User login
Meet the new members of the CHEST Physician® Editorial Board
We’re happy to introduce these new board members whose primary responsibility is the active review each month of potential articles for publication that could have an impact on or be of interest to our health-care professional readership.
Carolyn M. D’Ambrosio, MD, FCCP, is the Program Director for the Harvard-Brigham and Women’s Hospital Fellowship in Pulmonary and Critical Care Medicine and is Associate Professor of Medicine at Harvard Medical School. Most recently, she was awarded the Pillar Award for Educational Program Leadership, the top award for program directors throughout the Mass General Brigham institutions. In addition to teaching and clinical work, Dr. D’Ambrosio has conducted research on sleep and menopause, sleep and breathing in infants, and participated as the sleep medicine expert in two systemic reviews on home sleep apnea testing and fixed vs auto-titrating CPAP. She continues her work in Medical Ethics as a Senior Ethics Consultant at Brigham and Women’s Hospital.
Jonathan (Jona) Ludmir, MD, FCCP
After completing internal medicine/pediatrics, cardiology, and critical care training, Dr. Ludmir joined the Massachusetts General Hospital staff as a cardiac intensivist and noninvasive cardiologist. His clinical focus is in the heart center ICU, the echocardiography lab, as well as in outpatient cardiology. Additionally, he is the lead physician for the Family-Centered Care Initiative, where he focuses on incorporating evidence-based guidelines and leads in the science of family-centered cardiovascular care delivery. Dr. Ludmir’s research focuses on identifying and addressing psychological symptoms in the ICU, optimizing ICU communication, and enhancing delivery of family-centered care.
Abbie Begnaud, MD, FCCP
Dr. Begnaud hails from south Louisiana and reveals that she attended her first CHEST Annual Meeting in 2011 in Hawaii, and she was “instantly hooked.” Clinically, she practices general pulmonology, critical care, and interventional pulmonology and focuses her research on lung cancer screening and health disparities. She has been on faculty at the University of Minnesota since 2013 and is passionate about lung cancer, health equity, and mentoring.
Shyam Subramanian, MD , FCCP
Dr. Subramanian is currently the Section Chief for Specialty Clinics and the Division Chief for Pulmonary/Critical Care and Sleep Medicine at Sutter Gould Medical Foundation, Tracy, California.
He previously was Systems Director at Baylor College of Medicine in Houston and Section Chief at Case Western Reserve University in Cleveland. Dr. Subramanian currently serves as Chair for the CHEST Clinical Pulmonary NetWork and has previously served as Chair of the Practice Operations NetWork. He is a member of the Executive Committee of the Council of NetWorks and the Scientific Program Committee for the CHEST Annual Meeting.
Mary Jo S. Farmer, MD, PhD, FCCP
Dr. Farmer is a pulmonary, critical care, and sleep medicine physician at Baystate Medical Center (Springfield, MA); Assistant Professor of Medicine University at Massachusetts Medical School – Baystate; and adjunct faculty Tufts University School of Medicine. Dr. Farmer serves as director of pulmonary hypertension services for the Pulmonary & Critical Care Division. Pulmonary vascular disease, interprofessional education, clinical trials research, endobronchial ultrasound, and medical student, resident, and fellow education are her major interests. She is a member of the CHEST Interprofessional NetWork and Clinical Pulmonary NetWork.
We’re happy to introduce these new board members whose primary responsibility is the active review each month of potential articles for publication that could have an impact on or be of interest to our health-care professional readership.
Carolyn M. D’Ambrosio, MD, FCCP, is the Program Director for the Harvard-Brigham and Women’s Hospital Fellowship in Pulmonary and Critical Care Medicine and is Associate Professor of Medicine at Harvard Medical School. Most recently, she was awarded the Pillar Award for Educational Program Leadership, the top award for program directors throughout the Mass General Brigham institutions. In addition to teaching and clinical work, Dr. D’Ambrosio has conducted research on sleep and menopause, sleep and breathing in infants, and participated as the sleep medicine expert in two systemic reviews on home sleep apnea testing and fixed vs auto-titrating CPAP. She continues her work in Medical Ethics as a Senior Ethics Consultant at Brigham and Women’s Hospital.
Jonathan (Jona) Ludmir, MD, FCCP
After completing internal medicine/pediatrics, cardiology, and critical care training, Dr. Ludmir joined the Massachusetts General Hospital staff as a cardiac intensivist and noninvasive cardiologist. His clinical focus is in the heart center ICU, the echocardiography lab, as well as in outpatient cardiology. Additionally, he is the lead physician for the Family-Centered Care Initiative, where he focuses on incorporating evidence-based guidelines and leads in the science of family-centered cardiovascular care delivery. Dr. Ludmir’s research focuses on identifying and addressing psychological symptoms in the ICU, optimizing ICU communication, and enhancing delivery of family-centered care.
Abbie Begnaud, MD, FCCP
Dr. Begnaud hails from south Louisiana and reveals that she attended her first CHEST Annual Meeting in 2011 in Hawaii, and she was “instantly hooked.” Clinically, she practices general pulmonology, critical care, and interventional pulmonology and focuses her research on lung cancer screening and health disparities. She has been on faculty at the University of Minnesota since 2013 and is passionate about lung cancer, health equity, and mentoring.
Shyam Subramanian, MD , FCCP
Dr. Subramanian is currently the Section Chief for Specialty Clinics and the Division Chief for Pulmonary/Critical Care and Sleep Medicine at Sutter Gould Medical Foundation, Tracy, California.
He previously was Systems Director at Baylor College of Medicine in Houston and Section Chief at Case Western Reserve University in Cleveland. Dr. Subramanian currently serves as Chair for the CHEST Clinical Pulmonary NetWork and has previously served as Chair of the Practice Operations NetWork. He is a member of the Executive Committee of the Council of NetWorks and the Scientific Program Committee for the CHEST Annual Meeting.
Mary Jo S. Farmer, MD, PhD, FCCP
Dr. Farmer is a pulmonary, critical care, and sleep medicine physician at Baystate Medical Center (Springfield, MA); Assistant Professor of Medicine University at Massachusetts Medical School – Baystate; and adjunct faculty Tufts University School of Medicine. Dr. Farmer serves as director of pulmonary hypertension services for the Pulmonary & Critical Care Division. Pulmonary vascular disease, interprofessional education, clinical trials research, endobronchial ultrasound, and medical student, resident, and fellow education are her major interests. She is a member of the CHEST Interprofessional NetWork and Clinical Pulmonary NetWork.
We’re happy to introduce these new board members whose primary responsibility is the active review each month of potential articles for publication that could have an impact on or be of interest to our health-care professional readership.
Carolyn M. D’Ambrosio, MD, FCCP, is the Program Director for the Harvard-Brigham and Women’s Hospital Fellowship in Pulmonary and Critical Care Medicine and is Associate Professor of Medicine at Harvard Medical School. Most recently, she was awarded the Pillar Award for Educational Program Leadership, the top award for program directors throughout the Mass General Brigham institutions. In addition to teaching and clinical work, Dr. D’Ambrosio has conducted research on sleep and menopause, sleep and breathing in infants, and participated as the sleep medicine expert in two systemic reviews on home sleep apnea testing and fixed vs auto-titrating CPAP. She continues her work in Medical Ethics as a Senior Ethics Consultant at Brigham and Women’s Hospital.
Jonathan (Jona) Ludmir, MD, FCCP
After completing internal medicine/pediatrics, cardiology, and critical care training, Dr. Ludmir joined the Massachusetts General Hospital staff as a cardiac intensivist and noninvasive cardiologist. His clinical focus is in the heart center ICU, the echocardiography lab, as well as in outpatient cardiology. Additionally, he is the lead physician for the Family-Centered Care Initiative, where he focuses on incorporating evidence-based guidelines and leads in the science of family-centered cardiovascular care delivery. Dr. Ludmir’s research focuses on identifying and addressing psychological symptoms in the ICU, optimizing ICU communication, and enhancing delivery of family-centered care.
Abbie Begnaud, MD, FCCP
Dr. Begnaud hails from south Louisiana and reveals that she attended her first CHEST Annual Meeting in 2011 in Hawaii, and she was “instantly hooked.” Clinically, she practices general pulmonology, critical care, and interventional pulmonology and focuses her research on lung cancer screening and health disparities. She has been on faculty at the University of Minnesota since 2013 and is passionate about lung cancer, health equity, and mentoring.
Shyam Subramanian, MD , FCCP
Dr. Subramanian is currently the Section Chief for Specialty Clinics and the Division Chief for Pulmonary/Critical Care and Sleep Medicine at Sutter Gould Medical Foundation, Tracy, California.
He previously was Systems Director at Baylor College of Medicine in Houston and Section Chief at Case Western Reserve University in Cleveland. Dr. Subramanian currently serves as Chair for the CHEST Clinical Pulmonary NetWork and has previously served as Chair of the Practice Operations NetWork. He is a member of the Executive Committee of the Council of NetWorks and the Scientific Program Committee for the CHEST Annual Meeting.
Mary Jo S. Farmer, MD, PhD, FCCP
Dr. Farmer is a pulmonary, critical care, and sleep medicine physician at Baystate Medical Center (Springfield, MA); Assistant Professor of Medicine University at Massachusetts Medical School – Baystate; and adjunct faculty Tufts University School of Medicine. Dr. Farmer serves as director of pulmonary hypertension services for the Pulmonary & Critical Care Division. Pulmonary vascular disease, interprofessional education, clinical trials research, endobronchial ultrasound, and medical student, resident, and fellow education are her major interests. She is a member of the CHEST Interprofessional NetWork and Clinical Pulmonary NetWork.
How the Foundation’s virtual listening tour aims to help patients like James
Constance Baker was juggling the dual stresses of mothering a newborn and raising a teenager when she noticed a skin patch on her father looked discolored. His breathing soon became labored, and the skin on his hands turned calloused. Then he passed out. Initially, doctors thought his problems were cardiovascular.
Since James didn’t have a primary doctor, Constance repeatedly took him to the emergency room to receive care. His frequent visits attracted the attention of a medical intern who ordered tests and asked James to see a specialist. More than half a year later, Constance and James met pulmonologist Dr. Demondes Haynes and learned the cause of James’ troubled breathing. James has a rare disease called scleroderma, which hardens patches of skin and created scarring of his lung tissue. He also had pulmonary hypertension. James needed rapid intervention with a complicated regimen of medication.
At first, James didn’t want to go along with the program, but Dr. Haynes’ attentive and gentle nature changed his mind. “Dr. Haynes always made us comfortable, taking the time to listen and show us his concern. He even explained that we wouldn’t have to worry about paying for anything, which was a huge relief.”
Before Dr. Haynes, James and Constance had never met a doctor who didn’t treat them like a case file. “He actually acknowledged our circumstances, which meant he acknowledged us.”
As a native Mississippian, Dr. Haynes knows the plight of many of his patients. “Not everyone with lung disease can access a pulmonologist, like me, and not everyone can afford appropriate treatment. You have to recognize these disparities in order to build a relationship of trust with your patients.”
James was ready to start treatment with Dr. Haynes’ guidance, but since he couldn’t read, he couldn’t understand how to put the medication together. That’s when Constance had to step up. They worked together to change and clean the tubing to the port by his heart and make his medication. “We leaned on each other a lot during that time, and you know what? We made it through.”
Even though James’ disease can be debilitating at times, and his care can seem completely overwhelming, Constance wouldn’t have it any other way. “It’s always been my father and I, just us two. He’s always taken care of me, and now it’s my turn to take care of him.”
Unfortunately, Constance and James’ story is not unique. So many patients don’t have access to doctors, specialists, and caregivers, and many aren’t empowered enough to take
their medications. These stories don’t get posted on Instagram and they don’t make the evening news. Underprivileged and underserved patients have been left behind – left without a voice.
That’s why the foundation launched its virtual listening tours across America in September. Our tours give patients, caregivers, and physicians the opportunity to raise issues that they believe are impacting health care in their communities.
How can physicians work to understand their patients better? How can patients learn to trust their providers? These are all the questions we aim to answer.
James is doing as well as he is because of his relationship with Dr. Haynes. What can we do with that information? We can listen, we can learn, and we can spread the word.
Read more about the work of the CHEST Foundation in its 2020 Impact Report at chestfoundation.org.
Constance Baker was juggling the dual stresses of mothering a newborn and raising a teenager when she noticed a skin patch on her father looked discolored. His breathing soon became labored, and the skin on his hands turned calloused. Then he passed out. Initially, doctors thought his problems were cardiovascular.
Since James didn’t have a primary doctor, Constance repeatedly took him to the emergency room to receive care. His frequent visits attracted the attention of a medical intern who ordered tests and asked James to see a specialist. More than half a year later, Constance and James met pulmonologist Dr. Demondes Haynes and learned the cause of James’ troubled breathing. James has a rare disease called scleroderma, which hardens patches of skin and created scarring of his lung tissue. He also had pulmonary hypertension. James needed rapid intervention with a complicated regimen of medication.
At first, James didn’t want to go along with the program, but Dr. Haynes’ attentive and gentle nature changed his mind. “Dr. Haynes always made us comfortable, taking the time to listen and show us his concern. He even explained that we wouldn’t have to worry about paying for anything, which was a huge relief.”
Before Dr. Haynes, James and Constance had never met a doctor who didn’t treat them like a case file. “He actually acknowledged our circumstances, which meant he acknowledged us.”
As a native Mississippian, Dr. Haynes knows the plight of many of his patients. “Not everyone with lung disease can access a pulmonologist, like me, and not everyone can afford appropriate treatment. You have to recognize these disparities in order to build a relationship of trust with your patients.”
James was ready to start treatment with Dr. Haynes’ guidance, but since he couldn’t read, he couldn’t understand how to put the medication together. That’s when Constance had to step up. They worked together to change and clean the tubing to the port by his heart and make his medication. “We leaned on each other a lot during that time, and you know what? We made it through.”
Even though James’ disease can be debilitating at times, and his care can seem completely overwhelming, Constance wouldn’t have it any other way. “It’s always been my father and I, just us two. He’s always taken care of me, and now it’s my turn to take care of him.”
Unfortunately, Constance and James’ story is not unique. So many patients don’t have access to doctors, specialists, and caregivers, and many aren’t empowered enough to take
their medications. These stories don’t get posted on Instagram and they don’t make the evening news. Underprivileged and underserved patients have been left behind – left without a voice.
That’s why the foundation launched its virtual listening tours across America in September. Our tours give patients, caregivers, and physicians the opportunity to raise issues that they believe are impacting health care in their communities.
How can physicians work to understand their patients better? How can patients learn to trust their providers? These are all the questions we aim to answer.
James is doing as well as he is because of his relationship with Dr. Haynes. What can we do with that information? We can listen, we can learn, and we can spread the word.
Read more about the work of the CHEST Foundation in its 2020 Impact Report at chestfoundation.org.
Constance Baker was juggling the dual stresses of mothering a newborn and raising a teenager when she noticed a skin patch on her father looked discolored. His breathing soon became labored, and the skin on his hands turned calloused. Then he passed out. Initially, doctors thought his problems were cardiovascular.
Since James didn’t have a primary doctor, Constance repeatedly took him to the emergency room to receive care. His frequent visits attracted the attention of a medical intern who ordered tests and asked James to see a specialist. More than half a year later, Constance and James met pulmonologist Dr. Demondes Haynes and learned the cause of James’ troubled breathing. James has a rare disease called scleroderma, which hardens patches of skin and created scarring of his lung tissue. He also had pulmonary hypertension. James needed rapid intervention with a complicated regimen of medication.
At first, James didn’t want to go along with the program, but Dr. Haynes’ attentive and gentle nature changed his mind. “Dr. Haynes always made us comfortable, taking the time to listen and show us his concern. He even explained that we wouldn’t have to worry about paying for anything, which was a huge relief.”
Before Dr. Haynes, James and Constance had never met a doctor who didn’t treat them like a case file. “He actually acknowledged our circumstances, which meant he acknowledged us.”
As a native Mississippian, Dr. Haynes knows the plight of many of his patients. “Not everyone with lung disease can access a pulmonologist, like me, and not everyone can afford appropriate treatment. You have to recognize these disparities in order to build a relationship of trust with your patients.”
James was ready to start treatment with Dr. Haynes’ guidance, but since he couldn’t read, he couldn’t understand how to put the medication together. That’s when Constance had to step up. They worked together to change and clean the tubing to the port by his heart and make his medication. “We leaned on each other a lot during that time, and you know what? We made it through.”
Even though James’ disease can be debilitating at times, and his care can seem completely overwhelming, Constance wouldn’t have it any other way. “It’s always been my father and I, just us two. He’s always taken care of me, and now it’s my turn to take care of him.”
Unfortunately, Constance and James’ story is not unique. So many patients don’t have access to doctors, specialists, and caregivers, and many aren’t empowered enough to take
their medications. These stories don’t get posted on Instagram and they don’t make the evening news. Underprivileged and underserved patients have been left behind – left without a voice.
That’s why the foundation launched its virtual listening tours across America in September. Our tours give patients, caregivers, and physicians the opportunity to raise issues that they believe are impacting health care in their communities.
How can physicians work to understand their patients better? How can patients learn to trust their providers? These are all the questions we aim to answer.
James is doing as well as he is because of his relationship with Dr. Haynes. What can we do with that information? We can listen, we can learn, and we can spread the word.
Read more about the work of the CHEST Foundation in its 2020 Impact Report at chestfoundation.org.
This month in CHEST
Editor’s picks
Original Research
A behaviour change intervention aimed at increasing physical activity improves clinical control in adults with asthma: a randomised controlled trial. By Dr. C. Carvalho, et al.
Critically ill adults with COVID-19 in New Orleans and care with an evidence-based protocol. By Dr. D. Janz, et al.
Mortality trends of idiopathic pulmonary fibrosis in the United States from 2004 to 2017.By Dr. N. Jeganathan, et al.
United States Pulmonary Hypertension Scientific Registry (USPHSR): Baseline characteristics. By Dr. J. Badlam, et al.
CHEST Review
Pulmonary exacerbations in adults with cystic fibrosis: A grown-up issue in a changing CF landscape. By Dr. G. Stanford, et al.
Computed tomography imaging and comorbidities in chronic obstructive pulmonary disease: Beyond lung cancer screening. By Dr. J. Bon, et al.
How I Do It
The PERT concept: A step-by-step approach to managing PE. By Dr. B. Rivera-Lebron, et al.
Special Feature
A brief overview of the national outbreak of e-cigarette, or vaping, product use-associated lung injury (EVALI) and the primary causes. By Dr. E. Kiernan, et al.
Editor’s picks
Editor’s picks
Original Research
A behaviour change intervention aimed at increasing physical activity improves clinical control in adults with asthma: a randomised controlled trial. By Dr. C. Carvalho, et al.
Critically ill adults with COVID-19 in New Orleans and care with an evidence-based protocol. By Dr. D. Janz, et al.
Mortality trends of idiopathic pulmonary fibrosis in the United States from 2004 to 2017.By Dr. N. Jeganathan, et al.
United States Pulmonary Hypertension Scientific Registry (USPHSR): Baseline characteristics. By Dr. J. Badlam, et al.
CHEST Review
Pulmonary exacerbations in adults with cystic fibrosis: A grown-up issue in a changing CF landscape. By Dr. G. Stanford, et al.
Computed tomography imaging and comorbidities in chronic obstructive pulmonary disease: Beyond lung cancer screening. By Dr. J. Bon, et al.
How I Do It
The PERT concept: A step-by-step approach to managing PE. By Dr. B. Rivera-Lebron, et al.
Special Feature
A brief overview of the national outbreak of e-cigarette, or vaping, product use-associated lung injury (EVALI) and the primary causes. By Dr. E. Kiernan, et al.
Original Research
A behaviour change intervention aimed at increasing physical activity improves clinical control in adults with asthma: a randomised controlled trial. By Dr. C. Carvalho, et al.
Critically ill adults with COVID-19 in New Orleans and care with an evidence-based protocol. By Dr. D. Janz, et al.
Mortality trends of idiopathic pulmonary fibrosis in the United States from 2004 to 2017.By Dr. N. Jeganathan, et al.
United States Pulmonary Hypertension Scientific Registry (USPHSR): Baseline characteristics. By Dr. J. Badlam, et al.
CHEST Review
Pulmonary exacerbations in adults with cystic fibrosis: A grown-up issue in a changing CF landscape. By Dr. G. Stanford, et al.
Computed tomography imaging and comorbidities in chronic obstructive pulmonary disease: Beyond lung cancer screening. By Dr. J. Bon, et al.
How I Do It
The PERT concept: A step-by-step approach to managing PE. By Dr. B. Rivera-Lebron, et al.
Special Feature
A brief overview of the national outbreak of e-cigarette, or vaping, product use-associated lung injury (EVALI) and the primary causes. By Dr. E. Kiernan, et al.
Cardiovascular Medicine and Surgery
Cardiovascular Medicine and Surgery
Use of hepatitis C donors in thoracic organ transplantation: Reportedly associated with increased risk of rejection
Mark Jay Zucker, MD, JD, FCCP
Vice-Chair
Transplanting organs from hepatitis C (HCV) antibody and/or antigen-positive donors is associated with a greater than 8%-90% likelihood that the recipient will acquire the infection. Several studies reported that if HCV conversion happened, the outcomes in both heart and lung recipients were worse, even if treated with interferon/ribavirin (Haji SA, et al J Heart Lung Transplant. 2004;23:277; Wang BY, et al. Ann Thorac Surg. 2010 May;89[5]:1645; Carreno MC, et al. J Heart Lung Transplant. 2001;20(2):224). Thus, despite the shortage of thoracic organ donors and high wait-list mortality, the practice was strongly discouraged.
In 2016, the successful use of a direct-acting antiviral (DAA) for 12 weeks to eliminate HCV in a lung transplant recipient of a seropositive organ was published (Khan B, et al. Am J Transplant. 2017;17:1129). Two years later, the outcomes of seronegative heart (n=8) or lung (n=36) transplant recipients receiving organs from seropositive donors were presented (Woolley AE, et al. N Engl J Med. 2019;380:1606). Forty-two of the patients had viremia within days of the operation. All patients were treated with 4 weeks of a DAA and, of the 35 patients available for 6-month analysis, viral load was undetectable in all. Of concern, however—more cellular rejection requiring treatment was seen in the lung recipients of HCV+ donors compared with recipients of HCV- donors. The difference was not statistically significant.
The largest analysis of the safety of HCV+ donors in HCV- thoracic organ transplant recipients involved 343 heart transplant recipients (Kilic A, et al. J Am Heart Assoc. 2020;9(2):e014495). No differences were noted in outcomes, strokes, need for dialysis, or incidence of treated rejection during the first year. However, the observation regarding rejection was not subsequently confirmed by the NYU team (Gidea CG, et al. J Heart Lung Transplant. 2020;39:1199). Of 22 HCV- recipients of an HCV donor with viremia, the rate of rejection was 64% vs 18% in 28 patients receiving a donor without viremia (through day 180 (P=.001)).
In summary, the ability of DAAs to render 97%-99% of immunosuppressed transplant recipients HCV seronegative has transformed the landscape and HCV viremia in the donor (or recipient) and is no longer an absolute contraindication to transplantation. However, more information is needed as to whether there is an increased incidence of rejection.
Cardiovascular Medicine and Surgery
Use of hepatitis C donors in thoracic organ transplantation: Reportedly associated with increased risk of rejection
Mark Jay Zucker, MD, JD, FCCP
Vice-Chair
Transplanting organs from hepatitis C (HCV) antibody and/or antigen-positive donors is associated with a greater than 8%-90% likelihood that the recipient will acquire the infection. Several studies reported that if HCV conversion happened, the outcomes in both heart and lung recipients were worse, even if treated with interferon/ribavirin (Haji SA, et al J Heart Lung Transplant. 2004;23:277; Wang BY, et al. Ann Thorac Surg. 2010 May;89[5]:1645; Carreno MC, et al. J Heart Lung Transplant. 2001;20(2):224). Thus, despite the shortage of thoracic organ donors and high wait-list mortality, the practice was strongly discouraged.
In 2016, the successful use of a direct-acting antiviral (DAA) for 12 weeks to eliminate HCV in a lung transplant recipient of a seropositive organ was published (Khan B, et al. Am J Transplant. 2017;17:1129). Two years later, the outcomes of seronegative heart (n=8) or lung (n=36) transplant recipients receiving organs from seropositive donors were presented (Woolley AE, et al. N Engl J Med. 2019;380:1606). Forty-two of the patients had viremia within days of the operation. All patients were treated with 4 weeks of a DAA and, of the 35 patients available for 6-month analysis, viral load was undetectable in all. Of concern, however—more cellular rejection requiring treatment was seen in the lung recipients of HCV+ donors compared with recipients of HCV- donors. The difference was not statistically significant.
The largest analysis of the safety of HCV+ donors in HCV- thoracic organ transplant recipients involved 343 heart transplant recipients (Kilic A, et al. J Am Heart Assoc. 2020;9(2):e014495). No differences were noted in outcomes, strokes, need for dialysis, or incidence of treated rejection during the first year. However, the observation regarding rejection was not subsequently confirmed by the NYU team (Gidea CG, et al. J Heart Lung Transplant. 2020;39:1199). Of 22 HCV- recipients of an HCV donor with viremia, the rate of rejection was 64% vs 18% in 28 patients receiving a donor without viremia (through day 180 (P=.001)).
In summary, the ability of DAAs to render 97%-99% of immunosuppressed transplant recipients HCV seronegative has transformed the landscape and HCV viremia in the donor (or recipient) and is no longer an absolute contraindication to transplantation. However, more information is needed as to whether there is an increased incidence of rejection.
Cardiovascular Medicine and Surgery
Use of hepatitis C donors in thoracic organ transplantation: Reportedly associated with increased risk of rejection
Mark Jay Zucker, MD, JD, FCCP
Vice-Chair
Transplanting organs from hepatitis C (HCV) antibody and/or antigen-positive donors is associated with a greater than 8%-90% likelihood that the recipient will acquire the infection. Several studies reported that if HCV conversion happened, the outcomes in both heart and lung recipients were worse, even if treated with interferon/ribavirin (Haji SA, et al J Heart Lung Transplant. 2004;23:277; Wang BY, et al. Ann Thorac Surg. 2010 May;89[5]:1645; Carreno MC, et al. J Heart Lung Transplant. 2001;20(2):224). Thus, despite the shortage of thoracic organ donors and high wait-list mortality, the practice was strongly discouraged.
In 2016, the successful use of a direct-acting antiviral (DAA) for 12 weeks to eliminate HCV in a lung transplant recipient of a seropositive organ was published (Khan B, et al. Am J Transplant. 2017;17:1129). Two years later, the outcomes of seronegative heart (n=8) or lung (n=36) transplant recipients receiving organs from seropositive donors were presented (Woolley AE, et al. N Engl J Med. 2019;380:1606). Forty-two of the patients had viremia within days of the operation. All patients were treated with 4 weeks of a DAA and, of the 35 patients available for 6-month analysis, viral load was undetectable in all. Of concern, however—more cellular rejection requiring treatment was seen in the lung recipients of HCV+ donors compared with recipients of HCV- donors. The difference was not statistically significant.
The largest analysis of the safety of HCV+ donors in HCV- thoracic organ transplant recipients involved 343 heart transplant recipients (Kilic A, et al. J Am Heart Assoc. 2020;9(2):e014495). No differences were noted in outcomes, strokes, need for dialysis, or incidence of treated rejection during the first year. However, the observation regarding rejection was not subsequently confirmed by the NYU team (Gidea CG, et al. J Heart Lung Transplant. 2020;39:1199). Of 22 HCV- recipients of an HCV donor with viremia, the rate of rejection was 64% vs 18% in 28 patients receiving a donor without viremia (through day 180 (P=.001)).
In summary, the ability of DAAs to render 97%-99% of immunosuppressed transplant recipients HCV seronegative has transformed the landscape and HCV viremia in the donor (or recipient) and is no longer an absolute contraindication to transplantation. However, more information is needed as to whether there is an increased incidence of rejection.
Finding meaning in ‘Lean’?
Using systems improvement strategies to support the Quadruple Aim
General background on well-being and burnout
With burnout increasingly recognized as a shared responsibility that requires addressing organizational drivers while supporting individuals to be well,1-4 practical strategies and examples of successful implementation of systems interventions to address burnout will be helpful for service directors to support their staff. The Charter on Physician Well-being, recently developed through collaborative input from multiple organizations, defines guiding principles and key commitments at the societal, organizational, interpersonal, and individual levels and may be a useful framework for organizations that are developing well-being initiatives.5
The charter advocates including physician well-being as a quality improvement metric for health systems, aligned with the concept of the Quadruple Aim of optimizing patient care by enhancing provider experience, promoting high-value care, and improving population health.6 Identifying areas of alignment between the charter’s recommendations and systems improvement strategies that seek to optimize efficiency and reduce waste, such as Lean Management, may help physician leaders to contextualize well-being initiatives more easily within ongoing systems improvement efforts. In this perspective, we provide one division’s experience using the Charter to assess successes and identify additional areas of improvement for well-being initiatives developed using Lean Management methodology.
Past and current state of affairs
In 2011, the division of hospital medicine at Zuckerberg San Francisco General Hospital was established and has seen continual expansion in terms of direct patient care, medical education, and hospital leadership.
In 2015, the division of hospital medicine experienced leadership transitions, faculty attrition, and insufficient recruitment resulting in staffing shortages, service line closure, schedule instability, and ultimately, low morale. A baseline survey conducted using the 2-Item Maslach Burnout Inventory. This survey, which uses one item in the domain of emotional exhaustion and one item in the domain of depersonalization, has shown good correlation with the full Maslach Burnout Inventory.7 At baseline, approximately one-third of the division’s physicians experienced burnout.
In response, a subsequent retreat focused on the three greatest areas of concern identified by the survey: scheduling, faculty development, and well-being.
Like many health systems, the hospital has adopted Lean as its preferred systems-improvement framework. The retreat was structured around the principles of Lean philosophy, and was designed to emulate that of a consolidated Kaizen workshop.
“Kaizen” in Japanese means “change for the better.” A typical Kaizen workshop revolves around rapid problem-solving over the course of 3-5 days, in which a team of people come together to identify and implement significant improvements for a selected process. To this end, the retreat was divided into subgroups for each area of concern. In turn, each subgroup mapped out existing workflows (“value stream”), identified areas of waste and non–value added time, and generated ideas of what an idealized process would be. Next, a root-cause analysis was performed and subsequent interventions (“countermeasures”) developed to address each problem. At the conclusion of the retreat, each subgroup shared a summary of their findings with the larger group.
Moving forward, this information served as a guiding framework for service and division leadership to run small tests of change. We enacted a series of countermeasures over the course of several years, and multiple cycles of improvement work addressed the three areas of concern. We developed an A3 report (a Lean project management tool that incorporates the plan-do-study-act cycle, organizes strategic efforts, and tracks progress on a single page) to summarize and present these initiatives to the Performance Improvement and Patient Safety Committee of the hospital executive leadership team. This structure illustrated alignment with the hospital’s core values (“true north”) of “developing people” and “care experience.”
In 2018, interval surveys demonstrated a gradual reduction of burnout to approximately one-fifth of division physicians as measured by the 2-item Maslach Burnout Inventory.
Initiatives in faculty well-being
The Charter of Physician Well-being outlines a framework to promote well-being among doctors by maximizing a sense of fulfillment and minimizing the harms of burnout. It shares this responsibility among societal, organizational, and interpersonal and individual commitments.5
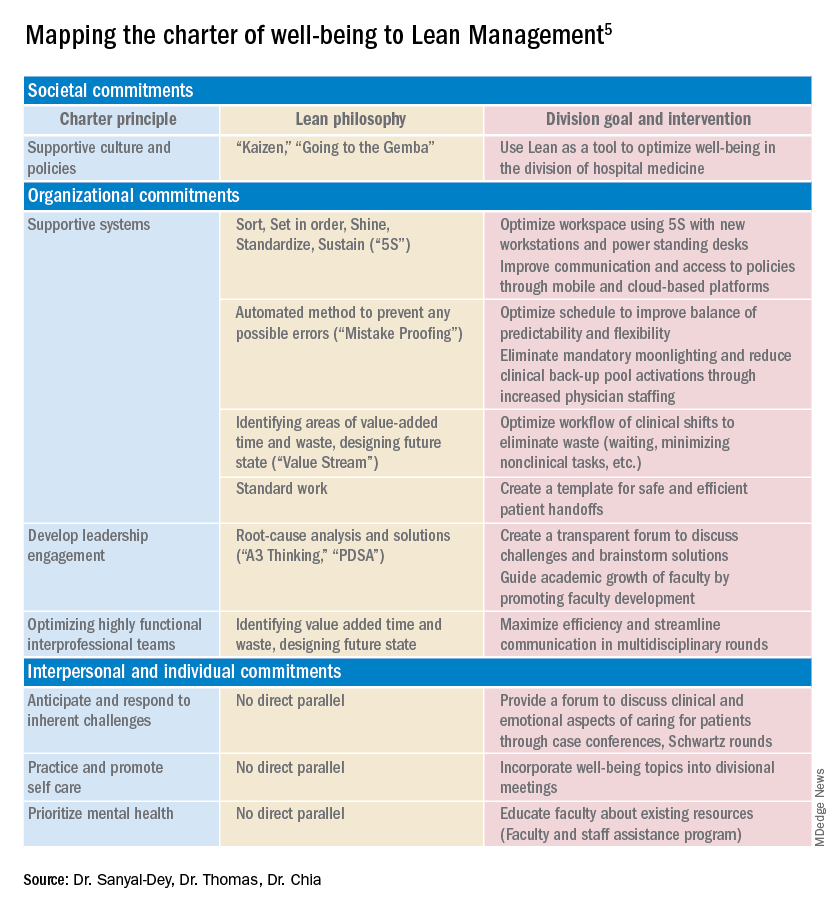
As illustrated above, we used principles of Lean Management to prospectively create initiatives to improve well-being in our division. Lean in health care is designed to optimize primarily the patient experience; its implementation has subsequently demonstrated mixed provider and staff experiences,8,9 and many providers are skeptical of Lean’s potential to improve their own well-being. If, however, Lean is aligned with best practice frameworks for well-being such as those outline in the charter, it may also help to meet the Quadruple Aim of optimizing both provider well-being and patient experience. To further test this hypothesis, we retrospectively categorized our Lean-based interventions into the commitments described by the charter to identify areas of alignment and gaps that were not initially addressed using Lean Management (Table).
Organizational commitments5Supportive systems
We optimized scheduling and enhanced physician staffing by budgeting for a physician staffing buffer each academic year in order to minimize mandatory moonlighting and jeopardy pool activations that result from operating on a thin staffing margin when expected personal leave and reductions in clinical effort occur. Furthermore, we revised scheduling principles to balance patient continuity and individual time off requests while setting limits on the maximum duration of clinical stretches and instituting mandatory minimum time off between them.
Leadership engagement
We initiated monthly operations meetings as a forum to discuss challenges, brainstorm solutions, and message new initiatives with group input. For example, as a result of these meetings, we designed and implemented an additional service line to address the high census, revised the distribution of new patient admissions to level-load clinical shifts, and established a maximum number of weekends worked per month and year. This approach aligns with recommendations to use participatory leadership strategies to enhance physician well-being.10 Engaging both executive level and service level management to focus on burnout and other related well-being metrics is necessary for sustaining such work.
Interprofessional teamwork
We revised multidisciplinary rounds with social work, utilization management, and physical therapy to maximize efficiency and streamline communication by developing standard approaches for each patient presentation.
Interpersonal and individual commitments5Address emotional challenges of physician work
Although these commitments did not have a direct corollary with Lean philosophy, some of these needs were identified by our physician group at our annual retreats. As a result, we initiated a monthly faculty-led noon conference series focused on the clinical challenges of caring for vulnerable populations, a particular source of distress in our practice setting, and revised the division schedule to encourage attendance at the hospital’s Schwartz rounds.
Mental health and self-care
We organized focus groups and faculty development sessions on provider well-being and burnout and dealing with challenging patients and invited the Faculty and Staff Assistance Program, our institution’s mental health service provider, to our weekly division meeting.
Future directions
After using Lean Management as an approach to prospectively improve physician well-being, we were able to use the Charter on Physician Well-being retrospectively as a “checklist” to identify additional gaps for targeted intervention to ensure all commitments are sufficiently addressed.
Overall, we found that, not surprisingly, Lean Management aligned best with the organizational commitments in the charter. Reviewing the organizational commitments, we found our biggest remaining challenges are in building supportive systems, namely ensuring sustainable workloads, offloading and delegating nonphysician tasks, and minimizing the burden of documentation and administration.
Reviewing the societal commitments helped us to identify opportunities for future directions that we may not have otherwise considered. As a safety-net institution, we benefit from a strong sense of mission and shared values within our hospital and division. However, we recognize the need to continue to be vigilant to ensure that our physicians perceive that their own values are aligned with the division’s stated mission. Devoting a Kaizen-style retreat to well-being likely helped, and allocating divisional resources to a well-being committee indirectly helped, to foster a culture of well-being; however, we could more deliberately identify local policies that may benefit from advocacy or revision. Although our faculty identified interventions to improve interpersonal and individual drivers of well-being, these charter commitments did not have direct parallels in Lean philosophy, and organizations may need to deliberately seek to address these commitments outside of a Lean approach. Specifically, by reviewing the charter, we identified opportunities to provide additional resources for peer support and protected time for mental health care and self-care.
Conclusion
Lean Management can be an effective strategy to address many of the organizational commitments outlined in the Charter on Physician Well-being. This approach may be particularly effective for solving local challenges with systems and workflows. Those who use Lean as a primary method to approach systems improvement in support of the Quadruple Aim may need to use additional strategies to address societal and interpersonal and individual commitments outlined in the charter.
Dr. Sanyal-Dey is visiting associate clinical professor of medicine at Zuckerberg San Francisco General Hospital and director of client services, LeanTaaS. Dr. Thomas is associate clinical professor of medicine at Zuckerberg San Francisco General Hospital. Dr. Chia is associate professor of clinical medicine at Zuckerberg San Francisco General Hospital.
References
1. West CP et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet. 2016;388(10057):2272-81.
2. Shanafelt TD, Noseworthy JH. Executive leadership and physician: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-46.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Shanafelt T et al. Building a program on well-being: Key design considerations to meet the unique needs of each organization. Acad Med. 2019 Feb;94(2):156-161.
5. Thomas LR et al. Charter on physician well-being. JAMA. 2018;319(15):1541-42.
6. Bodenheimer T, Sinsky C. From triple to quadruple aim: Care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-6.
7. West CP et al. Concurrent Validity of Single-Item Measures of Emotional Exhaustion and Depersonalization in Burnout Assessment. J Gen Intern Med. 2012;27(11):1445-52.
8. Hung DY et al. Experiences of primary care physicians and staff following lean workflow redesign. BMC Health Serv Res. 2018 Apr 10;18(1):274.
9. Zibrowski E et al. Easier and faster is not always better: Grounded theory of the impact of large-scale system transformation on the clinical work of emergency medicine nurses and physicians. JMIR Hum Factors. 2018. doi: 10.2196/11013.
10. Shanafelt TD et al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015;90(4):432-40.
Using systems improvement strategies to support the Quadruple Aim
Using systems improvement strategies to support the Quadruple Aim
General background on well-being and burnout
With burnout increasingly recognized as a shared responsibility that requires addressing organizational drivers while supporting individuals to be well,1-4 practical strategies and examples of successful implementation of systems interventions to address burnout will be helpful for service directors to support their staff. The Charter on Physician Well-being, recently developed through collaborative input from multiple organizations, defines guiding principles and key commitments at the societal, organizational, interpersonal, and individual levels and may be a useful framework for organizations that are developing well-being initiatives.5
The charter advocates including physician well-being as a quality improvement metric for health systems, aligned with the concept of the Quadruple Aim of optimizing patient care by enhancing provider experience, promoting high-value care, and improving population health.6 Identifying areas of alignment between the charter’s recommendations and systems improvement strategies that seek to optimize efficiency and reduce waste, such as Lean Management, may help physician leaders to contextualize well-being initiatives more easily within ongoing systems improvement efforts. In this perspective, we provide one division’s experience using the Charter to assess successes and identify additional areas of improvement for well-being initiatives developed using Lean Management methodology.
Past and current state of affairs
In 2011, the division of hospital medicine at Zuckerberg San Francisco General Hospital was established and has seen continual expansion in terms of direct patient care, medical education, and hospital leadership.
In 2015, the division of hospital medicine experienced leadership transitions, faculty attrition, and insufficient recruitment resulting in staffing shortages, service line closure, schedule instability, and ultimately, low morale. A baseline survey conducted using the 2-Item Maslach Burnout Inventory. This survey, which uses one item in the domain of emotional exhaustion and one item in the domain of depersonalization, has shown good correlation with the full Maslach Burnout Inventory.7 At baseline, approximately one-third of the division’s physicians experienced burnout.
In response, a subsequent retreat focused on the three greatest areas of concern identified by the survey: scheduling, faculty development, and well-being.
Like many health systems, the hospital has adopted Lean as its preferred systems-improvement framework. The retreat was structured around the principles of Lean philosophy, and was designed to emulate that of a consolidated Kaizen workshop.
“Kaizen” in Japanese means “change for the better.” A typical Kaizen workshop revolves around rapid problem-solving over the course of 3-5 days, in which a team of people come together to identify and implement significant improvements for a selected process. To this end, the retreat was divided into subgroups for each area of concern. In turn, each subgroup mapped out existing workflows (“value stream”), identified areas of waste and non–value added time, and generated ideas of what an idealized process would be. Next, a root-cause analysis was performed and subsequent interventions (“countermeasures”) developed to address each problem. At the conclusion of the retreat, each subgroup shared a summary of their findings with the larger group.
Moving forward, this information served as a guiding framework for service and division leadership to run small tests of change. We enacted a series of countermeasures over the course of several years, and multiple cycles of improvement work addressed the three areas of concern. We developed an A3 report (a Lean project management tool that incorporates the plan-do-study-act cycle, organizes strategic efforts, and tracks progress on a single page) to summarize and present these initiatives to the Performance Improvement and Patient Safety Committee of the hospital executive leadership team. This structure illustrated alignment with the hospital’s core values (“true north”) of “developing people” and “care experience.”
In 2018, interval surveys demonstrated a gradual reduction of burnout to approximately one-fifth of division physicians as measured by the 2-item Maslach Burnout Inventory.
Initiatives in faculty well-being
The Charter of Physician Well-being outlines a framework to promote well-being among doctors by maximizing a sense of fulfillment and minimizing the harms of burnout. It shares this responsibility among societal, organizational, and interpersonal and individual commitments.5

As illustrated above, we used principles of Lean Management to prospectively create initiatives to improve well-being in our division. Lean in health care is designed to optimize primarily the patient experience; its implementation has subsequently demonstrated mixed provider and staff experiences,8,9 and many providers are skeptical of Lean’s potential to improve their own well-being. If, however, Lean is aligned with best practice frameworks for well-being such as those outline in the charter, it may also help to meet the Quadruple Aim of optimizing both provider well-being and patient experience. To further test this hypothesis, we retrospectively categorized our Lean-based interventions into the commitments described by the charter to identify areas of alignment and gaps that were not initially addressed using Lean Management (Table).
Organizational commitments5Supportive systems
We optimized scheduling and enhanced physician staffing by budgeting for a physician staffing buffer each academic year in order to minimize mandatory moonlighting and jeopardy pool activations that result from operating on a thin staffing margin when expected personal leave and reductions in clinical effort occur. Furthermore, we revised scheduling principles to balance patient continuity and individual time off requests while setting limits on the maximum duration of clinical stretches and instituting mandatory minimum time off between them.
Leadership engagement
We initiated monthly operations meetings as a forum to discuss challenges, brainstorm solutions, and message new initiatives with group input. For example, as a result of these meetings, we designed and implemented an additional service line to address the high census, revised the distribution of new patient admissions to level-load clinical shifts, and established a maximum number of weekends worked per month and year. This approach aligns with recommendations to use participatory leadership strategies to enhance physician well-being.10 Engaging both executive level and service level management to focus on burnout and other related well-being metrics is necessary for sustaining such work.
Interprofessional teamwork
We revised multidisciplinary rounds with social work, utilization management, and physical therapy to maximize efficiency and streamline communication by developing standard approaches for each patient presentation.
Interpersonal and individual commitments5Address emotional challenges of physician work
Although these commitments did not have a direct corollary with Lean philosophy, some of these needs were identified by our physician group at our annual retreats. As a result, we initiated a monthly faculty-led noon conference series focused on the clinical challenges of caring for vulnerable populations, a particular source of distress in our practice setting, and revised the division schedule to encourage attendance at the hospital’s Schwartz rounds.
Mental health and self-care
We organized focus groups and faculty development sessions on provider well-being and burnout and dealing with challenging patients and invited the Faculty and Staff Assistance Program, our institution’s mental health service provider, to our weekly division meeting.
Future directions
After using Lean Management as an approach to prospectively improve physician well-being, we were able to use the Charter on Physician Well-being retrospectively as a “checklist” to identify additional gaps for targeted intervention to ensure all commitments are sufficiently addressed.
Overall, we found that, not surprisingly, Lean Management aligned best with the organizational commitments in the charter. Reviewing the organizational commitments, we found our biggest remaining challenges are in building supportive systems, namely ensuring sustainable workloads, offloading and delegating nonphysician tasks, and minimizing the burden of documentation and administration.
Reviewing the societal commitments helped us to identify opportunities for future directions that we may not have otherwise considered. As a safety-net institution, we benefit from a strong sense of mission and shared values within our hospital and division. However, we recognize the need to continue to be vigilant to ensure that our physicians perceive that their own values are aligned with the division’s stated mission. Devoting a Kaizen-style retreat to well-being likely helped, and allocating divisional resources to a well-being committee indirectly helped, to foster a culture of well-being; however, we could more deliberately identify local policies that may benefit from advocacy or revision. Although our faculty identified interventions to improve interpersonal and individual drivers of well-being, these charter commitments did not have direct parallels in Lean philosophy, and organizations may need to deliberately seek to address these commitments outside of a Lean approach. Specifically, by reviewing the charter, we identified opportunities to provide additional resources for peer support and protected time for mental health care and self-care.
Conclusion
Lean Management can be an effective strategy to address many of the organizational commitments outlined in the Charter on Physician Well-being. This approach may be particularly effective for solving local challenges with systems and workflows. Those who use Lean as a primary method to approach systems improvement in support of the Quadruple Aim may need to use additional strategies to address societal and interpersonal and individual commitments outlined in the charter.
Dr. Sanyal-Dey is visiting associate clinical professor of medicine at Zuckerberg San Francisco General Hospital and director of client services, LeanTaaS. Dr. Thomas is associate clinical professor of medicine at Zuckerberg San Francisco General Hospital. Dr. Chia is associate professor of clinical medicine at Zuckerberg San Francisco General Hospital.
References
1. West CP et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet. 2016;388(10057):2272-81.
2. Shanafelt TD, Noseworthy JH. Executive leadership and physician: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-46.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Shanafelt T et al. Building a program on well-being: Key design considerations to meet the unique needs of each organization. Acad Med. 2019 Feb;94(2):156-161.
5. Thomas LR et al. Charter on physician well-being. JAMA. 2018;319(15):1541-42.
6. Bodenheimer T, Sinsky C. From triple to quadruple aim: Care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-6.
7. West CP et al. Concurrent Validity of Single-Item Measures of Emotional Exhaustion and Depersonalization in Burnout Assessment. J Gen Intern Med. 2012;27(11):1445-52.
8. Hung DY et al. Experiences of primary care physicians and staff following lean workflow redesign. BMC Health Serv Res. 2018 Apr 10;18(1):274.
9. Zibrowski E et al. Easier and faster is not always better: Grounded theory of the impact of large-scale system transformation on the clinical work of emergency medicine nurses and physicians. JMIR Hum Factors. 2018. doi: 10.2196/11013.
10. Shanafelt TD et al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015;90(4):432-40.
General background on well-being and burnout
With burnout increasingly recognized as a shared responsibility that requires addressing organizational drivers while supporting individuals to be well,1-4 practical strategies and examples of successful implementation of systems interventions to address burnout will be helpful for service directors to support their staff. The Charter on Physician Well-being, recently developed through collaborative input from multiple organizations, defines guiding principles and key commitments at the societal, organizational, interpersonal, and individual levels and may be a useful framework for organizations that are developing well-being initiatives.5
The charter advocates including physician well-being as a quality improvement metric for health systems, aligned with the concept of the Quadruple Aim of optimizing patient care by enhancing provider experience, promoting high-value care, and improving population health.6 Identifying areas of alignment between the charter’s recommendations and systems improvement strategies that seek to optimize efficiency and reduce waste, such as Lean Management, may help physician leaders to contextualize well-being initiatives more easily within ongoing systems improvement efforts. In this perspective, we provide one division’s experience using the Charter to assess successes and identify additional areas of improvement for well-being initiatives developed using Lean Management methodology.
Past and current state of affairs
In 2011, the division of hospital medicine at Zuckerberg San Francisco General Hospital was established and has seen continual expansion in terms of direct patient care, medical education, and hospital leadership.
In 2015, the division of hospital medicine experienced leadership transitions, faculty attrition, and insufficient recruitment resulting in staffing shortages, service line closure, schedule instability, and ultimately, low morale. A baseline survey conducted using the 2-Item Maslach Burnout Inventory. This survey, which uses one item in the domain of emotional exhaustion and one item in the domain of depersonalization, has shown good correlation with the full Maslach Burnout Inventory.7 At baseline, approximately one-third of the division’s physicians experienced burnout.
In response, a subsequent retreat focused on the three greatest areas of concern identified by the survey: scheduling, faculty development, and well-being.
Like many health systems, the hospital has adopted Lean as its preferred systems-improvement framework. The retreat was structured around the principles of Lean philosophy, and was designed to emulate that of a consolidated Kaizen workshop.
“Kaizen” in Japanese means “change for the better.” A typical Kaizen workshop revolves around rapid problem-solving over the course of 3-5 days, in which a team of people come together to identify and implement significant improvements for a selected process. To this end, the retreat was divided into subgroups for each area of concern. In turn, each subgroup mapped out existing workflows (“value stream”), identified areas of waste and non–value added time, and generated ideas of what an idealized process would be. Next, a root-cause analysis was performed and subsequent interventions (“countermeasures”) developed to address each problem. At the conclusion of the retreat, each subgroup shared a summary of their findings with the larger group.
Moving forward, this information served as a guiding framework for service and division leadership to run small tests of change. We enacted a series of countermeasures over the course of several years, and multiple cycles of improvement work addressed the three areas of concern. We developed an A3 report (a Lean project management tool that incorporates the plan-do-study-act cycle, organizes strategic efforts, and tracks progress on a single page) to summarize and present these initiatives to the Performance Improvement and Patient Safety Committee of the hospital executive leadership team. This structure illustrated alignment with the hospital’s core values (“true north”) of “developing people” and “care experience.”
In 2018, interval surveys demonstrated a gradual reduction of burnout to approximately one-fifth of division physicians as measured by the 2-item Maslach Burnout Inventory.
Initiatives in faculty well-being
The Charter of Physician Well-being outlines a framework to promote well-being among doctors by maximizing a sense of fulfillment and minimizing the harms of burnout. It shares this responsibility among societal, organizational, and interpersonal and individual commitments.5

As illustrated above, we used principles of Lean Management to prospectively create initiatives to improve well-being in our division. Lean in health care is designed to optimize primarily the patient experience; its implementation has subsequently demonstrated mixed provider and staff experiences,8,9 and many providers are skeptical of Lean’s potential to improve their own well-being. If, however, Lean is aligned with best practice frameworks for well-being such as those outline in the charter, it may also help to meet the Quadruple Aim of optimizing both provider well-being and patient experience. To further test this hypothesis, we retrospectively categorized our Lean-based interventions into the commitments described by the charter to identify areas of alignment and gaps that were not initially addressed using Lean Management (Table).
Organizational commitments5Supportive systems
We optimized scheduling and enhanced physician staffing by budgeting for a physician staffing buffer each academic year in order to minimize mandatory moonlighting and jeopardy pool activations that result from operating on a thin staffing margin when expected personal leave and reductions in clinical effort occur. Furthermore, we revised scheduling principles to balance patient continuity and individual time off requests while setting limits on the maximum duration of clinical stretches and instituting mandatory minimum time off between them.
Leadership engagement
We initiated monthly operations meetings as a forum to discuss challenges, brainstorm solutions, and message new initiatives with group input. For example, as a result of these meetings, we designed and implemented an additional service line to address the high census, revised the distribution of new patient admissions to level-load clinical shifts, and established a maximum number of weekends worked per month and year. This approach aligns with recommendations to use participatory leadership strategies to enhance physician well-being.10 Engaging both executive level and service level management to focus on burnout and other related well-being metrics is necessary for sustaining such work.
Interprofessional teamwork
We revised multidisciplinary rounds with social work, utilization management, and physical therapy to maximize efficiency and streamline communication by developing standard approaches for each patient presentation.
Interpersonal and individual commitments5Address emotional challenges of physician work
Although these commitments did not have a direct corollary with Lean philosophy, some of these needs were identified by our physician group at our annual retreats. As a result, we initiated a monthly faculty-led noon conference series focused on the clinical challenges of caring for vulnerable populations, a particular source of distress in our practice setting, and revised the division schedule to encourage attendance at the hospital’s Schwartz rounds.
Mental health and self-care
We organized focus groups and faculty development sessions on provider well-being and burnout and dealing with challenging patients and invited the Faculty and Staff Assistance Program, our institution’s mental health service provider, to our weekly division meeting.
Future directions
After using Lean Management as an approach to prospectively improve physician well-being, we were able to use the Charter on Physician Well-being retrospectively as a “checklist” to identify additional gaps for targeted intervention to ensure all commitments are sufficiently addressed.
Overall, we found that, not surprisingly, Lean Management aligned best with the organizational commitments in the charter. Reviewing the organizational commitments, we found our biggest remaining challenges are in building supportive systems, namely ensuring sustainable workloads, offloading and delegating nonphysician tasks, and minimizing the burden of documentation and administration.
Reviewing the societal commitments helped us to identify opportunities for future directions that we may not have otherwise considered. As a safety-net institution, we benefit from a strong sense of mission and shared values within our hospital and division. However, we recognize the need to continue to be vigilant to ensure that our physicians perceive that their own values are aligned with the division’s stated mission. Devoting a Kaizen-style retreat to well-being likely helped, and allocating divisional resources to a well-being committee indirectly helped, to foster a culture of well-being; however, we could more deliberately identify local policies that may benefit from advocacy or revision. Although our faculty identified interventions to improve interpersonal and individual drivers of well-being, these charter commitments did not have direct parallels in Lean philosophy, and organizations may need to deliberately seek to address these commitments outside of a Lean approach. Specifically, by reviewing the charter, we identified opportunities to provide additional resources for peer support and protected time for mental health care and self-care.
Conclusion
Lean Management can be an effective strategy to address many of the organizational commitments outlined in the Charter on Physician Well-being. This approach may be particularly effective for solving local challenges with systems and workflows. Those who use Lean as a primary method to approach systems improvement in support of the Quadruple Aim may need to use additional strategies to address societal and interpersonal and individual commitments outlined in the charter.
Dr. Sanyal-Dey is visiting associate clinical professor of medicine at Zuckerberg San Francisco General Hospital and director of client services, LeanTaaS. Dr. Thomas is associate clinical professor of medicine at Zuckerberg San Francisco General Hospital. Dr. Chia is associate professor of clinical medicine at Zuckerberg San Francisco General Hospital.
References
1. West CP et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet. 2016;388(10057):2272-81.
2. Shanafelt TD, Noseworthy JH. Executive leadership and physician: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-46.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Shanafelt T et al. Building a program on well-being: Key design considerations to meet the unique needs of each organization. Acad Med. 2019 Feb;94(2):156-161.
5. Thomas LR et al. Charter on physician well-being. JAMA. 2018;319(15):1541-42.
6. Bodenheimer T, Sinsky C. From triple to quadruple aim: Care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-6.
7. West CP et al. Concurrent Validity of Single-Item Measures of Emotional Exhaustion and Depersonalization in Burnout Assessment. J Gen Intern Med. 2012;27(11):1445-52.
8. Hung DY et al. Experiences of primary care physicians and staff following lean workflow redesign. BMC Health Serv Res. 2018 Apr 10;18(1):274.
9. Zibrowski E et al. Easier and faster is not always better: Grounded theory of the impact of large-scale system transformation on the clinical work of emergency medicine nurses and physicians. JMIR Hum Factors. 2018. doi: 10.2196/11013.
10. Shanafelt TD et al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015;90(4):432-40.
Case study: Maternal cervical cancer linked to neonate lung cancer
That’s the conclusion of two ground-breaking cases from Japan in which investigators describe lung cancer in two boys that “probably developed” from their respective mothers via vaginal transmission during birth.
“Transmission of maternal cancer to offspring is extremely rare and is estimated to occur in approximately 1 infant per every 500,000 mothers with cancer,” wrote Ayumu Arakawa, MD, of the National Cancer Center Hospital in Japan, and colleagues, in a paper published Jan. 7 in The New England Journal of Medicine.
Previous cases, of which only 18 have been recorded, have been presumed to occur via transplacental transmission, they said.
In the two new cases, genetic analyses and other evidence suggest that both boys’ lung cancers developed after aspirating uterine cervical cancer tumor cells into their lungs during passage through the birth canal.
Tragically, both mothers, each of whom was diagnosed with cervical cancer after the births, died while their boys were still infants.
“Most of the maternal-to-infant cases reported have been leukemia or melanoma,” said Mel Greaves, PhD, of the Institute of Cancer Research, London, who was asked for comment. In 2009, Dr. Greaves and colleagues published a case study of maternal-to-infant cancer transmission (presumably via the placenta). “It attracted an enormous amount of publicity and no doubt some alarm,” he said in an interview. He emphasized that the phenomenon is “incredibly rare.”
Dr. Greaves explains why such transmission is so rare. “We suspect that cancer cells do transit from mum to unborn child more often, but the foreign (aka paternal) antigens (HLA) on the tumor cells prompt immunological rejection. The extremely rare cases of successful transmission probably do depend on the fortuitous loss of paternal HLA.”
Advances in genetic technology may allow such cases, which have been recorded since 1950, to be rapidly identified now, he said.
“Where there is an adult-type cancer in an infant or child whose mother carried cancer when pregnant, then whole-genome sequencing should quickly tell if the infant’s tumor was of maternal origin,” Dr. Greaves explained.
“I think we will be seeing more reports like this in the future, now that this phenomenon has been described and next-generation sequencing is more readily available,” added Mae Zakhour, MD, of the University of California, Los Angeles, Jonsson Comprehensive Cancer Center, when asked for comment.
In the case of the Japanese boys, both cases were discovered incidentally during an analysis of the results of routine next-generation sequencing testing in a prospective gene-profiling trial in cancer patients, known as TOP-GEAR.
How do the investigators know that the spread happened vaginally and not via the placenta?
They explained that, in other cases of mother-to-fetus transmission, the offspring presented with multiple metastases in the brain, bones, liver, lungs, and soft tissues, which were “consistent with presumed hematogenous spread from the placenta.” However, in the two boys, tumors were observed only in the lungs and were localized along the bronchi.
That peribronchial pattern of tumor growth “suggested that the tumors arose from mother-to-infant vaginal transmission through aspiration of tumor-contaminated vaginal fluids during birth.”
In addition, the tumors in both boys lacked the Y chromosome and shared multiple somatic mutations, an HPV genome, and SNP alleles with tumors from the mothers.
“The identical molecular profiles of maternal and pediatric tumors demonstrated by next-generation sequencing, as well as the location of the tumors in the children, provides strong evidence for cancer transmission during delivery,” Dr. Zakhour summarized.
C-section question
The first of the cases reported by the Japanese team was a toddler (23 months) who presented to a local hospital with a 2-week history of a productive cough. Computed tomography revealed multiple masses scattered along the bronchi in both lungs, and a biopsy revealed neuroendocrine carcinoma of the lung.
Notably, the mother’s cervical cancer was not diagnosed during her pregnancy. A cervical cytologic test performed in the mother 7 months before the birth was negative. The infant was delivered transvaginally at 39 weeks of gestation.
It was only 3 months after the birth that the 35-year-old mother received a diagnosis of squamous cell carcinoma of the cervix. She then underwent radical hysterectomy with pelvic lymphadenectomy, followed by chemotherapy.
Had it been known that she had cervical cancer, she may have been advised to have a cesarean section.
The study authors propose, on the basis of their paper, that all women with cervical cancer should have a cesarean section.
But a U.S. expert questioned this, and said the situation is “a bit nuanced.”
William Grobman, MD, of Northwestern University in Chicago, said the current standard recommendation for many pregnant women known to have cervical cancer is to have a cesarean section and that “the strength of the recommendation is dependent on factors such as stage and size.”
However, in an interview, he added that “it may be premature to make a blanket recommendation for all people based on two reports without any idea of the frequency of this event, and with such uncertainty, it seems that disclosure of all information and shared decision-making would be a key approach.”
In this case report, the authors also noted that the cancer found in the toddler looked similar to the cancer in the mother.
“Histologic similarities between the tumor samples from the mother and child prompted us to compare the results of their next-generation sequencing tests,” they said.
The result? “The comparison of the gene profiles in the samples of tumor and normal tissue confirmed that transmission of maternal tumor to the child had occurred.”
The lung cancer in the toddler progressed despite two chemotherapy regimens, so he was enrolled in a clinical trial of nivolumab therapy. He had a response that continued for 7 months, with no appearance of new lesions. Lobectomy was performed to resect a single remaining nodule. The boy had no evidence of disease recurrence at 12 months after lobectomy.
His mother was also enrolled in a nivolumab trial, but her cervical cancer had spread, and she died 5 months after disease progression.
Second case
In the second reported case, a 6-year-old boy presented to a local hospital with chest pain on the left side. Computed tomography revealed a mass in the left lung, and mucinous adenocarcinoma was eventually diagnosed.
In this case, the mother had a cervical polypoid tumor detected during pregnancy. But, as in the other case, cervical cytologic analysis was negative. Because the tumor was stable without any intervention, the mother delivered the boy vaginally at 38 weeks of gestation.
However, after the delivery, biopsy of the cervical lesion revealed adenocarcinoma. The mother underwent radical hysterectomy and bilateral salpingo-oophorectomy 3 months after delivery. She died of the disease 2 years after the surgery.
The boy received chemotherapy and had a partial response, with a reduction in levels of the tumor marker CA19-9 to normal levels. But 3 months later, the disease recurred in the left lung. After more chemotherapy, he underwent total left pneumonectomy and was subsequently free of disease.
The study authors said that they did not suspect maternal transmission of the cancer when her child received a diagnosis at 6 years of age. They explained that metastatic cervical cancer is typically a fast-growing tumor and the slow growth in the child seemed inconsistent with the idea that the cancer had been transmitted to him.
However, the pathology exam showed that the boy had mucinous adenocarcinoma, “which is an unusual morphologic finding for a primary lung tumor, but it was similar to the uterine cervical tumor in the mother,” the authors reported.
Samples of the cervical tumor from the mother and from the lung tumor of the child were submitted for next-generation sequencing tests and, said the authors, indicated mother-to-infant transmission.
The study was supported by grants from the Japan Agency for Medical Research and Development; the National Cancer Center Research and Development Fund; and the Ministry of Education, Culture, Sports, Science and Technology; and funding from Ono Pharmaceutical.
A version of this article first appeared on Medscape.com.
That’s the conclusion of two ground-breaking cases from Japan in which investigators describe lung cancer in two boys that “probably developed” from their respective mothers via vaginal transmission during birth.
“Transmission of maternal cancer to offspring is extremely rare and is estimated to occur in approximately 1 infant per every 500,000 mothers with cancer,” wrote Ayumu Arakawa, MD, of the National Cancer Center Hospital in Japan, and colleagues, in a paper published Jan. 7 in The New England Journal of Medicine.
Previous cases, of which only 18 have been recorded, have been presumed to occur via transplacental transmission, they said.
In the two new cases, genetic analyses and other evidence suggest that both boys’ lung cancers developed after aspirating uterine cervical cancer tumor cells into their lungs during passage through the birth canal.
Tragically, both mothers, each of whom was diagnosed with cervical cancer after the births, died while their boys were still infants.
“Most of the maternal-to-infant cases reported have been leukemia or melanoma,” said Mel Greaves, PhD, of the Institute of Cancer Research, London, who was asked for comment. In 2009, Dr. Greaves and colleagues published a case study of maternal-to-infant cancer transmission (presumably via the placenta). “It attracted an enormous amount of publicity and no doubt some alarm,” he said in an interview. He emphasized that the phenomenon is “incredibly rare.”
Dr. Greaves explains why such transmission is so rare. “We suspect that cancer cells do transit from mum to unborn child more often, but the foreign (aka paternal) antigens (HLA) on the tumor cells prompt immunological rejection. The extremely rare cases of successful transmission probably do depend on the fortuitous loss of paternal HLA.”
Advances in genetic technology may allow such cases, which have been recorded since 1950, to be rapidly identified now, he said.
“Where there is an adult-type cancer in an infant or child whose mother carried cancer when pregnant, then whole-genome sequencing should quickly tell if the infant’s tumor was of maternal origin,” Dr. Greaves explained.
“I think we will be seeing more reports like this in the future, now that this phenomenon has been described and next-generation sequencing is more readily available,” added Mae Zakhour, MD, of the University of California, Los Angeles, Jonsson Comprehensive Cancer Center, when asked for comment.
In the case of the Japanese boys, both cases were discovered incidentally during an analysis of the results of routine next-generation sequencing testing in a prospective gene-profiling trial in cancer patients, known as TOP-GEAR.
How do the investigators know that the spread happened vaginally and not via the placenta?
They explained that, in other cases of mother-to-fetus transmission, the offspring presented with multiple metastases in the brain, bones, liver, lungs, and soft tissues, which were “consistent with presumed hematogenous spread from the placenta.” However, in the two boys, tumors were observed only in the lungs and were localized along the bronchi.
That peribronchial pattern of tumor growth “suggested that the tumors arose from mother-to-infant vaginal transmission through aspiration of tumor-contaminated vaginal fluids during birth.”
In addition, the tumors in both boys lacked the Y chromosome and shared multiple somatic mutations, an HPV genome, and SNP alleles with tumors from the mothers.
“The identical molecular profiles of maternal and pediatric tumors demonstrated by next-generation sequencing, as well as the location of the tumors in the children, provides strong evidence for cancer transmission during delivery,” Dr. Zakhour summarized.
C-section question
The first of the cases reported by the Japanese team was a toddler (23 months) who presented to a local hospital with a 2-week history of a productive cough. Computed tomography revealed multiple masses scattered along the bronchi in both lungs, and a biopsy revealed neuroendocrine carcinoma of the lung.
Notably, the mother’s cervical cancer was not diagnosed during her pregnancy. A cervical cytologic test performed in the mother 7 months before the birth was negative. The infant was delivered transvaginally at 39 weeks of gestation.
It was only 3 months after the birth that the 35-year-old mother received a diagnosis of squamous cell carcinoma of the cervix. She then underwent radical hysterectomy with pelvic lymphadenectomy, followed by chemotherapy.
Had it been known that she had cervical cancer, she may have been advised to have a cesarean section.
The study authors propose, on the basis of their paper, that all women with cervical cancer should have a cesarean section.
But a U.S. expert questioned this, and said the situation is “a bit nuanced.”
William Grobman, MD, of Northwestern University in Chicago, said the current standard recommendation for many pregnant women known to have cervical cancer is to have a cesarean section and that “the strength of the recommendation is dependent on factors such as stage and size.”
However, in an interview, he added that “it may be premature to make a blanket recommendation for all people based on two reports without any idea of the frequency of this event, and with such uncertainty, it seems that disclosure of all information and shared decision-making would be a key approach.”
In this case report, the authors also noted that the cancer found in the toddler looked similar to the cancer in the mother.
“Histologic similarities between the tumor samples from the mother and child prompted us to compare the results of their next-generation sequencing tests,” they said.
The result? “The comparison of the gene profiles in the samples of tumor and normal tissue confirmed that transmission of maternal tumor to the child had occurred.”
The lung cancer in the toddler progressed despite two chemotherapy regimens, so he was enrolled in a clinical trial of nivolumab therapy. He had a response that continued for 7 months, with no appearance of new lesions. Lobectomy was performed to resect a single remaining nodule. The boy had no evidence of disease recurrence at 12 months after lobectomy.
His mother was also enrolled in a nivolumab trial, but her cervical cancer had spread, and she died 5 months after disease progression.
Second case
In the second reported case, a 6-year-old boy presented to a local hospital with chest pain on the left side. Computed tomography revealed a mass in the left lung, and mucinous adenocarcinoma was eventually diagnosed.
In this case, the mother had a cervical polypoid tumor detected during pregnancy. But, as in the other case, cervical cytologic analysis was negative. Because the tumor was stable without any intervention, the mother delivered the boy vaginally at 38 weeks of gestation.
However, after the delivery, biopsy of the cervical lesion revealed adenocarcinoma. The mother underwent radical hysterectomy and bilateral salpingo-oophorectomy 3 months after delivery. She died of the disease 2 years after the surgery.
The boy received chemotherapy and had a partial response, with a reduction in levels of the tumor marker CA19-9 to normal levels. But 3 months later, the disease recurred in the left lung. After more chemotherapy, he underwent total left pneumonectomy and was subsequently free of disease.
The study authors said that they did not suspect maternal transmission of the cancer when her child received a diagnosis at 6 years of age. They explained that metastatic cervical cancer is typically a fast-growing tumor and the slow growth in the child seemed inconsistent with the idea that the cancer had been transmitted to him.
However, the pathology exam showed that the boy had mucinous adenocarcinoma, “which is an unusual morphologic finding for a primary lung tumor, but it was similar to the uterine cervical tumor in the mother,” the authors reported.
Samples of the cervical tumor from the mother and from the lung tumor of the child were submitted for next-generation sequencing tests and, said the authors, indicated mother-to-infant transmission.
The study was supported by grants from the Japan Agency for Medical Research and Development; the National Cancer Center Research and Development Fund; and the Ministry of Education, Culture, Sports, Science and Technology; and funding from Ono Pharmaceutical.
A version of this article first appeared on Medscape.com.
That’s the conclusion of two ground-breaking cases from Japan in which investigators describe lung cancer in two boys that “probably developed” from their respective mothers via vaginal transmission during birth.
“Transmission of maternal cancer to offspring is extremely rare and is estimated to occur in approximately 1 infant per every 500,000 mothers with cancer,” wrote Ayumu Arakawa, MD, of the National Cancer Center Hospital in Japan, and colleagues, in a paper published Jan. 7 in The New England Journal of Medicine.
Previous cases, of which only 18 have been recorded, have been presumed to occur via transplacental transmission, they said.
In the two new cases, genetic analyses and other evidence suggest that both boys’ lung cancers developed after aspirating uterine cervical cancer tumor cells into their lungs during passage through the birth canal.
Tragically, both mothers, each of whom was diagnosed with cervical cancer after the births, died while their boys were still infants.
“Most of the maternal-to-infant cases reported have been leukemia or melanoma,” said Mel Greaves, PhD, of the Institute of Cancer Research, London, who was asked for comment. In 2009, Dr. Greaves and colleagues published a case study of maternal-to-infant cancer transmission (presumably via the placenta). “It attracted an enormous amount of publicity and no doubt some alarm,” he said in an interview. He emphasized that the phenomenon is “incredibly rare.”
Dr. Greaves explains why such transmission is so rare. “We suspect that cancer cells do transit from mum to unborn child more often, but the foreign (aka paternal) antigens (HLA) on the tumor cells prompt immunological rejection. The extremely rare cases of successful transmission probably do depend on the fortuitous loss of paternal HLA.”
Advances in genetic technology may allow such cases, which have been recorded since 1950, to be rapidly identified now, he said.
“Where there is an adult-type cancer in an infant or child whose mother carried cancer when pregnant, then whole-genome sequencing should quickly tell if the infant’s tumor was of maternal origin,” Dr. Greaves explained.
“I think we will be seeing more reports like this in the future, now that this phenomenon has been described and next-generation sequencing is more readily available,” added Mae Zakhour, MD, of the University of California, Los Angeles, Jonsson Comprehensive Cancer Center, when asked for comment.
In the case of the Japanese boys, both cases were discovered incidentally during an analysis of the results of routine next-generation sequencing testing in a prospective gene-profiling trial in cancer patients, known as TOP-GEAR.
How do the investigators know that the spread happened vaginally and not via the placenta?
They explained that, in other cases of mother-to-fetus transmission, the offspring presented with multiple metastases in the brain, bones, liver, lungs, and soft tissues, which were “consistent with presumed hematogenous spread from the placenta.” However, in the two boys, tumors were observed only in the lungs and were localized along the bronchi.
That peribronchial pattern of tumor growth “suggested that the tumors arose from mother-to-infant vaginal transmission through aspiration of tumor-contaminated vaginal fluids during birth.”
In addition, the tumors in both boys lacked the Y chromosome and shared multiple somatic mutations, an HPV genome, and SNP alleles with tumors from the mothers.
“The identical molecular profiles of maternal and pediatric tumors demonstrated by next-generation sequencing, as well as the location of the tumors in the children, provides strong evidence for cancer transmission during delivery,” Dr. Zakhour summarized.
C-section question
The first of the cases reported by the Japanese team was a toddler (23 months) who presented to a local hospital with a 2-week history of a productive cough. Computed tomography revealed multiple masses scattered along the bronchi in both lungs, and a biopsy revealed neuroendocrine carcinoma of the lung.
Notably, the mother’s cervical cancer was not diagnosed during her pregnancy. A cervical cytologic test performed in the mother 7 months before the birth was negative. The infant was delivered transvaginally at 39 weeks of gestation.
It was only 3 months after the birth that the 35-year-old mother received a diagnosis of squamous cell carcinoma of the cervix. She then underwent radical hysterectomy with pelvic lymphadenectomy, followed by chemotherapy.
Had it been known that she had cervical cancer, she may have been advised to have a cesarean section.
The study authors propose, on the basis of their paper, that all women with cervical cancer should have a cesarean section.
But a U.S. expert questioned this, and said the situation is “a bit nuanced.”
William Grobman, MD, of Northwestern University in Chicago, said the current standard recommendation for many pregnant women known to have cervical cancer is to have a cesarean section and that “the strength of the recommendation is dependent on factors such as stage and size.”
However, in an interview, he added that “it may be premature to make a blanket recommendation for all people based on two reports without any idea of the frequency of this event, and with such uncertainty, it seems that disclosure of all information and shared decision-making would be a key approach.”
In this case report, the authors also noted that the cancer found in the toddler looked similar to the cancer in the mother.
“Histologic similarities between the tumor samples from the mother and child prompted us to compare the results of their next-generation sequencing tests,” they said.
The result? “The comparison of the gene profiles in the samples of tumor and normal tissue confirmed that transmission of maternal tumor to the child had occurred.”
The lung cancer in the toddler progressed despite two chemotherapy regimens, so he was enrolled in a clinical trial of nivolumab therapy. He had a response that continued for 7 months, with no appearance of new lesions. Lobectomy was performed to resect a single remaining nodule. The boy had no evidence of disease recurrence at 12 months after lobectomy.
His mother was also enrolled in a nivolumab trial, but her cervical cancer had spread, and she died 5 months after disease progression.
Second case
In the second reported case, a 6-year-old boy presented to a local hospital with chest pain on the left side. Computed tomography revealed a mass in the left lung, and mucinous adenocarcinoma was eventually diagnosed.
In this case, the mother had a cervical polypoid tumor detected during pregnancy. But, as in the other case, cervical cytologic analysis was negative. Because the tumor was stable without any intervention, the mother delivered the boy vaginally at 38 weeks of gestation.
However, after the delivery, biopsy of the cervical lesion revealed adenocarcinoma. The mother underwent radical hysterectomy and bilateral salpingo-oophorectomy 3 months after delivery. She died of the disease 2 years after the surgery.
The boy received chemotherapy and had a partial response, with a reduction in levels of the tumor marker CA19-9 to normal levels. But 3 months later, the disease recurred in the left lung. After more chemotherapy, he underwent total left pneumonectomy and was subsequently free of disease.
The study authors said that they did not suspect maternal transmission of the cancer when her child received a diagnosis at 6 years of age. They explained that metastatic cervical cancer is typically a fast-growing tumor and the slow growth in the child seemed inconsistent with the idea that the cancer had been transmitted to him.
However, the pathology exam showed that the boy had mucinous adenocarcinoma, “which is an unusual morphologic finding for a primary lung tumor, but it was similar to the uterine cervical tumor in the mother,” the authors reported.
Samples of the cervical tumor from the mother and from the lung tumor of the child were submitted for next-generation sequencing tests and, said the authors, indicated mother-to-infant transmission.
The study was supported by grants from the Japan Agency for Medical Research and Development; the National Cancer Center Research and Development Fund; and the Ministry of Education, Culture, Sports, Science and Technology; and funding from Ono Pharmaceutical.
A version of this article first appeared on Medscape.com.
Arthritis drugs ‘impressive’ for severe COVID but not ‘magic cure’
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
An introduction to Naikan
The list of things to be ungrateful for last year is long. You’re not supposed to make this list, though. The best practice is to list what you’re grateful for, even when living in trying times. That’s a long list too, but I find making it similarly unfruitful.
Of course, I’m grateful I don’t have COVID-19, thankful my practice hasn’t been significantly impacted, grateful I got the vaccine. But simply repeating these gratitudes daily seems ineffective. I’ve learned a different “gratefulness practice” that perhaps works better.
It’s a Japanese method called Naikan (pronounced “nye-kan”). The word means introspection and the practice is one of self-reflection. But . Yoshimoto Ishin developed Naikan in the 1940s. He was a Japanese businessman and devout Buddhist who wanted to make a difficult form of meditation more accessible. He removed the ascetic bits like sleep deprivation and refined the exercises such that they better see how others see us. The result is a way to reframe your life experiences and help you understand how much others do for us and how our actions and attitudes impact others. It can be done alone or with a partner. You can do it at the beginning or end of your day.
The method is simple. You ask three questions:
What have I received today from ___________?
What have I given today to ___________?
What difficulty or trouble have I caused to ___________?
The first question is similar to most gratitude practices. For example, you might ask, “What have I received from (my husband or nurse or friend, etc.)? Today, I received a beautifully tidied-up office from my wife who spent time last night sorting things. This made it easy for me to sit down and start writing this piece.
The second question is better. What have I given today to (my wife, or patient, or mom, etc.)? It can be simple as: Today, I slowed down to let everyone who was in the closed highway lane back into traffic (even though some were clearly undeserving of my generosity). Or last night, I worked to coordinate with anesthesia and scheduling to help a little girl who would benefit from conscious sedation for her procedure.
Combined, these two questions pull you 180 degrees from our default mode, which is complaining. We are wired to find, and talk about, all the inconveniences in our lives: Roadway construction caused a traffic backup that led to running late for clinic. First patient was peeved and had a list of complaints, the last of which was hair loss. Isn’t it much better to rave about how our dermatology nurse volunteered to work the hospital COVID-19 unit to give her colleagues a break? Or how my 10:15 patient came early to be sure she was on time? (It happens.)
The last question is the best. We all spend time thinking about what others think of us. We should spend time thinking about what impact we’ve had on them. Like a cold shower, it’s both briskly awakening and easy to do. Go back through your day and reflect on what you did that made things difficult for others. It can be as simple as I started whining about how a patient waylaid me with her silly complaints. That led to my colleague’s joining in about difficult patients. Or I was late turning in my article, which made my editor have to work harder to get it completed in time.
There’s plenty of things we should be grateful for. In doing these exercises you’ll learn just how much others have cared for you and, I hope, how you might do things to make them grateful for you.
If you’re interested in learning more about Naikan, I discovered this from Brett McKay’s The Art of Manliness podcast and the teaching of Gregg Krech, summarized in his book, “Naikan: Gratitude, Grace, and the Japanese Art of Self-Reflection.”
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected] .
The list of things to be ungrateful for last year is long. You’re not supposed to make this list, though. The best practice is to list what you’re grateful for, even when living in trying times. That’s a long list too, but I find making it similarly unfruitful.
Of course, I’m grateful I don’t have COVID-19, thankful my practice hasn’t been significantly impacted, grateful I got the vaccine. But simply repeating these gratitudes daily seems ineffective. I’ve learned a different “gratefulness practice” that perhaps works better.
It’s a Japanese method called Naikan (pronounced “nye-kan”). The word means introspection and the practice is one of self-reflection. But . Yoshimoto Ishin developed Naikan in the 1940s. He was a Japanese businessman and devout Buddhist who wanted to make a difficult form of meditation more accessible. He removed the ascetic bits like sleep deprivation and refined the exercises such that they better see how others see us. The result is a way to reframe your life experiences and help you understand how much others do for us and how our actions and attitudes impact others. It can be done alone or with a partner. You can do it at the beginning or end of your day.
The method is simple. You ask three questions:
What have I received today from ___________?
What have I given today to ___________?
What difficulty or trouble have I caused to ___________?
The first question is similar to most gratitude practices. For example, you might ask, “What have I received from (my husband or nurse or friend, etc.)? Today, I received a beautifully tidied-up office from my wife who spent time last night sorting things. This made it easy for me to sit down and start writing this piece.
The second question is better. What have I given today to (my wife, or patient, or mom, etc.)? It can be simple as: Today, I slowed down to let everyone who was in the closed highway lane back into traffic (even though some were clearly undeserving of my generosity). Or last night, I worked to coordinate with anesthesia and scheduling to help a little girl who would benefit from conscious sedation for her procedure.
Combined, these two questions pull you 180 degrees from our default mode, which is complaining. We are wired to find, and talk about, all the inconveniences in our lives: Roadway construction caused a traffic backup that led to running late for clinic. First patient was peeved and had a list of complaints, the last of which was hair loss. Isn’t it much better to rave about how our dermatology nurse volunteered to work the hospital COVID-19 unit to give her colleagues a break? Or how my 10:15 patient came early to be sure she was on time? (It happens.)
The last question is the best. We all spend time thinking about what others think of us. We should spend time thinking about what impact we’ve had on them. Like a cold shower, it’s both briskly awakening and easy to do. Go back through your day and reflect on what you did that made things difficult for others. It can be as simple as I started whining about how a patient waylaid me with her silly complaints. That led to my colleague’s joining in about difficult patients. Or I was late turning in my article, which made my editor have to work harder to get it completed in time.
There’s plenty of things we should be grateful for. In doing these exercises you’ll learn just how much others have cared for you and, I hope, how you might do things to make them grateful for you.
If you’re interested in learning more about Naikan, I discovered this from Brett McKay’s The Art of Manliness podcast and the teaching of Gregg Krech, summarized in his book, “Naikan: Gratitude, Grace, and the Japanese Art of Self-Reflection.”
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected] .
The list of things to be ungrateful for last year is long. You’re not supposed to make this list, though. The best practice is to list what you’re grateful for, even when living in trying times. That’s a long list too, but I find making it similarly unfruitful.
Of course, I’m grateful I don’t have COVID-19, thankful my practice hasn’t been significantly impacted, grateful I got the vaccine. But simply repeating these gratitudes daily seems ineffective. I’ve learned a different “gratefulness practice” that perhaps works better.
It’s a Japanese method called Naikan (pronounced “nye-kan”). The word means introspection and the practice is one of self-reflection. But . Yoshimoto Ishin developed Naikan in the 1940s. He was a Japanese businessman and devout Buddhist who wanted to make a difficult form of meditation more accessible. He removed the ascetic bits like sleep deprivation and refined the exercises such that they better see how others see us. The result is a way to reframe your life experiences and help you understand how much others do for us and how our actions and attitudes impact others. It can be done alone or with a partner. You can do it at the beginning or end of your day.
The method is simple. You ask three questions:
What have I received today from ___________?
What have I given today to ___________?
What difficulty or trouble have I caused to ___________?
The first question is similar to most gratitude practices. For example, you might ask, “What have I received from (my husband or nurse or friend, etc.)? Today, I received a beautifully tidied-up office from my wife who spent time last night sorting things. This made it easy for me to sit down and start writing this piece.
The second question is better. What have I given today to (my wife, or patient, or mom, etc.)? It can be simple as: Today, I slowed down to let everyone who was in the closed highway lane back into traffic (even though some were clearly undeserving of my generosity). Or last night, I worked to coordinate with anesthesia and scheduling to help a little girl who would benefit from conscious sedation for her procedure.
Combined, these two questions pull you 180 degrees from our default mode, which is complaining. We are wired to find, and talk about, all the inconveniences in our lives: Roadway construction caused a traffic backup that led to running late for clinic. First patient was peeved and had a list of complaints, the last of which was hair loss. Isn’t it much better to rave about how our dermatology nurse volunteered to work the hospital COVID-19 unit to give her colleagues a break? Or how my 10:15 patient came early to be sure she was on time? (It happens.)
The last question is the best. We all spend time thinking about what others think of us. We should spend time thinking about what impact we’ve had on them. Like a cold shower, it’s both briskly awakening and easy to do. Go back through your day and reflect on what you did that made things difficult for others. It can be as simple as I started whining about how a patient waylaid me with her silly complaints. That led to my colleague’s joining in about difficult patients. Or I was late turning in my article, which made my editor have to work harder to get it completed in time.
There’s plenty of things we should be grateful for. In doing these exercises you’ll learn just how much others have cared for you and, I hope, how you might do things to make them grateful for you.
If you’re interested in learning more about Naikan, I discovered this from Brett McKay’s The Art of Manliness podcast and the teaching of Gregg Krech, summarized in his book, “Naikan: Gratitude, Grace, and the Japanese Art of Self-Reflection.”
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected] .
Could an osteoporosis drug reduce need for hip revision surgery?
A single injection of denosumab (Prolia, Amgen), frequently used to treat osteoporosis, may reduce the need for revision surgery in patients with symptomatic osteolysis following total hip arthroplasty, a new proof-of-concept study suggests.
Aseptic loosening is the result of wear-induced osteolysis caused by the prosthetic hip and is a major contributor to the need for revision surgery in many parts of the world.
“The only established treatment for prosthesis-related osteolysis after joint replacement is revision surgery, which carries substantially greater morbidity and mortality than primary joint replacement,” Mohit M. Mahatma, MRes, of the University of Sheffield, England, and colleagues wrote in their article, published online Jan. 11 in The Lancet Rheumatology.
As well as an increased risk of infection and other complications, revision surgery is much more costly than a first-time operation, they added.
“The results of this proof-of-concept clinical trial indicate that denosumab is effective at reducing bone resorption activity within osteolytic lesion tissue and is well tolerated within the limitations of the single dose used here,” they concluded.
Commenting on the findings, Antonia Chen, MD, associate professor of orthopedic surgery, Harvard Medical School, Boston, emphasized that further studies are needed to assess the effectiveness of this strategy to reduce the need for hip revision surgery.
Nevertheless, “osteolysis is still unfortunately a problem we do have to deal with and we do not have any other way to prevent it,” she said in an interview. “So it’s a good start ... although further studies are definitely needed,” Dr. Chen added.
In an accompanying editorial, Hannu Aro, MD, Turku University Hospital in Finland, agreed: “Without a doubt, the trial is a breakthrough, but it represents only the first step in the development of pharmacological therapy aiming to slow, prevent, or even reverse the process of wear-induced periprosthetic osteolysis.”
Small single-center study
The phase 2, single-center, randomized, controlled trial involved 22 patients who had previously undergone hip replacement surgery at Sheffield Teaching Hospitals and were scheduled for revision surgery due to symptomatic osteolysis. They were randomized to a single subcutaneous injection of denosumab at a dose of 60 mg, or placebo, on their second hospital visit.
“The primary outcome was the between-group difference in the number of osteoclasts per mm of osteolytic membrane at the osteolytic membrane-bone interface at week 8,” the authors noted.
At this time point, there were 83% fewer osteoclasts at the interface in the denosumab group compared with placebo, at a median of 0.05 per mm in the treatment group compared with 0.30 per mm in the placebo group (P = .011).
Secondary histological outcomes were also significantly improved in favor of the denosumab group compared with placebo.
Potential to prevent half of all hip revision surgeries?
Patients who received denosumab also demonstrated an acute fall in serum and urinary markers of bone resorption following administration of the drug, reaching a nadir at week 4, which was maintained until revision surgery at week 8.
In contrast, “no change in these markers was observed in the placebo group [P < .0003 for all biomarkers],” the investigators noted. Rates of adverse events were comparable in both treatment groups.
As the authors explained, osteolysis occurs following joint replacement surgery when particles of plastic wear off from the prosthesis, triggering an immune reaction that attacks the bone around the implant, causing the joint to loosen.
“It is very clear from our bone biopsies and bone imaging that the [denosumab] injection stops the bone absorbing the microplastic particles from the replacement joint and therefore could prevent the bone from being eaten away and the need for revision surgery,” senior author Mark Wilkinson, MBChB, PhD, honorary consultant orthopedic surgeon, Sheffield Teaching Hospitals, said in a press release from his institution.
“This study is a significant breakthrough as we’ve demonstrated that there is a drug, already available and successful in the treatment of osteoporosis, that has the potential to prevent up to half of all revised replacement surgeries which are caused by osteolysis,” he added.
Dr. Wilkinson and coauthors said their results justify the need for future trials targeting earlier-stage disease to further test the use of denosumab to prevent or reduce the need for revision surgery.
In 2018, aseptic loosening accounted for over half of all revision procedures, as reported to the National Joint Registry in England and Wales.
Older polyethylene prostheses are the main culprit
Commenting further on the study, Dr. Chen noted that osteolysis still plagues orthopedic surgeons because the original polyethylene prostheses were not very good. A better prosthesis developed at Massachusetts General Hospital is made up of highly crossed-link polyethylene and still wears over time but to a much lesser extent than the older polyethylene prostheses.
Metal and ceramic prostheses also can induce osteolysis, but again to a much lesser extent than the older polyethylene implants.
“Any particle can technically cause osteolysis but plastic produces the most particles,” Dr. Chen explained. Although hip revision rates in the United States are low to begin with, aseptic loosening is still one of the main reasons that patients need to undergo revision surgery, she observed.
“A lot of patients are still living with the old plastic [implants] so there is still a need for something like this,” she stressed.
However, many questions about this potential new strategy remain to be answered, including when best to initiate treatment and how to manage patients at risk for osteolysis 20-30 years after they have received their original implant.
In his editorial, Dr. Aro said that serious adverse consequences often become evident 10-20 years after patients have undergone the original hip replacement procedures, when they are potentially less physically fit than they were at the time of the operation and thus less able to withstand the rigors of a difficult revision surgery.
“In this context, the concept of nonsurgical pharmacological treatment of periprosthetic osteolysis ... brings a new hope for the ever-increasing population of patients with total hip arthroplasty to avoid revision surgery,” Dr. Aro suggested.
However, Dr. Aro cautioned that reduction of bone turnover by antiresorptive agents such as denosumab has been associated with the development of atypical femoral fractures.
The study was funded by Amgen. Dr. Wilkinson has reported receiving a grant from Amgen. Dr. Chen has reported serving as a consultant for Striker and b-One Ortho. Dr. Aro has reported receiving a grant to his institution from Amgen Finland and the Academy of Finland. He has also served as a member of an advisory scientific board for Amgen Finland.
A version of this article first appeared on Medscape.com.
A single injection of denosumab (Prolia, Amgen), frequently used to treat osteoporosis, may reduce the need for revision surgery in patients with symptomatic osteolysis following total hip arthroplasty, a new proof-of-concept study suggests.
Aseptic loosening is the result of wear-induced osteolysis caused by the prosthetic hip and is a major contributor to the need for revision surgery in many parts of the world.
“The only established treatment for prosthesis-related osteolysis after joint replacement is revision surgery, which carries substantially greater morbidity and mortality than primary joint replacement,” Mohit M. Mahatma, MRes, of the University of Sheffield, England, and colleagues wrote in their article, published online Jan. 11 in The Lancet Rheumatology.
As well as an increased risk of infection and other complications, revision surgery is much more costly than a first-time operation, they added.
“The results of this proof-of-concept clinical trial indicate that denosumab is effective at reducing bone resorption activity within osteolytic lesion tissue and is well tolerated within the limitations of the single dose used here,” they concluded.
Commenting on the findings, Antonia Chen, MD, associate professor of orthopedic surgery, Harvard Medical School, Boston, emphasized that further studies are needed to assess the effectiveness of this strategy to reduce the need for hip revision surgery.
Nevertheless, “osteolysis is still unfortunately a problem we do have to deal with and we do not have any other way to prevent it,” she said in an interview. “So it’s a good start ... although further studies are definitely needed,” Dr. Chen added.
In an accompanying editorial, Hannu Aro, MD, Turku University Hospital in Finland, agreed: “Without a doubt, the trial is a breakthrough, but it represents only the first step in the development of pharmacological therapy aiming to slow, prevent, or even reverse the process of wear-induced periprosthetic osteolysis.”
Small single-center study
The phase 2, single-center, randomized, controlled trial involved 22 patients who had previously undergone hip replacement surgery at Sheffield Teaching Hospitals and were scheduled for revision surgery due to symptomatic osteolysis. They were randomized to a single subcutaneous injection of denosumab at a dose of 60 mg, or placebo, on their second hospital visit.
“The primary outcome was the between-group difference in the number of osteoclasts per mm of osteolytic membrane at the osteolytic membrane-bone interface at week 8,” the authors noted.
At this time point, there were 83% fewer osteoclasts at the interface in the denosumab group compared with placebo, at a median of 0.05 per mm in the treatment group compared with 0.30 per mm in the placebo group (P = .011).
Secondary histological outcomes were also significantly improved in favor of the denosumab group compared with placebo.
Potential to prevent half of all hip revision surgeries?
Patients who received denosumab also demonstrated an acute fall in serum and urinary markers of bone resorption following administration of the drug, reaching a nadir at week 4, which was maintained until revision surgery at week 8.
In contrast, “no change in these markers was observed in the placebo group [P < .0003 for all biomarkers],” the investigators noted. Rates of adverse events were comparable in both treatment groups.
As the authors explained, osteolysis occurs following joint replacement surgery when particles of plastic wear off from the prosthesis, triggering an immune reaction that attacks the bone around the implant, causing the joint to loosen.
“It is very clear from our bone biopsies and bone imaging that the [denosumab] injection stops the bone absorbing the microplastic particles from the replacement joint and therefore could prevent the bone from being eaten away and the need for revision surgery,” senior author Mark Wilkinson, MBChB, PhD, honorary consultant orthopedic surgeon, Sheffield Teaching Hospitals, said in a press release from his institution.
“This study is a significant breakthrough as we’ve demonstrated that there is a drug, already available and successful in the treatment of osteoporosis, that has the potential to prevent up to half of all revised replacement surgeries which are caused by osteolysis,” he added.
Dr. Wilkinson and coauthors said their results justify the need for future trials targeting earlier-stage disease to further test the use of denosumab to prevent or reduce the need for revision surgery.
In 2018, aseptic loosening accounted for over half of all revision procedures, as reported to the National Joint Registry in England and Wales.
Older polyethylene prostheses are the main culprit
Commenting further on the study, Dr. Chen noted that osteolysis still plagues orthopedic surgeons because the original polyethylene prostheses were not very good. A better prosthesis developed at Massachusetts General Hospital is made up of highly crossed-link polyethylene and still wears over time but to a much lesser extent than the older polyethylene prostheses.
Metal and ceramic prostheses also can induce osteolysis, but again to a much lesser extent than the older polyethylene implants.
“Any particle can technically cause osteolysis but plastic produces the most particles,” Dr. Chen explained. Although hip revision rates in the United States are low to begin with, aseptic loosening is still one of the main reasons that patients need to undergo revision surgery, she observed.
“A lot of patients are still living with the old plastic [implants] so there is still a need for something like this,” she stressed.
However, many questions about this potential new strategy remain to be answered, including when best to initiate treatment and how to manage patients at risk for osteolysis 20-30 years after they have received their original implant.
In his editorial, Dr. Aro said that serious adverse consequences often become evident 10-20 years after patients have undergone the original hip replacement procedures, when they are potentially less physically fit than they were at the time of the operation and thus less able to withstand the rigors of a difficult revision surgery.
“In this context, the concept of nonsurgical pharmacological treatment of periprosthetic osteolysis ... brings a new hope for the ever-increasing population of patients with total hip arthroplasty to avoid revision surgery,” Dr. Aro suggested.
However, Dr. Aro cautioned that reduction of bone turnover by antiresorptive agents such as denosumab has been associated with the development of atypical femoral fractures.
The study was funded by Amgen. Dr. Wilkinson has reported receiving a grant from Amgen. Dr. Chen has reported serving as a consultant for Striker and b-One Ortho. Dr. Aro has reported receiving a grant to his institution from Amgen Finland and the Academy of Finland. He has also served as a member of an advisory scientific board for Amgen Finland.
A version of this article first appeared on Medscape.com.
A single injection of denosumab (Prolia, Amgen), frequently used to treat osteoporosis, may reduce the need for revision surgery in patients with symptomatic osteolysis following total hip arthroplasty, a new proof-of-concept study suggests.
Aseptic loosening is the result of wear-induced osteolysis caused by the prosthetic hip and is a major contributor to the need for revision surgery in many parts of the world.
“The only established treatment for prosthesis-related osteolysis after joint replacement is revision surgery, which carries substantially greater morbidity and mortality than primary joint replacement,” Mohit M. Mahatma, MRes, of the University of Sheffield, England, and colleagues wrote in their article, published online Jan. 11 in The Lancet Rheumatology.
As well as an increased risk of infection and other complications, revision surgery is much more costly than a first-time operation, they added.
“The results of this proof-of-concept clinical trial indicate that denosumab is effective at reducing bone resorption activity within osteolytic lesion tissue and is well tolerated within the limitations of the single dose used here,” they concluded.
Commenting on the findings, Antonia Chen, MD, associate professor of orthopedic surgery, Harvard Medical School, Boston, emphasized that further studies are needed to assess the effectiveness of this strategy to reduce the need for hip revision surgery.
Nevertheless, “osteolysis is still unfortunately a problem we do have to deal with and we do not have any other way to prevent it,” she said in an interview. “So it’s a good start ... although further studies are definitely needed,” Dr. Chen added.
In an accompanying editorial, Hannu Aro, MD, Turku University Hospital in Finland, agreed: “Without a doubt, the trial is a breakthrough, but it represents only the first step in the development of pharmacological therapy aiming to slow, prevent, or even reverse the process of wear-induced periprosthetic osteolysis.”
Small single-center study
The phase 2, single-center, randomized, controlled trial involved 22 patients who had previously undergone hip replacement surgery at Sheffield Teaching Hospitals and were scheduled for revision surgery due to symptomatic osteolysis. They were randomized to a single subcutaneous injection of denosumab at a dose of 60 mg, or placebo, on their second hospital visit.
“The primary outcome was the between-group difference in the number of osteoclasts per mm of osteolytic membrane at the osteolytic membrane-bone interface at week 8,” the authors noted.
At this time point, there were 83% fewer osteoclasts at the interface in the denosumab group compared with placebo, at a median of 0.05 per mm in the treatment group compared with 0.30 per mm in the placebo group (P = .011).
Secondary histological outcomes were also significantly improved in favor of the denosumab group compared with placebo.
Potential to prevent half of all hip revision surgeries?
Patients who received denosumab also demonstrated an acute fall in serum and urinary markers of bone resorption following administration of the drug, reaching a nadir at week 4, which was maintained until revision surgery at week 8.
In contrast, “no change in these markers was observed in the placebo group [P < .0003 for all biomarkers],” the investigators noted. Rates of adverse events were comparable in both treatment groups.
As the authors explained, osteolysis occurs following joint replacement surgery when particles of plastic wear off from the prosthesis, triggering an immune reaction that attacks the bone around the implant, causing the joint to loosen.
“It is very clear from our bone biopsies and bone imaging that the [denosumab] injection stops the bone absorbing the microplastic particles from the replacement joint and therefore could prevent the bone from being eaten away and the need for revision surgery,” senior author Mark Wilkinson, MBChB, PhD, honorary consultant orthopedic surgeon, Sheffield Teaching Hospitals, said in a press release from his institution.
“This study is a significant breakthrough as we’ve demonstrated that there is a drug, already available and successful in the treatment of osteoporosis, that has the potential to prevent up to half of all revised replacement surgeries which are caused by osteolysis,” he added.
Dr. Wilkinson and coauthors said their results justify the need for future trials targeting earlier-stage disease to further test the use of denosumab to prevent or reduce the need for revision surgery.
In 2018, aseptic loosening accounted for over half of all revision procedures, as reported to the National Joint Registry in England and Wales.
Older polyethylene prostheses are the main culprit
Commenting further on the study, Dr. Chen noted that osteolysis still plagues orthopedic surgeons because the original polyethylene prostheses were not very good. A better prosthesis developed at Massachusetts General Hospital is made up of highly crossed-link polyethylene and still wears over time but to a much lesser extent than the older polyethylene prostheses.
Metal and ceramic prostheses also can induce osteolysis, but again to a much lesser extent than the older polyethylene implants.
“Any particle can technically cause osteolysis but plastic produces the most particles,” Dr. Chen explained. Although hip revision rates in the United States are low to begin with, aseptic loosening is still one of the main reasons that patients need to undergo revision surgery, she observed.
“A lot of patients are still living with the old plastic [implants] so there is still a need for something like this,” she stressed.
However, many questions about this potential new strategy remain to be answered, including when best to initiate treatment and how to manage patients at risk for osteolysis 20-30 years after they have received their original implant.
In his editorial, Dr. Aro said that serious adverse consequences often become evident 10-20 years after patients have undergone the original hip replacement procedures, when they are potentially less physically fit than they were at the time of the operation and thus less able to withstand the rigors of a difficult revision surgery.
“In this context, the concept of nonsurgical pharmacological treatment of periprosthetic osteolysis ... brings a new hope for the ever-increasing population of patients with total hip arthroplasty to avoid revision surgery,” Dr. Aro suggested.
However, Dr. Aro cautioned that reduction of bone turnover by antiresorptive agents such as denosumab has been associated with the development of atypical femoral fractures.
The study was funded by Amgen. Dr. Wilkinson has reported receiving a grant from Amgen. Dr. Chen has reported serving as a consultant for Striker and b-One Ortho. Dr. Aro has reported receiving a grant to his institution from Amgen Finland and the Academy of Finland. He has also served as a member of an advisory scientific board for Amgen Finland.
A version of this article first appeared on Medscape.com.
Migraine: Helping patients through a difficult journey
Most clinicians who treat migraine know the statistics associated with this debilitating condition and can recite them almost verbatim. Likewise, we all know the long journey a patient experiencing headache can take before finding relief.
The question is, what can we do about it? It can sometimes feel easy to look at the statistics, accept them with little objection, and move on, but we must do the best we can for our patients in the face of the migraine challenge.
It’s a journey that I welcome you to take with me as we evaluate important migraine trends, treatments, and controversies, and figure out how to leverage these developments to improve outcomes and help our patients improve and feel and function better.
There are upwards of 39 million migraine sufferers in the US who are on a journey of their own that is often perplexing, frustrating, and can feel fruitless. According to the Migraine Research Foundation, most migraine sufferers do not seek medical treatment. More than half of patients who experience migraine are never diagnosed. Moreover, only 4% receive care from headache and pain specialists. Perhaps most disheartening, an estimated 5 million migraine sufferers, who we believe can benefit from preventive treatment, are not receiving it.
The patient journey can be extremely discouraging and often maddening. Results from the American Migraine Study show that nearly four in every 10 migraine patients suffer 3 years or more before being diagnosed. A cross-sectional study published in 2019 analyzed treatments, procedures, and follow-up approaches experienced by 456 migraine sufferers until their initial consult with a headache specialist. Patients reported an average headache frequency of approximately 16 days per month. More than half were found to have chronic migraine, and 3 in every 10 had migraine with aura. Despite these characteristics—which were apparently hiding in plain sight—it took patients in this study an average of about 17 years from pain onset to make the journey to an appointment with a headache specialist. That is hard to believe, let alone to understand. Along the way, many migraineurs—particularly those with chronic migraine—were subjected to unnecessary exams and treatments.
Results like these do not come cheaply for families, society, and the health care system. Migraine is estimated to cost more than $20 million per year in direct medical expenses and lost productivity in the US, according to the American Migraine Foundation. Others have estimated double that number. Sufferers, meanwhile, face the prospect of significant pain, stigma and ongoing disability. More than 8 in every 10participants in the American Migraine Study had at least some headache-related disability. More than half say their pain caused severe impairment, even requiring bed rest.
I believe we can help our migraine patients along this journey—and we can make it less arduous for them. We have education, tools, and treatments to help them. Learn how by joining me here each month. We will address the practical relevance of topics such as acute and preventive care (including the new gepants and CGRP-targeted treatments), new and more effective treatments for medication overuse headache, new treatment devices, behavioral approaches to migraine, and our role in headache advocacy, including stigma avoidance. We will not shy away from controversial topics such as the changing definition of chronic migraine, monoclonal antibody safety , high and low cerebrospinal fluid pressure syndromes, and more. See you next month.
Most clinicians who treat migraine know the statistics associated with this debilitating condition and can recite them almost verbatim. Likewise, we all know the long journey a patient experiencing headache can take before finding relief.
The question is, what can we do about it? It can sometimes feel easy to look at the statistics, accept them with little objection, and move on, but we must do the best we can for our patients in the face of the migraine challenge.
It’s a journey that I welcome you to take with me as we evaluate important migraine trends, treatments, and controversies, and figure out how to leverage these developments to improve outcomes and help our patients improve and feel and function better.
There are upwards of 39 million migraine sufferers in the US who are on a journey of their own that is often perplexing, frustrating, and can feel fruitless. According to the Migraine Research Foundation, most migraine sufferers do not seek medical treatment. More than half of patients who experience migraine are never diagnosed. Moreover, only 4% receive care from headache and pain specialists. Perhaps most disheartening, an estimated 5 million migraine sufferers, who we believe can benefit from preventive treatment, are not receiving it.
The patient journey can be extremely discouraging and often maddening. Results from the American Migraine Study show that nearly four in every 10 migraine patients suffer 3 years or more before being diagnosed. A cross-sectional study published in 2019 analyzed treatments, procedures, and follow-up approaches experienced by 456 migraine sufferers until their initial consult with a headache specialist. Patients reported an average headache frequency of approximately 16 days per month. More than half were found to have chronic migraine, and 3 in every 10 had migraine with aura. Despite these characteristics—which were apparently hiding in plain sight—it took patients in this study an average of about 17 years from pain onset to make the journey to an appointment with a headache specialist. That is hard to believe, let alone to understand. Along the way, many migraineurs—particularly those with chronic migraine—were subjected to unnecessary exams and treatments.
Results like these do not come cheaply for families, society, and the health care system. Migraine is estimated to cost more than $20 million per year in direct medical expenses and lost productivity in the US, according to the American Migraine Foundation. Others have estimated double that number. Sufferers, meanwhile, face the prospect of significant pain, stigma and ongoing disability. More than 8 in every 10participants in the American Migraine Study had at least some headache-related disability. More than half say their pain caused severe impairment, even requiring bed rest.
I believe we can help our migraine patients along this journey—and we can make it less arduous for them. We have education, tools, and treatments to help them. Learn how by joining me here each month. We will address the practical relevance of topics such as acute and preventive care (including the new gepants and CGRP-targeted treatments), new and more effective treatments for medication overuse headache, new treatment devices, behavioral approaches to migraine, and our role in headache advocacy, including stigma avoidance. We will not shy away from controversial topics such as the changing definition of chronic migraine, monoclonal antibody safety , high and low cerebrospinal fluid pressure syndromes, and more. See you next month.
Most clinicians who treat migraine know the statistics associated with this debilitating condition and can recite them almost verbatim. Likewise, we all know the long journey a patient experiencing headache can take before finding relief.
The question is, what can we do about it? It can sometimes feel easy to look at the statistics, accept them with little objection, and move on, but we must do the best we can for our patients in the face of the migraine challenge.
It’s a journey that I welcome you to take with me as we evaluate important migraine trends, treatments, and controversies, and figure out how to leverage these developments to improve outcomes and help our patients improve and feel and function better.
There are upwards of 39 million migraine sufferers in the US who are on a journey of their own that is often perplexing, frustrating, and can feel fruitless. According to the Migraine Research Foundation, most migraine sufferers do not seek medical treatment. More than half of patients who experience migraine are never diagnosed. Moreover, only 4% receive care from headache and pain specialists. Perhaps most disheartening, an estimated 5 million migraine sufferers, who we believe can benefit from preventive treatment, are not receiving it.
The patient journey can be extremely discouraging and often maddening. Results from the American Migraine Study show that nearly four in every 10 migraine patients suffer 3 years or more before being diagnosed. A cross-sectional study published in 2019 analyzed treatments, procedures, and follow-up approaches experienced by 456 migraine sufferers until their initial consult with a headache specialist. Patients reported an average headache frequency of approximately 16 days per month. More than half were found to have chronic migraine, and 3 in every 10 had migraine with aura. Despite these characteristics—which were apparently hiding in plain sight—it took patients in this study an average of about 17 years from pain onset to make the journey to an appointment with a headache specialist. That is hard to believe, let alone to understand. Along the way, many migraineurs—particularly those with chronic migraine—were subjected to unnecessary exams and treatments.
Results like these do not come cheaply for families, society, and the health care system. Migraine is estimated to cost more than $20 million per year in direct medical expenses and lost productivity in the US, according to the American Migraine Foundation. Others have estimated double that number. Sufferers, meanwhile, face the prospect of significant pain, stigma and ongoing disability. More than 8 in every 10participants in the American Migraine Study had at least some headache-related disability. More than half say their pain caused severe impairment, even requiring bed rest.
I believe we can help our migraine patients along this journey—and we can make it less arduous for them. We have education, tools, and treatments to help them. Learn how by joining me here each month. We will address the practical relevance of topics such as acute and preventive care (including the new gepants and CGRP-targeted treatments), new and more effective treatments for medication overuse headache, new treatment devices, behavioral approaches to migraine, and our role in headache advocacy, including stigma avoidance. We will not shy away from controversial topics such as the changing definition of chronic migraine, monoclonal antibody safety , high and low cerebrospinal fluid pressure syndromes, and more. See you next month.
















