User login
Study offers snapshot of esophageal strictures in EB patients
LAKE TAHOE, CALIF. – and direct visualization of these strictures is the preferred method of diagnosis. Those are key findings from a multicenter study that lead author Elena Pope, MD, discussed at the annual meeting of the Society for Pediatric Dermatology.
According to Dr. Pope, who heads the section of dermatology at the Hospital for Sick Children, Toronto, an estimated 10%-17% of epidermolysis bullosa (EB) patients experience strictures, with an overrepresentation in the recessive dystrophic EB subtype in up to 80% of cases. The risk increases with age. “What remains unknown is the best short- and long-term intervention to manage the strictures and predictors/associations for stricture-free episodes,” Dr. Pope said. “The objectives of the current study were to determine the prevalence and predisposing factors for strictures in EB, management options, patient outcomes, and predictors for recurrences and stricture-free intervals.”
She and her associates at seven centers worldwide collected data on 125 EB patients who experienced at least one episode of esophageal stricture. Data was analyzed descriptively and with ANOVA regression analysis for associations/predictors for recurrences/episode-free intervals.
The researchers evaluated 497 stricture events in the 125 patients. A slight female predominance was noted (53%), and the mean age of the first episode was 12.7 years, “which is a little bit older” than the age found in previously published data, Dr. Pope said. As expected, dystrophic EB patients made up most of the sample (98.4%); of these 123 patients, recessive dystrophic EB severe generalized subtype – approaching 50% – was the most common, followed by the recessive dystrophic EB severe intermediate subtype (almost 21%), the dominant dystrophic EB generalized subtype (7%), and other types of dystrophic EB (almost 26%).
The median body mass index percentile for age was 6.3, “so these were patients who were severely malnourished, probably as a result of their strictures as well as their underlying disease,” Dr. Pope said.
As expected, dysphagia was a presenting symptom in most patients (85.5%), while 29.8% presented with inability to swallow solids. The preferred method of evaluation was video fluoroscopy (57.7%), and less commonly with barium swallow (22.3%) or with clinical symptoms alone (0.1%). The mean number of strictures was 1.69; 76.7% were located in the cervical area, 56.7% were located in the thoracic area, and 9.7% were located in the abdominal area. Most patients (76%) had lesions that were 1 cm or longer in size.
Fluoroscopy guidance was the most common method of dilatation (in 45.2% of cases), followed by retrograde endoscopy was (33%), antegrade endoscopy (19.1%), and bougienage (0.1%). General anesthesia was used in most cases (87.6%), and corticosteroids were used around the dilatation in 90.4% of patients. The mean duration of medication use was about 5 days.
As for outcomes after dilatation, 92.2% of strictures completely resolved, 3.8% were partially resolved, 3.9% were not resolved, and 2.7% had complications. The median interval between dilatations was 7 months. Fluoroscopy-guided balloon dilatation was associated with the longest esophageal stricture-free duration (mean of 13.83 months vs. 8.75 months; P less than .001), followed by retrograde endoscopy (mean of 13.10 months vs. 7.85 months; P less than .001), and antegrade endoscopy (mean of 7.63 months vs. 11.46 months; P = .024). “I think this is interesting,” said Dr. Pope, who is also a professor of pediatrics at the University of Toronto. “I think the difference occurs because if you use the endoscopy, which a rigid tube, you can potentially cause more damage, and more long-term scarring.”
Another predictor of esophageal stricture-free episodes was systemic corticosteroid use (a mean of 25.28 months vs. 10.24 months; P less than .001) around the time of the dilatation procedure. “By using systemic steroids, you’re actually decreasing some of the inflammation associated with the trauma of the procedure decreasing the chances of strictures formation,” she said.
Dr. Pope recommended that future studies evaluate the benefit of periprocedural medical interventions on increasing the intervals between esophageal stricture occurrences.
The study was supported by an unrestricted grant from the Epidermolysis Bullosa Research Foundation. She reported having no financial disclosures.
LAKE TAHOE, CALIF. – and direct visualization of these strictures is the preferred method of diagnosis. Those are key findings from a multicenter study that lead author Elena Pope, MD, discussed at the annual meeting of the Society for Pediatric Dermatology.
According to Dr. Pope, who heads the section of dermatology at the Hospital for Sick Children, Toronto, an estimated 10%-17% of epidermolysis bullosa (EB) patients experience strictures, with an overrepresentation in the recessive dystrophic EB subtype in up to 80% of cases. The risk increases with age. “What remains unknown is the best short- and long-term intervention to manage the strictures and predictors/associations for stricture-free episodes,” Dr. Pope said. “The objectives of the current study were to determine the prevalence and predisposing factors for strictures in EB, management options, patient outcomes, and predictors for recurrences and stricture-free intervals.”
She and her associates at seven centers worldwide collected data on 125 EB patients who experienced at least one episode of esophageal stricture. Data was analyzed descriptively and with ANOVA regression analysis for associations/predictors for recurrences/episode-free intervals.
The researchers evaluated 497 stricture events in the 125 patients. A slight female predominance was noted (53%), and the mean age of the first episode was 12.7 years, “which is a little bit older” than the age found in previously published data, Dr. Pope said. As expected, dystrophic EB patients made up most of the sample (98.4%); of these 123 patients, recessive dystrophic EB severe generalized subtype – approaching 50% – was the most common, followed by the recessive dystrophic EB severe intermediate subtype (almost 21%), the dominant dystrophic EB generalized subtype (7%), and other types of dystrophic EB (almost 26%).
The median body mass index percentile for age was 6.3, “so these were patients who were severely malnourished, probably as a result of their strictures as well as their underlying disease,” Dr. Pope said.
As expected, dysphagia was a presenting symptom in most patients (85.5%), while 29.8% presented with inability to swallow solids. The preferred method of evaluation was video fluoroscopy (57.7%), and less commonly with barium swallow (22.3%) or with clinical symptoms alone (0.1%). The mean number of strictures was 1.69; 76.7% were located in the cervical area, 56.7% were located in the thoracic area, and 9.7% were located in the abdominal area. Most patients (76%) had lesions that were 1 cm or longer in size.
Fluoroscopy guidance was the most common method of dilatation (in 45.2% of cases), followed by retrograde endoscopy was (33%), antegrade endoscopy (19.1%), and bougienage (0.1%). General anesthesia was used in most cases (87.6%), and corticosteroids were used around the dilatation in 90.4% of patients. The mean duration of medication use was about 5 days.
As for outcomes after dilatation, 92.2% of strictures completely resolved, 3.8% were partially resolved, 3.9% were not resolved, and 2.7% had complications. The median interval between dilatations was 7 months. Fluoroscopy-guided balloon dilatation was associated with the longest esophageal stricture-free duration (mean of 13.83 months vs. 8.75 months; P less than .001), followed by retrograde endoscopy (mean of 13.10 months vs. 7.85 months; P less than .001), and antegrade endoscopy (mean of 7.63 months vs. 11.46 months; P = .024). “I think this is interesting,” said Dr. Pope, who is also a professor of pediatrics at the University of Toronto. “I think the difference occurs because if you use the endoscopy, which a rigid tube, you can potentially cause more damage, and more long-term scarring.”
Another predictor of esophageal stricture-free episodes was systemic corticosteroid use (a mean of 25.28 months vs. 10.24 months; P less than .001) around the time of the dilatation procedure. “By using systemic steroids, you’re actually decreasing some of the inflammation associated with the trauma of the procedure decreasing the chances of strictures formation,” she said.
Dr. Pope recommended that future studies evaluate the benefit of periprocedural medical interventions on increasing the intervals between esophageal stricture occurrences.
The study was supported by an unrestricted grant from the Epidermolysis Bullosa Research Foundation. She reported having no financial disclosures.
LAKE TAHOE, CALIF. – and direct visualization of these strictures is the preferred method of diagnosis. Those are key findings from a multicenter study that lead author Elena Pope, MD, discussed at the annual meeting of the Society for Pediatric Dermatology.
According to Dr. Pope, who heads the section of dermatology at the Hospital for Sick Children, Toronto, an estimated 10%-17% of epidermolysis bullosa (EB) patients experience strictures, with an overrepresentation in the recessive dystrophic EB subtype in up to 80% of cases. The risk increases with age. “What remains unknown is the best short- and long-term intervention to manage the strictures and predictors/associations for stricture-free episodes,” Dr. Pope said. “The objectives of the current study were to determine the prevalence and predisposing factors for strictures in EB, management options, patient outcomes, and predictors for recurrences and stricture-free intervals.”
She and her associates at seven centers worldwide collected data on 125 EB patients who experienced at least one episode of esophageal stricture. Data was analyzed descriptively and with ANOVA regression analysis for associations/predictors for recurrences/episode-free intervals.
The researchers evaluated 497 stricture events in the 125 patients. A slight female predominance was noted (53%), and the mean age of the first episode was 12.7 years, “which is a little bit older” than the age found in previously published data, Dr. Pope said. As expected, dystrophic EB patients made up most of the sample (98.4%); of these 123 patients, recessive dystrophic EB severe generalized subtype – approaching 50% – was the most common, followed by the recessive dystrophic EB severe intermediate subtype (almost 21%), the dominant dystrophic EB generalized subtype (7%), and other types of dystrophic EB (almost 26%).
The median body mass index percentile for age was 6.3, “so these were patients who were severely malnourished, probably as a result of their strictures as well as their underlying disease,” Dr. Pope said.
As expected, dysphagia was a presenting symptom in most patients (85.5%), while 29.8% presented with inability to swallow solids. The preferred method of evaluation was video fluoroscopy (57.7%), and less commonly with barium swallow (22.3%) or with clinical symptoms alone (0.1%). The mean number of strictures was 1.69; 76.7% were located in the cervical area, 56.7% were located in the thoracic area, and 9.7% were located in the abdominal area. Most patients (76%) had lesions that were 1 cm or longer in size.
Fluoroscopy guidance was the most common method of dilatation (in 45.2% of cases), followed by retrograde endoscopy was (33%), antegrade endoscopy (19.1%), and bougienage (0.1%). General anesthesia was used in most cases (87.6%), and corticosteroids were used around the dilatation in 90.4% of patients. The mean duration of medication use was about 5 days.
As for outcomes after dilatation, 92.2% of strictures completely resolved, 3.8% were partially resolved, 3.9% were not resolved, and 2.7% had complications. The median interval between dilatations was 7 months. Fluoroscopy-guided balloon dilatation was associated with the longest esophageal stricture-free duration (mean of 13.83 months vs. 8.75 months; P less than .001), followed by retrograde endoscopy (mean of 13.10 months vs. 7.85 months; P less than .001), and antegrade endoscopy (mean of 7.63 months vs. 11.46 months; P = .024). “I think this is interesting,” said Dr. Pope, who is also a professor of pediatrics at the University of Toronto. “I think the difference occurs because if you use the endoscopy, which a rigid tube, you can potentially cause more damage, and more long-term scarring.”
Another predictor of esophageal stricture-free episodes was systemic corticosteroid use (a mean of 25.28 months vs. 10.24 months; P less than .001) around the time of the dilatation procedure. “By using systemic steroids, you’re actually decreasing some of the inflammation associated with the trauma of the procedure decreasing the chances of strictures formation,” she said.
Dr. Pope recommended that future studies evaluate the benefit of periprocedural medical interventions on increasing the intervals between esophageal stricture occurrences.
The study was supported by an unrestricted grant from the Epidermolysis Bullosa Research Foundation. She reported having no financial disclosures.
REPORTING FROM SPD 2018
Key clinical point: Esophageal strictures are common complications of patients with severe types of epidermolysis bullosa.
Major finding: Most epidermolysis bullosa patients (85.5%) presented with dysphagia, while the preferred method of evaluation was video fluoroscopy (57.7%).
Study details: A multicenter study of 497 stricture events in 125 patients with epidermolysis bullosa.
Disclosures: The study was supported by an unrestricted grant from the Epidermolysis Bullosa Research Foundation. Dr. Pope reported having no financial disclosures.
More female authors than ever in cardiology journals
Also this week, valsartan recall and risk, synergy DES shines in acute MI, and incident heart failure is linked to HIV infection.
Also this week, valsartan recall and risk, synergy DES shines in acute MI, and incident heart failure is linked to HIV infection.
Also this week, valsartan recall and risk, synergy DES shines in acute MI, and incident heart failure is linked to HIV infection.
Interns Get IHS Work Experience—Virtually
Indian Health Service (IHS) is taking applications for students to “take part in enriching projects to further the IHS mission of raising the physical, mental, social, and spiritual health of American Indians and Alaska Natives to the highest level.” The twist? The students can do it remotely.
The IHS is a new partner with the Virtual Federal Service, the largest virtual internship program in the world, making it the 31st federal agency to participate. Other agencies include the Peace Corps and The National Aeronautics and Space Administration.
The “einterns” spend 10 hours a week from September through May working remotely. The work is unpaid, although they may get course credit. For some, it is the first time they have worked on issues affecting Native people. Those projects have included producing bilingual Navajo and English videos for rural health clinics, developing Navajo-specific health education materials on palliative care, creating a sexual assault locator map, and creating social media strategies and campaigns for health promotion.
IHS welcomed more than 15 interns, both undergraduates and graduate students, for the 2017-2018 academic year.
Indian Health Service (IHS) is taking applications for students to “take part in enriching projects to further the IHS mission of raising the physical, mental, social, and spiritual health of American Indians and Alaska Natives to the highest level.” The twist? The students can do it remotely.
The IHS is a new partner with the Virtual Federal Service, the largest virtual internship program in the world, making it the 31st federal agency to participate. Other agencies include the Peace Corps and The National Aeronautics and Space Administration.
The “einterns” spend 10 hours a week from September through May working remotely. The work is unpaid, although they may get course credit. For some, it is the first time they have worked on issues affecting Native people. Those projects have included producing bilingual Navajo and English videos for rural health clinics, developing Navajo-specific health education materials on palliative care, creating a sexual assault locator map, and creating social media strategies and campaigns for health promotion.
IHS welcomed more than 15 interns, both undergraduates and graduate students, for the 2017-2018 academic year.
Indian Health Service (IHS) is taking applications for students to “take part in enriching projects to further the IHS mission of raising the physical, mental, social, and spiritual health of American Indians and Alaska Natives to the highest level.” The twist? The students can do it remotely.
The IHS is a new partner with the Virtual Federal Service, the largest virtual internship program in the world, making it the 31st federal agency to participate. Other agencies include the Peace Corps and The National Aeronautics and Space Administration.
The “einterns” spend 10 hours a week from September through May working remotely. The work is unpaid, although they may get course credit. For some, it is the first time they have worked on issues affecting Native people. Those projects have included producing bilingual Navajo and English videos for rural health clinics, developing Navajo-specific health education materials on palliative care, creating a sexual assault locator map, and creating social media strategies and campaigns for health promotion.
IHS welcomed more than 15 interns, both undergraduates and graduate students, for the 2017-2018 academic year.
Work explains link between hypoxia and thrombosis
Researchers say they have discovered how hypoxia increases the risk of thrombosis.
The team noted that the cellular response to hypoxia is mediated by hypoxia-inducible factor 1 (HIF1).
The researchers were able to show that HIF1 downregulates expression of protein S (PS), a natural anticoagulant, which increases the risk of thrombosis.
“Our earlier work found that PS inhibits a key clotting protein, factor IXa,” said Rinku Majumder, PhD, of LSU Health Sciences Center in New Orleans, Louisiana.
“We knew that PS deficiency could occur in hypoxia but not why. With this study, our group identified the gene regulatory mechanism by which oxygen concentration controls PS production.”
Dr Majumder and her colleagues described this discovery in a letter published in Blood.
Because PS is primarily produced in the liver, the researchers cultured human hepatocarcinoma cells in normoxic and hypoxic conditions and then measured levels of PS.
The team found that increasing hypoxia reduced PS levels and increased stability of the HIF1α subunit of HIF1. The researchers said this inverse relationship between HIF1α and PS levels suggests HIF1 might regulate PS expression, and this theory was confirmed via experiments with mice.
Dr Majumder and her colleagues pointed out that an oxygen-dependent signaling system degrades HIF1α, and oxygen deficiency prevents HIF1α degradation. The HIF1α P564A mutant (HIF1α dPA) is resistant to degradation, which results in elevated HIF1 even in normoxic conditions.
The researchers conducted experiments with knockout mice expressing HIF1α dPA in the liver, HIF1α liver-specific knockout mice, and control mice.
When compared to PS levels in liver samples from control mice (100%), PS levels were elevated in liver samples from the HIF1α liver-specific knockout mice (220%) and reduced in samples from the HIF1α dPA mice (50%).
PS messenger RNA was 2-fold higher in HIF1α knockout mice than in controls. In HIF1α dPA mice, PS messenger RNA was 0.3-fold that of controls.
PS levels in plasma from HIF1α knockout mice were double the levels of controls, while PS levels in plasma from HIF1α dPA mice were half that of controls.
Plasma from HIF1α knockout mice produced 5-fold less thrombin and plasma from HIF1α dPA mice produced 1.5-fold more thrombin than control plasma.
Subsequent experiments confirmed that the variations in thrombin generation were due to changes in plasma PS levels, the researchers said.
The team concluded that stabilization of HIF1 in the liver, which is a normal response to hypoxia, is associated with reduced PS expression. This results in lower plasma PS levels and an increased risk of thrombosis.
Researchers say they have discovered how hypoxia increases the risk of thrombosis.
The team noted that the cellular response to hypoxia is mediated by hypoxia-inducible factor 1 (HIF1).
The researchers were able to show that HIF1 downregulates expression of protein S (PS), a natural anticoagulant, which increases the risk of thrombosis.
“Our earlier work found that PS inhibits a key clotting protein, factor IXa,” said Rinku Majumder, PhD, of LSU Health Sciences Center in New Orleans, Louisiana.
“We knew that PS deficiency could occur in hypoxia but not why. With this study, our group identified the gene regulatory mechanism by which oxygen concentration controls PS production.”
Dr Majumder and her colleagues described this discovery in a letter published in Blood.
Because PS is primarily produced in the liver, the researchers cultured human hepatocarcinoma cells in normoxic and hypoxic conditions and then measured levels of PS.
The team found that increasing hypoxia reduced PS levels and increased stability of the HIF1α subunit of HIF1. The researchers said this inverse relationship between HIF1α and PS levels suggests HIF1 might regulate PS expression, and this theory was confirmed via experiments with mice.
Dr Majumder and her colleagues pointed out that an oxygen-dependent signaling system degrades HIF1α, and oxygen deficiency prevents HIF1α degradation. The HIF1α P564A mutant (HIF1α dPA) is resistant to degradation, which results in elevated HIF1 even in normoxic conditions.
The researchers conducted experiments with knockout mice expressing HIF1α dPA in the liver, HIF1α liver-specific knockout mice, and control mice.
When compared to PS levels in liver samples from control mice (100%), PS levels were elevated in liver samples from the HIF1α liver-specific knockout mice (220%) and reduced in samples from the HIF1α dPA mice (50%).
PS messenger RNA was 2-fold higher in HIF1α knockout mice than in controls. In HIF1α dPA mice, PS messenger RNA was 0.3-fold that of controls.
PS levels in plasma from HIF1α knockout mice were double the levels of controls, while PS levels in plasma from HIF1α dPA mice were half that of controls.
Plasma from HIF1α knockout mice produced 5-fold less thrombin and plasma from HIF1α dPA mice produced 1.5-fold more thrombin than control plasma.
Subsequent experiments confirmed that the variations in thrombin generation were due to changes in plasma PS levels, the researchers said.
The team concluded that stabilization of HIF1 in the liver, which is a normal response to hypoxia, is associated with reduced PS expression. This results in lower plasma PS levels and an increased risk of thrombosis.
Researchers say they have discovered how hypoxia increases the risk of thrombosis.
The team noted that the cellular response to hypoxia is mediated by hypoxia-inducible factor 1 (HIF1).
The researchers were able to show that HIF1 downregulates expression of protein S (PS), a natural anticoagulant, which increases the risk of thrombosis.
“Our earlier work found that PS inhibits a key clotting protein, factor IXa,” said Rinku Majumder, PhD, of LSU Health Sciences Center in New Orleans, Louisiana.
“We knew that PS deficiency could occur in hypoxia but not why. With this study, our group identified the gene regulatory mechanism by which oxygen concentration controls PS production.”
Dr Majumder and her colleagues described this discovery in a letter published in Blood.
Because PS is primarily produced in the liver, the researchers cultured human hepatocarcinoma cells in normoxic and hypoxic conditions and then measured levels of PS.
The team found that increasing hypoxia reduced PS levels and increased stability of the HIF1α subunit of HIF1. The researchers said this inverse relationship between HIF1α and PS levels suggests HIF1 might regulate PS expression, and this theory was confirmed via experiments with mice.
Dr Majumder and her colleagues pointed out that an oxygen-dependent signaling system degrades HIF1α, and oxygen deficiency prevents HIF1α degradation. The HIF1α P564A mutant (HIF1α dPA) is resistant to degradation, which results in elevated HIF1 even in normoxic conditions.
The researchers conducted experiments with knockout mice expressing HIF1α dPA in the liver, HIF1α liver-specific knockout mice, and control mice.
When compared to PS levels in liver samples from control mice (100%), PS levels were elevated in liver samples from the HIF1α liver-specific knockout mice (220%) and reduced in samples from the HIF1α dPA mice (50%).
PS messenger RNA was 2-fold higher in HIF1α knockout mice than in controls. In HIF1α dPA mice, PS messenger RNA was 0.3-fold that of controls.
PS levels in plasma from HIF1α knockout mice were double the levels of controls, while PS levels in plasma from HIF1α dPA mice were half that of controls.
Plasma from HIF1α knockout mice produced 5-fold less thrombin and plasma from HIF1α dPA mice produced 1.5-fold more thrombin than control plasma.
Subsequent experiments confirmed that the variations in thrombin generation were due to changes in plasma PS levels, the researchers said.
The team concluded that stabilization of HIF1 in the liver, which is a normal response to hypoxia, is associated with reduced PS expression. This results in lower plasma PS levels and an increased risk of thrombosis.
Method may enable eradication of LSCs in AML
Disrupting mitophagy may be a “promising strategy” for eliminating leukemia stem cells (LSCs) in acute myeloid leukemia (AML), according to researchers.
The team found that AML LSCs depend on mitophagy to maintain their “stemness,” but targeting the central metabolic stress regulator AMPK or the mitochondrial dynamics regulator FIS1 can disrupt mitophagy and impair LSC function.
Craig T. Jordan, PhD, of the University of Colorado in Aurora, and his colleagues reported these findings in Cell Stem Cell.
The researchers said in vitro experiments showed that LSCs have elevated levels of FIS1 and “distinct mitochondrial morphology.”
When the team inhibited FIS1 in the AML cell line MOLM-13 and primary AML cells, they observed disruption of mitochondrial dynamics. Experiments in mouse models indicated that FIS1 is required for LSC self-renewal.
Specifically, the researchers said they found that depletion of FIS1 hinders mitophagy and leads to inactivation of GSK3, myeloid differentiation, cell-cycle arrest, and loss of LSC function.
Dr Jordan and his colleagues also found that AMPK is an upstream regulator of FIS1, and targeting AMPK produces similar effects as targeting FIS1—namely, disrupting mitophagy and impairing LSC self-renewal.
The researchers said their findings suggest that mitochondrial stress generated from oncogenic transformation may activate AMPK signaling in LSCs. And the AMPK signaling drives FIS1-mediated mitophagy, which eliminates stressed mitochondria and allows LSCs to thrive.
However, when AMPK or FIS1 is inhibited, the damaged mitochondria are not eliminated. This leads to “GSK3 inhibition and other unknown events” that prompt differentiation and hinder LSC function.
“Leukemia stem cells require AMPK for their survival, but normal hematopoietic cells can do without it,” Dr Jordan noted. “The reason this study is so important is that, so far, nobody’s come up with a good way to kill leukemia stem cells while sparing normal blood-forming cells. If we can translate this concept to patients, the potential for improved therapy is very exciting.”
Disrupting mitophagy may be a “promising strategy” for eliminating leukemia stem cells (LSCs) in acute myeloid leukemia (AML), according to researchers.
The team found that AML LSCs depend on mitophagy to maintain their “stemness,” but targeting the central metabolic stress regulator AMPK or the mitochondrial dynamics regulator FIS1 can disrupt mitophagy and impair LSC function.
Craig T. Jordan, PhD, of the University of Colorado in Aurora, and his colleagues reported these findings in Cell Stem Cell.
The researchers said in vitro experiments showed that LSCs have elevated levels of FIS1 and “distinct mitochondrial morphology.”
When the team inhibited FIS1 in the AML cell line MOLM-13 and primary AML cells, they observed disruption of mitochondrial dynamics. Experiments in mouse models indicated that FIS1 is required for LSC self-renewal.
Specifically, the researchers said they found that depletion of FIS1 hinders mitophagy and leads to inactivation of GSK3, myeloid differentiation, cell-cycle arrest, and loss of LSC function.
Dr Jordan and his colleagues also found that AMPK is an upstream regulator of FIS1, and targeting AMPK produces similar effects as targeting FIS1—namely, disrupting mitophagy and impairing LSC self-renewal.
The researchers said their findings suggest that mitochondrial stress generated from oncogenic transformation may activate AMPK signaling in LSCs. And the AMPK signaling drives FIS1-mediated mitophagy, which eliminates stressed mitochondria and allows LSCs to thrive.
However, when AMPK or FIS1 is inhibited, the damaged mitochondria are not eliminated. This leads to “GSK3 inhibition and other unknown events” that prompt differentiation and hinder LSC function.
“Leukemia stem cells require AMPK for their survival, but normal hematopoietic cells can do without it,” Dr Jordan noted. “The reason this study is so important is that, so far, nobody’s come up with a good way to kill leukemia stem cells while sparing normal blood-forming cells. If we can translate this concept to patients, the potential for improved therapy is very exciting.”
Disrupting mitophagy may be a “promising strategy” for eliminating leukemia stem cells (LSCs) in acute myeloid leukemia (AML), according to researchers.
The team found that AML LSCs depend on mitophagy to maintain their “stemness,” but targeting the central metabolic stress regulator AMPK or the mitochondrial dynamics regulator FIS1 can disrupt mitophagy and impair LSC function.
Craig T. Jordan, PhD, of the University of Colorado in Aurora, and his colleagues reported these findings in Cell Stem Cell.
The researchers said in vitro experiments showed that LSCs have elevated levels of FIS1 and “distinct mitochondrial morphology.”
When the team inhibited FIS1 in the AML cell line MOLM-13 and primary AML cells, they observed disruption of mitochondrial dynamics. Experiments in mouse models indicated that FIS1 is required for LSC self-renewal.
Specifically, the researchers said they found that depletion of FIS1 hinders mitophagy and leads to inactivation of GSK3, myeloid differentiation, cell-cycle arrest, and loss of LSC function.
Dr Jordan and his colleagues also found that AMPK is an upstream regulator of FIS1, and targeting AMPK produces similar effects as targeting FIS1—namely, disrupting mitophagy and impairing LSC self-renewal.
The researchers said their findings suggest that mitochondrial stress generated from oncogenic transformation may activate AMPK signaling in LSCs. And the AMPK signaling drives FIS1-mediated mitophagy, which eliminates stressed mitochondria and allows LSCs to thrive.
However, when AMPK or FIS1 is inhibited, the damaged mitochondria are not eliminated. This leads to “GSK3 inhibition and other unknown events” that prompt differentiation and hinder LSC function.
“Leukemia stem cells require AMPK for their survival, but normal hematopoietic cells can do without it,” Dr Jordan noted. “The reason this study is so important is that, so far, nobody’s come up with a good way to kill leukemia stem cells while sparing normal blood-forming cells. If we can translate this concept to patients, the potential for improved therapy is very exciting.”
Protein ‘atlas’ could aid study, treatment of diseases
New technology has enabled researchers to create a “genomic atlas of the human plasma proteome,” according to an article published in Nature.
The researchers identified nearly 2000 genetic associations with close to 1500 proteins, and they believe these discoveries will improve our understanding of diseases and aid drug development.
“Compared to genes, proteins have been relatively understudied in human blood, even though they are the ‘effectors’ of human biology, are disrupted in many diseases, and are the targets of most medicines,” said study author Adam Butterworth, PhD, of the University of Cambridge in the UK.
“Novel technologies are now allowing us to start addressing this gap in our knowledge.”
Dr Butterworth and his colleagues used an assay called SOMAscan (developed by the company SomaLogic) to measure 3622 proteins in the blood of 3301 people. The team then analyzed the DNA of these individuals to see which regions of their genomes were associated with protein levels.
In this way, the researchers found 1927 significant associations between 1478 proteins and 764 genomic regions. These findings are publicly available via the University of Cambridge website.
The researchers said one way to use this information is to identify biological pathways that cause diseases.
“Thanks to the genomics revolution over the past decade, we’ve been good at finding statistical associations between the genome and disease, but the difficulty has been then identifying the disease-causing genes and pathways,” said study author James Peters, PhD, of the University of Cambridge.
“Now, by combining our database with what we know about associations between genetic variants and disease, we are able to say a lot more about the biology of disease.”
In some cases, the researchers identified multiple genetic variants influencing levels of a protein. By combining these variants into a “score” for that protein, they were able to identify new associations between proteins and disease.
The team also said the proteomic genetic data can be used to aid drug development. In addition to highlighting potential side effects of drugs, the findings can provide insights on protein targets of new and existing drugs.
By linking drugs, proteins, genetic variation, and diseases, the researchers have already suggested existing drugs that could potentially be used to treat different diseases and increased confidence that certain drugs currently in development might be successful in clinical trials.
“Our database is really just a starting point,” said study author Benjamin Sun, an MB/PhD student at the University of Cambridge.
“We’ve given some examples in this study of how it might be used, but now it’s over to the research community to begin using it and finding new applications.”
The research was funded by MSD, National Institute for Health Research, NHS Blood and Transplant, British Heart Foundation, Medical Research Council, UK Research and Innovation, and SomaLogic.
New technology has enabled researchers to create a “genomic atlas of the human plasma proteome,” according to an article published in Nature.
The researchers identified nearly 2000 genetic associations with close to 1500 proteins, and they believe these discoveries will improve our understanding of diseases and aid drug development.
“Compared to genes, proteins have been relatively understudied in human blood, even though they are the ‘effectors’ of human biology, are disrupted in many diseases, and are the targets of most medicines,” said study author Adam Butterworth, PhD, of the University of Cambridge in the UK.
“Novel technologies are now allowing us to start addressing this gap in our knowledge.”
Dr Butterworth and his colleagues used an assay called SOMAscan (developed by the company SomaLogic) to measure 3622 proteins in the blood of 3301 people. The team then analyzed the DNA of these individuals to see which regions of their genomes were associated with protein levels.
In this way, the researchers found 1927 significant associations between 1478 proteins and 764 genomic regions. These findings are publicly available via the University of Cambridge website.
The researchers said one way to use this information is to identify biological pathways that cause diseases.
“Thanks to the genomics revolution over the past decade, we’ve been good at finding statistical associations between the genome and disease, but the difficulty has been then identifying the disease-causing genes and pathways,” said study author James Peters, PhD, of the University of Cambridge.
“Now, by combining our database with what we know about associations between genetic variants and disease, we are able to say a lot more about the biology of disease.”
In some cases, the researchers identified multiple genetic variants influencing levels of a protein. By combining these variants into a “score” for that protein, they were able to identify new associations between proteins and disease.
The team also said the proteomic genetic data can be used to aid drug development. In addition to highlighting potential side effects of drugs, the findings can provide insights on protein targets of new and existing drugs.
By linking drugs, proteins, genetic variation, and diseases, the researchers have already suggested existing drugs that could potentially be used to treat different diseases and increased confidence that certain drugs currently in development might be successful in clinical trials.
“Our database is really just a starting point,” said study author Benjamin Sun, an MB/PhD student at the University of Cambridge.
“We’ve given some examples in this study of how it might be used, but now it’s over to the research community to begin using it and finding new applications.”
The research was funded by MSD, National Institute for Health Research, NHS Blood and Transplant, British Heart Foundation, Medical Research Council, UK Research and Innovation, and SomaLogic.
New technology has enabled researchers to create a “genomic atlas of the human plasma proteome,” according to an article published in Nature.
The researchers identified nearly 2000 genetic associations with close to 1500 proteins, and they believe these discoveries will improve our understanding of diseases and aid drug development.
“Compared to genes, proteins have been relatively understudied in human blood, even though they are the ‘effectors’ of human biology, are disrupted in many diseases, and are the targets of most medicines,” said study author Adam Butterworth, PhD, of the University of Cambridge in the UK.
“Novel technologies are now allowing us to start addressing this gap in our knowledge.”
Dr Butterworth and his colleagues used an assay called SOMAscan (developed by the company SomaLogic) to measure 3622 proteins in the blood of 3301 people. The team then analyzed the DNA of these individuals to see which regions of their genomes were associated with protein levels.
In this way, the researchers found 1927 significant associations between 1478 proteins and 764 genomic regions. These findings are publicly available via the University of Cambridge website.
The researchers said one way to use this information is to identify biological pathways that cause diseases.
“Thanks to the genomics revolution over the past decade, we’ve been good at finding statistical associations between the genome and disease, but the difficulty has been then identifying the disease-causing genes and pathways,” said study author James Peters, PhD, of the University of Cambridge.
“Now, by combining our database with what we know about associations between genetic variants and disease, we are able to say a lot more about the biology of disease.”
In some cases, the researchers identified multiple genetic variants influencing levels of a protein. By combining these variants into a “score” for that protein, they were able to identify new associations between proteins and disease.
The team also said the proteomic genetic data can be used to aid drug development. In addition to highlighting potential side effects of drugs, the findings can provide insights on protein targets of new and existing drugs.
By linking drugs, proteins, genetic variation, and diseases, the researchers have already suggested existing drugs that could potentially be used to treat different diseases and increased confidence that certain drugs currently in development might be successful in clinical trials.
“Our database is really just a starting point,” said study author Benjamin Sun, an MB/PhD student at the University of Cambridge.
“We’ve given some examples in this study of how it might be used, but now it’s over to the research community to begin using it and finding new applications.”
The research was funded by MSD, National Institute for Health Research, NHS Blood and Transplant, British Heart Foundation, Medical Research Council, UK Research and Innovation, and SomaLogic.
Treating substance use disorders: What do you do after withdrawal?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PE is rare in patients presenting to the ED with syncope
Clinical question: What is the prevalence of pulmonary embolism (PE) in patients presenting to the ED with syncope?
Study design: Retrospective, observational study.
Setting: Canada, Denmark, Italy, and the United States, from January 2010 to September 2016.
Synopsis: Longitudinal administrative databases were used to identify patients with ICD codes for syncope at discharge from the ED or hospital. Those with an ICD code for PE were included to calculate the prevalence of PE in this population (primary outcome).
The prevalence of PE in all patients ranged from 0.06% (95% confidence interval, 0.05%-0.06%) to 0.55% (95% CI, 0.50%-0.61%); and in hospitalized patients from 0.15% (95% CI, 0.14%-0.16%) to 2.10% (95% CI, 1.84%-2.39%). This is a much lower than the estimated 17.3% prevalence of PE in patients presenting with syncope estimated by the PESIT study published in the New England Journal of Medicine in 2016. Further definitive research is needed to better characterize prevalence rates.
Limitations of this study include the potential for information bias: The inclusion criteria of patients coded for syncope at discharge likely omits some patients who initially presented with syncope but were coded for a primary diagnosis that caused syncope.
Bottom line: PE in patients presenting to the ED with syncope may be rare.
Citation: Costantino G et al. Prevalence of pulmonary embolism in patients with syncope. JAMA. 2018;178(3):356-62.
Dr. Roy is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: What is the prevalence of pulmonary embolism (PE) in patients presenting to the ED with syncope?
Study design: Retrospective, observational study.
Setting: Canada, Denmark, Italy, and the United States, from January 2010 to September 2016.
Synopsis: Longitudinal administrative databases were used to identify patients with ICD codes for syncope at discharge from the ED or hospital. Those with an ICD code for PE were included to calculate the prevalence of PE in this population (primary outcome).
The prevalence of PE in all patients ranged from 0.06% (95% confidence interval, 0.05%-0.06%) to 0.55% (95% CI, 0.50%-0.61%); and in hospitalized patients from 0.15% (95% CI, 0.14%-0.16%) to 2.10% (95% CI, 1.84%-2.39%). This is a much lower than the estimated 17.3% prevalence of PE in patients presenting with syncope estimated by the PESIT study published in the New England Journal of Medicine in 2016. Further definitive research is needed to better characterize prevalence rates.
Limitations of this study include the potential for information bias: The inclusion criteria of patients coded for syncope at discharge likely omits some patients who initially presented with syncope but were coded for a primary diagnosis that caused syncope.
Bottom line: PE in patients presenting to the ED with syncope may be rare.
Citation: Costantino G et al. Prevalence of pulmonary embolism in patients with syncope. JAMA. 2018;178(3):356-62.
Dr. Roy is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: What is the prevalence of pulmonary embolism (PE) in patients presenting to the ED with syncope?
Study design: Retrospective, observational study.
Setting: Canada, Denmark, Italy, and the United States, from January 2010 to September 2016.
Synopsis: Longitudinal administrative databases were used to identify patients with ICD codes for syncope at discharge from the ED or hospital. Those with an ICD code for PE were included to calculate the prevalence of PE in this population (primary outcome).
The prevalence of PE in all patients ranged from 0.06% (95% confidence interval, 0.05%-0.06%) to 0.55% (95% CI, 0.50%-0.61%); and in hospitalized patients from 0.15% (95% CI, 0.14%-0.16%) to 2.10% (95% CI, 1.84%-2.39%). This is a much lower than the estimated 17.3% prevalence of PE in patients presenting with syncope estimated by the PESIT study published in the New England Journal of Medicine in 2016. Further definitive research is needed to better characterize prevalence rates.
Limitations of this study include the potential for information bias: The inclusion criteria of patients coded for syncope at discharge likely omits some patients who initially presented with syncope but were coded for a primary diagnosis that caused syncope.
Bottom line: PE in patients presenting to the ED with syncope may be rare.
Citation: Costantino G et al. Prevalence of pulmonary embolism in patients with syncope. JAMA. 2018;178(3):356-62.
Dr. Roy is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Weight gain linked to progression of fibrosis in NAFLD patients
according to recent research published in Clinical Gastroenterology and Hepatology.
Researchers evaluated 40,700 Korean adults (minimum age, 18 years) with NAFLD who underwent health screenings during 2002-2016 with a median 6-year follow-up. Patients were categorized and placed into weight quintiles based on whether they lost weight (quintile 1, 2.3-kg or greater weight loss; quintile 2, 2.2-kg to 0.6-kg weight loss), gained weight (quintile 4, 0.7- to 2.1-kg weight gain; quintile 5, at least 2.2-kg or greater weight gain) or whether their weight remained stable (quintile 3, 0.5-kg weight loss to 0.6-kg weight gain). Researchers followed patients from baseline to fibrosis progression or last visit, calculated as person-years, and used the aspartate aminotransferase to platelet ratio index (APRI) to measure outcomes. They defined body mass index based on criteria specific to Asian populations, with underweight categorized as less than 18.5 kg/m2, normal weight as 18.5-23 kg/m2, overweight as 23-25 kg/m2, and obese as at least 25 kg/m2.
“Our findings from mostly asymptomatic, relatively young individuals with ultrasonographically detected steatosis, possibly reflecting low-risk NAFLD patients, are less likely to be affected by survivor bias and biases related to comorbidities, compared with previous findings from cohorts of high-risk groups that underwent liver biopsy,” Seungho Ryu, MD, PhD, from Kangbuk Samsung Hospital in Seoul, South Korea, and colleagues wrote in the study.
There were 5,454 participants who progressed from a low APRI to an intermediate or high APRI within 275,451.5 person-years, researchers said. Compared with the stable-weight group, hazard ratios for APRI progression in the first weight-change quintile were 0.68 (95% confidence interval, 0.62-0.74) and 0.86 in the second weight-change quintile (95% CI, 0.78-0.94). In the weight-gain groups, an increase in weight was associated with APRI progression in the fourth quintile (HR, 1.17; 95% CI, 1.07-1.28) and fifth quintile (HR, 1.71; 95% CI, 1.58-1.85) groups.
After multivariable adjustment, there was an increase in APRI progression among patients with BMIs between 23 and 24.9 kg/m2 (HR, 1.13; 95% CI, 1.02-1.26), between 25 and 29.9 kg/m2 (HR, 1.41; 95% CI, 1.28-1.55), and greater than or equal to 30 kg/m2 (HR, 2.09; 95% CI, 1.86-2.36) compared with patients with a BMI between 18.5 and 22.9 kg/m2,.
Limitations of the study included the use of ultrasonography in place of liver biopsy for diagnosing NAFLD and the use of APRI to predict fibrosis in individuals with NAFLD, researchers said.
“APRI has demonstrated a reasonable utility as a noninvasive method for the prediction of histologically confirmed advanced fibrosis,” Dr. Ryu and colleagues wrote. “Nonetheless, we acknowledge that there is no currently available longitudinal data to support the use of worsening noninvasive fibrosis markers as an indicator of histological progression of fibrosis stage over time.”
Other limitations included the study’s retrospective design, lack of availability of medication use and dietary intake, and lack of generalization based on a young, healthy population of mostly Korean employees who were employed by companies or local government. However, researchers said clinicians should encourage their patients with NAFLD to maintain a healthy weight to avoid progression of fibrosis.
The authors reported no relevant financial disclosures.
SOURCE: Kim Y et al. Clin Gastroenterol Hepatol. 2018. doi: 10.1016/j.cgh.2018.07.006.
according to recent research published in Clinical Gastroenterology and Hepatology.
Researchers evaluated 40,700 Korean adults (minimum age, 18 years) with NAFLD who underwent health screenings during 2002-2016 with a median 6-year follow-up. Patients were categorized and placed into weight quintiles based on whether they lost weight (quintile 1, 2.3-kg or greater weight loss; quintile 2, 2.2-kg to 0.6-kg weight loss), gained weight (quintile 4, 0.7- to 2.1-kg weight gain; quintile 5, at least 2.2-kg or greater weight gain) or whether their weight remained stable (quintile 3, 0.5-kg weight loss to 0.6-kg weight gain). Researchers followed patients from baseline to fibrosis progression or last visit, calculated as person-years, and used the aspartate aminotransferase to platelet ratio index (APRI) to measure outcomes. They defined body mass index based on criteria specific to Asian populations, with underweight categorized as less than 18.5 kg/m2, normal weight as 18.5-23 kg/m2, overweight as 23-25 kg/m2, and obese as at least 25 kg/m2.
“Our findings from mostly asymptomatic, relatively young individuals with ultrasonographically detected steatosis, possibly reflecting low-risk NAFLD patients, are less likely to be affected by survivor bias and biases related to comorbidities, compared with previous findings from cohorts of high-risk groups that underwent liver biopsy,” Seungho Ryu, MD, PhD, from Kangbuk Samsung Hospital in Seoul, South Korea, and colleagues wrote in the study.
There were 5,454 participants who progressed from a low APRI to an intermediate or high APRI within 275,451.5 person-years, researchers said. Compared with the stable-weight group, hazard ratios for APRI progression in the first weight-change quintile were 0.68 (95% confidence interval, 0.62-0.74) and 0.86 in the second weight-change quintile (95% CI, 0.78-0.94). In the weight-gain groups, an increase in weight was associated with APRI progression in the fourth quintile (HR, 1.17; 95% CI, 1.07-1.28) and fifth quintile (HR, 1.71; 95% CI, 1.58-1.85) groups.
After multivariable adjustment, there was an increase in APRI progression among patients with BMIs between 23 and 24.9 kg/m2 (HR, 1.13; 95% CI, 1.02-1.26), between 25 and 29.9 kg/m2 (HR, 1.41; 95% CI, 1.28-1.55), and greater than or equal to 30 kg/m2 (HR, 2.09; 95% CI, 1.86-2.36) compared with patients with a BMI between 18.5 and 22.9 kg/m2,.
Limitations of the study included the use of ultrasonography in place of liver biopsy for diagnosing NAFLD and the use of APRI to predict fibrosis in individuals with NAFLD, researchers said.
“APRI has demonstrated a reasonable utility as a noninvasive method for the prediction of histologically confirmed advanced fibrosis,” Dr. Ryu and colleagues wrote. “Nonetheless, we acknowledge that there is no currently available longitudinal data to support the use of worsening noninvasive fibrosis markers as an indicator of histological progression of fibrosis stage over time.”
Other limitations included the study’s retrospective design, lack of availability of medication use and dietary intake, and lack of generalization based on a young, healthy population of mostly Korean employees who were employed by companies or local government. However, researchers said clinicians should encourage their patients with NAFLD to maintain a healthy weight to avoid progression of fibrosis.
The authors reported no relevant financial disclosures.
SOURCE: Kim Y et al. Clin Gastroenterol Hepatol. 2018. doi: 10.1016/j.cgh.2018.07.006.
according to recent research published in Clinical Gastroenterology and Hepatology.
Researchers evaluated 40,700 Korean adults (minimum age, 18 years) with NAFLD who underwent health screenings during 2002-2016 with a median 6-year follow-up. Patients were categorized and placed into weight quintiles based on whether they lost weight (quintile 1, 2.3-kg or greater weight loss; quintile 2, 2.2-kg to 0.6-kg weight loss), gained weight (quintile 4, 0.7- to 2.1-kg weight gain; quintile 5, at least 2.2-kg or greater weight gain) or whether their weight remained stable (quintile 3, 0.5-kg weight loss to 0.6-kg weight gain). Researchers followed patients from baseline to fibrosis progression or last visit, calculated as person-years, and used the aspartate aminotransferase to platelet ratio index (APRI) to measure outcomes. They defined body mass index based on criteria specific to Asian populations, with underweight categorized as less than 18.5 kg/m2, normal weight as 18.5-23 kg/m2, overweight as 23-25 kg/m2, and obese as at least 25 kg/m2.
“Our findings from mostly asymptomatic, relatively young individuals with ultrasonographically detected steatosis, possibly reflecting low-risk NAFLD patients, are less likely to be affected by survivor bias and biases related to comorbidities, compared with previous findings from cohorts of high-risk groups that underwent liver biopsy,” Seungho Ryu, MD, PhD, from Kangbuk Samsung Hospital in Seoul, South Korea, and colleagues wrote in the study.
There were 5,454 participants who progressed from a low APRI to an intermediate or high APRI within 275,451.5 person-years, researchers said. Compared with the stable-weight group, hazard ratios for APRI progression in the first weight-change quintile were 0.68 (95% confidence interval, 0.62-0.74) and 0.86 in the second weight-change quintile (95% CI, 0.78-0.94). In the weight-gain groups, an increase in weight was associated with APRI progression in the fourth quintile (HR, 1.17; 95% CI, 1.07-1.28) and fifth quintile (HR, 1.71; 95% CI, 1.58-1.85) groups.
After multivariable adjustment, there was an increase in APRI progression among patients with BMIs between 23 and 24.9 kg/m2 (HR, 1.13; 95% CI, 1.02-1.26), between 25 and 29.9 kg/m2 (HR, 1.41; 95% CI, 1.28-1.55), and greater than or equal to 30 kg/m2 (HR, 2.09; 95% CI, 1.86-2.36) compared with patients with a BMI between 18.5 and 22.9 kg/m2,.
Limitations of the study included the use of ultrasonography in place of liver biopsy for diagnosing NAFLD and the use of APRI to predict fibrosis in individuals with NAFLD, researchers said.
“APRI has demonstrated a reasonable utility as a noninvasive method for the prediction of histologically confirmed advanced fibrosis,” Dr. Ryu and colleagues wrote. “Nonetheless, we acknowledge that there is no currently available longitudinal data to support the use of worsening noninvasive fibrosis markers as an indicator of histological progression of fibrosis stage over time.”
Other limitations included the study’s retrospective design, lack of availability of medication use and dietary intake, and lack of generalization based on a young, healthy population of mostly Korean employees who were employed by companies or local government. However, researchers said clinicians should encourage their patients with NAFLD to maintain a healthy weight to avoid progression of fibrosis.
The authors reported no relevant financial disclosures.
SOURCE: Kim Y et al. Clin Gastroenterol Hepatol. 2018. doi: 10.1016/j.cgh.2018.07.006.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Obesity and weight gain were linked to progression of fibrosis in adults with NAFLD.
Major finding: Degree of weight change was associated with risk of fibrosis progression; patients who gained weight in quintile 4 and quintile 5 had hazard ratios of 1.17 and 1.71, respectively, when compared with the quintile of patients whose weight remained stable.
Data source: A retrospective study of 40,700 Korean adults with NAFLD who underwent health screenings during 2002-2016 with a median 6-year follow-up.
Disclosures: The authors reported no relevant financial disclosures.
Source: Kim Y et al. Clin Gastroenterol Hepatol. 2018. doi: 10.1016/j.cgh.2018.07.006.
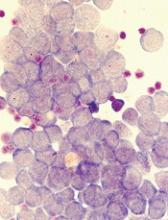
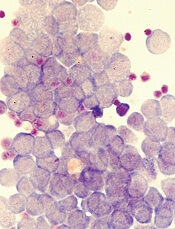
Vadastuximab talirine gives big boost to AML remission
For elderly patients with CD33-positive acute myeloid leukemia (AML), vadastuximab talirine in combination with a hypomethylating agent (HMA) improves remission rates, compared with HMA therapy alone, according to a phase 1 trial.
More than half of the patients treated with combination therapy achieved deep remission, defined as a negative-flow cytometry test for minimal residual disease. Despite these promising results, hematologic toxicity concerns may limit future trials.
“Outcomes for patients with acute myeloid leukemia (AML) remain poor, particularly in older patients,” wrote Amir T. Fathi, MD, of the division of hematology and oncology at Massachusetts General Hospital Cancer Center, Boston, and his coauthors.
Many elderly patients currently receive hypomethylating agents HMAs as a form of low-intensity therapy, but associated remission rates are low. “The development of novel, well-tolerated therapies to enhance the efficacy of HMAs could meaningfully improve the standard of care for older patients with AML,” the investigators wrote in Blood. Vadastuximab talirine is a novel antibody therapy that targets CD33; preclinical data suggested that it could be an effective combination with HMA therapy.
The phase 1 trial involved 53 patients with newly diagnosed, CD33-positive AML and a median age of 75 years. Patients were naive to HMA therapy but could have previously received other low-intensity treatments. HMA therapy was administered first; either azacitidine (75 mg/m2 subcutaneous IV for 7 days) or decitabine (20 mg/m2 IV for 5 days), according to institutional standards. On the last day of HMA therapy, vadastuximab talirine (10 mcg/kg IV) was given. This protocol was repeated in 28-day cycles for up to four cycles. Patients who tolerated the combination and showed a clinical response were eligible to continue therapy.
The composite remission rate (CRc: complete remission and complete remission with incomplete blood count recovery) with combination therapy was 70%. Historically, HMA monotherapies have much lower composite remission rates (decitabine, 17.8%; azacytidine, 27.8%). Of all patients achieving remission, 51% tested negative by flow cytometry for minimal residual disease. Median overall survival was 11.3 months and median relapse-free survival was 7.7 months.
“Nevertheless, the increased response rate with the addition of vadastuximab talirine to HMAs was also associated with increased toxicity when compared to single-agent HMA therapy – indicative of the greater degree of myelosuppression,” the researchers wrote. The most common grade 3 or higher adverse events were thrombocytopenia (57%), febrile neutropenia (49%), anemia (45%), neutropenia (42%), and fatigue (15%).
The investigators stated that “the overall safety profile was similar for patients treated with vadastuximab talirine in combination with azacitidine versus decitabine (with the exception of incidence of febrile neutropenia).”
Following the encouraging results of this phase 1 trial, the CASCADE phase 3 trial was launched to again compare this combination with HMA monotherapy; however, the trial was halted early because of deaths in the combination arm. The investigators cited the need for stricter protocols to ensure safety during future trials.
“With such guidance and precaution, promising combinations for AML, a disease affecting predominantly older and more frail patients, may be more effectively studied so as to enhance our current suboptimal therapeutic options,” they wrote.
Seattle Genetics provided study funding and author compensation.
SOURCE: Fathi AT et al. Blood. 2018 Jul 25. doi: 10.1182/blood-2018-03-841171.
For elderly patients with CD33-positive acute myeloid leukemia (AML), vadastuximab talirine in combination with a hypomethylating agent (HMA) improves remission rates, compared with HMA therapy alone, according to a phase 1 trial.
More than half of the patients treated with combination therapy achieved deep remission, defined as a negative-flow cytometry test for minimal residual disease. Despite these promising results, hematologic toxicity concerns may limit future trials.
“Outcomes for patients with acute myeloid leukemia (AML) remain poor, particularly in older patients,” wrote Amir T. Fathi, MD, of the division of hematology and oncology at Massachusetts General Hospital Cancer Center, Boston, and his coauthors.
Many elderly patients currently receive hypomethylating agents HMAs as a form of low-intensity therapy, but associated remission rates are low. “The development of novel, well-tolerated therapies to enhance the efficacy of HMAs could meaningfully improve the standard of care for older patients with AML,” the investigators wrote in Blood. Vadastuximab talirine is a novel antibody therapy that targets CD33; preclinical data suggested that it could be an effective combination with HMA therapy.
The phase 1 trial involved 53 patients with newly diagnosed, CD33-positive AML and a median age of 75 years. Patients were naive to HMA therapy but could have previously received other low-intensity treatments. HMA therapy was administered first; either azacitidine (75 mg/m2 subcutaneous IV for 7 days) or decitabine (20 mg/m2 IV for 5 days), according to institutional standards. On the last day of HMA therapy, vadastuximab talirine (10 mcg/kg IV) was given. This protocol was repeated in 28-day cycles for up to four cycles. Patients who tolerated the combination and showed a clinical response were eligible to continue therapy.
The composite remission rate (CRc: complete remission and complete remission with incomplete blood count recovery) with combination therapy was 70%. Historically, HMA monotherapies have much lower composite remission rates (decitabine, 17.8%; azacytidine, 27.8%). Of all patients achieving remission, 51% tested negative by flow cytometry for minimal residual disease. Median overall survival was 11.3 months and median relapse-free survival was 7.7 months.
“Nevertheless, the increased response rate with the addition of vadastuximab talirine to HMAs was also associated with increased toxicity when compared to single-agent HMA therapy – indicative of the greater degree of myelosuppression,” the researchers wrote. The most common grade 3 or higher adverse events were thrombocytopenia (57%), febrile neutropenia (49%), anemia (45%), neutropenia (42%), and fatigue (15%).
The investigators stated that “the overall safety profile was similar for patients treated with vadastuximab talirine in combination with azacitidine versus decitabine (with the exception of incidence of febrile neutropenia).”
Following the encouraging results of this phase 1 trial, the CASCADE phase 3 trial was launched to again compare this combination with HMA monotherapy; however, the trial was halted early because of deaths in the combination arm. The investigators cited the need for stricter protocols to ensure safety during future trials.
“With such guidance and precaution, promising combinations for AML, a disease affecting predominantly older and more frail patients, may be more effectively studied so as to enhance our current suboptimal therapeutic options,” they wrote.
Seattle Genetics provided study funding and author compensation.
SOURCE: Fathi AT et al. Blood. 2018 Jul 25. doi: 10.1182/blood-2018-03-841171.
For elderly patients with CD33-positive acute myeloid leukemia (AML), vadastuximab talirine in combination with a hypomethylating agent (HMA) improves remission rates, compared with HMA therapy alone, according to a phase 1 trial.
More than half of the patients treated with combination therapy achieved deep remission, defined as a negative-flow cytometry test for minimal residual disease. Despite these promising results, hematologic toxicity concerns may limit future trials.
“Outcomes for patients with acute myeloid leukemia (AML) remain poor, particularly in older patients,” wrote Amir T. Fathi, MD, of the division of hematology and oncology at Massachusetts General Hospital Cancer Center, Boston, and his coauthors.
Many elderly patients currently receive hypomethylating agents HMAs as a form of low-intensity therapy, but associated remission rates are low. “The development of novel, well-tolerated therapies to enhance the efficacy of HMAs could meaningfully improve the standard of care for older patients with AML,” the investigators wrote in Blood. Vadastuximab talirine is a novel antibody therapy that targets CD33; preclinical data suggested that it could be an effective combination with HMA therapy.
The phase 1 trial involved 53 patients with newly diagnosed, CD33-positive AML and a median age of 75 years. Patients were naive to HMA therapy but could have previously received other low-intensity treatments. HMA therapy was administered first; either azacitidine (75 mg/m2 subcutaneous IV for 7 days) or decitabine (20 mg/m2 IV for 5 days), according to institutional standards. On the last day of HMA therapy, vadastuximab talirine (10 mcg/kg IV) was given. This protocol was repeated in 28-day cycles for up to four cycles. Patients who tolerated the combination and showed a clinical response were eligible to continue therapy.
The composite remission rate (CRc: complete remission and complete remission with incomplete blood count recovery) with combination therapy was 70%. Historically, HMA monotherapies have much lower composite remission rates (decitabine, 17.8%; azacytidine, 27.8%). Of all patients achieving remission, 51% tested negative by flow cytometry for minimal residual disease. Median overall survival was 11.3 months and median relapse-free survival was 7.7 months.
“Nevertheless, the increased response rate with the addition of vadastuximab talirine to HMAs was also associated with increased toxicity when compared to single-agent HMA therapy – indicative of the greater degree of myelosuppression,” the researchers wrote. The most common grade 3 or higher adverse events were thrombocytopenia (57%), febrile neutropenia (49%), anemia (45%), neutropenia (42%), and fatigue (15%).
The investigators stated that “the overall safety profile was similar for patients treated with vadastuximab talirine in combination with azacitidine versus decitabine (with the exception of incidence of febrile neutropenia).”
Following the encouraging results of this phase 1 trial, the CASCADE phase 3 trial was launched to again compare this combination with HMA monotherapy; however, the trial was halted early because of deaths in the combination arm. The investigators cited the need for stricter protocols to ensure safety during future trials.
“With such guidance and precaution, promising combinations for AML, a disease affecting predominantly older and more frail patients, may be more effectively studied so as to enhance our current suboptimal therapeutic options,” they wrote.
Seattle Genetics provided study funding and author compensation.
SOURCE: Fathi AT et al. Blood. 2018 Jul 25. doi: 10.1182/blood-2018-03-841171.
FROM BLOOD
Key clinical point:
Major finding: The composite remission rate in patients treated with vadastuximab talirine and HMA therapy was 70%, compared with 17.8%-27.8% for patients treated with HMA therapy alone historically.
Study details: A prospective, phase 1 trial involving 53 elderly patients with CD33-positive AML at 14 treatment centers.
Disclosures: Seattle Genetics provided study funding and author compensation.
Source: Fathi AT et al. Blood. 2018 Jul 25. doi: 10.1182/blood-2018-03-841171.