User login
Tocilizumab (Actemra) scores FDA approval for systemic sclerosis–associated interstitial lung disease
The Food and Drug Administration has approved subcutaneously-injected tocilizumab (Actemra) to reduce the rate of pulmonary function decline in systemic sclerosis–associated interstitial lung disease (SSc-ILD) patients, according to a press release from manufacturer Genentech.
Tocilizumab is the first biologic to be approved by the agency for adults with SSc-ILD, a rare and potentially life-threatening condition that may affect up to 80% of SSc patients and lead to lung inflammation and scarring.
The approval was based primarily on data from a phase 3 randomized, double-blind, placebo-controlled clinical trial (the focuSSced trial) that included 212 adults with SSc. Although that study failed to meet its primary endpoint of change from baseline to 48 weeks in the modified Rodnan Skin Score, the researchers observed a significantly reduced lung function decline as measured by forced vital capacity (FVC) and percent predicted forced vital capacity (ppFVC) among tocilizumab-treated patients, compared with those who received placebo. A total of 68 patients (65%) in the tocilizumab group and 68 patients (64%) in the placebo group had SSc-ILD at baseline.
In a subgroup analysis, patients taking tocilizumab had a smaller decline in mean ppFVC, compared with placebo patients (0.07% vs. –6.4%; mean difference, 6.47%), and a smaller decline in FVC (mean change –14 mL vs. –255 mL with placebo; mean difference, 241 mL).
The mean change from baseline to week 48 in modified Rodnan Skin Score was –5.88 for patients on tocilizumab and –3.77 with placebo.
Safety data were similar between tocilizumab and placebo groups through 48 weeks, and similar for patients with and without SSc-ILD. In general, tocilizumab side effects include increased susceptibility to infections, and serious side effects may include stomach tears, hepatotoxicity, and increased risk of cancer and hepatitis B, according to the prescribing information. However, the most common side effects are upper respiratory tract infections, headache, hypertension, and injection-site reactions.
Tocilizumab, an interleukin-6 receptor antagonist, is already approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis, as well as for adult patients with giant cell arteritis; patients aged 2 years and older with active polyarticular juvenile idiopathic arthritis or active systemic juvenile idiopathic arthritis; and adults and pediatric patients 2 years of age and older with chimeric antigen receptor T-cell–induced severe or life-threatening cytokine release syndrome.
Prescribing information is available here.
The Food and Drug Administration has approved subcutaneously-injected tocilizumab (Actemra) to reduce the rate of pulmonary function decline in systemic sclerosis–associated interstitial lung disease (SSc-ILD) patients, according to a press release from manufacturer Genentech.
Tocilizumab is the first biologic to be approved by the agency for adults with SSc-ILD, a rare and potentially life-threatening condition that may affect up to 80% of SSc patients and lead to lung inflammation and scarring.
The approval was based primarily on data from a phase 3 randomized, double-blind, placebo-controlled clinical trial (the focuSSced trial) that included 212 adults with SSc. Although that study failed to meet its primary endpoint of change from baseline to 48 weeks in the modified Rodnan Skin Score, the researchers observed a significantly reduced lung function decline as measured by forced vital capacity (FVC) and percent predicted forced vital capacity (ppFVC) among tocilizumab-treated patients, compared with those who received placebo. A total of 68 patients (65%) in the tocilizumab group and 68 patients (64%) in the placebo group had SSc-ILD at baseline.
In a subgroup analysis, patients taking tocilizumab had a smaller decline in mean ppFVC, compared with placebo patients (0.07% vs. –6.4%; mean difference, 6.47%), and a smaller decline in FVC (mean change –14 mL vs. –255 mL with placebo; mean difference, 241 mL).
The mean change from baseline to week 48 in modified Rodnan Skin Score was –5.88 for patients on tocilizumab and –3.77 with placebo.
Safety data were similar between tocilizumab and placebo groups through 48 weeks, and similar for patients with and without SSc-ILD. In general, tocilizumab side effects include increased susceptibility to infections, and serious side effects may include stomach tears, hepatotoxicity, and increased risk of cancer and hepatitis B, according to the prescribing information. However, the most common side effects are upper respiratory tract infections, headache, hypertension, and injection-site reactions.
Tocilizumab, an interleukin-6 receptor antagonist, is already approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis, as well as for adult patients with giant cell arteritis; patients aged 2 years and older with active polyarticular juvenile idiopathic arthritis or active systemic juvenile idiopathic arthritis; and adults and pediatric patients 2 years of age and older with chimeric antigen receptor T-cell–induced severe or life-threatening cytokine release syndrome.
Prescribing information is available here.
The Food and Drug Administration has approved subcutaneously-injected tocilizumab (Actemra) to reduce the rate of pulmonary function decline in systemic sclerosis–associated interstitial lung disease (SSc-ILD) patients, according to a press release from manufacturer Genentech.
Tocilizumab is the first biologic to be approved by the agency for adults with SSc-ILD, a rare and potentially life-threatening condition that may affect up to 80% of SSc patients and lead to lung inflammation and scarring.
The approval was based primarily on data from a phase 3 randomized, double-blind, placebo-controlled clinical trial (the focuSSced trial) that included 212 adults with SSc. Although that study failed to meet its primary endpoint of change from baseline to 48 weeks in the modified Rodnan Skin Score, the researchers observed a significantly reduced lung function decline as measured by forced vital capacity (FVC) and percent predicted forced vital capacity (ppFVC) among tocilizumab-treated patients, compared with those who received placebo. A total of 68 patients (65%) in the tocilizumab group and 68 patients (64%) in the placebo group had SSc-ILD at baseline.
In a subgroup analysis, patients taking tocilizumab had a smaller decline in mean ppFVC, compared with placebo patients (0.07% vs. –6.4%; mean difference, 6.47%), and a smaller decline in FVC (mean change –14 mL vs. –255 mL with placebo; mean difference, 241 mL).
The mean change from baseline to week 48 in modified Rodnan Skin Score was –5.88 for patients on tocilizumab and –3.77 with placebo.
Safety data were similar between tocilizumab and placebo groups through 48 weeks, and similar for patients with and without SSc-ILD. In general, tocilizumab side effects include increased susceptibility to infections, and serious side effects may include stomach tears, hepatotoxicity, and increased risk of cancer and hepatitis B, according to the prescribing information. However, the most common side effects are upper respiratory tract infections, headache, hypertension, and injection-site reactions.
Tocilizumab, an interleukin-6 receptor antagonist, is already approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis, as well as for adult patients with giant cell arteritis; patients aged 2 years and older with active polyarticular juvenile idiopathic arthritis or active systemic juvenile idiopathic arthritis; and adults and pediatric patients 2 years of age and older with chimeric antigen receptor T-cell–induced severe or life-threatening cytokine release syndrome.
Prescribing information is available here.
Confirmed: Diet influences colorectal cancer risk
It’s now confirmed: What you eat does affect your risk of developing colorectal cancer (CRC).
An umbrella review of studies and meta-analyses found “convincing evidence of an association between a lower CRC risk and higher intakes of dietary fiber, dietary calcium, and yogurt and lower intakes of alcohol and red meat.”
However, more research is needed to address the link between CRC and other foods, including dairy products, whole grains, processed meat, and specific dietary patterns, the authors conclude.
“We can say that the existing recommendations for diet in the primary prevention of colorectal cancer is confirmed,” said lead author Nathorn Chaiyakunapruk, PharmD, PhD, professor of pharmacology at the University of Utah, Salt Lake City.
“It makes sense to encourage healthy diet, including those rich in fruits, vegetables, grains, and low-fat dairy, and reducing red meat and alcohol intake,” he said in an interview. “However, some of them may not yet have convincing evidence to fully support the claim.”
Other lifestyle factors, including excess weight and physical inactivity, also play a role in cancer risk. Dr. Chaiyakunapruk pointed out that their review was focused only on diet and that they had set out to confirm factors for which there was strong and convincing evidence.
The review was published online in JAMA Network Open.
The umbrella review of 45 meta-analyses found 109 associations. Overall, 35 of these 109 associations (32.1%) were nominally statistically significant, as determined on the basis of random-effects meta-analysis models, the researchers explained.
Convincing evidence was found for an increase in the risk for CRC with higher versus lower red meat consumption and with heavy alcohol intake (defined as more than four drinks per day, compared with no drinks per day or occasional drinks).
In addition, convincing evidence was found for three inverse associations: a decrease in the risk for CRC was associated with higher versus lower intake of total dietary fiber, calcium, and yogurt.
The researchers noted that, although not completely convincing, there was highly suggestive evidence for another association: a link between diet and CRC incidence. A higher intake of total dairy products (e.g., milk, cheese, and yogurt) was associated with significant risk reduction, in comparison with lower intake. A moderate intake of alcohol (from one to three drinks but not more than four per day) was associated with an increase in incidence in comparison with no drinks or an occasional drink.
Evidence suggested a reduced risk in association with several lifestyle behaviors, including adherence to a Mediterranean diet, a healthy diet, a pesco-vegetarian or semivegetarian diet, and the intake of whole grains, nonfermented milk, and supplemental calcium.
The evidence suggested that adherence to a Western diet and intake of processed meat were associated with an increased risk for CRC.
There was weak or no evidence for the remaining associations.
Existing cancer prevention guidelines
The findings support the existing cancer prevention dietary guidance and recommendations from the American Institute for Cancer Research, commented the institute’s director of nutrition programs, Sheena Swanner Patel, MS, RDN. The study confirms that dietary factors play a strong role in lowering CRC risk.
“AICR’s report found strong evidence for whole grains, foods containing dietary fiber, dairy products, and calcium supplements decreasing risk for colorectal cancer,” she said. “Specifically, eating 90 g or three servings of whole grains per day is associated with a 17% decrease in colorectal cancer risk.”
Ms. Patel added that the AICR’s report also suggested there was strong evidence that eating large amounts of red and processed meat, drinking alcohol excessively, and carrying extra body weight increased the risk for CRC.
Many previous studies have suggested a link between diet and CRC risk. One recent study suggested that, among all cancers, CRC has the highest proportion of diet-related cases (38.3%). The next highest were cancers of the mouth, pharynx, and larynx, for which almost 26% of cases were linked to diet, followed by endometrial cancer, postmenopausal breast cancer, and cancers of the kidney, stomach, liver, pancreas, and esophagus.
Neither Dr. Chaiyakunapruk and coauthors nor Ms. Patel disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s now confirmed: What you eat does affect your risk of developing colorectal cancer (CRC).
An umbrella review of studies and meta-analyses found “convincing evidence of an association between a lower CRC risk and higher intakes of dietary fiber, dietary calcium, and yogurt and lower intakes of alcohol and red meat.”
However, more research is needed to address the link between CRC and other foods, including dairy products, whole grains, processed meat, and specific dietary patterns, the authors conclude.
“We can say that the existing recommendations for diet in the primary prevention of colorectal cancer is confirmed,” said lead author Nathorn Chaiyakunapruk, PharmD, PhD, professor of pharmacology at the University of Utah, Salt Lake City.
“It makes sense to encourage healthy diet, including those rich in fruits, vegetables, grains, and low-fat dairy, and reducing red meat and alcohol intake,” he said in an interview. “However, some of them may not yet have convincing evidence to fully support the claim.”
Other lifestyle factors, including excess weight and physical inactivity, also play a role in cancer risk. Dr. Chaiyakunapruk pointed out that their review was focused only on diet and that they had set out to confirm factors for which there was strong and convincing evidence.
The review was published online in JAMA Network Open.
The umbrella review of 45 meta-analyses found 109 associations. Overall, 35 of these 109 associations (32.1%) were nominally statistically significant, as determined on the basis of random-effects meta-analysis models, the researchers explained.
Convincing evidence was found for an increase in the risk for CRC with higher versus lower red meat consumption and with heavy alcohol intake (defined as more than four drinks per day, compared with no drinks per day or occasional drinks).
In addition, convincing evidence was found for three inverse associations: a decrease in the risk for CRC was associated with higher versus lower intake of total dietary fiber, calcium, and yogurt.
The researchers noted that, although not completely convincing, there was highly suggestive evidence for another association: a link between diet and CRC incidence. A higher intake of total dairy products (e.g., milk, cheese, and yogurt) was associated with significant risk reduction, in comparison with lower intake. A moderate intake of alcohol (from one to three drinks but not more than four per day) was associated with an increase in incidence in comparison with no drinks or an occasional drink.
Evidence suggested a reduced risk in association with several lifestyle behaviors, including adherence to a Mediterranean diet, a healthy diet, a pesco-vegetarian or semivegetarian diet, and the intake of whole grains, nonfermented milk, and supplemental calcium.
The evidence suggested that adherence to a Western diet and intake of processed meat were associated with an increased risk for CRC.
There was weak or no evidence for the remaining associations.
Existing cancer prevention guidelines
The findings support the existing cancer prevention dietary guidance and recommendations from the American Institute for Cancer Research, commented the institute’s director of nutrition programs, Sheena Swanner Patel, MS, RDN. The study confirms that dietary factors play a strong role in lowering CRC risk.
“AICR’s report found strong evidence for whole grains, foods containing dietary fiber, dairy products, and calcium supplements decreasing risk for colorectal cancer,” she said. “Specifically, eating 90 g or three servings of whole grains per day is associated with a 17% decrease in colorectal cancer risk.”
Ms. Patel added that the AICR’s report also suggested there was strong evidence that eating large amounts of red and processed meat, drinking alcohol excessively, and carrying extra body weight increased the risk for CRC.
Many previous studies have suggested a link between diet and CRC risk. One recent study suggested that, among all cancers, CRC has the highest proportion of diet-related cases (38.3%). The next highest were cancers of the mouth, pharynx, and larynx, for which almost 26% of cases were linked to diet, followed by endometrial cancer, postmenopausal breast cancer, and cancers of the kidney, stomach, liver, pancreas, and esophagus.
Neither Dr. Chaiyakunapruk and coauthors nor Ms. Patel disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s now confirmed: What you eat does affect your risk of developing colorectal cancer (CRC).
An umbrella review of studies and meta-analyses found “convincing evidence of an association between a lower CRC risk and higher intakes of dietary fiber, dietary calcium, and yogurt and lower intakes of alcohol and red meat.”
However, more research is needed to address the link between CRC and other foods, including dairy products, whole grains, processed meat, and specific dietary patterns, the authors conclude.
“We can say that the existing recommendations for diet in the primary prevention of colorectal cancer is confirmed,” said lead author Nathorn Chaiyakunapruk, PharmD, PhD, professor of pharmacology at the University of Utah, Salt Lake City.
“It makes sense to encourage healthy diet, including those rich in fruits, vegetables, grains, and low-fat dairy, and reducing red meat and alcohol intake,” he said in an interview. “However, some of them may not yet have convincing evidence to fully support the claim.”
Other lifestyle factors, including excess weight and physical inactivity, also play a role in cancer risk. Dr. Chaiyakunapruk pointed out that their review was focused only on diet and that they had set out to confirm factors for which there was strong and convincing evidence.
The review was published online in JAMA Network Open.
The umbrella review of 45 meta-analyses found 109 associations. Overall, 35 of these 109 associations (32.1%) were nominally statistically significant, as determined on the basis of random-effects meta-analysis models, the researchers explained.
Convincing evidence was found for an increase in the risk for CRC with higher versus lower red meat consumption and with heavy alcohol intake (defined as more than four drinks per day, compared with no drinks per day or occasional drinks).
In addition, convincing evidence was found for three inverse associations: a decrease in the risk for CRC was associated with higher versus lower intake of total dietary fiber, calcium, and yogurt.
The researchers noted that, although not completely convincing, there was highly suggestive evidence for another association: a link between diet and CRC incidence. A higher intake of total dairy products (e.g., milk, cheese, and yogurt) was associated with significant risk reduction, in comparison with lower intake. A moderate intake of alcohol (from one to three drinks but not more than four per day) was associated with an increase in incidence in comparison with no drinks or an occasional drink.
Evidence suggested a reduced risk in association with several lifestyle behaviors, including adherence to a Mediterranean diet, a healthy diet, a pesco-vegetarian or semivegetarian diet, and the intake of whole grains, nonfermented milk, and supplemental calcium.
The evidence suggested that adherence to a Western diet and intake of processed meat were associated with an increased risk for CRC.
There was weak or no evidence for the remaining associations.
Existing cancer prevention guidelines
The findings support the existing cancer prevention dietary guidance and recommendations from the American Institute for Cancer Research, commented the institute’s director of nutrition programs, Sheena Swanner Patel, MS, RDN. The study confirms that dietary factors play a strong role in lowering CRC risk.
“AICR’s report found strong evidence for whole grains, foods containing dietary fiber, dairy products, and calcium supplements decreasing risk for colorectal cancer,” she said. “Specifically, eating 90 g or three servings of whole grains per day is associated with a 17% decrease in colorectal cancer risk.”
Ms. Patel added that the AICR’s report also suggested there was strong evidence that eating large amounts of red and processed meat, drinking alcohol excessively, and carrying extra body weight increased the risk for CRC.
Many previous studies have suggested a link between diet and CRC risk. One recent study suggested that, among all cancers, CRC has the highest proportion of diet-related cases (38.3%). The next highest were cancers of the mouth, pharynx, and larynx, for which almost 26% of cases were linked to diet, followed by endometrial cancer, postmenopausal breast cancer, and cancers of the kidney, stomach, liver, pancreas, and esophagus.
Neither Dr. Chaiyakunapruk and coauthors nor Ms. Patel disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
What drives treatment satisfaction among adults with atopic dermatitis?
.
Satisfaction scores were higher when specialists prescribed systemic therapy, but were lower when nonspecialists prescribed systemic therapy and when specialists prescribed only topical therapy.
Those are among key findings from an analysis of the Medical Expenditure Panel Surveys reported by Brian T. Cheng during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium.
“AD management is complex,” said Mr. Cheng, a medical student at Northwestern University, Chicago. “It includes patient education about trigger avoidance, over-the-counter and prescription topical therapies, as well as systemic therapies. Previous studies have shown major decrements to quality of life as well as atopic and non-atopic comorbidities in these patients. The burden of AD and their comorbidities, as well as their management, may impact patient satisfaction.”
Prior studies have demonstrated that patient satisfaction is associated with improvements in clinical outcomes, increased patient retention, and reduced malpractice claims (Br J Dermatol. 2001 Oct;145[4]:617-23, Arch Dermatol 2008 Feb;144[2]:263-5). However, since data on patient satisfaction in AD are limited, Mr. Cheng and the study’s senior author, Jonathan I. Silverberg, MD, PhD, MPH, set out to examine overall patient satisfaction among adults with AD, to determine associations of patient satisfaction with patterns of health care utilization, and to identify predictors of higher satisfaction among these adults.
The researchers conducted a cross-sectional retrospective analysis of 3,810 patients from the 2000-2015 Medical Expenditure Panel Surveys, representative surveys of the U.S. noninstitutionalized population conducted annually by the Agency for Healthcare Research and Quality. They used ICD-9 codes 691 and 692 to determine AD diagnosis and five Consumer Assessment of Health Plans Survey (CAHPS) questions to assess patients’ satisfaction with their clinicians. “These questions have been extensively validated to correlate with global satisfaction,” Mr. Cheng said. “These are not disease-specific and allow for comparison across multiple diseases.”
Next, the researchers created a composite satisfaction score based on the methods of Anthony Jerant, MD, of the University of California, Davis, and colleagues. They adjusted each question in the CAHPS survey to have an equal weight and then summed these into a composite satisfaction score. “We examined patient satisfaction comparing across diseases, and based on the guidelines from the AHRQ to isolate that impact of patient-physician interaction, we adjusted for sociodemographics, mental and physical health status, self-reported health rating, as well as multimorbidity and comorbid diseases.”
Compared with adults who are healthy, adults with AD had lower patient satisfaction overall. “Moreover, people with AD had lower satisfaction compared to those with psoriasis, which may reflect more substantial itch burden as well as the greater comorbid disease challenges in management,” Mr. Cheng said. “It may also reflect the renaissance in psoriasis treatment over the last 10-20 years, giving a wider spectrum of treatment and thus a higher patient satisfaction.”
Among adults with AD, lower satisfaction was consistent across all domains of CAHPS. For the question of “How often health providers listen carefully to you” the adjusted OR (aOR) was 0.87 (P = .008). For the question of “How often health providers explain things in a way that was easy to understand” the aOR was 0.89 (P = .003). For the question of “How often health providers spent enough time with you” the aOR was 0.86 (P = .0001). For “How often providers showed respect for what you had to say” the aOR was 0.91 (P = .02).
Recognizing that treatment regimens are complex and used differently by provider type, the researchers examined interactions between specialists (dermatologists and allergists) and treatment type. “Previous studies found dermatologists treat more severe, chronic AD,” Mr. Cheng said. “We found here that there was lower satisfaction among those treated with topical therapy and by specialists, which may reflect inadequate disease control. We also found lower satisfaction among those treated with systemic therapy by primary care physicians. This may reflect that these patients are not achieving optimal therapy. We found that satisfaction was highest among those treated with systemic therapy and by dermatologists and allergists.”
Socioeconomic, racial/ethnic, and health care disparities were observed in terms of satisfaction among this cohort. The following characteristics were significantly associated with lower patient satisfaction, compared with the general cohort of adults with AD: poor to low income (aOR, –1.82; P less than .0001), multiracial/other race (aOR, –2.34; P = .0001), Hispanic ethnicity (aOR, –1.40; P = .007), and having no insurance coverage (aOR, –4.53; P less than .0001).
“Moreover, those with multimorbidity had even lower satisfaction,” Mr. Cheng said. “In previous studies, AD has been linked with many other comorbidities. This may reflect that these patients are not being adequately managed overall. So, there’s a need here for multidisciplinary care to ensure that all of these comorbidities and the full spectrum of symptoms are being managed adequately.”
He concluded that future research is needed to determine strategies to optimize patient satisfaction in adults with AD.
“I’m not sure how much more provocative you can get in terms of data,” added Dr. Silverberg, director of clinical research and contact dermatitis at George Washington University, Washington. “It’s really eye-opening. I think many clinicians may feel like they’re doing a perfect job in managing this disease. These data suggest that at least at the national level that may not be the case.”
Mr. Cheng reported having no financial disclosures. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
.
Satisfaction scores were higher when specialists prescribed systemic therapy, but were lower when nonspecialists prescribed systemic therapy and when specialists prescribed only topical therapy.
Those are among key findings from an analysis of the Medical Expenditure Panel Surveys reported by Brian T. Cheng during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium.
“AD management is complex,” said Mr. Cheng, a medical student at Northwestern University, Chicago. “It includes patient education about trigger avoidance, over-the-counter and prescription topical therapies, as well as systemic therapies. Previous studies have shown major decrements to quality of life as well as atopic and non-atopic comorbidities in these patients. The burden of AD and their comorbidities, as well as their management, may impact patient satisfaction.”
Prior studies have demonstrated that patient satisfaction is associated with improvements in clinical outcomes, increased patient retention, and reduced malpractice claims (Br J Dermatol. 2001 Oct;145[4]:617-23, Arch Dermatol 2008 Feb;144[2]:263-5). However, since data on patient satisfaction in AD are limited, Mr. Cheng and the study’s senior author, Jonathan I. Silverberg, MD, PhD, MPH, set out to examine overall patient satisfaction among adults with AD, to determine associations of patient satisfaction with patterns of health care utilization, and to identify predictors of higher satisfaction among these adults.
The researchers conducted a cross-sectional retrospective analysis of 3,810 patients from the 2000-2015 Medical Expenditure Panel Surveys, representative surveys of the U.S. noninstitutionalized population conducted annually by the Agency for Healthcare Research and Quality. They used ICD-9 codes 691 and 692 to determine AD diagnosis and five Consumer Assessment of Health Plans Survey (CAHPS) questions to assess patients’ satisfaction with their clinicians. “These questions have been extensively validated to correlate with global satisfaction,” Mr. Cheng said. “These are not disease-specific and allow for comparison across multiple diseases.”
Next, the researchers created a composite satisfaction score based on the methods of Anthony Jerant, MD, of the University of California, Davis, and colleagues. They adjusted each question in the CAHPS survey to have an equal weight and then summed these into a composite satisfaction score. “We examined patient satisfaction comparing across diseases, and based on the guidelines from the AHRQ to isolate that impact of patient-physician interaction, we adjusted for sociodemographics, mental and physical health status, self-reported health rating, as well as multimorbidity and comorbid diseases.”
Compared with adults who are healthy, adults with AD had lower patient satisfaction overall. “Moreover, people with AD had lower satisfaction compared to those with psoriasis, which may reflect more substantial itch burden as well as the greater comorbid disease challenges in management,” Mr. Cheng said. “It may also reflect the renaissance in psoriasis treatment over the last 10-20 years, giving a wider spectrum of treatment and thus a higher patient satisfaction.”
Among adults with AD, lower satisfaction was consistent across all domains of CAHPS. For the question of “How often health providers listen carefully to you” the adjusted OR (aOR) was 0.87 (P = .008). For the question of “How often health providers explain things in a way that was easy to understand” the aOR was 0.89 (P = .003). For the question of “How often health providers spent enough time with you” the aOR was 0.86 (P = .0001). For “How often providers showed respect for what you had to say” the aOR was 0.91 (P = .02).
Recognizing that treatment regimens are complex and used differently by provider type, the researchers examined interactions between specialists (dermatologists and allergists) and treatment type. “Previous studies found dermatologists treat more severe, chronic AD,” Mr. Cheng said. “We found here that there was lower satisfaction among those treated with topical therapy and by specialists, which may reflect inadequate disease control. We also found lower satisfaction among those treated with systemic therapy by primary care physicians. This may reflect that these patients are not achieving optimal therapy. We found that satisfaction was highest among those treated with systemic therapy and by dermatologists and allergists.”
Socioeconomic, racial/ethnic, and health care disparities were observed in terms of satisfaction among this cohort. The following characteristics were significantly associated with lower patient satisfaction, compared with the general cohort of adults with AD: poor to low income (aOR, –1.82; P less than .0001), multiracial/other race (aOR, –2.34; P = .0001), Hispanic ethnicity (aOR, –1.40; P = .007), and having no insurance coverage (aOR, –4.53; P less than .0001).
“Moreover, those with multimorbidity had even lower satisfaction,” Mr. Cheng said. “In previous studies, AD has been linked with many other comorbidities. This may reflect that these patients are not being adequately managed overall. So, there’s a need here for multidisciplinary care to ensure that all of these comorbidities and the full spectrum of symptoms are being managed adequately.”
He concluded that future research is needed to determine strategies to optimize patient satisfaction in adults with AD.
“I’m not sure how much more provocative you can get in terms of data,” added Dr. Silverberg, director of clinical research and contact dermatitis at George Washington University, Washington. “It’s really eye-opening. I think many clinicians may feel like they’re doing a perfect job in managing this disease. These data suggest that at least at the national level that may not be the case.”
Mr. Cheng reported having no financial disclosures. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
.
Satisfaction scores were higher when specialists prescribed systemic therapy, but were lower when nonspecialists prescribed systemic therapy and when specialists prescribed only topical therapy.
Those are among key findings from an analysis of the Medical Expenditure Panel Surveys reported by Brian T. Cheng during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium.
“AD management is complex,” said Mr. Cheng, a medical student at Northwestern University, Chicago. “It includes patient education about trigger avoidance, over-the-counter and prescription topical therapies, as well as systemic therapies. Previous studies have shown major decrements to quality of life as well as atopic and non-atopic comorbidities in these patients. The burden of AD and their comorbidities, as well as their management, may impact patient satisfaction.”
Prior studies have demonstrated that patient satisfaction is associated with improvements in clinical outcomes, increased patient retention, and reduced malpractice claims (Br J Dermatol. 2001 Oct;145[4]:617-23, Arch Dermatol 2008 Feb;144[2]:263-5). However, since data on patient satisfaction in AD are limited, Mr. Cheng and the study’s senior author, Jonathan I. Silverberg, MD, PhD, MPH, set out to examine overall patient satisfaction among adults with AD, to determine associations of patient satisfaction with patterns of health care utilization, and to identify predictors of higher satisfaction among these adults.
The researchers conducted a cross-sectional retrospective analysis of 3,810 patients from the 2000-2015 Medical Expenditure Panel Surveys, representative surveys of the U.S. noninstitutionalized population conducted annually by the Agency for Healthcare Research and Quality. They used ICD-9 codes 691 and 692 to determine AD diagnosis and five Consumer Assessment of Health Plans Survey (CAHPS) questions to assess patients’ satisfaction with their clinicians. “These questions have been extensively validated to correlate with global satisfaction,” Mr. Cheng said. “These are not disease-specific and allow for comparison across multiple diseases.”
Next, the researchers created a composite satisfaction score based on the methods of Anthony Jerant, MD, of the University of California, Davis, and colleagues. They adjusted each question in the CAHPS survey to have an equal weight and then summed these into a composite satisfaction score. “We examined patient satisfaction comparing across diseases, and based on the guidelines from the AHRQ to isolate that impact of patient-physician interaction, we adjusted for sociodemographics, mental and physical health status, self-reported health rating, as well as multimorbidity and comorbid diseases.”
Compared with adults who are healthy, adults with AD had lower patient satisfaction overall. “Moreover, people with AD had lower satisfaction compared to those with psoriasis, which may reflect more substantial itch burden as well as the greater comorbid disease challenges in management,” Mr. Cheng said. “It may also reflect the renaissance in psoriasis treatment over the last 10-20 years, giving a wider spectrum of treatment and thus a higher patient satisfaction.”
Among adults with AD, lower satisfaction was consistent across all domains of CAHPS. For the question of “How often health providers listen carefully to you” the adjusted OR (aOR) was 0.87 (P = .008). For the question of “How often health providers explain things in a way that was easy to understand” the aOR was 0.89 (P = .003). For the question of “How often health providers spent enough time with you” the aOR was 0.86 (P = .0001). For “How often providers showed respect for what you had to say” the aOR was 0.91 (P = .02).
Recognizing that treatment regimens are complex and used differently by provider type, the researchers examined interactions between specialists (dermatologists and allergists) and treatment type. “Previous studies found dermatologists treat more severe, chronic AD,” Mr. Cheng said. “We found here that there was lower satisfaction among those treated with topical therapy and by specialists, which may reflect inadequate disease control. We also found lower satisfaction among those treated with systemic therapy by primary care physicians. This may reflect that these patients are not achieving optimal therapy. We found that satisfaction was highest among those treated with systemic therapy and by dermatologists and allergists.”
Socioeconomic, racial/ethnic, and health care disparities were observed in terms of satisfaction among this cohort. The following characteristics were significantly associated with lower patient satisfaction, compared with the general cohort of adults with AD: poor to low income (aOR, –1.82; P less than .0001), multiracial/other race (aOR, –2.34; P = .0001), Hispanic ethnicity (aOR, –1.40; P = .007), and having no insurance coverage (aOR, –4.53; P less than .0001).
“Moreover, those with multimorbidity had even lower satisfaction,” Mr. Cheng said. “In previous studies, AD has been linked with many other comorbidities. This may reflect that these patients are not being adequately managed overall. So, there’s a need here for multidisciplinary care to ensure that all of these comorbidities and the full spectrum of symptoms are being managed adequately.”
He concluded that future research is needed to determine strategies to optimize patient satisfaction in adults with AD.
“I’m not sure how much more provocative you can get in terms of data,” added Dr. Silverberg, director of clinical research and contact dermatitis at George Washington University, Washington. “It’s really eye-opening. I think many clinicians may feel like they’re doing a perfect job in managing this disease. These data suggest that at least at the national level that may not be the case.”
Mr. Cheng reported having no financial disclosures. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
FROM REVOLUTIONIZING AD 2020
Rather Than Bash the VA, Let’s Learn From Its Successes
A new report by the Veterans Healthcare Policy Institute (VHPI) documents how elements included in many bills passed on Capitol Hill have failed to improve the efficacy of mental health services for our nation’s former service members.1 The authors argue that while these efforts may be well intended, they often compound problems by squandering precious financial resources and stretching an already overtaxed workforce. Clearly, there are shortcomings in the US Department of Veterans Affairs (VA), our nation’s largest integrated health care system, but rather than bash the VA, as the media and Congress tend to favor, let’s learn from its successes as we improve its services.
To do this we must avoid several policy pitfalls. Consider, for example, the VA MISSION Act (38 USC § 1703), which aimed to increase veteran access to quality health care outside the VA system. Studies confirmed that private sector mental health providers are not ready to deliver veteran-specific mental health care.2,3 Indeed, a RAND report found that psychotherapists in the private sector were unlikely to have the requisite skills necessary to deliver high-quality mental health care to service members or veterans.4
The MISSION Act meant to fix this clinical deficit by directing that competency standards be set for non-VA mental health providers who treat veterans for posttraumatic stress disorder (PTSD), traumatic brain injury, and military sexual trauma. But to date, no minimum competency standards have been set for non-VA mental health providers who treat veterans’ common psychological conditions. A license is all they need.
Legislation like the MISSION Act and the newly passed Commander John Scott Hannon Act (38 USC § 101) also assume that veterans who are suicidal or have mental health problems and don’t go to the VA will seek care from private sector providers. Nothing is further from the truth. Many veterans are deeply resistant to seeking mental health care no matter where that care is delivered.4,5 Sometimes veterans believe that mental health problems are a sign of weakness and are loathe to seek help.
To address this issue, the VA pioneered models of integrated mental health and primary care services.6 This means that if a veteran goes to an outpatient primary care clinic at a VA medical facility or community-based outpatient clinic and discusses a mental health or substance abuse problem, the veteran can get immediate care with a mental health provider without making a separate mental health appointment. In addition, the VA already provides routine, annual screening for PTSD and sexual assault as well as depression and substance abuse at all its primary care clinics nationwide. Thanks to comprehensive screening (at a level unknown in most other health care systems) even if a veteran doesn’t spontaneously report a trauma history or mental health distress, VA is able to identify the problem and offer help right in the primary care clinic. This one-stop shopping reduces the shame and stigma of having to make an appointment with a mental health provider, allows treatment to begin immediately, and reduces no shows at follow-up appointments.
Other health care systems are trying to copy the VA model of integrated primary and mental health care, but given our fragmented insurance system, it’s not easy to replicate.7 According to Suzanne Gordon coauthor of the VHPI study, “This VA innovation encourages veterans, socialized by the military to conceal serious mental health problems, to get immediate help. So do many other VA programs, like peer support groups and networks. Legislation needs to strengthen, not weaken, such programs that are almost impossible to reproduce in the private sector.” Outside of VA, mental health challenges faced by veterans likely go undetected, and many veterans will not receive the care that might change, or even save, their lives.
VA best practices include an unprecedented national training initiative on 16 evidence-based psychotherapies that has been in operation for more than a decade.8 These high-quality treatments target debilitating conditions such as depression, PTSD, substance use disorders, insomnia, and chronic pain.9-13 More than 12,700 VA mental health providers have received training in these evidence-based psychotherapies.
“There is no way that non-VA health care systems can ever duplicate the quality of training and supervision that has now been provided, nationally, to VA mental health professionals,” Josef Ruzek, PhD, former Director of the VA National Center for PTSD Dissemination and Training Division told me in a phone conversation (January 14, 2021). “Their program of training and implementation in the very best treatments for veteran mental health conditions stands as an international model of a complex, well-executed, large-scale program to improve mental health service delivery and improve the outcomes of treatment.”
The VA not only paid for the training of these mental health providers, but also contributed substantial efforts to assist in the implementation and sustainability of such practices. These include policy changes mandating their availability at all VA facilities, designation of local evidence-based coordinators at each medical center, and even a nationwide PTSD mentoring program to help PTSD clinic managers make organizational changes and to guide the efforts of any VA clinician seeking advice on how to engage and work with a veteran living with PTSD.14 All these incredible dissemination and implementation endeavors have resulted in a substantial overall decrease in mental health symptoms and substance misuse behaviors and increase in functional outcomes, like improvement in relationship functioning and increase in quality of life for many veterans.
As a trauma psychologist and former VA employee, I urge lawmakers to assure that veterans are not sent to private sector providers who don’t understand their unique needs and aren’t trained to serve them well, and to similarly assure that systems of care are carefully designed to meet the specific needs of veterans.
1. Gordon S, Lemle RB, Ruzek JI, Kudler H. Creating effective solutions, programs, and policies to improve veterans’ mental health care. Published January 2021. Accessed February 22, 2021. https://static1.squarespace.com/static/5b19e25e89c1722037f0fdab/t/6018731daf20e7024b5d6aa8/1612215071469/VHPI_MHReport.pdf
2. Tanielian T, Farris C, Batka C, et al. Ready to serve: community-based provider capacity to deliver culturally competent, quality mental health care to veterans and their families. Published 2014. Accessed February 22, 2021. https://www.rand.org/pubs/research_reports/RR806.html
3. Tanielian T, Farmer CM, Burns RM, et al, Ready or not? Assessing the capacity of New York State health care providers to meet the needs of veterans. Published 2018. Accessed February 22, 2021. https://www.rand.org/pubs/research_reports/RR2298.html.
4. Crawford EF, Elbogen EB, Wagner HR, Kudler H, Calhoun PS, Brancu M, Straits-Troster KA. Surveying treatment preferences in U.S. Iraq-Afghanistan Veterans with PTSD symptoms: a step toward veteran-centered care. J Trauma Stress. 2015 Apr;28(2):118-26. doi: 10.1002/jts.21993.
5. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine 351:13–22, 2004
6. Zeiss AM, Karlin BE. Integrating mental health and primary care services in the Department of Veterans Affairs Health Care System. J Clin Psychol Med Settings. 2008;15(1):73-78. doi:10.1007/s10880-008-9100-4
7. Gordon S. Wounds of War: How the VA Delivers Health, Healing and Hope to the Nation’s Veterans. Cornell University Press; 2018.
8. Karlin BE, Cross G. From the laboratory to the therapy room: national dissemination and implementation of evidence-based psychotherapies in the U.S. Department of Veterans Affairs Health Care System. Am Psychol. 2014;69(1):19-33. doi:10.1037/a0033888
9. Stewart MO, Raffa SD, Steele JL, et al. National dissemination of interpersonal psychotherapy for depression in veterans: therapist and patient-level outcomes. J Consult Clin Psychol. 2014;82(6):1201-1206. doi:10.1037/a0037410
10. Karlin BE, Ruzek JI, Chard KM, et al. Dissemination of evidence-based psychological treatments for posttraumatic stress disorder in the Veterans Health Administration. J Trauma Stress. 2010;23(6):663-673. doi:10.1002/jts.20588
11. DeMarce JM, Gnys M, Raffa SD, Kumpula M, Karlin BE. Dissemination of cognitive behavioral therapy for substance use disorders in the Department of Veterans Affairs Health Care System: description and evaluation of veteran outcomes [published online ahead of print, 2019 Oct 23]. Subst Abus. 2019;1-7. doi:10.1080/08897077.2019.1674238
12. Karlin BE, Trockel M, Spira AP, Taylor CB, Manber R. National evaluation of the effectiveness of cognitive behavioral therapy for insomnia among older versus younger veterans. Int J Geriatr Psychiatry. 2015;30(3):308-315. doi:10.1002/gps.4143
13. Stewart MO, Karlin BE, Murphy JL, et al. National dissemination of cognitive-behavioral therapy for chronic pain in veterans: therapist and patient-level outcomes. Clin J Pain. 2015;31(8):722-729. doi:10.1097/AJP.0000000000000151
14. Bernardy NC, Hamblen JL, Friedman MJ, Ruzek JI, McFall ME. Implementation of a posttraumatic stress disorder mentoring program to improve treatment services. Psycholog Trauma. 2011;3(3):292-299. doi:10.1037/a0024847
A new report by the Veterans Healthcare Policy Institute (VHPI) documents how elements included in many bills passed on Capitol Hill have failed to improve the efficacy of mental health services for our nation’s former service members.1 The authors argue that while these efforts may be well intended, they often compound problems by squandering precious financial resources and stretching an already overtaxed workforce. Clearly, there are shortcomings in the US Department of Veterans Affairs (VA), our nation’s largest integrated health care system, but rather than bash the VA, as the media and Congress tend to favor, let’s learn from its successes as we improve its services.
To do this we must avoid several policy pitfalls. Consider, for example, the VA MISSION Act (38 USC § 1703), which aimed to increase veteran access to quality health care outside the VA system. Studies confirmed that private sector mental health providers are not ready to deliver veteran-specific mental health care.2,3 Indeed, a RAND report found that psychotherapists in the private sector were unlikely to have the requisite skills necessary to deliver high-quality mental health care to service members or veterans.4
The MISSION Act meant to fix this clinical deficit by directing that competency standards be set for non-VA mental health providers who treat veterans for posttraumatic stress disorder (PTSD), traumatic brain injury, and military sexual trauma. But to date, no minimum competency standards have been set for non-VA mental health providers who treat veterans’ common psychological conditions. A license is all they need.
Legislation like the MISSION Act and the newly passed Commander John Scott Hannon Act (38 USC § 101) also assume that veterans who are suicidal or have mental health problems and don’t go to the VA will seek care from private sector providers. Nothing is further from the truth. Many veterans are deeply resistant to seeking mental health care no matter where that care is delivered.4,5 Sometimes veterans believe that mental health problems are a sign of weakness and are loathe to seek help.
To address this issue, the VA pioneered models of integrated mental health and primary care services.6 This means that if a veteran goes to an outpatient primary care clinic at a VA medical facility or community-based outpatient clinic and discusses a mental health or substance abuse problem, the veteran can get immediate care with a mental health provider without making a separate mental health appointment. In addition, the VA already provides routine, annual screening for PTSD and sexual assault as well as depression and substance abuse at all its primary care clinics nationwide. Thanks to comprehensive screening (at a level unknown in most other health care systems) even if a veteran doesn’t spontaneously report a trauma history or mental health distress, VA is able to identify the problem and offer help right in the primary care clinic. This one-stop shopping reduces the shame and stigma of having to make an appointment with a mental health provider, allows treatment to begin immediately, and reduces no shows at follow-up appointments.
Other health care systems are trying to copy the VA model of integrated primary and mental health care, but given our fragmented insurance system, it’s not easy to replicate.7 According to Suzanne Gordon coauthor of the VHPI study, “This VA innovation encourages veterans, socialized by the military to conceal serious mental health problems, to get immediate help. So do many other VA programs, like peer support groups and networks. Legislation needs to strengthen, not weaken, such programs that are almost impossible to reproduce in the private sector.” Outside of VA, mental health challenges faced by veterans likely go undetected, and many veterans will not receive the care that might change, or even save, their lives.
VA best practices include an unprecedented national training initiative on 16 evidence-based psychotherapies that has been in operation for more than a decade.8 These high-quality treatments target debilitating conditions such as depression, PTSD, substance use disorders, insomnia, and chronic pain.9-13 More than 12,700 VA mental health providers have received training in these evidence-based psychotherapies.
“There is no way that non-VA health care systems can ever duplicate the quality of training and supervision that has now been provided, nationally, to VA mental health professionals,” Josef Ruzek, PhD, former Director of the VA National Center for PTSD Dissemination and Training Division told me in a phone conversation (January 14, 2021). “Their program of training and implementation in the very best treatments for veteran mental health conditions stands as an international model of a complex, well-executed, large-scale program to improve mental health service delivery and improve the outcomes of treatment.”
The VA not only paid for the training of these mental health providers, but also contributed substantial efforts to assist in the implementation and sustainability of such practices. These include policy changes mandating their availability at all VA facilities, designation of local evidence-based coordinators at each medical center, and even a nationwide PTSD mentoring program to help PTSD clinic managers make organizational changes and to guide the efforts of any VA clinician seeking advice on how to engage and work with a veteran living with PTSD.14 All these incredible dissemination and implementation endeavors have resulted in a substantial overall decrease in mental health symptoms and substance misuse behaviors and increase in functional outcomes, like improvement in relationship functioning and increase in quality of life for many veterans.
As a trauma psychologist and former VA employee, I urge lawmakers to assure that veterans are not sent to private sector providers who don’t understand their unique needs and aren’t trained to serve them well, and to similarly assure that systems of care are carefully designed to meet the specific needs of veterans.
A new report by the Veterans Healthcare Policy Institute (VHPI) documents how elements included in many bills passed on Capitol Hill have failed to improve the efficacy of mental health services for our nation’s former service members.1 The authors argue that while these efforts may be well intended, they often compound problems by squandering precious financial resources and stretching an already overtaxed workforce. Clearly, there are shortcomings in the US Department of Veterans Affairs (VA), our nation’s largest integrated health care system, but rather than bash the VA, as the media and Congress tend to favor, let’s learn from its successes as we improve its services.
To do this we must avoid several policy pitfalls. Consider, for example, the VA MISSION Act (38 USC § 1703), which aimed to increase veteran access to quality health care outside the VA system. Studies confirmed that private sector mental health providers are not ready to deliver veteran-specific mental health care.2,3 Indeed, a RAND report found that psychotherapists in the private sector were unlikely to have the requisite skills necessary to deliver high-quality mental health care to service members or veterans.4
The MISSION Act meant to fix this clinical deficit by directing that competency standards be set for non-VA mental health providers who treat veterans for posttraumatic stress disorder (PTSD), traumatic brain injury, and military sexual trauma. But to date, no minimum competency standards have been set for non-VA mental health providers who treat veterans’ common psychological conditions. A license is all they need.
Legislation like the MISSION Act and the newly passed Commander John Scott Hannon Act (38 USC § 101) also assume that veterans who are suicidal or have mental health problems and don’t go to the VA will seek care from private sector providers. Nothing is further from the truth. Many veterans are deeply resistant to seeking mental health care no matter where that care is delivered.4,5 Sometimes veterans believe that mental health problems are a sign of weakness and are loathe to seek help.
To address this issue, the VA pioneered models of integrated mental health and primary care services.6 This means that if a veteran goes to an outpatient primary care clinic at a VA medical facility or community-based outpatient clinic and discusses a mental health or substance abuse problem, the veteran can get immediate care with a mental health provider without making a separate mental health appointment. In addition, the VA already provides routine, annual screening for PTSD and sexual assault as well as depression and substance abuse at all its primary care clinics nationwide. Thanks to comprehensive screening (at a level unknown in most other health care systems) even if a veteran doesn’t spontaneously report a trauma history or mental health distress, VA is able to identify the problem and offer help right in the primary care clinic. This one-stop shopping reduces the shame and stigma of having to make an appointment with a mental health provider, allows treatment to begin immediately, and reduces no shows at follow-up appointments.
Other health care systems are trying to copy the VA model of integrated primary and mental health care, but given our fragmented insurance system, it’s not easy to replicate.7 According to Suzanne Gordon coauthor of the VHPI study, “This VA innovation encourages veterans, socialized by the military to conceal serious mental health problems, to get immediate help. So do many other VA programs, like peer support groups and networks. Legislation needs to strengthen, not weaken, such programs that are almost impossible to reproduce in the private sector.” Outside of VA, mental health challenges faced by veterans likely go undetected, and many veterans will not receive the care that might change, or even save, their lives.
VA best practices include an unprecedented national training initiative on 16 evidence-based psychotherapies that has been in operation for more than a decade.8 These high-quality treatments target debilitating conditions such as depression, PTSD, substance use disorders, insomnia, and chronic pain.9-13 More than 12,700 VA mental health providers have received training in these evidence-based psychotherapies.
“There is no way that non-VA health care systems can ever duplicate the quality of training and supervision that has now been provided, nationally, to VA mental health professionals,” Josef Ruzek, PhD, former Director of the VA National Center for PTSD Dissemination and Training Division told me in a phone conversation (January 14, 2021). “Their program of training and implementation in the very best treatments for veteran mental health conditions stands as an international model of a complex, well-executed, large-scale program to improve mental health service delivery and improve the outcomes of treatment.”
The VA not only paid for the training of these mental health providers, but also contributed substantial efforts to assist in the implementation and sustainability of such practices. These include policy changes mandating their availability at all VA facilities, designation of local evidence-based coordinators at each medical center, and even a nationwide PTSD mentoring program to help PTSD clinic managers make organizational changes and to guide the efforts of any VA clinician seeking advice on how to engage and work with a veteran living with PTSD.14 All these incredible dissemination and implementation endeavors have resulted in a substantial overall decrease in mental health symptoms and substance misuse behaviors and increase in functional outcomes, like improvement in relationship functioning and increase in quality of life for many veterans.
As a trauma psychologist and former VA employee, I urge lawmakers to assure that veterans are not sent to private sector providers who don’t understand their unique needs and aren’t trained to serve them well, and to similarly assure that systems of care are carefully designed to meet the specific needs of veterans.
1. Gordon S, Lemle RB, Ruzek JI, Kudler H. Creating effective solutions, programs, and policies to improve veterans’ mental health care. Published January 2021. Accessed February 22, 2021. https://static1.squarespace.com/static/5b19e25e89c1722037f0fdab/t/6018731daf20e7024b5d6aa8/1612215071469/VHPI_MHReport.pdf
2. Tanielian T, Farris C, Batka C, et al. Ready to serve: community-based provider capacity to deliver culturally competent, quality mental health care to veterans and their families. Published 2014. Accessed February 22, 2021. https://www.rand.org/pubs/research_reports/RR806.html
3. Tanielian T, Farmer CM, Burns RM, et al, Ready or not? Assessing the capacity of New York State health care providers to meet the needs of veterans. Published 2018. Accessed February 22, 2021. https://www.rand.org/pubs/research_reports/RR2298.html.
4. Crawford EF, Elbogen EB, Wagner HR, Kudler H, Calhoun PS, Brancu M, Straits-Troster KA. Surveying treatment preferences in U.S. Iraq-Afghanistan Veterans with PTSD symptoms: a step toward veteran-centered care. J Trauma Stress. 2015 Apr;28(2):118-26. doi: 10.1002/jts.21993.
5. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine 351:13–22, 2004
6. Zeiss AM, Karlin BE. Integrating mental health and primary care services in the Department of Veterans Affairs Health Care System. J Clin Psychol Med Settings. 2008;15(1):73-78. doi:10.1007/s10880-008-9100-4
7. Gordon S. Wounds of War: How the VA Delivers Health, Healing and Hope to the Nation’s Veterans. Cornell University Press; 2018.
8. Karlin BE, Cross G. From the laboratory to the therapy room: national dissemination and implementation of evidence-based psychotherapies in the U.S. Department of Veterans Affairs Health Care System. Am Psychol. 2014;69(1):19-33. doi:10.1037/a0033888
9. Stewart MO, Raffa SD, Steele JL, et al. National dissemination of interpersonal psychotherapy for depression in veterans: therapist and patient-level outcomes. J Consult Clin Psychol. 2014;82(6):1201-1206. doi:10.1037/a0037410
10. Karlin BE, Ruzek JI, Chard KM, et al. Dissemination of evidence-based psychological treatments for posttraumatic stress disorder in the Veterans Health Administration. J Trauma Stress. 2010;23(6):663-673. doi:10.1002/jts.20588
11. DeMarce JM, Gnys M, Raffa SD, Kumpula M, Karlin BE. Dissemination of cognitive behavioral therapy for substance use disorders in the Department of Veterans Affairs Health Care System: description and evaluation of veteran outcomes [published online ahead of print, 2019 Oct 23]. Subst Abus. 2019;1-7. doi:10.1080/08897077.2019.1674238
12. Karlin BE, Trockel M, Spira AP, Taylor CB, Manber R. National evaluation of the effectiveness of cognitive behavioral therapy for insomnia among older versus younger veterans. Int J Geriatr Psychiatry. 2015;30(3):308-315. doi:10.1002/gps.4143
13. Stewart MO, Karlin BE, Murphy JL, et al. National dissemination of cognitive-behavioral therapy for chronic pain in veterans: therapist and patient-level outcomes. Clin J Pain. 2015;31(8):722-729. doi:10.1097/AJP.0000000000000151
14. Bernardy NC, Hamblen JL, Friedman MJ, Ruzek JI, McFall ME. Implementation of a posttraumatic stress disorder mentoring program to improve treatment services. Psycholog Trauma. 2011;3(3):292-299. doi:10.1037/a0024847
1. Gordon S, Lemle RB, Ruzek JI, Kudler H. Creating effective solutions, programs, and policies to improve veterans’ mental health care. Published January 2021. Accessed February 22, 2021. https://static1.squarespace.com/static/5b19e25e89c1722037f0fdab/t/6018731daf20e7024b5d6aa8/1612215071469/VHPI_MHReport.pdf
2. Tanielian T, Farris C, Batka C, et al. Ready to serve: community-based provider capacity to deliver culturally competent, quality mental health care to veterans and their families. Published 2014. Accessed February 22, 2021. https://www.rand.org/pubs/research_reports/RR806.html
3. Tanielian T, Farmer CM, Burns RM, et al, Ready or not? Assessing the capacity of New York State health care providers to meet the needs of veterans. Published 2018. Accessed February 22, 2021. https://www.rand.org/pubs/research_reports/RR2298.html.
4. Crawford EF, Elbogen EB, Wagner HR, Kudler H, Calhoun PS, Brancu M, Straits-Troster KA. Surveying treatment preferences in U.S. Iraq-Afghanistan Veterans with PTSD symptoms: a step toward veteran-centered care. J Trauma Stress. 2015 Apr;28(2):118-26. doi: 10.1002/jts.21993.
5. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine 351:13–22, 2004
6. Zeiss AM, Karlin BE. Integrating mental health and primary care services in the Department of Veterans Affairs Health Care System. J Clin Psychol Med Settings. 2008;15(1):73-78. doi:10.1007/s10880-008-9100-4
7. Gordon S. Wounds of War: How the VA Delivers Health, Healing and Hope to the Nation’s Veterans. Cornell University Press; 2018.
8. Karlin BE, Cross G. From the laboratory to the therapy room: national dissemination and implementation of evidence-based psychotherapies in the U.S. Department of Veterans Affairs Health Care System. Am Psychol. 2014;69(1):19-33. doi:10.1037/a0033888
9. Stewart MO, Raffa SD, Steele JL, et al. National dissemination of interpersonal psychotherapy for depression in veterans: therapist and patient-level outcomes. J Consult Clin Psychol. 2014;82(6):1201-1206. doi:10.1037/a0037410
10. Karlin BE, Ruzek JI, Chard KM, et al. Dissemination of evidence-based psychological treatments for posttraumatic stress disorder in the Veterans Health Administration. J Trauma Stress. 2010;23(6):663-673. doi:10.1002/jts.20588
11. DeMarce JM, Gnys M, Raffa SD, Kumpula M, Karlin BE. Dissemination of cognitive behavioral therapy for substance use disorders in the Department of Veterans Affairs Health Care System: description and evaluation of veteran outcomes [published online ahead of print, 2019 Oct 23]. Subst Abus. 2019;1-7. doi:10.1080/08897077.2019.1674238
12. Karlin BE, Trockel M, Spira AP, Taylor CB, Manber R. National evaluation of the effectiveness of cognitive behavioral therapy for insomnia among older versus younger veterans. Int J Geriatr Psychiatry. 2015;30(3):308-315. doi:10.1002/gps.4143
13. Stewart MO, Karlin BE, Murphy JL, et al. National dissemination of cognitive-behavioral therapy for chronic pain in veterans: therapist and patient-level outcomes. Clin J Pain. 2015;31(8):722-729. doi:10.1097/AJP.0000000000000151
14. Bernardy NC, Hamblen JL, Friedman MJ, Ruzek JI, McFall ME. Implementation of a posttraumatic stress disorder mentoring program to improve treatment services. Psycholog Trauma. 2011;3(3):292-299. doi:10.1037/a0024847
Anticipating the care adolescents will need
Adolescents are an increasingly diverse population reflecting changes in the racial, ethnic, and geopolitical milieus of the United States. The World Health Organization classifies adolescence as ages 10 to 19 years.1 However, given the complexity of adolescent development physically, behaviorally, emotionally, and socially, others propose that adolescence may extend to age 24.2
Recognizing the specific challenges adolescents face is key to providing comprehensive longitudinal health care. Moreover, creating an environment of trust helps to ensure open 2-way communication that can facilitate anticipatory guidance.
Our review focuses on common adolescent issues, including injury from vehicles and firearms, tobacco and substance misuse, obesity, behavioral health, sexual health, and social media use. We discuss current trends and recommend strategies to maximize health and wellness.
Start by framing the visit
Confidentiality
Laws governing confidentiality in adolescent health care vary by state. Be aware of the laws pertaining to your practice setting. In addition, health care facilities may have their own policies regarding consent and confidentiality in adolescent care. Discuss confidentiality with both an adolescent and the parent/guardian at the initial visit. And, to help avoid potential misunderstandings, let them know in advance what will (and will not) be divulged.
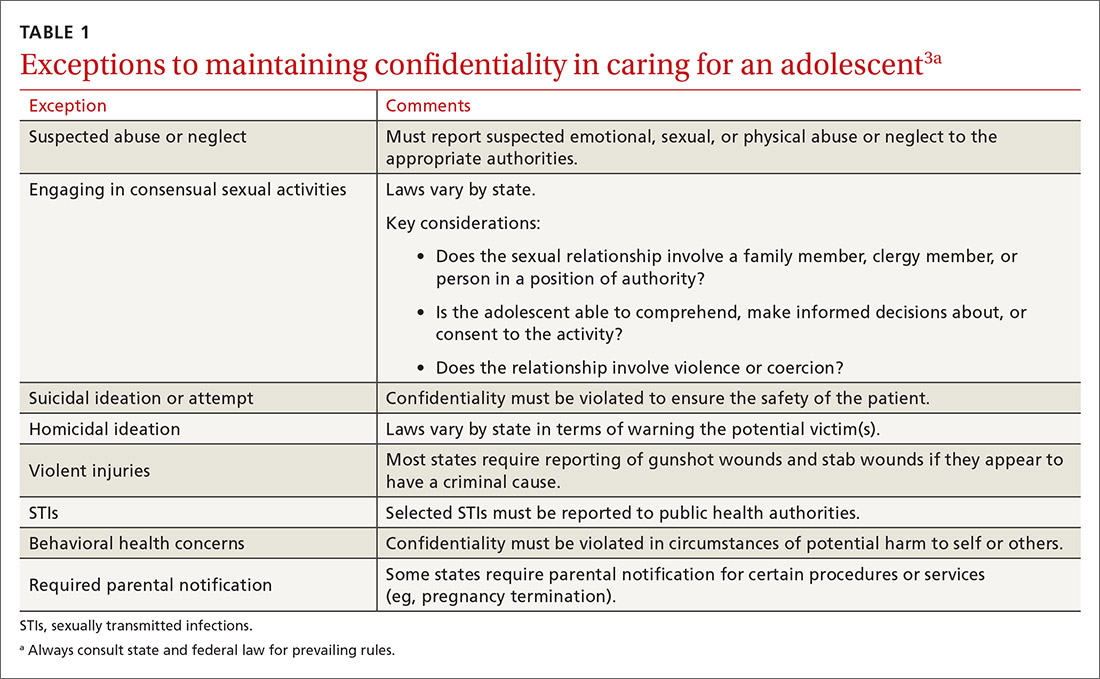
The American Academy of Pediatrics has developed a useful tip sheet regarding confidentiality laws (www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/healthy-foster-care-america/Documents/Confidentiality_Laws.pdf). Examples of required (conditional) disclosure include abuse and suicidal or homicidal ideations. Patients should understand that sexually transmitted infections (STIs) are reportable to public health authorities and that potentially injurious behaviors to self or others (eg, excessive drinking prior to driving) may also warrant disclosure(TABLE 13).
Privacy and general visit structure
Create a safe atmosphere where adolescents can discuss personal issues without fear of repercussion or judgment. While parents may prefer to be present during the visit, allowing for time to visit independently with an adolescent offers the opportunity to reinforce issues of privacy and confidentiality. Also discuss your office policies regarding electronic communication, phone communication, and relaying test results.

A useful paradigm for organizing a visit for routine adolescent care is to use an expanded version of the HEADSS mnemonic (TABLE 24,5), which includes questions about an adolescent’s Home, Education, Activities, Drug and alcohol use, Sexual behavior, Suicidality and depression, and other topics. Other validated screening tools include RAAPS (Rapid Adolescent Prevention Screening)6 (www.possibilitiesforchange.com/raaps/); the Guidelines for Adolescent Preventive Services7; and the Bright Futures recommendations for preventive care from the American Academy of Pediatrics.8 Below, we consider important topics addressed with the HEADSS approach.

Continue to: Injury from vehicles and firearms
Injury from vehicles and firearms
Motor vehicle accidents and firearm wounds are the 2 leading causes of adolescent injury. In 2016, of the more than 20,000 deaths in children and adolescents (ages 1-19 years), 20% were due to motor vehicle accidents (4074) and 15% were a result of firearm-related injuries (3143). Among firearm-related deaths, 60% were homicides, 35% were suicides, and 4% were due to accidental discharge.9 The rate of firearm-related deaths among American teens is 36 times greater than that of any other developed nation.9 Currently, 1 of every 3 US households with children younger than 18 has a firearm. Data suggest that in 43% of these households, the firearm is loaded and kept in an unlocked location.10
To aid anticipatory guidance, ask adolescents about firearm and seat belt use, drinking and driving, and suicidal thoughts (TABLE 24,5). Advise them to always wear seat belts whether driving or riding as a passenger. They should never drink and drive (or get in a car with someone who has been drinking). Advise parents that if firearms are present in the household, they should be kept in a secure, locked location. Weapons should be separated from ammunition and safety mechanisms should be engaged on all devices.
Tobacco and substance misuse
Tobacco use, the leading preventable cause of death in the United States,11 is responsible for more deaths than alcohol, motor vehicle accidents, suicides, homicides, and HIV disease combined.12 Most tobacco-associated mortality occurs in individuals who began smoking before the age of 18.12 Individuals who start smoking early are also more likely to continue smoking through adulthood.
Encouragingly, tobacco use has declined significantly among adolescents over the past several decades. Roughly 1 in 25 high school seniors reports daily tobacco use.13 Adolescent smoking behaviors are also changing dramatically with the increasing popularity of electronic cigarettes (“vaping”). Currently, more adolescents vape than smoke cigarettes.13 Vaping has additional health risks including toxic lung injury.
Multiple resources can help combat tobacco and nicotine use in adolescents. The US Preventive Services Task Force recommends that primary care clinicians intervene through education or brief counselling to prevent initiation of tobacco use in school-aged children and adolescents.14 Ask teens about tobacco and electronic cigarette use and encourage them to quit when use is acknowledged. Other helpful office-based tools are the “Quit Line” 800-QUIT-NOW and texting “Quit” to 47848. Smokefree teen (https://teen.smokefree.gov/) is a website that reviews the risks of tobacco and nicotine use and provides age-appropriate cessation tools and tips (including a smartphone app and a live-chat feature). Other useful information is available in a report from the Surgeon General on preventing tobacco use among young adults.15
Continue to: Alcohol use
Alcohol use. Three in 5 high school students report ever having used alcohol.13 As with tobacco, adolescent alcohol use has declined over the past decade. However, binge drinking (≥ 5 drinks on 1 occasion for males; ≥ 4 drinks on 1 occasion for females) remains a common high-risk behavior among adolescents (particularly college students). Based on the Monitoring the Future Survey, 1 in 6 high school seniors reported binge drinking in the past 2 weeks.13 While historically more common among males, rates of binge drinking are now basically similar between male and female adolescents.13
The National Institute on Alcohol Abuse and Alcoholism has a screening and intervention guide specifically for adolescents.16
Illicit drug use. Half of adolescents report using an illicit drug by their senior year in high school.13 Marijuana is the most commonly used substance, and laws governing its use are rapidly changing across the United States. Marijuana is illegal in 10 states and legal in 10 states (and the District of Columbia). The remaining states have varying policies on the medical use of marijuana and the decriminalization of marijuana. In addition, cannabinoid (CBD) products are increasingly available. Frequent cannabis use in adolescence has an adverse impact on general executive function (compared with adult users) and learning.17 Marijuana may serve as a gateway drug in the abuse of other substances,18 and its use should be strongly discouraged in adolescents.
Of note, there has been a sharp rise in the illicit use of prescription drugs, particularly opioids, creating a public health emergency across the United States.19 In 2015, more than 4000 young people, ages 15 to 24, died from a drug-related overdose (> 50% of these attributable to opioids).20 Adolescents with a history of substance abuse and behavioral illness are at particular risk. Many adolescents who misuse opioids and other prescription drugs obtain them from friends and relatives.21
The Substance Abuse and Mental Health Services Administration (SAMHSA) recommends universal screening of adolescents for substance abuse. This screening should be accompanied by a brief intervention to prevent, mitigate, or eliminate substance use, or a referral to appropriate treatment sources. This process of screening, brief intervention, and referral to treatment (SBIRT) is recommended as part of routine health care.22
Continue to: Obesity and physical activity
Obesity and physical activity
The percentage of overweight and obese adolescents in the United States has more than tripled over the past 40 years,23 and 1 in 5 US adolescents is obese.23 Obese teens are at higher risk for multiple chronic diseases, including type 2 diabetes, sleep apnea, and heart disease.24 They are also more likely to be bullied and to have poor self-esteem.25 Only 1 in 5 American high school students engages in 60 or more minutes of moderate-to-vigorous physical activity on 5 or more days per week.26
Regular physical activity is, of course, beneficial for cardiorespiratory fitness, bone health, weight control, and improved indices of behavioral health.26 Adolescents who are physically active consistently demonstrate better school attendance and grades.17 Higher levels of physical fitness are also associated with improved overall cognitive performance.24
General recommendations. The Department of Health and Human Services recommends that adolescents get at least 60 minutes of mostly moderate physical activity every day.26 Encourage adolescents to engage in vigorous physical activity (heavy breathing, sweating) at least 3 days a week. As part of their physical activity patterns, adolescents should also engage in muscle-strengthening and bone-strengthening activities on at least 3 days per week.
Behavioral health
As young people develop their sense of personal identity, they also strive for independence. It can be difficult, at times, to differentiate normal adolescent rebellion from true mental illness. An estimated 17% to 19% of adolescents meet criteria for mental illness, and about 7% have a severe psychiatric disorder.27 Only one-third of adolescents with mental illness receive any mental health services.28
Depression. The 1-year incidence of major depression in adolescents is 3% to 4%, and the lifetime prevalence of depressive symptoms is 25% in all high school students.27 Risk factors include ethnic minority status, poor self-esteem, poor health, recent personal crisis, insomnia, and alcohol/substance abuse. Depression in adolescent girls is correlated with becoming sexually active at a younger age, failure to use contraception, having an STI, and suicide attempts. Depressed boys are more likely to have unprotected intercourse and participate in physical fights.29 Depressed teens have a 2- to 3-fold greater risk for behavioral disorders, anxiety, and attention-deficit/hyperactivity disorder (ADHD).30
Continue to: Suicide
Suicide. Among individuals 15 to 29 years of age, suicide is the second leading cause of death globally, with an annual incidence of 11 to 15 per 100,000.31 Suicide attempts are 10 to 20 times more common than completed suicide.31 Males are more likely than females to die by suicide,32 and boys with a history of attempted suicide have a 30-fold increased risk of subsequent successful suicide.31 Hanging, drug poisoning, and firearms (particularly for males) are the most common means of suicide in adolescents. More than half of adolescents dying by suicide have coexisting depression.31
Characteristics associated with suicidal behaviors in adolescents include impulsivity, poor problem-solving skills, and dichotomous thinking.31 There may be a genetic component as well. In 1 of 5 teenage suicides, a precipitating life event such as the break-up of a relationship, cyber-bullying, or peer rejection is felt to contribute.31
ADHD. The prevalence of ADHD is 7% to 9% in US school-aged children.33 Boys more commonly exhibit hyperactive behaviors, while girls have more inattention. Hyperactivity often diminishes in teens, but inattention and impulsivity persist. Sequelae of ADHD include high-risk sexual behaviors, motor vehicle accidents, incarceration, and substance abuse.34 Poor self-esteem, suicidal ideation, smoking, and obesity are also increased.34 ADHD often persists into adulthood, with implications for social relationships and job performance.34
Eating disorders. The distribution of eating disorders is now known to increasingly include more minorities and males, the latter representing 5% to 10% of cases.35 Eating disorders show a strong genetic tendency and appear to be accelerated by puberty. The most common eating disorder (diagnosed in 0.8%-14% of teens) is eating disorder not otherwise specified (NOS).35 Anorexia nervosa is diagnosed in 0.5% of adolescent girls, and bulimia nervosa in 1% to 2%—particularly among athletes and performers.35 Unanticipated loss of weight, amenorrhea, excessive concern about weight, and deceleration in height/weight curves are potential indicators of an eating disorder. When identified, eating disorders are best managed by a trusted family physician, acting as a coordinator of a multidisciplinary team.
Sexual health
Girls begin to menstruate at an average age of 12, and it takes about 4 years for them to reach reproductive maturity.36 Puberty has been documented to start at younger ages over the past 30 years, likely due to an increase in average body mass index and a decrease in levels of physical activity.37 Girls with early maturation are often insecure and self-conscious, with higher levels of psychological distress.38 In boys, the average age for spermarche (first ejaculation) is 13.39 Boys who mature early tend to be taller, be more confident, and express a good body image.40 Those who have early puberty are more likely to be sexually active or participate in high-risk behaviors.41
Continue to: Pregnancy and contraception
Pregnancy and contraception
Over the past several decades, more US teens have been abstaining from sexual intercourse or have been using effective forms of birth control, particularly condoms and long-acting reversible contraceptives (LARCs).42 Teenage birth rates in girls ages 15 to 19 have declined significantly since the 1980s.42 Despite this, the teenage birth rate in the United States remains higher than in other industrialized nations, and most teen pregnancies are unintended.
There are numerous interventions to reduce teen pregnancy, including sex education, contraceptive counseling, the use of mobile apps that track a user’s monthly fertility cycle or issue reminders to take oral contraceptives,45 and the liberal distribution of contraceptives and condoms. The Contraceptive CHOICE Project shows that providing free (or low-cost) LARCs influences young women to choose these as their preferred contraceptive method.46 Other programs specifically empower girls to convince partners to use condoms and to resist unwanted sexual advances or intimate partner violence.
Adolescents prefer to have their health care providers address the topic of sexual health. Teens are more likely to share information with providers if asked directly about sexual behaviors.47TABLE 24,5 offers tips for anticipatory guidance and potential ways to frame questions with adolescents in this context. State laws vary with regard to the ability of minors to seek contraception, pregnancy testing, or care/screening for STIs without parental consent. Contraceptive counseling combined with effective screening decrease the incidence of STIs and pelvic inflammatory disease for sexually active teens.48

Sexually transmitted infections
Young adolescents often have a limited ability to imagine consequences related to specific actions. In general, there is also an increased desire to engage in experimental behaviors as an expression of developing autonomy, which may expose them to STIs. About half of all STIs contracted in the United States occur in individuals 15 to 24 years of age.49 Girls are at particular risk for the sequelae of these infections, including cervical dysplasia and infertility. Many teens erroneously believe that sexual activities other than intercourse decrease their risk of contracting an STI.50
Human papillomavirus (HPV) infection is the most common STI in adolescence.51 In most cases, HPV is transient and asymptomatic. Oncogenic strains may cause cervical cancer or cancers of the anogenital or oropharyngeal systems. Due to viral latency, it is not recommended to perform HPV typing in men or in women younger than 30 years of age; however, Pap tests are recommended every 3 years for women ages 21 to 29. Primary care providers are pivotal in the public health struggle to prevent HPV infection.
Continue to: Universal immunization of all children...
Universal immunization of all children older than 11 years of age against HPV is strongly advised as part of routine well-child care. Emphasize the proven role of HPV vaccination in preventing cervical52 and oropharyngeal53 cancers. And be prepared to address concerns raised by parents in the context of vaccine safety and the initiation of sexual behaviors (www.cdc.gov/hpv/hcp/answering-questions.html).
Chlamydia is the second most common STI in the United States, usually occurring in individuals younger than 24.54 The CDC estimates that more than 3 million new chlamydial infections occur yearly. These infections are often asymptomatic, particularly in females, but may cause urethritis, cervicitis, epididymitis, proctitis, or pelvic inflammatory disease. Indolent chlamydial infection is the leading cause of tubal infertility in women.54 Routine annual screening for chlamydia is recommended for all sexually active females ≤ 25 years (and for older women with specific risks).55 Annual screening is also recommended for men who have sex with men (MSM).55
Chlamydial infection may be diagnosed with first-catch urine sampling (men or women), urethral swab (men), endocervical swab (women), or self-collected vaginal swab. Nucleic acid amplification testing is the most sensitive test that is widely available.56 First-line treatment includes either azithromycin (1 g orally, single dose) or doxycycline (100 mg orally, twice daily for 7 days).56
Gonorrhea. In 2018, there were more than 500,000 annual cases of gonorrhea, with the majority occurring in those between 15 and 24 years of age.57 Gonorrhea may increase rates of HIV infection transmission up to 5-fold.57 As more adolescents practice oral sex, cases of pharyngeal gonorrhea (and oropharyngeal HPV) have increased. Symptoms of urethritis occur more frequently in men. Screening is recommended for all sexually active women younger than 25.56 Importantly, the organism Neisseria gonorrhoeae has developed significant antibiotic resistance over the past decade. The CDC currently recommends dual therapy for the treatment of gonorrhea using 250 mg of intramuscular ceftriaxone and 1 g of oral azithromycin.56
Syphilis. Rates of syphilis are increasing among individuals ages 15 to 24.51 Screening is particularly recommended for MSM and individuals infected with HIV. Benzathine penicillin G, 50,000 U/kg IM, remains the treatment of choice.56
Continue to: HIV
HIV. Globally, HIV impacts young people disproportionately. HIV infection also facilitates infection with other STIs. In the United States, the highest burden of HIV infection is borne by young MSM, with prevalence among those 18 to 24 years old varying between 26% to 30% (black) and 3% to 5.5% (non-Hispanic white).51 The use of emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis (PrEP) has recently been approved for the prevention of HIV. PrEP reduces risk by up to 92% for MSM and transgender women.58
Sexual identity
One in 10 high school students self-identifies as “nonheterosexual,” and 1 in 15 reports same-sex sexual contact.59 The term LGBTQ+ includes the communities of lesbian, gay, bisexual, transgender, transsexual, queer, questioning, intersex, and asexual individuals. Developing a safe sense of sexual identity is fundamental to adolescent psychological development, and many adolescents struggle to develop a positive sexual identity. Suicide rates and self-harm behaviors among LGBTQ+ adolescents can be 4 times higher than among their heterosexual peers.60 Rates of mood disorders, substance abuse, and high-risk sexual behaviors are also increased in the LGBTQ+ population.61
The LGBTQ+ community often seeks health care advice and affirmation from primary care providers. Resources to enhance this care are available at www.lgbthealtheducation.org.
Social media
Adolescents today have more media exposure than any prior generation, with smartphone and computer use increasing exponentially. Most (95%) teens have access to a smartphone,62 45% describe themselves as constantly connected to the Internet, and 14% feel that social media is “addictive.”62 Most manage their social media portfolio on multiple sites. Patterns of adolescents' online activities show that boys prefer online gaming, while girls tend to spend more time on social networking.62
Whether extensive media use is psychologically beneficial or deleterious has been widely debated. Increased time online correlates with decreased levels of physical activity.63 And sleep disturbances have been associated with excessive screen time and the presence of mobile devices in the bedroom.64 The use of social media prior to bedtime also has an adverse impact on academic performance—particularly for girls. This adverse impact on academics persists after correcting for daytime sleepiness, body mass index, and number of hours spent on homework.64
Continue to: Due to growing concerns...
Due to growing concerns about the risks of social media in children and adolescents, the American Academy of Pediatrics has developed the Family Media Plan (www.healthychildren.org/English/media/Pages/default.aspx). Some specific questions that providers may ask are outlined in TABLE 3.64 The Family Media Plan can provide age-specific guidelines to assist parents or caregivers in answering these questions.
Cyber-bullying. One in 3 adolescents (primarily female) has been a victim of cyber-bullying.65 Sadly, 1 in 5 teens has received some form of electronic sexual solicitation.66 The likelihood of unsolicited stranger contact correlates with teens’ online habits and the amount of information disclosed. Predictors include female sex, visiting chat rooms, posting photos, and disclosing personal information. Restricting computer use to an area with parental supervision or installing monitoring programs does not seem to exert any protective influence on cyber-bullying or unsolicited stranger contact.65 While 63% of cyber-bullying victims feel upset, embarrassed, or stressed by these contacts,66 few events are actually reported. To address this, some states have adopted laws adding cyber-bullying to school disciplinary codes.
Negative health impacts associated with cyber-bullying include anxiety, sadness, and greater difficulty in concentrating on school work.65 Victims of bullying are more likely to have school disciplinary actions and depression and to be truant or to carry weapons to school.66 Cyber-bullying is uniquely destructive due to its ubiquitous presence. A sense of relative anonymity online may encourage perpetrators to act more cruelly, with less concern for punishment.
Young people are also more likely to share passwords as a sign of friendship. This may result in others assuming their identity online. Adolescents rarely disclose bullying to parents or other adults, fearing restriction of Internet access, and many of them think that adults may downplay the seriousness of the events.66
CORRESPONDENCE
Mark B. Stephens, MD, Penn State Health Medical Group, 1850 East Park Avenue, State College, PA 16803; [email protected].
1. World Health Organization. Adolescent health. Accessed February 23, 2021. www.who.int/maternal_child_adolescent/adolescence/en/
2. Sawyer SM, Azzopardi PS, Wickremarathne D, et al. The age of adolescence. Lancet Child Adolesc Health. 2018;2:223-228.
3. Pathak PR, Chou A. Confidential care for adoloscents in the U.S. healthcare system. J Patient Cent Res Rev. 2019;6:46-50.
4. AMA Journal of Ethics. HEADSS: the “review of systems” for adolescents. Accessed February 23, 2021. https://journalofethics.ama-assn.org/article/headss-review-systems-adolescents/2005-03
5. Cohen E, MacKenzie RG, Yates GL. HEADSS, a psychosocial risk assessment instrument: implications for designing effective intervention programs for runaway youth. J Adolesc Health. 1991;12:539-544.
6. Possibilities for Change. Rapid Adolescent Prevention Screening (RAAPS). Accessed February 23, 2021. www.possibilitiesforchange.com/raaps/
7. Elster AB, Kuznets NJ. AMA Guidelines for Adolescent Preventive Services (GAPS): Recommendations and Rationale. Williams & Wilkins; 1994.
8. AAP. Engaging patients and families - periodicity schedule. Accessed February 23, 2021. www.aap.org/en-us/professional-resources/practice-support/Pages/PeriodicitySchedule.aspx
9. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Eng J Med. 2018;379:2468-2475.
10. Schuster MA, Franke TM, Bastian AM, et al. Firearm storage patterns in US homes with children. Am J Public Health. 2000;90:588-594.
11. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States. JAMA. 2004;291:1238-1245.
12. HHS. Health consequences of smoking, surgeon general fact sheet. Accessed February 23, 2021. www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/consequences-smoking-factsheet/index.html
13. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future: national survey results on drug use, 1975-2017. The University of Michigan. 2018. Accessed February 23, 2021. https://eric.ed.gov/?id=ED589762
14. US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
15. HHS. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: HHS, CDC, NCCDPHP, OSH; 2012. Accessed February 23, 2021. www.ncbi.nlm.nih.gov/books/NBK99237/
16. NIH. Alcohol screening and brief intervention for youth: a pocket guide. Accessed February 23, 2021. https://pubs.niaaa.nih.gov/publications/Practitioner/YouthGuide/YouthGuidePocket.pdf
17. Gorey C, Kuhns L, Smaragdi E, et al. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269:37-58.
18. Secades-Villa R, Garcia-Rodriguez O, Jin CJ, et al. Probability and predictors of the cannabis gateway effect: a national study. Int J Drug Policy. 2015;26:135-142.
19. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance—United States, 2017. MMWR Surveill Summ. 2018;67:1-114.
20. NIH. Drug overdoses in youth. How do drug overdoses happen?. Accessed February 23, 2021. https://teens.drugabuse.gov/drug-facts/drug-overdoses-youth
21. Branstetter SA, Low S, Furman W. The influence of parents and friends on adolescent substance use: a multidimensional approach. J Subst Use. 2011;162:150-160.
22. AAP. Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161210.
23. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;288:1-8.
24. Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13:6-13.
25. Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes. 2010;5:282-304.
26. National Physical Activity Plan Alliance. The 2018 United States report card on physical activity for children and youth. Accessed February 23, 2021. http://physicalactivityplan.org/projects/PA/2018/2018%20US%20Report%20Card%20Full%20Version_WEB.PDF?pdf=page-link
27. HHS. NIMH. Child and adolescent mental health. Accessed February 23, 2021. www.nimh.nih.gov/health/topics/child-and-adolescent-mental-health/index.shtml
28. Yonek JC, Jordan N, Dunlop D, et al. Patient-centered medical home care for adolescents in need of mental health treatment. J Adolesc Health. 2018;63:172-180.
29. Brooks TL, Harris SK, Thrall JS, et al. Association of adolescent risk behaviors with mental health symptoms in high school students. |J Adolesc Health. 2002;31:240-246.
30. Weller BE, Blanford KL, Butler AM. Estimated prevalence of psychiatric comorbidities in US adolescents with depression by race/ethnicity, 2011-2012. J Adolesc Health. 2018;62:716-721.
31. Bilsen J. Suicide and youth: risk factors. Front Psychiatry. 2018;9:540.
32. Shain B, AAP Committee on Adolescence. Suicide and suicide attempts in adolescents. Pediatrics. 2016;138:e20161420.
33. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: review and future directions. J Adolesc Health. 2016;59:135-143.
34. Bravender T. Attention-deficit/hyperactivity disorder and disordered eating. [editorial] J Adolesc Health. 2017;61:125-126.
35. Rosen DS, AAP Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics. 2010;126:1240-1253.
36. Susman EJ, Houts RM, Steinberg L, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 ½ and 15 ½ years. Arch Pediatr Adolesc Med. 2010;164:166-173.
37. Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(suppl 3):S208-S217.
38. Ge X, Conger RD, Elder GH. Coming of age too early: pubertal influences on girl’s vulnerability to psychologic distress. Child Dev. 1996;67:3386-3400.
39. Jørgensen M, Keiding N, Skakkebaek NE. Estimation of spermarche from longitudinal spermaturia data. Biometrics. 1991;47:177-193.
40. Kar SK, Choudhury A, Singh AP. Understanding normal development of adolescent sexuality: a bumpy ride. J Hum Reprod Sci. 2015;8:70-74.
41. Susman EJ, Dorn LD, Schiefelbein VL. Puberty, sexuality and health. In: Lerner MA, Easterbrooks MA, Mistry J (eds). Comprehensive Handbook of Psychology. Wiley; 2003.
42. Lindberg LD, Santelli JS, Desai S. Changing patterns of contraceptive use and the decline in rates of pregnancy and birth among U.S. adolescents, 2007-2014. J Adolesc Health. 2018;63:253-256.
43. Guttmacher Institute. Teen pregnancy. www.guttmacher.org/united-states/teens/teen-pregnancy. Accessed February 23, 2021.
44. CDC. Social determinants and eliminating disparities in teen pregnancy. Accessed February 23, 2021. www.cdc.gov/teenpregnancy/about/social-determinants-disparities-teen-pregnancy.htm
45. Widman L, Nesi J, Kamke K, et al. Technology-based interventions to reduce sexually transmitted infection and unintended pregnancy among youth. J Adolesc Health. 2018;62:651-660.
46. Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-115.e7.
47. Ham P, Allen C. Adolescent health screening and counseling. Am Fam Physician. 2012;86:1109-1116.
48. ACOG. Committee on Adolescent Health Care. Adolescent pregnancy, contraception and sexual activity. 2017. Accessed February 23, 2021. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/adolescent-pregnancy-contraception-and-sexual-activity
49. Wangu Z, Burstein GR. Adolescent sexuality: updates to the sexually transmitted infection guidelines. Pediatr Clin N Am. 2017;64:389-411.
50. Holway GV, Hernandez SM. Oral sex and condom use in a U.S. national sample of adolescents and young adults. J Adolesc Health. 2018;62:402-410.
51. CDC. STDs in adults and adolescents. Accessed February 23, 2021. www.cdc.gov/std/stats17/adolescents.htm
52. McClung N, Gargano J, Bennett N, et al. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008-2014. Accessed February 23, 2021. https://cebp.aacrjournals.org/content/28/3/602
53. Timbang MR, Sim MW, Bewley AF, et al. HPV-related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum Vaccin Immunother. 2019;15:1920-1928.
54. Carey AJ, Beagley KW. Chlamydia trachomatis, a hidden epidemic: effects on female reproduction and options for treatment. Am J Reprod Immunol. 2010;63:576-586.
55. USPSTF. Chlamydia and gonorrhea screening. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening
56. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1-135.
57. CDC. Sexually transmitted disease surveillance 2018. Accessed February 23, 2021. www.cdc.gov/std/stats18/gonorrhea.htm
5
59. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance–United States, 2015. MMWR Surveill Summ. 2016;65:1-174.
60. CDC. LGBT youth. Accessed February 23, 2021. www.cdc.gov/lgbthealth/youth.htm
61. Johns MM, Lowry R, Rasberry CN, et al. Violence victimization, substance use, and suicide risk among sexual minority high school students – United States, 2015-2017. MMWR Morb Mortal Wkly Rep. 2018;67:1211-1215.
62. Pew Research Center. Teens, social media & technology 2018. . Accessed February 23, 2021. www.pewinternet.org/2018/05/31/teens-social-media-technology-2018/
63. Chassiakos YLR, Radesky J, Christakis D, et al. Children and adolescents and digital media. Pediatrics. 2016;138:e20162593.
64. Arora T, Albahri A, Omar OM, et al. The prospective association between electronic device use before bedtime and academic attainment in adolescents. J Adolesc Health. 2018;63:451-458.
65. Mishna F, Saini M, Solomon S. Ongoing and online: children and youth’s perceptions of cyber bullying. Child Youth Serv Rev. 2009;31:1222-1228.
66. Sengupta A, Chaudhuri A. Are social networking sites a source of online harassment for teens? Evidence from survey data. Child Youth Serv Rev. 2011;33:284-290.
Adolescents are an increasingly diverse population reflecting changes in the racial, ethnic, and geopolitical milieus of the United States. The World Health Organization classifies adolescence as ages 10 to 19 years.1 However, given the complexity of adolescent development physically, behaviorally, emotionally, and socially, others propose that adolescence may extend to age 24.2
Recognizing the specific challenges adolescents face is key to providing comprehensive longitudinal health care. Moreover, creating an environment of trust helps to ensure open 2-way communication that can facilitate anticipatory guidance.
Our review focuses on common adolescent issues, including injury from vehicles and firearms, tobacco and substance misuse, obesity, behavioral health, sexual health, and social media use. We discuss current trends and recommend strategies to maximize health and wellness.
Start by framing the visit
Confidentiality
Laws governing confidentiality in adolescent health care vary by state. Be aware of the laws pertaining to your practice setting. In addition, health care facilities may have their own policies regarding consent and confidentiality in adolescent care. Discuss confidentiality with both an adolescent and the parent/guardian at the initial visit. And, to help avoid potential misunderstandings, let them know in advance what will (and will not) be divulged.
The American Academy of Pediatrics has developed a useful tip sheet regarding confidentiality laws (www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/healthy-foster-care-america/Documents/Confidentiality_Laws.pdf). Examples of required (conditional) disclosure include abuse and suicidal or homicidal ideations. Patients should understand that sexually transmitted infections (STIs) are reportable to public health authorities and that potentially injurious behaviors to self or others (eg, excessive drinking prior to driving) may also warrant disclosure(TABLE 13).

Privacy and general visit structure
Create a safe atmosphere where adolescents can discuss personal issues without fear of repercussion or judgment. While parents may prefer to be present during the visit, allowing for time to visit independently with an adolescent offers the opportunity to reinforce issues of privacy and confidentiality. Also discuss your office policies regarding electronic communication, phone communication, and relaying test results.

A useful paradigm for organizing a visit for routine adolescent care is to use an expanded version of the HEADSS mnemonic (TABLE 24,5), which includes questions about an adolescent’s Home, Education, Activities, Drug and alcohol use, Sexual behavior, Suicidality and depression, and other topics. Other validated screening tools include RAAPS (Rapid Adolescent Prevention Screening)6 (www.possibilitiesforchange.com/raaps/); the Guidelines for Adolescent Preventive Services7; and the Bright Futures recommendations for preventive care from the American Academy of Pediatrics.8 Below, we consider important topics addressed with the HEADSS approach.

Continue to: Injury from vehicles and firearms
Injury from vehicles and firearms
Motor vehicle accidents and firearm wounds are the 2 leading causes of adolescent injury. In 2016, of the more than 20,000 deaths in children and adolescents (ages 1-19 years), 20% were due to motor vehicle accidents (4074) and 15% were a result of firearm-related injuries (3143). Among firearm-related deaths, 60% were homicides, 35% were suicides, and 4% were due to accidental discharge.9 The rate of firearm-related deaths among American teens is 36 times greater than that of any other developed nation.9 Currently, 1 of every 3 US households with children younger than 18 has a firearm. Data suggest that in 43% of these households, the firearm is loaded and kept in an unlocked location.10
To aid anticipatory guidance, ask adolescents about firearm and seat belt use, drinking and driving, and suicidal thoughts (TABLE 24,5). Advise them to always wear seat belts whether driving or riding as a passenger. They should never drink and drive (or get in a car with someone who has been drinking). Advise parents that if firearms are present in the household, they should be kept in a secure, locked location. Weapons should be separated from ammunition and safety mechanisms should be engaged on all devices.
Tobacco and substance misuse
Tobacco use, the leading preventable cause of death in the United States,11 is responsible for more deaths than alcohol, motor vehicle accidents, suicides, homicides, and HIV disease combined.12 Most tobacco-associated mortality occurs in individuals who began smoking before the age of 18.12 Individuals who start smoking early are also more likely to continue smoking through adulthood.
Encouragingly, tobacco use has declined significantly among adolescents over the past several decades. Roughly 1 in 25 high school seniors reports daily tobacco use.13 Adolescent smoking behaviors are also changing dramatically with the increasing popularity of electronic cigarettes (“vaping”). Currently, more adolescents vape than smoke cigarettes.13 Vaping has additional health risks including toxic lung injury.
Multiple resources can help combat tobacco and nicotine use in adolescents. The US Preventive Services Task Force recommends that primary care clinicians intervene through education or brief counselling to prevent initiation of tobacco use in school-aged children and adolescents.14 Ask teens about tobacco and electronic cigarette use and encourage them to quit when use is acknowledged. Other helpful office-based tools are the “Quit Line” 800-QUIT-NOW and texting “Quit” to 47848. Smokefree teen (https://teen.smokefree.gov/) is a website that reviews the risks of tobacco and nicotine use and provides age-appropriate cessation tools and tips (including a smartphone app and a live-chat feature). Other useful information is available in a report from the Surgeon General on preventing tobacco use among young adults.15
Continue to: Alcohol use
Alcohol use. Three in 5 high school students report ever having used alcohol.13 As with tobacco, adolescent alcohol use has declined over the past decade. However, binge drinking (≥ 5 drinks on 1 occasion for males; ≥ 4 drinks on 1 occasion for females) remains a common high-risk behavior among adolescents (particularly college students). Based on the Monitoring the Future Survey, 1 in 6 high school seniors reported binge drinking in the past 2 weeks.13 While historically more common among males, rates of binge drinking are now basically similar between male and female adolescents.13
The National Institute on Alcohol Abuse and Alcoholism has a screening and intervention guide specifically for adolescents.16
Illicit drug use. Half of adolescents report using an illicit drug by their senior year in high school.13 Marijuana is the most commonly used substance, and laws governing its use are rapidly changing across the United States. Marijuana is illegal in 10 states and legal in 10 states (and the District of Columbia). The remaining states have varying policies on the medical use of marijuana and the decriminalization of marijuana. In addition, cannabinoid (CBD) products are increasingly available. Frequent cannabis use in adolescence has an adverse impact on general executive function (compared with adult users) and learning.17 Marijuana may serve as a gateway drug in the abuse of other substances,18 and its use should be strongly discouraged in adolescents.
Of note, there has been a sharp rise in the illicit use of prescription drugs, particularly opioids, creating a public health emergency across the United States.19 In 2015, more than 4000 young people, ages 15 to 24, died from a drug-related overdose (> 50% of these attributable to opioids).20 Adolescents with a history of substance abuse and behavioral illness are at particular risk. Many adolescents who misuse opioids and other prescription drugs obtain them from friends and relatives.21
The Substance Abuse and Mental Health Services Administration (SAMHSA) recommends universal screening of adolescents for substance abuse. This screening should be accompanied by a brief intervention to prevent, mitigate, or eliminate substance use, or a referral to appropriate treatment sources. This process of screening, brief intervention, and referral to treatment (SBIRT) is recommended as part of routine health care.22
Continue to: Obesity and physical activity
Obesity and physical activity
The percentage of overweight and obese adolescents in the United States has more than tripled over the past 40 years,23 and 1 in 5 US adolescents is obese.23 Obese teens are at higher risk for multiple chronic diseases, including type 2 diabetes, sleep apnea, and heart disease.24 They are also more likely to be bullied and to have poor self-esteem.25 Only 1 in 5 American high school students engages in 60 or more minutes of moderate-to-vigorous physical activity on 5 or more days per week.26
Regular physical activity is, of course, beneficial for cardiorespiratory fitness, bone health, weight control, and improved indices of behavioral health.26 Adolescents who are physically active consistently demonstrate better school attendance and grades.17 Higher levels of physical fitness are also associated with improved overall cognitive performance.24
General recommendations. The Department of Health and Human Services recommends that adolescents get at least 60 minutes of mostly moderate physical activity every day.26 Encourage adolescents to engage in vigorous physical activity (heavy breathing, sweating) at least 3 days a week. As part of their physical activity patterns, adolescents should also engage in muscle-strengthening and bone-strengthening activities on at least 3 days per week.
Behavioral health
As young people develop their sense of personal identity, they also strive for independence. It can be difficult, at times, to differentiate normal adolescent rebellion from true mental illness. An estimated 17% to 19% of adolescents meet criteria for mental illness, and about 7% have a severe psychiatric disorder.27 Only one-third of adolescents with mental illness receive any mental health services.28
Depression. The 1-year incidence of major depression in adolescents is 3% to 4%, and the lifetime prevalence of depressive symptoms is 25% in all high school students.27 Risk factors include ethnic minority status, poor self-esteem, poor health, recent personal crisis, insomnia, and alcohol/substance abuse. Depression in adolescent girls is correlated with becoming sexually active at a younger age, failure to use contraception, having an STI, and suicide attempts. Depressed boys are more likely to have unprotected intercourse and participate in physical fights.29 Depressed teens have a 2- to 3-fold greater risk for behavioral disorders, anxiety, and attention-deficit/hyperactivity disorder (ADHD).30
Continue to: Suicide
Suicide. Among individuals 15 to 29 years of age, suicide is the second leading cause of death globally, with an annual incidence of 11 to 15 per 100,000.31 Suicide attempts are 10 to 20 times more common than completed suicide.31 Males are more likely than females to die by suicide,32 and boys with a history of attempted suicide have a 30-fold increased risk of subsequent successful suicide.31 Hanging, drug poisoning, and firearms (particularly for males) are the most common means of suicide in adolescents. More than half of adolescents dying by suicide have coexisting depression.31
Characteristics associated with suicidal behaviors in adolescents include impulsivity, poor problem-solving skills, and dichotomous thinking.31 There may be a genetic component as well. In 1 of 5 teenage suicides, a precipitating life event such as the break-up of a relationship, cyber-bullying, or peer rejection is felt to contribute.31
ADHD. The prevalence of ADHD is 7% to 9% in US school-aged children.33 Boys more commonly exhibit hyperactive behaviors, while girls have more inattention. Hyperactivity often diminishes in teens, but inattention and impulsivity persist. Sequelae of ADHD include high-risk sexual behaviors, motor vehicle accidents, incarceration, and substance abuse.34 Poor self-esteem, suicidal ideation, smoking, and obesity are also increased.34 ADHD often persists into adulthood, with implications for social relationships and job performance.34
Eating disorders. The distribution of eating disorders is now known to increasingly include more minorities and males, the latter representing 5% to 10% of cases.35 Eating disorders show a strong genetic tendency and appear to be accelerated by puberty. The most common eating disorder (diagnosed in 0.8%-14% of teens) is eating disorder not otherwise specified (NOS).35 Anorexia nervosa is diagnosed in 0.5% of adolescent girls, and bulimia nervosa in 1% to 2%—particularly among athletes and performers.35 Unanticipated loss of weight, amenorrhea, excessive concern about weight, and deceleration in height/weight curves are potential indicators of an eating disorder. When identified, eating disorders are best managed by a trusted family physician, acting as a coordinator of a multidisciplinary team.
Sexual health
Girls begin to menstruate at an average age of 12, and it takes about 4 years for them to reach reproductive maturity.36 Puberty has been documented to start at younger ages over the past 30 years, likely due to an increase in average body mass index and a decrease in levels of physical activity.37 Girls with early maturation are often insecure and self-conscious, with higher levels of psychological distress.38 In boys, the average age for spermarche (first ejaculation) is 13.39 Boys who mature early tend to be taller, be more confident, and express a good body image.40 Those who have early puberty are more likely to be sexually active or participate in high-risk behaviors.41
Continue to: Pregnancy and contraception
Pregnancy and contraception
Over the past several decades, more US teens have been abstaining from sexual intercourse or have been using effective forms of birth control, particularly condoms and long-acting reversible contraceptives (LARCs).42 Teenage birth rates in girls ages 15 to 19 have declined significantly since the 1980s.42 Despite this, the teenage birth rate in the United States remains higher than in other industrialized nations, and most teen pregnancies are unintended.
There are numerous interventions to reduce teen pregnancy, including sex education, contraceptive counseling, the use of mobile apps that track a user’s monthly fertility cycle or issue reminders to take oral contraceptives,45 and the liberal distribution of contraceptives and condoms. The Contraceptive CHOICE Project shows that providing free (or low-cost) LARCs influences young women to choose these as their preferred contraceptive method.46 Other programs specifically empower girls to convince partners to use condoms and to resist unwanted sexual advances or intimate partner violence.
Adolescents prefer to have their health care providers address the topic of sexual health. Teens are more likely to share information with providers if asked directly about sexual behaviors.47TABLE 24,5 offers tips for anticipatory guidance and potential ways to frame questions with adolescents in this context. State laws vary with regard to the ability of minors to seek contraception, pregnancy testing, or care/screening for STIs without parental consent. Contraceptive counseling combined with effective screening decrease the incidence of STIs and pelvic inflammatory disease for sexually active teens.48

Sexually transmitted infections
Young adolescents often have a limited ability to imagine consequences related to specific actions. In general, there is also an increased desire to engage in experimental behaviors as an expression of developing autonomy, which may expose them to STIs. About half of all STIs contracted in the United States occur in individuals 15 to 24 years of age.49 Girls are at particular risk for the sequelae of these infections, including cervical dysplasia and infertility. Many teens erroneously believe that sexual activities other than intercourse decrease their risk of contracting an STI.50
Human papillomavirus (HPV) infection is the most common STI in adolescence.51 In most cases, HPV is transient and asymptomatic. Oncogenic strains may cause cervical cancer or cancers of the anogenital or oropharyngeal systems. Due to viral latency, it is not recommended to perform HPV typing in men or in women younger than 30 years of age; however, Pap tests are recommended every 3 years for women ages 21 to 29. Primary care providers are pivotal in the public health struggle to prevent HPV infection.
Continue to: Universal immunization of all children...
Universal immunization of all children older than 11 years of age against HPV is strongly advised as part of routine well-child care. Emphasize the proven role of HPV vaccination in preventing cervical52 and oropharyngeal53 cancers. And be prepared to address concerns raised by parents in the context of vaccine safety and the initiation of sexual behaviors (www.cdc.gov/hpv/hcp/answering-questions.html).
Chlamydia is the second most common STI in the United States, usually occurring in individuals younger than 24.54 The CDC estimates that more than 3 million new chlamydial infections occur yearly. These infections are often asymptomatic, particularly in females, but may cause urethritis, cervicitis, epididymitis, proctitis, or pelvic inflammatory disease. Indolent chlamydial infection is the leading cause of tubal infertility in women.54 Routine annual screening for chlamydia is recommended for all sexually active females ≤ 25 years (and for older women with specific risks).55 Annual screening is also recommended for men who have sex with men (MSM).55
Chlamydial infection may be diagnosed with first-catch urine sampling (men or women), urethral swab (men), endocervical swab (women), or self-collected vaginal swab. Nucleic acid amplification testing is the most sensitive test that is widely available.56 First-line treatment includes either azithromycin (1 g orally, single dose) or doxycycline (100 mg orally, twice daily for 7 days).56
Gonorrhea. In 2018, there were more than 500,000 annual cases of gonorrhea, with the majority occurring in those between 15 and 24 years of age.57 Gonorrhea may increase rates of HIV infection transmission up to 5-fold.57 As more adolescents practice oral sex, cases of pharyngeal gonorrhea (and oropharyngeal HPV) have increased. Symptoms of urethritis occur more frequently in men. Screening is recommended for all sexually active women younger than 25.56 Importantly, the organism Neisseria gonorrhoeae has developed significant antibiotic resistance over the past decade. The CDC currently recommends dual therapy for the treatment of gonorrhea using 250 mg of intramuscular ceftriaxone and 1 g of oral azithromycin.56
Syphilis. Rates of syphilis are increasing among individuals ages 15 to 24.51 Screening is particularly recommended for MSM and individuals infected with HIV. Benzathine penicillin G, 50,000 U/kg IM, remains the treatment of choice.56
Continue to: HIV
HIV. Globally, HIV impacts young people disproportionately. HIV infection also facilitates infection with other STIs. In the United States, the highest burden of HIV infection is borne by young MSM, with prevalence among those 18 to 24 years old varying between 26% to 30% (black) and 3% to 5.5% (non-Hispanic white).51 The use of emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis (PrEP) has recently been approved for the prevention of HIV. PrEP reduces risk by up to 92% for MSM and transgender women.58
Sexual identity
One in 10 high school students self-identifies as “nonheterosexual,” and 1 in 15 reports same-sex sexual contact.59 The term LGBTQ+ includes the communities of lesbian, gay, bisexual, transgender, transsexual, queer, questioning, intersex, and asexual individuals. Developing a safe sense of sexual identity is fundamental to adolescent psychological development, and many adolescents struggle to develop a positive sexual identity. Suicide rates and self-harm behaviors among LGBTQ+ adolescents can be 4 times higher than among their heterosexual peers.60 Rates of mood disorders, substance abuse, and high-risk sexual behaviors are also increased in the LGBTQ+ population.61
The LGBTQ+ community often seeks health care advice and affirmation from primary care providers. Resources to enhance this care are available at www.lgbthealtheducation.org.
Social media
Adolescents today have more media exposure than any prior generation, with smartphone and computer use increasing exponentially. Most (95%) teens have access to a smartphone,62 45% describe themselves as constantly connected to the Internet, and 14% feel that social media is “addictive.”62 Most manage their social media portfolio on multiple sites. Patterns of adolescents' online activities show that boys prefer online gaming, while girls tend to spend more time on social networking.62
Whether extensive media use is psychologically beneficial or deleterious has been widely debated. Increased time online correlates with decreased levels of physical activity.63 And sleep disturbances have been associated with excessive screen time and the presence of mobile devices in the bedroom.64 The use of social media prior to bedtime also has an adverse impact on academic performance—particularly for girls. This adverse impact on academics persists after correcting for daytime sleepiness, body mass index, and number of hours spent on homework.64
Continue to: Due to growing concerns...
Due to growing concerns about the risks of social media in children and adolescents, the American Academy of Pediatrics has developed the Family Media Plan (www.healthychildren.org/English/media/Pages/default.aspx). Some specific questions that providers may ask are outlined in TABLE 3.64 The Family Media Plan can provide age-specific guidelines to assist parents or caregivers in answering these questions.
Cyber-bullying. One in 3 adolescents (primarily female) has been a victim of cyber-bullying.65 Sadly, 1 in 5 teens has received some form of electronic sexual solicitation.66 The likelihood of unsolicited stranger contact correlates with teens’ online habits and the amount of information disclosed. Predictors include female sex, visiting chat rooms, posting photos, and disclosing personal information. Restricting computer use to an area with parental supervision or installing monitoring programs does not seem to exert any protective influence on cyber-bullying or unsolicited stranger contact.65 While 63% of cyber-bullying victims feel upset, embarrassed, or stressed by these contacts,66 few events are actually reported. To address this, some states have adopted laws adding cyber-bullying to school disciplinary codes.
Negative health impacts associated with cyber-bullying include anxiety, sadness, and greater difficulty in concentrating on school work.65 Victims of bullying are more likely to have school disciplinary actions and depression and to be truant or to carry weapons to school.66 Cyber-bullying is uniquely destructive due to its ubiquitous presence. A sense of relative anonymity online may encourage perpetrators to act more cruelly, with less concern for punishment.
Young people are also more likely to share passwords as a sign of friendship. This may result in others assuming their identity online. Adolescents rarely disclose bullying to parents or other adults, fearing restriction of Internet access, and many of them think that adults may downplay the seriousness of the events.66
CORRESPONDENCE
Mark B. Stephens, MD, Penn State Health Medical Group, 1850 East Park Avenue, State College, PA 16803; [email protected].
Adolescents are an increasingly diverse population reflecting changes in the racial, ethnic, and geopolitical milieus of the United States. The World Health Organization classifies adolescence as ages 10 to 19 years.1 However, given the complexity of adolescent development physically, behaviorally, emotionally, and socially, others propose that adolescence may extend to age 24.2
Recognizing the specific challenges adolescents face is key to providing comprehensive longitudinal health care. Moreover, creating an environment of trust helps to ensure open 2-way communication that can facilitate anticipatory guidance.
Our review focuses on common adolescent issues, including injury from vehicles and firearms, tobacco and substance misuse, obesity, behavioral health, sexual health, and social media use. We discuss current trends and recommend strategies to maximize health and wellness.
Start by framing the visit
Confidentiality
Laws governing confidentiality in adolescent health care vary by state. Be aware of the laws pertaining to your practice setting. In addition, health care facilities may have their own policies regarding consent and confidentiality in adolescent care. Discuss confidentiality with both an adolescent and the parent/guardian at the initial visit. And, to help avoid potential misunderstandings, let them know in advance what will (and will not) be divulged.
The American Academy of Pediatrics has developed a useful tip sheet regarding confidentiality laws (www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/healthy-foster-care-america/Documents/Confidentiality_Laws.pdf). Examples of required (conditional) disclosure include abuse and suicidal or homicidal ideations. Patients should understand that sexually transmitted infections (STIs) are reportable to public health authorities and that potentially injurious behaviors to self or others (eg, excessive drinking prior to driving) may also warrant disclosure(TABLE 13).

Privacy and general visit structure
Create a safe atmosphere where adolescents can discuss personal issues without fear of repercussion or judgment. While parents may prefer to be present during the visit, allowing for time to visit independently with an adolescent offers the opportunity to reinforce issues of privacy and confidentiality. Also discuss your office policies regarding electronic communication, phone communication, and relaying test results.

A useful paradigm for organizing a visit for routine adolescent care is to use an expanded version of the HEADSS mnemonic (TABLE 24,5), which includes questions about an adolescent’s Home, Education, Activities, Drug and alcohol use, Sexual behavior, Suicidality and depression, and other topics. Other validated screening tools include RAAPS (Rapid Adolescent Prevention Screening)6 (www.possibilitiesforchange.com/raaps/); the Guidelines for Adolescent Preventive Services7; and the Bright Futures recommendations for preventive care from the American Academy of Pediatrics.8 Below, we consider important topics addressed with the HEADSS approach.

Continue to: Injury from vehicles and firearms
Injury from vehicles and firearms
Motor vehicle accidents and firearm wounds are the 2 leading causes of adolescent injury. In 2016, of the more than 20,000 deaths in children and adolescents (ages 1-19 years), 20% were due to motor vehicle accidents (4074) and 15% were a result of firearm-related injuries (3143). Among firearm-related deaths, 60% were homicides, 35% were suicides, and 4% were due to accidental discharge.9 The rate of firearm-related deaths among American teens is 36 times greater than that of any other developed nation.9 Currently, 1 of every 3 US households with children younger than 18 has a firearm. Data suggest that in 43% of these households, the firearm is loaded and kept in an unlocked location.10
To aid anticipatory guidance, ask adolescents about firearm and seat belt use, drinking and driving, and suicidal thoughts (TABLE 24,5). Advise them to always wear seat belts whether driving or riding as a passenger. They should never drink and drive (or get in a car with someone who has been drinking). Advise parents that if firearms are present in the household, they should be kept in a secure, locked location. Weapons should be separated from ammunition and safety mechanisms should be engaged on all devices.
Tobacco and substance misuse
Tobacco use, the leading preventable cause of death in the United States,11 is responsible for more deaths than alcohol, motor vehicle accidents, suicides, homicides, and HIV disease combined.12 Most tobacco-associated mortality occurs in individuals who began smoking before the age of 18.12 Individuals who start smoking early are also more likely to continue smoking through adulthood.
Encouragingly, tobacco use has declined significantly among adolescents over the past several decades. Roughly 1 in 25 high school seniors reports daily tobacco use.13 Adolescent smoking behaviors are also changing dramatically with the increasing popularity of electronic cigarettes (“vaping”). Currently, more adolescents vape than smoke cigarettes.13 Vaping has additional health risks including toxic lung injury.
Multiple resources can help combat tobacco and nicotine use in adolescents. The US Preventive Services Task Force recommends that primary care clinicians intervene through education or brief counselling to prevent initiation of tobacco use in school-aged children and adolescents.14 Ask teens about tobacco and electronic cigarette use and encourage them to quit when use is acknowledged. Other helpful office-based tools are the “Quit Line” 800-QUIT-NOW and texting “Quit” to 47848. Smokefree teen (https://teen.smokefree.gov/) is a website that reviews the risks of tobacco and nicotine use and provides age-appropriate cessation tools and tips (including a smartphone app and a live-chat feature). Other useful information is available in a report from the Surgeon General on preventing tobacco use among young adults.15
Continue to: Alcohol use
Alcohol use. Three in 5 high school students report ever having used alcohol.13 As with tobacco, adolescent alcohol use has declined over the past decade. However, binge drinking (≥ 5 drinks on 1 occasion for males; ≥ 4 drinks on 1 occasion for females) remains a common high-risk behavior among adolescents (particularly college students). Based on the Monitoring the Future Survey, 1 in 6 high school seniors reported binge drinking in the past 2 weeks.13 While historically more common among males, rates of binge drinking are now basically similar between male and female adolescents.13
The National Institute on Alcohol Abuse and Alcoholism has a screening and intervention guide specifically for adolescents.16
Illicit drug use. Half of adolescents report using an illicit drug by their senior year in high school.13 Marijuana is the most commonly used substance, and laws governing its use are rapidly changing across the United States. Marijuana is illegal in 10 states and legal in 10 states (and the District of Columbia). The remaining states have varying policies on the medical use of marijuana and the decriminalization of marijuana. In addition, cannabinoid (CBD) products are increasingly available. Frequent cannabis use in adolescence has an adverse impact on general executive function (compared with adult users) and learning.17 Marijuana may serve as a gateway drug in the abuse of other substances,18 and its use should be strongly discouraged in adolescents.
Of note, there has been a sharp rise in the illicit use of prescription drugs, particularly opioids, creating a public health emergency across the United States.19 In 2015, more than 4000 young people, ages 15 to 24, died from a drug-related overdose (> 50% of these attributable to opioids).20 Adolescents with a history of substance abuse and behavioral illness are at particular risk. Many adolescents who misuse opioids and other prescription drugs obtain them from friends and relatives.21
The Substance Abuse and Mental Health Services Administration (SAMHSA) recommends universal screening of adolescents for substance abuse. This screening should be accompanied by a brief intervention to prevent, mitigate, or eliminate substance use, or a referral to appropriate treatment sources. This process of screening, brief intervention, and referral to treatment (SBIRT) is recommended as part of routine health care.22
Continue to: Obesity and physical activity
Obesity and physical activity
The percentage of overweight and obese adolescents in the United States has more than tripled over the past 40 years,23 and 1 in 5 US adolescents is obese.23 Obese teens are at higher risk for multiple chronic diseases, including type 2 diabetes, sleep apnea, and heart disease.24 They are also more likely to be bullied and to have poor self-esteem.25 Only 1 in 5 American high school students engages in 60 or more minutes of moderate-to-vigorous physical activity on 5 or more days per week.26
Regular physical activity is, of course, beneficial for cardiorespiratory fitness, bone health, weight control, and improved indices of behavioral health.26 Adolescents who are physically active consistently demonstrate better school attendance and grades.17 Higher levels of physical fitness are also associated with improved overall cognitive performance.24
General recommendations. The Department of Health and Human Services recommends that adolescents get at least 60 minutes of mostly moderate physical activity every day.26 Encourage adolescents to engage in vigorous physical activity (heavy breathing, sweating) at least 3 days a week. As part of their physical activity patterns, adolescents should also engage in muscle-strengthening and bone-strengthening activities on at least 3 days per week.
Behavioral health
As young people develop their sense of personal identity, they also strive for independence. It can be difficult, at times, to differentiate normal adolescent rebellion from true mental illness. An estimated 17% to 19% of adolescents meet criteria for mental illness, and about 7% have a severe psychiatric disorder.27 Only one-third of adolescents with mental illness receive any mental health services.28
Depression. The 1-year incidence of major depression in adolescents is 3% to 4%, and the lifetime prevalence of depressive symptoms is 25% in all high school students.27 Risk factors include ethnic minority status, poor self-esteem, poor health, recent personal crisis, insomnia, and alcohol/substance abuse. Depression in adolescent girls is correlated with becoming sexually active at a younger age, failure to use contraception, having an STI, and suicide attempts. Depressed boys are more likely to have unprotected intercourse and participate in physical fights.29 Depressed teens have a 2- to 3-fold greater risk for behavioral disorders, anxiety, and attention-deficit/hyperactivity disorder (ADHD).30
Continue to: Suicide
Suicide. Among individuals 15 to 29 years of age, suicide is the second leading cause of death globally, with an annual incidence of 11 to 15 per 100,000.31 Suicide attempts are 10 to 20 times more common than completed suicide.31 Males are more likely than females to die by suicide,32 and boys with a history of attempted suicide have a 30-fold increased risk of subsequent successful suicide.31 Hanging, drug poisoning, and firearms (particularly for males) are the most common means of suicide in adolescents. More than half of adolescents dying by suicide have coexisting depression.31
Characteristics associated with suicidal behaviors in adolescents include impulsivity, poor problem-solving skills, and dichotomous thinking.31 There may be a genetic component as well. In 1 of 5 teenage suicides, a precipitating life event such as the break-up of a relationship, cyber-bullying, or peer rejection is felt to contribute.31
ADHD. The prevalence of ADHD is 7% to 9% in US school-aged children.33 Boys more commonly exhibit hyperactive behaviors, while girls have more inattention. Hyperactivity often diminishes in teens, but inattention and impulsivity persist. Sequelae of ADHD include high-risk sexual behaviors, motor vehicle accidents, incarceration, and substance abuse.34 Poor self-esteem, suicidal ideation, smoking, and obesity are also increased.34 ADHD often persists into adulthood, with implications for social relationships and job performance.34
Eating disorders. The distribution of eating disorders is now known to increasingly include more minorities and males, the latter representing 5% to 10% of cases.35 Eating disorders show a strong genetic tendency and appear to be accelerated by puberty. The most common eating disorder (diagnosed in 0.8%-14% of teens) is eating disorder not otherwise specified (NOS).35 Anorexia nervosa is diagnosed in 0.5% of adolescent girls, and bulimia nervosa in 1% to 2%—particularly among athletes and performers.35 Unanticipated loss of weight, amenorrhea, excessive concern about weight, and deceleration in height/weight curves are potential indicators of an eating disorder. When identified, eating disorders are best managed by a trusted family physician, acting as a coordinator of a multidisciplinary team.
Sexual health
Girls begin to menstruate at an average age of 12, and it takes about 4 years for them to reach reproductive maturity.36 Puberty has been documented to start at younger ages over the past 30 years, likely due to an increase in average body mass index and a decrease in levels of physical activity.37 Girls with early maturation are often insecure and self-conscious, with higher levels of psychological distress.38 In boys, the average age for spermarche (first ejaculation) is 13.39 Boys who mature early tend to be taller, be more confident, and express a good body image.40 Those who have early puberty are more likely to be sexually active or participate in high-risk behaviors.41
Continue to: Pregnancy and contraception
Pregnancy and contraception
Over the past several decades, more US teens have been abstaining from sexual intercourse or have been using effective forms of birth control, particularly condoms and long-acting reversible contraceptives (LARCs).42 Teenage birth rates in girls ages 15 to 19 have declined significantly since the 1980s.42 Despite this, the teenage birth rate in the United States remains higher than in other industrialized nations, and most teen pregnancies are unintended.
There are numerous interventions to reduce teen pregnancy, including sex education, contraceptive counseling, the use of mobile apps that track a user’s monthly fertility cycle or issue reminders to take oral contraceptives,45 and the liberal distribution of contraceptives and condoms. The Contraceptive CHOICE Project shows that providing free (or low-cost) LARCs influences young women to choose these as their preferred contraceptive method.46 Other programs specifically empower girls to convince partners to use condoms and to resist unwanted sexual advances or intimate partner violence.
Adolescents prefer to have their health care providers address the topic of sexual health. Teens are more likely to share information with providers if asked directly about sexual behaviors.47TABLE 24,5 offers tips for anticipatory guidance and potential ways to frame questions with adolescents in this context. State laws vary with regard to the ability of minors to seek contraception, pregnancy testing, or care/screening for STIs without parental consent. Contraceptive counseling combined with effective screening decrease the incidence of STIs and pelvic inflammatory disease for sexually active teens.48

Sexually transmitted infections
Young adolescents often have a limited ability to imagine consequences related to specific actions. In general, there is also an increased desire to engage in experimental behaviors as an expression of developing autonomy, which may expose them to STIs. About half of all STIs contracted in the United States occur in individuals 15 to 24 years of age.49 Girls are at particular risk for the sequelae of these infections, including cervical dysplasia and infertility. Many teens erroneously believe that sexual activities other than intercourse decrease their risk of contracting an STI.50
Human papillomavirus (HPV) infection is the most common STI in adolescence.51 In most cases, HPV is transient and asymptomatic. Oncogenic strains may cause cervical cancer or cancers of the anogenital or oropharyngeal systems. Due to viral latency, it is not recommended to perform HPV typing in men or in women younger than 30 years of age; however, Pap tests are recommended every 3 years for women ages 21 to 29. Primary care providers are pivotal in the public health struggle to prevent HPV infection.
Continue to: Universal immunization of all children...
Universal immunization of all children older than 11 years of age against HPV is strongly advised as part of routine well-child care. Emphasize the proven role of HPV vaccination in preventing cervical52 and oropharyngeal53 cancers. And be prepared to address concerns raised by parents in the context of vaccine safety and the initiation of sexual behaviors (www.cdc.gov/hpv/hcp/answering-questions.html).
Chlamydia is the second most common STI in the United States, usually occurring in individuals younger than 24.54 The CDC estimates that more than 3 million new chlamydial infections occur yearly. These infections are often asymptomatic, particularly in females, but may cause urethritis, cervicitis, epididymitis, proctitis, or pelvic inflammatory disease. Indolent chlamydial infection is the leading cause of tubal infertility in women.54 Routine annual screening for chlamydia is recommended for all sexually active females ≤ 25 years (and for older women with specific risks).55 Annual screening is also recommended for men who have sex with men (MSM).55
Chlamydial infection may be diagnosed with first-catch urine sampling (men or women), urethral swab (men), endocervical swab (women), or self-collected vaginal swab. Nucleic acid amplification testing is the most sensitive test that is widely available.56 First-line treatment includes either azithromycin (1 g orally, single dose) or doxycycline (100 mg orally, twice daily for 7 days).56
Gonorrhea. In 2018, there were more than 500,000 annual cases of gonorrhea, with the majority occurring in those between 15 and 24 years of age.57 Gonorrhea may increase rates of HIV infection transmission up to 5-fold.57 As more adolescents practice oral sex, cases of pharyngeal gonorrhea (and oropharyngeal HPV) have increased. Symptoms of urethritis occur more frequently in men. Screening is recommended for all sexually active women younger than 25.56 Importantly, the organism Neisseria gonorrhoeae has developed significant antibiotic resistance over the past decade. The CDC currently recommends dual therapy for the treatment of gonorrhea using 250 mg of intramuscular ceftriaxone and 1 g of oral azithromycin.56
Syphilis. Rates of syphilis are increasing among individuals ages 15 to 24.51 Screening is particularly recommended for MSM and individuals infected with HIV. Benzathine penicillin G, 50,000 U/kg IM, remains the treatment of choice.56
Continue to: HIV
HIV. Globally, HIV impacts young people disproportionately. HIV infection also facilitates infection with other STIs. In the United States, the highest burden of HIV infection is borne by young MSM, with prevalence among those 18 to 24 years old varying between 26% to 30% (black) and 3% to 5.5% (non-Hispanic white).51 The use of emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis (PrEP) has recently been approved for the prevention of HIV. PrEP reduces risk by up to 92% for MSM and transgender women.58
Sexual identity
One in 10 high school students self-identifies as “nonheterosexual,” and 1 in 15 reports same-sex sexual contact.59 The term LGBTQ+ includes the communities of lesbian, gay, bisexual, transgender, transsexual, queer, questioning, intersex, and asexual individuals. Developing a safe sense of sexual identity is fundamental to adolescent psychological development, and many adolescents struggle to develop a positive sexual identity. Suicide rates and self-harm behaviors among LGBTQ+ adolescents can be 4 times higher than among their heterosexual peers.60 Rates of mood disorders, substance abuse, and high-risk sexual behaviors are also increased in the LGBTQ+ population.61
The LGBTQ+ community often seeks health care advice and affirmation from primary care providers. Resources to enhance this care are available at www.lgbthealtheducation.org.
Social media
Adolescents today have more media exposure than any prior generation, with smartphone and computer use increasing exponentially. Most (95%) teens have access to a smartphone,62 45% describe themselves as constantly connected to the Internet, and 14% feel that social media is “addictive.”62 Most manage their social media portfolio on multiple sites. Patterns of adolescents' online activities show that boys prefer online gaming, while girls tend to spend more time on social networking.62
Whether extensive media use is psychologically beneficial or deleterious has been widely debated. Increased time online correlates with decreased levels of physical activity.63 And sleep disturbances have been associated with excessive screen time and the presence of mobile devices in the bedroom.64 The use of social media prior to bedtime also has an adverse impact on academic performance—particularly for girls. This adverse impact on academics persists after correcting for daytime sleepiness, body mass index, and number of hours spent on homework.64
Continue to: Due to growing concerns...
Due to growing concerns about the risks of social media in children and adolescents, the American Academy of Pediatrics has developed the Family Media Plan (www.healthychildren.org/English/media/Pages/default.aspx). Some specific questions that providers may ask are outlined in TABLE 3.64 The Family Media Plan can provide age-specific guidelines to assist parents or caregivers in answering these questions.
Cyber-bullying. One in 3 adolescents (primarily female) has been a victim of cyber-bullying.65 Sadly, 1 in 5 teens has received some form of electronic sexual solicitation.66 The likelihood of unsolicited stranger contact correlates with teens’ online habits and the amount of information disclosed. Predictors include female sex, visiting chat rooms, posting photos, and disclosing personal information. Restricting computer use to an area with parental supervision or installing monitoring programs does not seem to exert any protective influence on cyber-bullying or unsolicited stranger contact.65 While 63% of cyber-bullying victims feel upset, embarrassed, or stressed by these contacts,66 few events are actually reported. To address this, some states have adopted laws adding cyber-bullying to school disciplinary codes.
Negative health impacts associated with cyber-bullying include anxiety, sadness, and greater difficulty in concentrating on school work.65 Victims of bullying are more likely to have school disciplinary actions and depression and to be truant or to carry weapons to school.66 Cyber-bullying is uniquely destructive due to its ubiquitous presence. A sense of relative anonymity online may encourage perpetrators to act more cruelly, with less concern for punishment.
Young people are also more likely to share passwords as a sign of friendship. This may result in others assuming their identity online. Adolescents rarely disclose bullying to parents or other adults, fearing restriction of Internet access, and many of them think that adults may downplay the seriousness of the events.66
CORRESPONDENCE
Mark B. Stephens, MD, Penn State Health Medical Group, 1850 East Park Avenue, State College, PA 16803; [email protected].
1. World Health Organization. Adolescent health. Accessed February 23, 2021. www.who.int/maternal_child_adolescent/adolescence/en/
2. Sawyer SM, Azzopardi PS, Wickremarathne D, et al. The age of adolescence. Lancet Child Adolesc Health. 2018;2:223-228.
3. Pathak PR, Chou A. Confidential care for adoloscents in the U.S. healthcare system. J Patient Cent Res Rev. 2019;6:46-50.
4. AMA Journal of Ethics. HEADSS: the “review of systems” for adolescents. Accessed February 23, 2021. https://journalofethics.ama-assn.org/article/headss-review-systems-adolescents/2005-03
5. Cohen E, MacKenzie RG, Yates GL. HEADSS, a psychosocial risk assessment instrument: implications for designing effective intervention programs for runaway youth. J Adolesc Health. 1991;12:539-544.
6. Possibilities for Change. Rapid Adolescent Prevention Screening (RAAPS). Accessed February 23, 2021. www.possibilitiesforchange.com/raaps/
7. Elster AB, Kuznets NJ. AMA Guidelines for Adolescent Preventive Services (GAPS): Recommendations and Rationale. Williams & Wilkins; 1994.
8. AAP. Engaging patients and families - periodicity schedule. Accessed February 23, 2021. www.aap.org/en-us/professional-resources/practice-support/Pages/PeriodicitySchedule.aspx
9. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Eng J Med. 2018;379:2468-2475.
10. Schuster MA, Franke TM, Bastian AM, et al. Firearm storage patterns in US homes with children. Am J Public Health. 2000;90:588-594.
11. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States. JAMA. 2004;291:1238-1245.
12. HHS. Health consequences of smoking, surgeon general fact sheet. Accessed February 23, 2021. www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/consequences-smoking-factsheet/index.html
13. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future: national survey results on drug use, 1975-2017. The University of Michigan. 2018. Accessed February 23, 2021. https://eric.ed.gov/?id=ED589762
14. US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
15. HHS. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: HHS, CDC, NCCDPHP, OSH; 2012. Accessed February 23, 2021. www.ncbi.nlm.nih.gov/books/NBK99237/
16. NIH. Alcohol screening and brief intervention for youth: a pocket guide. Accessed February 23, 2021. https://pubs.niaaa.nih.gov/publications/Practitioner/YouthGuide/YouthGuidePocket.pdf
17. Gorey C, Kuhns L, Smaragdi E, et al. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269:37-58.
18. Secades-Villa R, Garcia-Rodriguez O, Jin CJ, et al. Probability and predictors of the cannabis gateway effect: a national study. Int J Drug Policy. 2015;26:135-142.
19. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance—United States, 2017. MMWR Surveill Summ. 2018;67:1-114.
20. NIH. Drug overdoses in youth. How do drug overdoses happen?. Accessed February 23, 2021. https://teens.drugabuse.gov/drug-facts/drug-overdoses-youth
21. Branstetter SA, Low S, Furman W. The influence of parents and friends on adolescent substance use: a multidimensional approach. J Subst Use. 2011;162:150-160.
22. AAP. Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161210.
23. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;288:1-8.
24. Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13:6-13.
25. Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes. 2010;5:282-304.
26. National Physical Activity Plan Alliance. The 2018 United States report card on physical activity for children and youth. Accessed February 23, 2021. http://physicalactivityplan.org/projects/PA/2018/2018%20US%20Report%20Card%20Full%20Version_WEB.PDF?pdf=page-link
27. HHS. NIMH. Child and adolescent mental health. Accessed February 23, 2021. www.nimh.nih.gov/health/topics/child-and-adolescent-mental-health/index.shtml
28. Yonek JC, Jordan N, Dunlop D, et al. Patient-centered medical home care for adolescents in need of mental health treatment. J Adolesc Health. 2018;63:172-180.
29. Brooks TL, Harris SK, Thrall JS, et al. Association of adolescent risk behaviors with mental health symptoms in high school students. |J Adolesc Health. 2002;31:240-246.
30. Weller BE, Blanford KL, Butler AM. Estimated prevalence of psychiatric comorbidities in US adolescents with depression by race/ethnicity, 2011-2012. J Adolesc Health. 2018;62:716-721.
31. Bilsen J. Suicide and youth: risk factors. Front Psychiatry. 2018;9:540.
32. Shain B, AAP Committee on Adolescence. Suicide and suicide attempts in adolescents. Pediatrics. 2016;138:e20161420.
33. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: review and future directions. J Adolesc Health. 2016;59:135-143.
34. Bravender T. Attention-deficit/hyperactivity disorder and disordered eating. [editorial] J Adolesc Health. 2017;61:125-126.
35. Rosen DS, AAP Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics. 2010;126:1240-1253.
36. Susman EJ, Houts RM, Steinberg L, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 ½ and 15 ½ years. Arch Pediatr Adolesc Med. 2010;164:166-173.
37. Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(suppl 3):S208-S217.
38. Ge X, Conger RD, Elder GH. Coming of age too early: pubertal influences on girl’s vulnerability to psychologic distress. Child Dev. 1996;67:3386-3400.
39. Jørgensen M, Keiding N, Skakkebaek NE. Estimation of spermarche from longitudinal spermaturia data. Biometrics. 1991;47:177-193.
40. Kar SK, Choudhury A, Singh AP. Understanding normal development of adolescent sexuality: a bumpy ride. J Hum Reprod Sci. 2015;8:70-74.
41. Susman EJ, Dorn LD, Schiefelbein VL. Puberty, sexuality and health. In: Lerner MA, Easterbrooks MA, Mistry J (eds). Comprehensive Handbook of Psychology. Wiley; 2003.
42. Lindberg LD, Santelli JS, Desai S. Changing patterns of contraceptive use and the decline in rates of pregnancy and birth among U.S. adolescents, 2007-2014. J Adolesc Health. 2018;63:253-256.
43. Guttmacher Institute. Teen pregnancy. www.guttmacher.org/united-states/teens/teen-pregnancy. Accessed February 23, 2021.
44. CDC. Social determinants and eliminating disparities in teen pregnancy. Accessed February 23, 2021. www.cdc.gov/teenpregnancy/about/social-determinants-disparities-teen-pregnancy.htm
45. Widman L, Nesi J, Kamke K, et al. Technology-based interventions to reduce sexually transmitted infection and unintended pregnancy among youth. J Adolesc Health. 2018;62:651-660.
46. Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-115.e7.
47. Ham P, Allen C. Adolescent health screening and counseling. Am Fam Physician. 2012;86:1109-1116.
48. ACOG. Committee on Adolescent Health Care. Adolescent pregnancy, contraception and sexual activity. 2017. Accessed February 23, 2021. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/adolescent-pregnancy-contraception-and-sexual-activity
49. Wangu Z, Burstein GR. Adolescent sexuality: updates to the sexually transmitted infection guidelines. Pediatr Clin N Am. 2017;64:389-411.
50. Holway GV, Hernandez SM. Oral sex and condom use in a U.S. national sample of adolescents and young adults. J Adolesc Health. 2018;62:402-410.
51. CDC. STDs in adults and adolescents. Accessed February 23, 2021. www.cdc.gov/std/stats17/adolescents.htm
52. McClung N, Gargano J, Bennett N, et al. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008-2014. Accessed February 23, 2021. https://cebp.aacrjournals.org/content/28/3/602
53. Timbang MR, Sim MW, Bewley AF, et al. HPV-related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum Vaccin Immunother. 2019;15:1920-1928.
54. Carey AJ, Beagley KW. Chlamydia trachomatis, a hidden epidemic: effects on female reproduction and options for treatment. Am J Reprod Immunol. 2010;63:576-586.
55. USPSTF. Chlamydia and gonorrhea screening. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening
56. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1-135.
57. CDC. Sexually transmitted disease surveillance 2018. Accessed February 23, 2021. www.cdc.gov/std/stats18/gonorrhea.htm
5
59. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance–United States, 2015. MMWR Surveill Summ. 2016;65:1-174.
60. CDC. LGBT youth. Accessed February 23, 2021. www.cdc.gov/lgbthealth/youth.htm
61. Johns MM, Lowry R, Rasberry CN, et al. Violence victimization, substance use, and suicide risk among sexual minority high school students – United States, 2015-2017. MMWR Morb Mortal Wkly Rep. 2018;67:1211-1215.
62. Pew Research Center. Teens, social media & technology 2018. . Accessed February 23, 2021. www.pewinternet.org/2018/05/31/teens-social-media-technology-2018/
63. Chassiakos YLR, Radesky J, Christakis D, et al. Children and adolescents and digital media. Pediatrics. 2016;138:e20162593.
64. Arora T, Albahri A, Omar OM, et al. The prospective association between electronic device use before bedtime and academic attainment in adolescents. J Adolesc Health. 2018;63:451-458.
65. Mishna F, Saini M, Solomon S. Ongoing and online: children and youth’s perceptions of cyber bullying. Child Youth Serv Rev. 2009;31:1222-1228.
66. Sengupta A, Chaudhuri A. Are social networking sites a source of online harassment for teens? Evidence from survey data. Child Youth Serv Rev. 2011;33:284-290.
1. World Health Organization. Adolescent health. Accessed February 23, 2021. www.who.int/maternal_child_adolescent/adolescence/en/
2. Sawyer SM, Azzopardi PS, Wickremarathne D, et al. The age of adolescence. Lancet Child Adolesc Health. 2018;2:223-228.
3. Pathak PR, Chou A. Confidential care for adoloscents in the U.S. healthcare system. J Patient Cent Res Rev. 2019;6:46-50.
4. AMA Journal of Ethics. HEADSS: the “review of systems” for adolescents. Accessed February 23, 2021. https://journalofethics.ama-assn.org/article/headss-review-systems-adolescents/2005-03
5. Cohen E, MacKenzie RG, Yates GL. HEADSS, a psychosocial risk assessment instrument: implications for designing effective intervention programs for runaway youth. J Adolesc Health. 1991;12:539-544.
6. Possibilities for Change. Rapid Adolescent Prevention Screening (RAAPS). Accessed February 23, 2021. www.possibilitiesforchange.com/raaps/
7. Elster AB, Kuznets NJ. AMA Guidelines for Adolescent Preventive Services (GAPS): Recommendations and Rationale. Williams & Wilkins; 1994.
8. AAP. Engaging patients and families - periodicity schedule. Accessed February 23, 2021. www.aap.org/en-us/professional-resources/practice-support/Pages/PeriodicitySchedule.aspx
9. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Eng J Med. 2018;379:2468-2475.
10. Schuster MA, Franke TM, Bastian AM, et al. Firearm storage patterns in US homes with children. Am J Public Health. 2000;90:588-594.
11. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States. JAMA. 2004;291:1238-1245.
12. HHS. Health consequences of smoking, surgeon general fact sheet. Accessed February 23, 2021. www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/consequences-smoking-factsheet/index.html
13. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future: national survey results on drug use, 1975-2017. The University of Michigan. 2018. Accessed February 23, 2021. https://eric.ed.gov/?id=ED589762
14. US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
15. HHS. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: HHS, CDC, NCCDPHP, OSH; 2012. Accessed February 23, 2021. www.ncbi.nlm.nih.gov/books/NBK99237/
16. NIH. Alcohol screening and brief intervention for youth: a pocket guide. Accessed February 23, 2021. https://pubs.niaaa.nih.gov/publications/Practitioner/YouthGuide/YouthGuidePocket.pdf
17. Gorey C, Kuhns L, Smaragdi E, et al. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269:37-58.
18. Secades-Villa R, Garcia-Rodriguez O, Jin CJ, et al. Probability and predictors of the cannabis gateway effect: a national study. Int J Drug Policy. 2015;26:135-142.
19. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance—United States, 2017. MMWR Surveill Summ. 2018;67:1-114.
20. NIH. Drug overdoses in youth. How do drug overdoses happen?. Accessed February 23, 2021. https://teens.drugabuse.gov/drug-facts/drug-overdoses-youth
21. Branstetter SA, Low S, Furman W. The influence of parents and friends on adolescent substance use: a multidimensional approach. J Subst Use. 2011;162:150-160.
22. AAP. Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161210.
23. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;288:1-8.
24. Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13:6-13.
25. Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes. 2010;5:282-304.
26. National Physical Activity Plan Alliance. The 2018 United States report card on physical activity for children and youth. Accessed February 23, 2021. http://physicalactivityplan.org/projects/PA/2018/2018%20US%20Report%20Card%20Full%20Version_WEB.PDF?pdf=page-link
27. HHS. NIMH. Child and adolescent mental health. Accessed February 23, 2021. www.nimh.nih.gov/health/topics/child-and-adolescent-mental-health/index.shtml
28. Yonek JC, Jordan N, Dunlop D, et al. Patient-centered medical home care for adolescents in need of mental health treatment. J Adolesc Health. 2018;63:172-180.
29. Brooks TL, Harris SK, Thrall JS, et al. Association of adolescent risk behaviors with mental health symptoms in high school students. |J Adolesc Health. 2002;31:240-246.
30. Weller BE, Blanford KL, Butler AM. Estimated prevalence of psychiatric comorbidities in US adolescents with depression by race/ethnicity, 2011-2012. J Adolesc Health. 2018;62:716-721.
31. Bilsen J. Suicide and youth: risk factors. Front Psychiatry. 2018;9:540.
32. Shain B, AAP Committee on Adolescence. Suicide and suicide attempts in adolescents. Pediatrics. 2016;138:e20161420.
33. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: review and future directions. J Adolesc Health. 2016;59:135-143.
34. Bravender T. Attention-deficit/hyperactivity disorder and disordered eating. [editorial] J Adolesc Health. 2017;61:125-126.
35. Rosen DS, AAP Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics. 2010;126:1240-1253.
36. Susman EJ, Houts RM, Steinberg L, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 ½ and 15 ½ years. Arch Pediatr Adolesc Med. 2010;164:166-173.
37. Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(suppl 3):S208-S217.
38. Ge X, Conger RD, Elder GH. Coming of age too early: pubertal influences on girl’s vulnerability to psychologic distress. Child Dev. 1996;67:3386-3400.
39. Jørgensen M, Keiding N, Skakkebaek NE. Estimation of spermarche from longitudinal spermaturia data. Biometrics. 1991;47:177-193.
40. Kar SK, Choudhury A, Singh AP. Understanding normal development of adolescent sexuality: a bumpy ride. J Hum Reprod Sci. 2015;8:70-74.
41. Susman EJ, Dorn LD, Schiefelbein VL. Puberty, sexuality and health. In: Lerner MA, Easterbrooks MA, Mistry J (eds). Comprehensive Handbook of Psychology. Wiley; 2003.
42. Lindberg LD, Santelli JS, Desai S. Changing patterns of contraceptive use and the decline in rates of pregnancy and birth among U.S. adolescents, 2007-2014. J Adolesc Health. 2018;63:253-256.
43. Guttmacher Institute. Teen pregnancy. www.guttmacher.org/united-states/teens/teen-pregnancy. Accessed February 23, 2021.
44. CDC. Social determinants and eliminating disparities in teen pregnancy. Accessed February 23, 2021. www.cdc.gov/teenpregnancy/about/social-determinants-disparities-teen-pregnancy.htm
45. Widman L, Nesi J, Kamke K, et al. Technology-based interventions to reduce sexually transmitted infection and unintended pregnancy among youth. J Adolesc Health. 2018;62:651-660.
46. Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-115.e7.
47. Ham P, Allen C. Adolescent health screening and counseling. Am Fam Physician. 2012;86:1109-1116.
48. ACOG. Committee on Adolescent Health Care. Adolescent pregnancy, contraception and sexual activity. 2017. Accessed February 23, 2021. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/adolescent-pregnancy-contraception-and-sexual-activity
49. Wangu Z, Burstein GR. Adolescent sexuality: updates to the sexually transmitted infection guidelines. Pediatr Clin N Am. 2017;64:389-411.
50. Holway GV, Hernandez SM. Oral sex and condom use in a U.S. national sample of adolescents and young adults. J Adolesc Health. 2018;62:402-410.
51. CDC. STDs in adults and adolescents. Accessed February 23, 2021. www.cdc.gov/std/stats17/adolescents.htm
52. McClung N, Gargano J, Bennett N, et al. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008-2014. Accessed February 23, 2021. https://cebp.aacrjournals.org/content/28/3/602
53. Timbang MR, Sim MW, Bewley AF, et al. HPV-related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum Vaccin Immunother. 2019;15:1920-1928.
54. Carey AJ, Beagley KW. Chlamydia trachomatis, a hidden epidemic: effects on female reproduction and options for treatment. Am J Reprod Immunol. 2010;63:576-586.
55. USPSTF. Chlamydia and gonorrhea screening. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening
56. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1-135.
57. CDC. Sexually transmitted disease surveillance 2018. Accessed February 23, 2021. www.cdc.gov/std/stats18/gonorrhea.htm
5
59. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance–United States, 2015. MMWR Surveill Summ. 2016;65:1-174.
60. CDC. LGBT youth. Accessed February 23, 2021. www.cdc.gov/lgbthealth/youth.htm
61. Johns MM, Lowry R, Rasberry CN, et al. Violence victimization, substance use, and suicide risk among sexual minority high school students – United States, 2015-2017. MMWR Morb Mortal Wkly Rep. 2018;67:1211-1215.
62. Pew Research Center. Teens, social media & technology 2018. . Accessed February 23, 2021. www.pewinternet.org/2018/05/31/teens-social-media-technology-2018/
63. Chassiakos YLR, Radesky J, Christakis D, et al. Children and adolescents and digital media. Pediatrics. 2016;138:e20162593.
64. Arora T, Albahri A, Omar OM, et al. The prospective association between electronic device use before bedtime and academic attainment in adolescents. J Adolesc Health. 2018;63:451-458.
65. Mishna F, Saini M, Solomon S. Ongoing and online: children and youth’s perceptions of cyber bullying. Child Youth Serv Rev. 2009;31:1222-1228.
66. Sengupta A, Chaudhuri A. Are social networking sites a source of online harassment for teens? Evidence from survey data. Child Youth Serv Rev. 2011;33:284-290.
PRACTICE RECOMMENDATIONS
› Consider using a 2-question screening tool for adolescents that asks about personal use of alcohol and use of alcohol by friends; this resource offers a risk assessment with recommendations. C
› Consider using the American Academy of Pediatrics Family Media Plan to provide age-specific guidelines to help parents or caregivers establish rules for online activities. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Certain DMTs in MS may attenuate COVID-19 vaccines
“There’s no reason to think any of the three authorized vaccines are in any way more dangerous in people with MS, or in the context of MS DMTs. It’s only a question of whether certain DMTs will influence the degree of benefit you get from the vaccine,” said Amit Bar-Or, MD, director of the Center for Neuroinflammation and Neurotherapeutics, chief of the multiple sclerosis division, and Melissa and Paul Anderson President’s Distinguished Professor at the University of Pennsylvania, Philadelphia. He spoke at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, and he also answered questions in a follow-up interview.
“The merits of being protected by the COVID-19 vaccines far outweigh any risks that one would consider associated with vaccines and individuals with MS,” said Dr. Bar-Or. “And there’s reason to think that the RNA vaccines may even be safer than prior, more traditional vaccines. They are nonlive, noninactivated vaccines, and there is no risk in terms of interacting with MS.”
Where do DMTs fit in? In an interview, Hesham Abboud, MD, PhD, of University Hospitals of Cleveland and Case Western Reserve University, also in Cleveland, said there’s reason for caution regarding DMTs that deplete immune cells or entrap them in the lymph nodes. “What is not clearly known is the effect of the fumarates, which do not act through cell depletion but can occasionally deplete immune cells as a side effect. These likely have no negative effect on vaccine efficacy in patients with normal immune cell count but may have a negative effect in those with significant immune cell reduction. Luckily, significant immune cell reduction is rare in patients taking fumarates.”
In addition, he said, “interferons and natalizumab are generally thought to have no impact on vaccine efficacy while glatiramer acetate and teriflunomide are thought to have no or only little impact on vaccines. Most of these concepts are derived from studies of non–COVID-19 vaccines.”
Dr. Bar-Or highlighted specific DMTs. Teriflunomide (Aubagio) “has a relatively mild effect on the immune system and is not thought to be particularly immune suppressive or deplete immune cells,” Dr. Bar-Or said, as shown in a 2015 study he led (Neurol Neuroimmunol Neuroinflamm. 2015 Feb 12;2[2]:e70). In contrast, a 2020 study, also led by Dr. Bar-Or, showed that nonlive vaccinations given after treatment with ocrelizumab (Ocrevus) – an anti-CD20 monoclonal antibody – are “attenuated, compared with untreated or interferon-beta–treated patients, but they can still be expected to be protective.”
Dr. Bar-Or pointed to National MS Society guidelines about the timing of the Pfizer and Moderna mRNA vaccines for patients with MS who are on DMT. In patients with stable MS, the society recommends no adjustments in timing for patients starting or remaining on several DMTs. The list includes teriflunomide, glatiramer acetate (Copaxone), and dimethyl fumarate, among others.
Patients shouldn’t start fingolimod (Gilenya), siponimod (Mayzent), or ozanimod (Zeposia) until 4 weeks or more after their second vaccine dose, the guidelines suggest. Vaccine doses are recommended 3-5 days after the final dose of high-dose steroids. And there are more complicated recommendations regarding a number of other DMTs – ocrelizumab, ofatumumab (Kesimpta), alemtuzumab (Lemtrada), cladribine (Mavenclad), and rituximab (Rituxan).
Dr. Bar-Or cautioned that the guidelines are an imperfect “first pass” and are being updated.
He added that the guidelines are not set in stone: “Scheduling is not always possible in terms of adjusting the vaccine timing. Patients in general are recommended to take the vaccine when it becomes available, as it may be more important for them to get the vaccine than to try to time the vaccine relative to the DMT.”
Guidance regarding the newly authorized Johnson & Johnson vaccine is expected soon, said neurologist Barbara Giesser, MD, of Pacific Neuroscience Institute in Santa Monica, Calif., in an interview. As for her advice to patients, she said that, “in general, I am recommending that patients get [vaccinated] as soon as it is available to them with adjustment of timing of some DMTs as may be appropriate.”
Dr. Bar-Or has received consulting fees and/or grant support from – or participated as a speaker in events sponsored by – Accure, Atara Biotherapeutics, Biogen, Bristol-Myer Squibb/Celgene/Receptos, GlaxoSmithKline, Gossamer, Janssen/Actelion, Medimmune, Merck/EMD Serono, Novartis, Roche/Genentech, and Sanofi-Genzyme. He also receives research funding from various organizations and agencies. Dr. Abboud reported receiving consulting fees from Biogen, Genentech, Bristol-Myer Squibb, Alexion, and Viela Bio. He receives research support from Novartis, Bristol-Myer Squibb, Genentech, and Sanofi-Genzyme. Dr. Giesser reports no disclosures.
“There’s no reason to think any of the three authorized vaccines are in any way more dangerous in people with MS, or in the context of MS DMTs. It’s only a question of whether certain DMTs will influence the degree of benefit you get from the vaccine,” said Amit Bar-Or, MD, director of the Center for Neuroinflammation and Neurotherapeutics, chief of the multiple sclerosis division, and Melissa and Paul Anderson President’s Distinguished Professor at the University of Pennsylvania, Philadelphia. He spoke at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, and he also answered questions in a follow-up interview.
“The merits of being protected by the COVID-19 vaccines far outweigh any risks that one would consider associated with vaccines and individuals with MS,” said Dr. Bar-Or. “And there’s reason to think that the RNA vaccines may even be safer than prior, more traditional vaccines. They are nonlive, noninactivated vaccines, and there is no risk in terms of interacting with MS.”
Where do DMTs fit in? In an interview, Hesham Abboud, MD, PhD, of University Hospitals of Cleveland and Case Western Reserve University, also in Cleveland, said there’s reason for caution regarding DMTs that deplete immune cells or entrap them in the lymph nodes. “What is not clearly known is the effect of the fumarates, which do not act through cell depletion but can occasionally deplete immune cells as a side effect. These likely have no negative effect on vaccine efficacy in patients with normal immune cell count but may have a negative effect in those with significant immune cell reduction. Luckily, significant immune cell reduction is rare in patients taking fumarates.”
In addition, he said, “interferons and natalizumab are generally thought to have no impact on vaccine efficacy while glatiramer acetate and teriflunomide are thought to have no or only little impact on vaccines. Most of these concepts are derived from studies of non–COVID-19 vaccines.”
Dr. Bar-Or highlighted specific DMTs. Teriflunomide (Aubagio) “has a relatively mild effect on the immune system and is not thought to be particularly immune suppressive or deplete immune cells,” Dr. Bar-Or said, as shown in a 2015 study he led (Neurol Neuroimmunol Neuroinflamm. 2015 Feb 12;2[2]:e70). In contrast, a 2020 study, also led by Dr. Bar-Or, showed that nonlive vaccinations given after treatment with ocrelizumab (Ocrevus) – an anti-CD20 monoclonal antibody – are “attenuated, compared with untreated or interferon-beta–treated patients, but they can still be expected to be protective.”
Dr. Bar-Or pointed to National MS Society guidelines about the timing of the Pfizer and Moderna mRNA vaccines for patients with MS who are on DMT. In patients with stable MS, the society recommends no adjustments in timing for patients starting or remaining on several DMTs. The list includes teriflunomide, glatiramer acetate (Copaxone), and dimethyl fumarate, among others.
Patients shouldn’t start fingolimod (Gilenya), siponimod (Mayzent), or ozanimod (Zeposia) until 4 weeks or more after their second vaccine dose, the guidelines suggest. Vaccine doses are recommended 3-5 days after the final dose of high-dose steroids. And there are more complicated recommendations regarding a number of other DMTs – ocrelizumab, ofatumumab (Kesimpta), alemtuzumab (Lemtrada), cladribine (Mavenclad), and rituximab (Rituxan).
Dr. Bar-Or cautioned that the guidelines are an imperfect “first pass” and are being updated.
He added that the guidelines are not set in stone: “Scheduling is not always possible in terms of adjusting the vaccine timing. Patients in general are recommended to take the vaccine when it becomes available, as it may be more important for them to get the vaccine than to try to time the vaccine relative to the DMT.”
Guidance regarding the newly authorized Johnson & Johnson vaccine is expected soon, said neurologist Barbara Giesser, MD, of Pacific Neuroscience Institute in Santa Monica, Calif., in an interview. As for her advice to patients, she said that, “in general, I am recommending that patients get [vaccinated] as soon as it is available to them with adjustment of timing of some DMTs as may be appropriate.”
Dr. Bar-Or has received consulting fees and/or grant support from – or participated as a speaker in events sponsored by – Accure, Atara Biotherapeutics, Biogen, Bristol-Myer Squibb/Celgene/Receptos, GlaxoSmithKline, Gossamer, Janssen/Actelion, Medimmune, Merck/EMD Serono, Novartis, Roche/Genentech, and Sanofi-Genzyme. He also receives research funding from various organizations and agencies. Dr. Abboud reported receiving consulting fees from Biogen, Genentech, Bristol-Myer Squibb, Alexion, and Viela Bio. He receives research support from Novartis, Bristol-Myer Squibb, Genentech, and Sanofi-Genzyme. Dr. Giesser reports no disclosures.
“There’s no reason to think any of the three authorized vaccines are in any way more dangerous in people with MS, or in the context of MS DMTs. It’s only a question of whether certain DMTs will influence the degree of benefit you get from the vaccine,” said Amit Bar-Or, MD, director of the Center for Neuroinflammation and Neurotherapeutics, chief of the multiple sclerosis division, and Melissa and Paul Anderson President’s Distinguished Professor at the University of Pennsylvania, Philadelphia. He spoke at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, and he also answered questions in a follow-up interview.
“The merits of being protected by the COVID-19 vaccines far outweigh any risks that one would consider associated with vaccines and individuals with MS,” said Dr. Bar-Or. “And there’s reason to think that the RNA vaccines may even be safer than prior, more traditional vaccines. They are nonlive, noninactivated vaccines, and there is no risk in terms of interacting with MS.”
Where do DMTs fit in? In an interview, Hesham Abboud, MD, PhD, of University Hospitals of Cleveland and Case Western Reserve University, also in Cleveland, said there’s reason for caution regarding DMTs that deplete immune cells or entrap them in the lymph nodes. “What is not clearly known is the effect of the fumarates, which do not act through cell depletion but can occasionally deplete immune cells as a side effect. These likely have no negative effect on vaccine efficacy in patients with normal immune cell count but may have a negative effect in those with significant immune cell reduction. Luckily, significant immune cell reduction is rare in patients taking fumarates.”
In addition, he said, “interferons and natalizumab are generally thought to have no impact on vaccine efficacy while glatiramer acetate and teriflunomide are thought to have no or only little impact on vaccines. Most of these concepts are derived from studies of non–COVID-19 vaccines.”
Dr. Bar-Or highlighted specific DMTs. Teriflunomide (Aubagio) “has a relatively mild effect on the immune system and is not thought to be particularly immune suppressive or deplete immune cells,” Dr. Bar-Or said, as shown in a 2015 study he led (Neurol Neuroimmunol Neuroinflamm. 2015 Feb 12;2[2]:e70). In contrast, a 2020 study, also led by Dr. Bar-Or, showed that nonlive vaccinations given after treatment with ocrelizumab (Ocrevus) – an anti-CD20 monoclonal antibody – are “attenuated, compared with untreated or interferon-beta–treated patients, but they can still be expected to be protective.”
Dr. Bar-Or pointed to National MS Society guidelines about the timing of the Pfizer and Moderna mRNA vaccines for patients with MS who are on DMT. In patients with stable MS, the society recommends no adjustments in timing for patients starting or remaining on several DMTs. The list includes teriflunomide, glatiramer acetate (Copaxone), and dimethyl fumarate, among others.
Patients shouldn’t start fingolimod (Gilenya), siponimod (Mayzent), or ozanimod (Zeposia) until 4 weeks or more after their second vaccine dose, the guidelines suggest. Vaccine doses are recommended 3-5 days after the final dose of high-dose steroids. And there are more complicated recommendations regarding a number of other DMTs – ocrelizumab, ofatumumab (Kesimpta), alemtuzumab (Lemtrada), cladribine (Mavenclad), and rituximab (Rituxan).
Dr. Bar-Or cautioned that the guidelines are an imperfect “first pass” and are being updated.
He added that the guidelines are not set in stone: “Scheduling is not always possible in terms of adjusting the vaccine timing. Patients in general are recommended to take the vaccine when it becomes available, as it may be more important for them to get the vaccine than to try to time the vaccine relative to the DMT.”
Guidance regarding the newly authorized Johnson & Johnson vaccine is expected soon, said neurologist Barbara Giesser, MD, of Pacific Neuroscience Institute in Santa Monica, Calif., in an interview. As for her advice to patients, she said that, “in general, I am recommending that patients get [vaccinated] as soon as it is available to them with adjustment of timing of some DMTs as may be appropriate.”
Dr. Bar-Or has received consulting fees and/or grant support from – or participated as a speaker in events sponsored by – Accure, Atara Biotherapeutics, Biogen, Bristol-Myer Squibb/Celgene/Receptos, GlaxoSmithKline, Gossamer, Janssen/Actelion, Medimmune, Merck/EMD Serono, Novartis, Roche/Genentech, and Sanofi-Genzyme. He also receives research funding from various organizations and agencies. Dr. Abboud reported receiving consulting fees from Biogen, Genentech, Bristol-Myer Squibb, Alexion, and Viela Bio. He receives research support from Novartis, Bristol-Myer Squibb, Genentech, and Sanofi-Genzyme. Dr. Giesser reports no disclosures.
FROM ACTRIMS FORUM 2021
An alternative regimen to reduce risk of asthma exacerbations
ILLUSTRATIVE CASE
A 37-year-old woman with moderate persistent asthma, controlled on the ICS fluticasone (110 μg twice a day) presents to you for an annual exam. She uses her rescue albuterol inhaler a few times per month. Her last exacerbation was 2 years ago. She has never smoked. She is concerned about continuing to take an ICS every day. What alternative regimen would you recommend for this patient?
According to the Centers for Disease Control and Prevention, asthma affected 24.7 million children and adults in the United States in 2018, accounting for 9.8 million physician visits and 1.6 million emergency department (ED) visits.2 The National Institutes of Health (NIH) asthma care guidelines, updated in 2020, recommend a SABA prn as step 1 for intermittent asthma, along with nonpharmacologic management.3 Once a patient has persistent asthma, treatment escalation to step 2 calls for use of daily maintenance inhalers as the preferred treatment option.3
However, the 2020 Global Initiative for Asthma (GINA) warns that an as-needed SABA does not protect patients from severe exacerbations, and regular use of a SABA alone (> 3 inhalers/year) can increase the risk of exacerbations.4 A meta-analysis and systematic review from 2018 showed that using an ICS/LABA—scheduled and prn for rescue—had lower risk of asthma exacerbations compared with scheduled ICS/LABA with SABA prn for rescue in patients with moderate-to-severe persistent asthma.5 Interestingly, the updated 2020 NIH guidelines have adopted this strategy. SABA use prn is no longer recommended for rescue in mild and moderate persistent asthma, and the guidelines now suggest that ICS/LABA be used as rescue in addition to daily medication.3
Although evidence has been mounting for adding the as-needed ICS/LABA for rescue in patients on daily medication, the mainstay has been to provide a SABA prn for rescue use.5 Confusing matters more, evidence is emerging that as-needed ICS/LABA for rescue alone in certain patients is safe and effective. The randomized controlled Novel START study, an open-label, parallel-group study, compared ICS/LABA prn vs scheduled ICS with SABA prn vs SABA alone prn in adult patients with intermittent or mild persistent asthma.6 ICS/LABA prn prevented more exacerbations and provided better daily control than as-needed SABA alone.6 In addition, ICS/LABA as needed resulted in fewer severe exacerbations but potentially poorer daily control than ICS with SABA as needed.6
The PRACTICAL study investigated treatment of patients with intermittent, mild persistent, and moderate persistent asthma.1
STUDY SUMMARY
ICS/LABA prn reduced risk of severe exacerbations
The randomized controlled PRACTICAL study was a 52-week, open-label, parallel-group, superiority trial in New Zealand that compared as-needed ICS/LABA (n = 437) to scheduled ICS plus as-needed SABA (n = 448). Patients were 18 to 75 years old, with a diagnosis of asthma. Applying NIH guideline definitions, these patients would fall into intermittent, mild persistent, or moderate persistent asthma categories, and were on either as-needed SABA alone or a scheduled low- to moderate-dose ICS plus an as-needed SABA in the previous 12 weeks.
Patients on an as-needed SABA prerandomization had to have at least 1 of the following: (1) asthma symptoms or need for a SABA at least twice in the past 4 weeks; (2) at least 1 nighttime awakening due to asthma in the past 4 weeks; or (3) a severe exacerbation requiring oral corticosteroids in the past year. Patients on scheduled ICS plus SABA prn prerandomization were required to have either: (1) low or moderate ICS dosing with partly or well-controlled asthma; or (2) if uncontrolled, poor inhaler technique or adherence.
Continue to: Patients in the ICS/LABA group...
Patients in the ICS/LABA group were given budesonide 200 µg/formoterol 6 µg, 1 puff prn, and patients in the ICS plus as-needed SABA group were given budesonide 200 µg, 1 puff twice daily, and terbutaline 250 µg, 2 puffs prn. All patients received an asthma action plan that provided guidance on when to seek medical care if asthma worsened, as well as a log to note urgent medical visits and use of systemic corticosteroids. A subset of patients had adherence and dosing monitored by electronic inhaler usage monitors. Patients were seen at 0, 4, 16, 28, 40, and 52 weeks.
Outcomes. The primary outcome was the number of severe exacerbations per patient per year, defined as treatment with oral corticosteroids for ≥ 3 days or ED visit or hospital admission requiring systemic corticosteroids. Among the secondary outcomes were number of moderate and severe exacerbations per patient per year (defined as an unplanned medical visit: primary care, ED, hospital admission, and any duration of steroids); time to first severe exacerbation; assessment with the Asthma Control Questionnaire (ACQ-5); adverse outcomes; and quantity of ICS used (analysis done only for the subset with electronic inhaler monitoring).
ACQ-5 takes the mean of 5 questions assessing asthma control in the previous week, with each question ranging from 0 (no impairment) to 6 (maximum impairment). The statistician was blinded to the primary outcome.
Results. The rate of severe exacerbations per patient per year was 0.119 in the as-needed ICS/LABA group vs 0.172 in the scheduled ICS plus as-needed SABA group (relative rate [RR] = 0.69; 95% confidence interval [CI], 0.48–1.00). Time to first severe asthma exacerbation was longer in the as-needed ICS/LABA group (hazard ratio = 0.60; 95% CI, 0.40–0.91). The rate of moderate and severe exacerbations per patient per year was lower in the as-needed ICS/LABA group: 0.165 vs 0.237 (RR = 0.70; 95% CI, 0.51–0.95).
ACQ-5 scores were similar at all time points (mean difference = 0.07; 95% CI, –0.03 to 0.17). Adverse events were similar between groups (most commonly nasopharyngitis in both groups). Less ICS was used in the ICS/LABA group (difference = –126.5 µg per day; 95% CI, –171.0 to –81.9).
Continue to: WHAT'S NEW
WHAT’S NEW
Study lends support to recent recommendations
This study represents a compelling, real-world look at emerging asthma recommendations. This was the first comprehensive study to show that as-needed ICS/LABA therapy prevents more moderate and severe exacerbations and lengthens the time to first severe exacerbation, compared with scheduled ICS plus SABA prn in intermittent, mild persistent, or moderate persistent asthma. These data have been incorporated into the GINA guidelines, which recommend ICS/LABA prn for step 2.
CAVEATS
Potential bias in study design
The LABA used in this study was formoterol, which has a quicker onset than other LABAs. It is likely that not all LABAs can be used the same way, and both the NIH and GINA guidelines call it out specifically. Additionally, the study’s open-label design can introduce bias but may be the only way to simulate the real-world actions of our patients. Prior studies used placebo inhalers to keep participants and providers blinded but then could not capitalize on the behavior of using only an inhaler prn (as with the ICS/LABA of this study). Finally, there is discordance between the NIH and GINA asthma guidelines on how to use these data.
CHALLENGES TO IMPLEMENTATION
Cost of ICS/LABA may limit its use
Cost is the largest barrier to implementation. Budesonide costs 6 to 10 times more than albuterol per inhaler (retail price of $281-$427 vs $17-$92, respectively).7,8 However, cost differences are likely negated for patients already on a maintenance inhaler.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Hardy J, Baggott C, Fingleton J, et al; PRACTICAL study team. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394:919-928. Published correction appears in Lancet. 2020;395:1422.
2. Centers for Disease Control and Prevention. Summary Health Statistics: National Health Interview Survey, 2018. Accessed February 17, 2021. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2018_SHS_Table_A-2.pdf
3. National Institutes of Health. National Heart, Lung, and Blood Institute. 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. December 2020. Accessed February 17, 2021. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines
4. Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention, 2020. Accessed February 17, 2021. www.ginasthma.org/
5. Sobieraj DM, Weeda ER, Nguyen E, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA. 2018;319:1485-1496.
6. Beasley R, Holliday M, Reddel HK, et al; Novel START Study Team. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med. 2019;380:2020-2030.
7. Albuterol. GoodRx. Accessed February 17, 2021. www.goodrx.com/albuterol
8. Budesonide/formoterol. GoodRx. Accessed February 17, 2021. www.goodrx.com/budesonide-formoterol
ILLUSTRATIVE CASE
A 37-year-old woman with moderate persistent asthma, controlled on the ICS fluticasone (110 μg twice a day) presents to you for an annual exam. She uses her rescue albuterol inhaler a few times per month. Her last exacerbation was 2 years ago. She has never smoked. She is concerned about continuing to take an ICS every day. What alternative regimen would you recommend for this patient?
According to the Centers for Disease Control and Prevention, asthma affected 24.7 million children and adults in the United States in 2018, accounting for 9.8 million physician visits and 1.6 million emergency department (ED) visits.2 The National Institutes of Health (NIH) asthma care guidelines, updated in 2020, recommend a SABA prn as step 1 for intermittent asthma, along with nonpharmacologic management.3 Once a patient has persistent asthma, treatment escalation to step 2 calls for use of daily maintenance inhalers as the preferred treatment option.3
However, the 2020 Global Initiative for Asthma (GINA) warns that an as-needed SABA does not protect patients from severe exacerbations, and regular use of a SABA alone (> 3 inhalers/year) can increase the risk of exacerbations.4 A meta-analysis and systematic review from 2018 showed that using an ICS/LABA—scheduled and prn for rescue—had lower risk of asthma exacerbations compared with scheduled ICS/LABA with SABA prn for rescue in patients with moderate-to-severe persistent asthma.5 Interestingly, the updated 2020 NIH guidelines have adopted this strategy. SABA use prn is no longer recommended for rescue in mild and moderate persistent asthma, and the guidelines now suggest that ICS/LABA be used as rescue in addition to daily medication.3
Although evidence has been mounting for adding the as-needed ICS/LABA for rescue in patients on daily medication, the mainstay has been to provide a SABA prn for rescue use.5 Confusing matters more, evidence is emerging that as-needed ICS/LABA for rescue alone in certain patients is safe and effective. The randomized controlled Novel START study, an open-label, parallel-group study, compared ICS/LABA prn vs scheduled ICS with SABA prn vs SABA alone prn in adult patients with intermittent or mild persistent asthma.6 ICS/LABA prn prevented more exacerbations and provided better daily control than as-needed SABA alone.6 In addition, ICS/LABA as needed resulted in fewer severe exacerbations but potentially poorer daily control than ICS with SABA as needed.6
The PRACTICAL study investigated treatment of patients with intermittent, mild persistent, and moderate persistent asthma.1
STUDY SUMMARY
ICS/LABA prn reduced risk of severe exacerbations
The randomized controlled PRACTICAL study was a 52-week, open-label, parallel-group, superiority trial in New Zealand that compared as-needed ICS/LABA (n = 437) to scheduled ICS plus as-needed SABA (n = 448). Patients were 18 to 75 years old, with a diagnosis of asthma. Applying NIH guideline definitions, these patients would fall into intermittent, mild persistent, or moderate persistent asthma categories, and were on either as-needed SABA alone or a scheduled low- to moderate-dose ICS plus an as-needed SABA in the previous 12 weeks.
Patients on an as-needed SABA prerandomization had to have at least 1 of the following: (1) asthma symptoms or need for a SABA at least twice in the past 4 weeks; (2) at least 1 nighttime awakening due to asthma in the past 4 weeks; or (3) a severe exacerbation requiring oral corticosteroids in the past year. Patients on scheduled ICS plus SABA prn prerandomization were required to have either: (1) low or moderate ICS dosing with partly or well-controlled asthma; or (2) if uncontrolled, poor inhaler technique or adherence.
Continue to: Patients in the ICS/LABA group...
Patients in the ICS/LABA group were given budesonide 200 µg/formoterol 6 µg, 1 puff prn, and patients in the ICS plus as-needed SABA group were given budesonide 200 µg, 1 puff twice daily, and terbutaline 250 µg, 2 puffs prn. All patients received an asthma action plan that provided guidance on when to seek medical care if asthma worsened, as well as a log to note urgent medical visits and use of systemic corticosteroids. A subset of patients had adherence and dosing monitored by electronic inhaler usage monitors. Patients were seen at 0, 4, 16, 28, 40, and 52 weeks.
Outcomes. The primary outcome was the number of severe exacerbations per patient per year, defined as treatment with oral corticosteroids for ≥ 3 days or ED visit or hospital admission requiring systemic corticosteroids. Among the secondary outcomes were number of moderate and severe exacerbations per patient per year (defined as an unplanned medical visit: primary care, ED, hospital admission, and any duration of steroids); time to first severe exacerbation; assessment with the Asthma Control Questionnaire (ACQ-5); adverse outcomes; and quantity of ICS used (analysis done only for the subset with electronic inhaler monitoring).
ACQ-5 takes the mean of 5 questions assessing asthma control in the previous week, with each question ranging from 0 (no impairment) to 6 (maximum impairment). The statistician was blinded to the primary outcome.
Results. The rate of severe exacerbations per patient per year was 0.119 in the as-needed ICS/LABA group vs 0.172 in the scheduled ICS plus as-needed SABA group (relative rate [RR] = 0.69; 95% confidence interval [CI], 0.48–1.00). Time to first severe asthma exacerbation was longer in the as-needed ICS/LABA group (hazard ratio = 0.60; 95% CI, 0.40–0.91). The rate of moderate and severe exacerbations per patient per year was lower in the as-needed ICS/LABA group: 0.165 vs 0.237 (RR = 0.70; 95% CI, 0.51–0.95).
ACQ-5 scores were similar at all time points (mean difference = 0.07; 95% CI, –0.03 to 0.17). Adverse events were similar between groups (most commonly nasopharyngitis in both groups). Less ICS was used in the ICS/LABA group (difference = –126.5 µg per day; 95% CI, –171.0 to –81.9).
Continue to: WHAT'S NEW
WHAT’S NEW
Study lends support to recent recommendations
This study represents a compelling, real-world look at emerging asthma recommendations. This was the first comprehensive study to show that as-needed ICS/LABA therapy prevents more moderate and severe exacerbations and lengthens the time to first severe exacerbation, compared with scheduled ICS plus SABA prn in intermittent, mild persistent, or moderate persistent asthma. These data have been incorporated into the GINA guidelines, which recommend ICS/LABA prn for step 2.
CAVEATS
Potential bias in study design
The LABA used in this study was formoterol, which has a quicker onset than other LABAs. It is likely that not all LABAs can be used the same way, and both the NIH and GINA guidelines call it out specifically. Additionally, the study’s open-label design can introduce bias but may be the only way to simulate the real-world actions of our patients. Prior studies used placebo inhalers to keep participants and providers blinded but then could not capitalize on the behavior of using only an inhaler prn (as with the ICS/LABA of this study). Finally, there is discordance between the NIH and GINA asthma guidelines on how to use these data.
CHALLENGES TO IMPLEMENTATION
Cost of ICS/LABA may limit its use
Cost is the largest barrier to implementation. Budesonide costs 6 to 10 times more than albuterol per inhaler (retail price of $281-$427 vs $17-$92, respectively).7,8 However, cost differences are likely negated for patients already on a maintenance inhaler.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 37-year-old woman with moderate persistent asthma, controlled on the ICS fluticasone (110 μg twice a day) presents to you for an annual exam. She uses her rescue albuterol inhaler a few times per month. Her last exacerbation was 2 years ago. She has never smoked. She is concerned about continuing to take an ICS every day. What alternative regimen would you recommend for this patient?
According to the Centers for Disease Control and Prevention, asthma affected 24.7 million children and adults in the United States in 2018, accounting for 9.8 million physician visits and 1.6 million emergency department (ED) visits.2 The National Institutes of Health (NIH) asthma care guidelines, updated in 2020, recommend a SABA prn as step 1 for intermittent asthma, along with nonpharmacologic management.3 Once a patient has persistent asthma, treatment escalation to step 2 calls for use of daily maintenance inhalers as the preferred treatment option.3
However, the 2020 Global Initiative for Asthma (GINA) warns that an as-needed SABA does not protect patients from severe exacerbations, and regular use of a SABA alone (> 3 inhalers/year) can increase the risk of exacerbations.4 A meta-analysis and systematic review from 2018 showed that using an ICS/LABA—scheduled and prn for rescue—had lower risk of asthma exacerbations compared with scheduled ICS/LABA with SABA prn for rescue in patients with moderate-to-severe persistent asthma.5 Interestingly, the updated 2020 NIH guidelines have adopted this strategy. SABA use prn is no longer recommended for rescue in mild and moderate persistent asthma, and the guidelines now suggest that ICS/LABA be used as rescue in addition to daily medication.3
Although evidence has been mounting for adding the as-needed ICS/LABA for rescue in patients on daily medication, the mainstay has been to provide a SABA prn for rescue use.5 Confusing matters more, evidence is emerging that as-needed ICS/LABA for rescue alone in certain patients is safe and effective. The randomized controlled Novel START study, an open-label, parallel-group study, compared ICS/LABA prn vs scheduled ICS with SABA prn vs SABA alone prn in adult patients with intermittent or mild persistent asthma.6 ICS/LABA prn prevented more exacerbations and provided better daily control than as-needed SABA alone.6 In addition, ICS/LABA as needed resulted in fewer severe exacerbations but potentially poorer daily control than ICS with SABA as needed.6
The PRACTICAL study investigated treatment of patients with intermittent, mild persistent, and moderate persistent asthma.1
STUDY SUMMARY
ICS/LABA prn reduced risk of severe exacerbations
The randomized controlled PRACTICAL study was a 52-week, open-label, parallel-group, superiority trial in New Zealand that compared as-needed ICS/LABA (n = 437) to scheduled ICS plus as-needed SABA (n = 448). Patients were 18 to 75 years old, with a diagnosis of asthma. Applying NIH guideline definitions, these patients would fall into intermittent, mild persistent, or moderate persistent asthma categories, and were on either as-needed SABA alone or a scheduled low- to moderate-dose ICS plus an as-needed SABA in the previous 12 weeks.
Patients on an as-needed SABA prerandomization had to have at least 1 of the following: (1) asthma symptoms or need for a SABA at least twice in the past 4 weeks; (2) at least 1 nighttime awakening due to asthma in the past 4 weeks; or (3) a severe exacerbation requiring oral corticosteroids in the past year. Patients on scheduled ICS plus SABA prn prerandomization were required to have either: (1) low or moderate ICS dosing with partly or well-controlled asthma; or (2) if uncontrolled, poor inhaler technique or adherence.
Continue to: Patients in the ICS/LABA group...
Patients in the ICS/LABA group were given budesonide 200 µg/formoterol 6 µg, 1 puff prn, and patients in the ICS plus as-needed SABA group were given budesonide 200 µg, 1 puff twice daily, and terbutaline 250 µg, 2 puffs prn. All patients received an asthma action plan that provided guidance on when to seek medical care if asthma worsened, as well as a log to note urgent medical visits and use of systemic corticosteroids. A subset of patients had adherence and dosing monitored by electronic inhaler usage monitors. Patients were seen at 0, 4, 16, 28, 40, and 52 weeks.
Outcomes. The primary outcome was the number of severe exacerbations per patient per year, defined as treatment with oral corticosteroids for ≥ 3 days or ED visit or hospital admission requiring systemic corticosteroids. Among the secondary outcomes were number of moderate and severe exacerbations per patient per year (defined as an unplanned medical visit: primary care, ED, hospital admission, and any duration of steroids); time to first severe exacerbation; assessment with the Asthma Control Questionnaire (ACQ-5); adverse outcomes; and quantity of ICS used (analysis done only for the subset with electronic inhaler monitoring).
ACQ-5 takes the mean of 5 questions assessing asthma control in the previous week, with each question ranging from 0 (no impairment) to 6 (maximum impairment). The statistician was blinded to the primary outcome.
Results. The rate of severe exacerbations per patient per year was 0.119 in the as-needed ICS/LABA group vs 0.172 in the scheduled ICS plus as-needed SABA group (relative rate [RR] = 0.69; 95% confidence interval [CI], 0.48–1.00). Time to first severe asthma exacerbation was longer in the as-needed ICS/LABA group (hazard ratio = 0.60; 95% CI, 0.40–0.91). The rate of moderate and severe exacerbations per patient per year was lower in the as-needed ICS/LABA group: 0.165 vs 0.237 (RR = 0.70; 95% CI, 0.51–0.95).
ACQ-5 scores were similar at all time points (mean difference = 0.07; 95% CI, –0.03 to 0.17). Adverse events were similar between groups (most commonly nasopharyngitis in both groups). Less ICS was used in the ICS/LABA group (difference = –126.5 µg per day; 95% CI, –171.0 to –81.9).
Continue to: WHAT'S NEW
WHAT’S NEW
Study lends support to recent recommendations
This study represents a compelling, real-world look at emerging asthma recommendations. This was the first comprehensive study to show that as-needed ICS/LABA therapy prevents more moderate and severe exacerbations and lengthens the time to first severe exacerbation, compared with scheduled ICS plus SABA prn in intermittent, mild persistent, or moderate persistent asthma. These data have been incorporated into the GINA guidelines, which recommend ICS/LABA prn for step 2.
CAVEATS
Potential bias in study design
The LABA used in this study was formoterol, which has a quicker onset than other LABAs. It is likely that not all LABAs can be used the same way, and both the NIH and GINA guidelines call it out specifically. Additionally, the study’s open-label design can introduce bias but may be the only way to simulate the real-world actions of our patients. Prior studies used placebo inhalers to keep participants and providers blinded but then could not capitalize on the behavior of using only an inhaler prn (as with the ICS/LABA of this study). Finally, there is discordance between the NIH and GINA asthma guidelines on how to use these data.
CHALLENGES TO IMPLEMENTATION
Cost of ICS/LABA may limit its use
Cost is the largest barrier to implementation. Budesonide costs 6 to 10 times more than albuterol per inhaler (retail price of $281-$427 vs $17-$92, respectively).7,8 However, cost differences are likely negated for patients already on a maintenance inhaler.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Hardy J, Baggott C, Fingleton J, et al; PRACTICAL study team. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394:919-928. Published correction appears in Lancet. 2020;395:1422.
2. Centers for Disease Control and Prevention. Summary Health Statistics: National Health Interview Survey, 2018. Accessed February 17, 2021. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2018_SHS_Table_A-2.pdf
3. National Institutes of Health. National Heart, Lung, and Blood Institute. 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. December 2020. Accessed February 17, 2021. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines
4. Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention, 2020. Accessed February 17, 2021. www.ginasthma.org/
5. Sobieraj DM, Weeda ER, Nguyen E, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA. 2018;319:1485-1496.
6. Beasley R, Holliday M, Reddel HK, et al; Novel START Study Team. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med. 2019;380:2020-2030.
7. Albuterol. GoodRx. Accessed February 17, 2021. www.goodrx.com/albuterol
8. Budesonide/formoterol. GoodRx. Accessed February 17, 2021. www.goodrx.com/budesonide-formoterol
1. Hardy J, Baggott C, Fingleton J, et al; PRACTICAL study team. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394:919-928. Published correction appears in Lancet. 2020;395:1422.
2. Centers for Disease Control and Prevention. Summary Health Statistics: National Health Interview Survey, 2018. Accessed February 17, 2021. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2018_SHS_Table_A-2.pdf
3. National Institutes of Health. National Heart, Lung, and Blood Institute. 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. December 2020. Accessed February 17, 2021. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines
4. Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention, 2020. Accessed February 17, 2021. www.ginasthma.org/
5. Sobieraj DM, Weeda ER, Nguyen E, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA. 2018;319:1485-1496.
6. Beasley R, Holliday M, Reddel HK, et al; Novel START Study Team. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med. 2019;380:2020-2030.
7. Albuterol. GoodRx. Accessed February 17, 2021. www.goodrx.com/albuterol
8. Budesonide/formoterol. GoodRx. Accessed February 17, 2021. www.goodrx.com/budesonide-formoterol
PRACTICE CHANGER
Use an inhaled corticosteroid plus long-acting beta-agonist (ICS/LABA) prn for intermittent, mild persistent, or moderate persistent asthma for fewer moderate and severe exacerbations and the same daily symptom control as scheduled ICS with a short-acting beta-agonist (SABA) prn.1
STRENGTH OF RECOMMENDATION
A: Based on a single, good-quality, multicenter, randomized controlled trial.1
Hardy J, Baggott C, Fingleton J, et al; PRACTICAL study team. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394:919-928. Published correction appears in Lancet. 2020;395:1422.1
Routine vaccinations missed by older adults during pandemic
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.
In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.

In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.

In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
FROM MMWR
Heart health in pregnancy tied to CV risk in adolescent offspring
Children born to mothers in poor cardiovascular health during pregnancy had an almost eight times higher risk for landing in the poorest cardiovascular health category in early adolescence than children born to mothers who had ideal cardiovascular health during pregnancy.
In an observational cohort study that involved 2,302 mother-child dyads, 6.0% of mothers and 2.6% of children were considered to be in the poorest category of cardiovascular health on the basis of specific risk factors.
The children of mothers with any “intermediate” cardiovascular health metrics in pregnancy – for example, being overweight but not obese – were at just more than two times higher risk for poor cardiovascular health in early adolescence.
Although acknowledging the limitations of observational data, Amanda M. Perak, MD, Northwestern University, Chicago, suggested that focusing on whether or not the relationships seen in this study are causal might be throwing the baby out with the bathwater.
“I would suggest that it may not actually matter whether there is causality or correlation here, because if you can identify newborns at birth who have an eight times higher risk for poor cardiovascular health in childhood based on mom’s health during pregnancy, that’s valuable information either way,” said Dr. Perak.
“Even if you don’t know why their risk is elevated, you might be able to target those children for more intensive preventative efforts throughout childhood to help them hold on to their cardiovascular health for longer.”
That said, she thinks it’s possible that the intrauterine environment might actually directly affect offspring health, either through epigenetics modifications to cardiometabolic regulatory genes or possibly through actual organ development. Her group is collecting epigenetic data to study this further.
“We also need to do a study to see if intervening during pregnancy with mothers leads to better cardiovascular health in offspring, and that’s a question we can answer with a clinical trial,” said Dr. Perak.
This study was published on Feb. 16, 2021, in JAMA.
Equal footing
“We’ve always talked about cardiovascular health as if everyone is born with ideal cardiovascular health and loses it from there, and I think what this article points out is that not everybody starts on equal footing,” said Stephen R. Daniels, MD, PhD, University of Colorado at Denver, Aurora, who wrote an editorial accompanying the study.
“We need to start upstream, working with mothers before and during pregnancy, but it’s also important to understand, from a pediatric standpoint, that with some of these kids the horse is kind of already out of the barn very early.”
Dr. Daniels is pediatrician in chief and chair of pediatrics at Children’s Hospital Colorado in Aurora.
This study is the first to examine the relevance of maternal gestational cardiovascular health to offspring cardiovascular health and an important first step toward developing new approaches to address the concept of primordial prevention, he said.
“If primary prevention is identifying risk factors and treating them, I think of primordial prevention as preventing the development of those risk factors in the first place,” said Dr. Daniels.
Future trials, he added, should focus on the various mechanistic pathways – biological effects, shared genetics, and lifestyle being the options – to better understand opportunities for intervention.
Mother-child pairs
Dr. Perak and colleagues used data from the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study and the HAPO Follow-up Study.
Participants were 2,302 mother-child pairs from nine field centers in Barbados, Canada, China, Thailand, United Kingdom, and the United States, and represented a racially and ethnically diverse cohort.
The mean ages were 29.6 years for pregnant mothers and 11.3 years for children. The pregnancies occurred between 2000 and 2006, and the children were examined from 2013 to 2016, when the children were aged 10-14 years.
Using the American Heart Association’s definition of cardiovascular health, the scientists categorized pregnancy health for mothers based on their measures of body mass index, blood pressure, total cholesterol, glucose level, and smoking status at 28 weeks’ gestation. These five metrics of gestational cardiovascular health have been significantly associated with adverse pregnancy outcomes.
They categorized cardiovascular health for offspring at age 10-14 years based on four of these five metrics: body mass index, blood pressure, cholesterol, and glucose.
Only 32.8% of mothers and 42.2% of children had ideal cardiovascular health.
In analyses adjusted for pregnancy and birth outcomes, the associations seen between poor gestational maternal health and offspring cardiovascular health persisted but were attenuated.
Dr. Perak reported receiving grants from the Woman’s Board of Northwestern Memorial Hospital; the Dixon Family; the American Heart Association; and the National Heart, Lung, and Blood Institute. Dr. Daniels reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Children born to mothers in poor cardiovascular health during pregnancy had an almost eight times higher risk for landing in the poorest cardiovascular health category in early adolescence than children born to mothers who had ideal cardiovascular health during pregnancy.
In an observational cohort study that involved 2,302 mother-child dyads, 6.0% of mothers and 2.6% of children were considered to be in the poorest category of cardiovascular health on the basis of specific risk factors.
The children of mothers with any “intermediate” cardiovascular health metrics in pregnancy – for example, being overweight but not obese – were at just more than two times higher risk for poor cardiovascular health in early adolescence.
Although acknowledging the limitations of observational data, Amanda M. Perak, MD, Northwestern University, Chicago, suggested that focusing on whether or not the relationships seen in this study are causal might be throwing the baby out with the bathwater.
“I would suggest that it may not actually matter whether there is causality or correlation here, because if you can identify newborns at birth who have an eight times higher risk for poor cardiovascular health in childhood based on mom’s health during pregnancy, that’s valuable information either way,” said Dr. Perak.
“Even if you don’t know why their risk is elevated, you might be able to target those children for more intensive preventative efforts throughout childhood to help them hold on to their cardiovascular health for longer.”
That said, she thinks it’s possible that the intrauterine environment might actually directly affect offspring health, either through epigenetics modifications to cardiometabolic regulatory genes or possibly through actual organ development. Her group is collecting epigenetic data to study this further.
“We also need to do a study to see if intervening during pregnancy with mothers leads to better cardiovascular health in offspring, and that’s a question we can answer with a clinical trial,” said Dr. Perak.
This study was published on Feb. 16, 2021, in JAMA.
Equal footing
“We’ve always talked about cardiovascular health as if everyone is born with ideal cardiovascular health and loses it from there, and I think what this article points out is that not everybody starts on equal footing,” said Stephen R. Daniels, MD, PhD, University of Colorado at Denver, Aurora, who wrote an editorial accompanying the study.
“We need to start upstream, working with mothers before and during pregnancy, but it’s also important to understand, from a pediatric standpoint, that with some of these kids the horse is kind of already out of the barn very early.”
Dr. Daniels is pediatrician in chief and chair of pediatrics at Children’s Hospital Colorado in Aurora.
This study is the first to examine the relevance of maternal gestational cardiovascular health to offspring cardiovascular health and an important first step toward developing new approaches to address the concept of primordial prevention, he said.
“If primary prevention is identifying risk factors and treating them, I think of primordial prevention as preventing the development of those risk factors in the first place,” said Dr. Daniels.
Future trials, he added, should focus on the various mechanistic pathways – biological effects, shared genetics, and lifestyle being the options – to better understand opportunities for intervention.
Mother-child pairs
Dr. Perak and colleagues used data from the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study and the HAPO Follow-up Study.
Participants were 2,302 mother-child pairs from nine field centers in Barbados, Canada, China, Thailand, United Kingdom, and the United States, and represented a racially and ethnically diverse cohort.
The mean ages were 29.6 years for pregnant mothers and 11.3 years for children. The pregnancies occurred between 2000 and 2006, and the children were examined from 2013 to 2016, when the children were aged 10-14 years.
Using the American Heart Association’s definition of cardiovascular health, the scientists categorized pregnancy health for mothers based on their measures of body mass index, blood pressure, total cholesterol, glucose level, and smoking status at 28 weeks’ gestation. These five metrics of gestational cardiovascular health have been significantly associated with adverse pregnancy outcomes.
They categorized cardiovascular health for offspring at age 10-14 years based on four of these five metrics: body mass index, blood pressure, cholesterol, and glucose.
Only 32.8% of mothers and 42.2% of children had ideal cardiovascular health.
In analyses adjusted for pregnancy and birth outcomes, the associations seen between poor gestational maternal health and offspring cardiovascular health persisted but were attenuated.
Dr. Perak reported receiving grants from the Woman’s Board of Northwestern Memorial Hospital; the Dixon Family; the American Heart Association; and the National Heart, Lung, and Blood Institute. Dr. Daniels reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Children born to mothers in poor cardiovascular health during pregnancy had an almost eight times higher risk for landing in the poorest cardiovascular health category in early adolescence than children born to mothers who had ideal cardiovascular health during pregnancy.
In an observational cohort study that involved 2,302 mother-child dyads, 6.0% of mothers and 2.6% of children were considered to be in the poorest category of cardiovascular health on the basis of specific risk factors.
The children of mothers with any “intermediate” cardiovascular health metrics in pregnancy – for example, being overweight but not obese – were at just more than two times higher risk for poor cardiovascular health in early adolescence.
Although acknowledging the limitations of observational data, Amanda M. Perak, MD, Northwestern University, Chicago, suggested that focusing on whether or not the relationships seen in this study are causal might be throwing the baby out with the bathwater.
“I would suggest that it may not actually matter whether there is causality or correlation here, because if you can identify newborns at birth who have an eight times higher risk for poor cardiovascular health in childhood based on mom’s health during pregnancy, that’s valuable information either way,” said Dr. Perak.
“Even if you don’t know why their risk is elevated, you might be able to target those children for more intensive preventative efforts throughout childhood to help them hold on to their cardiovascular health for longer.”
That said, she thinks it’s possible that the intrauterine environment might actually directly affect offspring health, either through epigenetics modifications to cardiometabolic regulatory genes or possibly through actual organ development. Her group is collecting epigenetic data to study this further.
“We also need to do a study to see if intervening during pregnancy with mothers leads to better cardiovascular health in offspring, and that’s a question we can answer with a clinical trial,” said Dr. Perak.
This study was published on Feb. 16, 2021, in JAMA.
Equal footing
“We’ve always talked about cardiovascular health as if everyone is born with ideal cardiovascular health and loses it from there, and I think what this article points out is that not everybody starts on equal footing,” said Stephen R. Daniels, MD, PhD, University of Colorado at Denver, Aurora, who wrote an editorial accompanying the study.
“We need to start upstream, working with mothers before and during pregnancy, but it’s also important to understand, from a pediatric standpoint, that with some of these kids the horse is kind of already out of the barn very early.”
Dr. Daniels is pediatrician in chief and chair of pediatrics at Children’s Hospital Colorado in Aurora.
This study is the first to examine the relevance of maternal gestational cardiovascular health to offspring cardiovascular health and an important first step toward developing new approaches to address the concept of primordial prevention, he said.
“If primary prevention is identifying risk factors and treating them, I think of primordial prevention as preventing the development of those risk factors in the first place,” said Dr. Daniels.
Future trials, he added, should focus on the various mechanistic pathways – biological effects, shared genetics, and lifestyle being the options – to better understand opportunities for intervention.
Mother-child pairs
Dr. Perak and colleagues used data from the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study and the HAPO Follow-up Study.
Participants were 2,302 mother-child pairs from nine field centers in Barbados, Canada, China, Thailand, United Kingdom, and the United States, and represented a racially and ethnically diverse cohort.
The mean ages were 29.6 years for pregnant mothers and 11.3 years for children. The pregnancies occurred between 2000 and 2006, and the children were examined from 2013 to 2016, when the children were aged 10-14 years.
Using the American Heart Association’s definition of cardiovascular health, the scientists categorized pregnancy health for mothers based on their measures of body mass index, blood pressure, total cholesterol, glucose level, and smoking status at 28 weeks’ gestation. These five metrics of gestational cardiovascular health have been significantly associated with adverse pregnancy outcomes.
They categorized cardiovascular health for offspring at age 10-14 years based on four of these five metrics: body mass index, blood pressure, cholesterol, and glucose.
Only 32.8% of mothers and 42.2% of children had ideal cardiovascular health.
In analyses adjusted for pregnancy and birth outcomes, the associations seen between poor gestational maternal health and offspring cardiovascular health persisted but were attenuated.
Dr. Perak reported receiving grants from the Woman’s Board of Northwestern Memorial Hospital; the Dixon Family; the American Heart Association; and the National Heart, Lung, and Blood Institute. Dr. Daniels reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
BMI, age, and sex affect COVID-19 vaccine antibody response
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.