User login
ACIP recommendations for COVID-19 vaccines—and more
The year 2020 was challenging for public health agencies and especially for the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP). In a normal year, the ACIP meets in person 3 times for a total of 6 days of deliberations. In 2020, there were 10 meetings (all but 1 using Zoom) covering 14 days. Much of the time was dedicated to the COVID-19 pandemic, the vaccines being developed to prevent COVID-19, and the prioritization of those who should receive the vaccines first.
The ACIP also made recommendations for the use of influenza vaccines in the 2020-2021 season, approved the adult and pediatric immunization schedules for 2021, and approved the use of 2 new vaccines, one to protect against meningococcal meningitis and the other to prevent Ebola virus disease. The influenza recommendations were covered in the October 2020 Practice Alert,1 and the immunization schedules can be found on the CDC website at www.cdc.gov/vaccines/schedules/hcp/index.html.
COVID-19 vaccines
Two COVID-19 vaccines have been approved for use in the United States. The first was the Pfizer-BioNTech COVID-19 vaccine, approved by the Food and Drug Administration (FDA) on December 11 and recommended for use by the ACIP on December 12.2 The second vaccine, from Moderna, was approved by the FDA on December 18 and recommended by the ACIP on December 19.3 Both were approved by the FDA under an Emergency Use Authorization (EUA) and were approved by the ACIP for use while the EUA is in effect. Both vaccines must eventually undergo regular approval by the FDA and will be reconsidered by the ACIP regarding use in non–public health emergency conditions. A description of the EUA process and measures taken to assure efficacy and safety, before and after approval, were discussed in the September 2020 audiocast.
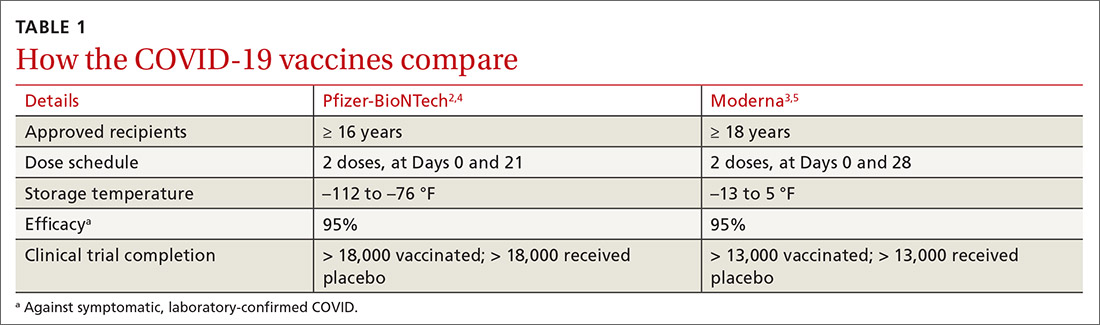
Both COVID-19 vaccines consist of nucleoside-modified mRNA encapsulated with lipid nanoparticles, which encode for a spike glycoprotein of SARS-CoV-2, the virus that causes COVID-19. Both vaccines require 2 doses (separated by 3 weeks for the Pfizer vaccine and 4 weeks for the Moderna vaccine) and are approved for use only in adults and older adolescents (ages ≥ 16 years for the Pfizer vaccine and ≥ 18 years for the Moderna vaccine) (TABLE 12-5).
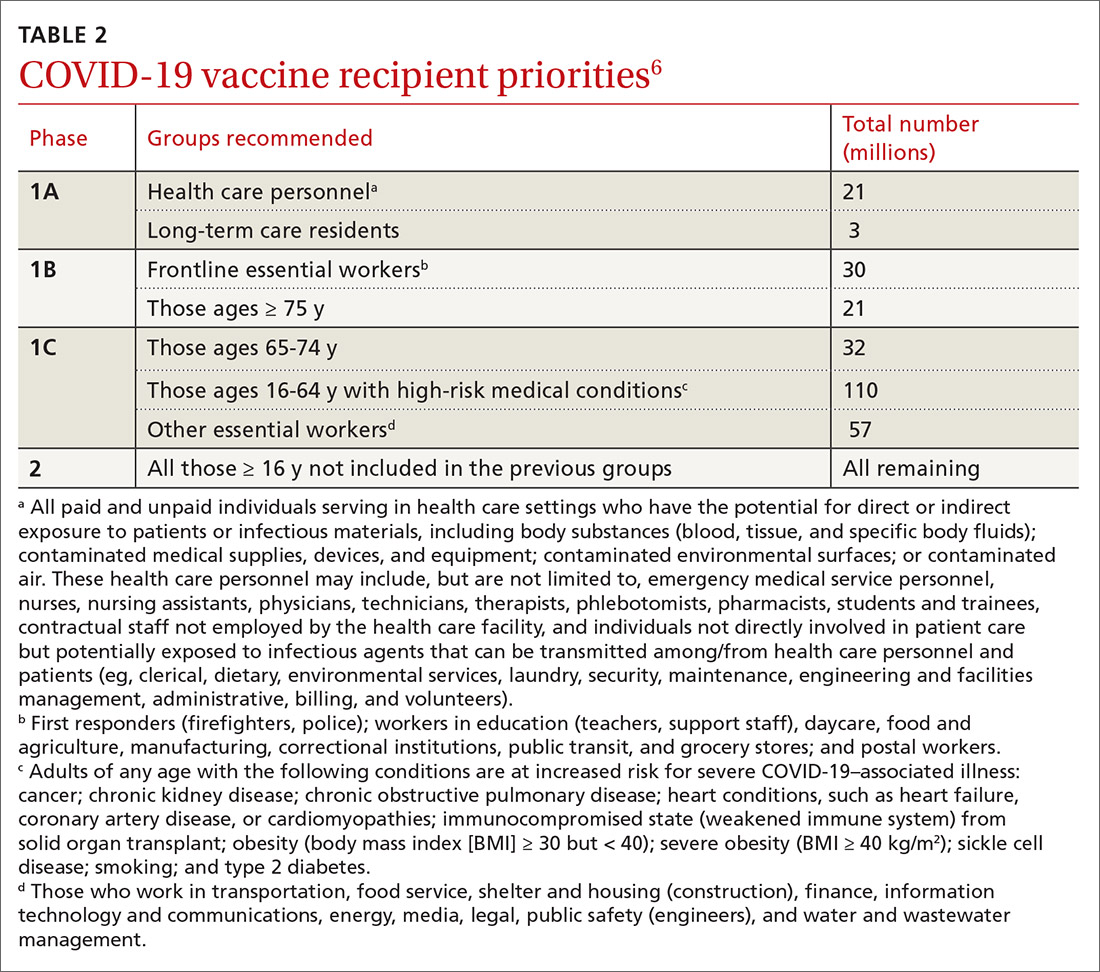
In anticipation of vaccine shortages immediately after approval for use and a high demand for the vaccine, the ACIP developed a list of high-priority groups who should receive the vaccine in ranked order.6 States are encouraged, but not required, to follow this priority list (TABLE 26).
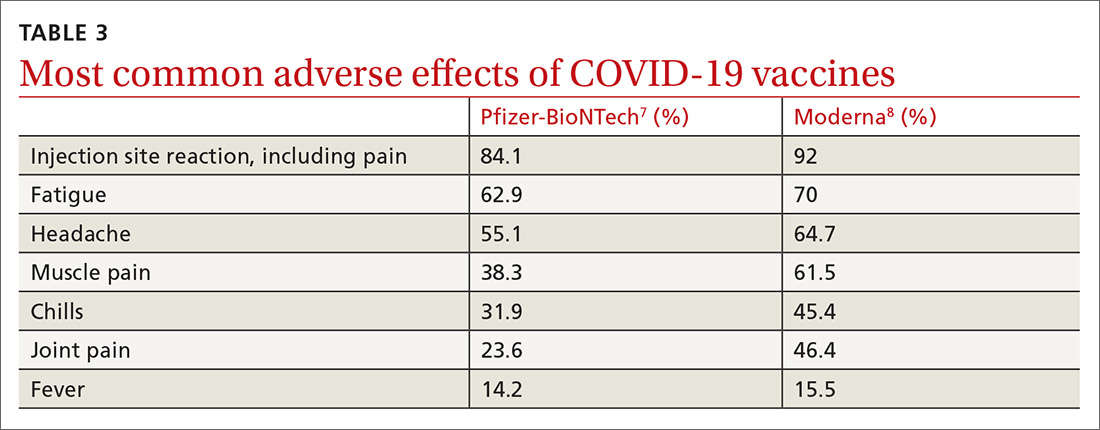
Caveats with usage. Both COVID-19 vaccines are very reactogenic, causing local and systemic adverse effects that patients should be warned about (TABLE 37,8). These reactions are usually mild to moderate and last 24 hours or less. Acetaminophen can alleviate these symptoms but should not be used to prevent them. In addition, both vaccines have stringent cold-storage requirements; once the vaccines are thawed, they must be used within a defined time-period.
Neither vaccine is listed as preferred. And they are not interchangeable; both recommended doses should be completed with the same vaccine. More details about the use of these vaccines were discussed in the January 2021 audiocast (www.mdedge.com/familymedicine/article/234239/coronavirus-updates/covid-19-vaccines-rollout-risks-and-reason-still) and can be located on the CDC website (www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html; www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html).
Continue to: Much remains unknown...
Much remains unknown regarding the use of these COVID-19 vaccines:
- What is their duration of protection, and will booster doses be needed?
- Will they protect against asymptomatic infection and carrier states, and thereby prevent transmission?
- Can they be co-administered with other vaccines?
- Will they be efficacious and safe to use during pregnancy and breastfeeding?
These issues will need to be addressed before they are recommended for non–public health emergency use.
Quadrivalent meningococcal conjugate vaccine (MenACWY)
In June 2020, the ACIP added a third quadrivalent meningococcal conjugate vaccine to its recommended list of vaccines that are FDA-approved for meningococcal disease (TABLE 49). The new vaccine fills a void left by the meningococcal polysaccharide vaccine (MPSV4), which is no longer marketed in the United States. MPSV4 was previously the only meningococcal vaccine approved for individuals 55 years and older.
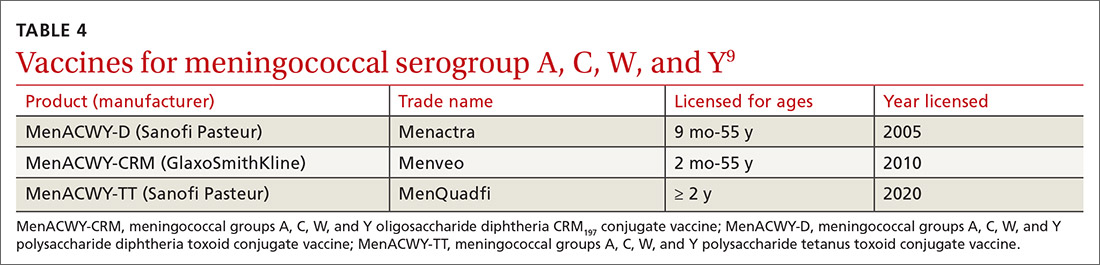
The new vaccine, MenACWY-TT (MenQuadfi), is approved for those ages 2 years and older, including those > 55 years. It is anticipated that MenQuadfi will, in the near future, be licensed and approved for individuals 6 months and older and will replace MenACWY-D (Menactra). (Both are manufactured by Sanofi Pasteur.)
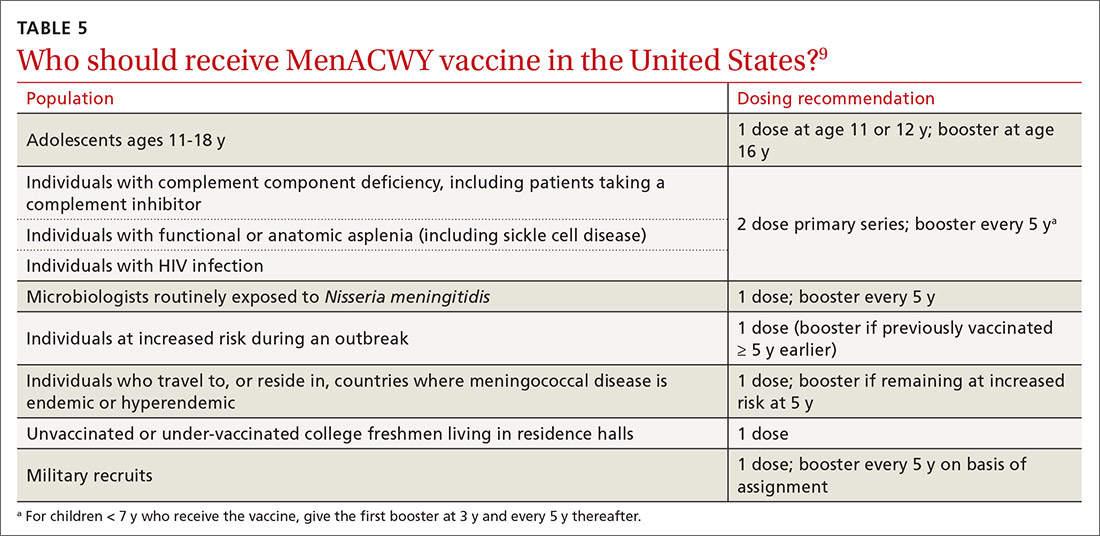
Groups for whom a MenACWY vaccine is recommended are listed in TABLE 5.9 A full description of current, updated recommendations for the prevention of meningococcal disease is also available.9
Continue to: Ebola virus (EBOV) vaccine
Ebola virus (EBOV) vaccine
A vaccine to prevent Ebola virus disease (EVD) is available by special request in the United States. Recombinant vesicular stomatitis virus-based Ebola virus vaccine, abbreviated as rVSVΔG-ZEBOV-GP (brand name, ERVBO) is manufactured by Merck and received approval by the FDA on December 19, 2019, for use in those ages 18 years and older. It is a live, attenuated vaccine.
The ACIP has recommended pre-exposure vaccination with rVSVΔG-ZEBOV-GP for adults 18 years or older who are at risk of exposure to EBOV while responding to an outbreak of EVD; while working as health care personnel at a federally designated Ebola Treatment Center; or while working at biosafety-level 4 facilities.10 The vaccine is protective against just 1 of 4 EBOV species, Zaire ebolavirus, which has been the cause of most reported EVD outbreaks, including the 2 largest EVD outbreaks in history that occurred in West Africa and the Republic of Congo.
It is estimated that EBOV outbreaks have infected more than 31,000 people and resulted in more than 12,000 deaths worldwide.11 Only 11 people infected with EBOV have been treated in the United States, all related to the 2014-2016 large outbreaks in West Africa. Nine of these cases were imported and only 1 resulted in transmission, to 2 people.10 The mammalian species that are suspected as intermediate hosts for EBOV are not present in the United States, which prevents EBOV from becoming endemic here.
The rVSVΔG-ZEBOV-GP vaccine was tested in a large trial in Africa during the 2014 outbreak. Its effectiveness was 100% (95% confidence interval, 63.5%-100%). The most common adverse effects were injection site pain, swelling, and redness. Mild-to-moderate systemic symptoms can occur within the first 2 days following vaccination, and include headache (37%), fever (34%), muscle pain (33%), fatigue (19%), joint pain (18%), nausea (8%), arthritis (5%), rash (4%), and
Since the vaccine contains a live virus that causes stomatitis in animals, it is possible that the virus could be transmitted to humans and other animals through close contact. Accordingly, the CDC has published some precautions including, but not limited to, not donating blood and, for 6 weeks after vaccination, avoiding contact with those who are immunosuppressed.10 The vaccine is not commercially available in the United States and must be obtained from the CDC. Information on requesting the vaccine is available at www.cdc.gov/vhf/ebola/clinicians/vaccine/.
1. Campos-Outcalt D. Prospects and challenges for the upcoming influenza season. J Fam Pract 2020;69:406-411.
2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922-1924.
3. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653-1656.
4. CDC. Pfizer-BioNTech COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
5. CDC. Moderna COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html#:~:text=How%20to%20Store%20the%20Moderna%20COVID%2D19%20Vaccine&text=Vaccine%20may%20be%20stored%20in,for%20this%20vaccine%20is%20tighter
6. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660.
7. FDA. Fact sheet for healthcare providers administering vaccine. [Pfizer–BioNTech]. Accessed February 17, 2021. www.fda.gov/media/144413/download
8. FDA. Fact sheet for healthcare providers administering vaccine. [Moderna]. Accessed February 17, 2021. www.fda.gov/media/144637/download
9. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1-41.
10. Choi MJ, Cossaboom CM, Whitesell AN, et al. Use of Ebola vaccine: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020. MMWR Recomm Rep. 2021;70:1-12.
11. CDC. Ebola background. Accessed February 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-02/Ebola-02-Choi-508.pdf
The year 2020 was challenging for public health agencies and especially for the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP). In a normal year, the ACIP meets in person 3 times for a total of 6 days of deliberations. In 2020, there were 10 meetings (all but 1 using Zoom) covering 14 days. Much of the time was dedicated to the COVID-19 pandemic, the vaccines being developed to prevent COVID-19, and the prioritization of those who should receive the vaccines first.
The ACIP also made recommendations for the use of influenza vaccines in the 2020-2021 season, approved the adult and pediatric immunization schedules for 2021, and approved the use of 2 new vaccines, one to protect against meningococcal meningitis and the other to prevent Ebola virus disease. The influenza recommendations were covered in the October 2020 Practice Alert,1 and the immunization schedules can be found on the CDC website at www.cdc.gov/vaccines/schedules/hcp/index.html.
COVID-19 vaccines
Two COVID-19 vaccines have been approved for use in the United States. The first was the Pfizer-BioNTech COVID-19 vaccine, approved by the Food and Drug Administration (FDA) on December 11 and recommended for use by the ACIP on December 12.2 The second vaccine, from Moderna, was approved by the FDA on December 18 and recommended by the ACIP on December 19.3 Both were approved by the FDA under an Emergency Use Authorization (EUA) and were approved by the ACIP for use while the EUA is in effect. Both vaccines must eventually undergo regular approval by the FDA and will be reconsidered by the ACIP regarding use in non–public health emergency conditions. A description of the EUA process and measures taken to assure efficacy and safety, before and after approval, were discussed in the September 2020 audiocast.
Both COVID-19 vaccines consist of nucleoside-modified mRNA encapsulated with lipid nanoparticles, which encode for a spike glycoprotein of SARS-CoV-2, the virus that causes COVID-19. Both vaccines require 2 doses (separated by 3 weeks for the Pfizer vaccine and 4 weeks for the Moderna vaccine) and are approved for use only in adults and older adolescents (ages ≥ 16 years for the Pfizer vaccine and ≥ 18 years for the Moderna vaccine) (TABLE 12-5).

In anticipation of vaccine shortages immediately after approval for use and a high demand for the vaccine, the ACIP developed a list of high-priority groups who should receive the vaccine in ranked order.6 States are encouraged, but not required, to follow this priority list (TABLE 26).

Caveats with usage. Both COVID-19 vaccines are very reactogenic, causing local and systemic adverse effects that patients should be warned about (TABLE 37,8). These reactions are usually mild to moderate and last 24 hours or less. Acetaminophen can alleviate these symptoms but should not be used to prevent them. In addition, both vaccines have stringent cold-storage requirements; once the vaccines are thawed, they must be used within a defined time-period.

Neither vaccine is listed as preferred. And they are not interchangeable; both recommended doses should be completed with the same vaccine. More details about the use of these vaccines were discussed in the January 2021 audiocast (www.mdedge.com/familymedicine/article/234239/coronavirus-updates/covid-19-vaccines-rollout-risks-and-reason-still) and can be located on the CDC website (www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html; www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html).
Continue to: Much remains unknown...
Much remains unknown regarding the use of these COVID-19 vaccines:
- What is their duration of protection, and will booster doses be needed?
- Will they protect against asymptomatic infection and carrier states, and thereby prevent transmission?
- Can they be co-administered with other vaccines?
- Will they be efficacious and safe to use during pregnancy and breastfeeding?
These issues will need to be addressed before they are recommended for non–public health emergency use.
Quadrivalent meningococcal conjugate vaccine (MenACWY)
In June 2020, the ACIP added a third quadrivalent meningococcal conjugate vaccine to its recommended list of vaccines that are FDA-approved for meningococcal disease (TABLE 49). The new vaccine fills a void left by the meningococcal polysaccharide vaccine (MPSV4), which is no longer marketed in the United States. MPSV4 was previously the only meningococcal vaccine approved for individuals 55 years and older.

The new vaccine, MenACWY-TT (MenQuadfi), is approved for those ages 2 years and older, including those > 55 years. It is anticipated that MenQuadfi will, in the near future, be licensed and approved for individuals 6 months and older and will replace MenACWY-D (Menactra). (Both are manufactured by Sanofi Pasteur.)
Groups for whom a MenACWY vaccine is recommended are listed in TABLE 5.9 A full description of current, updated recommendations for the prevention of meningococcal disease is also available.9

Continue to: Ebola virus (EBOV) vaccine
Ebola virus (EBOV) vaccine
A vaccine to prevent Ebola virus disease (EVD) is available by special request in the United States. Recombinant vesicular stomatitis virus-based Ebola virus vaccine, abbreviated as rVSVΔG-ZEBOV-GP (brand name, ERVBO) is manufactured by Merck and received approval by the FDA on December 19, 2019, for use in those ages 18 years and older. It is a live, attenuated vaccine.
The ACIP has recommended pre-exposure vaccination with rVSVΔG-ZEBOV-GP for adults 18 years or older who are at risk of exposure to EBOV while responding to an outbreak of EVD; while working as health care personnel at a federally designated Ebola Treatment Center; or while working at biosafety-level 4 facilities.10 The vaccine is protective against just 1 of 4 EBOV species, Zaire ebolavirus, which has been the cause of most reported EVD outbreaks, including the 2 largest EVD outbreaks in history that occurred in West Africa and the Republic of Congo.
It is estimated that EBOV outbreaks have infected more than 31,000 people and resulted in more than 12,000 deaths worldwide.11 Only 11 people infected with EBOV have been treated in the United States, all related to the 2014-2016 large outbreaks in West Africa. Nine of these cases were imported and only 1 resulted in transmission, to 2 people.10 The mammalian species that are suspected as intermediate hosts for EBOV are not present in the United States, which prevents EBOV from becoming endemic here.
The rVSVΔG-ZEBOV-GP vaccine was tested in a large trial in Africa during the 2014 outbreak. Its effectiveness was 100% (95% confidence interval, 63.5%-100%). The most common adverse effects were injection site pain, swelling, and redness. Mild-to-moderate systemic symptoms can occur within the first 2 days following vaccination, and include headache (37%), fever (34%), muscle pain (33%), fatigue (19%), joint pain (18%), nausea (8%), arthritis (5%), rash (4%), and
Since the vaccine contains a live virus that causes stomatitis in animals, it is possible that the virus could be transmitted to humans and other animals through close contact. Accordingly, the CDC has published some precautions including, but not limited to, not donating blood and, for 6 weeks after vaccination, avoiding contact with those who are immunosuppressed.10 The vaccine is not commercially available in the United States and must be obtained from the CDC. Information on requesting the vaccine is available at www.cdc.gov/vhf/ebola/clinicians/vaccine/.
The year 2020 was challenging for public health agencies and especially for the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP). In a normal year, the ACIP meets in person 3 times for a total of 6 days of deliberations. In 2020, there were 10 meetings (all but 1 using Zoom) covering 14 days. Much of the time was dedicated to the COVID-19 pandemic, the vaccines being developed to prevent COVID-19, and the prioritization of those who should receive the vaccines first.
The ACIP also made recommendations for the use of influenza vaccines in the 2020-2021 season, approved the adult and pediatric immunization schedules for 2021, and approved the use of 2 new vaccines, one to protect against meningococcal meningitis and the other to prevent Ebola virus disease. The influenza recommendations were covered in the October 2020 Practice Alert,1 and the immunization schedules can be found on the CDC website at www.cdc.gov/vaccines/schedules/hcp/index.html.
COVID-19 vaccines
Two COVID-19 vaccines have been approved for use in the United States. The first was the Pfizer-BioNTech COVID-19 vaccine, approved by the Food and Drug Administration (FDA) on December 11 and recommended for use by the ACIP on December 12.2 The second vaccine, from Moderna, was approved by the FDA on December 18 and recommended by the ACIP on December 19.3 Both were approved by the FDA under an Emergency Use Authorization (EUA) and were approved by the ACIP for use while the EUA is in effect. Both vaccines must eventually undergo regular approval by the FDA and will be reconsidered by the ACIP regarding use in non–public health emergency conditions. A description of the EUA process and measures taken to assure efficacy and safety, before and after approval, were discussed in the September 2020 audiocast.
Both COVID-19 vaccines consist of nucleoside-modified mRNA encapsulated with lipid nanoparticles, which encode for a spike glycoprotein of SARS-CoV-2, the virus that causes COVID-19. Both vaccines require 2 doses (separated by 3 weeks for the Pfizer vaccine and 4 weeks for the Moderna vaccine) and are approved for use only in adults and older adolescents (ages ≥ 16 years for the Pfizer vaccine and ≥ 18 years for the Moderna vaccine) (TABLE 12-5).

In anticipation of vaccine shortages immediately after approval for use and a high demand for the vaccine, the ACIP developed a list of high-priority groups who should receive the vaccine in ranked order.6 States are encouraged, but not required, to follow this priority list (TABLE 26).

Caveats with usage. Both COVID-19 vaccines are very reactogenic, causing local and systemic adverse effects that patients should be warned about (TABLE 37,8). These reactions are usually mild to moderate and last 24 hours or less. Acetaminophen can alleviate these symptoms but should not be used to prevent them. In addition, both vaccines have stringent cold-storage requirements; once the vaccines are thawed, they must be used within a defined time-period.

Neither vaccine is listed as preferred. And they are not interchangeable; both recommended doses should be completed with the same vaccine. More details about the use of these vaccines were discussed in the January 2021 audiocast (www.mdedge.com/familymedicine/article/234239/coronavirus-updates/covid-19-vaccines-rollout-risks-and-reason-still) and can be located on the CDC website (www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html; www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html).
Continue to: Much remains unknown...
Much remains unknown regarding the use of these COVID-19 vaccines:
- What is their duration of protection, and will booster doses be needed?
- Will they protect against asymptomatic infection and carrier states, and thereby prevent transmission?
- Can they be co-administered with other vaccines?
- Will they be efficacious and safe to use during pregnancy and breastfeeding?
These issues will need to be addressed before they are recommended for non–public health emergency use.
Quadrivalent meningococcal conjugate vaccine (MenACWY)
In June 2020, the ACIP added a third quadrivalent meningococcal conjugate vaccine to its recommended list of vaccines that are FDA-approved for meningococcal disease (TABLE 49). The new vaccine fills a void left by the meningococcal polysaccharide vaccine (MPSV4), which is no longer marketed in the United States. MPSV4 was previously the only meningococcal vaccine approved for individuals 55 years and older.

The new vaccine, MenACWY-TT (MenQuadfi), is approved for those ages 2 years and older, including those > 55 years. It is anticipated that MenQuadfi will, in the near future, be licensed and approved for individuals 6 months and older and will replace MenACWY-D (Menactra). (Both are manufactured by Sanofi Pasteur.)
Groups for whom a MenACWY vaccine is recommended are listed in TABLE 5.9 A full description of current, updated recommendations for the prevention of meningococcal disease is also available.9

Continue to: Ebola virus (EBOV) vaccine
Ebola virus (EBOV) vaccine
A vaccine to prevent Ebola virus disease (EVD) is available by special request in the United States. Recombinant vesicular stomatitis virus-based Ebola virus vaccine, abbreviated as rVSVΔG-ZEBOV-GP (brand name, ERVBO) is manufactured by Merck and received approval by the FDA on December 19, 2019, for use in those ages 18 years and older. It is a live, attenuated vaccine.
The ACIP has recommended pre-exposure vaccination with rVSVΔG-ZEBOV-GP for adults 18 years or older who are at risk of exposure to EBOV while responding to an outbreak of EVD; while working as health care personnel at a federally designated Ebola Treatment Center; or while working at biosafety-level 4 facilities.10 The vaccine is protective against just 1 of 4 EBOV species, Zaire ebolavirus, which has been the cause of most reported EVD outbreaks, including the 2 largest EVD outbreaks in history that occurred in West Africa and the Republic of Congo.
It is estimated that EBOV outbreaks have infected more than 31,000 people and resulted in more than 12,000 deaths worldwide.11 Only 11 people infected with EBOV have been treated in the United States, all related to the 2014-2016 large outbreaks in West Africa. Nine of these cases were imported and only 1 resulted in transmission, to 2 people.10 The mammalian species that are suspected as intermediate hosts for EBOV are not present in the United States, which prevents EBOV from becoming endemic here.
The rVSVΔG-ZEBOV-GP vaccine was tested in a large trial in Africa during the 2014 outbreak. Its effectiveness was 100% (95% confidence interval, 63.5%-100%). The most common adverse effects were injection site pain, swelling, and redness. Mild-to-moderate systemic symptoms can occur within the first 2 days following vaccination, and include headache (37%), fever (34%), muscle pain (33%), fatigue (19%), joint pain (18%), nausea (8%), arthritis (5%), rash (4%), and
Since the vaccine contains a live virus that causes stomatitis in animals, it is possible that the virus could be transmitted to humans and other animals through close contact. Accordingly, the CDC has published some precautions including, but not limited to, not donating blood and, for 6 weeks after vaccination, avoiding contact with those who are immunosuppressed.10 The vaccine is not commercially available in the United States and must be obtained from the CDC. Information on requesting the vaccine is available at www.cdc.gov/vhf/ebola/clinicians/vaccine/.
1. Campos-Outcalt D. Prospects and challenges for the upcoming influenza season. J Fam Pract 2020;69:406-411.
2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922-1924.
3. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653-1656.
4. CDC. Pfizer-BioNTech COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
5. CDC. Moderna COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html#:~:text=How%20to%20Store%20the%20Moderna%20COVID%2D19%20Vaccine&text=Vaccine%20may%20be%20stored%20in,for%20this%20vaccine%20is%20tighter
6. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660.
7. FDA. Fact sheet for healthcare providers administering vaccine. [Pfizer–BioNTech]. Accessed February 17, 2021. www.fda.gov/media/144413/download
8. FDA. Fact sheet for healthcare providers administering vaccine. [Moderna]. Accessed February 17, 2021. www.fda.gov/media/144637/download
9. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1-41.
10. Choi MJ, Cossaboom CM, Whitesell AN, et al. Use of Ebola vaccine: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020. MMWR Recomm Rep. 2021;70:1-12.
11. CDC. Ebola background. Accessed February 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-02/Ebola-02-Choi-508.pdf
1. Campos-Outcalt D. Prospects and challenges for the upcoming influenza season. J Fam Pract 2020;69:406-411.
2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922-1924.
3. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653-1656.
4. CDC. Pfizer-BioNTech COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
5. CDC. Moderna COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html#:~:text=How%20to%20Store%20the%20Moderna%20COVID%2D19%20Vaccine&text=Vaccine%20may%20be%20stored%20in,for%20this%20vaccine%20is%20tighter
6. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660.
7. FDA. Fact sheet for healthcare providers administering vaccine. [Pfizer–BioNTech]. Accessed February 17, 2021. www.fda.gov/media/144413/download
8. FDA. Fact sheet for healthcare providers administering vaccine. [Moderna]. Accessed February 17, 2021. www.fda.gov/media/144637/download
9. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1-41.
10. Choi MJ, Cossaboom CM, Whitesell AN, et al. Use of Ebola vaccine: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020. MMWR Recomm Rep. 2021;70:1-12.
11. CDC. Ebola background. Accessed February 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-02/Ebola-02-Choi-508.pdf
AT PRESS TIME
The US Food and Drug Administration issued an Emergency Use Authorization for a third COVID-19 vaccine. The single-dose vaccine was developed by the Janssen Pharmaceutical Companies of Johnson & Johnson. For more information, go to www.mdedge.com/familymedicine
Conservative or surgical management for that shoulder dislocation?
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected]
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected]
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected]
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
PRACTICE RECOMMENDATIONS
› Start with conservative management of shoulder dislocation in patients older than 30 years and those with uncomplicated injuries. B
› Discourage strict immobilization; its utility is debated and it may not change outcomes. B
› Recommend a progressive rehabilitative program after the initial acute shoulder injury. B
› Consider surgical management for patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability or for the highly physically active individual. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Functional neurological disorder: A practical guide to an elusive Dx
CASE
John D,* a 25-year-old patient with an otherwise unremarkable medical history, describes 2 months of daily headache, lower-extremity weakness, and unsteady gait that began fairly suddenly during his first deployment in the US Army. He explains that these symptoms affected his ability to perform his duties and necessitated an early return stateside for evaluation and treatment.
Mr. D denies precipitating trauma or unusual environmental exposures. He reports that, stateside now, symptoms continue to affect his ability to work and attend to personal and family responsibilities.
Asked about stressors, Mr. D notes the birth of his first child approximately 3 months ago, while he was deployed, and marital stressors. He denies suicidal or homicidal ideation.
* The patient’s name has been changed to protect his identity.
The challenge of identifying and managing FND
A functional neurological disorder (FND) is a constellation of psychological, physiological, and neurological symptoms, without an identifiable organic etiology, a conscious decision, or secondary gain for the patient,1 that adversely impacts functioning in 1 or more significant life domains.
Given the high throughput of patients in primary care practices, family physicians can expect to encounter suspected cases of FND in their practices. Regrettably, however, a lack of familiarity with the disorder and its related problems (eg, nonorganic paralysis, sensory loss, nonepileptic seizures, and abnormal movements) can add as much as $20,000 in excess direct and indirect costs of care for every such patient.1 In this article, we synthesize the recent literature on FND so that family physicians can expand their acumen in understanding, identifying, and evaluating patients whose presentation suggests FND.
An underrecognized entity
A precise estimate of the prevalence of FND is difficult to determine because the disorder is underrecognized and misdiagnosed and because it is often accompanied by the confounding of psychological and physiological comorbidities. A 2012 study estimated the annual incidence of FND to be 4 to 12 cases for every 100,000 people2; in primary care and outpatient neurology settings, prevalence is 6% to 22% of all patients.3,4 Stone and colleagues identified functional neurological symptoms as the second most common reason for outpatient neurology consultation,5 with 1 nonepileptic seizure patient seen for every 6 epileptic patients, and functional weakness presenting at the same rate as multiple sclerosis.6
Continue to: Demographics of patients with FND...
Demographics of patients with FND vary, depending on presenting neurological symptoms and disorder subtype. Existing data indicate a correlation between FND and younger age, female sex, physical disability,7 and a history of abuse or trauma.3,8 A challenge in concretely ascertaining the prevalence of FND is that conditions such as fibromyalgia, chronic pelvic pain, globus hystericus, and nonepileptic seizures can also be characterized as medically unexplained functional disorders, even within the network of neurology care.4
Misdiagnosis and bias are not uncommon
Ambiguity in classifying and evaluating FND can affect physicians’ perceptions, assessment, and care of patients with suggestive presenting symptoms. A major early challenge in diagnosing FND is the inconsistency of characterizing terminology (pseudoneurological, somatic, dissociative, conversion, psychogenic, hysterical, factitious, functional, medically unexplained9,10) and definitions in the literature. Neurological symptoms of unidentifiable organic cause can greatly diminish quality of life4; FND is a scientifically and clinically useful diagnosis for many combinations of nonrandomly co-occurring symptoms and clinical signs.
The pitfall of misdiagnosis. Remain cautious about making a diagnosis of FND by exclusion, which might yield an incorrect or false-negative finding because of an atypical presentation. It is important to avoid misdiagnosis by prematurely closing the differential diagnosis; instead, keep in mind that a medically unexplained diagnosis might be better explained by conducting a robust social and medical history and obtaining additional or collateral data, or both, along with appropriate consultation.4,9
Misdiagnosis can lead to a circuitous and costly work-up, with the potential to increase the patient’s distress. You can reduce this burden with early recognition of FND and centralized management of multidisciplinary care, which are more likely to lead to an accurate and timely diagnosis—paramount to empowering patients with access to the correct information and meaningful support needed to enhance treatment and self-care.9
Bias, haste, and dismissal are unproductive. Even with a clear definition of FND, it is not uncommon for a physician to rapidly assess a patient’s clinical signs, make a diagnosis of “unknown etiology,” or openly question the veracity of complaints. Furthermore, be aware of inadvertently characterizing FND using the prefix “pseudo” or the term “hysterical,” which can be psychologically discomforting for many patients, who legitimately experience inexplicable symptoms. Such pejoratives can lead to stigmatizing and misleading assessments and treatment paths4—courses of action that can cause early and, possibly, irreparable harm to the patient–physician relationship and increase the patient’s inclination to go “doctor-shopping,” with associated loss of continuity of care.
Why is it difficult to diagnose FND?
The latest (5th) edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) describes conversion, somatoform disorder, and FND synonymously.DSM-5 diagnostic criteria for conversion disorderare11:
- a specified type of symptom or deficit of altered voluntary motor or sensory function (eg, weakness, difficulty swallowing, slurred speech, seizures)
- clinical evidence of the incompatibility of the symptom or deficit and any recognized neurological or medical disorder
- incapability of better explaining the symptom or deficit as another medical or mental disorder.
- The symptom or deficit causes distress or impairment that (1) is clinically significant in occupational, social, or other important areas of function or (2) warrants medical evaluation.
The overarching feature of these criteria is the inconsistency of symptoms with recognized neurological, physiological, or psychiatric conditions. Although identification of psychological factors can help clarify and provide a treatment direction, such identification is not essential for making a diagnosis of FND. Malingering does not need to be refuted as part of establishing the diagnosis.12
Continue to: In contrast...
In contrast, the World Health Organization’s ICD-10 Classification of Mental and Behavioural Disorders groups diagnostic criteria for FND among the dissociative disorders13:
- Clinical features are specified for the individual dissociative disorder (motor, sensory, convulsions, mixed).
- Evidence is absent of a physical disorder that might explain symptoms.
- Evidence of psychological causation is present in clear temporal association with stressful events and problems or disturbed relationships, even if the patient denies such association.
Note the emphasis on psychological causation and exclusion of purposeful simulation of symptoms, as opposed to a primarily unconscious disconnection from the patient’s body or environment.
ICD-10 guidelines acknowledge the difficulty of finding definitive evidence of a psychological cause and recommend provisional diagnosis of FND if psychological factors are not readily apparent.14 Of note, many patients with FND are affected psychologically by their condition, with an impact on mood, behaviors, and interpersonal interactions, although not necessarily to a clinically diagnostic degree. Therefore, a psychiatric diagnosis alone is not a necessary precursor for the diagnosis of an FND.
CASE
History. Mr. D’s history is positive for light alcohol consumption (“2 or 3 cans of beer on weekends”) and chewing tobacco (he reports stopping 6 months earlier) and negative for substance abuse. The family history is positive for maternal hypertension and paternal suicide when the patient was 10 years old (no other known paternal history).
Physical findings. The review of systems is positive for intermittent palpitations, lower-extremity weakness causing unsteady gait, and generalized headache.
Vital signs are within normal limits, including blood pressure (120/82 mm Hg) and heart rate (110 beats/min). The patient is not in acute distress; he is awake, alert, and oriented × 3. No murmurs are heard; lungs are clear bilaterally to auscultation. There is no tenderness on abdominal palpation, and no hepatomegaly or splenomegaly; bowel sounds are normal. No significant bruising or lacerations are noted.
Neurology exam. Cranial nerves II-XII are intact. Pupils are equal and reactive to light. Reflexes are 2+ bilaterally. Muscle strength and tone are normal; no tremors are noted. Babinski signs are normal. A Romberg test is positive (swaying).
Continue to: Mr. D has an antalgic gait...
Mr. D has an antalgic gait with significant swaying (without falling); bent posture; and unsteadiness that requires a cane. However, he is able to get up and off the exam table without assistance, and to propel himself, by rolling a chair forward and backward, without difficulty.
Conducting a diagnostic examination
Taking the history. Certain clues can aid in the diagnosis of FND (TABLE 1).15 For example, the patient might have been seen in multiple specialty practices for a multitude of vague symptoms indicative of potentially related conditions (eg, chronic fatigue, allergies and sensitivities, fibromyalgia, and other chronic pain). The history might include repeated surgeries to investigate those symptoms (eg, laparoscopy, or hysterectomy at an early age). Taking time and care to explore all clinical clues, patient reports, and collateral data are therefore key to making an accurate diagnosis.
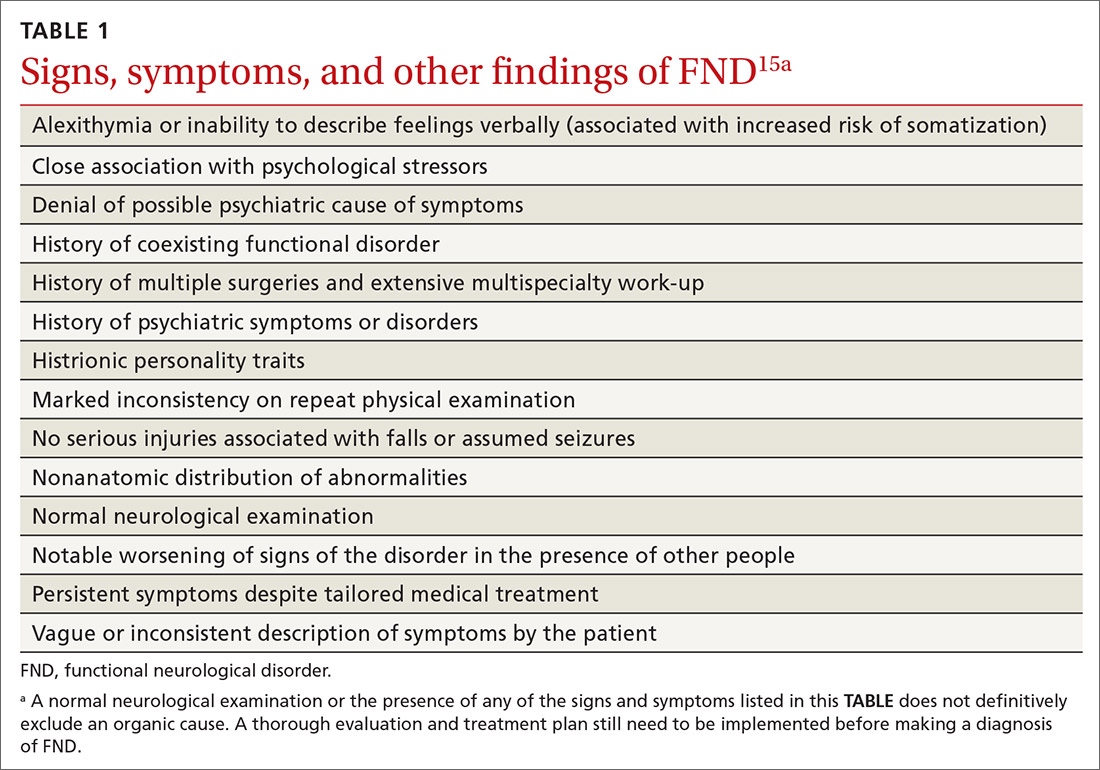
Note any discrepancies between the severity of reported symptoms and functional ability. A technique that can help elucidate a complex or ambiguous medical presentation is to ask the patient to list all their symptoms at the beginning of the interview. This has threefold benefit: You get a broad picture of the problem; the patient is unburdened of their concerns and experiences your validation; and a long list of symptoms can be an early clue to a diagnosis of FND.
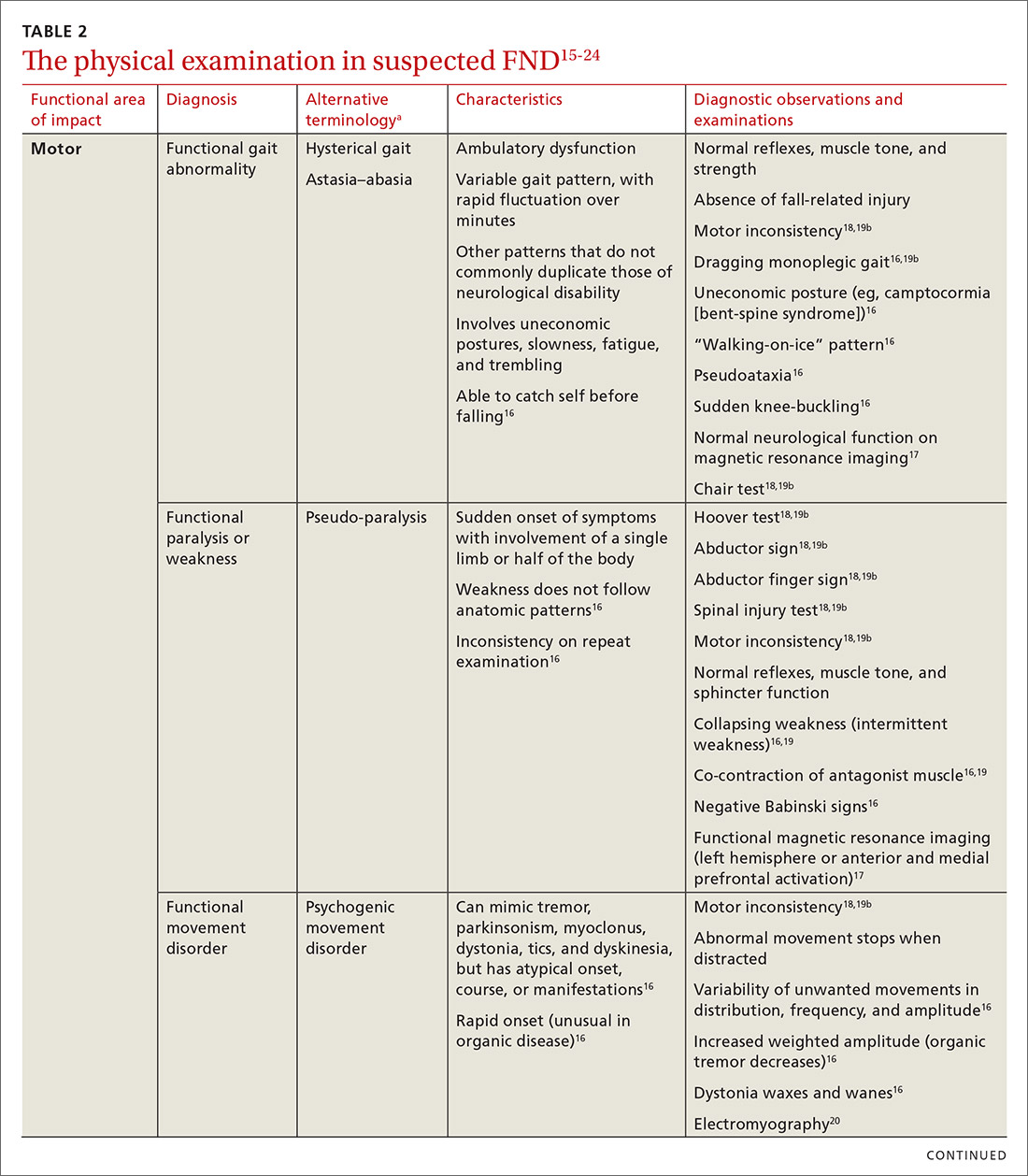
Other helpful questions to determine the impact of symptoms on the patient’s well-being include inquiries about16:
- functional impairment
- onset and course of symptoms
- potential causal or correlating events
- dissociative episodes
- previous diagnoses and treatments
- the patient’s perceptions of, and emotional response to, their illness
- a history of abuse.
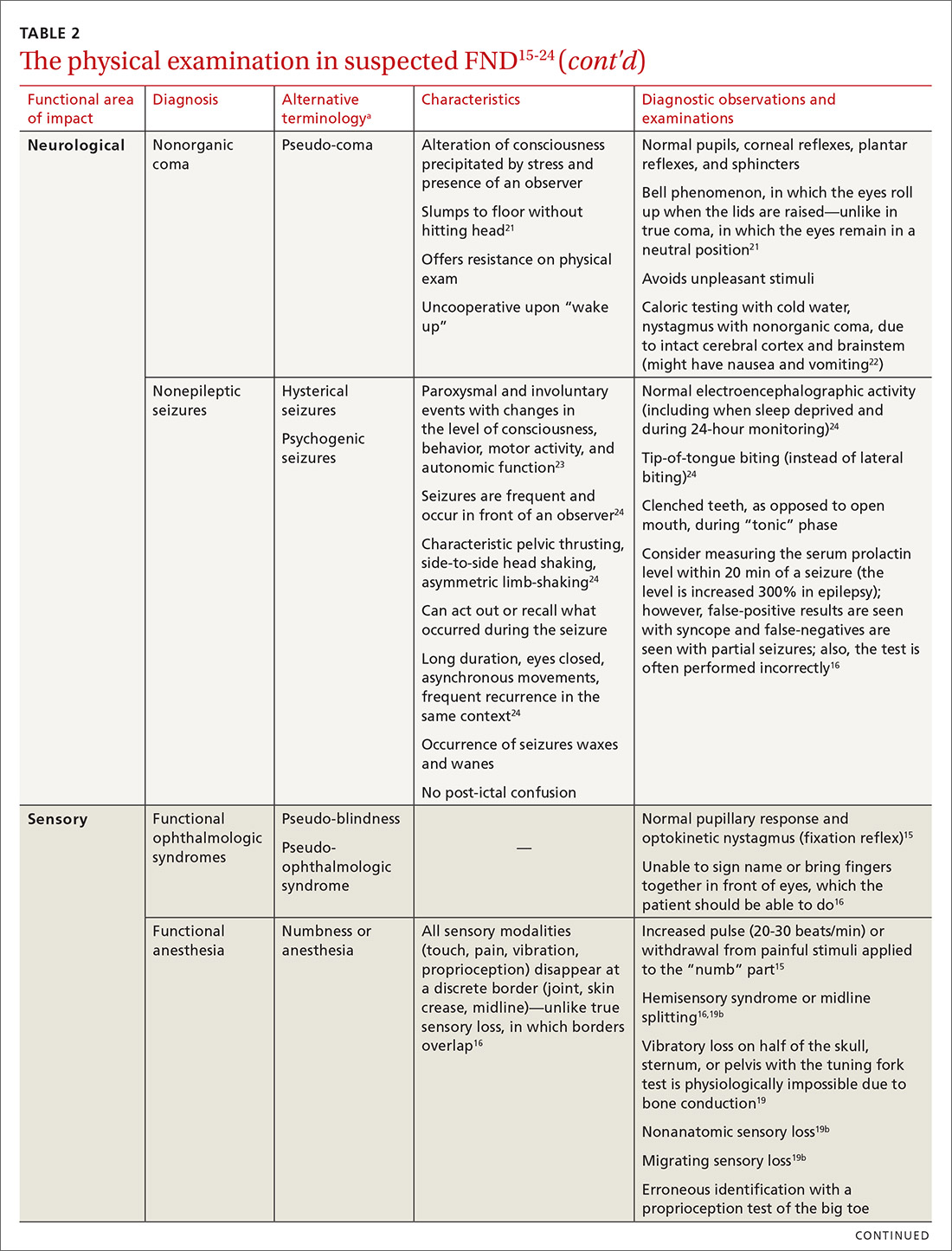
The physical examination to determine the presence of FND varies, depending on the functional area of impact (eg, motor, neurological, sensory, speech and swallowing). Pay particular attention to presenting signs and clues, and balance them with the patient’s report (or lack of report). Endeavor to demonstrate positive functional signs, such as a positive Hoover test, which relies on the principle of synergistic muscle contraction. You might see evidence of inconsistency, such as weakness or a change in gait, under observation, that seemingly resolves when the patient is getting on and off the exam table.16Table 215-24 describes areas affected by FND, characteristics of the disorder, and related diagnostic examinations.
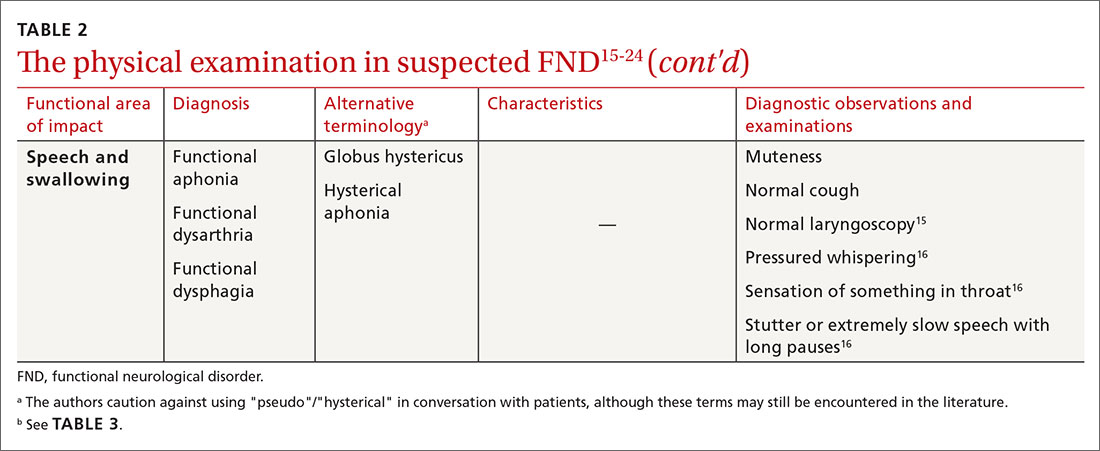
Table 315,18,19 reviews validated special exams that can aid in making the diagnosis. Additional special tests are discussed in the literature.15-24 These tests can be helpful in narrowing the differential diagnosis but have not been validated and should be used with caution.

Some clinical signs associated with FND might be affected by other factors, including socioeconomic status, limited access to health care, low health literacy, poor communication skills, and physician bias. Keep these factors in mind during the visit, to avoid contributing further to health disparities among groups of patients affected by these problems.
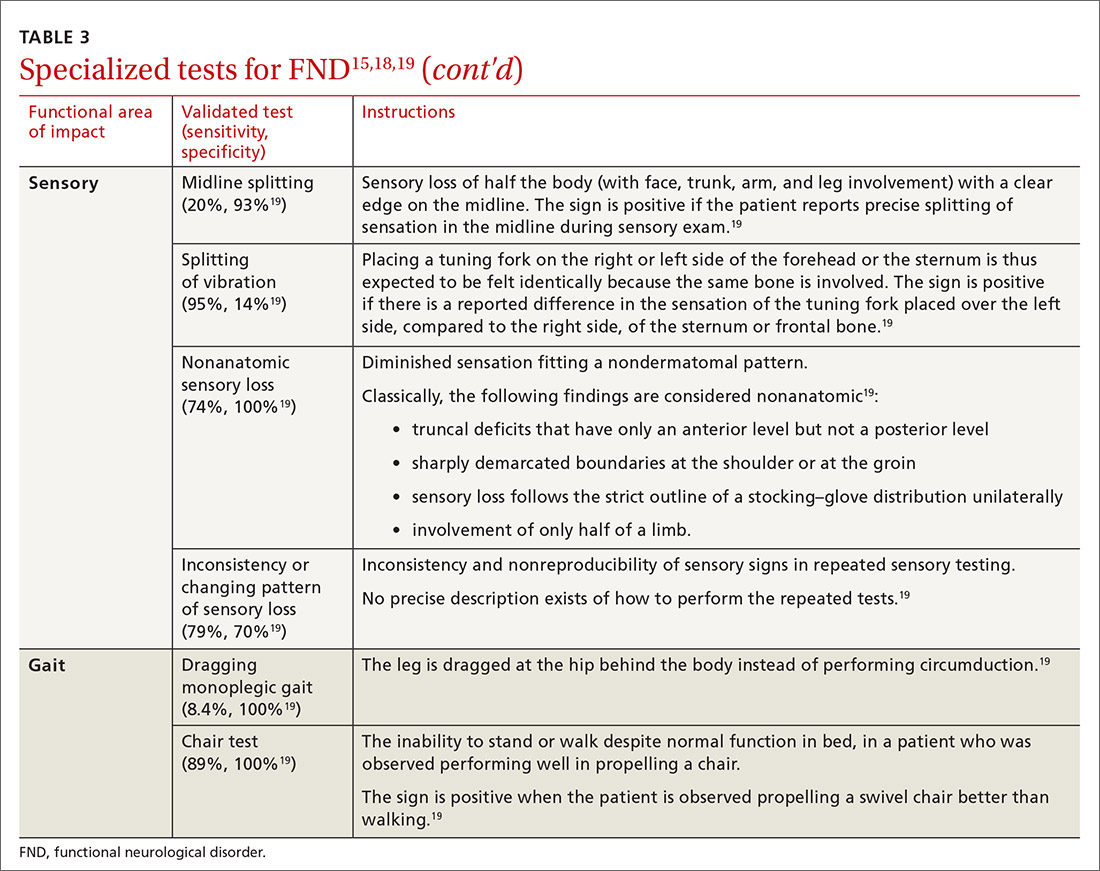
Continue to: CASE
CASE
The work-up over the next month for Mr. D includes numerous studies, all yielding results that are negative or within normal limits: visual acuity; electrocardiography and an event monitor; laboratory testing (including a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, folate, and vitamin D); magnetic resonance imaging of the brain and lumbar spine; lumbar puncture; and electromyography.
The score on the 9-item Patient Health Questionnaire for depression is 4 (severity: “none or minimal”); on the 7-item Generalized Anxiety Disorder scale, 0 (“no anxiety disorder”).
Referral. A neurology work-up of headache, lower extremity weakness, and unsteady gait to address several diagnostic possibilities, including migraine and multiple sclerosis, is within normal limits. A cardiology work-up of palpitations is negative for arrhythmias and other concerning findings.
Mr. D declines psychiatric and psychological evaluations.
Building a differential diagnosisis a formidable task
The differential diagnosis of FND is vast. It includes neurological, physiological, and psychiatric symptoms and disorders; somatization; and malingering (Table 4).6 Any disorder or condition in these areas that is in the differential diagnosis can be precipitated or exacerbated by stress; most, however, do not involve loss of physical function.12 In addition, the diagnosis of an FND does not necessarily exclude an organic disorder.
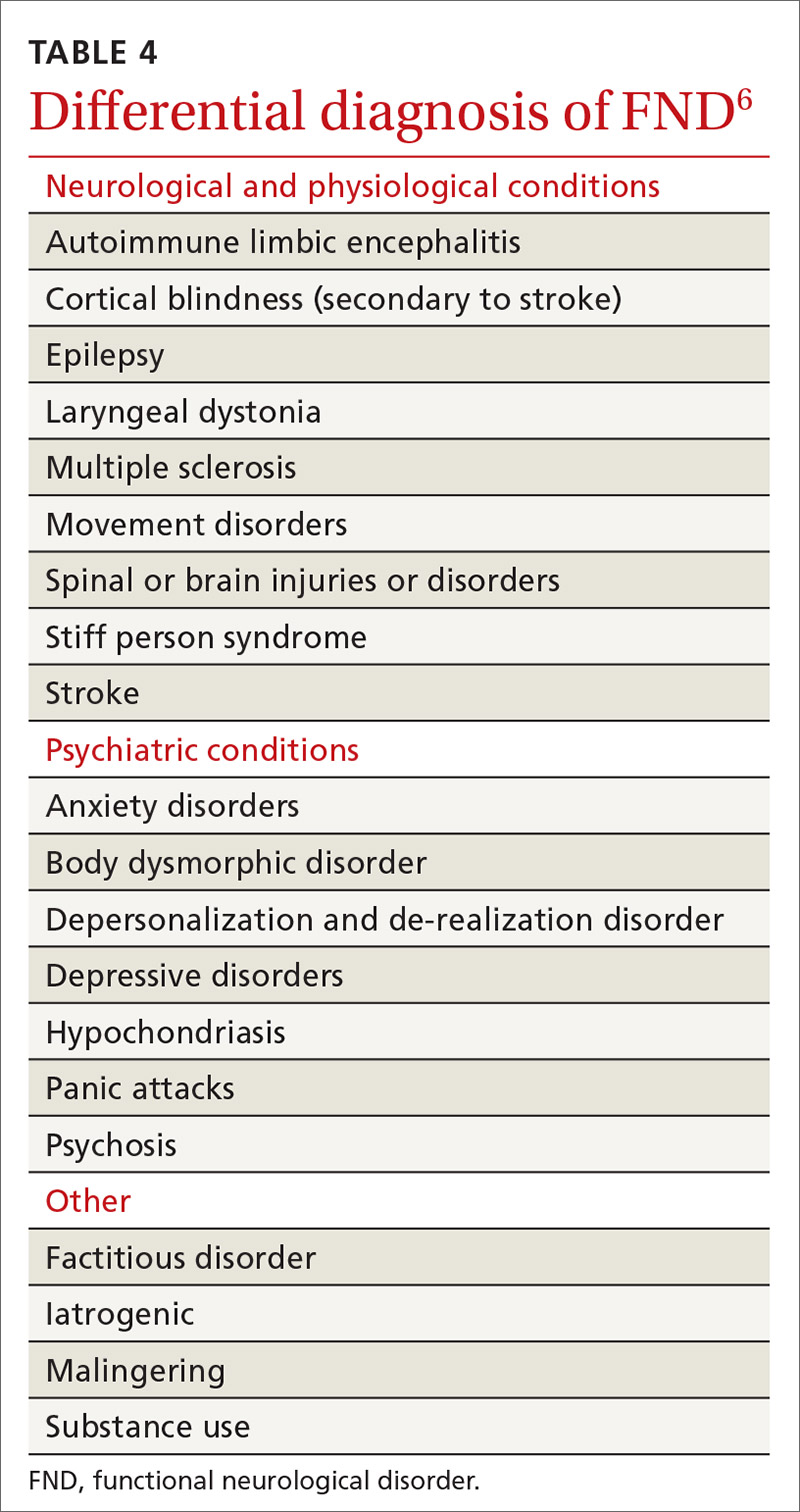
A patient’s presentation becomes complicated—and more difficult to treat—when functional symptoms and an unrelated underlying or early-stage neurological condition coexist. For example, a patient with epilepsy might also have dissociative seizures atop their organic disorder. Neurological disease is considered a risk factor for an overlying FND—just as the risk of depression or anxiety runs concurrently with other chronic diseases.14
Focus on clinical signs to narrow the differential. A thorough social and medical history and physical examination, as discussed earlier, help narrow the differential diagnosis of organic and medically unexplained disorders. Well-defined imaging or laboratory protocols do not exist to guide physicians to a definitive diagnosis, however.
Continue to: Psychiatric conditions
Psychiatric conditions can coexist with the diagnosis of FND, but might be unrelated. A systematic review of the literature showed that 17% to 42% of patients with FND had a concurrent anxiety disorder. Depression disorders were co-diagnosed in 19% to 71% of patients with FND; dissociative and personality disorders were noted, as well.25 However, coexisting psychiatric diagnosis might more likely be associated with distress from the presenting functional neurological symptoms, not linked to the FND diagnosis itself.12 This shift in understanding is reflected in the description of FND in the DSM-5.11
CASE
Mr. D reports debilitating headaches at return office visits. Trials of abortive triptans provide no relief; neither do control medications (beta-blockers, coenzyme Q10, magnesium, onabotulinumtoxinA [Botox], topiramate, and valproate). Lower-extremity weakness and unsteadiness are managed with supportive devices, including a cane, and physical therapy.
Importance of establishing a multidisciplinary approach
The complexity of FND lends itself to a multidisciplinary approach during evaluation and, eventually, for treatment. The assessment and diagnostic intervention that you provide, along with the contributions of consulted specialists (including neurology, physical and occupational therapy, psychiatry, psychology, and other mental health professionals) establishes a team-based approach that can increase the patient’s sense of support and reduce excessive testing and unnecessary medications, surgeries, and other treatments.26
Family physicians are in the ideal position to recognize the patient’s functional capacity and the quality of symptoms and to provide timely referral (eg, to Neurology and Psychiatry) for confirmation of the diagnosis and then treatment.
Evidence-based treatment options include:
- psychotherapy, with an emphasis on cognitive behavioral therapy
- physical therapy
- psychopharmacology
- promising combinations of physical and psychological treatment to improve long-term functionality.27
A promising diagnostic tool
The most significant update in the FND literature is on functional neuroimaging for assessing the disorder. Early findings suggest an intricate relationship between mind and body regarding the pathological distortion in FND. And, there is clear evidence that neuroimaging—specifically, functional magnetic resonance imaging—shows changes in brain activity that correspond to the patient’s symptom report. That said, imaging is not the recommended standard of care in the initial work-up of FND because of its cost and the fact that the diagnosis is principally a clinical undertaking.17,28
Call to action
Offer a generous ear. Begin the diagnostic pursuit by listening carefully and fully to the patient’s complaints, without arriving at a diagnosis with unwarranted bias or haste. This endeavor might require support from other clinical staff (eg, nurses, social workers, case managers) because the diagnostic process can be arduous and lengthy.
Continue to: Convey the diagnosis with sensitivity
Convey the diagnosis with sensitivity. Inquire about the patient’s perceptions and impairments to best personalize your diagnostic explanations. Delivery of the diagnosis might affect the patient’s acceptance and compliance with further testing and treatment of what is generally a persistent and treatment-resistant disorder; poor delivery of diagnostic information can impair the patient–physician relationship and increase the risk of disjointed care. Many patients find that improved patient–physician communication is therapeutic.29
Let the patient know that you’re taking her seriously. Validate patient concerns with a nonstigmatizing diagnostic label; discuss the diagnostic parameters and cause of symptoms in layman’s terms; and emphasize the potential for reversibility.30 Some patients are not satisfied with having a diagnosis of FND until they are reassured with normal results of testing and provided with referral; even then, some seek further reassurance.
Key tenets of managing care for patients who have been given a diagnosis of FND include:
- nonjudgmental, positive regard
- meaningful expression of empathy
- multidisciplinary coordination
- avoidance of unnecessary testing and harmful treatments
- descriptive and contextual explanations of the diagnosis.
Last, keep in mind that the course of treatment for FND is potentially prolonged and multilayered.
CASE
After many visits with his family physician and the neurology and cardiology specialists, as well as an extensive work-up, the physician approaches Mr. D with the possibility of a diagnosis of FND and proposes a multidisciplinary plan that includes:
- a course of physical and occupational therapy
- development of individualized cognitive behavioral tools
- weekly personal and marital counseling
- initiation of a selective serotonin reuptake inhibitor for anxiety
- monthly visits with his family physician.
Months after his return from deployment for evaluation and treatment, Mr. D is able to return to military duty. He reports that his quality of life has improved.
CORRESPONDENCE
Roselyn W. Clemente Fuentes, MD, FAAFP, Eglin Family Medicine Residency, 307 Boatner Road, Eglin AFB, FL 32547; [email protected].
1. Konnopka A, Schaefert R, Heinrich S, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265-275.
2. Carson AJ, Brown R, David AS, et al; on behalf of UK-FNS. Functional (conversion) neurological symptoms: research since the millennium. J Neurol Neurosurg Psychiatry. 2012;83:842-850.
3. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
4. Evens A, Vendetta L, Krebs K, et al. Medically unexplained neurologic symptoms: a primer for physicians who make the initial encounter. Am J Med. 2015;128:1059-1064.
5. Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol. 2013;13:104-113.
6. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537-1551.
7. Carson A, Stone J, Hibberd C, et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J Neurol Neurosurg Psychiatry. 2011;82:810-813.
8. Fink P, Hansen MS, Oxhøj M-L. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res. 2004;56:413-418.
9. Thomas LE. Are your patient’s medically unexplained symptoms really “all in her head”? Med Hypotheses. 2012;78:542-547.
10. Ding JM, Kanaan RAA. What should we say to patients with unexplained neurological symptoms? How explanation affects offence. J Psychosom Res. 2016;91:55-60.
11. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association; 2013.
12. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM-5: Conversion disorder. Am J Psychiatry. 2010;167:626-627.
13. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1994. Accessed January 21, 2021. www.who.int/classifications/icd/en/bluebook.pdf
14. Stone J, Carson A, Duncan R, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease.” J Neurol. 2012;259:33-38.
15. Shaibani A, Sabbagh M. Pseudoneurologic syndromes: recognition and diagnosis. Am Fam Physician. 1998;57:2485-2494.
16. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
17. Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323-337.
18. McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics. 2018;59:358-368.
19. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190.
20. Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001;16:595-599.
21. Ludwig L, McWhirter L, Williams S, et al. Functional coma. In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology: Volume 139: Functional Neurologic Disorders. 1st ed. Academic Press; 2016:313.
22. Miller NR, Subramanian PS, Patel VR. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 3rd ed. Wolters Kluwer; 2016:512-513.
23. Takazaki K, Stransky AD, Miller G. Psychogenic nonepileptic seizures: diagnosis, management, and bioethics. Pediatr Neurol. 2016;62:3-8.
24. Sahaya K, Dholakia SA, Sahota PK. Psychogenic non-epileptic seizures: a challenging entity. J Clin Neurosci. 2011;18:1602-1607.
25. Gelauff J, Stone J, Edwards M, et al. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220-226.
26. Kranick SM, Gorrindo T, Hallett M. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics. 2011;52:109-116.
27. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250-260.
28. Burgmer M, Kugel H, Pfleiderer B, et al. The mirror neuron system under hypnosis—brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex. 2013;49:437-445.
29. van Bokhoven MA, Koch H, van der Weijden T, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints, Ann Fam Med. 2009;7:112-120.
30. Stone J, Carson A, Hallet M. Explanation as treatment for functional neurologic disorders. Handb Clin Neurol. 2016;139:543-553.
CASE
John D,* a 25-year-old patient with an otherwise unremarkable medical history, describes 2 months of daily headache, lower-extremity weakness, and unsteady gait that began fairly suddenly during his first deployment in the US Army. He explains that these symptoms affected his ability to perform his duties and necessitated an early return stateside for evaluation and treatment.
Mr. D denies precipitating trauma or unusual environmental exposures. He reports that, stateside now, symptoms continue to affect his ability to work and attend to personal and family responsibilities.
Asked about stressors, Mr. D notes the birth of his first child approximately 3 months ago, while he was deployed, and marital stressors. He denies suicidal or homicidal ideation.
* The patient’s name has been changed to protect his identity.
The challenge of identifying and managing FND
A functional neurological disorder (FND) is a constellation of psychological, physiological, and neurological symptoms, without an identifiable organic etiology, a conscious decision, or secondary gain for the patient,1 that adversely impacts functioning in 1 or more significant life domains.
Given the high throughput of patients in primary care practices, family physicians can expect to encounter suspected cases of FND in their practices. Regrettably, however, a lack of familiarity with the disorder and its related problems (eg, nonorganic paralysis, sensory loss, nonepileptic seizures, and abnormal movements) can add as much as $20,000 in excess direct and indirect costs of care for every such patient.1 In this article, we synthesize the recent literature on FND so that family physicians can expand their acumen in understanding, identifying, and evaluating patients whose presentation suggests FND.
An underrecognized entity
A precise estimate of the prevalence of FND is difficult to determine because the disorder is underrecognized and misdiagnosed and because it is often accompanied by the confounding of psychological and physiological comorbidities. A 2012 study estimated the annual incidence of FND to be 4 to 12 cases for every 100,000 people2; in primary care and outpatient neurology settings, prevalence is 6% to 22% of all patients.3,4 Stone and colleagues identified functional neurological symptoms as the second most common reason for outpatient neurology consultation,5 with 1 nonepileptic seizure patient seen for every 6 epileptic patients, and functional weakness presenting at the same rate as multiple sclerosis.6
Continue to: Demographics of patients with FND...
Demographics of patients with FND vary, depending on presenting neurological symptoms and disorder subtype. Existing data indicate a correlation between FND and younger age, female sex, physical disability,7 and a history of abuse or trauma.3,8 A challenge in concretely ascertaining the prevalence of FND is that conditions such as fibromyalgia, chronic pelvic pain, globus hystericus, and nonepileptic seizures can also be characterized as medically unexplained functional disorders, even within the network of neurology care.4
Misdiagnosis and bias are not uncommon
Ambiguity in classifying and evaluating FND can affect physicians’ perceptions, assessment, and care of patients with suggestive presenting symptoms. A major early challenge in diagnosing FND is the inconsistency of characterizing terminology (pseudoneurological, somatic, dissociative, conversion, psychogenic, hysterical, factitious, functional, medically unexplained9,10) and definitions in the literature. Neurological symptoms of unidentifiable organic cause can greatly diminish quality of life4; FND is a scientifically and clinically useful diagnosis for many combinations of nonrandomly co-occurring symptoms and clinical signs.
The pitfall of misdiagnosis. Remain cautious about making a diagnosis of FND by exclusion, which might yield an incorrect or false-negative finding because of an atypical presentation. It is important to avoid misdiagnosis by prematurely closing the differential diagnosis; instead, keep in mind that a medically unexplained diagnosis might be better explained by conducting a robust social and medical history and obtaining additional or collateral data, or both, along with appropriate consultation.4,9
Misdiagnosis can lead to a circuitous and costly work-up, with the potential to increase the patient’s distress. You can reduce this burden with early recognition of FND and centralized management of multidisciplinary care, which are more likely to lead to an accurate and timely diagnosis—paramount to empowering patients with access to the correct information and meaningful support needed to enhance treatment and self-care.9
Bias, haste, and dismissal are unproductive. Even with a clear definition of FND, it is not uncommon for a physician to rapidly assess a patient’s clinical signs, make a diagnosis of “unknown etiology,” or openly question the veracity of complaints. Furthermore, be aware of inadvertently characterizing FND using the prefix “pseudo” or the term “hysterical,” which can be psychologically discomforting for many patients, who legitimately experience inexplicable symptoms. Such pejoratives can lead to stigmatizing and misleading assessments and treatment paths4—courses of action that can cause early and, possibly, irreparable harm to the patient–physician relationship and increase the patient’s inclination to go “doctor-shopping,” with associated loss of continuity of care.
Why is it difficult to diagnose FND?
The latest (5th) edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) describes conversion, somatoform disorder, and FND synonymously.DSM-5 diagnostic criteria for conversion disorderare11:
- a specified type of symptom or deficit of altered voluntary motor or sensory function (eg, weakness, difficulty swallowing, slurred speech, seizures)
- clinical evidence of the incompatibility of the symptom or deficit and any recognized neurological or medical disorder
- incapability of better explaining the symptom or deficit as another medical or mental disorder.
- The symptom or deficit causes distress or impairment that (1) is clinically significant in occupational, social, or other important areas of function or (2) warrants medical evaluation.
The overarching feature of these criteria is the inconsistency of symptoms with recognized neurological, physiological, or psychiatric conditions. Although identification of psychological factors can help clarify and provide a treatment direction, such identification is not essential for making a diagnosis of FND. Malingering does not need to be refuted as part of establishing the diagnosis.12
Continue to: In contrast...
In contrast, the World Health Organization’s ICD-10 Classification of Mental and Behavioural Disorders groups diagnostic criteria for FND among the dissociative disorders13:
- Clinical features are specified for the individual dissociative disorder (motor, sensory, convulsions, mixed).
- Evidence is absent of a physical disorder that might explain symptoms.
- Evidence of psychological causation is present in clear temporal association with stressful events and problems or disturbed relationships, even if the patient denies such association.
Note the emphasis on psychological causation and exclusion of purposeful simulation of symptoms, as opposed to a primarily unconscious disconnection from the patient’s body or environment.
ICD-10 guidelines acknowledge the difficulty of finding definitive evidence of a psychological cause and recommend provisional diagnosis of FND if psychological factors are not readily apparent.14 Of note, many patients with FND are affected psychologically by their condition, with an impact on mood, behaviors, and interpersonal interactions, although not necessarily to a clinically diagnostic degree. Therefore, a psychiatric diagnosis alone is not a necessary precursor for the diagnosis of an FND.
CASE
History. Mr. D’s history is positive for light alcohol consumption (“2 or 3 cans of beer on weekends”) and chewing tobacco (he reports stopping 6 months earlier) and negative for substance abuse. The family history is positive for maternal hypertension and paternal suicide when the patient was 10 years old (no other known paternal history).
Physical findings. The review of systems is positive for intermittent palpitations, lower-extremity weakness causing unsteady gait, and generalized headache.
Vital signs are within normal limits, including blood pressure (120/82 mm Hg) and heart rate (110 beats/min). The patient is not in acute distress; he is awake, alert, and oriented × 3. No murmurs are heard; lungs are clear bilaterally to auscultation. There is no tenderness on abdominal palpation, and no hepatomegaly or splenomegaly; bowel sounds are normal. No significant bruising or lacerations are noted.
Neurology exam. Cranial nerves II-XII are intact. Pupils are equal and reactive to light. Reflexes are 2+ bilaterally. Muscle strength and tone are normal; no tremors are noted. Babinski signs are normal. A Romberg test is positive (swaying).
Continue to: Mr. D has an antalgic gait...
Mr. D has an antalgic gait with significant swaying (without falling); bent posture; and unsteadiness that requires a cane. However, he is able to get up and off the exam table without assistance, and to propel himself, by rolling a chair forward and backward, without difficulty.
Conducting a diagnostic examination
Taking the history. Certain clues can aid in the diagnosis of FND (TABLE 1).15 For example, the patient might have been seen in multiple specialty practices for a multitude of vague symptoms indicative of potentially related conditions (eg, chronic fatigue, allergies and sensitivities, fibromyalgia, and other chronic pain). The history might include repeated surgeries to investigate those symptoms (eg, laparoscopy, or hysterectomy at an early age). Taking time and care to explore all clinical clues, patient reports, and collateral data are therefore key to making an accurate diagnosis.

Note any discrepancies between the severity of reported symptoms and functional ability. A technique that can help elucidate a complex or ambiguous medical presentation is to ask the patient to list all their symptoms at the beginning of the interview. This has threefold benefit: You get a broad picture of the problem; the patient is unburdened of their concerns and experiences your validation; and a long list of symptoms can be an early clue to a diagnosis of FND.

Other helpful questions to determine the impact of symptoms on the patient’s well-being include inquiries about16:
- functional impairment
- onset and course of symptoms
- potential causal or correlating events
- dissociative episodes
- previous diagnoses and treatments
- the patient’s perceptions of, and emotional response to, their illness
- a history of abuse.

The physical examination to determine the presence of FND varies, depending on the functional area of impact (eg, motor, neurological, sensory, speech and swallowing). Pay particular attention to presenting signs and clues, and balance them with the patient’s report (or lack of report). Endeavor to demonstrate positive functional signs, such as a positive Hoover test, which relies on the principle of synergistic muscle contraction. You might see evidence of inconsistency, such as weakness or a change in gait, under observation, that seemingly resolves when the patient is getting on and off the exam table.16Table 215-24 describes areas affected by FND, characteristics of the disorder, and related diagnostic examinations.

Table 315,18,19 reviews validated special exams that can aid in making the diagnosis. Additional special tests are discussed in the literature.15-24 These tests can be helpful in narrowing the differential diagnosis but have not been validated and should be used with caution.

Some clinical signs associated with FND might be affected by other factors, including socioeconomic status, limited access to health care, low health literacy, poor communication skills, and physician bias. Keep these factors in mind during the visit, to avoid contributing further to health disparities among groups of patients affected by these problems.

Continue to: CASE
CASE
The work-up over the next month for Mr. D includes numerous studies, all yielding results that are negative or within normal limits: visual acuity; electrocardiography and an event monitor; laboratory testing (including a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, folate, and vitamin D); magnetic resonance imaging of the brain and lumbar spine; lumbar puncture; and electromyography.
The score on the 9-item Patient Health Questionnaire for depression is 4 (severity: “none or minimal”); on the 7-item Generalized Anxiety Disorder scale, 0 (“no anxiety disorder”).
Referral. A neurology work-up of headache, lower extremity weakness, and unsteady gait to address several diagnostic possibilities, including migraine and multiple sclerosis, is within normal limits. A cardiology work-up of palpitations is negative for arrhythmias and other concerning findings.
Mr. D declines psychiatric and psychological evaluations.
Building a differential diagnosisis a formidable task
The differential diagnosis of FND is vast. It includes neurological, physiological, and psychiatric symptoms and disorders; somatization; and malingering (Table 4).6 Any disorder or condition in these areas that is in the differential diagnosis can be precipitated or exacerbated by stress; most, however, do not involve loss of physical function.12 In addition, the diagnosis of an FND does not necessarily exclude an organic disorder.

A patient’s presentation becomes complicated—and more difficult to treat—when functional symptoms and an unrelated underlying or early-stage neurological condition coexist. For example, a patient with epilepsy might also have dissociative seizures atop their organic disorder. Neurological disease is considered a risk factor for an overlying FND—just as the risk of depression or anxiety runs concurrently with other chronic diseases.14
Focus on clinical signs to narrow the differential. A thorough social and medical history and physical examination, as discussed earlier, help narrow the differential diagnosis of organic and medically unexplained disorders. Well-defined imaging or laboratory protocols do not exist to guide physicians to a definitive diagnosis, however.
Continue to: Psychiatric conditions
Psychiatric conditions can coexist with the diagnosis of FND, but might be unrelated. A systematic review of the literature showed that 17% to 42% of patients with FND had a concurrent anxiety disorder. Depression disorders were co-diagnosed in 19% to 71% of patients with FND; dissociative and personality disorders were noted, as well.25 However, coexisting psychiatric diagnosis might more likely be associated with distress from the presenting functional neurological symptoms, not linked to the FND diagnosis itself.12 This shift in understanding is reflected in the description of FND in the DSM-5.11
CASE
Mr. D reports debilitating headaches at return office visits. Trials of abortive triptans provide no relief; neither do control medications (beta-blockers, coenzyme Q10, magnesium, onabotulinumtoxinA [Botox], topiramate, and valproate). Lower-extremity weakness and unsteadiness are managed with supportive devices, including a cane, and physical therapy.
Importance of establishing a multidisciplinary approach
The complexity of FND lends itself to a multidisciplinary approach during evaluation and, eventually, for treatment. The assessment and diagnostic intervention that you provide, along with the contributions of consulted specialists (including neurology, physical and occupational therapy, psychiatry, psychology, and other mental health professionals) establishes a team-based approach that can increase the patient’s sense of support and reduce excessive testing and unnecessary medications, surgeries, and other treatments.26
Family physicians are in the ideal position to recognize the patient’s functional capacity and the quality of symptoms and to provide timely referral (eg, to Neurology and Psychiatry) for confirmation of the diagnosis and then treatment.
Evidence-based treatment options include:
- psychotherapy, with an emphasis on cognitive behavioral therapy
- physical therapy
- psychopharmacology
- promising combinations of physical and psychological treatment to improve long-term functionality.27
A promising diagnostic tool
The most significant update in the FND literature is on functional neuroimaging for assessing the disorder. Early findings suggest an intricate relationship between mind and body regarding the pathological distortion in FND. And, there is clear evidence that neuroimaging—specifically, functional magnetic resonance imaging—shows changes in brain activity that correspond to the patient’s symptom report. That said, imaging is not the recommended standard of care in the initial work-up of FND because of its cost and the fact that the diagnosis is principally a clinical undertaking.17,28
Call to action
Offer a generous ear. Begin the diagnostic pursuit by listening carefully and fully to the patient’s complaints, without arriving at a diagnosis with unwarranted bias or haste. This endeavor might require support from other clinical staff (eg, nurses, social workers, case managers) because the diagnostic process can be arduous and lengthy.
Continue to: Convey the diagnosis with sensitivity
Convey the diagnosis with sensitivity. Inquire about the patient’s perceptions and impairments to best personalize your diagnostic explanations. Delivery of the diagnosis might affect the patient’s acceptance and compliance with further testing and treatment of what is generally a persistent and treatment-resistant disorder; poor delivery of diagnostic information can impair the patient–physician relationship and increase the risk of disjointed care. Many patients find that improved patient–physician communication is therapeutic.29
Let the patient know that you’re taking her seriously. Validate patient concerns with a nonstigmatizing diagnostic label; discuss the diagnostic parameters and cause of symptoms in layman’s terms; and emphasize the potential for reversibility.30 Some patients are not satisfied with having a diagnosis of FND until they are reassured with normal results of testing and provided with referral; even then, some seek further reassurance.
Key tenets of managing care for patients who have been given a diagnosis of FND include:
- nonjudgmental, positive regard
- meaningful expression of empathy
- multidisciplinary coordination
- avoidance of unnecessary testing and harmful treatments
- descriptive and contextual explanations of the diagnosis.
Last, keep in mind that the course of treatment for FND is potentially prolonged and multilayered.
CASE
After many visits with his family physician and the neurology and cardiology specialists, as well as an extensive work-up, the physician approaches Mr. D with the possibility of a diagnosis of FND and proposes a multidisciplinary plan that includes:
- a course of physical and occupational therapy
- development of individualized cognitive behavioral tools
- weekly personal and marital counseling
- initiation of a selective serotonin reuptake inhibitor for anxiety
- monthly visits with his family physician.
Months after his return from deployment for evaluation and treatment, Mr. D is able to return to military duty. He reports that his quality of life has improved.
CORRESPONDENCE
Roselyn W. Clemente Fuentes, MD, FAAFP, Eglin Family Medicine Residency, 307 Boatner Road, Eglin AFB, FL 32547; [email protected].
CASE
John D,* a 25-year-old patient with an otherwise unremarkable medical history, describes 2 months of daily headache, lower-extremity weakness, and unsteady gait that began fairly suddenly during his first deployment in the US Army. He explains that these symptoms affected his ability to perform his duties and necessitated an early return stateside for evaluation and treatment.
Mr. D denies precipitating trauma or unusual environmental exposures. He reports that, stateside now, symptoms continue to affect his ability to work and attend to personal and family responsibilities.
Asked about stressors, Mr. D notes the birth of his first child approximately 3 months ago, while he was deployed, and marital stressors. He denies suicidal or homicidal ideation.
* The patient’s name has been changed to protect his identity.
The challenge of identifying and managing FND
A functional neurological disorder (FND) is a constellation of psychological, physiological, and neurological symptoms, without an identifiable organic etiology, a conscious decision, or secondary gain for the patient,1 that adversely impacts functioning in 1 or more significant life domains.
Given the high throughput of patients in primary care practices, family physicians can expect to encounter suspected cases of FND in their practices. Regrettably, however, a lack of familiarity with the disorder and its related problems (eg, nonorganic paralysis, sensory loss, nonepileptic seizures, and abnormal movements) can add as much as $20,000 in excess direct and indirect costs of care for every such patient.1 In this article, we synthesize the recent literature on FND so that family physicians can expand their acumen in understanding, identifying, and evaluating patients whose presentation suggests FND.
An underrecognized entity
A precise estimate of the prevalence of FND is difficult to determine because the disorder is underrecognized and misdiagnosed and because it is often accompanied by the confounding of psychological and physiological comorbidities. A 2012 study estimated the annual incidence of FND to be 4 to 12 cases for every 100,000 people2; in primary care and outpatient neurology settings, prevalence is 6% to 22% of all patients.3,4 Stone and colleagues identified functional neurological symptoms as the second most common reason for outpatient neurology consultation,5 with 1 nonepileptic seizure patient seen for every 6 epileptic patients, and functional weakness presenting at the same rate as multiple sclerosis.6
Continue to: Demographics of patients with FND...
Demographics of patients with FND vary, depending on presenting neurological symptoms and disorder subtype. Existing data indicate a correlation between FND and younger age, female sex, physical disability,7 and a history of abuse or trauma.3,8 A challenge in concretely ascertaining the prevalence of FND is that conditions such as fibromyalgia, chronic pelvic pain, globus hystericus, and nonepileptic seizures can also be characterized as medically unexplained functional disorders, even within the network of neurology care.4
Misdiagnosis and bias are not uncommon
Ambiguity in classifying and evaluating FND can affect physicians’ perceptions, assessment, and care of patients with suggestive presenting symptoms. A major early challenge in diagnosing FND is the inconsistency of characterizing terminology (pseudoneurological, somatic, dissociative, conversion, psychogenic, hysterical, factitious, functional, medically unexplained9,10) and definitions in the literature. Neurological symptoms of unidentifiable organic cause can greatly diminish quality of life4; FND is a scientifically and clinically useful diagnosis for many combinations of nonrandomly co-occurring symptoms and clinical signs.
The pitfall of misdiagnosis. Remain cautious about making a diagnosis of FND by exclusion, which might yield an incorrect or false-negative finding because of an atypical presentation. It is important to avoid misdiagnosis by prematurely closing the differential diagnosis; instead, keep in mind that a medically unexplained diagnosis might be better explained by conducting a robust social and medical history and obtaining additional or collateral data, or both, along with appropriate consultation.4,9
Misdiagnosis can lead to a circuitous and costly work-up, with the potential to increase the patient’s distress. You can reduce this burden with early recognition of FND and centralized management of multidisciplinary care, which are more likely to lead to an accurate and timely diagnosis—paramount to empowering patients with access to the correct information and meaningful support needed to enhance treatment and self-care.9
Bias, haste, and dismissal are unproductive. Even with a clear definition of FND, it is not uncommon for a physician to rapidly assess a patient’s clinical signs, make a diagnosis of “unknown etiology,” or openly question the veracity of complaints. Furthermore, be aware of inadvertently characterizing FND using the prefix “pseudo” or the term “hysterical,” which can be psychologically discomforting for many patients, who legitimately experience inexplicable symptoms. Such pejoratives can lead to stigmatizing and misleading assessments and treatment paths4—courses of action that can cause early and, possibly, irreparable harm to the patient–physician relationship and increase the patient’s inclination to go “doctor-shopping,” with associated loss of continuity of care.
Why is it difficult to diagnose FND?
The latest (5th) edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) describes conversion, somatoform disorder, and FND synonymously.DSM-5 diagnostic criteria for conversion disorderare11:
- a specified type of symptom or deficit of altered voluntary motor or sensory function (eg, weakness, difficulty swallowing, slurred speech, seizures)
- clinical evidence of the incompatibility of the symptom or deficit and any recognized neurological or medical disorder
- incapability of better explaining the symptom or deficit as another medical or mental disorder.
- The symptom or deficit causes distress or impairment that (1) is clinically significant in occupational, social, or other important areas of function or (2) warrants medical evaluation.
The overarching feature of these criteria is the inconsistency of symptoms with recognized neurological, physiological, or psychiatric conditions. Although identification of psychological factors can help clarify and provide a treatment direction, such identification is not essential for making a diagnosis of FND. Malingering does not need to be refuted as part of establishing the diagnosis.12
Continue to: In contrast...
In contrast, the World Health Organization’s ICD-10 Classification of Mental and Behavioural Disorders groups diagnostic criteria for FND among the dissociative disorders13:
- Clinical features are specified for the individual dissociative disorder (motor, sensory, convulsions, mixed).
- Evidence is absent of a physical disorder that might explain symptoms.
- Evidence of psychological causation is present in clear temporal association with stressful events and problems or disturbed relationships, even if the patient denies such association.
Note the emphasis on psychological causation and exclusion of purposeful simulation of symptoms, as opposed to a primarily unconscious disconnection from the patient’s body or environment.
ICD-10 guidelines acknowledge the difficulty of finding definitive evidence of a psychological cause and recommend provisional diagnosis of FND if psychological factors are not readily apparent.14 Of note, many patients with FND are affected psychologically by their condition, with an impact on mood, behaviors, and interpersonal interactions, although not necessarily to a clinically diagnostic degree. Therefore, a psychiatric diagnosis alone is not a necessary precursor for the diagnosis of an FND.
CASE
History. Mr. D’s history is positive for light alcohol consumption (“2 or 3 cans of beer on weekends”) and chewing tobacco (he reports stopping 6 months earlier) and negative for substance abuse. The family history is positive for maternal hypertension and paternal suicide when the patient was 10 years old (no other known paternal history).
Physical findings. The review of systems is positive for intermittent palpitations, lower-extremity weakness causing unsteady gait, and generalized headache.
Vital signs are within normal limits, including blood pressure (120/82 mm Hg) and heart rate (110 beats/min). The patient is not in acute distress; he is awake, alert, and oriented × 3. No murmurs are heard; lungs are clear bilaterally to auscultation. There is no tenderness on abdominal palpation, and no hepatomegaly or splenomegaly; bowel sounds are normal. No significant bruising or lacerations are noted.
Neurology exam. Cranial nerves II-XII are intact. Pupils are equal and reactive to light. Reflexes are 2+ bilaterally. Muscle strength and tone are normal; no tremors are noted. Babinski signs are normal. A Romberg test is positive (swaying).
Continue to: Mr. D has an antalgic gait...
Mr. D has an antalgic gait with significant swaying (without falling); bent posture; and unsteadiness that requires a cane. However, he is able to get up and off the exam table without assistance, and to propel himself, by rolling a chair forward and backward, without difficulty.
Conducting a diagnostic examination
Taking the history. Certain clues can aid in the diagnosis of FND (TABLE 1).15 For example, the patient might have been seen in multiple specialty practices for a multitude of vague symptoms indicative of potentially related conditions (eg, chronic fatigue, allergies and sensitivities, fibromyalgia, and other chronic pain). The history might include repeated surgeries to investigate those symptoms (eg, laparoscopy, or hysterectomy at an early age). Taking time and care to explore all clinical clues, patient reports, and collateral data are therefore key to making an accurate diagnosis.

Note any discrepancies between the severity of reported symptoms and functional ability. A technique that can help elucidate a complex or ambiguous medical presentation is to ask the patient to list all their symptoms at the beginning of the interview. This has threefold benefit: You get a broad picture of the problem; the patient is unburdened of their concerns and experiences your validation; and a long list of symptoms can be an early clue to a diagnosis of FND.

Other helpful questions to determine the impact of symptoms on the patient’s well-being include inquiries about16:
- functional impairment
- onset and course of symptoms
- potential causal or correlating events
- dissociative episodes
- previous diagnoses and treatments
- the patient’s perceptions of, and emotional response to, their illness
- a history of abuse.

The physical examination to determine the presence of FND varies, depending on the functional area of impact (eg, motor, neurological, sensory, speech and swallowing). Pay particular attention to presenting signs and clues, and balance them with the patient’s report (or lack of report). Endeavor to demonstrate positive functional signs, such as a positive Hoover test, which relies on the principle of synergistic muscle contraction. You might see evidence of inconsistency, such as weakness or a change in gait, under observation, that seemingly resolves when the patient is getting on and off the exam table.16Table 215-24 describes areas affected by FND, characteristics of the disorder, and related diagnostic examinations.

Table 315,18,19 reviews validated special exams that can aid in making the diagnosis. Additional special tests are discussed in the literature.15-24 These tests can be helpful in narrowing the differential diagnosis but have not been validated and should be used with caution.

Some clinical signs associated with FND might be affected by other factors, including socioeconomic status, limited access to health care, low health literacy, poor communication skills, and physician bias. Keep these factors in mind during the visit, to avoid contributing further to health disparities among groups of patients affected by these problems.

Continue to: CASE
CASE
The work-up over the next month for Mr. D includes numerous studies, all yielding results that are negative or within normal limits: visual acuity; electrocardiography and an event monitor; laboratory testing (including a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, folate, and vitamin D); magnetic resonance imaging of the brain and lumbar spine; lumbar puncture; and electromyography.
The score on the 9-item Patient Health Questionnaire for depression is 4 (severity: “none or minimal”); on the 7-item Generalized Anxiety Disorder scale, 0 (“no anxiety disorder”).
Referral. A neurology work-up of headache, lower extremity weakness, and unsteady gait to address several diagnostic possibilities, including migraine and multiple sclerosis, is within normal limits. A cardiology work-up of palpitations is negative for arrhythmias and other concerning findings.
Mr. D declines psychiatric and psychological evaluations.
Building a differential diagnosisis a formidable task
The differential diagnosis of FND is vast. It includes neurological, physiological, and psychiatric symptoms and disorders; somatization; and malingering (Table 4).6 Any disorder or condition in these areas that is in the differential diagnosis can be precipitated or exacerbated by stress; most, however, do not involve loss of physical function.12 In addition, the diagnosis of an FND does not necessarily exclude an organic disorder.

A patient’s presentation becomes complicated—and more difficult to treat—when functional symptoms and an unrelated underlying or early-stage neurological condition coexist. For example, a patient with epilepsy might also have dissociative seizures atop their organic disorder. Neurological disease is considered a risk factor for an overlying FND—just as the risk of depression or anxiety runs concurrently with other chronic diseases.14
Focus on clinical signs to narrow the differential. A thorough social and medical history and physical examination, as discussed earlier, help narrow the differential diagnosis of organic and medically unexplained disorders. Well-defined imaging or laboratory protocols do not exist to guide physicians to a definitive diagnosis, however.
Continue to: Psychiatric conditions
Psychiatric conditions can coexist with the diagnosis of FND, but might be unrelated. A systematic review of the literature showed that 17% to 42% of patients with FND had a concurrent anxiety disorder. Depression disorders were co-diagnosed in 19% to 71% of patients with FND; dissociative and personality disorders were noted, as well.25 However, coexisting psychiatric diagnosis might more likely be associated with distress from the presenting functional neurological symptoms, not linked to the FND diagnosis itself.12 This shift in understanding is reflected in the description of FND in the DSM-5.11
CASE
Mr. D reports debilitating headaches at return office visits. Trials of abortive triptans provide no relief; neither do control medications (beta-blockers, coenzyme Q10, magnesium, onabotulinumtoxinA [Botox], topiramate, and valproate). Lower-extremity weakness and unsteadiness are managed with supportive devices, including a cane, and physical therapy.
Importance of establishing a multidisciplinary approach
The complexity of FND lends itself to a multidisciplinary approach during evaluation and, eventually, for treatment. The assessment and diagnostic intervention that you provide, along with the contributions of consulted specialists (including neurology, physical and occupational therapy, psychiatry, psychology, and other mental health professionals) establishes a team-based approach that can increase the patient’s sense of support and reduce excessive testing and unnecessary medications, surgeries, and other treatments.26
Family physicians are in the ideal position to recognize the patient’s functional capacity and the quality of symptoms and to provide timely referral (eg, to Neurology and Psychiatry) for confirmation of the diagnosis and then treatment.
Evidence-based treatment options include:
- psychotherapy, with an emphasis on cognitive behavioral therapy
- physical therapy
- psychopharmacology
- promising combinations of physical and psychological treatment to improve long-term functionality.27
A promising diagnostic tool
The most significant update in the FND literature is on functional neuroimaging for assessing the disorder. Early findings suggest an intricate relationship between mind and body regarding the pathological distortion in FND. And, there is clear evidence that neuroimaging—specifically, functional magnetic resonance imaging—shows changes in brain activity that correspond to the patient’s symptom report. That said, imaging is not the recommended standard of care in the initial work-up of FND because of its cost and the fact that the diagnosis is principally a clinical undertaking.17,28
Call to action
Offer a generous ear. Begin the diagnostic pursuit by listening carefully and fully to the patient’s complaints, without arriving at a diagnosis with unwarranted bias or haste. This endeavor might require support from other clinical staff (eg, nurses, social workers, case managers) because the diagnostic process can be arduous and lengthy.
Continue to: Convey the diagnosis with sensitivity
Convey the diagnosis with sensitivity. Inquire about the patient’s perceptions and impairments to best personalize your diagnostic explanations. Delivery of the diagnosis might affect the patient’s acceptance and compliance with further testing and treatment of what is generally a persistent and treatment-resistant disorder; poor delivery of diagnostic information can impair the patient–physician relationship and increase the risk of disjointed care. Many patients find that improved patient–physician communication is therapeutic.29
Let the patient know that you’re taking her seriously. Validate patient concerns with a nonstigmatizing diagnostic label; discuss the diagnostic parameters and cause of symptoms in layman’s terms; and emphasize the potential for reversibility.30 Some patients are not satisfied with having a diagnosis of FND until they are reassured with normal results of testing and provided with referral; even then, some seek further reassurance.
Key tenets of managing care for patients who have been given a diagnosis of FND include:
- nonjudgmental, positive regard
- meaningful expression of empathy
- multidisciplinary coordination
- avoidance of unnecessary testing and harmful treatments
- descriptive and contextual explanations of the diagnosis.
Last, keep in mind that the course of treatment for FND is potentially prolonged and multilayered.
CASE
After many visits with his family physician and the neurology and cardiology specialists, as well as an extensive work-up, the physician approaches Mr. D with the possibility of a diagnosis of FND and proposes a multidisciplinary plan that includes:
- a course of physical and occupational therapy
- development of individualized cognitive behavioral tools
- weekly personal and marital counseling
- initiation of a selective serotonin reuptake inhibitor for anxiety
- monthly visits with his family physician.
Months after his return from deployment for evaluation and treatment, Mr. D is able to return to military duty. He reports that his quality of life has improved.
CORRESPONDENCE
Roselyn W. Clemente Fuentes, MD, FAAFP, Eglin Family Medicine Residency, 307 Boatner Road, Eglin AFB, FL 32547; [email protected].
1. Konnopka A, Schaefert R, Heinrich S, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265-275.
2. Carson AJ, Brown R, David AS, et al; on behalf of UK-FNS. Functional (conversion) neurological symptoms: research since the millennium. J Neurol Neurosurg Psychiatry. 2012;83:842-850.
3. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
4. Evens A, Vendetta L, Krebs K, et al. Medically unexplained neurologic symptoms: a primer for physicians who make the initial encounter. Am J Med. 2015;128:1059-1064.
5. Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol. 2013;13:104-113.
6. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537-1551.
7. Carson A, Stone J, Hibberd C, et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J Neurol Neurosurg Psychiatry. 2011;82:810-813.
8. Fink P, Hansen MS, Oxhøj M-L. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res. 2004;56:413-418.
9. Thomas LE. Are your patient’s medically unexplained symptoms really “all in her head”? Med Hypotheses. 2012;78:542-547.
10. Ding JM, Kanaan RAA. What should we say to patients with unexplained neurological symptoms? How explanation affects offence. J Psychosom Res. 2016;91:55-60.
11. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association; 2013.
12. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM-5: Conversion disorder. Am J Psychiatry. 2010;167:626-627.
13. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1994. Accessed January 21, 2021. www.who.int/classifications/icd/en/bluebook.pdf
14. Stone J, Carson A, Duncan R, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease.” J Neurol. 2012;259:33-38.
15. Shaibani A, Sabbagh M. Pseudoneurologic syndromes: recognition and diagnosis. Am Fam Physician. 1998;57:2485-2494.
16. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
17. Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323-337.
18. McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics. 2018;59:358-368.
19. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190.
20. Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001;16:595-599.
21. Ludwig L, McWhirter L, Williams S, et al. Functional coma. In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology: Volume 139: Functional Neurologic Disorders. 1st ed. Academic Press; 2016:313.
22. Miller NR, Subramanian PS, Patel VR. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 3rd ed. Wolters Kluwer; 2016:512-513.
23. Takazaki K, Stransky AD, Miller G. Psychogenic nonepileptic seizures: diagnosis, management, and bioethics. Pediatr Neurol. 2016;62:3-8.
24. Sahaya K, Dholakia SA, Sahota PK. Psychogenic non-epileptic seizures: a challenging entity. J Clin Neurosci. 2011;18:1602-1607.
25. Gelauff J, Stone J, Edwards M, et al. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220-226.
26. Kranick SM, Gorrindo T, Hallett M. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics. 2011;52:109-116.
27. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250-260.
28. Burgmer M, Kugel H, Pfleiderer B, et al. The mirror neuron system under hypnosis—brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex. 2013;49:437-445.
29. van Bokhoven MA, Koch H, van der Weijden T, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints, Ann Fam Med. 2009;7:112-120.
30. Stone J, Carson A, Hallet M. Explanation as treatment for functional neurologic disorders. Handb Clin Neurol. 2016;139:543-553.
1. Konnopka A, Schaefert R, Heinrich S, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265-275.
2. Carson AJ, Brown R, David AS, et al; on behalf of UK-FNS. Functional (conversion) neurological symptoms: research since the millennium. J Neurol Neurosurg Psychiatry. 2012;83:842-850.
3. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
4. Evens A, Vendetta L, Krebs K, et al. Medically unexplained neurologic symptoms: a primer for physicians who make the initial encounter. Am J Med. 2015;128:1059-1064.
5. Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol. 2013;13:104-113.
6. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537-1551.
7. Carson A, Stone J, Hibberd C, et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J Neurol Neurosurg Psychiatry. 2011;82:810-813.
8. Fink P, Hansen MS, Oxhøj M-L. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res. 2004;56:413-418.
9. Thomas LE. Are your patient’s medically unexplained symptoms really “all in her head”? Med Hypotheses. 2012;78:542-547.
10. Ding JM, Kanaan RAA. What should we say to patients with unexplained neurological symptoms? How explanation affects offence. J Psychosom Res. 2016;91:55-60.
11. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association; 2013.
12. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM-5: Conversion disorder. Am J Psychiatry. 2010;167:626-627.
13. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1994. Accessed January 21, 2021. www.who.int/classifications/icd/en/bluebook.pdf
14. Stone J, Carson A, Duncan R, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease.” J Neurol. 2012;259:33-38.
15. Shaibani A, Sabbagh M. Pseudoneurologic syndromes: recognition and diagnosis. Am Fam Physician. 1998;57:2485-2494.
16. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
17. Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323-337.
18. McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics. 2018;59:358-368.
19. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190.
20. Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001;16:595-599.
21. Ludwig L, McWhirter L, Williams S, et al. Functional coma. In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology: Volume 139: Functional Neurologic Disorders. 1st ed. Academic Press; 2016:313.
22. Miller NR, Subramanian PS, Patel VR. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 3rd ed. Wolters Kluwer; 2016:512-513.
23. Takazaki K, Stransky AD, Miller G. Psychogenic nonepileptic seizures: diagnosis, management, and bioethics. Pediatr Neurol. 2016;62:3-8.
24. Sahaya K, Dholakia SA, Sahota PK. Psychogenic non-epileptic seizures: a challenging entity. J Clin Neurosci. 2011;18:1602-1607.
25. Gelauff J, Stone J, Edwards M, et al. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220-226.
26. Kranick SM, Gorrindo T, Hallett M. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics. 2011;52:109-116.
27. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250-260.
28. Burgmer M, Kugel H, Pfleiderer B, et al. The mirror neuron system under hypnosis—brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex. 2013;49:437-445.
29. van Bokhoven MA, Koch H, van der Weijden T, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints, Ann Fam Med. 2009;7:112-120.
30. Stone J, Carson A, Hallet M. Explanation as treatment for functional neurologic disorders. Handb Clin Neurol. 2016;139:543-553.
PRACTICE RECOMMENDATIONS
› Avoid using stigmatizing terminology (eg, adding the prefix “pseudo” or the adjective “hysterical”) to characterize a suspected functional neurological disorder (FND) or a medically unexplained disorder. C
› Refrain from ordering functional magnetic resonance imaging as part of the routine evaluation of suspected FND. C
› Validate the patient‘s concerns with an appropriate diagnostic label; use layman’s terms to discuss the diagnostic parameters of FND and the cause of symptoms; and emphasize treatment possibilities and plans. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Is an underlying cardiac condition causing your patient’s palpitations?
Palpitations, the sensory perception of one’s heartbeat, are reported in 16% of primary care patients, from causes that are both cardiac (ie, arrhythmias) and noncardiac.1 Palpitations are usually benign; overall mortality is approximately 1% annually. In fact, a retrospective study found no difference in mortality and morbidity between patients with palpitations and control patients without palpitations.2 However, palpitations can reflect a life-threatening cardiac condition, as we discuss in this article, making careful assessment and targeted, sometimes urgent, intervention important.3
Here, we review the clinical work-up of palpitations, recommended diagnostic testing, and the range of interventions for cardiac arrhythmias—ectopic beats, ventricular tachycardia (VT), and atrial fibrillation (AF).
Cardiac and noncardiac causes of palpitations
In a prospective cohort study of 190 consecutive patients presenting with palpitations, the cause was cardiac in 43%, psychiatric in 31%, and of a miscellaneous nature (including medication, thyrotoxicosis, caffeine, cocaine, anemia, amphetamine, and mastocytosis) in 10%; in 16%, the cause was undetermined.2 In this study, 77% of patients experienced a recurrence of palpitations after their first episode.2
Cardiac arrhythmias, a common cause of palpitations, are differentiated by site of origin—supraventricular and ventricular. Noncardiac causes of palpitations, which we do not discuss here, include metabolic and psychiatric conditions, medications, and substance use. (For a summary of the causes of palpitations, see TABLE 1.2-4)
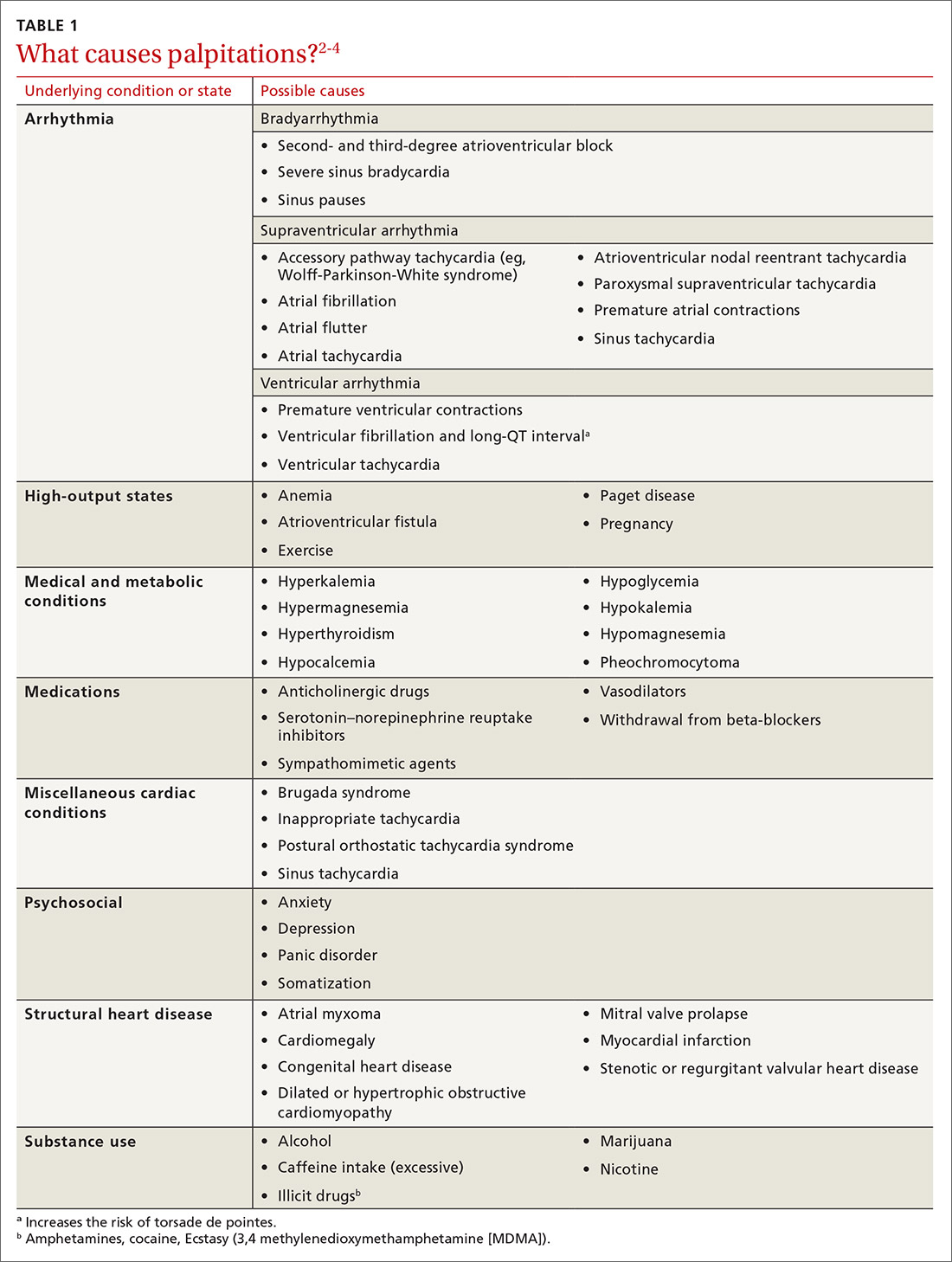
Common complaint: ectopic beats. Premature atrial contractions (PACs; also known as premature atrial beats, atrial premature complexes, and atrial premature beats) and premature ventricular contractions (PVCs; also known as ventricular premature complexes and ventricular premature beats, and also of a variety of possible causes) result in a feeling of a skipped heartbeat or a flipping sensation in the chest.
The burden of PACs is independently associated with mortality, cardiovascular hospitalization, new-onset AF, and pacemaker implantation. In a multivariate analysis, a PAC burden > 76 beats/d was an independent predictor of mortality (hazard ratio [HR] = 1.4; 95% CI, 1.2-16); cardiovascular hospitalization (HR = 1.3; 95% CI, 1.1-1.5); new-onset AF (HR = 1.8; 95% CI, 1.4-2.2); and pacemaker implantation (HR = 2.8; 95% CI, 1.9-4.2). Frequent PACs can lead to cardiac remodeling, so more intense follow-up of patients with a high PAC burden might allow for early detection of AF or subclinical cardiac disease.5,6
A burden of PVCs > 24% is associated with an increased risk of PVC-induced cardiomyopathy and heart failure. Polymorphic PVCs are more concerning than monomorphic PVCs because the former suggests the presence of more diffuse, rather than localized, myocardial injury. The presence of frequent (> 1000 beats/d) PVCs warrants evaluation and treatment for underlying structural heart disease and ischemic heart disease. Therapy directed toward underlying heart disease can reduce the frequency of PVCs.7-9
Continue to: The diagnostic work-up
The diagnostic work-up
The most important goal of the evaluation of palpitations is to determine the presence, or risk, of structural heart or coronary artery disease (CAD) by means of the history, physical examination, and electrocardiography (EKG). Patients who have an increased risk of structural heart disease need further evaluation with echocardiography; those at increased risk of CAD should have stress testing.
Hemodynamically unstable patients need admission; patients who have a history of syncope with palpitations usually should be admitted for cardiac monitoring. Patients who have had a single episode of palpitations and have normal baseline results of laboratory testing and a normal EKG, and no risk factors for structural heart disease or known CAD, can usually be observed.3,4,10 Patients with an abnormal baseline EKG, recurrent palpitations (especially tachyarrhythmia), or significant symptoms during palpitations (syncope, presyncope, dyspnea) need further evaluation with ambulatory monitoring3,4,10 (Figure).
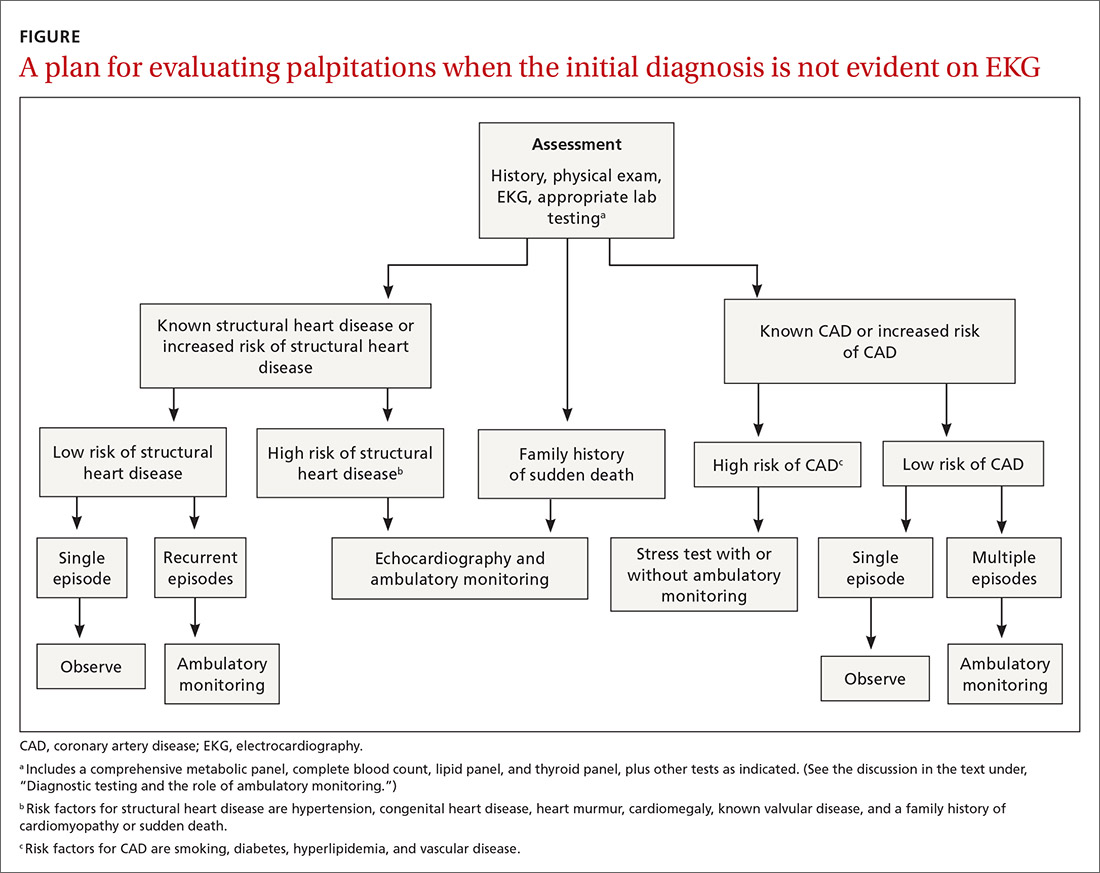
Take a thorough history; ask these questions
Have the patient describe the palpitations. The history should include the patient’s detailed characterization of the palpitations (sudden or gradual onset, rhythm, duration, frequency). Certain descriptions provide possible diagnostic clues:
- Palpitations lasting < 5 minutes are less likely to be of cardiac origin (likelihood ratio [LR] = 0.38; 95% CI, 0.2-0.6).4
- A patient who has a regular, rapid-pounding sensation in the neck has an increased probability of atrioventricular (AV) nodal reentrant tachycardia (AVNRT) (LR = 177; 95% CI, 25-1251); absence of this sensation decreases the likelihood of AVNRT (LR = 0.07; 95% CI, 0.03-0.2).4
- PACs and PVCs cause a sensation of a skipped heartbeat or a flipping sensation in the chest; they are not reported as a sustained rapid heartbeat.
- Patients with a supraventricular arrhythmia often report sudden onset and cessation of palpitations.
- Patients with palpitations since childhood are more likely to have supraventricular tachycardia (SVT).4
Elicit apparent precipitating and alleviating factors. The history should include notation of situations that appear to the patient to lead to palpitations (eg, context, positional variation). Palpitations that affect sleep (LR = 2.3; 95% CI, 1.3-3.9) and palpitations that occur at work (LR = 2.2; 95% CI, 1.3-5) increase the likelihood of a cardiac cause.4 Palpitations associated with sudden change in position, such as bending forward or squatting, are more likely due to AVNRT.11
Ask about aggravating factors (eg, exercise) and relieving factors (eg, rest, performing a Valsalva maneuver). Patients with SVT are often able to have palpitations terminated with a Valsalva maneuver, such as carotid sinus massage. Palpitations and syncope during exertion can be associated with hypertrophic cardiomyopathy, congenital coronary anomalies, and ion channelopathies, and can cause sudden cardiac death in athletes (estimated incidence, 1-3/100,000 person–years12).
Endeavor to identify underlying cardiac disease. A comprehensive history should also evaluate for risk factors and symptoms (chest pain, dyspnea, diaphoresis, lightheadedness, syncope) of cardiac disease, such as CAD, valvular disease, cardiomyopathy, and congenital heart disease, which increase the likelihood that the presenting complaint is a cardiac arrhythmia (LR = 2; 95% CI, 1.3-3.1).4 A history of syncope in a patient with palpitations should prompt evaluation for structural heart disease, such as aortic stenosis or hypertrophic cardiomyopathy, in which outflow-tract obstruction impairs cardiac output and, subsequently, cerebral blood flow.
Obtain additional key information. Determine the following in taking the history:
- Is there a family history of inherited cardiac disorders or sudden cardiac death?
- What prescription and over-the-counter medications is the patient taking? How does the patient characterize his or her use/intake of recreational drugs, nicotine, caffeine, and alcohol?
- Does the patient have a history of panic disorder, which lessens concern about a cardiac cause (LR = 0.2; 95% CI, 0.07-1.01)?4 (Of note: A nonpsychiatric cause can coexist in such patients, and should be considered.)
Continue to: Physical examination clues, and the utility of vagal maneuvers
Physical examination clues, and the utility of vagal maneuvers
Although most patients in whom palpitations are the presenting complaint are, in fact, asymptomatic during clinical assessment, cardiovascular examination can assist in diagnosing the arrhythmia or structural heart disease:
- Resting bradycardia increases the likelihood of a clinically significant arrhythmia (LR = 3; 95% CI, 1.27-7.0).11
- A murmur, such as a midsystolic click or holosystolic murmur, detected during the cardiac exam can indicate mitral valve prolapse; a holosystolic murmur, exacerbated upon performing a Valsalva maneuver, suggests hypertrophic cardiomyopathy.
- Visible neck pulsations detected during assessment of the jugular venous pressure, known as cannon atrial (cannon A) waves, reflect abnormal contraction of the right atrium against a closed tricuspid valve during AV dissociation. Cannon A waves have an LR of 2.68 (95% CI, 1.25-5.78) for predicting AVNRT.4
Vagal nerve stimulation. In the rare circumstance that a patient complaining of palpitations is symptomatic during assessment, several tachycardias can be detected with the use of vagal maneuvers. Interruption of the tachycardia during carotid massage suggests a tachycardia involving the AV junction (AVNRT), whereas only a temporary pause or reduction in frequency is more common in atrial flutter, AF, and atrial tachycardias. Carotid massage has no effect on the presentation of ventricular arrhythmias.10
Diagnostic testing and the role of ambulatory monitoring
Electrocardiography. All patients with palpitations should have a 12-lead EKG, which may provide diagnostic clues (TABLE 210).
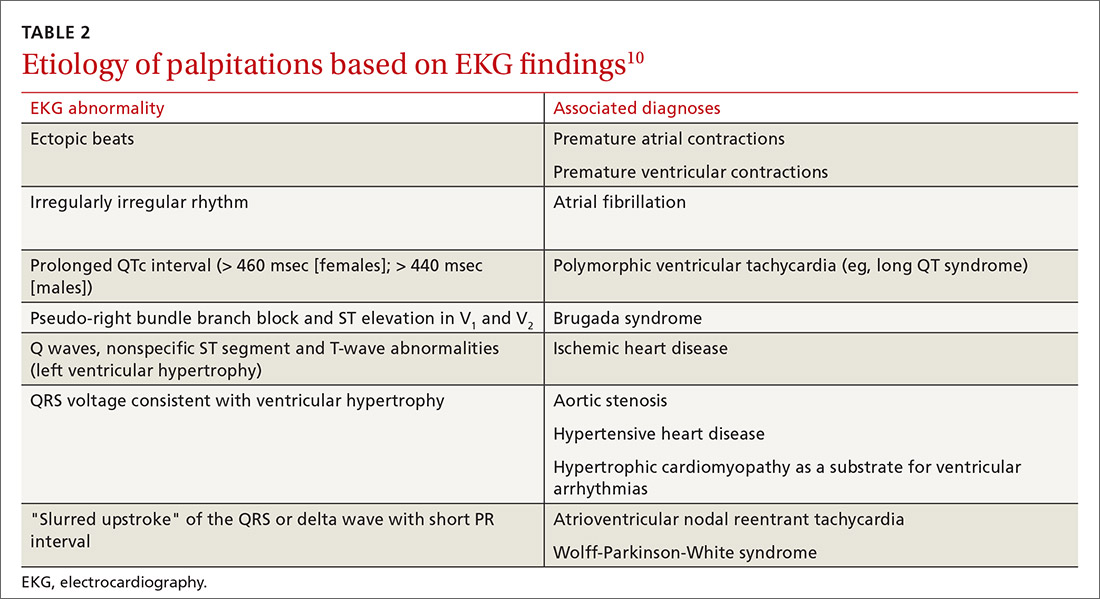
Ambulatory monitoring. When the EKG is nondiagnostic, ambulatory cardiac monitoring has an established role in the diagnosis of recurrent palpitations. In a small study of patients presenting with palpitations to a general practitioner, the deduction of those practitioners was wrong more than half the time when they predicted a ≤ 20% chance of an arrhythmia based on the history, physical exam, and EKG alone13—emphasizing the importance of ambulatory monitoring in patients with recurrent palpitations.
Which monitoring system is most suitable depends on symptom frequency, availability, cost, and patient competence. Twenty-four- to 48-hour Holter monitoring can be used in cases of frequent (eg, daily) palpitations. An automatic external loop recorder can be used for less frequent (eg, every 30 days) symptoms. Most ambulatory EKG is now automatic, and therefore does not require patient activation; older manual systems require patient activation during symptoms.
Two weeks of ambulatory EKG have proved sufficient for determining that there is a cardiac basis to palpitations. The diagnostic yield of ambulatory EKG is highest during Week 1 (1.04 diagnoses per patient), compared to Week 3 (0.17 diagnoses per patient).14
Implantable loop recorders are placed subcutaneously to provide EKG monitoring for approximately 3 years. They are better suited for diagnosing infrequent palpitations. The diagnostic yield of an implantable loop recorder over the course of 1 year for the detection of an arrhythmia is 73%, compared to 21% for a 24-hour Holter monitor, electrophysiology studies, and 4 weeks of an external loop recorder.15 Implantable loop recorders are often reserved for patients with palpitations associated with unexplained recurrent syncope.15
Continue to: Lab work
Lab work. A comprehensive metabolic panel, complete blood count, lipid panel, and thyroid panel should be ordered for all patients with palpitations. Possible additional tests include a urine drug screen (when recreational drug use is suspected); cardiac enzymes; N-terminal-pro hormone B-type natriuretic peptide (when there is evidence of CAD or heart failure); and urinary catecholamines (when pheochromocytoma is suspected).
Other investigations. Echocardiography is indicated when structural heart disease is suspected (TABLE 12-4). Patients who have multiple risk factors for CAD or exertional symptoms might warrant a stress test.
Management
PACs and PVCs
Typically, patients are counseled to minimize potential adrenergic precipitants, such as smoking, alcohol, stress, and caffeine. However, limited studies have demonstrated no significant arrhythmogenic potential of a modest dose of caffeine (200 mg), even in patients with known life-threatening ventricular arrhythmias.16 Beta-blockers and nondihydropyridine calcium channel blockers (CCBs) can reduce the severity of symptoms related to premature ectopic beats and might reduce their frequency, although response is inconsistent. Use of these medications for PACs is largely based on expert opinion and extrapolated from use in other supraventricular and ventricular arrhythmias.
Implantable cardioverter defibrillator therapy is indicated in patients with nonsustained VT due to prior myocardial infarction, left ventricular ejection fraction ≤ 40%, and inducible ventricular fibrillation or sustained VT on electrophysiological study.7
Patients with a high burden of ectopy who do not respond to treatment with AV nodal-blocking agents should be referred to Cardiology for other antiarrhythmic agents or catheter ablation. Last, asymptomatic ectopy does not need to be treated; there is no clear evidence that suppression with pharmacotherapy improves overall survival.15,17
Supraventricular tachycardia
The priority when evaluating any tachycardia is to assess the patient’s stability. Unstable patients should be treated immediately, usually with cardioversion, before an extensive diagnostic evaluation.18 Patients with wide-complex tachycardia (QRS > 120 ms) are generally more unstable and require more urgent therapy and cardiac consultation or referral. Hemodynamically stable patients with narrow-complex SVT (QRS < 120 ms) can be treated with IV adenosine, which has an 89.7% success rate.18,19 If adenosine is unsuccessful, cardioversion is indicated.
Stable patients with minimal symptoms and short episodes do not need treatment.
Continue to: Vagal maneuvers
Vagal maneuvers (eg, Valsalva maneuver; unilateral carotid massage after exclusion of a carotid bruit, with head tilted to the side opposite the massage, and not for longer than 10 seconds; or applying an ice-cold wet towel to the face) have a success rate of about 25% and are most effective when performed shortly after onset of arrhythmia. Vagal maneuvers can be used in all patients while preparing to administer medications.20
Patients who need treatment can take the “pill-in-the-pocket” approach with single-dose oral flecainide (3 mg/kg) or combined diltiazem and propranolol. Flecainide has a 94% success rate; diltiazem–propranolol has a lower success rate (61%) but a shorter time to conversion to sinus rhythm.21 Patients with sustained or recurrent episodes of SVT should be referred to a cardiologist for chronic prophylactic drug therapy or radiofrequency ablation.
Atrial fibrillation
Hemodynamically unstable patients with AF or atrial flutter, defined by the presence of angina, decompensated heart failure, hypotension, pulmonary edema, or evidence of organ hypoperfusion, should be electrically cardioverted using synchronized direct current.
Hemodynamically stable patients with a rapid ventricular rate should be treated with an IV or oral beta-blocker, CCB, or amiodarone, or electrically cardioverted. IV medications are typically preferred in the acute setting for ease and rapidity of administration; however, there is no evidence that IV formulations of beta-blockers and CCBs are superior to oral formulations. Once the ventricular rate is controlled, patients can be transitioned to an oral short-acting preparation of the selected agent, then converted to an appropriate dosage of an extended-release preparation.22
Cardioversion can be performed in patients with AF < 48 hours. In patients with AF > 48 hours, either 4 weeks of anticoagulation can be given, followed by cardioversion, or transesophageal echocardiography should be performed to evaluate for atrial thrombus; if atrial thrombus is absent, cardioversion can be performed.22 Transesophageal echocardiography might be unnecessary in patients known to have been on sustained anticoagulation.
Rate control is noninferior to rhythm control and does not decrease survival, functional capacity, or quality of life. Rate-control medications include beta-blockers, nondihydropyridine CCBs, amiodarone, and digoxin.
In the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial of 4060 patients, mortality was the same with rhythm control (21.3%) and rate control (23.8%) (HR = 1.15; 95% CI, 0.99-1.34), with no difference in the incidence of cardiac death, arrhythmic death, or death due to stroke.23 In the RACE (RAte Control versus Electrical cardioversion for persistent atrial fibrillation) trial of 522 patients with persistent AF, rate control was noninferior to rhythm control (by cardioversion and drugs) for reducing morbidity and preventing cardiovascular death.24 One possible reason why the rhythm control strategy in the RACE trial did not show superiority is the low number of patients who achieved sustained sinus rhythm.25
Continue to: The recommended ventricular rate...
The recommended ventricular rate has traditionally been 60 to 80 beats/min at rest and < 110 beats/min during daily activities. However, a recent trial found fewer adverse outcomes and no change in symptoms or the outcome of hospitalization in patients randomized to more lenient control (target resting heart rate, < 110 beats/min), although the mean of the actual lenient rate achieved was 86 beats/minute.24
Rhythm control. Antiarrhythmic agents or procedural interventions can be used in patients who fail or remain symptomatic despite rate control.26 Surgical measures include AV node ablation with placement of a pacemaker; atrial pacing with an implantable atrial defibrillator; the Maze procedure (open-heart surgery) to interrupt reentrant circuits in the left atrium; and percutaneous radiofrequency or cryotherapy ablation of arrhythmogenic foci in and around the junction of the pulmonary veins and left atrium.27
There is no significant benefit to immediate catheter ablation over standard medical therapy in adults with symptomatic AF in reducing the composite outcome of death, stroke, serious bleeding, and cardiac arrest. Catheter ablation is associated with a lower AF recurrence rate (50%) than drug therapy (69%) at 3 years.28
Anticoagulation. Patients at high risk of embolic stroke based on their score on the CHA2DS2-VASca risk stratification tool (ie, a score ≥ 2) should be anticoagulated.29,30 Options include a novel oral anticoagulant (dabigatran, rivaroxaban, apixaban, or edoxaban), the preferred class of agents for nonvalvular AF, and warfarin, with a target International Normalized Ratio of 2 to 3. Novel oral anticoagulants have been compared to warfarin for prevention of stroke in AF and were found more effective than warfarin, although at the expense of an increased risk of gastrointestinal bleeding.31 Percutaneous left atrial appendage closure, using a device such as the Watchman implant, is a noninferior surgical method to prevent embolic stroke in patients who are intolerant of, or have a contraindication to, anticoagulation.32
CORRESPONDENCE
Anne Mounsey, MD, Department of Family Medicine, University of North Carolina, 590 Manning Drive, Chapel Hill, NC 27599; [email protected].
1. Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685-1689.
2. Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. 1996;100:138-148.
3. Giada F, Raviele A. Clinical approach to patients with palpitations. Card Electrophysiol Clin. 2018;10:387-396.
4. Thavendiranathan P, Bagai A, Khoo C, et al. Does this patient with palpitations have a cardiac arrhythmia? JAMA. 2009;302:2135-2143.
5. Lin C-Y, Lin Y-J, Chen Y-Y, et al. Prognostic significance of premature atrial complexes burden in prediction of long-term outcome. J Am Heart Assoc. 2015;4:e002192.
6. Murakoshi N, Xu D, Sairenchi T, et al. Prognostic impact of supraventricular premature complexes in community-based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J. 2015;36:170-178.
7. Ahn M-S. Current concepts of premature ventricular contractions. J Lifestyle Med. 2013;3:26-33.
8. Panizo JG, Barra S, Mellor G, et al. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. 2018;7:128-134.
9. Ng GA. Treating patients with ventricular ectopic beats. Heart. 2006;92:1707-1712.
10 Raviele A, Giada F, Bergfeldt L, et al; European Heart Rhythm Association. Management of patients with palpitations: a position paper from the European Heart Rhythm Association. Europace. 2011;13:920-934.
11. Chiou C-W, Chen S-A, Kung M-H, et al. Effects of continuous enhanced vagal tone on dual atrioventricular node and accessory pathways. Circulation. 2003;107:2583-2588.
12 Borjesson M, Pelliccia A. Incidence and aetiology of sudden cardiac death in young athletes: an international perspective. Br J Sports Med. 2009;43:644-648.
13. Hoefman E, Boer KR, van Weert HCPM, et al. Predictive value of history taking and physical examination in diagnosing arrhythmias in general practice. Fam Pract. 2007;24:636-641.
14 Zimetbaum PJ, Kim KY, Josephson ME, et al. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med. 1998;128:890-895.
15. Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study: comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol. 2007;49:1951-1956.
16. Reiter MJ, Reiffel JA. Importance of beta blockade in the therapy of serious ventricular arrhythmias. Am J Cardiol. 1998;82:9I-19I.
17. Sheldon SH, Latchamsetty R, Morady F, et al. Catheter ablation in patients with pleomorphic, idiopathic, premature ventricular complexes. Heart Rhythm. 2017;14:1623-1628.
18. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e506-e574.
19. Alabed S, Sabouni A, Providencia R, et al. Adenosine versus intravenous calcium channel antagonists for supraventricular tachycardia. Cochrane Database Syst Rev. 2017;10:CD005154.
20. Smith GD, Fry MM, Taylor D, et al. Effectiveness of the Valsalva manoeuvre for reversion of supraventricular tachycardia. Cochrane Database Syst Rev. 2015;2015:CD009502.
21. Alboni P, Tomasi C, Menozzi C, et al. Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2001;37:548-553.
22. King DE, Dickerson LM, Sack JL. Acute management of atrial fibrillation: Part I. Rate and rhythm control. Am Fam Physician. 2002;66:249-256.
23. Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
24. Van Gelder IC, Groenveld HF, Crijns HJGM, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
25. Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
26. Lafuente-Lafuente C, Valembois L, Bergmann J-F, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;(3):CD005049.
27. Ramlawi B, Bedeir K. Surgical options in atrial fibrillation. J Thorac Dis. 2015;7:204-213.
28. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274.
29. Dooley P, Doolittle J, Knauss K, et al. Atrial fibrillation: effective strategies using the latest tools. J Fam Pract. 2017;66:16-26.
30. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):CD006186.
31. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
32. Reddy VY, Sievert H, Halperin J, et al; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
Palpitations, the sensory perception of one’s heartbeat, are reported in 16% of primary care patients, from causes that are both cardiac (ie, arrhythmias) and noncardiac.1 Palpitations are usually benign; overall mortality is approximately 1% annually. In fact, a retrospective study found no difference in mortality and morbidity between patients with palpitations and control patients without palpitations.2 However, palpitations can reflect a life-threatening cardiac condition, as we discuss in this article, making careful assessment and targeted, sometimes urgent, intervention important.3
Here, we review the clinical work-up of palpitations, recommended diagnostic testing, and the range of interventions for cardiac arrhythmias—ectopic beats, ventricular tachycardia (VT), and atrial fibrillation (AF).
Cardiac and noncardiac causes of palpitations
In a prospective cohort study of 190 consecutive patients presenting with palpitations, the cause was cardiac in 43%, psychiatric in 31%, and of a miscellaneous nature (including medication, thyrotoxicosis, caffeine, cocaine, anemia, amphetamine, and mastocytosis) in 10%; in 16%, the cause was undetermined.2 In this study, 77% of patients experienced a recurrence of palpitations after their first episode.2
Cardiac arrhythmias, a common cause of palpitations, are differentiated by site of origin—supraventricular and ventricular. Noncardiac causes of palpitations, which we do not discuss here, include metabolic and psychiatric conditions, medications, and substance use. (For a summary of the causes of palpitations, see TABLE 1.2-4)

Common complaint: ectopic beats. Premature atrial contractions (PACs; also known as premature atrial beats, atrial premature complexes, and atrial premature beats) and premature ventricular contractions (PVCs; also known as ventricular premature complexes and ventricular premature beats, and also of a variety of possible causes) result in a feeling of a skipped heartbeat or a flipping sensation in the chest.
The burden of PACs is independently associated with mortality, cardiovascular hospitalization, new-onset AF, and pacemaker implantation. In a multivariate analysis, a PAC burden > 76 beats/d was an independent predictor of mortality (hazard ratio [HR] = 1.4; 95% CI, 1.2-16); cardiovascular hospitalization (HR = 1.3; 95% CI, 1.1-1.5); new-onset AF (HR = 1.8; 95% CI, 1.4-2.2); and pacemaker implantation (HR = 2.8; 95% CI, 1.9-4.2). Frequent PACs can lead to cardiac remodeling, so more intense follow-up of patients with a high PAC burden might allow for early detection of AF or subclinical cardiac disease.5,6
A burden of PVCs > 24% is associated with an increased risk of PVC-induced cardiomyopathy and heart failure. Polymorphic PVCs are more concerning than monomorphic PVCs because the former suggests the presence of more diffuse, rather than localized, myocardial injury. The presence of frequent (> 1000 beats/d) PVCs warrants evaluation and treatment for underlying structural heart disease and ischemic heart disease. Therapy directed toward underlying heart disease can reduce the frequency of PVCs.7-9
Continue to: The diagnostic work-up
The diagnostic work-up
The most important goal of the evaluation of palpitations is to determine the presence, or risk, of structural heart or coronary artery disease (CAD) by means of the history, physical examination, and electrocardiography (EKG). Patients who have an increased risk of structural heart disease need further evaluation with echocardiography; those at increased risk of CAD should have stress testing.
Hemodynamically unstable patients need admission; patients who have a history of syncope with palpitations usually should be admitted for cardiac monitoring. Patients who have had a single episode of palpitations and have normal baseline results of laboratory testing and a normal EKG, and no risk factors for structural heart disease or known CAD, can usually be observed.3,4,10 Patients with an abnormal baseline EKG, recurrent palpitations (especially tachyarrhythmia), or significant symptoms during palpitations (syncope, presyncope, dyspnea) need further evaluation with ambulatory monitoring3,4,10 (Figure).

Take a thorough history; ask these questions
Have the patient describe the palpitations. The history should include the patient’s detailed characterization of the palpitations (sudden or gradual onset, rhythm, duration, frequency). Certain descriptions provide possible diagnostic clues:
- Palpitations lasting < 5 minutes are less likely to be of cardiac origin (likelihood ratio [LR] = 0.38; 95% CI, 0.2-0.6).4
- A patient who has a regular, rapid-pounding sensation in the neck has an increased probability of atrioventricular (AV) nodal reentrant tachycardia (AVNRT) (LR = 177; 95% CI, 25-1251); absence of this sensation decreases the likelihood of AVNRT (LR = 0.07; 95% CI, 0.03-0.2).4
- PACs and PVCs cause a sensation of a skipped heartbeat or a flipping sensation in the chest; they are not reported as a sustained rapid heartbeat.
- Patients with a supraventricular arrhythmia often report sudden onset and cessation of palpitations.
- Patients with palpitations since childhood are more likely to have supraventricular tachycardia (SVT).4
Elicit apparent precipitating and alleviating factors. The history should include notation of situations that appear to the patient to lead to palpitations (eg, context, positional variation). Palpitations that affect sleep (LR = 2.3; 95% CI, 1.3-3.9) and palpitations that occur at work (LR = 2.2; 95% CI, 1.3-5) increase the likelihood of a cardiac cause.4 Palpitations associated with sudden change in position, such as bending forward or squatting, are more likely due to AVNRT.11
Ask about aggravating factors (eg, exercise) and relieving factors (eg, rest, performing a Valsalva maneuver). Patients with SVT are often able to have palpitations terminated with a Valsalva maneuver, such as carotid sinus massage. Palpitations and syncope during exertion can be associated with hypertrophic cardiomyopathy, congenital coronary anomalies, and ion channelopathies, and can cause sudden cardiac death in athletes (estimated incidence, 1-3/100,000 person–years12).
Endeavor to identify underlying cardiac disease. A comprehensive history should also evaluate for risk factors and symptoms (chest pain, dyspnea, diaphoresis, lightheadedness, syncope) of cardiac disease, such as CAD, valvular disease, cardiomyopathy, and congenital heart disease, which increase the likelihood that the presenting complaint is a cardiac arrhythmia (LR = 2; 95% CI, 1.3-3.1).4 A history of syncope in a patient with palpitations should prompt evaluation for structural heart disease, such as aortic stenosis or hypertrophic cardiomyopathy, in which outflow-tract obstruction impairs cardiac output and, subsequently, cerebral blood flow.
Obtain additional key information. Determine the following in taking the history:
- Is there a family history of inherited cardiac disorders or sudden cardiac death?
- What prescription and over-the-counter medications is the patient taking? How does the patient characterize his or her use/intake of recreational drugs, nicotine, caffeine, and alcohol?
- Does the patient have a history of panic disorder, which lessens concern about a cardiac cause (LR = 0.2; 95% CI, 0.07-1.01)?4 (Of note: A nonpsychiatric cause can coexist in such patients, and should be considered.)
Continue to: Physical examination clues, and the utility of vagal maneuvers
Physical examination clues, and the utility of vagal maneuvers
Although most patients in whom palpitations are the presenting complaint are, in fact, asymptomatic during clinical assessment, cardiovascular examination can assist in diagnosing the arrhythmia or structural heart disease:
- Resting bradycardia increases the likelihood of a clinically significant arrhythmia (LR = 3; 95% CI, 1.27-7.0).11
- A murmur, such as a midsystolic click or holosystolic murmur, detected during the cardiac exam can indicate mitral valve prolapse; a holosystolic murmur, exacerbated upon performing a Valsalva maneuver, suggests hypertrophic cardiomyopathy.
- Visible neck pulsations detected during assessment of the jugular venous pressure, known as cannon atrial (cannon A) waves, reflect abnormal contraction of the right atrium against a closed tricuspid valve during AV dissociation. Cannon A waves have an LR of 2.68 (95% CI, 1.25-5.78) for predicting AVNRT.4
Vagal nerve stimulation. In the rare circumstance that a patient complaining of palpitations is symptomatic during assessment, several tachycardias can be detected with the use of vagal maneuvers. Interruption of the tachycardia during carotid massage suggests a tachycardia involving the AV junction (AVNRT), whereas only a temporary pause or reduction in frequency is more common in atrial flutter, AF, and atrial tachycardias. Carotid massage has no effect on the presentation of ventricular arrhythmias.10
Diagnostic testing and the role of ambulatory monitoring
Electrocardiography. All patients with palpitations should have a 12-lead EKG, which may provide diagnostic clues (TABLE 210).

Ambulatory monitoring. When the EKG is nondiagnostic, ambulatory cardiac monitoring has an established role in the diagnosis of recurrent palpitations. In a small study of patients presenting with palpitations to a general practitioner, the deduction of those practitioners was wrong more than half the time when they predicted a ≤ 20% chance of an arrhythmia based on the history, physical exam, and EKG alone13—emphasizing the importance of ambulatory monitoring in patients with recurrent palpitations.
Which monitoring system is most suitable depends on symptom frequency, availability, cost, and patient competence. Twenty-four- to 48-hour Holter monitoring can be used in cases of frequent (eg, daily) palpitations. An automatic external loop recorder can be used for less frequent (eg, every 30 days) symptoms. Most ambulatory EKG is now automatic, and therefore does not require patient activation; older manual systems require patient activation during symptoms.
Two weeks of ambulatory EKG have proved sufficient for determining that there is a cardiac basis to palpitations. The diagnostic yield of ambulatory EKG is highest during Week 1 (1.04 diagnoses per patient), compared to Week 3 (0.17 diagnoses per patient).14
Implantable loop recorders are placed subcutaneously to provide EKG monitoring for approximately 3 years. They are better suited for diagnosing infrequent palpitations. The diagnostic yield of an implantable loop recorder over the course of 1 year for the detection of an arrhythmia is 73%, compared to 21% for a 24-hour Holter monitor, electrophysiology studies, and 4 weeks of an external loop recorder.15 Implantable loop recorders are often reserved for patients with palpitations associated with unexplained recurrent syncope.15
Continue to: Lab work
Lab work. A comprehensive metabolic panel, complete blood count, lipid panel, and thyroid panel should be ordered for all patients with palpitations. Possible additional tests include a urine drug screen (when recreational drug use is suspected); cardiac enzymes; N-terminal-pro hormone B-type natriuretic peptide (when there is evidence of CAD or heart failure); and urinary catecholamines (when pheochromocytoma is suspected).
Other investigations. Echocardiography is indicated when structural heart disease is suspected (TABLE 12-4). Patients who have multiple risk factors for CAD or exertional symptoms might warrant a stress test.
Management
PACs and PVCs
Typically, patients are counseled to minimize potential adrenergic precipitants, such as smoking, alcohol, stress, and caffeine. However, limited studies have demonstrated no significant arrhythmogenic potential of a modest dose of caffeine (200 mg), even in patients with known life-threatening ventricular arrhythmias.16 Beta-blockers and nondihydropyridine calcium channel blockers (CCBs) can reduce the severity of symptoms related to premature ectopic beats and might reduce their frequency, although response is inconsistent. Use of these medications for PACs is largely based on expert opinion and extrapolated from use in other supraventricular and ventricular arrhythmias.
Implantable cardioverter defibrillator therapy is indicated in patients with nonsustained VT due to prior myocardial infarction, left ventricular ejection fraction ≤ 40%, and inducible ventricular fibrillation or sustained VT on electrophysiological study.7
Patients with a high burden of ectopy who do not respond to treatment with AV nodal-blocking agents should be referred to Cardiology for other antiarrhythmic agents or catheter ablation. Last, asymptomatic ectopy does not need to be treated; there is no clear evidence that suppression with pharmacotherapy improves overall survival.15,17
Supraventricular tachycardia
The priority when evaluating any tachycardia is to assess the patient’s stability. Unstable patients should be treated immediately, usually with cardioversion, before an extensive diagnostic evaluation.18 Patients with wide-complex tachycardia (QRS > 120 ms) are generally more unstable and require more urgent therapy and cardiac consultation or referral. Hemodynamically stable patients with narrow-complex SVT (QRS < 120 ms) can be treated with IV adenosine, which has an 89.7% success rate.18,19 If adenosine is unsuccessful, cardioversion is indicated.
Stable patients with minimal symptoms and short episodes do not need treatment.
Continue to: Vagal maneuvers
Vagal maneuvers (eg, Valsalva maneuver; unilateral carotid massage after exclusion of a carotid bruit, with head tilted to the side opposite the massage, and not for longer than 10 seconds; or applying an ice-cold wet towel to the face) have a success rate of about 25% and are most effective when performed shortly after onset of arrhythmia. Vagal maneuvers can be used in all patients while preparing to administer medications.20
Patients who need treatment can take the “pill-in-the-pocket” approach with single-dose oral flecainide (3 mg/kg) or combined diltiazem and propranolol. Flecainide has a 94% success rate; diltiazem–propranolol has a lower success rate (61%) but a shorter time to conversion to sinus rhythm.21 Patients with sustained or recurrent episodes of SVT should be referred to a cardiologist for chronic prophylactic drug therapy or radiofrequency ablation.
Atrial fibrillation
Hemodynamically unstable patients with AF or atrial flutter, defined by the presence of angina, decompensated heart failure, hypotension, pulmonary edema, or evidence of organ hypoperfusion, should be electrically cardioverted using synchronized direct current.
Hemodynamically stable patients with a rapid ventricular rate should be treated with an IV or oral beta-blocker, CCB, or amiodarone, or electrically cardioverted. IV medications are typically preferred in the acute setting for ease and rapidity of administration; however, there is no evidence that IV formulations of beta-blockers and CCBs are superior to oral formulations. Once the ventricular rate is controlled, patients can be transitioned to an oral short-acting preparation of the selected agent, then converted to an appropriate dosage of an extended-release preparation.22
Cardioversion can be performed in patients with AF < 48 hours. In patients with AF > 48 hours, either 4 weeks of anticoagulation can be given, followed by cardioversion, or transesophageal echocardiography should be performed to evaluate for atrial thrombus; if atrial thrombus is absent, cardioversion can be performed.22 Transesophageal echocardiography might be unnecessary in patients known to have been on sustained anticoagulation.
Rate control is noninferior to rhythm control and does not decrease survival, functional capacity, or quality of life. Rate-control medications include beta-blockers, nondihydropyridine CCBs, amiodarone, and digoxin.
In the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial of 4060 patients, mortality was the same with rhythm control (21.3%) and rate control (23.8%) (HR = 1.15; 95% CI, 0.99-1.34), with no difference in the incidence of cardiac death, arrhythmic death, or death due to stroke.23 In the RACE (RAte Control versus Electrical cardioversion for persistent atrial fibrillation) trial of 522 patients with persistent AF, rate control was noninferior to rhythm control (by cardioversion and drugs) for reducing morbidity and preventing cardiovascular death.24 One possible reason why the rhythm control strategy in the RACE trial did not show superiority is the low number of patients who achieved sustained sinus rhythm.25
Continue to: The recommended ventricular rate...
The recommended ventricular rate has traditionally been 60 to 80 beats/min at rest and < 110 beats/min during daily activities. However, a recent trial found fewer adverse outcomes and no change in symptoms or the outcome of hospitalization in patients randomized to more lenient control (target resting heart rate, < 110 beats/min), although the mean of the actual lenient rate achieved was 86 beats/minute.24
Rhythm control. Antiarrhythmic agents or procedural interventions can be used in patients who fail or remain symptomatic despite rate control.26 Surgical measures include AV node ablation with placement of a pacemaker; atrial pacing with an implantable atrial defibrillator; the Maze procedure (open-heart surgery) to interrupt reentrant circuits in the left atrium; and percutaneous radiofrequency or cryotherapy ablation of arrhythmogenic foci in and around the junction of the pulmonary veins and left atrium.27
There is no significant benefit to immediate catheter ablation over standard medical therapy in adults with symptomatic AF in reducing the composite outcome of death, stroke, serious bleeding, and cardiac arrest. Catheter ablation is associated with a lower AF recurrence rate (50%) than drug therapy (69%) at 3 years.28
Anticoagulation. Patients at high risk of embolic stroke based on their score on the CHA2DS2-VASca risk stratification tool (ie, a score ≥ 2) should be anticoagulated.29,30 Options include a novel oral anticoagulant (dabigatran, rivaroxaban, apixaban, or edoxaban), the preferred class of agents for nonvalvular AF, and warfarin, with a target International Normalized Ratio of 2 to 3. Novel oral anticoagulants have been compared to warfarin for prevention of stroke in AF and were found more effective than warfarin, although at the expense of an increased risk of gastrointestinal bleeding.31 Percutaneous left atrial appendage closure, using a device such as the Watchman implant, is a noninferior surgical method to prevent embolic stroke in patients who are intolerant of, or have a contraindication to, anticoagulation.32
CORRESPONDENCE
Anne Mounsey, MD, Department of Family Medicine, University of North Carolina, 590 Manning Drive, Chapel Hill, NC 27599; [email protected].
Palpitations, the sensory perception of one’s heartbeat, are reported in 16% of primary care patients, from causes that are both cardiac (ie, arrhythmias) and noncardiac.1 Palpitations are usually benign; overall mortality is approximately 1% annually. In fact, a retrospective study found no difference in mortality and morbidity between patients with palpitations and control patients without palpitations.2 However, palpitations can reflect a life-threatening cardiac condition, as we discuss in this article, making careful assessment and targeted, sometimes urgent, intervention important.3
Here, we review the clinical work-up of palpitations, recommended diagnostic testing, and the range of interventions for cardiac arrhythmias—ectopic beats, ventricular tachycardia (VT), and atrial fibrillation (AF).
Cardiac and noncardiac causes of palpitations
In a prospective cohort study of 190 consecutive patients presenting with palpitations, the cause was cardiac in 43%, psychiatric in 31%, and of a miscellaneous nature (including medication, thyrotoxicosis, caffeine, cocaine, anemia, amphetamine, and mastocytosis) in 10%; in 16%, the cause was undetermined.2 In this study, 77% of patients experienced a recurrence of palpitations after their first episode.2
Cardiac arrhythmias, a common cause of palpitations, are differentiated by site of origin—supraventricular and ventricular. Noncardiac causes of palpitations, which we do not discuss here, include metabolic and psychiatric conditions, medications, and substance use. (For a summary of the causes of palpitations, see TABLE 1.2-4)

Common complaint: ectopic beats. Premature atrial contractions (PACs; also known as premature atrial beats, atrial premature complexes, and atrial premature beats) and premature ventricular contractions (PVCs; also known as ventricular premature complexes and ventricular premature beats, and also of a variety of possible causes) result in a feeling of a skipped heartbeat or a flipping sensation in the chest.
The burden of PACs is independently associated with mortality, cardiovascular hospitalization, new-onset AF, and pacemaker implantation. In a multivariate analysis, a PAC burden > 76 beats/d was an independent predictor of mortality (hazard ratio [HR] = 1.4; 95% CI, 1.2-16); cardiovascular hospitalization (HR = 1.3; 95% CI, 1.1-1.5); new-onset AF (HR = 1.8; 95% CI, 1.4-2.2); and pacemaker implantation (HR = 2.8; 95% CI, 1.9-4.2). Frequent PACs can lead to cardiac remodeling, so more intense follow-up of patients with a high PAC burden might allow for early detection of AF or subclinical cardiac disease.5,6
A burden of PVCs > 24% is associated with an increased risk of PVC-induced cardiomyopathy and heart failure. Polymorphic PVCs are more concerning than monomorphic PVCs because the former suggests the presence of more diffuse, rather than localized, myocardial injury. The presence of frequent (> 1000 beats/d) PVCs warrants evaluation and treatment for underlying structural heart disease and ischemic heart disease. Therapy directed toward underlying heart disease can reduce the frequency of PVCs.7-9
Continue to: The diagnostic work-up
The diagnostic work-up
The most important goal of the evaluation of palpitations is to determine the presence, or risk, of structural heart or coronary artery disease (CAD) by means of the history, physical examination, and electrocardiography (EKG). Patients who have an increased risk of structural heart disease need further evaluation with echocardiography; those at increased risk of CAD should have stress testing.
Hemodynamically unstable patients need admission; patients who have a history of syncope with palpitations usually should be admitted for cardiac monitoring. Patients who have had a single episode of palpitations and have normal baseline results of laboratory testing and a normal EKG, and no risk factors for structural heart disease or known CAD, can usually be observed.3,4,10 Patients with an abnormal baseline EKG, recurrent palpitations (especially tachyarrhythmia), or significant symptoms during palpitations (syncope, presyncope, dyspnea) need further evaluation with ambulatory monitoring3,4,10 (Figure).

Take a thorough history; ask these questions
Have the patient describe the palpitations. The history should include the patient’s detailed characterization of the palpitations (sudden or gradual onset, rhythm, duration, frequency). Certain descriptions provide possible diagnostic clues:
- Palpitations lasting < 5 minutes are less likely to be of cardiac origin (likelihood ratio [LR] = 0.38; 95% CI, 0.2-0.6).4
- A patient who has a regular, rapid-pounding sensation in the neck has an increased probability of atrioventricular (AV) nodal reentrant tachycardia (AVNRT) (LR = 177; 95% CI, 25-1251); absence of this sensation decreases the likelihood of AVNRT (LR = 0.07; 95% CI, 0.03-0.2).4
- PACs and PVCs cause a sensation of a skipped heartbeat or a flipping sensation in the chest; they are not reported as a sustained rapid heartbeat.
- Patients with a supraventricular arrhythmia often report sudden onset and cessation of palpitations.
- Patients with palpitations since childhood are more likely to have supraventricular tachycardia (SVT).4
Elicit apparent precipitating and alleviating factors. The history should include notation of situations that appear to the patient to lead to palpitations (eg, context, positional variation). Palpitations that affect sleep (LR = 2.3; 95% CI, 1.3-3.9) and palpitations that occur at work (LR = 2.2; 95% CI, 1.3-5) increase the likelihood of a cardiac cause.4 Palpitations associated with sudden change in position, such as bending forward or squatting, are more likely due to AVNRT.11
Ask about aggravating factors (eg, exercise) and relieving factors (eg, rest, performing a Valsalva maneuver). Patients with SVT are often able to have palpitations terminated with a Valsalva maneuver, such as carotid sinus massage. Palpitations and syncope during exertion can be associated with hypertrophic cardiomyopathy, congenital coronary anomalies, and ion channelopathies, and can cause sudden cardiac death in athletes (estimated incidence, 1-3/100,000 person–years12).
Endeavor to identify underlying cardiac disease. A comprehensive history should also evaluate for risk factors and symptoms (chest pain, dyspnea, diaphoresis, lightheadedness, syncope) of cardiac disease, such as CAD, valvular disease, cardiomyopathy, and congenital heart disease, which increase the likelihood that the presenting complaint is a cardiac arrhythmia (LR = 2; 95% CI, 1.3-3.1).4 A history of syncope in a patient with palpitations should prompt evaluation for structural heart disease, such as aortic stenosis or hypertrophic cardiomyopathy, in which outflow-tract obstruction impairs cardiac output and, subsequently, cerebral blood flow.
Obtain additional key information. Determine the following in taking the history:
- Is there a family history of inherited cardiac disorders or sudden cardiac death?
- What prescription and over-the-counter medications is the patient taking? How does the patient characterize his or her use/intake of recreational drugs, nicotine, caffeine, and alcohol?
- Does the patient have a history of panic disorder, which lessens concern about a cardiac cause (LR = 0.2; 95% CI, 0.07-1.01)?4 (Of note: A nonpsychiatric cause can coexist in such patients, and should be considered.)
Continue to: Physical examination clues, and the utility of vagal maneuvers
Physical examination clues, and the utility of vagal maneuvers
Although most patients in whom palpitations are the presenting complaint are, in fact, asymptomatic during clinical assessment, cardiovascular examination can assist in diagnosing the arrhythmia or structural heart disease:
- Resting bradycardia increases the likelihood of a clinically significant arrhythmia (LR = 3; 95% CI, 1.27-7.0).11
- A murmur, such as a midsystolic click or holosystolic murmur, detected during the cardiac exam can indicate mitral valve prolapse; a holosystolic murmur, exacerbated upon performing a Valsalva maneuver, suggests hypertrophic cardiomyopathy.
- Visible neck pulsations detected during assessment of the jugular venous pressure, known as cannon atrial (cannon A) waves, reflect abnormal contraction of the right atrium against a closed tricuspid valve during AV dissociation. Cannon A waves have an LR of 2.68 (95% CI, 1.25-5.78) for predicting AVNRT.4
Vagal nerve stimulation. In the rare circumstance that a patient complaining of palpitations is symptomatic during assessment, several tachycardias can be detected with the use of vagal maneuvers. Interruption of the tachycardia during carotid massage suggests a tachycardia involving the AV junction (AVNRT), whereas only a temporary pause or reduction in frequency is more common in atrial flutter, AF, and atrial tachycardias. Carotid massage has no effect on the presentation of ventricular arrhythmias.10
Diagnostic testing and the role of ambulatory monitoring
Electrocardiography. All patients with palpitations should have a 12-lead EKG, which may provide diagnostic clues (TABLE 210).

Ambulatory monitoring. When the EKG is nondiagnostic, ambulatory cardiac monitoring has an established role in the diagnosis of recurrent palpitations. In a small study of patients presenting with palpitations to a general practitioner, the deduction of those practitioners was wrong more than half the time when they predicted a ≤ 20% chance of an arrhythmia based on the history, physical exam, and EKG alone13—emphasizing the importance of ambulatory monitoring in patients with recurrent palpitations.
Which monitoring system is most suitable depends on symptom frequency, availability, cost, and patient competence. Twenty-four- to 48-hour Holter monitoring can be used in cases of frequent (eg, daily) palpitations. An automatic external loop recorder can be used for less frequent (eg, every 30 days) symptoms. Most ambulatory EKG is now automatic, and therefore does not require patient activation; older manual systems require patient activation during symptoms.
Two weeks of ambulatory EKG have proved sufficient for determining that there is a cardiac basis to palpitations. The diagnostic yield of ambulatory EKG is highest during Week 1 (1.04 diagnoses per patient), compared to Week 3 (0.17 diagnoses per patient).14
Implantable loop recorders are placed subcutaneously to provide EKG monitoring for approximately 3 years. They are better suited for diagnosing infrequent palpitations. The diagnostic yield of an implantable loop recorder over the course of 1 year for the detection of an arrhythmia is 73%, compared to 21% for a 24-hour Holter monitor, electrophysiology studies, and 4 weeks of an external loop recorder.15 Implantable loop recorders are often reserved for patients with palpitations associated with unexplained recurrent syncope.15
Continue to: Lab work
Lab work. A comprehensive metabolic panel, complete blood count, lipid panel, and thyroid panel should be ordered for all patients with palpitations. Possible additional tests include a urine drug screen (when recreational drug use is suspected); cardiac enzymes; N-terminal-pro hormone B-type natriuretic peptide (when there is evidence of CAD or heart failure); and urinary catecholamines (when pheochromocytoma is suspected).
Other investigations. Echocardiography is indicated when structural heart disease is suspected (TABLE 12-4). Patients who have multiple risk factors for CAD or exertional symptoms might warrant a stress test.
Management
PACs and PVCs
Typically, patients are counseled to minimize potential adrenergic precipitants, such as smoking, alcohol, stress, and caffeine. However, limited studies have demonstrated no significant arrhythmogenic potential of a modest dose of caffeine (200 mg), even in patients with known life-threatening ventricular arrhythmias.16 Beta-blockers and nondihydropyridine calcium channel blockers (CCBs) can reduce the severity of symptoms related to premature ectopic beats and might reduce their frequency, although response is inconsistent. Use of these medications for PACs is largely based on expert opinion and extrapolated from use in other supraventricular and ventricular arrhythmias.
Implantable cardioverter defibrillator therapy is indicated in patients with nonsustained VT due to prior myocardial infarction, left ventricular ejection fraction ≤ 40%, and inducible ventricular fibrillation or sustained VT on electrophysiological study.7
Patients with a high burden of ectopy who do not respond to treatment with AV nodal-blocking agents should be referred to Cardiology for other antiarrhythmic agents or catheter ablation. Last, asymptomatic ectopy does not need to be treated; there is no clear evidence that suppression with pharmacotherapy improves overall survival.15,17
Supraventricular tachycardia
The priority when evaluating any tachycardia is to assess the patient’s stability. Unstable patients should be treated immediately, usually with cardioversion, before an extensive diagnostic evaluation.18 Patients with wide-complex tachycardia (QRS > 120 ms) are generally more unstable and require more urgent therapy and cardiac consultation or referral. Hemodynamically stable patients with narrow-complex SVT (QRS < 120 ms) can be treated with IV adenosine, which has an 89.7% success rate.18,19 If adenosine is unsuccessful, cardioversion is indicated.
Stable patients with minimal symptoms and short episodes do not need treatment.
Continue to: Vagal maneuvers
Vagal maneuvers (eg, Valsalva maneuver; unilateral carotid massage after exclusion of a carotid bruit, with head tilted to the side opposite the massage, and not for longer than 10 seconds; or applying an ice-cold wet towel to the face) have a success rate of about 25% and are most effective when performed shortly after onset of arrhythmia. Vagal maneuvers can be used in all patients while preparing to administer medications.20
Patients who need treatment can take the “pill-in-the-pocket” approach with single-dose oral flecainide (3 mg/kg) or combined diltiazem and propranolol. Flecainide has a 94% success rate; diltiazem–propranolol has a lower success rate (61%) but a shorter time to conversion to sinus rhythm.21 Patients with sustained or recurrent episodes of SVT should be referred to a cardiologist for chronic prophylactic drug therapy or radiofrequency ablation.
Atrial fibrillation
Hemodynamically unstable patients with AF or atrial flutter, defined by the presence of angina, decompensated heart failure, hypotension, pulmonary edema, or evidence of organ hypoperfusion, should be electrically cardioverted using synchronized direct current.
Hemodynamically stable patients with a rapid ventricular rate should be treated with an IV or oral beta-blocker, CCB, or amiodarone, or electrically cardioverted. IV medications are typically preferred in the acute setting for ease and rapidity of administration; however, there is no evidence that IV formulations of beta-blockers and CCBs are superior to oral formulations. Once the ventricular rate is controlled, patients can be transitioned to an oral short-acting preparation of the selected agent, then converted to an appropriate dosage of an extended-release preparation.22
Cardioversion can be performed in patients with AF < 48 hours. In patients with AF > 48 hours, either 4 weeks of anticoagulation can be given, followed by cardioversion, or transesophageal echocardiography should be performed to evaluate for atrial thrombus; if atrial thrombus is absent, cardioversion can be performed.22 Transesophageal echocardiography might be unnecessary in patients known to have been on sustained anticoagulation.
Rate control is noninferior to rhythm control and does not decrease survival, functional capacity, or quality of life. Rate-control medications include beta-blockers, nondihydropyridine CCBs, amiodarone, and digoxin.
In the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial of 4060 patients, mortality was the same with rhythm control (21.3%) and rate control (23.8%) (HR = 1.15; 95% CI, 0.99-1.34), with no difference in the incidence of cardiac death, arrhythmic death, or death due to stroke.23 In the RACE (RAte Control versus Electrical cardioversion for persistent atrial fibrillation) trial of 522 patients with persistent AF, rate control was noninferior to rhythm control (by cardioversion and drugs) for reducing morbidity and preventing cardiovascular death.24 One possible reason why the rhythm control strategy in the RACE trial did not show superiority is the low number of patients who achieved sustained sinus rhythm.25
Continue to: The recommended ventricular rate...
The recommended ventricular rate has traditionally been 60 to 80 beats/min at rest and < 110 beats/min during daily activities. However, a recent trial found fewer adverse outcomes and no change in symptoms or the outcome of hospitalization in patients randomized to more lenient control (target resting heart rate, < 110 beats/min), although the mean of the actual lenient rate achieved was 86 beats/minute.24
Rhythm control. Antiarrhythmic agents or procedural interventions can be used in patients who fail or remain symptomatic despite rate control.26 Surgical measures include AV node ablation with placement of a pacemaker; atrial pacing with an implantable atrial defibrillator; the Maze procedure (open-heart surgery) to interrupt reentrant circuits in the left atrium; and percutaneous radiofrequency or cryotherapy ablation of arrhythmogenic foci in and around the junction of the pulmonary veins and left atrium.27
There is no significant benefit to immediate catheter ablation over standard medical therapy in adults with symptomatic AF in reducing the composite outcome of death, stroke, serious bleeding, and cardiac arrest. Catheter ablation is associated with a lower AF recurrence rate (50%) than drug therapy (69%) at 3 years.28
Anticoagulation. Patients at high risk of embolic stroke based on their score on the CHA2DS2-VASca risk stratification tool (ie, a score ≥ 2) should be anticoagulated.29,30 Options include a novel oral anticoagulant (dabigatran, rivaroxaban, apixaban, or edoxaban), the preferred class of agents for nonvalvular AF, and warfarin, with a target International Normalized Ratio of 2 to 3. Novel oral anticoagulants have been compared to warfarin for prevention of stroke in AF and were found more effective than warfarin, although at the expense of an increased risk of gastrointestinal bleeding.31 Percutaneous left atrial appendage closure, using a device such as the Watchman implant, is a noninferior surgical method to prevent embolic stroke in patients who are intolerant of, or have a contraindication to, anticoagulation.32
CORRESPONDENCE
Anne Mounsey, MD, Department of Family Medicine, University of North Carolina, 590 Manning Drive, Chapel Hill, NC 27599; [email protected].
1. Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685-1689.
2. Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. 1996;100:138-148.
3. Giada F, Raviele A. Clinical approach to patients with palpitations. Card Electrophysiol Clin. 2018;10:387-396.
4. Thavendiranathan P, Bagai A, Khoo C, et al. Does this patient with palpitations have a cardiac arrhythmia? JAMA. 2009;302:2135-2143.
5. Lin C-Y, Lin Y-J, Chen Y-Y, et al. Prognostic significance of premature atrial complexes burden in prediction of long-term outcome. J Am Heart Assoc. 2015;4:e002192.
6. Murakoshi N, Xu D, Sairenchi T, et al. Prognostic impact of supraventricular premature complexes in community-based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J. 2015;36:170-178.
7. Ahn M-S. Current concepts of premature ventricular contractions. J Lifestyle Med. 2013;3:26-33.
8. Panizo JG, Barra S, Mellor G, et al. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. 2018;7:128-134.
9. Ng GA. Treating patients with ventricular ectopic beats. Heart. 2006;92:1707-1712.
10 Raviele A, Giada F, Bergfeldt L, et al; European Heart Rhythm Association. Management of patients with palpitations: a position paper from the European Heart Rhythm Association. Europace. 2011;13:920-934.
11. Chiou C-W, Chen S-A, Kung M-H, et al. Effects of continuous enhanced vagal tone on dual atrioventricular node and accessory pathways. Circulation. 2003;107:2583-2588.
12 Borjesson M, Pelliccia A. Incidence and aetiology of sudden cardiac death in young athletes: an international perspective. Br J Sports Med. 2009;43:644-648.
13. Hoefman E, Boer KR, van Weert HCPM, et al. Predictive value of history taking and physical examination in diagnosing arrhythmias in general practice. Fam Pract. 2007;24:636-641.
14 Zimetbaum PJ, Kim KY, Josephson ME, et al. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med. 1998;128:890-895.
15. Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study: comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol. 2007;49:1951-1956.
16. Reiter MJ, Reiffel JA. Importance of beta blockade in the therapy of serious ventricular arrhythmias. Am J Cardiol. 1998;82:9I-19I.
17. Sheldon SH, Latchamsetty R, Morady F, et al. Catheter ablation in patients with pleomorphic, idiopathic, premature ventricular complexes. Heart Rhythm. 2017;14:1623-1628.
18. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e506-e574.
19. Alabed S, Sabouni A, Providencia R, et al. Adenosine versus intravenous calcium channel antagonists for supraventricular tachycardia. Cochrane Database Syst Rev. 2017;10:CD005154.
20. Smith GD, Fry MM, Taylor D, et al. Effectiveness of the Valsalva manoeuvre for reversion of supraventricular tachycardia. Cochrane Database Syst Rev. 2015;2015:CD009502.
21. Alboni P, Tomasi C, Menozzi C, et al. Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2001;37:548-553.
22. King DE, Dickerson LM, Sack JL. Acute management of atrial fibrillation: Part I. Rate and rhythm control. Am Fam Physician. 2002;66:249-256.
23. Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
24. Van Gelder IC, Groenveld HF, Crijns HJGM, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
25. Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
26. Lafuente-Lafuente C, Valembois L, Bergmann J-F, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;(3):CD005049.
27. Ramlawi B, Bedeir K. Surgical options in atrial fibrillation. J Thorac Dis. 2015;7:204-213.
28. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274.
29. Dooley P, Doolittle J, Knauss K, et al. Atrial fibrillation: effective strategies using the latest tools. J Fam Pract. 2017;66:16-26.
30. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):CD006186.
31. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
32. Reddy VY, Sievert H, Halperin J, et al; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
1. Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685-1689.
2. Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. 1996;100:138-148.
3. Giada F, Raviele A. Clinical approach to patients with palpitations. Card Electrophysiol Clin. 2018;10:387-396.
4. Thavendiranathan P, Bagai A, Khoo C, et al. Does this patient with palpitations have a cardiac arrhythmia? JAMA. 2009;302:2135-2143.
5. Lin C-Y, Lin Y-J, Chen Y-Y, et al. Prognostic significance of premature atrial complexes burden in prediction of long-term outcome. J Am Heart Assoc. 2015;4:e002192.
6. Murakoshi N, Xu D, Sairenchi T, et al. Prognostic impact of supraventricular premature complexes in community-based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J. 2015;36:170-178.
7. Ahn M-S. Current concepts of premature ventricular contractions. J Lifestyle Med. 2013;3:26-33.
8. Panizo JG, Barra S, Mellor G, et al. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. 2018;7:128-134.
9. Ng GA. Treating patients with ventricular ectopic beats. Heart. 2006;92:1707-1712.
10 Raviele A, Giada F, Bergfeldt L, et al; European Heart Rhythm Association. Management of patients with palpitations: a position paper from the European Heart Rhythm Association. Europace. 2011;13:920-934.
11. Chiou C-W, Chen S-A, Kung M-H, et al. Effects of continuous enhanced vagal tone on dual atrioventricular node and accessory pathways. Circulation. 2003;107:2583-2588.
12 Borjesson M, Pelliccia A. Incidence and aetiology of sudden cardiac death in young athletes: an international perspective. Br J Sports Med. 2009;43:644-648.
13. Hoefman E, Boer KR, van Weert HCPM, et al. Predictive value of history taking and physical examination in diagnosing arrhythmias in general practice. Fam Pract. 2007;24:636-641.
14 Zimetbaum PJ, Kim KY, Josephson ME, et al. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med. 1998;128:890-895.
15. Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study: comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol. 2007;49:1951-1956.
16. Reiter MJ, Reiffel JA. Importance of beta blockade in the therapy of serious ventricular arrhythmias. Am J Cardiol. 1998;82:9I-19I.
17. Sheldon SH, Latchamsetty R, Morady F, et al. Catheter ablation in patients with pleomorphic, idiopathic, premature ventricular complexes. Heart Rhythm. 2017;14:1623-1628.
18. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e506-e574.
19. Alabed S, Sabouni A, Providencia R, et al. Adenosine versus intravenous calcium channel antagonists for supraventricular tachycardia. Cochrane Database Syst Rev. 2017;10:CD005154.
20. Smith GD, Fry MM, Taylor D, et al. Effectiveness of the Valsalva manoeuvre for reversion of supraventricular tachycardia. Cochrane Database Syst Rev. 2015;2015:CD009502.
21. Alboni P, Tomasi C, Menozzi C, et al. Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2001;37:548-553.
22. King DE, Dickerson LM, Sack JL. Acute management of atrial fibrillation: Part I. Rate and rhythm control. Am Fam Physician. 2002;66:249-256.
23. Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
24. Van Gelder IC, Groenveld HF, Crijns HJGM, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
25. Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
26. Lafuente-Lafuente C, Valembois L, Bergmann J-F, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;(3):CD005049.
27. Ramlawi B, Bedeir K. Surgical options in atrial fibrillation. J Thorac Dis. 2015;7:204-213.
28. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274.
29. Dooley P, Doolittle J, Knauss K, et al. Atrial fibrillation: effective strategies using the latest tools. J Fam Pract. 2017;66:16-26.
30. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):CD006186.
31. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
32. Reddy VY, Sievert H, Halperin J, et al; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
PRACTICE RECOMMENDATIONS
› Order echocardiography for patients who have palpitations and risk factors for structural heart disease. C
› Order stress testing for patients who have exertional symptoms or multiple risk factors for coronary artery disease. C
› Evaluate all patients who have syncope associated with their palpitations for a cardiac cause. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Happy National Hospitalist Day!
Hospitalists across the United States have been and continue to be a critical part of our nation’s response to COVID-19. On National Hospitalist Day, Thursday, March 4, 2021, the Society of Hospital Medicine invites you to celebrate the individuals and teams that make up the hospital medicine community.

On this special day, SHM encourages you to share your story, showcase your team’s efforts to improve patient care, express your pride for the specialty, or share how you are making a difference in your hospital and in the lives of patients.
Here are just a few of the ways you can celebrate:
- Register for our live roundtable, featuring Mark Shapiro, MD, hospitalist and host of the Explore the Space podcast, and four hospitalist panelists, on March 4 at 7 p.m. ET/4 p.m. PT.
- Download shareable graphics, posters, Zoom backgrounds, and coloring book pages
- Enter our social media photo contest and follow the #HowWeHospitalist hashtag across all platforms
- Read special hospitalist profiles in the Hospitalist, including: Eric E. Howell, MD, MHM; Grace Huang, MD; Bridget McGrath, PA-C, FHM; and Harry Cho, MD, SFHM
Thank you for all you do and continue to do for hospital medicine. We hope you take some time today to celebrate you and your colleagues, as well as your commendable contributions to health care and the future of the specialty.
To learn more about National Hospitalist Day, visit hospitalmedicine.org/hospitalistday.
Hospitalists across the United States have been and continue to be a critical part of our nation’s response to COVID-19. On National Hospitalist Day, Thursday, March 4, 2021, the Society of Hospital Medicine invites you to celebrate the individuals and teams that make up the hospital medicine community.

On this special day, SHM encourages you to share your story, showcase your team’s efforts to improve patient care, express your pride for the specialty, or share how you are making a difference in your hospital and in the lives of patients.
Here are just a few of the ways you can celebrate:
- Register for our live roundtable, featuring Mark Shapiro, MD, hospitalist and host of the Explore the Space podcast, and four hospitalist panelists, on March 4 at 7 p.m. ET/4 p.m. PT.
- Download shareable graphics, posters, Zoom backgrounds, and coloring book pages
- Enter our social media photo contest and follow the #HowWeHospitalist hashtag across all platforms
- Read special hospitalist profiles in the Hospitalist, including: Eric E. Howell, MD, MHM; Grace Huang, MD; Bridget McGrath, PA-C, FHM; and Harry Cho, MD, SFHM
Thank you for all you do and continue to do for hospital medicine. We hope you take some time today to celebrate you and your colleagues, as well as your commendable contributions to health care and the future of the specialty.
To learn more about National Hospitalist Day, visit hospitalmedicine.org/hospitalistday.
Hospitalists across the United States have been and continue to be a critical part of our nation’s response to COVID-19. On National Hospitalist Day, Thursday, March 4, 2021, the Society of Hospital Medicine invites you to celebrate the individuals and teams that make up the hospital medicine community.

On this special day, SHM encourages you to share your story, showcase your team’s efforts to improve patient care, express your pride for the specialty, or share how you are making a difference in your hospital and in the lives of patients.
Here are just a few of the ways you can celebrate:
- Register for our live roundtable, featuring Mark Shapiro, MD, hospitalist and host of the Explore the Space podcast, and four hospitalist panelists, on March 4 at 7 p.m. ET/4 p.m. PT.
- Download shareable graphics, posters, Zoom backgrounds, and coloring book pages
- Enter our social media photo contest and follow the #HowWeHospitalist hashtag across all platforms
- Read special hospitalist profiles in the Hospitalist, including: Eric E. Howell, MD, MHM; Grace Huang, MD; Bridget McGrath, PA-C, FHM; and Harry Cho, MD, SFHM
Thank you for all you do and continue to do for hospital medicine. We hope you take some time today to celebrate you and your colleagues, as well as your commendable contributions to health care and the future of the specialty.
To learn more about National Hospitalist Day, visit hospitalmedicine.org/hospitalistday.
More competition for docs as insurers boost new telehealth plans?
Initially, the service will be part of some employer-sponsored insurance plans in 11 states. United intends to expand its footprint next year.
United is using the platform and the medical group of American Well, a telehealth service, to provide virtual primary care. Besides minor acute care, United’s virtual service covers annual wellness visits, routine follow-ups for chronic conditions, lab tests, and specialist referrals with little or no cost sharing.
The giant insurer is now offering its virtual primary care plan in Arizona, Colorado, Illinois, Maryland, North Carolina, Ohio, South Carolina, Texas, Virginia, Washington, D.C., and West Virginia.
Other insurers are offering similar virtual primary care plans. For example, Humana has partnered with Doctor on Demand, and Cigna is working with MDLive to offer virtual primary care plans. Both of these plans encourage consumers to form ongoing relationships with physicians hired by the telehealth services. Similarly, Harvard Pilgrim, which has also joined with Doctor on Demand, said that consumers get “virtual PCPs” along with a full care team.
Humana has priced the premiums for its virtual service at about half the cost of Humana’s most popular traditional plan. There are no copays for telehealth visits; there are $5 copays for common lab tests and prescriptions. Cigna said that its virtual plan makes coverage “more affordable,” but doesn’t provide any specifics.
According to United spokeswoman Maria Shydlo, the insurer’s virtual primary care service is not cheaper than its traditional products.
Increased telehealth adoption
When the COVID-19 pandemic first struck last year, telehealth was a lifesaver for primary care practices. Physicians were able to treat half or more of their patients through telehealth, including video and phone consultations.
That initial romance with telehealth did not last. Today, telehealth represents 9% of adult primary care visits. However, that’s still a much higher percentage than before 2020, and telehealth has become a fixture of primary care.
Prior to the pandemic, telehealth services dominated the virtual care space. Some large groups experimented with having their doctors conduct virtual consults with their patients. Other physicians dabbled with telehealth or stayed out of it entirely because health plans paid much less for virtual visits than for in-person visits.
That began to change as more and more states passed laws requiring payment parity. (Today, 36 states do.) Then as the pandemic took hold, Medicare loosened its regulations, allowing coverage of telehealth everywhere and establishing parity. But it’s unclear what will happen after the public health emergency ends.
United and other insurers portray their virtual primary care plans as an effort to connect more consumers with primary care physicians. Having a relationship with a primary care doctor, United noted in a press release, increases access to care, including preventive services. Moreover, a United survey found that a quarter of respondents preferred a virtual relationship with a primary care doctor.
Physician have mostly positive but mixed reactions
This news organization interviewed several physicians who practice in states where United has introduced its new offering. Only one doctor had heard about it, and another, solo family physician Will Sawyer, MD, of Cincinnati no longer contracts with United. Nevertheless, they all had strong opinions about virtual primary care plans from United and other insurers.
Dr. Sawyer is a big proponent of telehealth and notes that it’s “incredibly convenient” for older people, many of whom are afraid to come to the office out of fear they might contract COVID-19. He has found that telehealth can be useful for many kinds of acute and chronic care. But he believes (although he admits he does not have evidence) that United started its virtual primary care service mainly to save money.
Peter Basch, MD, an internist with MedStar Health in Washington, D.C., says he’s willing to give United the benefit of the doubt. Increasing access to care while lowering its cost, he says, is the right thing to do, and “it makes financial sense. So I wouldn’t question their motives.”
Dr. Basch is concerned, however, that insurers such as United might eventually cover some services virtually but not in the office. “I can imagine a situation where doctors feel their judgment is being disregarded and that this person really needs to come in. And there might be pressure from the employer or the manager of the medical group, telling the doctor that if you’re not careful about how you manage these visits, you may be losing money for the practice.”
Kenneth Kubitschek, MD, an internist in a medium-sized group in Asheville, N.C., was less enamored of telehealth than Dr. Basch and Dr. Sawyer are, although it currently accounts for 15%-20% of his group’s visits. “There’s definitely something you lose with telehealth in terms of the nuances of the interaction.”
No to some kinds of telehealth doctors
The physicians we spoke with were unified in their opposition to virtual primary care plans that mainly use physicians hired by telehealth services. Dr. Sawyer noted that one-off consultations with telehealth doctors might be okay for urgent care. “But what we’re trying to do with patients is change their behavior for better health outcomes, and that doesn’t happen in these one-off contacts,” he said.
Even if a patient were able to develop an online relationship with a telehealth doctor, Dr. Basch said, there are any number of situations in which an in-person visit might be necessary. “Whether it’s a urologic visit, a cardiac visit, or an allergy visit, do I need to listen to you or put my hands on you to palpate your liver? Or is this just a conversation with someone I know to see how they’re doing, how they’re managing their meds? Ninety percent of a diagnosis is history.”
Although the virtual plans allow a telehealth physician to refer a patient to an in-network specialist for an office visit, this isn’t the same as their primary care physician asking them to come in to be examined.
Moreover, Dr. Basch noted, people with chronic conditions can’t be treated only virtually. “I wouldn’t say that primary care should be done predominantly through virtual visits. It may be okay for young and healthy patients, but not for older people with chronic conditions. There are times when they should see their doctor in person.”
What can be done via telehealth
On the other hand, Dr. Basch heartily approves of conducting routine follow-up visits virtually for patients with chronic diseases, as long as the physician knows the patient’s history. Telehealth can also be used to coach patients on exercise, nutrition, and other lifestyle changes.
Dr. Kubitschek estimates that around 40%-50% of primary care can be delivered through telehealth. But the remainder encompasses potentially serious conditions that should be diagnosed and treated in face-to-face encounters, he said. “For example, if a patient has abdominal pain, you have to examine the person to get a clue of what they’re talking about. The pains are often diffuse, but they might be painful locally, which could indicate a mass or a bladder distension.”
For that reason, he doesn’t support the idea of patients depending on telehealth doctors in virtual primary care plans. “These doctors would not be available to care for the patient in an urgent situation without sending them to a costly emergency room or urgent care clinic. In those settings, excess testing is done because of a lack of familiarity with the patient and his or her history and exam. I think a combination of in-person and telehealth visits presents the best circumstance for the patient and the physician. Having said that, I do believe that telehealth alone is better than no interaction with a health care provider.”
United approach can help with prevention
Donny Aga, MD, an internist with Kelsey-Seybold, a multispecialty group in Houston, has been a member of United’s virtual health advisory group for the past 2 years. In his view, United’s virtual primary care service is moving in the right direction by covering preventive and chronic care. Noting that 25%-30% of patients nationally have put off wellness and chronic care visits out of fear of COVID-19, he said that,“if health plans like United are willing to cover preventive services through telehealth, that will allow us to catch up on a lot of the needed screening tests and exams. So it’s a very positive step forward.”
On the other hand, he said, virtual plans that depend solely on telehealth doctors are not the way to manage chronic conditions. “Primary care is best done by your own primary care physician, not by someone who doesn’t know you from a distance.”
Regarding the virtual plans in which patients can establish relationships with telehealth physicians, Dr. Aga said that this approach can benefit some patients, especially those who live in rural areas and don’t have access to primary care. But there are drawbacks, including the telehealth providers’ lack of knowledge about local specialists.
“The negative is that you don’t have a [primary care physicians] who’s local, who knows you, who has examined you before, and who has a good relationship with those specialists and knows who is the right specialist to see for your problem,” Dr. Aga said. “It’s very difficult, if you don’t live and work in that area, to know the best places to send people.”
Virtual visits cost less
Like Dr. Basch, Dr. Aga said it’s possible that some insurance companies might begin to cover office visits only for certain conditions or services if they can be managed more cheaply via telehealth. He hopes that doesn’t happen; if it does, he predicts that patients and doctors will push back hard.
Why would a virtual primary care visit cost a health plan less than an in-person visit if it’s paying doctors the same for both? Dr. Aga said it’s because fewer prescriptions and lab tests are ordered in telehealth encounters. He bases this assertion on the quarter of a million virtual visits that Kelsey-Seybold has conducted and also alludes to published studies.
The characteristics of telehealth visits might explain this phenomenon, he said. “These visits are typically much shorter, and it’s easy to be problem-centric and problem based. Physicians use more of their intuitive skills, rather than just lab everybody up and get an x-ray, because that patient’s not there, and it’s easier to draw blood or get an x-ray if somebody is there.”
Cutting practice overhead
From the perspective of Kelsey-Seybold, which is now conducting about a fifth of its visits virtually, “infrastructure costs are less” for telehealth, Aga notes. Although Dr. Kubitschek and Dr. Sawyer say it doesn’t take less time to conduct a telehealth visit than an office visit, other practice costs may decrease in relationship to the percentage of a doctor’s visits that are virtual.
“If implemented appropriately, telehealth consults should cost less in terms of the ancillary costs surrounding care,” said Dr. Basch. He recalls that, some years ago, a five-doctor primary care group in Portland, Ore., began charging small monthly fees to patients for full-service care that included email access. After a while, 40% of their patients were coming in, and the rest received care by email or phone. As a result, the doctors were able to downsize to a smaller office space because they didn’t need a waiting room.
Although Dr. Basch doesn’t believe it would be appropriate for practices to do something like this in the midst of a pandemic, he sees the possibility of it happening in the future. “Eventually, a group might be able to say: ‘Yes, our practice expenses can be lower if we do this smartly. We could do as well as we’ve done on whatever insurance pays for office visits, knowing that we can deliver care to the same patient panel at, say, 10% lower overhead with telehealth.’ ”
A version of this article first appeared on Medscape.com.
Initially, the service will be part of some employer-sponsored insurance plans in 11 states. United intends to expand its footprint next year.
United is using the platform and the medical group of American Well, a telehealth service, to provide virtual primary care. Besides minor acute care, United’s virtual service covers annual wellness visits, routine follow-ups for chronic conditions, lab tests, and specialist referrals with little or no cost sharing.
The giant insurer is now offering its virtual primary care plan in Arizona, Colorado, Illinois, Maryland, North Carolina, Ohio, South Carolina, Texas, Virginia, Washington, D.C., and West Virginia.
Other insurers are offering similar virtual primary care plans. For example, Humana has partnered with Doctor on Demand, and Cigna is working with MDLive to offer virtual primary care plans. Both of these plans encourage consumers to form ongoing relationships with physicians hired by the telehealth services. Similarly, Harvard Pilgrim, which has also joined with Doctor on Demand, said that consumers get “virtual PCPs” along with a full care team.
Humana has priced the premiums for its virtual service at about half the cost of Humana’s most popular traditional plan. There are no copays for telehealth visits; there are $5 copays for common lab tests and prescriptions. Cigna said that its virtual plan makes coverage “more affordable,” but doesn’t provide any specifics.
According to United spokeswoman Maria Shydlo, the insurer’s virtual primary care service is not cheaper than its traditional products.
Increased telehealth adoption
When the COVID-19 pandemic first struck last year, telehealth was a lifesaver for primary care practices. Physicians were able to treat half or more of their patients through telehealth, including video and phone consultations.
That initial romance with telehealth did not last. Today, telehealth represents 9% of adult primary care visits. However, that’s still a much higher percentage than before 2020, and telehealth has become a fixture of primary care.
Prior to the pandemic, telehealth services dominated the virtual care space. Some large groups experimented with having their doctors conduct virtual consults with their patients. Other physicians dabbled with telehealth or stayed out of it entirely because health plans paid much less for virtual visits than for in-person visits.
That began to change as more and more states passed laws requiring payment parity. (Today, 36 states do.) Then as the pandemic took hold, Medicare loosened its regulations, allowing coverage of telehealth everywhere and establishing parity. But it’s unclear what will happen after the public health emergency ends.
United and other insurers portray their virtual primary care plans as an effort to connect more consumers with primary care physicians. Having a relationship with a primary care doctor, United noted in a press release, increases access to care, including preventive services. Moreover, a United survey found that a quarter of respondents preferred a virtual relationship with a primary care doctor.
Physician have mostly positive but mixed reactions
This news organization interviewed several physicians who practice in states where United has introduced its new offering. Only one doctor had heard about it, and another, solo family physician Will Sawyer, MD, of Cincinnati no longer contracts with United. Nevertheless, they all had strong opinions about virtual primary care plans from United and other insurers.
Dr. Sawyer is a big proponent of telehealth and notes that it’s “incredibly convenient” for older people, many of whom are afraid to come to the office out of fear they might contract COVID-19. He has found that telehealth can be useful for many kinds of acute and chronic care. But he believes (although he admits he does not have evidence) that United started its virtual primary care service mainly to save money.
Peter Basch, MD, an internist with MedStar Health in Washington, D.C., says he’s willing to give United the benefit of the doubt. Increasing access to care while lowering its cost, he says, is the right thing to do, and “it makes financial sense. So I wouldn’t question their motives.”
Dr. Basch is concerned, however, that insurers such as United might eventually cover some services virtually but not in the office. “I can imagine a situation where doctors feel their judgment is being disregarded and that this person really needs to come in. And there might be pressure from the employer or the manager of the medical group, telling the doctor that if you’re not careful about how you manage these visits, you may be losing money for the practice.”
Kenneth Kubitschek, MD, an internist in a medium-sized group in Asheville, N.C., was less enamored of telehealth than Dr. Basch and Dr. Sawyer are, although it currently accounts for 15%-20% of his group’s visits. “There’s definitely something you lose with telehealth in terms of the nuances of the interaction.”
No to some kinds of telehealth doctors
The physicians we spoke with were unified in their opposition to virtual primary care plans that mainly use physicians hired by telehealth services. Dr. Sawyer noted that one-off consultations with telehealth doctors might be okay for urgent care. “But what we’re trying to do with patients is change their behavior for better health outcomes, and that doesn’t happen in these one-off contacts,” he said.
Even if a patient were able to develop an online relationship with a telehealth doctor, Dr. Basch said, there are any number of situations in which an in-person visit might be necessary. “Whether it’s a urologic visit, a cardiac visit, or an allergy visit, do I need to listen to you or put my hands on you to palpate your liver? Or is this just a conversation with someone I know to see how they’re doing, how they’re managing their meds? Ninety percent of a diagnosis is history.”
Although the virtual plans allow a telehealth physician to refer a patient to an in-network specialist for an office visit, this isn’t the same as their primary care physician asking them to come in to be examined.
Moreover, Dr. Basch noted, people with chronic conditions can’t be treated only virtually. “I wouldn’t say that primary care should be done predominantly through virtual visits. It may be okay for young and healthy patients, but not for older people with chronic conditions. There are times when they should see their doctor in person.”
What can be done via telehealth
On the other hand, Dr. Basch heartily approves of conducting routine follow-up visits virtually for patients with chronic diseases, as long as the physician knows the patient’s history. Telehealth can also be used to coach patients on exercise, nutrition, and other lifestyle changes.
Dr. Kubitschek estimates that around 40%-50% of primary care can be delivered through telehealth. But the remainder encompasses potentially serious conditions that should be diagnosed and treated in face-to-face encounters, he said. “For example, if a patient has abdominal pain, you have to examine the person to get a clue of what they’re talking about. The pains are often diffuse, but they might be painful locally, which could indicate a mass or a bladder distension.”
For that reason, he doesn’t support the idea of patients depending on telehealth doctors in virtual primary care plans. “These doctors would not be available to care for the patient in an urgent situation without sending them to a costly emergency room or urgent care clinic. In those settings, excess testing is done because of a lack of familiarity with the patient and his or her history and exam. I think a combination of in-person and telehealth visits presents the best circumstance for the patient and the physician. Having said that, I do believe that telehealth alone is better than no interaction with a health care provider.”
United approach can help with prevention
Donny Aga, MD, an internist with Kelsey-Seybold, a multispecialty group in Houston, has been a member of United’s virtual health advisory group for the past 2 years. In his view, United’s virtual primary care service is moving in the right direction by covering preventive and chronic care. Noting that 25%-30% of patients nationally have put off wellness and chronic care visits out of fear of COVID-19, he said that,“if health plans like United are willing to cover preventive services through telehealth, that will allow us to catch up on a lot of the needed screening tests and exams. So it’s a very positive step forward.”
On the other hand, he said, virtual plans that depend solely on telehealth doctors are not the way to manage chronic conditions. “Primary care is best done by your own primary care physician, not by someone who doesn’t know you from a distance.”
Regarding the virtual plans in which patients can establish relationships with telehealth physicians, Dr. Aga said that this approach can benefit some patients, especially those who live in rural areas and don’t have access to primary care. But there are drawbacks, including the telehealth providers’ lack of knowledge about local specialists.
“The negative is that you don’t have a [primary care physicians] who’s local, who knows you, who has examined you before, and who has a good relationship with those specialists and knows who is the right specialist to see for your problem,” Dr. Aga said. “It’s very difficult, if you don’t live and work in that area, to know the best places to send people.”
Virtual visits cost less
Like Dr. Basch, Dr. Aga said it’s possible that some insurance companies might begin to cover office visits only for certain conditions or services if they can be managed more cheaply via telehealth. He hopes that doesn’t happen; if it does, he predicts that patients and doctors will push back hard.
Why would a virtual primary care visit cost a health plan less than an in-person visit if it’s paying doctors the same for both? Dr. Aga said it’s because fewer prescriptions and lab tests are ordered in telehealth encounters. He bases this assertion on the quarter of a million virtual visits that Kelsey-Seybold has conducted and also alludes to published studies.
The characteristics of telehealth visits might explain this phenomenon, he said. “These visits are typically much shorter, and it’s easy to be problem-centric and problem based. Physicians use more of their intuitive skills, rather than just lab everybody up and get an x-ray, because that patient’s not there, and it’s easier to draw blood or get an x-ray if somebody is there.”
Cutting practice overhead
From the perspective of Kelsey-Seybold, which is now conducting about a fifth of its visits virtually, “infrastructure costs are less” for telehealth, Aga notes. Although Dr. Kubitschek and Dr. Sawyer say it doesn’t take less time to conduct a telehealth visit than an office visit, other practice costs may decrease in relationship to the percentage of a doctor’s visits that are virtual.
“If implemented appropriately, telehealth consults should cost less in terms of the ancillary costs surrounding care,” said Dr. Basch. He recalls that, some years ago, a five-doctor primary care group in Portland, Ore., began charging small monthly fees to patients for full-service care that included email access. After a while, 40% of their patients were coming in, and the rest received care by email or phone. As a result, the doctors were able to downsize to a smaller office space because they didn’t need a waiting room.
Although Dr. Basch doesn’t believe it would be appropriate for practices to do something like this in the midst of a pandemic, he sees the possibility of it happening in the future. “Eventually, a group might be able to say: ‘Yes, our practice expenses can be lower if we do this smartly. We could do as well as we’ve done on whatever insurance pays for office visits, knowing that we can deliver care to the same patient panel at, say, 10% lower overhead with telehealth.’ ”
A version of this article first appeared on Medscape.com.
Initially, the service will be part of some employer-sponsored insurance plans in 11 states. United intends to expand its footprint next year.
United is using the platform and the medical group of American Well, a telehealth service, to provide virtual primary care. Besides minor acute care, United’s virtual service covers annual wellness visits, routine follow-ups for chronic conditions, lab tests, and specialist referrals with little or no cost sharing.
The giant insurer is now offering its virtual primary care plan in Arizona, Colorado, Illinois, Maryland, North Carolina, Ohio, South Carolina, Texas, Virginia, Washington, D.C., and West Virginia.
Other insurers are offering similar virtual primary care plans. For example, Humana has partnered with Doctor on Demand, and Cigna is working with MDLive to offer virtual primary care plans. Both of these plans encourage consumers to form ongoing relationships with physicians hired by the telehealth services. Similarly, Harvard Pilgrim, which has also joined with Doctor on Demand, said that consumers get “virtual PCPs” along with a full care team.
Humana has priced the premiums for its virtual service at about half the cost of Humana’s most popular traditional plan. There are no copays for telehealth visits; there are $5 copays for common lab tests and prescriptions. Cigna said that its virtual plan makes coverage “more affordable,” but doesn’t provide any specifics.
According to United spokeswoman Maria Shydlo, the insurer’s virtual primary care service is not cheaper than its traditional products.
Increased telehealth adoption
When the COVID-19 pandemic first struck last year, telehealth was a lifesaver for primary care practices. Physicians were able to treat half or more of their patients through telehealth, including video and phone consultations.
That initial romance with telehealth did not last. Today, telehealth represents 9% of adult primary care visits. However, that’s still a much higher percentage than before 2020, and telehealth has become a fixture of primary care.
Prior to the pandemic, telehealth services dominated the virtual care space. Some large groups experimented with having their doctors conduct virtual consults with their patients. Other physicians dabbled with telehealth or stayed out of it entirely because health plans paid much less for virtual visits than for in-person visits.
That began to change as more and more states passed laws requiring payment parity. (Today, 36 states do.) Then as the pandemic took hold, Medicare loosened its regulations, allowing coverage of telehealth everywhere and establishing parity. But it’s unclear what will happen after the public health emergency ends.
United and other insurers portray their virtual primary care plans as an effort to connect more consumers with primary care physicians. Having a relationship with a primary care doctor, United noted in a press release, increases access to care, including preventive services. Moreover, a United survey found that a quarter of respondents preferred a virtual relationship with a primary care doctor.
Physician have mostly positive but mixed reactions
This news organization interviewed several physicians who practice in states where United has introduced its new offering. Only one doctor had heard about it, and another, solo family physician Will Sawyer, MD, of Cincinnati no longer contracts with United. Nevertheless, they all had strong opinions about virtual primary care plans from United and other insurers.
Dr. Sawyer is a big proponent of telehealth and notes that it’s “incredibly convenient” for older people, many of whom are afraid to come to the office out of fear they might contract COVID-19. He has found that telehealth can be useful for many kinds of acute and chronic care. But he believes (although he admits he does not have evidence) that United started its virtual primary care service mainly to save money.
Peter Basch, MD, an internist with MedStar Health in Washington, D.C., says he’s willing to give United the benefit of the doubt. Increasing access to care while lowering its cost, he says, is the right thing to do, and “it makes financial sense. So I wouldn’t question their motives.”
Dr. Basch is concerned, however, that insurers such as United might eventually cover some services virtually but not in the office. “I can imagine a situation where doctors feel their judgment is being disregarded and that this person really needs to come in. And there might be pressure from the employer or the manager of the medical group, telling the doctor that if you’re not careful about how you manage these visits, you may be losing money for the practice.”
Kenneth Kubitschek, MD, an internist in a medium-sized group in Asheville, N.C., was less enamored of telehealth than Dr. Basch and Dr. Sawyer are, although it currently accounts for 15%-20% of his group’s visits. “There’s definitely something you lose with telehealth in terms of the nuances of the interaction.”
No to some kinds of telehealth doctors
The physicians we spoke with were unified in their opposition to virtual primary care plans that mainly use physicians hired by telehealth services. Dr. Sawyer noted that one-off consultations with telehealth doctors might be okay for urgent care. “But what we’re trying to do with patients is change their behavior for better health outcomes, and that doesn’t happen in these one-off contacts,” he said.
Even if a patient were able to develop an online relationship with a telehealth doctor, Dr. Basch said, there are any number of situations in which an in-person visit might be necessary. “Whether it’s a urologic visit, a cardiac visit, or an allergy visit, do I need to listen to you or put my hands on you to palpate your liver? Or is this just a conversation with someone I know to see how they’re doing, how they’re managing their meds? Ninety percent of a diagnosis is history.”
Although the virtual plans allow a telehealth physician to refer a patient to an in-network specialist for an office visit, this isn’t the same as their primary care physician asking them to come in to be examined.
Moreover, Dr. Basch noted, people with chronic conditions can’t be treated only virtually. “I wouldn’t say that primary care should be done predominantly through virtual visits. It may be okay for young and healthy patients, but not for older people with chronic conditions. There are times when they should see their doctor in person.”
What can be done via telehealth
On the other hand, Dr. Basch heartily approves of conducting routine follow-up visits virtually for patients with chronic diseases, as long as the physician knows the patient’s history. Telehealth can also be used to coach patients on exercise, nutrition, and other lifestyle changes.
Dr. Kubitschek estimates that around 40%-50% of primary care can be delivered through telehealth. But the remainder encompasses potentially serious conditions that should be diagnosed and treated in face-to-face encounters, he said. “For example, if a patient has abdominal pain, you have to examine the person to get a clue of what they’re talking about. The pains are often diffuse, but they might be painful locally, which could indicate a mass or a bladder distension.”
For that reason, he doesn’t support the idea of patients depending on telehealth doctors in virtual primary care plans. “These doctors would not be available to care for the patient in an urgent situation without sending them to a costly emergency room or urgent care clinic. In those settings, excess testing is done because of a lack of familiarity with the patient and his or her history and exam. I think a combination of in-person and telehealth visits presents the best circumstance for the patient and the physician. Having said that, I do believe that telehealth alone is better than no interaction with a health care provider.”
United approach can help with prevention
Donny Aga, MD, an internist with Kelsey-Seybold, a multispecialty group in Houston, has been a member of United’s virtual health advisory group for the past 2 years. In his view, United’s virtual primary care service is moving in the right direction by covering preventive and chronic care. Noting that 25%-30% of patients nationally have put off wellness and chronic care visits out of fear of COVID-19, he said that,“if health plans like United are willing to cover preventive services through telehealth, that will allow us to catch up on a lot of the needed screening tests and exams. So it’s a very positive step forward.”
On the other hand, he said, virtual plans that depend solely on telehealth doctors are not the way to manage chronic conditions. “Primary care is best done by your own primary care physician, not by someone who doesn’t know you from a distance.”
Regarding the virtual plans in which patients can establish relationships with telehealth physicians, Dr. Aga said that this approach can benefit some patients, especially those who live in rural areas and don’t have access to primary care. But there are drawbacks, including the telehealth providers’ lack of knowledge about local specialists.
“The negative is that you don’t have a [primary care physicians] who’s local, who knows you, who has examined you before, and who has a good relationship with those specialists and knows who is the right specialist to see for your problem,” Dr. Aga said. “It’s very difficult, if you don’t live and work in that area, to know the best places to send people.”
Virtual visits cost less
Like Dr. Basch, Dr. Aga said it’s possible that some insurance companies might begin to cover office visits only for certain conditions or services if they can be managed more cheaply via telehealth. He hopes that doesn’t happen; if it does, he predicts that patients and doctors will push back hard.
Why would a virtual primary care visit cost a health plan less than an in-person visit if it’s paying doctors the same for both? Dr. Aga said it’s because fewer prescriptions and lab tests are ordered in telehealth encounters. He bases this assertion on the quarter of a million virtual visits that Kelsey-Seybold has conducted and also alludes to published studies.
The characteristics of telehealth visits might explain this phenomenon, he said. “These visits are typically much shorter, and it’s easy to be problem-centric and problem based. Physicians use more of their intuitive skills, rather than just lab everybody up and get an x-ray, because that patient’s not there, and it’s easier to draw blood or get an x-ray if somebody is there.”
Cutting practice overhead
From the perspective of Kelsey-Seybold, which is now conducting about a fifth of its visits virtually, “infrastructure costs are less” for telehealth, Aga notes. Although Dr. Kubitschek and Dr. Sawyer say it doesn’t take less time to conduct a telehealth visit than an office visit, other practice costs may decrease in relationship to the percentage of a doctor’s visits that are virtual.
“If implemented appropriately, telehealth consults should cost less in terms of the ancillary costs surrounding care,” said Dr. Basch. He recalls that, some years ago, a five-doctor primary care group in Portland, Ore., began charging small monthly fees to patients for full-service care that included email access. After a while, 40% of their patients were coming in, and the rest received care by email or phone. As a result, the doctors were able to downsize to a smaller office space because they didn’t need a waiting room.
Although Dr. Basch doesn’t believe it would be appropriate for practices to do something like this in the midst of a pandemic, he sees the possibility of it happening in the future. “Eventually, a group might be able to say: ‘Yes, our practice expenses can be lower if we do this smartly. We could do as well as we’ve done on whatever insurance pays for office visits, knowing that we can deliver care to the same patient panel at, say, 10% lower overhead with telehealth.’ ”
A version of this article first appeared on Medscape.com.
Rural women receive antibiotics for longer than necessary for UTIs
Women living in rural areas were significantly more likely than were those in urban areas to receive inappropriate antibiotic prescriptions for urinary tract infections, based on data from an observational cohort study of more than 600,000 women.
Uncomplicated urinary tract infections (UTIs) are common among otherwise healthy women in the United States, and certain antibiotics are recommended as first-line therapy, wrote Abbye W. Clark, MD, of Washington University, St. Louis, and colleagues.
“However, the majority of antibiotic prescriptions for uncomplicated UTI are suboptimal because they are written for nonrecommended agents and durations,” they said.
Addressing rural health disparities has become a focus in the United States, and previous studies of respiratory tract infections have shown differences in antibiotic prescribing based on geographic region; “however, no large-scale studies have evaluated rural-urban differences in inappropriate outpatient prescribing for UTI,” they added.
In a study published in Infection Control & Hospital Epidemiology, the researchers identified 670,450 women aged 18-44 years who received oral antibiotics for uncomplicated UTIs between 2010 to 2015, using a commercial insurance database to determine diagnosis and antibiotic prescription information. Women were defined as urban if they lived in a metropolitan statistical area of at least 50,000 inhabitants (86.2%); all other women were defined as rural (13.8%). The median age was 30 years for both groups.
Overall, 46.7% of the women received prescriptions for inappropriate antibiotics, and 76.1% received antibiotics for inappropriate durations.
Antibiotics and durations were defined as appropriate or inappropriate based on current clinical guidelines. “We classified first-line agents (nitrofurantoin, TMP-SMX, fosfomycin) as appropriate and non–first-line agents (fluoroquinolones, beta-lactams) as inappropriate,” the researchers said.
The regimens classified as appropriate duration were “nitrofurantoin 5-day regimen, TMP-SMX (including TMP monotherapy) 3-day regimen, fosfomycin 1-day regimen, fluoroquinolones 3-day regimen, and beta-lactams 3- to 7-day regimen. All other regimens were classified as inappropriate duration,” they noted.
More rural women receive long-duration antibiotics
In a multivariate analysis, similar percentages of antibiotics for rural and urban women consisted of inappropriate agents (45.9% vs. 46.9%) including use of fluoroquinolones (41.0% vs. 41.7%) and beta-lactams (4.8% vs. 5.0%).
However, across all antibiotics, women in rural areas were more likely than were women in urban areas to receive prescriptions for inappropriately long durations (83.9% vs. 75.9%, adjusted risk ratio 1.10).
The percentage of women who received inappropriate antibiotic agents was not significantly different based on geographic region of the country.
From 2011 to 2015, the quarterly proportion of women overall who received inappropriate agents and antibiotics for inappropriate durations decreased slightly (48.5% to 43.7% and 78.3% to 73.4%, respectively), the researchers noted.
The study findings were limited by several factors including the potentially lenient definition of antibiotic duration, a study population that disproportionately oversampled from the South and undersampled from the West, use of ZIP codes to determine rural vs. urban status, lack of data on race and income, and lack of access to urine culture results, the researchers noted.
However, “our study identified rural-urban differences in antibiotic prescribing, including an actionable disparity in the duration of antibiotics that disproportionately affects women who live in rural locations,” they said.
“Given the large quantity of inappropriate prescriptions annually in the U.S., as well as the negative patient- and society-level consequences of unnecessary exposure to antibiotics, antimicrobial stewardship interventions are needed to improve outpatient UTI antibiotic prescribing, particularly in rural settings,” they concluded.
Data support need for education and stewardship
“This manuscript provides valuable information to all women’s health providers regarding the importance of antibiotic stewardship,” David M. Jaspan, DO, and Natasha Abdullah, MD, Einstein Medical Center, Philadelphia, said in an interview. Whether urban or rural, over 45% of the patients received inappropriate non–first-line treatment and 76% of the prescriptions were for an inappropriate duration (98.8% for longer than recommended), they emphasized.
“The potential negative impact of antibiotic resistance, coupled with the potential for increased side effects, should prompt providers to ensure that when treating uncomplicated UTIs in women, that the choice of treatment and the duration of treatment is tailored to the patient’s needs,” the Dr. Jaspan and Dr. Abdullah said.
To improve antibiotic prescribing, especially at the local and regional level, “We encourage providers to familiarize themselves with local information as it pertains to known resistance when prescribing empiric treatment regimens for uncomplicated UTIs,” they said.
The study was supported by the National Center for Advancing Translational Sciences at the National Institutes of Health. Lead author Dr. Clark, as well as Dr. Jaspan and Dr. Abdullah, had no financial conflicts to disclose.
Women living in rural areas were significantly more likely than were those in urban areas to receive inappropriate antibiotic prescriptions for urinary tract infections, based on data from an observational cohort study of more than 600,000 women.
Uncomplicated urinary tract infections (UTIs) are common among otherwise healthy women in the United States, and certain antibiotics are recommended as first-line therapy, wrote Abbye W. Clark, MD, of Washington University, St. Louis, and colleagues.
“However, the majority of antibiotic prescriptions for uncomplicated UTI are suboptimal because they are written for nonrecommended agents and durations,” they said.
Addressing rural health disparities has become a focus in the United States, and previous studies of respiratory tract infections have shown differences in antibiotic prescribing based on geographic region; “however, no large-scale studies have evaluated rural-urban differences in inappropriate outpatient prescribing for UTI,” they added.
In a study published in Infection Control & Hospital Epidemiology, the researchers identified 670,450 women aged 18-44 years who received oral antibiotics for uncomplicated UTIs between 2010 to 2015, using a commercial insurance database to determine diagnosis and antibiotic prescription information. Women were defined as urban if they lived in a metropolitan statistical area of at least 50,000 inhabitants (86.2%); all other women were defined as rural (13.8%). The median age was 30 years for both groups.
Overall, 46.7% of the women received prescriptions for inappropriate antibiotics, and 76.1% received antibiotics for inappropriate durations.
Antibiotics and durations were defined as appropriate or inappropriate based on current clinical guidelines. “We classified first-line agents (nitrofurantoin, TMP-SMX, fosfomycin) as appropriate and non–first-line agents (fluoroquinolones, beta-lactams) as inappropriate,” the researchers said.
The regimens classified as appropriate duration were “nitrofurantoin 5-day regimen, TMP-SMX (including TMP monotherapy) 3-day regimen, fosfomycin 1-day regimen, fluoroquinolones 3-day regimen, and beta-lactams 3- to 7-day regimen. All other regimens were classified as inappropriate duration,” they noted.
More rural women receive long-duration antibiotics
In a multivariate analysis, similar percentages of antibiotics for rural and urban women consisted of inappropriate agents (45.9% vs. 46.9%) including use of fluoroquinolones (41.0% vs. 41.7%) and beta-lactams (4.8% vs. 5.0%).
However, across all antibiotics, women in rural areas were more likely than were women in urban areas to receive prescriptions for inappropriately long durations (83.9% vs. 75.9%, adjusted risk ratio 1.10).
The percentage of women who received inappropriate antibiotic agents was not significantly different based on geographic region of the country.
From 2011 to 2015, the quarterly proportion of women overall who received inappropriate agents and antibiotics for inappropriate durations decreased slightly (48.5% to 43.7% and 78.3% to 73.4%, respectively), the researchers noted.
The study findings were limited by several factors including the potentially lenient definition of antibiotic duration, a study population that disproportionately oversampled from the South and undersampled from the West, use of ZIP codes to determine rural vs. urban status, lack of data on race and income, and lack of access to urine culture results, the researchers noted.
However, “our study identified rural-urban differences in antibiotic prescribing, including an actionable disparity in the duration of antibiotics that disproportionately affects women who live in rural locations,” they said.
“Given the large quantity of inappropriate prescriptions annually in the U.S., as well as the negative patient- and society-level consequences of unnecessary exposure to antibiotics, antimicrobial stewardship interventions are needed to improve outpatient UTI antibiotic prescribing, particularly in rural settings,” they concluded.
Data support need for education and stewardship
“This manuscript provides valuable information to all women’s health providers regarding the importance of antibiotic stewardship,” David M. Jaspan, DO, and Natasha Abdullah, MD, Einstein Medical Center, Philadelphia, said in an interview. Whether urban or rural, over 45% of the patients received inappropriate non–first-line treatment and 76% of the prescriptions were for an inappropriate duration (98.8% for longer than recommended), they emphasized.
“The potential negative impact of antibiotic resistance, coupled with the potential for increased side effects, should prompt providers to ensure that when treating uncomplicated UTIs in women, that the choice of treatment and the duration of treatment is tailored to the patient’s needs,” the Dr. Jaspan and Dr. Abdullah said.
To improve antibiotic prescribing, especially at the local and regional level, “We encourage providers to familiarize themselves with local information as it pertains to known resistance when prescribing empiric treatment regimens for uncomplicated UTIs,” they said.
The study was supported by the National Center for Advancing Translational Sciences at the National Institutes of Health. Lead author Dr. Clark, as well as Dr. Jaspan and Dr. Abdullah, had no financial conflicts to disclose.
Women living in rural areas were significantly more likely than were those in urban areas to receive inappropriate antibiotic prescriptions for urinary tract infections, based on data from an observational cohort study of more than 600,000 women.
Uncomplicated urinary tract infections (UTIs) are common among otherwise healthy women in the United States, and certain antibiotics are recommended as first-line therapy, wrote Abbye W. Clark, MD, of Washington University, St. Louis, and colleagues.
“However, the majority of antibiotic prescriptions for uncomplicated UTI are suboptimal because they are written for nonrecommended agents and durations,” they said.
Addressing rural health disparities has become a focus in the United States, and previous studies of respiratory tract infections have shown differences in antibiotic prescribing based on geographic region; “however, no large-scale studies have evaluated rural-urban differences in inappropriate outpatient prescribing for UTI,” they added.
In a study published in Infection Control & Hospital Epidemiology, the researchers identified 670,450 women aged 18-44 years who received oral antibiotics for uncomplicated UTIs between 2010 to 2015, using a commercial insurance database to determine diagnosis and antibiotic prescription information. Women were defined as urban if they lived in a metropolitan statistical area of at least 50,000 inhabitants (86.2%); all other women were defined as rural (13.8%). The median age was 30 years for both groups.
Overall, 46.7% of the women received prescriptions for inappropriate antibiotics, and 76.1% received antibiotics for inappropriate durations.
Antibiotics and durations were defined as appropriate or inappropriate based on current clinical guidelines. “We classified first-line agents (nitrofurantoin, TMP-SMX, fosfomycin) as appropriate and non–first-line agents (fluoroquinolones, beta-lactams) as inappropriate,” the researchers said.
The regimens classified as appropriate duration were “nitrofurantoin 5-day regimen, TMP-SMX (including TMP monotherapy) 3-day regimen, fosfomycin 1-day regimen, fluoroquinolones 3-day regimen, and beta-lactams 3- to 7-day regimen. All other regimens were classified as inappropriate duration,” they noted.
More rural women receive long-duration antibiotics
In a multivariate analysis, similar percentages of antibiotics for rural and urban women consisted of inappropriate agents (45.9% vs. 46.9%) including use of fluoroquinolones (41.0% vs. 41.7%) and beta-lactams (4.8% vs. 5.0%).
However, across all antibiotics, women in rural areas were more likely than were women in urban areas to receive prescriptions for inappropriately long durations (83.9% vs. 75.9%, adjusted risk ratio 1.10).
The percentage of women who received inappropriate antibiotic agents was not significantly different based on geographic region of the country.
From 2011 to 2015, the quarterly proportion of women overall who received inappropriate agents and antibiotics for inappropriate durations decreased slightly (48.5% to 43.7% and 78.3% to 73.4%, respectively), the researchers noted.
The study findings were limited by several factors including the potentially lenient definition of antibiotic duration, a study population that disproportionately oversampled from the South and undersampled from the West, use of ZIP codes to determine rural vs. urban status, lack of data on race and income, and lack of access to urine culture results, the researchers noted.
However, “our study identified rural-urban differences in antibiotic prescribing, including an actionable disparity in the duration of antibiotics that disproportionately affects women who live in rural locations,” they said.
“Given the large quantity of inappropriate prescriptions annually in the U.S., as well as the negative patient- and society-level consequences of unnecessary exposure to antibiotics, antimicrobial stewardship interventions are needed to improve outpatient UTI antibiotic prescribing, particularly in rural settings,” they concluded.
Data support need for education and stewardship
“This manuscript provides valuable information to all women’s health providers regarding the importance of antibiotic stewardship,” David M. Jaspan, DO, and Natasha Abdullah, MD, Einstein Medical Center, Philadelphia, said in an interview. Whether urban or rural, over 45% of the patients received inappropriate non–first-line treatment and 76% of the prescriptions were for an inappropriate duration (98.8% for longer than recommended), they emphasized.
“The potential negative impact of antibiotic resistance, coupled with the potential for increased side effects, should prompt providers to ensure that when treating uncomplicated UTIs in women, that the choice of treatment and the duration of treatment is tailored to the patient’s needs,” the Dr. Jaspan and Dr. Abdullah said.
To improve antibiotic prescribing, especially at the local and regional level, “We encourage providers to familiarize themselves with local information as it pertains to known resistance when prescribing empiric treatment regimens for uncomplicated UTIs,” they said.
The study was supported by the National Center for Advancing Translational Sciences at the National Institutes of Health. Lead author Dr. Clark, as well as Dr. Jaspan and Dr. Abdullah, had no financial conflicts to disclose.
FROM INFECTION CONTROL & HOSPITAL EPIDEMIOLOGY
Ovarian cancer prevention: How patients decide on surgery
Investigators interviewed 24 premenopausal women with BRCA mutations who were considering prophylactic surgery. Responses showed that women who prioritized cancer risk reduction opted for risk-reducing salpingo-oophorectomy (RRSO), while those who were more concerned about the effects of surgical menopause opted for risk-reducing early salpingectomy with delayed oophorectomy (RRESDO). Factors such as past surgical experience influenced patients’ decisions as well.
The women were participants in the PROTECTOR trial, which was designed to determine the impact of RRESDO on sexual function by comparing RRESDO, RRSO, and no surgery. Study participants made their own decisions regarding surgery, and the interviews with 24 participants provided insight into how patients made their decisions.
Faiza Gaba, MBBS, PhD, of Queen Mary University of London, and colleagues reviewed the results in the Journal of Medical Genetics.
“I think what is most important is that even though this is a tough decision women are being given, they are being given options that allow them to make up their own minds with support from health care providers,” said Barbara A. Goff, MD, of the University of Washington, Seattle, when asked to comment on this research.
Study details and results
The investigators interviewed 24 women aged 34-46 years. Fourteen patients were BRCA1 carriers, and 10 were BRCA2 carriers. Twenty-two women were White, and two were Asian. Nineteen women were married, and four were nulliparous.
The interviews lasted a mean of 55 minutes and were conducted with a predeveloped topic guide.
Eleven of the women interviewed opted for RRESDO, seven opted for RRSO, and six decided against prophylactic surgery. Four women who had previous breast cancer all opted for ovarian cancer prevention surgery.
Among the 18 women who chose surgery, 16 had completed childbearing, and 2 didn’t want children. Among the six women who decided against surgery, four had not completed childbearing, one was unsure of which procedure to choose, and the sixth had strong feelings against removing healthy tissue.
The 11 women who chose RRESDO did so because of concerns about early menopause following oophorectomy, particularly low mood, sexual dysfunction, and poorer quality of life. They were also more likely to have had a previous positive surgical experience, so they were not as concerned about a two-step surgery.
Women who chose RRSO were motivated by a strong family history of ovarian cancer, fear of dying, concurrent benign gynecological issues, lack of screening for ovarian cancer, and physician advice.
Nine women also opted for risk-reducing mastectomy (RRM), which was deemed a more difficult decision than prophylactic ovarian cancer surgery.
Women who decided against RRM did so because they hadn’t completed childbearing, they were concerned about recovery time and the psychological effects of the surgery, or they had confidence in breast cancer screening and treatment.
Among women who chose RRM, two highlighted lack of health care professional support for deciding against reconstruction. One interviewee regretted opting for flap reconstruction due to the resultant cosmetic appearance and chronic pain but did not regret RRM.
Fifteen women said they would have preferred combined RRM and ovarian cancer prevention in one surgery, due to less anxiety, less waiting, fewer appointments, less time off work, and a single surgical recovery.
The women advised fellow BRCA carriers to avoid time pressure with surgical decision-making, talk to other BRCA carriers, do personal research, and ask for second opinions if not satisfied. They also said the opportunity to ask experts questions and meet other BRCA carriers face-to-face was more helpful than online support groups. Women managed in specialist settings said they received better care, better access to hormone replacement therapy, and were more satisfied.
High-risk women need to be supported by a multidisciplinary team of geneticists, gynecologists/oncologists, oncoplastic surgeons, menopause specialists, fertility specialists, psychologists, and specialist nurses, the investigators wrote.
This study was funded by the Rosetrees Trust and Barts Charity. The authors disclosed relationships with AstraZeneca, Merck, Cancer Research UK, The Eve Appeal, Israel National Institute for Health Policy Research, and the UK National Health Service. Dr. Goff has no relevant disclosures.
Investigators interviewed 24 premenopausal women with BRCA mutations who were considering prophylactic surgery. Responses showed that women who prioritized cancer risk reduction opted for risk-reducing salpingo-oophorectomy (RRSO), while those who were more concerned about the effects of surgical menopause opted for risk-reducing early salpingectomy with delayed oophorectomy (RRESDO). Factors such as past surgical experience influenced patients’ decisions as well.
The women were participants in the PROTECTOR trial, which was designed to determine the impact of RRESDO on sexual function by comparing RRESDO, RRSO, and no surgery. Study participants made their own decisions regarding surgery, and the interviews with 24 participants provided insight into how patients made their decisions.
Faiza Gaba, MBBS, PhD, of Queen Mary University of London, and colleagues reviewed the results in the Journal of Medical Genetics.
“I think what is most important is that even though this is a tough decision women are being given, they are being given options that allow them to make up their own minds with support from health care providers,” said Barbara A. Goff, MD, of the University of Washington, Seattle, when asked to comment on this research.
Study details and results
The investigators interviewed 24 women aged 34-46 years. Fourteen patients were BRCA1 carriers, and 10 were BRCA2 carriers. Twenty-two women were White, and two were Asian. Nineteen women were married, and four were nulliparous.
The interviews lasted a mean of 55 minutes and were conducted with a predeveloped topic guide.
Eleven of the women interviewed opted for RRESDO, seven opted for RRSO, and six decided against prophylactic surgery. Four women who had previous breast cancer all opted for ovarian cancer prevention surgery.
Among the 18 women who chose surgery, 16 had completed childbearing, and 2 didn’t want children. Among the six women who decided against surgery, four had not completed childbearing, one was unsure of which procedure to choose, and the sixth had strong feelings against removing healthy tissue.
The 11 women who chose RRESDO did so because of concerns about early menopause following oophorectomy, particularly low mood, sexual dysfunction, and poorer quality of life. They were also more likely to have had a previous positive surgical experience, so they were not as concerned about a two-step surgery.
Women who chose RRSO were motivated by a strong family history of ovarian cancer, fear of dying, concurrent benign gynecological issues, lack of screening for ovarian cancer, and physician advice.
Nine women also opted for risk-reducing mastectomy (RRM), which was deemed a more difficult decision than prophylactic ovarian cancer surgery.
Women who decided against RRM did so because they hadn’t completed childbearing, they were concerned about recovery time and the psychological effects of the surgery, or they had confidence in breast cancer screening and treatment.
Among women who chose RRM, two highlighted lack of health care professional support for deciding against reconstruction. One interviewee regretted opting for flap reconstruction due to the resultant cosmetic appearance and chronic pain but did not regret RRM.
Fifteen women said they would have preferred combined RRM and ovarian cancer prevention in one surgery, due to less anxiety, less waiting, fewer appointments, less time off work, and a single surgical recovery.
The women advised fellow BRCA carriers to avoid time pressure with surgical decision-making, talk to other BRCA carriers, do personal research, and ask for second opinions if not satisfied. They also said the opportunity to ask experts questions and meet other BRCA carriers face-to-face was more helpful than online support groups. Women managed in specialist settings said they received better care, better access to hormone replacement therapy, and were more satisfied.
High-risk women need to be supported by a multidisciplinary team of geneticists, gynecologists/oncologists, oncoplastic surgeons, menopause specialists, fertility specialists, psychologists, and specialist nurses, the investigators wrote.
This study was funded by the Rosetrees Trust and Barts Charity. The authors disclosed relationships with AstraZeneca, Merck, Cancer Research UK, The Eve Appeal, Israel National Institute for Health Policy Research, and the UK National Health Service. Dr. Goff has no relevant disclosures.
Investigators interviewed 24 premenopausal women with BRCA mutations who were considering prophylactic surgery. Responses showed that women who prioritized cancer risk reduction opted for risk-reducing salpingo-oophorectomy (RRSO), while those who were more concerned about the effects of surgical menopause opted for risk-reducing early salpingectomy with delayed oophorectomy (RRESDO). Factors such as past surgical experience influenced patients’ decisions as well.
The women were participants in the PROTECTOR trial, which was designed to determine the impact of RRESDO on sexual function by comparing RRESDO, RRSO, and no surgery. Study participants made their own decisions regarding surgery, and the interviews with 24 participants provided insight into how patients made their decisions.
Faiza Gaba, MBBS, PhD, of Queen Mary University of London, and colleagues reviewed the results in the Journal of Medical Genetics.
“I think what is most important is that even though this is a tough decision women are being given, they are being given options that allow them to make up their own minds with support from health care providers,” said Barbara A. Goff, MD, of the University of Washington, Seattle, when asked to comment on this research.
Study details and results
The investigators interviewed 24 women aged 34-46 years. Fourteen patients were BRCA1 carriers, and 10 were BRCA2 carriers. Twenty-two women were White, and two were Asian. Nineteen women were married, and four were nulliparous.
The interviews lasted a mean of 55 minutes and were conducted with a predeveloped topic guide.
Eleven of the women interviewed opted for RRESDO, seven opted for RRSO, and six decided against prophylactic surgery. Four women who had previous breast cancer all opted for ovarian cancer prevention surgery.
Among the 18 women who chose surgery, 16 had completed childbearing, and 2 didn’t want children. Among the six women who decided against surgery, four had not completed childbearing, one was unsure of which procedure to choose, and the sixth had strong feelings against removing healthy tissue.
The 11 women who chose RRESDO did so because of concerns about early menopause following oophorectomy, particularly low mood, sexual dysfunction, and poorer quality of life. They were also more likely to have had a previous positive surgical experience, so they were not as concerned about a two-step surgery.
Women who chose RRSO were motivated by a strong family history of ovarian cancer, fear of dying, concurrent benign gynecological issues, lack of screening for ovarian cancer, and physician advice.
Nine women also opted for risk-reducing mastectomy (RRM), which was deemed a more difficult decision than prophylactic ovarian cancer surgery.
Women who decided against RRM did so because they hadn’t completed childbearing, they were concerned about recovery time and the psychological effects of the surgery, or they had confidence in breast cancer screening and treatment.
Among women who chose RRM, two highlighted lack of health care professional support for deciding against reconstruction. One interviewee regretted opting for flap reconstruction due to the resultant cosmetic appearance and chronic pain but did not regret RRM.
Fifteen women said they would have preferred combined RRM and ovarian cancer prevention in one surgery, due to less anxiety, less waiting, fewer appointments, less time off work, and a single surgical recovery.
The women advised fellow BRCA carriers to avoid time pressure with surgical decision-making, talk to other BRCA carriers, do personal research, and ask for second opinions if not satisfied. They also said the opportunity to ask experts questions and meet other BRCA carriers face-to-face was more helpful than online support groups. Women managed in specialist settings said they received better care, better access to hormone replacement therapy, and were more satisfied.
High-risk women need to be supported by a multidisciplinary team of geneticists, gynecologists/oncologists, oncoplastic surgeons, menopause specialists, fertility specialists, psychologists, and specialist nurses, the investigators wrote.
This study was funded by the Rosetrees Trust and Barts Charity. The authors disclosed relationships with AstraZeneca, Merck, Cancer Research UK, The Eve Appeal, Israel National Institute for Health Policy Research, and the UK National Health Service. Dr. Goff has no relevant disclosures.
FROM THE JOURNAL OF MEDICAL GENETICS
Owning all aspects of patient care: Bridget McGrath, PA-C, FHM
Editor’s note: This profile is part of the Society of Hospital Medicine’s celebration of National Hospitalist Day on March 4. National Hospitalist Day occurs the first Thursday in March annually and celebrates the fastest growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape.
Bridget McGrath, PA-C, FHM, is a physician assistant and director of the nurse practitioner/physician assistant service line for the section of hospital medicine at the University of Chicago. She is a cochair of SHM’s NP/PA Special Interest Group.
Where did you receive your PA education/training? Was your intention always to be a PA?
I graduated from the PA program at Butler University, Indianapolis, in 2014. In college, whenever I shadowed a PA, I was always impressed that each one loved their job and said they would never change it. That universal passion for the PA profession really made an impression on me.
At what point in your PA education/training did you decide to practice hospital medicine? What about it appealed to you?
That occurred during my clinical rotation year at Butler. I had always thought I wanted to practice neonatology, but during my clinical rotation I really fell in love with adult medicine. I recall that during my clinical rotation, the preceptor said to me that the goal was not to have me understand every aspect of medicine, but to learn how to exist in a hospital setting. I was exposed to the breadth of hospital medicine practice and I fell in love with the complexity, the variety, and the environment itself.
I initially accepted a job as a med-peds hospitalist PA – which brought both of my passions together at that time – at Schneck Medical Center in Seymour, Ind. During that time, Schneck was a 100-bed rural community hospital which had recently been the recipient of the Malcolm Baldrige National Quality Award. It was there that I was able to practice with a phenomenal group of physicians, nurses, and social workers who really took me under their wing and taught me how to be a hospitalist PA. I practiced at Schneck for 3 years, and then moved to the University of Chicago in 2017.
I am now the director of NP/PA services for the section of hospital medicine, overseeing a group of seven on our NP/PA team, within a larger group of about 60 physicians.
What are your favorite areas of clinical practice?
Like many hospitalists, I enjoy the variety of medicine that hospitalists practice. One area that I find especially rewarding is my time in our transplant comanagement services. To be able to walk with patients on their transplant journey is very rewarding, and I am very appreciative of the mentoring I have received from some of my colleagues with a deeper understanding of transplant medicine.
In my administrative role, I have the privilege of helping to expand the professional education and training of my colleagues. I have a passion for medical education, and we have been working to develop interprofessional educational opportunities within our section. I have had time to think about the imprint of NPs and PAs in academic medicine, and how we can continue to meet the professional educational needs of our section while improving the care of our patients.
What are the most challenging aspects of practicing hospital medicine?
The volume of diagnoses that we are expected to manage on a daily basis can be challenging. This challenges you to continue learning. The complexity of discharge planning, particularly for patients in underserved communities, can also be challenging. You have to make sure your patients are ready mentally, physically and emotionally for discharge. As a hospitalist, you are continuously thinking about how to optimize patients to leave your care. For example, patients have different insurance situations, different access to care at home – you are always managing the medical needs of your patient in the context of these other issues.
How does a hospitalist PA work differently from a PA in other care settings?
We are meant to be generalists. We serve as the main provider in owning our patients’ care. A hospitalist PA serves as a cog in the wheel, with connections to specialists, consultants, nurses, social workers, pharmacists, etc., and we are tasked with synthesizing all aspects of patient care to ensure the best outcome.
What has your experience taught you about how NPs and PAs can best fit into hospital medicine groups?
Each hospital medicine group will know how to best integrate their NPs and PAs based on the skillsets of their NPs and PAs, and the needs of the section and the hospital. I personally feel that the best way to utilize NPs and PAs is to allow them to own all aspects of patient care and work at the highest scope of practice. By doing this you empower the NP or PA to continue to develop their skill set and set a precedent of collaboration and respect for interprofessional care models within your section’s culture.
Scope of practice for an NP or PA is going to be based on a conglomeration of roles and bylaws. We are certified nationally, and our scope of practice is determined at the state level and the hospital by level. For the individual NP and PA, it really depends on the hospital medicine group, and how well a practice incorporates a sense of collegiality.
What kind of resources do hospitalist PAs need to succeed, either from SHM or from their own institutions?
There are a few key things that need to happen in order for hospital medicine groups to set up their NPs and PAs for success. The first is for PAs to have exposure to inpatient rotations during clinical rotations. A hospital medicine group also should have a very intentional onboarding process for NPs and PAs. They should also establish a culture of acceptance. To do this, they should utilize resources like SHM’s NP/PA Hospital Medicine Onboarding Toolkit and the SHM/American Academy of Physician Assistants Hospitalist Bootcamp On Demand.
Mentoring is also remarkably important. I have been incredibly blessed to have mentors that helped make me into the PA that I am. I could not have done what I did in the field without people taking a chance on me, and it is important to pass that on to the next generation of PAs.
How has COVID-19 changed the practice of hospital medicine, specifically for advanced practice providers?
The pandemic has demonstrated opportunities for teamwork and utilization of NPs and PAs. The COVID pandemic forced everyone to reflect on why they originally got into medicine – to help patients. I think there will be many doors opening for NPs and PAs, and many pathways for leadership.
The hospitalist leadership at the University of Chicago truly identified that we needed to make wellness a main priority during the beginning of the pandemic. We developed a wellness work group that I have been coleading.
What’s on the horizon for NPs and PAs in hospital medicine?
We are seeing significant increases in hospitalist program utilization, so this is a time where NPs and PAs can be advocates for our profession and articulate how we can use our backgrounds and training to build better care models in order to meet the needs of our patients.
I hope we will see more NPs and PAs assuming leadership roles to ensure that our voices are heard. We should also be advocating for more collaboration and teamwork with our MD and DO colleagues.
Do you have any advice for PA students interested in hospital medicine?
I always tell my students that they should be sponges – you are not expected to know everything as a hospitalist PA, but you are expected to continue learning in order to develop into the best PA you can be. Always be open to where your career path can take you. Hospital medicine is a relatively young field within medicine, and the diversity of our field is very exciting looking forward.
Editor’s note: This profile is part of the Society of Hospital Medicine’s celebration of National Hospitalist Day on March 4. National Hospitalist Day occurs the first Thursday in March annually and celebrates the fastest growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape.
Bridget McGrath, PA-C, FHM, is a physician assistant and director of the nurse practitioner/physician assistant service line for the section of hospital medicine at the University of Chicago. She is a cochair of SHM’s NP/PA Special Interest Group.
Where did you receive your PA education/training? Was your intention always to be a PA?
I graduated from the PA program at Butler University, Indianapolis, in 2014. In college, whenever I shadowed a PA, I was always impressed that each one loved their job and said they would never change it. That universal passion for the PA profession really made an impression on me.
At what point in your PA education/training did you decide to practice hospital medicine? What about it appealed to you?
That occurred during my clinical rotation year at Butler. I had always thought I wanted to practice neonatology, but during my clinical rotation I really fell in love with adult medicine. I recall that during my clinical rotation, the preceptor said to me that the goal was not to have me understand every aspect of medicine, but to learn how to exist in a hospital setting. I was exposed to the breadth of hospital medicine practice and I fell in love with the complexity, the variety, and the environment itself.
I initially accepted a job as a med-peds hospitalist PA – which brought both of my passions together at that time – at Schneck Medical Center in Seymour, Ind. During that time, Schneck was a 100-bed rural community hospital which had recently been the recipient of the Malcolm Baldrige National Quality Award. It was there that I was able to practice with a phenomenal group of physicians, nurses, and social workers who really took me under their wing and taught me how to be a hospitalist PA. I practiced at Schneck for 3 years, and then moved to the University of Chicago in 2017.
I am now the director of NP/PA services for the section of hospital medicine, overseeing a group of seven on our NP/PA team, within a larger group of about 60 physicians.
What are your favorite areas of clinical practice?
Like many hospitalists, I enjoy the variety of medicine that hospitalists practice. One area that I find especially rewarding is my time in our transplant comanagement services. To be able to walk with patients on their transplant journey is very rewarding, and I am very appreciative of the mentoring I have received from some of my colleagues with a deeper understanding of transplant medicine.
In my administrative role, I have the privilege of helping to expand the professional education and training of my colleagues. I have a passion for medical education, and we have been working to develop interprofessional educational opportunities within our section. I have had time to think about the imprint of NPs and PAs in academic medicine, and how we can continue to meet the professional educational needs of our section while improving the care of our patients.
What are the most challenging aspects of practicing hospital medicine?
The volume of diagnoses that we are expected to manage on a daily basis can be challenging. This challenges you to continue learning. The complexity of discharge planning, particularly for patients in underserved communities, can also be challenging. You have to make sure your patients are ready mentally, physically and emotionally for discharge. As a hospitalist, you are continuously thinking about how to optimize patients to leave your care. For example, patients have different insurance situations, different access to care at home – you are always managing the medical needs of your patient in the context of these other issues.
How does a hospitalist PA work differently from a PA in other care settings?
We are meant to be generalists. We serve as the main provider in owning our patients’ care. A hospitalist PA serves as a cog in the wheel, with connections to specialists, consultants, nurses, social workers, pharmacists, etc., and we are tasked with synthesizing all aspects of patient care to ensure the best outcome.
What has your experience taught you about how NPs and PAs can best fit into hospital medicine groups?
Each hospital medicine group will know how to best integrate their NPs and PAs based on the skillsets of their NPs and PAs, and the needs of the section and the hospital. I personally feel that the best way to utilize NPs and PAs is to allow them to own all aspects of patient care and work at the highest scope of practice. By doing this you empower the NP or PA to continue to develop their skill set and set a precedent of collaboration and respect for interprofessional care models within your section’s culture.
Scope of practice for an NP or PA is going to be based on a conglomeration of roles and bylaws. We are certified nationally, and our scope of practice is determined at the state level and the hospital by level. For the individual NP and PA, it really depends on the hospital medicine group, and how well a practice incorporates a sense of collegiality.
What kind of resources do hospitalist PAs need to succeed, either from SHM or from their own institutions?
There are a few key things that need to happen in order for hospital medicine groups to set up their NPs and PAs for success. The first is for PAs to have exposure to inpatient rotations during clinical rotations. A hospital medicine group also should have a very intentional onboarding process for NPs and PAs. They should also establish a culture of acceptance. To do this, they should utilize resources like SHM’s NP/PA Hospital Medicine Onboarding Toolkit and the SHM/American Academy of Physician Assistants Hospitalist Bootcamp On Demand.
Mentoring is also remarkably important. I have been incredibly blessed to have mentors that helped make me into the PA that I am. I could not have done what I did in the field without people taking a chance on me, and it is important to pass that on to the next generation of PAs.
How has COVID-19 changed the practice of hospital medicine, specifically for advanced practice providers?
The pandemic has demonstrated opportunities for teamwork and utilization of NPs and PAs. The COVID pandemic forced everyone to reflect on why they originally got into medicine – to help patients. I think there will be many doors opening for NPs and PAs, and many pathways for leadership.
The hospitalist leadership at the University of Chicago truly identified that we needed to make wellness a main priority during the beginning of the pandemic. We developed a wellness work group that I have been coleading.
What’s on the horizon for NPs and PAs in hospital medicine?
We are seeing significant increases in hospitalist program utilization, so this is a time where NPs and PAs can be advocates for our profession and articulate how we can use our backgrounds and training to build better care models in order to meet the needs of our patients.
I hope we will see more NPs and PAs assuming leadership roles to ensure that our voices are heard. We should also be advocating for more collaboration and teamwork with our MD and DO colleagues.
Do you have any advice for PA students interested in hospital medicine?
I always tell my students that they should be sponges – you are not expected to know everything as a hospitalist PA, but you are expected to continue learning in order to develop into the best PA you can be. Always be open to where your career path can take you. Hospital medicine is a relatively young field within medicine, and the diversity of our field is very exciting looking forward.
Editor’s note: This profile is part of the Society of Hospital Medicine’s celebration of National Hospitalist Day on March 4. National Hospitalist Day occurs the first Thursday in March annually and celebrates the fastest growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape.
Bridget McGrath, PA-C, FHM, is a physician assistant and director of the nurse practitioner/physician assistant service line for the section of hospital medicine at the University of Chicago. She is a cochair of SHM’s NP/PA Special Interest Group.
Where did you receive your PA education/training? Was your intention always to be a PA?
I graduated from the PA program at Butler University, Indianapolis, in 2014. In college, whenever I shadowed a PA, I was always impressed that each one loved their job and said they would never change it. That universal passion for the PA profession really made an impression on me.
At what point in your PA education/training did you decide to practice hospital medicine? What about it appealed to you?
That occurred during my clinical rotation year at Butler. I had always thought I wanted to practice neonatology, but during my clinical rotation I really fell in love with adult medicine. I recall that during my clinical rotation, the preceptor said to me that the goal was not to have me understand every aspect of medicine, but to learn how to exist in a hospital setting. I was exposed to the breadth of hospital medicine practice and I fell in love with the complexity, the variety, and the environment itself.
I initially accepted a job as a med-peds hospitalist PA – which brought both of my passions together at that time – at Schneck Medical Center in Seymour, Ind. During that time, Schneck was a 100-bed rural community hospital which had recently been the recipient of the Malcolm Baldrige National Quality Award. It was there that I was able to practice with a phenomenal group of physicians, nurses, and social workers who really took me under their wing and taught me how to be a hospitalist PA. I practiced at Schneck for 3 years, and then moved to the University of Chicago in 2017.
I am now the director of NP/PA services for the section of hospital medicine, overseeing a group of seven on our NP/PA team, within a larger group of about 60 physicians.
What are your favorite areas of clinical practice?
Like many hospitalists, I enjoy the variety of medicine that hospitalists practice. One area that I find especially rewarding is my time in our transplant comanagement services. To be able to walk with patients on their transplant journey is very rewarding, and I am very appreciative of the mentoring I have received from some of my colleagues with a deeper understanding of transplant medicine.
In my administrative role, I have the privilege of helping to expand the professional education and training of my colleagues. I have a passion for medical education, and we have been working to develop interprofessional educational opportunities within our section. I have had time to think about the imprint of NPs and PAs in academic medicine, and how we can continue to meet the professional educational needs of our section while improving the care of our patients.
What are the most challenging aspects of practicing hospital medicine?
The volume of diagnoses that we are expected to manage on a daily basis can be challenging. This challenges you to continue learning. The complexity of discharge planning, particularly for patients in underserved communities, can also be challenging. You have to make sure your patients are ready mentally, physically and emotionally for discharge. As a hospitalist, you are continuously thinking about how to optimize patients to leave your care. For example, patients have different insurance situations, different access to care at home – you are always managing the medical needs of your patient in the context of these other issues.
How does a hospitalist PA work differently from a PA in other care settings?
We are meant to be generalists. We serve as the main provider in owning our patients’ care. A hospitalist PA serves as a cog in the wheel, with connections to specialists, consultants, nurses, social workers, pharmacists, etc., and we are tasked with synthesizing all aspects of patient care to ensure the best outcome.
What has your experience taught you about how NPs and PAs can best fit into hospital medicine groups?
Each hospital medicine group will know how to best integrate their NPs and PAs based on the skillsets of their NPs and PAs, and the needs of the section and the hospital. I personally feel that the best way to utilize NPs and PAs is to allow them to own all aspects of patient care and work at the highest scope of practice. By doing this you empower the NP or PA to continue to develop their skill set and set a precedent of collaboration and respect for interprofessional care models within your section’s culture.
Scope of practice for an NP or PA is going to be based on a conglomeration of roles and bylaws. We are certified nationally, and our scope of practice is determined at the state level and the hospital by level. For the individual NP and PA, it really depends on the hospital medicine group, and how well a practice incorporates a sense of collegiality.
What kind of resources do hospitalist PAs need to succeed, either from SHM or from their own institutions?
There are a few key things that need to happen in order for hospital medicine groups to set up their NPs and PAs for success. The first is for PAs to have exposure to inpatient rotations during clinical rotations. A hospital medicine group also should have a very intentional onboarding process for NPs and PAs. They should also establish a culture of acceptance. To do this, they should utilize resources like SHM’s NP/PA Hospital Medicine Onboarding Toolkit and the SHM/American Academy of Physician Assistants Hospitalist Bootcamp On Demand.
Mentoring is also remarkably important. I have been incredibly blessed to have mentors that helped make me into the PA that I am. I could not have done what I did in the field without people taking a chance on me, and it is important to pass that on to the next generation of PAs.
How has COVID-19 changed the practice of hospital medicine, specifically for advanced practice providers?
The pandemic has demonstrated opportunities for teamwork and utilization of NPs and PAs. The COVID pandemic forced everyone to reflect on why they originally got into medicine – to help patients. I think there will be many doors opening for NPs and PAs, and many pathways for leadership.
The hospitalist leadership at the University of Chicago truly identified that we needed to make wellness a main priority during the beginning of the pandemic. We developed a wellness work group that I have been coleading.
What’s on the horizon for NPs and PAs in hospital medicine?
We are seeing significant increases in hospitalist program utilization, so this is a time where NPs and PAs can be advocates for our profession and articulate how we can use our backgrounds and training to build better care models in order to meet the needs of our patients.
I hope we will see more NPs and PAs assuming leadership roles to ensure that our voices are heard. We should also be advocating for more collaboration and teamwork with our MD and DO colleagues.
Do you have any advice for PA students interested in hospital medicine?
I always tell my students that they should be sponges – you are not expected to know everything as a hospitalist PA, but you are expected to continue learning in order to develop into the best PA you can be. Always be open to where your career path can take you. Hospital medicine is a relatively young field within medicine, and the diversity of our field is very exciting looking forward.
Maribavir seen as superior to other antivirals for CMV clearance post transplant
Maribavir, an investigational antiviral agent with a novel mechanism of action, was superior to other antiviral strategies at clearing cytomegalovirus (CMV) viremia and controlling symptoms in hematopoietic cell or solid-organ transplant recipients, results of a phase 3 clinical trial showed.
CMV viremia clearance at study week 8 was seen in 55.7% of all patients randomized to receive maribavir, compared with 23.9% for patients assigned to receive investigator-assigned therapy (IAT), Francisco Marty, MD, from the Dana-Farber Cancer Institute in Boston reported at the Transplant & Cellular Therapies Meetings.
“Maribavir’s benefit was driven by lower incidence of treatment-limiting toxicities, compared with IAT,” he said a late-breaking abstract session during the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
“Available anti-CMV antivirals are limited by development of resistance and toxicities, particularly myelosuppression with the use of valganciclovir and nephrotoxicity with the use of foscarnet and cidofovir. Alternative treatment options are required to address this unmet medical need,” he said.
Maribavir inhibits the CMV UL97 protein kinase and is thought to affect several critical processes in CMV replication, including viral DNA synthesis, viral gene expression, encapsidation, and egress of mature capsids from the nucleus.
Details of trial
In the phase 3 SHP620-30e trial (NCT02931539), Dr. Marty and colleagues enrolled patients with relapsed or refractory CMV infections after hematopoietic cell transplant (HCT) or solid-organ transplant (SOT) and after stratification by transplant type and screening CMV DNA level randomly assigned them on a 2:1 basis to receive either maribavir 400 mg twice daily (235 patients) or IAT (117 patients), consisting of either ganciclovir/valganciclovir, foscarnet, cidofovir, or combined foscarnet and val/ganciclovir.
The primary endpoint of viremia clearance at 8 weeks was defined as plasma CMV DNA less than 137 IU/mL in two consecutive tests at a central laboratory at least 5 days apart beginning at the end of week 8.
The trial met its primary endpoint, with a viremia clearance rate of 55.7% with maribavir versus 23.9% with IAT.
The viremia clearance rates were similar in each of the transplant groups: 55.9% versus 20.8%, respectively, in patients who underwent HCT, and 55.6% versus 26.1% in patients who underwent SOT (P < .001).
Clearance rates among patients with CMV DNA below 9,100 IU/mL at baseline were 62.1% with maribavir versus 24.7% with IAT. Among patients with baseline CMV DNA of 9100 IU/mL or above, the respective rates were 43.9% versus 21.9%.
CMV viremia clearance continued from week 8 to week 16 in 18.7% of patients assigned to maribavir and to 10.3% of patients randomized to IAT (P < .013).
The median time to first CMV viremia clearance as 22 days with maribavir versus 27 days with IAT (P = .039).
All-cause mortality was similar between the groups, at 11.5% versus 11.1%, respectively.
The incidences of serious and severe treatment-emergent adverse events (TEAE) were 38.5% and 32.1%, respectively, in the maribavir group, and 37.1% and 37.9% in the IAT group.
Any TEAE leading to study drug discontinuation was less common with maribavir, occurring in 13.2% of patients, compared with 31.9% of patients on IAT. Serious TEAEs leading to drug discontinuation occurred in 8.5% versus 14.7%, respectively.
Serious TEAEs leading to death occurred in 6.8% of patients on maribavir versus 5.2% of those on IAT.
Role of letermovir
In the question-and-answer session following the presentation, comoderator Monalisa Ghosh, MD, from the University of Michigan, Ann Arbor, asked whether any patients in the study were currently on letermovir (Prevymis) prophylaxis, and whether any patients had previously been treated with letermovir but had CMV reactivation and were then treated on study.
Dr. Marty noted that the trial was designed before letermovir was approved for CMV prophylaxis in adults who have undergone an allogeneic HCT.
“Nobody was on letermovir at the beginning of the trial,” he replied, but noted that some patients who were enrolled and had infections that were refractory or resistant to valganciclovir, foscarnet, or a combination of the two received letermovir as secondary prophylaxis.
“I haven’t got the data to tell you how often [letermovir] was used; I think part of the lack of mortality benefit [with maribavir] may be due to the fact that people jumped into secondary prophylaxis with letermovir to minimize the toxicities that we saw,” he said.
Although maribavir has not as of this writing received Food and Drug Administration approval, the drug may be available to some patients through a compassionate-use program from Takeda, Dr. Marty noted.
The study was funded by Shire ViroPharma. Dr. Marty disclosed research funding from Shire and from others. Dr. Ghosh had no relevant disclosures.
Maribavir, an investigational antiviral agent with a novel mechanism of action, was superior to other antiviral strategies at clearing cytomegalovirus (CMV) viremia and controlling symptoms in hematopoietic cell or solid-organ transplant recipients, results of a phase 3 clinical trial showed.
CMV viremia clearance at study week 8 was seen in 55.7% of all patients randomized to receive maribavir, compared with 23.9% for patients assigned to receive investigator-assigned therapy (IAT), Francisco Marty, MD, from the Dana-Farber Cancer Institute in Boston reported at the Transplant & Cellular Therapies Meetings.
“Maribavir’s benefit was driven by lower incidence of treatment-limiting toxicities, compared with IAT,” he said a late-breaking abstract session during the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
“Available anti-CMV antivirals are limited by development of resistance and toxicities, particularly myelosuppression with the use of valganciclovir and nephrotoxicity with the use of foscarnet and cidofovir. Alternative treatment options are required to address this unmet medical need,” he said.
Maribavir inhibits the CMV UL97 protein kinase and is thought to affect several critical processes in CMV replication, including viral DNA synthesis, viral gene expression, encapsidation, and egress of mature capsids from the nucleus.
Details of trial
In the phase 3 SHP620-30e trial (NCT02931539), Dr. Marty and colleagues enrolled patients with relapsed or refractory CMV infections after hematopoietic cell transplant (HCT) or solid-organ transplant (SOT) and after stratification by transplant type and screening CMV DNA level randomly assigned them on a 2:1 basis to receive either maribavir 400 mg twice daily (235 patients) or IAT (117 patients), consisting of either ganciclovir/valganciclovir, foscarnet, cidofovir, or combined foscarnet and val/ganciclovir.
The primary endpoint of viremia clearance at 8 weeks was defined as plasma CMV DNA less than 137 IU/mL in two consecutive tests at a central laboratory at least 5 days apart beginning at the end of week 8.
The trial met its primary endpoint, with a viremia clearance rate of 55.7% with maribavir versus 23.9% with IAT.
The viremia clearance rates were similar in each of the transplant groups: 55.9% versus 20.8%, respectively, in patients who underwent HCT, and 55.6% versus 26.1% in patients who underwent SOT (P < .001).
Clearance rates among patients with CMV DNA below 9,100 IU/mL at baseline were 62.1% with maribavir versus 24.7% with IAT. Among patients with baseline CMV DNA of 9100 IU/mL or above, the respective rates were 43.9% versus 21.9%.
CMV viremia clearance continued from week 8 to week 16 in 18.7% of patients assigned to maribavir and to 10.3% of patients randomized to IAT (P < .013).
The median time to first CMV viremia clearance as 22 days with maribavir versus 27 days with IAT (P = .039).
All-cause mortality was similar between the groups, at 11.5% versus 11.1%, respectively.
The incidences of serious and severe treatment-emergent adverse events (TEAE) were 38.5% and 32.1%, respectively, in the maribavir group, and 37.1% and 37.9% in the IAT group.
Any TEAE leading to study drug discontinuation was less common with maribavir, occurring in 13.2% of patients, compared with 31.9% of patients on IAT. Serious TEAEs leading to drug discontinuation occurred in 8.5% versus 14.7%, respectively.
Serious TEAEs leading to death occurred in 6.8% of patients on maribavir versus 5.2% of those on IAT.
Role of letermovir
In the question-and-answer session following the presentation, comoderator Monalisa Ghosh, MD, from the University of Michigan, Ann Arbor, asked whether any patients in the study were currently on letermovir (Prevymis) prophylaxis, and whether any patients had previously been treated with letermovir but had CMV reactivation and were then treated on study.
Dr. Marty noted that the trial was designed before letermovir was approved for CMV prophylaxis in adults who have undergone an allogeneic HCT.
“Nobody was on letermovir at the beginning of the trial,” he replied, but noted that some patients who were enrolled and had infections that were refractory or resistant to valganciclovir, foscarnet, or a combination of the two received letermovir as secondary prophylaxis.
“I haven’t got the data to tell you how often [letermovir] was used; I think part of the lack of mortality benefit [with maribavir] may be due to the fact that people jumped into secondary prophylaxis with letermovir to minimize the toxicities that we saw,” he said.
Although maribavir has not as of this writing received Food and Drug Administration approval, the drug may be available to some patients through a compassionate-use program from Takeda, Dr. Marty noted.
The study was funded by Shire ViroPharma. Dr. Marty disclosed research funding from Shire and from others. Dr. Ghosh had no relevant disclosures.
Maribavir, an investigational antiviral agent with a novel mechanism of action, was superior to other antiviral strategies at clearing cytomegalovirus (CMV) viremia and controlling symptoms in hematopoietic cell or solid-organ transplant recipients, results of a phase 3 clinical trial showed.
CMV viremia clearance at study week 8 was seen in 55.7% of all patients randomized to receive maribavir, compared with 23.9% for patients assigned to receive investigator-assigned therapy (IAT), Francisco Marty, MD, from the Dana-Farber Cancer Institute in Boston reported at the Transplant & Cellular Therapies Meetings.
“Maribavir’s benefit was driven by lower incidence of treatment-limiting toxicities, compared with IAT,” he said a late-breaking abstract session during the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
“Available anti-CMV antivirals are limited by development of resistance and toxicities, particularly myelosuppression with the use of valganciclovir and nephrotoxicity with the use of foscarnet and cidofovir. Alternative treatment options are required to address this unmet medical need,” he said.
Maribavir inhibits the CMV UL97 protein kinase and is thought to affect several critical processes in CMV replication, including viral DNA synthesis, viral gene expression, encapsidation, and egress of mature capsids from the nucleus.
Details of trial
In the phase 3 SHP620-30e trial (NCT02931539), Dr. Marty and colleagues enrolled patients with relapsed or refractory CMV infections after hematopoietic cell transplant (HCT) or solid-organ transplant (SOT) and after stratification by transplant type and screening CMV DNA level randomly assigned them on a 2:1 basis to receive either maribavir 400 mg twice daily (235 patients) or IAT (117 patients), consisting of either ganciclovir/valganciclovir, foscarnet, cidofovir, or combined foscarnet and val/ganciclovir.
The primary endpoint of viremia clearance at 8 weeks was defined as plasma CMV DNA less than 137 IU/mL in two consecutive tests at a central laboratory at least 5 days apart beginning at the end of week 8.
The trial met its primary endpoint, with a viremia clearance rate of 55.7% with maribavir versus 23.9% with IAT.
The viremia clearance rates were similar in each of the transplant groups: 55.9% versus 20.8%, respectively, in patients who underwent HCT, and 55.6% versus 26.1% in patients who underwent SOT (P < .001).
Clearance rates among patients with CMV DNA below 9,100 IU/mL at baseline were 62.1% with maribavir versus 24.7% with IAT. Among patients with baseline CMV DNA of 9100 IU/mL or above, the respective rates were 43.9% versus 21.9%.
CMV viremia clearance continued from week 8 to week 16 in 18.7% of patients assigned to maribavir and to 10.3% of patients randomized to IAT (P < .013).
The median time to first CMV viremia clearance as 22 days with maribavir versus 27 days with IAT (P = .039).
All-cause mortality was similar between the groups, at 11.5% versus 11.1%, respectively.
The incidences of serious and severe treatment-emergent adverse events (TEAE) were 38.5% and 32.1%, respectively, in the maribavir group, and 37.1% and 37.9% in the IAT group.
Any TEAE leading to study drug discontinuation was less common with maribavir, occurring in 13.2% of patients, compared with 31.9% of patients on IAT. Serious TEAEs leading to drug discontinuation occurred in 8.5% versus 14.7%, respectively.
Serious TEAEs leading to death occurred in 6.8% of patients on maribavir versus 5.2% of those on IAT.
Role of letermovir
In the question-and-answer session following the presentation, comoderator Monalisa Ghosh, MD, from the University of Michigan, Ann Arbor, asked whether any patients in the study were currently on letermovir (Prevymis) prophylaxis, and whether any patients had previously been treated with letermovir but had CMV reactivation and were then treated on study.
Dr. Marty noted that the trial was designed before letermovir was approved for CMV prophylaxis in adults who have undergone an allogeneic HCT.
“Nobody was on letermovir at the beginning of the trial,” he replied, but noted that some patients who were enrolled and had infections that were refractory or resistant to valganciclovir, foscarnet, or a combination of the two received letermovir as secondary prophylaxis.
“I haven’t got the data to tell you how often [letermovir] was used; I think part of the lack of mortality benefit [with maribavir] may be due to the fact that people jumped into secondary prophylaxis with letermovir to minimize the toxicities that we saw,” he said.
Although maribavir has not as of this writing received Food and Drug Administration approval, the drug may be available to some patients through a compassionate-use program from Takeda, Dr. Marty noted.
The study was funded by Shire ViroPharma. Dr. Marty disclosed research funding from Shire and from others. Dr. Ghosh had no relevant disclosures.
FROM TCT 2021