reports, and natural language processing. J Thorac Oncol. 2012;7(8):1257-1262.
11. US Department of Veterans Affairs, Office of Rural Health. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp. Updated January 28, 2020. Accessed April 8, 2020.
12. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2016;13(2 suppl):R18-R24.
13. Eisenberg RL, Fleischner S. Ways to improve radiologists’ adherence to Fleischner Society guidelines for management of pulmonary nodules. J Am Coll Radiol. 2013;10(6):439-441.
14. Aberle DR. Implementing lung cancer screening: the US experience. Clin Radiol. 2017;72(5):401-406.
15. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S-e120S.
16. Callister ME, Baldwin DR. How should pulmonary nodules be optimally investigated and managed? Lung Cancer. 2016;91:48-55.
1. Jacobs PC, Mali WP, Grobbee DE, van der Graaf Y. Prevalence of incidental findings in computed tomographic screening of the chest: a systematic review. Journal of computer assisted tomography. 2008;32(2):214-221.
2. Frank L, Quint LE. Chest CT incidentalomas: thyroid lesions, enlarged mediastinal lymph nodes, and lung nodules. Cancer Imaging. 2012;12(1):41-48.
3. National Institutes of Health, National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed April 8, 2020.
4. Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e1S-e29S.
5. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
6. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
7. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406.
8. Iqbal MN, Stott E, Huml AM, et al. What’s in a name? Factors associated with documentation and evaluation of incidental pulmonary nodules. Ann Am Thorac Soc. 2016;13(10):1704-1711.
9. Farjah F, Halgrim S, Buist DS, et al. An automated method for identifying individuals with a lung nodule can be feasibly implemented across health systems. Egems (Wash DC). 2016;4(1):1254.
10. Danforth KN, Early MI, Ngan S, Kosco AE, Zheng C, Gould MK. Automated identification of patients with pulmonary nodules in an integrated health system using administrative health plan data, radiology reports, and natural language processing. J Thorac Oncol. 2012;7(8):1257-1262.
11. US Department of Veterans Affairs, Office of Rural Health. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp. Updated January 28, 2020. Accessed April 8, 2020.
12. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2016;13(2 suppl):R18-R24.
13. Eisenberg RL, Fleischner S. Ways to improve radiologists’ adherence to Fleischner Society guidelines for management of pulmonary nodules. J Am Coll Radiol. 2013;10(6):439-441.
14. Aberle DR. Implementing lung cancer screening: the US experience. Clin Radiol. 2017;72(5):401-406.
15. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S-e120S.
16. Callister ME, Baldwin DR. How should pulmonary nodules be optimally investigated and managed? Lung Cancer. 2016;91:48-55.
Incidental Findings of Pulmonary and Hilar Malignancy by Low-Resolution Computed Tomography Used in Myocardial Perfusion Imaging (FULL)
Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) is a well-established technique for the evaluation of coronary artery disease (CAD).1 To improve image quality, low-resolution computed tomography (CT) is used commonly for anatomical correct and artifact attenuation during SPECT MPI.2 The low resolution, unenhanced CT images are considered low quality and are, therefore, labeled by the manufacturer as nondiagnostic. The CT portion of the MPI in many centers is used only for image fusion and attenuation correction, and these images are not routinely reviewed or reported by cardiologists.
Incidental findings by these low-resolution CT were frequent. However, clinically significant findings, including lung cancer, although relatively infrequent, were serious enough for major clinical management.3-5 Currently, there are no consensus recommendations for reviewing low-resolution CT images or the interpretation of such incidental findings during cardiac MPI.6 Clinically, low-dose CT were used for early detection and screening of lung cancer and were associated with reduced lung-cancer and any cause mortality in National Lung Screening Trial (NLST).7,8 Therefore, low-dose CT is recommended for lung cancer screening of high-risk patients by the US Preventive Service Task Force (USPSTF).9 In the veteran population, current and past smoking history are more common when compared with the general population; therefore, veterans are potentially at increased risk of lung cancer.10 In this study, we did not intend to use low-resolution CT for lung cancer screening or detection but rather to identify and report incidental findings of pulmonary/hilar malignancy detected during cardiac MPI.
Methods
The Siemens’ (Munich, Germany) Symbia Intevo Excel SPECT/CT MPI cameras with dedicated cardiac collimators were used at both the Dwight D. Eisenhower VA Medical Center (VAMC) in Leavenworth, Kansas and Colmery-O'Neil VAMC in Topeka, Kansas. The integrated CT scanner (x-ray tube current 30 to 240 mA; voltage 110 Kv with a 40 kW power generator) has the capability to image up to a 2-slice/rotation, each of 5.0 mm per slice with a scan time of about 30 seconds. The SPECT/CT gamma camera has a low energy (140 KeV), high resolution, parallel hole collimator with IQ SPECT capabilities.
The radiation dose received by the patients were expressed in dose length product (DLP), which reflects the total energy absorbed by the patient and represents integrated dose in terms of the total scan length. Additionally, each patients received 2 injections of Technetium Tc 99m sestamibi (1-day Protocol: 10 mCi rest injection, 30 mCi stress injection: 2-day Protocol for patients weighing > 350 pounds: 30 mCi at rest injection and 30 mCi at stress injection) for myocardial perfusion imaging.
All CT images and cardiac MPI findings were reviewed and reported contemporaneously by 1 of 2 experienced, board-certified radiologists who were blinded to patients’ clinical information except the indication for the cardiac stress testing. When suspicious pulmonary/hilar nodules or masses were detected, these findings and recommendations for further evaluation were conveyed to primary care provider or ordering physician via the electronic health record system.
All CT images were reviewed with cardiac MPI from September 1, 2017 to August 31, 2018. When pulmonary/hilar malignancies were identified, the health records were reviewed. Patients with known history of prior pulmonary malignancy were excluded from the study.
Results
A total of 1,098 patients underwent cardiac MPI during the study period. When the CT imaging and cardiac MPI were reviewed, incidental findings led to the diagnosis of lung cancer in 5 patients and hilar mantle cell lymphoma in 1 patient. Their clinical characteristics, CT findings, and types of malignancies for these 6 patients are summarized in the Table and Figure. Only 0.55% (6 of 1,098) patients were found to have incidental pulmonary/hilar malignancy with the cardiac evaluation low-resolution CT. Four patients with prior, known history of lung cancer were excluded from the study.
For the 6 patients found to have cancer, the average CT radiation dose during the cardiac MPI was 100 mGy-cm (range, 77 -133 mCy-cm). The subsequent chest CT with or without contrast delivered a radiation dose of 726.4 mGy-cm (range, 279.4 - 1,075 mGy-cm).
A total of 79 (7.2%) patients were found to have significant pulmonary nodules that required further evaluation; after CT examination, 32 patients had findings of benign nature and required no further follow-up; the other 47 patients are being followed according to the Fleischner Society 2017 guidelines for pulmonary nodules.11 The follow-up findings on these patients are not within the scope of this report.
Discussion
Although incidental findings on low-resolution CT during cardiac MPI are frequent, clinically significant findings are less common. However, some incidental findings may be of important clinical significance.3-5 A multicenter analysis by Coward and colleagues reported that 2.4% findings on low-resolution CT were significant enough to warrant follow-up tests, but only 0.2% were deemed potentially detrimental to patient outcomes (ie, pathology confirmed malignancies).12 Thus, the authors suggested that routine reporting of incidental findings on low-dose CT images was not beneficial.12,13
Currently, the majority of cardiac MPIs are reviewed and interpreted by nuclear cardiologists, the use of hybrid SPECT/CT for attenuation correction give rise of issue of reviewing and interpreting these CT images during cardiac MPI. Since low-dose, low-resolution CT are considered nondiagnostic, these images are not routinely and readily reviewed by cardiologists who are not trained or skilled in CT interpretations.
Studies of high-resolution cardiac CT (including multidetector CT with contrast) suggest that incidental extracardiac findings should always be reported as there was a 0.7% incidence of previously unknown malignancies, while others have argued against “performing large field reconstructionsfor the explicit purpose of screening as it will lead to additional cost, liability and anxiety without proven benefits.”14-16 A review of incidental findings of cardiac CT by Earls suggested that all cardiac CT should be reconstructed in the maximal field of view available and images should be adequately reviewed to detect pathological findings.17 This led to an interesting discussion by Douglas and colleagues regarding the role of cardiologists and radiologists in this issue.18 Currently there is no uniform or consensus recommendations regarding incidental findings during cardiac CT imaging. Guidances range from no recommendations to optional reporting or mandatory reporting.19-23
Risk Factors for Veterans
Lung cancer is the second most common cancer and the leading cause of cancer-related death in the US.24 Smoking is the most important risk factor for lung cancer and CAD.25 Current or past smoking are more common among the veterans.10 According to a report for the US Centers for Disease Control and Prevention report, about 29.2% US veterans use tobacco products between 2010-2015, which is similar to the rate reported in 1997.26
When low-dose CT was used for lung cancer screening, it was associated with a 20.0% reduction in lung cancer mortality and a 6.7% reduction in any cause mortality.7 Currently, the US Preventive Services Task Force (USPSTF) recommends annual low-dose CT screening for lung cancer in high-risk adults that includes patients aged 55 to 80 years who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years.8
It is likely that the cardiac patients in this study might have pulmonary malignancy mortality similar to those reported in the NLST. While other studies have shown a low incidence (0.2%) of detection of malignancy by low-resolution CT during cardiac MPI,12,13 in this study we found pulmonary or hilar malignancy in 0.55% of patients.The higher incidence of malignancy in our study might be due in part to differences in the patient population studied (ie, our veterans patients have a higher proportion of current or past smoking history).10
The CT used in this study is part of the cardiac imaging process. Therefore, there was no additional radiation exposure besides that of the cardiac MPI for patients. Despite the limitations of low-resolution CT, which may miss small lesions, this study showed 0.55% incidence of incidental detection of pulmonary/hilar malignancy. This is comparable with 0.65%/year of diagnosing lung cancer using low-dose CT for lung cancer screening in NLST.8
Two of the 5 study patients who were found to have lung cancer, had quit smoking > 15 years previously and thus would not be considered as high-risk for lung cancer screening according to USPSTF guideline. These patients would not have been candidates for annual low-dose CT lung cancer screening. This study suggests that it is appropriate and necessary to review the low-resolution CT images for incidental findings during cardiac MPI.
Limitations
The study was retrospective in nature and limited by its small number of patients. The CT modality used in the study also has limitations, including low resolution, respiratory motion artifacts, and scans that did not include the entire chest area. Therefore, small and apical lesions may have been missed. However, both sets of CT at rest and after stress were reviewed to reduce or minimize the effects of respiratory motion artifacts. The true prevalence or incidence of pulmonary/hilar malignancies may have been higher than reported here. Our study population of veterans may not be representative of the general population with regards to gender (as most of our veteran patient population are of male gender, vs general population), smoking history, or lung cancer risk, thus the results should be interpreted with caution.
Conclusion
Low-resolution CTs used for attenuation correction during cardiac MPI should be routinely reviewed and interpreted by a physician or radiologist skilled in CT interpretation in order to identify incidental findings of pulmonary/hilar malignancy. This would require close collaboration between cardiologists and radiologists in the field to ensure unfragmented and high-quality patient care.
Acknowledgements
We want to thank all the staffs in cardiology and radiology department on both campuses for their dedication for our patients. Special thanks to Laura Knox, Radiation Safety Officer, Nuclear Medicine Supervisor for her technical assistance.
1. Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation. 2009;119(22):e561-e587.
2. Hendel RC, Corbett JR, Cullom SJ, DePuey EG, Garcia EV, Bateman TM. The value and practice of attenuation correction for myocardial perfusion SPECT imaging: a joint position statement from the American Society of Nuclear Cardiology and the Society of Nuclear Medicine. J Nucl Cardiol. 2002;9(1):135–143.
3. Coward J, Nightingale J, Hogg P. The clinical dilemma of incidental findings on the low-resolution CT images from SPECT/CT MPI studies. J Nucl Med Technol. 2016;44(3):167-172.
4. Osman MM, Cohade C, Fishman E, Wahl RL. Clinically significant incidental findings on the unenhanced CT portion of PET/CT studies: frequency in 250 patients. J Nucl Med. 2005;46(8):1352-1355.
5. Goetze S, Pannu HK, Wahl RL. Clinically significant abnormal findings on the “nondiagnostic” CT portion of low-amperage-CT attenuation-corrected myocardial perfusion SPECT/CT studies. J Nucl Med. 2006;47(8):1312-1318.
6. American College of Cardiology Foundation Task Force on Expert Consensus Documents, Mark DB, Berman DS, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55(23):2663-2699.
7. Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology. 2002;222(3):773-781.
8. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
9. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338.
10. McKinney WP, McIntire DD, Carmody TJ, Joseph A. Comparing the smoking behavior of veterans and nonveterans. Public Health Rep. 1997;112(3):212-218.
11. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
12. Coward J, Lawson R, Kane T, et al. Multi-centre analysis of incidental findings on low-resolution CT attenuation correction images. Br J Radiol. 2014;87(1042):20130701.
13. Coward J, Lawson R, Kane T, et al. Multicentre analysis of incidental findings on low-resolution CT attenuation correction images: an extended study. Br J Radiol. 2015;88(1056):20150555.
14. Haller S, Kaiser C, Buser P, Bongartz G, Bremerich J. Coronary artery imaging with contrast-enhanced MDCT: extracardiac findings. AJR Am J Roentgenol. 2006;187(1):105-110.
15. Flor N, Di Leo G, Squarza SA, et al. Malignant incidental extracardiac findings on cardiac CT: systematic review and meta-analysis. AJR Am J Roentgenol. 2013;201(3):555-564.
16. Budoff MJ, Gopal A. Incidental findings on cardiac computed tomography. Should we look? J Cardiovasc Comput Tomogr. 2007;1(2):97-105.
17. Earls JP. The pros and cons of searching for extracardiac findings at cardiac CT: studies should be reconstructed in the maximum field of view and adequately reviewed to detect pathologic findings. Radiology. 2011;261(2):342-346.
18. Douglas PS, Cerqueria M, Rubin GD, Chin AS. Extracardiac findings: what is a cardiologist to do? JACC Cardiovasc Imaging. 2008;1(5):682-687.
19. Holly TA, Abbott BG, Al-Mallah M, et al. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17(5):941-973.
20. Dorbala S, Ananthasubramaniam K, Armstrong IS, et al. Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol. 2018;25(5):1784-1846.
21. Tilkemeier PL, Bourque J, Doukky R, Sanghani R, Weinberg RL. ASNC imaging guidelines for nuclear cardiology procedures : Standardized reporting of nuclear cardiology procedures. J Nucl Cardiol. 2017;24(6):2064-2128.
22. Dorbala S, Di Carli MF, Delbeke D, et al. SNMMI/ASNC/SCCT guideline for cardiac SPECT/CT and PET/CT 1.0. J Nucl Med. 2013;54(8):1485-1507.
23. Dilsizian V, Bacharach SL, Beanlands RS, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23(5):1187-1226.
24. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109(9):djx030.
25. US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Printed with corrections, January 2014.
26. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco Product Use Among Military Veterans - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12.
Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) is a well-established technique for the evaluation of coronary artery disease (CAD).1 To improve image quality, low-resolution computed tomography (CT) is used commonly for anatomical correct and artifact attenuation during SPECT MPI.2 The low resolution, unenhanced CT images are considered low quality and are, therefore, labeled by the manufacturer as nondiagnostic. The CT portion of the MPI in many centers is used only for image fusion and attenuation correction, and these images are not routinely reviewed or reported by cardiologists.
Incidental findings by these low-resolution CT were frequent. However, clinically significant findings, including lung cancer, although relatively infrequent, were serious enough for major clinical management.3-5 Currently, there are no consensus recommendations for reviewing low-resolution CT images or the interpretation of such incidental findings during cardiac MPI.6 Clinically, low-dose CT were used for early detection and screening of lung cancer and were associated with reduced lung-cancer and any cause mortality in National Lung Screening Trial (NLST).7,8 Therefore, low-dose CT is recommended for lung cancer screening of high-risk patients by the US Preventive Service Task Force (USPSTF).9 In the veteran population, current and past smoking history are more common when compared with the general population; therefore, veterans are potentially at increased risk of lung cancer.10 In this study, we did not intend to use low-resolution CT for lung cancer screening or detection but rather to identify and report incidental findings of pulmonary/hilar malignancy detected during cardiac MPI.
Methods
The Siemens’ (Munich, Germany) Symbia Intevo Excel SPECT/CT MPI cameras with dedicated cardiac collimators were used at both the Dwight D. Eisenhower VA Medical Center (VAMC) in Leavenworth, Kansas and Colmery-O'Neil VAMC in Topeka, Kansas. The integrated CT scanner (x-ray tube current 30 to 240 mA; voltage 110 Kv with a 40 kW power generator) has the capability to image up to a 2-slice/rotation, each of 5.0 mm per slice with a scan time of about 30 seconds. The SPECT/CT gamma camera has a low energy (140 KeV), high resolution, parallel hole collimator with IQ SPECT capabilities.
The radiation dose received by the patients were expressed in dose length product (DLP), which reflects the total energy absorbed by the patient and represents integrated dose in terms of the total scan length. Additionally, each patients received 2 injections of Technetium Tc 99m sestamibi (1-day Protocol: 10 mCi rest injection, 30 mCi stress injection: 2-day Protocol for patients weighing > 350 pounds: 30 mCi at rest injection and 30 mCi at stress injection) for myocardial perfusion imaging.
All CT images and cardiac MPI findings were reviewed and reported contemporaneously by 1 of 2 experienced, board-certified radiologists who were blinded to patients’ clinical information except the indication for the cardiac stress testing. When suspicious pulmonary/hilar nodules or masses were detected, these findings and recommendations for further evaluation were conveyed to primary care provider or ordering physician via the electronic health record system.
All CT images were reviewed with cardiac MPI from September 1, 2017 to August 31, 2018. When pulmonary/hilar malignancies were identified, the health records were reviewed. Patients with known history of prior pulmonary malignancy were excluded from the study.
Results
A total of 1,098 patients underwent cardiac MPI during the study period. When the CT imaging and cardiac MPI were reviewed, incidental findings led to the diagnosis of lung cancer in 5 patients and hilar mantle cell lymphoma in 1 patient. Their clinical characteristics, CT findings, and types of malignancies for these 6 patients are summarized in the Table and Figure. Only 0.55% (6 of 1,098) patients were found to have incidental pulmonary/hilar malignancy with the cardiac evaluation low-resolution CT. Four patients with prior, known history of lung cancer were excluded from the study.
For the 6 patients found to have cancer, the average CT radiation dose during the cardiac MPI was 100 mGy-cm (range, 77 -133 mCy-cm). The subsequent chest CT with or without contrast delivered a radiation dose of 726.4 mGy-cm (range, 279.4 - 1,075 mGy-cm).
A total of 79 (7.2%) patients were found to have significant pulmonary nodules that required further evaluation; after CT examination, 32 patients had findings of benign nature and required no further follow-up; the other 47 patients are being followed according to the Fleischner Society 2017 guidelines for pulmonary nodules.11 The follow-up findings on these patients are not within the scope of this report.
Discussion
Although incidental findings on low-resolution CT during cardiac MPI are frequent, clinically significant findings are less common. However, some incidental findings may be of important clinical significance.3-5 A multicenter analysis by Coward and colleagues reported that 2.4% findings on low-resolution CT were significant enough to warrant follow-up tests, but only 0.2% were deemed potentially detrimental to patient outcomes (ie, pathology confirmed malignancies).12 Thus, the authors suggested that routine reporting of incidental findings on low-dose CT images was not beneficial.12,13
Currently, the majority of cardiac MPIs are reviewed and interpreted by nuclear cardiologists, the use of hybrid SPECT/CT for attenuation correction give rise of issue of reviewing and interpreting these CT images during cardiac MPI. Since low-dose, low-resolution CT are considered nondiagnostic, these images are not routinely and readily reviewed by cardiologists who are not trained or skilled in CT interpretations.
Studies of high-resolution cardiac CT (including multidetector CT with contrast) suggest that incidental extracardiac findings should always be reported as there was a 0.7% incidence of previously unknown malignancies, while others have argued against “performing large field reconstructionsfor the explicit purpose of screening as it will lead to additional cost, liability and anxiety without proven benefits.”14-16 A review of incidental findings of cardiac CT by Earls suggested that all cardiac CT should be reconstructed in the maximal field of view available and images should be adequately reviewed to detect pathological findings.17 This led to an interesting discussion by Douglas and colleagues regarding the role of cardiologists and radiologists in this issue.18 Currently there is no uniform or consensus recommendations regarding incidental findings during cardiac CT imaging. Guidances range from no recommendations to optional reporting or mandatory reporting.19-23
Risk Factors for Veterans
Lung cancer is the second most common cancer and the leading cause of cancer-related death in the US.24 Smoking is the most important risk factor for lung cancer and CAD.25 Current or past smoking are more common among the veterans.10 According to a report for the US Centers for Disease Control and Prevention report, about 29.2% US veterans use tobacco products between 2010-2015, which is similar to the rate reported in 1997.26
When low-dose CT was used for lung cancer screening, it was associated with a 20.0% reduction in lung cancer mortality and a 6.7% reduction in any cause mortality.7 Currently, the US Preventive Services Task Force (USPSTF) recommends annual low-dose CT screening for lung cancer in high-risk adults that includes patients aged 55 to 80 years who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years.8
It is likely that the cardiac patients in this study might have pulmonary malignancy mortality similar to those reported in the NLST. While other studies have shown a low incidence (0.2%) of detection of malignancy by low-resolution CT during cardiac MPI,12,13 in this study we found pulmonary or hilar malignancy in 0.55% of patients.The higher incidence of malignancy in our study might be due in part to differences in the patient population studied (ie, our veterans patients have a higher proportion of current or past smoking history).10
The CT used in this study is part of the cardiac imaging process. Therefore, there was no additional radiation exposure besides that of the cardiac MPI for patients. Despite the limitations of low-resolution CT, which may miss small lesions, this study showed 0.55% incidence of incidental detection of pulmonary/hilar malignancy. This is comparable with 0.65%/year of diagnosing lung cancer using low-dose CT for lung cancer screening in NLST.8
Two of the 5 study patients who were found to have lung cancer, had quit smoking > 15 years previously and thus would not be considered as high-risk for lung cancer screening according to USPSTF guideline. These patients would not have been candidates for annual low-dose CT lung cancer screening. This study suggests that it is appropriate and necessary to review the low-resolution CT images for incidental findings during cardiac MPI.
Limitations
The study was retrospective in nature and limited by its small number of patients. The CT modality used in the study also has limitations, including low resolution, respiratory motion artifacts, and scans that did not include the entire chest area. Therefore, small and apical lesions may have been missed. However, both sets of CT at rest and after stress were reviewed to reduce or minimize the effects of respiratory motion artifacts. The true prevalence or incidence of pulmonary/hilar malignancies may have been higher than reported here. Our study population of veterans may not be representative of the general population with regards to gender (as most of our veteran patient population are of male gender, vs general population), smoking history, or lung cancer risk, thus the results should be interpreted with caution.
Conclusion
Low-resolution CTs used for attenuation correction during cardiac MPI should be routinely reviewed and interpreted by a physician or radiologist skilled in CT interpretation in order to identify incidental findings of pulmonary/hilar malignancy. This would require close collaboration between cardiologists and radiologists in the field to ensure unfragmented and high-quality patient care.
Acknowledgements
We want to thank all the staffs in cardiology and radiology department on both campuses for their dedication for our patients. Special thanks to Laura Knox, Radiation Safety Officer, Nuclear Medicine Supervisor for her technical assistance.
Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) is a well-established technique for the evaluation of coronary artery disease (CAD).1 To improve image quality, low-resolution computed tomography (CT) is used commonly for anatomical correct and artifact attenuation during SPECT MPI.2 The low resolution, unenhanced CT images are considered low quality and are, therefore, labeled by the manufacturer as nondiagnostic. The CT portion of the MPI in many centers is used only for image fusion and attenuation correction, and these images are not routinely reviewed or reported by cardiologists.
Incidental findings by these low-resolution CT were frequent. However, clinically significant findings, including lung cancer, although relatively infrequent, were serious enough for major clinical management.3-5 Currently, there are no consensus recommendations for reviewing low-resolution CT images or the interpretation of such incidental findings during cardiac MPI.6 Clinically, low-dose CT were used for early detection and screening of lung cancer and were associated with reduced lung-cancer and any cause mortality in National Lung Screening Trial (NLST).7,8 Therefore, low-dose CT is recommended for lung cancer screening of high-risk patients by the US Preventive Service Task Force (USPSTF).9 In the veteran population, current and past smoking history are more common when compared with the general population; therefore, veterans are potentially at increased risk of lung cancer.10 In this study, we did not intend to use low-resolution CT for lung cancer screening or detection but rather to identify and report incidental findings of pulmonary/hilar malignancy detected during cardiac MPI.
Methods
The Siemens’ (Munich, Germany) Symbia Intevo Excel SPECT/CT MPI cameras with dedicated cardiac collimators were used at both the Dwight D. Eisenhower VA Medical Center (VAMC) in Leavenworth, Kansas and Colmery-O'Neil VAMC in Topeka, Kansas. The integrated CT scanner (x-ray tube current 30 to 240 mA; voltage 110 Kv with a 40 kW power generator) has the capability to image up to a 2-slice/rotation, each of 5.0 mm per slice with a scan time of about 30 seconds. The SPECT/CT gamma camera has a low energy (140 KeV), high resolution, parallel hole collimator with IQ SPECT capabilities.
The radiation dose received by the patients were expressed in dose length product (DLP), which reflects the total energy absorbed by the patient and represents integrated dose in terms of the total scan length. Additionally, each patients received 2 injections of Technetium Tc 99m sestamibi (1-day Protocol: 10 mCi rest injection, 30 mCi stress injection: 2-day Protocol for patients weighing > 350 pounds: 30 mCi at rest injection and 30 mCi at stress injection) for myocardial perfusion imaging.
All CT images and cardiac MPI findings were reviewed and reported contemporaneously by 1 of 2 experienced, board-certified radiologists who were blinded to patients’ clinical information except the indication for the cardiac stress testing. When suspicious pulmonary/hilar nodules or masses were detected, these findings and recommendations for further evaluation were conveyed to primary care provider or ordering physician via the electronic health record system.
All CT images were reviewed with cardiac MPI from September 1, 2017 to August 31, 2018. When pulmonary/hilar malignancies were identified, the health records were reviewed. Patients with known history of prior pulmonary malignancy were excluded from the study.
Results
A total of 1,098 patients underwent cardiac MPI during the study period. When the CT imaging and cardiac MPI were reviewed, incidental findings led to the diagnosis of lung cancer in 5 patients and hilar mantle cell lymphoma in 1 patient. Their clinical characteristics, CT findings, and types of malignancies for these 6 patients are summarized in the Table and Figure. Only 0.55% (6 of 1,098) patients were found to have incidental pulmonary/hilar malignancy with the cardiac evaluation low-resolution CT. Four patients with prior, known history of lung cancer were excluded from the study.
For the 6 patients found to have cancer, the average CT radiation dose during the cardiac MPI was 100 mGy-cm (range, 77 -133 mCy-cm). The subsequent chest CT with or without contrast delivered a radiation dose of 726.4 mGy-cm (range, 279.4 - 1,075 mGy-cm).
A total of 79 (7.2%) patients were found to have significant pulmonary nodules that required further evaluation; after CT examination, 32 patients had findings of benign nature and required no further follow-up; the other 47 patients are being followed according to the Fleischner Society 2017 guidelines for pulmonary nodules.11 The follow-up findings on these patients are not within the scope of this report.
Discussion
Although incidental findings on low-resolution CT during cardiac MPI are frequent, clinically significant findings are less common. However, some incidental findings may be of important clinical significance.3-5 A multicenter analysis by Coward and colleagues reported that 2.4% findings on low-resolution CT were significant enough to warrant follow-up tests, but only 0.2% were deemed potentially detrimental to patient outcomes (ie, pathology confirmed malignancies).12 Thus, the authors suggested that routine reporting of incidental findings on low-dose CT images was not beneficial.12,13
Currently, the majority of cardiac MPIs are reviewed and interpreted by nuclear cardiologists, the use of hybrid SPECT/CT for attenuation correction give rise of issue of reviewing and interpreting these CT images during cardiac MPI. Since low-dose, low-resolution CT are considered nondiagnostic, these images are not routinely and readily reviewed by cardiologists who are not trained or skilled in CT interpretations.
Studies of high-resolution cardiac CT (including multidetector CT with contrast) suggest that incidental extracardiac findings should always be reported as there was a 0.7% incidence of previously unknown malignancies, while others have argued against “performing large field reconstructionsfor the explicit purpose of screening as it will lead to additional cost, liability and anxiety without proven benefits.”14-16 A review of incidental findings of cardiac CT by Earls suggested that all cardiac CT should be reconstructed in the maximal field of view available and images should be adequately reviewed to detect pathological findings.17 This led to an interesting discussion by Douglas and colleagues regarding the role of cardiologists and radiologists in this issue.18 Currently there is no uniform or consensus recommendations regarding incidental findings during cardiac CT imaging. Guidances range from no recommendations to optional reporting or mandatory reporting.19-23
Risk Factors for Veterans
Lung cancer is the second most common cancer and the leading cause of cancer-related death in the US.24 Smoking is the most important risk factor for lung cancer and CAD.25 Current or past smoking are more common among the veterans.10 According to a report for the US Centers for Disease Control and Prevention report, about 29.2% US veterans use tobacco products between 2010-2015, which is similar to the rate reported in 1997.26
When low-dose CT was used for lung cancer screening, it was associated with a 20.0% reduction in lung cancer mortality and a 6.7% reduction in any cause mortality.7 Currently, the US Preventive Services Task Force (USPSTF) recommends annual low-dose CT screening for lung cancer in high-risk adults that includes patients aged 55 to 80 years who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years.8
It is likely that the cardiac patients in this study might have pulmonary malignancy mortality similar to those reported in the NLST. While other studies have shown a low incidence (0.2%) of detection of malignancy by low-resolution CT during cardiac MPI,12,13 in this study we found pulmonary or hilar malignancy in 0.55% of patients.The higher incidence of malignancy in our study might be due in part to differences in the patient population studied (ie, our veterans patients have a higher proportion of current or past smoking history).10
The CT used in this study is part of the cardiac imaging process. Therefore, there was no additional radiation exposure besides that of the cardiac MPI for patients. Despite the limitations of low-resolution CT, which may miss small lesions, this study showed 0.55% incidence of incidental detection of pulmonary/hilar malignancy. This is comparable with 0.65%/year of diagnosing lung cancer using low-dose CT for lung cancer screening in NLST.8
Two of the 5 study patients who were found to have lung cancer, had quit smoking > 15 years previously and thus would not be considered as high-risk for lung cancer screening according to USPSTF guideline. These patients would not have been candidates for annual low-dose CT lung cancer screening. This study suggests that it is appropriate and necessary to review the low-resolution CT images for incidental findings during cardiac MPI.
Limitations
The study was retrospective in nature and limited by its small number of patients. The CT modality used in the study also has limitations, including low resolution, respiratory motion artifacts, and scans that did not include the entire chest area. Therefore, small and apical lesions may have been missed. However, both sets of CT at rest and after stress were reviewed to reduce or minimize the effects of respiratory motion artifacts. The true prevalence or incidence of pulmonary/hilar malignancies may have been higher than reported here. Our study population of veterans may not be representative of the general population with regards to gender (as most of our veteran patient population are of male gender, vs general population), smoking history, or lung cancer risk, thus the results should be interpreted with caution.
Conclusion
Low-resolution CTs used for attenuation correction during cardiac MPI should be routinely reviewed and interpreted by a physician or radiologist skilled in CT interpretation in order to identify incidental findings of pulmonary/hilar malignancy. This would require close collaboration between cardiologists and radiologists in the field to ensure unfragmented and high-quality patient care.
Acknowledgements
We want to thank all the staffs in cardiology and radiology department on both campuses for their dedication for our patients. Special thanks to Laura Knox, Radiation Safety Officer, Nuclear Medicine Supervisor for her technical assistance.
1. Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation. 2009;119(22):e561-e587.
2. Hendel RC, Corbett JR, Cullom SJ, DePuey EG, Garcia EV, Bateman TM. The value and practice of attenuation correction for myocardial perfusion SPECT imaging: a joint position statement from the American Society of Nuclear Cardiology and the Society of Nuclear Medicine. J Nucl Cardiol. 2002;9(1):135–143.
3. Coward J, Nightingale J, Hogg P. The clinical dilemma of incidental findings on the low-resolution CT images from SPECT/CT MPI studies. J Nucl Med Technol. 2016;44(3):167-172.
4. Osman MM, Cohade C, Fishman E, Wahl RL. Clinically significant incidental findings on the unenhanced CT portion of PET/CT studies: frequency in 250 patients. J Nucl Med. 2005;46(8):1352-1355.
5. Goetze S, Pannu HK, Wahl RL. Clinically significant abnormal findings on the “nondiagnostic” CT portion of low-amperage-CT attenuation-corrected myocardial perfusion SPECT/CT studies. J Nucl Med. 2006;47(8):1312-1318.
6. American College of Cardiology Foundation Task Force on Expert Consensus Documents, Mark DB, Berman DS, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55(23):2663-2699.
7. Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology. 2002;222(3):773-781.
8. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
9. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338.
10. McKinney WP, McIntire DD, Carmody TJ, Joseph A. Comparing the smoking behavior of veterans and nonveterans. Public Health Rep. 1997;112(3):212-218.
11. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
12. Coward J, Lawson R, Kane T, et al. Multi-centre analysis of incidental findings on low-resolution CT attenuation correction images. Br J Radiol. 2014;87(1042):20130701.
13. Coward J, Lawson R, Kane T, et al. Multicentre analysis of incidental findings on low-resolution CT attenuation correction images: an extended study. Br J Radiol. 2015;88(1056):20150555.
14. Haller S, Kaiser C, Buser P, Bongartz G, Bremerich J. Coronary artery imaging with contrast-enhanced MDCT: extracardiac findings. AJR Am J Roentgenol. 2006;187(1):105-110.
15. Flor N, Di Leo G, Squarza SA, et al. Malignant incidental extracardiac findings on cardiac CT: systematic review and meta-analysis. AJR Am J Roentgenol. 2013;201(3):555-564.
16. Budoff MJ, Gopal A. Incidental findings on cardiac computed tomography. Should we look? J Cardiovasc Comput Tomogr. 2007;1(2):97-105.
17. Earls JP. The pros and cons of searching for extracardiac findings at cardiac CT: studies should be reconstructed in the maximum field of view and adequately reviewed to detect pathologic findings. Radiology. 2011;261(2):342-346.
18. Douglas PS, Cerqueria M, Rubin GD, Chin AS. Extracardiac findings: what is a cardiologist to do? JACC Cardiovasc Imaging. 2008;1(5):682-687.
19. Holly TA, Abbott BG, Al-Mallah M, et al. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17(5):941-973.
20. Dorbala S, Ananthasubramaniam K, Armstrong IS, et al. Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol. 2018;25(5):1784-1846.
21. Tilkemeier PL, Bourque J, Doukky R, Sanghani R, Weinberg RL. ASNC imaging guidelines for nuclear cardiology procedures : Standardized reporting of nuclear cardiology procedures. J Nucl Cardiol. 2017;24(6):2064-2128.
22. Dorbala S, Di Carli MF, Delbeke D, et al. SNMMI/ASNC/SCCT guideline for cardiac SPECT/CT and PET/CT 1.0. J Nucl Med. 2013;54(8):1485-1507.
23. Dilsizian V, Bacharach SL, Beanlands RS, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23(5):1187-1226.
24. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109(9):djx030.
25. US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Printed with corrections, January 2014.
26. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco Product Use Among Military Veterans - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12.
1. Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation. 2009;119(22):e561-e587.
2. Hendel RC, Corbett JR, Cullom SJ, DePuey EG, Garcia EV, Bateman TM. The value and practice of attenuation correction for myocardial perfusion SPECT imaging: a joint position statement from the American Society of Nuclear Cardiology and the Society of Nuclear Medicine. J Nucl Cardiol. 2002;9(1):135–143.
3. Coward J, Nightingale J, Hogg P. The clinical dilemma of incidental findings on the low-resolution CT images from SPECT/CT MPI studies. J Nucl Med Technol. 2016;44(3):167-172.
4. Osman MM, Cohade C, Fishman E, Wahl RL. Clinically significant incidental findings on the unenhanced CT portion of PET/CT studies: frequency in 250 patients. J Nucl Med. 2005;46(8):1352-1355.
5. Goetze S, Pannu HK, Wahl RL. Clinically significant abnormal findings on the “nondiagnostic” CT portion of low-amperage-CT attenuation-corrected myocardial perfusion SPECT/CT studies. J Nucl Med. 2006;47(8):1312-1318.
6. American College of Cardiology Foundation Task Force on Expert Consensus Documents, Mark DB, Berman DS, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55(23):2663-2699.
7. Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology. 2002;222(3):773-781.
8. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
9. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338.
10. McKinney WP, McIntire DD, Carmody TJ, Joseph A. Comparing the smoking behavior of veterans and nonveterans. Public Health Rep. 1997;112(3):212-218.
11. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
12. Coward J, Lawson R, Kane T, et al. Multi-centre analysis of incidental findings on low-resolution CT attenuation correction images. Br J Radiol. 2014;87(1042):20130701.
13. Coward J, Lawson R, Kane T, et al. Multicentre analysis of incidental findings on low-resolution CT attenuation correction images: an extended study. Br J Radiol. 2015;88(1056):20150555.
14. Haller S, Kaiser C, Buser P, Bongartz G, Bremerich J. Coronary artery imaging with contrast-enhanced MDCT: extracardiac findings. AJR Am J Roentgenol. 2006;187(1):105-110.
15. Flor N, Di Leo G, Squarza SA, et al. Malignant incidental extracardiac findings on cardiac CT: systematic review and meta-analysis. AJR Am J Roentgenol. 2013;201(3):555-564.
16. Budoff MJ, Gopal A. Incidental findings on cardiac computed tomography. Should we look? J Cardiovasc Comput Tomogr. 2007;1(2):97-105.
17. Earls JP. The pros and cons of searching for extracardiac findings at cardiac CT: studies should be reconstructed in the maximum field of view and adequately reviewed to detect pathologic findings. Radiology. 2011;261(2):342-346.
18. Douglas PS, Cerqueria M, Rubin GD, Chin AS. Extracardiac findings: what is a cardiologist to do? JACC Cardiovasc Imaging. 2008;1(5):682-687.
19. Holly TA, Abbott BG, Al-Mallah M, et al. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17(5):941-973.
20. Dorbala S, Ananthasubramaniam K, Armstrong IS, et al. Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol. 2018;25(5):1784-1846.
21. Tilkemeier PL, Bourque J, Doukky R, Sanghani R, Weinberg RL. ASNC imaging guidelines for nuclear cardiology procedures : Standardized reporting of nuclear cardiology procedures. J Nucl Cardiol. 2017;24(6):2064-2128.
22. Dorbala S, Di Carli MF, Delbeke D, et al. SNMMI/ASNC/SCCT guideline for cardiac SPECT/CT and PET/CT 1.0. J Nucl Med. 2013;54(8):1485-1507.
23. Dilsizian V, Bacharach SL, Beanlands RS, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23(5):1187-1226.
24. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109(9):djx030.
25. US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Printed with corrections, January 2014.
26. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco Product Use Among Military Veterans - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12.