User login
Telltale dermoscopic features of melanomas lacking pigment reviewed
by familiarity with a handful of dermoscopic features specific to melanomas lacking significant pigment, Steven Q. Wang, MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
These features emerged from a major study conducted on five continents by members of the International Dermoscopy Society. The investigators developed a simple, eight-variable model, which demonstrated a sensitivity of 70% and specificity of 56% for diagnosis of melanoma. And while that’s a markedly worse performance than when dermoscopy is used for detection of pigmented melanomas, where sensitivities in excess of 90% and specificities greater than 70% are typical, it’s nonetheless a significant improvement over naked-eye evaluation of these challenging pigment-deprived melanomas, noted Dr. Wang, director of dermatologic surgery and dermatology at Memorial Sloan Kettering Basking Ridge (N.J.)
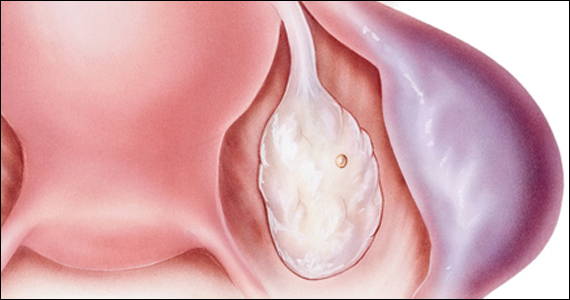
Using the predictive model developed in the international study to evaluate lesions lacking pigment, a diagnosis of melanoma is made provided two conditions are met: The lesion can have no more than three milia-like cysts, and it has to possess one or more of seven positive dermoscopic findings. The strongest predictor of melanoma in the study was the presence of a blue-white veil, which in univariate analysis was associated with a 13-fold increased likelihood of melanoma.
The other positive predictors were irregularly shaped depigmentation, more than one shade of pink, predominant central vessels, irregularly sized or distributed brown dots or globules, multiple blue-gray dots, and dotted and linear irregular vessels.
Dr. Wang emphasized that, when dermoscopy and clinical skin examination of a featureless hypomelanotic or amelanotic lesion yield ambiguous findings, frequent vigilant follow-up is a viable strategy to detect early melanoma – provided the lesion is superficial.
“The reality is not all melanomas are the same. The superficial spreading melanomas and lentigo melanomas grow very, very slowly: less than 0.1 mm per month. Those are the types of lesions you can monitor. But there is one type of lesion you should never, ever monitor: nodular lesions. They are the type of lesions that can do your patient harm because nodular melanomas can grow really fast. So my key takeaway message is, if you see a nodule and you don’t know what it is, take it off,” the dermatologist said.
Dermoscopy in the hands of experienced users has repeatedly been shown to improve diagnostic accuracy by more than 25%. But there is an additional very important reason to embrace dermoscopy in daily clinical practice, according to Dr. Wang: “When you put the scope on an individual, you slow down the exam and patients feels like you’re paying more attention to them.”
That’s worthwhile because the No. 1 complaint voiced by patients who make their way to Sloan Kettering for a second opinion is that their prior skin examination by an outside physician wasn’t thorough. They’re often angry about it. And while it’s true that incorporating dermoscopy does make for a lengthier skin examination, the additional time involved is actually minimal. Dr. Wang cited a randomized, prospective, multicenter study which documented that the median time required to conduct a thorough complete skin examination without dermoscopy was 70 seconds versus 142 seconds with dermoscopy.
Dr. Wang reported having no financial conflicts regarding his presentation.
MedscapeLive and this news organization are owned by the same parent company.
by familiarity with a handful of dermoscopic features specific to melanomas lacking significant pigment, Steven Q. Wang, MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
These features emerged from a major study conducted on five continents by members of the International Dermoscopy Society. The investigators developed a simple, eight-variable model, which demonstrated a sensitivity of 70% and specificity of 56% for diagnosis of melanoma. And while that’s a markedly worse performance than when dermoscopy is used for detection of pigmented melanomas, where sensitivities in excess of 90% and specificities greater than 70% are typical, it’s nonetheless a significant improvement over naked-eye evaluation of these challenging pigment-deprived melanomas, noted Dr. Wang, director of dermatologic surgery and dermatology at Memorial Sloan Kettering Basking Ridge (N.J.)
Using the predictive model developed in the international study to evaluate lesions lacking pigment, a diagnosis of melanoma is made provided two conditions are met: The lesion can have no more than three milia-like cysts, and it has to possess one or more of seven positive dermoscopic findings. The strongest predictor of melanoma in the study was the presence of a blue-white veil, which in univariate analysis was associated with a 13-fold increased likelihood of melanoma.
The other positive predictors were irregularly shaped depigmentation, more than one shade of pink, predominant central vessels, irregularly sized or distributed brown dots or globules, multiple blue-gray dots, and dotted and linear irregular vessels.
Dr. Wang emphasized that, when dermoscopy and clinical skin examination of a featureless hypomelanotic or amelanotic lesion yield ambiguous findings, frequent vigilant follow-up is a viable strategy to detect early melanoma – provided the lesion is superficial.
“The reality is not all melanomas are the same. The superficial spreading melanomas and lentigo melanomas grow very, very slowly: less than 0.1 mm per month. Those are the types of lesions you can monitor. But there is one type of lesion you should never, ever monitor: nodular lesions. They are the type of lesions that can do your patient harm because nodular melanomas can grow really fast. So my key takeaway message is, if you see a nodule and you don’t know what it is, take it off,” the dermatologist said.
Dermoscopy in the hands of experienced users has repeatedly been shown to improve diagnostic accuracy by more than 25%. But there is an additional very important reason to embrace dermoscopy in daily clinical practice, according to Dr. Wang: “When you put the scope on an individual, you slow down the exam and patients feels like you’re paying more attention to them.”
That’s worthwhile because the No. 1 complaint voiced by patients who make their way to Sloan Kettering for a second opinion is that their prior skin examination by an outside physician wasn’t thorough. They’re often angry about it. And while it’s true that incorporating dermoscopy does make for a lengthier skin examination, the additional time involved is actually minimal. Dr. Wang cited a randomized, prospective, multicenter study which documented that the median time required to conduct a thorough complete skin examination without dermoscopy was 70 seconds versus 142 seconds with dermoscopy.
Dr. Wang reported having no financial conflicts regarding his presentation.
MedscapeLive and this news organization are owned by the same parent company.
by familiarity with a handful of dermoscopic features specific to melanomas lacking significant pigment, Steven Q. Wang, MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
These features emerged from a major study conducted on five continents by members of the International Dermoscopy Society. The investigators developed a simple, eight-variable model, which demonstrated a sensitivity of 70% and specificity of 56% for diagnosis of melanoma. And while that’s a markedly worse performance than when dermoscopy is used for detection of pigmented melanomas, where sensitivities in excess of 90% and specificities greater than 70% are typical, it’s nonetheless a significant improvement over naked-eye evaluation of these challenging pigment-deprived melanomas, noted Dr. Wang, director of dermatologic surgery and dermatology at Memorial Sloan Kettering Basking Ridge (N.J.)
Using the predictive model developed in the international study to evaluate lesions lacking pigment, a diagnosis of melanoma is made provided two conditions are met: The lesion can have no more than three milia-like cysts, and it has to possess one or more of seven positive dermoscopic findings. The strongest predictor of melanoma in the study was the presence of a blue-white veil, which in univariate analysis was associated with a 13-fold increased likelihood of melanoma.
The other positive predictors were irregularly shaped depigmentation, more than one shade of pink, predominant central vessels, irregularly sized or distributed brown dots or globules, multiple blue-gray dots, and dotted and linear irregular vessels.
Dr. Wang emphasized that, when dermoscopy and clinical skin examination of a featureless hypomelanotic or amelanotic lesion yield ambiguous findings, frequent vigilant follow-up is a viable strategy to detect early melanoma – provided the lesion is superficial.
“The reality is not all melanomas are the same. The superficial spreading melanomas and lentigo melanomas grow very, very slowly: less than 0.1 mm per month. Those are the types of lesions you can monitor. But there is one type of lesion you should never, ever monitor: nodular lesions. They are the type of lesions that can do your patient harm because nodular melanomas can grow really fast. So my key takeaway message is, if you see a nodule and you don’t know what it is, take it off,” the dermatologist said.
Dermoscopy in the hands of experienced users has repeatedly been shown to improve diagnostic accuracy by more than 25%. But there is an additional very important reason to embrace dermoscopy in daily clinical practice, according to Dr. Wang: “When you put the scope on an individual, you slow down the exam and patients feels like you’re paying more attention to them.”
That’s worthwhile because the No. 1 complaint voiced by patients who make their way to Sloan Kettering for a second opinion is that their prior skin examination by an outside physician wasn’t thorough. They’re often angry about it. And while it’s true that incorporating dermoscopy does make for a lengthier skin examination, the additional time involved is actually minimal. Dr. Wang cited a randomized, prospective, multicenter study which documented that the median time required to conduct a thorough complete skin examination without dermoscopy was 70 seconds versus 142 seconds with dermoscopy.
Dr. Wang reported having no financial conflicts regarding his presentation.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Higher dose maximizes effects of magnesium sulfate for obese women
Obese women may benefit from a higher dose of magnesium sulfate to protect against preeclampsia, based on data from a randomized trial.
Pharmacokinetic models have shown that, “in women who received a 4-g intravenous loading dose followed by a 2-g/h IV maintenance dose, obese women took approximately twice as long as women of mean body weight in the sample to achieve these previously accepted therapeutic serum magnesium concentrations,” which suggests the need for alternate dosing based on body mass index, wrote Kathleen F. Brookfield, MD, of Oregon Health & Science University, Portland, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers randomized 37 women aged 15-45 years with a BMI of 35 kg/m2 or higher who were at least 32 weeks’ gestation to receive the standard Zuspan regimen of magnesium sulfate (4 g intravenous loading dose, followed by a 1-g/hour infusion) or to higher dosing (6 g IV loading dose, followed by a 2-g/hour infusion).
Higher dose increases effectiveness
Serum magnesium concentrations were measured at baseline, and after administration of magnesium sulfate at 1 hour, 4 hours, and delivery; the primary outcome was the proportion of women with subtherapeutic serum magnesium concentrations (less than 4.8 mg/dL) 4 hours after administration.
After 4 hours, the average magnesium sulfate concentrations were significantly higher for women in the high-dose group vs. the standard group (4.41 mg/dL vs. 3.53 mg/dL). In addition, 100% of women in the standard group had subtherapeutic serum magnesium concentrations compared with 63% of the high-dose group.
No significant differences in maternal side effects or neonatal outcomes occurred between the groups. However, rates of nausea and flushing were higher in the higher dose group, compared with the standard group (10.5% vs. 5.5% and 5.2% vs. 0%, respectively).
The study findings were limited by several factors including the lack of statistical power to evaluate clinical outcomes and lack of generalizability to extremely obese patients, as well as to settings in which the higher-dose regimen is already the standard treatment, the researchers noted. However, the results were strengthened by the use of prospective pharmacokinetic data to determine dosing.
The researchers also noted that the study was not powered to examine preeclampsia as an outcome “and there is no evidence to date to suggest women in the United States with higher BMIs are more likely to experience eclampsia,” they said. “Therefore, we caution against universally applying the study findings to obese women without also considering the potential for increased toxicity with higher dosing regimens,” they added.
Current results may not affect practice
The study objectives are unclear, as they do not change the dosing for magnesium sulfate already in use, said Baha M. Sibai, MD, of the University of Texas Health Science Center at Houston, in an interview.
Dr. Sibai said he was not surprised by the findings. “This information has been known for almost 30 years as to serum levels with different dosing irrespective of BMI,” he said. Based on current evidence, Dr. Sibai advised clinicians “not to change your practice, since there are no therapeutic levels for preventing seizures.” In fact, “the largest trial that included 10,000 women showed no difference in the rate of eclampsia between 4 grams loading with 1 g/hour [magnesium sulfate] and 6 g loading and 2 g/hour,” he explained.
Future research should focus on different outcomes, said Dr. Sibai. “The outcome should be eclampsia and not serum levels. This requires studying over 6,000 women,” he emphasized.
The study was supported by the National Institutes of Health Loan Repayment Program and a Mission Support Award from Oregon Health & Science University to Dr. Brookfield and by the Oregon Clinical & Translational Research Institute grant. Dr. Brookfield also disclosed funding from the World Health Organization. Dr. Sibai had no financial conflicts to disclose.
SOURCE: Brookfield KF et al. Obstet Gynecol. 2020 Dec. doi: 10.1097/AOG.0000000000004137.
Obese women may benefit from a higher dose of magnesium sulfate to protect against preeclampsia, based on data from a randomized trial.
Pharmacokinetic models have shown that, “in women who received a 4-g intravenous loading dose followed by a 2-g/h IV maintenance dose, obese women took approximately twice as long as women of mean body weight in the sample to achieve these previously accepted therapeutic serum magnesium concentrations,” which suggests the need for alternate dosing based on body mass index, wrote Kathleen F. Brookfield, MD, of Oregon Health & Science University, Portland, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers randomized 37 women aged 15-45 years with a BMI of 35 kg/m2 or higher who were at least 32 weeks’ gestation to receive the standard Zuspan regimen of magnesium sulfate (4 g intravenous loading dose, followed by a 1-g/hour infusion) or to higher dosing (6 g IV loading dose, followed by a 2-g/hour infusion).
Higher dose increases effectiveness
Serum magnesium concentrations were measured at baseline, and after administration of magnesium sulfate at 1 hour, 4 hours, and delivery; the primary outcome was the proportion of women with subtherapeutic serum magnesium concentrations (less than 4.8 mg/dL) 4 hours after administration.
After 4 hours, the average magnesium sulfate concentrations were significantly higher for women in the high-dose group vs. the standard group (4.41 mg/dL vs. 3.53 mg/dL). In addition, 100% of women in the standard group had subtherapeutic serum magnesium concentrations compared with 63% of the high-dose group.
No significant differences in maternal side effects or neonatal outcomes occurred between the groups. However, rates of nausea and flushing were higher in the higher dose group, compared with the standard group (10.5% vs. 5.5% and 5.2% vs. 0%, respectively).
The study findings were limited by several factors including the lack of statistical power to evaluate clinical outcomes and lack of generalizability to extremely obese patients, as well as to settings in which the higher-dose regimen is already the standard treatment, the researchers noted. However, the results were strengthened by the use of prospective pharmacokinetic data to determine dosing.
The researchers also noted that the study was not powered to examine preeclampsia as an outcome “and there is no evidence to date to suggest women in the United States with higher BMIs are more likely to experience eclampsia,” they said. “Therefore, we caution against universally applying the study findings to obese women without also considering the potential for increased toxicity with higher dosing regimens,” they added.
Current results may not affect practice
The study objectives are unclear, as they do not change the dosing for magnesium sulfate already in use, said Baha M. Sibai, MD, of the University of Texas Health Science Center at Houston, in an interview.
Dr. Sibai said he was not surprised by the findings. “This information has been known for almost 30 years as to serum levels with different dosing irrespective of BMI,” he said. Based on current evidence, Dr. Sibai advised clinicians “not to change your practice, since there are no therapeutic levels for preventing seizures.” In fact, “the largest trial that included 10,000 women showed no difference in the rate of eclampsia between 4 grams loading with 1 g/hour [magnesium sulfate] and 6 g loading and 2 g/hour,” he explained.
Future research should focus on different outcomes, said Dr. Sibai. “The outcome should be eclampsia and not serum levels. This requires studying over 6,000 women,” he emphasized.
The study was supported by the National Institutes of Health Loan Repayment Program and a Mission Support Award from Oregon Health & Science University to Dr. Brookfield and by the Oregon Clinical & Translational Research Institute grant. Dr. Brookfield also disclosed funding from the World Health Organization. Dr. Sibai had no financial conflicts to disclose.
SOURCE: Brookfield KF et al. Obstet Gynecol. 2020 Dec. doi: 10.1097/AOG.0000000000004137.
Obese women may benefit from a higher dose of magnesium sulfate to protect against preeclampsia, based on data from a randomized trial.
Pharmacokinetic models have shown that, “in women who received a 4-g intravenous loading dose followed by a 2-g/h IV maintenance dose, obese women took approximately twice as long as women of mean body weight in the sample to achieve these previously accepted therapeutic serum magnesium concentrations,” which suggests the need for alternate dosing based on body mass index, wrote Kathleen F. Brookfield, MD, of Oregon Health & Science University, Portland, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers randomized 37 women aged 15-45 years with a BMI of 35 kg/m2 or higher who were at least 32 weeks’ gestation to receive the standard Zuspan regimen of magnesium sulfate (4 g intravenous loading dose, followed by a 1-g/hour infusion) or to higher dosing (6 g IV loading dose, followed by a 2-g/hour infusion).
Higher dose increases effectiveness
Serum magnesium concentrations were measured at baseline, and after administration of magnesium sulfate at 1 hour, 4 hours, and delivery; the primary outcome was the proportion of women with subtherapeutic serum magnesium concentrations (less than 4.8 mg/dL) 4 hours after administration.
After 4 hours, the average magnesium sulfate concentrations were significantly higher for women in the high-dose group vs. the standard group (4.41 mg/dL vs. 3.53 mg/dL). In addition, 100% of women in the standard group had subtherapeutic serum magnesium concentrations compared with 63% of the high-dose group.
No significant differences in maternal side effects or neonatal outcomes occurred between the groups. However, rates of nausea and flushing were higher in the higher dose group, compared with the standard group (10.5% vs. 5.5% and 5.2% vs. 0%, respectively).
The study findings were limited by several factors including the lack of statistical power to evaluate clinical outcomes and lack of generalizability to extremely obese patients, as well as to settings in which the higher-dose regimen is already the standard treatment, the researchers noted. However, the results were strengthened by the use of prospective pharmacokinetic data to determine dosing.
The researchers also noted that the study was not powered to examine preeclampsia as an outcome “and there is no evidence to date to suggest women in the United States with higher BMIs are more likely to experience eclampsia,” they said. “Therefore, we caution against universally applying the study findings to obese women without also considering the potential for increased toxicity with higher dosing regimens,” they added.
Current results may not affect practice
The study objectives are unclear, as they do not change the dosing for magnesium sulfate already in use, said Baha M. Sibai, MD, of the University of Texas Health Science Center at Houston, in an interview.
Dr. Sibai said he was not surprised by the findings. “This information has been known for almost 30 years as to serum levels with different dosing irrespective of BMI,” he said. Based on current evidence, Dr. Sibai advised clinicians “not to change your practice, since there are no therapeutic levels for preventing seizures.” In fact, “the largest trial that included 10,000 women showed no difference in the rate of eclampsia between 4 grams loading with 1 g/hour [magnesium sulfate] and 6 g loading and 2 g/hour,” he explained.
Future research should focus on different outcomes, said Dr. Sibai. “The outcome should be eclampsia and not serum levels. This requires studying over 6,000 women,” he emphasized.
The study was supported by the National Institutes of Health Loan Repayment Program and a Mission Support Award from Oregon Health & Science University to Dr. Brookfield and by the Oregon Clinical & Translational Research Institute grant. Dr. Brookfield also disclosed funding from the World Health Organization. Dr. Sibai had no financial conflicts to disclose.
SOURCE: Brookfield KF et al. Obstet Gynecol. 2020 Dec. doi: 10.1097/AOG.0000000000004137.
FROM OBSTETRICS & GYNECOLOGY
Caring for Patients at a COVID-19 Field Hospital
During the initial peak of coronavirus disease 2019 (COVID-19) cases, US models suggested hospital bed shortages, hinting at the dire possibility of an overwhelmed healthcare system.1,2 Such projections invoked widespread uncertainty and fear of massive loss of life secondary to an undersupply of treatment resources. This led many state governments to rush into a series of historically unprecedented interventions, including the rapid deployment of field hospitals. US state governments, in partnership with the Army Corps of Engineers, invested more than $660 million to transform convention halls, university campus buildings, and even abandoned industrial warehouses, into overflow hospitals for the care of COVID-19 patients.1 Such a national scale of field hospital construction is truly historic, never before having occurred at this speed and on this scale. The only other time field hospitals were deployed nearly as widely in the United States was during the Civil War.3
FIELD HOSPITALS DURING THE COVID-19 PANDEMIC
The use of COVID-19 field hospital resources has been variable, with patient volumes ranging from 0 at many to more than 1,000 at the Javits Center field hospital in New York City.1 In fact, most field hospitals did not treat any patients because early public health measures, such as stay-at-home orders, helped contain the virus in most states.1 As of this writing, the United States has seen a dramatic surge in COVID-19 transmission and hospitalizations. This has led many states to re-introduce field hospitals into their COVID emergency response.
Our site, the Baltimore Convention Center Field Hospital (BCCFH), is one of few sites that is still operational and, to our knowledge, is the longest-running US COVID-19 field hospital. We have cared for 543 patients since opening and have had no cardiac arrests or on-site deaths. To safely offload lower-acuity COVID-19 patients from Maryland hospitals, we designed admission criteria and care processes to provide medical care on site until patients are ready for discharge. However, we anticipated that some patients would decompensate and need to return to a higher level of care. Here, we share our experience with identifying, assessing, resuscitating, and transporting unstable patients. We believe that this process has allowed us to treat about 80% of our patients in place with successful discharge to outpatient care. We have safely transferred about 20% to a higher level of care, having learned from our early cases to refine and improve our rapid response process.
CASES
Case 1
A 39-year-old man was transferred to the BCCFH on his 9th day of symptoms following a 3-day hospital admission for COVID-19. On BCCFH day 1, he developed an oxygen requirement of 2 L/min and a fever of 39.9 oC. Testing revealed worsening hyponatremia and new proteinuria, and a chest radiograph showed increased bilateral interstitial infiltrates. Cefdinir and fluid restriction were initiated. On BCCFH day 2, the patient developed hypotension (88/55 mm Hg), tachycardia (180 bpm), an oxygen requirement of 3 L/min, and a brief syncopal episode while sitting in bed. The charge physician and nurse were directed to the bedside. They instructed staff to bring a stretcher and intravenous (IV) supplies. Unable to locate these supplies in the triage bay, the staff found them in various locations. An IV line was inserted, and fluids administered, after which vital signs improved. Emergency medical services (EMS), which were on standby outside the field hospital, were alerted via radio; they donned personal protective equipment (PPE) and arrived at the triage bay. They were redirected to patient bedside, whence they transported the patient to the hospital.
Case 2
A 64-year-old man with a history of homelessness, myocardial infarctions, cerebrovascular accident, and paroxysmal atrial fibrillation was transferred to the BCCFH on his 6th day of symptoms after a 2-day hospitalization with COVID-19 respiratory illness. On BCCFH day 1, he had a temperature of 39.3 oC and atypical chest pain. A laboratory workup was unrevealing. On BCCFH day 2, he had asymptomatic hypotension and a heart rate of 60-85 bpm while receiving his usual metoprolol dose. On BCCFH day 3, he reported dizziness and was found to be hypotensive (83/41 mm Hg) and febrile (38.6 oC). The rapid response team (RRT) was called over radio, and they quickly assessed the patient and transported him to the triage bay. EMS, signaled through the RRT radio announcement, arrived at the triage bay and transported the patient to a traditional hospital.
ABOUT THE BCCFH
The BCCFH, which opened in April 2020, is a 252-bed facility that’s spread over a single exhibit hall floor and cares for stable adult COVID-19 patients from any hospital or emergency department in Maryland (Appendix A). The site offers basic laboratory tests, radiography, a limited on-site pharmacy, and spot vital sign monitoring without telemetry. Both EMS and a certified registered nurse anesthetist are on standby in the nonclinical area and must don PPE before entering the patient care area when called. The appendices show the patient beds (Appendix B) and triage area (Appendix C) used for patient evaluation and resuscitation. Unlike conventional hospitals, the BCCFH has limited consultant access, and there are frequent changes in clinical teams. In addition to clinicians, our site has physical therapists, occupational therapists, and social work teams to assist in patient care and discharge planning. As of this writing, we have cared for 543 patients, sent to us from one-third of Maryland’s hospitals. Use during the first wave of COVID was variable, with some hospitals sending us just a few patients. One Baltimore hospital sent us 8% of its COVID-19 patients. Because the patients have an average 5-day stay, the BCCFH has offloaded 2,600 bed-days of care from acute hospitals.
ROLE OF THE RRT IN A FIELD HOSPITAL
COVID-19 field hospitals must be prepared to respond effectively to decompensating patients. In our experience, effective RRTs provide a standard and reproducible approach to patient emergencies. In the conventional hospital setting, these teams consist of clinicians who can be called on by any healthcare worker to quickly assess deteriorating patients and intervene with treatment. The purpose of an RRT is to provide immediate care to a patient before progression to respiratory or cardiac arrest. RRTs proliferated in US hospitals after 2004 when the Institute for Healthcare Improvement in Boston, Massachusetts, recommended such teams for improved quality of care. Though studies report conflicting findings on the impact of RRTs on mortality rates, these studies were performed in traditional hospitals with ample resources, consultants, and clinicians familiar with their patients rather than in resource-limited field hospitals.4-13 Our field hospital has found RRTs, and the principles behind them, useful in the identification and management of decompensating COVID-19 patients.
A FOUR-STEP RAPID RESPONSE FRAMEWORK: CASE CORRELATION
An approach to managing decompensating patients in a COVID-19 field hospital can be considered in four phases: identification, assessment, resuscitation, and transport. Referring to these phases, the first case shows opportunities for improvement in resuscitation and transport. Although decompensation was identified, the patient was not transported to the triage bay for resuscitation, and there was confusion when trying to obtain the proper equipment. Additionally, EMS awaited the patient in the triage bay, while he remained in his cubicle, which delayed transport to an acute care hospital. The second case shows opportunities for improvement in identification and assessment. The patient had signs of impending decompensation that were not immediately recognized and treated. However, once decompensation occurred, the RRT was called and the patient was transported quickly to the triage bay, and then to the hospital via EMS.
In our experience at the BCCFH, identification is a key phase in COVID-19 care at a field hospital. Identification involves recognizing impending deterioration, as well as understanding risk factors for decompensation. For COVID-19 specifically, this requires heightened awareness of patients who are in the 2nd to 3rd week of symptoms. Data from Wuhan, China, suggest that decompensation occurs predictably around symptom day 9.14,15 At the BCCFH, the median symptom duration for patients who decompensated and returned to a hospital was 13 days. In both introductory cases, patients were in the high-risk 2nd week of symptoms when decompensation occurred. Clinicians at the BCCFH now discuss patient symptom day during their handoffs, when rounding, and when making decisions regarding acute care transfer. Our team has also integrated clinical information from our electronic health record to create a dashboard describing those patients requiring acute care transfer to assist in identifying other trends or predictive factors (Appendix D).
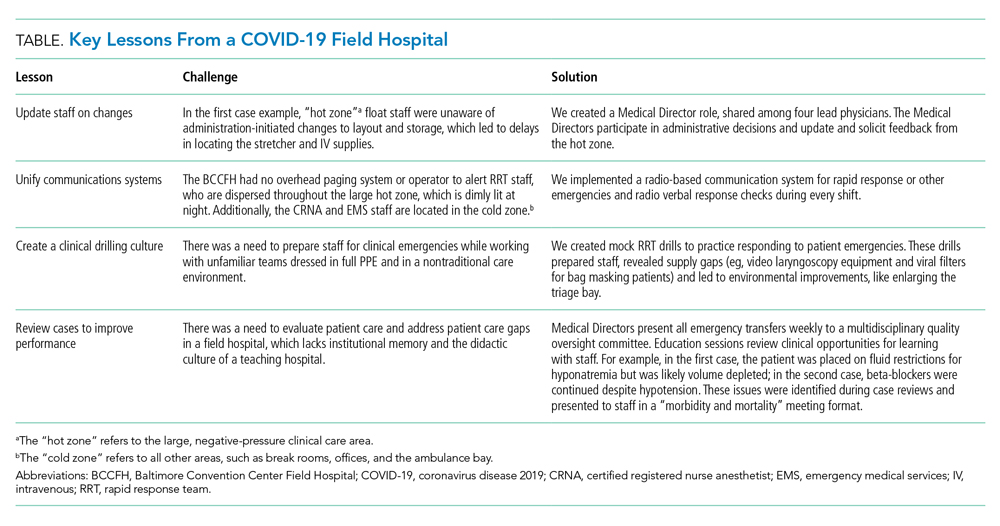
LESSONS FROM THE FIELD HOSPITAL: IMPROVING CLINICAL PERFORMANCE
Although RRTs are designed to activate when an individual patient decompensates, they should fit within a larger operational framework for patient safety. Our experience with emergencies at the BCCFH has yielded four opportunities for learning relevant to COVID-19 care in nontraditional settings (Table). These lessons include how to update staff on clinical process changes, unify communication systems, create a clinical drilling culture, and review cases to improve performance. They illustrate the importance of standardizing emergency processes, conducting frequent updates and drills, and ensuring continuous improvement. We found that, while caring for patients with an unpredictable, novel disease in a nontraditional setting and while wearing PPE and working with new colleagues during every shift, the best approach to support patients and staff is to anticipate emergencies rather than relying on individual staff to develop on-the-spot solutions.
CONCLUSION
The COVID-19 era has seen the unprecedented construction and utilization of emergency field hospital facilities. Such facilities can serve to offload some COVID-19 patients from strained healthcare infrastructure and provide essential care to these patients. We share many of the unique physical and logistical considerations specific to a nontraditional site. We optimized our space, our equipment, and our communication system. We learned how to identify, assess, resuscitate, and transport decompensating COVID-19 patients. Ultimately, our field hospital has been well utilized and successful at caring for patients because of its adaptability, accessibility, and safety record. Of the 15% of patients we transferred to a hospital for care, 81% were successfully stabilized and were willing to return to the BCCFH to complete their care. Our design included supportive care such as social work, physical and occupational therapy, and treatment of comorbidities, such as diabetes and substance use disorder. Our model demonstrates an effective nonhospital option for the care of lower-acuity, medically complex COVID-19 patients. If such facilities are used in subsequent COVID-19 outbreaks, we advise structured planning for the care of decompensating patients that takes into account the need for effective communication, drilling, and ongoing process improvement.
1. Rose J. U.S. Field Hospitals Stand Down, Most Without Treating Any COVID-19 Patients. All Things Considered. NPR; May 7, 2020. Accessed July 21, 2020. https://www.npr.org/2020/05/07/851712311/u-s-field-hospitals-stand-down-most-without-treating-any-covid-19-patients
2. Chen S, Zhang Z, Yang J, et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395(10232):1305-1314. https://doi.org/10.1016/s0140-6736(20)30744-3
3. Reilly RF. Medical and surgical care during the American Civil War, 1861-1865. Proc (Bayl Univ Med Cent). 2016;29(2):138-142. https://doi.org/10.1080/08998280.2016.11929390
4. Bellomo R, Goldsmith D, Uchino S, et al. Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Care Med. 2004;32(4):916-21. https://doi.org/10.1097/01.ccm.0000119428.02968.9e
5. Bellomo R, Goldsmith D, Uchino S, et al. A prospective before-and-after trial of a medical emergency team. Med J Aust. 2003;179(6):283-287.
6. Bristow PJ, Hillman KM, Chey T, et al. Rates of in-hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team. Med J Aust. 2000;173(5):236-240.
7. Buist MD, Moore GE, Bernard SA, Waxman BP, Anderson JN, Nguyen TV. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387-390. https://doi.org/10.1136/bmj.324.7334.387
8. DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons RL; Medical Emergency Response Improvement Team (MERIT). Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care. 2004;13(4):251-254. https://doi.org/10.1136/qhc.13.4.251
9. Goldhill DR, Worthington L, Mulcahy A, Tarling M, Sumner A. The patient-at-risk team: identifying and managing seriously ill ward patients. Anaesthesia. 1999;54(9):853-860. https://doi.org/10.1046/j.1365-2044.1999.00996.x
10. Hillman K, Chen J, Cretikos M, et al; MERIT study investigators. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005;365(9477):2091-2097. https://doi.org/10.1016/s0140-6736(05)66733-5
11. Kenward G, Castle N, Hodgetts T, Shaikh L. Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61(3):257-263. https://doi.org/10.1016/j.resuscitation.2004.01.021
12. Pittard AJ. Out of our reach? assessing the impact of introducing a critical care outreach service. Anaesthesia. 2003;58(9):882-885. https://doi.org/10.1046/j.1365-2044.2003.03331.x
13. Priestley G, Watson W, Rashidian A, et al. Introducing critical care outreach: a ward-randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398-1404. https://doi.org/10.1007/s00134-004-2268-7
14. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3
15. Zhou Y, Li W, Wang D, et al. Clinical time course of COVID-19, its neurological manifestation and some thoughts on its management. Stroke Vasc Neurol. 2020;5(2):177-179. https://doi.org/10.1136/svn-2020-000398
During the initial peak of coronavirus disease 2019 (COVID-19) cases, US models suggested hospital bed shortages, hinting at the dire possibility of an overwhelmed healthcare system.1,2 Such projections invoked widespread uncertainty and fear of massive loss of life secondary to an undersupply of treatment resources. This led many state governments to rush into a series of historically unprecedented interventions, including the rapid deployment of field hospitals. US state governments, in partnership with the Army Corps of Engineers, invested more than $660 million to transform convention halls, university campus buildings, and even abandoned industrial warehouses, into overflow hospitals for the care of COVID-19 patients.1 Such a national scale of field hospital construction is truly historic, never before having occurred at this speed and on this scale. The only other time field hospitals were deployed nearly as widely in the United States was during the Civil War.3
FIELD HOSPITALS DURING THE COVID-19 PANDEMIC
The use of COVID-19 field hospital resources has been variable, with patient volumes ranging from 0 at many to more than 1,000 at the Javits Center field hospital in New York City.1 In fact, most field hospitals did not treat any patients because early public health measures, such as stay-at-home orders, helped contain the virus in most states.1 As of this writing, the United States has seen a dramatic surge in COVID-19 transmission and hospitalizations. This has led many states to re-introduce field hospitals into their COVID emergency response.
Our site, the Baltimore Convention Center Field Hospital (BCCFH), is one of few sites that is still operational and, to our knowledge, is the longest-running US COVID-19 field hospital. We have cared for 543 patients since opening and have had no cardiac arrests or on-site deaths. To safely offload lower-acuity COVID-19 patients from Maryland hospitals, we designed admission criteria and care processes to provide medical care on site until patients are ready for discharge. However, we anticipated that some patients would decompensate and need to return to a higher level of care. Here, we share our experience with identifying, assessing, resuscitating, and transporting unstable patients. We believe that this process has allowed us to treat about 80% of our patients in place with successful discharge to outpatient care. We have safely transferred about 20% to a higher level of care, having learned from our early cases to refine and improve our rapid response process.
CASES
Case 1
A 39-year-old man was transferred to the BCCFH on his 9th day of symptoms following a 3-day hospital admission for COVID-19. On BCCFH day 1, he developed an oxygen requirement of 2 L/min and a fever of 39.9 oC. Testing revealed worsening hyponatremia and new proteinuria, and a chest radiograph showed increased bilateral interstitial infiltrates. Cefdinir and fluid restriction were initiated. On BCCFH day 2, the patient developed hypotension (88/55 mm Hg), tachycardia (180 bpm), an oxygen requirement of 3 L/min, and a brief syncopal episode while sitting in bed. The charge physician and nurse were directed to the bedside. They instructed staff to bring a stretcher and intravenous (IV) supplies. Unable to locate these supplies in the triage bay, the staff found them in various locations. An IV line was inserted, and fluids administered, after which vital signs improved. Emergency medical services (EMS), which were on standby outside the field hospital, were alerted via radio; they donned personal protective equipment (PPE) and arrived at the triage bay. They were redirected to patient bedside, whence they transported the patient to the hospital.
Case 2
A 64-year-old man with a history of homelessness, myocardial infarctions, cerebrovascular accident, and paroxysmal atrial fibrillation was transferred to the BCCFH on his 6th day of symptoms after a 2-day hospitalization with COVID-19 respiratory illness. On BCCFH day 1, he had a temperature of 39.3 oC and atypical chest pain. A laboratory workup was unrevealing. On BCCFH day 2, he had asymptomatic hypotension and a heart rate of 60-85 bpm while receiving his usual metoprolol dose. On BCCFH day 3, he reported dizziness and was found to be hypotensive (83/41 mm Hg) and febrile (38.6 oC). The rapid response team (RRT) was called over radio, and they quickly assessed the patient and transported him to the triage bay. EMS, signaled through the RRT radio announcement, arrived at the triage bay and transported the patient to a traditional hospital.
ABOUT THE BCCFH
The BCCFH, which opened in April 2020, is a 252-bed facility that’s spread over a single exhibit hall floor and cares for stable adult COVID-19 patients from any hospital or emergency department in Maryland (Appendix A). The site offers basic laboratory tests, radiography, a limited on-site pharmacy, and spot vital sign monitoring without telemetry. Both EMS and a certified registered nurse anesthetist are on standby in the nonclinical area and must don PPE before entering the patient care area when called. The appendices show the patient beds (Appendix B) and triage area (Appendix C) used for patient evaluation and resuscitation. Unlike conventional hospitals, the BCCFH has limited consultant access, and there are frequent changes in clinical teams. In addition to clinicians, our site has physical therapists, occupational therapists, and social work teams to assist in patient care and discharge planning. As of this writing, we have cared for 543 patients, sent to us from one-third of Maryland’s hospitals. Use during the first wave of COVID was variable, with some hospitals sending us just a few patients. One Baltimore hospital sent us 8% of its COVID-19 patients. Because the patients have an average 5-day stay, the BCCFH has offloaded 2,600 bed-days of care from acute hospitals.
ROLE OF THE RRT IN A FIELD HOSPITAL
COVID-19 field hospitals must be prepared to respond effectively to decompensating patients. In our experience, effective RRTs provide a standard and reproducible approach to patient emergencies. In the conventional hospital setting, these teams consist of clinicians who can be called on by any healthcare worker to quickly assess deteriorating patients and intervene with treatment. The purpose of an RRT is to provide immediate care to a patient before progression to respiratory or cardiac arrest. RRTs proliferated in US hospitals after 2004 when the Institute for Healthcare Improvement in Boston, Massachusetts, recommended such teams for improved quality of care. Though studies report conflicting findings on the impact of RRTs on mortality rates, these studies were performed in traditional hospitals with ample resources, consultants, and clinicians familiar with their patients rather than in resource-limited field hospitals.4-13 Our field hospital has found RRTs, and the principles behind them, useful in the identification and management of decompensating COVID-19 patients.
A FOUR-STEP RAPID RESPONSE FRAMEWORK: CASE CORRELATION
An approach to managing decompensating patients in a COVID-19 field hospital can be considered in four phases: identification, assessment, resuscitation, and transport. Referring to these phases, the first case shows opportunities for improvement in resuscitation and transport. Although decompensation was identified, the patient was not transported to the triage bay for resuscitation, and there was confusion when trying to obtain the proper equipment. Additionally, EMS awaited the patient in the triage bay, while he remained in his cubicle, which delayed transport to an acute care hospital. The second case shows opportunities for improvement in identification and assessment. The patient had signs of impending decompensation that were not immediately recognized and treated. However, once decompensation occurred, the RRT was called and the patient was transported quickly to the triage bay, and then to the hospital via EMS.
In our experience at the BCCFH, identification is a key phase in COVID-19 care at a field hospital. Identification involves recognizing impending deterioration, as well as understanding risk factors for decompensation. For COVID-19 specifically, this requires heightened awareness of patients who are in the 2nd to 3rd week of symptoms. Data from Wuhan, China, suggest that decompensation occurs predictably around symptom day 9.14,15 At the BCCFH, the median symptom duration for patients who decompensated and returned to a hospital was 13 days. In both introductory cases, patients were in the high-risk 2nd week of symptoms when decompensation occurred. Clinicians at the BCCFH now discuss patient symptom day during their handoffs, when rounding, and when making decisions regarding acute care transfer. Our team has also integrated clinical information from our electronic health record to create a dashboard describing those patients requiring acute care transfer to assist in identifying other trends or predictive factors (Appendix D).
LESSONS FROM THE FIELD HOSPITAL: IMPROVING CLINICAL PERFORMANCE
Although RRTs are designed to activate when an individual patient decompensates, they should fit within a larger operational framework for patient safety. Our experience with emergencies at the BCCFH has yielded four opportunities for learning relevant to COVID-19 care in nontraditional settings (Table). These lessons include how to update staff on clinical process changes, unify communication systems, create a clinical drilling culture, and review cases to improve performance. They illustrate the importance of standardizing emergency processes, conducting frequent updates and drills, and ensuring continuous improvement. We found that, while caring for patients with an unpredictable, novel disease in a nontraditional setting and while wearing PPE and working with new colleagues during every shift, the best approach to support patients and staff is to anticipate emergencies rather than relying on individual staff to develop on-the-spot solutions.

CONCLUSION
The COVID-19 era has seen the unprecedented construction and utilization of emergency field hospital facilities. Such facilities can serve to offload some COVID-19 patients from strained healthcare infrastructure and provide essential care to these patients. We share many of the unique physical and logistical considerations specific to a nontraditional site. We optimized our space, our equipment, and our communication system. We learned how to identify, assess, resuscitate, and transport decompensating COVID-19 patients. Ultimately, our field hospital has been well utilized and successful at caring for patients because of its adaptability, accessibility, and safety record. Of the 15% of patients we transferred to a hospital for care, 81% were successfully stabilized and were willing to return to the BCCFH to complete their care. Our design included supportive care such as social work, physical and occupational therapy, and treatment of comorbidities, such as diabetes and substance use disorder. Our model demonstrates an effective nonhospital option for the care of lower-acuity, medically complex COVID-19 patients. If such facilities are used in subsequent COVID-19 outbreaks, we advise structured planning for the care of decompensating patients that takes into account the need for effective communication, drilling, and ongoing process improvement.
During the initial peak of coronavirus disease 2019 (COVID-19) cases, US models suggested hospital bed shortages, hinting at the dire possibility of an overwhelmed healthcare system.1,2 Such projections invoked widespread uncertainty and fear of massive loss of life secondary to an undersupply of treatment resources. This led many state governments to rush into a series of historically unprecedented interventions, including the rapid deployment of field hospitals. US state governments, in partnership with the Army Corps of Engineers, invested more than $660 million to transform convention halls, university campus buildings, and even abandoned industrial warehouses, into overflow hospitals for the care of COVID-19 patients.1 Such a national scale of field hospital construction is truly historic, never before having occurred at this speed and on this scale. The only other time field hospitals were deployed nearly as widely in the United States was during the Civil War.3
FIELD HOSPITALS DURING THE COVID-19 PANDEMIC
The use of COVID-19 field hospital resources has been variable, with patient volumes ranging from 0 at many to more than 1,000 at the Javits Center field hospital in New York City.1 In fact, most field hospitals did not treat any patients because early public health measures, such as stay-at-home orders, helped contain the virus in most states.1 As of this writing, the United States has seen a dramatic surge in COVID-19 transmission and hospitalizations. This has led many states to re-introduce field hospitals into their COVID emergency response.
Our site, the Baltimore Convention Center Field Hospital (BCCFH), is one of few sites that is still operational and, to our knowledge, is the longest-running US COVID-19 field hospital. We have cared for 543 patients since opening and have had no cardiac arrests or on-site deaths. To safely offload lower-acuity COVID-19 patients from Maryland hospitals, we designed admission criteria and care processes to provide medical care on site until patients are ready for discharge. However, we anticipated that some patients would decompensate and need to return to a higher level of care. Here, we share our experience with identifying, assessing, resuscitating, and transporting unstable patients. We believe that this process has allowed us to treat about 80% of our patients in place with successful discharge to outpatient care. We have safely transferred about 20% to a higher level of care, having learned from our early cases to refine and improve our rapid response process.
CASES
Case 1
A 39-year-old man was transferred to the BCCFH on his 9th day of symptoms following a 3-day hospital admission for COVID-19. On BCCFH day 1, he developed an oxygen requirement of 2 L/min and a fever of 39.9 oC. Testing revealed worsening hyponatremia and new proteinuria, and a chest radiograph showed increased bilateral interstitial infiltrates. Cefdinir and fluid restriction were initiated. On BCCFH day 2, the patient developed hypotension (88/55 mm Hg), tachycardia (180 bpm), an oxygen requirement of 3 L/min, and a brief syncopal episode while sitting in bed. The charge physician and nurse were directed to the bedside. They instructed staff to bring a stretcher and intravenous (IV) supplies. Unable to locate these supplies in the triage bay, the staff found them in various locations. An IV line was inserted, and fluids administered, after which vital signs improved. Emergency medical services (EMS), which were on standby outside the field hospital, were alerted via radio; they donned personal protective equipment (PPE) and arrived at the triage bay. They were redirected to patient bedside, whence they transported the patient to the hospital.
Case 2
A 64-year-old man with a history of homelessness, myocardial infarctions, cerebrovascular accident, and paroxysmal atrial fibrillation was transferred to the BCCFH on his 6th day of symptoms after a 2-day hospitalization with COVID-19 respiratory illness. On BCCFH day 1, he had a temperature of 39.3 oC and atypical chest pain. A laboratory workup was unrevealing. On BCCFH day 2, he had asymptomatic hypotension and a heart rate of 60-85 bpm while receiving his usual metoprolol dose. On BCCFH day 3, he reported dizziness and was found to be hypotensive (83/41 mm Hg) and febrile (38.6 oC). The rapid response team (RRT) was called over radio, and they quickly assessed the patient and transported him to the triage bay. EMS, signaled through the RRT radio announcement, arrived at the triage bay and transported the patient to a traditional hospital.
ABOUT THE BCCFH
The BCCFH, which opened in April 2020, is a 252-bed facility that’s spread over a single exhibit hall floor and cares for stable adult COVID-19 patients from any hospital or emergency department in Maryland (Appendix A). The site offers basic laboratory tests, radiography, a limited on-site pharmacy, and spot vital sign monitoring without telemetry. Both EMS and a certified registered nurse anesthetist are on standby in the nonclinical area and must don PPE before entering the patient care area when called. The appendices show the patient beds (Appendix B) and triage area (Appendix C) used for patient evaluation and resuscitation. Unlike conventional hospitals, the BCCFH has limited consultant access, and there are frequent changes in clinical teams. In addition to clinicians, our site has physical therapists, occupational therapists, and social work teams to assist in patient care and discharge planning. As of this writing, we have cared for 543 patients, sent to us from one-third of Maryland’s hospitals. Use during the first wave of COVID was variable, with some hospitals sending us just a few patients. One Baltimore hospital sent us 8% of its COVID-19 patients. Because the patients have an average 5-day stay, the BCCFH has offloaded 2,600 bed-days of care from acute hospitals.
ROLE OF THE RRT IN A FIELD HOSPITAL
COVID-19 field hospitals must be prepared to respond effectively to decompensating patients. In our experience, effective RRTs provide a standard and reproducible approach to patient emergencies. In the conventional hospital setting, these teams consist of clinicians who can be called on by any healthcare worker to quickly assess deteriorating patients and intervene with treatment. The purpose of an RRT is to provide immediate care to a patient before progression to respiratory or cardiac arrest. RRTs proliferated in US hospitals after 2004 when the Institute for Healthcare Improvement in Boston, Massachusetts, recommended such teams for improved quality of care. Though studies report conflicting findings on the impact of RRTs on mortality rates, these studies were performed in traditional hospitals with ample resources, consultants, and clinicians familiar with their patients rather than in resource-limited field hospitals.4-13 Our field hospital has found RRTs, and the principles behind them, useful in the identification and management of decompensating COVID-19 patients.
A FOUR-STEP RAPID RESPONSE FRAMEWORK: CASE CORRELATION
An approach to managing decompensating patients in a COVID-19 field hospital can be considered in four phases: identification, assessment, resuscitation, and transport. Referring to these phases, the first case shows opportunities for improvement in resuscitation and transport. Although decompensation was identified, the patient was not transported to the triage bay for resuscitation, and there was confusion when trying to obtain the proper equipment. Additionally, EMS awaited the patient in the triage bay, while he remained in his cubicle, which delayed transport to an acute care hospital. The second case shows opportunities for improvement in identification and assessment. The patient had signs of impending decompensation that were not immediately recognized and treated. However, once decompensation occurred, the RRT was called and the patient was transported quickly to the triage bay, and then to the hospital via EMS.
In our experience at the BCCFH, identification is a key phase in COVID-19 care at a field hospital. Identification involves recognizing impending deterioration, as well as understanding risk factors for decompensation. For COVID-19 specifically, this requires heightened awareness of patients who are in the 2nd to 3rd week of symptoms. Data from Wuhan, China, suggest that decompensation occurs predictably around symptom day 9.14,15 At the BCCFH, the median symptom duration for patients who decompensated and returned to a hospital was 13 days. In both introductory cases, patients were in the high-risk 2nd week of symptoms when decompensation occurred. Clinicians at the BCCFH now discuss patient symptom day during their handoffs, when rounding, and when making decisions regarding acute care transfer. Our team has also integrated clinical information from our electronic health record to create a dashboard describing those patients requiring acute care transfer to assist in identifying other trends or predictive factors (Appendix D).
LESSONS FROM THE FIELD HOSPITAL: IMPROVING CLINICAL PERFORMANCE
Although RRTs are designed to activate when an individual patient decompensates, they should fit within a larger operational framework for patient safety. Our experience with emergencies at the BCCFH has yielded four opportunities for learning relevant to COVID-19 care in nontraditional settings (Table). These lessons include how to update staff on clinical process changes, unify communication systems, create a clinical drilling culture, and review cases to improve performance. They illustrate the importance of standardizing emergency processes, conducting frequent updates and drills, and ensuring continuous improvement. We found that, while caring for patients with an unpredictable, novel disease in a nontraditional setting and while wearing PPE and working with new colleagues during every shift, the best approach to support patients and staff is to anticipate emergencies rather than relying on individual staff to develop on-the-spot solutions.

CONCLUSION
The COVID-19 era has seen the unprecedented construction and utilization of emergency field hospital facilities. Such facilities can serve to offload some COVID-19 patients from strained healthcare infrastructure and provide essential care to these patients. We share many of the unique physical and logistical considerations specific to a nontraditional site. We optimized our space, our equipment, and our communication system. We learned how to identify, assess, resuscitate, and transport decompensating COVID-19 patients. Ultimately, our field hospital has been well utilized and successful at caring for patients because of its adaptability, accessibility, and safety record. Of the 15% of patients we transferred to a hospital for care, 81% were successfully stabilized and were willing to return to the BCCFH to complete their care. Our design included supportive care such as social work, physical and occupational therapy, and treatment of comorbidities, such as diabetes and substance use disorder. Our model demonstrates an effective nonhospital option for the care of lower-acuity, medically complex COVID-19 patients. If such facilities are used in subsequent COVID-19 outbreaks, we advise structured planning for the care of decompensating patients that takes into account the need for effective communication, drilling, and ongoing process improvement.
1. Rose J. U.S. Field Hospitals Stand Down, Most Without Treating Any COVID-19 Patients. All Things Considered. NPR; May 7, 2020. Accessed July 21, 2020. https://www.npr.org/2020/05/07/851712311/u-s-field-hospitals-stand-down-most-without-treating-any-covid-19-patients
2. Chen S, Zhang Z, Yang J, et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395(10232):1305-1314. https://doi.org/10.1016/s0140-6736(20)30744-3
3. Reilly RF. Medical and surgical care during the American Civil War, 1861-1865. Proc (Bayl Univ Med Cent). 2016;29(2):138-142. https://doi.org/10.1080/08998280.2016.11929390
4. Bellomo R, Goldsmith D, Uchino S, et al. Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Care Med. 2004;32(4):916-21. https://doi.org/10.1097/01.ccm.0000119428.02968.9e
5. Bellomo R, Goldsmith D, Uchino S, et al. A prospective before-and-after trial of a medical emergency team. Med J Aust. 2003;179(6):283-287.
6. Bristow PJ, Hillman KM, Chey T, et al. Rates of in-hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team. Med J Aust. 2000;173(5):236-240.
7. Buist MD, Moore GE, Bernard SA, Waxman BP, Anderson JN, Nguyen TV. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387-390. https://doi.org/10.1136/bmj.324.7334.387
8. DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons RL; Medical Emergency Response Improvement Team (MERIT). Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care. 2004;13(4):251-254. https://doi.org/10.1136/qhc.13.4.251
9. Goldhill DR, Worthington L, Mulcahy A, Tarling M, Sumner A. The patient-at-risk team: identifying and managing seriously ill ward patients. Anaesthesia. 1999;54(9):853-860. https://doi.org/10.1046/j.1365-2044.1999.00996.x
10. Hillman K, Chen J, Cretikos M, et al; MERIT study investigators. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005;365(9477):2091-2097. https://doi.org/10.1016/s0140-6736(05)66733-5
11. Kenward G, Castle N, Hodgetts T, Shaikh L. Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61(3):257-263. https://doi.org/10.1016/j.resuscitation.2004.01.021
12. Pittard AJ. Out of our reach? assessing the impact of introducing a critical care outreach service. Anaesthesia. 2003;58(9):882-885. https://doi.org/10.1046/j.1365-2044.2003.03331.x
13. Priestley G, Watson W, Rashidian A, et al. Introducing critical care outreach: a ward-randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398-1404. https://doi.org/10.1007/s00134-004-2268-7
14. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3
15. Zhou Y, Li W, Wang D, et al. Clinical time course of COVID-19, its neurological manifestation and some thoughts on its management. Stroke Vasc Neurol. 2020;5(2):177-179. https://doi.org/10.1136/svn-2020-000398
1. Rose J. U.S. Field Hospitals Stand Down, Most Without Treating Any COVID-19 Patients. All Things Considered. NPR; May 7, 2020. Accessed July 21, 2020. https://www.npr.org/2020/05/07/851712311/u-s-field-hospitals-stand-down-most-without-treating-any-covid-19-patients
2. Chen S, Zhang Z, Yang J, et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395(10232):1305-1314. https://doi.org/10.1016/s0140-6736(20)30744-3
3. Reilly RF. Medical and surgical care during the American Civil War, 1861-1865. Proc (Bayl Univ Med Cent). 2016;29(2):138-142. https://doi.org/10.1080/08998280.2016.11929390
4. Bellomo R, Goldsmith D, Uchino S, et al. Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Care Med. 2004;32(4):916-21. https://doi.org/10.1097/01.ccm.0000119428.02968.9e
5. Bellomo R, Goldsmith D, Uchino S, et al. A prospective before-and-after trial of a medical emergency team. Med J Aust. 2003;179(6):283-287.
6. Bristow PJ, Hillman KM, Chey T, et al. Rates of in-hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team. Med J Aust. 2000;173(5):236-240.
7. Buist MD, Moore GE, Bernard SA, Waxman BP, Anderson JN, Nguyen TV. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387-390. https://doi.org/10.1136/bmj.324.7334.387
8. DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons RL; Medical Emergency Response Improvement Team (MERIT). Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care. 2004;13(4):251-254. https://doi.org/10.1136/qhc.13.4.251
9. Goldhill DR, Worthington L, Mulcahy A, Tarling M, Sumner A. The patient-at-risk team: identifying and managing seriously ill ward patients. Anaesthesia. 1999;54(9):853-860. https://doi.org/10.1046/j.1365-2044.1999.00996.x
10. Hillman K, Chen J, Cretikos M, et al; MERIT study investigators. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005;365(9477):2091-2097. https://doi.org/10.1016/s0140-6736(05)66733-5
11. Kenward G, Castle N, Hodgetts T, Shaikh L. Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61(3):257-263. https://doi.org/10.1016/j.resuscitation.2004.01.021
12. Pittard AJ. Out of our reach? assessing the impact of introducing a critical care outreach service. Anaesthesia. 2003;58(9):882-885. https://doi.org/10.1046/j.1365-2044.2003.03331.x
13. Priestley G, Watson W, Rashidian A, et al. Introducing critical care outreach: a ward-randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398-1404. https://doi.org/10.1007/s00134-004-2268-7
14. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3
15. Zhou Y, Li W, Wang D, et al. Clinical time course of COVID-19, its neurological manifestation and some thoughts on its management. Stroke Vasc Neurol. 2020;5(2):177-179. https://doi.org/10.1136/svn-2020-000398
© 2021 Society of Hospital Medicine
ADHD through the retrospectoscope
Isolation in response to COVID-19 pandemic has driven many people to reestablish long forgotten connections between old friends and geographically distant relatives. Fed by the ease in which Zoom and other electronic miracles can bring once familiar voices and faces into our homes, we no longer need to wait until our high school or college reunions to reconnect.
The Class of 1962 at Pleasantville (N.Y.) High School has always attracted an unusually large number of attendees at its reunions, and its exuberant response to pandemic-fueled mini Zoom reunions is not surprising. With each virtual gathering we learn and relearn more about each other. I had always felt that because my birthday was in December that I was among the very youngest in my class. (New York’s school enrollment calendar cutoff is in December.) However, I recently learned that some of my classmates were even younger, having been born in the following spring.
This revelation prompted a discussion among the younger septuagenarians about whether we felt that our relative immaturity, at least as measured by the calendar, affected us. It was generally agreed that for the women, being younger seemed to present little problem. For, the men there were a few for whom immaturity put them at an athletic disadvantage. But, there was uniform agreement that social immaturity made dating an uncomfortable adventure. No one felt that his or her immaturity placed them at an academic disadvantage. Of course, all of these observations are heavily colored by the bias of those who have chosen to maintain contact with classmates.
A recent flurry of papers and commentaries about relative age at school entry and the diagnosis of attention deficit/hyperactivity disorder prompted me to ask my Zoom mates if they could recall anyone whom they would label as having exhibited the behavior we have all come to associate with ADHD (Vuori M et al. Children’s relative age and ADHD medication use: A Finnish population-based study. Pediatrics 2020 Oct. doi: 10.1542/peds.2019-4046, and Butter EM. Keeping relative age effects and ADHD care in context. Pediatrics. 2020;146[4]:e2020022798).
We could all recall classmates who struggled academically and seemed to not be paying attention. However, when one includes the hyperactivity descriptor we couldn’t recall anyone whose in-classroom physical activity drew our attention. Of course, there were many shared anecdotes about note passing, spitball throwing, and out-of-class shenanigans. But, from the perspective of behavior that disrupted the classroom there were very few. And, not surprisingly, given the intervening 6 decades, none of us could make an association between immaturity and the behavior.
While I have very few memories of what happened when I was in grade school, many of my classmates have vivid recollections of events both mundane and dramatic even as far back as first and second grade. Why do none of them recall classmates whose behavior would in current terminology be labeled as ADHD?
Were most of us that age bouncing off the walls and so there were no outliers? Were the teachers more tolerant because they expected that many children, particularly the younger ones, would be more physically active? Or, maybe we arrived at school, even those who were chronologically less mature, having already been settled down by home environments that neither fostered nor tolerated hyperactivity?
If you ask a pediatrician over the age of 70 if he or she recalls being taught anything about ADHD in medical school or seeing any children in his or her first years of practice who would fit the current diagnostic criteria, you will see them simply shrug. ADHD was simply not on our radar in the 1970s and 1980s. And it’s not because radar hadn’t been invented. We pediatricians were paying attention, and I trust in my high school classmates’ observations. I am sure there were isolated cases that could easily have been labeled as ADHD if the term had existed. But, the volume of hyperactive children a pediatrician sees today in the course of a normal office day just didn’t exist.
I have trouble believing that this dramatic increase in frequency is the result of accumulating genetic damage from Teflon cookware or climate change or air pollution. Although I am open to any serious attempt to explain the phenomenon I think we should look first into the home environment in which children are being raised. Sleep schedules, activity, and amusement opportunities as well as discipline styles – just to name a few – are far different now than before the ADHD diagnosis overtook the landscape.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Isolation in response to COVID-19 pandemic has driven many people to reestablish long forgotten connections between old friends and geographically distant relatives. Fed by the ease in which Zoom and other electronic miracles can bring once familiar voices and faces into our homes, we no longer need to wait until our high school or college reunions to reconnect.
The Class of 1962 at Pleasantville (N.Y.) High School has always attracted an unusually large number of attendees at its reunions, and its exuberant response to pandemic-fueled mini Zoom reunions is not surprising. With each virtual gathering we learn and relearn more about each other. I had always felt that because my birthday was in December that I was among the very youngest in my class. (New York’s school enrollment calendar cutoff is in December.) However, I recently learned that some of my classmates were even younger, having been born in the following spring.
This revelation prompted a discussion among the younger septuagenarians about whether we felt that our relative immaturity, at least as measured by the calendar, affected us. It was generally agreed that for the women, being younger seemed to present little problem. For, the men there were a few for whom immaturity put them at an athletic disadvantage. But, there was uniform agreement that social immaturity made dating an uncomfortable adventure. No one felt that his or her immaturity placed them at an academic disadvantage. Of course, all of these observations are heavily colored by the bias of those who have chosen to maintain contact with classmates.
A recent flurry of papers and commentaries about relative age at school entry and the diagnosis of attention deficit/hyperactivity disorder prompted me to ask my Zoom mates if they could recall anyone whom they would label as having exhibited the behavior we have all come to associate with ADHD (Vuori M et al. Children’s relative age and ADHD medication use: A Finnish population-based study. Pediatrics 2020 Oct. doi: 10.1542/peds.2019-4046, and Butter EM. Keeping relative age effects and ADHD care in context. Pediatrics. 2020;146[4]:e2020022798).
We could all recall classmates who struggled academically and seemed to not be paying attention. However, when one includes the hyperactivity descriptor we couldn’t recall anyone whose in-classroom physical activity drew our attention. Of course, there were many shared anecdotes about note passing, spitball throwing, and out-of-class shenanigans. But, from the perspective of behavior that disrupted the classroom there were very few. And, not surprisingly, given the intervening 6 decades, none of us could make an association between immaturity and the behavior.
While I have very few memories of what happened when I was in grade school, many of my classmates have vivid recollections of events both mundane and dramatic even as far back as first and second grade. Why do none of them recall classmates whose behavior would in current terminology be labeled as ADHD?
Were most of us that age bouncing off the walls and so there were no outliers? Were the teachers more tolerant because they expected that many children, particularly the younger ones, would be more physically active? Or, maybe we arrived at school, even those who were chronologically less mature, having already been settled down by home environments that neither fostered nor tolerated hyperactivity?
If you ask a pediatrician over the age of 70 if he or she recalls being taught anything about ADHD in medical school or seeing any children in his or her first years of practice who would fit the current diagnostic criteria, you will see them simply shrug. ADHD was simply not on our radar in the 1970s and 1980s. And it’s not because radar hadn’t been invented. We pediatricians were paying attention, and I trust in my high school classmates’ observations. I am sure there were isolated cases that could easily have been labeled as ADHD if the term had existed. But, the volume of hyperactive children a pediatrician sees today in the course of a normal office day just didn’t exist.
I have trouble believing that this dramatic increase in frequency is the result of accumulating genetic damage from Teflon cookware or climate change or air pollution. Although I am open to any serious attempt to explain the phenomenon I think we should look first into the home environment in which children are being raised. Sleep schedules, activity, and amusement opportunities as well as discipline styles – just to name a few – are far different now than before the ADHD diagnosis overtook the landscape.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Isolation in response to COVID-19 pandemic has driven many people to reestablish long forgotten connections between old friends and geographically distant relatives. Fed by the ease in which Zoom and other electronic miracles can bring once familiar voices and faces into our homes, we no longer need to wait until our high school or college reunions to reconnect.
The Class of 1962 at Pleasantville (N.Y.) High School has always attracted an unusually large number of attendees at its reunions, and its exuberant response to pandemic-fueled mini Zoom reunions is not surprising. With each virtual gathering we learn and relearn more about each other. I had always felt that because my birthday was in December that I was among the very youngest in my class. (New York’s school enrollment calendar cutoff is in December.) However, I recently learned that some of my classmates were even younger, having been born in the following spring.
This revelation prompted a discussion among the younger septuagenarians about whether we felt that our relative immaturity, at least as measured by the calendar, affected us. It was generally agreed that for the women, being younger seemed to present little problem. For, the men there were a few for whom immaturity put them at an athletic disadvantage. But, there was uniform agreement that social immaturity made dating an uncomfortable adventure. No one felt that his or her immaturity placed them at an academic disadvantage. Of course, all of these observations are heavily colored by the bias of those who have chosen to maintain contact with classmates.
A recent flurry of papers and commentaries about relative age at school entry and the diagnosis of attention deficit/hyperactivity disorder prompted me to ask my Zoom mates if they could recall anyone whom they would label as having exhibited the behavior we have all come to associate with ADHD (Vuori M et al. Children’s relative age and ADHD medication use: A Finnish population-based study. Pediatrics 2020 Oct. doi: 10.1542/peds.2019-4046, and Butter EM. Keeping relative age effects and ADHD care in context. Pediatrics. 2020;146[4]:e2020022798).
We could all recall classmates who struggled academically and seemed to not be paying attention. However, when one includes the hyperactivity descriptor we couldn’t recall anyone whose in-classroom physical activity drew our attention. Of course, there were many shared anecdotes about note passing, spitball throwing, and out-of-class shenanigans. But, from the perspective of behavior that disrupted the classroom there were very few. And, not surprisingly, given the intervening 6 decades, none of us could make an association between immaturity and the behavior.
While I have very few memories of what happened when I was in grade school, many of my classmates have vivid recollections of events both mundane and dramatic even as far back as first and second grade. Why do none of them recall classmates whose behavior would in current terminology be labeled as ADHD?
Were most of us that age bouncing off the walls and so there were no outliers? Were the teachers more tolerant because they expected that many children, particularly the younger ones, would be more physically active? Or, maybe we arrived at school, even those who were chronologically less mature, having already been settled down by home environments that neither fostered nor tolerated hyperactivity?
If you ask a pediatrician over the age of 70 if he or she recalls being taught anything about ADHD in medical school or seeing any children in his or her first years of practice who would fit the current diagnostic criteria, you will see them simply shrug. ADHD was simply not on our radar in the 1970s and 1980s. And it’s not because radar hadn’t been invented. We pediatricians were paying attention, and I trust in my high school classmates’ observations. I am sure there were isolated cases that could easily have been labeled as ADHD if the term had existed. But, the volume of hyperactive children a pediatrician sees today in the course of a normal office day just didn’t exist.
I have trouble believing that this dramatic increase in frequency is the result of accumulating genetic damage from Teflon cookware or climate change or air pollution. Although I am open to any serious attempt to explain the phenomenon I think we should look first into the home environment in which children are being raised. Sleep schedules, activity, and amusement opportunities as well as discipline styles – just to name a few – are far different now than before the ADHD diagnosis overtook the landscape.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Osteoporosis prevalence in PsA similar to general population
The rates of osteopenia and osteoporosis among individuals with psoriatic arthritis are comparable to those seen in the general population, research suggests.
The cohort study, published in Arthritis Care & Research, also found that clinicians are likely to refer patients for bone mineral density (BMD) testing based on osteoporosis risk factors or psoriatic arthritis disease severity markers.
Timothy S.H. Kwok, MD, of the University of Toronto, and coauthors wrote that previous research suggested a possible link between psoriatic arthritis and osteoporosis or osteopenia. However, no cohort studies appear to have examined this association.
The study involved 201 individuals with psoriatic arthritis attending a single specialist clinic, who were enrolled in a longitudinal study of psoriatic arthritis (PsA) and who were also referred for BMD testing with dual-energy x-ray absorptiometry.
Of these participants, 13% had a BMD in the osteoporotic range, 45% were in the osteopenic range, and 42% were in the normal range for BMD. The prevalence of osteoporosis observed in the general population aged 50 or above, observed in an earlier large prospective study, ranged from 7% to 16%, and osteopenia ranged from 27% to 46%.
“Our study suggests that patients with PsA have similar BMDs compared to the general population,” the authors wrote.
Researchers did note the suggestion that patients with polyarthritis had lower BMDs over time. Because of the small number of events, this did not achieve statistical significance, but “this relationship warrants further research, given that multiple cohort studies have independently demonstrated polyarticular onset of disease predicting clinical deformities and erosive disease in PsA,” they wrote.
They also saw that patients with increased body mass index had a significant 21% lower odds of having a BMD in the osteoporotic range, while those using biologics had a significant 83% lower odds.
Among participants with BMD scores in the osteopenic or osteoporotic range, these scores were seen in the lumbar spine in 63% of measurements, the femoral neck in 88%, and the total hip in 39%. Mean T-scores for the lumbar spine were –0.30±0.32, and for the femoral neck were –1.10±1.04 and the total hip, –0.45±0.42.
The study also examined what factors were associated with referral for BMD testing. They found that increasing age, menopause, elevated acute phase reactants, or use of biologics, methotrexate, and systemic glucocorticoids were associated with a higher likelihood of undergoing BMD testing.
Noting that the latest Canadian clinical practice guidelines on BMD testing advise that age, menopause, and use of systemic glucocorticoids use are risk factors that should prompt testing, the authors suggested clinicians were using a combination of traditional osteoporosis risk factors and markers of psoriatic disease severity to underpin their decision to refer.
However, they commented that none of the factors associated with a higher likelihood of having a BMD test were actually associated with lower BMD scores.
“This suggests that clinicians may be over-screening patients with PsA for osteopenia/osteoporosis, as they do not appear to be at baseline higher risk for lower BMD scores than the general population,” they wrote. “This is of importance, as there are currently no formal recommendations with regards to the optimal interval or time to commence BMD testing within the recent major PsA guidelines.”
The study was supported by a grant from the Krembil Foundation. No conflicts of interest were declared.
SOURCE: Kwok TSH et al. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24538.
The rates of osteopenia and osteoporosis among individuals with psoriatic arthritis are comparable to those seen in the general population, research suggests.
The cohort study, published in Arthritis Care & Research, also found that clinicians are likely to refer patients for bone mineral density (BMD) testing based on osteoporosis risk factors or psoriatic arthritis disease severity markers.
Timothy S.H. Kwok, MD, of the University of Toronto, and coauthors wrote that previous research suggested a possible link between psoriatic arthritis and osteoporosis or osteopenia. However, no cohort studies appear to have examined this association.
The study involved 201 individuals with psoriatic arthritis attending a single specialist clinic, who were enrolled in a longitudinal study of psoriatic arthritis (PsA) and who were also referred for BMD testing with dual-energy x-ray absorptiometry.
Of these participants, 13% had a BMD in the osteoporotic range, 45% were in the osteopenic range, and 42% were in the normal range for BMD. The prevalence of osteoporosis observed in the general population aged 50 or above, observed in an earlier large prospective study, ranged from 7% to 16%, and osteopenia ranged from 27% to 46%.
“Our study suggests that patients with PsA have similar BMDs compared to the general population,” the authors wrote.
Researchers did note the suggestion that patients with polyarthritis had lower BMDs over time. Because of the small number of events, this did not achieve statistical significance, but “this relationship warrants further research, given that multiple cohort studies have independently demonstrated polyarticular onset of disease predicting clinical deformities and erosive disease in PsA,” they wrote.
They also saw that patients with increased body mass index had a significant 21% lower odds of having a BMD in the osteoporotic range, while those using biologics had a significant 83% lower odds.
Among participants with BMD scores in the osteopenic or osteoporotic range, these scores were seen in the lumbar spine in 63% of measurements, the femoral neck in 88%, and the total hip in 39%. Mean T-scores for the lumbar spine were –0.30±0.32, and for the femoral neck were –1.10±1.04 and the total hip, –0.45±0.42.
The study also examined what factors were associated with referral for BMD testing. They found that increasing age, menopause, elevated acute phase reactants, or use of biologics, methotrexate, and systemic glucocorticoids were associated with a higher likelihood of undergoing BMD testing.
Noting that the latest Canadian clinical practice guidelines on BMD testing advise that age, menopause, and use of systemic glucocorticoids use are risk factors that should prompt testing, the authors suggested clinicians were using a combination of traditional osteoporosis risk factors and markers of psoriatic disease severity to underpin their decision to refer.
However, they commented that none of the factors associated with a higher likelihood of having a BMD test were actually associated with lower BMD scores.
“This suggests that clinicians may be over-screening patients with PsA for osteopenia/osteoporosis, as they do not appear to be at baseline higher risk for lower BMD scores than the general population,” they wrote. “This is of importance, as there are currently no formal recommendations with regards to the optimal interval or time to commence BMD testing within the recent major PsA guidelines.”
The study was supported by a grant from the Krembil Foundation. No conflicts of interest were declared.
SOURCE: Kwok TSH et al. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24538.
The rates of osteopenia and osteoporosis among individuals with psoriatic arthritis are comparable to those seen in the general population, research suggests.
The cohort study, published in Arthritis Care & Research, also found that clinicians are likely to refer patients for bone mineral density (BMD) testing based on osteoporosis risk factors or psoriatic arthritis disease severity markers.
Timothy S.H. Kwok, MD, of the University of Toronto, and coauthors wrote that previous research suggested a possible link between psoriatic arthritis and osteoporosis or osteopenia. However, no cohort studies appear to have examined this association.
The study involved 201 individuals with psoriatic arthritis attending a single specialist clinic, who were enrolled in a longitudinal study of psoriatic arthritis (PsA) and who were also referred for BMD testing with dual-energy x-ray absorptiometry.
Of these participants, 13% had a BMD in the osteoporotic range, 45% were in the osteopenic range, and 42% were in the normal range for BMD. The prevalence of osteoporosis observed in the general population aged 50 or above, observed in an earlier large prospective study, ranged from 7% to 16%, and osteopenia ranged from 27% to 46%.
“Our study suggests that patients with PsA have similar BMDs compared to the general population,” the authors wrote.
Researchers did note the suggestion that patients with polyarthritis had lower BMDs over time. Because of the small number of events, this did not achieve statistical significance, but “this relationship warrants further research, given that multiple cohort studies have independently demonstrated polyarticular onset of disease predicting clinical deformities and erosive disease in PsA,” they wrote.
They also saw that patients with increased body mass index had a significant 21% lower odds of having a BMD in the osteoporotic range, while those using biologics had a significant 83% lower odds.
Among participants with BMD scores in the osteopenic or osteoporotic range, these scores were seen in the lumbar spine in 63% of measurements, the femoral neck in 88%, and the total hip in 39%. Mean T-scores for the lumbar spine were –0.30±0.32, and for the femoral neck were –1.10±1.04 and the total hip, –0.45±0.42.
The study also examined what factors were associated with referral for BMD testing. They found that increasing age, menopause, elevated acute phase reactants, or use of biologics, methotrexate, and systemic glucocorticoids were associated with a higher likelihood of undergoing BMD testing.
Noting that the latest Canadian clinical practice guidelines on BMD testing advise that age, menopause, and use of systemic glucocorticoids use are risk factors that should prompt testing, the authors suggested clinicians were using a combination of traditional osteoporosis risk factors and markers of psoriatic disease severity to underpin their decision to refer.
However, they commented that none of the factors associated with a higher likelihood of having a BMD test were actually associated with lower BMD scores.
“This suggests that clinicians may be over-screening patients with PsA for osteopenia/osteoporosis, as they do not appear to be at baseline higher risk for lower BMD scores than the general population,” they wrote. “This is of importance, as there are currently no formal recommendations with regards to the optimal interval or time to commence BMD testing within the recent major PsA guidelines.”
The study was supported by a grant from the Krembil Foundation. No conflicts of interest were declared.
SOURCE: Kwok TSH et al. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24538.
FROM ARTHRITIS CARE & RESEARCH
Home pregnancy tests—Is ectopic always on your mind?
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
2021 Update on obstetrics
While 2020 was a challenge to say the least, obstetrician-gynecologists remained on the frontline caring for women through it all. Life continued despite the COVID-19 pandemic: prenatal care was delivered, albeit at times in different ways; babies were born; and our role in improving outcomes for women and their children became even more important. This year’s Update focuses on clinical guidelines centered on safety and optimal outcomes for women and children.
ACOG and SMFM update guidance on FGR management
American College of Obstetricians and Gynecologists. Practice advisory: Updated guidance regarding fetal growth restriction. September 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/09/updated-guidance-regarding-fetal-growth-restriction. Accessed December 18, 2020.
Fetal growth restriction (FGR) affects up to 10% of pregnancies and is a leading cause of infant morbidity and mortality. Suboptimal fetal growth can have lasting negative effects on development into early childhood and, some hypothesize, even into adulthood.1,2 Antenatal detection of fetuses with FGR is critical so that antenatal testing can be implemented in an attempt to deliver improved clinical outcomes. FGR is defined by several different diagnostic criteria, and many studies have been conducted to determine how best to diagnose this condition.
In September 2020, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory regarding guidance on FGR in an effort to align the ACOG Practice Bulletin No. 204, ACOG Committee Opinion No. 764, and SMFM (Society for Maternal-Fetal Medicine) Consult Series No. 52.3-5 This guidance updates and replaces prior guidelines, with an emphasis on 3 notable changes.
FGR definition, workup have changed
While the original definition of FGR was an estimated fetal weight (EFW) of less than the 10th percentile for gestational age, a similar level of accuracy in prediction of subsequent small for gestational age (SGA) at birth has been shown when this or an abdominal circumference (AC) of less than the 10th percentile is used. Based on these findings, SMFM now recommends that FGR be defined as an EFW or AC of less than the 10th percentile for gestational age.
Recent studies have done head-to-head comparisons of different methods of estimating fetal weight to determine the best detection and pregnancy outcome improvement in FGR. In all instances, the Hadlock formula has continued to more accurately estimate fetal weight, prediction of SGA, and composite neonatal morbidity. As such, new guidelines recommend that population-based fetal growth references (that is, the Hadlock formula) should be used to determine ultrasonography-derived fetal weight percentiles.
The new guidance also suggests classification of FGR based on gestational age at onset, with early FGR at less than 32 weeks and late FGR at 32 or more weeks. The definition of severe FGR is reserved for fetuses with an EFW of less than the 3rd percentile. A diagnosis of FGR should prompt the recommendation for a detailed obstetric ultrasonography. Diagnostic genetic testing should be offered in cases of early-onset FGR, concomitant sonographic abnormalities, and/or polyhydramnios. Routine serum screening for toxoplasmosis, rubella, herpes, or cytomegalovirus (CMV) should not be done unless there are risk factors for infection. If amniocentesis is performed for genetic diagnostic testing, consideration can be made for polymerase chain reaction for CMV in the amniotic fluid.
Continue to: Timing of delivery in isolated FGR...
Timing of delivery in isolated FGR
A complicating factor in diagnosing FGR is distinguishing between the pathologically growth-restricted fetus and the constitutionally small fetus. Antenatal testing and serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for evidence of fetal compromise until delivery is planned.
The current ACOG Practice Bulletin No. 204 and Committee Opinion No. 764 recommend delivery between 38 0/7 and 39 6/7 weeks in the setting of isolated FGR with reassuring fetal testing and umbilical artery Doppler assessment.To further refine this, the new recommendations use the growth percentiles. In cases of isolated FGR with EFW between the 3rd and 10th percentile in the setting of normal umbilical artery Doppler, delivery is recommended between 38 and 39 weeks’ gestation. In cases of isolated FGR with EFW of less than the 3rd percentile (severe FGR) in the setting of normal umbilical artery Doppler, delivery is recommended at 37 weeks.
Timing of delivery in complicated FGR
A normal umbilical artery Doppler reflects the low impedance that is necessary for continuous forward flow of blood to the fetus. Abnormal umbilical artery Doppler signifies aberrations of this low-pressure system that affect the amount of continuous forward flow during diastole of the cardiac cycle. With continued compromise, there is progression to absent end-diastolic velocity (AEDV) and, most concerning, reversed end-diastolic velocity (REDV).
Serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for progression that is associated with perinatal mortality, since intervention can be initiated in the form of delivery. Delivery at 37 weeks is recommended for FGR with elevated umbilical artery Doppler of greater than the 95th percentile for gestational age. For FGR with AEDV, delivery is recommended between 33 and 34 weeks of gestation and for FGR with REDV between 30 and 32 weeks, as the neonatal morbidity and mortality associated with continuing the pregnancy outweighs the risks of prematurity in this setting. Because of the abnormal placental-fetal circulation in FGR complicated by AEDV/REDV, there may be a higher likelihood of fetal intolerance of labor and cesarean delivery (CD) may be considered.
- Fetal growth restriction is now defined as EFW of less than the 10th percentile or AC of less than the 10th percentile.
- Evaluation of FGR includes detailed anatomic survey and consideration of genetic evaluation, but infection screening should be done only if the patient is at risk for infection.
- With reassuring antenatal testing and normal umbilical artery Doppler studies, delivery is recommended at 38 to 39 weeks for isolated FGR with EFW in the 3rd to 10th percentile and at 37 weeks for FGR with EFW of less than the 3rd percentile.
- Umbilical artery Doppler studies are used to decrease the risk of perinatal mortality and further guide timing of delivery
Continue to: New recommendations for PROM management...
New recommendations for PROM management
American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
Rupture of membranes prior to the onset of labor occurs at term in 8% of pregnancies and in the preterm period in 2% to 3% of pregnancies.6 Accurate diagnosis, gestational age, evidence of infection, and discussion of the risks and benefits to the mother and fetus/neonate are necessary to optimize outcomes. In the absence of other indications for delivery, a gestational age of 34 or more weeks traditionally has been the cutoff to proceed with delivery, although this has not been globally agreed on and/or practiced.
ACOG has published a comprehensive update that incorporates the results of the PPROMT trial and other recommendations for the diagnosis and management of both term and preterm prelabor rupture of membranes (PROM).6,7
Making the diagnosis
Diagnosis of PROM usually can be made clinically via history and the classic triad of physical exam findings—pooling of fluid, basic pH, and ferning; some institutions also use commercially available tests that detect placental-derived proteins. Both ACOG and the US Food and Drug Administration caution against using these tests alone without clinical evaluation due to concern for false-positives and false-negatives that lead to adverse maternal and fetal/neonatal outcomes. For equivocal cases, ultrasonography for amniotic fluid evaluation and ultrasonography-guided dye tests can be used to assist in accurate diagnosis, especially in the preterm period in which there are significant implications for pregnancy management.
PROM management depends on gestational age
All management recommendations require reassuring fetal testing, evaluation for infection, and no other contraindications to expectant management. Once these are established, the most important determinant of PROM management then becomes gestational age.
Previable PROM
Previable PROM (usually defined as less than 23–24 weeks) has high risks of both maternal and fetal/neonatal morbidity and mortality from infection, hemorrhage, pulmonary hypoplasia, and extreme prematurity. These very difficult cases benefit from a multidisciplinary approach to patient counseling regarding expectant management versus immediate delivery.
If expectant management is chosen, outpatient management with close monitoring for signs of maternal infection may be done until an agreed on gestational age of viability. Then inpatient management with fetal monitoring, corticosteroids, tocolysis, magnesium for neuroprotection, and group B streptococcus (GBS) prophylaxis may be considered as appropriate.
Preterm PROM at less than 34 weeks
If the mother and fetus are otherwise stable, PROM at less than 34 weeks warrants inpatient expectant management with close maternal and fetal monitoring for signs of infection and labor. Management includes latency antibiotics, antenatal corticosteroids, magnesium for neuroprotection if less than 32 weeks’ gestation and at risk for imminent delivery, and GBS prophylaxis. While tocolysis may increase latency and help with steroid course completion, it should be used cautiously and avoided in cases of abruption or chorioamnionitis. Although there is no definitive recommendation published, a rescue course of steroids may be considered as appropriate but should not delay an indicated delivery.
Continue to: Late preterm PROM...
Late preterm PROM
The biggest change to clinical management in this ACOG Practice Bulletin is for late preterm (34–36 6/7 weeks) PROM, with the recommendation for either immediate delivery or expectant management up to 37 weeks stemming from the PPROMPT study by Morris and colleagues.7
From the neonatal perspective, no difference has been demonstrated between immediate delivery and expectant management for neonatal sepsis or a composite neonatal morbidity and mortality. Expectant management may be preferred from the neonatal point of view as immediate delivery was associated with an increased rate of neonatal respiratory distress, mechanical ventilation, and length of stay in the neonatal intensive care unit. The potential for long-term neurodevelopmental outcomes of delivery at 34 versus 37 weeks also should be considered.
From the maternal perspective, expectant management has an increased risk of antepartum and postpartum hemorrhage, fever, antibiotic use, and maternal length of stay, but a decreased risk of CD.
A late preterm steroid course can be considered if delivery is planned in no less than 24 hours and likely to occur in the next 7 days and if the patient has not already received a course of steroids. A rescue course of steroids is not indicated if the patient received a steroid course prior in the pregnancy. While appropriate GBS prophylaxis is recommended, latency antibiotics and tocolysis are not, and delivery should not be delayed if chorioamnionitis is diagnosed.
Ultimately, preterm PROM management with a stable mother and fetus at or beyond 34 weeks requires comprehensive counseling of the risks and benefits for both mother and fetus/neonate. A multidisciplinary team that together counsels the patient also may help with this shared decision making.
Term PROM
For patients with term PROM, delivery is recommended. Although a short period of expectant management for 12 to 24 hours is reported as “reasonable,” the risk of infection increases with the length of rupture of membranes. Therefore, induction of labor or CD soon after rupture of membranes is recommended for patients who are GBS positive and is preferred for all others.
- Accurate diagnosis is necessary for appropriate counseling and management of PROM.
- Delivery is recommended for term PROM, chorioamnionitis, and for patients with previable PROM who do not desire expectant management.
- If the mother and fetus are otherwise stable, expectant management of preterm PROM until 34 to 37 weeks is recommended.
- The decision of when to deliver between 34 and 37 weeks is best made with multidisciplinary counseling and shared decision making with the patient.
VTE prophylaxis in pregnancy: Regimen adjustments, CD strategies, and COVID-19 considerations
Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series No. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17
Venous thromboembolism (VTE) prophylaxis is a timely topic for a number of reasons. First, a shortage of unfractionated heparin prompted an ACOG Practice Advisory, endorsed by SMFM and the Society for Obstetric Anesthesia and Perinatology, regarding use of low molecular weight heparin (LMWH) in the peripartum period.8 In addition, SMFM released updated recommendations for VTE prophylaxis for CD as part of the SMFM Consult Series.9 Finally, there is evidence that COVID-19 infection may increase the risk of coagulopathy, leading to consideration of additional VTE prophylaxis for pregnant and postpartum women with COVID-19.
Candidates for prophylaxis
As recommended by the ACOG Practice Bulletin on thromboembolism in pregnancy, women who may require VTE prophylaxis during pregnancy and/or the postpartum period include those with10:
- VTE diagnosed during pregnancy
- a history of VTE, including during pregnancy or with use of hormonal contraception
- a history of thrombophilia with or without a personal or family history of VTE.
For these patients, LMWH has many advantages over unfractionated heparin, including ease of use and reliability of dosing. It generally is preferred in pregnancy and postpartum (for both prophylactic and therapeutic anticoagulation) by patients and providers.
The Practice Bulletin references a strategy that describes converting LMWH to unfractionated heparin at around 36 weeks’ gestation in preparation for delivery because unfractionated heparin has the advantage of a shorter half-life and the option for anticoagulation reversal with protamine sulfate. In the Practice Advisory, a global shortage of unfractionated heparin and an argument that the above conversion was less about concern for maternal hemorrhage and more about avoiding spinal and epidural hematomas led to the following recommendations for continued use of LMWH through delivery:
- LMWH heparin can be discontinued in a planned fashion prior to scheduled induction of labor or CD (generally 12 hours for prophylactic dosing and 24 hours for intermediate dosing).
- Patients in spontaneous labor may receive neuraxial anesthesia 12 hours after the last prophylactic dose and 24 hours after the last intermediate dose of LMWH.
- Patients who require anticoagulation during pregnancy should be counseled that if they have vaginal bleeding, leakage of fluid, or regular contractions they should be evaluated prior to taking their next dose of anticoagulant.
- In the absence of other complications, delivery should not be before 39 weeks for the indication of anticoagulation requirement alone.
Continue to: Managing VTE risk in CD...
Managing VTE risk in CD
Recognizing that VTE is a major cause of maternal morbidity and mortality, as well as the variety of the published guidelines for VTE prophylaxis after CD, the SMFM Consult Series provides recommendations to assist clinicians caring for postpartum women after CD. As reviewed in the ACOG Practice Bulletin, there are good data to support pharmacologic prophylaxis during pregnancy and the postpartum period for women with a history of VTE or a thrombophilia. Solid evidence is lacking, however, for what to do for women who have a CD without this history but may have other potential risk factors for VTE, such as obesity, preeclampsia, and transfusion requirement. Universal pharmacologic prophylaxis also is not yet supported by evidence. SMFM supports LMWH as the preferred medication in pregnancy and postpartum and provides these additional recommendations:
- All women who have a CD should have sequential compression devices (SCDs) placed prior to surgery and continued until they are ambulatory.
- Women with a history of VTE or thrombophilia without history of VTE should have SCDs and pharmacologic VTE prophylaxis for 6 weeks postpartum.
- Intermediate dosing of LMWH is recommended for patients with class III obesity.
- Institutions should develop patient safety bundles for VTE prophylaxis to identify additional risk factors that may warrant pharmacologic prophylaxis after CD in select patients.
Our approach to patients with COVID-19 infection
At our institution, we recently incorporated a VTE prophylaxis protocol into our electronic medical record that provides risk stratification for each patient. In addition to the above recommendations, our patients may qualify for short-term in-house or longer postpartum prophylaxis depending on risk factors.
A new risk factor in recent months is COVID-19 infection, which appears to increase the risk of coagulopathy, especially in patients with disease severe enough to warrant hospitalization. Given the potential for additive risk in pregnancy, in consult with our medicine colleagues, we have placed some of our more ill hospitalized pregnant patients on a course of prophylactic LMWH both in the hospital and after discharge independent of delivery status or mode of delivery. ●
- Pregnant patients with a history of VTE or a thrombophilia may be candidates for pharmacologic anticoagulation during pregnancy and/or postpartum.
- LMWH is the preferred method of pharmacologic VTE prophylaxis during pregnancy and postpartum.
- For most patients, CD and neuraxial anesthesia safely can be performed 12 to 24 hours after the last dose of prophylactic or intermediate LMWH, respectively.
- All patients undergoing CD should have at least mechanical VTE prophylaxis with SCDs.
- All women who have a CD should be evaluated via institutional patient safety bundles for VTE prophylaxis for additional risk factors that potentially warrant postpartum pharmacologic VTE prophylaxis.
- More data are needed to determine recommendations for universal/ near universal pharmacologic VTE prophylaxis in the postpartum period.
- Pregnant or postpartum patients with moderate to severe COVID-19 infection may be at increased risk for VTE, warranting consideration of additional pharmacologic prophylaxis.
- Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571-577.
- Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect. 2011;25:153-172.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics and Society for Maternal-Fetal Medicine. ACOG practice bulletin no. 204: Fetal growth restriction. Obstet Gynecol. 2019;133: e97-e109.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice and Society for Maternal-Fetal Medicine. ACOG committee opinion no. 764: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155.
- Society for Maternal-Fetal Medicine; Martins JG, Biggio FR, Abuhamad A. SMFM consult series no. 52: diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2020;223:B2-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
- Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
- Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
- Pacheco LD, Saade G, Metz TD. Society for MaternalFetal Medicine consult series no. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 196: Thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
While 2020 was a challenge to say the least, obstetrician-gynecologists remained on the frontline caring for women through it all. Life continued despite the COVID-19 pandemic: prenatal care was delivered, albeit at times in different ways; babies were born; and our role in improving outcomes for women and their children became even more important. This year’s Update focuses on clinical guidelines centered on safety and optimal outcomes for women and children.
ACOG and SMFM update guidance on FGR management
American College of Obstetricians and Gynecologists. Practice advisory: Updated guidance regarding fetal growth restriction. September 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/09/updated-guidance-regarding-fetal-growth-restriction. Accessed December 18, 2020.
Fetal growth restriction (FGR) affects up to 10% of pregnancies and is a leading cause of infant morbidity and mortality. Suboptimal fetal growth can have lasting negative effects on development into early childhood and, some hypothesize, even into adulthood.1,2 Antenatal detection of fetuses with FGR is critical so that antenatal testing can be implemented in an attempt to deliver improved clinical outcomes. FGR is defined by several different diagnostic criteria, and many studies have been conducted to determine how best to diagnose this condition.
In September 2020, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory regarding guidance on FGR in an effort to align the ACOG Practice Bulletin No. 204, ACOG Committee Opinion No. 764, and SMFM (Society for Maternal-Fetal Medicine) Consult Series No. 52.3-5 This guidance updates and replaces prior guidelines, with an emphasis on 3 notable changes.
FGR definition, workup have changed
While the original definition of FGR was an estimated fetal weight (EFW) of less than the 10th percentile for gestational age, a similar level of accuracy in prediction of subsequent small for gestational age (SGA) at birth has been shown when this or an abdominal circumference (AC) of less than the 10th percentile is used. Based on these findings, SMFM now recommends that FGR be defined as an EFW or AC of less than the 10th percentile for gestational age.
Recent studies have done head-to-head comparisons of different methods of estimating fetal weight to determine the best detection and pregnancy outcome improvement in FGR. In all instances, the Hadlock formula has continued to more accurately estimate fetal weight, prediction of SGA, and composite neonatal morbidity. As such, new guidelines recommend that population-based fetal growth references (that is, the Hadlock formula) should be used to determine ultrasonography-derived fetal weight percentiles.
The new guidance also suggests classification of FGR based on gestational age at onset, with early FGR at less than 32 weeks and late FGR at 32 or more weeks. The definition of severe FGR is reserved for fetuses with an EFW of less than the 3rd percentile. A diagnosis of FGR should prompt the recommendation for a detailed obstetric ultrasonography. Diagnostic genetic testing should be offered in cases of early-onset FGR, concomitant sonographic abnormalities, and/or polyhydramnios. Routine serum screening for toxoplasmosis, rubella, herpes, or cytomegalovirus (CMV) should not be done unless there are risk factors for infection. If amniocentesis is performed for genetic diagnostic testing, consideration can be made for polymerase chain reaction for CMV in the amniotic fluid.
Continue to: Timing of delivery in isolated FGR...
Timing of delivery in isolated FGR
A complicating factor in diagnosing FGR is distinguishing between the pathologically growth-restricted fetus and the constitutionally small fetus. Antenatal testing and serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for evidence of fetal compromise until delivery is planned.
The current ACOG Practice Bulletin No. 204 and Committee Opinion No. 764 recommend delivery between 38 0/7 and 39 6/7 weeks in the setting of isolated FGR with reassuring fetal testing and umbilical artery Doppler assessment.To further refine this, the new recommendations use the growth percentiles. In cases of isolated FGR with EFW between the 3rd and 10th percentile in the setting of normal umbilical artery Doppler, delivery is recommended between 38 and 39 weeks’ gestation. In cases of isolated FGR with EFW of less than the 3rd percentile (severe FGR) in the setting of normal umbilical artery Doppler, delivery is recommended at 37 weeks.
Timing of delivery in complicated FGR
A normal umbilical artery Doppler reflects the low impedance that is necessary for continuous forward flow of blood to the fetus. Abnormal umbilical artery Doppler signifies aberrations of this low-pressure system that affect the amount of continuous forward flow during diastole of the cardiac cycle. With continued compromise, there is progression to absent end-diastolic velocity (AEDV) and, most concerning, reversed end-diastolic velocity (REDV).
Serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for progression that is associated with perinatal mortality, since intervention can be initiated in the form of delivery. Delivery at 37 weeks is recommended for FGR with elevated umbilical artery Doppler of greater than the 95th percentile for gestational age. For FGR with AEDV, delivery is recommended between 33 and 34 weeks of gestation and for FGR with REDV between 30 and 32 weeks, as the neonatal morbidity and mortality associated with continuing the pregnancy outweighs the risks of prematurity in this setting. Because of the abnormal placental-fetal circulation in FGR complicated by AEDV/REDV, there may be a higher likelihood of fetal intolerance of labor and cesarean delivery (CD) may be considered.
- Fetal growth restriction is now defined as EFW of less than the 10th percentile or AC of less than the 10th percentile.
- Evaluation of FGR includes detailed anatomic survey and consideration of genetic evaluation, but infection screening should be done only if the patient is at risk for infection.
- With reassuring antenatal testing and normal umbilical artery Doppler studies, delivery is recommended at 38 to 39 weeks for isolated FGR with EFW in the 3rd to 10th percentile and at 37 weeks for FGR with EFW of less than the 3rd percentile.
- Umbilical artery Doppler studies are used to decrease the risk of perinatal mortality and further guide timing of delivery
Continue to: New recommendations for PROM management...
New recommendations for PROM management
American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
Rupture of membranes prior to the onset of labor occurs at term in 8% of pregnancies and in the preterm period in 2% to 3% of pregnancies.6 Accurate diagnosis, gestational age, evidence of infection, and discussion of the risks and benefits to the mother and fetus/neonate are necessary to optimize outcomes. In the absence of other indications for delivery, a gestational age of 34 or more weeks traditionally has been the cutoff to proceed with delivery, although this has not been globally agreed on and/or practiced.
ACOG has published a comprehensive update that incorporates the results of the PPROMT trial and other recommendations for the diagnosis and management of both term and preterm prelabor rupture of membranes (PROM).6,7
Making the diagnosis
Diagnosis of PROM usually can be made clinically via history and the classic triad of physical exam findings—pooling of fluid, basic pH, and ferning; some institutions also use commercially available tests that detect placental-derived proteins. Both ACOG and the US Food and Drug Administration caution against using these tests alone without clinical evaluation due to concern for false-positives and false-negatives that lead to adverse maternal and fetal/neonatal outcomes. For equivocal cases, ultrasonography for amniotic fluid evaluation and ultrasonography-guided dye tests can be used to assist in accurate diagnosis, especially in the preterm period in which there are significant implications for pregnancy management.
PROM management depends on gestational age
All management recommendations require reassuring fetal testing, evaluation for infection, and no other contraindications to expectant management. Once these are established, the most important determinant of PROM management then becomes gestational age.
Previable PROM
Previable PROM (usually defined as less than 23–24 weeks) has high risks of both maternal and fetal/neonatal morbidity and mortality from infection, hemorrhage, pulmonary hypoplasia, and extreme prematurity. These very difficult cases benefit from a multidisciplinary approach to patient counseling regarding expectant management versus immediate delivery.
If expectant management is chosen, outpatient management with close monitoring for signs of maternal infection may be done until an agreed on gestational age of viability. Then inpatient management with fetal monitoring, corticosteroids, tocolysis, magnesium for neuroprotection, and group B streptococcus (GBS) prophylaxis may be considered as appropriate.
Preterm PROM at less than 34 weeks
If the mother and fetus are otherwise stable, PROM at less than 34 weeks warrants inpatient expectant management with close maternal and fetal monitoring for signs of infection and labor. Management includes latency antibiotics, antenatal corticosteroids, magnesium for neuroprotection if less than 32 weeks’ gestation and at risk for imminent delivery, and GBS prophylaxis. While tocolysis may increase latency and help with steroid course completion, it should be used cautiously and avoided in cases of abruption or chorioamnionitis. Although there is no definitive recommendation published, a rescue course of steroids may be considered as appropriate but should not delay an indicated delivery.
Continue to: Late preterm PROM...
Late preterm PROM
The biggest change to clinical management in this ACOG Practice Bulletin is for late preterm (34–36 6/7 weeks) PROM, with the recommendation for either immediate delivery or expectant management up to 37 weeks stemming from the PPROMPT study by Morris and colleagues.7
From the neonatal perspective, no difference has been demonstrated between immediate delivery and expectant management for neonatal sepsis or a composite neonatal morbidity and mortality. Expectant management may be preferred from the neonatal point of view as immediate delivery was associated with an increased rate of neonatal respiratory distress, mechanical ventilation, and length of stay in the neonatal intensive care unit. The potential for long-term neurodevelopmental outcomes of delivery at 34 versus 37 weeks also should be considered.
From the maternal perspective, expectant management has an increased risk of antepartum and postpartum hemorrhage, fever, antibiotic use, and maternal length of stay, but a decreased risk of CD.
A late preterm steroid course can be considered if delivery is planned in no less than 24 hours and likely to occur in the next 7 days and if the patient has not already received a course of steroids. A rescue course of steroids is not indicated if the patient received a steroid course prior in the pregnancy. While appropriate GBS prophylaxis is recommended, latency antibiotics and tocolysis are not, and delivery should not be delayed if chorioamnionitis is diagnosed.
Ultimately, preterm PROM management with a stable mother and fetus at or beyond 34 weeks requires comprehensive counseling of the risks and benefits for both mother and fetus/neonate. A multidisciplinary team that together counsels the patient also may help with this shared decision making.
Term PROM
For patients with term PROM, delivery is recommended. Although a short period of expectant management for 12 to 24 hours is reported as “reasonable,” the risk of infection increases with the length of rupture of membranes. Therefore, induction of labor or CD soon after rupture of membranes is recommended for patients who are GBS positive and is preferred for all others.
- Accurate diagnosis is necessary for appropriate counseling and management of PROM.
- Delivery is recommended for term PROM, chorioamnionitis, and for patients with previable PROM who do not desire expectant management.
- If the mother and fetus are otherwise stable, expectant management of preterm PROM until 34 to 37 weeks is recommended.
- The decision of when to deliver between 34 and 37 weeks is best made with multidisciplinary counseling and shared decision making with the patient.
VTE prophylaxis in pregnancy: Regimen adjustments, CD strategies, and COVID-19 considerations
Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series No. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17
Venous thromboembolism (VTE) prophylaxis is a timely topic for a number of reasons. First, a shortage of unfractionated heparin prompted an ACOG Practice Advisory, endorsed by SMFM and the Society for Obstetric Anesthesia and Perinatology, regarding use of low molecular weight heparin (LMWH) in the peripartum period.8 In addition, SMFM released updated recommendations for VTE prophylaxis for CD as part of the SMFM Consult Series.9 Finally, there is evidence that COVID-19 infection may increase the risk of coagulopathy, leading to consideration of additional VTE prophylaxis for pregnant and postpartum women with COVID-19.
Candidates for prophylaxis
As recommended by the ACOG Practice Bulletin on thromboembolism in pregnancy, women who may require VTE prophylaxis during pregnancy and/or the postpartum period include those with10:
- VTE diagnosed during pregnancy
- a history of VTE, including during pregnancy or with use of hormonal contraception
- a history of thrombophilia with or without a personal or family history of VTE.
For these patients, LMWH has many advantages over unfractionated heparin, including ease of use and reliability of dosing. It generally is preferred in pregnancy and postpartum (for both prophylactic and therapeutic anticoagulation) by patients and providers.
The Practice Bulletin references a strategy that describes converting LMWH to unfractionated heparin at around 36 weeks’ gestation in preparation for delivery because unfractionated heparin has the advantage of a shorter half-life and the option for anticoagulation reversal with protamine sulfate. In the Practice Advisory, a global shortage of unfractionated heparin and an argument that the above conversion was less about concern for maternal hemorrhage and more about avoiding spinal and epidural hematomas led to the following recommendations for continued use of LMWH through delivery:
- LMWH heparin can be discontinued in a planned fashion prior to scheduled induction of labor or CD (generally 12 hours for prophylactic dosing and 24 hours for intermediate dosing).
- Patients in spontaneous labor may receive neuraxial anesthesia 12 hours after the last prophylactic dose and 24 hours after the last intermediate dose of LMWH.
- Patients who require anticoagulation during pregnancy should be counseled that if they have vaginal bleeding, leakage of fluid, or regular contractions they should be evaluated prior to taking their next dose of anticoagulant.
- In the absence of other complications, delivery should not be before 39 weeks for the indication of anticoagulation requirement alone.
Continue to: Managing VTE risk in CD...
Managing VTE risk in CD
Recognizing that VTE is a major cause of maternal morbidity and mortality, as well as the variety of the published guidelines for VTE prophylaxis after CD, the SMFM Consult Series provides recommendations to assist clinicians caring for postpartum women after CD. As reviewed in the ACOG Practice Bulletin, there are good data to support pharmacologic prophylaxis during pregnancy and the postpartum period for women with a history of VTE or a thrombophilia. Solid evidence is lacking, however, for what to do for women who have a CD without this history but may have other potential risk factors for VTE, such as obesity, preeclampsia, and transfusion requirement. Universal pharmacologic prophylaxis also is not yet supported by evidence. SMFM supports LMWH as the preferred medication in pregnancy and postpartum and provides these additional recommendations:
- All women who have a CD should have sequential compression devices (SCDs) placed prior to surgery and continued until they are ambulatory.
- Women with a history of VTE or thrombophilia without history of VTE should have SCDs and pharmacologic VTE prophylaxis for 6 weeks postpartum.
- Intermediate dosing of LMWH is recommended for patients with class III obesity.
- Institutions should develop patient safety bundles for VTE prophylaxis to identify additional risk factors that may warrant pharmacologic prophylaxis after CD in select patients.
Our approach to patients with COVID-19 infection
At our institution, we recently incorporated a VTE prophylaxis protocol into our electronic medical record that provides risk stratification for each patient. In addition to the above recommendations, our patients may qualify for short-term in-house or longer postpartum prophylaxis depending on risk factors.
A new risk factor in recent months is COVID-19 infection, which appears to increase the risk of coagulopathy, especially in patients with disease severe enough to warrant hospitalization. Given the potential for additive risk in pregnancy, in consult with our medicine colleagues, we have placed some of our more ill hospitalized pregnant patients on a course of prophylactic LMWH both in the hospital and after discharge independent of delivery status or mode of delivery. ●
- Pregnant patients with a history of VTE or a thrombophilia may be candidates for pharmacologic anticoagulation during pregnancy and/or postpartum.
- LMWH is the preferred method of pharmacologic VTE prophylaxis during pregnancy and postpartum.
- For most patients, CD and neuraxial anesthesia safely can be performed 12 to 24 hours after the last dose of prophylactic or intermediate LMWH, respectively.
- All patients undergoing CD should have at least mechanical VTE prophylaxis with SCDs.
- All women who have a CD should be evaluated via institutional patient safety bundles for VTE prophylaxis for additional risk factors that potentially warrant postpartum pharmacologic VTE prophylaxis.
- More data are needed to determine recommendations for universal/ near universal pharmacologic VTE prophylaxis in the postpartum period.
- Pregnant or postpartum patients with moderate to severe COVID-19 infection may be at increased risk for VTE, warranting consideration of additional pharmacologic prophylaxis.
While 2020 was a challenge to say the least, obstetrician-gynecologists remained on the frontline caring for women through it all. Life continued despite the COVID-19 pandemic: prenatal care was delivered, albeit at times in different ways; babies were born; and our role in improving outcomes for women and their children became even more important. This year’s Update focuses on clinical guidelines centered on safety and optimal outcomes for women and children.
ACOG and SMFM update guidance on FGR management
American College of Obstetricians and Gynecologists. Practice advisory: Updated guidance regarding fetal growth restriction. September 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/09/updated-guidance-regarding-fetal-growth-restriction. Accessed December 18, 2020.
Fetal growth restriction (FGR) affects up to 10% of pregnancies and is a leading cause of infant morbidity and mortality. Suboptimal fetal growth can have lasting negative effects on development into early childhood and, some hypothesize, even into adulthood.1,2 Antenatal detection of fetuses with FGR is critical so that antenatal testing can be implemented in an attempt to deliver improved clinical outcomes. FGR is defined by several different diagnostic criteria, and many studies have been conducted to determine how best to diagnose this condition.
In September 2020, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory regarding guidance on FGR in an effort to align the ACOG Practice Bulletin No. 204, ACOG Committee Opinion No. 764, and SMFM (Society for Maternal-Fetal Medicine) Consult Series No. 52.3-5 This guidance updates and replaces prior guidelines, with an emphasis on 3 notable changes.
FGR definition, workup have changed
While the original definition of FGR was an estimated fetal weight (EFW) of less than the 10th percentile for gestational age, a similar level of accuracy in prediction of subsequent small for gestational age (SGA) at birth has been shown when this or an abdominal circumference (AC) of less than the 10th percentile is used. Based on these findings, SMFM now recommends that FGR be defined as an EFW or AC of less than the 10th percentile for gestational age.
Recent studies have done head-to-head comparisons of different methods of estimating fetal weight to determine the best detection and pregnancy outcome improvement in FGR. In all instances, the Hadlock formula has continued to more accurately estimate fetal weight, prediction of SGA, and composite neonatal morbidity. As such, new guidelines recommend that population-based fetal growth references (that is, the Hadlock formula) should be used to determine ultrasonography-derived fetal weight percentiles.
The new guidance also suggests classification of FGR based on gestational age at onset, with early FGR at less than 32 weeks and late FGR at 32 or more weeks. The definition of severe FGR is reserved for fetuses with an EFW of less than the 3rd percentile. A diagnosis of FGR should prompt the recommendation for a detailed obstetric ultrasonography. Diagnostic genetic testing should be offered in cases of early-onset FGR, concomitant sonographic abnormalities, and/or polyhydramnios. Routine serum screening for toxoplasmosis, rubella, herpes, or cytomegalovirus (CMV) should not be done unless there are risk factors for infection. If amniocentesis is performed for genetic diagnostic testing, consideration can be made for polymerase chain reaction for CMV in the amniotic fluid.
Continue to: Timing of delivery in isolated FGR...
Timing of delivery in isolated FGR
A complicating factor in diagnosing FGR is distinguishing between the pathologically growth-restricted fetus and the constitutionally small fetus. Antenatal testing and serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for evidence of fetal compromise until delivery is planned.
The current ACOG Practice Bulletin No. 204 and Committee Opinion No. 764 recommend delivery between 38 0/7 and 39 6/7 weeks in the setting of isolated FGR with reassuring fetal testing and umbilical artery Doppler assessment.To further refine this, the new recommendations use the growth percentiles. In cases of isolated FGR with EFW between the 3rd and 10th percentile in the setting of normal umbilical artery Doppler, delivery is recommended between 38 and 39 weeks’ gestation. In cases of isolated FGR with EFW of less than the 3rd percentile (severe FGR) in the setting of normal umbilical artery Doppler, delivery is recommended at 37 weeks.
Timing of delivery in complicated FGR
A normal umbilical artery Doppler reflects the low impedance that is necessary for continuous forward flow of blood to the fetus. Abnormal umbilical artery Doppler signifies aberrations of this low-pressure system that affect the amount of continuous forward flow during diastole of the cardiac cycle. With continued compromise, there is progression to absent end-diastolic velocity (AEDV) and, most concerning, reversed end-diastolic velocity (REDV).
Serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for progression that is associated with perinatal mortality, since intervention can be initiated in the form of delivery. Delivery at 37 weeks is recommended for FGR with elevated umbilical artery Doppler of greater than the 95th percentile for gestational age. For FGR with AEDV, delivery is recommended between 33 and 34 weeks of gestation and for FGR with REDV between 30 and 32 weeks, as the neonatal morbidity and mortality associated with continuing the pregnancy outweighs the risks of prematurity in this setting. Because of the abnormal placental-fetal circulation in FGR complicated by AEDV/REDV, there may be a higher likelihood of fetal intolerance of labor and cesarean delivery (CD) may be considered.
- Fetal growth restriction is now defined as EFW of less than the 10th percentile or AC of less than the 10th percentile.
- Evaluation of FGR includes detailed anatomic survey and consideration of genetic evaluation, but infection screening should be done only if the patient is at risk for infection.
- With reassuring antenatal testing and normal umbilical artery Doppler studies, delivery is recommended at 38 to 39 weeks for isolated FGR with EFW in the 3rd to 10th percentile and at 37 weeks for FGR with EFW of less than the 3rd percentile.
- Umbilical artery Doppler studies are used to decrease the risk of perinatal mortality and further guide timing of delivery
Continue to: New recommendations for PROM management...
New recommendations for PROM management
American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
Rupture of membranes prior to the onset of labor occurs at term in 8% of pregnancies and in the preterm period in 2% to 3% of pregnancies.6 Accurate diagnosis, gestational age, evidence of infection, and discussion of the risks and benefits to the mother and fetus/neonate are necessary to optimize outcomes. In the absence of other indications for delivery, a gestational age of 34 or more weeks traditionally has been the cutoff to proceed with delivery, although this has not been globally agreed on and/or practiced.
ACOG has published a comprehensive update that incorporates the results of the PPROMT trial and other recommendations for the diagnosis and management of both term and preterm prelabor rupture of membranes (PROM).6,7
Making the diagnosis
Diagnosis of PROM usually can be made clinically via history and the classic triad of physical exam findings—pooling of fluid, basic pH, and ferning; some institutions also use commercially available tests that detect placental-derived proteins. Both ACOG and the US Food and Drug Administration caution against using these tests alone without clinical evaluation due to concern for false-positives and false-negatives that lead to adverse maternal and fetal/neonatal outcomes. For equivocal cases, ultrasonography for amniotic fluid evaluation and ultrasonography-guided dye tests can be used to assist in accurate diagnosis, especially in the preterm period in which there are significant implications for pregnancy management.
PROM management depends on gestational age
All management recommendations require reassuring fetal testing, evaluation for infection, and no other contraindications to expectant management. Once these are established, the most important determinant of PROM management then becomes gestational age.
Previable PROM
Previable PROM (usually defined as less than 23–24 weeks) has high risks of both maternal and fetal/neonatal morbidity and mortality from infection, hemorrhage, pulmonary hypoplasia, and extreme prematurity. These very difficult cases benefit from a multidisciplinary approach to patient counseling regarding expectant management versus immediate delivery.
If expectant management is chosen, outpatient management with close monitoring for signs of maternal infection may be done until an agreed on gestational age of viability. Then inpatient management with fetal monitoring, corticosteroids, tocolysis, magnesium for neuroprotection, and group B streptococcus (GBS) prophylaxis may be considered as appropriate.
Preterm PROM at less than 34 weeks
If the mother and fetus are otherwise stable, PROM at less than 34 weeks warrants inpatient expectant management with close maternal and fetal monitoring for signs of infection and labor. Management includes latency antibiotics, antenatal corticosteroids, magnesium for neuroprotection if less than 32 weeks’ gestation and at risk for imminent delivery, and GBS prophylaxis. While tocolysis may increase latency and help with steroid course completion, it should be used cautiously and avoided in cases of abruption or chorioamnionitis. Although there is no definitive recommendation published, a rescue course of steroids may be considered as appropriate but should not delay an indicated delivery.
Continue to: Late preterm PROM...
Late preterm PROM
The biggest change to clinical management in this ACOG Practice Bulletin is for late preterm (34–36 6/7 weeks) PROM, with the recommendation for either immediate delivery or expectant management up to 37 weeks stemming from the PPROMPT study by Morris and colleagues.7
From the neonatal perspective, no difference has been demonstrated between immediate delivery and expectant management for neonatal sepsis or a composite neonatal morbidity and mortality. Expectant management may be preferred from the neonatal point of view as immediate delivery was associated with an increased rate of neonatal respiratory distress, mechanical ventilation, and length of stay in the neonatal intensive care unit. The potential for long-term neurodevelopmental outcomes of delivery at 34 versus 37 weeks also should be considered.
From the maternal perspective, expectant management has an increased risk of antepartum and postpartum hemorrhage, fever, antibiotic use, and maternal length of stay, but a decreased risk of CD.
A late preterm steroid course can be considered if delivery is planned in no less than 24 hours and likely to occur in the next 7 days and if the patient has not already received a course of steroids. A rescue course of steroids is not indicated if the patient received a steroid course prior in the pregnancy. While appropriate GBS prophylaxis is recommended, latency antibiotics and tocolysis are not, and delivery should not be delayed if chorioamnionitis is diagnosed.
Ultimately, preterm PROM management with a stable mother and fetus at or beyond 34 weeks requires comprehensive counseling of the risks and benefits for both mother and fetus/neonate. A multidisciplinary team that together counsels the patient also may help with this shared decision making.
Term PROM
For patients with term PROM, delivery is recommended. Although a short period of expectant management for 12 to 24 hours is reported as “reasonable,” the risk of infection increases with the length of rupture of membranes. Therefore, induction of labor or CD soon after rupture of membranes is recommended for patients who are GBS positive and is preferred for all others.
- Accurate diagnosis is necessary for appropriate counseling and management of PROM.
- Delivery is recommended for term PROM, chorioamnionitis, and for patients with previable PROM who do not desire expectant management.
- If the mother and fetus are otherwise stable, expectant management of preterm PROM until 34 to 37 weeks is recommended.
- The decision of when to deliver between 34 and 37 weeks is best made with multidisciplinary counseling and shared decision making with the patient.
VTE prophylaxis in pregnancy: Regimen adjustments, CD strategies, and COVID-19 considerations
Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series No. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17
Venous thromboembolism (VTE) prophylaxis is a timely topic for a number of reasons. First, a shortage of unfractionated heparin prompted an ACOG Practice Advisory, endorsed by SMFM and the Society for Obstetric Anesthesia and Perinatology, regarding use of low molecular weight heparin (LMWH) in the peripartum period.8 In addition, SMFM released updated recommendations for VTE prophylaxis for CD as part of the SMFM Consult Series.9 Finally, there is evidence that COVID-19 infection may increase the risk of coagulopathy, leading to consideration of additional VTE prophylaxis for pregnant and postpartum women with COVID-19.
Candidates for prophylaxis
As recommended by the ACOG Practice Bulletin on thromboembolism in pregnancy, women who may require VTE prophylaxis during pregnancy and/or the postpartum period include those with10:
- VTE diagnosed during pregnancy
- a history of VTE, including during pregnancy or with use of hormonal contraception
- a history of thrombophilia with or without a personal or family history of VTE.
For these patients, LMWH has many advantages over unfractionated heparin, including ease of use and reliability of dosing. It generally is preferred in pregnancy and postpartum (for both prophylactic and therapeutic anticoagulation) by patients and providers.
The Practice Bulletin references a strategy that describes converting LMWH to unfractionated heparin at around 36 weeks’ gestation in preparation for delivery because unfractionated heparin has the advantage of a shorter half-life and the option for anticoagulation reversal with protamine sulfate. In the Practice Advisory, a global shortage of unfractionated heparin and an argument that the above conversion was less about concern for maternal hemorrhage and more about avoiding spinal and epidural hematomas led to the following recommendations for continued use of LMWH through delivery:
- LMWH heparin can be discontinued in a planned fashion prior to scheduled induction of labor or CD (generally 12 hours for prophylactic dosing and 24 hours for intermediate dosing).
- Patients in spontaneous labor may receive neuraxial anesthesia 12 hours after the last prophylactic dose and 24 hours after the last intermediate dose of LMWH.
- Patients who require anticoagulation during pregnancy should be counseled that if they have vaginal bleeding, leakage of fluid, or regular contractions they should be evaluated prior to taking their next dose of anticoagulant.
- In the absence of other complications, delivery should not be before 39 weeks for the indication of anticoagulation requirement alone.
Continue to: Managing VTE risk in CD...
Managing VTE risk in CD
Recognizing that VTE is a major cause of maternal morbidity and mortality, as well as the variety of the published guidelines for VTE prophylaxis after CD, the SMFM Consult Series provides recommendations to assist clinicians caring for postpartum women after CD. As reviewed in the ACOG Practice Bulletin, there are good data to support pharmacologic prophylaxis during pregnancy and the postpartum period for women with a history of VTE or a thrombophilia. Solid evidence is lacking, however, for what to do for women who have a CD without this history but may have other potential risk factors for VTE, such as obesity, preeclampsia, and transfusion requirement. Universal pharmacologic prophylaxis also is not yet supported by evidence. SMFM supports LMWH as the preferred medication in pregnancy and postpartum and provides these additional recommendations:
- All women who have a CD should have sequential compression devices (SCDs) placed prior to surgery and continued until they are ambulatory.
- Women with a history of VTE or thrombophilia without history of VTE should have SCDs and pharmacologic VTE prophylaxis for 6 weeks postpartum.
- Intermediate dosing of LMWH is recommended for patients with class III obesity.
- Institutions should develop patient safety bundles for VTE prophylaxis to identify additional risk factors that may warrant pharmacologic prophylaxis after CD in select patients.
Our approach to patients with COVID-19 infection
At our institution, we recently incorporated a VTE prophylaxis protocol into our electronic medical record that provides risk stratification for each patient. In addition to the above recommendations, our patients may qualify for short-term in-house or longer postpartum prophylaxis depending on risk factors.
A new risk factor in recent months is COVID-19 infection, which appears to increase the risk of coagulopathy, especially in patients with disease severe enough to warrant hospitalization. Given the potential for additive risk in pregnancy, in consult with our medicine colleagues, we have placed some of our more ill hospitalized pregnant patients on a course of prophylactic LMWH both in the hospital and after discharge independent of delivery status or mode of delivery. ●
- Pregnant patients with a history of VTE or a thrombophilia may be candidates for pharmacologic anticoagulation during pregnancy and/or postpartum.
- LMWH is the preferred method of pharmacologic VTE prophylaxis during pregnancy and postpartum.
- For most patients, CD and neuraxial anesthesia safely can be performed 12 to 24 hours after the last dose of prophylactic or intermediate LMWH, respectively.
- All patients undergoing CD should have at least mechanical VTE prophylaxis with SCDs.
- All women who have a CD should be evaluated via institutional patient safety bundles for VTE prophylaxis for additional risk factors that potentially warrant postpartum pharmacologic VTE prophylaxis.
- More data are needed to determine recommendations for universal/ near universal pharmacologic VTE prophylaxis in the postpartum period.
- Pregnant or postpartum patients with moderate to severe COVID-19 infection may be at increased risk for VTE, warranting consideration of additional pharmacologic prophylaxis.
- Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571-577.
- Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect. 2011;25:153-172.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics and Society for Maternal-Fetal Medicine. ACOG practice bulletin no. 204: Fetal growth restriction. Obstet Gynecol. 2019;133: e97-e109.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice and Society for Maternal-Fetal Medicine. ACOG committee opinion no. 764: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155.
- Society for Maternal-Fetal Medicine; Martins JG, Biggio FR, Abuhamad A. SMFM consult series no. 52: diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2020;223:B2-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
- Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
- Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
- Pacheco LD, Saade G, Metz TD. Society for MaternalFetal Medicine consult series no. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 196: Thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
- Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571-577.
- Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect. 2011;25:153-172.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics and Society for Maternal-Fetal Medicine. ACOG practice bulletin no. 204: Fetal growth restriction. Obstet Gynecol. 2019;133: e97-e109.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice and Society for Maternal-Fetal Medicine. ACOG committee opinion no. 764: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155.
- Society for Maternal-Fetal Medicine; Martins JG, Biggio FR, Abuhamad A. SMFM consult series no. 52: diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2020;223:B2-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
- Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
- Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
- Pacheco LD, Saade G, Metz TD. Society for MaternalFetal Medicine consult series no. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 196: Thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
When ultrasonography reveals a fetal abdominal wall defect
CASE Fetal anomalies detected on ultrasonography
A 34-year-old woman (G2P1) at 19 weeks’ gestation presented for fetal anatomy ultrasonography evaluation. Ultrasonography demonstrated fetal demise with fetal size less than dates, oligohydramnios, and what appeared to be a full-thickness herniation of the thoracic and abdominal contents. Due to the positioning of the fetus and the oligohydramnios, the fetus appeared to have ectopia cordis and herniated liver and bowel; the bladder was not visualized. The patient was counseled regarding the findings and the suspected diagnosis of pentalogy of Cantrell. After counseling, the patient expressed desire to bury the fetus intact according to her religious custom. She underwent a successful uterine evacuation with misoprostol administration and delivered a nonviable fetus that had a closed thoracic cage without ectopia cordis. Key findings were a very short 2-vessel umbilical cord without coiling that was tethered to the intra-abdominal organs, “pulling” the internal organs out of the abdomen, and lack of an anterior abdominal wall (FIGURE 1). Given these findings, a final diagnosis of body-stalk anomaly was made.
Fetal abdominal wall defects (AWDs) encompass a wide array of congenital defects, although they all involve herniation of 1 or more intra-abdominal content through a ventral abdominal defect.1 Overall, the estimated incidence of AWDs is approximately 6 per 10,000 births.1 Gastroschisis and omphalocele are the most common of these defect types.2
The majority of AWDs can be diagnosed during the first trimester of pregnancy via ultrasonography; however, during the first trimester the physiologic midgut herniation resolves by 12 weeks of gestation. It is therefore important to repeat imaging at a later gestational age to confirm the suspicion. Furthermore, the differential diagnosis should include the relatively benign condition of umbilical hernia.
While many AWDs share similarities, they differ significantly in prognosis and management. Early detection is therefore crucial for fetal surveillance, prenatal testing, perinatal planning, and patient counseling (TABLE). In this article, we outline antenatal surveillance and management of AWDs based on recommendations from the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine as well as on our experience and practice.
Gastroschisis is an increasingly prevalent AWD
Gastroschisis is a full-thickness, ventral wall defect that results in bowel evisceration; it typically occurs to the right of the umbilical cord insertion.3 It is one of the most common AWDs and its prevalence has increased in the past few decades, from 2 to 3 cases per 10,000 live births in 1995 to as high as 6 cases per 10,000 live births in 2011.2,4,5
The cause of gastroschisis remains unclear. The main theory is that there is an ischemic disruption of the closure of the abdominal wall at or near the omphalomesenteric artery or the right umbilical vein.6,7 In addition, investigators have reported an increased incidence of gastroschisis in mothers exposed to cigarette smoking and certain medications, such as pseudoephedrine, salicylates, ibuprofen, and acetaminophen.8,9
Continue to: Making the diagnosis...
Making the diagnosis
Prenatal diagnosis using ultrasonography is possible at around 10 weeks of gestation. As previously mentioned, however, physiologic herniation of the midgut must be excluded by performing follow-up imaging at a later gestational age. In our practice, we typically do this at around 16 weeks of gestation.
Ultrasonographic features of gastroschisis include loops of bowel herniating through a small paraumbilical wall defect (usually 2–3 cm) floating in amniotic fluid without a covering membrane4 (FIGURE 2). Direct exposure to amniotic fluid causes small bowel inflammation and fibrin deposition, leading to a thickened, echogenic appearance. Polyhydramnios and intra-abdominal bowel dilation have been associated with the presence of intestinal atresia.10
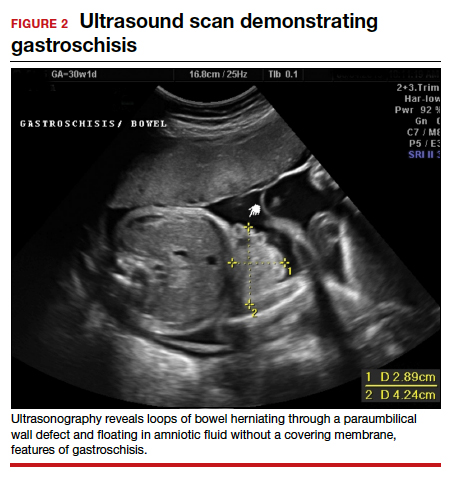
Management
There is no expert consensus regarding optimal prenatal management of gastroschisis.11-17 Prenatal care, patient counseling, and delivery planning should be individualized based on the defect and should be determined in a multidisciplinary discussion with specialists in maternal-fetal medicine, neonatology, and pediatric surgery, as necessary. In our practice, if the gastroschisis is isolated and uncomplicated, our generalist obstetricians manage the patient with maternal-fetal medicine consultation, increased fetal surveillance as described below, and delivery at our tertiary care institution.
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of the defect, measure the nuchal translucency, and evaluate for additional abnormalities. Serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth.10
As gastroschisis is a full-thickness defect of the anterior abdominal wall, the abdominal contents are exposed to amniotic fluid. This exposure causes progressive intestinal damage, which can be identified on ultrasonography as bowel thickening and dilation.12-14 Currently, intestinal thickening and dilation is not considered an indication for delivery as it is assumed that the intestinal damage has already occurred. It is debatable whether delivery around 37 weeks compared with delayed delivery beyond 37 weeks improves outcomes and decreases the stillbirth rate.11,13 Studies show that neonates delivered prior to 37 weeks have worse outcomes compared with those delivered after 37 weeks.14,15
Fetal surveillance. As standard practice, we evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, which is associated with 25% of cases,16,17 our standard practice includes performing serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Fetal echocardiography can be offered. However, unlike with omphalocele, which has a high incidence of associated cardiac structural anomalies, gastroschisis has a low incidence of congenital cardiac anomalies, estimated to be between 2.5% and 4%.18,19
Delivery considerations. Little agreement exists regarding when and how to deliver pregnancies complicated by fetal gastroschisis. While some advocate for induction of labor at 36 to 38 weeks, most infants with gastroschisis can be delivered safely at term via either vaginal or cesarean delivery.14,15
Delivery timing should consider the clinical picture and incorporate performance on antenatal testing, fetal growth, the size and contents of the gastroschisis, and consultation with maternal-fetal medicine. Fetuses with gastroschisis often have non-reassuring antenatal testing. This can necessitate early delivery, although cesarean delivery should be reserved for obstetric indications, with the caveat that if there is large liver involvement, some pediatric surgeons recommend cesarean delivery due to the risk of hepatic rupture.
Neonate management. The survival rate of gastroschisis is reported to be as high as 91% to 94%.2 Morbidity is related to intestinal complications, such as strictures, adhesions, and volvulus.
In the case of simple gastroschisis, when the bowel is in good condition, the treatment method of choice is primary reduction.20 If performed in the operating room, an immediate sutured closure of the defect can be done. The benefits of primary repair include decreased length of stay, fewer intensive care bed days, and less time to achieve full feeds.20,21 Primary reduction has a reported success rate of 50% to 83%.22 A reduction with a delayed spontaneous closure also can be performed at bedside in the neonatal intensive care unit.22
For complex gastroschisis, characterized by bowel complications such as inflammation, perforation, ischemia, atresia, necrosis, or volvulus, primary closure may not be possible and reduction may need to be achieved through silo application.22-25 Additionally, further bowel surgery, such as stoma formation and bowel resection, may be required.25
Continue to: Omphalocele often is associated with abnormal karyotype...
Omphalocele often is associated with abnormal karyotype
Also known as exomphalos, omphalocele is a relatively common defect, with an estimated prevalence of 2 to 3 cases per 10,000 live births.2 In this condition, there is a midline defect in which intra-abdominal contents herniate through the base of the umbilical cord. Omphaloceles are covered by amniotic membranes, making them distinguishable from gastroschisis, which has no covering, and congenital umbilical hernias, which are covered by intact skin and subcutaneous tissue.26-33
Additionally, in omphalocele the umbilical cord insertion site varies, whereas in gastroschisis the umbilical cord insertion is usually to the right of midline. An omphalocele is often categorized based on whether or not it contains the liver (extracorporeal liver) or only the bowel (intracorporeal liver).
Genetic studies
Approximately 67% to 88% of all pregnancies with omphalocele have an abnormal karyotype and/or associated malformations, including Beckwith-Wiedemann syndrome.31 Of the aneuploidies, trisomy 18 is the one most commonly associated with omphalocele, accounting for approximately 62% to 75%, while trisomy 13 accounts for approximately 11% to 24%.32,33 The presence of other anomalies is strongly associated with poor prognosis, and increased defect size is an independent predictor of neonatal morbidity and mortality, as neonates with large omphaloceles with extracorporeal livers can develop respiratory insufficiency and require more complex surgical repairs. It is interesting, however, that the absence of an extracorporeal liver is associated with a higher risk of aneuploidy than are cases with an intracorporeal liver.33
We offer chorionic villus sampling or amniocentesis to all patients with omphalocele. If the patient undergoes invasive diagnostic testing, the sample then undergoes karyotyping, chromosomal microarray, and testing for Beckwith-Wiedemann syndrome. If the patient declines diagnostic sampling, we perform a cell-free DNA screening to rule out aneuploidy.
Continue to: Making the diagnosis...
Making the diagnosis
Omphaloceles can be diagnosed via prenatal ultrasonography as early as 11 to 14 weeks’ gestation.26 They are classified based on size, location, and contents of the sac.26,27 A small omphalocele is defined as a defect less than 5 cm with a sac that may contain a few loops of intestines (FIGURE 3).27 A giant omphalocele is a defect with more than 75% of the liver contained in the sac.29
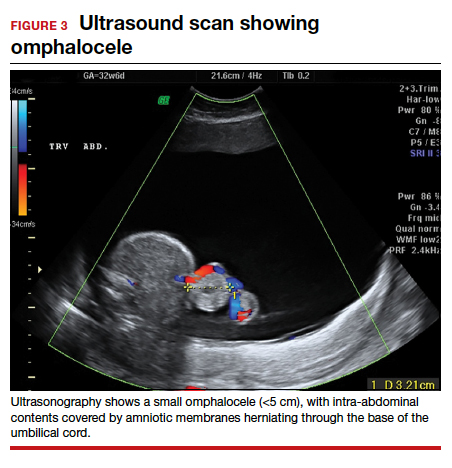
Location can be epigastric, umbilical, or hypogastric, and both small and giant omphaloceles may have ruptured membranes that will result in exposure of the contained viscera.27 Omphaloceles are associated with such structural anomalies as cardiac, gastrointestinal, genitourinary, diaphragmatic, and neural tube defects. We do not routinely perform magnetic resonance imaging (MRI) for evaluation of omphaloceles, but MRI may be used to help predict postnatal outcomes in the case of giant omphaloceles.26
Management
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of defect, measure the nuchal translucency, and evaluate for additional abnormalities. As in cases of gastroschisis, serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth. We typically evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, we recommend serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Additionally, we routinely obtain a fetal echocardiogram to rule out cardiac structural abnormalities.
Delivery considerations. Fetuses that do not undergo spontaneous abortion or medical termination of pregnancy often are born at term.26 We recommend expectant management until spontaneous labor, another indication for delivery arises, or at least 39 weeks’ estimated gestational age. There are no evidence-based guidelines for the optimal mode of delivery in fetuses with omphalocele, although we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage. Preterm induction of labor is not indicated as infants born preterm have about a 50% mortality rate.26,27
Children born with isolated omphalocele typically have a good prognosis, with an estimated survival rate of 50% to 90%.32,33 However, compared to gastroschisis, omphaloceles are often associated with other anomalies.32,33
Management of omphaloceles depends on the size of the defect. In our institution, our generalist obstetricians manage the standard prenatal care with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning, and delivery is performed at our tertiary care center.
Neonate management. Small omphaloceles are amenable to primary early fascial closure.26-30 However, attempted primary closure of giant omphaloceles carries significant risks, including abdominal compartment syndrome and postoperative herniation.29,30 Instead, several options exist for staged surgical closure, in which there are multiple operations prior to final fascial closure, as well as nonoperative delayed closure for management of giant omphaloceles.29,30
Conservative management of giant omphaloceles has certain benefits, such as earlier first feeds, decreased risk of abdominal compartment syndrome, and lower risk of infection.30 Ruptured omphaloceles can be repaired through primary repair, employment of a synthetic or biologic mesh fascial bridge, or silo placement with delayed closure.28
Body-stalk anomaly: Multiple defects and poor prognosis
Also known as limb body wall complex, body-stalk anomaly is a rare malformation that has a reported prevalence of approximately 0.12 cases per 10,000 births (both live and stillbirths).34 Body-stalk anomaly is characterized by multiple defects, including severe kyphosis or scoliosis, a short or absent umbilical cord, and a large anterior abdominal wall defect.34-36 This malformation is almost entirely incompatible with life, resulting in abortion or stillbirth.35 Survival is extremely rare and limited to case reports.
While the exact etiology of body-stalk anomaly is unknown, 3 possible causes have been hypothesized: early amnion rupture, vascular compromise, and embryonic dysgenesis.37-40
Continue to: Making the diagnosis...
Making the diagnosis
Body-stalk anomaly typically can be diagnosed by 10 to 14 weeks’ gestation via ultrasonography.34-41 We currently follow the diagnostic criteria proposed by Van Allen and colleagues, which requires 2 of the following 3 anomalies34:
- exencephaly/encephalocele with facial clefts
- thoraco- and/or abdominoschisis (midline defect)
- limb defect.
Additional ultrasonographic findings can include the identification of evisceration of the abdominal contents, a short umbilical cord, and increased nuchal thickness.36,42 During the second and third trimesters, oligohydramnios may be seen.2
Management
Body-stalk anomaly is considered a fatal condition without specific therapeutic interventions. Maternal risks include an increased risk of preterm labor and gestational hypertension.35 Research on body-stalk anomaly has not shown any correlation with patients’ age, fetal sex, or abnormal karyotype, and the reported risk of recurrence for this anomaly is very low.42,43 Early diagnosis therefore is essential to provide families with information and counseling. Given the poor fetal prognosis, increased maternal risk, and low recurrence rates, mothers can be advised toward elective termination of pregnancy.
Should a patient desire expectant management, care can be provided by generalist obstetricians or care can be transferred to maternal-fetal medicine, with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning; delivery should be performed at a tertiary care center.
Pentalogy of Cantrell: Very rare, with variable prognosis
Pentalogy of Cantrell is characterized by a collection of defects in the midline abdominal wall, lower sternum, anterior diaphragm, diaphragmatic pericardium, and some manifestation of intra-cardiac defect.44 It is thought to arise early in gestation due to abnormal differentiation, migration, and fusion of the embryonic mesoderm.44 The condition is rare, with an incidence of about 1 in 5.5 million live births.45
Making the diagnosis
The diagnosis of pentalogy of Cantrell can be made via prenatal ultrasonography as early as the first trimester, although it is diagnosed more commonly in the second trimester.46 Three-dimensional ultrasonography and fetal MRI have been used to confirm the diagnosis.47
Management
Typically, corrective operations are performed during the neonatal period, and cases of successful staged and one-stage operations have been reported.48 Surgical treatment is determined based on the complexity of the condition and the presence of coexistent heart defects.49,50 However, very few patients survive surgical repair; mortality rates are estimated at around 50% to 60%, with high postsurgical morbidity risks for those who do survive.45
Prognosis varies depending on the type and severity of the associated malformations and intracardiac anomalies.46 Patients with partial ectopia cordis and incomplete presentation may have more favorable outcomes, but for patients with severe ectopia cordis, the survival rate is only 5% to 10%.47
Depending on the severity of the defects, mothers can be advised toward elective termination of pregnancy. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
OEIS complex comprises abdominal, pelvic, and spinal defects
Omphalocele-exstrophy-imperforate anus-spinal defects (OEIS) complex is a congenital malformation syndrome characterized by the combination of midline abdominal and pelvic defects (including omphalocele, exstrophy of the cloaca, and imperforate anus) and spinal defects.51 The condition’s etiology is unknown but is thought to be multifactorial.51-53 It is a rare condition, with an incidence of around 1 in 200,000 to 400,000 pregnancies.52
Making the diagnosis
Prenatal diagnosis of OEIS complex can be made as early as the first trimester via ultrasonographic identification of an infraumbilical abdominal wall defect with protruding mass, absent bladder, and spinal defects.52 When OEIS complex is suspected, fetal MRI can play a critical role in the diagnosis.
Management
As OEIS complex is rare, there are no evidence-based guidelines for optimal mode and timing of delivery. Cases are individualized based on their specific pathology, and we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage.
The prognosis for infants with OEIS complex depends on the spectrum and severity of the structural defects.52,53 The many surgeries involved in the repair of OEIS have potential complications, such as urogenital and gastrointestinal dysfunction.52,53 Advances in medical and surgical treatment have resulted in improved survival and quality of life, and survival rates for OEIS complex are now close to 100%.53 While many OEIS patients live with a permanent colostomy, improvements in management mean that more patients are now candidates for gastrointestinal pull-through procedures, which allow for natural bowel control and a higher degree of bowel cleanliness.53
Prenatal care, patient counseling, and delivery planning should be individualized based on the defects present and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, and pediatric surgery as necessary. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
Multidisciplinary team strategy is essential
Based on our experience, when faced with an anterior AWD in utero, prenatal imaging, genetic testing, increased fetal surveillance, and a multidisciplinary team approach improves outcomes. We must emphasize that careful patient counseling is paramount in our practice. ●
Acknowledgement: The authors would like to thank Ashley Tran, BS, for her assistance in the literature review and drafting of this article.
- Patients with fetuses with anterior wall defects should be referred to a maternal-fetal medicine specialist for co-management and advanced fetal imaging.
- The American College of Obstetricians and Gynecologists recommends microarray for all major fetal structural abnormalities, with the qualifier that karyotype can be offered if a specific aneuploidy is suspected based on the abnormality or prior genetic screening tests.
- If confirmatory testing is performed (amniocentesis or chorionic villus sampling), the sample should undergo karyotyping, chromosomal microarray, and if indicated, testing for Beckwith-Wiedemann syndrome. If the patient declines confirmatory sampling, performing cell-free DNA screening to rule out aneuploidy is recommended.
- Fetal echocardiography is recommended.
- Fetal magnetic resonance imaging should be considered in complex cases.
- Management should be individualized based on the type and severity of defect(s).
- Delivery timing and method should be individualized based on the defect(s) and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, pediatric surgery, and pediatric cardiology, as necessary.
- The most common fetal abdominal wall defect is omphalocele, followed by gastroschisis.
- Maternal serum α-fetoprotein is usually elevated in all of the disorders.
- Victoria T, Andronikou S, Bowen D, et al. Fetal anterior abdominal wall defects: prenatal imaging by magnetic resonance imaging. Pediatr Radiol. 2018;48:499-512.
- Pakdaman R, Woodward PJ, Kennedy A. Complex abdominal wall defects: appearances at prenatal imaging. Radiographics. 2015;35:636-649.
- Oakes MC, Porto M, Chung JH. Advances in prenatal and perinatal diagnosis and management of gastroschisis. Semin Pediatr Surg. 2018;27:289-299.
- Mastroiacovo P, Lisi A, Castilla EE. The incidence of gastroschisis: research urgently needs resources. BMJ. 2006;332:423-424.
- Boyd PA, Haeusler M, Barisic I. EUROCAT report 9: surveillance of congenital anomalies in Europe 1980-2008. Birth Defects Res A Clin Mol Teratol. 2011;91(suppl 1):S1.
- Gamba P, Midrio P. Abdominal wall defects: prenatal diagnosis, newborn management, and long-term outcomes. Semin Pediatr Surg. 2014;23:283-290.
- Beaudoin S. Insights into the etiology and embryology of gastroschisis. Semin Pediatr Surg. 2018;27:283-288.
- Yazdy MM, Mitchell AA, Werler MM. Maternal genitourinary infections and the risk of gastroschisis. Am J Epidemiol. 2014;180:518-525.
- Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
- D’Antonio F, Virgone C, Rizzo G, et al. Prenatal risk factors and outcomes in gastroschisis: a meta-analysis. Pediatrics. 2015;136:e159-e169.
- Baud D, Lausman A, Alfaraj MA, et al. Expectant management compared with elective delivery at 37 weeks for gastroschisis. Obstet Gynecol. 2013;121:990-998.
- Goetzinger KR, Tuuli MG, Longman RE, et al. Sonographic predictors of postnatal bowel atresia in fetal gastroschisis. Ultrasound Obstet Gynecol. 2014;43:420-425.
- Overton TG, Pierce MR, Gao H, et al. Antenatal management and outcomes of gastroschisis in the UK. Prenat Diagn. 2012;32:1256-1262.
- Ergün O, Barksdale E, Ergün FS, et al. The timing of delivery of infants with gastroschisis influences outcome. J Pediatr Surg. 2005;40:424-428.
- Overcash RT, DeUgarte DA, Stephenson ML, et al; University of California Fetal Consortium. Factors associated with gastroschisis outcomes. Obstet Gynecol. 2014;124:551-557.
- Wissanji H, Puligandla PS. Risk stratification and outcome determinants in gastroschisis. Semin Pediatr Surg. 2018;27: 300-303.
- Raynor BD, Richards D. Growth retardation in fetuses with gastroschisis. J Ultrasound Med. 1997;16:13-16.
- Mastroiacovo P, Lisi A, Castilla EE, et al. Gastroschisis and associated defects: an international study. Am J Med Genet A. 2007;143A:660-671.
- Kunz LH, Gilbert WM, Towner DR. Increased incidence of cardiac anomalies in pregnancies complicated by gastroschisis. Am J Obstet Gynecol. 2005;193(3 pt 2): 1248-1252.
- Lakshminarayanan B, Lakhoo K. Abdominal wall defects. Early Hum Dev. 2014;90:917-920.
- Prefumo F, Izzi C. Fetal abdominal wall defects. Best Pract Res Clin Obstet Gynaecol. 2014;28:391-402.
- Petrosyan M, Sandler AD. Closure methods in gastroschisis. Semin Pediatr Surg. 2018;27:304-308.
- Skarsgard ED. Management of gastroschisis. Curr Opin Pediatr. 2016;28:363-369.
- Bergholz R, Boettcher M, Reinshagen K, et al. Complex gastroschisis is a different entity to simple gastroschisis affecting morbidity and mortality—a systematic review and meta-analysis. J Pediatr Surg. 2014;49:1527-1532.
- Emil S. Surgical strategies in complex gastroschisis. Semin Pediatr Surg. 2018;27:309-315.
- Verla MA, Style CC, Olutoye OO. Prenatal diagnosis and management of omphalocele. Semin Pediatr Surg. 2019;28:84-88.
- Gonzalez KW, Chandler NM. Ruptured omphalocele: diagnosis and management. Semin Pediatr Surg. 2019;28:101-105.
- Sugandhi N, Saha M, Bhatnagar V, et al. Repair of ruptured omphalocele sac in the neonatal period and beyond. J Indian Assoc Pediatr Surg. 2020;25:46-48.
- Bauman B, Stephens D, Gershone H, et al. Management of giant omphaloceles: a systematic review of methods of staged surgical vs nonoperative delayed closure. J Pediatr Surg. 2016;51:1725-1730.
- Kogut KA, Fiore NF. Nonoperative management of giant omphalocele leading to early fascial closure. J Pediatr Surg. 2018;53:2404-2408.
- Conner P, Vejde JH, Burgos CM. Accuracy and impact of prenatal diagnosis in infants with omphalocele. Pediatr Surg Int. 2018;34:629-633.
- Iacovella C, Contro E, Ghi T, et al. The effect of the contents of exomphalos and nuchal translucency at 11-14 weeks on the likelihood of associated chromosomal abnormality. Prenat Diagn. 2012;32:1066-1070.
- Getachew MM, Goldstein RB, Edge V, et al. Correlation between omphalocele contents and karyotypic abnormalities: sonographic study in 37 cases. AJR Am J Roentgenol. 1992;158:133-136.
- Singh A, Singh J, Gupta K. Body stalk anomaly: antenatal sonographic diagnosis of this rare entity with review of literature. J Ultrason. 2017;17:133-135.
- Lazaroni TL, Cruzeiro PC, Piçarro C, et al. Body stalk anomaly: Three months of survival. Case report and literature review. J Pediatr Surg Case Rep. 2016;14:22-25.
- Gajzer DC, Hirzel AC, Saigal G, et al. Possible genetic origin of limb-body wall complex. Fetal Pediatr Pathol. 2015;34: 257–270.
- Maruyama H, Inagaki T, Nakata Y, et al. Minimally conjoined omphalopagus twins with a body stalk anomaly. AJP Rep. 2015;5:e124-e128.
- Bhat A, Ilyas M, Dev G. Prenatal sonographic diagnosis of limb-body wall complex: case series of a rare congenital anomaly. Radiol Case Rep. 2016;11:116-120.
- Quijano FE, Rey MM, Echeverry M, et al. Body stalk anomaly in a 9-week pregnancy. Case Rep Obstet Gynecol. 2014;2014:357285.
- Kocherla K, Kumari V, Kocherla PR. Prenatal diagnosis of body stalk complex: a rare entity and review of literature. Indian J Radiol Imaging. 2015;25:67-70.
- Panaitescu AM, Ushakov F, Kalaskar A, et al. Ultrasound features and management of body stalk anomaly. Fetal Diagn Ther. 2016;40:285-290.
- Routhu M, Thakkallapelli S, Mohan P, et al. Role of ultrasound in body stalk anomaly and amniotic band syndrome. Int J Reprod Med. 2016;2016:3974139.
- Costa ML, Couto E, Furlan E, et al. Body stalk anomaly: adverse maternal outcomes in a series of 21 cases. Prenat Diagn. 2012;32:264-267.
- Hubbard R, Hayes S, Gillis H, et al. Management challenges in an infant with pentalogy of Cantrell, giant anterior encephalocele, and craniofacial anomalies: a case report. A A Pract. 2018;11:238-240.
- Jnah AJ, Newberry DM, England A. Pentalogy of Cantrell: case report with review of the literature. Adv Neonatal Care. 2015;15:261-268.
- Williams AP, Marayati R, Beierle EA. Pentalogy of Cantrell. Semin Pediatr Surg. 2019;28:106-110.
- Restrepo MS, Cerqua A, Turek JW. Pentalogy of Cantrell with ectopia cordis totalis, total anomalous pulmonary venous connection, and tetralogy of Fallot: a case report and review of the literature. Congenit Heart Dis. 2014;9:E129–E134.
- Zhang X, Xing Q, Sun J, et al. Surgical treatment and outcomes of pentalogy of Cantrell in eight patients. J Pediatr Surg. 2014;49:1335-1340.
- Harring G, Weil J, Thiel C, et al. Management of pentalogy of Cantrell with complete ectopia cordis and double outlet right ventricle. Congenit Anom (Kyoto). 2015;55:121- 123.
- Mallula KK, Sosnowski C, Awad S. Spectrum of Cantrell’s pentalogy: case series from a single tertiary care center and review of the literature. Pediatr Cardiol. 2013;34:1703- 1710.
- Allam ES, Shetty VS, Farmakis SG. Fetal and neonatal presentation of OEIS complex. J Pediatr Surg. 2015;50:2155-2158.
- Neel N, Tarabay MS. Omphalocele, exstrophy of cloaca, imperforate anus, and spinal defect complex, multiple major reconstructive surgeries needed. Urol Ann. 2018;10:118-121.
- Sawaya D, Gearhart JP. Gastrointestinal reconstruction and outcomes for patients with the OEIS complex. Semin Pediatr Surg. 2011;20:123-125.

CASE Fetal anomalies detected on ultrasonography
A 34-year-old woman (G2P1) at 19 weeks’ gestation presented for fetal anatomy ultrasonography evaluation. Ultrasonography demonstrated fetal demise with fetal size less than dates, oligohydramnios, and what appeared to be a full-thickness herniation of the thoracic and abdominal contents. Due to the positioning of the fetus and the oligohydramnios, the fetus appeared to have ectopia cordis and herniated liver and bowel; the bladder was not visualized. The patient was counseled regarding the findings and the suspected diagnosis of pentalogy of Cantrell. After counseling, the patient expressed desire to bury the fetus intact according to her religious custom. She underwent a successful uterine evacuation with misoprostol administration and delivered a nonviable fetus that had a closed thoracic cage without ectopia cordis. Key findings were a very short 2-vessel umbilical cord without coiling that was tethered to the intra-abdominal organs, “pulling” the internal organs out of the abdomen, and lack of an anterior abdominal wall (FIGURE 1). Given these findings, a final diagnosis of body-stalk anomaly was made.

Fetal abdominal wall defects (AWDs) encompass a wide array of congenital defects, although they all involve herniation of 1 or more intra-abdominal content through a ventral abdominal defect.1 Overall, the estimated incidence of AWDs is approximately 6 per 10,000 births.1 Gastroschisis and omphalocele are the most common of these defect types.2
The majority of AWDs can be diagnosed during the first trimester of pregnancy via ultrasonography; however, during the first trimester the physiologic midgut herniation resolves by 12 weeks of gestation. It is therefore important to repeat imaging at a later gestational age to confirm the suspicion. Furthermore, the differential diagnosis should include the relatively benign condition of umbilical hernia.
While many AWDs share similarities, they differ significantly in prognosis and management. Early detection is therefore crucial for fetal surveillance, prenatal testing, perinatal planning, and patient counseling (TABLE). In this article, we outline antenatal surveillance and management of AWDs based on recommendations from the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine as well as on our experience and practice.

Gastroschisis is an increasingly prevalent AWD
Gastroschisis is a full-thickness, ventral wall defect that results in bowel evisceration; it typically occurs to the right of the umbilical cord insertion.3 It is one of the most common AWDs and its prevalence has increased in the past few decades, from 2 to 3 cases per 10,000 live births in 1995 to as high as 6 cases per 10,000 live births in 2011.2,4,5
The cause of gastroschisis remains unclear. The main theory is that there is an ischemic disruption of the closure of the abdominal wall at or near the omphalomesenteric artery or the right umbilical vein.6,7 In addition, investigators have reported an increased incidence of gastroschisis in mothers exposed to cigarette smoking and certain medications, such as pseudoephedrine, salicylates, ibuprofen, and acetaminophen.8,9
Continue to: Making the diagnosis...
Making the diagnosis
Prenatal diagnosis using ultrasonography is possible at around 10 weeks of gestation. As previously mentioned, however, physiologic herniation of the midgut must be excluded by performing follow-up imaging at a later gestational age. In our practice, we typically do this at around 16 weeks of gestation.
Ultrasonographic features of gastroschisis include loops of bowel herniating through a small paraumbilical wall defect (usually 2–3 cm) floating in amniotic fluid without a covering membrane4 (FIGURE 2). Direct exposure to amniotic fluid causes small bowel inflammation and fibrin deposition, leading to a thickened, echogenic appearance. Polyhydramnios and intra-abdominal bowel dilation have been associated with the presence of intestinal atresia.10

Management
There is no expert consensus regarding optimal prenatal management of gastroschisis.11-17 Prenatal care, patient counseling, and delivery planning should be individualized based on the defect and should be determined in a multidisciplinary discussion with specialists in maternal-fetal medicine, neonatology, and pediatric surgery, as necessary. In our practice, if the gastroschisis is isolated and uncomplicated, our generalist obstetricians manage the patient with maternal-fetal medicine consultation, increased fetal surveillance as described below, and delivery at our tertiary care institution.
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of the defect, measure the nuchal translucency, and evaluate for additional abnormalities. Serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth.10
As gastroschisis is a full-thickness defect of the anterior abdominal wall, the abdominal contents are exposed to amniotic fluid. This exposure causes progressive intestinal damage, which can be identified on ultrasonography as bowel thickening and dilation.12-14 Currently, intestinal thickening and dilation is not considered an indication for delivery as it is assumed that the intestinal damage has already occurred. It is debatable whether delivery around 37 weeks compared with delayed delivery beyond 37 weeks improves outcomes and decreases the stillbirth rate.11,13 Studies show that neonates delivered prior to 37 weeks have worse outcomes compared with those delivered after 37 weeks.14,15
Fetal surveillance. As standard practice, we evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, which is associated with 25% of cases,16,17 our standard practice includes performing serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Fetal echocardiography can be offered. However, unlike with omphalocele, which has a high incidence of associated cardiac structural anomalies, gastroschisis has a low incidence of congenital cardiac anomalies, estimated to be between 2.5% and 4%.18,19
Delivery considerations. Little agreement exists regarding when and how to deliver pregnancies complicated by fetal gastroschisis. While some advocate for induction of labor at 36 to 38 weeks, most infants with gastroschisis can be delivered safely at term via either vaginal or cesarean delivery.14,15
Delivery timing should consider the clinical picture and incorporate performance on antenatal testing, fetal growth, the size and contents of the gastroschisis, and consultation with maternal-fetal medicine. Fetuses with gastroschisis often have non-reassuring antenatal testing. This can necessitate early delivery, although cesarean delivery should be reserved for obstetric indications, with the caveat that if there is large liver involvement, some pediatric surgeons recommend cesarean delivery due to the risk of hepatic rupture.
Neonate management. The survival rate of gastroschisis is reported to be as high as 91% to 94%.2 Morbidity is related to intestinal complications, such as strictures, adhesions, and volvulus.
In the case of simple gastroschisis, when the bowel is in good condition, the treatment method of choice is primary reduction.20 If performed in the operating room, an immediate sutured closure of the defect can be done. The benefits of primary repair include decreased length of stay, fewer intensive care bed days, and less time to achieve full feeds.20,21 Primary reduction has a reported success rate of 50% to 83%.22 A reduction with a delayed spontaneous closure also can be performed at bedside in the neonatal intensive care unit.22
For complex gastroschisis, characterized by bowel complications such as inflammation, perforation, ischemia, atresia, necrosis, or volvulus, primary closure may not be possible and reduction may need to be achieved through silo application.22-25 Additionally, further bowel surgery, such as stoma formation and bowel resection, may be required.25
Continue to: Omphalocele often is associated with abnormal karyotype...
Omphalocele often is associated with abnormal karyotype
Also known as exomphalos, omphalocele is a relatively common defect, with an estimated prevalence of 2 to 3 cases per 10,000 live births.2 In this condition, there is a midline defect in which intra-abdominal contents herniate through the base of the umbilical cord. Omphaloceles are covered by amniotic membranes, making them distinguishable from gastroschisis, which has no covering, and congenital umbilical hernias, which are covered by intact skin and subcutaneous tissue.26-33
Additionally, in omphalocele the umbilical cord insertion site varies, whereas in gastroschisis the umbilical cord insertion is usually to the right of midline. An omphalocele is often categorized based on whether or not it contains the liver (extracorporeal liver) or only the bowel (intracorporeal liver).
Genetic studies
Approximately 67% to 88% of all pregnancies with omphalocele have an abnormal karyotype and/or associated malformations, including Beckwith-Wiedemann syndrome.31 Of the aneuploidies, trisomy 18 is the one most commonly associated with omphalocele, accounting for approximately 62% to 75%, while trisomy 13 accounts for approximately 11% to 24%.32,33 The presence of other anomalies is strongly associated with poor prognosis, and increased defect size is an independent predictor of neonatal morbidity and mortality, as neonates with large omphaloceles with extracorporeal livers can develop respiratory insufficiency and require more complex surgical repairs. It is interesting, however, that the absence of an extracorporeal liver is associated with a higher risk of aneuploidy than are cases with an intracorporeal liver.33
We offer chorionic villus sampling or amniocentesis to all patients with omphalocele. If the patient undergoes invasive diagnostic testing, the sample then undergoes karyotyping, chromosomal microarray, and testing for Beckwith-Wiedemann syndrome. If the patient declines diagnostic sampling, we perform a cell-free DNA screening to rule out aneuploidy.
Continue to: Making the diagnosis...
Making the diagnosis
Omphaloceles can be diagnosed via prenatal ultrasonography as early as 11 to 14 weeks’ gestation.26 They are classified based on size, location, and contents of the sac.26,27 A small omphalocele is defined as a defect less than 5 cm with a sac that may contain a few loops of intestines (FIGURE 3).27 A giant omphalocele is a defect with more than 75% of the liver contained in the sac.29

Location can be epigastric, umbilical, or hypogastric, and both small and giant omphaloceles may have ruptured membranes that will result in exposure of the contained viscera.27 Omphaloceles are associated with such structural anomalies as cardiac, gastrointestinal, genitourinary, diaphragmatic, and neural tube defects. We do not routinely perform magnetic resonance imaging (MRI) for evaluation of omphaloceles, but MRI may be used to help predict postnatal outcomes in the case of giant omphaloceles.26
Management
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of defect, measure the nuchal translucency, and evaluate for additional abnormalities. As in cases of gastroschisis, serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth. We typically evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, we recommend serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Additionally, we routinely obtain a fetal echocardiogram to rule out cardiac structural abnormalities.
Delivery considerations. Fetuses that do not undergo spontaneous abortion or medical termination of pregnancy often are born at term.26 We recommend expectant management until spontaneous labor, another indication for delivery arises, or at least 39 weeks’ estimated gestational age. There are no evidence-based guidelines for the optimal mode of delivery in fetuses with omphalocele, although we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage. Preterm induction of labor is not indicated as infants born preterm have about a 50% mortality rate.26,27
Children born with isolated omphalocele typically have a good prognosis, with an estimated survival rate of 50% to 90%.32,33 However, compared to gastroschisis, omphaloceles are often associated with other anomalies.32,33
Management of omphaloceles depends on the size of the defect. In our institution, our generalist obstetricians manage the standard prenatal care with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning, and delivery is performed at our tertiary care center.
Neonate management. Small omphaloceles are amenable to primary early fascial closure.26-30 However, attempted primary closure of giant omphaloceles carries significant risks, including abdominal compartment syndrome and postoperative herniation.29,30 Instead, several options exist for staged surgical closure, in which there are multiple operations prior to final fascial closure, as well as nonoperative delayed closure for management of giant omphaloceles.29,30
Conservative management of giant omphaloceles has certain benefits, such as earlier first feeds, decreased risk of abdominal compartment syndrome, and lower risk of infection.30 Ruptured omphaloceles can be repaired through primary repair, employment of a synthetic or biologic mesh fascial bridge, or silo placement with delayed closure.28
Body-stalk anomaly: Multiple defects and poor prognosis
Also known as limb body wall complex, body-stalk anomaly is a rare malformation that has a reported prevalence of approximately 0.12 cases per 10,000 births (both live and stillbirths).34 Body-stalk anomaly is characterized by multiple defects, including severe kyphosis or scoliosis, a short or absent umbilical cord, and a large anterior abdominal wall defect.34-36 This malformation is almost entirely incompatible with life, resulting in abortion or stillbirth.35 Survival is extremely rare and limited to case reports.
While the exact etiology of body-stalk anomaly is unknown, 3 possible causes have been hypothesized: early amnion rupture, vascular compromise, and embryonic dysgenesis.37-40
Continue to: Making the diagnosis...
Making the diagnosis
Body-stalk anomaly typically can be diagnosed by 10 to 14 weeks’ gestation via ultrasonography.34-41 We currently follow the diagnostic criteria proposed by Van Allen and colleagues, which requires 2 of the following 3 anomalies34:
- exencephaly/encephalocele with facial clefts
- thoraco- and/or abdominoschisis (midline defect)
- limb defect.
Additional ultrasonographic findings can include the identification of evisceration of the abdominal contents, a short umbilical cord, and increased nuchal thickness.36,42 During the second and third trimesters, oligohydramnios may be seen.2
Management
Body-stalk anomaly is considered a fatal condition without specific therapeutic interventions. Maternal risks include an increased risk of preterm labor and gestational hypertension.35 Research on body-stalk anomaly has not shown any correlation with patients’ age, fetal sex, or abnormal karyotype, and the reported risk of recurrence for this anomaly is very low.42,43 Early diagnosis therefore is essential to provide families with information and counseling. Given the poor fetal prognosis, increased maternal risk, and low recurrence rates, mothers can be advised toward elective termination of pregnancy.
Should a patient desire expectant management, care can be provided by generalist obstetricians or care can be transferred to maternal-fetal medicine, with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning; delivery should be performed at a tertiary care center.
Pentalogy of Cantrell: Very rare, with variable prognosis
Pentalogy of Cantrell is characterized by a collection of defects in the midline abdominal wall, lower sternum, anterior diaphragm, diaphragmatic pericardium, and some manifestation of intra-cardiac defect.44 It is thought to arise early in gestation due to abnormal differentiation, migration, and fusion of the embryonic mesoderm.44 The condition is rare, with an incidence of about 1 in 5.5 million live births.45
Making the diagnosis
The diagnosis of pentalogy of Cantrell can be made via prenatal ultrasonography as early as the first trimester, although it is diagnosed more commonly in the second trimester.46 Three-dimensional ultrasonography and fetal MRI have been used to confirm the diagnosis.47
Management
Typically, corrective operations are performed during the neonatal period, and cases of successful staged and one-stage operations have been reported.48 Surgical treatment is determined based on the complexity of the condition and the presence of coexistent heart defects.49,50 However, very few patients survive surgical repair; mortality rates are estimated at around 50% to 60%, with high postsurgical morbidity risks for those who do survive.45
Prognosis varies depending on the type and severity of the associated malformations and intracardiac anomalies.46 Patients with partial ectopia cordis and incomplete presentation may have more favorable outcomes, but for patients with severe ectopia cordis, the survival rate is only 5% to 10%.47
Depending on the severity of the defects, mothers can be advised toward elective termination of pregnancy. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
OEIS complex comprises abdominal, pelvic, and spinal defects
Omphalocele-exstrophy-imperforate anus-spinal defects (OEIS) complex is a congenital malformation syndrome characterized by the combination of midline abdominal and pelvic defects (including omphalocele, exstrophy of the cloaca, and imperforate anus) and spinal defects.51 The condition’s etiology is unknown but is thought to be multifactorial.51-53 It is a rare condition, with an incidence of around 1 in 200,000 to 400,000 pregnancies.52
Making the diagnosis
Prenatal diagnosis of OEIS complex can be made as early as the first trimester via ultrasonographic identification of an infraumbilical abdominal wall defect with protruding mass, absent bladder, and spinal defects.52 When OEIS complex is suspected, fetal MRI can play a critical role in the diagnosis.
Management
As OEIS complex is rare, there are no evidence-based guidelines for optimal mode and timing of delivery. Cases are individualized based on their specific pathology, and we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage.
The prognosis for infants with OEIS complex depends on the spectrum and severity of the structural defects.52,53 The many surgeries involved in the repair of OEIS have potential complications, such as urogenital and gastrointestinal dysfunction.52,53 Advances in medical and surgical treatment have resulted in improved survival and quality of life, and survival rates for OEIS complex are now close to 100%.53 While many OEIS patients live with a permanent colostomy, improvements in management mean that more patients are now candidates for gastrointestinal pull-through procedures, which allow for natural bowel control and a higher degree of bowel cleanliness.53
Prenatal care, patient counseling, and delivery planning should be individualized based on the defects present and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, and pediatric surgery as necessary. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
Multidisciplinary team strategy is essential
Based on our experience, when faced with an anterior AWD in utero, prenatal imaging, genetic testing, increased fetal surveillance, and a multidisciplinary team approach improves outcomes. We must emphasize that careful patient counseling is paramount in our practice. ●
Acknowledgement: The authors would like to thank Ashley Tran, BS, for her assistance in the literature review and drafting of this article.
- Patients with fetuses with anterior wall defects should be referred to a maternal-fetal medicine specialist for co-management and advanced fetal imaging.
- The American College of Obstetricians and Gynecologists recommends microarray for all major fetal structural abnormalities, with the qualifier that karyotype can be offered if a specific aneuploidy is suspected based on the abnormality or prior genetic screening tests.
- If confirmatory testing is performed (amniocentesis or chorionic villus sampling), the sample should undergo karyotyping, chromosomal microarray, and if indicated, testing for Beckwith-Wiedemann syndrome. If the patient declines confirmatory sampling, performing cell-free DNA screening to rule out aneuploidy is recommended.
- Fetal echocardiography is recommended.
- Fetal magnetic resonance imaging should be considered in complex cases.
- Management should be individualized based on the type and severity of defect(s).
- Delivery timing and method should be individualized based on the defect(s) and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, pediatric surgery, and pediatric cardiology, as necessary.
- The most common fetal abdominal wall defect is omphalocele, followed by gastroschisis.
- Maternal serum α-fetoprotein is usually elevated in all of the disorders.

CASE Fetal anomalies detected on ultrasonography
A 34-year-old woman (G2P1) at 19 weeks’ gestation presented for fetal anatomy ultrasonography evaluation. Ultrasonography demonstrated fetal demise with fetal size less than dates, oligohydramnios, and what appeared to be a full-thickness herniation of the thoracic and abdominal contents. Due to the positioning of the fetus and the oligohydramnios, the fetus appeared to have ectopia cordis and herniated liver and bowel; the bladder was not visualized. The patient was counseled regarding the findings and the suspected diagnosis of pentalogy of Cantrell. After counseling, the patient expressed desire to bury the fetus intact according to her religious custom. She underwent a successful uterine evacuation with misoprostol administration and delivered a nonviable fetus that had a closed thoracic cage without ectopia cordis. Key findings were a very short 2-vessel umbilical cord without coiling that was tethered to the intra-abdominal organs, “pulling” the internal organs out of the abdomen, and lack of an anterior abdominal wall (FIGURE 1). Given these findings, a final diagnosis of body-stalk anomaly was made.

Fetal abdominal wall defects (AWDs) encompass a wide array of congenital defects, although they all involve herniation of 1 or more intra-abdominal content through a ventral abdominal defect.1 Overall, the estimated incidence of AWDs is approximately 6 per 10,000 births.1 Gastroschisis and omphalocele are the most common of these defect types.2
The majority of AWDs can be diagnosed during the first trimester of pregnancy via ultrasonography; however, during the first trimester the physiologic midgut herniation resolves by 12 weeks of gestation. It is therefore important to repeat imaging at a later gestational age to confirm the suspicion. Furthermore, the differential diagnosis should include the relatively benign condition of umbilical hernia.
While many AWDs share similarities, they differ significantly in prognosis and management. Early detection is therefore crucial for fetal surveillance, prenatal testing, perinatal planning, and patient counseling (TABLE). In this article, we outline antenatal surveillance and management of AWDs based on recommendations from the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine as well as on our experience and practice.

Gastroschisis is an increasingly prevalent AWD
Gastroschisis is a full-thickness, ventral wall defect that results in bowel evisceration; it typically occurs to the right of the umbilical cord insertion.3 It is one of the most common AWDs and its prevalence has increased in the past few decades, from 2 to 3 cases per 10,000 live births in 1995 to as high as 6 cases per 10,000 live births in 2011.2,4,5
The cause of gastroschisis remains unclear. The main theory is that there is an ischemic disruption of the closure of the abdominal wall at or near the omphalomesenteric artery or the right umbilical vein.6,7 In addition, investigators have reported an increased incidence of gastroschisis in mothers exposed to cigarette smoking and certain medications, such as pseudoephedrine, salicylates, ibuprofen, and acetaminophen.8,9
Continue to: Making the diagnosis...
Making the diagnosis
Prenatal diagnosis using ultrasonography is possible at around 10 weeks of gestation. As previously mentioned, however, physiologic herniation of the midgut must be excluded by performing follow-up imaging at a later gestational age. In our practice, we typically do this at around 16 weeks of gestation.
Ultrasonographic features of gastroschisis include loops of bowel herniating through a small paraumbilical wall defect (usually 2–3 cm) floating in amniotic fluid without a covering membrane4 (FIGURE 2). Direct exposure to amniotic fluid causes small bowel inflammation and fibrin deposition, leading to a thickened, echogenic appearance. Polyhydramnios and intra-abdominal bowel dilation have been associated with the presence of intestinal atresia.10

Management
There is no expert consensus regarding optimal prenatal management of gastroschisis.11-17 Prenatal care, patient counseling, and delivery planning should be individualized based on the defect and should be determined in a multidisciplinary discussion with specialists in maternal-fetal medicine, neonatology, and pediatric surgery, as necessary. In our practice, if the gastroschisis is isolated and uncomplicated, our generalist obstetricians manage the patient with maternal-fetal medicine consultation, increased fetal surveillance as described below, and delivery at our tertiary care institution.
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of the defect, measure the nuchal translucency, and evaluate for additional abnormalities. Serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth.10
As gastroschisis is a full-thickness defect of the anterior abdominal wall, the abdominal contents are exposed to amniotic fluid. This exposure causes progressive intestinal damage, which can be identified on ultrasonography as bowel thickening and dilation.12-14 Currently, intestinal thickening and dilation is not considered an indication for delivery as it is assumed that the intestinal damage has already occurred. It is debatable whether delivery around 37 weeks compared with delayed delivery beyond 37 weeks improves outcomes and decreases the stillbirth rate.11,13 Studies show that neonates delivered prior to 37 weeks have worse outcomes compared with those delivered after 37 weeks.14,15
Fetal surveillance. As standard practice, we evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, which is associated with 25% of cases,16,17 our standard practice includes performing serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Fetal echocardiography can be offered. However, unlike with omphalocele, which has a high incidence of associated cardiac structural anomalies, gastroschisis has a low incidence of congenital cardiac anomalies, estimated to be between 2.5% and 4%.18,19
Delivery considerations. Little agreement exists regarding when and how to deliver pregnancies complicated by fetal gastroschisis. While some advocate for induction of labor at 36 to 38 weeks, most infants with gastroschisis can be delivered safely at term via either vaginal or cesarean delivery.14,15
Delivery timing should consider the clinical picture and incorporate performance on antenatal testing, fetal growth, the size and contents of the gastroschisis, and consultation with maternal-fetal medicine. Fetuses with gastroschisis often have non-reassuring antenatal testing. This can necessitate early delivery, although cesarean delivery should be reserved for obstetric indications, with the caveat that if there is large liver involvement, some pediatric surgeons recommend cesarean delivery due to the risk of hepatic rupture.
Neonate management. The survival rate of gastroschisis is reported to be as high as 91% to 94%.2 Morbidity is related to intestinal complications, such as strictures, adhesions, and volvulus.
In the case of simple gastroschisis, when the bowel is in good condition, the treatment method of choice is primary reduction.20 If performed in the operating room, an immediate sutured closure of the defect can be done. The benefits of primary repair include decreased length of stay, fewer intensive care bed days, and less time to achieve full feeds.20,21 Primary reduction has a reported success rate of 50% to 83%.22 A reduction with a delayed spontaneous closure also can be performed at bedside in the neonatal intensive care unit.22
For complex gastroschisis, characterized by bowel complications such as inflammation, perforation, ischemia, atresia, necrosis, or volvulus, primary closure may not be possible and reduction may need to be achieved through silo application.22-25 Additionally, further bowel surgery, such as stoma formation and bowel resection, may be required.25
Continue to: Omphalocele often is associated with abnormal karyotype...
Omphalocele often is associated with abnormal karyotype
Also known as exomphalos, omphalocele is a relatively common defect, with an estimated prevalence of 2 to 3 cases per 10,000 live births.2 In this condition, there is a midline defect in which intra-abdominal contents herniate through the base of the umbilical cord. Omphaloceles are covered by amniotic membranes, making them distinguishable from gastroschisis, which has no covering, and congenital umbilical hernias, which are covered by intact skin and subcutaneous tissue.26-33
Additionally, in omphalocele the umbilical cord insertion site varies, whereas in gastroschisis the umbilical cord insertion is usually to the right of midline. An omphalocele is often categorized based on whether or not it contains the liver (extracorporeal liver) or only the bowel (intracorporeal liver).
Genetic studies
Approximately 67% to 88% of all pregnancies with omphalocele have an abnormal karyotype and/or associated malformations, including Beckwith-Wiedemann syndrome.31 Of the aneuploidies, trisomy 18 is the one most commonly associated with omphalocele, accounting for approximately 62% to 75%, while trisomy 13 accounts for approximately 11% to 24%.32,33 The presence of other anomalies is strongly associated with poor prognosis, and increased defect size is an independent predictor of neonatal morbidity and mortality, as neonates with large omphaloceles with extracorporeal livers can develop respiratory insufficiency and require more complex surgical repairs. It is interesting, however, that the absence of an extracorporeal liver is associated with a higher risk of aneuploidy than are cases with an intracorporeal liver.33
We offer chorionic villus sampling or amniocentesis to all patients with omphalocele. If the patient undergoes invasive diagnostic testing, the sample then undergoes karyotyping, chromosomal microarray, and testing for Beckwith-Wiedemann syndrome. If the patient declines diagnostic sampling, we perform a cell-free DNA screening to rule out aneuploidy.
Continue to: Making the diagnosis...
Making the diagnosis
Omphaloceles can be diagnosed via prenatal ultrasonography as early as 11 to 14 weeks’ gestation.26 They are classified based on size, location, and contents of the sac.26,27 A small omphalocele is defined as a defect less than 5 cm with a sac that may contain a few loops of intestines (FIGURE 3).27 A giant omphalocele is a defect with more than 75% of the liver contained in the sac.29

Location can be epigastric, umbilical, or hypogastric, and both small and giant omphaloceles may have ruptured membranes that will result in exposure of the contained viscera.27 Omphaloceles are associated with such structural anomalies as cardiac, gastrointestinal, genitourinary, diaphragmatic, and neural tube defects. We do not routinely perform magnetic resonance imaging (MRI) for evaluation of omphaloceles, but MRI may be used to help predict postnatal outcomes in the case of giant omphaloceles.26
Management
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of defect, measure the nuchal translucency, and evaluate for additional abnormalities. As in cases of gastroschisis, serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth. We typically evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, we recommend serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Additionally, we routinely obtain a fetal echocardiogram to rule out cardiac structural abnormalities.
Delivery considerations. Fetuses that do not undergo spontaneous abortion or medical termination of pregnancy often are born at term.26 We recommend expectant management until spontaneous labor, another indication for delivery arises, or at least 39 weeks’ estimated gestational age. There are no evidence-based guidelines for the optimal mode of delivery in fetuses with omphalocele, although we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage. Preterm induction of labor is not indicated as infants born preterm have about a 50% mortality rate.26,27
Children born with isolated omphalocele typically have a good prognosis, with an estimated survival rate of 50% to 90%.32,33 However, compared to gastroschisis, omphaloceles are often associated with other anomalies.32,33
Management of omphaloceles depends on the size of the defect. In our institution, our generalist obstetricians manage the standard prenatal care with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning, and delivery is performed at our tertiary care center.
Neonate management. Small omphaloceles are amenable to primary early fascial closure.26-30 However, attempted primary closure of giant omphaloceles carries significant risks, including abdominal compartment syndrome and postoperative herniation.29,30 Instead, several options exist for staged surgical closure, in which there are multiple operations prior to final fascial closure, as well as nonoperative delayed closure for management of giant omphaloceles.29,30
Conservative management of giant omphaloceles has certain benefits, such as earlier first feeds, decreased risk of abdominal compartment syndrome, and lower risk of infection.30 Ruptured omphaloceles can be repaired through primary repair, employment of a synthetic or biologic mesh fascial bridge, or silo placement with delayed closure.28
Body-stalk anomaly: Multiple defects and poor prognosis
Also known as limb body wall complex, body-stalk anomaly is a rare malformation that has a reported prevalence of approximately 0.12 cases per 10,000 births (both live and stillbirths).34 Body-stalk anomaly is characterized by multiple defects, including severe kyphosis or scoliosis, a short or absent umbilical cord, and a large anterior abdominal wall defect.34-36 This malformation is almost entirely incompatible with life, resulting in abortion or stillbirth.35 Survival is extremely rare and limited to case reports.
While the exact etiology of body-stalk anomaly is unknown, 3 possible causes have been hypothesized: early amnion rupture, vascular compromise, and embryonic dysgenesis.37-40
Continue to: Making the diagnosis...
Making the diagnosis
Body-stalk anomaly typically can be diagnosed by 10 to 14 weeks’ gestation via ultrasonography.34-41 We currently follow the diagnostic criteria proposed by Van Allen and colleagues, which requires 2 of the following 3 anomalies34:
- exencephaly/encephalocele with facial clefts
- thoraco- and/or abdominoschisis (midline defect)
- limb defect.
Additional ultrasonographic findings can include the identification of evisceration of the abdominal contents, a short umbilical cord, and increased nuchal thickness.36,42 During the second and third trimesters, oligohydramnios may be seen.2
Management
Body-stalk anomaly is considered a fatal condition without specific therapeutic interventions. Maternal risks include an increased risk of preterm labor and gestational hypertension.35 Research on body-stalk anomaly has not shown any correlation with patients’ age, fetal sex, or abnormal karyotype, and the reported risk of recurrence for this anomaly is very low.42,43 Early diagnosis therefore is essential to provide families with information and counseling. Given the poor fetal prognosis, increased maternal risk, and low recurrence rates, mothers can be advised toward elective termination of pregnancy.
Should a patient desire expectant management, care can be provided by generalist obstetricians or care can be transferred to maternal-fetal medicine, with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning; delivery should be performed at a tertiary care center.
Pentalogy of Cantrell: Very rare, with variable prognosis
Pentalogy of Cantrell is characterized by a collection of defects in the midline abdominal wall, lower sternum, anterior diaphragm, diaphragmatic pericardium, and some manifestation of intra-cardiac defect.44 It is thought to arise early in gestation due to abnormal differentiation, migration, and fusion of the embryonic mesoderm.44 The condition is rare, with an incidence of about 1 in 5.5 million live births.45
Making the diagnosis
The diagnosis of pentalogy of Cantrell can be made via prenatal ultrasonography as early as the first trimester, although it is diagnosed more commonly in the second trimester.46 Three-dimensional ultrasonography and fetal MRI have been used to confirm the diagnosis.47
Management
Typically, corrective operations are performed during the neonatal period, and cases of successful staged and one-stage operations have been reported.48 Surgical treatment is determined based on the complexity of the condition and the presence of coexistent heart defects.49,50 However, very few patients survive surgical repair; mortality rates are estimated at around 50% to 60%, with high postsurgical morbidity risks for those who do survive.45
Prognosis varies depending on the type and severity of the associated malformations and intracardiac anomalies.46 Patients with partial ectopia cordis and incomplete presentation may have more favorable outcomes, but for patients with severe ectopia cordis, the survival rate is only 5% to 10%.47
Depending on the severity of the defects, mothers can be advised toward elective termination of pregnancy. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
OEIS complex comprises abdominal, pelvic, and spinal defects
Omphalocele-exstrophy-imperforate anus-spinal defects (OEIS) complex is a congenital malformation syndrome characterized by the combination of midline abdominal and pelvic defects (including omphalocele, exstrophy of the cloaca, and imperforate anus) and spinal defects.51 The condition’s etiology is unknown but is thought to be multifactorial.51-53 It is a rare condition, with an incidence of around 1 in 200,000 to 400,000 pregnancies.52
Making the diagnosis
Prenatal diagnosis of OEIS complex can be made as early as the first trimester via ultrasonographic identification of an infraumbilical abdominal wall defect with protruding mass, absent bladder, and spinal defects.52 When OEIS complex is suspected, fetal MRI can play a critical role in the diagnosis.
Management
As OEIS complex is rare, there are no evidence-based guidelines for optimal mode and timing of delivery. Cases are individualized based on their specific pathology, and we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage.
The prognosis for infants with OEIS complex depends on the spectrum and severity of the structural defects.52,53 The many surgeries involved in the repair of OEIS have potential complications, such as urogenital and gastrointestinal dysfunction.52,53 Advances in medical and surgical treatment have resulted in improved survival and quality of life, and survival rates for OEIS complex are now close to 100%.53 While many OEIS patients live with a permanent colostomy, improvements in management mean that more patients are now candidates for gastrointestinal pull-through procedures, which allow for natural bowel control and a higher degree of bowel cleanliness.53
Prenatal care, patient counseling, and delivery planning should be individualized based on the defects present and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, and pediatric surgery as necessary. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
Multidisciplinary team strategy is essential
Based on our experience, when faced with an anterior AWD in utero, prenatal imaging, genetic testing, increased fetal surveillance, and a multidisciplinary team approach improves outcomes. We must emphasize that careful patient counseling is paramount in our practice. ●
Acknowledgement: The authors would like to thank Ashley Tran, BS, for her assistance in the literature review and drafting of this article.
- Patients with fetuses with anterior wall defects should be referred to a maternal-fetal medicine specialist for co-management and advanced fetal imaging.
- The American College of Obstetricians and Gynecologists recommends microarray for all major fetal structural abnormalities, with the qualifier that karyotype can be offered if a specific aneuploidy is suspected based on the abnormality or prior genetic screening tests.
- If confirmatory testing is performed (amniocentesis or chorionic villus sampling), the sample should undergo karyotyping, chromosomal microarray, and if indicated, testing for Beckwith-Wiedemann syndrome. If the patient declines confirmatory sampling, performing cell-free DNA screening to rule out aneuploidy is recommended.
- Fetal echocardiography is recommended.
- Fetal magnetic resonance imaging should be considered in complex cases.
- Management should be individualized based on the type and severity of defect(s).
- Delivery timing and method should be individualized based on the defect(s) and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, pediatric surgery, and pediatric cardiology, as necessary.
- The most common fetal abdominal wall defect is omphalocele, followed by gastroschisis.
- Maternal serum α-fetoprotein is usually elevated in all of the disorders.
- Victoria T, Andronikou S, Bowen D, et al. Fetal anterior abdominal wall defects: prenatal imaging by magnetic resonance imaging. Pediatr Radiol. 2018;48:499-512.
- Pakdaman R, Woodward PJ, Kennedy A. Complex abdominal wall defects: appearances at prenatal imaging. Radiographics. 2015;35:636-649.
- Oakes MC, Porto M, Chung JH. Advances in prenatal and perinatal diagnosis and management of gastroschisis. Semin Pediatr Surg. 2018;27:289-299.
- Mastroiacovo P, Lisi A, Castilla EE. The incidence of gastroschisis: research urgently needs resources. BMJ. 2006;332:423-424.
- Boyd PA, Haeusler M, Barisic I. EUROCAT report 9: surveillance of congenital anomalies in Europe 1980-2008. Birth Defects Res A Clin Mol Teratol. 2011;91(suppl 1):S1.
- Gamba P, Midrio P. Abdominal wall defects: prenatal diagnosis, newborn management, and long-term outcomes. Semin Pediatr Surg. 2014;23:283-290.
- Beaudoin S. Insights into the etiology and embryology of gastroschisis. Semin Pediatr Surg. 2018;27:283-288.
- Yazdy MM, Mitchell AA, Werler MM. Maternal genitourinary infections and the risk of gastroschisis. Am J Epidemiol. 2014;180:518-525.
- Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
- D’Antonio F, Virgone C, Rizzo G, et al. Prenatal risk factors and outcomes in gastroschisis: a meta-analysis. Pediatrics. 2015;136:e159-e169.
- Baud D, Lausman A, Alfaraj MA, et al. Expectant management compared with elective delivery at 37 weeks for gastroschisis. Obstet Gynecol. 2013;121:990-998.
- Goetzinger KR, Tuuli MG, Longman RE, et al. Sonographic predictors of postnatal bowel atresia in fetal gastroschisis. Ultrasound Obstet Gynecol. 2014;43:420-425.
- Overton TG, Pierce MR, Gao H, et al. Antenatal management and outcomes of gastroschisis in the UK. Prenat Diagn. 2012;32:1256-1262.
- Ergün O, Barksdale E, Ergün FS, et al. The timing of delivery of infants with gastroschisis influences outcome. J Pediatr Surg. 2005;40:424-428.
- Overcash RT, DeUgarte DA, Stephenson ML, et al; University of California Fetal Consortium. Factors associated with gastroschisis outcomes. Obstet Gynecol. 2014;124:551-557.
- Wissanji H, Puligandla PS. Risk stratification and outcome determinants in gastroschisis. Semin Pediatr Surg. 2018;27: 300-303.
- Raynor BD, Richards D. Growth retardation in fetuses with gastroschisis. J Ultrasound Med. 1997;16:13-16.
- Mastroiacovo P, Lisi A, Castilla EE, et al. Gastroschisis and associated defects: an international study. Am J Med Genet A. 2007;143A:660-671.
- Kunz LH, Gilbert WM, Towner DR. Increased incidence of cardiac anomalies in pregnancies complicated by gastroschisis. Am J Obstet Gynecol. 2005;193(3 pt 2): 1248-1252.
- Lakshminarayanan B, Lakhoo K. Abdominal wall defects. Early Hum Dev. 2014;90:917-920.
- Prefumo F, Izzi C. Fetal abdominal wall defects. Best Pract Res Clin Obstet Gynaecol. 2014;28:391-402.
- Petrosyan M, Sandler AD. Closure methods in gastroschisis. Semin Pediatr Surg. 2018;27:304-308.
- Skarsgard ED. Management of gastroschisis. Curr Opin Pediatr. 2016;28:363-369.
- Bergholz R, Boettcher M, Reinshagen K, et al. Complex gastroschisis is a different entity to simple gastroschisis affecting morbidity and mortality—a systematic review and meta-analysis. J Pediatr Surg. 2014;49:1527-1532.
- Emil S. Surgical strategies in complex gastroschisis. Semin Pediatr Surg. 2018;27:309-315.
- Verla MA, Style CC, Olutoye OO. Prenatal diagnosis and management of omphalocele. Semin Pediatr Surg. 2019;28:84-88.
- Gonzalez KW, Chandler NM. Ruptured omphalocele: diagnosis and management. Semin Pediatr Surg. 2019;28:101-105.
- Sugandhi N, Saha M, Bhatnagar V, et al. Repair of ruptured omphalocele sac in the neonatal period and beyond. J Indian Assoc Pediatr Surg. 2020;25:46-48.
- Bauman B, Stephens D, Gershone H, et al. Management of giant omphaloceles: a systematic review of methods of staged surgical vs nonoperative delayed closure. J Pediatr Surg. 2016;51:1725-1730.
- Kogut KA, Fiore NF. Nonoperative management of giant omphalocele leading to early fascial closure. J Pediatr Surg. 2018;53:2404-2408.
- Conner P, Vejde JH, Burgos CM. Accuracy and impact of prenatal diagnosis in infants with omphalocele. Pediatr Surg Int. 2018;34:629-633.
- Iacovella C, Contro E, Ghi T, et al. The effect of the contents of exomphalos and nuchal translucency at 11-14 weeks on the likelihood of associated chromosomal abnormality. Prenat Diagn. 2012;32:1066-1070.
- Getachew MM, Goldstein RB, Edge V, et al. Correlation between omphalocele contents and karyotypic abnormalities: sonographic study in 37 cases. AJR Am J Roentgenol. 1992;158:133-136.
- Singh A, Singh J, Gupta K. Body stalk anomaly: antenatal sonographic diagnosis of this rare entity with review of literature. J Ultrason. 2017;17:133-135.
- Lazaroni TL, Cruzeiro PC, Piçarro C, et al. Body stalk anomaly: Three months of survival. Case report and literature review. J Pediatr Surg Case Rep. 2016;14:22-25.
- Gajzer DC, Hirzel AC, Saigal G, et al. Possible genetic origin of limb-body wall complex. Fetal Pediatr Pathol. 2015;34: 257–270.
- Maruyama H, Inagaki T, Nakata Y, et al. Minimally conjoined omphalopagus twins with a body stalk anomaly. AJP Rep. 2015;5:e124-e128.
- Bhat A, Ilyas M, Dev G. Prenatal sonographic diagnosis of limb-body wall complex: case series of a rare congenital anomaly. Radiol Case Rep. 2016;11:116-120.
- Quijano FE, Rey MM, Echeverry M, et al. Body stalk anomaly in a 9-week pregnancy. Case Rep Obstet Gynecol. 2014;2014:357285.
- Kocherla K, Kumari V, Kocherla PR. Prenatal diagnosis of body stalk complex: a rare entity and review of literature. Indian J Radiol Imaging. 2015;25:67-70.
- Panaitescu AM, Ushakov F, Kalaskar A, et al. Ultrasound features and management of body stalk anomaly. Fetal Diagn Ther. 2016;40:285-290.
- Routhu M, Thakkallapelli S, Mohan P, et al. Role of ultrasound in body stalk anomaly and amniotic band syndrome. Int J Reprod Med. 2016;2016:3974139.
- Costa ML, Couto E, Furlan E, et al. Body stalk anomaly: adverse maternal outcomes in a series of 21 cases. Prenat Diagn. 2012;32:264-267.
- Hubbard R, Hayes S, Gillis H, et al. Management challenges in an infant with pentalogy of Cantrell, giant anterior encephalocele, and craniofacial anomalies: a case report. A A Pract. 2018;11:238-240.
- Jnah AJ, Newberry DM, England A. Pentalogy of Cantrell: case report with review of the literature. Adv Neonatal Care. 2015;15:261-268.
- Williams AP, Marayati R, Beierle EA. Pentalogy of Cantrell. Semin Pediatr Surg. 2019;28:106-110.
- Restrepo MS, Cerqua A, Turek JW. Pentalogy of Cantrell with ectopia cordis totalis, total anomalous pulmonary venous connection, and tetralogy of Fallot: a case report and review of the literature. Congenit Heart Dis. 2014;9:E129–E134.
- Zhang X, Xing Q, Sun J, et al. Surgical treatment and outcomes of pentalogy of Cantrell in eight patients. J Pediatr Surg. 2014;49:1335-1340.
- Harring G, Weil J, Thiel C, et al. Management of pentalogy of Cantrell with complete ectopia cordis and double outlet right ventricle. Congenit Anom (Kyoto). 2015;55:121- 123.
- Mallula KK, Sosnowski C, Awad S. Spectrum of Cantrell’s pentalogy: case series from a single tertiary care center and review of the literature. Pediatr Cardiol. 2013;34:1703- 1710.
- Allam ES, Shetty VS, Farmakis SG. Fetal and neonatal presentation of OEIS complex. J Pediatr Surg. 2015;50:2155-2158.
- Neel N, Tarabay MS. Omphalocele, exstrophy of cloaca, imperforate anus, and spinal defect complex, multiple major reconstructive surgeries needed. Urol Ann. 2018;10:118-121.
- Sawaya D, Gearhart JP. Gastrointestinal reconstruction and outcomes for patients with the OEIS complex. Semin Pediatr Surg. 2011;20:123-125.
- Victoria T, Andronikou S, Bowen D, et al. Fetal anterior abdominal wall defects: prenatal imaging by magnetic resonance imaging. Pediatr Radiol. 2018;48:499-512.
- Pakdaman R, Woodward PJ, Kennedy A. Complex abdominal wall defects: appearances at prenatal imaging. Radiographics. 2015;35:636-649.
- Oakes MC, Porto M, Chung JH. Advances in prenatal and perinatal diagnosis and management of gastroschisis. Semin Pediatr Surg. 2018;27:289-299.
- Mastroiacovo P, Lisi A, Castilla EE. The incidence of gastroschisis: research urgently needs resources. BMJ. 2006;332:423-424.
- Boyd PA, Haeusler M, Barisic I. EUROCAT report 9: surveillance of congenital anomalies in Europe 1980-2008. Birth Defects Res A Clin Mol Teratol. 2011;91(suppl 1):S1.
- Gamba P, Midrio P. Abdominal wall defects: prenatal diagnosis, newborn management, and long-term outcomes. Semin Pediatr Surg. 2014;23:283-290.
- Beaudoin S. Insights into the etiology and embryology of gastroschisis. Semin Pediatr Surg. 2018;27:283-288.
- Yazdy MM, Mitchell AA, Werler MM. Maternal genitourinary infections and the risk of gastroschisis. Am J Epidemiol. 2014;180:518-525.
- Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
- D’Antonio F, Virgone C, Rizzo G, et al. Prenatal risk factors and outcomes in gastroschisis: a meta-analysis. Pediatrics. 2015;136:e159-e169.
- Baud D, Lausman A, Alfaraj MA, et al. Expectant management compared with elective delivery at 37 weeks for gastroschisis. Obstet Gynecol. 2013;121:990-998.
- Goetzinger KR, Tuuli MG, Longman RE, et al. Sonographic predictors of postnatal bowel atresia in fetal gastroschisis. Ultrasound Obstet Gynecol. 2014;43:420-425.
- Overton TG, Pierce MR, Gao H, et al. Antenatal management and outcomes of gastroschisis in the UK. Prenat Diagn. 2012;32:1256-1262.
- Ergün O, Barksdale E, Ergün FS, et al. The timing of delivery of infants with gastroschisis influences outcome. J Pediatr Surg. 2005;40:424-428.
- Overcash RT, DeUgarte DA, Stephenson ML, et al; University of California Fetal Consortium. Factors associated with gastroschisis outcomes. Obstet Gynecol. 2014;124:551-557.
- Wissanji H, Puligandla PS. Risk stratification and outcome determinants in gastroschisis. Semin Pediatr Surg. 2018;27: 300-303.
- Raynor BD, Richards D. Growth retardation in fetuses with gastroschisis. J Ultrasound Med. 1997;16:13-16.
- Mastroiacovo P, Lisi A, Castilla EE, et al. Gastroschisis and associated defects: an international study. Am J Med Genet A. 2007;143A:660-671.
- Kunz LH, Gilbert WM, Towner DR. Increased incidence of cardiac anomalies in pregnancies complicated by gastroschisis. Am J Obstet Gynecol. 2005;193(3 pt 2): 1248-1252.
- Lakshminarayanan B, Lakhoo K. Abdominal wall defects. Early Hum Dev. 2014;90:917-920.
- Prefumo F, Izzi C. Fetal abdominal wall defects. Best Pract Res Clin Obstet Gynaecol. 2014;28:391-402.
- Petrosyan M, Sandler AD. Closure methods in gastroschisis. Semin Pediatr Surg. 2018;27:304-308.
- Skarsgard ED. Management of gastroschisis. Curr Opin Pediatr. 2016;28:363-369.
- Bergholz R, Boettcher M, Reinshagen K, et al. Complex gastroschisis is a different entity to simple gastroschisis affecting morbidity and mortality—a systematic review and meta-analysis. J Pediatr Surg. 2014;49:1527-1532.
- Emil S. Surgical strategies in complex gastroschisis. Semin Pediatr Surg. 2018;27:309-315.
- Verla MA, Style CC, Olutoye OO. Prenatal diagnosis and management of omphalocele. Semin Pediatr Surg. 2019;28:84-88.
- Gonzalez KW, Chandler NM. Ruptured omphalocele: diagnosis and management. Semin Pediatr Surg. 2019;28:101-105.
- Sugandhi N, Saha M, Bhatnagar V, et al. Repair of ruptured omphalocele sac in the neonatal period and beyond. J Indian Assoc Pediatr Surg. 2020;25:46-48.
- Bauman B, Stephens D, Gershone H, et al. Management of giant omphaloceles: a systematic review of methods of staged surgical vs nonoperative delayed closure. J Pediatr Surg. 2016;51:1725-1730.
- Kogut KA, Fiore NF. Nonoperative management of giant omphalocele leading to early fascial closure. J Pediatr Surg. 2018;53:2404-2408.
- Conner P, Vejde JH, Burgos CM. Accuracy and impact of prenatal diagnosis in infants with omphalocele. Pediatr Surg Int. 2018;34:629-633.
- Iacovella C, Contro E, Ghi T, et al. The effect of the contents of exomphalos and nuchal translucency at 11-14 weeks on the likelihood of associated chromosomal abnormality. Prenat Diagn. 2012;32:1066-1070.
- Getachew MM, Goldstein RB, Edge V, et al. Correlation between omphalocele contents and karyotypic abnormalities: sonographic study in 37 cases. AJR Am J Roentgenol. 1992;158:133-136.
- Singh A, Singh J, Gupta K. Body stalk anomaly: antenatal sonographic diagnosis of this rare entity with review of literature. J Ultrason. 2017;17:133-135.
- Lazaroni TL, Cruzeiro PC, Piçarro C, et al. Body stalk anomaly: Three months of survival. Case report and literature review. J Pediatr Surg Case Rep. 2016;14:22-25.
- Gajzer DC, Hirzel AC, Saigal G, et al. Possible genetic origin of limb-body wall complex. Fetal Pediatr Pathol. 2015;34: 257–270.
- Maruyama H, Inagaki T, Nakata Y, et al. Minimally conjoined omphalopagus twins with a body stalk anomaly. AJP Rep. 2015;5:e124-e128.
- Bhat A, Ilyas M, Dev G. Prenatal sonographic diagnosis of limb-body wall complex: case series of a rare congenital anomaly. Radiol Case Rep. 2016;11:116-120.
- Quijano FE, Rey MM, Echeverry M, et al. Body stalk anomaly in a 9-week pregnancy. Case Rep Obstet Gynecol. 2014;2014:357285.
- Kocherla K, Kumari V, Kocherla PR. Prenatal diagnosis of body stalk complex: a rare entity and review of literature. Indian J Radiol Imaging. 2015;25:67-70.
- Panaitescu AM, Ushakov F, Kalaskar A, et al. Ultrasound features and management of body stalk anomaly. Fetal Diagn Ther. 2016;40:285-290.
- Routhu M, Thakkallapelli S, Mohan P, et al. Role of ultrasound in body stalk anomaly and amniotic band syndrome. Int J Reprod Med. 2016;2016:3974139.
- Costa ML, Couto E, Furlan E, et al. Body stalk anomaly: adverse maternal outcomes in a series of 21 cases. Prenat Diagn. 2012;32:264-267.
- Hubbard R, Hayes S, Gillis H, et al. Management challenges in an infant with pentalogy of Cantrell, giant anterior encephalocele, and craniofacial anomalies: a case report. A A Pract. 2018;11:238-240.
- Jnah AJ, Newberry DM, England A. Pentalogy of Cantrell: case report with review of the literature. Adv Neonatal Care. 2015;15:261-268.
- Williams AP, Marayati R, Beierle EA. Pentalogy of Cantrell. Semin Pediatr Surg. 2019;28:106-110.
- Restrepo MS, Cerqua A, Turek JW. Pentalogy of Cantrell with ectopia cordis totalis, total anomalous pulmonary venous connection, and tetralogy of Fallot: a case report and review of the literature. Congenit Heart Dis. 2014;9:E129–E134.
- Zhang X, Xing Q, Sun J, et al. Surgical treatment and outcomes of pentalogy of Cantrell in eight patients. J Pediatr Surg. 2014;49:1335-1340.
- Harring G, Weil J, Thiel C, et al. Management of pentalogy of Cantrell with complete ectopia cordis and double outlet right ventricle. Congenit Anom (Kyoto). 2015;55:121- 123.
- Mallula KK, Sosnowski C, Awad S. Spectrum of Cantrell’s pentalogy: case series from a single tertiary care center and review of the literature. Pediatr Cardiol. 2013;34:1703- 1710.
- Allam ES, Shetty VS, Farmakis SG. Fetal and neonatal presentation of OEIS complex. J Pediatr Surg. 2015;50:2155-2158.
- Neel N, Tarabay MS. Omphalocele, exstrophy of cloaca, imperforate anus, and spinal defect complex, multiple major reconstructive surgeries needed. Urol Ann. 2018;10:118-121.
- Sawaya D, Gearhart JP. Gastrointestinal reconstruction and outcomes for patients with the OEIS complex. Semin Pediatr Surg. 2011;20:123-125.
COVID-19 vaccines: The rollout, the risks, and the reason to still wear a mask
REFERENCES
- Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm6950e2.htm
- 2. Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm695152e1.htm
- CDC. COVID-19 vaccines: update on allergic reactions, contraindications, and precautions [webinar]. December 30, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp
- CDC. What clinicians need to know about the Pfizer-BioNTech and Moderna COVID-19 vaccines [webinar]. December 18, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_121820.asp
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. ePub: January 6, 2021. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm
REFERENCES
- Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm6950e2.htm
- 2. Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm695152e1.htm
- CDC. COVID-19 vaccines: update on allergic reactions, contraindications, and precautions [webinar]. December 30, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp
- CDC. What clinicians need to know about the Pfizer-BioNTech and Moderna COVID-19 vaccines [webinar]. December 18, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_121820.asp
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. ePub: January 6, 2021. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm
REFERENCES
- Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm6950e2.htm
- 2. Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm695152e1.htm
- CDC. COVID-19 vaccines: update on allergic reactions, contraindications, and precautions [webinar]. December 30, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp
- CDC. What clinicians need to know about the Pfizer-BioNTech and Moderna COVID-19 vaccines [webinar]. December 18, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_121820.asp
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. ePub: January 6, 2021. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm
Does last contraceptive method used impact the return of normal fertility?
Yland JJ, Bresnick KA, Hatch EE, et al. Pregravid contraceptive use and fecundability: prospective cohort study. BMJ. 2020;371:m3966.
EXPERT COMMENTARY
Most US women aged 15 to 49 currently use contraception, with long-acting reversible contraception (LARC)—IUDs and the contraceptive implant—increasing in popularity over the last decade.1 Oral contraceptive pills, male condoms, and LARC are the most common reversible methods used.1 While the efficacy and safety of contraception have been established, few studies have examined the effect of recent contraceptive use on fertility.
Fecundability is the probability of pregnancy during a single menstrual cycle for a couple engaging in regular intercourse and not using contraception.2 Small studies have found short-term reductions in fecundability after discontinuing combined oral contraceptives and larger reductions after stopping injectable contraceptives, with no long-term differences among methods.3,4
Data are limited regarding the effects of other forms of contraception on fecundability, particularly LARC methods. A recent study was designed to evaluate the association between the last contraceptive method used and subsequent fecundability.2
Details of the study
Yland and colleagues pooled data from 3 prospective cohort studies of 17,954 women planning pregnancies in Denmark, Canada, and the United States. Participants reported the contraceptive method used most recently before trying to conceive. They completed questionnaires every 2 months for 12 months or until they reported a pregnancy. Women were excluded if they tried to conceive for more than 6 menstrual cycles at study entry.
The authors calculated the fecundability ratio—the average probability of conception per cycle for a specific contraceptive method compared with a reference method—using proportional probability models adjusted for potential confounders. They also calculated pregnancy attempt time using participant-reported menstrual cycle length and date of last menstrual period during follow-up questionnaires.
Continue to: Injectable contraceptives associated with longest delayed fertility return...
Injectable contraceptives associated with longest delayed fertility return
After adjusting for personal factors, medical history, lifestyle characteristics, and indicators of underlying fertility, the authors found that injectable contraceptive use was associated with decreased fecundability compared with barrier method use (fecundability ratio [FR], 0.65; 95% confidence interval [CI], 0.47–0.89). Hormonal IUD use was associated with slight increases in fecundability compared with barrier method use (FR, 1.14; 95% CI, 1.07–1.22) and copper IUD use (FR, 1.18; 95% CI, 1.05–1.33). All other contraceptive methods were not significantly different from barrier methods.
LARC method use was associated with the shortest delay in return of normal fertility (2 cycles), followed by oral and ring contraceptives (3 cycles) and patch (4 cycles). Women using injectable contraceptives experienced the longest delay (5–8 menstrual cycles). Lifetime duration of contraceptive use did not impact fecundability in the North American cohort.
Study strengths and limitations
This large, prospective study contributes useful information about fecundability after stopping contraceptive methods. It confirms earlier studies’ findings that showed decreased fecundability after stopping injectable contraceptives. Study participants’ most recent method used was similar to overall US method distribution.1
Study limitations include online recruitment of self-selecting participants, which introduces selection bias. The study population was overwhelmingly white (92%) and highly educated (70% with college degrees), quite different from the US population. These findings may therefore have limited generalizability. Additionally, injectable contraceptive users had higher body mass index and were more likely to smoke and have diabetes, infertility, or irregular menstrual cycles. IUD users were more likely to be parous and have a history of unplanned pregnancy, indicating possible higher baseline fertility. Even after adjusting, possible unmeasured factors could impact study results. ●
This is the largest study to date to evaluate fecundability after stopping different contraceptive methods among women planning pregnancies. The study confirms previous research that associated injectable contraceptives with delayed return of normal fertility. It provides reassurance for counseling users of IUDs, implants, oral contraception, ring, and patch: those methods were not associated with reduced fecundability compared with barrier methods. The study also suggests long-term contraceptive use does not decrease fecundability.
Women may ask when to stop their contraceptive method to optimally time a pregnancy. In this study, measurements of return to normal fertility were imprecise. Individualized counseling, accounting for personal circumstances, is still best when advising when to stop contraception for couples planning pregnancy.
LISA HOFLER, MD, MPH, MBA, AND LINDSAY DALE, MD
- Daniels K, Abma JC. Current contraceptive status among women aged 15–49: United States, 2017–2019. NCHS Data Brief, no. 388. Hyattsville, MD: National Center for Health Statistics; 2020.
- Yland JJ, Bresnick KA, Hatch EE, et al. Pregravid contraceptive use and fecundability: prospective cohort study. BMJ. 2020;371:m3966.
- Hassan MA, Killick SR. Is previous use of hormonal contraception associated with a detrimental effect on subsequent fecundity? Hum Reprod. 2004;19:344-351.
- Mansour D, Gemzell-Danielsson K, Inki P, et al. Fertility after discontinuation of contraception: a comprehensive review of the literature. Contraception. 2011;84:465-477.
Yland JJ, Bresnick KA, Hatch EE, et al. Pregravid contraceptive use and fecundability: prospective cohort study. BMJ. 2020;371:m3966.
EXPERT COMMENTARY
Most US women aged 15 to 49 currently use contraception, with long-acting reversible contraception (LARC)—IUDs and the contraceptive implant—increasing in popularity over the last decade.1 Oral contraceptive pills, male condoms, and LARC are the most common reversible methods used.1 While the efficacy and safety of contraception have been established, few studies have examined the effect of recent contraceptive use on fertility.
Fecundability is the probability of pregnancy during a single menstrual cycle for a couple engaging in regular intercourse and not using contraception.2 Small studies have found short-term reductions in fecundability after discontinuing combined oral contraceptives and larger reductions after stopping injectable contraceptives, with no long-term differences among methods.3,4
Data are limited regarding the effects of other forms of contraception on fecundability, particularly LARC methods. A recent study was designed to evaluate the association between the last contraceptive method used and subsequent fecundability.2
Details of the study
Yland and colleagues pooled data from 3 prospective cohort studies of 17,954 women planning pregnancies in Denmark, Canada, and the United States. Participants reported the contraceptive method used most recently before trying to conceive. They completed questionnaires every 2 months for 12 months or until they reported a pregnancy. Women were excluded if they tried to conceive for more than 6 menstrual cycles at study entry.
The authors calculated the fecundability ratio—the average probability of conception per cycle for a specific contraceptive method compared with a reference method—using proportional probability models adjusted for potential confounders. They also calculated pregnancy attempt time using participant-reported menstrual cycle length and date of last menstrual period during follow-up questionnaires.
Continue to: Injectable contraceptives associated with longest delayed fertility return...
Injectable contraceptives associated with longest delayed fertility return
After adjusting for personal factors, medical history, lifestyle characteristics, and indicators of underlying fertility, the authors found that injectable contraceptive use was associated with decreased fecundability compared with barrier method use (fecundability ratio [FR], 0.65; 95% confidence interval [CI], 0.47–0.89). Hormonal IUD use was associated with slight increases in fecundability compared with barrier method use (FR, 1.14; 95% CI, 1.07–1.22) and copper IUD use (FR, 1.18; 95% CI, 1.05–1.33). All other contraceptive methods were not significantly different from barrier methods.
LARC method use was associated with the shortest delay in return of normal fertility (2 cycles), followed by oral and ring contraceptives (3 cycles) and patch (4 cycles). Women using injectable contraceptives experienced the longest delay (5–8 menstrual cycles). Lifetime duration of contraceptive use did not impact fecundability in the North American cohort.
Study strengths and limitations
This large, prospective study contributes useful information about fecundability after stopping contraceptive methods. It confirms earlier studies’ findings that showed decreased fecundability after stopping injectable contraceptives. Study participants’ most recent method used was similar to overall US method distribution.1
Study limitations include online recruitment of self-selecting participants, which introduces selection bias. The study population was overwhelmingly white (92%) and highly educated (70% with college degrees), quite different from the US population. These findings may therefore have limited generalizability. Additionally, injectable contraceptive users had higher body mass index and were more likely to smoke and have diabetes, infertility, or irregular menstrual cycles. IUD users were more likely to be parous and have a history of unplanned pregnancy, indicating possible higher baseline fertility. Even after adjusting, possible unmeasured factors could impact study results. ●
This is the largest study to date to evaluate fecundability after stopping different contraceptive methods among women planning pregnancies. The study confirms previous research that associated injectable contraceptives with delayed return of normal fertility. It provides reassurance for counseling users of IUDs, implants, oral contraception, ring, and patch: those methods were not associated with reduced fecundability compared with barrier methods. The study also suggests long-term contraceptive use does not decrease fecundability.
Women may ask when to stop their contraceptive method to optimally time a pregnancy. In this study, measurements of return to normal fertility were imprecise. Individualized counseling, accounting for personal circumstances, is still best when advising when to stop contraception for couples planning pregnancy.
LISA HOFLER, MD, MPH, MBA, AND LINDSAY DALE, MD
Yland JJ, Bresnick KA, Hatch EE, et al. Pregravid contraceptive use and fecundability: prospective cohort study. BMJ. 2020;371:m3966.
EXPERT COMMENTARY
Most US women aged 15 to 49 currently use contraception, with long-acting reversible contraception (LARC)—IUDs and the contraceptive implant—increasing in popularity over the last decade.1 Oral contraceptive pills, male condoms, and LARC are the most common reversible methods used.1 While the efficacy and safety of contraception have been established, few studies have examined the effect of recent contraceptive use on fertility.
Fecundability is the probability of pregnancy during a single menstrual cycle for a couple engaging in regular intercourse and not using contraception.2 Small studies have found short-term reductions in fecundability after discontinuing combined oral contraceptives and larger reductions after stopping injectable contraceptives, with no long-term differences among methods.3,4
Data are limited regarding the effects of other forms of contraception on fecundability, particularly LARC methods. A recent study was designed to evaluate the association between the last contraceptive method used and subsequent fecundability.2
Details of the study
Yland and colleagues pooled data from 3 prospective cohort studies of 17,954 women planning pregnancies in Denmark, Canada, and the United States. Participants reported the contraceptive method used most recently before trying to conceive. They completed questionnaires every 2 months for 12 months or until they reported a pregnancy. Women were excluded if they tried to conceive for more than 6 menstrual cycles at study entry.
The authors calculated the fecundability ratio—the average probability of conception per cycle for a specific contraceptive method compared with a reference method—using proportional probability models adjusted for potential confounders. They also calculated pregnancy attempt time using participant-reported menstrual cycle length and date of last menstrual period during follow-up questionnaires.
Continue to: Injectable contraceptives associated with longest delayed fertility return...
Injectable contraceptives associated with longest delayed fertility return
After adjusting for personal factors, medical history, lifestyle characteristics, and indicators of underlying fertility, the authors found that injectable contraceptive use was associated with decreased fecundability compared with barrier method use (fecundability ratio [FR], 0.65; 95% confidence interval [CI], 0.47–0.89). Hormonal IUD use was associated with slight increases in fecundability compared with barrier method use (FR, 1.14; 95% CI, 1.07–1.22) and copper IUD use (FR, 1.18; 95% CI, 1.05–1.33). All other contraceptive methods were not significantly different from barrier methods.
LARC method use was associated with the shortest delay in return of normal fertility (2 cycles), followed by oral and ring contraceptives (3 cycles) and patch (4 cycles). Women using injectable contraceptives experienced the longest delay (5–8 menstrual cycles). Lifetime duration of contraceptive use did not impact fecundability in the North American cohort.
Study strengths and limitations
This large, prospective study contributes useful information about fecundability after stopping contraceptive methods. It confirms earlier studies’ findings that showed decreased fecundability after stopping injectable contraceptives. Study participants’ most recent method used was similar to overall US method distribution.1
Study limitations include online recruitment of self-selecting participants, which introduces selection bias. The study population was overwhelmingly white (92%) and highly educated (70% with college degrees), quite different from the US population. These findings may therefore have limited generalizability. Additionally, injectable contraceptive users had higher body mass index and were more likely to smoke and have diabetes, infertility, or irregular menstrual cycles. IUD users were more likely to be parous and have a history of unplanned pregnancy, indicating possible higher baseline fertility. Even after adjusting, possible unmeasured factors could impact study results. ●
This is the largest study to date to evaluate fecundability after stopping different contraceptive methods among women planning pregnancies. The study confirms previous research that associated injectable contraceptives with delayed return of normal fertility. It provides reassurance for counseling users of IUDs, implants, oral contraception, ring, and patch: those methods were not associated with reduced fecundability compared with barrier methods. The study also suggests long-term contraceptive use does not decrease fecundability.
Women may ask when to stop their contraceptive method to optimally time a pregnancy. In this study, measurements of return to normal fertility were imprecise. Individualized counseling, accounting for personal circumstances, is still best when advising when to stop contraception for couples planning pregnancy.
LISA HOFLER, MD, MPH, MBA, AND LINDSAY DALE, MD
- Daniels K, Abma JC. Current contraceptive status among women aged 15–49: United States, 2017–2019. NCHS Data Brief, no. 388. Hyattsville, MD: National Center for Health Statistics; 2020.
- Yland JJ, Bresnick KA, Hatch EE, et al. Pregravid contraceptive use and fecundability: prospective cohort study. BMJ. 2020;371:m3966.
- Hassan MA, Killick SR. Is previous use of hormonal contraception associated with a detrimental effect on subsequent fecundity? Hum Reprod. 2004;19:344-351.
- Mansour D, Gemzell-Danielsson K, Inki P, et al. Fertility after discontinuation of contraception: a comprehensive review of the literature. Contraception. 2011;84:465-477.
- Daniels K, Abma JC. Current contraceptive status among women aged 15–49: United States, 2017–2019. NCHS Data Brief, no. 388. Hyattsville, MD: National Center for Health Statistics; 2020.
- Yland JJ, Bresnick KA, Hatch EE, et al. Pregravid contraceptive use and fecundability: prospective cohort study. BMJ. 2020;371:m3966.
- Hassan MA, Killick SR. Is previous use of hormonal contraception associated with a detrimental effect on subsequent fecundity? Hum Reprod. 2004;19:344-351.
- Mansour D, Gemzell-Danielsson K, Inki P, et al. Fertility after discontinuation of contraception: a comprehensive review of the literature. Contraception. 2011;84:465-477.