User login
Drug receives orphan designation for ALL
The US Food and Drug Administration (FDA) has granted orphan drug designation to LBS-007 as a treatment for acute lymphoblastic leukemia (ALL).
LBS-007 is a non-ATP cell-cycle inhibitor targeting a range of cancers.
LBS-007 functions by blocking the kinase activity of CDC7, a key regulator of the cancer cell cycle.
Inhibiting CDC7 stops the proliferation of tumor cells and results in cell death.
Lin BioScience, Inc., the company developing LBS-007, said the drug has demonstrated “very potent activity” against leukemia and solid tumors in preclinical studies.
The company is expected to launch a phase 1 trial of LBS-007 in drug-resistant and refractory acute leukemia in the fourth quarter of 2018.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan drug designation to LBS-007 as a treatment for acute lymphoblastic leukemia (ALL).
LBS-007 is a non-ATP cell-cycle inhibitor targeting a range of cancers.
LBS-007 functions by blocking the kinase activity of CDC7, a key regulator of the cancer cell cycle.
Inhibiting CDC7 stops the proliferation of tumor cells and results in cell death.
Lin BioScience, Inc., the company developing LBS-007, said the drug has demonstrated “very potent activity” against leukemia and solid tumors in preclinical studies.
The company is expected to launch a phase 1 trial of LBS-007 in drug-resistant and refractory acute leukemia in the fourth quarter of 2018.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan drug designation to LBS-007 as a treatment for acute lymphoblastic leukemia (ALL).
LBS-007 is a non-ATP cell-cycle inhibitor targeting a range of cancers.
LBS-007 functions by blocking the kinase activity of CDC7, a key regulator of the cancer cell cycle.
Inhibiting CDC7 stops the proliferation of tumor cells and results in cell death.
Lin BioScience, Inc., the company developing LBS-007, said the drug has demonstrated “very potent activity” against leukemia and solid tumors in preclinical studies.
The company is expected to launch a phase 1 trial of LBS-007 in drug-resistant and refractory acute leukemia in the fourth quarter of 2018.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
Psoriasis duration reflects cardiovascular event risk
KAUAI, HAWAII – The recent report that the risk of a major adverse cardiovascular event increases by 1% more than in the general population for each additional year of psoriasis duration is sobering news for physicians who treat pediatric psoriasis.
“If I have a 16-year-old who has a 5-year history of psoriasis, what does that mean for when she’s 30 or 40? And should we be intervening more aggressively?” Lawrence F. Eichenfield, MD, asked at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even though there’s not a great deal of evidence, there’s some evidence to rationalize early screening in psoriasis,” according to Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego.
Psoriasis develops during childhood in almost one-third of patients.
The pediatric psoriasis screening guidelines describe a simple routine screening program and timeline for early identification of overweight or obesity, type 2 diabetes, hypertension, nonalcoholic fatty liver disease, anxiety, depression, substance abuse, inflammatory bowel disease, and quality of life issues, all of which are encountered with increased frequency in pediatric psoriasis patients. A fasting lipid panel is recommended in children aged 9-11 years with psoriasis and again at age 17-21 years.
“Don’t forget arthritis. For a kid with psoriasis, at every office visit, I ask about morning stiffness or limp. Those are probably the two most sensitive questions in screening for psoriatic arthritis,” according to Dr. Eichenfield.
It has been clear for some time that the skin is not the only organ affected by psoriatic inflammation. The study that quantified the relationship between psoriasis duration and cardiovascular risk – a 1% increase for each year of psoriasis – was a collaboration between investigators at the University of Copenhagen and the University of Pennsylvania, Philadelphia.
The two-part project included aortal imaging of 190 psoriasis patients using fludeoxyglucose F 18 PET/CT scan, which showed a strong relationship between duration of psoriasis and the degree of vascular inflammation. This was bolstered by a population-based study using Danish national registry data on 87,161 psoriasis patients and 4.2 million controls from the general Danish population (J Am Acad Dermatol. 2017 Oct;77[4]:650-56.e3).
Dr. Eichenfield reported serving as a consultant to and/or recipient of research grants from more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – The recent report that the risk of a major adverse cardiovascular event increases by 1% more than in the general population for each additional year of psoriasis duration is sobering news for physicians who treat pediatric psoriasis.
“If I have a 16-year-old who has a 5-year history of psoriasis, what does that mean for when she’s 30 or 40? And should we be intervening more aggressively?” Lawrence F. Eichenfield, MD, asked at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even though there’s not a great deal of evidence, there’s some evidence to rationalize early screening in psoriasis,” according to Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego.
Psoriasis develops during childhood in almost one-third of patients.
The pediatric psoriasis screening guidelines describe a simple routine screening program and timeline for early identification of overweight or obesity, type 2 diabetes, hypertension, nonalcoholic fatty liver disease, anxiety, depression, substance abuse, inflammatory bowel disease, and quality of life issues, all of which are encountered with increased frequency in pediatric psoriasis patients. A fasting lipid panel is recommended in children aged 9-11 years with psoriasis and again at age 17-21 years.
“Don’t forget arthritis. For a kid with psoriasis, at every office visit, I ask about morning stiffness or limp. Those are probably the two most sensitive questions in screening for psoriatic arthritis,” according to Dr. Eichenfield.
It has been clear for some time that the skin is not the only organ affected by psoriatic inflammation. The study that quantified the relationship between psoriasis duration and cardiovascular risk – a 1% increase for each year of psoriasis – was a collaboration between investigators at the University of Copenhagen and the University of Pennsylvania, Philadelphia.
The two-part project included aortal imaging of 190 psoriasis patients using fludeoxyglucose F 18 PET/CT scan, which showed a strong relationship between duration of psoriasis and the degree of vascular inflammation. This was bolstered by a population-based study using Danish national registry data on 87,161 psoriasis patients and 4.2 million controls from the general Danish population (J Am Acad Dermatol. 2017 Oct;77[4]:650-56.e3).
Dr. Eichenfield reported serving as a consultant to and/or recipient of research grants from more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – The recent report that the risk of a major adverse cardiovascular event increases by 1% more than in the general population for each additional year of psoriasis duration is sobering news for physicians who treat pediatric psoriasis.
“If I have a 16-year-old who has a 5-year history of psoriasis, what does that mean for when she’s 30 or 40? And should we be intervening more aggressively?” Lawrence F. Eichenfield, MD, asked at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even though there’s not a great deal of evidence, there’s some evidence to rationalize early screening in psoriasis,” according to Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego.
Psoriasis develops during childhood in almost one-third of patients.
The pediatric psoriasis screening guidelines describe a simple routine screening program and timeline for early identification of overweight or obesity, type 2 diabetes, hypertension, nonalcoholic fatty liver disease, anxiety, depression, substance abuse, inflammatory bowel disease, and quality of life issues, all of which are encountered with increased frequency in pediatric psoriasis patients. A fasting lipid panel is recommended in children aged 9-11 years with psoriasis and again at age 17-21 years.
“Don’t forget arthritis. For a kid with psoriasis, at every office visit, I ask about morning stiffness or limp. Those are probably the two most sensitive questions in screening for psoriatic arthritis,” according to Dr. Eichenfield.
It has been clear for some time that the skin is not the only organ affected by psoriatic inflammation. The study that quantified the relationship between psoriasis duration and cardiovascular risk – a 1% increase for each year of psoriasis – was a collaboration between investigators at the University of Copenhagen and the University of Pennsylvania, Philadelphia.
The two-part project included aortal imaging of 190 psoriasis patients using fludeoxyglucose F 18 PET/CT scan, which showed a strong relationship between duration of psoriasis and the degree of vascular inflammation. This was bolstered by a population-based study using Danish national registry data on 87,161 psoriasis patients and 4.2 million controls from the general Danish population (J Am Acad Dermatol. 2017 Oct;77[4]:650-56.e3).
Dr. Eichenfield reported serving as a consultant to and/or recipient of research grants from more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
FDA advisory committee recommends baricitinib 2 mg to treat rheumatoid arthritis
The Food and Drug Administration Arthritis Advisory Committee has voted to recommend the 2-mg dose of baricitinib, an orally administered Janus kinase (JAK) inhibitor, to treat adults with moderate to severe rheumatoid arthritis who have responded inadequately or poorly to methotrexate but rejected a 4-mg dose of the same drug.
In separate votes on efficacy of the 2-mg and 4-mg doses of baricitinib (14-1 and 15-0, respectively), on their safety (9-6 and 4-11), and on their benefit-risk ratio (10-5 and 5-10), the advisory committee consistently backed the 2-mg dose, but the committee rejected the 4-mg dose despite its effectiveness in improving the symptoms of rheumatoid arthritis. Although the FDA does not always follow the advice of its advisory committees, it generally does.
The New Drug Application was resubmitted by Eli Lilly and Incyte. The proposed doses included a 2-mg once-daily dose and a 4-mg dose for some patients. The original submission was filed in January of 2016 with an indication to treat moderate to severe rheumatoid arthritis, but that application was rejected primarily because of concerns about thrombotic events. Other issues with the original application included inadequate safety exposure for the 2-mg dose of baricitinib, as well as inconsistent findings concerning the efficacy of the higher 4-mg dose.
The resubmission addressed several of the issues noted by the FDA, and changed the indication to treat patients with moderate to severe RA who have had an inadequate response to methotrexate. Along with the different indication, the dosing regimen shifted to 2 mg once daily. For patients who did not adequately respond to disease-modifying antirheumatic drugs (DMARDS) or had an intolerance for one or more of these drugs, a 4-mg dose was recommended; after disease activity had been controlled, a taper to 2 mg once daily could be considered.
Apart from changes in the drug dosage and indication, the resubmission also included accumulated safety data, comparative epidemiologic data concerning venous thromboembolism and pulmonary embolism, and efficacy analyses to support the new dosing recommendations.
“The risk-benefit ratio may be less good here,” stated Donald Miller, PharmD, a professor of pharmacy practice at North Dakota State University, Fargo. “If there is a safety issue, it’s more likely to be a problem with the 4-mg dose.”
Jon Russell, MD, PhD, medical director of Fibromyalgia Research and Consulting, San Antonio, felt that the manufacturer understood that the safety signal indicated that the benefits outweighed the risks with the 4-mg dose of baricitinib.
“The drug is efficacious in resistant rheumatoid arthritis, and rheumatoid arthritis is a devastating disease. Organs are being destroyed, joints as well as organs, and it’s war. We need to make the patient aware that it’s war and then fight it like it is,” he said.
Many of the committee members mentioned that the efficacies of the 2-mg and 4-mg doses were not in question, primarily based on the data from four phase 3 clinical trials.
The studies RA-BEACON (JADW), RA-BUILD (JADX), RA-BEGIN (JADZ), and RA-BEAM (JADV) were all randomized phase 3 trials that evaluated the efficacy of baricitinib in patients with moderate to severe RA.
RA-BEACON and RA-BUILD both had similar designs and compared 2-mg and 4-mg doses of baricitinib with placebo; the trials primarily differed in their patient populations.
RA-BEACON. Investigators looked at 527 randomized patients who had failed treatment with a biologic DMARD with nearly half failing multiple classes of this drug. The primary endpoint for this study was met, with the 4-mg dose showing superior results, compared with placebo, based on American College of Rheumatology (ACR) 20 scores (P less than .0001). As early as week 1 of the trial, both patients receiving 2 mg and those receiving 4 mg of baricitinib showed significant improvement, compared with placebo. By week 4, the 4-mg dose produced as much improvement as the 2-mg dose achieved over nearly 6 months. While the 4-mg dose was considered more effective, the 2-mg dose showed improvement in ACR 20 scores, change in Disease Activity Score 28 C-reactive protein (DAS28-CRP), and change in health assessment questionnaire disability index (HAQ-DI) (P less than .001). Neither the 4-mg nor the 2-mg dose was able to reduce Simple Disease Activity Index (SDAI) scores, a difficult endpoint to achieve, particularly in such a short time frame.
RA-BUILD. The researchers looked at patients who had failed treatment with conventional DMARDs and had not been treated with biologic DMARDS. The investigators looked at 684 randomized patients and saw similar results to RA-BEACON, with both the 2-mg and 4-mg doses displaying significant improvement in ACR 20, change in DAS28-CRP, and change in HAQ-DI, as well as SDAI remission which had been absent in RA-BEACON. Patients taking the 4-mg dose showed improvement in morning joint stiffness duration and severity (P less than .0001), as well as improvements in worst joint pain (P less than .0001) and tiredness (P less than .027).
RA-BEGIN. The investigators took a different approach and compared various drug combinations, including baricitinib 4 mg alone or in combination with oral methotrexate or in patients taking methotrexate who were DMARD-naive. Ultimately, this trial displayed that baricitinib 4 mg alone was superior to methotrexate, according to ACR 20 scores. This held true across all clinical measures at week 24 whether baricitinib was administered alone or in combination with methotrexate. As it had in the previously discussed trials, the 4-mg dose improved all of the previously mentioned test scores, compared with methotrexate (P less than .0001) except for modified Total Sharp Score (mTSS) (P = 0.158). When baricitinib 4 mg was used in conjunction with methotrexate, improvements in test scores, including mTSS, were statistically significant.
RA-BEAM. The researchers compared baricitinib 4 mg with placebo and adalimumab in 1,305 patients. All patients maintained a background level of methotrexate to improve the efficacy of adalimumab. Consistent with previous studies, baricitinib 4 mg outperformed other therapies and placebo in improvement in ACR 20, change in DAS28-CRP, change in HAQ-DI, and SDAI remission, as well as improvements in morning joint stiffness duration and severity, worst joint pain, and worst tiredness (P less than .0001).
Despite the clear efficacy of baricitinib 4 mg, the primary issue of contention was safety and benefit-to-risk ratio. The primary safety concerns were serious infection from opportunistic pathogens, herpes zoster, various malignancies, arterial and venous thrombosis, and laboratory abnormalities including elevated platelet counts and liver test elevations.
The Food and Drug Administration Arthritis Advisory Committee has voted to recommend the 2-mg dose of baricitinib, an orally administered Janus kinase (JAK) inhibitor, to treat adults with moderate to severe rheumatoid arthritis who have responded inadequately or poorly to methotrexate but rejected a 4-mg dose of the same drug.
In separate votes on efficacy of the 2-mg and 4-mg doses of baricitinib (14-1 and 15-0, respectively), on their safety (9-6 and 4-11), and on their benefit-risk ratio (10-5 and 5-10), the advisory committee consistently backed the 2-mg dose, but the committee rejected the 4-mg dose despite its effectiveness in improving the symptoms of rheumatoid arthritis. Although the FDA does not always follow the advice of its advisory committees, it generally does.
The New Drug Application was resubmitted by Eli Lilly and Incyte. The proposed doses included a 2-mg once-daily dose and a 4-mg dose for some patients. The original submission was filed in January of 2016 with an indication to treat moderate to severe rheumatoid arthritis, but that application was rejected primarily because of concerns about thrombotic events. Other issues with the original application included inadequate safety exposure for the 2-mg dose of baricitinib, as well as inconsistent findings concerning the efficacy of the higher 4-mg dose.
The resubmission addressed several of the issues noted by the FDA, and changed the indication to treat patients with moderate to severe RA who have had an inadequate response to methotrexate. Along with the different indication, the dosing regimen shifted to 2 mg once daily. For patients who did not adequately respond to disease-modifying antirheumatic drugs (DMARDS) or had an intolerance for one or more of these drugs, a 4-mg dose was recommended; after disease activity had been controlled, a taper to 2 mg once daily could be considered.
Apart from changes in the drug dosage and indication, the resubmission also included accumulated safety data, comparative epidemiologic data concerning venous thromboembolism and pulmonary embolism, and efficacy analyses to support the new dosing recommendations.
“The risk-benefit ratio may be less good here,” stated Donald Miller, PharmD, a professor of pharmacy practice at North Dakota State University, Fargo. “If there is a safety issue, it’s more likely to be a problem with the 4-mg dose.”
Jon Russell, MD, PhD, medical director of Fibromyalgia Research and Consulting, San Antonio, felt that the manufacturer understood that the safety signal indicated that the benefits outweighed the risks with the 4-mg dose of baricitinib.
“The drug is efficacious in resistant rheumatoid arthritis, and rheumatoid arthritis is a devastating disease. Organs are being destroyed, joints as well as organs, and it’s war. We need to make the patient aware that it’s war and then fight it like it is,” he said.
Many of the committee members mentioned that the efficacies of the 2-mg and 4-mg doses were not in question, primarily based on the data from four phase 3 clinical trials.
The studies RA-BEACON (JADW), RA-BUILD (JADX), RA-BEGIN (JADZ), and RA-BEAM (JADV) were all randomized phase 3 trials that evaluated the efficacy of baricitinib in patients with moderate to severe RA.
RA-BEACON and RA-BUILD both had similar designs and compared 2-mg and 4-mg doses of baricitinib with placebo; the trials primarily differed in their patient populations.
RA-BEACON. Investigators looked at 527 randomized patients who had failed treatment with a biologic DMARD with nearly half failing multiple classes of this drug. The primary endpoint for this study was met, with the 4-mg dose showing superior results, compared with placebo, based on American College of Rheumatology (ACR) 20 scores (P less than .0001). As early as week 1 of the trial, both patients receiving 2 mg and those receiving 4 mg of baricitinib showed significant improvement, compared with placebo. By week 4, the 4-mg dose produced as much improvement as the 2-mg dose achieved over nearly 6 months. While the 4-mg dose was considered more effective, the 2-mg dose showed improvement in ACR 20 scores, change in Disease Activity Score 28 C-reactive protein (DAS28-CRP), and change in health assessment questionnaire disability index (HAQ-DI) (P less than .001). Neither the 4-mg nor the 2-mg dose was able to reduce Simple Disease Activity Index (SDAI) scores, a difficult endpoint to achieve, particularly in such a short time frame.
RA-BUILD. The researchers looked at patients who had failed treatment with conventional DMARDs and had not been treated with biologic DMARDS. The investigators looked at 684 randomized patients and saw similar results to RA-BEACON, with both the 2-mg and 4-mg doses displaying significant improvement in ACR 20, change in DAS28-CRP, and change in HAQ-DI, as well as SDAI remission which had been absent in RA-BEACON. Patients taking the 4-mg dose showed improvement in morning joint stiffness duration and severity (P less than .0001), as well as improvements in worst joint pain (P less than .0001) and tiredness (P less than .027).
RA-BEGIN. The investigators took a different approach and compared various drug combinations, including baricitinib 4 mg alone or in combination with oral methotrexate or in patients taking methotrexate who were DMARD-naive. Ultimately, this trial displayed that baricitinib 4 mg alone was superior to methotrexate, according to ACR 20 scores. This held true across all clinical measures at week 24 whether baricitinib was administered alone or in combination with methotrexate. As it had in the previously discussed trials, the 4-mg dose improved all of the previously mentioned test scores, compared with methotrexate (P less than .0001) except for modified Total Sharp Score (mTSS) (P = 0.158). When baricitinib 4 mg was used in conjunction with methotrexate, improvements in test scores, including mTSS, were statistically significant.
RA-BEAM. The researchers compared baricitinib 4 mg with placebo and adalimumab in 1,305 patients. All patients maintained a background level of methotrexate to improve the efficacy of adalimumab. Consistent with previous studies, baricitinib 4 mg outperformed other therapies and placebo in improvement in ACR 20, change in DAS28-CRP, change in HAQ-DI, and SDAI remission, as well as improvements in morning joint stiffness duration and severity, worst joint pain, and worst tiredness (P less than .0001).
Despite the clear efficacy of baricitinib 4 mg, the primary issue of contention was safety and benefit-to-risk ratio. The primary safety concerns were serious infection from opportunistic pathogens, herpes zoster, various malignancies, arterial and venous thrombosis, and laboratory abnormalities including elevated platelet counts and liver test elevations.
The Food and Drug Administration Arthritis Advisory Committee has voted to recommend the 2-mg dose of baricitinib, an orally administered Janus kinase (JAK) inhibitor, to treat adults with moderate to severe rheumatoid arthritis who have responded inadequately or poorly to methotrexate but rejected a 4-mg dose of the same drug.
In separate votes on efficacy of the 2-mg and 4-mg doses of baricitinib (14-1 and 15-0, respectively), on their safety (9-6 and 4-11), and on their benefit-risk ratio (10-5 and 5-10), the advisory committee consistently backed the 2-mg dose, but the committee rejected the 4-mg dose despite its effectiveness in improving the symptoms of rheumatoid arthritis. Although the FDA does not always follow the advice of its advisory committees, it generally does.
The New Drug Application was resubmitted by Eli Lilly and Incyte. The proposed doses included a 2-mg once-daily dose and a 4-mg dose for some patients. The original submission was filed in January of 2016 with an indication to treat moderate to severe rheumatoid arthritis, but that application was rejected primarily because of concerns about thrombotic events. Other issues with the original application included inadequate safety exposure for the 2-mg dose of baricitinib, as well as inconsistent findings concerning the efficacy of the higher 4-mg dose.
The resubmission addressed several of the issues noted by the FDA, and changed the indication to treat patients with moderate to severe RA who have had an inadequate response to methotrexate. Along with the different indication, the dosing regimen shifted to 2 mg once daily. For patients who did not adequately respond to disease-modifying antirheumatic drugs (DMARDS) or had an intolerance for one or more of these drugs, a 4-mg dose was recommended; after disease activity had been controlled, a taper to 2 mg once daily could be considered.
Apart from changes in the drug dosage and indication, the resubmission also included accumulated safety data, comparative epidemiologic data concerning venous thromboembolism and pulmonary embolism, and efficacy analyses to support the new dosing recommendations.
“The risk-benefit ratio may be less good here,” stated Donald Miller, PharmD, a professor of pharmacy practice at North Dakota State University, Fargo. “If there is a safety issue, it’s more likely to be a problem with the 4-mg dose.”
Jon Russell, MD, PhD, medical director of Fibromyalgia Research and Consulting, San Antonio, felt that the manufacturer understood that the safety signal indicated that the benefits outweighed the risks with the 4-mg dose of baricitinib.
“The drug is efficacious in resistant rheumatoid arthritis, and rheumatoid arthritis is a devastating disease. Organs are being destroyed, joints as well as organs, and it’s war. We need to make the patient aware that it’s war and then fight it like it is,” he said.
Many of the committee members mentioned that the efficacies of the 2-mg and 4-mg doses were not in question, primarily based on the data from four phase 3 clinical trials.
The studies RA-BEACON (JADW), RA-BUILD (JADX), RA-BEGIN (JADZ), and RA-BEAM (JADV) were all randomized phase 3 trials that evaluated the efficacy of baricitinib in patients with moderate to severe RA.
RA-BEACON and RA-BUILD both had similar designs and compared 2-mg and 4-mg doses of baricitinib with placebo; the trials primarily differed in their patient populations.
RA-BEACON. Investigators looked at 527 randomized patients who had failed treatment with a biologic DMARD with nearly half failing multiple classes of this drug. The primary endpoint for this study was met, with the 4-mg dose showing superior results, compared with placebo, based on American College of Rheumatology (ACR) 20 scores (P less than .0001). As early as week 1 of the trial, both patients receiving 2 mg and those receiving 4 mg of baricitinib showed significant improvement, compared with placebo. By week 4, the 4-mg dose produced as much improvement as the 2-mg dose achieved over nearly 6 months. While the 4-mg dose was considered more effective, the 2-mg dose showed improvement in ACR 20 scores, change in Disease Activity Score 28 C-reactive protein (DAS28-CRP), and change in health assessment questionnaire disability index (HAQ-DI) (P less than .001). Neither the 4-mg nor the 2-mg dose was able to reduce Simple Disease Activity Index (SDAI) scores, a difficult endpoint to achieve, particularly in such a short time frame.
RA-BUILD. The researchers looked at patients who had failed treatment with conventional DMARDs and had not been treated with biologic DMARDS. The investigators looked at 684 randomized patients and saw similar results to RA-BEACON, with both the 2-mg and 4-mg doses displaying significant improvement in ACR 20, change in DAS28-CRP, and change in HAQ-DI, as well as SDAI remission which had been absent in RA-BEACON. Patients taking the 4-mg dose showed improvement in morning joint stiffness duration and severity (P less than .0001), as well as improvements in worst joint pain (P less than .0001) and tiredness (P less than .027).
RA-BEGIN. The investigators took a different approach and compared various drug combinations, including baricitinib 4 mg alone or in combination with oral methotrexate or in patients taking methotrexate who were DMARD-naive. Ultimately, this trial displayed that baricitinib 4 mg alone was superior to methotrexate, according to ACR 20 scores. This held true across all clinical measures at week 24 whether baricitinib was administered alone or in combination with methotrexate. As it had in the previously discussed trials, the 4-mg dose improved all of the previously mentioned test scores, compared with methotrexate (P less than .0001) except for modified Total Sharp Score (mTSS) (P = 0.158). When baricitinib 4 mg was used in conjunction with methotrexate, improvements in test scores, including mTSS, were statistically significant.
RA-BEAM. The researchers compared baricitinib 4 mg with placebo and adalimumab in 1,305 patients. All patients maintained a background level of methotrexate to improve the efficacy of adalimumab. Consistent with previous studies, baricitinib 4 mg outperformed other therapies and placebo in improvement in ACR 20, change in DAS28-CRP, change in HAQ-DI, and SDAI remission, as well as improvements in morning joint stiffness duration and severity, worst joint pain, and worst tiredness (P less than .0001).
Despite the clear efficacy of baricitinib 4 mg, the primary issue of contention was safety and benefit-to-risk ratio. The primary safety concerns were serious infection from opportunistic pathogens, herpes zoster, various malignancies, arterial and venous thrombosis, and laboratory abnormalities including elevated platelet counts and liver test elevations.
Ertapenem slashes surgical site infections in carriers of ESBL-producing bacteria
MADRID – A targeted antibiotic strategy that employed ertapenem in carriers of extended-spectrum beta-lactamase–producing Enterobacteriaceae reduced infections after colorectal surgery by 41%, compared with routine treatment with cefuroxime and metronidazole.
The strategy was even more effective at preventing surgical site infections caused by ESBL-producing bacteria, cutting the rate by 87%, Amir Nutman, MD, said at the European Congress of Clinical Microbiology and Infectious Diseases.
He presented the results of the WP4 study, which was carried out in three hospitals in Serbia, Switzerland, and Israel. Designed as a before-and-after trial, it tested the theory that identifying ESBL carriers and targeting presurgical antibiotic prophylaxis could improve their surgical outcomes.
WP4 was one of five studies in the multinational R-GNOSIS project. “Resistance in gram-negative organisms: Studying intervention strategies” is a 12 million euro, 5-year European collaborative research project designed to identify effective interventions for reducing the carriage, infection, and spread of multidrug resistant Gram-negative bacteria. Several of the studies reported at ECCMID 2018.
During 2012-2017, WP4 enrolled almost 4,000 adults scheduled to undergo colorectal surgery (excluding appendectomy or minor anorectal procedures). All patients were screened for ESBL-producing bacteria from 2 weeks to 2 days before their operation. In the first phase, carriers were treated with the standard presurgical prophylaxis of 1.5 g cefuroxime and 500 mg metronidazole intravenously. In phase 2, carriers received targeted prophylaxis with IV ertapenem 1 g. Both interventions were given 30 minutes before surgery commenced.
All patients underwent regular surgical site infection surveillance until hospital discharge, then followed up 30 days later by phone or in person.
The primary outcome was surgical site infection at 30 days. Secondary outcomes were the type of any surgical site infection (superficial, deep, or organ/space), and infections caused by ESBL-producing bacteria.
ESBL screening was carried out on 3,626 patients; carriage prevalence was 13.8%, but varied by center from 9% to 29%. Of the carriers, 468 were included in the study; 247 received routine prophylaxis and 221 received ertapenem.
Patients were a mean of 63 years old; 98% were living at home before admission. About 20% had diabetes; 5% had some type of immunodeficiency. The most common surgical indication was colon cancer (68%), and about a third had undergone prior colon surgery. Most of the surgeries were open, and about half involved a colectomy.
Patients in the ertapenem group had overall better scores on the National Nosocomial Infections Surveillance Basic SSI Risk Index and were less likely to have an intraoperative finding of colon dilation (20.8% vs. 27%).There were no other clinically compelling intraoperative differences between the two groups, including bleeding, bowel spillage, the need for drains, or stoma placement.
Patients who received prophylactic ertapenem had significantly better 30-day outcomes on all measures of infection than did patients who had standard prophylaxis, Dr. Nutman said.
There were 34 surgical site infections in the routine prophylaxis group and 19 in the ertapenem group. Among these, 17 in the routine group and three in the ertapenem group were caused by ESBL-producing bacteria. The ESBL-positive infections were as follows:
- E. coli (thirteen in the routine and one in the ertapenem group).
- Klebsiella species (four and one, respectively).
- Proteus species (one in the ertapenem group).
Other infections were caused by ESBL-nonproducers, including E. coli, Klebsiella, Proteus, Enterococci, Pseudomonas aeruginosa, Staphylococcus aureus, and other unspecified organisms. Polymicrobial infections occurred in 25 patients.
In an analysis that controlled for National Nosocomial Infections Surveillance score and colon dilation, patients who received ertapenem were 41% less likely to develop any surgical site infection (15.8% vs. 22.7%; odds ratio, 0.59); 17% less likely to develop a deep infection (9.5% vs. 11.3%; OR, 0.83); and 87% less likely to develop an infection caused by an ESBL-producing bacteria (0.9% vs. 6.5%; OR, 0.13).
Dr. Nutman made no financial declarations.
SOURCE: Nutman et al. ECCMID 2018, Abstract O1129.
MADRID – A targeted antibiotic strategy that employed ertapenem in carriers of extended-spectrum beta-lactamase–producing Enterobacteriaceae reduced infections after colorectal surgery by 41%, compared with routine treatment with cefuroxime and metronidazole.
The strategy was even more effective at preventing surgical site infections caused by ESBL-producing bacteria, cutting the rate by 87%, Amir Nutman, MD, said at the European Congress of Clinical Microbiology and Infectious Diseases.
He presented the results of the WP4 study, which was carried out in three hospitals in Serbia, Switzerland, and Israel. Designed as a before-and-after trial, it tested the theory that identifying ESBL carriers and targeting presurgical antibiotic prophylaxis could improve their surgical outcomes.
WP4 was one of five studies in the multinational R-GNOSIS project. “Resistance in gram-negative organisms: Studying intervention strategies” is a 12 million euro, 5-year European collaborative research project designed to identify effective interventions for reducing the carriage, infection, and spread of multidrug resistant Gram-negative bacteria. Several of the studies reported at ECCMID 2018.
During 2012-2017, WP4 enrolled almost 4,000 adults scheduled to undergo colorectal surgery (excluding appendectomy or minor anorectal procedures). All patients were screened for ESBL-producing bacteria from 2 weeks to 2 days before their operation. In the first phase, carriers were treated with the standard presurgical prophylaxis of 1.5 g cefuroxime and 500 mg metronidazole intravenously. In phase 2, carriers received targeted prophylaxis with IV ertapenem 1 g. Both interventions were given 30 minutes before surgery commenced.
All patients underwent regular surgical site infection surveillance until hospital discharge, then followed up 30 days later by phone or in person.
The primary outcome was surgical site infection at 30 days. Secondary outcomes were the type of any surgical site infection (superficial, deep, or organ/space), and infections caused by ESBL-producing bacteria.
ESBL screening was carried out on 3,626 patients; carriage prevalence was 13.8%, but varied by center from 9% to 29%. Of the carriers, 468 were included in the study; 247 received routine prophylaxis and 221 received ertapenem.
Patients were a mean of 63 years old; 98% were living at home before admission. About 20% had diabetes; 5% had some type of immunodeficiency. The most common surgical indication was colon cancer (68%), and about a third had undergone prior colon surgery. Most of the surgeries were open, and about half involved a colectomy.
Patients in the ertapenem group had overall better scores on the National Nosocomial Infections Surveillance Basic SSI Risk Index and were less likely to have an intraoperative finding of colon dilation (20.8% vs. 27%).There were no other clinically compelling intraoperative differences between the two groups, including bleeding, bowel spillage, the need for drains, or stoma placement.
Patients who received prophylactic ertapenem had significantly better 30-day outcomes on all measures of infection than did patients who had standard prophylaxis, Dr. Nutman said.
There were 34 surgical site infections in the routine prophylaxis group and 19 in the ertapenem group. Among these, 17 in the routine group and three in the ertapenem group were caused by ESBL-producing bacteria. The ESBL-positive infections were as follows:
- E. coli (thirteen in the routine and one in the ertapenem group).
- Klebsiella species (four and one, respectively).
- Proteus species (one in the ertapenem group).
Other infections were caused by ESBL-nonproducers, including E. coli, Klebsiella, Proteus, Enterococci, Pseudomonas aeruginosa, Staphylococcus aureus, and other unspecified organisms. Polymicrobial infections occurred in 25 patients.
In an analysis that controlled for National Nosocomial Infections Surveillance score and colon dilation, patients who received ertapenem were 41% less likely to develop any surgical site infection (15.8% vs. 22.7%; odds ratio, 0.59); 17% less likely to develop a deep infection (9.5% vs. 11.3%; OR, 0.83); and 87% less likely to develop an infection caused by an ESBL-producing bacteria (0.9% vs. 6.5%; OR, 0.13).
Dr. Nutman made no financial declarations.
SOURCE: Nutman et al. ECCMID 2018, Abstract O1129.
MADRID – A targeted antibiotic strategy that employed ertapenem in carriers of extended-spectrum beta-lactamase–producing Enterobacteriaceae reduced infections after colorectal surgery by 41%, compared with routine treatment with cefuroxime and metronidazole.
The strategy was even more effective at preventing surgical site infections caused by ESBL-producing bacteria, cutting the rate by 87%, Amir Nutman, MD, said at the European Congress of Clinical Microbiology and Infectious Diseases.
He presented the results of the WP4 study, which was carried out in three hospitals in Serbia, Switzerland, and Israel. Designed as a before-and-after trial, it tested the theory that identifying ESBL carriers and targeting presurgical antibiotic prophylaxis could improve their surgical outcomes.
WP4 was one of five studies in the multinational R-GNOSIS project. “Resistance in gram-negative organisms: Studying intervention strategies” is a 12 million euro, 5-year European collaborative research project designed to identify effective interventions for reducing the carriage, infection, and spread of multidrug resistant Gram-negative bacteria. Several of the studies reported at ECCMID 2018.
During 2012-2017, WP4 enrolled almost 4,000 adults scheduled to undergo colorectal surgery (excluding appendectomy or minor anorectal procedures). All patients were screened for ESBL-producing bacteria from 2 weeks to 2 days before their operation. In the first phase, carriers were treated with the standard presurgical prophylaxis of 1.5 g cefuroxime and 500 mg metronidazole intravenously. In phase 2, carriers received targeted prophylaxis with IV ertapenem 1 g. Both interventions were given 30 minutes before surgery commenced.
All patients underwent regular surgical site infection surveillance until hospital discharge, then followed up 30 days later by phone or in person.
The primary outcome was surgical site infection at 30 days. Secondary outcomes were the type of any surgical site infection (superficial, deep, or organ/space), and infections caused by ESBL-producing bacteria.
ESBL screening was carried out on 3,626 patients; carriage prevalence was 13.8%, but varied by center from 9% to 29%. Of the carriers, 468 were included in the study; 247 received routine prophylaxis and 221 received ertapenem.
Patients were a mean of 63 years old; 98% were living at home before admission. About 20% had diabetes; 5% had some type of immunodeficiency. The most common surgical indication was colon cancer (68%), and about a third had undergone prior colon surgery. Most of the surgeries were open, and about half involved a colectomy.
Patients in the ertapenem group had overall better scores on the National Nosocomial Infections Surveillance Basic SSI Risk Index and were less likely to have an intraoperative finding of colon dilation (20.8% vs. 27%).There were no other clinically compelling intraoperative differences between the two groups, including bleeding, bowel spillage, the need for drains, or stoma placement.
Patients who received prophylactic ertapenem had significantly better 30-day outcomes on all measures of infection than did patients who had standard prophylaxis, Dr. Nutman said.
There were 34 surgical site infections in the routine prophylaxis group and 19 in the ertapenem group. Among these, 17 in the routine group and three in the ertapenem group were caused by ESBL-producing bacteria. The ESBL-positive infections were as follows:
- E. coli (thirteen in the routine and one in the ertapenem group).
- Klebsiella species (four and one, respectively).
- Proteus species (one in the ertapenem group).
Other infections were caused by ESBL-nonproducers, including E. coli, Klebsiella, Proteus, Enterococci, Pseudomonas aeruginosa, Staphylococcus aureus, and other unspecified organisms. Polymicrobial infections occurred in 25 patients.
In an analysis that controlled for National Nosocomial Infections Surveillance score and colon dilation, patients who received ertapenem were 41% less likely to develop any surgical site infection (15.8% vs. 22.7%; odds ratio, 0.59); 17% less likely to develop a deep infection (9.5% vs. 11.3%; OR, 0.83); and 87% less likely to develop an infection caused by an ESBL-producing bacteria (0.9% vs. 6.5%; OR, 0.13).
Dr. Nutman made no financial declarations.
SOURCE: Nutman et al. ECCMID 2018, Abstract O1129.
REPORTING FROM ECCMID 2018
Key clinical point: A targeted presurgical antibiotic prophylaxis significantly cut rates of surgical site infections in carriers of extended beta-lactamase–producing bacteria.
Major finding: Ertapenem reduced the rate of surgical site infection by 41% , and the rate of ESBL-producing infections by 87%, compared to routine prophylaxis.
Study details: The study comprised 468 patients.
Disclosures: The study was funded by the European Commission under the Seventh Framework Programme (FP7) for Research and Technology. Dr. Nutman had no financial disclosures.
Source: Nutman A et al. ECCMID 2018, Abstract O1129
Registration Now Open for 2018 ACS Quality and Safety Conference
Registration Now Open for 2018 ACS Quality and Safety Conference
Health care professionals dedicated to raising the bar on the quality of surgical care and patient safety are invited to attend the 2018 American College of Surgeons (ACS) Quality and Safety Conference, July 21–24 at the Hyatt Regency Orlando, FL.
• ACS National Surgical Quality Improvement Program (ACS NSQIP®)
• ACS Children’s Surgery Verification Program and ACS NSQIP Pediatric
• Cancer Programs, including the Commission on Cancer and National Accreditation Program for Breast Centers
• Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program
• Trauma Quality Programs, including Pediatrics, Trauma Center Verification, the Trauma Quality Improvement Program, and Performance Improvement and Patient Safety
The conference theme, Partnering for Improvement, will set the tone for the meeting, as presenters and organizers strive to achieve the following:
• Provide a professional forum to discuss and apply the most recent knowledge on quality and safety initiatives in surgery
• Present methods used to analyze data from ACS Quality Programs and demonstrate practical ways to use the data for quality improvement (QI)
• Assist hospitals and providers in managing, analyzing, and interpreting data
• Enhance the learning experience by offering breakout sessions that educate attendees on topic areas of their interest
An additional track will be dedicated to Optimal Resources for Surgical Quality and Safety, the “red book,” which will explore concepts and resources from the manual, information on QI tools, methodology, nomenclature, and organizational design and infrastructure. Other notable sessions will highlight important clinical topics, including efficiency in surgical care, the opioid epidemic, and preoperative optimization.
Keynote speaker Rolf Benirschke, a former placekicker in the NFL, will discuss his experience collapsing on a cross-country team flight while battling ulcerative colitis and subsequently undergoing two emergency operations within six days.
Further details about the conference can be viewed on the Quality and Safety Conference website at www.facs.org/quality-programs/quality-safety-conference.
Registration Now Open for 2018 ACS Quality and Safety Conference
Health care professionals dedicated to raising the bar on the quality of surgical care and patient safety are invited to attend the 2018 American College of Surgeons (ACS) Quality and Safety Conference, July 21–24 at the Hyatt Regency Orlando, FL.
• ACS National Surgical Quality Improvement Program (ACS NSQIP®)
• ACS Children’s Surgery Verification Program and ACS NSQIP Pediatric
• Cancer Programs, including the Commission on Cancer and National Accreditation Program for Breast Centers
• Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program
• Trauma Quality Programs, including Pediatrics, Trauma Center Verification, the Trauma Quality Improvement Program, and Performance Improvement and Patient Safety
The conference theme, Partnering for Improvement, will set the tone for the meeting, as presenters and organizers strive to achieve the following:
• Provide a professional forum to discuss and apply the most recent knowledge on quality and safety initiatives in surgery
• Present methods used to analyze data from ACS Quality Programs and demonstrate practical ways to use the data for quality improvement (QI)
• Assist hospitals and providers in managing, analyzing, and interpreting data
• Enhance the learning experience by offering breakout sessions that educate attendees on topic areas of their interest
An additional track will be dedicated to Optimal Resources for Surgical Quality and Safety, the “red book,” which will explore concepts and resources from the manual, information on QI tools, methodology, nomenclature, and organizational design and infrastructure. Other notable sessions will highlight important clinical topics, including efficiency in surgical care, the opioid epidemic, and preoperative optimization.
Keynote speaker Rolf Benirschke, a former placekicker in the NFL, will discuss his experience collapsing on a cross-country team flight while battling ulcerative colitis and subsequently undergoing two emergency operations within six days.
Further details about the conference can be viewed on the Quality and Safety Conference website at www.facs.org/quality-programs/quality-safety-conference.
Registration Now Open for 2018 ACS Quality and Safety Conference
Health care professionals dedicated to raising the bar on the quality of surgical care and patient safety are invited to attend the 2018 American College of Surgeons (ACS) Quality and Safety Conference, July 21–24 at the Hyatt Regency Orlando, FL.
• ACS National Surgical Quality Improvement Program (ACS NSQIP®)
• ACS Children’s Surgery Verification Program and ACS NSQIP Pediatric
• Cancer Programs, including the Commission on Cancer and National Accreditation Program for Breast Centers
• Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program
• Trauma Quality Programs, including Pediatrics, Trauma Center Verification, the Trauma Quality Improvement Program, and Performance Improvement and Patient Safety
The conference theme, Partnering for Improvement, will set the tone for the meeting, as presenters and organizers strive to achieve the following:
• Provide a professional forum to discuss and apply the most recent knowledge on quality and safety initiatives in surgery
• Present methods used to analyze data from ACS Quality Programs and demonstrate practical ways to use the data for quality improvement (QI)
• Assist hospitals and providers in managing, analyzing, and interpreting data
• Enhance the learning experience by offering breakout sessions that educate attendees on topic areas of their interest
An additional track will be dedicated to Optimal Resources for Surgical Quality and Safety, the “red book,” which will explore concepts and resources from the manual, information on QI tools, methodology, nomenclature, and organizational design and infrastructure. Other notable sessions will highlight important clinical topics, including efficiency in surgical care, the opioid epidemic, and preoperative optimization.
Keynote speaker Rolf Benirschke, a former placekicker in the NFL, will discuss his experience collapsing on a cross-country team flight while battling ulcerative colitis and subsequently undergoing two emergency operations within six days.
Further details about the conference can be viewed on the Quality and Safety Conference website at www.facs.org/quality-programs/quality-safety-conference.
Endometriosis pain stemming from pelvic spasms improved with botulinum toxin
LOS ANGELES – Women treated surgically and with hormones for endometriosis may continue to experience pain, say investigators, and that pain frequently extends beyond the pelvis.
At the annual meeting of the American Academy of Neurology, Barbara Karp, MD, of the National Institute of Neurological Disorders and Stroke, presented results from an ongoing randomized trial of women with endometriosis receiving botulinum toxin to treat endometriosis-related chronic pelvic pain and pelvic spasm.
All 28 women currently enrolled in the trial (median age, 29 years) were evaluated by a gynecologist to confirm pelvic muscle spasm as their primary source of pain. Each also underwent a neuromuscular examination to identify pain points beyond the pelvis.
All subjects had myofascial dysfunction. Most reported headaches and half reported orofacial pain, while 13 subjects reported myofascial trigger points in all the 26 spots assessed, which included head and facial muscles, shoulder and back muscles, and muscles in the buttocks, abdomen, and upper legs.
Dr. Karp said her group hypothesized that for patients with endometriosis, the widespread pain seen in the study “probably has some origin in sensitization initiated by pain associated with the endometriosis lesions, and that gives us a mechanism to think about peripheral and central sensitization.” But she noted that such sensitization can be easily missed in the clinic.
“One of the things that’s really underappreciated is how much women with chronic pelvic pain have pain elsewhere. So the neurologist or pain specialist may say, ‘that’s not my body territory, there’s something going on with your pelvis.’ And the gynecologist may be focused on the endometriosis and the endometriosis lesions. So you have these women with really widespread pain problems whose care is being fractionated.”
In another aspect of the study she also presented at the meeting, Dr. Karp, a neurologist who has studied the therapeutic use of neurotoxins such as botulinum for 30 years, showed results from an open-label extension of a randomized trial of botulinum toxin injections to treat pelvic spasm in the same cohort of women with confirmed endometriosis and confirmed pelvic muscle spasm.
A month after the open-label injection, spasm was reduced or absent in all subjects (P = .0005), with 11 of 13 rating pain as absent or mild (P = .0001), Dr. Karp and her colleagues reported. Between 5 and 11 months post injection, five women requested a repeat of the treatment.
Besides the data on pain and disability collected as part of the trial, Dr. Karp and her colleagues are also looking at biomarkers for pain and inflammation, and changes in medication and hormone use. They are preparing a separate literature review on injection techniques and dosages of toxin to the pelvic floor muscles.
“It’s an area of the body neurologists don’t feel comfortable injecting, and I don’t necessarily feel comfortable doing it myself,” Dr. Karp said.
The researchers had to develop their own procedure because at the time they started the research there was almost nothing in the literature on how to inject botulinum toxin for pelvic pain in women. “People in different specialties have been doing it [to relieve pelvic pain] and it’s really widespread, but they’re doing it all different ways,” Dr. Karp said. “We’re hoping to find a best approach.”
Dr. Karp and her colleagues’ research is supported by the NINDS and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. OnabotulinumtoxinA for the clinical trial is supplied by Allergan. Dr. Karp has received research support from Allergan and Merz.
SOURCE: Karp B et al. AAN 2018, Abstract P2.096; Karp B et al. AAN 2018, Abstract P2.098.
LOS ANGELES – Women treated surgically and with hormones for endometriosis may continue to experience pain, say investigators, and that pain frequently extends beyond the pelvis.
At the annual meeting of the American Academy of Neurology, Barbara Karp, MD, of the National Institute of Neurological Disorders and Stroke, presented results from an ongoing randomized trial of women with endometriosis receiving botulinum toxin to treat endometriosis-related chronic pelvic pain and pelvic spasm.
All 28 women currently enrolled in the trial (median age, 29 years) were evaluated by a gynecologist to confirm pelvic muscle spasm as their primary source of pain. Each also underwent a neuromuscular examination to identify pain points beyond the pelvis.
All subjects had myofascial dysfunction. Most reported headaches and half reported orofacial pain, while 13 subjects reported myofascial trigger points in all the 26 spots assessed, which included head and facial muscles, shoulder and back muscles, and muscles in the buttocks, abdomen, and upper legs.
Dr. Karp said her group hypothesized that for patients with endometriosis, the widespread pain seen in the study “probably has some origin in sensitization initiated by pain associated with the endometriosis lesions, and that gives us a mechanism to think about peripheral and central sensitization.” But she noted that such sensitization can be easily missed in the clinic.
“One of the things that’s really underappreciated is how much women with chronic pelvic pain have pain elsewhere. So the neurologist or pain specialist may say, ‘that’s not my body territory, there’s something going on with your pelvis.’ And the gynecologist may be focused on the endometriosis and the endometriosis lesions. So you have these women with really widespread pain problems whose care is being fractionated.”
In another aspect of the study she also presented at the meeting, Dr. Karp, a neurologist who has studied the therapeutic use of neurotoxins such as botulinum for 30 years, showed results from an open-label extension of a randomized trial of botulinum toxin injections to treat pelvic spasm in the same cohort of women with confirmed endometriosis and confirmed pelvic muscle spasm.
A month after the open-label injection, spasm was reduced or absent in all subjects (P = .0005), with 11 of 13 rating pain as absent or mild (P = .0001), Dr. Karp and her colleagues reported. Between 5 and 11 months post injection, five women requested a repeat of the treatment.
Besides the data on pain and disability collected as part of the trial, Dr. Karp and her colleagues are also looking at biomarkers for pain and inflammation, and changes in medication and hormone use. They are preparing a separate literature review on injection techniques and dosages of toxin to the pelvic floor muscles.
“It’s an area of the body neurologists don’t feel comfortable injecting, and I don’t necessarily feel comfortable doing it myself,” Dr. Karp said.
The researchers had to develop their own procedure because at the time they started the research there was almost nothing in the literature on how to inject botulinum toxin for pelvic pain in women. “People in different specialties have been doing it [to relieve pelvic pain] and it’s really widespread, but they’re doing it all different ways,” Dr. Karp said. “We’re hoping to find a best approach.”
Dr. Karp and her colleagues’ research is supported by the NINDS and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. OnabotulinumtoxinA for the clinical trial is supplied by Allergan. Dr. Karp has received research support from Allergan and Merz.
SOURCE: Karp B et al. AAN 2018, Abstract P2.096; Karp B et al. AAN 2018, Abstract P2.098.
LOS ANGELES – Women treated surgically and with hormones for endometriosis may continue to experience pain, say investigators, and that pain frequently extends beyond the pelvis.
At the annual meeting of the American Academy of Neurology, Barbara Karp, MD, of the National Institute of Neurological Disorders and Stroke, presented results from an ongoing randomized trial of women with endometriosis receiving botulinum toxin to treat endometriosis-related chronic pelvic pain and pelvic spasm.
All 28 women currently enrolled in the trial (median age, 29 years) were evaluated by a gynecologist to confirm pelvic muscle spasm as their primary source of pain. Each also underwent a neuromuscular examination to identify pain points beyond the pelvis.
All subjects had myofascial dysfunction. Most reported headaches and half reported orofacial pain, while 13 subjects reported myofascial trigger points in all the 26 spots assessed, which included head and facial muscles, shoulder and back muscles, and muscles in the buttocks, abdomen, and upper legs.
Dr. Karp said her group hypothesized that for patients with endometriosis, the widespread pain seen in the study “probably has some origin in sensitization initiated by pain associated with the endometriosis lesions, and that gives us a mechanism to think about peripheral and central sensitization.” But she noted that such sensitization can be easily missed in the clinic.
“One of the things that’s really underappreciated is how much women with chronic pelvic pain have pain elsewhere. So the neurologist or pain specialist may say, ‘that’s not my body territory, there’s something going on with your pelvis.’ And the gynecologist may be focused on the endometriosis and the endometriosis lesions. So you have these women with really widespread pain problems whose care is being fractionated.”
In another aspect of the study she also presented at the meeting, Dr. Karp, a neurologist who has studied the therapeutic use of neurotoxins such as botulinum for 30 years, showed results from an open-label extension of a randomized trial of botulinum toxin injections to treat pelvic spasm in the same cohort of women with confirmed endometriosis and confirmed pelvic muscle spasm.
A month after the open-label injection, spasm was reduced or absent in all subjects (P = .0005), with 11 of 13 rating pain as absent or mild (P = .0001), Dr. Karp and her colleagues reported. Between 5 and 11 months post injection, five women requested a repeat of the treatment.
Besides the data on pain and disability collected as part of the trial, Dr. Karp and her colleagues are also looking at biomarkers for pain and inflammation, and changes in medication and hormone use. They are preparing a separate literature review on injection techniques and dosages of toxin to the pelvic floor muscles.
“It’s an area of the body neurologists don’t feel comfortable injecting, and I don’t necessarily feel comfortable doing it myself,” Dr. Karp said.
The researchers had to develop their own procedure because at the time they started the research there was almost nothing in the literature on how to inject botulinum toxin for pelvic pain in women. “People in different specialties have been doing it [to relieve pelvic pain] and it’s really widespread, but they’re doing it all different ways,” Dr. Karp said. “We’re hoping to find a best approach.”
Dr. Karp and her colleagues’ research is supported by the NINDS and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. OnabotulinumtoxinA for the clinical trial is supplied by Allergan. Dr. Karp has received research support from Allergan and Merz.
SOURCE: Karp B et al. AAN 2018, Abstract P2.096; Karp B et al. AAN 2018, Abstract P2.098.
REPORTING FROM AAN 2018
Key clinical point:
Major finding: A month after the open-label injection, spasm was reduced or absent in all subjects (P = .0005), with 11 of 13 rating pain as absent or mild (P = .0001).
Study details: An open-label extension in 13 of 28 patients enrolled in a randomized trial.
Disclosures: Dr. Karp and her colleagues’ research is supported by the NINDS and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. OnabotulinumtoxinA for the clinical trial is supplied by Allergan. Dr. Karp has received research support from Allergan and Merz.
Source: Karp B et al. AAN 2018, Abstract P2.096; Karp B et al. AAN 2018, Abstract P2.098.
Hormone therapy raises diabetes risk in breast cancer survivors
Hormone therapy for breast cancer more than doubles a woman’s risk for developing type 2 diabetes, results of a case-cohort study suggest.
Hormone therapy with tamoxifen was associated with a more than twofold increase in risk of diabetes, and aromatase inhibitors were associated with a more than fourfold increase, reported Hatem Hamood, MD, of Leumit Health Services in Karmiel, Israel, and colleagues.
Among 2,246 women with breast cancer and no diabetes at baseline, followed for a mean of 5.9 years (longest follow-up 13 years), the crude cumulative lifetime incidence rate of diabetes was 20.9%, the investigators wrote. The report was published in the Journal of Clinical Oncology.
“[Hormone therapy] is a significant risk factor of diabetes among breast cancer survivors. The underlying mechanism is unclear, and additional research is warranted. Although cessation of treatment is not recommended and progression of breast cancer often is inevitable, devised strategies aimed at lifestyle modifications in patients at high risk of diabetes could at least preserve the natural history of breast cancer,” they wrote.
Diabetes has previously been identified as a possible risk factor for breast cancer, but the potential for breast cancer therapy as a precipitating factor for diabetes is uncertain, the authors said.
“Given the detrimental impact of diabetes on breast cancer survival, additional exploration of the role of breast cancer treatment in the development of diabetes is important not only because it would add valuable information on the etiology of diabetes but also because it would help to identify high-risk patients in need of accentuated clinical care,” they wrote.
To explore the possible association between hormone therapy and diabetes risk, the investigators performed a retrospective case-cohort study of 2,246 women who had been diagnosed with primary nonmetastatic breast cancer treated with hormone therapy from 2002 through 2012.
They examined data on a randomly selected cohort of 448 breast cancer survivors and all patients in the parent (no diabetes at baseline) cohort who developed diabetes during the study period (324 patients).
They found that the prevalence of diabetes among their source population of 2,644 breast cancer survivors (including those with baseline diabetes) increased “drastically” from 6% in 2002 to 28% in 2015. The prevalence exceeded Israeli national norms from 2010 through 2013, with standardized prevalence ratios of 1.61 to 1.81 (P less than .001).
As noted, in the population without baseline diabetes, the crude cumulative incidence rate of diabetes in the presence of death as a competing risk factor was 20.9%.
In multivariate analyses controlling for demographic and socioeconomic factors, and for chemotherapy type, hypertension, outpatient visits, use of corticosteroids, thiazide diuretics, beta-blockers, statins, and year of breast cancer diagnosis, factors significantly associated with diabetes risk were use of hormone therapy (adjusted hazard ratio [HR] 2.40, P = .008), tamoxifen (aHR 2.25, P = .013), aromatase inhibitors (aHR 4.27, P = .013), therapy duration more than 1 year (aHR 2.36, P = .009), and 1 year or less (aHR 6.48, P = .004).
The investigators noted that although other reports have found no association between aromatase inhibitors and diabetes risk, those studies had small samples or offered no explanation of the lack of association.
In contrast, a 2016 joint ACS/ASCO breast cancer survivorship-care guideline notes that aromatase inhibitors may raise the risk of diabetes, the investigators noted.
The study was supported by grants from the Israeli Council for Higher Education. The investigators reported no conflicts of interest.
SOURCE: Hamood H et al. J Clin Oncol. 2018 Apr 24. doi: 10.1200/JCO.2017.76.3524.
Hormone therapy for breast cancer more than doubles a woman’s risk for developing type 2 diabetes, results of a case-cohort study suggest.
Hormone therapy with tamoxifen was associated with a more than twofold increase in risk of diabetes, and aromatase inhibitors were associated with a more than fourfold increase, reported Hatem Hamood, MD, of Leumit Health Services in Karmiel, Israel, and colleagues.
Among 2,246 women with breast cancer and no diabetes at baseline, followed for a mean of 5.9 years (longest follow-up 13 years), the crude cumulative lifetime incidence rate of diabetes was 20.9%, the investigators wrote. The report was published in the Journal of Clinical Oncology.
“[Hormone therapy] is a significant risk factor of diabetes among breast cancer survivors. The underlying mechanism is unclear, and additional research is warranted. Although cessation of treatment is not recommended and progression of breast cancer often is inevitable, devised strategies aimed at lifestyle modifications in patients at high risk of diabetes could at least preserve the natural history of breast cancer,” they wrote.
Diabetes has previously been identified as a possible risk factor for breast cancer, but the potential for breast cancer therapy as a precipitating factor for diabetes is uncertain, the authors said.
“Given the detrimental impact of diabetes on breast cancer survival, additional exploration of the role of breast cancer treatment in the development of diabetes is important not only because it would add valuable information on the etiology of diabetes but also because it would help to identify high-risk patients in need of accentuated clinical care,” they wrote.
To explore the possible association between hormone therapy and diabetes risk, the investigators performed a retrospective case-cohort study of 2,246 women who had been diagnosed with primary nonmetastatic breast cancer treated with hormone therapy from 2002 through 2012.
They examined data on a randomly selected cohort of 448 breast cancer survivors and all patients in the parent (no diabetes at baseline) cohort who developed diabetes during the study period (324 patients).
They found that the prevalence of diabetes among their source population of 2,644 breast cancer survivors (including those with baseline diabetes) increased “drastically” from 6% in 2002 to 28% in 2015. The prevalence exceeded Israeli national norms from 2010 through 2013, with standardized prevalence ratios of 1.61 to 1.81 (P less than .001).
As noted, in the population without baseline diabetes, the crude cumulative incidence rate of diabetes in the presence of death as a competing risk factor was 20.9%.
In multivariate analyses controlling for demographic and socioeconomic factors, and for chemotherapy type, hypertension, outpatient visits, use of corticosteroids, thiazide diuretics, beta-blockers, statins, and year of breast cancer diagnosis, factors significantly associated with diabetes risk were use of hormone therapy (adjusted hazard ratio [HR] 2.40, P = .008), tamoxifen (aHR 2.25, P = .013), aromatase inhibitors (aHR 4.27, P = .013), therapy duration more than 1 year (aHR 2.36, P = .009), and 1 year or less (aHR 6.48, P = .004).
The investigators noted that although other reports have found no association between aromatase inhibitors and diabetes risk, those studies had small samples or offered no explanation of the lack of association.
In contrast, a 2016 joint ACS/ASCO breast cancer survivorship-care guideline notes that aromatase inhibitors may raise the risk of diabetes, the investigators noted.
The study was supported by grants from the Israeli Council for Higher Education. The investigators reported no conflicts of interest.
SOURCE: Hamood H et al. J Clin Oncol. 2018 Apr 24. doi: 10.1200/JCO.2017.76.3524.
Hormone therapy for breast cancer more than doubles a woman’s risk for developing type 2 diabetes, results of a case-cohort study suggest.
Hormone therapy with tamoxifen was associated with a more than twofold increase in risk of diabetes, and aromatase inhibitors were associated with a more than fourfold increase, reported Hatem Hamood, MD, of Leumit Health Services in Karmiel, Israel, and colleagues.
Among 2,246 women with breast cancer and no diabetes at baseline, followed for a mean of 5.9 years (longest follow-up 13 years), the crude cumulative lifetime incidence rate of diabetes was 20.9%, the investigators wrote. The report was published in the Journal of Clinical Oncology.
“[Hormone therapy] is a significant risk factor of diabetes among breast cancer survivors. The underlying mechanism is unclear, and additional research is warranted. Although cessation of treatment is not recommended and progression of breast cancer often is inevitable, devised strategies aimed at lifestyle modifications in patients at high risk of diabetes could at least preserve the natural history of breast cancer,” they wrote.
Diabetes has previously been identified as a possible risk factor for breast cancer, but the potential for breast cancer therapy as a precipitating factor for diabetes is uncertain, the authors said.
“Given the detrimental impact of diabetes on breast cancer survival, additional exploration of the role of breast cancer treatment in the development of diabetes is important not only because it would add valuable information on the etiology of diabetes but also because it would help to identify high-risk patients in need of accentuated clinical care,” they wrote.
To explore the possible association between hormone therapy and diabetes risk, the investigators performed a retrospective case-cohort study of 2,246 women who had been diagnosed with primary nonmetastatic breast cancer treated with hormone therapy from 2002 through 2012.
They examined data on a randomly selected cohort of 448 breast cancer survivors and all patients in the parent (no diabetes at baseline) cohort who developed diabetes during the study period (324 patients).
They found that the prevalence of diabetes among their source population of 2,644 breast cancer survivors (including those with baseline diabetes) increased “drastically” from 6% in 2002 to 28% in 2015. The prevalence exceeded Israeli national norms from 2010 through 2013, with standardized prevalence ratios of 1.61 to 1.81 (P less than .001).
As noted, in the population without baseline diabetes, the crude cumulative incidence rate of diabetes in the presence of death as a competing risk factor was 20.9%.
In multivariate analyses controlling for demographic and socioeconomic factors, and for chemotherapy type, hypertension, outpatient visits, use of corticosteroids, thiazide diuretics, beta-blockers, statins, and year of breast cancer diagnosis, factors significantly associated with diabetes risk were use of hormone therapy (adjusted hazard ratio [HR] 2.40, P = .008), tamoxifen (aHR 2.25, P = .013), aromatase inhibitors (aHR 4.27, P = .013), therapy duration more than 1 year (aHR 2.36, P = .009), and 1 year or less (aHR 6.48, P = .004).
The investigators noted that although other reports have found no association between aromatase inhibitors and diabetes risk, those studies had small samples or offered no explanation of the lack of association.
In contrast, a 2016 joint ACS/ASCO breast cancer survivorship-care guideline notes that aromatase inhibitors may raise the risk of diabetes, the investigators noted.
The study was supported by grants from the Israeli Council for Higher Education. The investigators reported no conflicts of interest.
SOURCE: Hamood H et al. J Clin Oncol. 2018 Apr 24. doi: 10.1200/JCO.2017.76.3524.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Consider screening survivors of nonmetastatic breast cancer for diabetes.
Major finding: The crude lifetime incidence of diabetes following hormone therapy for breast cancer was 20.9%.
Study details: Case-cohort study of 2,246 women with nonmetastatic breast cancer and no baseline diabetes treated with hormone therapy.
Disclosures: The study was supported by grants from the Israeli Council for Higher Education. The investigators reported no conflicts of interest.
Source: Hamood H et al. J Clin Oncol. 2018 Apr 24. doi: 10.1200/JCO.2017.76.3524.
Is PASI 100 the new benchmark in psoriasis?
KAUAI, HAWAII – I think we should just do away with PASI 90 [90% improvement in Psoriasis Area and Severity Index score] and look at how well our drugs do against the metric of PASI 100. The whole ball of wax. Let’s just go for complete clearance,” Craig L. Leonardi, MD, declared in a provocative presentation at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
He advocates using number needed to treat (NNT) as a performance yardstick. He finds it helpful in translating sometimes-arcane clinical trial results into useful information to guide everyday practice. The NNT is the average number of patients who need to be treated with a drug or procedure in order to achieve one additional good outcome, compared with a control intervention or placebo. It’s the inverse of the absolute risk reduction. The lower the NNT, the better an intervention is performing.
He presented a chart that summarized the NNTs to achieve a PASI 100 response for various systemic agents commonly used in treating moderate to severe psoriasis. He obtained the data from Food and Drug Administration–regulated product labeling and phase 3 clinical trials.
Dr. Leonardi drew attention to the worst performers on the list: methotrexate, with an NNT of 25 to achieve a PASI 100 response, and etanercept, with an NNT of 23.3.
“Methotrexate is a drug that the insurance industry says we have to flow through on our way to biologic drugs. But if complete clearance is your goal, this is an exercise in futility. These patients will never, ever get to complete clearance – or it’s at least very unlikely. We shouldn’t be asked to go through methotrexate on our way to anything. We shouldn’t be asked to use methotrexate at all. We should be bypassing it. And some of us are working on this,” he said.
Ustekinumab and adalimumab are the current market leaders in biologic therapy for psoriasis, but they don’t stack up so well when viewed through the filter of PASI 100 response, with NNTs of 9.2 and 5.3, respectively.
“These market leaders may not be the most relevant drugs in the current era,” according to the dermatologist.
In contrast, the high-performance biologics – the interleukin-17 inhibitors secukinumab, ixekizumab, and brodalumab and the interleukin-23 antagonist guselkumab – have impressively low NNTs of 2.4-3.6 in order to achieve complete clearance.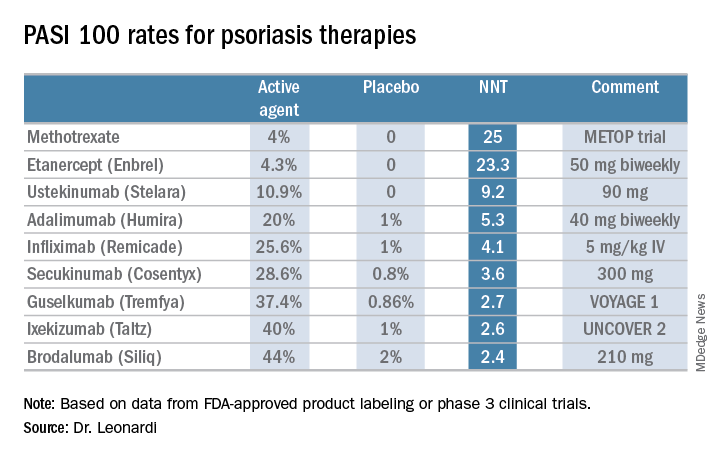
“But our IL-17 and IL-23 antagonists are markedly different from all other therapies, with NNTs of 1.3-1.1. With an NNT of 1.1, if you treated 11 patients with ixekizumab, 10 of them would achieve a PASI 75,” he explained.
“This is really quite remarkable,” Dr. Leonardi commented. “Our first drug back in 2002 was alefacept, and that drug was a ‘twenty-one percenter’: 21% of patients achieved a PASI 75. And quite frankly, we thought that was rocking voodoo science back in the day. Well, we’re really out there now. This is utterly amazing data: a PASI 75 of 81.6% for secukinumab, 86% for brodalumab, 90% for ixekizumab, and 91.2% for guselkumab. This is why we’re publishing this stuff in the best medical journals, because these results are absolutely amazing. So many different medical specialties are interested in what we’re doing with these drugs.”
He reported serving as a consultant to AbbVie, Amgen, Boehringer Ingelheim, Dermira, Eli Lilly, Janssen, Leo, Pfizer, Sandoz, and UCB and receiving research funding from 21 pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – I think we should just do away with PASI 90 [90% improvement in Psoriasis Area and Severity Index score] and look at how well our drugs do against the metric of PASI 100. The whole ball of wax. Let’s just go for complete clearance,” Craig L. Leonardi, MD, declared in a provocative presentation at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
He advocates using number needed to treat (NNT) as a performance yardstick. He finds it helpful in translating sometimes-arcane clinical trial results into useful information to guide everyday practice. The NNT is the average number of patients who need to be treated with a drug or procedure in order to achieve one additional good outcome, compared with a control intervention or placebo. It’s the inverse of the absolute risk reduction. The lower the NNT, the better an intervention is performing.
He presented a chart that summarized the NNTs to achieve a PASI 100 response for various systemic agents commonly used in treating moderate to severe psoriasis. He obtained the data from Food and Drug Administration–regulated product labeling and phase 3 clinical trials.
Dr. Leonardi drew attention to the worst performers on the list: methotrexate, with an NNT of 25 to achieve a PASI 100 response, and etanercept, with an NNT of 23.3.
“Methotrexate is a drug that the insurance industry says we have to flow through on our way to biologic drugs. But if complete clearance is your goal, this is an exercise in futility. These patients will never, ever get to complete clearance – or it’s at least very unlikely. We shouldn’t be asked to go through methotrexate on our way to anything. We shouldn’t be asked to use methotrexate at all. We should be bypassing it. And some of us are working on this,” he said.
Ustekinumab and adalimumab are the current market leaders in biologic therapy for psoriasis, but they don’t stack up so well when viewed through the filter of PASI 100 response, with NNTs of 9.2 and 5.3, respectively.
“These market leaders may not be the most relevant drugs in the current era,” according to the dermatologist.
In contrast, the high-performance biologics – the interleukin-17 inhibitors secukinumab, ixekizumab, and brodalumab and the interleukin-23 antagonist guselkumab – have impressively low NNTs of 2.4-3.6 in order to achieve complete clearance.
“But our IL-17 and IL-23 antagonists are markedly different from all other therapies, with NNTs of 1.3-1.1. With an NNT of 1.1, if you treated 11 patients with ixekizumab, 10 of them would achieve a PASI 75,” he explained.
“This is really quite remarkable,” Dr. Leonardi commented. “Our first drug back in 2002 was alefacept, and that drug was a ‘twenty-one percenter’: 21% of patients achieved a PASI 75. And quite frankly, we thought that was rocking voodoo science back in the day. Well, we’re really out there now. This is utterly amazing data: a PASI 75 of 81.6% for secukinumab, 86% for brodalumab, 90% for ixekizumab, and 91.2% for guselkumab. This is why we’re publishing this stuff in the best medical journals, because these results are absolutely amazing. So many different medical specialties are interested in what we’re doing with these drugs.”
He reported serving as a consultant to AbbVie, Amgen, Boehringer Ingelheim, Dermira, Eli Lilly, Janssen, Leo, Pfizer, Sandoz, and UCB and receiving research funding from 21 pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – I think we should just do away with PASI 90 [90% improvement in Psoriasis Area and Severity Index score] and look at how well our drugs do against the metric of PASI 100. The whole ball of wax. Let’s just go for complete clearance,” Craig L. Leonardi, MD, declared in a provocative presentation at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
He advocates using number needed to treat (NNT) as a performance yardstick. He finds it helpful in translating sometimes-arcane clinical trial results into useful information to guide everyday practice. The NNT is the average number of patients who need to be treated with a drug or procedure in order to achieve one additional good outcome, compared with a control intervention or placebo. It’s the inverse of the absolute risk reduction. The lower the NNT, the better an intervention is performing.
He presented a chart that summarized the NNTs to achieve a PASI 100 response for various systemic agents commonly used in treating moderate to severe psoriasis. He obtained the data from Food and Drug Administration–regulated product labeling and phase 3 clinical trials.
Dr. Leonardi drew attention to the worst performers on the list: methotrexate, with an NNT of 25 to achieve a PASI 100 response, and etanercept, with an NNT of 23.3.
“Methotrexate is a drug that the insurance industry says we have to flow through on our way to biologic drugs. But if complete clearance is your goal, this is an exercise in futility. These patients will never, ever get to complete clearance – or it’s at least very unlikely. We shouldn’t be asked to go through methotrexate on our way to anything. We shouldn’t be asked to use methotrexate at all. We should be bypassing it. And some of us are working on this,” he said.
Ustekinumab and adalimumab are the current market leaders in biologic therapy for psoriasis, but they don’t stack up so well when viewed through the filter of PASI 100 response, with NNTs of 9.2 and 5.3, respectively.
“These market leaders may not be the most relevant drugs in the current era,” according to the dermatologist.
In contrast, the high-performance biologics – the interleukin-17 inhibitors secukinumab, ixekizumab, and brodalumab and the interleukin-23 antagonist guselkumab – have impressively low NNTs of 2.4-3.6 in order to achieve complete clearance.
“But our IL-17 and IL-23 antagonists are markedly different from all other therapies, with NNTs of 1.3-1.1. With an NNT of 1.1, if you treated 11 patients with ixekizumab, 10 of them would achieve a PASI 75,” he explained.
“This is really quite remarkable,” Dr. Leonardi commented. “Our first drug back in 2002 was alefacept, and that drug was a ‘twenty-one percenter’: 21% of patients achieved a PASI 75. And quite frankly, we thought that was rocking voodoo science back in the day. Well, we’re really out there now. This is utterly amazing data: a PASI 75 of 81.6% for secukinumab, 86% for brodalumab, 90% for ixekizumab, and 91.2% for guselkumab. This is why we’re publishing this stuff in the best medical journals, because these results are absolutely amazing. So many different medical specialties are interested in what we’re doing with these drugs.”
He reported serving as a consultant to AbbVie, Amgen, Boehringer Ingelheim, Dermira, Eli Lilly, Janssen, Leo, Pfizer, Sandoz, and UCB and receiving research funding from 21 pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Vehicle crash risk linked to various sleep disorders
Individuals with certain sleeping disorders may have a higher risk of crashes, near-crashes or unsafe maneuvering prior to such events, suggests a study.
“The results confirm that some sleep disorders generally increase driving risk as defined by our dependent measures,” wrote Shu-Yuan Liu, a doctoral student, and two colleagues at Virginia Tech, Blacksburg (Sleep. 2018 Apr 1. doi: 10.1093/sleep/zsy023). “Furthermore, the results also provide some insights into how risk varies across specific types of sleep disorder and some moderating factors.”
The study involved licensed drivers who drove at least 3 days a week, had an eligible vehicle in good working condition, and agreed to participate for 1 to 2 years. At the start and end of the study, participants filled out a questionnaire on any medical conditions they had or had been treated for in the past year, any medications they were taking, and any aids they were using for a medical condition.
Among the conditions they were able to select were narcolepsy, sleep apnea, insomnia, shift work sleep disorder, restless legs syndrome (RLS), periodic limb movement disorder, and migraine. All of these conditions have been linked in previous studies to a higher risk of vehicle collisions.
A total of 646 participants, 18.2% of the sample, had one of those disorders: 0.14% had narcolepsy, 7.4% had sleep apnea, 4.8% had insomnia, 3.4% had RLS, 0.37% had shift work sleep disorder, 0.23% had periodic limb movement disorder, and 8.4% had migraine.
Analysis of vehicle data found that female drivers with RLS and any drivers with insomnia had a higher risk of crashes or near-crashes (adjusted odds ratio [AOR] = 2.26 and 1.49, respectively, P less than .05 for both). Drivers with narcolepsy had 9 times greater odds of being involved in a crash or near-crash, but the finding was not statistically significant (AOR = 10.24, P less than .1).
“Drivers who reported frequency of sleepy driving as ‘never,’ ‘rarely,’ and ‘sometimes’ also had higher a risk, indicating that crash or near-crash risk is also associated with sources other than these sleeping disorders,” the authors noted. These drivers’ increased odds of getting into or nearly getting into a crash ranged from 31% to 53% greater (P less than .05).
All drivers with shift work sleep disorder, except for those aged 20-24, had a crash or near-crash rate that was 7.5 times greater than that of drivers without any sleeping disorders. The rate among drivers aged 20-24 with this disorder had a 90% lower rate (risk ratio [RR] = 0.1, P less than .05) compared with control drivers.
When the researchers analyzed the drivers’ maneuvers just before a crash or near-crash, they found females with sleep apnea had a 36% greater odds of doing an unsafe maneuver in crash/near-crash circumstances (AOR = 1.36). (AOR = 3.38 and 3.53, respectively, P less than .05).
The only drivers with a sleeping disorder who were more likely to be involved in crashes of greater severity were those with periodic limb movement disorder (AOR = 1.43, P less than .05).
However, young drivers, senior drivers, and nighttime drivers also all had higher odds of being involved in more severe crashes and in performing unsafe maneuvers prior to a crash or near-crash. Nighttime drivers seemed to be most at risk for these, and they were linked to having more than 5 times greater odds of unsafely maneuvering their vehicles prior to getting into a crash or near crash (AOR = 6.71, P less than .05).
“This is a strong piece of evidence that nighttime driving is less safe than daytime driving and limiting amount of nighttime driving could be one method to moderate road risk for some individuals,” the authors wrote.
The study’s limitations include its observational nature, low numbers of participants with several of the sleeping disorders (at levels below the disorder’s prevalence in the general population), and the complexities involved in what causes a crash or near crash.
One limitation of this study was that sleep hygiene and sleep quality were not examined, even though these might contribute significantly to roadway safety, the researchers noted. This study also did not take into account what medications or other treatment (such as continuous positive airway pressure for those with sleep apnea) the participants might be receiving for their condition.
The study’s implications include the need for physicians to advise patients with insomnia or females with sleep apnea to use caution while driving without “exaggerating risks that introduce undue fear to patients with other sleep disorders and thereby limiting mobility unnecessarily,” the authors wrote. The researchers also suggested that employers consider providing alternative transportation to shift workers and/or that insurance companies offer employers lower rates for offering such alternatives.
SOURCE: Liu Shu-Yuan et al. Sleep J. 2018 Apr 1. doi: 10.1093/sleep/zsy023.
Individuals with certain sleeping disorders may have a higher risk of crashes, near-crashes or unsafe maneuvering prior to such events, suggests a study.
“The results confirm that some sleep disorders generally increase driving risk as defined by our dependent measures,” wrote Shu-Yuan Liu, a doctoral student, and two colleagues at Virginia Tech, Blacksburg (Sleep. 2018 Apr 1. doi: 10.1093/sleep/zsy023). “Furthermore, the results also provide some insights into how risk varies across specific types of sleep disorder and some moderating factors.”
The study involved licensed drivers who drove at least 3 days a week, had an eligible vehicle in good working condition, and agreed to participate for 1 to 2 years. At the start and end of the study, participants filled out a questionnaire on any medical conditions they had or had been treated for in the past year, any medications they were taking, and any aids they were using for a medical condition.
Among the conditions they were able to select were narcolepsy, sleep apnea, insomnia, shift work sleep disorder, restless legs syndrome (RLS), periodic limb movement disorder, and migraine. All of these conditions have been linked in previous studies to a higher risk of vehicle collisions.
A total of 646 participants, 18.2% of the sample, had one of those disorders: 0.14% had narcolepsy, 7.4% had sleep apnea, 4.8% had insomnia, 3.4% had RLS, 0.37% had shift work sleep disorder, 0.23% had periodic limb movement disorder, and 8.4% had migraine.
Analysis of vehicle data found that female drivers with RLS and any drivers with insomnia had a higher risk of crashes or near-crashes (adjusted odds ratio [AOR] = 2.26 and 1.49, respectively, P less than .05 for both). Drivers with narcolepsy had 9 times greater odds of being involved in a crash or near-crash, but the finding was not statistically significant (AOR = 10.24, P less than .1).
“Drivers who reported frequency of sleepy driving as ‘never,’ ‘rarely,’ and ‘sometimes’ also had higher a risk, indicating that crash or near-crash risk is also associated with sources other than these sleeping disorders,” the authors noted. These drivers’ increased odds of getting into or nearly getting into a crash ranged from 31% to 53% greater (P less than .05).
All drivers with shift work sleep disorder, except for those aged 20-24, had a crash or near-crash rate that was 7.5 times greater than that of drivers without any sleeping disorders. The rate among drivers aged 20-24 with this disorder had a 90% lower rate (risk ratio [RR] = 0.1, P less than .05) compared with control drivers.
When the researchers analyzed the drivers’ maneuvers just before a crash or near-crash, they found females with sleep apnea had a 36% greater odds of doing an unsafe maneuver in crash/near-crash circumstances (AOR = 1.36). (AOR = 3.38 and 3.53, respectively, P less than .05).
The only drivers with a sleeping disorder who were more likely to be involved in crashes of greater severity were those with periodic limb movement disorder (AOR = 1.43, P less than .05).
However, young drivers, senior drivers, and nighttime drivers also all had higher odds of being involved in more severe crashes and in performing unsafe maneuvers prior to a crash or near-crash. Nighttime drivers seemed to be most at risk for these, and they were linked to having more than 5 times greater odds of unsafely maneuvering their vehicles prior to getting into a crash or near crash (AOR = 6.71, P less than .05).
“This is a strong piece of evidence that nighttime driving is less safe than daytime driving and limiting amount of nighttime driving could be one method to moderate road risk for some individuals,” the authors wrote.
The study’s limitations include its observational nature, low numbers of participants with several of the sleeping disorders (at levels below the disorder’s prevalence in the general population), and the complexities involved in what causes a crash or near crash.
One limitation of this study was that sleep hygiene and sleep quality were not examined, even though these might contribute significantly to roadway safety, the researchers noted. This study also did not take into account what medications or other treatment (such as continuous positive airway pressure for those with sleep apnea) the participants might be receiving for their condition.
The study’s implications include the need for physicians to advise patients with insomnia or females with sleep apnea to use caution while driving without “exaggerating risks that introduce undue fear to patients with other sleep disorders and thereby limiting mobility unnecessarily,” the authors wrote. The researchers also suggested that employers consider providing alternative transportation to shift workers and/or that insurance companies offer employers lower rates for offering such alternatives.
SOURCE: Liu Shu-Yuan et al. Sleep J. 2018 Apr 1. doi: 10.1093/sleep/zsy023.
Individuals with certain sleeping disorders may have a higher risk of crashes, near-crashes or unsafe maneuvering prior to such events, suggests a study.
“The results confirm that some sleep disorders generally increase driving risk as defined by our dependent measures,” wrote Shu-Yuan Liu, a doctoral student, and two colleagues at Virginia Tech, Blacksburg (Sleep. 2018 Apr 1. doi: 10.1093/sleep/zsy023). “Furthermore, the results also provide some insights into how risk varies across specific types of sleep disorder and some moderating factors.”
The study involved licensed drivers who drove at least 3 days a week, had an eligible vehicle in good working condition, and agreed to participate for 1 to 2 years. At the start and end of the study, participants filled out a questionnaire on any medical conditions they had or had been treated for in the past year, any medications they were taking, and any aids they were using for a medical condition.
Among the conditions they were able to select were narcolepsy, sleep apnea, insomnia, shift work sleep disorder, restless legs syndrome (RLS), periodic limb movement disorder, and migraine. All of these conditions have been linked in previous studies to a higher risk of vehicle collisions.
A total of 646 participants, 18.2% of the sample, had one of those disorders: 0.14% had narcolepsy, 7.4% had sleep apnea, 4.8% had insomnia, 3.4% had RLS, 0.37% had shift work sleep disorder, 0.23% had periodic limb movement disorder, and 8.4% had migraine.
Analysis of vehicle data found that female drivers with RLS and any drivers with insomnia had a higher risk of crashes or near-crashes (adjusted odds ratio [AOR] = 2.26 and 1.49, respectively, P less than .05 for both). Drivers with narcolepsy had 9 times greater odds of being involved in a crash or near-crash, but the finding was not statistically significant (AOR = 10.24, P less than .1).
“Drivers who reported frequency of sleepy driving as ‘never,’ ‘rarely,’ and ‘sometimes’ also had higher a risk, indicating that crash or near-crash risk is also associated with sources other than these sleeping disorders,” the authors noted. These drivers’ increased odds of getting into or nearly getting into a crash ranged from 31% to 53% greater (P less than .05).
All drivers with shift work sleep disorder, except for those aged 20-24, had a crash or near-crash rate that was 7.5 times greater than that of drivers without any sleeping disorders. The rate among drivers aged 20-24 with this disorder had a 90% lower rate (risk ratio [RR] = 0.1, P less than .05) compared with control drivers.
When the researchers analyzed the drivers’ maneuvers just before a crash or near-crash, they found females with sleep apnea had a 36% greater odds of doing an unsafe maneuver in crash/near-crash circumstances (AOR = 1.36). (AOR = 3.38 and 3.53, respectively, P less than .05).
The only drivers with a sleeping disorder who were more likely to be involved in crashes of greater severity were those with periodic limb movement disorder (AOR = 1.43, P less than .05).
However, young drivers, senior drivers, and nighttime drivers also all had higher odds of being involved in more severe crashes and in performing unsafe maneuvers prior to a crash or near-crash. Nighttime drivers seemed to be most at risk for these, and they were linked to having more than 5 times greater odds of unsafely maneuvering their vehicles prior to getting into a crash or near crash (AOR = 6.71, P less than .05).
“This is a strong piece of evidence that nighttime driving is less safe than daytime driving and limiting amount of nighttime driving could be one method to moderate road risk for some individuals,” the authors wrote.
The study’s limitations include its observational nature, low numbers of participants with several of the sleeping disorders (at levels below the disorder’s prevalence in the general population), and the complexities involved in what causes a crash or near crash.
One limitation of this study was that sleep hygiene and sleep quality were not examined, even though these might contribute significantly to roadway safety, the researchers noted. This study also did not take into account what medications or other treatment (such as continuous positive airway pressure for those with sleep apnea) the participants might be receiving for their condition.
The study’s implications include the need for physicians to advise patients with insomnia or females with sleep apnea to use caution while driving without “exaggerating risks that introduce undue fear to patients with other sleep disorders and thereby limiting mobility unnecessarily,” the authors wrote. The researchers also suggested that employers consider providing alternative transportation to shift workers and/or that insurance companies offer employers lower rates for offering such alternatives.
SOURCE: Liu Shu-Yuan et al. Sleep J. 2018 Apr 1. doi: 10.1093/sleep/zsy023.
FROM SLEEP
Key clinical point: Individuals with certain sleeping disorders may have a higher risk of crashes, near-crashes or unsafe maneuvering prior to such events.
Major finding: Drivers with insomnia and female drivers with sleep apnea have 49% and 126% greater odds, respectively, of a crash or near-crash.
Data source: The findings are based on an analysis of naturalistic driving data from 3,541 U.S. drivers between ages 16 and 98.
Disclosures: The data were provided by the Transportation Research Board of the National Academy of Sciences. No external funding was noted. The authors reported having no disclosures.
Source: Liu Shu-Yuan et al. Sleep J. 2018 Apr 1. doi: 10.1093/sleep/zsy023.
Drs. O'Neil, Meadows, and Patterson Earn Annual AJO Resident Writer's Awards

Bryan Hanypsiak, MD, Editor-in-Chief of The American Journal of Orthopedics, along with Darla Conrad, Senior Director, North America, and Mindy Edgar, Manager, Academic Alliances from the Johnson & Johnson Institute presented the 2017 Resident Writer’s Award to the three winners at the American Academy of Orthopaedic Surgeons (AAOS) annual meeting in New Orleans. All articles published in 2017 with a resident as the first-listed author and accepted through the journal’s standard blinded-review process were eligible for this award. The annual Resident Writer's Award is sponsored by Johnson & Johnson. Papers published in 2018 will be judged by The American Journal of Orthopedics Editorial Board, and honoraria will be presented to the winners at the 2019 AAOS annual meeting.
Joseph T. O’Neil, MD
Molly C. Meadows, MD
Joseph T. Patterson, MD
See more information about the 2018 Resident Writer's Award.
Supported by Johnson & Johnson

Bryan Hanypsiak, MD, Editor-in-Chief of The American Journal of Orthopedics, along with Darla Conrad, Senior Director, North America, and Mindy Edgar, Manager, Academic Alliances from the Johnson & Johnson Institute presented the 2017 Resident Writer’s Award to the three winners at the American Academy of Orthopaedic Surgeons (AAOS) annual meeting in New Orleans. All articles published in 2017 with a resident as the first-listed author and accepted through the journal’s standard blinded-review process were eligible for this award. The annual Resident Writer's Award is sponsored by Johnson & Johnson. Papers published in 2018 will be judged by The American Journal of Orthopedics Editorial Board, and honoraria will be presented to the winners at the 2019 AAOS annual meeting.
Joseph T. O’Neil, MD
Molly C. Meadows, MD
Joseph T. Patterson, MD
See more information about the 2018 Resident Writer's Award.
Supported by Johnson & Johnson

Bryan Hanypsiak, MD, Editor-in-Chief of The American Journal of Orthopedics, along with Darla Conrad, Senior Director, North America, and Mindy Edgar, Manager, Academic Alliances from the Johnson & Johnson Institute presented the 2017 Resident Writer’s Award to the three winners at the American Academy of Orthopaedic Surgeons (AAOS) annual meeting in New Orleans. All articles published in 2017 with a resident as the first-listed author and accepted through the journal’s standard blinded-review process were eligible for this award. The annual Resident Writer's Award is sponsored by Johnson & Johnson. Papers published in 2018 will be judged by The American Journal of Orthopedics Editorial Board, and honoraria will be presented to the winners at the 2019 AAOS annual meeting.
Joseph T. O’Neil, MD
Molly C. Meadows, MD
Joseph T. Patterson, MD
See more information about the 2018 Resident Writer's Award.
Supported by Johnson & Johnson















