User login
Short-Course Vasoconstrictors After EVL: Time for a New Standard of Care?
PHILADELPHIA — without raising the risk for rebleeding, if the initial ligation successfully controls bleeding.
“This approach would allow earlier discharge from the hospital and reduce the risk of adverse events, all without sacrificing treatment efficacy or compromising patient safety,” Sushrut Ingawale, MD, MBBS, Quinnipiac University School of Medicine, North Haven, and St. Vincent’s Medical Center, Bridgeport, both in Connecticut, said in a presentation at the annual meeting of the American College of Gastroenterology (ACG).
Ingawale called for a “re-evaluation of existing protocols, emphasizing the potential to update current protocols to reflect shorter, more personalized” duration of vasoconstrictor therapy in these patients.
Commenting on this research, Nancy Reau, MD, AGAF, of Rush University in Chicago, Illinois, said: “We should always question the standard of care.”
“Vasoconstrictors for 5 days is the standard of care, but this could lead to prolonged hospitalization in patients who are otherwise doing well after endoscopic intervention. Recognizing that a shorter course of vasoconstrictor treatment may have equal outcome is very important though it may not be appropriate for all patients, especially those at high risk for rebleeding,” said Reau.
Outdated Guidelines?
In his presentation, Ingawale noted that current guidelines that recommend continuing vasoconstrictors, like octreotide or terlipressin, for at least 3-5 days after EVL for acute variceal bleeding are based primarily on old studies in which sclerotherapy was the primary hemostatic method.
The study team assessed comparative outcomes based on the duration of vasoconstrictors after EVL for acute variceal bleeding in a systematic review and network meta-analysis of 11 randomized controlled trials.
The studies had a total of 816 patients who were grouped based on the duration vasoconstrictor therapy: 24 hours or less (group 1), 24-72 hours (group 2), and 72-120 hours (group 3).
There was no significant difference in the risk for rebleeding in group 1 (risk ratio [RR], 1.36; 95% CI, 0.48-3.52) and group 2 (RR, 1.34; 95% CI, 0.42-4.54) vs group 3.
“This finding was even consistent when we compared individual durations” of 0, 12, 24, 48, and 72 hours vs 120 hours, Ingawale said.
There was also no statistically significant difference in the 5-day mortality risk between group 1 (RR, 0.66; 95% CI, 0.09-2.52) and group 2 (RR, 1.08; 95% CI, 0.15-6.43) or the 30-day mortality risk between group 1 (RR, 1.18; 95% CI, 0.51-2.51) and group 2 (RR, 0.98; 95% CI, 0.36-2.52) vs group 3.
Rapidly Evolving Area
“Our network meta-analysis did not show any benefit of continuing vasoconstrictors after EVL,” the researchers wrote in their conference abstract. Despite historical precedent, shorter durations may be adequate, “potentially enabling earlier hospital discharge without compromising patient outcomes.”
Ingawale suggested future research should look to identify the subset of patients at a risk for failure to control bleeding who might benefit from the continuation of vasoconstrictors.
“Management of complications of portal hypertension are rapidly evolving and this study will add to the data that drives our guidelines. Seeing this data in a peer reviewed publication will add the necessary validity to impact a change in the treatment paradigm,” Reau said.
The study had no specific funding. Ingawale had no relevant financial relationships. Reau disclosed various relationships with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — without raising the risk for rebleeding, if the initial ligation successfully controls bleeding.
“This approach would allow earlier discharge from the hospital and reduce the risk of adverse events, all without sacrificing treatment efficacy or compromising patient safety,” Sushrut Ingawale, MD, MBBS, Quinnipiac University School of Medicine, North Haven, and St. Vincent’s Medical Center, Bridgeport, both in Connecticut, said in a presentation at the annual meeting of the American College of Gastroenterology (ACG).
Ingawale called for a “re-evaluation of existing protocols, emphasizing the potential to update current protocols to reflect shorter, more personalized” duration of vasoconstrictor therapy in these patients.
Commenting on this research, Nancy Reau, MD, AGAF, of Rush University in Chicago, Illinois, said: “We should always question the standard of care.”
“Vasoconstrictors for 5 days is the standard of care, but this could lead to prolonged hospitalization in patients who are otherwise doing well after endoscopic intervention. Recognizing that a shorter course of vasoconstrictor treatment may have equal outcome is very important though it may not be appropriate for all patients, especially those at high risk for rebleeding,” said Reau.
Outdated Guidelines?
In his presentation, Ingawale noted that current guidelines that recommend continuing vasoconstrictors, like octreotide or terlipressin, for at least 3-5 days after EVL for acute variceal bleeding are based primarily on old studies in which sclerotherapy was the primary hemostatic method.
The study team assessed comparative outcomes based on the duration of vasoconstrictors after EVL for acute variceal bleeding in a systematic review and network meta-analysis of 11 randomized controlled trials.
The studies had a total of 816 patients who were grouped based on the duration vasoconstrictor therapy: 24 hours or less (group 1), 24-72 hours (group 2), and 72-120 hours (group 3).
There was no significant difference in the risk for rebleeding in group 1 (risk ratio [RR], 1.36; 95% CI, 0.48-3.52) and group 2 (RR, 1.34; 95% CI, 0.42-4.54) vs group 3.
“This finding was even consistent when we compared individual durations” of 0, 12, 24, 48, and 72 hours vs 120 hours, Ingawale said.
There was also no statistically significant difference in the 5-day mortality risk between group 1 (RR, 0.66; 95% CI, 0.09-2.52) and group 2 (RR, 1.08; 95% CI, 0.15-6.43) or the 30-day mortality risk between group 1 (RR, 1.18; 95% CI, 0.51-2.51) and group 2 (RR, 0.98; 95% CI, 0.36-2.52) vs group 3.
Rapidly Evolving Area
“Our network meta-analysis did not show any benefit of continuing vasoconstrictors after EVL,” the researchers wrote in their conference abstract. Despite historical precedent, shorter durations may be adequate, “potentially enabling earlier hospital discharge without compromising patient outcomes.”
Ingawale suggested future research should look to identify the subset of patients at a risk for failure to control bleeding who might benefit from the continuation of vasoconstrictors.
“Management of complications of portal hypertension are rapidly evolving and this study will add to the data that drives our guidelines. Seeing this data in a peer reviewed publication will add the necessary validity to impact a change in the treatment paradigm,” Reau said.
The study had no specific funding. Ingawale had no relevant financial relationships. Reau disclosed various relationships with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — without raising the risk for rebleeding, if the initial ligation successfully controls bleeding.
“This approach would allow earlier discharge from the hospital and reduce the risk of adverse events, all without sacrificing treatment efficacy or compromising patient safety,” Sushrut Ingawale, MD, MBBS, Quinnipiac University School of Medicine, North Haven, and St. Vincent’s Medical Center, Bridgeport, both in Connecticut, said in a presentation at the annual meeting of the American College of Gastroenterology (ACG).
Ingawale called for a “re-evaluation of existing protocols, emphasizing the potential to update current protocols to reflect shorter, more personalized” duration of vasoconstrictor therapy in these patients.
Commenting on this research, Nancy Reau, MD, AGAF, of Rush University in Chicago, Illinois, said: “We should always question the standard of care.”
“Vasoconstrictors for 5 days is the standard of care, but this could lead to prolonged hospitalization in patients who are otherwise doing well after endoscopic intervention. Recognizing that a shorter course of vasoconstrictor treatment may have equal outcome is very important though it may not be appropriate for all patients, especially those at high risk for rebleeding,” said Reau.
Outdated Guidelines?
In his presentation, Ingawale noted that current guidelines that recommend continuing vasoconstrictors, like octreotide or terlipressin, for at least 3-5 days after EVL for acute variceal bleeding are based primarily on old studies in which sclerotherapy was the primary hemostatic method.
The study team assessed comparative outcomes based on the duration of vasoconstrictors after EVL for acute variceal bleeding in a systematic review and network meta-analysis of 11 randomized controlled trials.
The studies had a total of 816 patients who were grouped based on the duration vasoconstrictor therapy: 24 hours or less (group 1), 24-72 hours (group 2), and 72-120 hours (group 3).
There was no significant difference in the risk for rebleeding in group 1 (risk ratio [RR], 1.36; 95% CI, 0.48-3.52) and group 2 (RR, 1.34; 95% CI, 0.42-4.54) vs group 3.
“This finding was even consistent when we compared individual durations” of 0, 12, 24, 48, and 72 hours vs 120 hours, Ingawale said.
There was also no statistically significant difference in the 5-day mortality risk between group 1 (RR, 0.66; 95% CI, 0.09-2.52) and group 2 (RR, 1.08; 95% CI, 0.15-6.43) or the 30-day mortality risk between group 1 (RR, 1.18; 95% CI, 0.51-2.51) and group 2 (RR, 0.98; 95% CI, 0.36-2.52) vs group 3.
Rapidly Evolving Area
“Our network meta-analysis did not show any benefit of continuing vasoconstrictors after EVL,” the researchers wrote in their conference abstract. Despite historical precedent, shorter durations may be adequate, “potentially enabling earlier hospital discharge without compromising patient outcomes.”
Ingawale suggested future research should look to identify the subset of patients at a risk for failure to control bleeding who might benefit from the continuation of vasoconstrictors.
“Management of complications of portal hypertension are rapidly evolving and this study will add to the data that drives our guidelines. Seeing this data in a peer reviewed publication will add the necessary validity to impact a change in the treatment paradigm,” Reau said.
The study had no specific funding. Ingawale had no relevant financial relationships. Reau disclosed various relationships with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article first appeared on Medscape.com.
FROM ACG 2024
Nutrition and Medical Education
How comfortable are you giving nutritional advice to your patients? When you offer it are you basing your advice on something you learned during medical school or your training? Was it included in a course devoted to nutrition? Did you learn it later as part of continuing medical education course (CME)? Or was it just something you just picked up from your experience seeing patients (osmosis)? It is very unlikely that a significant portion, or any part for that matter, of your medical training was devoted to nutrition. It certainly wasn’t during my training.
I recently read an interview with Emily M. Broad Leib, JD, faculty director of the Harvard School Center for Health Law and Policy Innovation, Cambridge, Massachusetts, who would like to correct that deficiency. She feels doctors need to know more about food and that acquiring that knowledge should be a significant component of their formal training.
In the interview, Leib said that “roughly 86% of physicians report they do not feel adequately trained to answer basic questions on diet or nutrition.” She also notes that while “72% of entering medical students report they believe food is important to health” less than 50% retained this belief after graduation.
Leib and associates feel they have recently reached a milestone in their efforts to include nutrition in the mainstream of medical education this fall by publishing a paper that demonstrates “consensus on doctor-approved nutritional standard for medical schools and residency programs.”
36 Recommended Competencies
Curious about what these nutrition experts chose to include in medical training, I decided to drill down into the list of 36 consensus-driven competencies they had agreed upon.
It was an interesting voyage into a forest of redundancies, many of which can be boiled down to having the student demonstrate that he/she understands that what we eat is important to our health and that there is a complex web of relationships connecting our society to the food consume.
Some of the recommended competencies I found make perfect sense. For example the student/trainee should be able to take a diet and food history and be able to interpret lab values and anthropometric measurements and be able to discuss the patient’s weight and diet with sensitivity while keeping in mind his/her own biases about food.
Some other recommendations are more problematic, for example, “performs a comprehensive nutrition-focused physical examination” or “demonstrates knowledge of how to create culinary nutrition SMART [Specific, Measurable, Achievable, Relevant, and Time-Bound] goals for personal use and for patient care” or “provides brief counseling interventions to help patients decrease visceral adiposity or reduce the risk of metabolic syndrome.” Including competencies like these demonstrates a lack of understanding of the time restraints and realities of a primary care physician’s life and training.
Instead of simply reinforcing the prospective physician’s preexisting assumption that food and health are entwined and discussing when and how to consult a nutrition expert, these 36 competencies seem to be an attempt to create fast-tracked part-time dietitians and nutrition advocates out of medical students and trainees who already believe that nutrition is important for health but also have a very full plate of clinical responsibilities ahead of them.
The study that Leib quotes — that 72% of medical students believed food was important in health while after graduation only 50% of agreed — doesn’t necessarily mean that professors are preaching that food was unimportant. It is more likely by the end of medical school the students have seen that food must share the spotlight with numerous other factors that influence their patients’ health.
‘A More Appropriate Focus’
In my experience, diet and lifestyle counseling done well is extremely time consuming and best done by people for whom that is their specialty. A more appropriate focus for a list of nutritional competencies for physicians in training would be for the student to achieve an understanding of when and how to consult a dietitian and then how to support and evaluate the dietitian’s recommendations to the patient.
Finally, I don’t think we can ignore a serious public relations problem that hangs like a cloud over the nutrition advocacy community. It is the same one that casts a shadow on the medical community as well. It is a common perception among the lay public that nutritionists (and physicians) are always changing their recommendations when it comes to food. What is believable? Just think about eggs, red wine, or introducing peanuts to infants, to name just a few. And what about the food pyramids that seem to have been rebuilt every several years? The problem is compounded when some “credentialed” nutritionists and physicians continue to make dietary pronouncements with only a shred of evidence or poorly documented anecdotal observations.
The first of the 36 competencies I reviewed reads: “Provide evidence-based, culturally sensitive nutrition and food recommendations for the prevention and treatment of disease.” When it comes to nutrition the “evidence” can be tough to come by. The natural experiments in which individuals and populations had extremely limited access to a certain nutrients (eg, scurvy) don’t occur very often. Animal studies don’t always extrapolate to humans. And, observational studies concerning diet often have co-factors that are difficult to control and must run over time courses that can tax even the most patient researchers.
I certainly applaud Leib and associates for promoting their primary goal of including more about of the relationship between food and health in the medical school and trainee curriculum. But I must voice a caution to be careful to keep it truly evidence-based and in a format that acknowledges the realities of the life and education of a primary care provider.
The best nutritional advice I ever received in my training was from an older pediatric professor who suggested that a healthy diet consisted of everything in moderation.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
How comfortable are you giving nutritional advice to your patients? When you offer it are you basing your advice on something you learned during medical school or your training? Was it included in a course devoted to nutrition? Did you learn it later as part of continuing medical education course (CME)? Or was it just something you just picked up from your experience seeing patients (osmosis)? It is very unlikely that a significant portion, or any part for that matter, of your medical training was devoted to nutrition. It certainly wasn’t during my training.
I recently read an interview with Emily M. Broad Leib, JD, faculty director of the Harvard School Center for Health Law and Policy Innovation, Cambridge, Massachusetts, who would like to correct that deficiency. She feels doctors need to know more about food and that acquiring that knowledge should be a significant component of their formal training.
In the interview, Leib said that “roughly 86% of physicians report they do not feel adequately trained to answer basic questions on diet or nutrition.” She also notes that while “72% of entering medical students report they believe food is important to health” less than 50% retained this belief after graduation.
Leib and associates feel they have recently reached a milestone in their efforts to include nutrition in the mainstream of medical education this fall by publishing a paper that demonstrates “consensus on doctor-approved nutritional standard for medical schools and residency programs.”
36 Recommended Competencies
Curious about what these nutrition experts chose to include in medical training, I decided to drill down into the list of 36 consensus-driven competencies they had agreed upon.
It was an interesting voyage into a forest of redundancies, many of which can be boiled down to having the student demonstrate that he/she understands that what we eat is important to our health and that there is a complex web of relationships connecting our society to the food consume.
Some of the recommended competencies I found make perfect sense. For example the student/trainee should be able to take a diet and food history and be able to interpret lab values and anthropometric measurements and be able to discuss the patient’s weight and diet with sensitivity while keeping in mind his/her own biases about food.
Some other recommendations are more problematic, for example, “performs a comprehensive nutrition-focused physical examination” or “demonstrates knowledge of how to create culinary nutrition SMART [Specific, Measurable, Achievable, Relevant, and Time-Bound] goals for personal use and for patient care” or “provides brief counseling interventions to help patients decrease visceral adiposity or reduce the risk of metabolic syndrome.” Including competencies like these demonstrates a lack of understanding of the time restraints and realities of a primary care physician’s life and training.
Instead of simply reinforcing the prospective physician’s preexisting assumption that food and health are entwined and discussing when and how to consult a nutrition expert, these 36 competencies seem to be an attempt to create fast-tracked part-time dietitians and nutrition advocates out of medical students and trainees who already believe that nutrition is important for health but also have a very full plate of clinical responsibilities ahead of them.
The study that Leib quotes — that 72% of medical students believed food was important in health while after graduation only 50% of agreed — doesn’t necessarily mean that professors are preaching that food was unimportant. It is more likely by the end of medical school the students have seen that food must share the spotlight with numerous other factors that influence their patients’ health.
‘A More Appropriate Focus’
In my experience, diet and lifestyle counseling done well is extremely time consuming and best done by people for whom that is their specialty. A more appropriate focus for a list of nutritional competencies for physicians in training would be for the student to achieve an understanding of when and how to consult a dietitian and then how to support and evaluate the dietitian’s recommendations to the patient.
Finally, I don’t think we can ignore a serious public relations problem that hangs like a cloud over the nutrition advocacy community. It is the same one that casts a shadow on the medical community as well. It is a common perception among the lay public that nutritionists (and physicians) are always changing their recommendations when it comes to food. What is believable? Just think about eggs, red wine, or introducing peanuts to infants, to name just a few. And what about the food pyramids that seem to have been rebuilt every several years? The problem is compounded when some “credentialed” nutritionists and physicians continue to make dietary pronouncements with only a shred of evidence or poorly documented anecdotal observations.
The first of the 36 competencies I reviewed reads: “Provide evidence-based, culturally sensitive nutrition and food recommendations for the prevention and treatment of disease.” When it comes to nutrition the “evidence” can be tough to come by. The natural experiments in which individuals and populations had extremely limited access to a certain nutrients (eg, scurvy) don’t occur very often. Animal studies don’t always extrapolate to humans. And, observational studies concerning diet often have co-factors that are difficult to control and must run over time courses that can tax even the most patient researchers.
I certainly applaud Leib and associates for promoting their primary goal of including more about of the relationship between food and health in the medical school and trainee curriculum. But I must voice a caution to be careful to keep it truly evidence-based and in a format that acknowledges the realities of the life and education of a primary care provider.
The best nutritional advice I ever received in my training was from an older pediatric professor who suggested that a healthy diet consisted of everything in moderation.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
How comfortable are you giving nutritional advice to your patients? When you offer it are you basing your advice on something you learned during medical school or your training? Was it included in a course devoted to nutrition? Did you learn it later as part of continuing medical education course (CME)? Or was it just something you just picked up from your experience seeing patients (osmosis)? It is very unlikely that a significant portion, or any part for that matter, of your medical training was devoted to nutrition. It certainly wasn’t during my training.
I recently read an interview with Emily M. Broad Leib, JD, faculty director of the Harvard School Center for Health Law and Policy Innovation, Cambridge, Massachusetts, who would like to correct that deficiency. She feels doctors need to know more about food and that acquiring that knowledge should be a significant component of their formal training.
In the interview, Leib said that “roughly 86% of physicians report they do not feel adequately trained to answer basic questions on diet or nutrition.” She also notes that while “72% of entering medical students report they believe food is important to health” less than 50% retained this belief after graduation.
Leib and associates feel they have recently reached a milestone in their efforts to include nutrition in the mainstream of medical education this fall by publishing a paper that demonstrates “consensus on doctor-approved nutritional standard for medical schools and residency programs.”
36 Recommended Competencies
Curious about what these nutrition experts chose to include in medical training, I decided to drill down into the list of 36 consensus-driven competencies they had agreed upon.
It was an interesting voyage into a forest of redundancies, many of which can be boiled down to having the student demonstrate that he/she understands that what we eat is important to our health and that there is a complex web of relationships connecting our society to the food consume.
Some of the recommended competencies I found make perfect sense. For example the student/trainee should be able to take a diet and food history and be able to interpret lab values and anthropometric measurements and be able to discuss the patient’s weight and diet with sensitivity while keeping in mind his/her own biases about food.
Some other recommendations are more problematic, for example, “performs a comprehensive nutrition-focused physical examination” or “demonstrates knowledge of how to create culinary nutrition SMART [Specific, Measurable, Achievable, Relevant, and Time-Bound] goals for personal use and for patient care” or “provides brief counseling interventions to help patients decrease visceral adiposity or reduce the risk of metabolic syndrome.” Including competencies like these demonstrates a lack of understanding of the time restraints and realities of a primary care physician’s life and training.
Instead of simply reinforcing the prospective physician’s preexisting assumption that food and health are entwined and discussing when and how to consult a nutrition expert, these 36 competencies seem to be an attempt to create fast-tracked part-time dietitians and nutrition advocates out of medical students and trainees who already believe that nutrition is important for health but also have a very full plate of clinical responsibilities ahead of them.
The study that Leib quotes — that 72% of medical students believed food was important in health while after graduation only 50% of agreed — doesn’t necessarily mean that professors are preaching that food was unimportant. It is more likely by the end of medical school the students have seen that food must share the spotlight with numerous other factors that influence their patients’ health.
‘A More Appropriate Focus’
In my experience, diet and lifestyle counseling done well is extremely time consuming and best done by people for whom that is their specialty. A more appropriate focus for a list of nutritional competencies for physicians in training would be for the student to achieve an understanding of when and how to consult a dietitian and then how to support and evaluate the dietitian’s recommendations to the patient.
Finally, I don’t think we can ignore a serious public relations problem that hangs like a cloud over the nutrition advocacy community. It is the same one that casts a shadow on the medical community as well. It is a common perception among the lay public that nutritionists (and physicians) are always changing their recommendations when it comes to food. What is believable? Just think about eggs, red wine, or introducing peanuts to infants, to name just a few. And what about the food pyramids that seem to have been rebuilt every several years? The problem is compounded when some “credentialed” nutritionists and physicians continue to make dietary pronouncements with only a shred of evidence or poorly documented anecdotal observations.
The first of the 36 competencies I reviewed reads: “Provide evidence-based, culturally sensitive nutrition and food recommendations for the prevention and treatment of disease.” When it comes to nutrition the “evidence” can be tough to come by. The natural experiments in which individuals and populations had extremely limited access to a certain nutrients (eg, scurvy) don’t occur very often. Animal studies don’t always extrapolate to humans. And, observational studies concerning diet often have co-factors that are difficult to control and must run over time courses that can tax even the most patient researchers.
I certainly applaud Leib and associates for promoting their primary goal of including more about of the relationship between food and health in the medical school and trainee curriculum. But I must voice a caution to be careful to keep it truly evidence-based and in a format that acknowledges the realities of the life and education of a primary care provider.
The best nutritional advice I ever received in my training was from an older pediatric professor who suggested that a healthy diet consisted of everything in moderation.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Sea Buckthorn
A member of the Elaeagnaceae family, Hippophae rhamnoides, better known as sea buckthorn, is a high-altitude wild shrub endemic to Europe and Asia with edible fruits and a lengthy record of use in traditional Chinese medicine.1-6 Used as a health supplement and consumed in the diet throughout the world,5 sea buckthorn berries, seeds, and leaves have been used in traditional medicine to treat burns/injuries, edema, hypertension, inflammation, skin grafts, ulcers, and wounds.4,7
This hardy plant is associated with a wide range of biologic activities, including anti-atherogenic, anti-atopic dermatitis, antibacterial, anticancer, antifungal, anti-inflammatory, antimicrobial, antioxidant, anti-psoriasis, anti-sebum, anti-stress, anti-tumor, cytoprotective, hepatoprotective, immunomodulatory, neuroprotective, radioprotective, and tissue regenerative functions.4,5,8-11
Key Constituents
Functional constituents identified in sea buckthorn include alkaloids, carotenoids, flavonoids, lignans, organic acids, phenolic acids, proanthocyanidins, polyunsaturated acids (including omega-3, -6, -7, and -9), steroids, tannins, terpenoids, and volatile oils, as well as nutritional compounds such as minerals, proteins, and vitamins.4,5,11 Sea buckthorn pericarp oil contains copious amounts of saturated palmitic acid (29%-36%) and omega-7 unsaturated palmitoleic acid (36%-48%), which fosters cutaneous and mucosal epithelialization, as well as linoleic (10%-12%) and oleic (4%-6%) acids.12,6 Significant amounts of carotenoids as well as alpha‐linolenic fatty acid (38%), linoleic (36%), oleic (13%), and palmitic (7%) acids are present in sea buckthorn seed oil.6
Polysaccharides
In an expansive review on the pharmacological activities of sea buckthorn polysaccharides, Teng and colleagues reported in April 2024 that 20 diverse polysaccharides have been culled from sea buckthorn and exhibited various healthy activities, including antioxidant, anti-fatigue, anti-inflammatory, anti-obesity, anti-tumor, hepatoprotective, hypoglycemic, and immunoregulation, and regulation of intestinal flora activities.1
Proanthocyanidins and Anti-Aging
In 2023, Liu and colleagues investigated the anti–skin aging impact of sea buckthorn proanthocyanidins in D-galactose-induced aging in mice given the known free radical scavenging activity of these compounds. They found the proanthocyanidins mitigated D-galactose-induced aging and can augment the total antioxidant capacity of the body. Sea buckthorn proanthocyanidins can further attenuate the effects of skin aging by regulating the TGF-beta1/Smads pathway and MMPs/TIMP system, thus amplifying collagen I and tropoelastin content.13
A year earlier, many of the same investigators assessed the possible protective activity of sea buckthorn proanthocyanidins against cutaneous aging engendered by oxidative stress from hydrogen peroxide. The compounds amplified superoxide dismutase and glutathione antioxidant functions. The extracts also fostered collagen I production in aging human skin fibroblasts via the TGF-beta1/Smads pathway and hindered collagen I degradation by regulating the MMPs/TIMPs system, which maintained extracellular matrix integrity. Senescent cell migration was also promoted with 100 mcg/mL of sea buckthorn proanthocyanidins. The researchers concluded that this sets the stage for investigating how sea buckthorn proanthocyanidins can be incorporated in cosmetic formulations.14 In a separate study, Liu and colleagues demonstrated that sea buckthorn proanthocyanidins can attenuate oxidative damage and protect mitochondrial function.9
Acne and Barrier Functions
The extracts of H rhamnoides and Cassia fistula in a combined formulation were found to be effective in lowering skin sebum content in humans with grade I and grade II acne vulgaris in a 2014 single-blind, randomized, placebo-controlled, split-face study with two groups of 25 patients each (aged 18-37 years).15 Khan and colleagues have also reported that a sea buckthorn oil-in-water emulsion improved barrier function in human skin as tested by a tewameter and corneometer (noninvasive probes) in 13 healthy males with a mean age of 27 ± 4.8 years.16
Anti-Aging, Antioxidant, Antibacterial, Skin-Whitening Activity
Zaman and colleagues reported in 2011 that results from an in vivo study of the effects of a sea buckthorn fruit extract topical cream on stratum corneum water content and transepidermal water loss indicated that the formulation enhanced cell surface integrin expression thus facilitating collagen contraction.17
In 2012, Khan and colleagues reported amelioration in skin elasticity, thus achieving an anti-aging result, from the use of a water-in-oil–based hydroalcoholic cream loaded with fruit extract of H rhamnoides, as measured with a Cutometer.18 The previous year, some of the same researchers reported that the antioxidants and flavonoids found in a topical sea buckthorn formulation could decrease cutaneous melanin and erythema levels.
More recently, Gęgotek and colleagues found that sea buckthorn seed oil prevented redox balance and lipid metabolism disturbances in skin fibroblasts and keratinocytes caused by UVA or UVB. They suggested that such findings point to the potential of this natural agent to confer anti-inflammatory properties and photoprotection to the skin.19
In 2020, Ivanišová and colleagues investigated the antioxidant and antimicrobial activities of H rhamnoides 100% oil, 100% juice, dry berries, and tea (dry berries, leaves, and twigs). They found that all of the studied sea buckthorn products displayed high antioxidant activity (identified through DPPH radical scavenging and molybdenum reducing antioxidant power tests). Sea buckthorn juice contained the highest total content of polyphenols, flavonoids, and carotenoids. All of the tested products also exhibited substantial antibacterial activity against the tested microbes.20
Burns and Wound Healing
In a preclinical study of the effects of sea buckthorn leaf extracts on wound healing in albino rats using an excision-punch wound model in 2005, Gupta and colleagues found that twice daily topical application of the aqueous leaf extract fostered wound healing. This was indicated by higher hydroxyproline and protein levels, a diminished wound area, and lower lipid peroxide levels. The investigators suggested that sea buckthorn may facilitate wound healing at least in part because of elevated antioxidant activity in the granulation tissue.3
A year later, Wang and colleagues reported on observations of using H rhamnoides oil, a traditional Chinese herbal medicine derived from sea buckthorn fruit, as a burn treatment. In the study, 151 burn patients received an H rhamnoides oil dressing (changed every other day until wound healing) that was covered with a disinfecting dressing. The dressing reduced swelling and effusion, and alleviated pain, with patients receiving the sea buckthorn dressing experiencing greater apparent exudation reduction, pain reduction, and more rapid epithelial cell growth and wound healing than controls (treated only with Vaseline gauze). The difference between the two groups was statistically significant.21
Conclusion
Sea buckthorn has been used for hundreds if not thousands of years in traditional medical applications, including for dermatologic purposes. Emerging data appear to support the use of this dynamic plant for consideration in dermatologic applications. As is often the case, much more work is necessary in the form of randomized controlled trials to determine the effectiveness of sea buckthorn formulations as well as the most appropriate avenues of research or uses for dermatologic application of this traditionally used botanical agent.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at [email protected].
References
1. Teng H et al. J Ethnopharmacol. 2024 Apr 24;324:117809. doi: 10.1016/j.jep.2024.117809.
2. Wang Z et al. Int J Biol Macromol. 2024 Apr;263(Pt 1):130206. doi: 10.1016/j.ijbiomac.2024.130206.
3. Gupta A et al. Int J Low Extrem Wounds. 2005 Jun;4(2):88-92. doi: 10.1177/1534734605277401.
4. Pundir S et al. J Ethnopharmacol. 2021 Feb 10;266:113434. doi: 10.1016/j.jep.2020.113434.
5. Ma QG et al. J Agric Food Chem. 2023 Mar 29;71(12):4769-4788. doi: 10.1021/acs.jafc.2c06916.
6. Poljšak N et al. Phytother Res. 2020 Feb;34(2):254-269. doi: 10.1002/ptr.6524. doi: 10.1002/ptr.6524.
7. Upadhyay NK et al. Evid Based Complement Alternat Med. 2011;2011:659705. doi: 10.1093/ecam/nep189.
8. Suryakumar G, Gupta A. J Ethnopharmacol. 2011 Nov 18;138(2):268-78. doi: 10.1016/j.jep.2011.09.024.
9. Liu K et al. Front Pharmacol. 2022 Jul 8;13:914146. doi: 10.3389/fphar.2022.914146.
10. Akhtar N et al. J Pharm Bioallied Sci. 2010 Jan;2(1):13-7. doi: 10.4103/0975-7406.62698.
11. Ren R et al. RSC Adv. 2020 Dec 17;10(73):44654-44671. doi: 10.1039/d0ra06488b.
12. Ito H et al. Burns. 2014 May;40(3):511-9. doi: 10.1016/j.burns.2013.08.011.
13. Liu X et al. Food Sci Nutr. 2023 Dec 7;12(2):1082-1094. doi: 10.1002/fsn3.3823.
14. Liu X at al. Antioxidants (Basel). 2022 Sep 25;11(10):1900. doi: 10.3390/antiox11101900.
15. Khan BA, Akhtar N. Postepy Dermatol Alergol. 2014 Aug;31(4):229-234. doi: 10.5114/pdia.2014.40934.
16. Khan BA, Akhtar N. Pak J Pharm Sci. 2014 Nov;27(6):1919-22.
17. Khan AB et al. African J Pharm Pharmacol. 2011 Aug;5(8):1092-5.
18. Khan BA, Akhtar N, Braga VA. Trop J Pharm Res. 2012;11(6):955-62.
19. Gęgotek A et al. Antioxidants (Basel). 2018 Aug 23;7(9):110. doi: 10.3390/antiox7090110.
20. Ivanišová E et al. Acta Sci Pol Technol Aliment. 2020 Apr-Jun;19(2):195-205. doi: 10.17306/J.AFS.0809.
21. Wang ZY, Luo XL, He CP. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Jan;26(1):124-5.
A member of the Elaeagnaceae family, Hippophae rhamnoides, better known as sea buckthorn, is a high-altitude wild shrub endemic to Europe and Asia with edible fruits and a lengthy record of use in traditional Chinese medicine.1-6 Used as a health supplement and consumed in the diet throughout the world,5 sea buckthorn berries, seeds, and leaves have been used in traditional medicine to treat burns/injuries, edema, hypertension, inflammation, skin grafts, ulcers, and wounds.4,7
This hardy plant is associated with a wide range of biologic activities, including anti-atherogenic, anti-atopic dermatitis, antibacterial, anticancer, antifungal, anti-inflammatory, antimicrobial, antioxidant, anti-psoriasis, anti-sebum, anti-stress, anti-tumor, cytoprotective, hepatoprotective, immunomodulatory, neuroprotective, radioprotective, and tissue regenerative functions.4,5,8-11
Key Constituents
Functional constituents identified in sea buckthorn include alkaloids, carotenoids, flavonoids, lignans, organic acids, phenolic acids, proanthocyanidins, polyunsaturated acids (including omega-3, -6, -7, and -9), steroids, tannins, terpenoids, and volatile oils, as well as nutritional compounds such as minerals, proteins, and vitamins.4,5,11 Sea buckthorn pericarp oil contains copious amounts of saturated palmitic acid (29%-36%) and omega-7 unsaturated palmitoleic acid (36%-48%), which fosters cutaneous and mucosal epithelialization, as well as linoleic (10%-12%) and oleic (4%-6%) acids.12,6 Significant amounts of carotenoids as well as alpha‐linolenic fatty acid (38%), linoleic (36%), oleic (13%), and palmitic (7%) acids are present in sea buckthorn seed oil.6
Polysaccharides
In an expansive review on the pharmacological activities of sea buckthorn polysaccharides, Teng and colleagues reported in April 2024 that 20 diverse polysaccharides have been culled from sea buckthorn and exhibited various healthy activities, including antioxidant, anti-fatigue, anti-inflammatory, anti-obesity, anti-tumor, hepatoprotective, hypoglycemic, and immunoregulation, and regulation of intestinal flora activities.1
Proanthocyanidins and Anti-Aging
In 2023, Liu and colleagues investigated the anti–skin aging impact of sea buckthorn proanthocyanidins in D-galactose-induced aging in mice given the known free radical scavenging activity of these compounds. They found the proanthocyanidins mitigated D-galactose-induced aging and can augment the total antioxidant capacity of the body. Sea buckthorn proanthocyanidins can further attenuate the effects of skin aging by regulating the TGF-beta1/Smads pathway and MMPs/TIMP system, thus amplifying collagen I and tropoelastin content.13
A year earlier, many of the same investigators assessed the possible protective activity of sea buckthorn proanthocyanidins against cutaneous aging engendered by oxidative stress from hydrogen peroxide. The compounds amplified superoxide dismutase and glutathione antioxidant functions. The extracts also fostered collagen I production in aging human skin fibroblasts via the TGF-beta1/Smads pathway and hindered collagen I degradation by regulating the MMPs/TIMPs system, which maintained extracellular matrix integrity. Senescent cell migration was also promoted with 100 mcg/mL of sea buckthorn proanthocyanidins. The researchers concluded that this sets the stage for investigating how sea buckthorn proanthocyanidins can be incorporated in cosmetic formulations.14 In a separate study, Liu and colleagues demonstrated that sea buckthorn proanthocyanidins can attenuate oxidative damage and protect mitochondrial function.9
Acne and Barrier Functions
The extracts of H rhamnoides and Cassia fistula in a combined formulation were found to be effective in lowering skin sebum content in humans with grade I and grade II acne vulgaris in a 2014 single-blind, randomized, placebo-controlled, split-face study with two groups of 25 patients each (aged 18-37 years).15 Khan and colleagues have also reported that a sea buckthorn oil-in-water emulsion improved barrier function in human skin as tested by a tewameter and corneometer (noninvasive probes) in 13 healthy males with a mean age of 27 ± 4.8 years.16
Anti-Aging, Antioxidant, Antibacterial, Skin-Whitening Activity
Zaman and colleagues reported in 2011 that results from an in vivo study of the effects of a sea buckthorn fruit extract topical cream on stratum corneum water content and transepidermal water loss indicated that the formulation enhanced cell surface integrin expression thus facilitating collagen contraction.17
In 2012, Khan and colleagues reported amelioration in skin elasticity, thus achieving an anti-aging result, from the use of a water-in-oil–based hydroalcoholic cream loaded with fruit extract of H rhamnoides, as measured with a Cutometer.18 The previous year, some of the same researchers reported that the antioxidants and flavonoids found in a topical sea buckthorn formulation could decrease cutaneous melanin and erythema levels.
More recently, Gęgotek and colleagues found that sea buckthorn seed oil prevented redox balance and lipid metabolism disturbances in skin fibroblasts and keratinocytes caused by UVA or UVB. They suggested that such findings point to the potential of this natural agent to confer anti-inflammatory properties and photoprotection to the skin.19
In 2020, Ivanišová and colleagues investigated the antioxidant and antimicrobial activities of H rhamnoides 100% oil, 100% juice, dry berries, and tea (dry berries, leaves, and twigs). They found that all of the studied sea buckthorn products displayed high antioxidant activity (identified through DPPH radical scavenging and molybdenum reducing antioxidant power tests). Sea buckthorn juice contained the highest total content of polyphenols, flavonoids, and carotenoids. All of the tested products also exhibited substantial antibacterial activity against the tested microbes.20
Burns and Wound Healing
In a preclinical study of the effects of sea buckthorn leaf extracts on wound healing in albino rats using an excision-punch wound model in 2005, Gupta and colleagues found that twice daily topical application of the aqueous leaf extract fostered wound healing. This was indicated by higher hydroxyproline and protein levels, a diminished wound area, and lower lipid peroxide levels. The investigators suggested that sea buckthorn may facilitate wound healing at least in part because of elevated antioxidant activity in the granulation tissue.3
A year later, Wang and colleagues reported on observations of using H rhamnoides oil, a traditional Chinese herbal medicine derived from sea buckthorn fruit, as a burn treatment. In the study, 151 burn patients received an H rhamnoides oil dressing (changed every other day until wound healing) that was covered with a disinfecting dressing. The dressing reduced swelling and effusion, and alleviated pain, with patients receiving the sea buckthorn dressing experiencing greater apparent exudation reduction, pain reduction, and more rapid epithelial cell growth and wound healing than controls (treated only with Vaseline gauze). The difference between the two groups was statistically significant.21
Conclusion
Sea buckthorn has been used for hundreds if not thousands of years in traditional medical applications, including for dermatologic purposes. Emerging data appear to support the use of this dynamic plant for consideration in dermatologic applications. As is often the case, much more work is necessary in the form of randomized controlled trials to determine the effectiveness of sea buckthorn formulations as well as the most appropriate avenues of research or uses for dermatologic application of this traditionally used botanical agent.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at [email protected].
References
1. Teng H et al. J Ethnopharmacol. 2024 Apr 24;324:117809. doi: 10.1016/j.jep.2024.117809.
2. Wang Z et al. Int J Biol Macromol. 2024 Apr;263(Pt 1):130206. doi: 10.1016/j.ijbiomac.2024.130206.
3. Gupta A et al. Int J Low Extrem Wounds. 2005 Jun;4(2):88-92. doi: 10.1177/1534734605277401.
4. Pundir S et al. J Ethnopharmacol. 2021 Feb 10;266:113434. doi: 10.1016/j.jep.2020.113434.
5. Ma QG et al. J Agric Food Chem. 2023 Mar 29;71(12):4769-4788. doi: 10.1021/acs.jafc.2c06916.
6. Poljšak N et al. Phytother Res. 2020 Feb;34(2):254-269. doi: 10.1002/ptr.6524. doi: 10.1002/ptr.6524.
7. Upadhyay NK et al. Evid Based Complement Alternat Med. 2011;2011:659705. doi: 10.1093/ecam/nep189.
8. Suryakumar G, Gupta A. J Ethnopharmacol. 2011 Nov 18;138(2):268-78. doi: 10.1016/j.jep.2011.09.024.
9. Liu K et al. Front Pharmacol. 2022 Jul 8;13:914146. doi: 10.3389/fphar.2022.914146.
10. Akhtar N et al. J Pharm Bioallied Sci. 2010 Jan;2(1):13-7. doi: 10.4103/0975-7406.62698.
11. Ren R et al. RSC Adv. 2020 Dec 17;10(73):44654-44671. doi: 10.1039/d0ra06488b.
12. Ito H et al. Burns. 2014 May;40(3):511-9. doi: 10.1016/j.burns.2013.08.011.
13. Liu X et al. Food Sci Nutr. 2023 Dec 7;12(2):1082-1094. doi: 10.1002/fsn3.3823.
14. Liu X at al. Antioxidants (Basel). 2022 Sep 25;11(10):1900. doi: 10.3390/antiox11101900.
15. Khan BA, Akhtar N. Postepy Dermatol Alergol. 2014 Aug;31(4):229-234. doi: 10.5114/pdia.2014.40934.
16. Khan BA, Akhtar N. Pak J Pharm Sci. 2014 Nov;27(6):1919-22.
17. Khan AB et al. African J Pharm Pharmacol. 2011 Aug;5(8):1092-5.
18. Khan BA, Akhtar N, Braga VA. Trop J Pharm Res. 2012;11(6):955-62.
19. Gęgotek A et al. Antioxidants (Basel). 2018 Aug 23;7(9):110. doi: 10.3390/antiox7090110.
20. Ivanišová E et al. Acta Sci Pol Technol Aliment. 2020 Apr-Jun;19(2):195-205. doi: 10.17306/J.AFS.0809.
21. Wang ZY, Luo XL, He CP. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Jan;26(1):124-5.
A member of the Elaeagnaceae family, Hippophae rhamnoides, better known as sea buckthorn, is a high-altitude wild shrub endemic to Europe and Asia with edible fruits and a lengthy record of use in traditional Chinese medicine.1-6 Used as a health supplement and consumed in the diet throughout the world,5 sea buckthorn berries, seeds, and leaves have been used in traditional medicine to treat burns/injuries, edema, hypertension, inflammation, skin grafts, ulcers, and wounds.4,7
This hardy plant is associated with a wide range of biologic activities, including anti-atherogenic, anti-atopic dermatitis, antibacterial, anticancer, antifungal, anti-inflammatory, antimicrobial, antioxidant, anti-psoriasis, anti-sebum, anti-stress, anti-tumor, cytoprotective, hepatoprotective, immunomodulatory, neuroprotective, radioprotective, and tissue regenerative functions.4,5,8-11
Key Constituents
Functional constituents identified in sea buckthorn include alkaloids, carotenoids, flavonoids, lignans, organic acids, phenolic acids, proanthocyanidins, polyunsaturated acids (including omega-3, -6, -7, and -9), steroids, tannins, terpenoids, and volatile oils, as well as nutritional compounds such as minerals, proteins, and vitamins.4,5,11 Sea buckthorn pericarp oil contains copious amounts of saturated palmitic acid (29%-36%) and omega-7 unsaturated palmitoleic acid (36%-48%), which fosters cutaneous and mucosal epithelialization, as well as linoleic (10%-12%) and oleic (4%-6%) acids.12,6 Significant amounts of carotenoids as well as alpha‐linolenic fatty acid (38%), linoleic (36%), oleic (13%), and palmitic (7%) acids are present in sea buckthorn seed oil.6
Polysaccharides
In an expansive review on the pharmacological activities of sea buckthorn polysaccharides, Teng and colleagues reported in April 2024 that 20 diverse polysaccharides have been culled from sea buckthorn and exhibited various healthy activities, including antioxidant, anti-fatigue, anti-inflammatory, anti-obesity, anti-tumor, hepatoprotective, hypoglycemic, and immunoregulation, and regulation of intestinal flora activities.1
Proanthocyanidins and Anti-Aging
In 2023, Liu and colleagues investigated the anti–skin aging impact of sea buckthorn proanthocyanidins in D-galactose-induced aging in mice given the known free radical scavenging activity of these compounds. They found the proanthocyanidins mitigated D-galactose-induced aging and can augment the total antioxidant capacity of the body. Sea buckthorn proanthocyanidins can further attenuate the effects of skin aging by regulating the TGF-beta1/Smads pathway and MMPs/TIMP system, thus amplifying collagen I and tropoelastin content.13
A year earlier, many of the same investigators assessed the possible protective activity of sea buckthorn proanthocyanidins against cutaneous aging engendered by oxidative stress from hydrogen peroxide. The compounds amplified superoxide dismutase and glutathione antioxidant functions. The extracts also fostered collagen I production in aging human skin fibroblasts via the TGF-beta1/Smads pathway and hindered collagen I degradation by regulating the MMPs/TIMPs system, which maintained extracellular matrix integrity. Senescent cell migration was also promoted with 100 mcg/mL of sea buckthorn proanthocyanidins. The researchers concluded that this sets the stage for investigating how sea buckthorn proanthocyanidins can be incorporated in cosmetic formulations.14 In a separate study, Liu and colleagues demonstrated that sea buckthorn proanthocyanidins can attenuate oxidative damage and protect mitochondrial function.9
Acne and Barrier Functions
The extracts of H rhamnoides and Cassia fistula in a combined formulation were found to be effective in lowering skin sebum content in humans with grade I and grade II acne vulgaris in a 2014 single-blind, randomized, placebo-controlled, split-face study with two groups of 25 patients each (aged 18-37 years).15 Khan and colleagues have also reported that a sea buckthorn oil-in-water emulsion improved barrier function in human skin as tested by a tewameter and corneometer (noninvasive probes) in 13 healthy males with a mean age of 27 ± 4.8 years.16
Anti-Aging, Antioxidant, Antibacterial, Skin-Whitening Activity
Zaman and colleagues reported in 2011 that results from an in vivo study of the effects of a sea buckthorn fruit extract topical cream on stratum corneum water content and transepidermal water loss indicated that the formulation enhanced cell surface integrin expression thus facilitating collagen contraction.17
In 2012, Khan and colleagues reported amelioration in skin elasticity, thus achieving an anti-aging result, from the use of a water-in-oil–based hydroalcoholic cream loaded with fruit extract of H rhamnoides, as measured with a Cutometer.18 The previous year, some of the same researchers reported that the antioxidants and flavonoids found in a topical sea buckthorn formulation could decrease cutaneous melanin and erythema levels.
More recently, Gęgotek and colleagues found that sea buckthorn seed oil prevented redox balance and lipid metabolism disturbances in skin fibroblasts and keratinocytes caused by UVA or UVB. They suggested that such findings point to the potential of this natural agent to confer anti-inflammatory properties and photoprotection to the skin.19
In 2020, Ivanišová and colleagues investigated the antioxidant and antimicrobial activities of H rhamnoides 100% oil, 100% juice, dry berries, and tea (dry berries, leaves, and twigs). They found that all of the studied sea buckthorn products displayed high antioxidant activity (identified through DPPH radical scavenging and molybdenum reducing antioxidant power tests). Sea buckthorn juice contained the highest total content of polyphenols, flavonoids, and carotenoids. All of the tested products also exhibited substantial antibacterial activity against the tested microbes.20
Burns and Wound Healing
In a preclinical study of the effects of sea buckthorn leaf extracts on wound healing in albino rats using an excision-punch wound model in 2005, Gupta and colleagues found that twice daily topical application of the aqueous leaf extract fostered wound healing. This was indicated by higher hydroxyproline and protein levels, a diminished wound area, and lower lipid peroxide levels. The investigators suggested that sea buckthorn may facilitate wound healing at least in part because of elevated antioxidant activity in the granulation tissue.3
A year later, Wang and colleagues reported on observations of using H rhamnoides oil, a traditional Chinese herbal medicine derived from sea buckthorn fruit, as a burn treatment. In the study, 151 burn patients received an H rhamnoides oil dressing (changed every other day until wound healing) that was covered with a disinfecting dressing. The dressing reduced swelling and effusion, and alleviated pain, with patients receiving the sea buckthorn dressing experiencing greater apparent exudation reduction, pain reduction, and more rapid epithelial cell growth and wound healing than controls (treated only with Vaseline gauze). The difference between the two groups was statistically significant.21
Conclusion
Sea buckthorn has been used for hundreds if not thousands of years in traditional medical applications, including for dermatologic purposes. Emerging data appear to support the use of this dynamic plant for consideration in dermatologic applications. As is often the case, much more work is necessary in the form of randomized controlled trials to determine the effectiveness of sea buckthorn formulations as well as the most appropriate avenues of research or uses for dermatologic application of this traditionally used botanical agent.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at [email protected].
References
1. Teng H et al. J Ethnopharmacol. 2024 Apr 24;324:117809. doi: 10.1016/j.jep.2024.117809.
2. Wang Z et al. Int J Biol Macromol. 2024 Apr;263(Pt 1):130206. doi: 10.1016/j.ijbiomac.2024.130206.
3. Gupta A et al. Int J Low Extrem Wounds. 2005 Jun;4(2):88-92. doi: 10.1177/1534734605277401.
4. Pundir S et al. J Ethnopharmacol. 2021 Feb 10;266:113434. doi: 10.1016/j.jep.2020.113434.
5. Ma QG et al. J Agric Food Chem. 2023 Mar 29;71(12):4769-4788. doi: 10.1021/acs.jafc.2c06916.
6. Poljšak N et al. Phytother Res. 2020 Feb;34(2):254-269. doi: 10.1002/ptr.6524. doi: 10.1002/ptr.6524.
7. Upadhyay NK et al. Evid Based Complement Alternat Med. 2011;2011:659705. doi: 10.1093/ecam/nep189.
8. Suryakumar G, Gupta A. J Ethnopharmacol. 2011 Nov 18;138(2):268-78. doi: 10.1016/j.jep.2011.09.024.
9. Liu K et al. Front Pharmacol. 2022 Jul 8;13:914146. doi: 10.3389/fphar.2022.914146.
10. Akhtar N et al. J Pharm Bioallied Sci. 2010 Jan;2(1):13-7. doi: 10.4103/0975-7406.62698.
11. Ren R et al. RSC Adv. 2020 Dec 17;10(73):44654-44671. doi: 10.1039/d0ra06488b.
12. Ito H et al. Burns. 2014 May;40(3):511-9. doi: 10.1016/j.burns.2013.08.011.
13. Liu X et al. Food Sci Nutr. 2023 Dec 7;12(2):1082-1094. doi: 10.1002/fsn3.3823.
14. Liu X at al. Antioxidants (Basel). 2022 Sep 25;11(10):1900. doi: 10.3390/antiox11101900.
15. Khan BA, Akhtar N. Postepy Dermatol Alergol. 2014 Aug;31(4):229-234. doi: 10.5114/pdia.2014.40934.
16. Khan BA, Akhtar N. Pak J Pharm Sci. 2014 Nov;27(6):1919-22.
17. Khan AB et al. African J Pharm Pharmacol. 2011 Aug;5(8):1092-5.
18. Khan BA, Akhtar N, Braga VA. Trop J Pharm Res. 2012;11(6):955-62.
19. Gęgotek A et al. Antioxidants (Basel). 2018 Aug 23;7(9):110. doi: 10.3390/antiox7090110.
20. Ivanišová E et al. Acta Sci Pol Technol Aliment. 2020 Apr-Jun;19(2):195-205. doi: 10.17306/J.AFS.0809.
21. Wang ZY, Luo XL, He CP. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Jan;26(1):124-5.
Experts Challenge New Diagnostic Criteria for Alzheimer’s disease
In a paper published online in JAMA Neurology, the International Working Group (IWG), which includes 46 experts from 17 countries, is recommending that the diagnosis of Alzheimer’s disease be limited to individuals with mild cognitive impairment or dementia and not be applied to cognitively normal individuals with Alzheimer’s disease biomarkers such as amyloid-beta 42/40 or p-tau.
Clinicians should be “very careful” about using the “A” word (Alzheimer’s) for cognitively unimpaired people with Alzheimer’s disease biomarkers, said the paper’s first author Bruno Dubois, MD, professor of neurology, Sorbonne University and Department of Neurology, Pitié-Salpêtrière Hospital, Paris, France.
Providing an Alzheimer’s disease diagnosis to those who have a high chance of never developing cognitive impairment can be psychologically harmful, said Dubois.
“It’s not something small like telling someone they have a fever. Just imagine you’re 65 years old and are amyloid positive, and you’re told you have Alzheimer’s disease. It affects the decisions you make for the rest of your life and changes your vision of your future, even though you may never develop the disease,” he added.
Divergent View
The IWG’s perspective on Alzheimer’s disease contrasts with a recent proposal from the Alzheimer’s Association. The Alzheimer’s Association criteria suggest that Alzheimer’s disease should be regarded solely as a biological entity, which could include cognitively normal individuals with one core Alzheimer’s disease biomarker.
The IWG noted that its concerns regarding the application of a purely biological definition of Alzheimer’s disease in clinical practice prompted the group to consider updating its guidelines, potentially offering “an alternative definitional view of Alzheimer’s disease as a clinical-biological construct for clinical use.”
The group conducted a PubMed search for relevant Alzheimer’s disease articles, and included references, published between July 2020 and March 2024. The research showed the majority of biomarker-positive, cognitively normal individuals will not become symptomatic during their lifetime.
The risk of a 55-year-old who is amyloid positive developing Alzheimer’s disease is not that much higher than that for an individual of a similar age who is amyloid negative, Dubois noted. “There’s an 83% chance that person will never develop Alzheimer’s disease.”
Disclosing a diagnosis of Alzheimer’s disease to cognitively normal people with only one core Alzheimer’s disease biomarker represents “the most problematic implication of a purely biological definition of the disease,” the authors noted.
“A biomarker is a marker of pathology, not a biomarker of disease,” said Dubois, adding that a person may have markers for several different brain diseases.
The IWG recommends the following nomenclature: At risk for Alzheimer’s disease for those with Alzheimer’s disease biomarkers but low lifetime risk and presymptomatic Alzheimer’s disease for those with Alzheimer’s disease biomarkers with a very high lifetime risk for progression such as individuals with autosomal dominant genetic mutations and other distinct biomarker profiles that put them at extremely high lifetime risk of developing the disease.
Dubois emphasized the difference between those showing typical Alzheimer’s disease symptoms with positive biomarkers who should be considered to have the disease and those with positive biomarkers but no typical Alzheimer’s disease symptoms who should be considered at risk.
This is an important distinction as it affects research approaches and assessment of risks, he said.
For low-risk asymptomatic individuals, the IWG does not recommend routine diagnostic testing outside of the research setting. “There’s no reason to send a 65-year-old cognitively normal subject off to collect biomarker information,” said Dubois.
He reiterated the importance of clinicians using appropriate and sensitive language surrounding Alzheimer’s disease when face to face with patients. This issue “is not purely semantic; this is real life.”
For these patients in the clinical setting, “we have to be very careful about proposing treatments that may have side effects,” he said.
However, this does not mean asymptomatic at-risk people should not be studied to determine what pharmacological interventions might prevent or delay the onset of clinical disease, he noted.
Presymptomatic individuals who are at a high risk of developing Alzheimer’s disease “should be the target for clinical trials in the future” to determine best ways to delay the conversion to Alzheimer’s disease, he said.
The main focus of such research should be to better understand the “biomarker pattern profile” that is associated with a high risk of developing Alzheimer’s disease, said Dubois.
Plea for Unity
In an accompanying editorial, Ronald C. Petersen, PhD, MD, director, Mayo Clinic Alzheimer’s Disease Research Center and Mayo Clinic Study of Aging, Rochester, Minnesota, and colleagues outline the difference between the IWG and Alzheimer’s Association positions.
As the IWG uses Alzheimer’s disease to define those with cognitive impairment and the Alzheimer’s Association group uses Alzheimer’s disease to define those with the pathology of the disease, the field is now at a crossroads. “Do we name the disease before clinical symptoms?” they asked.
They note that Alzheimer’s Association criteria distinguish between a disease and an illness, whereas the IWG does not. “As such, although the primary disagreement between the groups is semantic, the ramifications of the labeling can be significant.”
It is “incumbent” that the field “come together” on an Alzheimer’s disease definition, the editorial concluded. “Neither the Alzheimer’s Association or IWG documents are appropriate to serve as a guide for how to apply biomarkers in a clinical setting. Appropriate-use criteria are needed to form a bridge between biological frameworks and real-world clinical practice so we can all maximally help all of our patients with this disorder.”
In a comment, Reisa Sperling, MD, professor of neurology, Harvard Medical School, and director, Center for Alzheimer Research and Treatment, Brigham and Women’s Hospital and Massachusetts General Hospital, all in Boston, who is part of the Alzheimer’s Association work group that published the revised criteria for diagnosis and staging of Alzheimer’s disease, likened Alzheimer’s disease, which begins in the brain many years before dementia onset, to cardiovascular disease in that it involves multiple processes. She noted the World Health Organization classifies cardiovascular disease as a “disease” prior to clinical manifestations such as stroke and myocardial infarction.
“If someone has Alzheimer’s disease pathology in their brain, they are at risk for dementia or clinical manifestations of the disease — just like vascular disease quantifies the risk of stroke or heart attack, not risk of developing ‘vascular disease’ if the underlying vascular disease is already present,” said Sperling.
A large part of the controversy is related to terminology and the “stigma” of the “A” word in the same way there used to be fear around using the “C” word — cancer, said Sperling.
“Once people began talking about cancer publicly as a potentially treatable disease and began getting screened and diagnosed before symptoms of cancer were manifest, this has had a tremendous impact on public health.”
She clarified that her work group does not recommend screening asymptomatic people with Alzheimer’s disease biomarkers. “We actually need to prove that treating at the preclinical stage of the disease is able to prevent clinical impairment and dementia,” she said, adding “hopefully, we are getting closer to this.”
Dubois reported no relevant disclosures. Petersen reported receiving personal fees from Roche, Genentech, Eli Lilly and Company, Eisai, and Novo Nordisk outside the submitted work and royalties from Oxford University Press, UpToDate, and Medscape educational activities.
A version of this article appeared on Medscape.com.
In a paper published online in JAMA Neurology, the International Working Group (IWG), which includes 46 experts from 17 countries, is recommending that the diagnosis of Alzheimer’s disease be limited to individuals with mild cognitive impairment or dementia and not be applied to cognitively normal individuals with Alzheimer’s disease biomarkers such as amyloid-beta 42/40 or p-tau.
Clinicians should be “very careful” about using the “A” word (Alzheimer’s) for cognitively unimpaired people with Alzheimer’s disease biomarkers, said the paper’s first author Bruno Dubois, MD, professor of neurology, Sorbonne University and Department of Neurology, Pitié-Salpêtrière Hospital, Paris, France.
Providing an Alzheimer’s disease diagnosis to those who have a high chance of never developing cognitive impairment can be psychologically harmful, said Dubois.
“It’s not something small like telling someone they have a fever. Just imagine you’re 65 years old and are amyloid positive, and you’re told you have Alzheimer’s disease. It affects the decisions you make for the rest of your life and changes your vision of your future, even though you may never develop the disease,” he added.
Divergent View
The IWG’s perspective on Alzheimer’s disease contrasts with a recent proposal from the Alzheimer’s Association. The Alzheimer’s Association criteria suggest that Alzheimer’s disease should be regarded solely as a biological entity, which could include cognitively normal individuals with one core Alzheimer’s disease biomarker.
The IWG noted that its concerns regarding the application of a purely biological definition of Alzheimer’s disease in clinical practice prompted the group to consider updating its guidelines, potentially offering “an alternative definitional view of Alzheimer’s disease as a clinical-biological construct for clinical use.”
The group conducted a PubMed search for relevant Alzheimer’s disease articles, and included references, published between July 2020 and March 2024. The research showed the majority of biomarker-positive, cognitively normal individuals will not become symptomatic during their lifetime.
The risk of a 55-year-old who is amyloid positive developing Alzheimer’s disease is not that much higher than that for an individual of a similar age who is amyloid negative, Dubois noted. “There’s an 83% chance that person will never develop Alzheimer’s disease.”
Disclosing a diagnosis of Alzheimer’s disease to cognitively normal people with only one core Alzheimer’s disease biomarker represents “the most problematic implication of a purely biological definition of the disease,” the authors noted.
“A biomarker is a marker of pathology, not a biomarker of disease,” said Dubois, adding that a person may have markers for several different brain diseases.
The IWG recommends the following nomenclature: At risk for Alzheimer’s disease for those with Alzheimer’s disease biomarkers but low lifetime risk and presymptomatic Alzheimer’s disease for those with Alzheimer’s disease biomarkers with a very high lifetime risk for progression such as individuals with autosomal dominant genetic mutations and other distinct biomarker profiles that put them at extremely high lifetime risk of developing the disease.
Dubois emphasized the difference between those showing typical Alzheimer’s disease symptoms with positive biomarkers who should be considered to have the disease and those with positive biomarkers but no typical Alzheimer’s disease symptoms who should be considered at risk.
This is an important distinction as it affects research approaches and assessment of risks, he said.
For low-risk asymptomatic individuals, the IWG does not recommend routine diagnostic testing outside of the research setting. “There’s no reason to send a 65-year-old cognitively normal subject off to collect biomarker information,” said Dubois.
He reiterated the importance of clinicians using appropriate and sensitive language surrounding Alzheimer’s disease when face to face with patients. This issue “is not purely semantic; this is real life.”
For these patients in the clinical setting, “we have to be very careful about proposing treatments that may have side effects,” he said.
However, this does not mean asymptomatic at-risk people should not be studied to determine what pharmacological interventions might prevent or delay the onset of clinical disease, he noted.
Presymptomatic individuals who are at a high risk of developing Alzheimer’s disease “should be the target for clinical trials in the future” to determine best ways to delay the conversion to Alzheimer’s disease, he said.
The main focus of such research should be to better understand the “biomarker pattern profile” that is associated with a high risk of developing Alzheimer’s disease, said Dubois.
Plea for Unity
In an accompanying editorial, Ronald C. Petersen, PhD, MD, director, Mayo Clinic Alzheimer’s Disease Research Center and Mayo Clinic Study of Aging, Rochester, Minnesota, and colleagues outline the difference between the IWG and Alzheimer’s Association positions.
As the IWG uses Alzheimer’s disease to define those with cognitive impairment and the Alzheimer’s Association group uses Alzheimer’s disease to define those with the pathology of the disease, the field is now at a crossroads. “Do we name the disease before clinical symptoms?” they asked.
They note that Alzheimer’s Association criteria distinguish between a disease and an illness, whereas the IWG does not. “As such, although the primary disagreement between the groups is semantic, the ramifications of the labeling can be significant.”
It is “incumbent” that the field “come together” on an Alzheimer’s disease definition, the editorial concluded. “Neither the Alzheimer’s Association or IWG documents are appropriate to serve as a guide for how to apply biomarkers in a clinical setting. Appropriate-use criteria are needed to form a bridge between biological frameworks and real-world clinical practice so we can all maximally help all of our patients with this disorder.”
In a comment, Reisa Sperling, MD, professor of neurology, Harvard Medical School, and director, Center for Alzheimer Research and Treatment, Brigham and Women’s Hospital and Massachusetts General Hospital, all in Boston, who is part of the Alzheimer’s Association work group that published the revised criteria for diagnosis and staging of Alzheimer’s disease, likened Alzheimer’s disease, which begins in the brain many years before dementia onset, to cardiovascular disease in that it involves multiple processes. She noted the World Health Organization classifies cardiovascular disease as a “disease” prior to clinical manifestations such as stroke and myocardial infarction.
“If someone has Alzheimer’s disease pathology in their brain, they are at risk for dementia or clinical manifestations of the disease — just like vascular disease quantifies the risk of stroke or heart attack, not risk of developing ‘vascular disease’ if the underlying vascular disease is already present,” said Sperling.
A large part of the controversy is related to terminology and the “stigma” of the “A” word in the same way there used to be fear around using the “C” word — cancer, said Sperling.
“Once people began talking about cancer publicly as a potentially treatable disease and began getting screened and diagnosed before symptoms of cancer were manifest, this has had a tremendous impact on public health.”
She clarified that her work group does not recommend screening asymptomatic people with Alzheimer’s disease biomarkers. “We actually need to prove that treating at the preclinical stage of the disease is able to prevent clinical impairment and dementia,” she said, adding “hopefully, we are getting closer to this.”
Dubois reported no relevant disclosures. Petersen reported receiving personal fees from Roche, Genentech, Eli Lilly and Company, Eisai, and Novo Nordisk outside the submitted work and royalties from Oxford University Press, UpToDate, and Medscape educational activities.
A version of this article appeared on Medscape.com.
In a paper published online in JAMA Neurology, the International Working Group (IWG), which includes 46 experts from 17 countries, is recommending that the diagnosis of Alzheimer’s disease be limited to individuals with mild cognitive impairment or dementia and not be applied to cognitively normal individuals with Alzheimer’s disease biomarkers such as amyloid-beta 42/40 or p-tau.
Clinicians should be “very careful” about using the “A” word (Alzheimer’s) for cognitively unimpaired people with Alzheimer’s disease biomarkers, said the paper’s first author Bruno Dubois, MD, professor of neurology, Sorbonne University and Department of Neurology, Pitié-Salpêtrière Hospital, Paris, France.
Providing an Alzheimer’s disease diagnosis to those who have a high chance of never developing cognitive impairment can be psychologically harmful, said Dubois.
“It’s not something small like telling someone they have a fever. Just imagine you’re 65 years old and are amyloid positive, and you’re told you have Alzheimer’s disease. It affects the decisions you make for the rest of your life and changes your vision of your future, even though you may never develop the disease,” he added.
Divergent View
The IWG’s perspective on Alzheimer’s disease contrasts with a recent proposal from the Alzheimer’s Association. The Alzheimer’s Association criteria suggest that Alzheimer’s disease should be regarded solely as a biological entity, which could include cognitively normal individuals with one core Alzheimer’s disease biomarker.
The IWG noted that its concerns regarding the application of a purely biological definition of Alzheimer’s disease in clinical practice prompted the group to consider updating its guidelines, potentially offering “an alternative definitional view of Alzheimer’s disease as a clinical-biological construct for clinical use.”
The group conducted a PubMed search for relevant Alzheimer’s disease articles, and included references, published between July 2020 and March 2024. The research showed the majority of biomarker-positive, cognitively normal individuals will not become symptomatic during their lifetime.
The risk of a 55-year-old who is amyloid positive developing Alzheimer’s disease is not that much higher than that for an individual of a similar age who is amyloid negative, Dubois noted. “There’s an 83% chance that person will never develop Alzheimer’s disease.”
Disclosing a diagnosis of Alzheimer’s disease to cognitively normal people with only one core Alzheimer’s disease biomarker represents “the most problematic implication of a purely biological definition of the disease,” the authors noted.
“A biomarker is a marker of pathology, not a biomarker of disease,” said Dubois, adding that a person may have markers for several different brain diseases.
The IWG recommends the following nomenclature: At risk for Alzheimer’s disease for those with Alzheimer’s disease biomarkers but low lifetime risk and presymptomatic Alzheimer’s disease for those with Alzheimer’s disease biomarkers with a very high lifetime risk for progression such as individuals with autosomal dominant genetic mutations and other distinct biomarker profiles that put them at extremely high lifetime risk of developing the disease.
Dubois emphasized the difference between those showing typical Alzheimer’s disease symptoms with positive biomarkers who should be considered to have the disease and those with positive biomarkers but no typical Alzheimer’s disease symptoms who should be considered at risk.
This is an important distinction as it affects research approaches and assessment of risks, he said.
For low-risk asymptomatic individuals, the IWG does not recommend routine diagnostic testing outside of the research setting. “There’s no reason to send a 65-year-old cognitively normal subject off to collect biomarker information,” said Dubois.
He reiterated the importance of clinicians using appropriate and sensitive language surrounding Alzheimer’s disease when face to face with patients. This issue “is not purely semantic; this is real life.”
For these patients in the clinical setting, “we have to be very careful about proposing treatments that may have side effects,” he said.
However, this does not mean asymptomatic at-risk people should not be studied to determine what pharmacological interventions might prevent or delay the onset of clinical disease, he noted.
Presymptomatic individuals who are at a high risk of developing Alzheimer’s disease “should be the target for clinical trials in the future” to determine best ways to delay the conversion to Alzheimer’s disease, he said.
The main focus of such research should be to better understand the “biomarker pattern profile” that is associated with a high risk of developing Alzheimer’s disease, said Dubois.
Plea for Unity
In an accompanying editorial, Ronald C. Petersen, PhD, MD, director, Mayo Clinic Alzheimer’s Disease Research Center and Mayo Clinic Study of Aging, Rochester, Minnesota, and colleagues outline the difference between the IWG and Alzheimer’s Association positions.
As the IWG uses Alzheimer’s disease to define those with cognitive impairment and the Alzheimer’s Association group uses Alzheimer’s disease to define those with the pathology of the disease, the field is now at a crossroads. “Do we name the disease before clinical symptoms?” they asked.
They note that Alzheimer’s Association criteria distinguish between a disease and an illness, whereas the IWG does not. “As such, although the primary disagreement between the groups is semantic, the ramifications of the labeling can be significant.”
It is “incumbent” that the field “come together” on an Alzheimer’s disease definition, the editorial concluded. “Neither the Alzheimer’s Association or IWG documents are appropriate to serve as a guide for how to apply biomarkers in a clinical setting. Appropriate-use criteria are needed to form a bridge between biological frameworks and real-world clinical practice so we can all maximally help all of our patients with this disorder.”
In a comment, Reisa Sperling, MD, professor of neurology, Harvard Medical School, and director, Center for Alzheimer Research and Treatment, Brigham and Women’s Hospital and Massachusetts General Hospital, all in Boston, who is part of the Alzheimer’s Association work group that published the revised criteria for diagnosis and staging of Alzheimer’s disease, likened Alzheimer’s disease, which begins in the brain many years before dementia onset, to cardiovascular disease in that it involves multiple processes. She noted the World Health Organization classifies cardiovascular disease as a “disease” prior to clinical manifestations such as stroke and myocardial infarction.
“If someone has Alzheimer’s disease pathology in their brain, they are at risk for dementia or clinical manifestations of the disease — just like vascular disease quantifies the risk of stroke or heart attack, not risk of developing ‘vascular disease’ if the underlying vascular disease is already present,” said Sperling.
A large part of the controversy is related to terminology and the “stigma” of the “A” word in the same way there used to be fear around using the “C” word — cancer, said Sperling.
“Once people began talking about cancer publicly as a potentially treatable disease and began getting screened and diagnosed before symptoms of cancer were manifest, this has had a tremendous impact on public health.”
She clarified that her work group does not recommend screening asymptomatic people with Alzheimer’s disease biomarkers. “We actually need to prove that treating at the preclinical stage of the disease is able to prevent clinical impairment and dementia,” she said, adding “hopefully, we are getting closer to this.”
Dubois reported no relevant disclosures. Petersen reported receiving personal fees from Roche, Genentech, Eli Lilly and Company, Eisai, and Novo Nordisk outside the submitted work and royalties from Oxford University Press, UpToDate, and Medscape educational activities.
A version of this article appeared on Medscape.com.
From JAMA Neurology
How Extreme Rainfall Amplifies Health Risks
Climate change is intensifying the variability of precipitation caused by extreme daily and overall rainfall events. Awareness of the effects of these events is crucial for understanding the complex health consequences of climate change. Physicians have often advised their patients to move to a better climate, and when they did, the recommendation was rarely based on precise scientific knowledge. However, the benefits of changing environments were often so evident that they were indisputable.
Today, advanced models, satellite imagery, and biological approaches such as environmental epigenetics are enhancing our understanding of health risks related to climate change.
Extreme Rainfall and Health
The increase in precipitation variability is linked to climate warming, which leads to higher atmospheric humidity and extreme rainfall events. These manifestations can cause rapid weather changes, increasing interactions with harmful aerosols and raising the risk for various cardiovascular and respiratory conditions. However, a full understanding of the association between rain and health has been hindered by conflicting results and methodological issues (limited geographical locations and short observation durations) in studies.
The association between rainfall intensity and health effects is likely nonlinear. Moderate precipitation can mitigate summer heat and help reduce air pollution, an effect that may lower some environmental health risks. Conversely, intense, low-frequency, short-duration rainfall events can have particularly harmful effects on health, as such events can trigger rapid weather changes, increased proliferation of pathogens, and a rise in the risk of various pollutants, potentially exacerbating health conditions.
Rain and Mortality
Using an intensity-duration-frequency model of three rainfall indices (high intensity, low frequency, short duration), a study published in October 2024 combined these with mortality data from 34 countries or regions. Researchers estimated associations between mortality (all cause, cardiovascular, and respiratory) and rainfall events with different return periods (the average time expected before an extreme event of a certain magnitude occurs again) and crucial effect modifiers, including climatic, socioeconomic, and urban environmental conditions.
The analysis included 109,954,744 deaths from all causes; 31,164,161 cardiovascular deaths; and 11,817,278 respiratory deaths. During the study period, from 1980 to 2020, a total of 50,913 rainfall events with a 1-year return period, 8362 events with a 2-year return period, and 3301 events with a 5-year return period were identified.
The most significant finding was a global positive association between all-cause mortality and extreme rainfall events with a 5-year return period. One day of extreme rainfall with a 5-year return period was associated with a cumulative relative risk (RRc) of 1.08 (95% CI, 1.05-1.11) for daily mortality from all causes. Rainfall events with a 2-year return period were associated with increased daily respiratory mortality (RRc, 1.14), while no significant effect was observed for cardiovascular mortality during the same period. Rainfall events with a 5-year return period were associated with an increased risk for both cardiovascular mortality (RRc, 1.05) and respiratory mortality (RRc, 1.29), with the respiratory mortality being significantly higher.
Points of Concern
According to the authors, moderate to high rainfall can exert protective effects through two main mechanisms: Improving air quality (rainfall can reduce the concentration of particulate matter 2.5 cm in diameter or less in the atmosphere) and behavioral changes in people (more time spent in enclosed environments, reducing direct exposure to outdoor air pollution and nonoptimal temperatures). As rainfall intensity increases, the initial protective effects may be overshadowed by a cascade of negative impacts including:
- Critical resource disruptions: Intense rainfall can cause severe disruptions to access to healthcare, infrastructure damage including power outages, and compromised water and food quality.
- Physiological effects: Increased humidity levels facilitate the growth of airborne pathogens, potentially triggering allergic reactions and respiratory issues, particularly in vulnerable individuals. Rapid shifts in atmospheric pressure and temperature fluctuations can lead to cardiovascular and respiratory complications.
- Indirect effects: Extreme rainfall can have profound effects on mental health, inducing stress and anxiety that may exacerbate pre-existing mental health conditions and indirectly contribute to increased overall mortality from nonexternal causes.
The intensity-response curves for the health effects of heavy rainfall showed a nonlinear trend, transitioning from a protective effect at moderate levels of rainfall to a risk for severe harm when rainfall intensity became extreme. Additionally, the significant effects of extreme events were modified by various types of climate and were more pronounced in areas characterized by low variability in precipitation or sparse vegetation cover.
The study demonstrated that various local factors, such as climatic conditions, climate type, and vegetation cover, can potentially influence cardiovascular and respiratory mortality and all-cause mortality related to precipitation. The findings may help physicians convey to their patients the impact of climate change on their health.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Climate change is intensifying the variability of precipitation caused by extreme daily and overall rainfall events. Awareness of the effects of these events is crucial for understanding the complex health consequences of climate change. Physicians have often advised their patients to move to a better climate, and when they did, the recommendation was rarely based on precise scientific knowledge. However, the benefits of changing environments were often so evident that they were indisputable.
Today, advanced models, satellite imagery, and biological approaches such as environmental epigenetics are enhancing our understanding of health risks related to climate change.
Extreme Rainfall and Health
The increase in precipitation variability is linked to climate warming, which leads to higher atmospheric humidity and extreme rainfall events. These manifestations can cause rapid weather changes, increasing interactions with harmful aerosols and raising the risk for various cardiovascular and respiratory conditions. However, a full understanding of the association between rain and health has been hindered by conflicting results and methodological issues (limited geographical locations and short observation durations) in studies.
The association between rainfall intensity and health effects is likely nonlinear. Moderate precipitation can mitigate summer heat and help reduce air pollution, an effect that may lower some environmental health risks. Conversely, intense, low-frequency, short-duration rainfall events can have particularly harmful effects on health, as such events can trigger rapid weather changes, increased proliferation of pathogens, and a rise in the risk of various pollutants, potentially exacerbating health conditions.
Rain and Mortality
Using an intensity-duration-frequency model of three rainfall indices (high intensity, low frequency, short duration), a study published in October 2024 combined these with mortality data from 34 countries or regions. Researchers estimated associations between mortality (all cause, cardiovascular, and respiratory) and rainfall events with different return periods (the average time expected before an extreme event of a certain magnitude occurs again) and crucial effect modifiers, including climatic, socioeconomic, and urban environmental conditions.
The analysis included 109,954,744 deaths from all causes; 31,164,161 cardiovascular deaths; and 11,817,278 respiratory deaths. During the study period, from 1980 to 2020, a total of 50,913 rainfall events with a 1-year return period, 8362 events with a 2-year return period, and 3301 events with a 5-year return period were identified.
The most significant finding was a global positive association between all-cause mortality and extreme rainfall events with a 5-year return period. One day of extreme rainfall with a 5-year return period was associated with a cumulative relative risk (RRc) of 1.08 (95% CI, 1.05-1.11) for daily mortality from all causes. Rainfall events with a 2-year return period were associated with increased daily respiratory mortality (RRc, 1.14), while no significant effect was observed for cardiovascular mortality during the same period. Rainfall events with a 5-year return period were associated with an increased risk for both cardiovascular mortality (RRc, 1.05) and respiratory mortality (RRc, 1.29), with the respiratory mortality being significantly higher.
Points of Concern
According to the authors, moderate to high rainfall can exert protective effects through two main mechanisms: Improving air quality (rainfall can reduce the concentration of particulate matter 2.5 cm in diameter or less in the atmosphere) and behavioral changes in people (more time spent in enclosed environments, reducing direct exposure to outdoor air pollution and nonoptimal temperatures). As rainfall intensity increases, the initial protective effects may be overshadowed by a cascade of negative impacts including:
- Critical resource disruptions: Intense rainfall can cause severe disruptions to access to healthcare, infrastructure damage including power outages, and compromised water and food quality.
- Physiological effects: Increased humidity levels facilitate the growth of airborne pathogens, potentially triggering allergic reactions and respiratory issues, particularly in vulnerable individuals. Rapid shifts in atmospheric pressure and temperature fluctuations can lead to cardiovascular and respiratory complications.
- Indirect effects: Extreme rainfall can have profound effects on mental health, inducing stress and anxiety that may exacerbate pre-existing mental health conditions and indirectly contribute to increased overall mortality from nonexternal causes.
The intensity-response curves for the health effects of heavy rainfall showed a nonlinear trend, transitioning from a protective effect at moderate levels of rainfall to a risk for severe harm when rainfall intensity became extreme. Additionally, the significant effects of extreme events were modified by various types of climate and were more pronounced in areas characterized by low variability in precipitation or sparse vegetation cover.
The study demonstrated that various local factors, such as climatic conditions, climate type, and vegetation cover, can potentially influence cardiovascular and respiratory mortality and all-cause mortality related to precipitation. The findings may help physicians convey to their patients the impact of climate change on their health.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Climate change is intensifying the variability of precipitation caused by extreme daily and overall rainfall events. Awareness of the effects of these events is crucial for understanding the complex health consequences of climate change. Physicians have often advised their patients to move to a better climate, and when they did, the recommendation was rarely based on precise scientific knowledge. However, the benefits of changing environments were often so evident that they were indisputable.
Today, advanced models, satellite imagery, and biological approaches such as environmental epigenetics are enhancing our understanding of health risks related to climate change.
Extreme Rainfall and Health
The increase in precipitation variability is linked to climate warming, which leads to higher atmospheric humidity and extreme rainfall events. These manifestations can cause rapid weather changes, increasing interactions with harmful aerosols and raising the risk for various cardiovascular and respiratory conditions. However, a full understanding of the association between rain and health has been hindered by conflicting results and methodological issues (limited geographical locations and short observation durations) in studies.
The association between rainfall intensity and health effects is likely nonlinear. Moderate precipitation can mitigate summer heat and help reduce air pollution, an effect that may lower some environmental health risks. Conversely, intense, low-frequency, short-duration rainfall events can have particularly harmful effects on health, as such events can trigger rapid weather changes, increased proliferation of pathogens, and a rise in the risk of various pollutants, potentially exacerbating health conditions.
Rain and Mortality
Using an intensity-duration-frequency model of three rainfall indices (high intensity, low frequency, short duration), a study published in October 2024 combined these with mortality data from 34 countries or regions. Researchers estimated associations between mortality (all cause, cardiovascular, and respiratory) and rainfall events with different return periods (the average time expected before an extreme event of a certain magnitude occurs again) and crucial effect modifiers, including climatic, socioeconomic, and urban environmental conditions.
The analysis included 109,954,744 deaths from all causes; 31,164,161 cardiovascular deaths; and 11,817,278 respiratory deaths. During the study period, from 1980 to 2020, a total of 50,913 rainfall events with a 1-year return period, 8362 events with a 2-year return period, and 3301 events with a 5-year return period were identified.
The most significant finding was a global positive association between all-cause mortality and extreme rainfall events with a 5-year return period. One day of extreme rainfall with a 5-year return period was associated with a cumulative relative risk (RRc) of 1.08 (95% CI, 1.05-1.11) for daily mortality from all causes. Rainfall events with a 2-year return period were associated with increased daily respiratory mortality (RRc, 1.14), while no significant effect was observed for cardiovascular mortality during the same period. Rainfall events with a 5-year return period were associated with an increased risk for both cardiovascular mortality (RRc, 1.05) and respiratory mortality (RRc, 1.29), with the respiratory mortality being significantly higher.
Points of Concern
According to the authors, moderate to high rainfall can exert protective effects through two main mechanisms: Improving air quality (rainfall can reduce the concentration of particulate matter 2.5 cm in diameter or less in the atmosphere) and behavioral changes in people (more time spent in enclosed environments, reducing direct exposure to outdoor air pollution and nonoptimal temperatures). As rainfall intensity increases, the initial protective effects may be overshadowed by a cascade of negative impacts including:
- Critical resource disruptions: Intense rainfall can cause severe disruptions to access to healthcare, infrastructure damage including power outages, and compromised water and food quality.
- Physiological effects: Increased humidity levels facilitate the growth of airborne pathogens, potentially triggering allergic reactions and respiratory issues, particularly in vulnerable individuals. Rapid shifts in atmospheric pressure and temperature fluctuations can lead to cardiovascular and respiratory complications.
- Indirect effects: Extreme rainfall can have profound effects on mental health, inducing stress and anxiety that may exacerbate pre-existing mental health conditions and indirectly contribute to increased overall mortality from nonexternal causes.
The intensity-response curves for the health effects of heavy rainfall showed a nonlinear trend, transitioning from a protective effect at moderate levels of rainfall to a risk for severe harm when rainfall intensity became extreme. Additionally, the significant effects of extreme events were modified by various types of climate and were more pronounced in areas characterized by low variability in precipitation or sparse vegetation cover.
The study demonstrated that various local factors, such as climatic conditions, climate type, and vegetation cover, can potentially influence cardiovascular and respiratory mortality and all-cause mortality related to precipitation. The findings may help physicians convey to their patients the impact of climate change on their health.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Myasthenia Gravis: Where Does Traditional Therapy Fit In?
SAVANNAH, GEORGIA —
In a debate at American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) 2024, a pair of neurologists who specialize in neuromuscular disorders laid out opposing evidence for each approach.
On one hand, Benjamin Claytor, MD, of Cleveland Clinic, Cleveland, argued that “traditional therapy is very effective for the majority of myasthenia gravis patients,” and he said it should be considered first-line.
But Amanda C. Guidon, MD, MPH, of Massachusetts General Hospital and Harvard Medical School, both in Boston, responded that “the immunosuppression of traditional therapies is too broad: The time to benefit is too long, the burden of side effects is too high, and the cancer risk is too elevated.”
Traditional Therapy: Affordable, Tolerable, and Safe?
Claytor said ideal myasthenia gravis therapies are effective, tolerable, and safe. They’re also affordable, convenient (such as a pill), lead to sustained remission, and can have dosages reduced.
Only traditional therapies — corticosteroids, azathioprine, mycophenolate, and rituximab — meet those last three criteria, he said. Newer therapies, he said, do not.
Claytor highlighted a 2023 Duke University study that tracked 367 patients with MG who were treated with traditional therapies after the year 2000. Of those, 72% reached the treatment goal of minimal manifestations in a median of less than 2 years.
In addition, Claytor noted that the percentage of patients with myasthenia gravis who reach minimal symptom expression ranges from 45% (6 months) to 60% or more (2 years), while studies suggest that newer treatments such as eculizumab (Soliris), efgartigimod (Vyvgart), rozanolixizumab (Rystiggo), and zilucoplan (Zilbrysq) haven’t reached those levels.
As for specific traditional therapies, Claytor said the corticosteroid prednisone is “extremely affordable,” effective, and takes fewer than 2 weeks to work. All patients with myasthenia gravis can take it, he said, and at least 75% of those with mild/moderate disease respond to low doses.
Nonsteroidal Agents, Immune Globulin, Rituximab
He acknowledged side effects from corticosteroids but said doses can be tapered once severity improves. Calcium and vitamin D can be helpful to support bone health, he added.
As for nonsteroidal immunosuppressive treatments, he said they’re easy to administer, increase the likelihood of reaching minimal manifestation status, can be effective at lower doses, and may allow patients to discontinue steroids.
Two other traditional therapies, immune globulin and plasmapheresis, can be appropriate in crisis or impending crisis situations, he said, or as an add-on therapy if steroids and nonsteroidal immunosuppressive therapies don’t work.
What about rituximab? “We’re learning that patients with new-onset disease and younger patients seem to respond better,” Claytor said. While rituximab is expensive, it’s “not even in the same realm” as newer agents if only a dose or two are given, he said.
Steroids Are Ideal in MG? Not So Fast
In her response, Guidon noted that she was assigned to offer a counter-perspective in her presentation, and “personal opinions are not being represented here fully.” She then listed the weaknesses of traditional therapy in myasthenia gravis.
For one thing, she said the drugs don’t work well. She highlighted a 2019 registry study that found “many myasthenia gravis patients remain negatively impacted despite treatment.”
In addition, “we can’t predict who will respond to which therapy. ... We start drugs and don’t know if we’ll have benefit from 6 months up to 18 months. We also can’t determine minimally effective dose a priori. Some patients require higher doses, and some subtherapeutic doses are actually therapeutic for our patients.”
Broad immunosuppression, she added, boosts the risk for serious infections. “We’ve all heard from our patients that the side effects can be worse than the myasthenia, and next we’re going to talk about the role of corticosteroids in myasthenia.”
As for corticosteroids in particular, “they’re really the best treatment and also the worst treatment.” Efficacy and side effects battle for supremacy in patients, she said, “and you don’t know which is going to win out.”
Kicking Traditional Therapy to the Curb
There are many possible side effects from steroids, she said, including steroid-induced diabetes, which is “profound.” Some patients never recover from it.
On top of all these risks, she said, 20%-30% of patients are resistant to steroids.
As for other treatments, immune globulin and plasmapheresis “aren’t really benign,” Guidon said. They come with potentially serious side effects of their own, as do nonsteroidal immunosuppressive treatments.
Guidon said better treatments are needed to minimize the risks from traditional therapies. “We need targeted therapies that drive disease into remission, can be tapered, are delivered orally or with infrequent self-injections, and don’t require frequent lab monitoring.”
In addition, ideal treatments should “have a good safety data in pregnancy and for breastfeeding and have a favorable side effect profile with no significant long-term cancer risks.”
Claytor had no disclosures. Guidon disclosed consulting/medical advisory board (Alexion Pharmaceuticals, argenx, Regeneron, and UCB), publishing royalties (Oakstone), and other research support (Myasthenia Gravis Foundation of America, Myasthenia Gravis Rare Disease Network, National Institutes of Health, and National Institute of Neurological Disorders and Stroke/BioSensics).
A version of this article appeared on Medscape.com.
SAVANNAH, GEORGIA —
In a debate at American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) 2024, a pair of neurologists who specialize in neuromuscular disorders laid out opposing evidence for each approach.
On one hand, Benjamin Claytor, MD, of Cleveland Clinic, Cleveland, argued that “traditional therapy is very effective for the majority of myasthenia gravis patients,” and he said it should be considered first-line.
But Amanda C. Guidon, MD, MPH, of Massachusetts General Hospital and Harvard Medical School, both in Boston, responded that “the immunosuppression of traditional therapies is too broad: The time to benefit is too long, the burden of side effects is too high, and the cancer risk is too elevated.”
Traditional Therapy: Affordable, Tolerable, and Safe?
Claytor said ideal myasthenia gravis therapies are effective, tolerable, and safe. They’re also affordable, convenient (such as a pill), lead to sustained remission, and can have dosages reduced.
Only traditional therapies — corticosteroids, azathioprine, mycophenolate, and rituximab — meet those last three criteria, he said. Newer therapies, he said, do not.
Claytor highlighted a 2023 Duke University study that tracked 367 patients with MG who were treated with traditional therapies after the year 2000. Of those, 72% reached the treatment goal of minimal manifestations in a median of less than 2 years.
In addition, Claytor noted that the percentage of patients with myasthenia gravis who reach minimal symptom expression ranges from 45% (6 months) to 60% or more (2 years), while studies suggest that newer treatments such as eculizumab (Soliris), efgartigimod (Vyvgart), rozanolixizumab (Rystiggo), and zilucoplan (Zilbrysq) haven’t reached those levels.
As for specific traditional therapies, Claytor said the corticosteroid prednisone is “extremely affordable,” effective, and takes fewer than 2 weeks to work. All patients with myasthenia gravis can take it, he said, and at least 75% of those with mild/moderate disease respond to low doses.
Nonsteroidal Agents, Immune Globulin, Rituximab
He acknowledged side effects from corticosteroids but said doses can be tapered once severity improves. Calcium and vitamin D can be helpful to support bone health, he added.
As for nonsteroidal immunosuppressive treatments, he said they’re easy to administer, increase the likelihood of reaching minimal manifestation status, can be effective at lower doses, and may allow patients to discontinue steroids.
Two other traditional therapies, immune globulin and plasmapheresis, can be appropriate in crisis or impending crisis situations, he said, or as an add-on therapy if steroids and nonsteroidal immunosuppressive therapies don’t work.
What about rituximab? “We’re learning that patients with new-onset disease and younger patients seem to respond better,” Claytor said. While rituximab is expensive, it’s “not even in the same realm” as newer agents if only a dose or two are given, he said.
Steroids Are Ideal in MG? Not So Fast
In her response, Guidon noted that she was assigned to offer a counter-perspective in her presentation, and “personal opinions are not being represented here fully.” She then listed the weaknesses of traditional therapy in myasthenia gravis.
For one thing, she said the drugs don’t work well. She highlighted a 2019 registry study that found “many myasthenia gravis patients remain negatively impacted despite treatment.”
In addition, “we can’t predict who will respond to which therapy. ... We start drugs and don’t know if we’ll have benefit from 6 months up to 18 months. We also can’t determine minimally effective dose a priori. Some patients require higher doses, and some subtherapeutic doses are actually therapeutic for our patients.”
Broad immunosuppression, she added, boosts the risk for serious infections. “We’ve all heard from our patients that the side effects can be worse than the myasthenia, and next we’re going to talk about the role of corticosteroids in myasthenia.”
As for corticosteroids in particular, “they’re really the best treatment and also the worst treatment.” Efficacy and side effects battle for supremacy in patients, she said, “and you don’t know which is going to win out.”
Kicking Traditional Therapy to the Curb
There are many possible side effects from steroids, she said, including steroid-induced diabetes, which is “profound.” Some patients never recover from it.
On top of all these risks, she said, 20%-30% of patients are resistant to steroids.
As for other treatments, immune globulin and plasmapheresis “aren’t really benign,” Guidon said. They come with potentially serious side effects of their own, as do nonsteroidal immunosuppressive treatments.
Guidon said better treatments are needed to minimize the risks from traditional therapies. “We need targeted therapies that drive disease into remission, can be tapered, are delivered orally or with infrequent self-injections, and don’t require frequent lab monitoring.”
In addition, ideal treatments should “have a good safety data in pregnancy and for breastfeeding and have a favorable side effect profile with no significant long-term cancer risks.”
Claytor had no disclosures. Guidon disclosed consulting/medical advisory board (Alexion Pharmaceuticals, argenx, Regeneron, and UCB), publishing royalties (Oakstone), and other research support (Myasthenia Gravis Foundation of America, Myasthenia Gravis Rare Disease Network, National Institutes of Health, and National Institute of Neurological Disorders and Stroke/BioSensics).
A version of this article appeared on Medscape.com.
SAVANNAH, GEORGIA —
In a debate at American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) 2024, a pair of neurologists who specialize in neuromuscular disorders laid out opposing evidence for each approach.
On one hand, Benjamin Claytor, MD, of Cleveland Clinic, Cleveland, argued that “traditional therapy is very effective for the majority of myasthenia gravis patients,” and he said it should be considered first-line.
But Amanda C. Guidon, MD, MPH, of Massachusetts General Hospital and Harvard Medical School, both in Boston, responded that “the immunosuppression of traditional therapies is too broad: The time to benefit is too long, the burden of side effects is too high, and the cancer risk is too elevated.”
Traditional Therapy: Affordable, Tolerable, and Safe?
Claytor said ideal myasthenia gravis therapies are effective, tolerable, and safe. They’re also affordable, convenient (such as a pill), lead to sustained remission, and can have dosages reduced.
Only traditional therapies — corticosteroids, azathioprine, mycophenolate, and rituximab — meet those last three criteria, he said. Newer therapies, he said, do not.
Claytor highlighted a 2023 Duke University study that tracked 367 patients with MG who were treated with traditional therapies after the year 2000. Of those, 72% reached the treatment goal of minimal manifestations in a median of less than 2 years.
In addition, Claytor noted that the percentage of patients with myasthenia gravis who reach minimal symptom expression ranges from 45% (6 months) to 60% or more (2 years), while studies suggest that newer treatments such as eculizumab (Soliris), efgartigimod (Vyvgart), rozanolixizumab (Rystiggo), and zilucoplan (Zilbrysq) haven’t reached those levels.
As for specific traditional therapies, Claytor said the corticosteroid prednisone is “extremely affordable,” effective, and takes fewer than 2 weeks to work. All patients with myasthenia gravis can take it, he said, and at least 75% of those with mild/moderate disease respond to low doses.
Nonsteroidal Agents, Immune Globulin, Rituximab
He acknowledged side effects from corticosteroids but said doses can be tapered once severity improves. Calcium and vitamin D can be helpful to support bone health, he added.
As for nonsteroidal immunosuppressive treatments, he said they’re easy to administer, increase the likelihood of reaching minimal manifestation status, can be effective at lower doses, and may allow patients to discontinue steroids.
Two other traditional therapies, immune globulin and plasmapheresis, can be appropriate in crisis or impending crisis situations, he said, or as an add-on therapy if steroids and nonsteroidal immunosuppressive therapies don’t work.
What about rituximab? “We’re learning that patients with new-onset disease and younger patients seem to respond better,” Claytor said. While rituximab is expensive, it’s “not even in the same realm” as newer agents if only a dose or two are given, he said.
Steroids Are Ideal in MG? Not So Fast
In her response, Guidon noted that she was assigned to offer a counter-perspective in her presentation, and “personal opinions are not being represented here fully.” She then listed the weaknesses of traditional therapy in myasthenia gravis.
For one thing, she said the drugs don’t work well. She highlighted a 2019 registry study that found “many myasthenia gravis patients remain negatively impacted despite treatment.”
In addition, “we can’t predict who will respond to which therapy. ... We start drugs and don’t know if we’ll have benefit from 6 months up to 18 months. We also can’t determine minimally effective dose a priori. Some patients require higher doses, and some subtherapeutic doses are actually therapeutic for our patients.”
Broad immunosuppression, she added, boosts the risk for serious infections. “We’ve all heard from our patients that the side effects can be worse than the myasthenia, and next we’re going to talk about the role of corticosteroids in myasthenia.”
As for corticosteroids in particular, “they’re really the best treatment and also the worst treatment.” Efficacy and side effects battle for supremacy in patients, she said, “and you don’t know which is going to win out.”
Kicking Traditional Therapy to the Curb
There are many possible side effects from steroids, she said, including steroid-induced diabetes, which is “profound.” Some patients never recover from it.
On top of all these risks, she said, 20%-30% of patients are resistant to steroids.
As for other treatments, immune globulin and plasmapheresis “aren’t really benign,” Guidon said. They come with potentially serious side effects of their own, as do nonsteroidal immunosuppressive treatments.
Guidon said better treatments are needed to minimize the risks from traditional therapies. “We need targeted therapies that drive disease into remission, can be tapered, are delivered orally or with infrequent self-injections, and don’t require frequent lab monitoring.”
In addition, ideal treatments should “have a good safety data in pregnancy and for breastfeeding and have a favorable side effect profile with no significant long-term cancer risks.”
Claytor had no disclosures. Guidon disclosed consulting/medical advisory board (Alexion Pharmaceuticals, argenx, Regeneron, and UCB), publishing royalties (Oakstone), and other research support (Myasthenia Gravis Foundation of America, Myasthenia Gravis Rare Disease Network, National Institutes of Health, and National Institute of Neurological Disorders and Stroke/BioSensics).
A version of this article appeared on Medscape.com.
FROM AANEM 2024
New Drug Options Abound for Duchenne Muscular Dystrophy
SAVANNAH, GEORGIA — When Ann & Robert H. Lurie Children’s Hospital of Chicago pediatric neurologist Nancy L. Kuntz, MD, was a fellow about 45 years ago, there were few more devastating diagnoses than Duchenne muscular dystrophy (DMD).
“The rule of thumb was that they would stop walking by age 10 and probably die around age 20, and there was not much we could do,” Kuntz told colleagues at the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) 2024.
Now, “In the last 8 years, we’ve seen eight different therapies that are FDA-approved specifically for Duchenne, and many more are in the pipeline,” said session moderator Kathryn Mosher, MD, a pediatric physical medicine and rehabilitation physician at Akron Children’s Hospital, Akron, Ohio.
This is both good news and a new challenge for clinicians: Which of these treatments are best for which patients? Kuntz said the traditional therapy of corticosteroids is still crucial. However, “there are still families begging to not use steroids, or refusing to use steroids, just not filling the prescriptions,” she said.
Beware of Parents Who Reject Steroids
The failure to use steroids “breaks your heart” because data show their impact on “really important functions like walking and being able to get up from the ground,” she said. “You can add months and years to life with this treatment.”
However, “while we have shown that using corticosteroids makes a difference, I don’t think that we’ve really worked out the best age at which to start the steroids, or the dosing schedule, or even the type of steroids,” she cautioned.
In an accompanying presentation about therapy for DMD, pediatric neurologist Craig M. Zaidman, MD, of Washington University in St. Louis, Missouri, cautioned that “daily steroids make a big impact on your growth and particularly on your height.”
In particular, the corticosteroid deflazacort has been linked to more cataracts than prednisone and less weight gain and height growth. “They really don’t grow, they don’t get taller, and they also don’t gain weight. They look like little boys when they’re 13 years old.”
Deflazacort or Vamorolone?
Vamorolone (Agamree) is a cheaper corticosteroid alternative to deflazacort (Emflaza), and a 2024 study showed no difference in functional outcomes over 48 weeks, he said. Also, daily vamorolone does a better job of preserving height growth than daily prednisone, he said, and he’s seen less risk for vertebral fractures.
Where do newer drugs fit in? One crucial thing to know about the new generation of targeted therapies is that they’re often mutation-dependent, Kuntz said. They may only work in patients with certain mutations, or mutations may lead to more side effects.
“You should have the exact mutation of your patient, and then you can look and see what they’re eligible for,” she said.
$700,000 a Year for Givinostat
Zaidman highlighted the newly approved givinostat (Duvyzat), a histone deacetylase inhibitor approved for boys 6 years or older. The cost is $700,000 a year, he said, and it’s been linked to less decline in four-stair climb per a double-blind, placebo-controlled, phase 3 trial.
The drug can cause side effects such as reducing platelets, boosting triglycerides, and inducing gastrointestinal problems. “When you drop the dose, these problems go away,” he said.
Does givinostat work? While trial data are challenging to interpret, they do suggest that patients “will lose skill, but they might not lose two or three skills they otherwise would have,” Zaidman said. “To me, that’s quite compelling.”
As for exon-skipping therapies, another new-generation option for DMD, he noted that “these drugs are on the market based on their accelerated approval. We will never have the perfect phase 3, randomized, controlled, long-term trial for these. It’s just not going to come. This is what we get.”
Mosher disclosed the advisory board (Sarepta Therapeutics, Pfizer, Reata Pharmaceuticals, and PTC). Kuntz disclosed advisory board (Astellas Pharma, Inc., argenx, Catalyst, Entrada Therapeutics, Genentech, and Novartis), exchange expert on-demand program (Sarepta Therapeutics), speaker (Genentech, Sarepta Therapeutics, and Solid), and research funding (Astellas Pharma, Inc., argenx, Biogen, Catalyst, Genentech, Novartis, and Sarepta Therapeutics). Zaidman disclosed speaking/advisor/consulting (Sarepta Therapeutics and Optum) and research funding (Novartis and Biogen).
A version of this article appeared on Medscape.com.
SAVANNAH, GEORGIA — When Ann & Robert H. Lurie Children’s Hospital of Chicago pediatric neurologist Nancy L. Kuntz, MD, was a fellow about 45 years ago, there were few more devastating diagnoses than Duchenne muscular dystrophy (DMD).
“The rule of thumb was that they would stop walking by age 10 and probably die around age 20, and there was not much we could do,” Kuntz told colleagues at the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) 2024.
Now, “In the last 8 years, we’ve seen eight different therapies that are FDA-approved specifically for Duchenne, and many more are in the pipeline,” said session moderator Kathryn Mosher, MD, a pediatric physical medicine and rehabilitation physician at Akron Children’s Hospital, Akron, Ohio.
This is both good news and a new challenge for clinicians: Which of these treatments are best for which patients? Kuntz said the traditional therapy of corticosteroids is still crucial. However, “there are still families begging to not use steroids, or refusing to use steroids, just not filling the prescriptions,” she said.
Beware of Parents Who Reject Steroids
The failure to use steroids “breaks your heart” because data show their impact on “really important functions like walking and being able to get up from the ground,” she said. “You can add months and years to life with this treatment.”
However, “while we have shown that using corticosteroids makes a difference, I don’t think that we’ve really worked out the best age at which to start the steroids, or the dosing schedule, or even the type of steroids,” she cautioned.
In an accompanying presentation about therapy for DMD, pediatric neurologist Craig M. Zaidman, MD, of Washington University in St. Louis, Missouri, cautioned that “daily steroids make a big impact on your growth and particularly on your height.”
In particular, the corticosteroid deflazacort has been linked to more cataracts than prednisone and less weight gain and height growth. “They really don’t grow, they don’t get taller, and they also don’t gain weight. They look like little boys when they’re 13 years old.”
Deflazacort or Vamorolone?
Vamorolone (Agamree) is a cheaper corticosteroid alternative to deflazacort (Emflaza), and a 2024 study showed no difference in functional outcomes over 48 weeks, he said. Also, daily vamorolone does a better job of preserving height growth than daily prednisone, he said, and he’s seen less risk for vertebral fractures.
Where do newer drugs fit in? One crucial thing to know about the new generation of targeted therapies is that they’re often mutation-dependent, Kuntz said. They may only work in patients with certain mutations, or mutations may lead to more side effects.
“You should have the exact mutation of your patient, and then you can look and see what they’re eligible for,” she said.
$700,000 a Year for Givinostat
Zaidman highlighted the newly approved givinostat (Duvyzat), a histone deacetylase inhibitor approved for boys 6 years or older. The cost is $700,000 a year, he said, and it’s been linked to less decline in four-stair climb per a double-blind, placebo-controlled, phase 3 trial.
The drug can cause side effects such as reducing platelets, boosting triglycerides, and inducing gastrointestinal problems. “When you drop the dose, these problems go away,” he said.
Does givinostat work? While trial data are challenging to interpret, they do suggest that patients “will lose skill, but they might not lose two or three skills they otherwise would have,” Zaidman said. “To me, that’s quite compelling.”
As for exon-skipping therapies, another new-generation option for DMD, he noted that “these drugs are on the market based on their accelerated approval. We will never have the perfect phase 3, randomized, controlled, long-term trial for these. It’s just not going to come. This is what we get.”
Mosher disclosed the advisory board (Sarepta Therapeutics, Pfizer, Reata Pharmaceuticals, and PTC). Kuntz disclosed advisory board (Astellas Pharma, Inc., argenx, Catalyst, Entrada Therapeutics, Genentech, and Novartis), exchange expert on-demand program (Sarepta Therapeutics), speaker (Genentech, Sarepta Therapeutics, and Solid), and research funding (Astellas Pharma, Inc., argenx, Biogen, Catalyst, Genentech, Novartis, and Sarepta Therapeutics). Zaidman disclosed speaking/advisor/consulting (Sarepta Therapeutics and Optum) and research funding (Novartis and Biogen).
A version of this article appeared on Medscape.com.
SAVANNAH, GEORGIA — When Ann & Robert H. Lurie Children’s Hospital of Chicago pediatric neurologist Nancy L. Kuntz, MD, was a fellow about 45 years ago, there were few more devastating diagnoses than Duchenne muscular dystrophy (DMD).
“The rule of thumb was that they would stop walking by age 10 and probably die around age 20, and there was not much we could do,” Kuntz told colleagues at the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) 2024.
Now, “In the last 8 years, we’ve seen eight different therapies that are FDA-approved specifically for Duchenne, and many more are in the pipeline,” said session moderator Kathryn Mosher, MD, a pediatric physical medicine and rehabilitation physician at Akron Children’s Hospital, Akron, Ohio.
This is both good news and a new challenge for clinicians: Which of these treatments are best for which patients? Kuntz said the traditional therapy of corticosteroids is still crucial. However, “there are still families begging to not use steroids, or refusing to use steroids, just not filling the prescriptions,” she said.
Beware of Parents Who Reject Steroids
The failure to use steroids “breaks your heart” because data show their impact on “really important functions like walking and being able to get up from the ground,” she said. “You can add months and years to life with this treatment.”
However, “while we have shown that using corticosteroids makes a difference, I don’t think that we’ve really worked out the best age at which to start the steroids, or the dosing schedule, or even the type of steroids,” she cautioned.
In an accompanying presentation about therapy for DMD, pediatric neurologist Craig M. Zaidman, MD, of Washington University in St. Louis, Missouri, cautioned that “daily steroids make a big impact on your growth and particularly on your height.”
In particular, the corticosteroid deflazacort has been linked to more cataracts than prednisone and less weight gain and height growth. “They really don’t grow, they don’t get taller, and they also don’t gain weight. They look like little boys when they’re 13 years old.”
Deflazacort or Vamorolone?
Vamorolone (Agamree) is a cheaper corticosteroid alternative to deflazacort (Emflaza), and a 2024 study showed no difference in functional outcomes over 48 weeks, he said. Also, daily vamorolone does a better job of preserving height growth than daily prednisone, he said, and he’s seen less risk for vertebral fractures.
Where do newer drugs fit in? One crucial thing to know about the new generation of targeted therapies is that they’re often mutation-dependent, Kuntz said. They may only work in patients with certain mutations, or mutations may lead to more side effects.
“You should have the exact mutation of your patient, and then you can look and see what they’re eligible for,” she said.
$700,000 a Year for Givinostat
Zaidman highlighted the newly approved givinostat (Duvyzat), a histone deacetylase inhibitor approved for boys 6 years or older. The cost is $700,000 a year, he said, and it’s been linked to less decline in four-stair climb per a double-blind, placebo-controlled, phase 3 trial.
The drug can cause side effects such as reducing platelets, boosting triglycerides, and inducing gastrointestinal problems. “When you drop the dose, these problems go away,” he said.
Does givinostat work? While trial data are challenging to interpret, they do suggest that patients “will lose skill, but they might not lose two or three skills they otherwise would have,” Zaidman said. “To me, that’s quite compelling.”
As for exon-skipping therapies, another new-generation option for DMD, he noted that “these drugs are on the market based on their accelerated approval. We will never have the perfect phase 3, randomized, controlled, long-term trial for these. It’s just not going to come. This is what we get.”
Mosher disclosed the advisory board (Sarepta Therapeutics, Pfizer, Reata Pharmaceuticals, and PTC). Kuntz disclosed advisory board (Astellas Pharma, Inc., argenx, Catalyst, Entrada Therapeutics, Genentech, and Novartis), exchange expert on-demand program (Sarepta Therapeutics), speaker (Genentech, Sarepta Therapeutics, and Solid), and research funding (Astellas Pharma, Inc., argenx, Biogen, Catalyst, Genentech, Novartis, and Sarepta Therapeutics). Zaidman disclosed speaking/advisor/consulting (Sarepta Therapeutics and Optum) and research funding (Novartis and Biogen).
A version of this article appeared on Medscape.com.
FROM AANEM 2024
Evaluating Use of Empagliflozin for Diabetes Management in Veterans With Chronic Kidney Disease
More than 37 million Americans have diabetes mellitus (DM), and approximately 90% have type 2 DM (T2DM), including about 25% of veterans.1,2 The current guidelines suggest that therapy depends on a patient's comorbidities, management needs, and patient-centered treatment factors.3 About 1 in 3 adults with DM have chronic kidney disease (CKD), defined as the presence of kidney damage or an estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2, persisting for ≥ 3 months.4
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are a class of antihyperglycemic agents acting on the SGLT-2 proteins expressed in the renal proximal convoluted tubules. They exert their effects by preventing the reabsorption of filtered glucose from the tubular lumen. There are 4 SGLT-2 inhibitors approved by the US Food and Drug Administration: canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. Empagliflozin is currently the preferred SGLT-2 inhibitor on the US Department of Veterans Affairs (VA) formulary.
According to the American Diabetes Association guidelines, empagliflozin is considered when an individual has or is at risk for atherosclerotic cardiovascular disease, heart failure, and CKD.3 SGLT-2 inhibitors are a favorable option due to their low risk for hypoglycemia while also promoting weight loss. The EMPEROR-Reduced trial demonstrated that, in addition to benefits for patients with heart failure, empagliflozin also slowed the progressive decline in kidney function in those with and without DM.5 The purpose of this study was to evaluate the effectiveness of empagliflozin on hemoglobin A1c (HbA1c) levels in patients with CKD at the Hershel “Woody” Williams VA Medical Center (HWWVAMC) in Huntington, West Virginia, along with other laboratory test markers.
Methods
The Marshall University Institutional Review Board #1 (Medical) and the HWWVAMC institutional review board and research and development committee each reviewed and approved this study. A retrospective chart review was conducted on patients diagnosed with T2DM and stage 3 CKD who were prescribed empagliflozin for DM management between January 1, 2015, and October 1, 2022, yielding 1771 patients. Data were obtained through the VHA Corporate Data Warehouse (CDW) and stored on the VA Informatics and Computing Infrastructure (VINCI) research server.
Patients were included if they were aged 18 to 89 years, prescribed empagliflozin by a VA clinician for the treatment of T2DM, had an eGFR between 30 and 59 mL/min/1.73 m2, and had an initial HbA1c between 7% and 10%. Using further random sampling, patients were either excluded or divided into, those with stage 3a CKD and those with stage 3b CKD. The primary endpoint of this study was the change in HbA1c levels in patients with stage 3b CKD (eGFR 30-44 mL/min/1.73 m2) compared with stage 3a (eGFR 45-59 mL/min/1.73 m2) after 12 months. The secondary endpoints included effects on renal function, weight, blood pressure, incidence of adverse drug events, and cardiovascular events. Of the excluded, 38 had HbA1c < 7%, 30 had HbA1c ≥ 10%, 21 did not have data at 1-year mark, 15 had the medication discontinued due to decline in renal function, 14 discontinued their medication without documented reason, 10 discontinued their medication due to adverse drug reactions (ADRs), 12 had eGFR > 60 mL/ min/1.73 m2, 9 died within 1 year of initiation, 4 had eGFR < 30 mL/min/1.73 m2, 1 had no baseline eGFR, and 1 was the spouse of a veteran.
Statistical Analysis
All statistical analyses were performed using STATA v.15. We used t tests to examine changes within each group, along with paired t tests to compare the 2 groups. Two-sample t tests were used to analyze the continuous data at both the primary and secondary endpoints.
Results
Of the 1771 patients included in the initial data set, a randomized sample of 255 charts were reviewed, 155 were excluded, and 100 were included. Fifty patients, had stage 3a CKD and 50 had stage 3b CKD. Baseline demographics were similar between the stage 3a and 3b groups (Table 1). Both groups were predominantly White and male, with mean age > 70 years.
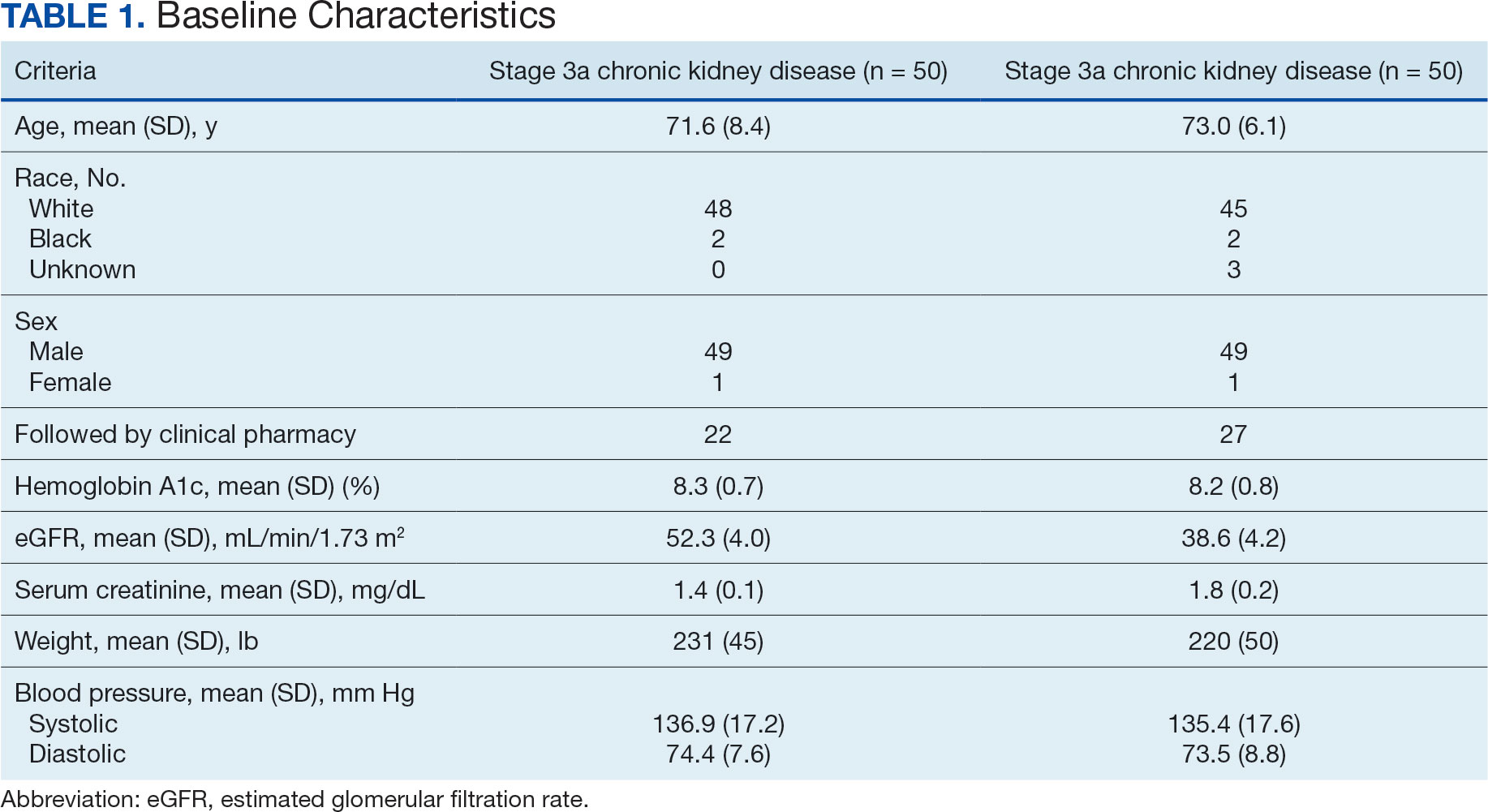
The primary endpoint was the differences in HbA1c levels over time and between groups for patients with stage 3a and stage 3b CKD 1 year after initiation of empagliflozin. The starting doses of empagliflozin were either 12.5 mg or 25.0 mg. For both groups, the changes in HbA1c levels were statistically significant (Table 2). HbA1c levels dropped 0.65% for the stage 3a group and 0.48% for the 3b group. When compared to one another, the results were not statistically significant (P = .51).
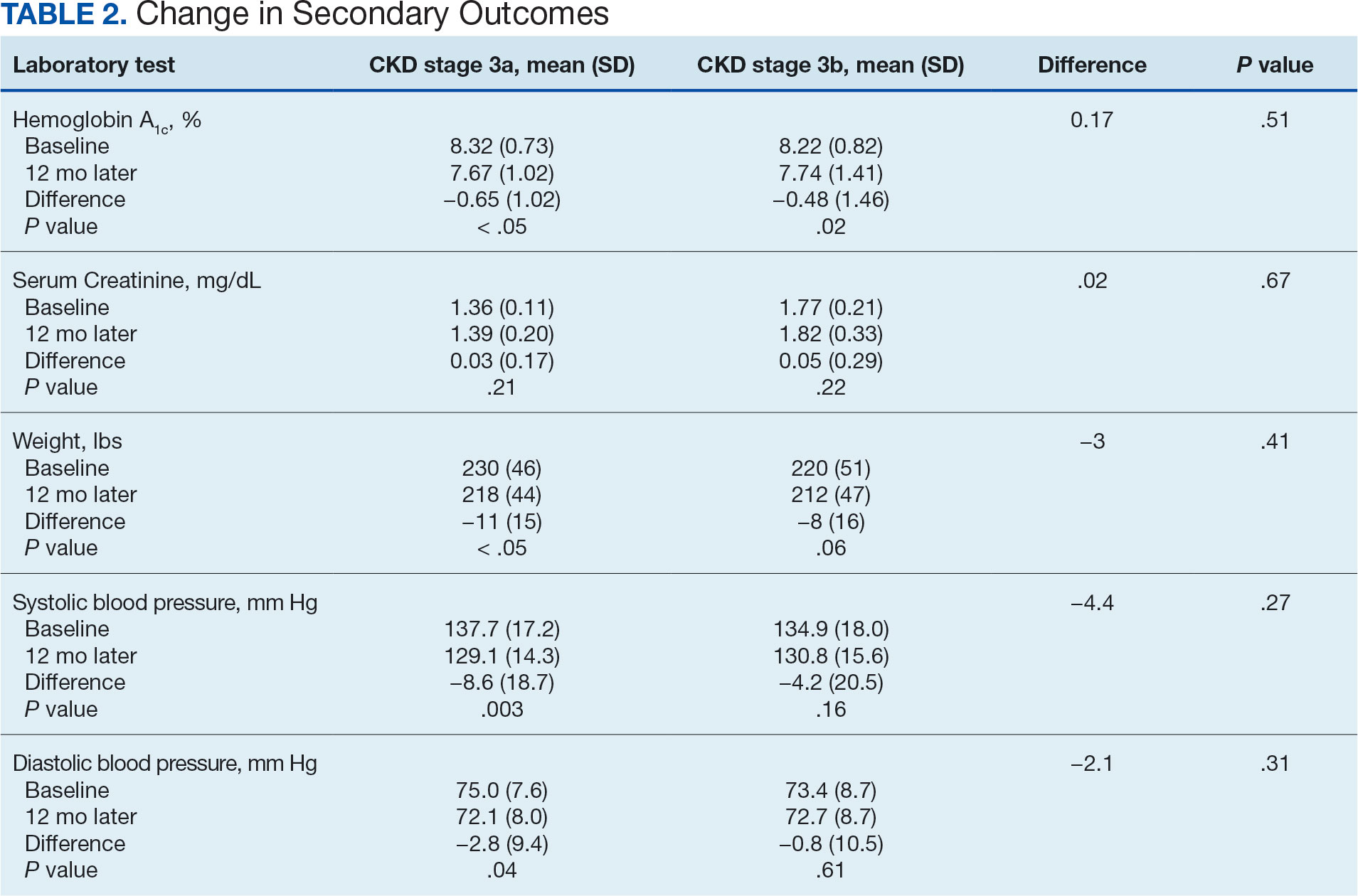
Secondary Endpoint
There was no statistically significant difference in serum creatinine levels within each group between baselines and 1 year later for the stage 3a (P = .21) and stage 3b (P = .22) groups, or when compared to each other (P = .67). There were statistically significant changes in weight for patients in the stage 3a group (P < .05), but not for stage 3b group (P = .06) or when compared to each other (P = .41). A statistically significant change in systolic blood pressure was observed for the stage 3a group (P = .003), but not the stage 3b group (P = .16) or when compared to each other (P = .27). There were statistically significant changes in diastolic blood pressure within the stage 3a group (P = .04), but not within the stage 3b group (P = .61) or when compared to each other (P = .31).
Ten patients discontinued empagliflozin before the 1-year mark due to ADRs, including dizziness, increased incidence of urinary tract infections, rash, and tachycardia (Table 3). Additionally, 3 ADRs resulted in the empagliflozin discontinuation after 1 year (Table 3).
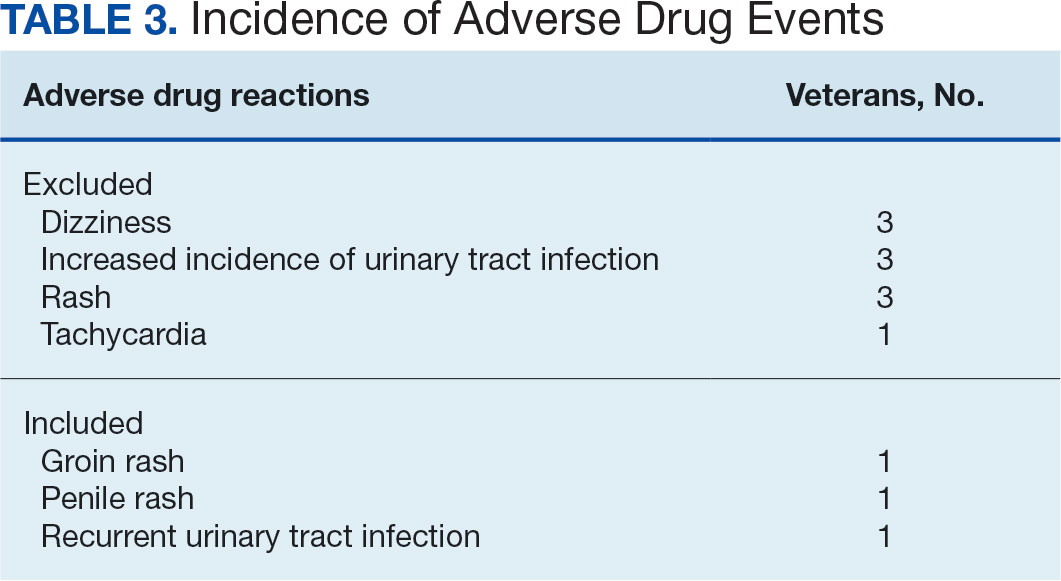
Discussion
This study showed a statistically significant change in HbA1c levels for patients with stage 3a and stage 3b CKD. With eGFR levels in these 2 groups > 30 mL/min/1.73 m2, patients were able to achieve glycemic benefits. There were no significant changes to the serum creatinine levels. Both groups saw statistically significant changes in weight loss within their own group; however, there were no statistically significant changes when compared to each other. With both systolic and diastolic blood pressure, the stage 3a group had statistically significant changes.
The EMPA-REG BP study demonstrated that empagliflozin was associated with significant and clinically meaningful reductions in blood pressure and HbA1c levels compared with placebo and was well tolerated in patients with T2DM and hypertension.6,7,8
Limitations
This study had a retrospective study design, which resulted in missing information for many patients and higher rates of exclusion. The population was predominantly older, White, and male and may not reflect other populations. The starting doses of empagliflozin varied between the groups. The VA employs tablet splitting for some patients, and the available doses were either 10.0 mg, 12.5 mg, or 25.0 mg. Some prescribers start veterans at lower doses and gradually increase to the higher dose of 25.0 mg, adding to the variability in starting doses.
Patients with eGFR < 30 mL/min/1.73 m2 make it difficult to determine any potential benefit in this population. The EMPA-KIDNEY trial demonstrated that the benefits of empagliflozin treatment were consistent among patients with or without DM and regardless of eGFR at randomization.9 Furthermore, many veterans had an initial HbA1c levels outside the inclusion criteria range, which was a factor in the smaller sample size.
Conclusions
While the reduction in HbA1c levels was less in patients with stage 3b CKD compared to patients stage 3a CKD, all patients experienced a benefit. The overall incidence of ADRs was low in the study population, showing empagliflozin as a favorable choice for those with T2DM and CKD. Based on the findings of this study, empagliflozin is a potentially beneficial option for reducing HbA1c levels in patients with CKD.
- Centers for Disease Control and Prevention. Type 2 diabetes. Updated May 25, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/about/about-type-2-diabetes.html?CDC_AAref_Val
- US Department of Veterans Affairs, VA research on diabetes. Updated September 2019. Accessed September 27, 2024. https://www.research.va.gov/pubs/docs/va_factsheets/Diabetes.pdf
- American Diabetes Association. Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin Diabetes. 2022;40(1):10-38. doi:10.2337/cd22-as01
- Centers for Disease Control and Prevention. Diabetes, chronic kidney disease. Updated May 15, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/diabetes-complications/diabetes-and-chronic-kidney-disease.html
- Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383(15):1413-1424. doi:10.1056/NEJMoa2022190
- Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428. doi:10.2337/dc14-1096
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/NEJMoa1504720
- Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180-1193. doi:10.1111/dom.12572
- The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, et al. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388(2):117-127. doi:10.1056/NEJMoa2204233
More than 37 million Americans have diabetes mellitus (DM), and approximately 90% have type 2 DM (T2DM), including about 25% of veterans.1,2 The current guidelines suggest that therapy depends on a patient's comorbidities, management needs, and patient-centered treatment factors.3 About 1 in 3 adults with DM have chronic kidney disease (CKD), defined as the presence of kidney damage or an estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2, persisting for ≥ 3 months.4
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are a class of antihyperglycemic agents acting on the SGLT-2 proteins expressed in the renal proximal convoluted tubules. They exert their effects by preventing the reabsorption of filtered glucose from the tubular lumen. There are 4 SGLT-2 inhibitors approved by the US Food and Drug Administration: canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. Empagliflozin is currently the preferred SGLT-2 inhibitor on the US Department of Veterans Affairs (VA) formulary.
According to the American Diabetes Association guidelines, empagliflozin is considered when an individual has or is at risk for atherosclerotic cardiovascular disease, heart failure, and CKD.3 SGLT-2 inhibitors are a favorable option due to their low risk for hypoglycemia while also promoting weight loss. The EMPEROR-Reduced trial demonstrated that, in addition to benefits for patients with heart failure, empagliflozin also slowed the progressive decline in kidney function in those with and without DM.5 The purpose of this study was to evaluate the effectiveness of empagliflozin on hemoglobin A1c (HbA1c) levels in patients with CKD at the Hershel “Woody” Williams VA Medical Center (HWWVAMC) in Huntington, West Virginia, along with other laboratory test markers.
Methods
The Marshall University Institutional Review Board #1 (Medical) and the HWWVAMC institutional review board and research and development committee each reviewed and approved this study. A retrospective chart review was conducted on patients diagnosed with T2DM and stage 3 CKD who were prescribed empagliflozin for DM management between January 1, 2015, and October 1, 2022, yielding 1771 patients. Data were obtained through the VHA Corporate Data Warehouse (CDW) and stored on the VA Informatics and Computing Infrastructure (VINCI) research server.
Patients were included if they were aged 18 to 89 years, prescribed empagliflozin by a VA clinician for the treatment of T2DM, had an eGFR between 30 and 59 mL/min/1.73 m2, and had an initial HbA1c between 7% and 10%. Using further random sampling, patients were either excluded or divided into, those with stage 3a CKD and those with stage 3b CKD. The primary endpoint of this study was the change in HbA1c levels in patients with stage 3b CKD (eGFR 30-44 mL/min/1.73 m2) compared with stage 3a (eGFR 45-59 mL/min/1.73 m2) after 12 months. The secondary endpoints included effects on renal function, weight, blood pressure, incidence of adverse drug events, and cardiovascular events. Of the excluded, 38 had HbA1c < 7%, 30 had HbA1c ≥ 10%, 21 did not have data at 1-year mark, 15 had the medication discontinued due to decline in renal function, 14 discontinued their medication without documented reason, 10 discontinued their medication due to adverse drug reactions (ADRs), 12 had eGFR > 60 mL/ min/1.73 m2, 9 died within 1 year of initiation, 4 had eGFR < 30 mL/min/1.73 m2, 1 had no baseline eGFR, and 1 was the spouse of a veteran.
Statistical Analysis
All statistical analyses were performed using STATA v.15. We used t tests to examine changes within each group, along with paired t tests to compare the 2 groups. Two-sample t tests were used to analyze the continuous data at both the primary and secondary endpoints.
Results
Of the 1771 patients included in the initial data set, a randomized sample of 255 charts were reviewed, 155 were excluded, and 100 were included. Fifty patients, had stage 3a CKD and 50 had stage 3b CKD. Baseline demographics were similar between the stage 3a and 3b groups (Table 1). Both groups were predominantly White and male, with mean age > 70 years.

The primary endpoint was the differences in HbA1c levels over time and between groups for patients with stage 3a and stage 3b CKD 1 year after initiation of empagliflozin. The starting doses of empagliflozin were either 12.5 mg or 25.0 mg. For both groups, the changes in HbA1c levels were statistically significant (Table 2). HbA1c levels dropped 0.65% for the stage 3a group and 0.48% for the 3b group. When compared to one another, the results were not statistically significant (P = .51).

Secondary Endpoint
There was no statistically significant difference in serum creatinine levels within each group between baselines and 1 year later for the stage 3a (P = .21) and stage 3b (P = .22) groups, or when compared to each other (P = .67). There were statistically significant changes in weight for patients in the stage 3a group (P < .05), but not for stage 3b group (P = .06) or when compared to each other (P = .41). A statistically significant change in systolic blood pressure was observed for the stage 3a group (P = .003), but not the stage 3b group (P = .16) or when compared to each other (P = .27). There were statistically significant changes in diastolic blood pressure within the stage 3a group (P = .04), but not within the stage 3b group (P = .61) or when compared to each other (P = .31).
Ten patients discontinued empagliflozin before the 1-year mark due to ADRs, including dizziness, increased incidence of urinary tract infections, rash, and tachycardia (Table 3). Additionally, 3 ADRs resulted in the empagliflozin discontinuation after 1 year (Table 3).

Discussion
This study showed a statistically significant change in HbA1c levels for patients with stage 3a and stage 3b CKD. With eGFR levels in these 2 groups > 30 mL/min/1.73 m2, patients were able to achieve glycemic benefits. There were no significant changes to the serum creatinine levels. Both groups saw statistically significant changes in weight loss within their own group; however, there were no statistically significant changes when compared to each other. With both systolic and diastolic blood pressure, the stage 3a group had statistically significant changes.
The EMPA-REG BP study demonstrated that empagliflozin was associated with significant and clinically meaningful reductions in blood pressure and HbA1c levels compared with placebo and was well tolerated in patients with T2DM and hypertension.6,7,8
Limitations
This study had a retrospective study design, which resulted in missing information for many patients and higher rates of exclusion. The population was predominantly older, White, and male and may not reflect other populations. The starting doses of empagliflozin varied between the groups. The VA employs tablet splitting for some patients, and the available doses were either 10.0 mg, 12.5 mg, or 25.0 mg. Some prescribers start veterans at lower doses and gradually increase to the higher dose of 25.0 mg, adding to the variability in starting doses.
Patients with eGFR < 30 mL/min/1.73 m2 make it difficult to determine any potential benefit in this population. The EMPA-KIDNEY trial demonstrated that the benefits of empagliflozin treatment were consistent among patients with or without DM and regardless of eGFR at randomization.9 Furthermore, many veterans had an initial HbA1c levels outside the inclusion criteria range, which was a factor in the smaller sample size.
Conclusions
While the reduction in HbA1c levels was less in patients with stage 3b CKD compared to patients stage 3a CKD, all patients experienced a benefit. The overall incidence of ADRs was low in the study population, showing empagliflozin as a favorable choice for those with T2DM and CKD. Based on the findings of this study, empagliflozin is a potentially beneficial option for reducing HbA1c levels in patients with CKD.
More than 37 million Americans have diabetes mellitus (DM), and approximately 90% have type 2 DM (T2DM), including about 25% of veterans.1,2 The current guidelines suggest that therapy depends on a patient's comorbidities, management needs, and patient-centered treatment factors.3 About 1 in 3 adults with DM have chronic kidney disease (CKD), defined as the presence of kidney damage or an estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2, persisting for ≥ 3 months.4
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are a class of antihyperglycemic agents acting on the SGLT-2 proteins expressed in the renal proximal convoluted tubules. They exert their effects by preventing the reabsorption of filtered glucose from the tubular lumen. There are 4 SGLT-2 inhibitors approved by the US Food and Drug Administration: canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. Empagliflozin is currently the preferred SGLT-2 inhibitor on the US Department of Veterans Affairs (VA) formulary.
According to the American Diabetes Association guidelines, empagliflozin is considered when an individual has or is at risk for atherosclerotic cardiovascular disease, heart failure, and CKD.3 SGLT-2 inhibitors are a favorable option due to their low risk for hypoglycemia while also promoting weight loss. The EMPEROR-Reduced trial demonstrated that, in addition to benefits for patients with heart failure, empagliflozin also slowed the progressive decline in kidney function in those with and without DM.5 The purpose of this study was to evaluate the effectiveness of empagliflozin on hemoglobin A1c (HbA1c) levels in patients with CKD at the Hershel “Woody” Williams VA Medical Center (HWWVAMC) in Huntington, West Virginia, along with other laboratory test markers.
Methods
The Marshall University Institutional Review Board #1 (Medical) and the HWWVAMC institutional review board and research and development committee each reviewed and approved this study. A retrospective chart review was conducted on patients diagnosed with T2DM and stage 3 CKD who were prescribed empagliflozin for DM management between January 1, 2015, and October 1, 2022, yielding 1771 patients. Data were obtained through the VHA Corporate Data Warehouse (CDW) and stored on the VA Informatics and Computing Infrastructure (VINCI) research server.
Patients were included if they were aged 18 to 89 years, prescribed empagliflozin by a VA clinician for the treatment of T2DM, had an eGFR between 30 and 59 mL/min/1.73 m2, and had an initial HbA1c between 7% and 10%. Using further random sampling, patients were either excluded or divided into, those with stage 3a CKD and those with stage 3b CKD. The primary endpoint of this study was the change in HbA1c levels in patients with stage 3b CKD (eGFR 30-44 mL/min/1.73 m2) compared with stage 3a (eGFR 45-59 mL/min/1.73 m2) after 12 months. The secondary endpoints included effects on renal function, weight, blood pressure, incidence of adverse drug events, and cardiovascular events. Of the excluded, 38 had HbA1c < 7%, 30 had HbA1c ≥ 10%, 21 did not have data at 1-year mark, 15 had the medication discontinued due to decline in renal function, 14 discontinued their medication without documented reason, 10 discontinued their medication due to adverse drug reactions (ADRs), 12 had eGFR > 60 mL/ min/1.73 m2, 9 died within 1 year of initiation, 4 had eGFR < 30 mL/min/1.73 m2, 1 had no baseline eGFR, and 1 was the spouse of a veteran.
Statistical Analysis
All statistical analyses were performed using STATA v.15. We used t tests to examine changes within each group, along with paired t tests to compare the 2 groups. Two-sample t tests were used to analyze the continuous data at both the primary and secondary endpoints.
Results
Of the 1771 patients included in the initial data set, a randomized sample of 255 charts were reviewed, 155 were excluded, and 100 were included. Fifty patients, had stage 3a CKD and 50 had stage 3b CKD. Baseline demographics were similar between the stage 3a and 3b groups (Table 1). Both groups were predominantly White and male, with mean age > 70 years.

The primary endpoint was the differences in HbA1c levels over time and between groups for patients with stage 3a and stage 3b CKD 1 year after initiation of empagliflozin. The starting doses of empagliflozin were either 12.5 mg or 25.0 mg. For both groups, the changes in HbA1c levels were statistically significant (Table 2). HbA1c levels dropped 0.65% for the stage 3a group and 0.48% for the 3b group. When compared to one another, the results were not statistically significant (P = .51).

Secondary Endpoint
There was no statistically significant difference in serum creatinine levels within each group between baselines and 1 year later for the stage 3a (P = .21) and stage 3b (P = .22) groups, or when compared to each other (P = .67). There were statistically significant changes in weight for patients in the stage 3a group (P < .05), but not for stage 3b group (P = .06) or when compared to each other (P = .41). A statistically significant change in systolic blood pressure was observed for the stage 3a group (P = .003), but not the stage 3b group (P = .16) or when compared to each other (P = .27). There were statistically significant changes in diastolic blood pressure within the stage 3a group (P = .04), but not within the stage 3b group (P = .61) or when compared to each other (P = .31).
Ten patients discontinued empagliflozin before the 1-year mark due to ADRs, including dizziness, increased incidence of urinary tract infections, rash, and tachycardia (Table 3). Additionally, 3 ADRs resulted in the empagliflozin discontinuation after 1 year (Table 3).

Discussion
This study showed a statistically significant change in HbA1c levels for patients with stage 3a and stage 3b CKD. With eGFR levels in these 2 groups > 30 mL/min/1.73 m2, patients were able to achieve glycemic benefits. There were no significant changes to the serum creatinine levels. Both groups saw statistically significant changes in weight loss within their own group; however, there were no statistically significant changes when compared to each other. With both systolic and diastolic blood pressure, the stage 3a group had statistically significant changes.
The EMPA-REG BP study demonstrated that empagliflozin was associated with significant and clinically meaningful reductions in blood pressure and HbA1c levels compared with placebo and was well tolerated in patients with T2DM and hypertension.6,7,8
Limitations
This study had a retrospective study design, which resulted in missing information for many patients and higher rates of exclusion. The population was predominantly older, White, and male and may not reflect other populations. The starting doses of empagliflozin varied between the groups. The VA employs tablet splitting for some patients, and the available doses were either 10.0 mg, 12.5 mg, or 25.0 mg. Some prescribers start veterans at lower doses and gradually increase to the higher dose of 25.0 mg, adding to the variability in starting doses.
Patients with eGFR < 30 mL/min/1.73 m2 make it difficult to determine any potential benefit in this population. The EMPA-KIDNEY trial demonstrated that the benefits of empagliflozin treatment were consistent among patients with or without DM and regardless of eGFR at randomization.9 Furthermore, many veterans had an initial HbA1c levels outside the inclusion criteria range, which was a factor in the smaller sample size.
Conclusions
While the reduction in HbA1c levels was less in patients with stage 3b CKD compared to patients stage 3a CKD, all patients experienced a benefit. The overall incidence of ADRs was low in the study population, showing empagliflozin as a favorable choice for those with T2DM and CKD. Based on the findings of this study, empagliflozin is a potentially beneficial option for reducing HbA1c levels in patients with CKD.
- Centers for Disease Control and Prevention. Type 2 diabetes. Updated May 25, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/about/about-type-2-diabetes.html?CDC_AAref_Val
- US Department of Veterans Affairs, VA research on diabetes. Updated September 2019. Accessed September 27, 2024. https://www.research.va.gov/pubs/docs/va_factsheets/Diabetes.pdf
- American Diabetes Association. Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin Diabetes. 2022;40(1):10-38. doi:10.2337/cd22-as01
- Centers for Disease Control and Prevention. Diabetes, chronic kidney disease. Updated May 15, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/diabetes-complications/diabetes-and-chronic-kidney-disease.html
- Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383(15):1413-1424. doi:10.1056/NEJMoa2022190
- Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428. doi:10.2337/dc14-1096
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/NEJMoa1504720
- Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180-1193. doi:10.1111/dom.12572
- The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, et al. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388(2):117-127. doi:10.1056/NEJMoa2204233
- Centers for Disease Control and Prevention. Type 2 diabetes. Updated May 25, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/about/about-type-2-diabetes.html?CDC_AAref_Val
- US Department of Veterans Affairs, VA research on diabetes. Updated September 2019. Accessed September 27, 2024. https://www.research.va.gov/pubs/docs/va_factsheets/Diabetes.pdf
- American Diabetes Association. Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin Diabetes. 2022;40(1):10-38. doi:10.2337/cd22-as01
- Centers for Disease Control and Prevention. Diabetes, chronic kidney disease. Updated May 15, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/diabetes-complications/diabetes-and-chronic-kidney-disease.html
- Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383(15):1413-1424. doi:10.1056/NEJMoa2022190
- Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428. doi:10.2337/dc14-1096
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/NEJMoa1504720
- Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180-1193. doi:10.1111/dom.12572
- The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, et al. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388(2):117-127. doi:10.1056/NEJMoa2204233
America’s PCPs: Take a Bow
Hi, everyone. I’m Dr. Kenny Lin. I am a family physician and associate director of the Lancaster General Hospital Family Medicine Residency, and I blog at Common Sense Family Doctor.
For the past 4 years, primary care clinicians have labored under a seemingly endless onslaught of bad news. A recent report estimated that there were over 1.3 million excess deaths in the United States from March 2020 to May 2023, including nearly half a million Americans younger than age 65. Social isolation and an ailing economy accelerated preexisting rises in drug overdoses and obesity, while teenage vaping threatened to hook a new generation on tobacco products even as adult smoking plummeted. Meanwhile, more than half of the nation’s physicians now report feelings of burnout, pay for family doctors appears to be stagnating, and our interactions with an increasing number of patients are fraught with suspicions about the value of vaccines— not just against COVID-19 but against flu and other viruses, too — and the medical system as a whole, doctors included.
Now, for the good news.
A year and a half since the end of the pandemic emergency, we are seeing gains on several fronts, and physicians deserve much of the credit. Preliminary data from the Centers for Disease Control and Prevention show that 10,000 fewer people died from drug overdoses than in the previous year. Although multiple factors contributed to this change, the elimination of the X-waiver, which had previously been required for physicians to prescribe buprenorphine for opioid use disorder, in January 2023 has improved access to medications for addiction treatment. In addition, the expansion of state requirements to check prescription drug monitoring programs when opioids or benzodiazepines are prescribed, and to prescribe naloxone to patients taking more than a certain number of morphine milligram equivalents per day, has probably reduced the harms of hazardous drug use.
On the obesity front, recent data from the National Health and Nutrition Examination Survey found that the prevalence of obesity in adults fell for the first time in more than a decade, from 41.9% to 40.3%. To be sure, obesity remains far too common, and this finding could be the result of statistical chance rather than representing a true decline. But the widespread prescribing of GLP-1 receptor agonists by primary care physicians, in particular, could have played a role in the encouraging trend.
Although more research is needed to prove causality, one analysis suggests that these drugs could easily have lowered the body mass index (BMI) of more than enough patients to account for the observed decline. What’s more, the rise in prevalence of BMIs above 40 (from 7.7% to 9.7%) could be explained by the mortality benefit of the drugs: More people remained in this severe obesity category because they didn’t die from complications of their weight. Whether future studies support keeping people on GLP-1s for life or eventually “off-ramping” them to other weight control strategies, family physicians are well positioned to help.
Finally, with little fanfare, the youth smoking rate has fallen precipitously. In 2023, 1.9% of high school students and 1.1% of middle-schoolers reported smoking cigarettes in the past 30 days. And they didn’t simply swap one form of nicotine delivery device for another. The 30-day prevalence of vaping among high school students fell from 27.5% in 2019 to 7.8% this year. Changing social norms and stricter federal regulation of tobacco products are probably more responsible for this positive trend than medical care, though the US Preventive Services Task Force recommends education or brief counseling to prevent initiation of tobacco use among school-aged children and adolescents. Should tobacco use in youth remain at these historically low levels, millions of premature deaths from lung cancer and heart disease will have been prevented.
America’s doctors have earned the right to take a bow. We have much more work to do, but our efforts are making a meaningful difference in three seemingly intractable health problems.
Dr. Lin, Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hi, everyone. I’m Dr. Kenny Lin. I am a family physician and associate director of the Lancaster General Hospital Family Medicine Residency, and I blog at Common Sense Family Doctor.
For the past 4 years, primary care clinicians have labored under a seemingly endless onslaught of bad news. A recent report estimated that there were over 1.3 million excess deaths in the United States from March 2020 to May 2023, including nearly half a million Americans younger than age 65. Social isolation and an ailing economy accelerated preexisting rises in drug overdoses and obesity, while teenage vaping threatened to hook a new generation on tobacco products even as adult smoking plummeted. Meanwhile, more than half of the nation’s physicians now report feelings of burnout, pay for family doctors appears to be stagnating, and our interactions with an increasing number of patients are fraught with suspicions about the value of vaccines— not just against COVID-19 but against flu and other viruses, too — and the medical system as a whole, doctors included.
Now, for the good news.
A year and a half since the end of the pandemic emergency, we are seeing gains on several fronts, and physicians deserve much of the credit. Preliminary data from the Centers for Disease Control and Prevention show that 10,000 fewer people died from drug overdoses than in the previous year. Although multiple factors contributed to this change, the elimination of the X-waiver, which had previously been required for physicians to prescribe buprenorphine for opioid use disorder, in January 2023 has improved access to medications for addiction treatment. In addition, the expansion of state requirements to check prescription drug monitoring programs when opioids or benzodiazepines are prescribed, and to prescribe naloxone to patients taking more than a certain number of morphine milligram equivalents per day, has probably reduced the harms of hazardous drug use.
On the obesity front, recent data from the National Health and Nutrition Examination Survey found that the prevalence of obesity in adults fell for the first time in more than a decade, from 41.9% to 40.3%. To be sure, obesity remains far too common, and this finding could be the result of statistical chance rather than representing a true decline. But the widespread prescribing of GLP-1 receptor agonists by primary care physicians, in particular, could have played a role in the encouraging trend.
Although more research is needed to prove causality, one analysis suggests that these drugs could easily have lowered the body mass index (BMI) of more than enough patients to account for the observed decline. What’s more, the rise in prevalence of BMIs above 40 (from 7.7% to 9.7%) could be explained by the mortality benefit of the drugs: More people remained in this severe obesity category because they didn’t die from complications of their weight. Whether future studies support keeping people on GLP-1s for life or eventually “off-ramping” them to other weight control strategies, family physicians are well positioned to help.
Finally, with little fanfare, the youth smoking rate has fallen precipitously. In 2023, 1.9% of high school students and 1.1% of middle-schoolers reported smoking cigarettes in the past 30 days. And they didn’t simply swap one form of nicotine delivery device for another. The 30-day prevalence of vaping among high school students fell from 27.5% in 2019 to 7.8% this year. Changing social norms and stricter federal regulation of tobacco products are probably more responsible for this positive trend than medical care, though the US Preventive Services Task Force recommends education or brief counseling to prevent initiation of tobacco use among school-aged children and adolescents. Should tobacco use in youth remain at these historically low levels, millions of premature deaths from lung cancer and heart disease will have been prevented.
America’s doctors have earned the right to take a bow. We have much more work to do, but our efforts are making a meaningful difference in three seemingly intractable health problems.
Dr. Lin, Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hi, everyone. I’m Dr. Kenny Lin. I am a family physician and associate director of the Lancaster General Hospital Family Medicine Residency, and I blog at Common Sense Family Doctor.
For the past 4 years, primary care clinicians have labored under a seemingly endless onslaught of bad news. A recent report estimated that there were over 1.3 million excess deaths in the United States from March 2020 to May 2023, including nearly half a million Americans younger than age 65. Social isolation and an ailing economy accelerated preexisting rises in drug overdoses and obesity, while teenage vaping threatened to hook a new generation on tobacco products even as adult smoking plummeted. Meanwhile, more than half of the nation’s physicians now report feelings of burnout, pay for family doctors appears to be stagnating, and our interactions with an increasing number of patients are fraught with suspicions about the value of vaccines— not just against COVID-19 but against flu and other viruses, too — and the medical system as a whole, doctors included.
Now, for the good news.
A year and a half since the end of the pandemic emergency, we are seeing gains on several fronts, and physicians deserve much of the credit. Preliminary data from the Centers for Disease Control and Prevention show that 10,000 fewer people died from drug overdoses than in the previous year. Although multiple factors contributed to this change, the elimination of the X-waiver, which had previously been required for physicians to prescribe buprenorphine for opioid use disorder, in January 2023 has improved access to medications for addiction treatment. In addition, the expansion of state requirements to check prescription drug monitoring programs when opioids or benzodiazepines are prescribed, and to prescribe naloxone to patients taking more than a certain number of morphine milligram equivalents per day, has probably reduced the harms of hazardous drug use.
On the obesity front, recent data from the National Health and Nutrition Examination Survey found that the prevalence of obesity in adults fell for the first time in more than a decade, from 41.9% to 40.3%. To be sure, obesity remains far too common, and this finding could be the result of statistical chance rather than representing a true decline. But the widespread prescribing of GLP-1 receptor agonists by primary care physicians, in particular, could have played a role in the encouraging trend.
Although more research is needed to prove causality, one analysis suggests that these drugs could easily have lowered the body mass index (BMI) of more than enough patients to account for the observed decline. What’s more, the rise in prevalence of BMIs above 40 (from 7.7% to 9.7%) could be explained by the mortality benefit of the drugs: More people remained in this severe obesity category because they didn’t die from complications of their weight. Whether future studies support keeping people on GLP-1s for life or eventually “off-ramping” them to other weight control strategies, family physicians are well positioned to help.
Finally, with little fanfare, the youth smoking rate has fallen precipitously. In 2023, 1.9% of high school students and 1.1% of middle-schoolers reported smoking cigarettes in the past 30 days. And they didn’t simply swap one form of nicotine delivery device for another. The 30-day prevalence of vaping among high school students fell from 27.5% in 2019 to 7.8% this year. Changing social norms and stricter federal regulation of tobacco products are probably more responsible for this positive trend than medical care, though the US Preventive Services Task Force recommends education or brief counseling to prevent initiation of tobacco use among school-aged children and adolescents. Should tobacco use in youth remain at these historically low levels, millions of premature deaths from lung cancer and heart disease will have been prevented.
America’s doctors have earned the right to take a bow. We have much more work to do, but our efforts are making a meaningful difference in three seemingly intractable health problems.
Dr. Lin, Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Weight Loss Interventions Improve Key Features of PCOS
TOPLINE:
Weight loss interventions using medication or behavioral changes can improve insulin resistance, hormonal markers, and menstrual frequency in women with polycystic ovary syndrome (PCOS), according to a new meta-analysis. Losing weight may not significantly reduce hirsutism or improve quality of life in women with the condition, however.
METHODOLOGY:
- Researchers systematically reviewed randomized controlled trials comparing weight loss interventions to usual care in women with PCOS.
- They focused on 12 studies with behavioral interventions (mainly diets with modest energy deficits), nine trials that used glucagon-like peptide 1 (GLP-1) receptor agonists, and eight studies using other weight loss medications.
- A total of 1529 participants were included in the analysis.
- The investigators synthesized the data using a random-effects meta-analysis with Knapp-Hartung adjustment to examine pooled mean differences.
TAKEAWAY:
- Menstrual frequency increased by 2.64 menses per year (95% CI, 0.65-4.63) with weight loss interventions.
- “To our knowledge, this is the first review to show a clinically significant association in improvement in menstrual frequency with weight loss interventions, an important indicator of subsequent fertility and an important outcome for women,” the researchers wrote.
- Glycemic control also improved, with a mean reduction in homeostatic model assessment of insulin resistance of 0.45 (95% CI, –0.75 to –0.15).
- Free androgen index decreased by an average of 2.03 (95% CI, –3.0 to –1.07).
IN PRACTICE:
“Clinicians may use these findings to counsel women with PCOS on the expected improvements in PCOS markers after weight loss and direct patients toward interventions,” the authors of the study wrote. “Because weight loss programs are cost-effective interventions to improve cardiometabolic risk, they may be particularly valuable for this population at elevated risk.”
SOURCE:
The study was led by Jadine Scragg, PhD, with the Nuffield Department of Primary Care Health Sciences at the University of Oxford in England. It was published online in Annals of Internal Medicine.
LIMITATIONS:
Interventions using GLP-1 agonists were dosed for glycemic control rather than weight management. The studies in the meta-analysis were relatively few and heterogeneous. Data were insufficient to assess ovulation and acne.
DISCLOSURES:
The meta-analysis was supported by grants from the National Institute for Health and Care Research School for Primary Care Research. Authors disclosed ties to Nestlé Health Science and Second Nature.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Weight loss interventions using medication or behavioral changes can improve insulin resistance, hormonal markers, and menstrual frequency in women with polycystic ovary syndrome (PCOS), according to a new meta-analysis. Losing weight may not significantly reduce hirsutism or improve quality of life in women with the condition, however.
METHODOLOGY:
- Researchers systematically reviewed randomized controlled trials comparing weight loss interventions to usual care in women with PCOS.
- They focused on 12 studies with behavioral interventions (mainly diets with modest energy deficits), nine trials that used glucagon-like peptide 1 (GLP-1) receptor agonists, and eight studies using other weight loss medications.
- A total of 1529 participants were included in the analysis.
- The investigators synthesized the data using a random-effects meta-analysis with Knapp-Hartung adjustment to examine pooled mean differences.
TAKEAWAY:
- Menstrual frequency increased by 2.64 menses per year (95% CI, 0.65-4.63) with weight loss interventions.
- “To our knowledge, this is the first review to show a clinically significant association in improvement in menstrual frequency with weight loss interventions, an important indicator of subsequent fertility and an important outcome for women,” the researchers wrote.
- Glycemic control also improved, with a mean reduction in homeostatic model assessment of insulin resistance of 0.45 (95% CI, –0.75 to –0.15).
- Free androgen index decreased by an average of 2.03 (95% CI, –3.0 to –1.07).
IN PRACTICE:
“Clinicians may use these findings to counsel women with PCOS on the expected improvements in PCOS markers after weight loss and direct patients toward interventions,” the authors of the study wrote. “Because weight loss programs are cost-effective interventions to improve cardiometabolic risk, they may be particularly valuable for this population at elevated risk.”
SOURCE:
The study was led by Jadine Scragg, PhD, with the Nuffield Department of Primary Care Health Sciences at the University of Oxford in England. It was published online in Annals of Internal Medicine.
LIMITATIONS:
Interventions using GLP-1 agonists were dosed for glycemic control rather than weight management. The studies in the meta-analysis were relatively few and heterogeneous. Data were insufficient to assess ovulation and acne.
DISCLOSURES:
The meta-analysis was supported by grants from the National Institute for Health and Care Research School for Primary Care Research. Authors disclosed ties to Nestlé Health Science and Second Nature.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Weight loss interventions using medication or behavioral changes can improve insulin resistance, hormonal markers, and menstrual frequency in women with polycystic ovary syndrome (PCOS), according to a new meta-analysis. Losing weight may not significantly reduce hirsutism or improve quality of life in women with the condition, however.
METHODOLOGY:
- Researchers systematically reviewed randomized controlled trials comparing weight loss interventions to usual care in women with PCOS.
- They focused on 12 studies with behavioral interventions (mainly diets with modest energy deficits), nine trials that used glucagon-like peptide 1 (GLP-1) receptor agonists, and eight studies using other weight loss medications.
- A total of 1529 participants were included in the analysis.
- The investigators synthesized the data using a random-effects meta-analysis with Knapp-Hartung adjustment to examine pooled mean differences.
TAKEAWAY:
- Menstrual frequency increased by 2.64 menses per year (95% CI, 0.65-4.63) with weight loss interventions.
- “To our knowledge, this is the first review to show a clinically significant association in improvement in menstrual frequency with weight loss interventions, an important indicator of subsequent fertility and an important outcome for women,” the researchers wrote.
- Glycemic control also improved, with a mean reduction in homeostatic model assessment of insulin resistance of 0.45 (95% CI, –0.75 to –0.15).
- Free androgen index decreased by an average of 2.03 (95% CI, –3.0 to –1.07).
IN PRACTICE:
“Clinicians may use these findings to counsel women with PCOS on the expected improvements in PCOS markers after weight loss and direct patients toward interventions,” the authors of the study wrote. “Because weight loss programs are cost-effective interventions to improve cardiometabolic risk, they may be particularly valuable for this population at elevated risk.”
SOURCE:
The study was led by Jadine Scragg, PhD, with the Nuffield Department of Primary Care Health Sciences at the University of Oxford in England. It was published online in Annals of Internal Medicine.
LIMITATIONS:
Interventions using GLP-1 agonists were dosed for glycemic control rather than weight management. The studies in the meta-analysis were relatively few and heterogeneous. Data were insufficient to assess ovulation and acne.
DISCLOSURES:
The meta-analysis was supported by grants from the National Institute for Health and Care Research School for Primary Care Research. Authors disclosed ties to Nestlé Health Science and Second Nature.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.






