User login
TNF inhibitors linked to threefold increased risk of psoriasis in JIA patients
Children with juvenile idiopathic arthritis (JIA) have nearly triple the risk of developing psoriasis after they begin therapy with tumor necrosis factor (TNF) inhibitors, according to preliminary research shared at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance (CARRA).
Previous retrospective research at the Children’s Hospital of Philadelphia had found similar results, so the goal of this study was to look at prospectively collected data from the CARRA registry that represented a broader patient population than that of a single institution, lead author Yongdong (Dan) Zhao, MD, PhD, assistant professor of rheumatology at the University of Washington, Seattle, and pediatric rheumatologist at Seattle Children’s Hospital, said in an interview.
“The take-home message is that we confirmed this finding, and everyone who prescribed this should be aware [of the risk] and also make the family aware because often the family just thinks this is eczema and they self-manage without reporting it to the physician,” Dr. Zhao said. He advised that physicians look for evidence of psoriasis at visits and, depending on the severity, be prepared with a management plan if needed.
The researchers analyzed data from patients with JIA enrolled in the CARRA registry during June 2015–January 2020. They excluded patients with a diagnosis of inflammatory bowel disease, psoriasis at or before their JIA diagnosis, or missing data regarding the timing of psoriasis diagnosis or starting TNF inhibitors.
Among 8,222 children (29% of whom were male), just over half (54%) had ever used TNF inhibitors. Most of the patients (76%) were White, and their average age at the time of JIA diagnosis was 7 years. Compared to those with no exposure to the drugs, patients who had ever been prescribed a TNF inhibitor were three times more likely to receive a diagnosis of psoriasis afterward (unadjusted hazard ratio [HR] = 3.01; P < .01). The risk dropped only slightly (HR = 2.93; P < .01) after adjustment for gender, race, family history of psoriasis, initial International League of Associations for Rheumatology classification category, and ever having taken methotrexate.
Overall median follow-up time for the cohort was 46.7 months. The overall incidence of psoriasis in the cohort was 5.28 cases per 1,000 person-years, which split into 3.24 cases for those never exposed to TNF inhibitors and 8.49 for those ever exposed. The incidence was similar (8.31 cases per 1,000 person-years) after only the first course of TNF inhibitors.
The risk appeared greatest for adalimumab, with an incidence of 12.2 cases per 1,000 person-years after a first course in TNF inhibitor-naive patients, compared to etanercept (6.31 cases) and infliximab (9.04 cases), which did not reach statistical significance. Incidence for cumulative exposure was greater for adalimumab: 13.17 cases per 1,000 person-years, compared to 5.19 cases for etanercept and 8.77 cases for infliximab.
TNF inhibitors are first-line biologic treatment for JIA and have a longer track record for safety and effectiveness than that of newer drugs, Dr. Zhao said. They’re also commonly used for children with psoriasis, said Pamela Weiss, MD, associate professor of pediatrics and epidemiology, at the University of Pennsylvania, Philadelphia, and clinical research director of rheumatology at Children’s Hospital of Philadelphia. She was not involved in the study.
“TNF inhibitors are an incredibly useful class of medications for children with arthritis, including psoriatic arthritis,” Dr. Weiss said in an interview. “I don’t think these findings impact the risk-benefit profile of TNF inhibitors as paradoxical psoriasis is a known side effect of the medication and something most of us already counsel our families and patients about before starting a TNF inhibitor medication.”
Dr. Zhao likewise did not think the findings changed these drugs’ benefit-risk profile as long as people are aware of it. If the psoriasis is mild, he said, it’s often possible to continue the TNF inhibitor therapy along with a topical medication for the psoriasis, “but if it’s really severe, or by patient preference, you may have to switch to a different TNF inhibitor or stop it,” he said. Occasionally, he has added an additional biologic to treat the psoriasis because the underlying JIA disease in the patient couldn’t be controlled without the TNF inhibitor.
Dr. Weiss similarly said that management will depend on the severity and on shared decision-making between the physician, patient, and family.
“If it’s a small area, it can often be managed with topical corticosteroids,” Dr. Weiss said. “If it involves a large area of the body or severely affects the scalp, then stopping the TNF inhibitor therapy and starting another therapy that targets a different pathway might be considered.”
The research was funded by CARRA. Dr. Zhao has received research funding from Bristol-Myers Squibb and has consulted for Novartis. Dr. Weiss has received consulting fees from Pfizer and Lilly.
Children with juvenile idiopathic arthritis (JIA) have nearly triple the risk of developing psoriasis after they begin therapy with tumor necrosis factor (TNF) inhibitors, according to preliminary research shared at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance (CARRA).
Previous retrospective research at the Children’s Hospital of Philadelphia had found similar results, so the goal of this study was to look at prospectively collected data from the CARRA registry that represented a broader patient population than that of a single institution, lead author Yongdong (Dan) Zhao, MD, PhD, assistant professor of rheumatology at the University of Washington, Seattle, and pediatric rheumatologist at Seattle Children’s Hospital, said in an interview.
“The take-home message is that we confirmed this finding, and everyone who prescribed this should be aware [of the risk] and also make the family aware because often the family just thinks this is eczema and they self-manage without reporting it to the physician,” Dr. Zhao said. He advised that physicians look for evidence of psoriasis at visits and, depending on the severity, be prepared with a management plan if needed.
The researchers analyzed data from patients with JIA enrolled in the CARRA registry during June 2015–January 2020. They excluded patients with a diagnosis of inflammatory bowel disease, psoriasis at or before their JIA diagnosis, or missing data regarding the timing of psoriasis diagnosis or starting TNF inhibitors.
Among 8,222 children (29% of whom were male), just over half (54%) had ever used TNF inhibitors. Most of the patients (76%) were White, and their average age at the time of JIA diagnosis was 7 years. Compared to those with no exposure to the drugs, patients who had ever been prescribed a TNF inhibitor were three times more likely to receive a diagnosis of psoriasis afterward (unadjusted hazard ratio [HR] = 3.01; P < .01). The risk dropped only slightly (HR = 2.93; P < .01) after adjustment for gender, race, family history of psoriasis, initial International League of Associations for Rheumatology classification category, and ever having taken methotrexate.
Overall median follow-up time for the cohort was 46.7 months. The overall incidence of psoriasis in the cohort was 5.28 cases per 1,000 person-years, which split into 3.24 cases for those never exposed to TNF inhibitors and 8.49 for those ever exposed. The incidence was similar (8.31 cases per 1,000 person-years) after only the first course of TNF inhibitors.
The risk appeared greatest for adalimumab, with an incidence of 12.2 cases per 1,000 person-years after a first course in TNF inhibitor-naive patients, compared to etanercept (6.31 cases) and infliximab (9.04 cases), which did not reach statistical significance. Incidence for cumulative exposure was greater for adalimumab: 13.17 cases per 1,000 person-years, compared to 5.19 cases for etanercept and 8.77 cases for infliximab.
TNF inhibitors are first-line biologic treatment for JIA and have a longer track record for safety and effectiveness than that of newer drugs, Dr. Zhao said. They’re also commonly used for children with psoriasis, said Pamela Weiss, MD, associate professor of pediatrics and epidemiology, at the University of Pennsylvania, Philadelphia, and clinical research director of rheumatology at Children’s Hospital of Philadelphia. She was not involved in the study.
“TNF inhibitors are an incredibly useful class of medications for children with arthritis, including psoriatic arthritis,” Dr. Weiss said in an interview. “I don’t think these findings impact the risk-benefit profile of TNF inhibitors as paradoxical psoriasis is a known side effect of the medication and something most of us already counsel our families and patients about before starting a TNF inhibitor medication.”
Dr. Zhao likewise did not think the findings changed these drugs’ benefit-risk profile as long as people are aware of it. If the psoriasis is mild, he said, it’s often possible to continue the TNF inhibitor therapy along with a topical medication for the psoriasis, “but if it’s really severe, or by patient preference, you may have to switch to a different TNF inhibitor or stop it,” he said. Occasionally, he has added an additional biologic to treat the psoriasis because the underlying JIA disease in the patient couldn’t be controlled without the TNF inhibitor.
Dr. Weiss similarly said that management will depend on the severity and on shared decision-making between the physician, patient, and family.
“If it’s a small area, it can often be managed with topical corticosteroids,” Dr. Weiss said. “If it involves a large area of the body or severely affects the scalp, then stopping the TNF inhibitor therapy and starting another therapy that targets a different pathway might be considered.”
The research was funded by CARRA. Dr. Zhao has received research funding from Bristol-Myers Squibb and has consulted for Novartis. Dr. Weiss has received consulting fees from Pfizer and Lilly.
Children with juvenile idiopathic arthritis (JIA) have nearly triple the risk of developing psoriasis after they begin therapy with tumor necrosis factor (TNF) inhibitors, according to preliminary research shared at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance (CARRA).
Previous retrospective research at the Children’s Hospital of Philadelphia had found similar results, so the goal of this study was to look at prospectively collected data from the CARRA registry that represented a broader patient population than that of a single institution, lead author Yongdong (Dan) Zhao, MD, PhD, assistant professor of rheumatology at the University of Washington, Seattle, and pediatric rheumatologist at Seattle Children’s Hospital, said in an interview.
“The take-home message is that we confirmed this finding, and everyone who prescribed this should be aware [of the risk] and also make the family aware because often the family just thinks this is eczema and they self-manage without reporting it to the physician,” Dr. Zhao said. He advised that physicians look for evidence of psoriasis at visits and, depending on the severity, be prepared with a management plan if needed.
The researchers analyzed data from patients with JIA enrolled in the CARRA registry during June 2015–January 2020. They excluded patients with a diagnosis of inflammatory bowel disease, psoriasis at or before their JIA diagnosis, or missing data regarding the timing of psoriasis diagnosis or starting TNF inhibitors.
Among 8,222 children (29% of whom were male), just over half (54%) had ever used TNF inhibitors. Most of the patients (76%) were White, and their average age at the time of JIA diagnosis was 7 years. Compared to those with no exposure to the drugs, patients who had ever been prescribed a TNF inhibitor were three times more likely to receive a diagnosis of psoriasis afterward (unadjusted hazard ratio [HR] = 3.01; P < .01). The risk dropped only slightly (HR = 2.93; P < .01) after adjustment for gender, race, family history of psoriasis, initial International League of Associations for Rheumatology classification category, and ever having taken methotrexate.
Overall median follow-up time for the cohort was 46.7 months. The overall incidence of psoriasis in the cohort was 5.28 cases per 1,000 person-years, which split into 3.24 cases for those never exposed to TNF inhibitors and 8.49 for those ever exposed. The incidence was similar (8.31 cases per 1,000 person-years) after only the first course of TNF inhibitors.
The risk appeared greatest for adalimumab, with an incidence of 12.2 cases per 1,000 person-years after a first course in TNF inhibitor-naive patients, compared to etanercept (6.31 cases) and infliximab (9.04 cases), which did not reach statistical significance. Incidence for cumulative exposure was greater for adalimumab: 13.17 cases per 1,000 person-years, compared to 5.19 cases for etanercept and 8.77 cases for infliximab.
TNF inhibitors are first-line biologic treatment for JIA and have a longer track record for safety and effectiveness than that of newer drugs, Dr. Zhao said. They’re also commonly used for children with psoriasis, said Pamela Weiss, MD, associate professor of pediatrics and epidemiology, at the University of Pennsylvania, Philadelphia, and clinical research director of rheumatology at Children’s Hospital of Philadelphia. She was not involved in the study.
“TNF inhibitors are an incredibly useful class of medications for children with arthritis, including psoriatic arthritis,” Dr. Weiss said in an interview. “I don’t think these findings impact the risk-benefit profile of TNF inhibitors as paradoxical psoriasis is a known side effect of the medication and something most of us already counsel our families and patients about before starting a TNF inhibitor medication.”
Dr. Zhao likewise did not think the findings changed these drugs’ benefit-risk profile as long as people are aware of it. If the psoriasis is mild, he said, it’s often possible to continue the TNF inhibitor therapy along with a topical medication for the psoriasis, “but if it’s really severe, or by patient preference, you may have to switch to a different TNF inhibitor or stop it,” he said. Occasionally, he has added an additional biologic to treat the psoriasis because the underlying JIA disease in the patient couldn’t be controlled without the TNF inhibitor.
Dr. Weiss similarly said that management will depend on the severity and on shared decision-making between the physician, patient, and family.
“If it’s a small area, it can often be managed with topical corticosteroids,” Dr. Weiss said. “If it involves a large area of the body or severely affects the scalp, then stopping the TNF inhibitor therapy and starting another therapy that targets a different pathway might be considered.”
The research was funded by CARRA. Dr. Zhao has received research funding from Bristol-Myers Squibb and has consulted for Novartis. Dr. Weiss has received consulting fees from Pfizer and Lilly.
FROM CARRA 2021
USPSTF reaffirms advice to screen all adults for hypertension
The U.S. Preventive Services Task Force continues to recommend that clinicians screen all adults aged 18 years and older for high blood pressure and that they confirm a diagnosis of hypertension with blood pressure measurements taken outside the office before starting treatment.
This grade A recommendation is consistent with the 2015 recommendation from the task force.
Hypertension affects approximately 45% of adults in the United States and is a major contributing risk factor for heart failure, myocardial infarction, stroke, and chronic kidney disease.
Using a reaffirmation deliberation process, the USPSTF concluded with high certainty that there was “substantial net benefit” from screening adults for hypertension in clinical office settings.
The reaffirmation recommendation clarifies that initial screening should be performed with office-based blood pressure measurement.
The task force found “convincing” evidence that screening for and treatment of hypertension detected in clinical office settings substantially reduces cardiovascular events and have few major harms.
To confirm a diagnosis of hypertension outside the office before starting treatment, ambulatory blood pressure monitoring or home blood pressure monitoring is recommended. Blood pressure measurements should be taken at the brachial artery with a validated and accurate device in a seated position after 5 minutes of rest.
Although evidence regarding optimal screening intervals is limited, the task force says “reasonable” options include screening for hypertension every year for adults aged 40 years or older and for adults who are at increased risk for hypertension, such as Black persons, persons with high-normal blood pressure, or those who are overweight or obese.
Screening less frequently (every 3-5 years) is appropriate for adults aged 18-39 years who are not at increased risk for hypertension and who have received a prior blood pressure reading that was in the normal range, said the task force, led by Alex Krist, MD, MPH, Virginia Commonwealth University, Richmond.
The recommendation and supporting evidence report were published online April 27, 2021, in JAMA.
‘Screening is just the first step’
In a JAMA editorial, Marwah Abdalla, MD, MPH, Columbia University Irving Medical Center, New York, and coauthors said the COVID-19 pandemic has demonstrated that “rapid and significant innovation in science, health care, and society is possible. Implementing the latest USPSTF recommendations will require widespread changes to how the health care system and other entities screen for hypertension.
“Yet screening is just the first step in a long road to controlling hypertension. Medicine and society need to implement a variety of interventions proven to be effective in controlling blood pressure at scale,” the editorialists said.
“Additionally, these efforts need to consider how to achieve success for all people. This will require working to address the roots of structural racism and reduce the racial disparities that increase hypertension-related morbidity and mortality for vulnerable populations,” they added.
“These changes will take innovation in how care delivery is provided at both the individual and population levels – lessons the health care system and society learned are achievable through the response to the COVID-19 pandemic,” Dr. Abdalla and colleagues concluded.
The USPSTF and Dr. Abdalla reported no relevant financial relationships. One editorialist reported receiving personal fees from Livongo and Cerner and grants from Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force continues to recommend that clinicians screen all adults aged 18 years and older for high blood pressure and that they confirm a diagnosis of hypertension with blood pressure measurements taken outside the office before starting treatment.
This grade A recommendation is consistent with the 2015 recommendation from the task force.
Hypertension affects approximately 45% of adults in the United States and is a major contributing risk factor for heart failure, myocardial infarction, stroke, and chronic kidney disease.
Using a reaffirmation deliberation process, the USPSTF concluded with high certainty that there was “substantial net benefit” from screening adults for hypertension in clinical office settings.
The reaffirmation recommendation clarifies that initial screening should be performed with office-based blood pressure measurement.
The task force found “convincing” evidence that screening for and treatment of hypertension detected in clinical office settings substantially reduces cardiovascular events and have few major harms.
To confirm a diagnosis of hypertension outside the office before starting treatment, ambulatory blood pressure monitoring or home blood pressure monitoring is recommended. Blood pressure measurements should be taken at the brachial artery with a validated and accurate device in a seated position after 5 minutes of rest.
Although evidence regarding optimal screening intervals is limited, the task force says “reasonable” options include screening for hypertension every year for adults aged 40 years or older and for adults who are at increased risk for hypertension, such as Black persons, persons with high-normal blood pressure, or those who are overweight or obese.
Screening less frequently (every 3-5 years) is appropriate for adults aged 18-39 years who are not at increased risk for hypertension and who have received a prior blood pressure reading that was in the normal range, said the task force, led by Alex Krist, MD, MPH, Virginia Commonwealth University, Richmond.
The recommendation and supporting evidence report were published online April 27, 2021, in JAMA.
‘Screening is just the first step’
In a JAMA editorial, Marwah Abdalla, MD, MPH, Columbia University Irving Medical Center, New York, and coauthors said the COVID-19 pandemic has demonstrated that “rapid and significant innovation in science, health care, and society is possible. Implementing the latest USPSTF recommendations will require widespread changes to how the health care system and other entities screen for hypertension.
“Yet screening is just the first step in a long road to controlling hypertension. Medicine and society need to implement a variety of interventions proven to be effective in controlling blood pressure at scale,” the editorialists said.
“Additionally, these efforts need to consider how to achieve success for all people. This will require working to address the roots of structural racism and reduce the racial disparities that increase hypertension-related morbidity and mortality for vulnerable populations,” they added.
“These changes will take innovation in how care delivery is provided at both the individual and population levels – lessons the health care system and society learned are achievable through the response to the COVID-19 pandemic,” Dr. Abdalla and colleagues concluded.
The USPSTF and Dr. Abdalla reported no relevant financial relationships. One editorialist reported receiving personal fees from Livongo and Cerner and grants from Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force continues to recommend that clinicians screen all adults aged 18 years and older for high blood pressure and that they confirm a diagnosis of hypertension with blood pressure measurements taken outside the office before starting treatment.
This grade A recommendation is consistent with the 2015 recommendation from the task force.
Hypertension affects approximately 45% of adults in the United States and is a major contributing risk factor for heart failure, myocardial infarction, stroke, and chronic kidney disease.
Using a reaffirmation deliberation process, the USPSTF concluded with high certainty that there was “substantial net benefit” from screening adults for hypertension in clinical office settings.
The reaffirmation recommendation clarifies that initial screening should be performed with office-based blood pressure measurement.
The task force found “convincing” evidence that screening for and treatment of hypertension detected in clinical office settings substantially reduces cardiovascular events and have few major harms.
To confirm a diagnosis of hypertension outside the office before starting treatment, ambulatory blood pressure monitoring or home blood pressure monitoring is recommended. Blood pressure measurements should be taken at the brachial artery with a validated and accurate device in a seated position after 5 minutes of rest.
Although evidence regarding optimal screening intervals is limited, the task force says “reasonable” options include screening for hypertension every year for adults aged 40 years or older and for adults who are at increased risk for hypertension, such as Black persons, persons with high-normal blood pressure, or those who are overweight or obese.
Screening less frequently (every 3-5 years) is appropriate for adults aged 18-39 years who are not at increased risk for hypertension and who have received a prior blood pressure reading that was in the normal range, said the task force, led by Alex Krist, MD, MPH, Virginia Commonwealth University, Richmond.
The recommendation and supporting evidence report were published online April 27, 2021, in JAMA.
‘Screening is just the first step’
In a JAMA editorial, Marwah Abdalla, MD, MPH, Columbia University Irving Medical Center, New York, and coauthors said the COVID-19 pandemic has demonstrated that “rapid and significant innovation in science, health care, and society is possible. Implementing the latest USPSTF recommendations will require widespread changes to how the health care system and other entities screen for hypertension.
“Yet screening is just the first step in a long road to controlling hypertension. Medicine and society need to implement a variety of interventions proven to be effective in controlling blood pressure at scale,” the editorialists said.
“Additionally, these efforts need to consider how to achieve success for all people. This will require working to address the roots of structural racism and reduce the racial disparities that increase hypertension-related morbidity and mortality for vulnerable populations,” they added.
“These changes will take innovation in how care delivery is provided at both the individual and population levels – lessons the health care system and society learned are achievable through the response to the COVID-19 pandemic,” Dr. Abdalla and colleagues concluded.
The USPSTF and Dr. Abdalla reported no relevant financial relationships. One editorialist reported receiving personal fees from Livongo and Cerner and grants from Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
Perimenopausal woman with adnexal mass
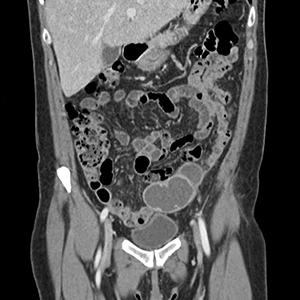
The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease (PID).
PID is an acute infection of the upper genital tract in women that is thought to be due to an ascending infection from the lower genital tract. Diagnosis of PID in middle-aged women is a challenge, given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. Delay in diagnosis in postmenopausal women can pose serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease.
The Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness. The CDC also suggests that the most specific criteria for PID include endometrial biopsy consistent with endometritis, imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or laparoscopic findings consistent with PID.
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.
This patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge. She made a full recovery and is doing well.
This case was adapted from: Khoo CP. Fever, abdominal pain, and adnexal mass. J Fam Pract. 2020;69:101-103

The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease (PID).
PID is an acute infection of the upper genital tract in women that is thought to be due to an ascending infection from the lower genital tract. Diagnosis of PID in middle-aged women is a challenge, given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. Delay in diagnosis in postmenopausal women can pose serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease.
The Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness. The CDC also suggests that the most specific criteria for PID include endometrial biopsy consistent with endometritis, imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or laparoscopic findings consistent with PID.
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.
This patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge. She made a full recovery and is doing well.
This case was adapted from: Khoo CP. Fever, abdominal pain, and adnexal mass. J Fam Pract. 2020;69:101-103

The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease (PID).
PID is an acute infection of the upper genital tract in women that is thought to be due to an ascending infection from the lower genital tract. Diagnosis of PID in middle-aged women is a challenge, given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. Delay in diagnosis in postmenopausal women can pose serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease.
The Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness. The CDC also suggests that the most specific criteria for PID include endometrial biopsy consistent with endometritis, imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or laparoscopic findings consistent with PID.
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.
This patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge. She made a full recovery and is doing well.
This case was adapted from: Khoo CP. Fever, abdominal pain, and adnexal mass. J Fam Pract. 2020;69:101-103
AHA statement flags CV risk of hormonal cancer therapies
Hormonal therapies for the treatment of hormone-dependent breast and prostate cancer could raise the risk for myocardial infarction and stroke, and patients need to be closely monitored to allow early detection and treatment of cardiovascular disease (CVD), the American Heart Association says in a new scientific statement.
“The statement provides data on the risks of each type of hormonal therapy so clinicians can use it as a guide to help manage cardiovascular risks during cancer treatment,” Tochi Okwuosa, DO, chair of the writing group, said in a news release.
“A team-based approach to patient care that includes the oncology team, cardiologist, primary care clinician, dietitian, endocrinologist, and other health care professionals as appropriate is needed to work with each patient to manage and reduce the increased risk of heart disease and strokes associated with hormonal therapy in breast and prostate cancer treatment,” said Dr. Okwuosa, director of cardio-oncology services, Rush University Medical Center, Chicago.
The scientific statement was published online April 26 in Circulation: Genomic and Precision Medicine.
Hormone-dependent cancers, such as prostate and breast cancer, are the most common noncutaneous cancers in the United States and around the world. As hormonal therapies have markedly improved survival in these patients, CVD has emerged as a leading cause illness and death.
The increased CVD burden might be explained by the increasing average age of cancer survivors, leading to higher rates of age-related CV risk factors and coronary artery disease.
The writing group reviewed existing evidence from observational studies and randomized controlled trials on the cardiovascular impact of anticancer hormonal therapies.
Among the key findings:
- In patients with breast cancer, has been shown to increase the risk for venous thromboembolic events, but to have somewhat protective to neutral effects on CVD risk burden and CVD events. Conversely, aromatase inhibitors have been shown to increase the risk for CVD risk factors and events, including MI and stroke.
- Androgen-deprivation therapy for prostate cancer appears to increase the risk for CV events, although gonadotrophin-releasing hormone (GnRH) antagonists are associated with a lower risk for CV events than are GnRH agonists. The oral antiandrogens appear to be associated with increased CVD risk as well, particularly when used for complete androgen blockade as combination GnRH/anti-androgen therapy.
- The duration of hormonal therapies has a significant impact on CVD risk; the longer patients receive hormonal therapy, the greater the risk. More research is needed to better define the risks associated with duration of treatment.
- The data are mixed on the impact of preexisting CV risk factors and CVD on CV events associated with hormonal therapy. Although the presence of baseline CV risk factors and CVD can increase CV events associated with aromatase inhibitors, it is not clear that tamoxifen does.
- Studies suggest that patients with prostate cancer and baseline CVD and CV risk factors have increased rates of CV events when treated with androgen-deprivation therapy.
- Although the prolonged use of some hormonal therapies worsens CV risk factors and , the effects of the duration of therapy on CV events are less clear.
The writing group noted that there are no definitive guidelines for the monitoring and management of hormonal therapy-related CVD risks.
The authors encourage clinicians to be alert for worsening CV problems in those with preexisting heart disease or risk factors, and to recognize that even patients without preexisting CV problems are at higher risk because of their exposure to hormonal therapies.
“For patients who have two or more cardiovascular risk factors, it is likely that referral to a cardiologist would be appropriate prior to beginning hormone treatment. For patients already receiving hormonal therapies, a discussion with the oncology team can help to determine if a cardiology referral is recommended,” Dr. Okwuosa said in the news release.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardio-Oncology Subcommittee of the Council on Clinical Cardiology and the Council on Genomic and Precision Medicine; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Council on Cardiovascular Radiology and Intervention.
The research had no commercial funding. Dr. Okwuosa has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hormonal therapies for the treatment of hormone-dependent breast and prostate cancer could raise the risk for myocardial infarction and stroke, and patients need to be closely monitored to allow early detection and treatment of cardiovascular disease (CVD), the American Heart Association says in a new scientific statement.
“The statement provides data on the risks of each type of hormonal therapy so clinicians can use it as a guide to help manage cardiovascular risks during cancer treatment,” Tochi Okwuosa, DO, chair of the writing group, said in a news release.
“A team-based approach to patient care that includes the oncology team, cardiologist, primary care clinician, dietitian, endocrinologist, and other health care professionals as appropriate is needed to work with each patient to manage and reduce the increased risk of heart disease and strokes associated with hormonal therapy in breast and prostate cancer treatment,” said Dr. Okwuosa, director of cardio-oncology services, Rush University Medical Center, Chicago.
The scientific statement was published online April 26 in Circulation: Genomic and Precision Medicine.
Hormone-dependent cancers, such as prostate and breast cancer, are the most common noncutaneous cancers in the United States and around the world. As hormonal therapies have markedly improved survival in these patients, CVD has emerged as a leading cause illness and death.
The increased CVD burden might be explained by the increasing average age of cancer survivors, leading to higher rates of age-related CV risk factors and coronary artery disease.
The writing group reviewed existing evidence from observational studies and randomized controlled trials on the cardiovascular impact of anticancer hormonal therapies.
Among the key findings:
- In patients with breast cancer, has been shown to increase the risk for venous thromboembolic events, but to have somewhat protective to neutral effects on CVD risk burden and CVD events. Conversely, aromatase inhibitors have been shown to increase the risk for CVD risk factors and events, including MI and stroke.
- Androgen-deprivation therapy for prostate cancer appears to increase the risk for CV events, although gonadotrophin-releasing hormone (GnRH) antagonists are associated with a lower risk for CV events than are GnRH agonists. The oral antiandrogens appear to be associated with increased CVD risk as well, particularly when used for complete androgen blockade as combination GnRH/anti-androgen therapy.
- The duration of hormonal therapies has a significant impact on CVD risk; the longer patients receive hormonal therapy, the greater the risk. More research is needed to better define the risks associated with duration of treatment.
- The data are mixed on the impact of preexisting CV risk factors and CVD on CV events associated with hormonal therapy. Although the presence of baseline CV risk factors and CVD can increase CV events associated with aromatase inhibitors, it is not clear that tamoxifen does.
- Studies suggest that patients with prostate cancer and baseline CVD and CV risk factors have increased rates of CV events when treated with androgen-deprivation therapy.
- Although the prolonged use of some hormonal therapies worsens CV risk factors and , the effects of the duration of therapy on CV events are less clear.
The writing group noted that there are no definitive guidelines for the monitoring and management of hormonal therapy-related CVD risks.
The authors encourage clinicians to be alert for worsening CV problems in those with preexisting heart disease or risk factors, and to recognize that even patients without preexisting CV problems are at higher risk because of their exposure to hormonal therapies.
“For patients who have two or more cardiovascular risk factors, it is likely that referral to a cardiologist would be appropriate prior to beginning hormone treatment. For patients already receiving hormonal therapies, a discussion with the oncology team can help to determine if a cardiology referral is recommended,” Dr. Okwuosa said in the news release.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardio-Oncology Subcommittee of the Council on Clinical Cardiology and the Council on Genomic and Precision Medicine; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Council on Cardiovascular Radiology and Intervention.
The research had no commercial funding. Dr. Okwuosa has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hormonal therapies for the treatment of hormone-dependent breast and prostate cancer could raise the risk for myocardial infarction and stroke, and patients need to be closely monitored to allow early detection and treatment of cardiovascular disease (CVD), the American Heart Association says in a new scientific statement.
“The statement provides data on the risks of each type of hormonal therapy so clinicians can use it as a guide to help manage cardiovascular risks during cancer treatment,” Tochi Okwuosa, DO, chair of the writing group, said in a news release.
“A team-based approach to patient care that includes the oncology team, cardiologist, primary care clinician, dietitian, endocrinologist, and other health care professionals as appropriate is needed to work with each patient to manage and reduce the increased risk of heart disease and strokes associated with hormonal therapy in breast and prostate cancer treatment,” said Dr. Okwuosa, director of cardio-oncology services, Rush University Medical Center, Chicago.
The scientific statement was published online April 26 in Circulation: Genomic and Precision Medicine.
Hormone-dependent cancers, such as prostate and breast cancer, are the most common noncutaneous cancers in the United States and around the world. As hormonal therapies have markedly improved survival in these patients, CVD has emerged as a leading cause illness and death.
The increased CVD burden might be explained by the increasing average age of cancer survivors, leading to higher rates of age-related CV risk factors and coronary artery disease.
The writing group reviewed existing evidence from observational studies and randomized controlled trials on the cardiovascular impact of anticancer hormonal therapies.
Among the key findings:
- In patients with breast cancer, has been shown to increase the risk for venous thromboembolic events, but to have somewhat protective to neutral effects on CVD risk burden and CVD events. Conversely, aromatase inhibitors have been shown to increase the risk for CVD risk factors and events, including MI and stroke.
- Androgen-deprivation therapy for prostate cancer appears to increase the risk for CV events, although gonadotrophin-releasing hormone (GnRH) antagonists are associated with a lower risk for CV events than are GnRH agonists. The oral antiandrogens appear to be associated with increased CVD risk as well, particularly when used for complete androgen blockade as combination GnRH/anti-androgen therapy.
- The duration of hormonal therapies has a significant impact on CVD risk; the longer patients receive hormonal therapy, the greater the risk. More research is needed to better define the risks associated with duration of treatment.
- The data are mixed on the impact of preexisting CV risk factors and CVD on CV events associated with hormonal therapy. Although the presence of baseline CV risk factors and CVD can increase CV events associated with aromatase inhibitors, it is not clear that tamoxifen does.
- Studies suggest that patients with prostate cancer and baseline CVD and CV risk factors have increased rates of CV events when treated with androgen-deprivation therapy.
- Although the prolonged use of some hormonal therapies worsens CV risk factors and , the effects of the duration of therapy on CV events are less clear.
The writing group noted that there are no definitive guidelines for the monitoring and management of hormonal therapy-related CVD risks.
The authors encourage clinicians to be alert for worsening CV problems in those with preexisting heart disease or risk factors, and to recognize that even patients without preexisting CV problems are at higher risk because of their exposure to hormonal therapies.
“For patients who have two or more cardiovascular risk factors, it is likely that referral to a cardiologist would be appropriate prior to beginning hormone treatment. For patients already receiving hormonal therapies, a discussion with the oncology team can help to determine if a cardiology referral is recommended,” Dr. Okwuosa said in the news release.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardio-Oncology Subcommittee of the Council on Clinical Cardiology and the Council on Genomic and Precision Medicine; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Council on Cardiovascular Radiology and Intervention.
The research had no commercial funding. Dr. Okwuosa has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Novel BRAF-inhibitor cream ameliorates rash from EGFR inhibitors
The results come from a first-in-human, phase 1 clinical trial conducted in 10 patients with metastatic colorectal cancer who were receiving treatment with either cetuximab or panitumumab and who developed a grade 1 or grade 2 rash while on treatment.
All were treated with the novel topical cream, dubbed LUTO14 (under development by Lutris Pharma).
For 6 of the 10 patients, the acneiform rash improved, according to investigator Mario Lacouture, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues.
The study was published online in Cancer Discovery.
“Based on preclinical modeling and early clinical trial testing, we conclude that improving a topmost adverse event of EGFR inhibitor therapy with topical LUT014 could allow [maintenance of] quality of life and dose intensity, thereby maximizing the antitumor effects [from EGFR inhibitor therapy] while locally inhibiting dose-limiting skin toxicities,” the investigators wrote.
The cream was well tolerated, and no dose-limiting toxicity or maximum tolerated dose was observed, although the cream did appear to be more effective at lower doses.
Rash is a common side effect of EGFR inhibitors. Previous studies have reported that 75%-90% of patients experience “some form of papulopustular, acneiform rash, which frequently leads to ... suboptimal anticancer treatment due to treatment interruptions, dose reductions, or permanent discontinuation of EGFR inhibitor therapy,” the investigators noted.
Paradoxical mechanism of action
How the novel cream containing a BRAF inhibitor helps ameliorate EGFR inhibitor–induced skin toxicity is complicated, but at a cellular level, the mechanism seems somewhat paradoxical, the team commented.
Skin toxicity experienced in the setting of EGFR inhibitor therapy is induced by inhibition of the mitogen-activated protein kinase (MAPK) pathway. Downstream inhibition of the MAPK pathway results in, among other effects, inflammatory changes in epithelial cells that mediate the acneiform rash on the skin.
In contrast, “BRAF inhibitors given systemically have an opposite effect on epithelial cells, resulting in paradoxical activation of the MAPK pathway,” the authors explained. They hypothesized that topical administration of BRAF inhibitors similarly activates the MAPK pathway in epithelial cells, although it was important to develop a specific BRAF inhibitor that would optimally induce paradoxical MAPK activation. That they managed to do so was shown when they evaluated LUT014 in cell culture systems.
The next phase of the study is designed to include approximately 120 patients recruited from centers in the United States and Israel. Interim results are expected by the end of 2021.
The study was funded by Lutris Pharma, the company developing LUT014.
A version of this article first appeared on Medscape.com.
The results come from a first-in-human, phase 1 clinical trial conducted in 10 patients with metastatic colorectal cancer who were receiving treatment with either cetuximab or panitumumab and who developed a grade 1 or grade 2 rash while on treatment.
All were treated with the novel topical cream, dubbed LUTO14 (under development by Lutris Pharma).
For 6 of the 10 patients, the acneiform rash improved, according to investigator Mario Lacouture, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues.
The study was published online in Cancer Discovery.
“Based on preclinical modeling and early clinical trial testing, we conclude that improving a topmost adverse event of EGFR inhibitor therapy with topical LUT014 could allow [maintenance of] quality of life and dose intensity, thereby maximizing the antitumor effects [from EGFR inhibitor therapy] while locally inhibiting dose-limiting skin toxicities,” the investigators wrote.
The cream was well tolerated, and no dose-limiting toxicity or maximum tolerated dose was observed, although the cream did appear to be more effective at lower doses.
Rash is a common side effect of EGFR inhibitors. Previous studies have reported that 75%-90% of patients experience “some form of papulopustular, acneiform rash, which frequently leads to ... suboptimal anticancer treatment due to treatment interruptions, dose reductions, or permanent discontinuation of EGFR inhibitor therapy,” the investigators noted.
Paradoxical mechanism of action
How the novel cream containing a BRAF inhibitor helps ameliorate EGFR inhibitor–induced skin toxicity is complicated, but at a cellular level, the mechanism seems somewhat paradoxical, the team commented.
Skin toxicity experienced in the setting of EGFR inhibitor therapy is induced by inhibition of the mitogen-activated protein kinase (MAPK) pathway. Downstream inhibition of the MAPK pathway results in, among other effects, inflammatory changes in epithelial cells that mediate the acneiform rash on the skin.
In contrast, “BRAF inhibitors given systemically have an opposite effect on epithelial cells, resulting in paradoxical activation of the MAPK pathway,” the authors explained. They hypothesized that topical administration of BRAF inhibitors similarly activates the MAPK pathway in epithelial cells, although it was important to develop a specific BRAF inhibitor that would optimally induce paradoxical MAPK activation. That they managed to do so was shown when they evaluated LUT014 in cell culture systems.
The next phase of the study is designed to include approximately 120 patients recruited from centers in the United States and Israel. Interim results are expected by the end of 2021.
The study was funded by Lutris Pharma, the company developing LUT014.
A version of this article first appeared on Medscape.com.
The results come from a first-in-human, phase 1 clinical trial conducted in 10 patients with metastatic colorectal cancer who were receiving treatment with either cetuximab or panitumumab and who developed a grade 1 or grade 2 rash while on treatment.
All were treated with the novel topical cream, dubbed LUTO14 (under development by Lutris Pharma).
For 6 of the 10 patients, the acneiform rash improved, according to investigator Mario Lacouture, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues.
The study was published online in Cancer Discovery.
“Based on preclinical modeling and early clinical trial testing, we conclude that improving a topmost adverse event of EGFR inhibitor therapy with topical LUT014 could allow [maintenance of] quality of life and dose intensity, thereby maximizing the antitumor effects [from EGFR inhibitor therapy] while locally inhibiting dose-limiting skin toxicities,” the investigators wrote.
The cream was well tolerated, and no dose-limiting toxicity or maximum tolerated dose was observed, although the cream did appear to be more effective at lower doses.
Rash is a common side effect of EGFR inhibitors. Previous studies have reported that 75%-90% of patients experience “some form of papulopustular, acneiform rash, which frequently leads to ... suboptimal anticancer treatment due to treatment interruptions, dose reductions, or permanent discontinuation of EGFR inhibitor therapy,” the investigators noted.
Paradoxical mechanism of action
How the novel cream containing a BRAF inhibitor helps ameliorate EGFR inhibitor–induced skin toxicity is complicated, but at a cellular level, the mechanism seems somewhat paradoxical, the team commented.
Skin toxicity experienced in the setting of EGFR inhibitor therapy is induced by inhibition of the mitogen-activated protein kinase (MAPK) pathway. Downstream inhibition of the MAPK pathway results in, among other effects, inflammatory changes in epithelial cells that mediate the acneiform rash on the skin.
In contrast, “BRAF inhibitors given systemically have an opposite effect on epithelial cells, resulting in paradoxical activation of the MAPK pathway,” the authors explained. They hypothesized that topical administration of BRAF inhibitors similarly activates the MAPK pathway in epithelial cells, although it was important to develop a specific BRAF inhibitor that would optimally induce paradoxical MAPK activation. That they managed to do so was shown when they evaluated LUT014 in cell culture systems.
The next phase of the study is designed to include approximately 120 patients recruited from centers in the United States and Israel. Interim results are expected by the end of 2021.
The study was funded by Lutris Pharma, the company developing LUT014.
A version of this article first appeared on Medscape.com.
Drinking your way to heart failure, and the fringe benefits of COVID-19 vaccination
Energy drink doom
Who doesn’t need some caffeine to get going in the morning and keep moving throughout the day? Whether it’s tea, coffee, or energy drinks, people can get addicted to caffeinated beverages when there are only so many hours in a day and way too much work to get done.
That’s what happened to a 21-year-old college student who powered down four 16-ounce cans of energy drink – each with double the amount of caffeine in an ordinary cup of coffee – every day for 2 years. Now, if you’ve ever overdone it with caffeine, you know there are some uncomfortable side effects, like shaking and anxiety. In this case, the student reported migraines, tremors, and heart palpitations. Instead of being able to focus better on his work, he had trouble concentrating.
Over time, after these side effects took a turn for the worse and became shortness of breath and weight loss, he visited St. Thomas’ Hospital in London, where physicians diagnosed him with both heart and renal failure.
Excessive consumption of energy drinks is known to cause issues such as high blood pressure and irregular heart beat, so if that’s your fuel of choice, it might be worth cutting down. Maybe take a morning run to get the blood pumping – in a good way – instead?
Loneliness may be hazardous to your health
Sometimes loneliness can feel like it affects your physical health, but according to a study there’s a possibility that it actually does.
Back in the 1980s, researchers from the University of Eastern Finland started monitoring almost 3,000 middle-aged men. They’ve kept up with the participants until the present day, and the results have been staggering. After an average follow-up of over 20 years, 25% of participants developed cancer and 11% died from cancer, and the increase in risk from loneliness was about 10%, regardless of age, lifestyle, and BMI.
What does that say about preventive care? The researchers think these data are cause enough to pay attention to loneliness as a health issue along with smoking and weight.
Social interactions and relationships play important roles in human mental health, of course, but this is pretty solid evidence that they play a role in physical health too. As the researchers said, “Awareness of the health effects of loneliness is constantly increasing. Therefore, it is important to examine, in more detail, the mechanisms by which loneliness causes adverse health effects.”
So, as we progress through this pandemic, maybe you should join that social group on Facebook? Who knows what kind of effect it could have on your health?
An ounce of prevention is worth 12 ounces of lager
COVID-19 vaccine refusal is now a thing, and there’s no law that says people have to be immunized against our newest, bestest buddy, SARS-CoV-2, but the folks who skip it are missing out. And no, we’re not talking about immunity against disease.
We’re talking … FREE STUFF!
Corporate America has stepped up and is now rewarding those who get the COVID-19 vaccine:
- Budweiser will give a free beer to anyone – anyone over age 21, that is – with proof of vaccination until May 16.
- Show a vaccination card at a Krispy Kreme and you can get a free glazed doughnut, every day. You don’t even need to buy anything.
- White Castle will give you a free dessert-on-a-stick just for showing proof of vaccination. No purchase is required, but the offer ends May 31.
But wait, there’s more!
Even the public sector is getting in on the giveaway action. Gov. Jim Justice announced April 26 that West Virginia will give a $100 savings bond to any resident aged 16-35 years who receives a COVID-19 vaccine. It must make sense, because the governor broke out a white board to show residents he’s done the math.
One closing thought: How cool would it be if he was named to the Supreme Court, so he could be Justice Justice?
Where no shirt has gone before
Space. The final frontier, for both humanity and for shirts. Specifically, it’s a new frontier for the Bio-Monitor smart shirt, a tank-top filled with sensors that monitor the wearer’s stats, such as heart and breathing rate, oxygen saturation, skin temperature, and blood pressure. And you thought space was just for finding a new human habitat and growing steak.
This shirt is already used by athletes to assess performance and by people with limited mobility to monitor health, so its potential impending usage by astronauts makes sense. Space is a pretty extreme environment, to put it mildly, and there’s a lot we still don’t know about how the human body reacts to it. Traditionally, astronauts hook themselves up to separate devices so their stats can be measured, a method which captures only snapshots of their bodies. By wearing the shirt constantly, the astronauts can be measured constantly, so scientists and doctors can see how the body deals with microgravity during normal activities and sleep. It also reduces stress, as there is no psychological impact of having to report in for constant health checks.
For the test, astronauts wore the shirt for 72 hours before flight and for 72 hours during flight. The shirts passed this first test with flying colors; in addition to providing accurate and more consistent stats monitoring than traditional methods, scientists on the ground determined that the astronauts recorded far less physical activity during flight than preflight, a finding in line with previous studies.
And before you question whether or not a tank top is really appropriate for space, just remember, Picard pulled it off at the end of “First Contact,” and that’s arguably the best Star Trek movie. So there’s certainly precedent.
Energy drink doom
Who doesn’t need some caffeine to get going in the morning and keep moving throughout the day? Whether it’s tea, coffee, or energy drinks, people can get addicted to caffeinated beverages when there are only so many hours in a day and way too much work to get done.
That’s what happened to a 21-year-old college student who powered down four 16-ounce cans of energy drink – each with double the amount of caffeine in an ordinary cup of coffee – every day for 2 years. Now, if you’ve ever overdone it with caffeine, you know there are some uncomfortable side effects, like shaking and anxiety. In this case, the student reported migraines, tremors, and heart palpitations. Instead of being able to focus better on his work, he had trouble concentrating.
Over time, after these side effects took a turn for the worse and became shortness of breath and weight loss, he visited St. Thomas’ Hospital in London, where physicians diagnosed him with both heart and renal failure.
Excessive consumption of energy drinks is known to cause issues such as high blood pressure and irregular heart beat, so if that’s your fuel of choice, it might be worth cutting down. Maybe take a morning run to get the blood pumping – in a good way – instead?
Loneliness may be hazardous to your health
Sometimes loneliness can feel like it affects your physical health, but according to a study there’s a possibility that it actually does.
Back in the 1980s, researchers from the University of Eastern Finland started monitoring almost 3,000 middle-aged men. They’ve kept up with the participants until the present day, and the results have been staggering. After an average follow-up of over 20 years, 25% of participants developed cancer and 11% died from cancer, and the increase in risk from loneliness was about 10%, regardless of age, lifestyle, and BMI.
What does that say about preventive care? The researchers think these data are cause enough to pay attention to loneliness as a health issue along with smoking and weight.
Social interactions and relationships play important roles in human mental health, of course, but this is pretty solid evidence that they play a role in physical health too. As the researchers said, “Awareness of the health effects of loneliness is constantly increasing. Therefore, it is important to examine, in more detail, the mechanisms by which loneliness causes adverse health effects.”
So, as we progress through this pandemic, maybe you should join that social group on Facebook? Who knows what kind of effect it could have on your health?
An ounce of prevention is worth 12 ounces of lager
COVID-19 vaccine refusal is now a thing, and there’s no law that says people have to be immunized against our newest, bestest buddy, SARS-CoV-2, but the folks who skip it are missing out. And no, we’re not talking about immunity against disease.
We’re talking … FREE STUFF!
Corporate America has stepped up and is now rewarding those who get the COVID-19 vaccine:
- Budweiser will give a free beer to anyone – anyone over age 21, that is – with proof of vaccination until May 16.
- Show a vaccination card at a Krispy Kreme and you can get a free glazed doughnut, every day. You don’t even need to buy anything.
- White Castle will give you a free dessert-on-a-stick just for showing proof of vaccination. No purchase is required, but the offer ends May 31.
But wait, there’s more!
Even the public sector is getting in on the giveaway action. Gov. Jim Justice announced April 26 that West Virginia will give a $100 savings bond to any resident aged 16-35 years who receives a COVID-19 vaccine. It must make sense, because the governor broke out a white board to show residents he’s done the math.
One closing thought: How cool would it be if he was named to the Supreme Court, so he could be Justice Justice?
Where no shirt has gone before
Space. The final frontier, for both humanity and for shirts. Specifically, it’s a new frontier for the Bio-Monitor smart shirt, a tank-top filled with sensors that monitor the wearer’s stats, such as heart and breathing rate, oxygen saturation, skin temperature, and blood pressure. And you thought space was just for finding a new human habitat and growing steak.
This shirt is already used by athletes to assess performance and by people with limited mobility to monitor health, so its potential impending usage by astronauts makes sense. Space is a pretty extreme environment, to put it mildly, and there’s a lot we still don’t know about how the human body reacts to it. Traditionally, astronauts hook themselves up to separate devices so their stats can be measured, a method which captures only snapshots of their bodies. By wearing the shirt constantly, the astronauts can be measured constantly, so scientists and doctors can see how the body deals with microgravity during normal activities and sleep. It also reduces stress, as there is no psychological impact of having to report in for constant health checks.
For the test, astronauts wore the shirt for 72 hours before flight and for 72 hours during flight. The shirts passed this first test with flying colors; in addition to providing accurate and more consistent stats monitoring than traditional methods, scientists on the ground determined that the astronauts recorded far less physical activity during flight than preflight, a finding in line with previous studies.
And before you question whether or not a tank top is really appropriate for space, just remember, Picard pulled it off at the end of “First Contact,” and that’s arguably the best Star Trek movie. So there’s certainly precedent.
Energy drink doom
Who doesn’t need some caffeine to get going in the morning and keep moving throughout the day? Whether it’s tea, coffee, or energy drinks, people can get addicted to caffeinated beverages when there are only so many hours in a day and way too much work to get done.
That’s what happened to a 21-year-old college student who powered down four 16-ounce cans of energy drink – each with double the amount of caffeine in an ordinary cup of coffee – every day for 2 years. Now, if you’ve ever overdone it with caffeine, you know there are some uncomfortable side effects, like shaking and anxiety. In this case, the student reported migraines, tremors, and heart palpitations. Instead of being able to focus better on his work, he had trouble concentrating.
Over time, after these side effects took a turn for the worse and became shortness of breath and weight loss, he visited St. Thomas’ Hospital in London, where physicians diagnosed him with both heart and renal failure.
Excessive consumption of energy drinks is known to cause issues such as high blood pressure and irregular heart beat, so if that’s your fuel of choice, it might be worth cutting down. Maybe take a morning run to get the blood pumping – in a good way – instead?
Loneliness may be hazardous to your health
Sometimes loneliness can feel like it affects your physical health, but according to a study there’s a possibility that it actually does.
Back in the 1980s, researchers from the University of Eastern Finland started monitoring almost 3,000 middle-aged men. They’ve kept up with the participants until the present day, and the results have been staggering. After an average follow-up of over 20 years, 25% of participants developed cancer and 11% died from cancer, and the increase in risk from loneliness was about 10%, regardless of age, lifestyle, and BMI.
What does that say about preventive care? The researchers think these data are cause enough to pay attention to loneliness as a health issue along with smoking and weight.
Social interactions and relationships play important roles in human mental health, of course, but this is pretty solid evidence that they play a role in physical health too. As the researchers said, “Awareness of the health effects of loneliness is constantly increasing. Therefore, it is important to examine, in more detail, the mechanisms by which loneliness causes adverse health effects.”
So, as we progress through this pandemic, maybe you should join that social group on Facebook? Who knows what kind of effect it could have on your health?
An ounce of prevention is worth 12 ounces of lager
COVID-19 vaccine refusal is now a thing, and there’s no law that says people have to be immunized against our newest, bestest buddy, SARS-CoV-2, but the folks who skip it are missing out. And no, we’re not talking about immunity against disease.
We’re talking … FREE STUFF!
Corporate America has stepped up and is now rewarding those who get the COVID-19 vaccine:
- Budweiser will give a free beer to anyone – anyone over age 21, that is – with proof of vaccination until May 16.
- Show a vaccination card at a Krispy Kreme and you can get a free glazed doughnut, every day. You don’t even need to buy anything.
- White Castle will give you a free dessert-on-a-stick just for showing proof of vaccination. No purchase is required, but the offer ends May 31.
But wait, there’s more!
Even the public sector is getting in on the giveaway action. Gov. Jim Justice announced April 26 that West Virginia will give a $100 savings bond to any resident aged 16-35 years who receives a COVID-19 vaccine. It must make sense, because the governor broke out a white board to show residents he’s done the math.
One closing thought: How cool would it be if he was named to the Supreme Court, so he could be Justice Justice?
Where no shirt has gone before
Space. The final frontier, for both humanity and for shirts. Specifically, it’s a new frontier for the Bio-Monitor smart shirt, a tank-top filled with sensors that monitor the wearer’s stats, such as heart and breathing rate, oxygen saturation, skin temperature, and blood pressure. And you thought space was just for finding a new human habitat and growing steak.
This shirt is already used by athletes to assess performance and by people with limited mobility to monitor health, so its potential impending usage by astronauts makes sense. Space is a pretty extreme environment, to put it mildly, and there’s a lot we still don’t know about how the human body reacts to it. Traditionally, astronauts hook themselves up to separate devices so their stats can be measured, a method which captures only snapshots of their bodies. By wearing the shirt constantly, the astronauts can be measured constantly, so scientists and doctors can see how the body deals with microgravity during normal activities and sleep. It also reduces stress, as there is no psychological impact of having to report in for constant health checks.
For the test, astronauts wore the shirt for 72 hours before flight and for 72 hours during flight. The shirts passed this first test with flying colors; in addition to providing accurate and more consistent stats monitoring than traditional methods, scientists on the ground determined that the astronauts recorded far less physical activity during flight than preflight, a finding in line with previous studies.
And before you question whether or not a tank top is really appropriate for space, just remember, Picard pulled it off at the end of “First Contact,” and that’s arguably the best Star Trek movie. So there’s certainly precedent.
FDA panel backs atezolizumab for mTNBC – at least for now
On the first day of a historic 3-day meeting about drugs that were granted an accelerated approval by the Food and Drug Administration for cancer indications, the first approval to come under discussion is staying in place, at least for now.
Members of the FDA’s Oncologic Drugs Advisory Committee voted 7-2 in favor of keeping in place the indication for atezolizumab (Tecentriq) for use in a certain form of breast cancer. At the same time, the committee urged the manufacturer, Genentech, to do the research needed to prove the medicine works for these patients.
The specific indication is for atezolizumab as part of a combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) whose tumors are PD-L1 positive.
The FDA granted accelerated approval in 2019 for this use of atezolizumab, expecting Genentech to produce more extensive evidence of this benefit. But so far, Genentech has not produced the data proving to the FDA that atezolizumab provides the expected benefit.
The drug was already available for use in bladder cancer, having been granted a full approval for this indication in 2016.
Other accelerated approvals withdrawn
This week’s 3-day ODAC meeting is part of the FDA’s broader reconsideration of what it has described as “dangling accelerated approvals.”
Earlier discussions between the FDA and drugmakers have already triggered four voluntary withdrawals of cancer indications with these accelerated approvals, noted Julia A. Beaver, MD, and Richard Pazdur, MD, two of the FDA’s top regulators of oncology medicine, in an April 21 perspective article in the New England Journal of Medicine.
“The small percentage of drugs whose clinical benefit is ultimately not confirmed should be viewed not as a failure of accelerated approval but rather as an expected trade-off in expediting drug development that benefits patients with severe or life-threatening diseases,” Dr. Beaver and Dr. Pazdur wrote.
But making these calls can be tough. On the first day of the meeting, even ODAC panelists who backed Genentech’s bid to maintain an mTNBC indication for atezolizumab expressed discomfort with this choice.
The FDA granted the accelerated approval for use of this drug in March 2019 based on improved progression-free survival from the IMpassion130 trial. But the drug fell short in subsequent efforts to confirm the results seen in that study. The confirmatory IMpassion131 trial failed to meet the primary endpoint, the FDA staff noted in briefing materials for the ODAC meeting.
ODAC panelist Stan Lipkowitz, MD, PhD, of the National Cancer Institute, said he expected this vote had been a tough one for all members serving on ODAC that day.
“In some ways, the purist in me said I should have voted no. But when I looked at the data, there are a couple of things that struck me,” said Dr. Lipkowitz, who is the chief of the Women’s Malignancies Branch at NCI’s Center for Cancer Research. “First of all, the landscape hasn’t changed. There’s really no therapy in the first line for triple-negative metastatic that is shown to improve survival.”
Dr. Lipkowitz emphasized that Genentech needs to continue to try to prove atezolizumab works in this setting.
“There needs to be confirmatory study,” Dr. Lipkowitz concluded.
ODAC panelist Matthew Ellis, MD, PhD, of Baylor College of Medicine, Houston, said he also understood the difficult outlook for women fighting this cancer, but he voted against maintaining the approval.
“It’s not that I don’t feel the tragedy of these women,” said Dr. Ellis, citing his own decades of clinical experience.
“I just think that the data are the data,” Dr. Ellis said, adding that, in his view, “the only correct interpretation” of the evidence supported a vote against allowing the indication to stay.
The FDA considers the recommendations of its advisory committees but is not bound by them.
In a statement issued after the vote, Genentech said it intends to work with the FDA to determine the next steps for this indication of atezolizumab because “the clinically meaningful benefit demonstrated in the IMpassion130 study remains.”
The ODAC meeting continues for 2 more days, and will consider five more cancer indications that have been granted an accelerated approval.
A version of this article first appeared on Medscape.com.
On the first day of a historic 3-day meeting about drugs that were granted an accelerated approval by the Food and Drug Administration for cancer indications, the first approval to come under discussion is staying in place, at least for now.
Members of the FDA’s Oncologic Drugs Advisory Committee voted 7-2 in favor of keeping in place the indication for atezolizumab (Tecentriq) for use in a certain form of breast cancer. At the same time, the committee urged the manufacturer, Genentech, to do the research needed to prove the medicine works for these patients.
The specific indication is for atezolizumab as part of a combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) whose tumors are PD-L1 positive.
The FDA granted accelerated approval in 2019 for this use of atezolizumab, expecting Genentech to produce more extensive evidence of this benefit. But so far, Genentech has not produced the data proving to the FDA that atezolizumab provides the expected benefit.
The drug was already available for use in bladder cancer, having been granted a full approval for this indication in 2016.
Other accelerated approvals withdrawn
This week’s 3-day ODAC meeting is part of the FDA’s broader reconsideration of what it has described as “dangling accelerated approvals.”
Earlier discussions between the FDA and drugmakers have already triggered four voluntary withdrawals of cancer indications with these accelerated approvals, noted Julia A. Beaver, MD, and Richard Pazdur, MD, two of the FDA’s top regulators of oncology medicine, in an April 21 perspective article in the New England Journal of Medicine.
“The small percentage of drugs whose clinical benefit is ultimately not confirmed should be viewed not as a failure of accelerated approval but rather as an expected trade-off in expediting drug development that benefits patients with severe or life-threatening diseases,” Dr. Beaver and Dr. Pazdur wrote.
But making these calls can be tough. On the first day of the meeting, even ODAC panelists who backed Genentech’s bid to maintain an mTNBC indication for atezolizumab expressed discomfort with this choice.
The FDA granted the accelerated approval for use of this drug in March 2019 based on improved progression-free survival from the IMpassion130 trial. But the drug fell short in subsequent efforts to confirm the results seen in that study. The confirmatory IMpassion131 trial failed to meet the primary endpoint, the FDA staff noted in briefing materials for the ODAC meeting.
ODAC panelist Stan Lipkowitz, MD, PhD, of the National Cancer Institute, said he expected this vote had been a tough one for all members serving on ODAC that day.
“In some ways, the purist in me said I should have voted no. But when I looked at the data, there are a couple of things that struck me,” said Dr. Lipkowitz, who is the chief of the Women’s Malignancies Branch at NCI’s Center for Cancer Research. “First of all, the landscape hasn’t changed. There’s really no therapy in the first line for triple-negative metastatic that is shown to improve survival.”
Dr. Lipkowitz emphasized that Genentech needs to continue to try to prove atezolizumab works in this setting.
“There needs to be confirmatory study,” Dr. Lipkowitz concluded.
ODAC panelist Matthew Ellis, MD, PhD, of Baylor College of Medicine, Houston, said he also understood the difficult outlook for women fighting this cancer, but he voted against maintaining the approval.
“It’s not that I don’t feel the tragedy of these women,” said Dr. Ellis, citing his own decades of clinical experience.
“I just think that the data are the data,” Dr. Ellis said, adding that, in his view, “the only correct interpretation” of the evidence supported a vote against allowing the indication to stay.
The FDA considers the recommendations of its advisory committees but is not bound by them.
In a statement issued after the vote, Genentech said it intends to work with the FDA to determine the next steps for this indication of atezolizumab because “the clinically meaningful benefit demonstrated in the IMpassion130 study remains.”
The ODAC meeting continues for 2 more days, and will consider five more cancer indications that have been granted an accelerated approval.
A version of this article first appeared on Medscape.com.
On the first day of a historic 3-day meeting about drugs that were granted an accelerated approval by the Food and Drug Administration for cancer indications, the first approval to come under discussion is staying in place, at least for now.
Members of the FDA’s Oncologic Drugs Advisory Committee voted 7-2 in favor of keeping in place the indication for atezolizumab (Tecentriq) for use in a certain form of breast cancer. At the same time, the committee urged the manufacturer, Genentech, to do the research needed to prove the medicine works for these patients.
The specific indication is for atezolizumab as part of a combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) whose tumors are PD-L1 positive.
The FDA granted accelerated approval in 2019 for this use of atezolizumab, expecting Genentech to produce more extensive evidence of this benefit. But so far, Genentech has not produced the data proving to the FDA that atezolizumab provides the expected benefit.
The drug was already available for use in bladder cancer, having been granted a full approval for this indication in 2016.
Other accelerated approvals withdrawn
This week’s 3-day ODAC meeting is part of the FDA’s broader reconsideration of what it has described as “dangling accelerated approvals.”
Earlier discussions between the FDA and drugmakers have already triggered four voluntary withdrawals of cancer indications with these accelerated approvals, noted Julia A. Beaver, MD, and Richard Pazdur, MD, two of the FDA’s top regulators of oncology medicine, in an April 21 perspective article in the New England Journal of Medicine.
“The small percentage of drugs whose clinical benefit is ultimately not confirmed should be viewed not as a failure of accelerated approval but rather as an expected trade-off in expediting drug development that benefits patients with severe or life-threatening diseases,” Dr. Beaver and Dr. Pazdur wrote.
But making these calls can be tough. On the first day of the meeting, even ODAC panelists who backed Genentech’s bid to maintain an mTNBC indication for atezolizumab expressed discomfort with this choice.
The FDA granted the accelerated approval for use of this drug in March 2019 based on improved progression-free survival from the IMpassion130 trial. But the drug fell short in subsequent efforts to confirm the results seen in that study. The confirmatory IMpassion131 trial failed to meet the primary endpoint, the FDA staff noted in briefing materials for the ODAC meeting.
ODAC panelist Stan Lipkowitz, MD, PhD, of the National Cancer Institute, said he expected this vote had been a tough one for all members serving on ODAC that day.
“In some ways, the purist in me said I should have voted no. But when I looked at the data, there are a couple of things that struck me,” said Dr. Lipkowitz, who is the chief of the Women’s Malignancies Branch at NCI’s Center for Cancer Research. “First of all, the landscape hasn’t changed. There’s really no therapy in the first line for triple-negative metastatic that is shown to improve survival.”
Dr. Lipkowitz emphasized that Genentech needs to continue to try to prove atezolizumab works in this setting.
“There needs to be confirmatory study,” Dr. Lipkowitz concluded.
ODAC panelist Matthew Ellis, MD, PhD, of Baylor College of Medicine, Houston, said he also understood the difficult outlook for women fighting this cancer, but he voted against maintaining the approval.
“It’s not that I don’t feel the tragedy of these women,” said Dr. Ellis, citing his own decades of clinical experience.
“I just think that the data are the data,” Dr. Ellis said, adding that, in his view, “the only correct interpretation” of the evidence supported a vote against allowing the indication to stay.
The FDA considers the recommendations of its advisory committees but is not bound by them.
In a statement issued after the vote, Genentech said it intends to work with the FDA to determine the next steps for this indication of atezolizumab because “the clinically meaningful benefit demonstrated in the IMpassion130 study remains.”
The ODAC meeting continues for 2 more days, and will consider five more cancer indications that have been granted an accelerated approval.
A version of this article first appeared on Medscape.com.
Pleural Effusion and an Axillary Mass in a Woman With Hypertension
Editor's Note:
The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us .
Background
A 58-year-old woman seeks medical attention after she discovered a new mass in her left axilla during a routine monthly breast self-examination while showering. She has not noted any changes in either of her breasts. The mass in her left axilla is not tender, and she has not felt any other abnormal masses, including in her right axilla. She reports no other symptoms and specifically has no pain anywhere in her body. She also does not have shortness of breath, fever, night sweats, fatigue, rash, or abdominal discomfort or bloating.
Fifteen years earlier, the patient was diagnosed with high-grade, stage 1 cervical cancer and underwent surgery and chemoradiation. She has been closely monitored since that time with physical examinations and abdominal CT, with no evidence of recurrent disease. The patient has not had any other surgical procedure, except for removal of two basal cell carcinomas on her neck 4 years ago. She has had yearly routine mammograms for at least the past 15 years.
The patient has hypertension, which has been well controlled with the same medications for the past 10 years. She also has a 25-year history of type 2 diabetes mellitus, which is currently managed with diet alone. She had a "silent myocardial infarction" sometime within the past 5 years but has had no cardiac symptoms and is not taking any cardiac medications. She smoked approximately one pack of cigarettes a day for less than 2 years when she was "in her teens" but has not had any tobacco products since that time.
Pancreatic cancer was diagnosed in the patient's father at age 49 years, and breast cancer was diagnosed in her aunt on her father's side at age 67 years. Her paternal grandmother is reported to have died in her 60s after diagnosis of a "cancer in her stomach." No further information is available regarding either the actual diagnosis or the medical care provided to this individual.
To the best of the patient's knowledge, her mother's side of the family and her two brothers have no history of cancer. She has no sisters. Her mother is in her 80s and has mild dementia. The patient is not aware of any member of her family having undergone genetic testing.
Physical Examination and Workup
The patient appears well and is in no acute distress. The patient is afebrile, with a blood pressure of 135/85 mm Hg, a respiratory rate of 16 breaths/min, and a pulse of 72 beats/min. Her weight is 148 lb (67 kg), and she has no reported recent weight loss.
Examination of the skin reveals no suspicious lesions. Scars from the previous removal of the basal cell carcinomas are noted, but no evidence suggests recurrence.
Results of the head and neck examination are unremarkable; specifically, no abnormal cervical lymphadenopathy is detected. The cardiac and chest examination results are normal. The lungs are clear to percussion and auscultation. The breast examination reveals no abnormal masses. The right axilla is unremarkable; however, a single 3 × 2 cm, nontender, firm, movable but partially fixed mass is noted in the left axilla.
The abdomen appears normal, with no ascites or enlargement of the liver. The pelvic examination reveals evidence of previous surgery and local radiation but no signs of recurrence of cervical cancer. The lymph nodes appear normal, except for the findings noted above. Results of the neurologic examination are unremarkable.
Complete blood cell count, serum electrolyte levels, renal function tests, and urinalysis are all normal. Liver function tests are normal except for a mildly elevated serum alkaline phosphatase level. The fecal occult blood test result is negative.
Chest radiography reveals a suspicious small left-sided pleural effusion. No other abnormalities are observed, and no prior chest radiographs are available to compare with the current findings.
Chest CT confirms the presence of a possible small pleural effusion, with no other abnormalities noted. The radiologist suggests it will not be possible to obtain fluid safely through an interventional procedure, owing to the limited (if any) amount of fluid present. Furthermore, the radiologist recommends PET/CT to look for other evidence of metastatic cancer in the lungs or elsewhere.
Bilateral mammograms reveal no suspicious abnormalities, and the results are unchanged from a previous examination 11 months earlier. Figure 1 shows a similar bilateral mammogram in another patient. Breast MRI shows no evidence of cancer. Figure 2 shows similar breast MRI findings in another patient.
CT of the abdomen and pelvis reveals no changes compared with a scan obtained 2 years earlier for follow-up of the previous diagnosis of cervical cancer. Specifically, no evidence suggests ascites or any pelvic masses.
An incisional biopsy sample is obtained from the left axillary mass. Light microscopy reveals a moderately well-differentiated adenocarcinoma. Immunostaining shows the cancer to be cytokeratin (CK) 7 positive and CK 20 negative (CK 7+/CK 20-, thyroid transcription factor 1 (TTF-1) negative, thyroglobulin negative, napsin A negative, and mammaglobin positive. The tumor is estrogen receptor positive (2% staining), progesterone receptor negative, and human epidermal growth factor receptor 2 (HER2) negative.
[polldaddy:10837180]
Discussion
The correct answer: Breast.
This case is a classic example of cancer of unknown primary site or origin (CUP). CUP represents approximately 5% of cancers diagnosed in the United States (50,000 to 60,000 cases each year), with various series reporting that the site of origin is not diagnosed in between 2% and 6% of all cancer cases.[1] Worldwide, the incidence of CUP is even higher, resulting from the limited availability of sophisticated (and expensive) diagnostic technology in many regions. The median age at diagnosis of CUP is 60 years, and men and women are equally likely to be affected.
A cancer is considered a CUP if, after routine clinical assessment, physical and laboratory examination, standard imaging studies, and routine pathologic evaluation (biopsy or surgical removal of a metastatic mass lesion), a site of origin cannot be defined. With the availability of more sophisticated imaging technologies (eg, MRI), the overall percentage of cancers that are defined as a CUP has been reduced. However, even at autopsy, the site of origin of such cancer is often unable to be determined if the location was unknown before the patient's death.
Several theories have been proposed for why a metastatic lesion becomes clinically evident despite the site of origin of the cancer remaining obscure. These include (1) very slow growth of the primary cancer compared with that of the metastasis; (2) spontaneous regression of the primary cancer; (3) a prominent vascular component of the cancer, which enhances the rate of spread; and (4) unique molecular events associated with the cancer, which result in rapid progression and the growth of metastatic lesions.
Approximately 60% of CUPs are adenocarcinomas (well or moderately well differentiated); 25%-30% are poorly differentiated (including poorly differentiated adenocarcinomas); 5% are completely undifferentiated, with no defining histologic features; 5% are squamous cell cancers; and approximately 1% are carcinomas, with evidence of neuroendocrine differentiation.[1]
Immunohistochemical staining of biopsy material can be helpful in narrowing the possible anatomical sites of origin. The results are particularly relevant in the selection of therapeutic strategies and in ensuring that a rare, potentially highly curable cancer is not missed (eg, lymphoma, germ cell tumor).[2]
A critical initial test is examination of several CK subtypes that are more likely to be expressed in certain carcinomas than in others. For example, the CK 7+/CK 20- staining seen in this patient is characteristic of breast and lung cancers (among others), whereas CK 7+/CK 20+ staining would be expected in pancreatic, gastric, and urothelial cancers. A CK 7-/CK 20+ finding would be more suggestive of colon or mucinous ovarian cancer. Furthermore, approximately 70% of lung adenocarcinomas are TTF-1 positive and 60%-80% are napsin A positive. The negative findings in this patient's case make the diagnosis of metastatic lung cancer less likely.
Examination for the presence (or absence) of well-established biomarkers for breast cancer can potentially be helpful in suggesting the site of origin or in helping to define subsequent therapy. These markers include estrogen and progesterone receptors and HER2 overexpression. An additional biomarker, mammaglobin, has been reported to be expressed in 48% of breast cancers but is absent in cancers of the lung, gastrointestinal tract, ovary, and head and neck region.[2]
Of note, mammaglobin was found to be expressed in this patient. Although only 2% of the cells were reported to stain for the estrogen receptor, this finding is still considered positive and supports breast cancer as the correct diagnosis.
Recognized relevant prognostic factors in CUP include baseline performance status, the number and location of metastatic lesions, and the response to cytotoxic chemotherapy.
Unfortunately, the overall prognosis associated with a diagnosis of CUP is poor, with median survival in various series reported to be less than 6 months. However, important exceptions to this outcome include women who present with an isolated metastatic axillary mass, as described in this case.
Previous reports of axillary adenopathy as the initial presentation of cancer in women revealed that the majority had evidence of cancer in the breast at the time of subsequent mastectomy.[3,4] As a result, in the absence of other indications found during routine workup (eg, a single pulmonary lesion suggestive of a primary lung cancer, pathologic findings inconsistent with breast cancer), an isolated adenocarcinoma in the breast (with no evidence of metastatic cancer elsewhere) should be treated as either stage II or stage III breast cancer. Note that this recommendation specifically relates to female patients. If a male patient has CUP with an isolated axillary mass, it is generally assumed that the lung is the origin of the malignancy.
In a female patient with negative mammographic findings, breast MRI can be helpful. In one series, 28 of 40 women (70%) with evidence of cancer in the axilla and a normal mammogram were found to have a breast abnormality on MRI.[5] Of note, and of considerable relevance to subsequent disease management, five of the 12 women with negative findings in this series underwent surgery, and in four of the cases no cancer was found. Although the number of participants in this series is limited, the absence of an MRI abnormality in the patient in this case can reasonably be considered in her future treatment plans.
Specifically, it might be suggested in this case that treatment include surgical removal of the axillary mass (if possible) followed by radiation to this area and the breast (rather than performing a mastectomy). Alternatively, treatment might begin with chemotherapy (a neoadjuvant approach) followed by surgery to remove any residual axillary mass and local/regional radiation or local/regional radiation alone. Adjuvant chemotherapy and/or hormonal therapy would then be administered.
The presence of a possible small pleural effusion is a concern because it potentially indicates more widespread metastatic disease, as does the mild elevation of the serum alkaline phosphatase level (eg, suggesting metastatic disease in bone or the liver). In the absence of other evidence of tumor spread, PET would not be unreasonable. A negative scan for evidence of metastatic disease would support a "curative" approach to the management of local disease in the axilla and presumably the breast, whereas a finding of other metastatic sites would lead to the conclusion that treatment should probably be delivered with more palliative intent.
The family history of cancer (father, paternal aunt with breast cancer, paternal grandmother with possible ovarian cancer) is intriguing and would suggest a role for genetic counseling and possibly genetic testing (eg, for BRCA mutation).
The patient in this case underwent PET. The only abnormality observed was in the left axilla. The axillary mass was subsequently resected. This was followed by curative radiation to both the axilla and left breast, adjuvant chemotherapy, and 5 years of hormonal therapy. The patient has showed no evidence of recurrence 2 years after completion of the hormonal treatment.
[polldaddy:10841207]
Discussion
The correct answer: Lung
The lungs are generally assumed to be the site of origin of the cancer in a male patient who has CUP with an isolated axillary mass. In contrast, the majority of women with axillary adenopathy as the initial presentation of cancer were found to have evidence of cancer in the breast at the time of subsequent mastectomy.[3,4]
[polldaddy:10837187]
Discussion
The correct answer: MRI
Breast MRI can be helpful in a female patient with negative mammographic findings. In one series, MRI detected a breast abnormality in 28 of 40 women (70%) with evidence of cancer in the axilla and a normal mammogram.[5]
Editor's Note:
The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us .
Background
A 58-year-old woman seeks medical attention after she discovered a new mass in her left axilla during a routine monthly breast self-examination while showering. She has not noted any changes in either of her breasts. The mass in her left axilla is not tender, and she has not felt any other abnormal masses, including in her right axilla. She reports no other symptoms and specifically has no pain anywhere in her body. She also does not have shortness of breath, fever, night sweats, fatigue, rash, or abdominal discomfort or bloating.
Fifteen years earlier, the patient was diagnosed with high-grade, stage 1 cervical cancer and underwent surgery and chemoradiation. She has been closely monitored since that time with physical examinations and abdominal CT, with no evidence of recurrent disease. The patient has not had any other surgical procedure, except for removal of two basal cell carcinomas on her neck 4 years ago. She has had yearly routine mammograms for at least the past 15 years.
The patient has hypertension, which has been well controlled with the same medications for the past 10 years. She also has a 25-year history of type 2 diabetes mellitus, which is currently managed with diet alone. She had a "silent myocardial infarction" sometime within the past 5 years but has had no cardiac symptoms and is not taking any cardiac medications. She smoked approximately one pack of cigarettes a day for less than 2 years when she was "in her teens" but has not had any tobacco products since that time.
Pancreatic cancer was diagnosed in the patient's father at age 49 years, and breast cancer was diagnosed in her aunt on her father's side at age 67 years. Her paternal grandmother is reported to have died in her 60s after diagnosis of a "cancer in her stomach." No further information is available regarding either the actual diagnosis or the medical care provided to this individual.
To the best of the patient's knowledge, her mother's side of the family and her two brothers have no history of cancer. She has no sisters. Her mother is in her 80s and has mild dementia. The patient is not aware of any member of her family having undergone genetic testing.
Physical Examination and Workup
The patient appears well and is in no acute distress. The patient is afebrile, with a blood pressure of 135/85 mm Hg, a respiratory rate of 16 breaths/min, and a pulse of 72 beats/min. Her weight is 148 lb (67 kg), and she has no reported recent weight loss.
Examination of the skin reveals no suspicious lesions. Scars from the previous removal of the basal cell carcinomas are noted, but no evidence suggests recurrence.
Results of the head and neck examination are unremarkable; specifically, no abnormal cervical lymphadenopathy is detected. The cardiac and chest examination results are normal. The lungs are clear to percussion and auscultation. The breast examination reveals no abnormal masses. The right axilla is unremarkable; however, a single 3 × 2 cm, nontender, firm, movable but partially fixed mass is noted in the left axilla.
The abdomen appears normal, with no ascites or enlargement of the liver. The pelvic examination reveals evidence of previous surgery and local radiation but no signs of recurrence of cervical cancer. The lymph nodes appear normal, except for the findings noted above. Results of the neurologic examination are unremarkable.
Complete blood cell count, serum electrolyte levels, renal function tests, and urinalysis are all normal. Liver function tests are normal except for a mildly elevated serum alkaline phosphatase level. The fecal occult blood test result is negative.
Chest radiography reveals a suspicious small left-sided pleural effusion. No other abnormalities are observed, and no prior chest radiographs are available to compare with the current findings.
Chest CT confirms the presence of a possible small pleural effusion, with no other abnormalities noted. The radiologist suggests it will not be possible to obtain fluid safely through an interventional procedure, owing to the limited (if any) amount of fluid present. Furthermore, the radiologist recommends PET/CT to look for other evidence of metastatic cancer in the lungs or elsewhere.
Bilateral mammograms reveal no suspicious abnormalities, and the results are unchanged from a previous examination 11 months earlier. Figure 1 shows a similar bilateral mammogram in another patient. Breast MRI shows no evidence of cancer. Figure 2 shows similar breast MRI findings in another patient.


CT of the abdomen and pelvis reveals no changes compared with a scan obtained 2 years earlier for follow-up of the previous diagnosis of cervical cancer. Specifically, no evidence suggests ascites or any pelvic masses.
An incisional biopsy sample is obtained from the left axillary mass. Light microscopy reveals a moderately well-differentiated adenocarcinoma. Immunostaining shows the cancer to be cytokeratin (CK) 7 positive and CK 20 negative (CK 7+/CK 20-, thyroid transcription factor 1 (TTF-1) negative, thyroglobulin negative, napsin A negative, and mammaglobin positive. The tumor is estrogen receptor positive (2% staining), progesterone receptor negative, and human epidermal growth factor receptor 2 (HER2) negative.
[polldaddy:10837180]
Discussion
The correct answer: Breast.
This case is a classic example of cancer of unknown primary site or origin (CUP). CUP represents approximately 5% of cancers diagnosed in the United States (50,000 to 60,000 cases each year), with various series reporting that the site of origin is not diagnosed in between 2% and 6% of all cancer cases.[1] Worldwide, the incidence of CUP is even higher, resulting from the limited availability of sophisticated (and expensive) diagnostic technology in many regions. The median age at diagnosis of CUP is 60 years, and men and women are equally likely to be affected.
A cancer is considered a CUP if, after routine clinical assessment, physical and laboratory examination, standard imaging studies, and routine pathologic evaluation (biopsy or surgical removal of a metastatic mass lesion), a site of origin cannot be defined. With the availability of more sophisticated imaging technologies (eg, MRI), the overall percentage of cancers that are defined as a CUP has been reduced. However, even at autopsy, the site of origin of such cancer is often unable to be determined if the location was unknown before the patient's death.
Several theories have been proposed for why a metastatic lesion becomes clinically evident despite the site of origin of the cancer remaining obscure. These include (1) very slow growth of the primary cancer compared with that of the metastasis; (2) spontaneous regression of the primary cancer; (3) a prominent vascular component of the cancer, which enhances the rate of spread; and (4) unique molecular events associated with the cancer, which result in rapid progression and the growth of metastatic lesions.
Approximately 60% of CUPs are adenocarcinomas (well or moderately well differentiated); 25%-30% are poorly differentiated (including poorly differentiated adenocarcinomas); 5% are completely undifferentiated, with no defining histologic features; 5% are squamous cell cancers; and approximately 1% are carcinomas, with evidence of neuroendocrine differentiation.[1]
Immunohistochemical staining of biopsy material can be helpful in narrowing the possible anatomical sites of origin. The results are particularly relevant in the selection of therapeutic strategies and in ensuring that a rare, potentially highly curable cancer is not missed (eg, lymphoma, germ cell tumor).[2]
A critical initial test is examination of several CK subtypes that are more likely to be expressed in certain carcinomas than in others. For example, the CK 7+/CK 20- staining seen in this patient is characteristic of breast and lung cancers (among others), whereas CK 7+/CK 20+ staining would be expected in pancreatic, gastric, and urothelial cancers. A CK 7-/CK 20+ finding would be more suggestive of colon or mucinous ovarian cancer. Furthermore, approximately 70% of lung adenocarcinomas are TTF-1 positive and 60%-80% are napsin A positive. The negative findings in this patient's case make the diagnosis of metastatic lung cancer less likely.
Examination for the presence (or absence) of well-established biomarkers for breast cancer can potentially be helpful in suggesting the site of origin or in helping to define subsequent therapy. These markers include estrogen and progesterone receptors and HER2 overexpression. An additional biomarker, mammaglobin, has been reported to be expressed in 48% of breast cancers but is absent in cancers of the lung, gastrointestinal tract, ovary, and head and neck region.[2]
Of note, mammaglobin was found to be expressed in this patient. Although only 2% of the cells were reported to stain for the estrogen receptor, this finding is still considered positive and supports breast cancer as the correct diagnosis.
Recognized relevant prognostic factors in CUP include baseline performance status, the number and location of metastatic lesions, and the response to cytotoxic chemotherapy.
Unfortunately, the overall prognosis associated with a diagnosis of CUP is poor, with median survival in various series reported to be less than 6 months. However, important exceptions to this outcome include women who present with an isolated metastatic axillary mass, as described in this case.
Previous reports of axillary adenopathy as the initial presentation of cancer in women revealed that the majority had evidence of cancer in the breast at the time of subsequent mastectomy.[3,4] As a result, in the absence of other indications found during routine workup (eg, a single pulmonary lesion suggestive of a primary lung cancer, pathologic findings inconsistent with breast cancer), an isolated adenocarcinoma in the breast (with no evidence of metastatic cancer elsewhere) should be treated as either stage II or stage III breast cancer. Note that this recommendation specifically relates to female patients. If a male patient has CUP with an isolated axillary mass, it is generally assumed that the lung is the origin of the malignancy.
In a female patient with negative mammographic findings, breast MRI can be helpful. In one series, 28 of 40 women (70%) with evidence of cancer in the axilla and a normal mammogram were found to have a breast abnormality on MRI.[5] Of note, and of considerable relevance to subsequent disease management, five of the 12 women with negative findings in this series underwent surgery, and in four of the cases no cancer was found. Although the number of participants in this series is limited, the absence of an MRI abnormality in the patient in this case can reasonably be considered in her future treatment plans.
Specifically, it might be suggested in this case that treatment include surgical removal of the axillary mass (if possible) followed by radiation to this area and the breast (rather than performing a mastectomy). Alternatively, treatment might begin with chemotherapy (a neoadjuvant approach) followed by surgery to remove any residual axillary mass and local/regional radiation or local/regional radiation alone. Adjuvant chemotherapy and/or hormonal therapy would then be administered.
The presence of a possible small pleural effusion is a concern because it potentially indicates more widespread metastatic disease, as does the mild elevation of the serum alkaline phosphatase level (eg, suggesting metastatic disease in bone or the liver). In the absence of other evidence of tumor spread, PET would not be unreasonable. A negative scan for evidence of metastatic disease would support a "curative" approach to the management of local disease in the axilla and presumably the breast, whereas a finding of other metastatic sites would lead to the conclusion that treatment should probably be delivered with more palliative intent.
The family history of cancer (father, paternal aunt with breast cancer, paternal grandmother with possible ovarian cancer) is intriguing and would suggest a role for genetic counseling and possibly genetic testing (eg, for BRCA mutation).
The patient in this case underwent PET. The only abnormality observed was in the left axilla. The axillary mass was subsequently resected. This was followed by curative radiation to both the axilla and left breast, adjuvant chemotherapy, and 5 years of hormonal therapy. The patient has showed no evidence of recurrence 2 years after completion of the hormonal treatment.
[polldaddy:10841207]
Discussion
The correct answer: Lung
The lungs are generally assumed to be the site of origin of the cancer in a male patient who has CUP with an isolated axillary mass. In contrast, the majority of women with axillary adenopathy as the initial presentation of cancer were found to have evidence of cancer in the breast at the time of subsequent mastectomy.[3,4]
[polldaddy:10837187]
Discussion
The correct answer: MRI
Breast MRI can be helpful in a female patient with negative mammographic findings. In one series, MRI detected a breast abnormality in 28 of 40 women (70%) with evidence of cancer in the axilla and a normal mammogram.[5]
Editor's Note:
The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us .
Background
A 58-year-old woman seeks medical attention after she discovered a new mass in her left axilla during a routine monthly breast self-examination while showering. She has not noted any changes in either of her breasts. The mass in her left axilla is not tender, and she has not felt any other abnormal masses, including in her right axilla. She reports no other symptoms and specifically has no pain anywhere in her body. She also does not have shortness of breath, fever, night sweats, fatigue, rash, or abdominal discomfort or bloating.
Fifteen years earlier, the patient was diagnosed with high-grade, stage 1 cervical cancer and underwent surgery and chemoradiation. She has been closely monitored since that time with physical examinations and abdominal CT, with no evidence of recurrent disease. The patient has not had any other surgical procedure, except for removal of two basal cell carcinomas on her neck 4 years ago. She has had yearly routine mammograms for at least the past 15 years.
The patient has hypertension, which has been well controlled with the same medications for the past 10 years. She also has a 25-year history of type 2 diabetes mellitus, which is currently managed with diet alone. She had a "silent myocardial infarction" sometime within the past 5 years but has had no cardiac symptoms and is not taking any cardiac medications. She smoked approximately one pack of cigarettes a day for less than 2 years when she was "in her teens" but has not had any tobacco products since that time.
Pancreatic cancer was diagnosed in the patient's father at age 49 years, and breast cancer was diagnosed in her aunt on her father's side at age 67 years. Her paternal grandmother is reported to have died in her 60s after diagnosis of a "cancer in her stomach." No further information is available regarding either the actual diagnosis or the medical care provided to this individual.
To the best of the patient's knowledge, her mother's side of the family and her two brothers have no history of cancer. She has no sisters. Her mother is in her 80s and has mild dementia. The patient is not aware of any member of her family having undergone genetic testing.
Physical Examination and Workup
The patient appears well and is in no acute distress. The patient is afebrile, with a blood pressure of 135/85 mm Hg, a respiratory rate of 16 breaths/min, and a pulse of 72 beats/min. Her weight is 148 lb (67 kg), and she has no reported recent weight loss.
Examination of the skin reveals no suspicious lesions. Scars from the previous removal of the basal cell carcinomas are noted, but no evidence suggests recurrence.
Results of the head and neck examination are unremarkable; specifically, no abnormal cervical lymphadenopathy is detected. The cardiac and chest examination results are normal. The lungs are clear to percussion and auscultation. The breast examination reveals no abnormal masses. The right axilla is unremarkable; however, a single 3 × 2 cm, nontender, firm, movable but partially fixed mass is noted in the left axilla.
The abdomen appears normal, with no ascites or enlargement of the liver. The pelvic examination reveals evidence of previous surgery and local radiation but no signs of recurrence of cervical cancer. The lymph nodes appear normal, except for the findings noted above. Results of the neurologic examination are unremarkable.
Complete blood cell count, serum electrolyte levels, renal function tests, and urinalysis are all normal. Liver function tests are normal except for a mildly elevated serum alkaline phosphatase level. The fecal occult blood test result is negative.
Chest radiography reveals a suspicious small left-sided pleural effusion. No other abnormalities are observed, and no prior chest radiographs are available to compare with the current findings.
Chest CT confirms the presence of a possible small pleural effusion, with no other abnormalities noted. The radiologist suggests it will not be possible to obtain fluid safely through an interventional procedure, owing to the limited (if any) amount of fluid present. Furthermore, the radiologist recommends PET/CT to look for other evidence of metastatic cancer in the lungs or elsewhere.
Bilateral mammograms reveal no suspicious abnormalities, and the results are unchanged from a previous examination 11 months earlier. Figure 1 shows a similar bilateral mammogram in another patient. Breast MRI shows no evidence of cancer. Figure 2 shows similar breast MRI findings in another patient.


CT of the abdomen and pelvis reveals no changes compared with a scan obtained 2 years earlier for follow-up of the previous diagnosis of cervical cancer. Specifically, no evidence suggests ascites or any pelvic masses.
An incisional biopsy sample is obtained from the left axillary mass. Light microscopy reveals a moderately well-differentiated adenocarcinoma. Immunostaining shows the cancer to be cytokeratin (CK) 7 positive and CK 20 negative (CK 7+/CK 20-, thyroid transcription factor 1 (TTF-1) negative, thyroglobulin negative, napsin A negative, and mammaglobin positive. The tumor is estrogen receptor positive (2% staining), progesterone receptor negative, and human epidermal growth factor receptor 2 (HER2) negative.
[polldaddy:10837180]
Discussion
The correct answer: Breast.
This case is a classic example of cancer of unknown primary site or origin (CUP). CUP represents approximately 5% of cancers diagnosed in the United States (50,000 to 60,000 cases each year), with various series reporting that the site of origin is not diagnosed in between 2% and 6% of all cancer cases.[1] Worldwide, the incidence of CUP is even higher, resulting from the limited availability of sophisticated (and expensive) diagnostic technology in many regions. The median age at diagnosis of CUP is 60 years, and men and women are equally likely to be affected.
A cancer is considered a CUP if, after routine clinical assessment, physical and laboratory examination, standard imaging studies, and routine pathologic evaluation (biopsy or surgical removal of a metastatic mass lesion), a site of origin cannot be defined. With the availability of more sophisticated imaging technologies (eg, MRI), the overall percentage of cancers that are defined as a CUP has been reduced. However, even at autopsy, the site of origin of such cancer is often unable to be determined if the location was unknown before the patient's death.
Several theories have been proposed for why a metastatic lesion becomes clinically evident despite the site of origin of the cancer remaining obscure. These include (1) very slow growth of the primary cancer compared with that of the metastasis; (2) spontaneous regression of the primary cancer; (3) a prominent vascular component of the cancer, which enhances the rate of spread; and (4) unique molecular events associated with the cancer, which result in rapid progression and the growth of metastatic lesions.
Approximately 60% of CUPs are adenocarcinomas (well or moderately well differentiated); 25%-30% are poorly differentiated (including poorly differentiated adenocarcinomas); 5% are completely undifferentiated, with no defining histologic features; 5% are squamous cell cancers; and approximately 1% are carcinomas, with evidence of neuroendocrine differentiation.[1]
Immunohistochemical staining of biopsy material can be helpful in narrowing the possible anatomical sites of origin. The results are particularly relevant in the selection of therapeutic strategies and in ensuring that a rare, potentially highly curable cancer is not missed (eg, lymphoma, germ cell tumor).[2]
A critical initial test is examination of several CK subtypes that are more likely to be expressed in certain carcinomas than in others. For example, the CK 7+/CK 20- staining seen in this patient is characteristic of breast and lung cancers (among others), whereas CK 7+/CK 20+ staining would be expected in pancreatic, gastric, and urothelial cancers. A CK 7-/CK 20+ finding would be more suggestive of colon or mucinous ovarian cancer. Furthermore, approximately 70% of lung adenocarcinomas are TTF-1 positive and 60%-80% are napsin A positive. The negative findings in this patient's case make the diagnosis of metastatic lung cancer less likely.
Examination for the presence (or absence) of well-established biomarkers for breast cancer can potentially be helpful in suggesting the site of origin or in helping to define subsequent therapy. These markers include estrogen and progesterone receptors and HER2 overexpression. An additional biomarker, mammaglobin, has been reported to be expressed in 48% of breast cancers but is absent in cancers of the lung, gastrointestinal tract, ovary, and head and neck region.[2]
Of note, mammaglobin was found to be expressed in this patient. Although only 2% of the cells were reported to stain for the estrogen receptor, this finding is still considered positive and supports breast cancer as the correct diagnosis.
Recognized relevant prognostic factors in CUP include baseline performance status, the number and location of metastatic lesions, and the response to cytotoxic chemotherapy.
Unfortunately, the overall prognosis associated with a diagnosis of CUP is poor, with median survival in various series reported to be less than 6 months. However, important exceptions to this outcome include women who present with an isolated metastatic axillary mass, as described in this case.
Previous reports of axillary adenopathy as the initial presentation of cancer in women revealed that the majority had evidence of cancer in the breast at the time of subsequent mastectomy.[3,4] As a result, in the absence of other indications found during routine workup (eg, a single pulmonary lesion suggestive of a primary lung cancer, pathologic findings inconsistent with breast cancer), an isolated adenocarcinoma in the breast (with no evidence of metastatic cancer elsewhere) should be treated as either stage II or stage III breast cancer. Note that this recommendation specifically relates to female patients. If a male patient has CUP with an isolated axillary mass, it is generally assumed that the lung is the origin of the malignancy.
In a female patient with negative mammographic findings, breast MRI can be helpful. In one series, 28 of 40 women (70%) with evidence of cancer in the axilla and a normal mammogram were found to have a breast abnormality on MRI.[5] Of note, and of considerable relevance to subsequent disease management, five of the 12 women with negative findings in this series underwent surgery, and in four of the cases no cancer was found. Although the number of participants in this series is limited, the absence of an MRI abnormality in the patient in this case can reasonably be considered in her future treatment plans.
Specifically, it might be suggested in this case that treatment include surgical removal of the axillary mass (if possible) followed by radiation to this area and the breast (rather than performing a mastectomy). Alternatively, treatment might begin with chemotherapy (a neoadjuvant approach) followed by surgery to remove any residual axillary mass and local/regional radiation or local/regional radiation alone. Adjuvant chemotherapy and/or hormonal therapy would then be administered.
The presence of a possible small pleural effusion is a concern because it potentially indicates more widespread metastatic disease, as does the mild elevation of the serum alkaline phosphatase level (eg, suggesting metastatic disease in bone or the liver). In the absence of other evidence of tumor spread, PET would not be unreasonable. A negative scan for evidence of metastatic disease would support a "curative" approach to the management of local disease in the axilla and presumably the breast, whereas a finding of other metastatic sites would lead to the conclusion that treatment should probably be delivered with more palliative intent.
The family history of cancer (father, paternal aunt with breast cancer, paternal grandmother with possible ovarian cancer) is intriguing and would suggest a role for genetic counseling and possibly genetic testing (eg, for BRCA mutation).
The patient in this case underwent PET. The only abnormality observed was in the left axilla. The axillary mass was subsequently resected. This was followed by curative radiation to both the axilla and left breast, adjuvant chemotherapy, and 5 years of hormonal therapy. The patient has showed no evidence of recurrence 2 years after completion of the hormonal treatment.
[polldaddy:10841207]
Discussion
The correct answer: Lung
The lungs are generally assumed to be the site of origin of the cancer in a male patient who has CUP with an isolated axillary mass. In contrast, the majority of women with axillary adenopathy as the initial presentation of cancer were found to have evidence of cancer in the breast at the time of subsequent mastectomy.[3,4]
[polldaddy:10837187]
Discussion
The correct answer: MRI
Breast MRI can be helpful in a female patient with negative mammographic findings. In one series, MRI detected a breast abnormality in 28 of 40 women (70%) with evidence of cancer in the axilla and a normal mammogram.[5]
Consider risk for Barrett’s esophagus after bariatric surgery
Barrett’s esophagus occurred in nearly 12% of patients who underwent esophagogastroduodenoscopy after sleeve gastrectomy, but it was not associated with postoperative gastroesophageal reflux disease (GERD), based on data from 10 studies that totaled 680 adult patients.
Sleeve gastrectomy has become more popular in recent years as an effective strategy for patients with severe obesity, wrote Bashar J. Qumseya, MD, of the University of Florida, Gainesville, and colleagues. However, GERD is a common concern for patients undergoing sleeve gastrectomy and is the major risk factor for Barrett’s esophagus. However, the prevalence of Barrett’s esophagus in the sleeve gastrectomy population has not been examined.
In a meta-analysis published in Gastrointestinal Endoscopy, the researchers reviewed 10 studies that totaled 680 patients who underwent esophagogastroduodenoscopy 6 months to 10 years after a sleeve gastrectomy procedure. The primary outcome was Barrett’s esophagus prevalence in sleeve gastrectomy patients, with the prevalence of erosive esophagitis and GERD at follow-up as secondary outcomes.
Overall, 54 patients developed Barrett’s esophagus, for a pooled prevalence of 11.6%, and all cases were nondysplastic and de novo. There was no significant association between Barrett’s esophagus and the presence of postoperative GERD, the researchers said (odds ratio, 1.74; P = .37).
However, the rate of erosive esophagitis increased by 86% in five studies with long-term follow-up and by 35% in two studies with short-term follow-up, which suggests an increased risk of 13% each year after sleeve gastrectomy, the researchers noted.
Besides the risk of Barrett’s esophagus after sleeve gastrectomy, “the risk of [erosive esophagitis] is also of significant interest and shares the same pathophysiology with [Barrett’s esophagus] and GERD,” they emphasized.
The study findings were limited by several factors including the small sample size and the focus on Barrett’s esophagus rather than erosive esophagitis or GERD as the primary outcome, the researchers noted. However, the results indicate that sleeve gastrectomy patients are at increased risk for Barrett’s esophagus, and larger studies are needed to better understand the pathophysiology. Furthermore, although there is some debate regarding the risk of GERD and erosive esophagitis after sleeve gastrectomy, the authors wrote that the data from their study showed a “consistent and substantial trend” toward more erosive esophagitis after sleeve gastrectomy.
“Gastroenterologists, primary care providers, and bariatric surgeons should be aware” of the data and should discuss the risks of sleeve gastrectomy with patients before the procedure, including the risks and benefits of postprocedure screening for Barrett’s esophagus, they concluded.
Consider surveillance for Barrett’s
The study is important because of the increased rates of GERD and potentially Barrett’s esophagus that have been noted after sleeve gastrectomy, Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, said in an interview.
“Many of these studies have been small, and the findings of meta-analyses have been limited by high heterogeneity,” he noted. “With the rise in popularity of sleeve gastrectomy, it is important to accurately assess potential long-term complications.”
Dr. Ketwaroo said he was not surprised by the study findings given several reports of increased GERD after sleeve gastrectomy. “Given the accepted pathophysiology of Barrett’s esophagus, I anticipated increased risk of Barrett’s esophagus after sleeve gastrectomy as well.
“Clinicians should consider surveillance for Barrett’s esophagus after sleeve gastrectomy, and possible early proton pump inhibitor use for both GERD/erosive esophagitis and Barrett’s esophagus chemoprophylaxis. Patients with longer-segment or dysplastic Barrett’s esophagus prior to sleeve gastrectomy may have to be monitored more closely after surgery,” he said.
Dr. Ketwaroo noted that the study was limited by the small sample size, “with only approximately 50 patients with Barrett’s esophagus after surgery among 680 overall.” He emphasized that “we will need a much larger prospective study to confirm this finding. Additionally, I would want to explore if sleeve gastrectomy increases rate of progression of dysplasia in those who develop Barrett’s esophagus.”
The study received no outside funding. Lead author Dr. Qumseya had no financial conflicts to disclose. Dr. Ketwaroo serves on the GI & Hepatology News editorial advisory board.
Barrett’s esophagus occurred in nearly 12% of patients who underwent esophagogastroduodenoscopy after sleeve gastrectomy, but it was not associated with postoperative gastroesophageal reflux disease (GERD), based on data from 10 studies that totaled 680 adult patients.
Sleeve gastrectomy has become more popular in recent years as an effective strategy for patients with severe obesity, wrote Bashar J. Qumseya, MD, of the University of Florida, Gainesville, and colleagues. However, GERD is a common concern for patients undergoing sleeve gastrectomy and is the major risk factor for Barrett’s esophagus. However, the prevalence of Barrett’s esophagus in the sleeve gastrectomy population has not been examined.
In a meta-analysis published in Gastrointestinal Endoscopy, the researchers reviewed 10 studies that totaled 680 patients who underwent esophagogastroduodenoscopy 6 months to 10 years after a sleeve gastrectomy procedure. The primary outcome was Barrett’s esophagus prevalence in sleeve gastrectomy patients, with the prevalence of erosive esophagitis and GERD at follow-up as secondary outcomes.
Overall, 54 patients developed Barrett’s esophagus, for a pooled prevalence of 11.6%, and all cases were nondysplastic and de novo. There was no significant association between Barrett’s esophagus and the presence of postoperative GERD, the researchers said (odds ratio, 1.74; P = .37).
However, the rate of erosive esophagitis increased by 86% in five studies with long-term follow-up and by 35% in two studies with short-term follow-up, which suggests an increased risk of 13% each year after sleeve gastrectomy, the researchers noted.
Besides the risk of Barrett’s esophagus after sleeve gastrectomy, “the risk of [erosive esophagitis] is also of significant interest and shares the same pathophysiology with [Barrett’s esophagus] and GERD,” they emphasized.
The study findings were limited by several factors including the small sample size and the focus on Barrett’s esophagus rather than erosive esophagitis or GERD as the primary outcome, the researchers noted. However, the results indicate that sleeve gastrectomy patients are at increased risk for Barrett’s esophagus, and larger studies are needed to better understand the pathophysiology. Furthermore, although there is some debate regarding the risk of GERD and erosive esophagitis after sleeve gastrectomy, the authors wrote that the data from their study showed a “consistent and substantial trend” toward more erosive esophagitis after sleeve gastrectomy.
“Gastroenterologists, primary care providers, and bariatric surgeons should be aware” of the data and should discuss the risks of sleeve gastrectomy with patients before the procedure, including the risks and benefits of postprocedure screening for Barrett’s esophagus, they concluded.
Consider surveillance for Barrett’s
The study is important because of the increased rates of GERD and potentially Barrett’s esophagus that have been noted after sleeve gastrectomy, Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, said in an interview.
“Many of these studies have been small, and the findings of meta-analyses have been limited by high heterogeneity,” he noted. “With the rise in popularity of sleeve gastrectomy, it is important to accurately assess potential long-term complications.”
Dr. Ketwaroo said he was not surprised by the study findings given several reports of increased GERD after sleeve gastrectomy. “Given the accepted pathophysiology of Barrett’s esophagus, I anticipated increased risk of Barrett’s esophagus after sleeve gastrectomy as well.
“Clinicians should consider surveillance for Barrett’s esophagus after sleeve gastrectomy, and possible early proton pump inhibitor use for both GERD/erosive esophagitis and Barrett’s esophagus chemoprophylaxis. Patients with longer-segment or dysplastic Barrett’s esophagus prior to sleeve gastrectomy may have to be monitored more closely after surgery,” he said.
Dr. Ketwaroo noted that the study was limited by the small sample size, “with only approximately 50 patients with Barrett’s esophagus after surgery among 680 overall.” He emphasized that “we will need a much larger prospective study to confirm this finding. Additionally, I would want to explore if sleeve gastrectomy increases rate of progression of dysplasia in those who develop Barrett’s esophagus.”
The study received no outside funding. Lead author Dr. Qumseya had no financial conflicts to disclose. Dr. Ketwaroo serves on the GI & Hepatology News editorial advisory board.
Barrett’s esophagus occurred in nearly 12% of patients who underwent esophagogastroduodenoscopy after sleeve gastrectomy, but it was not associated with postoperative gastroesophageal reflux disease (GERD), based on data from 10 studies that totaled 680 adult patients.
Sleeve gastrectomy has become more popular in recent years as an effective strategy for patients with severe obesity, wrote Bashar J. Qumseya, MD, of the University of Florida, Gainesville, and colleagues. However, GERD is a common concern for patients undergoing sleeve gastrectomy and is the major risk factor for Barrett’s esophagus. However, the prevalence of Barrett’s esophagus in the sleeve gastrectomy population has not been examined.
In a meta-analysis published in Gastrointestinal Endoscopy, the researchers reviewed 10 studies that totaled 680 patients who underwent esophagogastroduodenoscopy 6 months to 10 years after a sleeve gastrectomy procedure. The primary outcome was Barrett’s esophagus prevalence in sleeve gastrectomy patients, with the prevalence of erosive esophagitis and GERD at follow-up as secondary outcomes.
Overall, 54 patients developed Barrett’s esophagus, for a pooled prevalence of 11.6%, and all cases were nondysplastic and de novo. There was no significant association between Barrett’s esophagus and the presence of postoperative GERD, the researchers said (odds ratio, 1.74; P = .37).
However, the rate of erosive esophagitis increased by 86% in five studies with long-term follow-up and by 35% in two studies with short-term follow-up, which suggests an increased risk of 13% each year after sleeve gastrectomy, the researchers noted.
Besides the risk of Barrett’s esophagus after sleeve gastrectomy, “the risk of [erosive esophagitis] is also of significant interest and shares the same pathophysiology with [Barrett’s esophagus] and GERD,” they emphasized.
The study findings were limited by several factors including the small sample size and the focus on Barrett’s esophagus rather than erosive esophagitis or GERD as the primary outcome, the researchers noted. However, the results indicate that sleeve gastrectomy patients are at increased risk for Barrett’s esophagus, and larger studies are needed to better understand the pathophysiology. Furthermore, although there is some debate regarding the risk of GERD and erosive esophagitis after sleeve gastrectomy, the authors wrote that the data from their study showed a “consistent and substantial trend” toward more erosive esophagitis after sleeve gastrectomy.
“Gastroenterologists, primary care providers, and bariatric surgeons should be aware” of the data and should discuss the risks of sleeve gastrectomy with patients before the procedure, including the risks and benefits of postprocedure screening for Barrett’s esophagus, they concluded.
Consider surveillance for Barrett’s
The study is important because of the increased rates of GERD and potentially Barrett’s esophagus that have been noted after sleeve gastrectomy, Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, said in an interview.
“Many of these studies have been small, and the findings of meta-analyses have been limited by high heterogeneity,” he noted. “With the rise in popularity of sleeve gastrectomy, it is important to accurately assess potential long-term complications.”
Dr. Ketwaroo said he was not surprised by the study findings given several reports of increased GERD after sleeve gastrectomy. “Given the accepted pathophysiology of Barrett’s esophagus, I anticipated increased risk of Barrett’s esophagus after sleeve gastrectomy as well.
“Clinicians should consider surveillance for Barrett’s esophagus after sleeve gastrectomy, and possible early proton pump inhibitor use for both GERD/erosive esophagitis and Barrett’s esophagus chemoprophylaxis. Patients with longer-segment or dysplastic Barrett’s esophagus prior to sleeve gastrectomy may have to be monitored more closely after surgery,” he said.
Dr. Ketwaroo noted that the study was limited by the small sample size, “with only approximately 50 patients with Barrett’s esophagus after surgery among 680 overall.” He emphasized that “we will need a much larger prospective study to confirm this finding. Additionally, I would want to explore if sleeve gastrectomy increases rate of progression of dysplasia in those who develop Barrett’s esophagus.”
The study received no outside funding. Lead author Dr. Qumseya had no financial conflicts to disclose. Dr. Ketwaroo serves on the GI & Hepatology News editorial advisory board.
FROM GASTROINTESTINAL ENDOSCOPY
Pregnancy increases risk for symptomatic kidney stones
Pregnancy increases the risk for first-time symptomatic kidney stone formation which peaks close to the time of delivery but can persist even a year later, a population-based, case-controlled study suggests.
“We suspected the risk of a kidney stone event would be high during pregnancy, but we were surprised that the risk remained high for up to a year after delivery,” senior author Andrew Rule, MD, a nephrologist at Mayo Clinic, Rochester, Minn, said in a statement from his institution.
“[So] while most kidney stones that form during pregnancy are detected early by painful passage, some may remain stable in the kidney undetected for a longer period before dislodging and [again] resulting in a painful passage,” he added.
The study was published online April 15, 2021, in the American Journal of Kidney Diseases by Charat Thongprayoon, MD, also of the Mayo Clinic, and colleagues.
“The results of this study indicate that prenatal counseling regarding kidney stones may be warranted, especially for women with other risk factors for kidney stones, such as obesity,” he noted.
First-time stone formers
The observational study included 945 first-time symptomatic kidney stone formers aged between 15 and 45 years who were compared with 1,890 age-matched female controls from the Rochester Epidemiology Project. The latter is a medical record linkage system for almost all medical care administered in Olmsted County in Minnesota.
Compared with nonpregnant women, the odds of a symptomatic kidney stone forming in a pregnant woman was similar in the first trimester (odds ratio, 0.92; P = .8), began to increase during the second trimester (OR, 2.00; P = .007), further increased during the third trimester (OR, 2.69; P = .001), and peaked at 0-3 months after delivery (OR, 3.53; P < .001). The risk returned to baseline by 1 year after delivery.
These associations persisted after adjustment for age and race or for diabetes, hypertension, and obesity. These results did not significantly differ by age, race, time period, or number of prior pregnancies.
The risk of a pregnant woman developing a symptomatic kidney stone was higher in women with obesity, compared with those of normal weight (P = .01).
And compared with women who had not been pregnant before, one prior pregnancy also increased the risk of having a symptomatic kidney stone by approximately 30% (OR, 1.29; P = .03), although two or more prior pregnancies did not significantly increase symptomatic kidney stone risk.
Thus, “it can be inferred that the odds of a symptomatic kidney stone peak around the time of delivery,” the authors emphasized. “The odds of a first-time symptomatic kidney stone then decreased over time and were fully attenuated and no longer statistically significant by 12 months after delivery.”
Dr. Thongprayoon said there are several physiologic reasons why pregnancy might contribute to kidney stone formation.
During pregnancy, ureteral compression and ureteral relaxation caused by elevated progesterone levels can cause urinary stasis.
Furthermore, increased urinary calcium excretion and elevated urine pH during pregnancy can promote calcium phosphate stone formation. It is noteworthy that almost all pregnant, first-time stone formers had calcium phosphate stones.
“During pregnancy, a kidney stone may contribute to serious complications,” Dr. Thongprayoon explained.
General dietary recommendations for preventing kidney stones include drinking abundant fluids and consuming a low-salt diet.
The study was supported by the Mayo Clinic O’Brien Urology Research Center and a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnancy increases the risk for first-time symptomatic kidney stone formation which peaks close to the time of delivery but can persist even a year later, a population-based, case-controlled study suggests.
“We suspected the risk of a kidney stone event would be high during pregnancy, but we were surprised that the risk remained high for up to a year after delivery,” senior author Andrew Rule, MD, a nephrologist at Mayo Clinic, Rochester, Minn, said in a statement from his institution.
“[So] while most kidney stones that form during pregnancy are detected early by painful passage, some may remain stable in the kidney undetected for a longer period before dislodging and [again] resulting in a painful passage,” he added.
The study was published online April 15, 2021, in the American Journal of Kidney Diseases by Charat Thongprayoon, MD, also of the Mayo Clinic, and colleagues.
“The results of this study indicate that prenatal counseling regarding kidney stones may be warranted, especially for women with other risk factors for kidney stones, such as obesity,” he noted.
First-time stone formers
The observational study included 945 first-time symptomatic kidney stone formers aged between 15 and 45 years who were compared with 1,890 age-matched female controls from the Rochester Epidemiology Project. The latter is a medical record linkage system for almost all medical care administered in Olmsted County in Minnesota.
Compared with nonpregnant women, the odds of a symptomatic kidney stone forming in a pregnant woman was similar in the first trimester (odds ratio, 0.92; P = .8), began to increase during the second trimester (OR, 2.00; P = .007), further increased during the third trimester (OR, 2.69; P = .001), and peaked at 0-3 months after delivery (OR, 3.53; P < .001). The risk returned to baseline by 1 year after delivery.
These associations persisted after adjustment for age and race or for diabetes, hypertension, and obesity. These results did not significantly differ by age, race, time period, or number of prior pregnancies.
The risk of a pregnant woman developing a symptomatic kidney stone was higher in women with obesity, compared with those of normal weight (P = .01).
And compared with women who had not been pregnant before, one prior pregnancy also increased the risk of having a symptomatic kidney stone by approximately 30% (OR, 1.29; P = .03), although two or more prior pregnancies did not significantly increase symptomatic kidney stone risk.
Thus, “it can be inferred that the odds of a symptomatic kidney stone peak around the time of delivery,” the authors emphasized. “The odds of a first-time symptomatic kidney stone then decreased over time and were fully attenuated and no longer statistically significant by 12 months after delivery.”
Dr. Thongprayoon said there are several physiologic reasons why pregnancy might contribute to kidney stone formation.
During pregnancy, ureteral compression and ureteral relaxation caused by elevated progesterone levels can cause urinary stasis.
Furthermore, increased urinary calcium excretion and elevated urine pH during pregnancy can promote calcium phosphate stone formation. It is noteworthy that almost all pregnant, first-time stone formers had calcium phosphate stones.
“During pregnancy, a kidney stone may contribute to serious complications,” Dr. Thongprayoon explained.
General dietary recommendations for preventing kidney stones include drinking abundant fluids and consuming a low-salt diet.
The study was supported by the Mayo Clinic O’Brien Urology Research Center and a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnancy increases the risk for first-time symptomatic kidney stone formation which peaks close to the time of delivery but can persist even a year later, a population-based, case-controlled study suggests.
“We suspected the risk of a kidney stone event would be high during pregnancy, but we were surprised that the risk remained high for up to a year after delivery,” senior author Andrew Rule, MD, a nephrologist at Mayo Clinic, Rochester, Minn, said in a statement from his institution.
“[So] while most kidney stones that form during pregnancy are detected early by painful passage, some may remain stable in the kidney undetected for a longer period before dislodging and [again] resulting in a painful passage,” he added.
The study was published online April 15, 2021, in the American Journal of Kidney Diseases by Charat Thongprayoon, MD, also of the Mayo Clinic, and colleagues.
“The results of this study indicate that prenatal counseling regarding kidney stones may be warranted, especially for women with other risk factors for kidney stones, such as obesity,” he noted.
First-time stone formers
The observational study included 945 first-time symptomatic kidney stone formers aged between 15 and 45 years who were compared with 1,890 age-matched female controls from the Rochester Epidemiology Project. The latter is a medical record linkage system for almost all medical care administered in Olmsted County in Minnesota.
Compared with nonpregnant women, the odds of a symptomatic kidney stone forming in a pregnant woman was similar in the first trimester (odds ratio, 0.92; P = .8), began to increase during the second trimester (OR, 2.00; P = .007), further increased during the third trimester (OR, 2.69; P = .001), and peaked at 0-3 months after delivery (OR, 3.53; P < .001). The risk returned to baseline by 1 year after delivery.
These associations persisted after adjustment for age and race or for diabetes, hypertension, and obesity. These results did not significantly differ by age, race, time period, or number of prior pregnancies.
The risk of a pregnant woman developing a symptomatic kidney stone was higher in women with obesity, compared with those of normal weight (P = .01).
And compared with women who had not been pregnant before, one prior pregnancy also increased the risk of having a symptomatic kidney stone by approximately 30% (OR, 1.29; P = .03), although two or more prior pregnancies did not significantly increase symptomatic kidney stone risk.
Thus, “it can be inferred that the odds of a symptomatic kidney stone peak around the time of delivery,” the authors emphasized. “The odds of a first-time symptomatic kidney stone then decreased over time and were fully attenuated and no longer statistically significant by 12 months after delivery.”
Dr. Thongprayoon said there are several physiologic reasons why pregnancy might contribute to kidney stone formation.
During pregnancy, ureteral compression and ureteral relaxation caused by elevated progesterone levels can cause urinary stasis.
Furthermore, increased urinary calcium excretion and elevated urine pH during pregnancy can promote calcium phosphate stone formation. It is noteworthy that almost all pregnant, first-time stone formers had calcium phosphate stones.
“During pregnancy, a kidney stone may contribute to serious complications,” Dr. Thongprayoon explained.
General dietary recommendations for preventing kidney stones include drinking abundant fluids and consuming a low-salt diet.
The study was supported by the Mayo Clinic O’Brien Urology Research Center and a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.