User login
GO approved to treat AML in Europe
The European Commission has authorized use of gemtuzumab ozogamicin (GO, Mylotarg™) as a treatment for patients with acute myeloid leukemia (AML).
GO is now approved for use in combination with daunorubicin and cytarabine to treat patients age 15 and older who have previously untreated, de novo, CD33-positive AML, not including acute promyelocytic leukemia.
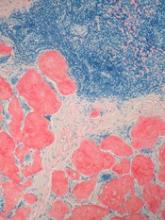
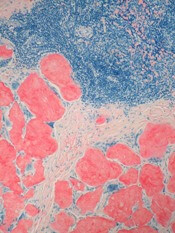
GO is an antibody-drug conjugate composed of the cytotoxic agent calicheamicin attached to a monoclonal antibody targeting CD33, an antigen expressed on the surface of myeloblasts in up to 90% of AML patients.
When GO binds to the CD33 antigen on the cell surface, it is absorbed into the cell, and calicheamicin is released, causing cell death.
Previous rejection
The European Commission’s approval of GO follows a positive opinion from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP). In February, the CHMP recommended that GO receive marketing authorization for the aforementioned indication.
However, the CHMP previously issued a negative opinion of GO (first in 2007, confirmed in 2008), saying the drug should not receive marketing authorization.
The proposed indication for GO at that time was as re-induction treatment in adults with CD33-positive AML in first relapse who were not candidates for other intensive re-induction chemotherapy regimens and were either older than 60 or had a duration of first remission lasting less than 12 months.
The CHMP said there was insufficient evidence to establish the effectiveness of GO in AML, and the drug’s benefits did not outweigh its risks.
Phase 3 trial
The current marketing authorization application for GO is supported by data from an investigator-led, phase 3, randomized trial known as ALFA-0701. Updated results from this trial are available in the US prescribing information for GO.
ALFA-0701 included 271 patients with newly diagnosed, de novo AML who were 50 to 70 years of age.
Patients were randomized (1:1) to receive induction consisting of daunorubicin (60 mg/m2 on days 1 to 3) and cytarabine (200 mg/m2 on days 1 to 7) with (n=135) or without (n=136) GO at 3 mg/m2 (up to a maximum of 1 vial) on days 1, 4, and 7. Patients who did not achieve a response after first induction could receive a second induction with daunorubicin and cytarabine alone.
Patients with a response received consolidation therapy with 2 courses of treatment including daunorubicin (60 mg/m2 on day 1 of first consolidation course; 60 mg/m2 on days 1 and 2 of second consolidation course) and cytarabine (1 g/m2 every 12 hours on days 1 to 4) with or without GO at 3 mg/m2 (up to a maximum of 1 vial) on day 1 according to their initial randomization.
Patients who achieved remission were also eligible for allogeneic transplant. An interval of at least 2 months between the last dose of GO and transplant was recommended.
Baseline characteristics were largely well balanced between the treatment arms, but there was a higher percentage of males in the GO arm than the control arm—55% and 44%, respectively.
The study’s primary endpoint was event-free survival. The median event-free survival was 17.3 months in the GO arm and 9.5 months in the control arm (hazard ratio=0.56; 95% CI: 0.42-0.76; P<0.001).
There was no significant difference in overall survival between the treatment arms. (Updated overall survival data have not been released).
All patients in this trial developed severe neutropenia, thrombocytopenia, and anemia. However, the incidence of prolonged, grade 3–4 thrombocytopenia in the absence of active leukemia was higher in the GO arm.
Treatment-emergent adverse events (AEs) considered most important for understanding the safety profile of GO were hemorrhage, veno-occlusive liver disease (VOD), and severe infections.
Treatment discontinuation due to any AE occurred in 31% of patients in the GO arm and 7% of those in the control arm. The most frequent AEs leading to discontinuation for patients on GO were thrombocytopenia (15%), VOD (3%), and septic shock (2%).
Fatal AEs occurred in 8 patients (6%) in the GO arm and 3 (2%) in the control arm. In the GO arm, 3 patients died of VOD, 4 died of hemorrhage-related events, and 1 died of a suspected cardiac cause. All 3 fatal AEs in the control arm were sepsis.
The European Commission has authorized use of gemtuzumab ozogamicin (GO, Mylotarg™) as a treatment for patients with acute myeloid leukemia (AML).
GO is now approved for use in combination with daunorubicin and cytarabine to treat patients age 15 and older who have previously untreated, de novo, CD33-positive AML, not including acute promyelocytic leukemia.
GO is an antibody-drug conjugate composed of the cytotoxic agent calicheamicin attached to a monoclonal antibody targeting CD33, an antigen expressed on the surface of myeloblasts in up to 90% of AML patients.
When GO binds to the CD33 antigen on the cell surface, it is absorbed into the cell, and calicheamicin is released, causing cell death.
Previous rejection
The European Commission’s approval of GO follows a positive opinion from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP). In February, the CHMP recommended that GO receive marketing authorization for the aforementioned indication.
However, the CHMP previously issued a negative opinion of GO (first in 2007, confirmed in 2008), saying the drug should not receive marketing authorization.
The proposed indication for GO at that time was as re-induction treatment in adults with CD33-positive AML in first relapse who were not candidates for other intensive re-induction chemotherapy regimens and were either older than 60 or had a duration of first remission lasting less than 12 months.
The CHMP said there was insufficient evidence to establish the effectiveness of GO in AML, and the drug’s benefits did not outweigh its risks.
Phase 3 trial
The current marketing authorization application for GO is supported by data from an investigator-led, phase 3, randomized trial known as ALFA-0701. Updated results from this trial are available in the US prescribing information for GO.
ALFA-0701 included 271 patients with newly diagnosed, de novo AML who were 50 to 70 years of age.
Patients were randomized (1:1) to receive induction consisting of daunorubicin (60 mg/m2 on days 1 to 3) and cytarabine (200 mg/m2 on days 1 to 7) with (n=135) or without (n=136) GO at 3 mg/m2 (up to a maximum of 1 vial) on days 1, 4, and 7. Patients who did not achieve a response after first induction could receive a second induction with daunorubicin and cytarabine alone.
Patients with a response received consolidation therapy with 2 courses of treatment including daunorubicin (60 mg/m2 on day 1 of first consolidation course; 60 mg/m2 on days 1 and 2 of second consolidation course) and cytarabine (1 g/m2 every 12 hours on days 1 to 4) with or without GO at 3 mg/m2 (up to a maximum of 1 vial) on day 1 according to their initial randomization.
Patients who achieved remission were also eligible for allogeneic transplant. An interval of at least 2 months between the last dose of GO and transplant was recommended.
Baseline characteristics were largely well balanced between the treatment arms, but there was a higher percentage of males in the GO arm than the control arm—55% and 44%, respectively.
The study’s primary endpoint was event-free survival. The median event-free survival was 17.3 months in the GO arm and 9.5 months in the control arm (hazard ratio=0.56; 95% CI: 0.42-0.76; P<0.001).
There was no significant difference in overall survival between the treatment arms. (Updated overall survival data have not been released).
All patients in this trial developed severe neutropenia, thrombocytopenia, and anemia. However, the incidence of prolonged, grade 3–4 thrombocytopenia in the absence of active leukemia was higher in the GO arm.
Treatment-emergent adverse events (AEs) considered most important for understanding the safety profile of GO were hemorrhage, veno-occlusive liver disease (VOD), and severe infections.
Treatment discontinuation due to any AE occurred in 31% of patients in the GO arm and 7% of those in the control arm. The most frequent AEs leading to discontinuation for patients on GO were thrombocytopenia (15%), VOD (3%), and septic shock (2%).
Fatal AEs occurred in 8 patients (6%) in the GO arm and 3 (2%) in the control arm. In the GO arm, 3 patients died of VOD, 4 died of hemorrhage-related events, and 1 died of a suspected cardiac cause. All 3 fatal AEs in the control arm were sepsis.
The European Commission has authorized use of gemtuzumab ozogamicin (GO, Mylotarg™) as a treatment for patients with acute myeloid leukemia (AML).
GO is now approved for use in combination with daunorubicin and cytarabine to treat patients age 15 and older who have previously untreated, de novo, CD33-positive AML, not including acute promyelocytic leukemia.
GO is an antibody-drug conjugate composed of the cytotoxic agent calicheamicin attached to a monoclonal antibody targeting CD33, an antigen expressed on the surface of myeloblasts in up to 90% of AML patients.
When GO binds to the CD33 antigen on the cell surface, it is absorbed into the cell, and calicheamicin is released, causing cell death.
Previous rejection
The European Commission’s approval of GO follows a positive opinion from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP). In February, the CHMP recommended that GO receive marketing authorization for the aforementioned indication.
However, the CHMP previously issued a negative opinion of GO (first in 2007, confirmed in 2008), saying the drug should not receive marketing authorization.
The proposed indication for GO at that time was as re-induction treatment in adults with CD33-positive AML in first relapse who were not candidates for other intensive re-induction chemotherapy regimens and were either older than 60 or had a duration of first remission lasting less than 12 months.
The CHMP said there was insufficient evidence to establish the effectiveness of GO in AML, and the drug’s benefits did not outweigh its risks.
Phase 3 trial
The current marketing authorization application for GO is supported by data from an investigator-led, phase 3, randomized trial known as ALFA-0701. Updated results from this trial are available in the US prescribing information for GO.
ALFA-0701 included 271 patients with newly diagnosed, de novo AML who were 50 to 70 years of age.
Patients were randomized (1:1) to receive induction consisting of daunorubicin (60 mg/m2 on days 1 to 3) and cytarabine (200 mg/m2 on days 1 to 7) with (n=135) or without (n=136) GO at 3 mg/m2 (up to a maximum of 1 vial) on days 1, 4, and 7. Patients who did not achieve a response after first induction could receive a second induction with daunorubicin and cytarabine alone.
Patients with a response received consolidation therapy with 2 courses of treatment including daunorubicin (60 mg/m2 on day 1 of first consolidation course; 60 mg/m2 on days 1 and 2 of second consolidation course) and cytarabine (1 g/m2 every 12 hours on days 1 to 4) with or without GO at 3 mg/m2 (up to a maximum of 1 vial) on day 1 according to their initial randomization.
Patients who achieved remission were also eligible for allogeneic transplant. An interval of at least 2 months between the last dose of GO and transplant was recommended.
Baseline characteristics were largely well balanced between the treatment arms, but there was a higher percentage of males in the GO arm than the control arm—55% and 44%, respectively.
The study’s primary endpoint was event-free survival. The median event-free survival was 17.3 months in the GO arm and 9.5 months in the control arm (hazard ratio=0.56; 95% CI: 0.42-0.76; P<0.001).
There was no significant difference in overall survival between the treatment arms. (Updated overall survival data have not been released).
All patients in this trial developed severe neutropenia, thrombocytopenia, and anemia. However, the incidence of prolonged, grade 3–4 thrombocytopenia in the absence of active leukemia was higher in the GO arm.
Treatment-emergent adverse events (AEs) considered most important for understanding the safety profile of GO were hemorrhage, veno-occlusive liver disease (VOD), and severe infections.
Treatment discontinuation due to any AE occurred in 31% of patients in the GO arm and 7% of those in the control arm. The most frequent AEs leading to discontinuation for patients on GO were thrombocytopenia (15%), VOD (3%), and septic shock (2%).
Fatal AEs occurred in 8 patients (6%) in the GO arm and 3 (2%) in the control arm. In the GO arm, 3 patients died of VOD, 4 died of hemorrhage-related events, and 1 died of a suspected cardiac cause. All 3 fatal AEs in the control arm were sepsis.
Exercise linked to risk of death in cancer patients
CHICAGO—Researchers have identified a link between habitual physical activity (PA) and mortality among cancer patients.
Engaging in regular PA, both pre- and post-diagnosis, was associated with a significantly lower risk of death for the entire population studied and for patients with 8 specific types of cancer.
However, the association was not significant for patients with other cancer types, including hematologic malignancies.
Rikki Cannioto, PhD, of Roswell Park Comprehensive Cancer Center in Buffalo, New York, and her colleagues presented these findings at the AACR Annual Meeting 2018 (abstract 5254*).
The researchers examined the association between habitual PA and outcomes in 5807 cancer patients enrolled in the Data Bank and BioRepository at Roswell Park between 2003 and 2016.
The population was 54.8% female and 93% white. The average age at diagnosis was 60.6 years.
The researchers looked at patterns of PA over time, from the decade before the cancer was diagnosed and continuing for up to 1 year after diagnosis.
Patients who engaged in regular, moderate- to vigorous-intensity PA (such as walking, running, aerobics, or other cardiovascular exercise) both before and after their diagnosis were considered habitually active, whereas those who did not exercise regularly were considered habitually inactive.
Overall, 52% of patients reported habitual activity, and 19% reported habitual inactivity. Twenty-three percent of patients said their activity level decreased after diagnosis, and 6% said their activity level increased.
Patients were followed through January 31, 2018. The median time to follow-up was 53 months, and 33.7% of patients (n=1956) died during the follow-up period.
Results
The researchers found that patients who were active before and after diagnosis were 40% more likely to survive than those who were habitually inactive (P<0.001). Habitually inactive patients had a 66% increased risk of mortality compared to active patients.
The habitually active patients had a 37-month mean survival advantage over the inactive patients.
In addition, patients whose activity level increased after diagnosis had a 25% lower risk of death than patients who remained inactive after diagnosis.
The researchers observed a significant (P<0.05) association between habitual PA and decreased mortality in patients with breast, colon, prostate, bladder, endometrial, ovarian, esophageal, and skin cancers.
However, the association between PA and mortality was not significant for patients with hematologic malignancies (P=0.59) or kidney, liver, lung, pancreas, stomach, or “other” cancers.
The researchers said the associations between habitual PA and decreased mortality remained consistent regardless of a patient’s sex, tumor stage, smoking status, or body mass index.
“[W]hen it comes to exercise, something is better than nothing, but regular, weekly exercise seems to really make a difference,” Dr Cannioto said.
“In fact, patients who were physically active 3 or 4 days a week experienced an even greater benefit than those who exercised daily, and patients who had only 1 or 2 days of regular activity per week did nearly as well. This is particularly encouraging, as cancer patients and survivors can be overwhelmed by current physical activity recommendations.”
*Information in the abstract differs from the presentation.
CHICAGO—Researchers have identified a link between habitual physical activity (PA) and mortality among cancer patients.
Engaging in regular PA, both pre- and post-diagnosis, was associated with a significantly lower risk of death for the entire population studied and for patients with 8 specific types of cancer.
However, the association was not significant for patients with other cancer types, including hematologic malignancies.
Rikki Cannioto, PhD, of Roswell Park Comprehensive Cancer Center in Buffalo, New York, and her colleagues presented these findings at the AACR Annual Meeting 2018 (abstract 5254*).
The researchers examined the association between habitual PA and outcomes in 5807 cancer patients enrolled in the Data Bank and BioRepository at Roswell Park between 2003 and 2016.
The population was 54.8% female and 93% white. The average age at diagnosis was 60.6 years.
The researchers looked at patterns of PA over time, from the decade before the cancer was diagnosed and continuing for up to 1 year after diagnosis.
Patients who engaged in regular, moderate- to vigorous-intensity PA (such as walking, running, aerobics, or other cardiovascular exercise) both before and after their diagnosis were considered habitually active, whereas those who did not exercise regularly were considered habitually inactive.
Overall, 52% of patients reported habitual activity, and 19% reported habitual inactivity. Twenty-three percent of patients said their activity level decreased after diagnosis, and 6% said their activity level increased.
Patients were followed through January 31, 2018. The median time to follow-up was 53 months, and 33.7% of patients (n=1956) died during the follow-up period.
Results
The researchers found that patients who were active before and after diagnosis were 40% more likely to survive than those who were habitually inactive (P<0.001). Habitually inactive patients had a 66% increased risk of mortality compared to active patients.
The habitually active patients had a 37-month mean survival advantage over the inactive patients.
In addition, patients whose activity level increased after diagnosis had a 25% lower risk of death than patients who remained inactive after diagnosis.
The researchers observed a significant (P<0.05) association between habitual PA and decreased mortality in patients with breast, colon, prostate, bladder, endometrial, ovarian, esophageal, and skin cancers.
However, the association between PA and mortality was not significant for patients with hematologic malignancies (P=0.59) or kidney, liver, lung, pancreas, stomach, or “other” cancers.
The researchers said the associations between habitual PA and decreased mortality remained consistent regardless of a patient’s sex, tumor stage, smoking status, or body mass index.
“[W]hen it comes to exercise, something is better than nothing, but regular, weekly exercise seems to really make a difference,” Dr Cannioto said.
“In fact, patients who were physically active 3 or 4 days a week experienced an even greater benefit than those who exercised daily, and patients who had only 1 or 2 days of regular activity per week did nearly as well. This is particularly encouraging, as cancer patients and survivors can be overwhelmed by current physical activity recommendations.”
*Information in the abstract differs from the presentation.
CHICAGO—Researchers have identified a link between habitual physical activity (PA) and mortality among cancer patients.
Engaging in regular PA, both pre- and post-diagnosis, was associated with a significantly lower risk of death for the entire population studied and for patients with 8 specific types of cancer.
However, the association was not significant for patients with other cancer types, including hematologic malignancies.
Rikki Cannioto, PhD, of Roswell Park Comprehensive Cancer Center in Buffalo, New York, and her colleagues presented these findings at the AACR Annual Meeting 2018 (abstract 5254*).
The researchers examined the association between habitual PA and outcomes in 5807 cancer patients enrolled in the Data Bank and BioRepository at Roswell Park between 2003 and 2016.
The population was 54.8% female and 93% white. The average age at diagnosis was 60.6 years.
The researchers looked at patterns of PA over time, from the decade before the cancer was diagnosed and continuing for up to 1 year after diagnosis.
Patients who engaged in regular, moderate- to vigorous-intensity PA (such as walking, running, aerobics, or other cardiovascular exercise) both before and after their diagnosis were considered habitually active, whereas those who did not exercise regularly were considered habitually inactive.
Overall, 52% of patients reported habitual activity, and 19% reported habitual inactivity. Twenty-three percent of patients said their activity level decreased after diagnosis, and 6% said their activity level increased.
Patients were followed through January 31, 2018. The median time to follow-up was 53 months, and 33.7% of patients (n=1956) died during the follow-up period.
Results
The researchers found that patients who were active before and after diagnosis were 40% more likely to survive than those who were habitually inactive (P<0.001). Habitually inactive patients had a 66% increased risk of mortality compared to active patients.
The habitually active patients had a 37-month mean survival advantage over the inactive patients.
In addition, patients whose activity level increased after diagnosis had a 25% lower risk of death than patients who remained inactive after diagnosis.
The researchers observed a significant (P<0.05) association between habitual PA and decreased mortality in patients with breast, colon, prostate, bladder, endometrial, ovarian, esophageal, and skin cancers.
However, the association between PA and mortality was not significant for patients with hematologic malignancies (P=0.59) or kidney, liver, lung, pancreas, stomach, or “other” cancers.
The researchers said the associations between habitual PA and decreased mortality remained consistent regardless of a patient’s sex, tumor stage, smoking status, or body mass index.
“[W]hen it comes to exercise, something is better than nothing, but regular, weekly exercise seems to really make a difference,” Dr Cannioto said.
“In fact, patients who were physically active 3 or 4 days a week experienced an even greater benefit than those who exercised daily, and patients who had only 1 or 2 days of regular activity per week did nearly as well. This is particularly encouraging, as cancer patients and survivors can be overwhelmed by current physical activity recommendations.”
*Information in the abstract differs from the presentation.
Company stops development of drug for AL amyloidosis
Prothena Corporation plc is discontinuing development of NEOD001, an investigational antibody intended for the treatment of AL amyloidosis.
The company’s decision was based on results from the phase 2b PRONTO study and a futility analysis of the phase 3 VITAL study.
NEOD001 did not meet the primary or secondary endpoints of the PRONTO study, so Prothena asked an independent data monitoring committee to review a futility analysis of the ongoing VITAL study.
The committee recommended discontinuation of the VITAL study, so Prothena decided to discontinue all development of NEOD001, including the VITAL study and open-label extension studies.
“We are deeply disappointed by this outcome, particularly for patients suffering from this devastating disease,” said Gene Kinney, PhD, president and chief executive officer of Prothena.
“We are surprised by the results from these 2 placebo-controlled studies and will continue to analyze the resulting data to share insights with our collaborators in the scientific, medical, and advocacy communities.”
Phase 3 VITAL study
The VITAL study was a multicenter, randomized, double-blind, placebo-controlled study of NEOD001 in treatment-naïve patients with AL amyloidosis and cardiac dysfunction.
The study enrolled 260 patients who were randomized on a 1:1 basis to receive 24 mg/kg of NEOD001 or placebo via intravenous infusion every 28 days. Patients in both arms received concurrent standard of care therapy.
The composite primary endpoint was event-based, with all-cause mortality or cardiac hospitalizations counted as events.
The futility analysis, based on 103 adjudicated events of the 156 events specified to complete the study, was not statistically significant. The hazard ratio was 0.84 favoring NEOD001 versus the control arm.
Phase 2b PRONTO study
The PRONTO study was a multicenter, randomized, double-blind, placebo-controlled study of NEOD001 in previously treated patients with AL amyloidosis and persistent cardiac dysfunction.
The study enrolled 129 patients who were randomized on a 1:1 basis to receive 24 mg/kg of NEOD001 (n=66) or placebo (n=63) via intravenous infusion every 28 days.
There was no significant difference between the treatment arms for any of the study’s endpoints.
The primary endpoint was cardiac best response, as assessed by N-terminal pro B-type natriuretic peptide (NT-proBNP) through 12 months of treatment. This endpoint was achieved by 39.4% of patients in the NEOD001 arm and 47.6% in the placebo arm.
The NT-proBNP rate of change (slope) through 12 months of treatment was 9.80 in the NEOD001 arm and 81.42 in the placebo arm.
The mean change in Short-form 36 Physical Component Summary Score after 12 months of treatment was 0.19 and 0.97, respectively.
The median change in 6-Minute Walk Test distance after 12 months of treatment was 19.25 m and 8.00 m, respectively.
And the mean change in Neuropathy Impairment Score of the Lower Limb after 12 months of treatment was -1.20 and -0.60, respectively.
The rate of renal best response (as assessed by proteinuria and estimated glomerular filtration rate) through 12 months of treatment was 53.8% in the NEOD001 arm and 33.3% in the placebo arm.
The rate of all-cause mortality was 4.5% (n=3) and 9.5% (n=6), respectively.
Prothena said NEOD001 was generally safe and well tolerated in this trial.
Prothena Corporation plc is discontinuing development of NEOD001, an investigational antibody intended for the treatment of AL amyloidosis.
The company’s decision was based on results from the phase 2b PRONTO study and a futility analysis of the phase 3 VITAL study.
NEOD001 did not meet the primary or secondary endpoints of the PRONTO study, so Prothena asked an independent data monitoring committee to review a futility analysis of the ongoing VITAL study.
The committee recommended discontinuation of the VITAL study, so Prothena decided to discontinue all development of NEOD001, including the VITAL study and open-label extension studies.
“We are deeply disappointed by this outcome, particularly for patients suffering from this devastating disease,” said Gene Kinney, PhD, president and chief executive officer of Prothena.
“We are surprised by the results from these 2 placebo-controlled studies and will continue to analyze the resulting data to share insights with our collaborators in the scientific, medical, and advocacy communities.”
Phase 3 VITAL study
The VITAL study was a multicenter, randomized, double-blind, placebo-controlled study of NEOD001 in treatment-naïve patients with AL amyloidosis and cardiac dysfunction.
The study enrolled 260 patients who were randomized on a 1:1 basis to receive 24 mg/kg of NEOD001 or placebo via intravenous infusion every 28 days. Patients in both arms received concurrent standard of care therapy.
The composite primary endpoint was event-based, with all-cause mortality or cardiac hospitalizations counted as events.
The futility analysis, based on 103 adjudicated events of the 156 events specified to complete the study, was not statistically significant. The hazard ratio was 0.84 favoring NEOD001 versus the control arm.
Phase 2b PRONTO study
The PRONTO study was a multicenter, randomized, double-blind, placebo-controlled study of NEOD001 in previously treated patients with AL amyloidosis and persistent cardiac dysfunction.
The study enrolled 129 patients who were randomized on a 1:1 basis to receive 24 mg/kg of NEOD001 (n=66) or placebo (n=63) via intravenous infusion every 28 days.
There was no significant difference between the treatment arms for any of the study’s endpoints.
The primary endpoint was cardiac best response, as assessed by N-terminal pro B-type natriuretic peptide (NT-proBNP) through 12 months of treatment. This endpoint was achieved by 39.4% of patients in the NEOD001 arm and 47.6% in the placebo arm.
The NT-proBNP rate of change (slope) through 12 months of treatment was 9.80 in the NEOD001 arm and 81.42 in the placebo arm.
The mean change in Short-form 36 Physical Component Summary Score after 12 months of treatment was 0.19 and 0.97, respectively.
The median change in 6-Minute Walk Test distance after 12 months of treatment was 19.25 m and 8.00 m, respectively.
And the mean change in Neuropathy Impairment Score of the Lower Limb after 12 months of treatment was -1.20 and -0.60, respectively.
The rate of renal best response (as assessed by proteinuria and estimated glomerular filtration rate) through 12 months of treatment was 53.8% in the NEOD001 arm and 33.3% in the placebo arm.
The rate of all-cause mortality was 4.5% (n=3) and 9.5% (n=6), respectively.
Prothena said NEOD001 was generally safe and well tolerated in this trial.
Prothena Corporation plc is discontinuing development of NEOD001, an investigational antibody intended for the treatment of AL amyloidosis.
The company’s decision was based on results from the phase 2b PRONTO study and a futility analysis of the phase 3 VITAL study.
NEOD001 did not meet the primary or secondary endpoints of the PRONTO study, so Prothena asked an independent data monitoring committee to review a futility analysis of the ongoing VITAL study.
The committee recommended discontinuation of the VITAL study, so Prothena decided to discontinue all development of NEOD001, including the VITAL study and open-label extension studies.
“We are deeply disappointed by this outcome, particularly for patients suffering from this devastating disease,” said Gene Kinney, PhD, president and chief executive officer of Prothena.
“We are surprised by the results from these 2 placebo-controlled studies and will continue to analyze the resulting data to share insights with our collaborators in the scientific, medical, and advocacy communities.”
Phase 3 VITAL study
The VITAL study was a multicenter, randomized, double-blind, placebo-controlled study of NEOD001 in treatment-naïve patients with AL amyloidosis and cardiac dysfunction.
The study enrolled 260 patients who were randomized on a 1:1 basis to receive 24 mg/kg of NEOD001 or placebo via intravenous infusion every 28 days. Patients in both arms received concurrent standard of care therapy.
The composite primary endpoint was event-based, with all-cause mortality or cardiac hospitalizations counted as events.
The futility analysis, based on 103 adjudicated events of the 156 events specified to complete the study, was not statistically significant. The hazard ratio was 0.84 favoring NEOD001 versus the control arm.
Phase 2b PRONTO study
The PRONTO study was a multicenter, randomized, double-blind, placebo-controlled study of NEOD001 in previously treated patients with AL amyloidosis and persistent cardiac dysfunction.
The study enrolled 129 patients who were randomized on a 1:1 basis to receive 24 mg/kg of NEOD001 (n=66) or placebo (n=63) via intravenous infusion every 28 days.
There was no significant difference between the treatment arms for any of the study’s endpoints.
The primary endpoint was cardiac best response, as assessed by N-terminal pro B-type natriuretic peptide (NT-proBNP) through 12 months of treatment. This endpoint was achieved by 39.4% of patients in the NEOD001 arm and 47.6% in the placebo arm.
The NT-proBNP rate of change (slope) through 12 months of treatment was 9.80 in the NEOD001 arm and 81.42 in the placebo arm.
The mean change in Short-form 36 Physical Component Summary Score after 12 months of treatment was 0.19 and 0.97, respectively.
The median change in 6-Minute Walk Test distance after 12 months of treatment was 19.25 m and 8.00 m, respectively.
And the mean change in Neuropathy Impairment Score of the Lower Limb after 12 months of treatment was -1.20 and -0.60, respectively.
The rate of renal best response (as assessed by proteinuria and estimated glomerular filtration rate) through 12 months of treatment was 53.8% in the NEOD001 arm and 33.3% in the placebo arm.
The rate of all-cause mortality was 4.5% (n=3) and 9.5% (n=6), respectively.
Prothena said NEOD001 was generally safe and well tolerated in this trial.
Inpatient care by PCPs associated with lower mortality than care by hospitalists
Clinical question: Are there differences in mortality and health care resource utilization in patients treated by hospitalists, primary care physicians, or other generalists?
Background: Most hospitalized patients now are being cared for by hospitalists rather than their primary care physicians (PCP). Covering generalists, who lack a prior relationship with the patient, also care for hospitalized patients when their PCP is unavailable. Although past studies have found some differences in outcomes in patients when care was provided by hospitalists vs. PCPs, those studies have grouped covering generalists with PCPs, which could affect the data.
Setting: Medicare admissions to acute care hospitals in all 50 states from January 2013 to December 2013.
Synopsis: Researchers analyzed data from 560,651 patients admitted with the 20 most common diagnoses looking for differences in health care utilization, length of stay, mortality, and discharge disposition depending on the type of provider: PCP, hospitalist, or other covering generalist. PCPs and other generalists consulted specialists more often than hospitalists. Length of stay was shorter in the hospitalist group. PCPs discharged patients to home more often than the other groups (68.5%, compared with 64% for hospitalists and 62% for other generalists). Readmission rates at 7 days were the same between hospitalists and PCPs but were higher in the other generalist group. PCPs also had lower 30-day mortality, compared with hospitalists (8.6% vs. 10.8%), while other generalists had higher mortality at 11%. Limitations include the use of administrative data and including only Medicare patients.
Bottom line: Inpatient care by PCP decreases mortality and increases likelihood of discharging home compared to care by hospitalists or other generalists.
Citation: Stevens JP et al. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017 Dec 1;177(12):1781-7.
Dr. Mathew is assistant professor of medicine, division of hospital medicine, University of Virginia.
Clinical question: Are there differences in mortality and health care resource utilization in patients treated by hospitalists, primary care physicians, or other generalists?
Background: Most hospitalized patients now are being cared for by hospitalists rather than their primary care physicians (PCP). Covering generalists, who lack a prior relationship with the patient, also care for hospitalized patients when their PCP is unavailable. Although past studies have found some differences in outcomes in patients when care was provided by hospitalists vs. PCPs, those studies have grouped covering generalists with PCPs, which could affect the data.
Setting: Medicare admissions to acute care hospitals in all 50 states from January 2013 to December 2013.
Synopsis: Researchers analyzed data from 560,651 patients admitted with the 20 most common diagnoses looking for differences in health care utilization, length of stay, mortality, and discharge disposition depending on the type of provider: PCP, hospitalist, or other covering generalist. PCPs and other generalists consulted specialists more often than hospitalists. Length of stay was shorter in the hospitalist group. PCPs discharged patients to home more often than the other groups (68.5%, compared with 64% for hospitalists and 62% for other generalists). Readmission rates at 7 days were the same between hospitalists and PCPs but were higher in the other generalist group. PCPs also had lower 30-day mortality, compared with hospitalists (8.6% vs. 10.8%), while other generalists had higher mortality at 11%. Limitations include the use of administrative data and including only Medicare patients.
Bottom line: Inpatient care by PCP decreases mortality and increases likelihood of discharging home compared to care by hospitalists or other generalists.
Citation: Stevens JP et al. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017 Dec 1;177(12):1781-7.
Dr. Mathew is assistant professor of medicine, division of hospital medicine, University of Virginia.
Clinical question: Are there differences in mortality and health care resource utilization in patients treated by hospitalists, primary care physicians, or other generalists?
Background: Most hospitalized patients now are being cared for by hospitalists rather than their primary care physicians (PCP). Covering generalists, who lack a prior relationship with the patient, also care for hospitalized patients when their PCP is unavailable. Although past studies have found some differences in outcomes in patients when care was provided by hospitalists vs. PCPs, those studies have grouped covering generalists with PCPs, which could affect the data.
Setting: Medicare admissions to acute care hospitals in all 50 states from January 2013 to December 2013.
Synopsis: Researchers analyzed data from 560,651 patients admitted with the 20 most common diagnoses looking for differences in health care utilization, length of stay, mortality, and discharge disposition depending on the type of provider: PCP, hospitalist, or other covering generalist. PCPs and other generalists consulted specialists more often than hospitalists. Length of stay was shorter in the hospitalist group. PCPs discharged patients to home more often than the other groups (68.5%, compared with 64% for hospitalists and 62% for other generalists). Readmission rates at 7 days were the same between hospitalists and PCPs but were higher in the other generalist group. PCPs also had lower 30-day mortality, compared with hospitalists (8.6% vs. 10.8%), while other generalists had higher mortality at 11%. Limitations include the use of administrative data and including only Medicare patients.
Bottom line: Inpatient care by PCP decreases mortality and increases likelihood of discharging home compared to care by hospitalists or other generalists.
Citation: Stevens JP et al. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017 Dec 1;177(12):1781-7.
Dr. Mathew is assistant professor of medicine, division of hospital medicine, University of Virginia.
ACOG advises earlier, more comprehensive postpartum care
It’s time to introduce a new paradigm for comprehensive care of women’s physical and mental health in the 3 months after giving birth, according to the American College of Obstetricians and Gynecologists.
In their newly revised committee opinion on postpartum care, ACOG encouraged doctors to think of a woman’s immediate postpartum period as a “fourth trimester” during which better care for women may help reduce maternal deaths and morbidity. That care includes a 3-week postpartum visit and a more comprehensive one within 3 months post partum.
Despite common practices in many other cultures that provide intense, dedicated support to women during the 30-40 days after giving birth, U.S. women typically only see their ob.gyn. at a single 6-week postpartum visit and receive little to no other formal maternal support. Beyond that visit, U.S. postpartum care typically is fragmented and inconsistent, split sporadically among pediatric and maternal providers and with little support in the transition from inpatient to outpatient care, the committee wrote.
Further, 40% of women do not attend a postpartum visit at all, and more than half of maternal deaths occur after the baby’s birth. The committee aims to overhaul maternal care and potentially help reduce those numbers. That process begins with prenatal discussions about the mother’s transition to parenthood, caring for herself and her health, her reproductive life plans, her desires related to future children, the timing of future pregnancies, and appropriate contraceptive options and decisions.
“Underutilization of postpartum care impedes management of chronic health conditions and access to effective contraception, which increases the risk of short interval pregnancy and preterm birth,” the committee wrote. “Attendance rates are lower among populations with limited resources, which contributes to health disparities.”
Components of comprehensive postpartum care
ACOG recommends the prenatal preparation for the postpartum period include discussions about infant feeding, “baby blues,” postpartum emotional health, parenting challenges, postpartum recovery from birth, long-term management of chronic health conditions, choosing a primary care provider for the mother’s ongoing care, her reproductive desires and choices, and any concerns about interpersonal or partner violence.
Before giving birth, a woman should develop a postpartum care plan with her physician and assemble a care team that includes her primary care providers along with family and friends who can provide support. The plan should include contact information for questions and written instructions about postpartum visits and follow-up care.
Prenatal planning also provides an opportunity to discuss a woman’s breastfeeding plans, goals, and questions as well as common physical problems that women may experience in the weeks after giving birth, such as heavy bleeding, pain, physical exhaustion, and urinary incontinence.
Physicians should inform women of the risks and benefits of becoming pregnant within 18 months and advise them not to have pregnancy intervals of less than 6 months. They should also ensure women know all their contraceptive options and should provide any information necessary for women to determine which methods best meet her needs.
The committee recommended a postpartum visit within the first 3 weeks after birth, instead of the current “6-week check,” that is timed and tailored to each woman’s particular needs. This visit allows assessment of postpartum depression risk and/or treatment and discussion of breastfeeding goals and/or difficulties. Approximately one in five women who stopped breastfeeding earlier than they wanted to had ceased within first 6 weeks post partum.
Woman-centered follow-up should be tailored to women’s individual needs and include a comprehensive postpartum visit no later than 12 weeks after giving birth. The comprehensive visit should include a complete assessment of the woman’s physical, social, and psychological well-being, including discussion of “mood and emotional well-being, infant care and feeding, sexuality, contraception, birth spacing, sleep and fatigue, physical recovery from birth, chronic disease management, and health maintenance,” the committee wrote.
The comprehensive visit should include the following components:
- Postpartum depression and anxiety screening.
- Screening for tobacco use and substance use.
- Follow-up on preexisting mental and physical health conditions.
- Assessment of mother’s confidence and comfort with newborn care, including feeding method, childcare strategy, identification of the child’s medical home, and recommended immunizations for all caregivers.
- Comfort and confidence with breastfeeding and management of any challenges, such as breastfeeding-associated pain; logistics and legal rights after returning to work or school; and fertility and contraception with breastfeeding.
- Assessment of material needs, including housing, utilities, food, and diapers.
- Guidance on sexuality, dyspareunia, reproductive life plans, contraception, and management of recurrent pregnancy complications, such as daily low-dose aspirin to reduce preeclampsia risk and 17a-hydroxyprogesterone caproate to reduce recurrent preterm birth.
- Sleep, fatigue, and coping options.
- Physical recovery from birth, including assessment of urinary and fecal continence and guidance on physical activity and a healthy weight.
- Chronic disease management and long-term implications of those conditions.
- Health maintenance, including review of vaccination history, needed vaccinations, and well-woman screenings, including Pap test and pelvic examination as indicated.
“However timed, the comprehensive postpartum visit is a medical appointment; it is not an ‘all-clear’ signal,” the authors wrote. “Obstetrician-gynecologists and other obstetric care providers should ensure that women, their families, and their employers understand that completion of the comprehensive postpartum visit does not obviate the need for continued recovery and support through 6 weeks’ post partum and beyond.”
Women with comorbidities or adverse birth outcomes
Women who had gestational diabetes, gestational hypertension, preeclampsia, eclampsia, or a preterm birth should be informed of their increased lifetime risk of cardiovascular and metabolic disease, the committee recommended. Women who have experienced a miscarriage, stillbirth, or neonatal death should also follow up with their provider, who can offer resources for emotional support and bereavement counseling, referrals as needed, a review of any laboratory or pathology results related to the loss and counseling regarding future risks and pregnancies.
The committee recommended that women with chronic medical conditions follow up with their ob.gyn. or other primary care providers to ensure ongoing coordinated care for hypertension, obesity, diabetes, thyroid disorders, renal disease, mood disorders, substance use disorders, seizure disorders, and any other chronic issues. Care should include assessment of medications, including antiepileptics and psychotropic drugs, that may require adjustment for postpartum physiology and, if relevant, breastfeeding.
Since half of postpartum strokes occur within the first 10 days after discharge, ACOG recommends women with other hypertensive disorders of pregnancy have a postpartum visit within 7-10 days after birth to assess blood pressure. A follow-up visit should occur within 72 hours for those with severe hypertension.
ACOG also recommended early postpartum follow-up for women with increased risk of complications, including postpartum depression, cesarean or perennial wound infections, lactation difficulties, or chronic conditions.
The committee opinion concluded with a call for public policy changes, including endorsement of guaranteed 100% paid parental leave for a minimum of 6 weeks with full benefits. Currently, 23% of employed mothers return to work in the first 10 days after giving birth, and another 22% return within 10-30 days, the committee cited. Close to half of employed mothers therefore go back to work before the 6-week postpartum follow-up visit.
“Obstetrician-gynecologists and other obstetric care providers should be in the forefront of policy efforts to enable all women to recover from birth and nurture their infants,” the committee wrote.
The ACOG Presidential Task Force on Redefining the Postpartum Visit and the Committee on Obstetrics Practice developed the new clinical opinion, which is endorsed by the Academy of Breastfeeding Medicine, the American College of Nurse-Midwives, the National Association of Nurse Practitioners in Women’s Health, the Society for Academic Specialists in General Obstetrics and Gynecology, and the Society for Maternal-Fetal Medicine. The committee opinion did not require external funding, and the authors did not report any disclosures.
SOURCE: Obstet Gynecol 2018;131:e140-50.
It’s time to introduce a new paradigm for comprehensive care of women’s physical and mental health in the 3 months after giving birth, according to the American College of Obstetricians and Gynecologists.
In their newly revised committee opinion on postpartum care, ACOG encouraged doctors to think of a woman’s immediate postpartum period as a “fourth trimester” during which better care for women may help reduce maternal deaths and morbidity. That care includes a 3-week postpartum visit and a more comprehensive one within 3 months post partum.
Despite common practices in many other cultures that provide intense, dedicated support to women during the 30-40 days after giving birth, U.S. women typically only see their ob.gyn. at a single 6-week postpartum visit and receive little to no other formal maternal support. Beyond that visit, U.S. postpartum care typically is fragmented and inconsistent, split sporadically among pediatric and maternal providers and with little support in the transition from inpatient to outpatient care, the committee wrote.
Further, 40% of women do not attend a postpartum visit at all, and more than half of maternal deaths occur after the baby’s birth. The committee aims to overhaul maternal care and potentially help reduce those numbers. That process begins with prenatal discussions about the mother’s transition to parenthood, caring for herself and her health, her reproductive life plans, her desires related to future children, the timing of future pregnancies, and appropriate contraceptive options and decisions.
“Underutilization of postpartum care impedes management of chronic health conditions and access to effective contraception, which increases the risk of short interval pregnancy and preterm birth,” the committee wrote. “Attendance rates are lower among populations with limited resources, which contributes to health disparities.”
Components of comprehensive postpartum care
ACOG recommends the prenatal preparation for the postpartum period include discussions about infant feeding, “baby blues,” postpartum emotional health, parenting challenges, postpartum recovery from birth, long-term management of chronic health conditions, choosing a primary care provider for the mother’s ongoing care, her reproductive desires and choices, and any concerns about interpersonal or partner violence.
Before giving birth, a woman should develop a postpartum care plan with her physician and assemble a care team that includes her primary care providers along with family and friends who can provide support. The plan should include contact information for questions and written instructions about postpartum visits and follow-up care.
Prenatal planning also provides an opportunity to discuss a woman’s breastfeeding plans, goals, and questions as well as common physical problems that women may experience in the weeks after giving birth, such as heavy bleeding, pain, physical exhaustion, and urinary incontinence.
Physicians should inform women of the risks and benefits of becoming pregnant within 18 months and advise them not to have pregnancy intervals of less than 6 months. They should also ensure women know all their contraceptive options and should provide any information necessary for women to determine which methods best meet her needs.
The committee recommended a postpartum visit within the first 3 weeks after birth, instead of the current “6-week check,” that is timed and tailored to each woman’s particular needs. This visit allows assessment of postpartum depression risk and/or treatment and discussion of breastfeeding goals and/or difficulties. Approximately one in five women who stopped breastfeeding earlier than they wanted to had ceased within first 6 weeks post partum.
Woman-centered follow-up should be tailored to women’s individual needs and include a comprehensive postpartum visit no later than 12 weeks after giving birth. The comprehensive visit should include a complete assessment of the woman’s physical, social, and psychological well-being, including discussion of “mood and emotional well-being, infant care and feeding, sexuality, contraception, birth spacing, sleep and fatigue, physical recovery from birth, chronic disease management, and health maintenance,” the committee wrote.
The comprehensive visit should include the following components:
- Postpartum depression and anxiety screening.
- Screening for tobacco use and substance use.
- Follow-up on preexisting mental and physical health conditions.
- Assessment of mother’s confidence and comfort with newborn care, including feeding method, childcare strategy, identification of the child’s medical home, and recommended immunizations for all caregivers.
- Comfort and confidence with breastfeeding and management of any challenges, such as breastfeeding-associated pain; logistics and legal rights after returning to work or school; and fertility and contraception with breastfeeding.
- Assessment of material needs, including housing, utilities, food, and diapers.
- Guidance on sexuality, dyspareunia, reproductive life plans, contraception, and management of recurrent pregnancy complications, such as daily low-dose aspirin to reduce preeclampsia risk and 17a-hydroxyprogesterone caproate to reduce recurrent preterm birth.
- Sleep, fatigue, and coping options.
- Physical recovery from birth, including assessment of urinary and fecal continence and guidance on physical activity and a healthy weight.
- Chronic disease management and long-term implications of those conditions.
- Health maintenance, including review of vaccination history, needed vaccinations, and well-woman screenings, including Pap test and pelvic examination as indicated.
“However timed, the comprehensive postpartum visit is a medical appointment; it is not an ‘all-clear’ signal,” the authors wrote. “Obstetrician-gynecologists and other obstetric care providers should ensure that women, their families, and their employers understand that completion of the comprehensive postpartum visit does not obviate the need for continued recovery and support through 6 weeks’ post partum and beyond.”
Women with comorbidities or adverse birth outcomes
Women who had gestational diabetes, gestational hypertension, preeclampsia, eclampsia, or a preterm birth should be informed of their increased lifetime risk of cardiovascular and metabolic disease, the committee recommended. Women who have experienced a miscarriage, stillbirth, or neonatal death should also follow up with their provider, who can offer resources for emotional support and bereavement counseling, referrals as needed, a review of any laboratory or pathology results related to the loss and counseling regarding future risks and pregnancies.
The committee recommended that women with chronic medical conditions follow up with their ob.gyn. or other primary care providers to ensure ongoing coordinated care for hypertension, obesity, diabetes, thyroid disorders, renal disease, mood disorders, substance use disorders, seizure disorders, and any other chronic issues. Care should include assessment of medications, including antiepileptics and psychotropic drugs, that may require adjustment for postpartum physiology and, if relevant, breastfeeding.
Since half of postpartum strokes occur within the first 10 days after discharge, ACOG recommends women with other hypertensive disorders of pregnancy have a postpartum visit within 7-10 days after birth to assess blood pressure. A follow-up visit should occur within 72 hours for those with severe hypertension.
ACOG also recommended early postpartum follow-up for women with increased risk of complications, including postpartum depression, cesarean or perennial wound infections, lactation difficulties, or chronic conditions.
The committee opinion concluded with a call for public policy changes, including endorsement of guaranteed 100% paid parental leave for a minimum of 6 weeks with full benefits. Currently, 23% of employed mothers return to work in the first 10 days after giving birth, and another 22% return within 10-30 days, the committee cited. Close to half of employed mothers therefore go back to work before the 6-week postpartum follow-up visit.
“Obstetrician-gynecologists and other obstetric care providers should be in the forefront of policy efforts to enable all women to recover from birth and nurture their infants,” the committee wrote.
The ACOG Presidential Task Force on Redefining the Postpartum Visit and the Committee on Obstetrics Practice developed the new clinical opinion, which is endorsed by the Academy of Breastfeeding Medicine, the American College of Nurse-Midwives, the National Association of Nurse Practitioners in Women’s Health, the Society for Academic Specialists in General Obstetrics and Gynecology, and the Society for Maternal-Fetal Medicine. The committee opinion did not require external funding, and the authors did not report any disclosures.
SOURCE: Obstet Gynecol 2018;131:e140-50.
It’s time to introduce a new paradigm for comprehensive care of women’s physical and mental health in the 3 months after giving birth, according to the American College of Obstetricians and Gynecologists.
In their newly revised committee opinion on postpartum care, ACOG encouraged doctors to think of a woman’s immediate postpartum period as a “fourth trimester” during which better care for women may help reduce maternal deaths and morbidity. That care includes a 3-week postpartum visit and a more comprehensive one within 3 months post partum.
Despite common practices in many other cultures that provide intense, dedicated support to women during the 30-40 days after giving birth, U.S. women typically only see their ob.gyn. at a single 6-week postpartum visit and receive little to no other formal maternal support. Beyond that visit, U.S. postpartum care typically is fragmented and inconsistent, split sporadically among pediatric and maternal providers and with little support in the transition from inpatient to outpatient care, the committee wrote.
Further, 40% of women do not attend a postpartum visit at all, and more than half of maternal deaths occur after the baby’s birth. The committee aims to overhaul maternal care and potentially help reduce those numbers. That process begins with prenatal discussions about the mother’s transition to parenthood, caring for herself and her health, her reproductive life plans, her desires related to future children, the timing of future pregnancies, and appropriate contraceptive options and decisions.
“Underutilization of postpartum care impedes management of chronic health conditions and access to effective contraception, which increases the risk of short interval pregnancy and preterm birth,” the committee wrote. “Attendance rates are lower among populations with limited resources, which contributes to health disparities.”
Components of comprehensive postpartum care
ACOG recommends the prenatal preparation for the postpartum period include discussions about infant feeding, “baby blues,” postpartum emotional health, parenting challenges, postpartum recovery from birth, long-term management of chronic health conditions, choosing a primary care provider for the mother’s ongoing care, her reproductive desires and choices, and any concerns about interpersonal or partner violence.
Before giving birth, a woman should develop a postpartum care plan with her physician and assemble a care team that includes her primary care providers along with family and friends who can provide support. The plan should include contact information for questions and written instructions about postpartum visits and follow-up care.
Prenatal planning also provides an opportunity to discuss a woman’s breastfeeding plans, goals, and questions as well as common physical problems that women may experience in the weeks after giving birth, such as heavy bleeding, pain, physical exhaustion, and urinary incontinence.
Physicians should inform women of the risks and benefits of becoming pregnant within 18 months and advise them not to have pregnancy intervals of less than 6 months. They should also ensure women know all their contraceptive options and should provide any information necessary for women to determine which methods best meet her needs.
The committee recommended a postpartum visit within the first 3 weeks after birth, instead of the current “6-week check,” that is timed and tailored to each woman’s particular needs. This visit allows assessment of postpartum depression risk and/or treatment and discussion of breastfeeding goals and/or difficulties. Approximately one in five women who stopped breastfeeding earlier than they wanted to had ceased within first 6 weeks post partum.
Woman-centered follow-up should be tailored to women’s individual needs and include a comprehensive postpartum visit no later than 12 weeks after giving birth. The comprehensive visit should include a complete assessment of the woman’s physical, social, and psychological well-being, including discussion of “mood and emotional well-being, infant care and feeding, sexuality, contraception, birth spacing, sleep and fatigue, physical recovery from birth, chronic disease management, and health maintenance,” the committee wrote.
The comprehensive visit should include the following components:
- Postpartum depression and anxiety screening.
- Screening for tobacco use and substance use.
- Follow-up on preexisting mental and physical health conditions.
- Assessment of mother’s confidence and comfort with newborn care, including feeding method, childcare strategy, identification of the child’s medical home, and recommended immunizations for all caregivers.
- Comfort and confidence with breastfeeding and management of any challenges, such as breastfeeding-associated pain; logistics and legal rights after returning to work or school; and fertility and contraception with breastfeeding.
- Assessment of material needs, including housing, utilities, food, and diapers.
- Guidance on sexuality, dyspareunia, reproductive life plans, contraception, and management of recurrent pregnancy complications, such as daily low-dose aspirin to reduce preeclampsia risk and 17a-hydroxyprogesterone caproate to reduce recurrent preterm birth.
- Sleep, fatigue, and coping options.
- Physical recovery from birth, including assessment of urinary and fecal continence and guidance on physical activity and a healthy weight.
- Chronic disease management and long-term implications of those conditions.
- Health maintenance, including review of vaccination history, needed vaccinations, and well-woman screenings, including Pap test and pelvic examination as indicated.
“However timed, the comprehensive postpartum visit is a medical appointment; it is not an ‘all-clear’ signal,” the authors wrote. “Obstetrician-gynecologists and other obstetric care providers should ensure that women, their families, and their employers understand that completion of the comprehensive postpartum visit does not obviate the need for continued recovery and support through 6 weeks’ post partum and beyond.”
Women with comorbidities or adverse birth outcomes
Women who had gestational diabetes, gestational hypertension, preeclampsia, eclampsia, or a preterm birth should be informed of their increased lifetime risk of cardiovascular and metabolic disease, the committee recommended. Women who have experienced a miscarriage, stillbirth, or neonatal death should also follow up with their provider, who can offer resources for emotional support and bereavement counseling, referrals as needed, a review of any laboratory or pathology results related to the loss and counseling regarding future risks and pregnancies.
The committee recommended that women with chronic medical conditions follow up with their ob.gyn. or other primary care providers to ensure ongoing coordinated care for hypertension, obesity, diabetes, thyroid disorders, renal disease, mood disorders, substance use disorders, seizure disorders, and any other chronic issues. Care should include assessment of medications, including antiepileptics and psychotropic drugs, that may require adjustment for postpartum physiology and, if relevant, breastfeeding.
Since half of postpartum strokes occur within the first 10 days after discharge, ACOG recommends women with other hypertensive disorders of pregnancy have a postpartum visit within 7-10 days after birth to assess blood pressure. A follow-up visit should occur within 72 hours for those with severe hypertension.
ACOG also recommended early postpartum follow-up for women with increased risk of complications, including postpartum depression, cesarean or perennial wound infections, lactation difficulties, or chronic conditions.
The committee opinion concluded with a call for public policy changes, including endorsement of guaranteed 100% paid parental leave for a minimum of 6 weeks with full benefits. Currently, 23% of employed mothers return to work in the first 10 days after giving birth, and another 22% return within 10-30 days, the committee cited. Close to half of employed mothers therefore go back to work before the 6-week postpartum follow-up visit.
“Obstetrician-gynecologists and other obstetric care providers should be in the forefront of policy efforts to enable all women to recover from birth and nurture their infants,” the committee wrote.
The ACOG Presidential Task Force on Redefining the Postpartum Visit and the Committee on Obstetrics Practice developed the new clinical opinion, which is endorsed by the Academy of Breastfeeding Medicine, the American College of Nurse-Midwives, the National Association of Nurse Practitioners in Women’s Health, the Society for Academic Specialists in General Obstetrics and Gynecology, and the Society for Maternal-Fetal Medicine. The committee opinion did not require external funding, and the authors did not report any disclosures.
SOURCE: Obstet Gynecol 2018;131:e140-50.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: New recommendations on postpartum care advise earlier and more comprehensive follow-up visits and propose a new paradigm for ensuring the physical, emotional, and mental health of women in the first 12 weeks after giving birth.
Major finding: Women should have a follow-up visit within 3 weeks post partum – earlier if they have chronic conditions or had pregnancy complications – and an additional comprehensive visit no later than 12 weeks post partum.
Data source: The findings are based on an assessment of existing evidence on postpartum care, postpartum risks, and currently unfulfilled needs that ob.gyns. can and should fulfill, according to ACOG.
Disclosures: The committee opinion did not require external funding, and the authors did not report any disclosures.
Source: Obstet Gynecol 2018;131:e140-50.
Self-administration of subcutaneous belimumab could eliminate hospital visits for SLE patients
Patients with systemic lupus erythematosus (SLE) who were hypocomplementemic and anti–double-stranded DNA (anti-dsDNA) positive took weekly subcutaneous belimumab in addition to their standard therapy and saw reduced disease activity and fatigue at 1 year, compared with patients taking a placebo, according to results of a phase 3, double-blinded study. These results suggest the subcutaneous version of the monoclonal antibody therapy could be administered at home without patients visiting a hospital, the investigators wrote in Arthritis and Rheumatology.
“Intravenous administration of belimumab is an obstacle to treatment for many patients due to the need to go to the hospital for drug infusions. Thus, a higher number of patients could benefit from this treatment,” Andrea Doria, MD, from the University of Padua (Italy) stated in a press release.
Researchers set a primary endpoint of response rate according to SLE Responder Index (SRI4), no new British Isles Lupus Assessment Group organ domain A or B scores, and less than a 0.3 increase in Physician’s Global Assessment score at 52 weeks, compared with baseline scores; the secondary endpoints included corticosteroid use reduction between week 40 and week 52 of 25% or more to 7.5 mg/day or less, change in Functional Assessment of Chronic Illness Therapy–Fatigue score, and measurement of time to severe flare as measured by the SLE Flare Index.
At 52 weeks, 64.6% of patients in the belimumab group responded according to SRI4, compared with 47.2% of patients in the placebo group (P = .0014). The researchers attributed the high SRI4 response rate for the placebo group to “administration of SoC [standard SLE therapy]; increased chance of receiving active treatment due to the unbalanced randomization schedule, thereby resulting in a psychological benefit; and the high frequency of visits and patient satisfaction associated with clinical trials.”
Patients had lower flare rates according to the SLE Flare Index in the belimumab group (31.5%), compared with placebo (14.1%), and those in the former group had a 62% reduction in severe flares, compared with the placebo group (hazard ratio, 0.38; 95% confidence interval, 0.24-0.61; P less than .0001). More patients taking belimumab reduced their use of corticosteroids (20.7%) than did those taking the placebo (11.4%) (odds ratio, 2.08; 95% CI, 0.91-4.77; P = .0844). Of patients taking belimumab, 44.8% had a Functional Assessment of Chronic Illness Therapy–Fatigue score of 4 or higher at week 52, compared with 33.3% of patients taking placebo (OR, 1.82; 95% CI, 1.10-3.01; P = .0199).
Regarding adverse events, there were 88 (81.5%) adverse events in the placebo group, with 29 (26.9%) of those events considered to have occurred during treatment, compared with 79 of 194 (31.9%) adverse events attributed to treatment in the belimumab group. The researchers reported 25 patients in the placebo group (23.1%) and 33 patients in the belimumab group (13.3%) had serious adverse events. Postinjection systemic reactions occurred in 21 patients (8.5%) and 13 patients (12.0%) in the belimumab and placebo groups, respectively.
“Some aspects of this study were identified as potential limitations,” Dr. Doria and his colleagues wrote. “Within the hypocomplementemic and anti-dsDNA positive subset population, only 65.7% of patients received steroids greater than 7.5 mg/day at baseline; thus (as in the overall population), this endpoint was not powered for statistical significance. In addition, this study excluded patients with SELENA-SLEDAI less than 8, active nephritis, or active CNS disease at screening.”
The study was funded, conducted, and designed by GlaxoSmithKline. Five authors have shares in and are employees of GSK; another was an employee of GSK at the time of the study. Seven authors declared consulting fees, grants and other remuneration from pharmaceutical companies, including GSK.
SOURCE: Doria A et al. Arthritis Rheumatol. 2018 Apr 18. doi: 10.1002/art.40511.
Patients with systemic lupus erythematosus (SLE) who were hypocomplementemic and anti–double-stranded DNA (anti-dsDNA) positive took weekly subcutaneous belimumab in addition to their standard therapy and saw reduced disease activity and fatigue at 1 year, compared with patients taking a placebo, according to results of a phase 3, double-blinded study. These results suggest the subcutaneous version of the monoclonal antibody therapy could be administered at home without patients visiting a hospital, the investigators wrote in Arthritis and Rheumatology.
“Intravenous administration of belimumab is an obstacle to treatment for many patients due to the need to go to the hospital for drug infusions. Thus, a higher number of patients could benefit from this treatment,” Andrea Doria, MD, from the University of Padua (Italy) stated in a press release.
Researchers set a primary endpoint of response rate according to SLE Responder Index (SRI4), no new British Isles Lupus Assessment Group organ domain A or B scores, and less than a 0.3 increase in Physician’s Global Assessment score at 52 weeks, compared with baseline scores; the secondary endpoints included corticosteroid use reduction between week 40 and week 52 of 25% or more to 7.5 mg/day or less, change in Functional Assessment of Chronic Illness Therapy–Fatigue score, and measurement of time to severe flare as measured by the SLE Flare Index.
At 52 weeks, 64.6% of patients in the belimumab group responded according to SRI4, compared with 47.2% of patients in the placebo group (P = .0014). The researchers attributed the high SRI4 response rate for the placebo group to “administration of SoC [standard SLE therapy]; increased chance of receiving active treatment due to the unbalanced randomization schedule, thereby resulting in a psychological benefit; and the high frequency of visits and patient satisfaction associated with clinical trials.”
Patients had lower flare rates according to the SLE Flare Index in the belimumab group (31.5%), compared with placebo (14.1%), and those in the former group had a 62% reduction in severe flares, compared with the placebo group (hazard ratio, 0.38; 95% confidence interval, 0.24-0.61; P less than .0001). More patients taking belimumab reduced their use of corticosteroids (20.7%) than did those taking the placebo (11.4%) (odds ratio, 2.08; 95% CI, 0.91-4.77; P = .0844). Of patients taking belimumab, 44.8% had a Functional Assessment of Chronic Illness Therapy–Fatigue score of 4 or higher at week 52, compared with 33.3% of patients taking placebo (OR, 1.82; 95% CI, 1.10-3.01; P = .0199).
Regarding adverse events, there were 88 (81.5%) adverse events in the placebo group, with 29 (26.9%) of those events considered to have occurred during treatment, compared with 79 of 194 (31.9%) adverse events attributed to treatment in the belimumab group. The researchers reported 25 patients in the placebo group (23.1%) and 33 patients in the belimumab group (13.3%) had serious adverse events. Postinjection systemic reactions occurred in 21 patients (8.5%) and 13 patients (12.0%) in the belimumab and placebo groups, respectively.
“Some aspects of this study were identified as potential limitations,” Dr. Doria and his colleagues wrote. “Within the hypocomplementemic and anti-dsDNA positive subset population, only 65.7% of patients received steroids greater than 7.5 mg/day at baseline; thus (as in the overall population), this endpoint was not powered for statistical significance. In addition, this study excluded patients with SELENA-SLEDAI less than 8, active nephritis, or active CNS disease at screening.”
The study was funded, conducted, and designed by GlaxoSmithKline. Five authors have shares in and are employees of GSK; another was an employee of GSK at the time of the study. Seven authors declared consulting fees, grants and other remuneration from pharmaceutical companies, including GSK.
SOURCE: Doria A et al. Arthritis Rheumatol. 2018 Apr 18. doi: 10.1002/art.40511.
Patients with systemic lupus erythematosus (SLE) who were hypocomplementemic and anti–double-stranded DNA (anti-dsDNA) positive took weekly subcutaneous belimumab in addition to their standard therapy and saw reduced disease activity and fatigue at 1 year, compared with patients taking a placebo, according to results of a phase 3, double-blinded study. These results suggest the subcutaneous version of the monoclonal antibody therapy could be administered at home without patients visiting a hospital, the investigators wrote in Arthritis and Rheumatology.
“Intravenous administration of belimumab is an obstacle to treatment for many patients due to the need to go to the hospital for drug infusions. Thus, a higher number of patients could benefit from this treatment,” Andrea Doria, MD, from the University of Padua (Italy) stated in a press release.
Researchers set a primary endpoint of response rate according to SLE Responder Index (SRI4), no new British Isles Lupus Assessment Group organ domain A or B scores, and less than a 0.3 increase in Physician’s Global Assessment score at 52 weeks, compared with baseline scores; the secondary endpoints included corticosteroid use reduction between week 40 and week 52 of 25% or more to 7.5 mg/day or less, change in Functional Assessment of Chronic Illness Therapy–Fatigue score, and measurement of time to severe flare as measured by the SLE Flare Index.
At 52 weeks, 64.6% of patients in the belimumab group responded according to SRI4, compared with 47.2% of patients in the placebo group (P = .0014). The researchers attributed the high SRI4 response rate for the placebo group to “administration of SoC [standard SLE therapy]; increased chance of receiving active treatment due to the unbalanced randomization schedule, thereby resulting in a psychological benefit; and the high frequency of visits and patient satisfaction associated with clinical trials.”
Patients had lower flare rates according to the SLE Flare Index in the belimumab group (31.5%), compared with placebo (14.1%), and those in the former group had a 62% reduction in severe flares, compared with the placebo group (hazard ratio, 0.38; 95% confidence interval, 0.24-0.61; P less than .0001). More patients taking belimumab reduced their use of corticosteroids (20.7%) than did those taking the placebo (11.4%) (odds ratio, 2.08; 95% CI, 0.91-4.77; P = .0844). Of patients taking belimumab, 44.8% had a Functional Assessment of Chronic Illness Therapy–Fatigue score of 4 or higher at week 52, compared with 33.3% of patients taking placebo (OR, 1.82; 95% CI, 1.10-3.01; P = .0199).
Regarding adverse events, there were 88 (81.5%) adverse events in the placebo group, with 29 (26.9%) of those events considered to have occurred during treatment, compared with 79 of 194 (31.9%) adverse events attributed to treatment in the belimumab group. The researchers reported 25 patients in the placebo group (23.1%) and 33 patients in the belimumab group (13.3%) had serious adverse events. Postinjection systemic reactions occurred in 21 patients (8.5%) and 13 patients (12.0%) in the belimumab and placebo groups, respectively.
“Some aspects of this study were identified as potential limitations,” Dr. Doria and his colleagues wrote. “Within the hypocomplementemic and anti-dsDNA positive subset population, only 65.7% of patients received steroids greater than 7.5 mg/day at baseline; thus (as in the overall population), this endpoint was not powered for statistical significance. In addition, this study excluded patients with SELENA-SLEDAI less than 8, active nephritis, or active CNS disease at screening.”
The study was funded, conducted, and designed by GlaxoSmithKline. Five authors have shares in and are employees of GSK; another was an employee of GSK at the time of the study. Seven authors declared consulting fees, grants and other remuneration from pharmaceutical companies, including GSK.
SOURCE: Doria A et al. Arthritis Rheumatol. 2018 Apr 18. doi: 10.1002/art.40511.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Subcutaneous belimumab reduced disease activity and fatigue in SLE patients, compared with placebo.
Major finding: In the belimumab group, 64.6% of patients were SLE Responder Index responders, 31.5% showed lower severe SLE Flare Index scores, and 20.7% of patients reduced use of corticosteroids to 7.5 mg/day or less between week 40 and week 52.
Study details: A phase 3, double-blinded, placebo-controlled study of 356 patients over 52 weeks.
Disclosures: The study was funded, conducted, and designed by GlaxoSmithKline. Five authors have shares in and are employees of GSK; Another was an employee of GSK at the time of the study. Seven authors declared consulting fees, grants and other remuneration from pharmaceutical companies, including GSK.
Source: Doria A et al. Arthritis Rheumatol. 2018 April 18. doi: 10.1002/art.40511.
Gene therapy for thalassemia normalizes hemoglobin
Most beta thalassemia patients were off transfusions at a median of 26 months after receiving gene therapy via a lentiviral vector, according to new results of two phase 1/2 studies regarding the use of LentiGlobin in transfusion-dependent beta thalassemia.
Of 13 patients who did not have the most severe beta0/beta0 genotype, all but 1 has become transfusion independent post transplant. Among patients who either had the beta0/beta0 genotype or had two copies of the IVS1-110 mutation, transfusions were down a median 73% annually, and three of these patients with more severe thalassemia became transfusion independent.
At the time of last data collection, hemoglobin levels in individual patients ranged from 8.2-13.7 g/dL.
“No clonal dominance related to vector integration was observed,” wrote Alexis Thompson, MD, and her collaborators, and replication-competent lentivirus had not been found in any patients.
Hematopoietic cell transplant (HCT) is an option primarily for younger beta thalassemia patients who have an HLA-matched sibling donor, the researchers wrote in the New England Journal of Medicine. Gene therapy represents an alternative to the current standard of care for patients who are not candidates for allogeneic HCT, which – without a good match – carries increased risk for rejection and graft-versus-host disease.
Patients with beta thalassemia aged 35 years or younger and without advanced organ damage were enrolled in the two studies, one conducted internationally and one conducted at a single site in France.
There were some protocol differences between the two studies; notably, the French study used enhanced red cell transfusion for 3 or more months before stem cell mobilization “to enrich for bona fide hematopoietic stem cells in the harvested CD34+ cell compartment by suppressing the erythroid lineage expansion and the skewing that is seen in beta thalassemia,” wrote Dr. Thompson, professor of pediatrics at Northwestern University, Chicago, and her colleagues.
In both studies, after mobilization, patients’ unmanipulated hematopoietic stem cells and progenitor cells were taken to a central processing facility, where CD34+ cells were enriched and then transduced with the lentiviral vector BB305, which encodes adult hemoglobin (HbA) with a T87Z amino acid substitution and thereby provides functioning Hb beta. Patients received the product via infusion after undergoing myeloablative conditioning with busulfan.
A total of 23 patients, 19 in the international study and 4 in the French study, went through mobilization and apheresis. One patient in the international study had apheresis failure, so a total of 22 patients received LentiGlobin, and all were followed for up to 2 years.
Patients were given the opportunity to participate in a follow-on open label study meant to continue for an additional 13 years after the initial 24-month period; 13 patients are currently enrolled in this long-term follow-up study.
When transfusion volume at baseline was assessed, patients in the international study were receiving a median annual red blood cell transfusion volume of 164 mL/kg per year, while the French study participants were receiving a median 182 mL/kg per year of red blood cell transfusion.
In both studies, blood HbAT87Q levels correlated with the vector copy numbers (R2, 0.75; P less than .001). Levels of HbAT87Q ranged from 3.4-10.0 g/dL.
“Other factors, such as age, genotype, and splenectomy status, did not appear to correlate with gene expression,” the researchers wrote.
An exploratory analysis looked at characteristics of patients who were able to stop transfusions after gene therapy. In this group, “the degree of hemolysis at first stabilized relative to pretransplantation levels and was fully corrected” in two patients by 36 months after treatment.
The researchers noted that the sponsor achieved “high-titer, large-scale, clinical-grade BB305 vector production and purification by ion-exchange chromatography” from a single site in the United States, which showed the feasibility of conducting this modality of gene therapy at scale.
The study was sponsored by bluebird bio, the National Institutes of Health, and by French national research organizations. Dr. Thompson reported research funding and fees from bluebird bio and other pharmaceutical companies.
SOURCE: Thompson A et al. N Engl J Med 2018;378:1479-93
Lentiviral vector hematopoietic stem cell (HSC) gene therapy represents a promising alternative to matched-sibling donor HSC transplants for treatment of beta thalassemia, with studies to date showing a safety profile that surpasses transplants from unrelated or alternative donors.
The prospect of a curative treatment raised by the work of Dr. Thompson and her colleagues also shows the feasibility of transfusion independence for beta0/betaE patients, who carry the most common beta thalassemia genotype, and a significant reduction in transfusions even for patients with the more severe beta0/beta0 genotype.
Beta thalassemia has greatest prevalence in North Africa, the Middle East, and Asia, where access to treatments is limited and patients’ prognoses are often grim. Gene therapy for beta thalassemia could thereby represent the first large-scale implementation of this intervention in developing countries.
Bringing HSC gene therapy to more patients will require not just the availability of autologous HSCs, but of high-quality vector and reliable, high-volume manufacturing of transduced cells.
Harnessing this still-evolving technology to bring a potentially curative treatment to patients in developing countries is an exciting, but challenging, frontier for physicians and researchers involved with gene therapy.
Alessandra Biffi, MD, is director of the gene therapy program at Dana Farber Cancer Institute/Boston Children’s Cancer and Blood Disorders Center, Boston. She serves on the board of directors of the American Society of Gene and Cell Therapy. These remarks were adapted from an accompanying editorial ( N Engl J Med. 2018;378[16]:1551-2 ).
Lentiviral vector hematopoietic stem cell (HSC) gene therapy represents a promising alternative to matched-sibling donor HSC transplants for treatment of beta thalassemia, with studies to date showing a safety profile that surpasses transplants from unrelated or alternative donors.
The prospect of a curative treatment raised by the work of Dr. Thompson and her colleagues also shows the feasibility of transfusion independence for beta0/betaE patients, who carry the most common beta thalassemia genotype, and a significant reduction in transfusions even for patients with the more severe beta0/beta0 genotype.
Beta thalassemia has greatest prevalence in North Africa, the Middle East, and Asia, where access to treatments is limited and patients’ prognoses are often grim. Gene therapy for beta thalassemia could thereby represent the first large-scale implementation of this intervention in developing countries.
Bringing HSC gene therapy to more patients will require not just the availability of autologous HSCs, but of high-quality vector and reliable, high-volume manufacturing of transduced cells.
Harnessing this still-evolving technology to bring a potentially curative treatment to patients in developing countries is an exciting, but challenging, frontier for physicians and researchers involved with gene therapy.
Alessandra Biffi, MD, is director of the gene therapy program at Dana Farber Cancer Institute/Boston Children’s Cancer and Blood Disorders Center, Boston. She serves on the board of directors of the American Society of Gene and Cell Therapy. These remarks were adapted from an accompanying editorial ( N Engl J Med. 2018;378[16]:1551-2 ).
Lentiviral vector hematopoietic stem cell (HSC) gene therapy represents a promising alternative to matched-sibling donor HSC transplants for treatment of beta thalassemia, with studies to date showing a safety profile that surpasses transplants from unrelated or alternative donors.
The prospect of a curative treatment raised by the work of Dr. Thompson and her colleagues also shows the feasibility of transfusion independence for beta0/betaE patients, who carry the most common beta thalassemia genotype, and a significant reduction in transfusions even for patients with the more severe beta0/beta0 genotype.
Beta thalassemia has greatest prevalence in North Africa, the Middle East, and Asia, where access to treatments is limited and patients’ prognoses are often grim. Gene therapy for beta thalassemia could thereby represent the first large-scale implementation of this intervention in developing countries.
Bringing HSC gene therapy to more patients will require not just the availability of autologous HSCs, but of high-quality vector and reliable, high-volume manufacturing of transduced cells.
Harnessing this still-evolving technology to bring a potentially curative treatment to patients in developing countries is an exciting, but challenging, frontier for physicians and researchers involved with gene therapy.
Alessandra Biffi, MD, is director of the gene therapy program at Dana Farber Cancer Institute/Boston Children’s Cancer and Blood Disorders Center, Boston. She serves on the board of directors of the American Society of Gene and Cell Therapy. These remarks were adapted from an accompanying editorial ( N Engl J Med. 2018;378[16]:1551-2 ).
Most beta thalassemia patients were off transfusions at a median of 26 months after receiving gene therapy via a lentiviral vector, according to new results of two phase 1/2 studies regarding the use of LentiGlobin in transfusion-dependent beta thalassemia.
Of 13 patients who did not have the most severe beta0/beta0 genotype, all but 1 has become transfusion independent post transplant. Among patients who either had the beta0/beta0 genotype or had two copies of the IVS1-110 mutation, transfusions were down a median 73% annually, and three of these patients with more severe thalassemia became transfusion independent.
At the time of last data collection, hemoglobin levels in individual patients ranged from 8.2-13.7 g/dL.
“No clonal dominance related to vector integration was observed,” wrote Alexis Thompson, MD, and her collaborators, and replication-competent lentivirus had not been found in any patients.
Hematopoietic cell transplant (HCT) is an option primarily for younger beta thalassemia patients who have an HLA-matched sibling donor, the researchers wrote in the New England Journal of Medicine. Gene therapy represents an alternative to the current standard of care for patients who are not candidates for allogeneic HCT, which – without a good match – carries increased risk for rejection and graft-versus-host disease.
Patients with beta thalassemia aged 35 years or younger and without advanced organ damage were enrolled in the two studies, one conducted internationally and one conducted at a single site in France.
There were some protocol differences between the two studies; notably, the French study used enhanced red cell transfusion for 3 or more months before stem cell mobilization “to enrich for bona fide hematopoietic stem cells in the harvested CD34+ cell compartment by suppressing the erythroid lineage expansion and the skewing that is seen in beta thalassemia,” wrote Dr. Thompson, professor of pediatrics at Northwestern University, Chicago, and her colleagues.
In both studies, after mobilization, patients’ unmanipulated hematopoietic stem cells and progenitor cells were taken to a central processing facility, where CD34+ cells were enriched and then transduced with the lentiviral vector BB305, which encodes adult hemoglobin (HbA) with a T87Z amino acid substitution and thereby provides functioning Hb beta. Patients received the product via infusion after undergoing myeloablative conditioning with busulfan.
A total of 23 patients, 19 in the international study and 4 in the French study, went through mobilization and apheresis. One patient in the international study had apheresis failure, so a total of 22 patients received LentiGlobin, and all were followed for up to 2 years.
Patients were given the opportunity to participate in a follow-on open label study meant to continue for an additional 13 years after the initial 24-month period; 13 patients are currently enrolled in this long-term follow-up study.
When transfusion volume at baseline was assessed, patients in the international study were receiving a median annual red blood cell transfusion volume of 164 mL/kg per year, while the French study participants were receiving a median 182 mL/kg per year of red blood cell transfusion.
In both studies, blood HbAT87Q levels correlated with the vector copy numbers (R2, 0.75; P less than .001). Levels of HbAT87Q ranged from 3.4-10.0 g/dL.
“Other factors, such as age, genotype, and splenectomy status, did not appear to correlate with gene expression,” the researchers wrote.
An exploratory analysis looked at characteristics of patients who were able to stop transfusions after gene therapy. In this group, “the degree of hemolysis at first stabilized relative to pretransplantation levels and was fully corrected” in two patients by 36 months after treatment.
The researchers noted that the sponsor achieved “high-titer, large-scale, clinical-grade BB305 vector production and purification by ion-exchange chromatography” from a single site in the United States, which showed the feasibility of conducting this modality of gene therapy at scale.
The study was sponsored by bluebird bio, the National Institutes of Health, and by French national research organizations. Dr. Thompson reported research funding and fees from bluebird bio and other pharmaceutical companies.
SOURCE: Thompson A et al. N Engl J Med 2018;378:1479-93
Most beta thalassemia patients were off transfusions at a median of 26 months after receiving gene therapy via a lentiviral vector, according to new results of two phase 1/2 studies regarding the use of LentiGlobin in transfusion-dependent beta thalassemia.
Of 13 patients who did not have the most severe beta0/beta0 genotype, all but 1 has become transfusion independent post transplant. Among patients who either had the beta0/beta0 genotype or had two copies of the IVS1-110 mutation, transfusions were down a median 73% annually, and three of these patients with more severe thalassemia became transfusion independent.
At the time of last data collection, hemoglobin levels in individual patients ranged from 8.2-13.7 g/dL.
“No clonal dominance related to vector integration was observed,” wrote Alexis Thompson, MD, and her collaborators, and replication-competent lentivirus had not been found in any patients.
Hematopoietic cell transplant (HCT) is an option primarily for younger beta thalassemia patients who have an HLA-matched sibling donor, the researchers wrote in the New England Journal of Medicine. Gene therapy represents an alternative to the current standard of care for patients who are not candidates for allogeneic HCT, which – without a good match – carries increased risk for rejection and graft-versus-host disease.
Patients with beta thalassemia aged 35 years or younger and without advanced organ damage were enrolled in the two studies, one conducted internationally and one conducted at a single site in France.
There were some protocol differences between the two studies; notably, the French study used enhanced red cell transfusion for 3 or more months before stem cell mobilization “to enrich for bona fide hematopoietic stem cells in the harvested CD34+ cell compartment by suppressing the erythroid lineage expansion and the skewing that is seen in beta thalassemia,” wrote Dr. Thompson, professor of pediatrics at Northwestern University, Chicago, and her colleagues.
In both studies, after mobilization, patients’ unmanipulated hematopoietic stem cells and progenitor cells were taken to a central processing facility, where CD34+ cells were enriched and then transduced with the lentiviral vector BB305, which encodes adult hemoglobin (HbA) with a T87Z amino acid substitution and thereby provides functioning Hb beta. Patients received the product via infusion after undergoing myeloablative conditioning with busulfan.
A total of 23 patients, 19 in the international study and 4 in the French study, went through mobilization and apheresis. One patient in the international study had apheresis failure, so a total of 22 patients received LentiGlobin, and all were followed for up to 2 years.
Patients were given the opportunity to participate in a follow-on open label study meant to continue for an additional 13 years after the initial 24-month period; 13 patients are currently enrolled in this long-term follow-up study.
When transfusion volume at baseline was assessed, patients in the international study were receiving a median annual red blood cell transfusion volume of 164 mL/kg per year, while the French study participants were receiving a median 182 mL/kg per year of red blood cell transfusion.
In both studies, blood HbAT87Q levels correlated with the vector copy numbers (R2, 0.75; P less than .001). Levels of HbAT87Q ranged from 3.4-10.0 g/dL.
“Other factors, such as age, genotype, and splenectomy status, did not appear to correlate with gene expression,” the researchers wrote.
An exploratory analysis looked at characteristics of patients who were able to stop transfusions after gene therapy. In this group, “the degree of hemolysis at first stabilized relative to pretransplantation levels and was fully corrected” in two patients by 36 months after treatment.
The researchers noted that the sponsor achieved “high-titer, large-scale, clinical-grade BB305 vector production and purification by ion-exchange chromatography” from a single site in the United States, which showed the feasibility of conducting this modality of gene therapy at scale.
The study was sponsored by bluebird bio, the National Institutes of Health, and by French national research organizations. Dr. Thompson reported research funding and fees from bluebird bio and other pharmaceutical companies.
SOURCE: Thompson A et al. N Engl J Med 2018;378:1479-93
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Transfusion requirements were down 73% annually in patients with the most severe thalassemia.
Study details: Data from 22 transfusion-dependent patients with beta thalassemia in ongoing phase 1/2 study of gene therapy delivered via lentiviral vector.
Disclosures: The study was sponsored by bluebird bio, the National Institutes of Health, and by French national research organizations. Dr. Thompson reported research funding and fees from bluebird bio and from other pharmaceutical companies.
Source: Thompson A et al. N Engl J Med. 2018;378:1479-93.
Impaired kidney function no problem for dabigatran reversal
ORLANDO – Idarucizumab, the reversal agent for the anticoagulant dabigatran, appeared as effective in quickly reversing dabigatran’s effects in patients with severe renal dysfunction as in patients with normally working kidneys, in a post hoc analysis of data collected in the drug’s pivotal trial.
A standard dose of idarucizumab “works just as well in patients with bad kidney function as it does in patients with preserved kidney function,” John W. Eikelboom, MD, said at the annual meeting of the American College of Cardiology. “The time to cessation of bleeding and the degree of normal hemostasis achieved was consistent” across the entire range of renal function examined, from severe renal dysfunction, with a creatinine clearance rate of less than 30 mL/min, to normal function, with an estimated rate of 80 mL/min or greater.
The ability of idarucizumab (Praxbind), conditionally approved by the Food and Drug Administration in 2015 and then fully approved in April 2018, to work in patients with impaired renal function has been an open question and concern because dabigatran (Pradaxa) is excreted renally, so it builds to unusually high levels in patients with poor kidney function. “Plasma dabigatran levels might be sky high, so a standard dose of idarucizumab might not work. That’s been a fear of clinicians,” explained Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont.
To examine whether idarucizumab’s activity varied by renal function he used data from the patients enrolled in the RE-VERSE AD (Reversal Effects of Idarucizumab on Active Dabigatran) study, the pivotal dataset that led to idarucizumab’s U.S. approval. The new, post hoc analysis divided patients into four subgroups based on their kidney function, and focused on the 489 patients for whom renal data were available out of the 503 patients in the study (N Engl J Med. 2017 Aug 3;377[5]:431-41). The subgroups included 91 patients with severe dysfunction with a creatinine clearance rate of less than 30 mL/min; 127 with moderate dysfunction and a clearance rate of 30-49 mL/min; 163 with mild dysfunction and a clearance rate of 50-79 mL/min; and 108 with normal function and a creatinine clearance of at least 80 mL/min.
Patients in the subgroup with severe renal dysfunction had the worst clinical profile overall, and as predicted, had a markedly elevated average plasma level of dabigatran, 231 ng/mL, nearly five times higher than the 47-ng/mL average level in patients with normal renal function.
The ability of a single, standard dose of idarucizumab to reverse the anticoagulant effects of dabigatran were essentially identical across the four strata of renal activity, with 98% of patients in both the severely impaired subgroup and the normal subgroup having 100% reversal within 4 hours of treatment, Dr. Eikelboom reported. Every patient included in the analysis had more than 50% reversal.
The study followed patients to 12-24 hours after they received idarucizumab, and 55% of patients with severe renal dysfunction showed a plasma dabigatran level that crept back toward a clinically meaningful level and so might need a second idarucizumab dose. In contrast, this happened in 8% of patients with normal renal function.
In patients with severe renal dysfunction given idarucizumab, “be alert for a recurrent bleed,” which could require a second dose of idarucizumab, Dr. Eikelboom suggested.
SOURCE: Eikelboom JW et al. ACC 18, Abstract 1231M-11.
ORLANDO – Idarucizumab, the reversal agent for the anticoagulant dabigatran, appeared as effective in quickly reversing dabigatran’s effects in patients with severe renal dysfunction as in patients with normally working kidneys, in a post hoc analysis of data collected in the drug’s pivotal trial.
A standard dose of idarucizumab “works just as well in patients with bad kidney function as it does in patients with preserved kidney function,” John W. Eikelboom, MD, said at the annual meeting of the American College of Cardiology. “The time to cessation of bleeding and the degree of normal hemostasis achieved was consistent” across the entire range of renal function examined, from severe renal dysfunction, with a creatinine clearance rate of less than 30 mL/min, to normal function, with an estimated rate of 80 mL/min or greater.
The ability of idarucizumab (Praxbind), conditionally approved by the Food and Drug Administration in 2015 and then fully approved in April 2018, to work in patients with impaired renal function has been an open question and concern because dabigatran (Pradaxa) is excreted renally, so it builds to unusually high levels in patients with poor kidney function. “Plasma dabigatran levels might be sky high, so a standard dose of idarucizumab might not work. That’s been a fear of clinicians,” explained Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont.
To examine whether idarucizumab’s activity varied by renal function he used data from the patients enrolled in the RE-VERSE AD (Reversal Effects of Idarucizumab on Active Dabigatran) study, the pivotal dataset that led to idarucizumab’s U.S. approval. The new, post hoc analysis divided patients into four subgroups based on their kidney function, and focused on the 489 patients for whom renal data were available out of the 503 patients in the study (N Engl J Med. 2017 Aug 3;377[5]:431-41). The subgroups included 91 patients with severe dysfunction with a creatinine clearance rate of less than 30 mL/min; 127 with moderate dysfunction and a clearance rate of 30-49 mL/min; 163 with mild dysfunction and a clearance rate of 50-79 mL/min; and 108 with normal function and a creatinine clearance of at least 80 mL/min.
Patients in the subgroup with severe renal dysfunction had the worst clinical profile overall, and as predicted, had a markedly elevated average plasma level of dabigatran, 231 ng/mL, nearly five times higher than the 47-ng/mL average level in patients with normal renal function.
The ability of a single, standard dose of idarucizumab to reverse the anticoagulant effects of dabigatran were essentially identical across the four strata of renal activity, with 98% of patients in both the severely impaired subgroup and the normal subgroup having 100% reversal within 4 hours of treatment, Dr. Eikelboom reported. Every patient included in the analysis had more than 50% reversal.
The study followed patients to 12-24 hours after they received idarucizumab, and 55% of patients with severe renal dysfunction showed a plasma dabigatran level that crept back toward a clinically meaningful level and so might need a second idarucizumab dose. In contrast, this happened in 8% of patients with normal renal function.
In patients with severe renal dysfunction given idarucizumab, “be alert for a recurrent bleed,” which could require a second dose of idarucizumab, Dr. Eikelboom suggested.
SOURCE: Eikelboom JW et al. ACC 18, Abstract 1231M-11.
ORLANDO – Idarucizumab, the reversal agent for the anticoagulant dabigatran, appeared as effective in quickly reversing dabigatran’s effects in patients with severe renal dysfunction as in patients with normally working kidneys, in a post hoc analysis of data collected in the drug’s pivotal trial.
A standard dose of idarucizumab “works just as well in patients with bad kidney function as it does in patients with preserved kidney function,” John W. Eikelboom, MD, said at the annual meeting of the American College of Cardiology. “The time to cessation of bleeding and the degree of normal hemostasis achieved was consistent” across the entire range of renal function examined, from severe renal dysfunction, with a creatinine clearance rate of less than 30 mL/min, to normal function, with an estimated rate of 80 mL/min or greater.
The ability of idarucizumab (Praxbind), conditionally approved by the Food and Drug Administration in 2015 and then fully approved in April 2018, to work in patients with impaired renal function has been an open question and concern because dabigatran (Pradaxa) is excreted renally, so it builds to unusually high levels in patients with poor kidney function. “Plasma dabigatran levels might be sky high, so a standard dose of idarucizumab might not work. That’s been a fear of clinicians,” explained Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont.
To examine whether idarucizumab’s activity varied by renal function he used data from the patients enrolled in the RE-VERSE AD (Reversal Effects of Idarucizumab on Active Dabigatran) study, the pivotal dataset that led to idarucizumab’s U.S. approval. The new, post hoc analysis divided patients into four subgroups based on their kidney function, and focused on the 489 patients for whom renal data were available out of the 503 patients in the study (N Engl J Med. 2017 Aug 3;377[5]:431-41). The subgroups included 91 patients with severe dysfunction with a creatinine clearance rate of less than 30 mL/min; 127 with moderate dysfunction and a clearance rate of 30-49 mL/min; 163 with mild dysfunction and a clearance rate of 50-79 mL/min; and 108 with normal function and a creatinine clearance of at least 80 mL/min.
Patients in the subgroup with severe renal dysfunction had the worst clinical profile overall, and as predicted, had a markedly elevated average plasma level of dabigatran, 231 ng/mL, nearly five times higher than the 47-ng/mL average level in patients with normal renal function.
The ability of a single, standard dose of idarucizumab to reverse the anticoagulant effects of dabigatran were essentially identical across the four strata of renal activity, with 98% of patients in both the severely impaired subgroup and the normal subgroup having 100% reversal within 4 hours of treatment, Dr. Eikelboom reported. Every patient included in the analysis had more than 50% reversal.
The study followed patients to 12-24 hours after they received idarucizumab, and 55% of patients with severe renal dysfunction showed a plasma dabigatran level that crept back toward a clinically meaningful level and so might need a second idarucizumab dose. In contrast, this happened in 8% of patients with normal renal function.
In patients with severe renal dysfunction given idarucizumab, “be alert for a recurrent bleed,” which could require a second dose of idarucizumab, Dr. Eikelboom suggested.
SOURCE: Eikelboom JW et al. ACC 18, Abstract 1231M-11.
REPORTING FROM ACC 18
Key clinical point: Renal function had no impact on idarucizumab’s efficacy for dabigatran reversal.
Major finding: Complete dabigatran reversal occurred in 98% of patients with severe renal dysfunction who received idarucizumab.
Study details: Post hoc analysis of data from RE-VERSE AD, idarucizumab’s pivotal trial with 503 patients.
Disclosures: RE-VERSE AD was funded by Boehringer Ingelheim, the company that markets idarucizumab (Praxbind) and dabigatran (Pradaxa). Dr. Eikelboom has been a consultant to and has received research support from Boehringer Ingelheim, as well as from Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Janssen, and Pfizer.
Source: Eikelboom JW et al. ACC 18, Abstract 1231M-11.
Updates of ongoing clinical trials
Randomized Phase 3 Trial Evaluating the Addition of the IGF-1R Monoclonal Antibody Ganitumab (AMG 479, NSC# 750008) to Multiagent Chemotherapy for Patients With Newly Diagnosed Metastatic Ewing Sarcoma
NCT02306161
Sponsor: National Cancer Institute (NCI)
Principal Investigator: Steven DuBois, Children’s Oncology Group and Dana-Farber Cancer Institute, Boston.
Study locations: Over 300 U.S. cancer centers
Study summary: This randomized phase 3 trial examines whether the monoclonal antibody ganitumab plus combination chemotherapy (vincristine sulfate, doxorubicin hydrochloride, cyclophosphamide, ifosfamide, and etoposide) improves event-free survival for patients with newly-diagnosed, metastatic Ewing sarcoma. Secondary outcomes include overall survival rate and comparative evaluations of toxicity.
Patients are randomized to induction and consolidation therapy with vincristine sulfate, doxorubicin hydrochloride and cyclophosphamide [VDC] and ifosfamide and etoposide [IE]) or to the same regimen plus ganitumab. Between weeks 13-18 of the trial, patients undergo surgery and/or radiation therapy for local control. Patients with lung metastases undergo definitive stereotactic body radiation therapy or external beam radiation therapy over 5 days.
Study inclusion summary: Patients up to 50 years old are eligible to participate in this trial if they have newly-diagnosed Ewing sarcoma or peripheral primitive neuroectodermal tumor (PNET) arising from bone or soft tissue and with metastatic disease involving lung, bone, bone marrow, or other metastatic site. Submission of pre-treatment serum, tumor tissue and whole blood is required. Patients should only have had a biopsy of the primary tumor without an attempt at complete or partial resection; patients will still be eligible if excision was attempted or accomplished as long as adequate anatomic imaging (MRI for most primary tumor sites) was obtained prior to surgery. Creatinine clearance or radioisotope glomerular filtration rate (GFR) must be at least 70 mL/min/1.73 m2 or greater. Total bilirubin must be less than 1.5 times the upper limit of normal, alanine aminotransferase must be less than 3 times the upper limit of normal, blood sugar must be normal, and heart ejection fraction must exceed 50%.
Induction therapy: Patients receive vincristine sulfate intravenously (IV) over 1 minute on day 1; doxorubicin hydrochloride IV over 1-15 minutes on days 1 and 2; and cyclophosphamide IV over 30-60 minutes on day 1 of weeks 1, 5, and 9; and ifosfamide IV over 1 hour on days 1 to 5 and etoposide IV over 1-2 hours on days 1 to 5 of weeks 3, 7, and 11. Patients in the control group receive induction therapy and placebo and patients in the treatment group receive induction therapy and ganitumab IV over 30-60 minutes or 60-120 minutes on day 1 of weeks 1, 3, 5, 7, 9, and 11.
Consolidation therapy: Patients receive vincristine sulfate IV over 1 minute on day 1 of weeks 1, 7, 9, and 13; doxorubicin hydrochloride IV over 1-15 minutes on days 1 and 2 of weeks 1 and 7; cyclophosphamide IV over 30-60 minutes on day 1 of weeks 1, 7, 9, and 13; ifosfamide IV over 1 hour on days 1 to 5 of weeks 3, 5, 11, and 15; and etoposide IV over 1-2 hours on days 1 to 5 of weeks 3, 5, 11, and 15. In addition to this standard consolidation therapy, pPatients in the active treatment group receive ganitumab IV over 30-60 minutes or 60-120 minutes on day 1 of weeks 7, 9, 11, 13, and 15.
Maintenance therapy: Patients receive ganitumab IV over 30-60 minutes or 60-120 minutes on day 1 in weeks 1, 4, 7, 10, 13, 16, 19, and 22.
Follow up: After completion of study treatment, patients are followed for 10 years.
Combination Chemotherapy With or Without Temsirolimus in Treating Patients With Intermediate Risk Rhabdomyosarcoma
NCT02567435
Sponsor: National Cancer Institute (NCI)
Principal Investigator: Abha Gupta, Children’s Oncology Group, The Hospital for Sick Children and Princess Margaret Cancer Centre.
Study locations: 293 cancer centers in the U.S. and Canada
Study summary: This randomized phase 3 trial compares standard combination chemotherapy with and without temsirolimus for patients with rhabdomyosarcoma that has an intermediate chance of recurrence after treatment. It is not yet known whether combination chemotherapy or combination chemotherapy plus temsirolimus is more effective in treating patients with intermediate-risk rhabdomyosarcoma.
Study inclusion summary: Patients up to age 40 with newly diagnosed RMS of any subtype, except adult-type pleomorphic, based upon institutional histopathologic classification, are eligible to enroll on the study. Lansky performance status score must be at least 50 for patients age 16 years and under; Karnofsky performance status score must be 50 or greater for patients over age 16. Peripheral absolute neutrophil count must be at least 750/uL and platelet count at least 75,000/uL. Creatinine clearance or radioisotope glomerular filtration rate must be at least 70 mL/min/1.73 m2. Total bilirubin must be no more than 1.5 times the upper limit of normal for patient age.
Treatment regimen: Patients are randomized to one of three study arms. One group receives vincristine sulfate IV over 1 minute on day 1 of weeks 1-13, 16, 17, 19, 20, 22-26, 28, 31-34, 37, 38, and 40, dactinomycin IV over 1-5 minutes on day 1 of weeks 1, 7, 13, 22, 28, 34, and 40, cyclophosphamide IV over 60 minutes on day 1 of weeks 1, 7, 13, 22, 28, 34, and 40, irinotecan hydrochloride IV over 90 minutes on days 1-5 of weeks 4, 10, 16, 19, 25, 31, and 37. The second group receives the same regimen plus temsirolimus IV over 30-60 minutes on day 1 of weeks 1-12 and 21-42. The third group receives vincristine sulfate IV over 1 minute on day 1 of weeks 1-10 and 13-22, dactinomycin IV over 1-5 minutes on day 1 of weeks 1, 4, 7, 10, 13, 16, 19, and 22, cyclophosphamide IV over 60 minutes on day 1 of weeks 1, 4, 7, and 10. Patients in all three groups also undergo radiation therapy beginning at week 13 for 6 weeks. Treatment continues in all three groups in the absence of disease progression or unacceptable toxicity.
Outcome Measures: The primary outcome measure is event-free survival (EFS) measured from study enrollment to the first occurrence of progression, relapse, second malignant neoplasm, or death as a first event. The secondary outcome measure is overall survival measured from study enrollment to death from any cause, assessed up to 10 years. TSJ
Randomized Phase 3 Trial Evaluating the Addition of the IGF-1R Monoclonal Antibody Ganitumab (AMG 479, NSC# 750008) to Multiagent Chemotherapy for Patients With Newly Diagnosed Metastatic Ewing Sarcoma
NCT02306161
Sponsor: National Cancer Institute (NCI)
Principal Investigator: Steven DuBois, Children’s Oncology Group and Dana-Farber Cancer Institute, Boston.
Study locations: Over 300 U.S. cancer centers
Study summary: This randomized phase 3 trial examines whether the monoclonal antibody ganitumab plus combination chemotherapy (vincristine sulfate, doxorubicin hydrochloride, cyclophosphamide, ifosfamide, and etoposide) improves event-free survival for patients with newly-diagnosed, metastatic Ewing sarcoma. Secondary outcomes include overall survival rate and comparative evaluations of toxicity.
Patients are randomized to induction and consolidation therapy with vincristine sulfate, doxorubicin hydrochloride and cyclophosphamide [VDC] and ifosfamide and etoposide [IE]) or to the same regimen plus ganitumab. Between weeks 13-18 of the trial, patients undergo surgery and/or radiation therapy for local control. Patients with lung metastases undergo definitive stereotactic body radiation therapy or external beam radiation therapy over 5 days.
Study inclusion summary: Patients up to 50 years old are eligible to participate in this trial if they have newly-diagnosed Ewing sarcoma or peripheral primitive neuroectodermal tumor (PNET) arising from bone or soft tissue and with metastatic disease involving lung, bone, bone marrow, or other metastatic site. Submission of pre-treatment serum, tumor tissue and whole blood is required. Patients should only have had a biopsy of the primary tumor without an attempt at complete or partial resection; patients will still be eligible if excision was attempted or accomplished as long as adequate anatomic imaging (MRI for most primary tumor sites) was obtained prior to surgery. Creatinine clearance or radioisotope glomerular filtration rate (GFR) must be at least 70 mL/min/1.73 m2 or greater. Total bilirubin must be less than 1.5 times the upper limit of normal, alanine aminotransferase must be less than 3 times the upper limit of normal, blood sugar must be normal, and heart ejection fraction must exceed 50%.
Induction therapy: Patients receive vincristine sulfate intravenously (IV) over 1 minute on day 1; doxorubicin hydrochloride IV over 1-15 minutes on days 1 and 2; and cyclophosphamide IV over 30-60 minutes on day 1 of weeks 1, 5, and 9; and ifosfamide IV over 1 hour on days 1 to 5 and etoposide IV over 1-2 hours on days 1 to 5 of weeks 3, 7, and 11. Patients in the control group receive induction therapy and placebo and patients in the treatment group receive induction therapy and ganitumab IV over 30-60 minutes or 60-120 minutes on day 1 of weeks 1, 3, 5, 7, 9, and 11.
Consolidation therapy: Patients receive vincristine sulfate IV over 1 minute on day 1 of weeks 1, 7, 9, and 13; doxorubicin hydrochloride IV over 1-15 minutes on days 1 and 2 of weeks 1 and 7; cyclophosphamide IV over 30-60 minutes on day 1 of weeks 1, 7, 9, and 13; ifosfamide IV over 1 hour on days 1 to 5 of weeks 3, 5, 11, and 15; and etoposide IV over 1-2 hours on days 1 to 5 of weeks 3, 5, 11, and 15. In addition to this standard consolidation therapy, pPatients in the active treatment group receive ganitumab IV over 30-60 minutes or 60-120 minutes on day 1 of weeks 7, 9, 11, 13, and 15.
Maintenance therapy: Patients receive ganitumab IV over 30-60 minutes or 60-120 minutes on day 1 in weeks 1, 4, 7, 10, 13, 16, 19, and 22.
Follow up: After completion of study treatment, patients are followed for 10 years.
Combination Chemotherapy With or Without Temsirolimus in Treating Patients With Intermediate Risk Rhabdomyosarcoma
NCT02567435
Sponsor: National Cancer Institute (NCI)
Principal Investigator: Abha Gupta, Children’s Oncology Group, The Hospital for Sick Children and Princess Margaret Cancer Centre.
Study locations: 293 cancer centers in the U.S. and Canada
Study summary: This randomized phase 3 trial compares standard combination chemotherapy with and without temsirolimus for patients with rhabdomyosarcoma that has an intermediate chance of recurrence after treatment. It is not yet known whether combination chemotherapy or combination chemotherapy plus temsirolimus is more effective in treating patients with intermediate-risk rhabdomyosarcoma.
Study inclusion summary: Patients up to age 40 with newly diagnosed RMS of any subtype, except adult-type pleomorphic, based upon institutional histopathologic classification, are eligible to enroll on the study. Lansky performance status score must be at least 50 for patients age 16 years and under; Karnofsky performance status score must be 50 or greater for patients over age 16. Peripheral absolute neutrophil count must be at least 750/uL and platelet count at least 75,000/uL. Creatinine clearance or radioisotope glomerular filtration rate must be at least 70 mL/min/1.73 m2. Total bilirubin must be no more than 1.5 times the upper limit of normal for patient age.
Treatment regimen: Patients are randomized to one of three study arms. One group receives vincristine sulfate IV over 1 minute on day 1 of weeks 1-13, 16, 17, 19, 20, 22-26, 28, 31-34, 37, 38, and 40, dactinomycin IV over 1-5 minutes on day 1 of weeks 1, 7, 13, 22, 28, 34, and 40, cyclophosphamide IV over 60 minutes on day 1 of weeks 1, 7, 13, 22, 28, 34, and 40, irinotecan hydrochloride IV over 90 minutes on days 1-5 of weeks 4, 10, 16, 19, 25, 31, and 37. The second group receives the same regimen plus temsirolimus IV over 30-60 minutes on day 1 of weeks 1-12 and 21-42. The third group receives vincristine sulfate IV over 1 minute on day 1 of weeks 1-10 and 13-22, dactinomycin IV over 1-5 minutes on day 1 of weeks 1, 4, 7, 10, 13, 16, 19, and 22, cyclophosphamide IV over 60 minutes on day 1 of weeks 1, 4, 7, and 10. Patients in all three groups also undergo radiation therapy beginning at week 13 for 6 weeks. Treatment continues in all three groups in the absence of disease progression or unacceptable toxicity.
Outcome Measures: The primary outcome measure is event-free survival (EFS) measured from study enrollment to the first occurrence of progression, relapse, second malignant neoplasm, or death as a first event. The secondary outcome measure is overall survival measured from study enrollment to death from any cause, assessed up to 10 years. TSJ
Randomized Phase 3 Trial Evaluating the Addition of the IGF-1R Monoclonal Antibody Ganitumab (AMG 479, NSC# 750008) to Multiagent Chemotherapy for Patients With Newly Diagnosed Metastatic Ewing Sarcoma
NCT02306161
Sponsor: National Cancer Institute (NCI)
Principal Investigator: Steven DuBois, Children’s Oncology Group and Dana-Farber Cancer Institute, Boston.
Study locations: Over 300 U.S. cancer centers
Study summary: This randomized phase 3 trial examines whether the monoclonal antibody ganitumab plus combination chemotherapy (vincristine sulfate, doxorubicin hydrochloride, cyclophosphamide, ifosfamide, and etoposide) improves event-free survival for patients with newly-diagnosed, metastatic Ewing sarcoma. Secondary outcomes include overall survival rate and comparative evaluations of toxicity.
Patients are randomized to induction and consolidation therapy with vincristine sulfate, doxorubicin hydrochloride and cyclophosphamide [VDC] and ifosfamide and etoposide [IE]) or to the same regimen plus ganitumab. Between weeks 13-18 of the trial, patients undergo surgery and/or radiation therapy for local control. Patients with lung metastases undergo definitive stereotactic body radiation therapy or external beam radiation therapy over 5 days.
Study inclusion summary: Patients up to 50 years old are eligible to participate in this trial if they have newly-diagnosed Ewing sarcoma or peripheral primitive neuroectodermal tumor (PNET) arising from bone or soft tissue and with metastatic disease involving lung, bone, bone marrow, or other metastatic site. Submission of pre-treatment serum, tumor tissue and whole blood is required. Patients should only have had a biopsy of the primary tumor without an attempt at complete or partial resection; patients will still be eligible if excision was attempted or accomplished as long as adequate anatomic imaging (MRI for most primary tumor sites) was obtained prior to surgery. Creatinine clearance or radioisotope glomerular filtration rate (GFR) must be at least 70 mL/min/1.73 m2 or greater. Total bilirubin must be less than 1.5 times the upper limit of normal, alanine aminotransferase must be less than 3 times the upper limit of normal, blood sugar must be normal, and heart ejection fraction must exceed 50%.
Induction therapy: Patients receive vincristine sulfate intravenously (IV) over 1 minute on day 1; doxorubicin hydrochloride IV over 1-15 minutes on days 1 and 2; and cyclophosphamide IV over 30-60 minutes on day 1 of weeks 1, 5, and 9; and ifosfamide IV over 1 hour on days 1 to 5 and etoposide IV over 1-2 hours on days 1 to 5 of weeks 3, 7, and 11. Patients in the control group receive induction therapy and placebo and patients in the treatment group receive induction therapy and ganitumab IV over 30-60 minutes or 60-120 minutes on day 1 of weeks 1, 3, 5, 7, 9, and 11.
Consolidation therapy: Patients receive vincristine sulfate IV over 1 minute on day 1 of weeks 1, 7, 9, and 13; doxorubicin hydrochloride IV over 1-15 minutes on days 1 and 2 of weeks 1 and 7; cyclophosphamide IV over 30-60 minutes on day 1 of weeks 1, 7, 9, and 13; ifosfamide IV over 1 hour on days 1 to 5 of weeks 3, 5, 11, and 15; and etoposide IV over 1-2 hours on days 1 to 5 of weeks 3, 5, 11, and 15. In addition to this standard consolidation therapy, pPatients in the active treatment group receive ganitumab IV over 30-60 minutes or 60-120 minutes on day 1 of weeks 7, 9, 11, 13, and 15.
Maintenance therapy: Patients receive ganitumab IV over 30-60 minutes or 60-120 minutes on day 1 in weeks 1, 4, 7, 10, 13, 16, 19, and 22.
Follow up: After completion of study treatment, patients are followed for 10 years.
Combination Chemotherapy With or Without Temsirolimus in Treating Patients With Intermediate Risk Rhabdomyosarcoma
NCT02567435
Sponsor: National Cancer Institute (NCI)
Principal Investigator: Abha Gupta, Children’s Oncology Group, The Hospital for Sick Children and Princess Margaret Cancer Centre.
Study locations: 293 cancer centers in the U.S. and Canada
Study summary: This randomized phase 3 trial compares standard combination chemotherapy with and without temsirolimus for patients with rhabdomyosarcoma that has an intermediate chance of recurrence after treatment. It is not yet known whether combination chemotherapy or combination chemotherapy plus temsirolimus is more effective in treating patients with intermediate-risk rhabdomyosarcoma.
Study inclusion summary: Patients up to age 40 with newly diagnosed RMS of any subtype, except adult-type pleomorphic, based upon institutional histopathologic classification, are eligible to enroll on the study. Lansky performance status score must be at least 50 for patients age 16 years and under; Karnofsky performance status score must be 50 or greater for patients over age 16. Peripheral absolute neutrophil count must be at least 750/uL and platelet count at least 75,000/uL. Creatinine clearance or radioisotope glomerular filtration rate must be at least 70 mL/min/1.73 m2. Total bilirubin must be no more than 1.5 times the upper limit of normal for patient age.
Treatment regimen: Patients are randomized to one of three study arms. One group receives vincristine sulfate IV over 1 minute on day 1 of weeks 1-13, 16, 17, 19, 20, 22-26, 28, 31-34, 37, 38, and 40, dactinomycin IV over 1-5 minutes on day 1 of weeks 1, 7, 13, 22, 28, 34, and 40, cyclophosphamide IV over 60 minutes on day 1 of weeks 1, 7, 13, 22, 28, 34, and 40, irinotecan hydrochloride IV over 90 minutes on days 1-5 of weeks 4, 10, 16, 19, 25, 31, and 37. The second group receives the same regimen plus temsirolimus IV over 30-60 minutes on day 1 of weeks 1-12 and 21-42. The third group receives vincristine sulfate IV over 1 minute on day 1 of weeks 1-10 and 13-22, dactinomycin IV over 1-5 minutes on day 1 of weeks 1, 4, 7, 10, 13, 16, 19, and 22, cyclophosphamide IV over 60 minutes on day 1 of weeks 1, 4, 7, and 10. Patients in all three groups also undergo radiation therapy beginning at week 13 for 6 weeks. Treatment continues in all three groups in the absence of disease progression or unacceptable toxicity.
Outcome Measures: The primary outcome measure is event-free survival (EFS) measured from study enrollment to the first occurrence of progression, relapse, second malignant neoplasm, or death as a first event. The secondary outcome measure is overall survival measured from study enrollment to death from any cause, assessed up to 10 years. TSJ
Picosecond 755-nm laser found effective for neck rejuvenation
DALLAS –
“It’s important to note that response was variable, but many patients were satisfied with the treatment,” Hana Jeon, MD said at the annual conference of the American Society for Laser Medicine and Surgery, Inc. “Further studies are needed to identify the clinical characteristics of neck laxity that would most benefit from this treatment.”
The researchers enrolled 25 patients with an average age of 58 years. The laser treatment settings were a 6-mm spot side-delivered at a fluence of 0.71 J/cm2 in a pulse width of 750 picoseconds. The patients were treated five times on the neck every 2-4 weeks, and follow-up visits were scheduled for 1 month and 3 months after the last treatment. Digital photos were taken at each visit. Formal assessment tools included patient and physician satisfaction scores and the Global Aesthetic Improvement Scale. In all, 21 women and 3 men completed the study. The majority (72%) had Fitzpatrick skin type II, while 16% had type III, 8% had type I, and 4% had type IV. An average of 5,042 pulses were delivered during each treatment session. The majority of patients (84%) required no anesthesia, while the rest used topical numbing medicine from 30 minutes to an hour prior to the procedure.
Dr. Jeon reported that the average pain score during the procedure was 4.7 on a 10-point scale. Forced-air cooling was used for comfort, and on average, mild redness following the treatment lasted less than 1 day (a mean of 0.6 days, with a range of 0-5 days). Mild pain also lasted less than 1 day (a mean of 0.1 days, with a range of 0-2 days). No swelling, crusting, bruising, bleeding, infection, blistering, scarring, burn, or dyspigmentation occurred.
On the Global Aesthetic Improvement Scale at 1 and 3 months, physicians described 43% and 23% of cases, respectively, as “improved,” 17% and 18% of cases as “much improved,” and 4% and 9% of cases as “extremely improved.”
At 3 months, 35% of patients said they would be “somewhat likely” to recommend the procedure, and 30% said they would be “extremely likely” to recommend it.
Dr. Jeon reported having no financial disclosures.
DALLAS –
“It’s important to note that response was variable, but many patients were satisfied with the treatment,” Hana Jeon, MD said at the annual conference of the American Society for Laser Medicine and Surgery, Inc. “Further studies are needed to identify the clinical characteristics of neck laxity that would most benefit from this treatment.”
The researchers enrolled 25 patients with an average age of 58 years. The laser treatment settings were a 6-mm spot side-delivered at a fluence of 0.71 J/cm2 in a pulse width of 750 picoseconds. The patients were treated five times on the neck every 2-4 weeks, and follow-up visits were scheduled for 1 month and 3 months after the last treatment. Digital photos were taken at each visit. Formal assessment tools included patient and physician satisfaction scores and the Global Aesthetic Improvement Scale. In all, 21 women and 3 men completed the study. The majority (72%) had Fitzpatrick skin type II, while 16% had type III, 8% had type I, and 4% had type IV. An average of 5,042 pulses were delivered during each treatment session. The majority of patients (84%) required no anesthesia, while the rest used topical numbing medicine from 30 minutes to an hour prior to the procedure.
Dr. Jeon reported that the average pain score during the procedure was 4.7 on a 10-point scale. Forced-air cooling was used for comfort, and on average, mild redness following the treatment lasted less than 1 day (a mean of 0.6 days, with a range of 0-5 days). Mild pain also lasted less than 1 day (a mean of 0.1 days, with a range of 0-2 days). No swelling, crusting, bruising, bleeding, infection, blistering, scarring, burn, or dyspigmentation occurred.
On the Global Aesthetic Improvement Scale at 1 and 3 months, physicians described 43% and 23% of cases, respectively, as “improved,” 17% and 18% of cases as “much improved,” and 4% and 9% of cases as “extremely improved.”
At 3 months, 35% of patients said they would be “somewhat likely” to recommend the procedure, and 30% said they would be “extremely likely” to recommend it.
Dr. Jeon reported having no financial disclosures.
DALLAS –
“It’s important to note that response was variable, but many patients were satisfied with the treatment,” Hana Jeon, MD said at the annual conference of the American Society for Laser Medicine and Surgery, Inc. “Further studies are needed to identify the clinical characteristics of neck laxity that would most benefit from this treatment.”
The researchers enrolled 25 patients with an average age of 58 years. The laser treatment settings were a 6-mm spot side-delivered at a fluence of 0.71 J/cm2 in a pulse width of 750 picoseconds. The patients were treated five times on the neck every 2-4 weeks, and follow-up visits were scheduled for 1 month and 3 months after the last treatment. Digital photos were taken at each visit. Formal assessment tools included patient and physician satisfaction scores and the Global Aesthetic Improvement Scale. In all, 21 women and 3 men completed the study. The majority (72%) had Fitzpatrick skin type II, while 16% had type III, 8% had type I, and 4% had type IV. An average of 5,042 pulses were delivered during each treatment session. The majority of patients (84%) required no anesthesia, while the rest used topical numbing medicine from 30 minutes to an hour prior to the procedure.
Dr. Jeon reported that the average pain score during the procedure was 4.7 on a 10-point scale. Forced-air cooling was used for comfort, and on average, mild redness following the treatment lasted less than 1 day (a mean of 0.6 days, with a range of 0-5 days). Mild pain also lasted less than 1 day (a mean of 0.1 days, with a range of 0-2 days). No swelling, crusting, bruising, bleeding, infection, blistering, scarring, burn, or dyspigmentation occurred.
On the Global Aesthetic Improvement Scale at 1 and 3 months, physicians described 43% and 23% of cases, respectively, as “improved,” 17% and 18% of cases as “much improved,” and 4% and 9% of cases as “extremely improved.”
At 3 months, 35% of patients said they would be “somewhat likely” to recommend the procedure, and 30% said they would be “extremely likely” to recommend it.
Dr. Jeon reported having no financial disclosures.
REPORTING FROM ASLMS 2018
Key clinical point: Response to using a picosecond 755-nm laser with focus lens array for neck rejuvenation was variable.
Major finding: On the Global Aesthetic Improvement Scale at 1 and 3 months, physicians described 43% and 23% of cases, respectively, as “improved.”
Study details: A single-center study of 25 patients treated for neck laxity.
Disclosures: Dr. Jeon reported having no financial disclosures.