User login
The Clinical Utility of Teledermatology in Triaging and Diagnosing Skin Malignancies: Case Series
With the increasing utilization of telemedicine since the COVID-19 pandemic, it is critical that clinicians have an appropriate understanding of the application of virtual care resources, including teledermatology. We present a case series of 3 patients to demonstrate the clinical utility of teledermatology in reducing the time to diagnosis of various rare and/or aggressive cutaneous malignancies, including Merkel cell carcinoma, malignant melanoma, and atypical fibroxanthoma. Cases were obtained from one large Midwestern medical center during the month of July 2021. Each case presented includes a description of the initial teledermatology presentation and reviews the clinical timeline from initial consultation submission to in-person clinic visit with lesion biopsy. This case series demonstrates real-world examples of how teledermatology can be utilized to expedite the care of specific vulnerable patient populations.
Teledermatology is a rapidly growing digital resource with specific utility in triaging patients to determine those requiring in-person evaluation for early and accurate detection of skin malignancies. Approximately one-third of teledermatology consultations result in face-to-face clinical encounters, with malignant neoplasms being the leading cause for biopsy.1,2 For specific populations, such as geriatric and immunocompromised patients, teledermatology may serve as a valuable tool, particularly in the wake of the COVID-19 pandemic. Furthermore, telemedicine may aid in addressing health disparities within the field of medicine and ultimately may improve access to care for vulnerable populations.3 Along with increasing access to specific subspecialty expertise, the use of teledermatology may reduce health care costs and improve the overall quality of care delivered to patients.4,5
We describe the clinical utility of teledermatology in triaging and diagnosing skin malignancies through a series of 3 cases obtained from digital image review at one large Midwestern medical center during the month of July 2021. Three unique cases with a final diagnosis of a rare or aggressive skin cancer were selected as examples, including a 75-year-old man with Merkle cell carcinoma, a 55-year-old man with aggressive pT3b malignant melanoma, and a 72-year-old man with an atypical fibroxanthoma. A clinical timeline of each case is presented, including the time intervals from initial image submission to image review, image submission to face-to-face clinical encounter, and image submission to final diagnosis. In all cases, the primary care provider submitted an order for teledermatology, and the teledermatology team obtained the images.
Case Series
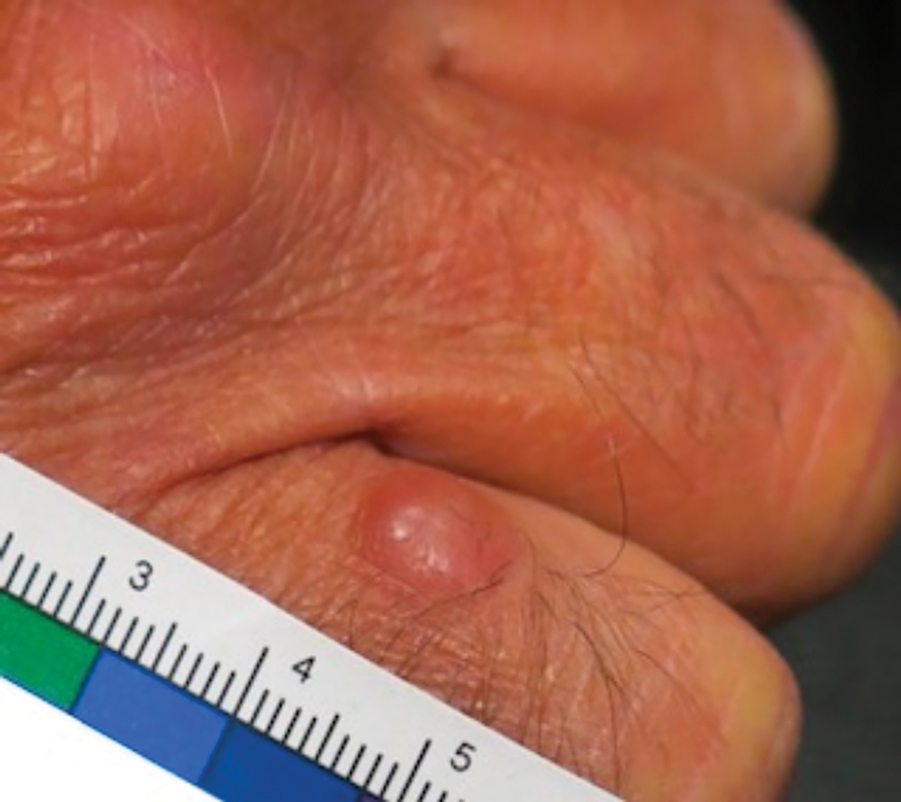
Patient 1—Images of the right hand of a 75-year-old man with a medical history of basal cell carcinoma were submitted for teledermatology consultation utilizing store-and-forward image-capturing technology (day 1). The patient history provided with image submission indicated that the lesion had been present for 6 months and there were no associated symptoms. Clinical imaging demonstrated a pink-red pearly papule located on the proximal fourth digit of the dorsal aspect of the right hand (Figure 1). One day following the teledermatology request (day 2), the patient’s case was reviewed and triaged for an in-person visit. The patient was brought to clinic on day 34, and a biopsy was performed. On day 36, dermatopathology results indicated a diagnosis of Merkel cell carcinoma. On day 37, the patient was referred to surgical oncology, and on day 44, the patient underwent an initial surgical oncology visit with a plan for wide local excision of the right fourth digit with right axillary sentinel lymph node biopsy.
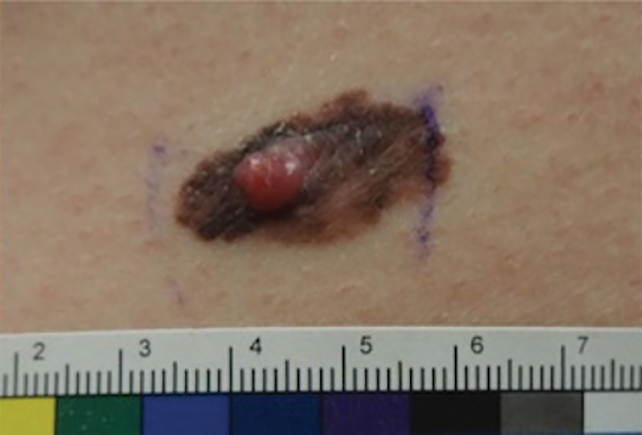
Patient 2—Images of the left flank of a 55-year-old man were submitted for teledermatology consultation via store-and-forward technology (day 1). A patient history provided with the image indicated that the lesion had been present for months to years and there were no associated symptoms, but the lesion recently had changed in color and size. Teledermatology images were reviewed on day 3 and demonstrated a 2- to 3-cm brown plaque on the left flank with color variegation and a prominent red papule protruding centrally (Figure 2). The patient was scheduled for an urgent in-person visit with biopsy. On day 6, the patient presented to clinic and an excision biopsy was performed. Dermatopathology was ordered with a RUSH indication, with results on day 7 revealing a pT3b malignant melanoma. An urgent consultation to surgical oncology was placed on the same day, and the patient underwent an initial surgical oncology visit on day 24 with a plan for wide local excision with left axillary and inguinal sentinel lymph node biopsy.
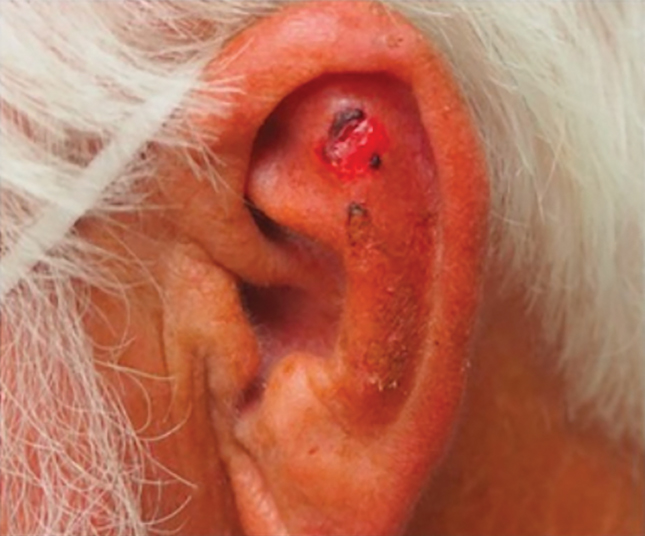
Patient 3—Images of the left ear of a 72-year-old man were submitted for teledermatology consultation utilizing review via store-and-forward technology (day 1). A patient history indicated that the lesion had been present for 3 months with associated bleeding. Image review demonstrated a solitary pearly pink papule located on the crura of the antihelix (Figure 3). Initial teledermatology consultation was reviewed on day 2 with notification of the need for in-person evaluation. The patient presented to clinic on day 33 for a biopsy, with dermatopathology results on day 36 consistent with an atypical fibroxanthoma. The patient was scheduled for Mohs micrographic surgery on day 37 and underwent surgical treatment on day 64.
Comment
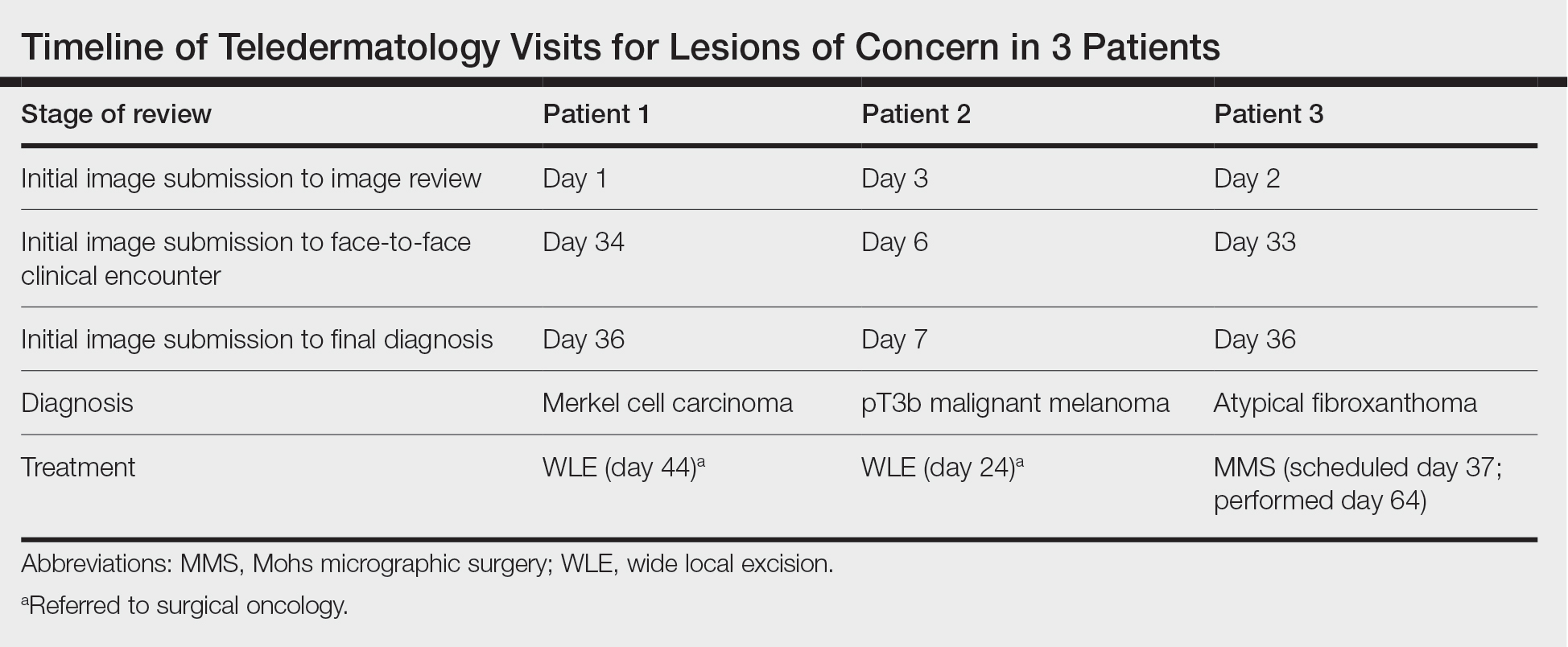
Teledermatology consultations from all patients demonstrated adequate image quality to be able to evaluate the lesion of concern and yielded a request for in-person evaluation with possible biopsy (Table). In this case series, the average time interval from teledermatology consultation placement to teledermatology image report was 2 days (range, 1–3 days). The average time from teledermatology consultation placement to face-to-face encounter with biopsy was 24.3 days for the 3 cases presented in this series (range, 6–34 days). The initial surgical oncology visits took place an average of 34 days after the initial teledermatology consultation was placed for the 2 patients requiring referral (44 days for patient 1; 24 days for patient 2). For patient 3, Mohs micrographic surgery was required for treatment, which was scheduled by day 37 and subsequently performed on day 64.
When specifically looking at the diagnosis of cutaneous malignancies, studies have found that the incidence of skin cancer detection is similar for teledermatology compared to in-person clinic visits.6,7 Creighton-Smith et al6 performed a retrospective cohort study comparing prebiopsy and postbiopsy diagnostic accuracy and detection rates of skin cancer between store-and-forward technology and face-to-face consultation. When adjusting for possible compounding factors including personal and family history of skin cancer, there was no notable difference in detection rates of any skin cancer, including melanoma and nonmelanoma skin cancers. Furthermore, the 2 cohorts of patients were found to have similar prebiopsy and postbiopsy diagnostic concordance, with similar times from consultation being placed to requested biopsy and time from biopsy to final treatment.6
Clarke et al7 similarly analyzed the accuracy of store-and-forward teledermatology and found that there was overall concordance in diagnosis when comparing clinical dermatologists to teledermatologists. Moreover, when melanocytic lesions were excluded from the study, the decision to biopsy did not differ substantially.7
Areas of further study include determining what percentage of teledermatology lesions of concern for malignancy were proven to be skin cancer after in-person evaluation and biopsy, as well as investigating the effectiveness of teledermatology for melanocytic lesions, which frequently are removed from analysis in large-scale teledermatology studies.
Although teledermatology has substantial clinical utility and may serve as a great resource for specific populations, including geriatric patients and those who are immunocompromised, it is important to recognize notable limitations. Specifically, brief history and image review should not serve as replacements for a face-to-face visit with physical examination in cases where the diagnosis remains uncertain or when high-risk skin malignancies are suspected or included in the differential. Certain aggressive cutaneous malignancies such as Merkel cell carcinoma may appear as less aggressive via teledermatology due to restrictions of technology.
Conclusion
Teledermatology has had a major impact on the way health care is delivered to patients and may increase access to care, reducing unnecessary in-person visits and decreasing the number of in-person visit no-shows. With the appropriate use of a brief clinical history and image review, teledermatology can be effective to evaluate specific lesions of concern. We report 3 unique cases identified during a 1-month period at a large Midwestern medical center. These cases serve as important examples of the application of teledermatology in reducing the time to diagnosis of aggressive skin malignancies. Further research on the clinical utility of teledermatology is warranted.
Acknowledgments—The authors thank the additional providers from the University of Wisconsin and William S. Middleton Memorial Veterans Hospital (both in Madison, Wisconsin) involved in the medical care of the patients included in this case series.
- Bianchi MG, Santos A, Cordioli E. Benefits of teledermatology for geriatric patients: population-based cross-sectional study. J Med Internet Res. 2020;22:E16700.
- Mortimer S, Rosin A. A retrospective review of incidental malignancies in veterans seen for face-to-face follow-up after teledermatology consultation. J Am Acad Dermatol. 2021;84:1130-1132.
- Costello CM, Cumsky HJL, Maly CJ, et al. Improving access to care through the establishment of a local, teledermatology network. Telemed J E Health. 2020;26:935-940. doi:10.1089/tmj.2019.0051
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260. doi:10.1007/s40257-017-0317-6
- Hadeler E, Beer J, Nouri K. The influence of teledermatology on health care access and equity. J Am Acad Dermatol. 2021;84:E219-E220. doi:10.1016/j.jaad.2020.12.036
- Creighton-Smith M, Murgia RD 3rd, Konnikov N, et al. Incidence of melanoma and keratinocytic carcinomas in patients evaluated by store-and-forward teledermatology vs dermatology clinic. Int J Dermatol. 2017;56:1026-1031. doi:10.1111/ijd.13672
- Clarke EL, Reichenberg JS, Ahmed AM, et al. The utility of teledermatology in the evaluation of skin lesions. J Telemed Telecare. 2023;29:382-389. doi:10.1177/1357633X20987423
With the increasing utilization of telemedicine since the COVID-19 pandemic, it is critical that clinicians have an appropriate understanding of the application of virtual care resources, including teledermatology. We present a case series of 3 patients to demonstrate the clinical utility of teledermatology in reducing the time to diagnosis of various rare and/or aggressive cutaneous malignancies, including Merkel cell carcinoma, malignant melanoma, and atypical fibroxanthoma. Cases were obtained from one large Midwestern medical center during the month of July 2021. Each case presented includes a description of the initial teledermatology presentation and reviews the clinical timeline from initial consultation submission to in-person clinic visit with lesion biopsy. This case series demonstrates real-world examples of how teledermatology can be utilized to expedite the care of specific vulnerable patient populations.
Teledermatology is a rapidly growing digital resource with specific utility in triaging patients to determine those requiring in-person evaluation for early and accurate detection of skin malignancies. Approximately one-third of teledermatology consultations result in face-to-face clinical encounters, with malignant neoplasms being the leading cause for biopsy.1,2 For specific populations, such as geriatric and immunocompromised patients, teledermatology may serve as a valuable tool, particularly in the wake of the COVID-19 pandemic. Furthermore, telemedicine may aid in addressing health disparities within the field of medicine and ultimately may improve access to care for vulnerable populations.3 Along with increasing access to specific subspecialty expertise, the use of teledermatology may reduce health care costs and improve the overall quality of care delivered to patients.4,5
We describe the clinical utility of teledermatology in triaging and diagnosing skin malignancies through a series of 3 cases obtained from digital image review at one large Midwestern medical center during the month of July 2021. Three unique cases with a final diagnosis of a rare or aggressive skin cancer were selected as examples, including a 75-year-old man with Merkle cell carcinoma, a 55-year-old man with aggressive pT3b malignant melanoma, and a 72-year-old man with an atypical fibroxanthoma. A clinical timeline of each case is presented, including the time intervals from initial image submission to image review, image submission to face-to-face clinical encounter, and image submission to final diagnosis. In all cases, the primary care provider submitted an order for teledermatology, and the teledermatology team obtained the images.
Case Series
Patient 1—Images of the right hand of a 75-year-old man with a medical history of basal cell carcinoma were submitted for teledermatology consultation utilizing store-and-forward image-capturing technology (day 1). The patient history provided with image submission indicated that the lesion had been present for 6 months and there were no associated symptoms. Clinical imaging demonstrated a pink-red pearly papule located on the proximal fourth digit of the dorsal aspect of the right hand (Figure 1). One day following the teledermatology request (day 2), the patient’s case was reviewed and triaged for an in-person visit. The patient was brought to clinic on day 34, and a biopsy was performed. On day 36, dermatopathology results indicated a diagnosis of Merkel cell carcinoma. On day 37, the patient was referred to surgical oncology, and on day 44, the patient underwent an initial surgical oncology visit with a plan for wide local excision of the right fourth digit with right axillary sentinel lymph node biopsy.

Patient 2—Images of the left flank of a 55-year-old man were submitted for teledermatology consultation via store-and-forward technology (day 1). A patient history provided with the image indicated that the lesion had been present for months to years and there were no associated symptoms, but the lesion recently had changed in color and size. Teledermatology images were reviewed on day 3 and demonstrated a 2- to 3-cm brown plaque on the left flank with color variegation and a prominent red papule protruding centrally (Figure 2). The patient was scheduled for an urgent in-person visit with biopsy. On day 6, the patient presented to clinic and an excision biopsy was performed. Dermatopathology was ordered with a RUSH indication, with results on day 7 revealing a pT3b malignant melanoma. An urgent consultation to surgical oncology was placed on the same day, and the patient underwent an initial surgical oncology visit on day 24 with a plan for wide local excision with left axillary and inguinal sentinel lymph node biopsy.

Patient 3—Images of the left ear of a 72-year-old man were submitted for teledermatology consultation utilizing review via store-and-forward technology (day 1). A patient history indicated that the lesion had been present for 3 months with associated bleeding. Image review demonstrated a solitary pearly pink papule located on the crura of the antihelix (Figure 3). Initial teledermatology consultation was reviewed on day 2 with notification of the need for in-person evaluation. The patient presented to clinic on day 33 for a biopsy, with dermatopathology results on day 36 consistent with an atypical fibroxanthoma. The patient was scheduled for Mohs micrographic surgery on day 37 and underwent surgical treatment on day 64.

Comment
Teledermatology consultations from all patients demonstrated adequate image quality to be able to evaluate the lesion of concern and yielded a request for in-person evaluation with possible biopsy (Table). In this case series, the average time interval from teledermatology consultation placement to teledermatology image report was 2 days (range, 1–3 days). The average time from teledermatology consultation placement to face-to-face encounter with biopsy was 24.3 days for the 3 cases presented in this series (range, 6–34 days). The initial surgical oncology visits took place an average of 34 days after the initial teledermatology consultation was placed for the 2 patients requiring referral (44 days for patient 1; 24 days for patient 2). For patient 3, Mohs micrographic surgery was required for treatment, which was scheduled by day 37 and subsequently performed on day 64.

When specifically looking at the diagnosis of cutaneous malignancies, studies have found that the incidence of skin cancer detection is similar for teledermatology compared to in-person clinic visits.6,7 Creighton-Smith et al6 performed a retrospective cohort study comparing prebiopsy and postbiopsy diagnostic accuracy and detection rates of skin cancer between store-and-forward technology and face-to-face consultation. When adjusting for possible compounding factors including personal and family history of skin cancer, there was no notable difference in detection rates of any skin cancer, including melanoma and nonmelanoma skin cancers. Furthermore, the 2 cohorts of patients were found to have similar prebiopsy and postbiopsy diagnostic concordance, with similar times from consultation being placed to requested biopsy and time from biopsy to final treatment.6
Clarke et al7 similarly analyzed the accuracy of store-and-forward teledermatology and found that there was overall concordance in diagnosis when comparing clinical dermatologists to teledermatologists. Moreover, when melanocytic lesions were excluded from the study, the decision to biopsy did not differ substantially.7
Areas of further study include determining what percentage of teledermatology lesions of concern for malignancy were proven to be skin cancer after in-person evaluation and biopsy, as well as investigating the effectiveness of teledermatology for melanocytic lesions, which frequently are removed from analysis in large-scale teledermatology studies.
Although teledermatology has substantial clinical utility and may serve as a great resource for specific populations, including geriatric patients and those who are immunocompromised, it is important to recognize notable limitations. Specifically, brief history and image review should not serve as replacements for a face-to-face visit with physical examination in cases where the diagnosis remains uncertain or when high-risk skin malignancies are suspected or included in the differential. Certain aggressive cutaneous malignancies such as Merkel cell carcinoma may appear as less aggressive via teledermatology due to restrictions of technology.
Conclusion
Teledermatology has had a major impact on the way health care is delivered to patients and may increase access to care, reducing unnecessary in-person visits and decreasing the number of in-person visit no-shows. With the appropriate use of a brief clinical history and image review, teledermatology can be effective to evaluate specific lesions of concern. We report 3 unique cases identified during a 1-month period at a large Midwestern medical center. These cases serve as important examples of the application of teledermatology in reducing the time to diagnosis of aggressive skin malignancies. Further research on the clinical utility of teledermatology is warranted.
Acknowledgments—The authors thank the additional providers from the University of Wisconsin and William S. Middleton Memorial Veterans Hospital (both in Madison, Wisconsin) involved in the medical care of the patients included in this case series.
With the increasing utilization of telemedicine since the COVID-19 pandemic, it is critical that clinicians have an appropriate understanding of the application of virtual care resources, including teledermatology. We present a case series of 3 patients to demonstrate the clinical utility of teledermatology in reducing the time to diagnosis of various rare and/or aggressive cutaneous malignancies, including Merkel cell carcinoma, malignant melanoma, and atypical fibroxanthoma. Cases were obtained from one large Midwestern medical center during the month of July 2021. Each case presented includes a description of the initial teledermatology presentation and reviews the clinical timeline from initial consultation submission to in-person clinic visit with lesion biopsy. This case series demonstrates real-world examples of how teledermatology can be utilized to expedite the care of specific vulnerable patient populations.
Teledermatology is a rapidly growing digital resource with specific utility in triaging patients to determine those requiring in-person evaluation for early and accurate detection of skin malignancies. Approximately one-third of teledermatology consultations result in face-to-face clinical encounters, with malignant neoplasms being the leading cause for biopsy.1,2 For specific populations, such as geriatric and immunocompromised patients, teledermatology may serve as a valuable tool, particularly in the wake of the COVID-19 pandemic. Furthermore, telemedicine may aid in addressing health disparities within the field of medicine and ultimately may improve access to care for vulnerable populations.3 Along with increasing access to specific subspecialty expertise, the use of teledermatology may reduce health care costs and improve the overall quality of care delivered to patients.4,5
We describe the clinical utility of teledermatology in triaging and diagnosing skin malignancies through a series of 3 cases obtained from digital image review at one large Midwestern medical center during the month of July 2021. Three unique cases with a final diagnosis of a rare or aggressive skin cancer were selected as examples, including a 75-year-old man with Merkle cell carcinoma, a 55-year-old man with aggressive pT3b malignant melanoma, and a 72-year-old man with an atypical fibroxanthoma. A clinical timeline of each case is presented, including the time intervals from initial image submission to image review, image submission to face-to-face clinical encounter, and image submission to final diagnosis. In all cases, the primary care provider submitted an order for teledermatology, and the teledermatology team obtained the images.
Case Series
Patient 1—Images of the right hand of a 75-year-old man with a medical history of basal cell carcinoma were submitted for teledermatology consultation utilizing store-and-forward image-capturing technology (day 1). The patient history provided with image submission indicated that the lesion had been present for 6 months and there were no associated symptoms. Clinical imaging demonstrated a pink-red pearly papule located on the proximal fourth digit of the dorsal aspect of the right hand (Figure 1). One day following the teledermatology request (day 2), the patient’s case was reviewed and triaged for an in-person visit. The patient was brought to clinic on day 34, and a biopsy was performed. On day 36, dermatopathology results indicated a diagnosis of Merkel cell carcinoma. On day 37, the patient was referred to surgical oncology, and on day 44, the patient underwent an initial surgical oncology visit with a plan for wide local excision of the right fourth digit with right axillary sentinel lymph node biopsy.

Patient 2—Images of the left flank of a 55-year-old man were submitted for teledermatology consultation via store-and-forward technology (day 1). A patient history provided with the image indicated that the lesion had been present for months to years and there were no associated symptoms, but the lesion recently had changed in color and size. Teledermatology images were reviewed on day 3 and demonstrated a 2- to 3-cm brown plaque on the left flank with color variegation and a prominent red papule protruding centrally (Figure 2). The patient was scheduled for an urgent in-person visit with biopsy. On day 6, the patient presented to clinic and an excision biopsy was performed. Dermatopathology was ordered with a RUSH indication, with results on day 7 revealing a pT3b malignant melanoma. An urgent consultation to surgical oncology was placed on the same day, and the patient underwent an initial surgical oncology visit on day 24 with a plan for wide local excision with left axillary and inguinal sentinel lymph node biopsy.

Patient 3—Images of the left ear of a 72-year-old man were submitted for teledermatology consultation utilizing review via store-and-forward technology (day 1). A patient history indicated that the lesion had been present for 3 months with associated bleeding. Image review demonstrated a solitary pearly pink papule located on the crura of the antihelix (Figure 3). Initial teledermatology consultation was reviewed on day 2 with notification of the need for in-person evaluation. The patient presented to clinic on day 33 for a biopsy, with dermatopathology results on day 36 consistent with an atypical fibroxanthoma. The patient was scheduled for Mohs micrographic surgery on day 37 and underwent surgical treatment on day 64.

Comment
Teledermatology consultations from all patients demonstrated adequate image quality to be able to evaluate the lesion of concern and yielded a request for in-person evaluation with possible biopsy (Table). In this case series, the average time interval from teledermatology consultation placement to teledermatology image report was 2 days (range, 1–3 days). The average time from teledermatology consultation placement to face-to-face encounter with biopsy was 24.3 days for the 3 cases presented in this series (range, 6–34 days). The initial surgical oncology visits took place an average of 34 days after the initial teledermatology consultation was placed for the 2 patients requiring referral (44 days for patient 1; 24 days for patient 2). For patient 3, Mohs micrographic surgery was required for treatment, which was scheduled by day 37 and subsequently performed on day 64.

When specifically looking at the diagnosis of cutaneous malignancies, studies have found that the incidence of skin cancer detection is similar for teledermatology compared to in-person clinic visits.6,7 Creighton-Smith et al6 performed a retrospective cohort study comparing prebiopsy and postbiopsy diagnostic accuracy and detection rates of skin cancer between store-and-forward technology and face-to-face consultation. When adjusting for possible compounding factors including personal and family history of skin cancer, there was no notable difference in detection rates of any skin cancer, including melanoma and nonmelanoma skin cancers. Furthermore, the 2 cohorts of patients were found to have similar prebiopsy and postbiopsy diagnostic concordance, with similar times from consultation being placed to requested biopsy and time from biopsy to final treatment.6
Clarke et al7 similarly analyzed the accuracy of store-and-forward teledermatology and found that there was overall concordance in diagnosis when comparing clinical dermatologists to teledermatologists. Moreover, when melanocytic lesions were excluded from the study, the decision to biopsy did not differ substantially.7
Areas of further study include determining what percentage of teledermatology lesions of concern for malignancy were proven to be skin cancer after in-person evaluation and biopsy, as well as investigating the effectiveness of teledermatology for melanocytic lesions, which frequently are removed from analysis in large-scale teledermatology studies.
Although teledermatology has substantial clinical utility and may serve as a great resource for specific populations, including geriatric patients and those who are immunocompromised, it is important to recognize notable limitations. Specifically, brief history and image review should not serve as replacements for a face-to-face visit with physical examination in cases where the diagnosis remains uncertain or when high-risk skin malignancies are suspected or included in the differential. Certain aggressive cutaneous malignancies such as Merkel cell carcinoma may appear as less aggressive via teledermatology due to restrictions of technology.
Conclusion
Teledermatology has had a major impact on the way health care is delivered to patients and may increase access to care, reducing unnecessary in-person visits and decreasing the number of in-person visit no-shows. With the appropriate use of a brief clinical history and image review, teledermatology can be effective to evaluate specific lesions of concern. We report 3 unique cases identified during a 1-month period at a large Midwestern medical center. These cases serve as important examples of the application of teledermatology in reducing the time to diagnosis of aggressive skin malignancies. Further research on the clinical utility of teledermatology is warranted.
Acknowledgments—The authors thank the additional providers from the University of Wisconsin and William S. Middleton Memorial Veterans Hospital (both in Madison, Wisconsin) involved in the medical care of the patients included in this case series.
- Bianchi MG, Santos A, Cordioli E. Benefits of teledermatology for geriatric patients: population-based cross-sectional study. J Med Internet Res. 2020;22:E16700.
- Mortimer S, Rosin A. A retrospective review of incidental malignancies in veterans seen for face-to-face follow-up after teledermatology consultation. J Am Acad Dermatol. 2021;84:1130-1132.
- Costello CM, Cumsky HJL, Maly CJ, et al. Improving access to care through the establishment of a local, teledermatology network. Telemed J E Health. 2020;26:935-940. doi:10.1089/tmj.2019.0051
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260. doi:10.1007/s40257-017-0317-6
- Hadeler E, Beer J, Nouri K. The influence of teledermatology on health care access and equity. J Am Acad Dermatol. 2021;84:E219-E220. doi:10.1016/j.jaad.2020.12.036
- Creighton-Smith M, Murgia RD 3rd, Konnikov N, et al. Incidence of melanoma and keratinocytic carcinomas in patients evaluated by store-and-forward teledermatology vs dermatology clinic. Int J Dermatol. 2017;56:1026-1031. doi:10.1111/ijd.13672
- Clarke EL, Reichenberg JS, Ahmed AM, et al. The utility of teledermatology in the evaluation of skin lesions. J Telemed Telecare. 2023;29:382-389. doi:10.1177/1357633X20987423
- Bianchi MG, Santos A, Cordioli E. Benefits of teledermatology for geriatric patients: population-based cross-sectional study. J Med Internet Res. 2020;22:E16700.
- Mortimer S, Rosin A. A retrospective review of incidental malignancies in veterans seen for face-to-face follow-up after teledermatology consultation. J Am Acad Dermatol. 2021;84:1130-1132.
- Costello CM, Cumsky HJL, Maly CJ, et al. Improving access to care through the establishment of a local, teledermatology network. Telemed J E Health. 2020;26:935-940. doi:10.1089/tmj.2019.0051
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260. doi:10.1007/s40257-017-0317-6
- Hadeler E, Beer J, Nouri K. The influence of teledermatology on health care access and equity. J Am Acad Dermatol. 2021;84:E219-E220. doi:10.1016/j.jaad.2020.12.036
- Creighton-Smith M, Murgia RD 3rd, Konnikov N, et al. Incidence of melanoma and keratinocytic carcinomas in patients evaluated by store-and-forward teledermatology vs dermatology clinic. Int J Dermatol. 2017;56:1026-1031. doi:10.1111/ijd.13672
- Clarke EL, Reichenberg JS, Ahmed AM, et al. The utility of teledermatology in the evaluation of skin lesions. J Telemed Telecare. 2023;29:382-389. doi:10.1177/1357633X20987423
Practice Points
- Teledermatology via store-and-forward technology has been demonstrated to be effective in assessing and triaging various cutaneous malignancies.
- The use of teledermatology has increased because of the COVID-19 pandemic and may be useful for specific vulnerable populations.
- When used appropriately, teledermatology may function as a useful resource to triage patients requiring in-person evaluation for the diagnosis of aggressive skin malignancies and may aid in reducing the time to diagnosis of various skin cancers.
Endoscopic Management of Barrett’s Esophagus
Introduction
Barrett’s esophagus (BE) is characterized by the replacement of squamous epithelium by columnar metaplasia of the distal esophagus (>1 cm length). It is a precancerous condition, with 3%-5% of patients with BE developing esophageal adenocarcinoma (EAC) in their lifetime. EAC is one of the cancers with high morbidity and mortality (5-year survival < 20%), and its incidence has been on the rise. Studies examining the natural history of BE have demonstrated that the progression happens through a metaplasia-dysplasia-neoplasia sequence. Therefore, early detection of BE and timely management to prevent progression to EAC is crucial.
Grades of Dysplasia
The current gold standard for the diagnosis of BE neoplasia includes a high-quality endoscopic evaluation and biopsies. Biopsies should be obtained from any visible lesions (nodules, ulcers) followed by a random 4-quadrant fashion (Seattle protocol) interval of the entire length of the BE segment. It is essential to pay attention to the results of the biopsy that have been obtained since it will not only determine the surveillance interval but is crucial in planning any necessary endoscopic therapy. The possible results of the biopsy and its implications are:
- No intestinal metaplasia (IM): This would rule out Barrett’s esophagus and no further surveillance would be necessary. A recent population-based study of over 1 million patients showed a 55% and 61% reduced risk of upper gastrointestinal (UGI) cancer and deaths respectively after a negative endoscopy.1
- Intestinal metaplasia with no dysplasia (non-dysplastic BE): Biopsies confirm presence of intestinal metaplasia in the biopsies without any evidence of dysplasia. While the rate of progression to EAC is low (0.07%-0.25%), it is not absent and thus surveillance would be indicated. Current guidelines suggest repeating an endoscopy with biopsy in 5 years if the length of BE is < 3 cm or 3 years if length of BE ≥ 3 cm.2
- Indeterminate for dysplasia (BE-IND): Biopsies confirm IM but are not able to definitively rule out dysplasia. This can be seen in about 4%-8% of the biopsies obtained. The progression rates to EAC are reported to be comparable or lower to low-grade dysplasia (LGD), so the current recommendation is to intensify acid reduction therapy and repeat endoscopy in 6 months. If repeat endoscopy downgrades to non-dysplastic, then can follow surveillance according to NDBE protocol; otherwise recommend continuing surveillance every 12 months.
- Low-grade dysplasia (BE-LGD): Biopsies confirm IM but also show tightly packed overlapping basal nuclei with hyperchromasia and irregular contours, basal stratification of nuclei, and diminished goblet and columnar cell mucus. There is significant inter-observer variability reported,3 and thus the slides must be reviewed by a second pathologist with experience in BE to confirm the findings. Once confirmed, based on risk factors such as presence of multifocal LGD, persistence of LGD, presence of visible lesions, etc., the patient can be offered Barrett’s endoscopic therapy (BET) or undergo continued surveillance. The decision of pursuing one or the other would be dependent on patient preference and shared decision-making between the patient and the provider.
- High-grade dysplasia (BE-HGD): Biopsies confirm IM with cells showing greater degree of cytologic and architectural alterations of dysplasia than LGD but without overt neoplastic features. Over 40% of the patients would progress to EAC and thus the current recommendations would be to recommend BET in these patients.4
- Esophageal adenocarcinoma (EAC): Biopsies demonstrate neoplasia. If the neoplastic changes are limited to the mucosa (T1a) on endoscopic ultrasound or cross-sectional imaging, then BET is suggested. If there is involvement of submucosa, then depending on the depth of invasion, absence of high-risk features (poor differentiation, lymphovascular invasion), BET can be considered as an alternative to esophagectomy.
Lesion Detection on Endoscopy
Data from large population-based studies with at least 3 years of follow-up reported that 58%-66% of EAC detected during endoscopy were diagnosed within 1 year of an index Barrett’s esophagus screening endoscopy, or post-endoscopy Barrett’s neoplasia, and were considered likely to have been missed during index endoscopy.5 This underscores the importance of careful and systematic endoscopic examination during an upper endoscopy.
Studies have also demonstrated that longer examination time was associated with significantly higher detection of HGD/EAC.6,7 Careful examination of the tubular esophagus and gastroesophageal junction (GEJ) should be performed in forward and retroflexed views looking for any subtle areas of nodularity, loop distortion, variability in vascular patterns, mucosal changes concerning for dysplasia or neoplasia. Use of high-definition white light endoscopy (HD-WLE) and virtual chromoendoscopy techniques such as narrow banding imaging (NBI) or blue laser imaging (BLI) are currently recommended in the guidelines.2 Spray chromoendoscopy using acetic acid can also be utilized. Another exciting development is the use of artificial intelligence (AI) in detecting and diagnosing BE associated lesions and neoplasia.
Barrett’s Endoscopic Therapy (BET)
Patients with visible lesions, dysplasia, or early EAC are candidates for BET (Table 1). 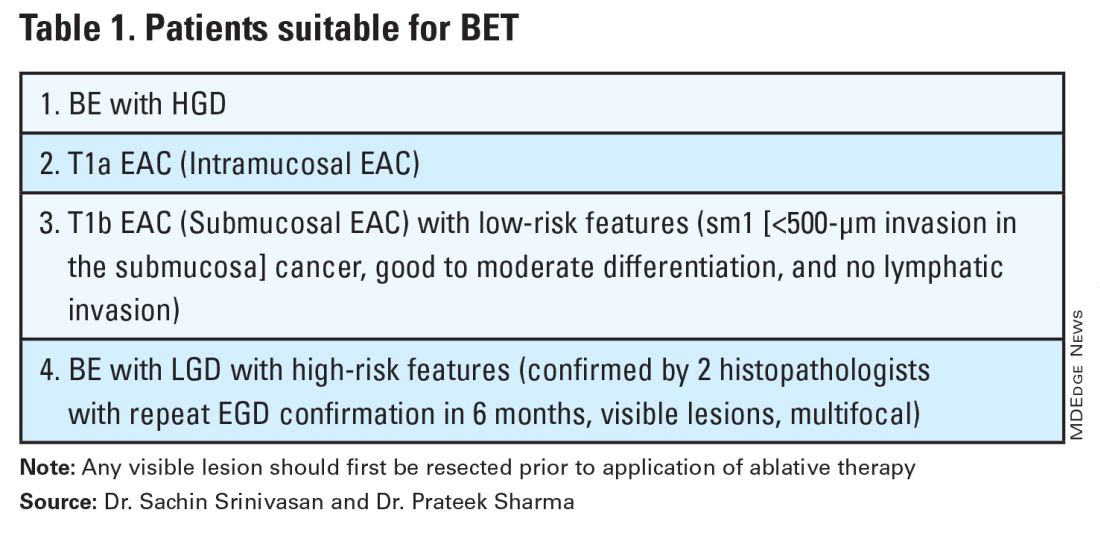
BET involves resective and ablative modalities. The resective modalities include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) and are the modalities of choice for nodular or raised lesions.
EMR involves endoscopic resection of abnormal mucosa using either lift-assisted technique or multi-band ligation (Figure 1). 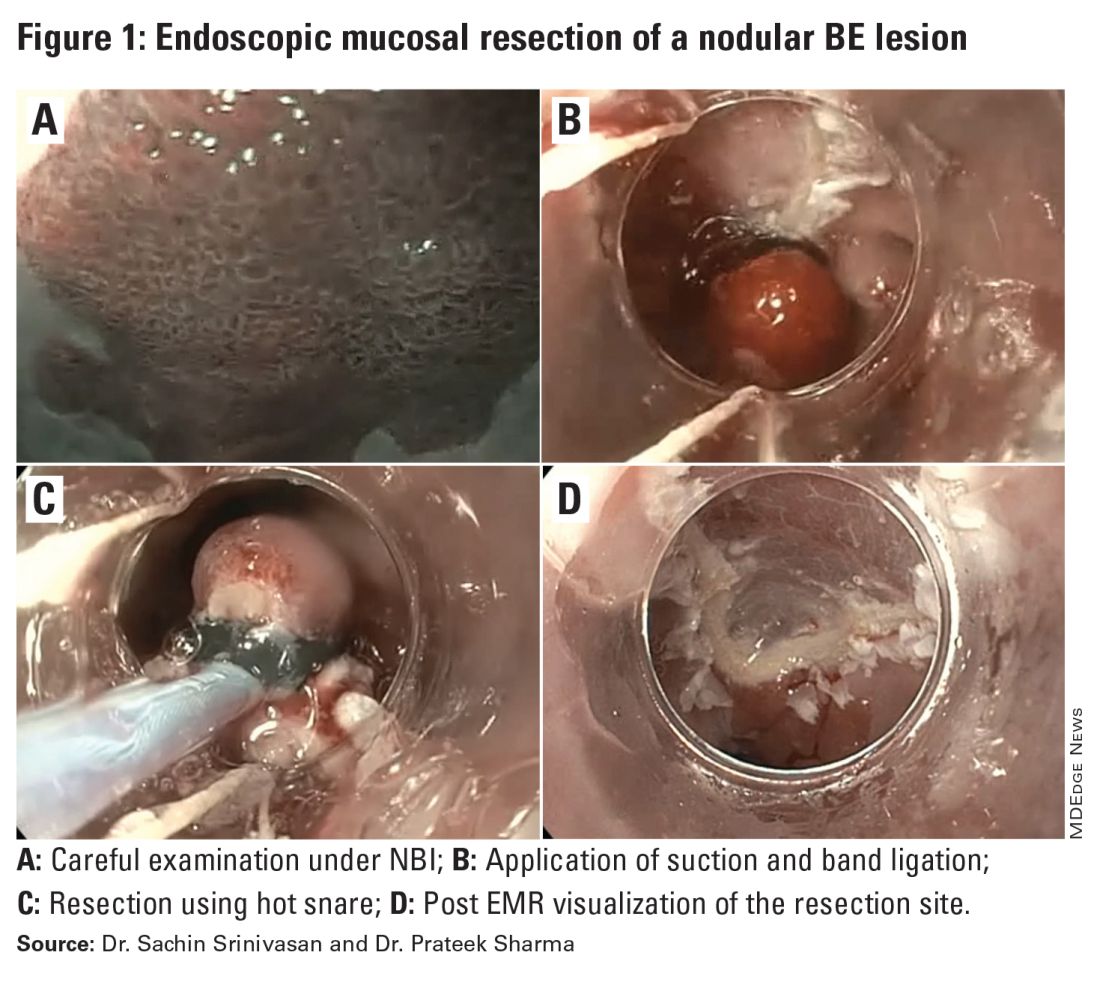
ESD, on the other hand, involves submucosal dissection and perimeter resection of the lesion, thus providing the advantage of an en-bloc resection. In a recent randomized controlled trial (RCT) of 40 patients undergoing ESD vs EMR for HGD/EAC, ESD was better for curative resection (R0) (58%) compared with EMR (12%); however, the remission rates at 3 months were comparable with two perforations reported in the ESD group while there were no complications in the EMR group.8
There is an apparent learning curve when it comes to these advanced techniques, and with more experience, we are seeing comparable results for both these modalities. However, given the complexity and time required for the procedure, current practices typically involve preserving ESD for lesions > 2 cm, those having a likelihood of cancer in the superficial submucosa, or those that EMR cannot remove due to underlying fibrosis or post-EMR recurrence.
The ablative modalities include radiofrequency ablation (RFA), cryotherapy, and hybrid argon plasma coagulation (hybrid APC). These modalities are used for flat lesions, and as therapy following endoscopic resection of nodular lesions to treat residual flat segment of BE. RFA, one of the earliest introduced endoscopic modalities, involves applying directed and controlled heat energy to ablate lesions. Current devices allow circumferential or focal application of RFA. It is a safe and effective modality with good complete eradication of IM (CE-IM) (71%-93%) and complete eradication of dysplasia (CE-D) (91%-100%) rates. These results have been sustained even at 2 years, with the most recent long-term data from a registry study showing a relapse rate of 6% for dysplasia and 19% for IM after 8 years, suggesting durability of this treatment.9
Cryotherapy involves the application of liquid nitrogen or rapidly expanding CO2 to the abnormal mucosa, leading to the rapid freezing and thawing that leads to the death of the cells. Cryogen can be applied as a spray or using a balloon with the spray nozzle in the center. This modality can be used to treat focal lesions and/or larger segments. While it has not been systematically compared with RFA, rates of CE-IM up to 81% and CE-D up to 97% are reported. Hybrid APC involves the use of submucosal saline injection to provide a protective cushion before APC is applied. It has CE-IM rate of 69% and CE-D rate of 67%-86%.10 In a recent RCT of 101 patients randomized to RFA or hybrid APC, CE-IM rates were similar (RFA:74.2% vs hAPC: 82.9%).11
Recently, another technique called radiofrequency vapor ablation (RFVA) is being evaluated, which involves ablating BE segment using vapor at 100° C generated with an RF electrode. A proof-of-concept study of 15 patients showed median squamous conversion of 55% (IQR 33-74) and 98% (IQR 56-99) for 1- and 3-second applications, respectively, with no reported adverse events.12
Barrett’s Refractory to Endoscopic Therapy
Failure of BET is defined as persistent columnar lined epithelium (intestinal metaplasia) with inadequate response, after adequate attempts at endoscopic ablation therapy (after resection) with at least four ablation sessions.13 If encountered, special attention must be given to check compliance with proton pump inhibitors (PPIs), previous incomplete resection, and presence of large hiatal hernia. If CE-IM is not achieved after multiple sessions, change of ablative modality is typically considered. In addition, careful examination for visible lesions should be performed and even if a small one is noted, this should be first resected prior to application of any ablative therapy.
Currently there are no guideline recommendations regarding the preference of one endoscopic modality over another or consideration of potential endoscopic or surgical fundoplication. These modalities primarily rely on technologies available at an institution and the preference of a provider based on their training and experience. Most studies indicate 1-3 sessions (~ 3 months apart) of ablative treatment before achieving CE-IM.
Success and Adverse Events of BET
In a recent real-world study of over 27,000 patients with dysplastic BE, 5295 underwent BET. Analysis showed that patients with HGD/EAC who had BET had a significantly lower 3-year mortality (HGD: RR, 0.59; 95%CI, 0.49-0.71; EAC: RR, 0.53; 95% CI, 0.44-0.65) compared with those who did not undergo BET. Esophageal strictures were the most common adverse event and were noted in 6.5%, followed by chest pain (1.8%), upper GI bleeding (0.47%), and esophageal perforation (0.2%).14
In general, adverse events can be divided into immediate and delayed adverse events. Immediate adverse events typically involve bleeding and perforation that can occur during or shortly after the procedure. These are reported at higher rates with resective modalities compared with ablative therapies. Standard endoscopic techniques involving coagulation grasper or clips can be used to achieve hemostasis. Endoscopic suturing devices offer the ability to contain any perforation. The need for surgical intervention is small and limited to adverse events not detected during the procedure.
Delayed adverse events such as stricture and stenosis are higher for resective modalities (up to 30%), especially when involving more significant than 75% of the esophageal circumference. Post-procedural pain/dysphagia is most common after ablative therapies. Dysphagia reported after any endoscopic therapy should be promptly evaluated, and sequential dilation until the goal esophageal lumen is achieved should be performed every 2-4 weeks.
Recurrences and Surveillance After BET
What is established is that recurrences can occur and may be subtle, therefore detailed endoscopic surveillance is required. In a prospective study, recurrence rates of 15%-16% for IM and 3%-5% for any dysplasia were reported, with the majority being in the first 2 years after achieving CE-IM.15 A systematic review of 21 studies looking at the location of recurrences suggested that the majority (56%) occur in the distal esophagus. Of those that occur in the esophagus, about 80% of them were in the distal 2 cm of the esophagus and only 50% of the recurrences were visible recurrences, thus reiterating the importance of meticulous examination and systematic biopsies.16
On the contrary, a recent single-center study of 217 patients who had achieved CE-IM with 5.5 years of follow-up demonstrated a 26% and 8% recurrence of IM and dysplasia, respectively. One hundred percent of the recurrence in the esophagus was reported as visible.17 Therefore, follow-up endoscopy surveillance protocol after CE-IM should still involve meticulous examination, biopsy of visible lesions, and systematic biopsies for non-visible lesions from the original BE segment, similar to those patients who have not needed BET.
Current guidelines based on expert consensus and evidence recommend surveillance after CE-IM based on original most advanced histology:2
1. LGD: 1 year, 3 years, and every 2 years after that.
2. HGD/EAC: 3 months, 6 months, 12 months, and annually after that.
There is no clear guideline on when to stop surveillance since the longest available follow-up is around 10 years, and recurrences are still detected. A potential surveillance endpoint may be based on age and comorbidities, especially those that would preclude a patient from being a candidate for BET.
When Should a Patient Be Referred?
BE patients with visible lesions and/or dysplastic changes in the biopsy who would require BET should be considered for referral to high-volume centers. Studies have shown higher success for CE-IM and lower rates of adverse events and recurrences in these patients managed at expert centers. The presence of a multidisciplinary team involving pathologists, surgeons, and oncologists is critical and offers a timely opportunity in case of need for a high-risk patient.
Conclusion
BE is a precursor to EAC, with rising incidence and poor 5-year survival. Endoscopic diagnosis is the gold standard and requires a high-quality examination and biopsies. Based on histopathology, a systematic surveillance and BET plan should be performed to achieve CE-IM in patients with dysplasia. Once CE-IM is achieved, regular surveillance should be performed with careful attention to recurrences and complications from the BET modalities.
Dr. Srinivasan and Dr. Sharma are based at the University of Kansas Medical Center, Kansas City, Kansas, and the Kansas City Veterans Affairs Medical Center, Kansas City, Missouri. Dr. Srinivasan has no relevant disclosures. Dr. Sharma disclosed research grants from ERBE, Ironwood Pharmaceuticals, Olympus, and Medtronic. He has served as a consultant for Takeda, Samsung Bioepis, Olympus, and Lumendi, and reports other funding from Medtronic, Fujifilm Medical Systems USA, and Salix.
References
1. Holmberg D, et al. Incidence and mortality in upper gastrointestinal cancer after negative endoscopy for gastroesophageal reflux disease. Gastroenterology. 2022;162(2):431-438.e4.
2. Shaheen NJ, et al. Diagnosis and management of Barrett’s esophagus: An updated ACG guideline. Am J Gastroenterol. 2022 Apr;117(4):559-587.
3. Pech O, et al. Inter-observer variability in the diagnosis of low-grade dysplasia in pathologists: A comparison between experienced and inexperienced pathologists. Gastrointest Endosc. 2006 Apr;63(5):AB130.
4. Krishnamoorthi R, et al. Factors associated with progression of Barrett’s esophagus: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1046-1055.e8.
5. Visrodia K, et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: A systematic review and meta-analysis. Gastroenterology. 2016 Mar;150(3):599-607.e7; quiz e14-5.
6. Perisetti A, Sharma P. Tips for improving the identification of neoplastic visible lesions in Barrett’s esophagus. Gastrointest Endosc. 2023 Feb;97(2):248-250.
7. Gupta N, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc. 2012 Sep;76(3):531-538.
8. Terheggen G, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut. 2017 May;66(5):783-793.
9. Wolfson P, et al. Endoscopic eradication therapy for Barrett’s esophagus-related neoplasia: A final 10-year report from the UK National HALO Radiofrequency Ablation Registry. Gastrointest Endosc. 2022 Aug;96(2):223-233.
10. Rösch T, et al. 1151 Multicenter feasibility study of combined injection and argon plasma coagulation (hybrid-APC) in the ablation therapy of neoplastic Barrett esophagus. Gastrointest Endosc. 2017;85(5):AB154.
11. Knabe M, et al. Radiofrequency ablation versus hybrid argon plasma coagulation in Barrett’s esophagus: A prospective randomised trial. Surg Endosc. 2023;37(10):7803-7811.
12. Van Munster SN, et al. Radiofrequency vapor ablation for Barrett’s esophagus: Feasibility, safety, and proof of concept in a stepwise study with in vitro, animal, and the first in-human application. Endoscopy. 2021 Nov;53(11):1162-1168.
13. Emura F, et al. Rio de Janeiro global consensus on landmarks, definitions, and classifications in Barrett’s esophagus: World Endoscopy Organization Delphi study. Gastroenterology. 2022 Jul;163(1):84-96.e2.
14. Singh RR, et al. Real-world evidence of safety and effectiveness of Barrett’s endoscopic therapy. Gastrointest Endosc. 2023 Aug;98(2):155-161.e1.
15. Wani S, et al. Recurrence Is rare following complete eradication of intestinal metaplasia in patients with Barrett’s esophagus and peaks at 18 months. Clin Gastroenterol Hepatol. 2020 Oct;18(11):2609-2617.e2.
16. Duvvuri A, et al. Mo1273 Location and pattern of recurrences in patients with Barrett’s esophagus after endoscopic therapy: A systematic review and critical analysis of the published literature. Gastrointest Endosc. 2020;91(6):AB410-1.
17. He T, et al. Location and appearance of dysplastic Barrett’s esophagus recurrence after endoscopic eradication therapy: No additional yield from random biopsy sampling neosquamous mucosa. Gastrointest Endosc. 2023 Nov;98(5):722-732.
Introduction
Barrett’s esophagus (BE) is characterized by the replacement of squamous epithelium by columnar metaplasia of the distal esophagus (>1 cm length). It is a precancerous condition, with 3%-5% of patients with BE developing esophageal adenocarcinoma (EAC) in their lifetime. EAC is one of the cancers with high morbidity and mortality (5-year survival < 20%), and its incidence has been on the rise. Studies examining the natural history of BE have demonstrated that the progression happens through a metaplasia-dysplasia-neoplasia sequence. Therefore, early detection of BE and timely management to prevent progression to EAC is crucial.
Grades of Dysplasia
The current gold standard for the diagnosis of BE neoplasia includes a high-quality endoscopic evaluation and biopsies. Biopsies should be obtained from any visible lesions (nodules, ulcers) followed by a random 4-quadrant fashion (Seattle protocol) interval of the entire length of the BE segment. It is essential to pay attention to the results of the biopsy that have been obtained since it will not only determine the surveillance interval but is crucial in planning any necessary endoscopic therapy. The possible results of the biopsy and its implications are:
- No intestinal metaplasia (IM): This would rule out Barrett’s esophagus and no further surveillance would be necessary. A recent population-based study of over 1 million patients showed a 55% and 61% reduced risk of upper gastrointestinal (UGI) cancer and deaths respectively after a negative endoscopy.1
- Intestinal metaplasia with no dysplasia (non-dysplastic BE): Biopsies confirm presence of intestinal metaplasia in the biopsies without any evidence of dysplasia. While the rate of progression to EAC is low (0.07%-0.25%), it is not absent and thus surveillance would be indicated. Current guidelines suggest repeating an endoscopy with biopsy in 5 years if the length of BE is < 3 cm or 3 years if length of BE ≥ 3 cm.2
- Indeterminate for dysplasia (BE-IND): Biopsies confirm IM but are not able to definitively rule out dysplasia. This can be seen in about 4%-8% of the biopsies obtained. The progression rates to EAC are reported to be comparable or lower to low-grade dysplasia (LGD), so the current recommendation is to intensify acid reduction therapy and repeat endoscopy in 6 months. If repeat endoscopy downgrades to non-dysplastic, then can follow surveillance according to NDBE protocol; otherwise recommend continuing surveillance every 12 months.
- Low-grade dysplasia (BE-LGD): Biopsies confirm IM but also show tightly packed overlapping basal nuclei with hyperchromasia and irregular contours, basal stratification of nuclei, and diminished goblet and columnar cell mucus. There is significant inter-observer variability reported,3 and thus the slides must be reviewed by a second pathologist with experience in BE to confirm the findings. Once confirmed, based on risk factors such as presence of multifocal LGD, persistence of LGD, presence of visible lesions, etc., the patient can be offered Barrett’s endoscopic therapy (BET) or undergo continued surveillance. The decision of pursuing one or the other would be dependent on patient preference and shared decision-making between the patient and the provider.
- High-grade dysplasia (BE-HGD): Biopsies confirm IM with cells showing greater degree of cytologic and architectural alterations of dysplasia than LGD but without overt neoplastic features. Over 40% of the patients would progress to EAC and thus the current recommendations would be to recommend BET in these patients.4
- Esophageal adenocarcinoma (EAC): Biopsies demonstrate neoplasia. If the neoplastic changes are limited to the mucosa (T1a) on endoscopic ultrasound or cross-sectional imaging, then BET is suggested. If there is involvement of submucosa, then depending on the depth of invasion, absence of high-risk features (poor differentiation, lymphovascular invasion), BET can be considered as an alternative to esophagectomy.
Lesion Detection on Endoscopy
Data from large population-based studies with at least 3 years of follow-up reported that 58%-66% of EAC detected during endoscopy were diagnosed within 1 year of an index Barrett’s esophagus screening endoscopy, or post-endoscopy Barrett’s neoplasia, and were considered likely to have been missed during index endoscopy.5 This underscores the importance of careful and systematic endoscopic examination during an upper endoscopy.
Studies have also demonstrated that longer examination time was associated with significantly higher detection of HGD/EAC.6,7 Careful examination of the tubular esophagus and gastroesophageal junction (GEJ) should be performed in forward and retroflexed views looking for any subtle areas of nodularity, loop distortion, variability in vascular patterns, mucosal changes concerning for dysplasia or neoplasia. Use of high-definition white light endoscopy (HD-WLE) and virtual chromoendoscopy techniques such as narrow banding imaging (NBI) or blue laser imaging (BLI) are currently recommended in the guidelines.2 Spray chromoendoscopy using acetic acid can also be utilized. Another exciting development is the use of artificial intelligence (AI) in detecting and diagnosing BE associated lesions and neoplasia.
Barrett’s Endoscopic Therapy (BET)
Patients with visible lesions, dysplasia, or early EAC are candidates for BET (Table 1). 
BET involves resective and ablative modalities. The resective modalities include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) and are the modalities of choice for nodular or raised lesions.
EMR involves endoscopic resection of abnormal mucosa using either lift-assisted technique or multi-band ligation (Figure 1). 
ESD, on the other hand, involves submucosal dissection and perimeter resection of the lesion, thus providing the advantage of an en-bloc resection. In a recent randomized controlled trial (RCT) of 40 patients undergoing ESD vs EMR for HGD/EAC, ESD was better for curative resection (R0) (58%) compared with EMR (12%); however, the remission rates at 3 months were comparable with two perforations reported in the ESD group while there were no complications in the EMR group.8
There is an apparent learning curve when it comes to these advanced techniques, and with more experience, we are seeing comparable results for both these modalities. However, given the complexity and time required for the procedure, current practices typically involve preserving ESD for lesions > 2 cm, those having a likelihood of cancer in the superficial submucosa, or those that EMR cannot remove due to underlying fibrosis or post-EMR recurrence.
The ablative modalities include radiofrequency ablation (RFA), cryotherapy, and hybrid argon plasma coagulation (hybrid APC). These modalities are used for flat lesions, and as therapy following endoscopic resection of nodular lesions to treat residual flat segment of BE. RFA, one of the earliest introduced endoscopic modalities, involves applying directed and controlled heat energy to ablate lesions. Current devices allow circumferential or focal application of RFA. It is a safe and effective modality with good complete eradication of IM (CE-IM) (71%-93%) and complete eradication of dysplasia (CE-D) (91%-100%) rates. These results have been sustained even at 2 years, with the most recent long-term data from a registry study showing a relapse rate of 6% for dysplasia and 19% for IM after 8 years, suggesting durability of this treatment.9
Cryotherapy involves the application of liquid nitrogen or rapidly expanding CO2 to the abnormal mucosa, leading to the rapid freezing and thawing that leads to the death of the cells. Cryogen can be applied as a spray or using a balloon with the spray nozzle in the center. This modality can be used to treat focal lesions and/or larger segments. While it has not been systematically compared with RFA, rates of CE-IM up to 81% and CE-D up to 97% are reported. Hybrid APC involves the use of submucosal saline injection to provide a protective cushion before APC is applied. It has CE-IM rate of 69% and CE-D rate of 67%-86%.10 In a recent RCT of 101 patients randomized to RFA or hybrid APC, CE-IM rates were similar (RFA:74.2% vs hAPC: 82.9%).11
Recently, another technique called radiofrequency vapor ablation (RFVA) is being evaluated, which involves ablating BE segment using vapor at 100° C generated with an RF electrode. A proof-of-concept study of 15 patients showed median squamous conversion of 55% (IQR 33-74) and 98% (IQR 56-99) for 1- and 3-second applications, respectively, with no reported adverse events.12
Barrett’s Refractory to Endoscopic Therapy
Failure of BET is defined as persistent columnar lined epithelium (intestinal metaplasia) with inadequate response, after adequate attempts at endoscopic ablation therapy (after resection) with at least four ablation sessions.13 If encountered, special attention must be given to check compliance with proton pump inhibitors (PPIs), previous incomplete resection, and presence of large hiatal hernia. If CE-IM is not achieved after multiple sessions, change of ablative modality is typically considered. In addition, careful examination for visible lesions should be performed and even if a small one is noted, this should be first resected prior to application of any ablative therapy.
Currently there are no guideline recommendations regarding the preference of one endoscopic modality over another or consideration of potential endoscopic or surgical fundoplication. These modalities primarily rely on technologies available at an institution and the preference of a provider based on their training and experience. Most studies indicate 1-3 sessions (~ 3 months apart) of ablative treatment before achieving CE-IM.
Success and Adverse Events of BET
In a recent real-world study of over 27,000 patients with dysplastic BE, 5295 underwent BET. Analysis showed that patients with HGD/EAC who had BET had a significantly lower 3-year mortality (HGD: RR, 0.59; 95%CI, 0.49-0.71; EAC: RR, 0.53; 95% CI, 0.44-0.65) compared with those who did not undergo BET. Esophageal strictures were the most common adverse event and were noted in 6.5%, followed by chest pain (1.8%), upper GI bleeding (0.47%), and esophageal perforation (0.2%).14
In general, adverse events can be divided into immediate and delayed adverse events. Immediate adverse events typically involve bleeding and perforation that can occur during or shortly after the procedure. These are reported at higher rates with resective modalities compared with ablative therapies. Standard endoscopic techniques involving coagulation grasper or clips can be used to achieve hemostasis. Endoscopic suturing devices offer the ability to contain any perforation. The need for surgical intervention is small and limited to adverse events not detected during the procedure.
Delayed adverse events such as stricture and stenosis are higher for resective modalities (up to 30%), especially when involving more significant than 75% of the esophageal circumference. Post-procedural pain/dysphagia is most common after ablative therapies. Dysphagia reported after any endoscopic therapy should be promptly evaluated, and sequential dilation until the goal esophageal lumen is achieved should be performed every 2-4 weeks.
Recurrences and Surveillance After BET
What is established is that recurrences can occur and may be subtle, therefore detailed endoscopic surveillance is required. In a prospective study, recurrence rates of 15%-16% for IM and 3%-5% for any dysplasia were reported, with the majority being in the first 2 years after achieving CE-IM.15 A systematic review of 21 studies looking at the location of recurrences suggested that the majority (56%) occur in the distal esophagus. Of those that occur in the esophagus, about 80% of them were in the distal 2 cm of the esophagus and only 50% of the recurrences were visible recurrences, thus reiterating the importance of meticulous examination and systematic biopsies.16
On the contrary, a recent single-center study of 217 patients who had achieved CE-IM with 5.5 years of follow-up demonstrated a 26% and 8% recurrence of IM and dysplasia, respectively. One hundred percent of the recurrence in the esophagus was reported as visible.17 Therefore, follow-up endoscopy surveillance protocol after CE-IM should still involve meticulous examination, biopsy of visible lesions, and systematic biopsies for non-visible lesions from the original BE segment, similar to those patients who have not needed BET.
Current guidelines based on expert consensus and evidence recommend surveillance after CE-IM based on original most advanced histology:2
1. LGD: 1 year, 3 years, and every 2 years after that.
2. HGD/EAC: 3 months, 6 months, 12 months, and annually after that.
There is no clear guideline on when to stop surveillance since the longest available follow-up is around 10 years, and recurrences are still detected. A potential surveillance endpoint may be based on age and comorbidities, especially those that would preclude a patient from being a candidate for BET.
When Should a Patient Be Referred?
BE patients with visible lesions and/or dysplastic changes in the biopsy who would require BET should be considered for referral to high-volume centers. Studies have shown higher success for CE-IM and lower rates of adverse events and recurrences in these patients managed at expert centers. The presence of a multidisciplinary team involving pathologists, surgeons, and oncologists is critical and offers a timely opportunity in case of need for a high-risk patient.
Conclusion
BE is a precursor to EAC, with rising incidence and poor 5-year survival. Endoscopic diagnosis is the gold standard and requires a high-quality examination and biopsies. Based on histopathology, a systematic surveillance and BET plan should be performed to achieve CE-IM in patients with dysplasia. Once CE-IM is achieved, regular surveillance should be performed with careful attention to recurrences and complications from the BET modalities.
Dr. Srinivasan and Dr. Sharma are based at the University of Kansas Medical Center, Kansas City, Kansas, and the Kansas City Veterans Affairs Medical Center, Kansas City, Missouri. Dr. Srinivasan has no relevant disclosures. Dr. Sharma disclosed research grants from ERBE, Ironwood Pharmaceuticals, Olympus, and Medtronic. He has served as a consultant for Takeda, Samsung Bioepis, Olympus, and Lumendi, and reports other funding from Medtronic, Fujifilm Medical Systems USA, and Salix.
References
1. Holmberg D, et al. Incidence and mortality in upper gastrointestinal cancer after negative endoscopy for gastroesophageal reflux disease. Gastroenterology. 2022;162(2):431-438.e4.
2. Shaheen NJ, et al. Diagnosis and management of Barrett’s esophagus: An updated ACG guideline. Am J Gastroenterol. 2022 Apr;117(4):559-587.
3. Pech O, et al. Inter-observer variability in the diagnosis of low-grade dysplasia in pathologists: A comparison between experienced and inexperienced pathologists. Gastrointest Endosc. 2006 Apr;63(5):AB130.
4. Krishnamoorthi R, et al. Factors associated with progression of Barrett’s esophagus: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1046-1055.e8.
5. Visrodia K, et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: A systematic review and meta-analysis. Gastroenterology. 2016 Mar;150(3):599-607.e7; quiz e14-5.
6. Perisetti A, Sharma P. Tips for improving the identification of neoplastic visible lesions in Barrett’s esophagus. Gastrointest Endosc. 2023 Feb;97(2):248-250.
7. Gupta N, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc. 2012 Sep;76(3):531-538.
8. Terheggen G, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut. 2017 May;66(5):783-793.
9. Wolfson P, et al. Endoscopic eradication therapy for Barrett’s esophagus-related neoplasia: A final 10-year report from the UK National HALO Radiofrequency Ablation Registry. Gastrointest Endosc. 2022 Aug;96(2):223-233.
10. Rösch T, et al. 1151 Multicenter feasibility study of combined injection and argon plasma coagulation (hybrid-APC) in the ablation therapy of neoplastic Barrett esophagus. Gastrointest Endosc. 2017;85(5):AB154.
11. Knabe M, et al. Radiofrequency ablation versus hybrid argon plasma coagulation in Barrett’s esophagus: A prospective randomised trial. Surg Endosc. 2023;37(10):7803-7811.
12. Van Munster SN, et al. Radiofrequency vapor ablation for Barrett’s esophagus: Feasibility, safety, and proof of concept in a stepwise study with in vitro, animal, and the first in-human application. Endoscopy. 2021 Nov;53(11):1162-1168.
13. Emura F, et al. Rio de Janeiro global consensus on landmarks, definitions, and classifications in Barrett’s esophagus: World Endoscopy Organization Delphi study. Gastroenterology. 2022 Jul;163(1):84-96.e2.
14. Singh RR, et al. Real-world evidence of safety and effectiveness of Barrett’s endoscopic therapy. Gastrointest Endosc. 2023 Aug;98(2):155-161.e1.
15. Wani S, et al. Recurrence Is rare following complete eradication of intestinal metaplasia in patients with Barrett’s esophagus and peaks at 18 months. Clin Gastroenterol Hepatol. 2020 Oct;18(11):2609-2617.e2.
16. Duvvuri A, et al. Mo1273 Location and pattern of recurrences in patients with Barrett’s esophagus after endoscopic therapy: A systematic review and critical analysis of the published literature. Gastrointest Endosc. 2020;91(6):AB410-1.
17. He T, et al. Location and appearance of dysplastic Barrett’s esophagus recurrence after endoscopic eradication therapy: No additional yield from random biopsy sampling neosquamous mucosa. Gastrointest Endosc. 2023 Nov;98(5):722-732.
Introduction
Barrett’s esophagus (BE) is characterized by the replacement of squamous epithelium by columnar metaplasia of the distal esophagus (>1 cm length). It is a precancerous condition, with 3%-5% of patients with BE developing esophageal adenocarcinoma (EAC) in their lifetime. EAC is one of the cancers with high morbidity and mortality (5-year survival < 20%), and its incidence has been on the rise. Studies examining the natural history of BE have demonstrated that the progression happens through a metaplasia-dysplasia-neoplasia sequence. Therefore, early detection of BE and timely management to prevent progression to EAC is crucial.
Grades of Dysplasia
The current gold standard for the diagnosis of BE neoplasia includes a high-quality endoscopic evaluation and biopsies. Biopsies should be obtained from any visible lesions (nodules, ulcers) followed by a random 4-quadrant fashion (Seattle protocol) interval of the entire length of the BE segment. It is essential to pay attention to the results of the biopsy that have been obtained since it will not only determine the surveillance interval but is crucial in planning any necessary endoscopic therapy. The possible results of the biopsy and its implications are:
- No intestinal metaplasia (IM): This would rule out Barrett’s esophagus and no further surveillance would be necessary. A recent population-based study of over 1 million patients showed a 55% and 61% reduced risk of upper gastrointestinal (UGI) cancer and deaths respectively after a negative endoscopy.1
- Intestinal metaplasia with no dysplasia (non-dysplastic BE): Biopsies confirm presence of intestinal metaplasia in the biopsies without any evidence of dysplasia. While the rate of progression to EAC is low (0.07%-0.25%), it is not absent and thus surveillance would be indicated. Current guidelines suggest repeating an endoscopy with biopsy in 5 years if the length of BE is < 3 cm or 3 years if length of BE ≥ 3 cm.2
- Indeterminate for dysplasia (BE-IND): Biopsies confirm IM but are not able to definitively rule out dysplasia. This can be seen in about 4%-8% of the biopsies obtained. The progression rates to EAC are reported to be comparable or lower to low-grade dysplasia (LGD), so the current recommendation is to intensify acid reduction therapy and repeat endoscopy in 6 months. If repeat endoscopy downgrades to non-dysplastic, then can follow surveillance according to NDBE protocol; otherwise recommend continuing surveillance every 12 months.
- Low-grade dysplasia (BE-LGD): Biopsies confirm IM but also show tightly packed overlapping basal nuclei with hyperchromasia and irregular contours, basal stratification of nuclei, and diminished goblet and columnar cell mucus. There is significant inter-observer variability reported,3 and thus the slides must be reviewed by a second pathologist with experience in BE to confirm the findings. Once confirmed, based on risk factors such as presence of multifocal LGD, persistence of LGD, presence of visible lesions, etc., the patient can be offered Barrett’s endoscopic therapy (BET) or undergo continued surveillance. The decision of pursuing one or the other would be dependent on patient preference and shared decision-making between the patient and the provider.
- High-grade dysplasia (BE-HGD): Biopsies confirm IM with cells showing greater degree of cytologic and architectural alterations of dysplasia than LGD but without overt neoplastic features. Over 40% of the patients would progress to EAC and thus the current recommendations would be to recommend BET in these patients.4
- Esophageal adenocarcinoma (EAC): Biopsies demonstrate neoplasia. If the neoplastic changes are limited to the mucosa (T1a) on endoscopic ultrasound or cross-sectional imaging, then BET is suggested. If there is involvement of submucosa, then depending on the depth of invasion, absence of high-risk features (poor differentiation, lymphovascular invasion), BET can be considered as an alternative to esophagectomy.
Lesion Detection on Endoscopy
Data from large population-based studies with at least 3 years of follow-up reported that 58%-66% of EAC detected during endoscopy were diagnosed within 1 year of an index Barrett’s esophagus screening endoscopy, or post-endoscopy Barrett’s neoplasia, and were considered likely to have been missed during index endoscopy.5 This underscores the importance of careful and systematic endoscopic examination during an upper endoscopy.
Studies have also demonstrated that longer examination time was associated with significantly higher detection of HGD/EAC.6,7 Careful examination of the tubular esophagus and gastroesophageal junction (GEJ) should be performed in forward and retroflexed views looking for any subtle areas of nodularity, loop distortion, variability in vascular patterns, mucosal changes concerning for dysplasia or neoplasia. Use of high-definition white light endoscopy (HD-WLE) and virtual chromoendoscopy techniques such as narrow banding imaging (NBI) or blue laser imaging (BLI) are currently recommended in the guidelines.2 Spray chromoendoscopy using acetic acid can also be utilized. Another exciting development is the use of artificial intelligence (AI) in detecting and diagnosing BE associated lesions and neoplasia.
Barrett’s Endoscopic Therapy (BET)
Patients with visible lesions, dysplasia, or early EAC are candidates for BET (Table 1). 
BET involves resective and ablative modalities. The resective modalities include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) and are the modalities of choice for nodular or raised lesions.
EMR involves endoscopic resection of abnormal mucosa using either lift-assisted technique or multi-band ligation (Figure 1). 
ESD, on the other hand, involves submucosal dissection and perimeter resection of the lesion, thus providing the advantage of an en-bloc resection. In a recent randomized controlled trial (RCT) of 40 patients undergoing ESD vs EMR for HGD/EAC, ESD was better for curative resection (R0) (58%) compared with EMR (12%); however, the remission rates at 3 months were comparable with two perforations reported in the ESD group while there were no complications in the EMR group.8
There is an apparent learning curve when it comes to these advanced techniques, and with more experience, we are seeing comparable results for both these modalities. However, given the complexity and time required for the procedure, current practices typically involve preserving ESD for lesions > 2 cm, those having a likelihood of cancer in the superficial submucosa, or those that EMR cannot remove due to underlying fibrosis or post-EMR recurrence.
The ablative modalities include radiofrequency ablation (RFA), cryotherapy, and hybrid argon plasma coagulation (hybrid APC). These modalities are used for flat lesions, and as therapy following endoscopic resection of nodular lesions to treat residual flat segment of BE. RFA, one of the earliest introduced endoscopic modalities, involves applying directed and controlled heat energy to ablate lesions. Current devices allow circumferential or focal application of RFA. It is a safe and effective modality with good complete eradication of IM (CE-IM) (71%-93%) and complete eradication of dysplasia (CE-D) (91%-100%) rates. These results have been sustained even at 2 years, with the most recent long-term data from a registry study showing a relapse rate of 6% for dysplasia and 19% for IM after 8 years, suggesting durability of this treatment.9
Cryotherapy involves the application of liquid nitrogen or rapidly expanding CO2 to the abnormal mucosa, leading to the rapid freezing and thawing that leads to the death of the cells. Cryogen can be applied as a spray or using a balloon with the spray nozzle in the center. This modality can be used to treat focal lesions and/or larger segments. While it has not been systematically compared with RFA, rates of CE-IM up to 81% and CE-D up to 97% are reported. Hybrid APC involves the use of submucosal saline injection to provide a protective cushion before APC is applied. It has CE-IM rate of 69% and CE-D rate of 67%-86%.10 In a recent RCT of 101 patients randomized to RFA or hybrid APC, CE-IM rates were similar (RFA:74.2% vs hAPC: 82.9%).11
Recently, another technique called radiofrequency vapor ablation (RFVA) is being evaluated, which involves ablating BE segment using vapor at 100° C generated with an RF electrode. A proof-of-concept study of 15 patients showed median squamous conversion of 55% (IQR 33-74) and 98% (IQR 56-99) for 1- and 3-second applications, respectively, with no reported adverse events.12
Barrett’s Refractory to Endoscopic Therapy
Failure of BET is defined as persistent columnar lined epithelium (intestinal metaplasia) with inadequate response, after adequate attempts at endoscopic ablation therapy (after resection) with at least four ablation sessions.13 If encountered, special attention must be given to check compliance with proton pump inhibitors (PPIs), previous incomplete resection, and presence of large hiatal hernia. If CE-IM is not achieved after multiple sessions, change of ablative modality is typically considered. In addition, careful examination for visible lesions should be performed and even if a small one is noted, this should be first resected prior to application of any ablative therapy.
Currently there are no guideline recommendations regarding the preference of one endoscopic modality over another or consideration of potential endoscopic or surgical fundoplication. These modalities primarily rely on technologies available at an institution and the preference of a provider based on their training and experience. Most studies indicate 1-3 sessions (~ 3 months apart) of ablative treatment before achieving CE-IM.
Success and Adverse Events of BET
In a recent real-world study of over 27,000 patients with dysplastic BE, 5295 underwent BET. Analysis showed that patients with HGD/EAC who had BET had a significantly lower 3-year mortality (HGD: RR, 0.59; 95%CI, 0.49-0.71; EAC: RR, 0.53; 95% CI, 0.44-0.65) compared with those who did not undergo BET. Esophageal strictures were the most common adverse event and were noted in 6.5%, followed by chest pain (1.8%), upper GI bleeding (0.47%), and esophageal perforation (0.2%).14
In general, adverse events can be divided into immediate and delayed adverse events. Immediate adverse events typically involve bleeding and perforation that can occur during or shortly after the procedure. These are reported at higher rates with resective modalities compared with ablative therapies. Standard endoscopic techniques involving coagulation grasper or clips can be used to achieve hemostasis. Endoscopic suturing devices offer the ability to contain any perforation. The need for surgical intervention is small and limited to adverse events not detected during the procedure.
Delayed adverse events such as stricture and stenosis are higher for resective modalities (up to 30%), especially when involving more significant than 75% of the esophageal circumference. Post-procedural pain/dysphagia is most common after ablative therapies. Dysphagia reported after any endoscopic therapy should be promptly evaluated, and sequential dilation until the goal esophageal lumen is achieved should be performed every 2-4 weeks.
Recurrences and Surveillance After BET
What is established is that recurrences can occur and may be subtle, therefore detailed endoscopic surveillance is required. In a prospective study, recurrence rates of 15%-16% for IM and 3%-5% for any dysplasia were reported, with the majority being in the first 2 years after achieving CE-IM.15 A systematic review of 21 studies looking at the location of recurrences suggested that the majority (56%) occur in the distal esophagus. Of those that occur in the esophagus, about 80% of them were in the distal 2 cm of the esophagus and only 50% of the recurrences were visible recurrences, thus reiterating the importance of meticulous examination and systematic biopsies.16
On the contrary, a recent single-center study of 217 patients who had achieved CE-IM with 5.5 years of follow-up demonstrated a 26% and 8% recurrence of IM and dysplasia, respectively. One hundred percent of the recurrence in the esophagus was reported as visible.17 Therefore, follow-up endoscopy surveillance protocol after CE-IM should still involve meticulous examination, biopsy of visible lesions, and systematic biopsies for non-visible lesions from the original BE segment, similar to those patients who have not needed BET.
Current guidelines based on expert consensus and evidence recommend surveillance after CE-IM based on original most advanced histology:2
1. LGD: 1 year, 3 years, and every 2 years after that.
2. HGD/EAC: 3 months, 6 months, 12 months, and annually after that.
There is no clear guideline on when to stop surveillance since the longest available follow-up is around 10 years, and recurrences are still detected. A potential surveillance endpoint may be based on age and comorbidities, especially those that would preclude a patient from being a candidate for BET.
When Should a Patient Be Referred?
BE patients with visible lesions and/or dysplastic changes in the biopsy who would require BET should be considered for referral to high-volume centers. Studies have shown higher success for CE-IM and lower rates of adverse events and recurrences in these patients managed at expert centers. The presence of a multidisciplinary team involving pathologists, surgeons, and oncologists is critical and offers a timely opportunity in case of need for a high-risk patient.
Conclusion
BE is a precursor to EAC, with rising incidence and poor 5-year survival. Endoscopic diagnosis is the gold standard and requires a high-quality examination and biopsies. Based on histopathology, a systematic surveillance and BET plan should be performed to achieve CE-IM in patients with dysplasia. Once CE-IM is achieved, regular surveillance should be performed with careful attention to recurrences and complications from the BET modalities.
Dr. Srinivasan and Dr. Sharma are based at the University of Kansas Medical Center, Kansas City, Kansas, and the Kansas City Veterans Affairs Medical Center, Kansas City, Missouri. Dr. Srinivasan has no relevant disclosures. Dr. Sharma disclosed research grants from ERBE, Ironwood Pharmaceuticals, Olympus, and Medtronic. He has served as a consultant for Takeda, Samsung Bioepis, Olympus, and Lumendi, and reports other funding from Medtronic, Fujifilm Medical Systems USA, and Salix.
References
1. Holmberg D, et al. Incidence and mortality in upper gastrointestinal cancer after negative endoscopy for gastroesophageal reflux disease. Gastroenterology. 2022;162(2):431-438.e4.
2. Shaheen NJ, et al. Diagnosis and management of Barrett’s esophagus: An updated ACG guideline. Am J Gastroenterol. 2022 Apr;117(4):559-587.
3. Pech O, et al. Inter-observer variability in the diagnosis of low-grade dysplasia in pathologists: A comparison between experienced and inexperienced pathologists. Gastrointest Endosc. 2006 Apr;63(5):AB130.
4. Krishnamoorthi R, et al. Factors associated with progression of Barrett’s esophagus: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1046-1055.e8.
5. Visrodia K, et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: A systematic review and meta-analysis. Gastroenterology. 2016 Mar;150(3):599-607.e7; quiz e14-5.
6. Perisetti A, Sharma P. Tips for improving the identification of neoplastic visible lesions in Barrett’s esophagus. Gastrointest Endosc. 2023 Feb;97(2):248-250.
7. Gupta N, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc. 2012 Sep;76(3):531-538.
8. Terheggen G, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut. 2017 May;66(5):783-793.
9. Wolfson P, et al. Endoscopic eradication therapy for Barrett’s esophagus-related neoplasia: A final 10-year report from the UK National HALO Radiofrequency Ablation Registry. Gastrointest Endosc. 2022 Aug;96(2):223-233.
10. Rösch T, et al. 1151 Multicenter feasibility study of combined injection and argon plasma coagulation (hybrid-APC) in the ablation therapy of neoplastic Barrett esophagus. Gastrointest Endosc. 2017;85(5):AB154.
11. Knabe M, et al. Radiofrequency ablation versus hybrid argon plasma coagulation in Barrett’s esophagus: A prospective randomised trial. Surg Endosc. 2023;37(10):7803-7811.
12. Van Munster SN, et al. Radiofrequency vapor ablation for Barrett’s esophagus: Feasibility, safety, and proof of concept in a stepwise study with in vitro, animal, and the first in-human application. Endoscopy. 2021 Nov;53(11):1162-1168.
13. Emura F, et al. Rio de Janeiro global consensus on landmarks, definitions, and classifications in Barrett’s esophagus: World Endoscopy Organization Delphi study. Gastroenterology. 2022 Jul;163(1):84-96.e2.
14. Singh RR, et al. Real-world evidence of safety and effectiveness of Barrett’s endoscopic therapy. Gastrointest Endosc. 2023 Aug;98(2):155-161.e1.
15. Wani S, et al. Recurrence Is rare following complete eradication of intestinal metaplasia in patients with Barrett’s esophagus and peaks at 18 months. Clin Gastroenterol Hepatol. 2020 Oct;18(11):2609-2617.e2.
16. Duvvuri A, et al. Mo1273 Location and pattern of recurrences in patients with Barrett’s esophagus after endoscopic therapy: A systematic review and critical analysis of the published literature. Gastrointest Endosc. 2020;91(6):AB410-1.
17. He T, et al. Location and appearance of dysplastic Barrett’s esophagus recurrence after endoscopic eradication therapy: No additional yield from random biopsy sampling neosquamous mucosa. Gastrointest Endosc. 2023 Nov;98(5):722-732.
Sunscreen Safety: 2024 Updates
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
Global Quest to Cut CAR T Costs
In the United States, a stand-alone device could greatly reduce the expense of producing modified immune cells. In India, researchers hope homegrown technology is the key to getting costs under control. In Latin America, a partnership between the Brazilian government and a US nonprofit may be just the ticket.
At stake is expanded access to CAR T-cell therapy, a form of immunotherapy that in just the past few years has revolutionized the care of hematologic cancers.
“Among patients with lymphoma, leukemia, and myeloma, anywhere between 30% to 50% reach long-term remission after one CAR T-cell infusion,” Mayo Clinic–Rochester hematologist/oncologist Saad J. Kenderian, MB, ChB, said in an interview. “It’s such an important therapy.”
However, only a small percentage of eligible patients in the United States — perhaps 20% or fewer — are receiving the treatment, he added.
A 2024 report suggested that many patients in the United States who may benefit aren’t being treated because of a range of possible reasons, including high prices, manufacturing logistics, and far distance from the limited number of institutions offering the therapy.
“Taken together, the real-world cost of CAR T-cell therapy can range from $700,000 to $1 million, which may make the treatment unaffordable to those patients without robust financial and/or social support,” the report authors noted.
Outside Western countries, access to the therapy is even more limited, because of its exorbitant price. The 2024 report noted that “there is a wide use of CAR T-cell therapy in Europe and China, but access is limited in developing countries in Southeast Asia, Africa, and Latin America.”
Harnessing the Power of T-Cells
Several types of CAR T-cell therapy have been approved by the US Food and Drug Administration (FDA) for patients with relapsed/refractory blood cancers such as follicular lymphoma, large B-cell lymphoma, multiple myeloma, and B-cell precursor acute lymphoblastic leukemia. A 2023 review analyzed clinical trials and reported that complete response rates were 40%-54% in aggressive B-cell lymphoma, 67% in mantle cell lymphoma, and 69%-74% in indolent B-cell lymphoma.
Pediatric hematologist/oncologist Kirsten Williams, MD, who specializes in pediatric blood and marrow transplant and cellular therapy at the Aflac Cancer and Blood Disorders Center of Children’s Healthcare of Atlanta, described CAR T-cell therapy as “a very unique form of immunotherapy” that harnesses the power of the immune system’s T-cells.
These cells are effective tumor killers, but they typically aren’t assigned to control cancer, she said in an interview. “We have very few of them, and most of our T cells are focused on killing various viruses,” she said. The therapy “allows us to take the T cell that would have killed the flu or mono and instead target leukemia, B-cell leukemia, or lymphoma.”
As she explained, “T cells are collected by a machine that reserves white blood cells and gives back the rest of the blood to the patient. We insert a gene into the T cells that encodes for a B-cell receptor. This receptor acts as a GPS signal, pulling T cells to the cancer so that they can kill it.”
In addition, “with this genetic change, we also add some things that allow the T cell to be stronger, to have a higher signal to kill the cancer cell once it locks on.”
The therapy is unique for each patient, Dr. Williams said. “We have collected and modified your specific T cells, and they can now only be infused into you. If we try to give your product to someone else, those cells would either cause harm by attacking the patient or would be immediately killed by that patient’s own immune system. This is very different than all the other kinds of therapies. When you take other medicines, it doesn’t matter who receives that pill.”
Treatment: Individual, Complex, and Costly
Why is CAR T-cell therapy so expensive? While only a single treatment is needed, the T cells have to go through an “individualized, bespoke manufacturing” process that’s “highly technical,” pediatric oncologist Stephan A. Grupp, MD, PhD, section chief of the Cellular Therapy and Transplant Section at Children’s Hospital of Philadelphia, said in an interview. As he explained, the cells for a single patient have to go through the same testing as with a drug that might be given to 1,000 people.
“The first thing we need to do is collect the cells from a patient,” said Dr. Williams. “For adults, that process involves putting in two big IVs — one in each arm — and then pulling the blood through a machine. This typically involves an 8-hour collection in the hospital and very highly specialized people to oversee the collection process.”
Secondly, at some institutions, “the cells get sent to a company where they undergo the process where the gene is inserted,” she said. “This process needs to be done in a very sterile environment so there’s no infections, and it needs to have a lot of oversight.”
Finally, “after the cells are generated, they are typically frozen and shipped back to the site where the patient is at the hospital,” she said. “Then we give chemotherapy to the patient, which prepares the patient’s blood system. It removes some of the T-cells that are there, allowing for the T cells that we’re about to infuse to quickly be activated, find the cancer, and kill it.”
Side effects can boost costs even more. “Unfortunately, some significant toxicities can occur after we infuse these cells,” Dr. Williams noted. “Patients can have trouble breathing and sometimes need ventilatory support. They can have trouble maintaining their blood pressure and become swollen as fluid seeps into tissues. Or they can have high fevers and organ dysfunction. Many of those patients go to the intensive care unit, which is obviously expensive as well.”
Taking Gene Therapy In-House
As Dr. Williams explained, one way to reduce costs is to “perform the genetic manipulation and expansion of the cells outside of a company.” Several academic institutions in the United States are embracing this approach, including Children’s Hospital of Philadelphia, which is experimenting with an automated device developed by the German company Miltenyi Biotec and known as the CliniMACS Prodigy machine.
“The current manufacturing process is very manual and requires a lot of interaction with the product and highly trained personnel,” Dr. Grupp said. “If you have an automated device, you have those cells in the device over the 7 to 12 days that you actually need to grow the cells. There’s much less interaction, so you need fewer trained personnel.”
The device is experimental and not yet FDA approved, Dr. Grupp noted, so that patients are all in clinical trials. Children’s Hospital of Philadelphia has treated more than a dozen patients with the device, he said.
Another member of Children’s Hospital of Philadelphia’s CAR T-cell team told WHYY-FM that a single patient’s treatment would run about $30,000 for labor and testing, but not other expenses such as facility costs.
Dr. Grupp estimated that about half a dozen of these devices are in use in the United States, and many more worldwide. “They’re all just like we are — at the absolute beginning. We’ve only been doing this for about a year.”
In the big picture, Dr. Grupp said, “this is where cell therapy is going. Whether it’s point of care or not, automated cell manufacturing is the obvious next step.”
India: Big Hopes for Homegrown Technology
In India, researchers are hoping that their homegrown approach to CAR T-cell therapy will expand access by greatly lowering treatment prices.
Last fall, India’s equivalent of the FDA-granted approval for actalycabtagene autoleucel (NexCAR19), which was developed by Indian scientists who worked closely with the US National Institutes of Health (NIH). The therapy’s developer is a company called ImmunoACT.
In an interview, ImmunoACT founder Rahul Purwar, PhD, MSc, associate professor at Indian Institute of Technology Bombay, said the treatment costs about $40,000. The price is much lower than in the United States because staffing, facility construction, and maintenance are less expensive in India, he said.
Results of small early clinical trials have been promising, with complete responses in 68% of 38 lymphoma patients and 72% of 15 leukemia patients. Updated data will be presented at the annual American Society of Hematology meeting in December 2024, Dr. Purwar said.
According to the NIH, at first ImmunoACT hopes to treat about 1,200 patients a year. The immediate goal is to “focus and stabilize our operation in India,” Dr. Purwar said. “Then, if opportunities come, we will try to bring CAR T to all who might benefit from these technologies. A majority of countries don’t have access to these technologies.”
A US-Brazil Partnership Holds Promise
Meanwhile, a US nonprofit known as Caring Cross announced this year that it has partnered with Fundação Oswaldo Cruz (Fiocruz), a Brazilian government foundation, to manufacture CAR T cells at point-of-care in South America.
“Our model is different than traditional biotech/pharma,” Boro Dropulic, PhD, MBA, cofounder and executive director of Caring Cross, said in an interview. “Our goal is to develop technologies and transfer them to organizations like Fiocruz to enable them to manufacture these transformative therapies for patients in their regions. We believe this model is an important solution for therapies that are priced so high that they are not accessible to many patients that need them, particularly underserved populations and those in low- and middle-income countries.”
According to Dr. Dropulic: “We have developed a production process where the material cost is about $20,000 per dose.” When labor and infrastructure costs are added, the total expense won’t be more than $37,000-$47,500, he said.
The research process for the CAR T-cell technology is at an earlier stage than in India. Scientists plan to start clinical trials of the technology in the United States by the end of 2024 and then begin them in Brazil in 2025, after safety and efficacy have been demonstrated. The first trial, a phase I/II study, will enroll about 20 patients, Dr. Dropulic said.
Dr. Kenderian reported ties with Novartis, Capstan Bio, Kite/Gilead, Juno/BMS, Humanigen, Tolero, Leah Labs, Lentigen, Luminary, Sunesis/Viracta, Morphosys, Troque, Carisma, Sendero, and LifEngine. Dr. Williams disclosed grants from National Institutes of Health and philanthropic organizations. Dr. Grupp reported relationships with Novartis, Kite, Vertex and Servier, Roche, GSK, Humanigen, CBMG, Eureka, Janssen/JNJ, Jazz, Adaptimmune, TCR2, Cellectis, Juno, Allogene, and Cabaletta. Dr. Purwar is the founder of ImmunoACT. Dr. Dropulic serves as executive director of Caring Cross and CEO of Vector BioMed, which provides vectors for gene therapy.
In the United States, a stand-alone device could greatly reduce the expense of producing modified immune cells. In India, researchers hope homegrown technology is the key to getting costs under control. In Latin America, a partnership between the Brazilian government and a US nonprofit may be just the ticket.
At stake is expanded access to CAR T-cell therapy, a form of immunotherapy that in just the past few years has revolutionized the care of hematologic cancers.
“Among patients with lymphoma, leukemia, and myeloma, anywhere between 30% to 50% reach long-term remission after one CAR T-cell infusion,” Mayo Clinic–Rochester hematologist/oncologist Saad J. Kenderian, MB, ChB, said in an interview. “It’s such an important therapy.”
However, only a small percentage of eligible patients in the United States — perhaps 20% or fewer — are receiving the treatment, he added.
A 2024 report suggested that many patients in the United States who may benefit aren’t being treated because of a range of possible reasons, including high prices, manufacturing logistics, and far distance from the limited number of institutions offering the therapy.
“Taken together, the real-world cost of CAR T-cell therapy can range from $700,000 to $1 million, which may make the treatment unaffordable to those patients without robust financial and/or social support,” the report authors noted.
Outside Western countries, access to the therapy is even more limited, because of its exorbitant price. The 2024 report noted that “there is a wide use of CAR T-cell therapy in Europe and China, but access is limited in developing countries in Southeast Asia, Africa, and Latin America.”
Harnessing the Power of T-Cells
Several types of CAR T-cell therapy have been approved by the US Food and Drug Administration (FDA) for patients with relapsed/refractory blood cancers such as follicular lymphoma, large B-cell lymphoma, multiple myeloma, and B-cell precursor acute lymphoblastic leukemia. A 2023 review analyzed clinical trials and reported that complete response rates were 40%-54% in aggressive B-cell lymphoma, 67% in mantle cell lymphoma, and 69%-74% in indolent B-cell lymphoma.
Pediatric hematologist/oncologist Kirsten Williams, MD, who specializes in pediatric blood and marrow transplant and cellular therapy at the Aflac Cancer and Blood Disorders Center of Children’s Healthcare of Atlanta, described CAR T-cell therapy as “a very unique form of immunotherapy” that harnesses the power of the immune system’s T-cells.
These cells are effective tumor killers, but they typically aren’t assigned to control cancer, she said in an interview. “We have very few of them, and most of our T cells are focused on killing various viruses,” she said. The therapy “allows us to take the T cell that would have killed the flu or mono and instead target leukemia, B-cell leukemia, or lymphoma.”
As she explained, “T cells are collected by a machine that reserves white blood cells and gives back the rest of the blood to the patient. We insert a gene into the T cells that encodes for a B-cell receptor. This receptor acts as a GPS signal, pulling T cells to the cancer so that they can kill it.”
In addition, “with this genetic change, we also add some things that allow the T cell to be stronger, to have a higher signal to kill the cancer cell once it locks on.”
The therapy is unique for each patient, Dr. Williams said. “We have collected and modified your specific T cells, and they can now only be infused into you. If we try to give your product to someone else, those cells would either cause harm by attacking the patient or would be immediately killed by that patient’s own immune system. This is very different than all the other kinds of therapies. When you take other medicines, it doesn’t matter who receives that pill.”
Treatment: Individual, Complex, and Costly
Why is CAR T-cell therapy so expensive? While only a single treatment is needed, the T cells have to go through an “individualized, bespoke manufacturing” process that’s “highly technical,” pediatric oncologist Stephan A. Grupp, MD, PhD, section chief of the Cellular Therapy and Transplant Section at Children’s Hospital of Philadelphia, said in an interview. As he explained, the cells for a single patient have to go through the same testing as with a drug that might be given to 1,000 people.
“The first thing we need to do is collect the cells from a patient,” said Dr. Williams. “For adults, that process involves putting in two big IVs — one in each arm — and then pulling the blood through a machine. This typically involves an 8-hour collection in the hospital and very highly specialized people to oversee the collection process.”
Secondly, at some institutions, “the cells get sent to a company where they undergo the process where the gene is inserted,” she said. “This process needs to be done in a very sterile environment so there’s no infections, and it needs to have a lot of oversight.”
Finally, “after the cells are generated, they are typically frozen and shipped back to the site where the patient is at the hospital,” she said. “Then we give chemotherapy to the patient, which prepares the patient’s blood system. It removes some of the T-cells that are there, allowing for the T cells that we’re about to infuse to quickly be activated, find the cancer, and kill it.”
Side effects can boost costs even more. “Unfortunately, some significant toxicities can occur after we infuse these cells,” Dr. Williams noted. “Patients can have trouble breathing and sometimes need ventilatory support. They can have trouble maintaining their blood pressure and become swollen as fluid seeps into tissues. Or they can have high fevers and organ dysfunction. Many of those patients go to the intensive care unit, which is obviously expensive as well.”
Taking Gene Therapy In-House
As Dr. Williams explained, one way to reduce costs is to “perform the genetic manipulation and expansion of the cells outside of a company.” Several academic institutions in the United States are embracing this approach, including Children’s Hospital of Philadelphia, which is experimenting with an automated device developed by the German company Miltenyi Biotec and known as the CliniMACS Prodigy machine.
“The current manufacturing process is very manual and requires a lot of interaction with the product and highly trained personnel,” Dr. Grupp said. “If you have an automated device, you have those cells in the device over the 7 to 12 days that you actually need to grow the cells. There’s much less interaction, so you need fewer trained personnel.”

The device is experimental and not yet FDA approved, Dr. Grupp noted, so that patients are all in clinical trials. Children’s Hospital of Philadelphia has treated more than a dozen patients with the device, he said.
Another member of Children’s Hospital of Philadelphia’s CAR T-cell team told WHYY-FM that a single patient’s treatment would run about $30,000 for labor and testing, but not other expenses such as facility costs.
Dr. Grupp estimated that about half a dozen of these devices are in use in the United States, and many more worldwide. “They’re all just like we are — at the absolute beginning. We’ve only been doing this for about a year.”
In the big picture, Dr. Grupp said, “this is where cell therapy is going. Whether it’s point of care or not, automated cell manufacturing is the obvious next step.”
India: Big Hopes for Homegrown Technology
In India, researchers are hoping that their homegrown approach to CAR T-cell therapy will expand access by greatly lowering treatment prices.
Last fall, India’s equivalent of the FDA-granted approval for actalycabtagene autoleucel (NexCAR19), which was developed by Indian scientists who worked closely with the US National Institutes of Health (NIH). The therapy’s developer is a company called ImmunoACT.
In an interview, ImmunoACT founder Rahul Purwar, PhD, MSc, associate professor at Indian Institute of Technology Bombay, said the treatment costs about $40,000. The price is much lower than in the United States because staffing, facility construction, and maintenance are less expensive in India, he said.
Results of small early clinical trials have been promising, with complete responses in 68% of 38 lymphoma patients and 72% of 15 leukemia patients. Updated data will be presented at the annual American Society of Hematology meeting in December 2024, Dr. Purwar said.
According to the NIH, at first ImmunoACT hopes to treat about 1,200 patients a year. The immediate goal is to “focus and stabilize our operation in India,” Dr. Purwar said. “Then, if opportunities come, we will try to bring CAR T to all who might benefit from these technologies. A majority of countries don’t have access to these technologies.”
A US-Brazil Partnership Holds Promise
Meanwhile, a US nonprofit known as Caring Cross announced this year that it has partnered with Fundação Oswaldo Cruz (Fiocruz), a Brazilian government foundation, to manufacture CAR T cells at point-of-care in South America.
“Our model is different than traditional biotech/pharma,” Boro Dropulic, PhD, MBA, cofounder and executive director of Caring Cross, said in an interview. “Our goal is to develop technologies and transfer them to organizations like Fiocruz to enable them to manufacture these transformative therapies for patients in their regions. We believe this model is an important solution for therapies that are priced so high that they are not accessible to many patients that need them, particularly underserved populations and those in low- and middle-income countries.”
According to Dr. Dropulic: “We have developed a production process where the material cost is about $20,000 per dose.” When labor and infrastructure costs are added, the total expense won’t be more than $37,000-$47,500, he said.
The research process for the CAR T-cell technology is at an earlier stage than in India. Scientists plan to start clinical trials of the technology in the United States by the end of 2024 and then begin them in Brazil in 2025, after safety and efficacy have been demonstrated. The first trial, a phase I/II study, will enroll about 20 patients, Dr. Dropulic said.
Dr. Kenderian reported ties with Novartis, Capstan Bio, Kite/Gilead, Juno/BMS, Humanigen, Tolero, Leah Labs, Lentigen, Luminary, Sunesis/Viracta, Morphosys, Troque, Carisma, Sendero, and LifEngine. Dr. Williams disclosed grants from National Institutes of Health and philanthropic organizations. Dr. Grupp reported relationships with Novartis, Kite, Vertex and Servier, Roche, GSK, Humanigen, CBMG, Eureka, Janssen/JNJ, Jazz, Adaptimmune, TCR2, Cellectis, Juno, Allogene, and Cabaletta. Dr. Purwar is the founder of ImmunoACT. Dr. Dropulic serves as executive director of Caring Cross and CEO of Vector BioMed, which provides vectors for gene therapy.
In the United States, a stand-alone device could greatly reduce the expense of producing modified immune cells. In India, researchers hope homegrown technology is the key to getting costs under control. In Latin America, a partnership between the Brazilian government and a US nonprofit may be just the ticket.
At stake is expanded access to CAR T-cell therapy, a form of immunotherapy that in just the past few years has revolutionized the care of hematologic cancers.
“Among patients with lymphoma, leukemia, and myeloma, anywhere between 30% to 50% reach long-term remission after one CAR T-cell infusion,” Mayo Clinic–Rochester hematologist/oncologist Saad J. Kenderian, MB, ChB, said in an interview. “It’s such an important therapy.”
However, only a small percentage of eligible patients in the United States — perhaps 20% or fewer — are receiving the treatment, he added.
A 2024 report suggested that many patients in the United States who may benefit aren’t being treated because of a range of possible reasons, including high prices, manufacturing logistics, and far distance from the limited number of institutions offering the therapy.
“Taken together, the real-world cost of CAR T-cell therapy can range from $700,000 to $1 million, which may make the treatment unaffordable to those patients without robust financial and/or social support,” the report authors noted.
Outside Western countries, access to the therapy is even more limited, because of its exorbitant price. The 2024 report noted that “there is a wide use of CAR T-cell therapy in Europe and China, but access is limited in developing countries in Southeast Asia, Africa, and Latin America.”
Harnessing the Power of T-Cells
Several types of CAR T-cell therapy have been approved by the US Food and Drug Administration (FDA) for patients with relapsed/refractory blood cancers such as follicular lymphoma, large B-cell lymphoma, multiple myeloma, and B-cell precursor acute lymphoblastic leukemia. A 2023 review analyzed clinical trials and reported that complete response rates were 40%-54% in aggressive B-cell lymphoma, 67% in mantle cell lymphoma, and 69%-74% in indolent B-cell lymphoma.
Pediatric hematologist/oncologist Kirsten Williams, MD, who specializes in pediatric blood and marrow transplant and cellular therapy at the Aflac Cancer and Blood Disorders Center of Children’s Healthcare of Atlanta, described CAR T-cell therapy as “a very unique form of immunotherapy” that harnesses the power of the immune system’s T-cells.
These cells are effective tumor killers, but they typically aren’t assigned to control cancer, she said in an interview. “We have very few of them, and most of our T cells are focused on killing various viruses,” she said. The therapy “allows us to take the T cell that would have killed the flu or mono and instead target leukemia, B-cell leukemia, or lymphoma.”
As she explained, “T cells are collected by a machine that reserves white blood cells and gives back the rest of the blood to the patient. We insert a gene into the T cells that encodes for a B-cell receptor. This receptor acts as a GPS signal, pulling T cells to the cancer so that they can kill it.”
In addition, “with this genetic change, we also add some things that allow the T cell to be stronger, to have a higher signal to kill the cancer cell once it locks on.”
The therapy is unique for each patient, Dr. Williams said. “We have collected and modified your specific T cells, and they can now only be infused into you. If we try to give your product to someone else, those cells would either cause harm by attacking the patient or would be immediately killed by that patient’s own immune system. This is very different than all the other kinds of therapies. When you take other medicines, it doesn’t matter who receives that pill.”
Treatment: Individual, Complex, and Costly
Why is CAR T-cell therapy so expensive? While only a single treatment is needed, the T cells have to go through an “individualized, bespoke manufacturing” process that’s “highly technical,” pediatric oncologist Stephan A. Grupp, MD, PhD, section chief of the Cellular Therapy and Transplant Section at Children’s Hospital of Philadelphia, said in an interview. As he explained, the cells for a single patient have to go through the same testing as with a drug that might be given to 1,000 people.
“The first thing we need to do is collect the cells from a patient,” said Dr. Williams. “For adults, that process involves putting in two big IVs — one in each arm — and then pulling the blood through a machine. This typically involves an 8-hour collection in the hospital and very highly specialized people to oversee the collection process.”
Secondly, at some institutions, “the cells get sent to a company where they undergo the process where the gene is inserted,” she said. “This process needs to be done in a very sterile environment so there’s no infections, and it needs to have a lot of oversight.”
Finally, “after the cells are generated, they are typically frozen and shipped back to the site where the patient is at the hospital,” she said. “Then we give chemotherapy to the patient, which prepares the patient’s blood system. It removes some of the T-cells that are there, allowing for the T cells that we’re about to infuse to quickly be activated, find the cancer, and kill it.”
Side effects can boost costs even more. “Unfortunately, some significant toxicities can occur after we infuse these cells,” Dr. Williams noted. “Patients can have trouble breathing and sometimes need ventilatory support. They can have trouble maintaining their blood pressure and become swollen as fluid seeps into tissues. Or they can have high fevers and organ dysfunction. Many of those patients go to the intensive care unit, which is obviously expensive as well.”
Taking Gene Therapy In-House
As Dr. Williams explained, one way to reduce costs is to “perform the genetic manipulation and expansion of the cells outside of a company.” Several academic institutions in the United States are embracing this approach, including Children’s Hospital of Philadelphia, which is experimenting with an automated device developed by the German company Miltenyi Biotec and known as the CliniMACS Prodigy machine.
“The current manufacturing process is very manual and requires a lot of interaction with the product and highly trained personnel,” Dr. Grupp said. “If you have an automated device, you have those cells in the device over the 7 to 12 days that you actually need to grow the cells. There’s much less interaction, so you need fewer trained personnel.”

The device is experimental and not yet FDA approved, Dr. Grupp noted, so that patients are all in clinical trials. Children’s Hospital of Philadelphia has treated more than a dozen patients with the device, he said.
Another member of Children’s Hospital of Philadelphia’s CAR T-cell team told WHYY-FM that a single patient’s treatment would run about $30,000 for labor and testing, but not other expenses such as facility costs.
Dr. Grupp estimated that about half a dozen of these devices are in use in the United States, and many more worldwide. “They’re all just like we are — at the absolute beginning. We’ve only been doing this for about a year.”
In the big picture, Dr. Grupp said, “this is where cell therapy is going. Whether it’s point of care or not, automated cell manufacturing is the obvious next step.”
India: Big Hopes for Homegrown Technology
In India, researchers are hoping that their homegrown approach to CAR T-cell therapy will expand access by greatly lowering treatment prices.
Last fall, India’s equivalent of the FDA-granted approval for actalycabtagene autoleucel (NexCAR19), which was developed by Indian scientists who worked closely with the US National Institutes of Health (NIH). The therapy’s developer is a company called ImmunoACT.
In an interview, ImmunoACT founder Rahul Purwar, PhD, MSc, associate professor at Indian Institute of Technology Bombay, said the treatment costs about $40,000. The price is much lower than in the United States because staffing, facility construction, and maintenance are less expensive in India, he said.
Results of small early clinical trials have been promising, with complete responses in 68% of 38 lymphoma patients and 72% of 15 leukemia patients. Updated data will be presented at the annual American Society of Hematology meeting in December 2024, Dr. Purwar said.
According to the NIH, at first ImmunoACT hopes to treat about 1,200 patients a year. The immediate goal is to “focus and stabilize our operation in India,” Dr. Purwar said. “Then, if opportunities come, we will try to bring CAR T to all who might benefit from these technologies. A majority of countries don’t have access to these technologies.”
A US-Brazil Partnership Holds Promise
Meanwhile, a US nonprofit known as Caring Cross announced this year that it has partnered with Fundação Oswaldo Cruz (Fiocruz), a Brazilian government foundation, to manufacture CAR T cells at point-of-care in South America.
“Our model is different than traditional biotech/pharma,” Boro Dropulic, PhD, MBA, cofounder and executive director of Caring Cross, said in an interview. “Our goal is to develop technologies and transfer them to organizations like Fiocruz to enable them to manufacture these transformative therapies for patients in their regions. We believe this model is an important solution for therapies that are priced so high that they are not accessible to many patients that need them, particularly underserved populations and those in low- and middle-income countries.”
According to Dr. Dropulic: “We have developed a production process where the material cost is about $20,000 per dose.” When labor and infrastructure costs are added, the total expense won’t be more than $37,000-$47,500, he said.
The research process for the CAR T-cell technology is at an earlier stage than in India. Scientists plan to start clinical trials of the technology in the United States by the end of 2024 and then begin them in Brazil in 2025, after safety and efficacy have been demonstrated. The first trial, a phase I/II study, will enroll about 20 patients, Dr. Dropulic said.
Dr. Kenderian reported ties with Novartis, Capstan Bio, Kite/Gilead, Juno/BMS, Humanigen, Tolero, Leah Labs, Lentigen, Luminary, Sunesis/Viracta, Morphosys, Troque, Carisma, Sendero, and LifEngine. Dr. Williams disclosed grants from National Institutes of Health and philanthropic organizations. Dr. Grupp reported relationships with Novartis, Kite, Vertex and Servier, Roche, GSK, Humanigen, CBMG, Eureka, Janssen/JNJ, Jazz, Adaptimmune, TCR2, Cellectis, Juno, Allogene, and Cabaletta. Dr. Purwar is the founder of ImmunoACT. Dr. Dropulic serves as executive director of Caring Cross and CEO of Vector BioMed, which provides vectors for gene therapy.
More Cases of Acute Diverticulitis Treated Outside Hospital
BOSTON — Patients with acute colonic diverticulitis are more likely to be seen by primary care providers than by emergency physicians, representing a shift in the way clinicians detect and treat the condition.
Acute colonic diverticulitis affects roughly 180 per 100,000 people per year in the United States.
CT of the abdomen and pelvis may not be a first-line method to detect diverticulitis in the primary care setting as it has been in emergent care, according to Kaveh Sharzehi, MD, MS, associate professor of medicine in the Division of Gastroenterology and Hepatology at Oregon Health & Science University in Portland.
Indeed, clinical guidelines by multiple physician groups recommend that providers use a more individualized approach to detecting and treating the condition.
“There is still great value in proper and thorough physical history and some adjunct testing,” Dr. Sharzehi told attendees during a presentation on April 20 at the American College of Physicians Internal Medicine Meeting 2024. These two methods can detect the disease up to 65% of the time, Dr. Sharzehi added.
An initial evaluation of a patient with suspected acute diverticulitis should first assess the patient’s history of abdominal pain, fever, and leukocytosis, Dr. Sharzehi said.
A C-reactive protein level > 50 mg/L “almost doubles the odds of having diverticulitis,” Dr. Sharzehi said. Studies also suggest increased levels of procalcitonin and fecal calprotectin can indicate the presence of the condition.
The American Gastroenterological Association (AGA) and the American College of Physicians recommend abdominal CT if clinicians are uncertain of the diagnosis, and to evaluate potential complications in severe cases. Ultrasound and MRI can be useful alternatives, according to guidelines from the American Society of Colon and Rectal Surgeons.
The chances of developing diverticulitis increase with age. More than 60% of Americans aged 60 years or older have diverticulosis, a condition characterized by small pouches in the colon lining that can weaken the colon wall. Less than 5% of people with diverticulosis go on to develop diverticulitis.
“Aspirin and opioid use are also risk factors, likely from their effect on the colonic transit time and causing constipation that might contribute to diverticulitis, but that›s not very well understood,” Dr. Sharzehi said.
Medical management has shifted from predominantly inpatient to predominantly outpatient care, Dr. Sharzehi told attendees
“Unfortunately, there are not that many supportive guidelines for what diet a patient should have in the acute setting of diverticulitis,” he said.
Patients with a mild case may benefit from a clear liquid diet; for some patients, high-fiber diets, regular physical activity, and statins may protect against recurrence.
Current guidelines recommend against prescribing antibiotics for most cases because evidence suggests that diverticulitis is primarily an inflammatory process that can result in small tears in the diverticulum, rather than the disease being a complication of existing tears.
Patients should also not be treated with probiotics or 5-aminosalicylic acid agents, Dr. Sharzehi said.
“My practice is in the Pacific Northwest, where there’s a lot of belief in naturopathic remedies, so we get a lot of questions about supplements and probiotics in preventing diverticulitis,” he said. “We don’t think it does help, and this is unanimous among all the main [physician] societies.”
The AGA recommends referring patients for a colonoscopy within a year after diverticulitis symptoms have resided.
Severe or unresolved cases could require inpatient procedures such as percutaneous drainage or surgery. An estimated 15%-30% of patients admitted to hospital with acute diverticulitis require surgery, Dr. Sharzehi said.
Surgery may become an option for patients who have recurrent cases of the disease, even if not severe, Dr. Sharzehi said.
Dr. Sharzehi reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
BOSTON — Patients with acute colonic diverticulitis are more likely to be seen by primary care providers than by emergency physicians, representing a shift in the way clinicians detect and treat the condition.
Acute colonic diverticulitis affects roughly 180 per 100,000 people per year in the United States.
CT of the abdomen and pelvis may not be a first-line method to detect diverticulitis in the primary care setting as it has been in emergent care, according to Kaveh Sharzehi, MD, MS, associate professor of medicine in the Division of Gastroenterology and Hepatology at Oregon Health & Science University in Portland.
Indeed, clinical guidelines by multiple physician groups recommend that providers use a more individualized approach to detecting and treating the condition.
“There is still great value in proper and thorough physical history and some adjunct testing,” Dr. Sharzehi told attendees during a presentation on April 20 at the American College of Physicians Internal Medicine Meeting 2024. These two methods can detect the disease up to 65% of the time, Dr. Sharzehi added.
An initial evaluation of a patient with suspected acute diverticulitis should first assess the patient’s history of abdominal pain, fever, and leukocytosis, Dr. Sharzehi said.
A C-reactive protein level > 50 mg/L “almost doubles the odds of having diverticulitis,” Dr. Sharzehi said. Studies also suggest increased levels of procalcitonin and fecal calprotectin can indicate the presence of the condition.
The American Gastroenterological Association (AGA) and the American College of Physicians recommend abdominal CT if clinicians are uncertain of the diagnosis, and to evaluate potential complications in severe cases. Ultrasound and MRI can be useful alternatives, according to guidelines from the American Society of Colon and Rectal Surgeons.
The chances of developing diverticulitis increase with age. More than 60% of Americans aged 60 years or older have diverticulosis, a condition characterized by small pouches in the colon lining that can weaken the colon wall. Less than 5% of people with diverticulosis go on to develop diverticulitis.
“Aspirin and opioid use are also risk factors, likely from their effect on the colonic transit time and causing constipation that might contribute to diverticulitis, but that›s not very well understood,” Dr. Sharzehi said.
Medical management has shifted from predominantly inpatient to predominantly outpatient care, Dr. Sharzehi told attendees
“Unfortunately, there are not that many supportive guidelines for what diet a patient should have in the acute setting of diverticulitis,” he said.
Patients with a mild case may benefit from a clear liquid diet; for some patients, high-fiber diets, regular physical activity, and statins may protect against recurrence.
Current guidelines recommend against prescribing antibiotics for most cases because evidence suggests that diverticulitis is primarily an inflammatory process that can result in small tears in the diverticulum, rather than the disease being a complication of existing tears.
Patients should also not be treated with probiotics or 5-aminosalicylic acid agents, Dr. Sharzehi said.
“My practice is in the Pacific Northwest, where there’s a lot of belief in naturopathic remedies, so we get a lot of questions about supplements and probiotics in preventing diverticulitis,” he said. “We don’t think it does help, and this is unanimous among all the main [physician] societies.”
The AGA recommends referring patients for a colonoscopy within a year after diverticulitis symptoms have resided.
Severe or unresolved cases could require inpatient procedures such as percutaneous drainage or surgery. An estimated 15%-30% of patients admitted to hospital with acute diverticulitis require surgery, Dr. Sharzehi said.
Surgery may become an option for patients who have recurrent cases of the disease, even if not severe, Dr. Sharzehi said.
Dr. Sharzehi reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
BOSTON — Patients with acute colonic diverticulitis are more likely to be seen by primary care providers than by emergency physicians, representing a shift in the way clinicians detect and treat the condition.
Acute colonic diverticulitis affects roughly 180 per 100,000 people per year in the United States.
CT of the abdomen and pelvis may not be a first-line method to detect diverticulitis in the primary care setting as it has been in emergent care, according to Kaveh Sharzehi, MD, MS, associate professor of medicine in the Division of Gastroenterology and Hepatology at Oregon Health & Science University in Portland.
Indeed, clinical guidelines by multiple physician groups recommend that providers use a more individualized approach to detecting and treating the condition.
“There is still great value in proper and thorough physical history and some adjunct testing,” Dr. Sharzehi told attendees during a presentation on April 20 at the American College of Physicians Internal Medicine Meeting 2024. These two methods can detect the disease up to 65% of the time, Dr. Sharzehi added.
An initial evaluation of a patient with suspected acute diverticulitis should first assess the patient’s history of abdominal pain, fever, and leukocytosis, Dr. Sharzehi said.
A C-reactive protein level > 50 mg/L “almost doubles the odds of having diverticulitis,” Dr. Sharzehi said. Studies also suggest increased levels of procalcitonin and fecal calprotectin can indicate the presence of the condition.
The American Gastroenterological Association (AGA) and the American College of Physicians recommend abdominal CT if clinicians are uncertain of the diagnosis, and to evaluate potential complications in severe cases. Ultrasound and MRI can be useful alternatives, according to guidelines from the American Society of Colon and Rectal Surgeons.
The chances of developing diverticulitis increase with age. More than 60% of Americans aged 60 years or older have diverticulosis, a condition characterized by small pouches in the colon lining that can weaken the colon wall. Less than 5% of people with diverticulosis go on to develop diverticulitis.
“Aspirin and opioid use are also risk factors, likely from their effect on the colonic transit time and causing constipation that might contribute to diverticulitis, but that›s not very well understood,” Dr. Sharzehi said.
Medical management has shifted from predominantly inpatient to predominantly outpatient care, Dr. Sharzehi told attendees
“Unfortunately, there are not that many supportive guidelines for what diet a patient should have in the acute setting of diverticulitis,” he said.
Patients with a mild case may benefit from a clear liquid diet; for some patients, high-fiber diets, regular physical activity, and statins may protect against recurrence.
Current guidelines recommend against prescribing antibiotics for most cases because evidence suggests that diverticulitis is primarily an inflammatory process that can result in small tears in the diverticulum, rather than the disease being a complication of existing tears.
Patients should also not be treated with probiotics or 5-aminosalicylic acid agents, Dr. Sharzehi said.
“My practice is in the Pacific Northwest, where there’s a lot of belief in naturopathic remedies, so we get a lot of questions about supplements and probiotics in preventing diverticulitis,” he said. “We don’t think it does help, and this is unanimous among all the main [physician] societies.”
The AGA recommends referring patients for a colonoscopy within a year after diverticulitis symptoms have resided.
Severe or unresolved cases could require inpatient procedures such as percutaneous drainage or surgery. An estimated 15%-30% of patients admitted to hospital with acute diverticulitis require surgery, Dr. Sharzehi said.
Surgery may become an option for patients who have recurrent cases of the disease, even if not severe, Dr. Sharzehi said.
Dr. Sharzehi reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Asthma, COPD inhaler price caps set for summer
In addition to warmer weather, June will usher in changes in asthma and COPD inhaler costs for many patients, potentially reducing barriers to those seeing high prescription prices. Price ceilings have been set by some companies, likely following action earlier this year by a Senate Committee which pointed to higher costs of US inhalers compared with other countries.
Senator Sanders stated: “In my view, Americans who have asthma and COPD should not be forced to pay, in many cases, 10-70 times more for the same exact inhalers as patients in Europe and other parts of the world.”
Starting June 1, Boehringer Ingelheim will cap out-of-pocket costs for the company’s inhaler products for chronic lung disease and asthma at $35 per month, according to a March 7, 2024, press release from the German drugmaker’s US headquarters in Ridgefield, Conn. The reductions cover the full range of the company’s inhaler products for asthma and chronic obstructive pulmonary disease (COPD) including Atrovent, Combivent Respimat and Spiriva HandiHaler and Respimat, Stiolto Respimat and Striverdi Respimat. In the release, Boehringer Ingelheim USA Corporation’s President and CEO Jean-Michel Boers stated, “The US health care system is complex and often doesn’t work for patients, especially the most vulnerable. While we can’t fix the entire system alone, we are bringing forward a solution to make it fairer. We want to do our part to help patients living with COPD or asthma who struggle to pay for their medications.”
Similar announcements were made by AstraZeneca and GSK. GSK’s cap will go into effect on January 1, 2025, and includes Advair Diskus, Advair HFA, Anoro Ellipta, Arnuity Ellipta, Breo Ellipta, Incruse Ellipta, Serevent Diskus, Trelegy Ellipta, and Ventolin HFA. The AstraZeneca cap, which covers Airsupra, Bevespi Aerosphere, Breztri Aeroshpere, and Symbicort, goes into effect on June 1, 2024.
Senate statement on pricing
These companies plus Teva had received letters sent on January 8, 2024, by the members of the Senate Committee on Health, Education, Labor, and Pensions: senators Sanders, Baldwin, Luján and Markey. The letters cited enormous inhaler price discrepancies, for example $489 for Combivent Respimat in the United States but just $7 in France, and announced the conduct of an investigation into efforts by these companies to artificially inflate and manipulate prices of asthma inhalers that have been on the market for decades. A statement from Sen. Sanders’ office noted that AstraZeneca, GSK, and Teva made more than $25 billion in revenue from inhalers alone in the past 5 years (Boehringer Ingelheim does not provide public US inhaler revenue information).
Suit claims generic delay
A federal lawsuit filed in Boston on March 6, according to a Reuters brief from March 7, cited Boehringer for improperly submitting patents to the US Food and Drug Administration (FDA). The purpose of those patents, the suit charges, was to delay generic competition and inflate Combivent Respimat and Spiriva Respimat inhaler prices.
Inhaler prices soared in the United States, according to a March 10 U.S. News & World Report commentary by The Conversation, a nonprofit news organization, after the 2008 FDA ban on chlorofluorocarbon (CFC)-propellants led to the phase-out of CFC-containing inhalers and their replacement with hydrofluoroalkane-propellant inhalers. For the insured that meant an average out-of-pocket inhaler cost increase from $13.60 per prescription in 2004 to $25 in 2015. The current rate for the now nongeneric HFA-propelled but otherwise identical albuterol inhaler is $98. Competition from a more recently FDA-approved (2020) generic version has not been robust enough to effect meaningful price reductions, the report stated. While good insurance generally covers most of inhaler costs, the more than 25 million uninsured in 2023 faced steep market prices that put strain even on some insured, the CDC found, driving many in the United States to purchase from Mexican, Canadian, or other foreign pharmacies. The Teva QVAR REdiHaler corticosteroid inhaler, costing $9 in Germany, costs $286 in the US. Dosages, however, may not be identical. A first FDA-authorization of drug importing this past January applied only to agents for a limited number of disease states and pertained only to Florida, but may serve as a model for other states, according to the commentary.
“The announced price cap from Boehringer Ingelheim,” stated Kenneth Mendez, president and CEO of the Asthma and Allergy Foundation of America (AAFA) in a press release, “is a step toward improving access to essential asthma medicine and demonstrates that the voice of the asthma patient community is being heard.” The AAFA release noted further that asthma death rates, while declining overall, are triple in Blacks compared with Whites. Death rates, asthma rates, and rates of being uninsured or underinsured are much higher in Black and Puerto Rican populations than in Whites. The complex layers of the current US system, composed of pharmaceutical manufacturers, pharmacy benefit managers, insurance companies, employers, and federal policies often conspire against those people who need asthma drugs the most. AAFA research has shown that when drug prices become a barrier to treatment, people with asthma ration or simply discontinue their essential asthma medications. Beyond saved lives, access to asthma medications can reduce hospitalizations and lower the more than $82 billion in annual asthma costs to the US economy.
Sen. Sanders, on March 20, applauded the GSK announcement: “As Chairman of the Senate Health, Education, Labor, and Pensions Committee, I very much appreciate GlaxoSmithKline’s announcement today that Americans throughout the country with asthma and COPD will pay no more than $35 for the brand name inhalers they manufacture. I look forward to working with GSK to make sure that this decision reaches as many patients as possible.”
“Inhaled medications continue to be an essential part of the therapy for patients with asthma, COPD, and other respiratory conditions,” said Diego J. Maselli, professor and chief, Division of Pulmonary Diseases & Critical Care, UT Health at San Antonio, San Antonio, Texas, in an interview with CHEST Physician. He added, “Unfortunately, with increasing cost of these and other treatments, access has been challenging for many patients. Patients, families, and providers constantly experience frustration with the difficulties of obtaining these lifesaving medications, and cost is the main barrier. Even those with ample insurance coverage face difficult challenges, as the high prices of these medications motivate insurance carriers to constantly adjust what is the ‘preferred’ option among inhalers. Regrettably, noncompliance and nonadherence to inhaled therapies has been linked to poor patient outcomes and increased health care utilization in both asthma and COPD. Because of the high prevalence of these diseases in the US and worldwide, efforts to increase the access of these vital medications has been a priority. With the leveling of the prices of these medications across the world, we hope that there will be both improved access and, as a consequence, better patient outcomes.”
In addition to warmer weather, June will usher in changes in asthma and COPD inhaler costs for many patients, potentially reducing barriers to those seeing high prescription prices. Price ceilings have been set by some companies, likely following action earlier this year by a Senate Committee which pointed to higher costs of US inhalers compared with other countries.
Senator Sanders stated: “In my view, Americans who have asthma and COPD should not be forced to pay, in many cases, 10-70 times more for the same exact inhalers as patients in Europe and other parts of the world.”
Starting June 1, Boehringer Ingelheim will cap out-of-pocket costs for the company’s inhaler products for chronic lung disease and asthma at $35 per month, according to a March 7, 2024, press release from the German drugmaker’s US headquarters in Ridgefield, Conn. The reductions cover the full range of the company’s inhaler products for asthma and chronic obstructive pulmonary disease (COPD) including Atrovent, Combivent Respimat and Spiriva HandiHaler and Respimat, Stiolto Respimat and Striverdi Respimat. In the release, Boehringer Ingelheim USA Corporation’s President and CEO Jean-Michel Boers stated, “The US health care system is complex and often doesn’t work for patients, especially the most vulnerable. While we can’t fix the entire system alone, we are bringing forward a solution to make it fairer. We want to do our part to help patients living with COPD or asthma who struggle to pay for their medications.”
Similar announcements were made by AstraZeneca and GSK. GSK’s cap will go into effect on January 1, 2025, and includes Advair Diskus, Advair HFA, Anoro Ellipta, Arnuity Ellipta, Breo Ellipta, Incruse Ellipta, Serevent Diskus, Trelegy Ellipta, and Ventolin HFA. The AstraZeneca cap, which covers Airsupra, Bevespi Aerosphere, Breztri Aeroshpere, and Symbicort, goes into effect on June 1, 2024.
Senate statement on pricing
These companies plus Teva had received letters sent on January 8, 2024, by the members of the Senate Committee on Health, Education, Labor, and Pensions: senators Sanders, Baldwin, Luján and Markey. The letters cited enormous inhaler price discrepancies, for example $489 for Combivent Respimat in the United States but just $7 in France, and announced the conduct of an investigation into efforts by these companies to artificially inflate and manipulate prices of asthma inhalers that have been on the market for decades. A statement from Sen. Sanders’ office noted that AstraZeneca, GSK, and Teva made more than $25 billion in revenue from inhalers alone in the past 5 years (Boehringer Ingelheim does not provide public US inhaler revenue information).
Suit claims generic delay
A federal lawsuit filed in Boston on March 6, according to a Reuters brief from March 7, cited Boehringer for improperly submitting patents to the US Food and Drug Administration (FDA). The purpose of those patents, the suit charges, was to delay generic competition and inflate Combivent Respimat and Spiriva Respimat inhaler prices.
Inhaler prices soared in the United States, according to a March 10 U.S. News & World Report commentary by The Conversation, a nonprofit news organization, after the 2008 FDA ban on chlorofluorocarbon (CFC)-propellants led to the phase-out of CFC-containing inhalers and their replacement with hydrofluoroalkane-propellant inhalers. For the insured that meant an average out-of-pocket inhaler cost increase from $13.60 per prescription in 2004 to $25 in 2015. The current rate for the now nongeneric HFA-propelled but otherwise identical albuterol inhaler is $98. Competition from a more recently FDA-approved (2020) generic version has not been robust enough to effect meaningful price reductions, the report stated. While good insurance generally covers most of inhaler costs, the more than 25 million uninsured in 2023 faced steep market prices that put strain even on some insured, the CDC found, driving many in the United States to purchase from Mexican, Canadian, or other foreign pharmacies. The Teva QVAR REdiHaler corticosteroid inhaler, costing $9 in Germany, costs $286 in the US. Dosages, however, may not be identical. A first FDA-authorization of drug importing this past January applied only to agents for a limited number of disease states and pertained only to Florida, but may serve as a model for other states, according to the commentary.
“The announced price cap from Boehringer Ingelheim,” stated Kenneth Mendez, president and CEO of the Asthma and Allergy Foundation of America (AAFA) in a press release, “is a step toward improving access to essential asthma medicine and demonstrates that the voice of the asthma patient community is being heard.” The AAFA release noted further that asthma death rates, while declining overall, are triple in Blacks compared with Whites. Death rates, asthma rates, and rates of being uninsured or underinsured are much higher in Black and Puerto Rican populations than in Whites. The complex layers of the current US system, composed of pharmaceutical manufacturers, pharmacy benefit managers, insurance companies, employers, and federal policies often conspire against those people who need asthma drugs the most. AAFA research has shown that when drug prices become a barrier to treatment, people with asthma ration or simply discontinue their essential asthma medications. Beyond saved lives, access to asthma medications can reduce hospitalizations and lower the more than $82 billion in annual asthma costs to the US economy.
Sen. Sanders, on March 20, applauded the GSK announcement: “As Chairman of the Senate Health, Education, Labor, and Pensions Committee, I very much appreciate GlaxoSmithKline’s announcement today that Americans throughout the country with asthma and COPD will pay no more than $35 for the brand name inhalers they manufacture. I look forward to working with GSK to make sure that this decision reaches as many patients as possible.”
“Inhaled medications continue to be an essential part of the therapy for patients with asthma, COPD, and other respiratory conditions,” said Diego J. Maselli, professor and chief, Division of Pulmonary Diseases & Critical Care, UT Health at San Antonio, San Antonio, Texas, in an interview with CHEST Physician. He added, “Unfortunately, with increasing cost of these and other treatments, access has been challenging for many patients. Patients, families, and providers constantly experience frustration with the difficulties of obtaining these lifesaving medications, and cost is the main barrier. Even those with ample insurance coverage face difficult challenges, as the high prices of these medications motivate insurance carriers to constantly adjust what is the ‘preferred’ option among inhalers. Regrettably, noncompliance and nonadherence to inhaled therapies has been linked to poor patient outcomes and increased health care utilization in both asthma and COPD. Because of the high prevalence of these diseases in the US and worldwide, efforts to increase the access of these vital medications has been a priority. With the leveling of the prices of these medications across the world, we hope that there will be both improved access and, as a consequence, better patient outcomes.”
In addition to warmer weather, June will usher in changes in asthma and COPD inhaler costs for many patients, potentially reducing barriers to those seeing high prescription prices. Price ceilings have been set by some companies, likely following action earlier this year by a Senate Committee which pointed to higher costs of US inhalers compared with other countries.
Senator Sanders stated: “In my view, Americans who have asthma and COPD should not be forced to pay, in many cases, 10-70 times more for the same exact inhalers as patients in Europe and other parts of the world.”
Starting June 1, Boehringer Ingelheim will cap out-of-pocket costs for the company’s inhaler products for chronic lung disease and asthma at $35 per month, according to a March 7, 2024, press release from the German drugmaker’s US headquarters in Ridgefield, Conn. The reductions cover the full range of the company’s inhaler products for asthma and chronic obstructive pulmonary disease (COPD) including Atrovent, Combivent Respimat and Spiriva HandiHaler and Respimat, Stiolto Respimat and Striverdi Respimat. In the release, Boehringer Ingelheim USA Corporation’s President and CEO Jean-Michel Boers stated, “The US health care system is complex and often doesn’t work for patients, especially the most vulnerable. While we can’t fix the entire system alone, we are bringing forward a solution to make it fairer. We want to do our part to help patients living with COPD or asthma who struggle to pay for their medications.”
Similar announcements were made by AstraZeneca and GSK. GSK’s cap will go into effect on January 1, 2025, and includes Advair Diskus, Advair HFA, Anoro Ellipta, Arnuity Ellipta, Breo Ellipta, Incruse Ellipta, Serevent Diskus, Trelegy Ellipta, and Ventolin HFA. The AstraZeneca cap, which covers Airsupra, Bevespi Aerosphere, Breztri Aeroshpere, and Symbicort, goes into effect on June 1, 2024.
Senate statement on pricing
These companies plus Teva had received letters sent on January 8, 2024, by the members of the Senate Committee on Health, Education, Labor, and Pensions: senators Sanders, Baldwin, Luján and Markey. The letters cited enormous inhaler price discrepancies, for example $489 for Combivent Respimat in the United States but just $7 in France, and announced the conduct of an investigation into efforts by these companies to artificially inflate and manipulate prices of asthma inhalers that have been on the market for decades. A statement from Sen. Sanders’ office noted that AstraZeneca, GSK, and Teva made more than $25 billion in revenue from inhalers alone in the past 5 years (Boehringer Ingelheim does not provide public US inhaler revenue information).
Suit claims generic delay
A federal lawsuit filed in Boston on March 6, according to a Reuters brief from March 7, cited Boehringer for improperly submitting patents to the US Food and Drug Administration (FDA). The purpose of those patents, the suit charges, was to delay generic competition and inflate Combivent Respimat and Spiriva Respimat inhaler prices.
Inhaler prices soared in the United States, according to a March 10 U.S. News & World Report commentary by The Conversation, a nonprofit news organization, after the 2008 FDA ban on chlorofluorocarbon (CFC)-propellants led to the phase-out of CFC-containing inhalers and their replacement with hydrofluoroalkane-propellant inhalers. For the insured that meant an average out-of-pocket inhaler cost increase from $13.60 per prescription in 2004 to $25 in 2015. The current rate for the now nongeneric HFA-propelled but otherwise identical albuterol inhaler is $98. Competition from a more recently FDA-approved (2020) generic version has not been robust enough to effect meaningful price reductions, the report stated. While good insurance generally covers most of inhaler costs, the more than 25 million uninsured in 2023 faced steep market prices that put strain even on some insured, the CDC found, driving many in the United States to purchase from Mexican, Canadian, or other foreign pharmacies. The Teva QVAR REdiHaler corticosteroid inhaler, costing $9 in Germany, costs $286 in the US. Dosages, however, may not be identical. A first FDA-authorization of drug importing this past January applied only to agents for a limited number of disease states and pertained only to Florida, but may serve as a model for other states, according to the commentary.
“The announced price cap from Boehringer Ingelheim,” stated Kenneth Mendez, president and CEO of the Asthma and Allergy Foundation of America (AAFA) in a press release, “is a step toward improving access to essential asthma medicine and demonstrates that the voice of the asthma patient community is being heard.” The AAFA release noted further that asthma death rates, while declining overall, are triple in Blacks compared with Whites. Death rates, asthma rates, and rates of being uninsured or underinsured are much higher in Black and Puerto Rican populations than in Whites. The complex layers of the current US system, composed of pharmaceutical manufacturers, pharmacy benefit managers, insurance companies, employers, and federal policies often conspire against those people who need asthma drugs the most. AAFA research has shown that when drug prices become a barrier to treatment, people with asthma ration or simply discontinue their essential asthma medications. Beyond saved lives, access to asthma medications can reduce hospitalizations and lower the more than $82 billion in annual asthma costs to the US economy.
Sen. Sanders, on March 20, applauded the GSK announcement: “As Chairman of the Senate Health, Education, Labor, and Pensions Committee, I very much appreciate GlaxoSmithKline’s announcement today that Americans throughout the country with asthma and COPD will pay no more than $35 for the brand name inhalers they manufacture. I look forward to working with GSK to make sure that this decision reaches as many patients as possible.”
“Inhaled medications continue to be an essential part of the therapy for patients with asthma, COPD, and other respiratory conditions,” said Diego J. Maselli, professor and chief, Division of Pulmonary Diseases & Critical Care, UT Health at San Antonio, San Antonio, Texas, in an interview with CHEST Physician. He added, “Unfortunately, with increasing cost of these and other treatments, access has been challenging for many patients. Patients, families, and providers constantly experience frustration with the difficulties of obtaining these lifesaving medications, and cost is the main barrier. Even those with ample insurance coverage face difficult challenges, as the high prices of these medications motivate insurance carriers to constantly adjust what is the ‘preferred’ option among inhalers. Regrettably, noncompliance and nonadherence to inhaled therapies has been linked to poor patient outcomes and increased health care utilization in both asthma and COPD. Because of the high prevalence of these diseases in the US and worldwide, efforts to increase the access of these vital medications has been a priority. With the leveling of the prices of these medications across the world, we hope that there will be both improved access and, as a consequence, better patient outcomes.”
In the Story of the Rubella Virus as a Source of Granulomas, the Plot Is Still Thickening
SAN DIEGO — Approximately 10 years ago in France, high throughput .
Based on accumulating evidence, the Centers for Disease Control and Prevention (CDC) through collaborations with others also recognized this in pediatric patients with inborn errors of immunity, and it is now appropriate for clinicians to consider this etiology when no other infectious agents can be identified, according to Karolyn A. Wanat, MD, professor of dermatology, Medical College of Wisconsin, Milwaukee, who spoke about rubella as a trigger in granulomatous disease at the meeting. “This is a huge evolving area of interest,” said Dr. Wanat, who has been the first author or coauthor on several published papers, including a review article published earlier this year.
In the earliest cases, including those reported in 2014, the cutaneous granulomas presumed to be causally related to vaccine-derived rubella virus were found only in those with a primary immunodeficiency. This is no longer the case. In a collaboration among US clinics, granulomas that had persisted for years in immunocompetent adults were identified, according to Dr. Wanat, the first author of a report on these findings in four adults in 2022. In addition, it now appears that wild-type rubella virus, like vaccine-derived rubella virus, can be the source of the antigenic response that underlies the development of rubella-associated cutaneous granulomas.
The phenotype of these granulomas is comparable to granulomas associated with other infectious agents. On dermatopathology, these commonly feature a robust granulomatous inflammation with multinucleated giant cells and lymphocytic infiltrate. Necrosis and fibrosis are also common.
“These are the types of granulomas that we would be thinking infection. If tissue cultures are negative, we would probably repeat them,” she said, suggesting that suspicion of an infectious etiology would probably remain high even after multiple negative tests.
Of the cases accruing in the United States and elsewhere, most but not all have been linked to inborn errors of immunity. In a 2020 CDC review, the risk of granulomas caused by compromised immunity, such as defects in T cell function, was estimated to be in the range of 0.6% to 2.5%, Dr. Wanat said.
It is now known that primary immunodeficiency is not a prerequisite, but this should not change the perception that the rubella vaccine, which was introduced in 1979, is effective and safe, according to Dr. Wanat. The vaccine is associated with few serious adverse events and is so effective that rubella was eliminated from the United States in 2004 and from the Americas in 2015.
This makes cases of granuloma associated with wild-type rubella virus surprising, but they appear to be exceedingly rare. Whether caused by vaccine exposure or another source, the mechanism of latent development of cutaneous granulomas is consistent with other infectious sources, and is not well understood.
“Rubella is a sneaky virus that can persist in some immunoprivileged sites indefinitely,” Dr. Wanat said. These sites include the eyes, joints, and placenta.
Many initial cases of rubella-associated granulomas occurred on the arms, presumably where the vaccine was administered, despite long intervals between exposure and lesion growth. This interval is often measured in years.
With more cases, it is now understood that involvement of other organs does occur even if the skin is the most common site of antigenic response in patients with immunodeficiency. The liver and lymph nodes represent other tissues that have been affected. Even lesions in the brain have been seen on autopsy.
Based on the benefit-to-risk ratio of a highly effective and successful vaccine, however, the association with a risk of granulomas “should not raise questions” about the value of the vaccine itself, Dr. Wanat noted.
“The proportion of patients who develop these granulomas is very, very low. Yet, the vaccine provides life-long immunity,” she said.
The discovery of granulomas associated with wild type rubella infection was “shocking” based on the supposition that the rubella virus had been eliminated, but this is just one of the unexpected discoveries as the still-evolving science has traced the story of rubella-associated granulomas over the past 10 years.
Cases now include children and adults through advanced ages.
Shedding of the virus and risk of infection to others has been studied but so far, the risk — if it exists — is very low. The evidence includes the many patients who have lived with the granulomas for years, even decades, without any known spread to others.
As for ongoing work in this area, Dr. Wanat said that a histopathological case definition for rubella-associated granulomas is being developed, and she and other investigators are actively seeking new cases to better characterize the disease.
So far, optimal treatment is not well defined. A number of strategies have had limited success or are considered impractical for routine use. One example is a stem cell transplant. In a case Dr. Wanat cited, complete resolution of the skin lesions was achieved with a transplant.
“I am not suggesting that those with localized disease in the skin should undergo a transplant, but it does support the role of the immune system and the potential for a reboot to clear the skin,” she said.
Other therapies associated with benefit in at least some patients include tumor necrosis factor (TNF) inhibitors with dapsone and ribavirin. The risk of adverse events for the latter might again limit its use, Dr. Wanat said.
With awareness, the number of granulomas found to be associated with rubella virus is expected to grow. Dr. Wanat speculated that those areas of the country that not yet have documented a case will do so over time. For idiopathic cases of cutaneous granulomas, rubella should be kept in mind, she said.
Characterizing rubella-associated cutaneous granulomas as “a public health concern,” Dr. Wanat urged clinicians to consider this etiology in lesions that match the phenotype, particularly when other more common infectious agents cannot be identified.
Asked for his perspective, Jeffrey P. North, MD, managing director of the UCSF Dermatopathology, and professor of dermatology and pathology at the University of California, San Francisco, agreed that rubella should be considered as a source of granulomas with a suspected infectious etiology when a pathogen cannot be found.
“It is likely much more common than we know as it has only been recently described and testing for it is limited. I suspected there are a lot of undiagnosed patients suffering from this disease,” Dr. North said in an interview.
“One of the important points for clinicians to consider is that while this has been reported mostly in patients with some form of immunodeficiency, there have also been patients reported to have this condition with no immunodeficiency,” he added. Even though the association between rubella and granulomas was made 10 years ago, awareness is only now spreading, which means the frequency with which rubella leads to granulomas remains uncertain.
“I think we will start to get a better idea of how common this is as more people learn about and testing for it expands,” Dr. North said.
Dr. Wanat reports no potential conflicts of interest. Dr. North reports financial relationships with AdviNow and Kiniksa Pharmaceuticals.
SAN DIEGO — Approximately 10 years ago in France, high throughput .
Based on accumulating evidence, the Centers for Disease Control and Prevention (CDC) through collaborations with others also recognized this in pediatric patients with inborn errors of immunity, and it is now appropriate for clinicians to consider this etiology when no other infectious agents can be identified, according to Karolyn A. Wanat, MD, professor of dermatology, Medical College of Wisconsin, Milwaukee, who spoke about rubella as a trigger in granulomatous disease at the meeting. “This is a huge evolving area of interest,” said Dr. Wanat, who has been the first author or coauthor on several published papers, including a review article published earlier this year.
In the earliest cases, including those reported in 2014, the cutaneous granulomas presumed to be causally related to vaccine-derived rubella virus were found only in those with a primary immunodeficiency. This is no longer the case. In a collaboration among US clinics, granulomas that had persisted for years in immunocompetent adults were identified, according to Dr. Wanat, the first author of a report on these findings in four adults in 2022. In addition, it now appears that wild-type rubella virus, like vaccine-derived rubella virus, can be the source of the antigenic response that underlies the development of rubella-associated cutaneous granulomas.
The phenotype of these granulomas is comparable to granulomas associated with other infectious agents. On dermatopathology, these commonly feature a robust granulomatous inflammation with multinucleated giant cells and lymphocytic infiltrate. Necrosis and fibrosis are also common.
“These are the types of granulomas that we would be thinking infection. If tissue cultures are negative, we would probably repeat them,” she said, suggesting that suspicion of an infectious etiology would probably remain high even after multiple negative tests.
Of the cases accruing in the United States and elsewhere, most but not all have been linked to inborn errors of immunity. In a 2020 CDC review, the risk of granulomas caused by compromised immunity, such as defects in T cell function, was estimated to be in the range of 0.6% to 2.5%, Dr. Wanat said.
It is now known that primary immunodeficiency is not a prerequisite, but this should not change the perception that the rubella vaccine, which was introduced in 1979, is effective and safe, according to Dr. Wanat. The vaccine is associated with few serious adverse events and is so effective that rubella was eliminated from the United States in 2004 and from the Americas in 2015.
This makes cases of granuloma associated with wild-type rubella virus surprising, but they appear to be exceedingly rare. Whether caused by vaccine exposure or another source, the mechanism of latent development of cutaneous granulomas is consistent with other infectious sources, and is not well understood.
“Rubella is a sneaky virus that can persist in some immunoprivileged sites indefinitely,” Dr. Wanat said. These sites include the eyes, joints, and placenta.
Many initial cases of rubella-associated granulomas occurred on the arms, presumably where the vaccine was administered, despite long intervals between exposure and lesion growth. This interval is often measured in years.
With more cases, it is now understood that involvement of other organs does occur even if the skin is the most common site of antigenic response in patients with immunodeficiency. The liver and lymph nodes represent other tissues that have been affected. Even lesions in the brain have been seen on autopsy.
Based on the benefit-to-risk ratio of a highly effective and successful vaccine, however, the association with a risk of granulomas “should not raise questions” about the value of the vaccine itself, Dr. Wanat noted.
“The proportion of patients who develop these granulomas is very, very low. Yet, the vaccine provides life-long immunity,” she said.
The discovery of granulomas associated with wild type rubella infection was “shocking” based on the supposition that the rubella virus had been eliminated, but this is just one of the unexpected discoveries as the still-evolving science has traced the story of rubella-associated granulomas over the past 10 years.
Cases now include children and adults through advanced ages.
Shedding of the virus and risk of infection to others has been studied but so far, the risk — if it exists — is very low. The evidence includes the many patients who have lived with the granulomas for years, even decades, without any known spread to others.
As for ongoing work in this area, Dr. Wanat said that a histopathological case definition for rubella-associated granulomas is being developed, and she and other investigators are actively seeking new cases to better characterize the disease.
So far, optimal treatment is not well defined. A number of strategies have had limited success or are considered impractical for routine use. One example is a stem cell transplant. In a case Dr. Wanat cited, complete resolution of the skin lesions was achieved with a transplant.
“I am not suggesting that those with localized disease in the skin should undergo a transplant, but it does support the role of the immune system and the potential for a reboot to clear the skin,” she said.
Other therapies associated with benefit in at least some patients include tumor necrosis factor (TNF) inhibitors with dapsone and ribavirin. The risk of adverse events for the latter might again limit its use, Dr. Wanat said.
With awareness, the number of granulomas found to be associated with rubella virus is expected to grow. Dr. Wanat speculated that those areas of the country that not yet have documented a case will do so over time. For idiopathic cases of cutaneous granulomas, rubella should be kept in mind, she said.
Characterizing rubella-associated cutaneous granulomas as “a public health concern,” Dr. Wanat urged clinicians to consider this etiology in lesions that match the phenotype, particularly when other more common infectious agents cannot be identified.
Asked for his perspective, Jeffrey P. North, MD, managing director of the UCSF Dermatopathology, and professor of dermatology and pathology at the University of California, San Francisco, agreed that rubella should be considered as a source of granulomas with a suspected infectious etiology when a pathogen cannot be found.
“It is likely much more common than we know as it has only been recently described and testing for it is limited. I suspected there are a lot of undiagnosed patients suffering from this disease,” Dr. North said in an interview.
“One of the important points for clinicians to consider is that while this has been reported mostly in patients with some form of immunodeficiency, there have also been patients reported to have this condition with no immunodeficiency,” he added. Even though the association between rubella and granulomas was made 10 years ago, awareness is only now spreading, which means the frequency with which rubella leads to granulomas remains uncertain.
“I think we will start to get a better idea of how common this is as more people learn about and testing for it expands,” Dr. North said.
Dr. Wanat reports no potential conflicts of interest. Dr. North reports financial relationships with AdviNow and Kiniksa Pharmaceuticals.
SAN DIEGO — Approximately 10 years ago in France, high throughput .
Based on accumulating evidence, the Centers for Disease Control and Prevention (CDC) through collaborations with others also recognized this in pediatric patients with inborn errors of immunity, and it is now appropriate for clinicians to consider this etiology when no other infectious agents can be identified, according to Karolyn A. Wanat, MD, professor of dermatology, Medical College of Wisconsin, Milwaukee, who spoke about rubella as a trigger in granulomatous disease at the meeting. “This is a huge evolving area of interest,” said Dr. Wanat, who has been the first author or coauthor on several published papers, including a review article published earlier this year.
In the earliest cases, including those reported in 2014, the cutaneous granulomas presumed to be causally related to vaccine-derived rubella virus were found only in those with a primary immunodeficiency. This is no longer the case. In a collaboration among US clinics, granulomas that had persisted for years in immunocompetent adults were identified, according to Dr. Wanat, the first author of a report on these findings in four adults in 2022. In addition, it now appears that wild-type rubella virus, like vaccine-derived rubella virus, can be the source of the antigenic response that underlies the development of rubella-associated cutaneous granulomas.
The phenotype of these granulomas is comparable to granulomas associated with other infectious agents. On dermatopathology, these commonly feature a robust granulomatous inflammation with multinucleated giant cells and lymphocytic infiltrate. Necrosis and fibrosis are also common.
“These are the types of granulomas that we would be thinking infection. If tissue cultures are negative, we would probably repeat them,” she said, suggesting that suspicion of an infectious etiology would probably remain high even after multiple negative tests.
Of the cases accruing in the United States and elsewhere, most but not all have been linked to inborn errors of immunity. In a 2020 CDC review, the risk of granulomas caused by compromised immunity, such as defects in T cell function, was estimated to be in the range of 0.6% to 2.5%, Dr. Wanat said.
It is now known that primary immunodeficiency is not a prerequisite, but this should not change the perception that the rubella vaccine, which was introduced in 1979, is effective and safe, according to Dr. Wanat. The vaccine is associated with few serious adverse events and is so effective that rubella was eliminated from the United States in 2004 and from the Americas in 2015.
This makes cases of granuloma associated with wild-type rubella virus surprising, but they appear to be exceedingly rare. Whether caused by vaccine exposure or another source, the mechanism of latent development of cutaneous granulomas is consistent with other infectious sources, and is not well understood.
“Rubella is a sneaky virus that can persist in some immunoprivileged sites indefinitely,” Dr. Wanat said. These sites include the eyes, joints, and placenta.
Many initial cases of rubella-associated granulomas occurred on the arms, presumably where the vaccine was administered, despite long intervals between exposure and lesion growth. This interval is often measured in years.
With more cases, it is now understood that involvement of other organs does occur even if the skin is the most common site of antigenic response in patients with immunodeficiency. The liver and lymph nodes represent other tissues that have been affected. Even lesions in the brain have been seen on autopsy.
Based on the benefit-to-risk ratio of a highly effective and successful vaccine, however, the association with a risk of granulomas “should not raise questions” about the value of the vaccine itself, Dr. Wanat noted.
“The proportion of patients who develop these granulomas is very, very low. Yet, the vaccine provides life-long immunity,” she said.
The discovery of granulomas associated with wild type rubella infection was “shocking” based on the supposition that the rubella virus had been eliminated, but this is just one of the unexpected discoveries as the still-evolving science has traced the story of rubella-associated granulomas over the past 10 years.
Cases now include children and adults through advanced ages.
Shedding of the virus and risk of infection to others has been studied but so far, the risk — if it exists — is very low. The evidence includes the many patients who have lived with the granulomas for years, even decades, without any known spread to others.
As for ongoing work in this area, Dr. Wanat said that a histopathological case definition for rubella-associated granulomas is being developed, and she and other investigators are actively seeking new cases to better characterize the disease.
So far, optimal treatment is not well defined. A number of strategies have had limited success or are considered impractical for routine use. One example is a stem cell transplant. In a case Dr. Wanat cited, complete resolution of the skin lesions was achieved with a transplant.
“I am not suggesting that those with localized disease in the skin should undergo a transplant, but it does support the role of the immune system and the potential for a reboot to clear the skin,” she said.
Other therapies associated with benefit in at least some patients include tumor necrosis factor (TNF) inhibitors with dapsone and ribavirin. The risk of adverse events for the latter might again limit its use, Dr. Wanat said.
With awareness, the number of granulomas found to be associated with rubella virus is expected to grow. Dr. Wanat speculated that those areas of the country that not yet have documented a case will do so over time. For idiopathic cases of cutaneous granulomas, rubella should be kept in mind, she said.
Characterizing rubella-associated cutaneous granulomas as “a public health concern,” Dr. Wanat urged clinicians to consider this etiology in lesions that match the phenotype, particularly when other more common infectious agents cannot be identified.
Asked for his perspective, Jeffrey P. North, MD, managing director of the UCSF Dermatopathology, and professor of dermatology and pathology at the University of California, San Francisco, agreed that rubella should be considered as a source of granulomas with a suspected infectious etiology when a pathogen cannot be found.
“It is likely much more common than we know as it has only been recently described and testing for it is limited. I suspected there are a lot of undiagnosed patients suffering from this disease,” Dr. North said in an interview.
“One of the important points for clinicians to consider is that while this has been reported mostly in patients with some form of immunodeficiency, there have also been patients reported to have this condition with no immunodeficiency,” he added. Even though the association between rubella and granulomas was made 10 years ago, awareness is only now spreading, which means the frequency with which rubella leads to granulomas remains uncertain.
“I think we will start to get a better idea of how common this is as more people learn about and testing for it expands,” Dr. North said.
Dr. Wanat reports no potential conflicts of interest. Dr. North reports financial relationships with AdviNow and Kiniksa Pharmaceuticals.
FROM AAD 2024
The Long, Controversial Search for a ‘Cancer Microbiome’
Last year, the controversy heightened when experts questioned a high-profile study — a 2020 analysis claiming that the tumors of 33 different cancers had their own unique microbiomes — on whether the “signature” of these bacterial compositions could help diagnose cancer.
The incident renewed the spotlight on “tumor microbiomes” because of the bold claims of the original paper and the strongly worded refutations of those claims. The broader field has focused primarily on ways the body’s microbiome interacts with cancers and cancer treatment.
This controversy has highlighted the challenges of making headway in a field where researchers may not even have the tools yet to puzzle-out the wide-ranging implications the microbiome holds for cancer diagnosis and treatment.
But it is also part of a provocative question within that larger field: whether tumors in the body, far from the natural microbiome in the gut, have their own thriving communities of bacteria, viruses, and fungi. And, if they do, how do those tumor microbiomes affect the development and progression of the cancer and the effectiveness of cancer therapies?
Cancer Controversy
The evidence is undeniable that some microbes can directly cause certain cancers and that the human gut microbiome can influence the effectiveness of certain therapies. Beyond that established science, however, the research has raised as many questions as answers about what we do and don’t know about microbiota and cancer.
The only confirmed microbiomes are on the skin and in the gut, mouth, and vagina, which are all areas with an easy direct route for bacteria to enter and grow in or on the body. A series of papers in recent years have suggested that other internal organs, and tumors within them, may have their own microbiomes.
“Whether microbes exist in tumors of internal organs beyond body surfaces exposed to the environment is a different matter,” said Ivan Vujkovic-Cvijin, PhD, an assistant professor of biomedical sciences and gastroenterology at Cedars-Sinai Medical Center in Los Angeles, whose lab studies how human gut microbes affect inflammatory diseases. “We’ve only recently had the tools to study that question on a molecular level, and the reported results have been conflicting.”
For example, research allegedly identified microbiota in the human placenta nearly one decade ago. But subsequent research contradicted those claims and showed that the source of the “placental microbiome” was actually contamination. Subsequent similar studies for other parts of the body faced the same scrutiny and, often, eventual debunking.
“Most likely, our immune system has undergone selective pressure to eliminate everything that crosses the gut barrier because there’s not much benefit to the body to have bacteria run amok in our internal organs,” Dr. Vujkovic-Cvijin said. “That can only disrupt the functioning of our tissues, to have an external organism living inside them.”
The controversy that erupted last summer, surrounding research from the lab of Rob Knight, PhD, at the University of California, San Diego, centered on a slightly different but related question: Could tumors harbor their own microbiomes?
This news organization spoke with two of the authors who published a paper contesting Dr. Knight’s findings: Steven Salzberg, PhD, a professor of biomedical engineering at John Hopkins Medicine, Baltimore, Maryland, and Abraham Gihawi, PhD, a research fellow at Norwich Medical School at the University of East Anglia in the United Kingdom.
Dr. Salzberg described two major problems with Dr. Knight’s study.
“What they found were false positives because of contamination in the database and flaws in their methods,” Dr. Salzberg said. “I can’t prove there’s no cancer microbiome, but I can say the cancer microbiomes that they reported don’t exist because the species they were finding aren’t there.”
Dr. Knight disagrees with Dr. Salzberg’s findings, noting that Dr. Salzberg and his co-authors did not examine the publicly available databases used in his study. In a written response, he said that his team’s examination of the database revealed that less than 1% of the microbial genomes overlapped with human ones and that removing them did not change their findings.
Dr. Knight also noted that his team could still “distinguish cancer types by their microbiome” even after running their analysis without the technique that Dr. Salzberg found fault with.
Dr. Salzberg said that the database linked above is not the one Dr. Knight’s study used, however. “The primary database in their study was never made public (it’s too large, they said), and it has/had about 69,000 genomes,” Dr. Salzberg said by email. “But even if we did, this is irrelevant. He’s trying to distract from the primary errors in their study,” which Dr. Salzberg said Dr. Knight’s team has not addressed.
The critiques Dr. Salzberg raised have been leveled at other studies investigating microbiomes specifically within tumors and independent of the body’s microbiome.
For example, a 2019 study in Nature described a fungal microbiome in pancreatic cancer that a Nature paper 4 years later directly contradicted, citing flaws that invalidated the original findings. A different 2019 study in Cell examined pancreatic tumor microbiota and patient outcomes, but it’s unclear whether the microorganisms moved from the gut to the pancreas or “constitute a durably colonized community that lives inside the tumor,” which remains a matter of debate, Dr. Vujkovic-Cvijin said.
A 2020 study in Science suggested diverse microbial communities in seven tumor types, but those findings were similarly called into question. That study stated that “bacteria were first detected in human tumors more than 100 years ago” and that “bacteria are well-known residents in human tumors,” but Dr. Salzberg considers those statements misleading.
It’s true that bacteria and viruses have been detected in tumors because “there’s very good evidence that an acute infection caused by a very small number of viruses and bacteria can cause a tumor,” Dr. Salzberg said. Human papillomavirus, for example, can cause six different types of cancer. Inflammation and ulcers caused by Helicobacter pylori may progress to stomach cancer, and Fusobacterium nucleatum and Enterococcus faecalis have been shown to contribute to colorectal cancer. Those examples differ from a microbiome; this “a community of bacteria and possibly other microscopic bugs, like fungi, that are happily living in the tumor” the same way microbes reside in our guts, he said.
Dr. Knight said that many bacteria his team identified “have been confirmed independently in subsequent work.” He acknowledged, however, that more research is needed.
Several of the contested studies above were among a lengthy list that Dr. Knight provided, noting that most of the disagreements “have two sides to them, and critiques from one particular group does not immediately invalidate a reported finding.”
Yet, many of the papers Dr. Knight listed are precisely the types that skeptics like Dr. Salzberg believe are too flawed to draw reliable conclusions.
“I think many agree that microbes may exist within tumors that are exposed to the environment, like tumors of the skin, gut, and mouth,” Dr. Vujkovic-Cvijin said. It’s less clear, however, whether tumors further from the body’s microbiome harbor any microbes or where they came from if they do. Microbial signals in organs elsewhere in the body become faint quickly, he said.
Underdeveloped Technology
Though Dr. Salzberg said that the concept of a tumor microbiome is “implausible” because there’s no easy route for bacteria to reach internal organs, it’s unclear whether scientists have the technology yet to adequately answer this question.
For one thing, samples in these types of studies are typically “ultra-low biomass samples, where the signal — the amount of microbes in the sample — is so low that it’s comparable to how much would be expected to be found in reagents and environmental contamination through processing,” Dr. Vujkovic-Cvijin explained. Many polymerases used to amplify a DNA signal, for example, are made in bacteria and may retain trace amounts identified in these studies.
Dr. Knight agreed that low biomass is a challenge in this field but is not an unsurmountable one.
Another challenge is that study samples, as with Dr. Knight’s work, were collected during routine surgeries without the intent to find a microbial signal. Simply using a scalpel to cut through the skin means cutting through a layer of bacteria, and surgery rooms are not designed to eliminate all bacteria. Some work has even shown there is a “hospital microbiome,” so “you can easily have that creep into your signal and mistake it for tumor-resident bacteria,” Dr. Vujkovic-Cvijin said.
Dr. Knight asserted that the samples are taken under sterile conditions, but other researchers do not think the level of sterility necessary for completely clean samples is possible.
“Just because it’s in your sample doesn’t mean it was in your tumor,” Dr. Gihawi said.
Even if scientists can retrieve a reliable sample without contamination, analyzing it requires comparing the genetic material to existing databases of microbial genomes. Yet, contamination and misclassification of genetic sequences can be problems in those reference genomes too, Dr. Gihawi explained.
Machine learning algorithms have a role in interpreting data, but “we need to be careful of what we use them for,” he added.
“These techniques are in their infancy, and we’re starting to chase them down, which is why we need to move microbiome research in a way that can be used clinically,” Dr. Gihawi said.
Influence on Cancer Treatment Outcomes
Again, however, the question of whether microbiomes exist within tumors is only one slice of the much larger field looking at microbiomes and cancer, including its influence on cancer treatment outcomes. Although much remains to be learned, less controversy exists over the thousands of studies in the past two decades that have gradually revealed how the body’s microbiome can affect both the course of a cancer and the effectiveness of different treatments.
The growing research showing the importance of the gut microbiome in cancer treatments is not surprising given its role in immunity more broadly. Because the human immune system must recognize and defend against microbes, the microbiome helps train it, Dr. Vujkovic-Cvijin said.
Some bacteria can escape the gut — a phenomenon called bacterial translocation — and may aid in fighting tumors. To grow large enough to be seen on imaging, tumors need to evolve several abilities, such as growing enough vascularization to receive blood flow and shutting down local immune responses.
“Any added boost, like immunotherapy, has a chance of breaking through that immune forcefield and killing the tumor cells,” Dr. Vujkovic-Cvijin said. Escaped gut bacteria may provide that boost.
“There’s a lot of evidence that depletion of the gut microbiome impairs immunotherapy and chemotherapy. The thinking behind some of those studies is that gut microbes can cross the gut barrier and when they do, they activate the immune system,” he said.
In mice engineered to have sterile guts, for example, the lack of bacteria results in less effective immune systems, Dr. Vujkovic-Cvijin pointed out. A host of research has shown that antibiotic exposure during and even 6 months before immunotherapy dramatically reduces survival rates. “That’s pretty convincing to me that gut microbes are important,” he said.
Dr. Vujkovic-Cvijin cautioned that there continues to be controversy on understanding which bacteria are important for response to immunotherapy. “The field is still in its infancy in terms of understanding which bacteria are most important for these effects,” he said.
Dr. Knight suggested that escaped bacteria may be the genesis of the ones that he and other researchers believe exist in tumors. “Because tumor microbes must come from somewhere, it is to be expected that some of those microbes will be co-opted from body-site specific commensals.”
It’s also possible that metabolites released from gut bacteria escape the gut and could theoretically affect distant tumor growth, Dr. Gihawi said. The most promising avenue of research in this area is metabolites being used as biomarkers, added Dr. Gihawi, whose lab published research on a link between bacteria detected in men’s urine and a more aggressive subset of prostate cancers. But that research is not far enough along to develop lab tests for clinical use, he noted.
No Consensus Yet
Even before the controversy erupted around Dr. Knight’s research, he co-founded the company Micronoma to develop cancer tests based on his microbe findings. The company has raised $17.5 million from private investors as of August 2023 and received the US Food and Drug Administration’s Breakthrough Device designation, allowing the firm to fast-track clinical trials testing the technology. The recent critiques have not changed the company’s plans.
It’s safe to say that scientists will continue to research and debate the possibility of tumor microbiomes until a consensus emerges.
“The field is evolving and studies testing the reproducibility of tumor-resident microbial signals are essential for developing our understanding in this area,” Dr. Vujkovic-Cvijin said.
Even if that path ultimately leads nowhere, as Dr. Salzberg expects, research into microbiomes and cancer has plenty of other directions to go.
“I’m actually quite an optimist,” Dr. Gihawi said. “I think there’s a lot of scope for some really good research here, especially in the sites where we know there is a strong microbiome, such as the gastrointestinal tract.”
A version of this article appeared on Medscape.com.
Last year, the controversy heightened when experts questioned a high-profile study — a 2020 analysis claiming that the tumors of 33 different cancers had their own unique microbiomes — on whether the “signature” of these bacterial compositions could help diagnose cancer.
The incident renewed the spotlight on “tumor microbiomes” because of the bold claims of the original paper and the strongly worded refutations of those claims. The broader field has focused primarily on ways the body’s microbiome interacts with cancers and cancer treatment.
This controversy has highlighted the challenges of making headway in a field where researchers may not even have the tools yet to puzzle-out the wide-ranging implications the microbiome holds for cancer diagnosis and treatment.
But it is also part of a provocative question within that larger field: whether tumors in the body, far from the natural microbiome in the gut, have their own thriving communities of bacteria, viruses, and fungi. And, if they do, how do those tumor microbiomes affect the development and progression of the cancer and the effectiveness of cancer therapies?
Cancer Controversy
The evidence is undeniable that some microbes can directly cause certain cancers and that the human gut microbiome can influence the effectiveness of certain therapies. Beyond that established science, however, the research has raised as many questions as answers about what we do and don’t know about microbiota and cancer.
The only confirmed microbiomes are on the skin and in the gut, mouth, and vagina, which are all areas with an easy direct route for bacteria to enter and grow in or on the body. A series of papers in recent years have suggested that other internal organs, and tumors within them, may have their own microbiomes.
“Whether microbes exist in tumors of internal organs beyond body surfaces exposed to the environment is a different matter,” said Ivan Vujkovic-Cvijin, PhD, an assistant professor of biomedical sciences and gastroenterology at Cedars-Sinai Medical Center in Los Angeles, whose lab studies how human gut microbes affect inflammatory diseases. “We’ve only recently had the tools to study that question on a molecular level, and the reported results have been conflicting.”
For example, research allegedly identified microbiota in the human placenta nearly one decade ago. But subsequent research contradicted those claims and showed that the source of the “placental microbiome” was actually contamination. Subsequent similar studies for other parts of the body faced the same scrutiny and, often, eventual debunking.
“Most likely, our immune system has undergone selective pressure to eliminate everything that crosses the gut barrier because there’s not much benefit to the body to have bacteria run amok in our internal organs,” Dr. Vujkovic-Cvijin said. “That can only disrupt the functioning of our tissues, to have an external organism living inside them.”
The controversy that erupted last summer, surrounding research from the lab of Rob Knight, PhD, at the University of California, San Diego, centered on a slightly different but related question: Could tumors harbor their own microbiomes?
This news organization spoke with two of the authors who published a paper contesting Dr. Knight’s findings: Steven Salzberg, PhD, a professor of biomedical engineering at John Hopkins Medicine, Baltimore, Maryland, and Abraham Gihawi, PhD, a research fellow at Norwich Medical School at the University of East Anglia in the United Kingdom.
Dr. Salzberg described two major problems with Dr. Knight’s study.
“What they found were false positives because of contamination in the database and flaws in their methods,” Dr. Salzberg said. “I can’t prove there’s no cancer microbiome, but I can say the cancer microbiomes that they reported don’t exist because the species they were finding aren’t there.”
Dr. Knight disagrees with Dr. Salzberg’s findings, noting that Dr. Salzberg and his co-authors did not examine the publicly available databases used in his study. In a written response, he said that his team’s examination of the database revealed that less than 1% of the microbial genomes overlapped with human ones and that removing them did not change their findings.
Dr. Knight also noted that his team could still “distinguish cancer types by their microbiome” even after running their analysis without the technique that Dr. Salzberg found fault with.
Dr. Salzberg said that the database linked above is not the one Dr. Knight’s study used, however. “The primary database in their study was never made public (it’s too large, they said), and it has/had about 69,000 genomes,” Dr. Salzberg said by email. “But even if we did, this is irrelevant. He’s trying to distract from the primary errors in their study,” which Dr. Salzberg said Dr. Knight’s team has not addressed.
The critiques Dr. Salzberg raised have been leveled at other studies investigating microbiomes specifically within tumors and independent of the body’s microbiome.
For example, a 2019 study in Nature described a fungal microbiome in pancreatic cancer that a Nature paper 4 years later directly contradicted, citing flaws that invalidated the original findings. A different 2019 study in Cell examined pancreatic tumor microbiota and patient outcomes, but it’s unclear whether the microorganisms moved from the gut to the pancreas or “constitute a durably colonized community that lives inside the tumor,” which remains a matter of debate, Dr. Vujkovic-Cvijin said.
A 2020 study in Science suggested diverse microbial communities in seven tumor types, but those findings were similarly called into question. That study stated that “bacteria were first detected in human tumors more than 100 years ago” and that “bacteria are well-known residents in human tumors,” but Dr. Salzberg considers those statements misleading.
It’s true that bacteria and viruses have been detected in tumors because “there’s very good evidence that an acute infection caused by a very small number of viruses and bacteria can cause a tumor,” Dr. Salzberg said. Human papillomavirus, for example, can cause six different types of cancer. Inflammation and ulcers caused by Helicobacter pylori may progress to stomach cancer, and Fusobacterium nucleatum and Enterococcus faecalis have been shown to contribute to colorectal cancer. Those examples differ from a microbiome; this “a community of bacteria and possibly other microscopic bugs, like fungi, that are happily living in the tumor” the same way microbes reside in our guts, he said.
Dr. Knight said that many bacteria his team identified “have been confirmed independently in subsequent work.” He acknowledged, however, that more research is needed.
Several of the contested studies above were among a lengthy list that Dr. Knight provided, noting that most of the disagreements “have two sides to them, and critiques from one particular group does not immediately invalidate a reported finding.”
Yet, many of the papers Dr. Knight listed are precisely the types that skeptics like Dr. Salzberg believe are too flawed to draw reliable conclusions.
“I think many agree that microbes may exist within tumors that are exposed to the environment, like tumors of the skin, gut, and mouth,” Dr. Vujkovic-Cvijin said. It’s less clear, however, whether tumors further from the body’s microbiome harbor any microbes or where they came from if they do. Microbial signals in organs elsewhere in the body become faint quickly, he said.
Underdeveloped Technology
Though Dr. Salzberg said that the concept of a tumor microbiome is “implausible” because there’s no easy route for bacteria to reach internal organs, it’s unclear whether scientists have the technology yet to adequately answer this question.
For one thing, samples in these types of studies are typically “ultra-low biomass samples, where the signal — the amount of microbes in the sample — is so low that it’s comparable to how much would be expected to be found in reagents and environmental contamination through processing,” Dr. Vujkovic-Cvijin explained. Many polymerases used to amplify a DNA signal, for example, are made in bacteria and may retain trace amounts identified in these studies.
Dr. Knight agreed that low biomass is a challenge in this field but is not an unsurmountable one.
Another challenge is that study samples, as with Dr. Knight’s work, were collected during routine surgeries without the intent to find a microbial signal. Simply using a scalpel to cut through the skin means cutting through a layer of bacteria, and surgery rooms are not designed to eliminate all bacteria. Some work has even shown there is a “hospital microbiome,” so “you can easily have that creep into your signal and mistake it for tumor-resident bacteria,” Dr. Vujkovic-Cvijin said.
Dr. Knight asserted that the samples are taken under sterile conditions, but other researchers do not think the level of sterility necessary for completely clean samples is possible.
“Just because it’s in your sample doesn’t mean it was in your tumor,” Dr. Gihawi said.
Even if scientists can retrieve a reliable sample without contamination, analyzing it requires comparing the genetic material to existing databases of microbial genomes. Yet, contamination and misclassification of genetic sequences can be problems in those reference genomes too, Dr. Gihawi explained.
Machine learning algorithms have a role in interpreting data, but “we need to be careful of what we use them for,” he added.
“These techniques are in their infancy, and we’re starting to chase them down, which is why we need to move microbiome research in a way that can be used clinically,” Dr. Gihawi said.
Influence on Cancer Treatment Outcomes
Again, however, the question of whether microbiomes exist within tumors is only one slice of the much larger field looking at microbiomes and cancer, including its influence on cancer treatment outcomes. Although much remains to be learned, less controversy exists over the thousands of studies in the past two decades that have gradually revealed how the body’s microbiome can affect both the course of a cancer and the effectiveness of different treatments.
The growing research showing the importance of the gut microbiome in cancer treatments is not surprising given its role in immunity more broadly. Because the human immune system must recognize and defend against microbes, the microbiome helps train it, Dr. Vujkovic-Cvijin said.
Some bacteria can escape the gut — a phenomenon called bacterial translocation — and may aid in fighting tumors. To grow large enough to be seen on imaging, tumors need to evolve several abilities, such as growing enough vascularization to receive blood flow and shutting down local immune responses.
“Any added boost, like immunotherapy, has a chance of breaking through that immune forcefield and killing the tumor cells,” Dr. Vujkovic-Cvijin said. Escaped gut bacteria may provide that boost.
“There’s a lot of evidence that depletion of the gut microbiome impairs immunotherapy and chemotherapy. The thinking behind some of those studies is that gut microbes can cross the gut barrier and when they do, they activate the immune system,” he said.
In mice engineered to have sterile guts, for example, the lack of bacteria results in less effective immune systems, Dr. Vujkovic-Cvijin pointed out. A host of research has shown that antibiotic exposure during and even 6 months before immunotherapy dramatically reduces survival rates. “That’s pretty convincing to me that gut microbes are important,” he said.
Dr. Vujkovic-Cvijin cautioned that there continues to be controversy on understanding which bacteria are important for response to immunotherapy. “The field is still in its infancy in terms of understanding which bacteria are most important for these effects,” he said.
Dr. Knight suggested that escaped bacteria may be the genesis of the ones that he and other researchers believe exist in tumors. “Because tumor microbes must come from somewhere, it is to be expected that some of those microbes will be co-opted from body-site specific commensals.”
It’s also possible that metabolites released from gut bacteria escape the gut and could theoretically affect distant tumor growth, Dr. Gihawi said. The most promising avenue of research in this area is metabolites being used as biomarkers, added Dr. Gihawi, whose lab published research on a link between bacteria detected in men’s urine and a more aggressive subset of prostate cancers. But that research is not far enough along to develop lab tests for clinical use, he noted.
No Consensus Yet
Even before the controversy erupted around Dr. Knight’s research, he co-founded the company Micronoma to develop cancer tests based on his microbe findings. The company has raised $17.5 million from private investors as of August 2023 and received the US Food and Drug Administration’s Breakthrough Device designation, allowing the firm to fast-track clinical trials testing the technology. The recent critiques have not changed the company’s plans.
It’s safe to say that scientists will continue to research and debate the possibility of tumor microbiomes until a consensus emerges.
“The field is evolving and studies testing the reproducibility of tumor-resident microbial signals are essential for developing our understanding in this area,” Dr. Vujkovic-Cvijin said.
Even if that path ultimately leads nowhere, as Dr. Salzberg expects, research into microbiomes and cancer has plenty of other directions to go.
“I’m actually quite an optimist,” Dr. Gihawi said. “I think there’s a lot of scope for some really good research here, especially in the sites where we know there is a strong microbiome, such as the gastrointestinal tract.”
A version of this article appeared on Medscape.com.
Last year, the controversy heightened when experts questioned a high-profile study — a 2020 analysis claiming that the tumors of 33 different cancers had their own unique microbiomes — on whether the “signature” of these bacterial compositions could help diagnose cancer.
The incident renewed the spotlight on “tumor microbiomes” because of the bold claims of the original paper and the strongly worded refutations of those claims. The broader field has focused primarily on ways the body’s microbiome interacts with cancers and cancer treatment.
This controversy has highlighted the challenges of making headway in a field where researchers may not even have the tools yet to puzzle-out the wide-ranging implications the microbiome holds for cancer diagnosis and treatment.
But it is also part of a provocative question within that larger field: whether tumors in the body, far from the natural microbiome in the gut, have their own thriving communities of bacteria, viruses, and fungi. And, if they do, how do those tumor microbiomes affect the development and progression of the cancer and the effectiveness of cancer therapies?
Cancer Controversy
The evidence is undeniable that some microbes can directly cause certain cancers and that the human gut microbiome can influence the effectiveness of certain therapies. Beyond that established science, however, the research has raised as many questions as answers about what we do and don’t know about microbiota and cancer.
The only confirmed microbiomes are on the skin and in the gut, mouth, and vagina, which are all areas with an easy direct route for bacteria to enter and grow in or on the body. A series of papers in recent years have suggested that other internal organs, and tumors within them, may have their own microbiomes.
“Whether microbes exist in tumors of internal organs beyond body surfaces exposed to the environment is a different matter,” said Ivan Vujkovic-Cvijin, PhD, an assistant professor of biomedical sciences and gastroenterology at Cedars-Sinai Medical Center in Los Angeles, whose lab studies how human gut microbes affect inflammatory diseases. “We’ve only recently had the tools to study that question on a molecular level, and the reported results have been conflicting.”
For example, research allegedly identified microbiota in the human placenta nearly one decade ago. But subsequent research contradicted those claims and showed that the source of the “placental microbiome” was actually contamination. Subsequent similar studies for other parts of the body faced the same scrutiny and, often, eventual debunking.
“Most likely, our immune system has undergone selective pressure to eliminate everything that crosses the gut barrier because there’s not much benefit to the body to have bacteria run amok in our internal organs,” Dr. Vujkovic-Cvijin said. “That can only disrupt the functioning of our tissues, to have an external organism living inside them.”
The controversy that erupted last summer, surrounding research from the lab of Rob Knight, PhD, at the University of California, San Diego, centered on a slightly different but related question: Could tumors harbor their own microbiomes?
This news organization spoke with two of the authors who published a paper contesting Dr. Knight’s findings: Steven Salzberg, PhD, a professor of biomedical engineering at John Hopkins Medicine, Baltimore, Maryland, and Abraham Gihawi, PhD, a research fellow at Norwich Medical School at the University of East Anglia in the United Kingdom.
Dr. Salzberg described two major problems with Dr. Knight’s study.
“What they found were false positives because of contamination in the database and flaws in their methods,” Dr. Salzberg said. “I can’t prove there’s no cancer microbiome, but I can say the cancer microbiomes that they reported don’t exist because the species they were finding aren’t there.”
Dr. Knight disagrees with Dr. Salzberg’s findings, noting that Dr. Salzberg and his co-authors did not examine the publicly available databases used in his study. In a written response, he said that his team’s examination of the database revealed that less than 1% of the microbial genomes overlapped with human ones and that removing them did not change their findings.
Dr. Knight also noted that his team could still “distinguish cancer types by their microbiome” even after running their analysis without the technique that Dr. Salzberg found fault with.
Dr. Salzberg said that the database linked above is not the one Dr. Knight’s study used, however. “The primary database in their study was never made public (it’s too large, they said), and it has/had about 69,000 genomes,” Dr. Salzberg said by email. “But even if we did, this is irrelevant. He’s trying to distract from the primary errors in their study,” which Dr. Salzberg said Dr. Knight’s team has not addressed.
The critiques Dr. Salzberg raised have been leveled at other studies investigating microbiomes specifically within tumors and independent of the body’s microbiome.
For example, a 2019 study in Nature described a fungal microbiome in pancreatic cancer that a Nature paper 4 years later directly contradicted, citing flaws that invalidated the original findings. A different 2019 study in Cell examined pancreatic tumor microbiota and patient outcomes, but it’s unclear whether the microorganisms moved from the gut to the pancreas or “constitute a durably colonized community that lives inside the tumor,” which remains a matter of debate, Dr. Vujkovic-Cvijin said.
A 2020 study in Science suggested diverse microbial communities in seven tumor types, but those findings were similarly called into question. That study stated that “bacteria were first detected in human tumors more than 100 years ago” and that “bacteria are well-known residents in human tumors,” but Dr. Salzberg considers those statements misleading.
It’s true that bacteria and viruses have been detected in tumors because “there’s very good evidence that an acute infection caused by a very small number of viruses and bacteria can cause a tumor,” Dr. Salzberg said. Human papillomavirus, for example, can cause six different types of cancer. Inflammation and ulcers caused by Helicobacter pylori may progress to stomach cancer, and Fusobacterium nucleatum and Enterococcus faecalis have been shown to contribute to colorectal cancer. Those examples differ from a microbiome; this “a community of bacteria and possibly other microscopic bugs, like fungi, that are happily living in the tumor” the same way microbes reside in our guts, he said.
Dr. Knight said that many bacteria his team identified “have been confirmed independently in subsequent work.” He acknowledged, however, that more research is needed.
Several of the contested studies above were among a lengthy list that Dr. Knight provided, noting that most of the disagreements “have two sides to them, and critiques from one particular group does not immediately invalidate a reported finding.”
Yet, many of the papers Dr. Knight listed are precisely the types that skeptics like Dr. Salzberg believe are too flawed to draw reliable conclusions.
“I think many agree that microbes may exist within tumors that are exposed to the environment, like tumors of the skin, gut, and mouth,” Dr. Vujkovic-Cvijin said. It’s less clear, however, whether tumors further from the body’s microbiome harbor any microbes or where they came from if they do. Microbial signals in organs elsewhere in the body become faint quickly, he said.
Underdeveloped Technology
Though Dr. Salzberg said that the concept of a tumor microbiome is “implausible” because there’s no easy route for bacteria to reach internal organs, it’s unclear whether scientists have the technology yet to adequately answer this question.
For one thing, samples in these types of studies are typically “ultra-low biomass samples, where the signal — the amount of microbes in the sample — is so low that it’s comparable to how much would be expected to be found in reagents and environmental contamination through processing,” Dr. Vujkovic-Cvijin explained. Many polymerases used to amplify a DNA signal, for example, are made in bacteria and may retain trace amounts identified in these studies.
Dr. Knight agreed that low biomass is a challenge in this field but is not an unsurmountable one.
Another challenge is that study samples, as with Dr. Knight’s work, were collected during routine surgeries without the intent to find a microbial signal. Simply using a scalpel to cut through the skin means cutting through a layer of bacteria, and surgery rooms are not designed to eliminate all bacteria. Some work has even shown there is a “hospital microbiome,” so “you can easily have that creep into your signal and mistake it for tumor-resident bacteria,” Dr. Vujkovic-Cvijin said.
Dr. Knight asserted that the samples are taken under sterile conditions, but other researchers do not think the level of sterility necessary for completely clean samples is possible.
“Just because it’s in your sample doesn’t mean it was in your tumor,” Dr. Gihawi said.
Even if scientists can retrieve a reliable sample without contamination, analyzing it requires comparing the genetic material to existing databases of microbial genomes. Yet, contamination and misclassification of genetic sequences can be problems in those reference genomes too, Dr. Gihawi explained.
Machine learning algorithms have a role in interpreting data, but “we need to be careful of what we use them for,” he added.
“These techniques are in their infancy, and we’re starting to chase them down, which is why we need to move microbiome research in a way that can be used clinically,” Dr. Gihawi said.
Influence on Cancer Treatment Outcomes
Again, however, the question of whether microbiomes exist within tumors is only one slice of the much larger field looking at microbiomes and cancer, including its influence on cancer treatment outcomes. Although much remains to be learned, less controversy exists over the thousands of studies in the past two decades that have gradually revealed how the body’s microbiome can affect both the course of a cancer and the effectiveness of different treatments.
The growing research showing the importance of the gut microbiome in cancer treatments is not surprising given its role in immunity more broadly. Because the human immune system must recognize and defend against microbes, the microbiome helps train it, Dr. Vujkovic-Cvijin said.
Some bacteria can escape the gut — a phenomenon called bacterial translocation — and may aid in fighting tumors. To grow large enough to be seen on imaging, tumors need to evolve several abilities, such as growing enough vascularization to receive blood flow and shutting down local immune responses.
“Any added boost, like immunotherapy, has a chance of breaking through that immune forcefield and killing the tumor cells,” Dr. Vujkovic-Cvijin said. Escaped gut bacteria may provide that boost.
“There’s a lot of evidence that depletion of the gut microbiome impairs immunotherapy and chemotherapy. The thinking behind some of those studies is that gut microbes can cross the gut barrier and when they do, they activate the immune system,” he said.
In mice engineered to have sterile guts, for example, the lack of bacteria results in less effective immune systems, Dr. Vujkovic-Cvijin pointed out. A host of research has shown that antibiotic exposure during and even 6 months before immunotherapy dramatically reduces survival rates. “That’s pretty convincing to me that gut microbes are important,” he said.
Dr. Vujkovic-Cvijin cautioned that there continues to be controversy on understanding which bacteria are important for response to immunotherapy. “The field is still in its infancy in terms of understanding which bacteria are most important for these effects,” he said.
Dr. Knight suggested that escaped bacteria may be the genesis of the ones that he and other researchers believe exist in tumors. “Because tumor microbes must come from somewhere, it is to be expected that some of those microbes will be co-opted from body-site specific commensals.”
It’s also possible that metabolites released from gut bacteria escape the gut and could theoretically affect distant tumor growth, Dr. Gihawi said. The most promising avenue of research in this area is metabolites being used as biomarkers, added Dr. Gihawi, whose lab published research on a link between bacteria detected in men’s urine and a more aggressive subset of prostate cancers. But that research is not far enough along to develop lab tests for clinical use, he noted.
No Consensus Yet
Even before the controversy erupted around Dr. Knight’s research, he co-founded the company Micronoma to develop cancer tests based on his microbe findings. The company has raised $17.5 million from private investors as of August 2023 and received the US Food and Drug Administration’s Breakthrough Device designation, allowing the firm to fast-track clinical trials testing the technology. The recent critiques have not changed the company’s plans.
It’s safe to say that scientists will continue to research and debate the possibility of tumor microbiomes until a consensus emerges.
“The field is evolving and studies testing the reproducibility of tumor-resident microbial signals are essential for developing our understanding in this area,” Dr. Vujkovic-Cvijin said.
Even if that path ultimately leads nowhere, as Dr. Salzberg expects, research into microbiomes and cancer has plenty of other directions to go.
“I’m actually quite an optimist,” Dr. Gihawi said. “I think there’s a lot of scope for some really good research here, especially in the sites where we know there is a strong microbiome, such as the gastrointestinal tract.”
A version of this article appeared on Medscape.com.
Cervical Cancer Screening: US Clinicians Unclear About Best Practices
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines, proposing two major changes: start cervical cancer screening at age 25, rather than 21, and perform primary human papillomavirus (HPV) testing, instead of a Pap test.
First, healthcare providers in the US may be unsure how to reconcile conflicting cervical cancer screening guidelines from another major organization — the US Preventive Services Task Force (USPSTF), which published guidelines in 2018.
Although the ACS guidelines are based on an analysis of the latest evidence,
the recommendations challenge those from the USPSTF, which dictates insurance coverage in the US. Last year, the American College of Obstetricians and Gynecologists (ACOG) aligned its guidelines with those from the USPSTF.
The USPSTF recommends average-risk individuals start Pap, not HPV, testing at age 21, and broadens the options to primary HPV testing, Pap testing, or both together starting at age 30. The ACS, on the other hand, says primary HPV testing is the preferred screening approach from the start, which should be age 25.
Because the ACS guidelines marked a notable departure from prevailing practice, a team of researchers from five US universities decided to find out if anyone was following them.
The results, published in the journal Cancer in March, revealed that most healthcare providers had not changed practice.
Lead author Rebecca Perkins, MD, MSc, and colleagues found that, among the 70 respondents, few were starting screening at age 25, and none had switched to primary HPV testing.
The survey then probed clinicians’ willingness to adopt the ACS guidelines as well as their reservations and barriers to doing so.
Notably, more than half of the survey participants said they would be willing to adopt the ACS guidelines if the best evidence supported the changes and other professional medical organizations endorsed them.
On the age change, participants highlighted a range of benefits to moving to a later screening age, including that earlier screening may not be valuable and delaying screening could reduce overtreatment.
One participant noted: “We know that cervical cancer is usually a slow‐growing, long‐term progressive disease that does not typically show up that early in life, and we also know that, if infected, oftentimes their immune system can fight off the virus. So, it sounds reasonable at first glance [to delay screening to age 25 years].”
Others, however, brought up barriers to initiating screening at age 25. Some mentioned that later screening may not work for high‐risk populations and others voiced concerns about missing high‐grade precancer or cancer. “It’s not unusual for us to see women in their early 20s that have already had 10 or 15 partners. … a lot of them smoke too … they just have a lot of bad habits that put them at more risk,” one respondent noted.
On the HPV vs Pap testing front, many participants described a growing confidence in HPV tests after trying co-testing. One participant said, “Honestly, I do look more at the HPV results than the cytology. I put more faith in knowing what their HPV status is than anything.”
The main barriers to primary HPV testing, however, included lack of autonomy when working in a large health system, concerns about the efficacy of HPV testing, and a belief that cytology was valuable.
Some clinicians were worried about missing high-grade lesions or cancer. One healthcare provider said, “My only concern with primary HPV screening is occasionally you will pick up endometrial abnormalities on a Pap that you’re not going to pick up with HPV screening.”
Logistics and finances also played a role in clinicians’ hesitancy to switch to the ACS recommendation. Labs that could handle primary HPV tests were not available to some participants, and lack of insurance coverage was a barrier for others. One respondent noted, for instance, that his institution has a “cytology infrastructure that already exists in the lab and I can’t really see them switching.”
Many survey respondents also said they were waiting for endorsement from organizations, such as ACOG and USPSTF. “We run by the USPSTF and … ACOG. We don’t run by the ACS guidelines,” one person said.
Finally, some participants were not aware of the ACS recommendations at all or the data behind them but said they would be willing to change to primary HPV testing in the future.
Overall, Dr. Perkins said she was happy to see that more than half of the respondents would be willing to shift to the ACS screening guidelines, but noted that many remain reluctant to do so until the USPSTF and ACOG change their guidelines.
“It’s really just a matter of the USPSTF and ACOG endorsing” the ACS guidelines, said Dr. Perkins, professor of obstetrics and gynecology at Boston University.
The USPSTF is currently updating its cervical screening guidelines, which could potentially help reconcile this discord between the guidelines and close the gaps in practice patterns.
The USPSTF’s review of the evidence, which led to the 2018 guidelines, did highlight the effectiveness of HPV testing. The review authors concluded that “the evidence was consistent across trials” that primary, high-risk HPV screening increased detection of grade 3 or worse cervical intraepithelial neoplasia in the initial round of screening “by as much as 2 to 3 times when compared with cytology.”
However, Joy Melnikow, MD, MPH, first author on the USPSTF evidence review, explained that the reviewers factored in access to HPV testing when making their final recommendations.
“The consideration was making sure that a recommendation could be inclusive of all providers and all populations and not restricting access for clinics that couldn’t afford or didn’t have the machine to do [HPV testing],” Dr. Melnikow, director of the Center for Healthcare Policy and Research and professor of family and community medicine at the University of California Davis, told this news organization.
The ACS, however, did not consider potential access problems in its analysis of the evidence.
Although the ACS evidence is “excellent,” Dr. Perkins said, “it’s really just a matter of the USPSTF and ACOG endorsing that, and then it seems like a lot of people are willing to make the change.”
Dr. Perkins reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines, proposing two major changes: start cervical cancer screening at age 25, rather than 21, and perform primary human papillomavirus (HPV) testing, instead of a Pap test.
First, healthcare providers in the US may be unsure how to reconcile conflicting cervical cancer screening guidelines from another major organization — the US Preventive Services Task Force (USPSTF), which published guidelines in 2018.
Although the ACS guidelines are based on an analysis of the latest evidence,
the recommendations challenge those from the USPSTF, which dictates insurance coverage in the US. Last year, the American College of Obstetricians and Gynecologists (ACOG) aligned its guidelines with those from the USPSTF.
The USPSTF recommends average-risk individuals start Pap, not HPV, testing at age 21, and broadens the options to primary HPV testing, Pap testing, or both together starting at age 30. The ACS, on the other hand, says primary HPV testing is the preferred screening approach from the start, which should be age 25.
Because the ACS guidelines marked a notable departure from prevailing practice, a team of researchers from five US universities decided to find out if anyone was following them.
The results, published in the journal Cancer in March, revealed that most healthcare providers had not changed practice.
Lead author Rebecca Perkins, MD, MSc, and colleagues found that, among the 70 respondents, few were starting screening at age 25, and none had switched to primary HPV testing.
The survey then probed clinicians’ willingness to adopt the ACS guidelines as well as their reservations and barriers to doing so.
Notably, more than half of the survey participants said they would be willing to adopt the ACS guidelines if the best evidence supported the changes and other professional medical organizations endorsed them.
On the age change, participants highlighted a range of benefits to moving to a later screening age, including that earlier screening may not be valuable and delaying screening could reduce overtreatment.
One participant noted: “We know that cervical cancer is usually a slow‐growing, long‐term progressive disease that does not typically show up that early in life, and we also know that, if infected, oftentimes their immune system can fight off the virus. So, it sounds reasonable at first glance [to delay screening to age 25 years].”
Others, however, brought up barriers to initiating screening at age 25. Some mentioned that later screening may not work for high‐risk populations and others voiced concerns about missing high‐grade precancer or cancer. “It’s not unusual for us to see women in their early 20s that have already had 10 or 15 partners. … a lot of them smoke too … they just have a lot of bad habits that put them at more risk,” one respondent noted.
On the HPV vs Pap testing front, many participants described a growing confidence in HPV tests after trying co-testing. One participant said, “Honestly, I do look more at the HPV results than the cytology. I put more faith in knowing what their HPV status is than anything.”
The main barriers to primary HPV testing, however, included lack of autonomy when working in a large health system, concerns about the efficacy of HPV testing, and a belief that cytology was valuable.
Some clinicians were worried about missing high-grade lesions or cancer. One healthcare provider said, “My only concern with primary HPV screening is occasionally you will pick up endometrial abnormalities on a Pap that you’re not going to pick up with HPV screening.”
Logistics and finances also played a role in clinicians’ hesitancy to switch to the ACS recommendation. Labs that could handle primary HPV tests were not available to some participants, and lack of insurance coverage was a barrier for others. One respondent noted, for instance, that his institution has a “cytology infrastructure that already exists in the lab and I can’t really see them switching.”
Many survey respondents also said they were waiting for endorsement from organizations, such as ACOG and USPSTF. “We run by the USPSTF and … ACOG. We don’t run by the ACS guidelines,” one person said.
Finally, some participants were not aware of the ACS recommendations at all or the data behind them but said they would be willing to change to primary HPV testing in the future.
Overall, Dr. Perkins said she was happy to see that more than half of the respondents would be willing to shift to the ACS screening guidelines, but noted that many remain reluctant to do so until the USPSTF and ACOG change their guidelines.
“It’s really just a matter of the USPSTF and ACOG endorsing” the ACS guidelines, said Dr. Perkins, professor of obstetrics and gynecology at Boston University.
The USPSTF is currently updating its cervical screening guidelines, which could potentially help reconcile this discord between the guidelines and close the gaps in practice patterns.
The USPSTF’s review of the evidence, which led to the 2018 guidelines, did highlight the effectiveness of HPV testing. The review authors concluded that “the evidence was consistent across trials” that primary, high-risk HPV screening increased detection of grade 3 or worse cervical intraepithelial neoplasia in the initial round of screening “by as much as 2 to 3 times when compared with cytology.”
However, Joy Melnikow, MD, MPH, first author on the USPSTF evidence review, explained that the reviewers factored in access to HPV testing when making their final recommendations.
“The consideration was making sure that a recommendation could be inclusive of all providers and all populations and not restricting access for clinics that couldn’t afford or didn’t have the machine to do [HPV testing],” Dr. Melnikow, director of the Center for Healthcare Policy and Research and professor of family and community medicine at the University of California Davis, told this news organization.
The ACS, however, did not consider potential access problems in its analysis of the evidence.
Although the ACS evidence is “excellent,” Dr. Perkins said, “it’s really just a matter of the USPSTF and ACOG endorsing that, and then it seems like a lot of people are willing to make the change.”
Dr. Perkins reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines, proposing two major changes: start cervical cancer screening at age 25, rather than 21, and perform primary human papillomavirus (HPV) testing, instead of a Pap test.
First, healthcare providers in the US may be unsure how to reconcile conflicting cervical cancer screening guidelines from another major organization — the US Preventive Services Task Force (USPSTF), which published guidelines in 2018.
Although the ACS guidelines are based on an analysis of the latest evidence,
the recommendations challenge those from the USPSTF, which dictates insurance coverage in the US. Last year, the American College of Obstetricians and Gynecologists (ACOG) aligned its guidelines with those from the USPSTF.
The USPSTF recommends average-risk individuals start Pap, not HPV, testing at age 21, and broadens the options to primary HPV testing, Pap testing, or both together starting at age 30. The ACS, on the other hand, says primary HPV testing is the preferred screening approach from the start, which should be age 25.
Because the ACS guidelines marked a notable departure from prevailing practice, a team of researchers from five US universities decided to find out if anyone was following them.
The results, published in the journal Cancer in March, revealed that most healthcare providers had not changed practice.
Lead author Rebecca Perkins, MD, MSc, and colleagues found that, among the 70 respondents, few were starting screening at age 25, and none had switched to primary HPV testing.
The survey then probed clinicians’ willingness to adopt the ACS guidelines as well as their reservations and barriers to doing so.
Notably, more than half of the survey participants said they would be willing to adopt the ACS guidelines if the best evidence supported the changes and other professional medical organizations endorsed them.
On the age change, participants highlighted a range of benefits to moving to a later screening age, including that earlier screening may not be valuable and delaying screening could reduce overtreatment.
One participant noted: “We know that cervical cancer is usually a slow‐growing, long‐term progressive disease that does not typically show up that early in life, and we also know that, if infected, oftentimes their immune system can fight off the virus. So, it sounds reasonable at first glance [to delay screening to age 25 years].”
Others, however, brought up barriers to initiating screening at age 25. Some mentioned that later screening may not work for high‐risk populations and others voiced concerns about missing high‐grade precancer or cancer. “It’s not unusual for us to see women in their early 20s that have already had 10 or 15 partners. … a lot of them smoke too … they just have a lot of bad habits that put them at more risk,” one respondent noted.
On the HPV vs Pap testing front, many participants described a growing confidence in HPV tests after trying co-testing. One participant said, “Honestly, I do look more at the HPV results than the cytology. I put more faith in knowing what their HPV status is than anything.”
The main barriers to primary HPV testing, however, included lack of autonomy when working in a large health system, concerns about the efficacy of HPV testing, and a belief that cytology was valuable.
Some clinicians were worried about missing high-grade lesions or cancer. One healthcare provider said, “My only concern with primary HPV screening is occasionally you will pick up endometrial abnormalities on a Pap that you’re not going to pick up with HPV screening.”
Logistics and finances also played a role in clinicians’ hesitancy to switch to the ACS recommendation. Labs that could handle primary HPV tests were not available to some participants, and lack of insurance coverage was a barrier for others. One respondent noted, for instance, that his institution has a “cytology infrastructure that already exists in the lab and I can’t really see them switching.”
Many survey respondents also said they were waiting for endorsement from organizations, such as ACOG and USPSTF. “We run by the USPSTF and … ACOG. We don’t run by the ACS guidelines,” one person said.
Finally, some participants were not aware of the ACS recommendations at all or the data behind them but said they would be willing to change to primary HPV testing in the future.
Overall, Dr. Perkins said she was happy to see that more than half of the respondents would be willing to shift to the ACS screening guidelines, but noted that many remain reluctant to do so until the USPSTF and ACOG change their guidelines.
“It’s really just a matter of the USPSTF and ACOG endorsing” the ACS guidelines, said Dr. Perkins, professor of obstetrics and gynecology at Boston University.
The USPSTF is currently updating its cervical screening guidelines, which could potentially help reconcile this discord between the guidelines and close the gaps in practice patterns.
The USPSTF’s review of the evidence, which led to the 2018 guidelines, did highlight the effectiveness of HPV testing. The review authors concluded that “the evidence was consistent across trials” that primary, high-risk HPV screening increased detection of grade 3 or worse cervical intraepithelial neoplasia in the initial round of screening “by as much as 2 to 3 times when compared with cytology.”
However, Joy Melnikow, MD, MPH, first author on the USPSTF evidence review, explained that the reviewers factored in access to HPV testing when making their final recommendations.
“The consideration was making sure that a recommendation could be inclusive of all providers and all populations and not restricting access for clinics that couldn’t afford or didn’t have the machine to do [HPV testing],” Dr. Melnikow, director of the Center for Healthcare Policy and Research and professor of family and community medicine at the University of California Davis, told this news organization.
The ACS, however, did not consider potential access problems in its analysis of the evidence.
Although the ACS evidence is “excellent,” Dr. Perkins said, “it’s really just a matter of the USPSTF and ACOG endorsing that, and then it seems like a lot of people are willing to make the change.”
Dr. Perkins reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
Potential Cure for Early BRCA-Mutated Breast Cancer?
SAN DIEGO —
In a small trial, 39 patients randomized to the regimen — a combination of standard chemotherapy with the poly(ADP-ribose)polymerase (PARP) inhibitor olaparib — were alive at 3 years vs 39 of 45 (87%) randomized to chemotherapy alone.
“A remarkable 100% of patients were still alive at 36 months, which is a significant landmark for these patients,” said chief investigator Jean Abraham, PhD, a breast oncologist at the University of Cambridge, England, who presented the findings at the American Association for Cancer Research annual meeting.
It’s a “small but very powerful signal” of “what could be a potentially curative regimen that definitely does need to be confirmed in a larger study,” Dr. Abraham added.
The study, a part of the PARTNER trial, included 84 patients with T1-2 tumors of any hormone status. Just over 70% in both arms had BRCA 1 mutations, and the rest had BRCA 2 mutations.
Past attempts at combining chemotherapy with PARP inhibitors have been hampered by excess bone marrow toxicity. To counter the problem, patients randomized to the combination therapy received olaparib 48 hours after carboplatin to give their bone marrow a chance to recover.
The median age was 38 years in the control group and 47 years in the olaparib arm. A greater proportion of patients in the control arm (42% vs 23%) had axillary node involvement.
Overall, patients received neoadjuvant carboplatin on day 1 and paclitaxel on days 1, 8, and 15 every 3 weeks for four cycles, followed by anthracycline every 3 weeks for three cycles. In the study arm, olaparib 150 mg was administered twice daily starting on day 3 continuing to day 14 during the first four cycles. Almost 90% of patients received at least 80% of their planned olaparib dose.
Despite the delay in olaparib dosing, 56.4% of patients in the combination arm required a transfusion vs 48.9% with chemotherapy alone.
At a median follow-up of 40.7 months, 96% of patients in the combination arm demonstrated event-free survival, with one patient relapsing, vs 80% in the chemotherapy-alone group, with nine patients relapsing.
In the final analysis, 64% of patients who received olaparib had a pathological complete response compared with almost 70% in the chemotherapy group, though the difference was not statistically significant.
The trial was stopped short at 50% enrollment after the data monitoring safety committee determined that olaparib add-on was unlikely to improve pathological complete response rates, the trial’s primary endpoint.
However, pathological complete response rates did not appear to affect overall survival.
“It didn’t seem to matter whether you had a non-pathological complete response, you still survived 100%” with the combination, Dr. Abraham said, adding that this is not the first study to show a disconnect between response rates and survival.
Perhaps, this disconnect could be due to “doomed cells” that look like residual disease but are, in fact, dying and unable to metastasize, she said.
No patients in the combination arm and two in the control arm received olaparib, immunotherapy, or capecitabine after surgery. Both control participants relapsed, and one died.
Toxicity was more severe for patients in the combination arm. More patients who received olaparib (76.9%) experienced a grade 3 or worse adverse event vs 60% of patients in the control arm.
Study discussant Hope S. Rugo, MD, a breast oncologist at the University of California San Francisco, highlighted a few limitations and remaining questions.
First, “this is a very small population, so small differences in the biology of the tumor, the patients, and even stage that we can’t assess in the neoadjuvant setting could make a difference that would affect event-free and overall survival,” she said.
Second, two patients with pathological complete responses relapsed in the control arm and died, “which is quite unusual,” Dr. Rugo said. “Patients who achieve a pathological complete response generally have an excellent outcome.”
Dr. Rugo noted that “gap sequencing doesn’t appear to avoid the toxicity of PARP inhibitors.”
However, she said, “the efficacy results are intriguing” and would need confirmation in a larger randomized trial, perhaps with newer, more selective PARP inhibitors.
The work was funded by AstraZeneca, maker of olaparib. Researchers included AstraZeneca employees. Dr. Abraham is an adviser to and disclosed grants, travel costs, and honoraria from the company. Dr. Rugo disclosed research funding from AstraZeneca and other companies.
A version of this article appeared on Medscape.com.
SAN DIEGO —
In a small trial, 39 patients randomized to the regimen — a combination of standard chemotherapy with the poly(ADP-ribose)polymerase (PARP) inhibitor olaparib — were alive at 3 years vs 39 of 45 (87%) randomized to chemotherapy alone.
“A remarkable 100% of patients were still alive at 36 months, which is a significant landmark for these patients,” said chief investigator Jean Abraham, PhD, a breast oncologist at the University of Cambridge, England, who presented the findings at the American Association for Cancer Research annual meeting.
It’s a “small but very powerful signal” of “what could be a potentially curative regimen that definitely does need to be confirmed in a larger study,” Dr. Abraham added.
The study, a part of the PARTNER trial, included 84 patients with T1-2 tumors of any hormone status. Just over 70% in both arms had BRCA 1 mutations, and the rest had BRCA 2 mutations.
Past attempts at combining chemotherapy with PARP inhibitors have been hampered by excess bone marrow toxicity. To counter the problem, patients randomized to the combination therapy received olaparib 48 hours after carboplatin to give their bone marrow a chance to recover.
The median age was 38 years in the control group and 47 years in the olaparib arm. A greater proportion of patients in the control arm (42% vs 23%) had axillary node involvement.
Overall, patients received neoadjuvant carboplatin on day 1 and paclitaxel on days 1, 8, and 15 every 3 weeks for four cycles, followed by anthracycline every 3 weeks for three cycles. In the study arm, olaparib 150 mg was administered twice daily starting on day 3 continuing to day 14 during the first four cycles. Almost 90% of patients received at least 80% of their planned olaparib dose.
Despite the delay in olaparib dosing, 56.4% of patients in the combination arm required a transfusion vs 48.9% with chemotherapy alone.
At a median follow-up of 40.7 months, 96% of patients in the combination arm demonstrated event-free survival, with one patient relapsing, vs 80% in the chemotherapy-alone group, with nine patients relapsing.
In the final analysis, 64% of patients who received olaparib had a pathological complete response compared with almost 70% in the chemotherapy group, though the difference was not statistically significant.
The trial was stopped short at 50% enrollment after the data monitoring safety committee determined that olaparib add-on was unlikely to improve pathological complete response rates, the trial’s primary endpoint.
However, pathological complete response rates did not appear to affect overall survival.
“It didn’t seem to matter whether you had a non-pathological complete response, you still survived 100%” with the combination, Dr. Abraham said, adding that this is not the first study to show a disconnect between response rates and survival.
Perhaps, this disconnect could be due to “doomed cells” that look like residual disease but are, in fact, dying and unable to metastasize, she said.
No patients in the combination arm and two in the control arm received olaparib, immunotherapy, or capecitabine after surgery. Both control participants relapsed, and one died.
Toxicity was more severe for patients in the combination arm. More patients who received olaparib (76.9%) experienced a grade 3 or worse adverse event vs 60% of patients in the control arm.
Study discussant Hope S. Rugo, MD, a breast oncologist at the University of California San Francisco, highlighted a few limitations and remaining questions.
First, “this is a very small population, so small differences in the biology of the tumor, the patients, and even stage that we can’t assess in the neoadjuvant setting could make a difference that would affect event-free and overall survival,” she said.
Second, two patients with pathological complete responses relapsed in the control arm and died, “which is quite unusual,” Dr. Rugo said. “Patients who achieve a pathological complete response generally have an excellent outcome.”
Dr. Rugo noted that “gap sequencing doesn’t appear to avoid the toxicity of PARP inhibitors.”
However, she said, “the efficacy results are intriguing” and would need confirmation in a larger randomized trial, perhaps with newer, more selective PARP inhibitors.
The work was funded by AstraZeneca, maker of olaparib. Researchers included AstraZeneca employees. Dr. Abraham is an adviser to and disclosed grants, travel costs, and honoraria from the company. Dr. Rugo disclosed research funding from AstraZeneca and other companies.
A version of this article appeared on Medscape.com.
SAN DIEGO —
In a small trial, 39 patients randomized to the regimen — a combination of standard chemotherapy with the poly(ADP-ribose)polymerase (PARP) inhibitor olaparib — were alive at 3 years vs 39 of 45 (87%) randomized to chemotherapy alone.
“A remarkable 100% of patients were still alive at 36 months, which is a significant landmark for these patients,” said chief investigator Jean Abraham, PhD, a breast oncologist at the University of Cambridge, England, who presented the findings at the American Association for Cancer Research annual meeting.
It’s a “small but very powerful signal” of “what could be a potentially curative regimen that definitely does need to be confirmed in a larger study,” Dr. Abraham added.
The study, a part of the PARTNER trial, included 84 patients with T1-2 tumors of any hormone status. Just over 70% in both arms had BRCA 1 mutations, and the rest had BRCA 2 mutations.
Past attempts at combining chemotherapy with PARP inhibitors have been hampered by excess bone marrow toxicity. To counter the problem, patients randomized to the combination therapy received olaparib 48 hours after carboplatin to give their bone marrow a chance to recover.
The median age was 38 years in the control group and 47 years in the olaparib arm. A greater proportion of patients in the control arm (42% vs 23%) had axillary node involvement.
Overall, patients received neoadjuvant carboplatin on day 1 and paclitaxel on days 1, 8, and 15 every 3 weeks for four cycles, followed by anthracycline every 3 weeks for three cycles. In the study arm, olaparib 150 mg was administered twice daily starting on day 3 continuing to day 14 during the first four cycles. Almost 90% of patients received at least 80% of their planned olaparib dose.
Despite the delay in olaparib dosing, 56.4% of patients in the combination arm required a transfusion vs 48.9% with chemotherapy alone.
At a median follow-up of 40.7 months, 96% of patients in the combination arm demonstrated event-free survival, with one patient relapsing, vs 80% in the chemotherapy-alone group, with nine patients relapsing.
In the final analysis, 64% of patients who received olaparib had a pathological complete response compared with almost 70% in the chemotherapy group, though the difference was not statistically significant.
The trial was stopped short at 50% enrollment after the data monitoring safety committee determined that olaparib add-on was unlikely to improve pathological complete response rates, the trial’s primary endpoint.
However, pathological complete response rates did not appear to affect overall survival.
“It didn’t seem to matter whether you had a non-pathological complete response, you still survived 100%” with the combination, Dr. Abraham said, adding that this is not the first study to show a disconnect between response rates and survival.
Perhaps, this disconnect could be due to “doomed cells” that look like residual disease but are, in fact, dying and unable to metastasize, she said.
No patients in the combination arm and two in the control arm received olaparib, immunotherapy, or capecitabine after surgery. Both control participants relapsed, and one died.
Toxicity was more severe for patients in the combination arm. More patients who received olaparib (76.9%) experienced a grade 3 or worse adverse event vs 60% of patients in the control arm.
Study discussant Hope S. Rugo, MD, a breast oncologist at the University of California San Francisco, highlighted a few limitations and remaining questions.
First, “this is a very small population, so small differences in the biology of the tumor, the patients, and even stage that we can’t assess in the neoadjuvant setting could make a difference that would affect event-free and overall survival,” she said.
Second, two patients with pathological complete responses relapsed in the control arm and died, “which is quite unusual,” Dr. Rugo said. “Patients who achieve a pathological complete response generally have an excellent outcome.”
Dr. Rugo noted that “gap sequencing doesn’t appear to avoid the toxicity of PARP inhibitors.”
However, she said, “the efficacy results are intriguing” and would need confirmation in a larger randomized trial, perhaps with newer, more selective PARP inhibitors.
The work was funded by AstraZeneca, maker of olaparib. Researchers included AstraZeneca employees. Dr. Abraham is an adviser to and disclosed grants, travel costs, and honoraria from the company. Dr. Rugo disclosed research funding from AstraZeneca and other companies.
A version of this article appeared on Medscape.com.
FROM AACR 2024


