User login
Mpox Presentation Compared in Different Racial, Ethnic Groups
TOPLINE:
.
METHODOLOGY:
- There is limited information on the populations disproportionately affected by the recent global mpox outbreak, particularly in individuals with HIV and racial and ethnic minorities.
- To investigate morphologic and clinical presentations of mpox in diverse populations, researchers conducted a review of the records of 54 individuals (mean age, 42.4 years) diagnosed with mpox at a San Francisco clinic for patients with HIV or at high risk for HIV, between June and October 2022.
- All patients were assigned male at birth, and three identified themselves as transgender women.
- Morphologic descriptions were documented through either photographic evidence or physical examination notes.
TAKEAWAY:
- Pustules or pseudopustules were the most common morphologic finding in 57.1% of the White non-Hispanic patients and 62.5% of the patients of color (P = .72).
- White non-Hispanic patients were more likely to have no prodromal symptoms (50.0% vs 17.5%; P = .02) and were more likely to have genital lesions (78.6% vs 40.0%; P = .01) than patients of color. These differences were significant or nearly significant when White non-Hispanic patients were compared with Hispanic patients but not in other ethnic or racial groups.
- There were no differences in HIV viral loads or CD4 counts between racial and ethnic groups, and no variations in clinical presentations were observed based on CD4 counts.
- Patients with higher HIV viral loads were more likely to have concurrent sexually transmitted infections (57.1% vs 25%; P = .03).
- Symptoms resolved in all patients, regardless of medical intervention, within weeks of initial presentation, and there were no hospitalizations or deaths.
IN PRACTICE:
Considering that HIV viral burden was not significantly different between White non-Hispanic patients and patients of color, the difference in presentation of the prodrome “may indicate disparities in vulnerable populations,” the authors wrote, noting that more research in large groups is needed to confirm their results.
SOURCE:
The study, led by Richard W. Kim, BS, from the University of California San Francisco, was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Inclusion of “other” racial category in the records highlighted potential inaccuracies in data representation.
DISCLOSURES:
The study received no external funding. The authors did not declare any competing interests.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- There is limited information on the populations disproportionately affected by the recent global mpox outbreak, particularly in individuals with HIV and racial and ethnic minorities.
- To investigate morphologic and clinical presentations of mpox in diverse populations, researchers conducted a review of the records of 54 individuals (mean age, 42.4 years) diagnosed with mpox at a San Francisco clinic for patients with HIV or at high risk for HIV, between June and October 2022.
- All patients were assigned male at birth, and three identified themselves as transgender women.
- Morphologic descriptions were documented through either photographic evidence or physical examination notes.
TAKEAWAY:
- Pustules or pseudopustules were the most common morphologic finding in 57.1% of the White non-Hispanic patients and 62.5% of the patients of color (P = .72).
- White non-Hispanic patients were more likely to have no prodromal symptoms (50.0% vs 17.5%; P = .02) and were more likely to have genital lesions (78.6% vs 40.0%; P = .01) than patients of color. These differences were significant or nearly significant when White non-Hispanic patients were compared with Hispanic patients but not in other ethnic or racial groups.
- There were no differences in HIV viral loads or CD4 counts between racial and ethnic groups, and no variations in clinical presentations were observed based on CD4 counts.
- Patients with higher HIV viral loads were more likely to have concurrent sexually transmitted infections (57.1% vs 25%; P = .03).
- Symptoms resolved in all patients, regardless of medical intervention, within weeks of initial presentation, and there were no hospitalizations or deaths.
IN PRACTICE:
Considering that HIV viral burden was not significantly different between White non-Hispanic patients and patients of color, the difference in presentation of the prodrome “may indicate disparities in vulnerable populations,” the authors wrote, noting that more research in large groups is needed to confirm their results.
SOURCE:
The study, led by Richard W. Kim, BS, from the University of California San Francisco, was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Inclusion of “other” racial category in the records highlighted potential inaccuracies in data representation.
DISCLOSURES:
The study received no external funding. The authors did not declare any competing interests.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- There is limited information on the populations disproportionately affected by the recent global mpox outbreak, particularly in individuals with HIV and racial and ethnic minorities.
- To investigate morphologic and clinical presentations of mpox in diverse populations, researchers conducted a review of the records of 54 individuals (mean age, 42.4 years) diagnosed with mpox at a San Francisco clinic for patients with HIV or at high risk for HIV, between June and October 2022.
- All patients were assigned male at birth, and three identified themselves as transgender women.
- Morphologic descriptions were documented through either photographic evidence or physical examination notes.
TAKEAWAY:
- Pustules or pseudopustules were the most common morphologic finding in 57.1% of the White non-Hispanic patients and 62.5% of the patients of color (P = .72).
- White non-Hispanic patients were more likely to have no prodromal symptoms (50.0% vs 17.5%; P = .02) and were more likely to have genital lesions (78.6% vs 40.0%; P = .01) than patients of color. These differences were significant or nearly significant when White non-Hispanic patients were compared with Hispanic patients but not in other ethnic or racial groups.
- There were no differences in HIV viral loads or CD4 counts between racial and ethnic groups, and no variations in clinical presentations were observed based on CD4 counts.
- Patients with higher HIV viral loads were more likely to have concurrent sexually transmitted infections (57.1% vs 25%; P = .03).
- Symptoms resolved in all patients, regardless of medical intervention, within weeks of initial presentation, and there were no hospitalizations or deaths.
IN PRACTICE:
Considering that HIV viral burden was not significantly different between White non-Hispanic patients and patients of color, the difference in presentation of the prodrome “may indicate disparities in vulnerable populations,” the authors wrote, noting that more research in large groups is needed to confirm their results.
SOURCE:
The study, led by Richard W. Kim, BS, from the University of California San Francisco, was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Inclusion of “other” racial category in the records highlighted potential inaccuracies in data representation.
DISCLOSURES:
The study received no external funding. The authors did not declare any competing interests.
A version of this article first appeared on Medscape.com.
Head and Neck Cancer in Spotlight at AVAHO Regional Meeting
In the US Department of Veterans Affairs (VA) health care system, head and neck cancer is one of the most complex oncologic conditions to treat because so many medical professionals are involved in its care. Specialists in speech therapy, nutrition, lymphedema, and dentistry are all part of the picture.
“It takes a complete team to treat cancer in a comprehensive manner, and specialists work hand-in-hand,” said Cindy Bowman, MSN, RN, OCN, president of the Association of VA Hematology/Oncology (AVAHO).
AVAHO held a regional meeting in Seattle on May 4, 2024, that was entirely devoted to head and neck cancer. “The goal was to help the VA oncology professionals gain a global view of how various team members can seamlessly work together,” said Bowman, an oncology nurse navigator and coordinator of the Cancer Care Navigation Program at Bay Pines VA Healthcare System in the Tampa-St. Petersburg, FL area.
According to a 2017 report, 2031 cases of head and neck cancer were diagnosed in 2010 among VA patients, accounting for 4.4% of all cancers. “Veterans are especially vulnerable to this type of cancer for several reasons, such as high rates of smoking and alcohol use,” Bowman said. In addition, she said veterans who served in parts of Southeast Asia, North Africa, and the Middle East are at higher risk of nasopharyngeal carcinoma, which has been linked to Epstein-Barr virus infections in those regions.
Radiation treatment were a significant topic at the regional meeting, and 1 session was focused on the importance of prompt care. “Head and neck cancers are very aggressive,” Bowman said. “The sooner we identify them, the sooner we get treatment started.”
Attendees also heard from a speech therapist and a dietician, who discussed a collaborative approach to improving treatment outcomes. “These are two very important pieces of the puzzle.” Bowman said.
On the nutrition front, a lot of newly diagnosed patients already have malnutrition because they have been having difficulty swallowing. So right up front, a registered dietician works with them and individualizes their nutrition treatment plans all the way into recovery. Some of these folks will end up with their relationship with their dietitian for many years.
“Speech therapists work with patients to design swallowing and tongue exercises that target their individual cancer.” Bowman said. The goal is to prevent the need for a feeding tube.
Another session at the regional conference focused on lymphedema—swelling that can develop due to radiation treatment. “All patients with head and neck cancer should be sent to a lymphedema specialist prior to starting treatment since the specialists can prevent this from happening by giving the patients tools, such as compression garments,” Bowman said. “This way, we don’t end up with somebody 15 or 20 years from now coming back and saying they’re not able to move their neck or unable to swallow the right way.”
Another session highlighted the important role of dental care for patients with head and neck cancer. “We send patients to the dentist prior to ever starting anything. We know that radiation therapy can cause osteoradionecrosis, in which people’s teeth begin to crumble. Fortunately, the VA is now covering dentures for these patients, and they automatically get dental care coverage.” Bowman said.
“In the big picture,” she said, “Attendees should come out of the regional meeting with new insight into the importance of teamwork in head and neck cancer care. We need to make sure that all the pieces to the puzzle are there, and everybody is working together to expedite care for the veterans so that they have the best outcomes possible.”
In the US Department of Veterans Affairs (VA) health care system, head and neck cancer is one of the most complex oncologic conditions to treat because so many medical professionals are involved in its care. Specialists in speech therapy, nutrition, lymphedema, and dentistry are all part of the picture.
“It takes a complete team to treat cancer in a comprehensive manner, and specialists work hand-in-hand,” said Cindy Bowman, MSN, RN, OCN, president of the Association of VA Hematology/Oncology (AVAHO).
AVAHO held a regional meeting in Seattle on May 4, 2024, that was entirely devoted to head and neck cancer. “The goal was to help the VA oncology professionals gain a global view of how various team members can seamlessly work together,” said Bowman, an oncology nurse navigator and coordinator of the Cancer Care Navigation Program at Bay Pines VA Healthcare System in the Tampa-St. Petersburg, FL area.
According to a 2017 report, 2031 cases of head and neck cancer were diagnosed in 2010 among VA patients, accounting for 4.4% of all cancers. “Veterans are especially vulnerable to this type of cancer for several reasons, such as high rates of smoking and alcohol use,” Bowman said. In addition, she said veterans who served in parts of Southeast Asia, North Africa, and the Middle East are at higher risk of nasopharyngeal carcinoma, which has been linked to Epstein-Barr virus infections in those regions.
Radiation treatment were a significant topic at the regional meeting, and 1 session was focused on the importance of prompt care. “Head and neck cancers are very aggressive,” Bowman said. “The sooner we identify them, the sooner we get treatment started.”
Attendees also heard from a speech therapist and a dietician, who discussed a collaborative approach to improving treatment outcomes. “These are two very important pieces of the puzzle.” Bowman said.
On the nutrition front, a lot of newly diagnosed patients already have malnutrition because they have been having difficulty swallowing. So right up front, a registered dietician works with them and individualizes their nutrition treatment plans all the way into recovery. Some of these folks will end up with their relationship with their dietitian for many years.
“Speech therapists work with patients to design swallowing and tongue exercises that target their individual cancer.” Bowman said. The goal is to prevent the need for a feeding tube.
Another session at the regional conference focused on lymphedema—swelling that can develop due to radiation treatment. “All patients with head and neck cancer should be sent to a lymphedema specialist prior to starting treatment since the specialists can prevent this from happening by giving the patients tools, such as compression garments,” Bowman said. “This way, we don’t end up with somebody 15 or 20 years from now coming back and saying they’re not able to move their neck or unable to swallow the right way.”
Another session highlighted the important role of dental care for patients with head and neck cancer. “We send patients to the dentist prior to ever starting anything. We know that radiation therapy can cause osteoradionecrosis, in which people’s teeth begin to crumble. Fortunately, the VA is now covering dentures for these patients, and they automatically get dental care coverage.” Bowman said.
“In the big picture,” she said, “Attendees should come out of the regional meeting with new insight into the importance of teamwork in head and neck cancer care. We need to make sure that all the pieces to the puzzle are there, and everybody is working together to expedite care for the veterans so that they have the best outcomes possible.”
In the US Department of Veterans Affairs (VA) health care system, head and neck cancer is one of the most complex oncologic conditions to treat because so many medical professionals are involved in its care. Specialists in speech therapy, nutrition, lymphedema, and dentistry are all part of the picture.
“It takes a complete team to treat cancer in a comprehensive manner, and specialists work hand-in-hand,” said Cindy Bowman, MSN, RN, OCN, president of the Association of VA Hematology/Oncology (AVAHO).
AVAHO held a regional meeting in Seattle on May 4, 2024, that was entirely devoted to head and neck cancer. “The goal was to help the VA oncology professionals gain a global view of how various team members can seamlessly work together,” said Bowman, an oncology nurse navigator and coordinator of the Cancer Care Navigation Program at Bay Pines VA Healthcare System in the Tampa-St. Petersburg, FL area.
According to a 2017 report, 2031 cases of head and neck cancer were diagnosed in 2010 among VA patients, accounting for 4.4% of all cancers. “Veterans are especially vulnerable to this type of cancer for several reasons, such as high rates of smoking and alcohol use,” Bowman said. In addition, she said veterans who served in parts of Southeast Asia, North Africa, and the Middle East are at higher risk of nasopharyngeal carcinoma, which has been linked to Epstein-Barr virus infections in those regions.
Radiation treatment were a significant topic at the regional meeting, and 1 session was focused on the importance of prompt care. “Head and neck cancers are very aggressive,” Bowman said. “The sooner we identify them, the sooner we get treatment started.”
Attendees also heard from a speech therapist and a dietician, who discussed a collaborative approach to improving treatment outcomes. “These are two very important pieces of the puzzle.” Bowman said.
On the nutrition front, a lot of newly diagnosed patients already have malnutrition because they have been having difficulty swallowing. So right up front, a registered dietician works with them and individualizes their nutrition treatment plans all the way into recovery. Some of these folks will end up with their relationship with their dietitian for many years.
“Speech therapists work with patients to design swallowing and tongue exercises that target their individual cancer.” Bowman said. The goal is to prevent the need for a feeding tube.
Another session at the regional conference focused on lymphedema—swelling that can develop due to radiation treatment. “All patients with head and neck cancer should be sent to a lymphedema specialist prior to starting treatment since the specialists can prevent this from happening by giving the patients tools, such as compression garments,” Bowman said. “This way, we don’t end up with somebody 15 or 20 years from now coming back and saying they’re not able to move their neck or unable to swallow the right way.”
Another session highlighted the important role of dental care for patients with head and neck cancer. “We send patients to the dentist prior to ever starting anything. We know that radiation therapy can cause osteoradionecrosis, in which people’s teeth begin to crumble. Fortunately, the VA is now covering dentures for these patients, and they automatically get dental care coverage.” Bowman said.
“In the big picture,” she said, “Attendees should come out of the regional meeting with new insight into the importance of teamwork in head and neck cancer care. We need to make sure that all the pieces to the puzzle are there, and everybody is working together to expedite care for the veterans so that they have the best outcomes possible.”
TMS May Be a Good Alternative to ECT in Depression
DENVER — , according to results from a retrospective study of patients treated in the past 20 years.
“We always learn in our textbooks that after about two or three medication trials is when you can start exploring more serious treatment protocols, such as ECT or TMS, but a lot of these patients weren’t going forward with it, and I was curious about it. I figured that TMS, which is a less expensive, less scary procedure that patients would more likely be open to, that is also approved for treatment resistant depression, would be a good alternative to ECT,” said Anuttham Kandhadai, a third-year medical student at University of Texas Medical Branch at Galveston, who presented the study at the 2024 annual meeting of the American Academy of Neurology.
Study Findings Lead to More Questions
The researchers found lower rates of depressive episodes, suicidal attempts, and suicidal ideation among patients treated with TMS, but an important limitation was that the researchers did not know the severity of the depression in the two patient groups, according to Branch Coslett, MD, who attended the session and has performed research with TMS to treat aphasia in stroke patients. “I think it’s a very interesting study, and certainly something worth pursuing, but given that ECT is only used as a last resort, whereas TMS is often used as a second-line therapy, I think you’re really talking about very different populations that have had these treatments,” said Dr. Coslett.
Mr. Kandhadai recognized the limitations of the study and looks forward to expanding the research. “I’d love to explore cost effectiveness of the treatments. I’d love to explore patient familiarity and patient comfort with different treatments. And I’d also love to explore a more controlled study that can determine how severe someone’s depression is, and then be able to control for that and explore the outcomes based on the treatment protocol,” he said.
The ideal comparative study would be prospective, “but that will never be done. One Flew Over the Cuckoo’s Nest and similar sources of information have really poisoned the well,” said Dr. Coslett. However, he noted that advances have been made in ECT, and that targeting the right hemisphere produces fewer side effects: “The outcomes from unilateral right hemisphere stimulation are said to be every bit as good or maybe better, and you don’t get the confusion, you don’t get the memory loss, you don’t get all that sort of stuff that you’d expect when somebody has a prolonged, generalized tonic-clonic seizure.”
Still, people are naturally reluctant to undergo ECT. “I’ve seen it. It’s pretty barbaric. It’s better now and at my institution, people do get it, but they really, really have to be intractable,” he said.
Comparing Treatment Options
Mr. Kandhadai and his co-authors used the TriNetX database to identify patients with treatment-resistant major depressive disorder who received TMS or ECT in the past 20 years. There were 2,916 patients in both cohorts, who were matched by age, sex, ethnicity, mood and behavioral disorders, endocrine disorders, intellectual disabilities, cerebrovascular disease, and other nervous system disorders. The mean age at treatment was 48.2 years, 38.5% were male, and 3.1% were Black or African American.
Short-term outcomes favored TMS, including the frequency of disorientation (0.41% vs 2.81%), retrograde amnesia (0.34% vs 0.65%), and headache (4.36% vs 7.20%). Long-term outcomes from 1 month to 5 years post treatment were also better in the TMS group, including depressive episodes (44.99% vs 53.77%), suicide attempts (3.98% vs 6.86%), and suicidal ideation (12.38% vs 23.49%). Kaplan-Meier curve analysis between 1 month and 5 years showed a benefit to TMS in probability of not experiencing a depressive episode, and not experiencing suicidal ideation.
“ECT has been the gold standard of treatment resistant depression for a long time, and it deserves to be. I think it’s something you should offer your patients. Not everyone might be comfortable with it, and if they’re not, I think it’s important to not stop the conversation there, but to offer something like TMS because TMS is something that might be more accessible to patients. It might be more affordable, and it might be less scary,” said Mr. Kandhadai
Mr. Kandhadai and Dr. Coslett have no relevant financial disclosures.
DENVER — , according to results from a retrospective study of patients treated in the past 20 years.
“We always learn in our textbooks that after about two or three medication trials is when you can start exploring more serious treatment protocols, such as ECT or TMS, but a lot of these patients weren’t going forward with it, and I was curious about it. I figured that TMS, which is a less expensive, less scary procedure that patients would more likely be open to, that is also approved for treatment resistant depression, would be a good alternative to ECT,” said Anuttham Kandhadai, a third-year medical student at University of Texas Medical Branch at Galveston, who presented the study at the 2024 annual meeting of the American Academy of Neurology.
Study Findings Lead to More Questions
The researchers found lower rates of depressive episodes, suicidal attempts, and suicidal ideation among patients treated with TMS, but an important limitation was that the researchers did not know the severity of the depression in the two patient groups, according to Branch Coslett, MD, who attended the session and has performed research with TMS to treat aphasia in stroke patients. “I think it’s a very interesting study, and certainly something worth pursuing, but given that ECT is only used as a last resort, whereas TMS is often used as a second-line therapy, I think you’re really talking about very different populations that have had these treatments,” said Dr. Coslett.
Mr. Kandhadai recognized the limitations of the study and looks forward to expanding the research. “I’d love to explore cost effectiveness of the treatments. I’d love to explore patient familiarity and patient comfort with different treatments. And I’d also love to explore a more controlled study that can determine how severe someone’s depression is, and then be able to control for that and explore the outcomes based on the treatment protocol,” he said.
The ideal comparative study would be prospective, “but that will never be done. One Flew Over the Cuckoo’s Nest and similar sources of information have really poisoned the well,” said Dr. Coslett. However, he noted that advances have been made in ECT, and that targeting the right hemisphere produces fewer side effects: “The outcomes from unilateral right hemisphere stimulation are said to be every bit as good or maybe better, and you don’t get the confusion, you don’t get the memory loss, you don’t get all that sort of stuff that you’d expect when somebody has a prolonged, generalized tonic-clonic seizure.”
Still, people are naturally reluctant to undergo ECT. “I’ve seen it. It’s pretty barbaric. It’s better now and at my institution, people do get it, but they really, really have to be intractable,” he said.
Comparing Treatment Options
Mr. Kandhadai and his co-authors used the TriNetX database to identify patients with treatment-resistant major depressive disorder who received TMS or ECT in the past 20 years. There were 2,916 patients in both cohorts, who were matched by age, sex, ethnicity, mood and behavioral disorders, endocrine disorders, intellectual disabilities, cerebrovascular disease, and other nervous system disorders. The mean age at treatment was 48.2 years, 38.5% were male, and 3.1% were Black or African American.
Short-term outcomes favored TMS, including the frequency of disorientation (0.41% vs 2.81%), retrograde amnesia (0.34% vs 0.65%), and headache (4.36% vs 7.20%). Long-term outcomes from 1 month to 5 years post treatment were also better in the TMS group, including depressive episodes (44.99% vs 53.77%), suicide attempts (3.98% vs 6.86%), and suicidal ideation (12.38% vs 23.49%). Kaplan-Meier curve analysis between 1 month and 5 years showed a benefit to TMS in probability of not experiencing a depressive episode, and not experiencing suicidal ideation.
“ECT has been the gold standard of treatment resistant depression for a long time, and it deserves to be. I think it’s something you should offer your patients. Not everyone might be comfortable with it, and if they’re not, I think it’s important to not stop the conversation there, but to offer something like TMS because TMS is something that might be more accessible to patients. It might be more affordable, and it might be less scary,” said Mr. Kandhadai
Mr. Kandhadai and Dr. Coslett have no relevant financial disclosures.
DENVER — , according to results from a retrospective study of patients treated in the past 20 years.
“We always learn in our textbooks that after about two or three medication trials is when you can start exploring more serious treatment protocols, such as ECT or TMS, but a lot of these patients weren’t going forward with it, and I was curious about it. I figured that TMS, which is a less expensive, less scary procedure that patients would more likely be open to, that is also approved for treatment resistant depression, would be a good alternative to ECT,” said Anuttham Kandhadai, a third-year medical student at University of Texas Medical Branch at Galveston, who presented the study at the 2024 annual meeting of the American Academy of Neurology.
Study Findings Lead to More Questions
The researchers found lower rates of depressive episodes, suicidal attempts, and suicidal ideation among patients treated with TMS, but an important limitation was that the researchers did not know the severity of the depression in the two patient groups, according to Branch Coslett, MD, who attended the session and has performed research with TMS to treat aphasia in stroke patients. “I think it’s a very interesting study, and certainly something worth pursuing, but given that ECT is only used as a last resort, whereas TMS is often used as a second-line therapy, I think you’re really talking about very different populations that have had these treatments,” said Dr. Coslett.
Mr. Kandhadai recognized the limitations of the study and looks forward to expanding the research. “I’d love to explore cost effectiveness of the treatments. I’d love to explore patient familiarity and patient comfort with different treatments. And I’d also love to explore a more controlled study that can determine how severe someone’s depression is, and then be able to control for that and explore the outcomes based on the treatment protocol,” he said.
The ideal comparative study would be prospective, “but that will never be done. One Flew Over the Cuckoo’s Nest and similar sources of information have really poisoned the well,” said Dr. Coslett. However, he noted that advances have been made in ECT, and that targeting the right hemisphere produces fewer side effects: “The outcomes from unilateral right hemisphere stimulation are said to be every bit as good or maybe better, and you don’t get the confusion, you don’t get the memory loss, you don’t get all that sort of stuff that you’d expect when somebody has a prolonged, generalized tonic-clonic seizure.”
Still, people are naturally reluctant to undergo ECT. “I’ve seen it. It’s pretty barbaric. It’s better now and at my institution, people do get it, but they really, really have to be intractable,” he said.
Comparing Treatment Options
Mr. Kandhadai and his co-authors used the TriNetX database to identify patients with treatment-resistant major depressive disorder who received TMS or ECT in the past 20 years. There were 2,916 patients in both cohorts, who were matched by age, sex, ethnicity, mood and behavioral disorders, endocrine disorders, intellectual disabilities, cerebrovascular disease, and other nervous system disorders. The mean age at treatment was 48.2 years, 38.5% were male, and 3.1% were Black or African American.
Short-term outcomes favored TMS, including the frequency of disorientation (0.41% vs 2.81%), retrograde amnesia (0.34% vs 0.65%), and headache (4.36% vs 7.20%). Long-term outcomes from 1 month to 5 years post treatment were also better in the TMS group, including depressive episodes (44.99% vs 53.77%), suicide attempts (3.98% vs 6.86%), and suicidal ideation (12.38% vs 23.49%). Kaplan-Meier curve analysis between 1 month and 5 years showed a benefit to TMS in probability of not experiencing a depressive episode, and not experiencing suicidal ideation.
“ECT has been the gold standard of treatment resistant depression for a long time, and it deserves to be. I think it’s something you should offer your patients. Not everyone might be comfortable with it, and if they’re not, I think it’s important to not stop the conversation there, but to offer something like TMS because TMS is something that might be more accessible to patients. It might be more affordable, and it might be less scary,” said Mr. Kandhadai
Mr. Kandhadai and Dr. Coslett have no relevant financial disclosures.
FROM AAN 2024
Most Homeless People Have Mental Health Disorders
Most people experiencing homelessness have mental health disorders, according to a systematic review and meta-analysis.
In an examination of studies that included nearly 50,000 participants, the current prevalence of mental health disorders among people experiencing homelessness was 67% and the lifetime prevalence was 77%.
“The relationship is likely bidirectional, where experiencing homelessness may exacerbate mental health symptoms or where having a mental health disorder may increase an individual’s risk for experiencing homelessness,” lead author Rebecca Barry, PhD, a postdoctoral fellow at the University of Calgary in Calgary, Alberta, Canada, told this news organization.
“There are also likely stressors that increase both risk for homelessness and risk for developing mental health disorders. This study examines prevalence but does not examine causal relationships,” she said.
The findings were published in JAMA Psychiatry.
A Growing Problem
To determine the current and lifetime prevalence of mental health disorders among the homeless population, the researchers analyzed 85 studies that examined this question in participants aged ≥ 18 years. The review included 48,414 participants, including 11,154 (23%) women and 37,260 (77%) men.
The lifetime prevalence of mental health disorders was significantly higher in men experiencing homelessness (86%) than in women (69%). The most common mental health disorder was substance use disorder (44%), followed by antisocial personality disorder (26%), major depression (19%), bipolar disorder (8%), and schizophrenia (7%).
The prevalence of current and lifetime mental health disorders among the homeless population was higher than that that observed in the general population (13%-15% and 12%-47%, respectively).
The results resembled those of a previous review that estimated that 76% of people experiencing homelessness living in high-income countries have mental health disorders.
“Even though our results are not surprising, they still are drawing attention to this issue because it is a big problem in Canada, the United States, Europe, and other places,” senior author Dallas Seitz, MD, PhD, professor of psychiatry at the University of Calgary’s Cumming School of Medicine, told this news organization. “The problem is concerning, and it’s not getting better. Addiction and mental health problems are becoming more common among people who are homeless.”
The bottom line is that people need affordable housing and mental health support, said Dr. Seitz. “It’s a housing problem and a health problem, and we need adequate resources to find better ways for those two systems to collaborate. There are public safety concerns, and we have to try and bring services to people experiencing homelessness. You have to come and meet people where they’re at. You have to try and establish a trusting relationship so that we can get people on the path to recovery.”
‘It’s Really About Income’
Commenting on the findings for this news organization, Stephen Hwang, MD, professor of medicine at the University of Toronto, Toronto, Ontario, Canada, said, “There have been previous studies of this type, but it is good to have an updated one.” Dr. Hwang, who is also chair in Homelessness, Housing, and Health at St. Michael’s Hospital, did not participate in the research.
The findings must be understood in the proper context, he added. For one thing, grouping together all mental health disorders and giving a single prevalence figure can be misleading. “They are including in that category a diverse group of conditions. Substance use disorder, personality disorder, schizophrenia, and depression are all lumped together. The 67% prevalence seems very high, but it is a combination of many different conditions. I just don’t want people to look at that number and think that this means that everyone is a substance user or everyone has schizophrenia,” said Dr. Hwang.
Also, some readers might interpret the findings to mean that mental problems are the reason people are homeless, he added. “That would be an incorrect interpretation because what this study is showing is that people with mental health disorders have a higher risk for becoming homeless. It doesn’t mean that it caused their homelessness. What really causes homelessness is a lack of affordable housing,” said Dr. Hwang.
“In a city or community where housing is very expensive, there’s not enough for everyone to be housed, there is a lot of competition for housing, and there’s not enough affordable housing for a number of reasons, we know that people with mental health conditions and substance use disorders will be among the first to lose their housing,” he said.
“It’s really about income. There are many reasons why a person cannot afford housing. So, not being able to earn enough money to afford it because you have a mental health disorder or substance use disorder is a common underlying reason for homelessness.”
Dr. Hwang also pointed out that people with mental illness who can access support, either through family members or through mental health care, and who also have the income to afford such services do not become homeless.
“Schizophrenia is seen in every population of the world at a rate of 1%. But you travel to certain cities and you see people who appear to have schizophrenia wandering the streets, and you go to other cities in the world and you don’t see anyone who looks like they’re homeless and have schizophrenia,” he said.
“It’s not because there are fewer people with schizophrenia in those cities or countries; it’s because people with schizophrenia are treated differently. The rate of homelessness is determined not by how many people have that condition [eg, schizophrenia] but by how we treat those people and how we set up our society to either support or not support people who have disabilities.”
The study was funded by the Precision Care With Information, Science and Experience – Mental Health grant funded by the Calgary Health Foundation. Dr. Barry is supported by the Harley Hotchkiss Samuel Weiss Postdoctoral Fellowship awarded by the Hotchkiss Brain Institute at the University of Calgary. Dr. Barry reported having no relevant financial relationships. Dr. Seitz reported grants from Calgary Health Foundation during the conduct of the study as well as grants from University Health Foundation, the Canadian Institutes of Health Research, the Public Health Agency of Canada, the Alzheimer’s Association, and the Hotchkiss Brain Institute. He received honoraria for guideline development from the Canadian Coalition for Seniors Mental Health outside the submitted work. Dr. Hwang reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Most people experiencing homelessness have mental health disorders, according to a systematic review and meta-analysis.
In an examination of studies that included nearly 50,000 participants, the current prevalence of mental health disorders among people experiencing homelessness was 67% and the lifetime prevalence was 77%.
“The relationship is likely bidirectional, where experiencing homelessness may exacerbate mental health symptoms or where having a mental health disorder may increase an individual’s risk for experiencing homelessness,” lead author Rebecca Barry, PhD, a postdoctoral fellow at the University of Calgary in Calgary, Alberta, Canada, told this news organization.
“There are also likely stressors that increase both risk for homelessness and risk for developing mental health disorders. This study examines prevalence but does not examine causal relationships,” she said.
The findings were published in JAMA Psychiatry.
A Growing Problem
To determine the current and lifetime prevalence of mental health disorders among the homeless population, the researchers analyzed 85 studies that examined this question in participants aged ≥ 18 years. The review included 48,414 participants, including 11,154 (23%) women and 37,260 (77%) men.
The lifetime prevalence of mental health disorders was significantly higher in men experiencing homelessness (86%) than in women (69%). The most common mental health disorder was substance use disorder (44%), followed by antisocial personality disorder (26%), major depression (19%), bipolar disorder (8%), and schizophrenia (7%).
The prevalence of current and lifetime mental health disorders among the homeless population was higher than that that observed in the general population (13%-15% and 12%-47%, respectively).
The results resembled those of a previous review that estimated that 76% of people experiencing homelessness living in high-income countries have mental health disorders.
“Even though our results are not surprising, they still are drawing attention to this issue because it is a big problem in Canada, the United States, Europe, and other places,” senior author Dallas Seitz, MD, PhD, professor of psychiatry at the University of Calgary’s Cumming School of Medicine, told this news organization. “The problem is concerning, and it’s not getting better. Addiction and mental health problems are becoming more common among people who are homeless.”
The bottom line is that people need affordable housing and mental health support, said Dr. Seitz. “It’s a housing problem and a health problem, and we need adequate resources to find better ways for those two systems to collaborate. There are public safety concerns, and we have to try and bring services to people experiencing homelessness. You have to come and meet people where they’re at. You have to try and establish a trusting relationship so that we can get people on the path to recovery.”
‘It’s Really About Income’
Commenting on the findings for this news organization, Stephen Hwang, MD, professor of medicine at the University of Toronto, Toronto, Ontario, Canada, said, “There have been previous studies of this type, but it is good to have an updated one.” Dr. Hwang, who is also chair in Homelessness, Housing, and Health at St. Michael’s Hospital, did not participate in the research.
The findings must be understood in the proper context, he added. For one thing, grouping together all mental health disorders and giving a single prevalence figure can be misleading. “They are including in that category a diverse group of conditions. Substance use disorder, personality disorder, schizophrenia, and depression are all lumped together. The 67% prevalence seems very high, but it is a combination of many different conditions. I just don’t want people to look at that number and think that this means that everyone is a substance user or everyone has schizophrenia,” said Dr. Hwang.
Also, some readers might interpret the findings to mean that mental problems are the reason people are homeless, he added. “That would be an incorrect interpretation because what this study is showing is that people with mental health disorders have a higher risk for becoming homeless. It doesn’t mean that it caused their homelessness. What really causes homelessness is a lack of affordable housing,” said Dr. Hwang.
“In a city or community where housing is very expensive, there’s not enough for everyone to be housed, there is a lot of competition for housing, and there’s not enough affordable housing for a number of reasons, we know that people with mental health conditions and substance use disorders will be among the first to lose their housing,” he said.
“It’s really about income. There are many reasons why a person cannot afford housing. So, not being able to earn enough money to afford it because you have a mental health disorder or substance use disorder is a common underlying reason for homelessness.”
Dr. Hwang also pointed out that people with mental illness who can access support, either through family members or through mental health care, and who also have the income to afford such services do not become homeless.
“Schizophrenia is seen in every population of the world at a rate of 1%. But you travel to certain cities and you see people who appear to have schizophrenia wandering the streets, and you go to other cities in the world and you don’t see anyone who looks like they’re homeless and have schizophrenia,” he said.
“It’s not because there are fewer people with schizophrenia in those cities or countries; it’s because people with schizophrenia are treated differently. The rate of homelessness is determined not by how many people have that condition [eg, schizophrenia] but by how we treat those people and how we set up our society to either support or not support people who have disabilities.”
The study was funded by the Precision Care With Information, Science and Experience – Mental Health grant funded by the Calgary Health Foundation. Dr. Barry is supported by the Harley Hotchkiss Samuel Weiss Postdoctoral Fellowship awarded by the Hotchkiss Brain Institute at the University of Calgary. Dr. Barry reported having no relevant financial relationships. Dr. Seitz reported grants from Calgary Health Foundation during the conduct of the study as well as grants from University Health Foundation, the Canadian Institutes of Health Research, the Public Health Agency of Canada, the Alzheimer’s Association, and the Hotchkiss Brain Institute. He received honoraria for guideline development from the Canadian Coalition for Seniors Mental Health outside the submitted work. Dr. Hwang reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Most people experiencing homelessness have mental health disorders, according to a systematic review and meta-analysis.
In an examination of studies that included nearly 50,000 participants, the current prevalence of mental health disorders among people experiencing homelessness was 67% and the lifetime prevalence was 77%.
“The relationship is likely bidirectional, where experiencing homelessness may exacerbate mental health symptoms or where having a mental health disorder may increase an individual’s risk for experiencing homelessness,” lead author Rebecca Barry, PhD, a postdoctoral fellow at the University of Calgary in Calgary, Alberta, Canada, told this news organization.
“There are also likely stressors that increase both risk for homelessness and risk for developing mental health disorders. This study examines prevalence but does not examine causal relationships,” she said.
The findings were published in JAMA Psychiatry.
A Growing Problem
To determine the current and lifetime prevalence of mental health disorders among the homeless population, the researchers analyzed 85 studies that examined this question in participants aged ≥ 18 years. The review included 48,414 participants, including 11,154 (23%) women and 37,260 (77%) men.
The lifetime prevalence of mental health disorders was significantly higher in men experiencing homelessness (86%) than in women (69%). The most common mental health disorder was substance use disorder (44%), followed by antisocial personality disorder (26%), major depression (19%), bipolar disorder (8%), and schizophrenia (7%).
The prevalence of current and lifetime mental health disorders among the homeless population was higher than that that observed in the general population (13%-15% and 12%-47%, respectively).
The results resembled those of a previous review that estimated that 76% of people experiencing homelessness living in high-income countries have mental health disorders.
“Even though our results are not surprising, they still are drawing attention to this issue because it is a big problem in Canada, the United States, Europe, and other places,” senior author Dallas Seitz, MD, PhD, professor of psychiatry at the University of Calgary’s Cumming School of Medicine, told this news organization. “The problem is concerning, and it’s not getting better. Addiction and mental health problems are becoming more common among people who are homeless.”
The bottom line is that people need affordable housing and mental health support, said Dr. Seitz. “It’s a housing problem and a health problem, and we need adequate resources to find better ways for those two systems to collaborate. There are public safety concerns, and we have to try and bring services to people experiencing homelessness. You have to come and meet people where they’re at. You have to try and establish a trusting relationship so that we can get people on the path to recovery.”
‘It’s Really About Income’
Commenting on the findings for this news organization, Stephen Hwang, MD, professor of medicine at the University of Toronto, Toronto, Ontario, Canada, said, “There have been previous studies of this type, but it is good to have an updated one.” Dr. Hwang, who is also chair in Homelessness, Housing, and Health at St. Michael’s Hospital, did not participate in the research.
The findings must be understood in the proper context, he added. For one thing, grouping together all mental health disorders and giving a single prevalence figure can be misleading. “They are including in that category a diverse group of conditions. Substance use disorder, personality disorder, schizophrenia, and depression are all lumped together. The 67% prevalence seems very high, but it is a combination of many different conditions. I just don’t want people to look at that number and think that this means that everyone is a substance user or everyone has schizophrenia,” said Dr. Hwang.
Also, some readers might interpret the findings to mean that mental problems are the reason people are homeless, he added. “That would be an incorrect interpretation because what this study is showing is that people with mental health disorders have a higher risk for becoming homeless. It doesn’t mean that it caused their homelessness. What really causes homelessness is a lack of affordable housing,” said Dr. Hwang.
“In a city or community where housing is very expensive, there’s not enough for everyone to be housed, there is a lot of competition for housing, and there’s not enough affordable housing for a number of reasons, we know that people with mental health conditions and substance use disorders will be among the first to lose their housing,” he said.
“It’s really about income. There are many reasons why a person cannot afford housing. So, not being able to earn enough money to afford it because you have a mental health disorder or substance use disorder is a common underlying reason for homelessness.”
Dr. Hwang also pointed out that people with mental illness who can access support, either through family members or through mental health care, and who also have the income to afford such services do not become homeless.
“Schizophrenia is seen in every population of the world at a rate of 1%. But you travel to certain cities and you see people who appear to have schizophrenia wandering the streets, and you go to other cities in the world and you don’t see anyone who looks like they’re homeless and have schizophrenia,” he said.
“It’s not because there are fewer people with schizophrenia in those cities or countries; it’s because people with schizophrenia are treated differently. The rate of homelessness is determined not by how many people have that condition [eg, schizophrenia] but by how we treat those people and how we set up our society to either support or not support people who have disabilities.”
The study was funded by the Precision Care With Information, Science and Experience – Mental Health grant funded by the Calgary Health Foundation. Dr. Barry is supported by the Harley Hotchkiss Samuel Weiss Postdoctoral Fellowship awarded by the Hotchkiss Brain Institute at the University of Calgary. Dr. Barry reported having no relevant financial relationships. Dr. Seitz reported grants from Calgary Health Foundation during the conduct of the study as well as grants from University Health Foundation, the Canadian Institutes of Health Research, the Public Health Agency of Canada, the Alzheimer’s Association, and the Hotchkiss Brain Institute. He received honoraria for guideline development from the Canadian Coalition for Seniors Mental Health outside the submitted work. Dr. Hwang reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Hereditary Amyloidosis: 5 Things to Know
Amyloidosis is a condition marked by the accumulation of insoluble beta-sheet fibrillar protein aggregates in tissues that can be acquired or hereditary. Hereditary amyloidogenic transthyretin (hATTR) amyloidosis is an autosomal-dominant disease caused by pathogenic variants in the TTR gene. The TTR protein is essential for transporting thyroxine and retinol-binding protein and is primarily synthesized in the liver, becoming unstable as a result of the pathogenic mutations. Inherited pathogenic variants lead to the protein’s misfolding, aggregation, and deposition as amyloid fibrils in different organs, resulting in progressive multisystem dysfunction. hATTR amyloidosis is a heterogenous disease, characterized by a wide range of clinical manifestations affecting the peripheral (both somatic and autonomic) nervous system, heart, kidneys, and central nervous system (CNS); however, the heart and peripheral nerves appear to be the main targets of the TTR-related pathologic process. Without treatment, the prognosis is poor, with an average life expectancy of 7-11 years; however, in recent years, the development of new therapeutics has brought new hope to patients.
Here are five things to know about hereditary amyloidosis.
1. Diagnosis of hereditary amyloidosis requires a high level of suspicion.
The diagnosis of hATTR amyloidosis presents a significant challenge, particularly in nonendemic regions where a lack of family history and heterogeneity of clinical presentation can delay diagnosis by 4-5 years. A timely diagnosis requires clinicians to maintain a high index of suspicion, especially when evaluating patients with neuropathic symptoms. Early diagnosis is crucial to begin patients on recently available disease-modifying therapies that can slow the disease course. Failure to recognize is the major barrier to improved patient outcomes.
Confirming the diagnosis involves detecting amyloid deposits in tissue biopsy specimens from various possible sites, including the skin, nerves, myocardium, and others. However, the diagnosis can be challenging owing to the uneven distribution of amyloid fibrils, sometimes requiring multiple biopsies or alternative diagnostic approaches, such as TTR gene sequencing, to confirm the presence of an amyloidogenic pathogenic variant. Biopsy for hATTR amyloidosis is not required if imaging of the clinical phenotype and genetic testing are consistent.
Once diagnosed, the assessment of organ involvement is essential, using nerve conduction studies, cardiac investigations (eg, echocardiography, ECG, scintigraphy), ophthalmologic assessments, and complete renal function evaluations to fully understand the extent of disease impact.
2. Hereditary amyloidosis diseases are classified into two primary categories.
Hereditary amyloidosis represents a group of diseases caused by inherited gene mutations and is classified into two main types: ATTR (transthyretin-related) and non-TTR. Most cases of hereditary amyloidosis are associated with the TTR gene. Mutations in this protein lead to different forms of ATTR amyloidosis, categorized on the basis of the specific mutation involved, such as hATTR50M (genotype Val50Met), which is the most prevalent form.
ATTR mutations result in a variety of health issues, manifesting in three primary forms:
- Neuropathic ATTR (genotype Val50Met): Early symptoms include sensorimotor polyneuropathy of the legs, carpal tunnel syndrome, autonomic dysfunction, constipation/diarrhea, and impotence; late symptoms include cardiomyopathy, vitreous opacities, glaucoma, nephropathy, and CNS symptoms.
- Cardiac ATTR (genotype Val142Ile): This type is characterized by cardiomegaly, conduction block, arrhythmia, anginal pain, congestive heart failure, and sudden death.
- Leptomeningeal ATTR (genotype Asp38Gly): This is characterized by transient focal neurologic episodes, intracerebral and/or subarachnoid hemorrhages, dementia, ataxia, and psychosis.
Non-TTR amyloidoses are rarer than are ATTR variations and involve mutations in different genes that also have significant health impacts. These include proteins such as apolipoprotein AI, fibrinogen A alpha, lysozyme, apolipoprotein AII, gelsolin, and cystatin C. Each type contributes to a range of symptoms and requires individualized management approaches.
3. Heightened disease awareness has increased the recognized prevalence of hereditary amyloidosis.
hATTR amyloidosis has historically been recognized as a rare disease, with significant clusters in Portugal, Brazil, Sweden, and Japan and alongside smaller foci in regions such as Cyprus and Majorca. This disease›s variable incidence across Europe is now perceived to be on the rise. It is attributed to heightened disease awareness among healthcare providers and the broader availability of genetic testing, extending its recognized impact to at least 29 countries globally. The genetic landscape of hATTR amyloidosis is diverse, with over 140 mutations identified in the TTR gene. Among these, the Val50Met mutation is particularly notable for its association with large patient clusters in the endemic regions.
Morbidity and mortality associated with hATTR amyloidosis are significant, with an average lifespan of 7-11 years post diagnosis; however, survival rates can vary widely depending on the specific genetic variant and organ involvement. Early diagnosis can substantially improve outcomes; yet, for many, the prognosis remains poor, especially in cases dominated by cardiomyopathy. Genetics play a central role in the disease›s transmission, with autosomal-dominant inheritance patterns and high penetrance among carriers of pathogenic mutations. Research continues to uncover the broad spectrum of genetic variations contributing to hATTR amyloidosis, with ongoing studies poised to expand our understanding of its molecular underpinnings and potential treatment options.
4. The effect on quality of life is significant both in patients living with hATTR amyloidosis and their caregivers.
hATTR amyloidosis imposes a multifaceted burden on patients and their caregivers as the disease progresses. Symptoms range from sensorimotor impairment and gastrointestinal or autonomic dysfunction to heart failure, leading to significant health-related quality-of-life deficits. The systemic nature of hATTR amyloidosis significantly affects patients› lifestyles, daily activities, and general well-being, especially because it typically manifests in adulthood — a crucial time for occupational changes. The progression of hATTR amyloidosis exacerbates the challenges in maintaining employment and managing household chores, with symptomatic patients often unable to work and experiencing difficulties with absenteeism and presenteeism when they are able to work.
hATTR amyloidosis leads to physical, mental, occupational, and social limitations for patients, and it also places a considerable strain on their families and caregivers, who report poor mental health, work impairment, and a high time commitment (mean, 45.9 h/wk) to providing care.
5. There have been significant advancements in therapeutic options for early-stage hATTR amyloidosis.
After diagnosis, prompt initiation of treatment is recommended to delay the progression of hATTR amyloidosis; a multidisciplinary approach is essential, incorporating anti-amyloid therapy to inhibit further production and/or deposition of amyloid aggregates. Treatment strategies also include addressing symptomatic therapy and managing cardiac, renal, and ocular involvement. Although many therapies have been developed, especially for the early stages of hATTR amyloidosis, therapeutic benefits for patients with advanced disease remain limited.
Recent advancements in the treatment of hATTR amyloidosis have introduced RNA-targeted therapies including patisiran, vutrisiran, and eplontersen, which have shown efficacy in reducing hepatic TTR synthesis and the aggregation of misfolded monomers into amyloid deposits. These therapies, ranging from small interfering RNA formulations to antisense oligonucleotides, offer benefits in managing both cardiomyopathy and neuropathy associated with hATTR amyloidosis , administered through various methods, including intravenous infusions and subcutaneous injections. In addition, the stabilization of TTR tetramers with the use of drugs such as tafamidis and diflunisal has effectively prevented the formation of amyloidogenic monomers. Moreover, other investigational agents, including TTR stabilizers like acoramidis and tolcapone, as well as novel compounds that inhibit amyloid formation and disrupt fibrils, are expanding the therapeutic landscape for hATTR amyloidosis , providing hope for improved management of this complex condition.
Dr. Gertz is a professor and consultant in the Department of Hematology, Mayo Clinic, Rochester, Minnesota. He has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from AstraZeneca, Ionis, and Alnylym.
A version of this article appeared on Medscape.com.
Amyloidosis is a condition marked by the accumulation of insoluble beta-sheet fibrillar protein aggregates in tissues that can be acquired or hereditary. Hereditary amyloidogenic transthyretin (hATTR) amyloidosis is an autosomal-dominant disease caused by pathogenic variants in the TTR gene. The TTR protein is essential for transporting thyroxine and retinol-binding protein and is primarily synthesized in the liver, becoming unstable as a result of the pathogenic mutations. Inherited pathogenic variants lead to the protein’s misfolding, aggregation, and deposition as amyloid fibrils in different organs, resulting in progressive multisystem dysfunction. hATTR amyloidosis is a heterogenous disease, characterized by a wide range of clinical manifestations affecting the peripheral (both somatic and autonomic) nervous system, heart, kidneys, and central nervous system (CNS); however, the heart and peripheral nerves appear to be the main targets of the TTR-related pathologic process. Without treatment, the prognosis is poor, with an average life expectancy of 7-11 years; however, in recent years, the development of new therapeutics has brought new hope to patients.
Here are five things to know about hereditary amyloidosis.
1. Diagnosis of hereditary amyloidosis requires a high level of suspicion.
The diagnosis of hATTR amyloidosis presents a significant challenge, particularly in nonendemic regions where a lack of family history and heterogeneity of clinical presentation can delay diagnosis by 4-5 years. A timely diagnosis requires clinicians to maintain a high index of suspicion, especially when evaluating patients with neuropathic symptoms. Early diagnosis is crucial to begin patients on recently available disease-modifying therapies that can slow the disease course. Failure to recognize is the major barrier to improved patient outcomes.
Confirming the diagnosis involves detecting amyloid deposits in tissue biopsy specimens from various possible sites, including the skin, nerves, myocardium, and others. However, the diagnosis can be challenging owing to the uneven distribution of amyloid fibrils, sometimes requiring multiple biopsies or alternative diagnostic approaches, such as TTR gene sequencing, to confirm the presence of an amyloidogenic pathogenic variant. Biopsy for hATTR amyloidosis is not required if imaging of the clinical phenotype and genetic testing are consistent.
Once diagnosed, the assessment of organ involvement is essential, using nerve conduction studies, cardiac investigations (eg, echocardiography, ECG, scintigraphy), ophthalmologic assessments, and complete renal function evaluations to fully understand the extent of disease impact.
2. Hereditary amyloidosis diseases are classified into two primary categories.
Hereditary amyloidosis represents a group of diseases caused by inherited gene mutations and is classified into two main types: ATTR (transthyretin-related) and non-TTR. Most cases of hereditary amyloidosis are associated with the TTR gene. Mutations in this protein lead to different forms of ATTR amyloidosis, categorized on the basis of the specific mutation involved, such as hATTR50M (genotype Val50Met), which is the most prevalent form.
ATTR mutations result in a variety of health issues, manifesting in three primary forms:
- Neuropathic ATTR (genotype Val50Met): Early symptoms include sensorimotor polyneuropathy of the legs, carpal tunnel syndrome, autonomic dysfunction, constipation/diarrhea, and impotence; late symptoms include cardiomyopathy, vitreous opacities, glaucoma, nephropathy, and CNS symptoms.
- Cardiac ATTR (genotype Val142Ile): This type is characterized by cardiomegaly, conduction block, arrhythmia, anginal pain, congestive heart failure, and sudden death.
- Leptomeningeal ATTR (genotype Asp38Gly): This is characterized by transient focal neurologic episodes, intracerebral and/or subarachnoid hemorrhages, dementia, ataxia, and psychosis.
Non-TTR amyloidoses are rarer than are ATTR variations and involve mutations in different genes that also have significant health impacts. These include proteins such as apolipoprotein AI, fibrinogen A alpha, lysozyme, apolipoprotein AII, gelsolin, and cystatin C. Each type contributes to a range of symptoms and requires individualized management approaches.
3. Heightened disease awareness has increased the recognized prevalence of hereditary amyloidosis.
hATTR amyloidosis has historically been recognized as a rare disease, with significant clusters in Portugal, Brazil, Sweden, and Japan and alongside smaller foci in regions such as Cyprus and Majorca. This disease›s variable incidence across Europe is now perceived to be on the rise. It is attributed to heightened disease awareness among healthcare providers and the broader availability of genetic testing, extending its recognized impact to at least 29 countries globally. The genetic landscape of hATTR amyloidosis is diverse, with over 140 mutations identified in the TTR gene. Among these, the Val50Met mutation is particularly notable for its association with large patient clusters in the endemic regions.
Morbidity and mortality associated with hATTR amyloidosis are significant, with an average lifespan of 7-11 years post diagnosis; however, survival rates can vary widely depending on the specific genetic variant and organ involvement. Early diagnosis can substantially improve outcomes; yet, for many, the prognosis remains poor, especially in cases dominated by cardiomyopathy. Genetics play a central role in the disease›s transmission, with autosomal-dominant inheritance patterns and high penetrance among carriers of pathogenic mutations. Research continues to uncover the broad spectrum of genetic variations contributing to hATTR amyloidosis, with ongoing studies poised to expand our understanding of its molecular underpinnings and potential treatment options.
4. The effect on quality of life is significant both in patients living with hATTR amyloidosis and their caregivers.
hATTR amyloidosis imposes a multifaceted burden on patients and their caregivers as the disease progresses. Symptoms range from sensorimotor impairment and gastrointestinal or autonomic dysfunction to heart failure, leading to significant health-related quality-of-life deficits. The systemic nature of hATTR amyloidosis significantly affects patients› lifestyles, daily activities, and general well-being, especially because it typically manifests in adulthood — a crucial time for occupational changes. The progression of hATTR amyloidosis exacerbates the challenges in maintaining employment and managing household chores, with symptomatic patients often unable to work and experiencing difficulties with absenteeism and presenteeism when they are able to work.
hATTR amyloidosis leads to physical, mental, occupational, and social limitations for patients, and it also places a considerable strain on their families and caregivers, who report poor mental health, work impairment, and a high time commitment (mean, 45.9 h/wk) to providing care.
5. There have been significant advancements in therapeutic options for early-stage hATTR amyloidosis.
After diagnosis, prompt initiation of treatment is recommended to delay the progression of hATTR amyloidosis; a multidisciplinary approach is essential, incorporating anti-amyloid therapy to inhibit further production and/or deposition of amyloid aggregates. Treatment strategies also include addressing symptomatic therapy and managing cardiac, renal, and ocular involvement. Although many therapies have been developed, especially for the early stages of hATTR amyloidosis, therapeutic benefits for patients with advanced disease remain limited.
Recent advancements in the treatment of hATTR amyloidosis have introduced RNA-targeted therapies including patisiran, vutrisiran, and eplontersen, which have shown efficacy in reducing hepatic TTR synthesis and the aggregation of misfolded monomers into amyloid deposits. These therapies, ranging from small interfering RNA formulations to antisense oligonucleotides, offer benefits in managing both cardiomyopathy and neuropathy associated with hATTR amyloidosis , administered through various methods, including intravenous infusions and subcutaneous injections. In addition, the stabilization of TTR tetramers with the use of drugs such as tafamidis and diflunisal has effectively prevented the formation of amyloidogenic monomers. Moreover, other investigational agents, including TTR stabilizers like acoramidis and tolcapone, as well as novel compounds that inhibit amyloid formation and disrupt fibrils, are expanding the therapeutic landscape for hATTR amyloidosis , providing hope for improved management of this complex condition.
Dr. Gertz is a professor and consultant in the Department of Hematology, Mayo Clinic, Rochester, Minnesota. He has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from AstraZeneca, Ionis, and Alnylym.
A version of this article appeared on Medscape.com.
Amyloidosis is a condition marked by the accumulation of insoluble beta-sheet fibrillar protein aggregates in tissues that can be acquired or hereditary. Hereditary amyloidogenic transthyretin (hATTR) amyloidosis is an autosomal-dominant disease caused by pathogenic variants in the TTR gene. The TTR protein is essential for transporting thyroxine and retinol-binding protein and is primarily synthesized in the liver, becoming unstable as a result of the pathogenic mutations. Inherited pathogenic variants lead to the protein’s misfolding, aggregation, and deposition as amyloid fibrils in different organs, resulting in progressive multisystem dysfunction. hATTR amyloidosis is a heterogenous disease, characterized by a wide range of clinical manifestations affecting the peripheral (both somatic and autonomic) nervous system, heart, kidneys, and central nervous system (CNS); however, the heart and peripheral nerves appear to be the main targets of the TTR-related pathologic process. Without treatment, the prognosis is poor, with an average life expectancy of 7-11 years; however, in recent years, the development of new therapeutics has brought new hope to patients.
Here are five things to know about hereditary amyloidosis.
1. Diagnosis of hereditary amyloidosis requires a high level of suspicion.
The diagnosis of hATTR amyloidosis presents a significant challenge, particularly in nonendemic regions where a lack of family history and heterogeneity of clinical presentation can delay diagnosis by 4-5 years. A timely diagnosis requires clinicians to maintain a high index of suspicion, especially when evaluating patients with neuropathic symptoms. Early diagnosis is crucial to begin patients on recently available disease-modifying therapies that can slow the disease course. Failure to recognize is the major barrier to improved patient outcomes.
Confirming the diagnosis involves detecting amyloid deposits in tissue biopsy specimens from various possible sites, including the skin, nerves, myocardium, and others. However, the diagnosis can be challenging owing to the uneven distribution of amyloid fibrils, sometimes requiring multiple biopsies or alternative diagnostic approaches, such as TTR gene sequencing, to confirm the presence of an amyloidogenic pathogenic variant. Biopsy for hATTR amyloidosis is not required if imaging of the clinical phenotype and genetic testing are consistent.
Once diagnosed, the assessment of organ involvement is essential, using nerve conduction studies, cardiac investigations (eg, echocardiography, ECG, scintigraphy), ophthalmologic assessments, and complete renal function evaluations to fully understand the extent of disease impact.
2. Hereditary amyloidosis diseases are classified into two primary categories.
Hereditary amyloidosis represents a group of diseases caused by inherited gene mutations and is classified into two main types: ATTR (transthyretin-related) and non-TTR. Most cases of hereditary amyloidosis are associated with the TTR gene. Mutations in this protein lead to different forms of ATTR amyloidosis, categorized on the basis of the specific mutation involved, such as hATTR50M (genotype Val50Met), which is the most prevalent form.
ATTR mutations result in a variety of health issues, manifesting in three primary forms:
- Neuropathic ATTR (genotype Val50Met): Early symptoms include sensorimotor polyneuropathy of the legs, carpal tunnel syndrome, autonomic dysfunction, constipation/diarrhea, and impotence; late symptoms include cardiomyopathy, vitreous opacities, glaucoma, nephropathy, and CNS symptoms.
- Cardiac ATTR (genotype Val142Ile): This type is characterized by cardiomegaly, conduction block, arrhythmia, anginal pain, congestive heart failure, and sudden death.
- Leptomeningeal ATTR (genotype Asp38Gly): This is characterized by transient focal neurologic episodes, intracerebral and/or subarachnoid hemorrhages, dementia, ataxia, and psychosis.
Non-TTR amyloidoses are rarer than are ATTR variations and involve mutations in different genes that also have significant health impacts. These include proteins such as apolipoprotein AI, fibrinogen A alpha, lysozyme, apolipoprotein AII, gelsolin, and cystatin C. Each type contributes to a range of symptoms and requires individualized management approaches.
3. Heightened disease awareness has increased the recognized prevalence of hereditary amyloidosis.
hATTR amyloidosis has historically been recognized as a rare disease, with significant clusters in Portugal, Brazil, Sweden, and Japan and alongside smaller foci in regions such as Cyprus and Majorca. This disease›s variable incidence across Europe is now perceived to be on the rise. It is attributed to heightened disease awareness among healthcare providers and the broader availability of genetic testing, extending its recognized impact to at least 29 countries globally. The genetic landscape of hATTR amyloidosis is diverse, with over 140 mutations identified in the TTR gene. Among these, the Val50Met mutation is particularly notable for its association with large patient clusters in the endemic regions.
Morbidity and mortality associated with hATTR amyloidosis are significant, with an average lifespan of 7-11 years post diagnosis; however, survival rates can vary widely depending on the specific genetic variant and organ involvement. Early diagnosis can substantially improve outcomes; yet, for many, the prognosis remains poor, especially in cases dominated by cardiomyopathy. Genetics play a central role in the disease›s transmission, with autosomal-dominant inheritance patterns and high penetrance among carriers of pathogenic mutations. Research continues to uncover the broad spectrum of genetic variations contributing to hATTR amyloidosis, with ongoing studies poised to expand our understanding of its molecular underpinnings and potential treatment options.
4. The effect on quality of life is significant both in patients living with hATTR amyloidosis and their caregivers.
hATTR amyloidosis imposes a multifaceted burden on patients and their caregivers as the disease progresses. Symptoms range from sensorimotor impairment and gastrointestinal or autonomic dysfunction to heart failure, leading to significant health-related quality-of-life deficits. The systemic nature of hATTR amyloidosis significantly affects patients› lifestyles, daily activities, and general well-being, especially because it typically manifests in adulthood — a crucial time for occupational changes. The progression of hATTR amyloidosis exacerbates the challenges in maintaining employment and managing household chores, with symptomatic patients often unable to work and experiencing difficulties with absenteeism and presenteeism when they are able to work.
hATTR amyloidosis leads to physical, mental, occupational, and social limitations for patients, and it also places a considerable strain on their families and caregivers, who report poor mental health, work impairment, and a high time commitment (mean, 45.9 h/wk) to providing care.
5. There have been significant advancements in therapeutic options for early-stage hATTR amyloidosis.
After diagnosis, prompt initiation of treatment is recommended to delay the progression of hATTR amyloidosis; a multidisciplinary approach is essential, incorporating anti-amyloid therapy to inhibit further production and/or deposition of amyloid aggregates. Treatment strategies also include addressing symptomatic therapy and managing cardiac, renal, and ocular involvement. Although many therapies have been developed, especially for the early stages of hATTR amyloidosis, therapeutic benefits for patients with advanced disease remain limited.
Recent advancements in the treatment of hATTR amyloidosis have introduced RNA-targeted therapies including patisiran, vutrisiran, and eplontersen, which have shown efficacy in reducing hepatic TTR synthesis and the aggregation of misfolded monomers into amyloid deposits. These therapies, ranging from small interfering RNA formulations to antisense oligonucleotides, offer benefits in managing both cardiomyopathy and neuropathy associated with hATTR amyloidosis , administered through various methods, including intravenous infusions and subcutaneous injections. In addition, the stabilization of TTR tetramers with the use of drugs such as tafamidis and diflunisal has effectively prevented the formation of amyloidogenic monomers. Moreover, other investigational agents, including TTR stabilizers like acoramidis and tolcapone, as well as novel compounds that inhibit amyloid formation and disrupt fibrils, are expanding the therapeutic landscape for hATTR amyloidosis , providing hope for improved management of this complex condition.
Dr. Gertz is a professor and consultant in the Department of Hematology, Mayo Clinic, Rochester, Minnesota. He has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from AstraZeneca, Ionis, and Alnylym.
A version of this article appeared on Medscape.com.
FDA Allows Implantable CGM to Integrate With Insulin Pumps
The US Food and Drug Administration (FDA) has designated the Eversense (Sensionics, Inc; Ascencia Diabetes Care) implanted continuous glucose monitor (CGM) an “integrated CGM,” meaning it can be used in conjunction with insulin pumps as part of an automated insulin delivery system (AID).
The Eversense now joins Dexcom’s G6 and G7 and the Freestyle Libre 2 Plus in being compatible with multiple different branded insulin pumps as part of AID systems, and it is the only implantable one.
The sensor device is inserted under the skin of the patient’s upper arm by a healthcare provider and a transmitter is worn over it on the skin. The FDA approved the Eversense in June 2018 for 3-month use and in February 2022 for use up to 6 months. It is indicated for people with diabetes aged 18 years and older.
Fingerstick blood glucose measurements are still required for calibration once a day after day 21, when symptoms don’t match the CGM information, or when taking tetracycline medications.
According to Sensionics, the Eversense is “the most accurate CGM in the critical low glucose ranges with essentially no compression lows.” The latter refers to ‘false low’ alarms that sometimes occur when a person presses on the device, such as during sleep.
“As we look ahead, we are focused on progressing our partnership discussions and software developments, and look forward to providing more updates,” Sensionics said in a statement.
A version of this article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has designated the Eversense (Sensionics, Inc; Ascencia Diabetes Care) implanted continuous glucose monitor (CGM) an “integrated CGM,” meaning it can be used in conjunction with insulin pumps as part of an automated insulin delivery system (AID).
The Eversense now joins Dexcom’s G6 and G7 and the Freestyle Libre 2 Plus in being compatible with multiple different branded insulin pumps as part of AID systems, and it is the only implantable one.
The sensor device is inserted under the skin of the patient’s upper arm by a healthcare provider and a transmitter is worn over it on the skin. The FDA approved the Eversense in June 2018 for 3-month use and in February 2022 for use up to 6 months. It is indicated for people with diabetes aged 18 years and older.
Fingerstick blood glucose measurements are still required for calibration once a day after day 21, when symptoms don’t match the CGM information, or when taking tetracycline medications.
According to Sensionics, the Eversense is “the most accurate CGM in the critical low glucose ranges with essentially no compression lows.” The latter refers to ‘false low’ alarms that sometimes occur when a person presses on the device, such as during sleep.
“As we look ahead, we are focused on progressing our partnership discussions and software developments, and look forward to providing more updates,” Sensionics said in a statement.
A version of this article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has designated the Eversense (Sensionics, Inc; Ascencia Diabetes Care) implanted continuous glucose monitor (CGM) an “integrated CGM,” meaning it can be used in conjunction with insulin pumps as part of an automated insulin delivery system (AID).
The Eversense now joins Dexcom’s G6 and G7 and the Freestyle Libre 2 Plus in being compatible with multiple different branded insulin pumps as part of AID systems, and it is the only implantable one.
The sensor device is inserted under the skin of the patient’s upper arm by a healthcare provider and a transmitter is worn over it on the skin. The FDA approved the Eversense in June 2018 for 3-month use and in February 2022 for use up to 6 months. It is indicated for people with diabetes aged 18 years and older.
Fingerstick blood glucose measurements are still required for calibration once a day after day 21, when symptoms don’t match the CGM information, or when taking tetracycline medications.
According to Sensionics, the Eversense is “the most accurate CGM in the critical low glucose ranges with essentially no compression lows.” The latter refers to ‘false low’ alarms that sometimes occur when a person presses on the device, such as during sleep.
“As we look ahead, we are focused on progressing our partnership discussions and software developments, and look forward to providing more updates,” Sensionics said in a statement.
A version of this article first appeared on Medscape.com.
What Underlies Sex Differences in CKD Cardiovascular Risk?
Older men with chronic kidney disease (CKD) show higher resting muscle sympathetic nerve activity, but not vascular stiffness, compared with older women, offering clues to the underlying reasons why men with CKD have a higher cardiovascular risk than do women with the disease.
“Although it is well established that sympathetic nerve system activity is chronically elevated in patients with impaired kidney function, we show for the first time that males with CKD have higher resting muscle sympathetic nerve activity compared with females with CKD,” report the authors on research published in the American Journal of Physiology-Renal Physiology.
“For clinicians, the key takeaway is the importance of recognizing sex-specific differences in sympathetic activity and vascular function when assessing cardiovascular risk in CKD patients,” first author Matias G. Zanuzzi, MD, of the Division of Renal Medicine, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, told this news organization.
In the general population, cardiovascular risk is lower in younger women vs men, but their risks converge in older age as women develop similar levels of sympathetic overactivity, vascular stiffness, and cardiovascular risk.
However, an exception to that pattern is seen in the CKD population, where men continue to have a higher cardiovascular mortality risk vs women even in older age.
Studies evaluating the reasons for that have been conflicting, with some reporting a tendency of higher muscle sympathetic nerve activity in older women compared with men and others suggest the opposite finding — lower activity vs men.
To further investigate, Dr. Zanuzzi and colleagues enrolled 129 participants, including 96 men and 33 women with stage III or IV CKD.
The mean age of the study participants was 64 years for men and65 years for women. Most had obesity, and importantly, more than 80% of participants in each group was Black. There were no significant differences between the groups in terms of body mass index or comorbidities, including smoking, diabetes, or hypertension.
At two separate study visits, vascular stiffness was assessed with carotid-femoral pulse wave velocity measurement, and resting muscle sympathetic nerve activity was measured using microneurography.
The results showed that men with CKD had significantly higher resting muscle sympathetic nerve activity compared with women with CKD (68 vs 55 bursts per 100 heartbeats; P = .005), whereas no differences in vascular stiffness were observed between the genders (P = .248).
“The findings suggest that the higher cardiovascular disease risk observed in older males with CKD may be influenced by elevated sympathetic activity,” Dr. Zanuzzi explained.
“However, the lack of significant differences in vascular stiffness between genders implies that additional factors beyond vascular remodeling may contribute to the observed sex-specific differences in cardiovascular risk,” he said.
Of note, resting vascular stiffness was not associated with muscular sympathetic nerve activity in either men or women, which was surprising to the authors, Dr. Zanuzzi noted.
“This underscores the multifactorial nature of vascular pathophysiology in CKD and underscores the need for further research to unravel the underlying mechanisms.”
In other findings, although prior studies have shown a positive correlation between age and resting muscle sympathetic nerve activity in White, healthy women and men without obesity,, no similar relationship was observed in men or women with CKD.
“These findings suggest that the protective effect of younger age on sympathetic function may not be present in the setting of decreased kidney function in both males and females,” the authors note.
In addition, whereas previous research has shown a clear association between sympathetic overactivity and a wide variety of measures of obesity, in the current study, that association was only observed in men with CKD.
Important limitations of the study include the cross-sectional design and that the population was predominantly Black, Dr. Zanuzzi noted.
“Generalizability to other demographic groups may be limited, and future longitudinal studies are needed to validate these findings and explore potential causal relationships,” he said.
The findings underscore “the need for novel therapeutic approaches targeting sympathetic overactivity and vascular stiffness in CKD patients, especially considering the observed sex-specific differences,” Dr. Zanuzzi added.
“Potential interventions may include pharmacological agents that modulate sympathetic tone or vascular remodeling pathways,” he said.
“Lifestyle modifications focusing on stress reduction and cardiovascular health promotion could also play a crucial role in mitigating cardiovascular risk.”
Dr. Zanuzzi concluded that “tailoring treatment strategies to address these differences may lead to more personalized and effective management approaches, ultimately improving clinical outcomes in this high-risk population.”
The authors had no disclosures to report.
A version of this article first appeared on Medscape.com.
Older men with chronic kidney disease (CKD) show higher resting muscle sympathetic nerve activity, but not vascular stiffness, compared with older women, offering clues to the underlying reasons why men with CKD have a higher cardiovascular risk than do women with the disease.
“Although it is well established that sympathetic nerve system activity is chronically elevated in patients with impaired kidney function, we show for the first time that males with CKD have higher resting muscle sympathetic nerve activity compared with females with CKD,” report the authors on research published in the American Journal of Physiology-Renal Physiology.
“For clinicians, the key takeaway is the importance of recognizing sex-specific differences in sympathetic activity and vascular function when assessing cardiovascular risk in CKD patients,” first author Matias G. Zanuzzi, MD, of the Division of Renal Medicine, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, told this news organization.
In the general population, cardiovascular risk is lower in younger women vs men, but their risks converge in older age as women develop similar levels of sympathetic overactivity, vascular stiffness, and cardiovascular risk.
However, an exception to that pattern is seen in the CKD population, where men continue to have a higher cardiovascular mortality risk vs women even in older age.
Studies evaluating the reasons for that have been conflicting, with some reporting a tendency of higher muscle sympathetic nerve activity in older women compared with men and others suggest the opposite finding — lower activity vs men.
To further investigate, Dr. Zanuzzi and colleagues enrolled 129 participants, including 96 men and 33 women with stage III or IV CKD.
The mean age of the study participants was 64 years for men and65 years for women. Most had obesity, and importantly, more than 80% of participants in each group was Black. There were no significant differences between the groups in terms of body mass index or comorbidities, including smoking, diabetes, or hypertension.
At two separate study visits, vascular stiffness was assessed with carotid-femoral pulse wave velocity measurement, and resting muscle sympathetic nerve activity was measured using microneurography.
The results showed that men with CKD had significantly higher resting muscle sympathetic nerve activity compared with women with CKD (68 vs 55 bursts per 100 heartbeats; P = .005), whereas no differences in vascular stiffness were observed between the genders (P = .248).
“The findings suggest that the higher cardiovascular disease risk observed in older males with CKD may be influenced by elevated sympathetic activity,” Dr. Zanuzzi explained.
“However, the lack of significant differences in vascular stiffness between genders implies that additional factors beyond vascular remodeling may contribute to the observed sex-specific differences in cardiovascular risk,” he said.
Of note, resting vascular stiffness was not associated with muscular sympathetic nerve activity in either men or women, which was surprising to the authors, Dr. Zanuzzi noted.
“This underscores the multifactorial nature of vascular pathophysiology in CKD and underscores the need for further research to unravel the underlying mechanisms.”
In other findings, although prior studies have shown a positive correlation between age and resting muscle sympathetic nerve activity in White, healthy women and men without obesity,, no similar relationship was observed in men or women with CKD.
“These findings suggest that the protective effect of younger age on sympathetic function may not be present in the setting of decreased kidney function in both males and females,” the authors note.
In addition, whereas previous research has shown a clear association between sympathetic overactivity and a wide variety of measures of obesity, in the current study, that association was only observed in men with CKD.
Important limitations of the study include the cross-sectional design and that the population was predominantly Black, Dr. Zanuzzi noted.
“Generalizability to other demographic groups may be limited, and future longitudinal studies are needed to validate these findings and explore potential causal relationships,” he said.
The findings underscore “the need for novel therapeutic approaches targeting sympathetic overactivity and vascular stiffness in CKD patients, especially considering the observed sex-specific differences,” Dr. Zanuzzi added.
“Potential interventions may include pharmacological agents that modulate sympathetic tone or vascular remodeling pathways,” he said.
“Lifestyle modifications focusing on stress reduction and cardiovascular health promotion could also play a crucial role in mitigating cardiovascular risk.”
Dr. Zanuzzi concluded that “tailoring treatment strategies to address these differences may lead to more personalized and effective management approaches, ultimately improving clinical outcomes in this high-risk population.”
The authors had no disclosures to report.
A version of this article first appeared on Medscape.com.
Older men with chronic kidney disease (CKD) show higher resting muscle sympathetic nerve activity, but not vascular stiffness, compared with older women, offering clues to the underlying reasons why men with CKD have a higher cardiovascular risk than do women with the disease.
“Although it is well established that sympathetic nerve system activity is chronically elevated in patients with impaired kidney function, we show for the first time that males with CKD have higher resting muscle sympathetic nerve activity compared with females with CKD,” report the authors on research published in the American Journal of Physiology-Renal Physiology.
“For clinicians, the key takeaway is the importance of recognizing sex-specific differences in sympathetic activity and vascular function when assessing cardiovascular risk in CKD patients,” first author Matias G. Zanuzzi, MD, of the Division of Renal Medicine, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, told this news organization.
In the general population, cardiovascular risk is lower in younger women vs men, but their risks converge in older age as women develop similar levels of sympathetic overactivity, vascular stiffness, and cardiovascular risk.
However, an exception to that pattern is seen in the CKD population, where men continue to have a higher cardiovascular mortality risk vs women even in older age.
Studies evaluating the reasons for that have been conflicting, with some reporting a tendency of higher muscle sympathetic nerve activity in older women compared with men and others suggest the opposite finding — lower activity vs men.
To further investigate, Dr. Zanuzzi and colleagues enrolled 129 participants, including 96 men and 33 women with stage III or IV CKD.
The mean age of the study participants was 64 years for men and65 years for women. Most had obesity, and importantly, more than 80% of participants in each group was Black. There were no significant differences between the groups in terms of body mass index or comorbidities, including smoking, diabetes, or hypertension.
At two separate study visits, vascular stiffness was assessed with carotid-femoral pulse wave velocity measurement, and resting muscle sympathetic nerve activity was measured using microneurography.
The results showed that men with CKD had significantly higher resting muscle sympathetic nerve activity compared with women with CKD (68 vs 55 bursts per 100 heartbeats; P = .005), whereas no differences in vascular stiffness were observed between the genders (P = .248).
“The findings suggest that the higher cardiovascular disease risk observed in older males with CKD may be influenced by elevated sympathetic activity,” Dr. Zanuzzi explained.
“However, the lack of significant differences in vascular stiffness between genders implies that additional factors beyond vascular remodeling may contribute to the observed sex-specific differences in cardiovascular risk,” he said.
Of note, resting vascular stiffness was not associated with muscular sympathetic nerve activity in either men or women, which was surprising to the authors, Dr. Zanuzzi noted.
“This underscores the multifactorial nature of vascular pathophysiology in CKD and underscores the need for further research to unravel the underlying mechanisms.”
In other findings, although prior studies have shown a positive correlation between age and resting muscle sympathetic nerve activity in White, healthy women and men without obesity,, no similar relationship was observed in men or women with CKD.
“These findings suggest that the protective effect of younger age on sympathetic function may not be present in the setting of decreased kidney function in both males and females,” the authors note.
In addition, whereas previous research has shown a clear association between sympathetic overactivity and a wide variety of measures of obesity, in the current study, that association was only observed in men with CKD.
Important limitations of the study include the cross-sectional design and that the population was predominantly Black, Dr. Zanuzzi noted.
“Generalizability to other demographic groups may be limited, and future longitudinal studies are needed to validate these findings and explore potential causal relationships,” he said.
The findings underscore “the need for novel therapeutic approaches targeting sympathetic overactivity and vascular stiffness in CKD patients, especially considering the observed sex-specific differences,” Dr. Zanuzzi added.
“Potential interventions may include pharmacological agents that modulate sympathetic tone or vascular remodeling pathways,” he said.
“Lifestyle modifications focusing on stress reduction and cardiovascular health promotion could also play a crucial role in mitigating cardiovascular risk.”
Dr. Zanuzzi concluded that “tailoring treatment strategies to address these differences may lead to more personalized and effective management approaches, ultimately improving clinical outcomes in this high-risk population.”
The authors had no disclosures to report.
A version of this article first appeared on Medscape.com.
The Burden of Skin Cancer in the Military Health System, 2017-2022
This retrospective observational study investigates skin cancer prevalence and care patterns within the Military Health System (MHS) from 2017 to 2022. Utilizing the MHS Management Analysis and Reporting Tool (most commonly called M2), we analyzed more than 5 million patient encounters and documented skin cancer prevalence in the MHS beneficiary population utilizing available demographic data. Notable findings included an increased prevalence of skin cancer in the military population compared with the civilian population, a substantial decline in direct care (DC) visits at military treatment facilities compared with civilian purchased care (PC) visits, and a decreased total number of visits during COVID-19 restrictions.
The Military Health System (MHS) is a worldwide health care delivery system that serves 9.6 million beneficiaries, including military service members, retirees, and their families.1 Its mission is 2-fold: provide a medically ready force, and provide a medical benefit in keeping with the service and sacrifice of active-duty personnel, military retirees, and their families. For fiscal year (FY) 2022, active-duty service members and their families comprised 16.7% and 19.9% of beneficiaries, respectively, while retired service members and their families comprised 27% and 32% of beneficiaries, respectively.
The MHS operates under the authority of the Department of Defense (DoD) and is supported by an annual budget of approximately $50 billion.1 Health care provision within the MHS is managed by TRICARE regional networks.2 Within these networks, MHS beneficiaries may receive health care in 2 categories: direct care (DC) and purchased care (PC). Direct care is rendered in military treatment facilities by military or civilian providers contracted by the DoD, and PC is administered by civilian providers at civilian health care facilities within the TRICARE network, which is comprised of individual providers, clinics, and hospitals that have agreed to accept TRICARE beneficiaries.1 Purchased care is fee-for-service and paid for by the MHS. Of note, the MHS differs from the Veterans Affairs health care system in that the MHS through DC and PC sees only active-duty service members, active-duty dependents, retirees, and retirees’ dependents (primarily spouses), whereas Veterans Affairs sees only veterans (not necessarily retirees) discharged from military service with compensable medical conditions or disabilities.
Skin cancer presents a notable concern for the US Military, as the risk for skin cancer is thought to be higher than in the general population.3,4 This elevated risk is attributed to numerous factors inherent to active-duty service, including time spent in tropical environments, increased exposure to UV radiation, time spent at high altitudes, and decreased rates of sun-protective behaviors.3 Although numerous studies have explored the mechanisms that contribute to service members’ increased skin cancer risk, there are few (if any) that discuss the burden of skin cancer on the MHS and where its beneficiaries receive their skin cancer care. This study evaluated the burden of skin cancer within the MHS, as demonstrated by the period prevalence of skin cancer among its beneficiaries and the number and distribution of patient visits for skin cancer across both DC and PC from 2017 to 2022.
Methods
Data Collection—This retrospective observational study was designed to describe trends in outpatient visits with a skin cancer diagnosis and annual prevalence of skin cancer types in the MHS. Data are from all MHS beneficiaries who were eligible or enrolled in the analysis year. Our data source was the MHS Management Analysis and Reporting Tool (most commonly called M2), a query tool that contains the current and most recent 5 full FYs of Defense Health Agency corporate health care data including aggregated FY and calendar-year counts of MHS beneficiaries from 2017 to 2022 using encounter and claims data tables from both DC and PC. Data in M2 are coded using a pseudo-person identification number, and queries performed for this study were limited to de-identified visit and patient counts.
Skin cancer diagnoses were defined by relevant International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes recorded from outpatient visits in DC and PC. The M2 database was queried to find aggregate counts of visits and unique MHS beneficiaries with one or more diagnoses of a skin cancer type of interest (defined by relevant ICD-10-CM code) over the study period stratified by year and by patient demographic characteristics. Skin cancer types by ICD-10-CM code group included basal cell carcinoma (BCC), squamous cell carcinoma (SCC), malignant melanoma (MM), and other (including Merkel cell carcinoma and sebaceous carcinoma). Demographic strata included age, sex, military status (active duty, dependents of active duty, retired, or all others), sponsor military rank, and sponsor branch (army, air force, marine corps, or navy). Visit counts included diagnoses from any ICD position (for encounters that contained multiple ICD codes) to describe the total volume of care that addressed a diagnosed skin cancer. Counts of unique patients in prevalence analyses included relevant diagnoses in the primary ICD position only to increase the specificity of prevalence estimates.
Data Analysis—Descriptive analyses included the total number of outpatient visits with a skin cancer diagnosis in DC and PC over the study period, with percentages of total visits by year and by demographic strata. Separate analyses estimated annual prevalences of skin cancer types in the MHS by study year and within 2022 by demographic strata. Numerators in prevalence analyses were defined as the number of unique individuals with one or more relevant ICD codes in the analysis year. Denominators were defined as the total number of MHS beneficiaries in the analysis year and resulting period prevalences reported. Observed prevalences were qualitatively described, and trends were compared with prevalences in nonmilitary populations reported in the literature.
Ethics—This study was conducted as part of a study using secondary analyses of de-identified data from the M2 database. The study was reviewed and approved by the Walter Reed National Military Medical Center institutional review board.
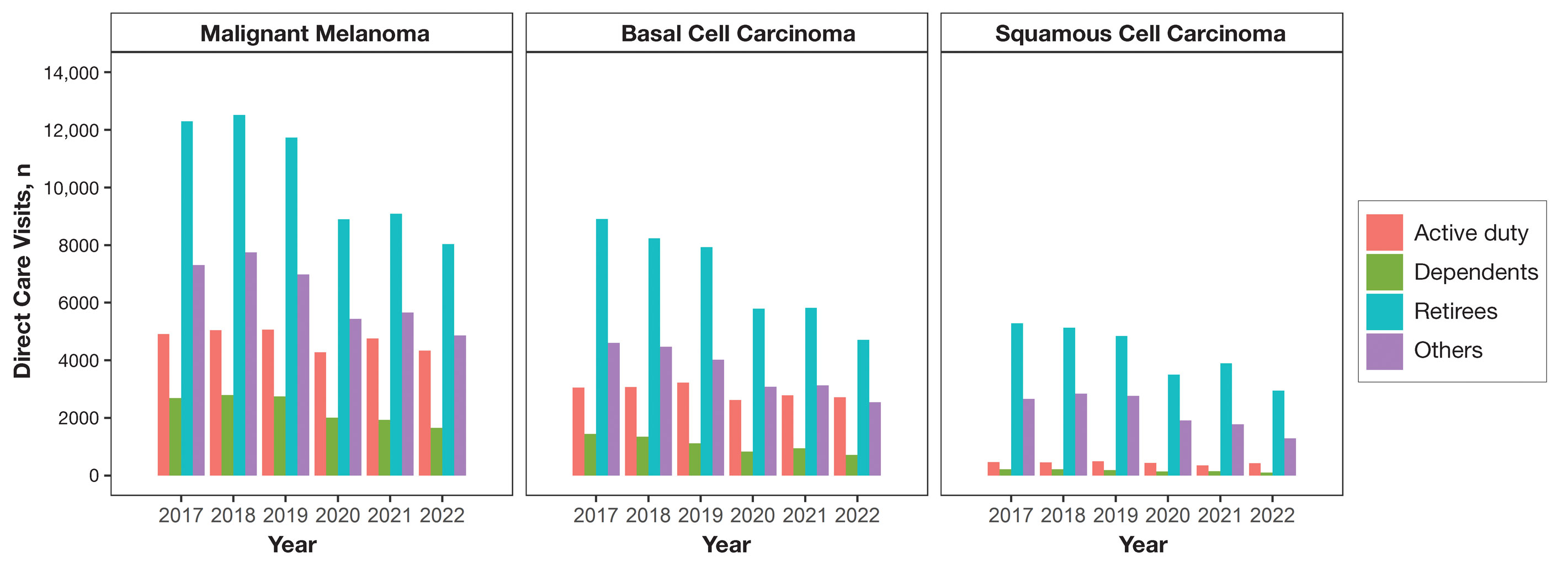
Results
Encounter data were analyzed from a total of 5,374,348 visits between DC and PC over the study period for each cancer type of interest. Figures 1 and 2 show temporal trends in DC visits compared with PC visits in each beneficiary category. The percentage of total DC visits subsequently declined each year throughout the study period, with percentage decreases from 2017 to 2022 of 1.45% or 8200 fewer visits for MM, 3.41% or 7280 fewer visits for BCC, and 2.26% or 3673 fewer visits for SCC.
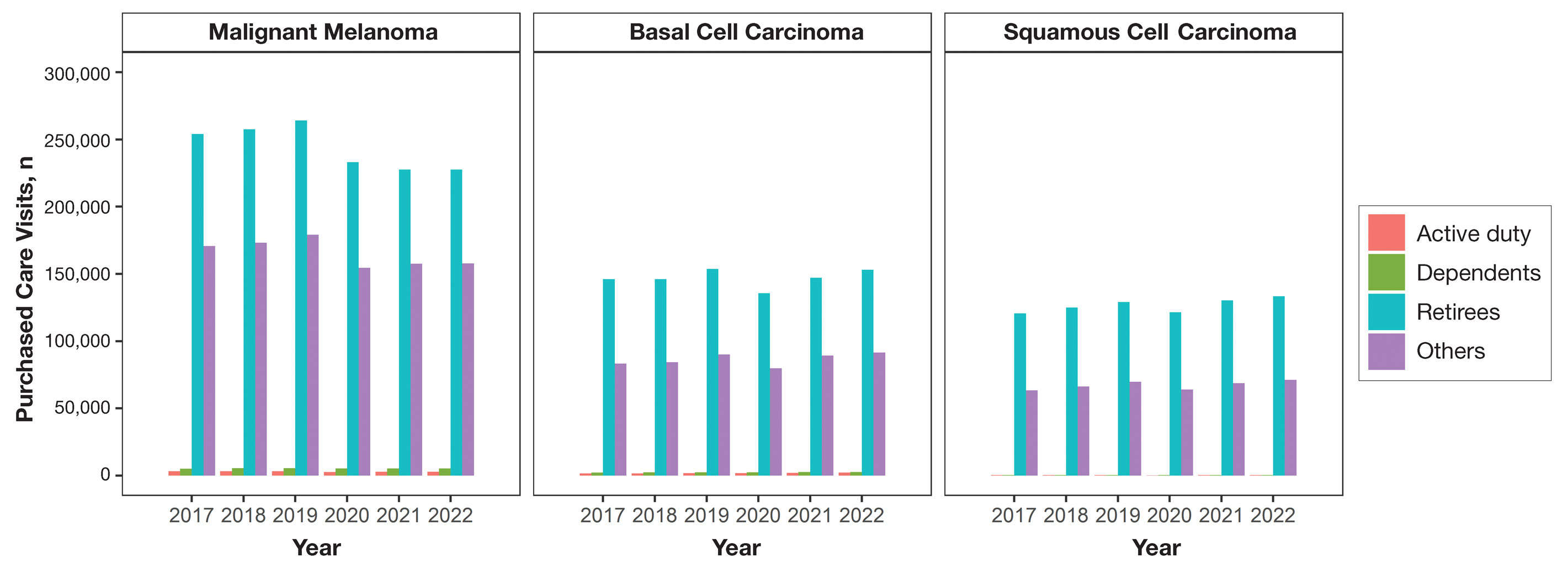
When stratified by beneficiary category, this trend remained consistent among dependents and retirees, with the most notable annual percentage decrease from 2019 to 2020. A higher proportion of younger adults and active-duty beneficiaries was seen in DC relative to PC, in which most visits were among retirees and others (primarily dependents of retirees, survivors, and Guard/Reserve on active duty, as well as inactive Guard/Reserve). No linear trends over time were apparent for active duty in DC and for dependents and retirees in PC. eTable 1 summarizes the demographic characteristics of MHS beneficiaries being seen in DC and PC over the study period for each cancer type of interest.
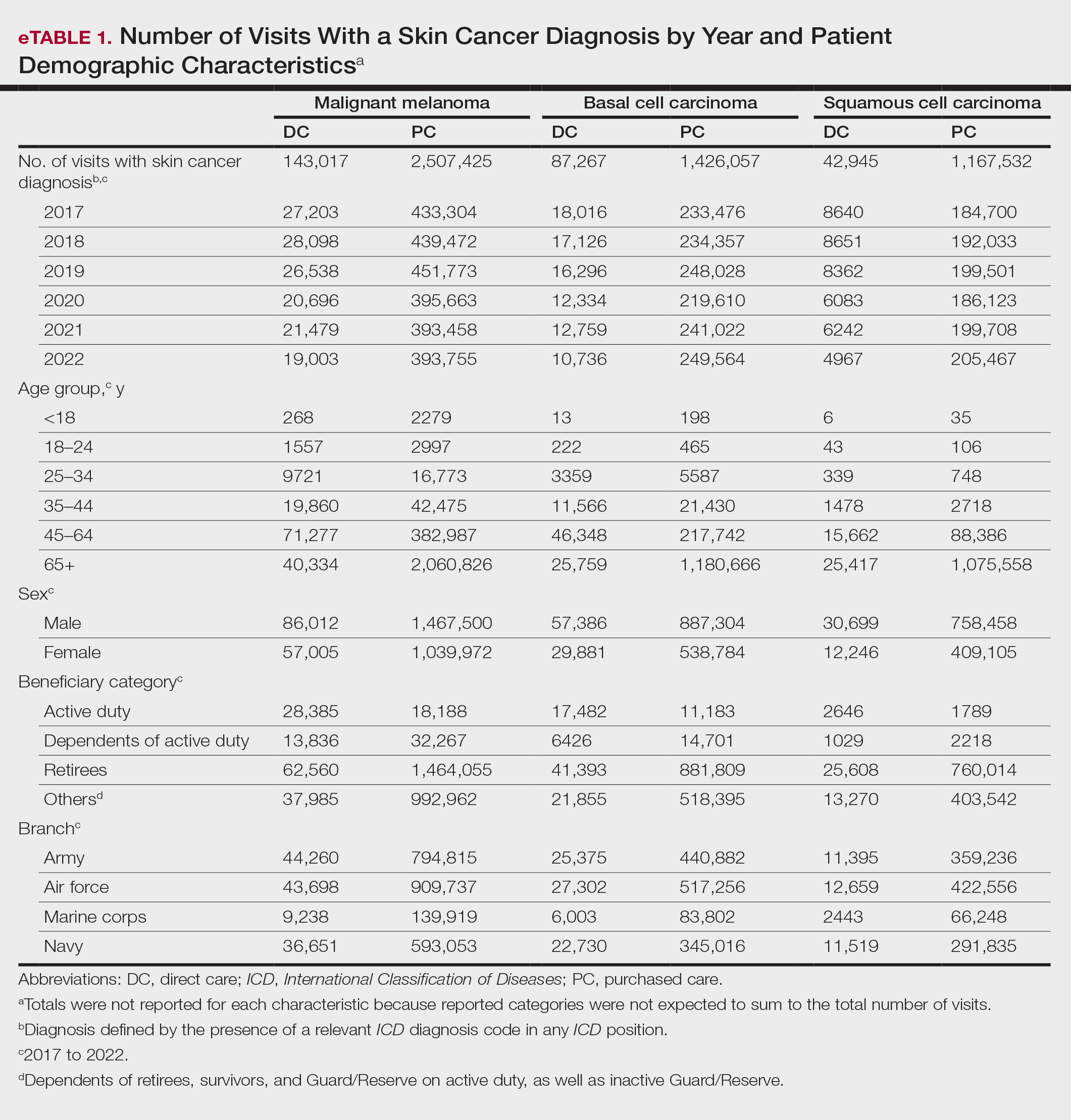
The Table shows the period prevalence of skin cancer diagnoses within the MHS beneficiary population from 2017 to 2022. These data were further analyzed by MM, BCC, and SCC (eTable 2) and demographics of interest for the year 2022. By beneficiary category, the period prevalence of MM was 0.08% in active duty, 0.06% in dependents, 0.48% in others, and 1.10% in retirees; the period prevalence of BCC was 0.12% in active duty, 0.07% in dependents, 0.91% in others, and 2.50% in retirees; and the period prevalence of SCC was 0.02% in active duty, 0.01% in dependents, 0.63% in others, and 1.87% in retirees. By sponsor branch, the period prevalence of MM was 0.35% in the army, 0.62% in the air force, 0.35% in the marine corps, and 0.65% in the navy; the period prevalence of BCC was 0.74% in the army, 1.30% in the air force, 0.74% in the marine corps, and 1.36% in the navy; and the period prevalence of SCC was 0.52% in the army, 0.92% in the air force, 0.51% in the marine corps, and 0.97% in the navy.
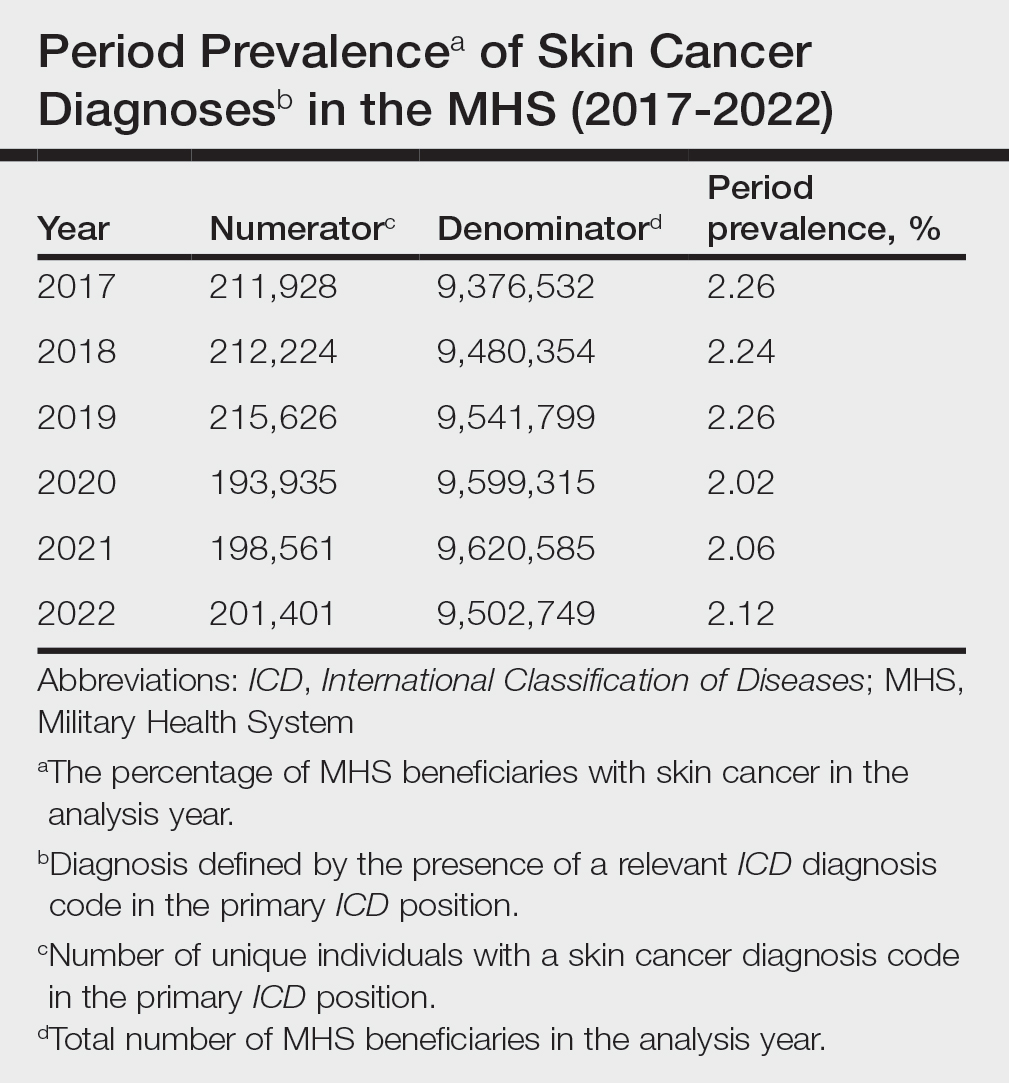
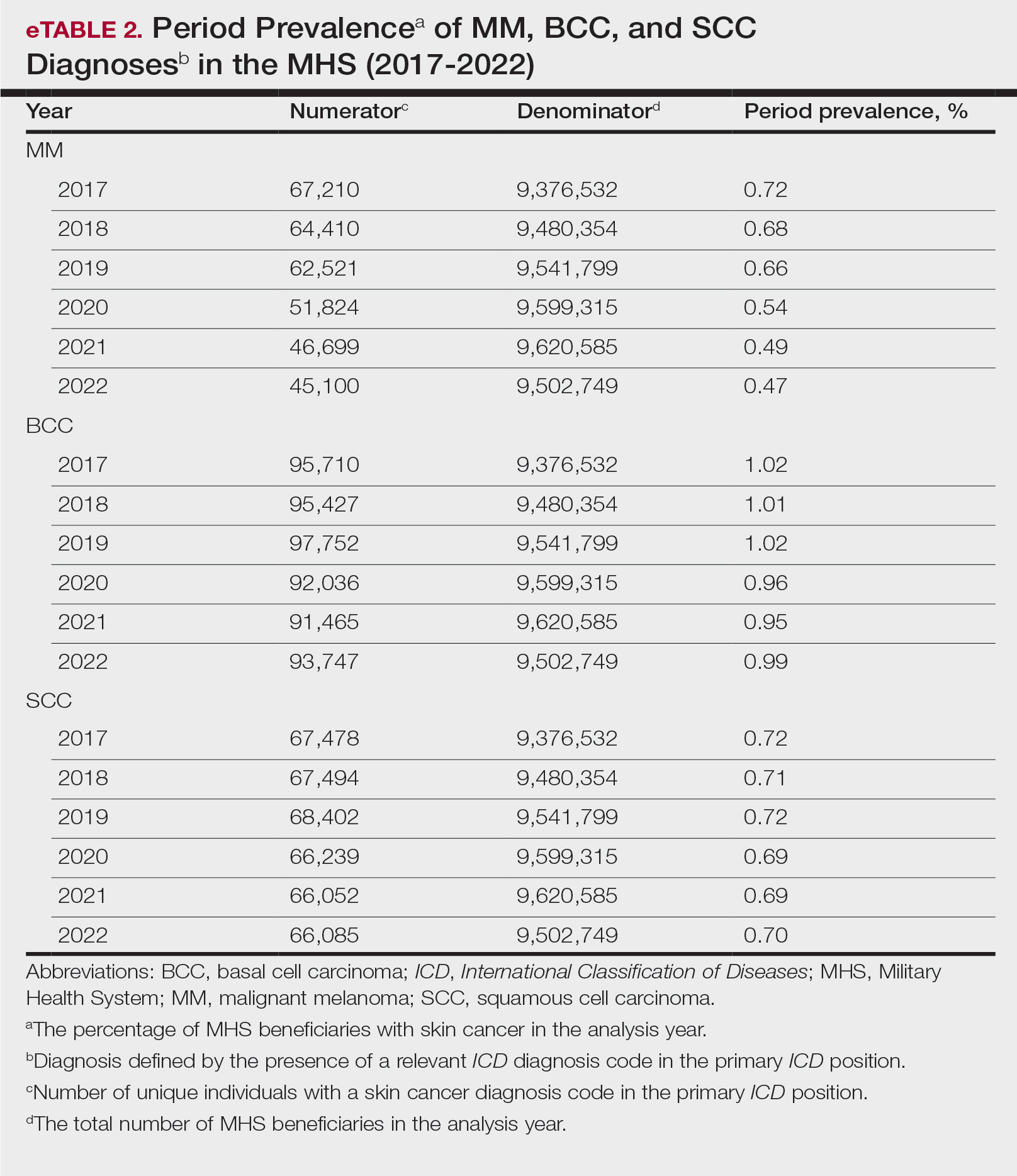
Comment
This study aimed to provide insight into the burden of skin cancer within the MHS beneficiary population and to identify temporal trends in where these beneficiaries receive their care. We examined patient encounter data from more than 9.6 million MHS beneficiaries.
The utilization of ICD codes from patient encounters to estimate the prevalence of nonmelanoma skin cancer (NMSC) has demonstrated a high positive predictive value. In one study, NMSC cases were confirmed in 96.5% of ICD code–identified patients.5 We presented an extensive collection of epidemiologic data on BCC and SCC, which posed unique challenges for tracking, as they are not reported to or monitored by cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) Program.6
MHS Compared to the US Population—A study using the Global Burden of Disease 2019 database revealed an increasing trend in the incidence and prevalence of NMSC and melanoma since 1990. The same study found the period prevalence in 2019 of MM, SCC, and BCC in the general US population to be 0.13%, 0.31%, and 0.05%, respectively.7 In contrast, among MHS beneficiaries, we observed a higher prevalence in the same year, with figures of 0.66% for MM, 0.72% for SCC, and 1.02% for BCC. According to the SEER database, the period prevalence of MM within the general US population in 2020 was 0.4%.8 That same year, we identified a higher period prevalence of MM—0.54%—within the MHS beneficiary population. Specifically, within the MHS retiree population, the prevalence in 2022 was double that of the general MHS population, with a rate of 1.10%, underscoring the importance of skin cancer screening in older, at-risk adult populations. Prior studies similarly found increased rates of skin cancer within the military beneficiary population. Further studies are needed to compare age-adjusted rates in the MHS vs US population.9-11
COVID-19 Trends—Our data showed an overall decreasing prevalence of skin cancer in the MHS from 2019 to 2021. We suspect that the apparent decrease in skin cancer prevalence may be attributed to underdiagnosis from COVID-19 pandemic restrictions. During that time, many dermatology clinics at military treatment facilities underwent temporary closures, and some dermatologists were sent on nondermatologic utilization tours. Likewise, a US multi-institutional study described declining rates of new melanomas from 2020 to 2021, with an increased proportion of patient presentations with advanced melanoma, suggesting an underdiagnosis of melanoma cases during pandemic restrictions. That study also noted an increased rate of patient-identified melanomas and a decreased rate of provider-identified melanomas during that time.12 Contributing factors may include excess hospital demand, increased patient complexity and acute care needs, and long outpatient clinic backlogs during this time.13Financial Burden—Over our 5-year study period, there were 5,374,348 patient encounters addressing skin cancer, both in DC and PC (Figures 1 and 2; eTable 1). In 2016 to 2018, the average annual cost of treating skin cancer in the US civilian, noninstitutionalized population was $1243 for NMSC (BCC and SCC) and $2430 for melanoma.6 Using this metric, the estimated total cost of care rendered in the MHS in 2018 for NMSC and melanoma was $202,510,803 and $156,516,300, respectively.
Trends in DC vs PC—In the years examined, we found a notable decrease in the number of beneficiaries receiving treatment for MM, BCC, and SCC in DC. Simultaneously, there has been an increase in the number of beneficiaries receiving PC for BCC and SCC, though this trend was not apparent for MM.
Our data provided interesting insights into the percentage of PC compared with DC offered within the MHS. Importantly, our findings suggested that the majority of skin cancer in active-duty service members is managed with DC within the military treatment facility setting (61% DC management over the period analyzed). This finding was true across all years of data analyzed, suggesting that the COVID-19 pandemic did not result in a quantifiable shift in care of skin cancer within the active-duty component to outside providers. One of the critical roles of dermatologists in the MHS is to diagnose and treat skin cancer, and our study suggested that the current global manning and staffing for MHS dermatologists may not be sufficient to meet the burden of skin cancers encountered within our active-duty troops, as only 61% are managed with DC. In particular, service members in more austere and/or overseas locations may not have ready access to a dermatologist.
The burden of skin cancer shifts dramatically when analyzing care of all other populations included in these data, including dependents of active-duty service members, retirees, and the category of “other” (ie, principally dependents of retirees). Within these populations, the rate of DC falls to 30%, with 70% of active-duty dependent care being deferred to network. The findings are even more noticeable for retirees and others within these 2 cohorts in all types of skin cancer analyzed, where DC only accounted for 5.2% of those skin cancers encountered and managed across TRICARE-eligible beneficiaries. For MM, BCC, and SCC, percentages of DC were 5.4%, 5.8%, and 3.5%, respectively. Although it is interesting to note the lower percentage of SCC managed via DC, our data did not allow for extrapolation as to why more SCC cases may be deferred to network. The shift to PC may align with DoD initiatives to increase the private sector’s involvement in military medicine and transition to civilianizing the MHS.14 In the end, the findings are remarkable, with approximately 95% of skin cancer care and management provided overall via PC.
These findings differ from previously published data regarding DC and PC from other specialty areas. Results from an analysis of DC vs PC for plastic surgery for the entire MHS from 2016 to 2019 found 83.2% of cases were deferred to network.15 A similar publication in the orthopedics literature examined TRICARE claims for patients who underwent total hip or knee arthroplasties between 2006 and 2019 and found 84.6% of cases were referred for PC. Notably, the authors utilized generalized linear models for cost analysis and found that DC was more expensive than PC, though this likely was a result of higher rates of hospital readmission within DC cases.16 Lastly, an article on the DC vs PC disposition of MHS patients with breast cancer from 2003 to 2008 found 46% of cases managed with DC vs 26.% with PC and 27.8% receiving a combination. In this case, the authors found a reduced cost associated with DC vs PC.17
Little additional literature exists regarding the costs of DC vs PC. An article published in 2016 designed to assess costs of DC vs PC showed that almost all military treatment facilities have higher costs than their private sector counterparts, with a few exceptions.18 This does not assess the costs of specific procedures, however, and only the overall cost to maintain a treatment facility. Importantly, this study was based on data from FY 2014 and has not been updated to reflect complex changes within the MHS system and the private health care system. Indeed, a US Government Accountability Office FY 2023 study highlighted staffing and efficiency issues within this transition to civilian medicine; subsequently, the 2024 President’s Budget suspended all planned clinical medical military end strength divestitures, underscoring the potential ineffectiveness of a civilianized MHS at meeting the health care needs of its beneficiaries.19,20 Future research on a national scale will be necessary to see if there is a reversal of this trend to PC and if doing so has any impact on access to DC for active-duty troops or active-duty dependents.
In addition to PC vs DC trends, we also can get a sense of the impact of the COVID pandemic restrictions on access to DC vs PC by assessing the change in rates seen in the data from the pre-COVID years (2017-2019) to the “post-COVID” years (2020-2022) included. Overall, rates of DC decreased uniformly from their already low percentages. In our study, rates of DC decreased from 5.8% in 2019 to 4.8% in 2022 for MM, from 6.6% to 4.3% for BCC, and from 4.2% to 2.9% for SCC. Although these changes seem small at first, they represent a 30.6% overall decrease in DC for BCC and an overall decrease of 55.4% in DC for SCC. Although our data do not allow us to extrapolate the real cost of this reduction across a nationwide health care system and more than 5 million care encounters, the financial and personal (ie, lost man-hours) costs of this decrease in DC likely are substantial.
In addition to costs, qualitative aspects that contribute to the burden of skin cancer include treatment-related morbidity, such as scarring, pain, and time spent away from family, work, and hobbies, as well as overall patient satisfaction with the quality of care they receive.21 Future work is critical to assess the real cost of this immense burden of PC for the treatment and management of skin cancers within the DoD beneficiary population.
Limitations—This study is limited by its observational nature. Given the mechanism of our data collection, we may have underestimated disease prevalence, as not all patients are seen for their diagnosis annually. Furthermore, reported demographic strata (eg, age, sex) were limited to those available and valid in the M2 reporting system. Finally, our study only collected data from those service members or former service members seen within the MHS and does not reflect any care rendered to those who are no longer active duty but did not officially retire from the military (ie, nonretired service members receiving care in the Veterans Affairs system for skin cancer).
Conclusion
We describe the annual burden of care for skin cancer in the MHS beneficiary population. Noteworthy findings observed were an overall decrease in beneficiaries being treated for skin cancer through DC; a decreasing annual prevalence of skin cancer diagnosis between 2019 and 2021, which may represent underdiagnosis or decreased follow-up in the setting of the COVID-19 pandemic; and a higher rate of skin cancer in the military beneficiary population compared to the civilian population.
- US Department of Defense. Military health. Accessed October 5, 2023. https://www.defense.gov/
- Wooten NR, Brittingham JA, Pitner RO, et al. Purchased behavioral health care received by Military Health System beneficiaries in civilian medical facilities, 2000-2014. Mil Med. 2018;183:E278-E290. doi:10.1093/milmed/usx101
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- American Academy of Dermatology. Skin cancer. Updated April 22, 2022. Accessed April 17, 2024. https://www.aad.org/media/stats-skin-cancer
- Eide MJ, Krajenta R, Johnson D, et al. Identification of patients with nonmelanoma skin cancer using health maintenance organization claims data. Am J Epidemiol. 2010;171:123-128. doi:10.1093/aje/kwp352
- Kao SYZ, Ekwueme DU, Holman DM, et al. Economic burden of skin cancer treatment in the USA: an analysis of the Medical Expenditure Panel Survey Data, 2012-2018. Cancer Causes Control. 2023;34:205-212. doi:10.1007/s10552-022-01644-0
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- SEER*Explorer. SEER Incidence Data, November 2023 Submission (1975-2021). National Cancer Institute; 2024. Accessed April 17, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=1&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=1
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663. doi:10.1111/j.1365-4362.1984.tb01228.x
- Page WF, Whiteman D, Murphy M. A comparison of melanoma mortality among WWII veterans of the Pacific and European theaters. Ann Epidemiol. 2000;10:192-195. doi:10.1016/s1047-2797(99)00050-2
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737. doi:10.1016/0190-9622(93)70102-Y
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Gibbs A. COVID-19 shutdowns caused delays in melanoma diagnoses, study finds. OHSU News. August 4, 2022. Accessed April 17, 2024. https://news.ohsu.edu/2022/08/04/covid-19-shutdowns-caused-delays-in-melanoma-diagnoses-study-finds
- Kime P. Pentagon budget calls for ‘civilianizing’ military hospitals. Military Times. Published February 10, 2020. Accessed April 17, 2024. https://www.militarytimes.com/news/your-military/2020/02/10/pentagon-budget-calls-for-civilianizing-military-hospitals/
- O’Reilly EB, Norris E, Ortiz-Pomales YT, et al. A comparison of direct care at military medical treatment facilities with purchased care in plastic surgery operative volume. Plast Reconstr Surg Glob Open. 2022;10(10 suppl):124-125. doi:10.1097/01.GOX.0000898976.03344.62
- Haag A, Hosein S, Lyon S, et al. Outcomes for arthroplasties in military health: a retrospective analysis of direct versus purchased care. Mil Med. 2023;188(suppl 6):45-51. doi:10.1093/milmed/usac441
- Eaglehouse YL, Georg MW, Richard P, et al. Cost-efficiency of breast cancer care in the US Military Health System: an economic evaluation in direct and purchased care. Mil Med. 2019;184:e494-e501. doi:10.1093/milmed/usz025
- Lurie PM. Comparing the cost of military treatment facilities with private sector care. Institute for Defense Analyses; February 2016. Accessed April 17, 2024. https://www.ida.org/research-and-publications/publications/all/c/co/comparing-the-costs-of-military-treatment-facilities-with-private-sector-care
- Defense Health Program. Fiscal Year (FY) 2024 President’s Budget: Operation and Maintenance Procurement Research, Development, Test and Evaluation. Department of Defense; March 2023. Accessed April 17, 2024. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2024/budget_justification/pdfs/09_Defense_Health_Program/00-DHP_Vols_I_II_and_III_PB24.pdf
- US Government Accountability Office. Defense Health Care. DOD should reevaluate market structure for military medical treatment facility management. Published August 21, 2023. Accessed April 17, 2024. https://www.gao.gov/products/gao-23-105441
- Rosenberg A, Cho S. We can do better at protecting our service members from skin cancer. Mil Med. 2022;187:311-313. doi:10.1093/milmed/usac198
This retrospective observational study investigates skin cancer prevalence and care patterns within the Military Health System (MHS) from 2017 to 2022. Utilizing the MHS Management Analysis and Reporting Tool (most commonly called M2), we analyzed more than 5 million patient encounters and documented skin cancer prevalence in the MHS beneficiary population utilizing available demographic data. Notable findings included an increased prevalence of skin cancer in the military population compared with the civilian population, a substantial decline in direct care (DC) visits at military treatment facilities compared with civilian purchased care (PC) visits, and a decreased total number of visits during COVID-19 restrictions.
The Military Health System (MHS) is a worldwide health care delivery system that serves 9.6 million beneficiaries, including military service members, retirees, and their families.1 Its mission is 2-fold: provide a medically ready force, and provide a medical benefit in keeping with the service and sacrifice of active-duty personnel, military retirees, and their families. For fiscal year (FY) 2022, active-duty service members and their families comprised 16.7% and 19.9% of beneficiaries, respectively, while retired service members and their families comprised 27% and 32% of beneficiaries, respectively.
The MHS operates under the authority of the Department of Defense (DoD) and is supported by an annual budget of approximately $50 billion.1 Health care provision within the MHS is managed by TRICARE regional networks.2 Within these networks, MHS beneficiaries may receive health care in 2 categories: direct care (DC) and purchased care (PC). Direct care is rendered in military treatment facilities by military or civilian providers contracted by the DoD, and PC is administered by civilian providers at civilian health care facilities within the TRICARE network, which is comprised of individual providers, clinics, and hospitals that have agreed to accept TRICARE beneficiaries.1 Purchased care is fee-for-service and paid for by the MHS. Of note, the MHS differs from the Veterans Affairs health care system in that the MHS through DC and PC sees only active-duty service members, active-duty dependents, retirees, and retirees’ dependents (primarily spouses), whereas Veterans Affairs sees only veterans (not necessarily retirees) discharged from military service with compensable medical conditions or disabilities.
Skin cancer presents a notable concern for the US Military, as the risk for skin cancer is thought to be higher than in the general population.3,4 This elevated risk is attributed to numerous factors inherent to active-duty service, including time spent in tropical environments, increased exposure to UV radiation, time spent at high altitudes, and decreased rates of sun-protective behaviors.3 Although numerous studies have explored the mechanisms that contribute to service members’ increased skin cancer risk, there are few (if any) that discuss the burden of skin cancer on the MHS and where its beneficiaries receive their skin cancer care. This study evaluated the burden of skin cancer within the MHS, as demonstrated by the period prevalence of skin cancer among its beneficiaries and the number and distribution of patient visits for skin cancer across both DC and PC from 2017 to 2022.
Methods
Data Collection—This retrospective observational study was designed to describe trends in outpatient visits with a skin cancer diagnosis and annual prevalence of skin cancer types in the MHS. Data are from all MHS beneficiaries who were eligible or enrolled in the analysis year. Our data source was the MHS Management Analysis and Reporting Tool (most commonly called M2), a query tool that contains the current and most recent 5 full FYs of Defense Health Agency corporate health care data including aggregated FY and calendar-year counts of MHS beneficiaries from 2017 to 2022 using encounter and claims data tables from both DC and PC. Data in M2 are coded using a pseudo-person identification number, and queries performed for this study were limited to de-identified visit and patient counts.
Skin cancer diagnoses were defined by relevant International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes recorded from outpatient visits in DC and PC. The M2 database was queried to find aggregate counts of visits and unique MHS beneficiaries with one or more diagnoses of a skin cancer type of interest (defined by relevant ICD-10-CM code) over the study period stratified by year and by patient demographic characteristics. Skin cancer types by ICD-10-CM code group included basal cell carcinoma (BCC), squamous cell carcinoma (SCC), malignant melanoma (MM), and other (including Merkel cell carcinoma and sebaceous carcinoma). Demographic strata included age, sex, military status (active duty, dependents of active duty, retired, or all others), sponsor military rank, and sponsor branch (army, air force, marine corps, or navy). Visit counts included diagnoses from any ICD position (for encounters that contained multiple ICD codes) to describe the total volume of care that addressed a diagnosed skin cancer. Counts of unique patients in prevalence analyses included relevant diagnoses in the primary ICD position only to increase the specificity of prevalence estimates.
Data Analysis—Descriptive analyses included the total number of outpatient visits with a skin cancer diagnosis in DC and PC over the study period, with percentages of total visits by year and by demographic strata. Separate analyses estimated annual prevalences of skin cancer types in the MHS by study year and within 2022 by demographic strata. Numerators in prevalence analyses were defined as the number of unique individuals with one or more relevant ICD codes in the analysis year. Denominators were defined as the total number of MHS beneficiaries in the analysis year and resulting period prevalences reported. Observed prevalences were qualitatively described, and trends were compared with prevalences in nonmilitary populations reported in the literature.
Ethics—This study was conducted as part of a study using secondary analyses of de-identified data from the M2 database. The study was reviewed and approved by the Walter Reed National Military Medical Center institutional review board.

Results
Encounter data were analyzed from a total of 5,374,348 visits between DC and PC over the study period for each cancer type of interest. Figures 1 and 2 show temporal trends in DC visits compared with PC visits in each beneficiary category. The percentage of total DC visits subsequently declined each year throughout the study period, with percentage decreases from 2017 to 2022 of 1.45% or 8200 fewer visits for MM, 3.41% or 7280 fewer visits for BCC, and 2.26% or 3673 fewer visits for SCC.

When stratified by beneficiary category, this trend remained consistent among dependents and retirees, with the most notable annual percentage decrease from 2019 to 2020. A higher proportion of younger adults and active-duty beneficiaries was seen in DC relative to PC, in which most visits were among retirees and others (primarily dependents of retirees, survivors, and Guard/Reserve on active duty, as well as inactive Guard/Reserve). No linear trends over time were apparent for active duty in DC and for dependents and retirees in PC. eTable 1 summarizes the demographic characteristics of MHS beneficiaries being seen in DC and PC over the study period for each cancer type of interest.

The Table shows the period prevalence of skin cancer diagnoses within the MHS beneficiary population from 2017 to 2022. These data were further analyzed by MM, BCC, and SCC (eTable 2) and demographics of interest for the year 2022. By beneficiary category, the period prevalence of MM was 0.08% in active duty, 0.06% in dependents, 0.48% in others, and 1.10% in retirees; the period prevalence of BCC was 0.12% in active duty, 0.07% in dependents, 0.91% in others, and 2.50% in retirees; and the period prevalence of SCC was 0.02% in active duty, 0.01% in dependents, 0.63% in others, and 1.87% in retirees. By sponsor branch, the period prevalence of MM was 0.35% in the army, 0.62% in the air force, 0.35% in the marine corps, and 0.65% in the navy; the period prevalence of BCC was 0.74% in the army, 1.30% in the air force, 0.74% in the marine corps, and 1.36% in the navy; and the period prevalence of SCC was 0.52% in the army, 0.92% in the air force, 0.51% in the marine corps, and 0.97% in the navy.


Comment
This study aimed to provide insight into the burden of skin cancer within the MHS beneficiary population and to identify temporal trends in where these beneficiaries receive their care. We examined patient encounter data from more than 9.6 million MHS beneficiaries.
The utilization of ICD codes from patient encounters to estimate the prevalence of nonmelanoma skin cancer (NMSC) has demonstrated a high positive predictive value. In one study, NMSC cases were confirmed in 96.5% of ICD code–identified patients.5 We presented an extensive collection of epidemiologic data on BCC and SCC, which posed unique challenges for tracking, as they are not reported to or monitored by cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) Program.6
MHS Compared to the US Population—A study using the Global Burden of Disease 2019 database revealed an increasing trend in the incidence and prevalence of NMSC and melanoma since 1990. The same study found the period prevalence in 2019 of MM, SCC, and BCC in the general US population to be 0.13%, 0.31%, and 0.05%, respectively.7 In contrast, among MHS beneficiaries, we observed a higher prevalence in the same year, with figures of 0.66% for MM, 0.72% for SCC, and 1.02% for BCC. According to the SEER database, the period prevalence of MM within the general US population in 2020 was 0.4%.8 That same year, we identified a higher period prevalence of MM—0.54%—within the MHS beneficiary population. Specifically, within the MHS retiree population, the prevalence in 2022 was double that of the general MHS population, with a rate of 1.10%, underscoring the importance of skin cancer screening in older, at-risk adult populations. Prior studies similarly found increased rates of skin cancer within the military beneficiary population. Further studies are needed to compare age-adjusted rates in the MHS vs US population.9-11
COVID-19 Trends—Our data showed an overall decreasing prevalence of skin cancer in the MHS from 2019 to 2021. We suspect that the apparent decrease in skin cancer prevalence may be attributed to underdiagnosis from COVID-19 pandemic restrictions. During that time, many dermatology clinics at military treatment facilities underwent temporary closures, and some dermatologists were sent on nondermatologic utilization tours. Likewise, a US multi-institutional study described declining rates of new melanomas from 2020 to 2021, with an increased proportion of patient presentations with advanced melanoma, suggesting an underdiagnosis of melanoma cases during pandemic restrictions. That study also noted an increased rate of patient-identified melanomas and a decreased rate of provider-identified melanomas during that time.12 Contributing factors may include excess hospital demand, increased patient complexity and acute care needs, and long outpatient clinic backlogs during this time.13Financial Burden—Over our 5-year study period, there were 5,374,348 patient encounters addressing skin cancer, both in DC and PC (Figures 1 and 2; eTable 1). In 2016 to 2018, the average annual cost of treating skin cancer in the US civilian, noninstitutionalized population was $1243 for NMSC (BCC and SCC) and $2430 for melanoma.6 Using this metric, the estimated total cost of care rendered in the MHS in 2018 for NMSC and melanoma was $202,510,803 and $156,516,300, respectively.
Trends in DC vs PC—In the years examined, we found a notable decrease in the number of beneficiaries receiving treatment for MM, BCC, and SCC in DC. Simultaneously, there has been an increase in the number of beneficiaries receiving PC for BCC and SCC, though this trend was not apparent for MM.
Our data provided interesting insights into the percentage of PC compared with DC offered within the MHS. Importantly, our findings suggested that the majority of skin cancer in active-duty service members is managed with DC within the military treatment facility setting (61% DC management over the period analyzed). This finding was true across all years of data analyzed, suggesting that the COVID-19 pandemic did not result in a quantifiable shift in care of skin cancer within the active-duty component to outside providers. One of the critical roles of dermatologists in the MHS is to diagnose and treat skin cancer, and our study suggested that the current global manning and staffing for MHS dermatologists may not be sufficient to meet the burden of skin cancers encountered within our active-duty troops, as only 61% are managed with DC. In particular, service members in more austere and/or overseas locations may not have ready access to a dermatologist.
The burden of skin cancer shifts dramatically when analyzing care of all other populations included in these data, including dependents of active-duty service members, retirees, and the category of “other” (ie, principally dependents of retirees). Within these populations, the rate of DC falls to 30%, with 70% of active-duty dependent care being deferred to network. The findings are even more noticeable for retirees and others within these 2 cohorts in all types of skin cancer analyzed, where DC only accounted for 5.2% of those skin cancers encountered and managed across TRICARE-eligible beneficiaries. For MM, BCC, and SCC, percentages of DC were 5.4%, 5.8%, and 3.5%, respectively. Although it is interesting to note the lower percentage of SCC managed via DC, our data did not allow for extrapolation as to why more SCC cases may be deferred to network. The shift to PC may align with DoD initiatives to increase the private sector’s involvement in military medicine and transition to civilianizing the MHS.14 In the end, the findings are remarkable, with approximately 95% of skin cancer care and management provided overall via PC.
These findings differ from previously published data regarding DC and PC from other specialty areas. Results from an analysis of DC vs PC for plastic surgery for the entire MHS from 2016 to 2019 found 83.2% of cases were deferred to network.15 A similar publication in the orthopedics literature examined TRICARE claims for patients who underwent total hip or knee arthroplasties between 2006 and 2019 and found 84.6% of cases were referred for PC. Notably, the authors utilized generalized linear models for cost analysis and found that DC was more expensive than PC, though this likely was a result of higher rates of hospital readmission within DC cases.16 Lastly, an article on the DC vs PC disposition of MHS patients with breast cancer from 2003 to 2008 found 46% of cases managed with DC vs 26.% with PC and 27.8% receiving a combination. In this case, the authors found a reduced cost associated with DC vs PC.17
Little additional literature exists regarding the costs of DC vs PC. An article published in 2016 designed to assess costs of DC vs PC showed that almost all military treatment facilities have higher costs than their private sector counterparts, with a few exceptions.18 This does not assess the costs of specific procedures, however, and only the overall cost to maintain a treatment facility. Importantly, this study was based on data from FY 2014 and has not been updated to reflect complex changes within the MHS system and the private health care system. Indeed, a US Government Accountability Office FY 2023 study highlighted staffing and efficiency issues within this transition to civilian medicine; subsequently, the 2024 President’s Budget suspended all planned clinical medical military end strength divestitures, underscoring the potential ineffectiveness of a civilianized MHS at meeting the health care needs of its beneficiaries.19,20 Future research on a national scale will be necessary to see if there is a reversal of this trend to PC and if doing so has any impact on access to DC for active-duty troops or active-duty dependents.
In addition to PC vs DC trends, we also can get a sense of the impact of the COVID pandemic restrictions on access to DC vs PC by assessing the change in rates seen in the data from the pre-COVID years (2017-2019) to the “post-COVID” years (2020-2022) included. Overall, rates of DC decreased uniformly from their already low percentages. In our study, rates of DC decreased from 5.8% in 2019 to 4.8% in 2022 for MM, from 6.6% to 4.3% for BCC, and from 4.2% to 2.9% for SCC. Although these changes seem small at first, they represent a 30.6% overall decrease in DC for BCC and an overall decrease of 55.4% in DC for SCC. Although our data do not allow us to extrapolate the real cost of this reduction across a nationwide health care system and more than 5 million care encounters, the financial and personal (ie, lost man-hours) costs of this decrease in DC likely are substantial.
In addition to costs, qualitative aspects that contribute to the burden of skin cancer include treatment-related morbidity, such as scarring, pain, and time spent away from family, work, and hobbies, as well as overall patient satisfaction with the quality of care they receive.21 Future work is critical to assess the real cost of this immense burden of PC for the treatment and management of skin cancers within the DoD beneficiary population.
Limitations—This study is limited by its observational nature. Given the mechanism of our data collection, we may have underestimated disease prevalence, as not all patients are seen for their diagnosis annually. Furthermore, reported demographic strata (eg, age, sex) were limited to those available and valid in the M2 reporting system. Finally, our study only collected data from those service members or former service members seen within the MHS and does not reflect any care rendered to those who are no longer active duty but did not officially retire from the military (ie, nonretired service members receiving care in the Veterans Affairs system for skin cancer).
Conclusion
We describe the annual burden of care for skin cancer in the MHS beneficiary population. Noteworthy findings observed were an overall decrease in beneficiaries being treated for skin cancer through DC; a decreasing annual prevalence of skin cancer diagnosis between 2019 and 2021, which may represent underdiagnosis or decreased follow-up in the setting of the COVID-19 pandemic; and a higher rate of skin cancer in the military beneficiary population compared to the civilian population.
This retrospective observational study investigates skin cancer prevalence and care patterns within the Military Health System (MHS) from 2017 to 2022. Utilizing the MHS Management Analysis and Reporting Tool (most commonly called M2), we analyzed more than 5 million patient encounters and documented skin cancer prevalence in the MHS beneficiary population utilizing available demographic data. Notable findings included an increased prevalence of skin cancer in the military population compared with the civilian population, a substantial decline in direct care (DC) visits at military treatment facilities compared with civilian purchased care (PC) visits, and a decreased total number of visits during COVID-19 restrictions.
The Military Health System (MHS) is a worldwide health care delivery system that serves 9.6 million beneficiaries, including military service members, retirees, and their families.1 Its mission is 2-fold: provide a medically ready force, and provide a medical benefit in keeping with the service and sacrifice of active-duty personnel, military retirees, and their families. For fiscal year (FY) 2022, active-duty service members and their families comprised 16.7% and 19.9% of beneficiaries, respectively, while retired service members and their families comprised 27% and 32% of beneficiaries, respectively.
The MHS operates under the authority of the Department of Defense (DoD) and is supported by an annual budget of approximately $50 billion.1 Health care provision within the MHS is managed by TRICARE regional networks.2 Within these networks, MHS beneficiaries may receive health care in 2 categories: direct care (DC) and purchased care (PC). Direct care is rendered in military treatment facilities by military or civilian providers contracted by the DoD, and PC is administered by civilian providers at civilian health care facilities within the TRICARE network, which is comprised of individual providers, clinics, and hospitals that have agreed to accept TRICARE beneficiaries.1 Purchased care is fee-for-service and paid for by the MHS. Of note, the MHS differs from the Veterans Affairs health care system in that the MHS through DC and PC sees only active-duty service members, active-duty dependents, retirees, and retirees’ dependents (primarily spouses), whereas Veterans Affairs sees only veterans (not necessarily retirees) discharged from military service with compensable medical conditions or disabilities.
Skin cancer presents a notable concern for the US Military, as the risk for skin cancer is thought to be higher than in the general population.3,4 This elevated risk is attributed to numerous factors inherent to active-duty service, including time spent in tropical environments, increased exposure to UV radiation, time spent at high altitudes, and decreased rates of sun-protective behaviors.3 Although numerous studies have explored the mechanisms that contribute to service members’ increased skin cancer risk, there are few (if any) that discuss the burden of skin cancer on the MHS and where its beneficiaries receive their skin cancer care. This study evaluated the burden of skin cancer within the MHS, as demonstrated by the period prevalence of skin cancer among its beneficiaries and the number and distribution of patient visits for skin cancer across both DC and PC from 2017 to 2022.
Methods
Data Collection—This retrospective observational study was designed to describe trends in outpatient visits with a skin cancer diagnosis and annual prevalence of skin cancer types in the MHS. Data are from all MHS beneficiaries who were eligible or enrolled in the analysis year. Our data source was the MHS Management Analysis and Reporting Tool (most commonly called M2), a query tool that contains the current and most recent 5 full FYs of Defense Health Agency corporate health care data including aggregated FY and calendar-year counts of MHS beneficiaries from 2017 to 2022 using encounter and claims data tables from both DC and PC. Data in M2 are coded using a pseudo-person identification number, and queries performed for this study were limited to de-identified visit and patient counts.
Skin cancer diagnoses were defined by relevant International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes recorded from outpatient visits in DC and PC. The M2 database was queried to find aggregate counts of visits and unique MHS beneficiaries with one or more diagnoses of a skin cancer type of interest (defined by relevant ICD-10-CM code) over the study period stratified by year and by patient demographic characteristics. Skin cancer types by ICD-10-CM code group included basal cell carcinoma (BCC), squamous cell carcinoma (SCC), malignant melanoma (MM), and other (including Merkel cell carcinoma and sebaceous carcinoma). Demographic strata included age, sex, military status (active duty, dependents of active duty, retired, or all others), sponsor military rank, and sponsor branch (army, air force, marine corps, or navy). Visit counts included diagnoses from any ICD position (for encounters that contained multiple ICD codes) to describe the total volume of care that addressed a diagnosed skin cancer. Counts of unique patients in prevalence analyses included relevant diagnoses in the primary ICD position only to increase the specificity of prevalence estimates.
Data Analysis—Descriptive analyses included the total number of outpatient visits with a skin cancer diagnosis in DC and PC over the study period, with percentages of total visits by year and by demographic strata. Separate analyses estimated annual prevalences of skin cancer types in the MHS by study year and within 2022 by demographic strata. Numerators in prevalence analyses were defined as the number of unique individuals with one or more relevant ICD codes in the analysis year. Denominators were defined as the total number of MHS beneficiaries in the analysis year and resulting period prevalences reported. Observed prevalences were qualitatively described, and trends were compared with prevalences in nonmilitary populations reported in the literature.
Ethics—This study was conducted as part of a study using secondary analyses of de-identified data from the M2 database. The study was reviewed and approved by the Walter Reed National Military Medical Center institutional review board.

Results
Encounter data were analyzed from a total of 5,374,348 visits between DC and PC over the study period for each cancer type of interest. Figures 1 and 2 show temporal trends in DC visits compared with PC visits in each beneficiary category. The percentage of total DC visits subsequently declined each year throughout the study period, with percentage decreases from 2017 to 2022 of 1.45% or 8200 fewer visits for MM, 3.41% or 7280 fewer visits for BCC, and 2.26% or 3673 fewer visits for SCC.

When stratified by beneficiary category, this trend remained consistent among dependents and retirees, with the most notable annual percentage decrease from 2019 to 2020. A higher proportion of younger adults and active-duty beneficiaries was seen in DC relative to PC, in which most visits were among retirees and others (primarily dependents of retirees, survivors, and Guard/Reserve on active duty, as well as inactive Guard/Reserve). No linear trends over time were apparent for active duty in DC and for dependents and retirees in PC. eTable 1 summarizes the demographic characteristics of MHS beneficiaries being seen in DC and PC over the study period for each cancer type of interest.

The Table shows the period prevalence of skin cancer diagnoses within the MHS beneficiary population from 2017 to 2022. These data were further analyzed by MM, BCC, and SCC (eTable 2) and demographics of interest for the year 2022. By beneficiary category, the period prevalence of MM was 0.08% in active duty, 0.06% in dependents, 0.48% in others, and 1.10% in retirees; the period prevalence of BCC was 0.12% in active duty, 0.07% in dependents, 0.91% in others, and 2.50% in retirees; and the period prevalence of SCC was 0.02% in active duty, 0.01% in dependents, 0.63% in others, and 1.87% in retirees. By sponsor branch, the period prevalence of MM was 0.35% in the army, 0.62% in the air force, 0.35% in the marine corps, and 0.65% in the navy; the period prevalence of BCC was 0.74% in the army, 1.30% in the air force, 0.74% in the marine corps, and 1.36% in the navy; and the period prevalence of SCC was 0.52% in the army, 0.92% in the air force, 0.51% in the marine corps, and 0.97% in the navy.


Comment
This study aimed to provide insight into the burden of skin cancer within the MHS beneficiary population and to identify temporal trends in where these beneficiaries receive their care. We examined patient encounter data from more than 9.6 million MHS beneficiaries.
The utilization of ICD codes from patient encounters to estimate the prevalence of nonmelanoma skin cancer (NMSC) has demonstrated a high positive predictive value. In one study, NMSC cases were confirmed in 96.5% of ICD code–identified patients.5 We presented an extensive collection of epidemiologic data on BCC and SCC, which posed unique challenges for tracking, as they are not reported to or monitored by cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) Program.6
MHS Compared to the US Population—A study using the Global Burden of Disease 2019 database revealed an increasing trend in the incidence and prevalence of NMSC and melanoma since 1990. The same study found the period prevalence in 2019 of MM, SCC, and BCC in the general US population to be 0.13%, 0.31%, and 0.05%, respectively.7 In contrast, among MHS beneficiaries, we observed a higher prevalence in the same year, with figures of 0.66% for MM, 0.72% for SCC, and 1.02% for BCC. According to the SEER database, the period prevalence of MM within the general US population in 2020 was 0.4%.8 That same year, we identified a higher period prevalence of MM—0.54%—within the MHS beneficiary population. Specifically, within the MHS retiree population, the prevalence in 2022 was double that of the general MHS population, with a rate of 1.10%, underscoring the importance of skin cancer screening in older, at-risk adult populations. Prior studies similarly found increased rates of skin cancer within the military beneficiary population. Further studies are needed to compare age-adjusted rates in the MHS vs US population.9-11
COVID-19 Trends—Our data showed an overall decreasing prevalence of skin cancer in the MHS from 2019 to 2021. We suspect that the apparent decrease in skin cancer prevalence may be attributed to underdiagnosis from COVID-19 pandemic restrictions. During that time, many dermatology clinics at military treatment facilities underwent temporary closures, and some dermatologists were sent on nondermatologic utilization tours. Likewise, a US multi-institutional study described declining rates of new melanomas from 2020 to 2021, with an increased proportion of patient presentations with advanced melanoma, suggesting an underdiagnosis of melanoma cases during pandemic restrictions. That study also noted an increased rate of patient-identified melanomas and a decreased rate of provider-identified melanomas during that time.12 Contributing factors may include excess hospital demand, increased patient complexity and acute care needs, and long outpatient clinic backlogs during this time.13Financial Burden—Over our 5-year study period, there were 5,374,348 patient encounters addressing skin cancer, both in DC and PC (Figures 1 and 2; eTable 1). In 2016 to 2018, the average annual cost of treating skin cancer in the US civilian, noninstitutionalized population was $1243 for NMSC (BCC and SCC) and $2430 for melanoma.6 Using this metric, the estimated total cost of care rendered in the MHS in 2018 for NMSC and melanoma was $202,510,803 and $156,516,300, respectively.
Trends in DC vs PC—In the years examined, we found a notable decrease in the number of beneficiaries receiving treatment for MM, BCC, and SCC in DC. Simultaneously, there has been an increase in the number of beneficiaries receiving PC for BCC and SCC, though this trend was not apparent for MM.
Our data provided interesting insights into the percentage of PC compared with DC offered within the MHS. Importantly, our findings suggested that the majority of skin cancer in active-duty service members is managed with DC within the military treatment facility setting (61% DC management over the period analyzed). This finding was true across all years of data analyzed, suggesting that the COVID-19 pandemic did not result in a quantifiable shift in care of skin cancer within the active-duty component to outside providers. One of the critical roles of dermatologists in the MHS is to diagnose and treat skin cancer, and our study suggested that the current global manning and staffing for MHS dermatologists may not be sufficient to meet the burden of skin cancers encountered within our active-duty troops, as only 61% are managed with DC. In particular, service members in more austere and/or overseas locations may not have ready access to a dermatologist.
The burden of skin cancer shifts dramatically when analyzing care of all other populations included in these data, including dependents of active-duty service members, retirees, and the category of “other” (ie, principally dependents of retirees). Within these populations, the rate of DC falls to 30%, with 70% of active-duty dependent care being deferred to network. The findings are even more noticeable for retirees and others within these 2 cohorts in all types of skin cancer analyzed, where DC only accounted for 5.2% of those skin cancers encountered and managed across TRICARE-eligible beneficiaries. For MM, BCC, and SCC, percentages of DC were 5.4%, 5.8%, and 3.5%, respectively. Although it is interesting to note the lower percentage of SCC managed via DC, our data did not allow for extrapolation as to why more SCC cases may be deferred to network. The shift to PC may align with DoD initiatives to increase the private sector’s involvement in military medicine and transition to civilianizing the MHS.14 In the end, the findings are remarkable, with approximately 95% of skin cancer care and management provided overall via PC.
These findings differ from previously published data regarding DC and PC from other specialty areas. Results from an analysis of DC vs PC for plastic surgery for the entire MHS from 2016 to 2019 found 83.2% of cases were deferred to network.15 A similar publication in the orthopedics literature examined TRICARE claims for patients who underwent total hip or knee arthroplasties between 2006 and 2019 and found 84.6% of cases were referred for PC. Notably, the authors utilized generalized linear models for cost analysis and found that DC was more expensive than PC, though this likely was a result of higher rates of hospital readmission within DC cases.16 Lastly, an article on the DC vs PC disposition of MHS patients with breast cancer from 2003 to 2008 found 46% of cases managed with DC vs 26.% with PC and 27.8% receiving a combination. In this case, the authors found a reduced cost associated with DC vs PC.17
Little additional literature exists regarding the costs of DC vs PC. An article published in 2016 designed to assess costs of DC vs PC showed that almost all military treatment facilities have higher costs than their private sector counterparts, with a few exceptions.18 This does not assess the costs of specific procedures, however, and only the overall cost to maintain a treatment facility. Importantly, this study was based on data from FY 2014 and has not been updated to reflect complex changes within the MHS system and the private health care system. Indeed, a US Government Accountability Office FY 2023 study highlighted staffing and efficiency issues within this transition to civilian medicine; subsequently, the 2024 President’s Budget suspended all planned clinical medical military end strength divestitures, underscoring the potential ineffectiveness of a civilianized MHS at meeting the health care needs of its beneficiaries.19,20 Future research on a national scale will be necessary to see if there is a reversal of this trend to PC and if doing so has any impact on access to DC for active-duty troops or active-duty dependents.
In addition to PC vs DC trends, we also can get a sense of the impact of the COVID pandemic restrictions on access to DC vs PC by assessing the change in rates seen in the data from the pre-COVID years (2017-2019) to the “post-COVID” years (2020-2022) included. Overall, rates of DC decreased uniformly from their already low percentages. In our study, rates of DC decreased from 5.8% in 2019 to 4.8% in 2022 for MM, from 6.6% to 4.3% for BCC, and from 4.2% to 2.9% for SCC. Although these changes seem small at first, they represent a 30.6% overall decrease in DC for BCC and an overall decrease of 55.4% in DC for SCC. Although our data do not allow us to extrapolate the real cost of this reduction across a nationwide health care system and more than 5 million care encounters, the financial and personal (ie, lost man-hours) costs of this decrease in DC likely are substantial.
In addition to costs, qualitative aspects that contribute to the burden of skin cancer include treatment-related morbidity, such as scarring, pain, and time spent away from family, work, and hobbies, as well as overall patient satisfaction with the quality of care they receive.21 Future work is critical to assess the real cost of this immense burden of PC for the treatment and management of skin cancers within the DoD beneficiary population.
Limitations—This study is limited by its observational nature. Given the mechanism of our data collection, we may have underestimated disease prevalence, as not all patients are seen for their diagnosis annually. Furthermore, reported demographic strata (eg, age, sex) were limited to those available and valid in the M2 reporting system. Finally, our study only collected data from those service members or former service members seen within the MHS and does not reflect any care rendered to those who are no longer active duty but did not officially retire from the military (ie, nonretired service members receiving care in the Veterans Affairs system for skin cancer).
Conclusion
We describe the annual burden of care for skin cancer in the MHS beneficiary population. Noteworthy findings observed were an overall decrease in beneficiaries being treated for skin cancer through DC; a decreasing annual prevalence of skin cancer diagnosis between 2019 and 2021, which may represent underdiagnosis or decreased follow-up in the setting of the COVID-19 pandemic; and a higher rate of skin cancer in the military beneficiary population compared to the civilian population.
- US Department of Defense. Military health. Accessed October 5, 2023. https://www.defense.gov/
- Wooten NR, Brittingham JA, Pitner RO, et al. Purchased behavioral health care received by Military Health System beneficiaries in civilian medical facilities, 2000-2014. Mil Med. 2018;183:E278-E290. doi:10.1093/milmed/usx101
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- American Academy of Dermatology. Skin cancer. Updated April 22, 2022. Accessed April 17, 2024. https://www.aad.org/media/stats-skin-cancer
- Eide MJ, Krajenta R, Johnson D, et al. Identification of patients with nonmelanoma skin cancer using health maintenance organization claims data. Am J Epidemiol. 2010;171:123-128. doi:10.1093/aje/kwp352
- Kao SYZ, Ekwueme DU, Holman DM, et al. Economic burden of skin cancer treatment in the USA: an analysis of the Medical Expenditure Panel Survey Data, 2012-2018. Cancer Causes Control. 2023;34:205-212. doi:10.1007/s10552-022-01644-0
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- SEER*Explorer. SEER Incidence Data, November 2023 Submission (1975-2021). National Cancer Institute; 2024. Accessed April 17, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=1&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=1
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663. doi:10.1111/j.1365-4362.1984.tb01228.x
- Page WF, Whiteman D, Murphy M. A comparison of melanoma mortality among WWII veterans of the Pacific and European theaters. Ann Epidemiol. 2000;10:192-195. doi:10.1016/s1047-2797(99)00050-2
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737. doi:10.1016/0190-9622(93)70102-Y
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Gibbs A. COVID-19 shutdowns caused delays in melanoma diagnoses, study finds. OHSU News. August 4, 2022. Accessed April 17, 2024. https://news.ohsu.edu/2022/08/04/covid-19-shutdowns-caused-delays-in-melanoma-diagnoses-study-finds
- Kime P. Pentagon budget calls for ‘civilianizing’ military hospitals. Military Times. Published February 10, 2020. Accessed April 17, 2024. https://www.militarytimes.com/news/your-military/2020/02/10/pentagon-budget-calls-for-civilianizing-military-hospitals/
- O’Reilly EB, Norris E, Ortiz-Pomales YT, et al. A comparison of direct care at military medical treatment facilities with purchased care in plastic surgery operative volume. Plast Reconstr Surg Glob Open. 2022;10(10 suppl):124-125. doi:10.1097/01.GOX.0000898976.03344.62
- Haag A, Hosein S, Lyon S, et al. Outcomes for arthroplasties in military health: a retrospective analysis of direct versus purchased care. Mil Med. 2023;188(suppl 6):45-51. doi:10.1093/milmed/usac441
- Eaglehouse YL, Georg MW, Richard P, et al. Cost-efficiency of breast cancer care in the US Military Health System: an economic evaluation in direct and purchased care. Mil Med. 2019;184:e494-e501. doi:10.1093/milmed/usz025
- Lurie PM. Comparing the cost of military treatment facilities with private sector care. Institute for Defense Analyses; February 2016. Accessed April 17, 2024. https://www.ida.org/research-and-publications/publications/all/c/co/comparing-the-costs-of-military-treatment-facilities-with-private-sector-care
- Defense Health Program. Fiscal Year (FY) 2024 President’s Budget: Operation and Maintenance Procurement Research, Development, Test and Evaluation. Department of Defense; March 2023. Accessed April 17, 2024. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2024/budget_justification/pdfs/09_Defense_Health_Program/00-DHP_Vols_I_II_and_III_PB24.pdf
- US Government Accountability Office. Defense Health Care. DOD should reevaluate market structure for military medical treatment facility management. Published August 21, 2023. Accessed April 17, 2024. https://www.gao.gov/products/gao-23-105441
- Rosenberg A, Cho S. We can do better at protecting our service members from skin cancer. Mil Med. 2022;187:311-313. doi:10.1093/milmed/usac198
- US Department of Defense. Military health. Accessed October 5, 2023. https://www.defense.gov/
- Wooten NR, Brittingham JA, Pitner RO, et al. Purchased behavioral health care received by Military Health System beneficiaries in civilian medical facilities, 2000-2014. Mil Med. 2018;183:E278-E290. doi:10.1093/milmed/usx101
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- American Academy of Dermatology. Skin cancer. Updated April 22, 2022. Accessed April 17, 2024. https://www.aad.org/media/stats-skin-cancer
- Eide MJ, Krajenta R, Johnson D, et al. Identification of patients with nonmelanoma skin cancer using health maintenance organization claims data. Am J Epidemiol. 2010;171:123-128. doi:10.1093/aje/kwp352
- Kao SYZ, Ekwueme DU, Holman DM, et al. Economic burden of skin cancer treatment in the USA: an analysis of the Medical Expenditure Panel Survey Data, 2012-2018. Cancer Causes Control. 2023;34:205-212. doi:10.1007/s10552-022-01644-0
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- SEER*Explorer. SEER Incidence Data, November 2023 Submission (1975-2021). National Cancer Institute; 2024. Accessed April 17, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=1&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=1
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663. doi:10.1111/j.1365-4362.1984.tb01228.x
- Page WF, Whiteman D, Murphy M. A comparison of melanoma mortality among WWII veterans of the Pacific and European theaters. Ann Epidemiol. 2000;10:192-195. doi:10.1016/s1047-2797(99)00050-2
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737. doi:10.1016/0190-9622(93)70102-Y
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Gibbs A. COVID-19 shutdowns caused delays in melanoma diagnoses, study finds. OHSU News. August 4, 2022. Accessed April 17, 2024. https://news.ohsu.edu/2022/08/04/covid-19-shutdowns-caused-delays-in-melanoma-diagnoses-study-finds
- Kime P. Pentagon budget calls for ‘civilianizing’ military hospitals. Military Times. Published February 10, 2020. Accessed April 17, 2024. https://www.militarytimes.com/news/your-military/2020/02/10/pentagon-budget-calls-for-civilianizing-military-hospitals/
- O’Reilly EB, Norris E, Ortiz-Pomales YT, et al. A comparison of direct care at military medical treatment facilities with purchased care in plastic surgery operative volume. Plast Reconstr Surg Glob Open. 2022;10(10 suppl):124-125. doi:10.1097/01.GOX.0000898976.03344.62
- Haag A, Hosein S, Lyon S, et al. Outcomes for arthroplasties in military health: a retrospective analysis of direct versus purchased care. Mil Med. 2023;188(suppl 6):45-51. doi:10.1093/milmed/usac441
- Eaglehouse YL, Georg MW, Richard P, et al. Cost-efficiency of breast cancer care in the US Military Health System: an economic evaluation in direct and purchased care. Mil Med. 2019;184:e494-e501. doi:10.1093/milmed/usz025
- Lurie PM. Comparing the cost of military treatment facilities with private sector care. Institute for Defense Analyses; February 2016. Accessed April 17, 2024. https://www.ida.org/research-and-publications/publications/all/c/co/comparing-the-costs-of-military-treatment-facilities-with-private-sector-care
- Defense Health Program. Fiscal Year (FY) 2024 President’s Budget: Operation and Maintenance Procurement Research, Development, Test and Evaluation. Department of Defense; March 2023. Accessed April 17, 2024. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2024/budget_justification/pdfs/09_Defense_Health_Program/00-DHP_Vols_I_II_and_III_PB24.pdf
- US Government Accountability Office. Defense Health Care. DOD should reevaluate market structure for military medical treatment facility management. Published August 21, 2023. Accessed April 17, 2024. https://www.gao.gov/products/gao-23-105441
- Rosenberg A, Cho S. We can do better at protecting our service members from skin cancer. Mil Med. 2022;187:311-313. doi:10.1093/milmed/usac198
PRACTICE POINTS
- Study data showed an overall decreasing prevalence of skin cancer in the Military Health System (MHS) from 2019 to 2021, possibly attributable to underdiagnosis resulting from the COVID-19 pandemic. Providers should be mindful of this trend when screening patients who have experienced interruptions in care.
- An overall increased prevalence of skin cancer was noted in the military beneficiary population compared with publicly available civilian data—and thus this diagnosis should be given special consideration within this population.
An Update on Cutaneous Angiosarcoma Diagnosis and Treatment
Angiosarcomas are aggressive endothelial cell tumors of vascular origin that account for 1% to 2% of all soft tissue sarcomas in the United States.1,2 They can affect any organ in the body but most commonly affect the skin and soft tissue. Cutaneous angiosarcoma (CAS) is a rare type of skin cancer that can present in 2 forms: primary and secondary.
Dermatologists may be responsible for the initial diagnosis and management of CAS. They must be familiar with its presentation, as this condition can be difficult to diagnose and mimics other diseases. Additionally, dermatologists must understand the role of varying treatment modalities including Mohs micrographic surgery (MMS) in the management of CAS. This review will provide an overview of the epidemiology, presentation, and pathologic features of CAS and will discuss both emerging and existing treatments.
Epidemiology
Cutaneous angiosarcoma may present in various locations in the body, predominantly on the head and neck.4,5 Approximately 85% of cases arise in patients older than 60 years, and most of these patients are White men.1,4,5 The risk factors for the development of CAS include prior radiation exposure; chronic lymphedema (ie, Stewart-Treves syndrome); and familial syndromes including neurofibromatosis 1, BRCA1 or BRCA2 mutations, Maffucci syndrome, and Klippel-Trenaunay syndrome. Exogenous exposure to toxins such as vinyl chloride, thorium dioxide, or anabolic steroids also is associated with angiosarcoma, primarily in the form of visceral disease such as hepatic angiosarcoma.6
The average tumor size is approximately 4 to 5 cm; however, some tumors may grow larger than 10 cm.7,8 Metastasis through hematogenous or lymphatic spread is fairly common, occurring in approximately 16% to 35% of patients. The lungs and liver are the most common sites of metastasis.9,10 The age-adjusted incidence rate of CAS is decreasing for patients younger than 50 years, from 1.30 in 1995 to 2004 to 1.10 in 2005 to 2014, but increasing for individuals older than 70 years, from 2.53 in 1995 to 2004 to 2.87 in 2005 to 2014.4 The incidence of angiosarcoma also has grown in the female population, likely due to the increasing use of radiotherapy for the treatment of breast cancer.11
The high rates of CAS on the head and neck may be explained by the increased vascularity and UV exposure in these locations.12 In a Surveillance, Epidemiology, and End Results population-based study (N=811), 43% of patients with CAS had a history of other malignancies such as breast, prostate, genitourinary, gastrointestinal tract, and respiratory tract cancers.4 Cutaneous angiosarcoma can develop secondary to the primary cancer treatment, as seen in patients who develop CAS following radiation therapy.11
The underlying mechanism of CAS is believed to involve dysregulation of angiogenesis due to the vascular origin of these tumors. Studies have identified overexpression of vascular endothelial growth factor (VEGF), TP53 mutations, and RAS pathway mutations as potential contributing factors to the pathogenesis of angiosarcoma.6 Molecular differences between primary and secondary angiosarcomas are not well documented; however, radiation-associated CAS has been found to have higher expression of LYN and PRKCΘ, while non–radiation-induced lesions express FTL1 and AKT3.2 Chromosomal abnormalities have been identified in a small set of primary CAS patients, but the specific role of these abnormalities in the pathogenesis of CAS remains unclear.7
Prognosis
Cutaneous angiosarcoma has a poor prognosis, with 3-year disease-specific survival rates as low as 40% and 5-year rates as low as 17%.4,5,13,14 Survival rates increased from 1985 to 2014, likely due to earlier diagnoses and more effective treatments.4 Several factors are associated with worse prognosis, including metastatic disease, increasing age, scalp and neck tumor location, tumor size greater than 5 cm, necrosis, multiple skin lesions, and nodular and epithelioid morphology.4,5,10,13-16 Factors including sex, race, and presence of another malignancy do not affect survival.4,5 Prognosis in CAS may be evaluated by TNM tumor staging. The American Joint Committee on Cancer Staging Manual (8th edition) for soft tissue sarcoma (STS) commonly is used; however, CAS is not included in this staging system because it does not share the same behavior and natural history as other types of STS. This staging system provides separate guidelines for STS of the head and neck and STS of the extremities and trunk because of the smaller size but paradoxically higher risk for head and neck tumors.17 Given that there is no agreed-upon staging system for CAS, prognosis and communication among providers may be complicated.
Clinical Presentation
Early CAS typically presents as single or multifocal ill-defined, enlarging, violaceous or dusky red macules or patches (Figure 1). Lesions often rapidly develop into raised nodules and plaques that may bleed and ulcerate. Other common symptoms include pain, edema, neuropathy, anemia, and weight loss; however, it is not uncommon for lesions to be asymptomatic.8,18-20 Nodular lesions are more common on the scalp, and patches are more common on the face and neck.16 Tumors typically extend into the dermis, and aggressive cancers may invade the subcutaneous tissue and fascia.2
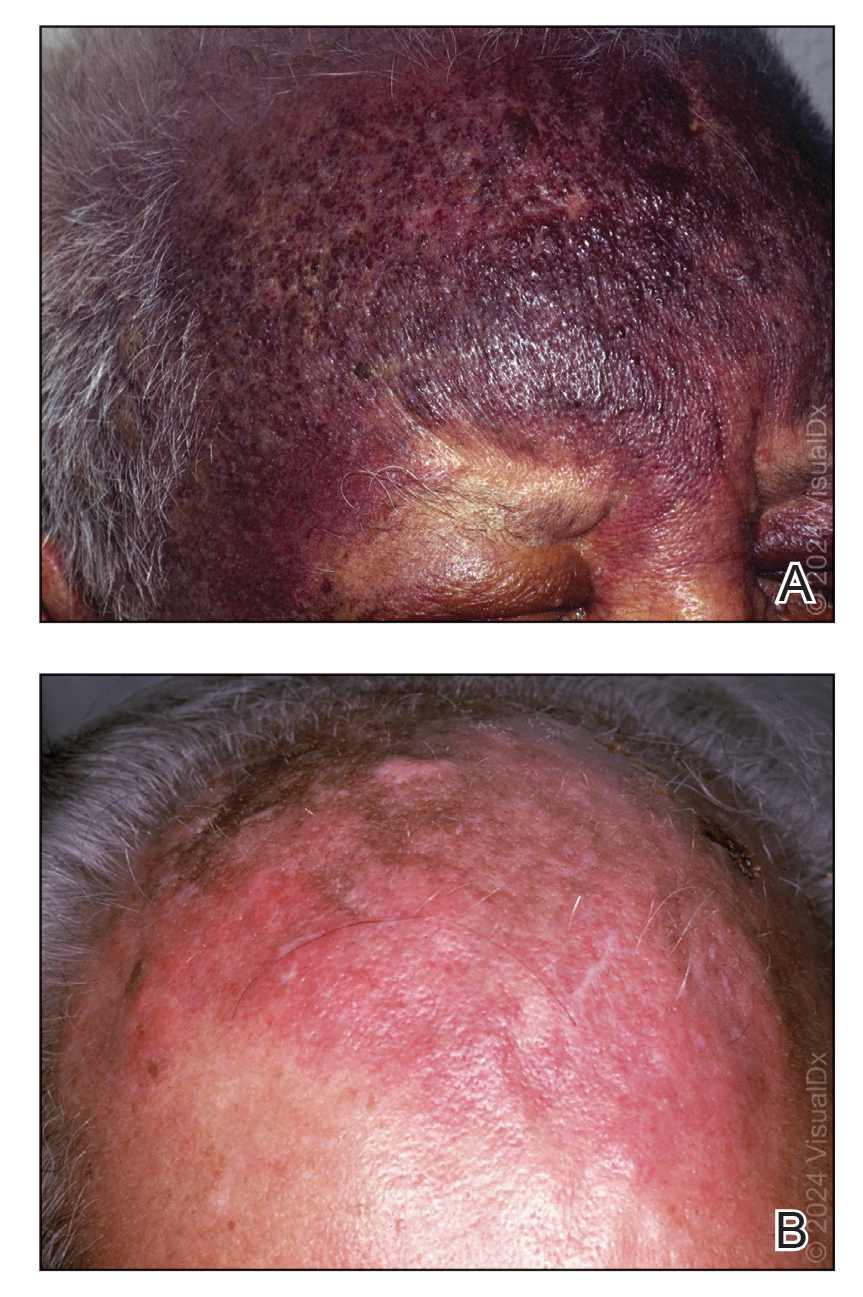
Cutaneous angiosarcoma may mimic ecchymosis, hemangioma, lymphangioma, edema, cellulitis, or scarring alopecia. Its nonspecific features make it difficult to recognize without dermoscopy or ultrasonography, which often results in delayed diagnosis and treatment. The median delay typically is 5 to 7 months and up to 1 year for some patients.7,16 Cutaneous angiosarcoma of the scalp tends to have a longer diagnostic delay than other areas of the body, which may be attributable to challenges in tumor identification and visualization by patients.16
Dermoscopy and ultrasonography can aid in the diagnosis of CAS. Dermoscopy may demonstrate a range of colors with yellow, brown, or red areas in a violaceous background. Other reported features include white veils and lines, purple ovals, pink-purple “steamlike” areas, and atypical vessels (Figure 2).21-23 Dermoscopic findings may appear similar to other vascular tumors, such as hemangioma and Kaposi sarcoma, or nonvascular tumors, including amelanotic melanoma, Merkel cell carcinoma, and primary cutaneous B-cell lymphoma. Ultrasonography may show ill-defined, hypoechoic areas with anechoic reticular channels and a hypoechoic subepidermal layer.21 Other radiologic modalities, such as computed tomography, magnetic resonance imaging, or positron emission tomography, are nonspecific and are more useful in evaluating the extent of tumor spread in visceral angiosarcoma. Magnetic resonance imaging in CAS may indicate malignancy with the presence of high T2 and T1 signal intensity and high-flow serpentine vessels.24
Histopathology
Histologically, angiosarcoma is characterized by anastomosing irregular vascular channels lined by a single layer of endothelial cells displaying slight to moderate atypia.25 These vascular channels dissect between collagen bundles and adipocytes. Monocyte infiltration may be observed.6 The neoplastic endothelial cells may present as spindle-shaped, round, polygonal, or epithelioid with eosinophilic cytoplasm. Histologic features differ based on the type of clinical lesion (Figure 3). In a study of CAS in Asian populations, nodular tumors showed solid sheets of pleomorphic spindle cells, many mitotic figures, and widely hemorrhagic spaces, whereas nonnodular tumors showed irregular vascular spaces dissecting collagen.16 Poorly differentiated tumors may present with hyperchromatic nuclei and prominent nucleoli, papillary endothelial formations, mitoses, and possible hemorrhage or necrosis.2,6,8 Histologic specimens also may reveal calcified bodies and hemosiderin particles.19 Angiosarcomas typically are invasive without a clear capsule or border.6
Secondary CAS in the setting of lymphedema and radiation therapy has MYC amplification and is positive for MYC via immunohistochemistry, which is uncommon in primary angiosarcoma.26 Immunohistochemical staining of tumor specimens is helpful to confirm the diagnosis of CAS. These markers include CD31, CD34, CD117, cytokeratin, vimentin, epithelial membrane antigen, factor VIII–related antigen, Ulex europaeus agglutinin-1, von Willebrand factor, and VEGF.6,19,27,28 Notably, advanced angiosarcomas with progressive dedifferentiation often lose these markers.
Treatment
Surgery—The majority of patients treated for CAS undergo surgical resection, as surgery has been shown to have the best prognosis for patients.5,9,10,13,15 Achieving R0 resection (microscopically negative margins) is the most important factor in determining the success of treatment, with incomplete surgical resection resulting in higher rates of systemic and local spread.29 Abraham et al8 found that the median disease-specific survival of patients with microscopically negative margins was 83.7 months; patients with microscopically positive and grossly positive margins had median disease-specific survival of 63.4 and 18.1 months, respectively. In a case series of patients undergoing resection with negative surgical margins, 4 patients demonstrated no evidence of local recurrence or systemic disease at an average of 4.3 years after therapy, and the other 4 patients each had 1 local recurrence but were disease free an average of 4.8 years after removal of the recurrent lesion. In a series of 27 patients with positive surgical margins, there was local recurrence within 2 years for most patients.12
Large tumors invading nearby structures may not be amenable to surgical resection because of extensive local growth, propensity for skip lesions, and localization near vital organs of the head and neck.5,7 The extended delay in diagnosis often seen in CAS allows for advanced local progression, resulting in large areas of resection. In a case series (N=8), the average surgical defect measured 14.3×11.8 cm, necessitating reconstruction with either a tissue flap or split-thickness skin graft in every case because primary closure was not possible. More than 80% of patients in this study still had positive margins after surgery, necessitating the use of additional chemotherapy or radiation to eradicate remaining disease.7 In several studies, multimodality therapy was associated with improved overall survival.7,14,30
Mohs Micrographic Surgery—Mohs micrographic surgery is the standard of care for many aggressive cutaneous malignancies on the head, but its utility for the treatment of CAS is uncertain. Only a few studies have compared the efficacy of MMS vs wide local excision (WLE). There have been reports of recurrence-free follow-up at 12, 16, 18, 20, and 72 months after MMS.31-36 The latter case showed a patient who underwent MMS with a 72-month relapse-free survival, whereas other patients who underwent WLE only survived 5 to 7 months without recurrence.36 In another study, there was a local recurrence rate of 42.9% after a median follow-up of 4 years in 7 patients with CAS treated with complete circumferential peripheral and deep margin assessment, which is less than the reported recurrence rates of 72% to 84% after standard excisional procedures.28,37
Houpe et al38 conducted a systematic review of the use of WLE vs MMS; the median overall survival was longest for WLE in conjunction with chemotherapy, radiotherapy, and immunotherapy at 39.3 months, followed by MMS alone at 37 months. Mohs micrographic surgery in conjunction with chemotherapy and radiotherapy was used in 1 patient, with a median overall survival of 82 months. Wide local excision alone resulted in a median overall survival of 19.8 months. Although these data are promising and suggest that the combination of surgery with adjuvant therapy may be more beneficial than surgery alone, it is important to note that there were only 9 cases treated with MMS compared with 825 cases treated with WLE.38
Several studies have documented that paraffin-embedded sections may be more useful than frozen sections in the determination of margin positivity from a surgical specimen, as frozen sections showed a poor negative predictive value of 33.3%.7,35 Mohs micrographic surgery has been proposed for tumors measuring less than 5 cm; however, the most recent appropriate use criteria for MMS of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery deemed the use of MMS for angiosarcoma uncertain.32,33,37 Further research is necessary to elucidate the role of MMS in the management of CAS.
Radiotherapy—Radiotherapy is a common adjuvant to surgical resection but has been used palliatively in patients with tumors that are unresectable. Improved local control and disease-free survival have been observed with the combination of radiation and surgery. A dose response to radiotherapy has been demonstrated,18,30 with 1 study showing that patients who received more than 5000 cGy of radiotherapy achieved better local control than patients who received 4500 cGy or less.18 Pawlik et al7 showed a decreased chance of death with the addition of adjunctive radiotherapy, and patients who underwent postoperative radiotherapy demonstrated a median survival almost 4-times longer than patients who did not receive radiation. Morrison et al39 reported that radiation therapy administered to patients with no clinically evident disease after surgical resection resulted in improved local control and overall survival vs patients who were irradiated with clinically evident disease.
Complications of radiotherapy for angiosarcoma have been reported, including xerostomia, nonfunctionally significant fibrosis, chronic ulceration/cellulitis of the scalp, necrosis requiring debridement, severe ocular complications, and fibrosis of the eyelids requiring surgical intervention.14 Radiation therapy also poses unique risks to patients with radiation-induced angiosarcoma of the breast, as many of these patients have already received the maximum recommended dose of radiation in the affected areas and additional radiation could exacerbate their CAS.
Chemotherapy—Chemotherapy occasionally is used as an adjunct to surgical resection with positive margins or as palliative care when surgical resection is not possible. Unfortunately, STSs have a response rate of less than 40% to standard chemotherapy.40 Studies in which the use of chemotherapy is evaluated for CAS have mixed results. Mark et al18 reported no significant overall survival benefit when comparing CAS treated with surgery plus radiotherapy with or without chemotherapy. Torres et al41 evaluated radiation-induced angiosarcoma of the breast and found a reduced risk for local recurrence in patients receiving chemotherapy in addition to surgery, indicating that chemotherapy may be useful in this subset of patients when radiation is not recommended.
Cytotoxic chemotherapy agents such as paclitaxel, doxorubicin, or doxorubicin in combination with mesna and ifosfamide (MAI) are common.39 Median progression-free survival is 5.4 months, 4 to 5.6 months, and 3.9 months for MAI, paclitaxel, and doxorubicin, respectively.8,9,42-46 Improved prognosis with MAI may indicate that combination chemotherapy regimens are more effective than single-agent regimens. Cutaneous angiosarcomas may respond better to paclitaxel than doxorubicin, and angiosarcomas of the scalp and face have shown a better response to paclitaxel.47,48
Other Therapies—Although there have not been large-scale studies performed on alternative treatments, there are several case reports on the use of immune modulators, biologics, β-blockers, and various other therapies in the treatment of CAS. The following studies include small sample sizes of patients with metastatic or locally aggressive disease not amenable to surgical resection, which may affect reported outcomes and survival times.49-57 In addition, several studies include patients with visceral angiosarcoma, which may not be generalizable to the CAS population. Even so, these treatment alternatives should not be overlooked because there are few agents that are truly efficacious in the treatment of CAS.
Results on the use of VEGF and tyrosine kinase inhibitors have been disappointing. There have been reports of median progression-free survival of only 3.8 months with sorafenib treatment, 3 months with pazopanib, and 6 months with bevacizumab.49-51 However, one study of patients who were treated with bevacizumab combined with radiation and surgery resulted in a complete response in 2 patients, with no evidence of residual disease at the last follow-up of 8.5 months and 2.1 years.52
Studies on the utility of β-blockers in the treatment of CAS have shown mixed results. Pasquier et al53 evaluated the use of adjunctive therapy with propranolol and vinblastine-based chemotherapy, with a promising median progression-free survival of 11 months compared with an average of 3 to 6 months with conventional chemotherapy regimens. However, in vitro studies reported by Pasquier et al53 indicated that the addition of propranolol to doxorubicin or paclitaxel did not result in increased efficacy. Chow et al54 demonstrated that propranolol monotherapy resulted in a reduction of the proliferative index of scalp angiosarcoma by 34% after only 1 week of treatment. This was followed by combination therapy of propranolol, paclitaxel, and radiation, which resulted in substantial tumor regression and no evidence of metastasis after 8 months of therapy.54
Immune checkpoint inhibitors have been a recent subject of interest in the treatment of angiosarcoma. Two case reports showed improvement in CAS of the face and primary pleural angiosarcoma with a course of pembrolizumab.55,56 In another case series, investigators used immune checkpoint inhibitors in 7 patients with cutaneous, breast, or radiation-associated angiosarcoma and found partial response in several patients treated with pembrolizumab and ipilimumab-nivolumab and complete response in 1 patient treated with anti–cytotoxic T-lymphocyte–associated protein 4 antibodies. The authors of this study hypothesized that treatment response was associated with the mutational profile of tumors, including mutational signatures of UV radiation with a large number of C-to-T substitutions similar to melanomas.57
Conclusion
Cutaneous angiosarcoma is a rare and aggressive tumor with a poor prognosis due to delayed detection. A thorough skin examination and heightened awareness of CAS by dermatologists may result in early biopsy and shortened time to a definitive diagnosis. Until quality evidence allows for the creation of consensus guidelines, care at a cancer center that specializes in rare and difficult-to-treat tumors and employs a multidisciplinary approach is essential to optimizing patient outcomes. Current knowledge supports surgery with negative margins as the mainstay of treatment, with adjuvant radiation, chemotherapy, and targeted therapies as possible additions for extensive disease. The role of MMS is uncertain, and because of the lack of contiguity in CAS, it may not be an optimal treatment.
- Rouhani P, Fletcher CD, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627.
- Goldblum JR, Folpe AL, Weiss SW. Enzinger & Weiss’s Soft Tissue Tumors. 7th ed. Elsevier Inc; 2020.
- Arora TK, Terracina KP, Soong J, et al. Primary and secondary angiosarcoma of the breast. Gland Surg. 2014;3:28-34.
- Conic RRZ, Damiani G, Frigerio A, et al. Incidence and outcomes of cutaneous angiosarcoma: a SEER population-based study. J Am Acad Dermatol. 2020;83:809-816.
- Chang C, Wu SP, Hu K, et al. Patterns of care and survival of cutaneous angiosarcoma of the head and neck. Otolaryngol Head Neck Surg. 2020;162:881-887.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953-1967.
- Fury MG, Antonescu CR, Van Zee KJ, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241-247.
- Lindet C, Neuville A, Penel N, et al. Localised angiosarcomas: the identification of prognostic factors and analysis of treatment impact. a retrospective analysis from the French Sarcoma Group (GSF/GETO). Eur J Cancer. 2013;49:369-376.
- Mery CM, George S, Bertagnolli MM, et al. Secondary sarcomas after radiotherapy for breast cancer: sustained risk and poor survival. Cancer. 2009;115:4055-4063.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Dettenborn T, Wermker K, Schulze HJ, et al. Prognostic features in angiosarcoma of the head and neck: a retrospective monocenter study. J Craniomaxillofac Surg. 2014;42:1623-1628.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Moon IJ, Kim YJ, Won CH, et al. Clinicopathological and survival analyses of primary cutaneous angiosarcoma in an Asian population: prognostic value of the clinical features of skin lesions. Int J Dermatol. 2020;59:582-589.
- Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma. a report of 67 patients and a review of the literature. Cancer. 1996;77:2400-2406.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. a review of 99 cases. Cancer. 1995;75:989-996.
- Fayette J, Martin E, Piperno-Neumann S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007;18:2030-2036.
- Oranges T, Janowska A, Vitali S, et al. Dermatoscopic and ultra-high frequency ultrasound evaluation in cutaneous postradiation angiosarcoma. J Eur Acad Dermatol Venereol. 2020;34:e741.
- Figueroa-Silva O, Argenziano G, Lallas A, et al. Dermoscopic pattern of radiation-induced angiosarcoma (RIA). J Am Acad Dermatol. 2015;73:E51-E55.
- Cole DW, Huerta T, Andea A, et al. Purpuric plaques-dermoscopic and histopathological correlation of cutaneous angiosarcoma. Dermatol Pract Concept. 2020;10:E2020084. doi:10.5826/dpc.1004a84
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. Vol 2. 4th ed. Elsevier; 2018.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39. doi:10.2353/ajpath.2010.090637
- Ohsawa M, Naka N, Tomita Y, et al. Use of immunohistochemical procedures in diagnosing angiosarcoma. Evaluation of 98 cases. Cancer. 1995;75:2867-2874.
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850.
- Lahat G, Dhuka AR, Lahat S, et al. Outcome of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol. 2009;16:2502-2509.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Kofler L, Breuninger H, Schulz C, et al. Local recurrence rates of skin tumors after resection with complete circumferential peripheral and deep margin assessment-identification of high-risk entities. Dermatol Surg. 2021;47:E31-E36.
- Houpe JE, Seger EW, Neill BC, et al. Treatment of angiosarcoma of the head and neck: a systematic review. Cutis. 2023;111:247-251. doi:10.12788/cutis.0767
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. a therapeutic dilemma. Cancer. 1995;76:319-327.
- Gonzalez MJ, Koehler MM, Satter EK. Angiosarcoma of the scalp: a case report and review of current and novel therapeutic regimens. Dermatol Surg. 2009;35:679-684.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26:5269-5274.
- Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann Oncol. 2012;23:517-523.
- Young RJ, Natukunda A, Litière S, et al. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer. 2014;50:3178-3186.
- Skubitz KM, Haddad PA. Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer. 2005;104:361-366.
- Fata F, O’Reilly E, Ilson D, et al. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer. 1999;86:2034-2037.
- Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer. 2012;118:3330-3336.
- Schlemmer M, Reichardt P, Verweij J, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2008;44:2433-2436.
- Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133-3140.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Kollár A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017;56:88-92.
- Koontz BF, Miles EF, Rubio MA, et al. Preoperative radiotherapy and bevacizumab for angiosarcoma of the head and neck: two case studies. Head Neck. 2008;30:262-266.
- Pasquier E, André N, Street J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine. 2016;6:87-95.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Wang X, Wei J, Zeng Z, et al. Primary pleural epithelioid angiosarcoma treated successfully with anti-PD-1 therapy: a rare case report. Medicine (Baltimore). 2021;100:E27132.
- Sindhu S, Gimber LH, Cranmer L, et al. Angiosarcoma treated successfully with anti-PD-1 therapy—a case report. J Immunother Cancer. 2017;5:58.
- Florou V, Rosenberg AE, Wieder E, et al. Angiosarcoma patients treated with immune checkpoint inhibitors: a case series of seven patients from a single institution. J Immunother Cancer. 2019;7:213.
Angiosarcomas are aggressive endothelial cell tumors of vascular origin that account for 1% to 2% of all soft tissue sarcomas in the United States.1,2 They can affect any organ in the body but most commonly affect the skin and soft tissue. Cutaneous angiosarcoma (CAS) is a rare type of skin cancer that can present in 2 forms: primary and secondary.
Dermatologists may be responsible for the initial diagnosis and management of CAS. They must be familiar with its presentation, as this condition can be difficult to diagnose and mimics other diseases. Additionally, dermatologists must understand the role of varying treatment modalities including Mohs micrographic surgery (MMS) in the management of CAS. This review will provide an overview of the epidemiology, presentation, and pathologic features of CAS and will discuss both emerging and existing treatments.
Epidemiology
Cutaneous angiosarcoma may present in various locations in the body, predominantly on the head and neck.4,5 Approximately 85% of cases arise in patients older than 60 years, and most of these patients are White men.1,4,5 The risk factors for the development of CAS include prior radiation exposure; chronic lymphedema (ie, Stewart-Treves syndrome); and familial syndromes including neurofibromatosis 1, BRCA1 or BRCA2 mutations, Maffucci syndrome, and Klippel-Trenaunay syndrome. Exogenous exposure to toxins such as vinyl chloride, thorium dioxide, or anabolic steroids also is associated with angiosarcoma, primarily in the form of visceral disease such as hepatic angiosarcoma.6
The average tumor size is approximately 4 to 5 cm; however, some tumors may grow larger than 10 cm.7,8 Metastasis through hematogenous or lymphatic spread is fairly common, occurring in approximately 16% to 35% of patients. The lungs and liver are the most common sites of metastasis.9,10 The age-adjusted incidence rate of CAS is decreasing for patients younger than 50 years, from 1.30 in 1995 to 2004 to 1.10 in 2005 to 2014, but increasing for individuals older than 70 years, from 2.53 in 1995 to 2004 to 2.87 in 2005 to 2014.4 The incidence of angiosarcoma also has grown in the female population, likely due to the increasing use of radiotherapy for the treatment of breast cancer.11
The high rates of CAS on the head and neck may be explained by the increased vascularity and UV exposure in these locations.12 In a Surveillance, Epidemiology, and End Results population-based study (N=811), 43% of patients with CAS had a history of other malignancies such as breast, prostate, genitourinary, gastrointestinal tract, and respiratory tract cancers.4 Cutaneous angiosarcoma can develop secondary to the primary cancer treatment, as seen in patients who develop CAS following radiation therapy.11
The underlying mechanism of CAS is believed to involve dysregulation of angiogenesis due to the vascular origin of these tumors. Studies have identified overexpression of vascular endothelial growth factor (VEGF), TP53 mutations, and RAS pathway mutations as potential contributing factors to the pathogenesis of angiosarcoma.6 Molecular differences between primary and secondary angiosarcomas are not well documented; however, radiation-associated CAS has been found to have higher expression of LYN and PRKCΘ, while non–radiation-induced lesions express FTL1 and AKT3.2 Chromosomal abnormalities have been identified in a small set of primary CAS patients, but the specific role of these abnormalities in the pathogenesis of CAS remains unclear.7
Prognosis
Cutaneous angiosarcoma has a poor prognosis, with 3-year disease-specific survival rates as low as 40% and 5-year rates as low as 17%.4,5,13,14 Survival rates increased from 1985 to 2014, likely due to earlier diagnoses and more effective treatments.4 Several factors are associated with worse prognosis, including metastatic disease, increasing age, scalp and neck tumor location, tumor size greater than 5 cm, necrosis, multiple skin lesions, and nodular and epithelioid morphology.4,5,10,13-16 Factors including sex, race, and presence of another malignancy do not affect survival.4,5 Prognosis in CAS may be evaluated by TNM tumor staging. The American Joint Committee on Cancer Staging Manual (8th edition) for soft tissue sarcoma (STS) commonly is used; however, CAS is not included in this staging system because it does not share the same behavior and natural history as other types of STS. This staging system provides separate guidelines for STS of the head and neck and STS of the extremities and trunk because of the smaller size but paradoxically higher risk for head and neck tumors.17 Given that there is no agreed-upon staging system for CAS, prognosis and communication among providers may be complicated.
Clinical Presentation
Early CAS typically presents as single or multifocal ill-defined, enlarging, violaceous or dusky red macules or patches (Figure 1). Lesions often rapidly develop into raised nodules and plaques that may bleed and ulcerate. Other common symptoms include pain, edema, neuropathy, anemia, and weight loss; however, it is not uncommon for lesions to be asymptomatic.8,18-20 Nodular lesions are more common on the scalp, and patches are more common on the face and neck.16 Tumors typically extend into the dermis, and aggressive cancers may invade the subcutaneous tissue and fascia.2

Cutaneous angiosarcoma may mimic ecchymosis, hemangioma, lymphangioma, edema, cellulitis, or scarring alopecia. Its nonspecific features make it difficult to recognize without dermoscopy or ultrasonography, which often results in delayed diagnosis and treatment. The median delay typically is 5 to 7 months and up to 1 year for some patients.7,16 Cutaneous angiosarcoma of the scalp tends to have a longer diagnostic delay than other areas of the body, which may be attributable to challenges in tumor identification and visualization by patients.16
Dermoscopy and ultrasonography can aid in the diagnosis of CAS. Dermoscopy may demonstrate a range of colors with yellow, brown, or red areas in a violaceous background. Other reported features include white veils and lines, purple ovals, pink-purple “steamlike” areas, and atypical vessels (Figure 2).21-23 Dermoscopic findings may appear similar to other vascular tumors, such as hemangioma and Kaposi sarcoma, or nonvascular tumors, including amelanotic melanoma, Merkel cell carcinoma, and primary cutaneous B-cell lymphoma. Ultrasonography may show ill-defined, hypoechoic areas with anechoic reticular channels and a hypoechoic subepidermal layer.21 Other radiologic modalities, such as computed tomography, magnetic resonance imaging, or positron emission tomography, are nonspecific and are more useful in evaluating the extent of tumor spread in visceral angiosarcoma. Magnetic resonance imaging in CAS may indicate malignancy with the presence of high T2 and T1 signal intensity and high-flow serpentine vessels.24

Histopathology
Histologically, angiosarcoma is characterized by anastomosing irregular vascular channels lined by a single layer of endothelial cells displaying slight to moderate atypia.25 These vascular channels dissect between collagen bundles and adipocytes. Monocyte infiltration may be observed.6 The neoplastic endothelial cells may present as spindle-shaped, round, polygonal, or epithelioid with eosinophilic cytoplasm. Histologic features differ based on the type of clinical lesion (Figure 3). In a study of CAS in Asian populations, nodular tumors showed solid sheets of pleomorphic spindle cells, many mitotic figures, and widely hemorrhagic spaces, whereas nonnodular tumors showed irregular vascular spaces dissecting collagen.16 Poorly differentiated tumors may present with hyperchromatic nuclei and prominent nucleoli, papillary endothelial formations, mitoses, and possible hemorrhage or necrosis.2,6,8 Histologic specimens also may reveal calcified bodies and hemosiderin particles.19 Angiosarcomas typically are invasive without a clear capsule or border.6

Secondary CAS in the setting of lymphedema and radiation therapy has MYC amplification and is positive for MYC via immunohistochemistry, which is uncommon in primary angiosarcoma.26 Immunohistochemical staining of tumor specimens is helpful to confirm the diagnosis of CAS. These markers include CD31, CD34, CD117, cytokeratin, vimentin, epithelial membrane antigen, factor VIII–related antigen, Ulex europaeus agglutinin-1, von Willebrand factor, and VEGF.6,19,27,28 Notably, advanced angiosarcomas with progressive dedifferentiation often lose these markers.
Treatment
Surgery—The majority of patients treated for CAS undergo surgical resection, as surgery has been shown to have the best prognosis for patients.5,9,10,13,15 Achieving R0 resection (microscopically negative margins) is the most important factor in determining the success of treatment, with incomplete surgical resection resulting in higher rates of systemic and local spread.29 Abraham et al8 found that the median disease-specific survival of patients with microscopically negative margins was 83.7 months; patients with microscopically positive and grossly positive margins had median disease-specific survival of 63.4 and 18.1 months, respectively. In a case series of patients undergoing resection with negative surgical margins, 4 patients demonstrated no evidence of local recurrence or systemic disease at an average of 4.3 years after therapy, and the other 4 patients each had 1 local recurrence but were disease free an average of 4.8 years after removal of the recurrent lesion. In a series of 27 patients with positive surgical margins, there was local recurrence within 2 years for most patients.12
Large tumors invading nearby structures may not be amenable to surgical resection because of extensive local growth, propensity for skip lesions, and localization near vital organs of the head and neck.5,7 The extended delay in diagnosis often seen in CAS allows for advanced local progression, resulting in large areas of resection. In a case series (N=8), the average surgical defect measured 14.3×11.8 cm, necessitating reconstruction with either a tissue flap or split-thickness skin graft in every case because primary closure was not possible. More than 80% of patients in this study still had positive margins after surgery, necessitating the use of additional chemotherapy or radiation to eradicate remaining disease.7 In several studies, multimodality therapy was associated with improved overall survival.7,14,30
Mohs Micrographic Surgery—Mohs micrographic surgery is the standard of care for many aggressive cutaneous malignancies on the head, but its utility for the treatment of CAS is uncertain. Only a few studies have compared the efficacy of MMS vs wide local excision (WLE). There have been reports of recurrence-free follow-up at 12, 16, 18, 20, and 72 months after MMS.31-36 The latter case showed a patient who underwent MMS with a 72-month relapse-free survival, whereas other patients who underwent WLE only survived 5 to 7 months without recurrence.36 In another study, there was a local recurrence rate of 42.9% after a median follow-up of 4 years in 7 patients with CAS treated with complete circumferential peripheral and deep margin assessment, which is less than the reported recurrence rates of 72% to 84% after standard excisional procedures.28,37
Houpe et al38 conducted a systematic review of the use of WLE vs MMS; the median overall survival was longest for WLE in conjunction with chemotherapy, radiotherapy, and immunotherapy at 39.3 months, followed by MMS alone at 37 months. Mohs micrographic surgery in conjunction with chemotherapy and radiotherapy was used in 1 patient, with a median overall survival of 82 months. Wide local excision alone resulted in a median overall survival of 19.8 months. Although these data are promising and suggest that the combination of surgery with adjuvant therapy may be more beneficial than surgery alone, it is important to note that there were only 9 cases treated with MMS compared with 825 cases treated with WLE.38
Several studies have documented that paraffin-embedded sections may be more useful than frozen sections in the determination of margin positivity from a surgical specimen, as frozen sections showed a poor negative predictive value of 33.3%.7,35 Mohs micrographic surgery has been proposed for tumors measuring less than 5 cm; however, the most recent appropriate use criteria for MMS of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery deemed the use of MMS for angiosarcoma uncertain.32,33,37 Further research is necessary to elucidate the role of MMS in the management of CAS.
Radiotherapy—Radiotherapy is a common adjuvant to surgical resection but has been used palliatively in patients with tumors that are unresectable. Improved local control and disease-free survival have been observed with the combination of radiation and surgery. A dose response to radiotherapy has been demonstrated,18,30 with 1 study showing that patients who received more than 5000 cGy of radiotherapy achieved better local control than patients who received 4500 cGy or less.18 Pawlik et al7 showed a decreased chance of death with the addition of adjunctive radiotherapy, and patients who underwent postoperative radiotherapy demonstrated a median survival almost 4-times longer than patients who did not receive radiation. Morrison et al39 reported that radiation therapy administered to patients with no clinically evident disease after surgical resection resulted in improved local control and overall survival vs patients who were irradiated with clinically evident disease.
Complications of radiotherapy for angiosarcoma have been reported, including xerostomia, nonfunctionally significant fibrosis, chronic ulceration/cellulitis of the scalp, necrosis requiring debridement, severe ocular complications, and fibrosis of the eyelids requiring surgical intervention.14 Radiation therapy also poses unique risks to patients with radiation-induced angiosarcoma of the breast, as many of these patients have already received the maximum recommended dose of radiation in the affected areas and additional radiation could exacerbate their CAS.
Chemotherapy—Chemotherapy occasionally is used as an adjunct to surgical resection with positive margins or as palliative care when surgical resection is not possible. Unfortunately, STSs have a response rate of less than 40% to standard chemotherapy.40 Studies in which the use of chemotherapy is evaluated for CAS have mixed results. Mark et al18 reported no significant overall survival benefit when comparing CAS treated with surgery plus radiotherapy with or without chemotherapy. Torres et al41 evaluated radiation-induced angiosarcoma of the breast and found a reduced risk for local recurrence in patients receiving chemotherapy in addition to surgery, indicating that chemotherapy may be useful in this subset of patients when radiation is not recommended.
Cytotoxic chemotherapy agents such as paclitaxel, doxorubicin, or doxorubicin in combination with mesna and ifosfamide (MAI) are common.39 Median progression-free survival is 5.4 months, 4 to 5.6 months, and 3.9 months for MAI, paclitaxel, and doxorubicin, respectively.8,9,42-46 Improved prognosis with MAI may indicate that combination chemotherapy regimens are more effective than single-agent regimens. Cutaneous angiosarcomas may respond better to paclitaxel than doxorubicin, and angiosarcomas of the scalp and face have shown a better response to paclitaxel.47,48
Other Therapies—Although there have not been large-scale studies performed on alternative treatments, there are several case reports on the use of immune modulators, biologics, β-blockers, and various other therapies in the treatment of CAS. The following studies include small sample sizes of patients with metastatic or locally aggressive disease not amenable to surgical resection, which may affect reported outcomes and survival times.49-57 In addition, several studies include patients with visceral angiosarcoma, which may not be generalizable to the CAS population. Even so, these treatment alternatives should not be overlooked because there are few agents that are truly efficacious in the treatment of CAS.
Results on the use of VEGF and tyrosine kinase inhibitors have been disappointing. There have been reports of median progression-free survival of only 3.8 months with sorafenib treatment, 3 months with pazopanib, and 6 months with bevacizumab.49-51 However, one study of patients who were treated with bevacizumab combined with radiation and surgery resulted in a complete response in 2 patients, with no evidence of residual disease at the last follow-up of 8.5 months and 2.1 years.52
Studies on the utility of β-blockers in the treatment of CAS have shown mixed results. Pasquier et al53 evaluated the use of adjunctive therapy with propranolol and vinblastine-based chemotherapy, with a promising median progression-free survival of 11 months compared with an average of 3 to 6 months with conventional chemotherapy regimens. However, in vitro studies reported by Pasquier et al53 indicated that the addition of propranolol to doxorubicin or paclitaxel did not result in increased efficacy. Chow et al54 demonstrated that propranolol monotherapy resulted in a reduction of the proliferative index of scalp angiosarcoma by 34% after only 1 week of treatment. This was followed by combination therapy of propranolol, paclitaxel, and radiation, which resulted in substantial tumor regression and no evidence of metastasis after 8 months of therapy.54
Immune checkpoint inhibitors have been a recent subject of interest in the treatment of angiosarcoma. Two case reports showed improvement in CAS of the face and primary pleural angiosarcoma with a course of pembrolizumab.55,56 In another case series, investigators used immune checkpoint inhibitors in 7 patients with cutaneous, breast, or radiation-associated angiosarcoma and found partial response in several patients treated with pembrolizumab and ipilimumab-nivolumab and complete response in 1 patient treated with anti–cytotoxic T-lymphocyte–associated protein 4 antibodies. The authors of this study hypothesized that treatment response was associated with the mutational profile of tumors, including mutational signatures of UV radiation with a large number of C-to-T substitutions similar to melanomas.57
Conclusion
Cutaneous angiosarcoma is a rare and aggressive tumor with a poor prognosis due to delayed detection. A thorough skin examination and heightened awareness of CAS by dermatologists may result in early biopsy and shortened time to a definitive diagnosis. Until quality evidence allows for the creation of consensus guidelines, care at a cancer center that specializes in rare and difficult-to-treat tumors and employs a multidisciplinary approach is essential to optimizing patient outcomes. Current knowledge supports surgery with negative margins as the mainstay of treatment, with adjuvant radiation, chemotherapy, and targeted therapies as possible additions for extensive disease. The role of MMS is uncertain, and because of the lack of contiguity in CAS, it may not be an optimal treatment.
Angiosarcomas are aggressive endothelial cell tumors of vascular origin that account for 1% to 2% of all soft tissue sarcomas in the United States.1,2 They can affect any organ in the body but most commonly affect the skin and soft tissue. Cutaneous angiosarcoma (CAS) is a rare type of skin cancer that can present in 2 forms: primary and secondary.
Dermatologists may be responsible for the initial diagnosis and management of CAS. They must be familiar with its presentation, as this condition can be difficult to diagnose and mimics other diseases. Additionally, dermatologists must understand the role of varying treatment modalities including Mohs micrographic surgery (MMS) in the management of CAS. This review will provide an overview of the epidemiology, presentation, and pathologic features of CAS and will discuss both emerging and existing treatments.
Epidemiology
Cutaneous angiosarcoma may present in various locations in the body, predominantly on the head and neck.4,5 Approximately 85% of cases arise in patients older than 60 years, and most of these patients are White men.1,4,5 The risk factors for the development of CAS include prior radiation exposure; chronic lymphedema (ie, Stewart-Treves syndrome); and familial syndromes including neurofibromatosis 1, BRCA1 or BRCA2 mutations, Maffucci syndrome, and Klippel-Trenaunay syndrome. Exogenous exposure to toxins such as vinyl chloride, thorium dioxide, or anabolic steroids also is associated with angiosarcoma, primarily in the form of visceral disease such as hepatic angiosarcoma.6
The average tumor size is approximately 4 to 5 cm; however, some tumors may grow larger than 10 cm.7,8 Metastasis through hematogenous or lymphatic spread is fairly common, occurring in approximately 16% to 35% of patients. The lungs and liver are the most common sites of metastasis.9,10 The age-adjusted incidence rate of CAS is decreasing for patients younger than 50 years, from 1.30 in 1995 to 2004 to 1.10 in 2005 to 2014, but increasing for individuals older than 70 years, from 2.53 in 1995 to 2004 to 2.87 in 2005 to 2014.4 The incidence of angiosarcoma also has grown in the female population, likely due to the increasing use of radiotherapy for the treatment of breast cancer.11
The high rates of CAS on the head and neck may be explained by the increased vascularity and UV exposure in these locations.12 In a Surveillance, Epidemiology, and End Results population-based study (N=811), 43% of patients with CAS had a history of other malignancies such as breast, prostate, genitourinary, gastrointestinal tract, and respiratory tract cancers.4 Cutaneous angiosarcoma can develop secondary to the primary cancer treatment, as seen in patients who develop CAS following radiation therapy.11
The underlying mechanism of CAS is believed to involve dysregulation of angiogenesis due to the vascular origin of these tumors. Studies have identified overexpression of vascular endothelial growth factor (VEGF), TP53 mutations, and RAS pathway mutations as potential contributing factors to the pathogenesis of angiosarcoma.6 Molecular differences between primary and secondary angiosarcomas are not well documented; however, radiation-associated CAS has been found to have higher expression of LYN and PRKCΘ, while non–radiation-induced lesions express FTL1 and AKT3.2 Chromosomal abnormalities have been identified in a small set of primary CAS patients, but the specific role of these abnormalities in the pathogenesis of CAS remains unclear.7
Prognosis
Cutaneous angiosarcoma has a poor prognosis, with 3-year disease-specific survival rates as low as 40% and 5-year rates as low as 17%.4,5,13,14 Survival rates increased from 1985 to 2014, likely due to earlier diagnoses and more effective treatments.4 Several factors are associated with worse prognosis, including metastatic disease, increasing age, scalp and neck tumor location, tumor size greater than 5 cm, necrosis, multiple skin lesions, and nodular and epithelioid morphology.4,5,10,13-16 Factors including sex, race, and presence of another malignancy do not affect survival.4,5 Prognosis in CAS may be evaluated by TNM tumor staging. The American Joint Committee on Cancer Staging Manual (8th edition) for soft tissue sarcoma (STS) commonly is used; however, CAS is not included in this staging system because it does not share the same behavior and natural history as other types of STS. This staging system provides separate guidelines for STS of the head and neck and STS of the extremities and trunk because of the smaller size but paradoxically higher risk for head and neck tumors.17 Given that there is no agreed-upon staging system for CAS, prognosis and communication among providers may be complicated.
Clinical Presentation
Early CAS typically presents as single or multifocal ill-defined, enlarging, violaceous or dusky red macules or patches (Figure 1). Lesions often rapidly develop into raised nodules and plaques that may bleed and ulcerate. Other common symptoms include pain, edema, neuropathy, anemia, and weight loss; however, it is not uncommon for lesions to be asymptomatic.8,18-20 Nodular lesions are more common on the scalp, and patches are more common on the face and neck.16 Tumors typically extend into the dermis, and aggressive cancers may invade the subcutaneous tissue and fascia.2

Cutaneous angiosarcoma may mimic ecchymosis, hemangioma, lymphangioma, edema, cellulitis, or scarring alopecia. Its nonspecific features make it difficult to recognize without dermoscopy or ultrasonography, which often results in delayed diagnosis and treatment. The median delay typically is 5 to 7 months and up to 1 year for some patients.7,16 Cutaneous angiosarcoma of the scalp tends to have a longer diagnostic delay than other areas of the body, which may be attributable to challenges in tumor identification and visualization by patients.16
Dermoscopy and ultrasonography can aid in the diagnosis of CAS. Dermoscopy may demonstrate a range of colors with yellow, brown, or red areas in a violaceous background. Other reported features include white veils and lines, purple ovals, pink-purple “steamlike” areas, and atypical vessels (Figure 2).21-23 Dermoscopic findings may appear similar to other vascular tumors, such as hemangioma and Kaposi sarcoma, or nonvascular tumors, including amelanotic melanoma, Merkel cell carcinoma, and primary cutaneous B-cell lymphoma. Ultrasonography may show ill-defined, hypoechoic areas with anechoic reticular channels and a hypoechoic subepidermal layer.21 Other radiologic modalities, such as computed tomography, magnetic resonance imaging, or positron emission tomography, are nonspecific and are more useful in evaluating the extent of tumor spread in visceral angiosarcoma. Magnetic resonance imaging in CAS may indicate malignancy with the presence of high T2 and T1 signal intensity and high-flow serpentine vessels.24

Histopathology
Histologically, angiosarcoma is characterized by anastomosing irregular vascular channels lined by a single layer of endothelial cells displaying slight to moderate atypia.25 These vascular channels dissect between collagen bundles and adipocytes. Monocyte infiltration may be observed.6 The neoplastic endothelial cells may present as spindle-shaped, round, polygonal, or epithelioid with eosinophilic cytoplasm. Histologic features differ based on the type of clinical lesion (Figure 3). In a study of CAS in Asian populations, nodular tumors showed solid sheets of pleomorphic spindle cells, many mitotic figures, and widely hemorrhagic spaces, whereas nonnodular tumors showed irregular vascular spaces dissecting collagen.16 Poorly differentiated tumors may present with hyperchromatic nuclei and prominent nucleoli, papillary endothelial formations, mitoses, and possible hemorrhage or necrosis.2,6,8 Histologic specimens also may reveal calcified bodies and hemosiderin particles.19 Angiosarcomas typically are invasive without a clear capsule or border.6

Secondary CAS in the setting of lymphedema and radiation therapy has MYC amplification and is positive for MYC via immunohistochemistry, which is uncommon in primary angiosarcoma.26 Immunohistochemical staining of tumor specimens is helpful to confirm the diagnosis of CAS. These markers include CD31, CD34, CD117, cytokeratin, vimentin, epithelial membrane antigen, factor VIII–related antigen, Ulex europaeus agglutinin-1, von Willebrand factor, and VEGF.6,19,27,28 Notably, advanced angiosarcomas with progressive dedifferentiation often lose these markers.
Treatment
Surgery—The majority of patients treated for CAS undergo surgical resection, as surgery has been shown to have the best prognosis for patients.5,9,10,13,15 Achieving R0 resection (microscopically negative margins) is the most important factor in determining the success of treatment, with incomplete surgical resection resulting in higher rates of systemic and local spread.29 Abraham et al8 found that the median disease-specific survival of patients with microscopically negative margins was 83.7 months; patients with microscopically positive and grossly positive margins had median disease-specific survival of 63.4 and 18.1 months, respectively. In a case series of patients undergoing resection with negative surgical margins, 4 patients demonstrated no evidence of local recurrence or systemic disease at an average of 4.3 years after therapy, and the other 4 patients each had 1 local recurrence but were disease free an average of 4.8 years after removal of the recurrent lesion. In a series of 27 patients with positive surgical margins, there was local recurrence within 2 years for most patients.12
Large tumors invading nearby structures may not be amenable to surgical resection because of extensive local growth, propensity for skip lesions, and localization near vital organs of the head and neck.5,7 The extended delay in diagnosis often seen in CAS allows for advanced local progression, resulting in large areas of resection. In a case series (N=8), the average surgical defect measured 14.3×11.8 cm, necessitating reconstruction with either a tissue flap or split-thickness skin graft in every case because primary closure was not possible. More than 80% of patients in this study still had positive margins after surgery, necessitating the use of additional chemotherapy or radiation to eradicate remaining disease.7 In several studies, multimodality therapy was associated with improved overall survival.7,14,30
Mohs Micrographic Surgery—Mohs micrographic surgery is the standard of care for many aggressive cutaneous malignancies on the head, but its utility for the treatment of CAS is uncertain. Only a few studies have compared the efficacy of MMS vs wide local excision (WLE). There have been reports of recurrence-free follow-up at 12, 16, 18, 20, and 72 months after MMS.31-36 The latter case showed a patient who underwent MMS with a 72-month relapse-free survival, whereas other patients who underwent WLE only survived 5 to 7 months without recurrence.36 In another study, there was a local recurrence rate of 42.9% after a median follow-up of 4 years in 7 patients with CAS treated with complete circumferential peripheral and deep margin assessment, which is less than the reported recurrence rates of 72% to 84% after standard excisional procedures.28,37
Houpe et al38 conducted a systematic review of the use of WLE vs MMS; the median overall survival was longest for WLE in conjunction with chemotherapy, radiotherapy, and immunotherapy at 39.3 months, followed by MMS alone at 37 months. Mohs micrographic surgery in conjunction with chemotherapy and radiotherapy was used in 1 patient, with a median overall survival of 82 months. Wide local excision alone resulted in a median overall survival of 19.8 months. Although these data are promising and suggest that the combination of surgery with adjuvant therapy may be more beneficial than surgery alone, it is important to note that there were only 9 cases treated with MMS compared with 825 cases treated with WLE.38
Several studies have documented that paraffin-embedded sections may be more useful than frozen sections in the determination of margin positivity from a surgical specimen, as frozen sections showed a poor negative predictive value of 33.3%.7,35 Mohs micrographic surgery has been proposed for tumors measuring less than 5 cm; however, the most recent appropriate use criteria for MMS of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery deemed the use of MMS for angiosarcoma uncertain.32,33,37 Further research is necessary to elucidate the role of MMS in the management of CAS.
Radiotherapy—Radiotherapy is a common adjuvant to surgical resection but has been used palliatively in patients with tumors that are unresectable. Improved local control and disease-free survival have been observed with the combination of radiation and surgery. A dose response to radiotherapy has been demonstrated,18,30 with 1 study showing that patients who received more than 5000 cGy of radiotherapy achieved better local control than patients who received 4500 cGy or less.18 Pawlik et al7 showed a decreased chance of death with the addition of adjunctive radiotherapy, and patients who underwent postoperative radiotherapy demonstrated a median survival almost 4-times longer than patients who did not receive radiation. Morrison et al39 reported that radiation therapy administered to patients with no clinically evident disease after surgical resection resulted in improved local control and overall survival vs patients who were irradiated with clinically evident disease.
Complications of radiotherapy for angiosarcoma have been reported, including xerostomia, nonfunctionally significant fibrosis, chronic ulceration/cellulitis of the scalp, necrosis requiring debridement, severe ocular complications, and fibrosis of the eyelids requiring surgical intervention.14 Radiation therapy also poses unique risks to patients with radiation-induced angiosarcoma of the breast, as many of these patients have already received the maximum recommended dose of radiation in the affected areas and additional radiation could exacerbate their CAS.
Chemotherapy—Chemotherapy occasionally is used as an adjunct to surgical resection with positive margins or as palliative care when surgical resection is not possible. Unfortunately, STSs have a response rate of less than 40% to standard chemotherapy.40 Studies in which the use of chemotherapy is evaluated for CAS have mixed results. Mark et al18 reported no significant overall survival benefit when comparing CAS treated with surgery plus radiotherapy with or without chemotherapy. Torres et al41 evaluated radiation-induced angiosarcoma of the breast and found a reduced risk for local recurrence in patients receiving chemotherapy in addition to surgery, indicating that chemotherapy may be useful in this subset of patients when radiation is not recommended.
Cytotoxic chemotherapy agents such as paclitaxel, doxorubicin, or doxorubicin in combination with mesna and ifosfamide (MAI) are common.39 Median progression-free survival is 5.4 months, 4 to 5.6 months, and 3.9 months for MAI, paclitaxel, and doxorubicin, respectively.8,9,42-46 Improved prognosis with MAI may indicate that combination chemotherapy regimens are more effective than single-agent regimens. Cutaneous angiosarcomas may respond better to paclitaxel than doxorubicin, and angiosarcomas of the scalp and face have shown a better response to paclitaxel.47,48
Other Therapies—Although there have not been large-scale studies performed on alternative treatments, there are several case reports on the use of immune modulators, biologics, β-blockers, and various other therapies in the treatment of CAS. The following studies include small sample sizes of patients with metastatic or locally aggressive disease not amenable to surgical resection, which may affect reported outcomes and survival times.49-57 In addition, several studies include patients with visceral angiosarcoma, which may not be generalizable to the CAS population. Even so, these treatment alternatives should not be overlooked because there are few agents that are truly efficacious in the treatment of CAS.
Results on the use of VEGF and tyrosine kinase inhibitors have been disappointing. There have been reports of median progression-free survival of only 3.8 months with sorafenib treatment, 3 months with pazopanib, and 6 months with bevacizumab.49-51 However, one study of patients who were treated with bevacizumab combined with radiation and surgery resulted in a complete response in 2 patients, with no evidence of residual disease at the last follow-up of 8.5 months and 2.1 years.52
Studies on the utility of β-blockers in the treatment of CAS have shown mixed results. Pasquier et al53 evaluated the use of adjunctive therapy with propranolol and vinblastine-based chemotherapy, with a promising median progression-free survival of 11 months compared with an average of 3 to 6 months with conventional chemotherapy regimens. However, in vitro studies reported by Pasquier et al53 indicated that the addition of propranolol to doxorubicin or paclitaxel did not result in increased efficacy. Chow et al54 demonstrated that propranolol monotherapy resulted in a reduction of the proliferative index of scalp angiosarcoma by 34% after only 1 week of treatment. This was followed by combination therapy of propranolol, paclitaxel, and radiation, which resulted in substantial tumor regression and no evidence of metastasis after 8 months of therapy.54
Immune checkpoint inhibitors have been a recent subject of interest in the treatment of angiosarcoma. Two case reports showed improvement in CAS of the face and primary pleural angiosarcoma with a course of pembrolizumab.55,56 In another case series, investigators used immune checkpoint inhibitors in 7 patients with cutaneous, breast, or radiation-associated angiosarcoma and found partial response in several patients treated with pembrolizumab and ipilimumab-nivolumab and complete response in 1 patient treated with anti–cytotoxic T-lymphocyte–associated protein 4 antibodies. The authors of this study hypothesized that treatment response was associated with the mutational profile of tumors, including mutational signatures of UV radiation with a large number of C-to-T substitutions similar to melanomas.57
Conclusion
Cutaneous angiosarcoma is a rare and aggressive tumor with a poor prognosis due to delayed detection. A thorough skin examination and heightened awareness of CAS by dermatologists may result in early biopsy and shortened time to a definitive diagnosis. Until quality evidence allows for the creation of consensus guidelines, care at a cancer center that specializes in rare and difficult-to-treat tumors and employs a multidisciplinary approach is essential to optimizing patient outcomes. Current knowledge supports surgery with negative margins as the mainstay of treatment, with adjuvant radiation, chemotherapy, and targeted therapies as possible additions for extensive disease. The role of MMS is uncertain, and because of the lack of contiguity in CAS, it may not be an optimal treatment.
- Rouhani P, Fletcher CD, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627.
- Goldblum JR, Folpe AL, Weiss SW. Enzinger & Weiss’s Soft Tissue Tumors. 7th ed. Elsevier Inc; 2020.
- Arora TK, Terracina KP, Soong J, et al. Primary and secondary angiosarcoma of the breast. Gland Surg. 2014;3:28-34.
- Conic RRZ, Damiani G, Frigerio A, et al. Incidence and outcomes of cutaneous angiosarcoma: a SEER population-based study. J Am Acad Dermatol. 2020;83:809-816.
- Chang C, Wu SP, Hu K, et al. Patterns of care and survival of cutaneous angiosarcoma of the head and neck. Otolaryngol Head Neck Surg. 2020;162:881-887.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953-1967.
- Fury MG, Antonescu CR, Van Zee KJ, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241-247.
- Lindet C, Neuville A, Penel N, et al. Localised angiosarcomas: the identification of prognostic factors and analysis of treatment impact. a retrospective analysis from the French Sarcoma Group (GSF/GETO). Eur J Cancer. 2013;49:369-376.
- Mery CM, George S, Bertagnolli MM, et al. Secondary sarcomas after radiotherapy for breast cancer: sustained risk and poor survival. Cancer. 2009;115:4055-4063.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Dettenborn T, Wermker K, Schulze HJ, et al. Prognostic features in angiosarcoma of the head and neck: a retrospective monocenter study. J Craniomaxillofac Surg. 2014;42:1623-1628.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Moon IJ, Kim YJ, Won CH, et al. Clinicopathological and survival analyses of primary cutaneous angiosarcoma in an Asian population: prognostic value of the clinical features of skin lesions. Int J Dermatol. 2020;59:582-589.
- Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma. a report of 67 patients and a review of the literature. Cancer. 1996;77:2400-2406.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. a review of 99 cases. Cancer. 1995;75:989-996.
- Fayette J, Martin E, Piperno-Neumann S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007;18:2030-2036.
- Oranges T, Janowska A, Vitali S, et al. Dermatoscopic and ultra-high frequency ultrasound evaluation in cutaneous postradiation angiosarcoma. J Eur Acad Dermatol Venereol. 2020;34:e741.
- Figueroa-Silva O, Argenziano G, Lallas A, et al. Dermoscopic pattern of radiation-induced angiosarcoma (RIA). J Am Acad Dermatol. 2015;73:E51-E55.
- Cole DW, Huerta T, Andea A, et al. Purpuric plaques-dermoscopic and histopathological correlation of cutaneous angiosarcoma. Dermatol Pract Concept. 2020;10:E2020084. doi:10.5826/dpc.1004a84
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. Vol 2. 4th ed. Elsevier; 2018.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39. doi:10.2353/ajpath.2010.090637
- Ohsawa M, Naka N, Tomita Y, et al. Use of immunohistochemical procedures in diagnosing angiosarcoma. Evaluation of 98 cases. Cancer. 1995;75:2867-2874.
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850.
- Lahat G, Dhuka AR, Lahat S, et al. Outcome of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol. 2009;16:2502-2509.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Kofler L, Breuninger H, Schulz C, et al. Local recurrence rates of skin tumors after resection with complete circumferential peripheral and deep margin assessment-identification of high-risk entities. Dermatol Surg. 2021;47:E31-E36.
- Houpe JE, Seger EW, Neill BC, et al. Treatment of angiosarcoma of the head and neck: a systematic review. Cutis. 2023;111:247-251. doi:10.12788/cutis.0767
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. a therapeutic dilemma. Cancer. 1995;76:319-327.
- Gonzalez MJ, Koehler MM, Satter EK. Angiosarcoma of the scalp: a case report and review of current and novel therapeutic regimens. Dermatol Surg. 2009;35:679-684.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26:5269-5274.
- Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann Oncol. 2012;23:517-523.
- Young RJ, Natukunda A, Litière S, et al. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer. 2014;50:3178-3186.
- Skubitz KM, Haddad PA. Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer. 2005;104:361-366.
- Fata F, O’Reilly E, Ilson D, et al. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer. 1999;86:2034-2037.
- Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer. 2012;118:3330-3336.
- Schlemmer M, Reichardt P, Verweij J, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2008;44:2433-2436.
- Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133-3140.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Kollár A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017;56:88-92.
- Koontz BF, Miles EF, Rubio MA, et al. Preoperative radiotherapy and bevacizumab for angiosarcoma of the head and neck: two case studies. Head Neck. 2008;30:262-266.
- Pasquier E, André N, Street J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine. 2016;6:87-95.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Wang X, Wei J, Zeng Z, et al. Primary pleural epithelioid angiosarcoma treated successfully with anti-PD-1 therapy: a rare case report. Medicine (Baltimore). 2021;100:E27132.
- Sindhu S, Gimber LH, Cranmer L, et al. Angiosarcoma treated successfully with anti-PD-1 therapy—a case report. J Immunother Cancer. 2017;5:58.
- Florou V, Rosenberg AE, Wieder E, et al. Angiosarcoma patients treated with immune checkpoint inhibitors: a case series of seven patients from a single institution. J Immunother Cancer. 2019;7:213.
- Rouhani P, Fletcher CD, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627.
- Goldblum JR, Folpe AL, Weiss SW. Enzinger & Weiss’s Soft Tissue Tumors. 7th ed. Elsevier Inc; 2020.
- Arora TK, Terracina KP, Soong J, et al. Primary and secondary angiosarcoma of the breast. Gland Surg. 2014;3:28-34.
- Conic RRZ, Damiani G, Frigerio A, et al. Incidence and outcomes of cutaneous angiosarcoma: a SEER population-based study. J Am Acad Dermatol. 2020;83:809-816.
- Chang C, Wu SP, Hu K, et al. Patterns of care and survival of cutaneous angiosarcoma of the head and neck. Otolaryngol Head Neck Surg. 2020;162:881-887.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953-1967.
- Fury MG, Antonescu CR, Van Zee KJ, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241-247.
- Lindet C, Neuville A, Penel N, et al. Localised angiosarcomas: the identification of prognostic factors and analysis of treatment impact. a retrospective analysis from the French Sarcoma Group (GSF/GETO). Eur J Cancer. 2013;49:369-376.
- Mery CM, George S, Bertagnolli MM, et al. Secondary sarcomas after radiotherapy for breast cancer: sustained risk and poor survival. Cancer. 2009;115:4055-4063.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Dettenborn T, Wermker K, Schulze HJ, et al. Prognostic features in angiosarcoma of the head and neck: a retrospective monocenter study. J Craniomaxillofac Surg. 2014;42:1623-1628.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Moon IJ, Kim YJ, Won CH, et al. Clinicopathological and survival analyses of primary cutaneous angiosarcoma in an Asian population: prognostic value of the clinical features of skin lesions. Int J Dermatol. 2020;59:582-589.
- Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma. a report of 67 patients and a review of the literature. Cancer. 1996;77:2400-2406.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. a review of 99 cases. Cancer. 1995;75:989-996.
- Fayette J, Martin E, Piperno-Neumann S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007;18:2030-2036.
- Oranges T, Janowska A, Vitali S, et al. Dermatoscopic and ultra-high frequency ultrasound evaluation in cutaneous postradiation angiosarcoma. J Eur Acad Dermatol Venereol. 2020;34:e741.
- Figueroa-Silva O, Argenziano G, Lallas A, et al. Dermoscopic pattern of radiation-induced angiosarcoma (RIA). J Am Acad Dermatol. 2015;73:E51-E55.
- Cole DW, Huerta T, Andea A, et al. Purpuric plaques-dermoscopic and histopathological correlation of cutaneous angiosarcoma. Dermatol Pract Concept. 2020;10:E2020084. doi:10.5826/dpc.1004a84
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. Vol 2. 4th ed. Elsevier; 2018.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39. doi:10.2353/ajpath.2010.090637
- Ohsawa M, Naka N, Tomita Y, et al. Use of immunohistochemical procedures in diagnosing angiosarcoma. Evaluation of 98 cases. Cancer. 1995;75:2867-2874.
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850.
- Lahat G, Dhuka AR, Lahat S, et al. Outcome of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol. 2009;16:2502-2509.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Kofler L, Breuninger H, Schulz C, et al. Local recurrence rates of skin tumors after resection with complete circumferential peripheral and deep margin assessment-identification of high-risk entities. Dermatol Surg. 2021;47:E31-E36.
- Houpe JE, Seger EW, Neill BC, et al. Treatment of angiosarcoma of the head and neck: a systematic review. Cutis. 2023;111:247-251. doi:10.12788/cutis.0767
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. a therapeutic dilemma. Cancer. 1995;76:319-327.
- Gonzalez MJ, Koehler MM, Satter EK. Angiosarcoma of the scalp: a case report and review of current and novel therapeutic regimens. Dermatol Surg. 2009;35:679-684.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26:5269-5274.
- Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann Oncol. 2012;23:517-523.
- Young RJ, Natukunda A, Litière S, et al. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer. 2014;50:3178-3186.
- Skubitz KM, Haddad PA. Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer. 2005;104:361-366.
- Fata F, O’Reilly E, Ilson D, et al. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer. 1999;86:2034-2037.
- Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer. 2012;118:3330-3336.
- Schlemmer M, Reichardt P, Verweij J, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2008;44:2433-2436.
- Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133-3140.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Kollár A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017;56:88-92.
- Koontz BF, Miles EF, Rubio MA, et al. Preoperative radiotherapy and bevacizumab for angiosarcoma of the head and neck: two case studies. Head Neck. 2008;30:262-266.
- Pasquier E, André N, Street J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine. 2016;6:87-95.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Wang X, Wei J, Zeng Z, et al. Primary pleural epithelioid angiosarcoma treated successfully with anti-PD-1 therapy: a rare case report. Medicine (Baltimore). 2021;100:E27132.
- Sindhu S, Gimber LH, Cranmer L, et al. Angiosarcoma treated successfully with anti-PD-1 therapy—a case report. J Immunother Cancer. 2017;5:58.
- Florou V, Rosenberg AE, Wieder E, et al. Angiosarcoma patients treated with immune checkpoint inhibitors: a case series of seven patients from a single institution. J Immunother Cancer. 2019;7:213.
PRACTICE POINTS
- Dermatologists should be aware of challenges in diagnosing cutaneous angiosarcoma (CAS) due to its clinical similarity to benign entities such as ecchymosis and hemangioma.
- Surgery with negative margins is the first-line treatment of CAS with the best prognosis.
- Mohs micrographic surgery is useful for well-defined lesions measuring less than 5 cm on the head and neck; however, further studies are needed to determine its use in other areas.
- Paraffin-embedded sections may be more reliable than frozen sections in determining margin clearance.
Recurrence Rates of Mohs Micrographic Surgery vs Radiation Therapy for Basal Cell Carcinoma of the Ear
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. In this retrospective analysis, we evaluated recurrence rates of BCC of the ear in 102 patients who underwent treatment with Mohs micrographic surgery (MMS) or radiation therapy (RT) at a single institution between January 2017 and December 2019. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. Recurrence rates were assessed over a mean follow-up time of 2.8 years. Although MMS is the gold standard for treatment of BCC of the ear, RT may be a suitable alternative for nonsurgical candidates.
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. Given that these aggressive histologic subtypes—defined as morpheaform, basosquamous, sclerosing, infiltrative, or micronodular in any portion of the tumor—have been reported as independent predictors of recurrence,1,2 BCC of the ear may be more likely to recur.
Mohs micrographic surgery (MMS) is the gold standard for the treatment of BCC of the ear. For nonsurgical candidates—those with high bleeding risk, low life expectancy, or other medical or social factors—definitive radiation therapy (RT) may be an option. Our study sought to examine recurrence rates in patients with BCC of the ear treated with MMS vs RT.
Methods
A retrospective review of patients undergoing treatment of BCC of the ear at Bighorn Mohs Surgery and Dermatology Center (San Diego, California) between January 2017 and December 2019 was conducted. A total of 507 medical records were reviewed, and 102 patients were included in the study. Inclusion criteria consisted of biopsy-confirmed BCC of the ear that was treated with MMS, RT, or both. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. This retrospective review of medical records was exempt from institutional review board approval, as it did not involve direct human research subjects, solely entailing a retrospective examination of existing data.
Results
Of the 102 patients included, 82 were male and 20 were female, with an average age of 71 years. All patients were White with the exception of 1 patient whose race was unknown. Two patients were immunocompromised. The helix was identified as the most frequently involved site on the ear (Table). Most of the tumors (56/102) exhibited aggressive histologic subtypes; 36 tumors had nonaggressive histology, and 10 had no subtype listed. Two of the BCCs demonstrated perineural invasion on biopsy. Mohs micrographic surgery was used to treat 96 BCCs, definitive RT was used to treat 5 BCCs (all of which occurred in nonsurgical candidates), and MMS and adjuvant RT were used in 1 patient given multifocal perineural involvement. All 5 patients treated with definitive RT received electron beam radiation therapy; the total dose ranged from 5100 to 6000 cGy divided into 17 to 24 fractions. The final MMS defects ranged from 6 to 55 mm in size. The average follow-up time was 2.8 years. One of the BCCs on the helix that was treated with MMS recurred after 1.3 years. The overall recurrence rate was 0.98%. None of the patients treated with definitive RT experienced recurrence after the mean follow-up time of 2.8 years.
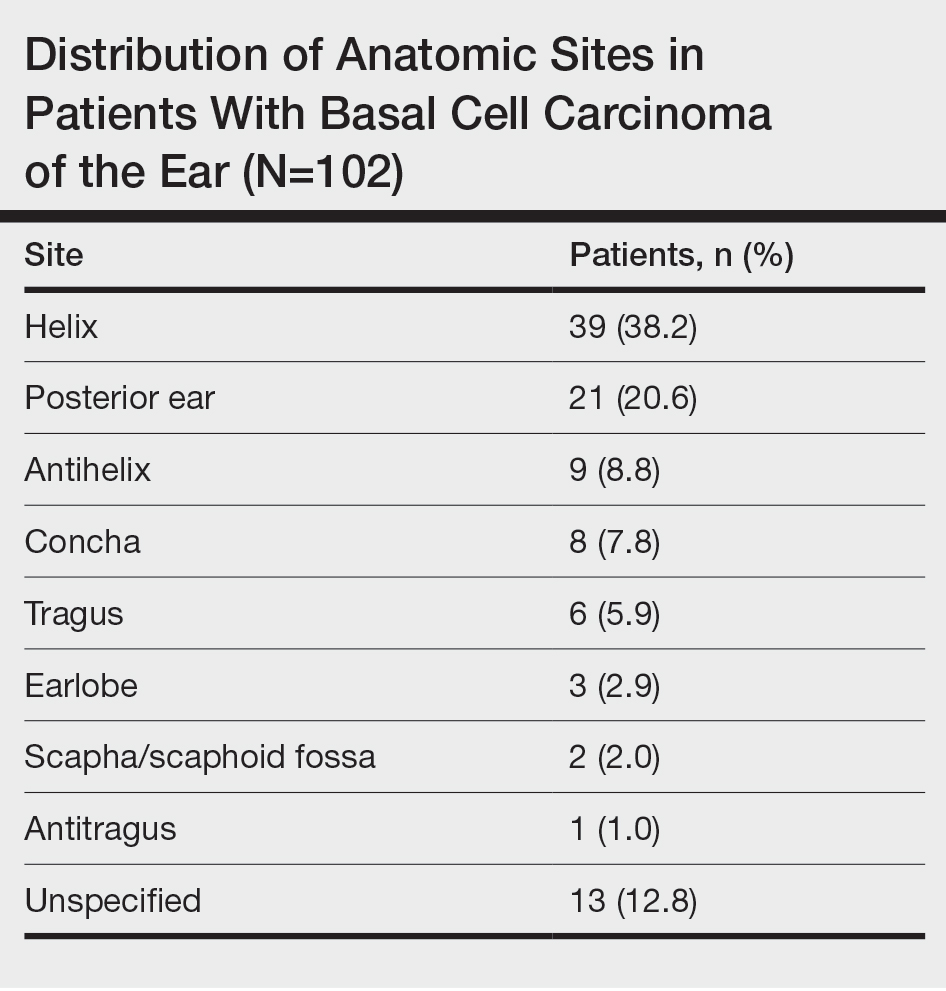
Comment
Basal cell carcinoma is the most commonly diagnosed cancer in the United States, with approximately 2 million new cases each year.1 Treatment modalities for localized BCC include MMS, surgical excision, electrodesiccation and curettage, topical and intralesional medications, laser therapy, and RT. For high-risk BCCs, MMS is associated with the lowest recurrence rates4 and remains the gold standard for treatment. For patients with contraindications to surgery, definitive RT is an alternative treatment for high-risk BCC.1
Definitive RT can be employed for patients who are poor surgical candidates or when surgery would result in substantial morbidity, impaired function, and/or poor cosmesis.3 Radiation therapy for skin cancers of the ear commonly is administered using high-energy electrons that produce double-strand breaks in the DNA of malignant cells, leading to cell death.4 Disadvantages of RT compared to MMS include a longer treatment course (3 to 6 weeks), possible minimal long-term cosmetic sequelae (eg, color or texture mismatch), lack of pathologic confirmation of margin control, and small risk for secondary malignancy in the treatment field over 2 to 3 decades. For patients with incurable or metastatic disease, palliative RT can provide local control and/or symptomatic relief to improve quality of life.4 Adjuvant RT may be indicated if there is substantial perineural involvement or positive margins after MMS when margins are unable to be achieved or in patients who may not tolerate prolonged or extensive surgical procedures.3
Basal cell carcinoma of the ear is considered a high-risk anatomic location independent of other prognostic factors. Basal cell carcinomas of the ear have a higher propensity for more aggressive histologic subtypes and subclinical spread.5 Our study demonstrated a higher proportion of aggressive histologic subtypes (56/102 [54.9%]) compared with nonaggressive subtypes (36/102 [35.3%]). There was 1 recurrence of a nodular, sclerosing, and infiltrative BCC on the helix treated with MMS after 1.3 years.
Limitations of our study include that it was conducted at a single institution with a homogenous study population and with relatively short follow-up.
Conclusion
Our study further validates the well-known utility of MMS for the treatment of BCC of the ears. Definitive RT is a suitable alternative for patients who are not surgical candidates. Adjuvant RT may be considered for substantial perineural involvement or positive margins after MMS.3
- Lee CT, Lehrer EJ, Aphale A, et al. Surgical excision, Mohs micrographic surgery, external-beam radiotherapy, or brachytherapy for indolent skin cancer: an international meta-analysis of 58 studies with 21,000 patients. Cancer. 2019;125:3582-3594.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part II: when is radiation therapy indicated? J Am Acad Dermatol. 2021;85:551-562.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part I: diagnostic modalities and applications. J Am Acad Dermatol. 2021;85:539-548.
- Bichakjian CK, Olencki T, Aasi SZ, et al. Basal cell skin cancer, version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:574-597.
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. In this retrospective analysis, we evaluated recurrence rates of BCC of the ear in 102 patients who underwent treatment with Mohs micrographic surgery (MMS) or radiation therapy (RT) at a single institution between January 2017 and December 2019. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. Recurrence rates were assessed over a mean follow-up time of 2.8 years. Although MMS is the gold standard for treatment of BCC of the ear, RT may be a suitable alternative for nonsurgical candidates.
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. Given that these aggressive histologic subtypes—defined as morpheaform, basosquamous, sclerosing, infiltrative, or micronodular in any portion of the tumor—have been reported as independent predictors of recurrence,1,2 BCC of the ear may be more likely to recur.
Mohs micrographic surgery (MMS) is the gold standard for the treatment of BCC of the ear. For nonsurgical candidates—those with high bleeding risk, low life expectancy, or other medical or social factors—definitive radiation therapy (RT) may be an option. Our study sought to examine recurrence rates in patients with BCC of the ear treated with MMS vs RT.
Methods
A retrospective review of patients undergoing treatment of BCC of the ear at Bighorn Mohs Surgery and Dermatology Center (San Diego, California) between January 2017 and December 2019 was conducted. A total of 507 medical records were reviewed, and 102 patients were included in the study. Inclusion criteria consisted of biopsy-confirmed BCC of the ear that was treated with MMS, RT, or both. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. This retrospective review of medical records was exempt from institutional review board approval, as it did not involve direct human research subjects, solely entailing a retrospective examination of existing data.
Results
Of the 102 patients included, 82 were male and 20 were female, with an average age of 71 years. All patients were White with the exception of 1 patient whose race was unknown. Two patients were immunocompromised. The helix was identified as the most frequently involved site on the ear (Table). Most of the tumors (56/102) exhibited aggressive histologic subtypes; 36 tumors had nonaggressive histology, and 10 had no subtype listed. Two of the BCCs demonstrated perineural invasion on biopsy. Mohs micrographic surgery was used to treat 96 BCCs, definitive RT was used to treat 5 BCCs (all of which occurred in nonsurgical candidates), and MMS and adjuvant RT were used in 1 patient given multifocal perineural involvement. All 5 patients treated with definitive RT received electron beam radiation therapy; the total dose ranged from 5100 to 6000 cGy divided into 17 to 24 fractions. The final MMS defects ranged from 6 to 55 mm in size. The average follow-up time was 2.8 years. One of the BCCs on the helix that was treated with MMS recurred after 1.3 years. The overall recurrence rate was 0.98%. None of the patients treated with definitive RT experienced recurrence after the mean follow-up time of 2.8 years.

Comment
Basal cell carcinoma is the most commonly diagnosed cancer in the United States, with approximately 2 million new cases each year.1 Treatment modalities for localized BCC include MMS, surgical excision, electrodesiccation and curettage, topical and intralesional medications, laser therapy, and RT. For high-risk BCCs, MMS is associated with the lowest recurrence rates4 and remains the gold standard for treatment. For patients with contraindications to surgery, definitive RT is an alternative treatment for high-risk BCC.1
Definitive RT can be employed for patients who are poor surgical candidates or when surgery would result in substantial morbidity, impaired function, and/or poor cosmesis.3 Radiation therapy for skin cancers of the ear commonly is administered using high-energy electrons that produce double-strand breaks in the DNA of malignant cells, leading to cell death.4 Disadvantages of RT compared to MMS include a longer treatment course (3 to 6 weeks), possible minimal long-term cosmetic sequelae (eg, color or texture mismatch), lack of pathologic confirmation of margin control, and small risk for secondary malignancy in the treatment field over 2 to 3 decades. For patients with incurable or metastatic disease, palliative RT can provide local control and/or symptomatic relief to improve quality of life.4 Adjuvant RT may be indicated if there is substantial perineural involvement or positive margins after MMS when margins are unable to be achieved or in patients who may not tolerate prolonged or extensive surgical procedures.3
Basal cell carcinoma of the ear is considered a high-risk anatomic location independent of other prognostic factors. Basal cell carcinomas of the ear have a higher propensity for more aggressive histologic subtypes and subclinical spread.5 Our study demonstrated a higher proportion of aggressive histologic subtypes (56/102 [54.9%]) compared with nonaggressive subtypes (36/102 [35.3%]). There was 1 recurrence of a nodular, sclerosing, and infiltrative BCC on the helix treated with MMS after 1.3 years.
Limitations of our study include that it was conducted at a single institution with a homogenous study population and with relatively short follow-up.
Conclusion
Our study further validates the well-known utility of MMS for the treatment of BCC of the ears. Definitive RT is a suitable alternative for patients who are not surgical candidates. Adjuvant RT may be considered for substantial perineural involvement or positive margins after MMS.3
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. In this retrospective analysis, we evaluated recurrence rates of BCC of the ear in 102 patients who underwent treatment with Mohs micrographic surgery (MMS) or radiation therapy (RT) at a single institution between January 2017 and December 2019. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. Recurrence rates were assessed over a mean follow-up time of 2.8 years. Although MMS is the gold standard for treatment of BCC of the ear, RT may be a suitable alternative for nonsurgical candidates.
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. Given that these aggressive histologic subtypes—defined as morpheaform, basosquamous, sclerosing, infiltrative, or micronodular in any portion of the tumor—have been reported as independent predictors of recurrence,1,2 BCC of the ear may be more likely to recur.
Mohs micrographic surgery (MMS) is the gold standard for the treatment of BCC of the ear. For nonsurgical candidates—those with high bleeding risk, low life expectancy, or other medical or social factors—definitive radiation therapy (RT) may be an option. Our study sought to examine recurrence rates in patients with BCC of the ear treated with MMS vs RT.
Methods
A retrospective review of patients undergoing treatment of BCC of the ear at Bighorn Mohs Surgery and Dermatology Center (San Diego, California) between January 2017 and December 2019 was conducted. A total of 507 medical records were reviewed, and 102 patients were included in the study. Inclusion criteria consisted of biopsy-confirmed BCC of the ear that was treated with MMS, RT, or both. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. This retrospective review of medical records was exempt from institutional review board approval, as it did not involve direct human research subjects, solely entailing a retrospective examination of existing data.
Results
Of the 102 patients included, 82 were male and 20 were female, with an average age of 71 years. All patients were White with the exception of 1 patient whose race was unknown. Two patients were immunocompromised. The helix was identified as the most frequently involved site on the ear (Table). Most of the tumors (56/102) exhibited aggressive histologic subtypes; 36 tumors had nonaggressive histology, and 10 had no subtype listed. Two of the BCCs demonstrated perineural invasion on biopsy. Mohs micrographic surgery was used to treat 96 BCCs, definitive RT was used to treat 5 BCCs (all of which occurred in nonsurgical candidates), and MMS and adjuvant RT were used in 1 patient given multifocal perineural involvement. All 5 patients treated with definitive RT received electron beam radiation therapy; the total dose ranged from 5100 to 6000 cGy divided into 17 to 24 fractions. The final MMS defects ranged from 6 to 55 mm in size. The average follow-up time was 2.8 years. One of the BCCs on the helix that was treated with MMS recurred after 1.3 years. The overall recurrence rate was 0.98%. None of the patients treated with definitive RT experienced recurrence after the mean follow-up time of 2.8 years.

Comment
Basal cell carcinoma is the most commonly diagnosed cancer in the United States, with approximately 2 million new cases each year.1 Treatment modalities for localized BCC include MMS, surgical excision, electrodesiccation and curettage, topical and intralesional medications, laser therapy, and RT. For high-risk BCCs, MMS is associated with the lowest recurrence rates4 and remains the gold standard for treatment. For patients with contraindications to surgery, definitive RT is an alternative treatment for high-risk BCC.1
Definitive RT can be employed for patients who are poor surgical candidates or when surgery would result in substantial morbidity, impaired function, and/or poor cosmesis.3 Radiation therapy for skin cancers of the ear commonly is administered using high-energy electrons that produce double-strand breaks in the DNA of malignant cells, leading to cell death.4 Disadvantages of RT compared to MMS include a longer treatment course (3 to 6 weeks), possible minimal long-term cosmetic sequelae (eg, color or texture mismatch), lack of pathologic confirmation of margin control, and small risk for secondary malignancy in the treatment field over 2 to 3 decades. For patients with incurable or metastatic disease, palliative RT can provide local control and/or symptomatic relief to improve quality of life.4 Adjuvant RT may be indicated if there is substantial perineural involvement or positive margins after MMS when margins are unable to be achieved or in patients who may not tolerate prolonged or extensive surgical procedures.3
Basal cell carcinoma of the ear is considered a high-risk anatomic location independent of other prognostic factors. Basal cell carcinomas of the ear have a higher propensity for more aggressive histologic subtypes and subclinical spread.5 Our study demonstrated a higher proportion of aggressive histologic subtypes (56/102 [54.9%]) compared with nonaggressive subtypes (36/102 [35.3%]). There was 1 recurrence of a nodular, sclerosing, and infiltrative BCC on the helix treated with MMS after 1.3 years.
Limitations of our study include that it was conducted at a single institution with a homogenous study population and with relatively short follow-up.
Conclusion
Our study further validates the well-known utility of MMS for the treatment of BCC of the ears. Definitive RT is a suitable alternative for patients who are not surgical candidates. Adjuvant RT may be considered for substantial perineural involvement or positive margins after MMS.3
- Lee CT, Lehrer EJ, Aphale A, et al. Surgical excision, Mohs micrographic surgery, external-beam radiotherapy, or brachytherapy for indolent skin cancer: an international meta-analysis of 58 studies with 21,000 patients. Cancer. 2019;125:3582-3594.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part II: when is radiation therapy indicated? J Am Acad Dermatol. 2021;85:551-562.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part I: diagnostic modalities and applications. J Am Acad Dermatol. 2021;85:539-548.
- Bichakjian CK, Olencki T, Aasi SZ, et al. Basal cell skin cancer, version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:574-597.
- Lee CT, Lehrer EJ, Aphale A, et al. Surgical excision, Mohs micrographic surgery, external-beam radiotherapy, or brachytherapy for indolent skin cancer: an international meta-analysis of 58 studies with 21,000 patients. Cancer. 2019;125:3582-3594.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part II: when is radiation therapy indicated? J Am Acad Dermatol. 2021;85:551-562.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part I: diagnostic modalities and applications. J Am Acad Dermatol. 2021;85:539-548.
- Bichakjian CK, Olencki T, Aasi SZ, et al. Basal cell skin cancer, version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:574-597.
PRACTICE POINTS
- Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations, highlighting the importance of careful management and follow-up.
- Although Mohs micrographic surgery remains the gold standard for treating BCC of the ear, radiation therapy can be considered as a suitable alternative for nonsurgical candidates.