User login
Antigen profiling may help prevent transfusion complications
BETHESDA, MD. – according to one researcher.
“We strongly feel that you should get an extended red cell type on the first encounter” for patients facing long-term transfusion support, Connie Westhoff, PhD, of the New York Blood Center, said at Sickle Cell in Focus, a conference held by the National Institutes of Health. “This can be a one-time test. It doesn’t have to be repeated.”
She also stressed the importance of ensuring that this information travels with patients, who may be seen at various hospitals. “One of the challenges here is making this part of the patient’s electronic medical record.”
Alloimmunization has been a major concern for chronically transfused patients, so there’s a real advantage to knowing a patient’s extended red cell antigen profile before the patient develops alloantibodies following transfusion, Dr. Westhoff said. Hemolytic transfusion reactions can sometimes destroy patients’ own RBCs in addition to the transfused RBCs. Having the patient’s profile to identify the potential cause of the incompatibility and guide transfusion support in an emergency can be lifesaving.
The implementation of genotyping by DNA-based methods has brought down the cost of this screening, making it no longer a barrier in care.
Dr. Westhoff cited a study she and her colleagues published on a study typing patients who have sickle cell disease (Transfusion. 2015 Jun;55[6 Pt 2]:1388-93). In that paper, they found that DNA-based RBC typing provided improved accuracy and expanded information on RBC antigens, compared with hemagglutination methods. This led to its implementation as the primary method for extended RBC typing for patients with sickle cell disease at the Children’s Hospital of Philadelphia.
About 65%-70% of antibodies drop to levels that are not detectable by routine assays, further demonstrating the need for DNA-based methods. The drugs used to dampen the immune response in many of these patients may also hinder or impact detection of antibodies that remain at levels that continue to cause in vivo hemolysis, Dr. Westhoff said in an interview.
For patients with sickle cell disease in many Western countries, antigen matching for CEK, at a minimum, is routine. The United States is now moving in this direction.
“Modern transfusion practice is moving to knowing what antigens the patient is at risk to become immunized against. ... What antigens does the patient lack and what antibodies could the patient make,” Dr. Westhoff said in an interview. “It’s a major advantage in your transfusion service to expedite work-ups and patient care by having that extended antigen profile.”
Dr. Westhoff reported no relevant financial disclosures.
BETHESDA, MD. – according to one researcher.
“We strongly feel that you should get an extended red cell type on the first encounter” for patients facing long-term transfusion support, Connie Westhoff, PhD, of the New York Blood Center, said at Sickle Cell in Focus, a conference held by the National Institutes of Health. “This can be a one-time test. It doesn’t have to be repeated.”
She also stressed the importance of ensuring that this information travels with patients, who may be seen at various hospitals. “One of the challenges here is making this part of the patient’s electronic medical record.”
Alloimmunization has been a major concern for chronically transfused patients, so there’s a real advantage to knowing a patient’s extended red cell antigen profile before the patient develops alloantibodies following transfusion, Dr. Westhoff said. Hemolytic transfusion reactions can sometimes destroy patients’ own RBCs in addition to the transfused RBCs. Having the patient’s profile to identify the potential cause of the incompatibility and guide transfusion support in an emergency can be lifesaving.
The implementation of genotyping by DNA-based methods has brought down the cost of this screening, making it no longer a barrier in care.
Dr. Westhoff cited a study she and her colleagues published on a study typing patients who have sickle cell disease (Transfusion. 2015 Jun;55[6 Pt 2]:1388-93). In that paper, they found that DNA-based RBC typing provided improved accuracy and expanded information on RBC antigens, compared with hemagglutination methods. This led to its implementation as the primary method for extended RBC typing for patients with sickle cell disease at the Children’s Hospital of Philadelphia.
About 65%-70% of antibodies drop to levels that are not detectable by routine assays, further demonstrating the need for DNA-based methods. The drugs used to dampen the immune response in many of these patients may also hinder or impact detection of antibodies that remain at levels that continue to cause in vivo hemolysis, Dr. Westhoff said in an interview.
For patients with sickle cell disease in many Western countries, antigen matching for CEK, at a minimum, is routine. The United States is now moving in this direction.
“Modern transfusion practice is moving to knowing what antigens the patient is at risk to become immunized against. ... What antigens does the patient lack and what antibodies could the patient make,” Dr. Westhoff said in an interview. “It’s a major advantage in your transfusion service to expedite work-ups and patient care by having that extended antigen profile.”
Dr. Westhoff reported no relevant financial disclosures.
BETHESDA, MD. – according to one researcher.
“We strongly feel that you should get an extended red cell type on the first encounter” for patients facing long-term transfusion support, Connie Westhoff, PhD, of the New York Blood Center, said at Sickle Cell in Focus, a conference held by the National Institutes of Health. “This can be a one-time test. It doesn’t have to be repeated.”
She also stressed the importance of ensuring that this information travels with patients, who may be seen at various hospitals. “One of the challenges here is making this part of the patient’s electronic medical record.”
Alloimmunization has been a major concern for chronically transfused patients, so there’s a real advantage to knowing a patient’s extended red cell antigen profile before the patient develops alloantibodies following transfusion, Dr. Westhoff said. Hemolytic transfusion reactions can sometimes destroy patients’ own RBCs in addition to the transfused RBCs. Having the patient’s profile to identify the potential cause of the incompatibility and guide transfusion support in an emergency can be lifesaving.
The implementation of genotyping by DNA-based methods has brought down the cost of this screening, making it no longer a barrier in care.
Dr. Westhoff cited a study she and her colleagues published on a study typing patients who have sickle cell disease (Transfusion. 2015 Jun;55[6 Pt 2]:1388-93). In that paper, they found that DNA-based RBC typing provided improved accuracy and expanded information on RBC antigens, compared with hemagglutination methods. This led to its implementation as the primary method for extended RBC typing for patients with sickle cell disease at the Children’s Hospital of Philadelphia.
About 65%-70% of antibodies drop to levels that are not detectable by routine assays, further demonstrating the need for DNA-based methods. The drugs used to dampen the immune response in many of these patients may also hinder or impact detection of antibodies that remain at levels that continue to cause in vivo hemolysis, Dr. Westhoff said in an interview.
For patients with sickle cell disease in many Western countries, antigen matching for CEK, at a minimum, is routine. The United States is now moving in this direction.
“Modern transfusion practice is moving to knowing what antigens the patient is at risk to become immunized against. ... What antigens does the patient lack and what antibodies could the patient make,” Dr. Westhoff said in an interview. “It’s a major advantage in your transfusion service to expedite work-ups and patient care by having that extended antigen profile.”
Dr. Westhoff reported no relevant financial disclosures.
REPORTING FROM SICKLE CELL IN FOCUS
DPP-4 drugs for diabetes may protect kidneys too
SAN DIEGO – Dipeptidyl peptidase–4 (DPP-4) inhibitors appear to delay the progression of chronic kidney disease (CKD) in patients with type 2 diabetes mellitus (T2DM), a new study has found. Researchers also found that all-cause long-term mortality dropped by an astonishing 78% in patients who took the drugs for an average of more than 3 years.
While the reasons for the impressive mortality results are a mystery, “these medications could have a beneficial effect on the kidneys, and it begins to show after 3 years,” said lead author Mariana Garcia-Touza, MD, of the Kansas City (Missouri) Department of Veterans Affairs Medical Center, in an interview. She presented the results at the meeting, sponsored by the American Society of Nephrology.
DPP-4 inhibitor drugs have been available for more than a decade in the United States. The medications, which include sitagliptin (Januvia) and linagliptin (Tradjenta), are used to treat patients with T2DM who are inadequately controlled by first-line treatments.
The drugs have critics. As UpToDate notes, they’re expensive and their effect on glucose levels is “modest.” In addition, UpToDate says, “some of the DPP-4 inhibitors have been associated with an increased risk of heart failure resulting in hospitalization.”
The authors of the new study sought to understand whether the drugs affect kidney function. As Dr. Garcia-Touza noted, metformin, which is processed in part by the kidneys, is considered harmful to certain patients with kidney disease. However, DPP-4 inhibitors are cleared through the liver. In fact, research has suggested the drugs may actually benefit the liver (Med Sci Monit. 2014 Sep 17;20:1662-7).
For the new study, researchers retrospectively analyzed 20,424 patients with T2DM in the VA system who took DPP-4 inhibitors (average age, 68 years) and compared them with a matched group of 52,118 patients with T2DM who didn’t take the drugs, tracking all patients for a mean of over 3 years.
T2DM control improved slightly in the DPP-4 group but remained worse than the non–DPP-4 group. However, “a significant reduction in progression of CKD was seen” in the DPP-4 group, she said.
The number of patients with creatinine levels above 1.5 mg/dL, 3 mg/dL, and 6 mg/dL was reduced by 7%, 41%, and 47%, respectively, in the DPP-4 group, compared with the other group (P less than .01). And the time to end-stage renal disease (creatinine above 6 mg/dL) was delayed by 144 days in the DPP-4 group (P less than .01).
All-cause mortality also fell by 78% in the DPP-4 group (P less than .0001). “Despite having worse glucose control [than the non–DPP-4 group], these patients have better overall mortality,” Dr. Garcia-Touza said.
The drugs may reduce the burden on the kidneys by decreasing inflammation, she said.
Could DPP-4 drugs be beneficial to patients with CKD even if they don’t have T2DM? Dr. Garcia-Touza wasn’t sure. However, she had a theory about why these kidney benefits didn’t show up in previous research. “My impression is that they didn’t go far enough [in time]. That was the main difference.”
Going forward, Dr. Garcia-Touza said her team plans to study the effects of the drugs on retinopathy and diabetic neuropathy.
The study was funded by the Midwest Biomedical Research Foundation and the VA. The study authors reported no relevant disclosures.
SOURCE: Garcia-Tourza M et al. Kidney Week 2018, Abstract TH-OR035.
SAN DIEGO – Dipeptidyl peptidase–4 (DPP-4) inhibitors appear to delay the progression of chronic kidney disease (CKD) in patients with type 2 diabetes mellitus (T2DM), a new study has found. Researchers also found that all-cause long-term mortality dropped by an astonishing 78% in patients who took the drugs for an average of more than 3 years.
While the reasons for the impressive mortality results are a mystery, “these medications could have a beneficial effect on the kidneys, and it begins to show after 3 years,” said lead author Mariana Garcia-Touza, MD, of the Kansas City (Missouri) Department of Veterans Affairs Medical Center, in an interview. She presented the results at the meeting, sponsored by the American Society of Nephrology.
DPP-4 inhibitor drugs have been available for more than a decade in the United States. The medications, which include sitagliptin (Januvia) and linagliptin (Tradjenta), are used to treat patients with T2DM who are inadequately controlled by first-line treatments.
The drugs have critics. As UpToDate notes, they’re expensive and their effect on glucose levels is “modest.” In addition, UpToDate says, “some of the DPP-4 inhibitors have been associated with an increased risk of heart failure resulting in hospitalization.”
The authors of the new study sought to understand whether the drugs affect kidney function. As Dr. Garcia-Touza noted, metformin, which is processed in part by the kidneys, is considered harmful to certain patients with kidney disease. However, DPP-4 inhibitors are cleared through the liver. In fact, research has suggested the drugs may actually benefit the liver (Med Sci Monit. 2014 Sep 17;20:1662-7).
For the new study, researchers retrospectively analyzed 20,424 patients with T2DM in the VA system who took DPP-4 inhibitors (average age, 68 years) and compared them with a matched group of 52,118 patients with T2DM who didn’t take the drugs, tracking all patients for a mean of over 3 years.
T2DM control improved slightly in the DPP-4 group but remained worse than the non–DPP-4 group. However, “a significant reduction in progression of CKD was seen” in the DPP-4 group, she said.
The number of patients with creatinine levels above 1.5 mg/dL, 3 mg/dL, and 6 mg/dL was reduced by 7%, 41%, and 47%, respectively, in the DPP-4 group, compared with the other group (P less than .01). And the time to end-stage renal disease (creatinine above 6 mg/dL) was delayed by 144 days in the DPP-4 group (P less than .01).
All-cause mortality also fell by 78% in the DPP-4 group (P less than .0001). “Despite having worse glucose control [than the non–DPP-4 group], these patients have better overall mortality,” Dr. Garcia-Touza said.
The drugs may reduce the burden on the kidneys by decreasing inflammation, she said.
Could DPP-4 drugs be beneficial to patients with CKD even if they don’t have T2DM? Dr. Garcia-Touza wasn’t sure. However, she had a theory about why these kidney benefits didn’t show up in previous research. “My impression is that they didn’t go far enough [in time]. That was the main difference.”
Going forward, Dr. Garcia-Touza said her team plans to study the effects of the drugs on retinopathy and diabetic neuropathy.
The study was funded by the Midwest Biomedical Research Foundation and the VA. The study authors reported no relevant disclosures.
SOURCE: Garcia-Tourza M et al. Kidney Week 2018, Abstract TH-OR035.
SAN DIEGO – Dipeptidyl peptidase–4 (DPP-4) inhibitors appear to delay the progression of chronic kidney disease (CKD) in patients with type 2 diabetes mellitus (T2DM), a new study has found. Researchers also found that all-cause long-term mortality dropped by an astonishing 78% in patients who took the drugs for an average of more than 3 years.
While the reasons for the impressive mortality results are a mystery, “these medications could have a beneficial effect on the kidneys, and it begins to show after 3 years,” said lead author Mariana Garcia-Touza, MD, of the Kansas City (Missouri) Department of Veterans Affairs Medical Center, in an interview. She presented the results at the meeting, sponsored by the American Society of Nephrology.
DPP-4 inhibitor drugs have been available for more than a decade in the United States. The medications, which include sitagliptin (Januvia) and linagliptin (Tradjenta), are used to treat patients with T2DM who are inadequately controlled by first-line treatments.
The drugs have critics. As UpToDate notes, they’re expensive and their effect on glucose levels is “modest.” In addition, UpToDate says, “some of the DPP-4 inhibitors have been associated with an increased risk of heart failure resulting in hospitalization.”
The authors of the new study sought to understand whether the drugs affect kidney function. As Dr. Garcia-Touza noted, metformin, which is processed in part by the kidneys, is considered harmful to certain patients with kidney disease. However, DPP-4 inhibitors are cleared through the liver. In fact, research has suggested the drugs may actually benefit the liver (Med Sci Monit. 2014 Sep 17;20:1662-7).
For the new study, researchers retrospectively analyzed 20,424 patients with T2DM in the VA system who took DPP-4 inhibitors (average age, 68 years) and compared them with a matched group of 52,118 patients with T2DM who didn’t take the drugs, tracking all patients for a mean of over 3 years.
T2DM control improved slightly in the DPP-4 group but remained worse than the non–DPP-4 group. However, “a significant reduction in progression of CKD was seen” in the DPP-4 group, she said.
The number of patients with creatinine levels above 1.5 mg/dL, 3 mg/dL, and 6 mg/dL was reduced by 7%, 41%, and 47%, respectively, in the DPP-4 group, compared with the other group (P less than .01). And the time to end-stage renal disease (creatinine above 6 mg/dL) was delayed by 144 days in the DPP-4 group (P less than .01).
All-cause mortality also fell by 78% in the DPP-4 group (P less than .0001). “Despite having worse glucose control [than the non–DPP-4 group], these patients have better overall mortality,” Dr. Garcia-Touza said.
The drugs may reduce the burden on the kidneys by decreasing inflammation, she said.
Could DPP-4 drugs be beneficial to patients with CKD even if they don’t have T2DM? Dr. Garcia-Touza wasn’t sure. However, she had a theory about why these kidney benefits didn’t show up in previous research. “My impression is that they didn’t go far enough [in time]. That was the main difference.”
Going forward, Dr. Garcia-Touza said her team plans to study the effects of the drugs on retinopathy and diabetic neuropathy.
The study was funded by the Midwest Biomedical Research Foundation and the VA. The study authors reported no relevant disclosures.
SOURCE: Garcia-Tourza M et al. Kidney Week 2018, Abstract TH-OR035.
REPORTING FROM KIDNEY WEEK 2018
Key clinical point: Dipeptidyl peptidase–4 (DPP-4) inhibitors may delay progression of chronic kidney disease in patients with type 2 diabetes mellitus (T2DM) and may dramatically reduce all-cause mortality.
Major finding: Compared with those who didn’t take the drugs, patients with T2DM who took DPP-4 inhibitors were much less likely to progress to creatinine levels above 1.5 mg/dL, 3 mg/dL, and 6 mg/dL (reduction of 7%, 41%, and 47%, respectively; P less than .01). All-cause mortality in the DPP-4 group fell by 78% (P less than .0001).
Study details: A retrospective study of 20,424 patients with T2DM in the Department of Veterans Affairs system who took DPP-4 inhibitors for mean of more than 3 years and a matched group of 52,118 patients with T2DM who didn’t take the drugs.
Disclosures: The study was funded by the Midwest Biomedical Research Foundation and the VA. The study authors reported no relevant disclosures.
Source: Garcia-Tourza M et al. Kidney Week 2018, Abstract TH-OR035.
Diffuse Pustular Eruption Following Computed Tomography
The Diagnosis: Acute Generalized Exanthematous Pustulosis
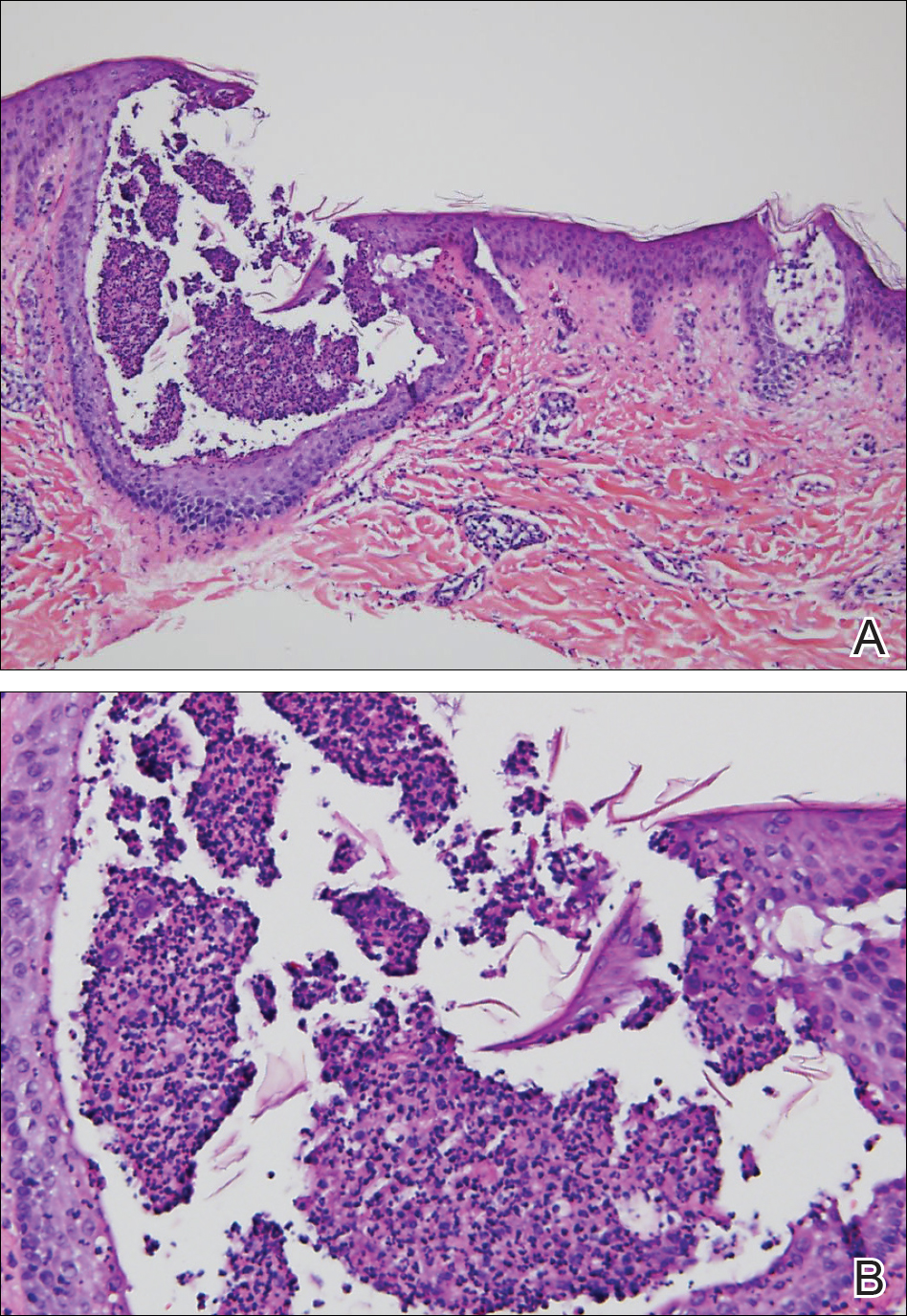
Histopathology demonstrated spongiosis with subcorneal pustules and an overlying basket-weave pattern stratum corneum. There was mild papillary dermal edema with scattered dermal neutrophils and rare eosinophils (Figure). The patient's clinical presentation and histopathology were consistent with acute generalized exanthematous pustulosis (AGEP). The inciting agent in this case was the contrast medium iopamidol. The patient was treated with a short course of prednisone, triamcinolone cream, diphenhydramine, and acetaminophen. Within 1 week the pustules and erythema had resolved.
Acute generalized exanthematous pustulosis is an uncommon T cell-mediated cutaneous reaction characterized by widespread progressive erythema with numerous nonfollicular pinpoint pustules. The patient usually is well appearing; however, he/she often will have concurrent fever and facial edema. Mucous membranes rarely are involved. Laboratory results typically are notable only for leukocytosis with neutrophilia.
The pustular eruption typically occurs within 1 to 2 days after exposure to an inciting agent1; however, this latent period can range from 1 hour to nearly 4 weeks in some studies.2 Systemic medications are the cause in approximately 90% of cases, with antibiotics being the most common category. Frequently implicated medications include β-lactams, macrolides, quinolones, sulfonamides, proton pump inhibitors, hydroxychloroquine, terbinafine, nonsteroidal anti-inflammatory drugs, diltiazem, ketoconazole, and fluconazole. Acute generalized exanthematous pustulosis also has been rarely reported following contact with mercury, viral and bacterial infections, and spider bites.3
Iodinated contrast agents have long been known to cause immediate and delayed adverse cutaneous reactions. However, one consensus study indicated that these reactions occur in only 0.05% to 0.10% of patients.4 Although rare, iodinated contrast media (eg, iopamidol, iohexol, ioversol, iodixanol, iomeprol, iobitridol, iopromide) have been reported as a cause of AGEP. A PubMed search of articles indexed for MEDLINE using the terms acute generalized exanthematous pustulosis, contrast, iodine, and iodinated revealed 10 adult cases reported in 6 articles in the English-language literature.1,5-9 The most recent articles focus on methods to identify the causative agent. If the etiology of the reaction is unclear, patch or intradermal testing can help to confirm the causative agent. These tests also can help determine similar agents to which the patient may cross-react.4,5
It can be difficult to differentiate AGEP from other cutaneous drug reactions and other nonfollicular pustular conditions. Drug-induced hypersensitivity syndrome typically presents with facial edema and a morbilliform rash. Although it can present with pustules, the latent period is longer (2-6 weeks), and there frequently are signs of multiorgan involvement including hepatic dysfunction, eosinophilia, atypical lymphocytosis, and lymphadenopathy. Patients with generalized pustular psoriasis often have a history of plaque psoriasis; the pustules are more concentrated in flexural sites; the eruption is gradual in onset; and histologically there tends to be features of psoriasis including parakeratosis, Munro microabscesses, and dilated blood vessels.10 Subcorneal pustular dermatosis also is more concentrated in flexural sites and frequently has an annular or serpiginous configuration. The onset also is gradual, and it follows a more chronic course than AGEP. Exfoliative erythroderma presents with widespread erythema and superficial desquamating scale. It often occurs in association with systemic symptoms and can be the result of a drug reaction or underlying inflammatory dermatosis such as psoriasis, mycosis fungoides, or pityriasis rubra pilaris.
Acute generalized exanthematous pustulosis usually resolves spontaneously within 2 weeks and is associated with a superficial desquamation as it clears. Appropriate treatment includes discontinuing the offending agent; monitoring for systemic involvement; and treating the patient's symptoms with antihistamines, analgesics, topical steroids, and emollients. In more severe or persistent cases, treatment with systemic steroids and tumor necrosis factor α inhibitors has been attempted, though their efficacy remains unclear. We report a case of iopamidol-induced AGEP that highlights the importance of eliciting a history of contrast exposure from a patient with suspected AGEP.
- Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis. Arch Dermatol. 2009;145:683-687.
- Thienvibul C, Vachiramon V, Chanprapaph K. Five-year retrospective review of acute generalized exanthematous pustulosis. Dermatol Res Pract. 2015;2015:1-8.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2016;73:843-848.
- Rosado Ingelmo A, Doña Diaz I, Cabañas Moreno R, et al. Clinical practice guidelines for diagnosis and management of hypersensitivity reactions to contrast media. J Investig Allergol Clin Immunol. 2016;26:144-155.
- Grandvuillemin A, Ripert C, Sgro C, et al. Iodinated contrast media-induced acute generalized exanthematous pustulosis confirmed by delayed skin tests. J Allergy Clin Immunol Pract. 2014;2:805-806.
- Bavbek S, Sözener ZÇ, Aydin Ö, et al. First case report of acute generalized exanthematous pustulosis due to intravenous iopromide. J Investig Allergol Clin Immunol. 2014;24:66-67.
- Kim SJ, Lee T, Lee YS, et al. Acute generalized exanthematous pustulosis caused by radiocontrast media. Ann Allergy Asthma Immunol. 2010;105:492-493.
- Peterson A, Katzberg RW, Fung MA, et al. Acute generalized exanthematous pustulosis as a delayed dermatotoxic reaction to IV-administered nonionic contrast media. Am J Roentgenol. 2006;187:198-201.
- Atasoy M, Erdem T, Sari RA. A case of acute generalized exanthematous pustulosis (AGEP) possibly induced by iohexol. J Dermatol. 2003;30:723-726.
- Halevy S, Kardaun S, Davidovici B, et al; EuroSCAR and RegiSCAR Study Group. The spectrum of histopathological features in acute generalized exanthematous pustulosis: a study of 102 cases. Br J Dermatol. 2010:163:1245-1252.
The Diagnosis: Acute Generalized Exanthematous Pustulosis
Histopathology demonstrated spongiosis with subcorneal pustules and an overlying basket-weave pattern stratum corneum. There was mild papillary dermal edema with scattered dermal neutrophils and rare eosinophils (Figure). The patient's clinical presentation and histopathology were consistent with acute generalized exanthematous pustulosis (AGEP). The inciting agent in this case was the contrast medium iopamidol. The patient was treated with a short course of prednisone, triamcinolone cream, diphenhydramine, and acetaminophen. Within 1 week the pustules and erythema had resolved.

Acute generalized exanthematous pustulosis is an uncommon T cell-mediated cutaneous reaction characterized by widespread progressive erythema with numerous nonfollicular pinpoint pustules. The patient usually is well appearing; however, he/she often will have concurrent fever and facial edema. Mucous membranes rarely are involved. Laboratory results typically are notable only for leukocytosis with neutrophilia.
The pustular eruption typically occurs within 1 to 2 days after exposure to an inciting agent1; however, this latent period can range from 1 hour to nearly 4 weeks in some studies.2 Systemic medications are the cause in approximately 90% of cases, with antibiotics being the most common category. Frequently implicated medications include β-lactams, macrolides, quinolones, sulfonamides, proton pump inhibitors, hydroxychloroquine, terbinafine, nonsteroidal anti-inflammatory drugs, diltiazem, ketoconazole, and fluconazole. Acute generalized exanthematous pustulosis also has been rarely reported following contact with mercury, viral and bacterial infections, and spider bites.3
Iodinated contrast agents have long been known to cause immediate and delayed adverse cutaneous reactions. However, one consensus study indicated that these reactions occur in only 0.05% to 0.10% of patients.4 Although rare, iodinated contrast media (eg, iopamidol, iohexol, ioversol, iodixanol, iomeprol, iobitridol, iopromide) have been reported as a cause of AGEP. A PubMed search of articles indexed for MEDLINE using the terms acute generalized exanthematous pustulosis, contrast, iodine, and iodinated revealed 10 adult cases reported in 6 articles in the English-language literature.1,5-9 The most recent articles focus on methods to identify the causative agent. If the etiology of the reaction is unclear, patch or intradermal testing can help to confirm the causative agent. These tests also can help determine similar agents to which the patient may cross-react.4,5
It can be difficult to differentiate AGEP from other cutaneous drug reactions and other nonfollicular pustular conditions. Drug-induced hypersensitivity syndrome typically presents with facial edema and a morbilliform rash. Although it can present with pustules, the latent period is longer (2-6 weeks), and there frequently are signs of multiorgan involvement including hepatic dysfunction, eosinophilia, atypical lymphocytosis, and lymphadenopathy. Patients with generalized pustular psoriasis often have a history of plaque psoriasis; the pustules are more concentrated in flexural sites; the eruption is gradual in onset; and histologically there tends to be features of psoriasis including parakeratosis, Munro microabscesses, and dilated blood vessels.10 Subcorneal pustular dermatosis also is more concentrated in flexural sites and frequently has an annular or serpiginous configuration. The onset also is gradual, and it follows a more chronic course than AGEP. Exfoliative erythroderma presents with widespread erythema and superficial desquamating scale. It often occurs in association with systemic symptoms and can be the result of a drug reaction or underlying inflammatory dermatosis such as psoriasis, mycosis fungoides, or pityriasis rubra pilaris.
Acute generalized exanthematous pustulosis usually resolves spontaneously within 2 weeks and is associated with a superficial desquamation as it clears. Appropriate treatment includes discontinuing the offending agent; monitoring for systemic involvement; and treating the patient's symptoms with antihistamines, analgesics, topical steroids, and emollients. In more severe or persistent cases, treatment with systemic steroids and tumor necrosis factor α inhibitors has been attempted, though their efficacy remains unclear. We report a case of iopamidol-induced AGEP that highlights the importance of eliciting a history of contrast exposure from a patient with suspected AGEP.
The Diagnosis: Acute Generalized Exanthematous Pustulosis
Histopathology demonstrated spongiosis with subcorneal pustules and an overlying basket-weave pattern stratum corneum. There was mild papillary dermal edema with scattered dermal neutrophils and rare eosinophils (Figure). The patient's clinical presentation and histopathology were consistent with acute generalized exanthematous pustulosis (AGEP). The inciting agent in this case was the contrast medium iopamidol. The patient was treated with a short course of prednisone, triamcinolone cream, diphenhydramine, and acetaminophen. Within 1 week the pustules and erythema had resolved.

Acute generalized exanthematous pustulosis is an uncommon T cell-mediated cutaneous reaction characterized by widespread progressive erythema with numerous nonfollicular pinpoint pustules. The patient usually is well appearing; however, he/she often will have concurrent fever and facial edema. Mucous membranes rarely are involved. Laboratory results typically are notable only for leukocytosis with neutrophilia.
The pustular eruption typically occurs within 1 to 2 days after exposure to an inciting agent1; however, this latent period can range from 1 hour to nearly 4 weeks in some studies.2 Systemic medications are the cause in approximately 90% of cases, with antibiotics being the most common category. Frequently implicated medications include β-lactams, macrolides, quinolones, sulfonamides, proton pump inhibitors, hydroxychloroquine, terbinafine, nonsteroidal anti-inflammatory drugs, diltiazem, ketoconazole, and fluconazole. Acute generalized exanthematous pustulosis also has been rarely reported following contact with mercury, viral and bacterial infections, and spider bites.3
Iodinated contrast agents have long been known to cause immediate and delayed adverse cutaneous reactions. However, one consensus study indicated that these reactions occur in only 0.05% to 0.10% of patients.4 Although rare, iodinated contrast media (eg, iopamidol, iohexol, ioversol, iodixanol, iomeprol, iobitridol, iopromide) have been reported as a cause of AGEP. A PubMed search of articles indexed for MEDLINE using the terms acute generalized exanthematous pustulosis, contrast, iodine, and iodinated revealed 10 adult cases reported in 6 articles in the English-language literature.1,5-9 The most recent articles focus on methods to identify the causative agent. If the etiology of the reaction is unclear, patch or intradermal testing can help to confirm the causative agent. These tests also can help determine similar agents to which the patient may cross-react.4,5
It can be difficult to differentiate AGEP from other cutaneous drug reactions and other nonfollicular pustular conditions. Drug-induced hypersensitivity syndrome typically presents with facial edema and a morbilliform rash. Although it can present with pustules, the latent period is longer (2-6 weeks), and there frequently are signs of multiorgan involvement including hepatic dysfunction, eosinophilia, atypical lymphocytosis, and lymphadenopathy. Patients with generalized pustular psoriasis often have a history of plaque psoriasis; the pustules are more concentrated in flexural sites; the eruption is gradual in onset; and histologically there tends to be features of psoriasis including parakeratosis, Munro microabscesses, and dilated blood vessels.10 Subcorneal pustular dermatosis also is more concentrated in flexural sites and frequently has an annular or serpiginous configuration. The onset also is gradual, and it follows a more chronic course than AGEP. Exfoliative erythroderma presents with widespread erythema and superficial desquamating scale. It often occurs in association with systemic symptoms and can be the result of a drug reaction or underlying inflammatory dermatosis such as psoriasis, mycosis fungoides, or pityriasis rubra pilaris.
Acute generalized exanthematous pustulosis usually resolves spontaneously within 2 weeks and is associated with a superficial desquamation as it clears. Appropriate treatment includes discontinuing the offending agent; monitoring for systemic involvement; and treating the patient's symptoms with antihistamines, analgesics, topical steroids, and emollients. In more severe or persistent cases, treatment with systemic steroids and tumor necrosis factor α inhibitors has been attempted, though their efficacy remains unclear. We report a case of iopamidol-induced AGEP that highlights the importance of eliciting a history of contrast exposure from a patient with suspected AGEP.
- Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis. Arch Dermatol. 2009;145:683-687.
- Thienvibul C, Vachiramon V, Chanprapaph K. Five-year retrospective review of acute generalized exanthematous pustulosis. Dermatol Res Pract. 2015;2015:1-8.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2016;73:843-848.
- Rosado Ingelmo A, Doña Diaz I, Cabañas Moreno R, et al. Clinical practice guidelines for diagnosis and management of hypersensitivity reactions to contrast media. J Investig Allergol Clin Immunol. 2016;26:144-155.
- Grandvuillemin A, Ripert C, Sgro C, et al. Iodinated contrast media-induced acute generalized exanthematous pustulosis confirmed by delayed skin tests. J Allergy Clin Immunol Pract. 2014;2:805-806.
- Bavbek S, Sözener ZÇ, Aydin Ö, et al. First case report of acute generalized exanthematous pustulosis due to intravenous iopromide. J Investig Allergol Clin Immunol. 2014;24:66-67.
- Kim SJ, Lee T, Lee YS, et al. Acute generalized exanthematous pustulosis caused by radiocontrast media. Ann Allergy Asthma Immunol. 2010;105:492-493.
- Peterson A, Katzberg RW, Fung MA, et al. Acute generalized exanthematous pustulosis as a delayed dermatotoxic reaction to IV-administered nonionic contrast media. Am J Roentgenol. 2006;187:198-201.
- Atasoy M, Erdem T, Sari RA. A case of acute generalized exanthematous pustulosis (AGEP) possibly induced by iohexol. J Dermatol. 2003;30:723-726.
- Halevy S, Kardaun S, Davidovici B, et al; EuroSCAR and RegiSCAR Study Group. The spectrum of histopathological features in acute generalized exanthematous pustulosis: a study of 102 cases. Br J Dermatol. 2010:163:1245-1252.
- Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis. Arch Dermatol. 2009;145:683-687.
- Thienvibul C, Vachiramon V, Chanprapaph K. Five-year retrospective review of acute generalized exanthematous pustulosis. Dermatol Res Pract. 2015;2015:1-8.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2016;73:843-848.
- Rosado Ingelmo A, Doña Diaz I, Cabañas Moreno R, et al. Clinical practice guidelines for diagnosis and management of hypersensitivity reactions to contrast media. J Investig Allergol Clin Immunol. 2016;26:144-155.
- Grandvuillemin A, Ripert C, Sgro C, et al. Iodinated contrast media-induced acute generalized exanthematous pustulosis confirmed by delayed skin tests. J Allergy Clin Immunol Pract. 2014;2:805-806.
- Bavbek S, Sözener ZÇ, Aydin Ö, et al. First case report of acute generalized exanthematous pustulosis due to intravenous iopromide. J Investig Allergol Clin Immunol. 2014;24:66-67.
- Kim SJ, Lee T, Lee YS, et al. Acute generalized exanthematous pustulosis caused by radiocontrast media. Ann Allergy Asthma Immunol. 2010;105:492-493.
- Peterson A, Katzberg RW, Fung MA, et al. Acute generalized exanthematous pustulosis as a delayed dermatotoxic reaction to IV-administered nonionic contrast media. Am J Roentgenol. 2006;187:198-201.
- Atasoy M, Erdem T, Sari RA. A case of acute generalized exanthematous pustulosis (AGEP) possibly induced by iohexol. J Dermatol. 2003;30:723-726.
- Halevy S, Kardaun S, Davidovici B, et al; EuroSCAR and RegiSCAR Study Group. The spectrum of histopathological features in acute generalized exanthematous pustulosis: a study of 102 cases. Br J Dermatol. 2010:163:1245-1252.
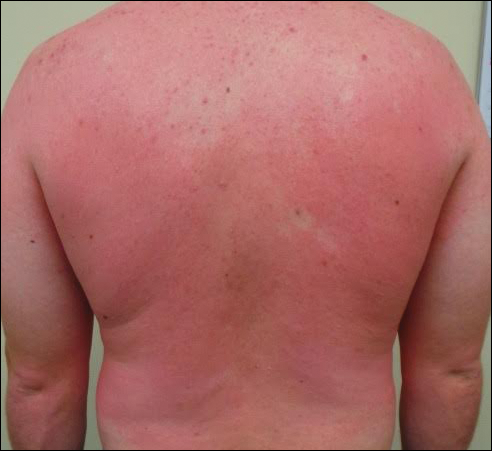
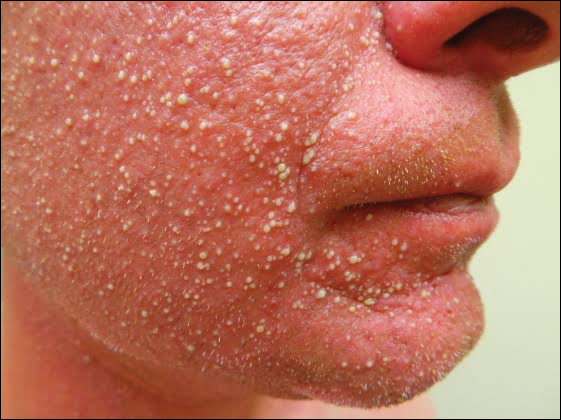
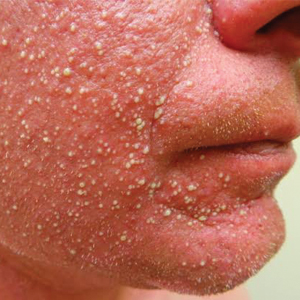
A 31-year-old man presented with a rapidly progressive, burning rash of 1 day's duration, along with malaise, nausea, and dizziness. At the time of presentation, he was hemodynamically stable and afebrile. Laboratory analysis revealed mild leukocytosis with neutrophilia. A complete metabolic panel was within normal limits. He had no chronic medical conditions and was taking no medications or supplements. One day prior to onset of the rash, he underwent contrast-enhanced (iopamidol) computed tomography of the abdomen. Physical examination revealed large edematous plaques on the face, neck, and trunk (top) that were studded with numerous pinpoint pustules (bottom). He also had subtle facial edema. There was relative sparing of the flexural sites and no involvement of the palms, soles, or mucous membranes. A shave biopsy was obtained from a pustular area on the neck.
Google Search Results for Diet and Psoriasis: Advice Patients Get on the Internet
Report details financial burden of blood cancers
with costs for acute leukemia almost tripling that amount, according to a new report from the Leukemia & Lymphoma Society (LLS).
Total allowed cost – the average amount paid by the insurer and patient combined – for acute leukemia was more than $463,000 for the 12 months after initial diagnosis. Averages for the other four cancers included in the analysis came in at $214,000 for multiple myeloma, $134,000 for bone marrow disorders, $131,000 for lymphoma, and $89,000 for chronic leukemia, the LLS said.
The cost figures are drawn from claims data for 2,332 patients diagnosed in 2014.
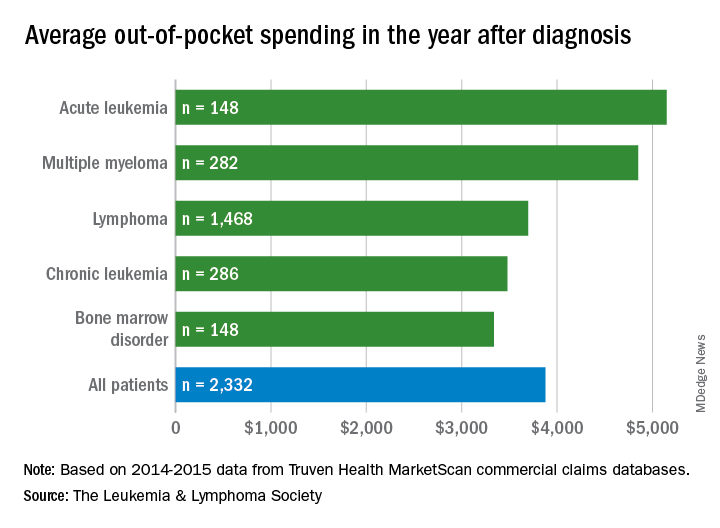
Differences in out-of-pocket (OOP) costs were smaller, with the average for all patients at almost $3,900 in the year after diagnosis and acute leukemia coming in the highest at $5,100. Over time, however, OOP costs for multiple myeloma patients became the highest, totaling $9,100 for the 3 years after diagnosis, compared with $8,800 for acute leukemia and an average of less than $7,800 for the other blood cancers, the LLS said in the report, which was prepared by the actuarial firm Milliman.
OOP costs also varied by the type of plan. Patients in high-deductible plans averaged nearly $5,400 for the first year after diagnosis, compared with $3,300 for those with traditional insurance, the LLS noted. For acute leukemia, the OOP costs of high-deductible plans were more than twice as high as those of traditional plans.
The study was based on data for adults aged 18-64 years from the Truven Health MarketScan commercial claims databases for the years from 2013 to 2016. The LLS received support for the study from Pfizer, Genentech, and Amgen.
with costs for acute leukemia almost tripling that amount, according to a new report from the Leukemia & Lymphoma Society (LLS).

Total allowed cost – the average amount paid by the insurer and patient combined – for acute leukemia was more than $463,000 for the 12 months after initial diagnosis. Averages for the other four cancers included in the analysis came in at $214,000 for multiple myeloma, $134,000 for bone marrow disorders, $131,000 for lymphoma, and $89,000 for chronic leukemia, the LLS said.
The cost figures are drawn from claims data for 2,332 patients diagnosed in 2014.
Differences in out-of-pocket (OOP) costs were smaller, with the average for all patients at almost $3,900 in the year after diagnosis and acute leukemia coming in the highest at $5,100. Over time, however, OOP costs for multiple myeloma patients became the highest, totaling $9,100 for the 3 years after diagnosis, compared with $8,800 for acute leukemia and an average of less than $7,800 for the other blood cancers, the LLS said in the report, which was prepared by the actuarial firm Milliman.
OOP costs also varied by the type of plan. Patients in high-deductible plans averaged nearly $5,400 for the first year after diagnosis, compared with $3,300 for those with traditional insurance, the LLS noted. For acute leukemia, the OOP costs of high-deductible plans were more than twice as high as those of traditional plans.
The study was based on data for adults aged 18-64 years from the Truven Health MarketScan commercial claims databases for the years from 2013 to 2016. The LLS received support for the study from Pfizer, Genentech, and Amgen.
with costs for acute leukemia almost tripling that amount, according to a new report from the Leukemia & Lymphoma Society (LLS).

Total allowed cost – the average amount paid by the insurer and patient combined – for acute leukemia was more than $463,000 for the 12 months after initial diagnosis. Averages for the other four cancers included in the analysis came in at $214,000 for multiple myeloma, $134,000 for bone marrow disorders, $131,000 for lymphoma, and $89,000 for chronic leukemia, the LLS said.
The cost figures are drawn from claims data for 2,332 patients diagnosed in 2014.
Differences in out-of-pocket (OOP) costs were smaller, with the average for all patients at almost $3,900 in the year after diagnosis and acute leukemia coming in the highest at $5,100. Over time, however, OOP costs for multiple myeloma patients became the highest, totaling $9,100 for the 3 years after diagnosis, compared with $8,800 for acute leukemia and an average of less than $7,800 for the other blood cancers, the LLS said in the report, which was prepared by the actuarial firm Milliman.
OOP costs also varied by the type of plan. Patients in high-deductible plans averaged nearly $5,400 for the first year after diagnosis, compared with $3,300 for those with traditional insurance, the LLS noted. For acute leukemia, the OOP costs of high-deductible plans were more than twice as high as those of traditional plans.
The study was based on data for adults aged 18-64 years from the Truven Health MarketScan commercial claims databases for the years from 2013 to 2016. The LLS received support for the study from Pfizer, Genentech, and Amgen.
Surgical repair of hip fractures in nursing home patients
Clinical question: Does surgical repair of hip fractures in nursing home residents with advanced dementia reduce adverse outcomes?
Background: Hip fractures are common in the advanced dementia nursing home population. The benefit of surgical repair is unclear in this population given significant baseline functional disability and limited life expectancy.
Study design: Retrospective cohort study.
Setting: Medicare claims data set.
Synopsis: Among 3,083 nursing home residents with advanced dementia and hip fracture, nearly 85% underwent surgical repair. The 30-day mortality rate in the nonsurgical group was 30.6%, compared with 11.5% in the surgical group. In an adjusted model, the surgical group had decreased risk of death, compared with the nonsurgical group (hazard ratio, 0.88; 95% confidence interval, 0.79-0.98). In additional adjusted models, surgical patients also had decreased risk of pressure ulcers (HR 0.64; 95% CI, 0.47-0.86) and less pain (HR, 0.78; 95% CI, 0.61-0.99). Limitations included the observational nature of the study. Although the models were adjusted, unmeasured confounding may have contributed to the findings.
Bottom line: Surgical repair of hip fractures in nursing home patients with advanced dementia reduces post-fracture mortality, pain, and pressure ulcer risk.
Citation: Berry SD et al. Association of clinical outcomes with surgical repair of hip fracture vs. nonsurgical management in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178(6):774-80.
Dr. Abramowicz is an assistant professor in the division of hospital medicine, University of Colorado, Denver.
Clinical question: Does surgical repair of hip fractures in nursing home residents with advanced dementia reduce adverse outcomes?
Background: Hip fractures are common in the advanced dementia nursing home population. The benefit of surgical repair is unclear in this population given significant baseline functional disability and limited life expectancy.
Study design: Retrospective cohort study.
Setting: Medicare claims data set.
Synopsis: Among 3,083 nursing home residents with advanced dementia and hip fracture, nearly 85% underwent surgical repair. The 30-day mortality rate in the nonsurgical group was 30.6%, compared with 11.5% in the surgical group. In an adjusted model, the surgical group had decreased risk of death, compared with the nonsurgical group (hazard ratio, 0.88; 95% confidence interval, 0.79-0.98). In additional adjusted models, surgical patients also had decreased risk of pressure ulcers (HR 0.64; 95% CI, 0.47-0.86) and less pain (HR, 0.78; 95% CI, 0.61-0.99). Limitations included the observational nature of the study. Although the models were adjusted, unmeasured confounding may have contributed to the findings.
Bottom line: Surgical repair of hip fractures in nursing home patients with advanced dementia reduces post-fracture mortality, pain, and pressure ulcer risk.
Citation: Berry SD et al. Association of clinical outcomes with surgical repair of hip fracture vs. nonsurgical management in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178(6):774-80.
Dr. Abramowicz is an assistant professor in the division of hospital medicine, University of Colorado, Denver.
Clinical question: Does surgical repair of hip fractures in nursing home residents with advanced dementia reduce adverse outcomes?
Background: Hip fractures are common in the advanced dementia nursing home population. The benefit of surgical repair is unclear in this population given significant baseline functional disability and limited life expectancy.
Study design: Retrospective cohort study.
Setting: Medicare claims data set.
Synopsis: Among 3,083 nursing home residents with advanced dementia and hip fracture, nearly 85% underwent surgical repair. The 30-day mortality rate in the nonsurgical group was 30.6%, compared with 11.5% in the surgical group. In an adjusted model, the surgical group had decreased risk of death, compared with the nonsurgical group (hazard ratio, 0.88; 95% confidence interval, 0.79-0.98). In additional adjusted models, surgical patients also had decreased risk of pressure ulcers (HR 0.64; 95% CI, 0.47-0.86) and less pain (HR, 0.78; 95% CI, 0.61-0.99). Limitations included the observational nature of the study. Although the models were adjusted, unmeasured confounding may have contributed to the findings.
Bottom line: Surgical repair of hip fractures in nursing home patients with advanced dementia reduces post-fracture mortality, pain, and pressure ulcer risk.
Citation: Berry SD et al. Association of clinical outcomes with surgical repair of hip fracture vs. nonsurgical management in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178(6):774-80.
Dr. Abramowicz is an assistant professor in the division of hospital medicine, University of Colorado, Denver.
Hypertension and CVD risk for young adults
The medical community is struggling to reach a vaccine for Hepatitis C virus, many teens don’t know that e-cigarettes contain nicotine, and there’s a duel in SLE classification criteria,
Amazon Alexa
Apple Podcasts
Spotify
The medical community is struggling to reach a vaccine for Hepatitis C virus, many teens don’t know that e-cigarettes contain nicotine, and there’s a duel in SLE classification criteria,
Amazon Alexa
Apple Podcasts
Spotify
The medical community is struggling to reach a vaccine for Hepatitis C virus, many teens don’t know that e-cigarettes contain nicotine, and there’s a duel in SLE classification criteria,
Amazon Alexa
Apple Podcasts
Spotify
Gastric Banding vs Metformin
Gastric banding was just as successful as metformin alone in stabilizing prediabetes or new-onset type 2 diabetes in the Beta Cell Restoration through Fat Mitigation study (BetaFat), an NIH-supported study.
The study is part of the Restoring Insulin Secretion (RISE) study, a set of 3 clinical trials designed to find ways to reverse or slow the loss of insulin production and release in people at risk for type 2 diabetes.
In this study, 44 patients were randomly assigned to have a gastric banding procedure, and 44 were taking metformin. After 2 years, people in the gastric banding group had lost an average of 23 lb , vs 4 lb in the metformin group. Insulin sensitivity improved similarly in both groups, as did function of insulin-producing cells. Both groups showed small improvements in blood glucose levels.
Gastric banding was just as successful as metformin alone in stabilizing prediabetes or new-onset type 2 diabetes in the Beta Cell Restoration through Fat Mitigation study (BetaFat), an NIH-supported study.
The study is part of the Restoring Insulin Secretion (RISE) study, a set of 3 clinical trials designed to find ways to reverse or slow the loss of insulin production and release in people at risk for type 2 diabetes.
In this study, 44 patients were randomly assigned to have a gastric banding procedure, and 44 were taking metformin. After 2 years, people in the gastric banding group had lost an average of 23 lb , vs 4 lb in the metformin group. Insulin sensitivity improved similarly in both groups, as did function of insulin-producing cells. Both groups showed small improvements in blood glucose levels.
Gastric banding was just as successful as metformin alone in stabilizing prediabetes or new-onset type 2 diabetes in the Beta Cell Restoration through Fat Mitigation study (BetaFat), an NIH-supported study.
The study is part of the Restoring Insulin Secretion (RISE) study, a set of 3 clinical trials designed to find ways to reverse or slow the loss of insulin production and release in people at risk for type 2 diabetes.
In this study, 44 patients were randomly assigned to have a gastric banding procedure, and 44 were taking metformin. After 2 years, people in the gastric banding group had lost an average of 23 lb , vs 4 lb in the metformin group. Insulin sensitivity improved similarly in both groups, as did function of insulin-producing cells. Both groups showed small improvements in blood glucose levels.
Combo worsens platelet recovery in MDS
Results from the phase 3 SUPPORT trial suggest there is no role for the combination of eltrombopag and azacitidine in patients with intermediate- or high-risk myelodysplastic syndromes (MDS), according to investigators.
Adding eltrombopag to azacitidine worsened platelet recovery, reduced the overall response rate, and did not improve overall or progression-free survival when compared to azacitidine plus placebo.
Investigators had hypothesized that eltrombopag would reduce the thrombocytopenia exacerbated by azacitidine treatment.
Not only was this not the case, but the investigators observed a trend toward increased progression to acute myeloid leukemia (AML) in eltrombopag-treated patients.
An independent monitoring committee recommended the trial be terminated early.
Michael Dickinson, MBBS, of Peter MacCallum Cancer Centre and Royal Melbourne Hospital in Melbourne, Australia, and his colleagues reported results from the trial in Blood.
The investigators conducted the SUPPORT study (NCT02158936) to investigate the efficacy and safety of eltrombopag as platelet supportive care in patients with intermediate- to high-risk MDS and thrombocytopenia who were receiving azacitidine.
Study design
Investigators randomized patients 1:1 to eltrombopag or placebo in combination with azacitidine. Patients had to have at least one platelet count less than 75 x 109/L within 28 days prior to the first azacitidine dose.
Patients received azacitidine subcutaneously at a dose of 75 mg/m2 once daily on 7 consecutive days for 28 days.
Patients received eltrombopag or placebo at a starting dose of 200 mg/day (100 mg/day for East Asians), adjusted by 100 mg increments (50 mg increments for East Asians) to a maximum of 300 mg/day (150 mg/day for East Asians).
Patient disposition
Investigators enrolled 356 patients from 30 countries between June 2014 and December 2015, randomizing 179 patients to eltrombopag and 177 to placebo.
Patients were a median age of 70, 66% were male, 83% were white, and 47% had platelet counts between 20 and 50 x 109/L.
At the start of the trial, 19% of patients (n=66) were platelet transfusion-dependent—16% (29/179) in the eltrombopag group and 21% (37/177) in the placebo group.
Treatment exposure
Patients received eltrombopag for a median of 83 days (range, 60 to 148) and placebo for a median of 149 days (range, 8 to 503).
For non-East Asian patients, the mean dose of eltrombopag was 205 mg/day (range, 65 to 293), and the mean dose of placebo was 245 mg/day (range, 107 to 316).
Sixty-eight patients (38%) in the eltrombopag arm received the recommended number of six or more azacitidine cycles, compared to 91 (51%) in the placebo arm.
Two patients in the eltrombopag arm did not receive treatment. Therefore, the safety analyses were conducted on the 177 treated patients in each arm.
Safety
The most common reason for treatment discontinuation was termination of the study. Thirty-two percent of patients in the eltrombopag cohort and 44% in the placebo cohort discontinued for this reason.
Patients had to discontinue eltrombopag or placebo if azacitidine was discontinued, and this occurred in 30% of patients in the eltrombopag group and 26% in the placebo group.
Twenty-two percent of eltrombopag-treated patients discontinued due to adverse events (AEs), compared with 14% in the placebo group.
The most common reasons for azacitidine discontinuation in the eltrombopag and placebo arms, respectively, were study termination (35% and 46%), AEs (25% and 19%), disease progression (15% and 14%), and patient decision (11% and 9%).
AEs with the greatest difference between the eltrombopag and placebo arms, respectively, were febrile neutropenia (31% and 21%), neutropenia (31% and 26%), nausea (31% and 26%), and diarrhea (25% and 14%).
Sixty-three of the 177 AEs in the eltrombopag group were suspected to be treatment-related, compared to 42 of 173 AEs in the placebo group.
One hundred twenty-eight (72%) serious AEs occurred in the eltrombopag arm, compared to 100 (56%) in the placebo arm.
Fifteen percent of the serious AEs were suspected to be related to eltrombopag treatment, compared to 4% of the serious AEs in the placebo group.
At the final analysis, 108 patients had died—57 (32%) in the eltrombopag group and 51 (29%) in the placebo group (hazard ratio [HR] 1.42; 95% CI 0.97, 2.08; nominal P=0.164).
The main causes of death were disease progression (28 in the eltrombopag group and 21 in the placebo group) and sepsis (18 in the eltrombopag group and 13 in the placebo group).
Efficacy
The study’s primary endpoint was the proportion of patients free of platelet transfusions during cycles one to four of azacitidine therapy.
At the final analysis, there were fewer patients in the eltrombopag arm than in the placebo arm who had achieved platelet transfusion independence—16% (28/179) and 31% (55/177), respectively. The odds ratio (OR) was 0.37 (95% CI 0.21, 0.65; two-sided P value=0.001).
Secondary efficacy endpoints included overall survival, disease response, duration of response, progression to AML, progression-free survival, and hematologic improvement.
The investigators pointed out that no formal statistical tests were performed for the secondary endpoints. Therefore, “statistical interpretation should be made with caution,” they wrote.
Overall response by IWG criteria occurred in 20% of patients in the eltrombopag group and 35% of those in the placebo group, according to investigator assessment (OR=0.51; 95% CI 0.30, 0.86; nominal P=0.005).
There was no significant difference in hematologic improvement, overall survival, or progression-free survival between the treatment arms.
The investigators found a higher rate of progression to AML in the eltrombopag arm than in the placebo arm—15% and 9%, respectively (OR=1.59; 95% CI 0.81, 3.14; nominal P=0.079).
They pointed out that these results contrasted with those of recent clinical studies of eltrombopag monotherapy1,2,3,4 in patients with MDS.
“[T]he findings of this trial were unexpected,” the investigators wrote.
They hypothesized that eltrombopag inhibits the effects of azacitidine when the drugs are given concomitantly. The issue is being studied further with ongoing research, they said.
The authors acknowledged financial support for medical editorial assistance provided by Novartis Pharmaceuticals Corporation.
Dr. Dickinson disclosed relationships with Celgene, Novartis, Janssen, Pfizer, and Roche. Senior study author Uwe Platzbecker, MD, disclosed relationships with Amgen, Celgene, GlaxoSmithKline, and Novartis.
1. Platzbecker U, et al Lancet Haematol. 2015;2(10):e417-e426. DOI: https://doi.org/10.1016/S2352-3026(15)00149-0
2. Oliva EN, et al. Lancet Haematol. 2017;4(3):e127-e136. DOI: https://doi.org/10.1016/S2352-3026(17)30012-1
3. Mittelman M, et al. Lancet Haematol. 2017;5(1):e34-e43. DOI: https://doi.org/10.1016/S2352-3026(17)30228-4
4. Buckstein R. Lancet Haematol. 2015;2(10):e396-e397. DOI: https://doi.org/10.1016/S2352-3026(15)00200-8
Results from the phase 3 SUPPORT trial suggest there is no role for the combination of eltrombopag and azacitidine in patients with intermediate- or high-risk myelodysplastic syndromes (MDS), according to investigators.
Adding eltrombopag to azacitidine worsened platelet recovery, reduced the overall response rate, and did not improve overall or progression-free survival when compared to azacitidine plus placebo.
Investigators had hypothesized that eltrombopag would reduce the thrombocytopenia exacerbated by azacitidine treatment.
Not only was this not the case, but the investigators observed a trend toward increased progression to acute myeloid leukemia (AML) in eltrombopag-treated patients.
An independent monitoring committee recommended the trial be terminated early.
Michael Dickinson, MBBS, of Peter MacCallum Cancer Centre and Royal Melbourne Hospital in Melbourne, Australia, and his colleagues reported results from the trial in Blood.
The investigators conducted the SUPPORT study (NCT02158936) to investigate the efficacy and safety of eltrombopag as platelet supportive care in patients with intermediate- to high-risk MDS and thrombocytopenia who were receiving azacitidine.
Study design
Investigators randomized patients 1:1 to eltrombopag or placebo in combination with azacitidine. Patients had to have at least one platelet count less than 75 x 109/L within 28 days prior to the first azacitidine dose.
Patients received azacitidine subcutaneously at a dose of 75 mg/m2 once daily on 7 consecutive days for 28 days.
Patients received eltrombopag or placebo at a starting dose of 200 mg/day (100 mg/day for East Asians), adjusted by 100 mg increments (50 mg increments for East Asians) to a maximum of 300 mg/day (150 mg/day for East Asians).
Patient disposition
Investigators enrolled 356 patients from 30 countries between June 2014 and December 2015, randomizing 179 patients to eltrombopag and 177 to placebo.
Patients were a median age of 70, 66% were male, 83% were white, and 47% had platelet counts between 20 and 50 x 109/L.
At the start of the trial, 19% of patients (n=66) were platelet transfusion-dependent—16% (29/179) in the eltrombopag group and 21% (37/177) in the placebo group.
Treatment exposure
Patients received eltrombopag for a median of 83 days (range, 60 to 148) and placebo for a median of 149 days (range, 8 to 503).
For non-East Asian patients, the mean dose of eltrombopag was 205 mg/day (range, 65 to 293), and the mean dose of placebo was 245 mg/day (range, 107 to 316).
Sixty-eight patients (38%) in the eltrombopag arm received the recommended number of six or more azacitidine cycles, compared to 91 (51%) in the placebo arm.
Two patients in the eltrombopag arm did not receive treatment. Therefore, the safety analyses were conducted on the 177 treated patients in each arm.
Safety
The most common reason for treatment discontinuation was termination of the study. Thirty-two percent of patients in the eltrombopag cohort and 44% in the placebo cohort discontinued for this reason.
Patients had to discontinue eltrombopag or placebo if azacitidine was discontinued, and this occurred in 30% of patients in the eltrombopag group and 26% in the placebo group.
Twenty-two percent of eltrombopag-treated patients discontinued due to adverse events (AEs), compared with 14% in the placebo group.
The most common reasons for azacitidine discontinuation in the eltrombopag and placebo arms, respectively, were study termination (35% and 46%), AEs (25% and 19%), disease progression (15% and 14%), and patient decision (11% and 9%).
AEs with the greatest difference between the eltrombopag and placebo arms, respectively, were febrile neutropenia (31% and 21%), neutropenia (31% and 26%), nausea (31% and 26%), and diarrhea (25% and 14%).
Sixty-three of the 177 AEs in the eltrombopag group were suspected to be treatment-related, compared to 42 of 173 AEs in the placebo group.
One hundred twenty-eight (72%) serious AEs occurred in the eltrombopag arm, compared to 100 (56%) in the placebo arm.
Fifteen percent of the serious AEs were suspected to be related to eltrombopag treatment, compared to 4% of the serious AEs in the placebo group.
At the final analysis, 108 patients had died—57 (32%) in the eltrombopag group and 51 (29%) in the placebo group (hazard ratio [HR] 1.42; 95% CI 0.97, 2.08; nominal P=0.164).
The main causes of death were disease progression (28 in the eltrombopag group and 21 in the placebo group) and sepsis (18 in the eltrombopag group and 13 in the placebo group).
Efficacy
The study’s primary endpoint was the proportion of patients free of platelet transfusions during cycles one to four of azacitidine therapy.
At the final analysis, there were fewer patients in the eltrombopag arm than in the placebo arm who had achieved platelet transfusion independence—16% (28/179) and 31% (55/177), respectively. The odds ratio (OR) was 0.37 (95% CI 0.21, 0.65; two-sided P value=0.001).
Secondary efficacy endpoints included overall survival, disease response, duration of response, progression to AML, progression-free survival, and hematologic improvement.
The investigators pointed out that no formal statistical tests were performed for the secondary endpoints. Therefore, “statistical interpretation should be made with caution,” they wrote.
Overall response by IWG criteria occurred in 20% of patients in the eltrombopag group and 35% of those in the placebo group, according to investigator assessment (OR=0.51; 95% CI 0.30, 0.86; nominal P=0.005).
There was no significant difference in hematologic improvement, overall survival, or progression-free survival between the treatment arms.
The investigators found a higher rate of progression to AML in the eltrombopag arm than in the placebo arm—15% and 9%, respectively (OR=1.59; 95% CI 0.81, 3.14; nominal P=0.079).
They pointed out that these results contrasted with those of recent clinical studies of eltrombopag monotherapy1,2,3,4 in patients with MDS.
“[T]he findings of this trial were unexpected,” the investigators wrote.
They hypothesized that eltrombopag inhibits the effects of azacitidine when the drugs are given concomitantly. The issue is being studied further with ongoing research, they said.
The authors acknowledged financial support for medical editorial assistance provided by Novartis Pharmaceuticals Corporation.
Dr. Dickinson disclosed relationships with Celgene, Novartis, Janssen, Pfizer, and Roche. Senior study author Uwe Platzbecker, MD, disclosed relationships with Amgen, Celgene, GlaxoSmithKline, and Novartis.
1. Platzbecker U, et al Lancet Haematol. 2015;2(10):e417-e426. DOI: https://doi.org/10.1016/S2352-3026(15)00149-0
2. Oliva EN, et al. Lancet Haematol. 2017;4(3):e127-e136. DOI: https://doi.org/10.1016/S2352-3026(17)30012-1
3. Mittelman M, et al. Lancet Haematol. 2017;5(1):e34-e43. DOI: https://doi.org/10.1016/S2352-3026(17)30228-4
4. Buckstein R. Lancet Haematol. 2015;2(10):e396-e397. DOI: https://doi.org/10.1016/S2352-3026(15)00200-8
Results from the phase 3 SUPPORT trial suggest there is no role for the combination of eltrombopag and azacitidine in patients with intermediate- or high-risk myelodysplastic syndromes (MDS), according to investigators.
Adding eltrombopag to azacitidine worsened platelet recovery, reduced the overall response rate, and did not improve overall or progression-free survival when compared to azacitidine plus placebo.
Investigators had hypothesized that eltrombopag would reduce the thrombocytopenia exacerbated by azacitidine treatment.
Not only was this not the case, but the investigators observed a trend toward increased progression to acute myeloid leukemia (AML) in eltrombopag-treated patients.
An independent monitoring committee recommended the trial be terminated early.
Michael Dickinson, MBBS, of Peter MacCallum Cancer Centre and Royal Melbourne Hospital in Melbourne, Australia, and his colleagues reported results from the trial in Blood.
The investigators conducted the SUPPORT study (NCT02158936) to investigate the efficacy and safety of eltrombopag as platelet supportive care in patients with intermediate- to high-risk MDS and thrombocytopenia who were receiving azacitidine.
Study design
Investigators randomized patients 1:1 to eltrombopag or placebo in combination with azacitidine. Patients had to have at least one platelet count less than 75 x 109/L within 28 days prior to the first azacitidine dose.
Patients received azacitidine subcutaneously at a dose of 75 mg/m2 once daily on 7 consecutive days for 28 days.
Patients received eltrombopag or placebo at a starting dose of 200 mg/day (100 mg/day for East Asians), adjusted by 100 mg increments (50 mg increments for East Asians) to a maximum of 300 mg/day (150 mg/day for East Asians).
Patient disposition
Investigators enrolled 356 patients from 30 countries between June 2014 and December 2015, randomizing 179 patients to eltrombopag and 177 to placebo.
Patients were a median age of 70, 66% were male, 83% were white, and 47% had platelet counts between 20 and 50 x 109/L.
At the start of the trial, 19% of patients (n=66) were platelet transfusion-dependent—16% (29/179) in the eltrombopag group and 21% (37/177) in the placebo group.
Treatment exposure
Patients received eltrombopag for a median of 83 days (range, 60 to 148) and placebo for a median of 149 days (range, 8 to 503).
For non-East Asian patients, the mean dose of eltrombopag was 205 mg/day (range, 65 to 293), and the mean dose of placebo was 245 mg/day (range, 107 to 316).
Sixty-eight patients (38%) in the eltrombopag arm received the recommended number of six or more azacitidine cycles, compared to 91 (51%) in the placebo arm.
Two patients in the eltrombopag arm did not receive treatment. Therefore, the safety analyses were conducted on the 177 treated patients in each arm.
Safety
The most common reason for treatment discontinuation was termination of the study. Thirty-two percent of patients in the eltrombopag cohort and 44% in the placebo cohort discontinued for this reason.
Patients had to discontinue eltrombopag or placebo if azacitidine was discontinued, and this occurred in 30% of patients in the eltrombopag group and 26% in the placebo group.
Twenty-two percent of eltrombopag-treated patients discontinued due to adverse events (AEs), compared with 14% in the placebo group.
The most common reasons for azacitidine discontinuation in the eltrombopag and placebo arms, respectively, were study termination (35% and 46%), AEs (25% and 19%), disease progression (15% and 14%), and patient decision (11% and 9%).
AEs with the greatest difference between the eltrombopag and placebo arms, respectively, were febrile neutropenia (31% and 21%), neutropenia (31% and 26%), nausea (31% and 26%), and diarrhea (25% and 14%).
Sixty-three of the 177 AEs in the eltrombopag group were suspected to be treatment-related, compared to 42 of 173 AEs in the placebo group.
One hundred twenty-eight (72%) serious AEs occurred in the eltrombopag arm, compared to 100 (56%) in the placebo arm.
Fifteen percent of the serious AEs were suspected to be related to eltrombopag treatment, compared to 4% of the serious AEs in the placebo group.
At the final analysis, 108 patients had died—57 (32%) in the eltrombopag group and 51 (29%) in the placebo group (hazard ratio [HR] 1.42; 95% CI 0.97, 2.08; nominal P=0.164).
The main causes of death were disease progression (28 in the eltrombopag group and 21 in the placebo group) and sepsis (18 in the eltrombopag group and 13 in the placebo group).
Efficacy
The study’s primary endpoint was the proportion of patients free of platelet transfusions during cycles one to four of azacitidine therapy.
At the final analysis, there were fewer patients in the eltrombopag arm than in the placebo arm who had achieved platelet transfusion independence—16% (28/179) and 31% (55/177), respectively. The odds ratio (OR) was 0.37 (95% CI 0.21, 0.65; two-sided P value=0.001).
Secondary efficacy endpoints included overall survival, disease response, duration of response, progression to AML, progression-free survival, and hematologic improvement.
The investigators pointed out that no formal statistical tests were performed for the secondary endpoints. Therefore, “statistical interpretation should be made with caution,” they wrote.
Overall response by IWG criteria occurred in 20% of patients in the eltrombopag group and 35% of those in the placebo group, according to investigator assessment (OR=0.51; 95% CI 0.30, 0.86; nominal P=0.005).
There was no significant difference in hematologic improvement, overall survival, or progression-free survival between the treatment arms.
The investigators found a higher rate of progression to AML in the eltrombopag arm than in the placebo arm—15% and 9%, respectively (OR=1.59; 95% CI 0.81, 3.14; nominal P=0.079).
They pointed out that these results contrasted with those of recent clinical studies of eltrombopag monotherapy1,2,3,4 in patients with MDS.
“[T]he findings of this trial were unexpected,” the investigators wrote.
They hypothesized that eltrombopag inhibits the effects of azacitidine when the drugs are given concomitantly. The issue is being studied further with ongoing research, they said.
The authors acknowledged financial support for medical editorial assistance provided by Novartis Pharmaceuticals Corporation.
Dr. Dickinson disclosed relationships with Celgene, Novartis, Janssen, Pfizer, and Roche. Senior study author Uwe Platzbecker, MD, disclosed relationships with Amgen, Celgene, GlaxoSmithKline, and Novartis.
1. Platzbecker U, et al Lancet Haematol. 2015;2(10):e417-e426. DOI: https://doi.org/10.1016/S2352-3026(15)00149-0
2. Oliva EN, et al. Lancet Haematol. 2017;4(3):e127-e136. DOI: https://doi.org/10.1016/S2352-3026(17)30012-1
3. Mittelman M, et al. Lancet Haematol. 2017;5(1):e34-e43. DOI: https://doi.org/10.1016/S2352-3026(17)30228-4
4. Buckstein R. Lancet Haematol. 2015;2(10):e396-e397. DOI: https://doi.org/10.1016/S2352-3026(15)00200-8
Molecule enhances PI activity in multiple myeloma
Researchers say they have identified a new class of protein disulfide isomerase (PDI) inhibitors that sensitize multiple myeloma (MM) cells to proteasome inhibitors (PIs).
The investigators screened approximately 20,000 compounds spanning multiple chemical libraries and found the compound E61 to be a “striking hit,” with a six-fold increase in bortezomib cytotoxicity and the ability to re-sensitize PI activity at low micromolar concentrations.
The researchers then synthesized and evaluated 150 E61 derivatives and discovered the lead candidate, E64FC26, which was highly synergistic with PIs at concentrations as low as 200 nM.
They reported that E64FC26 has “several advantages over previously reported PDI inhibitors, including superior potency and a pan-style mode of inhibition.”
“PDI is an attractive target in oncology, but good PDI inhibitors have been hard to find,” said Nathan G. Dolloff, PhD, of the Medical University of South Carolina (MUSC) in Charleston.
“The compounds we discovered have a lot of advantages, including high potency and good drug-like properties. We hope that those strengths translate into an effective new drug that can ultimately help patients.”
Dr. Dolloff and his colleagues reported their discovery in Leukemia.
The investigators detected the synergistic effects of E61 in combination with next-generation PIs, including carfilzomib, ixazomib, and oprozomib in both PI-sensitive and -resistant MM cell lines.
On the other hand, E61 had no effect on dexamethasone activity in dexamethasone-resistant cells. And E61 did not affect lenalidomide or doxorubicin cytotoxicity in PI-resistant cells.
The researchers also determined that E61 was only active in MM cells. E61 did not enhance the cytotoxic effects of PIs in normal cells.
This selective toxicity suggests that E61 may have “a wide therapeutic index in vivo,” the investigators wrote.
In vivo activity
To investigate the anti-MM activity and tolerability of E61 in vivo, the researchers treated a NOD-SCID IL2Rγ−/− mouse model with E61 at a continuous dose of 50 mg/kg/day.
E61 prolonged survival by 11 days in the treated mice (P=0.0007), and four of the 11 treated mice survived to the experiment’s end.
In another experiment, two of eight mice achieved a complete response.
After continuous dosing for 40 or more days, E61 was well tolerated, the investigators reported. The mice showed no overt signs of distress and did not lose weight.
Molecular target of E61
Using click (Cu(I)-catalyzed azide-alkyne cycloaddition) chemistry and a proteomics approach, the researchers then confirmed that PDI family members are the molecular target of E61.
Functional studies indicated that E61 inhibited PDI reductase activity in vitro. E61 also enhanced the accumulation of ubiquitinylated proteins and produced strong endoplasmic reticulum (ER) and oxidative stress responses when combined with PIs.
Anti-MM activity of E64FC26
The investigators used a structure activity relationship program to narrow the candidate molecules down to E64FC26.
E64FC26 demonstrated pan-inhibition in that it inhibited all members of the PDI family tested, including PDIA3, PDIA4, TXNDC5, and PDIA6.
E64FC26 also had greater in vitro potency against PDIA1 and the other PDI isoforms. It was the only compound to sensitize MM cells to PIs, with an average increase in PI sensitivity ranging from six- to seven-fold.
The researchers also noted that E64FC26 was superior to other PDI inhibitors they tested in activating ER stress.
The investigators tested the activity of E64FC26 in vivo using Vk*MYC transgenic mice, a model that closely resembles human MM.
Mice treated with E64FC26 had an immediate anti-MM response. Serum M-protein decreased in all mice by an average of 33 ± 7.9% (P=0.0135).
The investigators observed similar effects in a human xenotransplant MM model.
These mice were randomized to receive treatment with vehicle, E64FC26 (2 mg/kg for 3 days/week), bortezomib (0.25 mg/kg for 2 days/week), or a combination of the two agents.
E64FC26 increased median survival by 2 weeks compared with vehicle-treated mice (P<0.0001). By day 36, no vehicle-treated mouse survived, compared with 100% of the E64FC26-treated mice.
Single-agent bortezomib increased survival by 6 days (P=0.0007).
And the combination produced the greatest improvement in median survival, increasing it by 20 days (P<0.0001).
The investigators reported no overt toxicity or body weight fluctuation for mice treated with E64FC26 or the combination.
“These results provide preclinical proof of concept for the strategy of targeting PDI with this new class of compound for the treatment of MM,” the researchers concluded.
“One of the strengths of this study is that we spanned almost the entire drug discovery process,” Dr. Dolloff said. “We screened thousands of compounds, found an exciting molecule, deconvoluted what its binding target was, synthesized hundreds of derivatives to make it better, and then conducted animal studies.”
“The study has everything from biochemistry and cell biology to medicinal chemistry and animal pharmacology in it. There is still a lot of work to be done before this drug is ready for clinical trials in humans, but it has been a rewarding project, and I’m looking forward to the next steps.”
Dr. Dolloff is founder of Leukogene Therapeutics, Inc., which has licensed patents from MUSC, and a second study author is an inventor on patents. The other authors declared no conflicts of interest.
The research was supported by the National Institutes of Health/National Cancer Institute, the South Carolina Clinical & Translational Research Institute, the MUSC Hollings Cancer Center, and by the Hollings Cancer Center T32 Ruth L. Kirschstein National Research Service Award Training Program.
Researchers say they have identified a new class of protein disulfide isomerase (PDI) inhibitors that sensitize multiple myeloma (MM) cells to proteasome inhibitors (PIs).
The investigators screened approximately 20,000 compounds spanning multiple chemical libraries and found the compound E61 to be a “striking hit,” with a six-fold increase in bortezomib cytotoxicity and the ability to re-sensitize PI activity at low micromolar concentrations.
The researchers then synthesized and evaluated 150 E61 derivatives and discovered the lead candidate, E64FC26, which was highly synergistic with PIs at concentrations as low as 200 nM.
They reported that E64FC26 has “several advantages over previously reported PDI inhibitors, including superior potency and a pan-style mode of inhibition.”
“PDI is an attractive target in oncology, but good PDI inhibitors have been hard to find,” said Nathan G. Dolloff, PhD, of the Medical University of South Carolina (MUSC) in Charleston.
“The compounds we discovered have a lot of advantages, including high potency and good drug-like properties. We hope that those strengths translate into an effective new drug that can ultimately help patients.”
Dr. Dolloff and his colleagues reported their discovery in Leukemia.
The investigators detected the synergistic effects of E61 in combination with next-generation PIs, including carfilzomib, ixazomib, and oprozomib in both PI-sensitive and -resistant MM cell lines.
On the other hand, E61 had no effect on dexamethasone activity in dexamethasone-resistant cells. And E61 did not affect lenalidomide or doxorubicin cytotoxicity in PI-resistant cells.
The researchers also determined that E61 was only active in MM cells. E61 did not enhance the cytotoxic effects of PIs in normal cells.
This selective toxicity suggests that E61 may have “a wide therapeutic index in vivo,” the investigators wrote.
In vivo activity
To investigate the anti-MM activity and tolerability of E61 in vivo, the researchers treated a NOD-SCID IL2Rγ−/− mouse model with E61 at a continuous dose of 50 mg/kg/day.
E61 prolonged survival by 11 days in the treated mice (P=0.0007), and four of the 11 treated mice survived to the experiment’s end.
In another experiment, two of eight mice achieved a complete response.
After continuous dosing for 40 or more days, E61 was well tolerated, the investigators reported. The mice showed no overt signs of distress and did not lose weight.
Molecular target of E61
Using click (Cu(I)-catalyzed azide-alkyne cycloaddition) chemistry and a proteomics approach, the researchers then confirmed that PDI family members are the molecular target of E61.
Functional studies indicated that E61 inhibited PDI reductase activity in vitro. E61 also enhanced the accumulation of ubiquitinylated proteins and produced strong endoplasmic reticulum (ER) and oxidative stress responses when combined with PIs.
Anti-MM activity of E64FC26
The investigators used a structure activity relationship program to narrow the candidate molecules down to E64FC26.
E64FC26 demonstrated pan-inhibition in that it inhibited all members of the PDI family tested, including PDIA3, PDIA4, TXNDC5, and PDIA6.
E64FC26 also had greater in vitro potency against PDIA1 and the other PDI isoforms. It was the only compound to sensitize MM cells to PIs, with an average increase in PI sensitivity ranging from six- to seven-fold.
The researchers also noted that E64FC26 was superior to other PDI inhibitors they tested in activating ER stress.
The investigators tested the activity of E64FC26 in vivo using Vk*MYC transgenic mice, a model that closely resembles human MM.
Mice treated with E64FC26 had an immediate anti-MM response. Serum M-protein decreased in all mice by an average of 33 ± 7.9% (P=0.0135).
The investigators observed similar effects in a human xenotransplant MM model.
These mice were randomized to receive treatment with vehicle, E64FC26 (2 mg/kg for 3 days/week), bortezomib (0.25 mg/kg for 2 days/week), or a combination of the two agents.
E64FC26 increased median survival by 2 weeks compared with vehicle-treated mice (P<0.0001). By day 36, no vehicle-treated mouse survived, compared with 100% of the E64FC26-treated mice.
Single-agent bortezomib increased survival by 6 days (P=0.0007).
And the combination produced the greatest improvement in median survival, increasing it by 20 days (P<0.0001).
The investigators reported no overt toxicity or body weight fluctuation for mice treated with E64FC26 or the combination.
“These results provide preclinical proof of concept for the strategy of targeting PDI with this new class of compound for the treatment of MM,” the researchers concluded.
“One of the strengths of this study is that we spanned almost the entire drug discovery process,” Dr. Dolloff said. “We screened thousands of compounds, found an exciting molecule, deconvoluted what its binding target was, synthesized hundreds of derivatives to make it better, and then conducted animal studies.”
“The study has everything from biochemistry and cell biology to medicinal chemistry and animal pharmacology in it. There is still a lot of work to be done before this drug is ready for clinical trials in humans, but it has been a rewarding project, and I’m looking forward to the next steps.”
Dr. Dolloff is founder of Leukogene Therapeutics, Inc., which has licensed patents from MUSC, and a second study author is an inventor on patents. The other authors declared no conflicts of interest.
The research was supported by the National Institutes of Health/National Cancer Institute, the South Carolina Clinical & Translational Research Institute, the MUSC Hollings Cancer Center, and by the Hollings Cancer Center T32 Ruth L. Kirschstein National Research Service Award Training Program.
Researchers say they have identified a new class of protein disulfide isomerase (PDI) inhibitors that sensitize multiple myeloma (MM) cells to proteasome inhibitors (PIs).
The investigators screened approximately 20,000 compounds spanning multiple chemical libraries and found the compound E61 to be a “striking hit,” with a six-fold increase in bortezomib cytotoxicity and the ability to re-sensitize PI activity at low micromolar concentrations.
The researchers then synthesized and evaluated 150 E61 derivatives and discovered the lead candidate, E64FC26, which was highly synergistic with PIs at concentrations as low as 200 nM.
They reported that E64FC26 has “several advantages over previously reported PDI inhibitors, including superior potency and a pan-style mode of inhibition.”
“PDI is an attractive target in oncology, but good PDI inhibitors have been hard to find,” said Nathan G. Dolloff, PhD, of the Medical University of South Carolina (MUSC) in Charleston.
“The compounds we discovered have a lot of advantages, including high potency and good drug-like properties. We hope that those strengths translate into an effective new drug that can ultimately help patients.”
Dr. Dolloff and his colleagues reported their discovery in Leukemia.
The investigators detected the synergistic effects of E61 in combination with next-generation PIs, including carfilzomib, ixazomib, and oprozomib in both PI-sensitive and -resistant MM cell lines.
On the other hand, E61 had no effect on dexamethasone activity in dexamethasone-resistant cells. And E61 did not affect lenalidomide or doxorubicin cytotoxicity in PI-resistant cells.
The researchers also determined that E61 was only active in MM cells. E61 did not enhance the cytotoxic effects of PIs in normal cells.
This selective toxicity suggests that E61 may have “a wide therapeutic index in vivo,” the investigators wrote.
In vivo activity
To investigate the anti-MM activity and tolerability of E61 in vivo, the researchers treated a NOD-SCID IL2Rγ−/− mouse model with E61 at a continuous dose of 50 mg/kg/day.
E61 prolonged survival by 11 days in the treated mice (P=0.0007), and four of the 11 treated mice survived to the experiment’s end.
In another experiment, two of eight mice achieved a complete response.
After continuous dosing for 40 or more days, E61 was well tolerated, the investigators reported. The mice showed no overt signs of distress and did not lose weight.
Molecular target of E61
Using click (Cu(I)-catalyzed azide-alkyne cycloaddition) chemistry and a proteomics approach, the researchers then confirmed that PDI family members are the molecular target of E61.
Functional studies indicated that E61 inhibited PDI reductase activity in vitro. E61 also enhanced the accumulation of ubiquitinylated proteins and produced strong endoplasmic reticulum (ER) and oxidative stress responses when combined with PIs.
Anti-MM activity of E64FC26
The investigators used a structure activity relationship program to narrow the candidate molecules down to E64FC26.
E64FC26 demonstrated pan-inhibition in that it inhibited all members of the PDI family tested, including PDIA3, PDIA4, TXNDC5, and PDIA6.
E64FC26 also had greater in vitro potency against PDIA1 and the other PDI isoforms. It was the only compound to sensitize MM cells to PIs, with an average increase in PI sensitivity ranging from six- to seven-fold.
The researchers also noted that E64FC26 was superior to other PDI inhibitors they tested in activating ER stress.
The investigators tested the activity of E64FC26 in vivo using Vk*MYC transgenic mice, a model that closely resembles human MM.
Mice treated with E64FC26 had an immediate anti-MM response. Serum M-protein decreased in all mice by an average of 33 ± 7.9% (P=0.0135).
The investigators observed similar effects in a human xenotransplant MM model.
These mice were randomized to receive treatment with vehicle, E64FC26 (2 mg/kg for 3 days/week), bortezomib (0.25 mg/kg for 2 days/week), or a combination of the two agents.
E64FC26 increased median survival by 2 weeks compared with vehicle-treated mice (P<0.0001). By day 36, no vehicle-treated mouse survived, compared with 100% of the E64FC26-treated mice.
Single-agent bortezomib increased survival by 6 days (P=0.0007).
And the combination produced the greatest improvement in median survival, increasing it by 20 days (P<0.0001).
The investigators reported no overt toxicity or body weight fluctuation for mice treated with E64FC26 or the combination.
“These results provide preclinical proof of concept for the strategy of targeting PDI with this new class of compound for the treatment of MM,” the researchers concluded.
“One of the strengths of this study is that we spanned almost the entire drug discovery process,” Dr. Dolloff said. “We screened thousands of compounds, found an exciting molecule, deconvoluted what its binding target was, synthesized hundreds of derivatives to make it better, and then conducted animal studies.”
“The study has everything from biochemistry and cell biology to medicinal chemistry and animal pharmacology in it. There is still a lot of work to be done before this drug is ready for clinical trials in humans, but it has been a rewarding project, and I’m looking forward to the next steps.”
Dr. Dolloff is founder of Leukogene Therapeutics, Inc., which has licensed patents from MUSC, and a second study author is an inventor on patents. The other authors declared no conflicts of interest.
The research was supported by the National Institutes of Health/National Cancer Institute, the South Carolina Clinical & Translational Research Institute, the MUSC Hollings Cancer Center, and by the Hollings Cancer Center T32 Ruth L. Kirschstein National Research Service Award Training Program.