User login
How much is enough?
How much do I make compared with other doctors?
I see questions like that on surveys I get, asking me to fill something out on the Internet, then I’ll get back a list of how well other docs in my field/city/state/blood type are doing.
Nah. I’ll pass.
Realistically, why? So I can feel I’m superior or inferior to others? Isn’t keeping up with the Joneses the purpose of the doctors’ parking lot at the hospital? (Actually, the number of pricey cars there has dropped off over time).
I really don’t want to know how much others make. It’s probably more than what I make, but that’s the trade-off I accepted when I went with a small solo practice instead of a large group 20 years ago.
We become so obsessed with the question of “how much money should I be making?” and comparing it with the salaries of others that we lose track of the real question: “How much money do I need?”
That should be the real number to look at. How much money do I really need to pay for a comfortable home, support my family, pay for my kids’ education, fund my retirement?
Enough should be as good as a feast.
Yet, even when content we get caught in the trap of comparing ourselves with others. This is human nature. We’re programmed to be competitive to survive. Whether that means anything when we don’t have to be hunters and gatherers is irrelevant. It is who we are.
But we’re also intelligent enough to realize that. I for one, don’t want to know, or care, how much money the neurologist down the street is earning.
To quote Sheryl Crow, “it’s not having what you want, it’s wanting what you’ve got.”
So I’ll skip the comparisons and focus on the only people that really matter to me.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
How much do I make compared with other doctors?
I see questions like that on surveys I get, asking me to fill something out on the Internet, then I’ll get back a list of how well other docs in my field/city/state/blood type are doing.
Nah. I’ll pass.
Realistically, why? So I can feel I’m superior or inferior to others? Isn’t keeping up with the Joneses the purpose of the doctors’ parking lot at the hospital? (Actually, the number of pricey cars there has dropped off over time).
I really don’t want to know how much others make. It’s probably more than what I make, but that’s the trade-off I accepted when I went with a small solo practice instead of a large group 20 years ago.
We become so obsessed with the question of “how much money should I be making?” and comparing it with the salaries of others that we lose track of the real question: “How much money do I need?”
That should be the real number to look at. How much money do I really need to pay for a comfortable home, support my family, pay for my kids’ education, fund my retirement?
Enough should be as good as a feast.
Yet, even when content we get caught in the trap of comparing ourselves with others. This is human nature. We’re programmed to be competitive to survive. Whether that means anything when we don’t have to be hunters and gatherers is irrelevant. It is who we are.
But we’re also intelligent enough to realize that. I for one, don’t want to know, or care, how much money the neurologist down the street is earning.
To quote Sheryl Crow, “it’s not having what you want, it’s wanting what you’ve got.”
So I’ll skip the comparisons and focus on the only people that really matter to me.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
How much do I make compared with other doctors?
I see questions like that on surveys I get, asking me to fill something out on the Internet, then I’ll get back a list of how well other docs in my field/city/state/blood type are doing.
Nah. I’ll pass.
Realistically, why? So I can feel I’m superior or inferior to others? Isn’t keeping up with the Joneses the purpose of the doctors’ parking lot at the hospital? (Actually, the number of pricey cars there has dropped off over time).
I really don’t want to know how much others make. It’s probably more than what I make, but that’s the trade-off I accepted when I went with a small solo practice instead of a large group 20 years ago.
We become so obsessed with the question of “how much money should I be making?” and comparing it with the salaries of others that we lose track of the real question: “How much money do I need?”
That should be the real number to look at. How much money do I really need to pay for a comfortable home, support my family, pay for my kids’ education, fund my retirement?
Enough should be as good as a feast.
Yet, even when content we get caught in the trap of comparing ourselves with others. This is human nature. We’re programmed to be competitive to survive. Whether that means anything when we don’t have to be hunters and gatherers is irrelevant. It is who we are.
But we’re also intelligent enough to realize that. I for one, don’t want to know, or care, how much money the neurologist down the street is earning.
To quote Sheryl Crow, “it’s not having what you want, it’s wanting what you’ve got.”
So I’ll skip the comparisons and focus on the only people that really matter to me.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Cutaneous Insulin-Derived Amyloidosis Presenting as Hyperkeratotic Nodules
Amyloidosis consists of approximately 30 protein-folding disorders sharing the common feature of abnormal extracellular amyloid deposition. In each condition, a specific soluble precursor protein aggregates to form the insoluble fibrils of amyloid, characterized by the beta-pleated sheet structure.1 Amyloidosis occurs as either a systemic or localized process. Insulin-derived (AIns) amyloidosis, a localized process occurring at insulin injection sites, was first reported in 1983.2 There were fewer than 20 reported cases until 2014, when 57 additional cases were reported by just 2 institutions,3,4 indicating that AIns amyloidosis may be more common than previously thought.3,5
Despite the increasing prevalence of diabetes mellitus and insulin use, there is a paucity of published cases of AIns amyloidosis. The lack of awareness of this condition among both dermatologists and general practitioners may be in part due to its variable clinical manifestations. We describe 2 patients with unique presentations of localized amyloidosis at repeated insulin injection sites.
Case Reports
Patient 1
A 39-year-old man with a history of type 1 diabetes mellitus presented with 4 asymptomatic nodules on the lateral thighs in areas of previous insulin injection. He first noticed the lesions 9 months prior to presentation and subsequently switched the injection site to the abdomen without development of new nodules. Despite being compliant with his insulin regimen, he had a long history of irregular glucose control, including frequent hypoglycemic episodes. The patient was using regular and neutral protamine hagedorn insulin.
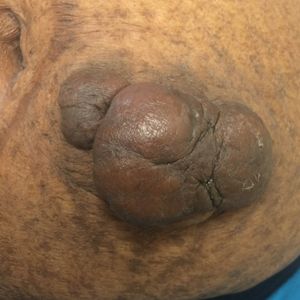
On physical examination, 2 soft, nontender, exophytic nodules were noted on each upper thigh with surrounding hyperpigmented and hyperkeratotic collarettes (Figure 1). The nodules ranged in size from 2 to 3.5 cm in diameter.
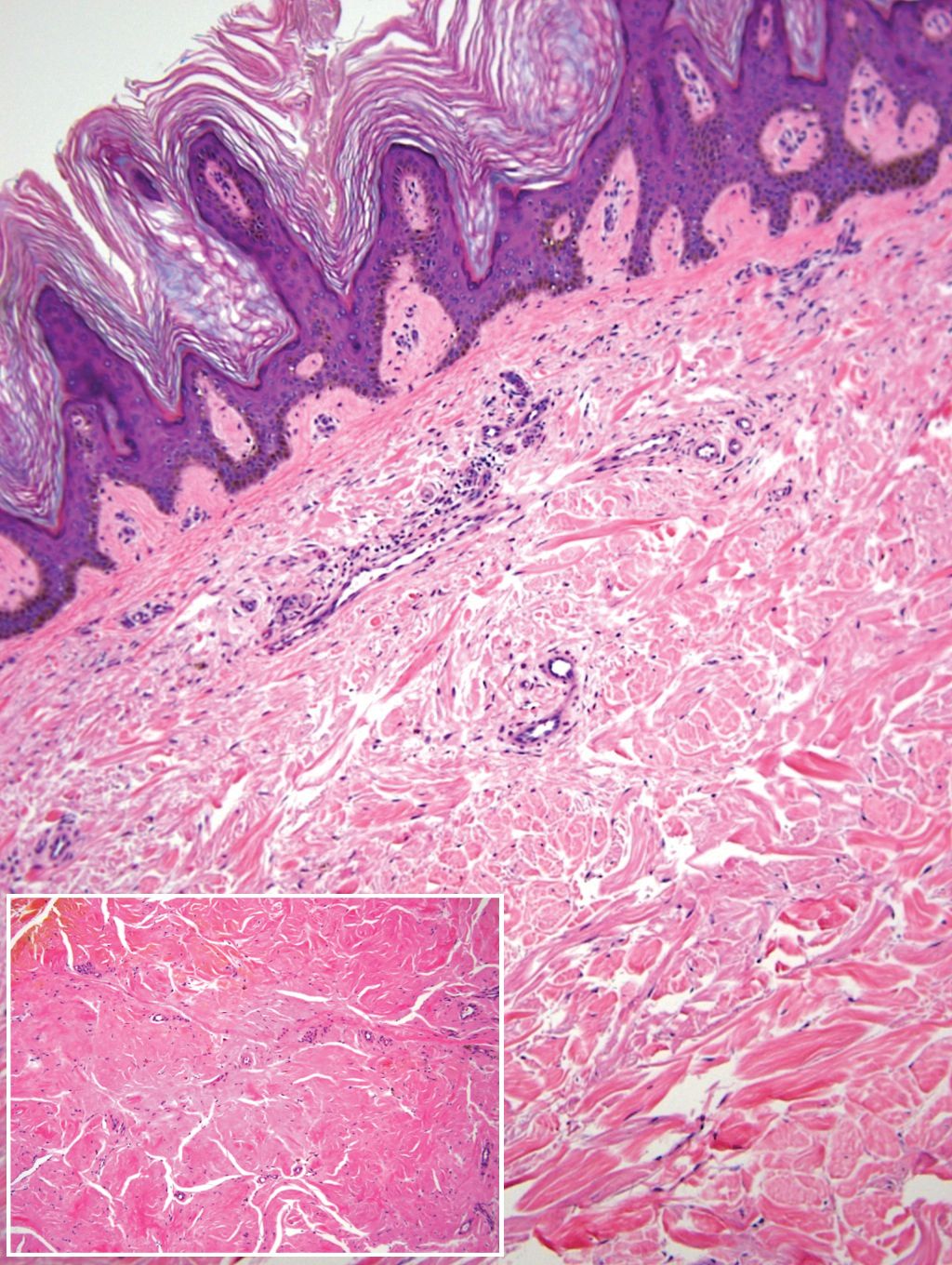
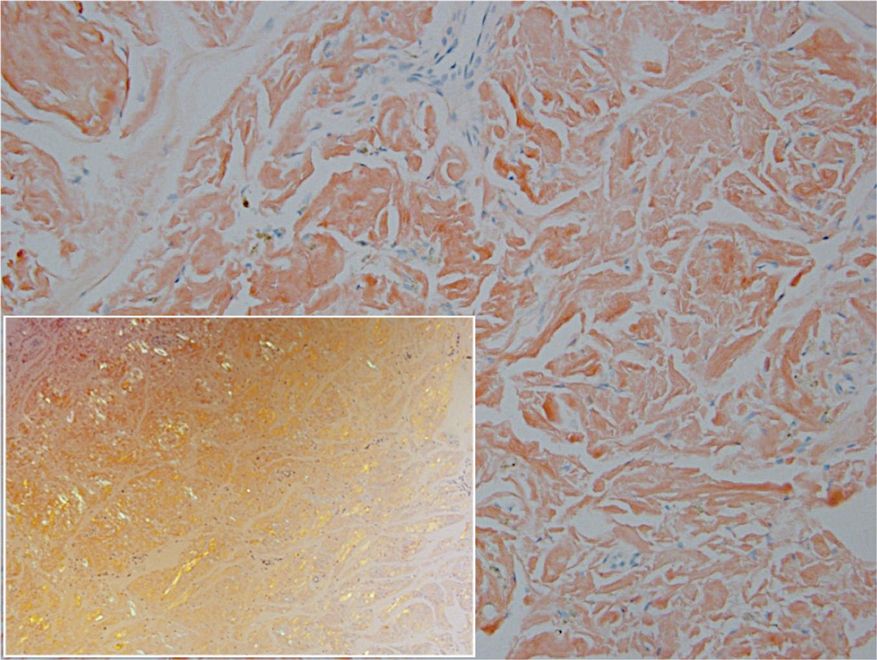
Remarkable laboratory data included a fasting glucose level of 207 mg/dL (reference range, 70–110 mg/dL) and a glycohemoglobin of 8.8% (reference range, <5.7%). Serum protein electrophoresis and immunofixation were normal. Histopathology of the lesions demonstrated diffuse deposition of pink amorphous material associated with prominent papillomatosis, hyperkeratosis, and acanthosis (Figure 2). Congo red staining was positive with green birefringence under polarized light, indicative of amyloid deposits (Figure 3). Liquid chromatography–tandem mass spectrometry of the specimens was consistent with deposition of AIns amyloidosis.
Due to the size and persistent nature of the lesions, the nodules were removed by tangential excision. In addition, the patient was advised to continue rotating injection sites frequently. His blood glucose levels are now well controlled, and he has not developed any new nodules.
Patient 2
A 53-year-old woman with a history of type 2 diabetes mellitus presented with painful subcutaneous nodules on the lower abdomen at sites of previous insulin injections. The nodules developed approximately 1 month after she started treatment with neutral protamine hagedorn insulin and had been slowly enlarging over the past year. She tried switching injection sites after noticing the lesions, but the nodules persisted. The patient had a long history of poor glucose control with chronically elevated glycohemoglobin and blood glucose levels.
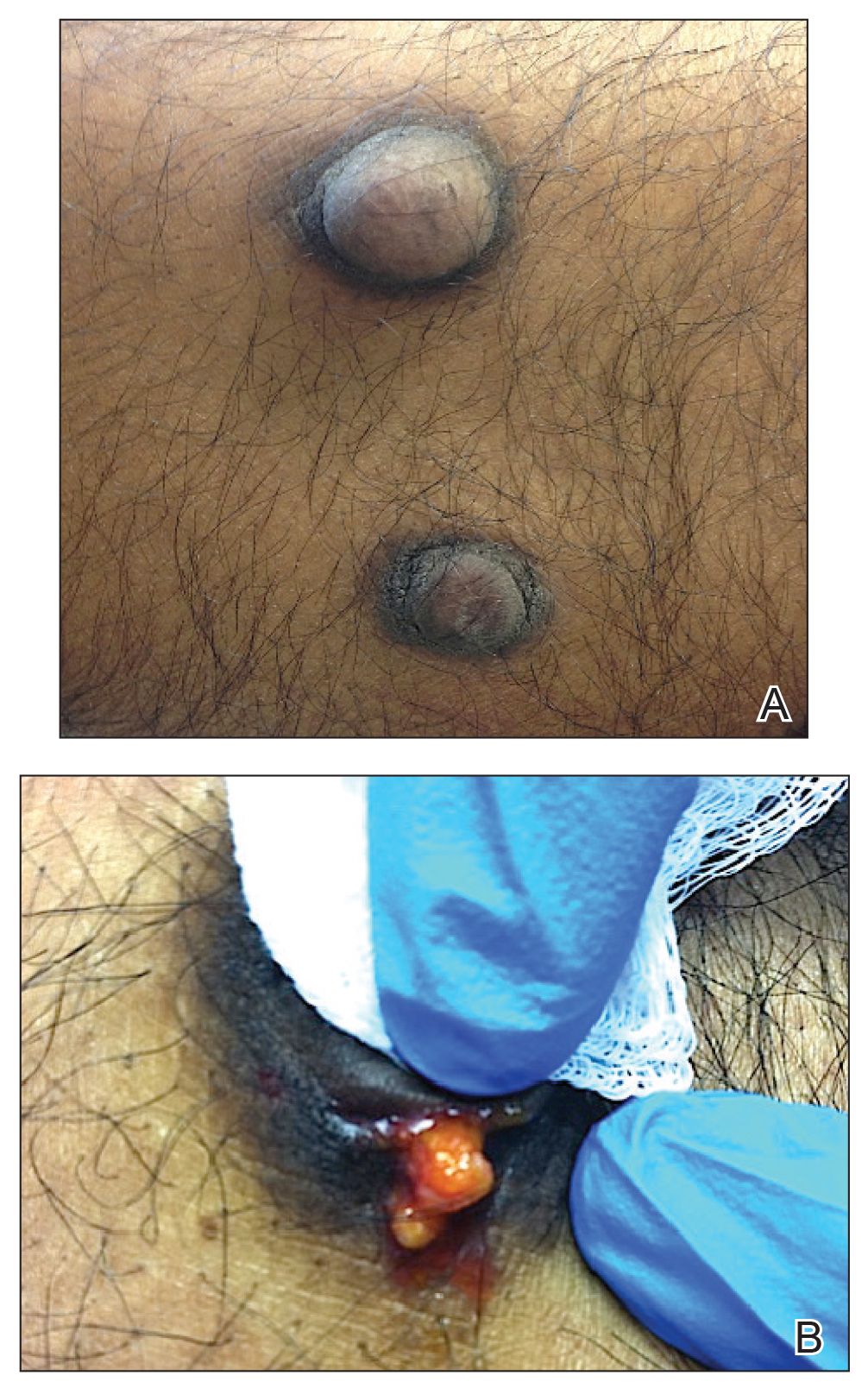
On physical examination, 2 hyperpigmented, exophytic, smooth nodules were noted on the right and left lower abdomen, ranging in size from 2.5 to 5.5 cm in diameter (Figure 4).
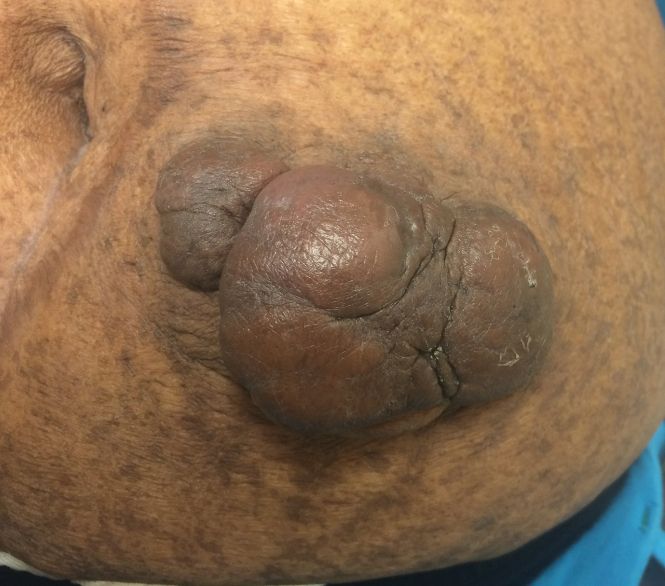
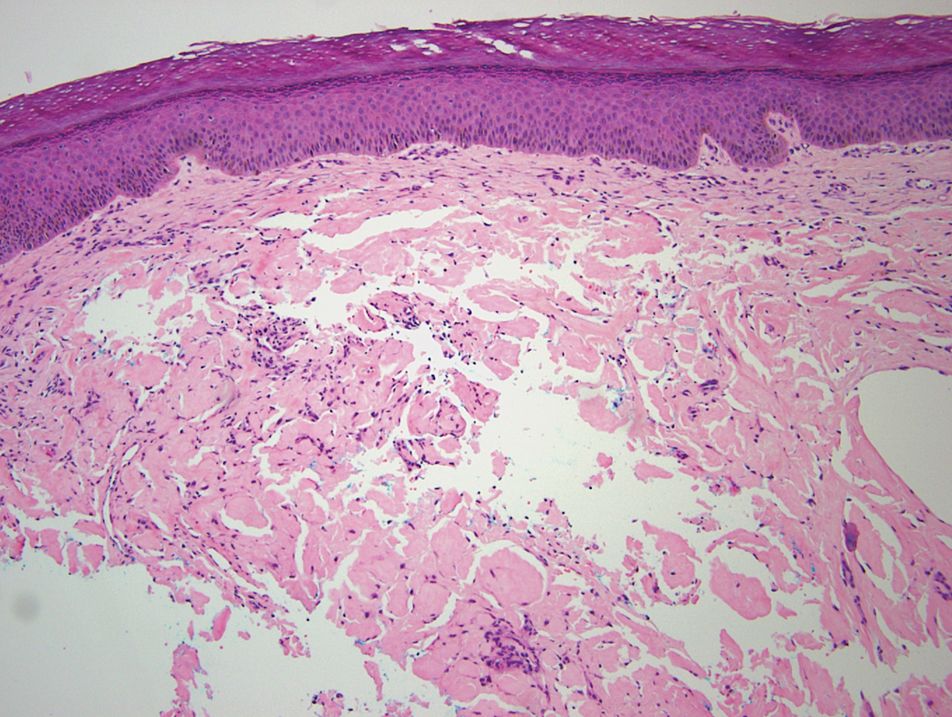
Relevant laboratory data included a fasting glucose level of 197 mg/dL and a glycohemoglobin of 9.3%. A biopsy of the lesion on the left lower abdomen revealed eosinophilic amorphous deposits with fissuring in the dermis (Figure 5). Congo red stain was positive with green birefringence under polarized light. Liquid chromatography–tandem mass spectrometry of the specimen showed deposition of AIns amyloid. The patient began injecting away from the amyloid nodules without development of any new lesions. The original nodules have persisted, and surgical excision is planned.
Comment
Insulin is the suspected precursor protein in AIns amyloidosis, but the exact pathogenesis is unknown. The protein that is derived from insulin in these tumors is now identified as AIns amyloidosis.5,6 It is hypothesized that insulin accumulates locally and is converted to amyloid by an unknown mechanism.7 Other potential contributory factors include chronic inflammation and foreign body reactions developing around amyloid deposits, as well as repeated trauma from injections into a single site.4,5 It appears that lesions may derive from a wide range of insulin types and occur after variable time periods.
A majority of cases of iatrogenic amyloid have been described as single, firm, subcutaneous masses at an injection site that commonly are misdiagnosed as lipomas or lipohypertrophy.7-11 To our knowledge, none of the reported cases resembled the multiple, discrete, exophytic nodules seen in our patients.3,4 The surrounding hyperkeratosis noted in patient 1 is another uncommon feature of AIns amyloidosis (Figures 1 and 2). Only 3 AIns amyloidosis cases described lesions with acanthosis nigricans–like changes, only 1 of which provided a clinical image.6,7,12The mechanism for the acanthosis nigricans–like changes may have been due to the high levels of insulin at the injection site. It has been suggested that the activation of insulinlike growth factor receptor by insulin leads to the proliferation of keratinocytes and fibroblasts.6 Histologic examination of AIns amyloidosis lesions generally demonstrates deposition of homogenous eosinophilic material consistent with amyloid, as well as positive Congo red staining with green birefringence by polarization. Immunohistologic staining with insulin antibody with or without proteomic analysis of the amyloid deposits can confirm the diagnosis. In both of our patients’ specimens, liquid chromatography–tandem mass spectrometry was performed for proteomic analysis, and results were consistent with AIns amyloidosis.
Reports in the literature have suggested that the deposition of amyloid at insulin injection sites has the potential to interfere with insulin absorption, leading to poor glucose control.4,11,13 Hence, injection site rotation is a crucial aspect of treatment and prevention of AIns amyloidosis. In their study of 4 patients, Nagase et al4 compared serum insulin levels after insulin injection into amyloid nodules vs insulin levels after injection into normal skin. Insulin absorption at the amyloid sites was 34% of that at normal sites. Given these results, patients should be instructed to inject away from the amyloid deposit once it is identified.6 Glucose levels should be monitored closely when patients first inject away from the amyloid mass, as injection of the same dosage to an area of normal skin can lead to increased insulin absorption and hypoglycemia.4,6 It is possible that the frequent hypoglycemic episodes noted in patient 1 were due to increased insulin sensitivity after switching to injection sites away from amyloid lesions.
Conclusion
Our patients demonstrate unique presentations of localized cutaneous amyloidosis at repeated insulin injection sites. We report these cases to complement the current data of iatrogenic amyloidosis and provide insight into this likely underreported phenomenon.
- Hazenberg BPC. Amyloidosis: a clinical overview. Rheum Dis Clin North Am. 2013;39:323-345.
- Storkel S, Schneider HM, Muntefering H, et al. Iatrogenic, insulin-dependent, local amyloidosis. Lab Invest. 1983;48:108-111.
- D’souza A, Theis JD, Vrana JA, et al. Pharmaceutical amyloidosis associated with subcutaneous insulin and enfuvirtide administration. Amyloid. 2014;21:71-75.
- Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127:450-454.
- Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19:174-177.
- Kudo-Watanuki S, Kurihara E, Yamamoto K, et al. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25-29.
- Yumlu S, Barany R, Eriksson M, et al. Localized insulin-derived amyloidosis in patients with diabetes mellitus: a case report. Hum Pathol. 2009;40:1655-1660.
- Okamura S, Hayashino Y, Kore-Eda S, et al. Localized amyloidosis at the site of repeated insulin injection in a patient with type 2 diabetes. Diabetes Care. 2013;36:E200.
- Dische FE, Wernstedt C, Westermark GT, et al. Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988;31:158-161.
- Swift B, Hawkins PN, Richards C, et al. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabetic Med. 2002;19:881-882.
- Albert SG, Obadiah J, Parseghian SA, et al. Severe insulin resistance associated with subcutaneous amyloid deposition. Diabetes Res Clin Pract. 2007;75:374-376.
- Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57:127-129.
- Endo JO, Rocken C, Lamb S, et al. Nodular amyloidosis in a diabetic patient with frequent hypoglycemia: sequelae of repeatedly injecting insulin without site rotation. J Am Acad Dermatol. 2010;63:E113-E114.
Amyloidosis consists of approximately 30 protein-folding disorders sharing the common feature of abnormal extracellular amyloid deposition. In each condition, a specific soluble precursor protein aggregates to form the insoluble fibrils of amyloid, characterized by the beta-pleated sheet structure.1 Amyloidosis occurs as either a systemic or localized process. Insulin-derived (AIns) amyloidosis, a localized process occurring at insulin injection sites, was first reported in 1983.2 There were fewer than 20 reported cases until 2014, when 57 additional cases were reported by just 2 institutions,3,4 indicating that AIns amyloidosis may be more common than previously thought.3,5
Despite the increasing prevalence of diabetes mellitus and insulin use, there is a paucity of published cases of AIns amyloidosis. The lack of awareness of this condition among both dermatologists and general practitioners may be in part due to its variable clinical manifestations. We describe 2 patients with unique presentations of localized amyloidosis at repeated insulin injection sites.
Case Reports
Patient 1
A 39-year-old man with a history of type 1 diabetes mellitus presented with 4 asymptomatic nodules on the lateral thighs in areas of previous insulin injection. He first noticed the lesions 9 months prior to presentation and subsequently switched the injection site to the abdomen without development of new nodules. Despite being compliant with his insulin regimen, he had a long history of irregular glucose control, including frequent hypoglycemic episodes. The patient was using regular and neutral protamine hagedorn insulin.
On physical examination, 2 soft, nontender, exophytic nodules were noted on each upper thigh with surrounding hyperpigmented and hyperkeratotic collarettes (Figure 1). The nodules ranged in size from 2 to 3.5 cm in diameter.

Remarkable laboratory data included a fasting glucose level of 207 mg/dL (reference range, 70–110 mg/dL) and a glycohemoglobin of 8.8% (reference range, <5.7%). Serum protein electrophoresis and immunofixation were normal. Histopathology of the lesions demonstrated diffuse deposition of pink amorphous material associated with prominent papillomatosis, hyperkeratosis, and acanthosis (Figure 2). Congo red staining was positive with green birefringence under polarized light, indicative of amyloid deposits (Figure 3). Liquid chromatography–tandem mass spectrometry of the specimens was consistent with deposition of AIns amyloidosis.


Due to the size and persistent nature of the lesions, the nodules were removed by tangential excision. In addition, the patient was advised to continue rotating injection sites frequently. His blood glucose levels are now well controlled, and he has not developed any new nodules.
Patient 2
A 53-year-old woman with a history of type 2 diabetes mellitus presented with painful subcutaneous nodules on the lower abdomen at sites of previous insulin injections. The nodules developed approximately 1 month after she started treatment with neutral protamine hagedorn insulin and had been slowly enlarging over the past year. She tried switching injection sites after noticing the lesions, but the nodules persisted. The patient had a long history of poor glucose control with chronically elevated glycohemoglobin and blood glucose levels.
On physical examination, 2 hyperpigmented, exophytic, smooth nodules were noted on the right and left lower abdomen, ranging in size from 2.5 to 5.5 cm in diameter (Figure 4).

Relevant laboratory data included a fasting glucose level of 197 mg/dL and a glycohemoglobin of 9.3%. A biopsy of the lesion on the left lower abdomen revealed eosinophilic amorphous deposits with fissuring in the dermis (Figure 5). Congo red stain was positive with green birefringence under polarized light. Liquid chromatography–tandem mass spectrometry of the specimen showed deposition of AIns amyloid. The patient began injecting away from the amyloid nodules without development of any new lesions. The original nodules have persisted, and surgical excision is planned.

Comment
Insulin is the suspected precursor protein in AIns amyloidosis, but the exact pathogenesis is unknown. The protein that is derived from insulin in these tumors is now identified as AIns amyloidosis.5,6 It is hypothesized that insulin accumulates locally and is converted to amyloid by an unknown mechanism.7 Other potential contributory factors include chronic inflammation and foreign body reactions developing around amyloid deposits, as well as repeated trauma from injections into a single site.4,5 It appears that lesions may derive from a wide range of insulin types and occur after variable time periods.
A majority of cases of iatrogenic amyloid have been described as single, firm, subcutaneous masses at an injection site that commonly are misdiagnosed as lipomas or lipohypertrophy.7-11 To our knowledge, none of the reported cases resembled the multiple, discrete, exophytic nodules seen in our patients.3,4 The surrounding hyperkeratosis noted in patient 1 is another uncommon feature of AIns amyloidosis (Figures 1 and 2). Only 3 AIns amyloidosis cases described lesions with acanthosis nigricans–like changes, only 1 of which provided a clinical image.6,7,12The mechanism for the acanthosis nigricans–like changes may have been due to the high levels of insulin at the injection site. It has been suggested that the activation of insulinlike growth factor receptor by insulin leads to the proliferation of keratinocytes and fibroblasts.6 Histologic examination of AIns amyloidosis lesions generally demonstrates deposition of homogenous eosinophilic material consistent with amyloid, as well as positive Congo red staining with green birefringence by polarization. Immunohistologic staining with insulin antibody with or without proteomic analysis of the amyloid deposits can confirm the diagnosis. In both of our patients’ specimens, liquid chromatography–tandem mass spectrometry was performed for proteomic analysis, and results were consistent with AIns amyloidosis.
Reports in the literature have suggested that the deposition of amyloid at insulin injection sites has the potential to interfere with insulin absorption, leading to poor glucose control.4,11,13 Hence, injection site rotation is a crucial aspect of treatment and prevention of AIns amyloidosis. In their study of 4 patients, Nagase et al4 compared serum insulin levels after insulin injection into amyloid nodules vs insulin levels after injection into normal skin. Insulin absorption at the amyloid sites was 34% of that at normal sites. Given these results, patients should be instructed to inject away from the amyloid deposit once it is identified.6 Glucose levels should be monitored closely when patients first inject away from the amyloid mass, as injection of the same dosage to an area of normal skin can lead to increased insulin absorption and hypoglycemia.4,6 It is possible that the frequent hypoglycemic episodes noted in patient 1 were due to increased insulin sensitivity after switching to injection sites away from amyloid lesions.
Conclusion
Our patients demonstrate unique presentations of localized cutaneous amyloidosis at repeated insulin injection sites. We report these cases to complement the current data of iatrogenic amyloidosis and provide insight into this likely underreported phenomenon.
Amyloidosis consists of approximately 30 protein-folding disorders sharing the common feature of abnormal extracellular amyloid deposition. In each condition, a specific soluble precursor protein aggregates to form the insoluble fibrils of amyloid, characterized by the beta-pleated sheet structure.1 Amyloidosis occurs as either a systemic or localized process. Insulin-derived (AIns) amyloidosis, a localized process occurring at insulin injection sites, was first reported in 1983.2 There were fewer than 20 reported cases until 2014, when 57 additional cases were reported by just 2 institutions,3,4 indicating that AIns amyloidosis may be more common than previously thought.3,5
Despite the increasing prevalence of diabetes mellitus and insulin use, there is a paucity of published cases of AIns amyloidosis. The lack of awareness of this condition among both dermatologists and general practitioners may be in part due to its variable clinical manifestations. We describe 2 patients with unique presentations of localized amyloidosis at repeated insulin injection sites.
Case Reports
Patient 1
A 39-year-old man with a history of type 1 diabetes mellitus presented with 4 asymptomatic nodules on the lateral thighs in areas of previous insulin injection. He first noticed the lesions 9 months prior to presentation and subsequently switched the injection site to the abdomen without development of new nodules. Despite being compliant with his insulin regimen, he had a long history of irregular glucose control, including frequent hypoglycemic episodes. The patient was using regular and neutral protamine hagedorn insulin.
On physical examination, 2 soft, nontender, exophytic nodules were noted on each upper thigh with surrounding hyperpigmented and hyperkeratotic collarettes (Figure 1). The nodules ranged in size from 2 to 3.5 cm in diameter.

Remarkable laboratory data included a fasting glucose level of 207 mg/dL (reference range, 70–110 mg/dL) and a glycohemoglobin of 8.8% (reference range, <5.7%). Serum protein electrophoresis and immunofixation were normal. Histopathology of the lesions demonstrated diffuse deposition of pink amorphous material associated with prominent papillomatosis, hyperkeratosis, and acanthosis (Figure 2). Congo red staining was positive with green birefringence under polarized light, indicative of amyloid deposits (Figure 3). Liquid chromatography–tandem mass spectrometry of the specimens was consistent with deposition of AIns amyloidosis.


Due to the size and persistent nature of the lesions, the nodules were removed by tangential excision. In addition, the patient was advised to continue rotating injection sites frequently. His blood glucose levels are now well controlled, and he has not developed any new nodules.
Patient 2
A 53-year-old woman with a history of type 2 diabetes mellitus presented with painful subcutaneous nodules on the lower abdomen at sites of previous insulin injections. The nodules developed approximately 1 month after she started treatment with neutral protamine hagedorn insulin and had been slowly enlarging over the past year. She tried switching injection sites after noticing the lesions, but the nodules persisted. The patient had a long history of poor glucose control with chronically elevated glycohemoglobin and blood glucose levels.
On physical examination, 2 hyperpigmented, exophytic, smooth nodules were noted on the right and left lower abdomen, ranging in size from 2.5 to 5.5 cm in diameter (Figure 4).

Relevant laboratory data included a fasting glucose level of 197 mg/dL and a glycohemoglobin of 9.3%. A biopsy of the lesion on the left lower abdomen revealed eosinophilic amorphous deposits with fissuring in the dermis (Figure 5). Congo red stain was positive with green birefringence under polarized light. Liquid chromatography–tandem mass spectrometry of the specimen showed deposition of AIns amyloid. The patient began injecting away from the amyloid nodules without development of any new lesions. The original nodules have persisted, and surgical excision is planned.

Comment
Insulin is the suspected precursor protein in AIns amyloidosis, but the exact pathogenesis is unknown. The protein that is derived from insulin in these tumors is now identified as AIns amyloidosis.5,6 It is hypothesized that insulin accumulates locally and is converted to amyloid by an unknown mechanism.7 Other potential contributory factors include chronic inflammation and foreign body reactions developing around amyloid deposits, as well as repeated trauma from injections into a single site.4,5 It appears that lesions may derive from a wide range of insulin types and occur after variable time periods.
A majority of cases of iatrogenic amyloid have been described as single, firm, subcutaneous masses at an injection site that commonly are misdiagnosed as lipomas or lipohypertrophy.7-11 To our knowledge, none of the reported cases resembled the multiple, discrete, exophytic nodules seen in our patients.3,4 The surrounding hyperkeratosis noted in patient 1 is another uncommon feature of AIns amyloidosis (Figures 1 and 2). Only 3 AIns amyloidosis cases described lesions with acanthosis nigricans–like changes, only 1 of which provided a clinical image.6,7,12The mechanism for the acanthosis nigricans–like changes may have been due to the high levels of insulin at the injection site. It has been suggested that the activation of insulinlike growth factor receptor by insulin leads to the proliferation of keratinocytes and fibroblasts.6 Histologic examination of AIns amyloidosis lesions generally demonstrates deposition of homogenous eosinophilic material consistent with amyloid, as well as positive Congo red staining with green birefringence by polarization. Immunohistologic staining with insulin antibody with or without proteomic analysis of the amyloid deposits can confirm the diagnosis. In both of our patients’ specimens, liquid chromatography–tandem mass spectrometry was performed for proteomic analysis, and results were consistent with AIns amyloidosis.
Reports in the literature have suggested that the deposition of amyloid at insulin injection sites has the potential to interfere with insulin absorption, leading to poor glucose control.4,11,13 Hence, injection site rotation is a crucial aspect of treatment and prevention of AIns amyloidosis. In their study of 4 patients, Nagase et al4 compared serum insulin levels after insulin injection into amyloid nodules vs insulin levels after injection into normal skin. Insulin absorption at the amyloid sites was 34% of that at normal sites. Given these results, patients should be instructed to inject away from the amyloid deposit once it is identified.6 Glucose levels should be monitored closely when patients first inject away from the amyloid mass, as injection of the same dosage to an area of normal skin can lead to increased insulin absorption and hypoglycemia.4,6 It is possible that the frequent hypoglycemic episodes noted in patient 1 were due to increased insulin sensitivity after switching to injection sites away from amyloid lesions.
Conclusion
Our patients demonstrate unique presentations of localized cutaneous amyloidosis at repeated insulin injection sites. We report these cases to complement the current data of iatrogenic amyloidosis and provide insight into this likely underreported phenomenon.
- Hazenberg BPC. Amyloidosis: a clinical overview. Rheum Dis Clin North Am. 2013;39:323-345.
- Storkel S, Schneider HM, Muntefering H, et al. Iatrogenic, insulin-dependent, local amyloidosis. Lab Invest. 1983;48:108-111.
- D’souza A, Theis JD, Vrana JA, et al. Pharmaceutical amyloidosis associated with subcutaneous insulin and enfuvirtide administration. Amyloid. 2014;21:71-75.
- Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127:450-454.
- Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19:174-177.
- Kudo-Watanuki S, Kurihara E, Yamamoto K, et al. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25-29.
- Yumlu S, Barany R, Eriksson M, et al. Localized insulin-derived amyloidosis in patients with diabetes mellitus: a case report. Hum Pathol. 2009;40:1655-1660.
- Okamura S, Hayashino Y, Kore-Eda S, et al. Localized amyloidosis at the site of repeated insulin injection in a patient with type 2 diabetes. Diabetes Care. 2013;36:E200.
- Dische FE, Wernstedt C, Westermark GT, et al. Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988;31:158-161.
- Swift B, Hawkins PN, Richards C, et al. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabetic Med. 2002;19:881-882.
- Albert SG, Obadiah J, Parseghian SA, et al. Severe insulin resistance associated with subcutaneous amyloid deposition. Diabetes Res Clin Pract. 2007;75:374-376.
- Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57:127-129.
- Endo JO, Rocken C, Lamb S, et al. Nodular amyloidosis in a diabetic patient with frequent hypoglycemia: sequelae of repeatedly injecting insulin without site rotation. J Am Acad Dermatol. 2010;63:E113-E114.
- Hazenberg BPC. Amyloidosis: a clinical overview. Rheum Dis Clin North Am. 2013;39:323-345.
- Storkel S, Schneider HM, Muntefering H, et al. Iatrogenic, insulin-dependent, local amyloidosis. Lab Invest. 1983;48:108-111.
- D’souza A, Theis JD, Vrana JA, et al. Pharmaceutical amyloidosis associated with subcutaneous insulin and enfuvirtide administration. Amyloid. 2014;21:71-75.
- Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127:450-454.
- Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19:174-177.
- Kudo-Watanuki S, Kurihara E, Yamamoto K, et al. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25-29.
- Yumlu S, Barany R, Eriksson M, et al. Localized insulin-derived amyloidosis in patients with diabetes mellitus: a case report. Hum Pathol. 2009;40:1655-1660.
- Okamura S, Hayashino Y, Kore-Eda S, et al. Localized amyloidosis at the site of repeated insulin injection in a patient with type 2 diabetes. Diabetes Care. 2013;36:E200.
- Dische FE, Wernstedt C, Westermark GT, et al. Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988;31:158-161.
- Swift B, Hawkins PN, Richards C, et al. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabetic Med. 2002;19:881-882.
- Albert SG, Obadiah J, Parseghian SA, et al. Severe insulin resistance associated with subcutaneous amyloid deposition. Diabetes Res Clin Pract. 2007;75:374-376.
- Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57:127-129.
- Endo JO, Rocken C, Lamb S, et al. Nodular amyloidosis in a diabetic patient with frequent hypoglycemia: sequelae of repeatedly injecting insulin without site rotation. J Am Acad Dermatol. 2010;63:E113-E114.
Practice Points
- Deposition of amyloid at insulin injection sites has the potential to interfere with insulin absorption, leading to poor glucose control.
- Patients with insulin-derived (AIns) amyloidosis may initially present after noticing nodular deposits.
- Insulin injection site rotation is a crucial aspect of treatment and prevention of AIns amyloidosis.
Boost dose reduces recurrence in high-risk DCIS
Giving a tumor bed boost (TBB) reduced the risk of local recurrence and overall disease recurrence, but there were no significant differences in recurrence rates between conventional WBI and hypofractionated WBI.
Boon Hui Chua, MD, of the University of New South Wales, Sydney, presented these results at the 2020 San Antonio Breast Cancer Symposium.
Dr. Chua and colleagues studied 1,608 women with DCIS resected by conservative surgery. Patients were either younger than 50 years or age 50 and older with at least one of the following risk factors: symptomatic presentation, palpable tumor, multifocal disease, tumor size 1.5 cm or larger, intermediate or high nuclear grade, central necrosis, comedo histology, and/or surgical margins less than 10 mm.
The patients were randomized to treatment in three categories. In randomization A (n = 503), patients were randomized to one of the following treatments:
- WBI at 50 Gy in 25 fractions
- WBI at 42.5 Gy in 16 fractions
- WBI at 50 Gy in 25 fractions plus TBB at 16 Gy in 8 fractions
- WBI at 42.5 Gy in 16 fractions plus TBB at 16 Gy in 8 fractions.
In randomization B (n = 581), patients received WBI at 50 Gy in 25 fractions, with or without TBB at 16 Gy in 8 fractions. In randomization C (n = 524), patients received WBI at 42.5 Gy in 16 fractions, with or without TBB at 16 Gy in 8 fractions.
All patients underwent CT-based radiation planning. WBI was delivered with tangential MV photon beams, and TBB was performed with CT contouring of protocol-defined tumor bed target volumes, with electron or photon energy. The median follow-up was 6.6 years.
Giving a boost to better outcomes
The 5-year rate of freedom from local recurrence was 97% for patients who received TBB and 93% for patients who did not (hazard ratio, 0.47; P < .001). The benefit of TBB was consistent across subgroups.
There were no significant differences in 5-year rates of freedom from local recurrence by WBI fractionation, either in randomization A (P = .837) or among all randomized patients (P = .887).
The tests for interactions between TBB and WBI fractionation on local recurrence were not significant in randomization A (P = .89) or in all randomized patients (P = .89).
The risk of overall disease recurrence was lower among patients who had received TBB, with an HR of 0.63 (P = .004). The 5-year rate of freedom from disease recurrence was 97% with TBB and 91% with no boost (P = .002).
There were no significant differences in freedom from disease recurrence rates by WBI fractionation either in randomization A (P = .443) or among all randomized patients (P = .605).
Acute radiation dermatitis occurred in significantly more patients who received TBB (P = .006), as did late breast pain (P = .003), induration or fibrosis (P < .0001), and telangiectasia (P = .02). There were no significant differences by boost status for acute or late fatigue, pneumonitis, cardiac complications, or second malignancies.
Reduce the boost dose?
A radiation oncologist who was not involved in this study said that, while the results confirm a benefit of boost dose for patients with non–low-risk DCIS, the doses used in the BIG-3-07 study may be higher than needed to achieve a protective effect.
“Here in America, we usually give 10 Gy in five fractions, and, in many countries, actually, it’s 10 Gy in five fractions, although a few European centers give 16 Gy in eight fractions,” said Alphose Taghian, MD, of Massachusetts General Hospital in Boston.
“I personally only give 10 Gy in five fractions. I am not under the impression that 16 Gy in eight fractions will give better results. The local failure rate is so low, it’s likely that 10 Gy will do the job,” Dr. Taghian said in an interview.
Dr. Taghian noted that raising the dose to 16 Gy increases the risk of fibrosis, as seen in the BIG-3-07 trial.
Nonetheless, the trial demonstrates the benefit of radiation boost dose in patients with high-risk DCIS, he said, adding that the local recurrence-free benefit curves may separate further with longer follow-up.
The study was sponsored by the Trans Tasman Radiation Oncology Group. Dr. Chua and Dr. Taghian reported no conflicts of interest.
Giving a tumor bed boost (TBB) reduced the risk of local recurrence and overall disease recurrence, but there were no significant differences in recurrence rates between conventional WBI and hypofractionated WBI.
Boon Hui Chua, MD, of the University of New South Wales, Sydney, presented these results at the 2020 San Antonio Breast Cancer Symposium.
Dr. Chua and colleagues studied 1,608 women with DCIS resected by conservative surgery. Patients were either younger than 50 years or age 50 and older with at least one of the following risk factors: symptomatic presentation, palpable tumor, multifocal disease, tumor size 1.5 cm or larger, intermediate or high nuclear grade, central necrosis, comedo histology, and/or surgical margins less than 10 mm.
The patients were randomized to treatment in three categories. In randomization A (n = 503), patients were randomized to one of the following treatments:
- WBI at 50 Gy in 25 fractions
- WBI at 42.5 Gy in 16 fractions
- WBI at 50 Gy in 25 fractions plus TBB at 16 Gy in 8 fractions
- WBI at 42.5 Gy in 16 fractions plus TBB at 16 Gy in 8 fractions.
In randomization B (n = 581), patients received WBI at 50 Gy in 25 fractions, with or without TBB at 16 Gy in 8 fractions. In randomization C (n = 524), patients received WBI at 42.5 Gy in 16 fractions, with or without TBB at 16 Gy in 8 fractions.
All patients underwent CT-based radiation planning. WBI was delivered with tangential MV photon beams, and TBB was performed with CT contouring of protocol-defined tumor bed target volumes, with electron or photon energy. The median follow-up was 6.6 years.
Giving a boost to better outcomes
The 5-year rate of freedom from local recurrence was 97% for patients who received TBB and 93% for patients who did not (hazard ratio, 0.47; P < .001). The benefit of TBB was consistent across subgroups.
There were no significant differences in 5-year rates of freedom from local recurrence by WBI fractionation, either in randomization A (P = .837) or among all randomized patients (P = .887).
The tests for interactions between TBB and WBI fractionation on local recurrence were not significant in randomization A (P = .89) or in all randomized patients (P = .89).
The risk of overall disease recurrence was lower among patients who had received TBB, with an HR of 0.63 (P = .004). The 5-year rate of freedom from disease recurrence was 97% with TBB and 91% with no boost (P = .002).
There were no significant differences in freedom from disease recurrence rates by WBI fractionation either in randomization A (P = .443) or among all randomized patients (P = .605).
Acute radiation dermatitis occurred in significantly more patients who received TBB (P = .006), as did late breast pain (P = .003), induration or fibrosis (P < .0001), and telangiectasia (P = .02). There were no significant differences by boost status for acute or late fatigue, pneumonitis, cardiac complications, or second malignancies.
Reduce the boost dose?
A radiation oncologist who was not involved in this study said that, while the results confirm a benefit of boost dose for patients with non–low-risk DCIS, the doses used in the BIG-3-07 study may be higher than needed to achieve a protective effect.
“Here in America, we usually give 10 Gy in five fractions, and, in many countries, actually, it’s 10 Gy in five fractions, although a few European centers give 16 Gy in eight fractions,” said Alphose Taghian, MD, of Massachusetts General Hospital in Boston.
“I personally only give 10 Gy in five fractions. I am not under the impression that 16 Gy in eight fractions will give better results. The local failure rate is so low, it’s likely that 10 Gy will do the job,” Dr. Taghian said in an interview.
Dr. Taghian noted that raising the dose to 16 Gy increases the risk of fibrosis, as seen in the BIG-3-07 trial.
Nonetheless, the trial demonstrates the benefit of radiation boost dose in patients with high-risk DCIS, he said, adding that the local recurrence-free benefit curves may separate further with longer follow-up.
The study was sponsored by the Trans Tasman Radiation Oncology Group. Dr. Chua and Dr. Taghian reported no conflicts of interest.
Giving a tumor bed boost (TBB) reduced the risk of local recurrence and overall disease recurrence, but there were no significant differences in recurrence rates between conventional WBI and hypofractionated WBI.
Boon Hui Chua, MD, of the University of New South Wales, Sydney, presented these results at the 2020 San Antonio Breast Cancer Symposium.
Dr. Chua and colleagues studied 1,608 women with DCIS resected by conservative surgery. Patients were either younger than 50 years or age 50 and older with at least one of the following risk factors: symptomatic presentation, palpable tumor, multifocal disease, tumor size 1.5 cm or larger, intermediate or high nuclear grade, central necrosis, comedo histology, and/or surgical margins less than 10 mm.
The patients were randomized to treatment in three categories. In randomization A (n = 503), patients were randomized to one of the following treatments:
- WBI at 50 Gy in 25 fractions
- WBI at 42.5 Gy in 16 fractions
- WBI at 50 Gy in 25 fractions plus TBB at 16 Gy in 8 fractions
- WBI at 42.5 Gy in 16 fractions plus TBB at 16 Gy in 8 fractions.
In randomization B (n = 581), patients received WBI at 50 Gy in 25 fractions, with or without TBB at 16 Gy in 8 fractions. In randomization C (n = 524), patients received WBI at 42.5 Gy in 16 fractions, with or without TBB at 16 Gy in 8 fractions.
All patients underwent CT-based radiation planning. WBI was delivered with tangential MV photon beams, and TBB was performed with CT contouring of protocol-defined tumor bed target volumes, with electron or photon energy. The median follow-up was 6.6 years.
Giving a boost to better outcomes
The 5-year rate of freedom from local recurrence was 97% for patients who received TBB and 93% for patients who did not (hazard ratio, 0.47; P < .001). The benefit of TBB was consistent across subgroups.
There were no significant differences in 5-year rates of freedom from local recurrence by WBI fractionation, either in randomization A (P = .837) or among all randomized patients (P = .887).
The tests for interactions between TBB and WBI fractionation on local recurrence were not significant in randomization A (P = .89) or in all randomized patients (P = .89).
The risk of overall disease recurrence was lower among patients who had received TBB, with an HR of 0.63 (P = .004). The 5-year rate of freedom from disease recurrence was 97% with TBB and 91% with no boost (P = .002).
There were no significant differences in freedom from disease recurrence rates by WBI fractionation either in randomization A (P = .443) or among all randomized patients (P = .605).
Acute radiation dermatitis occurred in significantly more patients who received TBB (P = .006), as did late breast pain (P = .003), induration or fibrosis (P < .0001), and telangiectasia (P = .02). There were no significant differences by boost status for acute or late fatigue, pneumonitis, cardiac complications, or second malignancies.
Reduce the boost dose?
A radiation oncologist who was not involved in this study said that, while the results confirm a benefit of boost dose for patients with non–low-risk DCIS, the doses used in the BIG-3-07 study may be higher than needed to achieve a protective effect.
“Here in America, we usually give 10 Gy in five fractions, and, in many countries, actually, it’s 10 Gy in five fractions, although a few European centers give 16 Gy in eight fractions,” said Alphose Taghian, MD, of Massachusetts General Hospital in Boston.
“I personally only give 10 Gy in five fractions. I am not under the impression that 16 Gy in eight fractions will give better results. The local failure rate is so low, it’s likely that 10 Gy will do the job,” Dr. Taghian said in an interview.
Dr. Taghian noted that raising the dose to 16 Gy increases the risk of fibrosis, as seen in the BIG-3-07 trial.
Nonetheless, the trial demonstrates the benefit of radiation boost dose in patients with high-risk DCIS, he said, adding that the local recurrence-free benefit curves may separate further with longer follow-up.
The study was sponsored by the Trans Tasman Radiation Oncology Group. Dr. Chua and Dr. Taghian reported no conflicts of interest.
FROM SABCS 2020
Immunotherapy response linked to low TMB in recurrent glioblastoma
In contrast to what has been seen in other tumor types, recurrent glioblastoma (rGBM) may respond better to immunotherapy when tumor mutational burden (TMB) is low, new research suggests.
There’s an “unexpected correlation between TMB, tumor-intrinsic inflammation, and survival after immunotherapy” in this patient population, researchers noted in a Nature Communications report.
Cases of rGBM in which TMB is low are more likely to respond to immunotherapy than cases in which TMB is higher, the investigators concluded from an analysis of tumor tissue from more than 100 patients.
“We need to do a prospective study and establish a threshold in a particular assay format,” senior author David Ashley, MBBS, PhD, a neurosurgery professor at Duke University, Durham, N.C., said in an interview.
Andrew Sloan, MD, a neurosurgery professor at the Seidman Cancer Center, Cleveland, said in a comment that “many have given up on immunotherapy for GBM because these tumors tend to have lower TMB than tumors that typically respond to immunotherapy, including checkpoint inhibitors.” (Examples include melanoma and lung cancer.)
“If the findings are confirmed, it would be very useful clinically to select” patients for immunotherapy, Dr. Sloan commented.
Correlation seen with rGBM, not primary tumor
Recurrence of GBM is almost inevitable, even when aggressive standard-of-care therapy is given initially, according to Dr. Ashley and colleagues. Studies have indicated that, in 10%-20% of patients with rGBM, disease responds to subsequent immunotherapy, and patients live beyond the predicted median survival of about 12 months. It’s been unclear, however, what distinguishes these survivors from the other patients.
Dr. Ashley and colleagues looked for common genetic factors that distinguish survivors.
The tumor tissue the team analyzed came from three studies. The first was a trial of an intratumoral infusion of a recombinant nonpathogenic poliorhinovirus chimera (PVSRIPO), developed at Duke University, that induces innate inflammation and T-cell attack. Among 61 patients, 21% were alive at 3 years versus 4% of historical control patients.
The second study was a review of 66 patients with GBM who underwent treatment with pembrolizumab or nivolumab. The median survival was 14.3 months among those who experienced a response versus10.1 months for those who did not.
The third study involved more than 1,000 patients with advanced cancer who underwent treatment with checkpoint inhibitors. There was no survival benefit among the 117 patients with glioma who were treated with checkpoint inhibitors.
In the PVSRIPO trial, rGBM tumors from patients who survived longer than 20 months harbored very low TMB, less than 0.6 mutations/Mb. In the two checkpoint inhibitor trials, among 110 patients with rGBM, survival was longer for those whose TMB was at or below the median level.
The differences in survival were not driven by differences in steroid dosing, unfavorable responses among patients with hypermutations, or the presence or absence of IDH1 or PTEN mutations or MGMT promoter methylation, according to Dr. Ashley and colleagues.
“Increased survival of immunotherapy-treated rGBM patients with very low TMB is due to immunotherapy response,” the investigators concluded.
As for the explanation, the team found that rGBM tumors with lower TMB levels had enriched inflammatory gene signatures, compared with tumors with higher TMB levels.
The correlation – and longer survival with low TMB – was not observed in primary GBM tumors, “indicating that a relationship between tumor-intrinsic inflammation and TMB develops upon recurrence. ... We postulate that the baseline inflammatory status of rGBM tumors determines their susceptibility to immunotherapy,” the authors wrote.
Because the correlation between tumor inflammation and TMB was robust in rGBM but not in primary tumors, it might well have been caused by standard-of-care therapy, which affects mutation levels.
“Chemotherapy, which is the standard of care for newly diagnosed glioblastoma, might be altering the inflammatory response in these tumors” and priming an inflammatory process that increases vulnerability to immunotherapy in recurrent tumors, Dr. Ashley said in a press release.
Shorter time to recurrence also correlated with lower TMB and favorable response to PVSRIPO, so shorter duration of standard therapy or shorter time from initial surgery might improve immunotherapy response, he speculated.
The study was funded by the National Institutes of Health and other organizations. Dr. Ashley and other investigators own intellectual property related to PVSRIPO, which has been licensed to Istari Oncology. Several investigators hold equity in and/or are paid consultants for Istari. Dr. Sloan is the Ohio principal investigator for an rGBM PVSRIPO and pembrolizumab study funded by the company.
A version of this article first appeared on Medscape.com.
In contrast to what has been seen in other tumor types, recurrent glioblastoma (rGBM) may respond better to immunotherapy when tumor mutational burden (TMB) is low, new research suggests.
There’s an “unexpected correlation between TMB, tumor-intrinsic inflammation, and survival after immunotherapy” in this patient population, researchers noted in a Nature Communications report.
Cases of rGBM in which TMB is low are more likely to respond to immunotherapy than cases in which TMB is higher, the investigators concluded from an analysis of tumor tissue from more than 100 patients.
“We need to do a prospective study and establish a threshold in a particular assay format,” senior author David Ashley, MBBS, PhD, a neurosurgery professor at Duke University, Durham, N.C., said in an interview.
Andrew Sloan, MD, a neurosurgery professor at the Seidman Cancer Center, Cleveland, said in a comment that “many have given up on immunotherapy for GBM because these tumors tend to have lower TMB than tumors that typically respond to immunotherapy, including checkpoint inhibitors.” (Examples include melanoma and lung cancer.)
“If the findings are confirmed, it would be very useful clinically to select” patients for immunotherapy, Dr. Sloan commented.
Correlation seen with rGBM, not primary tumor
Recurrence of GBM is almost inevitable, even when aggressive standard-of-care therapy is given initially, according to Dr. Ashley and colleagues. Studies have indicated that, in 10%-20% of patients with rGBM, disease responds to subsequent immunotherapy, and patients live beyond the predicted median survival of about 12 months. It’s been unclear, however, what distinguishes these survivors from the other patients.
Dr. Ashley and colleagues looked for common genetic factors that distinguish survivors.
The tumor tissue the team analyzed came from three studies. The first was a trial of an intratumoral infusion of a recombinant nonpathogenic poliorhinovirus chimera (PVSRIPO), developed at Duke University, that induces innate inflammation and T-cell attack. Among 61 patients, 21% were alive at 3 years versus 4% of historical control patients.
The second study was a review of 66 patients with GBM who underwent treatment with pembrolizumab or nivolumab. The median survival was 14.3 months among those who experienced a response versus10.1 months for those who did not.
The third study involved more than 1,000 patients with advanced cancer who underwent treatment with checkpoint inhibitors. There was no survival benefit among the 117 patients with glioma who were treated with checkpoint inhibitors.
In the PVSRIPO trial, rGBM tumors from patients who survived longer than 20 months harbored very low TMB, less than 0.6 mutations/Mb. In the two checkpoint inhibitor trials, among 110 patients with rGBM, survival was longer for those whose TMB was at or below the median level.
The differences in survival were not driven by differences in steroid dosing, unfavorable responses among patients with hypermutations, or the presence or absence of IDH1 or PTEN mutations or MGMT promoter methylation, according to Dr. Ashley and colleagues.
“Increased survival of immunotherapy-treated rGBM patients with very low TMB is due to immunotherapy response,” the investigators concluded.
As for the explanation, the team found that rGBM tumors with lower TMB levels had enriched inflammatory gene signatures, compared with tumors with higher TMB levels.
The correlation – and longer survival with low TMB – was not observed in primary GBM tumors, “indicating that a relationship between tumor-intrinsic inflammation and TMB develops upon recurrence. ... We postulate that the baseline inflammatory status of rGBM tumors determines their susceptibility to immunotherapy,” the authors wrote.
Because the correlation between tumor inflammation and TMB was robust in rGBM but not in primary tumors, it might well have been caused by standard-of-care therapy, which affects mutation levels.
“Chemotherapy, which is the standard of care for newly diagnosed glioblastoma, might be altering the inflammatory response in these tumors” and priming an inflammatory process that increases vulnerability to immunotherapy in recurrent tumors, Dr. Ashley said in a press release.
Shorter time to recurrence also correlated with lower TMB and favorable response to PVSRIPO, so shorter duration of standard therapy or shorter time from initial surgery might improve immunotherapy response, he speculated.
The study was funded by the National Institutes of Health and other organizations. Dr. Ashley and other investigators own intellectual property related to PVSRIPO, which has been licensed to Istari Oncology. Several investigators hold equity in and/or are paid consultants for Istari. Dr. Sloan is the Ohio principal investigator for an rGBM PVSRIPO and pembrolizumab study funded by the company.
A version of this article first appeared on Medscape.com.
In contrast to what has been seen in other tumor types, recurrent glioblastoma (rGBM) may respond better to immunotherapy when tumor mutational burden (TMB) is low, new research suggests.
There’s an “unexpected correlation between TMB, tumor-intrinsic inflammation, and survival after immunotherapy” in this patient population, researchers noted in a Nature Communications report.
Cases of rGBM in which TMB is low are more likely to respond to immunotherapy than cases in which TMB is higher, the investigators concluded from an analysis of tumor tissue from more than 100 patients.
“We need to do a prospective study and establish a threshold in a particular assay format,” senior author David Ashley, MBBS, PhD, a neurosurgery professor at Duke University, Durham, N.C., said in an interview.
Andrew Sloan, MD, a neurosurgery professor at the Seidman Cancer Center, Cleveland, said in a comment that “many have given up on immunotherapy for GBM because these tumors tend to have lower TMB than tumors that typically respond to immunotherapy, including checkpoint inhibitors.” (Examples include melanoma and lung cancer.)
“If the findings are confirmed, it would be very useful clinically to select” patients for immunotherapy, Dr. Sloan commented.
Correlation seen with rGBM, not primary tumor
Recurrence of GBM is almost inevitable, even when aggressive standard-of-care therapy is given initially, according to Dr. Ashley and colleagues. Studies have indicated that, in 10%-20% of patients with rGBM, disease responds to subsequent immunotherapy, and patients live beyond the predicted median survival of about 12 months. It’s been unclear, however, what distinguishes these survivors from the other patients.
Dr. Ashley and colleagues looked for common genetic factors that distinguish survivors.
The tumor tissue the team analyzed came from three studies. The first was a trial of an intratumoral infusion of a recombinant nonpathogenic poliorhinovirus chimera (PVSRIPO), developed at Duke University, that induces innate inflammation and T-cell attack. Among 61 patients, 21% were alive at 3 years versus 4% of historical control patients.
The second study was a review of 66 patients with GBM who underwent treatment with pembrolizumab or nivolumab. The median survival was 14.3 months among those who experienced a response versus10.1 months for those who did not.
The third study involved more than 1,000 patients with advanced cancer who underwent treatment with checkpoint inhibitors. There was no survival benefit among the 117 patients with glioma who were treated with checkpoint inhibitors.
In the PVSRIPO trial, rGBM tumors from patients who survived longer than 20 months harbored very low TMB, less than 0.6 mutations/Mb. In the two checkpoint inhibitor trials, among 110 patients with rGBM, survival was longer for those whose TMB was at or below the median level.
The differences in survival were not driven by differences in steroid dosing, unfavorable responses among patients with hypermutations, or the presence or absence of IDH1 or PTEN mutations or MGMT promoter methylation, according to Dr. Ashley and colleagues.
“Increased survival of immunotherapy-treated rGBM patients with very low TMB is due to immunotherapy response,” the investigators concluded.
As for the explanation, the team found that rGBM tumors with lower TMB levels had enriched inflammatory gene signatures, compared with tumors with higher TMB levels.
The correlation – and longer survival with low TMB – was not observed in primary GBM tumors, “indicating that a relationship between tumor-intrinsic inflammation and TMB develops upon recurrence. ... We postulate that the baseline inflammatory status of rGBM tumors determines their susceptibility to immunotherapy,” the authors wrote.
Because the correlation between tumor inflammation and TMB was robust in rGBM but not in primary tumors, it might well have been caused by standard-of-care therapy, which affects mutation levels.
“Chemotherapy, which is the standard of care for newly diagnosed glioblastoma, might be altering the inflammatory response in these tumors” and priming an inflammatory process that increases vulnerability to immunotherapy in recurrent tumors, Dr. Ashley said in a press release.
Shorter time to recurrence also correlated with lower TMB and favorable response to PVSRIPO, so shorter duration of standard therapy or shorter time from initial surgery might improve immunotherapy response, he speculated.
The study was funded by the National Institutes of Health and other organizations. Dr. Ashley and other investigators own intellectual property related to PVSRIPO, which has been licensed to Istari Oncology. Several investigators hold equity in and/or are paid consultants for Istari. Dr. Sloan is the Ohio principal investigator for an rGBM PVSRIPO and pembrolizumab study funded by the company.
A version of this article first appeared on Medscape.com.
Surprise medical billing may eliminate restrictive networks
Certainly, this has been a tumultuous year for health care, as well as the nation in general. There is so much to cover it is hard to know where to begin.
Against a background of a swelling pandemic, I remain confused about the new evaluation and management coding system, and suspect there will be much more training to be rolled out. It is excellent news that the Paycheck Protection Program has been renewed for a second pass, if you can demonstrate that you suffered at least a 25% drop in income for at least one quarter last year, and have fewer than 300 employees – which covers most dermatology practices. I plan to discuss the impact of price transparency in a future column, but today will discuss one area, where we have had the passage of major health care legislation, that may have been overlooked.
Starting in January 2022, patients are protected from surprise medical bills. For nonemergency services and services outside hospitals and other facilities, a patient can only be billed for the coinsurance/copay that they would have had if the patient had been in network unless you go through a consent process by which you inform the patient that you are out-of-network, inform them of the costs, and inform them of other in-network providers. It also requires that patients’ in-network cost-sharing payments for out-of-network surprise bills are attributed to a patient’s in-network deductible.
In section 103, it further states that, where out-of-network rates are determined, there will be a 30-day open negotiation period for providers and payers to settle out-of-network claims. It also states that if the parties are unable to reach a negotiated agreement, they may access a binding arbitration process – referred to as an independent dispute resolution (IDR) – in which one offer prevails. Providers may batch similar services in one proceeding when claims are from the same payer. The IDR process will be administered by independent, unbiased entities with no affiliation to providers or payers.
The IDR entity is required to consider the market-based median in-network rate, alongside relevant information brought by either party, information requested by the reviewer, as well as factors such as the provider’s training and experience patient acuity, and the complexity of furnishing the item or service, in the case of a provider that is a facility. Other factors include the teaching status, case mix and scope of services of such facility, demonstrations of good faith efforts (or lack of good faith efforts) to enter into a network agreement, prior contracted rates during the previous 4 plan-years, and other items. Billed charges and public payer (Medicare and Medicaid) rates are excluded from consideration. This should result in a payment closer to private insurance rates.
As many of you know, another one of the long-term outrages by insurers has been the closure of their networks and delisting of dermatologists. I have written about this situation before in this column. Insurers have also refused to update their provider lists, effectively denying care by the magical process of not having to pay for medical care, because there aren’t any medical providers.
Inaccurate physician rosters
Obviously, one source of surprise medical bills that is easily correctable are inaccurate insurance company physician rosters. The Centers for Medicare & Medicaid Services implemented new rules with stiff fines instructing Medicare advantage plans to improve the accuracy of physician rosters, after a scathing General Accounting Office report 5 years ago. This process, however, was effectively neutered by the last administration by referring all enforcement action to the states, which did not have the manpower or political will to enforce them. This new surprise billing law directly addresses this issue, requiring insurers to update their provider directories every 90 days and keeping them available to patients on line.
This law also eliminates gag clauses between physicians and patients regarding insurer policies.
In short, this bill solves many problems for dermatologists in their constant struggle with insurers. In particular, accurate provider directories will allow patients and companies buying insurance for their employees, to see what they are getting. I suspect the revelation of the paucity of dermatologists in many of these networks will result in increased demand for your services and perhaps provide you a little negotiating leverage.
Also, if I read this law correctly, and I inform patients of our out-of-network status and give them a reasonable estimate of the cost of their care, network participation will no longer restrict patients who want to see me. I acknowledge that we will have to make good-faith efforts to join their networks (which most of us have repeatedly) and learn how to navigate the arbitration process, but this could be a boon for small-practice dermatologists who have been shut out of participating. In fact, it may be less trouble for insurers to simply invite us in, than going through repeated arbitration.
In the bigger picture, I would remind you of the importance of your legislative participation at the past American Academy of Dermatology Association Washington fly-ins, your support of the American Medical Association, and your support of SkinPac. These issues were always in our top three asks in Washington. All this favorable language was suggested, supported, and aided by your efforts and support of organized medicine.
There is a sign on my desk my wife gave me that reads “Never, Never, Never, Give Up.” I am proud of all of you for never giving up, and think you all deserve a “way to go” and a pat on the back. This law, which is a far walk from abusive air ambulance bills and unexpected anesthesia charges, amply and happily demonstrates that things can be changed for the better, and that access to care for our patients can be improved.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Certainly, this has been a tumultuous year for health care, as well as the nation in general. There is so much to cover it is hard to know where to begin.
Against a background of a swelling pandemic, I remain confused about the new evaluation and management coding system, and suspect there will be much more training to be rolled out. It is excellent news that the Paycheck Protection Program has been renewed for a second pass, if you can demonstrate that you suffered at least a 25% drop in income for at least one quarter last year, and have fewer than 300 employees – which covers most dermatology practices. I plan to discuss the impact of price transparency in a future column, but today will discuss one area, where we have had the passage of major health care legislation, that may have been overlooked.
Starting in January 2022, patients are protected from surprise medical bills. For nonemergency services and services outside hospitals and other facilities, a patient can only be billed for the coinsurance/copay that they would have had if the patient had been in network unless you go through a consent process by which you inform the patient that you are out-of-network, inform them of the costs, and inform them of other in-network providers. It also requires that patients’ in-network cost-sharing payments for out-of-network surprise bills are attributed to a patient’s in-network deductible.
In section 103, it further states that, where out-of-network rates are determined, there will be a 30-day open negotiation period for providers and payers to settle out-of-network claims. It also states that if the parties are unable to reach a negotiated agreement, they may access a binding arbitration process – referred to as an independent dispute resolution (IDR) – in which one offer prevails. Providers may batch similar services in one proceeding when claims are from the same payer. The IDR process will be administered by independent, unbiased entities with no affiliation to providers or payers.
The IDR entity is required to consider the market-based median in-network rate, alongside relevant information brought by either party, information requested by the reviewer, as well as factors such as the provider’s training and experience patient acuity, and the complexity of furnishing the item or service, in the case of a provider that is a facility. Other factors include the teaching status, case mix and scope of services of such facility, demonstrations of good faith efforts (or lack of good faith efforts) to enter into a network agreement, prior contracted rates during the previous 4 plan-years, and other items. Billed charges and public payer (Medicare and Medicaid) rates are excluded from consideration. This should result in a payment closer to private insurance rates.
As many of you know, another one of the long-term outrages by insurers has been the closure of their networks and delisting of dermatologists. I have written about this situation before in this column. Insurers have also refused to update their provider lists, effectively denying care by the magical process of not having to pay for medical care, because there aren’t any medical providers.
Inaccurate physician rosters
Obviously, one source of surprise medical bills that is easily correctable are inaccurate insurance company physician rosters. The Centers for Medicare & Medicaid Services implemented new rules with stiff fines instructing Medicare advantage plans to improve the accuracy of physician rosters, after a scathing General Accounting Office report 5 years ago. This process, however, was effectively neutered by the last administration by referring all enforcement action to the states, which did not have the manpower or political will to enforce them. This new surprise billing law directly addresses this issue, requiring insurers to update their provider directories every 90 days and keeping them available to patients on line.
This law also eliminates gag clauses between physicians and patients regarding insurer policies.
In short, this bill solves many problems for dermatologists in their constant struggle with insurers. In particular, accurate provider directories will allow patients and companies buying insurance for their employees, to see what they are getting. I suspect the revelation of the paucity of dermatologists in many of these networks will result in increased demand for your services and perhaps provide you a little negotiating leverage.
Also, if I read this law correctly, and I inform patients of our out-of-network status and give them a reasonable estimate of the cost of their care, network participation will no longer restrict patients who want to see me. I acknowledge that we will have to make good-faith efforts to join their networks (which most of us have repeatedly) and learn how to navigate the arbitration process, but this could be a boon for small-practice dermatologists who have been shut out of participating. In fact, it may be less trouble for insurers to simply invite us in, than going through repeated arbitration.
In the bigger picture, I would remind you of the importance of your legislative participation at the past American Academy of Dermatology Association Washington fly-ins, your support of the American Medical Association, and your support of SkinPac. These issues were always in our top three asks in Washington. All this favorable language was suggested, supported, and aided by your efforts and support of organized medicine.
There is a sign on my desk my wife gave me that reads “Never, Never, Never, Give Up.” I am proud of all of you for never giving up, and think you all deserve a “way to go” and a pat on the back. This law, which is a far walk from abusive air ambulance bills and unexpected anesthesia charges, amply and happily demonstrates that things can be changed for the better, and that access to care for our patients can be improved.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Certainly, this has been a tumultuous year for health care, as well as the nation in general. There is so much to cover it is hard to know where to begin.
Against a background of a swelling pandemic, I remain confused about the new evaluation and management coding system, and suspect there will be much more training to be rolled out. It is excellent news that the Paycheck Protection Program has been renewed for a second pass, if you can demonstrate that you suffered at least a 25% drop in income for at least one quarter last year, and have fewer than 300 employees – which covers most dermatology practices. I plan to discuss the impact of price transparency in a future column, but today will discuss one area, where we have had the passage of major health care legislation, that may have been overlooked.
Starting in January 2022, patients are protected from surprise medical bills. For nonemergency services and services outside hospitals and other facilities, a patient can only be billed for the coinsurance/copay that they would have had if the patient had been in network unless you go through a consent process by which you inform the patient that you are out-of-network, inform them of the costs, and inform them of other in-network providers. It also requires that patients’ in-network cost-sharing payments for out-of-network surprise bills are attributed to a patient’s in-network deductible.
In section 103, it further states that, where out-of-network rates are determined, there will be a 30-day open negotiation period for providers and payers to settle out-of-network claims. It also states that if the parties are unable to reach a negotiated agreement, they may access a binding arbitration process – referred to as an independent dispute resolution (IDR) – in which one offer prevails. Providers may batch similar services in one proceeding when claims are from the same payer. The IDR process will be administered by independent, unbiased entities with no affiliation to providers or payers.
The IDR entity is required to consider the market-based median in-network rate, alongside relevant information brought by either party, information requested by the reviewer, as well as factors such as the provider’s training and experience patient acuity, and the complexity of furnishing the item or service, in the case of a provider that is a facility. Other factors include the teaching status, case mix and scope of services of such facility, demonstrations of good faith efforts (or lack of good faith efforts) to enter into a network agreement, prior contracted rates during the previous 4 plan-years, and other items. Billed charges and public payer (Medicare and Medicaid) rates are excluded from consideration. This should result in a payment closer to private insurance rates.
As many of you know, another one of the long-term outrages by insurers has been the closure of their networks and delisting of dermatologists. I have written about this situation before in this column. Insurers have also refused to update their provider lists, effectively denying care by the magical process of not having to pay for medical care, because there aren’t any medical providers.
Inaccurate physician rosters
Obviously, one source of surprise medical bills that is easily correctable are inaccurate insurance company physician rosters. The Centers for Medicare & Medicaid Services implemented new rules with stiff fines instructing Medicare advantage plans to improve the accuracy of physician rosters, after a scathing General Accounting Office report 5 years ago. This process, however, was effectively neutered by the last administration by referring all enforcement action to the states, which did not have the manpower or political will to enforce them. This new surprise billing law directly addresses this issue, requiring insurers to update their provider directories every 90 days and keeping them available to patients on line.
This law also eliminates gag clauses between physicians and patients regarding insurer policies.
In short, this bill solves many problems for dermatologists in their constant struggle with insurers. In particular, accurate provider directories will allow patients and companies buying insurance for their employees, to see what they are getting. I suspect the revelation of the paucity of dermatologists in many of these networks will result in increased demand for your services and perhaps provide you a little negotiating leverage.
Also, if I read this law correctly, and I inform patients of our out-of-network status and give them a reasonable estimate of the cost of their care, network participation will no longer restrict patients who want to see me. I acknowledge that we will have to make good-faith efforts to join their networks (which most of us have repeatedly) and learn how to navigate the arbitration process, but this could be a boon for small-practice dermatologists who have been shut out of participating. In fact, it may be less trouble for insurers to simply invite us in, than going through repeated arbitration.
In the bigger picture, I would remind you of the importance of your legislative participation at the past American Academy of Dermatology Association Washington fly-ins, your support of the American Medical Association, and your support of SkinPac. These issues were always in our top three asks in Washington. All this favorable language was suggested, supported, and aided by your efforts and support of organized medicine.
There is a sign on my desk my wife gave me that reads “Never, Never, Never, Give Up.” I am proud of all of you for never giving up, and think you all deserve a “way to go” and a pat on the back. This law, which is a far walk from abusive air ambulance bills and unexpected anesthesia charges, amply and happily demonstrates that things can be changed for the better, and that access to care for our patients can be improved.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Early Head Start program boosts healthy eating, self-regulation
Home-based preventive interventions not only improve healthy eating habits and self-regulation in toddlers but also guide their parents toward better food presentation and response to picky behaviors, reported Robert L. Nix, PhD, of the University of Wisconsin, Madison, and his associates.
In a small, randomized controlled trial of 73 families with toddlers aged 18-36 months enrolled in home-based Early Head Start (EHS), the researchers evaluated four protective factors, including toddlers’ healthy eating habits, toddlers’ self-regulation, parents’ responsive feeding practices, and parents’ sensitive scaffolding. The study, conducted from April to October 2013, is the first clinical trial of Recipe 4 Success, a preschool-focused intervention created by administrators and home visitors of EHS that promotes healthy eating and self-regulation in toddlers living in poverty who may otherwise face weight challenges and obesity later in life. Integrating the intervention into EHS allowed the researchers to take full advantage of its national infrastructure and to make dissemination more efficient.
Of the families selected to participate, all of whom were living below the Federal poverty threshold, 66 were retained through post treatment. Most participating parents were biological mothers; 61% were single; 29% were not high school graduates; just 11% were employed full time. The toddlers averaged 30.72 months; 44% were female. Roughly 48% of families were non-Hispanic White; 29% were Black; and 23% were Hispanic or Latinx, the investigators reported in Pediatrics. More than three-quarters of participants were enrolled in the Special Supplemental Nutrition Program for Women, Infants, and Children or the Supplemental Nutrition Assistance Program.
The program allowed parents to transform toddler eating habits quickly
The study, which was designed to evaluate for posttreatment differences in the primary outcomes, involved 10 consecutive weekly lessons implemented by regularly assigned EHS home visitors. Parents were required to adhere to feeding practices carefully targeted with sensitive, structured scaffolding designed to keep toddlers from becoming overwhelmed. Parents were guided to understand, for instance, that toddlers frequently need to be exposed to a new food 10-20 times before eating it, and that poor sleep can have a detrimental influence on emotional and behavioral controls that can progress to weight gain.
Parent recall of what food and drinks their toddlers consumed in the previous 24-hour period was collected by interviewers. The percentage of all meals that included a fruit and/or vegetable, a protein source, and the absence of sweets and junk food were noted. Toddler self-regulation was assessed in accordance with delay of gratification, task orientation, and emotional/behavioral control. Parents were asked to rate toddler ability to cease desired activities to comply with parental requests. Parental responsive feeding practices were also recorded to observe how they introduce unique healthy foods and how they responded to their toddlers’ reactions. Parental sensitive scaffolding was similarly observed for their ability to structure activities in a developmentally appropriate manner promoting self-regulation.
The researchers noted no statistically significant differences between families in the treatment and control groups, nor were there differences in outcome measures or covariates. Study findings showed that, compared with toddlers who continued to receive just EHS support, the toddlers randomly assigned to Recipe 4 Success were more likely to consume snacks and meals that contained fruits, vegetables, protein, and no sweets or junk food.
As the results of this study and others have shown, early food preferences offer the strongest indication of later diet and healthy eating habits throughout life. The program targeted in this study is significant in its ability to accelerate the adoption of better toddler eating habits in just a 10-week period.
Recipe 4 Success along with other successful preventive interventions for young children are most effective when parents drive the change. “In the present trial, the quality of parenting was most highly related to healthy eating habits and self-regulation at baseline,” the researchers noted.
Specifically, the authors attributed the success of the program to “targeting specific interrelated outcomes with an integrated, theoretically driven intervention model,” which allowed Recipe 4 Success to boost the effectiveness of EHS substantially “in just 10 weeks with a minimal increase in funding,” the authors added.
The authors noted several weaknesses as well as strengths of the study. Its primary weakness was a baseline-posttreatment design, which made it impossible to assert that intervention effects can be sustained. The study was also limited to English-speaking families. Given that most home visitors attended to families in both Recipe 4 Success and EHS, the researchers noted the possibility for contamination across conditions, but they added that this would have actually reduced the intervention effects. The study’s primary strength was the evidenced-based nature of the randomized control. That Recipe 4 Success was operated as an intervention only strengthen the benefits of normal EHS visits.
Patient parents who promote self-regulation have the best chance of success
“This small study emphasizes the importance of parent education and support in setting the toddlers’ palate for lifelong eating habits and self-regulation,” observed Silver Spring, MD, private practice pediatrician and associate clinical professor of pediatrics at George Washington University, Washington, Lillian M. Beard, MD, in a separate interview.
“With the goal of promoting eating habits and self-regulation, I try to guide parents’ choices of what they offer to their toddler. I applaud parents’ patience as I encourage them not to give in and quickly resort to offering salty or sweet snacks. I suggest that if during the course of a day, a palette of colorful healthy choices is offered, most toddlers will graze independently as they go about their play. The challenge is to really support the parent through this quirky stage of their child’s development,” she explained.
“The ultimate challenge today with so much food insecurity, COVID-19 related job losses, and shrinking dollars to feed families is that too many families are feeling a food crisis! A program such as Recipe 4 Success can provide invaluable education for families on how to best stretch their few dollars, with knowledge of which items to seek from their community food pantries, how to best utilize items from the State WIC programs and still seek nutrition tips from their pediatricians while avoiding expensive fast foods that only offer immediate satiety and gratification. The Recipe 4 Success educator, pediatrician, or any community educator can give recommendations about which fresh produce may be inexpensive, but nutritional,” Dr. Beard suggested.
Dr. Nix and colleagues as well as Dr. Beard had no conflicts of interest and no relevant financial disclosures.
Home-based preventive interventions not only improve healthy eating habits and self-regulation in toddlers but also guide their parents toward better food presentation and response to picky behaviors, reported Robert L. Nix, PhD, of the University of Wisconsin, Madison, and his associates.
In a small, randomized controlled trial of 73 families with toddlers aged 18-36 months enrolled in home-based Early Head Start (EHS), the researchers evaluated four protective factors, including toddlers’ healthy eating habits, toddlers’ self-regulation, parents’ responsive feeding practices, and parents’ sensitive scaffolding. The study, conducted from April to October 2013, is the first clinical trial of Recipe 4 Success, a preschool-focused intervention created by administrators and home visitors of EHS that promotes healthy eating and self-regulation in toddlers living in poverty who may otherwise face weight challenges and obesity later in life. Integrating the intervention into EHS allowed the researchers to take full advantage of its national infrastructure and to make dissemination more efficient.
Of the families selected to participate, all of whom were living below the Federal poverty threshold, 66 were retained through post treatment. Most participating parents were biological mothers; 61% were single; 29% were not high school graduates; just 11% were employed full time. The toddlers averaged 30.72 months; 44% were female. Roughly 48% of families were non-Hispanic White; 29% were Black; and 23% were Hispanic or Latinx, the investigators reported in Pediatrics. More than three-quarters of participants were enrolled in the Special Supplemental Nutrition Program for Women, Infants, and Children or the Supplemental Nutrition Assistance Program.
The program allowed parents to transform toddler eating habits quickly
The study, which was designed to evaluate for posttreatment differences in the primary outcomes, involved 10 consecutive weekly lessons implemented by regularly assigned EHS home visitors. Parents were required to adhere to feeding practices carefully targeted with sensitive, structured scaffolding designed to keep toddlers from becoming overwhelmed. Parents were guided to understand, for instance, that toddlers frequently need to be exposed to a new food 10-20 times before eating it, and that poor sleep can have a detrimental influence on emotional and behavioral controls that can progress to weight gain.
Parent recall of what food and drinks their toddlers consumed in the previous 24-hour period was collected by interviewers. The percentage of all meals that included a fruit and/or vegetable, a protein source, and the absence of sweets and junk food were noted. Toddler self-regulation was assessed in accordance with delay of gratification, task orientation, and emotional/behavioral control. Parents were asked to rate toddler ability to cease desired activities to comply with parental requests. Parental responsive feeding practices were also recorded to observe how they introduce unique healthy foods and how they responded to their toddlers’ reactions. Parental sensitive scaffolding was similarly observed for their ability to structure activities in a developmentally appropriate manner promoting self-regulation.
The researchers noted no statistically significant differences between families in the treatment and control groups, nor were there differences in outcome measures or covariates. Study findings showed that, compared with toddlers who continued to receive just EHS support, the toddlers randomly assigned to Recipe 4 Success were more likely to consume snacks and meals that contained fruits, vegetables, protein, and no sweets or junk food.
As the results of this study and others have shown, early food preferences offer the strongest indication of later diet and healthy eating habits throughout life. The program targeted in this study is significant in its ability to accelerate the adoption of better toddler eating habits in just a 10-week period.
Recipe 4 Success along with other successful preventive interventions for young children are most effective when parents drive the change. “In the present trial, the quality of parenting was most highly related to healthy eating habits and self-regulation at baseline,” the researchers noted.
Specifically, the authors attributed the success of the program to “targeting specific interrelated outcomes with an integrated, theoretically driven intervention model,” which allowed Recipe 4 Success to boost the effectiveness of EHS substantially “in just 10 weeks with a minimal increase in funding,” the authors added.
The authors noted several weaknesses as well as strengths of the study. Its primary weakness was a baseline-posttreatment design, which made it impossible to assert that intervention effects can be sustained. The study was also limited to English-speaking families. Given that most home visitors attended to families in both Recipe 4 Success and EHS, the researchers noted the possibility for contamination across conditions, but they added that this would have actually reduced the intervention effects. The study’s primary strength was the evidenced-based nature of the randomized control. That Recipe 4 Success was operated as an intervention only strengthen the benefits of normal EHS visits.
Patient parents who promote self-regulation have the best chance of success
“This small study emphasizes the importance of parent education and support in setting the toddlers’ palate for lifelong eating habits and self-regulation,” observed Silver Spring, MD, private practice pediatrician and associate clinical professor of pediatrics at George Washington University, Washington, Lillian M. Beard, MD, in a separate interview.
“With the goal of promoting eating habits and self-regulation, I try to guide parents’ choices of what they offer to their toddler. I applaud parents’ patience as I encourage them not to give in and quickly resort to offering salty or sweet snacks. I suggest that if during the course of a day, a palette of colorful healthy choices is offered, most toddlers will graze independently as they go about their play. The challenge is to really support the parent through this quirky stage of their child’s development,” she explained.
“The ultimate challenge today with so much food insecurity, COVID-19 related job losses, and shrinking dollars to feed families is that too many families are feeling a food crisis! A program such as Recipe 4 Success can provide invaluable education for families on how to best stretch their few dollars, with knowledge of which items to seek from their community food pantries, how to best utilize items from the State WIC programs and still seek nutrition tips from their pediatricians while avoiding expensive fast foods that only offer immediate satiety and gratification. The Recipe 4 Success educator, pediatrician, or any community educator can give recommendations about which fresh produce may be inexpensive, but nutritional,” Dr. Beard suggested.
Dr. Nix and colleagues as well as Dr. Beard had no conflicts of interest and no relevant financial disclosures.
Home-based preventive interventions not only improve healthy eating habits and self-regulation in toddlers but also guide their parents toward better food presentation and response to picky behaviors, reported Robert L. Nix, PhD, of the University of Wisconsin, Madison, and his associates.
In a small, randomized controlled trial of 73 families with toddlers aged 18-36 months enrolled in home-based Early Head Start (EHS), the researchers evaluated four protective factors, including toddlers’ healthy eating habits, toddlers’ self-regulation, parents’ responsive feeding practices, and parents’ sensitive scaffolding. The study, conducted from April to October 2013, is the first clinical trial of Recipe 4 Success, a preschool-focused intervention created by administrators and home visitors of EHS that promotes healthy eating and self-regulation in toddlers living in poverty who may otherwise face weight challenges and obesity later in life. Integrating the intervention into EHS allowed the researchers to take full advantage of its national infrastructure and to make dissemination more efficient.
Of the families selected to participate, all of whom were living below the Federal poverty threshold, 66 were retained through post treatment. Most participating parents were biological mothers; 61% were single; 29% were not high school graduates; just 11% were employed full time. The toddlers averaged 30.72 months; 44% were female. Roughly 48% of families were non-Hispanic White; 29% were Black; and 23% were Hispanic or Latinx, the investigators reported in Pediatrics. More than three-quarters of participants were enrolled in the Special Supplemental Nutrition Program for Women, Infants, and Children or the Supplemental Nutrition Assistance Program.
The program allowed parents to transform toddler eating habits quickly
The study, which was designed to evaluate for posttreatment differences in the primary outcomes, involved 10 consecutive weekly lessons implemented by regularly assigned EHS home visitors. Parents were required to adhere to feeding practices carefully targeted with sensitive, structured scaffolding designed to keep toddlers from becoming overwhelmed. Parents were guided to understand, for instance, that toddlers frequently need to be exposed to a new food 10-20 times before eating it, and that poor sleep can have a detrimental influence on emotional and behavioral controls that can progress to weight gain.
Parent recall of what food and drinks their toddlers consumed in the previous 24-hour period was collected by interviewers. The percentage of all meals that included a fruit and/or vegetable, a protein source, and the absence of sweets and junk food were noted. Toddler self-regulation was assessed in accordance with delay of gratification, task orientation, and emotional/behavioral control. Parents were asked to rate toddler ability to cease desired activities to comply with parental requests. Parental responsive feeding practices were also recorded to observe how they introduce unique healthy foods and how they responded to their toddlers’ reactions. Parental sensitive scaffolding was similarly observed for their ability to structure activities in a developmentally appropriate manner promoting self-regulation.
The researchers noted no statistically significant differences between families in the treatment and control groups, nor were there differences in outcome measures or covariates. Study findings showed that, compared with toddlers who continued to receive just EHS support, the toddlers randomly assigned to Recipe 4 Success were more likely to consume snacks and meals that contained fruits, vegetables, protein, and no sweets or junk food.
As the results of this study and others have shown, early food preferences offer the strongest indication of later diet and healthy eating habits throughout life. The program targeted in this study is significant in its ability to accelerate the adoption of better toddler eating habits in just a 10-week period.
Recipe 4 Success along with other successful preventive interventions for young children are most effective when parents drive the change. “In the present trial, the quality of parenting was most highly related to healthy eating habits and self-regulation at baseline,” the researchers noted.
Specifically, the authors attributed the success of the program to “targeting specific interrelated outcomes with an integrated, theoretically driven intervention model,” which allowed Recipe 4 Success to boost the effectiveness of EHS substantially “in just 10 weeks with a minimal increase in funding,” the authors added.
The authors noted several weaknesses as well as strengths of the study. Its primary weakness was a baseline-posttreatment design, which made it impossible to assert that intervention effects can be sustained. The study was also limited to English-speaking families. Given that most home visitors attended to families in both Recipe 4 Success and EHS, the researchers noted the possibility for contamination across conditions, but they added that this would have actually reduced the intervention effects. The study’s primary strength was the evidenced-based nature of the randomized control. That Recipe 4 Success was operated as an intervention only strengthen the benefits of normal EHS visits.
Patient parents who promote self-regulation have the best chance of success
“This small study emphasizes the importance of parent education and support in setting the toddlers’ palate for lifelong eating habits and self-regulation,” observed Silver Spring, MD, private practice pediatrician and associate clinical professor of pediatrics at George Washington University, Washington, Lillian M. Beard, MD, in a separate interview.
“With the goal of promoting eating habits and self-regulation, I try to guide parents’ choices of what they offer to their toddler. I applaud parents’ patience as I encourage them not to give in and quickly resort to offering salty or sweet snacks. I suggest that if during the course of a day, a palette of colorful healthy choices is offered, most toddlers will graze independently as they go about their play. The challenge is to really support the parent through this quirky stage of their child’s development,” she explained.
“The ultimate challenge today with so much food insecurity, COVID-19 related job losses, and shrinking dollars to feed families is that too many families are feeling a food crisis! A program such as Recipe 4 Success can provide invaluable education for families on how to best stretch their few dollars, with knowledge of which items to seek from their community food pantries, how to best utilize items from the State WIC programs and still seek nutrition tips from their pediatricians while avoiding expensive fast foods that only offer immediate satiety and gratification. The Recipe 4 Success educator, pediatrician, or any community educator can give recommendations about which fresh produce may be inexpensive, but nutritional,” Dr. Beard suggested.
Dr. Nix and colleagues as well as Dr. Beard had no conflicts of interest and no relevant financial disclosures.
FROM PEDIATRICS
Women physicians and the pandemic: A snapshot
“Women physicians do not have trouble balancing competing demands any more than men physicians do. It is simply a more common expectation that women physicians will adjust their professional lives,” she observed.
The daily grind of caring for patients during a global pandemic is taking an emotional and mental toll on doctors as well as a physical one. “The recently publicized suicide of emergency physician Lorna Breen, MD, following her intense work during the pandemic in New York should cause every physician to reflect on their culture in medicine,” Dr. Brubaker wrote in the article. In an interview, she expounded on the current climate for women psychiatrists and physicians in general, offering some coping techniques.
Question: The pandemic has amplified disparities among men and women physicians. What may be the repercussions from this, not just for patient care, but for work-life balance among women physicians?
Answer: Focusing on women in academic roles, both research and clinical productivity have changed in the professional arena. Many women continue to bear a disproportionate share of family responsibilities and have reduced paid work to accommodate these needs. These changes can impact academic promotion and, therefore, subsequent academic opportunities for leadership. These gaps will add to the well-recognized gender wage gap. Women physicians are more likely to experience reduced wages associated with reduced professional activities. This reduces their annual earnings, which reduces their contributions to Social Security and other retirement programs. This can adversely impact their financial security later in life, at a time when women are already disadvantaged, compared with men.
Q: Are women psychiatrists facing additional burdens, given that many patients are suffering from anxiety and depression right now, and seeking out prescriptions?
A: We know that mental health concerns are on the rise. Although I cannot point to specific evidence, as a result. Similar to those on the more well-recognized “front lines” in the ED and critical care units, I consider my psychiatric colleagues to be on the front lines as well, as they are addressing this marked increase in care needs, for patients and for other members of the health care team.
Q: You mentioned the suicide of Dr. Breen. What might women psychiatrists take away from this incident?
A: Physicians are drawn to our vocation with a commitment to be of service to others. During such demanding times as these, the “safety” rails between service to others and self-care shift – clearly this can endanger individual doctors.
Q: What advice might you have for women in this profession? Any resources that could provide support?
A: My advice is to ensure your own well-being, knowing that this differs for each woman. Be realistic with your time and commitments, allowing time for restoration and rest. Sometimes I tell my peers to meditate or do some other form of contemplative practice. Exercise (preferably outdoors) and sleep, including preparing for good sleep, such as not reading emails or patient charts right up until sleep time, are all important. Most importantly, identify your support team and check in regularly with them. Never hesitate to reach out for help. People truly do care and want to help you.
“Women physicians do not have trouble balancing competing demands any more than men physicians do. It is simply a more common expectation that women physicians will adjust their professional lives,” she observed.
The daily grind of caring for patients during a global pandemic is taking an emotional and mental toll on doctors as well as a physical one. “The recently publicized suicide of emergency physician Lorna Breen, MD, following her intense work during the pandemic in New York should cause every physician to reflect on their culture in medicine,” Dr. Brubaker wrote in the article. In an interview, she expounded on the current climate for women psychiatrists and physicians in general, offering some coping techniques.
Question: The pandemic has amplified disparities among men and women physicians. What may be the repercussions from this, not just for patient care, but for work-life balance among women physicians?
Answer: Focusing on women in academic roles, both research and clinical productivity have changed in the professional arena. Many women continue to bear a disproportionate share of family responsibilities and have reduced paid work to accommodate these needs. These changes can impact academic promotion and, therefore, subsequent academic opportunities for leadership. These gaps will add to the well-recognized gender wage gap. Women physicians are more likely to experience reduced wages associated with reduced professional activities. This reduces their annual earnings, which reduces their contributions to Social Security and other retirement programs. This can adversely impact their financial security later in life, at a time when women are already disadvantaged, compared with men.
Q: Are women psychiatrists facing additional burdens, given that many patients are suffering from anxiety and depression right now, and seeking out prescriptions?
A: We know that mental health concerns are on the rise. Although I cannot point to specific evidence, as a result. Similar to those on the more well-recognized “front lines” in the ED and critical care units, I consider my psychiatric colleagues to be on the front lines as well, as they are addressing this marked increase in care needs, for patients and for other members of the health care team.
Q: You mentioned the suicide of Dr. Breen. What might women psychiatrists take away from this incident?
A: Physicians are drawn to our vocation with a commitment to be of service to others. During such demanding times as these, the “safety” rails between service to others and self-care shift – clearly this can endanger individual doctors.
Q: What advice might you have for women in this profession? Any resources that could provide support?
A: My advice is to ensure your own well-being, knowing that this differs for each woman. Be realistic with your time and commitments, allowing time for restoration and rest. Sometimes I tell my peers to meditate or do some other form of contemplative practice. Exercise (preferably outdoors) and sleep, including preparing for good sleep, such as not reading emails or patient charts right up until sleep time, are all important. Most importantly, identify your support team and check in regularly with them. Never hesitate to reach out for help. People truly do care and want to help you.
“Women physicians do not have trouble balancing competing demands any more than men physicians do. It is simply a more common expectation that women physicians will adjust their professional lives,” she observed.
The daily grind of caring for patients during a global pandemic is taking an emotional and mental toll on doctors as well as a physical one. “The recently publicized suicide of emergency physician Lorna Breen, MD, following her intense work during the pandemic in New York should cause every physician to reflect on their culture in medicine,” Dr. Brubaker wrote in the article. In an interview, she expounded on the current climate for women psychiatrists and physicians in general, offering some coping techniques.
Question: The pandemic has amplified disparities among men and women physicians. What may be the repercussions from this, not just for patient care, but for work-life balance among women physicians?
Answer: Focusing on women in academic roles, both research and clinical productivity have changed in the professional arena. Many women continue to bear a disproportionate share of family responsibilities and have reduced paid work to accommodate these needs. These changes can impact academic promotion and, therefore, subsequent academic opportunities for leadership. These gaps will add to the well-recognized gender wage gap. Women physicians are more likely to experience reduced wages associated with reduced professional activities. This reduces their annual earnings, which reduces their contributions to Social Security and other retirement programs. This can adversely impact their financial security later in life, at a time when women are already disadvantaged, compared with men.
Q: Are women psychiatrists facing additional burdens, given that many patients are suffering from anxiety and depression right now, and seeking out prescriptions?
A: We know that mental health concerns are on the rise. Although I cannot point to specific evidence, as a result. Similar to those on the more well-recognized “front lines” in the ED and critical care units, I consider my psychiatric colleagues to be on the front lines as well, as they are addressing this marked increase in care needs, for patients and for other members of the health care team.
Q: You mentioned the suicide of Dr. Breen. What might women psychiatrists take away from this incident?
A: Physicians are drawn to our vocation with a commitment to be of service to others. During such demanding times as these, the “safety” rails between service to others and self-care shift – clearly this can endanger individual doctors.
Q: What advice might you have for women in this profession? Any resources that could provide support?
A: My advice is to ensure your own well-being, knowing that this differs for each woman. Be realistic with your time and commitments, allowing time for restoration and rest. Sometimes I tell my peers to meditate or do some other form of contemplative practice. Exercise (preferably outdoors) and sleep, including preparing for good sleep, such as not reading emails or patient charts right up until sleep time, are all important. Most importantly, identify your support team and check in regularly with them. Never hesitate to reach out for help. People truly do care and want to help you.
Opportunities for Stewardship in the Transition From Intravenous to Enteral Antibiotics in Hospitalized Pediatric Patients
Bacterial infections are a common reason for pediatric hospital admissions in the United States.1 Antibiotics are the mainstay of treatment, and whether to administer them intravenously (IV) or enterally is an important and, at times, challenging decision. Not all hospitalized patients with infections require IV antibiotics, and safe, effective early transitions to enteral therapy have been described for numerous infections.2-7 However, guidelines describing the ideal initial route of antibiotic administration and when to transition to oral therapy are lacking.5,7,8 This lack of high-quality evidence-based guidance may contribute to overuse of IV antibiotics for many hospitalized pediatric patients, even when safe and effective enteral options exist.9
Significant costs and harms are associated with the use of IV antibiotics. In particular, studies have demonstrated longer length of stay (LOS), increased costs, and worsened pain or anxiety related to complications (eg, phlebitis, extravasation injury, thrombosis, catheter-associated bloodstream infections) associated with IV antibiotics.3,4,10-13 Earlier transition to enteral therapy, however, can mitigate these increased risks and costs.
The Centers for Disease Control and Prevention lists the transition from IV to oral antibiotics as a key stewardship intervention for improving antibiotic use.14 The Infectious Diseases Society of America (IDSA) antibiotic stewardship program guidelines strongly recommend the timely conversion from IV to oral antibiotics, stating that efforts focusing on this transition should be integrated into routine practice.15 There are a few metrics in the literature to measure this intervention, but none is universally used, and a modified delphi process could not reach consensus on IV-to-oral transition metrics.16
Few studies describe the opportunity to transition to enteral antibiotics in hospitalized patients with common bacterial infections or explore variation across hospitals. It is critical to understand current practice of antibiotic administration in order to identify opportunities to optimize patient outcomes and promote high-value care. Furthermore, few studies have evaluated the feasibility of IV-to-oral transition metrics using an administrative database. Thus, the aims of this study were to (1) determine opportunities to transition from IV to enteral antibiotics for pediatric patients hospitalized with common bacterial infections based on their ability to tolerate other enteral medications, (2) describe variation in transition practices among children’s hospitals, and (3) evaluate the feasibility of novel IV-to-oral transition metrics using an administrative database to inform stewardship efforts.
METHODS
Study Design and Setting
This multicenter, retrospective cohort study used data from the Pediatric Health Information System (PHIS), an administrative and billing database containing encounter-level data from 52 tertiary care pediatric hospitals across the United States affiliated with the Children’s Hospital Association (Lenexa, Kansas). Hospitals submit encounter-level data, including demographics, medications, and diagnoses based on International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) codes. Data were de-identified at the time of submission, and data quality and reliability were assured by joint efforts between the Children’s Hospital Association and participating hospitals.
Study Population
This study included pediatric patients aged 60 days to 18 years who were hospitalized (inpatient or observation status) at one of the participating hospitals between January 1, 2017, and December 31, 2018, for one of the following seven common bacterial infections: community-acquired pneumonia (CAP), neck infection (superficial and deep), periorbital/orbital infection, urinary tract infection (UTI), osteomyelitis, septic arthritis, or skin and soft tissue infection (SSTI). The diagnosis cohorts were defined based on ICD-10-CM discharge diagnoses adapted from previous studies (Appendix Table 1).3,17-23 To define a cohort of generally healthy pediatric patients with an acute infection, we excluded patients hospitalized in the intensive care unit, patients with nonhome discharges, and patients with complex chronic conditions.24 We also excluded hospitals with incomplete data during the study period (n=1). The Institutional Review Board at Cincinnati Children’s Hospital Medical Center determined this study to be non–human-subjects research.
Outcomes
The primary outcomes were the number of opportunity days and the percent of days with opportunity to transition from IV to enteral therapy. Opportunity days, or days in which there was a potential opportunity to transition from IV to enteral antibiotics, were defined as days patients received only IV antibiotic doses and at least one enteral nonantibiotic medication, suggesting an ability to take enteral medications.13 We excluded days patients received IV antibiotics for which there was no enteral alternative (eg, vancomycin, Appendix Table 2). When measuring opportunity, to be conservative (ie, to underestimate rather than overestimate opportunity), we did not count as an opportunity day any day in which patients received both IV and enteral antibiotics. Percent opportunity, or the percent of days patients received antibiotics in which there was potential opportunity to transition from IV to enteral antibiotics, was defined as the number of opportunity days divided by number of inpatient days patients received enteral antibiotics or IV antibiotics with at least one enteral nonantibiotic medication (antibiotic days). Similar to opportunity days, antibiotic days excluded days patients were on IV antibiotics for which there was no enteral alternative. Based on our definition, a lower percent opportunity indicates that a hospital is using enteral antibiotics earlier during the hospitalization (earlier transition), while a higher percent opportunity represents later enteral antibiotic use (later transition).
Statistical Analysis
Demographic and clinical characteristics were summarized by diagnosis with descriptive statistics, including frequency with percentage, mean with standard deviation, and median with interquartile range (IQR). For each diagnosis, we evaluated aggregate opportunity days (sum of opportunity days among all hospitals), opportunity days per encounter, and aggregate percent opportunity using frequencies, mean with standard deviation, and percentages, respectively. We also calculated aggregate opportunity days for diagnosis-antibiotic combinations. To visually show variation in the percent opportunity across hospitals, we displayed the percent opportunity on a heat map, and evaluated percent opportunity across hospitals using chi-square tests. To compare the variability in the percent opportunity across and within hospitals, we used a generalized linear model with two fixed effects (hospital and diagnosis), and parsed the variability using the sum of squares. We performed a sensitivity analysis and excluded days that patients received antiemetic medications (eg, ondansetron, granisetron, prochlorperazine, promethazine), as these suggest potential intolerance of enteral medications. All statistical analyses were performed using SAS v.9.4 (SAS Institute Inc, Cary, North Carolina) and GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, California), and P values < .05 were considered statistically significant.
RESULTS
During the 2-year study period, 100,103 hospitalizations met our inclusion criteria across 51 hospitals and seven diagnosis categories (Table 1). Diagnosis cohorts ranged in size from 1,462 encounters for septic arthritis to 35,665 encounters for neck infections. Overall, we identified 88,522 aggregate opportunity days on which there was an opportunity to switch from IV to enteral treatment in the majority of participants (percent opportunity, 57%).
Opportunity by Diagnosis
The number of opportunity days (aggregate and mean per encounter) and percent opportunity varied by diagnosis (Table 2). The aggregate number of opportunity days ranged from 3,693 in patients with septic arthritis to 25,359 in patients with SSTI, and mean opportunity days per encounter ranged from 0.9 in CAP to 2.8 in septic arthritis. Percent opportunity was highest for septic arthritis at 72.7% and lowest for CAP at 39.7%.
Variation in Opportunity Among Hospitals
The variation in the percent opportunity across hospitals was statistically significant for all diagnoses (Figure). Within hospitals, we observed similar practice patterns across diagnoses. For example, hospitals with a higher percent opportunity for one diagnosis tended to have higher percent opportunity for the other diagnoses (as noted in the top portion of the Figure), and those with lower percent opportunity for one diagnosis tended to also have lower percent opportunity for the other diagnoses studied (as noted in the bottom portion of the Figure). When evaluating variability in the percent opportunity, 45% of the variability was attributable to the hospital-effect and 35% to the diagnosis; the remainder was unexplained variability. Sensitivity analysis excluding days when patients received an antiemetic medication yielded no differences in our results.

Opportunity by Antibiotic
The aggregate number of opportunity days varied by antibiotic (Table 3). Intravenous antibiotics with the largest number of opportunity days included clindamycin (44,293), ceftriaxone (23,896), and ampicillin-sulbactam (15,484). Antibiotic-diagnosis combinations with the largest number of opportunity days for each diagnosis included ceftriaxone and ampicillin in CAP; clindamycin in cellulitis, SSTI, and neck infections; ceftriaxone in UTI; and cefazolin in osteomyelitis and septic arthritis.
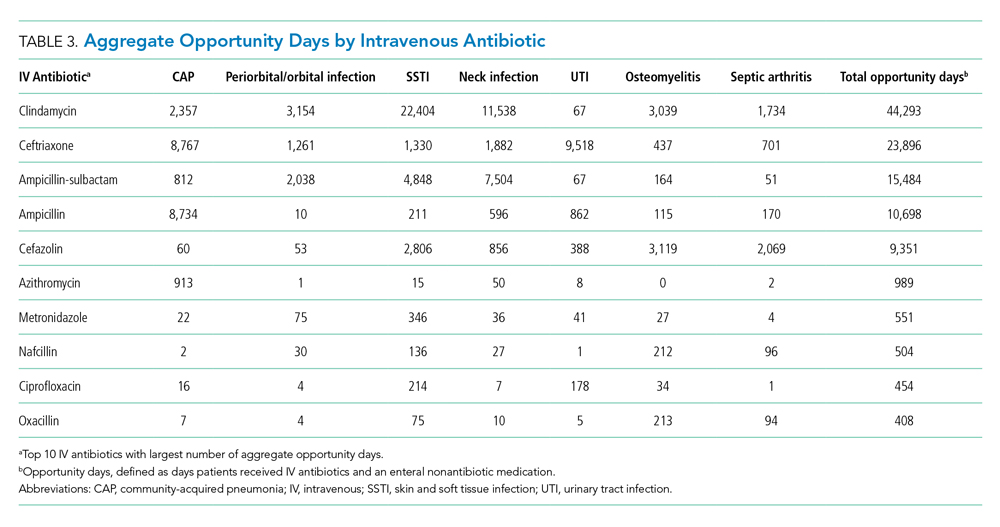
DISCUSSION
In this multicenter study of pediatric patients hospitalized with common bacterial infections, there was the potential to transition from IV to enteral treatment in over half of the antibiotic days. The degree of opportunity varied by infection, antibiotic, and hospital. Antibiotics with a large aggregate number of opportunity days for enteral transition included clindamycin, which has excellent bioavailability; and ampicillin and ampicillin-sulbactam, which can achieve pharmacodynamic targets with oral equivalents.25-29 The across-hospital variation for a given diagnosis suggests that certain hospitals have strategies in place which permit an earlier transition to enteral antibiotics compared to other institutions in which there were likely missed opportunities to do so. This variability is likely due to limited evidence, emphasizing the need for robust studies to better understand the optimal initial antibiotic route and transition time. Our findings highlight the need for, and large potential impact of, stewardship efforts to promote earlier transition for specific drug targets. This study also demonstrates the feasibility of obtaining two metrics—percent opportunity and opportunity days—from administrative databases to inform stewardship efforts within and across hospitals.
Opportunity days and percent opportunity varied among diagnoses. The variation in aggregate opportunity days was largely a reflection of the number of encounters: Diagnoses such as SSTI, neck infections, and CAP had a large number of both aggregate opportunity days and encounters. The range of opportunity days per encounter (0.9-2.5) suggests potential missed opportunities to transition to enteral antibiotics across all diagnoses (Table 2). The higher opportunity days per encounter in osteomyelitis and septic arthritis may be related to longer LOS and higher percent opportunity. Percent opportunity likely varied among diagnoses due to differences in admission and discharge readiness criteria, diagnostic evaluation, frequency of antibiotic administration, and evidence on the optimal route of initial antibiotics and when to transition to oral formulations. For example, we hypothesize that certain diagnoses, such as osteomyelitis and septic arthritis, have admission and discharge readiness criteria directly tied to the perceived need for IV antibiotics, which may limit in-hospital days on enteral antibiotics and explain the high percent opportunity that we observed. The high percent opportunity seen in musculoskeletal infections also may be due to delays in initiating targeted treatment until culture results were available. Encounters for CAP had the lowest percent opportunity; we hypothesize that this is because admission and discharge readiness may be determined by factors other than the need for IV antibiotics (eg, need for supplemental oxygen), which may increase days on enteral antibiotics and lead to a lower percent opportunity.30
Urinary tract infection encounters had a high percent opportunity. As with musculoskeletal infection, this may be related to delays in initiating targeted treatment until culture results became available. Another reason for the high percent opportunity in UTI could be the common use of ceftriaxone, which, dosed every 24 hours, likely reduced the opportunity to transition to enteral antibiotics. There is strong evidence demonstrating no difference in outcomes based on antibiotic routes for UTI, and we would expect this to result in a low percent opportunity.2,31 While the observed high opportunity in UTI may relate to an initial unknown diagnosis or concern for systemic infection, this highlights potential opportunities for quality improvement initiatives to promote empiric oral antibiotics in clinically stable patients hospitalized with suspected UTI.
There was substantial variation in percent opportunity across hospitals for a given diagnosis, with less variation across diagnoses for a given hospital. Variation across hospitals but consistency within individual hospitals suggests that some hospitals may promote earlier transition from IV to enteral antibiotics as standard practice for all diagnoses, while other hospitals continue IV antibiotics for the entire hospitalization, highlighting potential missed opportunities at some institutions. While emerging data suggest that traditional long durations of IV antibiotics are not necessary for many infections, the limited evidence on the optimal time to switch to oral antibiotics may have influenced this variation.2-7 Many guidelines recommend initial IV antibiotics for hospitalized pediatric patients, but there are few studies comparing IV and enteral therapy.2,5,9 Limited evidence leaves significant room for hospital culture, antibiotic stewardship efforts, reimbursement considerations, and/or hospital workflow to influence transition timing and overall opportunity at individual hospitals.7,8,32-34 These findings emphasize the importance of research to identify optimal transition time and comparative effectiveness studies to evaluate whether initial IV antibiotics are truly needed for mild—and even severe—disease presentations. Since many patients are admitted for the perceived need for IV antibiotics, earlier use of enteral antibiotics could reduce rates of hospitalizations, LOS, healthcare costs, and resource utilization.
Antibiotics with a high number of opportunity days included clindamycin, ceftriaxone, ampicillin-sublactam, and ampicillin. Our findings are consistent with another study which found that most bioavailable drugs, including clindamycin, were administered via the IV route and accounted for a large number of antibiotic days.35 The Infectious Diseases Society of America recommends that hospitals promote earlier transition to oral formulations for highly bioavailable drugs.7 Given the high bioavailability of clindamycin, its common use in high-frequency encounters such as SSTI and neck infections, and the fact that it accounted for a large number of opportunity days, quality improvement initiatives promoting earlier transition to oral clindamycin could have a large impact across health systems.25,26 Additionally, although beta-lactam antibiotics such as amoxicillin and amoxicillin-sulbactam are not highly bioavailable, oral dosing can achieve sufficient serum concentrations to reach pharmacodynamic targets for common clinical indications; this could be an important quality improvement initiative.27-29 Several single-site studies have successfully implemented quality improvement initiatives to promote earlier IV-to-enteral transition, with resulting reductions in costs and no adverse events noted, highlighting the feasibility and impact of such efforts.13,36-38
This study also demonstrates the feasibility of collecting two metrics (percent opportunity and opportunity days) from administrative databases to inform IV-to-oral transition benchmarking and stewardship efforts. While there are several metrics in the literature for evaluating antibiotic transition (eg, days of IV or oral therapy, percentage of antibiotics given via the oral route, time to switch from IV to oral, and acceptance rate of suggested changes to antibiotic route), none are universally used or agreed upon.15,16,39 The opportunity metrics used in this study have several strengths, including the feasibility of obtaining them from existing databases and the ability to account for intake of other enteral medications; the latter is not evaluated in other metrics. These opportunity metrics can be used together to identify the percent of time in which there is opportunity to transition and total number of days to understand the full extent of potential opportunity for future interventions. As demonstrated in this study, these metrics can be measured by diagnosis, antibiotic, or diagnosis-antibiotic combination, and they can be used to evaluate stewardship efforts at a single institution over time or compare efforts across hospitals.
These findings should be interpreted in the context of important limitations. First, we attempted to characterize potential opportunity to transition to enteral medications based on a patient’s ability to tolerate nonenteral medications. However, there are other factors that could limit the opportunity to transition that we could not account for with an administrative dataset, including the use of antibiotics prior to admission, disease progression, severity of illness, and malabsorptive concerns. Thus, though we may have overestimated the true opportunity to transition to enteral antibiotics, it is unlikely that this would account for all of the variation in transition times that we observed across hospitals. Second, while our study required patients to have one of seven types of infection, we did not exclude any additional infectious diagnoses (eg, concurrent bacteremia, Clostridioides difficile, otitis media) that could have driven the choice of antibiotic type and modality. Although emerging evidence is supporting earlier transitions to oral therapy, bacteremia is typically treated with IV antibiotics; this may have led to an overestimation of true opportunity.40 “Clostridioides” difficile and otitis media are typically treated with enteral therapy; concurrent infections such as these may have led to an underestimation of opportunity given the fact that, based on our definition, the days on which patients received both IV and enteral antibiotics were not counted as opportunity days. Third, because PHIS uses billing days to capture medication use, we were unable to distinguish transitions that occurred early in the day vs those that took place later in the day. This could have led to an underestimation of percent opportunity, particularly for diagnoses with a short LOS; it also likely led to an underestimation of the variability observed across hospitals. Fourth, because we used an administrative dataset, we are unable to understand reasoning behind transitioning time from IV to oral antibiotics, as well as provider, patient, and institutional level factors that influenced these decisions.
CONCLUSION
Children hospitalized with bacterial infections often receive IV antibiotics, and the timing of transition from IV to enteral antibiotics varies significantly across hospitals. Further research is needed to compare the effectiveness of IV and enteral antibiotics and better define criteria for transition to enteral therapy. We identified ample opportunities for quality improvement initiatives to promote earlier transition, which have the potential to reduce healthcare utilization and promote optimal patient-directed high-value care.
1. Keren R, Luan X, Localio R, et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X
3. Keren R, Shah SS, Srivastava R, et al; for the Pediatric Research Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e201692. https://doi.org/10.1542/peds.2016-1692
5. Li HK, Agweyu A, English M, Bejon P. An unsupported preference for intravenous antibiotics. PLoS Med. 2015;12(5):e1001825. https://dx.doi.org/10.1371%2Fjournal.pmed.1001825
6. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393
7. Bradley JS, Byington CL, Shah SS, et al; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Management of community-acquired pneumonia (CAP) in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1542/peds.2011-2385
8. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8-S14. https://doi.org/10.1093/cid/cir363
9. Schroeder AR, Ralston SL. Intravenous antibiotic durations for common bacterial infections in children: when is enough? J Hosp Med. 2014;9(9):604-609. https://doi.org/10.1002/jhm.2239
10. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatric Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003
11. van Zanten AR, Engelfriet PM, van Dillen K, van Veen M, Nuijten MJ, Polderman KH. Importance of nondrug costs of intravenous antibiotic therapy. Crit Care. 2003;7(6):R184-R190. https://doi.org/10.1186/cc2388
12. Ruebner R, Keren R, Coffin S, Chu J, Horn D, Zaoutis TE. Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006;117(4):1210-1215. https://doi.org/10.1542/peds.2005-1465
13. Girdwood SCT, Sellas MN, Courter JD, et al. Improving the transition of intravenous to enteral antibiotics in pediatric patients with pneumonia or skin and soft tissue infections. J Hosp Med. 2020;15(1):10-15. https://doi.org/10.12788/jhm.3253
14. Core Elements of Hospital Antibiotic Stewardship Programs. Centers for Disease Control and Prevention. Published 2019. Accessed May 30, 2020. https://www.cdc.gov/antibiotic-use/core-elements/hospital.html
15. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. https://doi.org/10.1093/cid/ciw118
16. Science M, Timberlake K, Morris A, Read S, Le Saux N; Groupe Antibiothérapie en Pédiatrie Canada Alliance for Stewardship of Antimicrobials in Pediatrics (GAP Can ASAP). Quality metrics for antimicrobial stewardship programs. Pediatrics. 2019;143(4):e20182372. https://doi.org/10.1542/peds.2018-2372
17. Tchou MJ, Hall M, Shah SS, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Patterns of electrolyte testing at children’s hospitals for common inpatient diagnoses. Pediatrics. 2019;144(1):e20181644. https://doi.org/10.1542/peds.2018-1644
18. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
19. Desai S, Shah SS, Hall M, Richardson TE, Thomson JE; Pediatric Research in Inpatient Settings (PRIS) Network. Imaging strategies and outcomes in children hospitalized with cervical lymphadenitis. J Hosp Med. 2020;15(4):197-203. https://doi.org/10.12788/jhm.3333
20. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
21. Tieder JS, Hall M, Auger KA, et al. Accuracy of administrative billing codes to detect urinary tract infection hospitalizations. Pediatrics. 2011;128(2):323-330. https://doi.org/10.1542/peds.2010-2064
22. Singh JA, Yu S. The burden of septic arthritis on the U.S. inpatient care: a national study. PLoS One. 2017;12(8):e0182577. https://doi.org/10.1371/journal.pone.0182577
23. Foradori DM, Lopez MA, Hall M, et al. Invasive bacterial infections in infants younger than 60 days with skin and soft tissue infections. Pediatr Emerg Care. 2018. https://doi.org/10.1097/pec.0000000000001584
24. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
25. Arancibia A, Icarte A, González C, Morasso I. Dose-dependent bioavailability of amoxycillin. Int J Clin Pharmacol Ther Toxicol. 1988;26(6):300-303.
26. Grayson ML, Cosgrove S, Crowe S, et al. Kucers’ the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs. 7th ed. CRC Press; 2018.
27. Downes KJ, Hahn A, Wiles J, Courter JD, Inks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in pediatrics’. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006
28. Gras-Le Guen C, Boscher C, Godon N, et al. Therapeutic amoxicillin levels achieved with oral administration in term neonates. Eur J Clin Pharmacol. 2007;63(7):657-662. https://doi.org/10.1007/s00228-007-0307-3
29. Sanchez Navarro A. New formulations of amoxicillin/clavulanic acid: a pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2005;44(11):1097-1115. https://doi.org/10.2165/00003088-200544110-00001
30. Fine MJ, Hough LJ, Medsger AR, et al. The hospital admission decision for patients with community-acquired pneumonia. Results from the pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 1997;157(1):36-44. https://doi.org/10.1001/archinte.1997.00440220040006
31. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007(4):CD003237. https://doi.org/10.1002/14651858.cd003237.pub2
32. Nageswaran S, Woods CR, Benjamin DK Jr, Givner LB, Shetty AK. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25(8):695-699. https://doi.org/10.1097/01.inf.0000227820.36036.f1
33. Al-Nammari S, Roberton B, Ferguson C. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Should a child with preseptal periorbital cellulitis be treated with intravenous or oral antibiotics? Emerg Med J. 2007;24(2):128-129. https://doi.org/10.1136/emj.2006.045245
34. Vieira F, Allen SM, Stocks RMS, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008;41(3):459-483, vii. https://doi.org/10.1016/j.otc.2008.01.002
35. Smith M, Shah S, Kronman M, Patel S, Thurm C, Hersh AL. Route of administration for highly orally bioavailable antibiotics. Open Forum Infect Dis. 2017;4(Suppl 1):S498-S499. https://doi.org/10.1093/ofid/ofx163.1291
36. Brady PW, Brinkman WB, Simmons JM, et al. Oral antibiotics at discharge for children with acute osteomyelitis: a rapid cycle improvement project. BMJ Qual Saf. 2014;23(6):499-507. https://doi.org/10.1136/bmjqs-2013-002179
37. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3
38. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585
39. Public Health Ontario. Antimicrobial stewardship programs metric examples. Published 2017. Accessed June 1, 2020. https://www.publichealthontario.ca/-/media/documents/A/2017/asp-metrics-examples.pdf?la=en
40. Desai S, Aronson PL, Shabanova V, et al; Febrile Young Infant Research Collaborative. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
Bacterial infections are a common reason for pediatric hospital admissions in the United States.1 Antibiotics are the mainstay of treatment, and whether to administer them intravenously (IV) or enterally is an important and, at times, challenging decision. Not all hospitalized patients with infections require IV antibiotics, and safe, effective early transitions to enteral therapy have been described for numerous infections.2-7 However, guidelines describing the ideal initial route of antibiotic administration and when to transition to oral therapy are lacking.5,7,8 This lack of high-quality evidence-based guidance may contribute to overuse of IV antibiotics for many hospitalized pediatric patients, even when safe and effective enteral options exist.9
Significant costs and harms are associated with the use of IV antibiotics. In particular, studies have demonstrated longer length of stay (LOS), increased costs, and worsened pain or anxiety related to complications (eg, phlebitis, extravasation injury, thrombosis, catheter-associated bloodstream infections) associated with IV antibiotics.3,4,10-13 Earlier transition to enteral therapy, however, can mitigate these increased risks and costs.
The Centers for Disease Control and Prevention lists the transition from IV to oral antibiotics as a key stewardship intervention for improving antibiotic use.14 The Infectious Diseases Society of America (IDSA) antibiotic stewardship program guidelines strongly recommend the timely conversion from IV to oral antibiotics, stating that efforts focusing on this transition should be integrated into routine practice.15 There are a few metrics in the literature to measure this intervention, but none is universally used, and a modified delphi process could not reach consensus on IV-to-oral transition metrics.16
Few studies describe the opportunity to transition to enteral antibiotics in hospitalized patients with common bacterial infections or explore variation across hospitals. It is critical to understand current practice of antibiotic administration in order to identify opportunities to optimize patient outcomes and promote high-value care. Furthermore, few studies have evaluated the feasibility of IV-to-oral transition metrics using an administrative database. Thus, the aims of this study were to (1) determine opportunities to transition from IV to enteral antibiotics for pediatric patients hospitalized with common bacterial infections based on their ability to tolerate other enteral medications, (2) describe variation in transition practices among children’s hospitals, and (3) evaluate the feasibility of novel IV-to-oral transition metrics using an administrative database to inform stewardship efforts.
METHODS
Study Design and Setting
This multicenter, retrospective cohort study used data from the Pediatric Health Information System (PHIS), an administrative and billing database containing encounter-level data from 52 tertiary care pediatric hospitals across the United States affiliated with the Children’s Hospital Association (Lenexa, Kansas). Hospitals submit encounter-level data, including demographics, medications, and diagnoses based on International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) codes. Data were de-identified at the time of submission, and data quality and reliability were assured by joint efforts between the Children’s Hospital Association and participating hospitals.
Study Population
This study included pediatric patients aged 60 days to 18 years who were hospitalized (inpatient or observation status) at one of the participating hospitals between January 1, 2017, and December 31, 2018, for one of the following seven common bacterial infections: community-acquired pneumonia (CAP), neck infection (superficial and deep), periorbital/orbital infection, urinary tract infection (UTI), osteomyelitis, septic arthritis, or skin and soft tissue infection (SSTI). The diagnosis cohorts were defined based on ICD-10-CM discharge diagnoses adapted from previous studies (Appendix Table 1).3,17-23 To define a cohort of generally healthy pediatric patients with an acute infection, we excluded patients hospitalized in the intensive care unit, patients with nonhome discharges, and patients with complex chronic conditions.24 We also excluded hospitals with incomplete data during the study period (n=1). The Institutional Review Board at Cincinnati Children’s Hospital Medical Center determined this study to be non–human-subjects research.
Outcomes
The primary outcomes were the number of opportunity days and the percent of days with opportunity to transition from IV to enteral therapy. Opportunity days, or days in which there was a potential opportunity to transition from IV to enteral antibiotics, were defined as days patients received only IV antibiotic doses and at least one enteral nonantibiotic medication, suggesting an ability to take enteral medications.13 We excluded days patients received IV antibiotics for which there was no enteral alternative (eg, vancomycin, Appendix Table 2). When measuring opportunity, to be conservative (ie, to underestimate rather than overestimate opportunity), we did not count as an opportunity day any day in which patients received both IV and enteral antibiotics. Percent opportunity, or the percent of days patients received antibiotics in which there was potential opportunity to transition from IV to enteral antibiotics, was defined as the number of opportunity days divided by number of inpatient days patients received enteral antibiotics or IV antibiotics with at least one enteral nonantibiotic medication (antibiotic days). Similar to opportunity days, antibiotic days excluded days patients were on IV antibiotics for which there was no enteral alternative. Based on our definition, a lower percent opportunity indicates that a hospital is using enteral antibiotics earlier during the hospitalization (earlier transition), while a higher percent opportunity represents later enteral antibiotic use (later transition).
Statistical Analysis
Demographic and clinical characteristics were summarized by diagnosis with descriptive statistics, including frequency with percentage, mean with standard deviation, and median with interquartile range (IQR). For each diagnosis, we evaluated aggregate opportunity days (sum of opportunity days among all hospitals), opportunity days per encounter, and aggregate percent opportunity using frequencies, mean with standard deviation, and percentages, respectively. We also calculated aggregate opportunity days for diagnosis-antibiotic combinations. To visually show variation in the percent opportunity across hospitals, we displayed the percent opportunity on a heat map, and evaluated percent opportunity across hospitals using chi-square tests. To compare the variability in the percent opportunity across and within hospitals, we used a generalized linear model with two fixed effects (hospital and diagnosis), and parsed the variability using the sum of squares. We performed a sensitivity analysis and excluded days that patients received antiemetic medications (eg, ondansetron, granisetron, prochlorperazine, promethazine), as these suggest potential intolerance of enteral medications. All statistical analyses were performed using SAS v.9.4 (SAS Institute Inc, Cary, North Carolina) and GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, California), and P values < .05 were considered statistically significant.
RESULTS
During the 2-year study period, 100,103 hospitalizations met our inclusion criteria across 51 hospitals and seven diagnosis categories (Table 1). Diagnosis cohorts ranged in size from 1,462 encounters for septic arthritis to 35,665 encounters for neck infections. Overall, we identified 88,522 aggregate opportunity days on which there was an opportunity to switch from IV to enteral treatment in the majority of participants (percent opportunity, 57%).

Opportunity by Diagnosis
The number of opportunity days (aggregate and mean per encounter) and percent opportunity varied by diagnosis (Table 2). The aggregate number of opportunity days ranged from 3,693 in patients with septic arthritis to 25,359 in patients with SSTI, and mean opportunity days per encounter ranged from 0.9 in CAP to 2.8 in septic arthritis. Percent opportunity was highest for septic arthritis at 72.7% and lowest for CAP at 39.7%.

Variation in Opportunity Among Hospitals
The variation in the percent opportunity across hospitals was statistically significant for all diagnoses (Figure). Within hospitals, we observed similar practice patterns across diagnoses. For example, hospitals with a higher percent opportunity for one diagnosis tended to have higher percent opportunity for the other diagnoses (as noted in the top portion of the Figure), and those with lower percent opportunity for one diagnosis tended to also have lower percent opportunity for the other diagnoses studied (as noted in the bottom portion of the Figure). When evaluating variability in the percent opportunity, 45% of the variability was attributable to the hospital-effect and 35% to the diagnosis; the remainder was unexplained variability. Sensitivity analysis excluding days when patients received an antiemetic medication yielded no differences in our results.

Opportunity by Antibiotic
The aggregate number of opportunity days varied by antibiotic (Table 3). Intravenous antibiotics with the largest number of opportunity days included clindamycin (44,293), ceftriaxone (23,896), and ampicillin-sulbactam (15,484). Antibiotic-diagnosis combinations with the largest number of opportunity days for each diagnosis included ceftriaxone and ampicillin in CAP; clindamycin in cellulitis, SSTI, and neck infections; ceftriaxone in UTI; and cefazolin in osteomyelitis and septic arthritis.

DISCUSSION
In this multicenter study of pediatric patients hospitalized with common bacterial infections, there was the potential to transition from IV to enteral treatment in over half of the antibiotic days. The degree of opportunity varied by infection, antibiotic, and hospital. Antibiotics with a large aggregate number of opportunity days for enteral transition included clindamycin, which has excellent bioavailability; and ampicillin and ampicillin-sulbactam, which can achieve pharmacodynamic targets with oral equivalents.25-29 The across-hospital variation for a given diagnosis suggests that certain hospitals have strategies in place which permit an earlier transition to enteral antibiotics compared to other institutions in which there were likely missed opportunities to do so. This variability is likely due to limited evidence, emphasizing the need for robust studies to better understand the optimal initial antibiotic route and transition time. Our findings highlight the need for, and large potential impact of, stewardship efforts to promote earlier transition for specific drug targets. This study also demonstrates the feasibility of obtaining two metrics—percent opportunity and opportunity days—from administrative databases to inform stewardship efforts within and across hospitals.
Opportunity days and percent opportunity varied among diagnoses. The variation in aggregate opportunity days was largely a reflection of the number of encounters: Diagnoses such as SSTI, neck infections, and CAP had a large number of both aggregate opportunity days and encounters. The range of opportunity days per encounter (0.9-2.5) suggests potential missed opportunities to transition to enteral antibiotics across all diagnoses (Table 2). The higher opportunity days per encounter in osteomyelitis and septic arthritis may be related to longer LOS and higher percent opportunity. Percent opportunity likely varied among diagnoses due to differences in admission and discharge readiness criteria, diagnostic evaluation, frequency of antibiotic administration, and evidence on the optimal route of initial antibiotics and when to transition to oral formulations. For example, we hypothesize that certain diagnoses, such as osteomyelitis and septic arthritis, have admission and discharge readiness criteria directly tied to the perceived need for IV antibiotics, which may limit in-hospital days on enteral antibiotics and explain the high percent opportunity that we observed. The high percent opportunity seen in musculoskeletal infections also may be due to delays in initiating targeted treatment until culture results were available. Encounters for CAP had the lowest percent opportunity; we hypothesize that this is because admission and discharge readiness may be determined by factors other than the need for IV antibiotics (eg, need for supplemental oxygen), which may increase days on enteral antibiotics and lead to a lower percent opportunity.30
Urinary tract infection encounters had a high percent opportunity. As with musculoskeletal infection, this may be related to delays in initiating targeted treatment until culture results became available. Another reason for the high percent opportunity in UTI could be the common use of ceftriaxone, which, dosed every 24 hours, likely reduced the opportunity to transition to enteral antibiotics. There is strong evidence demonstrating no difference in outcomes based on antibiotic routes for UTI, and we would expect this to result in a low percent opportunity.2,31 While the observed high opportunity in UTI may relate to an initial unknown diagnosis or concern for systemic infection, this highlights potential opportunities for quality improvement initiatives to promote empiric oral antibiotics in clinically stable patients hospitalized with suspected UTI.
There was substantial variation in percent opportunity across hospitals for a given diagnosis, with less variation across diagnoses for a given hospital. Variation across hospitals but consistency within individual hospitals suggests that some hospitals may promote earlier transition from IV to enteral antibiotics as standard practice for all diagnoses, while other hospitals continue IV antibiotics for the entire hospitalization, highlighting potential missed opportunities at some institutions. While emerging data suggest that traditional long durations of IV antibiotics are not necessary for many infections, the limited evidence on the optimal time to switch to oral antibiotics may have influenced this variation.2-7 Many guidelines recommend initial IV antibiotics for hospitalized pediatric patients, but there are few studies comparing IV and enteral therapy.2,5,9 Limited evidence leaves significant room for hospital culture, antibiotic stewardship efforts, reimbursement considerations, and/or hospital workflow to influence transition timing and overall opportunity at individual hospitals.7,8,32-34 These findings emphasize the importance of research to identify optimal transition time and comparative effectiveness studies to evaluate whether initial IV antibiotics are truly needed for mild—and even severe—disease presentations. Since many patients are admitted for the perceived need for IV antibiotics, earlier use of enteral antibiotics could reduce rates of hospitalizations, LOS, healthcare costs, and resource utilization.
Antibiotics with a high number of opportunity days included clindamycin, ceftriaxone, ampicillin-sublactam, and ampicillin. Our findings are consistent with another study which found that most bioavailable drugs, including clindamycin, were administered via the IV route and accounted for a large number of antibiotic days.35 The Infectious Diseases Society of America recommends that hospitals promote earlier transition to oral formulations for highly bioavailable drugs.7 Given the high bioavailability of clindamycin, its common use in high-frequency encounters such as SSTI and neck infections, and the fact that it accounted for a large number of opportunity days, quality improvement initiatives promoting earlier transition to oral clindamycin could have a large impact across health systems.25,26 Additionally, although beta-lactam antibiotics such as amoxicillin and amoxicillin-sulbactam are not highly bioavailable, oral dosing can achieve sufficient serum concentrations to reach pharmacodynamic targets for common clinical indications; this could be an important quality improvement initiative.27-29 Several single-site studies have successfully implemented quality improvement initiatives to promote earlier IV-to-enteral transition, with resulting reductions in costs and no adverse events noted, highlighting the feasibility and impact of such efforts.13,36-38
This study also demonstrates the feasibility of collecting two metrics (percent opportunity and opportunity days) from administrative databases to inform IV-to-oral transition benchmarking and stewardship efforts. While there are several metrics in the literature for evaluating antibiotic transition (eg, days of IV or oral therapy, percentage of antibiotics given via the oral route, time to switch from IV to oral, and acceptance rate of suggested changes to antibiotic route), none are universally used or agreed upon.15,16,39 The opportunity metrics used in this study have several strengths, including the feasibility of obtaining them from existing databases and the ability to account for intake of other enteral medications; the latter is not evaluated in other metrics. These opportunity metrics can be used together to identify the percent of time in which there is opportunity to transition and total number of days to understand the full extent of potential opportunity for future interventions. As demonstrated in this study, these metrics can be measured by diagnosis, antibiotic, or diagnosis-antibiotic combination, and they can be used to evaluate stewardship efforts at a single institution over time or compare efforts across hospitals.
These findings should be interpreted in the context of important limitations. First, we attempted to characterize potential opportunity to transition to enteral medications based on a patient’s ability to tolerate nonenteral medications. However, there are other factors that could limit the opportunity to transition that we could not account for with an administrative dataset, including the use of antibiotics prior to admission, disease progression, severity of illness, and malabsorptive concerns. Thus, though we may have overestimated the true opportunity to transition to enteral antibiotics, it is unlikely that this would account for all of the variation in transition times that we observed across hospitals. Second, while our study required patients to have one of seven types of infection, we did not exclude any additional infectious diagnoses (eg, concurrent bacteremia, Clostridioides difficile, otitis media) that could have driven the choice of antibiotic type and modality. Although emerging evidence is supporting earlier transitions to oral therapy, bacteremia is typically treated with IV antibiotics; this may have led to an overestimation of true opportunity.40 “Clostridioides” difficile and otitis media are typically treated with enteral therapy; concurrent infections such as these may have led to an underestimation of opportunity given the fact that, based on our definition, the days on which patients received both IV and enteral antibiotics were not counted as opportunity days. Third, because PHIS uses billing days to capture medication use, we were unable to distinguish transitions that occurred early in the day vs those that took place later in the day. This could have led to an underestimation of percent opportunity, particularly for diagnoses with a short LOS; it also likely led to an underestimation of the variability observed across hospitals. Fourth, because we used an administrative dataset, we are unable to understand reasoning behind transitioning time from IV to oral antibiotics, as well as provider, patient, and institutional level factors that influenced these decisions.
CONCLUSION
Children hospitalized with bacterial infections often receive IV antibiotics, and the timing of transition from IV to enteral antibiotics varies significantly across hospitals. Further research is needed to compare the effectiveness of IV and enteral antibiotics and better define criteria for transition to enteral therapy. We identified ample opportunities for quality improvement initiatives to promote earlier transition, which have the potential to reduce healthcare utilization and promote optimal patient-directed high-value care.
Bacterial infections are a common reason for pediatric hospital admissions in the United States.1 Antibiotics are the mainstay of treatment, and whether to administer them intravenously (IV) or enterally is an important and, at times, challenging decision. Not all hospitalized patients with infections require IV antibiotics, and safe, effective early transitions to enteral therapy have been described for numerous infections.2-7 However, guidelines describing the ideal initial route of antibiotic administration and when to transition to oral therapy are lacking.5,7,8 This lack of high-quality evidence-based guidance may contribute to overuse of IV antibiotics for many hospitalized pediatric patients, even when safe and effective enteral options exist.9
Significant costs and harms are associated with the use of IV antibiotics. In particular, studies have demonstrated longer length of stay (LOS), increased costs, and worsened pain or anxiety related to complications (eg, phlebitis, extravasation injury, thrombosis, catheter-associated bloodstream infections) associated with IV antibiotics.3,4,10-13 Earlier transition to enteral therapy, however, can mitigate these increased risks and costs.
The Centers for Disease Control and Prevention lists the transition from IV to oral antibiotics as a key stewardship intervention for improving antibiotic use.14 The Infectious Diseases Society of America (IDSA) antibiotic stewardship program guidelines strongly recommend the timely conversion from IV to oral antibiotics, stating that efforts focusing on this transition should be integrated into routine practice.15 There are a few metrics in the literature to measure this intervention, but none is universally used, and a modified delphi process could not reach consensus on IV-to-oral transition metrics.16
Few studies describe the opportunity to transition to enteral antibiotics in hospitalized patients with common bacterial infections or explore variation across hospitals. It is critical to understand current practice of antibiotic administration in order to identify opportunities to optimize patient outcomes and promote high-value care. Furthermore, few studies have evaluated the feasibility of IV-to-oral transition metrics using an administrative database. Thus, the aims of this study were to (1) determine opportunities to transition from IV to enteral antibiotics for pediatric patients hospitalized with common bacterial infections based on their ability to tolerate other enteral medications, (2) describe variation in transition practices among children’s hospitals, and (3) evaluate the feasibility of novel IV-to-oral transition metrics using an administrative database to inform stewardship efforts.
METHODS
Study Design and Setting
This multicenter, retrospective cohort study used data from the Pediatric Health Information System (PHIS), an administrative and billing database containing encounter-level data from 52 tertiary care pediatric hospitals across the United States affiliated with the Children’s Hospital Association (Lenexa, Kansas). Hospitals submit encounter-level data, including demographics, medications, and diagnoses based on International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) codes. Data were de-identified at the time of submission, and data quality and reliability were assured by joint efforts between the Children’s Hospital Association and participating hospitals.
Study Population
This study included pediatric patients aged 60 days to 18 years who were hospitalized (inpatient or observation status) at one of the participating hospitals between January 1, 2017, and December 31, 2018, for one of the following seven common bacterial infections: community-acquired pneumonia (CAP), neck infection (superficial and deep), periorbital/orbital infection, urinary tract infection (UTI), osteomyelitis, septic arthritis, or skin and soft tissue infection (SSTI). The diagnosis cohorts were defined based on ICD-10-CM discharge diagnoses adapted from previous studies (Appendix Table 1).3,17-23 To define a cohort of generally healthy pediatric patients with an acute infection, we excluded patients hospitalized in the intensive care unit, patients with nonhome discharges, and patients with complex chronic conditions.24 We also excluded hospitals with incomplete data during the study period (n=1). The Institutional Review Board at Cincinnati Children’s Hospital Medical Center determined this study to be non–human-subjects research.
Outcomes
The primary outcomes were the number of opportunity days and the percent of days with opportunity to transition from IV to enteral therapy. Opportunity days, or days in which there was a potential opportunity to transition from IV to enteral antibiotics, were defined as days patients received only IV antibiotic doses and at least one enteral nonantibiotic medication, suggesting an ability to take enteral medications.13 We excluded days patients received IV antibiotics for which there was no enteral alternative (eg, vancomycin, Appendix Table 2). When measuring opportunity, to be conservative (ie, to underestimate rather than overestimate opportunity), we did not count as an opportunity day any day in which patients received both IV and enteral antibiotics. Percent opportunity, or the percent of days patients received antibiotics in which there was potential opportunity to transition from IV to enteral antibiotics, was defined as the number of opportunity days divided by number of inpatient days patients received enteral antibiotics or IV antibiotics with at least one enteral nonantibiotic medication (antibiotic days). Similar to opportunity days, antibiotic days excluded days patients were on IV antibiotics for which there was no enteral alternative. Based on our definition, a lower percent opportunity indicates that a hospital is using enteral antibiotics earlier during the hospitalization (earlier transition), while a higher percent opportunity represents later enteral antibiotic use (later transition).
Statistical Analysis
Demographic and clinical characteristics were summarized by diagnosis with descriptive statistics, including frequency with percentage, mean with standard deviation, and median with interquartile range (IQR). For each diagnosis, we evaluated aggregate opportunity days (sum of opportunity days among all hospitals), opportunity days per encounter, and aggregate percent opportunity using frequencies, mean with standard deviation, and percentages, respectively. We also calculated aggregate opportunity days for diagnosis-antibiotic combinations. To visually show variation in the percent opportunity across hospitals, we displayed the percent opportunity on a heat map, and evaluated percent opportunity across hospitals using chi-square tests. To compare the variability in the percent opportunity across and within hospitals, we used a generalized linear model with two fixed effects (hospital and diagnosis), and parsed the variability using the sum of squares. We performed a sensitivity analysis and excluded days that patients received antiemetic medications (eg, ondansetron, granisetron, prochlorperazine, promethazine), as these suggest potential intolerance of enteral medications. All statistical analyses were performed using SAS v.9.4 (SAS Institute Inc, Cary, North Carolina) and GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, California), and P values < .05 were considered statistically significant.
RESULTS
During the 2-year study period, 100,103 hospitalizations met our inclusion criteria across 51 hospitals and seven diagnosis categories (Table 1). Diagnosis cohorts ranged in size from 1,462 encounters for septic arthritis to 35,665 encounters for neck infections. Overall, we identified 88,522 aggregate opportunity days on which there was an opportunity to switch from IV to enteral treatment in the majority of participants (percent opportunity, 57%).

Opportunity by Diagnosis
The number of opportunity days (aggregate and mean per encounter) and percent opportunity varied by diagnosis (Table 2). The aggregate number of opportunity days ranged from 3,693 in patients with septic arthritis to 25,359 in patients with SSTI, and mean opportunity days per encounter ranged from 0.9 in CAP to 2.8 in septic arthritis. Percent opportunity was highest for septic arthritis at 72.7% and lowest for CAP at 39.7%.

Variation in Opportunity Among Hospitals
The variation in the percent opportunity across hospitals was statistically significant for all diagnoses (Figure). Within hospitals, we observed similar practice patterns across diagnoses. For example, hospitals with a higher percent opportunity for one diagnosis tended to have higher percent opportunity for the other diagnoses (as noted in the top portion of the Figure), and those with lower percent opportunity for one diagnosis tended to also have lower percent opportunity for the other diagnoses studied (as noted in the bottom portion of the Figure). When evaluating variability in the percent opportunity, 45% of the variability was attributable to the hospital-effect and 35% to the diagnosis; the remainder was unexplained variability. Sensitivity analysis excluding days when patients received an antiemetic medication yielded no differences in our results.

Opportunity by Antibiotic
The aggregate number of opportunity days varied by antibiotic (Table 3). Intravenous antibiotics with the largest number of opportunity days included clindamycin (44,293), ceftriaxone (23,896), and ampicillin-sulbactam (15,484). Antibiotic-diagnosis combinations with the largest number of opportunity days for each diagnosis included ceftriaxone and ampicillin in CAP; clindamycin in cellulitis, SSTI, and neck infections; ceftriaxone in UTI; and cefazolin in osteomyelitis and septic arthritis.

DISCUSSION
In this multicenter study of pediatric patients hospitalized with common bacterial infections, there was the potential to transition from IV to enteral treatment in over half of the antibiotic days. The degree of opportunity varied by infection, antibiotic, and hospital. Antibiotics with a large aggregate number of opportunity days for enteral transition included clindamycin, which has excellent bioavailability; and ampicillin and ampicillin-sulbactam, which can achieve pharmacodynamic targets with oral equivalents.25-29 The across-hospital variation for a given diagnosis suggests that certain hospitals have strategies in place which permit an earlier transition to enteral antibiotics compared to other institutions in which there were likely missed opportunities to do so. This variability is likely due to limited evidence, emphasizing the need for robust studies to better understand the optimal initial antibiotic route and transition time. Our findings highlight the need for, and large potential impact of, stewardship efforts to promote earlier transition for specific drug targets. This study also demonstrates the feasibility of obtaining two metrics—percent opportunity and opportunity days—from administrative databases to inform stewardship efforts within and across hospitals.
Opportunity days and percent opportunity varied among diagnoses. The variation in aggregate opportunity days was largely a reflection of the number of encounters: Diagnoses such as SSTI, neck infections, and CAP had a large number of both aggregate opportunity days and encounters. The range of opportunity days per encounter (0.9-2.5) suggests potential missed opportunities to transition to enteral antibiotics across all diagnoses (Table 2). The higher opportunity days per encounter in osteomyelitis and septic arthritis may be related to longer LOS and higher percent opportunity. Percent opportunity likely varied among diagnoses due to differences in admission and discharge readiness criteria, diagnostic evaluation, frequency of antibiotic administration, and evidence on the optimal route of initial antibiotics and when to transition to oral formulations. For example, we hypothesize that certain diagnoses, such as osteomyelitis and septic arthritis, have admission and discharge readiness criteria directly tied to the perceived need for IV antibiotics, which may limit in-hospital days on enteral antibiotics and explain the high percent opportunity that we observed. The high percent opportunity seen in musculoskeletal infections also may be due to delays in initiating targeted treatment until culture results were available. Encounters for CAP had the lowest percent opportunity; we hypothesize that this is because admission and discharge readiness may be determined by factors other than the need for IV antibiotics (eg, need for supplemental oxygen), which may increase days on enteral antibiotics and lead to a lower percent opportunity.30
Urinary tract infection encounters had a high percent opportunity. As with musculoskeletal infection, this may be related to delays in initiating targeted treatment until culture results became available. Another reason for the high percent opportunity in UTI could be the common use of ceftriaxone, which, dosed every 24 hours, likely reduced the opportunity to transition to enteral antibiotics. There is strong evidence demonstrating no difference in outcomes based on antibiotic routes for UTI, and we would expect this to result in a low percent opportunity.2,31 While the observed high opportunity in UTI may relate to an initial unknown diagnosis or concern for systemic infection, this highlights potential opportunities for quality improvement initiatives to promote empiric oral antibiotics in clinically stable patients hospitalized with suspected UTI.
There was substantial variation in percent opportunity across hospitals for a given diagnosis, with less variation across diagnoses for a given hospital. Variation across hospitals but consistency within individual hospitals suggests that some hospitals may promote earlier transition from IV to enteral antibiotics as standard practice for all diagnoses, while other hospitals continue IV antibiotics for the entire hospitalization, highlighting potential missed opportunities at some institutions. While emerging data suggest that traditional long durations of IV antibiotics are not necessary for many infections, the limited evidence on the optimal time to switch to oral antibiotics may have influenced this variation.2-7 Many guidelines recommend initial IV antibiotics for hospitalized pediatric patients, but there are few studies comparing IV and enteral therapy.2,5,9 Limited evidence leaves significant room for hospital culture, antibiotic stewardship efforts, reimbursement considerations, and/or hospital workflow to influence transition timing and overall opportunity at individual hospitals.7,8,32-34 These findings emphasize the importance of research to identify optimal transition time and comparative effectiveness studies to evaluate whether initial IV antibiotics are truly needed for mild—and even severe—disease presentations. Since many patients are admitted for the perceived need for IV antibiotics, earlier use of enteral antibiotics could reduce rates of hospitalizations, LOS, healthcare costs, and resource utilization.
Antibiotics with a high number of opportunity days included clindamycin, ceftriaxone, ampicillin-sublactam, and ampicillin. Our findings are consistent with another study which found that most bioavailable drugs, including clindamycin, were administered via the IV route and accounted for a large number of antibiotic days.35 The Infectious Diseases Society of America recommends that hospitals promote earlier transition to oral formulations for highly bioavailable drugs.7 Given the high bioavailability of clindamycin, its common use in high-frequency encounters such as SSTI and neck infections, and the fact that it accounted for a large number of opportunity days, quality improvement initiatives promoting earlier transition to oral clindamycin could have a large impact across health systems.25,26 Additionally, although beta-lactam antibiotics such as amoxicillin and amoxicillin-sulbactam are not highly bioavailable, oral dosing can achieve sufficient serum concentrations to reach pharmacodynamic targets for common clinical indications; this could be an important quality improvement initiative.27-29 Several single-site studies have successfully implemented quality improvement initiatives to promote earlier IV-to-enteral transition, with resulting reductions in costs and no adverse events noted, highlighting the feasibility and impact of such efforts.13,36-38
This study also demonstrates the feasibility of collecting two metrics (percent opportunity and opportunity days) from administrative databases to inform IV-to-oral transition benchmarking and stewardship efforts. While there are several metrics in the literature for evaluating antibiotic transition (eg, days of IV or oral therapy, percentage of antibiotics given via the oral route, time to switch from IV to oral, and acceptance rate of suggested changes to antibiotic route), none are universally used or agreed upon.15,16,39 The opportunity metrics used in this study have several strengths, including the feasibility of obtaining them from existing databases and the ability to account for intake of other enteral medications; the latter is not evaluated in other metrics. These opportunity metrics can be used together to identify the percent of time in which there is opportunity to transition and total number of days to understand the full extent of potential opportunity for future interventions. As demonstrated in this study, these metrics can be measured by diagnosis, antibiotic, or diagnosis-antibiotic combination, and they can be used to evaluate stewardship efforts at a single institution over time or compare efforts across hospitals.
These findings should be interpreted in the context of important limitations. First, we attempted to characterize potential opportunity to transition to enteral medications based on a patient’s ability to tolerate nonenteral medications. However, there are other factors that could limit the opportunity to transition that we could not account for with an administrative dataset, including the use of antibiotics prior to admission, disease progression, severity of illness, and malabsorptive concerns. Thus, though we may have overestimated the true opportunity to transition to enteral antibiotics, it is unlikely that this would account for all of the variation in transition times that we observed across hospitals. Second, while our study required patients to have one of seven types of infection, we did not exclude any additional infectious diagnoses (eg, concurrent bacteremia, Clostridioides difficile, otitis media) that could have driven the choice of antibiotic type and modality. Although emerging evidence is supporting earlier transitions to oral therapy, bacteremia is typically treated with IV antibiotics; this may have led to an overestimation of true opportunity.40 “Clostridioides” difficile and otitis media are typically treated with enteral therapy; concurrent infections such as these may have led to an underestimation of opportunity given the fact that, based on our definition, the days on which patients received both IV and enteral antibiotics were not counted as opportunity days. Third, because PHIS uses billing days to capture medication use, we were unable to distinguish transitions that occurred early in the day vs those that took place later in the day. This could have led to an underestimation of percent opportunity, particularly for diagnoses with a short LOS; it also likely led to an underestimation of the variability observed across hospitals. Fourth, because we used an administrative dataset, we are unable to understand reasoning behind transitioning time from IV to oral antibiotics, as well as provider, patient, and institutional level factors that influenced these decisions.
CONCLUSION
Children hospitalized with bacterial infections often receive IV antibiotics, and the timing of transition from IV to enteral antibiotics varies significantly across hospitals. Further research is needed to compare the effectiveness of IV and enteral antibiotics and better define criteria for transition to enteral therapy. We identified ample opportunities for quality improvement initiatives to promote earlier transition, which have the potential to reduce healthcare utilization and promote optimal patient-directed high-value care.
1. Keren R, Luan X, Localio R, et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X
3. Keren R, Shah SS, Srivastava R, et al; for the Pediatric Research Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e201692. https://doi.org/10.1542/peds.2016-1692
5. Li HK, Agweyu A, English M, Bejon P. An unsupported preference for intravenous antibiotics. PLoS Med. 2015;12(5):e1001825. https://dx.doi.org/10.1371%2Fjournal.pmed.1001825
6. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393
7. Bradley JS, Byington CL, Shah SS, et al; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Management of community-acquired pneumonia (CAP) in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1542/peds.2011-2385
8. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8-S14. https://doi.org/10.1093/cid/cir363
9. Schroeder AR, Ralston SL. Intravenous antibiotic durations for common bacterial infections in children: when is enough? J Hosp Med. 2014;9(9):604-609. https://doi.org/10.1002/jhm.2239
10. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatric Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003
11. van Zanten AR, Engelfriet PM, van Dillen K, van Veen M, Nuijten MJ, Polderman KH. Importance of nondrug costs of intravenous antibiotic therapy. Crit Care. 2003;7(6):R184-R190. https://doi.org/10.1186/cc2388
12. Ruebner R, Keren R, Coffin S, Chu J, Horn D, Zaoutis TE. Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006;117(4):1210-1215. https://doi.org/10.1542/peds.2005-1465
13. Girdwood SCT, Sellas MN, Courter JD, et al. Improving the transition of intravenous to enteral antibiotics in pediatric patients with pneumonia or skin and soft tissue infections. J Hosp Med. 2020;15(1):10-15. https://doi.org/10.12788/jhm.3253
14. Core Elements of Hospital Antibiotic Stewardship Programs. Centers for Disease Control and Prevention. Published 2019. Accessed May 30, 2020. https://www.cdc.gov/antibiotic-use/core-elements/hospital.html
15. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. https://doi.org/10.1093/cid/ciw118
16. Science M, Timberlake K, Morris A, Read S, Le Saux N; Groupe Antibiothérapie en Pédiatrie Canada Alliance for Stewardship of Antimicrobials in Pediatrics (GAP Can ASAP). Quality metrics for antimicrobial stewardship programs. Pediatrics. 2019;143(4):e20182372. https://doi.org/10.1542/peds.2018-2372
17. Tchou MJ, Hall M, Shah SS, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Patterns of electrolyte testing at children’s hospitals for common inpatient diagnoses. Pediatrics. 2019;144(1):e20181644. https://doi.org/10.1542/peds.2018-1644
18. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
19. Desai S, Shah SS, Hall M, Richardson TE, Thomson JE; Pediatric Research in Inpatient Settings (PRIS) Network. Imaging strategies and outcomes in children hospitalized with cervical lymphadenitis. J Hosp Med. 2020;15(4):197-203. https://doi.org/10.12788/jhm.3333
20. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
21. Tieder JS, Hall M, Auger KA, et al. Accuracy of administrative billing codes to detect urinary tract infection hospitalizations. Pediatrics. 2011;128(2):323-330. https://doi.org/10.1542/peds.2010-2064
22. Singh JA, Yu S. The burden of septic arthritis on the U.S. inpatient care: a national study. PLoS One. 2017;12(8):e0182577. https://doi.org/10.1371/journal.pone.0182577
23. Foradori DM, Lopez MA, Hall M, et al. Invasive bacterial infections in infants younger than 60 days with skin and soft tissue infections. Pediatr Emerg Care. 2018. https://doi.org/10.1097/pec.0000000000001584
24. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
25. Arancibia A, Icarte A, González C, Morasso I. Dose-dependent bioavailability of amoxycillin. Int J Clin Pharmacol Ther Toxicol. 1988;26(6):300-303.
26. Grayson ML, Cosgrove S, Crowe S, et al. Kucers’ the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs. 7th ed. CRC Press; 2018.
27. Downes KJ, Hahn A, Wiles J, Courter JD, Inks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in pediatrics’. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006
28. Gras-Le Guen C, Boscher C, Godon N, et al. Therapeutic amoxicillin levels achieved with oral administration in term neonates. Eur J Clin Pharmacol. 2007;63(7):657-662. https://doi.org/10.1007/s00228-007-0307-3
29. Sanchez Navarro A. New formulations of amoxicillin/clavulanic acid: a pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2005;44(11):1097-1115. https://doi.org/10.2165/00003088-200544110-00001
30. Fine MJ, Hough LJ, Medsger AR, et al. The hospital admission decision for patients with community-acquired pneumonia. Results from the pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 1997;157(1):36-44. https://doi.org/10.1001/archinte.1997.00440220040006
31. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007(4):CD003237. https://doi.org/10.1002/14651858.cd003237.pub2
32. Nageswaran S, Woods CR, Benjamin DK Jr, Givner LB, Shetty AK. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25(8):695-699. https://doi.org/10.1097/01.inf.0000227820.36036.f1
33. Al-Nammari S, Roberton B, Ferguson C. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Should a child with preseptal periorbital cellulitis be treated with intravenous or oral antibiotics? Emerg Med J. 2007;24(2):128-129. https://doi.org/10.1136/emj.2006.045245
34. Vieira F, Allen SM, Stocks RMS, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008;41(3):459-483, vii. https://doi.org/10.1016/j.otc.2008.01.002
35. Smith M, Shah S, Kronman M, Patel S, Thurm C, Hersh AL. Route of administration for highly orally bioavailable antibiotics. Open Forum Infect Dis. 2017;4(Suppl 1):S498-S499. https://doi.org/10.1093/ofid/ofx163.1291
36. Brady PW, Brinkman WB, Simmons JM, et al. Oral antibiotics at discharge for children with acute osteomyelitis: a rapid cycle improvement project. BMJ Qual Saf. 2014;23(6):499-507. https://doi.org/10.1136/bmjqs-2013-002179
37. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3
38. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585
39. Public Health Ontario. Antimicrobial stewardship programs metric examples. Published 2017. Accessed June 1, 2020. https://www.publichealthontario.ca/-/media/documents/A/2017/asp-metrics-examples.pdf?la=en
40. Desai S, Aronson PL, Shabanova V, et al; Febrile Young Infant Research Collaborative. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
1. Keren R, Luan X, Localio R, et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X
3. Keren R, Shah SS, Srivastava R, et al; for the Pediatric Research Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e201692. https://doi.org/10.1542/peds.2016-1692
5. Li HK, Agweyu A, English M, Bejon P. An unsupported preference for intravenous antibiotics. PLoS Med. 2015;12(5):e1001825. https://dx.doi.org/10.1371%2Fjournal.pmed.1001825
6. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393
7. Bradley JS, Byington CL, Shah SS, et al; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Management of community-acquired pneumonia (CAP) in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1542/peds.2011-2385
8. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8-S14. https://doi.org/10.1093/cid/cir363
9. Schroeder AR, Ralston SL. Intravenous antibiotic durations for common bacterial infections in children: when is enough? J Hosp Med. 2014;9(9):604-609. https://doi.org/10.1002/jhm.2239
10. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatric Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003
11. van Zanten AR, Engelfriet PM, van Dillen K, van Veen M, Nuijten MJ, Polderman KH. Importance of nondrug costs of intravenous antibiotic therapy. Crit Care. 2003;7(6):R184-R190. https://doi.org/10.1186/cc2388
12. Ruebner R, Keren R, Coffin S, Chu J, Horn D, Zaoutis TE. Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006;117(4):1210-1215. https://doi.org/10.1542/peds.2005-1465
13. Girdwood SCT, Sellas MN, Courter JD, et al. Improving the transition of intravenous to enteral antibiotics in pediatric patients with pneumonia or skin and soft tissue infections. J Hosp Med. 2020;15(1):10-15. https://doi.org/10.12788/jhm.3253
14. Core Elements of Hospital Antibiotic Stewardship Programs. Centers for Disease Control and Prevention. Published 2019. Accessed May 30, 2020. https://www.cdc.gov/antibiotic-use/core-elements/hospital.html
15. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. https://doi.org/10.1093/cid/ciw118
16. Science M, Timberlake K, Morris A, Read S, Le Saux N; Groupe Antibiothérapie en Pédiatrie Canada Alliance for Stewardship of Antimicrobials in Pediatrics (GAP Can ASAP). Quality metrics for antimicrobial stewardship programs. Pediatrics. 2019;143(4):e20182372. https://doi.org/10.1542/peds.2018-2372
17. Tchou MJ, Hall M, Shah SS, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Patterns of electrolyte testing at children’s hospitals for common inpatient diagnoses. Pediatrics. 2019;144(1):e20181644. https://doi.org/10.1542/peds.2018-1644
18. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
19. Desai S, Shah SS, Hall M, Richardson TE, Thomson JE; Pediatric Research in Inpatient Settings (PRIS) Network. Imaging strategies and outcomes in children hospitalized with cervical lymphadenitis. J Hosp Med. 2020;15(4):197-203. https://doi.org/10.12788/jhm.3333
20. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
21. Tieder JS, Hall M, Auger KA, et al. Accuracy of administrative billing codes to detect urinary tract infection hospitalizations. Pediatrics. 2011;128(2):323-330. https://doi.org/10.1542/peds.2010-2064
22. Singh JA, Yu S. The burden of septic arthritis on the U.S. inpatient care: a national study. PLoS One. 2017;12(8):e0182577. https://doi.org/10.1371/journal.pone.0182577
23. Foradori DM, Lopez MA, Hall M, et al. Invasive bacterial infections in infants younger than 60 days with skin and soft tissue infections. Pediatr Emerg Care. 2018. https://doi.org/10.1097/pec.0000000000001584
24. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
25. Arancibia A, Icarte A, González C, Morasso I. Dose-dependent bioavailability of amoxycillin. Int J Clin Pharmacol Ther Toxicol. 1988;26(6):300-303.
26. Grayson ML, Cosgrove S, Crowe S, et al. Kucers’ the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs. 7th ed. CRC Press; 2018.
27. Downes KJ, Hahn A, Wiles J, Courter JD, Inks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in pediatrics’. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006
28. Gras-Le Guen C, Boscher C, Godon N, et al. Therapeutic amoxicillin levels achieved with oral administration in term neonates. Eur J Clin Pharmacol. 2007;63(7):657-662. https://doi.org/10.1007/s00228-007-0307-3
29. Sanchez Navarro A. New formulations of amoxicillin/clavulanic acid: a pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2005;44(11):1097-1115. https://doi.org/10.2165/00003088-200544110-00001
30. Fine MJ, Hough LJ, Medsger AR, et al. The hospital admission decision for patients with community-acquired pneumonia. Results from the pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 1997;157(1):36-44. https://doi.org/10.1001/archinte.1997.00440220040006
31. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007(4):CD003237. https://doi.org/10.1002/14651858.cd003237.pub2
32. Nageswaran S, Woods CR, Benjamin DK Jr, Givner LB, Shetty AK. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25(8):695-699. https://doi.org/10.1097/01.inf.0000227820.36036.f1
33. Al-Nammari S, Roberton B, Ferguson C. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Should a child with preseptal periorbital cellulitis be treated with intravenous or oral antibiotics? Emerg Med J. 2007;24(2):128-129. https://doi.org/10.1136/emj.2006.045245
34. Vieira F, Allen SM, Stocks RMS, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008;41(3):459-483, vii. https://doi.org/10.1016/j.otc.2008.01.002
35. Smith M, Shah S, Kronman M, Patel S, Thurm C, Hersh AL. Route of administration for highly orally bioavailable antibiotics. Open Forum Infect Dis. 2017;4(Suppl 1):S498-S499. https://doi.org/10.1093/ofid/ofx163.1291
36. Brady PW, Brinkman WB, Simmons JM, et al. Oral antibiotics at discharge for children with acute osteomyelitis: a rapid cycle improvement project. BMJ Qual Saf. 2014;23(6):499-507. https://doi.org/10.1136/bmjqs-2013-002179
37. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3
38. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585
39. Public Health Ontario. Antimicrobial stewardship programs metric examples. Published 2017. Accessed June 1, 2020. https://www.publichealthontario.ca/-/media/documents/A/2017/asp-metrics-examples.pdf?la=en
40. Desai S, Aronson PL, Shabanova V, et al; Febrile Young Infant Research Collaborative. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
© 2021 Society of Hospital Medicine
Development of a Simple Index to Measure Overuse of Diagnostic Testing at the Hospital Level Using Administrative Data
There is substantial geographic variation in intensity of healthcare use in the United States,1 yet areas with higher healthcare utilization do not demonstrate superior clinical outcomes.2 Low-value care exposes patients to unnecessary anxiety, radiation, and risk for adverse events.
Previous research has focused on measuring low-value care at the level of hospital referral regions,3-6 metropolitan statistical areas,7 provider organizations,8 and individual physicians.9,10 Hospital referral regions designate regional healthcare markets for tertiary care and generally include at least one major referral center.11 Well-calibrated and validated hospital-level measures of diagnostic overuse are lacking.
We sought to construct a novel index to measure hospital level overuse of diagnostic testing. We focused on diagnostic intensity rather than other forms of overuse such as screening or treatment intensity. Moreover, we aimed to create a parsimonious index—one that is simple, relies on a small number of inputs, is derived from readily available administrative data without the need for chart review or complex logic, and does not require exclusion criteria.
METHODS
Conceptual Framework for Choosing Index Components
To create our overuse index, we took advantage of the requirements for International Classification of Diseases, 9th Revision-Clinical Modification (ICD-9-CM) billing codes 780-796; these codes are based on “symptoms, signs, and ill-defined conditions” and can only be listed as the primary discharge diagnosis if no more specific diagnosis is made.12 As such, when coupled with expensive tests, a high prevalence of these symptom-based diagnosis codes at discharge may serve as a proxy for low-value care. One of the candidate metrics we selected was based on Choosing Wisely® recommendations.13 The other candidate metrics were based on clinical experience and consensus of the study team.
Data Sources
We used hospital-level data on primary discharge diagnosis codes and utilization of testing data from the State Inpatient Databases (SID), which are part of the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Our derivation cohort used data from acute care hospitals in Maryland, New Jersey, and Washington state. Our validation cohort used data from acute care hospitals in Kentucky, North Carolina, New York, and West Virginia. States were selected based on availability of data (certain states lacked complete testing utilization data) and cost of data acquisition. The SID contains hospital-level utilization of computed tomography (CT) scans (CT of the body and head) and diagnostic testing, including stress testing and esophagogastroduodenoscopy (EGD).
Data on three prespecified Dartmouth Atlas of Health Care metrics at the hospital service area (HSA) level were obtained from the Dartmouth Atlas website.14 These metrics were (1) rate of inpatient coronary angiograms per 1,000 Medicare enrollees, (2) price-adjusted physician reimbursement per fee-for-service Medicare enrollee per year (adjusted for patient sex, race, and age), and (3) mean inpatient spending per decedent in the last 6 months of life.15 Data on three prespecified Medicare metrics at the county level were obtained from the Centers for Medicare & Medicaid Services (CMS) website.16 These metrics were standardized per capita cost per (1) procedure, (2) imaging, and (3) test of Medicare fee-for-service patients. The CMS uses the Berenson-Eggers Type of Service Codes to classify fee-generating interventions into a number of categories, including procedure, imaging, and test.17
Components of the Overuse Index
We tested five candidate metrics for index inclusion (Table 1). We utilized Clinical Classifications Software (CCS) codes provided by HCUP, which combine several ICD-9-CM codes into a single primary CCS discharge code for ease of use. The components were (1) primary CCS diagnosis of “nausea and vomiting” coupled with body CT scan or EGD, (2) primary CCS diagnosis of abdominal pain and body CT scan or EGD, (3) primary CCS diagnosis of “nonspecific chest pain” and body CT scan or stress test, (4) primary CCS diagnosis of syncope and stress test, and (5) primary CCS diagnosis for syncope and CT of the brain. For a given metric, the denominator was all patients with the particular primary CCS discharge diagnosis code. The numerator was patients with the diagnostic code who also had the specific test or procedure. We characterized the denominators of each metric in terms of mean, SD, and range.
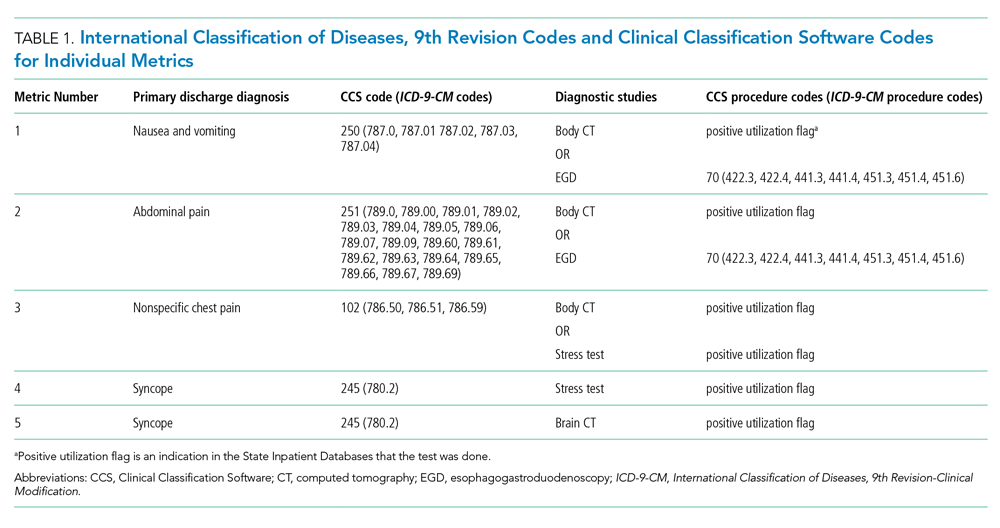
Index Inclusion Criteria and Construction
Specialty, pediatric, rehabilitation, and long-term care hospitals were excluded. Moreover, any hospital with an overall denominator (for the entire index, not an individual metric) of five or fewer observations was excluded. Admissions to acute care hospitals between January 2011 and September 2015 (time of transition from ICD-9-CM to ICD-10-CM) that had one of the specified diagnosis codes were included. For a given hospital, the value of each of the five candidate metrics was defined as the ratio of all admissions that had the given testing and all admissions during the observation period with inclusion CCS diagnosis codes.
Derivation and Validation of the Index
In our derivation cohort (hospitals in Maryland, New Jersey, and Washington state), we tested the temporal stability of each candidate metric by year using the intraclass correlation coefficient (ICC). Using exploratory factor analysis (EFA) and Cronbach’s alpha, we then tested internal consistency of the index candidate components to ensure that all measured a common underlying factor (ie, diagnostic overuse). To standardize data, test rates for both of these analyses were converted to z-scores. For the EFA, we expected that if the index was reflecting only a single underlying factor, the Eigenvalue for one factor should be much higher (typically above 1.0) than that for multiple factors. We calculated item-test correlation for each candidate metric and Cronbach’s alpha for the entire index. A high and stable value for item-test correlation for each index component, as well as a high Cronbach’s alpha, suggests that index components measure a single common factor. Given the small number of test items, we considered a Cronbach’s alpha above 0.6 to be satisfactory.
This analysis showed satisfactory temporal stability of each candidate metric and good internal consistency of the candidate metrics in the derivation cohort. Therefore, we decided to keep all metrics rather than discard any of them. This same process was repeated with the validation cohort (Kentucky, New York, North Carolina, and West Virginia) and then with the combined group of seven states. Tests on the validation and entire cohort further supported our decision to keep all five metrics.
To determine the overall index value for a hospital, all of its metric numerators and denominators were added to calculate one fraction. In this way for a given hospital, a metric for which there were no observations was effectively excluded from the index. This essentially weights each index component by frequency. We chose to count syncope admissions only once in the denominator to avoid the index being unduly influenced by this diagnosis. The hospital index values were combined into their HSAs by adding numerators and denominators from each hospital to calculate HSA index values, effectively giving higher weight to hospitals with more observations. Spearman’s correlation coefficients were measured for these Dartmouth Atlas metrics, also at the HSA level. For the county level analysis, we used a hospital-county crosswalk (available from the American Hospital Association [AHA] Annual Survey; https://www.ahadata.com/aha-annual-survey-database) to link a hospital overuse index value to a county level cost value rather than aggregating data at the county level. We felt this was appropriate, as HSAs were constructed to represent a local healthcare market, whereas counties are less likely to be homogenous from a healthcare perspective.
Analysis of Entire Hospital Sample
The mean index value and SD were calculated for the entire sample of hospitals and for each state. The mean index value for each year of data was calculated to measure the temporal change of the index (representing a change in diagnostic intensity over the study period) using linear regression. We divided the cohort of hospitals into tertiles based on their index value. This is consistent with the CMS categorization of hospital payments and value of care as being “at,” “significantly above,” or “significantly below” a mean value.18 The characteristics of hospitals by tertile were described by mean total hospital beds, mean annual admissions, teaching status (nonteaching hospital, minor teaching hospital, major teaching hospital), and critical access hospital (yes/no). We utilized the AHA Annual Survey for data on hospital characteristics. We calculated P values using analysis of variance for hospital bed size and a chi-square test for teaching status and critical access hospital.
The entire group of hospitals from seven states was then used to apply the index to the HSA level. Numerators and denominators for each hospital in an HSA were added to calculate an HSA-level proportion. Thus, the HSA level index value, though unweighted, is dominated by hospitals with larger numbers of observations. For each of the Dartmouth metrics, the adjusted odds ratio of being in a higher diagnostic overuse index tertile given being in a certain Dartmouth Atlas metric tertile was calculated using ordinal logistic regression. This model controlled for the mean number of beds of hospitals in the HSA (continuous variable), mean Elixhauser Comorbidity Index (ECI) score (continuous variable; unweighted average among hospitals in an HSA), whether the HSA had a major or minor teaching hospital (yes/no) or was a critical access hospital (yes/no), and state fixed effects. The ECI score is a validated score that uses the presence or absence of 29 comorbidities to predict in-hospital mortality.19 For discriminant validity, we also tested two variables not expected to be associated with overuse—hospital ownership and affiliation with the Catholic Church.
For the county-level analysis, ordinal logistic regression was used to predict the adjusted odds ratio of being in a higher diagnostic overuse index tertile given being in a certain tertile of a given county-level spending metric. This model controlled for hospital bed size (continuous variable), hospital ECI score (continuous variable), teaching status (major, minor, nonteaching), critical access hospital status (yes/no), and state fixed effects.
RESULTS
Descriptive Statistics for Metrics
A total of 620 acute care hospitals were included in the index. Thirteen hospitals were excluded because their denominator was five or fewer. The vast majority of HSAs (85.9%) had only one hospital, 8.2% had two hospitals, and 2.4% had three hospitals. Similarly, the majority of counties (68.7%) had only one hospital, 15.1% had two hospitals, and 6.6% had three hospitals (Appendix Tables 1.1 and 1.2). Nonspecific chest pain was the metric with largest denominator mean (650), SD (1,012), and range (0-10,725) (Appendix Table 2). Overall, the metric denominators were a small fraction of total hospital discharges, with means at the hospital level ranging from 0.69% for nausea and vomiting to 5.81% for nonspecific chest pain, suggesting that our index relies on a relatively small fraction of discharges.
Tests for Temporal Stability and Internal Consistency by Derivation and Validation Strategy
Overall, the ICCs for the derivation, validation, and entire cohort suggested strong temporal stability (Appendix Table 3). The EFA of the derivation, validation, and entire cohort showed high Eigenvalues for one principal component, with no other factors close to 1, indicating strong internal consistency (Appendix Table 4). The Cronbach’s alpha analysis also suggested strong internal consistency, with alpha values ranging from 0.73 for the validation cohort to 0.80 for the derivation cohort (Table 2).

Correlation With External Validation Measures
For the entire cohort, the Spearman’s rho for correlation between our overuse index and inpatient rate of coronary angiography at the HSA level was 0.186 (95% CI, 0.089-0.283), Medicare reimbursement at the HSA level was 0.355 (95% CI, 0.272-0.437), and Medicare spending during the last 6 months of life at the HSA level was 0.149 (95% CI, 0.061-0.236) (Appendix Figures 5.1-5.3). The Spearman’s rho for correlation between our overuse index and county level standardized procedure cost was 0.284 (95% CI, 0.210-0.358), imaging cost was 0.268 (95% CI, 0.195-0.342), and testing cost was 0.226 (95% CI, 0.152-0.300) (Appendix Figures 6.1-6.3).
Overall Index Values and Change Over Time
The mean hospital index value was 0.541 (SD, 0.178) (Appendix Table 7). There was a slight but statistically significant annual increase in the overall mean index value over the study period, suggesting a small rise in overuse of diagnostic testing (coefficient 0.011; P <.001) (Appendix Figure 8).
Diagnostic Overuse Index Tertiles
Hospitals in the lowest tertile of the index tended to be smaller (based on number of beds) (P < .0001) and were more likely to be critical access hospitals (P <.0001). There was a significant difference in the proportion of nonteaching, minor teaching, and major teaching hospitals, with more nonteaching hospitals in tertile 1 (P = .001) (Table 3). The median ECI score was not significantly different among tertiles. Neither of the variables tested for discriminant validity (hospital ownership and Catholic Church affiliation) was associated with our index.
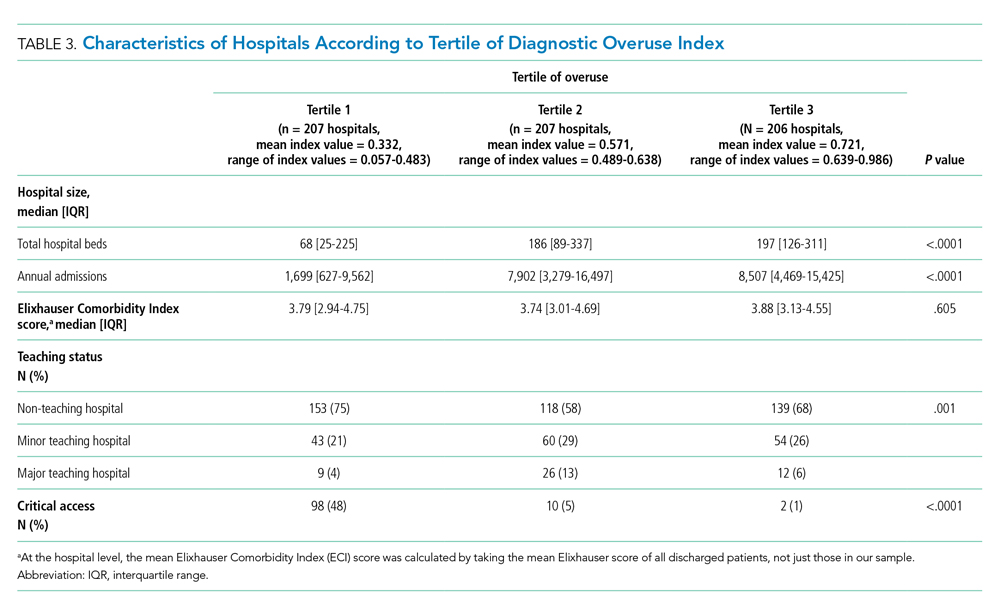
Adjusted Multilevel Mixed-Effects Ordinal Logistic Regression
Our overuse index correlated most closely with physician reimbursement, with an odds ratio of 2.02 (95% CI, 1.11-3.66) of being in a higher tertile of the overuse index when comparing tertiles 3 and 1 of this Dartmouth metric. Of the Medicare county-level metrics, our index correlated most closely with cost of procedures, with an odds ratio of 2.03 (95% CI, 1.21-3.39) of being in a higher overuse index tertile when comparing tertiles 3 and 1 of the cost per procedure metric (Figure 1).
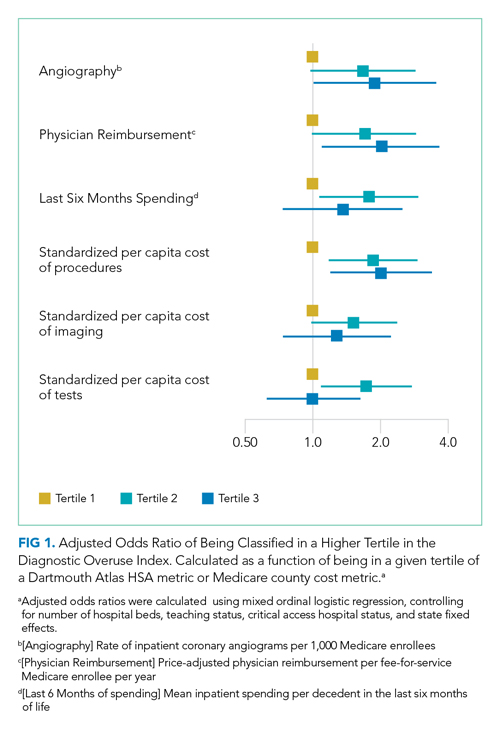
DISCUSSION
Previous research shows variation among hospitals for overall physician spending,20 noninvasive cardiac imaging,21 and the rate of finding obstructive lesions during elective coronary angiography.22 However, there is a lack of standardized methods to study a broad range of diagnostic overuse at the hospital level. To our knowledge, no studies have attempted to develop a diagnostic overuse index at the hospital level. We used a derivation-validation approach to achieve our goal. Although the five metrics represent a range of conditions, the EFA and Cronbach’s alpha tests suggest that they measure a common phenomenon. To avoid systematically excluding smaller hospitals, we limited the extent to which we eliminated hospitals with few observations. Our findings suggest that it may be reasonable to make generalizations on the diagnostic intensity of a hospital based on a relatively small number of discharges. Moreover, our index is a proof of concept that rates of negative diagnostic testing can serve as a proxy for estimating diagnostic overuse.
Our hospital-level index values extrapolated to the HSA level weakly correlated with prespecified Dartmouth Atlas metrics. In a multivariate ordinal regression, there was a significant though weak association between hospitals in higher tertiles of the Dartmouth Atlas metrics and categorization in higher tertiles of our diagnostic overuse index. Similarly, our hospital-level index correlated with two of the three county-level metrics in a multivariate ordinal regression.
We do not assume that all of the metrics in our index track together. However, our results, including the wide dispersion of index values among the tertiles (Table 3), suggest that at least some hospitals are outliers in multiple metrics. We did not assume ex ante that our index should correlate with Dartmouth overuse metrics or Medicare county-level spending; however, we did believe that an association with these measures would assist in validating our index. Given that our index utilizes four common diagnoses, while the Dartmouth and Medicare cost metrics are based on a much broader range of conditions, we would not expect more than a weak correlation even if our index is a valid way to measure overuse.
All of the metrics were based on the concept that hospitals with high rates of negative testing are likely providing large amounts of low-value care. Prior studies on diagnostic yield of CT scans in the emergency department for pulmonary embolus (PE) found an increase in testing and decrease in yield over time; these studies also showed that physicians with more experience ordered fewer CT scans and had a higher yield.23 A review of electronic health records and billing data also showed that hospitals with higher rates of D-dimer testing had higher yields on CT scans ordered to test for PE.24
We took advantage of the coding convention that certain diagnoses only be listed as the primary discharge diagnosis if no more specific diagnosis is made. This allowed us to identify hospitals that likely had high rates of negative tests without granular data. Of course, the metrics are not measuring rates of negative testing per se, but a proxy for this, based instead on the proportion of patients with a symptom-based primary discharge diagnosis who underwent diagnostic testing.
Measuring diagnostic overuse at the hospital level may help to understand factors that drive overuse, given that institutional incentives and culture likely play important roles in ordering tests. There is evidence that financial incentives drive physicians’ decisions,25-27 and there is also evidence that institutional culture impacts outcomes.28 Further, quality improvement projects are typically designed at the hospital level and may be an effective way to curb overuse.29,30
Previous studies have focused on measuring variation among providers and identifying outlier physicians.9,10,20 Providing feedback to underperforming physicians has been shown to change practice habits.31,32 Efforts to improve the practice habits of outlier hospitals may have a number of advantages, including economies of scale and scope and the added benefit of improving the habits of all providers—not just those who are underperforming.
Ordering expensive diagnostic tests on patients with a low pretest probability of having an organic etiology for their symptoms contributes to high healthcare costs. Of course, we do not believe that the ideal rate of negative testing is zero. However, hospitals with high rates of negative diagnostic testing are more likely to be those with clinicians who use expensive tests as a substitute for clinical judgment or less-expensive tests (eg, D-dimer testing to rule out PE).
One challenge we faced is that there is no gold standard of hospital-level overuse with which to validate our index. Our index is weakly correlated with a number of regional metrics that may be proxies for overuse. We are reassured that there is a statistically significant correlation with measures at both HSA and county levels. These correlations are weak, but these regional metrics are themselves imperfect surrogates for overuse. Furthermore, our index is preliminary and will need refinement in future studies.
Limitations
Our analysis has multiple limitations. First, since it relies heavily on primary ICD discharge diagnosis codes, biases could exist due to variations in coding practices. Second, the SID does not include observation stays or tests conducted in the ED, so differential use of observation stays among hospitals might impact results. Finally, based on utilization data, we were not able to distinguish between CT scans of the chest, abdomen, and pelvis because the SID labels each of these as body CT.
CONCLUSION
We developed a novel index to measure diagnostic intensity at the hospital level. This index relies on the concept that high rates of negative diagnostic testing likely indicate some degree of overuse. Our index is parsimonious, does not require granular claims data, and measures a range of potentially overused tests for common clinical scenarios. Our next steps include further refining the index, testing it with granular data, and validating it with other datasets. Thereafter, this index may be useful at identifying positive and negative outliers to understand what processes of care contribute to outlier high and low levels of diagnostic testing. We suspect our index is more useful at identifying extremes than comparing hospitals in the middle of the utilization curve. Additionally, exploring the relationship among individual metrics and the relationship between our index and quality measures like mortality and readmissions may be informative.
1. Fisher ES, Wennberg JE, Stukel TA, et al. Associations among hospital capacity, utilization, and mortality of US Medicare beneficiaries, controlling for sociodemographic factors. Health Serv Res. 2000;34(6):1351-1362.
2. Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder ÉL. The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138(4):288-298. https://doi.org/10.7326/0003-4819-138-4-200302180-00007
3. Segal JB, Nassery N, Chang H-Y, Chang E, Chan K, Bridges JFP. An index for measuring overuse of health care resources with Medicare claims. Med Care. 2015;53(3):230-236. https://doi.org/10.1097/mlr.0000000000000304
4. Colla CH, Morden NE, Sequist TD, Schpero WL, Rosenthal MB. Choosing wisely: prevalence and correlates of low-value health care services in the United States. J Gen Intern Med. 2014;30(2):221-228. https://doi.org/10.1007/s11606-014-3070-z
5. Colla CH, Morden NE, Sequist TD, Mainor AJ, Li Z, Rosenthal MB. Payer type and low-value care: comparing Choosing Wisely services across commercial and Medicare populations. Health Serv Res. 2018;53(2):730-746. https://doi.org/10.1111/1475-6773.12665
6. Schwartz AL, Landon BE, Elshaug AG, Chernew ME, McWilliams JM. Measuring low-value care in Medicare. JAMA Intern Med. 2014;174(7):1067-1076. https://doi.org/10.1001/jamainternmed.2014.1541
7. Oakes AH, Chang H-Y, Segal JB. Systemic overuse of health care in a commercially insured US population, 2010–2015. BMC Health Serv Res. 2019;19(1). https://doi.org/10.1186/s12913-019-4079-0
8. Schwartz AL, Zaslavsky AM, Landon BE, Chernew ME, McWilliams JM. Low-value service use in provider organizations. Health Serv Res. 2018;53(1):87-119. https://doi.org/10.1111/1475-6773.12597
9. Schwartz AL, Jena AB, Zaslavsky AM, McWilliams JM. Analysis of physician variation in provision of low-value services. JAMA Intern Med. 2019;179(1):16-25. https://doi.org/10.1001/jamainternmed.2018.5086
10. Bouck Z, Ferguson J, Ivers NM, et al. Physician characteristics associated with ordering 4 low-value screening tests in primary care. JAMA Netw Open. 2018;1(6):e183506. https://doi.org/10.1001/jamanetworkopen.2018.3506
11. Dartmouth Atlas Project. Data By Region - Dartmouth Atlas of Health Care. Accessed August 29, 2019. http://archive.dartmouthatlas.org/data/region/
12. ICD-9-CM Official Guidelines for Coding and Reporting (Effective October 11, 2011). Accessed March 1, 2018. https://www.cdc.gov/nchs/data/icd/icd9cm_guidelines_2011.pdf
13. Cassel CK, Guest JA. Choosing wisely - helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801-1802. https://doi.org/10.1001/jama.2012.476
14. The Dartmouth Atlas of Health Care. Accessed July 17, 2018. http://www.dartmouthatlas.org/
15. The Dartmouth Atlas of Healthcare. Research Methods. Accessed January 27, 2019. http://archive.dartmouthatlas.org/downloads/methods/research_methods.pdf
16. Centers for Medicare & Medicaid Services. Medicare geographic variation, public use file. Accessed January 5, 2020. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Geographic-Variation/GV_PUF
17. Centers for Medicare & Medicaid Services. Berenson-Eggers Type of Service (BETOS) codes. Accessed January 10, 2020. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareFeeforSvcPartsAB/downloads/betosdesccodes.pdf
18. Data.Medicare.gov. Payment and value of care – hospital: hospital compare. Accessed August 21, 2019. https://data.medicare.gov/Hospital-Compare/Payment-and-value-of-care-Hospital/c7us-v4mf
19. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser comorbidity index. Med Care. 2017;55(7):698-705. https://doi.org/10.1097/mlr.0000000000000735
20. Tsugawa Y, Jha AK, Newhouse JP, Zaslavsky AM, Jena AB. Variation in physician spending and association with patient outcomes. JAMA Intern Med. 2017;177(5):675-682. https://doi.org/10.1001/jamainternmed.2017.0059
21. Safavi KC, Li S-X, Dharmarajan K, et al. Hospital variation in the use of noninvasive cardiac imaging and its association with downstream testing, interventions, and outcomes. JAMA Intern Med. 2014;174(4):546-553. https://doi.org/10.1001/jamainternmed.2013.14407
22. Douglas PS, Patel MR, Bailey SR, et al. Hospital variability in the rate of finding obstructive coronary artery disease at elective, diagnostic coronary angiography. J Am Coll Cardiol. 2011;58(8):801-809. https://doi.org/10.1016/j.jacc.2011.05.019
23. Venkatesh AK, Agha L, Abaluck J, Rothenberg C, Kabrhel C, Raja AS. Trends and variation in the utilization and diagnostic yield of chest imaging for Medicare patients with suspected pulmonary embolism in the emergency department. Am J Roentgenol. 2018;210(3):572-577. https://doi.org/10.2214/ajr.17.18586
24. Kline JA, Garrett JS, Sarmiento EJ, Strachan CC, Courtney DM. Over-testing for suspected pulmonary embolism in american emergency departments: the continuing epidemic. Circ Cardiovasc Qual Outcomes. 2020;13(1):e005753. https://doi.org/10.1161/circoutcomes.119.005753
25. Welch HG, Fisher ES. Income and cancer overdiagnosis – when too much care is harmful. N Engl J Med. 2017;376(23):2208-2209. https://doi.org/10.1056/nejmp1615069
26. Nicholson S. Physician specialty choice under uncertainty. J Labor Econ. 2002;20(4):816-847. https://doi.org/10.1086/342039
27. Chang R-KR, Halfon N. Geographic distribution of pediatricians in the United States: an analysis of the fifty states and Washington, DC. Pediatrics. 1997;100(2 pt 1):172-179. https://doi.org/10.1542/peds.100.2.172
28. Braithwaite J, Herkes J, Ludlow K, Lamprell G, Testa L. Association between organisational and workplace cultures, and patient outcomes: systematic review protocol. BMJ Open. 2016;6(12):e013758. https://doi.org/10.1136/bmjopen-2016-013758
29. Bhatia RS, Milford CE, Picard MH, Weiner RB. An educational intervention reduces the rate of inappropriate echocardiograms on an inpatient medical service. JACC Cardiovasc Imaging. 2013;6(5):545-555. https://doi.org/10.1016/j.jcmg.2013.01.010
30. Blackmore CC, Watt D, Sicuro PL. The success and failure of a radiology quality metric: the case of OP-10. J Am Coll Radiol. 2016;13(6):630-637. https://doi.org/10.1016/j.jacr.2016.01.006
31. Albertini JG, Wang P, Fahim C, et al. Evaluation of a peer-to-peer data transparency intervention for Mohs micrographic surgery overuse. JAMA Dermatol. 2019;155(8):906-913. https://dx.doi.org/10.1001%2Fjamadermatol.2019.1259
32. Sacarny A, Barnett ML, Le J, Tetkoski F, Yokum D, Agrawal S. Effect of peer comparison letters for high-volume primary care prescribers of quetiapine in older and disabled adults: a randomized clinical trial. JAMA Psychiatry. 2018;75(10):1003-1011. https://doi.org/10.1001/jamapsychiatry.2018.1867
There is substantial geographic variation in intensity of healthcare use in the United States,1 yet areas with higher healthcare utilization do not demonstrate superior clinical outcomes.2 Low-value care exposes patients to unnecessary anxiety, radiation, and risk for adverse events.
Previous research has focused on measuring low-value care at the level of hospital referral regions,3-6 metropolitan statistical areas,7 provider organizations,8 and individual physicians.9,10 Hospital referral regions designate regional healthcare markets for tertiary care and generally include at least one major referral center.11 Well-calibrated and validated hospital-level measures of diagnostic overuse are lacking.
We sought to construct a novel index to measure hospital level overuse of diagnostic testing. We focused on diagnostic intensity rather than other forms of overuse such as screening or treatment intensity. Moreover, we aimed to create a parsimonious index—one that is simple, relies on a small number of inputs, is derived from readily available administrative data without the need for chart review or complex logic, and does not require exclusion criteria.
METHODS
Conceptual Framework for Choosing Index Components
To create our overuse index, we took advantage of the requirements for International Classification of Diseases, 9th Revision-Clinical Modification (ICD-9-CM) billing codes 780-796; these codes are based on “symptoms, signs, and ill-defined conditions” and can only be listed as the primary discharge diagnosis if no more specific diagnosis is made.12 As such, when coupled with expensive tests, a high prevalence of these symptom-based diagnosis codes at discharge may serve as a proxy for low-value care. One of the candidate metrics we selected was based on Choosing Wisely® recommendations.13 The other candidate metrics were based on clinical experience and consensus of the study team.
Data Sources
We used hospital-level data on primary discharge diagnosis codes and utilization of testing data from the State Inpatient Databases (SID), which are part of the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Our derivation cohort used data from acute care hospitals in Maryland, New Jersey, and Washington state. Our validation cohort used data from acute care hospitals in Kentucky, North Carolina, New York, and West Virginia. States were selected based on availability of data (certain states lacked complete testing utilization data) and cost of data acquisition. The SID contains hospital-level utilization of computed tomography (CT) scans (CT of the body and head) and diagnostic testing, including stress testing and esophagogastroduodenoscopy (EGD).
Data on three prespecified Dartmouth Atlas of Health Care metrics at the hospital service area (HSA) level were obtained from the Dartmouth Atlas website.14 These metrics were (1) rate of inpatient coronary angiograms per 1,000 Medicare enrollees, (2) price-adjusted physician reimbursement per fee-for-service Medicare enrollee per year (adjusted for patient sex, race, and age), and (3) mean inpatient spending per decedent in the last 6 months of life.15 Data on three prespecified Medicare metrics at the county level were obtained from the Centers for Medicare & Medicaid Services (CMS) website.16 These metrics were standardized per capita cost per (1) procedure, (2) imaging, and (3) test of Medicare fee-for-service patients. The CMS uses the Berenson-Eggers Type of Service Codes to classify fee-generating interventions into a number of categories, including procedure, imaging, and test.17
Components of the Overuse Index
We tested five candidate metrics for index inclusion (Table 1). We utilized Clinical Classifications Software (CCS) codes provided by HCUP, which combine several ICD-9-CM codes into a single primary CCS discharge code for ease of use. The components were (1) primary CCS diagnosis of “nausea and vomiting” coupled with body CT scan or EGD, (2) primary CCS diagnosis of abdominal pain and body CT scan or EGD, (3) primary CCS diagnosis of “nonspecific chest pain” and body CT scan or stress test, (4) primary CCS diagnosis of syncope and stress test, and (5) primary CCS diagnosis for syncope and CT of the brain. For a given metric, the denominator was all patients with the particular primary CCS discharge diagnosis code. The numerator was patients with the diagnostic code who also had the specific test or procedure. We characterized the denominators of each metric in terms of mean, SD, and range.

Index Inclusion Criteria and Construction
Specialty, pediatric, rehabilitation, and long-term care hospitals were excluded. Moreover, any hospital with an overall denominator (for the entire index, not an individual metric) of five or fewer observations was excluded. Admissions to acute care hospitals between January 2011 and September 2015 (time of transition from ICD-9-CM to ICD-10-CM) that had one of the specified diagnosis codes were included. For a given hospital, the value of each of the five candidate metrics was defined as the ratio of all admissions that had the given testing and all admissions during the observation period with inclusion CCS diagnosis codes.
Derivation and Validation of the Index
In our derivation cohort (hospitals in Maryland, New Jersey, and Washington state), we tested the temporal stability of each candidate metric by year using the intraclass correlation coefficient (ICC). Using exploratory factor analysis (EFA) and Cronbach’s alpha, we then tested internal consistency of the index candidate components to ensure that all measured a common underlying factor (ie, diagnostic overuse). To standardize data, test rates for both of these analyses were converted to z-scores. For the EFA, we expected that if the index was reflecting only a single underlying factor, the Eigenvalue for one factor should be much higher (typically above 1.0) than that for multiple factors. We calculated item-test correlation for each candidate metric and Cronbach’s alpha for the entire index. A high and stable value for item-test correlation for each index component, as well as a high Cronbach’s alpha, suggests that index components measure a single common factor. Given the small number of test items, we considered a Cronbach’s alpha above 0.6 to be satisfactory.
This analysis showed satisfactory temporal stability of each candidate metric and good internal consistency of the candidate metrics in the derivation cohort. Therefore, we decided to keep all metrics rather than discard any of them. This same process was repeated with the validation cohort (Kentucky, New York, North Carolina, and West Virginia) and then with the combined group of seven states. Tests on the validation and entire cohort further supported our decision to keep all five metrics.
To determine the overall index value for a hospital, all of its metric numerators and denominators were added to calculate one fraction. In this way for a given hospital, a metric for which there were no observations was effectively excluded from the index. This essentially weights each index component by frequency. We chose to count syncope admissions only once in the denominator to avoid the index being unduly influenced by this diagnosis. The hospital index values were combined into their HSAs by adding numerators and denominators from each hospital to calculate HSA index values, effectively giving higher weight to hospitals with more observations. Spearman’s correlation coefficients were measured for these Dartmouth Atlas metrics, also at the HSA level. For the county level analysis, we used a hospital-county crosswalk (available from the American Hospital Association [AHA] Annual Survey; https://www.ahadata.com/aha-annual-survey-database) to link a hospital overuse index value to a county level cost value rather than aggregating data at the county level. We felt this was appropriate, as HSAs were constructed to represent a local healthcare market, whereas counties are less likely to be homogenous from a healthcare perspective.
Analysis of Entire Hospital Sample
The mean index value and SD were calculated for the entire sample of hospitals and for each state. The mean index value for each year of data was calculated to measure the temporal change of the index (representing a change in diagnostic intensity over the study period) using linear regression. We divided the cohort of hospitals into tertiles based on their index value. This is consistent with the CMS categorization of hospital payments and value of care as being “at,” “significantly above,” or “significantly below” a mean value.18 The characteristics of hospitals by tertile were described by mean total hospital beds, mean annual admissions, teaching status (nonteaching hospital, minor teaching hospital, major teaching hospital), and critical access hospital (yes/no). We utilized the AHA Annual Survey for data on hospital characteristics. We calculated P values using analysis of variance for hospital bed size and a chi-square test for teaching status and critical access hospital.
The entire group of hospitals from seven states was then used to apply the index to the HSA level. Numerators and denominators for each hospital in an HSA were added to calculate an HSA-level proportion. Thus, the HSA level index value, though unweighted, is dominated by hospitals with larger numbers of observations. For each of the Dartmouth metrics, the adjusted odds ratio of being in a higher diagnostic overuse index tertile given being in a certain Dartmouth Atlas metric tertile was calculated using ordinal logistic regression. This model controlled for the mean number of beds of hospitals in the HSA (continuous variable), mean Elixhauser Comorbidity Index (ECI) score (continuous variable; unweighted average among hospitals in an HSA), whether the HSA had a major or minor teaching hospital (yes/no) or was a critical access hospital (yes/no), and state fixed effects. The ECI score is a validated score that uses the presence or absence of 29 comorbidities to predict in-hospital mortality.19 For discriminant validity, we also tested two variables not expected to be associated with overuse—hospital ownership and affiliation with the Catholic Church.
For the county-level analysis, ordinal logistic regression was used to predict the adjusted odds ratio of being in a higher diagnostic overuse index tertile given being in a certain tertile of a given county-level spending metric. This model controlled for hospital bed size (continuous variable), hospital ECI score (continuous variable), teaching status (major, minor, nonteaching), critical access hospital status (yes/no), and state fixed effects.
RESULTS
Descriptive Statistics for Metrics
A total of 620 acute care hospitals were included in the index. Thirteen hospitals were excluded because their denominator was five or fewer. The vast majority of HSAs (85.9%) had only one hospital, 8.2% had two hospitals, and 2.4% had three hospitals. Similarly, the majority of counties (68.7%) had only one hospital, 15.1% had two hospitals, and 6.6% had three hospitals (Appendix Tables 1.1 and 1.2). Nonspecific chest pain was the metric with largest denominator mean (650), SD (1,012), and range (0-10,725) (Appendix Table 2). Overall, the metric denominators were a small fraction of total hospital discharges, with means at the hospital level ranging from 0.69% for nausea and vomiting to 5.81% for nonspecific chest pain, suggesting that our index relies on a relatively small fraction of discharges.
Tests for Temporal Stability and Internal Consistency by Derivation and Validation Strategy
Overall, the ICCs for the derivation, validation, and entire cohort suggested strong temporal stability (Appendix Table 3). The EFA of the derivation, validation, and entire cohort showed high Eigenvalues for one principal component, with no other factors close to 1, indicating strong internal consistency (Appendix Table 4). The Cronbach’s alpha analysis also suggested strong internal consistency, with alpha values ranging from 0.73 for the validation cohort to 0.80 for the derivation cohort (Table 2).

Correlation With External Validation Measures
For the entire cohort, the Spearman’s rho for correlation between our overuse index and inpatient rate of coronary angiography at the HSA level was 0.186 (95% CI, 0.089-0.283), Medicare reimbursement at the HSA level was 0.355 (95% CI, 0.272-0.437), and Medicare spending during the last 6 months of life at the HSA level was 0.149 (95% CI, 0.061-0.236) (Appendix Figures 5.1-5.3). The Spearman’s rho for correlation between our overuse index and county level standardized procedure cost was 0.284 (95% CI, 0.210-0.358), imaging cost was 0.268 (95% CI, 0.195-0.342), and testing cost was 0.226 (95% CI, 0.152-0.300) (Appendix Figures 6.1-6.3).
Overall Index Values and Change Over Time
The mean hospital index value was 0.541 (SD, 0.178) (Appendix Table 7). There was a slight but statistically significant annual increase in the overall mean index value over the study period, suggesting a small rise in overuse of diagnostic testing (coefficient 0.011; P <.001) (Appendix Figure 8).
Diagnostic Overuse Index Tertiles
Hospitals in the lowest tertile of the index tended to be smaller (based on number of beds) (P < .0001) and were more likely to be critical access hospitals (P <.0001). There was a significant difference in the proportion of nonteaching, minor teaching, and major teaching hospitals, with more nonteaching hospitals in tertile 1 (P = .001) (Table 3). The median ECI score was not significantly different among tertiles. Neither of the variables tested for discriminant validity (hospital ownership and Catholic Church affiliation) was associated with our index.

Adjusted Multilevel Mixed-Effects Ordinal Logistic Regression
Our overuse index correlated most closely with physician reimbursement, with an odds ratio of 2.02 (95% CI, 1.11-3.66) of being in a higher tertile of the overuse index when comparing tertiles 3 and 1 of this Dartmouth metric. Of the Medicare county-level metrics, our index correlated most closely with cost of procedures, with an odds ratio of 2.03 (95% CI, 1.21-3.39) of being in a higher overuse index tertile when comparing tertiles 3 and 1 of the cost per procedure metric (Figure 1).

DISCUSSION
Previous research shows variation among hospitals for overall physician spending,20 noninvasive cardiac imaging,21 and the rate of finding obstructive lesions during elective coronary angiography.22 However, there is a lack of standardized methods to study a broad range of diagnostic overuse at the hospital level. To our knowledge, no studies have attempted to develop a diagnostic overuse index at the hospital level. We used a derivation-validation approach to achieve our goal. Although the five metrics represent a range of conditions, the EFA and Cronbach’s alpha tests suggest that they measure a common phenomenon. To avoid systematically excluding smaller hospitals, we limited the extent to which we eliminated hospitals with few observations. Our findings suggest that it may be reasonable to make generalizations on the diagnostic intensity of a hospital based on a relatively small number of discharges. Moreover, our index is a proof of concept that rates of negative diagnostic testing can serve as a proxy for estimating diagnostic overuse.
Our hospital-level index values extrapolated to the HSA level weakly correlated with prespecified Dartmouth Atlas metrics. In a multivariate ordinal regression, there was a significant though weak association between hospitals in higher tertiles of the Dartmouth Atlas metrics and categorization in higher tertiles of our diagnostic overuse index. Similarly, our hospital-level index correlated with two of the three county-level metrics in a multivariate ordinal regression.
We do not assume that all of the metrics in our index track together. However, our results, including the wide dispersion of index values among the tertiles (Table 3), suggest that at least some hospitals are outliers in multiple metrics. We did not assume ex ante that our index should correlate with Dartmouth overuse metrics or Medicare county-level spending; however, we did believe that an association with these measures would assist in validating our index. Given that our index utilizes four common diagnoses, while the Dartmouth and Medicare cost metrics are based on a much broader range of conditions, we would not expect more than a weak correlation even if our index is a valid way to measure overuse.
All of the metrics were based on the concept that hospitals with high rates of negative testing are likely providing large amounts of low-value care. Prior studies on diagnostic yield of CT scans in the emergency department for pulmonary embolus (PE) found an increase in testing and decrease in yield over time; these studies also showed that physicians with more experience ordered fewer CT scans and had a higher yield.23 A review of electronic health records and billing data also showed that hospitals with higher rates of D-dimer testing had higher yields on CT scans ordered to test for PE.24
We took advantage of the coding convention that certain diagnoses only be listed as the primary discharge diagnosis if no more specific diagnosis is made. This allowed us to identify hospitals that likely had high rates of negative tests without granular data. Of course, the metrics are not measuring rates of negative testing per se, but a proxy for this, based instead on the proportion of patients with a symptom-based primary discharge diagnosis who underwent diagnostic testing.
Measuring diagnostic overuse at the hospital level may help to understand factors that drive overuse, given that institutional incentives and culture likely play important roles in ordering tests. There is evidence that financial incentives drive physicians’ decisions,25-27 and there is also evidence that institutional culture impacts outcomes.28 Further, quality improvement projects are typically designed at the hospital level and may be an effective way to curb overuse.29,30
Previous studies have focused on measuring variation among providers and identifying outlier physicians.9,10,20 Providing feedback to underperforming physicians has been shown to change practice habits.31,32 Efforts to improve the practice habits of outlier hospitals may have a number of advantages, including economies of scale and scope and the added benefit of improving the habits of all providers—not just those who are underperforming.
Ordering expensive diagnostic tests on patients with a low pretest probability of having an organic etiology for their symptoms contributes to high healthcare costs. Of course, we do not believe that the ideal rate of negative testing is zero. However, hospitals with high rates of negative diagnostic testing are more likely to be those with clinicians who use expensive tests as a substitute for clinical judgment or less-expensive tests (eg, D-dimer testing to rule out PE).
One challenge we faced is that there is no gold standard of hospital-level overuse with which to validate our index. Our index is weakly correlated with a number of regional metrics that may be proxies for overuse. We are reassured that there is a statistically significant correlation with measures at both HSA and county levels. These correlations are weak, but these regional metrics are themselves imperfect surrogates for overuse. Furthermore, our index is preliminary and will need refinement in future studies.
Limitations
Our analysis has multiple limitations. First, since it relies heavily on primary ICD discharge diagnosis codes, biases could exist due to variations in coding practices. Second, the SID does not include observation stays or tests conducted in the ED, so differential use of observation stays among hospitals might impact results. Finally, based on utilization data, we were not able to distinguish between CT scans of the chest, abdomen, and pelvis because the SID labels each of these as body CT.
CONCLUSION
We developed a novel index to measure diagnostic intensity at the hospital level. This index relies on the concept that high rates of negative diagnostic testing likely indicate some degree of overuse. Our index is parsimonious, does not require granular claims data, and measures a range of potentially overused tests for common clinical scenarios. Our next steps include further refining the index, testing it with granular data, and validating it with other datasets. Thereafter, this index may be useful at identifying positive and negative outliers to understand what processes of care contribute to outlier high and low levels of diagnostic testing. We suspect our index is more useful at identifying extremes than comparing hospitals in the middle of the utilization curve. Additionally, exploring the relationship among individual metrics and the relationship between our index and quality measures like mortality and readmissions may be informative.
There is substantial geographic variation in intensity of healthcare use in the United States,1 yet areas with higher healthcare utilization do not demonstrate superior clinical outcomes.2 Low-value care exposes patients to unnecessary anxiety, radiation, and risk for adverse events.
Previous research has focused on measuring low-value care at the level of hospital referral regions,3-6 metropolitan statistical areas,7 provider organizations,8 and individual physicians.9,10 Hospital referral regions designate regional healthcare markets for tertiary care and generally include at least one major referral center.11 Well-calibrated and validated hospital-level measures of diagnostic overuse are lacking.
We sought to construct a novel index to measure hospital level overuse of diagnostic testing. We focused on diagnostic intensity rather than other forms of overuse such as screening or treatment intensity. Moreover, we aimed to create a parsimonious index—one that is simple, relies on a small number of inputs, is derived from readily available administrative data without the need for chart review or complex logic, and does not require exclusion criteria.
METHODS
Conceptual Framework for Choosing Index Components
To create our overuse index, we took advantage of the requirements for International Classification of Diseases, 9th Revision-Clinical Modification (ICD-9-CM) billing codes 780-796; these codes are based on “symptoms, signs, and ill-defined conditions” and can only be listed as the primary discharge diagnosis if no more specific diagnosis is made.12 As such, when coupled with expensive tests, a high prevalence of these symptom-based diagnosis codes at discharge may serve as a proxy for low-value care. One of the candidate metrics we selected was based on Choosing Wisely® recommendations.13 The other candidate metrics were based on clinical experience and consensus of the study team.
Data Sources
We used hospital-level data on primary discharge diagnosis codes and utilization of testing data from the State Inpatient Databases (SID), which are part of the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Our derivation cohort used data from acute care hospitals in Maryland, New Jersey, and Washington state. Our validation cohort used data from acute care hospitals in Kentucky, North Carolina, New York, and West Virginia. States were selected based on availability of data (certain states lacked complete testing utilization data) and cost of data acquisition. The SID contains hospital-level utilization of computed tomography (CT) scans (CT of the body and head) and diagnostic testing, including stress testing and esophagogastroduodenoscopy (EGD).
Data on three prespecified Dartmouth Atlas of Health Care metrics at the hospital service area (HSA) level were obtained from the Dartmouth Atlas website.14 These metrics were (1) rate of inpatient coronary angiograms per 1,000 Medicare enrollees, (2) price-adjusted physician reimbursement per fee-for-service Medicare enrollee per year (adjusted for patient sex, race, and age), and (3) mean inpatient spending per decedent in the last 6 months of life.15 Data on three prespecified Medicare metrics at the county level were obtained from the Centers for Medicare & Medicaid Services (CMS) website.16 These metrics were standardized per capita cost per (1) procedure, (2) imaging, and (3) test of Medicare fee-for-service patients. The CMS uses the Berenson-Eggers Type of Service Codes to classify fee-generating interventions into a number of categories, including procedure, imaging, and test.17
Components of the Overuse Index
We tested five candidate metrics for index inclusion (Table 1). We utilized Clinical Classifications Software (CCS) codes provided by HCUP, which combine several ICD-9-CM codes into a single primary CCS discharge code for ease of use. The components were (1) primary CCS diagnosis of “nausea and vomiting” coupled with body CT scan or EGD, (2) primary CCS diagnosis of abdominal pain and body CT scan or EGD, (3) primary CCS diagnosis of “nonspecific chest pain” and body CT scan or stress test, (4) primary CCS diagnosis of syncope and stress test, and (5) primary CCS diagnosis for syncope and CT of the brain. For a given metric, the denominator was all patients with the particular primary CCS discharge diagnosis code. The numerator was patients with the diagnostic code who also had the specific test or procedure. We characterized the denominators of each metric in terms of mean, SD, and range.

Index Inclusion Criteria and Construction
Specialty, pediatric, rehabilitation, and long-term care hospitals were excluded. Moreover, any hospital with an overall denominator (for the entire index, not an individual metric) of five or fewer observations was excluded. Admissions to acute care hospitals between January 2011 and September 2015 (time of transition from ICD-9-CM to ICD-10-CM) that had one of the specified diagnosis codes were included. For a given hospital, the value of each of the five candidate metrics was defined as the ratio of all admissions that had the given testing and all admissions during the observation period with inclusion CCS diagnosis codes.
Derivation and Validation of the Index
In our derivation cohort (hospitals in Maryland, New Jersey, and Washington state), we tested the temporal stability of each candidate metric by year using the intraclass correlation coefficient (ICC). Using exploratory factor analysis (EFA) and Cronbach’s alpha, we then tested internal consistency of the index candidate components to ensure that all measured a common underlying factor (ie, diagnostic overuse). To standardize data, test rates for both of these analyses were converted to z-scores. For the EFA, we expected that if the index was reflecting only a single underlying factor, the Eigenvalue for one factor should be much higher (typically above 1.0) than that for multiple factors. We calculated item-test correlation for each candidate metric and Cronbach’s alpha for the entire index. A high and stable value for item-test correlation for each index component, as well as a high Cronbach’s alpha, suggests that index components measure a single common factor. Given the small number of test items, we considered a Cronbach’s alpha above 0.6 to be satisfactory.
This analysis showed satisfactory temporal stability of each candidate metric and good internal consistency of the candidate metrics in the derivation cohort. Therefore, we decided to keep all metrics rather than discard any of them. This same process was repeated with the validation cohort (Kentucky, New York, North Carolina, and West Virginia) and then with the combined group of seven states. Tests on the validation and entire cohort further supported our decision to keep all five metrics.
To determine the overall index value for a hospital, all of its metric numerators and denominators were added to calculate one fraction. In this way for a given hospital, a metric for which there were no observations was effectively excluded from the index. This essentially weights each index component by frequency. We chose to count syncope admissions only once in the denominator to avoid the index being unduly influenced by this diagnosis. The hospital index values were combined into their HSAs by adding numerators and denominators from each hospital to calculate HSA index values, effectively giving higher weight to hospitals with more observations. Spearman’s correlation coefficients were measured for these Dartmouth Atlas metrics, also at the HSA level. For the county level analysis, we used a hospital-county crosswalk (available from the American Hospital Association [AHA] Annual Survey; https://www.ahadata.com/aha-annual-survey-database) to link a hospital overuse index value to a county level cost value rather than aggregating data at the county level. We felt this was appropriate, as HSAs were constructed to represent a local healthcare market, whereas counties are less likely to be homogenous from a healthcare perspective.
Analysis of Entire Hospital Sample
The mean index value and SD were calculated for the entire sample of hospitals and for each state. The mean index value for each year of data was calculated to measure the temporal change of the index (representing a change in diagnostic intensity over the study period) using linear regression. We divided the cohort of hospitals into tertiles based on their index value. This is consistent with the CMS categorization of hospital payments and value of care as being “at,” “significantly above,” or “significantly below” a mean value.18 The characteristics of hospitals by tertile were described by mean total hospital beds, mean annual admissions, teaching status (nonteaching hospital, minor teaching hospital, major teaching hospital), and critical access hospital (yes/no). We utilized the AHA Annual Survey for data on hospital characteristics. We calculated P values using analysis of variance for hospital bed size and a chi-square test for teaching status and critical access hospital.
The entire group of hospitals from seven states was then used to apply the index to the HSA level. Numerators and denominators for each hospital in an HSA were added to calculate an HSA-level proportion. Thus, the HSA level index value, though unweighted, is dominated by hospitals with larger numbers of observations. For each of the Dartmouth metrics, the adjusted odds ratio of being in a higher diagnostic overuse index tertile given being in a certain Dartmouth Atlas metric tertile was calculated using ordinal logistic regression. This model controlled for the mean number of beds of hospitals in the HSA (continuous variable), mean Elixhauser Comorbidity Index (ECI) score (continuous variable; unweighted average among hospitals in an HSA), whether the HSA had a major or minor teaching hospital (yes/no) or was a critical access hospital (yes/no), and state fixed effects. The ECI score is a validated score that uses the presence or absence of 29 comorbidities to predict in-hospital mortality.19 For discriminant validity, we also tested two variables not expected to be associated with overuse—hospital ownership and affiliation with the Catholic Church.
For the county-level analysis, ordinal logistic regression was used to predict the adjusted odds ratio of being in a higher diagnostic overuse index tertile given being in a certain tertile of a given county-level spending metric. This model controlled for hospital bed size (continuous variable), hospital ECI score (continuous variable), teaching status (major, minor, nonteaching), critical access hospital status (yes/no), and state fixed effects.
RESULTS
Descriptive Statistics for Metrics
A total of 620 acute care hospitals were included in the index. Thirteen hospitals were excluded because their denominator was five or fewer. The vast majority of HSAs (85.9%) had only one hospital, 8.2% had two hospitals, and 2.4% had three hospitals. Similarly, the majority of counties (68.7%) had only one hospital, 15.1% had two hospitals, and 6.6% had three hospitals (Appendix Tables 1.1 and 1.2). Nonspecific chest pain was the metric with largest denominator mean (650), SD (1,012), and range (0-10,725) (Appendix Table 2). Overall, the metric denominators were a small fraction of total hospital discharges, with means at the hospital level ranging from 0.69% for nausea and vomiting to 5.81% for nonspecific chest pain, suggesting that our index relies on a relatively small fraction of discharges.
Tests for Temporal Stability and Internal Consistency by Derivation and Validation Strategy
Overall, the ICCs for the derivation, validation, and entire cohort suggested strong temporal stability (Appendix Table 3). The EFA of the derivation, validation, and entire cohort showed high Eigenvalues for one principal component, with no other factors close to 1, indicating strong internal consistency (Appendix Table 4). The Cronbach’s alpha analysis also suggested strong internal consistency, with alpha values ranging from 0.73 for the validation cohort to 0.80 for the derivation cohort (Table 2).

Correlation With External Validation Measures
For the entire cohort, the Spearman’s rho for correlation between our overuse index and inpatient rate of coronary angiography at the HSA level was 0.186 (95% CI, 0.089-0.283), Medicare reimbursement at the HSA level was 0.355 (95% CI, 0.272-0.437), and Medicare spending during the last 6 months of life at the HSA level was 0.149 (95% CI, 0.061-0.236) (Appendix Figures 5.1-5.3). The Spearman’s rho for correlation between our overuse index and county level standardized procedure cost was 0.284 (95% CI, 0.210-0.358), imaging cost was 0.268 (95% CI, 0.195-0.342), and testing cost was 0.226 (95% CI, 0.152-0.300) (Appendix Figures 6.1-6.3).
Overall Index Values and Change Over Time
The mean hospital index value was 0.541 (SD, 0.178) (Appendix Table 7). There was a slight but statistically significant annual increase in the overall mean index value over the study period, suggesting a small rise in overuse of diagnostic testing (coefficient 0.011; P <.001) (Appendix Figure 8).
Diagnostic Overuse Index Tertiles
Hospitals in the lowest tertile of the index tended to be smaller (based on number of beds) (P < .0001) and were more likely to be critical access hospitals (P <.0001). There was a significant difference in the proportion of nonteaching, minor teaching, and major teaching hospitals, with more nonteaching hospitals in tertile 1 (P = .001) (Table 3). The median ECI score was not significantly different among tertiles. Neither of the variables tested for discriminant validity (hospital ownership and Catholic Church affiliation) was associated with our index.

Adjusted Multilevel Mixed-Effects Ordinal Logistic Regression
Our overuse index correlated most closely with physician reimbursement, with an odds ratio of 2.02 (95% CI, 1.11-3.66) of being in a higher tertile of the overuse index when comparing tertiles 3 and 1 of this Dartmouth metric. Of the Medicare county-level metrics, our index correlated most closely with cost of procedures, with an odds ratio of 2.03 (95% CI, 1.21-3.39) of being in a higher overuse index tertile when comparing tertiles 3 and 1 of the cost per procedure metric (Figure 1).

DISCUSSION
Previous research shows variation among hospitals for overall physician spending,20 noninvasive cardiac imaging,21 and the rate of finding obstructive lesions during elective coronary angiography.22 However, there is a lack of standardized methods to study a broad range of diagnostic overuse at the hospital level. To our knowledge, no studies have attempted to develop a diagnostic overuse index at the hospital level. We used a derivation-validation approach to achieve our goal. Although the five metrics represent a range of conditions, the EFA and Cronbach’s alpha tests suggest that they measure a common phenomenon. To avoid systematically excluding smaller hospitals, we limited the extent to which we eliminated hospitals with few observations. Our findings suggest that it may be reasonable to make generalizations on the diagnostic intensity of a hospital based on a relatively small number of discharges. Moreover, our index is a proof of concept that rates of negative diagnostic testing can serve as a proxy for estimating diagnostic overuse.
Our hospital-level index values extrapolated to the HSA level weakly correlated with prespecified Dartmouth Atlas metrics. In a multivariate ordinal regression, there was a significant though weak association between hospitals in higher tertiles of the Dartmouth Atlas metrics and categorization in higher tertiles of our diagnostic overuse index. Similarly, our hospital-level index correlated with two of the three county-level metrics in a multivariate ordinal regression.
We do not assume that all of the metrics in our index track together. However, our results, including the wide dispersion of index values among the tertiles (Table 3), suggest that at least some hospitals are outliers in multiple metrics. We did not assume ex ante that our index should correlate with Dartmouth overuse metrics or Medicare county-level spending; however, we did believe that an association with these measures would assist in validating our index. Given that our index utilizes four common diagnoses, while the Dartmouth and Medicare cost metrics are based on a much broader range of conditions, we would not expect more than a weak correlation even if our index is a valid way to measure overuse.
All of the metrics were based on the concept that hospitals with high rates of negative testing are likely providing large amounts of low-value care. Prior studies on diagnostic yield of CT scans in the emergency department for pulmonary embolus (PE) found an increase in testing and decrease in yield over time; these studies also showed that physicians with more experience ordered fewer CT scans and had a higher yield.23 A review of electronic health records and billing data also showed that hospitals with higher rates of D-dimer testing had higher yields on CT scans ordered to test for PE.24
We took advantage of the coding convention that certain diagnoses only be listed as the primary discharge diagnosis if no more specific diagnosis is made. This allowed us to identify hospitals that likely had high rates of negative tests without granular data. Of course, the metrics are not measuring rates of negative testing per se, but a proxy for this, based instead on the proportion of patients with a symptom-based primary discharge diagnosis who underwent diagnostic testing.
Measuring diagnostic overuse at the hospital level may help to understand factors that drive overuse, given that institutional incentives and culture likely play important roles in ordering tests. There is evidence that financial incentives drive physicians’ decisions,25-27 and there is also evidence that institutional culture impacts outcomes.28 Further, quality improvement projects are typically designed at the hospital level and may be an effective way to curb overuse.29,30
Previous studies have focused on measuring variation among providers and identifying outlier physicians.9,10,20 Providing feedback to underperforming physicians has been shown to change practice habits.31,32 Efforts to improve the practice habits of outlier hospitals may have a number of advantages, including economies of scale and scope and the added benefit of improving the habits of all providers—not just those who are underperforming.
Ordering expensive diagnostic tests on patients with a low pretest probability of having an organic etiology for their symptoms contributes to high healthcare costs. Of course, we do not believe that the ideal rate of negative testing is zero. However, hospitals with high rates of negative diagnostic testing are more likely to be those with clinicians who use expensive tests as a substitute for clinical judgment or less-expensive tests (eg, D-dimer testing to rule out PE).
One challenge we faced is that there is no gold standard of hospital-level overuse with which to validate our index. Our index is weakly correlated with a number of regional metrics that may be proxies for overuse. We are reassured that there is a statistically significant correlation with measures at both HSA and county levels. These correlations are weak, but these regional metrics are themselves imperfect surrogates for overuse. Furthermore, our index is preliminary and will need refinement in future studies.
Limitations
Our analysis has multiple limitations. First, since it relies heavily on primary ICD discharge diagnosis codes, biases could exist due to variations in coding practices. Second, the SID does not include observation stays or tests conducted in the ED, so differential use of observation stays among hospitals might impact results. Finally, based on utilization data, we were not able to distinguish between CT scans of the chest, abdomen, and pelvis because the SID labels each of these as body CT.
CONCLUSION
We developed a novel index to measure diagnostic intensity at the hospital level. This index relies on the concept that high rates of negative diagnostic testing likely indicate some degree of overuse. Our index is parsimonious, does not require granular claims data, and measures a range of potentially overused tests for common clinical scenarios. Our next steps include further refining the index, testing it with granular data, and validating it with other datasets. Thereafter, this index may be useful at identifying positive and negative outliers to understand what processes of care contribute to outlier high and low levels of diagnostic testing. We suspect our index is more useful at identifying extremes than comparing hospitals in the middle of the utilization curve. Additionally, exploring the relationship among individual metrics and the relationship between our index and quality measures like mortality and readmissions may be informative.
1. Fisher ES, Wennberg JE, Stukel TA, et al. Associations among hospital capacity, utilization, and mortality of US Medicare beneficiaries, controlling for sociodemographic factors. Health Serv Res. 2000;34(6):1351-1362.
2. Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder ÉL. The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138(4):288-298. https://doi.org/10.7326/0003-4819-138-4-200302180-00007
3. Segal JB, Nassery N, Chang H-Y, Chang E, Chan K, Bridges JFP. An index for measuring overuse of health care resources with Medicare claims. Med Care. 2015;53(3):230-236. https://doi.org/10.1097/mlr.0000000000000304
4. Colla CH, Morden NE, Sequist TD, Schpero WL, Rosenthal MB. Choosing wisely: prevalence and correlates of low-value health care services in the United States. J Gen Intern Med. 2014;30(2):221-228. https://doi.org/10.1007/s11606-014-3070-z
5. Colla CH, Morden NE, Sequist TD, Mainor AJ, Li Z, Rosenthal MB. Payer type and low-value care: comparing Choosing Wisely services across commercial and Medicare populations. Health Serv Res. 2018;53(2):730-746. https://doi.org/10.1111/1475-6773.12665
6. Schwartz AL, Landon BE, Elshaug AG, Chernew ME, McWilliams JM. Measuring low-value care in Medicare. JAMA Intern Med. 2014;174(7):1067-1076. https://doi.org/10.1001/jamainternmed.2014.1541
7. Oakes AH, Chang H-Y, Segal JB. Systemic overuse of health care in a commercially insured US population, 2010–2015. BMC Health Serv Res. 2019;19(1). https://doi.org/10.1186/s12913-019-4079-0
8. Schwartz AL, Zaslavsky AM, Landon BE, Chernew ME, McWilliams JM. Low-value service use in provider organizations. Health Serv Res. 2018;53(1):87-119. https://doi.org/10.1111/1475-6773.12597
9. Schwartz AL, Jena AB, Zaslavsky AM, McWilliams JM. Analysis of physician variation in provision of low-value services. JAMA Intern Med. 2019;179(1):16-25. https://doi.org/10.1001/jamainternmed.2018.5086
10. Bouck Z, Ferguson J, Ivers NM, et al. Physician characteristics associated with ordering 4 low-value screening tests in primary care. JAMA Netw Open. 2018;1(6):e183506. https://doi.org/10.1001/jamanetworkopen.2018.3506
11. Dartmouth Atlas Project. Data By Region - Dartmouth Atlas of Health Care. Accessed August 29, 2019. http://archive.dartmouthatlas.org/data/region/
12. ICD-9-CM Official Guidelines for Coding and Reporting (Effective October 11, 2011). Accessed March 1, 2018. https://www.cdc.gov/nchs/data/icd/icd9cm_guidelines_2011.pdf
13. Cassel CK, Guest JA. Choosing wisely - helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801-1802. https://doi.org/10.1001/jama.2012.476
14. The Dartmouth Atlas of Health Care. Accessed July 17, 2018. http://www.dartmouthatlas.org/
15. The Dartmouth Atlas of Healthcare. Research Methods. Accessed January 27, 2019. http://archive.dartmouthatlas.org/downloads/methods/research_methods.pdf
16. Centers for Medicare & Medicaid Services. Medicare geographic variation, public use file. Accessed January 5, 2020. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Geographic-Variation/GV_PUF
17. Centers for Medicare & Medicaid Services. Berenson-Eggers Type of Service (BETOS) codes. Accessed January 10, 2020. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareFeeforSvcPartsAB/downloads/betosdesccodes.pdf
18. Data.Medicare.gov. Payment and value of care – hospital: hospital compare. Accessed August 21, 2019. https://data.medicare.gov/Hospital-Compare/Payment-and-value-of-care-Hospital/c7us-v4mf
19. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser comorbidity index. Med Care. 2017;55(7):698-705. https://doi.org/10.1097/mlr.0000000000000735
20. Tsugawa Y, Jha AK, Newhouse JP, Zaslavsky AM, Jena AB. Variation in physician spending and association with patient outcomes. JAMA Intern Med. 2017;177(5):675-682. https://doi.org/10.1001/jamainternmed.2017.0059
21. Safavi KC, Li S-X, Dharmarajan K, et al. Hospital variation in the use of noninvasive cardiac imaging and its association with downstream testing, interventions, and outcomes. JAMA Intern Med. 2014;174(4):546-553. https://doi.org/10.1001/jamainternmed.2013.14407
22. Douglas PS, Patel MR, Bailey SR, et al. Hospital variability in the rate of finding obstructive coronary artery disease at elective, diagnostic coronary angiography. J Am Coll Cardiol. 2011;58(8):801-809. https://doi.org/10.1016/j.jacc.2011.05.019
23. Venkatesh AK, Agha L, Abaluck J, Rothenberg C, Kabrhel C, Raja AS. Trends and variation in the utilization and diagnostic yield of chest imaging for Medicare patients with suspected pulmonary embolism in the emergency department. Am J Roentgenol. 2018;210(3):572-577. https://doi.org/10.2214/ajr.17.18586
24. Kline JA, Garrett JS, Sarmiento EJ, Strachan CC, Courtney DM. Over-testing for suspected pulmonary embolism in american emergency departments: the continuing epidemic. Circ Cardiovasc Qual Outcomes. 2020;13(1):e005753. https://doi.org/10.1161/circoutcomes.119.005753
25. Welch HG, Fisher ES. Income and cancer overdiagnosis – when too much care is harmful. N Engl J Med. 2017;376(23):2208-2209. https://doi.org/10.1056/nejmp1615069
26. Nicholson S. Physician specialty choice under uncertainty. J Labor Econ. 2002;20(4):816-847. https://doi.org/10.1086/342039
27. Chang R-KR, Halfon N. Geographic distribution of pediatricians in the United States: an analysis of the fifty states and Washington, DC. Pediatrics. 1997;100(2 pt 1):172-179. https://doi.org/10.1542/peds.100.2.172
28. Braithwaite J, Herkes J, Ludlow K, Lamprell G, Testa L. Association between organisational and workplace cultures, and patient outcomes: systematic review protocol. BMJ Open. 2016;6(12):e013758. https://doi.org/10.1136/bmjopen-2016-013758
29. Bhatia RS, Milford CE, Picard MH, Weiner RB. An educational intervention reduces the rate of inappropriate echocardiograms on an inpatient medical service. JACC Cardiovasc Imaging. 2013;6(5):545-555. https://doi.org/10.1016/j.jcmg.2013.01.010
30. Blackmore CC, Watt D, Sicuro PL. The success and failure of a radiology quality metric: the case of OP-10. J Am Coll Radiol. 2016;13(6):630-637. https://doi.org/10.1016/j.jacr.2016.01.006
31. Albertini JG, Wang P, Fahim C, et al. Evaluation of a peer-to-peer data transparency intervention for Mohs micrographic surgery overuse. JAMA Dermatol. 2019;155(8):906-913. https://dx.doi.org/10.1001%2Fjamadermatol.2019.1259
32. Sacarny A, Barnett ML, Le J, Tetkoski F, Yokum D, Agrawal S. Effect of peer comparison letters for high-volume primary care prescribers of quetiapine in older and disabled adults: a randomized clinical trial. JAMA Psychiatry. 2018;75(10):1003-1011. https://doi.org/10.1001/jamapsychiatry.2018.1867
1. Fisher ES, Wennberg JE, Stukel TA, et al. Associations among hospital capacity, utilization, and mortality of US Medicare beneficiaries, controlling for sociodemographic factors. Health Serv Res. 2000;34(6):1351-1362.
2. Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder ÉL. The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138(4):288-298. https://doi.org/10.7326/0003-4819-138-4-200302180-00007
3. Segal JB, Nassery N, Chang H-Y, Chang E, Chan K, Bridges JFP. An index for measuring overuse of health care resources with Medicare claims. Med Care. 2015;53(3):230-236. https://doi.org/10.1097/mlr.0000000000000304
4. Colla CH, Morden NE, Sequist TD, Schpero WL, Rosenthal MB. Choosing wisely: prevalence and correlates of low-value health care services in the United States. J Gen Intern Med. 2014;30(2):221-228. https://doi.org/10.1007/s11606-014-3070-z
5. Colla CH, Morden NE, Sequist TD, Mainor AJ, Li Z, Rosenthal MB. Payer type and low-value care: comparing Choosing Wisely services across commercial and Medicare populations. Health Serv Res. 2018;53(2):730-746. https://doi.org/10.1111/1475-6773.12665
6. Schwartz AL, Landon BE, Elshaug AG, Chernew ME, McWilliams JM. Measuring low-value care in Medicare. JAMA Intern Med. 2014;174(7):1067-1076. https://doi.org/10.1001/jamainternmed.2014.1541
7. Oakes AH, Chang H-Y, Segal JB. Systemic overuse of health care in a commercially insured US population, 2010–2015. BMC Health Serv Res. 2019;19(1). https://doi.org/10.1186/s12913-019-4079-0
8. Schwartz AL, Zaslavsky AM, Landon BE, Chernew ME, McWilliams JM. Low-value service use in provider organizations. Health Serv Res. 2018;53(1):87-119. https://doi.org/10.1111/1475-6773.12597
9. Schwartz AL, Jena AB, Zaslavsky AM, McWilliams JM. Analysis of physician variation in provision of low-value services. JAMA Intern Med. 2019;179(1):16-25. https://doi.org/10.1001/jamainternmed.2018.5086
10. Bouck Z, Ferguson J, Ivers NM, et al. Physician characteristics associated with ordering 4 low-value screening tests in primary care. JAMA Netw Open. 2018;1(6):e183506. https://doi.org/10.1001/jamanetworkopen.2018.3506
11. Dartmouth Atlas Project. Data By Region - Dartmouth Atlas of Health Care. Accessed August 29, 2019. http://archive.dartmouthatlas.org/data/region/
12. ICD-9-CM Official Guidelines for Coding and Reporting (Effective October 11, 2011). Accessed March 1, 2018. https://www.cdc.gov/nchs/data/icd/icd9cm_guidelines_2011.pdf
13. Cassel CK, Guest JA. Choosing wisely - helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801-1802. https://doi.org/10.1001/jama.2012.476
14. The Dartmouth Atlas of Health Care. Accessed July 17, 2018. http://www.dartmouthatlas.org/
15. The Dartmouth Atlas of Healthcare. Research Methods. Accessed January 27, 2019. http://archive.dartmouthatlas.org/downloads/methods/research_methods.pdf
16. Centers for Medicare & Medicaid Services. Medicare geographic variation, public use file. Accessed January 5, 2020. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Geographic-Variation/GV_PUF
17. Centers for Medicare & Medicaid Services. Berenson-Eggers Type of Service (BETOS) codes. Accessed January 10, 2020. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareFeeforSvcPartsAB/downloads/betosdesccodes.pdf
18. Data.Medicare.gov. Payment and value of care – hospital: hospital compare. Accessed August 21, 2019. https://data.medicare.gov/Hospital-Compare/Payment-and-value-of-care-Hospital/c7us-v4mf
19. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser comorbidity index. Med Care. 2017;55(7):698-705. https://doi.org/10.1097/mlr.0000000000000735
20. Tsugawa Y, Jha AK, Newhouse JP, Zaslavsky AM, Jena AB. Variation in physician spending and association with patient outcomes. JAMA Intern Med. 2017;177(5):675-682. https://doi.org/10.1001/jamainternmed.2017.0059
21. Safavi KC, Li S-X, Dharmarajan K, et al. Hospital variation in the use of noninvasive cardiac imaging and its association with downstream testing, interventions, and outcomes. JAMA Intern Med. 2014;174(4):546-553. https://doi.org/10.1001/jamainternmed.2013.14407
22. Douglas PS, Patel MR, Bailey SR, et al. Hospital variability in the rate of finding obstructive coronary artery disease at elective, diagnostic coronary angiography. J Am Coll Cardiol. 2011;58(8):801-809. https://doi.org/10.1016/j.jacc.2011.05.019
23. Venkatesh AK, Agha L, Abaluck J, Rothenberg C, Kabrhel C, Raja AS. Trends and variation in the utilization and diagnostic yield of chest imaging for Medicare patients with suspected pulmonary embolism in the emergency department. Am J Roentgenol. 2018;210(3):572-577. https://doi.org/10.2214/ajr.17.18586
24. Kline JA, Garrett JS, Sarmiento EJ, Strachan CC, Courtney DM. Over-testing for suspected pulmonary embolism in american emergency departments: the continuing epidemic. Circ Cardiovasc Qual Outcomes. 2020;13(1):e005753. https://doi.org/10.1161/circoutcomes.119.005753
25. Welch HG, Fisher ES. Income and cancer overdiagnosis – when too much care is harmful. N Engl J Med. 2017;376(23):2208-2209. https://doi.org/10.1056/nejmp1615069
26. Nicholson S. Physician specialty choice under uncertainty. J Labor Econ. 2002;20(4):816-847. https://doi.org/10.1086/342039
27. Chang R-KR, Halfon N. Geographic distribution of pediatricians in the United States: an analysis of the fifty states and Washington, DC. Pediatrics. 1997;100(2 pt 1):172-179. https://doi.org/10.1542/peds.100.2.172
28. Braithwaite J, Herkes J, Ludlow K, Lamprell G, Testa L. Association between organisational and workplace cultures, and patient outcomes: systematic review protocol. BMJ Open. 2016;6(12):e013758. https://doi.org/10.1136/bmjopen-2016-013758
29. Bhatia RS, Milford CE, Picard MH, Weiner RB. An educational intervention reduces the rate of inappropriate echocardiograms on an inpatient medical service. JACC Cardiovasc Imaging. 2013;6(5):545-555. https://doi.org/10.1016/j.jcmg.2013.01.010
30. Blackmore CC, Watt D, Sicuro PL. The success and failure of a radiology quality metric: the case of OP-10. J Am Coll Radiol. 2016;13(6):630-637. https://doi.org/10.1016/j.jacr.2016.01.006
31. Albertini JG, Wang P, Fahim C, et al. Evaluation of a peer-to-peer data transparency intervention for Mohs micrographic surgery overuse. JAMA Dermatol. 2019;155(8):906-913. https://dx.doi.org/10.1001%2Fjamadermatol.2019.1259
32. Sacarny A, Barnett ML, Le J, Tetkoski F, Yokum D, Agrawal S. Effect of peer comparison letters for high-volume primary care prescribers of quetiapine in older and disabled adults: a randomized clinical trial. JAMA Psychiatry. 2018;75(10):1003-1011. https://doi.org/10.1001/jamapsychiatry.2018.1867
© 2021 Society of Hospital Medicine
Gender-Based Discrimination and Sexual Harassment Among Academic Internal Medicine Hospitalists
Gender-based discrimination refers to “any distinction, exclusion or restriction made on the basis of socially constructed gender roles and norms which prevents a person from enjoying full human rights.”1 Similarly, sexual harassment encompasses a spectrum of sexual conduct that includes “unwelcome sexual advances, requests for sexual favors, and other verbal or physical harassment of a sexual nature,” as defined by the US Equal Employment Opportunity Commission.2 Gender-based discrimination and sexual harassment can be “overt,” which includes explicitly recognizable behaviors, or they can be “implicit,” which includes verbal and nonverbal behaviors that often go unrecognized but convey hostility, objectification, or exclusion of another person. Gender-based discrimination and sexual harassment are commonly described and likely more prevalent in academic settings.3-6 Female physicians are disproportionately affected by gender-based discrimination and sexual harassment, compared with their male peers.4,7
Female physicians face workplace harassment from both patients and coworkers. A study in Canada reported that more than 75% of female physicians experienced sexual harassment from their patients.8 A recent study showed almost half of the physicians who reported harassment, which was three times more often among female physicians, described other physician colleagues as perpetrators.9 In a study among clinician-researchers in the field of academic medicine, 30% of females reported having experienced sexual harassment, compared with 4% of males.7 Among females who reported harassment in this study, 47% stated that these experiences adversely affected their opportunities for career advancement. Career stage may also affect experiences or perceptions of gender-based discrimination and sexual harassment, with females in earlier career stages reporting a less favorable environment of gender equity.10
Hospital medicine is a young and evolving specialty, and the number of hospitalists has increased substantially from a few hundred at the time of inception to over 50,000 as of 2016.11 The proportion of female hospitalists increased from 31% in 2012 to 52% in 2014, reflecting equal gender representation in hospital medicine.12 Available evidence shows gender disparities exist in hospital medicine disproportionately affecting female hospitalists in their career advancement, including leadership and scholarship opportunities.13 However, there remains a knowledge gap regarding the prevalence of gender-based discrimination and sexual harassment experienced by hospitalists.
Our study examines the experiences of academic hospitalists regarding gender-based discrimination and sexual harassment and the impact of gender on career satisfaction and advancement.
METHODS
Study Design and Participants
An online survey was developed and approved by the institutional board review (IRB) at the Medical College of Wisconsin in Milwaukee. University-based academic centers with hospitalist programs, identified through personal connections, from across the continental United Stated were identified as potential study sites, and leaders at each institution were contacted to ascertain potential participation in the survey. The survey was distributed to Internal Medicine hospitalists at 18 participating academic institutions from January 2019 to June 2019. Participation was voluntary. The cover letter explained the purpose of the study and provided a link to the survey. To maintain anonymity, none of the questionnaires requested identifying information from participants. A web-based Qualtrics online-based survey platform was used.
Survey Elements
The survey aimed to assess several elements of gender-based discrimination and sexual harassment. All questions about these experiences distinguished encounters with patients from those with colleagues, and questions about occurrences either over a career or in the last 30 days were intended to capture both distant and recent timeframes. The theme for the questions for the survey was based on previous studies.4,7,8 The wording of questions was simplified to make them easily understandable, and the brevity of the survey was maintained to prevent possible nonresponses.14 Additional questions (mistaken for a healthcare provider other than a physician, feeling respected by patients and colleagues, referred to by terms such as “honey,” “dear,” “sweetheart,” “sugar,” or equivalent), which were deemed relevant in day-to-day clinical practice through consensus among study investigators and discussions among peer hospitalists, were incorporated into the final survey (Appendix). Survey questions were intended to capture several elements regarding interactions with patients and with colleagues or other healthcare providers (HCPs).
Questions on gender-based discrimination included:
- Has a patient [colleague or other healthcare provider] mistaken you for a healthcare provider other than a physician?
- Has a patient [colleague or other healthcare provider] asked you to do something not at your level of training?
- Do you feel respected? Do you perceive your gender has impacted opportunities for your career advancement?
Questions on sexual harassment included:
- Has a patient [colleague or other healthcare provider] touched you inappropriately, made sexual remarks or gestures, or made suggestive looks?
- Has a patient [colleague or other healthcare provider] referred to you by terms such as “honey,” “dear,” “sweetheart,” “sugar,” or equivalent?
In addition, the survey sought demographic information of the participants (age, gender, and race/ethnicity) and information about their individual institutions (names and locations) (Appendix). The geographical locations of the institutions were further categorized into four different regions according to the United States Census Bureau (Northwest, Midwest, South, and West). At the end of the survey, participants were given an opportunity to provide any additional comments.
Statistical Analysis
Standard descriptive summary statistics were used for demographic data. Associations between the variables were analyzed using chi-square test or Fischer’s exact test, as appropriate, for categorical variables and t test for continuous variables. The variations among institution-based responses were presented in the form of inter-quartile range (IQR). All tests were 2-sided, and P values less than .05 were considered statistically significant. All analyses were performed using IBM® SPSS® Statistics software version 24. Relevant responses representative of the overall respondents’ sentiments as provided under additional comment section were discussed and cited.
RESULTS
Eighteen different academic institutions across the United States participated in the study, with 336 individual responses. The majority of respondents were females (57%), in younger age categories (58% were 30-39 years old), Caucasian (59%), and early-career hospitalists (>50% working as hospitalists for ≤5 years) (Table 1). Regarding the overall geographic distribution, the largest number of responses were from the Midwest (n = 115; 35.6%) (Table 1 and Appendix).
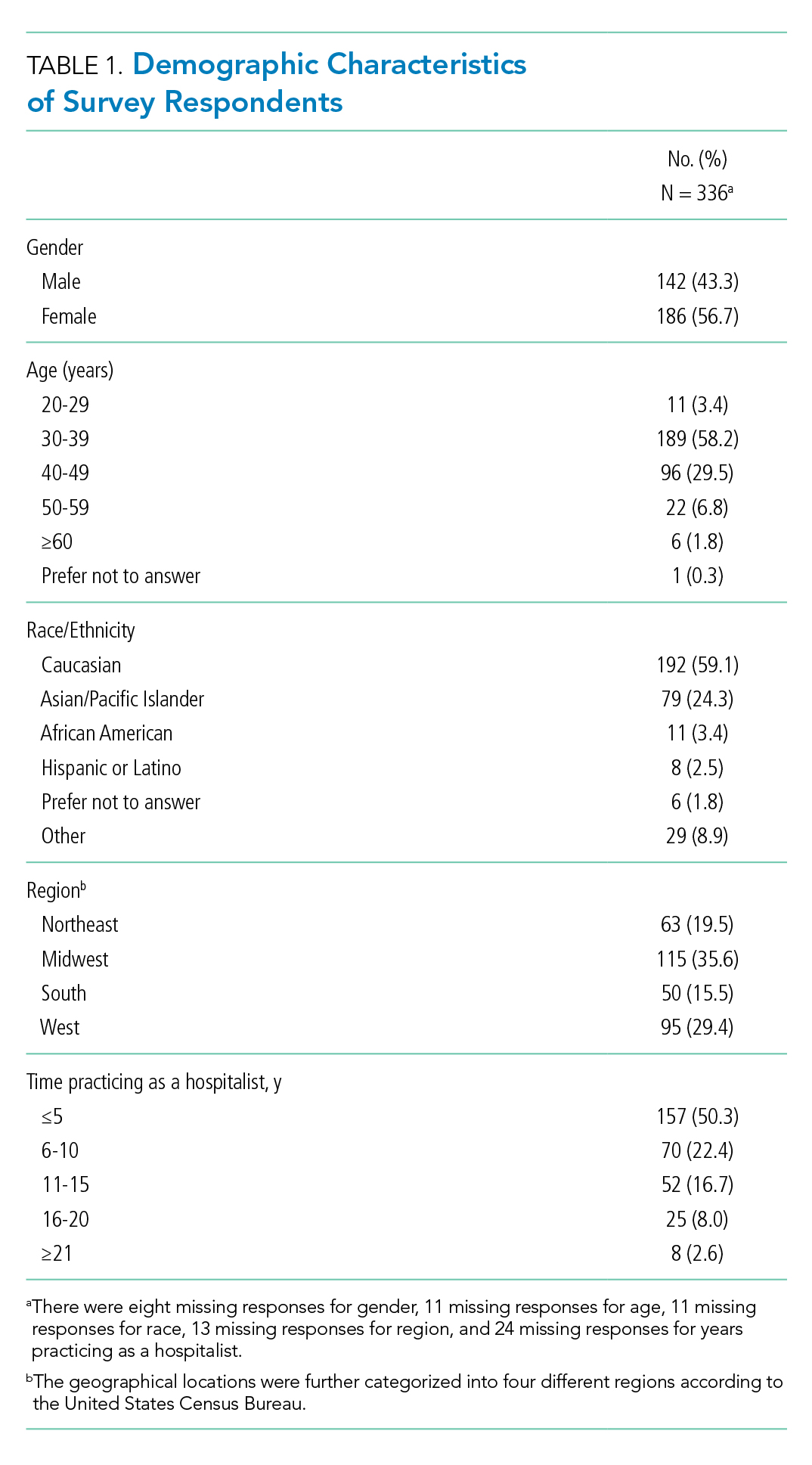
Gender Discrimination
Interactions With Patients
Over their careers, 69% of hospitalists reported being mistaken for an HCP other than a physician by patients. This was more common among females than among males (99% vs 29%, respectively; P < .001) (Table 2). Almost half (48%) reported this had occurred in the last 30 days, more frequently by females (76% vs 10%; P < .001).
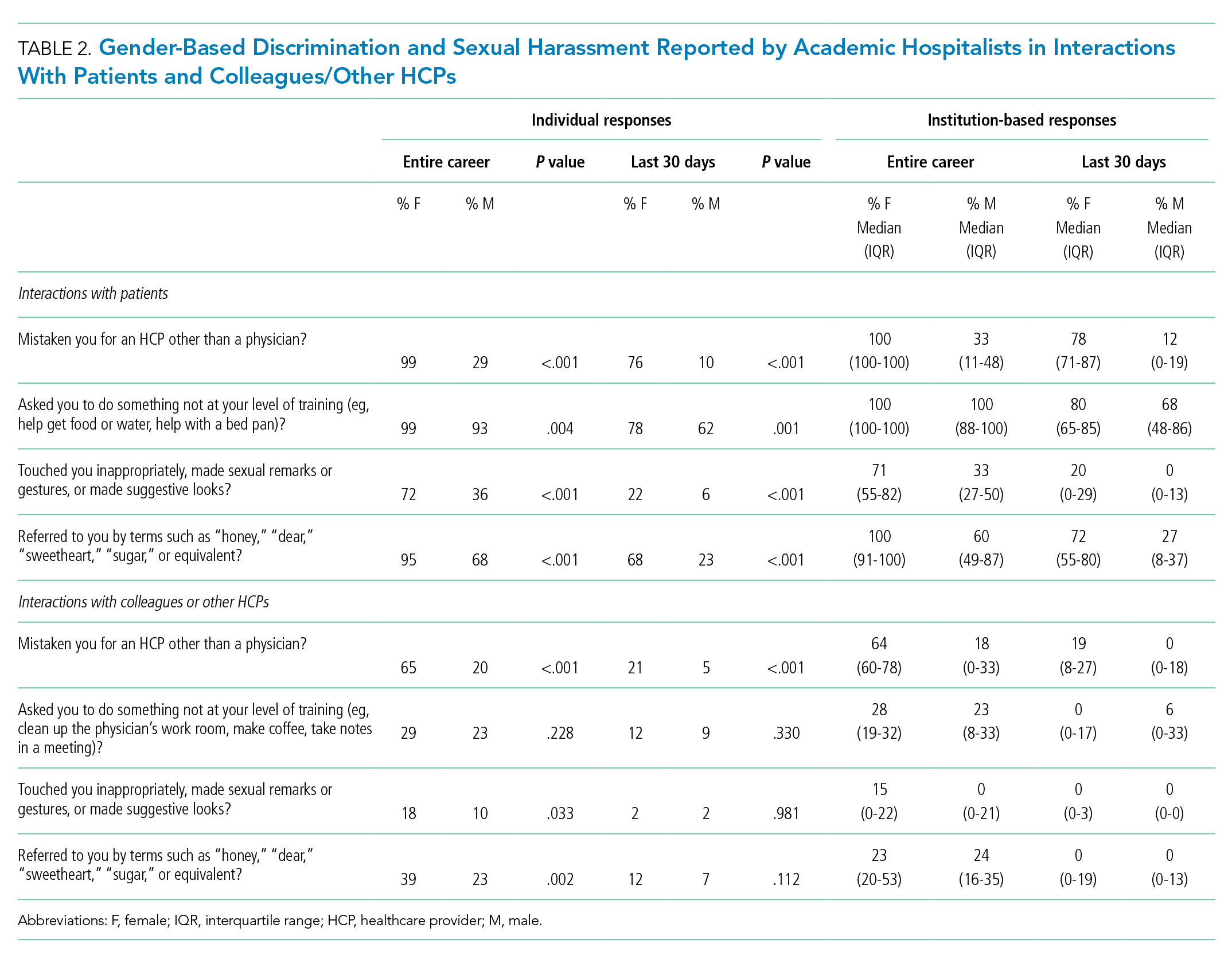
Of responding hospitalists, 96% stated that, over their careers, they have been asked by patients to do something they did not consider to be at their level of training (eg, help get food or water, help with a bed pan), with a higher prevalence of such experiences among females than males (99% vs 93%, respectively; P = .004) (Table 2). Most (71%) said they had experienced this in the last 30 days, which was again more frequently reported by females (78% vs 62%; P = .001).
The responses from female hospitalists regarding their career-long experiences of being mistaken for an HCP or asked to do something not at their level of training by their patients had both the highest number of positive responses across institutions (median of hospital proportions, 100%) and the least institutional variation since both had the narrowest IQR) (Table 2).
Interactions With Colleagues or Other HCPs
Among hospitalists responding to the survey, 46% felt that, over their careers, they had been mistaken for nonphysician HCPs by colleagues or other HCPs. This was more prevalent among females than among males (65% vs 20%; P < .001) (Table 2). Among respondents, 14% reported these events had occurred in the last 30 days, which was again more common among females (21% vs 5%; P < .001).
Over their careers, 26% of hospitalists reported they have been asked by a colleague or HCP to do something not at their level of training (eg, clean up the physician’s work room, make coffee, take notes in a meeting), with similar prevalence among females and males (29% vs 23%; P = .228). Ten percent reported these occurrences in the last 30 days, which was similar between females and males (12% vs 9%; P = .330).
Feelings of Respect and Opportunities for Career Advancement
When asked to rate the statement “I feel respected by patients” on a 5-point Likert scale, female hospitalists overall scored significantly lower as compared with their male counterparts (mean score, 3.73 vs 4.04; P < .001) (Table 3); this was also true when asked about feelings of respect by physicians (mean score, 3.84 vs 4.15; P < .001). Female hospitalists were more likely than males to report that their gender has more negatively impacted their opportunities for career advancement (mean score, 2.73 vs 3.34; P < .001).

Sexual Harassment
Interactions With Patients
Over half (57%) of hospitalists reported career-long experiences of patient(s) touching them inappropriately, making sexual remarks or gestures, or making suggestive looks. These experiences were more prevalent among females than among males (72% vs 36%, respectively; P < .001) (Table 2). Fifteen percent said they had such experience in rhe last 30 days, which was also more common among females (22% vs 6%; P < .001).
Most hospitalists (84%) reported that patients have referred to them by inappropriately familiar terms such as “honey,” “dear,” “sweetheart,” “sugar,” or equivalent over their careers, with females more frequently reporting these behaviors (95% vs 68%; P < .001). Experiencing this during the last 30 days was reported by 48%, which was again more common among females (68% vs 23%; P < .001).
Interactions With Colleagues or Other HCPs
Within their careers, 15% of hospitalists reported at least one experience of a colleague or HCP touching them inappropriately or making sexual remarks, gestures, or suggestive looks. This was more prevalent for females than males (18% vs 10%, respectively; P = .033). Only 2% of both females and males reported these experiences in the last 30 days (2% vs 2%; P = .981).
Almost one-third of participants (32%) affirmed that another HCP has referred to them by terms such as “honey,” “dear,” “sweetheart,” “sugar,” or equivalent in their career, with a higher proportion of females than males reporting these events (39% vs 23%; P = .002) (Table 2). Experiencing this during the last 30 days was reported by 10%, which was similar between females and males (12% vs 7%; P = .112).
Additional Comments From Respondents
- “Throughout my training and now into my professional career, there are nearly weekly incidents of elderly male patients referring to me as “honey/dear/sweetie” or even by my first name, even though I introduce myself as their physician and politely correct them. They will often refer to me as a nurse and ask me to do something not at my level of training. Sometimes even despite correcting the patient, they continue to refer to me as such. Throughout the years, other female MDs and I have discussed that this is ‘status quo’ for female physicians and observe that this is not an experience that male MDs share.”
- “I frequently round with a male nurse practitioner and the patients almost always, despite introducing ourselves and our roles, turn to him and ask him questions instead of addressing them to me.”
- “Our institution allows female faculty to be interviewed about childcare, household labor division, plans for pregnancy. One professor asks women private details about their private relationships such as what they do with spouse on date night or weekends away.”
- “It’s hard to answer questions related to my level of training. I don’t think it’s unreasonable for people to ask me to do things, no matter my level of training. . . . I don’t think being a doctor means that I am above this, or that it is inappropriate to be asked to do this.”
DISCUSSION
This survey demonstrated that gender-based discrimination and sexual harassment in the academic hospitalist healthcare environment are common, both in more distant and recent time frames. Notably, these experiences are shared by female and male physicians in interactions with both patients and colleagues, though male hospitalists report most of these experiences at significantly lower frequencies than females. These results support past work showing that female physicians are significantly more likely to be subjected to gender-based discrimination and sexual harassment, but also challenges the perception that gender-based discrimination and sexual harassment are uniquely experienced by females.
A startling number of females and males in the study reported sexual harassment (inappropriate touching, remarks, gestures, and looks) when interacting with patients throughout their careers and in last 30 days. Many males and females reported that patients had referred to them with inappropriately familiar, and potentially demeaning, terms of endearment. For both overt and implicit sexual harassment, females were significantly more likely than males to report experiencing these behaviors when interacting with patients. Although some of these experiences may seem less harmful than others, a meta-analysis demonstrated that frequent, less intense experiences of gender-based discrimination and sexual harassment have a similar impact on female’s well-being as do less frequent, more intense experiences.15 Although the person using the terms of endearment like “honey,” “sugar,” or “sweetheart” may feel the terms are harmless, such expressions can be inappropriate and constitute sexual harassment according to the U.S. Department of the Interior’s Office of Civil Rights.16 The Sexual Harassment/Assault Response and Prevention Program (SHARP) also classifies such terms into verbal categories of sexual harrassment.17
Of female physicians surveyed, 99% reported that they had been mistaken for HCPs other than physicians by their patients over their careers. Although this was also reported by male physicians, the experience was 3.4 times as likely for female physicians. Misidentification by patients may represent a disconnect between the growing female representation in the physician workforce and patients’ conceptions of the traditional image of a physician.
In parallel with this finding of misidentification, an interesting area of the study was the question regarding being asked to do “something not at your level of training.” A recurring theme in the comments was a rejection of the notion that certain tasks were “beneath a level of training,” suggesting a common view that acts of caregiving are not bounded by hierarchy. Analysis of qualitative responses showed that 40% of these responses had comments regarding this question. An example was “It’s hard to answer questions related to my level of training. . . . I don’t think being a doctor means that I am above this, or that it is inappropriate to be asked to do this.” Notably, however, a larger number of female than male physicians responded yes to this question in both study time frames. This points to a differential in how female physicians are viewed by patients, both in frequent misidentification and in behaviors more frequently asked of female physicians than their male counterparts. Given the comments, it may also suggest a difference in how female and male physicians perceive the fluidity of bounds on their care-taking roles set by their “level of training.”
A large number of study participants were early-career hospitalists, which may in part explain some of the study results. In a previous study of gender equity in an Internal Medicine department, physicians practicing medicine for more than 15 years perceived the departmental culture as more favorable than physicians with shorter careers.10 Additionally, the perception of cultures was most discordant between senior male physicians and junior female physicians.10 Because many hospitalists are early-career physicians, they may have trained in an environment that had heightened awareness surrounding gender-based discrimination and sexual harassment, which affects the overall study results.
Multiple qualitative comments, mentioned above, were submitted by participants describing their experiences in all categories. Such comments paint a picture of insidious bias and cultural norms affecting the quality of female physicians’ work lives.
Two questions focused on career satisfaction and the sense of respect from patients and colleagues. In both responses, there was a statistically different response between males and females, with females less likely to report that they felt respected and that their gender adversely impacted their opportunities for career advancement. This is disturbing information and warrants more investigation.
The reasons for the observed prevalence of gender-based discrimination and sexual harassment in this broad survey of academic hospitalists are uncertain. Multiple studies to date have demonstrated that gender-based discrimination and sexual harassment have historically existed in medicine and continue to even today. Unlike physicians with long-term relationships with patients, hospitalists may face more exposure due to a lack of long-term continuity with patients. The absence of an established trust in the relationship also may make them more vulnerable to inappropriate behaviors when interacting with patients. Hospital medicine, however, is a young specialty with equal gender representation and should be at the forefront of addressing and solving these issues of gender-based discrimination and sexual harassment.
The survey had a good distribution between female and male participants. Additionally, the survey reflected the general distribution of the national hospitalist workforce in gender, age, and ethnic/racial distribution, as well as number of years in practice.12 The study surveyed respondents regarding experiences in both long- and short-term time frames, as well as experiences with patients and colleagues.
Our study reflects a cross-sectional snapshot of hospitalists’ perceptions with no longitudinal follow-up. Since the survey was limited to academic medical centers, it may not reflect experiences in community/private practice settings. The small number of participants limited the ability to perform subgroup analyses by age, race, or years in practice, which may play a role in interactions with patients and colleagues. Since the number of respondents varied greatly by institution, a minority of institutions could have influenced some of the findings. Narrow IQRs of the hospital proportions as shown in Table 2 would suggest similar responses across institutions, whereas wide IQRs would suggest that a smaller number of institutions were possibly driving the findings. Because of the survey distribution method, it is unknown how many physicians received the survey and a response rate could not be calculated. Further, selection, recall, and detection biases cannot be ruled out.
CONCLUSION
This survey shows that gender-based discrimination and sexual harassment in the academic hospitalist healthcare environment are common and more frequently experienced by female physicians, both in interactions with patients and colleagues. Our study highlights the need to address this prevalent issue among academic hospitalists.
1. WHO Department of Reproductive Health and Research. Transforming health systems: gender and rights in reproductive health. A training manual for health managers. World Health Organization; 2001. https://www.who.int/reproductivehealth/publications/gender_rights/RHR_01_29/en/
2. Sexual Harassment. U.S. Equal Employment Opportunity Commission. Accessed Jan 5, 2020. https://www.eeoc.gov/laws/types/sexual_harassment.cfm
3. Frank E, Brogan D, Schiffman M. Prevalence and correlates of harassment among US women physicians. Arch Intern Med. 1998;158(4):352-358. https://doi.org/10.1001/archinte.158.4.352
4. Carr PL, Ash AS, Friedman RH, et al. Faculty perceptions of gender discrimination and sexual harassment in academic medicine. Ann Intern Med. 2000;132(11):889-96. https://doi.org/10.7326/0003-4819-132-11-200006060-00007
5. Bates CK, Jagsi R, Gordon LK, et al. It is time for zero tolerance for sexual harassment in academic medicine. Acad Med. 2018;93(2):163-165. https://doi.org/10.1097/acm.0000000000002050
6. Dzau VJ, Johnson PA. Ending sexual harassment in academic medicine. N Engl J Med. 2018;379(17):1589-1591. https://doi.org/10.1056/nejmp1809846
7. Jagsi R, Griffith KA, Jones R, Perumalswami CR, Ubel P, Stewart A. Sexual harassment and discrimination experiences of academic medical faculty. JAMA. 2016;315(19):2120-2121. https://doi.org/10.1001/jama.2016.2188
8. Phillips SP, Schneider MS. Sexual harassment of female doctors by patients. N Engl J Med. 1993;329(26):1936-1939. https://doi.org/10.1056/nejm199312233292607
9. Kane L. Sexual Harassment of Physicians: Report 2018. Medscape. June 13, 2018. Accessed Jan 24, 2020. https://www.medscape.com/slideshow/sexual-harassment-of-physicians-6010304
10. Ruzycki SM, Freeman G, Bharwani A, Brown A. Association of physician characteristics with perceptions and experiences of gender equity in an academic internal medicine department. JAMA Netw Open. 2019;2(11):e1915165. https://doi.org/10.1001/jamanetworkopen.2019.15165
11. Wachter RM, Goldman L. Zero to 50,000 - the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/nejmp1607958
12. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the society of general internal medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
13. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
14. Sahlqvist S, Song Y, Bull F, Adams E, Preston J, Ogilvie D; iConnect consortium. Effect of questionnaire length, personalisation and reminder type on response rate to a complex postal survey: randomised controlled trial. BMC Med Res Methodol. 2011;11:62. https://doi.org/10.1186/1471-2288-11-62
15 Sojo VE, Wood RE, Genat AE. Harmful Workplace Experiences and Women’s Occupational Well-Being: A Meta-Analysis. Psychol Women Q. 2016;40(1):10-40. https://doi.org/10.1177/0361684315599346
16. Office of Civil Rights: Sexual Harassment. U.S. Department of the Interior. Accessed April 20, 2020. https://www.doi.gov/pmb/eeo/Sexual-Harassment
17. Sexual Harassment: Categories of Sexual Harassment. Sexual Harassment/Assault Response and Prevention Program (SHARP). Accessed April 20, 2020. https://www.sexualassault.army.mil/categories_of_harassment.aspx
Gender-based discrimination refers to “any distinction, exclusion or restriction made on the basis of socially constructed gender roles and norms which prevents a person from enjoying full human rights.”1 Similarly, sexual harassment encompasses a spectrum of sexual conduct that includes “unwelcome sexual advances, requests for sexual favors, and other verbal or physical harassment of a sexual nature,” as defined by the US Equal Employment Opportunity Commission.2 Gender-based discrimination and sexual harassment can be “overt,” which includes explicitly recognizable behaviors, or they can be “implicit,” which includes verbal and nonverbal behaviors that often go unrecognized but convey hostility, objectification, or exclusion of another person. Gender-based discrimination and sexual harassment are commonly described and likely more prevalent in academic settings.3-6 Female physicians are disproportionately affected by gender-based discrimination and sexual harassment, compared with their male peers.4,7
Female physicians face workplace harassment from both patients and coworkers. A study in Canada reported that more than 75% of female physicians experienced sexual harassment from their patients.8 A recent study showed almost half of the physicians who reported harassment, which was three times more often among female physicians, described other physician colleagues as perpetrators.9 In a study among clinician-researchers in the field of academic medicine, 30% of females reported having experienced sexual harassment, compared with 4% of males.7 Among females who reported harassment in this study, 47% stated that these experiences adversely affected their opportunities for career advancement. Career stage may also affect experiences or perceptions of gender-based discrimination and sexual harassment, with females in earlier career stages reporting a less favorable environment of gender equity.10
Hospital medicine is a young and evolving specialty, and the number of hospitalists has increased substantially from a few hundred at the time of inception to over 50,000 as of 2016.11 The proportion of female hospitalists increased from 31% in 2012 to 52% in 2014, reflecting equal gender representation in hospital medicine.12 Available evidence shows gender disparities exist in hospital medicine disproportionately affecting female hospitalists in their career advancement, including leadership and scholarship opportunities.13 However, there remains a knowledge gap regarding the prevalence of gender-based discrimination and sexual harassment experienced by hospitalists.
Our study examines the experiences of academic hospitalists regarding gender-based discrimination and sexual harassment and the impact of gender on career satisfaction and advancement.
METHODS
Study Design and Participants
An online survey was developed and approved by the institutional board review (IRB) at the Medical College of Wisconsin in Milwaukee. University-based academic centers with hospitalist programs, identified through personal connections, from across the continental United Stated were identified as potential study sites, and leaders at each institution were contacted to ascertain potential participation in the survey. The survey was distributed to Internal Medicine hospitalists at 18 participating academic institutions from January 2019 to June 2019. Participation was voluntary. The cover letter explained the purpose of the study and provided a link to the survey. To maintain anonymity, none of the questionnaires requested identifying information from participants. A web-based Qualtrics online-based survey platform was used.
Survey Elements
The survey aimed to assess several elements of gender-based discrimination and sexual harassment. All questions about these experiences distinguished encounters with patients from those with colleagues, and questions about occurrences either over a career or in the last 30 days were intended to capture both distant and recent timeframes. The theme for the questions for the survey was based on previous studies.4,7,8 The wording of questions was simplified to make them easily understandable, and the brevity of the survey was maintained to prevent possible nonresponses.14 Additional questions (mistaken for a healthcare provider other than a physician, feeling respected by patients and colleagues, referred to by terms such as “honey,” “dear,” “sweetheart,” “sugar,” or equivalent), which were deemed relevant in day-to-day clinical practice through consensus among study investigators and discussions among peer hospitalists, were incorporated into the final survey (Appendix). Survey questions were intended to capture several elements regarding interactions with patients and with colleagues or other healthcare providers (HCPs).
Questions on gender-based discrimination included:
- Has a patient [colleague or other healthcare provider] mistaken you for a healthcare provider other than a physician?
- Has a patient [colleague or other healthcare provider] asked you to do something not at your level of training?
- Do you feel respected? Do you perceive your gender has impacted opportunities for your career advancement?
Questions on sexual harassment included:
- Has a patient [colleague or other healthcare provider] touched you inappropriately, made sexual remarks or gestures, or made suggestive looks?
- Has a patient [colleague or other healthcare provider] referred to you by terms such as “honey,” “dear,” “sweetheart,” “sugar,” or equivalent?
In addition, the survey sought demographic information of the participants (age, gender, and race/ethnicity) and information about their individual institutions (names and locations) (Appendix). The geographical locations of the institutions were further categorized into four different regions according to the United States Census Bureau (Northwest, Midwest, South, and West). At the end of the survey, participants were given an opportunity to provide any additional comments.
Statistical Analysis
Standard descriptive summary statistics were used for demographic data. Associations between the variables were analyzed using chi-square test or Fischer’s exact test, as appropriate, for categorical variables and t test for continuous variables. The variations among institution-based responses were presented in the form of inter-quartile range (IQR). All tests were 2-sided, and P values less than .05 were considered statistically significant. All analyses were performed using IBM® SPSS® Statistics software version 24. Relevant responses representative of the overall respondents’ sentiments as provided under additional comment section were discussed and cited.
RESULTS
Eighteen different academic institutions across the United States participated in the study, with 336 individual responses. The majority of respondents were females (57%), in younger age categories (58% were 30-39 years old), Caucasian (59%), and early-career hospitalists (>50% working as hospitalists for ≤5 years) (Table 1). Regarding the overall geographic distribution, the largest number of responses were from the Midwest (n = 115; 35.6%) (Table 1 and Appendix).

Gender Discrimination
Interactions With Patients
Over their careers, 69% of hospitalists reported being mistaken for an HCP other than a physician by patients. This was more common among females than among males (99% vs 29%, respectively; P < .001) (Table 2). Almost half (48%) reported this had occurred in the last 30 days, more frequently by females (76% vs 10%; P < .001).

Of responding hospitalists, 96% stated that, over their careers, they have been asked by patients to do something they did not consider to be at their level of training (eg, help get food or water, help with a bed pan), with a higher prevalence of such experiences among females than males (99% vs 93%, respectively; P = .004) (Table 2). Most (71%) said they had experienced this in the last 30 days, which was again more frequently reported by females (78% vs 62%; P = .001).
The responses from female hospitalists regarding their career-long experiences of being mistaken for an HCP or asked to do something not at their level of training by their patients had both the highest number of positive responses across institutions (median of hospital proportions, 100%) and the least institutional variation since both had the narrowest IQR) (Table 2).
Interactions With Colleagues or Other HCPs
Among hospitalists responding to the survey, 46% felt that, over their careers, they had been mistaken for nonphysician HCPs by colleagues or other HCPs. This was more prevalent among females than among males (65% vs 20%; P < .001) (Table 2). Among respondents, 14% reported these events had occurred in the last 30 days, which was again more common among females (21% vs 5%; P < .001).
Over their careers, 26% of hospitalists reported they have been asked by a colleague or HCP to do something not at their level of training (eg, clean up the physician’s work room, make coffee, take notes in a meeting), with similar prevalence among females and males (29% vs 23%; P = .228). Ten percent reported these occurrences in the last 30 days, which was similar between females and males (12% vs 9%; P = .330).
Feelings of Respect and Opportunities for Career Advancement
When asked to rate the statement “I feel respected by patients” on a 5-point Likert scale, female hospitalists overall scored significantly lower as compared with their male counterparts (mean score, 3.73 vs 4.04; P < .001) (Table 3); this was also true when asked about feelings of respect by physicians (mean score, 3.84 vs 4.15; P < .001). Female hospitalists were more likely than males to report that their gender has more negatively impacted their opportunities for career advancement (mean score, 2.73 vs 3.34; P < .001).

Sexual Harassment
Interactions With Patients
Over half (57%) of hospitalists reported career-long experiences of patient(s) touching them inappropriately, making sexual remarks or gestures, or making suggestive looks. These experiences were more prevalent among females than among males (72% vs 36%, respectively; P < .001) (Table 2). Fifteen percent said they had such experience in rhe last 30 days, which was also more common among females (22% vs 6%; P < .001).
Most hospitalists (84%) reported that patients have referred to them by inappropriately familiar terms such as “honey,” “dear,” “sweetheart,” “sugar,” or equivalent over their careers, with females more frequently reporting these behaviors (95% vs 68%; P < .001). Experiencing this during the last 30 days was reported by 48%, which was again more common among females (68% vs 23%; P < .001).
Interactions With Colleagues or Other HCPs
Within their careers, 15% of hospitalists reported at least one experience of a colleague or HCP touching them inappropriately or making sexual remarks, gestures, or suggestive looks. This was more prevalent for females than males (18% vs 10%, respectively; P = .033). Only 2% of both females and males reported these experiences in the last 30 days (2% vs 2%; P = .981).
Almost one-third of participants (32%) affirmed that another HCP has referred to them by terms such as “honey,” “dear,” “sweetheart,” “sugar,” or equivalent in their career, with a higher proportion of females than males reporting these events (39% vs 23%; P = .002) (Table 2). Experiencing this during the last 30 days was reported by 10%, which was similar between females and males (12% vs 7%; P = .112).
Additional Comments From Respondents
- “Throughout my training and now into my professional career, there are nearly weekly incidents of elderly male patients referring to me as “honey/dear/sweetie” or even by my first name, even though I introduce myself as their physician and politely correct them. They will often refer to me as a nurse and ask me to do something not at my level of training. Sometimes even despite correcting the patient, they continue to refer to me as such. Throughout the years, other female MDs and I have discussed that this is ‘status quo’ for female physicians and observe that this is not an experience that male MDs share.”
- “I frequently round with a male nurse practitioner and the patients almost always, despite introducing ourselves and our roles, turn to him and ask him questions instead of addressing them to me.”
- “Our institution allows female faculty to be interviewed about childcare, household labor division, plans for pregnancy. One professor asks women private details about their private relationships such as what they do with spouse on date night or weekends away.”
- “It’s hard to answer questions related to my level of training. I don’t think it’s unreasonable for people to ask me to do things, no matter my level of training. . . . I don’t think being a doctor means that I am above this, or that it is inappropriate to be asked to do this.”
DISCUSSION
This survey demonstrated that gender-based discrimination and sexual harassment in the academic hospitalist healthcare environment are common, both in more distant and recent time frames. Notably, these experiences are shared by female and male physicians in interactions with both patients and colleagues, though male hospitalists report most of these experiences at significantly lower frequencies than females. These results support past work showing that female physicians are significantly more likely to be subjected to gender-based discrimination and sexual harassment, but also challenges the perception that gender-based discrimination and sexual harassment are uniquely experienced by females.
A startling number of females and males in the study reported sexual harassment (inappropriate touching, remarks, gestures, and looks) when interacting with patients throughout their careers and in last 30 days. Many males and females reported that patients had referred to them with inappropriately familiar, and potentially demeaning, terms of endearment. For both overt and implicit sexual harassment, females were significantly more likely than males to report experiencing these behaviors when interacting with patients. Although some of these experiences may seem less harmful than others, a meta-analysis demonstrated that frequent, less intense experiences of gender-based discrimination and sexual harassment have a similar impact on female’s well-being as do less frequent, more intense experiences.15 Although the person using the terms of endearment like “honey,” “sugar,” or “sweetheart” may feel the terms are harmless, such expressions can be inappropriate and constitute sexual harassment according to the U.S. Department of the Interior’s Office of Civil Rights.16 The Sexual Harassment/Assault Response and Prevention Program (SHARP) also classifies such terms into verbal categories of sexual harrassment.17
Of female physicians surveyed, 99% reported that they had been mistaken for HCPs other than physicians by their patients over their careers. Although this was also reported by male physicians, the experience was 3.4 times as likely for female physicians. Misidentification by patients may represent a disconnect between the growing female representation in the physician workforce and patients’ conceptions of the traditional image of a physician.
In parallel with this finding of misidentification, an interesting area of the study was the question regarding being asked to do “something not at your level of training.” A recurring theme in the comments was a rejection of the notion that certain tasks were “beneath a level of training,” suggesting a common view that acts of caregiving are not bounded by hierarchy. Analysis of qualitative responses showed that 40% of these responses had comments regarding this question. An example was “It’s hard to answer questions related to my level of training. . . . I don’t think being a doctor means that I am above this, or that it is inappropriate to be asked to do this.” Notably, however, a larger number of female than male physicians responded yes to this question in both study time frames. This points to a differential in how female physicians are viewed by patients, both in frequent misidentification and in behaviors more frequently asked of female physicians than their male counterparts. Given the comments, it may also suggest a difference in how female and male physicians perceive the fluidity of bounds on their care-taking roles set by their “level of training.”
A large number of study participants were early-career hospitalists, which may in part explain some of the study results. In a previous study of gender equity in an Internal Medicine department, physicians practicing medicine for more than 15 years perceived the departmental culture as more favorable than physicians with shorter careers.10 Additionally, the perception of cultures was most discordant between senior male physicians and junior female physicians.10 Because many hospitalists are early-career physicians, they may have trained in an environment that had heightened awareness surrounding gender-based discrimination and sexual harassment, which affects the overall study results.
Multiple qualitative comments, mentioned above, were submitted by participants describing their experiences in all categories. Such comments paint a picture of insidious bias and cultural norms affecting the quality of female physicians’ work lives.
Two questions focused on career satisfaction and the sense of respect from patients and colleagues. In both responses, there was a statistically different response between males and females, with females less likely to report that they felt respected and that their gender adversely impacted their opportunities for career advancement. This is disturbing information and warrants more investigation.
The reasons for the observed prevalence of gender-based discrimination and sexual harassment in this broad survey of academic hospitalists are uncertain. Multiple studies to date have demonstrated that gender-based discrimination and sexual harassment have historically existed in medicine and continue to even today. Unlike physicians with long-term relationships with patients, hospitalists may face more exposure due to a lack of long-term continuity with patients. The absence of an established trust in the relationship also may make them more vulnerable to inappropriate behaviors when interacting with patients. Hospital medicine, however, is a young specialty with equal gender representation and should be at the forefront of addressing and solving these issues of gender-based discrimination and sexual harassment.
The survey had a good distribution between female and male participants. Additionally, the survey reflected the general distribution of the national hospitalist workforce in gender, age, and ethnic/racial distribution, as well as number of years in practice.12 The study surveyed respondents regarding experiences in both long- and short-term time frames, as well as experiences with patients and colleagues.
Our study reflects a cross-sectional snapshot of hospitalists’ perceptions with no longitudinal follow-up. Since the survey was limited to academic medical centers, it may not reflect experiences in community/private practice settings. The small number of participants limited the ability to perform subgroup analyses by age, race, or years in practice, which may play a role in interactions with patients and colleagues. Since the number of respondents varied greatly by institution, a minority of institutions could have influenced some of the findings. Narrow IQRs of the hospital proportions as shown in Table 2 would suggest similar responses across institutions, whereas wide IQRs would suggest that a smaller number of institutions were possibly driving the findings. Because of the survey distribution method, it is unknown how many physicians received the survey and a response rate could not be calculated. Further, selection, recall, and detection biases cannot be ruled out.
CONCLUSION
This survey shows that gender-based discrimination and sexual harassment in the academic hospitalist healthcare environment are common and more frequently experienced by female physicians, both in interactions with patients and colleagues. Our study highlights the need to address this prevalent issue among academic hospitalists.
Gender-based discrimination refers to “any distinction, exclusion or restriction made on the basis of socially constructed gender roles and norms which prevents a person from enjoying full human rights.”1 Similarly, sexual harassment encompasses a spectrum of sexual conduct that includes “unwelcome sexual advances, requests for sexual favors, and other verbal or physical harassment of a sexual nature,” as defined by the US Equal Employment Opportunity Commission.2 Gender-based discrimination and sexual harassment can be “overt,” which includes explicitly recognizable behaviors, or they can be “implicit,” which includes verbal and nonverbal behaviors that often go unrecognized but convey hostility, objectification, or exclusion of another person. Gender-based discrimination and sexual harassment are commonly described and likely more prevalent in academic settings.3-6 Female physicians are disproportionately affected by gender-based discrimination and sexual harassment, compared with their male peers.4,7
Female physicians face workplace harassment from both patients and coworkers. A study in Canada reported that more than 75% of female physicians experienced sexual harassment from their patients.8 A recent study showed almost half of the physicians who reported harassment, which was three times more often among female physicians, described other physician colleagues as perpetrators.9 In a study among clinician-researchers in the field of academic medicine, 30% of females reported having experienced sexual harassment, compared with 4% of males.7 Among females who reported harassment in this study, 47% stated that these experiences adversely affected their opportunities for career advancement. Career stage may also affect experiences or perceptions of gender-based discrimination and sexual harassment, with females in earlier career stages reporting a less favorable environment of gender equity.10
Hospital medicine is a young and evolving specialty, and the number of hospitalists has increased substantially from a few hundred at the time of inception to over 50,000 as of 2016.11 The proportion of female hospitalists increased from 31% in 2012 to 52% in 2014, reflecting equal gender representation in hospital medicine.12 Available evidence shows gender disparities exist in hospital medicine disproportionately affecting female hospitalists in their career advancement, including leadership and scholarship opportunities.13 However, there remains a knowledge gap regarding the prevalence of gender-based discrimination and sexual harassment experienced by hospitalists.
Our study examines the experiences of academic hospitalists regarding gender-based discrimination and sexual harassment and the impact of gender on career satisfaction and advancement.
METHODS
Study Design and Participants
An online survey was developed and approved by the institutional board review (IRB) at the Medical College of Wisconsin in Milwaukee. University-based academic centers with hospitalist programs, identified through personal connections, from across the continental United Stated were identified as potential study sites, and leaders at each institution were contacted to ascertain potential participation in the survey. The survey was distributed to Internal Medicine hospitalists at 18 participating academic institutions from January 2019 to June 2019. Participation was voluntary. The cover letter explained the purpose of the study and provided a link to the survey. To maintain anonymity, none of the questionnaires requested identifying information from participants. A web-based Qualtrics online-based survey platform was used.
Survey Elements
The survey aimed to assess several elements of gender-based discrimination and sexual harassment. All questions about these experiences distinguished encounters with patients from those with colleagues, and questions about occurrences either over a career or in the last 30 days were intended to capture both distant and recent timeframes. The theme for the questions for the survey was based on previous studies.4,7,8 The wording of questions was simplified to make them easily understandable, and the brevity of the survey was maintained to prevent possible nonresponses.14 Additional questions (mistaken for a healthcare provider other than a physician, feeling respected by patients and colleagues, referred to by terms such as “honey,” “dear,” “sweetheart,” “sugar,” or equivalent), which were deemed relevant in day-to-day clinical practice through consensus among study investigators and discussions among peer hospitalists, were incorporated into the final survey (Appendix). Survey questions were intended to capture several elements regarding interactions with patients and with colleagues or other healthcare providers (HCPs).
Questions on gender-based discrimination included:
- Has a patient [colleague or other healthcare provider] mistaken you for a healthcare provider other than a physician?
- Has a patient [colleague or other healthcare provider] asked you to do something not at your level of training?
- Do you feel respected? Do you perceive your gender has impacted opportunities for your career advancement?
Questions on sexual harassment included:
- Has a patient [colleague or other healthcare provider] touched you inappropriately, made sexual remarks or gestures, or made suggestive looks?
- Has a patient [colleague or other healthcare provider] referred to you by terms such as “honey,” “dear,” “sweetheart,” “sugar,” or equivalent?
In addition, the survey sought demographic information of the participants (age, gender, and race/ethnicity) and information about their individual institutions (names and locations) (Appendix). The geographical locations of the institutions were further categorized into four different regions according to the United States Census Bureau (Northwest, Midwest, South, and West). At the end of the survey, participants were given an opportunity to provide any additional comments.
Statistical Analysis
Standard descriptive summary statistics were used for demographic data. Associations between the variables were analyzed using chi-square test or Fischer’s exact test, as appropriate, for categorical variables and t test for continuous variables. The variations among institution-based responses were presented in the form of inter-quartile range (IQR). All tests were 2-sided, and P values less than .05 were considered statistically significant. All analyses were performed using IBM® SPSS® Statistics software version 24. Relevant responses representative of the overall respondents’ sentiments as provided under additional comment section were discussed and cited.
RESULTS
Eighteen different academic institutions across the United States participated in the study, with 336 individual responses. The majority of respondents were females (57%), in younger age categories (58% were 30-39 years old), Caucasian (59%), and early-career hospitalists (>50% working as hospitalists for ≤5 years) (Table 1). Regarding the overall geographic distribution, the largest number of responses were from the Midwest (n = 115; 35.6%) (Table 1 and Appendix).

Gender Discrimination
Interactions With Patients
Over their careers, 69% of hospitalists reported being mistaken for an HCP other than a physician by patients. This was more common among females than among males (99% vs 29%, respectively; P < .001) (Table 2). Almost half (48%) reported this had occurred in the last 30 days, more frequently by females (76% vs 10%; P < .001).

Of responding hospitalists, 96% stated that, over their careers, they have been asked by patients to do something they did not consider to be at their level of training (eg, help get food or water, help with a bed pan), with a higher prevalence of such experiences among females than males (99% vs 93%, respectively; P = .004) (Table 2). Most (71%) said they had experienced this in the last 30 days, which was again more frequently reported by females (78% vs 62%; P = .001).
The responses from female hospitalists regarding their career-long experiences of being mistaken for an HCP or asked to do something not at their level of training by their patients had both the highest number of positive responses across institutions (median of hospital proportions, 100%) and the least institutional variation since both had the narrowest IQR) (Table 2).
Interactions With Colleagues or Other HCPs
Among hospitalists responding to the survey, 46% felt that, over their careers, they had been mistaken for nonphysician HCPs by colleagues or other HCPs. This was more prevalent among females than among males (65% vs 20%; P < .001) (Table 2). Among respondents, 14% reported these events had occurred in the last 30 days, which was again more common among females (21% vs 5%; P < .001).
Over their careers, 26% of hospitalists reported they have been asked by a colleague or HCP to do something not at their level of training (eg, clean up the physician’s work room, make coffee, take notes in a meeting), with similar prevalence among females and males (29% vs 23%; P = .228). Ten percent reported these occurrences in the last 30 days, which was similar between females and males (12% vs 9%; P = .330).
Feelings of Respect and Opportunities for Career Advancement
When asked to rate the statement “I feel respected by patients” on a 5-point Likert scale, female hospitalists overall scored significantly lower as compared with their male counterparts (mean score, 3.73 vs 4.04; P < .001) (Table 3); this was also true when asked about feelings of respect by physicians (mean score, 3.84 vs 4.15; P < .001). Female hospitalists were more likely than males to report that their gender has more negatively impacted their opportunities for career advancement (mean score, 2.73 vs 3.34; P < .001).

Sexual Harassment
Interactions With Patients
Over half (57%) of hospitalists reported career-long experiences of patient(s) touching them inappropriately, making sexual remarks or gestures, or making suggestive looks. These experiences were more prevalent among females than among males (72% vs 36%, respectively; P < .001) (Table 2). Fifteen percent said they had such experience in rhe last 30 days, which was also more common among females (22% vs 6%; P < .001).
Most hospitalists (84%) reported that patients have referred to them by inappropriately familiar terms such as “honey,” “dear,” “sweetheart,” “sugar,” or equivalent over their careers, with females more frequently reporting these behaviors (95% vs 68%; P < .001). Experiencing this during the last 30 days was reported by 48%, which was again more common among females (68% vs 23%; P < .001).
Interactions With Colleagues or Other HCPs
Within their careers, 15% of hospitalists reported at least one experience of a colleague or HCP touching them inappropriately or making sexual remarks, gestures, or suggestive looks. This was more prevalent for females than males (18% vs 10%, respectively; P = .033). Only 2% of both females and males reported these experiences in the last 30 days (2% vs 2%; P = .981).
Almost one-third of participants (32%) affirmed that another HCP has referred to them by terms such as “honey,” “dear,” “sweetheart,” “sugar,” or equivalent in their career, with a higher proportion of females than males reporting these events (39% vs 23%; P = .002) (Table 2). Experiencing this during the last 30 days was reported by 10%, which was similar between females and males (12% vs 7%; P = .112).
Additional Comments From Respondents
- “Throughout my training and now into my professional career, there are nearly weekly incidents of elderly male patients referring to me as “honey/dear/sweetie” or even by my first name, even though I introduce myself as their physician and politely correct them. They will often refer to me as a nurse and ask me to do something not at my level of training. Sometimes even despite correcting the patient, they continue to refer to me as such. Throughout the years, other female MDs and I have discussed that this is ‘status quo’ for female physicians and observe that this is not an experience that male MDs share.”
- “I frequently round with a male nurse practitioner and the patients almost always, despite introducing ourselves and our roles, turn to him and ask him questions instead of addressing them to me.”
- “Our institution allows female faculty to be interviewed about childcare, household labor division, plans for pregnancy. One professor asks women private details about their private relationships such as what they do with spouse on date night or weekends away.”
- “It’s hard to answer questions related to my level of training. I don’t think it’s unreasonable for people to ask me to do things, no matter my level of training. . . . I don’t think being a doctor means that I am above this, or that it is inappropriate to be asked to do this.”
DISCUSSION
This survey demonstrated that gender-based discrimination and sexual harassment in the academic hospitalist healthcare environment are common, both in more distant and recent time frames. Notably, these experiences are shared by female and male physicians in interactions with both patients and colleagues, though male hospitalists report most of these experiences at significantly lower frequencies than females. These results support past work showing that female physicians are significantly more likely to be subjected to gender-based discrimination and sexual harassment, but also challenges the perception that gender-based discrimination and sexual harassment are uniquely experienced by females.
A startling number of females and males in the study reported sexual harassment (inappropriate touching, remarks, gestures, and looks) when interacting with patients throughout their careers and in last 30 days. Many males and females reported that patients had referred to them with inappropriately familiar, and potentially demeaning, terms of endearment. For both overt and implicit sexual harassment, females were significantly more likely than males to report experiencing these behaviors when interacting with patients. Although some of these experiences may seem less harmful than others, a meta-analysis demonstrated that frequent, less intense experiences of gender-based discrimination and sexual harassment have a similar impact on female’s well-being as do less frequent, more intense experiences.15 Although the person using the terms of endearment like “honey,” “sugar,” or “sweetheart” may feel the terms are harmless, such expressions can be inappropriate and constitute sexual harassment according to the U.S. Department of the Interior’s Office of Civil Rights.16 The Sexual Harassment/Assault Response and Prevention Program (SHARP) also classifies such terms into verbal categories of sexual harrassment.17
Of female physicians surveyed, 99% reported that they had been mistaken for HCPs other than physicians by their patients over their careers. Although this was also reported by male physicians, the experience was 3.4 times as likely for female physicians. Misidentification by patients may represent a disconnect between the growing female representation in the physician workforce and patients’ conceptions of the traditional image of a physician.
In parallel with this finding of misidentification, an interesting area of the study was the question regarding being asked to do “something not at your level of training.” A recurring theme in the comments was a rejection of the notion that certain tasks were “beneath a level of training,” suggesting a common view that acts of caregiving are not bounded by hierarchy. Analysis of qualitative responses showed that 40% of these responses had comments regarding this question. An example was “It’s hard to answer questions related to my level of training. . . . I don’t think being a doctor means that I am above this, or that it is inappropriate to be asked to do this.” Notably, however, a larger number of female than male physicians responded yes to this question in both study time frames. This points to a differential in how female physicians are viewed by patients, both in frequent misidentification and in behaviors more frequently asked of female physicians than their male counterparts. Given the comments, it may also suggest a difference in how female and male physicians perceive the fluidity of bounds on their care-taking roles set by their “level of training.”
A large number of study participants were early-career hospitalists, which may in part explain some of the study results. In a previous study of gender equity in an Internal Medicine department, physicians practicing medicine for more than 15 years perceived the departmental culture as more favorable than physicians with shorter careers.10 Additionally, the perception of cultures was most discordant between senior male physicians and junior female physicians.10 Because many hospitalists are early-career physicians, they may have trained in an environment that had heightened awareness surrounding gender-based discrimination and sexual harassment, which affects the overall study results.
Multiple qualitative comments, mentioned above, were submitted by participants describing their experiences in all categories. Such comments paint a picture of insidious bias and cultural norms affecting the quality of female physicians’ work lives.
Two questions focused on career satisfaction and the sense of respect from patients and colleagues. In both responses, there was a statistically different response between males and females, with females less likely to report that they felt respected and that their gender adversely impacted their opportunities for career advancement. This is disturbing information and warrants more investigation.
The reasons for the observed prevalence of gender-based discrimination and sexual harassment in this broad survey of academic hospitalists are uncertain. Multiple studies to date have demonstrated that gender-based discrimination and sexual harassment have historically existed in medicine and continue to even today. Unlike physicians with long-term relationships with patients, hospitalists may face more exposure due to a lack of long-term continuity with patients. The absence of an established trust in the relationship also may make them more vulnerable to inappropriate behaviors when interacting with patients. Hospital medicine, however, is a young specialty with equal gender representation and should be at the forefront of addressing and solving these issues of gender-based discrimination and sexual harassment.
The survey had a good distribution between female and male participants. Additionally, the survey reflected the general distribution of the national hospitalist workforce in gender, age, and ethnic/racial distribution, as well as number of years in practice.12 The study surveyed respondents regarding experiences in both long- and short-term time frames, as well as experiences with patients and colleagues.
Our study reflects a cross-sectional snapshot of hospitalists’ perceptions with no longitudinal follow-up. Since the survey was limited to academic medical centers, it may not reflect experiences in community/private practice settings. The small number of participants limited the ability to perform subgroup analyses by age, race, or years in practice, which may play a role in interactions with patients and colleagues. Since the number of respondents varied greatly by institution, a minority of institutions could have influenced some of the findings. Narrow IQRs of the hospital proportions as shown in Table 2 would suggest similar responses across institutions, whereas wide IQRs would suggest that a smaller number of institutions were possibly driving the findings. Because of the survey distribution method, it is unknown how many physicians received the survey and a response rate could not be calculated. Further, selection, recall, and detection biases cannot be ruled out.
CONCLUSION
This survey shows that gender-based discrimination and sexual harassment in the academic hospitalist healthcare environment are common and more frequently experienced by female physicians, both in interactions with patients and colleagues. Our study highlights the need to address this prevalent issue among academic hospitalists.
1. WHO Department of Reproductive Health and Research. Transforming health systems: gender and rights in reproductive health. A training manual for health managers. World Health Organization; 2001. https://www.who.int/reproductivehealth/publications/gender_rights/RHR_01_29/en/
2. Sexual Harassment. U.S. Equal Employment Opportunity Commission. Accessed Jan 5, 2020. https://www.eeoc.gov/laws/types/sexual_harassment.cfm
3. Frank E, Brogan D, Schiffman M. Prevalence and correlates of harassment among US women physicians. Arch Intern Med. 1998;158(4):352-358. https://doi.org/10.1001/archinte.158.4.352
4. Carr PL, Ash AS, Friedman RH, et al. Faculty perceptions of gender discrimination and sexual harassment in academic medicine. Ann Intern Med. 2000;132(11):889-96. https://doi.org/10.7326/0003-4819-132-11-200006060-00007
5. Bates CK, Jagsi R, Gordon LK, et al. It is time for zero tolerance for sexual harassment in academic medicine. Acad Med. 2018;93(2):163-165. https://doi.org/10.1097/acm.0000000000002050
6. Dzau VJ, Johnson PA. Ending sexual harassment in academic medicine. N Engl J Med. 2018;379(17):1589-1591. https://doi.org/10.1056/nejmp1809846
7. Jagsi R, Griffith KA, Jones R, Perumalswami CR, Ubel P, Stewart A. Sexual harassment and discrimination experiences of academic medical faculty. JAMA. 2016;315(19):2120-2121. https://doi.org/10.1001/jama.2016.2188
8. Phillips SP, Schneider MS. Sexual harassment of female doctors by patients. N Engl J Med. 1993;329(26):1936-1939. https://doi.org/10.1056/nejm199312233292607
9. Kane L. Sexual Harassment of Physicians: Report 2018. Medscape. June 13, 2018. Accessed Jan 24, 2020. https://www.medscape.com/slideshow/sexual-harassment-of-physicians-6010304
10. Ruzycki SM, Freeman G, Bharwani A, Brown A. Association of physician characteristics with perceptions and experiences of gender equity in an academic internal medicine department. JAMA Netw Open. 2019;2(11):e1915165. https://doi.org/10.1001/jamanetworkopen.2019.15165
11. Wachter RM, Goldman L. Zero to 50,000 - the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/nejmp1607958
12. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the society of general internal medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
13. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
14. Sahlqvist S, Song Y, Bull F, Adams E, Preston J, Ogilvie D; iConnect consortium. Effect of questionnaire length, personalisation and reminder type on response rate to a complex postal survey: randomised controlled trial. BMC Med Res Methodol. 2011;11:62. https://doi.org/10.1186/1471-2288-11-62
15 Sojo VE, Wood RE, Genat AE. Harmful Workplace Experiences and Women’s Occupational Well-Being: A Meta-Analysis. Psychol Women Q. 2016;40(1):10-40. https://doi.org/10.1177/0361684315599346
16. Office of Civil Rights: Sexual Harassment. U.S. Department of the Interior. Accessed April 20, 2020. https://www.doi.gov/pmb/eeo/Sexual-Harassment
17. Sexual Harassment: Categories of Sexual Harassment. Sexual Harassment/Assault Response and Prevention Program (SHARP). Accessed April 20, 2020. https://www.sexualassault.army.mil/categories_of_harassment.aspx
1. WHO Department of Reproductive Health and Research. Transforming health systems: gender and rights in reproductive health. A training manual for health managers. World Health Organization; 2001. https://www.who.int/reproductivehealth/publications/gender_rights/RHR_01_29/en/
2. Sexual Harassment. U.S. Equal Employment Opportunity Commission. Accessed Jan 5, 2020. https://www.eeoc.gov/laws/types/sexual_harassment.cfm
3. Frank E, Brogan D, Schiffman M. Prevalence and correlates of harassment among US women physicians. Arch Intern Med. 1998;158(4):352-358. https://doi.org/10.1001/archinte.158.4.352
4. Carr PL, Ash AS, Friedman RH, et al. Faculty perceptions of gender discrimination and sexual harassment in academic medicine. Ann Intern Med. 2000;132(11):889-96. https://doi.org/10.7326/0003-4819-132-11-200006060-00007
5. Bates CK, Jagsi R, Gordon LK, et al. It is time for zero tolerance for sexual harassment in academic medicine. Acad Med. 2018;93(2):163-165. https://doi.org/10.1097/acm.0000000000002050
6. Dzau VJ, Johnson PA. Ending sexual harassment in academic medicine. N Engl J Med. 2018;379(17):1589-1591. https://doi.org/10.1056/nejmp1809846
7. Jagsi R, Griffith KA, Jones R, Perumalswami CR, Ubel P, Stewart A. Sexual harassment and discrimination experiences of academic medical faculty. JAMA. 2016;315(19):2120-2121. https://doi.org/10.1001/jama.2016.2188
8. Phillips SP, Schneider MS. Sexual harassment of female doctors by patients. N Engl J Med. 1993;329(26):1936-1939. https://doi.org/10.1056/nejm199312233292607
9. Kane L. Sexual Harassment of Physicians: Report 2018. Medscape. June 13, 2018. Accessed Jan 24, 2020. https://www.medscape.com/slideshow/sexual-harassment-of-physicians-6010304
10. Ruzycki SM, Freeman G, Bharwani A, Brown A. Association of physician characteristics with perceptions and experiences of gender equity in an academic internal medicine department. JAMA Netw Open. 2019;2(11):e1915165. https://doi.org/10.1001/jamanetworkopen.2019.15165
11. Wachter RM, Goldman L. Zero to 50,000 - the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/nejmp1607958
12. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the society of general internal medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
13. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
14. Sahlqvist S, Song Y, Bull F, Adams E, Preston J, Ogilvie D; iConnect consortium. Effect of questionnaire length, personalisation and reminder type on response rate to a complex postal survey: randomised controlled trial. BMC Med Res Methodol. 2011;11:62. https://doi.org/10.1186/1471-2288-11-62
15 Sojo VE, Wood RE, Genat AE. Harmful Workplace Experiences and Women’s Occupational Well-Being: A Meta-Analysis. Psychol Women Q. 2016;40(1):10-40. https://doi.org/10.1177/0361684315599346
16. Office of Civil Rights: Sexual Harassment. U.S. Department of the Interior. Accessed April 20, 2020. https://www.doi.gov/pmb/eeo/Sexual-Harassment
17. Sexual Harassment: Categories of Sexual Harassment. Sexual Harassment/Assault Response and Prevention Program (SHARP). Accessed April 20, 2020. https://www.sexualassault.army.mil/categories_of_harassment.aspx
© 2021 Society of Hospital Medicine