User login
ACP: Average-risk women under 50 can postpone mammogram
(CBE) for screening in such women of any age, according to a new guideline from the American College of Physicians.
Further, clinicians should discuss whether to screen with mammography in average-risk women aged 40-49 years and consider potential harms and benefits, as well as patient preferences. Providers should discontinue screening average-risk women at age 75 years and women with a life expectancy of 10 years or less, Amir Qaseem, MD, PhD, of the ACP and colleagues wrote on behalf of the ACP Clinical Guidelines Committee.
The ACP guidance also addresses the varying recommendations from other organizations on the age at which to start and stop screening and on screening intervals, noting that “areas of disagreement include screening in women aged 40 to 49 years, screening in women aged 75 years or older, and recommended screening intervals,” and stresses the importance of patient input.
“Women should be informed participants in personalized decisions about breast cancer screening,” the authors wrote, adding that those under age 50 years without a clear preference for screening should not be screened.
However, the evidence shows that most average-risk women with no symptoms will benefit from mammography every other year beginning at age 50 years, they said.
The statement, published online April 8 in the Annals of Internal Medicine, was derived from a review of seven existing English-language breast cancer screening guidelines and the evidence cited in those guidelines. It’s intended to be a resource for all clinicians.
It differs from the 2017 American College of Obstetricians and Gynecologists (ACOG) guidelines in that ACOG recommends CBE and does not address screening in those with a life expectancy of less than 10 years. It also differs from the 2016 U.S. Preventive Services Task Force (USPSTF) guidelines, which make no recommendation on CBE and also do not address screening in those with a life expectancy of less than 10 years.
Other guidelines, such as those from the American College of Radiology, American Cancer Society (ACS), the Canadian Task Force on Preventive Health Care, and the National Comprehensive Cancer Network, recommend CBE, and the World Health Organization guidelines recommend CBE in low resource settings.
“Although CBE continues to be used as part of the examination of symptomatic women, data are sparse on screening asymptomatic women using CBE alone or combined with mammography,” the ACP guideline authors wrote. “The ACS recommends against CBE in average-risk women of any age because of the lack of demonstrated benefit and the potential for false-positive results.”
The guidance, which does not apply to patients with prior abnormal screening results or those at higher breast cancer risk, also includes an evidence-driven “talking points with patients” section based on frequently asked questions.
An important goal of the ACP Clinical Guidelines Committee in developing the guidance is to reduce overdiagnosis and overtreatment, which affects about 20% of women diagnosed over a 10-year period.
The committee reviewed all national guidelines published in English between January 1, 2013, and November 15, 2017, in the National Guideline Clearinghouse or Guidelines International Network library, and it also selected other guidelines commonly used in clinical practice. The committee evaluated the quality of each by using the Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument.
Alex Krist, MD, the USPSTF vice-chairperson, offered support for the “shift toward shared decision making that is emerging” and added it’s “part of a larger movement toward empowering people with information not only about the potential benefits but also the potential harms of screening tests.”
“In its 2016 recommendation, the Task Force found that the value of mammography increases with age, with women ages 50-74 benefiting most from screening. For women in their 40s, the Task Force also found that mammography screening every two years can be effective,” he told this publication. “We recommend that the decision to start screening should be an individual one, taking into account a woman’s health history, preferences, and how she values the different potential benefits and harms.”
Dr. Krist further noted that the USPSTF, ACP, and many others “have all affirmed that mammography is an important tool to reduce breast cancer mortality and that the benefits of mammography increase with age.”
Likewise, Robert Smith, PhD, vice president of cancer screening for the ACS, noted that the ACP guidance generally aligns with ACS and USPSTF guidelines because all “support informed decision making starting at age 40, and screening every two years starting at age 50 (USPSTF) or 55 (ACS).”
“The fact that all guidelines are not totally in sync is not unexpected. ... The most important thing to recognize is that all of these guidelines stress that regular mammography plays an important role in breast cancer early detection, and women should be aware of its benefits and limitations, and also remain vigilant and report any breast changes,” he said.
The guidance authors reported having no conflicts of interest.
SOURCE: Qaseem A et al., Ann Intern Med. 2019. doi: 10.7326/M18-2147.
The ACP guidance statements provide “clarity and simplicity amidst the chaos of diverging guidelines,” Joann G. Elmore, MD, and Christoph I. Lee, MD, wrote in an editorial that accompanied the guideline (Ann Intern Med. 2019. doi: 10.7326/M19-0726).
The four statements included in the guidance represent the convergence of differing recommendations, but they also highlight points for physicians to consider in shared decision making with patients, the editorial authors wrote.
Lacking, however, is advice on how clinicians should go about stopping screening in certain patients, they noted.
“We need reliable ways to determine life expectancy given comorbid conditions, as well as methods to appropriately manage the discussion about stopping screening. ... The cessation of routine screening is a highly uncomfortable situation for which we as clinicians currently have little guidance and few tools. At this crossroads of confusion, we need a clear path toward informed, tailored, risk-based screening for breast cancer,” they wrote adding that future guidance statements should “move beyond emphasizing variation across guidelines and instead provide more advice on how to implement high-value screening and deimplement low-value screening.”
Dr. Elmore is with the University of California, Los Angeles. Dr. Lee is with the University of Washington, Seattle.
The ACP guidance statements provide “clarity and simplicity amidst the chaos of diverging guidelines,” Joann G. Elmore, MD, and Christoph I. Lee, MD, wrote in an editorial that accompanied the guideline (Ann Intern Med. 2019. doi: 10.7326/M19-0726).
The four statements included in the guidance represent the convergence of differing recommendations, but they also highlight points for physicians to consider in shared decision making with patients, the editorial authors wrote.
Lacking, however, is advice on how clinicians should go about stopping screening in certain patients, they noted.
“We need reliable ways to determine life expectancy given comorbid conditions, as well as methods to appropriately manage the discussion about stopping screening. ... The cessation of routine screening is a highly uncomfortable situation for which we as clinicians currently have little guidance and few tools. At this crossroads of confusion, we need a clear path toward informed, tailored, risk-based screening for breast cancer,” they wrote adding that future guidance statements should “move beyond emphasizing variation across guidelines and instead provide more advice on how to implement high-value screening and deimplement low-value screening.”
Dr. Elmore is with the University of California, Los Angeles. Dr. Lee is with the University of Washington, Seattle.
The ACP guidance statements provide “clarity and simplicity amidst the chaos of diverging guidelines,” Joann G. Elmore, MD, and Christoph I. Lee, MD, wrote in an editorial that accompanied the guideline (Ann Intern Med. 2019. doi: 10.7326/M19-0726).
The four statements included in the guidance represent the convergence of differing recommendations, but they also highlight points for physicians to consider in shared decision making with patients, the editorial authors wrote.
Lacking, however, is advice on how clinicians should go about stopping screening in certain patients, they noted.
“We need reliable ways to determine life expectancy given comorbid conditions, as well as methods to appropriately manage the discussion about stopping screening. ... The cessation of routine screening is a highly uncomfortable situation for which we as clinicians currently have little guidance and few tools. At this crossroads of confusion, we need a clear path toward informed, tailored, risk-based screening for breast cancer,” they wrote adding that future guidance statements should “move beyond emphasizing variation across guidelines and instead provide more advice on how to implement high-value screening and deimplement low-value screening.”
Dr. Elmore is with the University of California, Los Angeles. Dr. Lee is with the University of Washington, Seattle.
(CBE) for screening in such women of any age, according to a new guideline from the American College of Physicians.
Further, clinicians should discuss whether to screen with mammography in average-risk women aged 40-49 years and consider potential harms and benefits, as well as patient preferences. Providers should discontinue screening average-risk women at age 75 years and women with a life expectancy of 10 years or less, Amir Qaseem, MD, PhD, of the ACP and colleagues wrote on behalf of the ACP Clinical Guidelines Committee.
The ACP guidance also addresses the varying recommendations from other organizations on the age at which to start and stop screening and on screening intervals, noting that “areas of disagreement include screening in women aged 40 to 49 years, screening in women aged 75 years or older, and recommended screening intervals,” and stresses the importance of patient input.
“Women should be informed participants in personalized decisions about breast cancer screening,” the authors wrote, adding that those under age 50 years without a clear preference for screening should not be screened.
However, the evidence shows that most average-risk women with no symptoms will benefit from mammography every other year beginning at age 50 years, they said.
The statement, published online April 8 in the Annals of Internal Medicine, was derived from a review of seven existing English-language breast cancer screening guidelines and the evidence cited in those guidelines. It’s intended to be a resource for all clinicians.
It differs from the 2017 American College of Obstetricians and Gynecologists (ACOG) guidelines in that ACOG recommends CBE and does not address screening in those with a life expectancy of less than 10 years. It also differs from the 2016 U.S. Preventive Services Task Force (USPSTF) guidelines, which make no recommendation on CBE and also do not address screening in those with a life expectancy of less than 10 years.
Other guidelines, such as those from the American College of Radiology, American Cancer Society (ACS), the Canadian Task Force on Preventive Health Care, and the National Comprehensive Cancer Network, recommend CBE, and the World Health Organization guidelines recommend CBE in low resource settings.
“Although CBE continues to be used as part of the examination of symptomatic women, data are sparse on screening asymptomatic women using CBE alone or combined with mammography,” the ACP guideline authors wrote. “The ACS recommends against CBE in average-risk women of any age because of the lack of demonstrated benefit and the potential for false-positive results.”
The guidance, which does not apply to patients with prior abnormal screening results or those at higher breast cancer risk, also includes an evidence-driven “talking points with patients” section based on frequently asked questions.
An important goal of the ACP Clinical Guidelines Committee in developing the guidance is to reduce overdiagnosis and overtreatment, which affects about 20% of women diagnosed over a 10-year period.
The committee reviewed all national guidelines published in English between January 1, 2013, and November 15, 2017, in the National Guideline Clearinghouse or Guidelines International Network library, and it also selected other guidelines commonly used in clinical practice. The committee evaluated the quality of each by using the Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument.
Alex Krist, MD, the USPSTF vice-chairperson, offered support for the “shift toward shared decision making that is emerging” and added it’s “part of a larger movement toward empowering people with information not only about the potential benefits but also the potential harms of screening tests.”
“In its 2016 recommendation, the Task Force found that the value of mammography increases with age, with women ages 50-74 benefiting most from screening. For women in their 40s, the Task Force also found that mammography screening every two years can be effective,” he told this publication. “We recommend that the decision to start screening should be an individual one, taking into account a woman’s health history, preferences, and how she values the different potential benefits and harms.”
Dr. Krist further noted that the USPSTF, ACP, and many others “have all affirmed that mammography is an important tool to reduce breast cancer mortality and that the benefits of mammography increase with age.”
Likewise, Robert Smith, PhD, vice president of cancer screening for the ACS, noted that the ACP guidance generally aligns with ACS and USPSTF guidelines because all “support informed decision making starting at age 40, and screening every two years starting at age 50 (USPSTF) or 55 (ACS).”
“The fact that all guidelines are not totally in sync is not unexpected. ... The most important thing to recognize is that all of these guidelines stress that regular mammography plays an important role in breast cancer early detection, and women should be aware of its benefits and limitations, and also remain vigilant and report any breast changes,” he said.
The guidance authors reported having no conflicts of interest.
SOURCE: Qaseem A et al., Ann Intern Med. 2019. doi: 10.7326/M18-2147.
(CBE) for screening in such women of any age, according to a new guideline from the American College of Physicians.
Further, clinicians should discuss whether to screen with mammography in average-risk women aged 40-49 years and consider potential harms and benefits, as well as patient preferences. Providers should discontinue screening average-risk women at age 75 years and women with a life expectancy of 10 years or less, Amir Qaseem, MD, PhD, of the ACP and colleagues wrote on behalf of the ACP Clinical Guidelines Committee.
The ACP guidance also addresses the varying recommendations from other organizations on the age at which to start and stop screening and on screening intervals, noting that “areas of disagreement include screening in women aged 40 to 49 years, screening in women aged 75 years or older, and recommended screening intervals,” and stresses the importance of patient input.
“Women should be informed participants in personalized decisions about breast cancer screening,” the authors wrote, adding that those under age 50 years without a clear preference for screening should not be screened.
However, the evidence shows that most average-risk women with no symptoms will benefit from mammography every other year beginning at age 50 years, they said.
The statement, published online April 8 in the Annals of Internal Medicine, was derived from a review of seven existing English-language breast cancer screening guidelines and the evidence cited in those guidelines. It’s intended to be a resource for all clinicians.
It differs from the 2017 American College of Obstetricians and Gynecologists (ACOG) guidelines in that ACOG recommends CBE and does not address screening in those with a life expectancy of less than 10 years. It also differs from the 2016 U.S. Preventive Services Task Force (USPSTF) guidelines, which make no recommendation on CBE and also do not address screening in those with a life expectancy of less than 10 years.
Other guidelines, such as those from the American College of Radiology, American Cancer Society (ACS), the Canadian Task Force on Preventive Health Care, and the National Comprehensive Cancer Network, recommend CBE, and the World Health Organization guidelines recommend CBE in low resource settings.
“Although CBE continues to be used as part of the examination of symptomatic women, data are sparse on screening asymptomatic women using CBE alone or combined with mammography,” the ACP guideline authors wrote. “The ACS recommends against CBE in average-risk women of any age because of the lack of demonstrated benefit and the potential for false-positive results.”
The guidance, which does not apply to patients with prior abnormal screening results or those at higher breast cancer risk, also includes an evidence-driven “talking points with patients” section based on frequently asked questions.
An important goal of the ACP Clinical Guidelines Committee in developing the guidance is to reduce overdiagnosis and overtreatment, which affects about 20% of women diagnosed over a 10-year period.
The committee reviewed all national guidelines published in English between January 1, 2013, and November 15, 2017, in the National Guideline Clearinghouse or Guidelines International Network library, and it also selected other guidelines commonly used in clinical practice. The committee evaluated the quality of each by using the Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument.
Alex Krist, MD, the USPSTF vice-chairperson, offered support for the “shift toward shared decision making that is emerging” and added it’s “part of a larger movement toward empowering people with information not only about the potential benefits but also the potential harms of screening tests.”
“In its 2016 recommendation, the Task Force found that the value of mammography increases with age, with women ages 50-74 benefiting most from screening. For women in their 40s, the Task Force also found that mammography screening every two years can be effective,” he told this publication. “We recommend that the decision to start screening should be an individual one, taking into account a woman’s health history, preferences, and how she values the different potential benefits and harms.”
Dr. Krist further noted that the USPSTF, ACP, and many others “have all affirmed that mammography is an important tool to reduce breast cancer mortality and that the benefits of mammography increase with age.”
Likewise, Robert Smith, PhD, vice president of cancer screening for the ACS, noted that the ACP guidance generally aligns with ACS and USPSTF guidelines because all “support informed decision making starting at age 40, and screening every two years starting at age 50 (USPSTF) or 55 (ACS).”
“The fact that all guidelines are not totally in sync is not unexpected. ... The most important thing to recognize is that all of these guidelines stress that regular mammography plays an important role in breast cancer early detection, and women should be aware of its benefits and limitations, and also remain vigilant and report any breast changes,” he said.
The guidance authors reported having no conflicts of interest.
SOURCE: Qaseem A et al., Ann Intern Med. 2019. doi: 10.7326/M18-2147.
REPORTING FROM THE ANNALS OF INTERNAL MEDICINE
Colchicine reduces inflammatory markers associated with metabolic syndrome
A small study offers a tantalizing hint that
The 3-month trial did not meet its primary endpoint – change in insulin sensitivity as measured by a glucose tolerance test – but it did hit several secondary goals, all of which were related to the inflammation that accompanies prediabetes, Jack A. Yanovski, MD, and colleagues wrote in Diabetes, Obesity, and Metabolism.
“Colchicine is well-known to have anti-inflammatory properties, although its effect on obesity-associated inflammation has not previously been investigated,” said Dr. Yanovski of the National institutes of Health and his coauthors. “Classically, it has been posited that colchicine blocks inflammation by impeding leukocyte locomotion, diapedesis, and, ultimately, recruitment to sites of inflammation. ... Recently, it has been shown that colchicine also inhibits the formation of the NLRP3 [NOD-like receptor family pyrin domain-containing 3] inflammasome, an important component of the obesity-associated inflammatory cascade.”
The NLRP3 inflammasome has been shown to play an important part in promoting the inflammatory state of obesity, the authors noted. When a cell senses danger, NLRP3 uses microtubules to create an inflammasome that then produces interleukin-1 beta gene and interleukin-18. One of colchicine’s known actions is to inhibit microtubule formation, suggesting that it could put the brakes on this process.
The study comprised 40 patients who had metabolic syndrome, significant insulin resistance, and elevated inflammatory markers. Among the exclusionary criteria were having a significant medical illness, a history of gout, and recent or current use of colchicine.
The patients were randomized to colchicine 0.6 mg or placebo twice daily for 3 months. No dietary advice was given during the study period. Of the 40 randomized patients, 37 completed the 3-month study, though none left because of adverse events.
Although there were no significant between-group differences in levels of fasting insulin, colchicine did significantly decrease inflammatory markers, compared with placebo. C-reactive protein dropped by 2.8 mg/L in the active group but increased slightly in the placebo group. The erythrocyte sedimentation rate also decreased in the colchicine group, compared with placebo (difference, –5.9 mm/hr; P = .07). The active group experienced an improvement in fasting insulin as measured by the homeostasis model assessment–estimated insulin resistance index and in glucose effectiveness, which suggests metabolic improvement.
“Larger trials are needed to investigate whether colchicine has efficacy in improving insulin resistance and/or preventing the onset of diabetes mellitus in at-risk individuals with obesity-associated inflammation,” the authors concluded.
The study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and by the National Institutes of Health. None of the authors reported any disclosures or conflicts of interest relating to this study.
SOURCE: Yanovski JA et al. Diabetes Obes Metab. 2019 Mar 14. doi: 10.1111/dom.13702.
A small study offers a tantalizing hint that
The 3-month trial did not meet its primary endpoint – change in insulin sensitivity as measured by a glucose tolerance test – but it did hit several secondary goals, all of which were related to the inflammation that accompanies prediabetes, Jack A. Yanovski, MD, and colleagues wrote in Diabetes, Obesity, and Metabolism.
“Colchicine is well-known to have anti-inflammatory properties, although its effect on obesity-associated inflammation has not previously been investigated,” said Dr. Yanovski of the National institutes of Health and his coauthors. “Classically, it has been posited that colchicine blocks inflammation by impeding leukocyte locomotion, diapedesis, and, ultimately, recruitment to sites of inflammation. ... Recently, it has been shown that colchicine also inhibits the formation of the NLRP3 [NOD-like receptor family pyrin domain-containing 3] inflammasome, an important component of the obesity-associated inflammatory cascade.”
The NLRP3 inflammasome has been shown to play an important part in promoting the inflammatory state of obesity, the authors noted. When a cell senses danger, NLRP3 uses microtubules to create an inflammasome that then produces interleukin-1 beta gene and interleukin-18. One of colchicine’s known actions is to inhibit microtubule formation, suggesting that it could put the brakes on this process.
The study comprised 40 patients who had metabolic syndrome, significant insulin resistance, and elevated inflammatory markers. Among the exclusionary criteria were having a significant medical illness, a history of gout, and recent or current use of colchicine.
The patients were randomized to colchicine 0.6 mg or placebo twice daily for 3 months. No dietary advice was given during the study period. Of the 40 randomized patients, 37 completed the 3-month study, though none left because of adverse events.
Although there were no significant between-group differences in levels of fasting insulin, colchicine did significantly decrease inflammatory markers, compared with placebo. C-reactive protein dropped by 2.8 mg/L in the active group but increased slightly in the placebo group. The erythrocyte sedimentation rate also decreased in the colchicine group, compared with placebo (difference, –5.9 mm/hr; P = .07). The active group experienced an improvement in fasting insulin as measured by the homeostasis model assessment–estimated insulin resistance index and in glucose effectiveness, which suggests metabolic improvement.
“Larger trials are needed to investigate whether colchicine has efficacy in improving insulin resistance and/or preventing the onset of diabetes mellitus in at-risk individuals with obesity-associated inflammation,” the authors concluded.
The study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and by the National Institutes of Health. None of the authors reported any disclosures or conflicts of interest relating to this study.
SOURCE: Yanovski JA et al. Diabetes Obes Metab. 2019 Mar 14. doi: 10.1111/dom.13702.
A small study offers a tantalizing hint that
The 3-month trial did not meet its primary endpoint – change in insulin sensitivity as measured by a glucose tolerance test – but it did hit several secondary goals, all of which were related to the inflammation that accompanies prediabetes, Jack A. Yanovski, MD, and colleagues wrote in Diabetes, Obesity, and Metabolism.
“Colchicine is well-known to have anti-inflammatory properties, although its effect on obesity-associated inflammation has not previously been investigated,” said Dr. Yanovski of the National institutes of Health and his coauthors. “Classically, it has been posited that colchicine blocks inflammation by impeding leukocyte locomotion, diapedesis, and, ultimately, recruitment to sites of inflammation. ... Recently, it has been shown that colchicine also inhibits the formation of the NLRP3 [NOD-like receptor family pyrin domain-containing 3] inflammasome, an important component of the obesity-associated inflammatory cascade.”
The NLRP3 inflammasome has been shown to play an important part in promoting the inflammatory state of obesity, the authors noted. When a cell senses danger, NLRP3 uses microtubules to create an inflammasome that then produces interleukin-1 beta gene and interleukin-18. One of colchicine’s known actions is to inhibit microtubule formation, suggesting that it could put the brakes on this process.
The study comprised 40 patients who had metabolic syndrome, significant insulin resistance, and elevated inflammatory markers. Among the exclusionary criteria were having a significant medical illness, a history of gout, and recent or current use of colchicine.
The patients were randomized to colchicine 0.6 mg or placebo twice daily for 3 months. No dietary advice was given during the study period. Of the 40 randomized patients, 37 completed the 3-month study, though none left because of adverse events.
Although there were no significant between-group differences in levels of fasting insulin, colchicine did significantly decrease inflammatory markers, compared with placebo. C-reactive protein dropped by 2.8 mg/L in the active group but increased slightly in the placebo group. The erythrocyte sedimentation rate also decreased in the colchicine group, compared with placebo (difference, –5.9 mm/hr; P = .07). The active group experienced an improvement in fasting insulin as measured by the homeostasis model assessment–estimated insulin resistance index and in glucose effectiveness, which suggests metabolic improvement.
“Larger trials are needed to investigate whether colchicine has efficacy in improving insulin resistance and/or preventing the onset of diabetes mellitus in at-risk individuals with obesity-associated inflammation,” the authors concluded.
The study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and by the National Institutes of Health. None of the authors reported any disclosures or conflicts of interest relating to this study.
SOURCE: Yanovski JA et al. Diabetes Obes Metab. 2019 Mar 14. doi: 10.1111/dom.13702.
FROM DIABETES, OBESITY, AND METABOLISM
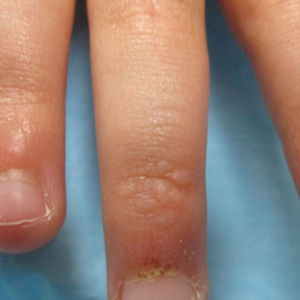
Papules and Telangiectases on the Distal Fingers of a Child
The Diagnosis: Juvenile Dermatomyositis
Juvenile dermatomyositis (JDM) is a rare idiopathic inflammatory myopathy of childhood that is autoimmune in nature with an annual incidence ranging from 2.5 to 4.1 cases per million children. Its peak incidence is between 5 and 10 years of age, and it affects girls more than boys at a 2-fold to 5-fold greater rate.1 Juvenile dermatomyositis is characterized by skeletal muscle weakness in the presence of distinctive rashes, including Gottron papules and heliotrope erythema. Muscle weakness typically is proximal and symmetrical, and eventually patients may have trouble rising from a seated position or lifting objects overhead. Other skin manifestations include nail fold capillary changes, calcinosis cutis, and less commonly ulcerations signifying vasculopathy of the skin.2 A subset of patients will present with juvenile amyopathic dermatomyositis. These children have the characteristic skin changes without the muscle weakness or elevated muscle enzymes for more than 6 months; however, one-quarter may go on to develop mysositis.3
Diagnosis of JDM traditionally was based on the following 5 diagnostic criteria: characteristic skin rash, proximal muscle weakness, elevated muscle enzymes, myopathic changes on electromyogram, and typical muscle biopsy.1 Current practice shows a broadening of diagnostic criteria using new techniques in the diagnosis of JDM. To make the diagnosis, the patient must have the characteristic skin manifestations with a minimum of 3 other criteria.4 A 2006 international consensus survey expanded the list of criteria to include typical findings on magnetic resonance imaging (MRI), nail fold capillaroscopy abnormalities, calcinosis, and
dysphonia.5
To assess muscle disease, MRI is utilized because it is a reliable noninvasive tool to assess muscle inflammation. Muscle biopsy is only recommended if the diagnosis is unclear.5 The results of the MRI in our patient displayed symmetric mild fatty atrophy of the gluteus maximus muscle, as well as edema in the right rectus femoris and left vastus lateralis muscles, suggesting early findings of myositis. Muscle enzymes may not be diagnostic because they are not always elevated at diagnosis. Our patient had a normal creatinine kinase level (92 U/L [reference range, <190 U/L]), and both aldolase and lactate dehydrogenase also were within reference range. Conversely, antinuclear antibodies frequently are positive in patients with JDM, such as in our patient at a 1:320 dilution, but are nonspecific and nondiagnostic. It is recommended to include nail fold capillaroscopy to evaluate periungual capillary changes because nailfold capillary density is a sensitive measure of both skin and muscle disease.5 Using dermoscopy, nail fold capillary dilation was observed in our patient.
Other differential diagnoses can have somewhat similar clinical features to JDM. Infantile papular acrodermatitis, commonly referred to as Gianotti-Crosti syndrome, is a viral exanthem that affects children (median age, 2 years).6 The rash appears as monomorphous, flat-topped, pink to brown papules affecting the face, buttocks, and arms; it typically spontaneously resolves in 10 days.6
Juvenile-onset lupus is a chronic autoimmune disorder that can involve any organ system and typically affects children aged 11 to 12 years with a female preponderance. Skin manifestations are similar to adult-onset lupus and include malar rash, discoid rash, oral ulcerations, petechiae, palpable purpura, and digital telangiectasia and ulcers. 7
Juvenile scleroderma is rare connective-tissue disorder that also has multiple organ involvement. Cutaneous involvement can range from isolated morphealike plaques to diffuse sclerotic lesions with growth disturbances, contractures, and facial atrophy.8
Verrucae planae, commonly referred to as flat warts, are papules caused primarily by human papillomavirus types 3, 10, 28, and 41. Children and young adults commonly are affected, and warts can appear on the hands, as in our patient.6
Treatment of JDM depends on disease severity at initial presentation and requires a multidisciplinary approach. The mainstay of treatment is high-dose oral prednisone in combination with disease-modifying drugs such as methotrexate and cyclosporin A. Patients with more severe presentations (eg, ulcerative skin disease) or life-threatening organ involvement are treated with cyclophosphamide, usually in combination with high-dose glucocorticoids.9
Early detection with aggressive treatment is vital to reduce morbidity and mortality from organ damage and disease complications. Mortality rates have dropped to 3%10 in recent decades with the use of systemic glucocorticoids. Delayed treatment is associated with a prolonged disease course and poorer outcomes. Disease complications in children with JDM include osteoporosis, calcinosis, and intestinal perforation; however, with early treatment, children with JDM can expect full recovery and to live a normal life as compared to adults with dermatomyositis.10
Prior to our patient's diagnosis, the family was assigned to move to an overseas location through the US Military with no direct access to advanced medical care. Early detection and diagnosis of JDM through an astute clinical examination allowed the patient and her family to remain in the continental United States to continue receiving specialty care.
- Mendez EP, Lipton R, Ramsey-Goldman R, et al. US incidence of juvenile dermatomyositis,1995-1998: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Registry. Arthritis Rheum. 2003;49:300-305.
- Shah M, Mamyrova G, Targoff IN, et al. The clinical phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine. 2013;92:25-41.
- Gerami P, Walling HW, Lewis J, et al. A systematic review of juvenile-onset clinically amyopathic dermatomyositis. Br J Dermatol. 2007;57:637-644.
- Enders FB, Bader-Meunier B, Baildam E, et al. Consensus-based recommendations for the management of juvenile dermatomyositis. Ann Rheum Dis. 2017;76:329-340.
- Brown VE, Pilkington CA, Feldman BM, et al. An international consensus survey of the diagnostic criteria for juvenile dermatomyositis (JDM). Rheumatology (Oxford). 2006;45:990-993.
- William JD, Berger TG, Elston DM. Viral diseases. In: William JD, Berger TG, Elston DM. Andrews' Diseases of the Skin: Clinical Dermatology. 11th ed. China: Saunders Elsevier; 2011:360-413.
- Levy DM, Kamphuis S. Systemic lupus erythematosus in children and adolescents. Pediatr Clin North Am. 2012;59:345-364.
- Li SC, Torok KS, Pope E, et al; Childhood Arthritis and Rheumatology Research Alliance (CARRA) Localized Scleroderma Workgroup. Development of consensus treatment plans for juvenile localized scleroderma: a roadmap toward comparative effectiveness studies in juvenile localized scleroderma. Arthritis Care Res (Hoboken). 2012;64:1175-1185.
- Stringer E, Ota S, Bohnsack J, et al. Treatment approaches to juvenile dermatomyositis (JDM) across North America: the Childhood Arthritis and Rheumatology Research Alliance (CARRA) JDM treatment study. J Rhematol. 2010;37:S1953-S1961.
- Huber AM, Feldman BM. Long-term outcomes in juvenile dermatomyositis: how did we get here and where are we going? Curr Rheumatol Rep. 2005;7:441-446.
The Diagnosis: Juvenile Dermatomyositis
Juvenile dermatomyositis (JDM) is a rare idiopathic inflammatory myopathy of childhood that is autoimmune in nature with an annual incidence ranging from 2.5 to 4.1 cases per million children. Its peak incidence is between 5 and 10 years of age, and it affects girls more than boys at a 2-fold to 5-fold greater rate.1 Juvenile dermatomyositis is characterized by skeletal muscle weakness in the presence of distinctive rashes, including Gottron papules and heliotrope erythema. Muscle weakness typically is proximal and symmetrical, and eventually patients may have trouble rising from a seated position or lifting objects overhead. Other skin manifestations include nail fold capillary changes, calcinosis cutis, and less commonly ulcerations signifying vasculopathy of the skin.2 A subset of patients will present with juvenile amyopathic dermatomyositis. These children have the characteristic skin changes without the muscle weakness or elevated muscle enzymes for more than 6 months; however, one-quarter may go on to develop mysositis.3
Diagnosis of JDM traditionally was based on the following 5 diagnostic criteria: characteristic skin rash, proximal muscle weakness, elevated muscle enzymes, myopathic changes on electromyogram, and typical muscle biopsy.1 Current practice shows a broadening of diagnostic criteria using new techniques in the diagnosis of JDM. To make the diagnosis, the patient must have the characteristic skin manifestations with a minimum of 3 other criteria.4 A 2006 international consensus survey expanded the list of criteria to include typical findings on magnetic resonance imaging (MRI), nail fold capillaroscopy abnormalities, calcinosis, and
dysphonia.5
To assess muscle disease, MRI is utilized because it is a reliable noninvasive tool to assess muscle inflammation. Muscle biopsy is only recommended if the diagnosis is unclear.5 The results of the MRI in our patient displayed symmetric mild fatty atrophy of the gluteus maximus muscle, as well as edema in the right rectus femoris and left vastus lateralis muscles, suggesting early findings of myositis. Muscle enzymes may not be diagnostic because they are not always elevated at diagnosis. Our patient had a normal creatinine kinase level (92 U/L [reference range, <190 U/L]), and both aldolase and lactate dehydrogenase also were within reference range. Conversely, antinuclear antibodies frequently are positive in patients with JDM, such as in our patient at a 1:320 dilution, but are nonspecific and nondiagnostic. It is recommended to include nail fold capillaroscopy to evaluate periungual capillary changes because nailfold capillary density is a sensitive measure of both skin and muscle disease.5 Using dermoscopy, nail fold capillary dilation was observed in our patient.
Other differential diagnoses can have somewhat similar clinical features to JDM. Infantile papular acrodermatitis, commonly referred to as Gianotti-Crosti syndrome, is a viral exanthem that affects children (median age, 2 years).6 The rash appears as monomorphous, flat-topped, pink to brown papules affecting the face, buttocks, and arms; it typically spontaneously resolves in 10 days.6
Juvenile-onset lupus is a chronic autoimmune disorder that can involve any organ system and typically affects children aged 11 to 12 years with a female preponderance. Skin manifestations are similar to adult-onset lupus and include malar rash, discoid rash, oral ulcerations, petechiae, palpable purpura, and digital telangiectasia and ulcers. 7
Juvenile scleroderma is rare connective-tissue disorder that also has multiple organ involvement. Cutaneous involvement can range from isolated morphealike plaques to diffuse sclerotic lesions with growth disturbances, contractures, and facial atrophy.8
Verrucae planae, commonly referred to as flat warts, are papules caused primarily by human papillomavirus types 3, 10, 28, and 41. Children and young adults commonly are affected, and warts can appear on the hands, as in our patient.6
Treatment of JDM depends on disease severity at initial presentation and requires a multidisciplinary approach. The mainstay of treatment is high-dose oral prednisone in combination with disease-modifying drugs such as methotrexate and cyclosporin A. Patients with more severe presentations (eg, ulcerative skin disease) or life-threatening organ involvement are treated with cyclophosphamide, usually in combination with high-dose glucocorticoids.9
Early detection with aggressive treatment is vital to reduce morbidity and mortality from organ damage and disease complications. Mortality rates have dropped to 3%10 in recent decades with the use of systemic glucocorticoids. Delayed treatment is associated with a prolonged disease course and poorer outcomes. Disease complications in children with JDM include osteoporosis, calcinosis, and intestinal perforation; however, with early treatment, children with JDM can expect full recovery and to live a normal life as compared to adults with dermatomyositis.10
Prior to our patient's diagnosis, the family was assigned to move to an overseas location through the US Military with no direct access to advanced medical care. Early detection and diagnosis of JDM through an astute clinical examination allowed the patient and her family to remain in the continental United States to continue receiving specialty care.
The Diagnosis: Juvenile Dermatomyositis
Juvenile dermatomyositis (JDM) is a rare idiopathic inflammatory myopathy of childhood that is autoimmune in nature with an annual incidence ranging from 2.5 to 4.1 cases per million children. Its peak incidence is between 5 and 10 years of age, and it affects girls more than boys at a 2-fold to 5-fold greater rate.1 Juvenile dermatomyositis is characterized by skeletal muscle weakness in the presence of distinctive rashes, including Gottron papules and heliotrope erythema. Muscle weakness typically is proximal and symmetrical, and eventually patients may have trouble rising from a seated position or lifting objects overhead. Other skin manifestations include nail fold capillary changes, calcinosis cutis, and less commonly ulcerations signifying vasculopathy of the skin.2 A subset of patients will present with juvenile amyopathic dermatomyositis. These children have the characteristic skin changes without the muscle weakness or elevated muscle enzymes for more than 6 months; however, one-quarter may go on to develop mysositis.3
Diagnosis of JDM traditionally was based on the following 5 diagnostic criteria: characteristic skin rash, proximal muscle weakness, elevated muscle enzymes, myopathic changes on electromyogram, and typical muscle biopsy.1 Current practice shows a broadening of diagnostic criteria using new techniques in the diagnosis of JDM. To make the diagnosis, the patient must have the characteristic skin manifestations with a minimum of 3 other criteria.4 A 2006 international consensus survey expanded the list of criteria to include typical findings on magnetic resonance imaging (MRI), nail fold capillaroscopy abnormalities, calcinosis, and
dysphonia.5
To assess muscle disease, MRI is utilized because it is a reliable noninvasive tool to assess muscle inflammation. Muscle biopsy is only recommended if the diagnosis is unclear.5 The results of the MRI in our patient displayed symmetric mild fatty atrophy of the gluteus maximus muscle, as well as edema in the right rectus femoris and left vastus lateralis muscles, suggesting early findings of myositis. Muscle enzymes may not be diagnostic because they are not always elevated at diagnosis. Our patient had a normal creatinine kinase level (92 U/L [reference range, <190 U/L]), and both aldolase and lactate dehydrogenase also were within reference range. Conversely, antinuclear antibodies frequently are positive in patients with JDM, such as in our patient at a 1:320 dilution, but are nonspecific and nondiagnostic. It is recommended to include nail fold capillaroscopy to evaluate periungual capillary changes because nailfold capillary density is a sensitive measure of both skin and muscle disease.5 Using dermoscopy, nail fold capillary dilation was observed in our patient.
Other differential diagnoses can have somewhat similar clinical features to JDM. Infantile papular acrodermatitis, commonly referred to as Gianotti-Crosti syndrome, is a viral exanthem that affects children (median age, 2 years).6 The rash appears as monomorphous, flat-topped, pink to brown papules affecting the face, buttocks, and arms; it typically spontaneously resolves in 10 days.6
Juvenile-onset lupus is a chronic autoimmune disorder that can involve any organ system and typically affects children aged 11 to 12 years with a female preponderance. Skin manifestations are similar to adult-onset lupus and include malar rash, discoid rash, oral ulcerations, petechiae, palpable purpura, and digital telangiectasia and ulcers. 7
Juvenile scleroderma is rare connective-tissue disorder that also has multiple organ involvement. Cutaneous involvement can range from isolated morphealike plaques to diffuse sclerotic lesions with growth disturbances, contractures, and facial atrophy.8
Verrucae planae, commonly referred to as flat warts, are papules caused primarily by human papillomavirus types 3, 10, 28, and 41. Children and young adults commonly are affected, and warts can appear on the hands, as in our patient.6
Treatment of JDM depends on disease severity at initial presentation and requires a multidisciplinary approach. The mainstay of treatment is high-dose oral prednisone in combination with disease-modifying drugs such as methotrexate and cyclosporin A. Patients with more severe presentations (eg, ulcerative skin disease) or life-threatening organ involvement are treated with cyclophosphamide, usually in combination with high-dose glucocorticoids.9
Early detection with aggressive treatment is vital to reduce morbidity and mortality from organ damage and disease complications. Mortality rates have dropped to 3%10 in recent decades with the use of systemic glucocorticoids. Delayed treatment is associated with a prolonged disease course and poorer outcomes. Disease complications in children with JDM include osteoporosis, calcinosis, and intestinal perforation; however, with early treatment, children with JDM can expect full recovery and to live a normal life as compared to adults with dermatomyositis.10
Prior to our patient's diagnosis, the family was assigned to move to an overseas location through the US Military with no direct access to advanced medical care. Early detection and diagnosis of JDM through an astute clinical examination allowed the patient and her family to remain in the continental United States to continue receiving specialty care.
- Mendez EP, Lipton R, Ramsey-Goldman R, et al. US incidence of juvenile dermatomyositis,1995-1998: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Registry. Arthritis Rheum. 2003;49:300-305.
- Shah M, Mamyrova G, Targoff IN, et al. The clinical phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine. 2013;92:25-41.
- Gerami P, Walling HW, Lewis J, et al. A systematic review of juvenile-onset clinically amyopathic dermatomyositis. Br J Dermatol. 2007;57:637-644.
- Enders FB, Bader-Meunier B, Baildam E, et al. Consensus-based recommendations for the management of juvenile dermatomyositis. Ann Rheum Dis. 2017;76:329-340.
- Brown VE, Pilkington CA, Feldman BM, et al. An international consensus survey of the diagnostic criteria for juvenile dermatomyositis (JDM). Rheumatology (Oxford). 2006;45:990-993.
- William JD, Berger TG, Elston DM. Viral diseases. In: William JD, Berger TG, Elston DM. Andrews' Diseases of the Skin: Clinical Dermatology. 11th ed. China: Saunders Elsevier; 2011:360-413.
- Levy DM, Kamphuis S. Systemic lupus erythematosus in children and adolescents. Pediatr Clin North Am. 2012;59:345-364.
- Li SC, Torok KS, Pope E, et al; Childhood Arthritis and Rheumatology Research Alliance (CARRA) Localized Scleroderma Workgroup. Development of consensus treatment plans for juvenile localized scleroderma: a roadmap toward comparative effectiveness studies in juvenile localized scleroderma. Arthritis Care Res (Hoboken). 2012;64:1175-1185.
- Stringer E, Ota S, Bohnsack J, et al. Treatment approaches to juvenile dermatomyositis (JDM) across North America: the Childhood Arthritis and Rheumatology Research Alliance (CARRA) JDM treatment study. J Rhematol. 2010;37:S1953-S1961.
- Huber AM, Feldman BM. Long-term outcomes in juvenile dermatomyositis: how did we get here and where are we going? Curr Rheumatol Rep. 2005;7:441-446.
- Mendez EP, Lipton R, Ramsey-Goldman R, et al. US incidence of juvenile dermatomyositis,1995-1998: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Registry. Arthritis Rheum. 2003;49:300-305.
- Shah M, Mamyrova G, Targoff IN, et al. The clinical phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine. 2013;92:25-41.
- Gerami P, Walling HW, Lewis J, et al. A systematic review of juvenile-onset clinically amyopathic dermatomyositis. Br J Dermatol. 2007;57:637-644.
- Enders FB, Bader-Meunier B, Baildam E, et al. Consensus-based recommendations for the management of juvenile dermatomyositis. Ann Rheum Dis. 2017;76:329-340.
- Brown VE, Pilkington CA, Feldman BM, et al. An international consensus survey of the diagnostic criteria for juvenile dermatomyositis (JDM). Rheumatology (Oxford). 2006;45:990-993.
- William JD, Berger TG, Elston DM. Viral diseases. In: William JD, Berger TG, Elston DM. Andrews' Diseases of the Skin: Clinical Dermatology. 11th ed. China: Saunders Elsevier; 2011:360-413.
- Levy DM, Kamphuis S. Systemic lupus erythematosus in children and adolescents. Pediatr Clin North Am. 2012;59:345-364.
- Li SC, Torok KS, Pope E, et al; Childhood Arthritis and Rheumatology Research Alliance (CARRA) Localized Scleroderma Workgroup. Development of consensus treatment plans for juvenile localized scleroderma: a roadmap toward comparative effectiveness studies in juvenile localized scleroderma. Arthritis Care Res (Hoboken). 2012;64:1175-1185.
- Stringer E, Ota S, Bohnsack J, et al. Treatment approaches to juvenile dermatomyositis (JDM) across North America: the Childhood Arthritis and Rheumatology Research Alliance (CARRA) JDM treatment study. J Rhematol. 2010;37:S1953-S1961.
- Huber AM, Feldman BM. Long-term outcomes in juvenile dermatomyositis: how did we get here and where are we going? Curr Rheumatol Rep. 2005;7:441-446.
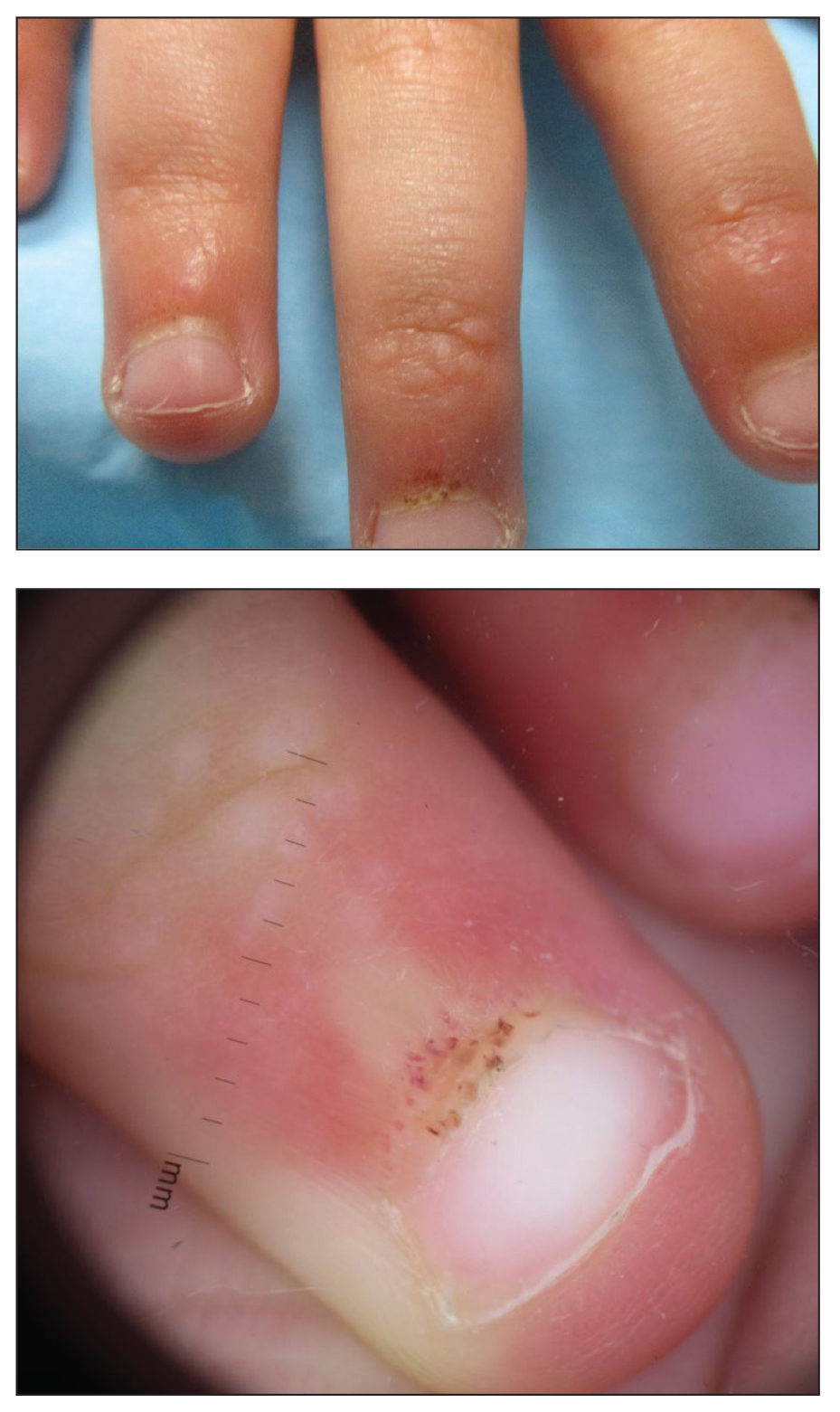
A 4-year-old girl presented to our dermatology clinic with asymptomatic flesh-colored bumps on the fingers of 2 to 3 months’ duration. Prior to presentation the patient was otherwise healthy with normal growth and development. She was referred to dermatology for recommended treatment options for suspected flat warts. On physical examination, grouped 1- to 3-mm, smooth, flat-topped papules were found on the dorsal aspects of the distal interphalangeal joints of all fingers (top). The papules were nonpruritic. Additionally, there were nail findings of ragged cuticles and dilated capillary loops in the proximal nail folds (bottom). The patient did not bite her nails, per the mother’s report, and no other rashes were noted. There were no systemic symptoms or reports of muscle fatigue. She was positive for antinuclear antibodies at 1:320 dilution. Magnetic resonance imaging of the thighs and pelvis was ordered.
Hormonal management strategies for hidradenitis suppurativa target androgens
WASHINGTON – Hidradenitis suppurativa (HS) management should be individualized in patients, with consideration of their comorbidities, and therapies should be layered and rotated to improve efficacy, Ginette Okoye, MD, said at the annual meeting of the American Academy of Dermatology.
, spironolactone, and oral contraceptives, said Dr. Okoye, professor and chair of dermatology at Howard University, Washington. A patient’s comorbidities can help tailor which treatments to use, so if a patient with HS also has androgenetic alopecia, finasteride can be considered, while spironolactone, with or without an OC, can be considered for a patient with acne – and metformin can be considered for a patient with diabetes or prediabetes, or polycystic ovary syndrome (PCOS), she commented.
The main goal behind hormonal and metabolic therapies in patients with HS is to decrease androgens. Metformin, the oral hypoglycemic drug, reduces ovarian androgen production, and increases insulin-receptor sensitivity, and is an option for patients with HS, and can also treat comorbid conditions these patients tend to have, such as obesity, insulin resistance, and PCOS, she noted. Metformin dosing is 1,500 to 2,000 mg a day, starting at 500 mg per day with an evening meal, titrating up 500 mg every 2-4 weeks based on how patients tolerate side effects such as diarrhea, nausea, vomiting, and flatulence. Lactic acidosis is a less common side effect, but the risk increases for patients with renal and hepatic impairment or excessive alcohol intake, and for those who are undergoing a radiological procedure with contrast or who are over 65 years of age. While metformin alone, in her experience, does not make a big difference, it can be helpful when combined with other treatments such as antibiotics and biologics, and in patients with these comorbidities, she said.
Pregnant women with HS can benefit from treatment with metformin, but dermatologists should consult with the patient’s obstetrician-gynecologist as the medication is classified as pregnancy category B. In addition, metformin should not be given to patients with a glomerular filtration rate (GFR) less than 45 mL/min, and long-term use is associated with low vitamin B12 levels, she said.
“I often layer this with the antibiotic therapy, so my patient may be on clindamycin, rifampin, and metformin,” said Dr. Okoye. “If they are, you can give them a much lower dose of metformin since rifampin increases the plasma concentration of metformin.”
Patients with HS may also respond well to finasteride at doses between 1 mg and 5 mg once daily, an off-label use for this medication. Finasteride, which targets type 2 5-alpha-reductase, reduces the levels of dihydrotestosterone within hair follicles, which can improve HS symptoms, she said. However, she discusses potential side effects of finasteride use with patients, which include reduced libido, abnormal ejaculation, breast tenderness, prostate cancer, and depression. She also referred to postmarketing data suggesting that finasteride can lead to post-finasteride syndrome, characterized by symptoms that include depression and anhedonia, even long after stopping treatment, she said.
“I still think that it’s worth a try,” Dr. Okoye commented. “Many of our HS patients already are dealing with depression because of their disease. ... In 3 months, we talk about their symptoms, [and] make sure that they’re feeling okay before continuing.”
While finasteride is not appropriate for women of childbearing potential (pregnancy category X), it can be an option for women with HS who are of childbearing age but are not at risk for becoming pregnant, Dr. Okoye added, which can be determined by discussing a patient’s family planning goals. For example, she said, “if you have a woman of childbearing age but she’s in a same-sex relationship and has no intention of having children, then maybe finasteride is an option for her.”
The mineralocorticoid- and aldosterone-receptor antagonist spironolactone, used off label for acne treatment, also has antiandrogenic properties and is an option for patients with HS “at the higher end of the dosing spectrum” with 100-200 mg daily. However, Dr. Okoye referred to a recently published single-center retrospective study that showed a low daily dose of 75 mg was effective for HS (J Am Acad Dermatol. 2019 Jan;80[1]:114-9).
While spironolactone increases the risk of hyperkalemia, in patients with no preexisting renal disease under 50 years of age, monitoring is not necessary because there is little to no risk of clinical hyperkalemia in these patients, she said. Combining spironolactone or finasteride with OCs may increase antiandrogenic activity, she noted.
The data on effectiveness of hormonal contraceptives are mixed with regard to treatment of HS, with some studies showing benefit or worsening of the disease with OC use. “I think one of the reasons the data is so ‘dirty’ is because OCs range widely in terms of their ingredients and in terms of how androgenic their progesterones are,” Dr. Okoye commented.
OCs increase the risk of venous thromboembolism (VTE), but Dr. Okoye noted the risk is less than a patient would experience during pregnancy. “When you talk to dermatologists, there are two camps: some dermatologists who are very comfortable prescribing OCs, and dermatologists who prefer not to, given the risk of VTEs,” she said. However, risk should also be applied to patient population and location, she noted.
“If you are in an area [where] you serve a patient population that has fewer options for access to care, and if you don’t prescribe the OCs, those patients have to wait several months before getting on therapy, said Dr. Okoye. “Maybe that’s a case where you might want to start the OC [with] one or two refills while they find an OB, but it’s really up to you and your risk aversion.”
Dietary factors may also contribute to HS, but more studies are needed to analyze how sugar and carbohydrates contribute to the condition. Instead of taking for granted that a patient will understand what reducing dietary carbohydrate and sugar intake means, Dr. Okoye said, “I like to get very specific; ask them what they’re drinking on a daily basis.”
With regard to weight loss, there is little to link significant weight loss and symptom improvement. However, weight loss could help with comorbid conditions in patients with HS, like metabolic syndrome, and subsequent skin reduction may reduce friction of intertriginous areas, she pointed out.
Dr. Okoye reports receiving grants and/or research funding from Eli Lilly.
WASHINGTON – Hidradenitis suppurativa (HS) management should be individualized in patients, with consideration of their comorbidities, and therapies should be layered and rotated to improve efficacy, Ginette Okoye, MD, said at the annual meeting of the American Academy of Dermatology.
, spironolactone, and oral contraceptives, said Dr. Okoye, professor and chair of dermatology at Howard University, Washington. A patient’s comorbidities can help tailor which treatments to use, so if a patient with HS also has androgenetic alopecia, finasteride can be considered, while spironolactone, with or without an OC, can be considered for a patient with acne – and metformin can be considered for a patient with diabetes or prediabetes, or polycystic ovary syndrome (PCOS), she commented.
The main goal behind hormonal and metabolic therapies in patients with HS is to decrease androgens. Metformin, the oral hypoglycemic drug, reduces ovarian androgen production, and increases insulin-receptor sensitivity, and is an option for patients with HS, and can also treat comorbid conditions these patients tend to have, such as obesity, insulin resistance, and PCOS, she noted. Metformin dosing is 1,500 to 2,000 mg a day, starting at 500 mg per day with an evening meal, titrating up 500 mg every 2-4 weeks based on how patients tolerate side effects such as diarrhea, nausea, vomiting, and flatulence. Lactic acidosis is a less common side effect, but the risk increases for patients with renal and hepatic impairment or excessive alcohol intake, and for those who are undergoing a radiological procedure with contrast or who are over 65 years of age. While metformin alone, in her experience, does not make a big difference, it can be helpful when combined with other treatments such as antibiotics and biologics, and in patients with these comorbidities, she said.
Pregnant women with HS can benefit from treatment with metformin, but dermatologists should consult with the patient’s obstetrician-gynecologist as the medication is classified as pregnancy category B. In addition, metformin should not be given to patients with a glomerular filtration rate (GFR) less than 45 mL/min, and long-term use is associated with low vitamin B12 levels, she said.
“I often layer this with the antibiotic therapy, so my patient may be on clindamycin, rifampin, and metformin,” said Dr. Okoye. “If they are, you can give them a much lower dose of metformin since rifampin increases the plasma concentration of metformin.”
Patients with HS may also respond well to finasteride at doses between 1 mg and 5 mg once daily, an off-label use for this medication. Finasteride, which targets type 2 5-alpha-reductase, reduces the levels of dihydrotestosterone within hair follicles, which can improve HS symptoms, she said. However, she discusses potential side effects of finasteride use with patients, which include reduced libido, abnormal ejaculation, breast tenderness, prostate cancer, and depression. She also referred to postmarketing data suggesting that finasteride can lead to post-finasteride syndrome, characterized by symptoms that include depression and anhedonia, even long after stopping treatment, she said.
“I still think that it’s worth a try,” Dr. Okoye commented. “Many of our HS patients already are dealing with depression because of their disease. ... In 3 months, we talk about their symptoms, [and] make sure that they’re feeling okay before continuing.”
While finasteride is not appropriate for women of childbearing potential (pregnancy category X), it can be an option for women with HS who are of childbearing age but are not at risk for becoming pregnant, Dr. Okoye added, which can be determined by discussing a patient’s family planning goals. For example, she said, “if you have a woman of childbearing age but she’s in a same-sex relationship and has no intention of having children, then maybe finasteride is an option for her.”
The mineralocorticoid- and aldosterone-receptor antagonist spironolactone, used off label for acne treatment, also has antiandrogenic properties and is an option for patients with HS “at the higher end of the dosing spectrum” with 100-200 mg daily. However, Dr. Okoye referred to a recently published single-center retrospective study that showed a low daily dose of 75 mg was effective for HS (J Am Acad Dermatol. 2019 Jan;80[1]:114-9).
While spironolactone increases the risk of hyperkalemia, in patients with no preexisting renal disease under 50 years of age, monitoring is not necessary because there is little to no risk of clinical hyperkalemia in these patients, she said. Combining spironolactone or finasteride with OCs may increase antiandrogenic activity, she noted.
The data on effectiveness of hormonal contraceptives are mixed with regard to treatment of HS, with some studies showing benefit or worsening of the disease with OC use. “I think one of the reasons the data is so ‘dirty’ is because OCs range widely in terms of their ingredients and in terms of how androgenic their progesterones are,” Dr. Okoye commented.
OCs increase the risk of venous thromboembolism (VTE), but Dr. Okoye noted the risk is less than a patient would experience during pregnancy. “When you talk to dermatologists, there are two camps: some dermatologists who are very comfortable prescribing OCs, and dermatologists who prefer not to, given the risk of VTEs,” she said. However, risk should also be applied to patient population and location, she noted.
“If you are in an area [where] you serve a patient population that has fewer options for access to care, and if you don’t prescribe the OCs, those patients have to wait several months before getting on therapy, said Dr. Okoye. “Maybe that’s a case where you might want to start the OC [with] one or two refills while they find an OB, but it’s really up to you and your risk aversion.”
Dietary factors may also contribute to HS, but more studies are needed to analyze how sugar and carbohydrates contribute to the condition. Instead of taking for granted that a patient will understand what reducing dietary carbohydrate and sugar intake means, Dr. Okoye said, “I like to get very specific; ask them what they’re drinking on a daily basis.”
With regard to weight loss, there is little to link significant weight loss and symptom improvement. However, weight loss could help with comorbid conditions in patients with HS, like metabolic syndrome, and subsequent skin reduction may reduce friction of intertriginous areas, she pointed out.
Dr. Okoye reports receiving grants and/or research funding from Eli Lilly.
WASHINGTON – Hidradenitis suppurativa (HS) management should be individualized in patients, with consideration of their comorbidities, and therapies should be layered and rotated to improve efficacy, Ginette Okoye, MD, said at the annual meeting of the American Academy of Dermatology.
, spironolactone, and oral contraceptives, said Dr. Okoye, professor and chair of dermatology at Howard University, Washington. A patient’s comorbidities can help tailor which treatments to use, so if a patient with HS also has androgenetic alopecia, finasteride can be considered, while spironolactone, with or without an OC, can be considered for a patient with acne – and metformin can be considered for a patient with diabetes or prediabetes, or polycystic ovary syndrome (PCOS), she commented.
The main goal behind hormonal and metabolic therapies in patients with HS is to decrease androgens. Metformin, the oral hypoglycemic drug, reduces ovarian androgen production, and increases insulin-receptor sensitivity, and is an option for patients with HS, and can also treat comorbid conditions these patients tend to have, such as obesity, insulin resistance, and PCOS, she noted. Metformin dosing is 1,500 to 2,000 mg a day, starting at 500 mg per day with an evening meal, titrating up 500 mg every 2-4 weeks based on how patients tolerate side effects such as diarrhea, nausea, vomiting, and flatulence. Lactic acidosis is a less common side effect, but the risk increases for patients with renal and hepatic impairment or excessive alcohol intake, and for those who are undergoing a radiological procedure with contrast or who are over 65 years of age. While metformin alone, in her experience, does not make a big difference, it can be helpful when combined with other treatments such as antibiotics and biologics, and in patients with these comorbidities, she said.
Pregnant women with HS can benefit from treatment with metformin, but dermatologists should consult with the patient’s obstetrician-gynecologist as the medication is classified as pregnancy category B. In addition, metformin should not be given to patients with a glomerular filtration rate (GFR) less than 45 mL/min, and long-term use is associated with low vitamin B12 levels, she said.
“I often layer this with the antibiotic therapy, so my patient may be on clindamycin, rifampin, and metformin,” said Dr. Okoye. “If they are, you can give them a much lower dose of metformin since rifampin increases the plasma concentration of metformin.”
Patients with HS may also respond well to finasteride at doses between 1 mg and 5 mg once daily, an off-label use for this medication. Finasteride, which targets type 2 5-alpha-reductase, reduces the levels of dihydrotestosterone within hair follicles, which can improve HS symptoms, she said. However, she discusses potential side effects of finasteride use with patients, which include reduced libido, abnormal ejaculation, breast tenderness, prostate cancer, and depression. She also referred to postmarketing data suggesting that finasteride can lead to post-finasteride syndrome, characterized by symptoms that include depression and anhedonia, even long after stopping treatment, she said.
“I still think that it’s worth a try,” Dr. Okoye commented. “Many of our HS patients already are dealing with depression because of their disease. ... In 3 months, we talk about their symptoms, [and] make sure that they’re feeling okay before continuing.”
While finasteride is not appropriate for women of childbearing potential (pregnancy category X), it can be an option for women with HS who are of childbearing age but are not at risk for becoming pregnant, Dr. Okoye added, which can be determined by discussing a patient’s family planning goals. For example, she said, “if you have a woman of childbearing age but she’s in a same-sex relationship and has no intention of having children, then maybe finasteride is an option for her.”
The mineralocorticoid- and aldosterone-receptor antagonist spironolactone, used off label for acne treatment, also has antiandrogenic properties and is an option for patients with HS “at the higher end of the dosing spectrum” with 100-200 mg daily. However, Dr. Okoye referred to a recently published single-center retrospective study that showed a low daily dose of 75 mg was effective for HS (J Am Acad Dermatol. 2019 Jan;80[1]:114-9).
While spironolactone increases the risk of hyperkalemia, in patients with no preexisting renal disease under 50 years of age, monitoring is not necessary because there is little to no risk of clinical hyperkalemia in these patients, she said. Combining spironolactone or finasteride with OCs may increase antiandrogenic activity, she noted.
The data on effectiveness of hormonal contraceptives are mixed with regard to treatment of HS, with some studies showing benefit or worsening of the disease with OC use. “I think one of the reasons the data is so ‘dirty’ is because OCs range widely in terms of their ingredients and in terms of how androgenic their progesterones are,” Dr. Okoye commented.
OCs increase the risk of venous thromboembolism (VTE), but Dr. Okoye noted the risk is less than a patient would experience during pregnancy. “When you talk to dermatologists, there are two camps: some dermatologists who are very comfortable prescribing OCs, and dermatologists who prefer not to, given the risk of VTEs,” she said. However, risk should also be applied to patient population and location, she noted.
“If you are in an area [where] you serve a patient population that has fewer options for access to care, and if you don’t prescribe the OCs, those patients have to wait several months before getting on therapy, said Dr. Okoye. “Maybe that’s a case where you might want to start the OC [with] one or two refills while they find an OB, but it’s really up to you and your risk aversion.”
Dietary factors may also contribute to HS, but more studies are needed to analyze how sugar and carbohydrates contribute to the condition. Instead of taking for granted that a patient will understand what reducing dietary carbohydrate and sugar intake means, Dr. Okoye said, “I like to get very specific; ask them what they’re drinking on a daily basis.”
With regard to weight loss, there is little to link significant weight loss and symptom improvement. However, weight loss could help with comorbid conditions in patients with HS, like metabolic syndrome, and subsequent skin reduction may reduce friction of intertriginous areas, she pointed out.
Dr. Okoye reports receiving grants and/or research funding from Eli Lilly.
EXPERT ANALYSIS FROM AAD 19
Acute Encephalopathy Following Hyperbaric Oxygen Therapy in a Patient on Metronidazole
Altered mental status (AMS) is a common presentation to the emergency department (ED) for older patients and is often due to underlying drug-associated adverse effects (AEs), medical or psychiatric illness, or neurologic disease. EDs often have protocols for diagnosing and managing AMS to assess the underlying etiology. A formal assessment with a full history and physical examination is paramount to diagnosing the cause of AMS.
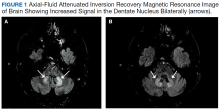
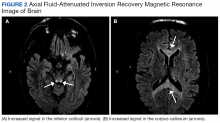
Oral metronidazole is a commonly used antibiotic for anaerobic bacterial infections and Clostridium difficile-associated diarrhea and colitis.1Metronidazole produces cytotoxic intermediates that cause DNA strand breakage and destabilization, resulting in bactericidal activity in host cells.2Common AEs include gastrointestinal symptoms such as nausea, vomiting, and diarrhea; less common AEs can involve the nervous system and include seizures, peripheral neuropathy, dizziness, ataxia, and encephalopathy.3,4A pattern of magnetic resonance image (MRI) abnormalities typically located at the cerebellar dentate nucleus midbrain, dorsal pons, medulla, and splenium of the corpus callosum have been associated with metronidazole usage.5
Hyperbaric oxygen therapy (HBOT) is a treatment modality used as the primary therapy for decompression sickness, arterial gas embolism, and carbon monoxide poisoning. HBOT is used as adjuvant therapy for osteonecrosis caused by radiation or bisphosphonate use.6,7 HBOT increases the partial pressure of oxygen in plasma and increases the amount of oxygen delivered to tissues throughout the body.8Hyperoxia, defined as an elevated partial pressure of oxygen leading to excess oxygenation to tissues and organs, increases production of reactive oxygen and nitrogen species, which are signaling factors in a variety of pathways that stimulate angiogenesis.8 AEs of HBOT include barotrauma-related injuries and oxygen toxicity, such as respiratory distress or central nervous system (CNS) symptoms.9 Severe CNS AEs occur in 1% to 2% of patients undergoing therapy and manifest as generalized tonic-clonic seizures, typically in patients with preexisting neurologic disorders, brain injury, or lowered seizure threshold.7,8,10 There have been no documented incidences of HBOT inducing acute encephalopathy.
Case Presentation
A 63-year-old male smoker with no history of alcohol use presented to the ED with an acute onset of lightheadedness, confusion, and poor coordination following his second HBOT for radiation-induced osteonecrosis of the mandible. The patient reported chronic, slowly progressive pain and numbness of the feet that began 4 years earlier. He noted marked worsening of pain and difficulty standing and walking 3 to 4 months prior to presentation.
Ten years prior, the patient was diagnosed with cancer of the right tonsil. A tonsillectomy with wide margins was performed, followed by 35 rounds of radiation treatment and 2 rounds of chemotherapy with cisplatin.
In May 2017, the patient presented with a lump in the right cheek that was diagnosed as osteonecrosis of the mandible. An oral surgeon prescribed metronidazole 500 mg qid and amoxicillin 500 mg tid. The patient was adherent until presentation in November 2017. Following lack of improvement of the osteonecrosis from antibiotic therapy, oral surgery was planned, and the patient was referred for HBOT with a planned 20 HBOT preoperative treatments and 10 postoperative treatments.
Following his first 2-hour HBOT treatment on November 13, 2017, the patient complained of light-headedness, confusion, and incoordination. While driving on a familiar route to his home, he collided with a tree that was 6 feet from the curb. The patient attempted to drive another vehicle later that day, resulting in a second motor vehicle accident. There was no significant injury reported in either accident.
His partner described the patient’s episode of disorientation lasting 6 to 8 hours, during which he “looked drunk” and was unable to sit in a chair without falling. The following morning, the patient had improved mental status but had not returned to baseline. His second HBOT treatment took place that day, and again, the patient acutely experienced light-headedness and confusion following completion. Therapy was suspended, and the patient was referred to the ED for further evaluation. Mild facial asymmetry without weakness, decreased sensation from toes to knees bilaterally, and absent Achilles reflexes bilaterally were found on neurologic examination. He exhibited past-pointing on finger-to-nose testing bilaterally. He was able to ambulate independently, but he could not perform tandem gait.
An MRI of the brain showed abnormal T2 hyperintensity found bilaterally at the dentate nuclei and inferior colliculi. The splenium of the corpus callosum also showed mild involvement with hyperintense lesions. Laboratory tests of the patient’s complete blood count; comprehensive metabolic panel; vitamins B1, B6, B12; and folic acid levels had no notable abnormalities and were within normal limits.
Metronidazole and HBOT therapy were discontinued, and all of the patient’s symptoms resolved within 2 weeks. A repeat examination and MRI performed 1 month later showed resolution of all the patient’s clinical findings and MRI abnormalities. HBOT was resumed without the recurrence of previously described symptoms.
Discussion
This patient’s encephalopathic symptoms correlate temporally with the onset of HBOT. There is no medical literature suggesting a relationship between HBOT and encephalopathic symptoms with MRI abnormalities, and in fact, some studies suggest HBOT as a treatment for hypoxic-ischemic encephalopathy in neonates.11 This led us to believe that the HBOT may have exacerbated some underlying condition, evidenced by the specific MRI findings of T2 fluid-attenuated inversion recovery (FLAIR) hyperintensities in the dentate nuclei and inferior colliculi (Figures 1 and 2).
Differential diagnoses for T2 hyperintense lesions in the dentate nuclei include metronidazole toxicity, acute Wernicke encephalopathy (WE), and methyl bromide intoxication. Diseases that would have presented in infancy with similar MRI findings (Canavan disease, maple-syrup urine disease, and glutaric aciduria type 1) were not considered plausible.12-14
Despite his denial of alcohol use, the patient was at risk for malnutrition secondary to his mandibular lesion and difficulty eating. Clinically, he presented with episodes of confusion and ataxia, consistent with 2 of the classic triad of symptoms of WE (no ocular abnormalities noted on exam). Typical MRI findings in WE include signal intensity alterations (including T2 hyperintensities) in the medial thalami, mammillary bodies, collicular bodies, and periaqueductal and periventricular regions.14,15 Atypical MRI findings in WE include symmetric signal intensity changes in the cerebellum, dentate nuclei, caudate nuclei, red nuclei, cranial nerve nuclei, and splenium.14 Of note, atypical MRI findings were more common in patients without alcohol use disorders and WE, and typical MRI findings were more common in patients with alcohol use disorders.14 However, this patient’s report of no alcohol use and the serum thiamine level being within normal limits (173 nmol/L; range 78-185 nmol/L) made acute WE less likely than metronidonazale-induced encephalopathy (MIE).
The most common neurologic AE of metronidazole is distal symmetric sensory polyneuropathy, which also can have motor or autonomic features.16,17 While our patient had a history of peripheral neuropathy, he noted marked worsening of foot pain 3 months after initiating metronidazole therapy. A potential mechanism involves metronidazole or its cytotoxic intermediates binding neuronal ribonucleic acids, thus inhibiting protein synthesis and resulting in degenerative neuronal changes and reversible axonal swelling (as opposed to the DNA interference attributed to the drug’s mechanism of bactericidal action).18 Neuropathies may result from prolonged high-dose metronidazole therapy (cumulative dose > 42 g),3 but they also have been seen in short-term use of high dosages.17
CNS AEs are much rarer and are thought to be associated with metronidazole’s ability to cross the blood-brain barrier. These patients present as a toxic encephalopathy with cerebellar dysfunction (dysarthria, ataxia) as the most common presentation, followed by AMS and seizures.4 Our patient presented with acute confusion and ataxia. Animal studies suggest that γ-aminobutyric acid (GABA) receptor modulation in the cerebellar and vestibular systems may contribute to this neurotoxicity, but no definitive mechanism of injury has been found.19
On MRI, MIE most commonly presents with hyperintense lesions in the bilateral cerebellar dentate nucleus on T2-weighted and FLAIR images.5,20 The midbrain, dorsal pons, medulla, and corpus callosum also can show increased signal intensity.5 This AE does not seem to be dose- or duration-dependent, and most cases report complete or partial resolution of symptoms following discontinuation of the drug, though this is not absolute.4,13,21 The patient’s MRI findings were highly consistent with MIE (Figure 2).
Conclusion
This patient’s highly specific MRI findings, neurologic examination consistent with confusion, ataxia, length-dependent sensory neuropathy, and 360-g cumulative dose of metronidazole over the previous 6 months suggest he experienced MIE. The mechanism of how HBOT precipitated the patient’s altered mental status, incoordination, and worsening of peripheral neuropathy is unknown. Although encephalopathy with MRI abnormalities as described is not a reported AE of HBOT, it may be unrecognized. It is possible that without HBOT the patient would have remained asymptomatic apart from his peripheral neuropathy.
We propose HBOT may exacerbate or increase the risk of a patient developing MIE. Our patient was able to safely resume HBOT after metronidazole was discontinued, suggesting that the combination was the causation for the development of encephalopathy. We do not believe any similar cases have been reported.
1. Samuelson J. Why metronidazole is active against both bacteria and parasites. Antimicrob Agents Chemother. 1999;43(7):1533-1541.
2. Edwards DI. The action of metronidazole on DNA. J Antimicrob Chemother. 1977;3(1):43-48.
3. Goolsby TA, Jakeman B, Gaynes RP. Clinical relevance of metronidazole and peripheral neuropathy: a systematic review of the literature. Int J Antimicrob Agents. 2018;51(3):319-325.
4. Kuriyama A, Jackson JL, Doi A, Kamiya T. Metronidazole-induced central nervous system toxicity: a systematic review. Clin Neuropharmacol. 2011;34(6):241-247.
5. Kim E, Na DG, Kim EY, Kim JH, Son KR, Chang KH. MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol. 2007;28(9):1652-1658.
6. Ceponis P, Keilman C, Guerry C, Freiberger JJ. Hyperbaric oxygen therapy and osteonecrosis. Oral Dis. 2017;23(2):141-151.
7. Leach R, Rees P, Wilmshurst P. Hyperbaric oxygen therapy. BMJ. 1998;317(7166):1140-1143.
8. Thom SR. Hyperbaric oxygen–its mechanisms and efficacy. Plastic Reconstr Surg. 2011;127(suppl 1):131S-141S.
9. Plafki C, Peters P, Almeling M, Welslau W, Busch R. Complications and side effects of hyperbaric oxygen therapy. Aviation Space Environ Med. 2000;71(2):119-124.
10. Hadanny A, Meir O, Bechor Y, Fishlev G, Bergan J, Efrati S. Seizures during hyperbaric oxygen therapy: retrospective analysis of 62,614 treatment sessions. Undersea Hyperb Med. 2016;43(1):21-28.
11. Liu Z, Xiong T, Meads C. Clinical effectiveness of treatment with hyperbaric oxygen for neonatal hypoxic-ischaemic encephalopathy: systematic review of Chinese literature. BMJ. 2006;333(7564):374.
12. Bond KM, Brinjikji W, Eckel LJ, Kallmes DF, McDonald RJ, Carr CM. Dentate update: imaging features of entities that affect the dentate nucleus. AJNR Am J Neuroradiol. 2017;38(8):1467-1474.
13. Agarwal A, Kanekar S, Sabat S, Thamburaj K. Metronidazole-induced cerebellar toxicity. Neurol Int. 2016;8(1):6365.
14. Zuccoli G, Pipitone N. Neuroimaging findings in acute Wernicke’s encephalopathy: review of the literature. AJR Am J Roentgenol. 2009;192(2):501-508.
15. Jung YC, Chanraud S, Sullivan EV. Neuroimaging of Wernicke’s encephalopathy and Korsakoff’s syndrome. Neuropsychol Rev. 2012;22(2):170-180.
16. Hobson-Webb LD, Roach ES, Donofrio PD. Metronidazole: newly recognized cause of autonomic neuropathy. J Child Neurol. 2006;21(5):429-431.
17. Nath Chaurasia R. Rapid onset metronidazole induced sensory neuropathy: case series and review of literature. Int J Neurorehabilitation. 2015;02:152.
18. Bradley WG, Karlsson IJ, Rassol CG. Metronidazole neuropathy. Br Med J. 1977;2(6087):610-611.
19. Evans J, Levesque D, Knowles K, Longshore R, Plummer S. Diazepam as a treatment for metronidazole toxicosis in dogs: a retrospective study of 21 cases. J Vet Intern Med. 2003;17(3):304-310.
20. Farmakiotis D, Zeluff B. Images in clinical medicine. Metronidazole-associated encephalopathy. N Engl J Med. 2016;374(15):1465.
21. Hobbs K, Stern-Nezer S, Buckwalter MS, Fischbein N, Finley Caulfield A. Metronidazole-induced encephalopathy: not always a reversible situation. Neurocrit Care. 2015;22(3):429-436.
Altered mental status (AMS) is a common presentation to the emergency department (ED) for older patients and is often due to underlying drug-associated adverse effects (AEs), medical or psychiatric illness, or neurologic disease. EDs often have protocols for diagnosing and managing AMS to assess the underlying etiology. A formal assessment with a full history and physical examination is paramount to diagnosing the cause of AMS.
Oral metronidazole is a commonly used antibiotic for anaerobic bacterial infections and Clostridium difficile-associated diarrhea and colitis.1Metronidazole produces cytotoxic intermediates that cause DNA strand breakage and destabilization, resulting in bactericidal activity in host cells.2Common AEs include gastrointestinal symptoms such as nausea, vomiting, and diarrhea; less common AEs can involve the nervous system and include seizures, peripheral neuropathy, dizziness, ataxia, and encephalopathy.3,4A pattern of magnetic resonance image (MRI) abnormalities typically located at the cerebellar dentate nucleus midbrain, dorsal pons, medulla, and splenium of the corpus callosum have been associated with metronidazole usage.5
Hyperbaric oxygen therapy (HBOT) is a treatment modality used as the primary therapy for decompression sickness, arterial gas embolism, and carbon monoxide poisoning. HBOT is used as adjuvant therapy for osteonecrosis caused by radiation or bisphosphonate use.6,7 HBOT increases the partial pressure of oxygen in plasma and increases the amount of oxygen delivered to tissues throughout the body.8Hyperoxia, defined as an elevated partial pressure of oxygen leading to excess oxygenation to tissues and organs, increases production of reactive oxygen and nitrogen species, which are signaling factors in a variety of pathways that stimulate angiogenesis.8 AEs of HBOT include barotrauma-related injuries and oxygen toxicity, such as respiratory distress or central nervous system (CNS) symptoms.9 Severe CNS AEs occur in 1% to 2% of patients undergoing therapy and manifest as generalized tonic-clonic seizures, typically in patients with preexisting neurologic disorders, brain injury, or lowered seizure threshold.7,8,10 There have been no documented incidences of HBOT inducing acute encephalopathy.
Case Presentation
A 63-year-old male smoker with no history of alcohol use presented to the ED with an acute onset of lightheadedness, confusion, and poor coordination following his second HBOT for radiation-induced osteonecrosis of the mandible. The patient reported chronic, slowly progressive pain and numbness of the feet that began 4 years earlier. He noted marked worsening of pain and difficulty standing and walking 3 to 4 months prior to presentation.
Ten years prior, the patient was diagnosed with cancer of the right tonsil. A tonsillectomy with wide margins was performed, followed by 35 rounds of radiation treatment and 2 rounds of chemotherapy with cisplatin.
In May 2017, the patient presented with a lump in the right cheek that was diagnosed as osteonecrosis of the mandible. An oral surgeon prescribed metronidazole 500 mg qid and amoxicillin 500 mg tid. The patient was adherent until presentation in November 2017. Following lack of improvement of the osteonecrosis from antibiotic therapy, oral surgery was planned, and the patient was referred for HBOT with a planned 20 HBOT preoperative treatments and 10 postoperative treatments.
Following his first 2-hour HBOT treatment on November 13, 2017, the patient complained of light-headedness, confusion, and incoordination. While driving on a familiar route to his home, he collided with a tree that was 6 feet from the curb. The patient attempted to drive another vehicle later that day, resulting in a second motor vehicle accident. There was no significant injury reported in either accident.
His partner described the patient’s episode of disorientation lasting 6 to 8 hours, during which he “looked drunk” and was unable to sit in a chair without falling. The following morning, the patient had improved mental status but had not returned to baseline. His second HBOT treatment took place that day, and again, the patient acutely experienced light-headedness and confusion following completion. Therapy was suspended, and the patient was referred to the ED for further evaluation. Mild facial asymmetry without weakness, decreased sensation from toes to knees bilaterally, and absent Achilles reflexes bilaterally were found on neurologic examination. He exhibited past-pointing on finger-to-nose testing bilaterally. He was able to ambulate independently, but he could not perform tandem gait.
An MRI of the brain showed abnormal T2 hyperintensity found bilaterally at the dentate nuclei and inferior colliculi. The splenium of the corpus callosum also showed mild involvement with hyperintense lesions. Laboratory tests of the patient’s complete blood count; comprehensive metabolic panel; vitamins B1, B6, B12; and folic acid levels had no notable abnormalities and were within normal limits.
Metronidazole and HBOT therapy were discontinued, and all of the patient’s symptoms resolved within 2 weeks. A repeat examination and MRI performed 1 month later showed resolution of all the patient’s clinical findings and MRI abnormalities. HBOT was resumed without the recurrence of previously described symptoms.
Discussion
This patient’s encephalopathic symptoms correlate temporally with the onset of HBOT. There is no medical literature suggesting a relationship between HBOT and encephalopathic symptoms with MRI abnormalities, and in fact, some studies suggest HBOT as a treatment for hypoxic-ischemic encephalopathy in neonates.11 This led us to believe that the HBOT may have exacerbated some underlying condition, evidenced by the specific MRI findings of T2 fluid-attenuated inversion recovery (FLAIR) hyperintensities in the dentate nuclei and inferior colliculi (Figures 1 and 2).
Differential diagnoses for T2 hyperintense lesions in the dentate nuclei include metronidazole toxicity, acute Wernicke encephalopathy (WE), and methyl bromide intoxication. Diseases that would have presented in infancy with similar MRI findings (Canavan disease, maple-syrup urine disease, and glutaric aciduria type 1) were not considered plausible.12-14
Despite his denial of alcohol use, the patient was at risk for malnutrition secondary to his mandibular lesion and difficulty eating. Clinically, he presented with episodes of confusion and ataxia, consistent with 2 of the classic triad of symptoms of WE (no ocular abnormalities noted on exam). Typical MRI findings in WE include signal intensity alterations (including T2 hyperintensities) in the medial thalami, mammillary bodies, collicular bodies, and periaqueductal and periventricular regions.14,15 Atypical MRI findings in WE include symmetric signal intensity changes in the cerebellum, dentate nuclei, caudate nuclei, red nuclei, cranial nerve nuclei, and splenium.14 Of note, atypical MRI findings were more common in patients without alcohol use disorders and WE, and typical MRI findings were more common in patients with alcohol use disorders.14 However, this patient’s report of no alcohol use and the serum thiamine level being within normal limits (173 nmol/L; range 78-185 nmol/L) made acute WE less likely than metronidonazale-induced encephalopathy (MIE).
The most common neurologic AE of metronidazole is distal symmetric sensory polyneuropathy, which also can have motor or autonomic features.16,17 While our patient had a history of peripheral neuropathy, he noted marked worsening of foot pain 3 months after initiating metronidazole therapy. A potential mechanism involves metronidazole or its cytotoxic intermediates binding neuronal ribonucleic acids, thus inhibiting protein synthesis and resulting in degenerative neuronal changes and reversible axonal swelling (as opposed to the DNA interference attributed to the drug’s mechanism of bactericidal action).18 Neuropathies may result from prolonged high-dose metronidazole therapy (cumulative dose > 42 g),3 but they also have been seen in short-term use of high dosages.17
CNS AEs are much rarer and are thought to be associated with metronidazole’s ability to cross the blood-brain barrier. These patients present as a toxic encephalopathy with cerebellar dysfunction (dysarthria, ataxia) as the most common presentation, followed by AMS and seizures.4 Our patient presented with acute confusion and ataxia. Animal studies suggest that γ-aminobutyric acid (GABA) receptor modulation in the cerebellar and vestibular systems may contribute to this neurotoxicity, but no definitive mechanism of injury has been found.19
On MRI, MIE most commonly presents with hyperintense lesions in the bilateral cerebellar dentate nucleus on T2-weighted and FLAIR images.5,20 The midbrain, dorsal pons, medulla, and corpus callosum also can show increased signal intensity.5 This AE does not seem to be dose- or duration-dependent, and most cases report complete or partial resolution of symptoms following discontinuation of the drug, though this is not absolute.4,13,21 The patient’s MRI findings were highly consistent with MIE (Figure 2).
Conclusion
This patient’s highly specific MRI findings, neurologic examination consistent with confusion, ataxia, length-dependent sensory neuropathy, and 360-g cumulative dose of metronidazole over the previous 6 months suggest he experienced MIE. The mechanism of how HBOT precipitated the patient’s altered mental status, incoordination, and worsening of peripheral neuropathy is unknown. Although encephalopathy with MRI abnormalities as described is not a reported AE of HBOT, it may be unrecognized. It is possible that without HBOT the patient would have remained asymptomatic apart from his peripheral neuropathy.
We propose HBOT may exacerbate or increase the risk of a patient developing MIE. Our patient was able to safely resume HBOT after metronidazole was discontinued, suggesting that the combination was the causation for the development of encephalopathy. We do not believe any similar cases have been reported.
Altered mental status (AMS) is a common presentation to the emergency department (ED) for older patients and is often due to underlying drug-associated adverse effects (AEs), medical or psychiatric illness, or neurologic disease. EDs often have protocols for diagnosing and managing AMS to assess the underlying etiology. A formal assessment with a full history and physical examination is paramount to diagnosing the cause of AMS.
Oral metronidazole is a commonly used antibiotic for anaerobic bacterial infections and Clostridium difficile-associated diarrhea and colitis.1Metronidazole produces cytotoxic intermediates that cause DNA strand breakage and destabilization, resulting in bactericidal activity in host cells.2Common AEs include gastrointestinal symptoms such as nausea, vomiting, and diarrhea; less common AEs can involve the nervous system and include seizures, peripheral neuropathy, dizziness, ataxia, and encephalopathy.3,4A pattern of magnetic resonance image (MRI) abnormalities typically located at the cerebellar dentate nucleus midbrain, dorsal pons, medulla, and splenium of the corpus callosum have been associated with metronidazole usage.5
Hyperbaric oxygen therapy (HBOT) is a treatment modality used as the primary therapy for decompression sickness, arterial gas embolism, and carbon monoxide poisoning. HBOT is used as adjuvant therapy for osteonecrosis caused by radiation or bisphosphonate use.6,7 HBOT increases the partial pressure of oxygen in plasma and increases the amount of oxygen delivered to tissues throughout the body.8Hyperoxia, defined as an elevated partial pressure of oxygen leading to excess oxygenation to tissues and organs, increases production of reactive oxygen and nitrogen species, which are signaling factors in a variety of pathways that stimulate angiogenesis.8 AEs of HBOT include barotrauma-related injuries and oxygen toxicity, such as respiratory distress or central nervous system (CNS) symptoms.9 Severe CNS AEs occur in 1% to 2% of patients undergoing therapy and manifest as generalized tonic-clonic seizures, typically in patients with preexisting neurologic disorders, brain injury, or lowered seizure threshold.7,8,10 There have been no documented incidences of HBOT inducing acute encephalopathy.
Case Presentation
A 63-year-old male smoker with no history of alcohol use presented to the ED with an acute onset of lightheadedness, confusion, and poor coordination following his second HBOT for radiation-induced osteonecrosis of the mandible. The patient reported chronic, slowly progressive pain and numbness of the feet that began 4 years earlier. He noted marked worsening of pain and difficulty standing and walking 3 to 4 months prior to presentation.
Ten years prior, the patient was diagnosed with cancer of the right tonsil. A tonsillectomy with wide margins was performed, followed by 35 rounds of radiation treatment and 2 rounds of chemotherapy with cisplatin.
In May 2017, the patient presented with a lump in the right cheek that was diagnosed as osteonecrosis of the mandible. An oral surgeon prescribed metronidazole 500 mg qid and amoxicillin 500 mg tid. The patient was adherent until presentation in November 2017. Following lack of improvement of the osteonecrosis from antibiotic therapy, oral surgery was planned, and the patient was referred for HBOT with a planned 20 HBOT preoperative treatments and 10 postoperative treatments.
Following his first 2-hour HBOT treatment on November 13, 2017, the patient complained of light-headedness, confusion, and incoordination. While driving on a familiar route to his home, he collided with a tree that was 6 feet from the curb. The patient attempted to drive another vehicle later that day, resulting in a second motor vehicle accident. There was no significant injury reported in either accident.
His partner described the patient’s episode of disorientation lasting 6 to 8 hours, during which he “looked drunk” and was unable to sit in a chair without falling. The following morning, the patient had improved mental status but had not returned to baseline. His second HBOT treatment took place that day, and again, the patient acutely experienced light-headedness and confusion following completion. Therapy was suspended, and the patient was referred to the ED for further evaluation. Mild facial asymmetry without weakness, decreased sensation from toes to knees bilaterally, and absent Achilles reflexes bilaterally were found on neurologic examination. He exhibited past-pointing on finger-to-nose testing bilaterally. He was able to ambulate independently, but he could not perform tandem gait.
An MRI of the brain showed abnormal T2 hyperintensity found bilaterally at the dentate nuclei and inferior colliculi. The splenium of the corpus callosum also showed mild involvement with hyperintense lesions. Laboratory tests of the patient’s complete blood count; comprehensive metabolic panel; vitamins B1, B6, B12; and folic acid levels had no notable abnormalities and were within normal limits.
Metronidazole and HBOT therapy were discontinued, and all of the patient’s symptoms resolved within 2 weeks. A repeat examination and MRI performed 1 month later showed resolution of all the patient’s clinical findings and MRI abnormalities. HBOT was resumed without the recurrence of previously described symptoms.
Discussion
This patient’s encephalopathic symptoms correlate temporally with the onset of HBOT. There is no medical literature suggesting a relationship between HBOT and encephalopathic symptoms with MRI abnormalities, and in fact, some studies suggest HBOT as a treatment for hypoxic-ischemic encephalopathy in neonates.11 This led us to believe that the HBOT may have exacerbated some underlying condition, evidenced by the specific MRI findings of T2 fluid-attenuated inversion recovery (FLAIR) hyperintensities in the dentate nuclei and inferior colliculi (Figures 1 and 2).
Differential diagnoses for T2 hyperintense lesions in the dentate nuclei include metronidazole toxicity, acute Wernicke encephalopathy (WE), and methyl bromide intoxication. Diseases that would have presented in infancy with similar MRI findings (Canavan disease, maple-syrup urine disease, and glutaric aciduria type 1) were not considered plausible.12-14
Despite his denial of alcohol use, the patient was at risk for malnutrition secondary to his mandibular lesion and difficulty eating. Clinically, he presented with episodes of confusion and ataxia, consistent with 2 of the classic triad of symptoms of WE (no ocular abnormalities noted on exam). Typical MRI findings in WE include signal intensity alterations (including T2 hyperintensities) in the medial thalami, mammillary bodies, collicular bodies, and periaqueductal and periventricular regions.14,15 Atypical MRI findings in WE include symmetric signal intensity changes in the cerebellum, dentate nuclei, caudate nuclei, red nuclei, cranial nerve nuclei, and splenium.14 Of note, atypical MRI findings were more common in patients without alcohol use disorders and WE, and typical MRI findings were more common in patients with alcohol use disorders.14 However, this patient’s report of no alcohol use and the serum thiamine level being within normal limits (173 nmol/L; range 78-185 nmol/L) made acute WE less likely than metronidonazale-induced encephalopathy (MIE).
The most common neurologic AE of metronidazole is distal symmetric sensory polyneuropathy, which also can have motor or autonomic features.16,17 While our patient had a history of peripheral neuropathy, he noted marked worsening of foot pain 3 months after initiating metronidazole therapy. A potential mechanism involves metronidazole or its cytotoxic intermediates binding neuronal ribonucleic acids, thus inhibiting protein synthesis and resulting in degenerative neuronal changes and reversible axonal swelling (as opposed to the DNA interference attributed to the drug’s mechanism of bactericidal action).18 Neuropathies may result from prolonged high-dose metronidazole therapy (cumulative dose > 42 g),3 but they also have been seen in short-term use of high dosages.17
CNS AEs are much rarer and are thought to be associated with metronidazole’s ability to cross the blood-brain barrier. These patients present as a toxic encephalopathy with cerebellar dysfunction (dysarthria, ataxia) as the most common presentation, followed by AMS and seizures.4 Our patient presented with acute confusion and ataxia. Animal studies suggest that γ-aminobutyric acid (GABA) receptor modulation in the cerebellar and vestibular systems may contribute to this neurotoxicity, but no definitive mechanism of injury has been found.19
On MRI, MIE most commonly presents with hyperintense lesions in the bilateral cerebellar dentate nucleus on T2-weighted and FLAIR images.5,20 The midbrain, dorsal pons, medulla, and corpus callosum also can show increased signal intensity.5 This AE does not seem to be dose- or duration-dependent, and most cases report complete or partial resolution of symptoms following discontinuation of the drug, though this is not absolute.4,13,21 The patient’s MRI findings were highly consistent with MIE (Figure 2).
Conclusion
This patient’s highly specific MRI findings, neurologic examination consistent with confusion, ataxia, length-dependent sensory neuropathy, and 360-g cumulative dose of metronidazole over the previous 6 months suggest he experienced MIE. The mechanism of how HBOT precipitated the patient’s altered mental status, incoordination, and worsening of peripheral neuropathy is unknown. Although encephalopathy with MRI abnormalities as described is not a reported AE of HBOT, it may be unrecognized. It is possible that without HBOT the patient would have remained asymptomatic apart from his peripheral neuropathy.
We propose HBOT may exacerbate or increase the risk of a patient developing MIE. Our patient was able to safely resume HBOT after metronidazole was discontinued, suggesting that the combination was the causation for the development of encephalopathy. We do not believe any similar cases have been reported.
1. Samuelson J. Why metronidazole is active against both bacteria and parasites. Antimicrob Agents Chemother. 1999;43(7):1533-1541.
2. Edwards DI. The action of metronidazole on DNA. J Antimicrob Chemother. 1977;3(1):43-48.
3. Goolsby TA, Jakeman B, Gaynes RP. Clinical relevance of metronidazole and peripheral neuropathy: a systematic review of the literature. Int J Antimicrob Agents. 2018;51(3):319-325.
4. Kuriyama A, Jackson JL, Doi A, Kamiya T. Metronidazole-induced central nervous system toxicity: a systematic review. Clin Neuropharmacol. 2011;34(6):241-247.
5. Kim E, Na DG, Kim EY, Kim JH, Son KR, Chang KH. MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol. 2007;28(9):1652-1658.
6. Ceponis P, Keilman C, Guerry C, Freiberger JJ. Hyperbaric oxygen therapy and osteonecrosis. Oral Dis. 2017;23(2):141-151.
7. Leach R, Rees P, Wilmshurst P. Hyperbaric oxygen therapy. BMJ. 1998;317(7166):1140-1143.
8. Thom SR. Hyperbaric oxygen–its mechanisms and efficacy. Plastic Reconstr Surg. 2011;127(suppl 1):131S-141S.
9. Plafki C, Peters P, Almeling M, Welslau W, Busch R. Complications and side effects of hyperbaric oxygen therapy. Aviation Space Environ Med. 2000;71(2):119-124.
10. Hadanny A, Meir O, Bechor Y, Fishlev G, Bergan J, Efrati S. Seizures during hyperbaric oxygen therapy: retrospective analysis of 62,614 treatment sessions. Undersea Hyperb Med. 2016;43(1):21-28.
11. Liu Z, Xiong T, Meads C. Clinical effectiveness of treatment with hyperbaric oxygen for neonatal hypoxic-ischaemic encephalopathy: systematic review of Chinese literature. BMJ. 2006;333(7564):374.
12. Bond KM, Brinjikji W, Eckel LJ, Kallmes DF, McDonald RJ, Carr CM. Dentate update: imaging features of entities that affect the dentate nucleus. AJNR Am J Neuroradiol. 2017;38(8):1467-1474.
13. Agarwal A, Kanekar S, Sabat S, Thamburaj K. Metronidazole-induced cerebellar toxicity. Neurol Int. 2016;8(1):6365.
14. Zuccoli G, Pipitone N. Neuroimaging findings in acute Wernicke’s encephalopathy: review of the literature. AJR Am J Roentgenol. 2009;192(2):501-508.
15. Jung YC, Chanraud S, Sullivan EV. Neuroimaging of Wernicke’s encephalopathy and Korsakoff’s syndrome. Neuropsychol Rev. 2012;22(2):170-180.
16. Hobson-Webb LD, Roach ES, Donofrio PD. Metronidazole: newly recognized cause of autonomic neuropathy. J Child Neurol. 2006;21(5):429-431.
17. Nath Chaurasia R. Rapid onset metronidazole induced sensory neuropathy: case series and review of literature. Int J Neurorehabilitation. 2015;02:152.
18. Bradley WG, Karlsson IJ, Rassol CG. Metronidazole neuropathy. Br Med J. 1977;2(6087):610-611.
19. Evans J, Levesque D, Knowles K, Longshore R, Plummer S. Diazepam as a treatment for metronidazole toxicosis in dogs: a retrospective study of 21 cases. J Vet Intern Med. 2003;17(3):304-310.
20. Farmakiotis D, Zeluff B. Images in clinical medicine. Metronidazole-associated encephalopathy. N Engl J Med. 2016;374(15):1465.
21. Hobbs K, Stern-Nezer S, Buckwalter MS, Fischbein N, Finley Caulfield A. Metronidazole-induced encephalopathy: not always a reversible situation. Neurocrit Care. 2015;22(3):429-436.
1. Samuelson J. Why metronidazole is active against both bacteria and parasites. Antimicrob Agents Chemother. 1999;43(7):1533-1541.
2. Edwards DI. The action of metronidazole on DNA. J Antimicrob Chemother. 1977;3(1):43-48.
3. Goolsby TA, Jakeman B, Gaynes RP. Clinical relevance of metronidazole and peripheral neuropathy: a systematic review of the literature. Int J Antimicrob Agents. 2018;51(3):319-325.
4. Kuriyama A, Jackson JL, Doi A, Kamiya T. Metronidazole-induced central nervous system toxicity: a systematic review. Clin Neuropharmacol. 2011;34(6):241-247.
5. Kim E, Na DG, Kim EY, Kim JH, Son KR, Chang KH. MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol. 2007;28(9):1652-1658.
6. Ceponis P, Keilman C, Guerry C, Freiberger JJ. Hyperbaric oxygen therapy and osteonecrosis. Oral Dis. 2017;23(2):141-151.
7. Leach R, Rees P, Wilmshurst P. Hyperbaric oxygen therapy. BMJ. 1998;317(7166):1140-1143.
8. Thom SR. Hyperbaric oxygen–its mechanisms and efficacy. Plastic Reconstr Surg. 2011;127(suppl 1):131S-141S.
9. Plafki C, Peters P, Almeling M, Welslau W, Busch R. Complications and side effects of hyperbaric oxygen therapy. Aviation Space Environ Med. 2000;71(2):119-124.
10. Hadanny A, Meir O, Bechor Y, Fishlev G, Bergan J, Efrati S. Seizures during hyperbaric oxygen therapy: retrospective analysis of 62,614 treatment sessions. Undersea Hyperb Med. 2016;43(1):21-28.
11. Liu Z, Xiong T, Meads C. Clinical effectiveness of treatment with hyperbaric oxygen for neonatal hypoxic-ischaemic encephalopathy: systematic review of Chinese literature. BMJ. 2006;333(7564):374.
12. Bond KM, Brinjikji W, Eckel LJ, Kallmes DF, McDonald RJ, Carr CM. Dentate update: imaging features of entities that affect the dentate nucleus. AJNR Am J Neuroradiol. 2017;38(8):1467-1474.
13. Agarwal A, Kanekar S, Sabat S, Thamburaj K. Metronidazole-induced cerebellar toxicity. Neurol Int. 2016;8(1):6365.
14. Zuccoli G, Pipitone N. Neuroimaging findings in acute Wernicke’s encephalopathy: review of the literature. AJR Am J Roentgenol. 2009;192(2):501-508.
15. Jung YC, Chanraud S, Sullivan EV. Neuroimaging of Wernicke’s encephalopathy and Korsakoff’s syndrome. Neuropsychol Rev. 2012;22(2):170-180.
16. Hobson-Webb LD, Roach ES, Donofrio PD. Metronidazole: newly recognized cause of autonomic neuropathy. J Child Neurol. 2006;21(5):429-431.
17. Nath Chaurasia R. Rapid onset metronidazole induced sensory neuropathy: case series and review of literature. Int J Neurorehabilitation. 2015;02:152.
18. Bradley WG, Karlsson IJ, Rassol CG. Metronidazole neuropathy. Br Med J. 1977;2(6087):610-611.
19. Evans J, Levesque D, Knowles K, Longshore R, Plummer S. Diazepam as a treatment for metronidazole toxicosis in dogs: a retrospective study of 21 cases. J Vet Intern Med. 2003;17(3):304-310.
20. Farmakiotis D, Zeluff B. Images in clinical medicine. Metronidazole-associated encephalopathy. N Engl J Med. 2016;374(15):1465.
21. Hobbs K, Stern-Nezer S, Buckwalter MS, Fischbein N, Finley Caulfield A. Metronidazole-induced encephalopathy: not always a reversible situation. Neurocrit Care. 2015;22(3):429-436.
Occupational Hazard: Disruptive Behavior in Patients
While private or other public health care organizations can refuse to care for patients who have displayed disruptive behavior (DB), the VA Response to Disruptive Behavior of Patients law (38 CFR §17.107) prohibits the Veterans Health Administration (VHA) of the Department of Veterans Affairs (VA) from refusing care to veterans who display DB.1 The VHA defines DB as any behavior that is intimidating, threatening, or dangerous or that has, or could, jeopardize the health or safety of patients, VHA staff, or others.2
VA Response to DB Law
The VA Response to Disruptive Behavior of Patients requires the VHA to provide alternative care options that minimize risk while ensuring services; for example, providing care at a different location and/or time when additional staff are available to assist and monitor the patient. This can provide a unique opportunity to capture data on DB and the results of alternative forms of caring for this population.
The reason public health care organizations refuse care to persons who display DB is clear: DBs hinder business operations, are financially taxing, and put health care workers at risk.3-10 “In 2009, the VHA spent close to $5.5 million on workers’ compensation and medical expenditures for 425 incidents–or about $130,000 per DB incident (Hodgson M, Drummond D, Van Male L. Unpublished data, 2010).” In another study, 106 of 762 nurses in 1 hospital system reported an assault by a patient, and 30 required medical attention, which resulted in a total cost of $94,156.8 From 2002 to 2013, incidents of serious workplace violence requiring days off for an injured worker to recover on average were 4 times more common in health care than in other industries.6-11 Incidents of patient violence and aggression toward staff transcend specialization; however, hospital nurses and staff from the emergency, rehabilitation and gerontology departments, psychiatric unit, and home-based services are more susceptible and vulnerable to DB incidents than are other types of employees.8,10-19
Data reported by health care staff suggest that patients rather than staff members or visitors initiate > 70% of serious physical attacks against health care workers.9,13,20-23 A 2015 study of VHA health care providers (HCPs) found that > 60% had experienced some form of DB, verbal abuse being the most prevalent, followed by sexual abuse and physical abuse.20 Of 72,000 VHA staff responding to a nationwide survey, 13% experienced, on average, ≥ 1 assault by a veteran (eg, something was thrown at them; they were pushed, kicked, slapped; or were threatened or injured by a weapon).8,21
To meet its legal obligations and deliver empathetic care, the VHA documents and analyzes data on all patients who exhibit DB. A local DB Committee (DBC) reviews the data, whether it occurs in an inpatient or outpatient setting, such as community-based outpatient clinics. Once a DB incident is reported, the DBC begins an evidence-based risk evaluation, including the option of contacting the persons who displayed or experienced the DB. Goals are to (1) prevent future DB incidents; (2) detect vulnerabilities in the environment; and (3) collaborate with HCPs and patients to provide optimal care while improving the patient/provider interactions.
Effects of Disruptive Behavior
DB has negative consequences for both patients and health care workers and results in poor evaluations of care from both groups.27-32 Aside from interfering with safe medical care, DB also impacts care for other patients by delaying access to care and increasing appointment wait times due to employee absenteeism and staff shortages.3,4,20,32,33 For HCPs, patient violence is associated with unwillingness to provide care, briefer treatment periods, and decreases in occupational satisfaction, performance, and commitment
Harmful health effects experienced by HCPs who have been victims of DB include fear, mood disorders, anxiety, all symptoms of psychological distress and posttraumatic stress disorder (PTSD).10,22,30,34-36 In a study of the impact on productivity of PTSD triggered by job-related DB, PTSD symptoms were associated with withdrawal from or minimizing encounters with patients, job turnover, and troubles with thinking
Reporting Disruptive Behavior
The literature suggests that consistent and effective DB reporting is pivotal to improving the outcome and quality of care for those displaying DB.37-39 To provide high-quality health services to veterans who display DB, the VHA must promote the management and reporting of DB. Without knowledge of the full spectrum of DB events at VHA facilities, efforts to prevent or manage DB and ensure safety may have limited impact.7,37 Reports can be used for clinical decision making to optimize staff training in delivery of quality care while assuring staff safety. More than 80% of DB incidents occur during interactions with patients, thus this is a clinical issue that can affect the outcome of patient care.8,21
Documented DB reports are used to analyze the degree, frequency, and nature of incidents, which might reveal risk factors and develop preventive efforts and training for specific hazards.8,39 Some have argued that implementing a standardized DB reporting system is a crucial first step toward minimizing hazards and improving health care.38,40,41
When DB incidents were recorded through a hospital electronic reporting system and discussed in meetings, staff reported: (1) increased awareness of DB; (2) improved ability to manage DB incidents; and (3) amplified reporting of incidents.38,41,42 These findings support similar results from studies of an intervention implemented at VA Community Living Centers (CLCs) from 2013 to 2017: Staff Training in Assisted Living Residences (STAR-VA).4,12,19 The aim of STAR-VA was to minimize challenging dementia-related DB in CLCs. The intervention initially was established to train direct-care, assisted-living staff to provide better care to older patients displaying DB. Data revealed that documentation of DBs was, the first step to ensuring staff and patient safety.18,40
VHA Reporting System
In 2013, the VA Office of Inspector General (OIG) found no standardized documentation of DB events across the VA health care system.42 Instead, DB events were documented in multiple records in various locations, including administrative and progress notes in the electronic health record (EHR), police reports, e-mails, or letters submitted to DBC chairs.42 This situation reduced administrators’ ability to consider all relevant information and render appropriate decisions in DB cases.42 In 2015, based on OIG recommendations, the VHA implemented the Disruptive Behavior Reporting System (DBRS) nationwide, which allowed all VHA staff to report DB events. The DBRS was designed to address factors likely to impede reporting and management of DB, namely, complexity of and lack of access to a central reporting system.43,44 The DBRS is currently the primary VHA tool to document DB events.
The DBRS consists of 32 questions in 5 sections relating to the (1) location and time of DB event; (2) reporter; (3) disrupter; (4) DB event details; and (5) the person who experienced (experiencer) the event. The system also provides a list of the types of DB, such as inappropriate communication, bullying and/or intimidation, verbal or written threat of physical harm, physical violence, sexual harassment, sexual assault, and property damage. The DBRS has the potential to provide useful data on DB and DB reporting, such as the typical staff entering data and the number and/or types of DB occurring.
The DBRS complements the preexisting VHA policies and committees for care of veterans who display DB.1-3,14,21,24,25 The VHA Workplace Violence Prevention Program (WVPP) required facilities to submit data on DB events through a Workplace Behavioral Risk report. Data for the report were obtained from police reports, patient safety reports, DBC records, and notes in the EHR. Following implementations of DBRS, the number of DB events per year became a part of facility performance standards.
VHA is creating novel approaches to handling DB that allow health care workers to render care in a safe and effective manner guided by documented information. For example, DBCs can recommend the use of Category I Patient Record Flags (PRFs) following documented DB, which informs staff of the potential risk of DB and provides guidance on protective methods to use when meeting with the patient.2,21,24 A survey of 140 VA hospital chiefs of staff indicated that DBC procedures were related to a decrease in the rates of assaults.1 Additionally, VA provides training for staff in techniques to promote personal safety, such as identifying signs that precede DB, using verbal deescalation, and practicing therapeutic containment.
Resistance to Reporting
Many health care employees and employers are reticent to report DBs.22,31,43,45-48 Studies suggest health care organizations can cultivate a culture that is resistant to reporting DB.49,50 This complicates the ability of the health care system to design and maintain safety protocols and safer treatment plans.3,41,51 Worldwide, < 30% of DBs are reported.47 One barrier may be that supervisors may not wish to acknowledge DBs on their units or may not provide sufficient staff time for training or reporting.31,46,47 HCPs may worry that a DB report will stigmatize patients, especially those who are elderly or have cognitive impairment, brain injury, psychological illness, or developmental disability. Patients with cognitive conditions are reportedly 20% more likely to be violent toward caregivers and providers.31 A dementia diagnosis, for example, is associated with a high likelihood for DB.30,52 More than 80% of DB events displayed by patients with dementia may go unreported.26,31,50,52
Some clinicians may attribute DB to physiologic conditions that need to be treated, not reported. However, employers can face various legal liabilities if steps are not taken to protect employees.47,51 Federal and state statutes require that organizations provide a healthy and safe employment environment for workers. This requires that employers institute reasonable protective measures, such as procedures to intervene, policies on addressing DB incidents, and/or training to minimize or deescalate DB.51,53 Also, employees may sue employers if security measures are inadequate or deficient in properly investigating current and past evidence of DB or identifying vulnerabilities in the workplace. Unwillingness to investigate DB and safety-related workplace concerns have contributed to increased workplace violence and legal liability.52,53 The mission of caring and trust is consistent with assuring a safe environment.
Training and Empathetic Care
To combat cultural resistance to reporting DBs, more and perhaps different contextual approaches to education and training may be needed that address ethical dilemmas and concerns of providers. The success of training relies on administrators supporting staff in reporting DB. Training must address providers’ conflicting beliefs and assist with identifying strategies to provide the best possible care for patients who display DB.1,38 HCPs are less likely to document a DB if they feel that administrators are creating documentation that will have negative consequences for a patient. Thus, leadership is responsible for ensuring that misconceptions are dispelled through training and other efforts and information on how reported DB data will be used is communicated through strategic channels.
Education and training must consider empathic care that attempts to understand why patients behave as they do through the information gathered.55 Empathy in health care is multifaceted: It involves comprehending a patient’s viewpoint, circumstances, and feelings and the capacity to analyze whether one is comprehending these accurately in order to demonstrate supportive care.54,55
Improving patient and staff interaction once a problematic behavior is identified is the aim of empathic care. Increasing empathic care can improve compassionate, patient-centered interactions that begin once the patient seeks care. This approach has proven to decrease DB by patients with dementia and improve their care, lessen staff problems during interactions, and increase staff morale.20 Experts call for the adoption of an interpersonal approach to patient encounters, and there is evidence that creating organizational change by moving toward compassionate care can lead to a positive impact for patients.54,55
Future Studies
There are growth opportunities in utilization of the DBRS. Analysis of the DBRS database by the VA Central Office (VACO) showed that the system is underutilized by facilities across the VA system.56 In response to this current underutilization, VACO is taking steps to close these gaps through increasing training to staff and promotion of the use of the DBRS. A 2015 pilot study of VHA providers showed that > 70% of providers had experienced a DB as defined by VHA, but only 34% of them reported their most recently experienced DB within the past 12 months.20 Thus, DBRS use must be studied within the context that patient-perpetrated DB is underreported in health care organizations.5,9,29,41,43,57,58 Studies addressing national DBRS utilization patterns and the cost associated with implementing the DBRS also are needed. One study suggests that there is an association between measures of facility complexity and staff perceptions of safety, which should be considered in analyzing DBRS usage.57 Studies addressing the role of the DBRS and misconceptions that the tool may represent a punitive tool also are needed. VHA should consider how the attribution “disruptive behavior” assigns a negative connotation and leads HCPs to avoid using the DBRS. Additionally, DB reporting may increase when HCPs understand that DB reporting is part of the comprehensive, consultative strategy to provide the best care to patients.
Conclusion
Accurate reporting of DB events enables the development of strategies for multidisciplinary teams to work together to minimize hazards and to provide interventions that provide for the safe delivery of health care to all patients. Improving reporting ensures there is an accurate representation of how disruptive events impact care provided within a facility—and what types of variables may be associated with increased risk for these types of events.
Additionally, ensuring that reporting is maximized also provides the VHA with opportunities for DBCs to offer evidence-based risk assessment of violence and consultation to staff members who may benefit from improved competencies in working with patients who display DB. These potential improvements are consistent with the VHA I CARE values and will provide data that can inform recommendations for health care in other agencies/health care organizations.
Acknowledgments
This work was supported by the Center of Innovation on Disability and Rehabilitation Research (CINDRR) of the Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs.
1. Hodgson MJ, Mohr DC, Drummond DJ, Bell M, Van Male L. Managing disruptive patients in health care: necessary solutions to a difficult problem. Am J Ind Med. 2012;55(11):1009-1017.
2. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 2010-053. Patient Record Flags. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2341 Published December 3, 2010. Accessed March 29, 2019.
3. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 2012-026. Sexual Assaults and Other Defined Public Safety Incidents in VHA Facilities. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2797. Published September 27, 2012. Accessed March 29, 2019.
4. Curyto KJ, McCurry SM, Luci K, Karlin BE, Teri L, Karel MJ. Managing challenging behaviors of dementia in veterans: identifying and changing activators and consequences using STAR-VA. J Gerontol Nurs. 2017;43(2):33-43.
5. Speroni KG, Fitch T, Dawson E, Dugan L, Atherton M. Incidence and cost of nurse workplace violence perpetrated by hospital patients or patient visitors. J Emerg Nurs. 2014;40(3):218-228.
6. Phillips JP. Workplace violence against health care workers in the United States. NEJM. 2016;374(17):1661-1669.
7. Janocha JA, Smith RT. Workplace safety and health in the health care and social assistance industry, 2003–07. https://www.bls.gov/opub/mlr/cwc/workplace-safety-and-health-in-the-health-care-and-social-assistance-industry-2003-07.pdf. Published August 30, 2010. Accessed February 19, 2019.
8. US Department of Labor, Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. https://www.osha.gov/Publications/OSHA3826.pdf. Published December 2015. Accessed February 19, 2019.
9. US Department of Labor, Occupational Safety and Health Administration. Prevention of Workplace Violence in Healthcare and Social Assistance. Occupational Safety and Health Administration, https://www.govinfo.gov/content/pkg/FR-2016-12-07/pdf/2016-29197.pdf. Accessed January 20, 2017.
10. Gerberich SG, Church TR, McGovern PM, et al. An epidemiological study of the magnitude and consequences of work related violence: the Minnesota Nurses’ Study. Occup Environ Med. 2004;61(6):495-503.
11. Sherman MF, Gershon RRM, Samar SM, Pearson JM, Canton AN, Damsky MR. Safety factors predictive of job satisfaction and job retention among home healthcare aides. J Occup Environ Med. 2008;50(12):1430-1441.
12. Karel MJ, Teri L, McConnell E, Visnic S, Karlin BE. Effectiveness of expanded implementation of STAR-VA for managing dementia-related behaviors among veterans. Gerontologist. 2016;56(1):126-134.
13. US Department of Labor, Bureau of Labor Statistics. Nonfatal occupational injuries and illnesses requiring days away from work. https://www.bls.gov/news.release/archives/osh2_11192015.htm. Published November 19, 2015.
14. Beech B, Leather P. Workplace violence in the health care sector: A review of staff training and integration of training evaluation models. Aggression Violent Behav. 2006;11(1):27-43.
15. Campbell CL, McCoy S, Burg MA, Hoffman N. Enhancing home care staff safety through reducing client aggression and violence in noninstitutional care settings: a systematic review. Home Health Care Manage Pract. 2014;26(1):3-10.
16. Gallant-Roman MA. Strategies and tools to reduce workplace violence. AAOHNJ. 2008;56(11):449-454.
17. Weinberger LE, Sreenivasan S, Smee DE, McGuire J, Garrick T. Balancing safety against obstruction to health care access: an examination of behavioral flags in the VA health care system. J Threat Assess Manage. 2018;5(1):35-41.
18. Elbogen EB, Johnson SC, Wagner HR, et al. Protective factors and risk modification of violence in Iraq and Afghanistan war veterans. J Clin Psychiatry. 2012;73(6):e767-e773.
19. Karlin BE, Visnic S, McGee JS, Teri L. Results from the multisite implementation of STAR-VA: a multicomponent psychosocial intervention for managing challenging dementia-related behaviors of veterans. Psychol Serv. 2014;11(2):200-208.
20. Semeah LM, Campbell CL, Cowper DC, Peet AC. Serving our homeless veterans: patient perpetrated violence as a barrier to health care access. J Pub Nonprofit Aff. 2017;3(2):223-234.
21. Hodgson MJ, Reed R, Craig T, et al. Violence in healthcare facilities: lessons from the Veterans Health Administration. J Occup Environ Med. 2004;46(11):1158-1165.
22. Farrell GA, Bobrowski C, Bobrowski P. Scoping workplace aggression in nursing: findings from an Australian study. J Adv Nurs. 2006;55(6):778-787.
23. Barling J, Rogers AG, Kelloway EK. Behind closed doors: in-home workers’ experience of sexual harassment and workplace violence. J Occup Health Psychol. 2001;6(3):255-269.
24. Pompeii LA, Schoenfisch AL, Lipscomb HJ, Dement JM, Smith CD, Upadhyaya M. Physical assault, physical threat, and verbal abuse perpetrated against hospital workers by patients or visitors in six U.S. hospitals. Am J Ind Med. 2015;58(11):1194-1204.
25. Sippel LM, Mota NP, Kachadourian LK, et al. The burden of hostility in U.S. veterans: results from the National Health and Resilience in Veterans Study. Psychiatry Res. 2016;243(suppl C):421-430.
26. Campbell C. Patient Violence and Aggression in Non-Institutional Health Care Settings: Predictors of Reporting By Healthcare Providers [doctoral dissertation]. Orlando: University of Central Florida; 2016.
27. Galinsky T, Feng HA, Streit J, et al. Risk factors associated with patient assaults of home healthcare workers. Rehabil Nurs. 2010;35(5):206-215.
28. Campbell CL. Incident reporting by health-care workers in noninstitutional care settings. Trauma, Violence Abuse. 2017;18(4):445-456.
29. Arnetz JE, Arnetz BB. Violence towards health care staff and possible effects on the quality of patient care. Soc Sci Med. 2001;52(3):417-427.
30. Gates D, Fitzwater E, Succop P. Relationships of stressors, strain, and anger to caregiver assaults. Issues Ment Health Nurs. 2003;24(8):775-793.
31. Brillhart B, Kruse B, Heard L. Safety concerns for rehabilitation nurses in home care. Rehabil Nurs. 2004;29(6):227-229.
32. Taylor H. Patient violence against clinicians: managing the risk. Innov Clin Neurosci. 2013;10(3):40-42.
33. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. The Joint Commission releases results of surveys of the VA health care system. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=2808. Updated August 5, 2014. Accessed February 19, 2019.
34. Büssing A, Höge T. Aggression and violence against home care workers. J Occup Health Psychol. 2004;9(3):206-219.
35. Geiger-Brown J, Muntaner C, McPhaul K, Lipscomb J, Trinkoff A. Abuse and violence during home care work as predictor of worker depression. Home Health Care Serv Q. 2007;26(1):59-77.
36. Gates DM, Gillespie GL, Succop P. Violence against nurses and its impact on stress and productivity. Nurs Econ. 2011;29(2):59-66.
37. Petterson IL, Arnetz BB. Psychosocial stressors and well-being in health care workers: the impact of an intervention program. Soc Sci Med. 1998;47(11):1763-1772.
38. Arnetz JE, Arnetz BB. Implementation and evaluation of a practical intervention programme for dealing with violence towards health care workers. J Adv Nurs. 2000;31(3):668-680.
39. Arnetz JE, Hamblin L, Russell J, et al. Preventing patient-to-worker violence in hospitals: outcome of a randomized controlled intervention. J Occup Environ Med. 2017;59(1):18-27.
40. Elbogen EB, Tomkins AJ, Pothuloori AP, Scalora MJ. Documentation of violence risk information in psychiatric hospital patient charts: an empirical examination. J Am Acad Psychiatry Law. 2003;31(1):58-64.
41. Winsvold Prang I, Jelson-Jorgensen LP. Should I report? A qualitative study of barriers to incident reporting among nurses working in nursing homes. Geriatr Nurs. 2014;35(6):441-447.
42. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection: management of disruptive patient behavior at VA medical facilities. Report No. 11-02585-129. https://www.va.gov/oig/pubs/VAOIG-11-02585-129.pdf. Published Mrach 7, 2013. Accessed February 21, 2019.
43. Lipscomb J, London M. Not Part of the Job: How to Take a Stand Against Violence in the Work Setting. Silver Spring, MD: American Nurses Association; 2015.
44. May DD, Grubbs LM. The extent, nature, and precipitating factors of nurse assault among three groups of registered nurses in a regional medical center. J Emerg Nurs. 2002;28(1):11-17.
45. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57(5):460-477.
46. Brennan C, Worrall-Davies A, McMillan D, Gilbody S, House A. The hospital anxiety and depression scale: a diagnostic meta-analysis of case-finding ability. J Psychosom Res. 2010;69(4):371-378.
47. McPhaul K, Lipscomb J, Johnson J. Assessing risk for violence on home health visits. Home Healthc Nurse. 2010;28(5):278-289.
48. McPhaul KM, London M, Murrett K, Flannery K, Rosen J, Lipscomb J. Environmental evaluation for workplace violence in healthcare and social services. J Safety Res. 2008;39(2):237-250.
49. Kelly JA, Somlai AM, DiFranceisco WJ, et al. Bridging the gap between the science and service of HIV prevention: transferring effective research-based HIV prevention interventions to community AIDS service providers. Am J Public Health. 2000;90(7):1082-1088.
50. Pawlin S. Reporting violence. Emerg Nurse. 2008;16(4):16-21.
51. Brakel SJ. Legal liability and workplace violence. J Am Acad Psychiatry Law. 1998;26(4):553-562.
52. Neuman JH, Baron RA. Workplace violence and workplace aggression: evidence concerning specific forms, potential causes, and preferred targets. J Manage. 1998;24(3):391-419.53. Ferns T, Chojnacka I. Angels and swingers, matrons and sinners: nursing stereotypes. Br J Nurs. 2005;14(19):1028-1032.
54. Mercer SW, Reynolds WJ. Empathy and quality of care. Br J Gen Pract 2002;52(suppl):S9-S12.
55. Lee TH. An Epidemic of Empathy in Healthcare: How to Deliver Compassionate, Connected Patient Care That Creates a Competitive Advantage. Columbus, OH: McGraw-Hill Education; 2015.
56. US Department of Veterans Affairs, Veterans Health Administrastion. Veterans Health Administration workplace violence prevention program (WVPP): disruptive behavior reporting system utilization report. Published 2017. https://vaww.portal2.va.gov/sites/wvpp/Shared%20Documents/DBRS%20Utilization%20Reports/FY2017%20DBRS%20Quarterly%20Utilization%20Report%20(Quarter%201).pdf. [Source not verified.]
57. Campbell CL, Burg, MA, Gammonley D. Measures for incident reporting of patient violence and aggression towards healthcare providers: a systematic review. Aggression Violent Behav. 2015;25(part B):314-322.
58. Carney PT, West P, Neily J, Mills PD, Bagian JP. The effect of facility complexity on perceptions of safety climate in the operating room: size matters. Am J Med Qual. 2010;25(6):457-461.
While private or other public health care organizations can refuse to care for patients who have displayed disruptive behavior (DB), the VA Response to Disruptive Behavior of Patients law (38 CFR §17.107) prohibits the Veterans Health Administration (VHA) of the Department of Veterans Affairs (VA) from refusing care to veterans who display DB.1 The VHA defines DB as any behavior that is intimidating, threatening, or dangerous or that has, or could, jeopardize the health or safety of patients, VHA staff, or others.2
VA Response to DB Law
The VA Response to Disruptive Behavior of Patients requires the VHA to provide alternative care options that minimize risk while ensuring services; for example, providing care at a different location and/or time when additional staff are available to assist and monitor the patient. This can provide a unique opportunity to capture data on DB and the results of alternative forms of caring for this population.
The reason public health care organizations refuse care to persons who display DB is clear: DBs hinder business operations, are financially taxing, and put health care workers at risk.3-10 “In 2009, the VHA spent close to $5.5 million on workers’ compensation and medical expenditures for 425 incidents–or about $130,000 per DB incident (Hodgson M, Drummond D, Van Male L. Unpublished data, 2010).” In another study, 106 of 762 nurses in 1 hospital system reported an assault by a patient, and 30 required medical attention, which resulted in a total cost of $94,156.8 From 2002 to 2013, incidents of serious workplace violence requiring days off for an injured worker to recover on average were 4 times more common in health care than in other industries.6-11 Incidents of patient violence and aggression toward staff transcend specialization; however, hospital nurses and staff from the emergency, rehabilitation and gerontology departments, psychiatric unit, and home-based services are more susceptible and vulnerable to DB incidents than are other types of employees.8,10-19
Data reported by health care staff suggest that patients rather than staff members or visitors initiate > 70% of serious physical attacks against health care workers.9,13,20-23 A 2015 study of VHA health care providers (HCPs) found that > 60% had experienced some form of DB, verbal abuse being the most prevalent, followed by sexual abuse and physical abuse.20 Of 72,000 VHA staff responding to a nationwide survey, 13% experienced, on average, ≥ 1 assault by a veteran (eg, something was thrown at them; they were pushed, kicked, slapped; or were threatened or injured by a weapon).8,21
To meet its legal obligations and deliver empathetic care, the VHA documents and analyzes data on all patients who exhibit DB. A local DB Committee (DBC) reviews the data, whether it occurs in an inpatient or outpatient setting, such as community-based outpatient clinics. Once a DB incident is reported, the DBC begins an evidence-based risk evaluation, including the option of contacting the persons who displayed or experienced the DB. Goals are to (1) prevent future DB incidents; (2) detect vulnerabilities in the environment; and (3) collaborate with HCPs and patients to provide optimal care while improving the patient/provider interactions.
Effects of Disruptive Behavior
DB has negative consequences for both patients and health care workers and results in poor evaluations of care from both groups.27-32 Aside from interfering with safe medical care, DB also impacts care for other patients by delaying access to care and increasing appointment wait times due to employee absenteeism and staff shortages.3,4,20,32,33 For HCPs, patient violence is associated with unwillingness to provide care, briefer treatment periods, and decreases in occupational satisfaction, performance, and commitment
Harmful health effects experienced by HCPs who have been victims of DB include fear, mood disorders, anxiety, all symptoms of psychological distress and posttraumatic stress disorder (PTSD).10,22,30,34-36 In a study of the impact on productivity of PTSD triggered by job-related DB, PTSD symptoms were associated with withdrawal from or minimizing encounters with patients, job turnover, and troubles with thinking
Reporting Disruptive Behavior
The literature suggests that consistent and effective DB reporting is pivotal to improving the outcome and quality of care for those displaying DB.37-39 To provide high-quality health services to veterans who display DB, the VHA must promote the management and reporting of DB. Without knowledge of the full spectrum of DB events at VHA facilities, efforts to prevent or manage DB and ensure safety may have limited impact.7,37 Reports can be used for clinical decision making to optimize staff training in delivery of quality care while assuring staff safety. More than 80% of DB incidents occur during interactions with patients, thus this is a clinical issue that can affect the outcome of patient care.8,21
Documented DB reports are used to analyze the degree, frequency, and nature of incidents, which might reveal risk factors and develop preventive efforts and training for specific hazards.8,39 Some have argued that implementing a standardized DB reporting system is a crucial first step toward minimizing hazards and improving health care.38,40,41
When DB incidents were recorded through a hospital electronic reporting system and discussed in meetings, staff reported: (1) increased awareness of DB; (2) improved ability to manage DB incidents; and (3) amplified reporting of incidents.38,41,42 These findings support similar results from studies of an intervention implemented at VA Community Living Centers (CLCs) from 2013 to 2017: Staff Training in Assisted Living Residences (STAR-VA).4,12,19 The aim of STAR-VA was to minimize challenging dementia-related DB in CLCs. The intervention initially was established to train direct-care, assisted-living staff to provide better care to older patients displaying DB. Data revealed that documentation of DBs was, the first step to ensuring staff and patient safety.18,40
VHA Reporting System
In 2013, the VA Office of Inspector General (OIG) found no standardized documentation of DB events across the VA health care system.42 Instead, DB events were documented in multiple records in various locations, including administrative and progress notes in the electronic health record (EHR), police reports, e-mails, or letters submitted to DBC chairs.42 This situation reduced administrators’ ability to consider all relevant information and render appropriate decisions in DB cases.42 In 2015, based on OIG recommendations, the VHA implemented the Disruptive Behavior Reporting System (DBRS) nationwide, which allowed all VHA staff to report DB events. The DBRS was designed to address factors likely to impede reporting and management of DB, namely, complexity of and lack of access to a central reporting system.43,44 The DBRS is currently the primary VHA tool to document DB events.
The DBRS consists of 32 questions in 5 sections relating to the (1) location and time of DB event; (2) reporter; (3) disrupter; (4) DB event details; and (5) the person who experienced (experiencer) the event. The system also provides a list of the types of DB, such as inappropriate communication, bullying and/or intimidation, verbal or written threat of physical harm, physical violence, sexual harassment, sexual assault, and property damage. The DBRS has the potential to provide useful data on DB and DB reporting, such as the typical staff entering data and the number and/or types of DB occurring.
The DBRS complements the preexisting VHA policies and committees for care of veterans who display DB.1-3,14,21,24,25 The VHA Workplace Violence Prevention Program (WVPP) required facilities to submit data on DB events through a Workplace Behavioral Risk report. Data for the report were obtained from police reports, patient safety reports, DBC records, and notes in the EHR. Following implementations of DBRS, the number of DB events per year became a part of facility performance standards.
VHA is creating novel approaches to handling DB that allow health care workers to render care in a safe and effective manner guided by documented information. For example, DBCs can recommend the use of Category I Patient Record Flags (PRFs) following documented DB, which informs staff of the potential risk of DB and provides guidance on protective methods to use when meeting with the patient.2,21,24 A survey of 140 VA hospital chiefs of staff indicated that DBC procedures were related to a decrease in the rates of assaults.1 Additionally, VA provides training for staff in techniques to promote personal safety, such as identifying signs that precede DB, using verbal deescalation, and practicing therapeutic containment.
Resistance to Reporting
Many health care employees and employers are reticent to report DBs.22,31,43,45-48 Studies suggest health care organizations can cultivate a culture that is resistant to reporting DB.49,50 This complicates the ability of the health care system to design and maintain safety protocols and safer treatment plans.3,41,51 Worldwide, < 30% of DBs are reported.47 One barrier may be that supervisors may not wish to acknowledge DBs on their units or may not provide sufficient staff time for training or reporting.31,46,47 HCPs may worry that a DB report will stigmatize patients, especially those who are elderly or have cognitive impairment, brain injury, psychological illness, or developmental disability. Patients with cognitive conditions are reportedly 20% more likely to be violent toward caregivers and providers.31 A dementia diagnosis, for example, is associated with a high likelihood for DB.30,52 More than 80% of DB events displayed by patients with dementia may go unreported.26,31,50,52
Some clinicians may attribute DB to physiologic conditions that need to be treated, not reported. However, employers can face various legal liabilities if steps are not taken to protect employees.47,51 Federal and state statutes require that organizations provide a healthy and safe employment environment for workers. This requires that employers institute reasonable protective measures, such as procedures to intervene, policies on addressing DB incidents, and/or training to minimize or deescalate DB.51,53 Also, employees may sue employers if security measures are inadequate or deficient in properly investigating current and past evidence of DB or identifying vulnerabilities in the workplace. Unwillingness to investigate DB and safety-related workplace concerns have contributed to increased workplace violence and legal liability.52,53 The mission of caring and trust is consistent with assuring a safe environment.
Training and Empathetic Care
To combat cultural resistance to reporting DBs, more and perhaps different contextual approaches to education and training may be needed that address ethical dilemmas and concerns of providers. The success of training relies on administrators supporting staff in reporting DB. Training must address providers’ conflicting beliefs and assist with identifying strategies to provide the best possible care for patients who display DB.1,38 HCPs are less likely to document a DB if they feel that administrators are creating documentation that will have negative consequences for a patient. Thus, leadership is responsible for ensuring that misconceptions are dispelled through training and other efforts and information on how reported DB data will be used is communicated through strategic channels.
Education and training must consider empathic care that attempts to understand why patients behave as they do through the information gathered.55 Empathy in health care is multifaceted: It involves comprehending a patient’s viewpoint, circumstances, and feelings and the capacity to analyze whether one is comprehending these accurately in order to demonstrate supportive care.54,55
Improving patient and staff interaction once a problematic behavior is identified is the aim of empathic care. Increasing empathic care can improve compassionate, patient-centered interactions that begin once the patient seeks care. This approach has proven to decrease DB by patients with dementia and improve their care, lessen staff problems during interactions, and increase staff morale.20 Experts call for the adoption of an interpersonal approach to patient encounters, and there is evidence that creating organizational change by moving toward compassionate care can lead to a positive impact for patients.54,55
Future Studies
There are growth opportunities in utilization of the DBRS. Analysis of the DBRS database by the VA Central Office (VACO) showed that the system is underutilized by facilities across the VA system.56 In response to this current underutilization, VACO is taking steps to close these gaps through increasing training to staff and promotion of the use of the DBRS. A 2015 pilot study of VHA providers showed that > 70% of providers had experienced a DB as defined by VHA, but only 34% of them reported their most recently experienced DB within the past 12 months.20 Thus, DBRS use must be studied within the context that patient-perpetrated DB is underreported in health care organizations.5,9,29,41,43,57,58 Studies addressing national DBRS utilization patterns and the cost associated with implementing the DBRS also are needed. One study suggests that there is an association between measures of facility complexity and staff perceptions of safety, which should be considered in analyzing DBRS usage.57 Studies addressing the role of the DBRS and misconceptions that the tool may represent a punitive tool also are needed. VHA should consider how the attribution “disruptive behavior” assigns a negative connotation and leads HCPs to avoid using the DBRS. Additionally, DB reporting may increase when HCPs understand that DB reporting is part of the comprehensive, consultative strategy to provide the best care to patients.
Conclusion
Accurate reporting of DB events enables the development of strategies for multidisciplinary teams to work together to minimize hazards and to provide interventions that provide for the safe delivery of health care to all patients. Improving reporting ensures there is an accurate representation of how disruptive events impact care provided within a facility—and what types of variables may be associated with increased risk for these types of events.
Additionally, ensuring that reporting is maximized also provides the VHA with opportunities for DBCs to offer evidence-based risk assessment of violence and consultation to staff members who may benefit from improved competencies in working with patients who display DB. These potential improvements are consistent with the VHA I CARE values and will provide data that can inform recommendations for health care in other agencies/health care organizations.
Acknowledgments
This work was supported by the Center of Innovation on Disability and Rehabilitation Research (CINDRR) of the Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs.
While private or other public health care organizations can refuse to care for patients who have displayed disruptive behavior (DB), the VA Response to Disruptive Behavior of Patients law (38 CFR §17.107) prohibits the Veterans Health Administration (VHA) of the Department of Veterans Affairs (VA) from refusing care to veterans who display DB.1 The VHA defines DB as any behavior that is intimidating, threatening, or dangerous or that has, or could, jeopardize the health or safety of patients, VHA staff, or others.2
VA Response to DB Law
The VA Response to Disruptive Behavior of Patients requires the VHA to provide alternative care options that minimize risk while ensuring services; for example, providing care at a different location and/or time when additional staff are available to assist and monitor the patient. This can provide a unique opportunity to capture data on DB and the results of alternative forms of caring for this population.
The reason public health care organizations refuse care to persons who display DB is clear: DBs hinder business operations, are financially taxing, and put health care workers at risk.3-10 “In 2009, the VHA spent close to $5.5 million on workers’ compensation and medical expenditures for 425 incidents–or about $130,000 per DB incident (Hodgson M, Drummond D, Van Male L. Unpublished data, 2010).” In another study, 106 of 762 nurses in 1 hospital system reported an assault by a patient, and 30 required medical attention, which resulted in a total cost of $94,156.8 From 2002 to 2013, incidents of serious workplace violence requiring days off for an injured worker to recover on average were 4 times more common in health care than in other industries.6-11 Incidents of patient violence and aggression toward staff transcend specialization; however, hospital nurses and staff from the emergency, rehabilitation and gerontology departments, psychiatric unit, and home-based services are more susceptible and vulnerable to DB incidents than are other types of employees.8,10-19
Data reported by health care staff suggest that patients rather than staff members or visitors initiate > 70% of serious physical attacks against health care workers.9,13,20-23 A 2015 study of VHA health care providers (HCPs) found that > 60% had experienced some form of DB, verbal abuse being the most prevalent, followed by sexual abuse and physical abuse.20 Of 72,000 VHA staff responding to a nationwide survey, 13% experienced, on average, ≥ 1 assault by a veteran (eg, something was thrown at them; they were pushed, kicked, slapped; or were threatened or injured by a weapon).8,21
To meet its legal obligations and deliver empathetic care, the VHA documents and analyzes data on all patients who exhibit DB. A local DB Committee (DBC) reviews the data, whether it occurs in an inpatient or outpatient setting, such as community-based outpatient clinics. Once a DB incident is reported, the DBC begins an evidence-based risk evaluation, including the option of contacting the persons who displayed or experienced the DB. Goals are to (1) prevent future DB incidents; (2) detect vulnerabilities in the environment; and (3) collaborate with HCPs and patients to provide optimal care while improving the patient/provider interactions.
Effects of Disruptive Behavior
DB has negative consequences for both patients and health care workers and results in poor evaluations of care from both groups.27-32 Aside from interfering with safe medical care, DB also impacts care for other patients by delaying access to care and increasing appointment wait times due to employee absenteeism and staff shortages.3,4,20,32,33 For HCPs, patient violence is associated with unwillingness to provide care, briefer treatment periods, and decreases in occupational satisfaction, performance, and commitment
Harmful health effects experienced by HCPs who have been victims of DB include fear, mood disorders, anxiety, all symptoms of psychological distress and posttraumatic stress disorder (PTSD).10,22,30,34-36 In a study of the impact on productivity of PTSD triggered by job-related DB, PTSD symptoms were associated with withdrawal from or minimizing encounters with patients, job turnover, and troubles with thinking
Reporting Disruptive Behavior
The literature suggests that consistent and effective DB reporting is pivotal to improving the outcome and quality of care for those displaying DB.37-39 To provide high-quality health services to veterans who display DB, the VHA must promote the management and reporting of DB. Without knowledge of the full spectrum of DB events at VHA facilities, efforts to prevent or manage DB and ensure safety may have limited impact.7,37 Reports can be used for clinical decision making to optimize staff training in delivery of quality care while assuring staff safety. More than 80% of DB incidents occur during interactions with patients, thus this is a clinical issue that can affect the outcome of patient care.8,21
Documented DB reports are used to analyze the degree, frequency, and nature of incidents, which might reveal risk factors and develop preventive efforts and training for specific hazards.8,39 Some have argued that implementing a standardized DB reporting system is a crucial first step toward minimizing hazards and improving health care.38,40,41
When DB incidents were recorded through a hospital electronic reporting system and discussed in meetings, staff reported: (1) increased awareness of DB; (2) improved ability to manage DB incidents; and (3) amplified reporting of incidents.38,41,42 These findings support similar results from studies of an intervention implemented at VA Community Living Centers (CLCs) from 2013 to 2017: Staff Training in Assisted Living Residences (STAR-VA).4,12,19 The aim of STAR-VA was to minimize challenging dementia-related DB in CLCs. The intervention initially was established to train direct-care, assisted-living staff to provide better care to older patients displaying DB. Data revealed that documentation of DBs was, the first step to ensuring staff and patient safety.18,40
VHA Reporting System
In 2013, the VA Office of Inspector General (OIG) found no standardized documentation of DB events across the VA health care system.42 Instead, DB events were documented in multiple records in various locations, including administrative and progress notes in the electronic health record (EHR), police reports, e-mails, or letters submitted to DBC chairs.42 This situation reduced administrators’ ability to consider all relevant information and render appropriate decisions in DB cases.42 In 2015, based on OIG recommendations, the VHA implemented the Disruptive Behavior Reporting System (DBRS) nationwide, which allowed all VHA staff to report DB events. The DBRS was designed to address factors likely to impede reporting and management of DB, namely, complexity of and lack of access to a central reporting system.43,44 The DBRS is currently the primary VHA tool to document DB events.
The DBRS consists of 32 questions in 5 sections relating to the (1) location and time of DB event; (2) reporter; (3) disrupter; (4) DB event details; and (5) the person who experienced (experiencer) the event. The system also provides a list of the types of DB, such as inappropriate communication, bullying and/or intimidation, verbal or written threat of physical harm, physical violence, sexual harassment, sexual assault, and property damage. The DBRS has the potential to provide useful data on DB and DB reporting, such as the typical staff entering data and the number and/or types of DB occurring.
The DBRS complements the preexisting VHA policies and committees for care of veterans who display DB.1-3,14,21,24,25 The VHA Workplace Violence Prevention Program (WVPP) required facilities to submit data on DB events through a Workplace Behavioral Risk report. Data for the report were obtained from police reports, patient safety reports, DBC records, and notes in the EHR. Following implementations of DBRS, the number of DB events per year became a part of facility performance standards.
VHA is creating novel approaches to handling DB that allow health care workers to render care in a safe and effective manner guided by documented information. For example, DBCs can recommend the use of Category I Patient Record Flags (PRFs) following documented DB, which informs staff of the potential risk of DB and provides guidance on protective methods to use when meeting with the patient.2,21,24 A survey of 140 VA hospital chiefs of staff indicated that DBC procedures were related to a decrease in the rates of assaults.1 Additionally, VA provides training for staff in techniques to promote personal safety, such as identifying signs that precede DB, using verbal deescalation, and practicing therapeutic containment.
Resistance to Reporting
Many health care employees and employers are reticent to report DBs.22,31,43,45-48 Studies suggest health care organizations can cultivate a culture that is resistant to reporting DB.49,50 This complicates the ability of the health care system to design and maintain safety protocols and safer treatment plans.3,41,51 Worldwide, < 30% of DBs are reported.47 One barrier may be that supervisors may not wish to acknowledge DBs on their units or may not provide sufficient staff time for training or reporting.31,46,47 HCPs may worry that a DB report will stigmatize patients, especially those who are elderly or have cognitive impairment, brain injury, psychological illness, or developmental disability. Patients with cognitive conditions are reportedly 20% more likely to be violent toward caregivers and providers.31 A dementia diagnosis, for example, is associated with a high likelihood for DB.30,52 More than 80% of DB events displayed by patients with dementia may go unreported.26,31,50,52
Some clinicians may attribute DB to physiologic conditions that need to be treated, not reported. However, employers can face various legal liabilities if steps are not taken to protect employees.47,51 Federal and state statutes require that organizations provide a healthy and safe employment environment for workers. This requires that employers institute reasonable protective measures, such as procedures to intervene, policies on addressing DB incidents, and/or training to minimize or deescalate DB.51,53 Also, employees may sue employers if security measures are inadequate or deficient in properly investigating current and past evidence of DB or identifying vulnerabilities in the workplace. Unwillingness to investigate DB and safety-related workplace concerns have contributed to increased workplace violence and legal liability.52,53 The mission of caring and trust is consistent with assuring a safe environment.
Training and Empathetic Care
To combat cultural resistance to reporting DBs, more and perhaps different contextual approaches to education and training may be needed that address ethical dilemmas and concerns of providers. The success of training relies on administrators supporting staff in reporting DB. Training must address providers’ conflicting beliefs and assist with identifying strategies to provide the best possible care for patients who display DB.1,38 HCPs are less likely to document a DB if they feel that administrators are creating documentation that will have negative consequences for a patient. Thus, leadership is responsible for ensuring that misconceptions are dispelled through training and other efforts and information on how reported DB data will be used is communicated through strategic channels.
Education and training must consider empathic care that attempts to understand why patients behave as they do through the information gathered.55 Empathy in health care is multifaceted: It involves comprehending a patient’s viewpoint, circumstances, and feelings and the capacity to analyze whether one is comprehending these accurately in order to demonstrate supportive care.54,55
Improving patient and staff interaction once a problematic behavior is identified is the aim of empathic care. Increasing empathic care can improve compassionate, patient-centered interactions that begin once the patient seeks care. This approach has proven to decrease DB by patients with dementia and improve their care, lessen staff problems during interactions, and increase staff morale.20 Experts call for the adoption of an interpersonal approach to patient encounters, and there is evidence that creating organizational change by moving toward compassionate care can lead to a positive impact for patients.54,55
Future Studies
There are growth opportunities in utilization of the DBRS. Analysis of the DBRS database by the VA Central Office (VACO) showed that the system is underutilized by facilities across the VA system.56 In response to this current underutilization, VACO is taking steps to close these gaps through increasing training to staff and promotion of the use of the DBRS. A 2015 pilot study of VHA providers showed that > 70% of providers had experienced a DB as defined by VHA, but only 34% of them reported their most recently experienced DB within the past 12 months.20 Thus, DBRS use must be studied within the context that patient-perpetrated DB is underreported in health care organizations.5,9,29,41,43,57,58 Studies addressing national DBRS utilization patterns and the cost associated with implementing the DBRS also are needed. One study suggests that there is an association between measures of facility complexity and staff perceptions of safety, which should be considered in analyzing DBRS usage.57 Studies addressing the role of the DBRS and misconceptions that the tool may represent a punitive tool also are needed. VHA should consider how the attribution “disruptive behavior” assigns a negative connotation and leads HCPs to avoid using the DBRS. Additionally, DB reporting may increase when HCPs understand that DB reporting is part of the comprehensive, consultative strategy to provide the best care to patients.
Conclusion
Accurate reporting of DB events enables the development of strategies for multidisciplinary teams to work together to minimize hazards and to provide interventions that provide for the safe delivery of health care to all patients. Improving reporting ensures there is an accurate representation of how disruptive events impact care provided within a facility—and what types of variables may be associated with increased risk for these types of events.
Additionally, ensuring that reporting is maximized also provides the VHA with opportunities for DBCs to offer evidence-based risk assessment of violence and consultation to staff members who may benefit from improved competencies in working with patients who display DB. These potential improvements are consistent with the VHA I CARE values and will provide data that can inform recommendations for health care in other agencies/health care organizations.
Acknowledgments
This work was supported by the Center of Innovation on Disability and Rehabilitation Research (CINDRR) of the Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs.
1. Hodgson MJ, Mohr DC, Drummond DJ, Bell M, Van Male L. Managing disruptive patients in health care: necessary solutions to a difficult problem. Am J Ind Med. 2012;55(11):1009-1017.
2. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 2010-053. Patient Record Flags. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2341 Published December 3, 2010. Accessed March 29, 2019.
3. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 2012-026. Sexual Assaults and Other Defined Public Safety Incidents in VHA Facilities. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2797. Published September 27, 2012. Accessed March 29, 2019.
4. Curyto KJ, McCurry SM, Luci K, Karlin BE, Teri L, Karel MJ. Managing challenging behaviors of dementia in veterans: identifying and changing activators and consequences using STAR-VA. J Gerontol Nurs. 2017;43(2):33-43.
5. Speroni KG, Fitch T, Dawson E, Dugan L, Atherton M. Incidence and cost of nurse workplace violence perpetrated by hospital patients or patient visitors. J Emerg Nurs. 2014;40(3):218-228.
6. Phillips JP. Workplace violence against health care workers in the United States. NEJM. 2016;374(17):1661-1669.
7. Janocha JA, Smith RT. Workplace safety and health in the health care and social assistance industry, 2003–07. https://www.bls.gov/opub/mlr/cwc/workplace-safety-and-health-in-the-health-care-and-social-assistance-industry-2003-07.pdf. Published August 30, 2010. Accessed February 19, 2019.
8. US Department of Labor, Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. https://www.osha.gov/Publications/OSHA3826.pdf. Published December 2015. Accessed February 19, 2019.
9. US Department of Labor, Occupational Safety and Health Administration. Prevention of Workplace Violence in Healthcare and Social Assistance. Occupational Safety and Health Administration, https://www.govinfo.gov/content/pkg/FR-2016-12-07/pdf/2016-29197.pdf. Accessed January 20, 2017.
10. Gerberich SG, Church TR, McGovern PM, et al. An epidemiological study of the magnitude and consequences of work related violence: the Minnesota Nurses’ Study. Occup Environ Med. 2004;61(6):495-503.
11. Sherman MF, Gershon RRM, Samar SM, Pearson JM, Canton AN, Damsky MR. Safety factors predictive of job satisfaction and job retention among home healthcare aides. J Occup Environ Med. 2008;50(12):1430-1441.
12. Karel MJ, Teri L, McConnell E, Visnic S, Karlin BE. Effectiveness of expanded implementation of STAR-VA for managing dementia-related behaviors among veterans. Gerontologist. 2016;56(1):126-134.
13. US Department of Labor, Bureau of Labor Statistics. Nonfatal occupational injuries and illnesses requiring days away from work. https://www.bls.gov/news.release/archives/osh2_11192015.htm. Published November 19, 2015.
14. Beech B, Leather P. Workplace violence in the health care sector: A review of staff training and integration of training evaluation models. Aggression Violent Behav. 2006;11(1):27-43.
15. Campbell CL, McCoy S, Burg MA, Hoffman N. Enhancing home care staff safety through reducing client aggression and violence in noninstitutional care settings: a systematic review. Home Health Care Manage Pract. 2014;26(1):3-10.
16. Gallant-Roman MA. Strategies and tools to reduce workplace violence. AAOHNJ. 2008;56(11):449-454.
17. Weinberger LE, Sreenivasan S, Smee DE, McGuire J, Garrick T. Balancing safety against obstruction to health care access: an examination of behavioral flags in the VA health care system. J Threat Assess Manage. 2018;5(1):35-41.
18. Elbogen EB, Johnson SC, Wagner HR, et al. Protective factors and risk modification of violence in Iraq and Afghanistan war veterans. J Clin Psychiatry. 2012;73(6):e767-e773.
19. Karlin BE, Visnic S, McGee JS, Teri L. Results from the multisite implementation of STAR-VA: a multicomponent psychosocial intervention for managing challenging dementia-related behaviors of veterans. Psychol Serv. 2014;11(2):200-208.
20. Semeah LM, Campbell CL, Cowper DC, Peet AC. Serving our homeless veterans: patient perpetrated violence as a barrier to health care access. J Pub Nonprofit Aff. 2017;3(2):223-234.
21. Hodgson MJ, Reed R, Craig T, et al. Violence in healthcare facilities: lessons from the Veterans Health Administration. J Occup Environ Med. 2004;46(11):1158-1165.
22. Farrell GA, Bobrowski C, Bobrowski P. Scoping workplace aggression in nursing: findings from an Australian study. J Adv Nurs. 2006;55(6):778-787.
23. Barling J, Rogers AG, Kelloway EK. Behind closed doors: in-home workers’ experience of sexual harassment and workplace violence. J Occup Health Psychol. 2001;6(3):255-269.
24. Pompeii LA, Schoenfisch AL, Lipscomb HJ, Dement JM, Smith CD, Upadhyaya M. Physical assault, physical threat, and verbal abuse perpetrated against hospital workers by patients or visitors in six U.S. hospitals. Am J Ind Med. 2015;58(11):1194-1204.
25. Sippel LM, Mota NP, Kachadourian LK, et al. The burden of hostility in U.S. veterans: results from the National Health and Resilience in Veterans Study. Psychiatry Res. 2016;243(suppl C):421-430.
26. Campbell C. Patient Violence and Aggression in Non-Institutional Health Care Settings: Predictors of Reporting By Healthcare Providers [doctoral dissertation]. Orlando: University of Central Florida; 2016.
27. Galinsky T, Feng HA, Streit J, et al. Risk factors associated with patient assaults of home healthcare workers. Rehabil Nurs. 2010;35(5):206-215.
28. Campbell CL. Incident reporting by health-care workers in noninstitutional care settings. Trauma, Violence Abuse. 2017;18(4):445-456.
29. Arnetz JE, Arnetz BB. Violence towards health care staff and possible effects on the quality of patient care. Soc Sci Med. 2001;52(3):417-427.
30. Gates D, Fitzwater E, Succop P. Relationships of stressors, strain, and anger to caregiver assaults. Issues Ment Health Nurs. 2003;24(8):775-793.
31. Brillhart B, Kruse B, Heard L. Safety concerns for rehabilitation nurses in home care. Rehabil Nurs. 2004;29(6):227-229.
32. Taylor H. Patient violence against clinicians: managing the risk. Innov Clin Neurosci. 2013;10(3):40-42.
33. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. The Joint Commission releases results of surveys of the VA health care system. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=2808. Updated August 5, 2014. Accessed February 19, 2019.
34. Büssing A, Höge T. Aggression and violence against home care workers. J Occup Health Psychol. 2004;9(3):206-219.
35. Geiger-Brown J, Muntaner C, McPhaul K, Lipscomb J, Trinkoff A. Abuse and violence during home care work as predictor of worker depression. Home Health Care Serv Q. 2007;26(1):59-77.
36. Gates DM, Gillespie GL, Succop P. Violence against nurses and its impact on stress and productivity. Nurs Econ. 2011;29(2):59-66.
37. Petterson IL, Arnetz BB. Psychosocial stressors and well-being in health care workers: the impact of an intervention program. Soc Sci Med. 1998;47(11):1763-1772.
38. Arnetz JE, Arnetz BB. Implementation and evaluation of a practical intervention programme for dealing with violence towards health care workers. J Adv Nurs. 2000;31(3):668-680.
39. Arnetz JE, Hamblin L, Russell J, et al. Preventing patient-to-worker violence in hospitals: outcome of a randomized controlled intervention. J Occup Environ Med. 2017;59(1):18-27.
40. Elbogen EB, Tomkins AJ, Pothuloori AP, Scalora MJ. Documentation of violence risk information in psychiatric hospital patient charts: an empirical examination. J Am Acad Psychiatry Law. 2003;31(1):58-64.
41. Winsvold Prang I, Jelson-Jorgensen LP. Should I report? A qualitative study of barriers to incident reporting among nurses working in nursing homes. Geriatr Nurs. 2014;35(6):441-447.
42. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection: management of disruptive patient behavior at VA medical facilities. Report No. 11-02585-129. https://www.va.gov/oig/pubs/VAOIG-11-02585-129.pdf. Published Mrach 7, 2013. Accessed February 21, 2019.
43. Lipscomb J, London M. Not Part of the Job: How to Take a Stand Against Violence in the Work Setting. Silver Spring, MD: American Nurses Association; 2015.
44. May DD, Grubbs LM. The extent, nature, and precipitating factors of nurse assault among three groups of registered nurses in a regional medical center. J Emerg Nurs. 2002;28(1):11-17.
45. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57(5):460-477.
46. Brennan C, Worrall-Davies A, McMillan D, Gilbody S, House A. The hospital anxiety and depression scale: a diagnostic meta-analysis of case-finding ability. J Psychosom Res. 2010;69(4):371-378.
47. McPhaul K, Lipscomb J, Johnson J. Assessing risk for violence on home health visits. Home Healthc Nurse. 2010;28(5):278-289.
48. McPhaul KM, London M, Murrett K, Flannery K, Rosen J, Lipscomb J. Environmental evaluation for workplace violence in healthcare and social services. J Safety Res. 2008;39(2):237-250.
49. Kelly JA, Somlai AM, DiFranceisco WJ, et al. Bridging the gap between the science and service of HIV prevention: transferring effective research-based HIV prevention interventions to community AIDS service providers. Am J Public Health. 2000;90(7):1082-1088.
50. Pawlin S. Reporting violence. Emerg Nurse. 2008;16(4):16-21.
51. Brakel SJ. Legal liability and workplace violence. J Am Acad Psychiatry Law. 1998;26(4):553-562.
52. Neuman JH, Baron RA. Workplace violence and workplace aggression: evidence concerning specific forms, potential causes, and preferred targets. J Manage. 1998;24(3):391-419.53. Ferns T, Chojnacka I. Angels and swingers, matrons and sinners: nursing stereotypes. Br J Nurs. 2005;14(19):1028-1032.
54. Mercer SW, Reynolds WJ. Empathy and quality of care. Br J Gen Pract 2002;52(suppl):S9-S12.
55. Lee TH. An Epidemic of Empathy in Healthcare: How to Deliver Compassionate, Connected Patient Care That Creates a Competitive Advantage. Columbus, OH: McGraw-Hill Education; 2015.
56. US Department of Veterans Affairs, Veterans Health Administrastion. Veterans Health Administration workplace violence prevention program (WVPP): disruptive behavior reporting system utilization report. Published 2017. https://vaww.portal2.va.gov/sites/wvpp/Shared%20Documents/DBRS%20Utilization%20Reports/FY2017%20DBRS%20Quarterly%20Utilization%20Report%20(Quarter%201).pdf. [Source not verified.]
57. Campbell CL, Burg, MA, Gammonley D. Measures for incident reporting of patient violence and aggression towards healthcare providers: a systematic review. Aggression Violent Behav. 2015;25(part B):314-322.
58. Carney PT, West P, Neily J, Mills PD, Bagian JP. The effect of facility complexity on perceptions of safety climate in the operating room: size matters. Am J Med Qual. 2010;25(6):457-461.
1. Hodgson MJ, Mohr DC, Drummond DJ, Bell M, Van Male L. Managing disruptive patients in health care: necessary solutions to a difficult problem. Am J Ind Med. 2012;55(11):1009-1017.
2. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 2010-053. Patient Record Flags. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2341 Published December 3, 2010. Accessed March 29, 2019.
3. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 2012-026. Sexual Assaults and Other Defined Public Safety Incidents in VHA Facilities. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2797. Published September 27, 2012. Accessed March 29, 2019.
4. Curyto KJ, McCurry SM, Luci K, Karlin BE, Teri L, Karel MJ. Managing challenging behaviors of dementia in veterans: identifying and changing activators and consequences using STAR-VA. J Gerontol Nurs. 2017;43(2):33-43.
5. Speroni KG, Fitch T, Dawson E, Dugan L, Atherton M. Incidence and cost of nurse workplace violence perpetrated by hospital patients or patient visitors. J Emerg Nurs. 2014;40(3):218-228.
6. Phillips JP. Workplace violence against health care workers in the United States. NEJM. 2016;374(17):1661-1669.
7. Janocha JA, Smith RT. Workplace safety and health in the health care and social assistance industry, 2003–07. https://www.bls.gov/opub/mlr/cwc/workplace-safety-and-health-in-the-health-care-and-social-assistance-industry-2003-07.pdf. Published August 30, 2010. Accessed February 19, 2019.
8. US Department of Labor, Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. https://www.osha.gov/Publications/OSHA3826.pdf. Published December 2015. Accessed February 19, 2019.
9. US Department of Labor, Occupational Safety and Health Administration. Prevention of Workplace Violence in Healthcare and Social Assistance. Occupational Safety and Health Administration, https://www.govinfo.gov/content/pkg/FR-2016-12-07/pdf/2016-29197.pdf. Accessed January 20, 2017.
10. Gerberich SG, Church TR, McGovern PM, et al. An epidemiological study of the magnitude and consequences of work related violence: the Minnesota Nurses’ Study. Occup Environ Med. 2004;61(6):495-503.
11. Sherman MF, Gershon RRM, Samar SM, Pearson JM, Canton AN, Damsky MR. Safety factors predictive of job satisfaction and job retention among home healthcare aides. J Occup Environ Med. 2008;50(12):1430-1441.
12. Karel MJ, Teri L, McConnell E, Visnic S, Karlin BE. Effectiveness of expanded implementation of STAR-VA for managing dementia-related behaviors among veterans. Gerontologist. 2016;56(1):126-134.
13. US Department of Labor, Bureau of Labor Statistics. Nonfatal occupational injuries and illnesses requiring days away from work. https://www.bls.gov/news.release/archives/osh2_11192015.htm. Published November 19, 2015.
14. Beech B, Leather P. Workplace violence in the health care sector: A review of staff training and integration of training evaluation models. Aggression Violent Behav. 2006;11(1):27-43.
15. Campbell CL, McCoy S, Burg MA, Hoffman N. Enhancing home care staff safety through reducing client aggression and violence in noninstitutional care settings: a systematic review. Home Health Care Manage Pract. 2014;26(1):3-10.
16. Gallant-Roman MA. Strategies and tools to reduce workplace violence. AAOHNJ. 2008;56(11):449-454.
17. Weinberger LE, Sreenivasan S, Smee DE, McGuire J, Garrick T. Balancing safety against obstruction to health care access: an examination of behavioral flags in the VA health care system. J Threat Assess Manage. 2018;5(1):35-41.
18. Elbogen EB, Johnson SC, Wagner HR, et al. Protective factors and risk modification of violence in Iraq and Afghanistan war veterans. J Clin Psychiatry. 2012;73(6):e767-e773.
19. Karlin BE, Visnic S, McGee JS, Teri L. Results from the multisite implementation of STAR-VA: a multicomponent psychosocial intervention for managing challenging dementia-related behaviors of veterans. Psychol Serv. 2014;11(2):200-208.
20. Semeah LM, Campbell CL, Cowper DC, Peet AC. Serving our homeless veterans: patient perpetrated violence as a barrier to health care access. J Pub Nonprofit Aff. 2017;3(2):223-234.
21. Hodgson MJ, Reed R, Craig T, et al. Violence in healthcare facilities: lessons from the Veterans Health Administration. J Occup Environ Med. 2004;46(11):1158-1165.
22. Farrell GA, Bobrowski C, Bobrowski P. Scoping workplace aggression in nursing: findings from an Australian study. J Adv Nurs. 2006;55(6):778-787.
23. Barling J, Rogers AG, Kelloway EK. Behind closed doors: in-home workers’ experience of sexual harassment and workplace violence. J Occup Health Psychol. 2001;6(3):255-269.
24. Pompeii LA, Schoenfisch AL, Lipscomb HJ, Dement JM, Smith CD, Upadhyaya M. Physical assault, physical threat, and verbal abuse perpetrated against hospital workers by patients or visitors in six U.S. hospitals. Am J Ind Med. 2015;58(11):1194-1204.
25. Sippel LM, Mota NP, Kachadourian LK, et al. The burden of hostility in U.S. veterans: results from the National Health and Resilience in Veterans Study. Psychiatry Res. 2016;243(suppl C):421-430.
26. Campbell C. Patient Violence and Aggression in Non-Institutional Health Care Settings: Predictors of Reporting By Healthcare Providers [doctoral dissertation]. Orlando: University of Central Florida; 2016.
27. Galinsky T, Feng HA, Streit J, et al. Risk factors associated with patient assaults of home healthcare workers. Rehabil Nurs. 2010;35(5):206-215.
28. Campbell CL. Incident reporting by health-care workers in noninstitutional care settings. Trauma, Violence Abuse. 2017;18(4):445-456.
29. Arnetz JE, Arnetz BB. Violence towards health care staff and possible effects on the quality of patient care. Soc Sci Med. 2001;52(3):417-427.
30. Gates D, Fitzwater E, Succop P. Relationships of stressors, strain, and anger to caregiver assaults. Issues Ment Health Nurs. 2003;24(8):775-793.
31. Brillhart B, Kruse B, Heard L. Safety concerns for rehabilitation nurses in home care. Rehabil Nurs. 2004;29(6):227-229.
32. Taylor H. Patient violence against clinicians: managing the risk. Innov Clin Neurosci. 2013;10(3):40-42.
33. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. The Joint Commission releases results of surveys of the VA health care system. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=2808. Updated August 5, 2014. Accessed February 19, 2019.
34. Büssing A, Höge T. Aggression and violence against home care workers. J Occup Health Psychol. 2004;9(3):206-219.
35. Geiger-Brown J, Muntaner C, McPhaul K, Lipscomb J, Trinkoff A. Abuse and violence during home care work as predictor of worker depression. Home Health Care Serv Q. 2007;26(1):59-77.
36. Gates DM, Gillespie GL, Succop P. Violence against nurses and its impact on stress and productivity. Nurs Econ. 2011;29(2):59-66.
37. Petterson IL, Arnetz BB. Psychosocial stressors and well-being in health care workers: the impact of an intervention program. Soc Sci Med. 1998;47(11):1763-1772.
38. Arnetz JE, Arnetz BB. Implementation and evaluation of a practical intervention programme for dealing with violence towards health care workers. J Adv Nurs. 2000;31(3):668-680.
39. Arnetz JE, Hamblin L, Russell J, et al. Preventing patient-to-worker violence in hospitals: outcome of a randomized controlled intervention. J Occup Environ Med. 2017;59(1):18-27.
40. Elbogen EB, Tomkins AJ, Pothuloori AP, Scalora MJ. Documentation of violence risk information in psychiatric hospital patient charts: an empirical examination. J Am Acad Psychiatry Law. 2003;31(1):58-64.
41. Winsvold Prang I, Jelson-Jorgensen LP. Should I report? A qualitative study of barriers to incident reporting among nurses working in nursing homes. Geriatr Nurs. 2014;35(6):441-447.
42. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection: management of disruptive patient behavior at VA medical facilities. Report No. 11-02585-129. https://www.va.gov/oig/pubs/VAOIG-11-02585-129.pdf. Published Mrach 7, 2013. Accessed February 21, 2019.
43. Lipscomb J, London M. Not Part of the Job: How to Take a Stand Against Violence in the Work Setting. Silver Spring, MD: American Nurses Association; 2015.
44. May DD, Grubbs LM. The extent, nature, and precipitating factors of nurse assault among three groups of registered nurses in a regional medical center. J Emerg Nurs. 2002;28(1):11-17.
45. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57(5):460-477.
46. Brennan C, Worrall-Davies A, McMillan D, Gilbody S, House A. The hospital anxiety and depression scale: a diagnostic meta-analysis of case-finding ability. J Psychosom Res. 2010;69(4):371-378.
47. McPhaul K, Lipscomb J, Johnson J. Assessing risk for violence on home health visits. Home Healthc Nurse. 2010;28(5):278-289.
48. McPhaul KM, London M, Murrett K, Flannery K, Rosen J, Lipscomb J. Environmental evaluation for workplace violence in healthcare and social services. J Safety Res. 2008;39(2):237-250.
49. Kelly JA, Somlai AM, DiFranceisco WJ, et al. Bridging the gap between the science and service of HIV prevention: transferring effective research-based HIV prevention interventions to community AIDS service providers. Am J Public Health. 2000;90(7):1082-1088.
50. Pawlin S. Reporting violence. Emerg Nurse. 2008;16(4):16-21.
51. Brakel SJ. Legal liability and workplace violence. J Am Acad Psychiatry Law. 1998;26(4):553-562.
52. Neuman JH, Baron RA. Workplace violence and workplace aggression: evidence concerning specific forms, potential causes, and preferred targets. J Manage. 1998;24(3):391-419.53. Ferns T, Chojnacka I. Angels and swingers, matrons and sinners: nursing stereotypes. Br J Nurs. 2005;14(19):1028-1032.
54. Mercer SW, Reynolds WJ. Empathy and quality of care. Br J Gen Pract 2002;52(suppl):S9-S12.
55. Lee TH. An Epidemic of Empathy in Healthcare: How to Deliver Compassionate, Connected Patient Care That Creates a Competitive Advantage. Columbus, OH: McGraw-Hill Education; 2015.
56. US Department of Veterans Affairs, Veterans Health Administrastion. Veterans Health Administration workplace violence prevention program (WVPP): disruptive behavior reporting system utilization report. Published 2017. https://vaww.portal2.va.gov/sites/wvpp/Shared%20Documents/DBRS%20Utilization%20Reports/FY2017%20DBRS%20Quarterly%20Utilization%20Report%20(Quarter%201).pdf. [Source not verified.]
57. Campbell CL, Burg, MA, Gammonley D. Measures for incident reporting of patient violence and aggression towards healthcare providers: a systematic review. Aggression Violent Behav. 2015;25(part B):314-322.
58. Carney PT, West P, Neily J, Mills PD, Bagian JP. The effect of facility complexity on perceptions of safety climate in the operating room: size matters. Am J Med Qual. 2010;25(6):457-461.
In situ vaccination produced responses in indolent NHL
A three-pronged treatment approach can produce responses in indolent non-Hodgkin lymphoma (iNHL), according to research published in Nature Medicine.
The approach – “in situ vaccination (ISV)” – involves intratumoral injections of Fms-like tyrosine kinase 3 ligand (Flt3L), local radiotherapy, and intratumoral injections of a TLR3 agonist (poly-ICLC).
ISV produced responses in patients with iNHL, prompting regression of tumors that were directly targeted with ISV, as well as untreated tumors.
In preclinical experiments, ISV induced tumor regression in mice but also overcame resistance to PD1 inhibition. This result led researchers to initiate a trial testing ISV in combination with pembrolizumab in patients with lymphoma and solid tumors.
“We discovered why some tumors do not respond to PD1 blockade: insufficient dendritic cells (DCs) and cross-presentation,” lead study author Joshua Brody, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview. “We developed a treatment, in situ vaccination (ISV), which brings DCs to the tumor, loads them with tumor antigens, and activates the DCs.”
Specifically, the researchers found that injecting Flt3L into a tumor recruits intratumoral DCs, local radiotherapy loads the DCs with tumor-associated antigens, and poly-ICLC activates DCs. This approach produced responses in mouse models of lymphoma and patients with iNHL.
Preclinical results
Dr. Brody and his colleagues tested ISV in A20 tumor-bearing mice. The mice received intratumoral injections of Flt3L, followed by local radiotherapy and poly-ICLC.
Tumor regression occurred within days of radiotherapy. About 40% of mice experienced tumor-free survival of at least 3 months, although most tumors recurred within 4 weeks of ISV administration.
However, the researchers observed increased PD1 and PD-L1 expression in ISV-treated mice, so the team theorized that an anti-PD1 monoclonal antibody (RMP1-14) could improve the efficacy of ISV.
The researchers found that ISV plus RMP1-14 delayed tumor growth when compared with ISV alone, and the rate of durable remissions increased from about 40% to about 80%.
Clinical results
Dr. Brody and his colleagues also tested ISV in a clinical trial. That trial included 11 iNHL patients – 9 with follicular lymphoma, 1 with marginal zone lymphoma, and 1 with small lymphocytic lymphoma.
The patients received nine daily injections of Flt3L (25 mcg/kg) into a target lesion, then two doses of radiation (2 Gy) to the same lesion, and eight intratumoral injections of poly-ICLC (2 mg).
“We ... have observed dramatic clinical responses; i.e., we administer ISV at one tumor site, and tumors throughout the body regress,” Dr. Brody said.
At the target lesion, there were two complete responses, six partial responses, and three cases of stable disease. At nontarget lesions, there was one complete response, two partial responses, six cases of stable disease, and two cases of progression.
ISV was considered well tolerated. One patient had grade 2 fever, three had grade 1 fever, and nine had grade 1 flu-like symptoms. Two patients did not have any adverse events.
This research was supported by Merck, Celldex Therapeutics, Oncovir, and Genentech. The authors reported relationships with Acerta Pharma, Bristol Myers Squibb, Genentech, Gilead Sciences, Seattle Genetics, Pharmacyclics, Celgene, Celldex Therapeutics, and Oncovir.
SOURCE: Hammerich L et al. Nat Med. 2019 Apr 8. doi: 10.1038/s41591-019-0410-x.
A three-pronged treatment approach can produce responses in indolent non-Hodgkin lymphoma (iNHL), according to research published in Nature Medicine.
The approach – “in situ vaccination (ISV)” – involves intratumoral injections of Fms-like tyrosine kinase 3 ligand (Flt3L), local radiotherapy, and intratumoral injections of a TLR3 agonist (poly-ICLC).
ISV produced responses in patients with iNHL, prompting regression of tumors that were directly targeted with ISV, as well as untreated tumors.
In preclinical experiments, ISV induced tumor regression in mice but also overcame resistance to PD1 inhibition. This result led researchers to initiate a trial testing ISV in combination with pembrolizumab in patients with lymphoma and solid tumors.
“We discovered why some tumors do not respond to PD1 blockade: insufficient dendritic cells (DCs) and cross-presentation,” lead study author Joshua Brody, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview. “We developed a treatment, in situ vaccination (ISV), which brings DCs to the tumor, loads them with tumor antigens, and activates the DCs.”
Specifically, the researchers found that injecting Flt3L into a tumor recruits intratumoral DCs, local radiotherapy loads the DCs with tumor-associated antigens, and poly-ICLC activates DCs. This approach produced responses in mouse models of lymphoma and patients with iNHL.
Preclinical results
Dr. Brody and his colleagues tested ISV in A20 tumor-bearing mice. The mice received intratumoral injections of Flt3L, followed by local radiotherapy and poly-ICLC.
Tumor regression occurred within days of radiotherapy. About 40% of mice experienced tumor-free survival of at least 3 months, although most tumors recurred within 4 weeks of ISV administration.
However, the researchers observed increased PD1 and PD-L1 expression in ISV-treated mice, so the team theorized that an anti-PD1 monoclonal antibody (RMP1-14) could improve the efficacy of ISV.
The researchers found that ISV plus RMP1-14 delayed tumor growth when compared with ISV alone, and the rate of durable remissions increased from about 40% to about 80%.
Clinical results
Dr. Brody and his colleagues also tested ISV in a clinical trial. That trial included 11 iNHL patients – 9 with follicular lymphoma, 1 with marginal zone lymphoma, and 1 with small lymphocytic lymphoma.
The patients received nine daily injections of Flt3L (25 mcg/kg) into a target lesion, then two doses of radiation (2 Gy) to the same lesion, and eight intratumoral injections of poly-ICLC (2 mg).
“We ... have observed dramatic clinical responses; i.e., we administer ISV at one tumor site, and tumors throughout the body regress,” Dr. Brody said.
At the target lesion, there were two complete responses, six partial responses, and three cases of stable disease. At nontarget lesions, there was one complete response, two partial responses, six cases of stable disease, and two cases of progression.
ISV was considered well tolerated. One patient had grade 2 fever, three had grade 1 fever, and nine had grade 1 flu-like symptoms. Two patients did not have any adverse events.
This research was supported by Merck, Celldex Therapeutics, Oncovir, and Genentech. The authors reported relationships with Acerta Pharma, Bristol Myers Squibb, Genentech, Gilead Sciences, Seattle Genetics, Pharmacyclics, Celgene, Celldex Therapeutics, and Oncovir.
SOURCE: Hammerich L et al. Nat Med. 2019 Apr 8. doi: 10.1038/s41591-019-0410-x.
A three-pronged treatment approach can produce responses in indolent non-Hodgkin lymphoma (iNHL), according to research published in Nature Medicine.
The approach – “in situ vaccination (ISV)” – involves intratumoral injections of Fms-like tyrosine kinase 3 ligand (Flt3L), local radiotherapy, and intratumoral injections of a TLR3 agonist (poly-ICLC).
ISV produced responses in patients with iNHL, prompting regression of tumors that were directly targeted with ISV, as well as untreated tumors.
In preclinical experiments, ISV induced tumor regression in mice but also overcame resistance to PD1 inhibition. This result led researchers to initiate a trial testing ISV in combination with pembrolizumab in patients with lymphoma and solid tumors.
“We discovered why some tumors do not respond to PD1 blockade: insufficient dendritic cells (DCs) and cross-presentation,” lead study author Joshua Brody, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview. “We developed a treatment, in situ vaccination (ISV), which brings DCs to the tumor, loads them with tumor antigens, and activates the DCs.”
Specifically, the researchers found that injecting Flt3L into a tumor recruits intratumoral DCs, local radiotherapy loads the DCs with tumor-associated antigens, and poly-ICLC activates DCs. This approach produced responses in mouse models of lymphoma and patients with iNHL.
Preclinical results
Dr. Brody and his colleagues tested ISV in A20 tumor-bearing mice. The mice received intratumoral injections of Flt3L, followed by local radiotherapy and poly-ICLC.
Tumor regression occurred within days of radiotherapy. About 40% of mice experienced tumor-free survival of at least 3 months, although most tumors recurred within 4 weeks of ISV administration.
However, the researchers observed increased PD1 and PD-L1 expression in ISV-treated mice, so the team theorized that an anti-PD1 monoclonal antibody (RMP1-14) could improve the efficacy of ISV.
The researchers found that ISV plus RMP1-14 delayed tumor growth when compared with ISV alone, and the rate of durable remissions increased from about 40% to about 80%.
Clinical results
Dr. Brody and his colleagues also tested ISV in a clinical trial. That trial included 11 iNHL patients – 9 with follicular lymphoma, 1 with marginal zone lymphoma, and 1 with small lymphocytic lymphoma.
The patients received nine daily injections of Flt3L (25 mcg/kg) into a target lesion, then two doses of radiation (2 Gy) to the same lesion, and eight intratumoral injections of poly-ICLC (2 mg).
“We ... have observed dramatic clinical responses; i.e., we administer ISV at one tumor site, and tumors throughout the body regress,” Dr. Brody said.
At the target lesion, there were two complete responses, six partial responses, and three cases of stable disease. At nontarget lesions, there was one complete response, two partial responses, six cases of stable disease, and two cases of progression.
ISV was considered well tolerated. One patient had grade 2 fever, three had grade 1 fever, and nine had grade 1 flu-like symptoms. Two patients did not have any adverse events.
This research was supported by Merck, Celldex Therapeutics, Oncovir, and Genentech. The authors reported relationships with Acerta Pharma, Bristol Myers Squibb, Genentech, Gilead Sciences, Seattle Genetics, Pharmacyclics, Celgene, Celldex Therapeutics, and Oncovir.
SOURCE: Hammerich L et al. Nat Med. 2019 Apr 8. doi: 10.1038/s41591-019-0410-x.
FROM NATURE MEDICINE
Nonopioid Alternatives to Addressing Pain Intensity: A Retrospective Look at 2 Noninvasive Pain Treatment Devices
Chronic pain is common among veterans treated in Veterans Health Administration (VHA) facilities, and optimal management remains challenging in the context of the national opioid misuse epidemic. The Eastern Oklahoma VA Health Care System (EOVAHCS) Pain Program offers a range of services that allow clinicians to tailor multimodal treatment strategies to a veteran’s needs. In 2014, a Modality Clinic was established to assess the utility of adding noninvasive treatment devices to the pain program’s armamentarium. This article addresses the context for introducing these devices and describes the EOVAHCS Pain Program and Modality Clinic. Also discussed are procedures and findings from an initial quality improvement evaluation designed to inform decision making regarding retention, expansion, or elimination of the EOVAHCS noninvasive, pain treatment device program.
Opioid prescriptions increased from 76 million in 1991 to 219 million in 2011. In 2011, the annual cost of chronic pain in the US was estimated at $635 billion.1-6 The confluence of an increasing concern about undertreatment of pain and overconfidence for the safety of opioids led to what former US Surgeon General Vivek H. Murthy, MD, called the opioid crisis.7 As awareness of its unintended consequences of opioid prescribing increased, the VHA began looking for nonopioid treatments that would decrease pain intensity. The 1993 article by Kehlet and Dahl was one of the first discussions of a multimodal nonpharmacologic strategy for addressing acute postoperative pain.8 Their pivotal literature review concluded that nonpharmacologic modalities, such as acupuncture, cranial manipulation, cranial electrostimulation treatment (CES), and low-level light technologies (LLLT), carried less risk and produced equal or greater clinical effects than those of drug therapies.8
Multimodal treatment approaches increasingly are encouraged, and nonopioid pain control has become more common across medical disciplines from physical therapy to anesthesiology.8-10 Innovative, noninvasive devices designed for self-use have appeared on the market. Many of these devices incorporate microcurrent electrical therapy (MET), CES, and/or LLLT (also known as cold laser).11-16 LLLT is a light modality that seems to lead to increased ATP production, resulting in improved healing and decreased inflammation.13-16 Although CES has been studied in a variety of patient populations, its effectiveness is not well understood.16 Research on the effects of CES on neurotransmitter levels as well as activation of parts of the brain involved in pain reception and transmission should clarify these mechanisms. Research has shown improvements in sleep and mood as well as overall pain reduction.11,16 Research has focused primarily on individual modalities rather than on combination devices and has been conducted on populations unlike the veteran population (eg, women with fibromyalgia).
Most of the devices that use electrical or LLLT cannot be used safely by patients who have implantable electrical devices or have medical conditions such as unstable seizures, pregnancy, and active malignancies.
The most common adverse effects (AEs) of CES—dizziness and headaches—are minimal compared with the AEs of pain medications. MET and LLLT AEs generally are limited to skin irritation and muscle soreness.11 Most devices require a prescription, and manufacturers provide training for purchase.
The Pain Program
EOVAHCS initially established its consultative pain program in 2013 to provide support, recommendations, and education about managing pain in veterans to primary care providers (PCPs). Veterans are referred to the pain program for a face-to-face assessment and set of recommendations to assist in developing a comprehensive pain treatment plan. Consistent with its multimodal, biopsychosocial rehabilitation model approach, the program also offers several chronic pain treatment services, including patient education courses, cognitive behavioral therapy (CBT) for chronic pain, chiropractic care, biofeedback, relaxation training, steroid injections, pain coaching, and a pain modality (noninvasive device) clinic. During their assessment, veterans are evaluated for the appropriateness of these programs, including treatment through the Pain Modality Clinic.
Pain Modality Clinic
The EOVAHCS Pain Modality Clinic was created in 2014 as a treatment and device-trial program to provide veterans access to newer noninvasive, patient-driven treatment devices as part of an active chronic pain self-management plan. A crucial innovation is that these devices are designed to be used by patients in their homes. These devices can be expensive, and not every patient will benefit from their use; therefore, clinic leaders recommended a trial before a device is issued to a veteran for home use.
The Pain Modality Clinic coordinator trains clinic facilitators on the device according to manufacturer’s guidelines. Each participating veteran takes part in a device trial to confirm that he or she is able to use the recommended device independently and is likely to benefit from its use. When appropriate, veterans who do not respond to the initial device trial could test the potential benefit of another device. Although data from these device trials are collected primarily to inform clinical decision making, this information also is useful in guiding local policy regarding continued support for each of the modalities.
Veterans who have chronic or persistent pain (≥ 3 months) that interferes with function or quality of life are considered good candidates for a device trial if they are actively involved in pain self-care, logistically able to participate, able to use a device long-term, and have no contraindications. “Active involvement” could be met by participation in any pain management effort, whether a specific exercise program, CBT, or other treatment.
The Modality Clinic currently offers device trials for persistent pain with Alpha-Stim-M (AS-M; Electromedical Products International, Mineral Wells, TX), Laser Touch One (LTO; Renewal Technologies, LLC, Phoenix, AZ), and Neurolumen (Oklahoma City, OK). Neurolumen devices were not available in the clinic initially and will not be discussed further in this article.
The first Alpha-Stim machine using MET and CES technology was created in 1981 for in-office pain management. In 2012, the currently used AS-M became available.11 AS-M is FDA approved for treating pain, anxiety, depression, and sleep problems and is the device used in the EOVAHCS Modality Clinic. AS-M uses probes or electrodes to send a MET waveform through the body area in pain. The device uses ear clips to provide CES, which is thought to increase alpha waves in the brain.11 The LTO is a device that combines LLLT and MET technologies in a home-use design.14 LTO is FDA approved for treating painand is a portable personal pain-relief device applied to the area of pain using electroconductive gel.
Both devices are designed for long-term, self-use, making them viable parts of a multimodal, chronic pain treatment plan. Contraindications for AS-M and LTO include having a pacemaker or an implantable defibrillator, pregnancy, current malignancy, or seizures. Eligible veterans with persistent pain and high levels of depression, anxiety, and/or sleep problems generally are triaged to AS-M, whereas those who have only pain intensity issues usually are assigned to LTO. Referral to the Modality Clinic is not limited to a specific type of pain; common pain conditions seen in the clinic are spine and joint pain, arthritis pain, myofascial pain, headaches, and neuropathy.
Training and Device Trials
Eligible veterans are educated about the device and complete clinical informed consent, which is documented in the electronic health record. The veterans’ primary care and/or specialist providers are contacted for concurrence regarding veterans’ participation in the treatment.
Protocols for the device trials are based on the manufacturers’ recommendations, adjusted to what is feasible in the clinic (manufacturers approved the changes). The number of treatments per trial varies by device. For AS-M, veterans come to the clinic 5 days a week for 2 weeks. For LTO, veterans attend the clinic 5 days a week for 1 week.
At the beginning of a device trial, a trained facilitator teaches each veteran and caregiver to use the device, sets functional goals for the trial, and provides education on the trial questionnaires and daily pain logs. The veteran then follows the device protocol in the clinic where the facilitator can respond to questions and address any issues. With support from their caregivers, veterans are expected to become independent on their device use by the end of the trial. Clinic staff or the veteran can stop the device trial at any point, without affecting the veteran’s participation in or eligibility for other EOVAHCS pain programs.
This project was submitted to the University of Oklahoma Health Sciences Center Institutional Review Board and was exempted from institutional review board oversight as a retrospective, quality improvement effort. Before data analysis, the EOVAHCS Coordinator for Research and Development reviewed the procedures to ensure that all policies were being followed.
Methods
Data for veterans who completed valid treatments of AS-M or LTO from May 9, 2014 to August 20, 2016, were included in the analyses. For an AS-M treatment to be considered valid, the veteran must have attended at least 8 sessions and completed assessment instruments at baseline (preintervention) and following completion (postintervention). For an LTO treatment to be considered valid, the veteran must have attended at least 4 sessions and completed assessment measures at baseline and after completion.
Measures
Veterans completed the following measures at baseline and after trial completion:
The Beck Depression Inventory (BDI-II) is a 21-item measure designed to assess depressive symptoms. Each item assesses intensity on a 0-to-3 scale. Scores from 0 to 13 indicate minimum depression; 14 to 19, mild depression; 20 to 28, moderate depression, and 29 to 63, severe depression.17
The Beck Anxiety Inventory (BAI) is a 21-item measure of anxiety symptoms that uses a 0-to-3 scale to assess severity of subjective, somatic, or panic-related symptoms of anxiety. Scores ranging from 0 to 9 indicate minimal anxiety; 10 to 16, mild anxiety; 17 to 29, moderate anxiety, and 30 to 63, severe anxiety.18
The Pain Catastrophizing Scale (PCS) is a 13-item measure of pain catastrophizing, a crucial marker of how individuals experience pain. Items are scored on a 0-to-4 scale; scores of ≥ 30 indicate a clinically relevant level of catastrophizing.19
The Subjective Units of Distress Scale (SUD) is a single-item measure of the subjective intensity of disturbance or distress currently being experienced. It is scored from 0 to 10; 1 to 4 is mild, 5 to 6 is moderate, and 7 to 10 is severe.20
The Brief Pain Inventory (BPI) measures pain intensity and the impact of pain on functioning. Four items assess pain intensity at its worst, least, and average over the previous 24 hours and at the time of assessment; responses are on a 0-to-10 scale with 10 being most severe. The pain intensity measure is the average of scores on these 4 items. Pain interference is measured with respect to 7 daily activities; general activity, walking, work, mood, enjoyment of life, relations with others, and sleep. Each of these items is scored on a 0-to-10 scale with 10 being the most severe. The pain interference measure is the average of scores on these 7 items.21
Participants completed a daily pain log and recorded self-ratings (0-to-10 scale) of pain and relaxation levels before and after using the device. These scores were primarily used to assist in determining whether goals, set collaboratively by the clinician and the veteran at the first session, had been met.
Analysis
Descriptive statistics were used to characterize the sample overall and by modality. Paired t tests were used to assess changes on each assessment measure over time and for each device separately. The significance of change was assessed for 8 outcomes for each device. In this context, using a conservative Bonferroni correction, significance was set at P < .006. Because AS-M is designed to address depression, anxiety, and sleep as well as pain, whereas LTO is not, device assignments were based on clinical considerations rather than randomization. Therefore, no comparisons were made between devices, and outcomes were assessed independently for the 2 devices. Analyses were performed using SAS 9.4 (Cary, NC).
Results
Device trials were initiated for 161 veterans (LTO, 70; AS-M, 91). Distribution of devices was unequal because veterans are assigned to 1 device or the other based on clinical presentation. Failure to complete a trial (n = 46; 28.6%) typically was because of travel barriers, lack of interest in continuing, and for 3 veterans, reports of headaches that they attributed to the AS-M treatment. Of the 115 participants who completed valid trials, 88 (76.5%) also completed assessment measures at pre- and postintervention (LTO = 38; AS-M = 50). None of the participants in this study completed trials with both the AS-M and LTO devices.
Most participants were male (84.1%) and rural residents (85.5%) (Table 1).
Pain Reduction
Treatment with AS-M or LTO was associated with statistically significant reductions in pain severity (BPI), pain interference (BPI), daily pain intensity scores (daily pain log), and pain catastrophizing (PCS) (Tables 2 and 3).
Impact on Mood
Use of AS-M was associated with statistically significant improvements in depression (BDI-II), anxiety (BAI), and distress (SUD) scores. In addition, veterans completing AS-M treatment showed a statistically significant improvement in self-reported relaxation scores. Interestingly, use of LTO also resulted in a statistically significant decrease in anxiety (BAI) and a nonstatistically significant decrease in depression (BDI-II).
Figure 1 and 2 illustrates the clinical impact of each device in shifting participants from 1 level of symptom severity to another.
Discussion
Use of both AS-M and LTO at EOVAHC was associated with reduced pain intensity. The devices also had positive effects beyond pain in areas such as depression, anxiety, and distress. Remission of depression and anxiety symptoms has been associated with significant decline in pain symptoms, suggesting that pain is best treated through multimodal approaches.22
In the context of the opioid crisis, the availability of effective nonopioid, nonpharmacologic, noninvasive treatments for chronic pain is needed. The Joint Commission recently expanded its pain management guidelines to support hospitals offering nonpharmacologic pain treatments.23 Integrating AS-M, LTO, or similar products into standard pain management practices allows for other treatment pathways with positive outcomes for providers and patients. The Joint Commission also recommends an interdisciplinary approach, defined as a process whereby health care professionals from different disciplines collaborate to diagnose and treat patients experiencing difficult pain conditions. This approach facilitates multimodal management because these disciplines contribute knowledge about a variety of treatment options. Devices such AS-M and LTO are well suited to interdisciplinary pain management because they are not seen as being under the purview of a specific health care specialty.
Limitations
Our findings are limited because they are derived from a retrospective, quality improvement evaluation of outcomes from a single clinic. Findings must be considered in the context of the relatively small samples of veterans. Because analyses were conducted as part of a quality improvement effort, veterans were offered a specific device based on clinical indications, there were no comparisons between devices, and there was no comparison group. Although most participants were using medication and other treatments as part of their pain treatment plan, all reported continued pain intensity before use of a device. Analyses did not control for variation in treatments received concurrently. Last, the logs used to collect self-report data on daily pain and relaxation levels were not validated.
The data highlight a clear need for research to better understand the long-term effects of these devices as well as the characteristics of patients who respond best to each device. Noninvasive treatments for pain often are dismissed as placebos. Rigorously designed, controlled studies will help demonstrate that these devices offer a statistically significant benefit beyond any placebo effect.
Conclusion
Understanding of chronic pain and its treatment will continue to evolve. It is clear that each person dealing with chronic pain requires a tailored combination of treatments and multimodal approaches, which is more effective than any single treatment. Nonpharmacologic, noninvasive devices pose fewer risks and seem to be more effective in reducing pain intensity than traditional treatments, including medications or surgical intervention. In light of the current emphasis on evidence-based health care and as the evidence for the effectiveness of noninvasive pain devices modalities grows, it is likely that treatments incorporating modalities such as MET, CES, and LLLT will become common options for managing chronic pain.
1. US Department of Veterans Affairs. Pain as the 5th Vital Sign Toolkit. https://www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf. Published October 2000. Accessed February 11, 2019.
2. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
3. Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16(5):405-416.
4. Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109(1):5-12.
5. Mosher HJ, Krebs EE, Carrel M, Kaboli PJ, Weg MW, Lund BC. Trends in prevalent and incident opioid receipt: an observational study in Veterans Health Administration 2004-2012. J Gen Intern Med. 2015;30(5):597-604.
6. Reuben DB, Alvanzo AAH, Ashikaga T, et al. National Institutes of Health Pathways to Prevention Workshop: The role of opioids in the treatment of chronic pain. Ann Intern Med. 2015;162(4):295-300.
7. Murthy VH. Opioid epidemic: we all have a role in turning the tide. https://obamawhitehouse.archives.gov/blog/2016/10/05/opioid-epidemic-we-all-have-role-turning-tide. Published October 5, 2016. Accessed February 12, 2019.
8. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048-1056.
9. Crane P, Feinberg L, Morris J. A multimodal physical therapy approach to the management of a patient with temporomandibular dysfunction and head and neck lymphedema: a case report. J Man Manip Ther. 2015;23(1): 37-42.
10. Arnstein P. Multimodal approaches to pain management. Nurs. 2011;41(3): 60-61.
11. Alpha-Stim. http://www.alpha-stim.com. Accessed March 22, 2019
12. Shekelle PG, Cook IA, Miake-Lye IM, Booth MS, Beroes JM, Mak S. Benefits and harms of cranial electrical stimulation for chronic painful conditions, depression, anxiety, and insomnia. Ann Intern Med. 2018;168(6):414-421.
13. Chow RT, Heller GZ, Barnsley L. The effect of 300 mW, 830 nm laser on chronic neck pain: a double-blind, randomized, placebo-controlled study. Pain. 2006;124(1):201-210.
14. Kulkarni AD, Smith RB. The use of microcurrent electrical therapy and cranial electrotherapy stimulation in pain control. Clin Pract Alternative Med. 2001;2(2):99-102.
15. Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897-1908.
16. Taylor AG, Anderson JG, Riedel SL, et al. Cranial electrical stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Manag Nurs. 2013;14(4):327-335.
17. Beck, AT, Steer, RA, Brown, GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
18. Beck AT, Steer RA. Beck Anxiety Inventory: Manual. San Antonio, TX: Psychological Corporation; 1993.
19. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524-532.
20. Wolpe J. The Practice of Behavior Therapy. 4th ed. Elmsford, NY: Pergamon; 1990.
21. Cleeland CS. The Brief Pain Inventory User Manual. https://www.mdanderson.org/research/departments-labs-institutes/departments-divisions/symptom-research/symptom-assessment-tools/brief-pain-inventory.html. Published 2009. Accessed February 12, 2019.
22. Gerrits MM, van Marwijk HW, van Oppen P, Horst HVD, Penninx BW. Longitudinal association between pain, and depression and anxiety over four years. J Psychosom Res. 2015;78(1):64-70.
23. The Joint Commission. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. https://www.jointcommission.org/assets/1/18/Joint_Commission_Enhances_Pain_Assessment_and_Management_Requirements_for_Accredited_Hospitals1.PDF. Published July 2017. Accessed March 21, 2019.
Chronic pain is common among veterans treated in Veterans Health Administration (VHA) facilities, and optimal management remains challenging in the context of the national opioid misuse epidemic. The Eastern Oklahoma VA Health Care System (EOVAHCS) Pain Program offers a range of services that allow clinicians to tailor multimodal treatment strategies to a veteran’s needs. In 2014, a Modality Clinic was established to assess the utility of adding noninvasive treatment devices to the pain program’s armamentarium. This article addresses the context for introducing these devices and describes the EOVAHCS Pain Program and Modality Clinic. Also discussed are procedures and findings from an initial quality improvement evaluation designed to inform decision making regarding retention, expansion, or elimination of the EOVAHCS noninvasive, pain treatment device program.
Opioid prescriptions increased from 76 million in 1991 to 219 million in 2011. In 2011, the annual cost of chronic pain in the US was estimated at $635 billion.1-6 The confluence of an increasing concern about undertreatment of pain and overconfidence for the safety of opioids led to what former US Surgeon General Vivek H. Murthy, MD, called the opioid crisis.7 As awareness of its unintended consequences of opioid prescribing increased, the VHA began looking for nonopioid treatments that would decrease pain intensity. The 1993 article by Kehlet and Dahl was one of the first discussions of a multimodal nonpharmacologic strategy for addressing acute postoperative pain.8 Their pivotal literature review concluded that nonpharmacologic modalities, such as acupuncture, cranial manipulation, cranial electrostimulation treatment (CES), and low-level light technologies (LLLT), carried less risk and produced equal or greater clinical effects than those of drug therapies.8
Multimodal treatment approaches increasingly are encouraged, and nonopioid pain control has become more common across medical disciplines from physical therapy to anesthesiology.8-10 Innovative, noninvasive devices designed for self-use have appeared on the market. Many of these devices incorporate microcurrent electrical therapy (MET), CES, and/or LLLT (also known as cold laser).11-16 LLLT is a light modality that seems to lead to increased ATP production, resulting in improved healing and decreased inflammation.13-16 Although CES has been studied in a variety of patient populations, its effectiveness is not well understood.16 Research on the effects of CES on neurotransmitter levels as well as activation of parts of the brain involved in pain reception and transmission should clarify these mechanisms. Research has shown improvements in sleep and mood as well as overall pain reduction.11,16 Research has focused primarily on individual modalities rather than on combination devices and has been conducted on populations unlike the veteran population (eg, women with fibromyalgia).
Most of the devices that use electrical or LLLT cannot be used safely by patients who have implantable electrical devices or have medical conditions such as unstable seizures, pregnancy, and active malignancies.
The most common adverse effects (AEs) of CES—dizziness and headaches—are minimal compared with the AEs of pain medications. MET and LLLT AEs generally are limited to skin irritation and muscle soreness.11 Most devices require a prescription, and manufacturers provide training for purchase.
The Pain Program
EOVAHCS initially established its consultative pain program in 2013 to provide support, recommendations, and education about managing pain in veterans to primary care providers (PCPs). Veterans are referred to the pain program for a face-to-face assessment and set of recommendations to assist in developing a comprehensive pain treatment plan. Consistent with its multimodal, biopsychosocial rehabilitation model approach, the program also offers several chronic pain treatment services, including patient education courses, cognitive behavioral therapy (CBT) for chronic pain, chiropractic care, biofeedback, relaxation training, steroid injections, pain coaching, and a pain modality (noninvasive device) clinic. During their assessment, veterans are evaluated for the appropriateness of these programs, including treatment through the Pain Modality Clinic.
Pain Modality Clinic
The EOVAHCS Pain Modality Clinic was created in 2014 as a treatment and device-trial program to provide veterans access to newer noninvasive, patient-driven treatment devices as part of an active chronic pain self-management plan. A crucial innovation is that these devices are designed to be used by patients in their homes. These devices can be expensive, and not every patient will benefit from their use; therefore, clinic leaders recommended a trial before a device is issued to a veteran for home use.
The Pain Modality Clinic coordinator trains clinic facilitators on the device according to manufacturer’s guidelines. Each participating veteran takes part in a device trial to confirm that he or she is able to use the recommended device independently and is likely to benefit from its use. When appropriate, veterans who do not respond to the initial device trial could test the potential benefit of another device. Although data from these device trials are collected primarily to inform clinical decision making, this information also is useful in guiding local policy regarding continued support for each of the modalities.
Veterans who have chronic or persistent pain (≥ 3 months) that interferes with function or quality of life are considered good candidates for a device trial if they are actively involved in pain self-care, logistically able to participate, able to use a device long-term, and have no contraindications. “Active involvement” could be met by participation in any pain management effort, whether a specific exercise program, CBT, or other treatment.
The Modality Clinic currently offers device trials for persistent pain with Alpha-Stim-M (AS-M; Electromedical Products International, Mineral Wells, TX), Laser Touch One (LTO; Renewal Technologies, LLC, Phoenix, AZ), and Neurolumen (Oklahoma City, OK). Neurolumen devices were not available in the clinic initially and will not be discussed further in this article.
The first Alpha-Stim machine using MET and CES technology was created in 1981 for in-office pain management. In 2012, the currently used AS-M became available.11 AS-M is FDA approved for treating pain, anxiety, depression, and sleep problems and is the device used in the EOVAHCS Modality Clinic. AS-M uses probes or electrodes to send a MET waveform through the body area in pain. The device uses ear clips to provide CES, which is thought to increase alpha waves in the brain.11 The LTO is a device that combines LLLT and MET technologies in a home-use design.14 LTO is FDA approved for treating painand is a portable personal pain-relief device applied to the area of pain using electroconductive gel.
Both devices are designed for long-term, self-use, making them viable parts of a multimodal, chronic pain treatment plan. Contraindications for AS-M and LTO include having a pacemaker or an implantable defibrillator, pregnancy, current malignancy, or seizures. Eligible veterans with persistent pain and high levels of depression, anxiety, and/or sleep problems generally are triaged to AS-M, whereas those who have only pain intensity issues usually are assigned to LTO. Referral to the Modality Clinic is not limited to a specific type of pain; common pain conditions seen in the clinic are spine and joint pain, arthritis pain, myofascial pain, headaches, and neuropathy.
Training and Device Trials
Eligible veterans are educated about the device and complete clinical informed consent, which is documented in the electronic health record. The veterans’ primary care and/or specialist providers are contacted for concurrence regarding veterans’ participation in the treatment.
Protocols for the device trials are based on the manufacturers’ recommendations, adjusted to what is feasible in the clinic (manufacturers approved the changes). The number of treatments per trial varies by device. For AS-M, veterans come to the clinic 5 days a week for 2 weeks. For LTO, veterans attend the clinic 5 days a week for 1 week.
At the beginning of a device trial, a trained facilitator teaches each veteran and caregiver to use the device, sets functional goals for the trial, and provides education on the trial questionnaires and daily pain logs. The veteran then follows the device protocol in the clinic where the facilitator can respond to questions and address any issues. With support from their caregivers, veterans are expected to become independent on their device use by the end of the trial. Clinic staff or the veteran can stop the device trial at any point, without affecting the veteran’s participation in or eligibility for other EOVAHCS pain programs.
This project was submitted to the University of Oklahoma Health Sciences Center Institutional Review Board and was exempted from institutional review board oversight as a retrospective, quality improvement effort. Before data analysis, the EOVAHCS Coordinator for Research and Development reviewed the procedures to ensure that all policies were being followed.
Methods
Data for veterans who completed valid treatments of AS-M or LTO from May 9, 2014 to August 20, 2016, were included in the analyses. For an AS-M treatment to be considered valid, the veteran must have attended at least 8 sessions and completed assessment instruments at baseline (preintervention) and following completion (postintervention). For an LTO treatment to be considered valid, the veteran must have attended at least 4 sessions and completed assessment measures at baseline and after completion.
Measures
Veterans completed the following measures at baseline and after trial completion:
The Beck Depression Inventory (BDI-II) is a 21-item measure designed to assess depressive symptoms. Each item assesses intensity on a 0-to-3 scale. Scores from 0 to 13 indicate minimum depression; 14 to 19, mild depression; 20 to 28, moderate depression, and 29 to 63, severe depression.17
The Beck Anxiety Inventory (BAI) is a 21-item measure of anxiety symptoms that uses a 0-to-3 scale to assess severity of subjective, somatic, or panic-related symptoms of anxiety. Scores ranging from 0 to 9 indicate minimal anxiety; 10 to 16, mild anxiety; 17 to 29, moderate anxiety, and 30 to 63, severe anxiety.18
The Pain Catastrophizing Scale (PCS) is a 13-item measure of pain catastrophizing, a crucial marker of how individuals experience pain. Items are scored on a 0-to-4 scale; scores of ≥ 30 indicate a clinically relevant level of catastrophizing.19
The Subjective Units of Distress Scale (SUD) is a single-item measure of the subjective intensity of disturbance or distress currently being experienced. It is scored from 0 to 10; 1 to 4 is mild, 5 to 6 is moderate, and 7 to 10 is severe.20
The Brief Pain Inventory (BPI) measures pain intensity and the impact of pain on functioning. Four items assess pain intensity at its worst, least, and average over the previous 24 hours and at the time of assessment; responses are on a 0-to-10 scale with 10 being most severe. The pain intensity measure is the average of scores on these 4 items. Pain interference is measured with respect to 7 daily activities; general activity, walking, work, mood, enjoyment of life, relations with others, and sleep. Each of these items is scored on a 0-to-10 scale with 10 being the most severe. The pain interference measure is the average of scores on these 7 items.21
Participants completed a daily pain log and recorded self-ratings (0-to-10 scale) of pain and relaxation levels before and after using the device. These scores were primarily used to assist in determining whether goals, set collaboratively by the clinician and the veteran at the first session, had been met.
Analysis
Descriptive statistics were used to characterize the sample overall and by modality. Paired t tests were used to assess changes on each assessment measure over time and for each device separately. The significance of change was assessed for 8 outcomes for each device. In this context, using a conservative Bonferroni correction, significance was set at P < .006. Because AS-M is designed to address depression, anxiety, and sleep as well as pain, whereas LTO is not, device assignments were based on clinical considerations rather than randomization. Therefore, no comparisons were made between devices, and outcomes were assessed independently for the 2 devices. Analyses were performed using SAS 9.4 (Cary, NC).
Results
Device trials were initiated for 161 veterans (LTO, 70; AS-M, 91). Distribution of devices was unequal because veterans are assigned to 1 device or the other based on clinical presentation. Failure to complete a trial (n = 46; 28.6%) typically was because of travel barriers, lack of interest in continuing, and for 3 veterans, reports of headaches that they attributed to the AS-M treatment. Of the 115 participants who completed valid trials, 88 (76.5%) also completed assessment measures at pre- and postintervention (LTO = 38; AS-M = 50). None of the participants in this study completed trials with both the AS-M and LTO devices.
Most participants were male (84.1%) and rural residents (85.5%) (Table 1).
Pain Reduction
Treatment with AS-M or LTO was associated with statistically significant reductions in pain severity (BPI), pain interference (BPI), daily pain intensity scores (daily pain log), and pain catastrophizing (PCS) (Tables 2 and 3).
Impact on Mood
Use of AS-M was associated with statistically significant improvements in depression (BDI-II), anxiety (BAI), and distress (SUD) scores. In addition, veterans completing AS-M treatment showed a statistically significant improvement in self-reported relaxation scores. Interestingly, use of LTO also resulted in a statistically significant decrease in anxiety (BAI) and a nonstatistically significant decrease in depression (BDI-II).
Figure 1 and 2 illustrates the clinical impact of each device in shifting participants from 1 level of symptom severity to another.
Discussion
Use of both AS-M and LTO at EOVAHC was associated with reduced pain intensity. The devices also had positive effects beyond pain in areas such as depression, anxiety, and distress. Remission of depression and anxiety symptoms has been associated with significant decline in pain symptoms, suggesting that pain is best treated through multimodal approaches.22
In the context of the opioid crisis, the availability of effective nonopioid, nonpharmacologic, noninvasive treatments for chronic pain is needed. The Joint Commission recently expanded its pain management guidelines to support hospitals offering nonpharmacologic pain treatments.23 Integrating AS-M, LTO, or similar products into standard pain management practices allows for other treatment pathways with positive outcomes for providers and patients. The Joint Commission also recommends an interdisciplinary approach, defined as a process whereby health care professionals from different disciplines collaborate to diagnose and treat patients experiencing difficult pain conditions. This approach facilitates multimodal management because these disciplines contribute knowledge about a variety of treatment options. Devices such AS-M and LTO are well suited to interdisciplinary pain management because they are not seen as being under the purview of a specific health care specialty.
Limitations
Our findings are limited because they are derived from a retrospective, quality improvement evaluation of outcomes from a single clinic. Findings must be considered in the context of the relatively small samples of veterans. Because analyses were conducted as part of a quality improvement effort, veterans were offered a specific device based on clinical indications, there were no comparisons between devices, and there was no comparison group. Although most participants were using medication and other treatments as part of their pain treatment plan, all reported continued pain intensity before use of a device. Analyses did not control for variation in treatments received concurrently. Last, the logs used to collect self-report data on daily pain and relaxation levels were not validated.
The data highlight a clear need for research to better understand the long-term effects of these devices as well as the characteristics of patients who respond best to each device. Noninvasive treatments for pain often are dismissed as placebos. Rigorously designed, controlled studies will help demonstrate that these devices offer a statistically significant benefit beyond any placebo effect.
Conclusion
Understanding of chronic pain and its treatment will continue to evolve. It is clear that each person dealing with chronic pain requires a tailored combination of treatments and multimodal approaches, which is more effective than any single treatment. Nonpharmacologic, noninvasive devices pose fewer risks and seem to be more effective in reducing pain intensity than traditional treatments, including medications or surgical intervention. In light of the current emphasis on evidence-based health care and as the evidence for the effectiveness of noninvasive pain devices modalities grows, it is likely that treatments incorporating modalities such as MET, CES, and LLLT will become common options for managing chronic pain.
Chronic pain is common among veterans treated in Veterans Health Administration (VHA) facilities, and optimal management remains challenging in the context of the national opioid misuse epidemic. The Eastern Oklahoma VA Health Care System (EOVAHCS) Pain Program offers a range of services that allow clinicians to tailor multimodal treatment strategies to a veteran’s needs. In 2014, a Modality Clinic was established to assess the utility of adding noninvasive treatment devices to the pain program’s armamentarium. This article addresses the context for introducing these devices and describes the EOVAHCS Pain Program and Modality Clinic. Also discussed are procedures and findings from an initial quality improvement evaluation designed to inform decision making regarding retention, expansion, or elimination of the EOVAHCS noninvasive, pain treatment device program.
Opioid prescriptions increased from 76 million in 1991 to 219 million in 2011. In 2011, the annual cost of chronic pain in the US was estimated at $635 billion.1-6 The confluence of an increasing concern about undertreatment of pain and overconfidence for the safety of opioids led to what former US Surgeon General Vivek H. Murthy, MD, called the opioid crisis.7 As awareness of its unintended consequences of opioid prescribing increased, the VHA began looking for nonopioid treatments that would decrease pain intensity. The 1993 article by Kehlet and Dahl was one of the first discussions of a multimodal nonpharmacologic strategy for addressing acute postoperative pain.8 Their pivotal literature review concluded that nonpharmacologic modalities, such as acupuncture, cranial manipulation, cranial electrostimulation treatment (CES), and low-level light technologies (LLLT), carried less risk and produced equal or greater clinical effects than those of drug therapies.8
Multimodal treatment approaches increasingly are encouraged, and nonopioid pain control has become more common across medical disciplines from physical therapy to anesthesiology.8-10 Innovative, noninvasive devices designed for self-use have appeared on the market. Many of these devices incorporate microcurrent electrical therapy (MET), CES, and/or LLLT (also known as cold laser).11-16 LLLT is a light modality that seems to lead to increased ATP production, resulting in improved healing and decreased inflammation.13-16 Although CES has been studied in a variety of patient populations, its effectiveness is not well understood.16 Research on the effects of CES on neurotransmitter levels as well as activation of parts of the brain involved in pain reception and transmission should clarify these mechanisms. Research has shown improvements in sleep and mood as well as overall pain reduction.11,16 Research has focused primarily on individual modalities rather than on combination devices and has been conducted on populations unlike the veteran population (eg, women with fibromyalgia).
Most of the devices that use electrical or LLLT cannot be used safely by patients who have implantable electrical devices or have medical conditions such as unstable seizures, pregnancy, and active malignancies.
The most common adverse effects (AEs) of CES—dizziness and headaches—are minimal compared with the AEs of pain medications. MET and LLLT AEs generally are limited to skin irritation and muscle soreness.11 Most devices require a prescription, and manufacturers provide training for purchase.
The Pain Program
EOVAHCS initially established its consultative pain program in 2013 to provide support, recommendations, and education about managing pain in veterans to primary care providers (PCPs). Veterans are referred to the pain program for a face-to-face assessment and set of recommendations to assist in developing a comprehensive pain treatment plan. Consistent with its multimodal, biopsychosocial rehabilitation model approach, the program also offers several chronic pain treatment services, including patient education courses, cognitive behavioral therapy (CBT) for chronic pain, chiropractic care, biofeedback, relaxation training, steroid injections, pain coaching, and a pain modality (noninvasive device) clinic. During their assessment, veterans are evaluated for the appropriateness of these programs, including treatment through the Pain Modality Clinic.
Pain Modality Clinic
The EOVAHCS Pain Modality Clinic was created in 2014 as a treatment and device-trial program to provide veterans access to newer noninvasive, patient-driven treatment devices as part of an active chronic pain self-management plan. A crucial innovation is that these devices are designed to be used by patients in their homes. These devices can be expensive, and not every patient will benefit from their use; therefore, clinic leaders recommended a trial before a device is issued to a veteran for home use.
The Pain Modality Clinic coordinator trains clinic facilitators on the device according to manufacturer’s guidelines. Each participating veteran takes part in a device trial to confirm that he or she is able to use the recommended device independently and is likely to benefit from its use. When appropriate, veterans who do not respond to the initial device trial could test the potential benefit of another device. Although data from these device trials are collected primarily to inform clinical decision making, this information also is useful in guiding local policy regarding continued support for each of the modalities.
Veterans who have chronic or persistent pain (≥ 3 months) that interferes with function or quality of life are considered good candidates for a device trial if they are actively involved in pain self-care, logistically able to participate, able to use a device long-term, and have no contraindications. “Active involvement” could be met by participation in any pain management effort, whether a specific exercise program, CBT, or other treatment.
The Modality Clinic currently offers device trials for persistent pain with Alpha-Stim-M (AS-M; Electromedical Products International, Mineral Wells, TX), Laser Touch One (LTO; Renewal Technologies, LLC, Phoenix, AZ), and Neurolumen (Oklahoma City, OK). Neurolumen devices were not available in the clinic initially and will not be discussed further in this article.
The first Alpha-Stim machine using MET and CES technology was created in 1981 for in-office pain management. In 2012, the currently used AS-M became available.11 AS-M is FDA approved for treating pain, anxiety, depression, and sleep problems and is the device used in the EOVAHCS Modality Clinic. AS-M uses probes or electrodes to send a MET waveform through the body area in pain. The device uses ear clips to provide CES, which is thought to increase alpha waves in the brain.11 The LTO is a device that combines LLLT and MET technologies in a home-use design.14 LTO is FDA approved for treating painand is a portable personal pain-relief device applied to the area of pain using electroconductive gel.
Both devices are designed for long-term, self-use, making them viable parts of a multimodal, chronic pain treatment plan. Contraindications for AS-M and LTO include having a pacemaker or an implantable defibrillator, pregnancy, current malignancy, or seizures. Eligible veterans with persistent pain and high levels of depression, anxiety, and/or sleep problems generally are triaged to AS-M, whereas those who have only pain intensity issues usually are assigned to LTO. Referral to the Modality Clinic is not limited to a specific type of pain; common pain conditions seen in the clinic are spine and joint pain, arthritis pain, myofascial pain, headaches, and neuropathy.
Training and Device Trials
Eligible veterans are educated about the device and complete clinical informed consent, which is documented in the electronic health record. The veterans’ primary care and/or specialist providers are contacted for concurrence regarding veterans’ participation in the treatment.
Protocols for the device trials are based on the manufacturers’ recommendations, adjusted to what is feasible in the clinic (manufacturers approved the changes). The number of treatments per trial varies by device. For AS-M, veterans come to the clinic 5 days a week for 2 weeks. For LTO, veterans attend the clinic 5 days a week for 1 week.
At the beginning of a device trial, a trained facilitator teaches each veteran and caregiver to use the device, sets functional goals for the trial, and provides education on the trial questionnaires and daily pain logs. The veteran then follows the device protocol in the clinic where the facilitator can respond to questions and address any issues. With support from their caregivers, veterans are expected to become independent on their device use by the end of the trial. Clinic staff or the veteran can stop the device trial at any point, without affecting the veteran’s participation in or eligibility for other EOVAHCS pain programs.
This project was submitted to the University of Oklahoma Health Sciences Center Institutional Review Board and was exempted from institutional review board oversight as a retrospective, quality improvement effort. Before data analysis, the EOVAHCS Coordinator for Research and Development reviewed the procedures to ensure that all policies were being followed.
Methods
Data for veterans who completed valid treatments of AS-M or LTO from May 9, 2014 to August 20, 2016, were included in the analyses. For an AS-M treatment to be considered valid, the veteran must have attended at least 8 sessions and completed assessment instruments at baseline (preintervention) and following completion (postintervention). For an LTO treatment to be considered valid, the veteran must have attended at least 4 sessions and completed assessment measures at baseline and after completion.
Measures
Veterans completed the following measures at baseline and after trial completion:
The Beck Depression Inventory (BDI-II) is a 21-item measure designed to assess depressive symptoms. Each item assesses intensity on a 0-to-3 scale. Scores from 0 to 13 indicate minimum depression; 14 to 19, mild depression; 20 to 28, moderate depression, and 29 to 63, severe depression.17
The Beck Anxiety Inventory (BAI) is a 21-item measure of anxiety symptoms that uses a 0-to-3 scale to assess severity of subjective, somatic, or panic-related symptoms of anxiety. Scores ranging from 0 to 9 indicate minimal anxiety; 10 to 16, mild anxiety; 17 to 29, moderate anxiety, and 30 to 63, severe anxiety.18
The Pain Catastrophizing Scale (PCS) is a 13-item measure of pain catastrophizing, a crucial marker of how individuals experience pain. Items are scored on a 0-to-4 scale; scores of ≥ 30 indicate a clinically relevant level of catastrophizing.19
The Subjective Units of Distress Scale (SUD) is a single-item measure of the subjective intensity of disturbance or distress currently being experienced. It is scored from 0 to 10; 1 to 4 is mild, 5 to 6 is moderate, and 7 to 10 is severe.20
The Brief Pain Inventory (BPI) measures pain intensity and the impact of pain on functioning. Four items assess pain intensity at its worst, least, and average over the previous 24 hours and at the time of assessment; responses are on a 0-to-10 scale with 10 being most severe. The pain intensity measure is the average of scores on these 4 items. Pain interference is measured with respect to 7 daily activities; general activity, walking, work, mood, enjoyment of life, relations with others, and sleep. Each of these items is scored on a 0-to-10 scale with 10 being the most severe. The pain interference measure is the average of scores on these 7 items.21
Participants completed a daily pain log and recorded self-ratings (0-to-10 scale) of pain and relaxation levels before and after using the device. These scores were primarily used to assist in determining whether goals, set collaboratively by the clinician and the veteran at the first session, had been met.
Analysis
Descriptive statistics were used to characterize the sample overall and by modality. Paired t tests were used to assess changes on each assessment measure over time and for each device separately. The significance of change was assessed for 8 outcomes for each device. In this context, using a conservative Bonferroni correction, significance was set at P < .006. Because AS-M is designed to address depression, anxiety, and sleep as well as pain, whereas LTO is not, device assignments were based on clinical considerations rather than randomization. Therefore, no comparisons were made between devices, and outcomes were assessed independently for the 2 devices. Analyses were performed using SAS 9.4 (Cary, NC).
Results
Device trials were initiated for 161 veterans (LTO, 70; AS-M, 91). Distribution of devices was unequal because veterans are assigned to 1 device or the other based on clinical presentation. Failure to complete a trial (n = 46; 28.6%) typically was because of travel barriers, lack of interest in continuing, and for 3 veterans, reports of headaches that they attributed to the AS-M treatment. Of the 115 participants who completed valid trials, 88 (76.5%) also completed assessment measures at pre- and postintervention (LTO = 38; AS-M = 50). None of the participants in this study completed trials with both the AS-M and LTO devices.
Most participants were male (84.1%) and rural residents (85.5%) (Table 1).
Pain Reduction
Treatment with AS-M or LTO was associated with statistically significant reductions in pain severity (BPI), pain interference (BPI), daily pain intensity scores (daily pain log), and pain catastrophizing (PCS) (Tables 2 and 3).
Impact on Mood
Use of AS-M was associated with statistically significant improvements in depression (BDI-II), anxiety (BAI), and distress (SUD) scores. In addition, veterans completing AS-M treatment showed a statistically significant improvement in self-reported relaxation scores. Interestingly, use of LTO also resulted in a statistically significant decrease in anxiety (BAI) and a nonstatistically significant decrease in depression (BDI-II).
Figure 1 and 2 illustrates the clinical impact of each device in shifting participants from 1 level of symptom severity to another.
Discussion
Use of both AS-M and LTO at EOVAHC was associated with reduced pain intensity. The devices also had positive effects beyond pain in areas such as depression, anxiety, and distress. Remission of depression and anxiety symptoms has been associated with significant decline in pain symptoms, suggesting that pain is best treated through multimodal approaches.22
In the context of the opioid crisis, the availability of effective nonopioid, nonpharmacologic, noninvasive treatments for chronic pain is needed. The Joint Commission recently expanded its pain management guidelines to support hospitals offering nonpharmacologic pain treatments.23 Integrating AS-M, LTO, or similar products into standard pain management practices allows for other treatment pathways with positive outcomes for providers and patients. The Joint Commission also recommends an interdisciplinary approach, defined as a process whereby health care professionals from different disciplines collaborate to diagnose and treat patients experiencing difficult pain conditions. This approach facilitates multimodal management because these disciplines contribute knowledge about a variety of treatment options. Devices such AS-M and LTO are well suited to interdisciplinary pain management because they are not seen as being under the purview of a specific health care specialty.
Limitations
Our findings are limited because they are derived from a retrospective, quality improvement evaluation of outcomes from a single clinic. Findings must be considered in the context of the relatively small samples of veterans. Because analyses were conducted as part of a quality improvement effort, veterans were offered a specific device based on clinical indications, there were no comparisons between devices, and there was no comparison group. Although most participants were using medication and other treatments as part of their pain treatment plan, all reported continued pain intensity before use of a device. Analyses did not control for variation in treatments received concurrently. Last, the logs used to collect self-report data on daily pain and relaxation levels were not validated.
The data highlight a clear need for research to better understand the long-term effects of these devices as well as the characteristics of patients who respond best to each device. Noninvasive treatments for pain often are dismissed as placebos. Rigorously designed, controlled studies will help demonstrate that these devices offer a statistically significant benefit beyond any placebo effect.
Conclusion
Understanding of chronic pain and its treatment will continue to evolve. It is clear that each person dealing with chronic pain requires a tailored combination of treatments and multimodal approaches, which is more effective than any single treatment. Nonpharmacologic, noninvasive devices pose fewer risks and seem to be more effective in reducing pain intensity than traditional treatments, including medications or surgical intervention. In light of the current emphasis on evidence-based health care and as the evidence for the effectiveness of noninvasive pain devices modalities grows, it is likely that treatments incorporating modalities such as MET, CES, and LLLT will become common options for managing chronic pain.
1. US Department of Veterans Affairs. Pain as the 5th Vital Sign Toolkit. https://www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf. Published October 2000. Accessed February 11, 2019.
2. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
3. Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16(5):405-416.
4. Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109(1):5-12.
5. Mosher HJ, Krebs EE, Carrel M, Kaboli PJ, Weg MW, Lund BC. Trends in prevalent and incident opioid receipt: an observational study in Veterans Health Administration 2004-2012. J Gen Intern Med. 2015;30(5):597-604.
6. Reuben DB, Alvanzo AAH, Ashikaga T, et al. National Institutes of Health Pathways to Prevention Workshop: The role of opioids in the treatment of chronic pain. Ann Intern Med. 2015;162(4):295-300.
7. Murthy VH. Opioid epidemic: we all have a role in turning the tide. https://obamawhitehouse.archives.gov/blog/2016/10/05/opioid-epidemic-we-all-have-role-turning-tide. Published October 5, 2016. Accessed February 12, 2019.
8. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048-1056.
9. Crane P, Feinberg L, Morris J. A multimodal physical therapy approach to the management of a patient with temporomandibular dysfunction and head and neck lymphedema: a case report. J Man Manip Ther. 2015;23(1): 37-42.
10. Arnstein P. Multimodal approaches to pain management. Nurs. 2011;41(3): 60-61.
11. Alpha-Stim. http://www.alpha-stim.com. Accessed March 22, 2019
12. Shekelle PG, Cook IA, Miake-Lye IM, Booth MS, Beroes JM, Mak S. Benefits and harms of cranial electrical stimulation for chronic painful conditions, depression, anxiety, and insomnia. Ann Intern Med. 2018;168(6):414-421.
13. Chow RT, Heller GZ, Barnsley L. The effect of 300 mW, 830 nm laser on chronic neck pain: a double-blind, randomized, placebo-controlled study. Pain. 2006;124(1):201-210.
14. Kulkarni AD, Smith RB. The use of microcurrent electrical therapy and cranial electrotherapy stimulation in pain control. Clin Pract Alternative Med. 2001;2(2):99-102.
15. Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897-1908.
16. Taylor AG, Anderson JG, Riedel SL, et al. Cranial electrical stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Manag Nurs. 2013;14(4):327-335.
17. Beck, AT, Steer, RA, Brown, GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
18. Beck AT, Steer RA. Beck Anxiety Inventory: Manual. San Antonio, TX: Psychological Corporation; 1993.
19. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524-532.
20. Wolpe J. The Practice of Behavior Therapy. 4th ed. Elmsford, NY: Pergamon; 1990.
21. Cleeland CS. The Brief Pain Inventory User Manual. https://www.mdanderson.org/research/departments-labs-institutes/departments-divisions/symptom-research/symptom-assessment-tools/brief-pain-inventory.html. Published 2009. Accessed February 12, 2019.
22. Gerrits MM, van Marwijk HW, van Oppen P, Horst HVD, Penninx BW. Longitudinal association between pain, and depression and anxiety over four years. J Psychosom Res. 2015;78(1):64-70.
23. The Joint Commission. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. https://www.jointcommission.org/assets/1/18/Joint_Commission_Enhances_Pain_Assessment_and_Management_Requirements_for_Accredited_Hospitals1.PDF. Published July 2017. Accessed March 21, 2019.
1. US Department of Veterans Affairs. Pain as the 5th Vital Sign Toolkit. https://www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf. Published October 2000. Accessed February 11, 2019.
2. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
3. Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16(5):405-416.
4. Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109(1):5-12.
5. Mosher HJ, Krebs EE, Carrel M, Kaboli PJ, Weg MW, Lund BC. Trends in prevalent and incident opioid receipt: an observational study in Veterans Health Administration 2004-2012. J Gen Intern Med. 2015;30(5):597-604.
6. Reuben DB, Alvanzo AAH, Ashikaga T, et al. National Institutes of Health Pathways to Prevention Workshop: The role of opioids in the treatment of chronic pain. Ann Intern Med. 2015;162(4):295-300.
7. Murthy VH. Opioid epidemic: we all have a role in turning the tide. https://obamawhitehouse.archives.gov/blog/2016/10/05/opioid-epidemic-we-all-have-role-turning-tide. Published October 5, 2016. Accessed February 12, 2019.
8. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048-1056.
9. Crane P, Feinberg L, Morris J. A multimodal physical therapy approach to the management of a patient with temporomandibular dysfunction and head and neck lymphedema: a case report. J Man Manip Ther. 2015;23(1): 37-42.
10. Arnstein P. Multimodal approaches to pain management. Nurs. 2011;41(3): 60-61.
11. Alpha-Stim. http://www.alpha-stim.com. Accessed March 22, 2019
12. Shekelle PG, Cook IA, Miake-Lye IM, Booth MS, Beroes JM, Mak S. Benefits and harms of cranial electrical stimulation for chronic painful conditions, depression, anxiety, and insomnia. Ann Intern Med. 2018;168(6):414-421.
13. Chow RT, Heller GZ, Barnsley L. The effect of 300 mW, 830 nm laser on chronic neck pain: a double-blind, randomized, placebo-controlled study. Pain. 2006;124(1):201-210.
14. Kulkarni AD, Smith RB. The use of microcurrent electrical therapy and cranial electrotherapy stimulation in pain control. Clin Pract Alternative Med. 2001;2(2):99-102.
15. Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897-1908.
16. Taylor AG, Anderson JG, Riedel SL, et al. Cranial electrical stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Manag Nurs. 2013;14(4):327-335.
17. Beck, AT, Steer, RA, Brown, GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
18. Beck AT, Steer RA. Beck Anxiety Inventory: Manual. San Antonio, TX: Psychological Corporation; 1993.
19. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524-532.
20. Wolpe J. The Practice of Behavior Therapy. 4th ed. Elmsford, NY: Pergamon; 1990.
21. Cleeland CS. The Brief Pain Inventory User Manual. https://www.mdanderson.org/research/departments-labs-institutes/departments-divisions/symptom-research/symptom-assessment-tools/brief-pain-inventory.html. Published 2009. Accessed February 12, 2019.
22. Gerrits MM, van Marwijk HW, van Oppen P, Horst HVD, Penninx BW. Longitudinal association between pain, and depression and anxiety over four years. J Psychosom Res. 2015;78(1):64-70.
23. The Joint Commission. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. https://www.jointcommission.org/assets/1/18/Joint_Commission_Enhances_Pain_Assessment_and_Management_Requirements_for_Accredited_Hospitals1.PDF. Published July 2017. Accessed March 21, 2019.
A Primary Care Provider’s Guide to Cataract Surgery in the Very Elderly
Cataract surgery is the most commonly performed surgical procedure in the US, including within the Veterans Health Administration (VHA).1,2 As the risk of surgical complications has decreased with improved techniques and instrumentation, the threshold for performing surgery has lowered.3 A substantial number of patients do not develop clinically significant cataracts until they are “very elderly,” defined as aged ≥ 85 years by the World Health Organization and National Institute of Aging.4
Should the general approach to cataract evaluation and surgery differ in this subset of patients? Advanced age is associated with a variety of systemic and ocular comorbidities that theoretically increase the risk of cataract surgery and reduce the potential visual benefit it might yield. However, the impact of age on the outcomes of cataract surgery differs even among the very elderly. There are no universally acknowledged guidelines that address the perioperative evaluation and management of cataracts in the very elderly, whose systemic and ocular health have greater variability than those of their younger counterparts. For very elderly patients who are found to have visually significant cataracts by their ophthalmologists, input from the primary care provider (PCP), who has insight into a patient’s health and well-being, is vital for formulating a management plan. Herein, we provide a framework for PCPs to assist very elderly patients and their ophthalmologists in making an informed decision regarding cataract surgery and in planning for perioperative care.
Cataract Surgery
Cataract surgeons recommend surgical extraction when there is a clinically significant lens opacity that imposes functional impairment, such as inability to read, perform near work, watch television, or drive.4 The standard of care for a clinically significant cataract is surgical removal of the crystalline lens and replacement with an artificial intraocular lens (IOL). At times, the onset of vision loss from a cataract is insidious such that patients may not be aware of their declining vision or the deterioration in quality of life (QOL) that it causes.
Despite the higher burden of ocular comorbidity (eg, age-related macular degeneration, glaucoma) relative to their younger counterparts, most very elderly patients obtain functionally important improvement in their vision, QOL, and cognitive function after surgery.5-16 Cataract surgery can also reduce the risk of dementia and the risk of falls and hip fractures.6,9,12-14,16-18 Ophthalmic complications of cataract surgery in the very elderly include posterior capsule tear (< 1%-9%), vitreous loss (< 1%-8%), zonular rupture (2%-5%), and retained lens fragments (≤ 1%).5,8-11,17,19-21 There is no evidence from well-controlled studies that suggests that very elderly cataract surgery patients are at higher risk of ocular complications relative to that of their younger counterparts.22
Surgery Alternatives
In some very elderly patients, cataract surgery may not be the best option, and PCPs can aid in establishing an alternative plan. Such patients include those with a limited life expectancy, incapacitating anxiety over surgery, or those in whom the potential for visual improvement is marginal because of ocular or systemic comorbidities—eg, vision-limiting glaucoma or age-related macular degeneration, history of stroke to the visual pathway, or restriction to bed. Alternatives to cataract surgery in these instances include changing environmental conditions to improve visual function, such as enhanced lighting and contrast, and/or use of low-vision aids (referring patients to low-vision professionals often improves QOL).23 Low-vision specialists also have a variety of nonvisual aids that can expand functional capabilities: large-print and talking versions of reading materials, telephones, remote controls, clocks, scales, calculators, and glucose monitors; glare-free lights for stairs, floors, and counters; and specialty glasses that use light-emitting diode screens and live video streams to magnify sight.23-25
Medical Evaluation
For patients who decide to proceed with surgery, it can be helpful to have a medical evaluation by their PCPs to minimize potential complications during surgery. The very elderly may be at increased risk of intraoperative transient hypertension, restlessness, and electrocardiogram abnormalities.5,7,17 Systemic comorbidities that become more prevalent with age, such as diabetes mellitus (DM), hypertension, heart disease, chronic obstructive pulmonary disease, and dementia, may adversely impact the risk of sedation and/or general anesthesia. In the VHA, providers also must be aware of combat-related disorders that can confound cataract surgery, such as posttraumatic stress disorder (PTSD), anxiety, and claustrophobia.26,27
Anesthesia in cataract surgery ranges from topical to general, and the selection largely rests on patient physical and psychological comfort and cooperation. Often, intracameral (inside the anterior eye) anesthetic is used with topical anesthesia to provide additional comfort.27 Patients who have high levels of anxiety about surgery may not tolerate topical anesthesia alone.28 In these cases, retrobulbar anesthesia may be performed to block all sensation and motility of the eye. IV sedation is performed prior to the retrobulbar injection to calm patients. Although cataract surgery is typically performed with topical or retrobulbar anesthesia (reducing the potential for systemic complications), there are cases in which general anesthesia may be considered.27 Very elderly patients may become confused or disoriented in the operating room (OR), leading to surgical complications and less than optimal outcomes.5 A higher rate of intraoperative “restlessness,” which occurred in patients who had comorbid dementia, and transient hypertension were found in a study on cataract surgery in the very elderly, but well-controlled studies are lacking.5 Dementia can impose problems with intraoperative cooperation, which is vital for successful surgery in patients who undergo topical or local anesthesia. If these potential problems are thought likely preoperatively, light sedation or general anesthesia—in conjunction with input from the patient’s PCP—are options to minimize disruptive behavior in the OR.
Additional features of the VHA population may influence the selection of anesthesia. The VHA has an important educational mission, and retrobulbar anesthesia may be preferred to minimize unpredictable intraoperative behavior in cases where resident surgeons are performing surgery under attending supervision.27,29,30 The prevalence of PTSD among veterans also may impact the selection of anesthesia. Patients with PTSD have displayed greater levels of anxiety and more discomfort, requiring more sedation and longer surgical times compared with that of a control group.28 Ophthalmic comorbidities prevalent among the predominantly older male population in the VHA include the use of α-1 antagonist prostate medications, such as tamsulosin and terazosin. These medications are associated with intraoperative floppy iris syndrome, which can increase case difficulty and prolong operative time.29
Surgery Preparation
Cataract surgery induces minimal physiologic stress since most surgeries are performed under local or topical anesthesia. Unless the preoperative medical history or physical examination detects an active or unattended medical condition that needs to be addressed, preoperative laboratory testing is generally not required.31-33 Current general guidelines for preoperative testing for cataract surgery exist but do not address specific issues facing very elderly patients. The American Academy of Ophthalmology advises against preoperative medical tests for eye surgery unless there are medical indications: an electrocardiogram for patients with a history of heart disease, a blood glucose test for those with DM, and a potassium test for patients who are on diuretics.31 The direct correlation of age with these comorbidities may translate into higher rates of preoperative testing among very elderly patients. In the VHA, 45% of ophthalmology services studied routinely performed preoperative electrocardiography, chemical analysis, and complete blood counts prior to performing cataract surgery.27 Patients who live with chronic bacterial colonization from indwelling catheters, ostomies, or bed sores need to be given instructions for proper hygienic practices to minimize risks of postoperative infection.34
Some patients undergoing cataract surgery may not be candidates for topical or local anesthesia alone. Sedation is often used to reduce anxiety and discomfort of surgery, but very elderly patients have narrower margins of therapeutic safety because of advanced aged or medical comorbidities. Since patients need to follow basic commands in the OR for ideal surgical execution, general anesthesia may need to be considered for those with dementia, deafness, anxiety attacks, or language barriers.
Postsurgical Care
Although cataract surgery is a less invasive procedure than it was in the past, full postoperative recovery regularly spans a month. During this time, proper healing relies on the regular administration of eye drops and a refrain from heavy lifting, straining, and eye rubbing. Very elderly patients may need varying degrees of assistance with postsurgical care. For example, adherence to the regimen of eye drops can be complicated by decreased dexterity from arthritis and difficulty remembering the administration schedule in some patients. Reliable transportation also is an important factor as patients are routinely scheduled for postoperative visits at the 1- day, 1-week, and 1-month mark. PCPs can assist in ensuring patients have prearranged assistance for eye care and transportation to and from appointments. Additionally, very elderly patients with a history of constipation may benefit from stool softeners and/or laxatives to help prevent straining.
Conclusion
The limited literature on clinical outcomes of cataract surgery in the very elderly indicates that most have successful surgery and improved postoperative QOL.22 Much of the benefits derived from cataract surgery in the very elderly can be ascribed to thoughtful preoperative evaluation and planning with the PCP.
1. US Census Bureau. An aging nation: the older population in the United States. https://www.census.gov/library/publications/2014/demo/p25-1140.html Published May 2014. Accessed March 18, 2019.
2. VA Office of Inspector General. Healthcare inspection: evaluation of cataract surgeries and outcomes in veterans health administration facilities. Report No. 11-02487-158. https://www.va.gov/oig/pubs/vaoig-11-02487-158.pdf. Published March 28, 2013. Accessed March 11, 2019.
3. Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. 2017;28(1):98-103.
4. American Academy of Ophthalmology. Cataract in the adult eye preferred practice pattern—2016. https://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-2016. Published October 2016. Accessed March 19, 2019.
5. Mutoh T, Isome S, Matsumoto Y, Chikuda M. Cataract surgery in patients older than 90 years of age. Can J Ophthalmol. 2012;47(2):140-144.
6. Monestam E, Wachmeister L. Impact of cataract surgery on the visual ability of the very old. Am J Ophthalmol. 2004;137(1):145-155.
7. Lai FH, Lok JY, Chow PP, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
8. Michalska-Malecka K, Nowak M, Gos´ciniewicz P, et al. Results of cataract surgery in the very elderly population. Clin Interv Aging. 2013;8:1041-1046.
9. Syam PP, Eleftheriadis H, Casswell AG, Brittain GP, McLeod BK, Liu CS. Clinical outcome following cataract surgery in very elderly patients. Eye (Lond). 2004;18(1):59-62.
10. Rosen E, Rubowitz A, Assia EI. Visual outcome following cataract extraction in patients aged 90 years and older. Eye (Lond). 2009;23(5):1120-1124.
11. Mehmet B, Abuzer G. Results of cataract surgery in the very elderly population. J Optom. 2009;2(3):138-141.
12. To KG, Meuleners L, Bulsara M, et al. A longitudinal cohort study of the impact of first- and both-eye cataract surgery on falls and other injuries in Vietnam. Clin Interv Aging. 2014;9:743-751.
13. Song E, Sun H, Xu Y, Ma Y, Zhu H, Pan CW. Age-related cataract, cataract surgery and subsequent mortality: a systematic review and meta-analysis. PLoS One. 2014;9(11):e112054.
14. Brannan S, Dewar C, Sen J, Clarke D, Marshall T, Murray PI. A prospective study of the rate of falls before and after cataract surgery. Br J Ophthalmol. 2003;87(5):560-562.
15. Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol. 2011;95(1):17-23.
16. Yu WK, Chen YT, Wang SJ, Kuo SC, Shia BC, Liu CJ. Cataract surgery is associated with a reduced risk of dementia: a nationwide population-based cohort study. Eur J Neurol. 2015;22(10):1370-1377, e1379-1380.
17. Tseng VL, Greenberg PB, Wu WC, et al. Cataract surgery complications in nonagenarians. Ophthalmology. 2011;118(7):1229-1235.
18. Jefferis JM, Clarke MP, Taylor JP. Effect of cataract surgery on cognition, mood, and visual hallucinations in older adults. J Cataract Refract Surg. 2015;41(6):1241-1247.
19. Celebi AR. The relationship between age and the intraoperative complication rate during phacoemulsification surgery. Aging Clin Exp Res. 2014;26(2):177-181.
20. Berler DK. Intraoperative complications during cataract surgery in the very old. Trans Am Ophthalmol Soc. 2000;98:127-130; discussion 130-132.
21. Lai FHP, Lok JYC, Chow PPC, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
22. Li E, Margo CE, Greenberg PB. Cataract surgery outcomes in the very elderly. J Cataract Refract Surg. 2018;44(9):1144-1149.
23. Young JS. Age-related eye diseases and recommendations for low-vision AIDS. Home Healthc Now. 2015;33(1):10-17; quiz 18-19.
24. Virgili G, Acosta R, Grover LL, Bentley SA, Giacomelli G. Reading aids for adults with low vision. Cochrane Database Syst Rev. 2013;(10):CD003303.
25. Young JS. Age-related eye diseases: a review of current treatment and recommendations for low-vision aids. Home Healthc Nurse. 2008;26(8):464-471; quiz 472-473.
26. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans study. Prim Care Companion CNS Disord. 2017;19(3):17m02118.
27. Havnaer AG, Greenberg PB, Cockerham GC, Clark MA, Chomsky A. Cataract surgery practices in the United States Veterans Health Administration. J Cataract Refract Surg. 2017;43(4):543-551.
28. Rapoport Y, Wayman LL, Chomsky AS. The effect of post-traumatic-stress-disorder on intra-operative analgesia in a veteran population during cataract procedures carried out using retrobulbar or topical anesthesia: a retrospective study. BMC Ophthalmol. 2017;17(1):85.
29. Payal AR, Gonzalez-Gonzalez LA, Chen X, et al. Outcomes of cataract surgery with residents as primary surgeons in the Veterans Affairs Healthcare System. J Cataract Refract Surg. 2016;42(3):370-384.
30. US Department of Veterans Affairs. Mission of the office of academic affiliations. https://www.va.gov/oaa/oaa_mission.asp. Updated November 30, 2018. Accessed March 18, 2019.
31. American Academy of Ophthalmology. Choosing wisely: five things ophthalmologists and patients should question. https://www.aao.org/choosing-wisely. Published February 2013. Accessed March 18, 2019.
32. Martin SK, Cifu AS. Routine preoperative laboratory tests for elective surgery. JAMA. 2017;318(6):567-568.
33. Schein OD, Katz J, Bass EB, et al; Study of Medical Testing for Cataract Surgery. The value of routine preoperative medical testing before cataract surgery. N Engl J Med. 2000;342(3):168-175.
34. Margo CE. Asymptomatic bacteriuria and acute-onset endophthalmitis after cataract surgery. Can J Ophthalmol. 2015;50(4):e51-52.
35. Fukui K, Fujioka M, Yamasaki K, Yamakawa S, Matsuo H, Noguchi M. Risk factors for postoperative complications among the elderly after plastic surgery procedures performed under general anesthesia. Plast Surg Int. 2018:7053839.
Cataract surgery is the most commonly performed surgical procedure in the US, including within the Veterans Health Administration (VHA).1,2 As the risk of surgical complications has decreased with improved techniques and instrumentation, the threshold for performing surgery has lowered.3 A substantial number of patients do not develop clinically significant cataracts until they are “very elderly,” defined as aged ≥ 85 years by the World Health Organization and National Institute of Aging.4
Should the general approach to cataract evaluation and surgery differ in this subset of patients? Advanced age is associated with a variety of systemic and ocular comorbidities that theoretically increase the risk of cataract surgery and reduce the potential visual benefit it might yield. However, the impact of age on the outcomes of cataract surgery differs even among the very elderly. There are no universally acknowledged guidelines that address the perioperative evaluation and management of cataracts in the very elderly, whose systemic and ocular health have greater variability than those of their younger counterparts. For very elderly patients who are found to have visually significant cataracts by their ophthalmologists, input from the primary care provider (PCP), who has insight into a patient’s health and well-being, is vital for formulating a management plan. Herein, we provide a framework for PCPs to assist very elderly patients and their ophthalmologists in making an informed decision regarding cataract surgery and in planning for perioperative care.
Cataract Surgery
Cataract surgeons recommend surgical extraction when there is a clinically significant lens opacity that imposes functional impairment, such as inability to read, perform near work, watch television, or drive.4 The standard of care for a clinically significant cataract is surgical removal of the crystalline lens and replacement with an artificial intraocular lens (IOL). At times, the onset of vision loss from a cataract is insidious such that patients may not be aware of their declining vision or the deterioration in quality of life (QOL) that it causes.
Despite the higher burden of ocular comorbidity (eg, age-related macular degeneration, glaucoma) relative to their younger counterparts, most very elderly patients obtain functionally important improvement in their vision, QOL, and cognitive function after surgery.5-16 Cataract surgery can also reduce the risk of dementia and the risk of falls and hip fractures.6,9,12-14,16-18 Ophthalmic complications of cataract surgery in the very elderly include posterior capsule tear (< 1%-9%), vitreous loss (< 1%-8%), zonular rupture (2%-5%), and retained lens fragments (≤ 1%).5,8-11,17,19-21 There is no evidence from well-controlled studies that suggests that very elderly cataract surgery patients are at higher risk of ocular complications relative to that of their younger counterparts.22
Surgery Alternatives
In some very elderly patients, cataract surgery may not be the best option, and PCPs can aid in establishing an alternative plan. Such patients include those with a limited life expectancy, incapacitating anxiety over surgery, or those in whom the potential for visual improvement is marginal because of ocular or systemic comorbidities—eg, vision-limiting glaucoma or age-related macular degeneration, history of stroke to the visual pathway, or restriction to bed. Alternatives to cataract surgery in these instances include changing environmental conditions to improve visual function, such as enhanced lighting and contrast, and/or use of low-vision aids (referring patients to low-vision professionals often improves QOL).23 Low-vision specialists also have a variety of nonvisual aids that can expand functional capabilities: large-print and talking versions of reading materials, telephones, remote controls, clocks, scales, calculators, and glucose monitors; glare-free lights for stairs, floors, and counters; and specialty glasses that use light-emitting diode screens and live video streams to magnify sight.23-25
Medical Evaluation
For patients who decide to proceed with surgery, it can be helpful to have a medical evaluation by their PCPs to minimize potential complications during surgery. The very elderly may be at increased risk of intraoperative transient hypertension, restlessness, and electrocardiogram abnormalities.5,7,17 Systemic comorbidities that become more prevalent with age, such as diabetes mellitus (DM), hypertension, heart disease, chronic obstructive pulmonary disease, and dementia, may adversely impact the risk of sedation and/or general anesthesia. In the VHA, providers also must be aware of combat-related disorders that can confound cataract surgery, such as posttraumatic stress disorder (PTSD), anxiety, and claustrophobia.26,27
Anesthesia in cataract surgery ranges from topical to general, and the selection largely rests on patient physical and psychological comfort and cooperation. Often, intracameral (inside the anterior eye) anesthetic is used with topical anesthesia to provide additional comfort.27 Patients who have high levels of anxiety about surgery may not tolerate topical anesthesia alone.28 In these cases, retrobulbar anesthesia may be performed to block all sensation and motility of the eye. IV sedation is performed prior to the retrobulbar injection to calm patients. Although cataract surgery is typically performed with topical or retrobulbar anesthesia (reducing the potential for systemic complications), there are cases in which general anesthesia may be considered.27 Very elderly patients may become confused or disoriented in the operating room (OR), leading to surgical complications and less than optimal outcomes.5 A higher rate of intraoperative “restlessness,” which occurred in patients who had comorbid dementia, and transient hypertension were found in a study on cataract surgery in the very elderly, but well-controlled studies are lacking.5 Dementia can impose problems with intraoperative cooperation, which is vital for successful surgery in patients who undergo topical or local anesthesia. If these potential problems are thought likely preoperatively, light sedation or general anesthesia—in conjunction with input from the patient’s PCP—are options to minimize disruptive behavior in the OR.
Additional features of the VHA population may influence the selection of anesthesia. The VHA has an important educational mission, and retrobulbar anesthesia may be preferred to minimize unpredictable intraoperative behavior in cases where resident surgeons are performing surgery under attending supervision.27,29,30 The prevalence of PTSD among veterans also may impact the selection of anesthesia. Patients with PTSD have displayed greater levels of anxiety and more discomfort, requiring more sedation and longer surgical times compared with that of a control group.28 Ophthalmic comorbidities prevalent among the predominantly older male population in the VHA include the use of α-1 antagonist prostate medications, such as tamsulosin and terazosin. These medications are associated with intraoperative floppy iris syndrome, which can increase case difficulty and prolong operative time.29
Surgery Preparation
Cataract surgery induces minimal physiologic stress since most surgeries are performed under local or topical anesthesia. Unless the preoperative medical history or physical examination detects an active or unattended medical condition that needs to be addressed, preoperative laboratory testing is generally not required.31-33 Current general guidelines for preoperative testing for cataract surgery exist but do not address specific issues facing very elderly patients. The American Academy of Ophthalmology advises against preoperative medical tests for eye surgery unless there are medical indications: an electrocardiogram for patients with a history of heart disease, a blood glucose test for those with DM, and a potassium test for patients who are on diuretics.31 The direct correlation of age with these comorbidities may translate into higher rates of preoperative testing among very elderly patients. In the VHA, 45% of ophthalmology services studied routinely performed preoperative electrocardiography, chemical analysis, and complete blood counts prior to performing cataract surgery.27 Patients who live with chronic bacterial colonization from indwelling catheters, ostomies, or bed sores need to be given instructions for proper hygienic practices to minimize risks of postoperative infection.34
Some patients undergoing cataract surgery may not be candidates for topical or local anesthesia alone. Sedation is often used to reduce anxiety and discomfort of surgery, but very elderly patients have narrower margins of therapeutic safety because of advanced aged or medical comorbidities. Since patients need to follow basic commands in the OR for ideal surgical execution, general anesthesia may need to be considered for those with dementia, deafness, anxiety attacks, or language barriers.
Postsurgical Care
Although cataract surgery is a less invasive procedure than it was in the past, full postoperative recovery regularly spans a month. During this time, proper healing relies on the regular administration of eye drops and a refrain from heavy lifting, straining, and eye rubbing. Very elderly patients may need varying degrees of assistance with postsurgical care. For example, adherence to the regimen of eye drops can be complicated by decreased dexterity from arthritis and difficulty remembering the administration schedule in some patients. Reliable transportation also is an important factor as patients are routinely scheduled for postoperative visits at the 1- day, 1-week, and 1-month mark. PCPs can assist in ensuring patients have prearranged assistance for eye care and transportation to and from appointments. Additionally, very elderly patients with a history of constipation may benefit from stool softeners and/or laxatives to help prevent straining.
Conclusion
The limited literature on clinical outcomes of cataract surgery in the very elderly indicates that most have successful surgery and improved postoperative QOL.22 Much of the benefits derived from cataract surgery in the very elderly can be ascribed to thoughtful preoperative evaluation and planning with the PCP.
Cataract surgery is the most commonly performed surgical procedure in the US, including within the Veterans Health Administration (VHA).1,2 As the risk of surgical complications has decreased with improved techniques and instrumentation, the threshold for performing surgery has lowered.3 A substantial number of patients do not develop clinically significant cataracts until they are “very elderly,” defined as aged ≥ 85 years by the World Health Organization and National Institute of Aging.4
Should the general approach to cataract evaluation and surgery differ in this subset of patients? Advanced age is associated with a variety of systemic and ocular comorbidities that theoretically increase the risk of cataract surgery and reduce the potential visual benefit it might yield. However, the impact of age on the outcomes of cataract surgery differs even among the very elderly. There are no universally acknowledged guidelines that address the perioperative evaluation and management of cataracts in the very elderly, whose systemic and ocular health have greater variability than those of their younger counterparts. For very elderly patients who are found to have visually significant cataracts by their ophthalmologists, input from the primary care provider (PCP), who has insight into a patient’s health and well-being, is vital for formulating a management plan. Herein, we provide a framework for PCPs to assist very elderly patients and their ophthalmologists in making an informed decision regarding cataract surgery and in planning for perioperative care.
Cataract Surgery
Cataract surgeons recommend surgical extraction when there is a clinically significant lens opacity that imposes functional impairment, such as inability to read, perform near work, watch television, or drive.4 The standard of care for a clinically significant cataract is surgical removal of the crystalline lens and replacement with an artificial intraocular lens (IOL). At times, the onset of vision loss from a cataract is insidious such that patients may not be aware of their declining vision or the deterioration in quality of life (QOL) that it causes.
Despite the higher burden of ocular comorbidity (eg, age-related macular degeneration, glaucoma) relative to their younger counterparts, most very elderly patients obtain functionally important improvement in their vision, QOL, and cognitive function after surgery.5-16 Cataract surgery can also reduce the risk of dementia and the risk of falls and hip fractures.6,9,12-14,16-18 Ophthalmic complications of cataract surgery in the very elderly include posterior capsule tear (< 1%-9%), vitreous loss (< 1%-8%), zonular rupture (2%-5%), and retained lens fragments (≤ 1%).5,8-11,17,19-21 There is no evidence from well-controlled studies that suggests that very elderly cataract surgery patients are at higher risk of ocular complications relative to that of their younger counterparts.22
Surgery Alternatives
In some very elderly patients, cataract surgery may not be the best option, and PCPs can aid in establishing an alternative plan. Such patients include those with a limited life expectancy, incapacitating anxiety over surgery, or those in whom the potential for visual improvement is marginal because of ocular or systemic comorbidities—eg, vision-limiting glaucoma or age-related macular degeneration, history of stroke to the visual pathway, or restriction to bed. Alternatives to cataract surgery in these instances include changing environmental conditions to improve visual function, such as enhanced lighting and contrast, and/or use of low-vision aids (referring patients to low-vision professionals often improves QOL).23 Low-vision specialists also have a variety of nonvisual aids that can expand functional capabilities: large-print and talking versions of reading materials, telephones, remote controls, clocks, scales, calculators, and glucose monitors; glare-free lights for stairs, floors, and counters; and specialty glasses that use light-emitting diode screens and live video streams to magnify sight.23-25
Medical Evaluation
For patients who decide to proceed with surgery, it can be helpful to have a medical evaluation by their PCPs to minimize potential complications during surgery. The very elderly may be at increased risk of intraoperative transient hypertension, restlessness, and electrocardiogram abnormalities.5,7,17 Systemic comorbidities that become more prevalent with age, such as diabetes mellitus (DM), hypertension, heart disease, chronic obstructive pulmonary disease, and dementia, may adversely impact the risk of sedation and/or general anesthesia. In the VHA, providers also must be aware of combat-related disorders that can confound cataract surgery, such as posttraumatic stress disorder (PTSD), anxiety, and claustrophobia.26,27
Anesthesia in cataract surgery ranges from topical to general, and the selection largely rests on patient physical and psychological comfort and cooperation. Often, intracameral (inside the anterior eye) anesthetic is used with topical anesthesia to provide additional comfort.27 Patients who have high levels of anxiety about surgery may not tolerate topical anesthesia alone.28 In these cases, retrobulbar anesthesia may be performed to block all sensation and motility of the eye. IV sedation is performed prior to the retrobulbar injection to calm patients. Although cataract surgery is typically performed with topical or retrobulbar anesthesia (reducing the potential for systemic complications), there are cases in which general anesthesia may be considered.27 Very elderly patients may become confused or disoriented in the operating room (OR), leading to surgical complications and less than optimal outcomes.5 A higher rate of intraoperative “restlessness,” which occurred in patients who had comorbid dementia, and transient hypertension were found in a study on cataract surgery in the very elderly, but well-controlled studies are lacking.5 Dementia can impose problems with intraoperative cooperation, which is vital for successful surgery in patients who undergo topical or local anesthesia. If these potential problems are thought likely preoperatively, light sedation or general anesthesia—in conjunction with input from the patient’s PCP—are options to minimize disruptive behavior in the OR.
Additional features of the VHA population may influence the selection of anesthesia. The VHA has an important educational mission, and retrobulbar anesthesia may be preferred to minimize unpredictable intraoperative behavior in cases where resident surgeons are performing surgery under attending supervision.27,29,30 The prevalence of PTSD among veterans also may impact the selection of anesthesia. Patients with PTSD have displayed greater levels of anxiety and more discomfort, requiring more sedation and longer surgical times compared with that of a control group.28 Ophthalmic comorbidities prevalent among the predominantly older male population in the VHA include the use of α-1 antagonist prostate medications, such as tamsulosin and terazosin. These medications are associated with intraoperative floppy iris syndrome, which can increase case difficulty and prolong operative time.29
Surgery Preparation
Cataract surgery induces minimal physiologic stress since most surgeries are performed under local or topical anesthesia. Unless the preoperative medical history or physical examination detects an active or unattended medical condition that needs to be addressed, preoperative laboratory testing is generally not required.31-33 Current general guidelines for preoperative testing for cataract surgery exist but do not address specific issues facing very elderly patients. The American Academy of Ophthalmology advises against preoperative medical tests for eye surgery unless there are medical indications: an electrocardiogram for patients with a history of heart disease, a blood glucose test for those with DM, and a potassium test for patients who are on diuretics.31 The direct correlation of age with these comorbidities may translate into higher rates of preoperative testing among very elderly patients. In the VHA, 45% of ophthalmology services studied routinely performed preoperative electrocardiography, chemical analysis, and complete blood counts prior to performing cataract surgery.27 Patients who live with chronic bacterial colonization from indwelling catheters, ostomies, or bed sores need to be given instructions for proper hygienic practices to minimize risks of postoperative infection.34
Some patients undergoing cataract surgery may not be candidates for topical or local anesthesia alone. Sedation is often used to reduce anxiety and discomfort of surgery, but very elderly patients have narrower margins of therapeutic safety because of advanced aged or medical comorbidities. Since patients need to follow basic commands in the OR for ideal surgical execution, general anesthesia may need to be considered for those with dementia, deafness, anxiety attacks, or language barriers.
Postsurgical Care
Although cataract surgery is a less invasive procedure than it was in the past, full postoperative recovery regularly spans a month. During this time, proper healing relies on the regular administration of eye drops and a refrain from heavy lifting, straining, and eye rubbing. Very elderly patients may need varying degrees of assistance with postsurgical care. For example, adherence to the regimen of eye drops can be complicated by decreased dexterity from arthritis and difficulty remembering the administration schedule in some patients. Reliable transportation also is an important factor as patients are routinely scheduled for postoperative visits at the 1- day, 1-week, and 1-month mark. PCPs can assist in ensuring patients have prearranged assistance for eye care and transportation to and from appointments. Additionally, very elderly patients with a history of constipation may benefit from stool softeners and/or laxatives to help prevent straining.
Conclusion
The limited literature on clinical outcomes of cataract surgery in the very elderly indicates that most have successful surgery and improved postoperative QOL.22 Much of the benefits derived from cataract surgery in the very elderly can be ascribed to thoughtful preoperative evaluation and planning with the PCP.
1. US Census Bureau. An aging nation: the older population in the United States. https://www.census.gov/library/publications/2014/demo/p25-1140.html Published May 2014. Accessed March 18, 2019.
2. VA Office of Inspector General. Healthcare inspection: evaluation of cataract surgeries and outcomes in veterans health administration facilities. Report No. 11-02487-158. https://www.va.gov/oig/pubs/vaoig-11-02487-158.pdf. Published March 28, 2013. Accessed March 11, 2019.
3. Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. 2017;28(1):98-103.
4. American Academy of Ophthalmology. Cataract in the adult eye preferred practice pattern—2016. https://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-2016. Published October 2016. Accessed March 19, 2019.
5. Mutoh T, Isome S, Matsumoto Y, Chikuda M. Cataract surgery in patients older than 90 years of age. Can J Ophthalmol. 2012;47(2):140-144.
6. Monestam E, Wachmeister L. Impact of cataract surgery on the visual ability of the very old. Am J Ophthalmol. 2004;137(1):145-155.
7. Lai FH, Lok JY, Chow PP, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
8. Michalska-Malecka K, Nowak M, Gos´ciniewicz P, et al. Results of cataract surgery in the very elderly population. Clin Interv Aging. 2013;8:1041-1046.
9. Syam PP, Eleftheriadis H, Casswell AG, Brittain GP, McLeod BK, Liu CS. Clinical outcome following cataract surgery in very elderly patients. Eye (Lond). 2004;18(1):59-62.
10. Rosen E, Rubowitz A, Assia EI. Visual outcome following cataract extraction in patients aged 90 years and older. Eye (Lond). 2009;23(5):1120-1124.
11. Mehmet B, Abuzer G. Results of cataract surgery in the very elderly population. J Optom. 2009;2(3):138-141.
12. To KG, Meuleners L, Bulsara M, et al. A longitudinal cohort study of the impact of first- and both-eye cataract surgery on falls and other injuries in Vietnam. Clin Interv Aging. 2014;9:743-751.
13. Song E, Sun H, Xu Y, Ma Y, Zhu H, Pan CW. Age-related cataract, cataract surgery and subsequent mortality: a systematic review and meta-analysis. PLoS One. 2014;9(11):e112054.
14. Brannan S, Dewar C, Sen J, Clarke D, Marshall T, Murray PI. A prospective study of the rate of falls before and after cataract surgery. Br J Ophthalmol. 2003;87(5):560-562.
15. Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol. 2011;95(1):17-23.
16. Yu WK, Chen YT, Wang SJ, Kuo SC, Shia BC, Liu CJ. Cataract surgery is associated with a reduced risk of dementia: a nationwide population-based cohort study. Eur J Neurol. 2015;22(10):1370-1377, e1379-1380.
17. Tseng VL, Greenberg PB, Wu WC, et al. Cataract surgery complications in nonagenarians. Ophthalmology. 2011;118(7):1229-1235.
18. Jefferis JM, Clarke MP, Taylor JP. Effect of cataract surgery on cognition, mood, and visual hallucinations in older adults. J Cataract Refract Surg. 2015;41(6):1241-1247.
19. Celebi AR. The relationship between age and the intraoperative complication rate during phacoemulsification surgery. Aging Clin Exp Res. 2014;26(2):177-181.
20. Berler DK. Intraoperative complications during cataract surgery in the very old. Trans Am Ophthalmol Soc. 2000;98:127-130; discussion 130-132.
21. Lai FHP, Lok JYC, Chow PPC, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
22. Li E, Margo CE, Greenberg PB. Cataract surgery outcomes in the very elderly. J Cataract Refract Surg. 2018;44(9):1144-1149.
23. Young JS. Age-related eye diseases and recommendations for low-vision AIDS. Home Healthc Now. 2015;33(1):10-17; quiz 18-19.
24. Virgili G, Acosta R, Grover LL, Bentley SA, Giacomelli G. Reading aids for adults with low vision. Cochrane Database Syst Rev. 2013;(10):CD003303.
25. Young JS. Age-related eye diseases: a review of current treatment and recommendations for low-vision aids. Home Healthc Nurse. 2008;26(8):464-471; quiz 472-473.
26. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans study. Prim Care Companion CNS Disord. 2017;19(3):17m02118.
27. Havnaer AG, Greenberg PB, Cockerham GC, Clark MA, Chomsky A. Cataract surgery practices in the United States Veterans Health Administration. J Cataract Refract Surg. 2017;43(4):543-551.
28. Rapoport Y, Wayman LL, Chomsky AS. The effect of post-traumatic-stress-disorder on intra-operative analgesia in a veteran population during cataract procedures carried out using retrobulbar or topical anesthesia: a retrospective study. BMC Ophthalmol. 2017;17(1):85.
29. Payal AR, Gonzalez-Gonzalez LA, Chen X, et al. Outcomes of cataract surgery with residents as primary surgeons in the Veterans Affairs Healthcare System. J Cataract Refract Surg. 2016;42(3):370-384.
30. US Department of Veterans Affairs. Mission of the office of academic affiliations. https://www.va.gov/oaa/oaa_mission.asp. Updated November 30, 2018. Accessed March 18, 2019.
31. American Academy of Ophthalmology. Choosing wisely: five things ophthalmologists and patients should question. https://www.aao.org/choosing-wisely. Published February 2013. Accessed March 18, 2019.
32. Martin SK, Cifu AS. Routine preoperative laboratory tests for elective surgery. JAMA. 2017;318(6):567-568.
33. Schein OD, Katz J, Bass EB, et al; Study of Medical Testing for Cataract Surgery. The value of routine preoperative medical testing before cataract surgery. N Engl J Med. 2000;342(3):168-175.
34. Margo CE. Asymptomatic bacteriuria and acute-onset endophthalmitis after cataract surgery. Can J Ophthalmol. 2015;50(4):e51-52.
35. Fukui K, Fujioka M, Yamasaki K, Yamakawa S, Matsuo H, Noguchi M. Risk factors for postoperative complications among the elderly after plastic surgery procedures performed under general anesthesia. Plast Surg Int. 2018:7053839.
1. US Census Bureau. An aging nation: the older population in the United States. https://www.census.gov/library/publications/2014/demo/p25-1140.html Published May 2014. Accessed March 18, 2019.
2. VA Office of Inspector General. Healthcare inspection: evaluation of cataract surgeries and outcomes in veterans health administration facilities. Report No. 11-02487-158. https://www.va.gov/oig/pubs/vaoig-11-02487-158.pdf. Published March 28, 2013. Accessed March 11, 2019.
3. Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. 2017;28(1):98-103.
4. American Academy of Ophthalmology. Cataract in the adult eye preferred practice pattern—2016. https://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-2016. Published October 2016. Accessed March 19, 2019.
5. Mutoh T, Isome S, Matsumoto Y, Chikuda M. Cataract surgery in patients older than 90 years of age. Can J Ophthalmol. 2012;47(2):140-144.
6. Monestam E, Wachmeister L. Impact of cataract surgery on the visual ability of the very old. Am J Ophthalmol. 2004;137(1):145-155.
7. Lai FH, Lok JY, Chow PP, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
8. Michalska-Malecka K, Nowak M, Gos´ciniewicz P, et al. Results of cataract surgery in the very elderly population. Clin Interv Aging. 2013;8:1041-1046.
9. Syam PP, Eleftheriadis H, Casswell AG, Brittain GP, McLeod BK, Liu CS. Clinical outcome following cataract surgery in very elderly patients. Eye (Lond). 2004;18(1):59-62.
10. Rosen E, Rubowitz A, Assia EI. Visual outcome following cataract extraction in patients aged 90 years and older. Eye (Lond). 2009;23(5):1120-1124.
11. Mehmet B, Abuzer G. Results of cataract surgery in the very elderly population. J Optom. 2009;2(3):138-141.
12. To KG, Meuleners L, Bulsara M, et al. A longitudinal cohort study of the impact of first- and both-eye cataract surgery on falls and other injuries in Vietnam. Clin Interv Aging. 2014;9:743-751.
13. Song E, Sun H, Xu Y, Ma Y, Zhu H, Pan CW. Age-related cataract, cataract surgery and subsequent mortality: a systematic review and meta-analysis. PLoS One. 2014;9(11):e112054.
14. Brannan S, Dewar C, Sen J, Clarke D, Marshall T, Murray PI. A prospective study of the rate of falls before and after cataract surgery. Br J Ophthalmol. 2003;87(5):560-562.
15. Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol. 2011;95(1):17-23.
16. Yu WK, Chen YT, Wang SJ, Kuo SC, Shia BC, Liu CJ. Cataract surgery is associated with a reduced risk of dementia: a nationwide population-based cohort study. Eur J Neurol. 2015;22(10):1370-1377, e1379-1380.
17. Tseng VL, Greenberg PB, Wu WC, et al. Cataract surgery complications in nonagenarians. Ophthalmology. 2011;118(7):1229-1235.
18. Jefferis JM, Clarke MP, Taylor JP. Effect of cataract surgery on cognition, mood, and visual hallucinations in older adults. J Cataract Refract Surg. 2015;41(6):1241-1247.
19. Celebi AR. The relationship between age and the intraoperative complication rate during phacoemulsification surgery. Aging Clin Exp Res. 2014;26(2):177-181.
20. Berler DK. Intraoperative complications during cataract surgery in the very old. Trans Am Ophthalmol Soc. 2000;98:127-130; discussion 130-132.
21. Lai FHP, Lok JYC, Chow PPC, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
22. Li E, Margo CE, Greenberg PB. Cataract surgery outcomes in the very elderly. J Cataract Refract Surg. 2018;44(9):1144-1149.
23. Young JS. Age-related eye diseases and recommendations for low-vision AIDS. Home Healthc Now. 2015;33(1):10-17; quiz 18-19.
24. Virgili G, Acosta R, Grover LL, Bentley SA, Giacomelli G. Reading aids for adults with low vision. Cochrane Database Syst Rev. 2013;(10):CD003303.
25. Young JS. Age-related eye diseases: a review of current treatment and recommendations for low-vision aids. Home Healthc Nurse. 2008;26(8):464-471; quiz 472-473.
26. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans study. Prim Care Companion CNS Disord. 2017;19(3):17m02118.
27. Havnaer AG, Greenberg PB, Cockerham GC, Clark MA, Chomsky A. Cataract surgery practices in the United States Veterans Health Administration. J Cataract Refract Surg. 2017;43(4):543-551.
28. Rapoport Y, Wayman LL, Chomsky AS. The effect of post-traumatic-stress-disorder on intra-operative analgesia in a veteran population during cataract procedures carried out using retrobulbar or topical anesthesia: a retrospective study. BMC Ophthalmol. 2017;17(1):85.
29. Payal AR, Gonzalez-Gonzalez LA, Chen X, et al. Outcomes of cataract surgery with residents as primary surgeons in the Veterans Affairs Healthcare System. J Cataract Refract Surg. 2016;42(3):370-384.
30. US Department of Veterans Affairs. Mission of the office of academic affiliations. https://www.va.gov/oaa/oaa_mission.asp. Updated November 30, 2018. Accessed March 18, 2019.
31. American Academy of Ophthalmology. Choosing wisely: five things ophthalmologists and patients should question. https://www.aao.org/choosing-wisely. Published February 2013. Accessed March 18, 2019.
32. Martin SK, Cifu AS. Routine preoperative laboratory tests for elective surgery. JAMA. 2017;318(6):567-568.
33. Schein OD, Katz J, Bass EB, et al; Study of Medical Testing for Cataract Surgery. The value of routine preoperative medical testing before cataract surgery. N Engl J Med. 2000;342(3):168-175.
34. Margo CE. Asymptomatic bacteriuria and acute-onset endophthalmitis after cataract surgery. Can J Ophthalmol. 2015;50(4):e51-52.
35. Fukui K, Fujioka M, Yamasaki K, Yamakawa S, Matsuo H, Noguchi M. Risk factors for postoperative complications among the elderly after plastic surgery procedures performed under general anesthesia. Plast Surg Int. 2018:7053839.
Effects of Process Improvement on Guideline-Concordant Cardiac Enzyme Testing
In recent years, driven by accelerating health care costs and desire for improved health care value, major specialty group guidelines have incorporated resource utilization and value calculations into their recommendations. High-value care has the characteristics of enhancing outcomes, safety, and patient satisfaction at a reasonable cost. As one example, the American College of Cardiology (ACC) recently published a consensus statement on its clinical practice guidelines with a specific focus on cost and value.1 This guideline acknowledges the difficulty in incorporating value into clinical decision making but stresses a need for increased transparency and consistency to boost value in everyday practice.
Chest pain and related symptoms were listed as the second leading principle reasons for emergency department visits in the US in 2011 with 14% of patients undergoing cardiac enzyme testing.2 The ACC guidelines advocate use of troponin as the preferred laboratory test for the initial evaluation of acute coronary syndrome (ACS). Fractionated creatine kinase (CK-MB) is an acceptable alternative only when a cardiac troponin test is not available.3 Furthermore, troponins should be obtained no more than 3 times for the initial evaluation of a single event, and further trending provides no additional benefit or prognostic information.
A recent study from an academic hospital showed that process improvement interventions focused on eliminating unnecessary cardiac enzyme testing led to a 1-year cost savings of $1.25 million while increasing the rate of ACS diagnosis.4 Common clinical practice at Naval Medical Center Portsmouth (NMCP) in Virginia still routinely includes both troponin as well as a CK panel comprised of CK, CK-MB, and a calculated CK-MB/CK index. Our study focuses on the implementation of quality improvement efforts described by Larochelle and colleagues at NMCP.4 The study aimed to determine the impact of implementing interventions designed to improve the ordering practices and reduce the cost of cardiac enzyme testing.
Methods
The primary focus of the intervention was on ordering practices of the emergency medicine department (EMD), internal medicine (IM) inpatient services, and cardiology inpatient services. Specific interventions were: (1) removal of the CK panel from the chest pain order set in the EMD electronic health record (EHR); (2) removal of the CK panel from the inpatient cardiology order set; (3) education of staff on the changes in CK panel utility via direct communication during IM academic seminars; (4) education of nursing staff ordering laboratory results on behalf of physicians on the cardiology service at the morning and evening huddles; and (5) addition of “max of 3 tests indicated” comment to the inpatient EHR ordering page of the troponin test
Data Source
The process improvement interventions were considered exempt from institutional review board (IRB) approval; however, we obtained expedited IRB approval with waiver of consent for the research aspect of the project. We obtained clinical administrative data from the Military Health System Data Repository (MDR). We identified all adult patients aged ≥ 18 years who had a troponin test, CK-MB, or both drawn at NMCP on the following services: the EMD, IM, and cardiology. A troponin or CK-MB test was defined using Current Procedural Terminology (CPT) codes and unique Logical Observation Identifiers Names and Codes (LOINC).
Measures
The study was divided into 3 periods: the preintervention period from August 1, 2013 to July 31, 2014; the intervention period from August 1, 2014 to January 31, 2015; and the postintervention period February 1, 2015 to January 31, 2016.
The primary outcomes measured were the frequency of guideline concordance and total costs for tests ordered per month using the Centers for Medicare and Medicaid Services (CMS) clinical laboratory fee schedule of $13.40 for troponin and $16.17 for CK-MB.5Concordance was defined as ≤ 3 troponin tests and no CK-MB tests ordered during 1 encounter for a patient without an ACS diagnosis in the preceding 7 days. Due to faster cellular release kinetics of CK-MB compared with that of troponin, this test has utility in evaluating new or worsening chest pain in the setting of a recent myocardial infarction (MI). Therefore, we excluded any patient who had a MI within the preceding 7 days of an order for either CK-MB or troponin tests. Additionally, the number of tests, both CK-MB and troponin, ordered per patient encounter (hereafter referred to as an episode) were measured. Finally, we measured the monthly prevalence of ACS diagnosis and percentage of visits having that diagnosis.
Data Analysis
Descriptive statistics were used to calculate population demographics of age group, sex, beneficiary category, sponsor service, and clinical setting. Monthly data were grouped into the preintervention and postintervention periods. The analysis was performed using t tests to compare mean values and CIs before and after the intervention. Simple linear regression with attention to correlation was used to create best fit lines with confidence bands before and after the intervention. Interrupted time series (ITS) regression was used to describe all data points throughout the study. Consistency between these various methods was verified. Mean values and CIs were reported from the t tests. Statistical significance was reported when appropriate. Equations and confidence predictions on the simple linear regressions were produced and reported. These were used to identify values at the start, midpoint, and end of the pre- and postintervention periods.
Results
There were a total of 6,281 patients in the study population. More patients were seen during the postintervention period than in the preintervention period. The mean age of patients was slightly higher during the preintervention period (Table 1).
Guideline Concordance
To determine whether ordering practices for cardiac enzyme testing improved, we assessed the changes in the frequency of guideline concordance during the pre- and postintervention period. On average during the preintervention year, the percentage of tests ordered that met guideline concordance was 10.1% (95% CI, 7.4%-12.9%), increasing by 0.80% (95% CI, 0.17%-1.42%) each month.
Costs
We assessed changes in total dollars spent on cardiac enzyme testing during the pre- and postintervention periods. During the preintervention year, $9,400 (95% CI, $8,700-$10,100) was spent on average each month, which did not change significantly throughout the period. During the postintervention year, the cost was stable at $5,000 (95% CI, $4,600-$5,300) on average each month, a reduction of $4,400 (95% CI, $3,700-$5,100) (Figure 2).
CK-MB and Troponin Tests per Patient
To further assess ordering practices for cardiac enzyme testing, we compared the changes in the monthly number of tests and the average number of CK-MB and troponin tests ordered per episode pre- and postintervention. On average during the preintervention year, 297 tests (95% CI, 278-315) were run per month, with an average of 1.21 CK tests (95% CI, 1.15-1.27) per episode (Table 2, Figure 3).
The changes in troponin testing were not as dramatic. The counts of tests each month remained similar, with a preintervention year average of 341 (95% CI, 306-377) and postintervention year average of 310 (95% CI, 287-332), which were not statistically different. However, there was a statistically significant decrease in the number of tests per episode. During the preintervention year, 1.38 troponin tests (95% CI, 1.31-1.45) were ordered per patient on average. This dropped by 0.17 (95% CI, 0.09-0.24) to the postintervention average of 1.21 (95% CI, 1.17-1.25) (Table 2, Figure 4).
ACS Prevalence
To determine whether there was an impact on ACS diagnoses, we looked at the numbers of ACS diagnoses and their prevalence among visits before and after the intervention. During the preintervention year, the average monthly number of diagnoses was 29.7 (95% CI, 26.1-33.2), and prevalence of ACS was 0.56% (95% CI, 0.48%-0.63%) of all episodes. Although the monthly rate was statistically decreasing by 0.022% (95% CI, 0.003-0.41), this has little meaning since the level of correlation (r2 = 0.2522, not displayed) was poor due to the essentially nonexistent correlation in number of visits each month (r2 = 0.0112, not displayed). During the postintervention year, the average number of diagnoses was 32.2 (95% CI, 27.9-36.6), and the prevalence of ACS was 0.62% (95% CI, 0.54-0.65). Neither of these values changed significantly between the pre- and postintervention period. All ICD-9 and ICD-10 diagnosis codes used for the analysis are available upon request from the authors.
Discussion
Our data demonstrate the ability of simple process improvement interventions to decrease unnecessary testing in the workup of ACS, increasing the rate of guideline concordant testing by > 70% at a single military treatment facility (MTF). In particular, with the now widespread use of EHR, the order set presents a high-yield target for process improvement in an easily implemented, durable fashion. We had expected to see some decrease in the efficacy of the intervention at a time of staff turnover in the summer of 2015 because ongoing dedicated teaching sessions were not performed. Despite that, the intervention remained effective without further dedicated teaching sessions. This outcome was certainly attributable to the hardwired interventions made (mainly via order sets), but possibly indicates an institutional memory that can take hold after an initial concerted effort is made.
We reduced the estimated preintervention annual cost of $113,000 by $53,000 (95% CI, $42,000-$64,000). Although on a much smaller scale than the study by Larochelle, our study represents a nearly 50% reduction in the total cost of initial testing for possible ACS and a > 80% reduction in unnecessary CK-MB testing.4 This result was achieved with no statistical change in the prevalence of ACS. The cost reduction does not account for the labor costs to clinically follow-up and address additional unnecessary lab results. The estimated cost of intervention was limited to the time required to educate residents, interns, and nursing staff as well as the implementation of the automated, reflexive laboratory results ordering process.
Unique to our study, we also demonstrated an intervention that satisfied all the major stakeholders in the ordering of these laboratory results. By instituting the reflexive ordering of CK-MB tests for positive troponins, we obtained the support of the facility’s interventional cardiology department, which finds value in that data. Appreciating the time-sensitive nature of an ACS diagnosis, the reflexive ordering minimized the delay in receiving these data while still greatly reducing the number of tests performed. That being said, if the current trend away from CK-MB in favor of exclusively testing troponin continues, removing the reflexive ordering for positive laboratory results protocol would be an easy follow-on intervention.
Limitations
Our study presented several limitations. First, reporting errors due to improper or insufficient medical coding as well as data entry errors may exist within the MDR; therefore, the results of this analysis may be over- or underestimated. Specifically, CPT codes for troponin and CK-MB were available only in 1 of the 2 data sets used for this study, which primarily contains outpatient patient encounters. For this reason, most of the laboratory testing comes from the EMD rather than from inpatient services. However, because we excluded all patients who eventually had an ACS diagnosis (patients who likely had more inpatient time and better indication for repeat troponin), we feel that our intervention was still thoroughly investigated. Second, the number of tests drawn per patient was significantly < 2, the expected minimum number of tests to rule out ACS in patients with appropriate symptoms.
This study was not designed to answer the source of variation from guidelines. Many patients had only 1 test, which we feel represents an opportunity for future study to identify other ways cardiac enzyme testing is being used clinically. These tests might be used for patients without convincing symptoms and signs of coronary syndromes or for patients with other primary problems. Third, by using the ITS analysis, we assumed that the outcome during each intervention period follows a linear pattern. However, changes may follow a nonlinear pattern over a long period. Finally, our intervention was limited to only a single MTF, which may limit generalizability to other facilities across military medicine. However, we feel this study should serve as a guide for other MTFs as well as US Department of Veterans Affairs facilities that could institute similar process improvements.
Conclusion
We made easily implemented and durable process improvement interventions that changed institution-wide ordering practices. These changes dramatically increased the rate of guideline-concordant testing, decreasing cost and furthering the goal of high-value medical care.
1. Anderson JL, Heidenreich PA, Barnett PG, et al; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129(22):2329-2345.
2. Centers for Disease Control and Prevention, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf. Accessed March 15, 2019.
3. Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356-e375.
4. Larochelle MR, Knight AM, Pantle H, Riedel S, Trost JC. Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468-1474.
5. Centers for Medicare and Medicaid Services. 2016 clinical laboratory fee schedule. https://www.cms.gov/Medicare/Medicare-Fee -for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files-Items/16CLAB.html?DLPage=1&DLEntries=10&DLSort=2&DLSortDir=descending. Accessed March 15, 2019.
In recent years, driven by accelerating health care costs and desire for improved health care value, major specialty group guidelines have incorporated resource utilization and value calculations into their recommendations. High-value care has the characteristics of enhancing outcomes, safety, and patient satisfaction at a reasonable cost. As one example, the American College of Cardiology (ACC) recently published a consensus statement on its clinical practice guidelines with a specific focus on cost and value.1 This guideline acknowledges the difficulty in incorporating value into clinical decision making but stresses a need for increased transparency and consistency to boost value in everyday practice.
Chest pain and related symptoms were listed as the second leading principle reasons for emergency department visits in the US in 2011 with 14% of patients undergoing cardiac enzyme testing.2 The ACC guidelines advocate use of troponin as the preferred laboratory test for the initial evaluation of acute coronary syndrome (ACS). Fractionated creatine kinase (CK-MB) is an acceptable alternative only when a cardiac troponin test is not available.3 Furthermore, troponins should be obtained no more than 3 times for the initial evaluation of a single event, and further trending provides no additional benefit or prognostic information.
A recent study from an academic hospital showed that process improvement interventions focused on eliminating unnecessary cardiac enzyme testing led to a 1-year cost savings of $1.25 million while increasing the rate of ACS diagnosis.4 Common clinical practice at Naval Medical Center Portsmouth (NMCP) in Virginia still routinely includes both troponin as well as a CK panel comprised of CK, CK-MB, and a calculated CK-MB/CK index. Our study focuses on the implementation of quality improvement efforts described by Larochelle and colleagues at NMCP.4 The study aimed to determine the impact of implementing interventions designed to improve the ordering practices and reduce the cost of cardiac enzyme testing.
Methods
The primary focus of the intervention was on ordering practices of the emergency medicine department (EMD), internal medicine (IM) inpatient services, and cardiology inpatient services. Specific interventions were: (1) removal of the CK panel from the chest pain order set in the EMD electronic health record (EHR); (2) removal of the CK panel from the inpatient cardiology order set; (3) education of staff on the changes in CK panel utility via direct communication during IM academic seminars; (4) education of nursing staff ordering laboratory results on behalf of physicians on the cardiology service at the morning and evening huddles; and (5) addition of “max of 3 tests indicated” comment to the inpatient EHR ordering page of the troponin test
Data Source
The process improvement interventions were considered exempt from institutional review board (IRB) approval; however, we obtained expedited IRB approval with waiver of consent for the research aspect of the project. We obtained clinical administrative data from the Military Health System Data Repository (MDR). We identified all adult patients aged ≥ 18 years who had a troponin test, CK-MB, or both drawn at NMCP on the following services: the EMD, IM, and cardiology. A troponin or CK-MB test was defined using Current Procedural Terminology (CPT) codes and unique Logical Observation Identifiers Names and Codes (LOINC).
Measures
The study was divided into 3 periods: the preintervention period from August 1, 2013 to July 31, 2014; the intervention period from August 1, 2014 to January 31, 2015; and the postintervention period February 1, 2015 to January 31, 2016.
The primary outcomes measured were the frequency of guideline concordance and total costs for tests ordered per month using the Centers for Medicare and Medicaid Services (CMS) clinical laboratory fee schedule of $13.40 for troponin and $16.17 for CK-MB.5Concordance was defined as ≤ 3 troponin tests and no CK-MB tests ordered during 1 encounter for a patient without an ACS diagnosis in the preceding 7 days. Due to faster cellular release kinetics of CK-MB compared with that of troponin, this test has utility in evaluating new or worsening chest pain in the setting of a recent myocardial infarction (MI). Therefore, we excluded any patient who had a MI within the preceding 7 days of an order for either CK-MB or troponin tests. Additionally, the number of tests, both CK-MB and troponin, ordered per patient encounter (hereafter referred to as an episode) were measured. Finally, we measured the monthly prevalence of ACS diagnosis and percentage of visits having that diagnosis.
Data Analysis
Descriptive statistics were used to calculate population demographics of age group, sex, beneficiary category, sponsor service, and clinical setting. Monthly data were grouped into the preintervention and postintervention periods. The analysis was performed using t tests to compare mean values and CIs before and after the intervention. Simple linear regression with attention to correlation was used to create best fit lines with confidence bands before and after the intervention. Interrupted time series (ITS) regression was used to describe all data points throughout the study. Consistency between these various methods was verified. Mean values and CIs were reported from the t tests. Statistical significance was reported when appropriate. Equations and confidence predictions on the simple linear regressions were produced and reported. These were used to identify values at the start, midpoint, and end of the pre- and postintervention periods.
Results
There were a total of 6,281 patients in the study population. More patients were seen during the postintervention period than in the preintervention period. The mean age of patients was slightly higher during the preintervention period (Table 1).
Guideline Concordance
To determine whether ordering practices for cardiac enzyme testing improved, we assessed the changes in the frequency of guideline concordance during the pre- and postintervention period. On average during the preintervention year, the percentage of tests ordered that met guideline concordance was 10.1% (95% CI, 7.4%-12.9%), increasing by 0.80% (95% CI, 0.17%-1.42%) each month.
Costs
We assessed changes in total dollars spent on cardiac enzyme testing during the pre- and postintervention periods. During the preintervention year, $9,400 (95% CI, $8,700-$10,100) was spent on average each month, which did not change significantly throughout the period. During the postintervention year, the cost was stable at $5,000 (95% CI, $4,600-$5,300) on average each month, a reduction of $4,400 (95% CI, $3,700-$5,100) (Figure 2).
CK-MB and Troponin Tests per Patient
To further assess ordering practices for cardiac enzyme testing, we compared the changes in the monthly number of tests and the average number of CK-MB and troponin tests ordered per episode pre- and postintervention. On average during the preintervention year, 297 tests (95% CI, 278-315) were run per month, with an average of 1.21 CK tests (95% CI, 1.15-1.27) per episode (Table 2, Figure 3).
The changes in troponin testing were not as dramatic. The counts of tests each month remained similar, with a preintervention year average of 341 (95% CI, 306-377) and postintervention year average of 310 (95% CI, 287-332), which were not statistically different. However, there was a statistically significant decrease in the number of tests per episode. During the preintervention year, 1.38 troponin tests (95% CI, 1.31-1.45) were ordered per patient on average. This dropped by 0.17 (95% CI, 0.09-0.24) to the postintervention average of 1.21 (95% CI, 1.17-1.25) (Table 2, Figure 4).
ACS Prevalence
To determine whether there was an impact on ACS diagnoses, we looked at the numbers of ACS diagnoses and their prevalence among visits before and after the intervention. During the preintervention year, the average monthly number of diagnoses was 29.7 (95% CI, 26.1-33.2), and prevalence of ACS was 0.56% (95% CI, 0.48%-0.63%) of all episodes. Although the monthly rate was statistically decreasing by 0.022% (95% CI, 0.003-0.41), this has little meaning since the level of correlation (r2 = 0.2522, not displayed) was poor due to the essentially nonexistent correlation in number of visits each month (r2 = 0.0112, not displayed). During the postintervention year, the average number of diagnoses was 32.2 (95% CI, 27.9-36.6), and the prevalence of ACS was 0.62% (95% CI, 0.54-0.65). Neither of these values changed significantly between the pre- and postintervention period. All ICD-9 and ICD-10 diagnosis codes used for the analysis are available upon request from the authors.
Discussion
Our data demonstrate the ability of simple process improvement interventions to decrease unnecessary testing in the workup of ACS, increasing the rate of guideline concordant testing by > 70% at a single military treatment facility (MTF). In particular, with the now widespread use of EHR, the order set presents a high-yield target for process improvement in an easily implemented, durable fashion. We had expected to see some decrease in the efficacy of the intervention at a time of staff turnover in the summer of 2015 because ongoing dedicated teaching sessions were not performed. Despite that, the intervention remained effective without further dedicated teaching sessions. This outcome was certainly attributable to the hardwired interventions made (mainly via order sets), but possibly indicates an institutional memory that can take hold after an initial concerted effort is made.
We reduced the estimated preintervention annual cost of $113,000 by $53,000 (95% CI, $42,000-$64,000). Although on a much smaller scale than the study by Larochelle, our study represents a nearly 50% reduction in the total cost of initial testing for possible ACS and a > 80% reduction in unnecessary CK-MB testing.4 This result was achieved with no statistical change in the prevalence of ACS. The cost reduction does not account for the labor costs to clinically follow-up and address additional unnecessary lab results. The estimated cost of intervention was limited to the time required to educate residents, interns, and nursing staff as well as the implementation of the automated, reflexive laboratory results ordering process.
Unique to our study, we also demonstrated an intervention that satisfied all the major stakeholders in the ordering of these laboratory results. By instituting the reflexive ordering of CK-MB tests for positive troponins, we obtained the support of the facility’s interventional cardiology department, which finds value in that data. Appreciating the time-sensitive nature of an ACS diagnosis, the reflexive ordering minimized the delay in receiving these data while still greatly reducing the number of tests performed. That being said, if the current trend away from CK-MB in favor of exclusively testing troponin continues, removing the reflexive ordering for positive laboratory results protocol would be an easy follow-on intervention.
Limitations
Our study presented several limitations. First, reporting errors due to improper or insufficient medical coding as well as data entry errors may exist within the MDR; therefore, the results of this analysis may be over- or underestimated. Specifically, CPT codes for troponin and CK-MB were available only in 1 of the 2 data sets used for this study, which primarily contains outpatient patient encounters. For this reason, most of the laboratory testing comes from the EMD rather than from inpatient services. However, because we excluded all patients who eventually had an ACS diagnosis (patients who likely had more inpatient time and better indication for repeat troponin), we feel that our intervention was still thoroughly investigated. Second, the number of tests drawn per patient was significantly < 2, the expected minimum number of tests to rule out ACS in patients with appropriate symptoms.
This study was not designed to answer the source of variation from guidelines. Many patients had only 1 test, which we feel represents an opportunity for future study to identify other ways cardiac enzyme testing is being used clinically. These tests might be used for patients without convincing symptoms and signs of coronary syndromes or for patients with other primary problems. Third, by using the ITS analysis, we assumed that the outcome during each intervention period follows a linear pattern. However, changes may follow a nonlinear pattern over a long period. Finally, our intervention was limited to only a single MTF, which may limit generalizability to other facilities across military medicine. However, we feel this study should serve as a guide for other MTFs as well as US Department of Veterans Affairs facilities that could institute similar process improvements.
Conclusion
We made easily implemented and durable process improvement interventions that changed institution-wide ordering practices. These changes dramatically increased the rate of guideline-concordant testing, decreasing cost and furthering the goal of high-value medical care.
In recent years, driven by accelerating health care costs and desire for improved health care value, major specialty group guidelines have incorporated resource utilization and value calculations into their recommendations. High-value care has the characteristics of enhancing outcomes, safety, and patient satisfaction at a reasonable cost. As one example, the American College of Cardiology (ACC) recently published a consensus statement on its clinical practice guidelines with a specific focus on cost and value.1 This guideline acknowledges the difficulty in incorporating value into clinical decision making but stresses a need for increased transparency and consistency to boost value in everyday practice.
Chest pain and related symptoms were listed as the second leading principle reasons for emergency department visits in the US in 2011 with 14% of patients undergoing cardiac enzyme testing.2 The ACC guidelines advocate use of troponin as the preferred laboratory test for the initial evaluation of acute coronary syndrome (ACS). Fractionated creatine kinase (CK-MB) is an acceptable alternative only when a cardiac troponin test is not available.3 Furthermore, troponins should be obtained no more than 3 times for the initial evaluation of a single event, and further trending provides no additional benefit or prognostic information.
A recent study from an academic hospital showed that process improvement interventions focused on eliminating unnecessary cardiac enzyme testing led to a 1-year cost savings of $1.25 million while increasing the rate of ACS diagnosis.4 Common clinical practice at Naval Medical Center Portsmouth (NMCP) in Virginia still routinely includes both troponin as well as a CK panel comprised of CK, CK-MB, and a calculated CK-MB/CK index. Our study focuses on the implementation of quality improvement efforts described by Larochelle and colleagues at NMCP.4 The study aimed to determine the impact of implementing interventions designed to improve the ordering practices and reduce the cost of cardiac enzyme testing.
Methods
The primary focus of the intervention was on ordering practices of the emergency medicine department (EMD), internal medicine (IM) inpatient services, and cardiology inpatient services. Specific interventions were: (1) removal of the CK panel from the chest pain order set in the EMD electronic health record (EHR); (2) removal of the CK panel from the inpatient cardiology order set; (3) education of staff on the changes in CK panel utility via direct communication during IM academic seminars; (4) education of nursing staff ordering laboratory results on behalf of physicians on the cardiology service at the morning and evening huddles; and (5) addition of “max of 3 tests indicated” comment to the inpatient EHR ordering page of the troponin test
Data Source
The process improvement interventions were considered exempt from institutional review board (IRB) approval; however, we obtained expedited IRB approval with waiver of consent for the research aspect of the project. We obtained clinical administrative data from the Military Health System Data Repository (MDR). We identified all adult patients aged ≥ 18 years who had a troponin test, CK-MB, or both drawn at NMCP on the following services: the EMD, IM, and cardiology. A troponin or CK-MB test was defined using Current Procedural Terminology (CPT) codes and unique Logical Observation Identifiers Names and Codes (LOINC).
Measures
The study was divided into 3 periods: the preintervention period from August 1, 2013 to July 31, 2014; the intervention period from August 1, 2014 to January 31, 2015; and the postintervention period February 1, 2015 to January 31, 2016.
The primary outcomes measured were the frequency of guideline concordance and total costs for tests ordered per month using the Centers for Medicare and Medicaid Services (CMS) clinical laboratory fee schedule of $13.40 for troponin and $16.17 for CK-MB.5Concordance was defined as ≤ 3 troponin tests and no CK-MB tests ordered during 1 encounter for a patient without an ACS diagnosis in the preceding 7 days. Due to faster cellular release kinetics of CK-MB compared with that of troponin, this test has utility in evaluating new or worsening chest pain in the setting of a recent myocardial infarction (MI). Therefore, we excluded any patient who had a MI within the preceding 7 days of an order for either CK-MB or troponin tests. Additionally, the number of tests, both CK-MB and troponin, ordered per patient encounter (hereafter referred to as an episode) were measured. Finally, we measured the monthly prevalence of ACS diagnosis and percentage of visits having that diagnosis.
Data Analysis
Descriptive statistics were used to calculate population demographics of age group, sex, beneficiary category, sponsor service, and clinical setting. Monthly data were grouped into the preintervention and postintervention periods. The analysis was performed using t tests to compare mean values and CIs before and after the intervention. Simple linear regression with attention to correlation was used to create best fit lines with confidence bands before and after the intervention. Interrupted time series (ITS) regression was used to describe all data points throughout the study. Consistency between these various methods was verified. Mean values and CIs were reported from the t tests. Statistical significance was reported when appropriate. Equations and confidence predictions on the simple linear regressions were produced and reported. These were used to identify values at the start, midpoint, and end of the pre- and postintervention periods.
Results
There were a total of 6,281 patients in the study population. More patients were seen during the postintervention period than in the preintervention period. The mean age of patients was slightly higher during the preintervention period (Table 1).
Guideline Concordance
To determine whether ordering practices for cardiac enzyme testing improved, we assessed the changes in the frequency of guideline concordance during the pre- and postintervention period. On average during the preintervention year, the percentage of tests ordered that met guideline concordance was 10.1% (95% CI, 7.4%-12.9%), increasing by 0.80% (95% CI, 0.17%-1.42%) each month.
Costs
We assessed changes in total dollars spent on cardiac enzyme testing during the pre- and postintervention periods. During the preintervention year, $9,400 (95% CI, $8,700-$10,100) was spent on average each month, which did not change significantly throughout the period. During the postintervention year, the cost was stable at $5,000 (95% CI, $4,600-$5,300) on average each month, a reduction of $4,400 (95% CI, $3,700-$5,100) (Figure 2).
CK-MB and Troponin Tests per Patient
To further assess ordering practices for cardiac enzyme testing, we compared the changes in the monthly number of tests and the average number of CK-MB and troponin tests ordered per episode pre- and postintervention. On average during the preintervention year, 297 tests (95% CI, 278-315) were run per month, with an average of 1.21 CK tests (95% CI, 1.15-1.27) per episode (Table 2, Figure 3).
The changes in troponin testing were not as dramatic. The counts of tests each month remained similar, with a preintervention year average of 341 (95% CI, 306-377) and postintervention year average of 310 (95% CI, 287-332), which were not statistically different. However, there was a statistically significant decrease in the number of tests per episode. During the preintervention year, 1.38 troponin tests (95% CI, 1.31-1.45) were ordered per patient on average. This dropped by 0.17 (95% CI, 0.09-0.24) to the postintervention average of 1.21 (95% CI, 1.17-1.25) (Table 2, Figure 4).
ACS Prevalence
To determine whether there was an impact on ACS diagnoses, we looked at the numbers of ACS diagnoses and their prevalence among visits before and after the intervention. During the preintervention year, the average monthly number of diagnoses was 29.7 (95% CI, 26.1-33.2), and prevalence of ACS was 0.56% (95% CI, 0.48%-0.63%) of all episodes. Although the monthly rate was statistically decreasing by 0.022% (95% CI, 0.003-0.41), this has little meaning since the level of correlation (r2 = 0.2522, not displayed) was poor due to the essentially nonexistent correlation in number of visits each month (r2 = 0.0112, not displayed). During the postintervention year, the average number of diagnoses was 32.2 (95% CI, 27.9-36.6), and the prevalence of ACS was 0.62% (95% CI, 0.54-0.65). Neither of these values changed significantly between the pre- and postintervention period. All ICD-9 and ICD-10 diagnosis codes used for the analysis are available upon request from the authors.
Discussion
Our data demonstrate the ability of simple process improvement interventions to decrease unnecessary testing in the workup of ACS, increasing the rate of guideline concordant testing by > 70% at a single military treatment facility (MTF). In particular, with the now widespread use of EHR, the order set presents a high-yield target for process improvement in an easily implemented, durable fashion. We had expected to see some decrease in the efficacy of the intervention at a time of staff turnover in the summer of 2015 because ongoing dedicated teaching sessions were not performed. Despite that, the intervention remained effective without further dedicated teaching sessions. This outcome was certainly attributable to the hardwired interventions made (mainly via order sets), but possibly indicates an institutional memory that can take hold after an initial concerted effort is made.
We reduced the estimated preintervention annual cost of $113,000 by $53,000 (95% CI, $42,000-$64,000). Although on a much smaller scale than the study by Larochelle, our study represents a nearly 50% reduction in the total cost of initial testing for possible ACS and a > 80% reduction in unnecessary CK-MB testing.4 This result was achieved with no statistical change in the prevalence of ACS. The cost reduction does not account for the labor costs to clinically follow-up and address additional unnecessary lab results. The estimated cost of intervention was limited to the time required to educate residents, interns, and nursing staff as well as the implementation of the automated, reflexive laboratory results ordering process.
Unique to our study, we also demonstrated an intervention that satisfied all the major stakeholders in the ordering of these laboratory results. By instituting the reflexive ordering of CK-MB tests for positive troponins, we obtained the support of the facility’s interventional cardiology department, which finds value in that data. Appreciating the time-sensitive nature of an ACS diagnosis, the reflexive ordering minimized the delay in receiving these data while still greatly reducing the number of tests performed. That being said, if the current trend away from CK-MB in favor of exclusively testing troponin continues, removing the reflexive ordering for positive laboratory results protocol would be an easy follow-on intervention.
Limitations
Our study presented several limitations. First, reporting errors due to improper or insufficient medical coding as well as data entry errors may exist within the MDR; therefore, the results of this analysis may be over- or underestimated. Specifically, CPT codes for troponin and CK-MB were available only in 1 of the 2 data sets used for this study, which primarily contains outpatient patient encounters. For this reason, most of the laboratory testing comes from the EMD rather than from inpatient services. However, because we excluded all patients who eventually had an ACS diagnosis (patients who likely had more inpatient time and better indication for repeat troponin), we feel that our intervention was still thoroughly investigated. Second, the number of tests drawn per patient was significantly < 2, the expected minimum number of tests to rule out ACS in patients with appropriate symptoms.
This study was not designed to answer the source of variation from guidelines. Many patients had only 1 test, which we feel represents an opportunity for future study to identify other ways cardiac enzyme testing is being used clinically. These tests might be used for patients without convincing symptoms and signs of coronary syndromes or for patients with other primary problems. Third, by using the ITS analysis, we assumed that the outcome during each intervention period follows a linear pattern. However, changes may follow a nonlinear pattern over a long period. Finally, our intervention was limited to only a single MTF, which may limit generalizability to other facilities across military medicine. However, we feel this study should serve as a guide for other MTFs as well as US Department of Veterans Affairs facilities that could institute similar process improvements.
Conclusion
We made easily implemented and durable process improvement interventions that changed institution-wide ordering practices. These changes dramatically increased the rate of guideline-concordant testing, decreasing cost and furthering the goal of high-value medical care.
1. Anderson JL, Heidenreich PA, Barnett PG, et al; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129(22):2329-2345.
2. Centers for Disease Control and Prevention, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf. Accessed March 15, 2019.
3. Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356-e375.
4. Larochelle MR, Knight AM, Pantle H, Riedel S, Trost JC. Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468-1474.
5. Centers for Medicare and Medicaid Services. 2016 clinical laboratory fee schedule. https://www.cms.gov/Medicare/Medicare-Fee -for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files-Items/16CLAB.html?DLPage=1&DLEntries=10&DLSort=2&DLSortDir=descending. Accessed March 15, 2019.
1. Anderson JL, Heidenreich PA, Barnett PG, et al; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129(22):2329-2345.
2. Centers for Disease Control and Prevention, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf. Accessed March 15, 2019.
3. Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356-e375.
4. Larochelle MR, Knight AM, Pantle H, Riedel S, Trost JC. Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468-1474.
5. Centers for Medicare and Medicaid Services. 2016 clinical laboratory fee schedule. https://www.cms.gov/Medicare/Medicare-Fee -for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files-Items/16CLAB.html?DLPage=1&DLEntries=10&DLSort=2&DLSortDir=descending. Accessed March 15, 2019.