User login
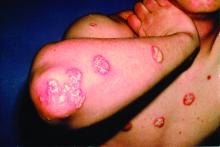
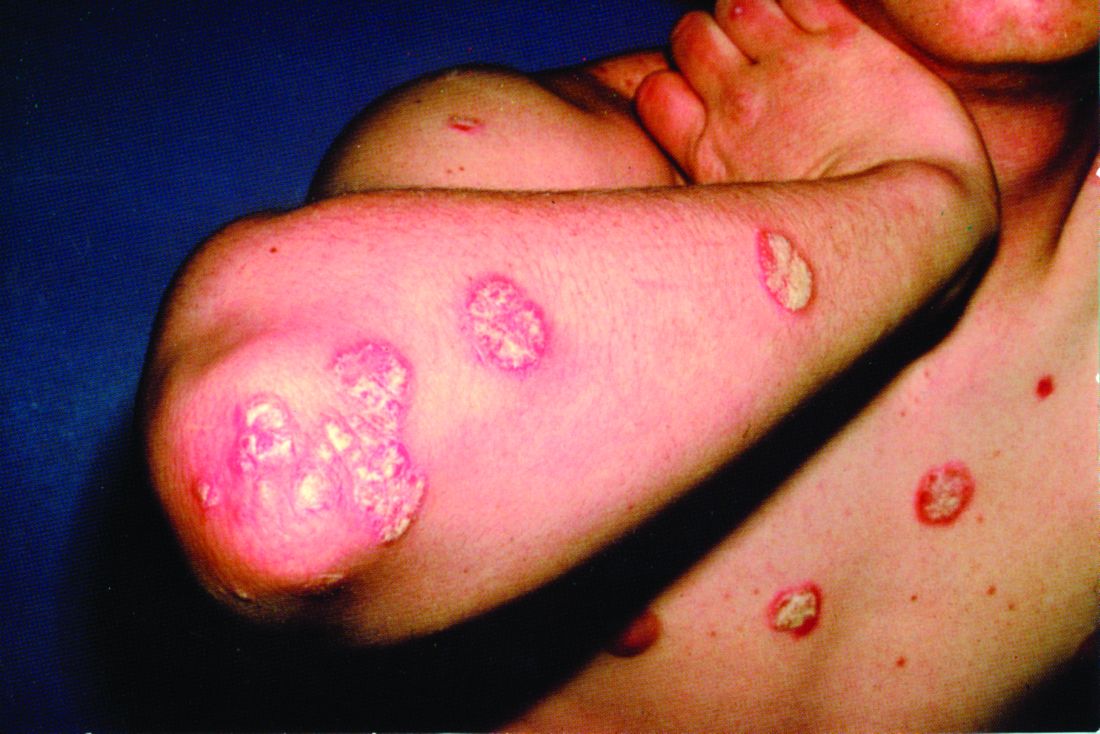
Dupilumab conjunctivitis does not always require an ophthalmologist referral
WAIKOLOA, HAWAII – Since its approval in 2017, dupilumab (Dupixent) has proven to be a solid addition to the atopic dermatitis (AD) armamentarium.

About 80% to 85% of patients treated with the biologic will achieve a 50% reduction in their Eczema Area and Severity Index score, and some will go on to a 90% reduction, according to Jonathan Silverberg, MD, PhD, of the department of dermatology, Northwestern University, Chicago.
But Dr. Silverberg has seen it in his own practice and said it can be hard to know whether or not to refer to ophthalmology. “We’re often left with this conundrum of ... ‘Is it a side effect of the medication, or is it just because they happen to have hay fever or keratoconjunctivitis or other ophthalmic comorbidities?’ And it’s not always easy to sort out.”
In an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation, he offered his advice on managing a patient who develops conjunctivitis during dupilumab treatment, including his treatment tips for when it is safe to handle in the dermatology clinic.
Dr. Silverberg, who was an investigator in the dupilumab phase 3 trials, said that, while dupilumab is the only systemic agent approved by the Food and Drug Administration for treating AD, and more are on the way for AD, there will always still be a role for traditional immunosuppressives. He explained why in the interview and why he favors methotrexate when old school options are in order.
This news organization and SDEF/Global Academy for Medical Education are owned by the same parent company.
WAIKOLOA, HAWAII – Since its approval in 2017, dupilumab (Dupixent) has proven to be a solid addition to the atopic dermatitis (AD) armamentarium.

About 80% to 85% of patients treated with the biologic will achieve a 50% reduction in their Eczema Area and Severity Index score, and some will go on to a 90% reduction, according to Jonathan Silverberg, MD, PhD, of the department of dermatology, Northwestern University, Chicago.
But Dr. Silverberg has seen it in his own practice and said it can be hard to know whether or not to refer to ophthalmology. “We’re often left with this conundrum of ... ‘Is it a side effect of the medication, or is it just because they happen to have hay fever or keratoconjunctivitis or other ophthalmic comorbidities?’ And it’s not always easy to sort out.”
In an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation, he offered his advice on managing a patient who develops conjunctivitis during dupilumab treatment, including his treatment tips for when it is safe to handle in the dermatology clinic.
Dr. Silverberg, who was an investigator in the dupilumab phase 3 trials, said that, while dupilumab is the only systemic agent approved by the Food and Drug Administration for treating AD, and more are on the way for AD, there will always still be a role for traditional immunosuppressives. He explained why in the interview and why he favors methotrexate when old school options are in order.
This news organization and SDEF/Global Academy for Medical Education are owned by the same parent company.
WAIKOLOA, HAWAII – Since its approval in 2017, dupilumab (Dupixent) has proven to be a solid addition to the atopic dermatitis (AD) armamentarium.

About 80% to 85% of patients treated with the biologic will achieve a 50% reduction in their Eczema Area and Severity Index score, and some will go on to a 90% reduction, according to Jonathan Silverberg, MD, PhD, of the department of dermatology, Northwestern University, Chicago.
But Dr. Silverberg has seen it in his own practice and said it can be hard to know whether or not to refer to ophthalmology. “We’re often left with this conundrum of ... ‘Is it a side effect of the medication, or is it just because they happen to have hay fever or keratoconjunctivitis or other ophthalmic comorbidities?’ And it’s not always easy to sort out.”
In an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation, he offered his advice on managing a patient who develops conjunctivitis during dupilumab treatment, including his treatment tips for when it is safe to handle in the dermatology clinic.
Dr. Silverberg, who was an investigator in the dupilumab phase 3 trials, said that, while dupilumab is the only systemic agent approved by the Food and Drug Administration for treating AD, and more are on the way for AD, there will always still be a role for traditional immunosuppressives. He explained why in the interview and why he favors methotrexate when old school options are in order.
This news organization and SDEF/Global Academy for Medical Education are owned by the same parent company.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
FDA clears first-of-its-kind phone app for insulin management
The Food and Drug Administration has granted 501(k) clearance to a phone app for managing insulin in patients with type 2 diabetes.
The app will enhance Hygieia’s d-Nav Insulin Guidance Service, which uses Cloud-based technology and a small group of health care professionals to support physicians and help patients with diabetes achieve better glycemic control by providing personalized insulin adjustments. The system has been shown, in a 90-day clinical study, to reduce both costs and levels of glycosylated hemoglobin. Patients can use the app to enter glucose-event data and receive a recommended insulin dose.
“Insulin therapy is critical to the health of more than 8 million people in the United States, but it is often ineffective, largely because it requires a continual, personalized adjustment that is not practical for physicians and not manageable for patients. The d-Nav service, including the user-friendly phone app, makes insulin therapy more effective, less time intensive, and less costly for everyone involved,” Eran Bashan, CEO of Hygieia, said in a press release.
This is the first insulin-mangement app that can titrate individualized doses for all insulin regimens and the first that can connect to any glucose meter that shares information with the cloud. It is available for both Android and iOS.
Find the full press release on the Hygieia website.
The Food and Drug Administration has granted 501(k) clearance to a phone app for managing insulin in patients with type 2 diabetes.
The app will enhance Hygieia’s d-Nav Insulin Guidance Service, which uses Cloud-based technology and a small group of health care professionals to support physicians and help patients with diabetes achieve better glycemic control by providing personalized insulin adjustments. The system has been shown, in a 90-day clinical study, to reduce both costs and levels of glycosylated hemoglobin. Patients can use the app to enter glucose-event data and receive a recommended insulin dose.
“Insulin therapy is critical to the health of more than 8 million people in the United States, but it is often ineffective, largely because it requires a continual, personalized adjustment that is not practical for physicians and not manageable for patients. The d-Nav service, including the user-friendly phone app, makes insulin therapy more effective, less time intensive, and less costly for everyone involved,” Eran Bashan, CEO of Hygieia, said in a press release.
This is the first insulin-mangement app that can titrate individualized doses for all insulin regimens and the first that can connect to any glucose meter that shares information with the cloud. It is available for both Android and iOS.
Find the full press release on the Hygieia website.
The Food and Drug Administration has granted 501(k) clearance to a phone app for managing insulin in patients with type 2 diabetes.
The app will enhance Hygieia’s d-Nav Insulin Guidance Service, which uses Cloud-based technology and a small group of health care professionals to support physicians and help patients with diabetes achieve better glycemic control by providing personalized insulin adjustments. The system has been shown, in a 90-day clinical study, to reduce both costs and levels of glycosylated hemoglobin. Patients can use the app to enter glucose-event data and receive a recommended insulin dose.
“Insulin therapy is critical to the health of more than 8 million people in the United States, but it is often ineffective, largely because it requires a continual, personalized adjustment that is not practical for physicians and not manageable for patients. The d-Nav service, including the user-friendly phone app, makes insulin therapy more effective, less time intensive, and less costly for everyone involved,” Eran Bashan, CEO of Hygieia, said in a press release.
This is the first insulin-mangement app that can titrate individualized doses for all insulin regimens and the first that can connect to any glucose meter that shares information with the cloud. It is available for both Android and iOS.
Find the full press release on the Hygieia website.
Haplo-HCT shows viability in DLBCL
For patients with diffuse large B-cell lymphoma (DLBCL) who need allogeneic hematopoietic cell transplantation (allo-HCT), a haploidentical family member could be a viable donor, according to a retrospective study of 1,438 patients.
When combined with nonmyeloablative/reduced intensity conditioning (NMC/RIC) and posttransplant cyclophosphamide (PTCy), patients treated with haploidentical HCT (haplo-HCT) had outcomes similar to those seen in patients with matched donors, reported Peter Dreger, MD, of the University of Heidelberg (Germany) and his colleagues.
“Using well-matched sibling donors (MSDs) or unrelated donors (MUDs), allo-HCT can result in sustained disease control in 30% to 45% of patients with DLBCL who have early disease recurrence after standard chemoimmunotherapy or have failed auto-HCT [autologous HCT],” the investigators wrote in Blood Advances. “However, the search for a well-matched unrelated donor could be time-consuming and unsuccessful in up to 50% of the patients in need.”
But the present findings suggest that haplo-HCT may one day improve these odds by providing a larger pool of potential donors.
The patients in the study were divided into four treatment groups: haplo-HCT (n = 132), MSD (n = 525), MUD with T-cell depletion (n = 403), and MUD without T-cell depletion (n = 378). For graft-versus-host disease (GVHD) prophylaxis, patients in the haplo-HCT group received PTCy, with or without a calcineurin inhibitor and mycophenolate mofetil, whereas all patients with matched donors received a calcineurin inhibitor. T-cell depletion was accomplished by in vivo antithymocyte globulin and alemtuzumab.
The primary end point was overall survival (OS). Secondary end points were progression-free survival (PFS), progression/relapse, and nonrelapse mortality (NRM).
After a median follow-up of 4.1 years, all groups had similar outcomes, without statistical differences in multivariable analysis.
In the haplo-HCT group, the 3-year OS rate was 46%, the NRM rate was 22%, the PFS rate was 38%, and the relapse/progression rate was 41%.
Of note, patients receiving haplo-HCT did have a lower cumulative incidence of chronic GVHD, at 15% after 1 year and 18% after 2 years. These rates were significantly lower than the other groups’ 1- and 2-year GVHD rates, which were as follows: MSD, 41% and 48%; MUD with T-cell depletion, 23% and 27%; and MUD without T-cell depletion, 48% and 57%.
The investigators noted that these disparities may actually be caused by the use of bone marrow grafts in the haplo-HCT group instead of peripheral blood grafts, which were used in most of the patients in the other groups.
Overall, the findings were encouraging, but the investigators cautioned that “additional studies are needed before haploidentical donors can be considered as equivalent to well-matched related or unrelated donors in patients with DLBCL.”
The study was funded by the Center for International Blood & Marrow Transplant Research (CIBMTR) and the European Society for Blood and Marrow Transplantation. CIBMTR is supported by grants from the U.S. government and the pharmaceutical industry. The authors reported having no competing financial interests.
SOURCE: Dreger P et al. Blood Adv. 2019 Feb 12;3(3):360-9.
For patients with diffuse large B-cell lymphoma (DLBCL) who need allogeneic hematopoietic cell transplantation (allo-HCT), a haploidentical family member could be a viable donor, according to a retrospective study of 1,438 patients.
When combined with nonmyeloablative/reduced intensity conditioning (NMC/RIC) and posttransplant cyclophosphamide (PTCy), patients treated with haploidentical HCT (haplo-HCT) had outcomes similar to those seen in patients with matched donors, reported Peter Dreger, MD, of the University of Heidelberg (Germany) and his colleagues.
“Using well-matched sibling donors (MSDs) or unrelated donors (MUDs), allo-HCT can result in sustained disease control in 30% to 45% of patients with DLBCL who have early disease recurrence after standard chemoimmunotherapy or have failed auto-HCT [autologous HCT],” the investigators wrote in Blood Advances. “However, the search for a well-matched unrelated donor could be time-consuming and unsuccessful in up to 50% of the patients in need.”
But the present findings suggest that haplo-HCT may one day improve these odds by providing a larger pool of potential donors.
The patients in the study were divided into four treatment groups: haplo-HCT (n = 132), MSD (n = 525), MUD with T-cell depletion (n = 403), and MUD without T-cell depletion (n = 378). For graft-versus-host disease (GVHD) prophylaxis, patients in the haplo-HCT group received PTCy, with or without a calcineurin inhibitor and mycophenolate mofetil, whereas all patients with matched donors received a calcineurin inhibitor. T-cell depletion was accomplished by in vivo antithymocyte globulin and alemtuzumab.
The primary end point was overall survival (OS). Secondary end points were progression-free survival (PFS), progression/relapse, and nonrelapse mortality (NRM).
After a median follow-up of 4.1 years, all groups had similar outcomes, without statistical differences in multivariable analysis.
In the haplo-HCT group, the 3-year OS rate was 46%, the NRM rate was 22%, the PFS rate was 38%, and the relapse/progression rate was 41%.
Of note, patients receiving haplo-HCT did have a lower cumulative incidence of chronic GVHD, at 15% after 1 year and 18% after 2 years. These rates were significantly lower than the other groups’ 1- and 2-year GVHD rates, which were as follows: MSD, 41% and 48%; MUD with T-cell depletion, 23% and 27%; and MUD without T-cell depletion, 48% and 57%.
The investigators noted that these disparities may actually be caused by the use of bone marrow grafts in the haplo-HCT group instead of peripheral blood grafts, which were used in most of the patients in the other groups.
Overall, the findings were encouraging, but the investigators cautioned that “additional studies are needed before haploidentical donors can be considered as equivalent to well-matched related or unrelated donors in patients with DLBCL.”
The study was funded by the Center for International Blood & Marrow Transplant Research (CIBMTR) and the European Society for Blood and Marrow Transplantation. CIBMTR is supported by grants from the U.S. government and the pharmaceutical industry. The authors reported having no competing financial interests.
SOURCE: Dreger P et al. Blood Adv. 2019 Feb 12;3(3):360-9.
For patients with diffuse large B-cell lymphoma (DLBCL) who need allogeneic hematopoietic cell transplantation (allo-HCT), a haploidentical family member could be a viable donor, according to a retrospective study of 1,438 patients.
When combined with nonmyeloablative/reduced intensity conditioning (NMC/RIC) and posttransplant cyclophosphamide (PTCy), patients treated with haploidentical HCT (haplo-HCT) had outcomes similar to those seen in patients with matched donors, reported Peter Dreger, MD, of the University of Heidelberg (Germany) and his colleagues.
“Using well-matched sibling donors (MSDs) or unrelated donors (MUDs), allo-HCT can result in sustained disease control in 30% to 45% of patients with DLBCL who have early disease recurrence after standard chemoimmunotherapy or have failed auto-HCT [autologous HCT],” the investigators wrote in Blood Advances. “However, the search for a well-matched unrelated donor could be time-consuming and unsuccessful in up to 50% of the patients in need.”
But the present findings suggest that haplo-HCT may one day improve these odds by providing a larger pool of potential donors.
The patients in the study were divided into four treatment groups: haplo-HCT (n = 132), MSD (n = 525), MUD with T-cell depletion (n = 403), and MUD without T-cell depletion (n = 378). For graft-versus-host disease (GVHD) prophylaxis, patients in the haplo-HCT group received PTCy, with or without a calcineurin inhibitor and mycophenolate mofetil, whereas all patients with matched donors received a calcineurin inhibitor. T-cell depletion was accomplished by in vivo antithymocyte globulin and alemtuzumab.
The primary end point was overall survival (OS). Secondary end points were progression-free survival (PFS), progression/relapse, and nonrelapse mortality (NRM).
After a median follow-up of 4.1 years, all groups had similar outcomes, without statistical differences in multivariable analysis.
In the haplo-HCT group, the 3-year OS rate was 46%, the NRM rate was 22%, the PFS rate was 38%, and the relapse/progression rate was 41%.
Of note, patients receiving haplo-HCT did have a lower cumulative incidence of chronic GVHD, at 15% after 1 year and 18% after 2 years. These rates were significantly lower than the other groups’ 1- and 2-year GVHD rates, which were as follows: MSD, 41% and 48%; MUD with T-cell depletion, 23% and 27%; and MUD without T-cell depletion, 48% and 57%.
The investigators noted that these disparities may actually be caused by the use of bone marrow grafts in the haplo-HCT group instead of peripheral blood grafts, which were used in most of the patients in the other groups.
Overall, the findings were encouraging, but the investigators cautioned that “additional studies are needed before haploidentical donors can be considered as equivalent to well-matched related or unrelated donors in patients with DLBCL.”
The study was funded by the Center for International Blood & Marrow Transplant Research (CIBMTR) and the European Society for Blood and Marrow Transplantation. CIBMTR is supported by grants from the U.S. government and the pharmaceutical industry. The authors reported having no competing financial interests.
SOURCE: Dreger P et al. Blood Adv. 2019 Feb 12;3(3):360-9.
FROM BLOOD ADVANCES
Stroke endovascular therapy: The more you do, the better you do
HONOLULU – The well-documented link between higher procedure volumes and better procedure efficacy also applies to endovascular thrombectomy for acute ischemic stroke.
in analysis of data from two different U.S. sources: the National Inpatient Sample (NIS) and the state of Florida, Sunil A. Sheth, MD, said at the International Stroke Conference sponsored by the American Heart Association.
The finding raises questions about how to best triage patients with an acute ischemic stroke, suggesting that, in at least some situations, patients might be better served being taken to a higher-volume center even if it’s not the closest, noted Dr. Sheth, The results also suggest that the findings in the trials proving the value of EST run primarily at large, tertiary care, referral centers might not be generalizable to all centers that start an endovascular program.
The study looked at data collected during 2006-2016 in Florida and during 2012-2016 in the NIS, and found that in both databases the rate of EST procedures performed showed steadily increasing use over time, with a sharp increase in the number of centers performing EST in 2015. Each of the two data sets also showed that better discharge outcomes occurred in patients treated at centers with the highest procedural volumes.
In the nationwide NIS data, for every 10 additional patients a center treated with EST annually, the incidence of a “good” hospital-discharge outcome (defined as either discharge home or to an acute rehabilitation hospital) rose by 30%, compared with lower-volume centers in a multivariate regression analysis, a statistically significant relationship, said Dr. Sheth, a neurologist at the University of Texas, Houston. This volume-outcome relationship held fairly constant through volumes up to about 50 EST cases annually. “The more the better,” he observed.
“The data suggest that EST outcomes are not always the same,” but right now most emergency medical service systems do not take EST case volume into account when deciding where to take an acute stroke patient, Dr. Sheth said in an interview. But he cautioned against an oversimplified focus on just EST case volume.
A link between volume and better outcomes “is easy to understand and not surprising. We see this relationship for a variety of procedures. The data suggest we need to consider procedure volumes. But volume is only part of makes for good outcomes; it’s not the only factor,” he stressed.
Dr. Sheth and his associates used data collected by the Florida Agency for Health Care Administration on 3,890 acute ischemic stroke patients treated with EST at 56 Florida hospitals and on 42,505 such patients in the NIS database treated at 2,260 U.S. hospitals. During the 11-year period for Florida data collection, the number of centers performing EST in the state rose steadily at an average rate of about four new centers per year. Although the number of EST procedures done also rose sharply, in general over time a higher percentage of patients underwent treatment at lower-volume centers. Similar patterns existed in the national data. The Florida data showed a statistically significant 10% improvement in good discharge outcomes for every 10 additional EST patients a center treated a year, consistent with the NIS data.
Concurrently with Dr. Sheth’s report, the results also appeared in an article published online (Stroke. 2019 Feb 6. doi: 10.1161/STROKEAHA.118.023967).
Dr. Sheth reported no disclosures.
SOURCE: Sheth SA et al. ISC 2019, Abstract 002.
The idea that when centers perform more of a procedure, such as endovascular thrombectomy for acute ischemic stroke, they more often do it better is intuitively plausible, which helps makes these findings believable. It’s also a relationship we’ve already seen for other types of endovascular therapies. What this study did not address were other factors beyond case volume also might also make important contributions to outcome, such as the speed of treatment delivery.
Volume thresholds for endovascular stroke programs will come eventually, but for the time being our focus should be on insuring wide access to endovascular treatment.
Bruce Ovbiagele, MD, a neurologist and chief of staff for the San Francisco Veteran Affairs Health Care System, made these comments in an interview. He reported no disclosures.
The idea that when centers perform more of a procedure, such as endovascular thrombectomy for acute ischemic stroke, they more often do it better is intuitively plausible, which helps makes these findings believable. It’s also a relationship we’ve already seen for other types of endovascular therapies. What this study did not address were other factors beyond case volume also might also make important contributions to outcome, such as the speed of treatment delivery.
Volume thresholds for endovascular stroke programs will come eventually, but for the time being our focus should be on insuring wide access to endovascular treatment.
Bruce Ovbiagele, MD, a neurologist and chief of staff for the San Francisco Veteran Affairs Health Care System, made these comments in an interview. He reported no disclosures.
The idea that when centers perform more of a procedure, such as endovascular thrombectomy for acute ischemic stroke, they more often do it better is intuitively plausible, which helps makes these findings believable. It’s also a relationship we’ve already seen for other types of endovascular therapies. What this study did not address were other factors beyond case volume also might also make important contributions to outcome, such as the speed of treatment delivery.
Volume thresholds for endovascular stroke programs will come eventually, but for the time being our focus should be on insuring wide access to endovascular treatment.
Bruce Ovbiagele, MD, a neurologist and chief of staff for the San Francisco Veteran Affairs Health Care System, made these comments in an interview. He reported no disclosures.
HONOLULU – The well-documented link between higher procedure volumes and better procedure efficacy also applies to endovascular thrombectomy for acute ischemic stroke.
in analysis of data from two different U.S. sources: the National Inpatient Sample (NIS) and the state of Florida, Sunil A. Sheth, MD, said at the International Stroke Conference sponsored by the American Heart Association.
The finding raises questions about how to best triage patients with an acute ischemic stroke, suggesting that, in at least some situations, patients might be better served being taken to a higher-volume center even if it’s not the closest, noted Dr. Sheth, The results also suggest that the findings in the trials proving the value of EST run primarily at large, tertiary care, referral centers might not be generalizable to all centers that start an endovascular program.
The study looked at data collected during 2006-2016 in Florida and during 2012-2016 in the NIS, and found that in both databases the rate of EST procedures performed showed steadily increasing use over time, with a sharp increase in the number of centers performing EST in 2015. Each of the two data sets also showed that better discharge outcomes occurred in patients treated at centers with the highest procedural volumes.
In the nationwide NIS data, for every 10 additional patients a center treated with EST annually, the incidence of a “good” hospital-discharge outcome (defined as either discharge home or to an acute rehabilitation hospital) rose by 30%, compared with lower-volume centers in a multivariate regression analysis, a statistically significant relationship, said Dr. Sheth, a neurologist at the University of Texas, Houston. This volume-outcome relationship held fairly constant through volumes up to about 50 EST cases annually. “The more the better,” he observed.
“The data suggest that EST outcomes are not always the same,” but right now most emergency medical service systems do not take EST case volume into account when deciding where to take an acute stroke patient, Dr. Sheth said in an interview. But he cautioned against an oversimplified focus on just EST case volume.
A link between volume and better outcomes “is easy to understand and not surprising. We see this relationship for a variety of procedures. The data suggest we need to consider procedure volumes. But volume is only part of makes for good outcomes; it’s not the only factor,” he stressed.
Dr. Sheth and his associates used data collected by the Florida Agency for Health Care Administration on 3,890 acute ischemic stroke patients treated with EST at 56 Florida hospitals and on 42,505 such patients in the NIS database treated at 2,260 U.S. hospitals. During the 11-year period for Florida data collection, the number of centers performing EST in the state rose steadily at an average rate of about four new centers per year. Although the number of EST procedures done also rose sharply, in general over time a higher percentage of patients underwent treatment at lower-volume centers. Similar patterns existed in the national data. The Florida data showed a statistically significant 10% improvement in good discharge outcomes for every 10 additional EST patients a center treated a year, consistent with the NIS data.
Concurrently with Dr. Sheth’s report, the results also appeared in an article published online (Stroke. 2019 Feb 6. doi: 10.1161/STROKEAHA.118.023967).
Dr. Sheth reported no disclosures.
SOURCE: Sheth SA et al. ISC 2019, Abstract 002.
HONOLULU – The well-documented link between higher procedure volumes and better procedure efficacy also applies to endovascular thrombectomy for acute ischemic stroke.
in analysis of data from two different U.S. sources: the National Inpatient Sample (NIS) and the state of Florida, Sunil A. Sheth, MD, said at the International Stroke Conference sponsored by the American Heart Association.
The finding raises questions about how to best triage patients with an acute ischemic stroke, suggesting that, in at least some situations, patients might be better served being taken to a higher-volume center even if it’s not the closest, noted Dr. Sheth, The results also suggest that the findings in the trials proving the value of EST run primarily at large, tertiary care, referral centers might not be generalizable to all centers that start an endovascular program.
The study looked at data collected during 2006-2016 in Florida and during 2012-2016 in the NIS, and found that in both databases the rate of EST procedures performed showed steadily increasing use over time, with a sharp increase in the number of centers performing EST in 2015. Each of the two data sets also showed that better discharge outcomes occurred in patients treated at centers with the highest procedural volumes.
In the nationwide NIS data, for every 10 additional patients a center treated with EST annually, the incidence of a “good” hospital-discharge outcome (defined as either discharge home or to an acute rehabilitation hospital) rose by 30%, compared with lower-volume centers in a multivariate regression analysis, a statistically significant relationship, said Dr. Sheth, a neurologist at the University of Texas, Houston. This volume-outcome relationship held fairly constant through volumes up to about 50 EST cases annually. “The more the better,” he observed.
“The data suggest that EST outcomes are not always the same,” but right now most emergency medical service systems do not take EST case volume into account when deciding where to take an acute stroke patient, Dr. Sheth said in an interview. But he cautioned against an oversimplified focus on just EST case volume.
A link between volume and better outcomes “is easy to understand and not surprising. We see this relationship for a variety of procedures. The data suggest we need to consider procedure volumes. But volume is only part of makes for good outcomes; it’s not the only factor,” he stressed.
Dr. Sheth and his associates used data collected by the Florida Agency for Health Care Administration on 3,890 acute ischemic stroke patients treated with EST at 56 Florida hospitals and on 42,505 such patients in the NIS database treated at 2,260 U.S. hospitals. During the 11-year period for Florida data collection, the number of centers performing EST in the state rose steadily at an average rate of about four new centers per year. Although the number of EST procedures done also rose sharply, in general over time a higher percentage of patients underwent treatment at lower-volume centers. Similar patterns existed in the national data. The Florida data showed a statistically significant 10% improvement in good discharge outcomes for every 10 additional EST patients a center treated a year, consistent with the NIS data.
Concurrently with Dr. Sheth’s report, the results also appeared in an article published online (Stroke. 2019 Feb 6. doi: 10.1161/STROKEAHA.118.023967).
Dr. Sheth reported no disclosures.
SOURCE: Sheth SA et al. ISC 2019, Abstract 002.
REPORTING FROM ISC 2019
HHS to target step therapy, Stark Law in 2019
WASHINGTON –
Speaking Feb. 12 at the American Medical Association’s National Advocacy Conference, Secretary Azar said the agency will be looking into ensuring that patients on medical plans who have found a working drug after going through a step-therapy protocol will not have to restart on a drug that has already failed for them if they switch insurance providers.
“I was very disturbed to hear that stable patients switching among insurance plans, like switching among Medicare Advantage plans, can often be required to start over again on a step therapy regimen,” he said.
“This is not just potentially injurious to their health, it’s also penny-wise and pound-foolish,” Secretary Azar continued. “We know that getting a patient on the right drug, at the right time, is one of the best investments we can make in their health, and we do not want to impede physicians from making that happen. We’re looking at how we can address that issue now.”
The other area Secretary Azar highlighted that the agency is working on is making changes to the Stark Law.
“The Stark Law was written with noble purposes in mind, but it was designed for a fee-for-service system, not the kind of system we are moving toward today,” he said. “We’ve heard from many, many stakeholders, including the AMA, about the need to update the enumerated exceptions in the Stark Law to include value-based approaches to care.”
He added that how care coordination interacts with the antikickback statutes and HIPAA are also going to be examined.
He used most of his speech to discuss recent regulatory actions around drug pricing and pushed for support for the Part B drug pricing model that the agency is preparing for a formal proposed rule, despite having received a critical reception from medical societies.
“If you have a small practice that uses infusions, and you don’t want to bear the risk of buy and bill, now you’re off the hook,” he said. “We’ll allow you to work with private vendors who can take the risk for buying the drugs in a way that isn’t possible today. But if you’re part of a much larger practice that’s able to drive a better deal than you could on your own, or want to band together with other practices to do the purchasing, then you can do that, too.”
He continued: “Next is the launch of the actual proposed rule, followed by the rule itself, which, I’ll remind you, is just a model.”
However, despite it being a model under test from the Center for Medicare & Medicaid Innovation, the advanced notice of proposed rule making that was issued in October 2018 suggested that participation in the so-called International Pricing Index model would be mandatory.
AGA is pleased about Secretary Azar’s commitment to ensuring Medicare beneficiaries will continue to have access to and coverage of medications that work for them. Patients should not be forced to switch to a therapy that they have already failed if they change insurance plans. Read more about AGA’s advocacy for similar federal legislation at http://ow.ly/2t3030nLbcB.
AGA, in conjunction with other physician specialty organizations, continues to advocate for changes in the Stark Law to allow physician practices to participate in advanced payment models in the Medicare program that will improve care coordination and patient outcomes.
WASHINGTON –
Speaking Feb. 12 at the American Medical Association’s National Advocacy Conference, Secretary Azar said the agency will be looking into ensuring that patients on medical plans who have found a working drug after going through a step-therapy protocol will not have to restart on a drug that has already failed for them if they switch insurance providers.
“I was very disturbed to hear that stable patients switching among insurance plans, like switching among Medicare Advantage plans, can often be required to start over again on a step therapy regimen,” he said.
“This is not just potentially injurious to their health, it’s also penny-wise and pound-foolish,” Secretary Azar continued. “We know that getting a patient on the right drug, at the right time, is one of the best investments we can make in their health, and we do not want to impede physicians from making that happen. We’re looking at how we can address that issue now.”
The other area Secretary Azar highlighted that the agency is working on is making changes to the Stark Law.
“The Stark Law was written with noble purposes in mind, but it was designed for a fee-for-service system, not the kind of system we are moving toward today,” he said. “We’ve heard from many, many stakeholders, including the AMA, about the need to update the enumerated exceptions in the Stark Law to include value-based approaches to care.”
He added that how care coordination interacts with the antikickback statutes and HIPAA are also going to be examined.
He used most of his speech to discuss recent regulatory actions around drug pricing and pushed for support for the Part B drug pricing model that the agency is preparing for a formal proposed rule, despite having received a critical reception from medical societies.
“If you have a small practice that uses infusions, and you don’t want to bear the risk of buy and bill, now you’re off the hook,” he said. “We’ll allow you to work with private vendors who can take the risk for buying the drugs in a way that isn’t possible today. But if you’re part of a much larger practice that’s able to drive a better deal than you could on your own, or want to band together with other practices to do the purchasing, then you can do that, too.”
He continued: “Next is the launch of the actual proposed rule, followed by the rule itself, which, I’ll remind you, is just a model.”
However, despite it being a model under test from the Center for Medicare & Medicaid Innovation, the advanced notice of proposed rule making that was issued in October 2018 suggested that participation in the so-called International Pricing Index model would be mandatory.
AGA is pleased about Secretary Azar’s commitment to ensuring Medicare beneficiaries will continue to have access to and coverage of medications that work for them. Patients should not be forced to switch to a therapy that they have already failed if they change insurance plans. Read more about AGA’s advocacy for similar federal legislation at http://ow.ly/2t3030nLbcB.
AGA, in conjunction with other physician specialty organizations, continues to advocate for changes in the Stark Law to allow physician practices to participate in advanced payment models in the Medicare program that will improve care coordination and patient outcomes.
WASHINGTON –
Speaking Feb. 12 at the American Medical Association’s National Advocacy Conference, Secretary Azar said the agency will be looking into ensuring that patients on medical plans who have found a working drug after going through a step-therapy protocol will not have to restart on a drug that has already failed for them if they switch insurance providers.
“I was very disturbed to hear that stable patients switching among insurance plans, like switching among Medicare Advantage plans, can often be required to start over again on a step therapy regimen,” he said.
“This is not just potentially injurious to their health, it’s also penny-wise and pound-foolish,” Secretary Azar continued. “We know that getting a patient on the right drug, at the right time, is one of the best investments we can make in their health, and we do not want to impede physicians from making that happen. We’re looking at how we can address that issue now.”
The other area Secretary Azar highlighted that the agency is working on is making changes to the Stark Law.
“The Stark Law was written with noble purposes in mind, but it was designed for a fee-for-service system, not the kind of system we are moving toward today,” he said. “We’ve heard from many, many stakeholders, including the AMA, about the need to update the enumerated exceptions in the Stark Law to include value-based approaches to care.”
He added that how care coordination interacts with the antikickback statutes and HIPAA are also going to be examined.
He used most of his speech to discuss recent regulatory actions around drug pricing and pushed for support for the Part B drug pricing model that the agency is preparing for a formal proposed rule, despite having received a critical reception from medical societies.
“If you have a small practice that uses infusions, and you don’t want to bear the risk of buy and bill, now you’re off the hook,” he said. “We’ll allow you to work with private vendors who can take the risk for buying the drugs in a way that isn’t possible today. But if you’re part of a much larger practice that’s able to drive a better deal than you could on your own, or want to band together with other practices to do the purchasing, then you can do that, too.”
He continued: “Next is the launch of the actual proposed rule, followed by the rule itself, which, I’ll remind you, is just a model.”
However, despite it being a model under test from the Center for Medicare & Medicaid Innovation, the advanced notice of proposed rule making that was issued in October 2018 suggested that participation in the so-called International Pricing Index model would be mandatory.
AGA is pleased about Secretary Azar’s commitment to ensuring Medicare beneficiaries will continue to have access to and coverage of medications that work for them. Patients should not be forced to switch to a therapy that they have already failed if they change insurance plans. Read more about AGA’s advocacy for similar federal legislation at http://ow.ly/2t3030nLbcB.
AGA, in conjunction with other physician specialty organizations, continues to advocate for changes in the Stark Law to allow physician practices to participate in advanced payment models in the Medicare program that will improve care coordination and patient outcomes.
First-line avelumab/axitinib for RCC benefits wide range of patients
SAN FRANCISCO – subgroup analyses of the JAVELIN Renal 101 trial have shown. Results were reported at the 2019 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Previous research had shown that avelumab (Bavencio), an immune checkpoint inhibitor targeting programmed death-ligand 1 (PD-L1), is active when used alone for advanced RCC, noted lead investigator Toni K. Choueiri, MD, director of the Lank Center for Genitourinary Oncology at the Dana-Farber Cancer Institute, Boston. And axitinib (Inlyta), a vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor, is approved for use in the second line. In an early-phase trial among patients being treated in the first line, a combination of the two drugs led to an impressive 58% objective response rate (ORR) and had a favorable safety profile (Lancet Oncol. 2018 Apr;19(4):451-60).
JAVELIN Renal 101 (NCT02684006), a phase 3 randomized, controlled trial, enrolled 886 patients with treatment-naive advanced RCC having a clear cell component regardless of their tumor’s PD-L1 status. They were randomized to the combination of avelumab/axitinib or to the VEGF tyrosine kinase inhibitor sunitinib (Sutent) alone.
Full trial results, published during the symposium in the New England Journal of Medicine (2019 Feb 16. doi: 10.1056/NEJMoa1816047), showed significant progression-free and overall survival benefits of avelumab/axitinib over sunitinib in the 63.2% of patients with PD-L1–positive tumors – the trial’s primary endpoints – as well as a progression-free survival benefit in the entire trial population.
In the subgroup analyses reported at the symposium, the combination reduced risk of progression or death by roughly 20%-50% across patients having different statuses in regard to International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk group, Memorial Sloan Kettering Cancer Center (MSKCC) risk group, and tumor PD-L1, as well as other characteristics. Findings were similar for ORR, with the combination roughly doubling to quadrupling the odds of response, irrespective of patient and disease characteristics.
“The progression-free survival and response rate benefit was observed in all patients, regardless of PD-L1 status, regardless of prognostic risk group. At this time, the study continues to follow up for overall survival,” Dr. Choueiri commented. Taken together, “the results do support avelumab plus axitinib as a new first-line standard of care for patients with advanced RCC.”
JAVELIN Renal 101 complemented two other noteworthy trials exploring first-line checkpoint inhibitors for which new data were reported at the symposium. One, KEYNOTE-426 (NCT02853331), established that the combination of the immune checkpoint inhibitor pembrolizumab (Keytruda) and axitinib was superior to sunitinib. The other, CheckMate 214 (NCT02231749), established that the combination of two immune checkpoint inhibitors, nivolumab (Opdivo) and ipilimumab (Yervoy), was superior to sunitinib.
Weighing new options
“So a new standard of care in 2019 is present: The majority of patients with advanced clear cell RCC will be eligible to receive the combination of a checkpoint inhibitor and axitinib,” commented invited discussant Lori Wood, MD, a professor in the division of medical oncology at Dalhousie University, Halifax, Canada. “The questions now are: Which treatment should we choose? can we afford it? and perhaps more importantly, can we safely deliver this therapy to all patients?”
When it comes to selecting among the three combinations above, “I don’t think PD-L1 expression is going to help us at all,” she said. In contrast, IMDC risk category is likely still helpful because, in CheckMate 214, there was no progression-free or overall survival benefit of ipilimumab/nivolumab in patients with favorable-risk disease.
Differences in rates of discontinuation of all treatment because of treatment-related adverse events are hard to assess because CheckMate 214 had restrictions on allowing patients in the combination group to receive single-agent nivolumab, according to Dr. Wood. Financial costs are a major consideration, but so are time and staffing costs: Compared with single-agent sunitinib, the combinations as much as triple physician visits, nurse visits, infusions, and unscheduled visits.
Safely administering the combinations – through use of education, judicious patient selection, and attention to logistics – is a challenge, she maintained. “I tell the residents, you can probably give cisplatin/gemcitabine to 10 patients and you can probably give sunitinib to 20 patients and get a good sense of what’s going to happen. But every single patient that I have put on immune therapy, I learn something new.”
Evolving issues, such as nuanced differences among the immune checkpoint inhibitors and whether the doses used in trials are really needed, have yet to be worked through. But combining these agents is likely better than sequencing them because only about half of RCC patients given first-line therapy go on to get second-line therapy, “so we might as well use our best therapy up front,” Dr. Wood said. Finally, it’s unclear whether cytoreductive nephrectomy is needed to achieve a complete response with these combinations because all trials predated the CARMENA trial (NCT00930033), so most patients underwent this surgery.
“These are exciting times. I think that for the first-line metastatic renal cell patient with favorable-, intermediate-, or poor-risk disease, a checkpoint inhibitor/axitinib combination will be a new standard of care in many parts of the world, not all,” she summarized. “For intermediate- and poor-risk patients, there’s no clear winner in my mind at this current time between ipilimumab/nivolumab and checkpoint inhibitor/axitinib. Decisions will need to be based on overall survival, complete response rates, toxicities, and then practical aspects, as well as costs.”
“But we cannot safely and effectively deliver this new standard of care without true infrastructure and system changes to accommodate more doctor and nurse visits, more infusion time, all of these extra visits, and more education for everybody who is both delivering and receiving these agents,” Dr. Wood concluded.
Study details
The subgroup analyses showed that, compared with sunitinib, avelumab/axitinib yielded better progression-free survival across patients differing with respect to IMDC risk group (range of hazard ratios, 0.539-0.736), MSKCC risk group (range of HRs, 0.495-0.715), tumor PD-L1 status (range of HRs, 0.626-0.827), prior nephrectomy status (range of HRs, 0.673-0.748), smoking status (range of HRs, 0.663-0.711), and body mass index (range of HRs, 0.667-0.674), Dr. Choueiri reported at the symposium. However, the 95% confidence intervals crossed 1 in some cases.
Overall, 20.8% of the avelumab/axitinib group and 39.2% of the sunitinib group went on to receive a follow-up anticancer drug therapy. The most common was cabozantinib (Cabometyx) in the former and nivolumab (Opdivo) in the latter.
The rate of progression-free survival 2 could not be estimated for the avelumab/axitinib group and was 18.4 months for the sunitinib group (HR, 0.56). “In theory, the first-line treatment could change the biology of the disease and therefore lead to substantially shorter benefit of second-line treatment, and progression-free survival 2 is actually a potentially important endpoint for regulatory and reimbursement evaluation,” Dr. Choueiri explained. “This suggests at least no negative impact of first-line treatment with the combination on subsequent benefit from second-line treatment.”
Compared with sunitinib, avelumab/axitinib also yielded better odds of objective response regardless of IMDC risk group (range of odds ratios, 3.099-3.556), MSKCC risk group (range of ORs, 3.061-4.686), PD-L1 status (range of ORs, 2.240-3.594), prior nephrectomy status (range of ORs, 2.592-3.249), smoking status (range of ORs, 2.649-3.798), and body mass index (range of ORs, 3.086-3.292). Here, virtually all 95% confidence intervals excluded 1.
Mean duration of response was more than 4 months longer with the combination than with sunitinib. Moreover, responses were deeper for the combination patients.
In updated safety results, the avelumab/axitinib group had higher rates of any-grade treatment-related diarrhea (54% vs. 45%) and hypothyroidism (24% vs. 13%). But there were few of these adverse events of grade 3 or 4 in either group.
Dr. Choueiri disclosed that he receives honoraria from, has a consulting or advisory role with, and receives institutional research funding from Merck and Pfizer – among other disclosures. The trial was sponsored by Pfizer.
SOURCE: Choueiri TK et al. GUCS 2019, Abstract 544.
“For first-line therapy of metastatic clear-cell renal cancer, we now have two regimens that have demonstrated a survival advantage over first-line sunitinib,” Walter M. Stadler, MD, said in an interview. For first-line therapy, there is the combination of nivolumab and ipilimumab in intermediate- and poor-risk patients and also the combination of pembrolizumab and axitinib.
Dr. Stadler is the Fred C. Buffett Professor of Medicine and Surgery, chief of the section of hematology/oncology, director of the genitourinary oncology program, and deputy director of the Comprehensive Cancer Center at the University of Chicago.
“For first-line therapy of metastatic clear-cell renal cancer, we now have two regimens that have demonstrated a survival advantage over first-line sunitinib,” Walter M. Stadler, MD, said in an interview. For first-line therapy, there is the combination of nivolumab and ipilimumab in intermediate- and poor-risk patients and also the combination of pembrolizumab and axitinib.
Dr. Stadler is the Fred C. Buffett Professor of Medicine and Surgery, chief of the section of hematology/oncology, director of the genitourinary oncology program, and deputy director of the Comprehensive Cancer Center at the University of Chicago.
“For first-line therapy of metastatic clear-cell renal cancer, we now have two regimens that have demonstrated a survival advantage over first-line sunitinib,” Walter M. Stadler, MD, said in an interview. For first-line therapy, there is the combination of nivolumab and ipilimumab in intermediate- and poor-risk patients and also the combination of pembrolizumab and axitinib.
Dr. Stadler is the Fred C. Buffett Professor of Medicine and Surgery, chief of the section of hematology/oncology, director of the genitourinary oncology program, and deputy director of the Comprehensive Cancer Center at the University of Chicago.
SAN FRANCISCO – subgroup analyses of the JAVELIN Renal 101 trial have shown. Results were reported at the 2019 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Previous research had shown that avelumab (Bavencio), an immune checkpoint inhibitor targeting programmed death-ligand 1 (PD-L1), is active when used alone for advanced RCC, noted lead investigator Toni K. Choueiri, MD, director of the Lank Center for Genitourinary Oncology at the Dana-Farber Cancer Institute, Boston. And axitinib (Inlyta), a vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor, is approved for use in the second line. In an early-phase trial among patients being treated in the first line, a combination of the two drugs led to an impressive 58% objective response rate (ORR) and had a favorable safety profile (Lancet Oncol. 2018 Apr;19(4):451-60).
JAVELIN Renal 101 (NCT02684006), a phase 3 randomized, controlled trial, enrolled 886 patients with treatment-naive advanced RCC having a clear cell component regardless of their tumor’s PD-L1 status. They were randomized to the combination of avelumab/axitinib or to the VEGF tyrosine kinase inhibitor sunitinib (Sutent) alone.
Full trial results, published during the symposium in the New England Journal of Medicine (2019 Feb 16. doi: 10.1056/NEJMoa1816047), showed significant progression-free and overall survival benefits of avelumab/axitinib over sunitinib in the 63.2% of patients with PD-L1–positive tumors – the trial’s primary endpoints – as well as a progression-free survival benefit in the entire trial population.
In the subgroup analyses reported at the symposium, the combination reduced risk of progression or death by roughly 20%-50% across patients having different statuses in regard to International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk group, Memorial Sloan Kettering Cancer Center (MSKCC) risk group, and tumor PD-L1, as well as other characteristics. Findings were similar for ORR, with the combination roughly doubling to quadrupling the odds of response, irrespective of patient and disease characteristics.
“The progression-free survival and response rate benefit was observed in all patients, regardless of PD-L1 status, regardless of prognostic risk group. At this time, the study continues to follow up for overall survival,” Dr. Choueiri commented. Taken together, “the results do support avelumab plus axitinib as a new first-line standard of care for patients with advanced RCC.”
JAVELIN Renal 101 complemented two other noteworthy trials exploring first-line checkpoint inhibitors for which new data were reported at the symposium. One, KEYNOTE-426 (NCT02853331), established that the combination of the immune checkpoint inhibitor pembrolizumab (Keytruda) and axitinib was superior to sunitinib. The other, CheckMate 214 (NCT02231749), established that the combination of two immune checkpoint inhibitors, nivolumab (Opdivo) and ipilimumab (Yervoy), was superior to sunitinib.
Weighing new options
“So a new standard of care in 2019 is present: The majority of patients with advanced clear cell RCC will be eligible to receive the combination of a checkpoint inhibitor and axitinib,” commented invited discussant Lori Wood, MD, a professor in the division of medical oncology at Dalhousie University, Halifax, Canada. “The questions now are: Which treatment should we choose? can we afford it? and perhaps more importantly, can we safely deliver this therapy to all patients?”
When it comes to selecting among the three combinations above, “I don’t think PD-L1 expression is going to help us at all,” she said. In contrast, IMDC risk category is likely still helpful because, in CheckMate 214, there was no progression-free or overall survival benefit of ipilimumab/nivolumab in patients with favorable-risk disease.
Differences in rates of discontinuation of all treatment because of treatment-related adverse events are hard to assess because CheckMate 214 had restrictions on allowing patients in the combination group to receive single-agent nivolumab, according to Dr. Wood. Financial costs are a major consideration, but so are time and staffing costs: Compared with single-agent sunitinib, the combinations as much as triple physician visits, nurse visits, infusions, and unscheduled visits.
Safely administering the combinations – through use of education, judicious patient selection, and attention to logistics – is a challenge, she maintained. “I tell the residents, you can probably give cisplatin/gemcitabine to 10 patients and you can probably give sunitinib to 20 patients and get a good sense of what’s going to happen. But every single patient that I have put on immune therapy, I learn something new.”
Evolving issues, such as nuanced differences among the immune checkpoint inhibitors and whether the doses used in trials are really needed, have yet to be worked through. But combining these agents is likely better than sequencing them because only about half of RCC patients given first-line therapy go on to get second-line therapy, “so we might as well use our best therapy up front,” Dr. Wood said. Finally, it’s unclear whether cytoreductive nephrectomy is needed to achieve a complete response with these combinations because all trials predated the CARMENA trial (NCT00930033), so most patients underwent this surgery.
“These are exciting times. I think that for the first-line metastatic renal cell patient with favorable-, intermediate-, or poor-risk disease, a checkpoint inhibitor/axitinib combination will be a new standard of care in many parts of the world, not all,” she summarized. “For intermediate- and poor-risk patients, there’s no clear winner in my mind at this current time between ipilimumab/nivolumab and checkpoint inhibitor/axitinib. Decisions will need to be based on overall survival, complete response rates, toxicities, and then practical aspects, as well as costs.”
“But we cannot safely and effectively deliver this new standard of care without true infrastructure and system changes to accommodate more doctor and nurse visits, more infusion time, all of these extra visits, and more education for everybody who is both delivering and receiving these agents,” Dr. Wood concluded.
Study details
The subgroup analyses showed that, compared with sunitinib, avelumab/axitinib yielded better progression-free survival across patients differing with respect to IMDC risk group (range of hazard ratios, 0.539-0.736), MSKCC risk group (range of HRs, 0.495-0.715), tumor PD-L1 status (range of HRs, 0.626-0.827), prior nephrectomy status (range of HRs, 0.673-0.748), smoking status (range of HRs, 0.663-0.711), and body mass index (range of HRs, 0.667-0.674), Dr. Choueiri reported at the symposium. However, the 95% confidence intervals crossed 1 in some cases.
Overall, 20.8% of the avelumab/axitinib group and 39.2% of the sunitinib group went on to receive a follow-up anticancer drug therapy. The most common was cabozantinib (Cabometyx) in the former and nivolumab (Opdivo) in the latter.
The rate of progression-free survival 2 could not be estimated for the avelumab/axitinib group and was 18.4 months for the sunitinib group (HR, 0.56). “In theory, the first-line treatment could change the biology of the disease and therefore lead to substantially shorter benefit of second-line treatment, and progression-free survival 2 is actually a potentially important endpoint for regulatory and reimbursement evaluation,” Dr. Choueiri explained. “This suggests at least no negative impact of first-line treatment with the combination on subsequent benefit from second-line treatment.”
Compared with sunitinib, avelumab/axitinib also yielded better odds of objective response regardless of IMDC risk group (range of odds ratios, 3.099-3.556), MSKCC risk group (range of ORs, 3.061-4.686), PD-L1 status (range of ORs, 2.240-3.594), prior nephrectomy status (range of ORs, 2.592-3.249), smoking status (range of ORs, 2.649-3.798), and body mass index (range of ORs, 3.086-3.292). Here, virtually all 95% confidence intervals excluded 1.
Mean duration of response was more than 4 months longer with the combination than with sunitinib. Moreover, responses were deeper for the combination patients.
In updated safety results, the avelumab/axitinib group had higher rates of any-grade treatment-related diarrhea (54% vs. 45%) and hypothyroidism (24% vs. 13%). But there were few of these adverse events of grade 3 or 4 in either group.
Dr. Choueiri disclosed that he receives honoraria from, has a consulting or advisory role with, and receives institutional research funding from Merck and Pfizer – among other disclosures. The trial was sponsored by Pfizer.
SOURCE: Choueiri TK et al. GUCS 2019, Abstract 544.
SAN FRANCISCO – subgroup analyses of the JAVELIN Renal 101 trial have shown. Results were reported at the 2019 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Previous research had shown that avelumab (Bavencio), an immune checkpoint inhibitor targeting programmed death-ligand 1 (PD-L1), is active when used alone for advanced RCC, noted lead investigator Toni K. Choueiri, MD, director of the Lank Center for Genitourinary Oncology at the Dana-Farber Cancer Institute, Boston. And axitinib (Inlyta), a vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor, is approved for use in the second line. In an early-phase trial among patients being treated in the first line, a combination of the two drugs led to an impressive 58% objective response rate (ORR) and had a favorable safety profile (Lancet Oncol. 2018 Apr;19(4):451-60).
JAVELIN Renal 101 (NCT02684006), a phase 3 randomized, controlled trial, enrolled 886 patients with treatment-naive advanced RCC having a clear cell component regardless of their tumor’s PD-L1 status. They were randomized to the combination of avelumab/axitinib or to the VEGF tyrosine kinase inhibitor sunitinib (Sutent) alone.
Full trial results, published during the symposium in the New England Journal of Medicine (2019 Feb 16. doi: 10.1056/NEJMoa1816047), showed significant progression-free and overall survival benefits of avelumab/axitinib over sunitinib in the 63.2% of patients with PD-L1–positive tumors – the trial’s primary endpoints – as well as a progression-free survival benefit in the entire trial population.
In the subgroup analyses reported at the symposium, the combination reduced risk of progression or death by roughly 20%-50% across patients having different statuses in regard to International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk group, Memorial Sloan Kettering Cancer Center (MSKCC) risk group, and tumor PD-L1, as well as other characteristics. Findings were similar for ORR, with the combination roughly doubling to quadrupling the odds of response, irrespective of patient and disease characteristics.
“The progression-free survival and response rate benefit was observed in all patients, regardless of PD-L1 status, regardless of prognostic risk group. At this time, the study continues to follow up for overall survival,” Dr. Choueiri commented. Taken together, “the results do support avelumab plus axitinib as a new first-line standard of care for patients with advanced RCC.”
JAVELIN Renal 101 complemented two other noteworthy trials exploring first-line checkpoint inhibitors for which new data were reported at the symposium. One, KEYNOTE-426 (NCT02853331), established that the combination of the immune checkpoint inhibitor pembrolizumab (Keytruda) and axitinib was superior to sunitinib. The other, CheckMate 214 (NCT02231749), established that the combination of two immune checkpoint inhibitors, nivolumab (Opdivo) and ipilimumab (Yervoy), was superior to sunitinib.
Weighing new options
“So a new standard of care in 2019 is present: The majority of patients with advanced clear cell RCC will be eligible to receive the combination of a checkpoint inhibitor and axitinib,” commented invited discussant Lori Wood, MD, a professor in the division of medical oncology at Dalhousie University, Halifax, Canada. “The questions now are: Which treatment should we choose? can we afford it? and perhaps more importantly, can we safely deliver this therapy to all patients?”
When it comes to selecting among the three combinations above, “I don’t think PD-L1 expression is going to help us at all,” she said. In contrast, IMDC risk category is likely still helpful because, in CheckMate 214, there was no progression-free or overall survival benefit of ipilimumab/nivolumab in patients with favorable-risk disease.
Differences in rates of discontinuation of all treatment because of treatment-related adverse events are hard to assess because CheckMate 214 had restrictions on allowing patients in the combination group to receive single-agent nivolumab, according to Dr. Wood. Financial costs are a major consideration, but so are time and staffing costs: Compared with single-agent sunitinib, the combinations as much as triple physician visits, nurse visits, infusions, and unscheduled visits.
Safely administering the combinations – through use of education, judicious patient selection, and attention to logistics – is a challenge, she maintained. “I tell the residents, you can probably give cisplatin/gemcitabine to 10 patients and you can probably give sunitinib to 20 patients and get a good sense of what’s going to happen. But every single patient that I have put on immune therapy, I learn something new.”
Evolving issues, such as nuanced differences among the immune checkpoint inhibitors and whether the doses used in trials are really needed, have yet to be worked through. But combining these agents is likely better than sequencing them because only about half of RCC patients given first-line therapy go on to get second-line therapy, “so we might as well use our best therapy up front,” Dr. Wood said. Finally, it’s unclear whether cytoreductive nephrectomy is needed to achieve a complete response with these combinations because all trials predated the CARMENA trial (NCT00930033), so most patients underwent this surgery.
“These are exciting times. I think that for the first-line metastatic renal cell patient with favorable-, intermediate-, or poor-risk disease, a checkpoint inhibitor/axitinib combination will be a new standard of care in many parts of the world, not all,” she summarized. “For intermediate- and poor-risk patients, there’s no clear winner in my mind at this current time between ipilimumab/nivolumab and checkpoint inhibitor/axitinib. Decisions will need to be based on overall survival, complete response rates, toxicities, and then practical aspects, as well as costs.”
“But we cannot safely and effectively deliver this new standard of care without true infrastructure and system changes to accommodate more doctor and nurse visits, more infusion time, all of these extra visits, and more education for everybody who is both delivering and receiving these agents,” Dr. Wood concluded.
Study details
The subgroup analyses showed that, compared with sunitinib, avelumab/axitinib yielded better progression-free survival across patients differing with respect to IMDC risk group (range of hazard ratios, 0.539-0.736), MSKCC risk group (range of HRs, 0.495-0.715), tumor PD-L1 status (range of HRs, 0.626-0.827), prior nephrectomy status (range of HRs, 0.673-0.748), smoking status (range of HRs, 0.663-0.711), and body mass index (range of HRs, 0.667-0.674), Dr. Choueiri reported at the symposium. However, the 95% confidence intervals crossed 1 in some cases.
Overall, 20.8% of the avelumab/axitinib group and 39.2% of the sunitinib group went on to receive a follow-up anticancer drug therapy. The most common was cabozantinib (Cabometyx) in the former and nivolumab (Opdivo) in the latter.
The rate of progression-free survival 2 could not be estimated for the avelumab/axitinib group and was 18.4 months for the sunitinib group (HR, 0.56). “In theory, the first-line treatment could change the biology of the disease and therefore lead to substantially shorter benefit of second-line treatment, and progression-free survival 2 is actually a potentially important endpoint for regulatory and reimbursement evaluation,” Dr. Choueiri explained. “This suggests at least no negative impact of first-line treatment with the combination on subsequent benefit from second-line treatment.”
Compared with sunitinib, avelumab/axitinib also yielded better odds of objective response regardless of IMDC risk group (range of odds ratios, 3.099-3.556), MSKCC risk group (range of ORs, 3.061-4.686), PD-L1 status (range of ORs, 2.240-3.594), prior nephrectomy status (range of ORs, 2.592-3.249), smoking status (range of ORs, 2.649-3.798), and body mass index (range of ORs, 3.086-3.292). Here, virtually all 95% confidence intervals excluded 1.
Mean duration of response was more than 4 months longer with the combination than with sunitinib. Moreover, responses were deeper for the combination patients.
In updated safety results, the avelumab/axitinib group had higher rates of any-grade treatment-related diarrhea (54% vs. 45%) and hypothyroidism (24% vs. 13%). But there were few of these adverse events of grade 3 or 4 in either group.
Dr. Choueiri disclosed that he receives honoraria from, has a consulting or advisory role with, and receives institutional research funding from Merck and Pfizer – among other disclosures. The trial was sponsored by Pfizer.
SOURCE: Choueiri TK et al. GUCS 2019, Abstract 544.
REPORTING FROM GUCS 2019
New handoff tool can improve safety
Standardization of process reduces variation
Hospitalists know all too well that a significant source of medical errors is miscommunication during transitions: By interrupting the continuity of care, handoffs can increase the risk of adverse events.
Yet the transfer of patients from the ED to the hospitalist inpatient service has not been well studied, said Carmen Gonzalez, MD, lead author of a recent paper that examined the issue. “The scope of this study was to develop and test a handoff communication tool and a standardized process for transitioning patients from the ED to the hospitalist service at a comprehensive cancer center,” she explained.
In the study, the researchers found that the number of ICU transfers within 24 hours of admission and the number of rapid-response calls decreased after the implementation of a customized handoff tool. “The tool was named DE-PASS (DE-PASS: Decisive problem requiring admission, Evaluation time, Patient summary, Acute issues/action list, Situation unfinished/awareness, Signed out to), which was a modification of the I-PASS, and adapted to our workflow,” reported Dr. Gonzalez, who is based at the University of Texas MD Anderson Cancer Center, Houston. DE-PASS stratifies patients as stable/urgent/emergent and establishes requirements for communications between providers.
Results from the 1-month pilot revealed that, within a 24-hour period, DE-PASS reduced the number of intensive care unit transfers by 58%, the number of rapid-response team calls by 39%, and time to inpatient order by 31%.
“The standardization of the language and format of the handoff process of admission from the ED to the hospitalist service reduced handoff variations, increased provider satisfaction, and improved patient safety,” she noted.
The hospitalists expressed satisfaction with the tool. “This handoff tool helps stratify newly admitted patients based on their illness acuity, hence, assists the busy admitting hospitalist in prioritizing which patient needs to be attended first,” said study coauthor Norman Brito-Dellan, MD, also of MD Anderson Cancer Center. “In this study, DE-PASS reduced admission-to-evaluation times for unstable patients. These patients tend to be evaluated earlier, improving safety.”
Reference
1. Gonzalez CE et al. Handoff tool enabling standardized transitions between the emergency department and the hospitalist inpatient service at a major cancer center. American Journal of Medical Quality. 2018 May 21. doi: 10.1177/1062860618776096.
Standardization of process reduces variation
Standardization of process reduces variation
Hospitalists know all too well that a significant source of medical errors is miscommunication during transitions: By interrupting the continuity of care, handoffs can increase the risk of adverse events.
Yet the transfer of patients from the ED to the hospitalist inpatient service has not been well studied, said Carmen Gonzalez, MD, lead author of a recent paper that examined the issue. “The scope of this study was to develop and test a handoff communication tool and a standardized process for transitioning patients from the ED to the hospitalist service at a comprehensive cancer center,” she explained.
In the study, the researchers found that the number of ICU transfers within 24 hours of admission and the number of rapid-response calls decreased after the implementation of a customized handoff tool. “The tool was named DE-PASS (DE-PASS: Decisive problem requiring admission, Evaluation time, Patient summary, Acute issues/action list, Situation unfinished/awareness, Signed out to), which was a modification of the I-PASS, and adapted to our workflow,” reported Dr. Gonzalez, who is based at the University of Texas MD Anderson Cancer Center, Houston. DE-PASS stratifies patients as stable/urgent/emergent and establishes requirements for communications between providers.
Results from the 1-month pilot revealed that, within a 24-hour period, DE-PASS reduced the number of intensive care unit transfers by 58%, the number of rapid-response team calls by 39%, and time to inpatient order by 31%.
“The standardization of the language and format of the handoff process of admission from the ED to the hospitalist service reduced handoff variations, increased provider satisfaction, and improved patient safety,” she noted.
The hospitalists expressed satisfaction with the tool. “This handoff tool helps stratify newly admitted patients based on their illness acuity, hence, assists the busy admitting hospitalist in prioritizing which patient needs to be attended first,” said study coauthor Norman Brito-Dellan, MD, also of MD Anderson Cancer Center. “In this study, DE-PASS reduced admission-to-evaluation times for unstable patients. These patients tend to be evaluated earlier, improving safety.”
Reference
1. Gonzalez CE et al. Handoff tool enabling standardized transitions between the emergency department and the hospitalist inpatient service at a major cancer center. American Journal of Medical Quality. 2018 May 21. doi: 10.1177/1062860618776096.
Hospitalists know all too well that a significant source of medical errors is miscommunication during transitions: By interrupting the continuity of care, handoffs can increase the risk of adverse events.
Yet the transfer of patients from the ED to the hospitalist inpatient service has not been well studied, said Carmen Gonzalez, MD, lead author of a recent paper that examined the issue. “The scope of this study was to develop and test a handoff communication tool and a standardized process for transitioning patients from the ED to the hospitalist service at a comprehensive cancer center,” she explained.
In the study, the researchers found that the number of ICU transfers within 24 hours of admission and the number of rapid-response calls decreased after the implementation of a customized handoff tool. “The tool was named DE-PASS (DE-PASS: Decisive problem requiring admission, Evaluation time, Patient summary, Acute issues/action list, Situation unfinished/awareness, Signed out to), which was a modification of the I-PASS, and adapted to our workflow,” reported Dr. Gonzalez, who is based at the University of Texas MD Anderson Cancer Center, Houston. DE-PASS stratifies patients as stable/urgent/emergent and establishes requirements for communications between providers.
Results from the 1-month pilot revealed that, within a 24-hour period, DE-PASS reduced the number of intensive care unit transfers by 58%, the number of rapid-response team calls by 39%, and time to inpatient order by 31%.
“The standardization of the language and format of the handoff process of admission from the ED to the hospitalist service reduced handoff variations, increased provider satisfaction, and improved patient safety,” she noted.
The hospitalists expressed satisfaction with the tool. “This handoff tool helps stratify newly admitted patients based on their illness acuity, hence, assists the busy admitting hospitalist in prioritizing which patient needs to be attended first,” said study coauthor Norman Brito-Dellan, MD, also of MD Anderson Cancer Center. “In this study, DE-PASS reduced admission-to-evaluation times for unstable patients. These patients tend to be evaluated earlier, improving safety.”
Reference
1. Gonzalez CE et al. Handoff tool enabling standardized transitions between the emergency department and the hospitalist inpatient service at a major cancer center. American Journal of Medical Quality. 2018 May 21. doi: 10.1177/1062860618776096.
AAD, NPF release two joint guidelines on treatment, management of psoriasis
The .
These guidelines are the first of two papers to be published in the Journal of the American Academy of Dermatology (JAAD), with four more guidelines on psoriasis to be published later this year in JAAD on phototherapy, topical therapy, nonbiologic systemic medications, and treatment of pediatric patients.
The guideline on biologics updates the 2008 AAD guidelines on psoriasis. In an interview, Alan Menter, MD, cochair of the guidelines work group and lead author of the biologics paper, said the guidelines for biologics were needed because of major advances with the availability of new biologics over the last decade. For example, three tumor necrosis factor–alpha (TNF-alpha) inhibitors were available in 2008, but that number has increased to 10 biologics and now includes agents such as those targeting interleukin (IL)-12/IL-23, IL-17 and IL-23.
In addition, the new guidelines from AAD were developed to represent improvements in the management of patients with moderate to severe psoriasis as well as the relationship between psoriasis and related comorbidities.
“Major advances in new biologic drugs [are] now available to patients, plus [there have been] significant advances in our understanding of comorbid conditions,” such as cardiovascular comorbidities, said Dr. Menter, chairman of the division of dermatology, Baylor University Medical Center, and clinical professor of dermatology, University of Texas, both in Dallas.
The working group for each set of guidelines consisted of dermatologists, patient representatives, a cardiologist, and a rheumatologist. The biologic guidelines working group analyzed studies published between January 2008 and December 2018 and issued a series of recommendations based on published evidence for the effectiveness, adverse events, and switching for Food and Drug Administration–approved TNF-alpha inhibitors (etanercept, infliximab, adalimumab, certolizumab, and TNF-alpha biosimilars); IL-12/IL-23 inhibitors (ustekinumab); IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab); and IL-23 inhibitors (guselkumab and tildrakizumab, and risankizumab, which is still under FDA review) for monotherapy or combination therapy in patients with moderate to severe psoriasis.
The biologic guidelines noted that, while FDA-approved biologics were deemed safe overall for patients with moderate to severe psoriasis, dermatologists should recognize the adverse effects of these therapies, monitor for infections, and counsel their patients against modifying or discontinuing therapy without first consulting a dermatologist. In general, the working group noted that failure with one biologic does not necessarily mean that a patient will experience failure with a different biologic, even among TNF-alpha and IL-12/IL-23 inhibitors. However, reduced efficacy for a patient receiving a specific TNF-alpha inhibitor may predict reduced efficacy when switching to a different TNF-alpha inhibitor, they said.
In the psoriasis comorbidity guideline, the working group examined the therapeutic interventions for psoriasis-related comorbidities such as psoriatic arthritis (PsA), cardiovascular disease, metabolic syndrome, and inflammatory bowel disease. They also provided recommendations on the effect of psoriasis on mental health, quality of life, and lifestyle choices such as smoking and alcohol use.
With respect to cardiovascular disease, the dermatologist should ensure that patients are aware of the association between risk factors for cardiovascular disease and psoriasis, and that they undergo screening for these risk factors, consider lifestyle changes to reduce risk of cardiovascular disease, and consult with cardiologists and primary care providers based on individual risk, the guideline states. The working group recommended that patients with psoriasis undergo screening for hypertension, diabetes, and hyperlipidemia based on national guidelines, with more frequent screening recommended for patients with psoriasis greater than 10% body surface area or who are eligible for systemic or phototherapy.
In both the biologic and the comorbidity guidelines, the working groups stressed the importance of patient education and the role of the dermatologist in educating patients so that shared decision-making can occur. They noted that education was related to improved quality of life for these patients.
“Both the comorbidities guidelines and the biologic guidelines will help educate the psoriasis population with input from dermatologists in clinical practices,” Dr. Menter said.
However, both working groups noted there are still significant gaps in research, such as the effects of treatment combinations for new biologics and the lack of biomarkers that would identify which biologics are best suited for individual psoriasis patients.
There is also little known about the complex relationship between psoriasis and its comorbidities, and how psoriasis treatment can potentially prevent future disease. To ensure treatment of psoriasis-related comorbidities, dermatologists should consider psoriasis as a systemic disease with multiple comorbidities and interact with primary care doctors, cardiologists, and other providers involved in the care of the patients, Dr. Menter said.
There were no specific funding sources reported for the guidelines. Several authors reported relationships with industry, including pharmaceutical companies with drugs and products involving psoriasis, during the development of the guidelines. If a potential conflict was noted, the working group member recused himself or herself from discussion and drafting of recommendations, according to the paper. Dr. Menter’s disclosure includes serving as a consultant, speaker, investigator, and adviser, and receiving honoraria, from multiple pharmaceutical companies.
SOURCE: Menter A et al. J Am Acad Dermatol. 2019 Feb 13. doi: 10.1016/j.jaad.2018.11.057. Elmets CA et al. J Am Acad Dermatol. 2019 Feb 13. doi: 10.1016/j.jaad.2018.11.058.
The .
These guidelines are the first of two papers to be published in the Journal of the American Academy of Dermatology (JAAD), with four more guidelines on psoriasis to be published later this year in JAAD on phototherapy, topical therapy, nonbiologic systemic medications, and treatment of pediatric patients.
The guideline on biologics updates the 2008 AAD guidelines on psoriasis. In an interview, Alan Menter, MD, cochair of the guidelines work group and lead author of the biologics paper, said the guidelines for biologics were needed because of major advances with the availability of new biologics over the last decade. For example, three tumor necrosis factor–alpha (TNF-alpha) inhibitors were available in 2008, but that number has increased to 10 biologics and now includes agents such as those targeting interleukin (IL)-12/IL-23, IL-17 and IL-23.
In addition, the new guidelines from AAD were developed to represent improvements in the management of patients with moderate to severe psoriasis as well as the relationship between psoriasis and related comorbidities.
“Major advances in new biologic drugs [are] now available to patients, plus [there have been] significant advances in our understanding of comorbid conditions,” such as cardiovascular comorbidities, said Dr. Menter, chairman of the division of dermatology, Baylor University Medical Center, and clinical professor of dermatology, University of Texas, both in Dallas.
The working group for each set of guidelines consisted of dermatologists, patient representatives, a cardiologist, and a rheumatologist. The biologic guidelines working group analyzed studies published between January 2008 and December 2018 and issued a series of recommendations based on published evidence for the effectiveness, adverse events, and switching for Food and Drug Administration–approved TNF-alpha inhibitors (etanercept, infliximab, adalimumab, certolizumab, and TNF-alpha biosimilars); IL-12/IL-23 inhibitors (ustekinumab); IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab); and IL-23 inhibitors (guselkumab and tildrakizumab, and risankizumab, which is still under FDA review) for monotherapy or combination therapy in patients with moderate to severe psoriasis.
The biologic guidelines noted that, while FDA-approved biologics were deemed safe overall for patients with moderate to severe psoriasis, dermatologists should recognize the adverse effects of these therapies, monitor for infections, and counsel their patients against modifying or discontinuing therapy without first consulting a dermatologist. In general, the working group noted that failure with one biologic does not necessarily mean that a patient will experience failure with a different biologic, even among TNF-alpha and IL-12/IL-23 inhibitors. However, reduced efficacy for a patient receiving a specific TNF-alpha inhibitor may predict reduced efficacy when switching to a different TNF-alpha inhibitor, they said.
In the psoriasis comorbidity guideline, the working group examined the therapeutic interventions for psoriasis-related comorbidities such as psoriatic arthritis (PsA), cardiovascular disease, metabolic syndrome, and inflammatory bowel disease. They also provided recommendations on the effect of psoriasis on mental health, quality of life, and lifestyle choices such as smoking and alcohol use.
With respect to cardiovascular disease, the dermatologist should ensure that patients are aware of the association between risk factors for cardiovascular disease and psoriasis, and that they undergo screening for these risk factors, consider lifestyle changes to reduce risk of cardiovascular disease, and consult with cardiologists and primary care providers based on individual risk, the guideline states. The working group recommended that patients with psoriasis undergo screening for hypertension, diabetes, and hyperlipidemia based on national guidelines, with more frequent screening recommended for patients with psoriasis greater than 10% body surface area or who are eligible for systemic or phototherapy.
In both the biologic and the comorbidity guidelines, the working groups stressed the importance of patient education and the role of the dermatologist in educating patients so that shared decision-making can occur. They noted that education was related to improved quality of life for these patients.
“Both the comorbidities guidelines and the biologic guidelines will help educate the psoriasis population with input from dermatologists in clinical practices,” Dr. Menter said.
However, both working groups noted there are still significant gaps in research, such as the effects of treatment combinations for new biologics and the lack of biomarkers that would identify which biologics are best suited for individual psoriasis patients.
There is also little known about the complex relationship between psoriasis and its comorbidities, and how psoriasis treatment can potentially prevent future disease. To ensure treatment of psoriasis-related comorbidities, dermatologists should consider psoriasis as a systemic disease with multiple comorbidities and interact with primary care doctors, cardiologists, and other providers involved in the care of the patients, Dr. Menter said.
There were no specific funding sources reported for the guidelines. Several authors reported relationships with industry, including pharmaceutical companies with drugs and products involving psoriasis, during the development of the guidelines. If a potential conflict was noted, the working group member recused himself or herself from discussion and drafting of recommendations, according to the paper. Dr. Menter’s disclosure includes serving as a consultant, speaker, investigator, and adviser, and receiving honoraria, from multiple pharmaceutical companies.
SOURCE: Menter A et al. J Am Acad Dermatol. 2019 Feb 13. doi: 10.1016/j.jaad.2018.11.057. Elmets CA et al. J Am Acad Dermatol. 2019 Feb 13. doi: 10.1016/j.jaad.2018.11.058.
The .
These guidelines are the first of two papers to be published in the Journal of the American Academy of Dermatology (JAAD), with four more guidelines on psoriasis to be published later this year in JAAD on phototherapy, topical therapy, nonbiologic systemic medications, and treatment of pediatric patients.
The guideline on biologics updates the 2008 AAD guidelines on psoriasis. In an interview, Alan Menter, MD, cochair of the guidelines work group and lead author of the biologics paper, said the guidelines for biologics were needed because of major advances with the availability of new biologics over the last decade. For example, three tumor necrosis factor–alpha (TNF-alpha) inhibitors were available in 2008, but that number has increased to 10 biologics and now includes agents such as those targeting interleukin (IL)-12/IL-23, IL-17 and IL-23.
In addition, the new guidelines from AAD were developed to represent improvements in the management of patients with moderate to severe psoriasis as well as the relationship between psoriasis and related comorbidities.
“Major advances in new biologic drugs [are] now available to patients, plus [there have been] significant advances in our understanding of comorbid conditions,” such as cardiovascular comorbidities, said Dr. Menter, chairman of the division of dermatology, Baylor University Medical Center, and clinical professor of dermatology, University of Texas, both in Dallas.
The working group for each set of guidelines consisted of dermatologists, patient representatives, a cardiologist, and a rheumatologist. The biologic guidelines working group analyzed studies published between January 2008 and December 2018 and issued a series of recommendations based on published evidence for the effectiveness, adverse events, and switching for Food and Drug Administration–approved TNF-alpha inhibitors (etanercept, infliximab, adalimumab, certolizumab, and TNF-alpha biosimilars); IL-12/IL-23 inhibitors (ustekinumab); IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab); and IL-23 inhibitors (guselkumab and tildrakizumab, and risankizumab, which is still under FDA review) for monotherapy or combination therapy in patients with moderate to severe psoriasis.
The biologic guidelines noted that, while FDA-approved biologics were deemed safe overall for patients with moderate to severe psoriasis, dermatologists should recognize the adverse effects of these therapies, monitor for infections, and counsel their patients against modifying or discontinuing therapy without first consulting a dermatologist. In general, the working group noted that failure with one biologic does not necessarily mean that a patient will experience failure with a different biologic, even among TNF-alpha and IL-12/IL-23 inhibitors. However, reduced efficacy for a patient receiving a specific TNF-alpha inhibitor may predict reduced efficacy when switching to a different TNF-alpha inhibitor, they said.
In the psoriasis comorbidity guideline, the working group examined the therapeutic interventions for psoriasis-related comorbidities such as psoriatic arthritis (PsA), cardiovascular disease, metabolic syndrome, and inflammatory bowel disease. They also provided recommendations on the effect of psoriasis on mental health, quality of life, and lifestyle choices such as smoking and alcohol use.
With respect to cardiovascular disease, the dermatologist should ensure that patients are aware of the association between risk factors for cardiovascular disease and psoriasis, and that they undergo screening for these risk factors, consider lifestyle changes to reduce risk of cardiovascular disease, and consult with cardiologists and primary care providers based on individual risk, the guideline states. The working group recommended that patients with psoriasis undergo screening for hypertension, diabetes, and hyperlipidemia based on national guidelines, with more frequent screening recommended for patients with psoriasis greater than 10% body surface area or who are eligible for systemic or phototherapy.
In both the biologic and the comorbidity guidelines, the working groups stressed the importance of patient education and the role of the dermatologist in educating patients so that shared decision-making can occur. They noted that education was related to improved quality of life for these patients.
“Both the comorbidities guidelines and the biologic guidelines will help educate the psoriasis population with input from dermatologists in clinical practices,” Dr. Menter said.
However, both working groups noted there are still significant gaps in research, such as the effects of treatment combinations for new biologics and the lack of biomarkers that would identify which biologics are best suited for individual psoriasis patients.
There is also little known about the complex relationship between psoriasis and its comorbidities, and how psoriasis treatment can potentially prevent future disease. To ensure treatment of psoriasis-related comorbidities, dermatologists should consider psoriasis as a systemic disease with multiple comorbidities and interact with primary care doctors, cardiologists, and other providers involved in the care of the patients, Dr. Menter said.
There were no specific funding sources reported for the guidelines. Several authors reported relationships with industry, including pharmaceutical companies with drugs and products involving psoriasis, during the development of the guidelines. If a potential conflict was noted, the working group member recused himself or herself from discussion and drafting of recommendations, according to the paper. Dr. Menter’s disclosure includes serving as a consultant, speaker, investigator, and adviser, and receiving honoraria, from multiple pharmaceutical companies.
SOURCE: Menter A et al. J Am Acad Dermatol. 2019 Feb 13. doi: 10.1016/j.jaad.2018.11.057. Elmets CA et al. J Am Acad Dermatol. 2019 Feb 13. doi: 10.1016/j.jaad.2018.11.058.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
New Topical Treatments for Psoriasis