User login
Neuro-politics: Will you vote with your cortex or limbic system?
It’s election season again. Every 4 years, October becomes the purgatory month of politics. But this year, it’s even more complicated, being juxtaposed against a chaotic mosaic of a viral pandemic, economic travails, social upheaval, and exceptionally toxic political hyperpartisanship.
The widespread expectation is that citizens will vote for their party’s candidates, but there is now a body of evidence suggesting that our brains may be pre-wired to be liberal or conservative.
Enter neuro-politics. This discipline is younger than neuro-economics, neuro-law, neuro-ethics, neuro-marketing, neuro-art, neuro-culture, or neuro-esthetics. Neuro-politics focuses on the intersection of politics with neuroscience.1 However, there are many antecedents to neuro-politics reflected in the writings of Plato, Aristotle, Niccolò Machiavelli, John Locke, Baruch Spinoza, Henri Bergson, William James, and others.
Neuro-politics attempts to generate data to answer a variety of questions about political behavior, such as:
- Is political orientation associated with differences in certain brain regions?
- Are there reliable neural biomarkers of political orientation?
- Is political orientation modifiable, and if so, why are some individuals ferociously entrenched to one political dogma while others are able to untether themselves and adopt another political doctrine?
- What are the brain characteristics of “swing voters” who may align themselves with different parties in different election cycles?
- Is there a “religification” of politics among the ardent fanatics who regard the tenets of their political beliefs as “articles of faith?”
- Is the brain modified by certain attributes (such as educational level, age, sex, marital status, race, ethnicity, and religious affiliation) that translate to political decision-making?
- Can neuro-politics explain the sprouting of psychiatric symptoms such as obsessions, anxiety, irritability, anger, hatred, and conspiracy theories?
- Is political extremism driven by cortical structures, limbic structures, or both?
Politics and the brain
Here is a brief review of some studies that examined the relationship of political orientation or voting behavior with brain structure and function:
1. Roger Sperry, the 1981 Nobel Laureate (for his studies on split-brain patients) reported that in patients who underwent callosotomy, both cerebral hemispheres gave the same ratings of politicians when their photos were shown to each hemisphere separately.2
2. A functional magnetic resonance imaging (fMRI) study found that the faces of candidates activated participants’ ventromedial and anterior prefrontal cortices. Amygdala activation was associated with the intensity of the emotion.3
Continue to: A skin conductance...
3. A skin conductance study reported that politically liberal individuals had low reactivity to sudden noises and threatening stimuli, while conservative counterparts demonstrated high physiological reactions to noises and stimuli.4
4. Images of a losing candidate elicited greater activation on fMRI in the insula and ventral anterior cingulate compared to no activation by exposure to an image of the winning candidate.5
5. Another fMRI study found that “individualism” was associated with activation of the medial prefrontal cortex and temporo-parietal junction when participants listened to a set of political statements. On the other hand, “conservatism” activated the dorsolateral prefrontal cortex, while “radicalism” activated the ventral striatum and posterior cingulate.6
6. An EEG activity study of healthy individuals revealed desynchronization in the alpha band related to the politicians who lost simulated elections and were judged as “less trustworthy” when the participant watched their faces.7
7. A structural MRI study of young adults reported that liberalism was associated with increased gray matter volume in the anterior cingulate, while conservatism was associated with increased volume of the right amygdala. The authors replicated their findings and concluded there is a possible link between brain structure and psychological mechanisms that mediate political attitudes.8
Continue to: To examine the effect of...
8. To examine the effect of a “first impression” based on the physical appearance of candidates, researchers compared individuals with damage to the lateral orbitofrontal cortex (OFC) with a group that had frontal damage that spared the lateral OFC and another group of matched healthy volunteers. They used a simulated elections paradigm in which participants voted based solely on photographs of the candidates’ faces. Only the group with OFC damage was influenced by attractiveness, while those with an intact frontal lobe or non-OFC frontal damage relied on other data, such as competence.9 These researchers concluded that an intact OFC is necessary for political decision-making.
9. A study using cognitive tasks reported that liberals are more adept at dealing with novel information than conservatives.10
What part of your brain will you use?
Regardless of the data generated by the neuro-politics studies, the bottom line is: What part of your brain do you use when you cast your vote for an issue, a representative, a senator, or a president? Is it a purely intellectual decision (ie, cortical), or is it driven by visceral emotions (ie, limbic)? Do you believe that every single item in your party’s platform is right and virtuous, while every item in the other party’s platform is wrong and evil? Can you think of any redeeming feature of the candidate you hate or the party you despise?
One attribute that we psychiatrists possess by virtue of our training and clinical work is that we are able to transcend dichotomies and to perceive nuances and shades of gray about controversial issues. So I hope we employ the circuits of our brain where wisdom putatively resides11 and which may develop further (via neuroplasticity) with the conduct of psychotherapy.12 Those brain circuits include:
- prefrontal cortex (for emotional regulation, decision-making, and value relativism)
- lateral prefrontal cortex (to facilitate calculated, reason-based decision-making)
- medial prefrontal cortex (for emotional valence and pro-social attitudes and behaviors).
However, being human, it is quite likely that our amygdala may “seep through” and color our judgment and decisions. But let us try to cast a vote that is not only good for the country but also good for our patients, many of whom may not even be able to vote. Election season is a time to make a positive difference in our patients’ lives, not just ours. Let’s hope our brains exploit this unique opportunity.
1. Schreiber D. Neuropolitics: twenty years later. Politics Life Sci. 2017;36(2):114-131.
2. Sperry RW, Zaidel E, Zaidel D. Self recognition and social awareness in the deconnected minor hemisphere. Neuropsychologia. 1979;17(2):153-166.
3. Knutson KM, Wood JN, Spampinato MV, et al. Politics on the brain: an FMRI investigation. Soc Neurosci. 2006;1(1):25-40.
4. Oxley DR, Smith KB, Alford JR, et al. Political attitudes vary with physiological traits. Science. 2008;321(5896):1667-1670.
5. Spezio ML, Rangel A, Alvarez RM, et al. A neural basis for the effect of candidate appearance on election outcomes. Soc Cogn Affect Neurosci. 2008;3(4):344-352.
6. Zamboni G, Gozzi M, Krueger F, et al. Individualism, conservatism, and radicalism as criteria for processing political beliefs: a parametric fMRI study. Soc Neurosci. 2009;4(5):367-383.
7. Vecchiato G, Toppi J, Cincotti F, et al. Neuropolitics: EEG spectral maps related to a political vote based on the first impression of the candidate’s face. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:2902-2905.
8. Kanai R, Feilden T, Firth C, et al. Political orientations are correlated with brain structure in young adults. Curr Biol. 2011;21(8):677-680.
9. Xia C, Stolle D, Gidengil E, et al. Lateral orbitofrontal cortex links social impressions to political choices. J Neurosci. 2015;35(22):8507-8514.
10. Bernabel RT, Oliveira A. Conservatism and liberalism predict performance in two nonideological cognitive tasks. Politics Life Sci. 2017;36(2):49-59.
11. Meeks TW, Jeste DV. Neurobiology of wisdom: a literature overview. Arch Gen Psychiatry. 2009;66(4):355-365.
12. Nasrallah HA. Does psychiatric practice make us wiser? Current Psychiatry. 2009;8(10):12,14.
It’s election season again. Every 4 years, October becomes the purgatory month of politics. But this year, it’s even more complicated, being juxtaposed against a chaotic mosaic of a viral pandemic, economic travails, social upheaval, and exceptionally toxic political hyperpartisanship.
The widespread expectation is that citizens will vote for their party’s candidates, but there is now a body of evidence suggesting that our brains may be pre-wired to be liberal or conservative.
Enter neuro-politics. This discipline is younger than neuro-economics, neuro-law, neuro-ethics, neuro-marketing, neuro-art, neuro-culture, or neuro-esthetics. Neuro-politics focuses on the intersection of politics with neuroscience.1 However, there are many antecedents to neuro-politics reflected in the writings of Plato, Aristotle, Niccolò Machiavelli, John Locke, Baruch Spinoza, Henri Bergson, William James, and others.
Neuro-politics attempts to generate data to answer a variety of questions about political behavior, such as:
- Is political orientation associated with differences in certain brain regions?
- Are there reliable neural biomarkers of political orientation?
- Is political orientation modifiable, and if so, why are some individuals ferociously entrenched to one political dogma while others are able to untether themselves and adopt another political doctrine?
- What are the brain characteristics of “swing voters” who may align themselves with different parties in different election cycles?
- Is there a “religification” of politics among the ardent fanatics who regard the tenets of their political beliefs as “articles of faith?”
- Is the brain modified by certain attributes (such as educational level, age, sex, marital status, race, ethnicity, and religious affiliation) that translate to political decision-making?
- Can neuro-politics explain the sprouting of psychiatric symptoms such as obsessions, anxiety, irritability, anger, hatred, and conspiracy theories?
- Is political extremism driven by cortical structures, limbic structures, or both?
Politics and the brain
Here is a brief review of some studies that examined the relationship of political orientation or voting behavior with brain structure and function:
1. Roger Sperry, the 1981 Nobel Laureate (for his studies on split-brain patients) reported that in patients who underwent callosotomy, both cerebral hemispheres gave the same ratings of politicians when their photos were shown to each hemisphere separately.2
2. A functional magnetic resonance imaging (fMRI) study found that the faces of candidates activated participants’ ventromedial and anterior prefrontal cortices. Amygdala activation was associated with the intensity of the emotion.3
Continue to: A skin conductance...
3. A skin conductance study reported that politically liberal individuals had low reactivity to sudden noises and threatening stimuli, while conservative counterparts demonstrated high physiological reactions to noises and stimuli.4
4. Images of a losing candidate elicited greater activation on fMRI in the insula and ventral anterior cingulate compared to no activation by exposure to an image of the winning candidate.5
5. Another fMRI study found that “individualism” was associated with activation of the medial prefrontal cortex and temporo-parietal junction when participants listened to a set of political statements. On the other hand, “conservatism” activated the dorsolateral prefrontal cortex, while “radicalism” activated the ventral striatum and posterior cingulate.6
6. An EEG activity study of healthy individuals revealed desynchronization in the alpha band related to the politicians who lost simulated elections and were judged as “less trustworthy” when the participant watched their faces.7
7. A structural MRI study of young adults reported that liberalism was associated with increased gray matter volume in the anterior cingulate, while conservatism was associated with increased volume of the right amygdala. The authors replicated their findings and concluded there is a possible link between brain structure and psychological mechanisms that mediate political attitudes.8
Continue to: To examine the effect of...
8. To examine the effect of a “first impression” based on the physical appearance of candidates, researchers compared individuals with damage to the lateral orbitofrontal cortex (OFC) with a group that had frontal damage that spared the lateral OFC and another group of matched healthy volunteers. They used a simulated elections paradigm in which participants voted based solely on photographs of the candidates’ faces. Only the group with OFC damage was influenced by attractiveness, while those with an intact frontal lobe or non-OFC frontal damage relied on other data, such as competence.9 These researchers concluded that an intact OFC is necessary for political decision-making.
9. A study using cognitive tasks reported that liberals are more adept at dealing with novel information than conservatives.10
What part of your brain will you use?
Regardless of the data generated by the neuro-politics studies, the bottom line is: What part of your brain do you use when you cast your vote for an issue, a representative, a senator, or a president? Is it a purely intellectual decision (ie, cortical), or is it driven by visceral emotions (ie, limbic)? Do you believe that every single item in your party’s platform is right and virtuous, while every item in the other party’s platform is wrong and evil? Can you think of any redeeming feature of the candidate you hate or the party you despise?
One attribute that we psychiatrists possess by virtue of our training and clinical work is that we are able to transcend dichotomies and to perceive nuances and shades of gray about controversial issues. So I hope we employ the circuits of our brain where wisdom putatively resides11 and which may develop further (via neuroplasticity) with the conduct of psychotherapy.12 Those brain circuits include:
- prefrontal cortex (for emotional regulation, decision-making, and value relativism)
- lateral prefrontal cortex (to facilitate calculated, reason-based decision-making)
- medial prefrontal cortex (for emotional valence and pro-social attitudes and behaviors).
However, being human, it is quite likely that our amygdala may “seep through” and color our judgment and decisions. But let us try to cast a vote that is not only good for the country but also good for our patients, many of whom may not even be able to vote. Election season is a time to make a positive difference in our patients’ lives, not just ours. Let’s hope our brains exploit this unique opportunity.
It’s election season again. Every 4 years, October becomes the purgatory month of politics. But this year, it’s even more complicated, being juxtaposed against a chaotic mosaic of a viral pandemic, economic travails, social upheaval, and exceptionally toxic political hyperpartisanship.
The widespread expectation is that citizens will vote for their party’s candidates, but there is now a body of evidence suggesting that our brains may be pre-wired to be liberal or conservative.
Enter neuro-politics. This discipline is younger than neuro-economics, neuro-law, neuro-ethics, neuro-marketing, neuro-art, neuro-culture, or neuro-esthetics. Neuro-politics focuses on the intersection of politics with neuroscience.1 However, there are many antecedents to neuro-politics reflected in the writings of Plato, Aristotle, Niccolò Machiavelli, John Locke, Baruch Spinoza, Henri Bergson, William James, and others.
Neuro-politics attempts to generate data to answer a variety of questions about political behavior, such as:
- Is political orientation associated with differences in certain brain regions?
- Are there reliable neural biomarkers of political orientation?
- Is political orientation modifiable, and if so, why are some individuals ferociously entrenched to one political dogma while others are able to untether themselves and adopt another political doctrine?
- What are the brain characteristics of “swing voters” who may align themselves with different parties in different election cycles?
- Is there a “religification” of politics among the ardent fanatics who regard the tenets of their political beliefs as “articles of faith?”
- Is the brain modified by certain attributes (such as educational level, age, sex, marital status, race, ethnicity, and religious affiliation) that translate to political decision-making?
- Can neuro-politics explain the sprouting of psychiatric symptoms such as obsessions, anxiety, irritability, anger, hatred, and conspiracy theories?
- Is political extremism driven by cortical structures, limbic structures, or both?
Politics and the brain
Here is a brief review of some studies that examined the relationship of political orientation or voting behavior with brain structure and function:
1. Roger Sperry, the 1981 Nobel Laureate (for his studies on split-brain patients) reported that in patients who underwent callosotomy, both cerebral hemispheres gave the same ratings of politicians when their photos were shown to each hemisphere separately.2
2. A functional magnetic resonance imaging (fMRI) study found that the faces of candidates activated participants’ ventromedial and anterior prefrontal cortices. Amygdala activation was associated with the intensity of the emotion.3
Continue to: A skin conductance...
3. A skin conductance study reported that politically liberal individuals had low reactivity to sudden noises and threatening stimuli, while conservative counterparts demonstrated high physiological reactions to noises and stimuli.4
4. Images of a losing candidate elicited greater activation on fMRI in the insula and ventral anterior cingulate compared to no activation by exposure to an image of the winning candidate.5
5. Another fMRI study found that “individualism” was associated with activation of the medial prefrontal cortex and temporo-parietal junction when participants listened to a set of political statements. On the other hand, “conservatism” activated the dorsolateral prefrontal cortex, while “radicalism” activated the ventral striatum and posterior cingulate.6
6. An EEG activity study of healthy individuals revealed desynchronization in the alpha band related to the politicians who lost simulated elections and were judged as “less trustworthy” when the participant watched their faces.7
7. A structural MRI study of young adults reported that liberalism was associated with increased gray matter volume in the anterior cingulate, while conservatism was associated with increased volume of the right amygdala. The authors replicated their findings and concluded there is a possible link between brain structure and psychological mechanisms that mediate political attitudes.8
Continue to: To examine the effect of...
8. To examine the effect of a “first impression” based on the physical appearance of candidates, researchers compared individuals with damage to the lateral orbitofrontal cortex (OFC) with a group that had frontal damage that spared the lateral OFC and another group of matched healthy volunteers. They used a simulated elections paradigm in which participants voted based solely on photographs of the candidates’ faces. Only the group with OFC damage was influenced by attractiveness, while those with an intact frontal lobe or non-OFC frontal damage relied on other data, such as competence.9 These researchers concluded that an intact OFC is necessary for political decision-making.
9. A study using cognitive tasks reported that liberals are more adept at dealing with novel information than conservatives.10
What part of your brain will you use?
Regardless of the data generated by the neuro-politics studies, the bottom line is: What part of your brain do you use when you cast your vote for an issue, a representative, a senator, or a president? Is it a purely intellectual decision (ie, cortical), or is it driven by visceral emotions (ie, limbic)? Do you believe that every single item in your party’s platform is right and virtuous, while every item in the other party’s platform is wrong and evil? Can you think of any redeeming feature of the candidate you hate or the party you despise?
One attribute that we psychiatrists possess by virtue of our training and clinical work is that we are able to transcend dichotomies and to perceive nuances and shades of gray about controversial issues. So I hope we employ the circuits of our brain where wisdom putatively resides11 and which may develop further (via neuroplasticity) with the conduct of psychotherapy.12 Those brain circuits include:
- prefrontal cortex (for emotional regulation, decision-making, and value relativism)
- lateral prefrontal cortex (to facilitate calculated, reason-based decision-making)
- medial prefrontal cortex (for emotional valence and pro-social attitudes and behaviors).
However, being human, it is quite likely that our amygdala may “seep through” and color our judgment and decisions. But let us try to cast a vote that is not only good for the country but also good for our patients, many of whom may not even be able to vote. Election season is a time to make a positive difference in our patients’ lives, not just ours. Let’s hope our brains exploit this unique opportunity.
1. Schreiber D. Neuropolitics: twenty years later. Politics Life Sci. 2017;36(2):114-131.
2. Sperry RW, Zaidel E, Zaidel D. Self recognition and social awareness in the deconnected minor hemisphere. Neuropsychologia. 1979;17(2):153-166.
3. Knutson KM, Wood JN, Spampinato MV, et al. Politics on the brain: an FMRI investigation. Soc Neurosci. 2006;1(1):25-40.
4. Oxley DR, Smith KB, Alford JR, et al. Political attitudes vary with physiological traits. Science. 2008;321(5896):1667-1670.
5. Spezio ML, Rangel A, Alvarez RM, et al. A neural basis for the effect of candidate appearance on election outcomes. Soc Cogn Affect Neurosci. 2008;3(4):344-352.
6. Zamboni G, Gozzi M, Krueger F, et al. Individualism, conservatism, and radicalism as criteria for processing political beliefs: a parametric fMRI study. Soc Neurosci. 2009;4(5):367-383.
7. Vecchiato G, Toppi J, Cincotti F, et al. Neuropolitics: EEG spectral maps related to a political vote based on the first impression of the candidate’s face. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:2902-2905.
8. Kanai R, Feilden T, Firth C, et al. Political orientations are correlated with brain structure in young adults. Curr Biol. 2011;21(8):677-680.
9. Xia C, Stolle D, Gidengil E, et al. Lateral orbitofrontal cortex links social impressions to political choices. J Neurosci. 2015;35(22):8507-8514.
10. Bernabel RT, Oliveira A. Conservatism and liberalism predict performance in two nonideological cognitive tasks. Politics Life Sci. 2017;36(2):49-59.
11. Meeks TW, Jeste DV. Neurobiology of wisdom: a literature overview. Arch Gen Psychiatry. 2009;66(4):355-365.
12. Nasrallah HA. Does psychiatric practice make us wiser? Current Psychiatry. 2009;8(10):12,14.
1. Schreiber D. Neuropolitics: twenty years later. Politics Life Sci. 2017;36(2):114-131.
2. Sperry RW, Zaidel E, Zaidel D. Self recognition and social awareness in the deconnected minor hemisphere. Neuropsychologia. 1979;17(2):153-166.
3. Knutson KM, Wood JN, Spampinato MV, et al. Politics on the brain: an FMRI investigation. Soc Neurosci. 2006;1(1):25-40.
4. Oxley DR, Smith KB, Alford JR, et al. Political attitudes vary with physiological traits. Science. 2008;321(5896):1667-1670.
5. Spezio ML, Rangel A, Alvarez RM, et al. A neural basis for the effect of candidate appearance on election outcomes. Soc Cogn Affect Neurosci. 2008;3(4):344-352.
6. Zamboni G, Gozzi M, Krueger F, et al. Individualism, conservatism, and radicalism as criteria for processing political beliefs: a parametric fMRI study. Soc Neurosci. 2009;4(5):367-383.
7. Vecchiato G, Toppi J, Cincotti F, et al. Neuropolitics: EEG spectral maps related to a political vote based on the first impression of the candidate’s face. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:2902-2905.
8. Kanai R, Feilden T, Firth C, et al. Political orientations are correlated with brain structure in young adults. Curr Biol. 2011;21(8):677-680.
9. Xia C, Stolle D, Gidengil E, et al. Lateral orbitofrontal cortex links social impressions to political choices. J Neurosci. 2015;35(22):8507-8514.
10. Bernabel RT, Oliveira A. Conservatism and liberalism predict performance in two nonideological cognitive tasks. Politics Life Sci. 2017;36(2):49-59.
11. Meeks TW, Jeste DV. Neurobiology of wisdom: a literature overview. Arch Gen Psychiatry. 2009;66(4):355-365.
12. Nasrallah HA. Does psychiatric practice make us wiser? Current Psychiatry. 2009;8(10):12,14.
Impact of the MTHFR C677T genetic variant on depression
Ms. T, age 55, presents to her psychiatrist’s clinic with a chief complaint of ongoing symptoms of anhedonia and lethargy related to her diagnosis of major depressive disorder (MDD). She also has a history of peripheral arterial disease, hypothyroidism, and generalized anxiety disorder. Her current antidepressant regimen is duloxetine, 60 mg/d, and mirtazapine, 15 mg at night. She recently elected to undergo pharmacogenetic testing, which showed that she is heterozygous for the methylenetetrahydrofolate reductase (MTHFR) C677T mutation (MTHFR C677T CT carrier). Her test report states that she may have impaired folate metabolism. Her psychiatrist adds L-methylfolate, 15 mg/d, to her current antidepressant regimen.
What is the relationship between folic acid and MTHFR?
Methylenetetrahydrofolate reductase is an intracellular enzyme responsible for one of several steps involved in converting dietary folic acid to its physiologically active form, L-methylfolate.1 Once active, L-methylfolate can be transported into the CNS, where it participates in one-carbon transfer reactions.2,3 Mutations in the MTHFR gene have been associated with decreased activity of the enzyme, which has been shown to result in accumulation of homocysteine and may lead to decreased synthesis of neurotransmitters.2,4Commercial pharmacogenetic testing panels may offer MTHFR genetic testing to assist with prescribing decisions for patients with mental illness. The most well-characterized mutation currently is C677T (rsID1801133), which is a single amino acid base pair change (cytosine [C] to thymine [T]) that leads to increased thermolability and instability of the enzyme.5 Carrying 1 or 2 T alleles can lead to a 35% or 70% reduction in enzyme activity, respectively. The T variant allele is most frequent in Hispanics (20% to 25%), Asians (up to 63%), and Caucasians (8% to 20%); however, it is relatively uncommon in African Americans (<2%).5,6 Another variant, A1289C (rs1801131), has also been associated with decreased enzyme function, particularly when analyzed in combination with C677T. However, carrying the 1289C variant allele does not appear to result in as large of a reduction of enzyme function as the 677T variant.7
What is the relationship between MTHFR C677T and depression?
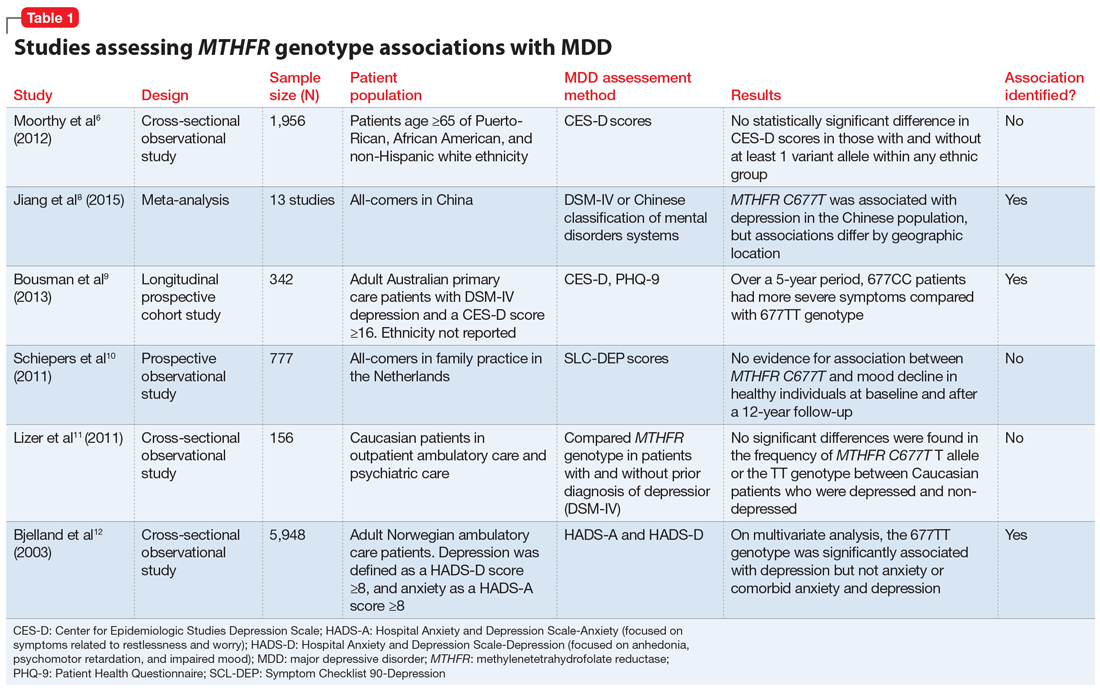
Some researchers have proposed that the C677T mutation in MTHFR may be associated with depression as a result of decreased neurotransmitter synthesis, but studies have not consistently supported this hypothesis. Several studies suggest an association between MTHFR mutations and MDD8-10:
Jiang et al8 performed a meta-analysis of 13 studies including 1,295 Chinese patients and found that having at least 1 C677T variant allele was significantly associated with an increased risk of depression (for T vs C odds ratio 1.52, 95% confidence interval 1.24 to 1.85). The authors noted a stronger association identified in the Northern Chinese population compared with the Southern Chinese population.8
Bousman et al9 found that American patients with MDD and the 677CC genotype had greater Patient Health Questionnaire-9 (PHQ-9) scores at assessments at 24, 36, and 48 months post-baseline compared with those with the 677TT genotype (P = .024), which was unexpected based on previously reported associations.9
Schiepers et al10 also assessed the association between the MTHFR genotype in a Dutch ambulatory care population over 12 years. There was no association identified between scores on the depression subscale of the Symptom Checklist 90 and C677T diplotype.10
Table 16,8-12 provides summaries of these and other selected studies on MTHFR and MDD. Overall, although a pathophysiological basis for depression and decreased MTHFR function has been proposed, the current body of literature does not indicate a consistent link between MTHFR C677T genetic variants alone and depression.
Continue to: Medication changes based on MTHFR: What is the evidence?
Medication changes based on MTHFR: What is the evidence?
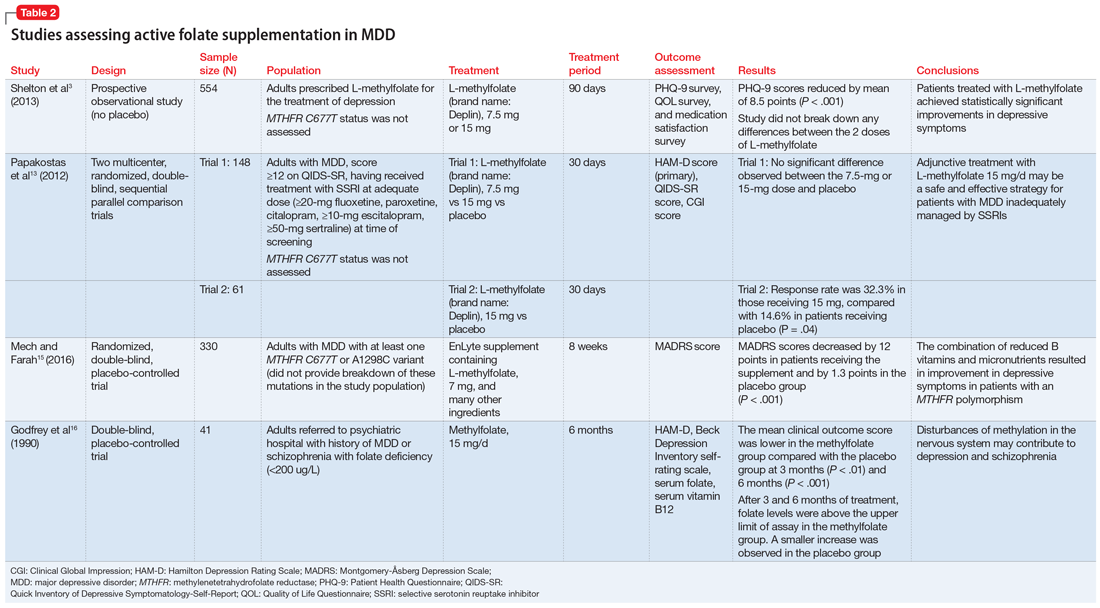
Some evidence supports the use of active folate supplementation to improve symptoms of MDD.
Shelton et al3 conducted an observational study that assessed the effects of adding L-methylfolate (brand name: Deplin), 7.5 or 15 mg, to existing antidepressant therapy in 502 patients with MDD who had baseline PHQ-9 scores of at least 5. After an average 95 days of therapy, PHQ-9 scores were reduced by a mean of 8.5 points, with 67.9% of patients achieving at least a 50% reduction in PHQ-9 scores. The study did not take into account patients’ MTHFR genotype or differentiate results between the 2 doses of L-methylfolate.3
Papakostas et al13 performed 2 randomized, double-blind, parallel-sequential, placebo-controlled trials of L-methylfolate for patients with MDD. The first compared L-methylfolate, 7.5 and 15 mg, to placebo, without regard to MTHFR genotype.13 There was no significant difference between the 7.5-mg dose and placebo, or the 15-mg dose and placebo. However, among the group receiving the 15-mg dose, the response rate was 24%, vs 9% in the placebo group, which approached significance (P = .1). Papakostas et al13 followed up with a smaller trial comparing the 15-mg dose alone to placebo, and found the response rate was 32.3% in patients treated with L-methylfolate compared with 14.6% in the placebo group (P = .04).13
Although the Shelton et al3 and Papakostas et al13 studies showed some improvement in depressive symptom scores among patients who received L-methylfolate supplementation, an important consideration is if MTHFR genotype may predict patient response to this therapy.
Papakostas et al14 performed a post hoc analysis of their earlier study to assess potential associations amongst multiple other biomarkers of inflammation and metabolic disturbances hypothesized by the authors to be associated with MDD, as well as body mass index (BMI), with treatment outcome.14 When change in the Hamilton Depression Rating Scale-28 (HDRS-28) was analyzed by C677T and A1298C variant groups (677 CT vs TT and 1298 AC vs CC), no statistically significant improvements were identified (C677T mean change from baseline −3.8 points, P = .087; A1298C mean change from baseline −0.5 points, P = .807).14 However, statistically significant improvements in HDRS-28 scores were observed compared with baseline when the C677T genotype was pooled with other biomarkers, including methionine synthase (MTR 2756 AG/GG, −23.3 points vs baseline, P < .001) and a voltage-dependent calcium channel (CACNAIC AG/AA, −9 points vs baseline, P < .001), as well as with BMI ≥ 30 kg/m2 (−9.9 points vs baseline, P = .001).14
Continue to: Mech and Farah...
Mech and Farah15 performed a randomized, double-blind, placebo-controlled study of the use of EnLyte, a supplement containing 7-mg L-methylfolate, in patients with at least 1 variant of MTHFR (either C677T or A1298C) over an 8-week period. In addition to L-methylfolate, this supplement contains other active ingredients, including leucovorin (or folinic acid), magnesium ascorbate, and ferrous glycine cysteinate. Montgomery-Åsberg Depression Scale (MADRS) scores improved by 12 points in patients who received the supplement and by 1.3 points in patients who received placebo. However, because the supplement contained many ingredients, the response observed in this study cannot be attributed to L-methylfolate alone.15
Table 23,13,15,16 contains summaries of these and other selected studies assessing active folate supplementation in MDD.
CASE CONTINUED
Over the next several weeks, Ms. T experiences some modest improvement in mood while taking L-methylfolate and her antidepressant regimen, and she experiences no notable adverse effects. Unfortunately, after 3 months, Ms. T discontinues the supplement due to the cost.
The value of MTHFR testing
Ms. T’s case is an example of how clinicians may respond to MTHFR pharmacogenetic testing. Although L-methylfolate has shown some benefit in several randomized clinical trials, available data do not confirm the relevance of MTHFR functional status to symptom response. Additionally, there is likely interplay among multiple factors affecting patients’ response to L-methylfolate. Larger randomized trials prospectively assessing other pharmacogenetic and lifestyle factors may shed more light on which patients would benefit.
Based on available data, the decision to prescribe L-methylfolate should not necessarily hinge on MTHFR genetics alone. Both patients and clinicians must be aware of the potentially prohibitive cost if L-methylfolate is recommended, as prescription insurance may not provide coverage (eg, a recent search on GoodRx.com showed that generic L-methylfolate was approximately $40 for 30 tablets; prices may vary). Additionally, clinicians should be aware that L-methylfolate is regulated as a medical food product and is not subject to strict quality standards required for prescription medications. Future prospective studies assessing the use of L-methylfolate specifically in patients with a MTHFR variants while investigating other relevant covariates may help identify which specific patient populations would benefit from supplementation.
Continue to: Related Resources
Related Resources
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
- Trimmer E. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Current Pharmaceutical Design. 2013;19(4):2574-3595.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
L-methylfolate • Deplin
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
1. Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480-488.
2. Jadavji N, Wieske F, Dirnagl U, et al. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Molecular Genetics and Metabolism Reports. 2015;3(Issue C):1-4.
3. Shelton R, Manning J, Barrentine L, et al. Assessing effects of L-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):pii:PCC.13m01520. doi: 10.4088/PCC.13m01520.
4. Brustolin S, Giugliani R, Felix T. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1-7.
5. Blom H, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81.
6. Moorthy D, Peter I, Scott T, et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012;142:1554-1560.
7. Lievers K, Boers G, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl). 2001;79(9):522-528.
8. Jiang W, Xu J, Lu X, et al. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2015;21(6):675-685.
9. Bousman C, Potiriadis M, Everall I, et al. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):68-76.
10. Schiepers O, Van Boxtel M, de Groot R, et al. Genetic variation in folate metabolism is not associated with cognitive functioning or mood in healthy adults. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(7):1682-1688.
11. Lizer M, Bogdan R, Kidd R. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in depressed versus nondepressed patients. J Psychiatr Pract. 2011;17(6):404-409.
12. Bjelland I, Tell G, Vollset S, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618-626.
13. Papakostas G, Shelton R, Zajecka J, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
14. Papakostas G, Shelton R, Zajecka J, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75(8):855-863.
15. Mech A, Farah A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(5):668-671.
16. Godfrey P, Toone B, Carney M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
Ms. T, age 55, presents to her psychiatrist’s clinic with a chief complaint of ongoing symptoms of anhedonia and lethargy related to her diagnosis of major depressive disorder (MDD). She also has a history of peripheral arterial disease, hypothyroidism, and generalized anxiety disorder. Her current antidepressant regimen is duloxetine, 60 mg/d, and mirtazapine, 15 mg at night. She recently elected to undergo pharmacogenetic testing, which showed that she is heterozygous for the methylenetetrahydrofolate reductase (MTHFR) C677T mutation (MTHFR C677T CT carrier). Her test report states that she may have impaired folate metabolism. Her psychiatrist adds L-methylfolate, 15 mg/d, to her current antidepressant regimen.
What is the relationship between folic acid and MTHFR?
Methylenetetrahydrofolate reductase is an intracellular enzyme responsible for one of several steps involved in converting dietary folic acid to its physiologically active form, L-methylfolate.1 Once active, L-methylfolate can be transported into the CNS, where it participates in one-carbon transfer reactions.2,3 Mutations in the MTHFR gene have been associated with decreased activity of the enzyme, which has been shown to result in accumulation of homocysteine and may lead to decreased synthesis of neurotransmitters.2,4Commercial pharmacogenetic testing panels may offer MTHFR genetic testing to assist with prescribing decisions for patients with mental illness. The most well-characterized mutation currently is C677T (rsID1801133), which is a single amino acid base pair change (cytosine [C] to thymine [T]) that leads to increased thermolability and instability of the enzyme.5 Carrying 1 or 2 T alleles can lead to a 35% or 70% reduction in enzyme activity, respectively. The T variant allele is most frequent in Hispanics (20% to 25%), Asians (up to 63%), and Caucasians (8% to 20%); however, it is relatively uncommon in African Americans (<2%).5,6 Another variant, A1289C (rs1801131), has also been associated with decreased enzyme function, particularly when analyzed in combination with C677T. However, carrying the 1289C variant allele does not appear to result in as large of a reduction of enzyme function as the 677T variant.7
What is the relationship between MTHFR C677T and depression?
Some researchers have proposed that the C677T mutation in MTHFR may be associated with depression as a result of decreased neurotransmitter synthesis, but studies have not consistently supported this hypothesis. Several studies suggest an association between MTHFR mutations and MDD8-10:
Jiang et al8 performed a meta-analysis of 13 studies including 1,295 Chinese patients and found that having at least 1 C677T variant allele was significantly associated with an increased risk of depression (for T vs C odds ratio 1.52, 95% confidence interval 1.24 to 1.85). The authors noted a stronger association identified in the Northern Chinese population compared with the Southern Chinese population.8
Bousman et al9 found that American patients with MDD and the 677CC genotype had greater Patient Health Questionnaire-9 (PHQ-9) scores at assessments at 24, 36, and 48 months post-baseline compared with those with the 677TT genotype (P = .024), which was unexpected based on previously reported associations.9
Schiepers et al10 also assessed the association between the MTHFR genotype in a Dutch ambulatory care population over 12 years. There was no association identified between scores on the depression subscale of the Symptom Checklist 90 and C677T diplotype.10
Table 16,8-12 provides summaries of these and other selected studies on MTHFR and MDD. Overall, although a pathophysiological basis for depression and decreased MTHFR function has been proposed, the current body of literature does not indicate a consistent link between MTHFR C677T genetic variants alone and depression.

Continue to: Medication changes based on MTHFR: What is the evidence?
Medication changes based on MTHFR: What is the evidence?
Some evidence supports the use of active folate supplementation to improve symptoms of MDD.
Shelton et al3 conducted an observational study that assessed the effects of adding L-methylfolate (brand name: Deplin), 7.5 or 15 mg, to existing antidepressant therapy in 502 patients with MDD who had baseline PHQ-9 scores of at least 5. After an average 95 days of therapy, PHQ-9 scores were reduced by a mean of 8.5 points, with 67.9% of patients achieving at least a 50% reduction in PHQ-9 scores. The study did not take into account patients’ MTHFR genotype or differentiate results between the 2 doses of L-methylfolate.3
Papakostas et al13 performed 2 randomized, double-blind, parallel-sequential, placebo-controlled trials of L-methylfolate for patients with MDD. The first compared L-methylfolate, 7.5 and 15 mg, to placebo, without regard to MTHFR genotype.13 There was no significant difference between the 7.5-mg dose and placebo, or the 15-mg dose and placebo. However, among the group receiving the 15-mg dose, the response rate was 24%, vs 9% in the placebo group, which approached significance (P = .1). Papakostas et al13 followed up with a smaller trial comparing the 15-mg dose alone to placebo, and found the response rate was 32.3% in patients treated with L-methylfolate compared with 14.6% in the placebo group (P = .04).13
Although the Shelton et al3 and Papakostas et al13 studies showed some improvement in depressive symptom scores among patients who received L-methylfolate supplementation, an important consideration is if MTHFR genotype may predict patient response to this therapy.
Papakostas et al14 performed a post hoc analysis of their earlier study to assess potential associations amongst multiple other biomarkers of inflammation and metabolic disturbances hypothesized by the authors to be associated with MDD, as well as body mass index (BMI), with treatment outcome.14 When change in the Hamilton Depression Rating Scale-28 (HDRS-28) was analyzed by C677T and A1298C variant groups (677 CT vs TT and 1298 AC vs CC), no statistically significant improvements were identified (C677T mean change from baseline −3.8 points, P = .087; A1298C mean change from baseline −0.5 points, P = .807).14 However, statistically significant improvements in HDRS-28 scores were observed compared with baseline when the C677T genotype was pooled with other biomarkers, including methionine synthase (MTR 2756 AG/GG, −23.3 points vs baseline, P < .001) and a voltage-dependent calcium channel (CACNAIC AG/AA, −9 points vs baseline, P < .001), as well as with BMI ≥ 30 kg/m2 (−9.9 points vs baseline, P = .001).14
Continue to: Mech and Farah...
Mech and Farah15 performed a randomized, double-blind, placebo-controlled study of the use of EnLyte, a supplement containing 7-mg L-methylfolate, in patients with at least 1 variant of MTHFR (either C677T or A1298C) over an 8-week period. In addition to L-methylfolate, this supplement contains other active ingredients, including leucovorin (or folinic acid), magnesium ascorbate, and ferrous glycine cysteinate. Montgomery-Åsberg Depression Scale (MADRS) scores improved by 12 points in patients who received the supplement and by 1.3 points in patients who received placebo. However, because the supplement contained many ingredients, the response observed in this study cannot be attributed to L-methylfolate alone.15
Table 23,13,15,16 contains summaries of these and other selected studies assessing active folate supplementation in MDD.

CASE CONTINUED
Over the next several weeks, Ms. T experiences some modest improvement in mood while taking L-methylfolate and her antidepressant regimen, and she experiences no notable adverse effects. Unfortunately, after 3 months, Ms. T discontinues the supplement due to the cost.
The value of MTHFR testing
Ms. T’s case is an example of how clinicians may respond to MTHFR pharmacogenetic testing. Although L-methylfolate has shown some benefit in several randomized clinical trials, available data do not confirm the relevance of MTHFR functional status to symptom response. Additionally, there is likely interplay among multiple factors affecting patients’ response to L-methylfolate. Larger randomized trials prospectively assessing other pharmacogenetic and lifestyle factors may shed more light on which patients would benefit.
Based on available data, the decision to prescribe L-methylfolate should not necessarily hinge on MTHFR genetics alone. Both patients and clinicians must be aware of the potentially prohibitive cost if L-methylfolate is recommended, as prescription insurance may not provide coverage (eg, a recent search on GoodRx.com showed that generic L-methylfolate was approximately $40 for 30 tablets; prices may vary). Additionally, clinicians should be aware that L-methylfolate is regulated as a medical food product and is not subject to strict quality standards required for prescription medications. Future prospective studies assessing the use of L-methylfolate specifically in patients with a MTHFR variants while investigating other relevant covariates may help identify which specific patient populations would benefit from supplementation.
Continue to: Related Resources
Related Resources
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
- Trimmer E. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Current Pharmaceutical Design. 2013;19(4):2574-3595.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
L-methylfolate • Deplin
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
Ms. T, age 55, presents to her psychiatrist’s clinic with a chief complaint of ongoing symptoms of anhedonia and lethargy related to her diagnosis of major depressive disorder (MDD). She also has a history of peripheral arterial disease, hypothyroidism, and generalized anxiety disorder. Her current antidepressant regimen is duloxetine, 60 mg/d, and mirtazapine, 15 mg at night. She recently elected to undergo pharmacogenetic testing, which showed that she is heterozygous for the methylenetetrahydrofolate reductase (MTHFR) C677T mutation (MTHFR C677T CT carrier). Her test report states that she may have impaired folate metabolism. Her psychiatrist adds L-methylfolate, 15 mg/d, to her current antidepressant regimen.
What is the relationship between folic acid and MTHFR?
Methylenetetrahydrofolate reductase is an intracellular enzyme responsible for one of several steps involved in converting dietary folic acid to its physiologically active form, L-methylfolate.1 Once active, L-methylfolate can be transported into the CNS, where it participates in one-carbon transfer reactions.2,3 Mutations in the MTHFR gene have been associated with decreased activity of the enzyme, which has been shown to result in accumulation of homocysteine and may lead to decreased synthesis of neurotransmitters.2,4Commercial pharmacogenetic testing panels may offer MTHFR genetic testing to assist with prescribing decisions for patients with mental illness. The most well-characterized mutation currently is C677T (rsID1801133), which is a single amino acid base pair change (cytosine [C] to thymine [T]) that leads to increased thermolability and instability of the enzyme.5 Carrying 1 or 2 T alleles can lead to a 35% or 70% reduction in enzyme activity, respectively. The T variant allele is most frequent in Hispanics (20% to 25%), Asians (up to 63%), and Caucasians (8% to 20%); however, it is relatively uncommon in African Americans (<2%).5,6 Another variant, A1289C (rs1801131), has also been associated with decreased enzyme function, particularly when analyzed in combination with C677T. However, carrying the 1289C variant allele does not appear to result in as large of a reduction of enzyme function as the 677T variant.7
What is the relationship between MTHFR C677T and depression?
Some researchers have proposed that the C677T mutation in MTHFR may be associated with depression as a result of decreased neurotransmitter synthesis, but studies have not consistently supported this hypothesis. Several studies suggest an association between MTHFR mutations and MDD8-10:
Jiang et al8 performed a meta-analysis of 13 studies including 1,295 Chinese patients and found that having at least 1 C677T variant allele was significantly associated with an increased risk of depression (for T vs C odds ratio 1.52, 95% confidence interval 1.24 to 1.85). The authors noted a stronger association identified in the Northern Chinese population compared with the Southern Chinese population.8
Bousman et al9 found that American patients with MDD and the 677CC genotype had greater Patient Health Questionnaire-9 (PHQ-9) scores at assessments at 24, 36, and 48 months post-baseline compared with those with the 677TT genotype (P = .024), which was unexpected based on previously reported associations.9
Schiepers et al10 also assessed the association between the MTHFR genotype in a Dutch ambulatory care population over 12 years. There was no association identified between scores on the depression subscale of the Symptom Checklist 90 and C677T diplotype.10
Table 16,8-12 provides summaries of these and other selected studies on MTHFR and MDD. Overall, although a pathophysiological basis for depression and decreased MTHFR function has been proposed, the current body of literature does not indicate a consistent link between MTHFR C677T genetic variants alone and depression.

Continue to: Medication changes based on MTHFR: What is the evidence?
Medication changes based on MTHFR: What is the evidence?
Some evidence supports the use of active folate supplementation to improve symptoms of MDD.
Shelton et al3 conducted an observational study that assessed the effects of adding L-methylfolate (brand name: Deplin), 7.5 or 15 mg, to existing antidepressant therapy in 502 patients with MDD who had baseline PHQ-9 scores of at least 5. After an average 95 days of therapy, PHQ-9 scores were reduced by a mean of 8.5 points, with 67.9% of patients achieving at least a 50% reduction in PHQ-9 scores. The study did not take into account patients’ MTHFR genotype or differentiate results between the 2 doses of L-methylfolate.3
Papakostas et al13 performed 2 randomized, double-blind, parallel-sequential, placebo-controlled trials of L-methylfolate for patients with MDD. The first compared L-methylfolate, 7.5 and 15 mg, to placebo, without regard to MTHFR genotype.13 There was no significant difference between the 7.5-mg dose and placebo, or the 15-mg dose and placebo. However, among the group receiving the 15-mg dose, the response rate was 24%, vs 9% in the placebo group, which approached significance (P = .1). Papakostas et al13 followed up with a smaller trial comparing the 15-mg dose alone to placebo, and found the response rate was 32.3% in patients treated with L-methylfolate compared with 14.6% in the placebo group (P = .04).13
Although the Shelton et al3 and Papakostas et al13 studies showed some improvement in depressive symptom scores among patients who received L-methylfolate supplementation, an important consideration is if MTHFR genotype may predict patient response to this therapy.
Papakostas et al14 performed a post hoc analysis of their earlier study to assess potential associations amongst multiple other biomarkers of inflammation and metabolic disturbances hypothesized by the authors to be associated with MDD, as well as body mass index (BMI), with treatment outcome.14 When change in the Hamilton Depression Rating Scale-28 (HDRS-28) was analyzed by C677T and A1298C variant groups (677 CT vs TT and 1298 AC vs CC), no statistically significant improvements were identified (C677T mean change from baseline −3.8 points, P = .087; A1298C mean change from baseline −0.5 points, P = .807).14 However, statistically significant improvements in HDRS-28 scores were observed compared with baseline when the C677T genotype was pooled with other biomarkers, including methionine synthase (MTR 2756 AG/GG, −23.3 points vs baseline, P < .001) and a voltage-dependent calcium channel (CACNAIC AG/AA, −9 points vs baseline, P < .001), as well as with BMI ≥ 30 kg/m2 (−9.9 points vs baseline, P = .001).14
Continue to: Mech and Farah...
Mech and Farah15 performed a randomized, double-blind, placebo-controlled study of the use of EnLyte, a supplement containing 7-mg L-methylfolate, in patients with at least 1 variant of MTHFR (either C677T or A1298C) over an 8-week period. In addition to L-methylfolate, this supplement contains other active ingredients, including leucovorin (or folinic acid), magnesium ascorbate, and ferrous glycine cysteinate. Montgomery-Åsberg Depression Scale (MADRS) scores improved by 12 points in patients who received the supplement and by 1.3 points in patients who received placebo. However, because the supplement contained many ingredients, the response observed in this study cannot be attributed to L-methylfolate alone.15
Table 23,13,15,16 contains summaries of these and other selected studies assessing active folate supplementation in MDD.

CASE CONTINUED
Over the next several weeks, Ms. T experiences some modest improvement in mood while taking L-methylfolate and her antidepressant regimen, and she experiences no notable adverse effects. Unfortunately, after 3 months, Ms. T discontinues the supplement due to the cost.
The value of MTHFR testing
Ms. T’s case is an example of how clinicians may respond to MTHFR pharmacogenetic testing. Although L-methylfolate has shown some benefit in several randomized clinical trials, available data do not confirm the relevance of MTHFR functional status to symptom response. Additionally, there is likely interplay among multiple factors affecting patients’ response to L-methylfolate. Larger randomized trials prospectively assessing other pharmacogenetic and lifestyle factors may shed more light on which patients would benefit.
Based on available data, the decision to prescribe L-methylfolate should not necessarily hinge on MTHFR genetics alone. Both patients and clinicians must be aware of the potentially prohibitive cost if L-methylfolate is recommended, as prescription insurance may not provide coverage (eg, a recent search on GoodRx.com showed that generic L-methylfolate was approximately $40 for 30 tablets; prices may vary). Additionally, clinicians should be aware that L-methylfolate is regulated as a medical food product and is not subject to strict quality standards required for prescription medications. Future prospective studies assessing the use of L-methylfolate specifically in patients with a MTHFR variants while investigating other relevant covariates may help identify which specific patient populations would benefit from supplementation.
Continue to: Related Resources
Related Resources
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
- Trimmer E. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Current Pharmaceutical Design. 2013;19(4):2574-3595.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
L-methylfolate • Deplin
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
1. Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480-488.
2. Jadavji N, Wieske F, Dirnagl U, et al. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Molecular Genetics and Metabolism Reports. 2015;3(Issue C):1-4.
3. Shelton R, Manning J, Barrentine L, et al. Assessing effects of L-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):pii:PCC.13m01520. doi: 10.4088/PCC.13m01520.
4. Brustolin S, Giugliani R, Felix T. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1-7.
5. Blom H, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81.
6. Moorthy D, Peter I, Scott T, et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012;142:1554-1560.
7. Lievers K, Boers G, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl). 2001;79(9):522-528.
8. Jiang W, Xu J, Lu X, et al. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2015;21(6):675-685.
9. Bousman C, Potiriadis M, Everall I, et al. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):68-76.
10. Schiepers O, Van Boxtel M, de Groot R, et al. Genetic variation in folate metabolism is not associated with cognitive functioning or mood in healthy adults. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(7):1682-1688.
11. Lizer M, Bogdan R, Kidd R. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in depressed versus nondepressed patients. J Psychiatr Pract. 2011;17(6):404-409.
12. Bjelland I, Tell G, Vollset S, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618-626.
13. Papakostas G, Shelton R, Zajecka J, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
14. Papakostas G, Shelton R, Zajecka J, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75(8):855-863.
15. Mech A, Farah A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(5):668-671.
16. Godfrey P, Toone B, Carney M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
1. Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480-488.
2. Jadavji N, Wieske F, Dirnagl U, et al. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Molecular Genetics and Metabolism Reports. 2015;3(Issue C):1-4.
3. Shelton R, Manning J, Barrentine L, et al. Assessing effects of L-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):pii:PCC.13m01520. doi: 10.4088/PCC.13m01520.
4. Brustolin S, Giugliani R, Felix T. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1-7.
5. Blom H, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81.
6. Moorthy D, Peter I, Scott T, et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012;142:1554-1560.
7. Lievers K, Boers G, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl). 2001;79(9):522-528.
8. Jiang W, Xu J, Lu X, et al. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2015;21(6):675-685.
9. Bousman C, Potiriadis M, Everall I, et al. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):68-76.
10. Schiepers O, Van Boxtel M, de Groot R, et al. Genetic variation in folate metabolism is not associated with cognitive functioning or mood in healthy adults. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(7):1682-1688.
11. Lizer M, Bogdan R, Kidd R. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in depressed versus nondepressed patients. J Psychiatr Pract. 2011;17(6):404-409.
12. Bjelland I, Tell G, Vollset S, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618-626.
13. Papakostas G, Shelton R, Zajecka J, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
14. Papakostas G, Shelton R, Zajecka J, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75(8):855-863.
15. Mech A, Farah A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(5):668-671.
16. Godfrey P, Toone B, Carney M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
The boy whose arm wouldn’t work
CASE Drooling, unsteady, and not himself
B, age 10, who is left handed and has autism spectrum disorder, is brought to the emergency department (ED) with a 1-day history of drooling, unsteady gait, and left wrist in sustained flexion. His parents report that for the past week, B has had cold symptoms, including rhinorrhea, a low-grade fever (100.0°F), and cough. Earlier in the day, he was seen at his pediatrician’s office, where he was diagnosed with an acute respiratory infection and started on amoxicillin, 500 mg twice daily for 7 days.
At baseline, B is nonverbal. He requires some assistance with his activities of daily living. He usually is able to walk without assistance and dress himself, but he is not toilet trained. His parents report that in the past day, he has had significant difficulties with tasks involving his left hand. Normally, B is able to feed himself “finger foods” but has been unable to do so today. His parents say that he has been unsteady on his feet, and has been “falling forward” when he tries to walk.
Two years ago, B was started on risperidone, 0.5 mg nightly, for behavioral aggression and self-mutilation. Over the next 12 months, the dosage was steadily increased to 1 mg twice daily, with good response. He has been taking his current dosage, 1 mg twice daily, for the past 12 months without adjustment. His parents report there have been no other medication changes, other than starting amoxicillin earlier that day.
As part of his initial ED evaluation, B is found to be mildly dehydrated, with an elevated sedimentation rate on urinalysis. His complete blood count (CBC) with differential is within normal limits. A comprehensive metabolic panel shows a slight increase in his creatinine level, indicating dehydration. B is administered IV fluid replacement because he is having difficulty drinking due to excessive drooling.
The ED physician is concerned that B may be experiencing an acute dystonic reaction from risperidone, so the team holds this medication, and gives B a one-time dose of IV diphenhydramine, 25 mg, for presumptive acute dystonic reaction. After several minutes, there is no improvement in the sustained flexion of his left wrist.
[polldaddy:10615848]
The authors’ observations
B presented with new-onset neurologic findings after a recently diagnosed upper respiratory viral illness. His symptoms appeared to be confined to his left upper extremity, specifically demonstrating left arm extension at the elbow with flexion of the left wrist. He also had new-onset unsteady gait with a stooped forward posture and required assistance with walking. Interestingly, despite B’s history of antipsychotic use, administering an anticholinergic agent did not lessen the dystonic posturing at his wrist and elbow.
EVALUATION Laboratory results reveal new clues
While in the ED, B undergoes MRI of the brain and spinal cord to rule out any mass lesions that could be impinging upon the motor pathways. Both brain and spinal cord imaging appear to be essentially normal, without evidence of impingement of the spinal nerves or lesions involving the brainstem or cerebellum.
Continue to: Due to concerns...
Due to concerns of possible airway obstruction, a CT scan of the neck is obtained to rule out any acute pathology, such as epiglottitis compromising his airway. The scan shows some inflammation and edema in the soft tissues that is thought to be secondary to his acute viral illness. B is able to maintain his airway and oxygenation, so intubation is not necessary.
A CPK test is ordered because there are concerns of sustained muscle contraction of B’s left wrist and elbow. The CPK level is 884 U/L (reference range 26 to 192 U/L). The elevation in CPK is consistent with prior laboratory findings of dehydration and indicating skeletal muscle breakdown from sustained muscle contraction. All other laboratory results, including a comprehensive metabolic panel, urine drug screen, and thyroid screening panel, are within normal limits.
[polldaddy:10615850]
EVALUATION No variation in facial expression
B is admitted to the general pediatrics service. Maintenance IV fluids are started due to concerns of dehydration and possible rhabdomyolysis due to his elevated CPK level. Risperidone is held throughout the hospital course due to concerns for an acute dystonic reaction. B is monitored for several days without clinical improvement and eventually discharged home with a diagnosis of inflammatory mononeuropathy due to viral infection. The patient is told to discontinue risperidone as part of discharge instructions.
Five days later, B returns to the hospital because there was no improvement in his left extremity or walking. His left elbow remains extended with left wrist in flexion. Psychiatry is consulted for further diagnostic clarity and evaluation.
On physical examination, B’s left arm remains unchanged. Despite discontinuing risperidone, there is evidence of cogwheel rigidity of the left wrist joint. Reflexes in the upper and lower extremities are 2+ and symmetrical bilaterally, suggesting intact upper and lower motor pathways. Babinski sign is absent bilaterally, which is a normal finding in B’s age group. B continues to have difficulty with ambulating and appears to “fall forward” while trying to walk with assistance. His parents also say that B is not laughing, smiling, or showing any variation in facial expression.
Continue to: Additional family history...
Additional family history is gathered from B’s parents for possible hereditary movement disorders such as Wilson’s disease. They report that no family members have developed involuntary movements or other neurologic syndromes. Additional considerations on the differential diagnosis for B include juvenile ALS or mononeuropathy involving the C5 and C6 nerve roots. B’s parents deny any recent shoulder trauma, and radiographic studies did not demonstrate any involvement of the nerve roots.
TREATMENT A trial of bromocriptine
At this point, B’s neurologic workup is essentially normal, and he is given a provisional diagnosis of antipsychotic-induced tardive dystonia vs tardive parkinsonism. Risperidone continues to be held, and B is monitored for clinical improvement. B is administered a one-time dose of diphenhydramine, 25 mg, for dystonia with no improvement in symptoms. He is then started on bromocriptine, 1.25 mg twice daily with meals, for parkinsonian symptoms secondary to antipsychotic medication use. After 1 day of treatment, B shows less sustained flexion of his left wrist. He is able to relax his left arm, shows improvements in ambulation, and requires less assistance. B continues to be observed closely and continues to improve toward his baseline.
At Day 4, he is discharged. B is able to walk mostly without assistance and demonstrates improvement in left wrist flexion. He is scheduled to see a movement disorders specialist a week after discharge. The initial diagnosis given by the movement disorder specialist is tardive dystonia.
The authors’ observations
Tardive dyskinesia is a well-known iatrogenic effect of antipsychotic medications that are commonly used to manage conditions such as schizophrenia or behavioral agitation associated with autism spectrum disorder. Symptoms of tardive dyskinesia typically emerge after 1 to 2 years of continuous exposure to dopamine receptor blocking agents (DRBAs). Tardive dyskinesia symptoms include involuntary, repetitive, purposeless movements of the tongue, jaw, lips, face, trunk, and upper and lower extremities, with significant functional impairment.1
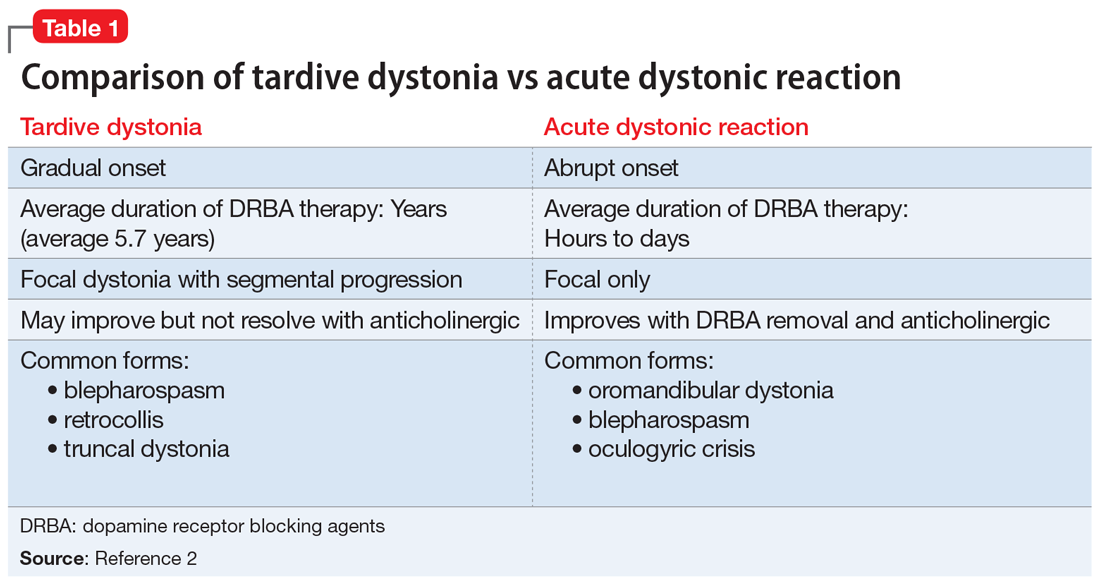
Tardive syndromes refer to a diverse array of hyperkinetic, hypokinetic, and sensory movement disorders resulting from at least 3 months of continuous DRBA therapy.2 Tardive dyskinesia is perhaps the most well-known of the tardive syndromes, but is not the only one to consider when assessing for antipsychotic-induced movement disorders. A key feature differentiating a tardive syndrome is the persistence of the movement disorder after the DRBA is discontinued. In this case, B had been receiving a stable dose of risperidone for >1 year. He developed dystonic posturing of his left wrist and elbow that was both unresponsive to anticholinergic medication and persisted after risperidone was discontinued. The term “tardive” emphasizes the delay in development of abnormal involuntary movement symptoms after initiating antipsychotic medications.3 Table 12 shows a comparison of tardive dystonia vs an acute dystonic reaction.
Continue to: Other tardive syndromes include...
Other tardive syndromes include:
- tardive tics
- tardive parkinsonism
- tardive pain
- tardive myoclonus
- tardive akathisia
- tardive tremors.
The incidence of tardive syndromes increases 5% annually for the first 5 years of treatment. At 10 years of treatment, the annual incidence is thought to be 49%, and at 25 years of treatment, 68%.4 The predominant theory of the pathophysiology of tardive syndromes is that the chronic use of DRBAs causes a gradual hypersensitization of dopamine receptors.4 The diagnosis of a tardive syndrome is based on history of exposure to a DRBA as well as clinical observation of symptoms.
Compared with classic tardive dyskinesia, tardive dystonia is more common among younger patients. The mean age of onset of tardive dystonia is 40, and it typically affects young males.5 Typical posturing observed in cases of tardive dystonia include extension of the arms and flexion at the wrists.6 In contrast to cases of primary dystonia, tardive dystonia is typically associated with stereotypies, akathisia, or other movement disorders. Anticholinergic agents, such as
The American Psychiatric Association has issued guidelines on screening for involuntary movement syndromes by using the Abnormal Involuntary Movement Scale (AIMS).7 The current recommendations include assessment every 6 months for patients receiving first-generation antipsychotics, and every 12 months for those receiving second-generation antipsychotics.7 Prescribers should also carefully assess for any pre-existing involuntary movements before prescribing a DRBA.7
[polldaddy:10615855]
The authors’ observations
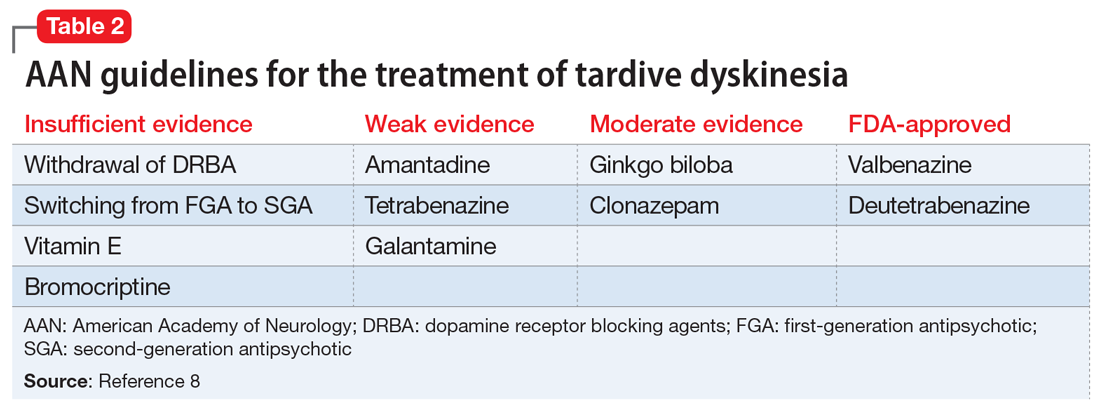
In 2013, the American Academy of Neurology (AAN) published guidelines on the treatment of tardive dyskinesia. According to these guidelines, at that time, the treatments with the most evidence supporting their use were clonazepam, ginkgo biloba,
Continue to: In 2017, valbenazine and deutetrabenazine...
In 2017, valbenazine and deutetrabenazine became the first FDA-approved treatments for tardive dyskinesia in adults. Both medications block the vesicular monoamine transporter 2 (VMAT2) system, which results in decreased synaptic dopamine and dopamine receptor stimulation. Both VMAT2 inhibitor medications have a category level A supporting their use for treating tardive dyskinesia.8-10
Currently, there are no published treatment guidelines on pharmacologic management of tardive dystonia. In B’s case, bromocriptine, a dopamine agonist, was used to counter the dopamine-blocking effects of risperidone on the nigrostriatal pathway and improve parkinsonian features of B’s presentation, including bradykinesia, stooped forward posture, and masked facies. Bromocriptine was found to be effective in alleviating parkinsonian features; however, to date there is no evidence demonstrating its effectiveness in countering delayed dystonic effects of DRBAs.
OUTCOME Improvement of dystonia symptoms
One week after discharge, B is seen for a follow-up visit. He continues taking bromocriptine, 1.25 mg twice daily, with meals after discharge. On examination, he has some evidence of tardive dystonia, including flexion of left wrist and posturing while ambulating. B’s parkinsonian features, including stooped forward posture, masked facies, and cogwheel rigidity of the left wrist muscle, have resolved. B is now able to walk on his own without unsteadiness. Bromocriptine is discontinued after 1 month, and his symptoms of dystonia continue to improve.
Two months after hospitalization, B is started on quetiapine, 25 mg twice daily, for behavioral aggression. Quetiapine is chosen because it has a lower dopamine receptor affinity compared with risperidone, and theoretically, quetiapine is associated with a lower risk of developing tardive symptoms. During the next 6 months, B is monitored closely for recurrence of tardive symptoms. Quetiapine is slowly titrated to 25 mg in the morning, and 50 mg at bedtime. His behavioral agitation improves significantly and he does not have a recurrence of tardive symptoms.
Bottom Line
Tardive dystonia is a possible iatrogenic adverse effect for patients receiving long-term dopamine receptor blocking agent (DRBA) therapy. Tardive syndromes encompass delayed-onset movement disorders caused by long-term blockade of the dopamine receptor by antipsychotic agents. Tardive dystonia can be contrasted from acute dystonic reaction based on the time course of development as well as by the persistence of symptoms after DRBAs are withheld.
Continue to: Related Resources
Related Resources
- American Academy of Neurology. Summary of evidence-based guideline for clinicians: treatment of tardive syndromes. https://www.aan.com/Guidelines/Home/GetGuidelineContent/613. Published 2013.
- Dystonia Medical Research Foundation. https://dystonia-foundation.org/.
Drug Brand Names
Amantadine • Gocovri, Symmetrel
Amoxicillin • Amoxil
Baclofen • Kemstro, Liroesal
Benztropine • Cogentin
Bromocriptine • Parlodel
Clonazepam • Klonopin
Deutetrabenazine • Austedo
Galantamine • Razadyne
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trihexyphenidyl • Artane, Tremin
Valbenazine • Ingrezza
1. Margolese HC, Chouinard G, Kolivakis TT, et al. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 1: pathophysiology and mechanisms of induction. Can J Psychiatr. 2005;50(9):541-547.
2. Truong D, Frei K. Setting the record straight: the nosology of tardive syndromes. Parkinsonism Relat Disord. 2019;59:146-150.
3. Cornett EM, Novitch M, Kaye AD, et al. Medication-induced tardive dyskinesia: a review and update. Ochsner J. 2017;17(2):162-174.
4. Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry. 1982;39(4):486-487.
5. Fahn S, Jankovic J, Hallett M. Principles and Practice of Movement Disorders. 2nd ed. Philadelphia, PA: Saunders; 2011:415-446.
6. Kang UJ, Burke RE, Fahn S. Natural history and treatment of tardive dystonia. Mov Disord. 1986;1(3):193-208.
7. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al, Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Ingrezza [package insert]. San Diego, CA: Neurocrine Biosciences, Inc.; 2020.
10. Austedo [package insert]. North Wales, PA: Teva Pharmaceuticals; 2017.
CASE Drooling, unsteady, and not himself
B, age 10, who is left handed and has autism spectrum disorder, is brought to the emergency department (ED) with a 1-day history of drooling, unsteady gait, and left wrist in sustained flexion. His parents report that for the past week, B has had cold symptoms, including rhinorrhea, a low-grade fever (100.0°F), and cough. Earlier in the day, he was seen at his pediatrician’s office, where he was diagnosed with an acute respiratory infection and started on amoxicillin, 500 mg twice daily for 7 days.
At baseline, B is nonverbal. He requires some assistance with his activities of daily living. He usually is able to walk without assistance and dress himself, but he is not toilet trained. His parents report that in the past day, he has had significant difficulties with tasks involving his left hand. Normally, B is able to feed himself “finger foods” but has been unable to do so today. His parents say that he has been unsteady on his feet, and has been “falling forward” when he tries to walk.
Two years ago, B was started on risperidone, 0.5 mg nightly, for behavioral aggression and self-mutilation. Over the next 12 months, the dosage was steadily increased to 1 mg twice daily, with good response. He has been taking his current dosage, 1 mg twice daily, for the past 12 months without adjustment. His parents report there have been no other medication changes, other than starting amoxicillin earlier that day.
As part of his initial ED evaluation, B is found to be mildly dehydrated, with an elevated sedimentation rate on urinalysis. His complete blood count (CBC) with differential is within normal limits. A comprehensive metabolic panel shows a slight increase in his creatinine level, indicating dehydration. B is administered IV fluid replacement because he is having difficulty drinking due to excessive drooling.
The ED physician is concerned that B may be experiencing an acute dystonic reaction from risperidone, so the team holds this medication, and gives B a one-time dose of IV diphenhydramine, 25 mg, for presumptive acute dystonic reaction. After several minutes, there is no improvement in the sustained flexion of his left wrist.
[polldaddy:10615848]
The authors’ observations
B presented with new-onset neurologic findings after a recently diagnosed upper respiratory viral illness. His symptoms appeared to be confined to his left upper extremity, specifically demonstrating left arm extension at the elbow with flexion of the left wrist. He also had new-onset unsteady gait with a stooped forward posture and required assistance with walking. Interestingly, despite B’s history of antipsychotic use, administering an anticholinergic agent did not lessen the dystonic posturing at his wrist and elbow.
EVALUATION Laboratory results reveal new clues
While in the ED, B undergoes MRI of the brain and spinal cord to rule out any mass lesions that could be impinging upon the motor pathways. Both brain and spinal cord imaging appear to be essentially normal, without evidence of impingement of the spinal nerves or lesions involving the brainstem or cerebellum.
Continue to: Due to concerns...
Due to concerns of possible airway obstruction, a CT scan of the neck is obtained to rule out any acute pathology, such as epiglottitis compromising his airway. The scan shows some inflammation and edema in the soft tissues that is thought to be secondary to his acute viral illness. B is able to maintain his airway and oxygenation, so intubation is not necessary.
A CPK test is ordered because there are concerns of sustained muscle contraction of B’s left wrist and elbow. The CPK level is 884 U/L (reference range 26 to 192 U/L). The elevation in CPK is consistent with prior laboratory findings of dehydration and indicating skeletal muscle breakdown from sustained muscle contraction. All other laboratory results, including a comprehensive metabolic panel, urine drug screen, and thyroid screening panel, are within normal limits.
[polldaddy:10615850]
EVALUATION No variation in facial expression
B is admitted to the general pediatrics service. Maintenance IV fluids are started due to concerns of dehydration and possible rhabdomyolysis due to his elevated CPK level. Risperidone is held throughout the hospital course due to concerns for an acute dystonic reaction. B is monitored for several days without clinical improvement and eventually discharged home with a diagnosis of inflammatory mononeuropathy due to viral infection. The patient is told to discontinue risperidone as part of discharge instructions.
Five days later, B returns to the hospital because there was no improvement in his left extremity or walking. His left elbow remains extended with left wrist in flexion. Psychiatry is consulted for further diagnostic clarity and evaluation.
On physical examination, B’s left arm remains unchanged. Despite discontinuing risperidone, there is evidence of cogwheel rigidity of the left wrist joint. Reflexes in the upper and lower extremities are 2+ and symmetrical bilaterally, suggesting intact upper and lower motor pathways. Babinski sign is absent bilaterally, which is a normal finding in B’s age group. B continues to have difficulty with ambulating and appears to “fall forward” while trying to walk with assistance. His parents also say that B is not laughing, smiling, or showing any variation in facial expression.
Continue to: Additional family history...
Additional family history is gathered from B’s parents for possible hereditary movement disorders such as Wilson’s disease. They report that no family members have developed involuntary movements or other neurologic syndromes. Additional considerations on the differential diagnosis for B include juvenile ALS or mononeuropathy involving the C5 and C6 nerve roots. B’s parents deny any recent shoulder trauma, and radiographic studies did not demonstrate any involvement of the nerve roots.
TREATMENT A trial of bromocriptine
At this point, B’s neurologic workup is essentially normal, and he is given a provisional diagnosis of antipsychotic-induced tardive dystonia vs tardive parkinsonism. Risperidone continues to be held, and B is monitored for clinical improvement. B is administered a one-time dose of diphenhydramine, 25 mg, for dystonia with no improvement in symptoms. He is then started on bromocriptine, 1.25 mg twice daily with meals, for parkinsonian symptoms secondary to antipsychotic medication use. After 1 day of treatment, B shows less sustained flexion of his left wrist. He is able to relax his left arm, shows improvements in ambulation, and requires less assistance. B continues to be observed closely and continues to improve toward his baseline.
At Day 4, he is discharged. B is able to walk mostly without assistance and demonstrates improvement in left wrist flexion. He is scheduled to see a movement disorders specialist a week after discharge. The initial diagnosis given by the movement disorder specialist is tardive dystonia.
The authors’ observations
Tardive dyskinesia is a well-known iatrogenic effect of antipsychotic medications that are commonly used to manage conditions such as schizophrenia or behavioral agitation associated with autism spectrum disorder. Symptoms of tardive dyskinesia typically emerge after 1 to 2 years of continuous exposure to dopamine receptor blocking agents (DRBAs). Tardive dyskinesia symptoms include involuntary, repetitive, purposeless movements of the tongue, jaw, lips, face, trunk, and upper and lower extremities, with significant functional impairment.1
Tardive syndromes refer to a diverse array of hyperkinetic, hypokinetic, and sensory movement disorders resulting from at least 3 months of continuous DRBA therapy.2 Tardive dyskinesia is perhaps the most well-known of the tardive syndromes, but is not the only one to consider when assessing for antipsychotic-induced movement disorders. A key feature differentiating a tardive syndrome is the persistence of the movement disorder after the DRBA is discontinued. In this case, B had been receiving a stable dose of risperidone for >1 year. He developed dystonic posturing of his left wrist and elbow that was both unresponsive to anticholinergic medication and persisted after risperidone was discontinued. The term “tardive” emphasizes the delay in development of abnormal involuntary movement symptoms after initiating antipsychotic medications.3 Table 12 shows a comparison of tardive dystonia vs an acute dystonic reaction.

Continue to: Other tardive syndromes include...
Other tardive syndromes include:
- tardive tics
- tardive parkinsonism
- tardive pain
- tardive myoclonus
- tardive akathisia
- tardive tremors.
The incidence of tardive syndromes increases 5% annually for the first 5 years of treatment. At 10 years of treatment, the annual incidence is thought to be 49%, and at 25 years of treatment, 68%.4 The predominant theory of the pathophysiology of tardive syndromes is that the chronic use of DRBAs causes a gradual hypersensitization of dopamine receptors.4 The diagnosis of a tardive syndrome is based on history of exposure to a DRBA as well as clinical observation of symptoms.
Compared with classic tardive dyskinesia, tardive dystonia is more common among younger patients. The mean age of onset of tardive dystonia is 40, and it typically affects young males.5 Typical posturing observed in cases of tardive dystonia include extension of the arms and flexion at the wrists.6 In contrast to cases of primary dystonia, tardive dystonia is typically associated with stereotypies, akathisia, or other movement disorders. Anticholinergic agents, such as
The American Psychiatric Association has issued guidelines on screening for involuntary movement syndromes by using the Abnormal Involuntary Movement Scale (AIMS).7 The current recommendations include assessment every 6 months for patients receiving first-generation antipsychotics, and every 12 months for those receiving second-generation antipsychotics.7 Prescribers should also carefully assess for any pre-existing involuntary movements before prescribing a DRBA.7
[polldaddy:10615855]
The authors’ observations
In 2013, the American Academy of Neurology (AAN) published guidelines on the treatment of tardive dyskinesia. According to these guidelines, at that time, the treatments with the most evidence supporting their use were clonazepam, ginkgo biloba,

Continue to: In 2017, valbenazine and deutetrabenazine...
In 2017, valbenazine and deutetrabenazine became the first FDA-approved treatments for tardive dyskinesia in adults. Both medications block the vesicular monoamine transporter 2 (VMAT2) system, which results in decreased synaptic dopamine and dopamine receptor stimulation. Both VMAT2 inhibitor medications have a category level A supporting their use for treating tardive dyskinesia.8-10
Currently, there are no published treatment guidelines on pharmacologic management of tardive dystonia. In B’s case, bromocriptine, a dopamine agonist, was used to counter the dopamine-blocking effects of risperidone on the nigrostriatal pathway and improve parkinsonian features of B’s presentation, including bradykinesia, stooped forward posture, and masked facies. Bromocriptine was found to be effective in alleviating parkinsonian features; however, to date there is no evidence demonstrating its effectiveness in countering delayed dystonic effects of DRBAs.
OUTCOME Improvement of dystonia symptoms
One week after discharge, B is seen for a follow-up visit. He continues taking bromocriptine, 1.25 mg twice daily, with meals after discharge. On examination, he has some evidence of tardive dystonia, including flexion of left wrist and posturing while ambulating. B’s parkinsonian features, including stooped forward posture, masked facies, and cogwheel rigidity of the left wrist muscle, have resolved. B is now able to walk on his own without unsteadiness. Bromocriptine is discontinued after 1 month, and his symptoms of dystonia continue to improve.
Two months after hospitalization, B is started on quetiapine, 25 mg twice daily, for behavioral aggression. Quetiapine is chosen because it has a lower dopamine receptor affinity compared with risperidone, and theoretically, quetiapine is associated with a lower risk of developing tardive symptoms. During the next 6 months, B is monitored closely for recurrence of tardive symptoms. Quetiapine is slowly titrated to 25 mg in the morning, and 50 mg at bedtime. His behavioral agitation improves significantly and he does not have a recurrence of tardive symptoms.
Bottom Line
Tardive dystonia is a possible iatrogenic adverse effect for patients receiving long-term dopamine receptor blocking agent (DRBA) therapy. Tardive syndromes encompass delayed-onset movement disorders caused by long-term blockade of the dopamine receptor by antipsychotic agents. Tardive dystonia can be contrasted from acute dystonic reaction based on the time course of development as well as by the persistence of symptoms after DRBAs are withheld.
Continue to: Related Resources
Related Resources
- American Academy of Neurology. Summary of evidence-based guideline for clinicians: treatment of tardive syndromes. https://www.aan.com/Guidelines/Home/GetGuidelineContent/613. Published 2013.
- Dystonia Medical Research Foundation. https://dystonia-foundation.org/.
Drug Brand Names
Amantadine • Gocovri, Symmetrel
Amoxicillin • Amoxil
Baclofen • Kemstro, Liroesal
Benztropine • Cogentin
Bromocriptine • Parlodel
Clonazepam • Klonopin
Deutetrabenazine • Austedo
Galantamine • Razadyne
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trihexyphenidyl • Artane, Tremin
Valbenazine • Ingrezza
CASE Drooling, unsteady, and not himself
B, age 10, who is left handed and has autism spectrum disorder, is brought to the emergency department (ED) with a 1-day history of drooling, unsteady gait, and left wrist in sustained flexion. His parents report that for the past week, B has had cold symptoms, including rhinorrhea, a low-grade fever (100.0°F), and cough. Earlier in the day, he was seen at his pediatrician’s office, where he was diagnosed with an acute respiratory infection and started on amoxicillin, 500 mg twice daily for 7 days.
At baseline, B is nonverbal. He requires some assistance with his activities of daily living. He usually is able to walk without assistance and dress himself, but he is not toilet trained. His parents report that in the past day, he has had significant difficulties with tasks involving his left hand. Normally, B is able to feed himself “finger foods” but has been unable to do so today. His parents say that he has been unsteady on his feet, and has been “falling forward” when he tries to walk.
Two years ago, B was started on risperidone, 0.5 mg nightly, for behavioral aggression and self-mutilation. Over the next 12 months, the dosage was steadily increased to 1 mg twice daily, with good response. He has been taking his current dosage, 1 mg twice daily, for the past 12 months without adjustment. His parents report there have been no other medication changes, other than starting amoxicillin earlier that day.
As part of his initial ED evaluation, B is found to be mildly dehydrated, with an elevated sedimentation rate on urinalysis. His complete blood count (CBC) with differential is within normal limits. A comprehensive metabolic panel shows a slight increase in his creatinine level, indicating dehydration. B is administered IV fluid replacement because he is having difficulty drinking due to excessive drooling.
The ED physician is concerned that B may be experiencing an acute dystonic reaction from risperidone, so the team holds this medication, and gives B a one-time dose of IV diphenhydramine, 25 mg, for presumptive acute dystonic reaction. After several minutes, there is no improvement in the sustained flexion of his left wrist.
[polldaddy:10615848]
The authors’ observations
B presented with new-onset neurologic findings after a recently diagnosed upper respiratory viral illness. His symptoms appeared to be confined to his left upper extremity, specifically demonstrating left arm extension at the elbow with flexion of the left wrist. He also had new-onset unsteady gait with a stooped forward posture and required assistance with walking. Interestingly, despite B’s history of antipsychotic use, administering an anticholinergic agent did not lessen the dystonic posturing at his wrist and elbow.
EVALUATION Laboratory results reveal new clues
While in the ED, B undergoes MRI of the brain and spinal cord to rule out any mass lesions that could be impinging upon the motor pathways. Both brain and spinal cord imaging appear to be essentially normal, without evidence of impingement of the spinal nerves or lesions involving the brainstem or cerebellum.
Continue to: Due to concerns...
Due to concerns of possible airway obstruction, a CT scan of the neck is obtained to rule out any acute pathology, such as epiglottitis compromising his airway. The scan shows some inflammation and edema in the soft tissues that is thought to be secondary to his acute viral illness. B is able to maintain his airway and oxygenation, so intubation is not necessary.
A CPK test is ordered because there are concerns of sustained muscle contraction of B’s left wrist and elbow. The CPK level is 884 U/L (reference range 26 to 192 U/L). The elevation in CPK is consistent with prior laboratory findings of dehydration and indicating skeletal muscle breakdown from sustained muscle contraction. All other laboratory results, including a comprehensive metabolic panel, urine drug screen, and thyroid screening panel, are within normal limits.
[polldaddy:10615850]
EVALUATION No variation in facial expression
B is admitted to the general pediatrics service. Maintenance IV fluids are started due to concerns of dehydration and possible rhabdomyolysis due to his elevated CPK level. Risperidone is held throughout the hospital course due to concerns for an acute dystonic reaction. B is monitored for several days without clinical improvement and eventually discharged home with a diagnosis of inflammatory mononeuropathy due to viral infection. The patient is told to discontinue risperidone as part of discharge instructions.
Five days later, B returns to the hospital because there was no improvement in his left extremity or walking. His left elbow remains extended with left wrist in flexion. Psychiatry is consulted for further diagnostic clarity and evaluation.
On physical examination, B’s left arm remains unchanged. Despite discontinuing risperidone, there is evidence of cogwheel rigidity of the left wrist joint. Reflexes in the upper and lower extremities are 2+ and symmetrical bilaterally, suggesting intact upper and lower motor pathways. Babinski sign is absent bilaterally, which is a normal finding in B’s age group. B continues to have difficulty with ambulating and appears to “fall forward” while trying to walk with assistance. His parents also say that B is not laughing, smiling, or showing any variation in facial expression.
Continue to: Additional family history...
Additional family history is gathered from B’s parents for possible hereditary movement disorders such as Wilson’s disease. They report that no family members have developed involuntary movements or other neurologic syndromes. Additional considerations on the differential diagnosis for B include juvenile ALS or mononeuropathy involving the C5 and C6 nerve roots. B’s parents deny any recent shoulder trauma, and radiographic studies did not demonstrate any involvement of the nerve roots.
TREATMENT A trial of bromocriptine
At this point, B’s neurologic workup is essentially normal, and he is given a provisional diagnosis of antipsychotic-induced tardive dystonia vs tardive parkinsonism. Risperidone continues to be held, and B is monitored for clinical improvement. B is administered a one-time dose of diphenhydramine, 25 mg, for dystonia with no improvement in symptoms. He is then started on bromocriptine, 1.25 mg twice daily with meals, for parkinsonian symptoms secondary to antipsychotic medication use. After 1 day of treatment, B shows less sustained flexion of his left wrist. He is able to relax his left arm, shows improvements in ambulation, and requires less assistance. B continues to be observed closely and continues to improve toward his baseline.
At Day 4, he is discharged. B is able to walk mostly without assistance and demonstrates improvement in left wrist flexion. He is scheduled to see a movement disorders specialist a week after discharge. The initial diagnosis given by the movement disorder specialist is tardive dystonia.
The authors’ observations
Tardive dyskinesia is a well-known iatrogenic effect of antipsychotic medications that are commonly used to manage conditions such as schizophrenia or behavioral agitation associated with autism spectrum disorder. Symptoms of tardive dyskinesia typically emerge after 1 to 2 years of continuous exposure to dopamine receptor blocking agents (DRBAs). Tardive dyskinesia symptoms include involuntary, repetitive, purposeless movements of the tongue, jaw, lips, face, trunk, and upper and lower extremities, with significant functional impairment.1
Tardive syndromes refer to a diverse array of hyperkinetic, hypokinetic, and sensory movement disorders resulting from at least 3 months of continuous DRBA therapy.2 Tardive dyskinesia is perhaps the most well-known of the tardive syndromes, but is not the only one to consider when assessing for antipsychotic-induced movement disorders. A key feature differentiating a tardive syndrome is the persistence of the movement disorder after the DRBA is discontinued. In this case, B had been receiving a stable dose of risperidone for >1 year. He developed dystonic posturing of his left wrist and elbow that was both unresponsive to anticholinergic medication and persisted after risperidone was discontinued. The term “tardive” emphasizes the delay in development of abnormal involuntary movement symptoms after initiating antipsychotic medications.3 Table 12 shows a comparison of tardive dystonia vs an acute dystonic reaction.

Continue to: Other tardive syndromes include...
Other tardive syndromes include:
- tardive tics
- tardive parkinsonism
- tardive pain
- tardive myoclonus
- tardive akathisia
- tardive tremors.
The incidence of tardive syndromes increases 5% annually for the first 5 years of treatment. At 10 years of treatment, the annual incidence is thought to be 49%, and at 25 years of treatment, 68%.4 The predominant theory of the pathophysiology of tardive syndromes is that the chronic use of DRBAs causes a gradual hypersensitization of dopamine receptors.4 The diagnosis of a tardive syndrome is based on history of exposure to a DRBA as well as clinical observation of symptoms.
Compared with classic tardive dyskinesia, tardive dystonia is more common among younger patients. The mean age of onset of tardive dystonia is 40, and it typically affects young males.5 Typical posturing observed in cases of tardive dystonia include extension of the arms and flexion at the wrists.6 In contrast to cases of primary dystonia, tardive dystonia is typically associated with stereotypies, akathisia, or other movement disorders. Anticholinergic agents, such as
The American Psychiatric Association has issued guidelines on screening for involuntary movement syndromes by using the Abnormal Involuntary Movement Scale (AIMS).7 The current recommendations include assessment every 6 months for patients receiving first-generation antipsychotics, and every 12 months for those receiving second-generation antipsychotics.7 Prescribers should also carefully assess for any pre-existing involuntary movements before prescribing a DRBA.7
[polldaddy:10615855]
The authors’ observations
In 2013, the American Academy of Neurology (AAN) published guidelines on the treatment of tardive dyskinesia. According to these guidelines, at that time, the treatments with the most evidence supporting their use were clonazepam, ginkgo biloba,

Continue to: In 2017, valbenazine and deutetrabenazine...
In 2017, valbenazine and deutetrabenazine became the first FDA-approved treatments for tardive dyskinesia in adults. Both medications block the vesicular monoamine transporter 2 (VMAT2) system, which results in decreased synaptic dopamine and dopamine receptor stimulation. Both VMAT2 inhibitor medications have a category level A supporting their use for treating tardive dyskinesia.8-10
Currently, there are no published treatment guidelines on pharmacologic management of tardive dystonia. In B’s case, bromocriptine, a dopamine agonist, was used to counter the dopamine-blocking effects of risperidone on the nigrostriatal pathway and improve parkinsonian features of B’s presentation, including bradykinesia, stooped forward posture, and masked facies. Bromocriptine was found to be effective in alleviating parkinsonian features; however, to date there is no evidence demonstrating its effectiveness in countering delayed dystonic effects of DRBAs.
OUTCOME Improvement of dystonia symptoms
One week after discharge, B is seen for a follow-up visit. He continues taking bromocriptine, 1.25 mg twice daily, with meals after discharge. On examination, he has some evidence of tardive dystonia, including flexion of left wrist and posturing while ambulating. B’s parkinsonian features, including stooped forward posture, masked facies, and cogwheel rigidity of the left wrist muscle, have resolved. B is now able to walk on his own without unsteadiness. Bromocriptine is discontinued after 1 month, and his symptoms of dystonia continue to improve.
Two months after hospitalization, B is started on quetiapine, 25 mg twice daily, for behavioral aggression. Quetiapine is chosen because it has a lower dopamine receptor affinity compared with risperidone, and theoretically, quetiapine is associated with a lower risk of developing tardive symptoms. During the next 6 months, B is monitored closely for recurrence of tardive symptoms. Quetiapine is slowly titrated to 25 mg in the morning, and 50 mg at bedtime. His behavioral agitation improves significantly and he does not have a recurrence of tardive symptoms.
Bottom Line
Tardive dystonia is a possible iatrogenic adverse effect for patients receiving long-term dopamine receptor blocking agent (DRBA) therapy. Tardive syndromes encompass delayed-onset movement disorders caused by long-term blockade of the dopamine receptor by antipsychotic agents. Tardive dystonia can be contrasted from acute dystonic reaction based on the time course of development as well as by the persistence of symptoms after DRBAs are withheld.
Continue to: Related Resources
Related Resources
- American Academy of Neurology. Summary of evidence-based guideline for clinicians: treatment of tardive syndromes. https://www.aan.com/Guidelines/Home/GetGuidelineContent/613. Published 2013.
- Dystonia Medical Research Foundation. https://dystonia-foundation.org/.
Drug Brand Names
Amantadine • Gocovri, Symmetrel
Amoxicillin • Amoxil
Baclofen • Kemstro, Liroesal
Benztropine • Cogentin
Bromocriptine • Parlodel
Clonazepam • Klonopin
Deutetrabenazine • Austedo
Galantamine • Razadyne
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trihexyphenidyl • Artane, Tremin
Valbenazine • Ingrezza
1. Margolese HC, Chouinard G, Kolivakis TT, et al. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 1: pathophysiology and mechanisms of induction. Can J Psychiatr. 2005;50(9):541-547.
2. Truong D, Frei K. Setting the record straight: the nosology of tardive syndromes. Parkinsonism Relat Disord. 2019;59:146-150.
3. Cornett EM, Novitch M, Kaye AD, et al. Medication-induced tardive dyskinesia: a review and update. Ochsner J. 2017;17(2):162-174.
4. Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry. 1982;39(4):486-487.
5. Fahn S, Jankovic J, Hallett M. Principles and Practice of Movement Disorders. 2nd ed. Philadelphia, PA: Saunders; 2011:415-446.
6. Kang UJ, Burke RE, Fahn S. Natural history and treatment of tardive dystonia. Mov Disord. 1986;1(3):193-208.
7. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al, Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Ingrezza [package insert]. San Diego, CA: Neurocrine Biosciences, Inc.; 2020.
10. Austedo [package insert]. North Wales, PA: Teva Pharmaceuticals; 2017.
1. Margolese HC, Chouinard G, Kolivakis TT, et al. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 1: pathophysiology and mechanisms of induction. Can J Psychiatr. 2005;50(9):541-547.
2. Truong D, Frei K. Setting the record straight: the nosology of tardive syndromes. Parkinsonism Relat Disord. 2019;59:146-150.
3. Cornett EM, Novitch M, Kaye AD, et al. Medication-induced tardive dyskinesia: a review and update. Ochsner J. 2017;17(2):162-174.
4. Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry. 1982;39(4):486-487.
5. Fahn S, Jankovic J, Hallett M. Principles and Practice of Movement Disorders. 2nd ed. Philadelphia, PA: Saunders; 2011:415-446.
6. Kang UJ, Burke RE, Fahn S. Natural history and treatment of tardive dystonia. Mov Disord. 1986;1(3):193-208.
7. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al, Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Ingrezza [package insert]. San Diego, CA: Neurocrine Biosciences, Inc.; 2020.
10. Austedo [package insert]. North Wales, PA: Teva Pharmaceuticals; 2017.
Legal concerns after a patient suicide
Most psychiatrists will care for at least one patient who dies by suicide. Many clinicians consider this to be one of the most stressful and formative events of their careers, prompting strong emotions, logistical questions, and legal concerns. Because the aftermath of a patient suicide can be difficult, we offer guidance on how to cope with such events, and specifically how to address the legal concerns.
Attend to self-care. “At a cardiac arrest, the first procedure is to take your own pulse.” This advice, from Samuel Shem’s The House of God, highlights the importance of self-awareness during highly stressful events.1 When facing the aftermath of a patient suicide, be sure to attend to your own needs, such as eating, staying hydrated, and getting enough sleep. Identify and reach out to your support systems, such as friends and family. Your colleagues can be a source of support, both formally or informally. Reaching out to other psychiatrists, who likely have their own experience with patient suicide, can help process the event. A support group consisting of other psychiatrists also may be beneficial. Finally, avoid blaming yourself. Although you might perceive your patient’s suicide as a personal failing, suicide is notoriously difficult to predict and an unfortunate reality of working in this specialty.
Report the event. Follow your institution’s guidelines for reporting adverse events. You may be required to inform your supervisor, the risk management department, legal services, your malpractice provider, and/or the police. Your risk management department and malpractice provider may have their own regulations and recommendations.
Review the case. Institutions often have established processes for reviewing adverse events and, if applicable, suggesting constructive feedback or general quality improvements. A review process may provide an opportunity to look for potential negligence that could be an issue if there is a malpractice suit. Ideally, such processes are constructive and a time for reflection, rather than punitive or blaming. Trainees may find their supervisors’ presence and guidance to be particularly helpful during this review process.
Assess malpractice risk. Although psychiatrists have a relatively low risk of being sued for malpractice, many lawsuits against psychiatrists occur after a completed patient suicide.2 In a successful malpractice suit, the plaintiff needs to establish all 4 “Ds” of medical malpractice:
1) Duty, or an established physician–patient relationship
2) Damages from an adverse event
3) Dereliction of duty (negligence)
4) Direct causality between the deviation and the damages.
In the event of a patient suicide, both a doctor–patient relationship (duty) and an adverse outcome (damages) exist.3 Establishing dereliction of duty and direct causality rests on the plaintiff to prove. Good documentation can serve as evidence against accusations of negligence.3
Typically, a patient’s medical record will be used as evidence in a malpractice suit. After a suicide, do not alter this record, such as by editing your past assessments of the patient. If an addendum must be made, such as to document a conversation with suicide survivors (family and friends of the deceased), be sure to label it as such with the current date. An addendum should contain only facts; avoid adding information that attempts to explain your patient’s suicide, justifying or apologizing for past treatment decisions, or otherwise editorializing.
Continue to: Consider reaching out to suicide survivors
Consider reaching out to suicide survivors. The Health Insurance Portability and Accountability Act permits clinicians to use their best judgment when identifying individuals to contact and deciding what information to share after a patient’s death.4 Some states and practice settings have stricter confidentiality laws. Consider seeking legal counsel before interacting with suicide survivors.
Suicide survivors may experience feelings such as guilt, shame, and anger, and these feelings may lead suicide survivors to file a malpractice suit.3 Speaking with suicide survivors can help to address these feelings and potentially decrease the likelihood of them pursuing a malpractice suit. In addition, suicide survivors are at high risk for developing mental health issues, including suicidality. Contacting them can be an opportunity to encourage them to seek mental health treatment. It is important to clarify that any recommendations you provide in such situations do not constitute a doctor–patient relationship.
Should you offer an apology? Consider seeking legal counsel if you wish to apologize. Some states have “apology laws” that render a clinician’s apologetic statements inadmissible if a malpractice suit should occur.5 These laws might include empathic statements (“I’m sorry for your loss”) or disclosures of error (“I’m sorry for causing your loss”).5 It is unclear whether these laws affect the likelihood and/or outcome of malpractice suits.5
Focus on empathy. Experiencing a patient suicide can be one of the most challenging events in a psychiatrist’s career. Empathy is crucial, both towards the suicide survivors and to oneself.
1. Shem S. The House of God. New York, NY: Berkley Books; 2010.
2. Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177(5):710-719.
3. Gutheil TG, Appelbaum PS. Clinical handbook of psychiatry and the law, 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2000.
4. Office of Civil Rights. How can a covered entity determine if a person is a family member prior to an individual’s death. US Department of Health and Human Services. https://www.hhs.gov/hipaa/for-professionals/faq/1505/how-can-a-covered-entity-determine-whether-a-person-is-a-family-member/index.html. Accessed September 9, 2020.
5. McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71(2):341-409.
Most psychiatrists will care for at least one patient who dies by suicide. Many clinicians consider this to be one of the most stressful and formative events of their careers, prompting strong emotions, logistical questions, and legal concerns. Because the aftermath of a patient suicide can be difficult, we offer guidance on how to cope with such events, and specifically how to address the legal concerns.
Attend to self-care. “At a cardiac arrest, the first procedure is to take your own pulse.” This advice, from Samuel Shem’s The House of God, highlights the importance of self-awareness during highly stressful events.1 When facing the aftermath of a patient suicide, be sure to attend to your own needs, such as eating, staying hydrated, and getting enough sleep. Identify and reach out to your support systems, such as friends and family. Your colleagues can be a source of support, both formally or informally. Reaching out to other psychiatrists, who likely have their own experience with patient suicide, can help process the event. A support group consisting of other psychiatrists also may be beneficial. Finally, avoid blaming yourself. Although you might perceive your patient’s suicide as a personal failing, suicide is notoriously difficult to predict and an unfortunate reality of working in this specialty.
Report the event. Follow your institution’s guidelines for reporting adverse events. You may be required to inform your supervisor, the risk management department, legal services, your malpractice provider, and/or the police. Your risk management department and malpractice provider may have their own regulations and recommendations.
Review the case. Institutions often have established processes for reviewing adverse events and, if applicable, suggesting constructive feedback or general quality improvements. A review process may provide an opportunity to look for potential negligence that could be an issue if there is a malpractice suit. Ideally, such processes are constructive and a time for reflection, rather than punitive or blaming. Trainees may find their supervisors’ presence and guidance to be particularly helpful during this review process.
Assess malpractice risk. Although psychiatrists have a relatively low risk of being sued for malpractice, many lawsuits against psychiatrists occur after a completed patient suicide.2 In a successful malpractice suit, the plaintiff needs to establish all 4 “Ds” of medical malpractice:
1) Duty, or an established physician–patient relationship
2) Damages from an adverse event
3) Dereliction of duty (negligence)
4) Direct causality between the deviation and the damages.
In the event of a patient suicide, both a doctor–patient relationship (duty) and an adverse outcome (damages) exist.3 Establishing dereliction of duty and direct causality rests on the plaintiff to prove. Good documentation can serve as evidence against accusations of negligence.3
Typically, a patient’s medical record will be used as evidence in a malpractice suit. After a suicide, do not alter this record, such as by editing your past assessments of the patient. If an addendum must be made, such as to document a conversation with suicide survivors (family and friends of the deceased), be sure to label it as such with the current date. An addendum should contain only facts; avoid adding information that attempts to explain your patient’s suicide, justifying or apologizing for past treatment decisions, or otherwise editorializing.
Continue to: Consider reaching out to suicide survivors
Consider reaching out to suicide survivors. The Health Insurance Portability and Accountability Act permits clinicians to use their best judgment when identifying individuals to contact and deciding what information to share after a patient’s death.4 Some states and practice settings have stricter confidentiality laws. Consider seeking legal counsel before interacting with suicide survivors.
Suicide survivors may experience feelings such as guilt, shame, and anger, and these feelings may lead suicide survivors to file a malpractice suit.3 Speaking with suicide survivors can help to address these feelings and potentially decrease the likelihood of them pursuing a malpractice suit. In addition, suicide survivors are at high risk for developing mental health issues, including suicidality. Contacting them can be an opportunity to encourage them to seek mental health treatment. It is important to clarify that any recommendations you provide in such situations do not constitute a doctor–patient relationship.
Should you offer an apology? Consider seeking legal counsel if you wish to apologize. Some states have “apology laws” that render a clinician’s apologetic statements inadmissible if a malpractice suit should occur.5 These laws might include empathic statements (“I’m sorry for your loss”) or disclosures of error (“I’m sorry for causing your loss”).5 It is unclear whether these laws affect the likelihood and/or outcome of malpractice suits.5
Focus on empathy. Experiencing a patient suicide can be one of the most challenging events in a psychiatrist’s career. Empathy is crucial, both towards the suicide survivors and to oneself.
Most psychiatrists will care for at least one patient who dies by suicide. Many clinicians consider this to be one of the most stressful and formative events of their careers, prompting strong emotions, logistical questions, and legal concerns. Because the aftermath of a patient suicide can be difficult, we offer guidance on how to cope with such events, and specifically how to address the legal concerns.
Attend to self-care. “At a cardiac arrest, the first procedure is to take your own pulse.” This advice, from Samuel Shem’s The House of God, highlights the importance of self-awareness during highly stressful events.1 When facing the aftermath of a patient suicide, be sure to attend to your own needs, such as eating, staying hydrated, and getting enough sleep. Identify and reach out to your support systems, such as friends and family. Your colleagues can be a source of support, both formally or informally. Reaching out to other psychiatrists, who likely have their own experience with patient suicide, can help process the event. A support group consisting of other psychiatrists also may be beneficial. Finally, avoid blaming yourself. Although you might perceive your patient’s suicide as a personal failing, suicide is notoriously difficult to predict and an unfortunate reality of working in this specialty.
Report the event. Follow your institution’s guidelines for reporting adverse events. You may be required to inform your supervisor, the risk management department, legal services, your malpractice provider, and/or the police. Your risk management department and malpractice provider may have their own regulations and recommendations.
Review the case. Institutions often have established processes for reviewing adverse events and, if applicable, suggesting constructive feedback or general quality improvements. A review process may provide an opportunity to look for potential negligence that could be an issue if there is a malpractice suit. Ideally, such processes are constructive and a time for reflection, rather than punitive or blaming. Trainees may find their supervisors’ presence and guidance to be particularly helpful during this review process.
Assess malpractice risk. Although psychiatrists have a relatively low risk of being sued for malpractice, many lawsuits against psychiatrists occur after a completed patient suicide.2 In a successful malpractice suit, the plaintiff needs to establish all 4 “Ds” of medical malpractice:
1) Duty, or an established physician–patient relationship
2) Damages from an adverse event
3) Dereliction of duty (negligence)
4) Direct causality between the deviation and the damages.
In the event of a patient suicide, both a doctor–patient relationship (duty) and an adverse outcome (damages) exist.3 Establishing dereliction of duty and direct causality rests on the plaintiff to prove. Good documentation can serve as evidence against accusations of negligence.3
Typically, a patient’s medical record will be used as evidence in a malpractice suit. After a suicide, do not alter this record, such as by editing your past assessments of the patient. If an addendum must be made, such as to document a conversation with suicide survivors (family and friends of the deceased), be sure to label it as such with the current date. An addendum should contain only facts; avoid adding information that attempts to explain your patient’s suicide, justifying or apologizing for past treatment decisions, or otherwise editorializing.
Continue to: Consider reaching out to suicide survivors
Consider reaching out to suicide survivors. The Health Insurance Portability and Accountability Act permits clinicians to use their best judgment when identifying individuals to contact and deciding what information to share after a patient’s death.4 Some states and practice settings have stricter confidentiality laws. Consider seeking legal counsel before interacting with suicide survivors.
Suicide survivors may experience feelings such as guilt, shame, and anger, and these feelings may lead suicide survivors to file a malpractice suit.3 Speaking with suicide survivors can help to address these feelings and potentially decrease the likelihood of them pursuing a malpractice suit. In addition, suicide survivors are at high risk for developing mental health issues, including suicidality. Contacting them can be an opportunity to encourage them to seek mental health treatment. It is important to clarify that any recommendations you provide in such situations do not constitute a doctor–patient relationship.
Should you offer an apology? Consider seeking legal counsel if you wish to apologize. Some states have “apology laws” that render a clinician’s apologetic statements inadmissible if a malpractice suit should occur.5 These laws might include empathic statements (“I’m sorry for your loss”) or disclosures of error (“I’m sorry for causing your loss”).5 It is unclear whether these laws affect the likelihood and/or outcome of malpractice suits.5
Focus on empathy. Experiencing a patient suicide can be one of the most challenging events in a psychiatrist’s career. Empathy is crucial, both towards the suicide survivors and to oneself.
1. Shem S. The House of God. New York, NY: Berkley Books; 2010.
2. Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177(5):710-719.
3. Gutheil TG, Appelbaum PS. Clinical handbook of psychiatry and the law, 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2000.
4. Office of Civil Rights. How can a covered entity determine if a person is a family member prior to an individual’s death. US Department of Health and Human Services. https://www.hhs.gov/hipaa/for-professionals/faq/1505/how-can-a-covered-entity-determine-whether-a-person-is-a-family-member/index.html. Accessed September 9, 2020.
5. McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71(2):341-409.
1. Shem S. The House of God. New York, NY: Berkley Books; 2010.
2. Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177(5):710-719.
3. Gutheil TG, Appelbaum PS. Clinical handbook of psychiatry and the law, 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2000.
4. Office of Civil Rights. How can a covered entity determine if a person is a family member prior to an individual’s death. US Department of Health and Human Services. https://www.hhs.gov/hipaa/for-professionals/faq/1505/how-can-a-covered-entity-determine-whether-a-person-is-a-family-member/index.html. Accessed September 9, 2020.
5. McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71(2):341-409.
Cannabis-derived compounds: What you need to know
Cannabis-derived compounds, such as cannabidiol (CBD), are popping up like weeds (so to speak) in retail and online stores, and are being marketed for a wide range of purported health benefits, most of which are unsubstantiated. Cannabidiol—a chemical component of the Cannabis sativa plant (marijuana)—does not produce intoxication or euphoria (ie, the “high”) that comes from delta-9-tetrahydrocannabinol (THC), which is the psychoactive component of marijuana.1 Cannabidiol has become popular partly due to increased cultural acceptance of marijuana. In a 2019 Pew Research Center survey, 67% of Americans supported marijuana legalization.2
In addition, changing laws have increased the interest in and availability of CBD. The Agricultural Improvement Act of 2018 legalized hemp, which is defined as cannabis and cannabis-derived compounds with significantly low concentrations of THC (<0.3% on a dry weight basis).1,3 However, this act also preserved the FDA’s authority to regulate products containing cannabis and cannabis-derived compounds.1
With the recent emphasis on CBD, it is easy to forget that the FDA has approved a few medications that are derived from or related to cannabis. In this article, I review the current FDA-approved cannabis-related treatments and their indications, and concerns regarding CBD products.
FDA-approved treatments
To date, the FDA has not approved cannabis for the treatment of any medical or psychiatric condition. However, the FDA has approved 1 cannabis-derived medication (CBD) and 2 cannabis-related medications (dronabinol and nabilone) for specific indications (these medications are available by prescription only):
Cannabidiol (brand name: Epidiolex) is approved for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients age ≥2, and for the treatment of seizures associated with tuberous sclerosis complex in patients age ≥1.1,4 There are no other FDA-approved medications that contain CBD.
Dronabinol (brand names: Marinol and Syndros) is an antiemetic agent that contains synthetic THC. It is approved for treating or preventing nausea and vomiting caused by cancer medications and for increasing the appetite of individuals with AIDS.1
Nabilone (brand name: Cesamet) is a synthetic compound that is structurally similar to THC. It is approved for treating or preventing nausea and vomiting caused by cancer medications.1
Continue to: Questionable claims about CBD
Questionable claims about CBD
Some manufacturers market CBD products as having a variety of health benefits for both humans and pets, but most of these claims are unsubstantiated.1 The FDA has issued warning letters to several manufacturers who have marketed CBD products as producing therapeutic effects.5
Under the Federal Food, Drug, and Cosmetic Act, any products intended to have a therapeutic effect are considered drugs, and unapproved drugs cannot be distributed or sold in interstate commerce.1 Cannabidiol products cannot be sold as dietary supplements.1 In addition, food products containing CBD cannot be introduced or delivered for introduction into interstate commerce.1 Many CBD products do not contain the amount of CBD advertised, and some contain contaminants such as pesticides and heavy metals.1 Also, CBD products can affect the therapeutic effectiveness of prescription medications.
Discuss CBD with your patients
Ask your patients if they use CBD and, if so, find out which product(s), the quantity and frequency of use, and any effects they have experienced from using them. Patients can report any adverse effects from CBD products to the FDA’s MedWatch program (www.accessdata.fda.gov/scripts/medwatch/). Tell your patients that there is limited or inconclusive evidence regarding the therapeutic efficacy of over-the-counter CBD products for any medical or psychiatric condition. Encourage your patients to be open with you about using these products, so you can make appropriate treatment decisions.
1. US Food and Drug Administration. FDA regulation of cannabis and cannabis-derived products, including cannabidiol (CBD). https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-questions-and-answers. Updated August 3, 2020. Accessed September 1, 2020.
2. Daniller A. Two-thirds of Americans support marijuana legalization. Pew Research Center. https://www.pewresearch.org/fact-tank/2018/10/08/americans-support-marijuana-legalization/. Updated November 14, 2019. Accessed September 1, 2020.
3. Agricultural Improvement Act of 2018, HR 2—115th Cong, Public L No. 115-334 (2018). https://www.congress.gov/bill/115th-congress/house-bill/2/text?overview=closed. Accessed September 1, 2020.
4. US Food and Drug Administration. FDA approves new indication for drug containing an active ingredient derived from cannabis to treat seizures in rare genetic disease. https://www.fda.gov/news-events/press-announcements/fda-approves-new-indication-drug-containing-active-ingredient-derived-cannabis-treat-seizures-rare. Published July 31, 2020. Accessed September 1, 2020.
5. US Food and Drug Administration. Warning letters and test results for cannabidiol-related products. https://www.fda.gov/news-events/public-health-focus/warning-letters-and-test-results-cannabidiol-related-products. Updated August 20, 2020. Accessed September 1, 2020.
Cannabis-derived compounds, such as cannabidiol (CBD), are popping up like weeds (so to speak) in retail and online stores, and are being marketed for a wide range of purported health benefits, most of which are unsubstantiated. Cannabidiol—a chemical component of the Cannabis sativa plant (marijuana)—does not produce intoxication or euphoria (ie, the “high”) that comes from delta-9-tetrahydrocannabinol (THC), which is the psychoactive component of marijuana.1 Cannabidiol has become popular partly due to increased cultural acceptance of marijuana. In a 2019 Pew Research Center survey, 67% of Americans supported marijuana legalization.2
In addition, changing laws have increased the interest in and availability of CBD. The Agricultural Improvement Act of 2018 legalized hemp, which is defined as cannabis and cannabis-derived compounds with significantly low concentrations of THC (<0.3% on a dry weight basis).1,3 However, this act also preserved the FDA’s authority to regulate products containing cannabis and cannabis-derived compounds.1
With the recent emphasis on CBD, it is easy to forget that the FDA has approved a few medications that are derived from or related to cannabis. In this article, I review the current FDA-approved cannabis-related treatments and their indications, and concerns regarding CBD products.
FDA-approved treatments
To date, the FDA has not approved cannabis for the treatment of any medical or psychiatric condition. However, the FDA has approved 1 cannabis-derived medication (CBD) and 2 cannabis-related medications (dronabinol and nabilone) for specific indications (these medications are available by prescription only):
Cannabidiol (brand name: Epidiolex) is approved for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients age ≥2, and for the treatment of seizures associated with tuberous sclerosis complex in patients age ≥1.1,4 There are no other FDA-approved medications that contain CBD.
Dronabinol (brand names: Marinol and Syndros) is an antiemetic agent that contains synthetic THC. It is approved for treating or preventing nausea and vomiting caused by cancer medications and for increasing the appetite of individuals with AIDS.1
Nabilone (brand name: Cesamet) is a synthetic compound that is structurally similar to THC. It is approved for treating or preventing nausea and vomiting caused by cancer medications.1
Continue to: Questionable claims about CBD
Questionable claims about CBD
Some manufacturers market CBD products as having a variety of health benefits for both humans and pets, but most of these claims are unsubstantiated.1 The FDA has issued warning letters to several manufacturers who have marketed CBD products as producing therapeutic effects.5
Under the Federal Food, Drug, and Cosmetic Act, any products intended to have a therapeutic effect are considered drugs, and unapproved drugs cannot be distributed or sold in interstate commerce.1 Cannabidiol products cannot be sold as dietary supplements.1 In addition, food products containing CBD cannot be introduced or delivered for introduction into interstate commerce.1 Many CBD products do not contain the amount of CBD advertised, and some contain contaminants such as pesticides and heavy metals.1 Also, CBD products can affect the therapeutic effectiveness of prescription medications.
Discuss CBD with your patients
Ask your patients if they use CBD and, if so, find out which product(s), the quantity and frequency of use, and any effects they have experienced from using them. Patients can report any adverse effects from CBD products to the FDA’s MedWatch program (www.accessdata.fda.gov/scripts/medwatch/). Tell your patients that there is limited or inconclusive evidence regarding the therapeutic efficacy of over-the-counter CBD products for any medical or psychiatric condition. Encourage your patients to be open with you about using these products, so you can make appropriate treatment decisions.
Cannabis-derived compounds, such as cannabidiol (CBD), are popping up like weeds (so to speak) in retail and online stores, and are being marketed for a wide range of purported health benefits, most of which are unsubstantiated. Cannabidiol—a chemical component of the Cannabis sativa plant (marijuana)—does not produce intoxication or euphoria (ie, the “high”) that comes from delta-9-tetrahydrocannabinol (THC), which is the psychoactive component of marijuana.1 Cannabidiol has become popular partly due to increased cultural acceptance of marijuana. In a 2019 Pew Research Center survey, 67% of Americans supported marijuana legalization.2
In addition, changing laws have increased the interest in and availability of CBD. The Agricultural Improvement Act of 2018 legalized hemp, which is defined as cannabis and cannabis-derived compounds with significantly low concentrations of THC (<0.3% on a dry weight basis).1,3 However, this act also preserved the FDA’s authority to regulate products containing cannabis and cannabis-derived compounds.1
With the recent emphasis on CBD, it is easy to forget that the FDA has approved a few medications that are derived from or related to cannabis. In this article, I review the current FDA-approved cannabis-related treatments and their indications, and concerns regarding CBD products.
FDA-approved treatments
To date, the FDA has not approved cannabis for the treatment of any medical or psychiatric condition. However, the FDA has approved 1 cannabis-derived medication (CBD) and 2 cannabis-related medications (dronabinol and nabilone) for specific indications (these medications are available by prescription only):
Cannabidiol (brand name: Epidiolex) is approved for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients age ≥2, and for the treatment of seizures associated with tuberous sclerosis complex in patients age ≥1.1,4 There are no other FDA-approved medications that contain CBD.
Dronabinol (brand names: Marinol and Syndros) is an antiemetic agent that contains synthetic THC. It is approved for treating or preventing nausea and vomiting caused by cancer medications and for increasing the appetite of individuals with AIDS.1
Nabilone (brand name: Cesamet) is a synthetic compound that is structurally similar to THC. It is approved for treating or preventing nausea and vomiting caused by cancer medications.1
Continue to: Questionable claims about CBD
Questionable claims about CBD
Some manufacturers market CBD products as having a variety of health benefits for both humans and pets, but most of these claims are unsubstantiated.1 The FDA has issued warning letters to several manufacturers who have marketed CBD products as producing therapeutic effects.5
Under the Federal Food, Drug, and Cosmetic Act, any products intended to have a therapeutic effect are considered drugs, and unapproved drugs cannot be distributed or sold in interstate commerce.1 Cannabidiol products cannot be sold as dietary supplements.1 In addition, food products containing CBD cannot be introduced or delivered for introduction into interstate commerce.1 Many CBD products do not contain the amount of CBD advertised, and some contain contaminants such as pesticides and heavy metals.1 Also, CBD products can affect the therapeutic effectiveness of prescription medications.
Discuss CBD with your patients
Ask your patients if they use CBD and, if so, find out which product(s), the quantity and frequency of use, and any effects they have experienced from using them. Patients can report any adverse effects from CBD products to the FDA’s MedWatch program (www.accessdata.fda.gov/scripts/medwatch/). Tell your patients that there is limited or inconclusive evidence regarding the therapeutic efficacy of over-the-counter CBD products for any medical or psychiatric condition. Encourage your patients to be open with you about using these products, so you can make appropriate treatment decisions.
1. US Food and Drug Administration. FDA regulation of cannabis and cannabis-derived products, including cannabidiol (CBD). https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-questions-and-answers. Updated August 3, 2020. Accessed September 1, 2020.
2. Daniller A. Two-thirds of Americans support marijuana legalization. Pew Research Center. https://www.pewresearch.org/fact-tank/2018/10/08/americans-support-marijuana-legalization/. Updated November 14, 2019. Accessed September 1, 2020.
3. Agricultural Improvement Act of 2018, HR 2—115th Cong, Public L No. 115-334 (2018). https://www.congress.gov/bill/115th-congress/house-bill/2/text?overview=closed. Accessed September 1, 2020.
4. US Food and Drug Administration. FDA approves new indication for drug containing an active ingredient derived from cannabis to treat seizures in rare genetic disease. https://www.fda.gov/news-events/press-announcements/fda-approves-new-indication-drug-containing-active-ingredient-derived-cannabis-treat-seizures-rare. Published July 31, 2020. Accessed September 1, 2020.
5. US Food and Drug Administration. Warning letters and test results for cannabidiol-related products. https://www.fda.gov/news-events/public-health-focus/warning-letters-and-test-results-cannabidiol-related-products. Updated August 20, 2020. Accessed September 1, 2020.
1. US Food and Drug Administration. FDA regulation of cannabis and cannabis-derived products, including cannabidiol (CBD). https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-questions-and-answers. Updated August 3, 2020. Accessed September 1, 2020.
2. Daniller A. Two-thirds of Americans support marijuana legalization. Pew Research Center. https://www.pewresearch.org/fact-tank/2018/10/08/americans-support-marijuana-legalization/. Updated November 14, 2019. Accessed September 1, 2020.
3. Agricultural Improvement Act of 2018, HR 2—115th Cong, Public L No. 115-334 (2018). https://www.congress.gov/bill/115th-congress/house-bill/2/text?overview=closed. Accessed September 1, 2020.
4. US Food and Drug Administration. FDA approves new indication for drug containing an active ingredient derived from cannabis to treat seizures in rare genetic disease. https://www.fda.gov/news-events/press-announcements/fda-approves-new-indication-drug-containing-active-ingredient-derived-cannabis-treat-seizures-rare. Published July 31, 2020. Accessed September 1, 2020.
5. US Food and Drug Administration. Warning letters and test results for cannabidiol-related products. https://www.fda.gov/news-events/public-health-focus/warning-letters-and-test-results-cannabidiol-related-products. Updated August 20, 2020. Accessed September 1, 2020.
Recurrent leg lesions
Tender erythematous nodules or plaques on the extensor surfaces—usually on the legs and occasionally on the arms—are the hallmarks for erythema nodosum, which was diagnosed in this case. It typically occurs in young women, ages 15 to 30, and the nodules or plaques are often accompanied by prodromal fever and malaise. The lesions often are painful and tender to pressure or palpation; they are thought to be caused by a reaction to a stimulus, leading to inflammation of the septa in the subcutaneous fat. While the trigger is often unknown, in some cases, an underlying infection, particularly Streptococcus or tuberculosis (TB), is identified. Sarcoidosis, malignancy, or an increase in estrogen (exogenous or endogenous) also can provoke the disorder.
Due to the risk of underlying disease or triggers, it is prudent to perform radiography of the chest, as well as obtain a complete blood count, sedimentation rate or C reactive protein, and an antistreptolysin O titer when you suspect erythema nodosum. TB testing is also advised. Biopsy typically is not performed because the diagnosis usually is made clinically. If the diagnosis is in doubt, a biopsy can offer confirmation or lead to a different diagnosis such as vasculitis—especially if the lesions are eroded. Since erythema nodosum is an inflammation of the subcutaneous fat, it is important to sample skin lesions deeper than the usual punch biopsy; an incisional biopsy may be required to get an adequate sample.
Erythema nodosum typically resolves spontaneously over a period of weeks, even if there is underlying disease. Therefore, it may be possible to defer treatment if minimal symptoms are present. Otherwise, first-line treatment for the pain and malaise is a nonsteroidal anti-inflammatory drug (NSAID). Oral potassium iodide (360-900 mg/d) is considered second-line treatment and systemic corticosteroids are a third-line option.
For this patient, biopsy was deferred and diagnostic tests were all negative. She had notable pain and a history of good resolution of symptoms with prednisone (5 mg/d), so this drug was prescribed for a 7-day course. She was counseled to avoid taking the NSAIDs and prednisone together due to increased risk of gastritis and ulceration. Recurrent disease can be treated with dapsone (100 mg/d) or hydroxychloroquine (200 mg bid).
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Blake T, Manahan M, Rodins K. Erythema nodosum - a review of an uncommon panniculitis. Dermatol Online J. 2014;20:22376.

Tender erythematous nodules or plaques on the extensor surfaces—usually on the legs and occasionally on the arms—are the hallmarks for erythema nodosum, which was diagnosed in this case. It typically occurs in young women, ages 15 to 30, and the nodules or plaques are often accompanied by prodromal fever and malaise. The lesions often are painful and tender to pressure or palpation; they are thought to be caused by a reaction to a stimulus, leading to inflammation of the septa in the subcutaneous fat. While the trigger is often unknown, in some cases, an underlying infection, particularly Streptococcus or tuberculosis (TB), is identified. Sarcoidosis, malignancy, or an increase in estrogen (exogenous or endogenous) also can provoke the disorder.
Due to the risk of underlying disease or triggers, it is prudent to perform radiography of the chest, as well as obtain a complete blood count, sedimentation rate or C reactive protein, and an antistreptolysin O titer when you suspect erythema nodosum. TB testing is also advised. Biopsy typically is not performed because the diagnosis usually is made clinically. If the diagnosis is in doubt, a biopsy can offer confirmation or lead to a different diagnosis such as vasculitis—especially if the lesions are eroded. Since erythema nodosum is an inflammation of the subcutaneous fat, it is important to sample skin lesions deeper than the usual punch biopsy; an incisional biopsy may be required to get an adequate sample.
Erythema nodosum typically resolves spontaneously over a period of weeks, even if there is underlying disease. Therefore, it may be possible to defer treatment if minimal symptoms are present. Otherwise, first-line treatment for the pain and malaise is a nonsteroidal anti-inflammatory drug (NSAID). Oral potassium iodide (360-900 mg/d) is considered second-line treatment and systemic corticosteroids are a third-line option.
For this patient, biopsy was deferred and diagnostic tests were all negative. She had notable pain and a history of good resolution of symptoms with prednisone (5 mg/d), so this drug was prescribed for a 7-day course. She was counseled to avoid taking the NSAIDs and prednisone together due to increased risk of gastritis and ulceration. Recurrent disease can be treated with dapsone (100 mg/d) or hydroxychloroquine (200 mg bid).
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

Tender erythematous nodules or plaques on the extensor surfaces—usually on the legs and occasionally on the arms—are the hallmarks for erythema nodosum, which was diagnosed in this case. It typically occurs in young women, ages 15 to 30, and the nodules or plaques are often accompanied by prodromal fever and malaise. The lesions often are painful and tender to pressure or palpation; they are thought to be caused by a reaction to a stimulus, leading to inflammation of the septa in the subcutaneous fat. While the trigger is often unknown, in some cases, an underlying infection, particularly Streptococcus or tuberculosis (TB), is identified. Sarcoidosis, malignancy, or an increase in estrogen (exogenous or endogenous) also can provoke the disorder.
Due to the risk of underlying disease or triggers, it is prudent to perform radiography of the chest, as well as obtain a complete blood count, sedimentation rate or C reactive protein, and an antistreptolysin O titer when you suspect erythema nodosum. TB testing is also advised. Biopsy typically is not performed because the diagnosis usually is made clinically. If the diagnosis is in doubt, a biopsy can offer confirmation or lead to a different diagnosis such as vasculitis—especially if the lesions are eroded. Since erythema nodosum is an inflammation of the subcutaneous fat, it is important to sample skin lesions deeper than the usual punch biopsy; an incisional biopsy may be required to get an adequate sample.
Erythema nodosum typically resolves spontaneously over a period of weeks, even if there is underlying disease. Therefore, it may be possible to defer treatment if minimal symptoms are present. Otherwise, first-line treatment for the pain and malaise is a nonsteroidal anti-inflammatory drug (NSAID). Oral potassium iodide (360-900 mg/d) is considered second-line treatment and systemic corticosteroids are a third-line option.
For this patient, biopsy was deferred and diagnostic tests were all negative. She had notable pain and a history of good resolution of symptoms with prednisone (5 mg/d), so this drug was prescribed for a 7-day course. She was counseled to avoid taking the NSAIDs and prednisone together due to increased risk of gastritis and ulceration. Recurrent disease can be treated with dapsone (100 mg/d) or hydroxychloroquine (200 mg bid).
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Blake T, Manahan M, Rodins K. Erythema nodosum - a review of an uncommon panniculitis. Dermatol Online J. 2014;20:22376.
Blake T, Manahan M, Rodins K. Erythema nodosum - a review of an uncommon panniculitis. Dermatol Online J. 2014;20:22376.
Despite overall exhaustion, health care workers continue on
I write this editorial in mid-September. Fires (and ash) are devastating the West and multiple hurricanes are pummeling the Gulf Coast states. We are struggling to admit how our democracy has systematically failed so many people and learn how we might rectify past inequities and abuses so we can create a better future together. All this with the backdrop of COVID-19, as we pass 200,000 American deaths. We will figure this out and be stronger, but for now it is exhausting, and many people are suffering.
The year 2020 will change gastroenterology forever. The economic fallout already has accelerated the disappearance of traditional medical practices, whose finances were based on steady cash flow. Medicaid rolls will increase from 70 million to over 80 million next year, putting State budgets in deficit and likely altering enrollment requirements. Currently, only half of Baby Boomers are enrolled in Medicare, a statistic that will change with loss of employment and early retirements. Many Americans are losing their employer-based insurance and shifting to government-based insurance (or losing insurance entirely). Providers will face enormous financial headwinds for years no matter how rapidly our economy recovers.
But not all news is bad. We can still read how scientific knowledge continues to progress (our issue this month is rich with examples). Our responses to COVID-19 have been breath-taking in their speed. The death rate per hospitalized patient has fallen dramatically, we continue to learn how to mitigate the effects of COVID-19, and we anticipate a vaccine in record time compared with past epidemics. Physicians and other health care providers are demonstrating daily their dedication to patients despite physical, emotional, and mental exhaustion.
I have no glib answers or words of advice. But I continue to be optimistic. In a nonpartisan tone, I quote Bill Clinton’s 1993 inaugural address: “There is nothing wrong with America that cannot be cured by what is right with America.”
John I. Allen, MD, MBA, AGAF
Editor in Chief
I write this editorial in mid-September. Fires (and ash) are devastating the West and multiple hurricanes are pummeling the Gulf Coast states. We are struggling to admit how our democracy has systematically failed so many people and learn how we might rectify past inequities and abuses so we can create a better future together. All this with the backdrop of COVID-19, as we pass 200,000 American deaths. We will figure this out and be stronger, but for now it is exhausting, and many people are suffering.
The year 2020 will change gastroenterology forever. The economic fallout already has accelerated the disappearance of traditional medical practices, whose finances were based on steady cash flow. Medicaid rolls will increase from 70 million to over 80 million next year, putting State budgets in deficit and likely altering enrollment requirements. Currently, only half of Baby Boomers are enrolled in Medicare, a statistic that will change with loss of employment and early retirements. Many Americans are losing their employer-based insurance and shifting to government-based insurance (or losing insurance entirely). Providers will face enormous financial headwinds for years no matter how rapidly our economy recovers.
But not all news is bad. We can still read how scientific knowledge continues to progress (our issue this month is rich with examples). Our responses to COVID-19 have been breath-taking in their speed. The death rate per hospitalized patient has fallen dramatically, we continue to learn how to mitigate the effects of COVID-19, and we anticipate a vaccine in record time compared with past epidemics. Physicians and other health care providers are demonstrating daily their dedication to patients despite physical, emotional, and mental exhaustion.
I have no glib answers or words of advice. But I continue to be optimistic. In a nonpartisan tone, I quote Bill Clinton’s 1993 inaugural address: “There is nothing wrong with America that cannot be cured by what is right with America.”
John I. Allen, MD, MBA, AGAF
Editor in Chief
I write this editorial in mid-September. Fires (and ash) are devastating the West and multiple hurricanes are pummeling the Gulf Coast states. We are struggling to admit how our democracy has systematically failed so many people and learn how we might rectify past inequities and abuses so we can create a better future together. All this with the backdrop of COVID-19, as we pass 200,000 American deaths. We will figure this out and be stronger, but for now it is exhausting, and many people are suffering.
The year 2020 will change gastroenterology forever. The economic fallout already has accelerated the disappearance of traditional medical practices, whose finances were based on steady cash flow. Medicaid rolls will increase from 70 million to over 80 million next year, putting State budgets in deficit and likely altering enrollment requirements. Currently, only half of Baby Boomers are enrolled in Medicare, a statistic that will change with loss of employment and early retirements. Many Americans are losing their employer-based insurance and shifting to government-based insurance (or losing insurance entirely). Providers will face enormous financial headwinds for years no matter how rapidly our economy recovers.
But not all news is bad. We can still read how scientific knowledge continues to progress (our issue this month is rich with examples). Our responses to COVID-19 have been breath-taking in their speed. The death rate per hospitalized patient has fallen dramatically, we continue to learn how to mitigate the effects of COVID-19, and we anticipate a vaccine in record time compared with past epidemics. Physicians and other health care providers are demonstrating daily their dedication to patients despite physical, emotional, and mental exhaustion.
I have no glib answers or words of advice. But I continue to be optimistic. In a nonpartisan tone, I quote Bill Clinton’s 1993 inaugural address: “There is nothing wrong with America that cannot be cured by what is right with America.”
John I. Allen, MD, MBA, AGAF
Editor in Chief
Orthopedic problems in children can be the first indication of acute lymphoblastic leukemia
The diagnosis of acute lymphoblastic leukemia (ALL) can be delayed because of vague presentation and normal hematological results. Orthopedic manifestations may be the primary presentation of ALL to physicians, and such symptoms in children should be cause for suspicion, even in the absence of hematological abnormalities, according to a report published in the Journal of Orthopaedics.
The study retrospectively assessed 250 consecutive ALL patients at a single institution to identify the frequency of ALL cases presented to the orthopedic department and to determine the number of these patients presenting with normal hematological results, according to Amrath Raj BK, MD, and colleagues at the Manipal (India) Academy of Higher Education.
Suspicion warranted
Twenty-two of the 250 patients (8.8%) presented primarily to the orthopedic department (4 with vertebral compression fractures, 12 with joint pain, and 6 with bone pain), but were subsequently diagnosed with ALL. These results were comparable to previous studies. The mean patient age at the first visit was 5.6 years; 13 patients were boys, and 9 were girls. Six of these 22 patients (27.3%) had a normal peripheral blood smear, according to the researchers.
“Acute leukemia should be considered strongly as a differential diagnosis in children with severe osteoporosis and vertebral fractures. Initial orthopedic manifestations are not uncommon, and the primary physician should maintain a high index of suspicion as a peripheral smear is not diagnostic in all patients,” the researchers concluded.
The authors reported that there was no outside funding source and that they had no conflicts.
SOURCE: Raj BK A et al. Journal of Orthopaedics. 2020;22:326-330.
The diagnosis of acute lymphoblastic leukemia (ALL) can be delayed because of vague presentation and normal hematological results. Orthopedic manifestations may be the primary presentation of ALL to physicians, and such symptoms in children should be cause for suspicion, even in the absence of hematological abnormalities, according to a report published in the Journal of Orthopaedics.
The study retrospectively assessed 250 consecutive ALL patients at a single institution to identify the frequency of ALL cases presented to the orthopedic department and to determine the number of these patients presenting with normal hematological results, according to Amrath Raj BK, MD, and colleagues at the Manipal (India) Academy of Higher Education.
Suspicion warranted
Twenty-two of the 250 patients (8.8%) presented primarily to the orthopedic department (4 with vertebral compression fractures, 12 with joint pain, and 6 with bone pain), but were subsequently diagnosed with ALL. These results were comparable to previous studies. The mean patient age at the first visit was 5.6 years; 13 patients were boys, and 9 were girls. Six of these 22 patients (27.3%) had a normal peripheral blood smear, according to the researchers.
“Acute leukemia should be considered strongly as a differential diagnosis in children with severe osteoporosis and vertebral fractures. Initial orthopedic manifestations are not uncommon, and the primary physician should maintain a high index of suspicion as a peripheral smear is not diagnostic in all patients,” the researchers concluded.
The authors reported that there was no outside funding source and that they had no conflicts.
SOURCE: Raj BK A et al. Journal of Orthopaedics. 2020;22:326-330.
The diagnosis of acute lymphoblastic leukemia (ALL) can be delayed because of vague presentation and normal hematological results. Orthopedic manifestations may be the primary presentation of ALL to physicians, and such symptoms in children should be cause for suspicion, even in the absence of hematological abnormalities, according to a report published in the Journal of Orthopaedics.
The study retrospectively assessed 250 consecutive ALL patients at a single institution to identify the frequency of ALL cases presented to the orthopedic department and to determine the number of these patients presenting with normal hematological results, according to Amrath Raj BK, MD, and colleagues at the Manipal (India) Academy of Higher Education.
Suspicion warranted
Twenty-two of the 250 patients (8.8%) presented primarily to the orthopedic department (4 with vertebral compression fractures, 12 with joint pain, and 6 with bone pain), but were subsequently diagnosed with ALL. These results were comparable to previous studies. The mean patient age at the first visit was 5.6 years; 13 patients were boys, and 9 were girls. Six of these 22 patients (27.3%) had a normal peripheral blood smear, according to the researchers.
“Acute leukemia should be considered strongly as a differential diagnosis in children with severe osteoporosis and vertebral fractures. Initial orthopedic manifestations are not uncommon, and the primary physician should maintain a high index of suspicion as a peripheral smear is not diagnostic in all patients,” the researchers concluded.
The authors reported that there was no outside funding source and that they had no conflicts.
SOURCE: Raj BK A et al. Journal of Orthopaedics. 2020;22:326-330.
FROM THE JOURNAL OF ORTHOPAEDICS
Cancer disparities: One of the most pressing public health issues
“The burden of cancer is not shouldered equally by all segments of the U.S. population,” the AACR adds. “The adverse differences in cancer burden that exist among certain population groups are one of the most pressing public health challenges that we face in the United States.”
AACR president Antoni Ribas, MD, PhD, gave some examples of these disparities at a September 16 Congressional briefing that focused on the inaugural AACR Cancer Disparities Progress Report 2020.
He noted that:
- Black men have more than double the rate of death from prostate cancer compared with men of other racial and ethnic groups.
- Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
- Non-Hispanic Black children and adolescents with cancer are more than 50% more likely to die from the cancer than non-Hispanic white children and adolescents with cancer.
- Women of low socioeconomic status with early stage ovarian cancer are 50% less likely to receive recommended care than are women of high socioeconomic status.
- In addition to racial and ethnic minority groups, other populations that bear a disproportionate burden when it comes to cancer include individuals lacking adequate health insurance coverage, immigrants, those with disabilities, residents in rural areas, and members of the lesbian, gay, bisexual, and transgender communities.
“It is absolutely unacceptable that advances in cancer care and treatment are not benefiting everyone equally,” Ribas commented.
Making progress against cancer
Progress being made against cancer was highlighted in another publication, the annual AACR Cancer Progress Report 2020.
U.S. cancer deaths declined by 29% between 1991 and 2017, translating to nearly 3 million cancer deaths avoided, the report notes. In addition, 5-year survival rates for all cancers combined increased from 49% in the mid-1970s to 70% for patients diagnosed from 2010-2016.
Between August 2019 and July 31 of this year, the U.S. Food and Drug Administration approved 20 new anticancer drugs for various cancer types and 15 new indications for previously approved cancer drugs, marking the highest number of approvals in one 12-month period since AACR started producing these reports 10 years ago.
A continuing reduction in the cigarette smoking rate among US adults, which is now below 14%, is contributing greatly to declines in lung cancer rates, which have largely driven the improvements in cancer survival, the AACR noted.
This report also notes that progress has been made toward reducing cancer disparities. Overall disparities in cancer death rates among racial and ethnic groups are less pronounced now than they have been in the past two decades. For example, the overall cancer death rate for African American patients was 33% higher than for White patients in 1990 but just 14% higher in 2016.
However, both reports agree that more must be done to reduce cancer disparities even further.
They highlight initiatives that are underway, including:
- The draft guidance issued by the FDA to promote diversification of clinical trial populations.
- The National Institutes of Health’s (NIH’s) Continuing Umbrella of Research Experiences (CURE) program supporting underrepresented students and scientists along their academic and research career pathway.
- The Centers for Disease Control and Prevention’s Racial and Ethnic Approaches to Community Health (REACH) program, a grant-making program focused on encouraging preventive behaviors in underserved communities.
- The NIH’s All of Us program, which is gathering information from the genomes of 1 million healthy individuals with a focus on recruitment from historically underrepresented populations.
Ribas also announced that AACR has established a task force to focus on racial inequalities in cancer research.
Eliminating disparities would save money, argued John D. Carpten, PhD, from the University of Southern California, Los Angeles, who chaired the steering committee that developed the AACR Cancer Disparities Progress Report.
Carpten noted research showing that eliminating disparities for racial and ethnic minorities between 2003 and 2006 would have reduced health care costs by more than $1 trillion in the United States. This underscores the potentially far-reaching impact of efforts to eliminate disparities, he said.
“Without a doubt, socioeconomics and inequities in access to quality care represent major factors influencing cancer health disparities, and these disparities will persist until we address these issues” he said.
Both progress reports culminate in a call to action, largely focused on the need for “unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for the NIH, NCI [National Cancer Institute], and FDA,” which is vital for accelerating the pace of progress.
The challenge is now compounded by the ongoing COVID-19 pandemic: Both progress reports note that racial and ethnic minorities, including African Americans, are not only affected disproportionately by cancer, but also by COVID-19, further highlighting the “stark inequities in health care.”
Ribas further called for action from national leadership and the scientific community.
“During this unprecedented time in our nation’s history, there is also a need for our nation’s leaders to take on a much bigger role in confronting and combating the structural and systemic racism that contributes to health disparities,” he said. The “pervasive racism and social injustices” that have contributed to disparities in both COVID-19 and cancer underscore the need for “the scientific community to step up and partner with Congress to assess and address this issue within the research community.”
This article first appeared on Medscape.com.
“The burden of cancer is not shouldered equally by all segments of the U.S. population,” the AACR adds. “The adverse differences in cancer burden that exist among certain population groups are one of the most pressing public health challenges that we face in the United States.”
AACR president Antoni Ribas, MD, PhD, gave some examples of these disparities at a September 16 Congressional briefing that focused on the inaugural AACR Cancer Disparities Progress Report 2020.
He noted that:
- Black men have more than double the rate of death from prostate cancer compared with men of other racial and ethnic groups.
- Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
- Non-Hispanic Black children and adolescents with cancer are more than 50% more likely to die from the cancer than non-Hispanic white children and adolescents with cancer.
- Women of low socioeconomic status with early stage ovarian cancer are 50% less likely to receive recommended care than are women of high socioeconomic status.
- In addition to racial and ethnic minority groups, other populations that bear a disproportionate burden when it comes to cancer include individuals lacking adequate health insurance coverage, immigrants, those with disabilities, residents in rural areas, and members of the lesbian, gay, bisexual, and transgender communities.
“It is absolutely unacceptable that advances in cancer care and treatment are not benefiting everyone equally,” Ribas commented.
Making progress against cancer
Progress being made against cancer was highlighted in another publication, the annual AACR Cancer Progress Report 2020.
U.S. cancer deaths declined by 29% between 1991 and 2017, translating to nearly 3 million cancer deaths avoided, the report notes. In addition, 5-year survival rates for all cancers combined increased from 49% in the mid-1970s to 70% for patients diagnosed from 2010-2016.
Between August 2019 and July 31 of this year, the U.S. Food and Drug Administration approved 20 new anticancer drugs for various cancer types and 15 new indications for previously approved cancer drugs, marking the highest number of approvals in one 12-month period since AACR started producing these reports 10 years ago.
A continuing reduction in the cigarette smoking rate among US adults, which is now below 14%, is contributing greatly to declines in lung cancer rates, which have largely driven the improvements in cancer survival, the AACR noted.
This report also notes that progress has been made toward reducing cancer disparities. Overall disparities in cancer death rates among racial and ethnic groups are less pronounced now than they have been in the past two decades. For example, the overall cancer death rate for African American patients was 33% higher than for White patients in 1990 but just 14% higher in 2016.
However, both reports agree that more must be done to reduce cancer disparities even further.
They highlight initiatives that are underway, including:
- The draft guidance issued by the FDA to promote diversification of clinical trial populations.
- The National Institutes of Health’s (NIH’s) Continuing Umbrella of Research Experiences (CURE) program supporting underrepresented students and scientists along their academic and research career pathway.
- The Centers for Disease Control and Prevention’s Racial and Ethnic Approaches to Community Health (REACH) program, a grant-making program focused on encouraging preventive behaviors in underserved communities.
- The NIH’s All of Us program, which is gathering information from the genomes of 1 million healthy individuals with a focus on recruitment from historically underrepresented populations.
Ribas also announced that AACR has established a task force to focus on racial inequalities in cancer research.
Eliminating disparities would save money, argued John D. Carpten, PhD, from the University of Southern California, Los Angeles, who chaired the steering committee that developed the AACR Cancer Disparities Progress Report.
Carpten noted research showing that eliminating disparities for racial and ethnic minorities between 2003 and 2006 would have reduced health care costs by more than $1 trillion in the United States. This underscores the potentially far-reaching impact of efforts to eliminate disparities, he said.
“Without a doubt, socioeconomics and inequities in access to quality care represent major factors influencing cancer health disparities, and these disparities will persist until we address these issues” he said.
Both progress reports culminate in a call to action, largely focused on the need for “unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for the NIH, NCI [National Cancer Institute], and FDA,” which is vital for accelerating the pace of progress.
The challenge is now compounded by the ongoing COVID-19 pandemic: Both progress reports note that racial and ethnic minorities, including African Americans, are not only affected disproportionately by cancer, but also by COVID-19, further highlighting the “stark inequities in health care.”
Ribas further called for action from national leadership and the scientific community.
“During this unprecedented time in our nation’s history, there is also a need for our nation’s leaders to take on a much bigger role in confronting and combating the structural and systemic racism that contributes to health disparities,” he said. The “pervasive racism and social injustices” that have contributed to disparities in both COVID-19 and cancer underscore the need for “the scientific community to step up and partner with Congress to assess and address this issue within the research community.”
This article first appeared on Medscape.com.
“The burden of cancer is not shouldered equally by all segments of the U.S. population,” the AACR adds. “The adverse differences in cancer burden that exist among certain population groups are one of the most pressing public health challenges that we face in the United States.”
AACR president Antoni Ribas, MD, PhD, gave some examples of these disparities at a September 16 Congressional briefing that focused on the inaugural AACR Cancer Disparities Progress Report 2020.
He noted that:
- Black men have more than double the rate of death from prostate cancer compared with men of other racial and ethnic groups.
- Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
- Non-Hispanic Black children and adolescents with cancer are more than 50% more likely to die from the cancer than non-Hispanic white children and adolescents with cancer.
- Women of low socioeconomic status with early stage ovarian cancer are 50% less likely to receive recommended care than are women of high socioeconomic status.
- In addition to racial and ethnic minority groups, other populations that bear a disproportionate burden when it comes to cancer include individuals lacking adequate health insurance coverage, immigrants, those with disabilities, residents in rural areas, and members of the lesbian, gay, bisexual, and transgender communities.
“It is absolutely unacceptable that advances in cancer care and treatment are not benefiting everyone equally,” Ribas commented.
Making progress against cancer
Progress being made against cancer was highlighted in another publication, the annual AACR Cancer Progress Report 2020.
U.S. cancer deaths declined by 29% between 1991 and 2017, translating to nearly 3 million cancer deaths avoided, the report notes. In addition, 5-year survival rates for all cancers combined increased from 49% in the mid-1970s to 70% for patients diagnosed from 2010-2016.
Between August 2019 and July 31 of this year, the U.S. Food and Drug Administration approved 20 new anticancer drugs for various cancer types and 15 new indications for previously approved cancer drugs, marking the highest number of approvals in one 12-month period since AACR started producing these reports 10 years ago.
A continuing reduction in the cigarette smoking rate among US adults, which is now below 14%, is contributing greatly to declines in lung cancer rates, which have largely driven the improvements in cancer survival, the AACR noted.
This report also notes that progress has been made toward reducing cancer disparities. Overall disparities in cancer death rates among racial and ethnic groups are less pronounced now than they have been in the past two decades. For example, the overall cancer death rate for African American patients was 33% higher than for White patients in 1990 but just 14% higher in 2016.
However, both reports agree that more must be done to reduce cancer disparities even further.
They highlight initiatives that are underway, including:
- The draft guidance issued by the FDA to promote diversification of clinical trial populations.
- The National Institutes of Health’s (NIH’s) Continuing Umbrella of Research Experiences (CURE) program supporting underrepresented students and scientists along their academic and research career pathway.
- The Centers for Disease Control and Prevention’s Racial and Ethnic Approaches to Community Health (REACH) program, a grant-making program focused on encouraging preventive behaviors in underserved communities.
- The NIH’s All of Us program, which is gathering information from the genomes of 1 million healthy individuals with a focus on recruitment from historically underrepresented populations.
Ribas also announced that AACR has established a task force to focus on racial inequalities in cancer research.
Eliminating disparities would save money, argued John D. Carpten, PhD, from the University of Southern California, Los Angeles, who chaired the steering committee that developed the AACR Cancer Disparities Progress Report.
Carpten noted research showing that eliminating disparities for racial and ethnic minorities between 2003 and 2006 would have reduced health care costs by more than $1 trillion in the United States. This underscores the potentially far-reaching impact of efforts to eliminate disparities, he said.
“Without a doubt, socioeconomics and inequities in access to quality care represent major factors influencing cancer health disparities, and these disparities will persist until we address these issues” he said.
Both progress reports culminate in a call to action, largely focused on the need for “unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for the NIH, NCI [National Cancer Institute], and FDA,” which is vital for accelerating the pace of progress.
The challenge is now compounded by the ongoing COVID-19 pandemic: Both progress reports note that racial and ethnic minorities, including African Americans, are not only affected disproportionately by cancer, but also by COVID-19, further highlighting the “stark inequities in health care.”
Ribas further called for action from national leadership and the scientific community.
“During this unprecedented time in our nation’s history, there is also a need for our nation’s leaders to take on a much bigger role in confronting and combating the structural and systemic racism that contributes to health disparities,” he said. The “pervasive racism and social injustices” that have contributed to disparities in both COVID-19 and cancer underscore the need for “the scientific community to step up and partner with Congress to assess and address this issue within the research community.”
This article first appeared on Medscape.com.
OTC ‘brain boosters’ may pose serious risks, experts say
, new research shows.
“Americans spend more than $600 million on over-the-counter smart pills every year, but we know very little about what is actually in these products,” said Pieter A. Cohen, MD, of the department of medicine at Harvard Medical School, Boston.
“Finding new combinations of drugs [that have] never been tested in humans in over-the-counter brain-boosting supplements is alarming,” said Dr. Cohen.
The study was published online Sept. 23 in Neurology Clinical Practice, a journal of the American Academy of Neurology.
Buyer beware
In a search of the National Institutes of Health Dietary Supplement Label Database and the Natural Medicines Database, Dr. Cohen and colleagues identified 10 supplements labeled as containing omberacetam, aniracetam, phenylpiracetam, or oxiracetam – four analogues of piracetam that are not approved for human use in the United States. Piracetam is also not approved in the United States.
In these 10 products, five unapproved drugs were discovered – omberacetam and aniracetam along with three others (phenibut, vinpocetine and picamilon).
By consuming the recommended serving size of these products, consumers could be exposed to pharmaceutical-level dosages of drugs including a maximum of 40.6 mg omberacetam (typical pharmacologic dose 10 mg), 502 mg of aniracetam (typical pharmacologic dose 200-750 mg), 15.4 mg of phenibut (typical dose 250-500 mg), 4.3 mg of vinpocetine (typical dose 5-40 mg), and 90.1 mg of picamilon (typical dose 50-200 mg), the study team reported.
Several drugs detected in these “smart” pills were not declared on the label, and several declared drugs were not detected in the products. For those products with drug quantities provided on the labels, three-quarters of declared quantities were inaccurate.
Consumers who use these cognitive enhancers could be exposed to amounts of these unapproved drugs that are fourfold greater than pharmaceutical dosages and combinations never tested in humans, the study team says. One product combined three different unapproved drugs and another product contained four different drugs.
“We have previously shown that these products may contain individual foreign drugs, but in our new study we found complex combinations of foreign drugs, up to four different drugs in a single product,” Dr. Cohen said.
The presence of these unapproved drugs in supplements, including at supratherapeutic dosages, suggests “serious risks to consumers and weaknesses in the regulatory framework under which supplements are permitted to be introduced in the U.S.,” Dr. Cohen and colleagues wrote.
“We should counsel our patients to avoid over-the-counter ‘smart pills’ until we can be assured as to the safety and efficacy of these products,” said Dr. Cohen.
Concerning findings
Glen R. Finney, MD, director of the Geisinger Memory and Cognition Program at the Neuroscience Institute, Geisinger Health System, Wilkes-Barre, Penn., said in an interview that two findings are very concerning: the lack of listed ingredients and especially the presence of unlisted drugs at active levels. “What if a person has a sensitivity or allergy to one of the unlisted drugs? This is a safety issue and a consumer issue,” Dr. Finney said.
Despite being widely promoted on television, “over-the-counter supplements are not regulated, so there is no guarantee that they contain what they claim, and there is very little evidence that they help memory and thinking even when they do have the ingredients they claim in the supplement,” said Dr. Finney,
“The best way to stay safe and help memory and thinking is to speak with your health providers about proven treatments that have good safety regulation, so you know what you’re getting, and what you’re getting from it,” Dr. Finney advised.
The study had no targeted funding. Dr. Cohen has collaborated in research with NSF International, received compensation from UptoDate, and received research support from Consumers Union and PEW Charitable Trusts. Dr. Finney has no relevant disclosures.
A version of this article originally appeared on Medscape.com.
, new research shows.
“Americans spend more than $600 million on over-the-counter smart pills every year, but we know very little about what is actually in these products,” said Pieter A. Cohen, MD, of the department of medicine at Harvard Medical School, Boston.
“Finding new combinations of drugs [that have] never been tested in humans in over-the-counter brain-boosting supplements is alarming,” said Dr. Cohen.
The study was published online Sept. 23 in Neurology Clinical Practice, a journal of the American Academy of Neurology.
Buyer beware
In a search of the National Institutes of Health Dietary Supplement Label Database and the Natural Medicines Database, Dr. Cohen and colleagues identified 10 supplements labeled as containing omberacetam, aniracetam, phenylpiracetam, or oxiracetam – four analogues of piracetam that are not approved for human use in the United States. Piracetam is also not approved in the United States.
In these 10 products, five unapproved drugs were discovered – omberacetam and aniracetam along with three others (phenibut, vinpocetine and picamilon).
By consuming the recommended serving size of these products, consumers could be exposed to pharmaceutical-level dosages of drugs including a maximum of 40.6 mg omberacetam (typical pharmacologic dose 10 mg), 502 mg of aniracetam (typical pharmacologic dose 200-750 mg), 15.4 mg of phenibut (typical dose 250-500 mg), 4.3 mg of vinpocetine (typical dose 5-40 mg), and 90.1 mg of picamilon (typical dose 50-200 mg), the study team reported.
Several drugs detected in these “smart” pills were not declared on the label, and several declared drugs were not detected in the products. For those products with drug quantities provided on the labels, three-quarters of declared quantities were inaccurate.
Consumers who use these cognitive enhancers could be exposed to amounts of these unapproved drugs that are fourfold greater than pharmaceutical dosages and combinations never tested in humans, the study team says. One product combined three different unapproved drugs and another product contained four different drugs.
“We have previously shown that these products may contain individual foreign drugs, but in our new study we found complex combinations of foreign drugs, up to four different drugs in a single product,” Dr. Cohen said.
The presence of these unapproved drugs in supplements, including at supratherapeutic dosages, suggests “serious risks to consumers and weaknesses in the regulatory framework under which supplements are permitted to be introduced in the U.S.,” Dr. Cohen and colleagues wrote.
“We should counsel our patients to avoid over-the-counter ‘smart pills’ until we can be assured as to the safety and efficacy of these products,” said Dr. Cohen.
Concerning findings
Glen R. Finney, MD, director of the Geisinger Memory and Cognition Program at the Neuroscience Institute, Geisinger Health System, Wilkes-Barre, Penn., said in an interview that two findings are very concerning: the lack of listed ingredients and especially the presence of unlisted drugs at active levels. “What if a person has a sensitivity or allergy to one of the unlisted drugs? This is a safety issue and a consumer issue,” Dr. Finney said.
Despite being widely promoted on television, “over-the-counter supplements are not regulated, so there is no guarantee that they contain what they claim, and there is very little evidence that they help memory and thinking even when they do have the ingredients they claim in the supplement,” said Dr. Finney,
“The best way to stay safe and help memory and thinking is to speak with your health providers about proven treatments that have good safety regulation, so you know what you’re getting, and what you’re getting from it,” Dr. Finney advised.
The study had no targeted funding. Dr. Cohen has collaborated in research with NSF International, received compensation from UptoDate, and received research support from Consumers Union and PEW Charitable Trusts. Dr. Finney has no relevant disclosures.
A version of this article originally appeared on Medscape.com.
, new research shows.
“Americans spend more than $600 million on over-the-counter smart pills every year, but we know very little about what is actually in these products,” said Pieter A. Cohen, MD, of the department of medicine at Harvard Medical School, Boston.
“Finding new combinations of drugs [that have] never been tested in humans in over-the-counter brain-boosting supplements is alarming,” said Dr. Cohen.
The study was published online Sept. 23 in Neurology Clinical Practice, a journal of the American Academy of Neurology.
Buyer beware
In a search of the National Institutes of Health Dietary Supplement Label Database and the Natural Medicines Database, Dr. Cohen and colleagues identified 10 supplements labeled as containing omberacetam, aniracetam, phenylpiracetam, or oxiracetam – four analogues of piracetam that are not approved for human use in the United States. Piracetam is also not approved in the United States.
In these 10 products, five unapproved drugs were discovered – omberacetam and aniracetam along with three others (phenibut, vinpocetine and picamilon).
By consuming the recommended serving size of these products, consumers could be exposed to pharmaceutical-level dosages of drugs including a maximum of 40.6 mg omberacetam (typical pharmacologic dose 10 mg), 502 mg of aniracetam (typical pharmacologic dose 200-750 mg), 15.4 mg of phenibut (typical dose 250-500 mg), 4.3 mg of vinpocetine (typical dose 5-40 mg), and 90.1 mg of picamilon (typical dose 50-200 mg), the study team reported.
Several drugs detected in these “smart” pills were not declared on the label, and several declared drugs were not detected in the products. For those products with drug quantities provided on the labels, three-quarters of declared quantities were inaccurate.
Consumers who use these cognitive enhancers could be exposed to amounts of these unapproved drugs that are fourfold greater than pharmaceutical dosages and combinations never tested in humans, the study team says. One product combined three different unapproved drugs and another product contained four different drugs.
“We have previously shown that these products may contain individual foreign drugs, but in our new study we found complex combinations of foreign drugs, up to four different drugs in a single product,” Dr. Cohen said.
The presence of these unapproved drugs in supplements, including at supratherapeutic dosages, suggests “serious risks to consumers and weaknesses in the regulatory framework under which supplements are permitted to be introduced in the U.S.,” Dr. Cohen and colleagues wrote.
“We should counsel our patients to avoid over-the-counter ‘smart pills’ until we can be assured as to the safety and efficacy of these products,” said Dr. Cohen.
Concerning findings
Glen R. Finney, MD, director of the Geisinger Memory and Cognition Program at the Neuroscience Institute, Geisinger Health System, Wilkes-Barre, Penn., said in an interview that two findings are very concerning: the lack of listed ingredients and especially the presence of unlisted drugs at active levels. “What if a person has a sensitivity or allergy to one of the unlisted drugs? This is a safety issue and a consumer issue,” Dr. Finney said.
Despite being widely promoted on television, “over-the-counter supplements are not regulated, so there is no guarantee that they contain what they claim, and there is very little evidence that they help memory and thinking even when they do have the ingredients they claim in the supplement,” said Dr. Finney,
“The best way to stay safe and help memory and thinking is to speak with your health providers about proven treatments that have good safety regulation, so you know what you’re getting, and what you’re getting from it,” Dr. Finney advised.
The study had no targeted funding. Dr. Cohen has collaborated in research with NSF International, received compensation from UptoDate, and received research support from Consumers Union and PEW Charitable Trusts. Dr. Finney has no relevant disclosures.
A version of this article originally appeared on Medscape.com.
FROM NEUROLOGY CLINICAL PRACTICE