User login
Gardasil 9 at 10 Years: Vaccine Protects Against Multiple Cancers
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Cannabis Use Linked to Brain Thinning in Adolescents
, research in mice and humans suggested.
The multilevel study demonstrated that tetrahydrocannabinol (THC), an active substance in cannabis, causes shrinkage of dendritic arborization — the neurons’ network of antennae that play a critical role in communication between brain cells.
The connection between dendritic arborization and cortical thickness was hinted at in an earlier study by Tomáš Paus, MD, PhD, professor of psychiatry and addictology at the University of Montreal, Quebec, Canada, and colleagues, who found that cannabis use in early adolescence was associated with lower cortical thickness in boys with a high genetic risk for schizophrenia.
“We speculated at that time that the differences in cortical thickness might be related to differences in dendritic arborization, and our current study confirmed it,” Paus said.
That confirmation came in the mouse part of the study, when coauthor Graciela Piñeyro, MD, PhD, also of the University of Montreal, counted the dendritic branches of mice exposed to THC and compared the total with the number of dendritic branches in unexposed mice. “What surprised me was finding that THC in the mice was targeting the same type of cells and structures that Dr. Paus had predicted would be affected from the human studies,” she said. “Structurally, they were mostly the neurons that contribute to synapses in the cortex, and their branching was reduced.”
Paus explained that in humans, a decrease in input from the affected dendrites “makes it harder for the brain to learn new things, interact with people, cope with new situations, et cetera. In other words, it makes the brain more vulnerable to everything that can happen in a young person’s life.”
The study was published online on October 9 in the Journal of Neuroscience.
Of Mice, Men, and Cannabis
Although associations between cannabis use by teenagers and variations in brain maturation have been well studied, the cellular and molecular underpinnings of these associations were unclear, according to the authors.
To investigate further, they conducted this three-step study. First, they exposed adolescent male mice to THC or a synthetic cannabinoid (WIN 55,212-2) and assessed differentially expressed genes, spine numbers, and the extent of dendritic complexity in the frontal cortex of each mouse.
Next, using MRI, they examined differences in cortical thickness in 34 brain regions in 140 male adolescents who experimented with cannabis before age 16 years and 327 who did not.
Then, they again conducted experiments in mice and found that 13 THC-related genes correlated with variations in cortical thickness. Virtual histology revealed that these 13 genes were coexpressed with cell markers of astrocytes, microglia, and a type of pyramidal cell enriched in genes that regulate dendritic expression.
Similarly, the WIN-related genes correlated with differences in cortical thickness and showed coexpression patterns with the same three cell types.
Furthermore, the affected genes were also found in humans, particularly in the thinner cortical regions of the adolescents who experimented with cannabis.
By acting on microglia, THC seems to promote the removal of synapses and, eventually, the reduction of the dendritic tree in mice, Piñeyro explained. That’s important not only because a similar mechanism may be at work in humans but also because “we now might have a model to test different types of cannabis products to see which ones are producing the greatest effect on neurons and therefore greater removal of synapses through the microglia. This could be a way of testing drugs that are out in the street to see which would be the most or least dangerous to the synapses in the brain.”
‘Significant Implications’
Commenting on the study, Yasmin Hurd, PhD, Ward-Coleman chair of translational neuroscience at the Icahn School of Medicine at Mount Sinai and director of the Addiction Institute of Mount Sinai in New York City, said, “These findings are in line with previous results, so they are feasible. This study adds more depth by showing that cortical genes that were differentially altered by adolescent THC correlated with cannabis-related changes in cortical thickness based on human neuroimaging data.” Hurd did not participate in the research.
“The results emphasize that consumption of potent cannabis products during adolescence can impact cortical function, which has significant implications for decision-making and risky behavior as well. It also can increase vulnerability to psychiatric disorders such as schizophrenia.”
Although a mouse model is “not truly the same as the human condition, the fact that the animal model also showed evidence of the morphological changes indicative of reduced cortical thickness, [like] the humans, is strong,” she said.
Additional research could include women and assess potential sex differences, she added.
Ronald Ellis, MD, PhD, an investigator in the Center for Medicinal Cannabis Research at the University of California, San Diego School of Medicine, said, “The findings are plausible and extend prior work showing evidence of increased risk for psychotic disorders later in life in adolescents who use cannabis.” Ellis did not participate in the research.
“Future studies should explore how these findings might vary across different demographic groups, which could provide a more inclusive understanding of how cannabis impacts the brain,” he said. “Additionally, longitudinal studies to track changes in the brain over time could help to establish causal relationships more robustly.
“The take-home message to clinicians at this point is to discuss cannabis use history carefully and confidentially with adolescent patients to better provide advice on its potential risks,” he concluded.
Paus added that he would tell patients, “If you’re going to use cannabis, don’t start early. If you have to, then do so in moderation. And if you have family history of mental illness, be very careful.”
No funding for the study was reported. Paus, Piñeyro, Hurd, and Ellis declared having no relevant financial relationships.
A version of this article appeared on Medscape.com.
, research in mice and humans suggested.
The multilevel study demonstrated that tetrahydrocannabinol (THC), an active substance in cannabis, causes shrinkage of dendritic arborization — the neurons’ network of antennae that play a critical role in communication between brain cells.
The connection between dendritic arborization and cortical thickness was hinted at in an earlier study by Tomáš Paus, MD, PhD, professor of psychiatry and addictology at the University of Montreal, Quebec, Canada, and colleagues, who found that cannabis use in early adolescence was associated with lower cortical thickness in boys with a high genetic risk for schizophrenia.
“We speculated at that time that the differences in cortical thickness might be related to differences in dendritic arborization, and our current study confirmed it,” Paus said.
That confirmation came in the mouse part of the study, when coauthor Graciela Piñeyro, MD, PhD, also of the University of Montreal, counted the dendritic branches of mice exposed to THC and compared the total with the number of dendritic branches in unexposed mice. “What surprised me was finding that THC in the mice was targeting the same type of cells and structures that Dr. Paus had predicted would be affected from the human studies,” she said. “Structurally, they were mostly the neurons that contribute to synapses in the cortex, and their branching was reduced.”
Paus explained that in humans, a decrease in input from the affected dendrites “makes it harder for the brain to learn new things, interact with people, cope with new situations, et cetera. In other words, it makes the brain more vulnerable to everything that can happen in a young person’s life.”
The study was published online on October 9 in the Journal of Neuroscience.
Of Mice, Men, and Cannabis
Although associations between cannabis use by teenagers and variations in brain maturation have been well studied, the cellular and molecular underpinnings of these associations were unclear, according to the authors.
To investigate further, they conducted this three-step study. First, they exposed adolescent male mice to THC or a synthetic cannabinoid (WIN 55,212-2) and assessed differentially expressed genes, spine numbers, and the extent of dendritic complexity in the frontal cortex of each mouse.
Next, using MRI, they examined differences in cortical thickness in 34 brain regions in 140 male adolescents who experimented with cannabis before age 16 years and 327 who did not.
Then, they again conducted experiments in mice and found that 13 THC-related genes correlated with variations in cortical thickness. Virtual histology revealed that these 13 genes were coexpressed with cell markers of astrocytes, microglia, and a type of pyramidal cell enriched in genes that regulate dendritic expression.
Similarly, the WIN-related genes correlated with differences in cortical thickness and showed coexpression patterns with the same three cell types.
Furthermore, the affected genes were also found in humans, particularly in the thinner cortical regions of the adolescents who experimented with cannabis.
By acting on microglia, THC seems to promote the removal of synapses and, eventually, the reduction of the dendritic tree in mice, Piñeyro explained. That’s important not only because a similar mechanism may be at work in humans but also because “we now might have a model to test different types of cannabis products to see which ones are producing the greatest effect on neurons and therefore greater removal of synapses through the microglia. This could be a way of testing drugs that are out in the street to see which would be the most or least dangerous to the synapses in the brain.”
‘Significant Implications’
Commenting on the study, Yasmin Hurd, PhD, Ward-Coleman chair of translational neuroscience at the Icahn School of Medicine at Mount Sinai and director of the Addiction Institute of Mount Sinai in New York City, said, “These findings are in line with previous results, so they are feasible. This study adds more depth by showing that cortical genes that were differentially altered by adolescent THC correlated with cannabis-related changes in cortical thickness based on human neuroimaging data.” Hurd did not participate in the research.
“The results emphasize that consumption of potent cannabis products during adolescence can impact cortical function, which has significant implications for decision-making and risky behavior as well. It also can increase vulnerability to psychiatric disorders such as schizophrenia.”
Although a mouse model is “not truly the same as the human condition, the fact that the animal model also showed evidence of the morphological changes indicative of reduced cortical thickness, [like] the humans, is strong,” she said.
Additional research could include women and assess potential sex differences, she added.
Ronald Ellis, MD, PhD, an investigator in the Center for Medicinal Cannabis Research at the University of California, San Diego School of Medicine, said, “The findings are plausible and extend prior work showing evidence of increased risk for psychotic disorders later in life in adolescents who use cannabis.” Ellis did not participate in the research.
“Future studies should explore how these findings might vary across different demographic groups, which could provide a more inclusive understanding of how cannabis impacts the brain,” he said. “Additionally, longitudinal studies to track changes in the brain over time could help to establish causal relationships more robustly.
“The take-home message to clinicians at this point is to discuss cannabis use history carefully and confidentially with adolescent patients to better provide advice on its potential risks,” he concluded.
Paus added that he would tell patients, “If you’re going to use cannabis, don’t start early. If you have to, then do so in moderation. And if you have family history of mental illness, be very careful.”
No funding for the study was reported. Paus, Piñeyro, Hurd, and Ellis declared having no relevant financial relationships.
A version of this article appeared on Medscape.com.
, research in mice and humans suggested.
The multilevel study demonstrated that tetrahydrocannabinol (THC), an active substance in cannabis, causes shrinkage of dendritic arborization — the neurons’ network of antennae that play a critical role in communication between brain cells.
The connection between dendritic arborization and cortical thickness was hinted at in an earlier study by Tomáš Paus, MD, PhD, professor of psychiatry and addictology at the University of Montreal, Quebec, Canada, and colleagues, who found that cannabis use in early adolescence was associated with lower cortical thickness in boys with a high genetic risk for schizophrenia.
“We speculated at that time that the differences in cortical thickness might be related to differences in dendritic arborization, and our current study confirmed it,” Paus said.
That confirmation came in the mouse part of the study, when coauthor Graciela Piñeyro, MD, PhD, also of the University of Montreal, counted the dendritic branches of mice exposed to THC and compared the total with the number of dendritic branches in unexposed mice. “What surprised me was finding that THC in the mice was targeting the same type of cells and structures that Dr. Paus had predicted would be affected from the human studies,” she said. “Structurally, they were mostly the neurons that contribute to synapses in the cortex, and their branching was reduced.”
Paus explained that in humans, a decrease in input from the affected dendrites “makes it harder for the brain to learn new things, interact with people, cope with new situations, et cetera. In other words, it makes the brain more vulnerable to everything that can happen in a young person’s life.”
The study was published online on October 9 in the Journal of Neuroscience.
Of Mice, Men, and Cannabis
Although associations between cannabis use by teenagers and variations in brain maturation have been well studied, the cellular and molecular underpinnings of these associations were unclear, according to the authors.
To investigate further, they conducted this three-step study. First, they exposed adolescent male mice to THC or a synthetic cannabinoid (WIN 55,212-2) and assessed differentially expressed genes, spine numbers, and the extent of dendritic complexity in the frontal cortex of each mouse.
Next, using MRI, they examined differences in cortical thickness in 34 brain regions in 140 male adolescents who experimented with cannabis before age 16 years and 327 who did not.
Then, they again conducted experiments in mice and found that 13 THC-related genes correlated with variations in cortical thickness. Virtual histology revealed that these 13 genes were coexpressed with cell markers of astrocytes, microglia, and a type of pyramidal cell enriched in genes that regulate dendritic expression.
Similarly, the WIN-related genes correlated with differences in cortical thickness and showed coexpression patterns with the same three cell types.
Furthermore, the affected genes were also found in humans, particularly in the thinner cortical regions of the adolescents who experimented with cannabis.
By acting on microglia, THC seems to promote the removal of synapses and, eventually, the reduction of the dendritic tree in mice, Piñeyro explained. That’s important not only because a similar mechanism may be at work in humans but also because “we now might have a model to test different types of cannabis products to see which ones are producing the greatest effect on neurons and therefore greater removal of synapses through the microglia. This could be a way of testing drugs that are out in the street to see which would be the most or least dangerous to the synapses in the brain.”
‘Significant Implications’
Commenting on the study, Yasmin Hurd, PhD, Ward-Coleman chair of translational neuroscience at the Icahn School of Medicine at Mount Sinai and director of the Addiction Institute of Mount Sinai in New York City, said, “These findings are in line with previous results, so they are feasible. This study adds more depth by showing that cortical genes that were differentially altered by adolescent THC correlated with cannabis-related changes in cortical thickness based on human neuroimaging data.” Hurd did not participate in the research.
“The results emphasize that consumption of potent cannabis products during adolescence can impact cortical function, which has significant implications for decision-making and risky behavior as well. It also can increase vulnerability to psychiatric disorders such as schizophrenia.”
Although a mouse model is “not truly the same as the human condition, the fact that the animal model also showed evidence of the morphological changes indicative of reduced cortical thickness, [like] the humans, is strong,” she said.
Additional research could include women and assess potential sex differences, she added.
Ronald Ellis, MD, PhD, an investigator in the Center for Medicinal Cannabis Research at the University of California, San Diego School of Medicine, said, “The findings are plausible and extend prior work showing evidence of increased risk for psychotic disorders later in life in adolescents who use cannabis.” Ellis did not participate in the research.
“Future studies should explore how these findings might vary across different demographic groups, which could provide a more inclusive understanding of how cannabis impacts the brain,” he said. “Additionally, longitudinal studies to track changes in the brain over time could help to establish causal relationships more robustly.
“The take-home message to clinicians at this point is to discuss cannabis use history carefully and confidentially with adolescent patients to better provide advice on its potential risks,” he concluded.
Paus added that he would tell patients, “If you’re going to use cannabis, don’t start early. If you have to, then do so in moderation. And if you have family history of mental illness, be very careful.”
No funding for the study was reported. Paus, Piñeyro, Hurd, and Ellis declared having no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM THE JOURNAL OF NEUROSCIENCE
Outpatient CAR T: Safe, Effective, Accessible
In one recent study, an industry-funded phase 2 trial, researchers found similar outcomes from outpatient and inpatient CAR T-cell therapy for relapsed/refractory large B-cell lymphoma with lisocabtagene maraleucel (Breyanzi).
Another recent study reported that outpatient treatment of B cell non-Hodgkin lymphoma with tisagenlecleucel (Kymriah) had similar efficacy to inpatient treatment. Meanwhile, a 2023 review of CAR T-cell therapy in various settings found similar outcomes in outpatient and inpatient treatment.
“The future of CAR T-cell therapy lies in balancing safety with accessibility,” said Rayne Rouce, MD, a pediatric oncologist at Texas Children’s Cancer Center in Houston, Texas, in an interview. “Expanding CAR T-cell therapy beyond large medical centers is a critical next step.”
Great Outcomes, Low Access
Since 2017, the FDA has approved six CAR T-cell therapies, which target cancer by harnessing the power of a patient’s own T cells. As an Oregon Health & Sciences University/Knight Cancer Center website explains, T cells are removed from the patient’s body, “genetically modified to make the chimeric antigen receptor, or CAR, [which] protein binds to specific proteins on the surface of cancer cells.”
Modified cells are grown and then infused back into the body, where they “multiply and may be able to destroy all the cancer cells.”
As Rouce puts it, “CAR T-cells have revolutionized the treatment of relapsed or refractory blood cancers.” One or more of the therapies have been approved to treat types of lymphoblastic leukemia, B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and multiple myeloma.
A 2023 review of clinical trial data reported complete response rates of 40%-54% in aggressive B-cell lymphoma, 67% in mantle cell lymphoma, and 69%-74% in indolent B cell lymphoma.
“Commercialization of CAR T-cell therapy brought hope that access would expand beyond the major academic medical centers with the highly specialized infrastructure and advanced laboratories required to manufacture and ultimately treat patients,” Rouce said. “However, it quickly became clear that patients who are underinsured or uninsured — or who live outside the network of the well-resourced institutions that house these therapies — are still unable to access these potentially life-saving therapies.”
A 2024 report estimated the cost of CAR T-cell therapy as $700,000-$1 million and said only a small percentage of those who could benefit from the treatment actually get it. For example, an estimated 10,000 patients with diffuse large B-cell lymphoma alone could benefit from CAR T therapy annually, but a survey of 200 US healthcare centers in 2021 found that 1900 procedures were performed overall for all indications.
Distance to Treatment Is a Major Obstacle
Even if patients have insurance plans willing to cover CAR T-cell therapy, they may not be able get care. While more than 150 US centers are certified to administer the therapy, “distance to major medical centers with CAR T capabilities is a major obstacle,” Yuliya Linhares, MD, chief of lymphoma at Miami Cancer Institute in Miami, Florida, said in an interview.
“I have had patients who chose to not proceed with CAR T therapy due to inability to travel the distance to the medical center for pre-CAR T appointments and assessments and a lack of caretakers who are available to stay nearby,” Linhares said.
Indeed, the challenges facing patients in rural and underserved urban areas can be overwhelming, Hoda Badr, PhD, professor of medicine at Baylor College of Medicine in Houston, Texas, said in an interview.
“They must take time off work, arrange accommodations near treatment sites, and manage travel costs, all of which strain limited financial resources. The inability to afford these additional expenses can lead to delays in receiving care or patients forgoing the treatment altogether,” Badr said. She added that “the psychological and social burden of being away from family and community support systems during treatment can intensify the stress of an already difficult situation.”
A statistic tells the story of the urban/community divide. CAR T-cell therapy administration at academic centers after leukapheresis — the separation and collection of white blood cells — is reported to be at around 90%, while it’s only 47% in community-based practices that have to refer patients elsewhere, Linhares noted.
Researchers Explore CAR T-Cell Therapy in the Community
Linhares is lead author of the phase 2 trial that explored administration of lisocabtagene maraleucel in 82 patients with relapsed/refractory large B-cell lymphoma. The findings were published Sept. 30 in Blood Advances.
The OUTREACH trial, funded by Juno/Bristol-Myers Squibb, treated patients in the third line and beyond at community medical centers (outpatient-monitored, 70%; inpatient-monitored, 30%). The trial didn’t require facilities to be certified by the Foundation for the Accreditation of Cellular Therapy (FACT); all had to be non-tertiary cancer centers that weren’t associated with a university. In order to administer therapy on the outpatient basis, the centers had to have phase 1 or hematopoietic stem cell transplant capabilities.
As Linhares explained, 72% of participating centers hadn’t provided CAR T-cell therapy before, and 44% did not have FACT accreditation. “About 32% of patients received CAR T at CAR T naive sites, while 70% of patients received CAR T as outpatients. Investigators had to decide whether patients qualified for the outpatient observation or had to be admitted for the inpatient observation,” she noted.
Community Outcomes Were Comparable to Major Trial
As for the results, grade 3 or higher adverse events occurred at a similar frequency among outpatients and inpatients at 74% and 76%, Linhares said. There were no grade 5 adverse events, and 25% of patients treated as outpatients were never hospitalized.
Response rates were similar to those in the major TRANSCEND trial with the objective response rates rate of 80% and complete response rates of 54%.
“Overall,” Linhares said, “our study demonstrated that with the availability of standard operating procedures, specially trained staff and a multidisciplinary team trained in CAR T toxicity management, inpatient and outpatient CAR T administration is feasible at specialized community medical centers.”
In 2023, another study examined patients with B-cell non-Hodgkin lymphoma who were treated on an outpatient basis with tisagenlecleucel. Researchers reported that outpatient therapy was “feasible and associated with similar efficacy outcomes as inpatient treatment.”
And a 2023 systematic literature review identified 11 studies that reported outpatient vs inpatient outcomes in CAR T-cell therapy and found “comparable response rates (80-82% in outpatient and 72-80% in inpatient).” Costs were cheaper in the outpatient setting.
Research findings like these are good news, Baylor College of Medicine’s Badr said. “Outpatient administration could help to scale the availability of this therapy to a broader range of healthcare settings, including those serving underserved populations. Findings indicate promising safety profiles, which is encouraging for expanding access.”
Not Every Patient Can Tolerate Outpatient Care
Linhares noted that the patients who received outpatient care in the lisocabtagene maraleucel study were in better shape than those in the inpatient group. Those selected for inpatient care had “higher disease risk characteristics, including high grade B cell lymphoma histology, higher disease burden, and having received bridging therapy. This points to the fact that the investigators properly selected patients who were at a higher risk of complications for inpatient observation. Additionally, some patients stayed as inpatient due to social factors, which increases length of stay independently of disease characteristics.”
Specifically, reasons for inpatient monitoring were disease characteristics (48%) including tumor burden and risk of adverse events; psychosocial factors (32%) including lack of caregiver support or transportation; COVID-19 precautions (8%); pre-infusion adverse events (8%) of fever and vasovagal reaction; and principal investigator decision (4%) due to limited hospital experience with CAR T-cell therapy.
Texas Children’s Cancer Center’s Rouce said “certain patients, particularly those with higher risk for complications or those who require intensive monitoring, may not be suited for outpatient CAR T-cell therapy. This may be due to other comorbidities or baseline factors known to predispose to CAR T-related toxicities. However, evidence-based risk mitigation algorithms may still allow closely monitored outpatient treatment, with recognition that hospital admission for incipient side effects may be necessary.”
What’s Next for Access to Therapy?
Rouce noted that her institution, like many others, is offering CAR T-cell therapy on an outpatient basis. “Additionally, continued scientific innovation, such as immediately available, off-the-shelf cell therapies and inducible safety switches, will ultimately improve access,” she said.
Linhares noted a recent advance and highlighted research that’s now in progress. “CAR Ts now have an indication as a second-line therapy in relapsed/refractory large B-cell lymphoma, and there are ongoing clinical trials that will potentially move CAR Ts into the first line,” she said. “Some trials are exploring allogeneic, readily available off-the-shelf CAR T for the treatment of minimal residual disease positive large B-cell lymphoma after completion of first-line therapy.”
These potential advances “are increasing the need for CAR T-capable medical centers,” Linhares noted. “More and more medical centers with expert hematology teams are becoming CAR T-certified, with more patients having access to CAR T.”
Still, she said, “I don’t think access is nearly as good as it should be. Many patients in rural areas are still unable to get this life-saving treatment. “However, “it is very possible that other novel targeted therapies, such as bispecific antibodies, will be used in place of CAR T in areas with poor CAR T access. Bispecific antibody efficacy in various B cell lymphoma histologies are being currently explored.”
Rouce discloses relationships with Novartis and Pfizer. Linhares reports ties with Kyowa Kirin, AbbVie, ADC, BeiGene, Genentech, Gilead, GlaxoSmithKline, Seagen, and TG. Badr has no disclosures.
A version of this article appeared on Medscape.com.
In one recent study, an industry-funded phase 2 trial, researchers found similar outcomes from outpatient and inpatient CAR T-cell therapy for relapsed/refractory large B-cell lymphoma with lisocabtagene maraleucel (Breyanzi).
Another recent study reported that outpatient treatment of B cell non-Hodgkin lymphoma with tisagenlecleucel (Kymriah) had similar efficacy to inpatient treatment. Meanwhile, a 2023 review of CAR T-cell therapy in various settings found similar outcomes in outpatient and inpatient treatment.
“The future of CAR T-cell therapy lies in balancing safety with accessibility,” said Rayne Rouce, MD, a pediatric oncologist at Texas Children’s Cancer Center in Houston, Texas, in an interview. “Expanding CAR T-cell therapy beyond large medical centers is a critical next step.”
Great Outcomes, Low Access
Since 2017, the FDA has approved six CAR T-cell therapies, which target cancer by harnessing the power of a patient’s own T cells. As an Oregon Health & Sciences University/Knight Cancer Center website explains, T cells are removed from the patient’s body, “genetically modified to make the chimeric antigen receptor, or CAR, [which] protein binds to specific proteins on the surface of cancer cells.”
Modified cells are grown and then infused back into the body, where they “multiply and may be able to destroy all the cancer cells.”
As Rouce puts it, “CAR T-cells have revolutionized the treatment of relapsed or refractory blood cancers.” One or more of the therapies have been approved to treat types of lymphoblastic leukemia, B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and multiple myeloma.
A 2023 review of clinical trial data reported complete response rates of 40%-54% in aggressive B-cell lymphoma, 67% in mantle cell lymphoma, and 69%-74% in indolent B cell lymphoma.
“Commercialization of CAR T-cell therapy brought hope that access would expand beyond the major academic medical centers with the highly specialized infrastructure and advanced laboratories required to manufacture and ultimately treat patients,” Rouce said. “However, it quickly became clear that patients who are underinsured or uninsured — or who live outside the network of the well-resourced institutions that house these therapies — are still unable to access these potentially life-saving therapies.”
A 2024 report estimated the cost of CAR T-cell therapy as $700,000-$1 million and said only a small percentage of those who could benefit from the treatment actually get it. For example, an estimated 10,000 patients with diffuse large B-cell lymphoma alone could benefit from CAR T therapy annually, but a survey of 200 US healthcare centers in 2021 found that 1900 procedures were performed overall for all indications.
Distance to Treatment Is a Major Obstacle
Even if patients have insurance plans willing to cover CAR T-cell therapy, they may not be able get care. While more than 150 US centers are certified to administer the therapy, “distance to major medical centers with CAR T capabilities is a major obstacle,” Yuliya Linhares, MD, chief of lymphoma at Miami Cancer Institute in Miami, Florida, said in an interview.
“I have had patients who chose to not proceed with CAR T therapy due to inability to travel the distance to the medical center for pre-CAR T appointments and assessments and a lack of caretakers who are available to stay nearby,” Linhares said.
Indeed, the challenges facing patients in rural and underserved urban areas can be overwhelming, Hoda Badr, PhD, professor of medicine at Baylor College of Medicine in Houston, Texas, said in an interview.
“They must take time off work, arrange accommodations near treatment sites, and manage travel costs, all of which strain limited financial resources. The inability to afford these additional expenses can lead to delays in receiving care or patients forgoing the treatment altogether,” Badr said. She added that “the psychological and social burden of being away from family and community support systems during treatment can intensify the stress of an already difficult situation.”
A statistic tells the story of the urban/community divide. CAR T-cell therapy administration at academic centers after leukapheresis — the separation and collection of white blood cells — is reported to be at around 90%, while it’s only 47% in community-based practices that have to refer patients elsewhere, Linhares noted.
Researchers Explore CAR T-Cell Therapy in the Community
Linhares is lead author of the phase 2 trial that explored administration of lisocabtagene maraleucel in 82 patients with relapsed/refractory large B-cell lymphoma. The findings were published Sept. 30 in Blood Advances.
The OUTREACH trial, funded by Juno/Bristol-Myers Squibb, treated patients in the third line and beyond at community medical centers (outpatient-monitored, 70%; inpatient-monitored, 30%). The trial didn’t require facilities to be certified by the Foundation for the Accreditation of Cellular Therapy (FACT); all had to be non-tertiary cancer centers that weren’t associated with a university. In order to administer therapy on the outpatient basis, the centers had to have phase 1 or hematopoietic stem cell transplant capabilities.
As Linhares explained, 72% of participating centers hadn’t provided CAR T-cell therapy before, and 44% did not have FACT accreditation. “About 32% of patients received CAR T at CAR T naive sites, while 70% of patients received CAR T as outpatients. Investigators had to decide whether patients qualified for the outpatient observation or had to be admitted for the inpatient observation,” she noted.
Community Outcomes Were Comparable to Major Trial
As for the results, grade 3 or higher adverse events occurred at a similar frequency among outpatients and inpatients at 74% and 76%, Linhares said. There were no grade 5 adverse events, and 25% of patients treated as outpatients were never hospitalized.
Response rates were similar to those in the major TRANSCEND trial with the objective response rates rate of 80% and complete response rates of 54%.
“Overall,” Linhares said, “our study demonstrated that with the availability of standard operating procedures, specially trained staff and a multidisciplinary team trained in CAR T toxicity management, inpatient and outpatient CAR T administration is feasible at specialized community medical centers.”
In 2023, another study examined patients with B-cell non-Hodgkin lymphoma who were treated on an outpatient basis with tisagenlecleucel. Researchers reported that outpatient therapy was “feasible and associated with similar efficacy outcomes as inpatient treatment.”
And a 2023 systematic literature review identified 11 studies that reported outpatient vs inpatient outcomes in CAR T-cell therapy and found “comparable response rates (80-82% in outpatient and 72-80% in inpatient).” Costs were cheaper in the outpatient setting.
Research findings like these are good news, Baylor College of Medicine’s Badr said. “Outpatient administration could help to scale the availability of this therapy to a broader range of healthcare settings, including those serving underserved populations. Findings indicate promising safety profiles, which is encouraging for expanding access.”
Not Every Patient Can Tolerate Outpatient Care
Linhares noted that the patients who received outpatient care in the lisocabtagene maraleucel study were in better shape than those in the inpatient group. Those selected for inpatient care had “higher disease risk characteristics, including high grade B cell lymphoma histology, higher disease burden, and having received bridging therapy. This points to the fact that the investigators properly selected patients who were at a higher risk of complications for inpatient observation. Additionally, some patients stayed as inpatient due to social factors, which increases length of stay independently of disease characteristics.”
Specifically, reasons for inpatient monitoring were disease characteristics (48%) including tumor burden and risk of adverse events; psychosocial factors (32%) including lack of caregiver support or transportation; COVID-19 precautions (8%); pre-infusion adverse events (8%) of fever and vasovagal reaction; and principal investigator decision (4%) due to limited hospital experience with CAR T-cell therapy.
Texas Children’s Cancer Center’s Rouce said “certain patients, particularly those with higher risk for complications or those who require intensive monitoring, may not be suited for outpatient CAR T-cell therapy. This may be due to other comorbidities or baseline factors known to predispose to CAR T-related toxicities. However, evidence-based risk mitigation algorithms may still allow closely monitored outpatient treatment, with recognition that hospital admission for incipient side effects may be necessary.”
What’s Next for Access to Therapy?
Rouce noted that her institution, like many others, is offering CAR T-cell therapy on an outpatient basis. “Additionally, continued scientific innovation, such as immediately available, off-the-shelf cell therapies and inducible safety switches, will ultimately improve access,” she said.
Linhares noted a recent advance and highlighted research that’s now in progress. “CAR Ts now have an indication as a second-line therapy in relapsed/refractory large B-cell lymphoma, and there are ongoing clinical trials that will potentially move CAR Ts into the first line,” she said. “Some trials are exploring allogeneic, readily available off-the-shelf CAR T for the treatment of minimal residual disease positive large B-cell lymphoma after completion of first-line therapy.”
These potential advances “are increasing the need for CAR T-capable medical centers,” Linhares noted. “More and more medical centers with expert hematology teams are becoming CAR T-certified, with more patients having access to CAR T.”
Still, she said, “I don’t think access is nearly as good as it should be. Many patients in rural areas are still unable to get this life-saving treatment. “However, “it is very possible that other novel targeted therapies, such as bispecific antibodies, will be used in place of CAR T in areas with poor CAR T access. Bispecific antibody efficacy in various B cell lymphoma histologies are being currently explored.”
Rouce discloses relationships with Novartis and Pfizer. Linhares reports ties with Kyowa Kirin, AbbVie, ADC, BeiGene, Genentech, Gilead, GlaxoSmithKline, Seagen, and TG. Badr has no disclosures.
A version of this article appeared on Medscape.com.
In one recent study, an industry-funded phase 2 trial, researchers found similar outcomes from outpatient and inpatient CAR T-cell therapy for relapsed/refractory large B-cell lymphoma with lisocabtagene maraleucel (Breyanzi).
Another recent study reported that outpatient treatment of B cell non-Hodgkin lymphoma with tisagenlecleucel (Kymriah) had similar efficacy to inpatient treatment. Meanwhile, a 2023 review of CAR T-cell therapy in various settings found similar outcomes in outpatient and inpatient treatment.
“The future of CAR T-cell therapy lies in balancing safety with accessibility,” said Rayne Rouce, MD, a pediatric oncologist at Texas Children’s Cancer Center in Houston, Texas, in an interview. “Expanding CAR T-cell therapy beyond large medical centers is a critical next step.”
Great Outcomes, Low Access
Since 2017, the FDA has approved six CAR T-cell therapies, which target cancer by harnessing the power of a patient’s own T cells. As an Oregon Health & Sciences University/Knight Cancer Center website explains, T cells are removed from the patient’s body, “genetically modified to make the chimeric antigen receptor, or CAR, [which] protein binds to specific proteins on the surface of cancer cells.”
Modified cells are grown and then infused back into the body, where they “multiply and may be able to destroy all the cancer cells.”
As Rouce puts it, “CAR T-cells have revolutionized the treatment of relapsed or refractory blood cancers.” One or more of the therapies have been approved to treat types of lymphoblastic leukemia, B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and multiple myeloma.
A 2023 review of clinical trial data reported complete response rates of 40%-54% in aggressive B-cell lymphoma, 67% in mantle cell lymphoma, and 69%-74% in indolent B cell lymphoma.
“Commercialization of CAR T-cell therapy brought hope that access would expand beyond the major academic medical centers with the highly specialized infrastructure and advanced laboratories required to manufacture and ultimately treat patients,” Rouce said. “However, it quickly became clear that patients who are underinsured or uninsured — or who live outside the network of the well-resourced institutions that house these therapies — are still unable to access these potentially life-saving therapies.”
A 2024 report estimated the cost of CAR T-cell therapy as $700,000-$1 million and said only a small percentage of those who could benefit from the treatment actually get it. For example, an estimated 10,000 patients with diffuse large B-cell lymphoma alone could benefit from CAR T therapy annually, but a survey of 200 US healthcare centers in 2021 found that 1900 procedures were performed overall for all indications.
Distance to Treatment Is a Major Obstacle
Even if patients have insurance plans willing to cover CAR T-cell therapy, they may not be able get care. While more than 150 US centers are certified to administer the therapy, “distance to major medical centers with CAR T capabilities is a major obstacle,” Yuliya Linhares, MD, chief of lymphoma at Miami Cancer Institute in Miami, Florida, said in an interview.
“I have had patients who chose to not proceed with CAR T therapy due to inability to travel the distance to the medical center for pre-CAR T appointments and assessments and a lack of caretakers who are available to stay nearby,” Linhares said.
Indeed, the challenges facing patients in rural and underserved urban areas can be overwhelming, Hoda Badr, PhD, professor of medicine at Baylor College of Medicine in Houston, Texas, said in an interview.
“They must take time off work, arrange accommodations near treatment sites, and manage travel costs, all of which strain limited financial resources. The inability to afford these additional expenses can lead to delays in receiving care or patients forgoing the treatment altogether,” Badr said. She added that “the psychological and social burden of being away from family and community support systems during treatment can intensify the stress of an already difficult situation.”
A statistic tells the story of the urban/community divide. CAR T-cell therapy administration at academic centers after leukapheresis — the separation and collection of white blood cells — is reported to be at around 90%, while it’s only 47% in community-based practices that have to refer patients elsewhere, Linhares noted.
Researchers Explore CAR T-Cell Therapy in the Community
Linhares is lead author of the phase 2 trial that explored administration of lisocabtagene maraleucel in 82 patients with relapsed/refractory large B-cell lymphoma. The findings were published Sept. 30 in Blood Advances.
The OUTREACH trial, funded by Juno/Bristol-Myers Squibb, treated patients in the third line and beyond at community medical centers (outpatient-monitored, 70%; inpatient-monitored, 30%). The trial didn’t require facilities to be certified by the Foundation for the Accreditation of Cellular Therapy (FACT); all had to be non-tertiary cancer centers that weren’t associated with a university. In order to administer therapy on the outpatient basis, the centers had to have phase 1 or hematopoietic stem cell transplant capabilities.
As Linhares explained, 72% of participating centers hadn’t provided CAR T-cell therapy before, and 44% did not have FACT accreditation. “About 32% of patients received CAR T at CAR T naive sites, while 70% of patients received CAR T as outpatients. Investigators had to decide whether patients qualified for the outpatient observation or had to be admitted for the inpatient observation,” she noted.
Community Outcomes Were Comparable to Major Trial
As for the results, grade 3 or higher adverse events occurred at a similar frequency among outpatients and inpatients at 74% and 76%, Linhares said. There were no grade 5 adverse events, and 25% of patients treated as outpatients were never hospitalized.
Response rates were similar to those in the major TRANSCEND trial with the objective response rates rate of 80% and complete response rates of 54%.
“Overall,” Linhares said, “our study demonstrated that with the availability of standard operating procedures, specially trained staff and a multidisciplinary team trained in CAR T toxicity management, inpatient and outpatient CAR T administration is feasible at specialized community medical centers.”
In 2023, another study examined patients with B-cell non-Hodgkin lymphoma who were treated on an outpatient basis with tisagenlecleucel. Researchers reported that outpatient therapy was “feasible and associated with similar efficacy outcomes as inpatient treatment.”
And a 2023 systematic literature review identified 11 studies that reported outpatient vs inpatient outcomes in CAR T-cell therapy and found “comparable response rates (80-82% in outpatient and 72-80% in inpatient).” Costs were cheaper in the outpatient setting.
Research findings like these are good news, Baylor College of Medicine’s Badr said. “Outpatient administration could help to scale the availability of this therapy to a broader range of healthcare settings, including those serving underserved populations. Findings indicate promising safety profiles, which is encouraging for expanding access.”
Not Every Patient Can Tolerate Outpatient Care
Linhares noted that the patients who received outpatient care in the lisocabtagene maraleucel study were in better shape than those in the inpatient group. Those selected for inpatient care had “higher disease risk characteristics, including high grade B cell lymphoma histology, higher disease burden, and having received bridging therapy. This points to the fact that the investigators properly selected patients who were at a higher risk of complications for inpatient observation. Additionally, some patients stayed as inpatient due to social factors, which increases length of stay independently of disease characteristics.”
Specifically, reasons for inpatient monitoring were disease characteristics (48%) including tumor burden and risk of adverse events; psychosocial factors (32%) including lack of caregiver support or transportation; COVID-19 precautions (8%); pre-infusion adverse events (8%) of fever and vasovagal reaction; and principal investigator decision (4%) due to limited hospital experience with CAR T-cell therapy.
Texas Children’s Cancer Center’s Rouce said “certain patients, particularly those with higher risk for complications or those who require intensive monitoring, may not be suited for outpatient CAR T-cell therapy. This may be due to other comorbidities or baseline factors known to predispose to CAR T-related toxicities. However, evidence-based risk mitigation algorithms may still allow closely monitored outpatient treatment, with recognition that hospital admission for incipient side effects may be necessary.”
What’s Next for Access to Therapy?
Rouce noted that her institution, like many others, is offering CAR T-cell therapy on an outpatient basis. “Additionally, continued scientific innovation, such as immediately available, off-the-shelf cell therapies and inducible safety switches, will ultimately improve access,” she said.
Linhares noted a recent advance and highlighted research that’s now in progress. “CAR Ts now have an indication as a second-line therapy in relapsed/refractory large B-cell lymphoma, and there are ongoing clinical trials that will potentially move CAR Ts into the first line,” she said. “Some trials are exploring allogeneic, readily available off-the-shelf CAR T for the treatment of minimal residual disease positive large B-cell lymphoma after completion of first-line therapy.”
These potential advances “are increasing the need for CAR T-capable medical centers,” Linhares noted. “More and more medical centers with expert hematology teams are becoming CAR T-certified, with more patients having access to CAR T.”
Still, she said, “I don’t think access is nearly as good as it should be. Many patients in rural areas are still unable to get this life-saving treatment. “However, “it is very possible that other novel targeted therapies, such as bispecific antibodies, will be used in place of CAR T in areas with poor CAR T access. Bispecific antibody efficacy in various B cell lymphoma histologies are being currently explored.”
Rouce discloses relationships with Novartis and Pfizer. Linhares reports ties with Kyowa Kirin, AbbVie, ADC, BeiGene, Genentech, Gilead, GlaxoSmithKline, Seagen, and TG. Badr has no disclosures.
A version of this article appeared on Medscape.com.
ATA: Updates on Risk, Diagnosis, and Treatment of Thyroid Cancer
The study, presented by Juan Brito Campana, MBBS, of the Mayo Clinic in Rochester, Minnesota, used Medicare records to perform a secondary analysis of 41,000 adults with type 2 diabetes and moderate cardiovascular risk who were new users of GLP-1 receptor agonists, compared to users of other diabetes medications.
“We took the innovative approach of applying the methodological rigor of a randomized clinical trial to the very large dataset of observational studies,” said Brito Campana.
The results showed a low absolute risk of thyroid cancer, with only 0.17% of patients in the GLP-1 group developing the disease. However, the data also showed a potential relative increase in risk during the first year of GLP-1 receptor agonist use.
“This is likely due to increased detection rather than true incidence, as the latency period for thyroid cancer development is typically longer,” Brito Campana said.
“We also note the limitations of the observational study design, including the short follow-up period and lack of detailed histological data. However, we believe the benefits of GLP-1 receptor agonists likely outweigh the risk of thyroid cancer.”
Malignancy in Bethesda III and IV Thyroid Nodules
At the same ATA session, Sapir Nachum Goldberg, MD, of the University of Pennsylvania, Philadelphia, presented the results of a retrospective record review that examined the prevalence of malignancy in Bethesda III and IV thyroid nodules with negative Thyrogen Receptor Signaling (ThyroSeq) version 3 molecular testing results.
Goldberg reported that 87% of patients with ThyroSeq negative subtype results were managed nonoperatively. “Based on our data, the true prevalence of malignancy likely lies between our low and high estimates of 3% and 23%,” she said. “We believe that the prevalence of malignancy may be higher in real-world practice than validation studies.”
Additionally, nodules with “currently negative” or “negative but limited” ThyroSeq results had a higher prevalence of malignancy (7%), compared with those with a “negative” result (2%). Factors like immediate vs delayed surgery, nodule size, and ultrasound pattern did not significantly impact malignancy prevalence.
The study results also indicated that surveillance ultrasonography is not routinely performed in up to one-third of patients, Goldberg said.
She closed by suggesting that colleagues consider the negative subtype in clinical decision-making. For “negative but limited” nodules, repeat the fine needle aspiration and, for “negative” and “currently negative” nodules, consider ultrasound follow-up as per ATA guidelines for Bethesda II cytology, she said.
RET-Mutated Medullary Thyroid Cancer
For patients with RET-mutated medullary thyroid cancer, Julien Hadoux, MD, PhD, of Institut de Cancérologie Gustave Roussy, Villejuif, France, presented a combined analysis of the efficacy of the RET inhibitor selpercatinib from the phase 1/2 LIBRETTO-001 and phase 3 LIBRETTO-531 trials.
This post hoc analysis used a combined cohort of 509 patients with RET-mutated advanced or metastatic medullary thyroid cancer who had received selpercatinib in the two trials.
Hadoux reported that robust and durable responses were seen across all mutation groups, including M918T, extracellular cysteine, and an “other” group composed of various uncommon RET mutations. “The median [progression-free survival] PFS was not reached for either the M918T or extracellular groups and it was 51.4 months for the Other group,” he said.
“Selpercatinib showed superior median PFS vs control, regardless of the RET mutation. This analysis constitutes the largest catalog of RET mutations in medullary thyroid cancers treated with RET-specific inhibitors.”
TRK-Fusion Differentiated Thyroid Cancer
Steven Waguespack, MD, of the University of Texas MD Anderson Cancer Center, Houston, shared updated efficacy and safety data from three phase 1/2 pooled clinical trials of the tropomyosin kinase receptor (TRK) inhibitor larotrectinib in thyroid cancer. These data updated results initially published in 2022.
“Larotrectinib continues to demonstrate rapid and durable responses, extended survival, and offers a favorable safety profile in patients with TRK fusion differentiated thyroid cancer, with limited activity in anaplastic thyroid cancer,” Waguespack said.
“Additionally, in a subset of patients, we identified some acquired on-target NTRK mutations and off-target GNAS and TP53 mutations that may give further insight into mechanisms of resistance.”
The primary endpoint was the investigator-assessed objective response rate (ORR); at 48 months, the ORR was 79% by independent review. The median PFS in patients with TRK fusion differentiated thyroid cancer was 44 months, while the median duration of response was 41 months. The 4-year overall survival rate was 86%.
Waguespack closed with a cautionary note to colleagues: “While circulating tumor DNA next-generation sequencing (NGS) analysis can be used to test for NTRK gene fusions, negative results should be followed up with tissue-based NGS,” he said.
Brito Campana and Goldberg disclosed no relevant financial relationships. Hadoux reported receiving honoraria for speaker engagements, advisory roles, or funding for CME from Eli Lilly, AAA, IPSEN, Roche, Pharma Mar, and EISAI, and research grants from Novartis, Sanofi, and Eli Lilly.
A version of this article appeared on Medscape.com.
The study, presented by Juan Brito Campana, MBBS, of the Mayo Clinic in Rochester, Minnesota, used Medicare records to perform a secondary analysis of 41,000 adults with type 2 diabetes and moderate cardiovascular risk who were new users of GLP-1 receptor agonists, compared to users of other diabetes medications.
“We took the innovative approach of applying the methodological rigor of a randomized clinical trial to the very large dataset of observational studies,” said Brito Campana.
The results showed a low absolute risk of thyroid cancer, with only 0.17% of patients in the GLP-1 group developing the disease. However, the data also showed a potential relative increase in risk during the first year of GLP-1 receptor agonist use.
“This is likely due to increased detection rather than true incidence, as the latency period for thyroid cancer development is typically longer,” Brito Campana said.
“We also note the limitations of the observational study design, including the short follow-up period and lack of detailed histological data. However, we believe the benefits of GLP-1 receptor agonists likely outweigh the risk of thyroid cancer.”
Malignancy in Bethesda III and IV Thyroid Nodules
At the same ATA session, Sapir Nachum Goldberg, MD, of the University of Pennsylvania, Philadelphia, presented the results of a retrospective record review that examined the prevalence of malignancy in Bethesda III and IV thyroid nodules with negative Thyrogen Receptor Signaling (ThyroSeq) version 3 molecular testing results.
Goldberg reported that 87% of patients with ThyroSeq negative subtype results were managed nonoperatively. “Based on our data, the true prevalence of malignancy likely lies between our low and high estimates of 3% and 23%,” she said. “We believe that the prevalence of malignancy may be higher in real-world practice than validation studies.”
Additionally, nodules with “currently negative” or “negative but limited” ThyroSeq results had a higher prevalence of malignancy (7%), compared with those with a “negative” result (2%). Factors like immediate vs delayed surgery, nodule size, and ultrasound pattern did not significantly impact malignancy prevalence.
The study results also indicated that surveillance ultrasonography is not routinely performed in up to one-third of patients, Goldberg said.
She closed by suggesting that colleagues consider the negative subtype in clinical decision-making. For “negative but limited” nodules, repeat the fine needle aspiration and, for “negative” and “currently negative” nodules, consider ultrasound follow-up as per ATA guidelines for Bethesda II cytology, she said.
RET-Mutated Medullary Thyroid Cancer
For patients with RET-mutated medullary thyroid cancer, Julien Hadoux, MD, PhD, of Institut de Cancérologie Gustave Roussy, Villejuif, France, presented a combined analysis of the efficacy of the RET inhibitor selpercatinib from the phase 1/2 LIBRETTO-001 and phase 3 LIBRETTO-531 trials.
This post hoc analysis used a combined cohort of 509 patients with RET-mutated advanced or metastatic medullary thyroid cancer who had received selpercatinib in the two trials.
Hadoux reported that robust and durable responses were seen across all mutation groups, including M918T, extracellular cysteine, and an “other” group composed of various uncommon RET mutations. “The median [progression-free survival] PFS was not reached for either the M918T or extracellular groups and it was 51.4 months for the Other group,” he said.
“Selpercatinib showed superior median PFS vs control, regardless of the RET mutation. This analysis constitutes the largest catalog of RET mutations in medullary thyroid cancers treated with RET-specific inhibitors.”
TRK-Fusion Differentiated Thyroid Cancer
Steven Waguespack, MD, of the University of Texas MD Anderson Cancer Center, Houston, shared updated efficacy and safety data from three phase 1/2 pooled clinical trials of the tropomyosin kinase receptor (TRK) inhibitor larotrectinib in thyroid cancer. These data updated results initially published in 2022.
“Larotrectinib continues to demonstrate rapid and durable responses, extended survival, and offers a favorable safety profile in patients with TRK fusion differentiated thyroid cancer, with limited activity in anaplastic thyroid cancer,” Waguespack said.
“Additionally, in a subset of patients, we identified some acquired on-target NTRK mutations and off-target GNAS and TP53 mutations that may give further insight into mechanisms of resistance.”
The primary endpoint was the investigator-assessed objective response rate (ORR); at 48 months, the ORR was 79% by independent review. The median PFS in patients with TRK fusion differentiated thyroid cancer was 44 months, while the median duration of response was 41 months. The 4-year overall survival rate was 86%.
Waguespack closed with a cautionary note to colleagues: “While circulating tumor DNA next-generation sequencing (NGS) analysis can be used to test for NTRK gene fusions, negative results should be followed up with tissue-based NGS,” he said.
Brito Campana and Goldberg disclosed no relevant financial relationships. Hadoux reported receiving honoraria for speaker engagements, advisory roles, or funding for CME from Eli Lilly, AAA, IPSEN, Roche, Pharma Mar, and EISAI, and research grants from Novartis, Sanofi, and Eli Lilly.
A version of this article appeared on Medscape.com.
The study, presented by Juan Brito Campana, MBBS, of the Mayo Clinic in Rochester, Minnesota, used Medicare records to perform a secondary analysis of 41,000 adults with type 2 diabetes and moderate cardiovascular risk who were new users of GLP-1 receptor agonists, compared to users of other diabetes medications.
“We took the innovative approach of applying the methodological rigor of a randomized clinical trial to the very large dataset of observational studies,” said Brito Campana.
The results showed a low absolute risk of thyroid cancer, with only 0.17% of patients in the GLP-1 group developing the disease. However, the data also showed a potential relative increase in risk during the first year of GLP-1 receptor agonist use.
“This is likely due to increased detection rather than true incidence, as the latency period for thyroid cancer development is typically longer,” Brito Campana said.
“We also note the limitations of the observational study design, including the short follow-up period and lack of detailed histological data. However, we believe the benefits of GLP-1 receptor agonists likely outweigh the risk of thyroid cancer.”
Malignancy in Bethesda III and IV Thyroid Nodules
At the same ATA session, Sapir Nachum Goldberg, MD, of the University of Pennsylvania, Philadelphia, presented the results of a retrospective record review that examined the prevalence of malignancy in Bethesda III and IV thyroid nodules with negative Thyrogen Receptor Signaling (ThyroSeq) version 3 molecular testing results.
Goldberg reported that 87% of patients with ThyroSeq negative subtype results were managed nonoperatively. “Based on our data, the true prevalence of malignancy likely lies between our low and high estimates of 3% and 23%,” she said. “We believe that the prevalence of malignancy may be higher in real-world practice than validation studies.”
Additionally, nodules with “currently negative” or “negative but limited” ThyroSeq results had a higher prevalence of malignancy (7%), compared with those with a “negative” result (2%). Factors like immediate vs delayed surgery, nodule size, and ultrasound pattern did not significantly impact malignancy prevalence.
The study results also indicated that surveillance ultrasonography is not routinely performed in up to one-third of patients, Goldberg said.
She closed by suggesting that colleagues consider the negative subtype in clinical decision-making. For “negative but limited” nodules, repeat the fine needle aspiration and, for “negative” and “currently negative” nodules, consider ultrasound follow-up as per ATA guidelines for Bethesda II cytology, she said.
RET-Mutated Medullary Thyroid Cancer
For patients with RET-mutated medullary thyroid cancer, Julien Hadoux, MD, PhD, of Institut de Cancérologie Gustave Roussy, Villejuif, France, presented a combined analysis of the efficacy of the RET inhibitor selpercatinib from the phase 1/2 LIBRETTO-001 and phase 3 LIBRETTO-531 trials.
This post hoc analysis used a combined cohort of 509 patients with RET-mutated advanced or metastatic medullary thyroid cancer who had received selpercatinib in the two trials.
Hadoux reported that robust and durable responses were seen across all mutation groups, including M918T, extracellular cysteine, and an “other” group composed of various uncommon RET mutations. “The median [progression-free survival] PFS was not reached for either the M918T or extracellular groups and it was 51.4 months for the Other group,” he said.
“Selpercatinib showed superior median PFS vs control, regardless of the RET mutation. This analysis constitutes the largest catalog of RET mutations in medullary thyroid cancers treated with RET-specific inhibitors.”
TRK-Fusion Differentiated Thyroid Cancer
Steven Waguespack, MD, of the University of Texas MD Anderson Cancer Center, Houston, shared updated efficacy and safety data from three phase 1/2 pooled clinical trials of the tropomyosin kinase receptor (TRK) inhibitor larotrectinib in thyroid cancer. These data updated results initially published in 2022.
“Larotrectinib continues to demonstrate rapid and durable responses, extended survival, and offers a favorable safety profile in patients with TRK fusion differentiated thyroid cancer, with limited activity in anaplastic thyroid cancer,” Waguespack said.
“Additionally, in a subset of patients, we identified some acquired on-target NTRK mutations and off-target GNAS and TP53 mutations that may give further insight into mechanisms of resistance.”
The primary endpoint was the investigator-assessed objective response rate (ORR); at 48 months, the ORR was 79% by independent review. The median PFS in patients with TRK fusion differentiated thyroid cancer was 44 months, while the median duration of response was 41 months. The 4-year overall survival rate was 86%.
Waguespack closed with a cautionary note to colleagues: “While circulating tumor DNA next-generation sequencing (NGS) analysis can be used to test for NTRK gene fusions, negative results should be followed up with tissue-based NGS,” he said.
Brito Campana and Goldberg disclosed no relevant financial relationships. Hadoux reported receiving honoraria for speaker engagements, advisory roles, or funding for CME from Eli Lilly, AAA, IPSEN, Roche, Pharma Mar, and EISAI, and research grants from Novartis, Sanofi, and Eli Lilly.
A version of this article appeared on Medscape.com.
FROM ATA 2024
Cosmetic Dermatology Product Recalls Still Common, Analysis Finds
TOPLINE:
Between 2011 and 2023, the US Food and Drug Administration (FDA) reported recalls of 334 cosmetic dermatology products in the United States, affecting over 77 million units, predominantly due to bacterial contamination.
METHODOLOGY:
- Researchers conducted a cross-sectional analysis of the FDA Enforcement Report database for cosmetic dermatology products from 2011 to 2023.
- Cosmetic products are any article “intended for body cleaning or beauty enhancement,” as defined by the FDA.
- Recalls were categorized by product type, reason for the recall, microbial contaminant, inorganic contaminant, distribution, and risk classification.
TAKEAWAY:
- During the study period, 334 voluntary and manufacturer-initiated recalls of cosmetic products were reported, affecting 77,135,700 units.
- A total of 297 recalls (88.9%) were categorized as Class II, indicating that they caused “medically reversible health consequences.” The median recall duration was 307 days.
- Hygiene and cleaning products accounted for most of the recalls (51.5%). Makeup gels, soaps, shampoos, tattoo ink, wipes, and lotions were the most recalled product categories. Nearly 51% of the products were distributed internationally.
- Microbial and inorganic contamination accounted for 76.8% and 10.2% of the recalls (the two most common reasons for the recall), respectively, with bacteria (80%) the most common contaminating pathogen (primarily Pseudomonas and Burkholderia species).
IN PRACTICE:
With 77 million units recalled by the FDA over 12 years, cosmetic recalls have remained common, the authors concluded, adding that “dermatologists should be key voices in pharmacovigilance given scientific expertise and frontline experience managing products and associated concerns.” Dermatologists, they added, “should also be aware of FDA enforcement reports for recall updates given that average recall termination took approximately 1 year.”
SOURCE:
The study was led by Kaushik P. Venkatesh, MBA, MPH, Harvard Medical School, Boston, and was published online on October 29 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations include the potential underreporting of Class III recalls (products that are unlikely to cause any adverse health reaction but violate FDA labeling or manufacturing laws) and lack of complete information on contaminants.
DISCLOSURES:
No information on funding was provided in the study. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Between 2011 and 2023, the US Food and Drug Administration (FDA) reported recalls of 334 cosmetic dermatology products in the United States, affecting over 77 million units, predominantly due to bacterial contamination.
METHODOLOGY:
- Researchers conducted a cross-sectional analysis of the FDA Enforcement Report database for cosmetic dermatology products from 2011 to 2023.
- Cosmetic products are any article “intended for body cleaning or beauty enhancement,” as defined by the FDA.
- Recalls were categorized by product type, reason for the recall, microbial contaminant, inorganic contaminant, distribution, and risk classification.
TAKEAWAY:
- During the study period, 334 voluntary and manufacturer-initiated recalls of cosmetic products were reported, affecting 77,135,700 units.
- A total of 297 recalls (88.9%) were categorized as Class II, indicating that they caused “medically reversible health consequences.” The median recall duration was 307 days.
- Hygiene and cleaning products accounted for most of the recalls (51.5%). Makeup gels, soaps, shampoos, tattoo ink, wipes, and lotions were the most recalled product categories. Nearly 51% of the products were distributed internationally.
- Microbial and inorganic contamination accounted for 76.8% and 10.2% of the recalls (the two most common reasons for the recall), respectively, with bacteria (80%) the most common contaminating pathogen (primarily Pseudomonas and Burkholderia species).
IN PRACTICE:
With 77 million units recalled by the FDA over 12 years, cosmetic recalls have remained common, the authors concluded, adding that “dermatologists should be key voices in pharmacovigilance given scientific expertise and frontline experience managing products and associated concerns.” Dermatologists, they added, “should also be aware of FDA enforcement reports for recall updates given that average recall termination took approximately 1 year.”
SOURCE:
The study was led by Kaushik P. Venkatesh, MBA, MPH, Harvard Medical School, Boston, and was published online on October 29 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations include the potential underreporting of Class III recalls (products that are unlikely to cause any adverse health reaction but violate FDA labeling or manufacturing laws) and lack of complete information on contaminants.
DISCLOSURES:
No information on funding was provided in the study. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Between 2011 and 2023, the US Food and Drug Administration (FDA) reported recalls of 334 cosmetic dermatology products in the United States, affecting over 77 million units, predominantly due to bacterial contamination.
METHODOLOGY:
- Researchers conducted a cross-sectional analysis of the FDA Enforcement Report database for cosmetic dermatology products from 2011 to 2023.
- Cosmetic products are any article “intended for body cleaning or beauty enhancement,” as defined by the FDA.
- Recalls were categorized by product type, reason for the recall, microbial contaminant, inorganic contaminant, distribution, and risk classification.
TAKEAWAY:
- During the study period, 334 voluntary and manufacturer-initiated recalls of cosmetic products were reported, affecting 77,135,700 units.
- A total of 297 recalls (88.9%) were categorized as Class II, indicating that they caused “medically reversible health consequences.” The median recall duration was 307 days.
- Hygiene and cleaning products accounted for most of the recalls (51.5%). Makeup gels, soaps, shampoos, tattoo ink, wipes, and lotions were the most recalled product categories. Nearly 51% of the products were distributed internationally.
- Microbial and inorganic contamination accounted for 76.8% and 10.2% of the recalls (the two most common reasons for the recall), respectively, with bacteria (80%) the most common contaminating pathogen (primarily Pseudomonas and Burkholderia species).
IN PRACTICE:
With 77 million units recalled by the FDA over 12 years, cosmetic recalls have remained common, the authors concluded, adding that “dermatologists should be key voices in pharmacovigilance given scientific expertise and frontline experience managing products and associated concerns.” Dermatologists, they added, “should also be aware of FDA enforcement reports for recall updates given that average recall termination took approximately 1 year.”
SOURCE:
The study was led by Kaushik P. Venkatesh, MBA, MPH, Harvard Medical School, Boston, and was published online on October 29 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations include the potential underreporting of Class III recalls (products that are unlikely to cause any adverse health reaction but violate FDA labeling or manufacturing laws) and lack of complete information on contaminants.
DISCLOSURES:
No information on funding was provided in the study. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
RA Prevention: A Decade of Trials Provides Insights on What’s to Come
With the discovery of autoantibodies and other risk factors for rheumatoid arthritis (RA), researchers developed clinical trials to see whether the disease can be prevented entirely. In the past 10 years, a number of these trials have concluded, with variable results.
While some trials demonstrated no effect at all, others showed that medical intervention can delay the onset of disease in certain populations and even reduce the rates of progression to RA. These completed trials also offer researchers the chance to identify opportunities to improve RA prevention trials moving forward.
“We’re looking at all that data and trying to figure out what the next step is going to be,” said Kevin Deane, MD, PhD, a professor of medicine and a rheumatologist at the University of Colorado School of Medicine, Aurora.
Key lessons include the need for improved risk stratification tools and better understanding of RA pathogenesis, he said.
The Research So Far
All RA prevention trials except for one have been completed and/or published within the past decade, bringing valuable insights to the field. (See chart below.)
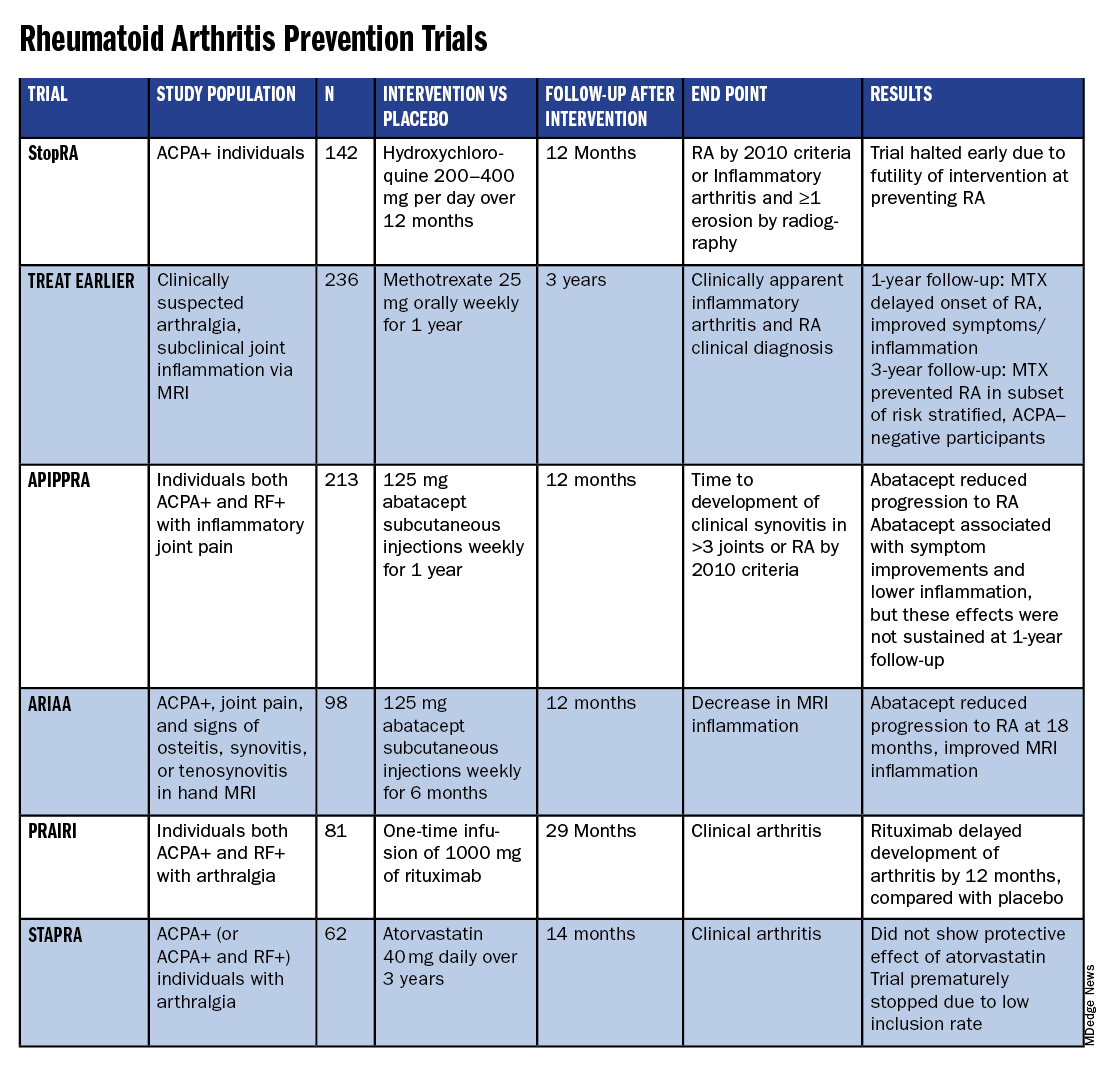
Atorvastatin (STAPRA) and hydroxychloroquine (StopRA) proved ineffective in preventing the onset of RA, and both trials were stopped early. Rituximab and methotrexate (MTX) both delayed the onset of RA, but the effect disappeared by the end of the follow-up periods.
However, the 2-year results from the TREAT EARLIER trial showed that compared with patients given placebo, those given MTX showed improved MRI-detected joint inflammation, physical functioning, and reported symptoms.
The 4-year analysis of the trial further risk stratified participants and found that MTX showed a preventive effect in anti–citrullinated protein antibody (ACPA)–negative participants at an increased risk for RA.
Abatacept also showed promise in preventing RA in two separate trials. In the ARIAA trial, compared with placebo, 6 months of treatment with abatacept reduced MRI inflammation and symptoms and lowered the rates of progression to RA. This treatment effect lessened during the 1-year follow-up period, but the difference between the two groups was still significant at 18 months.
In the APIPPRA trial, 12 months of treatment with abatacept improved subclinical inflammation and quality-of-life measures in participants and reduced the rates of progression to RA through another 12 months of observation. However, during this post-treatment follow-up period, the treatment effect began to diminish.
While there have been some promising findings — not only in disease prevention but also in disease modification — these studies all looked at different patient groups, noted Kulveer Mankia, MA, DM, an associate professor and consulting rheumatologist at the Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds in England.
“You have disparate, different inclusion criteria in different studies, all of which take years to complete,” he said. For example, while the TREAT EARLIER trial recruited patients with joint pain and subclinical joint inflammation via MRI, regardless of autoantibody status, the APIPPRA trial enrolled patients that were both ACPA+ and rheumatoid factor (RF)+ with joint pain.
“You’re left extrapolating as to whether [these interventions] will work in different at-risk populations,” he said.
Even with specific inclusion criteria in each study, there can still be heterogeneity in risk within a study group, Deane said. In the TREAT EARLIER study, 18%-20% of participants ultimately developed RA over the study period, which is lower than expected.
“While it seemed like a pretty high-risk group, it wasn’t as high risk as we thought,” he said, “and that’s why we’ve gone back to the drawing board.”
Risk Stratification Efforts
There are now two ongoing joint efforts by the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) to define these populations and “bring some consensus to the field,” Mankia said.
The first aims to create a unanimous risk stratification tool for future RA prevention studies. The proposed system, devised for individuals with new joint symptoms who are at a risk for RA, was presented at the EULAR 2024 annual meeting and will be further discussed at the upcoming ACR 2024 annual meeting in Washington, DC.
The system uses a point system based on six criteria — three lab tests and three criteria commonly assessed in clinical practice:
- Morning stiffness
- Patient-reported joint swelling
- Difficulty making a fist
- Increased C-reactive protein
- RF positivity
- ACPA positivity
These criteria were picked so that the risk stratification tool can be used without imaging; however, the inclusion of MRI can further refine the score.
The ACR-EULAR task force that created the tool has emphasized that this criterion is specifically designed for research purposes and should not be used in clinical practice. Using this stratification tool should allow future clinical studies to group patients by similar risk, Deane said.
“Not that all studies have to look at exactly the same people, but each study should have similar risk stratification,” he said.
The second ACR-EULAR joint effort is taking a population-based approach to risk stratification, Deane said, to better predict RA risk in individuals without common symptoms like joint pain.
The aim is to create something analogous to the Framingham Risk Score in predicting cardiovascular disease, in which simple variables like total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and smoking status can be used to calculate an individual’s 10-year risk for CVD, Deane explained.
The second approach could also identify patients earlier in the progression to RA, which may be easier to treat than later stages of disease.
Understanding RA Origins
However, treating an earlier stage of disease might require a different approach. Up to this point, medical interventions for RA prevention used drugs approved to treat RA, but inventions during the pre-RA stage — before any joint symptoms appear — might require targeting different immunologic pathways.
“The general concept is if there is a pre-RA stage when joints are not involved, that means all the immunologic abnormalities are probably happening somewhere else in the body,” he said. “The big question is: Where is that, and how exactly is that happening?”
One theory is that RA begins to develop in mucosal sites, such as the intestines or lungs, before it involves synovial joints.
“In the absence of resolution, these localized immune processes transition into a systemic process that targets the joints, either by direct effects of microbiota, molecular mimicry, and/or immune amplification,” wrote Deane and coauthors in a recent review article in Annals of the Rheumatic Diseases. “This, in turn, leads to inappropriate engagement of a range of effector mechanisms in both synovium and periarticular sites.”
Following this logic, the progression of the at-risk stage of RA could be considered a continuum along which there are multiple possible points for intervention. It’s also probable that the disease can develop through multiple pathways, Deane said.
“If you look at all the people who get rheumatoid arthritis, there’s probably no way those could have the same exact pathways,” he said. “There’s probably going to be different endotypes and understanding that is going to help us prevent disease in a better way.”
Looking Forward
Beyond improving risk stratification and understanding RA pathogenesis, researchers are also considering novel therapeutic approaches for future trials. Glucagon-like peptide 1 (GLP-1) receptor agonists could be worth exploring in RA prevention and treatment, said Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women’s Hospital, Boston, Massachusetts.
These drugs — initially developed for diabetes — have already shown anti-inflammatory effects, and one study suggested that GLP-1s lowered the risk for major adverse cardiovascular events and all-cause mortality in individuals with immune-mediated inflammatory diseases. Obesity is a known risk factor for RA, so weight loss aided by GLP-1 drugs could also help reduce risk in certain patients. Clinical trials are needed to explore GLP-1s for both RA prevention and treatment, he said.
While prevention trials up to this point have used one-time, time-limited interventions, longer durations of medication or multiple rounds of therapy may be more efficacious. Even for trials that demonstrated the intervention arms had less progression to RA, this effect diminished once participants stopped the medication. In the ARIAA and APIPPRA trials using abatacept, “it wasn’t like we hit a reset button and [patients] just permanently now did not get rheumatoid arthritis,” Deane said, suggesting that alternative approaches should be explored.
“Future studies need to look at potentially longer doses of drug or lower doses of drug, or some combination that might be effective,” he said.
Deane received honoraria from Bristol-Myers Squibb, Thermo Fisher, and Werfen and grant funding from Janssen Research and Development and Gilead Sciences. Mankia received grant support from Gilead, Lilly, AstraZeneca, and Serac Life Sciences and honoraria or consultant fees from AbbVie, UCB, Lilly, Galapagos, DeepCure, Serac Life Sciences, AstraZeneca, and Zura Bio. Sparks received research support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Sonoma Biotherapeutics. He consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Merck, Mustang, Optum, Pfizer, ReCor Medical, Sana, Sobi, and UCB.
A version of this article first appeared on Medscape.com.
With the discovery of autoantibodies and other risk factors for rheumatoid arthritis (RA), researchers developed clinical trials to see whether the disease can be prevented entirely. In the past 10 years, a number of these trials have concluded, with variable results.
While some trials demonstrated no effect at all, others showed that medical intervention can delay the onset of disease in certain populations and even reduce the rates of progression to RA. These completed trials also offer researchers the chance to identify opportunities to improve RA prevention trials moving forward.
“We’re looking at all that data and trying to figure out what the next step is going to be,” said Kevin Deane, MD, PhD, a professor of medicine and a rheumatologist at the University of Colorado School of Medicine, Aurora.
Key lessons include the need for improved risk stratification tools and better understanding of RA pathogenesis, he said.
The Research So Far
All RA prevention trials except for one have been completed and/or published within the past decade, bringing valuable insights to the field. (See chart below.)

Atorvastatin (STAPRA) and hydroxychloroquine (StopRA) proved ineffective in preventing the onset of RA, and both trials were stopped early. Rituximab and methotrexate (MTX) both delayed the onset of RA, but the effect disappeared by the end of the follow-up periods.
However, the 2-year results from the TREAT EARLIER trial showed that compared with patients given placebo, those given MTX showed improved MRI-detected joint inflammation, physical functioning, and reported symptoms.
The 4-year analysis of the trial further risk stratified participants and found that MTX showed a preventive effect in anti–citrullinated protein antibody (ACPA)–negative participants at an increased risk for RA.
Abatacept also showed promise in preventing RA in two separate trials. In the ARIAA trial, compared with placebo, 6 months of treatment with abatacept reduced MRI inflammation and symptoms and lowered the rates of progression to RA. This treatment effect lessened during the 1-year follow-up period, but the difference between the two groups was still significant at 18 months.
In the APIPPRA trial, 12 months of treatment with abatacept improved subclinical inflammation and quality-of-life measures in participants and reduced the rates of progression to RA through another 12 months of observation. However, during this post-treatment follow-up period, the treatment effect began to diminish.
While there have been some promising findings — not only in disease prevention but also in disease modification — these studies all looked at different patient groups, noted Kulveer Mankia, MA, DM, an associate professor and consulting rheumatologist at the Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds in England.
“You have disparate, different inclusion criteria in different studies, all of which take years to complete,” he said. For example, while the TREAT EARLIER trial recruited patients with joint pain and subclinical joint inflammation via MRI, regardless of autoantibody status, the APIPPRA trial enrolled patients that were both ACPA+ and rheumatoid factor (RF)+ with joint pain.
“You’re left extrapolating as to whether [these interventions] will work in different at-risk populations,” he said.
Even with specific inclusion criteria in each study, there can still be heterogeneity in risk within a study group, Deane said. In the TREAT EARLIER study, 18%-20% of participants ultimately developed RA over the study period, which is lower than expected.
“While it seemed like a pretty high-risk group, it wasn’t as high risk as we thought,” he said, “and that’s why we’ve gone back to the drawing board.”
Risk Stratification Efforts
There are now two ongoing joint efforts by the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) to define these populations and “bring some consensus to the field,” Mankia said.
The first aims to create a unanimous risk stratification tool for future RA prevention studies. The proposed system, devised for individuals with new joint symptoms who are at a risk for RA, was presented at the EULAR 2024 annual meeting and will be further discussed at the upcoming ACR 2024 annual meeting in Washington, DC.
The system uses a point system based on six criteria — three lab tests and three criteria commonly assessed in clinical practice:
- Morning stiffness
- Patient-reported joint swelling
- Difficulty making a fist
- Increased C-reactive protein
- RF positivity
- ACPA positivity
These criteria were picked so that the risk stratification tool can be used without imaging; however, the inclusion of MRI can further refine the score.
The ACR-EULAR task force that created the tool has emphasized that this criterion is specifically designed for research purposes and should not be used in clinical practice. Using this stratification tool should allow future clinical studies to group patients by similar risk, Deane said.
“Not that all studies have to look at exactly the same people, but each study should have similar risk stratification,” he said.
The second ACR-EULAR joint effort is taking a population-based approach to risk stratification, Deane said, to better predict RA risk in individuals without common symptoms like joint pain.
The aim is to create something analogous to the Framingham Risk Score in predicting cardiovascular disease, in which simple variables like total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and smoking status can be used to calculate an individual’s 10-year risk for CVD, Deane explained.
The second approach could also identify patients earlier in the progression to RA, which may be easier to treat than later stages of disease.
Understanding RA Origins
However, treating an earlier stage of disease might require a different approach. Up to this point, medical interventions for RA prevention used drugs approved to treat RA, but inventions during the pre-RA stage — before any joint symptoms appear — might require targeting different immunologic pathways.
“The general concept is if there is a pre-RA stage when joints are not involved, that means all the immunologic abnormalities are probably happening somewhere else in the body,” he said. “The big question is: Where is that, and how exactly is that happening?”
One theory is that RA begins to develop in mucosal sites, such as the intestines or lungs, before it involves synovial joints.
“In the absence of resolution, these localized immune processes transition into a systemic process that targets the joints, either by direct effects of microbiota, molecular mimicry, and/or immune amplification,” wrote Deane and coauthors in a recent review article in Annals of the Rheumatic Diseases. “This, in turn, leads to inappropriate engagement of a range of effector mechanisms in both synovium and periarticular sites.”
Following this logic, the progression of the at-risk stage of RA could be considered a continuum along which there are multiple possible points for intervention. It’s also probable that the disease can develop through multiple pathways, Deane said.
“If you look at all the people who get rheumatoid arthritis, there’s probably no way those could have the same exact pathways,” he said. “There’s probably going to be different endotypes and understanding that is going to help us prevent disease in a better way.”
Looking Forward
Beyond improving risk stratification and understanding RA pathogenesis, researchers are also considering novel therapeutic approaches for future trials. Glucagon-like peptide 1 (GLP-1) receptor agonists could be worth exploring in RA prevention and treatment, said Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women’s Hospital, Boston, Massachusetts.
These drugs — initially developed for diabetes — have already shown anti-inflammatory effects, and one study suggested that GLP-1s lowered the risk for major adverse cardiovascular events and all-cause mortality in individuals with immune-mediated inflammatory diseases. Obesity is a known risk factor for RA, so weight loss aided by GLP-1 drugs could also help reduce risk in certain patients. Clinical trials are needed to explore GLP-1s for both RA prevention and treatment, he said.
While prevention trials up to this point have used one-time, time-limited interventions, longer durations of medication or multiple rounds of therapy may be more efficacious. Even for trials that demonstrated the intervention arms had less progression to RA, this effect diminished once participants stopped the medication. In the ARIAA and APIPPRA trials using abatacept, “it wasn’t like we hit a reset button and [patients] just permanently now did not get rheumatoid arthritis,” Deane said, suggesting that alternative approaches should be explored.
“Future studies need to look at potentially longer doses of drug or lower doses of drug, or some combination that might be effective,” he said.
Deane received honoraria from Bristol-Myers Squibb, Thermo Fisher, and Werfen and grant funding from Janssen Research and Development and Gilead Sciences. Mankia received grant support from Gilead, Lilly, AstraZeneca, and Serac Life Sciences and honoraria or consultant fees from AbbVie, UCB, Lilly, Galapagos, DeepCure, Serac Life Sciences, AstraZeneca, and Zura Bio. Sparks received research support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Sonoma Biotherapeutics. He consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Merck, Mustang, Optum, Pfizer, ReCor Medical, Sana, Sobi, and UCB.
A version of this article first appeared on Medscape.com.
With the discovery of autoantibodies and other risk factors for rheumatoid arthritis (RA), researchers developed clinical trials to see whether the disease can be prevented entirely. In the past 10 years, a number of these trials have concluded, with variable results.
While some trials demonstrated no effect at all, others showed that medical intervention can delay the onset of disease in certain populations and even reduce the rates of progression to RA. These completed trials also offer researchers the chance to identify opportunities to improve RA prevention trials moving forward.
“We’re looking at all that data and trying to figure out what the next step is going to be,” said Kevin Deane, MD, PhD, a professor of medicine and a rheumatologist at the University of Colorado School of Medicine, Aurora.
Key lessons include the need for improved risk stratification tools and better understanding of RA pathogenesis, he said.
The Research So Far
All RA prevention trials except for one have been completed and/or published within the past decade, bringing valuable insights to the field. (See chart below.)

Atorvastatin (STAPRA) and hydroxychloroquine (StopRA) proved ineffective in preventing the onset of RA, and both trials were stopped early. Rituximab and methotrexate (MTX) both delayed the onset of RA, but the effect disappeared by the end of the follow-up periods.
However, the 2-year results from the TREAT EARLIER trial showed that compared with patients given placebo, those given MTX showed improved MRI-detected joint inflammation, physical functioning, and reported symptoms.
The 4-year analysis of the trial further risk stratified participants and found that MTX showed a preventive effect in anti–citrullinated protein antibody (ACPA)–negative participants at an increased risk for RA.
Abatacept also showed promise in preventing RA in two separate trials. In the ARIAA trial, compared with placebo, 6 months of treatment with abatacept reduced MRI inflammation and symptoms and lowered the rates of progression to RA. This treatment effect lessened during the 1-year follow-up period, but the difference between the two groups was still significant at 18 months.
In the APIPPRA trial, 12 months of treatment with abatacept improved subclinical inflammation and quality-of-life measures in participants and reduced the rates of progression to RA through another 12 months of observation. However, during this post-treatment follow-up period, the treatment effect began to diminish.
While there have been some promising findings — not only in disease prevention but also in disease modification — these studies all looked at different patient groups, noted Kulveer Mankia, MA, DM, an associate professor and consulting rheumatologist at the Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds in England.
“You have disparate, different inclusion criteria in different studies, all of which take years to complete,” he said. For example, while the TREAT EARLIER trial recruited patients with joint pain and subclinical joint inflammation via MRI, regardless of autoantibody status, the APIPPRA trial enrolled patients that were both ACPA+ and rheumatoid factor (RF)+ with joint pain.
“You’re left extrapolating as to whether [these interventions] will work in different at-risk populations,” he said.
Even with specific inclusion criteria in each study, there can still be heterogeneity in risk within a study group, Deane said. In the TREAT EARLIER study, 18%-20% of participants ultimately developed RA over the study period, which is lower than expected.
“While it seemed like a pretty high-risk group, it wasn’t as high risk as we thought,” he said, “and that’s why we’ve gone back to the drawing board.”
Risk Stratification Efforts
There are now two ongoing joint efforts by the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) to define these populations and “bring some consensus to the field,” Mankia said.
The first aims to create a unanimous risk stratification tool for future RA prevention studies. The proposed system, devised for individuals with new joint symptoms who are at a risk for RA, was presented at the EULAR 2024 annual meeting and will be further discussed at the upcoming ACR 2024 annual meeting in Washington, DC.
The system uses a point system based on six criteria — three lab tests and three criteria commonly assessed in clinical practice:
- Morning stiffness
- Patient-reported joint swelling
- Difficulty making a fist
- Increased C-reactive protein
- RF positivity
- ACPA positivity
These criteria were picked so that the risk stratification tool can be used without imaging; however, the inclusion of MRI can further refine the score.
The ACR-EULAR task force that created the tool has emphasized that this criterion is specifically designed for research purposes and should not be used in clinical practice. Using this stratification tool should allow future clinical studies to group patients by similar risk, Deane said.
“Not that all studies have to look at exactly the same people, but each study should have similar risk stratification,” he said.
The second ACR-EULAR joint effort is taking a population-based approach to risk stratification, Deane said, to better predict RA risk in individuals without common symptoms like joint pain.
The aim is to create something analogous to the Framingham Risk Score in predicting cardiovascular disease, in which simple variables like total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and smoking status can be used to calculate an individual’s 10-year risk for CVD, Deane explained.
The second approach could also identify patients earlier in the progression to RA, which may be easier to treat than later stages of disease.
Understanding RA Origins
However, treating an earlier stage of disease might require a different approach. Up to this point, medical interventions for RA prevention used drugs approved to treat RA, but inventions during the pre-RA stage — before any joint symptoms appear — might require targeting different immunologic pathways.
“The general concept is if there is a pre-RA stage when joints are not involved, that means all the immunologic abnormalities are probably happening somewhere else in the body,” he said. “The big question is: Where is that, and how exactly is that happening?”
One theory is that RA begins to develop in mucosal sites, such as the intestines or lungs, before it involves synovial joints.
“In the absence of resolution, these localized immune processes transition into a systemic process that targets the joints, either by direct effects of microbiota, molecular mimicry, and/or immune amplification,” wrote Deane and coauthors in a recent review article in Annals of the Rheumatic Diseases. “This, in turn, leads to inappropriate engagement of a range of effector mechanisms in both synovium and periarticular sites.”
Following this logic, the progression of the at-risk stage of RA could be considered a continuum along which there are multiple possible points for intervention. It’s also probable that the disease can develop through multiple pathways, Deane said.
“If you look at all the people who get rheumatoid arthritis, there’s probably no way those could have the same exact pathways,” he said. “There’s probably going to be different endotypes and understanding that is going to help us prevent disease in a better way.”
Looking Forward
Beyond improving risk stratification and understanding RA pathogenesis, researchers are also considering novel therapeutic approaches for future trials. Glucagon-like peptide 1 (GLP-1) receptor agonists could be worth exploring in RA prevention and treatment, said Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women’s Hospital, Boston, Massachusetts.
These drugs — initially developed for diabetes — have already shown anti-inflammatory effects, and one study suggested that GLP-1s lowered the risk for major adverse cardiovascular events and all-cause mortality in individuals with immune-mediated inflammatory diseases. Obesity is a known risk factor for RA, so weight loss aided by GLP-1 drugs could also help reduce risk in certain patients. Clinical trials are needed to explore GLP-1s for both RA prevention and treatment, he said.
While prevention trials up to this point have used one-time, time-limited interventions, longer durations of medication or multiple rounds of therapy may be more efficacious. Even for trials that demonstrated the intervention arms had less progression to RA, this effect diminished once participants stopped the medication. In the ARIAA and APIPPRA trials using abatacept, “it wasn’t like we hit a reset button and [patients] just permanently now did not get rheumatoid arthritis,” Deane said, suggesting that alternative approaches should be explored.
“Future studies need to look at potentially longer doses of drug or lower doses of drug, or some combination that might be effective,” he said.
Deane received honoraria from Bristol-Myers Squibb, Thermo Fisher, and Werfen and grant funding from Janssen Research and Development and Gilead Sciences. Mankia received grant support from Gilead, Lilly, AstraZeneca, and Serac Life Sciences and honoraria or consultant fees from AbbVie, UCB, Lilly, Galapagos, DeepCure, Serac Life Sciences, AstraZeneca, and Zura Bio. Sparks received research support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Sonoma Biotherapeutics. He consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Merck, Mustang, Optum, Pfizer, ReCor Medical, Sana, Sobi, and UCB.
A version of this article first appeared on Medscape.com.
Lifestyle Medicine Trends to Keep an Eye On
Our current healthcare system, which is a costly and unending cycle of merely managing chronic disease symptoms, is failing us. What we truly need is a patient-centered approach that restores health by addressing not just diagnoses but also the physical, emotional, and social needs of each individual. This is the essence of whole-person health, and transformation toward this model of care is already underway.
This shift underscores why clinicians like me support placing lifestyle medicine at the foundation of health and healthcare. Evidence-based lifestyle medicine — which applies interventions in nutrition, physical activity, restorative sleep, stress management, positive social connections, and avoidance of risky substances to prevent, treat, and when used intensively, even reverse lifestyle-related chronic disease — is a medical specialty equipped to successfully address patients’ whole-person health in an effective, high-value clinical care delivery model.
As this transformation continues, here are four key lifestyle medicine trends for 2025.
Lifestyle Medicine Becomes More Ingrained in Primary Care
The 2021 National Academies of Science, Engineering, and Medicine report, “Implementing High-Quality Primary Care” sounded the alarm about the state of primary care and outlined a comprehensive approach to transform it. Lifestyle medicine emerged as a solution as clinicians found innovative ways to integrate lifestyle behavior interventions into existing care models in a financially sustainable, scalable manner. Examples include Blue Zones Health, a new delivery model that aligns lifestyle medicine–certified clinicians with community and payers in California, and the University of Pittsburgh Medical Center lifestyle medicine program, where primary care patients are referred to virtual group coaching, a teaching kitchen, and classes on food as medicine, obesity, type 2 diabetes, and more.
Organizations dedicated to advancing primary care are paying close attention to the potential of lifestyle medicine. Currently, The Primary Care Collaborative has launched a new multi-year initiative on whole-person care and lifestyle medicine. This initiative aims to broaden the primary care community’s understanding of whole health and lifestyle medicine concepts and the evidence behind them, as well as lay the groundwork for future work to promote whole-person primary care and lifestyle medicine among an engaged and committed community of members.
Digital Tools and AI Spark Lifestyle Medicine Innovations
American College of Lifestyle Medicine partner organizations are increasingly utilizing digital tools, such as health apps tailored to lifestyle behavior interventions, to expand access to care and support behavior change. One of the biggest challenges in lifestyle interventions is the limited time during patient encounters. But artificial intelligence (AI) tools can record (with patient permission) and summarize encounters, enabling clinicians to turn away from their keyboards and be more present to learn about the unique living, environmental, and societal factors that impact every individual’s lifestyle choices. AI tools can create individualized whole-food, plant-predominant meal plans or physical activity schedules for patients in just a few seconds. The potential for AI in lifestyle medicine is vast, and its applications were further explored at the American College of Lifestyle Medicine’s annual conference in October.
Behavior Change and Sustainability of the Food-as-Medicine Movement
Significant investments have been made in food as medicine to address diet-related chronic diseases. But merely providing medically tailored meals or produce prescriptions is not enough because once the prescriptions end, so will the health benefits. Clinicians certified in lifestyle medicine are prepared to coach patients into long-term behavior change, supporting them with education and information to shop for and prepare tasty, nutritious, and affordable food. The same applies to the use of glucagon-like peptide 1 drugs. Although the initial weight loss offers motivation, lifestyle changes are necessary to sustain long-term health benefits beyond medications.
Lifestyle Medicine Emerges as a Strategy to Achieve Health Equity
Lifestyle behavior interventions have the unique ability to address health status and social drivers of health. For example, food as medicine affects an individual’s health while also addressing nutrition security. Certainly, no medication can both improve health status and feed someone. The addition of payment for the screening of social drivers of health to the 2024 Medicare Physician Fee Schedule is an important step toward connecting clinicians with community health–based organizations that can address factors that influence patients’ ability to adhere to lifestyle behavior care plans. Lifestyle medicine clinicians are poised to lead this effort because they are already having conversations with patients about their environment, living conditions, and access to nutritious food.
The changes coming to our healthcare system are exciting and long overdue. Lifestyle medicine is positioned to be at the forefront of this transformation now and in the future.
Dr. Patel, president of the American College of Lifestyle Medicine in St. Louis, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Our current healthcare system, which is a costly and unending cycle of merely managing chronic disease symptoms, is failing us. What we truly need is a patient-centered approach that restores health by addressing not just diagnoses but also the physical, emotional, and social needs of each individual. This is the essence of whole-person health, and transformation toward this model of care is already underway.
This shift underscores why clinicians like me support placing lifestyle medicine at the foundation of health and healthcare. Evidence-based lifestyle medicine — which applies interventions in nutrition, physical activity, restorative sleep, stress management, positive social connections, and avoidance of risky substances to prevent, treat, and when used intensively, even reverse lifestyle-related chronic disease — is a medical specialty equipped to successfully address patients’ whole-person health in an effective, high-value clinical care delivery model.
As this transformation continues, here are four key lifestyle medicine trends for 2025.
Lifestyle Medicine Becomes More Ingrained in Primary Care
The 2021 National Academies of Science, Engineering, and Medicine report, “Implementing High-Quality Primary Care” sounded the alarm about the state of primary care and outlined a comprehensive approach to transform it. Lifestyle medicine emerged as a solution as clinicians found innovative ways to integrate lifestyle behavior interventions into existing care models in a financially sustainable, scalable manner. Examples include Blue Zones Health, a new delivery model that aligns lifestyle medicine–certified clinicians with community and payers in California, and the University of Pittsburgh Medical Center lifestyle medicine program, where primary care patients are referred to virtual group coaching, a teaching kitchen, and classes on food as medicine, obesity, type 2 diabetes, and more.
Organizations dedicated to advancing primary care are paying close attention to the potential of lifestyle medicine. Currently, The Primary Care Collaborative has launched a new multi-year initiative on whole-person care and lifestyle medicine. This initiative aims to broaden the primary care community’s understanding of whole health and lifestyle medicine concepts and the evidence behind them, as well as lay the groundwork for future work to promote whole-person primary care and lifestyle medicine among an engaged and committed community of members.
Digital Tools and AI Spark Lifestyle Medicine Innovations
American College of Lifestyle Medicine partner organizations are increasingly utilizing digital tools, such as health apps tailored to lifestyle behavior interventions, to expand access to care and support behavior change. One of the biggest challenges in lifestyle interventions is the limited time during patient encounters. But artificial intelligence (AI) tools can record (with patient permission) and summarize encounters, enabling clinicians to turn away from their keyboards and be more present to learn about the unique living, environmental, and societal factors that impact every individual’s lifestyle choices. AI tools can create individualized whole-food, plant-predominant meal plans or physical activity schedules for patients in just a few seconds. The potential for AI in lifestyle medicine is vast, and its applications were further explored at the American College of Lifestyle Medicine’s annual conference in October.
Behavior Change and Sustainability of the Food-as-Medicine Movement
Significant investments have been made in food as medicine to address diet-related chronic diseases. But merely providing medically tailored meals or produce prescriptions is not enough because once the prescriptions end, so will the health benefits. Clinicians certified in lifestyle medicine are prepared to coach patients into long-term behavior change, supporting them with education and information to shop for and prepare tasty, nutritious, and affordable food. The same applies to the use of glucagon-like peptide 1 drugs. Although the initial weight loss offers motivation, lifestyle changes are necessary to sustain long-term health benefits beyond medications.
Lifestyle Medicine Emerges as a Strategy to Achieve Health Equity
Lifestyle behavior interventions have the unique ability to address health status and social drivers of health. For example, food as medicine affects an individual’s health while also addressing nutrition security. Certainly, no medication can both improve health status and feed someone. The addition of payment for the screening of social drivers of health to the 2024 Medicare Physician Fee Schedule is an important step toward connecting clinicians with community health–based organizations that can address factors that influence patients’ ability to adhere to lifestyle behavior care plans. Lifestyle medicine clinicians are poised to lead this effort because they are already having conversations with patients about their environment, living conditions, and access to nutritious food.
The changes coming to our healthcare system are exciting and long overdue. Lifestyle medicine is positioned to be at the forefront of this transformation now and in the future.
Dr. Patel, president of the American College of Lifestyle Medicine in St. Louis, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Our current healthcare system, which is a costly and unending cycle of merely managing chronic disease symptoms, is failing us. What we truly need is a patient-centered approach that restores health by addressing not just diagnoses but also the physical, emotional, and social needs of each individual. This is the essence of whole-person health, and transformation toward this model of care is already underway.
This shift underscores why clinicians like me support placing lifestyle medicine at the foundation of health and healthcare. Evidence-based lifestyle medicine — which applies interventions in nutrition, physical activity, restorative sleep, stress management, positive social connections, and avoidance of risky substances to prevent, treat, and when used intensively, even reverse lifestyle-related chronic disease — is a medical specialty equipped to successfully address patients’ whole-person health in an effective, high-value clinical care delivery model.
As this transformation continues, here are four key lifestyle medicine trends for 2025.
Lifestyle Medicine Becomes More Ingrained in Primary Care
The 2021 National Academies of Science, Engineering, and Medicine report, “Implementing High-Quality Primary Care” sounded the alarm about the state of primary care and outlined a comprehensive approach to transform it. Lifestyle medicine emerged as a solution as clinicians found innovative ways to integrate lifestyle behavior interventions into existing care models in a financially sustainable, scalable manner. Examples include Blue Zones Health, a new delivery model that aligns lifestyle medicine–certified clinicians with community and payers in California, and the University of Pittsburgh Medical Center lifestyle medicine program, where primary care patients are referred to virtual group coaching, a teaching kitchen, and classes on food as medicine, obesity, type 2 diabetes, and more.
Organizations dedicated to advancing primary care are paying close attention to the potential of lifestyle medicine. Currently, The Primary Care Collaborative has launched a new multi-year initiative on whole-person care and lifestyle medicine. This initiative aims to broaden the primary care community’s understanding of whole health and lifestyle medicine concepts and the evidence behind them, as well as lay the groundwork for future work to promote whole-person primary care and lifestyle medicine among an engaged and committed community of members.
Digital Tools and AI Spark Lifestyle Medicine Innovations
American College of Lifestyle Medicine partner organizations are increasingly utilizing digital tools, such as health apps tailored to lifestyle behavior interventions, to expand access to care and support behavior change. One of the biggest challenges in lifestyle interventions is the limited time during patient encounters. But artificial intelligence (AI) tools can record (with patient permission) and summarize encounters, enabling clinicians to turn away from their keyboards and be more present to learn about the unique living, environmental, and societal factors that impact every individual’s lifestyle choices. AI tools can create individualized whole-food, plant-predominant meal plans or physical activity schedules for patients in just a few seconds. The potential for AI in lifestyle medicine is vast, and its applications were further explored at the American College of Lifestyle Medicine’s annual conference in October.
Behavior Change and Sustainability of the Food-as-Medicine Movement
Significant investments have been made in food as medicine to address diet-related chronic diseases. But merely providing medically tailored meals or produce prescriptions is not enough because once the prescriptions end, so will the health benefits. Clinicians certified in lifestyle medicine are prepared to coach patients into long-term behavior change, supporting them with education and information to shop for and prepare tasty, nutritious, and affordable food. The same applies to the use of glucagon-like peptide 1 drugs. Although the initial weight loss offers motivation, lifestyle changes are necessary to sustain long-term health benefits beyond medications.
Lifestyle Medicine Emerges as a Strategy to Achieve Health Equity
Lifestyle behavior interventions have the unique ability to address health status and social drivers of health. For example, food as medicine affects an individual’s health while also addressing nutrition security. Certainly, no medication can both improve health status and feed someone. The addition of payment for the screening of social drivers of health to the 2024 Medicare Physician Fee Schedule is an important step toward connecting clinicians with community health–based organizations that can address factors that influence patients’ ability to adhere to lifestyle behavior care plans. Lifestyle medicine clinicians are poised to lead this effort because they are already having conversations with patients about their environment, living conditions, and access to nutritious food.
The changes coming to our healthcare system are exciting and long overdue. Lifestyle medicine is positioned to be at the forefront of this transformation now and in the future.
Dr. Patel, president of the American College of Lifestyle Medicine in St. Louis, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Novel Treatment Promising for Cutaneous Lupus in Phase 2 Trial
TOPLINE:
particularly in subacute and chronic cases.
METHODOLOGY:
- Researchers conducted a randomized phase 2 trial to evaluate the efficacy and safety of iberdomide in 288 patients with CLE (mean age, 45 years; 97% women). Iberdomide is a cereblon modulator, which results in degradation of two transcription factors of immune cell development and homeostasis — Ikaros and Aiolos — that have been implicated in the genetic predisposition of systemic lupus.
- CLE Disease Area and Severity Index Activity (CLASI-A) endpoints included mean percent change from baseline and ≥ 50% reduction from baseline (CLASI-50), which were evaluated in all patients with baseline CLASI-A scores ≥ 8 and by CLE subtypes (acute, subacute, and chronic).
- At baseline, 56% of patients had acute CLE, 29% had chronic CLE, and 16% had subacute CLE; 28% of patients had a baseline CLASI-A score ≥ 8.
- Patients were randomly assigned to receive oral iberdomide (0.45 mg, 0.30 mg, 0.15 mg, or placebo daily) for 24 weeks while continuing standard lupus medications. At week 24, patients on placebo were rerandomized to iberdomide 0.45 mg or 0.30 mg once a day, while those on iberdomide continued their assigned dose through week 52.
TAKEAWAY:
- Among patients with baseline CLASI-A ≥ 8, the mean change in CLASI-A score from baseline at week 24 was −66.7% for those on iberdomide 0.45 mg vs −54.2% for placebo (P = .295).
- At week 24, patients with subacute CLE showed a significantly greater mean percent change from baseline in CLASI-A with iberdomide 0.45 mg vs placebo (−90.5% vs −51.2%; P = .007), while no significant differences were observed with the 0.45-mg dose vs placebo in patients with chronic or acute CLE.
- Overall, CLASI-50 responses were not significantly different among those on 0.45 mg vs placebo (55.6% vs 44.6%). The proportions of patients achieving CLASI-50 at week 24 were significantly greater for iberdomide 0.45 mg vs placebo for those with subacute CLE (91.7% vs 52.9%; P = .035) and chronic CLE (62.1% vs 27.8%; P = .029), but not for those with baseline CLASI-A ≥ 8 (66.7% vs 50%).
- More than 80% of patients had treatment-emergent adverse events (TEAEs), which were mostly mild to moderate. Over 2 years, the most common were urinary tract infections, upper respiratory tract infections, neutropenia, and nasopharyngitis. TEAEs leading to iberdomide discontinuation in one or more patients were neutropenia (n = 7), rash (n = 7), increased hepatic enzymes (n = 4), and deep vein thrombosis (n = 3).
IN PRACTICE:
“Data from this phase 2 trial of iberdomide in patients with SLE suggest that a greater proportion of patients with subacute or chronic CLE who received the higher dose of 0.45 mg iberdomide achieved CLASI-50 vs placebo. For the overall population, CLASI-50 response was not significantly different between treatment groups at week 24, partly due to a high placebo response that may have been driven by patients with acute CLE,” the authors wrote.
SOURCE:
The study was led by Victoria P. Werth, MD, of the University of Pennsylvania and the Veteran’s Administration Medical Center, both in Philadelphia, and was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study included small patient subgroups for different CLE subtypes, which may affect the generalizability of the findings. CLE subtype was determined by the investigator without additional photographic adjudication. Additionally, the use of background lupus medications could have influenced the placebo group’s response, limiting the ability to observe the treatment effect of iberdomide monotherapy.
DISCLOSURES:
The study was funded by Bristol-Myers Squibb. Six authors reported being employed by Bristol-Myers Squibb, and several others reported consultancy and research support from various sources including Bristol-Myers Squibb.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
particularly in subacute and chronic cases.
METHODOLOGY:
- Researchers conducted a randomized phase 2 trial to evaluate the efficacy and safety of iberdomide in 288 patients with CLE (mean age, 45 years; 97% women). Iberdomide is a cereblon modulator, which results in degradation of two transcription factors of immune cell development and homeostasis — Ikaros and Aiolos — that have been implicated in the genetic predisposition of systemic lupus.
- CLE Disease Area and Severity Index Activity (CLASI-A) endpoints included mean percent change from baseline and ≥ 50% reduction from baseline (CLASI-50), which were evaluated in all patients with baseline CLASI-A scores ≥ 8 and by CLE subtypes (acute, subacute, and chronic).
- At baseline, 56% of patients had acute CLE, 29% had chronic CLE, and 16% had subacute CLE; 28% of patients had a baseline CLASI-A score ≥ 8.
- Patients were randomly assigned to receive oral iberdomide (0.45 mg, 0.30 mg, 0.15 mg, or placebo daily) for 24 weeks while continuing standard lupus medications. At week 24, patients on placebo were rerandomized to iberdomide 0.45 mg or 0.30 mg once a day, while those on iberdomide continued their assigned dose through week 52.
TAKEAWAY:
- Among patients with baseline CLASI-A ≥ 8, the mean change in CLASI-A score from baseline at week 24 was −66.7% for those on iberdomide 0.45 mg vs −54.2% for placebo (P = .295).
- At week 24, patients with subacute CLE showed a significantly greater mean percent change from baseline in CLASI-A with iberdomide 0.45 mg vs placebo (−90.5% vs −51.2%; P = .007), while no significant differences were observed with the 0.45-mg dose vs placebo in patients with chronic or acute CLE.
- Overall, CLASI-50 responses were not significantly different among those on 0.45 mg vs placebo (55.6% vs 44.6%). The proportions of patients achieving CLASI-50 at week 24 were significantly greater for iberdomide 0.45 mg vs placebo for those with subacute CLE (91.7% vs 52.9%; P = .035) and chronic CLE (62.1% vs 27.8%; P = .029), but not for those with baseline CLASI-A ≥ 8 (66.7% vs 50%).
- More than 80% of patients had treatment-emergent adverse events (TEAEs), which were mostly mild to moderate. Over 2 years, the most common were urinary tract infections, upper respiratory tract infections, neutropenia, and nasopharyngitis. TEAEs leading to iberdomide discontinuation in one or more patients were neutropenia (n = 7), rash (n = 7), increased hepatic enzymes (n = 4), and deep vein thrombosis (n = 3).
IN PRACTICE:
“Data from this phase 2 trial of iberdomide in patients with SLE suggest that a greater proportion of patients with subacute or chronic CLE who received the higher dose of 0.45 mg iberdomide achieved CLASI-50 vs placebo. For the overall population, CLASI-50 response was not significantly different between treatment groups at week 24, partly due to a high placebo response that may have been driven by patients with acute CLE,” the authors wrote.
SOURCE:
The study was led by Victoria P. Werth, MD, of the University of Pennsylvania and the Veteran’s Administration Medical Center, both in Philadelphia, and was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study included small patient subgroups for different CLE subtypes, which may affect the generalizability of the findings. CLE subtype was determined by the investigator without additional photographic adjudication. Additionally, the use of background lupus medications could have influenced the placebo group’s response, limiting the ability to observe the treatment effect of iberdomide monotherapy.
DISCLOSURES:
The study was funded by Bristol-Myers Squibb. Six authors reported being employed by Bristol-Myers Squibb, and several others reported consultancy and research support from various sources including Bristol-Myers Squibb.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
particularly in subacute and chronic cases.
METHODOLOGY:
- Researchers conducted a randomized phase 2 trial to evaluate the efficacy and safety of iberdomide in 288 patients with CLE (mean age, 45 years; 97% women). Iberdomide is a cereblon modulator, which results in degradation of two transcription factors of immune cell development and homeostasis — Ikaros and Aiolos — that have been implicated in the genetic predisposition of systemic lupus.
- CLE Disease Area and Severity Index Activity (CLASI-A) endpoints included mean percent change from baseline and ≥ 50% reduction from baseline (CLASI-50), which were evaluated in all patients with baseline CLASI-A scores ≥ 8 and by CLE subtypes (acute, subacute, and chronic).
- At baseline, 56% of patients had acute CLE, 29% had chronic CLE, and 16% had subacute CLE; 28% of patients had a baseline CLASI-A score ≥ 8.
- Patients were randomly assigned to receive oral iberdomide (0.45 mg, 0.30 mg, 0.15 mg, or placebo daily) for 24 weeks while continuing standard lupus medications. At week 24, patients on placebo were rerandomized to iberdomide 0.45 mg or 0.30 mg once a day, while those on iberdomide continued their assigned dose through week 52.
TAKEAWAY:
- Among patients with baseline CLASI-A ≥ 8, the mean change in CLASI-A score from baseline at week 24 was −66.7% for those on iberdomide 0.45 mg vs −54.2% for placebo (P = .295).
- At week 24, patients with subacute CLE showed a significantly greater mean percent change from baseline in CLASI-A with iberdomide 0.45 mg vs placebo (−90.5% vs −51.2%; P = .007), while no significant differences were observed with the 0.45-mg dose vs placebo in patients with chronic or acute CLE.
- Overall, CLASI-50 responses were not significantly different among those on 0.45 mg vs placebo (55.6% vs 44.6%). The proportions of patients achieving CLASI-50 at week 24 were significantly greater for iberdomide 0.45 mg vs placebo for those with subacute CLE (91.7% vs 52.9%; P = .035) and chronic CLE (62.1% vs 27.8%; P = .029), but not for those with baseline CLASI-A ≥ 8 (66.7% vs 50%).
- More than 80% of patients had treatment-emergent adverse events (TEAEs), which were mostly mild to moderate. Over 2 years, the most common were urinary tract infections, upper respiratory tract infections, neutropenia, and nasopharyngitis. TEAEs leading to iberdomide discontinuation in one or more patients were neutropenia (n = 7), rash (n = 7), increased hepatic enzymes (n = 4), and deep vein thrombosis (n = 3).
IN PRACTICE:
“Data from this phase 2 trial of iberdomide in patients with SLE suggest that a greater proportion of patients with subacute or chronic CLE who received the higher dose of 0.45 mg iberdomide achieved CLASI-50 vs placebo. For the overall population, CLASI-50 response was not significantly different between treatment groups at week 24, partly due to a high placebo response that may have been driven by patients with acute CLE,” the authors wrote.
SOURCE:
The study was led by Victoria P. Werth, MD, of the University of Pennsylvania and the Veteran’s Administration Medical Center, both in Philadelphia, and was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study included small patient subgroups for different CLE subtypes, which may affect the generalizability of the findings. CLE subtype was determined by the investigator without additional photographic adjudication. Additionally, the use of background lupus medications could have influenced the placebo group’s response, limiting the ability to observe the treatment effect of iberdomide monotherapy.
DISCLOSURES:
The study was funded by Bristol-Myers Squibb. Six authors reported being employed by Bristol-Myers Squibb, and several others reported consultancy and research support from various sources including Bristol-Myers Squibb.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Social Adversity Increases Mortality Risk in Patients With Pulmonary Hypertension
BOSTON — Social adversity is associated with worse survival among patients with pulmonary hypertension (PH), according to a new retrospective study of a New York City population.
A sub-analysis of both HIV+ and HIV– patients showed worse mortality outcomes with social adversity in both groups.
“Almost the majority of patients that we treat have either some social adversity or no insurance or are undocumented, so as a group of residents, we decided to study the impact of these factors on their health and the care that can be provided. We started using the two cohorts and now we keep it going with every new resident,” said Luca Biavati, MD, who presented the study at the CHEST Annual Meeting.
“The presence of any form of socioeconomic disadvantage is negatively impacting care and for a large part of the population, there are some factors that could probably be addressed by either an institutional or hospital policy,” said Dr. Biavati, who is an internal medicine resident at Jacobi Medical Center, New York.
Other factors are more difficult to address, such as lack of education. “[Some patients] don’t understand the gravity of their issue and medical condition until it’s too late, and then they’re not fit enough for the treatment, or just because of the social situation, they cannot qualify for advanced therapies,” said Dr. Biavati.
The researchers established two cohorts: One consisting of patients with HIV and heart failure who may or may not have had PH and one comprising patients with PH with or without HIV and heart failure. In the HIV/heart failure group, PH without social adversity was associated with a nearly threefold increase in all-cause mortality (hazard ratio [HR], 2.83; P = .004), whereas PH with social adversity was linked to a more than sevenfold increase in all-cause mortality (HR, 7.14; P < .001). Social adversity without PA was associated with a more than fourfold increase (HR, 4.47; P < .001).
Within the PH cohort, social adversity was associated with lower survival (P < .001). When the researchers broke down the results by types of social adversity, they found statistically significant relationships between greater mortality risk and economic instability within the HIV+ population (HR, 2.59; P = .040), transportation issues within the HIV– population (HR, 12.8; P < .001), and lack of social or family support within both the HIV– (HR, 5.49; P < .001) and the HIV+ population (HR, 2.03; P = .028).
The research has prompted interventions, which are now being studied at the institution, according to Dr. Biavati. “We have a policy of giving medications in bags when we discharge a patient with a social adversity. We literally go to the pharmacy, bring up the bag of medication, and we [put it] in their hands before they leave the hospital. They get a 1- or 3-month supply, depending on the medication, and then we usually discharge them with a clinical appointment already scheduled with either a pulmonary or primary care provider, and we usually call them before every appointment to confirm that they’re coming. That increases the chances of some success, but there’s still a very long way to go,” said Dr. Biavati.
Dr. Biavati was blinded to the results of the intervention, so he could not report on whether it was working. “But I can tell you that I’ve had busier clinics, so hopefully that means that they’re showing up more,” he said.
The problem is complex, according to Sandeep Jain, MD, who moderated the session. “Social adversity means lack of education. Lack of education means lack of compliance. Lack of compliance means what can you do if people are not taking medications? So it’s all matched together. It’s all lack of education and lack of money, lack of family support. And these drugs they have to take every single day. It’s not that easy. It’s very easy for us to say I had antiretroviral treatment for 6 months. It is almost impossible to continue regular treatment for that long [for a patient with social adversity]. You can’t blame them if they aren’t taking treatments. It’s very difficult for them,” said Dr. Jain.
That underscores the need for interventions that can address the needs of patients with social adversity. “We have to [practice] medicine considering the social situation of the patient and not just the medicine that we study in books. That’s kind of what we are faced with every day. We have therapies, and then life happens. It’s much harder to care for those patients,” said Dr. Biavati.
Dr. Biavati and Dr. Jain reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON — Social adversity is associated with worse survival among patients with pulmonary hypertension (PH), according to a new retrospective study of a New York City population.
A sub-analysis of both HIV+ and HIV– patients showed worse mortality outcomes with social adversity in both groups.
“Almost the majority of patients that we treat have either some social adversity or no insurance or are undocumented, so as a group of residents, we decided to study the impact of these factors on their health and the care that can be provided. We started using the two cohorts and now we keep it going with every new resident,” said Luca Biavati, MD, who presented the study at the CHEST Annual Meeting.
“The presence of any form of socioeconomic disadvantage is negatively impacting care and for a large part of the population, there are some factors that could probably be addressed by either an institutional or hospital policy,” said Dr. Biavati, who is an internal medicine resident at Jacobi Medical Center, New York.
Other factors are more difficult to address, such as lack of education. “[Some patients] don’t understand the gravity of their issue and medical condition until it’s too late, and then they’re not fit enough for the treatment, or just because of the social situation, they cannot qualify for advanced therapies,” said Dr. Biavati.
The researchers established two cohorts: One consisting of patients with HIV and heart failure who may or may not have had PH and one comprising patients with PH with or without HIV and heart failure. In the HIV/heart failure group, PH without social adversity was associated with a nearly threefold increase in all-cause mortality (hazard ratio [HR], 2.83; P = .004), whereas PH with social adversity was linked to a more than sevenfold increase in all-cause mortality (HR, 7.14; P < .001). Social adversity without PA was associated with a more than fourfold increase (HR, 4.47; P < .001).
Within the PH cohort, social adversity was associated with lower survival (P < .001). When the researchers broke down the results by types of social adversity, they found statistically significant relationships between greater mortality risk and economic instability within the HIV+ population (HR, 2.59; P = .040), transportation issues within the HIV– population (HR, 12.8; P < .001), and lack of social or family support within both the HIV– (HR, 5.49; P < .001) and the HIV+ population (HR, 2.03; P = .028).
The research has prompted interventions, which are now being studied at the institution, according to Dr. Biavati. “We have a policy of giving medications in bags when we discharge a patient with a social adversity. We literally go to the pharmacy, bring up the bag of medication, and we [put it] in their hands before they leave the hospital. They get a 1- or 3-month supply, depending on the medication, and then we usually discharge them with a clinical appointment already scheduled with either a pulmonary or primary care provider, and we usually call them before every appointment to confirm that they’re coming. That increases the chances of some success, but there’s still a very long way to go,” said Dr. Biavati.
Dr. Biavati was blinded to the results of the intervention, so he could not report on whether it was working. “But I can tell you that I’ve had busier clinics, so hopefully that means that they’re showing up more,” he said.
The problem is complex, according to Sandeep Jain, MD, who moderated the session. “Social adversity means lack of education. Lack of education means lack of compliance. Lack of compliance means what can you do if people are not taking medications? So it’s all matched together. It’s all lack of education and lack of money, lack of family support. And these drugs they have to take every single day. It’s not that easy. It’s very easy for us to say I had antiretroviral treatment for 6 months. It is almost impossible to continue regular treatment for that long [for a patient with social adversity]. You can’t blame them if they aren’t taking treatments. It’s very difficult for them,” said Dr. Jain.
That underscores the need for interventions that can address the needs of patients with social adversity. “We have to [practice] medicine considering the social situation of the patient and not just the medicine that we study in books. That’s kind of what we are faced with every day. We have therapies, and then life happens. It’s much harder to care for those patients,” said Dr. Biavati.
Dr. Biavati and Dr. Jain reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON — Social adversity is associated with worse survival among patients with pulmonary hypertension (PH), according to a new retrospective study of a New York City population.
A sub-analysis of both HIV+ and HIV– patients showed worse mortality outcomes with social adversity in both groups.
“Almost the majority of patients that we treat have either some social adversity or no insurance or are undocumented, so as a group of residents, we decided to study the impact of these factors on their health and the care that can be provided. We started using the two cohorts and now we keep it going with every new resident,” said Luca Biavati, MD, who presented the study at the CHEST Annual Meeting.
“The presence of any form of socioeconomic disadvantage is negatively impacting care and for a large part of the population, there are some factors that could probably be addressed by either an institutional or hospital policy,” said Dr. Biavati, who is an internal medicine resident at Jacobi Medical Center, New York.
Other factors are more difficult to address, such as lack of education. “[Some patients] don’t understand the gravity of their issue and medical condition until it’s too late, and then they’re not fit enough for the treatment, or just because of the social situation, they cannot qualify for advanced therapies,” said Dr. Biavati.
The researchers established two cohorts: One consisting of patients with HIV and heart failure who may or may not have had PH and one comprising patients with PH with or without HIV and heart failure. In the HIV/heart failure group, PH without social adversity was associated with a nearly threefold increase in all-cause mortality (hazard ratio [HR], 2.83; P = .004), whereas PH with social adversity was linked to a more than sevenfold increase in all-cause mortality (HR, 7.14; P < .001). Social adversity without PA was associated with a more than fourfold increase (HR, 4.47; P < .001).
Within the PH cohort, social adversity was associated with lower survival (P < .001). When the researchers broke down the results by types of social adversity, they found statistically significant relationships between greater mortality risk and economic instability within the HIV+ population (HR, 2.59; P = .040), transportation issues within the HIV– population (HR, 12.8; P < .001), and lack of social or family support within both the HIV– (HR, 5.49; P < .001) and the HIV+ population (HR, 2.03; P = .028).
The research has prompted interventions, which are now being studied at the institution, according to Dr. Biavati. “We have a policy of giving medications in bags when we discharge a patient with a social adversity. We literally go to the pharmacy, bring up the bag of medication, and we [put it] in their hands before they leave the hospital. They get a 1- or 3-month supply, depending on the medication, and then we usually discharge them with a clinical appointment already scheduled with either a pulmonary or primary care provider, and we usually call them before every appointment to confirm that they’re coming. That increases the chances of some success, but there’s still a very long way to go,” said Dr. Biavati.
Dr. Biavati was blinded to the results of the intervention, so he could not report on whether it was working. “But I can tell you that I’ve had busier clinics, so hopefully that means that they’re showing up more,” he said.
The problem is complex, according to Sandeep Jain, MD, who moderated the session. “Social adversity means lack of education. Lack of education means lack of compliance. Lack of compliance means what can you do if people are not taking medications? So it’s all matched together. It’s all lack of education and lack of money, lack of family support. And these drugs they have to take every single day. It’s not that easy. It’s very easy for us to say I had antiretroviral treatment for 6 months. It is almost impossible to continue regular treatment for that long [for a patient with social adversity]. You can’t blame them if they aren’t taking treatments. It’s very difficult for them,” said Dr. Jain.
That underscores the need for interventions that can address the needs of patients with social adversity. “We have to [practice] medicine considering the social situation of the patient and not just the medicine that we study in books. That’s kind of what we are faced with every day. We have therapies, and then life happens. It’s much harder to care for those patients,” said Dr. Biavati.
Dr. Biavati and Dr. Jain reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CHEST 2024
Virtual Yoga Classes Improve Chronic Low Back Pain
TOPLINE:
Virtual yoga classes significantly reduced chronic low back pain intensity and improved back-related function in health system employees. Improvements were sustained at 24 weeks, with reduced pain medication use and better sleep quality.
METHODOLOGY:
- A single-blinded, 24-week, 2-arm, randomized clinical trial was conducted from May 3, 2022, through May 23, 2023, comparing live-streamed yoga classes with a wait-list control among adults with chronic low back pain.
- A total of 140 participants aged 18-64 years with chronic low back pain were recruited from the Cleveland Clinic Employee Health Plan.
- Inclusion criteria included a mean low back pain intensity score of at least 4 on an 11-point numerical rating scale and daily back pain interference about half or more of the days.
- The intervention consisted of 12 consecutive weekly, 60-minute, virtual, live-streamed hatha yoga group classes.
Coprimary outcomes were mean pain intensity in the previous week on the 11-point numerical rating scale and back-related function as assessed using the 23-point modified Roland Morris Disability Questionnaire at 12 weeks.
TAKEAWAY:
- Participants in the virtual yoga group showed greater reductions in mean pain intensity at 12 weeks (mean change, –1.5 points; P < .001) and 24 weeks (mean change, –2.3 points; P < .001) compared to the wait-list control group.
- Back-related function improved significantly in the virtual yoga group at 12 weeks (mean change, –2.8 points; P < .001) and 24 weeks (mean change, –4.6 points; P < .001), compared with the control group.
- Virtual yoga participants reported 21.2 percentage points less use of any analgesic medication during the past week at 24 weeks, compared with the control group.
- Sleep quality improved more in the virtual yoga group at 12 weeks (mean change, 0.4 points; P = .008) and 24 weeks (mean change, 0.4 points; P = .005), compared with the control group.
IN PRACTICE:
“Given the demonstrated noninferiority of yoga to physical therapy, structured virtual yoga programs and physical therapy are reasonable choices for patients with [chronic low back pain] depending on accessibility, cost, and patient preference. These findings support the call by the National Academy of Medicine for increased evidenced-based pain treatments that can be disseminated via technology-based platforms,” wrote the authors of the study.
SOURCE:
The study was led by Hallie Tankha, PhD, Cleveland Clinic in Ohio. It was published online on November 1, 2024, in JAMA Network Open.
LIMITATIONS:
The study had a low adherence rate, with only 36.6% of participants attending at least 50% of the yoga classes. There was also a higher rate of missing data in the yoga group compared to the control group. The study did not include a longer-term follow-up assessment beyond 24 weeks.
DISCLOSURES:
This study was supported by grants from Cleveland Clinic Healthcare Delivery and Implementation Science Center. One coauthor disclosed receiving personal fees from the Blue Cross Blue Shield Association. Eric Roseen, DC, PhD, reported receiving grants from the National Institutes of Health National Center for Complementary and Integrative Health. One coauthor disclosed receiving personal fees from UpToDate and grants from NCCIH related to yoga and tai chi for treatment of pain. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Virtual yoga classes significantly reduced chronic low back pain intensity and improved back-related function in health system employees. Improvements were sustained at 24 weeks, with reduced pain medication use and better sleep quality.
METHODOLOGY:
- A single-blinded, 24-week, 2-arm, randomized clinical trial was conducted from May 3, 2022, through May 23, 2023, comparing live-streamed yoga classes with a wait-list control among adults with chronic low back pain.
- A total of 140 participants aged 18-64 years with chronic low back pain were recruited from the Cleveland Clinic Employee Health Plan.
- Inclusion criteria included a mean low back pain intensity score of at least 4 on an 11-point numerical rating scale and daily back pain interference about half or more of the days.
- The intervention consisted of 12 consecutive weekly, 60-minute, virtual, live-streamed hatha yoga group classes.
Coprimary outcomes were mean pain intensity in the previous week on the 11-point numerical rating scale and back-related function as assessed using the 23-point modified Roland Morris Disability Questionnaire at 12 weeks.
TAKEAWAY:
- Participants in the virtual yoga group showed greater reductions in mean pain intensity at 12 weeks (mean change, –1.5 points; P < .001) and 24 weeks (mean change, –2.3 points; P < .001) compared to the wait-list control group.
- Back-related function improved significantly in the virtual yoga group at 12 weeks (mean change, –2.8 points; P < .001) and 24 weeks (mean change, –4.6 points; P < .001), compared with the control group.
- Virtual yoga participants reported 21.2 percentage points less use of any analgesic medication during the past week at 24 weeks, compared with the control group.
- Sleep quality improved more in the virtual yoga group at 12 weeks (mean change, 0.4 points; P = .008) and 24 weeks (mean change, 0.4 points; P = .005), compared with the control group.
IN PRACTICE:
“Given the demonstrated noninferiority of yoga to physical therapy, structured virtual yoga programs and physical therapy are reasonable choices for patients with [chronic low back pain] depending on accessibility, cost, and patient preference. These findings support the call by the National Academy of Medicine for increased evidenced-based pain treatments that can be disseminated via technology-based platforms,” wrote the authors of the study.
SOURCE:
The study was led by Hallie Tankha, PhD, Cleveland Clinic in Ohio. It was published online on November 1, 2024, in JAMA Network Open.
LIMITATIONS:
The study had a low adherence rate, with only 36.6% of participants attending at least 50% of the yoga classes. There was also a higher rate of missing data in the yoga group compared to the control group. The study did not include a longer-term follow-up assessment beyond 24 weeks.
DISCLOSURES:
This study was supported by grants from Cleveland Clinic Healthcare Delivery and Implementation Science Center. One coauthor disclosed receiving personal fees from the Blue Cross Blue Shield Association. Eric Roseen, DC, PhD, reported receiving grants from the National Institutes of Health National Center for Complementary and Integrative Health. One coauthor disclosed receiving personal fees from UpToDate and grants from NCCIH related to yoga and tai chi for treatment of pain. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Virtual yoga classes significantly reduced chronic low back pain intensity and improved back-related function in health system employees. Improvements were sustained at 24 weeks, with reduced pain medication use and better sleep quality.
METHODOLOGY:
- A single-blinded, 24-week, 2-arm, randomized clinical trial was conducted from May 3, 2022, through May 23, 2023, comparing live-streamed yoga classes with a wait-list control among adults with chronic low back pain.
- A total of 140 participants aged 18-64 years with chronic low back pain were recruited from the Cleveland Clinic Employee Health Plan.
- Inclusion criteria included a mean low back pain intensity score of at least 4 on an 11-point numerical rating scale and daily back pain interference about half or more of the days.
- The intervention consisted of 12 consecutive weekly, 60-minute, virtual, live-streamed hatha yoga group classes.
Coprimary outcomes were mean pain intensity in the previous week on the 11-point numerical rating scale and back-related function as assessed using the 23-point modified Roland Morris Disability Questionnaire at 12 weeks.
TAKEAWAY:
- Participants in the virtual yoga group showed greater reductions in mean pain intensity at 12 weeks (mean change, –1.5 points; P < .001) and 24 weeks (mean change, –2.3 points; P < .001) compared to the wait-list control group.
- Back-related function improved significantly in the virtual yoga group at 12 weeks (mean change, –2.8 points; P < .001) and 24 weeks (mean change, –4.6 points; P < .001), compared with the control group.
- Virtual yoga participants reported 21.2 percentage points less use of any analgesic medication during the past week at 24 weeks, compared with the control group.
- Sleep quality improved more in the virtual yoga group at 12 weeks (mean change, 0.4 points; P = .008) and 24 weeks (mean change, 0.4 points; P = .005), compared with the control group.
IN PRACTICE:
“Given the demonstrated noninferiority of yoga to physical therapy, structured virtual yoga programs and physical therapy are reasonable choices for patients with [chronic low back pain] depending on accessibility, cost, and patient preference. These findings support the call by the National Academy of Medicine for increased evidenced-based pain treatments that can be disseminated via technology-based platforms,” wrote the authors of the study.
SOURCE:
The study was led by Hallie Tankha, PhD, Cleveland Clinic in Ohio. It was published online on November 1, 2024, in JAMA Network Open.
LIMITATIONS:
The study had a low adherence rate, with only 36.6% of participants attending at least 50% of the yoga classes. There was also a higher rate of missing data in the yoga group compared to the control group. The study did not include a longer-term follow-up assessment beyond 24 weeks.
DISCLOSURES:
This study was supported by grants from Cleveland Clinic Healthcare Delivery and Implementation Science Center. One coauthor disclosed receiving personal fees from the Blue Cross Blue Shield Association. Eric Roseen, DC, PhD, reported receiving grants from the National Institutes of Health National Center for Complementary and Integrative Health. One coauthor disclosed receiving personal fees from UpToDate and grants from NCCIH related to yoga and tai chi for treatment of pain. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
