User login
Azacytidine-treated MDS patients at risk for invasive fungal infection
Key clinical point: Azacytidine-treated patients with myelodysplastic syndrome (MDS) are at significant risk for invasive fungal infection (IFI) with a corresponding higher risk for mortality, warranting mold-spectrum prophylaxis in these patients.
Major finding: Overall, 7.7% of patients developed IFI at a rate of 10.9% in patients who did not receive fungal prophylaxis. IFI was associated with a significantly higher risk for death (hazard ratio, 8.37; P less than .0001).
Study details: Findings are from a retrospective cohort study of 117 patients receiving 5-azacytidine for MDS and low blast count acute myeloid leukemia.
Disclosures: The study was funded by a Monash Haematology research grant. B Rogers and J Shortt declared being on advisory boards and receiving research grants, speaker, and consultation fees from various sources.
Source: Tey A et al. Eur J Haematol. 2021 Apr 7. doi: 10.1111/ejh.13631
Key clinical point: Azacytidine-treated patients with myelodysplastic syndrome (MDS) are at significant risk for invasive fungal infection (IFI) with a corresponding higher risk for mortality, warranting mold-spectrum prophylaxis in these patients.
Major finding: Overall, 7.7% of patients developed IFI at a rate of 10.9% in patients who did not receive fungal prophylaxis. IFI was associated with a significantly higher risk for death (hazard ratio, 8.37; P less than .0001).
Study details: Findings are from a retrospective cohort study of 117 patients receiving 5-azacytidine for MDS and low blast count acute myeloid leukemia.
Disclosures: The study was funded by a Monash Haematology research grant. B Rogers and J Shortt declared being on advisory boards and receiving research grants, speaker, and consultation fees from various sources.
Source: Tey A et al. Eur J Haematol. 2021 Apr 7. doi: 10.1111/ejh.13631
Key clinical point: Azacytidine-treated patients with myelodysplastic syndrome (MDS) are at significant risk for invasive fungal infection (IFI) with a corresponding higher risk for mortality, warranting mold-spectrum prophylaxis in these patients.
Major finding: Overall, 7.7% of patients developed IFI at a rate of 10.9% in patients who did not receive fungal prophylaxis. IFI was associated with a significantly higher risk for death (hazard ratio, 8.37; P less than .0001).
Study details: Findings are from a retrospective cohort study of 117 patients receiving 5-azacytidine for MDS and low blast count acute myeloid leukemia.
Disclosures: The study was funded by a Monash Haematology research grant. B Rogers and J Shortt declared being on advisory boards and receiving research grants, speaker, and consultation fees from various sources.
Source: Tey A et al. Eur J Haematol. 2021 Apr 7. doi: 10.1111/ejh.13631
MDS: Low lymphocyte-to-monocyte ratio predicts better outcomes
Key clinical point: Low lymphocyte-to-monocyte ratio (LMR) in patients with myelodysplastic syndrome (MDS) is associated with a favorable prognosis.
Major finding: LMR lesser than vs. greater than 5 was associated with a lower risk for leukemic transformation (median time not reached; P = .003) and better leukemia-free survival (median, 48 months vs. 21 months; P = .03).
Study details: Findings are from a retrospective study of 201 patients with a new diagnosis of MDS.
Disclosures: No source of funding was declared. The authors declared no potential conflicts of interest.
Source: Pénichoux J et al. Leuk Lymphoma. 2021 Apr 2. doi: 10.1080/10428194.2021.1907381
Key clinical point: Low lymphocyte-to-monocyte ratio (LMR) in patients with myelodysplastic syndrome (MDS) is associated with a favorable prognosis.
Major finding: LMR lesser than vs. greater than 5 was associated with a lower risk for leukemic transformation (median time not reached; P = .003) and better leukemia-free survival (median, 48 months vs. 21 months; P = .03).
Study details: Findings are from a retrospective study of 201 patients with a new diagnosis of MDS.
Disclosures: No source of funding was declared. The authors declared no potential conflicts of interest.
Source: Pénichoux J et al. Leuk Lymphoma. 2021 Apr 2. doi: 10.1080/10428194.2021.1907381
Key clinical point: Low lymphocyte-to-monocyte ratio (LMR) in patients with myelodysplastic syndrome (MDS) is associated with a favorable prognosis.
Major finding: LMR lesser than vs. greater than 5 was associated with a lower risk for leukemic transformation (median time not reached; P = .003) and better leukemia-free survival (median, 48 months vs. 21 months; P = .03).
Study details: Findings are from a retrospective study of 201 patients with a new diagnosis of MDS.
Disclosures: No source of funding was declared. The authors declared no potential conflicts of interest.
Source: Pénichoux J et al. Leuk Lymphoma. 2021 Apr 2. doi: 10.1080/10428194.2021.1907381
MDS-associated autoimmune manifestations predict poor prognosis
Key clinical point: Presence of autoimmune manifestations (AIMs) predicts poor prognosis irrespective of disease severity in patients with myelodysplastic syndrome (MDS).
Major finding: MDS-associated AIMs were identified in 20% of patients, with overall survival being shorter in patients with vs. without AIMs (P log-rank = .03). The prognosis was poor and comparable among patients with low-risk MDS and associated AIMs vs. those with high-risk MDS without AIMs (P log-rank = .9).
Study details: Findings are from a retrospective study of 61 patients with a new diagnosis of MDS.
Disclosures: This research was supported by Grant-in-Aid for Scientific Research(C). The authors declared no conflicts of interest.
Source: Arinobu Y et al. Medicine (Baltimore). 2021 Apr 2. doi: 10.1097/MD.0000000000025406
Key clinical point: Presence of autoimmune manifestations (AIMs) predicts poor prognosis irrespective of disease severity in patients with myelodysplastic syndrome (MDS).
Major finding: MDS-associated AIMs were identified in 20% of patients, with overall survival being shorter in patients with vs. without AIMs (P log-rank = .03). The prognosis was poor and comparable among patients with low-risk MDS and associated AIMs vs. those with high-risk MDS without AIMs (P log-rank = .9).
Study details: Findings are from a retrospective study of 61 patients with a new diagnosis of MDS.
Disclosures: This research was supported by Grant-in-Aid for Scientific Research(C). The authors declared no conflicts of interest.
Source: Arinobu Y et al. Medicine (Baltimore). 2021 Apr 2. doi: 10.1097/MD.0000000000025406
Key clinical point: Presence of autoimmune manifestations (AIMs) predicts poor prognosis irrespective of disease severity in patients with myelodysplastic syndrome (MDS).
Major finding: MDS-associated AIMs were identified in 20% of patients, with overall survival being shorter in patients with vs. without AIMs (P log-rank = .03). The prognosis was poor and comparable among patients with low-risk MDS and associated AIMs vs. those with high-risk MDS without AIMs (P log-rank = .9).
Study details: Findings are from a retrospective study of 61 patients with a new diagnosis of MDS.
Disclosures: This research was supported by Grant-in-Aid for Scientific Research(C). The authors declared no conflicts of interest.
Source: Arinobu Y et al. Medicine (Baltimore). 2021 Apr 2. doi: 10.1097/MD.0000000000025406
Azacitidine may allow bridging to salvage allo-HSCT after hematologic relapse
Key clinical point: Azacitidine treatment for hematological relapse of myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) is associated with poor outcomes; however, patients receiving subsequent allogeneic hematopoietic stem cell transplantation (allo-HSCT) may benefit from it.
Major finding: With a median follow-up of 4.7 and 13.6 months, the median overall survival (OS) was 5.9 (95% confidence interval [CI], 3.4-13) months and 9.5 (95% CI, 5.6-NA) months in patients receiving azacitidine as the first-line treatment of relapse and those receiving it after other treatment of relapse, respectively. In addition, the median OS was 11.6 (95% CI, 5.5-NA) months and not reached in patients who proceeded to salvage allo-HSCT in both groups, respectively.
Study details: This was a retrospective multicenter study of 31 patients with MDS or AML who had a hematological relapse after allo-HSCT and were treated with azacitidine.
Disclosures: This research did not receive any specific grant from funding agencies. The authors declared no conflicts of interest.
Source: Drozd-Sokołowska J et al. Eur J Haematol. 2021 Mar 25. doi: 10.1111/ejh.13628.
Key clinical point: Azacitidine treatment for hematological relapse of myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) is associated with poor outcomes; however, patients receiving subsequent allogeneic hematopoietic stem cell transplantation (allo-HSCT) may benefit from it.
Major finding: With a median follow-up of 4.7 and 13.6 months, the median overall survival (OS) was 5.9 (95% confidence interval [CI], 3.4-13) months and 9.5 (95% CI, 5.6-NA) months in patients receiving azacitidine as the first-line treatment of relapse and those receiving it after other treatment of relapse, respectively. In addition, the median OS was 11.6 (95% CI, 5.5-NA) months and not reached in patients who proceeded to salvage allo-HSCT in both groups, respectively.
Study details: This was a retrospective multicenter study of 31 patients with MDS or AML who had a hematological relapse after allo-HSCT and were treated with azacitidine.
Disclosures: This research did not receive any specific grant from funding agencies. The authors declared no conflicts of interest.
Source: Drozd-Sokołowska J et al. Eur J Haematol. 2021 Mar 25. doi: 10.1111/ejh.13628.
Key clinical point: Azacitidine treatment for hematological relapse of myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) is associated with poor outcomes; however, patients receiving subsequent allogeneic hematopoietic stem cell transplantation (allo-HSCT) may benefit from it.
Major finding: With a median follow-up of 4.7 and 13.6 months, the median overall survival (OS) was 5.9 (95% confidence interval [CI], 3.4-13) months and 9.5 (95% CI, 5.6-NA) months in patients receiving azacitidine as the first-line treatment of relapse and those receiving it after other treatment of relapse, respectively. In addition, the median OS was 11.6 (95% CI, 5.5-NA) months and not reached in patients who proceeded to salvage allo-HSCT in both groups, respectively.
Study details: This was a retrospective multicenter study of 31 patients with MDS or AML who had a hematological relapse after allo-HSCT and were treated with azacitidine.
Disclosures: This research did not receive any specific grant from funding agencies. The authors declared no conflicts of interest.
Source: Drozd-Sokołowska J et al. Eur J Haematol. 2021 Mar 25. doi: 10.1111/ejh.13628.
The cloudy role of cannabis as a neuropsychiatric treatment
Although the healing properties of cannabis have been touted for millennia, research into its potential neuropsychiatric applications truly began to take off in the 1990s following the discovery of the cannabinoid system in the brain. This led to speculation that cannabis could play a therapeutic role in regulating dopamine, serotonin, and other neurotransmitters and offer a new means of treating various ailments.
At the same time, efforts to liberalize marijuana laws have successfully played out in several nations, including the United States, where, as of April 29, 36 states provide some access to cannabis. These dual tracks – medical and political – have made cannabis an increasingly accepted part of the cultural fabric.
Yet with this development has come a new quandary for clinicians. Medical cannabis has been made widely available to patients and has largely outpaced the clinical evidence, leaving it unclear how and for which indications it should be used.
The many forms of medical cannabis
Cannabis is a genus of plants that includes marijuana (Cannabis sativa) and hemp. These plants contain over 100 compounds, including terpenes, flavonoids, and – most importantly for medicinal applications – cannabinoids.
The most abundant cannabinoid in marijuana is the psychotropic delta-9-tetrahydrocannabinol (THC), which imparts the “high” sensation. The next most abundant cannabinoid is cannabidiol (CBD), which is the nonpsychotropic. THC and CBD are the most extensively studied cannabinoids, together and in isolation. Evidence suggests that other cannabinoids and terpenoids may also hold medical promise and that cannabis’ various compounds can work synergistically to produce a so-called entourage effect.
Patients walking into a typical medical cannabis dispensary will be faced with several plant-derived and synthetic options, which can differ considerably in terms of the ratios and amounts of THC and CBD they contain, as well in how they are consumed (i.e., via smoke, vapor, ingestion, topical administration, or oromucosal spray), all of which can alter their effects. Further complicating matters is the varying level of oversight each state and country has in how and whether they test for and accurately label products’ potency, cannabinoid content, and possible impurities.
Medically authorized, prescription cannabis products go through an official regulatory review process, and indications/contraindications have been established for them. To date, the Food and Drug Administration has approved one cannabis-derived drug product – Epidiolex (purified CBD) – for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients aged 2 years and older. The FDA has also approved three synthetic cannabis-related drug products – Marinol, Syndros (or dronabinol, created from synthetic THC), and Cesamet (or nabilone, a synthetic cannabinoid similar to THC) – all of which are indicated for treatment-related nausea and anorexia associated with weight loss in AIDS patients.
Surveys of medical cannabis consumers indicate that most people cannot distinguish between THC and CBD, so the first role that physicians find themselves in when recommending this treatment may be in helping patients navigate the volume of options.
Promising treatment for pain
Chronic pain is the leading reason patients seek out medical cannabis. It is also the indication that most researchers agree has the strongest evidence to support its use.
“In my mind, the most promising immediate use for medical cannabis is with THC for pain,” Diana M. Martinez, MD, a professor of psychiatry at Columbia University, New York, who specializes in addiction research, said in a recent MDedge podcast. “THC could be added to the armamentarium of pain medications that we use today.”
In a 2015 systematic literature review, researchers assessed 28 randomized, controlled trials (RCTs) of the use of cannabinoids for chronic pain. They reported that a variety of formulations resulted in at least a 30% reduction in the odds of pain, compared with placebo. A meta-analysis of five RCTs involving patients with neuropathic pain found a 30% reduction in pain over placebo with inhaled, vaporized cannabis. Varying results have been reported in additional studies for this indication. The National Academies of Sciences, Engineering, and Medicine concluded that there was a substantial body of evidence that cannabis is an effective treatment for chronic pain in adults.
The ongoing opioid epidemic has lent these results additional relevance.
Seeing this firsthand has caused Mark Steven Wallace, MD, a pain management specialist and chair of the division of pain medicine at the University of California San Diego Health, to reconsider offering cannabis to his patients.
“I think it’s probably more efficacious, just from my personal experience, and it’s a much lower risk of abuse and dependence than the opioids,” he said.
Dr. Wallace advised that clinicians who treat pain consider the ratios of cannabinoids.
“This is anecdotal, but we do find that with the combination of the two, CBD reduces the psychoactive effects of the THC. The ratios we use during the daytime range around 20 mg of CBD to 1 mg of THC,” he said.
In a recent secondary analysis of an RCT involving patients with painful diabetic peripheral neuropathy, Dr. Wallace and colleagues showed that THC’s effects appear to reverse themselves at a certain level.
“As the THC level goes up, the pain reduces until you reach about 16 ng/mL; then it starts going in the opposite direction, and pain will start to increase,” he said. “Even recreational cannabis users have reported that they avoid high doses because it’s very aversive. Using cannabis is all about, start low and go slow.”
A mixed bag for neurologic indications
There are relatively limited data on the use of medical cannabis for other neurologic conditions, and results have varied. For uses other than pain management, the evidence that does exist is strongest regarding epilepsy, said Daniel Freedman, DO, assistant professor of neurology at the University of Texas at Austin. He noted “multiple high-quality RCTs showing that pharmaceutical-grade CBD can reduce seizures associated with two particular epilepsy syndromes: Dravet Syndrome and Lennox Gastaut.”
These findings led to the FDA’s 2018 approval of Epidiolex for these syndromes. In earlier years, interest in CBD for pediatric seizures was largely driven by anecdotal parental reports of its benefits. NASEM’s 2017 overview on medical cannabis found evidence from subsequent RCTs in this indication to be insufficient. Clinicians who prescribe CBD for this indication must be vigilant because it can interact with several commonly used antiepileptic drugs.
Cannabinoid treatments have also shown success in alleviating muscle spasticity resulting from multiple sclerosis, most prominently in the form of nabiximols (Sativex), a standardized oralmucosal spray containing approximately equal quantities of THC and CBD. Nabiximols is approved in Europe but not in the United States. Moderate evidence supports the efficacy of these and other treatments over placebo in reducing muscle spasticity. Patient ratings of its effects tend to be higher than clinician assessment.
Parkinson’s disease has not yet been approved as an indication for treatment with cannabis or cannabinoids, yet a growing body of preclinical data suggests these could influence the dopaminergic system, said Carsten Buhmann, MD, from the department of neurology at the University Medical Center Hamburg-Eppendorf (Germany).
“In general, cannabinoids modulate basal-ganglia function on two levels which are especially relevant in Parkinson’s disease, i.e., the glutamatergic/dopaminergic synaptic neurotransmission and the corticostriatal plasticity,” he said. “Furthermore, activation of the endocannabinoid system might induce neuroprotective effects related to direct receptor-independent mechanisms, activation of anti-inflammatory cascades in glial cells via the cannabinoid receptor type 2, and antiglutamatergic antiexcitotoxic properties.”
Dr. Buhmann said that currently, clinical evidence is scarce, consisting of only four double-blind, placebo-controlled RCTs involving 49 patients. Various cannabinoids and methods of administering treatment were employed. Improvement was only observed in one of these RCTs, which found that the cannabinoid receptor agonist nabilone significantly reduced levodopa-induced dyskinesia for patients with Parkinson’s disease. Subjective data support a beneficial effect. In a nationwide survey of 1,348 respondents conducted by Dr. Buhmann and colleagues, the majority of medical cannabis users reported that it improved their symptoms (54% with oral CBD and 68% with inhaled THC-containing cannabis).
NASEM concluded that there was insufficient evidence to support the efficacy of medical cannabis for other neurologic conditions, including Tourette syndrome, amyotrophic lateral sclerosis, Huntington disease, dystonia, or dementia. A 2020 position statement from the American Academy of Neurology cited the lack of sufficient peer-reviewed research as the reason it could not currently support the use of cannabis for neurologic disorders.
Yet, according to Dr. Freedman, who served as a coauthor of the AAN position statement, this hasn’t stymied research interest in the topic. He’s seen a substantial uptick in studies of CBD over the past 2 years. “The body of evidence grows, but I still see many claims being made without evidence. And no one seems to care about all the negative trials.”
Cannabis as a treatment for, and cause of, psychiatric disorders
Mental health problems – such as anxiety, depression, and PTSD – are among the most common reasons patients seek out medical cannabis. There is an understandable interest in using cannabis and cannabinoids to treat psychiatric disorders. Preclinical studies suggest that the endocannabinoid system plays a prominent role in modulating feelings of anxiety, mood, and fear. As with opioids and chronic pain management, there is hope that medical cannabis may provide a means of reducing prescription anxiolytics and their associated risks.
The authors of the first systematic review (BMC Psychiatry. 2020 Jan 16;20[1]:24) of the use of medical cannabis for major psychiatric disorders noted that the current evidence was “encouraging, albeit embryonic.”
Meta-analyses have indicated a small but positive association between cannabis use and anxiety, although this may reflect the fact that patients with anxiety sought out this treatment. Given the risks for substance use disorders among patients with anxiety, CBD may present a more viable option. Positive results have been shown as treatment for generalized social anxiety disorder.
Limited but encouraging results have also been reported regarding the alleviation of PTSD symptoms with both cannabis and CBD, although the body of high-quality evidence hasn’t notably progressed since 2017, when NASEM declared that the evidence was insufficient. Supportive evidence is similarly lacking regarding the treatment of depression. Longitudinal studies suggest that cannabis use, particularly heavy use, may increase the risk of developing this disorder. Because THC is psychoactive, it is advised that it be avoided by patients at risk for psychotic disorders. However, CBD has yielded limited benefits for patients with treatment-resistant schizophrenia and for young people at risk for psychosis.
The use of medical cannabis for psychiatric conditions requires a complex balancing act, inasmuch as these treatments may exacerbate the very problems they are intended to alleviate.
Marta Di Forti, MD, PhD, professor of psychiatric research at Kings College London, has been at the forefront of determining the mental health risks of continued cannabis use. In 2019, Dr. Di Forti developed the first and only Cannabis Clinic for Patients With Psychosis in London where she and her colleagues have continued to elucidate this connection.
Dr. Di Forti and colleagues have linked daily cannabis use to an increase in the risk of experiencing psychotic disorder, compared with never using it. That risk was further increased among users of high-potency cannabis (≥10% THC). The latter finding has troubling implications, because concentrations of THC have steadily risen since 1970. By contrast, CBD concentrations have remained generally stable. High-potency cannabis products are common in both recreational and medicinal settings.
“For somebody prescribing medicinal cannabis that has a ≥10% concentration of THC, I’d be particularly wary of the risk of psychosis,” said Dr. Di Forti. “If you’re expecting people to use a high content of THC daily to medicate pain or a chronic condition, you even more so need to be aware that this is a potential side effect.”
Dr. Di Forti noted that her findings come from a cohort of recreational users, most of whom were aged 18-35 years.
“There have actually not been studies developed from collecting data in this area from groups specifically using cannabis for medicinal rather than recreational purposes,” she said.
She added that she personally has no concerns about the use of medical cannabis but wants clinicians to be aware of the risk for psychosis, to structure their patient conversations to identify risk factors or family histories of psychosis, and to become knowledgeable in detecting the often subtle signs of its initial onset.
When cannabis-associated psychosis occurs, Dr. Di Forti said it is primarily treated with conventional means, such as antipsychotics and therapeutic interventions and by refraining from using cannabis. Achieving the latter goal can be a challenge for patients who are daily users of high-potency cannabis. Currently, there are no treatment options such as those offered to patients withdrawing from the use of alcohol or opioids. Dr. Di Forti and colleagues are currently researching a solution to that problem through the use of another medical cannabis, the oromucosal spray Sativex, which has been approved in the European Union.
The regulatory obstacles to clarifying cannabis’ role in medicine
That currently there is limited or no evidence to support the use of medical cannabis for the treatment of neuropsychiatric conditions points to the inherent difficulties in conducting high-level research in this area.
“There’s a tremendous shortage of reliable data, largely due to regulatory barriers,” said Dr. Martinez.
Since 1970, cannabis has been listed as a Schedule I drug that is illegal to prescribe (the Agriculture Improvement Act of 2018 removed hemp from such restrictions). The FDA has issued guidance for researchers who wish to investigate treatments using Cannabis sativa or its derivatives in which the THC content is greater than 0.3%. Such research requires regular interactions with several federal agencies, including the Drug Enforcement Administration.
“It’s impossible to do multicenter RCTs with large numbers of patients, because you can’t transport cannabis across state lines,” said Dr. Wallace.
Regulatory restrictions regarding medical cannabis vary considerably throughout the world (the European Monitoring Center for Drugs and Drug Addiction provides a useful breakdown of this on their website). The lack of consistency in regulatory oversight acts as an impediment for conducting large-scale international multicenter studies on the topic.
Dr. Buhmann noted that, in Germany, cannabis has been broadly approved for treatment-resistant conditions with severe symptoms that impair quality of life. In addition, it is easy to be reimbursed for the use of cannabis as a medical treatment. These factors serve as disincentives for the funding of high-quality studies.
“It’s likely that no pharmaceutical company will do an expensive RCT to get an approval for Parkinson’s disease because it is already possible to prescribe medical cannabis of any type of THC-containing cannabinoid, dose, or route of application,” Dr. Buhmann said.
In the face of such restrictions and barriers, researchers are turning to ambitious real-world data projects to better understand medical cannabis’ efficacy and safety. A notable example is ProjectTwenty21, which is supported by the Royal College of Psychiatrists. The project is collecting outcomes of the use of medical cannabis among 20,000 U.K. patients whose conventional treatments of chronic pain, anxiety disorder, epilepsy, multiple sclerosis, PTSD, substance use disorder, and Tourette syndrome failed.
Dr. Freedman noted that the continued lack of high-quality data creates a void that commercial interests fill with unfounded claims.
“The danger is that patients might abandon a medication or intervention backed by robust science in favor of something without any science or evidence behind it,” he said. “There is no reason not to expect the same level of data for claims about cannabis products as we would expect from pharmaceutical products.”
Getting to that point, however, will require that the authorities governing clinical trials begin to view cannabis as the research community does, as a possible treatment with potential value, rather than as an illicit drug that needs to be tamped down.
A version of this article first appeared on Medscape.com.
Although the healing properties of cannabis have been touted for millennia, research into its potential neuropsychiatric applications truly began to take off in the 1990s following the discovery of the cannabinoid system in the brain. This led to speculation that cannabis could play a therapeutic role in regulating dopamine, serotonin, and other neurotransmitters and offer a new means of treating various ailments.
At the same time, efforts to liberalize marijuana laws have successfully played out in several nations, including the United States, where, as of April 29, 36 states provide some access to cannabis. These dual tracks – medical and political – have made cannabis an increasingly accepted part of the cultural fabric.
Yet with this development has come a new quandary for clinicians. Medical cannabis has been made widely available to patients and has largely outpaced the clinical evidence, leaving it unclear how and for which indications it should be used.
The many forms of medical cannabis
Cannabis is a genus of plants that includes marijuana (Cannabis sativa) and hemp. These plants contain over 100 compounds, including terpenes, flavonoids, and – most importantly for medicinal applications – cannabinoids.
The most abundant cannabinoid in marijuana is the psychotropic delta-9-tetrahydrocannabinol (THC), which imparts the “high” sensation. The next most abundant cannabinoid is cannabidiol (CBD), which is the nonpsychotropic. THC and CBD are the most extensively studied cannabinoids, together and in isolation. Evidence suggests that other cannabinoids and terpenoids may also hold medical promise and that cannabis’ various compounds can work synergistically to produce a so-called entourage effect.
Patients walking into a typical medical cannabis dispensary will be faced with several plant-derived and synthetic options, which can differ considerably in terms of the ratios and amounts of THC and CBD they contain, as well in how they are consumed (i.e., via smoke, vapor, ingestion, topical administration, or oromucosal spray), all of which can alter their effects. Further complicating matters is the varying level of oversight each state and country has in how and whether they test for and accurately label products’ potency, cannabinoid content, and possible impurities.
Medically authorized, prescription cannabis products go through an official regulatory review process, and indications/contraindications have been established for them. To date, the Food and Drug Administration has approved one cannabis-derived drug product – Epidiolex (purified CBD) – for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients aged 2 years and older. The FDA has also approved three synthetic cannabis-related drug products – Marinol, Syndros (or dronabinol, created from synthetic THC), and Cesamet (or nabilone, a synthetic cannabinoid similar to THC) – all of which are indicated for treatment-related nausea and anorexia associated with weight loss in AIDS patients.
Surveys of medical cannabis consumers indicate that most people cannot distinguish between THC and CBD, so the first role that physicians find themselves in when recommending this treatment may be in helping patients navigate the volume of options.
Promising treatment for pain
Chronic pain is the leading reason patients seek out medical cannabis. It is also the indication that most researchers agree has the strongest evidence to support its use.
“In my mind, the most promising immediate use for medical cannabis is with THC for pain,” Diana M. Martinez, MD, a professor of psychiatry at Columbia University, New York, who specializes in addiction research, said in a recent MDedge podcast. “THC could be added to the armamentarium of pain medications that we use today.”
In a 2015 systematic literature review, researchers assessed 28 randomized, controlled trials (RCTs) of the use of cannabinoids for chronic pain. They reported that a variety of formulations resulted in at least a 30% reduction in the odds of pain, compared with placebo. A meta-analysis of five RCTs involving patients with neuropathic pain found a 30% reduction in pain over placebo with inhaled, vaporized cannabis. Varying results have been reported in additional studies for this indication. The National Academies of Sciences, Engineering, and Medicine concluded that there was a substantial body of evidence that cannabis is an effective treatment for chronic pain in adults.
The ongoing opioid epidemic has lent these results additional relevance.
Seeing this firsthand has caused Mark Steven Wallace, MD, a pain management specialist and chair of the division of pain medicine at the University of California San Diego Health, to reconsider offering cannabis to his patients.
“I think it’s probably more efficacious, just from my personal experience, and it’s a much lower risk of abuse and dependence than the opioids,” he said.
Dr. Wallace advised that clinicians who treat pain consider the ratios of cannabinoids.
“This is anecdotal, but we do find that with the combination of the two, CBD reduces the psychoactive effects of the THC. The ratios we use during the daytime range around 20 mg of CBD to 1 mg of THC,” he said.
In a recent secondary analysis of an RCT involving patients with painful diabetic peripheral neuropathy, Dr. Wallace and colleagues showed that THC’s effects appear to reverse themselves at a certain level.
“As the THC level goes up, the pain reduces until you reach about 16 ng/mL; then it starts going in the opposite direction, and pain will start to increase,” he said. “Even recreational cannabis users have reported that they avoid high doses because it’s very aversive. Using cannabis is all about, start low and go slow.”
A mixed bag for neurologic indications
There are relatively limited data on the use of medical cannabis for other neurologic conditions, and results have varied. For uses other than pain management, the evidence that does exist is strongest regarding epilepsy, said Daniel Freedman, DO, assistant professor of neurology at the University of Texas at Austin. He noted “multiple high-quality RCTs showing that pharmaceutical-grade CBD can reduce seizures associated with two particular epilepsy syndromes: Dravet Syndrome and Lennox Gastaut.”
These findings led to the FDA’s 2018 approval of Epidiolex for these syndromes. In earlier years, interest in CBD for pediatric seizures was largely driven by anecdotal parental reports of its benefits. NASEM’s 2017 overview on medical cannabis found evidence from subsequent RCTs in this indication to be insufficient. Clinicians who prescribe CBD for this indication must be vigilant because it can interact with several commonly used antiepileptic drugs.
Cannabinoid treatments have also shown success in alleviating muscle spasticity resulting from multiple sclerosis, most prominently in the form of nabiximols (Sativex), a standardized oralmucosal spray containing approximately equal quantities of THC and CBD. Nabiximols is approved in Europe but not in the United States. Moderate evidence supports the efficacy of these and other treatments over placebo in reducing muscle spasticity. Patient ratings of its effects tend to be higher than clinician assessment.
Parkinson’s disease has not yet been approved as an indication for treatment with cannabis or cannabinoids, yet a growing body of preclinical data suggests these could influence the dopaminergic system, said Carsten Buhmann, MD, from the department of neurology at the University Medical Center Hamburg-Eppendorf (Germany).
“In general, cannabinoids modulate basal-ganglia function on two levels which are especially relevant in Parkinson’s disease, i.e., the glutamatergic/dopaminergic synaptic neurotransmission and the corticostriatal plasticity,” he said. “Furthermore, activation of the endocannabinoid system might induce neuroprotective effects related to direct receptor-independent mechanisms, activation of anti-inflammatory cascades in glial cells via the cannabinoid receptor type 2, and antiglutamatergic antiexcitotoxic properties.”
Dr. Buhmann said that currently, clinical evidence is scarce, consisting of only four double-blind, placebo-controlled RCTs involving 49 patients. Various cannabinoids and methods of administering treatment were employed. Improvement was only observed in one of these RCTs, which found that the cannabinoid receptor agonist nabilone significantly reduced levodopa-induced dyskinesia for patients with Parkinson’s disease. Subjective data support a beneficial effect. In a nationwide survey of 1,348 respondents conducted by Dr. Buhmann and colleagues, the majority of medical cannabis users reported that it improved their symptoms (54% with oral CBD and 68% with inhaled THC-containing cannabis).
NASEM concluded that there was insufficient evidence to support the efficacy of medical cannabis for other neurologic conditions, including Tourette syndrome, amyotrophic lateral sclerosis, Huntington disease, dystonia, or dementia. A 2020 position statement from the American Academy of Neurology cited the lack of sufficient peer-reviewed research as the reason it could not currently support the use of cannabis for neurologic disorders.
Yet, according to Dr. Freedman, who served as a coauthor of the AAN position statement, this hasn’t stymied research interest in the topic. He’s seen a substantial uptick in studies of CBD over the past 2 years. “The body of evidence grows, but I still see many claims being made without evidence. And no one seems to care about all the negative trials.”
Cannabis as a treatment for, and cause of, psychiatric disorders
Mental health problems – such as anxiety, depression, and PTSD – are among the most common reasons patients seek out medical cannabis. There is an understandable interest in using cannabis and cannabinoids to treat psychiatric disorders. Preclinical studies suggest that the endocannabinoid system plays a prominent role in modulating feelings of anxiety, mood, and fear. As with opioids and chronic pain management, there is hope that medical cannabis may provide a means of reducing prescription anxiolytics and their associated risks.
The authors of the first systematic review (BMC Psychiatry. 2020 Jan 16;20[1]:24) of the use of medical cannabis for major psychiatric disorders noted that the current evidence was “encouraging, albeit embryonic.”
Meta-analyses have indicated a small but positive association between cannabis use and anxiety, although this may reflect the fact that patients with anxiety sought out this treatment. Given the risks for substance use disorders among patients with anxiety, CBD may present a more viable option. Positive results have been shown as treatment for generalized social anxiety disorder.
Limited but encouraging results have also been reported regarding the alleviation of PTSD symptoms with both cannabis and CBD, although the body of high-quality evidence hasn’t notably progressed since 2017, when NASEM declared that the evidence was insufficient. Supportive evidence is similarly lacking regarding the treatment of depression. Longitudinal studies suggest that cannabis use, particularly heavy use, may increase the risk of developing this disorder. Because THC is psychoactive, it is advised that it be avoided by patients at risk for psychotic disorders. However, CBD has yielded limited benefits for patients with treatment-resistant schizophrenia and for young people at risk for psychosis.
The use of medical cannabis for psychiatric conditions requires a complex balancing act, inasmuch as these treatments may exacerbate the very problems they are intended to alleviate.
Marta Di Forti, MD, PhD, professor of psychiatric research at Kings College London, has been at the forefront of determining the mental health risks of continued cannabis use. In 2019, Dr. Di Forti developed the first and only Cannabis Clinic for Patients With Psychosis in London where she and her colleagues have continued to elucidate this connection.
Dr. Di Forti and colleagues have linked daily cannabis use to an increase in the risk of experiencing psychotic disorder, compared with never using it. That risk was further increased among users of high-potency cannabis (≥10% THC). The latter finding has troubling implications, because concentrations of THC have steadily risen since 1970. By contrast, CBD concentrations have remained generally stable. High-potency cannabis products are common in both recreational and medicinal settings.
“For somebody prescribing medicinal cannabis that has a ≥10% concentration of THC, I’d be particularly wary of the risk of psychosis,” said Dr. Di Forti. “If you’re expecting people to use a high content of THC daily to medicate pain or a chronic condition, you even more so need to be aware that this is a potential side effect.”
Dr. Di Forti noted that her findings come from a cohort of recreational users, most of whom were aged 18-35 years.
“There have actually not been studies developed from collecting data in this area from groups specifically using cannabis for medicinal rather than recreational purposes,” she said.
She added that she personally has no concerns about the use of medical cannabis but wants clinicians to be aware of the risk for psychosis, to structure their patient conversations to identify risk factors or family histories of psychosis, and to become knowledgeable in detecting the often subtle signs of its initial onset.
When cannabis-associated psychosis occurs, Dr. Di Forti said it is primarily treated with conventional means, such as antipsychotics and therapeutic interventions and by refraining from using cannabis. Achieving the latter goal can be a challenge for patients who are daily users of high-potency cannabis. Currently, there are no treatment options such as those offered to patients withdrawing from the use of alcohol or opioids. Dr. Di Forti and colleagues are currently researching a solution to that problem through the use of another medical cannabis, the oromucosal spray Sativex, which has been approved in the European Union.
The regulatory obstacles to clarifying cannabis’ role in medicine
That currently there is limited or no evidence to support the use of medical cannabis for the treatment of neuropsychiatric conditions points to the inherent difficulties in conducting high-level research in this area.
“There’s a tremendous shortage of reliable data, largely due to regulatory barriers,” said Dr. Martinez.
Since 1970, cannabis has been listed as a Schedule I drug that is illegal to prescribe (the Agriculture Improvement Act of 2018 removed hemp from such restrictions). The FDA has issued guidance for researchers who wish to investigate treatments using Cannabis sativa or its derivatives in which the THC content is greater than 0.3%. Such research requires regular interactions with several federal agencies, including the Drug Enforcement Administration.
“It’s impossible to do multicenter RCTs with large numbers of patients, because you can’t transport cannabis across state lines,” said Dr. Wallace.
Regulatory restrictions regarding medical cannabis vary considerably throughout the world (the European Monitoring Center for Drugs and Drug Addiction provides a useful breakdown of this on their website). The lack of consistency in regulatory oversight acts as an impediment for conducting large-scale international multicenter studies on the topic.
Dr. Buhmann noted that, in Germany, cannabis has been broadly approved for treatment-resistant conditions with severe symptoms that impair quality of life. In addition, it is easy to be reimbursed for the use of cannabis as a medical treatment. These factors serve as disincentives for the funding of high-quality studies.
“It’s likely that no pharmaceutical company will do an expensive RCT to get an approval for Parkinson’s disease because it is already possible to prescribe medical cannabis of any type of THC-containing cannabinoid, dose, or route of application,” Dr. Buhmann said.
In the face of such restrictions and barriers, researchers are turning to ambitious real-world data projects to better understand medical cannabis’ efficacy and safety. A notable example is ProjectTwenty21, which is supported by the Royal College of Psychiatrists. The project is collecting outcomes of the use of medical cannabis among 20,000 U.K. patients whose conventional treatments of chronic pain, anxiety disorder, epilepsy, multiple sclerosis, PTSD, substance use disorder, and Tourette syndrome failed.
Dr. Freedman noted that the continued lack of high-quality data creates a void that commercial interests fill with unfounded claims.
“The danger is that patients might abandon a medication or intervention backed by robust science in favor of something without any science or evidence behind it,” he said. “There is no reason not to expect the same level of data for claims about cannabis products as we would expect from pharmaceutical products.”
Getting to that point, however, will require that the authorities governing clinical trials begin to view cannabis as the research community does, as a possible treatment with potential value, rather than as an illicit drug that needs to be tamped down.
A version of this article first appeared on Medscape.com.
Although the healing properties of cannabis have been touted for millennia, research into its potential neuropsychiatric applications truly began to take off in the 1990s following the discovery of the cannabinoid system in the brain. This led to speculation that cannabis could play a therapeutic role in regulating dopamine, serotonin, and other neurotransmitters and offer a new means of treating various ailments.
At the same time, efforts to liberalize marijuana laws have successfully played out in several nations, including the United States, where, as of April 29, 36 states provide some access to cannabis. These dual tracks – medical and political – have made cannabis an increasingly accepted part of the cultural fabric.
Yet with this development has come a new quandary for clinicians. Medical cannabis has been made widely available to patients and has largely outpaced the clinical evidence, leaving it unclear how and for which indications it should be used.
The many forms of medical cannabis
Cannabis is a genus of plants that includes marijuana (Cannabis sativa) and hemp. These plants contain over 100 compounds, including terpenes, flavonoids, and – most importantly for medicinal applications – cannabinoids.
The most abundant cannabinoid in marijuana is the psychotropic delta-9-tetrahydrocannabinol (THC), which imparts the “high” sensation. The next most abundant cannabinoid is cannabidiol (CBD), which is the nonpsychotropic. THC and CBD are the most extensively studied cannabinoids, together and in isolation. Evidence suggests that other cannabinoids and terpenoids may also hold medical promise and that cannabis’ various compounds can work synergistically to produce a so-called entourage effect.
Patients walking into a typical medical cannabis dispensary will be faced with several plant-derived and synthetic options, which can differ considerably in terms of the ratios and amounts of THC and CBD they contain, as well in how they are consumed (i.e., via smoke, vapor, ingestion, topical administration, or oromucosal spray), all of which can alter their effects. Further complicating matters is the varying level of oversight each state and country has in how and whether they test for and accurately label products’ potency, cannabinoid content, and possible impurities.
Medically authorized, prescription cannabis products go through an official regulatory review process, and indications/contraindications have been established for them. To date, the Food and Drug Administration has approved one cannabis-derived drug product – Epidiolex (purified CBD) – for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients aged 2 years and older. The FDA has also approved three synthetic cannabis-related drug products – Marinol, Syndros (or dronabinol, created from synthetic THC), and Cesamet (or nabilone, a synthetic cannabinoid similar to THC) – all of which are indicated for treatment-related nausea and anorexia associated with weight loss in AIDS patients.
Surveys of medical cannabis consumers indicate that most people cannot distinguish between THC and CBD, so the first role that physicians find themselves in when recommending this treatment may be in helping patients navigate the volume of options.
Promising treatment for pain
Chronic pain is the leading reason patients seek out medical cannabis. It is also the indication that most researchers agree has the strongest evidence to support its use.
“In my mind, the most promising immediate use for medical cannabis is with THC for pain,” Diana M. Martinez, MD, a professor of psychiatry at Columbia University, New York, who specializes in addiction research, said in a recent MDedge podcast. “THC could be added to the armamentarium of pain medications that we use today.”
In a 2015 systematic literature review, researchers assessed 28 randomized, controlled trials (RCTs) of the use of cannabinoids for chronic pain. They reported that a variety of formulations resulted in at least a 30% reduction in the odds of pain, compared with placebo. A meta-analysis of five RCTs involving patients with neuropathic pain found a 30% reduction in pain over placebo with inhaled, vaporized cannabis. Varying results have been reported in additional studies for this indication. The National Academies of Sciences, Engineering, and Medicine concluded that there was a substantial body of evidence that cannabis is an effective treatment for chronic pain in adults.
The ongoing opioid epidemic has lent these results additional relevance.
Seeing this firsthand has caused Mark Steven Wallace, MD, a pain management specialist and chair of the division of pain medicine at the University of California San Diego Health, to reconsider offering cannabis to his patients.
“I think it’s probably more efficacious, just from my personal experience, and it’s a much lower risk of abuse and dependence than the opioids,” he said.
Dr. Wallace advised that clinicians who treat pain consider the ratios of cannabinoids.
“This is anecdotal, but we do find that with the combination of the two, CBD reduces the psychoactive effects of the THC. The ratios we use during the daytime range around 20 mg of CBD to 1 mg of THC,” he said.
In a recent secondary analysis of an RCT involving patients with painful diabetic peripheral neuropathy, Dr. Wallace and colleagues showed that THC’s effects appear to reverse themselves at a certain level.
“As the THC level goes up, the pain reduces until you reach about 16 ng/mL; then it starts going in the opposite direction, and pain will start to increase,” he said. “Even recreational cannabis users have reported that they avoid high doses because it’s very aversive. Using cannabis is all about, start low and go slow.”
A mixed bag for neurologic indications
There are relatively limited data on the use of medical cannabis for other neurologic conditions, and results have varied. For uses other than pain management, the evidence that does exist is strongest regarding epilepsy, said Daniel Freedman, DO, assistant professor of neurology at the University of Texas at Austin. He noted “multiple high-quality RCTs showing that pharmaceutical-grade CBD can reduce seizures associated with two particular epilepsy syndromes: Dravet Syndrome and Lennox Gastaut.”
These findings led to the FDA’s 2018 approval of Epidiolex for these syndromes. In earlier years, interest in CBD for pediatric seizures was largely driven by anecdotal parental reports of its benefits. NASEM’s 2017 overview on medical cannabis found evidence from subsequent RCTs in this indication to be insufficient. Clinicians who prescribe CBD for this indication must be vigilant because it can interact with several commonly used antiepileptic drugs.
Cannabinoid treatments have also shown success in alleviating muscle spasticity resulting from multiple sclerosis, most prominently in the form of nabiximols (Sativex), a standardized oralmucosal spray containing approximately equal quantities of THC and CBD. Nabiximols is approved in Europe but not in the United States. Moderate evidence supports the efficacy of these and other treatments over placebo in reducing muscle spasticity. Patient ratings of its effects tend to be higher than clinician assessment.
Parkinson’s disease has not yet been approved as an indication for treatment with cannabis or cannabinoids, yet a growing body of preclinical data suggests these could influence the dopaminergic system, said Carsten Buhmann, MD, from the department of neurology at the University Medical Center Hamburg-Eppendorf (Germany).
“In general, cannabinoids modulate basal-ganglia function on two levels which are especially relevant in Parkinson’s disease, i.e., the glutamatergic/dopaminergic synaptic neurotransmission and the corticostriatal plasticity,” he said. “Furthermore, activation of the endocannabinoid system might induce neuroprotective effects related to direct receptor-independent mechanisms, activation of anti-inflammatory cascades in glial cells via the cannabinoid receptor type 2, and antiglutamatergic antiexcitotoxic properties.”
Dr. Buhmann said that currently, clinical evidence is scarce, consisting of only four double-blind, placebo-controlled RCTs involving 49 patients. Various cannabinoids and methods of administering treatment were employed. Improvement was only observed in one of these RCTs, which found that the cannabinoid receptor agonist nabilone significantly reduced levodopa-induced dyskinesia for patients with Parkinson’s disease. Subjective data support a beneficial effect. In a nationwide survey of 1,348 respondents conducted by Dr. Buhmann and colleagues, the majority of medical cannabis users reported that it improved their symptoms (54% with oral CBD and 68% with inhaled THC-containing cannabis).
NASEM concluded that there was insufficient evidence to support the efficacy of medical cannabis for other neurologic conditions, including Tourette syndrome, amyotrophic lateral sclerosis, Huntington disease, dystonia, or dementia. A 2020 position statement from the American Academy of Neurology cited the lack of sufficient peer-reviewed research as the reason it could not currently support the use of cannabis for neurologic disorders.
Yet, according to Dr. Freedman, who served as a coauthor of the AAN position statement, this hasn’t stymied research interest in the topic. He’s seen a substantial uptick in studies of CBD over the past 2 years. “The body of evidence grows, but I still see many claims being made without evidence. And no one seems to care about all the negative trials.”
Cannabis as a treatment for, and cause of, psychiatric disorders
Mental health problems – such as anxiety, depression, and PTSD – are among the most common reasons patients seek out medical cannabis. There is an understandable interest in using cannabis and cannabinoids to treat psychiatric disorders. Preclinical studies suggest that the endocannabinoid system plays a prominent role in modulating feelings of anxiety, mood, and fear. As with opioids and chronic pain management, there is hope that medical cannabis may provide a means of reducing prescription anxiolytics and their associated risks.
The authors of the first systematic review (BMC Psychiatry. 2020 Jan 16;20[1]:24) of the use of medical cannabis for major psychiatric disorders noted that the current evidence was “encouraging, albeit embryonic.”
Meta-analyses have indicated a small but positive association between cannabis use and anxiety, although this may reflect the fact that patients with anxiety sought out this treatment. Given the risks for substance use disorders among patients with anxiety, CBD may present a more viable option. Positive results have been shown as treatment for generalized social anxiety disorder.
Limited but encouraging results have also been reported regarding the alleviation of PTSD symptoms with both cannabis and CBD, although the body of high-quality evidence hasn’t notably progressed since 2017, when NASEM declared that the evidence was insufficient. Supportive evidence is similarly lacking regarding the treatment of depression. Longitudinal studies suggest that cannabis use, particularly heavy use, may increase the risk of developing this disorder. Because THC is psychoactive, it is advised that it be avoided by patients at risk for psychotic disorders. However, CBD has yielded limited benefits for patients with treatment-resistant schizophrenia and for young people at risk for psychosis.
The use of medical cannabis for psychiatric conditions requires a complex balancing act, inasmuch as these treatments may exacerbate the very problems they are intended to alleviate.
Marta Di Forti, MD, PhD, professor of psychiatric research at Kings College London, has been at the forefront of determining the mental health risks of continued cannabis use. In 2019, Dr. Di Forti developed the first and only Cannabis Clinic for Patients With Psychosis in London where she and her colleagues have continued to elucidate this connection.
Dr. Di Forti and colleagues have linked daily cannabis use to an increase in the risk of experiencing psychotic disorder, compared with never using it. That risk was further increased among users of high-potency cannabis (≥10% THC). The latter finding has troubling implications, because concentrations of THC have steadily risen since 1970. By contrast, CBD concentrations have remained generally stable. High-potency cannabis products are common in both recreational and medicinal settings.
“For somebody prescribing medicinal cannabis that has a ≥10% concentration of THC, I’d be particularly wary of the risk of psychosis,” said Dr. Di Forti. “If you’re expecting people to use a high content of THC daily to medicate pain or a chronic condition, you even more so need to be aware that this is a potential side effect.”
Dr. Di Forti noted that her findings come from a cohort of recreational users, most of whom were aged 18-35 years.
“There have actually not been studies developed from collecting data in this area from groups specifically using cannabis for medicinal rather than recreational purposes,” she said.
She added that she personally has no concerns about the use of medical cannabis but wants clinicians to be aware of the risk for psychosis, to structure their patient conversations to identify risk factors or family histories of psychosis, and to become knowledgeable in detecting the often subtle signs of its initial onset.
When cannabis-associated psychosis occurs, Dr. Di Forti said it is primarily treated with conventional means, such as antipsychotics and therapeutic interventions and by refraining from using cannabis. Achieving the latter goal can be a challenge for patients who are daily users of high-potency cannabis. Currently, there are no treatment options such as those offered to patients withdrawing from the use of alcohol or opioids. Dr. Di Forti and colleagues are currently researching a solution to that problem through the use of another medical cannabis, the oromucosal spray Sativex, which has been approved in the European Union.
The regulatory obstacles to clarifying cannabis’ role in medicine
That currently there is limited or no evidence to support the use of medical cannabis for the treatment of neuropsychiatric conditions points to the inherent difficulties in conducting high-level research in this area.
“There’s a tremendous shortage of reliable data, largely due to regulatory barriers,” said Dr. Martinez.
Since 1970, cannabis has been listed as a Schedule I drug that is illegal to prescribe (the Agriculture Improvement Act of 2018 removed hemp from such restrictions). The FDA has issued guidance for researchers who wish to investigate treatments using Cannabis sativa or its derivatives in which the THC content is greater than 0.3%. Such research requires regular interactions with several federal agencies, including the Drug Enforcement Administration.
“It’s impossible to do multicenter RCTs with large numbers of patients, because you can’t transport cannabis across state lines,” said Dr. Wallace.
Regulatory restrictions regarding medical cannabis vary considerably throughout the world (the European Monitoring Center for Drugs and Drug Addiction provides a useful breakdown of this on their website). The lack of consistency in regulatory oversight acts as an impediment for conducting large-scale international multicenter studies on the topic.
Dr. Buhmann noted that, in Germany, cannabis has been broadly approved for treatment-resistant conditions with severe symptoms that impair quality of life. In addition, it is easy to be reimbursed for the use of cannabis as a medical treatment. These factors serve as disincentives for the funding of high-quality studies.
“It’s likely that no pharmaceutical company will do an expensive RCT to get an approval for Parkinson’s disease because it is already possible to prescribe medical cannabis of any type of THC-containing cannabinoid, dose, or route of application,” Dr. Buhmann said.
In the face of such restrictions and barriers, researchers are turning to ambitious real-world data projects to better understand medical cannabis’ efficacy and safety. A notable example is ProjectTwenty21, which is supported by the Royal College of Psychiatrists. The project is collecting outcomes of the use of medical cannabis among 20,000 U.K. patients whose conventional treatments of chronic pain, anxiety disorder, epilepsy, multiple sclerosis, PTSD, substance use disorder, and Tourette syndrome failed.
Dr. Freedman noted that the continued lack of high-quality data creates a void that commercial interests fill with unfounded claims.
“The danger is that patients might abandon a medication or intervention backed by robust science in favor of something without any science or evidence behind it,” he said. “There is no reason not to expect the same level of data for claims about cannabis products as we would expect from pharmaceutical products.”
Getting to that point, however, will require that the authorities governing clinical trials begin to view cannabis as the research community does, as a possible treatment with potential value, rather than as an illicit drug that needs to be tamped down.
A version of this article first appeared on Medscape.com.
Innovations in Dermatology Spring Abstract Compendium
Progressive Fibrosing Interstitial Lung Diseases
Insights gained over the past two decades about idiopathic pulmonary fibrosis (IPF) and other interstitial lung diseases (ILD) have greatly advanced our understanding of these conditions and have helped facilitate earlier diagnosis and intervention and improvements to patient care. Recently, the concept of progressive fibrosing ILD has emerged, as many patients with fibrosing ILDs show rapid deterioration similar to IPF, thereby requiring close monitoring.
This publication explores fibrosing ILDs, in recognition of the need for further education about these conditions.
Read More
Insights gained over the past two decades about idiopathic pulmonary fibrosis (IPF) and other interstitial lung diseases (ILD) have greatly advanced our understanding of these conditions and have helped facilitate earlier diagnosis and intervention and improvements to patient care. Recently, the concept of progressive fibrosing ILD has emerged, as many patients with fibrosing ILDs show rapid deterioration similar to IPF, thereby requiring close monitoring.
This publication explores fibrosing ILDs, in recognition of the need for further education about these conditions.
Read More
Insights gained over the past two decades about idiopathic pulmonary fibrosis (IPF) and other interstitial lung diseases (ILD) have greatly advanced our understanding of these conditions and have helped facilitate earlier diagnosis and intervention and improvements to patient care. Recently, the concept of progressive fibrosing ILD has emerged, as many patients with fibrosing ILDs show rapid deterioration similar to IPF, thereby requiring close monitoring.
This publication explores fibrosing ILDs, in recognition of the need for further education about these conditions.
Read More
Doctors lose jobs after speaking out about unsafe conditions
In April 2020, hospitalist Samantha Houston, MD, lost her job at Baptist Memorial Hospital–North, in Oxford, Miss., after she publicly campaigned to get donations of N95 masks for nurses. Dr. Houston filed a lawsuit against the hospital, saying she was improperly fired for speaking out. The lawsuit has not yet gone to trial.
In January 2017, emergency physician Raymond Brovont, MD, was fired by EmCare, an emergency physician staffing company, after reporting understaffing at hospitals with which it contracted in the Kansas City, Mo., area. Dr. Brovont sued EmCare, and the company lost the case. In February 2019, it was ordered to pay him $13.1 million in damages.
These are just two of several cases in recent years in which physicians have spoken out about problems involving patient care and have been sanctioned. Other physicians who see problems choose to stay silent.
Doctors often hesitate to speak out because of the prospect of losing their jobs. A 2013 study of emergency physicians found that nearly 20% reported a possible or real threat to their employment if they expressed concerns about quality of care.
When physicians do not speak openly about important medical issues, the quality of care in their institutions suffers, said a coauthor of the study, Larry D. Weiss, MD, JD, a retired professor of emergency medicine at the University of Maryland, Baltimore.
“Physicians can’t effectively represent patients if they are always thinking they can get fired for what they say,” Dr. Weiss said. “If you don’t have protections like due process, which is often the case, you are less likely to speak out.”
In the first few weeks, health care facilities were struggling to obtain personal protective equipment (PPE) and to create policies that would keep patients and caregivers safe.
Physicians such as Dr. Houston took the initiative to make sure their institutions were taking the right steps against COVID-19 and found themselves at loggerheads with administrators who were concerned that their organizations were being portrayed as unsafe.
The case of one physician who spoke out
One of the highest-profile cases of a physician speaking out and being removed from work during the pandemic is that of Ming Lin, MD, an emergency physician who lost a job he had held for 17 years at St. Joseph Medical Center, in Bellingham, Wash. Dr. Lin lost his job after he made a series of Facebook posts that criticized the hospital’s COVID-19 preparedness efforts.
In an interview, Dr. Lin discussed the details of his situation to a degree that rarely occurs in such cases. This is one of the most extensive interviews he has granted.
Postings on Facebook
Dr. Lin said that on the basis of an intense study of the virus at the onset of the pandemic, he developed many ideas as to what could be done to mitigate its spread. While working as a locum tenens physician on his time off, he could see how others dealt with COVID-19.
Dr. Lin said from past experiences he did not feel that he could present his ideas directly to administration and be heard, so he decided to air his ideas about how his hospital could handle COVID-19 on his Facebook page, which drew a large audience.
He said he was certain that hospital administrators were reading his posts. He said receptionists at this hospital were advised not to wear masks, evidently because it would alarm patients. Dr. Lin said he posted concerns about their safety and called for them to wear masks. Soon after, the hospital directed receptionists to wear masks.
Dr. Lin’s Facebook posts also criticized the hospital for taking what he felt was too long to get results on COVID-19 tests. “It was taking them up to 10 days to get test results, because samples were being sent to a lab in California,” he said. He suggested it would be faster to send samples to the University of Washington. Soon after, the hospital started sending samples there.
In just a couple of weeks, Dr. Lin said, he voiced almost a dozen concerns. Each time the hospital made changes in line with his recommendations. Although he didn’t get any direct acknowledgment from the hospital for his help, he said he felt he was making a positive impact.
How employers react to physicians who speak out
Physicians who speak out about conditions tend to deeply disturb administrators, said William P. Sullivan, DO, JD, an emergency physician and lawyer in Frankfort, Ill., who has written about physicians being terminated by hospitals.
“These physicians go to the news media or they use social media,” Dr. Sullivan said, “but hospital administrators don’t want the public to hear bad things about their hospital.”
Then the public might not come to the hospital, which is an administrator’s worst nightmare. Even if physicians think their criticisms are reasonable, administrators may still fear a resulting drop in patients.
Dr. Houston, for example, was helping her Mississippi hospital by collecting donations of N95 masks for nurses, but to administrators, it showed that the hospital did not have enough masks.
“It is not helpful to stoke fear and anxiety, even if the intent is sincere,” a spokesperson for the hospital said.
Administrator fires back
Dr. Lin’s posts were deeply concerning to Richard DeCarlo, chief operating officer of PeaceHealth, which runs St. Joseph Hospital. Mr. DeCarlo discussed his concerns in a video interview in April with the blogger Zubin Damania, MD, known as ZDoggMD.
Comments on Dr. Lin’s Facebook posts showed that people “were fearful to go to the hospital,” he told Dr. Damania. “They were concluding that they would need to drive to another hospital.”
Mr. DeCarlo said he was also unhappy that Dr. Lin did not directly contact administrators about his concerns. “He didn’t communicate with his medical director,” Mr. DeCarlo said in the interview. “The ED staff had been meeting three times a week with the chief medical officer to make sure they had everything they needed, but he only attended one of these meetings and didn’t ask any questions.”
Dr. Lin maintains he did ask questions at the first meeting but stopped attending because he felt he wasn’t being heeded. “I found their tone not very receptive,” he said.
Doctor allegedly offered “misinformation”
At the start of the pandemic, some hospitals made it clear what would happen to doctors who brought up lack of PPE or other problems to the media. For example, NYU Langone Medical Center in New York sent an email to staff warning that speaking to the media without permission “will be subject to disciplinary action, including termination.”
PeaceHealth took a different tack. “It’s not that we have a policy that says don’t ever talk to the media,” Mr. DeCarlo said in the ZDoggMD interview, but in Dr. Lin’s case, “what was at issue was the misinformation. His leader went to him and said, ‘Look, you’re posting things that aren’t accurate.’ ”
Dr. Lin disputes that he provided any misinformation. In the interview, Mr. DeCarlo cited just one example of alleged misinformation. He said Dr. Lin called for a tent outside the emergency department (ED) to protect patients entering the department from aerosol exposure to COVID-19. Mr. DeCarlo said the tent was not needed because fewer people were using the ED.
“To put it in an extreme way,” Mr. DeCarlo said of Dr. Lin’s posts, “it was like yelling fire in a theater where there is not a fire.”
Dr. Lin said the hospital did briefly erect a tent and then removed it, and he still insisted that a tent was a good idea. He added that Mr. DeCarlo never mentioned any of the other suggestions Dr. Lin made, nor did he state that the hospital adopted them.
Doctor gets a warning
Dr. Lin said that after he started posting his concerns, he got a call from the emergency department director who worked for TeamHealth, an emergency medicine staffing firm that contracted with PeaceHealth and employed Dr. Lin, too.
Dr. Lin said his immediate supervisor at TeamHealth told him the hospital was unhappy with his posts and that he should take them down and suggested he might be fired. Dr. Lin said the supervisor also asked him to apologize to the hospital administration for these posts, but he refused to do so.
“Retracting and apologizing was not only wrong but would have left me vulnerable to being terminated with no repercussions,” he said.
“At that point, I realized I had crossed the Rubicon,” Dr. Lin said. He thought he might well be fired, no matter what he did, so he took his story to The Seattle Times, which had a much wider platform than his Facebook page had.
Dr. Lin lost his job at St. Joseph a week after The Seattle Times story about him appeared. “About 10 minutes before my shift was supposed to start, I received a text message from TeamHealth saying that someone else would be taking the shift,” he said.
In a release, TeamHealth insisted Dr. Lin was not fired and that he was scheduled to be reassigned to work at other hospitals. Dr. Lin, however, said he was not told this at the time and that he found out later that the new assignment would involve a pay cut and a significant commute. He said he has not taken any new assignments from TeamHealth since he lost his job at St. Joseph.
Dr. Lin has filed a lawsuit against PeaceHealth, TeamHealth, and Mr. DeCarlo, asking for his job back and for an apology. He said he has not asked for any financial damages at this point.
Since leaving St. Joseph, Dr. Lin has been working as an administrator for the Indian Health Service in the upper plains states. He said he can do some of the work at home in Washington State, which allows him to be with his wife and three young children.
Dr. Lin no longer sees patients. “I feel I have lost my confidence as a clinician,” he said. “I’m not sure why, but I find it hard to make quick judgments when taking care of patients.”
He said many doctors have told him about their own troubles with speaking out, but they did not want to come forward and talk about it because they feared more repercussions.
Do doctors who speak out have any rights?
Because TeamHealth, Dr. Lin’s actual employer, asserts he was never actually terminated, Dr. Lin has not been able to appeal his case internally in accordance with due process, an option that allows doctors to get a fair hearing and to appeal decisions against them.
The American Academy of Emergency Medicine pointed out this problem. “Dr. Lin, as a member of the medical staff, is entitled to full due process and a fair hearing from his peers on the medical staff,” the academy said in a statement supporting him.
The Joint Commission, the hospital accreditor, requires that hospitals provide due process to doctors before they can be terminated. However, Dr. Sullivan said employers often make physicians waive their due process rights in the employment contract. “The result is that the employer can terminate doctors for no reason,” he said.
In the 2013 survey of emergency physicians, 62% reported that their employers could terminate them without full due process.
Dr. Weiss, the Maryland MD-JD, said that when he advises doctors on their contracts, he generally tells them to cross out the waiver language. The applicant, he says, may also tell the employer that the waivers are considered unethical by many physician professional societies. In some cases, he said, the hospital will back down.
Conclusion
To maintain quality of care, it is essential that physicians feel free to speak out about issues that concern them. They can improve their chances of being heard by working directly with management and attending meetings, but in some cases, management may be unwilling to listen.
A version of this article first appeared on Medscape.com.
In April 2020, hospitalist Samantha Houston, MD, lost her job at Baptist Memorial Hospital–North, in Oxford, Miss., after she publicly campaigned to get donations of N95 masks for nurses. Dr. Houston filed a lawsuit against the hospital, saying she was improperly fired for speaking out. The lawsuit has not yet gone to trial.
In January 2017, emergency physician Raymond Brovont, MD, was fired by EmCare, an emergency physician staffing company, after reporting understaffing at hospitals with which it contracted in the Kansas City, Mo., area. Dr. Brovont sued EmCare, and the company lost the case. In February 2019, it was ordered to pay him $13.1 million in damages.
These are just two of several cases in recent years in which physicians have spoken out about problems involving patient care and have been sanctioned. Other physicians who see problems choose to stay silent.
Doctors often hesitate to speak out because of the prospect of losing their jobs. A 2013 study of emergency physicians found that nearly 20% reported a possible or real threat to their employment if they expressed concerns about quality of care.
When physicians do not speak openly about important medical issues, the quality of care in their institutions suffers, said a coauthor of the study, Larry D. Weiss, MD, JD, a retired professor of emergency medicine at the University of Maryland, Baltimore.
“Physicians can’t effectively represent patients if they are always thinking they can get fired for what they say,” Dr. Weiss said. “If you don’t have protections like due process, which is often the case, you are less likely to speak out.”
In the first few weeks, health care facilities were struggling to obtain personal protective equipment (PPE) and to create policies that would keep patients and caregivers safe.
Physicians such as Dr. Houston took the initiative to make sure their institutions were taking the right steps against COVID-19 and found themselves at loggerheads with administrators who were concerned that their organizations were being portrayed as unsafe.
The case of one physician who spoke out
One of the highest-profile cases of a physician speaking out and being removed from work during the pandemic is that of Ming Lin, MD, an emergency physician who lost a job he had held for 17 years at St. Joseph Medical Center, in Bellingham, Wash. Dr. Lin lost his job after he made a series of Facebook posts that criticized the hospital’s COVID-19 preparedness efforts.
In an interview, Dr. Lin discussed the details of his situation to a degree that rarely occurs in such cases. This is one of the most extensive interviews he has granted.
Postings on Facebook
Dr. Lin said that on the basis of an intense study of the virus at the onset of the pandemic, he developed many ideas as to what could be done to mitigate its spread. While working as a locum tenens physician on his time off, he could see how others dealt with COVID-19.
Dr. Lin said from past experiences he did not feel that he could present his ideas directly to administration and be heard, so he decided to air his ideas about how his hospital could handle COVID-19 on his Facebook page, which drew a large audience.
He said he was certain that hospital administrators were reading his posts. He said receptionists at this hospital were advised not to wear masks, evidently because it would alarm patients. Dr. Lin said he posted concerns about their safety and called for them to wear masks. Soon after, the hospital directed receptionists to wear masks.
Dr. Lin’s Facebook posts also criticized the hospital for taking what he felt was too long to get results on COVID-19 tests. “It was taking them up to 10 days to get test results, because samples were being sent to a lab in California,” he said. He suggested it would be faster to send samples to the University of Washington. Soon after, the hospital started sending samples there.
In just a couple of weeks, Dr. Lin said, he voiced almost a dozen concerns. Each time the hospital made changes in line with his recommendations. Although he didn’t get any direct acknowledgment from the hospital for his help, he said he felt he was making a positive impact.
How employers react to physicians who speak out
Physicians who speak out about conditions tend to deeply disturb administrators, said William P. Sullivan, DO, JD, an emergency physician and lawyer in Frankfort, Ill., who has written about physicians being terminated by hospitals.
“These physicians go to the news media or they use social media,” Dr. Sullivan said, “but hospital administrators don’t want the public to hear bad things about their hospital.”
Then the public might not come to the hospital, which is an administrator’s worst nightmare. Even if physicians think their criticisms are reasonable, administrators may still fear a resulting drop in patients.
Dr. Houston, for example, was helping her Mississippi hospital by collecting donations of N95 masks for nurses, but to administrators, it showed that the hospital did not have enough masks.
“It is not helpful to stoke fear and anxiety, even if the intent is sincere,” a spokesperson for the hospital said.
Administrator fires back
Dr. Lin’s posts were deeply concerning to Richard DeCarlo, chief operating officer of PeaceHealth, which runs St. Joseph Hospital. Mr. DeCarlo discussed his concerns in a video interview in April with the blogger Zubin Damania, MD, known as ZDoggMD.
Comments on Dr. Lin’s Facebook posts showed that people “were fearful to go to the hospital,” he told Dr. Damania. “They were concluding that they would need to drive to another hospital.”
Mr. DeCarlo said he was also unhappy that Dr. Lin did not directly contact administrators about his concerns. “He didn’t communicate with his medical director,” Mr. DeCarlo said in the interview. “The ED staff had been meeting three times a week with the chief medical officer to make sure they had everything they needed, but he only attended one of these meetings and didn’t ask any questions.”
Dr. Lin maintains he did ask questions at the first meeting but stopped attending because he felt he wasn’t being heeded. “I found their tone not very receptive,” he said.
Doctor allegedly offered “misinformation”
At the start of the pandemic, some hospitals made it clear what would happen to doctors who brought up lack of PPE or other problems to the media. For example, NYU Langone Medical Center in New York sent an email to staff warning that speaking to the media without permission “will be subject to disciplinary action, including termination.”
PeaceHealth took a different tack. “It’s not that we have a policy that says don’t ever talk to the media,” Mr. DeCarlo said in the ZDoggMD interview, but in Dr. Lin’s case, “what was at issue was the misinformation. His leader went to him and said, ‘Look, you’re posting things that aren’t accurate.’ ”
Dr. Lin disputes that he provided any misinformation. In the interview, Mr. DeCarlo cited just one example of alleged misinformation. He said Dr. Lin called for a tent outside the emergency department (ED) to protect patients entering the department from aerosol exposure to COVID-19. Mr. DeCarlo said the tent was not needed because fewer people were using the ED.
“To put it in an extreme way,” Mr. DeCarlo said of Dr. Lin’s posts, “it was like yelling fire in a theater where there is not a fire.”
Dr. Lin said the hospital did briefly erect a tent and then removed it, and he still insisted that a tent was a good idea. He added that Mr. DeCarlo never mentioned any of the other suggestions Dr. Lin made, nor did he state that the hospital adopted them.
Doctor gets a warning
Dr. Lin said that after he started posting his concerns, he got a call from the emergency department director who worked for TeamHealth, an emergency medicine staffing firm that contracted with PeaceHealth and employed Dr. Lin, too.
Dr. Lin said his immediate supervisor at TeamHealth told him the hospital was unhappy with his posts and that he should take them down and suggested he might be fired. Dr. Lin said the supervisor also asked him to apologize to the hospital administration for these posts, but he refused to do so.
“Retracting and apologizing was not only wrong but would have left me vulnerable to being terminated with no repercussions,” he said.
“At that point, I realized I had crossed the Rubicon,” Dr. Lin said. He thought he might well be fired, no matter what he did, so he took his story to The Seattle Times, which had a much wider platform than his Facebook page had.
Dr. Lin lost his job at St. Joseph a week after The Seattle Times story about him appeared. “About 10 minutes before my shift was supposed to start, I received a text message from TeamHealth saying that someone else would be taking the shift,” he said.
In a release, TeamHealth insisted Dr. Lin was not fired and that he was scheduled to be reassigned to work at other hospitals. Dr. Lin, however, said he was not told this at the time and that he found out later that the new assignment would involve a pay cut and a significant commute. He said he has not taken any new assignments from TeamHealth since he lost his job at St. Joseph.
Dr. Lin has filed a lawsuit against PeaceHealth, TeamHealth, and Mr. DeCarlo, asking for his job back and for an apology. He said he has not asked for any financial damages at this point.
Since leaving St. Joseph, Dr. Lin has been working as an administrator for the Indian Health Service in the upper plains states. He said he can do some of the work at home in Washington State, which allows him to be with his wife and three young children.
Dr. Lin no longer sees patients. “I feel I have lost my confidence as a clinician,” he said. “I’m not sure why, but I find it hard to make quick judgments when taking care of patients.”
He said many doctors have told him about their own troubles with speaking out, but they did not want to come forward and talk about it because they feared more repercussions.
Do doctors who speak out have any rights?
Because TeamHealth, Dr. Lin’s actual employer, asserts he was never actually terminated, Dr. Lin has not been able to appeal his case internally in accordance with due process, an option that allows doctors to get a fair hearing and to appeal decisions against them.
The American Academy of Emergency Medicine pointed out this problem. “Dr. Lin, as a member of the medical staff, is entitled to full due process and a fair hearing from his peers on the medical staff,” the academy said in a statement supporting him.
The Joint Commission, the hospital accreditor, requires that hospitals provide due process to doctors before they can be terminated. However, Dr. Sullivan said employers often make physicians waive their due process rights in the employment contract. “The result is that the employer can terminate doctors for no reason,” he said.
In the 2013 survey of emergency physicians, 62% reported that their employers could terminate them without full due process.
Dr. Weiss, the Maryland MD-JD, said that when he advises doctors on their contracts, he generally tells them to cross out the waiver language. The applicant, he says, may also tell the employer that the waivers are considered unethical by many physician professional societies. In some cases, he said, the hospital will back down.
Conclusion
To maintain quality of care, it is essential that physicians feel free to speak out about issues that concern them. They can improve their chances of being heard by working directly with management and attending meetings, but in some cases, management may be unwilling to listen.
A version of this article first appeared on Medscape.com.
In April 2020, hospitalist Samantha Houston, MD, lost her job at Baptist Memorial Hospital–North, in Oxford, Miss., after she publicly campaigned to get donations of N95 masks for nurses. Dr. Houston filed a lawsuit against the hospital, saying she was improperly fired for speaking out. The lawsuit has not yet gone to trial.
In January 2017, emergency physician Raymond Brovont, MD, was fired by EmCare, an emergency physician staffing company, after reporting understaffing at hospitals with which it contracted in the Kansas City, Mo., area. Dr. Brovont sued EmCare, and the company lost the case. In February 2019, it was ordered to pay him $13.1 million in damages.
These are just two of several cases in recent years in which physicians have spoken out about problems involving patient care and have been sanctioned. Other physicians who see problems choose to stay silent.
Doctors often hesitate to speak out because of the prospect of losing their jobs. A 2013 study of emergency physicians found that nearly 20% reported a possible or real threat to their employment if they expressed concerns about quality of care.
When physicians do not speak openly about important medical issues, the quality of care in their institutions suffers, said a coauthor of the study, Larry D. Weiss, MD, JD, a retired professor of emergency medicine at the University of Maryland, Baltimore.
“Physicians can’t effectively represent patients if they are always thinking they can get fired for what they say,” Dr. Weiss said. “If you don’t have protections like due process, which is often the case, you are less likely to speak out.”
In the first few weeks, health care facilities were struggling to obtain personal protective equipment (PPE) and to create policies that would keep patients and caregivers safe.
Physicians such as Dr. Houston took the initiative to make sure their institutions were taking the right steps against COVID-19 and found themselves at loggerheads with administrators who were concerned that their organizations were being portrayed as unsafe.
The case of one physician who spoke out
One of the highest-profile cases of a physician speaking out and being removed from work during the pandemic is that of Ming Lin, MD, an emergency physician who lost a job he had held for 17 years at St. Joseph Medical Center, in Bellingham, Wash. Dr. Lin lost his job after he made a series of Facebook posts that criticized the hospital’s COVID-19 preparedness efforts.
In an interview, Dr. Lin discussed the details of his situation to a degree that rarely occurs in such cases. This is one of the most extensive interviews he has granted.
Postings on Facebook
Dr. Lin said that on the basis of an intense study of the virus at the onset of the pandemic, he developed many ideas as to what could be done to mitigate its spread. While working as a locum tenens physician on his time off, he could see how others dealt with COVID-19.
Dr. Lin said from past experiences he did not feel that he could present his ideas directly to administration and be heard, so he decided to air his ideas about how his hospital could handle COVID-19 on his Facebook page, which drew a large audience.
He said he was certain that hospital administrators were reading his posts. He said receptionists at this hospital were advised not to wear masks, evidently because it would alarm patients. Dr. Lin said he posted concerns about their safety and called for them to wear masks. Soon after, the hospital directed receptionists to wear masks.
Dr. Lin’s Facebook posts also criticized the hospital for taking what he felt was too long to get results on COVID-19 tests. “It was taking them up to 10 days to get test results, because samples were being sent to a lab in California,” he said. He suggested it would be faster to send samples to the University of Washington. Soon after, the hospital started sending samples there.
In just a couple of weeks, Dr. Lin said, he voiced almost a dozen concerns. Each time the hospital made changes in line with his recommendations. Although he didn’t get any direct acknowledgment from the hospital for his help, he said he felt he was making a positive impact.
How employers react to physicians who speak out
Physicians who speak out about conditions tend to deeply disturb administrators, said William P. Sullivan, DO, JD, an emergency physician and lawyer in Frankfort, Ill., who has written about physicians being terminated by hospitals.
“These physicians go to the news media or they use social media,” Dr. Sullivan said, “but hospital administrators don’t want the public to hear bad things about their hospital.”
Then the public might not come to the hospital, which is an administrator’s worst nightmare. Even if physicians think their criticisms are reasonable, administrators may still fear a resulting drop in patients.
Dr. Houston, for example, was helping her Mississippi hospital by collecting donations of N95 masks for nurses, but to administrators, it showed that the hospital did not have enough masks.
“It is not helpful to stoke fear and anxiety, even if the intent is sincere,” a spokesperson for the hospital said.
Administrator fires back
Dr. Lin’s posts were deeply concerning to Richard DeCarlo, chief operating officer of PeaceHealth, which runs St. Joseph Hospital. Mr. DeCarlo discussed his concerns in a video interview in April with the blogger Zubin Damania, MD, known as ZDoggMD.
Comments on Dr. Lin’s Facebook posts showed that people “were fearful to go to the hospital,” he told Dr. Damania. “They were concluding that they would need to drive to another hospital.”
Mr. DeCarlo said he was also unhappy that Dr. Lin did not directly contact administrators about his concerns. “He didn’t communicate with his medical director,” Mr. DeCarlo said in the interview. “The ED staff had been meeting three times a week with the chief medical officer to make sure they had everything they needed, but he only attended one of these meetings and didn’t ask any questions.”
Dr. Lin maintains he did ask questions at the first meeting but stopped attending because he felt he wasn’t being heeded. “I found their tone not very receptive,” he said.
Doctor allegedly offered “misinformation”
At the start of the pandemic, some hospitals made it clear what would happen to doctors who brought up lack of PPE or other problems to the media. For example, NYU Langone Medical Center in New York sent an email to staff warning that speaking to the media without permission “will be subject to disciplinary action, including termination.”
PeaceHealth took a different tack. “It’s not that we have a policy that says don’t ever talk to the media,” Mr. DeCarlo said in the ZDoggMD interview, but in Dr. Lin’s case, “what was at issue was the misinformation. His leader went to him and said, ‘Look, you’re posting things that aren’t accurate.’ ”
Dr. Lin disputes that he provided any misinformation. In the interview, Mr. DeCarlo cited just one example of alleged misinformation. He said Dr. Lin called for a tent outside the emergency department (ED) to protect patients entering the department from aerosol exposure to COVID-19. Mr. DeCarlo said the tent was not needed because fewer people were using the ED.
“To put it in an extreme way,” Mr. DeCarlo said of Dr. Lin’s posts, “it was like yelling fire in a theater where there is not a fire.”
Dr. Lin said the hospital did briefly erect a tent and then removed it, and he still insisted that a tent was a good idea. He added that Mr. DeCarlo never mentioned any of the other suggestions Dr. Lin made, nor did he state that the hospital adopted them.
Doctor gets a warning
Dr. Lin said that after he started posting his concerns, he got a call from the emergency department director who worked for TeamHealth, an emergency medicine staffing firm that contracted with PeaceHealth and employed Dr. Lin, too.
Dr. Lin said his immediate supervisor at TeamHealth told him the hospital was unhappy with his posts and that he should take them down and suggested he might be fired. Dr. Lin said the supervisor also asked him to apologize to the hospital administration for these posts, but he refused to do so.
“Retracting and apologizing was not only wrong but would have left me vulnerable to being terminated with no repercussions,” he said.
“At that point, I realized I had crossed the Rubicon,” Dr. Lin said. He thought he might well be fired, no matter what he did, so he took his story to The Seattle Times, which had a much wider platform than his Facebook page had.
Dr. Lin lost his job at St. Joseph a week after The Seattle Times story about him appeared. “About 10 minutes before my shift was supposed to start, I received a text message from TeamHealth saying that someone else would be taking the shift,” he said.
In a release, TeamHealth insisted Dr. Lin was not fired and that he was scheduled to be reassigned to work at other hospitals. Dr. Lin, however, said he was not told this at the time and that he found out later that the new assignment would involve a pay cut and a significant commute. He said he has not taken any new assignments from TeamHealth since he lost his job at St. Joseph.
Dr. Lin has filed a lawsuit against PeaceHealth, TeamHealth, and Mr. DeCarlo, asking for his job back and for an apology. He said he has not asked for any financial damages at this point.
Since leaving St. Joseph, Dr. Lin has been working as an administrator for the Indian Health Service in the upper plains states. He said he can do some of the work at home in Washington State, which allows him to be with his wife and three young children.
Dr. Lin no longer sees patients. “I feel I have lost my confidence as a clinician,” he said. “I’m not sure why, but I find it hard to make quick judgments when taking care of patients.”
He said many doctors have told him about their own troubles with speaking out, but they did not want to come forward and talk about it because they feared more repercussions.
Do doctors who speak out have any rights?
Because TeamHealth, Dr. Lin’s actual employer, asserts he was never actually terminated, Dr. Lin has not been able to appeal his case internally in accordance with due process, an option that allows doctors to get a fair hearing and to appeal decisions against them.
The American Academy of Emergency Medicine pointed out this problem. “Dr. Lin, as a member of the medical staff, is entitled to full due process and a fair hearing from his peers on the medical staff,” the academy said in a statement supporting him.
The Joint Commission, the hospital accreditor, requires that hospitals provide due process to doctors before they can be terminated. However, Dr. Sullivan said employers often make physicians waive their due process rights in the employment contract. “The result is that the employer can terminate doctors for no reason,” he said.
In the 2013 survey of emergency physicians, 62% reported that their employers could terminate them without full due process.
Dr. Weiss, the Maryland MD-JD, said that when he advises doctors on their contracts, he generally tells them to cross out the waiver language. The applicant, he says, may also tell the employer that the waivers are considered unethical by many physician professional societies. In some cases, he said, the hospital will back down.
Conclusion
To maintain quality of care, it is essential that physicians feel free to speak out about issues that concern them. They can improve their chances of being heard by working directly with management and attending meetings, but in some cases, management may be unwilling to listen.
A version of this article first appeared on Medscape.com.
Measuring cotinine to monitor tobacco use and smoking cessation
Cigarette smoking is common among patients with schizophrenia, mood disorders, anxiety disorders,1-3 substance use disorders (SUDs),4 and other psychiatric disorders. Research suggests that compared with the general population, patients with SUDs consume more nicotine products and are more vulnerable to the effects of smoking.5 Despite the availability of effective treatments, many mental health professionals are reluctant to identify and treat tobacco use disorder,6-8 or they prioritize other disorders over tobacco use. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illness.
Cotinine is a biomarker that can be used to detect tobacco use. It can be measured in routine clinical practice by collecting urinary, serum, or salivary specimens, and used to monitor psychiatric patients’ tobacco use. Monitoring cotinine levels is similar to using other biomarkers to assess medication adherence or identify illicit substance use. A growing body of evidence supports the utility of cotinine screening as a part of a comprehensive substance use disorder treatment plan,5,9,10 especially for:
- patients who have comorbid conditions that can be exacerbated by tobacco use, such as chronic obstructive pulmonary disease
- patients who are pregnant11,12
- patients who are less reliable in self-report or who require objective testing for validation.
Routine clinical screening of tobacco use is recommended for all patients and early detection may facilitate earlier treatment. Several FDA-approved medications are available for smoking cessation13; however, discussion of treatment options is beyond the scope of this review. In this article, we describe how cotinine is measured and analyzed, 3 case vignettes that illustrate its potential clinical utility, and limitations to its use as a biomarker of tobacco use.
Methods of measuring cotinine
Cigarette smoking is associated with the absorption of nicotine, which is mainly metabolized by cytochrome P450 (CYP) 2A6 to 6 primary metabolites: cotinine, hydroxycotinine, norcotinine, nornicotine, cotinine oxide, and nicotine oxide.14,15 Cotinine is the biomarker of choice for detecting use of tobacco/nicotine products due to its stability (it is not influenced by dietary or environmental factors), extended half-life (16 to 19 hours, compared with 2 hours for nicotine), and stable concentration throughout the day. Samples from saliva, urine, or blood can be analyzed through radioimmunoassay, enzyme-linked immunosorbent assay (ELISA), and gas/liquid chromatography.16 The specificity of cotinine for tobacco use is excellent, except for persons who are taking medications that contain nicotine.17
An advantage of cotinine over other biomarkers for smoking (such as carbon monoxide in expired air) is that the optimal cut-off points for cotinine are relatively uninfluenced by the prevalence of smoking in the population. The optimal cut-off levels used to detect current tobacco use may vary based on the sample or test used (saliva, urine, or plasma) and certain patient-specific factors (Box 111,16,18-21). However, for plasma or saliva cotinine, 16 ng/mL is the generally accepted cut-off level for detecting current tobacco use. A urinary cotinine cut-off level of 50 ng/mL is likely appropriate for most circumstances.17 Users of electronic nicotine delivery systems (electronic cigarettes) have been found to have cotinine levels similar to those of cigarette smokers.22
Box 1
Daily smokers typically have a serum/plasma cotinine concentration of ≥100 ng/mL. Individuals with heavy exposure to secondhand smoking may have plasma cotinine concentrations up to 25 ng/mL, and urine samples tend to be much more specific.16 However, serum cotinine has a wide cut-off range due to diverse racial/ethnic, gender, and pregnancy-related variations; the wide range is also associated with genetic polymorphisms of cytochrome P450 2A6 alleles and nicotine’s numerous metabolic pathways.11,18
Traditionally a serum/plasma cut-off point of approximately 15 ng/mL has been accepted to detect current tobacco use; however, recent studies21 recommend an average optimal cut-off point for US adults of 3 ng/mL. This possibly reflects differences in national cigarette smoking patterns and exposure.21 One study suggested optimal cut-off differences for men (1.78 ng/mL) and women (4.47 ng/mL).19 The same study also suggested different optimal cut-off levels for non-Hispanic White men (6.79 ng/ mL), non-Hispanic Black men (13.3 ng/mL), and Mexican-American men (0.79 ng/mL).19 These researchers also suggested different optimal cut-off levels for non-Hispanic White women (4.73 ng/mL), non-Hispanic Black women (5.91 ng/mL), and Mexican-American women (0.84 ng/mL).19 Genetic factors may also play a role in the progression of nicotine dependence and pose challenges that impact smoking persistence.20
Assessment of cotinine levels in saliva may be considered for outpatient monitoring due to its noninvasive nature, tolerability, and the ability to collect multiple samples over a limited period.23 Saliva cotinine levels correlate closely with blood concentrations. Urine cotinine levels offer some advantage because concentrations are 6 times higher in urine than in blood or saliva. For this reason, urine cotinine is the most widely used biomarker in individuals who use tobacco due to its high sensitivity, specificity, reliability, and noninvasive collection.23 By using a lower urinary cut-off of ≥2.47 ng/mL, ELISA kits detect the highest sensitivity and specificity, which is useful for monitoring daily tobacco use.24 This cut-off value was associated with 100% sensitivity and specificity, and these numbers declined with increases in the cut-off threshold.23
The following 3 clinical vignettes illustrate the impact of tobacco use disorder on patients, and how cotinine might help with their treatment.
Continue to: Vignette 1
Vignette 1
Mr. D, age 44, has a history of schizophrenia and has smoked 1 pack of cigarettes per day for the last 15 years. He was recently discharged from an inpatient psychiatric facility after his symptoms were stabilized. During his hospitalization, Mr. D used a nicotine-replacement product to comply with the hospital’s smoke-free policy. Unfortunately, since discharge, Mr. D reports worsening auditory hallucinations despite adherence with his antipsychotic medication, clozapine, 600 mg at bedtime. Collateral information gathered from Mr. D’s mother confirms that he has been adherent with the discharge medication regimen; however, Mr. D has resumed smoking 1 pack of cigarettes daily. The treatment team suspects that his worsening psychosis is related to the decrease of blood clozapine level due to CYP induction by cigarette smoke.
Cotinine and smoking-related drug interactions
Vignette 1 illustrates the significant impact tobacco smoke can have on the effectiveness of a psychotropic medication. This is caused by polycyclic aromatic hydrocarbons induction of hepatic CYP1A2 isoenzymes. Clinicians should routinely screen patients for smoking status due to the potential for drug interactions. Common major CYP1A2 substrates include
Vignette 2
Mr. B, age 34, has a history of cocaine use disorder and tobacco use disorder. He is referred to a treatment program and participates in a contingency management program for his substance use disorders. Biomarkers, including salivary cotinine, are used to assess Mr. B’s exposure to tobacco use. Mr. B and other participants in his program are eligible for prize draws if they are found to have samples that are negative for tobacco and other substances. There are other incentives in place for patients who show a reduced cotinine concentration.
Cotinine monitoring and contingency management
Clinicians can incorporate cotinine monitoring into existing SUD treatment. This is similar to the utilization of other biomarkers that are commonly used to identify recent illicit substance use or monitor adherence to treatment medications. For example, benzoylecgonine, a metabolite of cocaine, is frequently used to monitor abstinence from cocaine.
Treatments based on contingency management principles involve giving patients tangible rewards to reinforce desired (positive) behaviors. Smoking cessation can be confirmed by monitoring cotinine levels. Gayman et al9 found twice-weekly salivary testing was compatible with monitoring and promoting abstinence in a prize-based contingency management smoking cessation program. Most prior studies used urine cotinine measures to verify abstinence. Although highly reliable, urine samples require close monitoring to ensure sample validity, which can be a burden on staff and unpleasant for patients.9 It is also important to note that the rate of elimination of cotinine from saliva and urine are comparable. The half-life of cotinine is approximately 18 hours, and therefore the specificity of salivary test strips may be impacted during the first 4 to 5 days of abstinence. In the first few days of smoking cessation, a more intensive approach, such as quantifying urine cotinine levels and monitoring decline, may be appropriate.23
Continue to: Vignette 3
Vignette 3
Ms. C, age 34 and pregnant, is admitted to an outpatient treatment program for alcohol use disorder. She also has generalized anxiety disorder and tobacco use disorder. In addition to attending group therapy sessions and self-reporting any recent alcohol consumption, Ms. C also undergoes alcohol breathalyzer tests and urine studies of alcohol metabolites to monitor abstinence from alcohol. She says that the regular laboratory screening for alcohol use gives her a sense of accountability and tangible evidence of change that positively impacts her treatment. When the treating psychiatrist recommends that Ms. C also consider addressing her tobacco use disorder, she asks if there is some way to include laboratory testing to monitor her smoking cessation.
Cotinine as a predictor of smoking status
Smoking abstinence rates during pregnancy are lower than that for other substances, and pregnant women may not be aware of the impact of smoking on fetal development.30 Cotinine can be used to verify self-report of smoking status and severity.10,31,32
Salivary cotinine tests are commercially available, relatively economical, and convenient to use when frequent monitoring is required.32 In general, based on established cut-off values that are unique to the specimen collected, the overall high specificity and sensitivity of salivary testing allows clinicians to predict smoker vs nonsmoker status with confidence. For example, a 2008 study reported a salivary cotinine cut-off level of 12 ng/mL for smokers.21 The sensitivity and specificity of this cut-off value for distinguishing cigarette smokers from never smokers were 96.7% and 96.9%, respectively.21
Additionally, some studies suggest that cotinine levels may be predictive of treatment outcomes and retention in SUD treatment programs.33,34 One study of smoking cessation using nicotine replacement products found that compared with patients with lower baseline cotinine levels prior to treatment, patients with higher baseline cotinine plasma levels had lower smoking cessation success rates.34
A few caveats
There are several limitations to quantitative measures of cotinine (Box 221,23). These include (but are not limited to) potential errors related to sample collection, storage, shipping, and analysis.23 Compared with other methods, point-of-care cotinine measurement in saliva is noninvasive, simple, and requires less training to properly use.23
Box 2
Challenges in the collection of samples, storage, shipping, and instrumentation may limit cotinine consistency as a dependable biomarker in the clinical setting.23 Overall, quantitative measurements of cotinine have relative constructive utility in separating smokers from nonsmokers, because daily smokers typically have serum concentrations of 100 ng/mL or higher, in contrast to light/non-daily smokers, who have cotinine concentrations <10 ng/mL. Even heavy exposure to secondhand smoke typically yields plasma concentrations up to approximately 25 ng/mL. However, cotinine is a general metabolite found with the use of all nicotine products, which makes it extremely difficult to differentiate tobacco use from the use of nicotine replacement products, which are frequently used to treat tobacco use disorders.
One potential solution is to measure nicotine-derived nitrosamine ketone (NNK) and its metabolite 4-(methylnitrosamino)- 1-(3-pyridyl)-1-butanol (NNAL). Both NNK and NNAL are tobacco-specific lung carcinogens. NNAL can be measured in the urine. Although total NNAL represents only 15% of NNK dose intake, it has been quantified, with urine concentrations of ≥1,000 fmol/mL for daily smokers. NNAL also has an extremely high specificity to tobacco smoke, and thus allows differentiation of tobacco use from nicotine replacement treatment. Unfortunately, measurement for this biomarker requires specific chemical expertise and expensive equipment.
Another potential barrier to using cotinine in the clinical setting is the variable cut-off levels used in the United States, based on differences in race/ethnicity. This may be secondary to differences in smoking behaviors and/or differences in cotinine metabolism.21
Continue to: Confirmation of smoking cessation...
Confirmation of smoking cessation can be monitored reliably within the clinical setting using cotinine monitoring. However, this is not a routine test, and there are no guidelines or consensus on how or when it should be used. The clinical feasibility of cotinine monitoring for psychiatric patients will depend on the cost of testing, methods used, amount of reimbursement for performing the tests, and how clinicians value such testing.35
Bottom Line
Cotinine is a biomarker that can be used to detect tobacco use. Cotinine measurement can be used to monitor tobacco use and smoking cessation in psychiatric patients. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illnesses. However, cotinine measurement is not a routine test, and there are no guidelines on how or when this test should be used.
Related Resources
- Peckham E, Brabyn S, Cook L, et al. Smoking cessation in severe mental ill health: what works? An updated systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):252.
- Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ. 2015;351:h4065. doi: 10.1136/bmj.h4065
Drug Brand Names
Asenapine • Saphris
Buprenorphine • Sublocade
Clozapine • Clozaril
Duloxetine • Cymbalta
Haloperidol • Haldol
Mirtazapine • Remeron
Olanzapine • Zyprexa
Ziprasidone • Geodon
Zolpidem • Ambien
1. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185.
2. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12.
3. Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285.
4. Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14(2):106-123.
5. Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. J Subst Abuse Treat. 2009;36(2):205-219.
6. Hall SM, Tsoh JY, Prochaska JJ, et al. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Public Health. 2006;96(10):1808-1814.
7. McHugh RK, Votaw VR, Fulciniti F, et al. Perceived barriers to smoking cessation among adults with substance use disorders. J Subst Abuse Treat. 2017;74:48-53.
8. Strong DR, Uebelacker L, Fokas K, et al. Utilization of evidence-based smoking cessation treatments by psychiatric inpatient smokers with depression. J Addict Med. 2014;8(2):77-83.
9. Gayman C, Anderson K, Pietras C. Saliva cotinine as a measure of smoking abstinence in contingency management – a feasibility study. The Psychological Record. 2017;67(2):261-272.
10. Schepis TS, Duhig AM, Liss T, et al. Contingency management for smoking cessation: enhancing feasibility through use of immunoassay test strips measuring cotinine. Nicotine Tob Res. 2008;10(9):1495-1501.
11. Stragierowicz J, Mikolajewska K, Zawadzka-Stolarz M, et al. Estimation of cutoff values of cotinine in urine and saliva for pregnant women in Poland. Biomed Res Int. 2013;2013:386784. doi.org/10.1155/2013/386784
12. Shipton D, Tappin DM, Vadiveloo T, et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. doi.org/10.1136/bmj.b4347
13. Aubin HJ, Karila L, Reynaud M. Pharmacotherapy for smoking cessation: present and future. Curr Pharm Des. 2011;17(14):1343-1350.
14. McGuffey JE, Wei B, Bernert JT, et al. Validation of a LC-MS/MS method for quantifying urinary nicotine, six nicotine metabolites and the minor tobacco alkaloids--anatabine and anabasine--in smokers’ urine. PLoS One. 2014;9(7):e101816. doi: 10.1371/journal.pone.0101816
15. Duque A, Martinez PJ, Giraldo A, et al. Accuracy of cotinine serum test to detect the smoking habit and its association with periodontal disease in a multicenter study. Med Oral Patol Oral Cir Bucal. 2017;22(4):e425-e431. doi: 10.4317/medoral.21292
16. Avila-Tang E, Elf JL, Cummings KM, et al. Assessing secondhand smoke exposure with reported measures. Tob Control. 2013;22(3):156-163.
17. Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 Update. Nicotine Tob Res. 2020;22(7):1086-1097.
18. Nakajima M TY. Interindividual variability in nicotine metabolism: c-oxidation and glucuronidation. Drug Metab Pharmaokinet. 2005;20(4):227-235.
19. Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236-248.
20. Schnoll R, Johnson TA, Lerman C. Genetics and smoking behavior. Curr Psychiatry Rep. 2007;9(5):349-357.
21. Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health. 2016;13(12):1236.
22. Etter JF, Bullen C. Saliva cotinine levels in users of electronic cigarettes. Eur Respir J. 2011;38(5):1219-1220.
23. Raja M, Garg A, Yadav P, et al. Diagnostic methods for detection of cotinine level in tobacco users: a review. J Clin Diagn Res. 2016;10(3):ZE04-06. doi: 10.7860/JCDR/2016/17360.7423
24. Balhara YP, Jain R. A receiver operated curve-based evaluation of change in sensitivity and specificity of cotinine urinalysis for detecting active tobacco use. J Cancer Res Ther. 2013;9(1):84-89.
25. Fankhauser M. Drug interactions with tobacco smoke: implications for patient care. Current Psychiatry. 2013;12(1):12-16.
26. Scheuermann TS, Richter KP, Rigotti NA, et al. Accuracy of self-reported smoking abstinence in clinical trials of hospital-initiated smoking interventions. Addiction. 2017;112(12):2227-2236.
27. Holtyn AF, Knealing TW, Jarvis BP, et al. Monitoring cocaine use and abstinence among cocaine users for contingency management interventions. Psychol Rec. 2017;67(2):253-259.
28. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51.
29. Sullivan M, Covey, LS. Current perspectives on smoking cessation among substance abusers. Curr Psychiatry Rep. 2002;4(5):388-396.
30. Forray A, Merry B, Lin H, et al. Perinatal substance use: a prospective evaluation of abstinence and relapse. Drug Alcohol Depend. 2015;150:147-155.
31. Parker DR, Lasater TM, Windsor R, et al. The accuracy of self-reported smoking status assessed by cotinine test strips. Nicotine Tob Res. 2002;4(3):305-309.
32. Asha V, Dhanya M. Immunochromatographic assessment of salivary cotinine and its correlation with nicotine dependence in tobacco chewers. J Cancer Prev. 2015;20(2):159-163.
33. Hall S, Herning RI, Jones RT, et al. Blood cotinine levels as indicators of smoking treatment outcome. Clin Pharmacol Ther. 1984;35(6):810-814.
34. Paoletti P, Fornai E, Maggiorelli F, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double-blind study with nicotine patch. Eur Respir J. 1996;9(4):643-651.
35. Montalto NJ, Wells WO. Validation of self-reported smoking status using saliva cotinine: a rapid semiquantitative dipstick method. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1858-1862.
Cigarette smoking is common among patients with schizophrenia, mood disorders, anxiety disorders,1-3 substance use disorders (SUDs),4 and other psychiatric disorders. Research suggests that compared with the general population, patients with SUDs consume more nicotine products and are more vulnerable to the effects of smoking.5 Despite the availability of effective treatments, many mental health professionals are reluctant to identify and treat tobacco use disorder,6-8 or they prioritize other disorders over tobacco use. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illness.
Cotinine is a biomarker that can be used to detect tobacco use. It can be measured in routine clinical practice by collecting urinary, serum, or salivary specimens, and used to monitor psychiatric patients’ tobacco use. Monitoring cotinine levels is similar to using other biomarkers to assess medication adherence or identify illicit substance use. A growing body of evidence supports the utility of cotinine screening as a part of a comprehensive substance use disorder treatment plan,5,9,10 especially for:
- patients who have comorbid conditions that can be exacerbated by tobacco use, such as chronic obstructive pulmonary disease
- patients who are pregnant11,12
- patients who are less reliable in self-report or who require objective testing for validation.
Routine clinical screening of tobacco use is recommended for all patients and early detection may facilitate earlier treatment. Several FDA-approved medications are available for smoking cessation13; however, discussion of treatment options is beyond the scope of this review. In this article, we describe how cotinine is measured and analyzed, 3 case vignettes that illustrate its potential clinical utility, and limitations to its use as a biomarker of tobacco use.
Methods of measuring cotinine
Cigarette smoking is associated with the absorption of nicotine, which is mainly metabolized by cytochrome P450 (CYP) 2A6 to 6 primary metabolites: cotinine, hydroxycotinine, norcotinine, nornicotine, cotinine oxide, and nicotine oxide.14,15 Cotinine is the biomarker of choice for detecting use of tobacco/nicotine products due to its stability (it is not influenced by dietary or environmental factors), extended half-life (16 to 19 hours, compared with 2 hours for nicotine), and stable concentration throughout the day. Samples from saliva, urine, or blood can be analyzed through radioimmunoassay, enzyme-linked immunosorbent assay (ELISA), and gas/liquid chromatography.16 The specificity of cotinine for tobacco use is excellent, except for persons who are taking medications that contain nicotine.17
An advantage of cotinine over other biomarkers for smoking (such as carbon monoxide in expired air) is that the optimal cut-off points for cotinine are relatively uninfluenced by the prevalence of smoking in the population. The optimal cut-off levels used to detect current tobacco use may vary based on the sample or test used (saliva, urine, or plasma) and certain patient-specific factors (Box 111,16,18-21). However, for plasma or saliva cotinine, 16 ng/mL is the generally accepted cut-off level for detecting current tobacco use. A urinary cotinine cut-off level of 50 ng/mL is likely appropriate for most circumstances.17 Users of electronic nicotine delivery systems (electronic cigarettes) have been found to have cotinine levels similar to those of cigarette smokers.22
Box 1
Daily smokers typically have a serum/plasma cotinine concentration of ≥100 ng/mL. Individuals with heavy exposure to secondhand smoking may have plasma cotinine concentrations up to 25 ng/mL, and urine samples tend to be much more specific.16 However, serum cotinine has a wide cut-off range due to diverse racial/ethnic, gender, and pregnancy-related variations; the wide range is also associated with genetic polymorphisms of cytochrome P450 2A6 alleles and nicotine’s numerous metabolic pathways.11,18
Traditionally a serum/plasma cut-off point of approximately 15 ng/mL has been accepted to detect current tobacco use; however, recent studies21 recommend an average optimal cut-off point for US adults of 3 ng/mL. This possibly reflects differences in national cigarette smoking patterns and exposure.21 One study suggested optimal cut-off differences for men (1.78 ng/mL) and women (4.47 ng/mL).19 The same study also suggested different optimal cut-off levels for non-Hispanic White men (6.79 ng/ mL), non-Hispanic Black men (13.3 ng/mL), and Mexican-American men (0.79 ng/mL).19 These researchers also suggested different optimal cut-off levels for non-Hispanic White women (4.73 ng/mL), non-Hispanic Black women (5.91 ng/mL), and Mexican-American women (0.84 ng/mL).19 Genetic factors may also play a role in the progression of nicotine dependence and pose challenges that impact smoking persistence.20
Assessment of cotinine levels in saliva may be considered for outpatient monitoring due to its noninvasive nature, tolerability, and the ability to collect multiple samples over a limited period.23 Saliva cotinine levels correlate closely with blood concentrations. Urine cotinine levels offer some advantage because concentrations are 6 times higher in urine than in blood or saliva. For this reason, urine cotinine is the most widely used biomarker in individuals who use tobacco due to its high sensitivity, specificity, reliability, and noninvasive collection.23 By using a lower urinary cut-off of ≥2.47 ng/mL, ELISA kits detect the highest sensitivity and specificity, which is useful for monitoring daily tobacco use.24 This cut-off value was associated with 100% sensitivity and specificity, and these numbers declined with increases in the cut-off threshold.23
The following 3 clinical vignettes illustrate the impact of tobacco use disorder on patients, and how cotinine might help with their treatment.
Continue to: Vignette 1
Vignette 1
Mr. D, age 44, has a history of schizophrenia and has smoked 1 pack of cigarettes per day for the last 15 years. He was recently discharged from an inpatient psychiatric facility after his symptoms were stabilized. During his hospitalization, Mr. D used a nicotine-replacement product to comply with the hospital’s smoke-free policy. Unfortunately, since discharge, Mr. D reports worsening auditory hallucinations despite adherence with his antipsychotic medication, clozapine, 600 mg at bedtime. Collateral information gathered from Mr. D’s mother confirms that he has been adherent with the discharge medication regimen; however, Mr. D has resumed smoking 1 pack of cigarettes daily. The treatment team suspects that his worsening psychosis is related to the decrease of blood clozapine level due to CYP induction by cigarette smoke.
Cotinine and smoking-related drug interactions
Vignette 1 illustrates the significant impact tobacco smoke can have on the effectiveness of a psychotropic medication. This is caused by polycyclic aromatic hydrocarbons induction of hepatic CYP1A2 isoenzymes. Clinicians should routinely screen patients for smoking status due to the potential for drug interactions. Common major CYP1A2 substrates include
Vignette 2
Mr. B, age 34, has a history of cocaine use disorder and tobacco use disorder. He is referred to a treatment program and participates in a contingency management program for his substance use disorders. Biomarkers, including salivary cotinine, are used to assess Mr. B’s exposure to tobacco use. Mr. B and other participants in his program are eligible for prize draws if they are found to have samples that are negative for tobacco and other substances. There are other incentives in place for patients who show a reduced cotinine concentration.
Cotinine monitoring and contingency management
Clinicians can incorporate cotinine monitoring into existing SUD treatment. This is similar to the utilization of other biomarkers that are commonly used to identify recent illicit substance use or monitor adherence to treatment medications. For example, benzoylecgonine, a metabolite of cocaine, is frequently used to monitor abstinence from cocaine.
Treatments based on contingency management principles involve giving patients tangible rewards to reinforce desired (positive) behaviors. Smoking cessation can be confirmed by monitoring cotinine levels. Gayman et al9 found twice-weekly salivary testing was compatible with monitoring and promoting abstinence in a prize-based contingency management smoking cessation program. Most prior studies used urine cotinine measures to verify abstinence. Although highly reliable, urine samples require close monitoring to ensure sample validity, which can be a burden on staff and unpleasant for patients.9 It is also important to note that the rate of elimination of cotinine from saliva and urine are comparable. The half-life of cotinine is approximately 18 hours, and therefore the specificity of salivary test strips may be impacted during the first 4 to 5 days of abstinence. In the first few days of smoking cessation, a more intensive approach, such as quantifying urine cotinine levels and monitoring decline, may be appropriate.23
Continue to: Vignette 3
Vignette 3
Ms. C, age 34 and pregnant, is admitted to an outpatient treatment program for alcohol use disorder. She also has generalized anxiety disorder and tobacco use disorder. In addition to attending group therapy sessions and self-reporting any recent alcohol consumption, Ms. C also undergoes alcohol breathalyzer tests and urine studies of alcohol metabolites to monitor abstinence from alcohol. She says that the regular laboratory screening for alcohol use gives her a sense of accountability and tangible evidence of change that positively impacts her treatment. When the treating psychiatrist recommends that Ms. C also consider addressing her tobacco use disorder, she asks if there is some way to include laboratory testing to monitor her smoking cessation.
Cotinine as a predictor of smoking status
Smoking abstinence rates during pregnancy are lower than that for other substances, and pregnant women may not be aware of the impact of smoking on fetal development.30 Cotinine can be used to verify self-report of smoking status and severity.10,31,32
Salivary cotinine tests are commercially available, relatively economical, and convenient to use when frequent monitoring is required.32 In general, based on established cut-off values that are unique to the specimen collected, the overall high specificity and sensitivity of salivary testing allows clinicians to predict smoker vs nonsmoker status with confidence. For example, a 2008 study reported a salivary cotinine cut-off level of 12 ng/mL for smokers.21 The sensitivity and specificity of this cut-off value for distinguishing cigarette smokers from never smokers were 96.7% and 96.9%, respectively.21
Additionally, some studies suggest that cotinine levels may be predictive of treatment outcomes and retention in SUD treatment programs.33,34 One study of smoking cessation using nicotine replacement products found that compared with patients with lower baseline cotinine levels prior to treatment, patients with higher baseline cotinine plasma levels had lower smoking cessation success rates.34
A few caveats
There are several limitations to quantitative measures of cotinine (Box 221,23). These include (but are not limited to) potential errors related to sample collection, storage, shipping, and analysis.23 Compared with other methods, point-of-care cotinine measurement in saliva is noninvasive, simple, and requires less training to properly use.23
Box 2
Challenges in the collection of samples, storage, shipping, and instrumentation may limit cotinine consistency as a dependable biomarker in the clinical setting.23 Overall, quantitative measurements of cotinine have relative constructive utility in separating smokers from nonsmokers, because daily smokers typically have serum concentrations of 100 ng/mL or higher, in contrast to light/non-daily smokers, who have cotinine concentrations <10 ng/mL. Even heavy exposure to secondhand smoke typically yields plasma concentrations up to approximately 25 ng/mL. However, cotinine is a general metabolite found with the use of all nicotine products, which makes it extremely difficult to differentiate tobacco use from the use of nicotine replacement products, which are frequently used to treat tobacco use disorders.
One potential solution is to measure nicotine-derived nitrosamine ketone (NNK) and its metabolite 4-(methylnitrosamino)- 1-(3-pyridyl)-1-butanol (NNAL). Both NNK and NNAL are tobacco-specific lung carcinogens. NNAL can be measured in the urine. Although total NNAL represents only 15% of NNK dose intake, it has been quantified, with urine concentrations of ≥1,000 fmol/mL for daily smokers. NNAL also has an extremely high specificity to tobacco smoke, and thus allows differentiation of tobacco use from nicotine replacement treatment. Unfortunately, measurement for this biomarker requires specific chemical expertise and expensive equipment.
Another potential barrier to using cotinine in the clinical setting is the variable cut-off levels used in the United States, based on differences in race/ethnicity. This may be secondary to differences in smoking behaviors and/or differences in cotinine metabolism.21
Continue to: Confirmation of smoking cessation...
Confirmation of smoking cessation can be monitored reliably within the clinical setting using cotinine monitoring. However, this is not a routine test, and there are no guidelines or consensus on how or when it should be used. The clinical feasibility of cotinine monitoring for psychiatric patients will depend on the cost of testing, methods used, amount of reimbursement for performing the tests, and how clinicians value such testing.35
Bottom Line
Cotinine is a biomarker that can be used to detect tobacco use. Cotinine measurement can be used to monitor tobacco use and smoking cessation in psychiatric patients. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illnesses. However, cotinine measurement is not a routine test, and there are no guidelines on how or when this test should be used.
Related Resources
- Peckham E, Brabyn S, Cook L, et al. Smoking cessation in severe mental ill health: what works? An updated systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):252.
- Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ. 2015;351:h4065. doi: 10.1136/bmj.h4065
Drug Brand Names
Asenapine • Saphris
Buprenorphine • Sublocade
Clozapine • Clozaril
Duloxetine • Cymbalta
Haloperidol • Haldol
Mirtazapine • Remeron
Olanzapine • Zyprexa
Ziprasidone • Geodon
Zolpidem • Ambien
Cigarette smoking is common among patients with schizophrenia, mood disorders, anxiety disorders,1-3 substance use disorders (SUDs),4 and other psychiatric disorders. Research suggests that compared with the general population, patients with SUDs consume more nicotine products and are more vulnerable to the effects of smoking.5 Despite the availability of effective treatments, many mental health professionals are reluctant to identify and treat tobacco use disorder,6-8 or they prioritize other disorders over tobacco use. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illness.
Cotinine is a biomarker that can be used to detect tobacco use. It can be measured in routine clinical practice by collecting urinary, serum, or salivary specimens, and used to monitor psychiatric patients’ tobacco use. Monitoring cotinine levels is similar to using other biomarkers to assess medication adherence or identify illicit substance use. A growing body of evidence supports the utility of cotinine screening as a part of a comprehensive substance use disorder treatment plan,5,9,10 especially for:
- patients who have comorbid conditions that can be exacerbated by tobacco use, such as chronic obstructive pulmonary disease
- patients who are pregnant11,12
- patients who are less reliable in self-report or who require objective testing for validation.
Routine clinical screening of tobacco use is recommended for all patients and early detection may facilitate earlier treatment. Several FDA-approved medications are available for smoking cessation13; however, discussion of treatment options is beyond the scope of this review. In this article, we describe how cotinine is measured and analyzed, 3 case vignettes that illustrate its potential clinical utility, and limitations to its use as a biomarker of tobacco use.
Methods of measuring cotinine
Cigarette smoking is associated with the absorption of nicotine, which is mainly metabolized by cytochrome P450 (CYP) 2A6 to 6 primary metabolites: cotinine, hydroxycotinine, norcotinine, nornicotine, cotinine oxide, and nicotine oxide.14,15 Cotinine is the biomarker of choice for detecting use of tobacco/nicotine products due to its stability (it is not influenced by dietary or environmental factors), extended half-life (16 to 19 hours, compared with 2 hours for nicotine), and stable concentration throughout the day. Samples from saliva, urine, or blood can be analyzed through radioimmunoassay, enzyme-linked immunosorbent assay (ELISA), and gas/liquid chromatography.16 The specificity of cotinine for tobacco use is excellent, except for persons who are taking medications that contain nicotine.17
An advantage of cotinine over other biomarkers for smoking (such as carbon monoxide in expired air) is that the optimal cut-off points for cotinine are relatively uninfluenced by the prevalence of smoking in the population. The optimal cut-off levels used to detect current tobacco use may vary based on the sample or test used (saliva, urine, or plasma) and certain patient-specific factors (Box 111,16,18-21). However, for plasma or saliva cotinine, 16 ng/mL is the generally accepted cut-off level for detecting current tobacco use. A urinary cotinine cut-off level of 50 ng/mL is likely appropriate for most circumstances.17 Users of electronic nicotine delivery systems (electronic cigarettes) have been found to have cotinine levels similar to those of cigarette smokers.22
Box 1
Daily smokers typically have a serum/plasma cotinine concentration of ≥100 ng/mL. Individuals with heavy exposure to secondhand smoking may have plasma cotinine concentrations up to 25 ng/mL, and urine samples tend to be much more specific.16 However, serum cotinine has a wide cut-off range due to diverse racial/ethnic, gender, and pregnancy-related variations; the wide range is also associated with genetic polymorphisms of cytochrome P450 2A6 alleles and nicotine’s numerous metabolic pathways.11,18
Traditionally a serum/plasma cut-off point of approximately 15 ng/mL has been accepted to detect current tobacco use; however, recent studies21 recommend an average optimal cut-off point for US adults of 3 ng/mL. This possibly reflects differences in national cigarette smoking patterns and exposure.21 One study suggested optimal cut-off differences for men (1.78 ng/mL) and women (4.47 ng/mL).19 The same study also suggested different optimal cut-off levels for non-Hispanic White men (6.79 ng/ mL), non-Hispanic Black men (13.3 ng/mL), and Mexican-American men (0.79 ng/mL).19 These researchers also suggested different optimal cut-off levels for non-Hispanic White women (4.73 ng/mL), non-Hispanic Black women (5.91 ng/mL), and Mexican-American women (0.84 ng/mL).19 Genetic factors may also play a role in the progression of nicotine dependence and pose challenges that impact smoking persistence.20
Assessment of cotinine levels in saliva may be considered for outpatient monitoring due to its noninvasive nature, tolerability, and the ability to collect multiple samples over a limited period.23 Saliva cotinine levels correlate closely with blood concentrations. Urine cotinine levels offer some advantage because concentrations are 6 times higher in urine than in blood or saliva. For this reason, urine cotinine is the most widely used biomarker in individuals who use tobacco due to its high sensitivity, specificity, reliability, and noninvasive collection.23 By using a lower urinary cut-off of ≥2.47 ng/mL, ELISA kits detect the highest sensitivity and specificity, which is useful for monitoring daily tobacco use.24 This cut-off value was associated with 100% sensitivity and specificity, and these numbers declined with increases in the cut-off threshold.23
The following 3 clinical vignettes illustrate the impact of tobacco use disorder on patients, and how cotinine might help with their treatment.
Continue to: Vignette 1
Vignette 1
Mr. D, age 44, has a history of schizophrenia and has smoked 1 pack of cigarettes per day for the last 15 years. He was recently discharged from an inpatient psychiatric facility after his symptoms were stabilized. During his hospitalization, Mr. D used a nicotine-replacement product to comply with the hospital’s smoke-free policy. Unfortunately, since discharge, Mr. D reports worsening auditory hallucinations despite adherence with his antipsychotic medication, clozapine, 600 mg at bedtime. Collateral information gathered from Mr. D’s mother confirms that he has been adherent with the discharge medication regimen; however, Mr. D has resumed smoking 1 pack of cigarettes daily. The treatment team suspects that his worsening psychosis is related to the decrease of blood clozapine level due to CYP induction by cigarette smoke.
Cotinine and smoking-related drug interactions
Vignette 1 illustrates the significant impact tobacco smoke can have on the effectiveness of a psychotropic medication. This is caused by polycyclic aromatic hydrocarbons induction of hepatic CYP1A2 isoenzymes. Clinicians should routinely screen patients for smoking status due to the potential for drug interactions. Common major CYP1A2 substrates include
Vignette 2
Mr. B, age 34, has a history of cocaine use disorder and tobacco use disorder. He is referred to a treatment program and participates in a contingency management program for his substance use disorders. Biomarkers, including salivary cotinine, are used to assess Mr. B’s exposure to tobacco use. Mr. B and other participants in his program are eligible for prize draws if they are found to have samples that are negative for tobacco and other substances. There are other incentives in place for patients who show a reduced cotinine concentration.
Cotinine monitoring and contingency management
Clinicians can incorporate cotinine monitoring into existing SUD treatment. This is similar to the utilization of other biomarkers that are commonly used to identify recent illicit substance use or monitor adherence to treatment medications. For example, benzoylecgonine, a metabolite of cocaine, is frequently used to monitor abstinence from cocaine.
Treatments based on contingency management principles involve giving patients tangible rewards to reinforce desired (positive) behaviors. Smoking cessation can be confirmed by monitoring cotinine levels. Gayman et al9 found twice-weekly salivary testing was compatible with monitoring and promoting abstinence in a prize-based contingency management smoking cessation program. Most prior studies used urine cotinine measures to verify abstinence. Although highly reliable, urine samples require close monitoring to ensure sample validity, which can be a burden on staff and unpleasant for patients.9 It is also important to note that the rate of elimination of cotinine from saliva and urine are comparable. The half-life of cotinine is approximately 18 hours, and therefore the specificity of salivary test strips may be impacted during the first 4 to 5 days of abstinence. In the first few days of smoking cessation, a more intensive approach, such as quantifying urine cotinine levels and monitoring decline, may be appropriate.23
Continue to: Vignette 3
Vignette 3
Ms. C, age 34 and pregnant, is admitted to an outpatient treatment program for alcohol use disorder. She also has generalized anxiety disorder and tobacco use disorder. In addition to attending group therapy sessions and self-reporting any recent alcohol consumption, Ms. C also undergoes alcohol breathalyzer tests and urine studies of alcohol metabolites to monitor abstinence from alcohol. She says that the regular laboratory screening for alcohol use gives her a sense of accountability and tangible evidence of change that positively impacts her treatment. When the treating psychiatrist recommends that Ms. C also consider addressing her tobacco use disorder, she asks if there is some way to include laboratory testing to monitor her smoking cessation.
Cotinine as a predictor of smoking status
Smoking abstinence rates during pregnancy are lower than that for other substances, and pregnant women may not be aware of the impact of smoking on fetal development.30 Cotinine can be used to verify self-report of smoking status and severity.10,31,32
Salivary cotinine tests are commercially available, relatively economical, and convenient to use when frequent monitoring is required.32 In general, based on established cut-off values that are unique to the specimen collected, the overall high specificity and sensitivity of salivary testing allows clinicians to predict smoker vs nonsmoker status with confidence. For example, a 2008 study reported a salivary cotinine cut-off level of 12 ng/mL for smokers.21 The sensitivity and specificity of this cut-off value for distinguishing cigarette smokers from never smokers were 96.7% and 96.9%, respectively.21
Additionally, some studies suggest that cotinine levels may be predictive of treatment outcomes and retention in SUD treatment programs.33,34 One study of smoking cessation using nicotine replacement products found that compared with patients with lower baseline cotinine levels prior to treatment, patients with higher baseline cotinine plasma levels had lower smoking cessation success rates.34
A few caveats
There are several limitations to quantitative measures of cotinine (Box 221,23). These include (but are not limited to) potential errors related to sample collection, storage, shipping, and analysis.23 Compared with other methods, point-of-care cotinine measurement in saliva is noninvasive, simple, and requires less training to properly use.23
Box 2
Challenges in the collection of samples, storage, shipping, and instrumentation may limit cotinine consistency as a dependable biomarker in the clinical setting.23 Overall, quantitative measurements of cotinine have relative constructive utility in separating smokers from nonsmokers, because daily smokers typically have serum concentrations of 100 ng/mL or higher, in contrast to light/non-daily smokers, who have cotinine concentrations <10 ng/mL. Even heavy exposure to secondhand smoke typically yields plasma concentrations up to approximately 25 ng/mL. However, cotinine is a general metabolite found with the use of all nicotine products, which makes it extremely difficult to differentiate tobacco use from the use of nicotine replacement products, which are frequently used to treat tobacco use disorders.
One potential solution is to measure nicotine-derived nitrosamine ketone (NNK) and its metabolite 4-(methylnitrosamino)- 1-(3-pyridyl)-1-butanol (NNAL). Both NNK and NNAL are tobacco-specific lung carcinogens. NNAL can be measured in the urine. Although total NNAL represents only 15% of NNK dose intake, it has been quantified, with urine concentrations of ≥1,000 fmol/mL for daily smokers. NNAL also has an extremely high specificity to tobacco smoke, and thus allows differentiation of tobacco use from nicotine replacement treatment. Unfortunately, measurement for this biomarker requires specific chemical expertise and expensive equipment.
Another potential barrier to using cotinine in the clinical setting is the variable cut-off levels used in the United States, based on differences in race/ethnicity. This may be secondary to differences in smoking behaviors and/or differences in cotinine metabolism.21
Continue to: Confirmation of smoking cessation...
Confirmation of smoking cessation can be monitored reliably within the clinical setting using cotinine monitoring. However, this is not a routine test, and there are no guidelines or consensus on how or when it should be used. The clinical feasibility of cotinine monitoring for psychiatric patients will depend on the cost of testing, methods used, amount of reimbursement for performing the tests, and how clinicians value such testing.35
Bottom Line
Cotinine is a biomarker that can be used to detect tobacco use. Cotinine measurement can be used to monitor tobacco use and smoking cessation in psychiatric patients. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illnesses. However, cotinine measurement is not a routine test, and there are no guidelines on how or when this test should be used.
Related Resources
- Peckham E, Brabyn S, Cook L, et al. Smoking cessation in severe mental ill health: what works? An updated systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):252.
- Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ. 2015;351:h4065. doi: 10.1136/bmj.h4065
Drug Brand Names
Asenapine • Saphris
Buprenorphine • Sublocade
Clozapine • Clozaril
Duloxetine • Cymbalta
Haloperidol • Haldol
Mirtazapine • Remeron
Olanzapine • Zyprexa
Ziprasidone • Geodon
Zolpidem • Ambien
1. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185.
2. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12.
3. Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285.
4. Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14(2):106-123.
5. Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. J Subst Abuse Treat. 2009;36(2):205-219.
6. Hall SM, Tsoh JY, Prochaska JJ, et al. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Public Health. 2006;96(10):1808-1814.
7. McHugh RK, Votaw VR, Fulciniti F, et al. Perceived barriers to smoking cessation among adults with substance use disorders. J Subst Abuse Treat. 2017;74:48-53.
8. Strong DR, Uebelacker L, Fokas K, et al. Utilization of evidence-based smoking cessation treatments by psychiatric inpatient smokers with depression. J Addict Med. 2014;8(2):77-83.
9. Gayman C, Anderson K, Pietras C. Saliva cotinine as a measure of smoking abstinence in contingency management – a feasibility study. The Psychological Record. 2017;67(2):261-272.
10. Schepis TS, Duhig AM, Liss T, et al. Contingency management for smoking cessation: enhancing feasibility through use of immunoassay test strips measuring cotinine. Nicotine Tob Res. 2008;10(9):1495-1501.
11. Stragierowicz J, Mikolajewska K, Zawadzka-Stolarz M, et al. Estimation of cutoff values of cotinine in urine and saliva for pregnant women in Poland. Biomed Res Int. 2013;2013:386784. doi.org/10.1155/2013/386784
12. Shipton D, Tappin DM, Vadiveloo T, et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. doi.org/10.1136/bmj.b4347
13. Aubin HJ, Karila L, Reynaud M. Pharmacotherapy for smoking cessation: present and future. Curr Pharm Des. 2011;17(14):1343-1350.
14. McGuffey JE, Wei B, Bernert JT, et al. Validation of a LC-MS/MS method for quantifying urinary nicotine, six nicotine metabolites and the minor tobacco alkaloids--anatabine and anabasine--in smokers’ urine. PLoS One. 2014;9(7):e101816. doi: 10.1371/journal.pone.0101816
15. Duque A, Martinez PJ, Giraldo A, et al. Accuracy of cotinine serum test to detect the smoking habit and its association with periodontal disease in a multicenter study. Med Oral Patol Oral Cir Bucal. 2017;22(4):e425-e431. doi: 10.4317/medoral.21292
16. Avila-Tang E, Elf JL, Cummings KM, et al. Assessing secondhand smoke exposure with reported measures. Tob Control. 2013;22(3):156-163.
17. Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 Update. Nicotine Tob Res. 2020;22(7):1086-1097.
18. Nakajima M TY. Interindividual variability in nicotine metabolism: c-oxidation and glucuronidation. Drug Metab Pharmaokinet. 2005;20(4):227-235.
19. Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236-248.
20. Schnoll R, Johnson TA, Lerman C. Genetics and smoking behavior. Curr Psychiatry Rep. 2007;9(5):349-357.
21. Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health. 2016;13(12):1236.
22. Etter JF, Bullen C. Saliva cotinine levels in users of electronic cigarettes. Eur Respir J. 2011;38(5):1219-1220.
23. Raja M, Garg A, Yadav P, et al. Diagnostic methods for detection of cotinine level in tobacco users: a review. J Clin Diagn Res. 2016;10(3):ZE04-06. doi: 10.7860/JCDR/2016/17360.7423
24. Balhara YP, Jain R. A receiver operated curve-based evaluation of change in sensitivity and specificity of cotinine urinalysis for detecting active tobacco use. J Cancer Res Ther. 2013;9(1):84-89.
25. Fankhauser M. Drug interactions with tobacco smoke: implications for patient care. Current Psychiatry. 2013;12(1):12-16.
26. Scheuermann TS, Richter KP, Rigotti NA, et al. Accuracy of self-reported smoking abstinence in clinical trials of hospital-initiated smoking interventions. Addiction. 2017;112(12):2227-2236.
27. Holtyn AF, Knealing TW, Jarvis BP, et al. Monitoring cocaine use and abstinence among cocaine users for contingency management interventions. Psychol Rec. 2017;67(2):253-259.
28. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51.
29. Sullivan M, Covey, LS. Current perspectives on smoking cessation among substance abusers. Curr Psychiatry Rep. 2002;4(5):388-396.
30. Forray A, Merry B, Lin H, et al. Perinatal substance use: a prospective evaluation of abstinence and relapse. Drug Alcohol Depend. 2015;150:147-155.
31. Parker DR, Lasater TM, Windsor R, et al. The accuracy of self-reported smoking status assessed by cotinine test strips. Nicotine Tob Res. 2002;4(3):305-309.
32. Asha V, Dhanya M. Immunochromatographic assessment of salivary cotinine and its correlation with nicotine dependence in tobacco chewers. J Cancer Prev. 2015;20(2):159-163.
33. Hall S, Herning RI, Jones RT, et al. Blood cotinine levels as indicators of smoking treatment outcome. Clin Pharmacol Ther. 1984;35(6):810-814.
34. Paoletti P, Fornai E, Maggiorelli F, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double-blind study with nicotine patch. Eur Respir J. 1996;9(4):643-651.
35. Montalto NJ, Wells WO. Validation of self-reported smoking status using saliva cotinine: a rapid semiquantitative dipstick method. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1858-1862.
1. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185.
2. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12.
3. Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285.
4. Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14(2):106-123.
5. Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. J Subst Abuse Treat. 2009;36(2):205-219.
6. Hall SM, Tsoh JY, Prochaska JJ, et al. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Public Health. 2006;96(10):1808-1814.
7. McHugh RK, Votaw VR, Fulciniti F, et al. Perceived barriers to smoking cessation among adults with substance use disorders. J Subst Abuse Treat. 2017;74:48-53.
8. Strong DR, Uebelacker L, Fokas K, et al. Utilization of evidence-based smoking cessation treatments by psychiatric inpatient smokers with depression. J Addict Med. 2014;8(2):77-83.
9. Gayman C, Anderson K, Pietras C. Saliva cotinine as a measure of smoking abstinence in contingency management – a feasibility study. The Psychological Record. 2017;67(2):261-272.
10. Schepis TS, Duhig AM, Liss T, et al. Contingency management for smoking cessation: enhancing feasibility through use of immunoassay test strips measuring cotinine. Nicotine Tob Res. 2008;10(9):1495-1501.
11. Stragierowicz J, Mikolajewska K, Zawadzka-Stolarz M, et al. Estimation of cutoff values of cotinine in urine and saliva for pregnant women in Poland. Biomed Res Int. 2013;2013:386784. doi.org/10.1155/2013/386784
12. Shipton D, Tappin DM, Vadiveloo T, et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. doi.org/10.1136/bmj.b4347
13. Aubin HJ, Karila L, Reynaud M. Pharmacotherapy for smoking cessation: present and future. Curr Pharm Des. 2011;17(14):1343-1350.
14. McGuffey JE, Wei B, Bernert JT, et al. Validation of a LC-MS/MS method for quantifying urinary nicotine, six nicotine metabolites and the minor tobacco alkaloids--anatabine and anabasine--in smokers’ urine. PLoS One. 2014;9(7):e101816. doi: 10.1371/journal.pone.0101816
15. Duque A, Martinez PJ, Giraldo A, et al. Accuracy of cotinine serum test to detect the smoking habit and its association with periodontal disease in a multicenter study. Med Oral Patol Oral Cir Bucal. 2017;22(4):e425-e431. doi: 10.4317/medoral.21292
16. Avila-Tang E, Elf JL, Cummings KM, et al. Assessing secondhand smoke exposure with reported measures. Tob Control. 2013;22(3):156-163.
17. Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 Update. Nicotine Tob Res. 2020;22(7):1086-1097.
18. Nakajima M TY. Interindividual variability in nicotine metabolism: c-oxidation and glucuronidation. Drug Metab Pharmaokinet. 2005;20(4):227-235.
19. Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236-248.
20. Schnoll R, Johnson TA, Lerman C. Genetics and smoking behavior. Curr Psychiatry Rep. 2007;9(5):349-357.
21. Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health. 2016;13(12):1236.
22. Etter JF, Bullen C. Saliva cotinine levels in users of electronic cigarettes. Eur Respir J. 2011;38(5):1219-1220.
23. Raja M, Garg A, Yadav P, et al. Diagnostic methods for detection of cotinine level in tobacco users: a review. J Clin Diagn Res. 2016;10(3):ZE04-06. doi: 10.7860/JCDR/2016/17360.7423
24. Balhara YP, Jain R. A receiver operated curve-based evaluation of change in sensitivity and specificity of cotinine urinalysis for detecting active tobacco use. J Cancer Res Ther. 2013;9(1):84-89.
25. Fankhauser M. Drug interactions with tobacco smoke: implications for patient care. Current Psychiatry. 2013;12(1):12-16.
26. Scheuermann TS, Richter KP, Rigotti NA, et al. Accuracy of self-reported smoking abstinence in clinical trials of hospital-initiated smoking interventions. Addiction. 2017;112(12):2227-2236.
27. Holtyn AF, Knealing TW, Jarvis BP, et al. Monitoring cocaine use and abstinence among cocaine users for contingency management interventions. Psychol Rec. 2017;67(2):253-259.
28. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51.
29. Sullivan M, Covey, LS. Current perspectives on smoking cessation among substance abusers. Curr Psychiatry Rep. 2002;4(5):388-396.
30. Forray A, Merry B, Lin H, et al. Perinatal substance use: a prospective evaluation of abstinence and relapse. Drug Alcohol Depend. 2015;150:147-155.
31. Parker DR, Lasater TM, Windsor R, et al. The accuracy of self-reported smoking status assessed by cotinine test strips. Nicotine Tob Res. 2002;4(3):305-309.
32. Asha V, Dhanya M. Immunochromatographic assessment of salivary cotinine and its correlation with nicotine dependence in tobacco chewers. J Cancer Prev. 2015;20(2):159-163.
33. Hall S, Herning RI, Jones RT, et al. Blood cotinine levels as indicators of smoking treatment outcome. Clin Pharmacol Ther. 1984;35(6):810-814.
34. Paoletti P, Fornai E, Maggiorelli F, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double-blind study with nicotine patch. Eur Respir J. 1996;9(4):643-651.
35. Montalto NJ, Wells WO. Validation of self-reported smoking status using saliva cotinine: a rapid semiquantitative dipstick method. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1858-1862.
Cannabinoid-based medications for pain
Against the backdrop of an increasing opioid use epidemic and a marked acceleration of prescription opioid–related deaths,1,2 there has been an impetus to explore the usefulness of alternative and co-analgesic agents to assist patients with chronic pain. Preclinical studies employing animal-based models of human pain syndromes have demonstrated that cannabis and chemicals derived from cannabis extracts may mitigate several pain conditions.3
Because there are significant comorbidities between psychiatric disorders and chronic pain, psychiatrists are likely to care for patients with chronic pain. As the availability of and interest in cannabinoid-based medications (CBM) increases, psychiatrists will need to be apprised of the utility, adverse effects, and potential drug interactions of these agents.
The endocannabinoid system and cannabis receptors
The endogenous cannabinoid (endocannabinoid) system is abundantly present within the peripheral and central nervous systems. The first identified, and best studied, endocannabinoids are N-arachidonoyl-ethanolamine (AEA; anandamide) and 2-arachidonoylglycerol (2-AG).4 Unlike typical neurotransmitters, AEA and 2-AG are not stored within vesicles within presynaptic neuron axons. Instead, they are lipophilic molecules produced on demand, synthesized from phospholipids (ie, arachidonic acid derivatives) at the membranes of post-synaptic neurons, and released into the synapse directly.5
Acting as retrograde messengers, the endocannabinoids traverse the synapse, binding to receptors located on the axons of the presynaptic neuron. Two receptors—CB1 and CB2—have been most extensively studied and characterized.6,7 These receptors couple to Gi/o-proteins to inhibit adenylate cyclase, decreasing Ca2+ conductance and increasing K+ conductance.8 Once activated, cannabinoid receptors modulate neurotransmitter release from presynaptic axon terminals. Evidence points to a similar retrograde signaling between neurons and glial cells. Shortly after receptor activation, the endocannabinoids are deactivated by the actions of a transporter mechanism and enzyme degradation.9,10
The endocannabinoid system and pain transmission
Cannabinoid receptors are present in pain transmission circuits spanning from the peripheral sensory nerve endings (from which pain signals originate) to the spinal cord and supraspinal regions within the brain.11-14 CB1 receptors are abundantly present within the CNS, including regions involved in pain transmission. Binding to CB1 receptors, endocannabinoids modulate neurotransmission that impacts pain transmission centrally. Endocannabinoids can also indirectly modulate opiate and N-methyl-
By contrast, CB2 receptors are predominantly localized to peripheral tissues and immune cells, although there has been some discovery of their presence within the CNS (eg, on microglia). Endocannabinoid activation of CB2 receptors is thought to modulate the activity of peripheral afferent pain fibers and immune-mediated neuroinflammatory processes—such as inhibition of prostaglandin synthesis and mast cell degranulation—that can precipitate and maintain chronic pain states.16-18
Evidence garnered from preclinical (animal) studies points to the role of the endocannabinoid system in modulating normal pain transmission (see Manzanares et al3 for details). These studies offer a putative basis for understanding how exogenous cannabinoid congeners might serve to ameliorate pain transmission in pathophysiologic states, including chronic pain.
Continue to: Cannabinoid-based medications
Cannabinoid-based medications
Marijuana contains multiple components (cannabinoids). The most extensively studied are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Because it predominantly binds CB1 receptors centrally, THC is the major psychoactive component of cannabis; it promotes sleep and appetite, influences anxiety, and produces the “high” associated with cannabis use. By contrast, CBD weakly binds CB1 and thus exerts minimal or no psychoactive effects.19
Cannabinoid absorption, metabolism, bioavailability, and clinical effects vary depending on the formulation and method of administration (Table 1).20-22 THC and CBD content and potency in inhaled cannabis can vary significantly depending on the strains of the cannabis plant and manner of cultivation.23 To standardize approaches for administering cannabinoids in clinical trials and for clinical use, researchers have developed pharmaceutical analogs that contain extracted chemicals or synthetic chemicals similar to THC and/or CBD.
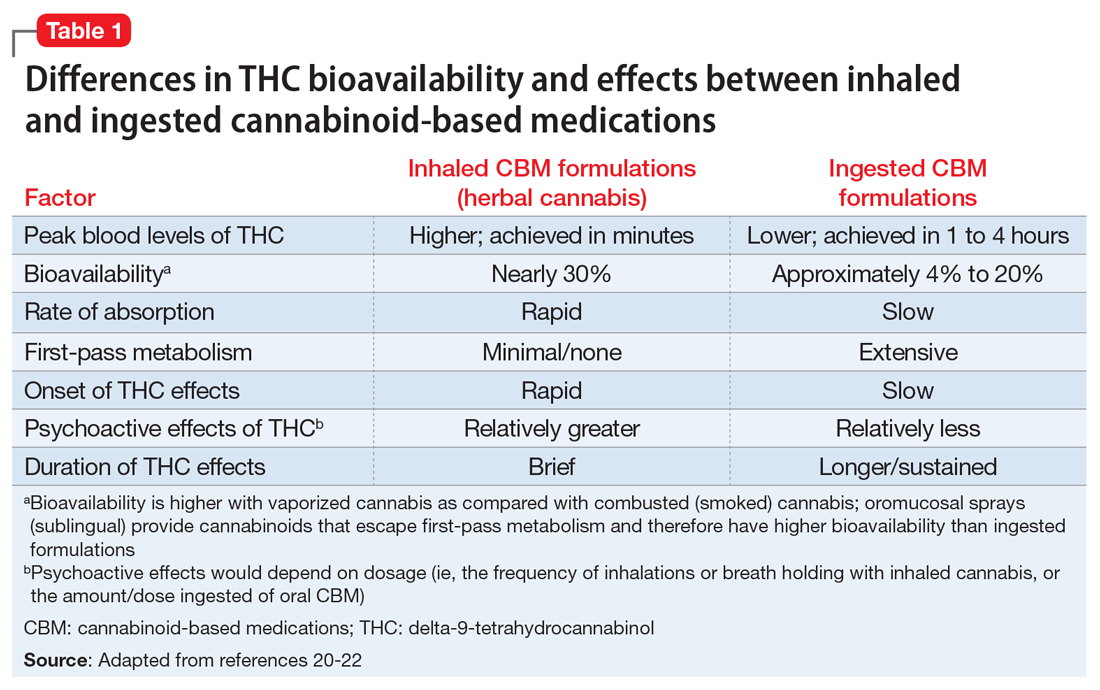
In this article, CBM refers to smoked/vaporized herbal cannabis as well as pharmaceutical cannabis analogs. Table 2 summarizes the characteristics of CBM commonly used in studies investigating their use for managing pain conditions.
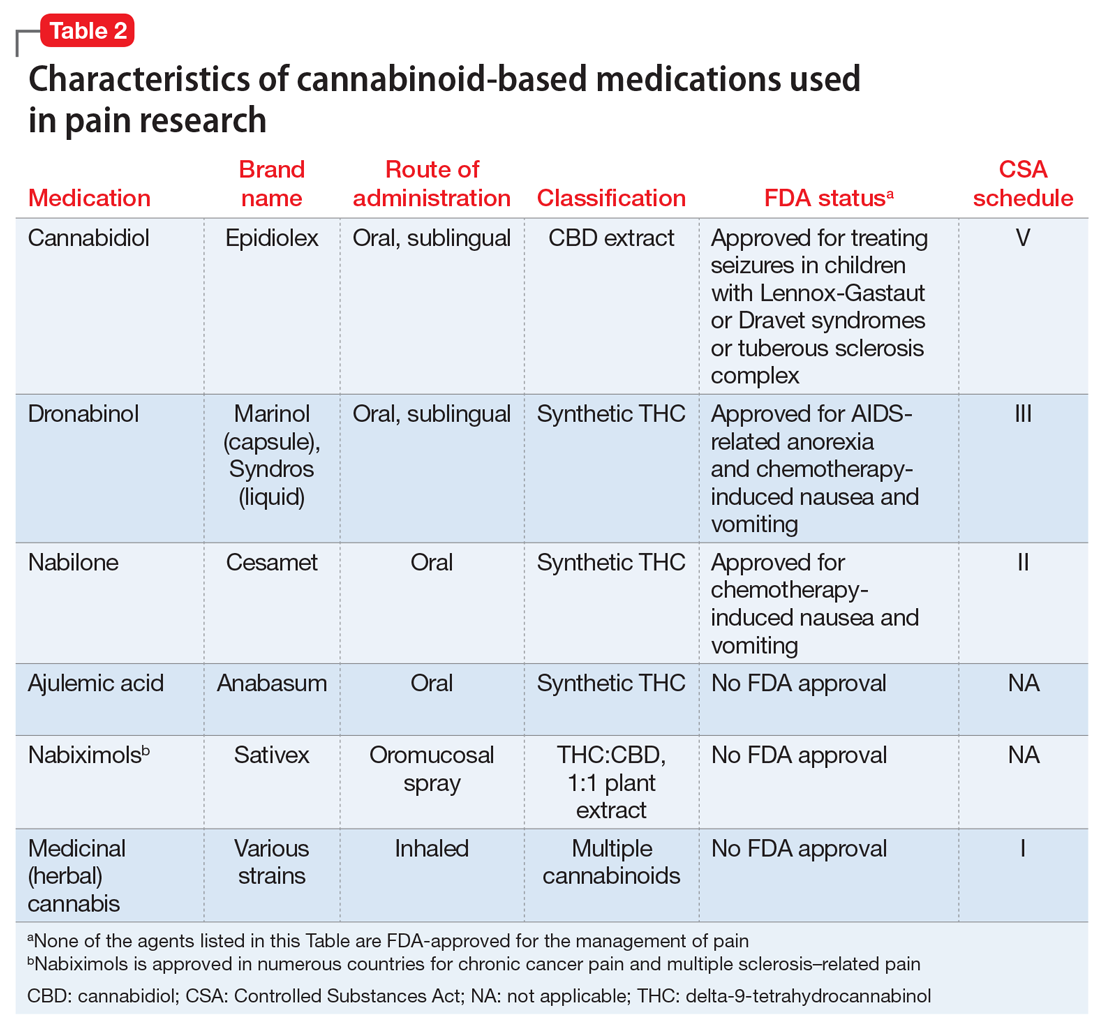
CBM for chronic pain
The literature base examining the role of CBM for managing chronic nonmalignant and malignant pain of varying etiologies is rapidly expanding. Randomized controlled trials (RCTs) have focused on inhaled/smoked products and related cannabinoid medications, some of which are FDA-approved (Table 2).
A multitude of other cannabinoid-based products are currently commercially available to consumers, including tincture and oil-based products; over-the-counter CBD products; and several other formulations of CBM (eg, edible and suppository products). Because such products are not standardized or quality-controlled,24 RCTs have not assessed their efficacy for mitigating pain. Consequently, the findings summarized in this article do not address the utility of these agents.
Continue to: CBM for non-cancer pain
CBM for non-cancer pain
Neuropathic pain. Randomized controlled trials have assessed the pain-mitigating effects of various CBM, including inhaled cannabis, synthetic THC, plant-extracted CBD, and a THC/CBD spray. Studies have shown that inhaled/vaporized cannabis can produce short-term pain reduction in patients with chronic neuropathic pain of diverse etiologies, including diabetes mellitus-, HIV-, trauma-, and medication-induced neuropathies.22,25,26 Similar beneficial effects have been observed with the use of cannabis analogues (eg, nabiximols).25,26-29
Meta-analyses and systematic reviews have determined that most of these RCTs were of low-to-moderate quality.26,30 Meta-analyses have revealed divergent and conflicting results because of differences in the inclusion and exclusion criteria used to select RCTs for analysis and differences in the standards with which the quality of evidence were determined.25,30
Overall, the benefit of CBM for mitigating neuropathic pain is promising, but the effectiveness may not be robust.30,31 Several noteworthy caveats limit the interpretation of the results of these RCTs:
- due to the small sample sizes and brief durations of study, questions remain regarding the extent to which effects are generalizable, whether the benefits are sustained, and whether adverse effects emerge over time with continued use
- most RCTs evaluated inhaled (herbal) cannabis and nabiximols; there is little data on the effectiveness of other CBM formulations25,26,30
- the pain-mitigating effects of CBM were usually compared with those of placebo; the comparative efficacy against agents commonly used to treat neuropathic pain remains largely unexamined
- these RCTs typically compared mean pain severity score differences between cannabis-treated and placebo groups using standard subjective rating scales of pain intensity, such as the Numerical Rating Scale or Visual Analogue Scale. Customarily, the pain literature has used a 30% or 50% reduction in pain severity from baseline as an indicator of significant clinical improvement.32,33 The RCTs of CBM for neuropathic pain rarely used this standard, which makes it unclear whether CBM results in clinically significant pain reductions30
- indirect measures of effectiveness (ie, whether using CBM reduces the need for opioids or other analgesics to manage pain) were seldom reported in these RCTs.
Due to these limitations, clinical guidelines and systematic reviews consider CBM as a third- or fourth-line therapy for patients experiencing chronic neuropathic pain for whom conventional agents such as anticonvulsants and antidepressants have failed.34,35
Spasticity in multiple sclerosis (MS). Several RCTs have assessed the use of CBM for MS-related spasticity, although few were deemed to be high quality. Nabiximols and synthetic THC were effective in managing spasticity and reducing pain severity associated with muscle spasms.36 Generally, investigations revealed that CBM were associated with improvements in subjective measures of spasticity, but these were not born out in clinical, objective measures.26,37 The efficacy of smoked cannabis was uncertain.37 The existing literature on CBM for MS-related spasticity does not address dosing, duration of effects, tolerability, or comparative effectiveness against conventional anti-spasm medications.36,37
Continue to: Other chronic pain conditions
Other chronic pain conditions. CBM have also been studied for their usefulness in several other noncancer chronic conditions, including Crohn’s disease, inflammatory bowel disease, fibromyalgia, and other rheumatologic pain conditions.22,31,38-40 However, a solid foundation of empirical work to inform their utility for managing pain in these conditions is lacking.
CBM for cancer pain
Anecdotal evidence suggests that inhaled cannabis has promising pain-mitigating effects in patients with advanced cancer.41-43 There is a dearth of high-quality RCTs assessing the utility of CBM in patients with cancer pain.43-45 The types of CBM used and dosing strategies varied across RCTs, which makes it difficult to infer how best to treat patients with cancer pain. The agents studied included nabiximols, THC spray, and synthetic THC capsules.43-45 Although some studies have demonstrated that synthetic THC and nabiximols have potential for reducing subjective pain ratings compared with placebo,46,47 these results were inconsistent.46,48 Oromucosal nabiximols did not appear to confer any additional analgesic benefit in patients who were already prescribed opioids.31,45
The benefit of CBM for mitigating cancer pain is promising, but it remains difficult to know how to position the use of CBM in managing cancer pain. Limitations in the cancer literature include:
- the RCTs addressing CBM use for cancer pain were often brief, which raises questions about the long-term effectiveness and adverse effects of these agents
- tolerability and dosing limits encountered due to adverse effects were seldom reported43,45
- the types of cancer pain that patients had were often quite diverse. The small sample sizes and the heterogeneity of conditions included in these RCTs limit the ability to determine whether pain-mitigating effects might vary according to type of cancer-related pain.31,45
Despite these limitations, some clinical guidelines and systematic reviews have suggested that CBM have some role in addressing refractory malignant pain conditions.49
Psychiatric considerations related to CBM
As of November 2020, 36 states had legalized the use of cannabis for medical purposes, typically for painful conditions, despite the fact that empirical evidence to support their efficacy is mixed.50 In light of recent changes in both the legal and popular attitudes regarding cannabis, the implications of legalizing CBM remains to be seen. For example, some research suggests that adults with pain are vulnerable to frequent nonmedical cannabis use and/or cannabis use disorder.51 Although well-intended, the legalization of CBM use might represent society’s next misstep in the quest to address the suffering of patients with chronic pain. Some evidence shows that cannabis use and cannabis use disorders increase in states that have legalized medical marijuana.52,53 Psychiatrists will be on the front lines of addressing any potential consequences arising from the use of CBM for treating pain.
Continue to: Psychiatric disorders and CBM
Psychiatric disorders and CBM. The psychological impact of CBM use among patients enduring chronic pain can include sedation, cognitive/attention disturbance, and fatigue. These adverse effects can limit the utility of such agents.22,29,45
Contraindications for CBM use, and conditions for which CBM ought to be used with caution, are listed in Table 354,55.The safety of CBM, particularly in patients with chronic pain and psychiatric disorders, has not been examined. Patients with psychiatric disorders may be poor candidates for medical cannabis. Epidemiologic data suggest that recreational cannabis use is positively associated both cross-sectionally and prospectively with psychotic spectrum disorders, depressive symptoms, and anxiety symptoms, including panic disorder.56 Psychotic reactions have also been associated with CBM (dronabinol and nabilone).57 Cannabis use also has been associated with an earlier onset of, and lower remission rates of, symptoms associated with bipolar disorder.58,59 Consequently, patients who have been diagnosed with or are at risk for developing any of the aforementioned conditions may not be suitable candidates for CBM. If CBM are used, patients should be closely monitored for the emergence/exacerbation of psychiatric symptoms. The frequency and extent of follow-up is not clear, however. Because of its reduced propensity to produce psychoactive effects, CBD may be safer than THC for managing pain in individuals who have or are vulnerable to developing psychiatric disorders.
There is a lack of evidence to support the use of CBM for treating primary depressive disorders, general anxiety disorder, posttraumatic stress disorder, or psychosis.60,61 Very low-quality evidence suggests that CBM could lead to a small improvement in anxiety among individuals with noncancer pain and MS.60 However, interpreting causality is complicated. It is plausible that, for some patients, subjective improvement in pain severity may be related to reduced anxiety.62 Conversely, it is equally plausible that reductions in emotional distress may reduce the propensity to attend to, and thus magnify, pain severity. In the latter case, the indirect impact of reducing pain by modifying emotional distress can be impacted by the type and dose of CBM used. For example, low concentrations of THC produce anxiolytic effects, but high concentrations may be anxiety-provoking.63,64
Several potential pharmacokinetic drug interactions may arise between herbal cannabis or CBM and other medications (Table 465,66). THC and CBD are both metabolized by cytochrome P450 (CYP) 2C19 and 3A4.65,66 In addition, THC is also metabolized by CYP2C9. Medications that inhibit or induce these enzymes can increase or decrease the bioavailability of THC and CBD.67
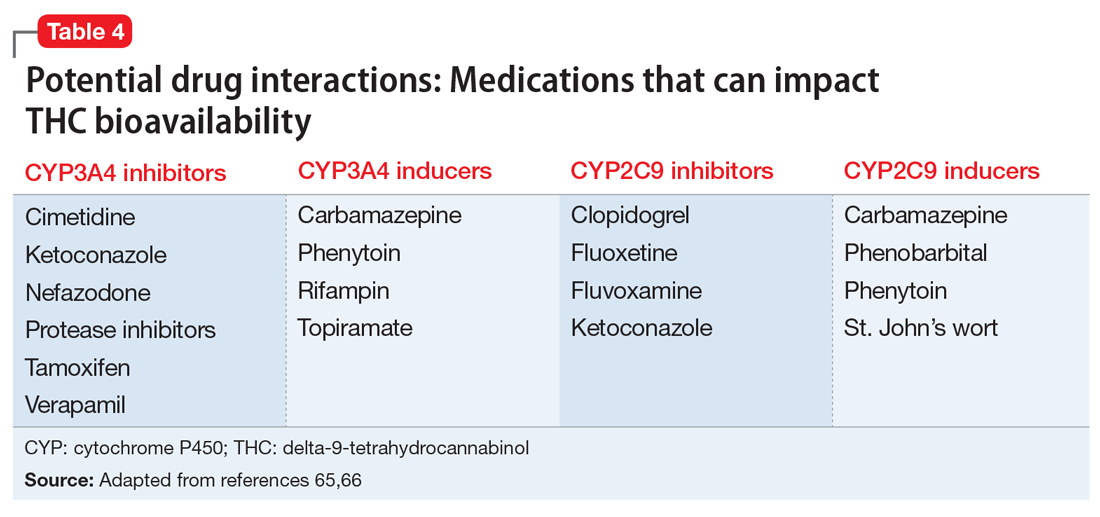
Simultaneously, cannabinoids can impact the bioavailability of co-prescribed medications (Table 566,68). Although such CYP enzyme interactions remain a theoretical possibility, it is uncertain whether significant perturbations in plasma concentrations (and clinical effects) have been encountered with prescription medications when co-administered with CBM.69 Nonetheless, patients receiving CBM should be closely monitored for their response to prescribed medications.70
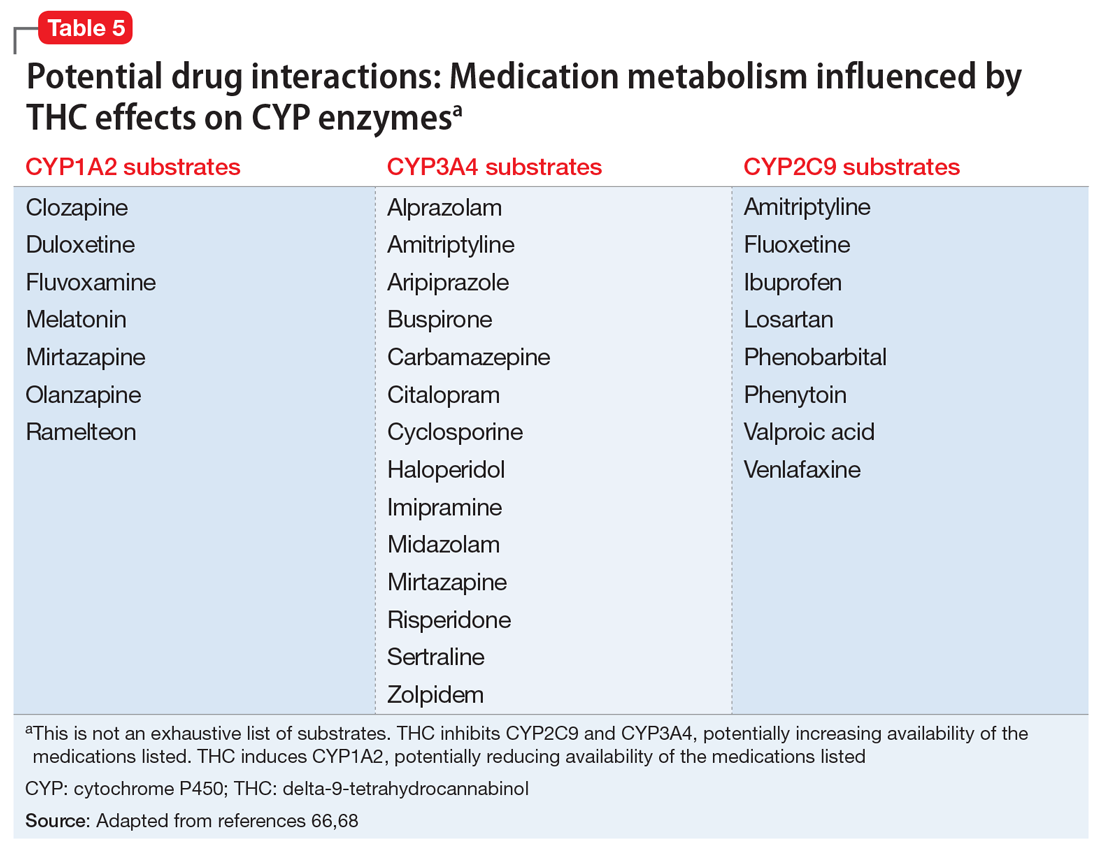
Continue to: Potential CYP enzyme interactions...
Potential CYP enzyme interactions aside, clinicians need to consider the additive effects that may occur when CBM are combined with sympathomimetic agents (eg, tachycardia, hypertension); CNS depressants such as alcohol, benzodiazepines, and opioids (eg, drowsiness, ataxia); or anticholinergics (eg, tachycardia, confusion).71 Inhaled herbal cannabis contains mutagens and can result in lung damage, exacerbations of chronic bronchitis, and certain types of cancer.54,72 Co-prescribing benzodiazepines may be contraindicated in light of their effects on respiratory rate and effort.
The THC contained in CBM produces hormonal effects (ie, significantly increases plasma levels of ghrelin and leptin and decreases peptide YY levels)73 that affect appetite and can produce weight gain. This may be problematic for patients receiving psychoactive medications associated with increased risk of weight gain and dyslipidemia. Because of the association between cannabis use and motor vehicle accidents, patients whose jobs require them to drive or operate industrial equipment may not be ideal candidates for CBM, especially if such patients also consume alcohol or are prescribed benzodiazepines and/or sedative hypnotics.74 Lastly, due to their lipophilicity, cannabinoids cross the placental barrier and can be found in breast milk75 and therefore can affect pregnancy outcomes and neurodevelopment.
Bottom Line
The popularity of cannabinoid-based medications (CBM) for the treatment of chronic pain conditions is growing, but the interest in their use may be outpacing the evidence supporting their analgesic benefits. High-quality, well-controlled randomized controlled trials are needed to decipher whether, and to what extent, these agents can be positioned in chronic pain management. Because psychiatrists are likely to encounter patients considering, or receiving, CBM, they must be aware of the potential benefits, risks, and adverse effects of such treatments.
Related Resources
- Joshi KG. Cannabis-derived compounds: what you need to know. Current Psychiatry. 2020;19(10):64-65. doi:10.12788/ cp.0050
- Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018; 17(1):34-41.
Drug Brand Names
Ajulemic acid • Anabasum
Alprazolam • Xanax
Amitriptyline • Elavil
Aripiprazole • Abilify, Abilify Maintena
Buspirone • BuSpar
Cannabidiol • Epidiolex
Carbamazepine • Tegretol, Equetro
Cimetidine • Tagamet HB
Citalopram • Celexa
Clopidogrel • Plavix
Clozapine • Clozaril
Cyclosporine • Neoral, Sandimmune
Dronabinol • Marinol, Syndros
Duloxetine • Cymbalta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Haloperidol • Haldol
Imipramine • Tofranil
Ketoconazole • Nizoral AD
Losartan • Cozaar
Midazolam • Versed
Mirtazapine • Remeron
Nabilone • Cesamet
Nabiximols • Sativex
Nefazodone • Serzone
Olanzapine • Zyprexa
Phenobarbital • Solfoton
Phenytoin • Dilantin
Ramelteon • Rozerem
Rifampin • Rifadin
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Topiramate • Topamax
Valproic acid • Depakote, Depakene
Venlafaxine • Effexor
Verapamil • Verelan
Zolpidem • Ambien
1. Okie S. A floor of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. doi:10.1056/NEJMp1011512
2. Powell D, Pacula RL, Taylor E. How increasing medical access to opioids contributes to the opioid epidemic: evidence from Medicare Part D. J Health Econ. 2020;71:102286. doi: 10.1016/j.jhealeco.2019.102286
3. Manzanares J, Julian MD, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006;4(3):239-257. doi: 10.2174/157015906778019527
4. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833. doi: 10.3390/ijms19030833
5. Huang WJ, Chen WW, Zhang X. Endocannabinoid system: role in depression, reward and pain control (Review). Mol Med Rep. 2016;14(4):2899-2903. doi:10.3892/mmr.2016.5585
6. Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83-90. doi:10.1016/0006-2952(95)00109-d
7. Walker JM, Krey JF, Chu CJ, et al. Endocannabinoids and related fatty acid derivatives in pain modulation. Chem Phys Lipids. 2002;121(1-2):159-172. doi: 10.1016/s0009-3084(02)00152-4
8. Howlett AC. Efficacy in CB1 receptor-mediated signal transduction. Br J Pharmacol. 2004;142(8):1209-1218. doi: 10.1038/sj.bjp.0705881
9. Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation, biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7-14.
10. Piomelli D, Beltramo M, Giuffrida A, et al. Endogenous cannabinoid signaling. Neurobiol Dis. 1998;5(6 Pt B):462-473. doi: 10.1006/nbdi.1998.0221
11. Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17(1):175-191. doi: 10.1093/cercor/bhj136
12. Jennings EA, Vaughan CW, Christie MJ. Cannabinoid actions on rat superficial medullary dorsal horn neurons in vitro. J Physiol. 2001;534(Pt 3):805-812. doi: 10.1111/j.1469-7793.2001.00805.x
13. Vaughan CW, Connor M, Bagley EE, et al. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol. 2000;57(2):288-295.
14. Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol. 1999;127(4):935-940. doi: 10.1038/sj.bjp.0702636
15. Raichlen DA, Foster AD, Gerdeman GI, et al. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner’s high.” J Exp Biol. 2012;215(Pt 8):1331-1336. doi: 10.1242/jeb.063677
16. Beltrano M. Cannabinoid type 2 receptor as a target for chronic pain. Mini Rev Chem. 2009;234:253-254.
17. Ibrahim MM, Deng H, Zvonok A, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100(18):10529-10533. doi: 10.1073/pnas.1834309100
18. Valenzano KJ, Tafessem L, Lee G, et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658-672.
19. Pertwee RG, Howlett AC, Abood ME, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588-631. doi: 10.1124/pr.110.003004
20. Carter GT, Weydt P, Kyashna-Tocha M, et al. Medicinal cannabis: rational guidelines for dosing. Drugs. 2004;7(5):464-470.
21. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804.
22. Johal H, Devji T, Chang Y, et al. cannabinoids in chronic non-cancer pain: a systematic review and meta-analysis. Clin Med Insights Arthritis Musculoskelet Disord. 2020;13:1179544120906461. doi: 10.1177/1179544120906461
23. Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am J Bot. 2004;91(6):966-975. doi: 10.3732/ajb.91.6.966
24. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids--an international cross-sectional survey on administration forms. J Psychoactive Drugs. 2013;45(3):199-210. doi: 10.1080/02791072.2013.805976
25. Andreae MH, Carter GM, Shaparin N, et al. inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain. 2015;16(12):1221-1232. doi: 10.1016/j.jpain.2015.07.009
26. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473. doi: 10.1001/jama.2015.6358
27. Boychuk DG, Goddard G, Mauro G, et al. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29(1):7-14. doi: 10.11607/ofph.1274
28. Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72(5):735-744. doi: 10.1111/j.1365-2125.2011.03970.x
29. Stockings E, Campbell G, Hall WD, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159(10):1932-1954. doi: 10.1097/j.pain.0000000000001293
30. Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3(3):CD012182. doi: 10.1002/14651858.CD012182.pub2
31. Häuser W, Fitzcharles MA, Radbruch L, et al. Cannabinoids in pain management and palliative medicine. Dtsch Arztebl Int. 2017;114(38):627-634. doi: 10.3238/arztebl.2017.0627
32. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. doi: 10.1016/j.jpain.2007.09.005
33. Farrar JT, Troxel AB, Stott C, et al. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther. 2008;30(5):974-985. doi: 10.1016/j.clinthera.2008.05.011
34. Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-335. doi: 10.1155/2014/754693
35. Petzke F, Enax-Krumova EK, Häuser W. Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: a systematic review of randomized controlled studies. Schmerz. 2016;30(1):62-88. doi: 10.1007/s00482-015-0089-y
36. Rice J, Cameron M. Cannabinoids for treatment of MS symptoms: state of the evidence. Curr Neurol Neurosci Rep. 2018;18(8):50. doi: 10.1007/s11910-018-0859-x
37. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556-1563. doi: 10.1212/WNL.0000000000000363
38. Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of Crohn’s disease and ulcerative colitis: evidence from Cochrane Reviews. Inflamm Bowel Dis. 2020;26(4):502-509. doi: 10.1093/ibd/izz233
39. Katz-Talmor D, Katz I, Porat-Katz BS, et al. Cannabinoids for the treatment of rheumatic diseases - where do we stand? Nat Rev Rheumatol. 2018;14(8):488-498. doi: 10.1038/s41584-018-0025-5
40. Walitt B, Klose P, Fitzcharles MA, et al. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016;7(7):CD011694. doi: 10.1002/14651858.CD011694.pub2
41. Bar-Lev Schleider L, Mechoulam R, Lederman V, et al. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur J Intern Med. 2018;49:37‐43. doi: 10.1016/j.ejim.2018.01.023
42. Bennett M, Paice JA, Wallace M. Pain and opioids in cancer care: benefits, risks, and alternatives. Am Soc Clin Oncol Educ Book. 2017;37:705‐713. doi:10.1200/EDBK_180469
43. Blake A, Wan BA, Malek L, et al. A selective review of medical cannabis in cancer pain management. Ann Palliat Med. 2017;6(Suppl 2):5215-5222. doi: 10.21037/apm.2017.08.05
44. Aviram J, Samuelly-Lechtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20(6):E755-E796.
45. Häuser W, Welsch P, Klose P, et al. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain: a systematic review with meta-analysis of randomised controlled trials. Schmerz. 2019;33(5):424-436. doi: 10.1007/s00482-019-0373-3
46. Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010; 39:167-179.
47. Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):438-449. doi: 10.1016/j.jpain.2012.01.003
48. Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014;47(1):166-173. doi: 10.1016/j.jpainsymman.2013.02.018
49. Kleckner AS, Kleckner IR, Kamen CS, et al. Opportunities for cannabis in supportive care in cancer. Ther Adv Med Oncol. 2019;11:1758835919866362. doi: 10.1177/1758835919866362
50. National Conference of State Legislatures (ncsl.org). State Medical Marijuana Laws. Accessed April 5, 2021. https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
51. Hasin DS, Shmulewitz D, Cerda M, et al. US adults with pain, a group increasingly vulnerable to nonmedical cannabis use and cannabis use disorder: 2001-2002 and 2012-2013. Am J Psychiatry. 2020;177(7):611-618. doi: 10.1176/appi.ajp.2019.19030284
52. Hasin DS, Sarvet AL, Cerdá M, et al. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991-1992 to 2012-2013. JAMA Psychiatry. 2017;74(6):579-588. doi: 10.1001/jamapsychiatry.2017.0724
53. National Institute on Drug Abuse. Illicit cannabis use and use disorders increase in states with medical marijuana laws. April 26, 2017. Accessed October 24, 2020. https://archives.drugabuse.gov/news-events/news-releases/2017/04/illicit-cannabis-use-use-disorders-increase-in-states-medical-marijuana-laws
54. National Academies of Sciences, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. The National Academies Press; 2017. https://doi.org/10.17226/24625
55. Stanford M. Physician recommended marijuana: contraindications & standards of care. A review of the literature. Accessed July 7, 2020. http://drneurosci.com/MedicalMarijuanaStandardsofCare.pdf
56. Repp K, Raich A. Marijuana and health: a comprehensive review of 20 years of research. Washington County Oregon Department of Health and Human Services. 2014. Accessed April 8, 2021. https://www.co.washington.or.us/CAO/upload/HHSmarijuana-review.pdf
57. Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Social Adm Pharm. 2016;12(4):638-654. doi: 10.1016/j.sapharm.2015.09.002.
58. Leite RT, Nogueira Sde O, do Nascimento JP, et al. The use of cannabis as a predictor of early onset of bipolar disorder and suicide attempts. Neural Plast. 2015;2015:434127. doi: 10.1155/2015/43412
59. Kim SW, Dodd S, Berk L, et al. Impact of cannabis use on long-term remission in bipolar I and schizoaffective disorder. Psychiatry Investig. 2015;12(3):349-355. doi: 10.4306/pi.2015.12.3.349
60. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(12):995-1010.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064. doi: 10.4088/JCP.15r10036.
62. Woolf CJ, American College of Physicians. American Physiological Society Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441-451.
63. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24(7):515‐523. doi: 10.1002/hup.1048
64. Sachs J, McGlade E, Yurgelun-Todd D. Safety and toxicology of cannabinoids. Neurotherapeutics. 2015;12(4):735‐746. doi: 10.1007/s13311-015-0380-8
65. Antoniou T, Bodkin J, Ho JMW. Drug interactions with cannabinoids. CMAJ. 2020;2;192:E206. doi: 10.1503/cmaj.191097
66. Brown JD. Potential adverse drug events with tetrahydrocannabinol (THC) due to drug-drug interactions. J Clin Med. 2020;9(4):919. doi: 10.3390/jcm9040919.
67. Maida V, Daeninck P. A user’s guide to cannabinoid therapy in oncology. Curr Oncol. 2016;23(6):398-406. doi: http://dx.doi.org/10.3747/co.23.3487
68. Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46(1):86-95. doi: 10.3109/03602532.2013.849268
69. Abrams DI. Integrating cannabis into clinical cancer care. Curr Oncol. 2016;23(52):S8-S14.
70. Alsherbiny MA, Li CG. Medicinal cannabis—potential drug interactions. Medicines. 2018;6(1):3. doi: 10.3390/medicines6010003
71. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84:2477-2482.
72. Ghasemiesfe M, Barrow B, Leonard S, et al. Association between marijuana use and risk of cancer: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(11):e1916318. doi: 10.1001/jamanetworkopen.2019.16318
73. Riggs PK, Vaida F, Rossi SS, et al. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res. 2012;1431:46-52. doi: 10.1016/j.brainres.2011.11.001
74. Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. doi: 10.1136/bmj.e536
75. Carlier J, Huestis MA, Zaami S, et al. Monitoring perinatal exposure to cannabis and synthetic cannabinoids. Ther Drug Monit. 2020;42(2):194-204.
Against the backdrop of an increasing opioid use epidemic and a marked acceleration of prescription opioid–related deaths,1,2 there has been an impetus to explore the usefulness of alternative and co-analgesic agents to assist patients with chronic pain. Preclinical studies employing animal-based models of human pain syndromes have demonstrated that cannabis and chemicals derived from cannabis extracts may mitigate several pain conditions.3
Because there are significant comorbidities between psychiatric disorders and chronic pain, psychiatrists are likely to care for patients with chronic pain. As the availability of and interest in cannabinoid-based medications (CBM) increases, psychiatrists will need to be apprised of the utility, adverse effects, and potential drug interactions of these agents.
The endocannabinoid system and cannabis receptors
The endogenous cannabinoid (endocannabinoid) system is abundantly present within the peripheral and central nervous systems. The first identified, and best studied, endocannabinoids are N-arachidonoyl-ethanolamine (AEA; anandamide) and 2-arachidonoylglycerol (2-AG).4 Unlike typical neurotransmitters, AEA and 2-AG are not stored within vesicles within presynaptic neuron axons. Instead, they are lipophilic molecules produced on demand, synthesized from phospholipids (ie, arachidonic acid derivatives) at the membranes of post-synaptic neurons, and released into the synapse directly.5
Acting as retrograde messengers, the endocannabinoids traverse the synapse, binding to receptors located on the axons of the presynaptic neuron. Two receptors—CB1 and CB2—have been most extensively studied and characterized.6,7 These receptors couple to Gi/o-proteins to inhibit adenylate cyclase, decreasing Ca2+ conductance and increasing K+ conductance.8 Once activated, cannabinoid receptors modulate neurotransmitter release from presynaptic axon terminals. Evidence points to a similar retrograde signaling between neurons and glial cells. Shortly after receptor activation, the endocannabinoids are deactivated by the actions of a transporter mechanism and enzyme degradation.9,10
The endocannabinoid system and pain transmission
Cannabinoid receptors are present in pain transmission circuits spanning from the peripheral sensory nerve endings (from which pain signals originate) to the spinal cord and supraspinal regions within the brain.11-14 CB1 receptors are abundantly present within the CNS, including regions involved in pain transmission. Binding to CB1 receptors, endocannabinoids modulate neurotransmission that impacts pain transmission centrally. Endocannabinoids can also indirectly modulate opiate and N-methyl-
By contrast, CB2 receptors are predominantly localized to peripheral tissues and immune cells, although there has been some discovery of their presence within the CNS (eg, on microglia). Endocannabinoid activation of CB2 receptors is thought to modulate the activity of peripheral afferent pain fibers and immune-mediated neuroinflammatory processes—such as inhibition of prostaglandin synthesis and mast cell degranulation—that can precipitate and maintain chronic pain states.16-18
Evidence garnered from preclinical (animal) studies points to the role of the endocannabinoid system in modulating normal pain transmission (see Manzanares et al3 for details). These studies offer a putative basis for understanding how exogenous cannabinoid congeners might serve to ameliorate pain transmission in pathophysiologic states, including chronic pain.
Continue to: Cannabinoid-based medications
Cannabinoid-based medications
Marijuana contains multiple components (cannabinoids). The most extensively studied are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Because it predominantly binds CB1 receptors centrally, THC is the major psychoactive component of cannabis; it promotes sleep and appetite, influences anxiety, and produces the “high” associated with cannabis use. By contrast, CBD weakly binds CB1 and thus exerts minimal or no psychoactive effects.19
Cannabinoid absorption, metabolism, bioavailability, and clinical effects vary depending on the formulation and method of administration (Table 1).20-22 THC and CBD content and potency in inhaled cannabis can vary significantly depending on the strains of the cannabis plant and manner of cultivation.23 To standardize approaches for administering cannabinoids in clinical trials and for clinical use, researchers have developed pharmaceutical analogs that contain extracted chemicals or synthetic chemicals similar to THC and/or CBD.

In this article, CBM refers to smoked/vaporized herbal cannabis as well as pharmaceutical cannabis analogs. Table 2 summarizes the characteristics of CBM commonly used in studies investigating their use for managing pain conditions.

CBM for chronic pain
The literature base examining the role of CBM for managing chronic nonmalignant and malignant pain of varying etiologies is rapidly expanding. Randomized controlled trials (RCTs) have focused on inhaled/smoked products and related cannabinoid medications, some of which are FDA-approved (Table 2).
A multitude of other cannabinoid-based products are currently commercially available to consumers, including tincture and oil-based products; over-the-counter CBD products; and several other formulations of CBM (eg, edible and suppository products). Because such products are not standardized or quality-controlled,24 RCTs have not assessed their efficacy for mitigating pain. Consequently, the findings summarized in this article do not address the utility of these agents.
Continue to: CBM for non-cancer pain
CBM for non-cancer pain
Neuropathic pain. Randomized controlled trials have assessed the pain-mitigating effects of various CBM, including inhaled cannabis, synthetic THC, plant-extracted CBD, and a THC/CBD spray. Studies have shown that inhaled/vaporized cannabis can produce short-term pain reduction in patients with chronic neuropathic pain of diverse etiologies, including diabetes mellitus-, HIV-, trauma-, and medication-induced neuropathies.22,25,26 Similar beneficial effects have been observed with the use of cannabis analogues (eg, nabiximols).25,26-29
Meta-analyses and systematic reviews have determined that most of these RCTs were of low-to-moderate quality.26,30 Meta-analyses have revealed divergent and conflicting results because of differences in the inclusion and exclusion criteria used to select RCTs for analysis and differences in the standards with which the quality of evidence were determined.25,30
Overall, the benefit of CBM for mitigating neuropathic pain is promising, but the effectiveness may not be robust.30,31 Several noteworthy caveats limit the interpretation of the results of these RCTs:
- due to the small sample sizes and brief durations of study, questions remain regarding the extent to which effects are generalizable, whether the benefits are sustained, and whether adverse effects emerge over time with continued use
- most RCTs evaluated inhaled (herbal) cannabis and nabiximols; there is little data on the effectiveness of other CBM formulations25,26,30
- the pain-mitigating effects of CBM were usually compared with those of placebo; the comparative efficacy against agents commonly used to treat neuropathic pain remains largely unexamined
- these RCTs typically compared mean pain severity score differences between cannabis-treated and placebo groups using standard subjective rating scales of pain intensity, such as the Numerical Rating Scale or Visual Analogue Scale. Customarily, the pain literature has used a 30% or 50% reduction in pain severity from baseline as an indicator of significant clinical improvement.32,33 The RCTs of CBM for neuropathic pain rarely used this standard, which makes it unclear whether CBM results in clinically significant pain reductions30
- indirect measures of effectiveness (ie, whether using CBM reduces the need for opioids or other analgesics to manage pain) were seldom reported in these RCTs.
Due to these limitations, clinical guidelines and systematic reviews consider CBM as a third- or fourth-line therapy for patients experiencing chronic neuropathic pain for whom conventional agents such as anticonvulsants and antidepressants have failed.34,35
Spasticity in multiple sclerosis (MS). Several RCTs have assessed the use of CBM for MS-related spasticity, although few were deemed to be high quality. Nabiximols and synthetic THC were effective in managing spasticity and reducing pain severity associated with muscle spasms.36 Generally, investigations revealed that CBM were associated with improvements in subjective measures of spasticity, but these were not born out in clinical, objective measures.26,37 The efficacy of smoked cannabis was uncertain.37 The existing literature on CBM for MS-related spasticity does not address dosing, duration of effects, tolerability, or comparative effectiveness against conventional anti-spasm medications.36,37
Continue to: Other chronic pain conditions
Other chronic pain conditions. CBM have also been studied for their usefulness in several other noncancer chronic conditions, including Crohn’s disease, inflammatory bowel disease, fibromyalgia, and other rheumatologic pain conditions.22,31,38-40 However, a solid foundation of empirical work to inform their utility for managing pain in these conditions is lacking.
CBM for cancer pain
Anecdotal evidence suggests that inhaled cannabis has promising pain-mitigating effects in patients with advanced cancer.41-43 There is a dearth of high-quality RCTs assessing the utility of CBM in patients with cancer pain.43-45 The types of CBM used and dosing strategies varied across RCTs, which makes it difficult to infer how best to treat patients with cancer pain. The agents studied included nabiximols, THC spray, and synthetic THC capsules.43-45 Although some studies have demonstrated that synthetic THC and nabiximols have potential for reducing subjective pain ratings compared with placebo,46,47 these results were inconsistent.46,48 Oromucosal nabiximols did not appear to confer any additional analgesic benefit in patients who were already prescribed opioids.31,45
The benefit of CBM for mitigating cancer pain is promising, but it remains difficult to know how to position the use of CBM in managing cancer pain. Limitations in the cancer literature include:
- the RCTs addressing CBM use for cancer pain were often brief, which raises questions about the long-term effectiveness and adverse effects of these agents
- tolerability and dosing limits encountered due to adverse effects were seldom reported43,45
- the types of cancer pain that patients had were often quite diverse. The small sample sizes and the heterogeneity of conditions included in these RCTs limit the ability to determine whether pain-mitigating effects might vary according to type of cancer-related pain.31,45
Despite these limitations, some clinical guidelines and systematic reviews have suggested that CBM have some role in addressing refractory malignant pain conditions.49
Psychiatric considerations related to CBM
As of November 2020, 36 states had legalized the use of cannabis for medical purposes, typically for painful conditions, despite the fact that empirical evidence to support their efficacy is mixed.50 In light of recent changes in both the legal and popular attitudes regarding cannabis, the implications of legalizing CBM remains to be seen. For example, some research suggests that adults with pain are vulnerable to frequent nonmedical cannabis use and/or cannabis use disorder.51 Although well-intended, the legalization of CBM use might represent society’s next misstep in the quest to address the suffering of patients with chronic pain. Some evidence shows that cannabis use and cannabis use disorders increase in states that have legalized medical marijuana.52,53 Psychiatrists will be on the front lines of addressing any potential consequences arising from the use of CBM for treating pain.
Continue to: Psychiatric disorders and CBM
Psychiatric disorders and CBM. The psychological impact of CBM use among patients enduring chronic pain can include sedation, cognitive/attention disturbance, and fatigue. These adverse effects can limit the utility of such agents.22,29,45
Contraindications for CBM use, and conditions for which CBM ought to be used with caution, are listed in Table 354,55.The safety of CBM, particularly in patients with chronic pain and psychiatric disorders, has not been examined. Patients with psychiatric disorders may be poor candidates for medical cannabis. Epidemiologic data suggest that recreational cannabis use is positively associated both cross-sectionally and prospectively with psychotic spectrum disorders, depressive symptoms, and anxiety symptoms, including panic disorder.56 Psychotic reactions have also been associated with CBM (dronabinol and nabilone).57 Cannabis use also has been associated with an earlier onset of, and lower remission rates of, symptoms associated with bipolar disorder.58,59 Consequently, patients who have been diagnosed with or are at risk for developing any of the aforementioned conditions may not be suitable candidates for CBM. If CBM are used, patients should be closely monitored for the emergence/exacerbation of psychiatric symptoms. The frequency and extent of follow-up is not clear, however. Because of its reduced propensity to produce psychoactive effects, CBD may be safer than THC for managing pain in individuals who have or are vulnerable to developing psychiatric disorders.
There is a lack of evidence to support the use of CBM for treating primary depressive disorders, general anxiety disorder, posttraumatic stress disorder, or psychosis.60,61 Very low-quality evidence suggests that CBM could lead to a small improvement in anxiety among individuals with noncancer pain and MS.60 However, interpreting causality is complicated. It is plausible that, for some patients, subjective improvement in pain severity may be related to reduced anxiety.62 Conversely, it is equally plausible that reductions in emotional distress may reduce the propensity to attend to, and thus magnify, pain severity. In the latter case, the indirect impact of reducing pain by modifying emotional distress can be impacted by the type and dose of CBM used. For example, low concentrations of THC produce anxiolytic effects, but high concentrations may be anxiety-provoking.63,64
Several potential pharmacokinetic drug interactions may arise between herbal cannabis or CBM and other medications (Table 465,66). THC and CBD are both metabolized by cytochrome P450 (CYP) 2C19 and 3A4.65,66 In addition, THC is also metabolized by CYP2C9. Medications that inhibit or induce these enzymes can increase or decrease the bioavailability of THC and CBD.67

Simultaneously, cannabinoids can impact the bioavailability of co-prescribed medications (Table 566,68). Although such CYP enzyme interactions remain a theoretical possibility, it is uncertain whether significant perturbations in plasma concentrations (and clinical effects) have been encountered with prescription medications when co-administered with CBM.69 Nonetheless, patients receiving CBM should be closely monitored for their response to prescribed medications.70

Continue to: Potential CYP enzyme interactions...
Potential CYP enzyme interactions aside, clinicians need to consider the additive effects that may occur when CBM are combined with sympathomimetic agents (eg, tachycardia, hypertension); CNS depressants such as alcohol, benzodiazepines, and opioids (eg, drowsiness, ataxia); or anticholinergics (eg, tachycardia, confusion).71 Inhaled herbal cannabis contains mutagens and can result in lung damage, exacerbations of chronic bronchitis, and certain types of cancer.54,72 Co-prescribing benzodiazepines may be contraindicated in light of their effects on respiratory rate and effort.
The THC contained in CBM produces hormonal effects (ie, significantly increases plasma levels of ghrelin and leptin and decreases peptide YY levels)73 that affect appetite and can produce weight gain. This may be problematic for patients receiving psychoactive medications associated with increased risk of weight gain and dyslipidemia. Because of the association between cannabis use and motor vehicle accidents, patients whose jobs require them to drive or operate industrial equipment may not be ideal candidates for CBM, especially if such patients also consume alcohol or are prescribed benzodiazepines and/or sedative hypnotics.74 Lastly, due to their lipophilicity, cannabinoids cross the placental barrier and can be found in breast milk75 and therefore can affect pregnancy outcomes and neurodevelopment.
Bottom Line
The popularity of cannabinoid-based medications (CBM) for the treatment of chronic pain conditions is growing, but the interest in their use may be outpacing the evidence supporting their analgesic benefits. High-quality, well-controlled randomized controlled trials are needed to decipher whether, and to what extent, these agents can be positioned in chronic pain management. Because psychiatrists are likely to encounter patients considering, or receiving, CBM, they must be aware of the potential benefits, risks, and adverse effects of such treatments.
Related Resources
- Joshi KG. Cannabis-derived compounds: what you need to know. Current Psychiatry. 2020;19(10):64-65. doi:10.12788/ cp.0050
- Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018; 17(1):34-41.
Drug Brand Names
Ajulemic acid • Anabasum
Alprazolam • Xanax
Amitriptyline • Elavil
Aripiprazole • Abilify, Abilify Maintena
Buspirone • BuSpar
Cannabidiol • Epidiolex
Carbamazepine • Tegretol, Equetro
Cimetidine • Tagamet HB
Citalopram • Celexa
Clopidogrel • Plavix
Clozapine • Clozaril
Cyclosporine • Neoral, Sandimmune
Dronabinol • Marinol, Syndros
Duloxetine • Cymbalta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Haloperidol • Haldol
Imipramine • Tofranil
Ketoconazole • Nizoral AD
Losartan • Cozaar
Midazolam • Versed
Mirtazapine • Remeron
Nabilone • Cesamet
Nabiximols • Sativex
Nefazodone • Serzone
Olanzapine • Zyprexa
Phenobarbital • Solfoton
Phenytoin • Dilantin
Ramelteon • Rozerem
Rifampin • Rifadin
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Topiramate • Topamax
Valproic acid • Depakote, Depakene
Venlafaxine • Effexor
Verapamil • Verelan
Zolpidem • Ambien
Against the backdrop of an increasing opioid use epidemic and a marked acceleration of prescription opioid–related deaths,1,2 there has been an impetus to explore the usefulness of alternative and co-analgesic agents to assist patients with chronic pain. Preclinical studies employing animal-based models of human pain syndromes have demonstrated that cannabis and chemicals derived from cannabis extracts may mitigate several pain conditions.3
Because there are significant comorbidities between psychiatric disorders and chronic pain, psychiatrists are likely to care for patients with chronic pain. As the availability of and interest in cannabinoid-based medications (CBM) increases, psychiatrists will need to be apprised of the utility, adverse effects, and potential drug interactions of these agents.
The endocannabinoid system and cannabis receptors
The endogenous cannabinoid (endocannabinoid) system is abundantly present within the peripheral and central nervous systems. The first identified, and best studied, endocannabinoids are N-arachidonoyl-ethanolamine (AEA; anandamide) and 2-arachidonoylglycerol (2-AG).4 Unlike typical neurotransmitters, AEA and 2-AG are not stored within vesicles within presynaptic neuron axons. Instead, they are lipophilic molecules produced on demand, synthesized from phospholipids (ie, arachidonic acid derivatives) at the membranes of post-synaptic neurons, and released into the synapse directly.5
Acting as retrograde messengers, the endocannabinoids traverse the synapse, binding to receptors located on the axons of the presynaptic neuron. Two receptors—CB1 and CB2—have been most extensively studied and characterized.6,7 These receptors couple to Gi/o-proteins to inhibit adenylate cyclase, decreasing Ca2+ conductance and increasing K+ conductance.8 Once activated, cannabinoid receptors modulate neurotransmitter release from presynaptic axon terminals. Evidence points to a similar retrograde signaling between neurons and glial cells. Shortly after receptor activation, the endocannabinoids are deactivated by the actions of a transporter mechanism and enzyme degradation.9,10
The endocannabinoid system and pain transmission
Cannabinoid receptors are present in pain transmission circuits spanning from the peripheral sensory nerve endings (from which pain signals originate) to the spinal cord and supraspinal regions within the brain.11-14 CB1 receptors are abundantly present within the CNS, including regions involved in pain transmission. Binding to CB1 receptors, endocannabinoids modulate neurotransmission that impacts pain transmission centrally. Endocannabinoids can also indirectly modulate opiate and N-methyl-
By contrast, CB2 receptors are predominantly localized to peripheral tissues and immune cells, although there has been some discovery of their presence within the CNS (eg, on microglia). Endocannabinoid activation of CB2 receptors is thought to modulate the activity of peripheral afferent pain fibers and immune-mediated neuroinflammatory processes—such as inhibition of prostaglandin synthesis and mast cell degranulation—that can precipitate and maintain chronic pain states.16-18
Evidence garnered from preclinical (animal) studies points to the role of the endocannabinoid system in modulating normal pain transmission (see Manzanares et al3 for details). These studies offer a putative basis for understanding how exogenous cannabinoid congeners might serve to ameliorate pain transmission in pathophysiologic states, including chronic pain.
Continue to: Cannabinoid-based medications
Cannabinoid-based medications
Marijuana contains multiple components (cannabinoids). The most extensively studied are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Because it predominantly binds CB1 receptors centrally, THC is the major psychoactive component of cannabis; it promotes sleep and appetite, influences anxiety, and produces the “high” associated with cannabis use. By contrast, CBD weakly binds CB1 and thus exerts minimal or no psychoactive effects.19
Cannabinoid absorption, metabolism, bioavailability, and clinical effects vary depending on the formulation and method of administration (Table 1).20-22 THC and CBD content and potency in inhaled cannabis can vary significantly depending on the strains of the cannabis plant and manner of cultivation.23 To standardize approaches for administering cannabinoids in clinical trials and for clinical use, researchers have developed pharmaceutical analogs that contain extracted chemicals or synthetic chemicals similar to THC and/or CBD.

In this article, CBM refers to smoked/vaporized herbal cannabis as well as pharmaceutical cannabis analogs. Table 2 summarizes the characteristics of CBM commonly used in studies investigating their use for managing pain conditions.

CBM for chronic pain
The literature base examining the role of CBM for managing chronic nonmalignant and malignant pain of varying etiologies is rapidly expanding. Randomized controlled trials (RCTs) have focused on inhaled/smoked products and related cannabinoid medications, some of which are FDA-approved (Table 2).
A multitude of other cannabinoid-based products are currently commercially available to consumers, including tincture and oil-based products; over-the-counter CBD products; and several other formulations of CBM (eg, edible and suppository products). Because such products are not standardized or quality-controlled,24 RCTs have not assessed their efficacy for mitigating pain. Consequently, the findings summarized in this article do not address the utility of these agents.
Continue to: CBM for non-cancer pain
CBM for non-cancer pain
Neuropathic pain. Randomized controlled trials have assessed the pain-mitigating effects of various CBM, including inhaled cannabis, synthetic THC, plant-extracted CBD, and a THC/CBD spray. Studies have shown that inhaled/vaporized cannabis can produce short-term pain reduction in patients with chronic neuropathic pain of diverse etiologies, including diabetes mellitus-, HIV-, trauma-, and medication-induced neuropathies.22,25,26 Similar beneficial effects have been observed with the use of cannabis analogues (eg, nabiximols).25,26-29
Meta-analyses and systematic reviews have determined that most of these RCTs were of low-to-moderate quality.26,30 Meta-analyses have revealed divergent and conflicting results because of differences in the inclusion and exclusion criteria used to select RCTs for analysis and differences in the standards with which the quality of evidence were determined.25,30
Overall, the benefit of CBM for mitigating neuropathic pain is promising, but the effectiveness may not be robust.30,31 Several noteworthy caveats limit the interpretation of the results of these RCTs:
- due to the small sample sizes and brief durations of study, questions remain regarding the extent to which effects are generalizable, whether the benefits are sustained, and whether adverse effects emerge over time with continued use
- most RCTs evaluated inhaled (herbal) cannabis and nabiximols; there is little data on the effectiveness of other CBM formulations25,26,30
- the pain-mitigating effects of CBM were usually compared with those of placebo; the comparative efficacy against agents commonly used to treat neuropathic pain remains largely unexamined
- these RCTs typically compared mean pain severity score differences between cannabis-treated and placebo groups using standard subjective rating scales of pain intensity, such as the Numerical Rating Scale or Visual Analogue Scale. Customarily, the pain literature has used a 30% or 50% reduction in pain severity from baseline as an indicator of significant clinical improvement.32,33 The RCTs of CBM for neuropathic pain rarely used this standard, which makes it unclear whether CBM results in clinically significant pain reductions30
- indirect measures of effectiveness (ie, whether using CBM reduces the need for opioids or other analgesics to manage pain) were seldom reported in these RCTs.
Due to these limitations, clinical guidelines and systematic reviews consider CBM as a third- or fourth-line therapy for patients experiencing chronic neuropathic pain for whom conventional agents such as anticonvulsants and antidepressants have failed.34,35
Spasticity in multiple sclerosis (MS). Several RCTs have assessed the use of CBM for MS-related spasticity, although few were deemed to be high quality. Nabiximols and synthetic THC were effective in managing spasticity and reducing pain severity associated with muscle spasms.36 Generally, investigations revealed that CBM were associated with improvements in subjective measures of spasticity, but these were not born out in clinical, objective measures.26,37 The efficacy of smoked cannabis was uncertain.37 The existing literature on CBM for MS-related spasticity does not address dosing, duration of effects, tolerability, or comparative effectiveness against conventional anti-spasm medications.36,37
Continue to: Other chronic pain conditions
Other chronic pain conditions. CBM have also been studied for their usefulness in several other noncancer chronic conditions, including Crohn’s disease, inflammatory bowel disease, fibromyalgia, and other rheumatologic pain conditions.22,31,38-40 However, a solid foundation of empirical work to inform their utility for managing pain in these conditions is lacking.
CBM for cancer pain
Anecdotal evidence suggests that inhaled cannabis has promising pain-mitigating effects in patients with advanced cancer.41-43 There is a dearth of high-quality RCTs assessing the utility of CBM in patients with cancer pain.43-45 The types of CBM used and dosing strategies varied across RCTs, which makes it difficult to infer how best to treat patients with cancer pain. The agents studied included nabiximols, THC spray, and synthetic THC capsules.43-45 Although some studies have demonstrated that synthetic THC and nabiximols have potential for reducing subjective pain ratings compared with placebo,46,47 these results were inconsistent.46,48 Oromucosal nabiximols did not appear to confer any additional analgesic benefit in patients who were already prescribed opioids.31,45
The benefit of CBM for mitigating cancer pain is promising, but it remains difficult to know how to position the use of CBM in managing cancer pain. Limitations in the cancer literature include:
- the RCTs addressing CBM use for cancer pain were often brief, which raises questions about the long-term effectiveness and adverse effects of these agents
- tolerability and dosing limits encountered due to adverse effects were seldom reported43,45
- the types of cancer pain that patients had were often quite diverse. The small sample sizes and the heterogeneity of conditions included in these RCTs limit the ability to determine whether pain-mitigating effects might vary according to type of cancer-related pain.31,45
Despite these limitations, some clinical guidelines and systematic reviews have suggested that CBM have some role in addressing refractory malignant pain conditions.49
Psychiatric considerations related to CBM
As of November 2020, 36 states had legalized the use of cannabis for medical purposes, typically for painful conditions, despite the fact that empirical evidence to support their efficacy is mixed.50 In light of recent changes in both the legal and popular attitudes regarding cannabis, the implications of legalizing CBM remains to be seen. For example, some research suggests that adults with pain are vulnerable to frequent nonmedical cannabis use and/or cannabis use disorder.51 Although well-intended, the legalization of CBM use might represent society’s next misstep in the quest to address the suffering of patients with chronic pain. Some evidence shows that cannabis use and cannabis use disorders increase in states that have legalized medical marijuana.52,53 Psychiatrists will be on the front lines of addressing any potential consequences arising from the use of CBM for treating pain.
Continue to: Psychiatric disorders and CBM
Psychiatric disorders and CBM. The psychological impact of CBM use among patients enduring chronic pain can include sedation, cognitive/attention disturbance, and fatigue. These adverse effects can limit the utility of such agents.22,29,45
Contraindications for CBM use, and conditions for which CBM ought to be used with caution, are listed in Table 354,55.The safety of CBM, particularly in patients with chronic pain and psychiatric disorders, has not been examined. Patients with psychiatric disorders may be poor candidates for medical cannabis. Epidemiologic data suggest that recreational cannabis use is positively associated both cross-sectionally and prospectively with psychotic spectrum disorders, depressive symptoms, and anxiety symptoms, including panic disorder.56 Psychotic reactions have also been associated with CBM (dronabinol and nabilone).57 Cannabis use also has been associated with an earlier onset of, and lower remission rates of, symptoms associated with bipolar disorder.58,59 Consequently, patients who have been diagnosed with or are at risk for developing any of the aforementioned conditions may not be suitable candidates for CBM. If CBM are used, patients should be closely monitored for the emergence/exacerbation of psychiatric symptoms. The frequency and extent of follow-up is not clear, however. Because of its reduced propensity to produce psychoactive effects, CBD may be safer than THC for managing pain in individuals who have or are vulnerable to developing psychiatric disorders.
There is a lack of evidence to support the use of CBM for treating primary depressive disorders, general anxiety disorder, posttraumatic stress disorder, or psychosis.60,61 Very low-quality evidence suggests that CBM could lead to a small improvement in anxiety among individuals with noncancer pain and MS.60 However, interpreting causality is complicated. It is plausible that, for some patients, subjective improvement in pain severity may be related to reduced anxiety.62 Conversely, it is equally plausible that reductions in emotional distress may reduce the propensity to attend to, and thus magnify, pain severity. In the latter case, the indirect impact of reducing pain by modifying emotional distress can be impacted by the type and dose of CBM used. For example, low concentrations of THC produce anxiolytic effects, but high concentrations may be anxiety-provoking.63,64
Several potential pharmacokinetic drug interactions may arise between herbal cannabis or CBM and other medications (Table 465,66). THC and CBD are both metabolized by cytochrome P450 (CYP) 2C19 and 3A4.65,66 In addition, THC is also metabolized by CYP2C9. Medications that inhibit or induce these enzymes can increase or decrease the bioavailability of THC and CBD.67

Simultaneously, cannabinoids can impact the bioavailability of co-prescribed medications (Table 566,68). Although such CYP enzyme interactions remain a theoretical possibility, it is uncertain whether significant perturbations in plasma concentrations (and clinical effects) have been encountered with prescription medications when co-administered with CBM.69 Nonetheless, patients receiving CBM should be closely monitored for their response to prescribed medications.70

Continue to: Potential CYP enzyme interactions...
Potential CYP enzyme interactions aside, clinicians need to consider the additive effects that may occur when CBM are combined with sympathomimetic agents (eg, tachycardia, hypertension); CNS depressants such as alcohol, benzodiazepines, and opioids (eg, drowsiness, ataxia); or anticholinergics (eg, tachycardia, confusion).71 Inhaled herbal cannabis contains mutagens and can result in lung damage, exacerbations of chronic bronchitis, and certain types of cancer.54,72 Co-prescribing benzodiazepines may be contraindicated in light of their effects on respiratory rate and effort.
The THC contained in CBM produces hormonal effects (ie, significantly increases plasma levels of ghrelin and leptin and decreases peptide YY levels)73 that affect appetite and can produce weight gain. This may be problematic for patients receiving psychoactive medications associated with increased risk of weight gain and dyslipidemia. Because of the association between cannabis use and motor vehicle accidents, patients whose jobs require them to drive or operate industrial equipment may not be ideal candidates for CBM, especially if such patients also consume alcohol or are prescribed benzodiazepines and/or sedative hypnotics.74 Lastly, due to their lipophilicity, cannabinoids cross the placental barrier and can be found in breast milk75 and therefore can affect pregnancy outcomes and neurodevelopment.
Bottom Line
The popularity of cannabinoid-based medications (CBM) for the treatment of chronic pain conditions is growing, but the interest in their use may be outpacing the evidence supporting their analgesic benefits. High-quality, well-controlled randomized controlled trials are needed to decipher whether, and to what extent, these agents can be positioned in chronic pain management. Because psychiatrists are likely to encounter patients considering, or receiving, CBM, they must be aware of the potential benefits, risks, and adverse effects of such treatments.
Related Resources
- Joshi KG. Cannabis-derived compounds: what you need to know. Current Psychiatry. 2020;19(10):64-65. doi:10.12788/ cp.0050
- Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018; 17(1):34-41.
Drug Brand Names
Ajulemic acid • Anabasum
Alprazolam • Xanax
Amitriptyline • Elavil
Aripiprazole • Abilify, Abilify Maintena
Buspirone • BuSpar
Cannabidiol • Epidiolex
Carbamazepine • Tegretol, Equetro
Cimetidine • Tagamet HB
Citalopram • Celexa
Clopidogrel • Plavix
Clozapine • Clozaril
Cyclosporine • Neoral, Sandimmune
Dronabinol • Marinol, Syndros
Duloxetine • Cymbalta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Haloperidol • Haldol
Imipramine • Tofranil
Ketoconazole • Nizoral AD
Losartan • Cozaar
Midazolam • Versed
Mirtazapine • Remeron
Nabilone • Cesamet
Nabiximols • Sativex
Nefazodone • Serzone
Olanzapine • Zyprexa
Phenobarbital • Solfoton
Phenytoin • Dilantin
Ramelteon • Rozerem
Rifampin • Rifadin
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Topiramate • Topamax
Valproic acid • Depakote, Depakene
Venlafaxine • Effexor
Verapamil • Verelan
Zolpidem • Ambien
1. Okie S. A floor of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. doi:10.1056/NEJMp1011512
2. Powell D, Pacula RL, Taylor E. How increasing medical access to opioids contributes to the opioid epidemic: evidence from Medicare Part D. J Health Econ. 2020;71:102286. doi: 10.1016/j.jhealeco.2019.102286
3. Manzanares J, Julian MD, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006;4(3):239-257. doi: 10.2174/157015906778019527
4. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833. doi: 10.3390/ijms19030833
5. Huang WJ, Chen WW, Zhang X. Endocannabinoid system: role in depression, reward and pain control (Review). Mol Med Rep. 2016;14(4):2899-2903. doi:10.3892/mmr.2016.5585
6. Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83-90. doi:10.1016/0006-2952(95)00109-d
7. Walker JM, Krey JF, Chu CJ, et al. Endocannabinoids and related fatty acid derivatives in pain modulation. Chem Phys Lipids. 2002;121(1-2):159-172. doi: 10.1016/s0009-3084(02)00152-4
8. Howlett AC. Efficacy in CB1 receptor-mediated signal transduction. Br J Pharmacol. 2004;142(8):1209-1218. doi: 10.1038/sj.bjp.0705881
9. Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation, biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7-14.
10. Piomelli D, Beltramo M, Giuffrida A, et al. Endogenous cannabinoid signaling. Neurobiol Dis. 1998;5(6 Pt B):462-473. doi: 10.1006/nbdi.1998.0221
11. Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17(1):175-191. doi: 10.1093/cercor/bhj136
12. Jennings EA, Vaughan CW, Christie MJ. Cannabinoid actions on rat superficial medullary dorsal horn neurons in vitro. J Physiol. 2001;534(Pt 3):805-812. doi: 10.1111/j.1469-7793.2001.00805.x
13. Vaughan CW, Connor M, Bagley EE, et al. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol. 2000;57(2):288-295.
14. Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol. 1999;127(4):935-940. doi: 10.1038/sj.bjp.0702636
15. Raichlen DA, Foster AD, Gerdeman GI, et al. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner’s high.” J Exp Biol. 2012;215(Pt 8):1331-1336. doi: 10.1242/jeb.063677
16. Beltrano M. Cannabinoid type 2 receptor as a target for chronic pain. Mini Rev Chem. 2009;234:253-254.
17. Ibrahim MM, Deng H, Zvonok A, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100(18):10529-10533. doi: 10.1073/pnas.1834309100
18. Valenzano KJ, Tafessem L, Lee G, et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658-672.
19. Pertwee RG, Howlett AC, Abood ME, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588-631. doi: 10.1124/pr.110.003004
20. Carter GT, Weydt P, Kyashna-Tocha M, et al. Medicinal cannabis: rational guidelines for dosing. Drugs. 2004;7(5):464-470.
21. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804.
22. Johal H, Devji T, Chang Y, et al. cannabinoids in chronic non-cancer pain: a systematic review and meta-analysis. Clin Med Insights Arthritis Musculoskelet Disord. 2020;13:1179544120906461. doi: 10.1177/1179544120906461
23. Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am J Bot. 2004;91(6):966-975. doi: 10.3732/ajb.91.6.966
24. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids--an international cross-sectional survey on administration forms. J Psychoactive Drugs. 2013;45(3):199-210. doi: 10.1080/02791072.2013.805976
25. Andreae MH, Carter GM, Shaparin N, et al. inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain. 2015;16(12):1221-1232. doi: 10.1016/j.jpain.2015.07.009
26. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473. doi: 10.1001/jama.2015.6358
27. Boychuk DG, Goddard G, Mauro G, et al. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29(1):7-14. doi: 10.11607/ofph.1274
28. Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72(5):735-744. doi: 10.1111/j.1365-2125.2011.03970.x
29. Stockings E, Campbell G, Hall WD, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159(10):1932-1954. doi: 10.1097/j.pain.0000000000001293
30. Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3(3):CD012182. doi: 10.1002/14651858.CD012182.pub2
31. Häuser W, Fitzcharles MA, Radbruch L, et al. Cannabinoids in pain management and palliative medicine. Dtsch Arztebl Int. 2017;114(38):627-634. doi: 10.3238/arztebl.2017.0627
32. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. doi: 10.1016/j.jpain.2007.09.005
33. Farrar JT, Troxel AB, Stott C, et al. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther. 2008;30(5):974-985. doi: 10.1016/j.clinthera.2008.05.011
34. Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-335. doi: 10.1155/2014/754693
35. Petzke F, Enax-Krumova EK, Häuser W. Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: a systematic review of randomized controlled studies. Schmerz. 2016;30(1):62-88. doi: 10.1007/s00482-015-0089-y
36. Rice J, Cameron M. Cannabinoids for treatment of MS symptoms: state of the evidence. Curr Neurol Neurosci Rep. 2018;18(8):50. doi: 10.1007/s11910-018-0859-x
37. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556-1563. doi: 10.1212/WNL.0000000000000363
38. Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of Crohn’s disease and ulcerative colitis: evidence from Cochrane Reviews. Inflamm Bowel Dis. 2020;26(4):502-509. doi: 10.1093/ibd/izz233
39. Katz-Talmor D, Katz I, Porat-Katz BS, et al. Cannabinoids for the treatment of rheumatic diseases - where do we stand? Nat Rev Rheumatol. 2018;14(8):488-498. doi: 10.1038/s41584-018-0025-5
40. Walitt B, Klose P, Fitzcharles MA, et al. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016;7(7):CD011694. doi: 10.1002/14651858.CD011694.pub2
41. Bar-Lev Schleider L, Mechoulam R, Lederman V, et al. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur J Intern Med. 2018;49:37‐43. doi: 10.1016/j.ejim.2018.01.023
42. Bennett M, Paice JA, Wallace M. Pain and opioids in cancer care: benefits, risks, and alternatives. Am Soc Clin Oncol Educ Book. 2017;37:705‐713. doi:10.1200/EDBK_180469
43. Blake A, Wan BA, Malek L, et al. A selective review of medical cannabis in cancer pain management. Ann Palliat Med. 2017;6(Suppl 2):5215-5222. doi: 10.21037/apm.2017.08.05
44. Aviram J, Samuelly-Lechtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20(6):E755-E796.
45. Häuser W, Welsch P, Klose P, et al. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain: a systematic review with meta-analysis of randomised controlled trials. Schmerz. 2019;33(5):424-436. doi: 10.1007/s00482-019-0373-3
46. Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010; 39:167-179.
47. Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):438-449. doi: 10.1016/j.jpain.2012.01.003
48. Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014;47(1):166-173. doi: 10.1016/j.jpainsymman.2013.02.018
49. Kleckner AS, Kleckner IR, Kamen CS, et al. Opportunities for cannabis in supportive care in cancer. Ther Adv Med Oncol. 2019;11:1758835919866362. doi: 10.1177/1758835919866362
50. National Conference of State Legislatures (ncsl.org). State Medical Marijuana Laws. Accessed April 5, 2021. https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
51. Hasin DS, Shmulewitz D, Cerda M, et al. US adults with pain, a group increasingly vulnerable to nonmedical cannabis use and cannabis use disorder: 2001-2002 and 2012-2013. Am J Psychiatry. 2020;177(7):611-618. doi: 10.1176/appi.ajp.2019.19030284
52. Hasin DS, Sarvet AL, Cerdá M, et al. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991-1992 to 2012-2013. JAMA Psychiatry. 2017;74(6):579-588. doi: 10.1001/jamapsychiatry.2017.0724
53. National Institute on Drug Abuse. Illicit cannabis use and use disorders increase in states with medical marijuana laws. April 26, 2017. Accessed October 24, 2020. https://archives.drugabuse.gov/news-events/news-releases/2017/04/illicit-cannabis-use-use-disorders-increase-in-states-medical-marijuana-laws
54. National Academies of Sciences, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. The National Academies Press; 2017. https://doi.org/10.17226/24625
55. Stanford M. Physician recommended marijuana: contraindications & standards of care. A review of the literature. Accessed July 7, 2020. http://drneurosci.com/MedicalMarijuanaStandardsofCare.pdf
56. Repp K, Raich A. Marijuana and health: a comprehensive review of 20 years of research. Washington County Oregon Department of Health and Human Services. 2014. Accessed April 8, 2021. https://www.co.washington.or.us/CAO/upload/HHSmarijuana-review.pdf
57. Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Social Adm Pharm. 2016;12(4):638-654. doi: 10.1016/j.sapharm.2015.09.002.
58. Leite RT, Nogueira Sde O, do Nascimento JP, et al. The use of cannabis as a predictor of early onset of bipolar disorder and suicide attempts. Neural Plast. 2015;2015:434127. doi: 10.1155/2015/43412
59. Kim SW, Dodd S, Berk L, et al. Impact of cannabis use on long-term remission in bipolar I and schizoaffective disorder. Psychiatry Investig. 2015;12(3):349-355. doi: 10.4306/pi.2015.12.3.349
60. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(12):995-1010.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064. doi: 10.4088/JCP.15r10036.
62. Woolf CJ, American College of Physicians. American Physiological Society Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441-451.
63. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24(7):515‐523. doi: 10.1002/hup.1048
64. Sachs J, McGlade E, Yurgelun-Todd D. Safety and toxicology of cannabinoids. Neurotherapeutics. 2015;12(4):735‐746. doi: 10.1007/s13311-015-0380-8
65. Antoniou T, Bodkin J, Ho JMW. Drug interactions with cannabinoids. CMAJ. 2020;2;192:E206. doi: 10.1503/cmaj.191097
66. Brown JD. Potential adverse drug events with tetrahydrocannabinol (THC) due to drug-drug interactions. J Clin Med. 2020;9(4):919. doi: 10.3390/jcm9040919.
67. Maida V, Daeninck P. A user’s guide to cannabinoid therapy in oncology. Curr Oncol. 2016;23(6):398-406. doi: http://dx.doi.org/10.3747/co.23.3487
68. Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46(1):86-95. doi: 10.3109/03602532.2013.849268
69. Abrams DI. Integrating cannabis into clinical cancer care. Curr Oncol. 2016;23(52):S8-S14.
70. Alsherbiny MA, Li CG. Medicinal cannabis—potential drug interactions. Medicines. 2018;6(1):3. doi: 10.3390/medicines6010003
71. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84:2477-2482.
72. Ghasemiesfe M, Barrow B, Leonard S, et al. Association between marijuana use and risk of cancer: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(11):e1916318. doi: 10.1001/jamanetworkopen.2019.16318
73. Riggs PK, Vaida F, Rossi SS, et al. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res. 2012;1431:46-52. doi: 10.1016/j.brainres.2011.11.001
74. Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. doi: 10.1136/bmj.e536
75. Carlier J, Huestis MA, Zaami S, et al. Monitoring perinatal exposure to cannabis and synthetic cannabinoids. Ther Drug Monit. 2020;42(2):194-204.
1. Okie S. A floor of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. doi:10.1056/NEJMp1011512
2. Powell D, Pacula RL, Taylor E. How increasing medical access to opioids contributes to the opioid epidemic: evidence from Medicare Part D. J Health Econ. 2020;71:102286. doi: 10.1016/j.jhealeco.2019.102286
3. Manzanares J, Julian MD, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006;4(3):239-257. doi: 10.2174/157015906778019527
4. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833. doi: 10.3390/ijms19030833
5. Huang WJ, Chen WW, Zhang X. Endocannabinoid system: role in depression, reward and pain control (Review). Mol Med Rep. 2016;14(4):2899-2903. doi:10.3892/mmr.2016.5585
6. Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83-90. doi:10.1016/0006-2952(95)00109-d
7. Walker JM, Krey JF, Chu CJ, et al. Endocannabinoids and related fatty acid derivatives in pain modulation. Chem Phys Lipids. 2002;121(1-2):159-172. doi: 10.1016/s0009-3084(02)00152-4
8. Howlett AC. Efficacy in CB1 receptor-mediated signal transduction. Br J Pharmacol. 2004;142(8):1209-1218. doi: 10.1038/sj.bjp.0705881
9. Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation, biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7-14.
10. Piomelli D, Beltramo M, Giuffrida A, et al. Endogenous cannabinoid signaling. Neurobiol Dis. 1998;5(6 Pt B):462-473. doi: 10.1006/nbdi.1998.0221
11. Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17(1):175-191. doi: 10.1093/cercor/bhj136
12. Jennings EA, Vaughan CW, Christie MJ. Cannabinoid actions on rat superficial medullary dorsal horn neurons in vitro. J Physiol. 2001;534(Pt 3):805-812. doi: 10.1111/j.1469-7793.2001.00805.x
13. Vaughan CW, Connor M, Bagley EE, et al. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol. 2000;57(2):288-295.
14. Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol. 1999;127(4):935-940. doi: 10.1038/sj.bjp.0702636
15. Raichlen DA, Foster AD, Gerdeman GI, et al. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner’s high.” J Exp Biol. 2012;215(Pt 8):1331-1336. doi: 10.1242/jeb.063677
16. Beltrano M. Cannabinoid type 2 receptor as a target for chronic pain. Mini Rev Chem. 2009;234:253-254.
17. Ibrahim MM, Deng H, Zvonok A, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100(18):10529-10533. doi: 10.1073/pnas.1834309100
18. Valenzano KJ, Tafessem L, Lee G, et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658-672.
19. Pertwee RG, Howlett AC, Abood ME, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588-631. doi: 10.1124/pr.110.003004
20. Carter GT, Weydt P, Kyashna-Tocha M, et al. Medicinal cannabis: rational guidelines for dosing. Drugs. 2004;7(5):464-470.
21. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804.
22. Johal H, Devji T, Chang Y, et al. cannabinoids in chronic non-cancer pain: a systematic review and meta-analysis. Clin Med Insights Arthritis Musculoskelet Disord. 2020;13:1179544120906461. doi: 10.1177/1179544120906461
23. Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am J Bot. 2004;91(6):966-975. doi: 10.3732/ajb.91.6.966
24. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids--an international cross-sectional survey on administration forms. J Psychoactive Drugs. 2013;45(3):199-210. doi: 10.1080/02791072.2013.805976
25. Andreae MH, Carter GM, Shaparin N, et al. inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain. 2015;16(12):1221-1232. doi: 10.1016/j.jpain.2015.07.009
26. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473. doi: 10.1001/jama.2015.6358
27. Boychuk DG, Goddard G, Mauro G, et al. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29(1):7-14. doi: 10.11607/ofph.1274
28. Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72(5):735-744. doi: 10.1111/j.1365-2125.2011.03970.x
29. Stockings E, Campbell G, Hall WD, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159(10):1932-1954. doi: 10.1097/j.pain.0000000000001293
30. Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3(3):CD012182. doi: 10.1002/14651858.CD012182.pub2
31. Häuser W, Fitzcharles MA, Radbruch L, et al. Cannabinoids in pain management and palliative medicine. Dtsch Arztebl Int. 2017;114(38):627-634. doi: 10.3238/arztebl.2017.0627
32. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. doi: 10.1016/j.jpain.2007.09.005
33. Farrar JT, Troxel AB, Stott C, et al. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther. 2008;30(5):974-985. doi: 10.1016/j.clinthera.2008.05.011
34. Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-335. doi: 10.1155/2014/754693
35. Petzke F, Enax-Krumova EK, Häuser W. Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: a systematic review of randomized controlled studies. Schmerz. 2016;30(1):62-88. doi: 10.1007/s00482-015-0089-y
36. Rice J, Cameron M. Cannabinoids for treatment of MS symptoms: state of the evidence. Curr Neurol Neurosci Rep. 2018;18(8):50. doi: 10.1007/s11910-018-0859-x
37. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556-1563. doi: 10.1212/WNL.0000000000000363
38. Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of Crohn’s disease and ulcerative colitis: evidence from Cochrane Reviews. Inflamm Bowel Dis. 2020;26(4):502-509. doi: 10.1093/ibd/izz233
39. Katz-Talmor D, Katz I, Porat-Katz BS, et al. Cannabinoids for the treatment of rheumatic diseases - where do we stand? Nat Rev Rheumatol. 2018;14(8):488-498. doi: 10.1038/s41584-018-0025-5
40. Walitt B, Klose P, Fitzcharles MA, et al. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016;7(7):CD011694. doi: 10.1002/14651858.CD011694.pub2
41. Bar-Lev Schleider L, Mechoulam R, Lederman V, et al. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur J Intern Med. 2018;49:37‐43. doi: 10.1016/j.ejim.2018.01.023
42. Bennett M, Paice JA, Wallace M. Pain and opioids in cancer care: benefits, risks, and alternatives. Am Soc Clin Oncol Educ Book. 2017;37:705‐713. doi:10.1200/EDBK_180469
43. Blake A, Wan BA, Malek L, et al. A selective review of medical cannabis in cancer pain management. Ann Palliat Med. 2017;6(Suppl 2):5215-5222. doi: 10.21037/apm.2017.08.05
44. Aviram J, Samuelly-Lechtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20(6):E755-E796.
45. Häuser W, Welsch P, Klose P, et al. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain: a systematic review with meta-analysis of randomised controlled trials. Schmerz. 2019;33(5):424-436. doi: 10.1007/s00482-019-0373-3
46. Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010; 39:167-179.
47. Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):438-449. doi: 10.1016/j.jpain.2012.01.003
48. Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014;47(1):166-173. doi: 10.1016/j.jpainsymman.2013.02.018
49. Kleckner AS, Kleckner IR, Kamen CS, et al. Opportunities for cannabis in supportive care in cancer. Ther Adv Med Oncol. 2019;11:1758835919866362. doi: 10.1177/1758835919866362
50. National Conference of State Legislatures (ncsl.org). State Medical Marijuana Laws. Accessed April 5, 2021. https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
51. Hasin DS, Shmulewitz D, Cerda M, et al. US adults with pain, a group increasingly vulnerable to nonmedical cannabis use and cannabis use disorder: 2001-2002 and 2012-2013. Am J Psychiatry. 2020;177(7):611-618. doi: 10.1176/appi.ajp.2019.19030284
52. Hasin DS, Sarvet AL, Cerdá M, et al. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991-1992 to 2012-2013. JAMA Psychiatry. 2017;74(6):579-588. doi: 10.1001/jamapsychiatry.2017.0724
53. National Institute on Drug Abuse. Illicit cannabis use and use disorders increase in states with medical marijuana laws. April 26, 2017. Accessed October 24, 2020. https://archives.drugabuse.gov/news-events/news-releases/2017/04/illicit-cannabis-use-use-disorders-increase-in-states-medical-marijuana-laws
54. National Academies of Sciences, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. The National Academies Press; 2017. https://doi.org/10.17226/24625
55. Stanford M. Physician recommended marijuana: contraindications & standards of care. A review of the literature. Accessed July 7, 2020. http://drneurosci.com/MedicalMarijuanaStandardsofCare.pdf
56. Repp K, Raich A. Marijuana and health: a comprehensive review of 20 years of research. Washington County Oregon Department of Health and Human Services. 2014. Accessed April 8, 2021. https://www.co.washington.or.us/CAO/upload/HHSmarijuana-review.pdf
57. Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Social Adm Pharm. 2016;12(4):638-654. doi: 10.1016/j.sapharm.2015.09.002.
58. Leite RT, Nogueira Sde O, do Nascimento JP, et al. The use of cannabis as a predictor of early onset of bipolar disorder and suicide attempts. Neural Plast. 2015;2015:434127. doi: 10.1155/2015/43412
59. Kim SW, Dodd S, Berk L, et al. Impact of cannabis use on long-term remission in bipolar I and schizoaffective disorder. Psychiatry Investig. 2015;12(3):349-355. doi: 10.4306/pi.2015.12.3.349
60. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(12):995-1010.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064. doi: 10.4088/JCP.15r10036.
62. Woolf CJ, American College of Physicians. American Physiological Society Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441-451.
63. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24(7):515‐523. doi: 10.1002/hup.1048
64. Sachs J, McGlade E, Yurgelun-Todd D. Safety and toxicology of cannabinoids. Neurotherapeutics. 2015;12(4):735‐746. doi: 10.1007/s13311-015-0380-8
65. Antoniou T, Bodkin J, Ho JMW. Drug interactions with cannabinoids. CMAJ. 2020;2;192:E206. doi: 10.1503/cmaj.191097
66. Brown JD. Potential adverse drug events with tetrahydrocannabinol (THC) due to drug-drug interactions. J Clin Med. 2020;9(4):919. doi: 10.3390/jcm9040919.
67. Maida V, Daeninck P. A user’s guide to cannabinoid therapy in oncology. Curr Oncol. 2016;23(6):398-406. doi: http://dx.doi.org/10.3747/co.23.3487
68. Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46(1):86-95. doi: 10.3109/03602532.2013.849268
69. Abrams DI. Integrating cannabis into clinical cancer care. Curr Oncol. 2016;23(52):S8-S14.
70. Alsherbiny MA, Li CG. Medicinal cannabis—potential drug interactions. Medicines. 2018;6(1):3. doi: 10.3390/medicines6010003
71. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84:2477-2482.
72. Ghasemiesfe M, Barrow B, Leonard S, et al. Association between marijuana use and risk of cancer: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(11):e1916318. doi: 10.1001/jamanetworkopen.2019.16318
73. Riggs PK, Vaida F, Rossi SS, et al. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res. 2012;1431:46-52. doi: 10.1016/j.brainres.2011.11.001
74. Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. doi: 10.1136/bmj.e536
75. Carlier J, Huestis MA, Zaami S, et al. Monitoring perinatal exposure to cannabis and synthetic cannabinoids. Ther Drug Monit. 2020;42(2):194-204.