User login
At what diameter does a scar form after a full-thickness wound?
DENVER – A clinically identifiable scar occurs after full-thickness skin wounds greater than 400-500 mcm in diameter, while wounds of smaller diameter heal with no clinically perceptible scar.
The findings come from a “The broader purpose of this work is to contribute to the development of techniques for harvesting skin tissue with less morbidity than conventional methods,” lead study author Amanda H. Champlain, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery. “The size threshold at which a full-thickness skin wound can heal without scarring had not been determined prior to this study.”
Dr. Champlain, a fellow at Massachusetts General Hospital and The Wellman Center for Photomedicine, both in Boston, and her colleagues designed a way to evaluate healing responses and safety after collecting skin microbiopsies of different sizes from preabdominoplasty skin. According to the study abstract, the concept “is based on fractional photothermolysis in which a multitude of small, full-thickness thermal burns are produced by a laser on the skin with rapid healing and no scarring.” Measures included the Patient and Observer Scar Assessment Scale (POSAS), donor site pain scale, subject satisfaction survey, and an assessment of side effects, clinical photographs, and histology.
Preliminary data are available for five subjects. The POSAS-Observer scale ranges from 5 to 50 while the POSAS-Patient scale ranges from 6 to 60. The researchers observed that average final POSAS-Observer scores were 5.6 for scars 200 mcm in diameter, 5.2 for scars 400 mcm in diameter, 7.0 for scars 500 mcm in diameter, 6.8 for scars 600 mcm in diameter, 8.2 for scars 800 mcm in diameter, 9.6 for scars 1 mm in diameter, and 13.2 for those 2 mm in diameter. Meanwhile, the average final POSAS-Subject scores were 6.0 for scars 200 mcm in diameter, 6.0 for scars 400 mcm in diameter, 6.6 for scars 500 mcm in diameter, 6.4 for those 600 mcm in diameter, 7.2 for scars 800 mcm in diameter, 7.4 for scars 1 mm in diameter, and 10.0 for those 2 mm in diameter.
The maximum donor site pain reported was 4 out of 10 in one subject. “The procedure was very well tolerated by the subjects,” Dr. Champlain said. “They healed quickly, and the majority were happy with the cosmetic outcome regardless of the diameter of the microbiopsy used.”
The most common side effects of the study procedures included mild bleeding, scabbing, redness, and hyper/hypopigmentation. “The majority of study participants strongly agree that the study procedure was safe, tolerable, and cosmetically sound,” she said.
Dr. Champlain does not have any disclosures, but she said that the study was funded by the Department of Defense.
DENVER – A clinically identifiable scar occurs after full-thickness skin wounds greater than 400-500 mcm in diameter, while wounds of smaller diameter heal with no clinically perceptible scar.
The findings come from a “The broader purpose of this work is to contribute to the development of techniques for harvesting skin tissue with less morbidity than conventional methods,” lead study author Amanda H. Champlain, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery. “The size threshold at which a full-thickness skin wound can heal without scarring had not been determined prior to this study.”
Dr. Champlain, a fellow at Massachusetts General Hospital and The Wellman Center for Photomedicine, both in Boston, and her colleagues designed a way to evaluate healing responses and safety after collecting skin microbiopsies of different sizes from preabdominoplasty skin. According to the study abstract, the concept “is based on fractional photothermolysis in which a multitude of small, full-thickness thermal burns are produced by a laser on the skin with rapid healing and no scarring.” Measures included the Patient and Observer Scar Assessment Scale (POSAS), donor site pain scale, subject satisfaction survey, and an assessment of side effects, clinical photographs, and histology.
Preliminary data are available for five subjects. The POSAS-Observer scale ranges from 5 to 50 while the POSAS-Patient scale ranges from 6 to 60. The researchers observed that average final POSAS-Observer scores were 5.6 for scars 200 mcm in diameter, 5.2 for scars 400 mcm in diameter, 7.0 for scars 500 mcm in diameter, 6.8 for scars 600 mcm in diameter, 8.2 for scars 800 mcm in diameter, 9.6 for scars 1 mm in diameter, and 13.2 for those 2 mm in diameter. Meanwhile, the average final POSAS-Subject scores were 6.0 for scars 200 mcm in diameter, 6.0 for scars 400 mcm in diameter, 6.6 for scars 500 mcm in diameter, 6.4 for those 600 mcm in diameter, 7.2 for scars 800 mcm in diameter, 7.4 for scars 1 mm in diameter, and 10.0 for those 2 mm in diameter.
The maximum donor site pain reported was 4 out of 10 in one subject. “The procedure was very well tolerated by the subjects,” Dr. Champlain said. “They healed quickly, and the majority were happy with the cosmetic outcome regardless of the diameter of the microbiopsy used.”
The most common side effects of the study procedures included mild bleeding, scabbing, redness, and hyper/hypopigmentation. “The majority of study participants strongly agree that the study procedure was safe, tolerable, and cosmetically sound,” she said.
Dr. Champlain does not have any disclosures, but she said that the study was funded by the Department of Defense.
DENVER – A clinically identifiable scar occurs after full-thickness skin wounds greater than 400-500 mcm in diameter, while wounds of smaller diameter heal with no clinically perceptible scar.
The findings come from a “The broader purpose of this work is to contribute to the development of techniques for harvesting skin tissue with less morbidity than conventional methods,” lead study author Amanda H. Champlain, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery. “The size threshold at which a full-thickness skin wound can heal without scarring had not been determined prior to this study.”
Dr. Champlain, a fellow at Massachusetts General Hospital and The Wellman Center for Photomedicine, both in Boston, and her colleagues designed a way to evaluate healing responses and safety after collecting skin microbiopsies of different sizes from preabdominoplasty skin. According to the study abstract, the concept “is based on fractional photothermolysis in which a multitude of small, full-thickness thermal burns are produced by a laser on the skin with rapid healing and no scarring.” Measures included the Patient and Observer Scar Assessment Scale (POSAS), donor site pain scale, subject satisfaction survey, and an assessment of side effects, clinical photographs, and histology.
Preliminary data are available for five subjects. The POSAS-Observer scale ranges from 5 to 50 while the POSAS-Patient scale ranges from 6 to 60. The researchers observed that average final POSAS-Observer scores were 5.6 for scars 200 mcm in diameter, 5.2 for scars 400 mcm in diameter, 7.0 for scars 500 mcm in diameter, 6.8 for scars 600 mcm in diameter, 8.2 for scars 800 mcm in diameter, 9.6 for scars 1 mm in diameter, and 13.2 for those 2 mm in diameter. Meanwhile, the average final POSAS-Subject scores were 6.0 for scars 200 mcm in diameter, 6.0 for scars 400 mcm in diameter, 6.6 for scars 500 mcm in diameter, 6.4 for those 600 mcm in diameter, 7.2 for scars 800 mcm in diameter, 7.4 for scars 1 mm in diameter, and 10.0 for those 2 mm in diameter.
The maximum donor site pain reported was 4 out of 10 in one subject. “The procedure was very well tolerated by the subjects,” Dr. Champlain said. “They healed quickly, and the majority were happy with the cosmetic outcome regardless of the diameter of the microbiopsy used.”
The most common side effects of the study procedures included mild bleeding, scabbing, redness, and hyper/hypopigmentation. “The majority of study participants strongly agree that the study procedure was safe, tolerable, and cosmetically sound,” she said.
Dr. Champlain does not have any disclosures, but she said that the study was funded by the Department of Defense.
REPORTING FROM ASLMS 2019
Key clinical point: Collecting skin microbiopsies of different sizes from preabdominoplasty skin is safe and highly tolerable.
Major finding: Full-thickness skin wounds greater than 400-500 mcm in diameter heal with a clinically identifiable scar.
Study details: A pilot trial in five individuals that set out to determine the biopsy size limit at which healing occurs without a scar, as well as demonstrate the safety of performing multiple skin microbiopsies.
Disclosures: Dr. Champlain does not have any disclosures, but she said that the study was funded by the Department of Defense.
Implementation of a population-based cirrhosis identification and management system
Cirrhosis-related morbidity and mortality is potentially preventable. Antiviral treatment in patients with cirrhosis-related to hepatitis C virus (HCV) or hepatitis B virus can prevent complications.1-3 Beta-blockers and endoscopic treatments of esophageal varices are effective in primary prophylaxis of variceal hemorrhage.4 Surveillance for hepatocellular cancer is associated with increased detection of early-stage cancer and improved survival.5 However, many patients with cirrhosis are either not diagnosed in a primary care setting, or even when diagnosed, not seen or referred to specialty clinics to receive disease-specific care,6 and thus remain at high risk for complications.
Our goal was to implement a population-based cirrhosis identification and management system (P-CIMS) to allow identification of all patients with potential cirrhosis in the health care system and to facilitate their linkage to specialty liver care. We describe the implementation of P-CIMS at a large Veterans Health Administration (VHA) hospital and present initial results about its impact on patient care.
P-CIMS Intervention
P-CIMS is a multicomponent intervention that includes a secure web-based tracking system, standardized communication templates, and care coordination protocols.
Web-based tracking system
An interdisciplinary team of clinicians, programmers, and informatics experts developed the P-CIMS software program by extending an existing comprehensive care tracking system.7 The P-CIMS program (referred to as cirrhosis tracker) extracts information from VHA’s national corporate data warehouse. VHA corporate data warehouse includes diagnosis codes, laboratory test results, vital status, and pharmacy data for each encounter in the VA since October 1999. We designed the cirrhosis tracker program to identify patients who had outpatient or inpatient encounters in the last 3 years with either at least 1 cirrhosis diagnosis (defined as any instance of previously validated International Classification of Diseases-9 and -10 codes)8; or possible cirrhosis (defined as either aspartate aminotransferase to platelet ratio index greater than 2.0 or Fibrosis-4 above 3.24 in patients with active HCV infection9 [defined based on positive HCV RNA or genotype test results]).
The user interface of the cirrhosis tracker is designed for easy patient lookup with live links to patient information extracted from the corporate data warehouse (recent laboratory test results, recent imaging studies, and appointments). The tracker also includes free-text fields that store follow-up information and alerting functions that remind the end user when to follow up with a patient. Supplementary Figure 1 shows screen-shots from the program.
We refined the program through an iterative process to ensure accuracy and completeness of data. Each data element (e.g., cirrhosis diagnosis, laboratory tests, clinic appointments) was validated using the full electronic medical record as the reference standard; this process occurred over a period of 9 months. The program can run to update patient data on a daily basis.
Standardized communication templates and care coordination protocols
Our interdisciplinary team created chart review note templates for use in the VHA electronic medical record to verify diagnosis of cirrhosis and to facilitate accurate communication with primary care providers (PCPs) and other specialty clinicians. We also designed standard patient letters to communicate the recommendations with patients. We established protocols for initial clinical reviews, patient outreach, scheduling, and follow-ups. These care coordination protocols were modified in an iterative manner during the implementation phase of P-CIMS.
Setting and patients
Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston provides care to more than 111,000 veterans, including more than 3,800 patients with cirrhosis. At the time of P-CIMS implementation, there were three hepatologists and four advanced practice providers (APP) who provided liver-related care at the MEDVAMC.
The primary goal of the initial phase of implementation was to link patients with cirrhosis to regular liver-related care. Thus, the sample was limited to patients who did not have ongoing specialty care (i.e., no liver clinic visits in the last 6 months, including patients who were never seen in liver clinics).
Implementation strategy
We used implementation facilitation (IF), an evidence-based strategy, to implement P-CIMS.10 The IF team included facilitators (F.K., D.S.), local champions (S.M., K.H.), and technical support personnel (e.g., tracker programmers). Core components of IF were leadership engagement, creation of and regular engagement with a local stakeholder group of clinicians, educational outreach to clinicians and support staff, and problem solving. The IF activities took part in two phases: preimplementation and implementation.
Preimplementation phase
We interviewed key stakeholders to identify facilitators and barriers to P-CIMS implementation. One of the implementation facilitators (F.K.) obtained facility and clinical section’s leadership support, engaged key stakeholders, and devised a local implementation plan. Stakeholders included leadership in several disciplines: hepatology, infectious diseases, and primary care. We developed a map of clinical workflow processes to describe optimal integration of P-CIMS into existing workflow (Supplementary Figure 2).
Implementation phase
The facilitators met regularly (biweekly for the first year) with the stakeholder group including local champions and clinical staff. One of the facilitators (D.S.) served as the liaison between the P-CIMS team (F.K., A.M., R.M., T.T.) and the clinic staff to ensure that no patients were getting missed and to follow through on patient referrals to care. The programmers troubleshot technical issues that arose, and both facilitators worked with clinical staff to modify workflow as needed. At the start of IF, the facilitator conducted an initial round of trainings through in-person training or with the use of screen-sharing software. The impact of P-CIMS on patient care was tracked and feedback was provided to clinical staff on a quarterly basis.
Implementation results: Linkage to liver specialty care
P-CIMS was successfully implemented at the MEDVAMC. Patient data were first extracted in October 2015 with five updates through March 2017. In total, four APP, one MD, and the facilitator used the cirrhosis tracker on a regular basis. The clinical team (APP) conducted the initial review, triage, and outreach. It took on average 7 minutes (range, 2–20 minutes) for the initial review and outreach. The APPs entered each follow-up reminder in the tracker. For example, if they negotiated a liver clinic appointment with the patient, then they entered a reminder to follow up with the date by which this step (patient seen in liver clinic) should be completed. The tracker has a built-in alerting function. The implementation team was notified (via the tracker) when these tasks were due to ensure timely receipt of recommended care processes.
We identified 2,065 patients who met the case definition of cirrhosis (diagnosed and potentially undiagnosed) and were not in regular liver care. Based on initial review, 1,507 patients had an indication to be seen in the liver clinic. Among the remaining 558, the most common reasons for not requiring liver clinic follow-up were: being seen in other facilities (138 in other VHA and 51 in outside hospitals), followed in other specialty clinics (e.g., liver transplant or infectious disease, n = 93), or absence of cirrhosis based on initial review (n = 165) (see Figure 1 for other reasons).
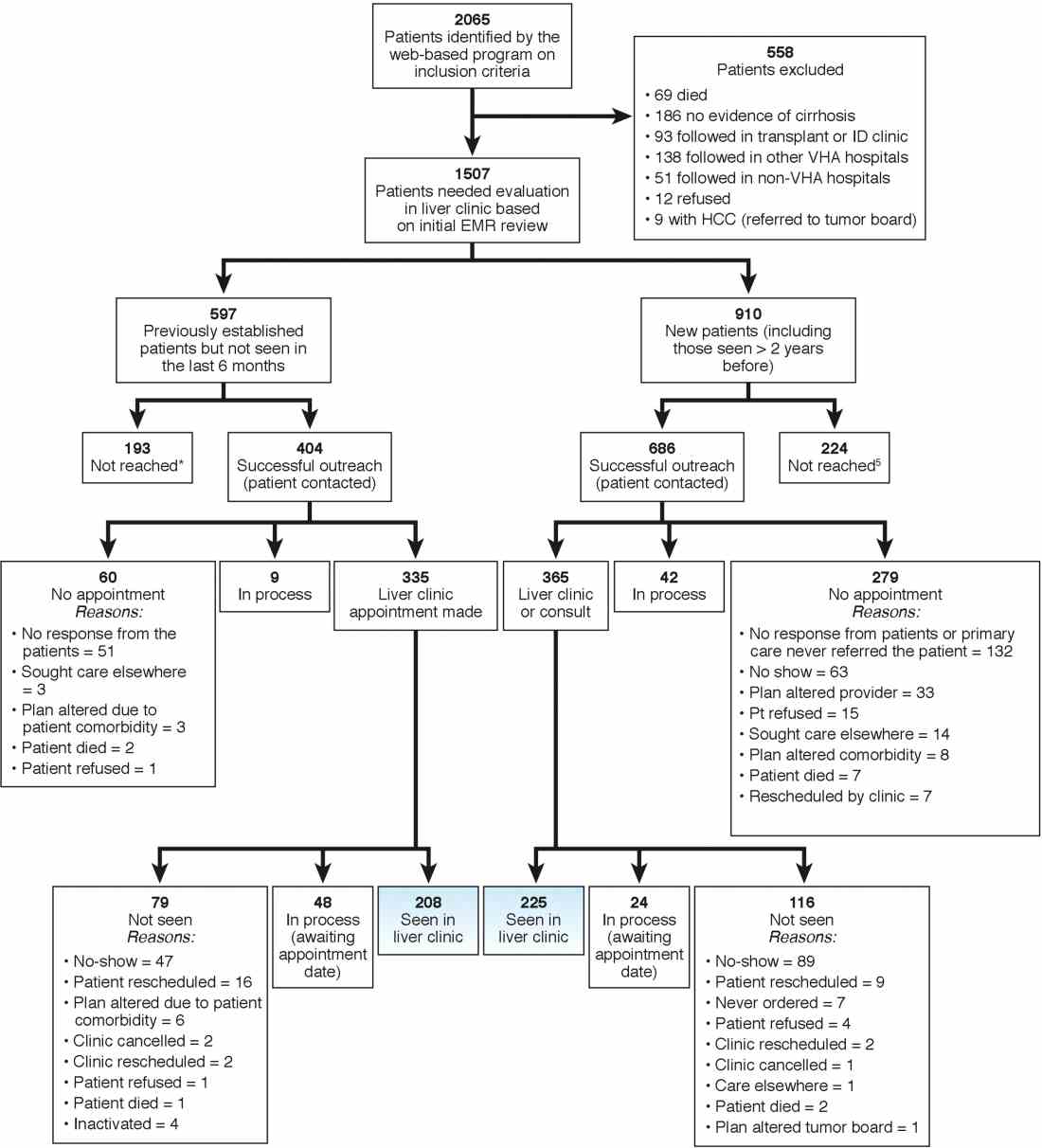
We used two different strategies to reach out to the patients. Of the 1,507 patients, 597 were previously seen in the liver clinics but were lost to follow-up. These patients were contacted directly by the liver clinic staff. The other 910 patients with cirrhosis (of 1,507) had never been seen in the ambulatory liver clinics (n = 559) or were seen more than 2 years before the implementation of cirrhosis tracker (n = 351). These patients were reached through their PCPs. We used standard electronic medical record templates to request PCP’s assistance in reviewing patients' records and submitting a liver consultation after they discussed the need for liver evaluation with the patient.
Of the 597 patients who were previously seen but lost to follow-up, we successfully contacted 404 (67.7%) patients via telephone and/or letters (for the latter, success was defined when patients called back); of these 335 (82.9%) patients had clinic appointments scheduled. In total, 208 (51.5% of 404; 34.8% of 597) patients were subsequently seen in the liver clinics during a median of 12-month follow-up. As shown in Figure 1, the most common reasons for inability to successfully link patients to the clinic were at the patient level, including no show, cancellation, and noninterest in seeking liver care. It took on average 1.5 attempts (range, 1–4) to link 214 patients to the liver clinic.
Of the other 910 patients with cirrhosis, 686 (75.4%) were successfully contacted; and of these 365 (53.2%) patients had liver clinic appointments scheduled. In total, 225 (61.7% of 365; 24.7% of 910) patients were seen in the liver clinics during a median of 12-month follow-up. The reasons underlying inability to link patients to liver specialty clinics are listed in Figure 1 and included shortfalls at the PCP and the patient levels. It took on average 2.4 attempts (range, 1–5) to link 225 patients to the liver clinic.
A total of 124 patients were initiated on direct-acting antiviral agents for HCV treatment and 18 new hepatocellular carcinoma cases were diagnosed as part of P-CIMS.
Discussion and future directions
We learned several lessons during this initiative. First, it was critical to allow time to iteratively revise the cirrhosis tracker program, with input from key stakeholders, including clinician end users. For example, based on feedback, the program was modified to exclude patients who had died or those who were seeking primary care at other VHA facilities. Second, merely having a program that accurately identifies patients with cirrhosis is not the same as knowing how to get organizations and providers to use it. We found that it was critical to involve local leadership and key stakeholders in the preimplementation phase to foster active ownership of P-CIMS and to encourage the rise of natural champions. Additionally, we focused on integrating P-CIMS in the existing workflow. We also had to be cognizant of the needs of patients, such as potential problems with communication relating to notification and appointments for evaluation. Third, several elements at the facility level played a key role in the successful implementation of P-CIMS, including the culture of the facility (commitment to quality improvement); leadership engagement; and perceived need for and relative priority of identifying and managing patients with cirrhosis, especially those with chronic HCV. We also had strong buy-in from the VHA National Program Office tasked with improving care for those with liver disease, which provided support for development of the cirrhosis tracker.
Overall, our early results show that about 30% of patients with cirrhosis without ongoing linkage to liver care were seen in the liver specialty clinics because of P-CIMS. This proportion should be interpreted in the context of the patient population and setting. Cirrhosis disproportionately affects vulnerable patients, including those who are impoverished, homeless, and with drug- and alcohol-related problems; a complex population who often have difficulty staying in care. Most patients in our sample had no linkage with specialty care. It is plausible that some patients with cirrhosis would have been seen in the liver clinics, regardless of P-CIMS. However, we expect this proportion would have been substantially lower than the 30% observed with P-CIMS.
We found several barriers to successful linkage and identified possible solutions. Our results suggest that a direct outreach to patients (without going through PCP) may result in fewer failures to linkage. In total, about 35% of patients who were contacted directly by the liver clinic met the endpoint compared with about 25% of patients who were contacted via their PCP. Future iterations of P-CIMS will rely on direct outreach for most patients. We also found that many patients were unable to keep scheduled appointments; some of this was because of inability to come on specific days and times. Open-access clinics may be one way to accommodate these high-risk patients. Although a full cost-effectiveness analysis is beyond the scope of this report, annual cost of maintaining P-CIMS was less than $100,000 (facilitator and programming support), which is equivalent to antiviral treatment cost of four to five HCV patients, suggesting that P-CIMS (with ability to reach out to hundreds of patients) may indeed be cost effective (if not cost saving).
In summary, we built and successfully implemented a population-based health management system with a structured care coordination strategy to facilitate identification and linkage to care of patients with cirrhosis. Our initial results suggest modest success in managing a complex population who often have difficulty staying in care. The next steps include comparing the rates of linkage to specialty care with rates in comparable facilities that did not use the tracker; broadening the scope to ensure patients are retained in care and receive guideline-concordant care over time. We will share these results in a subsequent manuscript. To our knowledge, cirrhosis tracker is the first informatics tool that leverages data from the electronic medical records with other tools and strategies to improve quality of cirrhosis care. We believe that the lessons that we learned can also help inform efforts to design programs that encourage use of administrative data–based risk screeners to identify patients with other chronic conditions who are at risk for suboptimal outcomes.
References
1. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-16.
2. Kanwal F, Kramer J, Asch SM, et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996-1005.
3. Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-31.
4. Gluud LL, Klingenberg S, Nikolova D, et al. Banding ligation versus beta-blockers as primary prophylaxis in esophageal varices: systematic review of randomized trials. Am J Gastroenterol. 2007;102:2842-8.
5. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130:1099-106.
6. Kanwal F, Volk M, Singal A, et al. Improving quality of health care for patients with cirrhosis. Gastroenterology. 2014;147:1204-7.
7. Taddei T, Hunnibell L, DeLorenzo A, et al. EMR-linked cancer tracker facilitates lung and liver cancer care. J Clin Oncol. 2012;30:77.
8. Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274-82.
9. Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. 2013;158:807-20.
10. Kirchner JE, Ritchie MJ, Pitcock JA, et al. Outcomes of a partnered facilitation strategy to implement primary care-mental health. J Gen Intern Med. 2014;29:904-12.
Dr. Kanwal is professor of medicine, chief of gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Mapakshi is a fellow in gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Centerof Excellence, Michael E. DeBakey VA Medical Center; Ms. Smith is project manager at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Taddei is director of the HCC Initiative, VA Connecticut Healthcare System, associate professor of medicine, digestive diseases, Yale University School of Medicine, director, liver cancer team, Smilow Cancer Hospital at Yale New Haven Hospital; Dr. Hussain is assistant professor, Baylor College of Medicine, Michael E. DeBakey VA Medical Center; Ms. Madu is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Ms. Duong is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Dr. White is assistant professor of medicine, health services research, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Cao is a statistical analyst at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Mehta is in Health Services Research at the VA Connecticut Healthcare System, Yale University School of Medicine, New Haven; Dr. El-Serag is Chairman and Professor Margaret M. and Albert B. Alkek, department of medicine, Baylor College of Medicine, Houston; Dr. Asch is chief of health service research, director of HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System , Palo Alto, Calif., professor of medicine, primary care and population health, Stanford, Calif.; Dr. Midboe is co-implementation research coordinator, HIV/Hepatitis QUERI, director VA patient safety center of inquiry, HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System, Palo Alto, Calif. The authors disclose no conflicts. This material is based on work supported by Department of Veterans Affairs, QUERI Program, QUE 15-284, VA HIV, Hepatitis C, and Related Conditions Program, and VA National Center for Patient Safety. The work is also supported in part by the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413); Michael E. DeBakey VA Medical Center, Houston, Tex.; and the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Cirrhosis-related morbidity and mortality is potentially preventable. Antiviral treatment in patients with cirrhosis-related to hepatitis C virus (HCV) or hepatitis B virus can prevent complications.1-3 Beta-blockers and endoscopic treatments of esophageal varices are effective in primary prophylaxis of variceal hemorrhage.4 Surveillance for hepatocellular cancer is associated with increased detection of early-stage cancer and improved survival.5 However, many patients with cirrhosis are either not diagnosed in a primary care setting, or even when diagnosed, not seen or referred to specialty clinics to receive disease-specific care,6 and thus remain at high risk for complications.
Our goal was to implement a population-based cirrhosis identification and management system (P-CIMS) to allow identification of all patients with potential cirrhosis in the health care system and to facilitate their linkage to specialty liver care. We describe the implementation of P-CIMS at a large Veterans Health Administration (VHA) hospital and present initial results about its impact on patient care.
P-CIMS Intervention
P-CIMS is a multicomponent intervention that includes a secure web-based tracking system, standardized communication templates, and care coordination protocols.
Web-based tracking system
An interdisciplinary team of clinicians, programmers, and informatics experts developed the P-CIMS software program by extending an existing comprehensive care tracking system.7 The P-CIMS program (referred to as cirrhosis tracker) extracts information from VHA’s national corporate data warehouse. VHA corporate data warehouse includes diagnosis codes, laboratory test results, vital status, and pharmacy data for each encounter in the VA since October 1999. We designed the cirrhosis tracker program to identify patients who had outpatient or inpatient encounters in the last 3 years with either at least 1 cirrhosis diagnosis (defined as any instance of previously validated International Classification of Diseases-9 and -10 codes)8; or possible cirrhosis (defined as either aspartate aminotransferase to platelet ratio index greater than 2.0 or Fibrosis-4 above 3.24 in patients with active HCV infection9 [defined based on positive HCV RNA or genotype test results]).
The user interface of the cirrhosis tracker is designed for easy patient lookup with live links to patient information extracted from the corporate data warehouse (recent laboratory test results, recent imaging studies, and appointments). The tracker also includes free-text fields that store follow-up information and alerting functions that remind the end user when to follow up with a patient. Supplementary Figure 1 shows screen-shots from the program.
We refined the program through an iterative process to ensure accuracy and completeness of data. Each data element (e.g., cirrhosis diagnosis, laboratory tests, clinic appointments) was validated using the full electronic medical record as the reference standard; this process occurred over a period of 9 months. The program can run to update patient data on a daily basis.
Standardized communication templates and care coordination protocols
Our interdisciplinary team created chart review note templates for use in the VHA electronic medical record to verify diagnosis of cirrhosis and to facilitate accurate communication with primary care providers (PCPs) and other specialty clinicians. We also designed standard patient letters to communicate the recommendations with patients. We established protocols for initial clinical reviews, patient outreach, scheduling, and follow-ups. These care coordination protocols were modified in an iterative manner during the implementation phase of P-CIMS.
Setting and patients
Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston provides care to more than 111,000 veterans, including more than 3,800 patients with cirrhosis. At the time of P-CIMS implementation, there were three hepatologists and four advanced practice providers (APP) who provided liver-related care at the MEDVAMC.
The primary goal of the initial phase of implementation was to link patients with cirrhosis to regular liver-related care. Thus, the sample was limited to patients who did not have ongoing specialty care (i.e., no liver clinic visits in the last 6 months, including patients who were never seen in liver clinics).
Implementation strategy
We used implementation facilitation (IF), an evidence-based strategy, to implement P-CIMS.10 The IF team included facilitators (F.K., D.S.), local champions (S.M., K.H.), and technical support personnel (e.g., tracker programmers). Core components of IF were leadership engagement, creation of and regular engagement with a local stakeholder group of clinicians, educational outreach to clinicians and support staff, and problem solving. The IF activities took part in two phases: preimplementation and implementation.
Preimplementation phase
We interviewed key stakeholders to identify facilitators and barriers to P-CIMS implementation. One of the implementation facilitators (F.K.) obtained facility and clinical section’s leadership support, engaged key stakeholders, and devised a local implementation plan. Stakeholders included leadership in several disciplines: hepatology, infectious diseases, and primary care. We developed a map of clinical workflow processes to describe optimal integration of P-CIMS into existing workflow (Supplementary Figure 2).
Implementation phase
The facilitators met regularly (biweekly for the first year) with the stakeholder group including local champions and clinical staff. One of the facilitators (D.S.) served as the liaison between the P-CIMS team (F.K., A.M., R.M., T.T.) and the clinic staff to ensure that no patients were getting missed and to follow through on patient referrals to care. The programmers troubleshot technical issues that arose, and both facilitators worked with clinical staff to modify workflow as needed. At the start of IF, the facilitator conducted an initial round of trainings through in-person training or with the use of screen-sharing software. The impact of P-CIMS on patient care was tracked and feedback was provided to clinical staff on a quarterly basis.
Implementation results: Linkage to liver specialty care
P-CIMS was successfully implemented at the MEDVAMC. Patient data were first extracted in October 2015 with five updates through March 2017. In total, four APP, one MD, and the facilitator used the cirrhosis tracker on a regular basis. The clinical team (APP) conducted the initial review, triage, and outreach. It took on average 7 minutes (range, 2–20 minutes) for the initial review and outreach. The APPs entered each follow-up reminder in the tracker. For example, if they negotiated a liver clinic appointment with the patient, then they entered a reminder to follow up with the date by which this step (patient seen in liver clinic) should be completed. The tracker has a built-in alerting function. The implementation team was notified (via the tracker) when these tasks were due to ensure timely receipt of recommended care processes.
We identified 2,065 patients who met the case definition of cirrhosis (diagnosed and potentially undiagnosed) and were not in regular liver care. Based on initial review, 1,507 patients had an indication to be seen in the liver clinic. Among the remaining 558, the most common reasons for not requiring liver clinic follow-up were: being seen in other facilities (138 in other VHA and 51 in outside hospitals), followed in other specialty clinics (e.g., liver transplant or infectious disease, n = 93), or absence of cirrhosis based on initial review (n = 165) (see Figure 1 for other reasons).

We used two different strategies to reach out to the patients. Of the 1,507 patients, 597 were previously seen in the liver clinics but were lost to follow-up. These patients were contacted directly by the liver clinic staff. The other 910 patients with cirrhosis (of 1,507) had never been seen in the ambulatory liver clinics (n = 559) or were seen more than 2 years before the implementation of cirrhosis tracker (n = 351). These patients were reached through their PCPs. We used standard electronic medical record templates to request PCP’s assistance in reviewing patients' records and submitting a liver consultation after they discussed the need for liver evaluation with the patient.
Of the 597 patients who were previously seen but lost to follow-up, we successfully contacted 404 (67.7%) patients via telephone and/or letters (for the latter, success was defined when patients called back); of these 335 (82.9%) patients had clinic appointments scheduled. In total, 208 (51.5% of 404; 34.8% of 597) patients were subsequently seen in the liver clinics during a median of 12-month follow-up. As shown in Figure 1, the most common reasons for inability to successfully link patients to the clinic were at the patient level, including no show, cancellation, and noninterest in seeking liver care. It took on average 1.5 attempts (range, 1–4) to link 214 patients to the liver clinic.
Of the other 910 patients with cirrhosis, 686 (75.4%) were successfully contacted; and of these 365 (53.2%) patients had liver clinic appointments scheduled. In total, 225 (61.7% of 365; 24.7% of 910) patients were seen in the liver clinics during a median of 12-month follow-up. The reasons underlying inability to link patients to liver specialty clinics are listed in Figure 1 and included shortfalls at the PCP and the patient levels. It took on average 2.4 attempts (range, 1–5) to link 225 patients to the liver clinic.
A total of 124 patients were initiated on direct-acting antiviral agents for HCV treatment and 18 new hepatocellular carcinoma cases were diagnosed as part of P-CIMS.
Discussion and future directions
We learned several lessons during this initiative. First, it was critical to allow time to iteratively revise the cirrhosis tracker program, with input from key stakeholders, including clinician end users. For example, based on feedback, the program was modified to exclude patients who had died or those who were seeking primary care at other VHA facilities. Second, merely having a program that accurately identifies patients with cirrhosis is not the same as knowing how to get organizations and providers to use it. We found that it was critical to involve local leadership and key stakeholders in the preimplementation phase to foster active ownership of P-CIMS and to encourage the rise of natural champions. Additionally, we focused on integrating P-CIMS in the existing workflow. We also had to be cognizant of the needs of patients, such as potential problems with communication relating to notification and appointments for evaluation. Third, several elements at the facility level played a key role in the successful implementation of P-CIMS, including the culture of the facility (commitment to quality improvement); leadership engagement; and perceived need for and relative priority of identifying and managing patients with cirrhosis, especially those with chronic HCV. We also had strong buy-in from the VHA National Program Office tasked with improving care for those with liver disease, which provided support for development of the cirrhosis tracker.
Overall, our early results show that about 30% of patients with cirrhosis without ongoing linkage to liver care were seen in the liver specialty clinics because of P-CIMS. This proportion should be interpreted in the context of the patient population and setting. Cirrhosis disproportionately affects vulnerable patients, including those who are impoverished, homeless, and with drug- and alcohol-related problems; a complex population who often have difficulty staying in care. Most patients in our sample had no linkage with specialty care. It is plausible that some patients with cirrhosis would have been seen in the liver clinics, regardless of P-CIMS. However, we expect this proportion would have been substantially lower than the 30% observed with P-CIMS.
We found several barriers to successful linkage and identified possible solutions. Our results suggest that a direct outreach to patients (without going through PCP) may result in fewer failures to linkage. In total, about 35% of patients who were contacted directly by the liver clinic met the endpoint compared with about 25% of patients who were contacted via their PCP. Future iterations of P-CIMS will rely on direct outreach for most patients. We also found that many patients were unable to keep scheduled appointments; some of this was because of inability to come on specific days and times. Open-access clinics may be one way to accommodate these high-risk patients. Although a full cost-effectiveness analysis is beyond the scope of this report, annual cost of maintaining P-CIMS was less than $100,000 (facilitator and programming support), which is equivalent to antiviral treatment cost of four to five HCV patients, suggesting that P-CIMS (with ability to reach out to hundreds of patients) may indeed be cost effective (if not cost saving).
In summary, we built and successfully implemented a population-based health management system with a structured care coordination strategy to facilitate identification and linkage to care of patients with cirrhosis. Our initial results suggest modest success in managing a complex population who often have difficulty staying in care. The next steps include comparing the rates of linkage to specialty care with rates in comparable facilities that did not use the tracker; broadening the scope to ensure patients are retained in care and receive guideline-concordant care over time. We will share these results in a subsequent manuscript. To our knowledge, cirrhosis tracker is the first informatics tool that leverages data from the electronic medical records with other tools and strategies to improve quality of cirrhosis care. We believe that the lessons that we learned can also help inform efforts to design programs that encourage use of administrative data–based risk screeners to identify patients with other chronic conditions who are at risk for suboptimal outcomes.
References
1. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-16.
2. Kanwal F, Kramer J, Asch SM, et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996-1005.
3. Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-31.
4. Gluud LL, Klingenberg S, Nikolova D, et al. Banding ligation versus beta-blockers as primary prophylaxis in esophageal varices: systematic review of randomized trials. Am J Gastroenterol. 2007;102:2842-8.
5. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130:1099-106.
6. Kanwal F, Volk M, Singal A, et al. Improving quality of health care for patients with cirrhosis. Gastroenterology. 2014;147:1204-7.
7. Taddei T, Hunnibell L, DeLorenzo A, et al. EMR-linked cancer tracker facilitates lung and liver cancer care. J Clin Oncol. 2012;30:77.
8. Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274-82.
9. Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. 2013;158:807-20.
10. Kirchner JE, Ritchie MJ, Pitcock JA, et al. Outcomes of a partnered facilitation strategy to implement primary care-mental health. J Gen Intern Med. 2014;29:904-12.
Dr. Kanwal is professor of medicine, chief of gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Mapakshi is a fellow in gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Centerof Excellence, Michael E. DeBakey VA Medical Center; Ms. Smith is project manager at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Taddei is director of the HCC Initiative, VA Connecticut Healthcare System, associate professor of medicine, digestive diseases, Yale University School of Medicine, director, liver cancer team, Smilow Cancer Hospital at Yale New Haven Hospital; Dr. Hussain is assistant professor, Baylor College of Medicine, Michael E. DeBakey VA Medical Center; Ms. Madu is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Ms. Duong is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Dr. White is assistant professor of medicine, health services research, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Cao is a statistical analyst at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Mehta is in Health Services Research at the VA Connecticut Healthcare System, Yale University School of Medicine, New Haven; Dr. El-Serag is Chairman and Professor Margaret M. and Albert B. Alkek, department of medicine, Baylor College of Medicine, Houston; Dr. Asch is chief of health service research, director of HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System , Palo Alto, Calif., professor of medicine, primary care and population health, Stanford, Calif.; Dr. Midboe is co-implementation research coordinator, HIV/Hepatitis QUERI, director VA patient safety center of inquiry, HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System, Palo Alto, Calif. The authors disclose no conflicts. This material is based on work supported by Department of Veterans Affairs, QUERI Program, QUE 15-284, VA HIV, Hepatitis C, and Related Conditions Program, and VA National Center for Patient Safety. The work is also supported in part by the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413); Michael E. DeBakey VA Medical Center, Houston, Tex.; and the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Cirrhosis-related morbidity and mortality is potentially preventable. Antiviral treatment in patients with cirrhosis-related to hepatitis C virus (HCV) or hepatitis B virus can prevent complications.1-3 Beta-blockers and endoscopic treatments of esophageal varices are effective in primary prophylaxis of variceal hemorrhage.4 Surveillance for hepatocellular cancer is associated with increased detection of early-stage cancer and improved survival.5 However, many patients with cirrhosis are either not diagnosed in a primary care setting, or even when diagnosed, not seen or referred to specialty clinics to receive disease-specific care,6 and thus remain at high risk for complications.
Our goal was to implement a population-based cirrhosis identification and management system (P-CIMS) to allow identification of all patients with potential cirrhosis in the health care system and to facilitate their linkage to specialty liver care. We describe the implementation of P-CIMS at a large Veterans Health Administration (VHA) hospital and present initial results about its impact on patient care.
P-CIMS Intervention
P-CIMS is a multicomponent intervention that includes a secure web-based tracking system, standardized communication templates, and care coordination protocols.
Web-based tracking system
An interdisciplinary team of clinicians, programmers, and informatics experts developed the P-CIMS software program by extending an existing comprehensive care tracking system.7 The P-CIMS program (referred to as cirrhosis tracker) extracts information from VHA’s national corporate data warehouse. VHA corporate data warehouse includes diagnosis codes, laboratory test results, vital status, and pharmacy data for each encounter in the VA since October 1999. We designed the cirrhosis tracker program to identify patients who had outpatient or inpatient encounters in the last 3 years with either at least 1 cirrhosis diagnosis (defined as any instance of previously validated International Classification of Diseases-9 and -10 codes)8; or possible cirrhosis (defined as either aspartate aminotransferase to platelet ratio index greater than 2.0 or Fibrosis-4 above 3.24 in patients with active HCV infection9 [defined based on positive HCV RNA or genotype test results]).
The user interface of the cirrhosis tracker is designed for easy patient lookup with live links to patient information extracted from the corporate data warehouse (recent laboratory test results, recent imaging studies, and appointments). The tracker also includes free-text fields that store follow-up information and alerting functions that remind the end user when to follow up with a patient. Supplementary Figure 1 shows screen-shots from the program.
We refined the program through an iterative process to ensure accuracy and completeness of data. Each data element (e.g., cirrhosis diagnosis, laboratory tests, clinic appointments) was validated using the full electronic medical record as the reference standard; this process occurred over a period of 9 months. The program can run to update patient data on a daily basis.
Standardized communication templates and care coordination protocols
Our interdisciplinary team created chart review note templates for use in the VHA electronic medical record to verify diagnosis of cirrhosis and to facilitate accurate communication with primary care providers (PCPs) and other specialty clinicians. We also designed standard patient letters to communicate the recommendations with patients. We established protocols for initial clinical reviews, patient outreach, scheduling, and follow-ups. These care coordination protocols were modified in an iterative manner during the implementation phase of P-CIMS.
Setting and patients
Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston provides care to more than 111,000 veterans, including more than 3,800 patients with cirrhosis. At the time of P-CIMS implementation, there were three hepatologists and four advanced practice providers (APP) who provided liver-related care at the MEDVAMC.
The primary goal of the initial phase of implementation was to link patients with cirrhosis to regular liver-related care. Thus, the sample was limited to patients who did not have ongoing specialty care (i.e., no liver clinic visits in the last 6 months, including patients who were never seen in liver clinics).
Implementation strategy
We used implementation facilitation (IF), an evidence-based strategy, to implement P-CIMS.10 The IF team included facilitators (F.K., D.S.), local champions (S.M., K.H.), and technical support personnel (e.g., tracker programmers). Core components of IF were leadership engagement, creation of and regular engagement with a local stakeholder group of clinicians, educational outreach to clinicians and support staff, and problem solving. The IF activities took part in two phases: preimplementation and implementation.
Preimplementation phase
We interviewed key stakeholders to identify facilitators and barriers to P-CIMS implementation. One of the implementation facilitators (F.K.) obtained facility and clinical section’s leadership support, engaged key stakeholders, and devised a local implementation plan. Stakeholders included leadership in several disciplines: hepatology, infectious diseases, and primary care. We developed a map of clinical workflow processes to describe optimal integration of P-CIMS into existing workflow (Supplementary Figure 2).
Implementation phase
The facilitators met regularly (biweekly for the first year) with the stakeholder group including local champions and clinical staff. One of the facilitators (D.S.) served as the liaison between the P-CIMS team (F.K., A.M., R.M., T.T.) and the clinic staff to ensure that no patients were getting missed and to follow through on patient referrals to care. The programmers troubleshot technical issues that arose, and both facilitators worked with clinical staff to modify workflow as needed. At the start of IF, the facilitator conducted an initial round of trainings through in-person training or with the use of screen-sharing software. The impact of P-CIMS on patient care was tracked and feedback was provided to clinical staff on a quarterly basis.
Implementation results: Linkage to liver specialty care
P-CIMS was successfully implemented at the MEDVAMC. Patient data were first extracted in October 2015 with five updates through March 2017. In total, four APP, one MD, and the facilitator used the cirrhosis tracker on a regular basis. The clinical team (APP) conducted the initial review, triage, and outreach. It took on average 7 minutes (range, 2–20 minutes) for the initial review and outreach. The APPs entered each follow-up reminder in the tracker. For example, if they negotiated a liver clinic appointment with the patient, then they entered a reminder to follow up with the date by which this step (patient seen in liver clinic) should be completed. The tracker has a built-in alerting function. The implementation team was notified (via the tracker) when these tasks were due to ensure timely receipt of recommended care processes.
We identified 2,065 patients who met the case definition of cirrhosis (diagnosed and potentially undiagnosed) and were not in regular liver care. Based on initial review, 1,507 patients had an indication to be seen in the liver clinic. Among the remaining 558, the most common reasons for not requiring liver clinic follow-up were: being seen in other facilities (138 in other VHA and 51 in outside hospitals), followed in other specialty clinics (e.g., liver transplant or infectious disease, n = 93), or absence of cirrhosis based on initial review (n = 165) (see Figure 1 for other reasons).

We used two different strategies to reach out to the patients. Of the 1,507 patients, 597 were previously seen in the liver clinics but were lost to follow-up. These patients were contacted directly by the liver clinic staff. The other 910 patients with cirrhosis (of 1,507) had never been seen in the ambulatory liver clinics (n = 559) or were seen more than 2 years before the implementation of cirrhosis tracker (n = 351). These patients were reached through their PCPs. We used standard electronic medical record templates to request PCP’s assistance in reviewing patients' records and submitting a liver consultation after they discussed the need for liver evaluation with the patient.
Of the 597 patients who were previously seen but lost to follow-up, we successfully contacted 404 (67.7%) patients via telephone and/or letters (for the latter, success was defined when patients called back); of these 335 (82.9%) patients had clinic appointments scheduled. In total, 208 (51.5% of 404; 34.8% of 597) patients were subsequently seen in the liver clinics during a median of 12-month follow-up. As shown in Figure 1, the most common reasons for inability to successfully link patients to the clinic were at the patient level, including no show, cancellation, and noninterest in seeking liver care. It took on average 1.5 attempts (range, 1–4) to link 214 patients to the liver clinic.
Of the other 910 patients with cirrhosis, 686 (75.4%) were successfully contacted; and of these 365 (53.2%) patients had liver clinic appointments scheduled. In total, 225 (61.7% of 365; 24.7% of 910) patients were seen in the liver clinics during a median of 12-month follow-up. The reasons underlying inability to link patients to liver specialty clinics are listed in Figure 1 and included shortfalls at the PCP and the patient levels. It took on average 2.4 attempts (range, 1–5) to link 225 patients to the liver clinic.
A total of 124 patients were initiated on direct-acting antiviral agents for HCV treatment and 18 new hepatocellular carcinoma cases were diagnosed as part of P-CIMS.
Discussion and future directions
We learned several lessons during this initiative. First, it was critical to allow time to iteratively revise the cirrhosis tracker program, with input from key stakeholders, including clinician end users. For example, based on feedback, the program was modified to exclude patients who had died or those who were seeking primary care at other VHA facilities. Second, merely having a program that accurately identifies patients with cirrhosis is not the same as knowing how to get organizations and providers to use it. We found that it was critical to involve local leadership and key stakeholders in the preimplementation phase to foster active ownership of P-CIMS and to encourage the rise of natural champions. Additionally, we focused on integrating P-CIMS in the existing workflow. We also had to be cognizant of the needs of patients, such as potential problems with communication relating to notification and appointments for evaluation. Third, several elements at the facility level played a key role in the successful implementation of P-CIMS, including the culture of the facility (commitment to quality improvement); leadership engagement; and perceived need for and relative priority of identifying and managing patients with cirrhosis, especially those with chronic HCV. We also had strong buy-in from the VHA National Program Office tasked with improving care for those with liver disease, which provided support for development of the cirrhosis tracker.
Overall, our early results show that about 30% of patients with cirrhosis without ongoing linkage to liver care were seen in the liver specialty clinics because of P-CIMS. This proportion should be interpreted in the context of the patient population and setting. Cirrhosis disproportionately affects vulnerable patients, including those who are impoverished, homeless, and with drug- and alcohol-related problems; a complex population who often have difficulty staying in care. Most patients in our sample had no linkage with specialty care. It is plausible that some patients with cirrhosis would have been seen in the liver clinics, regardless of P-CIMS. However, we expect this proportion would have been substantially lower than the 30% observed with P-CIMS.
We found several barriers to successful linkage and identified possible solutions. Our results suggest that a direct outreach to patients (without going through PCP) may result in fewer failures to linkage. In total, about 35% of patients who were contacted directly by the liver clinic met the endpoint compared with about 25% of patients who were contacted via their PCP. Future iterations of P-CIMS will rely on direct outreach for most patients. We also found that many patients were unable to keep scheduled appointments; some of this was because of inability to come on specific days and times. Open-access clinics may be one way to accommodate these high-risk patients. Although a full cost-effectiveness analysis is beyond the scope of this report, annual cost of maintaining P-CIMS was less than $100,000 (facilitator and programming support), which is equivalent to antiviral treatment cost of four to five HCV patients, suggesting that P-CIMS (with ability to reach out to hundreds of patients) may indeed be cost effective (if not cost saving).
In summary, we built and successfully implemented a population-based health management system with a structured care coordination strategy to facilitate identification and linkage to care of patients with cirrhosis. Our initial results suggest modest success in managing a complex population who often have difficulty staying in care. The next steps include comparing the rates of linkage to specialty care with rates in comparable facilities that did not use the tracker; broadening the scope to ensure patients are retained in care and receive guideline-concordant care over time. We will share these results in a subsequent manuscript. To our knowledge, cirrhosis tracker is the first informatics tool that leverages data from the electronic medical records with other tools and strategies to improve quality of cirrhosis care. We believe that the lessons that we learned can also help inform efforts to design programs that encourage use of administrative data–based risk screeners to identify patients with other chronic conditions who are at risk for suboptimal outcomes.
References
1. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-16.
2. Kanwal F, Kramer J, Asch SM, et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996-1005.
3. Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-31.
4. Gluud LL, Klingenberg S, Nikolova D, et al. Banding ligation versus beta-blockers as primary prophylaxis in esophageal varices: systematic review of randomized trials. Am J Gastroenterol. 2007;102:2842-8.
5. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130:1099-106.
6. Kanwal F, Volk M, Singal A, et al. Improving quality of health care for patients with cirrhosis. Gastroenterology. 2014;147:1204-7.
7. Taddei T, Hunnibell L, DeLorenzo A, et al. EMR-linked cancer tracker facilitates lung and liver cancer care. J Clin Oncol. 2012;30:77.
8. Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274-82.
9. Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. 2013;158:807-20.
10. Kirchner JE, Ritchie MJ, Pitcock JA, et al. Outcomes of a partnered facilitation strategy to implement primary care-mental health. J Gen Intern Med. 2014;29:904-12.
Dr. Kanwal is professor of medicine, chief of gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Mapakshi is a fellow in gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Centerof Excellence, Michael E. DeBakey VA Medical Center; Ms. Smith is project manager at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Taddei is director of the HCC Initiative, VA Connecticut Healthcare System, associate professor of medicine, digestive diseases, Yale University School of Medicine, director, liver cancer team, Smilow Cancer Hospital at Yale New Haven Hospital; Dr. Hussain is assistant professor, Baylor College of Medicine, Michael E. DeBakey VA Medical Center; Ms. Madu is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Ms. Duong is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Dr. White is assistant professor of medicine, health services research, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Cao is a statistical analyst at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Mehta is in Health Services Research at the VA Connecticut Healthcare System, Yale University School of Medicine, New Haven; Dr. El-Serag is Chairman and Professor Margaret M. and Albert B. Alkek, department of medicine, Baylor College of Medicine, Houston; Dr. Asch is chief of health service research, director of HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System , Palo Alto, Calif., professor of medicine, primary care and population health, Stanford, Calif.; Dr. Midboe is co-implementation research coordinator, HIV/Hepatitis QUERI, director VA patient safety center of inquiry, HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System, Palo Alto, Calif. The authors disclose no conflicts. This material is based on work supported by Department of Veterans Affairs, QUERI Program, QUE 15-284, VA HIV, Hepatitis C, and Related Conditions Program, and VA National Center for Patient Safety. The work is also supported in part by the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413); Michael E. DeBakey VA Medical Center, Houston, Tex.; and the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Top AGA Community patient cases
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses.
In case you missed it, here are the most popular clinical discussions shared in the forum recently:
1. Refractory lymphocytic colitis and diarrhea
An elderly female with lymphocytic colitis wasn’t responding to any treatment provided by her physician, who was trying to avoid a colectomy due to her advanced age. The GI community shared their support with recommendations for therapy options and next steps.
2. Atypical case of enteropathy
This physician found mild erosive gastritis, villous blunting and mucosal accumulation of eosinophils up to 55/hpf in an 18-year-old female with a history of nausea, vomiting, nonbloody diarrhea, abdominal pain, and weight loss over the past year. She tested negative for celiac disease and a gluten-free diet only provided partial improvement. The conversation in the Community forum covered potential diagnoses to be considered and recommendations for therapy.
3. Eosinophilic esophagitis with aperistalsis
A 21-year-old male presented with progressive dysphagia due to eosinophilic esophagitis with a weight loss of 17 pounds in two months. A panendoscopy revealed a hiatal hernia and aperistalsis of the esophagus, with normal inferior and superior sphincter pressures. No changes were observed recently; he is being managed with prokinetics and remains asymptomatic.
More clinical cases and discussions are at https://community.gastro.org/discussions.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses.
In case you missed it, here are the most popular clinical discussions shared in the forum recently:
1. Refractory lymphocytic colitis and diarrhea
An elderly female with lymphocytic colitis wasn’t responding to any treatment provided by her physician, who was trying to avoid a colectomy due to her advanced age. The GI community shared their support with recommendations for therapy options and next steps.
2. Atypical case of enteropathy
This physician found mild erosive gastritis, villous blunting and mucosal accumulation of eosinophils up to 55/hpf in an 18-year-old female with a history of nausea, vomiting, nonbloody diarrhea, abdominal pain, and weight loss over the past year. She tested negative for celiac disease and a gluten-free diet only provided partial improvement. The conversation in the Community forum covered potential diagnoses to be considered and recommendations for therapy.
3. Eosinophilic esophagitis with aperistalsis
A 21-year-old male presented with progressive dysphagia due to eosinophilic esophagitis with a weight loss of 17 pounds in two months. A panendoscopy revealed a hiatal hernia and aperistalsis of the esophagus, with normal inferior and superior sphincter pressures. No changes were observed recently; he is being managed with prokinetics and remains asymptomatic.
More clinical cases and discussions are at https://community.gastro.org/discussions.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses.
In case you missed it, here are the most popular clinical discussions shared in the forum recently:
1. Refractory lymphocytic colitis and diarrhea
An elderly female with lymphocytic colitis wasn’t responding to any treatment provided by her physician, who was trying to avoid a colectomy due to her advanced age. The GI community shared their support with recommendations for therapy options and next steps.
2. Atypical case of enteropathy
This physician found mild erosive gastritis, villous blunting and mucosal accumulation of eosinophils up to 55/hpf in an 18-year-old female with a history of nausea, vomiting, nonbloody diarrhea, abdominal pain, and weight loss over the past year. She tested negative for celiac disease and a gluten-free diet only provided partial improvement. The conversation in the Community forum covered potential diagnoses to be considered and recommendations for therapy.
3. Eosinophilic esophagitis with aperistalsis
A 21-year-old male presented with progressive dysphagia due to eosinophilic esophagitis with a weight loss of 17 pounds in two months. A panendoscopy revealed a hiatal hernia and aperistalsis of the esophagus, with normal inferior and superior sphincter pressures. No changes were observed recently; he is being managed with prokinetics and remains asymptomatic.
More clinical cases and discussions are at https://community.gastro.org/discussions.
Continuing board certification vision report includes many sound recommendations on MOC
Five key wins will help lay the foundation for new assessment pathway to maintaining board certification.
The Continuing Board Certification: Vision for the Future Commission submitted its final report to the American Board of Medical Specialties (ABMS) Board of Directors. The draft reflected many of the issues AGA has raised with ABIM over the years and our comments on the draft report.
Having this report in hand puts AGA in a position of strength as we begin working with ABIM and the other GI societies to explore the development of a new assessment pathway through which gastroenterologists and hepatologists can maintain board certification.
Here’s a link to the full report: https://visioninitiative.org/commission/final-report/
Key wins:
- Commission recommended the term “Maintenance of Certification” be abandoned.
- “Emphasis on continuing certification must be focused on the availability of curated information that helps diplomates deliver improved clinical care ... traditional infrequent high-stakes assessments no matter how psychometrically valid, is viewed as inappropriate as the future direction for continuing certification.”
- The Commission believes ABMS Boards need to engage with diplomates on an ongoing basis instead of every 2, 5, or 10 years.
- The ABMS Board must offer an alternative to burdensome highly secure, point-in-time examinations of knowledge.
- ABMS and ABMS Boards must facilitate and encourage independent research to build on the existing evidence base about the value of continuing certification.
MOC and AGA’s approach to reform are topics of much discussion on the AGA Community.
Five key wins will help lay the foundation for new assessment pathway to maintaining board certification.
The Continuing Board Certification: Vision for the Future Commission submitted its final report to the American Board of Medical Specialties (ABMS) Board of Directors. The draft reflected many of the issues AGA has raised with ABIM over the years and our comments on the draft report.
Having this report in hand puts AGA in a position of strength as we begin working with ABIM and the other GI societies to explore the development of a new assessment pathway through which gastroenterologists and hepatologists can maintain board certification.
Here’s a link to the full report: https://visioninitiative.org/commission/final-report/
Key wins:
- Commission recommended the term “Maintenance of Certification” be abandoned.
- “Emphasis on continuing certification must be focused on the availability of curated information that helps diplomates deliver improved clinical care ... traditional infrequent high-stakes assessments no matter how psychometrically valid, is viewed as inappropriate as the future direction for continuing certification.”
- The Commission believes ABMS Boards need to engage with diplomates on an ongoing basis instead of every 2, 5, or 10 years.
- The ABMS Board must offer an alternative to burdensome highly secure, point-in-time examinations of knowledge.
- ABMS and ABMS Boards must facilitate and encourage independent research to build on the existing evidence base about the value of continuing certification.
MOC and AGA’s approach to reform are topics of much discussion on the AGA Community.
Five key wins will help lay the foundation for new assessment pathway to maintaining board certification.
The Continuing Board Certification: Vision for the Future Commission submitted its final report to the American Board of Medical Specialties (ABMS) Board of Directors. The draft reflected many of the issues AGA has raised with ABIM over the years and our comments on the draft report.
Having this report in hand puts AGA in a position of strength as we begin working with ABIM and the other GI societies to explore the development of a new assessment pathway through which gastroenterologists and hepatologists can maintain board certification.
Here’s a link to the full report: https://visioninitiative.org/commission/final-report/
Key wins:
- Commission recommended the term “Maintenance of Certification” be abandoned.
- “Emphasis on continuing certification must be focused on the availability of curated information that helps diplomates deliver improved clinical care ... traditional infrequent high-stakes assessments no matter how psychometrically valid, is viewed as inappropriate as the future direction for continuing certification.”
- The Commission believes ABMS Boards need to engage with diplomates on an ongoing basis instead of every 2, 5, or 10 years.
- The ABMS Board must offer an alternative to burdensome highly secure, point-in-time examinations of knowledge.
- ABMS and ABMS Boards must facilitate and encourage independent research to build on the existing evidence base about the value of continuing certification.
MOC and AGA’s approach to reform are topics of much discussion on the AGA Community.
Sperm quality linked to some recurrent pregnancy loss
NEW ORLEANS – Couples with recurrent pregnancy loss (RPL) might benefit from seminal parameter testing, based on results from a study presented at the annual meeting of the Endocrine Society.
Sperm quality was more likely to be impaired in the 50 male partners of women with recurrent pregnancy loss than it was in a control group of 63 similar-age men, reported Anastasia P. Dimakopoulou, MBBS, a clinical research fellow at Imperial College, London.
Recurrent pregnancy loss is defined as three pregnancy losses before 20 weeks of gestation.
The reported prevalence of RPL has been estimated at less than 2% in couples attempting pregnancy. About half of those cases are considered to be idiopathic, she said.
Sperm DNA plays a role in placentation, and previous study findings have shown that men in RPL couples are more likely to have higher rates of DNA fragmentation in their sperm. Male partners are not routinely evaluated when seeking a cause for RPL, however.
In this study, 50 men from RPL couples and 63 control men were screened for factors known to affect sperm quality, such as previous testicular surgery, sexually transmitted diseases, alcohol intake, and smoking. In patients and controls, semen reactive oxidative stress, a novel biomarker of sperm function, was measured with a chemiluminescence luminol assay.
The proportion of men with abnormal sperm morphology, although modest in both groups, was significantly more common in men from RPL couples than in controls (4.5% vs. 3.4%, respectively; P less than .001). In addition, the mean reactive oxidative stress levels were four times greater in the RPL men (9.3 vs. 2.3 relative light units/sec per 106 sperm; P less than .05).
Consistent with the higher median reactive oxidative stress levels, the median DNA fragmentation index, which is likely to be linked to increased reactive oxidative stress, was more than twice as high in the RPL men, compared with the controls (16.3 vs. 7.4; P less than .0001).
In addition, the sperm volume was significantly lower in men from the RPL couples, compared with controls. The levels of morning serum testosterone also were lower in men from RPL couples, but the difference did not reach significance relative to controls.
There has been relatively little attention directed toward the male partner in the evaluation and treatment of RPL, but that should change, according to Dr. Dimakopoulou. She said data encourage a new direction of study, including the effort to look for treatable causes of RPL in the male partner.
“By pursuing drugs that stop sperm DNA damage, it may be possible to identify new therapeutic pathways for couples who experience RPL,” Dr. Dimakopoulou maintained. However, even in advance of targeted therapies, she suggested these data encourage investigation of male partners in couples with RPL. Although evidence of reactive oxidative stress may not define a cause, it broadens the scope of investigation and might have value when counseling patients.
Dr. Dimakopoulou reported no relevant financial relationships to disclose.
SOURCE: Dimakopoulou AP et al. ENDO 2019, Session OR18-5.
NEW ORLEANS – Couples with recurrent pregnancy loss (RPL) might benefit from seminal parameter testing, based on results from a study presented at the annual meeting of the Endocrine Society.
Sperm quality was more likely to be impaired in the 50 male partners of women with recurrent pregnancy loss than it was in a control group of 63 similar-age men, reported Anastasia P. Dimakopoulou, MBBS, a clinical research fellow at Imperial College, London.
Recurrent pregnancy loss is defined as three pregnancy losses before 20 weeks of gestation.
The reported prevalence of RPL has been estimated at less than 2% in couples attempting pregnancy. About half of those cases are considered to be idiopathic, she said.
Sperm DNA plays a role in placentation, and previous study findings have shown that men in RPL couples are more likely to have higher rates of DNA fragmentation in their sperm. Male partners are not routinely evaluated when seeking a cause for RPL, however.
In this study, 50 men from RPL couples and 63 control men were screened for factors known to affect sperm quality, such as previous testicular surgery, sexually transmitted diseases, alcohol intake, and smoking. In patients and controls, semen reactive oxidative stress, a novel biomarker of sperm function, was measured with a chemiluminescence luminol assay.
The proportion of men with abnormal sperm morphology, although modest in both groups, was significantly more common in men from RPL couples than in controls (4.5% vs. 3.4%, respectively; P less than .001). In addition, the mean reactive oxidative stress levels were four times greater in the RPL men (9.3 vs. 2.3 relative light units/sec per 106 sperm; P less than .05).
Consistent with the higher median reactive oxidative stress levels, the median DNA fragmentation index, which is likely to be linked to increased reactive oxidative stress, was more than twice as high in the RPL men, compared with the controls (16.3 vs. 7.4; P less than .0001).
In addition, the sperm volume was significantly lower in men from the RPL couples, compared with controls. The levels of morning serum testosterone also were lower in men from RPL couples, but the difference did not reach significance relative to controls.
There has been relatively little attention directed toward the male partner in the evaluation and treatment of RPL, but that should change, according to Dr. Dimakopoulou. She said data encourage a new direction of study, including the effort to look for treatable causes of RPL in the male partner.
“By pursuing drugs that stop sperm DNA damage, it may be possible to identify new therapeutic pathways for couples who experience RPL,” Dr. Dimakopoulou maintained. However, even in advance of targeted therapies, she suggested these data encourage investigation of male partners in couples with RPL. Although evidence of reactive oxidative stress may not define a cause, it broadens the scope of investigation and might have value when counseling patients.
Dr. Dimakopoulou reported no relevant financial relationships to disclose.
SOURCE: Dimakopoulou AP et al. ENDO 2019, Session OR18-5.
NEW ORLEANS – Couples with recurrent pregnancy loss (RPL) might benefit from seminal parameter testing, based on results from a study presented at the annual meeting of the Endocrine Society.
Sperm quality was more likely to be impaired in the 50 male partners of women with recurrent pregnancy loss than it was in a control group of 63 similar-age men, reported Anastasia P. Dimakopoulou, MBBS, a clinical research fellow at Imperial College, London.
Recurrent pregnancy loss is defined as three pregnancy losses before 20 weeks of gestation.
The reported prevalence of RPL has been estimated at less than 2% in couples attempting pregnancy. About half of those cases are considered to be idiopathic, she said.
Sperm DNA plays a role in placentation, and previous study findings have shown that men in RPL couples are more likely to have higher rates of DNA fragmentation in their sperm. Male partners are not routinely evaluated when seeking a cause for RPL, however.
In this study, 50 men from RPL couples and 63 control men were screened for factors known to affect sperm quality, such as previous testicular surgery, sexually transmitted diseases, alcohol intake, and smoking. In patients and controls, semen reactive oxidative stress, a novel biomarker of sperm function, was measured with a chemiluminescence luminol assay.
The proportion of men with abnormal sperm morphology, although modest in both groups, was significantly more common in men from RPL couples than in controls (4.5% vs. 3.4%, respectively; P less than .001). In addition, the mean reactive oxidative stress levels were four times greater in the RPL men (9.3 vs. 2.3 relative light units/sec per 106 sperm; P less than .05).
Consistent with the higher median reactive oxidative stress levels, the median DNA fragmentation index, which is likely to be linked to increased reactive oxidative stress, was more than twice as high in the RPL men, compared with the controls (16.3 vs. 7.4; P less than .0001).
In addition, the sperm volume was significantly lower in men from the RPL couples, compared with controls. The levels of morning serum testosterone also were lower in men from RPL couples, but the difference did not reach significance relative to controls.
There has been relatively little attention directed toward the male partner in the evaluation and treatment of RPL, but that should change, according to Dr. Dimakopoulou. She said data encourage a new direction of study, including the effort to look for treatable causes of RPL in the male partner.
“By pursuing drugs that stop sperm DNA damage, it may be possible to identify new therapeutic pathways for couples who experience RPL,” Dr. Dimakopoulou maintained. However, even in advance of targeted therapies, she suggested these data encourage investigation of male partners in couples with RPL. Although evidence of reactive oxidative stress may not define a cause, it broadens the scope of investigation and might have value when counseling patients.
Dr. Dimakopoulou reported no relevant financial relationships to disclose.
SOURCE: Dimakopoulou AP et al. ENDO 2019, Session OR18-5.
REPORTING FROM ENDO 2019
Consider 9-mm surgical margins for MIS
WAIKOLOA, HAWAII – The widely utilized 5-mm surgical margins for excision of melanoma in situ are inadequate in many cases, Christopher B. Zachary, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“You probably should be considering more like 9- or 10-mm margins for melanoma in situ,” advised Dr. Zachary, professor and chair of the department of dermatology at the University of California, Irvine.
This has been a controversial matter. The recommendation for the long-standard 5-mm margins for excision of melanoma in situ (MIS) date back to a 1992 consensus opinion. Since then, however, persuasive data have emerged showing that 5-mm margins are often inadequate for clearance, and the latest American Academy of Dermatology guidelines for the management of primary cutaneous melanoma recommend margins of 5-10 mm (J Am Acad Dermatol. 2019 Jan;80[1]:208-50).
Dr. Zachary’s advice to go on the high side of that 5- to 10-mm zone is based in large part on studies led by John A Zitelli, MD, of the University of Pittsburgh. More than 20 years ago, Dr. Zitelli and his coinvestigators published a provocative prospective series of 535 patients whose melanomas – in situ or invasive – were excised via Mohs micrographic surgery with frozen section examination of the margins. A 9-mm margin successfully removed 95% of the melanomas, a 12-mm margin removed 97%, and a 6-mm margin successfully excised only 83% of the lesions (J Am Acad Dermatol. 1997 Sep;37(3 Pt 1):422-9).
In a follow-up study, Dr. Zitelli and his colleagues reported on a prospective series of 1,072 patients with 1,120 MIS, all excised by Mohs micrographic surgery with frozen sections (J Am Acad Dermatol. 2012 Mar;66[3]:438-44). They determined that 86% of the MIS were completely cleared using a 6-mm margin, compared with 98.9% excised with a 9 mm margin, a statistically significant difference (P less than .001).
Support for Dr. Zitelli’s stance that 5-mm margins for MIS are inadequate was provided by dermatologic surgeons at the Mayo Clinic in Scottsdale, Ariz. Of 46 patients who underwent Mohs micrographic surgery with immunostaining for excision of MIS, margins of 6 mm achieved clearance in only half of them. Surgical excision margins of 15 mm were required to successfully clear 96% of the MIS (Dermatol Surg. 2000 Aug;26[8]:771-84).
Quite a few hands shot up when Dr. Zachary asked how many members of his audience utilize 5-mm margins for surgical excision of MIS.
“That had been my practice as well until quite recently,” he said.
Dr. Zachary reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The widely utilized 5-mm surgical margins for excision of melanoma in situ are inadequate in many cases, Christopher B. Zachary, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“You probably should be considering more like 9- or 10-mm margins for melanoma in situ,” advised Dr. Zachary, professor and chair of the department of dermatology at the University of California, Irvine.
This has been a controversial matter. The recommendation for the long-standard 5-mm margins for excision of melanoma in situ (MIS) date back to a 1992 consensus opinion. Since then, however, persuasive data have emerged showing that 5-mm margins are often inadequate for clearance, and the latest American Academy of Dermatology guidelines for the management of primary cutaneous melanoma recommend margins of 5-10 mm (J Am Acad Dermatol. 2019 Jan;80[1]:208-50).
Dr. Zachary’s advice to go on the high side of that 5- to 10-mm zone is based in large part on studies led by John A Zitelli, MD, of the University of Pittsburgh. More than 20 years ago, Dr. Zitelli and his coinvestigators published a provocative prospective series of 535 patients whose melanomas – in situ or invasive – were excised via Mohs micrographic surgery with frozen section examination of the margins. A 9-mm margin successfully removed 95% of the melanomas, a 12-mm margin removed 97%, and a 6-mm margin successfully excised only 83% of the lesions (J Am Acad Dermatol. 1997 Sep;37(3 Pt 1):422-9).
In a follow-up study, Dr. Zitelli and his colleagues reported on a prospective series of 1,072 patients with 1,120 MIS, all excised by Mohs micrographic surgery with frozen sections (J Am Acad Dermatol. 2012 Mar;66[3]:438-44). They determined that 86% of the MIS were completely cleared using a 6-mm margin, compared with 98.9% excised with a 9 mm margin, a statistically significant difference (P less than .001).
Support for Dr. Zitelli’s stance that 5-mm margins for MIS are inadequate was provided by dermatologic surgeons at the Mayo Clinic in Scottsdale, Ariz. Of 46 patients who underwent Mohs micrographic surgery with immunostaining for excision of MIS, margins of 6 mm achieved clearance in only half of them. Surgical excision margins of 15 mm were required to successfully clear 96% of the MIS (Dermatol Surg. 2000 Aug;26[8]:771-84).
Quite a few hands shot up when Dr. Zachary asked how many members of his audience utilize 5-mm margins for surgical excision of MIS.
“That had been my practice as well until quite recently,” he said.
Dr. Zachary reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The widely utilized 5-mm surgical margins for excision of melanoma in situ are inadequate in many cases, Christopher B. Zachary, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“You probably should be considering more like 9- or 10-mm margins for melanoma in situ,” advised Dr. Zachary, professor and chair of the department of dermatology at the University of California, Irvine.
This has been a controversial matter. The recommendation for the long-standard 5-mm margins for excision of melanoma in situ (MIS) date back to a 1992 consensus opinion. Since then, however, persuasive data have emerged showing that 5-mm margins are often inadequate for clearance, and the latest American Academy of Dermatology guidelines for the management of primary cutaneous melanoma recommend margins of 5-10 mm (J Am Acad Dermatol. 2019 Jan;80[1]:208-50).
Dr. Zachary’s advice to go on the high side of that 5- to 10-mm zone is based in large part on studies led by John A Zitelli, MD, of the University of Pittsburgh. More than 20 years ago, Dr. Zitelli and his coinvestigators published a provocative prospective series of 535 patients whose melanomas – in situ or invasive – were excised via Mohs micrographic surgery with frozen section examination of the margins. A 9-mm margin successfully removed 95% of the melanomas, a 12-mm margin removed 97%, and a 6-mm margin successfully excised only 83% of the lesions (J Am Acad Dermatol. 1997 Sep;37(3 Pt 1):422-9).
In a follow-up study, Dr. Zitelli and his colleagues reported on a prospective series of 1,072 patients with 1,120 MIS, all excised by Mohs micrographic surgery with frozen sections (J Am Acad Dermatol. 2012 Mar;66[3]:438-44). They determined that 86% of the MIS were completely cleared using a 6-mm margin, compared with 98.9% excised with a 9 mm margin, a statistically significant difference (P less than .001).
Support for Dr. Zitelli’s stance that 5-mm margins for MIS are inadequate was provided by dermatologic surgeons at the Mayo Clinic in Scottsdale, Ariz. Of 46 patients who underwent Mohs micrographic surgery with immunostaining for excision of MIS, margins of 6 mm achieved clearance in only half of them. Surgical excision margins of 15 mm were required to successfully clear 96% of the MIS (Dermatol Surg. 2000 Aug;26[8]:771-84).
Quite a few hands shot up when Dr. Zachary asked how many members of his audience utilize 5-mm margins for surgical excision of MIS.
“That had been my practice as well until quite recently,” he said.
Dr. Zachary reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Performance-based pay linked to anxiety, depression
A study from researchers at Washington University in St. Louis and Aarhus University in Denmark charts the relationship of payment based on work done and employee mental health. According to phys.org, an aggregator of science, research, and technology news, performance-based pay is in place in 70% of U.S. companies. This means that employee income is based on a combination of bonuses, commission, profit sharing, and individual/team incentives, rather than guaranteed salaries. For some employees, performance-based pay can prove lucrative. But for others, such systems can lead to poor mental health.
The study, published in the Academy of Management Discoveries, charted the use of prescription medications for anxiety and depression by nearly 319,000 employees at about 1,300 companies in Denmark (2019 Feb 26. doi: 10.5465/amd.2018.0007). Those in lower-paid positions and older employees were most vulnerable.
“Basically, older workers seem to be driving all of this effect,” said coauthor Lamar Pierce, PhD, professor of organization and strategy, and associate dean at Washington University. “One, it’s harder for them to move, so they have less labor mobility. And, two, they have less flexibility: Learning new roles, adapting to change, they have more fully formed preferences at this point.”
A gender link also was evident; women were more likely to leave companies that adopted a pay-for-performance system. “Our study expands existing work by showing that the mental health costs of performance-based pay can be severe enough to necessitate pharmaceutical treatment,” the authors wrote.
Once a firm switched to the pay-for-performance system, the number of employees using anxiety and depression medications, which included Xanax and Zoloft, increased by 5.7%. The actual number of affected employees is almost certainly much higher, according to Dr. Pierce. Projecting the data to the United States, Dr. Pierce and his coauthor, Michael S. Dahl, PhD, estimated that 100,000 Americans could be affected.
“These types of mental health problems are incredibly costly to both the individual and firm. If this is reflective of a broader increase in stress and depression in employees, the costs are very high,” added Dr. Pierce. The study highlights the broader health and wellness implications of the companies’ compensation policies, he said. phys.org.
More and more people in the United States with severe mental illness and addictions reportedly are homeless, particularly in the Pacific Northwest and on the West Coast. Legislation aimed at addressing that problem is under discussion by Washington state lawmakers and appears to have broad support. The bill, which would authorize creation of a teaching hospital with 150 beds for people with mental illness, garnered unanimous support in the state’s House of Representatives and now has passed a Senate committee.
“The need for mental health care across our state has outgrown our facilities and our supply of trained health care professionals,” said State Sen. Annette Cleveland, chair of the Senate Health & Long Term Care Committee. “This important facility will address those needs head-on by expanding our physical capacity, enlarging our skilled workforce and increasing access through the use of telehealth services. The establishment of this dedicated behavioral health facility at the University of Washington would be the first of its kind in the nation.”
Jürgen Unützer, MD, MPH told KOMO News that the facility would be accredited and modern. “We would have a facility that’s from 2021 that’s state of the art, that’s approved, that’s a safe, welcoming environment where people would take their family members and say ‘this is a place that can give me some hope,’ ” said Dr. Unützer, who chairs the department of psychiatry and behavioral services at the University of Washington, Seattle.
The legislation, H.B. 1593, is part of efforts by Gov. Jay Inslee to tackle mental health issues in the state. The state’s aging mental health infrastructure has been losing federal funding, and patient safety issues have been identified at state-run mental health hospitals. KOMO News.
The New York Police Department recently reported a near-doubling of 911 calls by people the city refers to as “emotionally disturbed persons” over the past decade. Encounters between police and people in need reportedly have resulted in the deaths of 14 people over the last 3 years.
“There is a serious problem in New York City in the manner in which the NYPD interacts with mentally ill people,” said attorney Sanford Rubenstein, who is representing seven families whose family members with mental illness have been shot by police since 2016. “The training of police officers with regard to that interaction is limited and the number of patrol officers who have been trained is small. That is unacceptable.”
The problem was recognized years ago, and a plan was put in place by the city to provide mental health training to every police officer. Flash ahead 4 years and less than one-third of the police force has received any mental health training – just 11,970 of the 36,753 uniformed police officers. What’s more, teams of mental health workers and police that were formed 3 years ago to help intervene in responses to emotionally disturbed people have not been brought into the loop of the 911 system. The result has been 911 responses by officers not trained to deal with such situations and without the support of those who could help. The number of 911 calls related to emotionally disturbed people rose from just over 91,000 in 2009 to nearly 180,000 in 2018, averaging almost 500 every day. The calls are disproportionately from predominantly black and Hispanic precincts.
A 2014 announcement of “diversion centers,” where people with emotional disturbances could be brought by police instead of ferrying them to hospitals or jail, has failed to materialize. “[The problem] is overwhelming in the neighborhoods that I represent,” said Bronx council member Ritchie J. Torres. “Whether it be Tremont or Fordham – you can feel it and see it on the ground. ... You see chemically addicted, mentally ill people, who either are languishing on the street or being cycled in and out of the criminal justice system. And I’m wondering to myself, there has to be a better approach. This is insane.” New York Magazine.
Iowa Gov. Kim Reynolds has signed a comprehensive bill that, among other things, aims to make sweeping changes in the state’s mental health system. The new law also requires suicide prevention training for school personnel in the state. “This legislation was pushed over the finish line by individuals and families who knew firsthand the importance of having a robust mental health system,” Gov. Reynolds said. Critics contend that the legislation does not go far enough in several respects, including specifying where funding will come from and ensuring full access to care. Meanwhile, the governor announced plans to sign an executive order “establishing a platform to begin developing a children’s mental health system.” Des Moines Register.
Emergency room staff at AdventHealth hospitals in Orlando and neighboring Kissimmee, Fla., will begin assessing the mental health of patients as part of a pilot project with the University of Central Florida, according to reporting by 90.7 WMFE, a National Public Radio affiliate in central Florida. “How do we start providing that preventive care like we would with a typical chest pain patient? The same type of health care probably doesn’t apply to those patients with that mental health disorder,” said Robert Geissler, director of emergency services at AdventHealth Kissimmee. “And that’s why we’re trying to change the landscape with this particular project.” Similar programs in Michigan and Tennessee have helped curb suicides and lowered costs associated with mental health–related emergency care. As part of the AdventHealth program in Florida, emergency room staff will ask patients about feelings of hopelessness or despair as part of routine assessments. Patients deemed at high risk of suicide will be paired with counselors for the next 3 months, with daily calls and possibly house visits. Other mental health care resources in the community will be enlisted. 90.7 WMFE.
A study from researchers at Washington University in St. Louis and Aarhus University in Denmark charts the relationship of payment based on work done and employee mental health. According to phys.org, an aggregator of science, research, and technology news, performance-based pay is in place in 70% of U.S. companies. This means that employee income is based on a combination of bonuses, commission, profit sharing, and individual/team incentives, rather than guaranteed salaries. For some employees, performance-based pay can prove lucrative. But for others, such systems can lead to poor mental health.
The study, published in the Academy of Management Discoveries, charted the use of prescription medications for anxiety and depression by nearly 319,000 employees at about 1,300 companies in Denmark (2019 Feb 26. doi: 10.5465/amd.2018.0007). Those in lower-paid positions and older employees were most vulnerable.
“Basically, older workers seem to be driving all of this effect,” said coauthor Lamar Pierce, PhD, professor of organization and strategy, and associate dean at Washington University. “One, it’s harder for them to move, so they have less labor mobility. And, two, they have less flexibility: Learning new roles, adapting to change, they have more fully formed preferences at this point.”
A gender link also was evident; women were more likely to leave companies that adopted a pay-for-performance system. “Our study expands existing work by showing that the mental health costs of performance-based pay can be severe enough to necessitate pharmaceutical treatment,” the authors wrote.
Once a firm switched to the pay-for-performance system, the number of employees using anxiety and depression medications, which included Xanax and Zoloft, increased by 5.7%. The actual number of affected employees is almost certainly much higher, according to Dr. Pierce. Projecting the data to the United States, Dr. Pierce and his coauthor, Michael S. Dahl, PhD, estimated that 100,000 Americans could be affected.
“These types of mental health problems are incredibly costly to both the individual and firm. If this is reflective of a broader increase in stress and depression in employees, the costs are very high,” added Dr. Pierce. The study highlights the broader health and wellness implications of the companies’ compensation policies, he said. phys.org.
More and more people in the United States with severe mental illness and addictions reportedly are homeless, particularly in the Pacific Northwest and on the West Coast. Legislation aimed at addressing that problem is under discussion by Washington state lawmakers and appears to have broad support. The bill, which would authorize creation of a teaching hospital with 150 beds for people with mental illness, garnered unanimous support in the state’s House of Representatives and now has passed a Senate committee.
“The need for mental health care across our state has outgrown our facilities and our supply of trained health care professionals,” said State Sen. Annette Cleveland, chair of the Senate Health & Long Term Care Committee. “This important facility will address those needs head-on by expanding our physical capacity, enlarging our skilled workforce and increasing access through the use of telehealth services. The establishment of this dedicated behavioral health facility at the University of Washington would be the first of its kind in the nation.”
Jürgen Unützer, MD, MPH told KOMO News that the facility would be accredited and modern. “We would have a facility that’s from 2021 that’s state of the art, that’s approved, that’s a safe, welcoming environment where people would take their family members and say ‘this is a place that can give me some hope,’ ” said Dr. Unützer, who chairs the department of psychiatry and behavioral services at the University of Washington, Seattle.
The legislation, H.B. 1593, is part of efforts by Gov. Jay Inslee to tackle mental health issues in the state. The state’s aging mental health infrastructure has been losing federal funding, and patient safety issues have been identified at state-run mental health hospitals. KOMO News.
The New York Police Department recently reported a near-doubling of 911 calls by people the city refers to as “emotionally disturbed persons” over the past decade. Encounters between police and people in need reportedly have resulted in the deaths of 14 people over the last 3 years.
“There is a serious problem in New York City in the manner in which the NYPD interacts with mentally ill people,” said attorney Sanford Rubenstein, who is representing seven families whose family members with mental illness have been shot by police since 2016. “The training of police officers with regard to that interaction is limited and the number of patrol officers who have been trained is small. That is unacceptable.”
The problem was recognized years ago, and a plan was put in place by the city to provide mental health training to every police officer. Flash ahead 4 years and less than one-third of the police force has received any mental health training – just 11,970 of the 36,753 uniformed police officers. What’s more, teams of mental health workers and police that were formed 3 years ago to help intervene in responses to emotionally disturbed people have not been brought into the loop of the 911 system. The result has been 911 responses by officers not trained to deal with such situations and without the support of those who could help. The number of 911 calls related to emotionally disturbed people rose from just over 91,000 in 2009 to nearly 180,000 in 2018, averaging almost 500 every day. The calls are disproportionately from predominantly black and Hispanic precincts.
A 2014 announcement of “diversion centers,” where people with emotional disturbances could be brought by police instead of ferrying them to hospitals or jail, has failed to materialize. “[The problem] is overwhelming in the neighborhoods that I represent,” said Bronx council member Ritchie J. Torres. “Whether it be Tremont or Fordham – you can feel it and see it on the ground. ... You see chemically addicted, mentally ill people, who either are languishing on the street or being cycled in and out of the criminal justice system. And I’m wondering to myself, there has to be a better approach. This is insane.” New York Magazine.
Iowa Gov. Kim Reynolds has signed a comprehensive bill that, among other things, aims to make sweeping changes in the state’s mental health system. The new law also requires suicide prevention training for school personnel in the state. “This legislation was pushed over the finish line by individuals and families who knew firsthand the importance of having a robust mental health system,” Gov. Reynolds said. Critics contend that the legislation does not go far enough in several respects, including specifying where funding will come from and ensuring full access to care. Meanwhile, the governor announced plans to sign an executive order “establishing a platform to begin developing a children’s mental health system.” Des Moines Register.
Emergency room staff at AdventHealth hospitals in Orlando and neighboring Kissimmee, Fla., will begin assessing the mental health of patients as part of a pilot project with the University of Central Florida, according to reporting by 90.7 WMFE, a National Public Radio affiliate in central Florida. “How do we start providing that preventive care like we would with a typical chest pain patient? The same type of health care probably doesn’t apply to those patients with that mental health disorder,” said Robert Geissler, director of emergency services at AdventHealth Kissimmee. “And that’s why we’re trying to change the landscape with this particular project.” Similar programs in Michigan and Tennessee have helped curb suicides and lowered costs associated with mental health–related emergency care. As part of the AdventHealth program in Florida, emergency room staff will ask patients about feelings of hopelessness or despair as part of routine assessments. Patients deemed at high risk of suicide will be paired with counselors for the next 3 months, with daily calls and possibly house visits. Other mental health care resources in the community will be enlisted. 90.7 WMFE.
A study from researchers at Washington University in St. Louis and Aarhus University in Denmark charts the relationship of payment based on work done and employee mental health. According to phys.org, an aggregator of science, research, and technology news, performance-based pay is in place in 70% of U.S. companies. This means that employee income is based on a combination of bonuses, commission, profit sharing, and individual/team incentives, rather than guaranteed salaries. For some employees, performance-based pay can prove lucrative. But for others, such systems can lead to poor mental health.
The study, published in the Academy of Management Discoveries, charted the use of prescription medications for anxiety and depression by nearly 319,000 employees at about 1,300 companies in Denmark (2019 Feb 26. doi: 10.5465/amd.2018.0007). Those in lower-paid positions and older employees were most vulnerable.
“Basically, older workers seem to be driving all of this effect,” said coauthor Lamar Pierce, PhD, professor of organization and strategy, and associate dean at Washington University. “One, it’s harder for them to move, so they have less labor mobility. And, two, they have less flexibility: Learning new roles, adapting to change, they have more fully formed preferences at this point.”
A gender link also was evident; women were more likely to leave companies that adopted a pay-for-performance system. “Our study expands existing work by showing that the mental health costs of performance-based pay can be severe enough to necessitate pharmaceutical treatment,” the authors wrote.
Once a firm switched to the pay-for-performance system, the number of employees using anxiety and depression medications, which included Xanax and Zoloft, increased by 5.7%. The actual number of affected employees is almost certainly much higher, according to Dr. Pierce. Projecting the data to the United States, Dr. Pierce and his coauthor, Michael S. Dahl, PhD, estimated that 100,000 Americans could be affected.
“These types of mental health problems are incredibly costly to both the individual and firm. If this is reflective of a broader increase in stress and depression in employees, the costs are very high,” added Dr. Pierce. The study highlights the broader health and wellness implications of the companies’ compensation policies, he said. phys.org.
More and more people in the United States with severe mental illness and addictions reportedly are homeless, particularly in the Pacific Northwest and on the West Coast. Legislation aimed at addressing that problem is under discussion by Washington state lawmakers and appears to have broad support. The bill, which would authorize creation of a teaching hospital with 150 beds for people with mental illness, garnered unanimous support in the state’s House of Representatives and now has passed a Senate committee.
“The need for mental health care across our state has outgrown our facilities and our supply of trained health care professionals,” said State Sen. Annette Cleveland, chair of the Senate Health & Long Term Care Committee. “This important facility will address those needs head-on by expanding our physical capacity, enlarging our skilled workforce and increasing access through the use of telehealth services. The establishment of this dedicated behavioral health facility at the University of Washington would be the first of its kind in the nation.”
Jürgen Unützer, MD, MPH told KOMO News that the facility would be accredited and modern. “We would have a facility that’s from 2021 that’s state of the art, that’s approved, that’s a safe, welcoming environment where people would take their family members and say ‘this is a place that can give me some hope,’ ” said Dr. Unützer, who chairs the department of psychiatry and behavioral services at the University of Washington, Seattle.
The legislation, H.B. 1593, is part of efforts by Gov. Jay Inslee to tackle mental health issues in the state. The state’s aging mental health infrastructure has been losing federal funding, and patient safety issues have been identified at state-run mental health hospitals. KOMO News.
The New York Police Department recently reported a near-doubling of 911 calls by people the city refers to as “emotionally disturbed persons” over the past decade. Encounters between police and people in need reportedly have resulted in the deaths of 14 people over the last 3 years.
“There is a serious problem in New York City in the manner in which the NYPD interacts with mentally ill people,” said attorney Sanford Rubenstein, who is representing seven families whose family members with mental illness have been shot by police since 2016. “The training of police officers with regard to that interaction is limited and the number of patrol officers who have been trained is small. That is unacceptable.”
The problem was recognized years ago, and a plan was put in place by the city to provide mental health training to every police officer. Flash ahead 4 years and less than one-third of the police force has received any mental health training – just 11,970 of the 36,753 uniformed police officers. What’s more, teams of mental health workers and police that were formed 3 years ago to help intervene in responses to emotionally disturbed people have not been brought into the loop of the 911 system. The result has been 911 responses by officers not trained to deal with such situations and without the support of those who could help. The number of 911 calls related to emotionally disturbed people rose from just over 91,000 in 2009 to nearly 180,000 in 2018, averaging almost 500 every day. The calls are disproportionately from predominantly black and Hispanic precincts.
A 2014 announcement of “diversion centers,” where people with emotional disturbances could be brought by police instead of ferrying them to hospitals or jail, has failed to materialize. “[The problem] is overwhelming in the neighborhoods that I represent,” said Bronx council member Ritchie J. Torres. “Whether it be Tremont or Fordham – you can feel it and see it on the ground. ... You see chemically addicted, mentally ill people, who either are languishing on the street or being cycled in and out of the criminal justice system. And I’m wondering to myself, there has to be a better approach. This is insane.” New York Magazine.
Iowa Gov. Kim Reynolds has signed a comprehensive bill that, among other things, aims to make sweeping changes in the state’s mental health system. The new law also requires suicide prevention training for school personnel in the state. “This legislation was pushed over the finish line by individuals and families who knew firsthand the importance of having a robust mental health system,” Gov. Reynolds said. Critics contend that the legislation does not go far enough in several respects, including specifying where funding will come from and ensuring full access to care. Meanwhile, the governor announced plans to sign an executive order “establishing a platform to begin developing a children’s mental health system.” Des Moines Register.
Emergency room staff at AdventHealth hospitals in Orlando and neighboring Kissimmee, Fla., will begin assessing the mental health of patients as part of a pilot project with the University of Central Florida, according to reporting by 90.7 WMFE, a National Public Radio affiliate in central Florida. “How do we start providing that preventive care like we would with a typical chest pain patient? The same type of health care probably doesn’t apply to those patients with that mental health disorder,” said Robert Geissler, director of emergency services at AdventHealth Kissimmee. “And that’s why we’re trying to change the landscape with this particular project.” Similar programs in Michigan and Tennessee have helped curb suicides and lowered costs associated with mental health–related emergency care. As part of the AdventHealth program in Florida, emergency room staff will ask patients about feelings of hopelessness or despair as part of routine assessments. Patients deemed at high risk of suicide will be paired with counselors for the next 3 months, with daily calls and possibly house visits. Other mental health care resources in the community will be enlisted. 90.7 WMFE.
Gastroenterology billing and coding: Just the basics
Understanding the business side of medicine helps physicians run a successful practice. However, the business side of medicine is not part of the normal curriculum in training and fellowship programs. Physicians come out of training with the knowledge to treat patients but with little or no knowledge of how to get reimbursed for their services. Gastroenterologists provide both medical and surgical services.
Listed below are some of the basic principles for both documentation and reimbursement policies. All reimbursement is based upon Relative Value Units (RVUs) assigned to every service provided. The services are based upon three factors: physician work value, malpractice cost, and practice expense. Those factors added together and multiplied by a conversion factor assigned by the Centers for Medicare & Medicaid Services (CMS) creates the national physician fee schedule. Each Medicare carrier has localities, and there is another percentage that is multiplied based upon geographic location, which will finalize the approved amount for each service. Your Medicare carrier has the actual approved amounts available on their websites with an effective date of Jan. 1. Commercial payers most commonly base contracts on the Medicare Fee Schedule, but each practice and payer relationship is different. For a better understanding, please contact your practice manager for more specific information based on your payer contracts.
Medical necessity is the key to success. If medical necessity is not demonstrated, payers can deny a claim, deny authorization for a lab test and/or diagnostic study, or recoup previously paid claims. Medicare and commercial payers will often have local coverage determinations (LCDs) for procedures and testing that include indications and restrictions along with approved diagnosis codes. Listed below are the four primary services that GI providers perform and provide interpretation for:
1. Evaluation and management (E&M) services: There are three criteria that have to be met to support any initial visit with patients: the history obtained, the examination performed, and the development of the treatment plan. There are five levels of service for office visits and three levels for inpatient visits, respectively. The levels are chosen based on the decision-making element of the visit, provided the documentation requirements are met for the level chosen. This is often not an easy selection unless the providers are educated on the E&M criteria. Auditors often see that visits are chosen by “guessing” the level, which leads to choosing either a lower or higher level of service than what was actually provided. Some providers have been instructed that E&M services are not that important since procedures are the major source of revenue for the practice. However, GI practices are visit-driven practices, and the initial visits are often worth more RVUs than some procedures. The E&M visit is truly vital and often the backbone for the medical necessity of any additional procedures and diagnostic services required in order for successful treatment of the patient.
2. Endoscopy and procedural billing: Here, medical necessity must be documented in order to submit charges for what was done. Gastroenterologists will often use multiple techniques when treating different areas within the gastrointestinal tract. Documentation has to include the location of lesions/abnormalities, method of treatment/removal, and the reason(s)/indication(s) for those procedures. There may be different instruments used in the colon (for example, snare in the sigmoid colon or biopsy forceps in the transverse colon). These may be separately reported with an appropriate modifier to indicate that these services were performed to different lesions/abnormalities. However, in order to bill for each of the procedures, all of this has to be documented in the endoscopy report. The physician is responsible for accurate and specific documentation and bringing charges back to the billing staff for claim submission. For a successful practice, a team approach is vital. Physicians and coding staff need to have an open line of communication to make sure that everything is submitted appropriately according to payer policies. Billing staff need to communicate any significant changes to the physicians/providers as these changes occur. Ignorance of payer policy is not considered an appropriate excuse when a payer investigates a claim and potential recoupment of moneys paid.
3. Diagnostic studies: Medical necessity/indication for the testing must be documented in order to submit charges for diagnostic studies. The terms “rule out” and “suspect” don’t completely give coders the reason why a physician suspects the patient might have a condition. Usually, abnormal lab tests, signs, and symptoms will often warrant the need for further investigation, and these are the most crucial indications for testing. Not only is this important for diagnostic studies but also for procedures. Make sure that the interpretation of the test results is clear along with a plan/recommendation(s).
4. Diagnosis codes: Assignment of codes per the International Classification of Diseases, 10th revision, Clinical Modification (ICD-10-CM) is the next and most important step after a visit, diagnostic study, and/or procedure. These codes support medical necessity for the services provided, and specificity of the diagnosis code is vital to successful submission and payment of a claim. Signs and symptoms are valid code choices when ruling out a more significant disease/diagnosis because these support medical necessity for a work-up to determine etiology. Comorbidities that impact the provider’s decision making should also be added as additional diagnoses to support the higher level of decision making. Up to 12 diagnosis codes can be assigned to any type of service provided. This also applies to preauthorization of all services, such as lab tests, radiology studies, GI diagnostic studies, and procedures. If specific information is not in the documentation for your staff to access, payers will often deny certain lab and radiology studies, as well as some procedures. There are 71,932 ICD-10-CM codes to choose from, and it is often difficult to find the “specific” code when doing a search in the electronic health record and billing system. Education and training are essential during the orientation sessions prior to active employment, as well as any time the system is upgraded. The providers should be willing to work with the IT representative(s) in the practice to help make the information easier to access. In other words, what “buzz” words would they like included in the description of the ICD-10-CM code in the practice’s list of favorites? For example, Crohn’s disease and ulcerative colitis have over a hundred choices. The choices are based on the location of the disease and whether the disease is without or with complications. If you are going to choose to provide a higher level of E&M service for a patient with Crohn’s disease of the large intestine because of exacerbation of the disease with bleeding, then the appropriate diagnosis code would be one of the following:
- K50.10 Crohn’s disease of large intestine without complications.
- K50.111 Crohn’s disease of large intestine with rectal bleeding.
- K50.112 Crohn’s disease of large intestine with intestinal obstruction.
- K50.113 Crohn’s disease of large intestine with fistula.
- K50.114 Crohn’s disease of large intestine with abscess.
- K50.118 Crohn’s disease of large intestine with other complication.
- K50.119 Crohn’s disease of large intestine with unspecified complications.
Getting paid for your provided services requires attention to detail and communication with your entire staffing team, including all providers. Make sure that your team is educated on all current issues and services pertaining to gastroenterology practices. If there is ever a question when reviewing a procedure note or any service, ask the provider who performed that service. Often, there will have to be legal corrections to the note before services can be billed. Making sure that the claim you are submitting is “clean” is essential for prompt payment. There are multiple resources available through the AGA that will help guide you with coding and billing. There are webinars, training sessions, and onsite services available via http://agau.gastro.org/diweb/catalog that can be provided for all providers, coding and billing staff, administrators, and clinical staff. Everyone needs to take an active role.
Ms. Mueller is a health care consultant with more than 35 years of experience in health care, including ICU/CCU nursing, physician office administration, GI claims submission and adjudication, and seminar instruction. She is president and owner of AskMueller Consulting in Lenzburg, Ill., which provides consulting services for physicians nationwide. Ms. Mueller is a nationally known speaker and the author of many multispecialty medical and surgical coding workbooks. She has a great amount of experience in gastroenterology, surgical subspecialties, and pediatric subspecialties. Her presentations have had audiences with the American Gastroenterological Association (AGA), North American Society for Pediatric Gastroenterology and Nutrition (NASPGAN), Society of Gastroenterology Nurses and Associates, Digestive Disease Week, American Pediatric Surgical Associations, and Decision Health and the Coding Institute. Ms. Mueller has written coding columns for ASGE, NASPGHAN, and AGA. She is the coeditor of the ASGE Coding Primer and also answers the coding hotline for the ASGE.
Understanding the business side of medicine helps physicians run a successful practice. However, the business side of medicine is not part of the normal curriculum in training and fellowship programs. Physicians come out of training with the knowledge to treat patients but with little or no knowledge of how to get reimbursed for their services. Gastroenterologists provide both medical and surgical services.
Listed below are some of the basic principles for both documentation and reimbursement policies. All reimbursement is based upon Relative Value Units (RVUs) assigned to every service provided. The services are based upon three factors: physician work value, malpractice cost, and practice expense. Those factors added together and multiplied by a conversion factor assigned by the Centers for Medicare & Medicaid Services (CMS) creates the national physician fee schedule. Each Medicare carrier has localities, and there is another percentage that is multiplied based upon geographic location, which will finalize the approved amount for each service. Your Medicare carrier has the actual approved amounts available on their websites with an effective date of Jan. 1. Commercial payers most commonly base contracts on the Medicare Fee Schedule, but each practice and payer relationship is different. For a better understanding, please contact your practice manager for more specific information based on your payer contracts.
Medical necessity is the key to success. If medical necessity is not demonstrated, payers can deny a claim, deny authorization for a lab test and/or diagnostic study, or recoup previously paid claims. Medicare and commercial payers will often have local coverage determinations (LCDs) for procedures and testing that include indications and restrictions along with approved diagnosis codes. Listed below are the four primary services that GI providers perform and provide interpretation for:
1. Evaluation and management (E&M) services: There are three criteria that have to be met to support any initial visit with patients: the history obtained, the examination performed, and the development of the treatment plan. There are five levels of service for office visits and three levels for inpatient visits, respectively. The levels are chosen based on the decision-making element of the visit, provided the documentation requirements are met for the level chosen. This is often not an easy selection unless the providers are educated on the E&M criteria. Auditors often see that visits are chosen by “guessing” the level, which leads to choosing either a lower or higher level of service than what was actually provided. Some providers have been instructed that E&M services are not that important since procedures are the major source of revenue for the practice. However, GI practices are visit-driven practices, and the initial visits are often worth more RVUs than some procedures. The E&M visit is truly vital and often the backbone for the medical necessity of any additional procedures and diagnostic services required in order for successful treatment of the patient.
2. Endoscopy and procedural billing: Here, medical necessity must be documented in order to submit charges for what was done. Gastroenterologists will often use multiple techniques when treating different areas within the gastrointestinal tract. Documentation has to include the location of lesions/abnormalities, method of treatment/removal, and the reason(s)/indication(s) for those procedures. There may be different instruments used in the colon (for example, snare in the sigmoid colon or biopsy forceps in the transverse colon). These may be separately reported with an appropriate modifier to indicate that these services were performed to different lesions/abnormalities. However, in order to bill for each of the procedures, all of this has to be documented in the endoscopy report. The physician is responsible for accurate and specific documentation and bringing charges back to the billing staff for claim submission. For a successful practice, a team approach is vital. Physicians and coding staff need to have an open line of communication to make sure that everything is submitted appropriately according to payer policies. Billing staff need to communicate any significant changes to the physicians/providers as these changes occur. Ignorance of payer policy is not considered an appropriate excuse when a payer investigates a claim and potential recoupment of moneys paid.
3. Diagnostic studies: Medical necessity/indication for the testing must be documented in order to submit charges for diagnostic studies. The terms “rule out” and “suspect” don’t completely give coders the reason why a physician suspects the patient might have a condition. Usually, abnormal lab tests, signs, and symptoms will often warrant the need for further investigation, and these are the most crucial indications for testing. Not only is this important for diagnostic studies but also for procedures. Make sure that the interpretation of the test results is clear along with a plan/recommendation(s).
4. Diagnosis codes: Assignment of codes per the International Classification of Diseases, 10th revision, Clinical Modification (ICD-10-CM) is the next and most important step after a visit, diagnostic study, and/or procedure. These codes support medical necessity for the services provided, and specificity of the diagnosis code is vital to successful submission and payment of a claim. Signs and symptoms are valid code choices when ruling out a more significant disease/diagnosis because these support medical necessity for a work-up to determine etiology. Comorbidities that impact the provider’s decision making should also be added as additional diagnoses to support the higher level of decision making. Up to 12 diagnosis codes can be assigned to any type of service provided. This also applies to preauthorization of all services, such as lab tests, radiology studies, GI diagnostic studies, and procedures. If specific information is not in the documentation for your staff to access, payers will often deny certain lab and radiology studies, as well as some procedures. There are 71,932 ICD-10-CM codes to choose from, and it is often difficult to find the “specific” code when doing a search in the electronic health record and billing system. Education and training are essential during the orientation sessions prior to active employment, as well as any time the system is upgraded. The providers should be willing to work with the IT representative(s) in the practice to help make the information easier to access. In other words, what “buzz” words would they like included in the description of the ICD-10-CM code in the practice’s list of favorites? For example, Crohn’s disease and ulcerative colitis have over a hundred choices. The choices are based on the location of the disease and whether the disease is without or with complications. If you are going to choose to provide a higher level of E&M service for a patient with Crohn’s disease of the large intestine because of exacerbation of the disease with bleeding, then the appropriate diagnosis code would be one of the following:
- K50.10 Crohn’s disease of large intestine without complications.
- K50.111 Crohn’s disease of large intestine with rectal bleeding.
- K50.112 Crohn’s disease of large intestine with intestinal obstruction.
- K50.113 Crohn’s disease of large intestine with fistula.
- K50.114 Crohn’s disease of large intestine with abscess.
- K50.118 Crohn’s disease of large intestine with other complication.
- K50.119 Crohn’s disease of large intestine with unspecified complications.
Getting paid for your provided services requires attention to detail and communication with your entire staffing team, including all providers. Make sure that your team is educated on all current issues and services pertaining to gastroenterology practices. If there is ever a question when reviewing a procedure note or any service, ask the provider who performed that service. Often, there will have to be legal corrections to the note before services can be billed. Making sure that the claim you are submitting is “clean” is essential for prompt payment. There are multiple resources available through the AGA that will help guide you with coding and billing. There are webinars, training sessions, and onsite services available via http://agau.gastro.org/diweb/catalog that can be provided for all providers, coding and billing staff, administrators, and clinical staff. Everyone needs to take an active role.
Ms. Mueller is a health care consultant with more than 35 years of experience in health care, including ICU/CCU nursing, physician office administration, GI claims submission and adjudication, and seminar instruction. She is president and owner of AskMueller Consulting in Lenzburg, Ill., which provides consulting services for physicians nationwide. Ms. Mueller is a nationally known speaker and the author of many multispecialty medical and surgical coding workbooks. She has a great amount of experience in gastroenterology, surgical subspecialties, and pediatric subspecialties. Her presentations have had audiences with the American Gastroenterological Association (AGA), North American Society for Pediatric Gastroenterology and Nutrition (NASPGAN), Society of Gastroenterology Nurses and Associates, Digestive Disease Week, American Pediatric Surgical Associations, and Decision Health and the Coding Institute. Ms. Mueller has written coding columns for ASGE, NASPGHAN, and AGA. She is the coeditor of the ASGE Coding Primer and also answers the coding hotline for the ASGE.
Understanding the business side of medicine helps physicians run a successful practice. However, the business side of medicine is not part of the normal curriculum in training and fellowship programs. Physicians come out of training with the knowledge to treat patients but with little or no knowledge of how to get reimbursed for their services. Gastroenterologists provide both medical and surgical services.
Listed below are some of the basic principles for both documentation and reimbursement policies. All reimbursement is based upon Relative Value Units (RVUs) assigned to every service provided. The services are based upon three factors: physician work value, malpractice cost, and practice expense. Those factors added together and multiplied by a conversion factor assigned by the Centers for Medicare & Medicaid Services (CMS) creates the national physician fee schedule. Each Medicare carrier has localities, and there is another percentage that is multiplied based upon geographic location, which will finalize the approved amount for each service. Your Medicare carrier has the actual approved amounts available on their websites with an effective date of Jan. 1. Commercial payers most commonly base contracts on the Medicare Fee Schedule, but each practice and payer relationship is different. For a better understanding, please contact your practice manager for more specific information based on your payer contracts.
Medical necessity is the key to success. If medical necessity is not demonstrated, payers can deny a claim, deny authorization for a lab test and/or diagnostic study, or recoup previously paid claims. Medicare and commercial payers will often have local coverage determinations (LCDs) for procedures and testing that include indications and restrictions along with approved diagnosis codes. Listed below are the four primary services that GI providers perform and provide interpretation for:
1. Evaluation and management (E&M) services: There are three criteria that have to be met to support any initial visit with patients: the history obtained, the examination performed, and the development of the treatment plan. There are five levels of service for office visits and three levels for inpatient visits, respectively. The levels are chosen based on the decision-making element of the visit, provided the documentation requirements are met for the level chosen. This is often not an easy selection unless the providers are educated on the E&M criteria. Auditors often see that visits are chosen by “guessing” the level, which leads to choosing either a lower or higher level of service than what was actually provided. Some providers have been instructed that E&M services are not that important since procedures are the major source of revenue for the practice. However, GI practices are visit-driven practices, and the initial visits are often worth more RVUs than some procedures. The E&M visit is truly vital and often the backbone for the medical necessity of any additional procedures and diagnostic services required in order for successful treatment of the patient.
2. Endoscopy and procedural billing: Here, medical necessity must be documented in order to submit charges for what was done. Gastroenterologists will often use multiple techniques when treating different areas within the gastrointestinal tract. Documentation has to include the location of lesions/abnormalities, method of treatment/removal, and the reason(s)/indication(s) for those procedures. There may be different instruments used in the colon (for example, snare in the sigmoid colon or biopsy forceps in the transverse colon). These may be separately reported with an appropriate modifier to indicate that these services were performed to different lesions/abnormalities. However, in order to bill for each of the procedures, all of this has to be documented in the endoscopy report. The physician is responsible for accurate and specific documentation and bringing charges back to the billing staff for claim submission. For a successful practice, a team approach is vital. Physicians and coding staff need to have an open line of communication to make sure that everything is submitted appropriately according to payer policies. Billing staff need to communicate any significant changes to the physicians/providers as these changes occur. Ignorance of payer policy is not considered an appropriate excuse when a payer investigates a claim and potential recoupment of moneys paid.
3. Diagnostic studies: Medical necessity/indication for the testing must be documented in order to submit charges for diagnostic studies. The terms “rule out” and “suspect” don’t completely give coders the reason why a physician suspects the patient might have a condition. Usually, abnormal lab tests, signs, and symptoms will often warrant the need for further investigation, and these are the most crucial indications for testing. Not only is this important for diagnostic studies but also for procedures. Make sure that the interpretation of the test results is clear along with a plan/recommendation(s).
4. Diagnosis codes: Assignment of codes per the International Classification of Diseases, 10th revision, Clinical Modification (ICD-10-CM) is the next and most important step after a visit, diagnostic study, and/or procedure. These codes support medical necessity for the services provided, and specificity of the diagnosis code is vital to successful submission and payment of a claim. Signs and symptoms are valid code choices when ruling out a more significant disease/diagnosis because these support medical necessity for a work-up to determine etiology. Comorbidities that impact the provider’s decision making should also be added as additional diagnoses to support the higher level of decision making. Up to 12 diagnosis codes can be assigned to any type of service provided. This also applies to preauthorization of all services, such as lab tests, radiology studies, GI diagnostic studies, and procedures. If specific information is not in the documentation for your staff to access, payers will often deny certain lab and radiology studies, as well as some procedures. There are 71,932 ICD-10-CM codes to choose from, and it is often difficult to find the “specific” code when doing a search in the electronic health record and billing system. Education and training are essential during the orientation sessions prior to active employment, as well as any time the system is upgraded. The providers should be willing to work with the IT representative(s) in the practice to help make the information easier to access. In other words, what “buzz” words would they like included in the description of the ICD-10-CM code in the practice’s list of favorites? For example, Crohn’s disease and ulcerative colitis have over a hundred choices. The choices are based on the location of the disease and whether the disease is without or with complications. If you are going to choose to provide a higher level of E&M service for a patient with Crohn’s disease of the large intestine because of exacerbation of the disease with bleeding, then the appropriate diagnosis code would be one of the following:
- K50.10 Crohn’s disease of large intestine without complications.
- K50.111 Crohn’s disease of large intestine with rectal bleeding.
- K50.112 Crohn’s disease of large intestine with intestinal obstruction.
- K50.113 Crohn’s disease of large intestine with fistula.
- K50.114 Crohn’s disease of large intestine with abscess.
- K50.118 Crohn’s disease of large intestine with other complication.
- K50.119 Crohn’s disease of large intestine with unspecified complications.
Getting paid for your provided services requires attention to detail and communication with your entire staffing team, including all providers. Make sure that your team is educated on all current issues and services pertaining to gastroenterology practices. If there is ever a question when reviewing a procedure note or any service, ask the provider who performed that service. Often, there will have to be legal corrections to the note before services can be billed. Making sure that the claim you are submitting is “clean” is essential for prompt payment. There are multiple resources available through the AGA that will help guide you with coding and billing. There are webinars, training sessions, and onsite services available via http://agau.gastro.org/diweb/catalog that can be provided for all providers, coding and billing staff, administrators, and clinical staff. Everyone needs to take an active role.
Ms. Mueller is a health care consultant with more than 35 years of experience in health care, including ICU/CCU nursing, physician office administration, GI claims submission and adjudication, and seminar instruction. She is president and owner of AskMueller Consulting in Lenzburg, Ill., which provides consulting services for physicians nationwide. Ms. Mueller is a nationally known speaker and the author of many multispecialty medical and surgical coding workbooks. She has a great amount of experience in gastroenterology, surgical subspecialties, and pediatric subspecialties. Her presentations have had audiences with the American Gastroenterological Association (AGA), North American Society for Pediatric Gastroenterology and Nutrition (NASPGAN), Society of Gastroenterology Nurses and Associates, Digestive Disease Week, American Pediatric Surgical Associations, and Decision Health and the Coding Institute. Ms. Mueller has written coding columns for ASGE, NASPGHAN, and AGA. She is the coeditor of the ASGE Coding Primer and also answers the coding hotline for the ASGE.
Better late than later: Lessons learned from an investigator-led clinical trial
As a second-year gastroenterology fellow, I designed a prospective, double-blind randomized, controlled trial for vitamin D repletion in patients with Crohn’s disease at a referral inflammatory bowel disease (IBD) center. I had the support of a dedicated research team, several mentors, and a 2-year time frame in which to complete this study. Intellectually curious and academically eager, I labored over a grant application that I did not receive. Under generous financial support from my department, I forged on and opened the trial for enrollment at the start of my advanced IBD fellowship year. However, we experienced recruitment challenges that ultimately led to the study’s premature termination. Through this journey, I gained invaluable experience that will continue to serve me – and, I hope, the reader – as I progress in my career. Below are some important insights gleaned from this experience that may benefit others interested in clinical trial design.
Know your “why” (personally and clinically)
Asked to reflect on their career path, experts tend to recount their “being in the right place at the right time” and having “good mentors.” While luck and good mentorship are necessary, I propose that doing your homework is equally as important. Before ambling down the path of an investigator-led trial, I urge a hard pause to reflect on your “why.” The personal “why” of “getting into fellowship,” “advancement in the department,” or “learning more about the principles of research,” are all valid. But I suggest a deeper dive is in order. Successful clinical trials require resources, a substantial time commitment, lots of sweat and maybe a few tears. In the ideal setting, your trial experience will serve as the foundation for a compelling personal narrative and might help launch a productive clinical research career. With stakes that high, asking the tough questions is critical.
The clinical “why” is just as critical. We press our attendings on why they used this drug or that clip, so don’t be afraid to ask whether this is a space in which others have succeeded. Or conversely, why is there such a large gap in the literature? I remember emailing the world’s expert about my topic because the published data were so murky. He had more questions than answers which, in retrospect, should have raised red flags about the ability to design a sound study. It is equally important to determine if patients are vested in the research question. A successful clinical trial hinges on subject participation, often outside their clinic visit. Patients with complex chronic diseases spend a lot of time navigating the health care system. Participating in a clinical trial needs to be meaningful to them if you want your patients to fully engage. Thoughtfully answering these questions on the front end – for yourself and the study in question – will improve both your experience and the ultimate outcome exponentially.
Identify your village (and listen to them)
Designing a clinical trial truly takes a village, and you need to identify the villagers early. As trainees, the value of mentorship is frequently underscored. But the importance of, and the nuance involved in managing, collaborating, and support cannot be overstated. Meet with a biostatistician to ensure your sample size calculations are correct. Work closely with the research pharmacist to ensure the medication formulation is available; decide on the manner of distribution so as not to inconvenience subjects; create a budget with an experienced manager. Seek out research coordinators often for assistance in creating case report forms, learning appropriate documentation, and crafting responses to Institutional Review Board concerns. Ask clinic personnel about arranging consent or follow-up visits around subjects’ clinic appointments. Present a draft of the protocol to your colleagues, as you will ultimately need them on board to recruit patients. And most importantly, listen to them ... all of them. Get your biases in check and write down all constructive criticism. Thoughtfully address each concern encountered to your satisfaction (and your mentor’s) and present to your village again. Rinse and repeat. Throw nothing under the rug, because if you do, it will eventually rear its head while you are in the throes of the study.
In retrospect, there were concerns raised by faculty and grant reviewers that I did not adequately address. First was the feasibility of screening, recruiting, and enrolling 80 patients during a busy clinical fellowship. While I took this criticism as a reflection of my personal commitment to the project, it was actually a call to consider the impact on clinical (and familial) responsibilities. But as I started enrolling patients, I realized there were logistic issues implied in this suggestion. I could not recruit subjects in Clinic A if I was assigned to see patients at the same time in Clinic B. Patients were not likely to come back another day for study-related discussions. Second, I designed eligibility criteria to make the data as clean as possible. Limiting the study to subjects with Crohn’s disease, in clinical and biochemical remission, without complications of their disease, who also have vitamin deficiency, may be an unrealistic recipe to recruit 80 people in a limited period of time. Finally, I designed laboratory follow-up schedules based on the pharmacokinetics of the drug alone, failing to consider the clinical milieu from which study subjects were recruited. Neglecting the fact that many patients obtain labs with their infusions, my study increased lab draw burden, heaped more patient reminders onto my plate – and more concerning – decreased overall study compliance. In short, trial design cannot be done in a vacuum or by just poring over published data. There are logistical and patient-related considerations that require early input from physicians and clinical staff in order for all the moving parts of a clinical trial to successfully work in harmony.
Create a timetable and follow it
Make a recruitment timetable very early on in the enrollment period. Set up biweekly meetings with mentors to discuss enrollment numbers and reflect on any unforeseen challenges. And be sure to celebrate the wins as well – not matter how small. In our study, falling behind on recruitment goals forced me to amend the stringent eligibility criteria and add additional manpower to help with reminders for laboratory follow-up and patient screening. These pivots caused study delays and cost resources. Ultimately, having a timetable forced me to take pause when it became clear I could not finish the study in the allotted time.
Know when to fold ’em
Knowing when to close a study is far easier said than done. The sunk cost fallacy says it is much harder to abandon a project after investing so many resources into it. For us, it was the recruitment timetable that gave us pause. Finishing trial accrual by the end of my advanced fellowship year was wholly unfeasible. When it became clear that nobody in the department could see it through to completion, I was propelled to terminate the study. If there is concern about termination, I suggest sending the protocol, recruitment numbers, and timeline to an outside colleague for a second, unbiased opinion. Review the already compiled data for any notable findings worthy of a smaller publication. It is said, we often learn more from our failures than our successes. The experience described herein – largely in part to my mentors, collaborators, and the patients who put their faith in me – translates to a lasting, invaluable win.
Mentor’s note
Clinical research is hard. Many trainees meet with me to “get involved” in clinical research, and the challenge as a mentor is to identify a project appropriate to the level of training and provide the infrastructure and resources to facilitate success for the motivated trainee. Trainees have various goals of their involvement in research – to foster a relationship in the hopes of receiving a strong letter of support, to facilitate getting into a competitive training program, and/or to publish. My goal as a mentor is to help my trainees reach their goals, but as a clinical researcher, I look for the trainee’s desire to engage with and learn the research process, with the ancillary potential for a letter, for acceptance to a program, or for publication.
This particular study, a randomized, controlled trial of vitamin D in patients with Crohn’s disease, involved an enormous undertaking by a very motivated trainee who took the project from its inception; to putting a thoughtful grant proposal together; to developing a full clinical trial protocol with its ancillary regulatory documents; and obtaining institutional review board approval, statistician input, pharmacy support, and buy-in from faculty and ancillary staff stakeholders. The study ultimately failed because of low enrollment – patients did not want to participate (for reasons elucidated above) – not because of poor design or execution of the myriad components of a prospective clinical trial. Low enrollment has led to the failure of many otherwise excellent studies, including several in our field of IBD.1,2 As a mentor, it is rational to accept blame for the failure of a trainee project; how could I have better foreseen the outcome of this study? Could this have been prevented with more support, more oversight, or more “micromanagement,” to the potential detriment of fostering independence?
Ultimately, the value of clinical research to trainees is multifaceted. If the goal was a first-author publication with high clinical impact, this trial failed. But if the goal was to learn about the clinical trial process, this study was a resounding success. Ultimately, it behooves trainees and their mentors to engage in early, upfront conversations about research. What are the goals? What does success look like? What if the trial fails? By shifting the focus from the success of the project to the success of the mentorship and educational process, even failed projects are resounding successes, upon which future careers can be further developed.
References
1. Kan S et al. When subjects violate the research covenant: Lessons learned from a failed clinical trial of fecal microbiota transplantation. Am J Gastroenterol 2016;111:1508-10.
2. Dassopoulos T et al. Randomised clinical trial: Individualised vs.weight-based dosing of azathioprine in Crohn’s disease. Aliment Pharmacol Ther. 2014 Jan;39(2):163-75.
Dr. Cohen is an inflammatory bowel disease fellow, inflammatory bowel disease center, division of gastroenterology and hepatology, department of medicine, Cedars-Sinai Medical Center, Los Angeles. Dr. Melmed is director, inflammatory bowel disease clinical research and codirector, clinical inflammatory bowel disease, inflammatory bowel disease center, division of gastroenterology and hepatology, department of medicine, Cedars-Sinai Medical Center, Los Angeles.
As a second-year gastroenterology fellow, I designed a prospective, double-blind randomized, controlled trial for vitamin D repletion in patients with Crohn’s disease at a referral inflammatory bowel disease (IBD) center. I had the support of a dedicated research team, several mentors, and a 2-year time frame in which to complete this study. Intellectually curious and academically eager, I labored over a grant application that I did not receive. Under generous financial support from my department, I forged on and opened the trial for enrollment at the start of my advanced IBD fellowship year. However, we experienced recruitment challenges that ultimately led to the study’s premature termination. Through this journey, I gained invaluable experience that will continue to serve me – and, I hope, the reader – as I progress in my career. Below are some important insights gleaned from this experience that may benefit others interested in clinical trial design.
Know your “why” (personally and clinically)
Asked to reflect on their career path, experts tend to recount their “being in the right place at the right time” and having “good mentors.” While luck and good mentorship are necessary, I propose that doing your homework is equally as important. Before ambling down the path of an investigator-led trial, I urge a hard pause to reflect on your “why.” The personal “why” of “getting into fellowship,” “advancement in the department,” or “learning more about the principles of research,” are all valid. But I suggest a deeper dive is in order. Successful clinical trials require resources, a substantial time commitment, lots of sweat and maybe a few tears. In the ideal setting, your trial experience will serve as the foundation for a compelling personal narrative and might help launch a productive clinical research career. With stakes that high, asking the tough questions is critical.
The clinical “why” is just as critical. We press our attendings on why they used this drug or that clip, so don’t be afraid to ask whether this is a space in which others have succeeded. Or conversely, why is there such a large gap in the literature? I remember emailing the world’s expert about my topic because the published data were so murky. He had more questions than answers which, in retrospect, should have raised red flags about the ability to design a sound study. It is equally important to determine if patients are vested in the research question. A successful clinical trial hinges on subject participation, often outside their clinic visit. Patients with complex chronic diseases spend a lot of time navigating the health care system. Participating in a clinical trial needs to be meaningful to them if you want your patients to fully engage. Thoughtfully answering these questions on the front end – for yourself and the study in question – will improve both your experience and the ultimate outcome exponentially.
Identify your village (and listen to them)
Designing a clinical trial truly takes a village, and you need to identify the villagers early. As trainees, the value of mentorship is frequently underscored. But the importance of, and the nuance involved in managing, collaborating, and support cannot be overstated. Meet with a biostatistician to ensure your sample size calculations are correct. Work closely with the research pharmacist to ensure the medication formulation is available; decide on the manner of distribution so as not to inconvenience subjects; create a budget with an experienced manager. Seek out research coordinators often for assistance in creating case report forms, learning appropriate documentation, and crafting responses to Institutional Review Board concerns. Ask clinic personnel about arranging consent or follow-up visits around subjects’ clinic appointments. Present a draft of the protocol to your colleagues, as you will ultimately need them on board to recruit patients. And most importantly, listen to them ... all of them. Get your biases in check and write down all constructive criticism. Thoughtfully address each concern encountered to your satisfaction (and your mentor’s) and present to your village again. Rinse and repeat. Throw nothing under the rug, because if you do, it will eventually rear its head while you are in the throes of the study.
In retrospect, there were concerns raised by faculty and grant reviewers that I did not adequately address. First was the feasibility of screening, recruiting, and enrolling 80 patients during a busy clinical fellowship. While I took this criticism as a reflection of my personal commitment to the project, it was actually a call to consider the impact on clinical (and familial) responsibilities. But as I started enrolling patients, I realized there were logistic issues implied in this suggestion. I could not recruit subjects in Clinic A if I was assigned to see patients at the same time in Clinic B. Patients were not likely to come back another day for study-related discussions. Second, I designed eligibility criteria to make the data as clean as possible. Limiting the study to subjects with Crohn’s disease, in clinical and biochemical remission, without complications of their disease, who also have vitamin deficiency, may be an unrealistic recipe to recruit 80 people in a limited period of time. Finally, I designed laboratory follow-up schedules based on the pharmacokinetics of the drug alone, failing to consider the clinical milieu from which study subjects were recruited. Neglecting the fact that many patients obtain labs with their infusions, my study increased lab draw burden, heaped more patient reminders onto my plate – and more concerning – decreased overall study compliance. In short, trial design cannot be done in a vacuum or by just poring over published data. There are logistical and patient-related considerations that require early input from physicians and clinical staff in order for all the moving parts of a clinical trial to successfully work in harmony.
Create a timetable and follow it
Make a recruitment timetable very early on in the enrollment period. Set up biweekly meetings with mentors to discuss enrollment numbers and reflect on any unforeseen challenges. And be sure to celebrate the wins as well – not matter how small. In our study, falling behind on recruitment goals forced me to amend the stringent eligibility criteria and add additional manpower to help with reminders for laboratory follow-up and patient screening. These pivots caused study delays and cost resources. Ultimately, having a timetable forced me to take pause when it became clear I could not finish the study in the allotted time.
Know when to fold ’em
Knowing when to close a study is far easier said than done. The sunk cost fallacy says it is much harder to abandon a project after investing so many resources into it. For us, it was the recruitment timetable that gave us pause. Finishing trial accrual by the end of my advanced fellowship year was wholly unfeasible. When it became clear that nobody in the department could see it through to completion, I was propelled to terminate the study. If there is concern about termination, I suggest sending the protocol, recruitment numbers, and timeline to an outside colleague for a second, unbiased opinion. Review the already compiled data for any notable findings worthy of a smaller publication. It is said, we often learn more from our failures than our successes. The experience described herein – largely in part to my mentors, collaborators, and the patients who put their faith in me – translates to a lasting, invaluable win.
Mentor’s note
Clinical research is hard. Many trainees meet with me to “get involved” in clinical research, and the challenge as a mentor is to identify a project appropriate to the level of training and provide the infrastructure and resources to facilitate success for the motivated trainee. Trainees have various goals of their involvement in research – to foster a relationship in the hopes of receiving a strong letter of support, to facilitate getting into a competitive training program, and/or to publish. My goal as a mentor is to help my trainees reach their goals, but as a clinical researcher, I look for the trainee’s desire to engage with and learn the research process, with the ancillary potential for a letter, for acceptance to a program, or for publication.
This particular study, a randomized, controlled trial of vitamin D in patients with Crohn’s disease, involved an enormous undertaking by a very motivated trainee who took the project from its inception; to putting a thoughtful grant proposal together; to developing a full clinical trial protocol with its ancillary regulatory documents; and obtaining institutional review board approval, statistician input, pharmacy support, and buy-in from faculty and ancillary staff stakeholders. The study ultimately failed because of low enrollment – patients did not want to participate (for reasons elucidated above) – not because of poor design or execution of the myriad components of a prospective clinical trial. Low enrollment has led to the failure of many otherwise excellent studies, including several in our field of IBD.1,2 As a mentor, it is rational to accept blame for the failure of a trainee project; how could I have better foreseen the outcome of this study? Could this have been prevented with more support, more oversight, or more “micromanagement,” to the potential detriment of fostering independence?
Ultimately, the value of clinical research to trainees is multifaceted. If the goal was a first-author publication with high clinical impact, this trial failed. But if the goal was to learn about the clinical trial process, this study was a resounding success. Ultimately, it behooves trainees and their mentors to engage in early, upfront conversations about research. What are the goals? What does success look like? What if the trial fails? By shifting the focus from the success of the project to the success of the mentorship and educational process, even failed projects are resounding successes, upon which future careers can be further developed.
References
1. Kan S et al. When subjects violate the research covenant: Lessons learned from a failed clinical trial of fecal microbiota transplantation. Am J Gastroenterol 2016;111:1508-10.
2. Dassopoulos T et al. Randomised clinical trial: Individualised vs.weight-based dosing of azathioprine in Crohn’s disease. Aliment Pharmacol Ther. 2014 Jan;39(2):163-75.
Dr. Cohen is an inflammatory bowel disease fellow, inflammatory bowel disease center, division of gastroenterology and hepatology, department of medicine, Cedars-Sinai Medical Center, Los Angeles. Dr. Melmed is director, inflammatory bowel disease clinical research and codirector, clinical inflammatory bowel disease, inflammatory bowel disease center, division of gastroenterology and hepatology, department of medicine, Cedars-Sinai Medical Center, Los Angeles.
As a second-year gastroenterology fellow, I designed a prospective, double-blind randomized, controlled trial for vitamin D repletion in patients with Crohn’s disease at a referral inflammatory bowel disease (IBD) center. I had the support of a dedicated research team, several mentors, and a 2-year time frame in which to complete this study. Intellectually curious and academically eager, I labored over a grant application that I did not receive. Under generous financial support from my department, I forged on and opened the trial for enrollment at the start of my advanced IBD fellowship year. However, we experienced recruitment challenges that ultimately led to the study’s premature termination. Through this journey, I gained invaluable experience that will continue to serve me – and, I hope, the reader – as I progress in my career. Below are some important insights gleaned from this experience that may benefit others interested in clinical trial design.
Know your “why” (personally and clinically)
Asked to reflect on their career path, experts tend to recount their “being in the right place at the right time” and having “good mentors.” While luck and good mentorship are necessary, I propose that doing your homework is equally as important. Before ambling down the path of an investigator-led trial, I urge a hard pause to reflect on your “why.” The personal “why” of “getting into fellowship,” “advancement in the department,” or “learning more about the principles of research,” are all valid. But I suggest a deeper dive is in order. Successful clinical trials require resources, a substantial time commitment, lots of sweat and maybe a few tears. In the ideal setting, your trial experience will serve as the foundation for a compelling personal narrative and might help launch a productive clinical research career. With stakes that high, asking the tough questions is critical.
The clinical “why” is just as critical. We press our attendings on why they used this drug or that clip, so don’t be afraid to ask whether this is a space in which others have succeeded. Or conversely, why is there such a large gap in the literature? I remember emailing the world’s expert about my topic because the published data were so murky. He had more questions than answers which, in retrospect, should have raised red flags about the ability to design a sound study. It is equally important to determine if patients are vested in the research question. A successful clinical trial hinges on subject participation, often outside their clinic visit. Patients with complex chronic diseases spend a lot of time navigating the health care system. Participating in a clinical trial needs to be meaningful to them if you want your patients to fully engage. Thoughtfully answering these questions on the front end – for yourself and the study in question – will improve both your experience and the ultimate outcome exponentially.
Identify your village (and listen to them)
Designing a clinical trial truly takes a village, and you need to identify the villagers early. As trainees, the value of mentorship is frequently underscored. But the importance of, and the nuance involved in managing, collaborating, and support cannot be overstated. Meet with a biostatistician to ensure your sample size calculations are correct. Work closely with the research pharmacist to ensure the medication formulation is available; decide on the manner of distribution so as not to inconvenience subjects; create a budget with an experienced manager. Seek out research coordinators often for assistance in creating case report forms, learning appropriate documentation, and crafting responses to Institutional Review Board concerns. Ask clinic personnel about arranging consent or follow-up visits around subjects’ clinic appointments. Present a draft of the protocol to your colleagues, as you will ultimately need them on board to recruit patients. And most importantly, listen to them ... all of them. Get your biases in check and write down all constructive criticism. Thoughtfully address each concern encountered to your satisfaction (and your mentor’s) and present to your village again. Rinse and repeat. Throw nothing under the rug, because if you do, it will eventually rear its head while you are in the throes of the study.
In retrospect, there were concerns raised by faculty and grant reviewers that I did not adequately address. First was the feasibility of screening, recruiting, and enrolling 80 patients during a busy clinical fellowship. While I took this criticism as a reflection of my personal commitment to the project, it was actually a call to consider the impact on clinical (and familial) responsibilities. But as I started enrolling patients, I realized there were logistic issues implied in this suggestion. I could not recruit subjects in Clinic A if I was assigned to see patients at the same time in Clinic B. Patients were not likely to come back another day for study-related discussions. Second, I designed eligibility criteria to make the data as clean as possible. Limiting the study to subjects with Crohn’s disease, in clinical and biochemical remission, without complications of their disease, who also have vitamin deficiency, may be an unrealistic recipe to recruit 80 people in a limited period of time. Finally, I designed laboratory follow-up schedules based on the pharmacokinetics of the drug alone, failing to consider the clinical milieu from which study subjects were recruited. Neglecting the fact that many patients obtain labs with their infusions, my study increased lab draw burden, heaped more patient reminders onto my plate – and more concerning – decreased overall study compliance. In short, trial design cannot be done in a vacuum or by just poring over published data. There are logistical and patient-related considerations that require early input from physicians and clinical staff in order for all the moving parts of a clinical trial to successfully work in harmony.
Create a timetable and follow it
Make a recruitment timetable very early on in the enrollment period. Set up biweekly meetings with mentors to discuss enrollment numbers and reflect on any unforeseen challenges. And be sure to celebrate the wins as well – not matter how small. In our study, falling behind on recruitment goals forced me to amend the stringent eligibility criteria and add additional manpower to help with reminders for laboratory follow-up and patient screening. These pivots caused study delays and cost resources. Ultimately, having a timetable forced me to take pause when it became clear I could not finish the study in the allotted time.
Know when to fold ’em
Knowing when to close a study is far easier said than done. The sunk cost fallacy says it is much harder to abandon a project after investing so many resources into it. For us, it was the recruitment timetable that gave us pause. Finishing trial accrual by the end of my advanced fellowship year was wholly unfeasible. When it became clear that nobody in the department could see it through to completion, I was propelled to terminate the study. If there is concern about termination, I suggest sending the protocol, recruitment numbers, and timeline to an outside colleague for a second, unbiased opinion. Review the already compiled data for any notable findings worthy of a smaller publication. It is said, we often learn more from our failures than our successes. The experience described herein – largely in part to my mentors, collaborators, and the patients who put their faith in me – translates to a lasting, invaluable win.
Mentor’s note
Clinical research is hard. Many trainees meet with me to “get involved” in clinical research, and the challenge as a mentor is to identify a project appropriate to the level of training and provide the infrastructure and resources to facilitate success for the motivated trainee. Trainees have various goals of their involvement in research – to foster a relationship in the hopes of receiving a strong letter of support, to facilitate getting into a competitive training program, and/or to publish. My goal as a mentor is to help my trainees reach their goals, but as a clinical researcher, I look for the trainee’s desire to engage with and learn the research process, with the ancillary potential for a letter, for acceptance to a program, or for publication.
This particular study, a randomized, controlled trial of vitamin D in patients with Crohn’s disease, involved an enormous undertaking by a very motivated trainee who took the project from its inception; to putting a thoughtful grant proposal together; to developing a full clinical trial protocol with its ancillary regulatory documents; and obtaining institutional review board approval, statistician input, pharmacy support, and buy-in from faculty and ancillary staff stakeholders. The study ultimately failed because of low enrollment – patients did not want to participate (for reasons elucidated above) – not because of poor design or execution of the myriad components of a prospective clinical trial. Low enrollment has led to the failure of many otherwise excellent studies, including several in our field of IBD.1,2 As a mentor, it is rational to accept blame for the failure of a trainee project; how could I have better foreseen the outcome of this study? Could this have been prevented with more support, more oversight, or more “micromanagement,” to the potential detriment of fostering independence?
Ultimately, the value of clinical research to trainees is multifaceted. If the goal was a first-author publication with high clinical impact, this trial failed. But if the goal was to learn about the clinical trial process, this study was a resounding success. Ultimately, it behooves trainees and their mentors to engage in early, upfront conversations about research. What are the goals? What does success look like? What if the trial fails? By shifting the focus from the success of the project to the success of the mentorship and educational process, even failed projects are resounding successes, upon which future careers can be further developed.
References
1. Kan S et al. When subjects violate the research covenant: Lessons learned from a failed clinical trial of fecal microbiota transplantation. Am J Gastroenterol 2016;111:1508-10.
2. Dassopoulos T et al. Randomised clinical trial: Individualised vs.weight-based dosing of azathioprine in Crohn’s disease. Aliment Pharmacol Ther. 2014 Jan;39(2):163-75.
Dr. Cohen is an inflammatory bowel disease fellow, inflammatory bowel disease center, division of gastroenterology and hepatology, department of medicine, Cedars-Sinai Medical Center, Los Angeles. Dr. Melmed is director, inflammatory bowel disease clinical research and codirector, clinical inflammatory bowel disease, inflammatory bowel disease center, division of gastroenterology and hepatology, department of medicine, Cedars-Sinai Medical Center, Los Angeles.
A career in industry: Is it right for me?
As gastroenterology fellows ponder their futures, one career path is often overlooked. Working in the pharmaceutical or biotechnology industry is a path that is not often at the top of career option lists. It is a rare occurrence for fellows to transition immediately into an industry position as opposed to a clinical or academic post. Initial clinical experience, caring for patients, and gaining experience with health economic challenges in today’s complex environment are considered invaluable assets for job applicants seeking industry positions. A minimum of 3-5 years of real-world clinical care experience will greatly enhance applicants’ marketability as “clinical experts” who can provide meaningful value to industry employers.
What exactly does “industry” mean? Traditionally it includes pharmaceutical and/or biotechnology (discovery, development, manufacture, sales, and marketing of small or large molecules), contract research organizations (CROs), and medical device companies. The variety in terms of size, scope, and reach of these companies is truly staggering and includes: entrepreneurial small startups (fewer than 20 employees, one location), midsize companies (more than 200 employees), and global multinational worldwide behemoths (“big pharma” with more than 50,000 employees and numerous facilities with diverse geographic locations). There are certain geographic regions of the United States where many companies’ headquarters are concentrated. At present (although this certainly can change over time), Cambridge, Mass.; New Jersey; Philadelphia; Raleigh-Durham, N.C.; and the San Francisco Bay Area are “hot areas.”
The breadth of “specialty” areas in industry for experienced clinicians is wide and includes: discovery, translational medicine, early- and late-stage clinical development, medical affairs, patient safety, epidemiology, and commercial development. For those interested in transitioning into industry, it is ideal to have a preferred area in mind so that training and education while in fellowship and clinical practice can be directed to that topic.
Discovery and translational medicine
These areas focus on preclinical development of small and large molecules from first concept until first-in-human studies and filing of an investigational new drug application (IND) with regulatory agencies. Translation of basic science concepts into potentially clinically useful “candidate” molecules requires a strong basic knowledge of science in addition to clinical experience. A passion for bridging novel concepts from “bench” to nonhuman studies is critical for success in this area.
Early-stage clinical development
Early-stage clinical development focuses on progressing discovery candidates to first-in-human studies (phase 1 in healthy volunteers) through phase 2 proof-of-concept studies (PoC). PoC studies typically involve first proof in a clinical trial in the target population that the drug under development may provide clinical benefit. These studies typically include 50-200 subjects with tight inclusion and exclusion controls. Intellectual rigor and scientific curiosity, as well as a passion for protecting patient safety, are essential for success as an early-stage drug developer.
Late-stage clinical development
Late-stage clinical development involves designing, conducting, and executing very large clinical studies (typically with hundreds to thousands of patients) that will provide the necessary rigorous pivotal clinical data supporting new drug marketing applications (NDAs). Relatively few drug candidates successfully make it to this stage of development and these studies are extremely expensive (sometimes hundreds of millions of dollars). This stage of development requires close collaboration with numerous company functions including regulatory, biostatistics, patient safety, clinical pharmacology, clinical operations, and manufacturing, as well as commercial colleagues. In addition to strong clinical expertise, this stage of drug development requires excellent communication, with leadership skills and attention to detail as well. Successfully shepherding a drug candidate through to Food and Drug Administration or other regulatory agency approval is an extremely satisfying experience, which can lead to meaningful differences for patients.
Medical affairs
This is a very important and challenging specialty area that, at its core, demands value demonstration of a medicine and communication to key stakeholders such as patients, physicians, and payers. This objective has become increasingly challenging over the past decade while evolving from a qualitative specialty to a rigorous quantitative one. Scientific and commercial success depends on efficient design, execution, and dissemination of results for real-world evidence and postapproval studies. Ideally, these data will demonstrate the medicine’s benefit-risk profile and how it fits into treatment algorithms. Communication requires leadership of physician and payer advisory boards, as well as publication planning. Close collaboration with marketing teams to advise on ethical and scientifically accurate promotional activities is another key component.
Patient safety
As the name implies, patient safety focuses on evaluating signals both from clinical trials and from literature that can accurately map out risks to patients that can arise from taking these medications. This is a critical function for proper and ethical prescription and use of medicine in today’s society. In addition to signal recognition and consultation with clinical development teams, collaboration with regulatory agencies is an important component.
Epidemiology
Epidemiologists with clinical expertise have become an increasingly important need for pharmaceutical and biotech companies over the past decade – specifically, for the design of real-world studies that demonstrate benefit-risk profiles for medicines in real-world use. These data are in demand for both private and governmental payers, as well as for regulatory agencies who are interested in evolving postapproval safety data. Successful epidemiologists often have acquired MPH degrees and expertise in study design and biostatistics.
Commercial development
Those with more financial or business acumen and clinical experience sometimes staff commercial careers. Typically commercial leads also have an MBA degree and are responsible for assessing commercial markets and forecasting and executing a path to commercial viability. Ultimately this career path can lead to a CEO position.
A career in industry is a perfect fit for some, but not so much for others. Table 1 outlines some pros and cons. Commercial factors do come into play with regard to corporate objectives and areas of focus. The top pharmaceutical product therapeutic categories, according to the number of drugs under development in 2017, were cancer, vaccines, diabetes, ophthalmology, gene therapy, anti-inflammatory, and antivirals and immunosuppressants; inflammatory bowel disease was 15th. Therapeutic research and development areas in gastroenterology that are relatively in demand in 2019 include IBD, irritable bowel syndrome, and nonalcoholic steatohepatitis. The high demand areas seem to change with the science and also payers’ willingness to reimburse.
Is industry a good career choice for you? Consider the following factors:
- Travel capabilities.
- Small biotech versus big pharma versus CRO.
- Capability to function in a team environment.
- Communication skills, resilience, self-awareness.
- Therapeutic area and category.
- Early stage versus late stage versus translational versus medical affairs.
- Additional education: MBA, MPH, PhD.
- Geography.
The pharmaceutical industry evolves and undergoes transformation extremely quickly in response to changes in the external environment. If you are considering a current or future career in industry, staying informed about changes in the delivery of health care and health economics is important. There is an ongoing need in industry for trainees and experienced gastroenterologists who can deploy their clinical expertise in development and communication of new medicines and devices that will make a positive difference in patients’ lives.
As gastroenterology fellows ponder their futures, one career path is often overlooked. Working in the pharmaceutical or biotechnology industry is a path that is not often at the top of career option lists. It is a rare occurrence for fellows to transition immediately into an industry position as opposed to a clinical or academic post. Initial clinical experience, caring for patients, and gaining experience with health economic challenges in today’s complex environment are considered invaluable assets for job applicants seeking industry positions. A minimum of 3-5 years of real-world clinical care experience will greatly enhance applicants’ marketability as “clinical experts” who can provide meaningful value to industry employers.
What exactly does “industry” mean? Traditionally it includes pharmaceutical and/or biotechnology (discovery, development, manufacture, sales, and marketing of small or large molecules), contract research organizations (CROs), and medical device companies. The variety in terms of size, scope, and reach of these companies is truly staggering and includes: entrepreneurial small startups (fewer than 20 employees, one location), midsize companies (more than 200 employees), and global multinational worldwide behemoths (“big pharma” with more than 50,000 employees and numerous facilities with diverse geographic locations). There are certain geographic regions of the United States where many companies’ headquarters are concentrated. At present (although this certainly can change over time), Cambridge, Mass.; New Jersey; Philadelphia; Raleigh-Durham, N.C.; and the San Francisco Bay Area are “hot areas.”
The breadth of “specialty” areas in industry for experienced clinicians is wide and includes: discovery, translational medicine, early- and late-stage clinical development, medical affairs, patient safety, epidemiology, and commercial development. For those interested in transitioning into industry, it is ideal to have a preferred area in mind so that training and education while in fellowship and clinical practice can be directed to that topic.
Discovery and translational medicine
These areas focus on preclinical development of small and large molecules from first concept until first-in-human studies and filing of an investigational new drug application (IND) with regulatory agencies. Translation of basic science concepts into potentially clinically useful “candidate” molecules requires a strong basic knowledge of science in addition to clinical experience. A passion for bridging novel concepts from “bench” to nonhuman studies is critical for success in this area.
Early-stage clinical development
Early-stage clinical development focuses on progressing discovery candidates to first-in-human studies (phase 1 in healthy volunteers) through phase 2 proof-of-concept studies (PoC). PoC studies typically involve first proof in a clinical trial in the target population that the drug under development may provide clinical benefit. These studies typically include 50-200 subjects with tight inclusion and exclusion controls. Intellectual rigor and scientific curiosity, as well as a passion for protecting patient safety, are essential for success as an early-stage drug developer.
Late-stage clinical development
Late-stage clinical development involves designing, conducting, and executing very large clinical studies (typically with hundreds to thousands of patients) that will provide the necessary rigorous pivotal clinical data supporting new drug marketing applications (NDAs). Relatively few drug candidates successfully make it to this stage of development and these studies are extremely expensive (sometimes hundreds of millions of dollars). This stage of development requires close collaboration with numerous company functions including regulatory, biostatistics, patient safety, clinical pharmacology, clinical operations, and manufacturing, as well as commercial colleagues. In addition to strong clinical expertise, this stage of drug development requires excellent communication, with leadership skills and attention to detail as well. Successfully shepherding a drug candidate through to Food and Drug Administration or other regulatory agency approval is an extremely satisfying experience, which can lead to meaningful differences for patients.
Medical affairs
This is a very important and challenging specialty area that, at its core, demands value demonstration of a medicine and communication to key stakeholders such as patients, physicians, and payers. This objective has become increasingly challenging over the past decade while evolving from a qualitative specialty to a rigorous quantitative one. Scientific and commercial success depends on efficient design, execution, and dissemination of results for real-world evidence and postapproval studies. Ideally, these data will demonstrate the medicine’s benefit-risk profile and how it fits into treatment algorithms. Communication requires leadership of physician and payer advisory boards, as well as publication planning. Close collaboration with marketing teams to advise on ethical and scientifically accurate promotional activities is another key component.
Patient safety
As the name implies, patient safety focuses on evaluating signals both from clinical trials and from literature that can accurately map out risks to patients that can arise from taking these medications. This is a critical function for proper and ethical prescription and use of medicine in today’s society. In addition to signal recognition and consultation with clinical development teams, collaboration with regulatory agencies is an important component.
Epidemiology
Epidemiologists with clinical expertise have become an increasingly important need for pharmaceutical and biotech companies over the past decade – specifically, for the design of real-world studies that demonstrate benefit-risk profiles for medicines in real-world use. These data are in demand for both private and governmental payers, as well as for regulatory agencies who are interested in evolving postapproval safety data. Successful epidemiologists often have acquired MPH degrees and expertise in study design and biostatistics.
Commercial development
Those with more financial or business acumen and clinical experience sometimes staff commercial careers. Typically commercial leads also have an MBA degree and are responsible for assessing commercial markets and forecasting and executing a path to commercial viability. Ultimately this career path can lead to a CEO position.

A career in industry is a perfect fit for some, but not so much for others. Table 1 outlines some pros and cons. Commercial factors do come into play with regard to corporate objectives and areas of focus. The top pharmaceutical product therapeutic categories, according to the number of drugs under development in 2017, were cancer, vaccines, diabetes, ophthalmology, gene therapy, anti-inflammatory, and antivirals and immunosuppressants; inflammatory bowel disease was 15th. Therapeutic research and development areas in gastroenterology that are relatively in demand in 2019 include IBD, irritable bowel syndrome, and nonalcoholic steatohepatitis. The high demand areas seem to change with the science and also payers’ willingness to reimburse.
Is industry a good career choice for you? Consider the following factors:
- Travel capabilities.
- Small biotech versus big pharma versus CRO.
- Capability to function in a team environment.
- Communication skills, resilience, self-awareness.
- Therapeutic area and category.
- Early stage versus late stage versus translational versus medical affairs.
- Additional education: MBA, MPH, PhD.
- Geography.
The pharmaceutical industry evolves and undergoes transformation extremely quickly in response to changes in the external environment. If you are considering a current or future career in industry, staying informed about changes in the delivery of health care and health economics is important. There is an ongoing need in industry for trainees and experienced gastroenterologists who can deploy their clinical expertise in development and communication of new medicines and devices that will make a positive difference in patients’ lives.
As gastroenterology fellows ponder their futures, one career path is often overlooked. Working in the pharmaceutical or biotechnology industry is a path that is not often at the top of career option lists. It is a rare occurrence for fellows to transition immediately into an industry position as opposed to a clinical or academic post. Initial clinical experience, caring for patients, and gaining experience with health economic challenges in today’s complex environment are considered invaluable assets for job applicants seeking industry positions. A minimum of 3-5 years of real-world clinical care experience will greatly enhance applicants’ marketability as “clinical experts” who can provide meaningful value to industry employers.
What exactly does “industry” mean? Traditionally it includes pharmaceutical and/or biotechnology (discovery, development, manufacture, sales, and marketing of small or large molecules), contract research organizations (CROs), and medical device companies. The variety in terms of size, scope, and reach of these companies is truly staggering and includes: entrepreneurial small startups (fewer than 20 employees, one location), midsize companies (more than 200 employees), and global multinational worldwide behemoths (“big pharma” with more than 50,000 employees and numerous facilities with diverse geographic locations). There are certain geographic regions of the United States where many companies’ headquarters are concentrated. At present (although this certainly can change over time), Cambridge, Mass.; New Jersey; Philadelphia; Raleigh-Durham, N.C.; and the San Francisco Bay Area are “hot areas.”
The breadth of “specialty” areas in industry for experienced clinicians is wide and includes: discovery, translational medicine, early- and late-stage clinical development, medical affairs, patient safety, epidemiology, and commercial development. For those interested in transitioning into industry, it is ideal to have a preferred area in mind so that training and education while in fellowship and clinical practice can be directed to that topic.
Discovery and translational medicine
These areas focus on preclinical development of small and large molecules from first concept until first-in-human studies and filing of an investigational new drug application (IND) with regulatory agencies. Translation of basic science concepts into potentially clinically useful “candidate” molecules requires a strong basic knowledge of science in addition to clinical experience. A passion for bridging novel concepts from “bench” to nonhuman studies is critical for success in this area.
Early-stage clinical development
Early-stage clinical development focuses on progressing discovery candidates to first-in-human studies (phase 1 in healthy volunteers) through phase 2 proof-of-concept studies (PoC). PoC studies typically involve first proof in a clinical trial in the target population that the drug under development may provide clinical benefit. These studies typically include 50-200 subjects with tight inclusion and exclusion controls. Intellectual rigor and scientific curiosity, as well as a passion for protecting patient safety, are essential for success as an early-stage drug developer.
Late-stage clinical development
Late-stage clinical development involves designing, conducting, and executing very large clinical studies (typically with hundreds to thousands of patients) that will provide the necessary rigorous pivotal clinical data supporting new drug marketing applications (NDAs). Relatively few drug candidates successfully make it to this stage of development and these studies are extremely expensive (sometimes hundreds of millions of dollars). This stage of development requires close collaboration with numerous company functions including regulatory, biostatistics, patient safety, clinical pharmacology, clinical operations, and manufacturing, as well as commercial colleagues. In addition to strong clinical expertise, this stage of drug development requires excellent communication, with leadership skills and attention to detail as well. Successfully shepherding a drug candidate through to Food and Drug Administration or other regulatory agency approval is an extremely satisfying experience, which can lead to meaningful differences for patients.
Medical affairs
This is a very important and challenging specialty area that, at its core, demands value demonstration of a medicine and communication to key stakeholders such as patients, physicians, and payers. This objective has become increasingly challenging over the past decade while evolving from a qualitative specialty to a rigorous quantitative one. Scientific and commercial success depends on efficient design, execution, and dissemination of results for real-world evidence and postapproval studies. Ideally, these data will demonstrate the medicine’s benefit-risk profile and how it fits into treatment algorithms. Communication requires leadership of physician and payer advisory boards, as well as publication planning. Close collaboration with marketing teams to advise on ethical and scientifically accurate promotional activities is another key component.
Patient safety
As the name implies, patient safety focuses on evaluating signals both from clinical trials and from literature that can accurately map out risks to patients that can arise from taking these medications. This is a critical function for proper and ethical prescription and use of medicine in today’s society. In addition to signal recognition and consultation with clinical development teams, collaboration with regulatory agencies is an important component.
Epidemiology
Epidemiologists with clinical expertise have become an increasingly important need for pharmaceutical and biotech companies over the past decade – specifically, for the design of real-world studies that demonstrate benefit-risk profiles for medicines in real-world use. These data are in demand for both private and governmental payers, as well as for regulatory agencies who are interested in evolving postapproval safety data. Successful epidemiologists often have acquired MPH degrees and expertise in study design and biostatistics.
Commercial development
Those with more financial or business acumen and clinical experience sometimes staff commercial careers. Typically commercial leads also have an MBA degree and are responsible for assessing commercial markets and forecasting and executing a path to commercial viability. Ultimately this career path can lead to a CEO position.

A career in industry is a perfect fit for some, but not so much for others. Table 1 outlines some pros and cons. Commercial factors do come into play with regard to corporate objectives and areas of focus. The top pharmaceutical product therapeutic categories, according to the number of drugs under development in 2017, were cancer, vaccines, diabetes, ophthalmology, gene therapy, anti-inflammatory, and antivirals and immunosuppressants; inflammatory bowel disease was 15th. Therapeutic research and development areas in gastroenterology that are relatively in demand in 2019 include IBD, irritable bowel syndrome, and nonalcoholic steatohepatitis. The high demand areas seem to change with the science and also payers’ willingness to reimburse.
Is industry a good career choice for you? Consider the following factors:
- Travel capabilities.
- Small biotech versus big pharma versus CRO.
- Capability to function in a team environment.
- Communication skills, resilience, self-awareness.
- Therapeutic area and category.
- Early stage versus late stage versus translational versus medical affairs.
- Additional education: MBA, MPH, PhD.
- Geography.
The pharmaceutical industry evolves and undergoes transformation extremely quickly in response to changes in the external environment. If you are considering a current or future career in industry, staying informed about changes in the delivery of health care and health economics is important. There is an ongoing need in industry for trainees and experienced gastroenterologists who can deploy their clinical expertise in development and communication of new medicines and devices that will make a positive difference in patients’ lives.