User login
Anti-infective update addresses SSSI choices
ORLANDO – What’s new in infectious disease therapeutics for dermatologists? He ran through an array of updates at the Orlando Dermatology Aesthetic and Clinical Conference.
While naturally occurring smallpox was globally eradicated in 1980, small research stores are held in the United States and Russia, and effective antivirals are part of a strategy to combat bioweapons. Tecovirimat (TPOXX) is an antiviral that inhibits a major envelope protein that poxviruses need to produce extracellular virus. Approved by the Food and Drug Administration in mid-2018, it is currently the only antiviral for treating variola virus infection approved in the United States, noted Dr. Finch of the University of Connecticut, Farmington. He added that 2 million doses are currently held in the U.S. Strategic National Stockpile.
Another anti-infective agent that won’t be used by those practicing in the United States, but which promises to alleviate a significant source of suffering in the developing world, is moxidectin. The anthelmintic had previously been approved for veterinary uses, but in June 2018, the FDA approved moxidectin to treat onchocerciasis, also known as river blindness. The drug defeats the parasitic worm by binding to glutamate-gated chloride ion channels; it is licensed by the nonprofit Medicines Development for Global Health.
Another antiparasitic drug, benznidazole, was approved to treat children aged 2-12 years with Chagas disease in 2017, Dr. Finch said.
Also in 2017, a topical quinolone, ozenoxacin (Xepi) was approved to treat impetigo in adults and children aged at least 2 months. Formulated as a 1% cream, ozenoxacin is applied twice daily for 5 days. In clinical trials, ozenoxacin was shown to be noninferior to retapamulin, he said.
A new topical choice is important as mupirocin resistance climbs, Dr. Finch added. A recent Greek study showed that 20% (437) of 2,137 staph infections studied were mupirocin resistant. Of the 20%, all but one were skin and skin structure infections (SSSIs), with 88% of these being impetigo.
In the United States, mupirocin resistance has been seen in one in three outpatients in a Florida study and in 31% of patients in a New York City sample. Other studies have shown mupirocin resistance in Staphylococcus aureus isolates with resistance in the 10%-15% range among children with SSSIs, Dr. Finch said.
Two other new antibiotics to fight SSSIs can each be administered orally or intravenously. One, omadacycline (Nuzyra), is a novel tetracycline that maintains efficacy against bacteria that express tetracycline resistance through efflux and ribosomal protection. Approved in late 2018 for acute bacterial SSSIs, omadacycline treats not just methicillin-sensitive and methicillin-resistant S. aureus, but also Streptococcus species and gram-negative rods such as Enterobacter and Klebsiella pneumoniae, Dr. Finch noted.
Another new fluorinated quinolone, approved in 2017, delafloxacin (Baxdela) has broad spectrum activity against gram-negative and gram-positive bacteria.
Dr. Finch reported that he has no relevant conflicts of interest.
ORLANDO – What’s new in infectious disease therapeutics for dermatologists? He ran through an array of updates at the Orlando Dermatology Aesthetic and Clinical Conference.
While naturally occurring smallpox was globally eradicated in 1980, small research stores are held in the United States and Russia, and effective antivirals are part of a strategy to combat bioweapons. Tecovirimat (TPOXX) is an antiviral that inhibits a major envelope protein that poxviruses need to produce extracellular virus. Approved by the Food and Drug Administration in mid-2018, it is currently the only antiviral for treating variola virus infection approved in the United States, noted Dr. Finch of the University of Connecticut, Farmington. He added that 2 million doses are currently held in the U.S. Strategic National Stockpile.
Another anti-infective agent that won’t be used by those practicing in the United States, but which promises to alleviate a significant source of suffering in the developing world, is moxidectin. The anthelmintic had previously been approved for veterinary uses, but in June 2018, the FDA approved moxidectin to treat onchocerciasis, also known as river blindness. The drug defeats the parasitic worm by binding to glutamate-gated chloride ion channels; it is licensed by the nonprofit Medicines Development for Global Health.
Another antiparasitic drug, benznidazole, was approved to treat children aged 2-12 years with Chagas disease in 2017, Dr. Finch said.
Also in 2017, a topical quinolone, ozenoxacin (Xepi) was approved to treat impetigo in adults and children aged at least 2 months. Formulated as a 1% cream, ozenoxacin is applied twice daily for 5 days. In clinical trials, ozenoxacin was shown to be noninferior to retapamulin, he said.
A new topical choice is important as mupirocin resistance climbs, Dr. Finch added. A recent Greek study showed that 20% (437) of 2,137 staph infections studied were mupirocin resistant. Of the 20%, all but one were skin and skin structure infections (SSSIs), with 88% of these being impetigo.
In the United States, mupirocin resistance has been seen in one in three outpatients in a Florida study and in 31% of patients in a New York City sample. Other studies have shown mupirocin resistance in Staphylococcus aureus isolates with resistance in the 10%-15% range among children with SSSIs, Dr. Finch said.
Two other new antibiotics to fight SSSIs can each be administered orally or intravenously. One, omadacycline (Nuzyra), is a novel tetracycline that maintains efficacy against bacteria that express tetracycline resistance through efflux and ribosomal protection. Approved in late 2018 for acute bacterial SSSIs, omadacycline treats not just methicillin-sensitive and methicillin-resistant S. aureus, but also Streptococcus species and gram-negative rods such as Enterobacter and Klebsiella pneumoniae, Dr. Finch noted.
Another new fluorinated quinolone, approved in 2017, delafloxacin (Baxdela) has broad spectrum activity against gram-negative and gram-positive bacteria.
Dr. Finch reported that he has no relevant conflicts of interest.
ORLANDO – What’s new in infectious disease therapeutics for dermatologists? He ran through an array of updates at the Orlando Dermatology Aesthetic and Clinical Conference.
While naturally occurring smallpox was globally eradicated in 1980, small research stores are held in the United States and Russia, and effective antivirals are part of a strategy to combat bioweapons. Tecovirimat (TPOXX) is an antiviral that inhibits a major envelope protein that poxviruses need to produce extracellular virus. Approved by the Food and Drug Administration in mid-2018, it is currently the only antiviral for treating variola virus infection approved in the United States, noted Dr. Finch of the University of Connecticut, Farmington. He added that 2 million doses are currently held in the U.S. Strategic National Stockpile.
Another anti-infective agent that won’t be used by those practicing in the United States, but which promises to alleviate a significant source of suffering in the developing world, is moxidectin. The anthelmintic had previously been approved for veterinary uses, but in June 2018, the FDA approved moxidectin to treat onchocerciasis, also known as river blindness. The drug defeats the parasitic worm by binding to glutamate-gated chloride ion channels; it is licensed by the nonprofit Medicines Development for Global Health.
Another antiparasitic drug, benznidazole, was approved to treat children aged 2-12 years with Chagas disease in 2017, Dr. Finch said.
Also in 2017, a topical quinolone, ozenoxacin (Xepi) was approved to treat impetigo in adults and children aged at least 2 months. Formulated as a 1% cream, ozenoxacin is applied twice daily for 5 days. In clinical trials, ozenoxacin was shown to be noninferior to retapamulin, he said.
A new topical choice is important as mupirocin resistance climbs, Dr. Finch added. A recent Greek study showed that 20% (437) of 2,137 staph infections studied were mupirocin resistant. Of the 20%, all but one were skin and skin structure infections (SSSIs), with 88% of these being impetigo.
In the United States, mupirocin resistance has been seen in one in three outpatients in a Florida study and in 31% of patients in a New York City sample. Other studies have shown mupirocin resistance in Staphylococcus aureus isolates with resistance in the 10%-15% range among children with SSSIs, Dr. Finch said.
Two other new antibiotics to fight SSSIs can each be administered orally or intravenously. One, omadacycline (Nuzyra), is a novel tetracycline that maintains efficacy against bacteria that express tetracycline resistance through efflux and ribosomal protection. Approved in late 2018 for acute bacterial SSSIs, omadacycline treats not just methicillin-sensitive and methicillin-resistant S. aureus, but also Streptococcus species and gram-negative rods such as Enterobacter and Klebsiella pneumoniae, Dr. Finch noted.
Another new fluorinated quinolone, approved in 2017, delafloxacin (Baxdela) has broad spectrum activity against gram-negative and gram-positive bacteria.
Dr. Finch reported that he has no relevant conflicts of interest.
EXPERT ANALYSIS FROM ODAC 2019
April 2019 - Question 2
Critique:
Factors raising suspicion for Zollinger-Ellison syndrome include recurrent peptic ulcer disease, multiple ulcers, post-bulbar ulcer, non-H. pylori/non-NSAID-related duodenal ulcer, diarrhea, erosive esophagitis, and family or personal history of multiple endocrine neoplasia type 1. The patient in this question presents with duodenal ulcer without H. pylori or NSAID use, erosive esophagitis, and diarrhea, which raises suspicion for hypergastrinemia.
His laboratory evaluation also showed hypercalcemia, which may be due to hyperparathyroidism, a condition related to MEN I. The initial test to obtain when gastrinoma is suspected includes a fasting serum gastrin level. In follow-up of gastrin elevations, a gastric pH assessment should be performed and, depending on these results, a secretin stimulation test may be useful. Routine repeat upper endoscopy is not indicated after hemostasis of duodenal ulcer bleeding.
A restrictive transfusion strategy with a hemoglobin threshold of 7 g/dL has been shown to result in improved clinical outcome compared to a liberal transfusion strategy. While sucralfate may help the healing of duodenal ulcers, it is not the first-line therapy for long-term secondary prevention.
References
1. Roy PK, Venzon DJ, Shojamanesh H, et al. Zollinger-Ellison syndrome. Clinical presentation in 261 patients. Medicine (Baltimore) 2000;79:379.
2. Murugesan SV, Varro A, Pritchard DM. Review article: Strategies to determine whether hypergastrinaemia is due to Zollinger-Ellison syndrome rather than a more common benign cause. Aliment Pharmacol Ther 2009;29:1055-68.
3. Villaneuva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013 Jan 3;368(1):11-21.
Critique:
Factors raising suspicion for Zollinger-Ellison syndrome include recurrent peptic ulcer disease, multiple ulcers, post-bulbar ulcer, non-H. pylori/non-NSAID-related duodenal ulcer, diarrhea, erosive esophagitis, and family or personal history of multiple endocrine neoplasia type 1. The patient in this question presents with duodenal ulcer without H. pylori or NSAID use, erosive esophagitis, and diarrhea, which raises suspicion for hypergastrinemia.
His laboratory evaluation also showed hypercalcemia, which may be due to hyperparathyroidism, a condition related to MEN I. The initial test to obtain when gastrinoma is suspected includes a fasting serum gastrin level. In follow-up of gastrin elevations, a gastric pH assessment should be performed and, depending on these results, a secretin stimulation test may be useful. Routine repeat upper endoscopy is not indicated after hemostasis of duodenal ulcer bleeding.
A restrictive transfusion strategy with a hemoglobin threshold of 7 g/dL has been shown to result in improved clinical outcome compared to a liberal transfusion strategy. While sucralfate may help the healing of duodenal ulcers, it is not the first-line therapy for long-term secondary prevention.
References
1. Roy PK, Venzon DJ, Shojamanesh H, et al. Zollinger-Ellison syndrome. Clinical presentation in 261 patients. Medicine (Baltimore) 2000;79:379.
2. Murugesan SV, Varro A, Pritchard DM. Review article: Strategies to determine whether hypergastrinaemia is due to Zollinger-Ellison syndrome rather than a more common benign cause. Aliment Pharmacol Ther 2009;29:1055-68.
3. Villaneuva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013 Jan 3;368(1):11-21.
Critique:
Factors raising suspicion for Zollinger-Ellison syndrome include recurrent peptic ulcer disease, multiple ulcers, post-bulbar ulcer, non-H. pylori/non-NSAID-related duodenal ulcer, diarrhea, erosive esophagitis, and family or personal history of multiple endocrine neoplasia type 1. The patient in this question presents with duodenal ulcer without H. pylori or NSAID use, erosive esophagitis, and diarrhea, which raises suspicion for hypergastrinemia.
His laboratory evaluation also showed hypercalcemia, which may be due to hyperparathyroidism, a condition related to MEN I. The initial test to obtain when gastrinoma is suspected includes a fasting serum gastrin level. In follow-up of gastrin elevations, a gastric pH assessment should be performed and, depending on these results, a secretin stimulation test may be useful. Routine repeat upper endoscopy is not indicated after hemostasis of duodenal ulcer bleeding.
A restrictive transfusion strategy with a hemoglobin threshold of 7 g/dL has been shown to result in improved clinical outcome compared to a liberal transfusion strategy. While sucralfate may help the healing of duodenal ulcers, it is not the first-line therapy for long-term secondary prevention.
References
1. Roy PK, Venzon DJ, Shojamanesh H, et al. Zollinger-Ellison syndrome. Clinical presentation in 261 patients. Medicine (Baltimore) 2000;79:379.
2. Murugesan SV, Varro A, Pritchard DM. Review article: Strategies to determine whether hypergastrinaemia is due to Zollinger-Ellison syndrome rather than a more common benign cause. Aliment Pharmacol Ther 2009;29:1055-68.
3. Villaneuva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013 Jan 3;368(1):11-21.
A 47-year-old man with a history of chronic diarrhea presents with black, tarry stools for 2 days. Laboratory evaluation shows hemoglobin 8.9 g/dL (normal: 14-17 g/dL), platelet 201 x 103/mcL (normal: 150-350 mcL), blood urea nitrogen 40 mg/dL (normal: 8-20 mg/dL), creatinine 0.8 mg/dL (normal: 0.7-1.3 mg/dL), and calcium 12.5 mg/dL (normal: 9-10.5 mg/dL). An upper endoscopy reveals LA grade C esophagitis and a 1-cm clean-based ulcer in the duodenal bulb. Gastric biopsies show no H. pylori on H&E stain. He denies any history of NSAID or aspirin use.
April 2019 - Question 1
Rationale:
ICP has a 60%-70% recurrence rate, and therefore, this patient is at high risk of recurrence. Ursodeoxycholic acid (UDCA) has been shown to reduce pruritus and improve bile acid levels and liver-associated enzymes. There is also evidence that UDCA is safe late in pregnancy and likely improves fetal outcomes. Cholestyramine is not as effective as UCDA at reducing pruritus, reducing bile acid levels, or normalizing aminotransferase. In addition, babies are delivered closer to term with UDCA as opposed to cholestyramine. Hydroxyzine improves pruritus but can aggravate respiratory issues in preterm babies and is not recommended in ICP. Given these findings, UDCA is considered first-line therapy in treatment of ICP. A recent study showed that the perinatal mortality is decreased with delivery of the baby at 36 weeks gestation, or if ICP develops past 36 weeks, delivery with onset of symptoms. Thus, optimal management if her current pregnancy mimics the previous pregnancy if UDCA is given with development of symptoms with planned delivery at approximately 36-37 weeks gestation.
References
1. Bacq Y, Sentilhes L, Reyes HB, et al. Efficacy of ursodeoxycholic acid in treating intrahepatic cholestasis of pregnancy: a meta-analysis. Gastroenterology. 2012;143(6):1492-501.
2. Kondrackiene J, Beurers U, Kupcinskas L. Efficacy and safety of ursodeoxycholic acid versus cholestyramine in intrahepatic cholestasis of pregnancy. Gastroenterology. 2005;129(3):894-901.
3. Puljic A, Kim E, Page J, et al. The risk of fetal death by each additional week of expectant management in intrahepatic cholestasis of pregnancy by gestational age. Am J Obstet Gynecol. 2015;212(5):667e1-5.
Rationale:
ICP has a 60%-70% recurrence rate, and therefore, this patient is at high risk of recurrence. Ursodeoxycholic acid (UDCA) has been shown to reduce pruritus and improve bile acid levels and liver-associated enzymes. There is also evidence that UDCA is safe late in pregnancy and likely improves fetal outcomes. Cholestyramine is not as effective as UCDA at reducing pruritus, reducing bile acid levels, or normalizing aminotransferase. In addition, babies are delivered closer to term with UDCA as opposed to cholestyramine. Hydroxyzine improves pruritus but can aggravate respiratory issues in preterm babies and is not recommended in ICP. Given these findings, UDCA is considered first-line therapy in treatment of ICP. A recent study showed that the perinatal mortality is decreased with delivery of the baby at 36 weeks gestation, or if ICP develops past 36 weeks, delivery with onset of symptoms. Thus, optimal management if her current pregnancy mimics the previous pregnancy if UDCA is given with development of symptoms with planned delivery at approximately 36-37 weeks gestation.
References
1. Bacq Y, Sentilhes L, Reyes HB, et al. Efficacy of ursodeoxycholic acid in treating intrahepatic cholestasis of pregnancy: a meta-analysis. Gastroenterology. 2012;143(6):1492-501.
2. Kondrackiene J, Beurers U, Kupcinskas L. Efficacy and safety of ursodeoxycholic acid versus cholestyramine in intrahepatic cholestasis of pregnancy. Gastroenterology. 2005;129(3):894-901.
3. Puljic A, Kim E, Page J, et al. The risk of fetal death by each additional week of expectant management in intrahepatic cholestasis of pregnancy by gestational age. Am J Obstet Gynecol. 2015;212(5):667e1-5.
Rationale:
ICP has a 60%-70% recurrence rate, and therefore, this patient is at high risk of recurrence. Ursodeoxycholic acid (UDCA) has been shown to reduce pruritus and improve bile acid levels and liver-associated enzymes. There is also evidence that UDCA is safe late in pregnancy and likely improves fetal outcomes. Cholestyramine is not as effective as UCDA at reducing pruritus, reducing bile acid levels, or normalizing aminotransferase. In addition, babies are delivered closer to term with UDCA as opposed to cholestyramine. Hydroxyzine improves pruritus but can aggravate respiratory issues in preterm babies and is not recommended in ICP. Given these findings, UDCA is considered first-line therapy in treatment of ICP. A recent study showed that the perinatal mortality is decreased with delivery of the baby at 36 weeks gestation, or if ICP develops past 36 weeks, delivery with onset of symptoms. Thus, optimal management if her current pregnancy mimics the previous pregnancy if UDCA is given with development of symptoms with planned delivery at approximately 36-37 weeks gestation.
References
1. Bacq Y, Sentilhes L, Reyes HB, et al. Efficacy of ursodeoxycholic acid in treating intrahepatic cholestasis of pregnancy: a meta-analysis. Gastroenterology. 2012;143(6):1492-501.
2. Kondrackiene J, Beurers U, Kupcinskas L. Efficacy and safety of ursodeoxycholic acid versus cholestyramine in intrahepatic cholestasis of pregnancy. Gastroenterology. 2005;129(3):894-901.
3. Puljic A, Kim E, Page J, et al. The risk of fetal death by each additional week of expectant management in intrahepatic cholestasis of pregnancy by gestational age. Am J Obstet Gynecol. 2015;212(5):667e1-5.
A 31-year-old G2P1 woman presents to your clinic for pregnancy counseling. She is currently 12 weeks pregnant, and states that her first pregnancy was complicated by intrahepatic cholestasis of pregnancy (ICP) development at 29 weeks. She developed severe pruritus, and the baby was delivered prematurely. She is concerned about complications with her current pregnancy and is wondering about therapy if ICP recurred at the same point in her pregnancy.
Who’s increasing health care costs? Not us!
Determining who is responsible for the increase in health care cost in the United States has seemingly limitless possibilities. There are enough culprits to go around, but a recent analysis points a finger at hospital-based care (Health Aff. 2019 Feb;38[2]:184-9).
The authors of the paper found that, during the period from 2007 to 2014, inpatient hospital care for surgical procedures increased 42% and outpatient hospital care increased 25%. In the same period, physician care increased only 6%. Much of this increase in hospital costs was associated with hospital consolidations and mergers.
We have been led to believe that hospital mergers will cut costs by eliminating duplication of both physical and personnel overhead costs. In fact that doesn’t seem to happen. It appears that hospital mergers were associated with increased per patient costs and is a result of decrease in competition in local health care markets. This observation has been made in the past (Am Econ Rev. 2015 Jan;105:172-203), and was reiterated by the most recent report. If there were decreases in overhead observed in the mergers, they were not passed on to the patients or insurers.
There was a time when community hospitals, large and small, were run by community leaders and local doctors, often under the aegis of religious and social groups. I can remember the medical and community leadership in Utica, N.Y., where I grew up and where I worked in a hospital as a summer intern. Their goal was to provide quality health care. The financial success or failures of the hospitals were the responsibility of the local community, and the profits and losses were kept at a minimum.
Fast forward to the 21st century and health care in general, and hospital care in particular, has become a “cash cow.” Community leadership has been minimized, and where it exists, it is under constant pressure to make a profit. Hospital mergers, arranged under the guise of economy of size, are now controlled by hedge funds and large health care corporations.
The community board of trustees has been replaced by investors, whose main concern is the return on their investments regardless of quality of care or need. If those profits fail to materialize, the hospitals are taken over by another investor group. So much for quality. As the corporations grow, they buy up the competition, particularly small community hospitals leaving many, particularly in rural America, without medical support.
There seems to be little recourse to consumers or insurers to mitigate this process. Investors have a right to a return on their investment, but until we have governmental control of competition, that incentive remains. We can see similar price increases in the pharmaceutical marketplace, where Congress has limited competition to preserve the drug monopoly. Americans will be asked to pay more to maintain a system that is inherently on the road to bankruptcy and fails to provide either quality or fair drug and hospital charges.
But what do we care, we can afford it.
Dr. Goldstein is professor of medicine at Wayne State University and the division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit.
Determining who is responsible for the increase in health care cost in the United States has seemingly limitless possibilities. There are enough culprits to go around, but a recent analysis points a finger at hospital-based care (Health Aff. 2019 Feb;38[2]:184-9).
The authors of the paper found that, during the period from 2007 to 2014, inpatient hospital care for surgical procedures increased 42% and outpatient hospital care increased 25%. In the same period, physician care increased only 6%. Much of this increase in hospital costs was associated with hospital consolidations and mergers.
We have been led to believe that hospital mergers will cut costs by eliminating duplication of both physical and personnel overhead costs. In fact that doesn’t seem to happen. It appears that hospital mergers were associated with increased per patient costs and is a result of decrease in competition in local health care markets. This observation has been made in the past (Am Econ Rev. 2015 Jan;105:172-203), and was reiterated by the most recent report. If there were decreases in overhead observed in the mergers, they were not passed on to the patients or insurers.
There was a time when community hospitals, large and small, were run by community leaders and local doctors, often under the aegis of religious and social groups. I can remember the medical and community leadership in Utica, N.Y., where I grew up and where I worked in a hospital as a summer intern. Their goal was to provide quality health care. The financial success or failures of the hospitals were the responsibility of the local community, and the profits and losses were kept at a minimum.
Fast forward to the 21st century and health care in general, and hospital care in particular, has become a “cash cow.” Community leadership has been minimized, and where it exists, it is under constant pressure to make a profit. Hospital mergers, arranged under the guise of economy of size, are now controlled by hedge funds and large health care corporations.
The community board of trustees has been replaced by investors, whose main concern is the return on their investments regardless of quality of care or need. If those profits fail to materialize, the hospitals are taken over by another investor group. So much for quality. As the corporations grow, they buy up the competition, particularly small community hospitals leaving many, particularly in rural America, without medical support.
There seems to be little recourse to consumers or insurers to mitigate this process. Investors have a right to a return on their investment, but until we have governmental control of competition, that incentive remains. We can see similar price increases in the pharmaceutical marketplace, where Congress has limited competition to preserve the drug monopoly. Americans will be asked to pay more to maintain a system that is inherently on the road to bankruptcy and fails to provide either quality or fair drug and hospital charges.
But what do we care, we can afford it.
Dr. Goldstein is professor of medicine at Wayne State University and the division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit.
Determining who is responsible for the increase in health care cost in the United States has seemingly limitless possibilities. There are enough culprits to go around, but a recent analysis points a finger at hospital-based care (Health Aff. 2019 Feb;38[2]:184-9).
The authors of the paper found that, during the period from 2007 to 2014, inpatient hospital care for surgical procedures increased 42% and outpatient hospital care increased 25%. In the same period, physician care increased only 6%. Much of this increase in hospital costs was associated with hospital consolidations and mergers.
We have been led to believe that hospital mergers will cut costs by eliminating duplication of both physical and personnel overhead costs. In fact that doesn’t seem to happen. It appears that hospital mergers were associated with increased per patient costs and is a result of decrease in competition in local health care markets. This observation has been made in the past (Am Econ Rev. 2015 Jan;105:172-203), and was reiterated by the most recent report. If there were decreases in overhead observed in the mergers, they were not passed on to the patients or insurers.
There was a time when community hospitals, large and small, were run by community leaders and local doctors, often under the aegis of religious and social groups. I can remember the medical and community leadership in Utica, N.Y., where I grew up and where I worked in a hospital as a summer intern. Their goal was to provide quality health care. The financial success or failures of the hospitals were the responsibility of the local community, and the profits and losses were kept at a minimum.
Fast forward to the 21st century and health care in general, and hospital care in particular, has become a “cash cow.” Community leadership has been minimized, and where it exists, it is under constant pressure to make a profit. Hospital mergers, arranged under the guise of economy of size, are now controlled by hedge funds and large health care corporations.
The community board of trustees has been replaced by investors, whose main concern is the return on their investments regardless of quality of care or need. If those profits fail to materialize, the hospitals are taken over by another investor group. So much for quality. As the corporations grow, they buy up the competition, particularly small community hospitals leaving many, particularly in rural America, without medical support.
There seems to be little recourse to consumers or insurers to mitigate this process. Investors have a right to a return on their investment, but until we have governmental control of competition, that incentive remains. We can see similar price increases in the pharmaceutical marketplace, where Congress has limited competition to preserve the drug monopoly. Americans will be asked to pay more to maintain a system that is inherently on the road to bankruptcy and fails to provide either quality or fair drug and hospital charges.
But what do we care, we can afford it.
Dr. Goldstein is professor of medicine at Wayne State University and the division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit.
Recognizing the Scale of the Problem
For more than 2 years, this 36-year-old woman has had a slightly itchy rash that waxes and wanes on her posterior neck. She has consulted several primary care providers and received multiple diagnoses, the most consistent of which has been fungal infection. However, despite use of a variety of antifungal creams (nystatin, clotrimazole, and combination clotrimazole/betamethasone), a 1-month course of oral terbinafine, and OTC tolnaftate, no improvement has occurred.
The patient asserts that she is otherwise in good health, with no joint pain or fever and no history of recent health crises. Family history is free of dermatologic complaints except for psoriasis in her father.
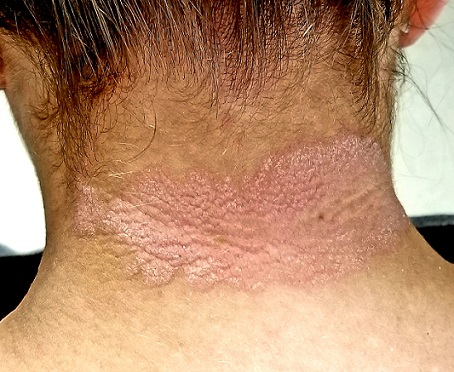
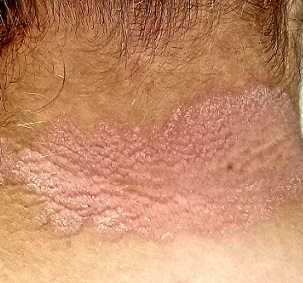
EXAMINATION
A pink plaque with white, fairly adherent scale covers most of the patient’s posterior neck/upper midline back. When a 3-mm section of scaling is peeled away, 2 tiny dots of pinpoint bleeding are immediately noted.
The rest of her scalp is free of any such changes, as are her elbows and knees. But a similar rash is seen in the upper intergluteal area, and 3 of 10 fingernails are mildly pitted.
What’s the diagnosis?
DISCUSSION
Psoriasis vulgaris (common psoriasis) affects around 3% of the white population in this country. That incidence almost doubles in northern Europe and Scandinavia.
Psoriasis is so common that you should expect to see it regularly; the important question is not “Will you see it?” but rather “Will you know it when you see it?” Sometimes the various clinical elements of psoriasis must be sought, and those dots connected, as this case demonstrates effectively.
For one thing, the nape of the neck is commonly affected, especially in women. It is pure speculation, but one imagines that the heat and sweat associated with longer hair might contribute to this predilection.
The pink color, whitish scale, and pinpoint bleeding (termed the Auspitz sign) all corroborate the diagnosis, as does the positive family history and nail pitting. The intergluteal involvement was the icing on the cake; this is seen in only 2 common conditions: psoriasis and seborrhea.
The lesson? Even though psoriasis is supposed to appear on elbows, knees, and other extensor surfaces, sometimes it breaks the rules. The posterior neck was the primary area of involvement in this case, but sometimes psoriasis is completely confined to the scalp or the palms. And, of course, there are different types of psoriasis, some of which bear scant resemblance to psoriasis vulgaris. That’s where biopsies and/or referrals prove to be useful.
It is true that this patient’s rash could have had a fungal origin. When in doubt, however, a punch or shave biopsy would most likely settle the matter, since the histologic picture is usually pathognomic.
TAKE-HOME LEARNING POINTS
- Psoriasis is often be easy to diagnose—but just as often, it takes a bit of detective work.
- This “investigation” consists of looking for and asking about findings that could corroborate the diagnosis.
- The morphology of the neck lesion, as well as the Auspitz sign, nail pitting, intergluteal involvement, and family history in this case all served quite well to establish the diagnosis of psoriasis.
- It is helpful to remember how utterly common psoriasis is, affecting around 10,000,000 Americans.
For more than 2 years, this 36-year-old woman has had a slightly itchy rash that waxes and wanes on her posterior neck. She has consulted several primary care providers and received multiple diagnoses, the most consistent of which has been fungal infection. However, despite use of a variety of antifungal creams (nystatin, clotrimazole, and combination clotrimazole/betamethasone), a 1-month course of oral terbinafine, and OTC tolnaftate, no improvement has occurred.
The patient asserts that she is otherwise in good health, with no joint pain or fever and no history of recent health crises. Family history is free of dermatologic complaints except for psoriasis in her father.

EXAMINATION
A pink plaque with white, fairly adherent scale covers most of the patient’s posterior neck/upper midline back. When a 3-mm section of scaling is peeled away, 2 tiny dots of pinpoint bleeding are immediately noted.
The rest of her scalp is free of any such changes, as are her elbows and knees. But a similar rash is seen in the upper intergluteal area, and 3 of 10 fingernails are mildly pitted.
What’s the diagnosis?
DISCUSSION
Psoriasis vulgaris (common psoriasis) affects around 3% of the white population in this country. That incidence almost doubles in northern Europe and Scandinavia.
Psoriasis is so common that you should expect to see it regularly; the important question is not “Will you see it?” but rather “Will you know it when you see it?” Sometimes the various clinical elements of psoriasis must be sought, and those dots connected, as this case demonstrates effectively.
For one thing, the nape of the neck is commonly affected, especially in women. It is pure speculation, but one imagines that the heat and sweat associated with longer hair might contribute to this predilection.
The pink color, whitish scale, and pinpoint bleeding (termed the Auspitz sign) all corroborate the diagnosis, as does the positive family history and nail pitting. The intergluteal involvement was the icing on the cake; this is seen in only 2 common conditions: psoriasis and seborrhea.
The lesson? Even though psoriasis is supposed to appear on elbows, knees, and other extensor surfaces, sometimes it breaks the rules. The posterior neck was the primary area of involvement in this case, but sometimes psoriasis is completely confined to the scalp or the palms. And, of course, there are different types of psoriasis, some of which bear scant resemblance to psoriasis vulgaris. That’s where biopsies and/or referrals prove to be useful.
It is true that this patient’s rash could have had a fungal origin. When in doubt, however, a punch or shave biopsy would most likely settle the matter, since the histologic picture is usually pathognomic.
TAKE-HOME LEARNING POINTS
- Psoriasis is often be easy to diagnose—but just as often, it takes a bit of detective work.
- This “investigation” consists of looking for and asking about findings that could corroborate the diagnosis.
- The morphology of the neck lesion, as well as the Auspitz sign, nail pitting, intergluteal involvement, and family history in this case all served quite well to establish the diagnosis of psoriasis.
- It is helpful to remember how utterly common psoriasis is, affecting around 10,000,000 Americans.
For more than 2 years, this 36-year-old woman has had a slightly itchy rash that waxes and wanes on her posterior neck. She has consulted several primary care providers and received multiple diagnoses, the most consistent of which has been fungal infection. However, despite use of a variety of antifungal creams (nystatin, clotrimazole, and combination clotrimazole/betamethasone), a 1-month course of oral terbinafine, and OTC tolnaftate, no improvement has occurred.
The patient asserts that she is otherwise in good health, with no joint pain or fever and no history of recent health crises. Family history is free of dermatologic complaints except for psoriasis in her father.

EXAMINATION
A pink plaque with white, fairly adherent scale covers most of the patient’s posterior neck/upper midline back. When a 3-mm section of scaling is peeled away, 2 tiny dots of pinpoint bleeding are immediately noted.
The rest of her scalp is free of any such changes, as are her elbows and knees. But a similar rash is seen in the upper intergluteal area, and 3 of 10 fingernails are mildly pitted.
What’s the diagnosis?
DISCUSSION
Psoriasis vulgaris (common psoriasis) affects around 3% of the white population in this country. That incidence almost doubles in northern Europe and Scandinavia.
Psoriasis is so common that you should expect to see it regularly; the important question is not “Will you see it?” but rather “Will you know it when you see it?” Sometimes the various clinical elements of psoriasis must be sought, and those dots connected, as this case demonstrates effectively.
For one thing, the nape of the neck is commonly affected, especially in women. It is pure speculation, but one imagines that the heat and sweat associated with longer hair might contribute to this predilection.
The pink color, whitish scale, and pinpoint bleeding (termed the Auspitz sign) all corroborate the diagnosis, as does the positive family history and nail pitting. The intergluteal involvement was the icing on the cake; this is seen in only 2 common conditions: psoriasis and seborrhea.
The lesson? Even though psoriasis is supposed to appear on elbows, knees, and other extensor surfaces, sometimes it breaks the rules. The posterior neck was the primary area of involvement in this case, but sometimes psoriasis is completely confined to the scalp or the palms. And, of course, there are different types of psoriasis, some of which bear scant resemblance to psoriasis vulgaris. That’s where biopsies and/or referrals prove to be useful.
It is true that this patient’s rash could have had a fungal origin. When in doubt, however, a punch or shave biopsy would most likely settle the matter, since the histologic picture is usually pathognomic.
TAKE-HOME LEARNING POINTS
- Psoriasis is often be easy to diagnose—but just as often, it takes a bit of detective work.
- This “investigation” consists of looking for and asking about findings that could corroborate the diagnosis.
- The morphology of the neck lesion, as well as the Auspitz sign, nail pitting, intergluteal involvement, and family history in this case all served quite well to establish the diagnosis of psoriasis.
- It is helpful to remember how utterly common psoriasis is, affecting around 10,000,000 Americans.
Western diet linked to lower microbiome diversity
MIAMI – Eating a Western diet correlated with significantly lower gut microbiome diversity in an observational study of 1,000 healthy men and women.
The chief culprits were fried foods, sodas, fatty sweets, processed meats, ready-cooked meals, and desserts, reported Valentin Partula, a PhD student at the Université Paris 13 Nord and his associates. The more often individuals reported consuming these, the fewer bacterial species were identified in their stool (P less than .05 for each association), the investigators wrote in a poster presented at the annual Gut Microbiota for Health World Summit.
Studies have linked decreased microbiota diversity with health conditions ranging from inflammatory bowel disease and colorectal cancer to diabetes mellitus. Obesity also is characterized by a less diverse microbiome and is linked to many of the same diseases, but the diversity (richness) of the gut microbiome appears to have more to do with diet than body mass index. However, interventional studies linking diet to microbiome shifts often have been small, narrow in scope, and short in duration, the researchers noted at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
To help fill these gaps, they administered a 19-item food-frequency questionnaire to 1,000 healthy men and women in France who were 20-69 years old. Each food question had six possible responses, ranging from “at least twice a day” to “never.” For 862 of these men and women, the researchers also analyzed stool samples using 16S rRNA sequencing – a standard test for microbiome diversity. These sequencing results were analyzed in terms of both alpha diversity (the number of species within a sample, and the relative abundance of each) and beta diversity (the degree of dissimilarity among different individuals).
The most significant correlate of low alpha diversity (that is, a less diverse gut microbiome) was frequent consumption of fried foods, followed by sodas or sugary drinks, fatty sweet products, processed meats, ready-cooked meals, and desserts (P less than .05 for each). Conversely, raw fruits and fish each correlated with having a richer microbiome (P less than .05). Consuming eggs and raw and cooked vegetables also correlated with greater diversity, but these associations did not reach statistical significance.
In terms of beta diversity (uniqueness of the microbiome signature), the strongest correlates were fresh fruit, fried products, ready-cooked meals, and cheese. The finding for fresh fruit might be an effect of weighting but needs further study, the researchers said. Taken together, however, the findings “extend and support mechanistic arguments linking Western diet to altered microbiota composition,” they said.
Next, they looked at how specific foods correlated with specific bacterial taxa. Consuming more dairy correlated with a greater abundance of Streptococcus salivarius, which disrupts S. pyogenes biofilms in the pharynx and thus might help prevent bacterial pharyngitis. Eating raw fruits was tied to increases in Eubacterium eligens, a nonpathogenic bacterium whose role in the gut remains unclear. Finally, frequent cheese consumption was linked to lower abundance of Akkermansia muciniphila, a bacterium that is thought to benefit metabolic pathways and immune signaling.
For the same 846 individuals, the researchers performed 1hydrogen nuclear magnetic resonance metabolomic tests on plasma Carr-Purcell-Meiboom-Gill (CPMG)–pulse sequence and nuclear Overhauser enhancement spectroscopy (NOESY). Increased creatinine was associated with the highest number of bacterial taxa and might reflect effects on kidney function or trimethylamine N-oxide, they wrote. Greater microbiome diversity correlated with higher plasma levels of amino acids, proteins, creatinine, choline, glucose, and citrate. Lower diversity was tied to the presence of lipid-based metabolites, including ketones and esters.
The next step is to confirm the findings in a separate population and establish which of these associations are probably causal, the researchers wrote. “Mechanistic studies elucidating the metabolic capability of the organisms [also] are needed.”
No external funding sources or conflicts of interest were reported.
MIAMI – Eating a Western diet correlated with significantly lower gut microbiome diversity in an observational study of 1,000 healthy men and women.
The chief culprits were fried foods, sodas, fatty sweets, processed meats, ready-cooked meals, and desserts, reported Valentin Partula, a PhD student at the Université Paris 13 Nord and his associates. The more often individuals reported consuming these, the fewer bacterial species were identified in their stool (P less than .05 for each association), the investigators wrote in a poster presented at the annual Gut Microbiota for Health World Summit.
Studies have linked decreased microbiota diversity with health conditions ranging from inflammatory bowel disease and colorectal cancer to diabetes mellitus. Obesity also is characterized by a less diverse microbiome and is linked to many of the same diseases, but the diversity (richness) of the gut microbiome appears to have more to do with diet than body mass index. However, interventional studies linking diet to microbiome shifts often have been small, narrow in scope, and short in duration, the researchers noted at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
To help fill these gaps, they administered a 19-item food-frequency questionnaire to 1,000 healthy men and women in France who were 20-69 years old. Each food question had six possible responses, ranging from “at least twice a day” to “never.” For 862 of these men and women, the researchers also analyzed stool samples using 16S rRNA sequencing – a standard test for microbiome diversity. These sequencing results were analyzed in terms of both alpha diversity (the number of species within a sample, and the relative abundance of each) and beta diversity (the degree of dissimilarity among different individuals).
The most significant correlate of low alpha diversity (that is, a less diverse gut microbiome) was frequent consumption of fried foods, followed by sodas or sugary drinks, fatty sweet products, processed meats, ready-cooked meals, and desserts (P less than .05 for each). Conversely, raw fruits and fish each correlated with having a richer microbiome (P less than .05). Consuming eggs and raw and cooked vegetables also correlated with greater diversity, but these associations did not reach statistical significance.
In terms of beta diversity (uniqueness of the microbiome signature), the strongest correlates were fresh fruit, fried products, ready-cooked meals, and cheese. The finding for fresh fruit might be an effect of weighting but needs further study, the researchers said. Taken together, however, the findings “extend and support mechanistic arguments linking Western diet to altered microbiota composition,” they said.
Next, they looked at how specific foods correlated with specific bacterial taxa. Consuming more dairy correlated with a greater abundance of Streptococcus salivarius, which disrupts S. pyogenes biofilms in the pharynx and thus might help prevent bacterial pharyngitis. Eating raw fruits was tied to increases in Eubacterium eligens, a nonpathogenic bacterium whose role in the gut remains unclear. Finally, frequent cheese consumption was linked to lower abundance of Akkermansia muciniphila, a bacterium that is thought to benefit metabolic pathways and immune signaling.
For the same 846 individuals, the researchers performed 1hydrogen nuclear magnetic resonance metabolomic tests on plasma Carr-Purcell-Meiboom-Gill (CPMG)–pulse sequence and nuclear Overhauser enhancement spectroscopy (NOESY). Increased creatinine was associated with the highest number of bacterial taxa and might reflect effects on kidney function or trimethylamine N-oxide, they wrote. Greater microbiome diversity correlated with higher plasma levels of amino acids, proteins, creatinine, choline, glucose, and citrate. Lower diversity was tied to the presence of lipid-based metabolites, including ketones and esters.
The next step is to confirm the findings in a separate population and establish which of these associations are probably causal, the researchers wrote. “Mechanistic studies elucidating the metabolic capability of the organisms [also] are needed.”
No external funding sources or conflicts of interest were reported.
MIAMI – Eating a Western diet correlated with significantly lower gut microbiome diversity in an observational study of 1,000 healthy men and women.
The chief culprits were fried foods, sodas, fatty sweets, processed meats, ready-cooked meals, and desserts, reported Valentin Partula, a PhD student at the Université Paris 13 Nord and his associates. The more often individuals reported consuming these, the fewer bacterial species were identified in their stool (P less than .05 for each association), the investigators wrote in a poster presented at the annual Gut Microbiota for Health World Summit.
Studies have linked decreased microbiota diversity with health conditions ranging from inflammatory bowel disease and colorectal cancer to diabetes mellitus. Obesity also is characterized by a less diverse microbiome and is linked to many of the same diseases, but the diversity (richness) of the gut microbiome appears to have more to do with diet than body mass index. However, interventional studies linking diet to microbiome shifts often have been small, narrow in scope, and short in duration, the researchers noted at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
To help fill these gaps, they administered a 19-item food-frequency questionnaire to 1,000 healthy men and women in France who were 20-69 years old. Each food question had six possible responses, ranging from “at least twice a day” to “never.” For 862 of these men and women, the researchers also analyzed stool samples using 16S rRNA sequencing – a standard test for microbiome diversity. These sequencing results were analyzed in terms of both alpha diversity (the number of species within a sample, and the relative abundance of each) and beta diversity (the degree of dissimilarity among different individuals).
The most significant correlate of low alpha diversity (that is, a less diverse gut microbiome) was frequent consumption of fried foods, followed by sodas or sugary drinks, fatty sweet products, processed meats, ready-cooked meals, and desserts (P less than .05 for each). Conversely, raw fruits and fish each correlated with having a richer microbiome (P less than .05). Consuming eggs and raw and cooked vegetables also correlated with greater diversity, but these associations did not reach statistical significance.
In terms of beta diversity (uniqueness of the microbiome signature), the strongest correlates were fresh fruit, fried products, ready-cooked meals, and cheese. The finding for fresh fruit might be an effect of weighting but needs further study, the researchers said. Taken together, however, the findings “extend and support mechanistic arguments linking Western diet to altered microbiota composition,” they said.
Next, they looked at how specific foods correlated with specific bacterial taxa. Consuming more dairy correlated with a greater abundance of Streptococcus salivarius, which disrupts S. pyogenes biofilms in the pharynx and thus might help prevent bacterial pharyngitis. Eating raw fruits was tied to increases in Eubacterium eligens, a nonpathogenic bacterium whose role in the gut remains unclear. Finally, frequent cheese consumption was linked to lower abundance of Akkermansia muciniphila, a bacterium that is thought to benefit metabolic pathways and immune signaling.
For the same 846 individuals, the researchers performed 1hydrogen nuclear magnetic resonance metabolomic tests on plasma Carr-Purcell-Meiboom-Gill (CPMG)–pulse sequence and nuclear Overhauser enhancement spectroscopy (NOESY). Increased creatinine was associated with the highest number of bacterial taxa and might reflect effects on kidney function or trimethylamine N-oxide, they wrote. Greater microbiome diversity correlated with higher plasma levels of amino acids, proteins, creatinine, choline, glucose, and citrate. Lower diversity was tied to the presence of lipid-based metabolites, including ketones and esters.
The next step is to confirm the findings in a separate population and establish which of these associations are probably causal, the researchers wrote. “Mechanistic studies elucidating the metabolic capability of the organisms [also] are needed.”
No external funding sources or conflicts of interest were reported.
REPORTING FROM GMFH 2019
Earlier diagnosis, treatment needed to curb dramatic rise in neonatal HSV
A 56% increase in neonatal herpes simplex virus (HSV) infection over 7 years was determined as part of a retrospective, multistate, longitudinal cohort study using information collected from the MarketScan Medicaid Database, reported Sanjay Mahant, MD, of the University of Toronto, and his associates.
Comprehensive coordinated care – as well as public health strategies targeting disease prevention, early diagnosis, and treatment – are needed to manage the growing number of neonates diagnosed with HSV, Dr. Mahant and his colleagues said.
A total of 900 newborn Medicaid enrollees aged 0-28 days were chosen from 2,107,124 births for inclusion in the study. All patients, who were diagnosed with HSV infection during hospital admission, were born during Jan. 1, 2009–Dec. 31, 2015.
Susceptibility to primary HSV-1 infection among younger women has been attributed to an increase in oral sex practices over the past 2 decades, which is putting adolescents and young adults at greater risk of genital HSV-1 infection (J Infect Dis. 2007;196[12]:1852-9). As a result, more “primary or nonprimary genital HSV-1 infections among childbearing women” are believed to be the likely cause for the increasing numbers of neonatal HSV cases, the authors speculated, citing a recent study (J Infect Dis. 2014 Feb 1;209[3]:315-7).
HSV, a rare infection typically contracted immediately before or after birth, has both high morbidity and mortality rates; transmission rates “after exposure and during delivery increase from 2% in recurrent infection to 25% and 60% in nonprimary and primary infections, respectively,” Dr. Mahant and his colleagues noted.
Over the study period, disease incidence grew from 3.4/10,000 births in 2009 (1/2,941 births) to 5.3/10,000 births in 2015 (1/1,886 births).
Dr. Mahant and his associates noted several limitations in the study that might explain the increase in incidence.
ICD diagnosis codes, which they characterized as imperfect in their ability to correctly identify neonatal HSV infections, may have led researchers to include infants who were not actually infected or (less likely) to have excluded infants who were infected. States participating in the MarketScan Medicaid Database also may have changed over the study period. Incomplete follow-up after hospitalization made it impossible to track infants who had changed insurers, moved to other states, or died during the study. They also cautioned that outcomes may not be transferable to the general population because outcomes were specific to Medicaid enrollees.
The total cost for initial hospitalization and treatments provided during 6 months of follow-up was $60,620,431 ($87,602 median cost per patient) for the cohort of 900 infants. This is significant given that the authors reported a median length of stay of 18 days for initial hospitalization. Of the 846 patients discharged (54, or 6%, died during initial hospitalization), follow-up data was available for 692 (81%). A total of 316 (46%) infants required at least one subsequent visit to the emergency room, and another 112 (16%) experienced at least one hospital readmission.
That Dr. Mahant and his colleagues “observed high health care use and associated payments over the first 6 months, including and after hospitalization for neonatal HSV” suggests that there is a need for comprehensive, coordinated care once neonatal patients receive a diagnosis of HSV.
“Public health strategies that are targeted on disease prevention and early diagnosis and treatment are needed,” they advised.
The authors had no relevant financial disclosures. The study was funded by the National Institutes of Health.
SOURCE: Mahant S et al. Pediatrics. 2019 Mar. doi: 10.1542/peds.2018-3233.
The rise in herpes simplex virus cases among neonates reported by Mahant et al. is significant, but there are other possible explanations that warrant additional research, James Gaensbauer, MD, and Joseph A. Grubenhoff, MD, wrote in an accompanying editorial.
Among those explanations, Dr. Gaensbauer and Dr. Grubenhoff cite recommendations made nationally in 2013 to screen asymptomatic infants who had been exposed to HSV at the time of delivery as one possible factor elevating the number of cases being reported. More widespread use of polymerase chain reaction (PCR)–based diagnostic testing, which is reported to be more sensitive, also could play a role in increasing the number of cases being identified.
As part of a larger diagnostic “conundrum” challenging clinicians, the editorialists noted that, at present, there is no uniform consensus for performing HSV testing and providing empirical treatment. “Current recommendations from the American Academy of Pediatrics identify and emphasize the importance of recognition of the factors associated with increased likelihood of HSV infection but do not specify a more comprehensive (e.g., all febrile infants) strategy.” Stakeholders should build flexibility into their recommended treatment approaches for the benefit of practitioners operating on the front lines, they advised.
Ultimately, if the increase in incidence of neonatal HSV cases proves largely attributable to the changing behaviors of young women, who have been engaging more frequently in oral sex, as Dr. Mahant and his colleagues suggest, further research will be warranted, cautioned Dr. Gaensbauer and Dr. Grubenhoff.
“With their work, the authors contribute further nuance to a complicated and ongoing question: How do we correctly identify all infants with neonatal HSV in a timely manner while avoiding subjecting large numbers of children to unnecessary tests and empirical treatments?” This debate “is likely to be transformed by increasing availability of rapid PCR testing for HSV,” they said.
The “pathway to better clarity will depend on researchers and clinicians such as Mahant et al., who continue to provide important data and ask critical questions,” Dr. Gaensbauer and Dr. Grubenhoff concluded.
Dr. Gaensbauer and Dr. Grubenhoff are affiliated with the Denver Health Medical Center; the Children’s Hospital Colorado, Aurora; and the department of pediatrics at University of Colorado at Denver, Aurora. This is a summarization of their editorial, which accompanied the article by Mahant et al. (Pediatrics. 2019 Mar. doi: 10.1542/peds.2019-0159). They received no external funding and had no relevant financial disclosures.
The rise in herpes simplex virus cases among neonates reported by Mahant et al. is significant, but there are other possible explanations that warrant additional research, James Gaensbauer, MD, and Joseph A. Grubenhoff, MD, wrote in an accompanying editorial.
Among those explanations, Dr. Gaensbauer and Dr. Grubenhoff cite recommendations made nationally in 2013 to screen asymptomatic infants who had been exposed to HSV at the time of delivery as one possible factor elevating the number of cases being reported. More widespread use of polymerase chain reaction (PCR)–based diagnostic testing, which is reported to be more sensitive, also could play a role in increasing the number of cases being identified.
As part of a larger diagnostic “conundrum” challenging clinicians, the editorialists noted that, at present, there is no uniform consensus for performing HSV testing and providing empirical treatment. “Current recommendations from the American Academy of Pediatrics identify and emphasize the importance of recognition of the factors associated with increased likelihood of HSV infection but do not specify a more comprehensive (e.g., all febrile infants) strategy.” Stakeholders should build flexibility into their recommended treatment approaches for the benefit of practitioners operating on the front lines, they advised.
Ultimately, if the increase in incidence of neonatal HSV cases proves largely attributable to the changing behaviors of young women, who have been engaging more frequently in oral sex, as Dr. Mahant and his colleagues suggest, further research will be warranted, cautioned Dr. Gaensbauer and Dr. Grubenhoff.
“With their work, the authors contribute further nuance to a complicated and ongoing question: How do we correctly identify all infants with neonatal HSV in a timely manner while avoiding subjecting large numbers of children to unnecessary tests and empirical treatments?” This debate “is likely to be transformed by increasing availability of rapid PCR testing for HSV,” they said.
The “pathway to better clarity will depend on researchers and clinicians such as Mahant et al., who continue to provide important data and ask critical questions,” Dr. Gaensbauer and Dr. Grubenhoff concluded.
Dr. Gaensbauer and Dr. Grubenhoff are affiliated with the Denver Health Medical Center; the Children’s Hospital Colorado, Aurora; and the department of pediatrics at University of Colorado at Denver, Aurora. This is a summarization of their editorial, which accompanied the article by Mahant et al. (Pediatrics. 2019 Mar. doi: 10.1542/peds.2019-0159). They received no external funding and had no relevant financial disclosures.
The rise in herpes simplex virus cases among neonates reported by Mahant et al. is significant, but there are other possible explanations that warrant additional research, James Gaensbauer, MD, and Joseph A. Grubenhoff, MD, wrote in an accompanying editorial.
Among those explanations, Dr. Gaensbauer and Dr. Grubenhoff cite recommendations made nationally in 2013 to screen asymptomatic infants who had been exposed to HSV at the time of delivery as one possible factor elevating the number of cases being reported. More widespread use of polymerase chain reaction (PCR)–based diagnostic testing, which is reported to be more sensitive, also could play a role in increasing the number of cases being identified.
As part of a larger diagnostic “conundrum” challenging clinicians, the editorialists noted that, at present, there is no uniform consensus for performing HSV testing and providing empirical treatment. “Current recommendations from the American Academy of Pediatrics identify and emphasize the importance of recognition of the factors associated with increased likelihood of HSV infection but do not specify a more comprehensive (e.g., all febrile infants) strategy.” Stakeholders should build flexibility into their recommended treatment approaches for the benefit of practitioners operating on the front lines, they advised.
Ultimately, if the increase in incidence of neonatal HSV cases proves largely attributable to the changing behaviors of young women, who have been engaging more frequently in oral sex, as Dr. Mahant and his colleagues suggest, further research will be warranted, cautioned Dr. Gaensbauer and Dr. Grubenhoff.
“With their work, the authors contribute further nuance to a complicated and ongoing question: How do we correctly identify all infants with neonatal HSV in a timely manner while avoiding subjecting large numbers of children to unnecessary tests and empirical treatments?” This debate “is likely to be transformed by increasing availability of rapid PCR testing for HSV,” they said.
The “pathway to better clarity will depend on researchers and clinicians such as Mahant et al., who continue to provide important data and ask critical questions,” Dr. Gaensbauer and Dr. Grubenhoff concluded.
Dr. Gaensbauer and Dr. Grubenhoff are affiliated with the Denver Health Medical Center; the Children’s Hospital Colorado, Aurora; and the department of pediatrics at University of Colorado at Denver, Aurora. This is a summarization of their editorial, which accompanied the article by Mahant et al. (Pediatrics. 2019 Mar. doi: 10.1542/peds.2019-0159). They received no external funding and had no relevant financial disclosures.
A 56% increase in neonatal herpes simplex virus (HSV) infection over 7 years was determined as part of a retrospective, multistate, longitudinal cohort study using information collected from the MarketScan Medicaid Database, reported Sanjay Mahant, MD, of the University of Toronto, and his associates.
Comprehensive coordinated care – as well as public health strategies targeting disease prevention, early diagnosis, and treatment – are needed to manage the growing number of neonates diagnosed with HSV, Dr. Mahant and his colleagues said.
A total of 900 newborn Medicaid enrollees aged 0-28 days were chosen from 2,107,124 births for inclusion in the study. All patients, who were diagnosed with HSV infection during hospital admission, were born during Jan. 1, 2009–Dec. 31, 2015.
Susceptibility to primary HSV-1 infection among younger women has been attributed to an increase in oral sex practices over the past 2 decades, which is putting adolescents and young adults at greater risk of genital HSV-1 infection (J Infect Dis. 2007;196[12]:1852-9). As a result, more “primary or nonprimary genital HSV-1 infections among childbearing women” are believed to be the likely cause for the increasing numbers of neonatal HSV cases, the authors speculated, citing a recent study (J Infect Dis. 2014 Feb 1;209[3]:315-7).
HSV, a rare infection typically contracted immediately before or after birth, has both high morbidity and mortality rates; transmission rates “after exposure and during delivery increase from 2% in recurrent infection to 25% and 60% in nonprimary and primary infections, respectively,” Dr. Mahant and his colleagues noted.
Over the study period, disease incidence grew from 3.4/10,000 births in 2009 (1/2,941 births) to 5.3/10,000 births in 2015 (1/1,886 births).
Dr. Mahant and his associates noted several limitations in the study that might explain the increase in incidence.
ICD diagnosis codes, which they characterized as imperfect in their ability to correctly identify neonatal HSV infections, may have led researchers to include infants who were not actually infected or (less likely) to have excluded infants who were infected. States participating in the MarketScan Medicaid Database also may have changed over the study period. Incomplete follow-up after hospitalization made it impossible to track infants who had changed insurers, moved to other states, or died during the study. They also cautioned that outcomes may not be transferable to the general population because outcomes were specific to Medicaid enrollees.
The total cost for initial hospitalization and treatments provided during 6 months of follow-up was $60,620,431 ($87,602 median cost per patient) for the cohort of 900 infants. This is significant given that the authors reported a median length of stay of 18 days for initial hospitalization. Of the 846 patients discharged (54, or 6%, died during initial hospitalization), follow-up data was available for 692 (81%). A total of 316 (46%) infants required at least one subsequent visit to the emergency room, and another 112 (16%) experienced at least one hospital readmission.
That Dr. Mahant and his colleagues “observed high health care use and associated payments over the first 6 months, including and after hospitalization for neonatal HSV” suggests that there is a need for comprehensive, coordinated care once neonatal patients receive a diagnosis of HSV.
“Public health strategies that are targeted on disease prevention and early diagnosis and treatment are needed,” they advised.
The authors had no relevant financial disclosures. The study was funded by the National Institutes of Health.
SOURCE: Mahant S et al. Pediatrics. 2019 Mar. doi: 10.1542/peds.2018-3233.
A 56% increase in neonatal herpes simplex virus (HSV) infection over 7 years was determined as part of a retrospective, multistate, longitudinal cohort study using information collected from the MarketScan Medicaid Database, reported Sanjay Mahant, MD, of the University of Toronto, and his associates.
Comprehensive coordinated care – as well as public health strategies targeting disease prevention, early diagnosis, and treatment – are needed to manage the growing number of neonates diagnosed with HSV, Dr. Mahant and his colleagues said.
A total of 900 newborn Medicaid enrollees aged 0-28 days were chosen from 2,107,124 births for inclusion in the study. All patients, who were diagnosed with HSV infection during hospital admission, were born during Jan. 1, 2009–Dec. 31, 2015.
Susceptibility to primary HSV-1 infection among younger women has been attributed to an increase in oral sex practices over the past 2 decades, which is putting adolescents and young adults at greater risk of genital HSV-1 infection (J Infect Dis. 2007;196[12]:1852-9). As a result, more “primary or nonprimary genital HSV-1 infections among childbearing women” are believed to be the likely cause for the increasing numbers of neonatal HSV cases, the authors speculated, citing a recent study (J Infect Dis. 2014 Feb 1;209[3]:315-7).
HSV, a rare infection typically contracted immediately before or after birth, has both high morbidity and mortality rates; transmission rates “after exposure and during delivery increase from 2% in recurrent infection to 25% and 60% in nonprimary and primary infections, respectively,” Dr. Mahant and his colleagues noted.
Over the study period, disease incidence grew from 3.4/10,000 births in 2009 (1/2,941 births) to 5.3/10,000 births in 2015 (1/1,886 births).
Dr. Mahant and his associates noted several limitations in the study that might explain the increase in incidence.
ICD diagnosis codes, which they characterized as imperfect in their ability to correctly identify neonatal HSV infections, may have led researchers to include infants who were not actually infected or (less likely) to have excluded infants who were infected. States participating in the MarketScan Medicaid Database also may have changed over the study period. Incomplete follow-up after hospitalization made it impossible to track infants who had changed insurers, moved to other states, or died during the study. They also cautioned that outcomes may not be transferable to the general population because outcomes were specific to Medicaid enrollees.
The total cost for initial hospitalization and treatments provided during 6 months of follow-up was $60,620,431 ($87,602 median cost per patient) for the cohort of 900 infants. This is significant given that the authors reported a median length of stay of 18 days for initial hospitalization. Of the 846 patients discharged (54, or 6%, died during initial hospitalization), follow-up data was available for 692 (81%). A total of 316 (46%) infants required at least one subsequent visit to the emergency room, and another 112 (16%) experienced at least one hospital readmission.
That Dr. Mahant and his colleagues “observed high health care use and associated payments over the first 6 months, including and after hospitalization for neonatal HSV” suggests that there is a need for comprehensive, coordinated care once neonatal patients receive a diagnosis of HSV.
“Public health strategies that are targeted on disease prevention and early diagnosis and treatment are needed,” they advised.
The authors had no relevant financial disclosures. The study was funded by the National Institutes of Health.
SOURCE: Mahant S et al. Pediatrics. 2019 Mar. doi: 10.1542/peds.2018-3233.
FROM PEDIATRICS
The 39th ASLMS meeting is now underway
WASHINGTON – At the annual meeting of the American Academy of Dermatology, the taking place March 27-31, 2019, in Denver.

“ASLMS is always an amazing meeting, and it’s a unique meeting,” said past president Mathew Avram, MD, director of the Dermatology Laser & Cosmetic Center at Massachusetts General Hospital, Boston. “At its core, it’s a scientific meeting ... you can take things back to your practice that change the practice of medicine.”
Current ASLMS president Eric Bernstein, MD, of Main Line Center for Laser Surgery, Ardmore, Pa., pointed out that, in addition to doctors and other health care practitioners, other available and accessible attendees include the engineers who build the lasers. And this year, injectables are being incorporated into the program.
MDedge reporter Doug Brunk will be reporting from the meeting.
WASHINGTON – At the annual meeting of the American Academy of Dermatology, the taking place March 27-31, 2019, in Denver.

“ASLMS is always an amazing meeting, and it’s a unique meeting,” said past president Mathew Avram, MD, director of the Dermatology Laser & Cosmetic Center at Massachusetts General Hospital, Boston. “At its core, it’s a scientific meeting ... you can take things back to your practice that change the practice of medicine.”
Current ASLMS president Eric Bernstein, MD, of Main Line Center for Laser Surgery, Ardmore, Pa., pointed out that, in addition to doctors and other health care practitioners, other available and accessible attendees include the engineers who build the lasers. And this year, injectables are being incorporated into the program.
MDedge reporter Doug Brunk will be reporting from the meeting.
WASHINGTON – At the annual meeting of the American Academy of Dermatology, the taking place March 27-31, 2019, in Denver.

“ASLMS is always an amazing meeting, and it’s a unique meeting,” said past president Mathew Avram, MD, director of the Dermatology Laser & Cosmetic Center at Massachusetts General Hospital, Boston. “At its core, it’s a scientific meeting ... you can take things back to your practice that change the practice of medicine.”
Current ASLMS president Eric Bernstein, MD, of Main Line Center for Laser Surgery, Ardmore, Pa., pointed out that, in addition to doctors and other health care practitioners, other available and accessible attendees include the engineers who build the lasers. And this year, injectables are being incorporated into the program.
MDedge reporter Doug Brunk will be reporting from the meeting.
REPORTING FROM AAD 2019
Cellulitis pearls
A 38-year-old man is admitted to the hospital with a painful, swollen left leg. This was not the first instance of this kind for him. He had been admitted for the same problem 3 months earlier. During the earlier admission, he was diagnosed with cellulitis and treated with intravenous cefazolin for 4 days, then discharged on cephalexin with resolution of his swelling and pain. Today, his blood pressure is 120/70, pulse is 90, temperature is 38.2°C, his left leg is edematous from the mid-calf to the ankle, and he has erythema and warmth over the calf. His white blood cell count is 13,000, and a diagnosis of cellulitis is made. Which of the following treatments is most likely to shorten his hospital stay?
A. Vancomycin therapy instead of cefazolin.
B. Piperacillin/tazobactam therapy instead of cefazolin.
C. Prednisolone therapy in addition to antibiotics.
D. Furosemide therapy in addition to antibiotics.
The correct answer is C, prednisolone therapy in addition to antibiotics. Corticosteroids have been used as therapy for a number of infectious diseases, and steroid use has been shown to improve survival in patients with bacterial meningitis, tuberculous meningitis, tuberculous pericarditis, severe typhoid fever, tetanus, or pneumocystis pneumonia with moderate to severe hypoxemia.1 Corticosteroid use in many other infections has been studied, and for many infections, symptomatic benefit has been shown. Berkvist and Sjobeck studied 112 patients admitted to the hospital with lower-extremity erysipelas/cellulitis and randomized the patients to receive prednisolone or placebo in addition to antibiotic treatment.2 The prednisolone-treated patients had a shorter hospital stay (5 days vs. 6 days; P less than .01), and had a shorter length of intravenous antibiotic treatment ( 3 days vs. 4 days; P less than .05). The same researchers followed up the study cohort a year later to see if there was any difference in relapse between the steroid- and placebo-treated patients.3 There was no statistically significant difference in relapse (six patients treated with prednisolone relapsed, compared with 13 who received placebo). Solomon et al. did a retrospective study of patients admitted with erysipelas/cellulitis over a 7-year period.4 The control group was defined as patients who received antibiotics but did not receive prednisone, while the other patients in the study received both antibiotics and prednisone. The patients who received antibiotics and prednisone had more severe cellulitis (most had bullous cellulitis) than the patients in the control group. Long-term follow-up showed a higher incidence of erythema and recurrence of cellulitis in the control group. The return to full function was faster in the prednisone-treated patients than in the control group.
Back to the case. Which of the following is most important to do for this patient to help prevent future episodes of cellulitis?
A. Daily penicillin.
B. Treatment of tinea pedis.
C. Hydrochlorothiazide treatment for leg edema.
D. Topical triamcinolone treatment of dry skin on legs.
The correct answer here is treatment of concurrent tinea pedis infection. Antibiotic prophylaxis is considered in patients who have multiple recurrent episodes. This patient’s unilateral edema is most likely attributable to the cellulitis and should resolve with therapy, so diuretics would not be indicated. Risk factors for recurrent cellulitis are tinea pedis, obesity, venous insufficiency, and lymphedema.5
Concheiro and colleagues did a retrospective study of 122 cases of cellulitis and found tinea pedis in 33% of the cases.6 Muller et al. studied the importance of toe web microorganisms and erysipelas and found that the presence of interdigital tinea pedis was correlated with recurrent infection.7 Treatment of tinea pedis is an easily modifiable risk factor in patients with recurrent cellulitis.
Pearls: Consider adding a short course of steroids in patients with more severe erysipelas/cellulitis, as it can decrease hospital stay and IV antibiotics.
Look for tinea pedis and treat if present in patients who have erysipelas/cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Arch Intern Med. 2008 May 26;168(10):1034-46.
2. Scand J Infect Dis 1997;29(4):377-82.
3. Scand J Infect Dis. 1998;30(2):206-7.
4. Isr Med Assoc J. 2018 Mar;20(3):137-40.
5. J Dtsch Dermatol Ges. 2004 Feb;2(2):89-95.
6. Actas Dermosifiliogr. 2009 Dec;100(10):888-94.
7. J Dtsch Dermatol Ges. 2014 Aug;12(8):691-5.
A 38-year-old man is admitted to the hospital with a painful, swollen left leg. This was not the first instance of this kind for him. He had been admitted for the same problem 3 months earlier. During the earlier admission, he was diagnosed with cellulitis and treated with intravenous cefazolin for 4 days, then discharged on cephalexin with resolution of his swelling and pain. Today, his blood pressure is 120/70, pulse is 90, temperature is 38.2°C, his left leg is edematous from the mid-calf to the ankle, and he has erythema and warmth over the calf. His white blood cell count is 13,000, and a diagnosis of cellulitis is made. Which of the following treatments is most likely to shorten his hospital stay?
A. Vancomycin therapy instead of cefazolin.
B. Piperacillin/tazobactam therapy instead of cefazolin.
C. Prednisolone therapy in addition to antibiotics.
D. Furosemide therapy in addition to antibiotics.
The correct answer is C, prednisolone therapy in addition to antibiotics. Corticosteroids have been used as therapy for a number of infectious diseases, and steroid use has been shown to improve survival in patients with bacterial meningitis, tuberculous meningitis, tuberculous pericarditis, severe typhoid fever, tetanus, or pneumocystis pneumonia with moderate to severe hypoxemia.1 Corticosteroid use in many other infections has been studied, and for many infections, symptomatic benefit has been shown. Berkvist and Sjobeck studied 112 patients admitted to the hospital with lower-extremity erysipelas/cellulitis and randomized the patients to receive prednisolone or placebo in addition to antibiotic treatment.2 The prednisolone-treated patients had a shorter hospital stay (5 days vs. 6 days; P less than .01), and had a shorter length of intravenous antibiotic treatment ( 3 days vs. 4 days; P less than .05). The same researchers followed up the study cohort a year later to see if there was any difference in relapse between the steroid- and placebo-treated patients.3 There was no statistically significant difference in relapse (six patients treated with prednisolone relapsed, compared with 13 who received placebo). Solomon et al. did a retrospective study of patients admitted with erysipelas/cellulitis over a 7-year period.4 The control group was defined as patients who received antibiotics but did not receive prednisone, while the other patients in the study received both antibiotics and prednisone. The patients who received antibiotics and prednisone had more severe cellulitis (most had bullous cellulitis) than the patients in the control group. Long-term follow-up showed a higher incidence of erythema and recurrence of cellulitis in the control group. The return to full function was faster in the prednisone-treated patients than in the control group.
Back to the case. Which of the following is most important to do for this patient to help prevent future episodes of cellulitis?
A. Daily penicillin.
B. Treatment of tinea pedis.
C. Hydrochlorothiazide treatment for leg edema.
D. Topical triamcinolone treatment of dry skin on legs.
The correct answer here is treatment of concurrent tinea pedis infection. Antibiotic prophylaxis is considered in patients who have multiple recurrent episodes. This patient’s unilateral edema is most likely attributable to the cellulitis and should resolve with therapy, so diuretics would not be indicated. Risk factors for recurrent cellulitis are tinea pedis, obesity, venous insufficiency, and lymphedema.5
Concheiro and colleagues did a retrospective study of 122 cases of cellulitis and found tinea pedis in 33% of the cases.6 Muller et al. studied the importance of toe web microorganisms and erysipelas and found that the presence of interdigital tinea pedis was correlated with recurrent infection.7 Treatment of tinea pedis is an easily modifiable risk factor in patients with recurrent cellulitis.
Pearls: Consider adding a short course of steroids in patients with more severe erysipelas/cellulitis, as it can decrease hospital stay and IV antibiotics.
Look for tinea pedis and treat if present in patients who have erysipelas/cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Arch Intern Med. 2008 May 26;168(10):1034-46.
2. Scand J Infect Dis 1997;29(4):377-82.
3. Scand J Infect Dis. 1998;30(2):206-7.
4. Isr Med Assoc J. 2018 Mar;20(3):137-40.
5. J Dtsch Dermatol Ges. 2004 Feb;2(2):89-95.
6. Actas Dermosifiliogr. 2009 Dec;100(10):888-94.
7. J Dtsch Dermatol Ges. 2014 Aug;12(8):691-5.
A 38-year-old man is admitted to the hospital with a painful, swollen left leg. This was not the first instance of this kind for him. He had been admitted for the same problem 3 months earlier. During the earlier admission, he was diagnosed with cellulitis and treated with intravenous cefazolin for 4 days, then discharged on cephalexin with resolution of his swelling and pain. Today, his blood pressure is 120/70, pulse is 90, temperature is 38.2°C, his left leg is edematous from the mid-calf to the ankle, and he has erythema and warmth over the calf. His white blood cell count is 13,000, and a diagnosis of cellulitis is made. Which of the following treatments is most likely to shorten his hospital stay?
A. Vancomycin therapy instead of cefazolin.
B. Piperacillin/tazobactam therapy instead of cefazolin.
C. Prednisolone therapy in addition to antibiotics.
D. Furosemide therapy in addition to antibiotics.
The correct answer is C, prednisolone therapy in addition to antibiotics. Corticosteroids have been used as therapy for a number of infectious diseases, and steroid use has been shown to improve survival in patients with bacterial meningitis, tuberculous meningitis, tuberculous pericarditis, severe typhoid fever, tetanus, or pneumocystis pneumonia with moderate to severe hypoxemia.1 Corticosteroid use in many other infections has been studied, and for many infections, symptomatic benefit has been shown. Berkvist and Sjobeck studied 112 patients admitted to the hospital with lower-extremity erysipelas/cellulitis and randomized the patients to receive prednisolone or placebo in addition to antibiotic treatment.2 The prednisolone-treated patients had a shorter hospital stay (5 days vs. 6 days; P less than .01), and had a shorter length of intravenous antibiotic treatment ( 3 days vs. 4 days; P less than .05). The same researchers followed up the study cohort a year later to see if there was any difference in relapse between the steroid- and placebo-treated patients.3 There was no statistically significant difference in relapse (six patients treated with prednisolone relapsed, compared with 13 who received placebo). Solomon et al. did a retrospective study of patients admitted with erysipelas/cellulitis over a 7-year period.4 The control group was defined as patients who received antibiotics but did not receive prednisone, while the other patients in the study received both antibiotics and prednisone. The patients who received antibiotics and prednisone had more severe cellulitis (most had bullous cellulitis) than the patients in the control group. Long-term follow-up showed a higher incidence of erythema and recurrence of cellulitis in the control group. The return to full function was faster in the prednisone-treated patients than in the control group.
Back to the case. Which of the following is most important to do for this patient to help prevent future episodes of cellulitis?
A. Daily penicillin.
B. Treatment of tinea pedis.
C. Hydrochlorothiazide treatment for leg edema.
D. Topical triamcinolone treatment of dry skin on legs.
The correct answer here is treatment of concurrent tinea pedis infection. Antibiotic prophylaxis is considered in patients who have multiple recurrent episodes. This patient’s unilateral edema is most likely attributable to the cellulitis and should resolve with therapy, so diuretics would not be indicated. Risk factors for recurrent cellulitis are tinea pedis, obesity, venous insufficiency, and lymphedema.5
Concheiro and colleagues did a retrospective study of 122 cases of cellulitis and found tinea pedis in 33% of the cases.6 Muller et al. studied the importance of toe web microorganisms and erysipelas and found that the presence of interdigital tinea pedis was correlated with recurrent infection.7 Treatment of tinea pedis is an easily modifiable risk factor in patients with recurrent cellulitis.
Pearls: Consider adding a short course of steroids in patients with more severe erysipelas/cellulitis, as it can decrease hospital stay and IV antibiotics.
Look for tinea pedis and treat if present in patients who have erysipelas/cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Arch Intern Med. 2008 May 26;168(10):1034-46.
2. Scand J Infect Dis 1997;29(4):377-82.
3. Scand J Infect Dis. 1998;30(2):206-7.
4. Isr Med Assoc J. 2018 Mar;20(3):137-40.
5. J Dtsch Dermatol Ges. 2004 Feb;2(2):89-95.
6. Actas Dermosifiliogr. 2009 Dec;100(10):888-94.
7. J Dtsch Dermatol Ges. 2014 Aug;12(8):691-5.
Artesunate to become first-line malaria treatment in U.S.
Starting April 1, 2019, intravenous artesunate will become the first-line treatment for malaria in the United States, following the discontinuation of quinidine, the only Food and Drug Administration–approved intravenous drug for severe malaria treatment.
Although artesunate is not approved or commercially available in the United States, it is recommended by the World Health Organization. The Centers for Disease Control and Prevention have made the drug available through an expanded use investigational new drug protocol, an FDA regulatory mechanism. Clinicians can obtain the medication through the CDC’s Malaria Hotline (770-488-7788); artesunate will be stocked at 10 quarantine stations and will be released to hospitals free of charge, according to a CDC announcement.
Clinical trials have illustrated that intravenous artesunate is safe, well tolerated, and can be administered even to infants, children, and pregnant women in the second and third trimester.
About 1,700 cases of malaria are reported in the United States per year, 300 of which are classified as severe. The CDC believes the supply of artesunate obtained will be sufficient to treat all cases of severe malaria in the country, according to a CDC press release.
Starting April 1, 2019, intravenous artesunate will become the first-line treatment for malaria in the United States, following the discontinuation of quinidine, the only Food and Drug Administration–approved intravenous drug for severe malaria treatment.
Although artesunate is not approved or commercially available in the United States, it is recommended by the World Health Organization. The Centers for Disease Control and Prevention have made the drug available through an expanded use investigational new drug protocol, an FDA regulatory mechanism. Clinicians can obtain the medication through the CDC’s Malaria Hotline (770-488-7788); artesunate will be stocked at 10 quarantine stations and will be released to hospitals free of charge, according to a CDC announcement.
Clinical trials have illustrated that intravenous artesunate is safe, well tolerated, and can be administered even to infants, children, and pregnant women in the second and third trimester.
About 1,700 cases of malaria are reported in the United States per year, 300 of which are classified as severe. The CDC believes the supply of artesunate obtained will be sufficient to treat all cases of severe malaria in the country, according to a CDC press release.
Starting April 1, 2019, intravenous artesunate will become the first-line treatment for malaria in the United States, following the discontinuation of quinidine, the only Food and Drug Administration–approved intravenous drug for severe malaria treatment.
Although artesunate is not approved or commercially available in the United States, it is recommended by the World Health Organization. The Centers for Disease Control and Prevention have made the drug available through an expanded use investigational new drug protocol, an FDA regulatory mechanism. Clinicians can obtain the medication through the CDC’s Malaria Hotline (770-488-7788); artesunate will be stocked at 10 quarantine stations and will be released to hospitals free of charge, according to a CDC announcement.
Clinical trials have illustrated that intravenous artesunate is safe, well tolerated, and can be administered even to infants, children, and pregnant women in the second and third trimester.
About 1,700 cases of malaria are reported in the United States per year, 300 of which are classified as severe. The CDC believes the supply of artesunate obtained will be sufficient to treat all cases of severe malaria in the country, according to a CDC press release.