User login
CPAP use associated with greater weight loss in obese patients with sleep apnea
NEW ORLEANS – Contrary to previously published data suggesting continuous positive airway pressure (CPAP) produces weight gain in patients with obstructive sleep apnea (OSA), new study findings presented at the annual meeting of the Endocrine Society provided data supporting the exact opposite conclusion.
“We think the data are strong enough to conclude that combining CPAP with a weight-loss program should be considered for all OSA patients. The weight-loss advantage is substantial,” reported Yuanjie Mao, MD, PhD, of the University of Arkansas for Medical Sciences, Little Rock.
Both weight loss and CPAP have been shown to be effective for the treatment of OSA, but concern that CPAP produces a counterproductive gain in weight was raised by findings in a meta-analysis in which CPAP was associated with increased body mass index (Thorax. 2015 Mar;70:258-64). As a result of that finding, some guidelines subsequently advised intensifying a weight-loss program at the time that CPAP is initiated to mitigate the weight gain effect, according to Dr. Mao. However, he noted that prospective data were never collected, so a causal relationship was never proven. Now, his data support the opposite conclusion.
In the more recent study, 300 patients who had participated in an intensive weight-loss program at his institution were divided into three groups: OSA patients who had been treated with CPAP, symptomatic OSA patients who had not been treated with CPAP, and asymptomatic OSA patients not treated with CPAP. They were compared retrospectively for weight change over a 16-week period.
“This was a very simple study,” said Dr. Mao, who explained that several exclusions, such as thyroid dysfunction, active infection, and uncontrolled diabetes, were used to reduce variables that might also affect weight change. At the end of 16 weeks, the median absolute weight loss in the CPAP group was 26.7 lb (12.1 kg), compared with 21 lb (9.5 kg) for the symptomatic OSA group and 19.2 lb (8.7 kg) for the asymptomatic OSA group. The weight loss was significantly greater for the CPAP group (P less than .01), compared with either of the other two groups, but not significantly different between the groups that were not treated with CPAP.
“The differences remained significant after adjusting for baseline BMI [body mass index], age, and gender,” Dr. Mao reported.
Asked why his data contradicted the previously reported data, Dr. Mao said that the previous studies were not evaluating CPAP in the context of a weight-loss program. He contends that when CPAP is combined with a rigorous weight-reduction regimen, there is an additive benefit from CPAP.
According to Dr. Mao, these data bring the value of CPAP for weight loss full circle. Before publication of the 2015 meta-analysis, it was widely assumed that CPAP helped with weight loss based on the expectation that better sleep quality would increase daytime activity. However, in the absence of strong data confirming that effect, Dr. Mao believes the unexpected results of the 2015 study easily pushed the pendulum in the opposite direction.
“The conclusion that CPAP increases weight was drawn from studies not designed to evaluate a weight-loss effect in those participating in a weight-loss program,” Dr. Mao explained. His study suggests that it is this combination that is important. He believes the observed effect from better sleep quality associated with CPAP is not necessarily related to better daytime function alone.
“Patients who sleep well also have more favorable diurnal changes in factors that might be important to weight change, such as leptin resistance and hormonal secretion,” he said. Although more work is needed to determine whether these purported mechanisms are important, he thinks his study has an immediate clinical message.
“Patients with OSA who are prescribed weight loss should also be considered for CPAP for the goal of weight loss,” Dr. Mao said. “We think this therapy should be started right away.”
SOURCE: Mao Y et al. ENDO 2019, Session SAT-095.
NEW ORLEANS – Contrary to previously published data suggesting continuous positive airway pressure (CPAP) produces weight gain in patients with obstructive sleep apnea (OSA), new study findings presented at the annual meeting of the Endocrine Society provided data supporting the exact opposite conclusion.
“We think the data are strong enough to conclude that combining CPAP with a weight-loss program should be considered for all OSA patients. The weight-loss advantage is substantial,” reported Yuanjie Mao, MD, PhD, of the University of Arkansas for Medical Sciences, Little Rock.
Both weight loss and CPAP have been shown to be effective for the treatment of OSA, but concern that CPAP produces a counterproductive gain in weight was raised by findings in a meta-analysis in which CPAP was associated with increased body mass index (Thorax. 2015 Mar;70:258-64). As a result of that finding, some guidelines subsequently advised intensifying a weight-loss program at the time that CPAP is initiated to mitigate the weight gain effect, according to Dr. Mao. However, he noted that prospective data were never collected, so a causal relationship was never proven. Now, his data support the opposite conclusion.
In the more recent study, 300 patients who had participated in an intensive weight-loss program at his institution were divided into three groups: OSA patients who had been treated with CPAP, symptomatic OSA patients who had not been treated with CPAP, and asymptomatic OSA patients not treated with CPAP. They were compared retrospectively for weight change over a 16-week period.
“This was a very simple study,” said Dr. Mao, who explained that several exclusions, such as thyroid dysfunction, active infection, and uncontrolled diabetes, were used to reduce variables that might also affect weight change. At the end of 16 weeks, the median absolute weight loss in the CPAP group was 26.7 lb (12.1 kg), compared with 21 lb (9.5 kg) for the symptomatic OSA group and 19.2 lb (8.7 kg) for the asymptomatic OSA group. The weight loss was significantly greater for the CPAP group (P less than .01), compared with either of the other two groups, but not significantly different between the groups that were not treated with CPAP.
“The differences remained significant after adjusting for baseline BMI [body mass index], age, and gender,” Dr. Mao reported.
Asked why his data contradicted the previously reported data, Dr. Mao said that the previous studies were not evaluating CPAP in the context of a weight-loss program. He contends that when CPAP is combined with a rigorous weight-reduction regimen, there is an additive benefit from CPAP.
According to Dr. Mao, these data bring the value of CPAP for weight loss full circle. Before publication of the 2015 meta-analysis, it was widely assumed that CPAP helped with weight loss based on the expectation that better sleep quality would increase daytime activity. However, in the absence of strong data confirming that effect, Dr. Mao believes the unexpected results of the 2015 study easily pushed the pendulum in the opposite direction.
“The conclusion that CPAP increases weight was drawn from studies not designed to evaluate a weight-loss effect in those participating in a weight-loss program,” Dr. Mao explained. His study suggests that it is this combination that is important. He believes the observed effect from better sleep quality associated with CPAP is not necessarily related to better daytime function alone.
“Patients who sleep well also have more favorable diurnal changes in factors that might be important to weight change, such as leptin resistance and hormonal secretion,” he said. Although more work is needed to determine whether these purported mechanisms are important, he thinks his study has an immediate clinical message.
“Patients with OSA who are prescribed weight loss should also be considered for CPAP for the goal of weight loss,” Dr. Mao said. “We think this therapy should be started right away.”
SOURCE: Mao Y et al. ENDO 2019, Session SAT-095.
NEW ORLEANS – Contrary to previously published data suggesting continuous positive airway pressure (CPAP) produces weight gain in patients with obstructive sleep apnea (OSA), new study findings presented at the annual meeting of the Endocrine Society provided data supporting the exact opposite conclusion.
“We think the data are strong enough to conclude that combining CPAP with a weight-loss program should be considered for all OSA patients. The weight-loss advantage is substantial,” reported Yuanjie Mao, MD, PhD, of the University of Arkansas for Medical Sciences, Little Rock.
Both weight loss and CPAP have been shown to be effective for the treatment of OSA, but concern that CPAP produces a counterproductive gain in weight was raised by findings in a meta-analysis in which CPAP was associated with increased body mass index (Thorax. 2015 Mar;70:258-64). As a result of that finding, some guidelines subsequently advised intensifying a weight-loss program at the time that CPAP is initiated to mitigate the weight gain effect, according to Dr. Mao. However, he noted that prospective data were never collected, so a causal relationship was never proven. Now, his data support the opposite conclusion.
In the more recent study, 300 patients who had participated in an intensive weight-loss program at his institution were divided into three groups: OSA patients who had been treated with CPAP, symptomatic OSA patients who had not been treated with CPAP, and asymptomatic OSA patients not treated with CPAP. They were compared retrospectively for weight change over a 16-week period.
“This was a very simple study,” said Dr. Mao, who explained that several exclusions, such as thyroid dysfunction, active infection, and uncontrolled diabetes, were used to reduce variables that might also affect weight change. At the end of 16 weeks, the median absolute weight loss in the CPAP group was 26.7 lb (12.1 kg), compared with 21 lb (9.5 kg) for the symptomatic OSA group and 19.2 lb (8.7 kg) for the asymptomatic OSA group. The weight loss was significantly greater for the CPAP group (P less than .01), compared with either of the other two groups, but not significantly different between the groups that were not treated with CPAP.
“The differences remained significant after adjusting for baseline BMI [body mass index], age, and gender,” Dr. Mao reported.
Asked why his data contradicted the previously reported data, Dr. Mao said that the previous studies were not evaluating CPAP in the context of a weight-loss program. He contends that when CPAP is combined with a rigorous weight-reduction regimen, there is an additive benefit from CPAP.
According to Dr. Mao, these data bring the value of CPAP for weight loss full circle. Before publication of the 2015 meta-analysis, it was widely assumed that CPAP helped with weight loss based on the expectation that better sleep quality would increase daytime activity. However, in the absence of strong data confirming that effect, Dr. Mao believes the unexpected results of the 2015 study easily pushed the pendulum in the opposite direction.
“The conclusion that CPAP increases weight was drawn from studies not designed to evaluate a weight-loss effect in those participating in a weight-loss program,” Dr. Mao explained. His study suggests that it is this combination that is important. He believes the observed effect from better sleep quality associated with CPAP is not necessarily related to better daytime function alone.
“Patients who sleep well also have more favorable diurnal changes in factors that might be important to weight change, such as leptin resistance and hormonal secretion,” he said. Although more work is needed to determine whether these purported mechanisms are important, he thinks his study has an immediate clinical message.
“Patients with OSA who are prescribed weight loss should also be considered for CPAP for the goal of weight loss,” Dr. Mao said. “We think this therapy should be started right away.”
SOURCE: Mao Y et al. ENDO 2019, Session SAT-095.
REPORTING FROM ENDO 2019
How to create your specialized niche in a private practice
Let’s imagine you landed your first job in a private gastroenterology practice or are trying to find the perfect job that allows you to put your energy toward your passions. And, like many GI doctors, you spent additional time in your fellowship training focusing on a specific interest – whether inflammatory bowel disease, advanced endoscopy, motility, hepatology, or maybe the lesser-traveled paths of weight management, geriatrics, or public policy.
Perhaps you haven’t taken an extra year of training, but you have a desire to specialize. What steps should you take to create your own niche in a private practice? How do you go about growing a practice that allows you to utilize your training?
Why specialize? Know your market!
Without a focus, unless you plan to work in an underserved area or to take over a retiring physician’s practice, a generalist position can be challenging because the demand for your skills may not be met with the supply of patients. Much like in any business, the more focused you are, the more you have a differentiator that separates you from your colleagues, increasing your chances of success.
With specialization, however, comes the importance of understanding your patient catchment area. If your focus is highly specialized and serves a less-diagnosed entity, you’ll need a larger catchment area or you won’t have the volume of patients. Also, be mindful about an oversupply of subspecialists in your given area. If you are the third or fourth subspecialist in your group, the only way you will get patients is if you are far superior in talent or personality (sorry – not typical!) or your more senior colleagues are looking to turn over work to you.
Economic considerations for subspecialties
Compensation for subspecializing is often a major factor. Understanding the economics of your specialty are important, as providers can become disappointed and disenchanted when they realize that their desire for income, especially when compared to other colleagues, differs from what their subspecialty can provide.
For instance, in GI, a physician pursuing a procedurally focused subspecialty like advanced endoscopy is likely to be compensated more highly than one who focuses on a more office-based, evaluation and management (E/M) billing-driven specialty, like motility, geriatric gastroenterology, or even hepatology. These office-based specialties are no less important, but the reality is that they create less revenue for a private practice.
Negotiating a fair contract at the beginning is critical, as you may need your income to be supplemented by your higher revenue-producing colleagues and partners for long-term success. Academic centers are often able to provide the supplement through endowments, grants, or better payer reimbursement for E/M codes, compared with a private practice.
Remember, everyone’s in sales
From my vantage point as a partner at Atlanta Gastroenterology Associates, there are several ways new GI physicians can set about a path toward specialization.
One of the first things that you should ask yourself during training is whether you want to spend another year beyond the typical 3 years. Best-case scenario would be figuring out a way to get the necessary training during the 3 years, possibly spending the third year dedicated to the specialty. Another possibility is to simply get on-the-job training during your first few years in practice without the extra year.
Whichever path you choose, building up a specialized niche within a private practice won’t come overnight. You have to create a plan and navigate a course. Here are a few ways to do that:
Take the case, especially the hard ones!
Have the mentality: “I will take care of it.” One of the best ways to specialize is to offer to help with all cases, but especially the most challenging ones. Be open to helping take on any patient. In the beginning, if you develop a reputation that you enjoy caring for all patients, even when the case requires more time and effort, this will translate into future referrals. Naturally, it may be slower in the beginning, as there may not be enough patients to treat within your specialty. Being willing to do everything will expedite the growth of your practice. No consult should be rebuffed, even when it appears unnecessary (i.e., heme-positive stool in an elderly, septic ICU patient – we all have gotten them); think of it as your opportunity to show off your skills and share your interests.
Market yourself.
This is perhaps one of the most important steps you can take. Get out in the community! This includes:
- Attend your hospital grand rounds and offer to be a presenter. There is no better way to show your enthusiasm and knowledge on a topic than to teach it. Many state GI societies have meetings, which provide opportunities to introduce yourself to physicians in other practices that can act as a good referral source if you are a local expert.
- Remember, as a subspecialist, always communicate back with the primary gastroenterologist. In doing so, feel out whether the referring doctor wants you to take over the patient’s management or send the patient back.
- Reach out to foundations, pharmaceutical companies, and advocacy groups in the area. Understand each specialty has an ecosystem beyond just a doctor-patient relationship. Participating in events that support the patient outside of the office will provide goodwill. Further, many patients rely on foundations for referrals.
- Consider research studies. Many pharmaceutical companies have the opportunity for you to register patients in investigational drug studies. By being a part of these studies, you will be included in publications, which will build your brand.
- Many disease processes need a multidisciplinary approach to treating them. Attending multidisciplinary conferences will allow you to lend your expertise. Also, presenting interesting cases and asking for help from more experienced physicians will show humility and leads to more referrals; it won’t be viewed as a weakness.
- Be creative. Develop relationships with providers who are not often considered to be a primary referral source. Motility experts may want to work closely with the local speech pathologists. An IBD specialist should develop a network of specialists for patients with extraintestinal manifestations. Advanced endoscopists and oncologists work closely together.
- Get involved in social media. Engage with other specialists and become part of the online community. Follow the subspecialty organizations or key thought leaders in your space on Twitter, Facebook, and LinkedIn. You should share relevant articles or interesting cases.
There are so many aspects of gastroenterology that present great opportunities to specialize. Following your passions will lead to long-term happiness and prevent burnout. Remember that, even once you’ve built your practice, you must continue to stay involved and nurture what you’ve built. Go to the conferences. Make connections. Continue your education. Your career will thank you.
Dr. Sonenshine joined Atlanta Gastroenterology Associates in 2012. An Atlanta native, he graduated magna cum laude from the University of Georgia in Athens where he received a bachelor’s degree in microbiology and was selected to the Phi Beta Kappa Academic Honor Society. He received his medical degree from the Medical College of Georgia in Augusta, where he was named to the Alpha Omega Alpha Medical Honor Society. He completed both his internship and residency through the Osler Housestaff Training Program at Johns Hopkins Hospital in Baltimore. Following his residency, Dr. Sonenshine completed a fellowship in digestive diseases at Emory University in Atlanta while earning a master of business administration degree from the Terry College of Business at the University of Georgia. He is a partner in United Digestive and the chairman of medicine at Northside Hospital.
Let’s imagine you landed your first job in a private gastroenterology practice or are trying to find the perfect job that allows you to put your energy toward your passions. And, like many GI doctors, you spent additional time in your fellowship training focusing on a specific interest – whether inflammatory bowel disease, advanced endoscopy, motility, hepatology, or maybe the lesser-traveled paths of weight management, geriatrics, or public policy.
Perhaps you haven’t taken an extra year of training, but you have a desire to specialize. What steps should you take to create your own niche in a private practice? How do you go about growing a practice that allows you to utilize your training?
Why specialize? Know your market!
Without a focus, unless you plan to work in an underserved area or to take over a retiring physician’s practice, a generalist position can be challenging because the demand for your skills may not be met with the supply of patients. Much like in any business, the more focused you are, the more you have a differentiator that separates you from your colleagues, increasing your chances of success.
With specialization, however, comes the importance of understanding your patient catchment area. If your focus is highly specialized and serves a less-diagnosed entity, you’ll need a larger catchment area or you won’t have the volume of patients. Also, be mindful about an oversupply of subspecialists in your given area. If you are the third or fourth subspecialist in your group, the only way you will get patients is if you are far superior in talent or personality (sorry – not typical!) or your more senior colleagues are looking to turn over work to you.
Economic considerations for subspecialties
Compensation for subspecializing is often a major factor. Understanding the economics of your specialty are important, as providers can become disappointed and disenchanted when they realize that their desire for income, especially when compared to other colleagues, differs from what their subspecialty can provide.
For instance, in GI, a physician pursuing a procedurally focused subspecialty like advanced endoscopy is likely to be compensated more highly than one who focuses on a more office-based, evaluation and management (E/M) billing-driven specialty, like motility, geriatric gastroenterology, or even hepatology. These office-based specialties are no less important, but the reality is that they create less revenue for a private practice.
Negotiating a fair contract at the beginning is critical, as you may need your income to be supplemented by your higher revenue-producing colleagues and partners for long-term success. Academic centers are often able to provide the supplement through endowments, grants, or better payer reimbursement for E/M codes, compared with a private practice.
Remember, everyone’s in sales
From my vantage point as a partner at Atlanta Gastroenterology Associates, there are several ways new GI physicians can set about a path toward specialization.
One of the first things that you should ask yourself during training is whether you want to spend another year beyond the typical 3 years. Best-case scenario would be figuring out a way to get the necessary training during the 3 years, possibly spending the third year dedicated to the specialty. Another possibility is to simply get on-the-job training during your first few years in practice without the extra year.
Whichever path you choose, building up a specialized niche within a private practice won’t come overnight. You have to create a plan and navigate a course. Here are a few ways to do that:
Take the case, especially the hard ones!
Have the mentality: “I will take care of it.” One of the best ways to specialize is to offer to help with all cases, but especially the most challenging ones. Be open to helping take on any patient. In the beginning, if you develop a reputation that you enjoy caring for all patients, even when the case requires more time and effort, this will translate into future referrals. Naturally, it may be slower in the beginning, as there may not be enough patients to treat within your specialty. Being willing to do everything will expedite the growth of your practice. No consult should be rebuffed, even when it appears unnecessary (i.e., heme-positive stool in an elderly, septic ICU patient – we all have gotten them); think of it as your opportunity to show off your skills and share your interests.
Market yourself.
This is perhaps one of the most important steps you can take. Get out in the community! This includes:
- Attend your hospital grand rounds and offer to be a presenter. There is no better way to show your enthusiasm and knowledge on a topic than to teach it. Many state GI societies have meetings, which provide opportunities to introduce yourself to physicians in other practices that can act as a good referral source if you are a local expert.
- Remember, as a subspecialist, always communicate back with the primary gastroenterologist. In doing so, feel out whether the referring doctor wants you to take over the patient’s management or send the patient back.
- Reach out to foundations, pharmaceutical companies, and advocacy groups in the area. Understand each specialty has an ecosystem beyond just a doctor-patient relationship. Participating in events that support the patient outside of the office will provide goodwill. Further, many patients rely on foundations for referrals.
- Consider research studies. Many pharmaceutical companies have the opportunity for you to register patients in investigational drug studies. By being a part of these studies, you will be included in publications, which will build your brand.
- Many disease processes need a multidisciplinary approach to treating them. Attending multidisciplinary conferences will allow you to lend your expertise. Also, presenting interesting cases and asking for help from more experienced physicians will show humility and leads to more referrals; it won’t be viewed as a weakness.
- Be creative. Develop relationships with providers who are not often considered to be a primary referral source. Motility experts may want to work closely with the local speech pathologists. An IBD specialist should develop a network of specialists for patients with extraintestinal manifestations. Advanced endoscopists and oncologists work closely together.
- Get involved in social media. Engage with other specialists and become part of the online community. Follow the subspecialty organizations or key thought leaders in your space on Twitter, Facebook, and LinkedIn. You should share relevant articles or interesting cases.
There are so many aspects of gastroenterology that present great opportunities to specialize. Following your passions will lead to long-term happiness and prevent burnout. Remember that, even once you’ve built your practice, you must continue to stay involved and nurture what you’ve built. Go to the conferences. Make connections. Continue your education. Your career will thank you.
Dr. Sonenshine joined Atlanta Gastroenterology Associates in 2012. An Atlanta native, he graduated magna cum laude from the University of Georgia in Athens where he received a bachelor’s degree in microbiology and was selected to the Phi Beta Kappa Academic Honor Society. He received his medical degree from the Medical College of Georgia in Augusta, where he was named to the Alpha Omega Alpha Medical Honor Society. He completed both his internship and residency through the Osler Housestaff Training Program at Johns Hopkins Hospital in Baltimore. Following his residency, Dr. Sonenshine completed a fellowship in digestive diseases at Emory University in Atlanta while earning a master of business administration degree from the Terry College of Business at the University of Georgia. He is a partner in United Digestive and the chairman of medicine at Northside Hospital.
Let’s imagine you landed your first job in a private gastroenterology practice or are trying to find the perfect job that allows you to put your energy toward your passions. And, like many GI doctors, you spent additional time in your fellowship training focusing on a specific interest – whether inflammatory bowel disease, advanced endoscopy, motility, hepatology, or maybe the lesser-traveled paths of weight management, geriatrics, or public policy.
Perhaps you haven’t taken an extra year of training, but you have a desire to specialize. What steps should you take to create your own niche in a private practice? How do you go about growing a practice that allows you to utilize your training?
Why specialize? Know your market!
Without a focus, unless you plan to work in an underserved area or to take over a retiring physician’s practice, a generalist position can be challenging because the demand for your skills may not be met with the supply of patients. Much like in any business, the more focused you are, the more you have a differentiator that separates you from your colleagues, increasing your chances of success.
With specialization, however, comes the importance of understanding your patient catchment area. If your focus is highly specialized and serves a less-diagnosed entity, you’ll need a larger catchment area or you won’t have the volume of patients. Also, be mindful about an oversupply of subspecialists in your given area. If you are the third or fourth subspecialist in your group, the only way you will get patients is if you are far superior in talent or personality (sorry – not typical!) or your more senior colleagues are looking to turn over work to you.
Economic considerations for subspecialties
Compensation for subspecializing is often a major factor. Understanding the economics of your specialty are important, as providers can become disappointed and disenchanted when they realize that their desire for income, especially when compared to other colleagues, differs from what their subspecialty can provide.
For instance, in GI, a physician pursuing a procedurally focused subspecialty like advanced endoscopy is likely to be compensated more highly than one who focuses on a more office-based, evaluation and management (E/M) billing-driven specialty, like motility, geriatric gastroenterology, or even hepatology. These office-based specialties are no less important, but the reality is that they create less revenue for a private practice.
Negotiating a fair contract at the beginning is critical, as you may need your income to be supplemented by your higher revenue-producing colleagues and partners for long-term success. Academic centers are often able to provide the supplement through endowments, grants, or better payer reimbursement for E/M codes, compared with a private practice.
Remember, everyone’s in sales
From my vantage point as a partner at Atlanta Gastroenterology Associates, there are several ways new GI physicians can set about a path toward specialization.
One of the first things that you should ask yourself during training is whether you want to spend another year beyond the typical 3 years. Best-case scenario would be figuring out a way to get the necessary training during the 3 years, possibly spending the third year dedicated to the specialty. Another possibility is to simply get on-the-job training during your first few years in practice without the extra year.
Whichever path you choose, building up a specialized niche within a private practice won’t come overnight. You have to create a plan and navigate a course. Here are a few ways to do that:
Take the case, especially the hard ones!
Have the mentality: “I will take care of it.” One of the best ways to specialize is to offer to help with all cases, but especially the most challenging ones. Be open to helping take on any patient. In the beginning, if you develop a reputation that you enjoy caring for all patients, even when the case requires more time and effort, this will translate into future referrals. Naturally, it may be slower in the beginning, as there may not be enough patients to treat within your specialty. Being willing to do everything will expedite the growth of your practice. No consult should be rebuffed, even when it appears unnecessary (i.e., heme-positive stool in an elderly, septic ICU patient – we all have gotten them); think of it as your opportunity to show off your skills and share your interests.
Market yourself.
This is perhaps one of the most important steps you can take. Get out in the community! This includes:
- Attend your hospital grand rounds and offer to be a presenter. There is no better way to show your enthusiasm and knowledge on a topic than to teach it. Many state GI societies have meetings, which provide opportunities to introduce yourself to physicians in other practices that can act as a good referral source if you are a local expert.
- Remember, as a subspecialist, always communicate back with the primary gastroenterologist. In doing so, feel out whether the referring doctor wants you to take over the patient’s management or send the patient back.
- Reach out to foundations, pharmaceutical companies, and advocacy groups in the area. Understand each specialty has an ecosystem beyond just a doctor-patient relationship. Participating in events that support the patient outside of the office will provide goodwill. Further, many patients rely on foundations for referrals.
- Consider research studies. Many pharmaceutical companies have the opportunity for you to register patients in investigational drug studies. By being a part of these studies, you will be included in publications, which will build your brand.
- Many disease processes need a multidisciplinary approach to treating them. Attending multidisciplinary conferences will allow you to lend your expertise. Also, presenting interesting cases and asking for help from more experienced physicians will show humility and leads to more referrals; it won’t be viewed as a weakness.
- Be creative. Develop relationships with providers who are not often considered to be a primary referral source. Motility experts may want to work closely with the local speech pathologists. An IBD specialist should develop a network of specialists for patients with extraintestinal manifestations. Advanced endoscopists and oncologists work closely together.
- Get involved in social media. Engage with other specialists and become part of the online community. Follow the subspecialty organizations or key thought leaders in your space on Twitter, Facebook, and LinkedIn. You should share relevant articles or interesting cases.
There are so many aspects of gastroenterology that present great opportunities to specialize. Following your passions will lead to long-term happiness and prevent burnout. Remember that, even once you’ve built your practice, you must continue to stay involved and nurture what you’ve built. Go to the conferences. Make connections. Continue your education. Your career will thank you.
Dr. Sonenshine joined Atlanta Gastroenterology Associates in 2012. An Atlanta native, he graduated magna cum laude from the University of Georgia in Athens where he received a bachelor’s degree in microbiology and was selected to the Phi Beta Kappa Academic Honor Society. He received his medical degree from the Medical College of Georgia in Augusta, where he was named to the Alpha Omega Alpha Medical Honor Society. He completed both his internship and residency through the Osler Housestaff Training Program at Johns Hopkins Hospital in Baltimore. Following his residency, Dr. Sonenshine completed a fellowship in digestive diseases at Emory University in Atlanta while earning a master of business administration degree from the Terry College of Business at the University of Georgia. He is a partner in United Digestive and the chairman of medicine at Northside Hospital.
First RCT with aromatase inhibitor for male hypogonadism shows promise
NEW ORLEANS – In obese men with hypogonadotropic hypogonadism, an experimental aromatase inhibitor (Ai) normalized testosterone, seemed to improve sperm function, and was not associated with any significant adverse safety signals, according to findings presented at the annual meeting of the Endocrine Society.
Unlike testosterone therapy, “leflutrozole was associated with positive effects on semen fertility parameters, such as semen volume and concentration,” reported Thomas Hugh Jones, MD, FRCP, of the Centre for Diabetes and Endocrinology, Barnsley Hospital NHS Foundation Trust, and the department of oncology and metabolism, University of Sheffield Medical School, both in England.
Although the impact of the experimental aromatase inhibitor leflutrozole on parameters of semen function was an exploratory analysis in this multicenter, placebo-controlled study, it is particularly noteworthy because it addresses one of the weaknesses of testosterone replacement, which is often the first choice in treating hypogonadism, Dr. Jones said.
“Testosterone replacement frequently results in negative feedback suppression of follicle stimulating hormone and luteinizing hormone so that along with lower sperm counts, these men have significant problems with fertility,” he explained.
In this phase 2, double-blind, randomized trial, 271 men with hypogonadism were randomized to placebo or to leflutrozole in a dose of 0.1 mg, 0.3 mg, or 1.0 mg taken orally once weekly. All patients had a serum testosterone level of less than 300 ng/dL at entry. The median body mass index was 38 kg/m2, and the average age was 50.9 years.
Results were presented after 24 weeks of treatment, but the blinded study continued for an additional 24 weeks.
Normalization of testosterone, defined as a level between 300 and 1,000 ng/dL, was the primary endpoint. The mean testosterone levels were essentially unchanged in the placebo group during the first 24-week phase of the study, but they climbed to means of 458 ng/dL in the 0.1-mg group, 512 ng/dL in the 0.3-mg group, and 586 ng/dL in the 1.0-mg group.
“Overall, 75% were in the normal range, but it reached 90% in the groups taking the two higher doses,” Dr. Jones reported. Testosterone levels never exceeded 1,500 ng/d.
For the effect on FSH and LH, which were secondary endpoints, both were increased in a dose-dependent manner at 12 and 20 weeks (P less than .001 for the highest dose relative to placebo).
For the semen analysis, also conducted at 12 and 20 weeks, all three doses were associated with a numerical increase in sperm count relative to placebo, with the highest dose achieving significant improvements in semen volume (P = .006), semen concentration (P = .01), and total motile sperm count (P = .03), Dr. Jones reported.
“The 48-week analysis has just been completed, and these types of improvements have been persistent,” Dr. Jones said in reference to the increase in sex hormones as well as measures of sperm function. Although he did not present the 48-week results in detail, he disclosed that this longer follow-up also supported favorable effects on bone density, which is among several prespecified substudies being performed.
Leflutrozole, which is chemically related to letrozole, has been well tolerated at the doses studied. An increase in hematocrit consistent with the rise in testosterone was observed, but Dr. Jones reported that there are no significant safety issues identified so far.
Aromatase inhibitors have been used off label to treat hypogonadism, but this is the first randomized controlled trial for this indication, Dr. Jones said.
Although leflutrozole was used in this study at far lower doses than the aromatase inhibitors currently available for treatment of breast cancer, it might provide an advance for a challenging condition, according to Dr. Jones. He did not speculate when a phase 3 registration trial might start, but he did say that the promise of this agent warrants further development.
Dr. Jones reported a financial relationship with Mereo BioPharma, the sponsor of this trial.
Source: Jones et al. ENDO 2019, Session OR18-4.
NEW ORLEANS – In obese men with hypogonadotropic hypogonadism, an experimental aromatase inhibitor (Ai) normalized testosterone, seemed to improve sperm function, and was not associated with any significant adverse safety signals, according to findings presented at the annual meeting of the Endocrine Society.
Unlike testosterone therapy, “leflutrozole was associated with positive effects on semen fertility parameters, such as semen volume and concentration,” reported Thomas Hugh Jones, MD, FRCP, of the Centre for Diabetes and Endocrinology, Barnsley Hospital NHS Foundation Trust, and the department of oncology and metabolism, University of Sheffield Medical School, both in England.
Although the impact of the experimental aromatase inhibitor leflutrozole on parameters of semen function was an exploratory analysis in this multicenter, placebo-controlled study, it is particularly noteworthy because it addresses one of the weaknesses of testosterone replacement, which is often the first choice in treating hypogonadism, Dr. Jones said.
“Testosterone replacement frequently results in negative feedback suppression of follicle stimulating hormone and luteinizing hormone so that along with lower sperm counts, these men have significant problems with fertility,” he explained.
In this phase 2, double-blind, randomized trial, 271 men with hypogonadism were randomized to placebo or to leflutrozole in a dose of 0.1 mg, 0.3 mg, or 1.0 mg taken orally once weekly. All patients had a serum testosterone level of less than 300 ng/dL at entry. The median body mass index was 38 kg/m2, and the average age was 50.9 years.
Results were presented after 24 weeks of treatment, but the blinded study continued for an additional 24 weeks.
Normalization of testosterone, defined as a level between 300 and 1,000 ng/dL, was the primary endpoint. The mean testosterone levels were essentially unchanged in the placebo group during the first 24-week phase of the study, but they climbed to means of 458 ng/dL in the 0.1-mg group, 512 ng/dL in the 0.3-mg group, and 586 ng/dL in the 1.0-mg group.
“Overall, 75% were in the normal range, but it reached 90% in the groups taking the two higher doses,” Dr. Jones reported. Testosterone levels never exceeded 1,500 ng/d.
For the effect on FSH and LH, which were secondary endpoints, both were increased in a dose-dependent manner at 12 and 20 weeks (P less than .001 for the highest dose relative to placebo).
For the semen analysis, also conducted at 12 and 20 weeks, all three doses were associated with a numerical increase in sperm count relative to placebo, with the highest dose achieving significant improvements in semen volume (P = .006), semen concentration (P = .01), and total motile sperm count (P = .03), Dr. Jones reported.
“The 48-week analysis has just been completed, and these types of improvements have been persistent,” Dr. Jones said in reference to the increase in sex hormones as well as measures of sperm function. Although he did not present the 48-week results in detail, he disclosed that this longer follow-up also supported favorable effects on bone density, which is among several prespecified substudies being performed.
Leflutrozole, which is chemically related to letrozole, has been well tolerated at the doses studied. An increase in hematocrit consistent with the rise in testosterone was observed, but Dr. Jones reported that there are no significant safety issues identified so far.
Aromatase inhibitors have been used off label to treat hypogonadism, but this is the first randomized controlled trial for this indication, Dr. Jones said.
Although leflutrozole was used in this study at far lower doses than the aromatase inhibitors currently available for treatment of breast cancer, it might provide an advance for a challenging condition, according to Dr. Jones. He did not speculate when a phase 3 registration trial might start, but he did say that the promise of this agent warrants further development.
Dr. Jones reported a financial relationship with Mereo BioPharma, the sponsor of this trial.
Source: Jones et al. ENDO 2019, Session OR18-4.
NEW ORLEANS – In obese men with hypogonadotropic hypogonadism, an experimental aromatase inhibitor (Ai) normalized testosterone, seemed to improve sperm function, and was not associated with any significant adverse safety signals, according to findings presented at the annual meeting of the Endocrine Society.
Unlike testosterone therapy, “leflutrozole was associated with positive effects on semen fertility parameters, such as semen volume and concentration,” reported Thomas Hugh Jones, MD, FRCP, of the Centre for Diabetes and Endocrinology, Barnsley Hospital NHS Foundation Trust, and the department of oncology and metabolism, University of Sheffield Medical School, both in England.
Although the impact of the experimental aromatase inhibitor leflutrozole on parameters of semen function was an exploratory analysis in this multicenter, placebo-controlled study, it is particularly noteworthy because it addresses one of the weaknesses of testosterone replacement, which is often the first choice in treating hypogonadism, Dr. Jones said.
“Testosterone replacement frequently results in negative feedback suppression of follicle stimulating hormone and luteinizing hormone so that along with lower sperm counts, these men have significant problems with fertility,” he explained.
In this phase 2, double-blind, randomized trial, 271 men with hypogonadism were randomized to placebo or to leflutrozole in a dose of 0.1 mg, 0.3 mg, or 1.0 mg taken orally once weekly. All patients had a serum testosterone level of less than 300 ng/dL at entry. The median body mass index was 38 kg/m2, and the average age was 50.9 years.
Results were presented after 24 weeks of treatment, but the blinded study continued for an additional 24 weeks.
Normalization of testosterone, defined as a level between 300 and 1,000 ng/dL, was the primary endpoint. The mean testosterone levels were essentially unchanged in the placebo group during the first 24-week phase of the study, but they climbed to means of 458 ng/dL in the 0.1-mg group, 512 ng/dL in the 0.3-mg group, and 586 ng/dL in the 1.0-mg group.
“Overall, 75% were in the normal range, but it reached 90% in the groups taking the two higher doses,” Dr. Jones reported. Testosterone levels never exceeded 1,500 ng/d.
For the effect on FSH and LH, which were secondary endpoints, both were increased in a dose-dependent manner at 12 and 20 weeks (P less than .001 for the highest dose relative to placebo).
For the semen analysis, also conducted at 12 and 20 weeks, all three doses were associated with a numerical increase in sperm count relative to placebo, with the highest dose achieving significant improvements in semen volume (P = .006), semen concentration (P = .01), and total motile sperm count (P = .03), Dr. Jones reported.
“The 48-week analysis has just been completed, and these types of improvements have been persistent,” Dr. Jones said in reference to the increase in sex hormones as well as measures of sperm function. Although he did not present the 48-week results in detail, he disclosed that this longer follow-up also supported favorable effects on bone density, which is among several prespecified substudies being performed.
Leflutrozole, which is chemically related to letrozole, has been well tolerated at the doses studied. An increase in hematocrit consistent with the rise in testosterone was observed, but Dr. Jones reported that there are no significant safety issues identified so far.
Aromatase inhibitors have been used off label to treat hypogonadism, but this is the first randomized controlled trial for this indication, Dr. Jones said.
Although leflutrozole was used in this study at far lower doses than the aromatase inhibitors currently available for treatment of breast cancer, it might provide an advance for a challenging condition, according to Dr. Jones. He did not speculate when a phase 3 registration trial might start, but he did say that the promise of this agent warrants further development.
Dr. Jones reported a financial relationship with Mereo BioPharma, the sponsor of this trial.
Source: Jones et al. ENDO 2019, Session OR18-4.
REPORTING FROM ENDO 2019
Electronic health records and the lost power of prose
“Don’t tell me the moon is shining; show me the glint of light on broken glass,” Anton Chekhov
In March 2006, four programmers turned entrepreneurs launched Twitter. This revolutionary tool experienced a monumental growth in scale over the next 10 years from a handful of users sharing a few thousand messages (known as “tweets”) each day to a global social network of over 300 million users valued at over $25 billion dollars. In fact, on Election Day 2016, Twitter was the No. 1 source of breaking news1, and it has been used as a launchpad for everything from social activism to national revolutions.
When Twitter was first conceived, it was designed to operate through wireless phone carriers’ SMS messaging functionality (aka “via text message”). SMS messages are limited to just 160 characters, so Twitter’s creators decided to restrict tweets to 140 characters, allowing 20 characters for a username. This decision created a necessity for communication efficiency that harks back to the days of the telegraph. From the liberal use of contractions and abbreviations to the tireless search for the shortest synonyms possible, Twitter users have employed countless techniques to enable them to say more with less. While clever and creative, this extreme verbal austerity has pervaded other media as well, becoming the hallmark literary style of the current generation.
Contemporaneous with the Twitter revolution, the medical field has allowed technology to dramatically change its style of communication as well, but in the opposite way. We have become far less efficient in our use of words, yet we seem to be doing a really poor job of expressing ourselves.
Saying less with more
I was once asked to provide expert testimony in a medical malpractice lawsuit. Working in support of the defense, I endured question after question from the plaintiff’s legal team as they picked apart every aspect of the case. Of particular interest was the physician’s documentation. Sadly – yet perhaps unsurprisingly – it was poor. The defendant had clearly used an EHR template and clicked checkboxes to create his note, documenting history, physical exam, assessment, and plan without having typed a single word. While adequate for billing purposes, the note was missing any narrative that could communicate the story of what had transpired during the patient’s visit. Sure, the presenting symptoms and vital signs were there, but the no description of the patient’s appearance had been recorded? What had the physician been thinking? What unspoken messages had led the physician to make the decisions he had made?
Like Twitter, the dawn of EHRs created an entirely new form of communication, but instead of limiting the content of physicians’ notes it expanded it. Objectively, this has made for more complete notes. Subjectively, this has led to notes packed with data, yet devoid of meaningful narrative. While handwritten notes from the previous generation were brief, they included the most important elements of the patient’s history and often the physician’s thought process in forming the differential. The electronically generated notes of today are quite the opposite; they are dense, yet far from illuminating. A clinician referring back to the record might have tremendous difficulty discerning salient features amidst all of the “note bloat.”This puts the patient (and the provider, as in the case above) at risk. Details may be present, but the diagnosis will be missed without the story that ties them all together.
Writing a new chapter
Physicians hoping to create meaningful notes are often stymied by the technology at their disposal or the demands placed on their time. These issues, combined with an ever-growing number of regulatory requirements, are what led to the decay of narrative in the first place. As a result, doctors are looking for alternative ways to buck the trend and bring patients’ stories back to their medical records. These methods are often expensive or involved, but in many cases they dramatically improve quality and efficiency.
An example of a tool that allows doctors to achieve these goals is speech recognition technology. Instead of typing or clicking, physicians dictate into the EHR, creating notes that are typically richer and more akin to a story than a list of symptoms or data points. When voice-to-text is properly deployed and utilized, documentation improves along with efficiency. Alternately, many providers are now employing scribes to accompany them in the exam room and complete the medical record. Taking this step leads to more descriptive notes, better productivity, and happier providers. The use of scribes also seems to result in happier patients, who report better therapeutic interactions when their doctors aren’t typing or staring at a computer screen.
The above-mentioned methods for recording information about a patient during a visit may be too expensive or complicated for some providers, but there are other simple techniques that can be used without incurring additional cost or resources. Previsit planning is one such possibility. By reviewing patient charts in advance of appointments, physicians can look over results, identify preventive health gaps, and anticipate follow-up needs and medication refills. They can then create skeleton notes and prepopulate orders to reduce the documentation burden during the visit. While time consuming at first, physicians have reported this practice actually saves time in the long run and allows them to focus on recording the patient narrative during the visit.
Another strategy is even more simple in concept, though may seem counter-intuitive at first: get better acquainted with the electronic records system. That is, take the time to really learn and understand the tools designed to improve productivity that are available in your EHR, then use them judiciously; take advantage of templates and macros when they’ll make you more efficient yet won’t inhibit your ability to tell the patient’s story; embrace optimization but don’t compromise on narrative. By carefully choosing your words, you’ll paint a clearer picture of every patient and enable safer and more personalized care.
Reference
1. “For Election Day Influence, Twitter Ruled Social Media” New York Times. Nov. 8, 2016.
“Don’t tell me the moon is shining; show me the glint of light on broken glass,” Anton Chekhov
In March 2006, four programmers turned entrepreneurs launched Twitter. This revolutionary tool experienced a monumental growth in scale over the next 10 years from a handful of users sharing a few thousand messages (known as “tweets”) each day to a global social network of over 300 million users valued at over $25 billion dollars. In fact, on Election Day 2016, Twitter was the No. 1 source of breaking news1, and it has been used as a launchpad for everything from social activism to national revolutions.
When Twitter was first conceived, it was designed to operate through wireless phone carriers’ SMS messaging functionality (aka “via text message”). SMS messages are limited to just 160 characters, so Twitter’s creators decided to restrict tweets to 140 characters, allowing 20 characters for a username. This decision created a necessity for communication efficiency that harks back to the days of the telegraph. From the liberal use of contractions and abbreviations to the tireless search for the shortest synonyms possible, Twitter users have employed countless techniques to enable them to say more with less. While clever and creative, this extreme verbal austerity has pervaded other media as well, becoming the hallmark literary style of the current generation.
Contemporaneous with the Twitter revolution, the medical field has allowed technology to dramatically change its style of communication as well, but in the opposite way. We have become far less efficient in our use of words, yet we seem to be doing a really poor job of expressing ourselves.
Saying less with more
I was once asked to provide expert testimony in a medical malpractice lawsuit. Working in support of the defense, I endured question after question from the plaintiff’s legal team as they picked apart every aspect of the case. Of particular interest was the physician’s documentation. Sadly – yet perhaps unsurprisingly – it was poor. The defendant had clearly used an EHR template and clicked checkboxes to create his note, documenting history, physical exam, assessment, and plan without having typed a single word. While adequate for billing purposes, the note was missing any narrative that could communicate the story of what had transpired during the patient’s visit. Sure, the presenting symptoms and vital signs were there, but the no description of the patient’s appearance had been recorded? What had the physician been thinking? What unspoken messages had led the physician to make the decisions he had made?
Like Twitter, the dawn of EHRs created an entirely new form of communication, but instead of limiting the content of physicians’ notes it expanded it. Objectively, this has made for more complete notes. Subjectively, this has led to notes packed with data, yet devoid of meaningful narrative. While handwritten notes from the previous generation were brief, they included the most important elements of the patient’s history and often the physician’s thought process in forming the differential. The electronically generated notes of today are quite the opposite; they are dense, yet far from illuminating. A clinician referring back to the record might have tremendous difficulty discerning salient features amidst all of the “note bloat.”This puts the patient (and the provider, as in the case above) at risk. Details may be present, but the diagnosis will be missed without the story that ties them all together.
Writing a new chapter
Physicians hoping to create meaningful notes are often stymied by the technology at their disposal or the demands placed on their time. These issues, combined with an ever-growing number of regulatory requirements, are what led to the decay of narrative in the first place. As a result, doctors are looking for alternative ways to buck the trend and bring patients’ stories back to their medical records. These methods are often expensive or involved, but in many cases they dramatically improve quality and efficiency.
An example of a tool that allows doctors to achieve these goals is speech recognition technology. Instead of typing or clicking, physicians dictate into the EHR, creating notes that are typically richer and more akin to a story than a list of symptoms or data points. When voice-to-text is properly deployed and utilized, documentation improves along with efficiency. Alternately, many providers are now employing scribes to accompany them in the exam room and complete the medical record. Taking this step leads to more descriptive notes, better productivity, and happier providers. The use of scribes also seems to result in happier patients, who report better therapeutic interactions when their doctors aren’t typing or staring at a computer screen.
The above-mentioned methods for recording information about a patient during a visit may be too expensive or complicated for some providers, but there are other simple techniques that can be used without incurring additional cost or resources. Previsit planning is one such possibility. By reviewing patient charts in advance of appointments, physicians can look over results, identify preventive health gaps, and anticipate follow-up needs and medication refills. They can then create skeleton notes and prepopulate orders to reduce the documentation burden during the visit. While time consuming at first, physicians have reported this practice actually saves time in the long run and allows them to focus on recording the patient narrative during the visit.
Another strategy is even more simple in concept, though may seem counter-intuitive at first: get better acquainted with the electronic records system. That is, take the time to really learn and understand the tools designed to improve productivity that are available in your EHR, then use them judiciously; take advantage of templates and macros when they’ll make you more efficient yet won’t inhibit your ability to tell the patient’s story; embrace optimization but don’t compromise on narrative. By carefully choosing your words, you’ll paint a clearer picture of every patient and enable safer and more personalized care.
Reference
1. “For Election Day Influence, Twitter Ruled Social Media” New York Times. Nov. 8, 2016.
“Don’t tell me the moon is shining; show me the glint of light on broken glass,” Anton Chekhov
In March 2006, four programmers turned entrepreneurs launched Twitter. This revolutionary tool experienced a monumental growth in scale over the next 10 years from a handful of users sharing a few thousand messages (known as “tweets”) each day to a global social network of over 300 million users valued at over $25 billion dollars. In fact, on Election Day 2016, Twitter was the No. 1 source of breaking news1, and it has been used as a launchpad for everything from social activism to national revolutions.
When Twitter was first conceived, it was designed to operate through wireless phone carriers’ SMS messaging functionality (aka “via text message”). SMS messages are limited to just 160 characters, so Twitter’s creators decided to restrict tweets to 140 characters, allowing 20 characters for a username. This decision created a necessity for communication efficiency that harks back to the days of the telegraph. From the liberal use of contractions and abbreviations to the tireless search for the shortest synonyms possible, Twitter users have employed countless techniques to enable them to say more with less. While clever and creative, this extreme verbal austerity has pervaded other media as well, becoming the hallmark literary style of the current generation.
Contemporaneous with the Twitter revolution, the medical field has allowed technology to dramatically change its style of communication as well, but in the opposite way. We have become far less efficient in our use of words, yet we seem to be doing a really poor job of expressing ourselves.
Saying less with more
I was once asked to provide expert testimony in a medical malpractice lawsuit. Working in support of the defense, I endured question after question from the plaintiff’s legal team as they picked apart every aspect of the case. Of particular interest was the physician’s documentation. Sadly – yet perhaps unsurprisingly – it was poor. The defendant had clearly used an EHR template and clicked checkboxes to create his note, documenting history, physical exam, assessment, and plan without having typed a single word. While adequate for billing purposes, the note was missing any narrative that could communicate the story of what had transpired during the patient’s visit. Sure, the presenting symptoms and vital signs were there, but the no description of the patient’s appearance had been recorded? What had the physician been thinking? What unspoken messages had led the physician to make the decisions he had made?
Like Twitter, the dawn of EHRs created an entirely new form of communication, but instead of limiting the content of physicians’ notes it expanded it. Objectively, this has made for more complete notes. Subjectively, this has led to notes packed with data, yet devoid of meaningful narrative. While handwritten notes from the previous generation were brief, they included the most important elements of the patient’s history and often the physician’s thought process in forming the differential. The electronically generated notes of today are quite the opposite; they are dense, yet far from illuminating. A clinician referring back to the record might have tremendous difficulty discerning salient features amidst all of the “note bloat.”This puts the patient (and the provider, as in the case above) at risk. Details may be present, but the diagnosis will be missed without the story that ties them all together.
Writing a new chapter
Physicians hoping to create meaningful notes are often stymied by the technology at their disposal or the demands placed on their time. These issues, combined with an ever-growing number of regulatory requirements, are what led to the decay of narrative in the first place. As a result, doctors are looking for alternative ways to buck the trend and bring patients’ stories back to their medical records. These methods are often expensive or involved, but in many cases they dramatically improve quality and efficiency.
An example of a tool that allows doctors to achieve these goals is speech recognition technology. Instead of typing or clicking, physicians dictate into the EHR, creating notes that are typically richer and more akin to a story than a list of symptoms or data points. When voice-to-text is properly deployed and utilized, documentation improves along with efficiency. Alternately, many providers are now employing scribes to accompany them in the exam room and complete the medical record. Taking this step leads to more descriptive notes, better productivity, and happier providers. The use of scribes also seems to result in happier patients, who report better therapeutic interactions when their doctors aren’t typing or staring at a computer screen.
The above-mentioned methods for recording information about a patient during a visit may be too expensive or complicated for some providers, but there are other simple techniques that can be used without incurring additional cost or resources. Previsit planning is one such possibility. By reviewing patient charts in advance of appointments, physicians can look over results, identify preventive health gaps, and anticipate follow-up needs and medication refills. They can then create skeleton notes and prepopulate orders to reduce the documentation burden during the visit. While time consuming at first, physicians have reported this practice actually saves time in the long run and allows them to focus on recording the patient narrative during the visit.
Another strategy is even more simple in concept, though may seem counter-intuitive at first: get better acquainted with the electronic records system. That is, take the time to really learn and understand the tools designed to improve productivity that are available in your EHR, then use them judiciously; take advantage of templates and macros when they’ll make you more efficient yet won’t inhibit your ability to tell the patient’s story; embrace optimization but don’t compromise on narrative. By carefully choosing your words, you’ll paint a clearer picture of every patient and enable safer and more personalized care.
Reference
1. “For Election Day Influence, Twitter Ruled Social Media” New York Times. Nov. 8, 2016.
ObGyn medical liability trends: A specialty poll
More than 1 in 10 dermatology residents report laser-associated adverse events in training
DENVER –
“Incorporating a formal laser safety education curriculum is an opportunity for residency programs and organizations like ASLMS,” study coauthor Daniel J. Bergman, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery.
In what is believed to be the first study of its kind, Dr. Bergman and his coauthor Shari A. Ochoa, MD, created an online survey intended to evaluate the safety education and number of adverse laser-associated events that occurred during dermatology residencies in the United States. After the coauthors sought input for content of the survey from dermatology faculty and their colleagues at the Mayo Clinic in Scottsdale, Ariz., they used the Association of Professors of Dermatology email database to distribute the survey to Accreditation Council for Graduate Medical Education (ACGME)–approved dermatology residency programs. “In general, most studies evaluate the models of education and the number of hours dedicated to learning a skill,” said Dr. Bergman, who is a first-year dermatology resident at the Mayo Clinic. “This study is unique because it identified adverse events experienced by dermatology residents and also evaluated their formal laser safety training.”
To date, 78 dermatology residents have completed responses to the survey. Of these, 10 (13%) identified an adverse event associated with use of a laser. Of those respondents, six respondents knew how to report the event, five felt comfortable operating the laser, three had formal laser safety training, five felt like they understood the risks associated with lasers, and all but one felt properly supervised. One identified plans for postresidency laser training. Of the 68 respondents who have not identified an adverse event, 39 (57%) reported formal laser safety training, and only 24 (35%) indicated that they knew how to report an adverse event.
“I was interested to find that 13% of dermatology residents have already experienced an adverse laser event,” Dr. Bergman said. “I was also surprised to discover that only 54% of all survey respondents identified or recognized formal laser safety training. The ACGME mandates that dermatology residents receive training in the theoretical and practical applications of lasers. This finding may indicate that additional training, focusing on laser safety, should be incorporated more formally into the curriculum at some programs.”
He acknowledged certain limitations of the study, including the relatively small number of respondents and the fact that only ACGME-accredited residencies were asked to participate. “Therefore, we are still missing a large amount of data,” Dr. Bergman said. “Most notably, the results are subject to recall bias and participants defined the nature of an adverse laser event.”
He reported having no financial disclosures.
DENVER –
“Incorporating a formal laser safety education curriculum is an opportunity for residency programs and organizations like ASLMS,” study coauthor Daniel J. Bergman, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery.
In what is believed to be the first study of its kind, Dr. Bergman and his coauthor Shari A. Ochoa, MD, created an online survey intended to evaluate the safety education and number of adverse laser-associated events that occurred during dermatology residencies in the United States. After the coauthors sought input for content of the survey from dermatology faculty and their colleagues at the Mayo Clinic in Scottsdale, Ariz., they used the Association of Professors of Dermatology email database to distribute the survey to Accreditation Council for Graduate Medical Education (ACGME)–approved dermatology residency programs. “In general, most studies evaluate the models of education and the number of hours dedicated to learning a skill,” said Dr. Bergman, who is a first-year dermatology resident at the Mayo Clinic. “This study is unique because it identified adverse events experienced by dermatology residents and also evaluated their formal laser safety training.”
To date, 78 dermatology residents have completed responses to the survey. Of these, 10 (13%) identified an adverse event associated with use of a laser. Of those respondents, six respondents knew how to report the event, five felt comfortable operating the laser, three had formal laser safety training, five felt like they understood the risks associated with lasers, and all but one felt properly supervised. One identified plans for postresidency laser training. Of the 68 respondents who have not identified an adverse event, 39 (57%) reported formal laser safety training, and only 24 (35%) indicated that they knew how to report an adverse event.
“I was interested to find that 13% of dermatology residents have already experienced an adverse laser event,” Dr. Bergman said. “I was also surprised to discover that only 54% of all survey respondents identified or recognized formal laser safety training. The ACGME mandates that dermatology residents receive training in the theoretical and practical applications of lasers. This finding may indicate that additional training, focusing on laser safety, should be incorporated more formally into the curriculum at some programs.”
He acknowledged certain limitations of the study, including the relatively small number of respondents and the fact that only ACGME-accredited residencies were asked to participate. “Therefore, we are still missing a large amount of data,” Dr. Bergman said. “Most notably, the results are subject to recall bias and participants defined the nature of an adverse laser event.”
He reported having no financial disclosures.
DENVER –
“Incorporating a formal laser safety education curriculum is an opportunity for residency programs and organizations like ASLMS,” study coauthor Daniel J. Bergman, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery.
In what is believed to be the first study of its kind, Dr. Bergman and his coauthor Shari A. Ochoa, MD, created an online survey intended to evaluate the safety education and number of adverse laser-associated events that occurred during dermatology residencies in the United States. After the coauthors sought input for content of the survey from dermatology faculty and their colleagues at the Mayo Clinic in Scottsdale, Ariz., they used the Association of Professors of Dermatology email database to distribute the survey to Accreditation Council for Graduate Medical Education (ACGME)–approved dermatology residency programs. “In general, most studies evaluate the models of education and the number of hours dedicated to learning a skill,” said Dr. Bergman, who is a first-year dermatology resident at the Mayo Clinic. “This study is unique because it identified adverse events experienced by dermatology residents and also evaluated their formal laser safety training.”
To date, 78 dermatology residents have completed responses to the survey. Of these, 10 (13%) identified an adverse event associated with use of a laser. Of those respondents, six respondents knew how to report the event, five felt comfortable operating the laser, three had formal laser safety training, five felt like they understood the risks associated with lasers, and all but one felt properly supervised. One identified plans for postresidency laser training. Of the 68 respondents who have not identified an adverse event, 39 (57%) reported formal laser safety training, and only 24 (35%) indicated that they knew how to report an adverse event.
“I was interested to find that 13% of dermatology residents have already experienced an adverse laser event,” Dr. Bergman said. “I was also surprised to discover that only 54% of all survey respondents identified or recognized formal laser safety training. The ACGME mandates that dermatology residents receive training in the theoretical and practical applications of lasers. This finding may indicate that additional training, focusing on laser safety, should be incorporated more formally into the curriculum at some programs.”
He acknowledged certain limitations of the study, including the relatively small number of respondents and the fact that only ACGME-accredited residencies were asked to participate. “Therefore, we are still missing a large amount of data,” Dr. Bergman said. “Most notably, the results are subject to recall bias and participants defined the nature of an adverse laser event.”
He reported having no financial disclosures.
REPORTING FROM ASLMS 2019
Key clinical point: Laser-associated adverse events experienced by dermatology residents are not uncommon.
Major finding: Of 78 dermatology residents, 10 (13%) identified an adverse events associated with use of a laser.
Study details: An online survey of 78 dermatology residents.
Disclosures: Dr. Bergman reported having no financial disclosures.
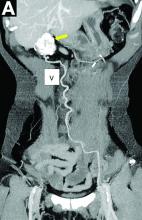
Periventricular Lesions and Migraine Distinction
Periventricular lesions (PVLs) play a key role in the differential diagnosis between migraine with aura (MA) and clinically isolated syndrome (CIS), particularly when there are greater than 3, according to a recent retrospective study. White matter hyperintensities (WMH) of 84 patients with MA and 79 patients with CIS were assessed using manual segmentation technique. Lesion probability maps (LPMs) and voxel-wise analysis of lesion distribution by diagnosis were obtained. Researchers also performed a logistic regression analysis based on lesion locations and volumes. They found:
- Compared to patients with MA, patients with CIS showed a significant overall higher T2 WMH mean number and volume (17.9 ± 16.9 vs 6.2 ± 11.9 and 3.1 ± 4.2 vs 0.3 ± 0.6 mL) and a significantly higher T2 WMH mean number in infratentorial, periventricular, and juxtacortical areas.
- LPMs identified the periventricular regions as the sites with the highest probability of detecting T2 WMH in patients with CIS.
- Voxel-wise analysis of lesion distribution by diagnosis revealed a statistically significant association exclusively between the diagnosis of CIS and the PVLs.
Lapucci C, Saitta L, Bommarito G, et al. How much do periventricular lesions assist in distinguishing migraine with aura from CIS? [Published online ahead of print March 8, 2019]. Neurology. doi:10.1212/WNL.0000000000007266.
Periventricular lesions (PVLs) play a key role in the differential diagnosis between migraine with aura (MA) and clinically isolated syndrome (CIS), particularly when there are greater than 3, according to a recent retrospective study. White matter hyperintensities (WMH) of 84 patients with MA and 79 patients with CIS were assessed using manual segmentation technique. Lesion probability maps (LPMs) and voxel-wise analysis of lesion distribution by diagnosis were obtained. Researchers also performed a logistic regression analysis based on lesion locations and volumes. They found:
- Compared to patients with MA, patients with CIS showed a significant overall higher T2 WMH mean number and volume (17.9 ± 16.9 vs 6.2 ± 11.9 and 3.1 ± 4.2 vs 0.3 ± 0.6 mL) and a significantly higher T2 WMH mean number in infratentorial, periventricular, and juxtacortical areas.
- LPMs identified the periventricular regions as the sites with the highest probability of detecting T2 WMH in patients with CIS.
- Voxel-wise analysis of lesion distribution by diagnosis revealed a statistically significant association exclusively between the diagnosis of CIS and the PVLs.
Lapucci C, Saitta L, Bommarito G, et al. How much do periventricular lesions assist in distinguishing migraine with aura from CIS? [Published online ahead of print March 8, 2019]. Neurology. doi:10.1212/WNL.0000000000007266.
Periventricular lesions (PVLs) play a key role in the differential diagnosis between migraine with aura (MA) and clinically isolated syndrome (CIS), particularly when there are greater than 3, according to a recent retrospective study. White matter hyperintensities (WMH) of 84 patients with MA and 79 patients with CIS were assessed using manual segmentation technique. Lesion probability maps (LPMs) and voxel-wise analysis of lesion distribution by diagnosis were obtained. Researchers also performed a logistic regression analysis based on lesion locations and volumes. They found:
- Compared to patients with MA, patients with CIS showed a significant overall higher T2 WMH mean number and volume (17.9 ± 16.9 vs 6.2 ± 11.9 and 3.1 ± 4.2 vs 0.3 ± 0.6 mL) and a significantly higher T2 WMH mean number in infratentorial, periventricular, and juxtacortical areas.
- LPMs identified the periventricular regions as the sites with the highest probability of detecting T2 WMH in patients with CIS.
- Voxel-wise analysis of lesion distribution by diagnosis revealed a statistically significant association exclusively between the diagnosis of CIS and the PVLs.
Lapucci C, Saitta L, Bommarito G, et al. How much do periventricular lesions assist in distinguishing migraine with aura from CIS? [Published online ahead of print March 8, 2019]. Neurology. doi:10.1212/WNL.0000000000007266.
Does Insomnia Affect Link Between CRP and Migraine?
Patients with migraine, in particular migraine with aura, were more likely to have elevated high sensitivity C-reactive protein (CRP), evident only among those with insomnia, according to a recent cross-sectional study. A total of 20,486 (63%) out of 32,591 individuals, aged 40 years and older, participated in the seventh wave of the Tromsø study conducted in 2015 and 2016, and had valid information on headache, insomnia, and high sensitivity CRP. Researchers found:
- A total of 6290 participants (30.7%) suffered from headache during the last year.
- Among these, 1736 (8.5%) fulfilled the criteria of migraine, 991 (4.8%) had migraine with aura, 746 (3.6%) migraine without aura (3.8%), and 4554 (22.2%) had non-migrainous headache.
- In the final multi-adjusted analysis, elevated high sensitivity CRP was associated with headache, migraine, and migraine with aura.
- No association was found between elevated high sensitivity CRP and migraine without aura or non-migrainous headache.
- The association between high sensitivity CRP and migraine was strongly dependent on insomnia status.
- Among individuals with insomnia, elevated high sensitivity CRP was associated with migraine, and migraine with aura, whereas no such relationship was found among those without insomnia.
Hagen K, Hopstock LA, Eggen AE, Mathiesen EB, Bilsen KB. Does insomnia modify the association between C-reactive protein and migraine? The Tromsø Study 2015–2016. [Published online ahead of print March 12, 2019]. Cephalalgia. doi:10.1177%2F0333102418825370.
Patients with migraine, in particular migraine with aura, were more likely to have elevated high sensitivity C-reactive protein (CRP), evident only among those with insomnia, according to a recent cross-sectional study. A total of 20,486 (63%) out of 32,591 individuals, aged 40 years and older, participated in the seventh wave of the Tromsø study conducted in 2015 and 2016, and had valid information on headache, insomnia, and high sensitivity CRP. Researchers found:
- A total of 6290 participants (30.7%) suffered from headache during the last year.
- Among these, 1736 (8.5%) fulfilled the criteria of migraine, 991 (4.8%) had migraine with aura, 746 (3.6%) migraine without aura (3.8%), and 4554 (22.2%) had non-migrainous headache.
- In the final multi-adjusted analysis, elevated high sensitivity CRP was associated with headache, migraine, and migraine with aura.
- No association was found between elevated high sensitivity CRP and migraine without aura or non-migrainous headache.
- The association between high sensitivity CRP and migraine was strongly dependent on insomnia status.
- Among individuals with insomnia, elevated high sensitivity CRP was associated with migraine, and migraine with aura, whereas no such relationship was found among those without insomnia.
Hagen K, Hopstock LA, Eggen AE, Mathiesen EB, Bilsen KB. Does insomnia modify the association between C-reactive protein and migraine? The Tromsø Study 2015–2016. [Published online ahead of print March 12, 2019]. Cephalalgia. doi:10.1177%2F0333102418825370.
Patients with migraine, in particular migraine with aura, were more likely to have elevated high sensitivity C-reactive protein (CRP), evident only among those with insomnia, according to a recent cross-sectional study. A total of 20,486 (63%) out of 32,591 individuals, aged 40 years and older, participated in the seventh wave of the Tromsø study conducted in 2015 and 2016, and had valid information on headache, insomnia, and high sensitivity CRP. Researchers found:
- A total of 6290 participants (30.7%) suffered from headache during the last year.
- Among these, 1736 (8.5%) fulfilled the criteria of migraine, 991 (4.8%) had migraine with aura, 746 (3.6%) migraine without aura (3.8%), and 4554 (22.2%) had non-migrainous headache.
- In the final multi-adjusted analysis, elevated high sensitivity CRP was associated with headache, migraine, and migraine with aura.
- No association was found between elevated high sensitivity CRP and migraine without aura or non-migrainous headache.
- The association between high sensitivity CRP and migraine was strongly dependent on insomnia status.
- Among individuals with insomnia, elevated high sensitivity CRP was associated with migraine, and migraine with aura, whereas no such relationship was found among those without insomnia.
Hagen K, Hopstock LA, Eggen AE, Mathiesen EB, Bilsen KB. Does insomnia modify the association between C-reactive protein and migraine? The Tromsø Study 2015–2016. [Published online ahead of print March 12, 2019]. Cephalalgia. doi:10.1177%2F0333102418825370.
Impact of Spinal Manipulation on Migraine Pain
Although results of a recent study are preliminary, spinal manipulation may be an effective therapeutic technique to reduce migraine days and pain/intensity. Literature databases were searched for clinical trials that evaluated spinal manipulation and migraine‐related outcomes through April 2017. Search terms included: migraine, spinal manipulation, manual therapy, chiropractic, and osteopathic. Meta‐analytic methods were employed to estimate the effect sizes (Hedges’ g) and heterogeneity (I2) for migraine days, pain, and disability. Researchers found:
- Six randomized clinical trials (RCTs) (pooled n=677; range of n=42‐218) were eligible for meta‐analysis.
- Intervention duration ranged from 2 to 6 months; outcomes included measures of migraine days (primary outcome), migraine pain/intensity, and migraine disability.
- Due to high levels of heterogeneity when all 6 studies were included in the meta‐analysis, the 1 RCT that was performed only among chronic migraineurs was excluded.
- Heterogeneity across the remaining studies was low.
- Spinal manipulation reduced migraine days with an overall small effect size (Hedges’ g=−0.35) as well as migraine pain/intensity.
Rist PM, Hernandez A, Bernstein, C, et al. The impact of spinal manipulation on migraine pain and disability: A systematic review and meta‐analysis. [Published online ahead of print March 14, 2019]. Headache. doi:10.1111/head.13501.
Although results of a recent study are preliminary, spinal manipulation may be an effective therapeutic technique to reduce migraine days and pain/intensity. Literature databases were searched for clinical trials that evaluated spinal manipulation and migraine‐related outcomes through April 2017. Search terms included: migraine, spinal manipulation, manual therapy, chiropractic, and osteopathic. Meta‐analytic methods were employed to estimate the effect sizes (Hedges’ g) and heterogeneity (I2) for migraine days, pain, and disability. Researchers found:
- Six randomized clinical trials (RCTs) (pooled n=677; range of n=42‐218) were eligible for meta‐analysis.
- Intervention duration ranged from 2 to 6 months; outcomes included measures of migraine days (primary outcome), migraine pain/intensity, and migraine disability.
- Due to high levels of heterogeneity when all 6 studies were included in the meta‐analysis, the 1 RCT that was performed only among chronic migraineurs was excluded.
- Heterogeneity across the remaining studies was low.
- Spinal manipulation reduced migraine days with an overall small effect size (Hedges’ g=−0.35) as well as migraine pain/intensity.
Rist PM, Hernandez A, Bernstein, C, et al. The impact of spinal manipulation on migraine pain and disability: A systematic review and meta‐analysis. [Published online ahead of print March 14, 2019]. Headache. doi:10.1111/head.13501.
Although results of a recent study are preliminary, spinal manipulation may be an effective therapeutic technique to reduce migraine days and pain/intensity. Literature databases were searched for clinical trials that evaluated spinal manipulation and migraine‐related outcomes through April 2017. Search terms included: migraine, spinal manipulation, manual therapy, chiropractic, and osteopathic. Meta‐analytic methods were employed to estimate the effect sizes (Hedges’ g) and heterogeneity (I2) for migraine days, pain, and disability. Researchers found:
- Six randomized clinical trials (RCTs) (pooled n=677; range of n=42‐218) were eligible for meta‐analysis.
- Intervention duration ranged from 2 to 6 months; outcomes included measures of migraine days (primary outcome), migraine pain/intensity, and migraine disability.
- Due to high levels of heterogeneity when all 6 studies were included in the meta‐analysis, the 1 RCT that was performed only among chronic migraineurs was excluded.
- Heterogeneity across the remaining studies was low.
- Spinal manipulation reduced migraine days with an overall small effect size (Hedges’ g=−0.35) as well as migraine pain/intensity.
Rist PM, Hernandez A, Bernstein, C, et al. The impact of spinal manipulation on migraine pain and disability: A systematic review and meta‐analysis. [Published online ahead of print March 14, 2019]. Headache. doi:10.1111/head.13501.
What is your diagnosis? - April 2019
Cystic and calcified PEComa of the ligamentum teres
PEComas are tumors derived from epithelioid perivascular cells that typically coexpress smooth muscle and melanocytic markers. The family of PEComas includes angiomyolipoma, clear cell “sugar” tumor, and lymphangioleiomyomatosis. Some of these tumors may be associated with tuberous sclerosis complex. PEComas of the ligamentum teres (also called in this location “clear cell myomelanocytic tumors”) are rare, but the ligamentum teres location is the most classic in children. Thirteen cases have been reported in the literature, within or in the immediate vicinity of falciform ligament/ligamentum teres.1-3 There was a marked female predominance with a mean age of 20 years (range, 3-54 years), a mean size of 8 cm (range, 5-20 cm), and a significant risk of metastasis (3 of 13 cases). Many of the lesions were calcified and had hemorrhagic and cystic alterations.
In the absence of established malignancy criteria, PEComas must be considered to have an uncertain malignant potential, requiring surgical resection and long-term monitoring. A mass of the ligamentum teres should always lead to consideration of the diagnosis of PEComa, even in adults, and even with cystic presentation. Moreover, most frequently, other tumors of the ligamentum teres are malignant (local extension of hepatocellular carcinoma, metastatic adenocarcinoma of gastrointestinal or gynecological origin). The patient was free of disease at 6 months’ follow-up.
References
1. Folpe AL, Goodman ZD, Ishak KG, et al. Clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres: a novel member of the perivascular epithelioid clear cell family of tumors with a predilection for children and young adults. Am J Surg Pathol. 2000;24:1239-46.
2. Folpe AL, Mentzel T, Lehr HA, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin. Am J Surg Pathol. 2005;29:1558-75.
3. Alaggio R, Cecchetto G, Martignoni G, et al. Malignant perivascular epithelioid cell tumor in children: description of a case and review of literature. J Pediatr Surg. 2012;47:31-40.
Cystic and calcified PEComa of the ligamentum teres
PEComas are tumors derived from epithelioid perivascular cells that typically coexpress smooth muscle and melanocytic markers. The family of PEComas includes angiomyolipoma, clear cell “sugar” tumor, and lymphangioleiomyomatosis. Some of these tumors may be associated with tuberous sclerosis complex. PEComas of the ligamentum teres (also called in this location “clear cell myomelanocytic tumors”) are rare, but the ligamentum teres location is the most classic in children. Thirteen cases have been reported in the literature, within or in the immediate vicinity of falciform ligament/ligamentum teres.1-3 There was a marked female predominance with a mean age of 20 years (range, 3-54 years), a mean size of 8 cm (range, 5-20 cm), and a significant risk of metastasis (3 of 13 cases). Many of the lesions were calcified and had hemorrhagic and cystic alterations.
In the absence of established malignancy criteria, PEComas must be considered to have an uncertain malignant potential, requiring surgical resection and long-term monitoring. A mass of the ligamentum teres should always lead to consideration of the diagnosis of PEComa, even in adults, and even with cystic presentation. Moreover, most frequently, other tumors of the ligamentum teres are malignant (local extension of hepatocellular carcinoma, metastatic adenocarcinoma of gastrointestinal or gynecological origin). The patient was free of disease at 6 months’ follow-up.
References
1. Folpe AL, Goodman ZD, Ishak KG, et al. Clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres: a novel member of the perivascular epithelioid clear cell family of tumors with a predilection for children and young adults. Am J Surg Pathol. 2000;24:1239-46.
2. Folpe AL, Mentzel T, Lehr HA, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin. Am J Surg Pathol. 2005;29:1558-75.
3. Alaggio R, Cecchetto G, Martignoni G, et al. Malignant perivascular epithelioid cell tumor in children: description of a case and review of literature. J Pediatr Surg. 2012;47:31-40.
Cystic and calcified PEComa of the ligamentum teres
PEComas are tumors derived from epithelioid perivascular cells that typically coexpress smooth muscle and melanocytic markers. The family of PEComas includes angiomyolipoma, clear cell “sugar” tumor, and lymphangioleiomyomatosis. Some of these tumors may be associated with tuberous sclerosis complex. PEComas of the ligamentum teres (also called in this location “clear cell myomelanocytic tumors”) are rare, but the ligamentum teres location is the most classic in children. Thirteen cases have been reported in the literature, within or in the immediate vicinity of falciform ligament/ligamentum teres.1-3 There was a marked female predominance with a mean age of 20 years (range, 3-54 years), a mean size of 8 cm (range, 5-20 cm), and a significant risk of metastasis (3 of 13 cases). Many of the lesions were calcified and had hemorrhagic and cystic alterations.
In the absence of established malignancy criteria, PEComas must be considered to have an uncertain malignant potential, requiring surgical resection and long-term monitoring. A mass of the ligamentum teres should always lead to consideration of the diagnosis of PEComa, even in adults, and even with cystic presentation. Moreover, most frequently, other tumors of the ligamentum teres are malignant (local extension of hepatocellular carcinoma, metastatic adenocarcinoma of gastrointestinal or gynecological origin). The patient was free of disease at 6 months’ follow-up.
References
1. Folpe AL, Goodman ZD, Ishak KG, et al. Clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres: a novel member of the perivascular epithelioid clear cell family of tumors with a predilection for children and young adults. Am J Surg Pathol. 2000;24:1239-46.
2. Folpe AL, Mentzel T, Lehr HA, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin. Am J Surg Pathol. 2005;29:1558-75.
3. Alaggio R, Cecchetto G, Martignoni G, et al. Malignant perivascular epithelioid cell tumor in children: description of a case and review of literature. J Pediatr Surg. 2012;47:31-40.
A laparoscopic resection of this mass was performed because of the risk of spontaneous hemorrhage linked to the dense tumoral vasculature and the lack of formal histologic diagnosis. During the procedure, the surgeon observed a cystic mass attached to the ligamentum teres between the liver and the umbilicus. At pathologic examination (Figure B), a well-circumscribed largely cystic mass, with a fibrous and calcified shell and hemorrhagic modifications (arrow) was observed.