User login
MicroRNAs May Predict Pancreatic Cancer Risk Years Before Diagnosis
TOPLINE:
METHODOLOGY:
- Early detection of pancreatic cancer could improve patient prognosis, but clinically viable biomarkers are lacking. In a two-stage study, researchers screened and validated circulating miRNAs as biomarkers for early detection using prediagnostic plasma samples from 462 case-control pairs across multiple cohorts.
- The discovery stage included 185 pairs from the PLCO Cancer Screening Trial, and the replication stage included 277 pairs from Shanghai Women’s/Men’s Health Study, Southern Community Cohort Study, and Multiethnic Cohort Study.
- Overall, 798 plasma microRNAs were measured using the NanoString nCounter Analysis System, and odds ratios (ORs) for pancreatic cancer risk were calculated on the basis of miRNA concentrations.
- Statistical analysis involved conditional logistic regression, stratified by age and time from sample collection to diagnosis.
TAKEAWAY:
- In the discovery stage, the researchers identified 120 miRNAs significantly associated with pancreatic cancer risk.
- Three of these miRNAs showed consistent significant associations in the replication stage. Specifically, hsa-miR-199a-3p/hsa-miR-199b-3p and hsa-miR-191-5p were associated with a 10%-11% lower risk for pancreatic cancer (OR, 0.89 and 0.90, respectively), and hsa-miR-767-5p was associated with an 8% higher risk for pancreatic cancer (OR, 1.08) within 5 years of the blood draw.
- In age-stratified analyses, hsa-miR-767-5p (OR, 1.23) along with four other miRNAs — hsa-miR-640 (OR, 1.33), hsa-miR-874-5p (OR, 1.25), hsa-miR-1299 (OR, 1.28), and hsa-miR-449b-5p (OR, 1.22) — were associated with an increased risk for pancreatic cancer among patients diagnosed at age 65 or older.
- One miRNA, hsa-miR-22-3p (OR, 0.76), was associated with a lower risk for pancreatic cancer in this older age group.
IN PRACTICE:
The findings provide evidence that miRNAs “have a potential utilization in clinical practice” to help “identify high-risk individuals who could subsequently undergo a more definitive but invasive diagnostic procedure,” the authors said. “Such a multistep strategy for pancreatic cancer screening and early detection, likely cost-efficient and low-risk, could be critical to improve survival.”
SOURCE:
The study, with first author Cong Wang, Vanderbilt University Medical Center, Nashville, Tennessee, was published online in the International Journal of Cancer.
LIMITATIONS:
The researchers lacked miRNA profiles of patients with pancreatic cancer at diagnosis and were not able to track the miRNA changes among pancreatic cancer cases at the time of clinical diagnosis. Sample collection protocols differed across study cohorts, and the researchers changed assay panels during the study.
DISCLOSURES:
The research was funded by grants from the National Cancer Institute. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Early detection of pancreatic cancer could improve patient prognosis, but clinically viable biomarkers are lacking. In a two-stage study, researchers screened and validated circulating miRNAs as biomarkers for early detection using prediagnostic plasma samples from 462 case-control pairs across multiple cohorts.
- The discovery stage included 185 pairs from the PLCO Cancer Screening Trial, and the replication stage included 277 pairs from Shanghai Women’s/Men’s Health Study, Southern Community Cohort Study, and Multiethnic Cohort Study.
- Overall, 798 plasma microRNAs were measured using the NanoString nCounter Analysis System, and odds ratios (ORs) for pancreatic cancer risk were calculated on the basis of miRNA concentrations.
- Statistical analysis involved conditional logistic regression, stratified by age and time from sample collection to diagnosis.
TAKEAWAY:
- In the discovery stage, the researchers identified 120 miRNAs significantly associated with pancreatic cancer risk.
- Three of these miRNAs showed consistent significant associations in the replication stage. Specifically, hsa-miR-199a-3p/hsa-miR-199b-3p and hsa-miR-191-5p were associated with a 10%-11% lower risk for pancreatic cancer (OR, 0.89 and 0.90, respectively), and hsa-miR-767-5p was associated with an 8% higher risk for pancreatic cancer (OR, 1.08) within 5 years of the blood draw.
- In age-stratified analyses, hsa-miR-767-5p (OR, 1.23) along with four other miRNAs — hsa-miR-640 (OR, 1.33), hsa-miR-874-5p (OR, 1.25), hsa-miR-1299 (OR, 1.28), and hsa-miR-449b-5p (OR, 1.22) — were associated with an increased risk for pancreatic cancer among patients diagnosed at age 65 or older.
- One miRNA, hsa-miR-22-3p (OR, 0.76), was associated with a lower risk for pancreatic cancer in this older age group.
IN PRACTICE:
The findings provide evidence that miRNAs “have a potential utilization in clinical practice” to help “identify high-risk individuals who could subsequently undergo a more definitive but invasive diagnostic procedure,” the authors said. “Such a multistep strategy for pancreatic cancer screening and early detection, likely cost-efficient and low-risk, could be critical to improve survival.”
SOURCE:
The study, with first author Cong Wang, Vanderbilt University Medical Center, Nashville, Tennessee, was published online in the International Journal of Cancer.
LIMITATIONS:
The researchers lacked miRNA profiles of patients with pancreatic cancer at diagnosis and were not able to track the miRNA changes among pancreatic cancer cases at the time of clinical diagnosis. Sample collection protocols differed across study cohorts, and the researchers changed assay panels during the study.
DISCLOSURES:
The research was funded by grants from the National Cancer Institute. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Early detection of pancreatic cancer could improve patient prognosis, but clinically viable biomarkers are lacking. In a two-stage study, researchers screened and validated circulating miRNAs as biomarkers for early detection using prediagnostic plasma samples from 462 case-control pairs across multiple cohorts.
- The discovery stage included 185 pairs from the PLCO Cancer Screening Trial, and the replication stage included 277 pairs from Shanghai Women’s/Men’s Health Study, Southern Community Cohort Study, and Multiethnic Cohort Study.
- Overall, 798 plasma microRNAs were measured using the NanoString nCounter Analysis System, and odds ratios (ORs) for pancreatic cancer risk were calculated on the basis of miRNA concentrations.
- Statistical analysis involved conditional logistic regression, stratified by age and time from sample collection to diagnosis.
TAKEAWAY:
- In the discovery stage, the researchers identified 120 miRNAs significantly associated with pancreatic cancer risk.
- Three of these miRNAs showed consistent significant associations in the replication stage. Specifically, hsa-miR-199a-3p/hsa-miR-199b-3p and hsa-miR-191-5p were associated with a 10%-11% lower risk for pancreatic cancer (OR, 0.89 and 0.90, respectively), and hsa-miR-767-5p was associated with an 8% higher risk for pancreatic cancer (OR, 1.08) within 5 years of the blood draw.
- In age-stratified analyses, hsa-miR-767-5p (OR, 1.23) along with four other miRNAs — hsa-miR-640 (OR, 1.33), hsa-miR-874-5p (OR, 1.25), hsa-miR-1299 (OR, 1.28), and hsa-miR-449b-5p (OR, 1.22) — were associated with an increased risk for pancreatic cancer among patients diagnosed at age 65 or older.
- One miRNA, hsa-miR-22-3p (OR, 0.76), was associated with a lower risk for pancreatic cancer in this older age group.
IN PRACTICE:
The findings provide evidence that miRNAs “have a potential utilization in clinical practice” to help “identify high-risk individuals who could subsequently undergo a more definitive but invasive diagnostic procedure,” the authors said. “Such a multistep strategy for pancreatic cancer screening and early detection, likely cost-efficient and low-risk, could be critical to improve survival.”
SOURCE:
The study, with first author Cong Wang, Vanderbilt University Medical Center, Nashville, Tennessee, was published online in the International Journal of Cancer.
LIMITATIONS:
The researchers lacked miRNA profiles of patients with pancreatic cancer at diagnosis and were not able to track the miRNA changes among pancreatic cancer cases at the time of clinical diagnosis. Sample collection protocols differed across study cohorts, and the researchers changed assay panels during the study.
DISCLOSURES:
The research was funded by grants from the National Cancer Institute. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
‘Bread and Butter’: Societies Issue T2D Management Guidance
Two professional societies have issued new guidance for type 2 diabetes management in primary care, with one focused specifically on the use of the newer medications.
On April 19, 2024, the American College of Physicians (ACP) published Newer Pharmacologic Treatments in Adults With Type 2 Diabetes: A Clinical Guideline From the American College of Physicians. The internal medicine group recommends the use of glucagon-like peptide-1 (GLP-1) agonists, and sodium–glucose cotransporter-2 (SGLT2) inhibitors as second-line treatment after metformin. They also advise against the use of dipeptidyl peptidase-4 (DPP-4) inhibitors.
The document was also presented simultaneously at the ACP annual meeting.
And on April 15, the American Diabetes Association (ADA) posted its comprehensive Standards of Care in Diabetes—2024 Abridged for Primary Care Professionals as a follow-up to the December 2023 publication of its full-length Standards. Section 9, Pharmacologic Approaches to Glycemic Treatment, covers the same ground as the ACP guidelines.
General Agreement but Some Differences
The recommendations generally agree regarding medication use, although there are some differences. Both societies continue to endorse metformin and lifestyle modification as first-line therapy for glycemic management in type 2 diabetes. However, while ADA also gives the option of initial combination therapy with prioritization of avoiding hypoglycemia, ACP advises adding new medications only if glycemic goals aren’t met with lifestyle and metformin alone.
The new ACP document gives two general recommendations:
1. Add an SGLT2 inhibitor or a GLP-1 agonist to metformin and lifestyle modifications in adults with type 2 diabetes and inadequate glycemic control.
*Use an SGLT2 inhibitor to reduce the risk for all-cause mortality, major adverse cardiovascular events, progression of chronic kidney disease, and hospitalization due to congestive heart failure.
*Use a GLP-1 agonist to reduce the risk for all-cause mortality, major adverse cardiovascular events, and stroke.
2. ACP recommends against adding a DPP-4 inhibitor to metformin and lifestyle modifications in adults with type 2 diabetes and inadequate glycemic control to reduce morbidity and all-cause mortality.
Both ADA and ACP advise using SGLT2 inhibitors in patients with congestive heart failure and/or chronic kidney disease, and using GLP-1 agonists in patients for whom weight management is a priority. The ADA also advises using agents of either drug class with proven cardiovascular benefit for people with type 2 diabetes who have established cardiovascular disease or who are at high risk.
ADA doesn’t advise against the use of DPP-4 inhibitors but doesn’t prioritize them either. Both insulin and sulfonylureas remain options for both, but they also are lower priority due to their potential for causing hypoglycemia. ACP says that sulfonylureas and long-acting insulin are “inferior to SGLT2 inhibitors and GLP-1 agonists in reducing all-cause mortality and morbidity but may still have some limited value for glycemic control.”
The two groups continue to differ regarding A1c goals, although both recommend individualization. The ACP generally advises levels between 7% and 8% for most adults with type 2 diabetes, and de-intensification of pharmacologic agents for those with A1c levels below 6.5%. On the other hand, ADA recommends A1c levels < 7% as long as that can be achieved safely.
This is the first time ACP has addressed this topic in a guideline, panel chair Carolyn J. Crandall, MD, told this news organization. “Diabetes treatment, of course, is our bread and butter…but what we had done before was based on the need to identify a target, like glycosylated hemoglobin. What patients and physicians really want to know now is, who should receive these new drugs? Should they receive these new drugs? And what benefits do they have?”
Added Dr. Crandall, who is professor of medicine at the David Geffen School of Medicine at the University of California, Los Angeles. “At ACP we have a complicated process that I’m actually very proud of, where we’ve asked a lay public panel, as well at the members of our guideline committee, to rank what’s most important in terms of the health outcomes for this condition…And then we look at how to balance those risks and benefits to make the recommendations.”
In the same Annals of Internal Medicine issue are two systematic reviews/meta-analyses that informed the new document, one on drug effectiveness and the other on cost-effectiveness.
In the accompanying editorial from Fatima Z. Syed, MD, an internist and medical weight management specialist at Duke University Division of General Internal Medicine, Durham, North Carolina, she notes, “the potential added benefits of these newer medications, including weight loss and cardiovascular and renal benefits, motivate their prescription, but cost and prior authorization hurdles can bar their use.”
Dr. Syed cites as “missing” from the ACP guidelines an analysis of comorbidities, including obesity. The reason for that, according to the document, is that “weight loss, as measured by percentage of participants who achieved at least 10% total body weight loss, was a prioritized outcome, but data were insufficient for network meta-analysis.”
However, Dr. Syed notes that factoring in weight loss could improve the cost-effectiveness of the newer medications. She points out that the ADA Standards suggest a GLP-1 agonist with or without metformin as initial therapy options for people with newly diagnosed type 2 diabetes who might benefit from weight loss.
“The ACP guidelines strengthen the case for metformin as first-line medication for diabetes when comorbid conditions are not present. Metformin is cost-effective and has excellent hemoglobin A1c reduction. The accompanying economic analysis tells us that in the absence of comorbidity, the newer medication classes do not seem to be cost-effective. However, given that many patients with type 2 diabetes have obesity or existing cardiovascular or renal disease, the choice and accessibility of newer medications can be nuanced. The cost-effectiveness of GLP1 agonists and SGLT2 inhibitors as initial diabetes therapy in the setting of various comorbid conditions warrants careful exploration.”
Dr. Crandall has no disclosures. Dr. Syed disclosed that her husband is employed by Blue Cross Blue Shield of North Carolina.
A version of this article first appeared on Medscape.com.
Two professional societies have issued new guidance for type 2 diabetes management in primary care, with one focused specifically on the use of the newer medications.
On April 19, 2024, the American College of Physicians (ACP) published Newer Pharmacologic Treatments in Adults With Type 2 Diabetes: A Clinical Guideline From the American College of Physicians. The internal medicine group recommends the use of glucagon-like peptide-1 (GLP-1) agonists, and sodium–glucose cotransporter-2 (SGLT2) inhibitors as second-line treatment after metformin. They also advise against the use of dipeptidyl peptidase-4 (DPP-4) inhibitors.
The document was also presented simultaneously at the ACP annual meeting.
And on April 15, the American Diabetes Association (ADA) posted its comprehensive Standards of Care in Diabetes—2024 Abridged for Primary Care Professionals as a follow-up to the December 2023 publication of its full-length Standards. Section 9, Pharmacologic Approaches to Glycemic Treatment, covers the same ground as the ACP guidelines.
General Agreement but Some Differences
The recommendations generally agree regarding medication use, although there are some differences. Both societies continue to endorse metformin and lifestyle modification as first-line therapy for glycemic management in type 2 diabetes. However, while ADA also gives the option of initial combination therapy with prioritization of avoiding hypoglycemia, ACP advises adding new medications only if glycemic goals aren’t met with lifestyle and metformin alone.
The new ACP document gives two general recommendations:
1. Add an SGLT2 inhibitor or a GLP-1 agonist to metformin and lifestyle modifications in adults with type 2 diabetes and inadequate glycemic control.
*Use an SGLT2 inhibitor to reduce the risk for all-cause mortality, major adverse cardiovascular events, progression of chronic kidney disease, and hospitalization due to congestive heart failure.
*Use a GLP-1 agonist to reduce the risk for all-cause mortality, major adverse cardiovascular events, and stroke.
2. ACP recommends against adding a DPP-4 inhibitor to metformin and lifestyle modifications in adults with type 2 diabetes and inadequate glycemic control to reduce morbidity and all-cause mortality.
Both ADA and ACP advise using SGLT2 inhibitors in patients with congestive heart failure and/or chronic kidney disease, and using GLP-1 agonists in patients for whom weight management is a priority. The ADA also advises using agents of either drug class with proven cardiovascular benefit for people with type 2 diabetes who have established cardiovascular disease or who are at high risk.
ADA doesn’t advise against the use of DPP-4 inhibitors but doesn’t prioritize them either. Both insulin and sulfonylureas remain options for both, but they also are lower priority due to their potential for causing hypoglycemia. ACP says that sulfonylureas and long-acting insulin are “inferior to SGLT2 inhibitors and GLP-1 agonists in reducing all-cause mortality and morbidity but may still have some limited value for glycemic control.”
The two groups continue to differ regarding A1c goals, although both recommend individualization. The ACP generally advises levels between 7% and 8% for most adults with type 2 diabetes, and de-intensification of pharmacologic agents for those with A1c levels below 6.5%. On the other hand, ADA recommends A1c levels < 7% as long as that can be achieved safely.
This is the first time ACP has addressed this topic in a guideline, panel chair Carolyn J. Crandall, MD, told this news organization. “Diabetes treatment, of course, is our bread and butter…but what we had done before was based on the need to identify a target, like glycosylated hemoglobin. What patients and physicians really want to know now is, who should receive these new drugs? Should they receive these new drugs? And what benefits do they have?”
Added Dr. Crandall, who is professor of medicine at the David Geffen School of Medicine at the University of California, Los Angeles. “At ACP we have a complicated process that I’m actually very proud of, where we’ve asked a lay public panel, as well at the members of our guideline committee, to rank what’s most important in terms of the health outcomes for this condition…And then we look at how to balance those risks and benefits to make the recommendations.”
In the same Annals of Internal Medicine issue are two systematic reviews/meta-analyses that informed the new document, one on drug effectiveness and the other on cost-effectiveness.
In the accompanying editorial from Fatima Z. Syed, MD, an internist and medical weight management specialist at Duke University Division of General Internal Medicine, Durham, North Carolina, she notes, “the potential added benefits of these newer medications, including weight loss and cardiovascular and renal benefits, motivate their prescription, but cost and prior authorization hurdles can bar their use.”
Dr. Syed cites as “missing” from the ACP guidelines an analysis of comorbidities, including obesity. The reason for that, according to the document, is that “weight loss, as measured by percentage of participants who achieved at least 10% total body weight loss, was a prioritized outcome, but data were insufficient for network meta-analysis.”
However, Dr. Syed notes that factoring in weight loss could improve the cost-effectiveness of the newer medications. She points out that the ADA Standards suggest a GLP-1 agonist with or without metformin as initial therapy options for people with newly diagnosed type 2 diabetes who might benefit from weight loss.
“The ACP guidelines strengthen the case for metformin as first-line medication for diabetes when comorbid conditions are not present. Metformin is cost-effective and has excellent hemoglobin A1c reduction. The accompanying economic analysis tells us that in the absence of comorbidity, the newer medication classes do not seem to be cost-effective. However, given that many patients with type 2 diabetes have obesity or existing cardiovascular or renal disease, the choice and accessibility of newer medications can be nuanced. The cost-effectiveness of GLP1 agonists and SGLT2 inhibitors as initial diabetes therapy in the setting of various comorbid conditions warrants careful exploration.”
Dr. Crandall has no disclosures. Dr. Syed disclosed that her husband is employed by Blue Cross Blue Shield of North Carolina.
A version of this article first appeared on Medscape.com.
Two professional societies have issued new guidance for type 2 diabetes management in primary care, with one focused specifically on the use of the newer medications.
On April 19, 2024, the American College of Physicians (ACP) published Newer Pharmacologic Treatments in Adults With Type 2 Diabetes: A Clinical Guideline From the American College of Physicians. The internal medicine group recommends the use of glucagon-like peptide-1 (GLP-1) agonists, and sodium–glucose cotransporter-2 (SGLT2) inhibitors as second-line treatment after metformin. They also advise against the use of dipeptidyl peptidase-4 (DPP-4) inhibitors.
The document was also presented simultaneously at the ACP annual meeting.
And on April 15, the American Diabetes Association (ADA) posted its comprehensive Standards of Care in Diabetes—2024 Abridged for Primary Care Professionals as a follow-up to the December 2023 publication of its full-length Standards. Section 9, Pharmacologic Approaches to Glycemic Treatment, covers the same ground as the ACP guidelines.
General Agreement but Some Differences
The recommendations generally agree regarding medication use, although there are some differences. Both societies continue to endorse metformin and lifestyle modification as first-line therapy for glycemic management in type 2 diabetes. However, while ADA also gives the option of initial combination therapy with prioritization of avoiding hypoglycemia, ACP advises adding new medications only if glycemic goals aren’t met with lifestyle and metformin alone.
The new ACP document gives two general recommendations:
1. Add an SGLT2 inhibitor or a GLP-1 agonist to metformin and lifestyle modifications in adults with type 2 diabetes and inadequate glycemic control.
*Use an SGLT2 inhibitor to reduce the risk for all-cause mortality, major adverse cardiovascular events, progression of chronic kidney disease, and hospitalization due to congestive heart failure.
*Use a GLP-1 agonist to reduce the risk for all-cause mortality, major adverse cardiovascular events, and stroke.
2. ACP recommends against adding a DPP-4 inhibitor to metformin and lifestyle modifications in adults with type 2 diabetes and inadequate glycemic control to reduce morbidity and all-cause mortality.
Both ADA and ACP advise using SGLT2 inhibitors in patients with congestive heart failure and/or chronic kidney disease, and using GLP-1 agonists in patients for whom weight management is a priority. The ADA also advises using agents of either drug class with proven cardiovascular benefit for people with type 2 diabetes who have established cardiovascular disease or who are at high risk.
ADA doesn’t advise against the use of DPP-4 inhibitors but doesn’t prioritize them either. Both insulin and sulfonylureas remain options for both, but they also are lower priority due to their potential for causing hypoglycemia. ACP says that sulfonylureas and long-acting insulin are “inferior to SGLT2 inhibitors and GLP-1 agonists in reducing all-cause mortality and morbidity but may still have some limited value for glycemic control.”
The two groups continue to differ regarding A1c goals, although both recommend individualization. The ACP generally advises levels between 7% and 8% for most adults with type 2 diabetes, and de-intensification of pharmacologic agents for those with A1c levels below 6.5%. On the other hand, ADA recommends A1c levels < 7% as long as that can be achieved safely.
This is the first time ACP has addressed this topic in a guideline, panel chair Carolyn J. Crandall, MD, told this news organization. “Diabetes treatment, of course, is our bread and butter…but what we had done before was based on the need to identify a target, like glycosylated hemoglobin. What patients and physicians really want to know now is, who should receive these new drugs? Should they receive these new drugs? And what benefits do they have?”
Added Dr. Crandall, who is professor of medicine at the David Geffen School of Medicine at the University of California, Los Angeles. “At ACP we have a complicated process that I’m actually very proud of, where we’ve asked a lay public panel, as well at the members of our guideline committee, to rank what’s most important in terms of the health outcomes for this condition…And then we look at how to balance those risks and benefits to make the recommendations.”
In the same Annals of Internal Medicine issue are two systematic reviews/meta-analyses that informed the new document, one on drug effectiveness and the other on cost-effectiveness.
In the accompanying editorial from Fatima Z. Syed, MD, an internist and medical weight management specialist at Duke University Division of General Internal Medicine, Durham, North Carolina, she notes, “the potential added benefits of these newer medications, including weight loss and cardiovascular and renal benefits, motivate their prescription, but cost and prior authorization hurdles can bar their use.”
Dr. Syed cites as “missing” from the ACP guidelines an analysis of comorbidities, including obesity. The reason for that, according to the document, is that “weight loss, as measured by percentage of participants who achieved at least 10% total body weight loss, was a prioritized outcome, but data were insufficient for network meta-analysis.”
However, Dr. Syed notes that factoring in weight loss could improve the cost-effectiveness of the newer medications. She points out that the ADA Standards suggest a GLP-1 agonist with or without metformin as initial therapy options for people with newly diagnosed type 2 diabetes who might benefit from weight loss.
“The ACP guidelines strengthen the case for metformin as first-line medication for diabetes when comorbid conditions are not present. Metformin is cost-effective and has excellent hemoglobin A1c reduction. The accompanying economic analysis tells us that in the absence of comorbidity, the newer medication classes do not seem to be cost-effective. However, given that many patients with type 2 diabetes have obesity or existing cardiovascular or renal disease, the choice and accessibility of newer medications can be nuanced. The cost-effectiveness of GLP1 agonists and SGLT2 inhibitors as initial diabetes therapy in the setting of various comorbid conditions warrants careful exploration.”
Dr. Crandall has no disclosures. Dr. Syed disclosed that her husband is employed by Blue Cross Blue Shield of North Carolina.
A version of this article first appeared on Medscape.com.
Pediatric Clinic Doubles Well-Visit Data Capture by Going Completely Paperless
TORONTO — , and, of course, it eliminated the work associated with managing paper records.
Due to the efficacy of the pre-visit capture, “all of the information — including individual responses and total scores — is available to the provider at a glance” by the time of the examination encounter, according to Brian T. Ketterman, MD, a third-year resident in pediatrics at the Monroe Carell Jr. Children’s Hospital, Vanderbilt University, Nashville, Tennessee.
Urgent issues, such as suicidality risk, “are flagged so that they are seen first,” he added.
The goal of going paperless was to improve the amount and the consistency of data captured with each well visit, according to Dr. Ketterman. He described a major undertaking involving fundamental changes in the electronic medical record (EMR) system to accommodate the new approach.
Characterizing screening at well visits as “one of the most important jobs of a pediatrician” when he presented these data at the Pediatric Academic Societies annual meeting, he added that a paperless approach comes with many advantages.
Raising the Rate of Complete Data Capture
The American Academy of Pediatrics (AAP) has identified more than 30 screening elements at well-child visits. At his institution several additional screening elements have been added. Prior to going paperless, about 45.6% on average of this well-visit data was being captured in any specific patient encounter, even if the institution was doing well overall in capturing essential information, such as key laboratory values and immunizations.
The vast majority of the information that was missing depended on patient or family input, such as social determinants of health (SDoH), which includes the aspects of home environment, such as nutrition and safety. Dr. Ketterman said that going paperless was inspired by positive experiences reported elsewhere.
“We wanted to build on this work,” he said.
The goal of the program was to raise the rate of complete data capture at well visits to at least 80% and do this across all languages. The first step was to create digital forms in English and Spanish for completion unique to each milestone well-child visit defined by age. For those who speak English or Spanish, these forms were supplied through the patient portal several days before the visit.
For those who spoke one of the more than 40 other languages encountered among patients at Dr. Ketterman’s institution, an interpreter was supplied. If patients arrived at the clinic without completing the digital entry, they completed it on a tablet with the help of an interpreter if one was needed.
Prior to going paperless, all screening data were captured on paper forms completed in the waiting room. The provider then manually reviewed and scored the forms before they were then scanned into the medical records. The paperless approach has eliminated all of these steps and the information is already available for review by the time the pediatrician enters the examination room.
With more than 50,000 well-child checkups captured over a recent 15-month period of paperless questionnaires, the proportion with 100% data capture using what Dr. Ketterman characterized as “strict criteria” was 84%, surpassing the goal at the initiation of the program.
“We improved in almost every screening measure,” Dr. Ketterman said, providing P values that were mostly < .001 for a range of standard tests such as M-CHAT-R (Modified Checklist for Autism in Toddlers), QPA (Quick Parenting Assessment), and PSC-17 (Pediatric Symptom Checklist) when compared to the baseline period.
Additional Advantages
The improvement in well-visit data capture was the goal, but the list of other advantages of the paperless system is long. For one, the EMR system now automatically uses the data to offer guidance that might improve patient outcomes. For example, if the family reports that the child does not see a dentist or does not know how to swim, these lead prompt the EMR to provide resources, such as the names of dentists of swim programs, to address the problem.
As another example, screening questions that reveal food insecurity automatically trigger guidance for enrolling in the U.S. Department of Agriculture’s WIC (Women, Infants, and Children) food program. According to Dr. Ketterman, the proportion of children now enrolled in WIC has increased 10-fold from baseline. He also reported there was a twofold increase in the proportion of patients enrolled in a free book program as a result of a screening questions that ask about reading at home.
The improvement in well-visit data capture was seen across all languages. Even if the gains were not quite as good in languages other than English or Spanish, they were still highly significant relative to baseline.
A ‘Life-Changing’ Improvement
The discussion following his talk made it clear that similar approaches are being actively pursued nationwide. Several in the audience working on similar programs identified such challenges as getting electronic medical record (EMR) systems to cooperate, ensuring patient enrollment in the portals, and avoiding form completion fatigue, but the comments were uniformly supportive of the benefits of this approach.
“This has been life-changing for us,” said Katie E. McPeak, MD, a primary care pediatrician and Medical Director for Health Equity at the Children’s Hospital of Philadelphia. “The information is more accurate in the digital format and it reduces time for the clinician reviewing the data in the exam room.” She also agreed that paperless completion of screen captures better information on more topics, like sleep, nutrition, and mood disorders.
However, Dr. McPeak was one of those who was concerned about form fatigue. Patients have to enter extensive information over multiple screens for each well-child visit. She said this problem might need to be addressed if the success of paperless screening leads to even greater expansion of data requested.
In addressing the work behind creating a system of the depth and scope of the one he described, Dr. Ketterman acknowledged that it involved a daunting development process with substantial coding and testing. Referring to the EMR system used at his hospital, he said the preparation required an “epic guru,” but he said that input fatigue has not yet arisen as a major issue.
“Many of the screens are mandatory, so you cannot advance without completing them, but some are optional,” he noted. “However, we are seeing a high rate of response even on the screens they could click past.”
Dr. Ketterman and Dr. McPeak report no potential conflicts of interest.
TORONTO — , and, of course, it eliminated the work associated with managing paper records.
Due to the efficacy of the pre-visit capture, “all of the information — including individual responses and total scores — is available to the provider at a glance” by the time of the examination encounter, according to Brian T. Ketterman, MD, a third-year resident in pediatrics at the Monroe Carell Jr. Children’s Hospital, Vanderbilt University, Nashville, Tennessee.
Urgent issues, such as suicidality risk, “are flagged so that they are seen first,” he added.
The goal of going paperless was to improve the amount and the consistency of data captured with each well visit, according to Dr. Ketterman. He described a major undertaking involving fundamental changes in the electronic medical record (EMR) system to accommodate the new approach.
Characterizing screening at well visits as “one of the most important jobs of a pediatrician” when he presented these data at the Pediatric Academic Societies annual meeting, he added that a paperless approach comes with many advantages.
Raising the Rate of Complete Data Capture
The American Academy of Pediatrics (AAP) has identified more than 30 screening elements at well-child visits. At his institution several additional screening elements have been added. Prior to going paperless, about 45.6% on average of this well-visit data was being captured in any specific patient encounter, even if the institution was doing well overall in capturing essential information, such as key laboratory values and immunizations.
The vast majority of the information that was missing depended on patient or family input, such as social determinants of health (SDoH), which includes the aspects of home environment, such as nutrition and safety. Dr. Ketterman said that going paperless was inspired by positive experiences reported elsewhere.
“We wanted to build on this work,” he said.
The goal of the program was to raise the rate of complete data capture at well visits to at least 80% and do this across all languages. The first step was to create digital forms in English and Spanish for completion unique to each milestone well-child visit defined by age. For those who speak English or Spanish, these forms were supplied through the patient portal several days before the visit.
For those who spoke one of the more than 40 other languages encountered among patients at Dr. Ketterman’s institution, an interpreter was supplied. If patients arrived at the clinic without completing the digital entry, they completed it on a tablet with the help of an interpreter if one was needed.
Prior to going paperless, all screening data were captured on paper forms completed in the waiting room. The provider then manually reviewed and scored the forms before they were then scanned into the medical records. The paperless approach has eliminated all of these steps and the information is already available for review by the time the pediatrician enters the examination room.
With more than 50,000 well-child checkups captured over a recent 15-month period of paperless questionnaires, the proportion with 100% data capture using what Dr. Ketterman characterized as “strict criteria” was 84%, surpassing the goal at the initiation of the program.
“We improved in almost every screening measure,” Dr. Ketterman said, providing P values that were mostly < .001 for a range of standard tests such as M-CHAT-R (Modified Checklist for Autism in Toddlers), QPA (Quick Parenting Assessment), and PSC-17 (Pediatric Symptom Checklist) when compared to the baseline period.
Additional Advantages
The improvement in well-visit data capture was the goal, but the list of other advantages of the paperless system is long. For one, the EMR system now automatically uses the data to offer guidance that might improve patient outcomes. For example, if the family reports that the child does not see a dentist or does not know how to swim, these lead prompt the EMR to provide resources, such as the names of dentists of swim programs, to address the problem.
As another example, screening questions that reveal food insecurity automatically trigger guidance for enrolling in the U.S. Department of Agriculture’s WIC (Women, Infants, and Children) food program. According to Dr. Ketterman, the proportion of children now enrolled in WIC has increased 10-fold from baseline. He also reported there was a twofold increase in the proportion of patients enrolled in a free book program as a result of a screening questions that ask about reading at home.
The improvement in well-visit data capture was seen across all languages. Even if the gains were not quite as good in languages other than English or Spanish, they were still highly significant relative to baseline.
A ‘Life-Changing’ Improvement
The discussion following his talk made it clear that similar approaches are being actively pursued nationwide. Several in the audience working on similar programs identified such challenges as getting electronic medical record (EMR) systems to cooperate, ensuring patient enrollment in the portals, and avoiding form completion fatigue, but the comments were uniformly supportive of the benefits of this approach.
“This has been life-changing for us,” said Katie E. McPeak, MD, a primary care pediatrician and Medical Director for Health Equity at the Children’s Hospital of Philadelphia. “The information is more accurate in the digital format and it reduces time for the clinician reviewing the data in the exam room.” She also agreed that paperless completion of screen captures better information on more topics, like sleep, nutrition, and mood disorders.
However, Dr. McPeak was one of those who was concerned about form fatigue. Patients have to enter extensive information over multiple screens for each well-child visit. She said this problem might need to be addressed if the success of paperless screening leads to even greater expansion of data requested.
In addressing the work behind creating a system of the depth and scope of the one he described, Dr. Ketterman acknowledged that it involved a daunting development process with substantial coding and testing. Referring to the EMR system used at his hospital, he said the preparation required an “epic guru,” but he said that input fatigue has not yet arisen as a major issue.
“Many of the screens are mandatory, so you cannot advance without completing them, but some are optional,” he noted. “However, we are seeing a high rate of response even on the screens they could click past.”
Dr. Ketterman and Dr. McPeak report no potential conflicts of interest.
TORONTO — , and, of course, it eliminated the work associated with managing paper records.
Due to the efficacy of the pre-visit capture, “all of the information — including individual responses and total scores — is available to the provider at a glance” by the time of the examination encounter, according to Brian T. Ketterman, MD, a third-year resident in pediatrics at the Monroe Carell Jr. Children’s Hospital, Vanderbilt University, Nashville, Tennessee.
Urgent issues, such as suicidality risk, “are flagged so that they are seen first,” he added.
The goal of going paperless was to improve the amount and the consistency of data captured with each well visit, according to Dr. Ketterman. He described a major undertaking involving fundamental changes in the electronic medical record (EMR) system to accommodate the new approach.
Characterizing screening at well visits as “one of the most important jobs of a pediatrician” when he presented these data at the Pediatric Academic Societies annual meeting, he added that a paperless approach comes with many advantages.
Raising the Rate of Complete Data Capture
The American Academy of Pediatrics (AAP) has identified more than 30 screening elements at well-child visits. At his institution several additional screening elements have been added. Prior to going paperless, about 45.6% on average of this well-visit data was being captured in any specific patient encounter, even if the institution was doing well overall in capturing essential information, such as key laboratory values and immunizations.
The vast majority of the information that was missing depended on patient or family input, such as social determinants of health (SDoH), which includes the aspects of home environment, such as nutrition and safety. Dr. Ketterman said that going paperless was inspired by positive experiences reported elsewhere.
“We wanted to build on this work,” he said.
The goal of the program was to raise the rate of complete data capture at well visits to at least 80% and do this across all languages. The first step was to create digital forms in English and Spanish for completion unique to each milestone well-child visit defined by age. For those who speak English or Spanish, these forms were supplied through the patient portal several days before the visit.
For those who spoke one of the more than 40 other languages encountered among patients at Dr. Ketterman’s institution, an interpreter was supplied. If patients arrived at the clinic without completing the digital entry, they completed it on a tablet with the help of an interpreter if one was needed.
Prior to going paperless, all screening data were captured on paper forms completed in the waiting room. The provider then manually reviewed and scored the forms before they were then scanned into the medical records. The paperless approach has eliminated all of these steps and the information is already available for review by the time the pediatrician enters the examination room.
With more than 50,000 well-child checkups captured over a recent 15-month period of paperless questionnaires, the proportion with 100% data capture using what Dr. Ketterman characterized as “strict criteria” was 84%, surpassing the goal at the initiation of the program.
“We improved in almost every screening measure,” Dr. Ketterman said, providing P values that were mostly < .001 for a range of standard tests such as M-CHAT-R (Modified Checklist for Autism in Toddlers), QPA (Quick Parenting Assessment), and PSC-17 (Pediatric Symptom Checklist) when compared to the baseline period.
Additional Advantages
The improvement in well-visit data capture was the goal, but the list of other advantages of the paperless system is long. For one, the EMR system now automatically uses the data to offer guidance that might improve patient outcomes. For example, if the family reports that the child does not see a dentist or does not know how to swim, these lead prompt the EMR to provide resources, such as the names of dentists of swim programs, to address the problem.
As another example, screening questions that reveal food insecurity automatically trigger guidance for enrolling in the U.S. Department of Agriculture’s WIC (Women, Infants, and Children) food program. According to Dr. Ketterman, the proportion of children now enrolled in WIC has increased 10-fold from baseline. He also reported there was a twofold increase in the proportion of patients enrolled in a free book program as a result of a screening questions that ask about reading at home.
The improvement in well-visit data capture was seen across all languages. Even if the gains were not quite as good in languages other than English or Spanish, they were still highly significant relative to baseline.
A ‘Life-Changing’ Improvement
The discussion following his talk made it clear that similar approaches are being actively pursued nationwide. Several in the audience working on similar programs identified such challenges as getting electronic medical record (EMR) systems to cooperate, ensuring patient enrollment in the portals, and avoiding form completion fatigue, but the comments were uniformly supportive of the benefits of this approach.
“This has been life-changing for us,” said Katie E. McPeak, MD, a primary care pediatrician and Medical Director for Health Equity at the Children’s Hospital of Philadelphia. “The information is more accurate in the digital format and it reduces time for the clinician reviewing the data in the exam room.” She also agreed that paperless completion of screen captures better information on more topics, like sleep, nutrition, and mood disorders.
However, Dr. McPeak was one of those who was concerned about form fatigue. Patients have to enter extensive information over multiple screens for each well-child visit. She said this problem might need to be addressed if the success of paperless screening leads to even greater expansion of data requested.
In addressing the work behind creating a system of the depth and scope of the one he described, Dr. Ketterman acknowledged that it involved a daunting development process with substantial coding and testing. Referring to the EMR system used at his hospital, he said the preparation required an “epic guru,” but he said that input fatigue has not yet arisen as a major issue.
“Many of the screens are mandatory, so you cannot advance without completing them, but some are optional,” he noted. “However, we are seeing a high rate of response even on the screens they could click past.”
Dr. Ketterman and Dr. McPeak report no potential conflicts of interest.
FROM PAS 2024
Multiple Asymptomatic Dome-Shaped Papules on the Scalp
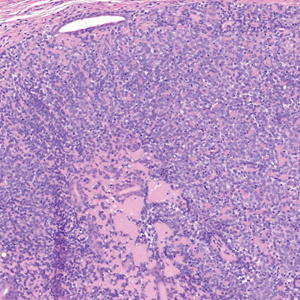
The Diagnosis: Spiradenocylindroma
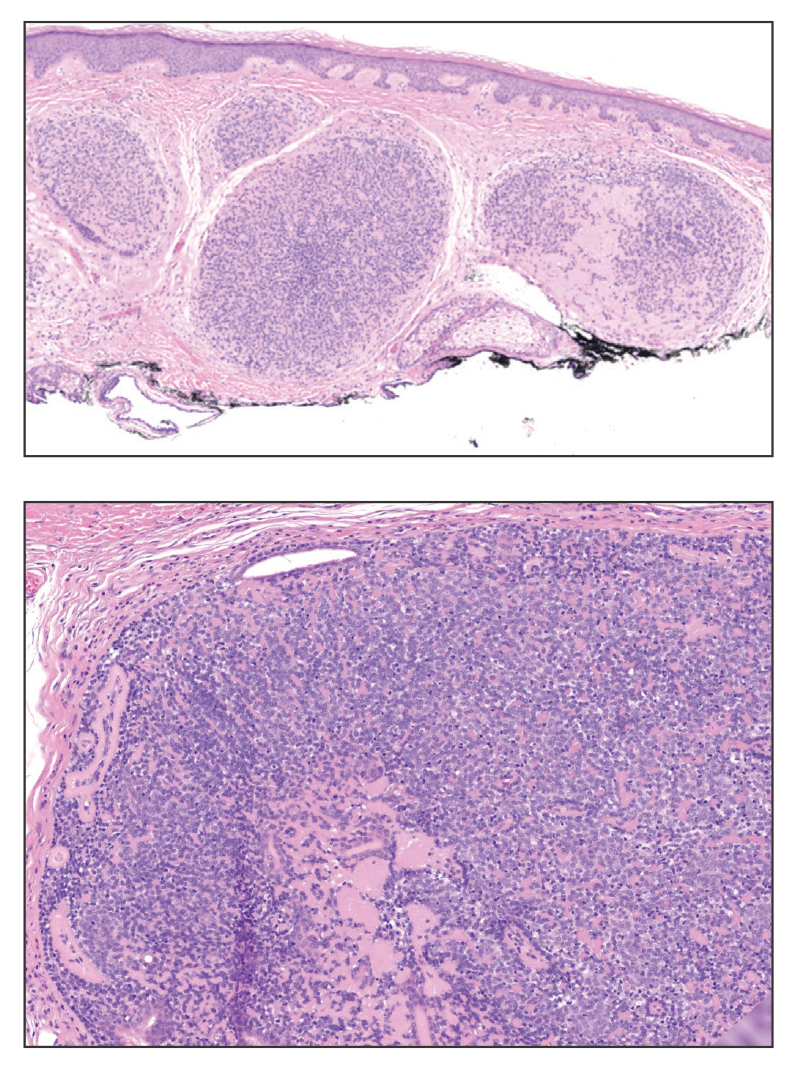
Shave biopsies of our patient’s lesions showed wellcircumscribed dermal nodules resembling a spiradenoma with 3 cell populations: those with lighter nuclei, darker nuclei, and scattered lymphocytes. However, the conspicuous globules of basement membrane material were reminiscent of a cylindroma. These overlapping features and the patient’s history of cylindroma were suggestive of a diagnosis of spiradenocylindroma.
Spiradenocylindroma is an uncommon dermal tumor with features that overlap with spiradenoma and cylindroma.1 It may manifest as a solitary lesion or multiple lesions and can occur sporadically or in the context of a family history. Histologically, it must be distinguished from other intradermal basaloid neoplasms including conventional cylindroma and spiradenoma, dermal duct tumor, hidradenoma, and trichoblastoma.
When patients present with multiple cylindromas, spiradenomas, or spiradenocylindromas, physicians should consider genetic testing and review of the family history to assess for cylindromatosis gene mutations or Brooke-Spiegler syndrome. Biopsy and histologic examination are important because malignant tumors can evolve from pre-existing spiradenocylindromas, cylindromas, and spiradenomas,2 with an increased risk in patients with Brooke-Spiegler syndrome.1 Our patient declined further genetic workup but continues to follow up with dermatology for monitoring of lesions.
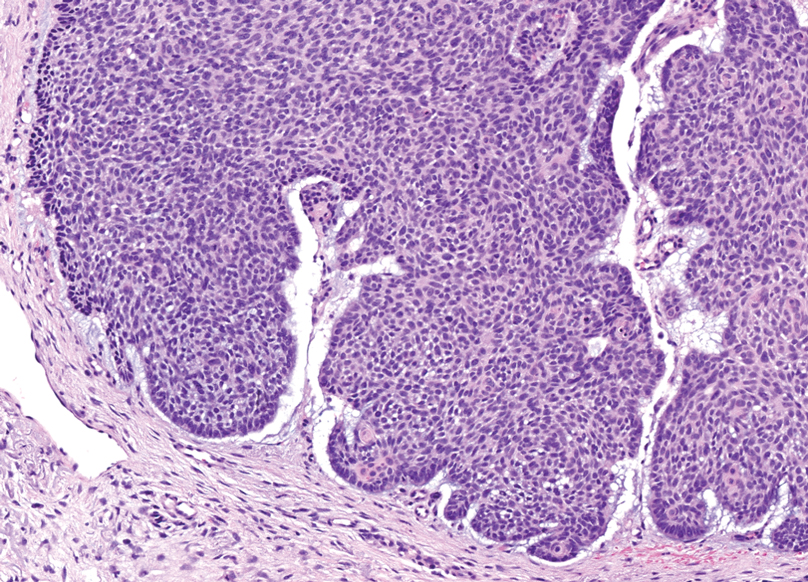
Dermal duct tumors are morphologic variants of poromas that are derived from sweat gland lineage and usually manifest as solitary dome-shaped papules, plaques, or nodules most often seen on acral surfaces as well as the head and neck.3 Clinically, they may be indistinguishable from spiradenocylindromas and require biopsy for histologic evaluation. They can be distinguished from spiradenocylindroma by the presence of small dermal nodules composed of cuboidal cells with ample pink cytoplasm and cuticle-lined ducts (Figure 1).
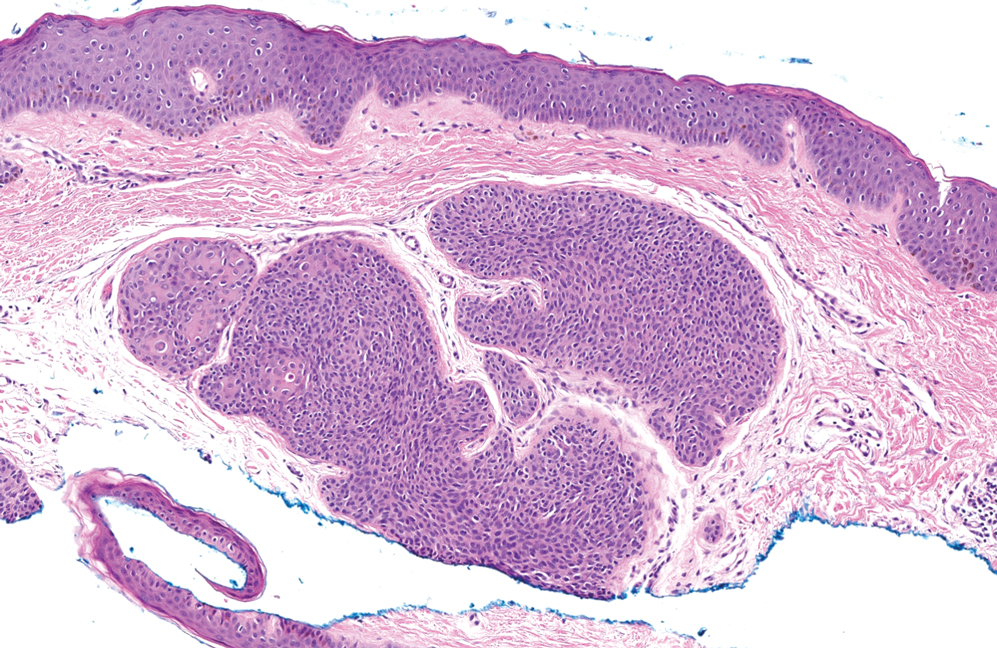
Trichoblastomas typically are deep-seated basaloid follicular neoplasms on the scalp with papillary mesenchyme resembling the normal fibrous sheath of the hair follicle, often replete with papillary mesenchymal bodies (Figure 2). There generally are no retraction spaces between its basaloid nests and the surrounding stroma, which is unlikely to contain mucin relative to basal cell carcinoma (BCC).4,5
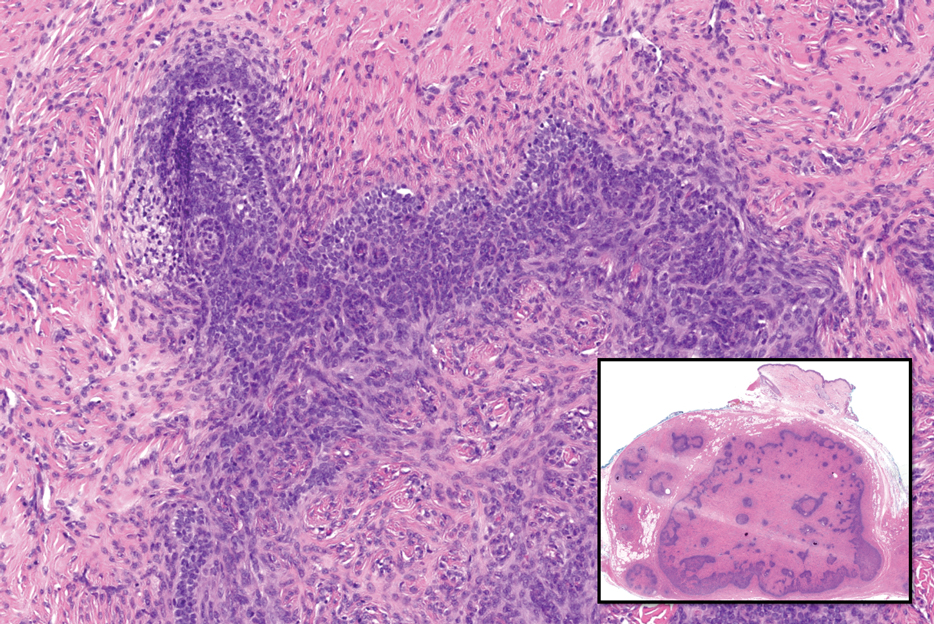
Adenoid cystic carcinoma is a rare salivary gland tumor that can metastasize to the skin and rarely arises as a primary skin adnexal tumor. It manifests as a slowgrowing mass that can be tender to palpation.6 Histologic examination shows dermal islands with cribriform blue and pink spaces. Compared to BCC, adenoid cystic carcinoma cells are enlarged and epithelioid with relatively scarce cytoplasm (Figure 3).6,7 Adenoid cystic carcinoma can show variable growth patterns including infiltrative nests and trabeculae. Perineural invasion is common, and there is a high risk for local recurrence.7 First-line therapy usually is surgical, and postoperative radiotherapy may be required.6,7
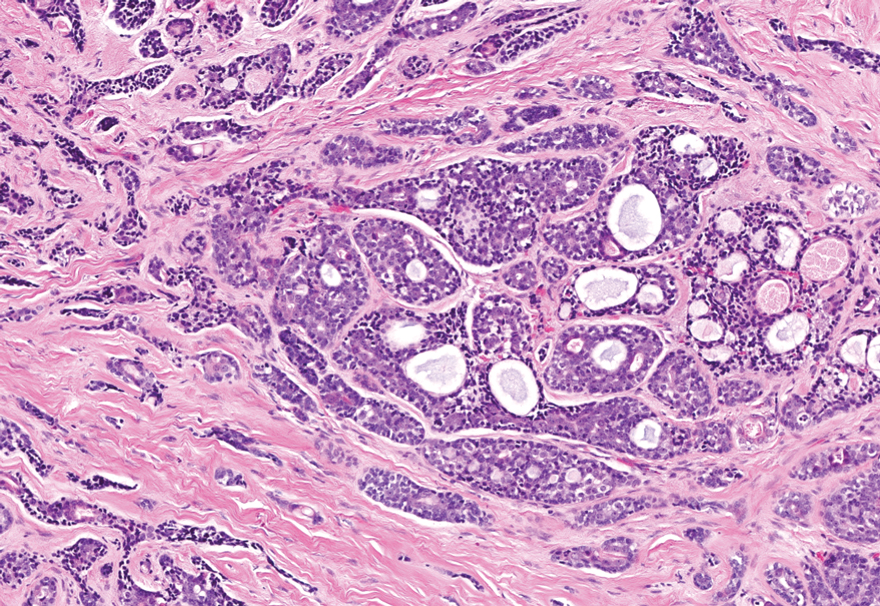
Nodular BCC commonly manifests as an enlarging nonhealing lesion on sun-exposed skin and has many subtypes, typically with arborizing telangiectases on dermoscopy. Histopathologic examination of nodular BCC reveals a nest of basaloid follicular germinative cells in the dermis with peripheral palisading and a fibromyxoid stroma (Figure 4).8 Patients with Brooke-Spiegler syndrome are at increased risk for nodular BCC, which may be clinically indistinguishable from spiradenoma, cylindroma, and spiradenocylindroma, necessitating histologic assessment.
- Facchini V, Colangeli W, Bozza F, et al. A rare histopathological spiradenocylindroma: a case report. Clin Ter. 2022;173:292-294. doi:10.7417/ CT.2022.2433
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update [published online March 14, 2016]. Head Neck Pathol. 2016;10:125-30. doi:10.1007/s12105-016-0705-x
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Elston DM. Pilar and sebaceous neoplasms. In: Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 3rd ed. Elsevier; 2018:71-85.
- McCalmont TH, Pincus LB. Adnexal neoplasms. In: Bolognia J, Schaffer J, Cerroni, L. Dermatology. 4th ed. Elsevier; 2017:1930-1953.
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update [published online May 2, 2015]. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251- 255. doi:10.1159/000431082
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations [published online May 18, 2018]. J Am Acad Dermatol. 2019;80:303-317. doi:10.1016/j.jaad.2018.03.060
The Diagnosis: Spiradenocylindroma
Shave biopsies of our patient’s lesions showed wellcircumscribed dermal nodules resembling a spiradenoma with 3 cell populations: those with lighter nuclei, darker nuclei, and scattered lymphocytes. However, the conspicuous globules of basement membrane material were reminiscent of a cylindroma. These overlapping features and the patient’s history of cylindroma were suggestive of a diagnosis of spiradenocylindroma.
Spiradenocylindroma is an uncommon dermal tumor with features that overlap with spiradenoma and cylindroma.1 It may manifest as a solitary lesion or multiple lesions and can occur sporadically or in the context of a family history. Histologically, it must be distinguished from other intradermal basaloid neoplasms including conventional cylindroma and spiradenoma, dermal duct tumor, hidradenoma, and trichoblastoma.
When patients present with multiple cylindromas, spiradenomas, or spiradenocylindromas, physicians should consider genetic testing and review of the family history to assess for cylindromatosis gene mutations or Brooke-Spiegler syndrome. Biopsy and histologic examination are important because malignant tumors can evolve from pre-existing spiradenocylindromas, cylindromas, and spiradenomas,2 with an increased risk in patients with Brooke-Spiegler syndrome.1 Our patient declined further genetic workup but continues to follow up with dermatology for monitoring of lesions.
Dermal duct tumors are morphologic variants of poromas that are derived from sweat gland lineage and usually manifest as solitary dome-shaped papules, plaques, or nodules most often seen on acral surfaces as well as the head and neck.3 Clinically, they may be indistinguishable from spiradenocylindromas and require biopsy for histologic evaluation. They can be distinguished from spiradenocylindroma by the presence of small dermal nodules composed of cuboidal cells with ample pink cytoplasm and cuticle-lined ducts (Figure 1).

Trichoblastomas typically are deep-seated basaloid follicular neoplasms on the scalp with papillary mesenchyme resembling the normal fibrous sheath of the hair follicle, often replete with papillary mesenchymal bodies (Figure 2). There generally are no retraction spaces between its basaloid nests and the surrounding stroma, which is unlikely to contain mucin relative to basal cell carcinoma (BCC).4,5

Adenoid cystic carcinoma is a rare salivary gland tumor that can metastasize to the skin and rarely arises as a primary skin adnexal tumor. It manifests as a slowgrowing mass that can be tender to palpation.6 Histologic examination shows dermal islands with cribriform blue and pink spaces. Compared to BCC, adenoid cystic carcinoma cells are enlarged and epithelioid with relatively scarce cytoplasm (Figure 3).6,7 Adenoid cystic carcinoma can show variable growth patterns including infiltrative nests and trabeculae. Perineural invasion is common, and there is a high risk for local recurrence.7 First-line therapy usually is surgical, and postoperative radiotherapy may be required.6,7

Nodular BCC commonly manifests as an enlarging nonhealing lesion on sun-exposed skin and has many subtypes, typically with arborizing telangiectases on dermoscopy. Histopathologic examination of nodular BCC reveals a nest of basaloid follicular germinative cells in the dermis with peripheral palisading and a fibromyxoid stroma (Figure 4).8 Patients with Brooke-Spiegler syndrome are at increased risk for nodular BCC, which may be clinically indistinguishable from spiradenoma, cylindroma, and spiradenocylindroma, necessitating histologic assessment.

The Diagnosis: Spiradenocylindroma
Shave biopsies of our patient’s lesions showed wellcircumscribed dermal nodules resembling a spiradenoma with 3 cell populations: those with lighter nuclei, darker nuclei, and scattered lymphocytes. However, the conspicuous globules of basement membrane material were reminiscent of a cylindroma. These overlapping features and the patient’s history of cylindroma were suggestive of a diagnosis of spiradenocylindroma.
Spiradenocylindroma is an uncommon dermal tumor with features that overlap with spiradenoma and cylindroma.1 It may manifest as a solitary lesion or multiple lesions and can occur sporadically or in the context of a family history. Histologically, it must be distinguished from other intradermal basaloid neoplasms including conventional cylindroma and spiradenoma, dermal duct tumor, hidradenoma, and trichoblastoma.
When patients present with multiple cylindromas, spiradenomas, or spiradenocylindromas, physicians should consider genetic testing and review of the family history to assess for cylindromatosis gene mutations or Brooke-Spiegler syndrome. Biopsy and histologic examination are important because malignant tumors can evolve from pre-existing spiradenocylindromas, cylindromas, and spiradenomas,2 with an increased risk in patients with Brooke-Spiegler syndrome.1 Our patient declined further genetic workup but continues to follow up with dermatology for monitoring of lesions.
Dermal duct tumors are morphologic variants of poromas that are derived from sweat gland lineage and usually manifest as solitary dome-shaped papules, plaques, or nodules most often seen on acral surfaces as well as the head and neck.3 Clinically, they may be indistinguishable from spiradenocylindromas and require biopsy for histologic evaluation. They can be distinguished from spiradenocylindroma by the presence of small dermal nodules composed of cuboidal cells with ample pink cytoplasm and cuticle-lined ducts (Figure 1).

Trichoblastomas typically are deep-seated basaloid follicular neoplasms on the scalp with papillary mesenchyme resembling the normal fibrous sheath of the hair follicle, often replete with papillary mesenchymal bodies (Figure 2). There generally are no retraction spaces between its basaloid nests and the surrounding stroma, which is unlikely to contain mucin relative to basal cell carcinoma (BCC).4,5

Adenoid cystic carcinoma is a rare salivary gland tumor that can metastasize to the skin and rarely arises as a primary skin adnexal tumor. It manifests as a slowgrowing mass that can be tender to palpation.6 Histologic examination shows dermal islands with cribriform blue and pink spaces. Compared to BCC, adenoid cystic carcinoma cells are enlarged and epithelioid with relatively scarce cytoplasm (Figure 3).6,7 Adenoid cystic carcinoma can show variable growth patterns including infiltrative nests and trabeculae. Perineural invasion is common, and there is a high risk for local recurrence.7 First-line therapy usually is surgical, and postoperative radiotherapy may be required.6,7

Nodular BCC commonly manifests as an enlarging nonhealing lesion on sun-exposed skin and has many subtypes, typically with arborizing telangiectases on dermoscopy. Histopathologic examination of nodular BCC reveals a nest of basaloid follicular germinative cells in the dermis with peripheral palisading and a fibromyxoid stroma (Figure 4).8 Patients with Brooke-Spiegler syndrome are at increased risk for nodular BCC, which may be clinically indistinguishable from spiradenoma, cylindroma, and spiradenocylindroma, necessitating histologic assessment.

- Facchini V, Colangeli W, Bozza F, et al. A rare histopathological spiradenocylindroma: a case report. Clin Ter. 2022;173:292-294. doi:10.7417/ CT.2022.2433
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update [published online March 14, 2016]. Head Neck Pathol. 2016;10:125-30. doi:10.1007/s12105-016-0705-x
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Elston DM. Pilar and sebaceous neoplasms. In: Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 3rd ed. Elsevier; 2018:71-85.
- McCalmont TH, Pincus LB. Adnexal neoplasms. In: Bolognia J, Schaffer J, Cerroni, L. Dermatology. 4th ed. Elsevier; 2017:1930-1953.
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update [published online May 2, 2015]. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251- 255. doi:10.1159/000431082
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations [published online May 18, 2018]. J Am Acad Dermatol. 2019;80:303-317. doi:10.1016/j.jaad.2018.03.060
- Facchini V, Colangeli W, Bozza F, et al. A rare histopathological spiradenocylindroma: a case report. Clin Ter. 2022;173:292-294. doi:10.7417/ CT.2022.2433
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update [published online March 14, 2016]. Head Neck Pathol. 2016;10:125-30. doi:10.1007/s12105-016-0705-x
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Elston DM. Pilar and sebaceous neoplasms. In: Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 3rd ed. Elsevier; 2018:71-85.
- McCalmont TH, Pincus LB. Adnexal neoplasms. In: Bolognia J, Schaffer J, Cerroni, L. Dermatology. 4th ed. Elsevier; 2017:1930-1953.
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update [published online May 2, 2015]. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251- 255. doi:10.1159/000431082
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations [published online May 18, 2018]. J Am Acad Dermatol. 2019;80:303-317. doi:10.1016/j.jaad.2018.03.060
A 62-year-old man with a history of cylindromas presented to our clinic with multiple asymptomatic, 3- to 4-mm, nonmobile, dome-shaped, telangiectatic, pink papules over the parietal and vertex scalp that had been present for more than 10 years without change. Several family members had similar lesions that had not been evaluated by a physician, and there had been no genetic evaluation. Shave biopsies of several lesions were performed.
Impact of the COVID-19 Pandemic on Care for Patients With Skin Cancer
To the Editor:
The most common malignancy in the United States is skin cancer, with melanoma accounting for the majority of skin cancer deaths.1 Despite the lack of established guidelines for routine total-body skin examinations, many patients regularly visit their dermatologist for assessment of pigmented skin lesions.2 During the COVID-19 pandemic, many patients were unable to attend in-person dermatology visits, which resulted in many high-risk individuals not receiving care or alternatively seeking virtual care for cutaneous lesions.3 There has been a lack of research in the United States exploring the utilization of teledermatology during the pandemic and its overall impact on the care of patients with a history of skin cancer. We explored the impact of the COVID-19 pandemic on care for patients with skin cancer in a large US population.
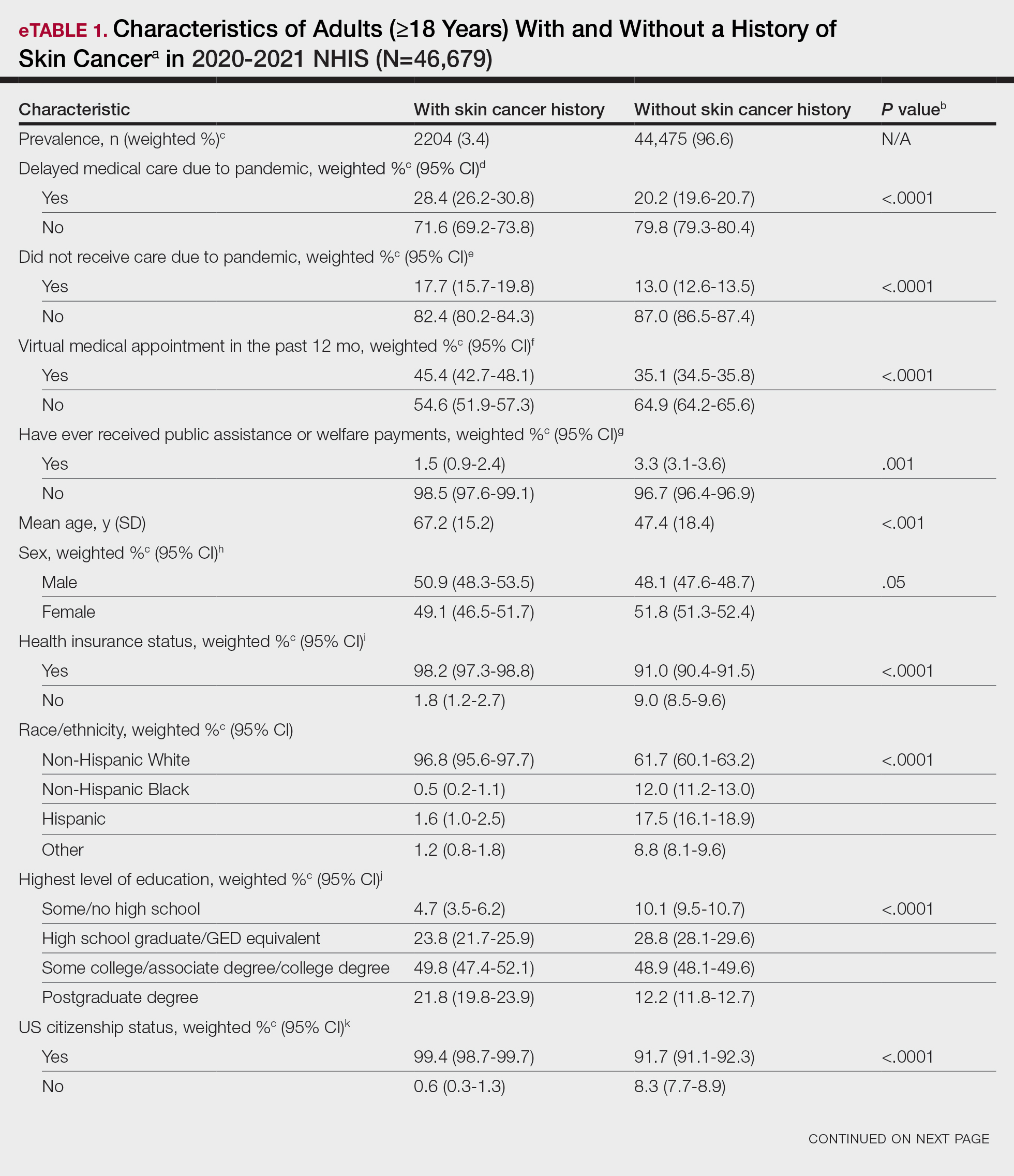

Using anonymous survey data from the 2020-2021 National Health Interview Survey,4 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with a self-reported history of skin cancer—melanoma, nonmelanoma skin cancer, or unknown skin cancer. The 3 outcome variables included having a virtual medical appointment in the past 12 months (yes/no), delaying medical care due to the COVID-19 pandemic (yes/no), and not receiving care due to the COVID-19 pandemic (yes/no). Multivariable logistic regression models evaluating the relationship between a history of skin cancer and access to care were constructed using Stata/MP 17.0 (StataCorp LLC). We controlled for patient age; education; race/ethnicity; received public assistance or welfare payments; sex; region; US citizenship status; health insurance status; comorbidities including history of hypertension, diabetes, and hypercholesterolemia; and birthplace in the United States in the logistic regression models.
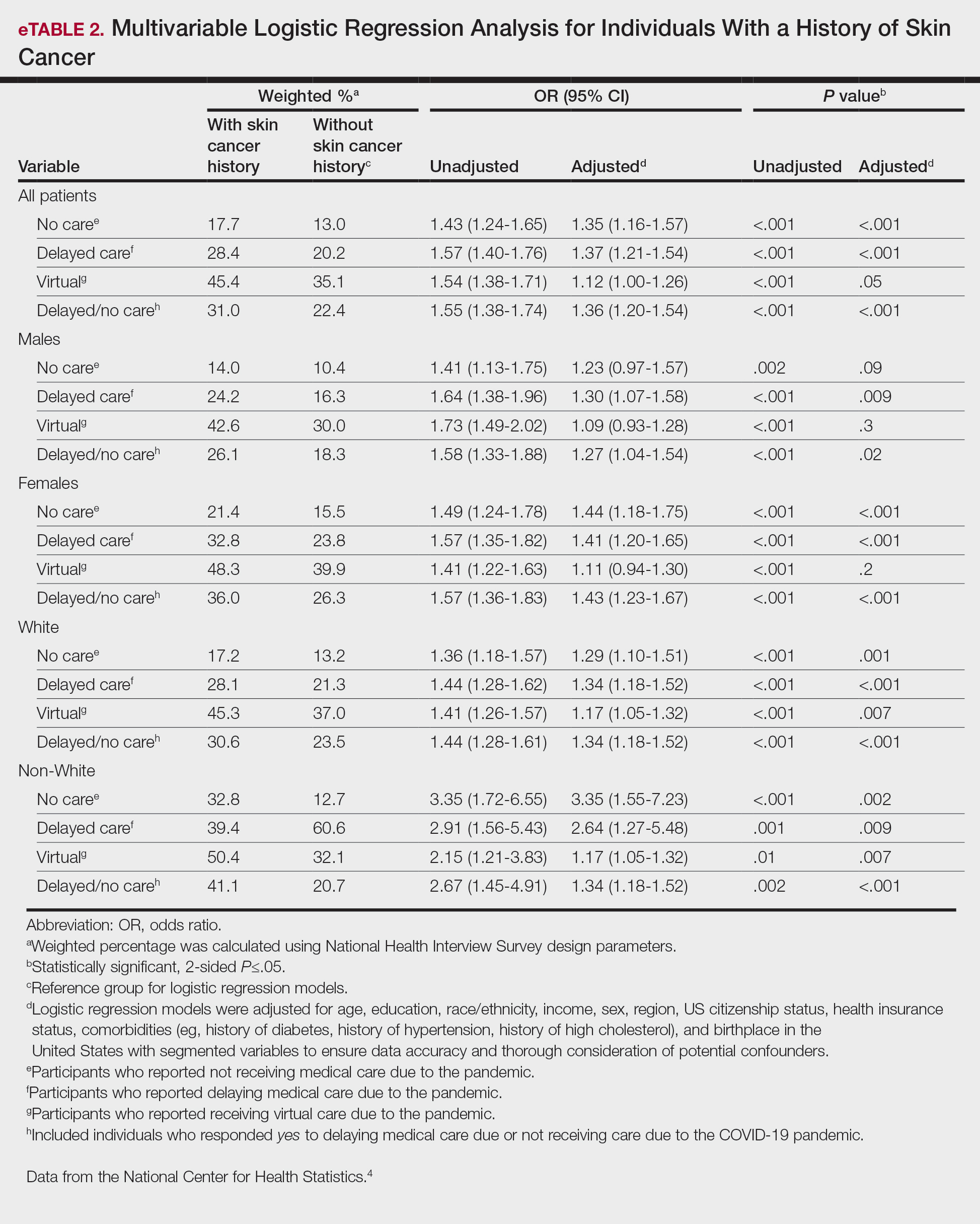
Our analysis included 46,679 patients aged 18 years or older, of whom 3.4% (weighted)(n=2204) reported a history of skin cancer (eTable 1). The weighted percentage was calculated using National Health Interview Survey design parameters (accounting for the multistage sampling design) to represent the general US population. Compared with those with no history of skin cancer, patients with a history of skin cancer were significantly more likely to delay medical care (adjusted odds ratio [AOR], 1.37; 95% CI, 1.21-1.54; P<.001) or not receive care (AOR, 1.35; 95% CI, 1.16-1.57; P<.001) due to the pandemic and were more likely to have had a virtual medical visit in the past 12 months (AOR, 1.12; 95% CI, 1.00-1.26; P=.05). Additionally, subgroup analysis revealed that females were more likely than males to forego medical care (eTable 2). β Coefficients for independent and dependent variables were further analyzed using logistic regression (eTable 3).
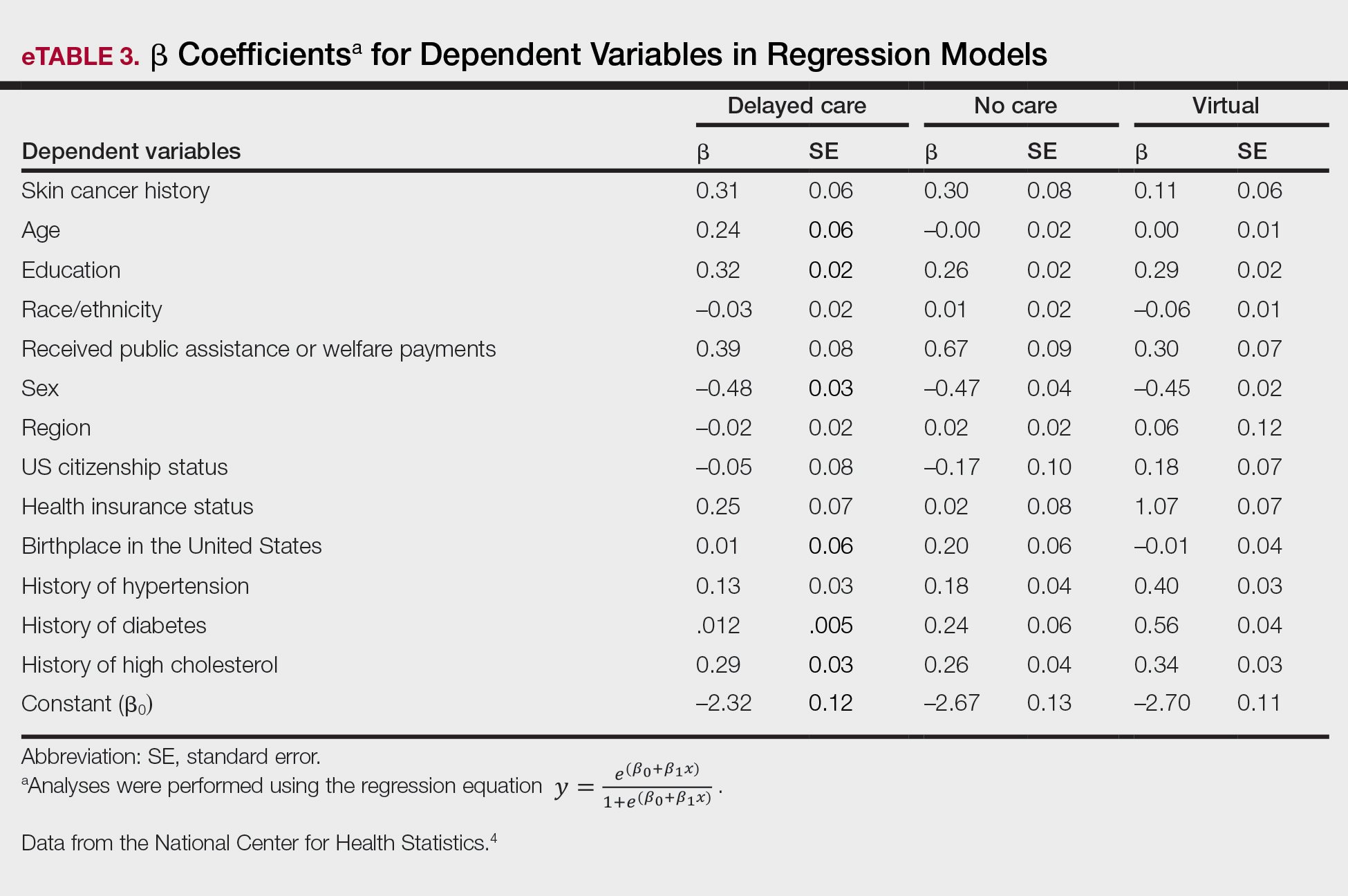
After adjusting for various potential confounders including comorbidities, our results revealed that patients with a history of skin cancer reported that they were less likely to receive in-person medical care due to the COVID-19 pandemic, as high-risk individuals with a history of skin cancer may have stopped receiving total-body skin examinations and dermatology care during the pandemic. Our findings showed that patients with a history of skin cancer were more likely than those without skin cancer to delay or forego care due to the pandemic, which may contribute to a higher incidence of advanced-stage melanomas postpandemic. Trepanowski et al5 reported an increased incidence of patients presenting with more advanced melanomas during the pandemic. Telemedicine was more commonly utilized by patients with a history of skin cancer during the pandemic.
In the future, virtual care may help limit advanced stages of skin cancer by serving as a viable alternative to in-person care.6 It has been reported that telemedicine can serve as a useful triage service reducing patient wait times.7 Teledermatology should not replace in-person care, as there is no evidence of the diagnostic accuracy of this service and many patients still will need to be seen in-person for confirmation of their diagnosis and potential biopsy. Further studies are needed to assess for missed skin cancer diagnoses due to the utilization of telemedicine.
Limitations of this study included a self-reported history of skin cancer, β coefficients that may suggest a high degree of collinearity, and lack of specific survey questions regarding dermatologic care during the COVID-19 pandemic. Further long-term studies exploring the clinical applicability and diagnostic accuracy of virtual medicine visits for cutaneous malignancies are vital, as teledermatology may play an essential role in curbing rising skin cancer rates even beyond the pandemic.
- Guy GP Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64:591-596.
- Whiteman DC, Olsen CM, MacGregor S, et al; QSkin Study. The effect of screening on melanoma incidence and biopsy rates. Br J Dermatol. 2022;187:515-522. doi:10.1111/bjd.21649
- Jobbágy A, Kiss N, Meznerics FA, et al. Emergency use and efficacy of an asynchronous teledermatology system as a novel tool for early diagnosis of skin cancer during the first wave of COVID-19 pandemic. Int J Environ Res Public Health. 2022;19:2699. doi:10.3390/ijerph19052699
- National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed April 19, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Chiru MR, Hindocha S, Burova E, et al. Management of the two-week wait pathway for skin cancer patients, before and during the pandemic: is virtual consultation an option? J Pers Med. 2022;12:1258. doi:10.3390/jpm12081258
- Finnane A Dallest K Janda M et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327. doi:10.1001/jamadermatol.2016.4361
To the Editor:
The most common malignancy in the United States is skin cancer, with melanoma accounting for the majority of skin cancer deaths.1 Despite the lack of established guidelines for routine total-body skin examinations, many patients regularly visit their dermatologist for assessment of pigmented skin lesions.2 During the COVID-19 pandemic, many patients were unable to attend in-person dermatology visits, which resulted in many high-risk individuals not receiving care or alternatively seeking virtual care for cutaneous lesions.3 There has been a lack of research in the United States exploring the utilization of teledermatology during the pandemic and its overall impact on the care of patients with a history of skin cancer. We explored the impact of the COVID-19 pandemic on care for patients with skin cancer in a large US population.


Using anonymous survey data from the 2020-2021 National Health Interview Survey,4 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with a self-reported history of skin cancer—melanoma, nonmelanoma skin cancer, or unknown skin cancer. The 3 outcome variables included having a virtual medical appointment in the past 12 months (yes/no), delaying medical care due to the COVID-19 pandemic (yes/no), and not receiving care due to the COVID-19 pandemic (yes/no). Multivariable logistic regression models evaluating the relationship between a history of skin cancer and access to care were constructed using Stata/MP 17.0 (StataCorp LLC). We controlled for patient age; education; race/ethnicity; received public assistance or welfare payments; sex; region; US citizenship status; health insurance status; comorbidities including history of hypertension, diabetes, and hypercholesterolemia; and birthplace in the United States in the logistic regression models.

Our analysis included 46,679 patients aged 18 years or older, of whom 3.4% (weighted)(n=2204) reported a history of skin cancer (eTable 1). The weighted percentage was calculated using National Health Interview Survey design parameters (accounting for the multistage sampling design) to represent the general US population. Compared with those with no history of skin cancer, patients with a history of skin cancer were significantly more likely to delay medical care (adjusted odds ratio [AOR], 1.37; 95% CI, 1.21-1.54; P<.001) or not receive care (AOR, 1.35; 95% CI, 1.16-1.57; P<.001) due to the pandemic and were more likely to have had a virtual medical visit in the past 12 months (AOR, 1.12; 95% CI, 1.00-1.26; P=.05). Additionally, subgroup analysis revealed that females were more likely than males to forego medical care (eTable 2). β Coefficients for independent and dependent variables were further analyzed using logistic regression (eTable 3).

After adjusting for various potential confounders including comorbidities, our results revealed that patients with a history of skin cancer reported that they were less likely to receive in-person medical care due to the COVID-19 pandemic, as high-risk individuals with a history of skin cancer may have stopped receiving total-body skin examinations and dermatology care during the pandemic. Our findings showed that patients with a history of skin cancer were more likely than those without skin cancer to delay or forego care due to the pandemic, which may contribute to a higher incidence of advanced-stage melanomas postpandemic. Trepanowski et al5 reported an increased incidence of patients presenting with more advanced melanomas during the pandemic. Telemedicine was more commonly utilized by patients with a history of skin cancer during the pandemic.
In the future, virtual care may help limit advanced stages of skin cancer by serving as a viable alternative to in-person care.6 It has been reported that telemedicine can serve as a useful triage service reducing patient wait times.7 Teledermatology should not replace in-person care, as there is no evidence of the diagnostic accuracy of this service and many patients still will need to be seen in-person for confirmation of their diagnosis and potential biopsy. Further studies are needed to assess for missed skin cancer diagnoses due to the utilization of telemedicine.
Limitations of this study included a self-reported history of skin cancer, β coefficients that may suggest a high degree of collinearity, and lack of specific survey questions regarding dermatologic care during the COVID-19 pandemic. Further long-term studies exploring the clinical applicability and diagnostic accuracy of virtual medicine visits for cutaneous malignancies are vital, as teledermatology may play an essential role in curbing rising skin cancer rates even beyond the pandemic.
To the Editor:
The most common malignancy in the United States is skin cancer, with melanoma accounting for the majority of skin cancer deaths.1 Despite the lack of established guidelines for routine total-body skin examinations, many patients regularly visit their dermatologist for assessment of pigmented skin lesions.2 During the COVID-19 pandemic, many patients were unable to attend in-person dermatology visits, which resulted in many high-risk individuals not receiving care or alternatively seeking virtual care for cutaneous lesions.3 There has been a lack of research in the United States exploring the utilization of teledermatology during the pandemic and its overall impact on the care of patients with a history of skin cancer. We explored the impact of the COVID-19 pandemic on care for patients with skin cancer in a large US population.


Using anonymous survey data from the 2020-2021 National Health Interview Survey,4 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with a self-reported history of skin cancer—melanoma, nonmelanoma skin cancer, or unknown skin cancer. The 3 outcome variables included having a virtual medical appointment in the past 12 months (yes/no), delaying medical care due to the COVID-19 pandemic (yes/no), and not receiving care due to the COVID-19 pandemic (yes/no). Multivariable logistic regression models evaluating the relationship between a history of skin cancer and access to care were constructed using Stata/MP 17.0 (StataCorp LLC). We controlled for patient age; education; race/ethnicity; received public assistance or welfare payments; sex; region; US citizenship status; health insurance status; comorbidities including history of hypertension, diabetes, and hypercholesterolemia; and birthplace in the United States in the logistic regression models.

Our analysis included 46,679 patients aged 18 years or older, of whom 3.4% (weighted)(n=2204) reported a history of skin cancer (eTable 1). The weighted percentage was calculated using National Health Interview Survey design parameters (accounting for the multistage sampling design) to represent the general US population. Compared with those with no history of skin cancer, patients with a history of skin cancer were significantly more likely to delay medical care (adjusted odds ratio [AOR], 1.37; 95% CI, 1.21-1.54; P<.001) or not receive care (AOR, 1.35; 95% CI, 1.16-1.57; P<.001) due to the pandemic and were more likely to have had a virtual medical visit in the past 12 months (AOR, 1.12; 95% CI, 1.00-1.26; P=.05). Additionally, subgroup analysis revealed that females were more likely than males to forego medical care (eTable 2). β Coefficients for independent and dependent variables were further analyzed using logistic regression (eTable 3).

After adjusting for various potential confounders including comorbidities, our results revealed that patients with a history of skin cancer reported that they were less likely to receive in-person medical care due to the COVID-19 pandemic, as high-risk individuals with a history of skin cancer may have stopped receiving total-body skin examinations and dermatology care during the pandemic. Our findings showed that patients with a history of skin cancer were more likely than those without skin cancer to delay or forego care due to the pandemic, which may contribute to a higher incidence of advanced-stage melanomas postpandemic. Trepanowski et al5 reported an increased incidence of patients presenting with more advanced melanomas during the pandemic. Telemedicine was more commonly utilized by patients with a history of skin cancer during the pandemic.
In the future, virtual care may help limit advanced stages of skin cancer by serving as a viable alternative to in-person care.6 It has been reported that telemedicine can serve as a useful triage service reducing patient wait times.7 Teledermatology should not replace in-person care, as there is no evidence of the diagnostic accuracy of this service and many patients still will need to be seen in-person for confirmation of their diagnosis and potential biopsy. Further studies are needed to assess for missed skin cancer diagnoses due to the utilization of telemedicine.
Limitations of this study included a self-reported history of skin cancer, β coefficients that may suggest a high degree of collinearity, and lack of specific survey questions regarding dermatologic care during the COVID-19 pandemic. Further long-term studies exploring the clinical applicability and diagnostic accuracy of virtual medicine visits for cutaneous malignancies are vital, as teledermatology may play an essential role in curbing rising skin cancer rates even beyond the pandemic.
- Guy GP Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64:591-596.
- Whiteman DC, Olsen CM, MacGregor S, et al; QSkin Study. The effect of screening on melanoma incidence and biopsy rates. Br J Dermatol. 2022;187:515-522. doi:10.1111/bjd.21649
- Jobbágy A, Kiss N, Meznerics FA, et al. Emergency use and efficacy of an asynchronous teledermatology system as a novel tool for early diagnosis of skin cancer during the first wave of COVID-19 pandemic. Int J Environ Res Public Health. 2022;19:2699. doi:10.3390/ijerph19052699
- National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed April 19, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Chiru MR, Hindocha S, Burova E, et al. Management of the two-week wait pathway for skin cancer patients, before and during the pandemic: is virtual consultation an option? J Pers Med. 2022;12:1258. doi:10.3390/jpm12081258
- Finnane A Dallest K Janda M et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327. doi:10.1001/jamadermatol.2016.4361
- Guy GP Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64:591-596.
- Whiteman DC, Olsen CM, MacGregor S, et al; QSkin Study. The effect of screening on melanoma incidence and biopsy rates. Br J Dermatol. 2022;187:515-522. doi:10.1111/bjd.21649
- Jobbágy A, Kiss N, Meznerics FA, et al. Emergency use and efficacy of an asynchronous teledermatology system as a novel tool for early diagnosis of skin cancer during the first wave of COVID-19 pandemic. Int J Environ Res Public Health. 2022;19:2699. doi:10.3390/ijerph19052699
- National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed April 19, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Chiru MR, Hindocha S, Burova E, et al. Management of the two-week wait pathway for skin cancer patients, before and during the pandemic: is virtual consultation an option? J Pers Med. 2022;12:1258. doi:10.3390/jpm12081258
- Finnane A Dallest K Janda M et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327. doi:10.1001/jamadermatol.2016.4361
PRACTICE POINTS
- The COVID-19 pandemic has altered the landscape of medicine, as many individuals are now utilizing telemedicine to receive care.
- Many individuals will continue to receive telemedicine moving forward, making it crucial to understand access to care.
Comment on “Skin Cancer Screening: The Paradox of Melanoma and Improved All-Cause Mortality”
To the Editor:
I was unsurprised and gratified by the information presented in the Viewpoint on skin cancer screening by Ngo1 (Cutis. 2024;113:94-96). In my 30 years as a community dermatologist, I have observed that patients who opt to have periodic full-body skin examinations usually are more health literate, more likely to have a primary care physician (PCP) who has encouraged them to do so (ie, a conscientious practitioner directing their preventive care), more likely to have a strong will to live, and less likely to have multiple stressors that preclude self-care (eg, may be less likely to have a spouse for whom they are a caregiver) compared to those who do not get screened.
Findings on a full-body skin examination may impact patients in many ways, not only by the detection of skin cancers. I have discovered the following:
- evidence of diabetes/insulin resistance in the form of acanthosis nigricans, tinea corporis, erythrasma;
- evidence of rosacea associated with excessive alcohol intake;
- evidence of smoking-related issues such as psoriasis or hidradenitis suppurativa;
- cutaneous evidence of other systemic diseases (eg, autoimmune disease, cancer);
- elucidation of other chronic health problems (eg, psoriasis of the skin as a clue for undiagnosed psoriatic arthritis); and
- detection of parasites on the skin (eg, ticks) or signs of infection that may have notable ramifications (eg, interdigital maceration of a diabetic patient with tinea pedis).
I even saw a patient who had been sent for magnetic resonance imaging for back pain by her internist without any physical examination when she actually had an erosion over the sacrum from a rug burn!
When conducting full-body skin examinations, dermatologists should not underestimate these principles:
- The “magic” of using a relatively noninvasive and sensitive screening tool—comfort and stress reduction for the patient from a thorough visual, tactile, olfactory, and auditory examination.
- Human interaction—especially when the patient is seen annually or even more frequently over a period of years or decades, and especially when an excellent patient-physician rapport has been established.
- The impact of improving a patient’s appearance on their overall sense of well-being (eg, by controlling rosacea).
- The opportunity to introduce concepts (ie, educate patients) such as alcohol avoidance, smoking cessation, weight reduction, hygiene, diet, and exercise in a more tangential way than a PCP, as well as to consider with patients the idea that lifestyle modification may be an adjunct, if not a replacement, for prescription treatments.
- The stress reduction that ensues when a variety of self-identified health issues are addressed, for which the only treatment may be reassurance.
I would add to Dr. Ngo’s argument that stratifying patients into skin cancer risk categories may be a useful measure if the only goal of periodic dermatologic evaluation is skin cancer detection. One size rarely fits all when it comes to health recommendations.
In sum, I believe that periodic full-body skin examination is absolutely beneficial to patient care, and I am not at all surprised that all-cause mortality was lower in patients who have those examinations. Furthermore, when I offer my healthy, low-risk patients the option to return in 2 years rather than 1, the vast majority insist on 1 year. My mother used to say, “It’s better to be looked over than to be overlooked,” and I tell my patients that, too—but it seems they already know that instinctively.
- Ngo BT. Skin cancer screening: the paradox of melanoma and improved all-cause mortality. Cutis. 2024;113:94-96. doi:10.12788/cutis.0948
To the Editor:
I was unsurprised and gratified by the information presented in the Viewpoint on skin cancer screening by Ngo1 (Cutis. 2024;113:94-96). In my 30 years as a community dermatologist, I have observed that patients who opt to have periodic full-body skin examinations usually are more health literate, more likely to have a primary care physician (PCP) who has encouraged them to do so (ie, a conscientious practitioner directing their preventive care), more likely to have a strong will to live, and less likely to have multiple stressors that preclude self-care (eg, may be less likely to have a spouse for whom they are a caregiver) compared to those who do not get screened.
Findings on a full-body skin examination may impact patients in many ways, not only by the detection of skin cancers. I have discovered the following:
- evidence of diabetes/insulin resistance in the form of acanthosis nigricans, tinea corporis, erythrasma;
- evidence of rosacea associated with excessive alcohol intake;
- evidence of smoking-related issues such as psoriasis or hidradenitis suppurativa;
- cutaneous evidence of other systemic diseases (eg, autoimmune disease, cancer);
- elucidation of other chronic health problems (eg, psoriasis of the skin as a clue for undiagnosed psoriatic arthritis); and
- detection of parasites on the skin (eg, ticks) or signs of infection that may have notable ramifications (eg, interdigital maceration of a diabetic patient with tinea pedis).
I even saw a patient who had been sent for magnetic resonance imaging for back pain by her internist without any physical examination when she actually had an erosion over the sacrum from a rug burn!
When conducting full-body skin examinations, dermatologists should not underestimate these principles:
- The “magic” of using a relatively noninvasive and sensitive screening tool—comfort and stress reduction for the patient from a thorough visual, tactile, olfactory, and auditory examination.
- Human interaction—especially when the patient is seen annually or even more frequently over a period of years or decades, and especially when an excellent patient-physician rapport has been established.
- The impact of improving a patient’s appearance on their overall sense of well-being (eg, by controlling rosacea).
- The opportunity to introduce concepts (ie, educate patients) such as alcohol avoidance, smoking cessation, weight reduction, hygiene, diet, and exercise in a more tangential way than a PCP, as well as to consider with patients the idea that lifestyle modification may be an adjunct, if not a replacement, for prescription treatments.
- The stress reduction that ensues when a variety of self-identified health issues are addressed, for which the only treatment may be reassurance.
I would add to Dr. Ngo’s argument that stratifying patients into skin cancer risk categories may be a useful measure if the only goal of periodic dermatologic evaluation is skin cancer detection. One size rarely fits all when it comes to health recommendations.
In sum, I believe that periodic full-body skin examination is absolutely beneficial to patient care, and I am not at all surprised that all-cause mortality was lower in patients who have those examinations. Furthermore, when I offer my healthy, low-risk patients the option to return in 2 years rather than 1, the vast majority insist on 1 year. My mother used to say, “It’s better to be looked over than to be overlooked,” and I tell my patients that, too—but it seems they already know that instinctively.
To the Editor:
I was unsurprised and gratified by the information presented in the Viewpoint on skin cancer screening by Ngo1 (Cutis. 2024;113:94-96). In my 30 years as a community dermatologist, I have observed that patients who opt to have periodic full-body skin examinations usually are more health literate, more likely to have a primary care physician (PCP) who has encouraged them to do so (ie, a conscientious practitioner directing their preventive care), more likely to have a strong will to live, and less likely to have multiple stressors that preclude self-care (eg, may be less likely to have a spouse for whom they are a caregiver) compared to those who do not get screened.
Findings on a full-body skin examination may impact patients in many ways, not only by the detection of skin cancers. I have discovered the following:
- evidence of diabetes/insulin resistance in the form of acanthosis nigricans, tinea corporis, erythrasma;
- evidence of rosacea associated with excessive alcohol intake;
- evidence of smoking-related issues such as psoriasis or hidradenitis suppurativa;
- cutaneous evidence of other systemic diseases (eg, autoimmune disease, cancer);
- elucidation of other chronic health problems (eg, psoriasis of the skin as a clue for undiagnosed psoriatic arthritis); and
- detection of parasites on the skin (eg, ticks) or signs of infection that may have notable ramifications (eg, interdigital maceration of a diabetic patient with tinea pedis).
I even saw a patient who had been sent for magnetic resonance imaging for back pain by her internist without any physical examination when she actually had an erosion over the sacrum from a rug burn!
When conducting full-body skin examinations, dermatologists should not underestimate these principles:
- The “magic” of using a relatively noninvasive and sensitive screening tool—comfort and stress reduction for the patient from a thorough visual, tactile, olfactory, and auditory examination.
- Human interaction—especially when the patient is seen annually or even more frequently over a period of years or decades, and especially when an excellent patient-physician rapport has been established.
- The impact of improving a patient’s appearance on their overall sense of well-being (eg, by controlling rosacea).
- The opportunity to introduce concepts (ie, educate patients) such as alcohol avoidance, smoking cessation, weight reduction, hygiene, diet, and exercise in a more tangential way than a PCP, as well as to consider with patients the idea that lifestyle modification may be an adjunct, if not a replacement, for prescription treatments.
- The stress reduction that ensues when a variety of self-identified health issues are addressed, for which the only treatment may be reassurance.
I would add to Dr. Ngo’s argument that stratifying patients into skin cancer risk categories may be a useful measure if the only goal of periodic dermatologic evaluation is skin cancer detection. One size rarely fits all when it comes to health recommendations.
In sum, I believe that periodic full-body skin examination is absolutely beneficial to patient care, and I am not at all surprised that all-cause mortality was lower in patients who have those examinations. Furthermore, when I offer my healthy, low-risk patients the option to return in 2 years rather than 1, the vast majority insist on 1 year. My mother used to say, “It’s better to be looked over than to be overlooked,” and I tell my patients that, too—but it seems they already know that instinctively.
- Ngo BT. Skin cancer screening: the paradox of melanoma and improved all-cause mortality. Cutis. 2024;113:94-96. doi:10.12788/cutis.0948
- Ngo BT. Skin cancer screening: the paradox of melanoma and improved all-cause mortality. Cutis. 2024;113:94-96. doi:10.12788/cutis.0948
Understanding the Evaluation and Management Add-on Complexity Code
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
PRACTICE POINTS
- The add-on code G2211 went into effect on January 1, 2024, and can be applied to outpatient evaluation and management visits that fulfill certain criteria.
- This code should be utilized when one is serving as the continuing focal point for all of the patient's health care needs or providing ongoing medical care related to a patient’s single, serious or complex condition.
A Structured Approach for the Management of Orodynia (Burning Mouth Syndrome)
Practice Gap
Orodynia (OD)—together with glossodynia colloquially termed “burning mouth syndrome”—is a chronic disorder characterized by a burning sensation within the oral cavity without objective clinical signs. It is most common in perimenopausal and postmenopausal women.1,2
Orodynia is a diagnosis of exclusion and is considered after 4 to 6 months of normal imaging and laboratory test results.1,2 Its pathophysiology is poorly understood, as it can be intermittent or continuous, manifest with a variety of symptoms, and affect various entities of the oral cavity.3,4 The most common structure affected is the tongue, and symptoms may include xerostomia, dysgeusia, and discomfort.1,2 Orodynia is a frustrating condition, as many patients do not respond to treatment and experience symptoms for years.1-4
The current approach to management of OD typically involves a combination of psychosocial strategies and pharmacologic agents. The psychosocial component consists of coping mechanisms (eg, stress management techniques and behavioral therapies) aimed at alleviating the psychological impact of the condition. Pharmacologic agents such as antidepressants, anticonvulsants, and topical medications often are prescribed to address neuropathic pain and dry mouth symptoms.1,2 Additionally, oral rinses, saliva substitutes, and dietary supplements may be recommended to counteract the discomfort associated with xerostomia.1,2 However, there is no stepwise protocol, leaving these treatments to be trialed in a disorganized manner.2
The Tools
In our unique approach to managing OD, physicians may employ a variety of tools, including autoantibody profiles, noninvasive salivary gland analysis, saliva analysis, patch testing for allergens, and—if deemed necessary—a minor salivary gland biopsy. The use of specific prescription medications is included in the later stages of our approach.
The Technique
First, exclude inflammatory conditions such as geographic tongue, oral lichen planus, autoimmune bullous disorders, and other treatable conditions such as dyspepsia and Sjögren syndrome using the tools described above. Noninvasive modalities should be exhausted first, and dermatologists/clinicians should exercise clinical judgement to determine whether all options should be trialed, including more invasive/costly ones.
If symptoms persist, clinicians may want to obtain a culture for oral candida. If results are positive, candida may be treated quickly with oral fluconazole. If that treatment fails and fissuring is present, advise the patient on treating the tongue; we recommend lightly brushing the tongue once daily with a hydrogen peroxide 3% solution, followed by rinsing. Next, the patient can allow an active probiotic yogurt to sit on the tongue for at least 1 minute to repopulate it with healthy oral bacteria.
If symptoms persist, prescribe gabapentin 100 to 300 mg to be taken at bedtime. Cevimeline 30 mg 3 times daily can be added to treat symptoms of xerostomia. As a last resort, a low daily dose of trifluoperazine 1 to 2 mg may alleviate the dysesthesia of OD. Because this medication is an antipsychotic, there is an increased risk for adverse effects such as tardive dyskinesia; however, given that we recommend using at most one-twentieth of the dose recommended for psychiatric illnesses such as schizophrenia, the risk appears to be minimal.5
We have found this protocol to be more structured, and in our practice, it has led to better outcomes than previously described therapeutic interventions.
Practice Implications
As a chronic condition, OD can be frustrating for patients, as many of them have attempted multiple treatments without success. It also may be challenging for dermatologists who are unfamiliar with its management. This approach to OD provides simple step-by-step diagnostic and therapeutic plans for a condition with an often-uncertain etiology and stubborn response to initial treatments. By following this protocol, dermatologists can be confident in their ability to help patients find relief from OD.
- Klein B, Thoppay JR, De Rossi SS, et al. Burning mouth syndrome. Dermatol Clin. 2020;38:477-483. doi:10.1016/j.det.2020.05.008
- Bender SD. Burning mouth syndrome. Dent Clin North Am. 2018;62:585-596. doi:10.1016/j.cden.2018.05.006
- Javali MA. Burning mouth syndrome: an enigmatic disorder. Kathmandu Univ Med J. 2013;11:175-178. doi:10.3126/kumj.v11i2.12498
- Sardella A, Lodi G, Demarosi F, et al. Burning mouth syndrome: a retrospective study investigating spontaneous remission and response to treatments. Oral Dis. 2006;12:152-155. doi:10.1111/j.1601-0825.2005.01174
- Macdonald R, Watts TP. Trifluoperazine dihydrochloride (stelazine) in paranoid schizophrenia. Br Med J. 1959;1:549-550. doi:10.1136/bmj.1.5121.549
Practice Gap
Orodynia (OD)—together with glossodynia colloquially termed “burning mouth syndrome”—is a chronic disorder characterized by a burning sensation within the oral cavity without objective clinical signs. It is most common in perimenopausal and postmenopausal women.1,2
Orodynia is a diagnosis of exclusion and is considered after 4 to 6 months of normal imaging and laboratory test results.1,2 Its pathophysiology is poorly understood, as it can be intermittent or continuous, manifest with a variety of symptoms, and affect various entities of the oral cavity.3,4 The most common structure affected is the tongue, and symptoms may include xerostomia, dysgeusia, and discomfort.1,2 Orodynia is a frustrating condition, as many patients do not respond to treatment and experience symptoms for years.1-4
The current approach to management of OD typically involves a combination of psychosocial strategies and pharmacologic agents. The psychosocial component consists of coping mechanisms (eg, stress management techniques and behavioral therapies) aimed at alleviating the psychological impact of the condition. Pharmacologic agents such as antidepressants, anticonvulsants, and topical medications often are prescribed to address neuropathic pain and dry mouth symptoms.1,2 Additionally, oral rinses, saliva substitutes, and dietary supplements may be recommended to counteract the discomfort associated with xerostomia.1,2 However, there is no stepwise protocol, leaving these treatments to be trialed in a disorganized manner.2
The Tools
In our unique approach to managing OD, physicians may employ a variety of tools, including autoantibody profiles, noninvasive salivary gland analysis, saliva analysis, patch testing for allergens, and—if deemed necessary—a minor salivary gland biopsy. The use of specific prescription medications is included in the later stages of our approach.
The Technique
First, exclude inflammatory conditions such as geographic tongue, oral lichen planus, autoimmune bullous disorders, and other treatable conditions such as dyspepsia and Sjögren syndrome using the tools described above. Noninvasive modalities should be exhausted first, and dermatologists/clinicians should exercise clinical judgement to determine whether all options should be trialed, including more invasive/costly ones.
If symptoms persist, clinicians may want to obtain a culture for oral candida. If results are positive, candida may be treated quickly with oral fluconazole. If that treatment fails and fissuring is present, advise the patient on treating the tongue; we recommend lightly brushing the tongue once daily with a hydrogen peroxide 3% solution, followed by rinsing. Next, the patient can allow an active probiotic yogurt to sit on the tongue for at least 1 minute to repopulate it with healthy oral bacteria.
If symptoms persist, prescribe gabapentin 100 to 300 mg to be taken at bedtime. Cevimeline 30 mg 3 times daily can be added to treat symptoms of xerostomia. As a last resort, a low daily dose of trifluoperazine 1 to 2 mg may alleviate the dysesthesia of OD. Because this medication is an antipsychotic, there is an increased risk for adverse effects such as tardive dyskinesia; however, given that we recommend using at most one-twentieth of the dose recommended for psychiatric illnesses such as schizophrenia, the risk appears to be minimal.5
We have found this protocol to be more structured, and in our practice, it has led to better outcomes than previously described therapeutic interventions.
Practice Implications
As a chronic condition, OD can be frustrating for patients, as many of them have attempted multiple treatments without success. It also may be challenging for dermatologists who are unfamiliar with its management. This approach to OD provides simple step-by-step diagnostic and therapeutic plans for a condition with an often-uncertain etiology and stubborn response to initial treatments. By following this protocol, dermatologists can be confident in their ability to help patients find relief from OD.
Practice Gap
Orodynia (OD)—together with glossodynia colloquially termed “burning mouth syndrome”—is a chronic disorder characterized by a burning sensation within the oral cavity without objective clinical signs. It is most common in perimenopausal and postmenopausal women.1,2
Orodynia is a diagnosis of exclusion and is considered after 4 to 6 months of normal imaging and laboratory test results.1,2 Its pathophysiology is poorly understood, as it can be intermittent or continuous, manifest with a variety of symptoms, and affect various entities of the oral cavity.3,4 The most common structure affected is the tongue, and symptoms may include xerostomia, dysgeusia, and discomfort.1,2 Orodynia is a frustrating condition, as many patients do not respond to treatment and experience symptoms for years.1-4
The current approach to management of OD typically involves a combination of psychosocial strategies and pharmacologic agents. The psychosocial component consists of coping mechanisms (eg, stress management techniques and behavioral therapies) aimed at alleviating the psychological impact of the condition. Pharmacologic agents such as antidepressants, anticonvulsants, and topical medications often are prescribed to address neuropathic pain and dry mouth symptoms.1,2 Additionally, oral rinses, saliva substitutes, and dietary supplements may be recommended to counteract the discomfort associated with xerostomia.1,2 However, there is no stepwise protocol, leaving these treatments to be trialed in a disorganized manner.2
The Tools
In our unique approach to managing OD, physicians may employ a variety of tools, including autoantibody profiles, noninvasive salivary gland analysis, saliva analysis, patch testing for allergens, and—if deemed necessary—a minor salivary gland biopsy. The use of specific prescription medications is included in the later stages of our approach.
The Technique
First, exclude inflammatory conditions such as geographic tongue, oral lichen planus, autoimmune bullous disorders, and other treatable conditions such as dyspepsia and Sjögren syndrome using the tools described above. Noninvasive modalities should be exhausted first, and dermatologists/clinicians should exercise clinical judgement to determine whether all options should be trialed, including more invasive/costly ones.
If symptoms persist, clinicians may want to obtain a culture for oral candida. If results are positive, candida may be treated quickly with oral fluconazole. If that treatment fails and fissuring is present, advise the patient on treating the tongue; we recommend lightly brushing the tongue once daily with a hydrogen peroxide 3% solution, followed by rinsing. Next, the patient can allow an active probiotic yogurt to sit on the tongue for at least 1 minute to repopulate it with healthy oral bacteria.
If symptoms persist, prescribe gabapentin 100 to 300 mg to be taken at bedtime. Cevimeline 30 mg 3 times daily can be added to treat symptoms of xerostomia. As a last resort, a low daily dose of trifluoperazine 1 to 2 mg may alleviate the dysesthesia of OD. Because this medication is an antipsychotic, there is an increased risk for adverse effects such as tardive dyskinesia; however, given that we recommend using at most one-twentieth of the dose recommended for psychiatric illnesses such as schizophrenia, the risk appears to be minimal.5
We have found this protocol to be more structured, and in our practice, it has led to better outcomes than previously described therapeutic interventions.
Practice Implications
As a chronic condition, OD can be frustrating for patients, as many of them have attempted multiple treatments without success. It also may be challenging for dermatologists who are unfamiliar with its management. This approach to OD provides simple step-by-step diagnostic and therapeutic plans for a condition with an often-uncertain etiology and stubborn response to initial treatments. By following this protocol, dermatologists can be confident in their ability to help patients find relief from OD.
- Klein B, Thoppay JR, De Rossi SS, et al. Burning mouth syndrome. Dermatol Clin. 2020;38:477-483. doi:10.1016/j.det.2020.05.008
- Bender SD. Burning mouth syndrome. Dent Clin North Am. 2018;62:585-596. doi:10.1016/j.cden.2018.05.006
- Javali MA. Burning mouth syndrome: an enigmatic disorder. Kathmandu Univ Med J. 2013;11:175-178. doi:10.3126/kumj.v11i2.12498
- Sardella A, Lodi G, Demarosi F, et al. Burning mouth syndrome: a retrospective study investigating spontaneous remission and response to treatments. Oral Dis. 2006;12:152-155. doi:10.1111/j.1601-0825.2005.01174
- Macdonald R, Watts TP. Trifluoperazine dihydrochloride (stelazine) in paranoid schizophrenia. Br Med J. 1959;1:549-550. doi:10.1136/bmj.1.5121.549
- Klein B, Thoppay JR, De Rossi SS, et al. Burning mouth syndrome. Dermatol Clin. 2020;38:477-483. doi:10.1016/j.det.2020.05.008
- Bender SD. Burning mouth syndrome. Dent Clin North Am. 2018;62:585-596. doi:10.1016/j.cden.2018.05.006
- Javali MA. Burning mouth syndrome: an enigmatic disorder. Kathmandu Univ Med J. 2013;11:175-178. doi:10.3126/kumj.v11i2.12498
- Sardella A, Lodi G, Demarosi F, et al. Burning mouth syndrome: a retrospective study investigating spontaneous remission and response to treatments. Oral Dis. 2006;12:152-155. doi:10.1111/j.1601-0825.2005.01174
- Macdonald R, Watts TP. Trifluoperazine dihydrochloride (stelazine) in paranoid schizophrenia. Br Med J. 1959;1:549-550. doi:10.1136/bmj.1.5121.549
The DEA Plans to Reschedule Marijuana: What Happens Next?
The US Drug Enforcement Agency (DEA) is moving forward with plans to move marijuana from a Schedule I to a Schedule III controlled substance under the Controlled Substance Act (CSA), the US Department of Justice officials announced this week.
First reported by the Associated Press and since confirmed by this news organization through a US Department of Justice spokesperson, the news made international headlines. Despite the media splash, the final rule is still months away.
How did we get here? What happens next? What impact might rescheduling have on clinicians, patients, researchers, and the medical cannabis industry?
Why Reschedule? Why Now?
The DEA’s decision is based on a 2023 determination from the US Food and Drug Administration (FDA) that marijuana has a legitimate medical use and should be moved to Schedule III.
This class includes ketamine, acetaminophen with codeine, and buprenorphine.
Even though the manufacturing, distribution, sale, and use of marijuana has long violated federal law, 38 states and Washington, DC, have legalized medical cannabis, and 24 states and DC have legalized its recreational use.
Congress has allowed states leeway for the distribution and use of medical marijuana, and current and previous presidential administrations have chosen not to aggressively pursue prosecution of state-allowed marijuana use, the Congressional Research Service (CRS) reports.
Pressure to address the conflict between federal and state laws and an increasing interest in drug development of cannabis and cannabis-derived products probably contributed to the DEA’s decision, said Stephen Strakowski, MD, professor, and vice chair of psychiatry at Indiana University in Indianapolis, and professor and associate vice president at University of Texas in Austin.
“The trend toward legalization is everywhere and even though nationally the feds in this instance are lagging the states, the pressure to legalize has been intense for 50 years and it’s not surprising that the DEA is finally following that lead,” Dr. Strakowski told this news organization.
How Does Rescheduling Work? What’s the Timeline?
The DEA will submit a formal rule proposing that marijuana be moved from Schedule I to Schedule III to the White House Office of Management and Budget. The timing of the submission is unclear.
Once the proposed rule is posted to the Federal Register, there will be a public comment period, which usually lasts 30-60 days.
“This will likely generate a lot of public comment,” Robert Mikos, JD, LaRoche Family Chair in Law at Vanderbilt University Law School in Nashville, Tennessee, told this news organization. “Then the agency has to go back and wade through those comments and decide if they want to proceed with the rule as proposed or modify it.”
A final rule will probably be posted before the end of the current presidential term in January, Mr. Mikos said. While a lawsuit blocking its implementation is possible, there is a “low chance that a court would block this,” he added.
How Will Rescheduling Affect Medical Marijuana?
For medical marijuana, changing the drug to a Schedule III means that it can legally be prescribed but only in states that have legalized medical cannabis, Mr. Mikos said.
“If you’re a patient in a state with a medical marijuana law and your physician gives you a prescription for medical marijuana and you possess it, you will no longer be guilty of a federal crime,” he said.
Rescheduling could also benefit patients who receive care through the Veterans Administration (VA), Mr. Mikos said. For several years, the VA has had a policy that blocked clinicians from prescribing medical marijuana because as a Schedule I drug, it was determined to have no accepted medical use.
“It’s possible the VA may drop that policy once the drug gets rescheduled. If you’re in a medical marijuana state, if you’re a VA patient, and you don’t want to spend the extra money to go outside that system, this will have meaningful impact on their lives,” Mr. Mikos said.
But what about patients living in states that have not legalized medical cannabis?
“You still wouldn’t be committing a federal crime, but you could be violating state law,” Mr. Mikos said. “That’s a much more salient consideration because if you look at who goes after individuals who possess small amounts of drugs, the state handles 99% of those cases.”
The manufacture, distribution, and possession of recreational marijuana would remain illegal under federal law.
What Does It Mean for Medical Marijuana Dispensaries?
Though rescheduling makes it legal for clinicians to prescribe medical marijuana and for patients to use it, the actual sale of the drug will remain illegal under federal law because rescheduling only changes prescribing under the CSA, Mr. Mikos said.
“If you’re a dispensary and you sell it, even if it’s to somebody who’s got a prescription, you’re still probably violating the Food, Drug and Cosmetics Act. Rescheduling doesn’t change that,” he said.
“Even assuming the DEA follows through with this and it doesn’t come undone at some future date, the industry is still going struggle to comply with the Controlled Substances Act post rescheduling because that statute is going to continue to impose a number of regulations on the industry,” Mr. Mikos added.
However, rescheduling would change the tax status of the estimated 12,000-15,000 state-licensed cannabis dispensaries in the United States, allowing access to certain tax deductions that are unavailable to sales involving Schedule I controlled substances, James Daily, JD, MS, with Center for Empirical Research in the Law at Washington University School of Law in St. Louis, told this news organization.
“Many cannabis businesses do in fact pay federal taxes, but the inability to take any federal tax credits or deductions means that their effective tax rate is much higher than it would otherwise be,” Mr. Daily said.
Although new federal tax deductions would likely available to cannabis businesses if marijuana were rescheduled to Schedule III, “their business would still be in violation of federal law,” he said.
“This creates a further tension between state and federal law, which could be resolved by further legalization or it could be resolved by extending the prohibition on tax deductions to include cannabis and not just Schedule I and II drugs,” he added.
Will Rescheduling Make It Easier to Conduct Cannabis-Related Research?
Research on medical cannabis has been stymied by FDA and DEA regulations regarding the study of Schedule I controlled substances. Although rescheduling could lift that barrier, other challenges would remain.
“Schedule III drugs can be more easily researched, but it’s unclear if, for example, a clinical trial could lawfully obtain the cannabis from a dispensary or if they would still have to go through the one legal federal supplier of cannabis,” Daily said.
The FDA reports having received more than 800 investigational new drug applications for and pre-investigational new drug applications related to cannabis and cannabis-derived products since the 1970s, the agency reports. To date, the FDA has not approved any marketing drug applications for cannabis for the treatment of any disease or condition.
In January 2023, the agency published updated guidelines for researchers and sponsors interested in developing drugs containing cannabis or cannabis-derived compounds.
It’s unclear whether those guidelines would be updated if the rescheduling moves forward.
Does Rescheduling Marijuana Pose Any Risk?
In its report to the DEA that marijuana be rescheduled, the FDA was careful to note that the agency’s recommendation is “not meant to imply that safety and effectiveness have been established for marijuana that would support FDA approval of a marijuana drug product for a particular indication.”
That’s a notation that clinicians and patients should take to heart, Dr. Strakowski said.
“It’s important to remind people that Schedule III drugs, by definition, have addiction and other side effect risks,” he said. “The celebrity marketing that sits behind a lot of this is incompletely informed. It’s portrayed as fun and harmless in almost every movie and conversation you see, and we know that’s not true.”
Previous studies have linked cannabis to increased risk for mania, anxiety disorders, and schizophrenia.
“It is increasingly clear that marijuana use is linked to poor outcomes in people who struggle with mental illness,” Dr. Strakowski said. “We have no evidence that it can help you but there is evidence that it can harm you.”
Dr. Strakowski likens cannabis use to alcohol, which is a known depressant that is associated with worse outcomes in people with mental illness.
“I think with cannabis, we don’t know enough about it yet, but we do know that it does have some anxiety risks,” he said. “The risks in people with mental illness are simply different than in people who don’t have mental illness.”
Dr. Strakowski, Mr. Mikos, and Mr. Daily report no relevant disclosures.
A version of this article appeared on Medscape.com.
The US Drug Enforcement Agency (DEA) is moving forward with plans to move marijuana from a Schedule I to a Schedule III controlled substance under the Controlled Substance Act (CSA), the US Department of Justice officials announced this week.
First reported by the Associated Press and since confirmed by this news organization through a US Department of Justice spokesperson, the news made international headlines. Despite the media splash, the final rule is still months away.
How did we get here? What happens next? What impact might rescheduling have on clinicians, patients, researchers, and the medical cannabis industry?
Why Reschedule? Why Now?
The DEA’s decision is based on a 2023 determination from the US Food and Drug Administration (FDA) that marijuana has a legitimate medical use and should be moved to Schedule III.
This class includes ketamine, acetaminophen with codeine, and buprenorphine.
Even though the manufacturing, distribution, sale, and use of marijuana has long violated federal law, 38 states and Washington, DC, have legalized medical cannabis, and 24 states and DC have legalized its recreational use.
Congress has allowed states leeway for the distribution and use of medical marijuana, and current and previous presidential administrations have chosen not to aggressively pursue prosecution of state-allowed marijuana use, the Congressional Research Service (CRS) reports.
Pressure to address the conflict between federal and state laws and an increasing interest in drug development of cannabis and cannabis-derived products probably contributed to the DEA’s decision, said Stephen Strakowski, MD, professor, and vice chair of psychiatry at Indiana University in Indianapolis, and professor and associate vice president at University of Texas in Austin.
“The trend toward legalization is everywhere and even though nationally the feds in this instance are lagging the states, the pressure to legalize has been intense for 50 years and it’s not surprising that the DEA is finally following that lead,” Dr. Strakowski told this news organization.
How Does Rescheduling Work? What’s the Timeline?
The DEA will submit a formal rule proposing that marijuana be moved from Schedule I to Schedule III to the White House Office of Management and Budget. The timing of the submission is unclear.
Once the proposed rule is posted to the Federal Register, there will be a public comment period, which usually lasts 30-60 days.
“This will likely generate a lot of public comment,” Robert Mikos, JD, LaRoche Family Chair in Law at Vanderbilt University Law School in Nashville, Tennessee, told this news organization. “Then the agency has to go back and wade through those comments and decide if they want to proceed with the rule as proposed or modify it.”
A final rule will probably be posted before the end of the current presidential term in January, Mr. Mikos said. While a lawsuit blocking its implementation is possible, there is a “low chance that a court would block this,” he added.
How Will Rescheduling Affect Medical Marijuana?
For medical marijuana, changing the drug to a Schedule III means that it can legally be prescribed but only in states that have legalized medical cannabis, Mr. Mikos said.
“If you’re a patient in a state with a medical marijuana law and your physician gives you a prescription for medical marijuana and you possess it, you will no longer be guilty of a federal crime,” he said.
Rescheduling could also benefit patients who receive care through the Veterans Administration (VA), Mr. Mikos said. For several years, the VA has had a policy that blocked clinicians from prescribing medical marijuana because as a Schedule I drug, it was determined to have no accepted medical use.
“It’s possible the VA may drop that policy once the drug gets rescheduled. If you’re in a medical marijuana state, if you’re a VA patient, and you don’t want to spend the extra money to go outside that system, this will have meaningful impact on their lives,” Mr. Mikos said.
But what about patients living in states that have not legalized medical cannabis?
“You still wouldn’t be committing a federal crime, but you could be violating state law,” Mr. Mikos said. “That’s a much more salient consideration because if you look at who goes after individuals who possess small amounts of drugs, the state handles 99% of those cases.”
The manufacture, distribution, and possession of recreational marijuana would remain illegal under federal law.
What Does It Mean for Medical Marijuana Dispensaries?
Though rescheduling makes it legal for clinicians to prescribe medical marijuana and for patients to use it, the actual sale of the drug will remain illegal under federal law because rescheduling only changes prescribing under the CSA, Mr. Mikos said.
“If you’re a dispensary and you sell it, even if it’s to somebody who’s got a prescription, you’re still probably violating the Food, Drug and Cosmetics Act. Rescheduling doesn’t change that,” he said.
“Even assuming the DEA follows through with this and it doesn’t come undone at some future date, the industry is still going struggle to comply with the Controlled Substances Act post rescheduling because that statute is going to continue to impose a number of regulations on the industry,” Mr. Mikos added.
However, rescheduling would change the tax status of the estimated 12,000-15,000 state-licensed cannabis dispensaries in the United States, allowing access to certain tax deductions that are unavailable to sales involving Schedule I controlled substances, James Daily, JD, MS, with Center for Empirical Research in the Law at Washington University School of Law in St. Louis, told this news organization.
“Many cannabis businesses do in fact pay federal taxes, but the inability to take any federal tax credits or deductions means that their effective tax rate is much higher than it would otherwise be,” Mr. Daily said.
Although new federal tax deductions would likely available to cannabis businesses if marijuana were rescheduled to Schedule III, “their business would still be in violation of federal law,” he said.
“This creates a further tension between state and federal law, which could be resolved by further legalization or it could be resolved by extending the prohibition on tax deductions to include cannabis and not just Schedule I and II drugs,” he added.
Will Rescheduling Make It Easier to Conduct Cannabis-Related Research?
Research on medical cannabis has been stymied by FDA and DEA regulations regarding the study of Schedule I controlled substances. Although rescheduling could lift that barrier, other challenges would remain.
“Schedule III drugs can be more easily researched, but it’s unclear if, for example, a clinical trial could lawfully obtain the cannabis from a dispensary or if they would still have to go through the one legal federal supplier of cannabis,” Daily said.
The FDA reports having received more than 800 investigational new drug applications for and pre-investigational new drug applications related to cannabis and cannabis-derived products since the 1970s, the agency reports. To date, the FDA has not approved any marketing drug applications for cannabis for the treatment of any disease or condition.
In January 2023, the agency published updated guidelines for researchers and sponsors interested in developing drugs containing cannabis or cannabis-derived compounds.
It’s unclear whether those guidelines would be updated if the rescheduling moves forward.
Does Rescheduling Marijuana Pose Any Risk?
In its report to the DEA that marijuana be rescheduled, the FDA was careful to note that the agency’s recommendation is “not meant to imply that safety and effectiveness have been established for marijuana that would support FDA approval of a marijuana drug product for a particular indication.”
That’s a notation that clinicians and patients should take to heart, Dr. Strakowski said.
“It’s important to remind people that Schedule III drugs, by definition, have addiction and other side effect risks,” he said. “The celebrity marketing that sits behind a lot of this is incompletely informed. It’s portrayed as fun and harmless in almost every movie and conversation you see, and we know that’s not true.”
Previous studies have linked cannabis to increased risk for mania, anxiety disorders, and schizophrenia.
“It is increasingly clear that marijuana use is linked to poor outcomes in people who struggle with mental illness,” Dr. Strakowski said. “We have no evidence that it can help you but there is evidence that it can harm you.”
Dr. Strakowski likens cannabis use to alcohol, which is a known depressant that is associated with worse outcomes in people with mental illness.
“I think with cannabis, we don’t know enough about it yet, but we do know that it does have some anxiety risks,” he said. “The risks in people with mental illness are simply different than in people who don’t have mental illness.”
Dr. Strakowski, Mr. Mikos, and Mr. Daily report no relevant disclosures.
A version of this article appeared on Medscape.com.
The US Drug Enforcement Agency (DEA) is moving forward with plans to move marijuana from a Schedule I to a Schedule III controlled substance under the Controlled Substance Act (CSA), the US Department of Justice officials announced this week.
First reported by the Associated Press and since confirmed by this news organization through a US Department of Justice spokesperson, the news made international headlines. Despite the media splash, the final rule is still months away.
How did we get here? What happens next? What impact might rescheduling have on clinicians, patients, researchers, and the medical cannabis industry?
Why Reschedule? Why Now?
The DEA’s decision is based on a 2023 determination from the US Food and Drug Administration (FDA) that marijuana has a legitimate medical use and should be moved to Schedule III.
This class includes ketamine, acetaminophen with codeine, and buprenorphine.
Even though the manufacturing, distribution, sale, and use of marijuana has long violated federal law, 38 states and Washington, DC, have legalized medical cannabis, and 24 states and DC have legalized its recreational use.
Congress has allowed states leeway for the distribution and use of medical marijuana, and current and previous presidential administrations have chosen not to aggressively pursue prosecution of state-allowed marijuana use, the Congressional Research Service (CRS) reports.
Pressure to address the conflict between federal and state laws and an increasing interest in drug development of cannabis and cannabis-derived products probably contributed to the DEA’s decision, said Stephen Strakowski, MD, professor, and vice chair of psychiatry at Indiana University in Indianapolis, and professor and associate vice president at University of Texas in Austin.
“The trend toward legalization is everywhere and even though nationally the feds in this instance are lagging the states, the pressure to legalize has been intense for 50 years and it’s not surprising that the DEA is finally following that lead,” Dr. Strakowski told this news organization.
How Does Rescheduling Work? What’s the Timeline?
The DEA will submit a formal rule proposing that marijuana be moved from Schedule I to Schedule III to the White House Office of Management and Budget. The timing of the submission is unclear.
Once the proposed rule is posted to the Federal Register, there will be a public comment period, which usually lasts 30-60 days.
“This will likely generate a lot of public comment,” Robert Mikos, JD, LaRoche Family Chair in Law at Vanderbilt University Law School in Nashville, Tennessee, told this news organization. “Then the agency has to go back and wade through those comments and decide if they want to proceed with the rule as proposed or modify it.”
A final rule will probably be posted before the end of the current presidential term in January, Mr. Mikos said. While a lawsuit blocking its implementation is possible, there is a “low chance that a court would block this,” he added.
How Will Rescheduling Affect Medical Marijuana?
For medical marijuana, changing the drug to a Schedule III means that it can legally be prescribed but only in states that have legalized medical cannabis, Mr. Mikos said.
“If you’re a patient in a state with a medical marijuana law and your physician gives you a prescription for medical marijuana and you possess it, you will no longer be guilty of a federal crime,” he said.
Rescheduling could also benefit patients who receive care through the Veterans Administration (VA), Mr. Mikos said. For several years, the VA has had a policy that blocked clinicians from prescribing medical marijuana because as a Schedule I drug, it was determined to have no accepted medical use.
“It’s possible the VA may drop that policy once the drug gets rescheduled. If you’re in a medical marijuana state, if you’re a VA patient, and you don’t want to spend the extra money to go outside that system, this will have meaningful impact on their lives,” Mr. Mikos said.
But what about patients living in states that have not legalized medical cannabis?
“You still wouldn’t be committing a federal crime, but you could be violating state law,” Mr. Mikos said. “That’s a much more salient consideration because if you look at who goes after individuals who possess small amounts of drugs, the state handles 99% of those cases.”
The manufacture, distribution, and possession of recreational marijuana would remain illegal under federal law.
What Does It Mean for Medical Marijuana Dispensaries?
Though rescheduling makes it legal for clinicians to prescribe medical marijuana and for patients to use it, the actual sale of the drug will remain illegal under federal law because rescheduling only changes prescribing under the CSA, Mr. Mikos said.
“If you’re a dispensary and you sell it, even if it’s to somebody who’s got a prescription, you’re still probably violating the Food, Drug and Cosmetics Act. Rescheduling doesn’t change that,” he said.
“Even assuming the DEA follows through with this and it doesn’t come undone at some future date, the industry is still going struggle to comply with the Controlled Substances Act post rescheduling because that statute is going to continue to impose a number of regulations on the industry,” Mr. Mikos added.
However, rescheduling would change the tax status of the estimated 12,000-15,000 state-licensed cannabis dispensaries in the United States, allowing access to certain tax deductions that are unavailable to sales involving Schedule I controlled substances, James Daily, JD, MS, with Center for Empirical Research in the Law at Washington University School of Law in St. Louis, told this news organization.
“Many cannabis businesses do in fact pay federal taxes, but the inability to take any federal tax credits or deductions means that their effective tax rate is much higher than it would otherwise be,” Mr. Daily said.
Although new federal tax deductions would likely available to cannabis businesses if marijuana were rescheduled to Schedule III, “their business would still be in violation of federal law,” he said.
“This creates a further tension between state and federal law, which could be resolved by further legalization or it could be resolved by extending the prohibition on tax deductions to include cannabis and not just Schedule I and II drugs,” he added.
Will Rescheduling Make It Easier to Conduct Cannabis-Related Research?
Research on medical cannabis has been stymied by FDA and DEA regulations regarding the study of Schedule I controlled substances. Although rescheduling could lift that barrier, other challenges would remain.
“Schedule III drugs can be more easily researched, but it’s unclear if, for example, a clinical trial could lawfully obtain the cannabis from a dispensary or if they would still have to go through the one legal federal supplier of cannabis,” Daily said.
The FDA reports having received more than 800 investigational new drug applications for and pre-investigational new drug applications related to cannabis and cannabis-derived products since the 1970s, the agency reports. To date, the FDA has not approved any marketing drug applications for cannabis for the treatment of any disease or condition.
In January 2023, the agency published updated guidelines for researchers and sponsors interested in developing drugs containing cannabis or cannabis-derived compounds.
It’s unclear whether those guidelines would be updated if the rescheduling moves forward.
Does Rescheduling Marijuana Pose Any Risk?
In its report to the DEA that marijuana be rescheduled, the FDA was careful to note that the agency’s recommendation is “not meant to imply that safety and effectiveness have been established for marijuana that would support FDA approval of a marijuana drug product for a particular indication.”
That’s a notation that clinicians and patients should take to heart, Dr. Strakowski said.
“It’s important to remind people that Schedule III drugs, by definition, have addiction and other side effect risks,” he said. “The celebrity marketing that sits behind a lot of this is incompletely informed. It’s portrayed as fun and harmless in almost every movie and conversation you see, and we know that’s not true.”
Previous studies have linked cannabis to increased risk for mania, anxiety disorders, and schizophrenia.
“It is increasingly clear that marijuana use is linked to poor outcomes in people who struggle with mental illness,” Dr. Strakowski said. “We have no evidence that it can help you but there is evidence that it can harm you.”
Dr. Strakowski likens cannabis use to alcohol, which is a known depressant that is associated with worse outcomes in people with mental illness.
“I think with cannabis, we don’t know enough about it yet, but we do know that it does have some anxiety risks,” he said. “The risks in people with mental illness are simply different than in people who don’t have mental illness.”
Dr. Strakowski, Mr. Mikos, and Mr. Daily report no relevant disclosures.
A version of this article appeared on Medscape.com.
Risk of Knee OA From Weight-Bearing Exercise Seen Only With Low Muscle Mass
Weight-bearing recreational activity was associated with a 22% increased odds of developing knee osteoarthritis (OA) in a large prospective cohort study in the Netherlands, but notably, the increased risk was seen only in those with low levels of lower-limb muscle mass.
The findings point toward the value of “tailored advice” for physical activity, and suggest that “caution is needed when engaging in weight-bearing activity, especially for individuals with low levels of lower-limb muscle mass,” Yahong Wu, MD, and coinvestigators, of the Erasmus Medical Center in Rotterdam, the Netherlands, wrote in JAMA Network Open.
Investigators used data from sequential cohorts of the longitudinal Rotterdam Study, which enrolled people aged 45 and older starting in 1990. The 5003 participants in this new analysis of physical activity and knee OA had complete records of baseline recreational physical activity, baseline knee pain, and knee radiographs from both baseline and at least one follow-up exam. Those with radiographically defined knee OA at baseline were excluded.
The incident rate of radiographically defined (x-ray) knee OA among all participants was 8.4%, with a mean follow-up time of 6.33 years. Among 3492 individuals without baseline knee pain, the researchers found no increased odds of incident radiographic OA with non–weight-bearing activity (odds ratio [OR], 1.04; 95% CI, 0.95-1.15; P = .37) but a significant association of weight-bearing activity with OA incidence (OR, 1.22; 95% CI, 1.10-1.35; P < .001).
A stratification analysis of a subset of participants whose lower-limb mass had been measured by dual-energy x-ray absorptiometry (DXA) showed, however, that the association of weight-bearing activity with incident OA was limited to patients in the lowest third of lower-limb muscle mass index (LMI), who had a 53% increased likelihood of developing knee OA (OR, 1.53; 95% CI, 1.15-2.04; P = .003).
For patients in the middle and upper tertiles, there was no significant association between weight-bearing activity and the odds of incident OA (OR, 0.93; P = .73, and OR, 1.15; P = .40, respectively).
The findings are reassuring overall, said Kelli D. Allen, PhD, research professor of medicine and exercise physiologist at the University of North Carolina at Chapel Hill, who was asked to comment on the study. “The study corroborates prior research showing that for most people, weight-bearing recreational activity does not increase the risk of knee osteoarthritis. This should be encouraging for people who want to increase their physical activity,” she said.
The study also suggests that “for people with low lower-limb muscle mass, there may be some considerations to make regarding the best type of physical activity to prevent future knee osteoarthritis,” she said in an e-mail. “The best approach may include non–weight-bearing activities, which could include biking, swimming, or other water exercises, along with strengthening exercises that help to increase muscle mass.”
Other studies, Dr. Allen said, have shown that low muscle mass itself is a risk factor for knee OA.
Physical Activity Types, Other Analyses
The researchers assessed total, weight-bearing, and non–weight-bearing physical activity using two validated questionnaires (an adapted version of the Zutphen Physical Activity Questionnaire and the Longitudinal Aging Study Amsterdam physical activity questionnaire) that asked participants about the frequency and duration of various types of physical activity. Activity was quantified as metabolic equivalent of task (MET) hours per week, and weight-bearing activities were defined as those in which the knee joint bears the body’s weight.
Walking, gardening, golf, dancing, and ball sports were among the activities qualifying as weight-bearing activities. Non–weight-bearing activities included cycling, rowing, and swimming.
Sex, body mass index, and follow-up time were among the covariates adjusted for in the primary analysis. Similar results were found when adjustments were also made for educational level, alcohol intake, lipid levels, and diabetes.
While incident radiographic knee OA (measured using the Kellgren & Lawrence grading system) was the primary outcome, the researchers also looked at symptomatic knee OA, as defined by x-ray and a knee pain questionnaire, and found no significant association of its incidence with any of the exercise categories (total, weight-bearing, or non-weight-bearing).
Coauthor Joyce B. J. van Meurs, PhD, of the departments of internal medicine and orthopedics & sports medicine at Erasmus Medical Center, told this news organization that “pain as a subjective, recurrent symptom is more difficult to study … [and] a larger sample size or more precise measurements [of pain] in future studies would help to better understand the true association” of symptomatic knee OA and physical activity.
Similarly, analyses of the 1511 patients (out of 5003) who had knee pain at baseline found no significant association of weight-bearing or non–weight-bearing physical activity with incident radiographic knee OA. The trends were similar to those found in the population without knee pain, however, which suggests the analysis was underpowered, the researchers wrote, noting too that patients with baseline pain had lower activity levels than those without pain. (Low case numbers precluded a stratification analysis on LMI for incident symptomatic OA.)
Thigh Circumference as an Indicator of Muscle Mass
The findings build upon an international meta-analysis published in 2021 that found no association between total physical activity and knee OA and align with other studies suggesting a link between greater mechanical stress/strain and greater OA risk, the researchers wrote. (The meta-analysis couldn’t investigate different types of activity.)
“Although we cannot establish a causal relationship … we hypothesize that the mechanical loading on joints and cartilage could explain the association of weight-bearing activity with osteoarthritis in the low LMI tertile group,” they said.
It is possible that thigh muscle-specific strength or mass may temper the risk of knee OA, they wrote, but the lack of thigh strength data in the Rotterdam Study precluded such evaluation. Still, in everyday practice, the researchers noted, lower limb muscle function could be assessed using thigh circumference.
Dr. Allen agreed. “ ‘Gold standard’ assessment of muscle mass is not common in routine practice, but clinicians can evaluate muscle mass in other ways, such as thigh circumference,” she told this news organization, noting that measurement should align with procedures described by the National Health and Nutrition Examination Survey in its anthropometry procedures manual.
“If low lower-limb muscle mass is suspected, a referral to a physical therapist can be helpful for more formally assessing muscle mass and muscle strength,” she added, “and for instructions for a safe and appropriate exercise program for building muscle and protecting joints.”
Among other limitations of the study, according to the researchers, are an ethnically nondiverse population, the unavailability of knee injury data, and the assessment of physical activity only at baseline.
Moving forward, Dr. van Meurs told this news organization, “the main question regarding physical activity and OA is still, if people already have pain or early OA complaints, what kinds of sports they can do without hurting their joints?” This “should be tested,” she said, “in a real-life, ideally trial-like intervention study.”
The study was funded by the Erasmus Medical Center and Erasmus University as well as through various government grants. Dr. Wu also had study support from the China Scholarship Council. Two of the authors reported relationships with arthritis-related organizations. Dr. Allen reported having no disclosures relevant to her comments.
Weight-bearing recreational activity was associated with a 22% increased odds of developing knee osteoarthritis (OA) in a large prospective cohort study in the Netherlands, but notably, the increased risk was seen only in those with low levels of lower-limb muscle mass.
The findings point toward the value of “tailored advice” for physical activity, and suggest that “caution is needed when engaging in weight-bearing activity, especially for individuals with low levels of lower-limb muscle mass,” Yahong Wu, MD, and coinvestigators, of the Erasmus Medical Center in Rotterdam, the Netherlands, wrote in JAMA Network Open.
Investigators used data from sequential cohorts of the longitudinal Rotterdam Study, which enrolled people aged 45 and older starting in 1990. The 5003 participants in this new analysis of physical activity and knee OA had complete records of baseline recreational physical activity, baseline knee pain, and knee radiographs from both baseline and at least one follow-up exam. Those with radiographically defined knee OA at baseline were excluded.
The incident rate of radiographically defined (x-ray) knee OA among all participants was 8.4%, with a mean follow-up time of 6.33 years. Among 3492 individuals without baseline knee pain, the researchers found no increased odds of incident radiographic OA with non–weight-bearing activity (odds ratio [OR], 1.04; 95% CI, 0.95-1.15; P = .37) but a significant association of weight-bearing activity with OA incidence (OR, 1.22; 95% CI, 1.10-1.35; P < .001).
A stratification analysis of a subset of participants whose lower-limb mass had been measured by dual-energy x-ray absorptiometry (DXA) showed, however, that the association of weight-bearing activity with incident OA was limited to patients in the lowest third of lower-limb muscle mass index (LMI), who had a 53% increased likelihood of developing knee OA (OR, 1.53; 95% CI, 1.15-2.04; P = .003).
For patients in the middle and upper tertiles, there was no significant association between weight-bearing activity and the odds of incident OA (OR, 0.93; P = .73, and OR, 1.15; P = .40, respectively).
The findings are reassuring overall, said Kelli D. Allen, PhD, research professor of medicine and exercise physiologist at the University of North Carolina at Chapel Hill, who was asked to comment on the study. “The study corroborates prior research showing that for most people, weight-bearing recreational activity does not increase the risk of knee osteoarthritis. This should be encouraging for people who want to increase their physical activity,” she said.
The study also suggests that “for people with low lower-limb muscle mass, there may be some considerations to make regarding the best type of physical activity to prevent future knee osteoarthritis,” she said in an e-mail. “The best approach may include non–weight-bearing activities, which could include biking, swimming, or other water exercises, along with strengthening exercises that help to increase muscle mass.”
Other studies, Dr. Allen said, have shown that low muscle mass itself is a risk factor for knee OA.
Physical Activity Types, Other Analyses
The researchers assessed total, weight-bearing, and non–weight-bearing physical activity using two validated questionnaires (an adapted version of the Zutphen Physical Activity Questionnaire and the Longitudinal Aging Study Amsterdam physical activity questionnaire) that asked participants about the frequency and duration of various types of physical activity. Activity was quantified as metabolic equivalent of task (MET) hours per week, and weight-bearing activities were defined as those in which the knee joint bears the body’s weight.
Walking, gardening, golf, dancing, and ball sports were among the activities qualifying as weight-bearing activities. Non–weight-bearing activities included cycling, rowing, and swimming.
Sex, body mass index, and follow-up time were among the covariates adjusted for in the primary analysis. Similar results were found when adjustments were also made for educational level, alcohol intake, lipid levels, and diabetes.
While incident radiographic knee OA (measured using the Kellgren & Lawrence grading system) was the primary outcome, the researchers also looked at symptomatic knee OA, as defined by x-ray and a knee pain questionnaire, and found no significant association of its incidence with any of the exercise categories (total, weight-bearing, or non-weight-bearing).
Coauthor Joyce B. J. van Meurs, PhD, of the departments of internal medicine and orthopedics & sports medicine at Erasmus Medical Center, told this news organization that “pain as a subjective, recurrent symptom is more difficult to study … [and] a larger sample size or more precise measurements [of pain] in future studies would help to better understand the true association” of symptomatic knee OA and physical activity.
Similarly, analyses of the 1511 patients (out of 5003) who had knee pain at baseline found no significant association of weight-bearing or non–weight-bearing physical activity with incident radiographic knee OA. The trends were similar to those found in the population without knee pain, however, which suggests the analysis was underpowered, the researchers wrote, noting too that patients with baseline pain had lower activity levels than those without pain. (Low case numbers precluded a stratification analysis on LMI for incident symptomatic OA.)
Thigh Circumference as an Indicator of Muscle Mass
The findings build upon an international meta-analysis published in 2021 that found no association between total physical activity and knee OA and align with other studies suggesting a link between greater mechanical stress/strain and greater OA risk, the researchers wrote. (The meta-analysis couldn’t investigate different types of activity.)
“Although we cannot establish a causal relationship … we hypothesize that the mechanical loading on joints and cartilage could explain the association of weight-bearing activity with osteoarthritis in the low LMI tertile group,” they said.
It is possible that thigh muscle-specific strength or mass may temper the risk of knee OA, they wrote, but the lack of thigh strength data in the Rotterdam Study precluded such evaluation. Still, in everyday practice, the researchers noted, lower limb muscle function could be assessed using thigh circumference.
Dr. Allen agreed. “ ‘Gold standard’ assessment of muscle mass is not common in routine practice, but clinicians can evaluate muscle mass in other ways, such as thigh circumference,” she told this news organization, noting that measurement should align with procedures described by the National Health and Nutrition Examination Survey in its anthropometry procedures manual.
“If low lower-limb muscle mass is suspected, a referral to a physical therapist can be helpful for more formally assessing muscle mass and muscle strength,” she added, “and for instructions for a safe and appropriate exercise program for building muscle and protecting joints.”
Among other limitations of the study, according to the researchers, are an ethnically nondiverse population, the unavailability of knee injury data, and the assessment of physical activity only at baseline.
Moving forward, Dr. van Meurs told this news organization, “the main question regarding physical activity and OA is still, if people already have pain or early OA complaints, what kinds of sports they can do without hurting their joints?” This “should be tested,” she said, “in a real-life, ideally trial-like intervention study.”
The study was funded by the Erasmus Medical Center and Erasmus University as well as through various government grants. Dr. Wu also had study support from the China Scholarship Council. Two of the authors reported relationships with arthritis-related organizations. Dr. Allen reported having no disclosures relevant to her comments.
Weight-bearing recreational activity was associated with a 22% increased odds of developing knee osteoarthritis (OA) in a large prospective cohort study in the Netherlands, but notably, the increased risk was seen only in those with low levels of lower-limb muscle mass.
The findings point toward the value of “tailored advice” for physical activity, and suggest that “caution is needed when engaging in weight-bearing activity, especially for individuals with low levels of lower-limb muscle mass,” Yahong Wu, MD, and coinvestigators, of the Erasmus Medical Center in Rotterdam, the Netherlands, wrote in JAMA Network Open.
Investigators used data from sequential cohorts of the longitudinal Rotterdam Study, which enrolled people aged 45 and older starting in 1990. The 5003 participants in this new analysis of physical activity and knee OA had complete records of baseline recreational physical activity, baseline knee pain, and knee radiographs from both baseline and at least one follow-up exam. Those with radiographically defined knee OA at baseline were excluded.
The incident rate of radiographically defined (x-ray) knee OA among all participants was 8.4%, with a mean follow-up time of 6.33 years. Among 3492 individuals without baseline knee pain, the researchers found no increased odds of incident radiographic OA with non–weight-bearing activity (odds ratio [OR], 1.04; 95% CI, 0.95-1.15; P = .37) but a significant association of weight-bearing activity with OA incidence (OR, 1.22; 95% CI, 1.10-1.35; P < .001).
A stratification analysis of a subset of participants whose lower-limb mass had been measured by dual-energy x-ray absorptiometry (DXA) showed, however, that the association of weight-bearing activity with incident OA was limited to patients in the lowest third of lower-limb muscle mass index (LMI), who had a 53% increased likelihood of developing knee OA (OR, 1.53; 95% CI, 1.15-2.04; P = .003).
For patients in the middle and upper tertiles, there was no significant association between weight-bearing activity and the odds of incident OA (OR, 0.93; P = .73, and OR, 1.15; P = .40, respectively).
The findings are reassuring overall, said Kelli D. Allen, PhD, research professor of medicine and exercise physiologist at the University of North Carolina at Chapel Hill, who was asked to comment on the study. “The study corroborates prior research showing that for most people, weight-bearing recreational activity does not increase the risk of knee osteoarthritis. This should be encouraging for people who want to increase their physical activity,” she said.
The study also suggests that “for people with low lower-limb muscle mass, there may be some considerations to make regarding the best type of physical activity to prevent future knee osteoarthritis,” she said in an e-mail. “The best approach may include non–weight-bearing activities, which could include biking, swimming, or other water exercises, along with strengthening exercises that help to increase muscle mass.”
Other studies, Dr. Allen said, have shown that low muscle mass itself is a risk factor for knee OA.
Physical Activity Types, Other Analyses
The researchers assessed total, weight-bearing, and non–weight-bearing physical activity using two validated questionnaires (an adapted version of the Zutphen Physical Activity Questionnaire and the Longitudinal Aging Study Amsterdam physical activity questionnaire) that asked participants about the frequency and duration of various types of physical activity. Activity was quantified as metabolic equivalent of task (MET) hours per week, and weight-bearing activities were defined as those in which the knee joint bears the body’s weight.
Walking, gardening, golf, dancing, and ball sports were among the activities qualifying as weight-bearing activities. Non–weight-bearing activities included cycling, rowing, and swimming.
Sex, body mass index, and follow-up time were among the covariates adjusted for in the primary analysis. Similar results were found when adjustments were also made for educational level, alcohol intake, lipid levels, and diabetes.
While incident radiographic knee OA (measured using the Kellgren & Lawrence grading system) was the primary outcome, the researchers also looked at symptomatic knee OA, as defined by x-ray and a knee pain questionnaire, and found no significant association of its incidence with any of the exercise categories (total, weight-bearing, or non-weight-bearing).
Coauthor Joyce B. J. van Meurs, PhD, of the departments of internal medicine and orthopedics & sports medicine at Erasmus Medical Center, told this news organization that “pain as a subjective, recurrent symptom is more difficult to study … [and] a larger sample size or more precise measurements [of pain] in future studies would help to better understand the true association” of symptomatic knee OA and physical activity.
Similarly, analyses of the 1511 patients (out of 5003) who had knee pain at baseline found no significant association of weight-bearing or non–weight-bearing physical activity with incident radiographic knee OA. The trends were similar to those found in the population without knee pain, however, which suggests the analysis was underpowered, the researchers wrote, noting too that patients with baseline pain had lower activity levels than those without pain. (Low case numbers precluded a stratification analysis on LMI for incident symptomatic OA.)
Thigh Circumference as an Indicator of Muscle Mass
The findings build upon an international meta-analysis published in 2021 that found no association between total physical activity and knee OA and align with other studies suggesting a link between greater mechanical stress/strain and greater OA risk, the researchers wrote. (The meta-analysis couldn’t investigate different types of activity.)
“Although we cannot establish a causal relationship … we hypothesize that the mechanical loading on joints and cartilage could explain the association of weight-bearing activity with osteoarthritis in the low LMI tertile group,” they said.
It is possible that thigh muscle-specific strength or mass may temper the risk of knee OA, they wrote, but the lack of thigh strength data in the Rotterdam Study precluded such evaluation. Still, in everyday practice, the researchers noted, lower limb muscle function could be assessed using thigh circumference.
Dr. Allen agreed. “ ‘Gold standard’ assessment of muscle mass is not common in routine practice, but clinicians can evaluate muscle mass in other ways, such as thigh circumference,” she told this news organization, noting that measurement should align with procedures described by the National Health and Nutrition Examination Survey in its anthropometry procedures manual.
“If low lower-limb muscle mass is suspected, a referral to a physical therapist can be helpful for more formally assessing muscle mass and muscle strength,” she added, “and for instructions for a safe and appropriate exercise program for building muscle and protecting joints.”
Among other limitations of the study, according to the researchers, are an ethnically nondiverse population, the unavailability of knee injury data, and the assessment of physical activity only at baseline.
Moving forward, Dr. van Meurs told this news organization, “the main question regarding physical activity and OA is still, if people already have pain or early OA complaints, what kinds of sports they can do without hurting their joints?” This “should be tested,” she said, “in a real-life, ideally trial-like intervention study.”
The study was funded by the Erasmus Medical Center and Erasmus University as well as through various government grants. Dr. Wu also had study support from the China Scholarship Council. Two of the authors reported relationships with arthritis-related organizations. Dr. Allen reported having no disclosures relevant to her comments.
FROM JAMA NETWORK OPEN