User login
ERRATUM TO: Cardiac Troponins in Low-Risk Pulmonary Embolism Patients: A Systematic Review and Meta-Analysis
The authors would like to make the following corrections to their manuscript, Cardiac Troponins in Low-Risk Pulmonary Embolism Patients: A Systematic Review and Meta-Analysis (doi: 10.12788/jhm.2961), published online first April 25, 2018 (all corrections in bold):
- The last sentence of the results section in the abstract should read: The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40].
- In the "All studies pooled" of the last row of Table 2, Tn+ is corrected to 463. See revised table below.
- On page E5, the first paragraph in the "Outcomes of Studies with Corresponding Troponin+ and Troponin-" section beginning with the fifth sentence should read as follows):
"In the pooled data, 463 (67%) patients tested negative for troponin and 228 (33%) tested positive. The overall mortality (from sensitivity analysis) including in-hospital, 30-day, and 90-day mortalities was 1.2%. The NPVs for all individual studies and the overall NPV are 1 or approximately 1. The overall PPVs and by study were low, ranging from 0 to 0.60. The PLRs and NLRs were not estimated for an outcome within an individual study if none of the patients experienced the outcome. When outcomes were only observed among troponin-negative patients, such as in the study of Moore (2009) who used 30-day all-cause mortality, the PLR had a value of zero. When outcomes were only observed among troponin-positive patients, as for 30-day all-cause mortality in the Hakemi9(2015), Lauque10 (2014), and Lankeit16 (2011) studies, the NLR had a value of zero. For zero cells, a continuity correction of 0.5 was applied. The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40]. The OR for all-cause mortality was 4.79 [95% CI 1.11 to 20.68, P = .0357].

The authors would like to make the following corrections to their manuscript, Cardiac Troponins in Low-Risk Pulmonary Embolism Patients: A Systematic Review and Meta-Analysis (doi: 10.12788/jhm.2961), published online first April 25, 2018 (all corrections in bold):
- The last sentence of the results section in the abstract should read: The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40].
- In the "All studies pooled" of the last row of Table 2, Tn+ is corrected to 463. See revised table below.
- On page E5, the first paragraph in the "Outcomes of Studies with Corresponding Troponin+ and Troponin-" section beginning with the fifth sentence should read as follows):
"In the pooled data, 463 (67%) patients tested negative for troponin and 228 (33%) tested positive. The overall mortality (from sensitivity analysis) including in-hospital, 30-day, and 90-day mortalities was 1.2%. The NPVs for all individual studies and the overall NPV are 1 or approximately 1. The overall PPVs and by study were low, ranging from 0 to 0.60. The PLRs and NLRs were not estimated for an outcome within an individual study if none of the patients experienced the outcome. When outcomes were only observed among troponin-negative patients, such as in the study of Moore (2009) who used 30-day all-cause mortality, the PLR had a value of zero. When outcomes were only observed among troponin-positive patients, as for 30-day all-cause mortality in the Hakemi9(2015), Lauque10 (2014), and Lankeit16 (2011) studies, the NLR had a value of zero. For zero cells, a continuity correction of 0.5 was applied. The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40]. The OR for all-cause mortality was 4.79 [95% CI 1.11 to 20.68, P = .0357].

The authors would like to make the following corrections to their manuscript, Cardiac Troponins in Low-Risk Pulmonary Embolism Patients: A Systematic Review and Meta-Analysis (doi: 10.12788/jhm.2961), published online first April 25, 2018 (all corrections in bold):
- The last sentence of the results section in the abstract should read: The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40].
- In the "All studies pooled" of the last row of Table 2, Tn+ is corrected to 463. See revised table below.
- On page E5, the first paragraph in the "Outcomes of Studies with Corresponding Troponin+ and Troponin-" section beginning with the fifth sentence should read as follows):
"In the pooled data, 463 (67%) patients tested negative for troponin and 228 (33%) tested positive. The overall mortality (from sensitivity analysis) including in-hospital, 30-day, and 90-day mortalities was 1.2%. The NPVs for all individual studies and the overall NPV are 1 or approximately 1. The overall PPVs and by study were low, ranging from 0 to 0.60. The PLRs and NLRs were not estimated for an outcome within an individual study if none of the patients experienced the outcome. When outcomes were only observed among troponin-negative patients, such as in the study of Moore (2009) who used 30-day all-cause mortality, the PLR had a value of zero. When outcomes were only observed among troponin-positive patients, as for 30-day all-cause mortality in the Hakemi9(2015), Lauque10 (2014), and Lankeit16 (2011) studies, the NLR had a value of zero. For zero cells, a continuity correction of 0.5 was applied. The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40]. The OR for all-cause mortality was 4.79 [95% CI 1.11 to 20.68, P = .0357].

© 2018 Society of Hospital Medicine
Salivary gland ultrasound is accurate diagnostic tool for Sjögren’s
AMSTERDAM – Ultrasound of the salivary glands is a readily available and inexpensive tool for the diagnosis of Sjögren’s syndrome, according to a study that evaluated this test in relation to the recent American College of Rheumatology and European League Against Rheumatism (ACR/EULAR) classification criteria.
In a video interview, Esther-Jellina Mossel reported that the sensitivity and specificity of a Sjögren’s syndrome diagnosis is essentially unchanged when ultrasound replaces a positive ocular staining score, the Schirmer test, or an unstimulated whole saliva flow test, without reducing diagnostic accuracy.
The sensitivity of the diagnosis is reduced only if ultrasound is used to replace either of the two remaining ACR/EULAR criteria, which are a labial gland biopsy or an anti-SSA antibody test. In relation to the three criteria that it can replace without loss of diagnostic accuracy, ultrasound might have advantages.
“People who don’t have access to an ophthalmologist performing an ocular staining score, for instance, could use an ultrasound of the salivary glands instead of the ocular staining score and still make a diagnosis,” said Ms. Mossel, a PhD student in the department of rheumatology at the University of Groningen (the Netherlands).
Ultrasound, which is commonly used to evaluate joints of patients with inflammatory diseases, is available in the offices of most rheumatologists, according to Ms. Mossel. She estimated that the evaluation of the salivary glands, which reveals characteristic hypoechogenic areas when Sjögren’s syndrome is present, takes about 10 minutes.
At Ms. Mossel’s center, ultrasound has already become a standard tool for the diagnosis of Sjögren’s syndrome. She said that other centers have also found this imaging tool to be accurate and useful for Sjögren’s syndrome diagnosis.
Based on the experience at the University of Groningen, Ms. Mossel believes that ultrasound will eventually be widely adopted for Sjögren’s syndrome diagnosis. Indeed, she expects that this strategy is likely to be added to the ACR/EULAR diagnostic criteria when its accuracy becomes more generally recognized.
AMSTERDAM – Ultrasound of the salivary glands is a readily available and inexpensive tool for the diagnosis of Sjögren’s syndrome, according to a study that evaluated this test in relation to the recent American College of Rheumatology and European League Against Rheumatism (ACR/EULAR) classification criteria.
In a video interview, Esther-Jellina Mossel reported that the sensitivity and specificity of a Sjögren’s syndrome diagnosis is essentially unchanged when ultrasound replaces a positive ocular staining score, the Schirmer test, or an unstimulated whole saliva flow test, without reducing diagnostic accuracy.
The sensitivity of the diagnosis is reduced only if ultrasound is used to replace either of the two remaining ACR/EULAR criteria, which are a labial gland biopsy or an anti-SSA antibody test. In relation to the three criteria that it can replace without loss of diagnostic accuracy, ultrasound might have advantages.
“People who don’t have access to an ophthalmologist performing an ocular staining score, for instance, could use an ultrasound of the salivary glands instead of the ocular staining score and still make a diagnosis,” said Ms. Mossel, a PhD student in the department of rheumatology at the University of Groningen (the Netherlands).
Ultrasound, which is commonly used to evaluate joints of patients with inflammatory diseases, is available in the offices of most rheumatologists, according to Ms. Mossel. She estimated that the evaluation of the salivary glands, which reveals characteristic hypoechogenic areas when Sjögren’s syndrome is present, takes about 10 minutes.
At Ms. Mossel’s center, ultrasound has already become a standard tool for the diagnosis of Sjögren’s syndrome. She said that other centers have also found this imaging tool to be accurate and useful for Sjögren’s syndrome diagnosis.
Based on the experience at the University of Groningen, Ms. Mossel believes that ultrasound will eventually be widely adopted for Sjögren’s syndrome diagnosis. Indeed, she expects that this strategy is likely to be added to the ACR/EULAR diagnostic criteria when its accuracy becomes more generally recognized.
AMSTERDAM – Ultrasound of the salivary glands is a readily available and inexpensive tool for the diagnosis of Sjögren’s syndrome, according to a study that evaluated this test in relation to the recent American College of Rheumatology and European League Against Rheumatism (ACR/EULAR) classification criteria.
In a video interview, Esther-Jellina Mossel reported that the sensitivity and specificity of a Sjögren’s syndrome diagnosis is essentially unchanged when ultrasound replaces a positive ocular staining score, the Schirmer test, or an unstimulated whole saliva flow test, without reducing diagnostic accuracy.
The sensitivity of the diagnosis is reduced only if ultrasound is used to replace either of the two remaining ACR/EULAR criteria, which are a labial gland biopsy or an anti-SSA antibody test. In relation to the three criteria that it can replace without loss of diagnostic accuracy, ultrasound might have advantages.
“People who don’t have access to an ophthalmologist performing an ocular staining score, for instance, could use an ultrasound of the salivary glands instead of the ocular staining score and still make a diagnosis,” said Ms. Mossel, a PhD student in the department of rheumatology at the University of Groningen (the Netherlands).
Ultrasound, which is commonly used to evaluate joints of patients with inflammatory diseases, is available in the offices of most rheumatologists, according to Ms. Mossel. She estimated that the evaluation of the salivary glands, which reveals characteristic hypoechogenic areas when Sjögren’s syndrome is present, takes about 10 minutes.
At Ms. Mossel’s center, ultrasound has already become a standard tool for the diagnosis of Sjögren’s syndrome. She said that other centers have also found this imaging tool to be accurate and useful for Sjögren’s syndrome diagnosis.
Based on the experience at the University of Groningen, Ms. Mossel believes that ultrasound will eventually be widely adopted for Sjögren’s syndrome diagnosis. Indeed, she expects that this strategy is likely to be added to the ACR/EULAR diagnostic criteria when its accuracy becomes more generally recognized.
REPORTING FROM THE EULAR 2018 CONGRESS
FDA approves Epidiolex for Lennox-Gastaut syndrome and Dravet syndrome
The Food and Drug Administration has approved cannabidiol oral solution (Epidiolex, GW Pharmaceuticals) for the treatment of two rare pediatric seizure disorders.
“This product approval demonstrates that advancing sound scientific research to investigate ingredients derived from marijuana can lead to important therapies. This new treatment provides new options for patients,” said FDA Commissioner Scott Gottlieb, MD, in a statement.
However, he cautioned, “This is an important medical advance. But it’s also important to note that this is not an approval of marijuana or all of its components. This is the approval of one specific CBD medication for a specific use. And it was based on well-controlled clinical trials evaluating the use of this compound in the treatment of a specific condition.”
The FDA Peripheral and Central Nervous System Drugs Advisory Committee’s earlier positive recommendation was based on three randomized, double-blind, placebo-controlled clinical trials. These trials showed a 50% reduction of drop seizure frequency in 40%-44% of patients with Lennox-Gastaut syndrome, and a 39% decrease in convulsive seizure frequency for trial participants with Dravet Syndrome. A total of 516 patients with one of the two seizure disorders participated in the clinical trials.
“In addition to another important treatment option for Lennox-Gastaut patients, this first-ever approval of a drug specifically for Dravet patients will provide a significant and needed improvement in the therapeutic approach to caring for people with this condition,” said Billy Dunn, MD, director of the Division of Neurology Products in the FDA Center for Drug Evaluation and Research, in a statement.
After reviewing information provided by the drug’s sponsor and the FDA, the advisory committee judged that CBD-OS, derived from a non-psychoactive chemical found in marijuana, was very unlikely to have potential for abuse.
Sedation, sleepiness, and lethargy were among the most frequently reported adverse events for the patients taking CBD-OS. In data pooled from the clinical trials, 16.3% of patients taking CBD-OS at the higher dose of 20 mg/kg/day had liver transaminase elevations above three times the upper limit of normal; this level of transaminase elevation was seen in 0.9% of patients taking placebo.
A patient medication guide detailing risks and how the drug should be used will accompany CBD-OS when it is dispensed, according to the FDA approval.
In his statement, Dr. Gottlieb put the approval in the context of the FDA’s broader efforts to encourage a strong clinical development program for marijuana-derived drugs that does not compromise standards for ensuring safety and efficacy of drugs approved by the agency. He also noted that ongoing efforts to support high quality research into marijuana-based therapies involve other federal agencies, including the National Institute on Drug Abuse and the Drug Enforcement Administration.
The FDA’s actions against companies distributing unapproved products that contain cannabidiol and making unproven marketing claims will continue, said Dr. Gottlieb. Still, “Today’s approval demonstrates our commitment to the scientific process and working with product developers to bring marijuana-based products to market,” he said.
The Food and Drug Administration has approved cannabidiol oral solution (Epidiolex, GW Pharmaceuticals) for the treatment of two rare pediatric seizure disorders.
“This product approval demonstrates that advancing sound scientific research to investigate ingredients derived from marijuana can lead to important therapies. This new treatment provides new options for patients,” said FDA Commissioner Scott Gottlieb, MD, in a statement.
However, he cautioned, “This is an important medical advance. But it’s also important to note that this is not an approval of marijuana or all of its components. This is the approval of one specific CBD medication for a specific use. And it was based on well-controlled clinical trials evaluating the use of this compound in the treatment of a specific condition.”
The FDA Peripheral and Central Nervous System Drugs Advisory Committee’s earlier positive recommendation was based on three randomized, double-blind, placebo-controlled clinical trials. These trials showed a 50% reduction of drop seizure frequency in 40%-44% of patients with Lennox-Gastaut syndrome, and a 39% decrease in convulsive seizure frequency for trial participants with Dravet Syndrome. A total of 516 patients with one of the two seizure disorders participated in the clinical trials.
“In addition to another important treatment option for Lennox-Gastaut patients, this first-ever approval of a drug specifically for Dravet patients will provide a significant and needed improvement in the therapeutic approach to caring for people with this condition,” said Billy Dunn, MD, director of the Division of Neurology Products in the FDA Center for Drug Evaluation and Research, in a statement.
After reviewing information provided by the drug’s sponsor and the FDA, the advisory committee judged that CBD-OS, derived from a non-psychoactive chemical found in marijuana, was very unlikely to have potential for abuse.
Sedation, sleepiness, and lethargy were among the most frequently reported adverse events for the patients taking CBD-OS. In data pooled from the clinical trials, 16.3% of patients taking CBD-OS at the higher dose of 20 mg/kg/day had liver transaminase elevations above three times the upper limit of normal; this level of transaminase elevation was seen in 0.9% of patients taking placebo.
A patient medication guide detailing risks and how the drug should be used will accompany CBD-OS when it is dispensed, according to the FDA approval.
In his statement, Dr. Gottlieb put the approval in the context of the FDA’s broader efforts to encourage a strong clinical development program for marijuana-derived drugs that does not compromise standards for ensuring safety and efficacy of drugs approved by the agency. He also noted that ongoing efforts to support high quality research into marijuana-based therapies involve other federal agencies, including the National Institute on Drug Abuse and the Drug Enforcement Administration.
The FDA’s actions against companies distributing unapproved products that contain cannabidiol and making unproven marketing claims will continue, said Dr. Gottlieb. Still, “Today’s approval demonstrates our commitment to the scientific process and working with product developers to bring marijuana-based products to market,” he said.
The Food and Drug Administration has approved cannabidiol oral solution (Epidiolex, GW Pharmaceuticals) for the treatment of two rare pediatric seizure disorders.
“This product approval demonstrates that advancing sound scientific research to investigate ingredients derived from marijuana can lead to important therapies. This new treatment provides new options for patients,” said FDA Commissioner Scott Gottlieb, MD, in a statement.
However, he cautioned, “This is an important medical advance. But it’s also important to note that this is not an approval of marijuana or all of its components. This is the approval of one specific CBD medication for a specific use. And it was based on well-controlled clinical trials evaluating the use of this compound in the treatment of a specific condition.”
The FDA Peripheral and Central Nervous System Drugs Advisory Committee’s earlier positive recommendation was based on three randomized, double-blind, placebo-controlled clinical trials. These trials showed a 50% reduction of drop seizure frequency in 40%-44% of patients with Lennox-Gastaut syndrome, and a 39% decrease in convulsive seizure frequency for trial participants with Dravet Syndrome. A total of 516 patients with one of the two seizure disorders participated in the clinical trials.
“In addition to another important treatment option for Lennox-Gastaut patients, this first-ever approval of a drug specifically for Dravet patients will provide a significant and needed improvement in the therapeutic approach to caring for people with this condition,” said Billy Dunn, MD, director of the Division of Neurology Products in the FDA Center for Drug Evaluation and Research, in a statement.
After reviewing information provided by the drug’s sponsor and the FDA, the advisory committee judged that CBD-OS, derived from a non-psychoactive chemical found in marijuana, was very unlikely to have potential for abuse.
Sedation, sleepiness, and lethargy were among the most frequently reported adverse events for the patients taking CBD-OS. In data pooled from the clinical trials, 16.3% of patients taking CBD-OS at the higher dose of 20 mg/kg/day had liver transaminase elevations above three times the upper limit of normal; this level of transaminase elevation was seen in 0.9% of patients taking placebo.
A patient medication guide detailing risks and how the drug should be used will accompany CBD-OS when it is dispensed, according to the FDA approval.
In his statement, Dr. Gottlieb put the approval in the context of the FDA’s broader efforts to encourage a strong clinical development program for marijuana-derived drugs that does not compromise standards for ensuring safety and efficacy of drugs approved by the agency. He also noted that ongoing efforts to support high quality research into marijuana-based therapies involve other federal agencies, including the National Institute on Drug Abuse and the Drug Enforcement Administration.
The FDA’s actions against companies distributing unapproved products that contain cannabidiol and making unproven marketing claims will continue, said Dr. Gottlieb. Still, “Today’s approval demonstrates our commitment to the scientific process and working with product developers to bring marijuana-based products to market,” he said.
Clomiphene citrate improves pregnancy outcomes for PCOS patients
Clomiphene citrate significantly improved markers of polycystic ovarian syndrome (PCOS) and improved ovulation and pregnancy outcomes in women with PCOS, according to data from 72 women.
Nitric oxide (NO), interleukin-10 (IL-10), and matrix metalloproteinase–9 (MMP-9) “are known to be involved in the pathogenesis as well as the complications of PCOS,” wrote Angel Mercy Sylus, MD, of the Jawaharlal Institute of Postgraduate Medical Education & Research, Puducherry, India, and colleagues.
Clomiphene citrate is used to treat infertility, including infertile women with PCOS, but its mechanism of action remains unclear, the researchers wrote.
In a study published in the European Journal of Obstetrics & Gynecology and Reproductive Biology, the researchers enrolled 72 women with PCOS. The women received 50 mg of oral clomiphene citrate daily on days 3-7 of their cycles to induce ovulation. Levels of NO, IL-10, and MMP-9 were measured at baseline and after 3 weeks. The average age of the women was 25 years, and the average body mass index was 26.4 kg/m2.
After the participants took clomiphene citrate, their levels of NO and IL-10 were significantly higher, compared with baseline (P = .03 and P less than .001, respectively), and MMP-9 levels were significantly lower, compared with baseline (P less than .001).
The ovulation rate in the study population was 52.8%, and the clinical pregnancy rate was 19.4%. Levels of MMP-9 were significantly reduced (P less than .001) in the ovulatory group, compared with the nonovulatory group, the researchers noted. “Although the mechanism through which CC [clomiphene citrate] reduces MMP-9 and increases IL-10 is not clear, our findings indicate that CC therapy improves ovulation by reducing inflammation and reducing MMP-9 levels,” they wrote.
The findings were limited by several factors, mainly by the timing of the 4-week assessment of NO, IL-10, and MPP-9 for ethical reasons, the researchers wrote. They did not get study approval to conduct a separate blood collection. In addition, the study did not measure the effect of increasing doses of clomiphene citrate.
However, the results have suggested that clomiphene citrate can help promote ovulation and pregnancy for infertile women with PCOS, and further studies are needed to assess the mechanism of action and the effect of higher doses on NO, IL-10, and MPP-9, the researchers wrote.
The study was supported by a grant from the Jawaharlal Institute of Postgraduate Medical Education and Research intramural fund. The researchers had no financial conflicts to disclose.
SOURCE: Sylus AM et al. Eur J Obstet Gynecol Reprod Biol. 2018 Sept; 228:27-31.
Clomiphene citrate significantly improved markers of polycystic ovarian syndrome (PCOS) and improved ovulation and pregnancy outcomes in women with PCOS, according to data from 72 women.
Nitric oxide (NO), interleukin-10 (IL-10), and matrix metalloproteinase–9 (MMP-9) “are known to be involved in the pathogenesis as well as the complications of PCOS,” wrote Angel Mercy Sylus, MD, of the Jawaharlal Institute of Postgraduate Medical Education & Research, Puducherry, India, and colleagues.
Clomiphene citrate is used to treat infertility, including infertile women with PCOS, but its mechanism of action remains unclear, the researchers wrote.
In a study published in the European Journal of Obstetrics & Gynecology and Reproductive Biology, the researchers enrolled 72 women with PCOS. The women received 50 mg of oral clomiphene citrate daily on days 3-7 of their cycles to induce ovulation. Levels of NO, IL-10, and MMP-9 were measured at baseline and after 3 weeks. The average age of the women was 25 years, and the average body mass index was 26.4 kg/m2.
After the participants took clomiphene citrate, their levels of NO and IL-10 were significantly higher, compared with baseline (P = .03 and P less than .001, respectively), and MMP-9 levels were significantly lower, compared with baseline (P less than .001).
The ovulation rate in the study population was 52.8%, and the clinical pregnancy rate was 19.4%. Levels of MMP-9 were significantly reduced (P less than .001) in the ovulatory group, compared with the nonovulatory group, the researchers noted. “Although the mechanism through which CC [clomiphene citrate] reduces MMP-9 and increases IL-10 is not clear, our findings indicate that CC therapy improves ovulation by reducing inflammation and reducing MMP-9 levels,” they wrote.
The findings were limited by several factors, mainly by the timing of the 4-week assessment of NO, IL-10, and MPP-9 for ethical reasons, the researchers wrote. They did not get study approval to conduct a separate blood collection. In addition, the study did not measure the effect of increasing doses of clomiphene citrate.
However, the results have suggested that clomiphene citrate can help promote ovulation and pregnancy for infertile women with PCOS, and further studies are needed to assess the mechanism of action and the effect of higher doses on NO, IL-10, and MPP-9, the researchers wrote.
The study was supported by a grant from the Jawaharlal Institute of Postgraduate Medical Education and Research intramural fund. The researchers had no financial conflicts to disclose.
SOURCE: Sylus AM et al. Eur J Obstet Gynecol Reprod Biol. 2018 Sept; 228:27-31.
Clomiphene citrate significantly improved markers of polycystic ovarian syndrome (PCOS) and improved ovulation and pregnancy outcomes in women with PCOS, according to data from 72 women.
Nitric oxide (NO), interleukin-10 (IL-10), and matrix metalloproteinase–9 (MMP-9) “are known to be involved in the pathogenesis as well as the complications of PCOS,” wrote Angel Mercy Sylus, MD, of the Jawaharlal Institute of Postgraduate Medical Education & Research, Puducherry, India, and colleagues.
Clomiphene citrate is used to treat infertility, including infertile women with PCOS, but its mechanism of action remains unclear, the researchers wrote.
In a study published in the European Journal of Obstetrics & Gynecology and Reproductive Biology, the researchers enrolled 72 women with PCOS. The women received 50 mg of oral clomiphene citrate daily on days 3-7 of their cycles to induce ovulation. Levels of NO, IL-10, and MMP-9 were measured at baseline and after 3 weeks. The average age of the women was 25 years, and the average body mass index was 26.4 kg/m2.
After the participants took clomiphene citrate, their levels of NO and IL-10 were significantly higher, compared with baseline (P = .03 and P less than .001, respectively), and MMP-9 levels were significantly lower, compared with baseline (P less than .001).
The ovulation rate in the study population was 52.8%, and the clinical pregnancy rate was 19.4%. Levels of MMP-9 were significantly reduced (P less than .001) in the ovulatory group, compared with the nonovulatory group, the researchers noted. “Although the mechanism through which CC [clomiphene citrate] reduces MMP-9 and increases IL-10 is not clear, our findings indicate that CC therapy improves ovulation by reducing inflammation and reducing MMP-9 levels,” they wrote.
The findings were limited by several factors, mainly by the timing of the 4-week assessment of NO, IL-10, and MPP-9 for ethical reasons, the researchers wrote. They did not get study approval to conduct a separate blood collection. In addition, the study did not measure the effect of increasing doses of clomiphene citrate.
However, the results have suggested that clomiphene citrate can help promote ovulation and pregnancy for infertile women with PCOS, and further studies are needed to assess the mechanism of action and the effect of higher doses on NO, IL-10, and MPP-9, the researchers wrote.
The study was supported by a grant from the Jawaharlal Institute of Postgraduate Medical Education and Research intramural fund. The researchers had no financial conflicts to disclose.
SOURCE: Sylus AM et al. Eur J Obstet Gynecol Reprod Biol. 2018 Sept; 228:27-31.
FROM THE EUROPEAN JOURNAL OF OBSTETRICS & GYNECOLOGY AND REPRODUCTIVE BIOLOGY
Key clinical point: Clomiphene citrate increased the levels of both nitric oxide and interleukin-10 and reduced levels of matrix metalloproteinase–9.
Major finding: The ovulation rate was 53%, and the clinical pregnancy rate was 19% in PCOS women given clomiphene citrate.
Study details: The data come from 72 women with PCOS.
Disclosures: The study was supported by a grant from the Jawaharlal Institute of Postgraduate Medical Education and Research intramural fund. The researchers had no financial conflicts to disclose.
Source: Sylus A et al. Eur J Obstet Gynecol Reprod Biol. 2018 Sep;228:27-31.
Risankizumab impresses in phase 2 psoriatic arthritis trial
AMSTERDAM – Phase 2 data with the IL-23 inhibitor risankizumab at week 24 were even more impressive than the week 16 data, showing that without any further dosing after week 16, all doses provided protection against radiographic progression relative to placebo at 24 weeks, according to data presented at the European Congress of Rheumatology.
In a video interview, first author Philip J. Mease, MD, a rheumatologist at Swedish Medical Center in Seattle, explained that it is not only the high rates of response to risankizumab but also the prolonged response that are attracting attention.
Risankizumab is among several monoclonal antibodies developed to target the p19 subunit of the proinflammatory cytokine IL-23. These drugs have already shown a high degree of efficacy for psoriasis, according to Dr. Mease. However, the new data with risankizumab confirm prolonged responses against a broad range of additional clinical targets specific to psoriatic arthritis, including bone destruction and enthesitis.
A prolonged response in patients treated with a single, relatively low dose of risankizumab is one of the intriguing findings. While three of the four active treatments arms received multiple infusions of 150 mg, the single-dose arm received only 75 mg of risankizumab once at baseline. At 16 weeks and 24 weeks, all arms, including the single-dose arm, met the primary endpoint of superiority to placebo for ACR20. At week 24, the single infusion of 75 mg was also providing significant benefit for several secondary endpoints, including radiographic progression.
However, the higher, more frequent doses did show greater efficacy overall. For example, patients in the arm with the most frequent dosing of risankizumab (every 4 weeks) and no dosing after week 16 continued to show significant improvement in enthesitis. A less frequent schedule of 150 mg risankizumab and the arm receiving a single dose of 75 mg risankizumab were not associated with a significant advantage over placebo for this endpoint.
Still, the prolonged responses at week 24 suggest that it may be possible to administer risankizumab at intervals that are less frequent than many other biologics.
So far, there “is nothing remarkable about safety,” Dr. Mease explained. A higher rate of infection relative to placebo was a treatment-emergent side effect in this study, but Dr. Mease said the drug is well tolerated.
Risankizumab is poised for evaluation in a phase 3 trial for psoriatic arthritis, and Dr. Mease was optimistic about its potential role, predicting that this, as well as other anti-IL23 p19 monoclonal antibodies, is likely to be an “important addition to our armamentarium.”
AbbVie and Boehringer Ingelheim funded the risankizumab study. Dr. Mease has received grant/research support from AbbVie and many other pharmaceutical companies. He also is a consultant to them and is on their speakers bureaus.
SOURCE: Mease P et al. Ann Rheum Dis. 2018;77(Suppl 2):200-1. Abstract OP0307
AMSTERDAM – Phase 2 data with the IL-23 inhibitor risankizumab at week 24 were even more impressive than the week 16 data, showing that without any further dosing after week 16, all doses provided protection against radiographic progression relative to placebo at 24 weeks, according to data presented at the European Congress of Rheumatology.
In a video interview, first author Philip J. Mease, MD, a rheumatologist at Swedish Medical Center in Seattle, explained that it is not only the high rates of response to risankizumab but also the prolonged response that are attracting attention.
Risankizumab is among several monoclonal antibodies developed to target the p19 subunit of the proinflammatory cytokine IL-23. These drugs have already shown a high degree of efficacy for psoriasis, according to Dr. Mease. However, the new data with risankizumab confirm prolonged responses against a broad range of additional clinical targets specific to psoriatic arthritis, including bone destruction and enthesitis.
A prolonged response in patients treated with a single, relatively low dose of risankizumab is one of the intriguing findings. While three of the four active treatments arms received multiple infusions of 150 mg, the single-dose arm received only 75 mg of risankizumab once at baseline. At 16 weeks and 24 weeks, all arms, including the single-dose arm, met the primary endpoint of superiority to placebo for ACR20. At week 24, the single infusion of 75 mg was also providing significant benefit for several secondary endpoints, including radiographic progression.
However, the higher, more frequent doses did show greater efficacy overall. For example, patients in the arm with the most frequent dosing of risankizumab (every 4 weeks) and no dosing after week 16 continued to show significant improvement in enthesitis. A less frequent schedule of 150 mg risankizumab and the arm receiving a single dose of 75 mg risankizumab were not associated with a significant advantage over placebo for this endpoint.
Still, the prolonged responses at week 24 suggest that it may be possible to administer risankizumab at intervals that are less frequent than many other biologics.
So far, there “is nothing remarkable about safety,” Dr. Mease explained. A higher rate of infection relative to placebo was a treatment-emergent side effect in this study, but Dr. Mease said the drug is well tolerated.
Risankizumab is poised for evaluation in a phase 3 trial for psoriatic arthritis, and Dr. Mease was optimistic about its potential role, predicting that this, as well as other anti-IL23 p19 monoclonal antibodies, is likely to be an “important addition to our armamentarium.”
AbbVie and Boehringer Ingelheim funded the risankizumab study. Dr. Mease has received grant/research support from AbbVie and many other pharmaceutical companies. He also is a consultant to them and is on their speakers bureaus.
SOURCE: Mease P et al. Ann Rheum Dis. 2018;77(Suppl 2):200-1. Abstract OP0307
AMSTERDAM – Phase 2 data with the IL-23 inhibitor risankizumab at week 24 were even more impressive than the week 16 data, showing that without any further dosing after week 16, all doses provided protection against radiographic progression relative to placebo at 24 weeks, according to data presented at the European Congress of Rheumatology.
In a video interview, first author Philip J. Mease, MD, a rheumatologist at Swedish Medical Center in Seattle, explained that it is not only the high rates of response to risankizumab but also the prolonged response that are attracting attention.
Risankizumab is among several monoclonal antibodies developed to target the p19 subunit of the proinflammatory cytokine IL-23. These drugs have already shown a high degree of efficacy for psoriasis, according to Dr. Mease. However, the new data with risankizumab confirm prolonged responses against a broad range of additional clinical targets specific to psoriatic arthritis, including bone destruction and enthesitis.
A prolonged response in patients treated with a single, relatively low dose of risankizumab is one of the intriguing findings. While three of the four active treatments arms received multiple infusions of 150 mg, the single-dose arm received only 75 mg of risankizumab once at baseline. At 16 weeks and 24 weeks, all arms, including the single-dose arm, met the primary endpoint of superiority to placebo for ACR20. At week 24, the single infusion of 75 mg was also providing significant benefit for several secondary endpoints, including radiographic progression.
However, the higher, more frequent doses did show greater efficacy overall. For example, patients in the arm with the most frequent dosing of risankizumab (every 4 weeks) and no dosing after week 16 continued to show significant improvement in enthesitis. A less frequent schedule of 150 mg risankizumab and the arm receiving a single dose of 75 mg risankizumab were not associated with a significant advantage over placebo for this endpoint.
Still, the prolonged responses at week 24 suggest that it may be possible to administer risankizumab at intervals that are less frequent than many other biologics.
So far, there “is nothing remarkable about safety,” Dr. Mease explained. A higher rate of infection relative to placebo was a treatment-emergent side effect in this study, but Dr. Mease said the drug is well tolerated.
Risankizumab is poised for evaluation in a phase 3 trial for psoriatic arthritis, and Dr. Mease was optimistic about its potential role, predicting that this, as well as other anti-IL23 p19 monoclonal antibodies, is likely to be an “important addition to our armamentarium.”
AbbVie and Boehringer Ingelheim funded the risankizumab study. Dr. Mease has received grant/research support from AbbVie and many other pharmaceutical companies. He also is a consultant to them and is on their speakers bureaus.
SOURCE: Mease P et al. Ann Rheum Dis. 2018;77(Suppl 2):200-1. Abstract OP0307
REPORTING FROM the EULAR 2018 Congress
Diabetes patients pushed into high-deductible plans
ORLANDO – The proportion of diabetes patients enrolled in high-deductible health plans jumped from 10% in 2005 to about 50% in 2014, according to a review of insurance data for 63 million Americans under age 65 years.
Diabetes patients often don’t have a choice. To cut costs, high-deductible plans are increasingly the only ones employers offer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
While that may be adequate for healthy people, it’s quite another issue for people with chronic conditions, especially ones with low income. Out-of-pocket expenses can be thousands of dollars more than with traditional health plans, and the extra costs aren’t always offset by lower premiums.
The trend is concerning, said senior investigator J. Frank Wharam, MB, MPH, an associate professor of population medicine at Harvard Medical School, Boston. He explained the problem, and what’s being done about it, in an interview at the annual scientific sessions of the American Diabetes Association.
SOURCE: Garabedian LF et al. ADA 2018. Abstract 175-OR.
ORLANDO – The proportion of diabetes patients enrolled in high-deductible health plans jumped from 10% in 2005 to about 50% in 2014, according to a review of insurance data for 63 million Americans under age 65 years.
Diabetes patients often don’t have a choice. To cut costs, high-deductible plans are increasingly the only ones employers offer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
While that may be adequate for healthy people, it’s quite another issue for people with chronic conditions, especially ones with low income. Out-of-pocket expenses can be thousands of dollars more than with traditional health plans, and the extra costs aren’t always offset by lower premiums.
The trend is concerning, said senior investigator J. Frank Wharam, MB, MPH, an associate professor of population medicine at Harvard Medical School, Boston. He explained the problem, and what’s being done about it, in an interview at the annual scientific sessions of the American Diabetes Association.
SOURCE: Garabedian LF et al. ADA 2018. Abstract 175-OR.
ORLANDO – The proportion of diabetes patients enrolled in high-deductible health plans jumped from 10% in 2005 to about 50% in 2014, according to a review of insurance data for 63 million Americans under age 65 years.
Diabetes patients often don’t have a choice. To cut costs, high-deductible plans are increasingly the only ones employers offer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
While that may be adequate for healthy people, it’s quite another issue for people with chronic conditions, especially ones with low income. Out-of-pocket expenses can be thousands of dollars more than with traditional health plans, and the extra costs aren’t always offset by lower premiums.
The trend is concerning, said senior investigator J. Frank Wharam, MB, MPH, an associate professor of population medicine at Harvard Medical School, Boston. He explained the problem, and what’s being done about it, in an interview at the annual scientific sessions of the American Diabetes Association.
SOURCE: Garabedian LF et al. ADA 2018. Abstract 175-OR.
REPORTING FROM ADA 2018
KEYNOTE-427: Pembrolizumab monotherapy shows promise in accRCC
CHICAGO – (accRCC), according to findings from the phase 2 KEYNOTE-427 study.
At a median follow-up of 12 months, the overall response rate in 110 study participants with at least one post-baseline assessment was 38%. Three patients (2.7%) achieved a complete response and 39 (35.5%) achieved a partial response, David F. McDermott, MD, reported at the annual meeting of the American Society of Clinical Oncology.
“The disease control rate was 59%,” he said.
Overall, 67% of the patients experienced a reduction in tumor burden, 14% experienced at least an 80% reduction, and 7% experienced a 100% reduction of their target lesion, said Dr. McDermott of Beth Israel Deaconess Medical Center, Boston.
“Most tumor responses occurred early in the course of therapy,” he noted.
The median time to response was 2.8 months, and the median duration of response was not reached at data cutoff, but 74.8% of responders had a response lasting at least 6 months.
An analysis by International Metastatic Renal Cell Carcinoma Database Criteria (IMDC) category showed a confirmed overall response rate (ORR) of 32% among 41 patients with favorable risk, and 42% in 69 patients with intermediate or poor risk.
“Nine of 17 patients in the poor risk group achieved a major response,” Dr. McDermott noted. “Complete and durable responders were seen in all IMDC subgroups.”
In 46 patients with increased PD-L1 expression or a combined positive score of at least 1 the confirmed ORR was 50.0%, and in 53 patients with low PD-L1 expression and a combined positive score less than 1 it was 26%. The ORR was 45% in the remaining patients in whom PD-L1testing could not be performed.
“Of note, all of the complete responses were seen in the PD-L1-high or CPS-greater-than-1 group,” he said.
Median progression-free survival was 8.7 months, and median overall survival has not been reached.
Tolerability of pembrolizumab in this study was acceptable and consistent with that seen with pembrolizumab monotherapy in other tumor types. Although 80% of patients experienced a treatment-related adverse event, the events mainly included fatigue, pruritus, diarrhea, rash, and arthralgia, occurring in 12.7% to 27.3% of patients, he said.
Grade 3/4 events occurred in 21.8% of patients and one patient experienced a fatal grade 5 case of pneumonitis, he added, noting that 11% of patients discontinued treatment because of a treatment-related adverse event.
Overall, 61 patients discontinued therapy, and 33 of those discontinued because of disease progression.
Programmed death-1 (PD-1) inhibitor-based combination therapies have been shown to have clinical benefit when used first-line in accRCC, but data with respect to the clinical impact of first-line PD-1 inhibitor monotherapy are lacking, Dr. McDermott explained.
KEYNOTE-427 was a single-arm, open-label, two-cohort study evaluating the efficacy and safety of pembrolizumab as first-line monotherapy in accRCC and advanced non–clear cell RCC (anccRCC). Patients had accRCC or anccRCC, measurable disease, no prior systemic therapy and Karnofsky Performance Status score of 70% or greater. They were treated with intravenous pembrolizumab at a dose of 200 mg every 3 weeks, and response was assessed at week 12, then every 6 weeks thereafter until week 54, then every 12 weeks.
The current analysis focused on the accRCC cohort and showed that in treatment-naive patients with histologically confirmed accRCC and measurable disease, pembrolizumab shows promising antitumor activity across IMDC risk groups, he said.
“Encouraging activity was also observed in key subgroups, such as the IMDC intermediate/poor risk group ... and patients with [programmed death-ligand 1]-positive tumors,” he said. “The findings ... provide support for the exploration of pembrolizumab in the adjuvant setting and will allow investigators to put the benefit of anti-PD-1-based combination therapies in better context,” he concluded, noting that KEYNOTE-564, a study of pembrolizumab in the adjuvant setting is currently enrolling, and the current study (KEYNOTE-427) cohort B exploring pembrolizumab monotherapy in anccRCC patients is ongoing.
Merck sponsored the study. Dr. McDermott reported consulting or advisory roles with Array BioPharma, Bristol-Myers Squibb, Exelixis, Genentech/Roche, Merck, Novartis, Pfizer, and X4 Pharma. His institution has received research funding from Prometheus Laboratories.
SOURCE: McDermott DF et al., ASCO 2018 Abstract 4500.
CHICAGO – (accRCC), according to findings from the phase 2 KEYNOTE-427 study.
At a median follow-up of 12 months, the overall response rate in 110 study participants with at least one post-baseline assessment was 38%. Three patients (2.7%) achieved a complete response and 39 (35.5%) achieved a partial response, David F. McDermott, MD, reported at the annual meeting of the American Society of Clinical Oncology.
“The disease control rate was 59%,” he said.
Overall, 67% of the patients experienced a reduction in tumor burden, 14% experienced at least an 80% reduction, and 7% experienced a 100% reduction of their target lesion, said Dr. McDermott of Beth Israel Deaconess Medical Center, Boston.
“Most tumor responses occurred early in the course of therapy,” he noted.
The median time to response was 2.8 months, and the median duration of response was not reached at data cutoff, but 74.8% of responders had a response lasting at least 6 months.
An analysis by International Metastatic Renal Cell Carcinoma Database Criteria (IMDC) category showed a confirmed overall response rate (ORR) of 32% among 41 patients with favorable risk, and 42% in 69 patients with intermediate or poor risk.
“Nine of 17 patients in the poor risk group achieved a major response,” Dr. McDermott noted. “Complete and durable responders were seen in all IMDC subgroups.”
In 46 patients with increased PD-L1 expression or a combined positive score of at least 1 the confirmed ORR was 50.0%, and in 53 patients with low PD-L1 expression and a combined positive score less than 1 it was 26%. The ORR was 45% in the remaining patients in whom PD-L1testing could not be performed.
“Of note, all of the complete responses were seen in the PD-L1-high or CPS-greater-than-1 group,” he said.
Median progression-free survival was 8.7 months, and median overall survival has not been reached.
Tolerability of pembrolizumab in this study was acceptable and consistent with that seen with pembrolizumab monotherapy in other tumor types. Although 80% of patients experienced a treatment-related adverse event, the events mainly included fatigue, pruritus, diarrhea, rash, and arthralgia, occurring in 12.7% to 27.3% of patients, he said.
Grade 3/4 events occurred in 21.8% of patients and one patient experienced a fatal grade 5 case of pneumonitis, he added, noting that 11% of patients discontinued treatment because of a treatment-related adverse event.
Overall, 61 patients discontinued therapy, and 33 of those discontinued because of disease progression.
Programmed death-1 (PD-1) inhibitor-based combination therapies have been shown to have clinical benefit when used first-line in accRCC, but data with respect to the clinical impact of first-line PD-1 inhibitor monotherapy are lacking, Dr. McDermott explained.
KEYNOTE-427 was a single-arm, open-label, two-cohort study evaluating the efficacy and safety of pembrolizumab as first-line monotherapy in accRCC and advanced non–clear cell RCC (anccRCC). Patients had accRCC or anccRCC, measurable disease, no prior systemic therapy and Karnofsky Performance Status score of 70% or greater. They were treated with intravenous pembrolizumab at a dose of 200 mg every 3 weeks, and response was assessed at week 12, then every 6 weeks thereafter until week 54, then every 12 weeks.
The current analysis focused on the accRCC cohort and showed that in treatment-naive patients with histologically confirmed accRCC and measurable disease, pembrolizumab shows promising antitumor activity across IMDC risk groups, he said.
“Encouraging activity was also observed in key subgroups, such as the IMDC intermediate/poor risk group ... and patients with [programmed death-ligand 1]-positive tumors,” he said. “The findings ... provide support for the exploration of pembrolizumab in the adjuvant setting and will allow investigators to put the benefit of anti-PD-1-based combination therapies in better context,” he concluded, noting that KEYNOTE-564, a study of pembrolizumab in the adjuvant setting is currently enrolling, and the current study (KEYNOTE-427) cohort B exploring pembrolizumab monotherapy in anccRCC patients is ongoing.
Merck sponsored the study. Dr. McDermott reported consulting or advisory roles with Array BioPharma, Bristol-Myers Squibb, Exelixis, Genentech/Roche, Merck, Novartis, Pfizer, and X4 Pharma. His institution has received research funding from Prometheus Laboratories.
SOURCE: McDermott DF et al., ASCO 2018 Abstract 4500.
CHICAGO – (accRCC), according to findings from the phase 2 KEYNOTE-427 study.
At a median follow-up of 12 months, the overall response rate in 110 study participants with at least one post-baseline assessment was 38%. Three patients (2.7%) achieved a complete response and 39 (35.5%) achieved a partial response, David F. McDermott, MD, reported at the annual meeting of the American Society of Clinical Oncology.
“The disease control rate was 59%,” he said.
Overall, 67% of the patients experienced a reduction in tumor burden, 14% experienced at least an 80% reduction, and 7% experienced a 100% reduction of their target lesion, said Dr. McDermott of Beth Israel Deaconess Medical Center, Boston.
“Most tumor responses occurred early in the course of therapy,” he noted.
The median time to response was 2.8 months, and the median duration of response was not reached at data cutoff, but 74.8% of responders had a response lasting at least 6 months.
An analysis by International Metastatic Renal Cell Carcinoma Database Criteria (IMDC) category showed a confirmed overall response rate (ORR) of 32% among 41 patients with favorable risk, and 42% in 69 patients with intermediate or poor risk.
“Nine of 17 patients in the poor risk group achieved a major response,” Dr. McDermott noted. “Complete and durable responders were seen in all IMDC subgroups.”
In 46 patients with increased PD-L1 expression or a combined positive score of at least 1 the confirmed ORR was 50.0%, and in 53 patients with low PD-L1 expression and a combined positive score less than 1 it was 26%. The ORR was 45% in the remaining patients in whom PD-L1testing could not be performed.
“Of note, all of the complete responses were seen in the PD-L1-high or CPS-greater-than-1 group,” he said.
Median progression-free survival was 8.7 months, and median overall survival has not been reached.
Tolerability of pembrolizumab in this study was acceptable and consistent with that seen with pembrolizumab monotherapy in other tumor types. Although 80% of patients experienced a treatment-related adverse event, the events mainly included fatigue, pruritus, diarrhea, rash, and arthralgia, occurring in 12.7% to 27.3% of patients, he said.
Grade 3/4 events occurred in 21.8% of patients and one patient experienced a fatal grade 5 case of pneumonitis, he added, noting that 11% of patients discontinued treatment because of a treatment-related adverse event.
Overall, 61 patients discontinued therapy, and 33 of those discontinued because of disease progression.
Programmed death-1 (PD-1) inhibitor-based combination therapies have been shown to have clinical benefit when used first-line in accRCC, but data with respect to the clinical impact of first-line PD-1 inhibitor monotherapy are lacking, Dr. McDermott explained.
KEYNOTE-427 was a single-arm, open-label, two-cohort study evaluating the efficacy and safety of pembrolizumab as first-line monotherapy in accRCC and advanced non–clear cell RCC (anccRCC). Patients had accRCC or anccRCC, measurable disease, no prior systemic therapy and Karnofsky Performance Status score of 70% or greater. They were treated with intravenous pembrolizumab at a dose of 200 mg every 3 weeks, and response was assessed at week 12, then every 6 weeks thereafter until week 54, then every 12 weeks.
The current analysis focused on the accRCC cohort and showed that in treatment-naive patients with histologically confirmed accRCC and measurable disease, pembrolizumab shows promising antitumor activity across IMDC risk groups, he said.
“Encouraging activity was also observed in key subgroups, such as the IMDC intermediate/poor risk group ... and patients with [programmed death-ligand 1]-positive tumors,” he said. “The findings ... provide support for the exploration of pembrolizumab in the adjuvant setting and will allow investigators to put the benefit of anti-PD-1-based combination therapies in better context,” he concluded, noting that KEYNOTE-564, a study of pembrolizumab in the adjuvant setting is currently enrolling, and the current study (KEYNOTE-427) cohort B exploring pembrolizumab monotherapy in anccRCC patients is ongoing.
Merck sponsored the study. Dr. McDermott reported consulting or advisory roles with Array BioPharma, Bristol-Myers Squibb, Exelixis, Genentech/Roche, Merck, Novartis, Pfizer, and X4 Pharma. His institution has received research funding from Prometheus Laboratories.
SOURCE: McDermott DF et al., ASCO 2018 Abstract 4500.
REPORTING FROM ASCO 2018
Key clinical point: Pembrolizumab monotherapy shows promising efficacy and tolerability in accRCC.
Major finding: Overall response rate was 38%.
Study details: The phase 2 KEYNOTE-427 trial of 110 patients from one of two study cohorts.
Disclosures: Merck sponsored the study. Dr. McDermott reported consulting or advisory roles with Array BioPharma, Bristol-Myers Squibb, Exelixis, Genentech/Roche, Merck, Novartis, Pfizer, and X4 Pharma. His institution has received research funding from Prometheus Laboratories.
Source: McDermott DF et al. ASCO 2018, Abstract 4500.
The fragile gray mass between your ears
He’s almost 10 years younger than me.
He’d been in the hospital for 3 weeks. The ICU room had been decorated, as many families do, with pictures of his life. His wedding. His kids. He and his wife dressed as Darth Vader and Princess Leia for a Halloween party. A few religious items.
He was off sedation. EEG didn’t show any seizures. Head CT just showed the extensive damage from his head injury. The neurosurgeons can evacuate clots and decrease intracranial pressure, but they can’t repair brain tissue.
His wife was long past the point of shock when I met with her. After 3 weeks, she understood what the new normal was and how the lives of both herself and their kids would never be the same. She held his hand at the bedside as we talked, asked me a few pointed questions, and then thanked me for coming in to see him.
For me, it was just another day on call. I walked back to the nurses station, got some coffee from the galley, and sat down to dictate a note. There are always other patients to see on the coverage list.
But it still reminds you.
The brain doesn’t weigh much, just 2-3 pounds; it’s about the size of your fists put together.
But it’s everything that we are, both as individuals and as a species. All that humanity has achieved, good and bad, came from the brain.
The rest of him was in good shape. A healthy guy in his 40s. Probably in better condition than me. But with his brain irreparably damaged, none of that meant anything.
Even after almost 20 years of doing this work, this sort of thing still reminds me how lucky I, and most of us, are – and to be grateful for what I have.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
He’s almost 10 years younger than me.
He’d been in the hospital for 3 weeks. The ICU room had been decorated, as many families do, with pictures of his life. His wedding. His kids. He and his wife dressed as Darth Vader and Princess Leia for a Halloween party. A few religious items.
He was off sedation. EEG didn’t show any seizures. Head CT just showed the extensive damage from his head injury. The neurosurgeons can evacuate clots and decrease intracranial pressure, but they can’t repair brain tissue.
His wife was long past the point of shock when I met with her. After 3 weeks, she understood what the new normal was and how the lives of both herself and their kids would never be the same. She held his hand at the bedside as we talked, asked me a few pointed questions, and then thanked me for coming in to see him.
For me, it was just another day on call. I walked back to the nurses station, got some coffee from the galley, and sat down to dictate a note. There are always other patients to see on the coverage list.
But it still reminds you.
The brain doesn’t weigh much, just 2-3 pounds; it’s about the size of your fists put together.
But it’s everything that we are, both as individuals and as a species. All that humanity has achieved, good and bad, came from the brain.
The rest of him was in good shape. A healthy guy in his 40s. Probably in better condition than me. But with his brain irreparably damaged, none of that meant anything.
Even after almost 20 years of doing this work, this sort of thing still reminds me how lucky I, and most of us, are – and to be grateful for what I have.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
He’s almost 10 years younger than me.
He’d been in the hospital for 3 weeks. The ICU room had been decorated, as many families do, with pictures of his life. His wedding. His kids. He and his wife dressed as Darth Vader and Princess Leia for a Halloween party. A few religious items.
He was off sedation. EEG didn’t show any seizures. Head CT just showed the extensive damage from his head injury. The neurosurgeons can evacuate clots and decrease intracranial pressure, but they can’t repair brain tissue.
His wife was long past the point of shock when I met with her. After 3 weeks, she understood what the new normal was and how the lives of both herself and their kids would never be the same. She held his hand at the bedside as we talked, asked me a few pointed questions, and then thanked me for coming in to see him.
For me, it was just another day on call. I walked back to the nurses station, got some coffee from the galley, and sat down to dictate a note. There are always other patients to see on the coverage list.
But it still reminds you.
The brain doesn’t weigh much, just 2-3 pounds; it’s about the size of your fists put together.
But it’s everything that we are, both as individuals and as a species. All that humanity has achieved, good and bad, came from the brain.
The rest of him was in good shape. A healthy guy in his 40s. Probably in better condition than me. But with his brain irreparably damaged, none of that meant anything.
Even after almost 20 years of doing this work, this sort of thing still reminds me how lucky I, and most of us, are – and to be grateful for what I have.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Hints of nivolumab efficacy seen in biliary tract cancers
BARCELONA – The immune checkpoint inhibitor nivolumab (Opdivo) shows activity against biliary tract cancers (BTC) that have progressed on prior systemic therapies, investigators report.
Among 27 patients with intra- and extrahepatic cholangiocarcinoma and cancers of the gallbladder for whom at least one prior line of therapy had failed, the overall response rate with nivolumab monotherapy was 18.5%, reported Richard Kim, MD of Moffitt Cancer Center, in Tampa.
“Nivolumab demonstrated clinical efficacy in BTC patients. It was very well tolerated, with few grade 3 or 4 adverse events,” he said at the European Society of Medical Oncology World Congress on Gastrointestinal Cancer.
The worldwide incidence of biliary tract cancers has grown over the last 4 decades.
“It is a very aggressive disease, with 5-year overall survival rate of advance disease of less than 2%,” he said.
The standard of care for first-line treatment of advanced disease is gemcitabine and cisplatin, but there is no standard treatment available for patients for whom first-line therapy fails.
Median survival of patients with biliary tract cancers who are receiving second- or third-line therapies is approximately 6-7 months, Dr. Kim said.
The rationale for using nivolumab in this setting comes from evidence suggesting that cholangiocarcinoma is related to dysregulated immunity, with carcinogenesis linked to autoimmune conditions such as primary sclerosing cholangitis, and to chronic parasitic infections.
“Immune regulatory protein PD-1 is upregulated more in intrahepatic cholangiocarcinoma tissues than in adjacent normal tissue, and patients with memory CD8 T cells had longer relapse-free survival and overall survival in extrahepatic cholangiocarcinoma after resection,” he explained.
To see whether the use of an immune checkpoint inhibitor could provide clinically meaningful benefit in patients with advance biliary tract cancers, the investigators conducted a phase 2, two-stage study. They first accrued 18 patients with histologically confirmed, treatment-refractory biliary tract malignancies and treated them with nivolumab 240 mg IV every 2 weeks for 16 weeks, followed by 480 mg IV every 4 weeks.
According to the study protocol, if one or more patients had a complete or partial response, additional patients would be enrolled. As of May 2018, 34 patients had been treated.
The median patient age was 64.5 years. Two-thirds of the patients (64.7%) had intrahepatic cholangiocarcinoma, 2.9% had extrahepatic cholangiocarcinoma, and 32.4% had tumors of the gallbladder.
Twenty patients were failed by their first-line therapies, and 14 were failed by two or more lines of therapy. All 34 received at least one dose of nivolumab.
Of this group, 10 patients remained on study at the time of Dr. Kim’s presentation. Fifteen were withdrawn for progressive disease according to Response Evaluation Criteria in Solid Tumors (RECIST) revision 1.1, and 9 due to clinical progression.
Of 27 patients evaluable for investigator-assessed overall responses – the primary endpoint – 5 patients (18.5%) had a partial response, and 11 (40.7%) had stable disease, for a disease-control rate of 59.3%. The remaining 11 evaluable patients had progressive disease.
“Of interest, of our five patients who had a partial response, three had a diagnosis of intrahepatic cholangiocarcinoma, and two had a diagnosis of a gallbladder tumor,” Dr. Kim said.
All five patients remained on treatment at the time of the presentation, with response duration ranging from 24 to 64 weeks. The median duration of response in these patients has not been reached.
Median progression-free survival for all 34 patients treated with at least one dose was 3.5 months. Overall survival with a median follow-up of 9.9 months has not been reached. The 6-months overall survival rate was 73.5%.
Approximately 20% of patients experienced grade 3 or 4 treatment-related adverse events. There were no grade 4 events and no treatment-related deaths.
The most common grade 3 events were hyponatremia in three patients (8.8%), and lymphopenia, colitis, and hyperbilirubinemia in one patient each (2.9%).
The investigators have collected tissues from all patients and plan to present data from biomarker studies at future meetings. Based on the results of this study, they plan to add 20 more patients to the phase 2 trial to confirm efficacy of nivolumab in this setting.
BARCELONA – The immune checkpoint inhibitor nivolumab (Opdivo) shows activity against biliary tract cancers (BTC) that have progressed on prior systemic therapies, investigators report.
Among 27 patients with intra- and extrahepatic cholangiocarcinoma and cancers of the gallbladder for whom at least one prior line of therapy had failed, the overall response rate with nivolumab monotherapy was 18.5%, reported Richard Kim, MD of Moffitt Cancer Center, in Tampa.
“Nivolumab demonstrated clinical efficacy in BTC patients. It was very well tolerated, with few grade 3 or 4 adverse events,” he said at the European Society of Medical Oncology World Congress on Gastrointestinal Cancer.
The worldwide incidence of biliary tract cancers has grown over the last 4 decades.
“It is a very aggressive disease, with 5-year overall survival rate of advance disease of less than 2%,” he said.
The standard of care for first-line treatment of advanced disease is gemcitabine and cisplatin, but there is no standard treatment available for patients for whom first-line therapy fails.
Median survival of patients with biliary tract cancers who are receiving second- or third-line therapies is approximately 6-7 months, Dr. Kim said.
The rationale for using nivolumab in this setting comes from evidence suggesting that cholangiocarcinoma is related to dysregulated immunity, with carcinogenesis linked to autoimmune conditions such as primary sclerosing cholangitis, and to chronic parasitic infections.
“Immune regulatory protein PD-1 is upregulated more in intrahepatic cholangiocarcinoma tissues than in adjacent normal tissue, and patients with memory CD8 T cells had longer relapse-free survival and overall survival in extrahepatic cholangiocarcinoma after resection,” he explained.
To see whether the use of an immune checkpoint inhibitor could provide clinically meaningful benefit in patients with advance biliary tract cancers, the investigators conducted a phase 2, two-stage study. They first accrued 18 patients with histologically confirmed, treatment-refractory biliary tract malignancies and treated them with nivolumab 240 mg IV every 2 weeks for 16 weeks, followed by 480 mg IV every 4 weeks.
According to the study protocol, if one or more patients had a complete or partial response, additional patients would be enrolled. As of May 2018, 34 patients had been treated.
The median patient age was 64.5 years. Two-thirds of the patients (64.7%) had intrahepatic cholangiocarcinoma, 2.9% had extrahepatic cholangiocarcinoma, and 32.4% had tumors of the gallbladder.
Twenty patients were failed by their first-line therapies, and 14 were failed by two or more lines of therapy. All 34 received at least one dose of nivolumab.
Of this group, 10 patients remained on study at the time of Dr. Kim’s presentation. Fifteen were withdrawn for progressive disease according to Response Evaluation Criteria in Solid Tumors (RECIST) revision 1.1, and 9 due to clinical progression.
Of 27 patients evaluable for investigator-assessed overall responses – the primary endpoint – 5 patients (18.5%) had a partial response, and 11 (40.7%) had stable disease, for a disease-control rate of 59.3%. The remaining 11 evaluable patients had progressive disease.
“Of interest, of our five patients who had a partial response, three had a diagnosis of intrahepatic cholangiocarcinoma, and two had a diagnosis of a gallbladder tumor,” Dr. Kim said.
All five patients remained on treatment at the time of the presentation, with response duration ranging from 24 to 64 weeks. The median duration of response in these patients has not been reached.
Median progression-free survival for all 34 patients treated with at least one dose was 3.5 months. Overall survival with a median follow-up of 9.9 months has not been reached. The 6-months overall survival rate was 73.5%.
Approximately 20% of patients experienced grade 3 or 4 treatment-related adverse events. There were no grade 4 events and no treatment-related deaths.
The most common grade 3 events were hyponatremia in three patients (8.8%), and lymphopenia, colitis, and hyperbilirubinemia in one patient each (2.9%).
The investigators have collected tissues from all patients and plan to present data from biomarker studies at future meetings. Based on the results of this study, they plan to add 20 more patients to the phase 2 trial to confirm efficacy of nivolumab in this setting.
BARCELONA – The immune checkpoint inhibitor nivolumab (Opdivo) shows activity against biliary tract cancers (BTC) that have progressed on prior systemic therapies, investigators report.
Among 27 patients with intra- and extrahepatic cholangiocarcinoma and cancers of the gallbladder for whom at least one prior line of therapy had failed, the overall response rate with nivolumab monotherapy was 18.5%, reported Richard Kim, MD of Moffitt Cancer Center, in Tampa.
“Nivolumab demonstrated clinical efficacy in BTC patients. It was very well tolerated, with few grade 3 or 4 adverse events,” he said at the European Society of Medical Oncology World Congress on Gastrointestinal Cancer.
The worldwide incidence of biliary tract cancers has grown over the last 4 decades.
“It is a very aggressive disease, with 5-year overall survival rate of advance disease of less than 2%,” he said.
The standard of care for first-line treatment of advanced disease is gemcitabine and cisplatin, but there is no standard treatment available for patients for whom first-line therapy fails.
Median survival of patients with biliary tract cancers who are receiving second- or third-line therapies is approximately 6-7 months, Dr. Kim said.
The rationale for using nivolumab in this setting comes from evidence suggesting that cholangiocarcinoma is related to dysregulated immunity, with carcinogenesis linked to autoimmune conditions such as primary sclerosing cholangitis, and to chronic parasitic infections.
“Immune regulatory protein PD-1 is upregulated more in intrahepatic cholangiocarcinoma tissues than in adjacent normal tissue, and patients with memory CD8 T cells had longer relapse-free survival and overall survival in extrahepatic cholangiocarcinoma after resection,” he explained.
To see whether the use of an immune checkpoint inhibitor could provide clinically meaningful benefit in patients with advance biliary tract cancers, the investigators conducted a phase 2, two-stage study. They first accrued 18 patients with histologically confirmed, treatment-refractory biliary tract malignancies and treated them with nivolumab 240 mg IV every 2 weeks for 16 weeks, followed by 480 mg IV every 4 weeks.
According to the study protocol, if one or more patients had a complete or partial response, additional patients would be enrolled. As of May 2018, 34 patients had been treated.
The median patient age was 64.5 years. Two-thirds of the patients (64.7%) had intrahepatic cholangiocarcinoma, 2.9% had extrahepatic cholangiocarcinoma, and 32.4% had tumors of the gallbladder.
Twenty patients were failed by their first-line therapies, and 14 were failed by two or more lines of therapy. All 34 received at least one dose of nivolumab.
Of this group, 10 patients remained on study at the time of Dr. Kim’s presentation. Fifteen were withdrawn for progressive disease according to Response Evaluation Criteria in Solid Tumors (RECIST) revision 1.1, and 9 due to clinical progression.
Of 27 patients evaluable for investigator-assessed overall responses – the primary endpoint – 5 patients (18.5%) had a partial response, and 11 (40.7%) had stable disease, for a disease-control rate of 59.3%. The remaining 11 evaluable patients had progressive disease.
“Of interest, of our five patients who had a partial response, three had a diagnosis of intrahepatic cholangiocarcinoma, and two had a diagnosis of a gallbladder tumor,” Dr. Kim said.
All five patients remained on treatment at the time of the presentation, with response duration ranging from 24 to 64 weeks. The median duration of response in these patients has not been reached.
Median progression-free survival for all 34 patients treated with at least one dose was 3.5 months. Overall survival with a median follow-up of 9.9 months has not been reached. The 6-months overall survival rate was 73.5%.
Approximately 20% of patients experienced grade 3 or 4 treatment-related adverse events. There were no grade 4 events and no treatment-related deaths.
The most common grade 3 events were hyponatremia in three patients (8.8%), and lymphopenia, colitis, and hyperbilirubinemia in one patient each (2.9%).
The investigators have collected tissues from all patients and plan to present data from biomarker studies at future meetings. Based on the results of this study, they plan to add 20 more patients to the phase 2 trial to confirm efficacy of nivolumab in this setting.
REPORTING FROM ESMO GI 2018
Key clinical point: Nivolumab monotherapy appears to have activity in treatment-refractory biliary tract cancers.
Major finding: Five of 27 evaluable patients had partial responses to nivolumab.
Study details: Two-stage phase 2 trial of 34 patients with intrahepatic or extrahepatic cholangiocarcinomas or gallbladder tumors.
Disclosures: Bristol-Myers Squibb sponsored the study. Dr. Kim disclosed honoraria and institutional research funding from that company and others.
Source: Kim R et al. European Society of Medical Oncology World Congress on Gastrointestinal Cancer. Abstract O-009.
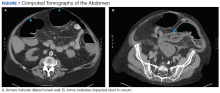
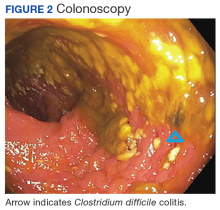
Clostridium difficile Colitis in a Patient With Abdominal Distention, Pain, and Severe Constipation
A 66-year-old man with steroid-dependent asthma, well-controlled diabetes mellitus (DM), and chronic pain on hospice presented to Georg
On presentation, the patient reported taking the following medications: daily oxycodone 20 to 30 mg, tramadol 200 mg, gabapentin 1,200 mg, and frequent doses of morphine concentrate. Due to episodes of constipation and diarrhea, the veteran had recently self-discontinued taking stool softener (Senna plus). One month prior to this admission, the patient was enrolled in hospice service by his primary physician for severe COPD due to chronic hypoxic respiratory failure and worsening frailty. His baseline oxygen requirement was 4 to 5 L of supplemental oxygen with continued dyspnea upon any ambulation. The patient reported frequent falls prior to admission. Despite chronic steroid use, the patient’s DM was well controlled with metformin His hemoglobin A1c ranged from 6.0 to 7.8.
The patient was supine and appeared to be uncomfortable but not in acute distress on exam. His body habitus was Cushingoid, and he appeared much older than his stated age. His vitals were as follows: temperature 100.2°F, heart rate of 104 beats per minute, blood pressure of 98/56 mm Hg, and 95% oxygen on 4L nasal cannula (baseline 4-5L). A respiratory exam revealed distant breath sounds without wheeze, rhonchi, or rales, and a cardiac exam revealed no murmurs. He was in sinus rhythm with tachycardia. The abdomen was obese with purple straie and markedly distended. On percussion, his abdomen was tympanic with tinkling bowel sounds. He had no rebound tenderness, peritoneal signs, or fluid wave.
Laboratory results revealed a white blood cell (WBC) count of 13,790 cells/μL with a neutrophilic shift of 82.0, and an elevated creatinine of 2.16 mg/dL up from a baseline of 1.12 mg/dL. The chemistry panel was abnormal with a 125 mmol/L sodium (reference range 137-145 mmol/L).
Diagnosis
On admission, the authors’ differential diagnosis included fecal impaction with large bowel obstruction, colitis, narcotic induced ileus, dehydration leading to severe constipation, and delayed gastric emptying secondary to long-standing DM. Ciprofloxacin and metronidazole antibiotics were initiated out of concern for possible colitis and potential bacterial translocation. Intravenous fluids were initiated, and the patient was instructed to have nothing by mouth (NPO) aside from the antibiotics. All opioids, including tramadol, were held. Out of concern for narcotic-induced constipation, a dose of methylnaltrexone to induce stooling was administered but had no effect on the constipation.
The gastroenterology department was consulted for a possible endoscopy to aid in decompression of the sigmoid. However, given the amount of distention and concern for perforation with endoscopy, the patient did not undergo endoscopy on admission. The patient remained afebrile on hospital day 3, and all antibiotics were discontinued. His WBC count normalized with complete resolution of the kidney injury. Antibiotic stewardship and infectious disease consults at George E. Wahlen VAMC reviewed the case and supported the decision to stop all antibiotics since it was not clear whether or not the patient was infected. Despite aggressive bowel care that included a nasogastric tube for large-volume polyethylene glycol and lactulose, various enemas and suppositories, the patient remained constipated.
On hospital day 5, still NPO, the patient had several bilious liquid stools that appeared to have a sediment quality to them. His abdomen remained distended, tympanic, and uncomfortable to palpation., He was examined frequently due to concern for possible perforation. On hospital day 8, gastroenterology reevaluated the need for endoscopy and proceeded with a flexible sigmoidoscopy.
Polymerase chain reaction analysis of the colonoscopy stool samples were positive for Clostridium difficile (C difficile). The patient was started on IV metronidazole and oral vancomycin. His diet advanced and over the next few days he began stooling. He was subsequently discharged back to an extended care facility for rehabilitation. During this hospitalization, he made it clear he wished to be discharged from hospice services. He wanted to regain his strength through aggressive physical and occupational therapies.
Conclusion
Typical clinical manifestations of fulminant colitis include fever, diarrhea, abdominal pain, distention, and frequently WBC counts > 20,000 cells/μL. However, C difficile colitis, also known as pseudomembranous colitis, occasionally can present as an acute ileus, with little or no diarrhea.1 This veteran had several risk factors for C difficile infection, which included long-term residence in an extended care facility, frequent asthma exacerbations that required antibiotics, severe chronic disease, aged > 65 years,and ciprofloxacin given the first 3 days of this hospitalization.2 Until the endoscopy results were presented, no one on the patient’s care team, including gastroenterology and infectious disease, had included an infectious etiology in the differential diagnosis. This case reinforces the need to broaden differential diagnoses and look beyond assumptions that opioids without an adequate bowel regime were the cause. Avoiding anchoring heuristics can be a challenge as this case demonstrates.
1. Kawsar HI, Gopal KV, Shahnewaz J, Daw HA. Constipation in Clostridium difficile infection. BMJ Case Rep. 2012;2012: pii: bcr0220125938.
2. Leffler D, Lamont T. Clostridium difficile infection. N Engl J Med. 2015;372(16)1539-1548.
A 66-year-old man with steroid-dependent asthma, well-controlled diabetes mellitus (DM), and chronic pain on hospice presented to Georg
On presentation, the patient reported taking the following medications: daily oxycodone 20 to 30 mg, tramadol 200 mg, gabapentin 1,200 mg, and frequent doses of morphine concentrate. Due to episodes of constipation and diarrhea, the veteran had recently self-discontinued taking stool softener (Senna plus). One month prior to this admission, the patient was enrolled in hospice service by his primary physician for severe COPD due to chronic hypoxic respiratory failure and worsening frailty. His baseline oxygen requirement was 4 to 5 L of supplemental oxygen with continued dyspnea upon any ambulation. The patient reported frequent falls prior to admission. Despite chronic steroid use, the patient’s DM was well controlled with metformin His hemoglobin A1c ranged from 6.0 to 7.8.
The patient was supine and appeared to be uncomfortable but not in acute distress on exam. His body habitus was Cushingoid, and he appeared much older than his stated age. His vitals were as follows: temperature 100.2°F, heart rate of 104 beats per minute, blood pressure of 98/56 mm Hg, and 95% oxygen on 4L nasal cannula (baseline 4-5L). A respiratory exam revealed distant breath sounds without wheeze, rhonchi, or rales, and a cardiac exam revealed no murmurs. He was in sinus rhythm with tachycardia. The abdomen was obese with purple straie and markedly distended. On percussion, his abdomen was tympanic with tinkling bowel sounds. He had no rebound tenderness, peritoneal signs, or fluid wave.
Laboratory results revealed a white blood cell (WBC) count of 13,790 cells/μL with a neutrophilic shift of 82.0, and an elevated creatinine of 2.16 mg/dL up from a baseline of 1.12 mg/dL. The chemistry panel was abnormal with a 125 mmol/L sodium (reference range 137-145 mmol/L).
Diagnosis
On admission, the authors’ differential diagnosis included fecal impaction with large bowel obstruction, colitis, narcotic induced ileus, dehydration leading to severe constipation, and delayed gastric emptying secondary to long-standing DM. Ciprofloxacin and metronidazole antibiotics were initiated out of concern for possible colitis and potential bacterial translocation. Intravenous fluids were initiated, and the patient was instructed to have nothing by mouth (NPO) aside from the antibiotics. All opioids, including tramadol, were held. Out of concern for narcotic-induced constipation, a dose of methylnaltrexone to induce stooling was administered but had no effect on the constipation.
The gastroenterology department was consulted for a possible endoscopy to aid in decompression of the sigmoid. However, given the amount of distention and concern for perforation with endoscopy, the patient did not undergo endoscopy on admission. The patient remained afebrile on hospital day 3, and all antibiotics were discontinued. His WBC count normalized with complete resolution of the kidney injury. Antibiotic stewardship and infectious disease consults at George E. Wahlen VAMC reviewed the case and supported the decision to stop all antibiotics since it was not clear whether or not the patient was infected. Despite aggressive bowel care that included a nasogastric tube for large-volume polyethylene glycol and lactulose, various enemas and suppositories, the patient remained constipated.
On hospital day 5, still NPO, the patient had several bilious liquid stools that appeared to have a sediment quality to them. His abdomen remained distended, tympanic, and uncomfortable to palpation., He was examined frequently due to concern for possible perforation. On hospital day 8, gastroenterology reevaluated the need for endoscopy and proceeded with a flexible sigmoidoscopy.
Polymerase chain reaction analysis of the colonoscopy stool samples were positive for Clostridium difficile (C difficile). The patient was started on IV metronidazole and oral vancomycin. His diet advanced and over the next few days he began stooling. He was subsequently discharged back to an extended care facility for rehabilitation. During this hospitalization, he made it clear he wished to be discharged from hospice services. He wanted to regain his strength through aggressive physical and occupational therapies.
Conclusion
Typical clinical manifestations of fulminant colitis include fever, diarrhea, abdominal pain, distention, and frequently WBC counts > 20,000 cells/μL. However, C difficile colitis, also known as pseudomembranous colitis, occasionally can present as an acute ileus, with little or no diarrhea.1 This veteran had several risk factors for C difficile infection, which included long-term residence in an extended care facility, frequent asthma exacerbations that required antibiotics, severe chronic disease, aged > 65 years,and ciprofloxacin given the first 3 days of this hospitalization.2 Until the endoscopy results were presented, no one on the patient’s care team, including gastroenterology and infectious disease, had included an infectious etiology in the differential diagnosis. This case reinforces the need to broaden differential diagnoses and look beyond assumptions that opioids without an adequate bowel regime were the cause. Avoiding anchoring heuristics can be a challenge as this case demonstrates.
A 66-year-old man with steroid-dependent asthma, well-controlled diabetes mellitus (DM), and chronic pain on hospice presented to Georg
On presentation, the patient reported taking the following medications: daily oxycodone 20 to 30 mg, tramadol 200 mg, gabapentin 1,200 mg, and frequent doses of morphine concentrate. Due to episodes of constipation and diarrhea, the veteran had recently self-discontinued taking stool softener (Senna plus). One month prior to this admission, the patient was enrolled in hospice service by his primary physician for severe COPD due to chronic hypoxic respiratory failure and worsening frailty. His baseline oxygen requirement was 4 to 5 L of supplemental oxygen with continued dyspnea upon any ambulation. The patient reported frequent falls prior to admission. Despite chronic steroid use, the patient’s DM was well controlled with metformin His hemoglobin A1c ranged from 6.0 to 7.8.
The patient was supine and appeared to be uncomfortable but not in acute distress on exam. His body habitus was Cushingoid, and he appeared much older than his stated age. His vitals were as follows: temperature 100.2°F, heart rate of 104 beats per minute, blood pressure of 98/56 mm Hg, and 95% oxygen on 4L nasal cannula (baseline 4-5L). A respiratory exam revealed distant breath sounds without wheeze, rhonchi, or rales, and a cardiac exam revealed no murmurs. He was in sinus rhythm with tachycardia. The abdomen was obese with purple straie and markedly distended. On percussion, his abdomen was tympanic with tinkling bowel sounds. He had no rebound tenderness, peritoneal signs, or fluid wave.
Laboratory results revealed a white blood cell (WBC) count of 13,790 cells/μL with a neutrophilic shift of 82.0, and an elevated creatinine of 2.16 mg/dL up from a baseline of 1.12 mg/dL. The chemistry panel was abnormal with a 125 mmol/L sodium (reference range 137-145 mmol/L).
Diagnosis
On admission, the authors’ differential diagnosis included fecal impaction with large bowel obstruction, colitis, narcotic induced ileus, dehydration leading to severe constipation, and delayed gastric emptying secondary to long-standing DM. Ciprofloxacin and metronidazole antibiotics were initiated out of concern for possible colitis and potential bacterial translocation. Intravenous fluids were initiated, and the patient was instructed to have nothing by mouth (NPO) aside from the antibiotics. All opioids, including tramadol, were held. Out of concern for narcotic-induced constipation, a dose of methylnaltrexone to induce stooling was administered but had no effect on the constipation.
The gastroenterology department was consulted for a possible endoscopy to aid in decompression of the sigmoid. However, given the amount of distention and concern for perforation with endoscopy, the patient did not undergo endoscopy on admission. The patient remained afebrile on hospital day 3, and all antibiotics were discontinued. His WBC count normalized with complete resolution of the kidney injury. Antibiotic stewardship and infectious disease consults at George E. Wahlen VAMC reviewed the case and supported the decision to stop all antibiotics since it was not clear whether or not the patient was infected. Despite aggressive bowel care that included a nasogastric tube for large-volume polyethylene glycol and lactulose, various enemas and suppositories, the patient remained constipated.
On hospital day 5, still NPO, the patient had several bilious liquid stools that appeared to have a sediment quality to them. His abdomen remained distended, tympanic, and uncomfortable to palpation., He was examined frequently due to concern for possible perforation. On hospital day 8, gastroenterology reevaluated the need for endoscopy and proceeded with a flexible sigmoidoscopy.
Polymerase chain reaction analysis of the colonoscopy stool samples were positive for Clostridium difficile (C difficile). The patient was started on IV metronidazole and oral vancomycin. His diet advanced and over the next few days he began stooling. He was subsequently discharged back to an extended care facility for rehabilitation. During this hospitalization, he made it clear he wished to be discharged from hospice services. He wanted to regain his strength through aggressive physical and occupational therapies.
Conclusion
Typical clinical manifestations of fulminant colitis include fever, diarrhea, abdominal pain, distention, and frequently WBC counts > 20,000 cells/μL. However, C difficile colitis, also known as pseudomembranous colitis, occasionally can present as an acute ileus, with little or no diarrhea.1 This veteran had several risk factors for C difficile infection, which included long-term residence in an extended care facility, frequent asthma exacerbations that required antibiotics, severe chronic disease, aged > 65 years,and ciprofloxacin given the first 3 days of this hospitalization.2 Until the endoscopy results were presented, no one on the patient’s care team, including gastroenterology and infectious disease, had included an infectious etiology in the differential diagnosis. This case reinforces the need to broaden differential diagnoses and look beyond assumptions that opioids without an adequate bowel regime were the cause. Avoiding anchoring heuristics can be a challenge as this case demonstrates.
1. Kawsar HI, Gopal KV, Shahnewaz J, Daw HA. Constipation in Clostridium difficile infection. BMJ Case Rep. 2012;2012: pii: bcr0220125938.
2. Leffler D, Lamont T. Clostridium difficile infection. N Engl J Med. 2015;372(16)1539-1548.
1. Kawsar HI, Gopal KV, Shahnewaz J, Daw HA. Constipation in Clostridium difficile infection. BMJ Case Rep. 2012;2012: pii: bcr0220125938.
2. Leffler D, Lamont T. Clostridium difficile infection. N Engl J Med. 2015;372(16)1539-1548.