User login
Ultraprocessed food again linked to increased CVD, death
Yet another study has linked the consumption of ultraprocessed, or “junk,” foods to bad health outcomes.
In a longitudinal analysis of more than 22,000 men and women from southern Italy, those who consumed the most ultraprocessed food (UPF) had the highest risk for cardiovascular disease (CVD) and all-cause mortality, likely mediated through a diet high in sugar, researchers said.
High consumption of UPF in this Mediterranean cohort was associated with a 58% increased risk for CVD mortality and 52% higher risk of dying from ischemic heart disease (IHD) and cerebrovascular causes, independently of known risk factors for CVD, even among individuals who otherwise adhered to the Mediterranean diet.
The findings “should serve as an incentive for limiting consumption of UPF and encouraging natural or minimally processed foods, as several national nutritional policies recommend,” Marialaura Bonaccio, PhD, department of epidemiology and prevention, IRCCS Neuromed, Pozzilli, Italy, and colleagues wrote. The results were published online Dec. 18 in the American Journal of Clinical Nutrition.
Earlier this year, as reported by this news organization, researchers found mounting evidence that the obesity epidemic and the increase in incidence of related chronic conditions corresponded with an increase in the intake of UPF.
A study that was conducted in a European cohort found that adults whose diet included more UPF and beverages, such as ice cream, soda, and hamburgers, were more likely to develop CVD or die sooner than others who had a more wholesome diet.
As reported previously by this news organization, among adults in France who had a 10% higher intake of UPF and beverages, the rate of CVD, coronary heart disease, and cerebrovascular disease was 11% to 13% higher over a period of about 5 years.
Similarly, university graduates in Spain who consumed more than four servings of UPF and beverages a day were 62% more likely to die of any cause over about a decade than those who consumed less than two servings per day.
Where’s the food?
There is very little actual food in UPF. “The NOVA classification provides 4 main classes of food and beverages, the last of which is represented by the ultraprocessed food group. This comprises products (e.g., snacks, drinks, and ready meals, ‘created mostly or entirely from substances extracted from foods or derived from food constituents with little, if any intact food, which often contain flavors, colors, and other additives that imitate or intensify the sensory qualities of foods or culinary preparations made from foods,’ ” Dr. Bonaccio and colleagues wrote.
Such foods are very convenient, tasty, inexpensive, and have a long shelf life. They are highly competitive with foods that are naturally ready to consume and freshly prepared dishes and meals, the authors add.
The researchers conducted a longitudinal analysis on 22,475 men and women (mean age, 55 years; range, 43-67 years) who were recruited from the Moli-sani Study, a population-based cohort of men and women aged 35 years and older in the Molise region of southern Italy, between 2005 and 2010. Participants were followed for 8.2 years.
Food intake was assessed with the Food Frequency Questionnaire; UPF was defined using the NOVA classification according to degree of processing.
UPF intakes were categorized as quartiles of the ratio of UPF to total food consumed.
Overall, study participants reported a median of 10% (interquartile range, 6.6%-14.6%) of dietary intake as UPF and a total of 181.5 g/d of UPF intake.
The foods that contributed most to total UPF consumed were processed meat, which accounted for 19.8% of UPF intake; pizza (16.8%); and cakes and pies (13.4%).
High consumers of UPF, defined as those for whom UPF constituted more than 14.6% of their total diet, were more likely to be women, to be younger, and to have a higher educational level. They also reported fewer risk factors and fewer baseline chronic diseases and health conditions than persons who consumed UPF less frequently.
In addition, high consumption of UPF was associated with lower adherence to the Mediterranean diet; higher intake of fat, sugar, dietary cholesterol, and sodium; and lower intake of fiber.
During a median follow-up of 8.2 years, 1,216 all-cause deaths occurred. Of these, 439 were attributed to CVD, 255 to IHD/cerebrovascular disease, 477 to cancer, and 300 to other causes.
The more UPF, the higher the risk for CVD, death
The researchers found a direct linear dose-response relation between a 5% increase in the proportion of UPF in the diet and risk for all-cause and CVD mortality.
Individuals who reported the highest intake of UPF (fourth quartile, 14.6% of total food) as opposed to the lowest (first quartile, UPF <6.6%) experienced increased risks for CVD mortality (hazard ratio, 1.58; 95% CI, 1.23-2.03), death from IHD/cerebrovascular disease (HR, 1.52, 95% CI, 1.10-2.09), and all-cause mortality (HR, 1.26; 95% CI, 1.09-1.46).
High sugar content accounted for 36.3% of the relation of UPF with IHD/cerebrovascular mortality. Other nutritional factors, such as saturated fats, were unlikely to play a role, the researchers wrote.
Biomarkers of renal function accounted for 20.1% of the association of UPF with all-cause mortality and 12.0% for that of UPF with CVD mortality.
Subgroup analyses indicated that the magnitude of the association between UPF and all-cause mortality risk was greater among high-risk individuals, such as those with a history of CVD or diabetes. UPF was also likely to be more strongly associated with CVD mortality among those high-risk groups.
The interesting finding that the association between UPF and CVD mortality was greater among individuals with good adherence to the Mediterranean diet, which is known to have health benefits, could be explained by the fact that people who may benefit from a Mediterranean diet are more susceptible to losing health advantages when they also include “detrimental dietary behavior,” whereas those who consume a poor-quality diet are less likely to be harmed by an additional unhealthy behavior such as eating UPF regularly, wrote Dr. Bonaccio and colleagues.
“This is an interesting study confirming that consumption of highly processed foods such as pizza, processed meats, and soda are associated with greater risks of cardiovascular disease,” Walter Willett, MD, professor of epidemiology and nutrition, Harvard School of Public Health, Boston, said in an interview.
“These higher risks appear to be mediated in part by high intakes of saturated fat and sugar, but lower intakes of health-promoting aspects of diet also likely contribute to the findings,” Dr. Willett said.
“Some processing of food can be useful for preservation and control of infectious agents, but in general, a diet emphasizing minimally processed fruits and vegetables, whole grains, nuts, legumes, and plant sources of fat will be best for long-term well-being,” he said.
The study was supported in part by the Italian Ministry of Health and the HYPERCAN Study Italian Association for Cancer Research. Dr. Bonaccio and Dr. Willett reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Yet another study has linked the consumption of ultraprocessed, or “junk,” foods to bad health outcomes.
In a longitudinal analysis of more than 22,000 men and women from southern Italy, those who consumed the most ultraprocessed food (UPF) had the highest risk for cardiovascular disease (CVD) and all-cause mortality, likely mediated through a diet high in sugar, researchers said.
High consumption of UPF in this Mediterranean cohort was associated with a 58% increased risk for CVD mortality and 52% higher risk of dying from ischemic heart disease (IHD) and cerebrovascular causes, independently of known risk factors for CVD, even among individuals who otherwise adhered to the Mediterranean diet.
The findings “should serve as an incentive for limiting consumption of UPF and encouraging natural or minimally processed foods, as several national nutritional policies recommend,” Marialaura Bonaccio, PhD, department of epidemiology and prevention, IRCCS Neuromed, Pozzilli, Italy, and colleagues wrote. The results were published online Dec. 18 in the American Journal of Clinical Nutrition.
Earlier this year, as reported by this news organization, researchers found mounting evidence that the obesity epidemic and the increase in incidence of related chronic conditions corresponded with an increase in the intake of UPF.
A study that was conducted in a European cohort found that adults whose diet included more UPF and beverages, such as ice cream, soda, and hamburgers, were more likely to develop CVD or die sooner than others who had a more wholesome diet.
As reported previously by this news organization, among adults in France who had a 10% higher intake of UPF and beverages, the rate of CVD, coronary heart disease, and cerebrovascular disease was 11% to 13% higher over a period of about 5 years.
Similarly, university graduates in Spain who consumed more than four servings of UPF and beverages a day were 62% more likely to die of any cause over about a decade than those who consumed less than two servings per day.
Where’s the food?
There is very little actual food in UPF. “The NOVA classification provides 4 main classes of food and beverages, the last of which is represented by the ultraprocessed food group. This comprises products (e.g., snacks, drinks, and ready meals, ‘created mostly or entirely from substances extracted from foods or derived from food constituents with little, if any intact food, which often contain flavors, colors, and other additives that imitate or intensify the sensory qualities of foods or culinary preparations made from foods,’ ” Dr. Bonaccio and colleagues wrote.
Such foods are very convenient, tasty, inexpensive, and have a long shelf life. They are highly competitive with foods that are naturally ready to consume and freshly prepared dishes and meals, the authors add.
The researchers conducted a longitudinal analysis on 22,475 men and women (mean age, 55 years; range, 43-67 years) who were recruited from the Moli-sani Study, a population-based cohort of men and women aged 35 years and older in the Molise region of southern Italy, between 2005 and 2010. Participants were followed for 8.2 years.
Food intake was assessed with the Food Frequency Questionnaire; UPF was defined using the NOVA classification according to degree of processing.
UPF intakes were categorized as quartiles of the ratio of UPF to total food consumed.
Overall, study participants reported a median of 10% (interquartile range, 6.6%-14.6%) of dietary intake as UPF and a total of 181.5 g/d of UPF intake.
The foods that contributed most to total UPF consumed were processed meat, which accounted for 19.8% of UPF intake; pizza (16.8%); and cakes and pies (13.4%).
High consumers of UPF, defined as those for whom UPF constituted more than 14.6% of their total diet, were more likely to be women, to be younger, and to have a higher educational level. They also reported fewer risk factors and fewer baseline chronic diseases and health conditions than persons who consumed UPF less frequently.
In addition, high consumption of UPF was associated with lower adherence to the Mediterranean diet; higher intake of fat, sugar, dietary cholesterol, and sodium; and lower intake of fiber.
During a median follow-up of 8.2 years, 1,216 all-cause deaths occurred. Of these, 439 were attributed to CVD, 255 to IHD/cerebrovascular disease, 477 to cancer, and 300 to other causes.
The more UPF, the higher the risk for CVD, death
The researchers found a direct linear dose-response relation between a 5% increase in the proportion of UPF in the diet and risk for all-cause and CVD mortality.
Individuals who reported the highest intake of UPF (fourth quartile, 14.6% of total food) as opposed to the lowest (first quartile, UPF <6.6%) experienced increased risks for CVD mortality (hazard ratio, 1.58; 95% CI, 1.23-2.03), death from IHD/cerebrovascular disease (HR, 1.52, 95% CI, 1.10-2.09), and all-cause mortality (HR, 1.26; 95% CI, 1.09-1.46).
High sugar content accounted for 36.3% of the relation of UPF with IHD/cerebrovascular mortality. Other nutritional factors, such as saturated fats, were unlikely to play a role, the researchers wrote.
Biomarkers of renal function accounted for 20.1% of the association of UPF with all-cause mortality and 12.0% for that of UPF with CVD mortality.
Subgroup analyses indicated that the magnitude of the association between UPF and all-cause mortality risk was greater among high-risk individuals, such as those with a history of CVD or diabetes. UPF was also likely to be more strongly associated with CVD mortality among those high-risk groups.
The interesting finding that the association between UPF and CVD mortality was greater among individuals with good adherence to the Mediterranean diet, which is known to have health benefits, could be explained by the fact that people who may benefit from a Mediterranean diet are more susceptible to losing health advantages when they also include “detrimental dietary behavior,” whereas those who consume a poor-quality diet are less likely to be harmed by an additional unhealthy behavior such as eating UPF regularly, wrote Dr. Bonaccio and colleagues.
“This is an interesting study confirming that consumption of highly processed foods such as pizza, processed meats, and soda are associated with greater risks of cardiovascular disease,” Walter Willett, MD, professor of epidemiology and nutrition, Harvard School of Public Health, Boston, said in an interview.
“These higher risks appear to be mediated in part by high intakes of saturated fat and sugar, but lower intakes of health-promoting aspects of diet also likely contribute to the findings,” Dr. Willett said.
“Some processing of food can be useful for preservation and control of infectious agents, but in general, a diet emphasizing minimally processed fruits and vegetables, whole grains, nuts, legumes, and plant sources of fat will be best for long-term well-being,” he said.
The study was supported in part by the Italian Ministry of Health and the HYPERCAN Study Italian Association for Cancer Research. Dr. Bonaccio and Dr. Willett reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Yet another study has linked the consumption of ultraprocessed, or “junk,” foods to bad health outcomes.
In a longitudinal analysis of more than 22,000 men and women from southern Italy, those who consumed the most ultraprocessed food (UPF) had the highest risk for cardiovascular disease (CVD) and all-cause mortality, likely mediated through a diet high in sugar, researchers said.
High consumption of UPF in this Mediterranean cohort was associated with a 58% increased risk for CVD mortality and 52% higher risk of dying from ischemic heart disease (IHD) and cerebrovascular causes, independently of known risk factors for CVD, even among individuals who otherwise adhered to the Mediterranean diet.
The findings “should serve as an incentive for limiting consumption of UPF and encouraging natural or minimally processed foods, as several national nutritional policies recommend,” Marialaura Bonaccio, PhD, department of epidemiology and prevention, IRCCS Neuromed, Pozzilli, Italy, and colleagues wrote. The results were published online Dec. 18 in the American Journal of Clinical Nutrition.
Earlier this year, as reported by this news organization, researchers found mounting evidence that the obesity epidemic and the increase in incidence of related chronic conditions corresponded with an increase in the intake of UPF.
A study that was conducted in a European cohort found that adults whose diet included more UPF and beverages, such as ice cream, soda, and hamburgers, were more likely to develop CVD or die sooner than others who had a more wholesome diet.
As reported previously by this news organization, among adults in France who had a 10% higher intake of UPF and beverages, the rate of CVD, coronary heart disease, and cerebrovascular disease was 11% to 13% higher over a period of about 5 years.
Similarly, university graduates in Spain who consumed more than four servings of UPF and beverages a day were 62% more likely to die of any cause over about a decade than those who consumed less than two servings per day.
Where’s the food?
There is very little actual food in UPF. “The NOVA classification provides 4 main classes of food and beverages, the last of which is represented by the ultraprocessed food group. This comprises products (e.g., snacks, drinks, and ready meals, ‘created mostly or entirely from substances extracted from foods or derived from food constituents with little, if any intact food, which often contain flavors, colors, and other additives that imitate or intensify the sensory qualities of foods or culinary preparations made from foods,’ ” Dr. Bonaccio and colleagues wrote.
Such foods are very convenient, tasty, inexpensive, and have a long shelf life. They are highly competitive with foods that are naturally ready to consume and freshly prepared dishes and meals, the authors add.
The researchers conducted a longitudinal analysis on 22,475 men and women (mean age, 55 years; range, 43-67 years) who were recruited from the Moli-sani Study, a population-based cohort of men and women aged 35 years and older in the Molise region of southern Italy, between 2005 and 2010. Participants were followed for 8.2 years.
Food intake was assessed with the Food Frequency Questionnaire; UPF was defined using the NOVA classification according to degree of processing.
UPF intakes were categorized as quartiles of the ratio of UPF to total food consumed.
Overall, study participants reported a median of 10% (interquartile range, 6.6%-14.6%) of dietary intake as UPF and a total of 181.5 g/d of UPF intake.
The foods that contributed most to total UPF consumed were processed meat, which accounted for 19.8% of UPF intake; pizza (16.8%); and cakes and pies (13.4%).
High consumers of UPF, defined as those for whom UPF constituted more than 14.6% of their total diet, were more likely to be women, to be younger, and to have a higher educational level. They also reported fewer risk factors and fewer baseline chronic diseases and health conditions than persons who consumed UPF less frequently.
In addition, high consumption of UPF was associated with lower adherence to the Mediterranean diet; higher intake of fat, sugar, dietary cholesterol, and sodium; and lower intake of fiber.
During a median follow-up of 8.2 years, 1,216 all-cause deaths occurred. Of these, 439 were attributed to CVD, 255 to IHD/cerebrovascular disease, 477 to cancer, and 300 to other causes.
The more UPF, the higher the risk for CVD, death
The researchers found a direct linear dose-response relation between a 5% increase in the proportion of UPF in the diet and risk for all-cause and CVD mortality.
Individuals who reported the highest intake of UPF (fourth quartile, 14.6% of total food) as opposed to the lowest (first quartile, UPF <6.6%) experienced increased risks for CVD mortality (hazard ratio, 1.58; 95% CI, 1.23-2.03), death from IHD/cerebrovascular disease (HR, 1.52, 95% CI, 1.10-2.09), and all-cause mortality (HR, 1.26; 95% CI, 1.09-1.46).
High sugar content accounted for 36.3% of the relation of UPF with IHD/cerebrovascular mortality. Other nutritional factors, such as saturated fats, were unlikely to play a role, the researchers wrote.
Biomarkers of renal function accounted for 20.1% of the association of UPF with all-cause mortality and 12.0% for that of UPF with CVD mortality.
Subgroup analyses indicated that the magnitude of the association between UPF and all-cause mortality risk was greater among high-risk individuals, such as those with a history of CVD or diabetes. UPF was also likely to be more strongly associated with CVD mortality among those high-risk groups.
The interesting finding that the association between UPF and CVD mortality was greater among individuals with good adherence to the Mediterranean diet, which is known to have health benefits, could be explained by the fact that people who may benefit from a Mediterranean diet are more susceptible to losing health advantages when they also include “detrimental dietary behavior,” whereas those who consume a poor-quality diet are less likely to be harmed by an additional unhealthy behavior such as eating UPF regularly, wrote Dr. Bonaccio and colleagues.
“This is an interesting study confirming that consumption of highly processed foods such as pizza, processed meats, and soda are associated with greater risks of cardiovascular disease,” Walter Willett, MD, professor of epidemiology and nutrition, Harvard School of Public Health, Boston, said in an interview.
“These higher risks appear to be mediated in part by high intakes of saturated fat and sugar, but lower intakes of health-promoting aspects of diet also likely contribute to the findings,” Dr. Willett said.
“Some processing of food can be useful for preservation and control of infectious agents, but in general, a diet emphasizing minimally processed fruits and vegetables, whole grains, nuts, legumes, and plant sources of fat will be best for long-term well-being,” he said.
The study was supported in part by the Italian Ministry of Health and the HYPERCAN Study Italian Association for Cancer Research. Dr. Bonaccio and Dr. Willett reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Older adults with myelodysplastic syndrome can benefit from transplants
Key clinical point: Overall survival improved by more than 20% in older adults with myelodysplastic syndrome who received allogeneic stem cell transplantation.
Major finding: Overall survival at 3 years was 47.9% for patients who received allogeneic hematopoietic cell transplantation compared to those who did not.
Study details: The data come from myelodysplastic syndrome patients with a median age of 66 years who were part of the prospective Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Study 1102 (NCT02016781) presented at the American Society of Hematology (ASH) 2020 virtual meeting.
Disclosures: Lead author Dr. Cutler disclosed serving as a consultant for Mesoblast, Generon, Medsenic, Jazz, Kadmon, and Incyte.
Source: Cutler C et al. ASH 2020.
Key clinical point: Overall survival improved by more than 20% in older adults with myelodysplastic syndrome who received allogeneic stem cell transplantation.
Major finding: Overall survival at 3 years was 47.9% for patients who received allogeneic hematopoietic cell transplantation compared to those who did not.
Study details: The data come from myelodysplastic syndrome patients with a median age of 66 years who were part of the prospective Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Study 1102 (NCT02016781) presented at the American Society of Hematology (ASH) 2020 virtual meeting.
Disclosures: Lead author Dr. Cutler disclosed serving as a consultant for Mesoblast, Generon, Medsenic, Jazz, Kadmon, and Incyte.
Source: Cutler C et al. ASH 2020.
Key clinical point: Overall survival improved by more than 20% in older adults with myelodysplastic syndrome who received allogeneic stem cell transplantation.
Major finding: Overall survival at 3 years was 47.9% for patients who received allogeneic hematopoietic cell transplantation compared to those who did not.
Study details: The data come from myelodysplastic syndrome patients with a median age of 66 years who were part of the prospective Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Study 1102 (NCT02016781) presented at the American Society of Hematology (ASH) 2020 virtual meeting.
Disclosures: Lead author Dr. Cutler disclosed serving as a consultant for Mesoblast, Generon, Medsenic, Jazz, Kadmon, and Incyte.
Source: Cutler C et al. ASH 2020.
Rabbit antithymocyte globulin improved outcomes in stem cell transplants
Key clinical point: Incidence of graft vs. host disease was significantly lower in patients undergoing matched sibling donor stem cell transplants who received low-dose rabbit antitymoctye globulin (rATG).
Major finding: The 2-year cumulative incidences of chronic GVHD (cGVHD) were 35.4% and 60.4%, respectively, for patients in the rATG and non-rATG groups (P=0.039).
Study details: The data come from a retrospective study of 79 patients aged 16 years and older with hematologic malignancies who were treated with matched sibling donor hematopoietic stem cell transplantation (MSD-HSCT); all received standard prophylaxis and 38 received 5 mg/kg of rATG in addition.
Disclosures: The study was supported in part by the Scientific Research Seed Fund of Peking University First Hospital. The researchers had no financial conflicts to disclose.
Source: Song ZY et al. Cancer Manag Res. 2020 Nov 30. doi: 10.2147/CMAR.S283855.
Key clinical point: Incidence of graft vs. host disease was significantly lower in patients undergoing matched sibling donor stem cell transplants who received low-dose rabbit antitymoctye globulin (rATG).
Major finding: The 2-year cumulative incidences of chronic GVHD (cGVHD) were 35.4% and 60.4%, respectively, for patients in the rATG and non-rATG groups (P=0.039).
Study details: The data come from a retrospective study of 79 patients aged 16 years and older with hematologic malignancies who were treated with matched sibling donor hematopoietic stem cell transplantation (MSD-HSCT); all received standard prophylaxis and 38 received 5 mg/kg of rATG in addition.
Disclosures: The study was supported in part by the Scientific Research Seed Fund of Peking University First Hospital. The researchers had no financial conflicts to disclose.
Source: Song ZY et al. Cancer Manag Res. 2020 Nov 30. doi: 10.2147/CMAR.S283855.
Key clinical point: Incidence of graft vs. host disease was significantly lower in patients undergoing matched sibling donor stem cell transplants who received low-dose rabbit antitymoctye globulin (rATG).
Major finding: The 2-year cumulative incidences of chronic GVHD (cGVHD) were 35.4% and 60.4%, respectively, for patients in the rATG and non-rATG groups (P=0.039).
Study details: The data come from a retrospective study of 79 patients aged 16 years and older with hematologic malignancies who were treated with matched sibling donor hematopoietic stem cell transplantation (MSD-HSCT); all received standard prophylaxis and 38 received 5 mg/kg of rATG in addition.
Disclosures: The study was supported in part by the Scientific Research Seed Fund of Peking University First Hospital. The researchers had no financial conflicts to disclose.
Source: Song ZY et al. Cancer Manag Res. 2020 Nov 30. doi: 10.2147/CMAR.S283855.
Experimental compound for myelodysplastic syndrome shows potential in phase III trial
Key clinical point: The compound APR‐246 (PRIMA‐1Met/Eprenetapopt) caused synergistic tumor death in myelodysplastic syndrome with combined with inhibitors of efflux pump MRP1/ABCC1.
Major finding: A combination of combination of APR‐246 with the MRP1 inhibitor MK‐571 significantly reduced IC50 values in 21 cell lines (P < 0.0001).
Study details: The data come from a combination of in vitro, ex vivo, and in vivo models, using combined treatment of APR‐246 and inhibitors of efflux pump MRP1/ABCC1.
Disclosures: The study was funded by multiple organizations including Vetenskapsrådet, Cancerfonden (Swedish Cancer Society), Barncancerfonden (Swedish Childhood Cancer Foundation), Radiumhemmets Forskningsfonder (Cancer Research Foundations of Radiumhemmet, Knut och Alice Wallenbergs Stiftelse (Knut and Alice Wallenberg Foundation), Aprea Therapeutics, Karolinska Institutet (KI), Department of Health, Australian Government/National Health and Medical Research Council (NHMRC), Department of Health and Human Services, State Government of Victoria (DHHS), Tour de Cure Foundation, UKRI/Medical Research Council (MRC), Australian Cancer Research Foundation (ACRF), Australian Government’s National Collaborative Research Infrastructure Strategy (NCRIS) program, Peter MacCallum Cancer Centre Foundation, University of Melbourne Research Collaborative Infrastructure Program (MCRIP), and Cancer Research UK.
Source: Ceder S et al. EMBO Mol Med. 2020 Dec 14. doi: 10.15252/emmm.201910852.
Key clinical point: The compound APR‐246 (PRIMA‐1Met/Eprenetapopt) caused synergistic tumor death in myelodysplastic syndrome with combined with inhibitors of efflux pump MRP1/ABCC1.
Major finding: A combination of combination of APR‐246 with the MRP1 inhibitor MK‐571 significantly reduced IC50 values in 21 cell lines (P < 0.0001).
Study details: The data come from a combination of in vitro, ex vivo, and in vivo models, using combined treatment of APR‐246 and inhibitors of efflux pump MRP1/ABCC1.
Disclosures: The study was funded by multiple organizations including Vetenskapsrådet, Cancerfonden (Swedish Cancer Society), Barncancerfonden (Swedish Childhood Cancer Foundation), Radiumhemmets Forskningsfonder (Cancer Research Foundations of Radiumhemmet, Knut och Alice Wallenbergs Stiftelse (Knut and Alice Wallenberg Foundation), Aprea Therapeutics, Karolinska Institutet (KI), Department of Health, Australian Government/National Health and Medical Research Council (NHMRC), Department of Health and Human Services, State Government of Victoria (DHHS), Tour de Cure Foundation, UKRI/Medical Research Council (MRC), Australian Cancer Research Foundation (ACRF), Australian Government’s National Collaborative Research Infrastructure Strategy (NCRIS) program, Peter MacCallum Cancer Centre Foundation, University of Melbourne Research Collaborative Infrastructure Program (MCRIP), and Cancer Research UK.
Source: Ceder S et al. EMBO Mol Med. 2020 Dec 14. doi: 10.15252/emmm.201910852.
Key clinical point: The compound APR‐246 (PRIMA‐1Met/Eprenetapopt) caused synergistic tumor death in myelodysplastic syndrome with combined with inhibitors of efflux pump MRP1/ABCC1.
Major finding: A combination of combination of APR‐246 with the MRP1 inhibitor MK‐571 significantly reduced IC50 values in 21 cell lines (P < 0.0001).
Study details: The data come from a combination of in vitro, ex vivo, and in vivo models, using combined treatment of APR‐246 and inhibitors of efflux pump MRP1/ABCC1.
Disclosures: The study was funded by multiple organizations including Vetenskapsrådet, Cancerfonden (Swedish Cancer Society), Barncancerfonden (Swedish Childhood Cancer Foundation), Radiumhemmets Forskningsfonder (Cancer Research Foundations of Radiumhemmet, Knut och Alice Wallenbergs Stiftelse (Knut and Alice Wallenberg Foundation), Aprea Therapeutics, Karolinska Institutet (KI), Department of Health, Australian Government/National Health and Medical Research Council (NHMRC), Department of Health and Human Services, State Government of Victoria (DHHS), Tour de Cure Foundation, UKRI/Medical Research Council (MRC), Australian Cancer Research Foundation (ACRF), Australian Government’s National Collaborative Research Infrastructure Strategy (NCRIS) program, Peter MacCallum Cancer Centre Foundation, University of Melbourne Research Collaborative Infrastructure Program (MCRIP), and Cancer Research UK.
Source: Ceder S et al. EMBO Mol Med. 2020 Dec 14. doi: 10.15252/emmm.201910852.
Flow score adds value to myelodysplastic syndrome prognosis
Key clinical point: Researchers developed a flow score based on three variables: developed a “flow score” with the three variables percentage of myeloid CD34+ cells > 2%; percentage of B-cell progenitors < 0.05%; and CD16+ monocytes/TNCs > 1.0%.
Major finding: Of the 95 patients in the study, 23 remained alive after the end of the study period. A high percentage of CD16+ monocytes/TNCs > 1.0% (in the highest quartile) was associated with significantly worse survival.
Study details: The data come from 95 adult patients with newly diagnosed myelodysplastic syndrome seen at a single center and followed for an average of 42 months.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Vido-Marques JR et al. Sci Rep. 2020 Nov 20. doi: 10.1038/s41598-020-77158-z.
Key clinical point: Researchers developed a flow score based on three variables: developed a “flow score” with the three variables percentage of myeloid CD34+ cells > 2%; percentage of B-cell progenitors < 0.05%; and CD16+ monocytes/TNCs > 1.0%.
Major finding: Of the 95 patients in the study, 23 remained alive after the end of the study period. A high percentage of CD16+ monocytes/TNCs > 1.0% (in the highest quartile) was associated with significantly worse survival.
Study details: The data come from 95 adult patients with newly diagnosed myelodysplastic syndrome seen at a single center and followed for an average of 42 months.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Vido-Marques JR et al. Sci Rep. 2020 Nov 20. doi: 10.1038/s41598-020-77158-z.
Key clinical point: Researchers developed a flow score based on three variables: developed a “flow score” with the three variables percentage of myeloid CD34+ cells > 2%; percentage of B-cell progenitors < 0.05%; and CD16+ monocytes/TNCs > 1.0%.
Major finding: Of the 95 patients in the study, 23 remained alive after the end of the study period. A high percentage of CD16+ monocytes/TNCs > 1.0% (in the highest quartile) was associated with significantly worse survival.
Study details: The data come from 95 adult patients with newly diagnosed myelodysplastic syndrome seen at a single center and followed for an average of 42 months.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Vido-Marques JR et al. Sci Rep. 2020 Nov 20. doi: 10.1038/s41598-020-77158-z.
Clinical Edge Journal Scans: MDS January 2021 Commentary
Higher expression of Wilms’ tumor 1 (WT-1) has been associated with poor survival in MDS. To further characterize the prognostic value of WT-1 expression level, peripheral WT-1 mRNA expression level at baseline was measured in MDS patients undergoing treatment with azacitidine. Lower WT-1 mRNA level was associated with higher overall response, as defined by CR, marrow CR, partial response, or HI (p=0.03), although WT-1 mRNA level did not show difference in overall survival. This was a small retrospective, single center study; role of WT-1 mRNA level as a biomarker for response to azacitidine should be further investigated.
Results of a multi-center trial comparing reduced intensity allogeneic stem cell transplant (SCT) to hypomethylating agents or best supportive care in higher risk MDS patients aged 50-75 was reported in the annual meeting of the American Society of Hematology in 2020. Patients with intermediate-2 or higher risk by IPSS were assigned based on SCT or no SCT arm based on donor status, and patients who had donor proceeded to reduced intensity SCT within 6 months of enrolment. In an intent-to-treat analysis, the study showed different in 3-year overall survival, which was the primary endpoint between SCT vs non-SCT arms (47.9% vs 26.6%, p=0.0001). Leukemia-free survival at 3 years was also greater for the SCT arm (35.8% vs 20.6%, p=0.003). Allogenic stem cell transplant is currently offered to patients with higher risk MDS given this is the only potential curable approach for MDS patients. This study further supports allogeneic stem cell transplant for older patients with higher risk MDS.
Higher expression of Wilms’ tumor 1 (WT-1) has been associated with poor survival in MDS. To further characterize the prognostic value of WT-1 expression level, peripheral WT-1 mRNA expression level at baseline was measured in MDS patients undergoing treatment with azacitidine. Lower WT-1 mRNA level was associated with higher overall response, as defined by CR, marrow CR, partial response, or HI (p=0.03), although WT-1 mRNA level did not show difference in overall survival. This was a small retrospective, single center study; role of WT-1 mRNA level as a biomarker for response to azacitidine should be further investigated.
Results of a multi-center trial comparing reduced intensity allogeneic stem cell transplant (SCT) to hypomethylating agents or best supportive care in higher risk MDS patients aged 50-75 was reported in the annual meeting of the American Society of Hematology in 2020. Patients with intermediate-2 or higher risk by IPSS were assigned based on SCT or no SCT arm based on donor status, and patients who had donor proceeded to reduced intensity SCT within 6 months of enrolment. In an intent-to-treat analysis, the study showed different in 3-year overall survival, which was the primary endpoint between SCT vs non-SCT arms (47.9% vs 26.6%, p=0.0001). Leukemia-free survival at 3 years was also greater for the SCT arm (35.8% vs 20.6%, p=0.003). Allogenic stem cell transplant is currently offered to patients with higher risk MDS given this is the only potential curable approach for MDS patients. This study further supports allogeneic stem cell transplant for older patients with higher risk MDS.
Higher expression of Wilms’ tumor 1 (WT-1) has been associated with poor survival in MDS. To further characterize the prognostic value of WT-1 expression level, peripheral WT-1 mRNA expression level at baseline was measured in MDS patients undergoing treatment with azacitidine. Lower WT-1 mRNA level was associated with higher overall response, as defined by CR, marrow CR, partial response, or HI (p=0.03), although WT-1 mRNA level did not show difference in overall survival. This was a small retrospective, single center study; role of WT-1 mRNA level as a biomarker for response to azacitidine should be further investigated.
Results of a multi-center trial comparing reduced intensity allogeneic stem cell transplant (SCT) to hypomethylating agents or best supportive care in higher risk MDS patients aged 50-75 was reported in the annual meeting of the American Society of Hematology in 2020. Patients with intermediate-2 or higher risk by IPSS were assigned based on SCT or no SCT arm based on donor status, and patients who had donor proceeded to reduced intensity SCT within 6 months of enrolment. In an intent-to-treat analysis, the study showed different in 3-year overall survival, which was the primary endpoint between SCT vs non-SCT arms (47.9% vs 26.6%, p=0.0001). Leukemia-free survival at 3 years was also greater for the SCT arm (35.8% vs 20.6%, p=0.003). Allogenic stem cell transplant is currently offered to patients with higher risk MDS given this is the only potential curable approach for MDS patients. This study further supports allogeneic stem cell transplant for older patients with higher risk MDS.
Many health plans now must cover full cost of expensive HIV prevention drugs
Ted Howard started taking Truvada a few years ago because he wanted to protect himself against HIV, the virus that causes AIDS. But the daily pill was so pricey he was seriously thinking about giving it up.
Under his insurance plan, the former flight attendant and customer service instructor owed $500 in copayments every month for the drug and an additional $250 every three months for lab work and clinic visits.
Luckily for Howard, his doctor at Las Vegas’ Huntridge Family Clinic, which specializes in LGBTQ care, enrolled him in a clinical trial that covered his medication and other costs in full.
“If I hadn’t been able to get into the trial, I wouldn’t have kept taking PrEP,” said Howard, 68, using the shorthand term for “preexposure prophylaxis.” Taken daily, these drugs — like Truvada — are more than 90% effective at preventing infection with HIV.
(some plans already began doing so last year).
Drugs in this category — Truvada, Descovy and, newly available, a generic version of Truvada — received an “A” recommendation by the U.S. Preventive Services Task Force. Under the Affordable Care Act, preventive services that receive an “A” or “B” rating by the task force, a group of medical experts in prevention and primary care, must be covered by most private health plans without making members share the cost, usually through copayments or deductibles. Only plans that are grandfathered under the health law are exempt.
The task force recommended PrEP for people at high risk of HIV infection, including men who have sex with men and injection drug users.
In the United States, more than 1 million people live with HIV, and nearly 40,000 new HIV cases are diagnosed every year. Yet fewer than 10% of people who could benefit from PrEP are taking it. One key reason is that out-of-pocket costs can exceed $1,000 annually, according to a study published in the American Journal of Public Health last year. Required periodic blood tests and doctor visits can add hundreds of dollars to the cost of the drug, and it’s not clear if insurers are required to pick up all those costs.
“Cost sharing has been a problem,” said Michael Crews, policy director at One Colorado, an advocacy group for the LGBTQ community. “It’s not just getting on PrEP and taking a pill. It’s the lab and clinical services. That’s a huge barrier for folks.”
Whether you’re shopping for a new plan during open enrollment or want to check out what your current plan covers, here are answers to questions you may have about the new preventive coverage requirement.
Q: How can people find out whether their health plan covers PrEP medications without charge?
The plan’s list of covered drugs, called a formulary, should spell out which drugs are covered, along with details about which drug tier they fall into. Drugs placed in higher tiers generally have higher cost sharing. That list should be online with the plan documents that give coverage details.
Sorting out coverage and cost sharing can be tricky. Both Truvada and Descovy can also be used to treat HIV, and if they are taken for that purpose, a plan may require members to pay some of the cost. But if the drugs are taken to prevent HIV infection, patients shouldn’t owe anything out-of-pocket, no matter which tier they are on.
In a recent analysis of online formularies for plans sold on the ACA marketplaces, Carl Schmid, executive director of the HIV + Hepatitis Policy Institute, found that many plans seemed out of compliance with the requirement to cover PrEP without cost sharing this year.
But representatives for Oscar and Kaiser Permanente, two insurers that were called out in the analysis for lack of compliance, said the drugs are covered without cost sharing in plans nationwide if they are taken to prevent HIV. Schmid later revised his analysis to reflect Oscar’s coverage.
Coverage and cost-sharing information needs to be transparent and easy to find, Schmid said.
“I acted like a shopper of insurance, just like any person would do,” he said. “Even when the information is correct, [it’s so] difficult to find [and there’s] no uniformity.”
It may be necessary to call the insurer directly to confirm coverage details if information on the website is unclear.
Q: Are all three drugs covered without cost sharing?
Health plans have to cover at least one of the drugs in this category — Descovy and the brand and generic versions of Truvada — without cost sharing. People may have to jump through some hoops to get approval for a specific drug, however. For example, Oscar plans sold in 18 states cover the three PrEP options without cost sharing. The generic version of Truvada doesn’t require prior authorization by the insurer. But if someone wants to take the name-brand drug, that person has to go through an approval process. Descovy, a newer drug, is available without cost sharing only if people are unable to use Truvada or its generic version because of clinical intolerance or other issues.
Q: What about the lab work and clinical visits that are necessary while taking PrEP? Are those services also covered without cost sharing?
That is the thousand-dollar question. People who are taking drugs to prevent HIV infection need to meet with a clinician and have blood work every three months to test for HIV, hepatitis B and sexually transmitted infections, and to check their kidney function.
The task force recommendation doesn’t specify whether these services must also be covered without cost sharing, and advocates say federal guidance is necessary to ensure they are free.
“If you’ve got a high-deductible plan and you’ve got to meet it before those services are covered, that’s going to add up,” said Amy Killelea, senior director of health systems and policy at the National Alliance of State & Territorial AIDS Directors. “We’re trying to emphasize that it’s integral to the intervention itself.”
A handful of states have programs that help people cover their out-of-pocket costs for lab and clinical visits, generally based on income.
There is precedent for including free ancillary care as part of a recommended preventive service. After consumers and advocates complained, the Centers for Medicare & Medicaid Services (CMS) clarified that under the ACA removing a polyp during a screening colonoscopy is considered an integral part of the procedure and patients shouldn’t be charged for it.
CMS officials declined to clarify whether PrEP services such as lab work and clinical visits are to be covered without cost sharing as part of the preventive service and noted that states generally enforce such insurance requirements. “CMS intends to contact state regulators, as appropriate, to discuss issuer’s compliance with the federal requirements and whether issuers need further guidance on which services associated with PrEP must be covered without cost sharing,” the agency said in a statement.
Q: What if someone runs into roadblocks getting a plan to cover PrEP or related services without cost sharing?
If an insurer charges for the medication or a follow-up visit, people may have to go through an appeals process to fight it.
“They’d have to appeal to the insurance company and then to the state if they don’t succeed,” said Nadeen Israel, vice president of policy and advocacy at the AIDS Foundation of Chicago. “Most people don’t know to do that.”
Q: Are uninsured people also protected by this new cost-sharing change for PrEP?
Unfortunately, no. The ACA requirement to cover recommended preventive services without charging patients applies only to private insurance plans. People without insurance don’t benefit. Gilead, which makes both Truvada and Descovy, has a patient assistance program for the uninsured.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Ted Howard started taking Truvada a few years ago because he wanted to protect himself against HIV, the virus that causes AIDS. But the daily pill was so pricey he was seriously thinking about giving it up.
Under his insurance plan, the former flight attendant and customer service instructor owed $500 in copayments every month for the drug and an additional $250 every three months for lab work and clinic visits.
Luckily for Howard, his doctor at Las Vegas’ Huntridge Family Clinic, which specializes in LGBTQ care, enrolled him in a clinical trial that covered his medication and other costs in full.
“If I hadn’t been able to get into the trial, I wouldn’t have kept taking PrEP,” said Howard, 68, using the shorthand term for “preexposure prophylaxis.” Taken daily, these drugs — like Truvada — are more than 90% effective at preventing infection with HIV.
(some plans already began doing so last year).
Drugs in this category — Truvada, Descovy and, newly available, a generic version of Truvada — received an “A” recommendation by the U.S. Preventive Services Task Force. Under the Affordable Care Act, preventive services that receive an “A” or “B” rating by the task force, a group of medical experts in prevention and primary care, must be covered by most private health plans without making members share the cost, usually through copayments or deductibles. Only plans that are grandfathered under the health law are exempt.
The task force recommended PrEP for people at high risk of HIV infection, including men who have sex with men and injection drug users.
In the United States, more than 1 million people live with HIV, and nearly 40,000 new HIV cases are diagnosed every year. Yet fewer than 10% of people who could benefit from PrEP are taking it. One key reason is that out-of-pocket costs can exceed $1,000 annually, according to a study published in the American Journal of Public Health last year. Required periodic blood tests and doctor visits can add hundreds of dollars to the cost of the drug, and it’s not clear if insurers are required to pick up all those costs.
“Cost sharing has been a problem,” said Michael Crews, policy director at One Colorado, an advocacy group for the LGBTQ community. “It’s not just getting on PrEP and taking a pill. It’s the lab and clinical services. That’s a huge barrier for folks.”
Whether you’re shopping for a new plan during open enrollment or want to check out what your current plan covers, here are answers to questions you may have about the new preventive coverage requirement.
Q: How can people find out whether their health plan covers PrEP medications without charge?
The plan’s list of covered drugs, called a formulary, should spell out which drugs are covered, along with details about which drug tier they fall into. Drugs placed in higher tiers generally have higher cost sharing. That list should be online with the plan documents that give coverage details.
Sorting out coverage and cost sharing can be tricky. Both Truvada and Descovy can also be used to treat HIV, and if they are taken for that purpose, a plan may require members to pay some of the cost. But if the drugs are taken to prevent HIV infection, patients shouldn’t owe anything out-of-pocket, no matter which tier they are on.
In a recent analysis of online formularies for plans sold on the ACA marketplaces, Carl Schmid, executive director of the HIV + Hepatitis Policy Institute, found that many plans seemed out of compliance with the requirement to cover PrEP without cost sharing this year.
But representatives for Oscar and Kaiser Permanente, two insurers that were called out in the analysis for lack of compliance, said the drugs are covered without cost sharing in plans nationwide if they are taken to prevent HIV. Schmid later revised his analysis to reflect Oscar’s coverage.
Coverage and cost-sharing information needs to be transparent and easy to find, Schmid said.
“I acted like a shopper of insurance, just like any person would do,” he said. “Even when the information is correct, [it’s so] difficult to find [and there’s] no uniformity.”
It may be necessary to call the insurer directly to confirm coverage details if information on the website is unclear.
Q: Are all three drugs covered without cost sharing?
Health plans have to cover at least one of the drugs in this category — Descovy and the brand and generic versions of Truvada — without cost sharing. People may have to jump through some hoops to get approval for a specific drug, however. For example, Oscar plans sold in 18 states cover the three PrEP options without cost sharing. The generic version of Truvada doesn’t require prior authorization by the insurer. But if someone wants to take the name-brand drug, that person has to go through an approval process. Descovy, a newer drug, is available without cost sharing only if people are unable to use Truvada or its generic version because of clinical intolerance or other issues.
Q: What about the lab work and clinical visits that are necessary while taking PrEP? Are those services also covered without cost sharing?
That is the thousand-dollar question. People who are taking drugs to prevent HIV infection need to meet with a clinician and have blood work every three months to test for HIV, hepatitis B and sexually transmitted infections, and to check their kidney function.
The task force recommendation doesn’t specify whether these services must also be covered without cost sharing, and advocates say federal guidance is necessary to ensure they are free.
“If you’ve got a high-deductible plan and you’ve got to meet it before those services are covered, that’s going to add up,” said Amy Killelea, senior director of health systems and policy at the National Alliance of State & Territorial AIDS Directors. “We’re trying to emphasize that it’s integral to the intervention itself.”
A handful of states have programs that help people cover their out-of-pocket costs for lab and clinical visits, generally based on income.
There is precedent for including free ancillary care as part of a recommended preventive service. After consumers and advocates complained, the Centers for Medicare & Medicaid Services (CMS) clarified that under the ACA removing a polyp during a screening colonoscopy is considered an integral part of the procedure and patients shouldn’t be charged for it.
CMS officials declined to clarify whether PrEP services such as lab work and clinical visits are to be covered without cost sharing as part of the preventive service and noted that states generally enforce such insurance requirements. “CMS intends to contact state regulators, as appropriate, to discuss issuer’s compliance with the federal requirements and whether issuers need further guidance on which services associated with PrEP must be covered without cost sharing,” the agency said in a statement.
Q: What if someone runs into roadblocks getting a plan to cover PrEP or related services without cost sharing?
If an insurer charges for the medication or a follow-up visit, people may have to go through an appeals process to fight it.
“They’d have to appeal to the insurance company and then to the state if they don’t succeed,” said Nadeen Israel, vice president of policy and advocacy at the AIDS Foundation of Chicago. “Most people don’t know to do that.”
Q: Are uninsured people also protected by this new cost-sharing change for PrEP?
Unfortunately, no. The ACA requirement to cover recommended preventive services without charging patients applies only to private insurance plans. People without insurance don’t benefit. Gilead, which makes both Truvada and Descovy, has a patient assistance program for the uninsured.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Ted Howard started taking Truvada a few years ago because he wanted to protect himself against HIV, the virus that causes AIDS. But the daily pill was so pricey he was seriously thinking about giving it up.
Under his insurance plan, the former flight attendant and customer service instructor owed $500 in copayments every month for the drug and an additional $250 every three months for lab work and clinic visits.
Luckily for Howard, his doctor at Las Vegas’ Huntridge Family Clinic, which specializes in LGBTQ care, enrolled him in a clinical trial that covered his medication and other costs in full.
“If I hadn’t been able to get into the trial, I wouldn’t have kept taking PrEP,” said Howard, 68, using the shorthand term for “preexposure prophylaxis.” Taken daily, these drugs — like Truvada — are more than 90% effective at preventing infection with HIV.
(some plans already began doing so last year).
Drugs in this category — Truvada, Descovy and, newly available, a generic version of Truvada — received an “A” recommendation by the U.S. Preventive Services Task Force. Under the Affordable Care Act, preventive services that receive an “A” or “B” rating by the task force, a group of medical experts in prevention and primary care, must be covered by most private health plans without making members share the cost, usually through copayments or deductibles. Only plans that are grandfathered under the health law are exempt.
The task force recommended PrEP for people at high risk of HIV infection, including men who have sex with men and injection drug users.
In the United States, more than 1 million people live with HIV, and nearly 40,000 new HIV cases are diagnosed every year. Yet fewer than 10% of people who could benefit from PrEP are taking it. One key reason is that out-of-pocket costs can exceed $1,000 annually, according to a study published in the American Journal of Public Health last year. Required periodic blood tests and doctor visits can add hundreds of dollars to the cost of the drug, and it’s not clear if insurers are required to pick up all those costs.
“Cost sharing has been a problem,” said Michael Crews, policy director at One Colorado, an advocacy group for the LGBTQ community. “It’s not just getting on PrEP and taking a pill. It’s the lab and clinical services. That’s a huge barrier for folks.”
Whether you’re shopping for a new plan during open enrollment or want to check out what your current plan covers, here are answers to questions you may have about the new preventive coverage requirement.
Q: How can people find out whether their health plan covers PrEP medications without charge?
The plan’s list of covered drugs, called a formulary, should spell out which drugs are covered, along with details about which drug tier they fall into. Drugs placed in higher tiers generally have higher cost sharing. That list should be online with the plan documents that give coverage details.
Sorting out coverage and cost sharing can be tricky. Both Truvada and Descovy can also be used to treat HIV, and if they are taken for that purpose, a plan may require members to pay some of the cost. But if the drugs are taken to prevent HIV infection, patients shouldn’t owe anything out-of-pocket, no matter which tier they are on.
In a recent analysis of online formularies for plans sold on the ACA marketplaces, Carl Schmid, executive director of the HIV + Hepatitis Policy Institute, found that many plans seemed out of compliance with the requirement to cover PrEP without cost sharing this year.
But representatives for Oscar and Kaiser Permanente, two insurers that were called out in the analysis for lack of compliance, said the drugs are covered without cost sharing in plans nationwide if they are taken to prevent HIV. Schmid later revised his analysis to reflect Oscar’s coverage.
Coverage and cost-sharing information needs to be transparent and easy to find, Schmid said.
“I acted like a shopper of insurance, just like any person would do,” he said. “Even when the information is correct, [it’s so] difficult to find [and there’s] no uniformity.”
It may be necessary to call the insurer directly to confirm coverage details if information on the website is unclear.
Q: Are all three drugs covered without cost sharing?
Health plans have to cover at least one of the drugs in this category — Descovy and the brand and generic versions of Truvada — without cost sharing. People may have to jump through some hoops to get approval for a specific drug, however. For example, Oscar plans sold in 18 states cover the three PrEP options without cost sharing. The generic version of Truvada doesn’t require prior authorization by the insurer. But if someone wants to take the name-brand drug, that person has to go through an approval process. Descovy, a newer drug, is available without cost sharing only if people are unable to use Truvada or its generic version because of clinical intolerance or other issues.
Q: What about the lab work and clinical visits that are necessary while taking PrEP? Are those services also covered without cost sharing?
That is the thousand-dollar question. People who are taking drugs to prevent HIV infection need to meet with a clinician and have blood work every three months to test for HIV, hepatitis B and sexually transmitted infections, and to check their kidney function.
The task force recommendation doesn’t specify whether these services must also be covered without cost sharing, and advocates say federal guidance is necessary to ensure they are free.
“If you’ve got a high-deductible plan and you’ve got to meet it before those services are covered, that’s going to add up,” said Amy Killelea, senior director of health systems and policy at the National Alliance of State & Territorial AIDS Directors. “We’re trying to emphasize that it’s integral to the intervention itself.”
A handful of states have programs that help people cover their out-of-pocket costs for lab and clinical visits, generally based on income.
There is precedent for including free ancillary care as part of a recommended preventive service. After consumers and advocates complained, the Centers for Medicare & Medicaid Services (CMS) clarified that under the ACA removing a polyp during a screening colonoscopy is considered an integral part of the procedure and patients shouldn’t be charged for it.
CMS officials declined to clarify whether PrEP services such as lab work and clinical visits are to be covered without cost sharing as part of the preventive service and noted that states generally enforce such insurance requirements. “CMS intends to contact state regulators, as appropriate, to discuss issuer’s compliance with the federal requirements and whether issuers need further guidance on which services associated with PrEP must be covered without cost sharing,” the agency said in a statement.
Q: What if someone runs into roadblocks getting a plan to cover PrEP or related services without cost sharing?
If an insurer charges for the medication or a follow-up visit, people may have to go through an appeals process to fight it.
“They’d have to appeal to the insurance company and then to the state if they don’t succeed,” said Nadeen Israel, vice president of policy and advocacy at the AIDS Foundation of Chicago. “Most people don’t know to do that.”
Q: Are uninsured people also protected by this new cost-sharing change for PrEP?
Unfortunately, no. The ACA requirement to cover recommended preventive services without charging patients applies only to private insurance plans. People without insurance don’t benefit. Gilead, which makes both Truvada and Descovy, has a patient assistance program for the uninsured.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Which imaging criteria identify progressive forms of MS?
The role of imaging in diagnosing progressive multiple sclerosis (MS) and in assessing prognosis is the subject of a new review.
MRI is central in the diagnostic work-up of patients suspected of having MS, given its high sensitivity in detecting disease dissemination in space and over time and its notable ability to exclude mimics of MS, the authors noted. However, diagnosis of primary progressive MS remains challenging and is only possible retrospectively on the basis of clinical assessment.
they wrote.
Diagnosis of progressive MS is limited by difficulties in distinguishing accumulating disability caused by inflammatory disease activity from that attributable to degenerative processes associated with secondary progressive MS. Moreover, there are no accepted clinical criteria for diagnosing secondary progressive MS, the authors explained.
This need has promoted extensive research in the field of imaging, facilitated by definition of novel MRI sequences, to identify imaging features reflecting pathophysiological mechanisms relevant to the pathobiology of progressive MS, the authors said.
The current review reports the conclusions of a workshop held in Milan in November 2019, at which an expert panel of neurologists and neuroradiologists addressed the role of MRI in progressive MS.
Massimo Filippi, MD, IRCCS San Raffaele Scientific Institute, Milan, was the lead author of the review, which was published online Dec. 14, 2020, in JAMA Neurology.
The authors concluded that no definitive, qualitative clinical, immunologic, histopathologic, or neuroimaging features differentiate primary progressive and secondary progressive forms of MS; both are characterized by neurodegenerative phenomena and a gradual and irreversible accumulation of clinical disability, which is also affected by aging and comorbidities.
A definitive diagnosis of primary progressive MS is more difficult than a diagnosis of relapsing remitting MS; in part, primary progressive MS is a diagnosis of exclusion because it can be mimicked by other conditions clinically and radiologically, the authors noted.
The writers did report that, although nonspecific, some spinal cord imaging features are typical of primary progressive MS. These include diffuse abnormalities and lesions involving gray matter and two or more white-matter columns, but confirmation of this is required.
In patients with primary progressive MS and those with relapse-onset MS, MRI features at disease onset predict long-term disability and a progressive disease course. These features include lesions in critical central nervous system regions (i.e., spinal cord, infratentorial regions, and gray matter) and high inflammatory activity in the first years after disease onset. These measures are evaluable in clinical practice, the authors said.
In patients with established MS, gray-matter involvement and neurodegeneration are associated with accelerated clinical worsening; however, detection validation and standardization need to be implemented at the individual patient level, they commented.
Novel candidate imaging biomarkers, such as subpial demyelination, and the presence of slowly expanding lesions or paramagnetic rim lesions may identify progressive MS but should be further investigated, they added.
Discovery of MRI markers capable of detecting evolution from relapsing-remitting to secondary progressive MS remains an unmet need that will probably require multiparametric MRI studies, because it is unlikely that a single MRI method will be able to allow clinicians to optimally distinguish among these stages, the authors said.
The contribution of these promising MRI measures combined with other biomarkers, such as quantification of serum neurofilament light chain levels or optical coherence tomography assessment, should be explored to improve the identification of patients with progressive MS, they concluded.
‘A comprehensive review’
In a comment, Jeffrey A. Cohen, MD, director of the Cleveland Clinic’s Mellen Center for MS Treatment and Research, said the article is a comprehensive review of the pathologic mechanisms that underlie progression in MS and the proxy measures of those processes (brain and spinal cord MRI, PET, optical coherence tomography, and biomarkers).
“The paper reports there is no qualitative difference between relapsing remitting and progressive MS; rather, the difference is quantitative,” Dr. Cohen noted. “In other words, the processes that underlie progression are present from the earliest stages of MS, becoming more prominent over time.”
The apparent transition to progressive MS, he added, “rather than representing a ‘transition,’ instead results from the accumulation of pathology over time, a shift from focal lesions to diffuse inflammation and damage, and unmasking of the damage due to decreased resiliency due to aging and failure of compensatory mechanisms (neuroplasticity and remyelination).”
Also commenting, Edward Fox, MD, director, MS Clinic of Central Texas and clinical associate professor, University of Texas, Austin, explained that loss of tissue is the main driver of progressive MS.
“We all look at imaging to confirm that the progressive symptoms expressed by the patient are related to demyelinating disease,” he said. “When I see MRI of the spinal cord showing multifocal lesions, especially if localized atrophy is seen in a region of the cord, I expect to hear a history of progressive deficits in gait and other signs of disability.”
Dr. Fox noted that, on MRI of the brain, gray matter atrophy both cortically and in the deep gray structures usually manifests as cognitive slowing and poorer performance in work and social situations.
“We hope that other biomarkers, such as neurofilament light chain, will add to this body of knowledge and give us a better grasp of the definition of neurodegeneration to confirm the clinical and radiographic findings,” he added.
Dr. Filippi has received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck Serono, Novartis, Roche, Sanofi, Genzyme, Takeda, and Teva Pharmaceutical Industries; and research support from ARiSLA, Biogen Idec, Fondazione Italiana Sclerosi Multipla, Italian Ministry of Health, Merck Serono, Novartis, Roche, and Teva.
A version of this article first appeared on Medscape.com.
The role of imaging in diagnosing progressive multiple sclerosis (MS) and in assessing prognosis is the subject of a new review.
MRI is central in the diagnostic work-up of patients suspected of having MS, given its high sensitivity in detecting disease dissemination in space and over time and its notable ability to exclude mimics of MS, the authors noted. However, diagnosis of primary progressive MS remains challenging and is only possible retrospectively on the basis of clinical assessment.
they wrote.
Diagnosis of progressive MS is limited by difficulties in distinguishing accumulating disability caused by inflammatory disease activity from that attributable to degenerative processes associated with secondary progressive MS. Moreover, there are no accepted clinical criteria for diagnosing secondary progressive MS, the authors explained.
This need has promoted extensive research in the field of imaging, facilitated by definition of novel MRI sequences, to identify imaging features reflecting pathophysiological mechanisms relevant to the pathobiology of progressive MS, the authors said.
The current review reports the conclusions of a workshop held in Milan in November 2019, at which an expert panel of neurologists and neuroradiologists addressed the role of MRI in progressive MS.
Massimo Filippi, MD, IRCCS San Raffaele Scientific Institute, Milan, was the lead author of the review, which was published online Dec. 14, 2020, in JAMA Neurology.
The authors concluded that no definitive, qualitative clinical, immunologic, histopathologic, or neuroimaging features differentiate primary progressive and secondary progressive forms of MS; both are characterized by neurodegenerative phenomena and a gradual and irreversible accumulation of clinical disability, which is also affected by aging and comorbidities.
A definitive diagnosis of primary progressive MS is more difficult than a diagnosis of relapsing remitting MS; in part, primary progressive MS is a diagnosis of exclusion because it can be mimicked by other conditions clinically and radiologically, the authors noted.
The writers did report that, although nonspecific, some spinal cord imaging features are typical of primary progressive MS. These include diffuse abnormalities and lesions involving gray matter and two or more white-matter columns, but confirmation of this is required.
In patients with primary progressive MS and those with relapse-onset MS, MRI features at disease onset predict long-term disability and a progressive disease course. These features include lesions in critical central nervous system regions (i.e., spinal cord, infratentorial regions, and gray matter) and high inflammatory activity in the first years after disease onset. These measures are evaluable in clinical practice, the authors said.
In patients with established MS, gray-matter involvement and neurodegeneration are associated with accelerated clinical worsening; however, detection validation and standardization need to be implemented at the individual patient level, they commented.
Novel candidate imaging biomarkers, such as subpial demyelination, and the presence of slowly expanding lesions or paramagnetic rim lesions may identify progressive MS but should be further investigated, they added.
Discovery of MRI markers capable of detecting evolution from relapsing-remitting to secondary progressive MS remains an unmet need that will probably require multiparametric MRI studies, because it is unlikely that a single MRI method will be able to allow clinicians to optimally distinguish among these stages, the authors said.
The contribution of these promising MRI measures combined with other biomarkers, such as quantification of serum neurofilament light chain levels or optical coherence tomography assessment, should be explored to improve the identification of patients with progressive MS, they concluded.
‘A comprehensive review’
In a comment, Jeffrey A. Cohen, MD, director of the Cleveland Clinic’s Mellen Center for MS Treatment and Research, said the article is a comprehensive review of the pathologic mechanisms that underlie progression in MS and the proxy measures of those processes (brain and spinal cord MRI, PET, optical coherence tomography, and biomarkers).
“The paper reports there is no qualitative difference between relapsing remitting and progressive MS; rather, the difference is quantitative,” Dr. Cohen noted. “In other words, the processes that underlie progression are present from the earliest stages of MS, becoming more prominent over time.”
The apparent transition to progressive MS, he added, “rather than representing a ‘transition,’ instead results from the accumulation of pathology over time, a shift from focal lesions to diffuse inflammation and damage, and unmasking of the damage due to decreased resiliency due to aging and failure of compensatory mechanisms (neuroplasticity and remyelination).”
Also commenting, Edward Fox, MD, director, MS Clinic of Central Texas and clinical associate professor, University of Texas, Austin, explained that loss of tissue is the main driver of progressive MS.
“We all look at imaging to confirm that the progressive symptoms expressed by the patient are related to demyelinating disease,” he said. “When I see MRI of the spinal cord showing multifocal lesions, especially if localized atrophy is seen in a region of the cord, I expect to hear a history of progressive deficits in gait and other signs of disability.”
Dr. Fox noted that, on MRI of the brain, gray matter atrophy both cortically and in the deep gray structures usually manifests as cognitive slowing and poorer performance in work and social situations.
“We hope that other biomarkers, such as neurofilament light chain, will add to this body of knowledge and give us a better grasp of the definition of neurodegeneration to confirm the clinical and radiographic findings,” he added.
Dr. Filippi has received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck Serono, Novartis, Roche, Sanofi, Genzyme, Takeda, and Teva Pharmaceutical Industries; and research support from ARiSLA, Biogen Idec, Fondazione Italiana Sclerosi Multipla, Italian Ministry of Health, Merck Serono, Novartis, Roche, and Teva.
A version of this article first appeared on Medscape.com.
The role of imaging in diagnosing progressive multiple sclerosis (MS) and in assessing prognosis is the subject of a new review.
MRI is central in the diagnostic work-up of patients suspected of having MS, given its high sensitivity in detecting disease dissemination in space and over time and its notable ability to exclude mimics of MS, the authors noted. However, diagnosis of primary progressive MS remains challenging and is only possible retrospectively on the basis of clinical assessment.
they wrote.
Diagnosis of progressive MS is limited by difficulties in distinguishing accumulating disability caused by inflammatory disease activity from that attributable to degenerative processes associated with secondary progressive MS. Moreover, there are no accepted clinical criteria for diagnosing secondary progressive MS, the authors explained.
This need has promoted extensive research in the field of imaging, facilitated by definition of novel MRI sequences, to identify imaging features reflecting pathophysiological mechanisms relevant to the pathobiology of progressive MS, the authors said.
The current review reports the conclusions of a workshop held in Milan in November 2019, at which an expert panel of neurologists and neuroradiologists addressed the role of MRI in progressive MS.
Massimo Filippi, MD, IRCCS San Raffaele Scientific Institute, Milan, was the lead author of the review, which was published online Dec. 14, 2020, in JAMA Neurology.
The authors concluded that no definitive, qualitative clinical, immunologic, histopathologic, or neuroimaging features differentiate primary progressive and secondary progressive forms of MS; both are characterized by neurodegenerative phenomena and a gradual and irreversible accumulation of clinical disability, which is also affected by aging and comorbidities.
A definitive diagnosis of primary progressive MS is more difficult than a diagnosis of relapsing remitting MS; in part, primary progressive MS is a diagnosis of exclusion because it can be mimicked by other conditions clinically and radiologically, the authors noted.
The writers did report that, although nonspecific, some spinal cord imaging features are typical of primary progressive MS. These include diffuse abnormalities and lesions involving gray matter and two or more white-matter columns, but confirmation of this is required.
In patients with primary progressive MS and those with relapse-onset MS, MRI features at disease onset predict long-term disability and a progressive disease course. These features include lesions in critical central nervous system regions (i.e., spinal cord, infratentorial regions, and gray matter) and high inflammatory activity in the first years after disease onset. These measures are evaluable in clinical practice, the authors said.
In patients with established MS, gray-matter involvement and neurodegeneration are associated with accelerated clinical worsening; however, detection validation and standardization need to be implemented at the individual patient level, they commented.
Novel candidate imaging biomarkers, such as subpial demyelination, and the presence of slowly expanding lesions or paramagnetic rim lesions may identify progressive MS but should be further investigated, they added.
Discovery of MRI markers capable of detecting evolution from relapsing-remitting to secondary progressive MS remains an unmet need that will probably require multiparametric MRI studies, because it is unlikely that a single MRI method will be able to allow clinicians to optimally distinguish among these stages, the authors said.
The contribution of these promising MRI measures combined with other biomarkers, such as quantification of serum neurofilament light chain levels or optical coherence tomography assessment, should be explored to improve the identification of patients with progressive MS, they concluded.
‘A comprehensive review’
In a comment, Jeffrey A. Cohen, MD, director of the Cleveland Clinic’s Mellen Center for MS Treatment and Research, said the article is a comprehensive review of the pathologic mechanisms that underlie progression in MS and the proxy measures of those processes (brain and spinal cord MRI, PET, optical coherence tomography, and biomarkers).
“The paper reports there is no qualitative difference between relapsing remitting and progressive MS; rather, the difference is quantitative,” Dr. Cohen noted. “In other words, the processes that underlie progression are present from the earliest stages of MS, becoming more prominent over time.”
The apparent transition to progressive MS, he added, “rather than representing a ‘transition,’ instead results from the accumulation of pathology over time, a shift from focal lesions to diffuse inflammation and damage, and unmasking of the damage due to decreased resiliency due to aging and failure of compensatory mechanisms (neuroplasticity and remyelination).”
Also commenting, Edward Fox, MD, director, MS Clinic of Central Texas and clinical associate professor, University of Texas, Austin, explained that loss of tissue is the main driver of progressive MS.
“We all look at imaging to confirm that the progressive symptoms expressed by the patient are related to demyelinating disease,” he said. “When I see MRI of the spinal cord showing multifocal lesions, especially if localized atrophy is seen in a region of the cord, I expect to hear a history of progressive deficits in gait and other signs of disability.”
Dr. Fox noted that, on MRI of the brain, gray matter atrophy both cortically and in the deep gray structures usually manifests as cognitive slowing and poorer performance in work and social situations.
“We hope that other biomarkers, such as neurofilament light chain, will add to this body of knowledge and give us a better grasp of the definition of neurodegeneration to confirm the clinical and radiographic findings,” he added.
Dr. Filippi has received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck Serono, Novartis, Roche, Sanofi, Genzyme, Takeda, and Teva Pharmaceutical Industries; and research support from ARiSLA, Biogen Idec, Fondazione Italiana Sclerosi Multipla, Italian Ministry of Health, Merck Serono, Novartis, Roche, and Teva.
A version of this article first appeared on Medscape.com.
Microplastics permeate human placentas
Researchers in Italy have identified microplastic (MP) fragments in four human placentas that were donated for study after delivery.
“The presence of MPs in the placenta tissue requires the reconsideration of the immunological mechanism of self-tolerance,” wrote Antonio Ragusa, MD, of San Giovanni Calibita Fatebenefratelli Hospital, Rome, and colleagues. “Placenta represents the interface between the fetus and the environment.”
In a pilot observational study published in Environment International, the researchers used Raman microspectroscopy to analyze placentas from six women with physiological pregnancies for the presence of MPs. MPs were defined as particles smaller than 5 mm resulting from the degradation of plastic in the environment, such as plastic objects, coatings, adhesives, paints, and personal care products. Data from previous studies have shown that MPs can move into living organisms, but this study is the first to identify MPs in human placentas, the researchers said.
Polypropylene and pigments identified
A total of 12 microplastic fragments were identified in tissue from the placentas of four women; 5 in the fetal side, 4 in the maternal side, and 3 in the chorioamniotic membranes, which suggests that MPs can reach all levels of placental tissue, the researchers said. Most of the MPs were approximately 10 mcm in size, but two were roughly 5 mcm.
All 12 of the MPs were pigmented; of these, 3 were identified as stained polypropylene and the other 9 contained pigments used in a variety of items including coatings, paints, adhesives, plasters, finger paints, polymers and cosmetics, and personal care products. The researchers used a software program to analyze the pigments and matched them with information from the European Chemical Agency for identification of the commercial name, chemical formula, International Union of Pure and Applied Chemistry name, and Color Index Constitution Number.
The mechanism by which MPs may enter the bloodstream and access the placenta remains unclear, the researchers said. “The most probable transport route for MPs is a mechanism of particle uptake and translocation, already described for the internalization from the gastrointestinal tract. Once MPs have reached the maternal surface of the placenta, as other exogenous materials, they can invade the tissue in depth by several transport mechanisms, both active and passive, that are not clearly understood yet.”
The range in location and characteristics of the particles found in the study suggest that passage of MPs into the placenta may be affected by physiological conditions and genetics, as well as food and lifestyle habits of the patients, the researchers said.
The study findings were limited by several factors including the small sample size and observational study design.
However, the presence of MPs in the placenta could affect the pregnancy in various ways, including immunity, growth factor signaling, maternal-fetal communication, and trafficking of various cell types and macrophages, the researchers wrote. In addition, MPs could have a transgenerational effect on metabolism and reproduction.
“Further studies need to be performed to assess if the presence of MPs in human placenta may trigger immune responses or may lead to the release of toxic contaminants, resulting harmful for pregnancy,” they concluded.
Cause for concern, but research gaps remain
“Microplastics are ubiquitous in the environment and are detectable in tissues of humans and wildlife,” Andrea C. Gore, PhD, of the University of Texas, Austin, said in an interview. “To my knowledge, this was never previously shown in the placenta.
“There are two reasons why detection of microplastics in placenta would be concerning,” Dr. Gore explained. “First, microplastics may be endocrine-disrupting chemicals (EDCs), or they may concentrate other chemicals that are EDCs. Second, the developing fetus is exquisitely sensitive to natural hormones, and disruptions by EDCs may lead to both immediate as well as latent health problems.
“Clinicians should be concerned about particulate matter in the placenta, “although the number of particles was very small,” said Dr. Gore. “Out of six women, four had particles in their placentas (total of 12) of which one was confirmed to be a plastic (polypropylene). For the other 11 particles, only the pigments could be identified, so more work is needed to confirm whether they were plastics.
“If I were a clinician discussing this article with my patients, I would point out that, although it is concerning that microparticles are present in placenta, few of them were found, and it is not known whether any chemical is released from the particles or actually reaches the fetal circulation,” Dr. Gore said. “I would use it as a starting point for a conversation about lifestyle during pregnancy and encouraging pregnant women to avoid eating foods stored and/or prepared in plastics.”
The limitations of the study include not only the small sample size, but also that “the type of chemicals in the microplastics is for the most part unknown, making it difficult to assess which (if any) might be EDCs,” Dr. Gore emphasized. In addition, “lifestyle and diet can greatly affect exposures to chemicals, so this needs to be carefully factored into the analysis.” Also, “most of the detected particles are pigments, so connections to plastics (other than the one polypropylene particle) need to be strengthened,” she explained.
“The pathways by which microplastics might get into tissues are still rather speculative, and the mechanisms proposed by the authors (endocytosis, paracellular diffusion, entry via airways) need to be demonstrated,” Dr. Gore concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Gore had no conflicts to disclose.
SOURCE: Ragusa A et al. Environ Int. 2020 Dec 2. doi: 10.1016/j.envint.2020.106274.
Researchers in Italy have identified microplastic (MP) fragments in four human placentas that were donated for study after delivery.
“The presence of MPs in the placenta tissue requires the reconsideration of the immunological mechanism of self-tolerance,” wrote Antonio Ragusa, MD, of San Giovanni Calibita Fatebenefratelli Hospital, Rome, and colleagues. “Placenta represents the interface between the fetus and the environment.”
In a pilot observational study published in Environment International, the researchers used Raman microspectroscopy to analyze placentas from six women with physiological pregnancies for the presence of MPs. MPs were defined as particles smaller than 5 mm resulting from the degradation of plastic in the environment, such as plastic objects, coatings, adhesives, paints, and personal care products. Data from previous studies have shown that MPs can move into living organisms, but this study is the first to identify MPs in human placentas, the researchers said.
Polypropylene and pigments identified
A total of 12 microplastic fragments were identified in tissue from the placentas of four women; 5 in the fetal side, 4 in the maternal side, and 3 in the chorioamniotic membranes, which suggests that MPs can reach all levels of placental tissue, the researchers said. Most of the MPs were approximately 10 mcm in size, but two were roughly 5 mcm.
All 12 of the MPs were pigmented; of these, 3 were identified as stained polypropylene and the other 9 contained pigments used in a variety of items including coatings, paints, adhesives, plasters, finger paints, polymers and cosmetics, and personal care products. The researchers used a software program to analyze the pigments and matched them with information from the European Chemical Agency for identification of the commercial name, chemical formula, International Union of Pure and Applied Chemistry name, and Color Index Constitution Number.
The mechanism by which MPs may enter the bloodstream and access the placenta remains unclear, the researchers said. “The most probable transport route for MPs is a mechanism of particle uptake and translocation, already described for the internalization from the gastrointestinal tract. Once MPs have reached the maternal surface of the placenta, as other exogenous materials, they can invade the tissue in depth by several transport mechanisms, both active and passive, that are not clearly understood yet.”
The range in location and characteristics of the particles found in the study suggest that passage of MPs into the placenta may be affected by physiological conditions and genetics, as well as food and lifestyle habits of the patients, the researchers said.
The study findings were limited by several factors including the small sample size and observational study design.
However, the presence of MPs in the placenta could affect the pregnancy in various ways, including immunity, growth factor signaling, maternal-fetal communication, and trafficking of various cell types and macrophages, the researchers wrote. In addition, MPs could have a transgenerational effect on metabolism and reproduction.
“Further studies need to be performed to assess if the presence of MPs in human placenta may trigger immune responses or may lead to the release of toxic contaminants, resulting harmful for pregnancy,” they concluded.
Cause for concern, but research gaps remain
“Microplastics are ubiquitous in the environment and are detectable in tissues of humans and wildlife,” Andrea C. Gore, PhD, of the University of Texas, Austin, said in an interview. “To my knowledge, this was never previously shown in the placenta.
“There are two reasons why detection of microplastics in placenta would be concerning,” Dr. Gore explained. “First, microplastics may be endocrine-disrupting chemicals (EDCs), or they may concentrate other chemicals that are EDCs. Second, the developing fetus is exquisitely sensitive to natural hormones, and disruptions by EDCs may lead to both immediate as well as latent health problems.
“Clinicians should be concerned about particulate matter in the placenta, “although the number of particles was very small,” said Dr. Gore. “Out of six women, four had particles in their placentas (total of 12) of which one was confirmed to be a plastic (polypropylene). For the other 11 particles, only the pigments could be identified, so more work is needed to confirm whether they were plastics.
“If I were a clinician discussing this article with my patients, I would point out that, although it is concerning that microparticles are present in placenta, few of them were found, and it is not known whether any chemical is released from the particles or actually reaches the fetal circulation,” Dr. Gore said. “I would use it as a starting point for a conversation about lifestyle during pregnancy and encouraging pregnant women to avoid eating foods stored and/or prepared in plastics.”
The limitations of the study include not only the small sample size, but also that “the type of chemicals in the microplastics is for the most part unknown, making it difficult to assess which (if any) might be EDCs,” Dr. Gore emphasized. In addition, “lifestyle and diet can greatly affect exposures to chemicals, so this needs to be carefully factored into the analysis.” Also, “most of the detected particles are pigments, so connections to plastics (other than the one polypropylene particle) need to be strengthened,” she explained.
“The pathways by which microplastics might get into tissues are still rather speculative, and the mechanisms proposed by the authors (endocytosis, paracellular diffusion, entry via airways) need to be demonstrated,” Dr. Gore concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Gore had no conflicts to disclose.
SOURCE: Ragusa A et al. Environ Int. 2020 Dec 2. doi: 10.1016/j.envint.2020.106274.
Researchers in Italy have identified microplastic (MP) fragments in four human placentas that were donated for study after delivery.
“The presence of MPs in the placenta tissue requires the reconsideration of the immunological mechanism of self-tolerance,” wrote Antonio Ragusa, MD, of San Giovanni Calibita Fatebenefratelli Hospital, Rome, and colleagues. “Placenta represents the interface between the fetus and the environment.”
In a pilot observational study published in Environment International, the researchers used Raman microspectroscopy to analyze placentas from six women with physiological pregnancies for the presence of MPs. MPs were defined as particles smaller than 5 mm resulting from the degradation of plastic in the environment, such as plastic objects, coatings, adhesives, paints, and personal care products. Data from previous studies have shown that MPs can move into living organisms, but this study is the first to identify MPs in human placentas, the researchers said.
Polypropylene and pigments identified
A total of 12 microplastic fragments were identified in tissue from the placentas of four women; 5 in the fetal side, 4 in the maternal side, and 3 in the chorioamniotic membranes, which suggests that MPs can reach all levels of placental tissue, the researchers said. Most of the MPs were approximately 10 mcm in size, but two were roughly 5 mcm.
All 12 of the MPs were pigmented; of these, 3 were identified as stained polypropylene and the other 9 contained pigments used in a variety of items including coatings, paints, adhesives, plasters, finger paints, polymers and cosmetics, and personal care products. The researchers used a software program to analyze the pigments and matched them with information from the European Chemical Agency for identification of the commercial name, chemical formula, International Union of Pure and Applied Chemistry name, and Color Index Constitution Number.
The mechanism by which MPs may enter the bloodstream and access the placenta remains unclear, the researchers said. “The most probable transport route for MPs is a mechanism of particle uptake and translocation, already described for the internalization from the gastrointestinal tract. Once MPs have reached the maternal surface of the placenta, as other exogenous materials, they can invade the tissue in depth by several transport mechanisms, both active and passive, that are not clearly understood yet.”
The range in location and characteristics of the particles found in the study suggest that passage of MPs into the placenta may be affected by physiological conditions and genetics, as well as food and lifestyle habits of the patients, the researchers said.
The study findings were limited by several factors including the small sample size and observational study design.
However, the presence of MPs in the placenta could affect the pregnancy in various ways, including immunity, growth factor signaling, maternal-fetal communication, and trafficking of various cell types and macrophages, the researchers wrote. In addition, MPs could have a transgenerational effect on metabolism and reproduction.
“Further studies need to be performed to assess if the presence of MPs in human placenta may trigger immune responses or may lead to the release of toxic contaminants, resulting harmful for pregnancy,” they concluded.
Cause for concern, but research gaps remain
“Microplastics are ubiquitous in the environment and are detectable in tissues of humans and wildlife,” Andrea C. Gore, PhD, of the University of Texas, Austin, said in an interview. “To my knowledge, this was never previously shown in the placenta.
“There are two reasons why detection of microplastics in placenta would be concerning,” Dr. Gore explained. “First, microplastics may be endocrine-disrupting chemicals (EDCs), or they may concentrate other chemicals that are EDCs. Second, the developing fetus is exquisitely sensitive to natural hormones, and disruptions by EDCs may lead to both immediate as well as latent health problems.
“Clinicians should be concerned about particulate matter in the placenta, “although the number of particles was very small,” said Dr. Gore. “Out of six women, four had particles in their placentas (total of 12) of which one was confirmed to be a plastic (polypropylene). For the other 11 particles, only the pigments could be identified, so more work is needed to confirm whether they were plastics.
“If I were a clinician discussing this article with my patients, I would point out that, although it is concerning that microparticles are present in placenta, few of them were found, and it is not known whether any chemical is released from the particles or actually reaches the fetal circulation,” Dr. Gore said. “I would use it as a starting point for a conversation about lifestyle during pregnancy and encouraging pregnant women to avoid eating foods stored and/or prepared in plastics.”
The limitations of the study include not only the small sample size, but also that “the type of chemicals in the microplastics is for the most part unknown, making it difficult to assess which (if any) might be EDCs,” Dr. Gore emphasized. In addition, “lifestyle and diet can greatly affect exposures to chemicals, so this needs to be carefully factored into the analysis.” Also, “most of the detected particles are pigments, so connections to plastics (other than the one polypropylene particle) need to be strengthened,” she explained.
“The pathways by which microplastics might get into tissues are still rather speculative, and the mechanisms proposed by the authors (endocytosis, paracellular diffusion, entry via airways) need to be demonstrated,” Dr. Gore concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Gore had no conflicts to disclose.
SOURCE: Ragusa A et al. Environ Int. 2020 Dec 2. doi: 10.1016/j.envint.2020.106274.
FROM ENVIRONMENT INTERNATIONAL
Pandemic packed a year of distress into 1 month
The first month of the coronavirus pandemic created almost as much psychological distress among American adults as they had experienced in the year before February 2019, according to the results of two representative surveys.
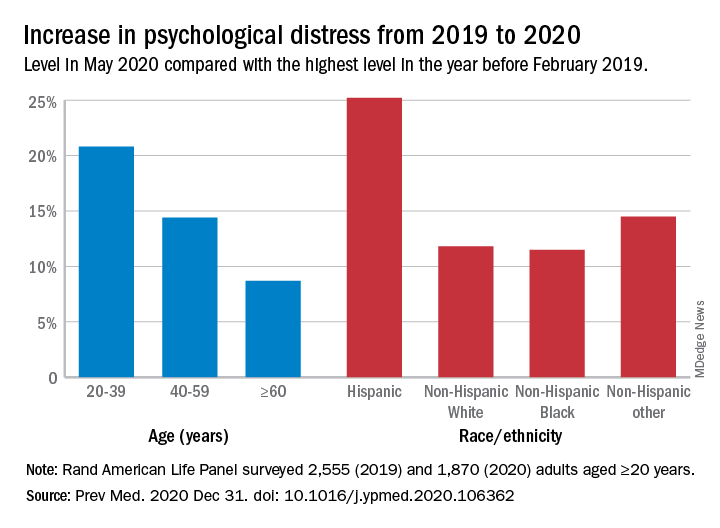
“The 30-day prevalence of SD [serious distress] in May 2020 did not differ from the past-year prevalence of SD assessed with the same instrument [the Kessler-6 distress scale] in February 2019. In other words, equal numbers of people experienced SD in 30-days during the pandemic as experienced SD over an entire year prior to the pandemic,” Joshua Breslau, PhD, and associates at the Rand Corporation wrote in Preventive Medicine.
In May of 2020, the prevalence of SD was 10.1% in the previous month among 1,870 adults aged 20 years and older who had participated in the two Rand American Life Panel surveys, the first occurring in February 2019. In that earlier poll, 10.9% of the 2,555 respondents said that they experienced SD in the worst month of the previous year, the investigators said.
The prevalence of overall psychological distress increased by 12.8% from February 2019 to May 2020, with increases higher among women (17.7%) than men (10.6%); adults under age 60 years, compared with those over 60 (see graph); and Hispanics, compared with other races/ethnicities. Disparities also were seen among income groups: Distress rose 10.2% for those earning over $100,000, compared with 15.4% for those making less than $35,000 and 18.2% for Americans earning between $35,000 and $60,000, the researchers reported.
A high level of stress in the prepandemic survey strongly predicted serious distress during the pandemic. “Risk for SD during the pandemic among those with SD during a year before the pandemic was almost 3 times higher than among those reporting mild/moderate distress and 15 times higher than among those reporting no/low distress during the prepandemic year,” they noted.
Distress levels often return to normal after a disaster, Dr. Breslau and associates pointed out, but “the pandemic’s influence on economic stressors, disruption of usual activities and subsequent effects on population health may continue for an extended period and affect different regions of the country at different points in time.”
SOURCE: Breslau J et al. Prev Med. 2020 Dec 31. doi: 10.1016/j.ypmed.2020.106362.
The first month of the coronavirus pandemic created almost as much psychological distress among American adults as they had experienced in the year before February 2019, according to the results of two representative surveys.

“The 30-day prevalence of SD [serious distress] in May 2020 did not differ from the past-year prevalence of SD assessed with the same instrument [the Kessler-6 distress scale] in February 2019. In other words, equal numbers of people experienced SD in 30-days during the pandemic as experienced SD over an entire year prior to the pandemic,” Joshua Breslau, PhD, and associates at the Rand Corporation wrote in Preventive Medicine.
In May of 2020, the prevalence of SD was 10.1% in the previous month among 1,870 adults aged 20 years and older who had participated in the two Rand American Life Panel surveys, the first occurring in February 2019. In that earlier poll, 10.9% of the 2,555 respondents said that they experienced SD in the worst month of the previous year, the investigators said.
The prevalence of overall psychological distress increased by 12.8% from February 2019 to May 2020, with increases higher among women (17.7%) than men (10.6%); adults under age 60 years, compared with those over 60 (see graph); and Hispanics, compared with other races/ethnicities. Disparities also were seen among income groups: Distress rose 10.2% for those earning over $100,000, compared with 15.4% for those making less than $35,000 and 18.2% for Americans earning between $35,000 and $60,000, the researchers reported.
A high level of stress in the prepandemic survey strongly predicted serious distress during the pandemic. “Risk for SD during the pandemic among those with SD during a year before the pandemic was almost 3 times higher than among those reporting mild/moderate distress and 15 times higher than among those reporting no/low distress during the prepandemic year,” they noted.
Distress levels often return to normal after a disaster, Dr. Breslau and associates pointed out, but “the pandemic’s influence on economic stressors, disruption of usual activities and subsequent effects on population health may continue for an extended period and affect different regions of the country at different points in time.”
SOURCE: Breslau J et al. Prev Med. 2020 Dec 31. doi: 10.1016/j.ypmed.2020.106362.
The first month of the coronavirus pandemic created almost as much psychological distress among American adults as they had experienced in the year before February 2019, according to the results of two representative surveys.

“The 30-day prevalence of SD [serious distress] in May 2020 did not differ from the past-year prevalence of SD assessed with the same instrument [the Kessler-6 distress scale] in February 2019. In other words, equal numbers of people experienced SD in 30-days during the pandemic as experienced SD over an entire year prior to the pandemic,” Joshua Breslau, PhD, and associates at the Rand Corporation wrote in Preventive Medicine.
In May of 2020, the prevalence of SD was 10.1% in the previous month among 1,870 adults aged 20 years and older who had participated in the two Rand American Life Panel surveys, the first occurring in February 2019. In that earlier poll, 10.9% of the 2,555 respondents said that they experienced SD in the worst month of the previous year, the investigators said.
The prevalence of overall psychological distress increased by 12.8% from February 2019 to May 2020, with increases higher among women (17.7%) than men (10.6%); adults under age 60 years, compared with those over 60 (see graph); and Hispanics, compared with other races/ethnicities. Disparities also were seen among income groups: Distress rose 10.2% for those earning over $100,000, compared with 15.4% for those making less than $35,000 and 18.2% for Americans earning between $35,000 and $60,000, the researchers reported.
A high level of stress in the prepandemic survey strongly predicted serious distress during the pandemic. “Risk for SD during the pandemic among those with SD during a year before the pandemic was almost 3 times higher than among those reporting mild/moderate distress and 15 times higher than among those reporting no/low distress during the prepandemic year,” they noted.
Distress levels often return to normal after a disaster, Dr. Breslau and associates pointed out, but “the pandemic’s influence on economic stressors, disruption of usual activities and subsequent effects on population health may continue for an extended period and affect different regions of the country at different points in time.”
SOURCE: Breslau J et al. Prev Med. 2020 Dec 31. doi: 10.1016/j.ypmed.2020.106362.
FROM PREVENTIVE MEDICINE





