User login
Medical marijuana: Do the benefits outweigh the risks?
There is a need for additional treatment options to improve symptoms, enhance the quality of life (QOL), and reduce suffering among patients who have chronic medical illness. Medical marijuana (MM) has the potential to help patients who have certain medical conditions in states where it is legal for prescription by a licensed medical provider.
Cannabis has a long history of medicinal use (Box 11-12). Two derivatives of the Cannabis plant—cannabinoid delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD)—are responsible for most of its effects. Some of these effects, including analgesia, decreased muscle spasticity, and reduced eye pressure, have been harnessed for their potential therapeutic effects (Box 213-19). As of November 2017, 29 states had legalized Cannabis for medical use, and several had legalized its recreational use.12
With the increasing availability of MM, psychiatrists are likely to encounter patients who are using it or who will ask them about it. This article reviews evidence related to using MM to treat patients with neuropathic pain; chemotherapyinduced nausea and vomiting (CINV); epilepsy; multiple sclerosis (MS); glaucoma; Crohn’s disease; Parkinson’s disease; amyotrophic lateral sclerosis; dementia-related behavioral disturbances; posttraumatic stress disorder (PTSD); and anxiety.
Box 1
Cannabis: A history of medicinal use
Cannabis has been cultivated since ancient times, beginning in China and India. The earliest reference of its use for healing purposes may have been in the Chinese Pharmacopeia, circa 1500 BC.1 In 1839, Dr. William Brooke O’Shaughnessy introduced Cannabis Indica, or “Indian hemp,” to the western world after a professorship in Calcutta, India.2 In the early 1840s, an English physician, Dr. John Clendinning, prescribed Cannabis for migraine headache.3 In the 19th and early 20th centuries, several prominent physicians advocated using Cannabis for migraines; Sir William Osler did so in his textbook, The principles and practice of medicine.4 It was listed in the U.S. Pharmacopeia in 1850 but removed in 1942.5,6
Until 1937, Cannabis was used in the United States for medicinal purposes, such as for treating inflamed skin, incontinence, and sexually transmitted diseases.7 In 1937, the Marihuana Tax Act, which prohibited the production, importation, possession, use, and dispersal of Cannabis, was passed.8 Cannabis became a Schedule I drug under the Controlled Substance Act of 1970.9
In 1999, based on available evidence, the Institute of Medicine (IOM) concluded Cannabis had less likelihood of dependence than benzodiazepines, opiates, cocaine, or nicotine. The IOM also concluded that the symptoms of withdrawal were mild in comparison with benzodiazepines or opiates. Finally, the IOM stated that Cannabis was not a “gateway” drug.10
In 1996, California was the first state to reimplement medicinal use of Cannabis under the Compassionate Use Act, also known as Proposition 215.11 This act allowed individuals to retain or produce Cannabis for personal consumption with a physician’s approval. Many states eventually followed California’s lead. As of November 2017, 29 states, the District of Columbia, Guam, and Puerto Rico had regulated Cannabis use for medical purposes,12 and recreational use had been approved in 7 states and the District of Columbia.
Medical illnesses
Neuropathic pain. Chronic neuropathic pain affects an estimated 7% to 8% of adults.20 Patients with neuropathic pain are often treated with anticonvulsants, antidepressants, opioids, and local anesthetics21; however, these medications may not provide substantial relief. Research has revealed that THC and CBD can improve central and peripheral neuropathic pain, as well as pain associated with rheumatoid arthritis and fibromyalgia.22
Wilsey et al23 evaluated the analgesic effects of smoked MM for neuropathic pain in a small (N = 38) double-blind, randomized controlled trial (RCT). Patients in this study had a preexisting diagnosis of complex regional pain syndrome, spinal cord injury, peripheral neuropathy, or nerve injury. To prevent any unforeseen adverse outcomes related to Cannabis use, participants were required to have previous exposure to Cannabis. Patients were excluded if they had major mental illness, substance abuse, or other major medical ailments.
Participants smoked high-dose Cannabis cigarettes (7% THC), low-dose Cannabis cigarettes (3.5% THC), or placebo cigarettes. Pain was measured on a visual analog scale (VAS) that ranged from 0 (no pain) to 100 (worst possible pain). Compared with the placebo group, significant analgesia was achieved in both Cannabis groups (P = .016). The high-dose group had greater neurocognitive impairment.
Ware et al24 conducted a crossover RCT (N = 23) to determine the efficacy of smoked MM for neuropathic pain. Participants had neuropathic pain for at least 3 months that was caused by trauma or surgery, with an average weekly pain intensity score >4 on scale of 0 to 10. Patients with pain due to cancer, nociceptive causes, unstable medical conditions, current substance abuse, history of a psychotic disorder, or suicidal ideation were excluded. Participants were assigned to a 9.4% THC group or a 0% THC group. Pain intensity was evaluated daily via telephone. Participants in the 9.4% THC group had statistically lower pain intensity compared with the 0% THC group (P = .023). Common adverse effects reported by those in the 9.4% group included headache, dry eyes, burning sensation, dizziness, numbness, and cough.
Box 2
The effects of Cannabis
Marijuana is harvested from the plant Cannabis sativa and composed of 400 lipophilic chemical compounds, including phytocannabinoids, terpenoids, and flavonoids.13 The plant contains compounds termed “cannabinoids.” Two of these derivatives in particular are responsible for most of the effects of marijuana: cannabinoid delta-9- tetrahydrocannabinol (THC) and cannabidiol (CBD). THC has a comparable structure and binding mechanism to anandamide, a naturally occurring fatty acid neurotransmitter present within the human brain.14-16 The endogenous endocannabinoid system and its receptors are found throughout the entire body (brain, organs, glands, immune cells, and connective tissues).
THC binds to cannabinoid receptors CB1 and CB2. CB1 is found predominantly in the CNS. CB2 is found predominantly outside the CNS and is associated with the immune system.14-16 The effects of THC include euphoria, relaxation, appetite stimulation, improvement of nausea and vomiting, analgesia, decreased muscle spasticity, and reduced eye pressure.14,15 CBD may have anxiolytic, antipsychotic, anticonvulsive, and analgesic effects.
The rate of absorption of THC and CBD depends both on the potency of the cannabinoid as well as the mechanism of consumption. Cannabis can be administered by multiple routes, including via smoking, oral ingestion, or IV.16 When Cannabis is smoked (the route for the most rapid delivery), THC is transported from the lungs to the bloodstream and reaches peak concentrations in 3 to 10 minutes. Oral ingestion (capsules, tinctures, sprays, and edibles) has a more flexible onset of action, usually occurring in 30 to 120 minutes, with effects lasting 5 to 6 hours. IV administration has rapid effects; the onset can occur within seconds to minutes, and effects can last 2 to 3 hours. The IV form allows 90% of THC to be distributed in plasma and can rapidly penetrate highly vascularized tissues, such as the liver, heart, fat, lungs, and muscles.
Pharmaceutical manufacturers have used cannabinoid derivatives to produce Cannabis-based medications for treating medical conditions. Nabilone, a potent agonist of the CB1 receptor, became available as a Schedule II medication in 1981 and was approved for patients with chemotherapy-induced nausea and vomiting (CINV).17 In 1985, dronabinol was introduced as an antiemetic for CINV as well as an appetite stimulant for patients with conditions associated with excessive weight loss.18 Another option, nabiximols, is an oral mucosal spray that consists of THC and CBD in a 1:1 ratio.19 Nabiximols is approved in Canada for pain relief in end-stage cancer patients and pain associated with multiple sclerosis.19
In an RCT of vaporized Cannabis, 39 patients with a diagnosis of complex regional pain syndrome, thalamic pain, spinal cord injury, peripheral neuropathy, radiculopathy, or nerve injury were assigned to a medium-dose (3.53% THC), low-dose (1.29% THC), or placebo group.25 Serious mental illness, substance abuse, and medical conditions were cause for exclusion. Participants received vaporized marijuana (average 8 to 12 puffs per visit) over 3 sessions. A 30% pain reduction was achieved by 26% of those in the placebo group, 57% of those in the low-dose group, and 61% of individuals in the high-dose group; the difference between placebo and each Cannabis group was statistically significant.
Chemotherapy-induced nausea and vomiting. Up to 80% of patients who receive chemotherapy experience CINV, which occurs from 24 hours to 7 days after receiving such therapy.26 CINV negatively influences a patient’s QOL and may impact the decision to continue with chemotherapy. Use of MM can help to diminish vomiting by binding to central CB1 receptors and averting the proemetic effects of dopamine and serotonin.27 Two synthetically derived cannabinoids, dronabinol and nabilone, are FDA-approved for treating CINV.
In a small (N = 64) parallel-group RCT, Meiri et al27 compared dronabinol with the commonly used antiemetic ondansetron and with a combination of dronabinol and ondansetron for treating CINV in adults. The primary outcome was prevention of delayed-onset CINV. Patients were eligible for this study if they had a malignancy that did not involve bone marrow, were receiving treatment with a moderately to highly emetogenic regimen, were not pregnant, and had an estimated life expectancy of at least 6 weeks after chemotherapy. The patients were randomized to 1 of 4 treatment groups: dronabinol alone, ondansetron alone, dronabinol plus ondansetron, or placebo. Overall, 47% to 58% of the active treatment groups improved, compared with 20% of the placebo group. Combination therapy did not provide any benefit beyond any single agent alone. All active treatments reduced nausea compared with placebo; there was no difference between active treatment groups. This study was limited by low enrollment.
Tramèr et al28 conducted a systematic review of 30 randomized comparisons of MM with placebo or antiemetics. The reviewed studies were completed between 1975 to 1997 and analyzed a total of 1,366 patients. Nabilone was evaluated in 16 trials; dronabinol was utilized in 13 trials; and IM levonantradol, a synthetic cannabinoid analog of dronabinol, was used in 1 trial. These agents were found to be more effective as an antiemetic compared with prochlorperazine, metoclopramide, chlorpromazine, thiethylperazine, haloperidol, domperidone, or alizapride. In addition, 38% to 90% of patients in these studies preferred MM over the traditional antiemetics.
A Cochrane review29 suggested that MM may be a viable option for treatment-resistant CINV; however, further studies are needed because current studies have methodological limitations.
Epilepsy. Maa and Figi30 reported a case of a 5-year-old girl who had Dravet syndrome, which resulted in 50 generalized tonic-clonic seizures daily; multiple anticonvulsants did not alleviate these seizures. Because of her recurring seizures, the patient had multiple cognitive and motor delays and needed a feeding tube. In addition to her existing antiepileptic drug regimen, she was started on adjunctive therapy with a sublingual Cannabis extract containing a high concentration of CBD. Her seizures decreased from 50 per day to 2 to 3 nocturnal convulsions per month. The treatment enabled her to stop using a feeding tube, resume walking and talking, and sleep soundly.
dos Santos et al31 reviewed studies of MM for treating epilepsy. One was a double-blind, placebo-controlled trial that included 15 patients ages 14 to 49 who had secondary generalized epilepsy with a temporal lobe focus. Eight patients received 200 to 300 mg/d of oral CBD for 8 to 18 weeks, and 7 received placebo. Seven patients had fewer seizures and 4 had no seizures. Only 1 patient in the placebo group demonstrated any improvement. Another study in this review included 19 children with treatment-resistant epilepsy: Dravet syndrome (n = 13), Doose syndrome (n = 4), Lennox-Gastaut syndrome (n = 1), or idiopathic epilepsy (n = 1). These patients experienced various types of seizures with a frequency ranging from 2 per week to 250 per day. Overall, 84% of children treated with CBD had fewer seizures: 11% were seizure-free, 42% had a >80% reduction in seizures, and 32% had a 25% to 60% reduction in seizures. Parents also noted additional benefits, including increased attention, improved mood, and improved sleep. CBD was well tolerated in most patients in both studies.
Despite these results, a Cochrane review32 found that no reliable conclusions can be drawn regarding the efficacy of MM for treating epilepsy.
Multiple sclerosis. According to American Academy of Neurology guidelines, physicians may provide MM as an alternative treatment for patients with MS-related spasticity.33 Multiple studies have tested MM and MM-related extracts for treating spasticity related to MS.34,35 In a placebo-controlled crossover study, Corey-Bloom et al34 reported a significant reduction in spasticity, measured using the modified Ashworth scale, in MS patients receiving Cannabis cigarettes vs placebo cigarettes (P < .0001). However, compared with the placebo group, patients who received MM had significant adverse effects, primarily cognitive impairment (P = .003).
In a multicenter RCT (N = 572 patients with refractory MS spasticity), Novotna et al36 evaluated nabiximols, an oral mucosal spray of a formulated extract of Cannabis that contains THC and CBD in a 1:1 ratio. They assessed spasticity using the Numerical Spasticity Rating Scale (NRS). Results were confirmed by measuring the number of daily spasms, self-report of sleep quality, and activities of daily living. After 4 weeks of single-blind treatment, patients who responded to nabiximols (≥20% improvement in spasticity) were randomized to a placebo group or nabiximols group for 12 additional weeks. After 12 weeks, compared with those who received placebo, those in the nabiximols group experienced a statistically significant reduction in spasticity based on NRS score (P = .0002).
For a summary of evidence on MM for treating glaucoma, Crohn’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis, see Box 3.37-43
Box 3
Cannabis for treating glaucoma, Crohn’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis
Glaucoma. In a placebo-controlled study, oromucosal administration of medical marijuana (MM) reduced intraocular pressure from 28 mm Hg to 22 mm Hg, with a duration of action of 3.5 hours.37However, the American Academy of Ophthalmologists does not recommend treating glaucoma with MM because the effect is short-lasting, and MM causes significant cognitive impairment compared with other standardized treatments.38 MM also leads to decreased blood pressure, which lowers blood flow to the optic nerve, thus increasing the risk of blindness.
Crohn’s disease. A randomized controlled trial (RCT) of MM for Crohn’s disease was conducted using the Crohn’s Disease Activity Index (CDAI) to assess for remission. In this 8-week study,21 individuals with Crohn’s disease were administered smoked MM (115 mg of delta-9-tetrahydrocannabinol [THC]) or placebo.39 Eligible patients were at least 20 years old, had active Crohn’s disease (CDAI >200), and had not responded to medical treatment for the illness. Compared with those who received placebo, patients who received MM experienced a statistically significant reduction in CDAI scores (P < .05). However, at follow-up 2 weeks after the study, when MM was no longer administered, there was no difference in mean CDAI scores between the 2 groups. Five of the 11 patients in the MM group achieved clinical remission, compared with 1 of 10 in the placebo group, but this difference was not statistically significant.
Parkinson’s disease (PD). According to the American Academy of Neurology, oral Cannabis extracts are “probably ineffective” for levodopa-induced dyskinesia in patients with PD.40 Reported benefits have come mainly from self-report studies. A 2014 survey (22 patients) found a significant reduction in PD symptoms—mainly relief from drug-induced tremor and pain—when measured using the Unified Parkinson’s Disease Rating Scale (UPDRS). Patients also reported better sleep and reduced pain (measured with a visual analog scale [VAS]). An exploratory double-blind placebo trial (N = 119) found no difference in mean UPDRS and no difference in any neuroprotective measures.41 However, the experimental group had a significantly higher quality of life (QOL; P = .05). A similar double-blind crossover study that included 19 patients found no significant difference in dyskinesia, as measured with the UPDRS, in the group receiving oral Cannabis extract compared with the placebo group.42
Amyotrophic lateral sclerosis (ALS). A randomized double-blind crossover trial of 27 ALS patients found that an oral THC extract (dronabinol, 5 mg, twice daily) had no significant effects on spasticity, as measured with the VAS.43 There was also no significant difference between the experimental and placebo groups on number of spasms (also measured with a VAS), quality of sleep (measured with the Sleep Disorders Questionnaire), or QOL (measured with the Amyotrophic Lateral Sclerosis Assessment questionnaire).
Psychiatric illnesses
Dementia-related behavioral disturbances. A few clinical trials with small sample sizes have found evidence supporting the use of MM compounds for alleviating neuropsychiatric symptoms of patients with dementia. An open-label pilot study of 6 individuals with late-stage dementia who received dronabinol, 2.5 mg/d, for 2 weeks, found a significant reduction (compared with baseline) in nighttime motor activity as measured with an actometer (P < .0028).44 The secondary Neuropsychiatric Inventory (NPI) assessment found reductions in aberrant motor behavior (P = .042), agitation (P = .042), and nighttime behaviors (P = .42).
A 2014 retrospective analysis of 40 inpatients with dementia-related agitation and appetite loss who were treated with dronabinol (mean dosage: 7.03 mg/d) found reductions in all aspects of agitation, including aberrant vocalization, motor agitation, aggressiveness, and treatment resistance, as measured with the Pittsburgh Agitation Scale (P < .0001).45 The study found no significant improvements in appetite, Global Assessment of Functioning mean score, or number of times patients awoke during the night. Adverse effects included sedation and delirium.
A RCT of 50 dementia patients with clinically relevant neuropsychiatric symptoms found no significant difference in mean NPI scores between patients given placebo and those who received nabiximols, 1.5 mg, 3 times daily.46 There were no significant differences found in agitation, QOL, life activities, or caregiver-scored Caregiver Global Impression of Change scale.
In a small RCT, THC was safe and well tolerated in 10 older patients with dementia.47 A 2009 Cochrane review48 concluded that there was no evidence for the efficacy of MM in treating the neuropsychiatric symptoms related to dementia.
PTSD. Preclinical evidence shows that the endocannabinoid system is involved in regulating emotional memory. Evidence also suggests that cannabinoids may facilitate the extinction of aversive memories.49,50
In 2009, New Mexico became the first state to authorize the use of MM for patients with PTSD. In a study of patients applying for the New Mexico Medical Cannabis Program, researchers used the Clinician Administered Posttraumatic Scale (CAPS) to assess PTSD symptoms.51 A retrospective chart review of the first 80 patients evaluated found significant (P < .0001) reductions of several PTSD symptoms, including intrusive memories, distressing dreams, flashbacks, numbing and avoidance, and hyperarousal, in the group using MM vs those not using MM. There also was a significant difference in CAPS total score (P < .0001). Patients reported a 75% reduction in PTSD symptoms while using MM. This study has several limitations: It was a retrospective review, not an RCT, and patients were prescreened and knew before the study began that MM helped their PTSD symptoms.
In another retrospective study, researchers evaluated treatment with nabilone, 0.5 to 6 mg/d, in 104 incarcerated men with various major mental illnesses; most (91%) met criteria for Cannabis dependence.52 They found significant improvements in sleep and PTSD symptoms.
A double-blind RCT evaluated MM in 10 Canadian male soldiers with PTSD who experienced nightmares despite standard medication treatment. Adjunctive nabilone (maximum dose: 3 mg/d) resulted in a reduction in nightmares as measured by the CAPS recurrent distressing dream of the event item score.53
Currently, there are no adequately powered RCTs of MM in a diverse group of PTSD patients. Most studies are open-label, enriched design, and included white male veterans. No well-conducted trials have evaluated patients with noncombat-related PTSD. Most of the relevant literature consists of case reports of Cannabis use by patients with PTSD.
Anxiety disorders.Patients frequently indicate that smoking Cannabis helps relieve their anxiety, although there is no replicated evidence based on double-blind RCTs to support this. However, in rat models CBD has been shown to facilitate extinction of conditioned fear via the endocannabinoid system.54-56 The mechanism of action is not completely understood. CBD has been shown to have antagonistic action at CB1 and CB2 receptors. It may have similar effects on memory extinction and may be an adjunct to exposure therapies for anxiety disorders.
Das et al57 studied the effects of CBD (32 mg) on extinction and consolidation of memory related to contextual fear in 48 individuals. They found that CBD can enhance extinction learning, and suggested it may have potential as an adjunct to extinction-based therapies for anxiety disorders.
Caveats: Adverse effects, lack of RCTs
Cannabis use causes impairment of learning, memory, attention, and working memory. Adolescents are particularly vulnerable to the effects of Cannabis on brain development at a time when synaptic pruning and increased myelination occur. Normal brain development could be disrupted. Some studies have linked Cannabis use to abnormalities in the amygdala, hippocampus, frontal lobe, and cerebellum. From 1995 to 2014, the potency of Cannabis (THC concentration) increased from 4% to 12%.58 This has substantial implications for increased abuse among adolescents and the deleterious effects of Cannabis on the brain.
Heavy Cannabis use impairs motivation and could precipitate psychosis in vulnerable individuals. Cannabis use may be linked to the development of schizophrenia.59
There are no well-conducted RCTs on the efficacy of MM, and adequate safety data are lacking. There is also lack of consensus among qualified experts. There is soft evidence that MM may be helpful in some medical conditions, including but not limited to CINV, neuropathic pain, epilepsy, and MS-related spasticity. Currently, the benefits of using MM do not appear to outweigh the risks.
Bottom Line
Limited evidence suggests medical marijuana (MM) may be beneficial for treating a few medical conditions, including neuropathic pain and chemotherapy-induced nausea and vomiting. There is no clear and convincing evidence MM is beneficial for psychiatric disorders, and Cannabis can impair cognition and attention and may precipitate psychosis. The risk of deleterious effects are greater in adolescents.
Related Resources
- Nguyen DH, Thant TM. Caring for medical marijuana patients who request controlled prescriptions. Current Psychiatry. 2017;16(8):50-51.
- National Institute on Drug Abuse. Marijuana as medicine. https://www.drugabuse.gov/publications/drugfacts/ marijuana-medicine.
Drug Brand Names
Alizapride • Litican, Superan
Chlorpromazine • Thorazine
Domperidone • Motilium
Dronabinol • Marinol, Syndros
Haloperidol • Haldol
Metoclopramide • Reglan
Nabilone • Cesamet
Nabiximols • Sativex
Ondansetron • Zofran, Zuplenz
Prochlorperazine • Compazine
Thiethylperazine • Torecan
1. National Institute on Drug Abuse (NIDA). Marijuana Research Findings 1976. NIDA research monograph 14. https://archives.drugabuse.gov/sites/default/files/monograph14.pdf. Published July 1977. Accessed November 15, 2017.
2. O’Shaughnessy WB. On the preparations of the Indian hemp, or gunjah- cannabis indica their effects on the animal system in health, and their utility in the treatment of tetanus and other convulsive diseases. Prov Med J Retrosp Med Sci. 1843;5(123):363-369.
3. Clendinning J. Observations on the medical properties of the Cannabis Sativa of India. Med Chir Trans. 1843;26:188-210.
4. Osler W, McCrae T. The principles and practice of medicine. 9th ed. New York, NY: D. Appleton and Company; 1921.
5. The pharmacopoeia of the United States of America. 3rd ed. Philadelphia, PA: Lippincott; 1851.
6. The pharmacopoeia of the United States of America. 12th ed. Easton, PA: Mack Printing Company; 1942.
7. Philipsen N, Butler RD, Simon C, et al. Medical marijuana: a primer on ethics, evidence, and politics. Journal Nurse Pract. 2014;10(9):633-640.
8. Marihuana Tax Act of 1937, Pub L No. 75-238, 75th Cong, 50 Stat 551 (1937).
9. Controlled Substances Act, 21 USC §812.
10. Watson SJ, Benson JA, Joy JE, eds. Marijuana and medicine: assessing the science base. Washington, DC: National Academy Press; 1999.
11. California Proposition 215, the medical marijuana initiative (1996). https://ballotpedia.org/California_Proposition_215,_the_Medical_Marijuana_Initiative_(1996). Accessed November 16, 2017.
12. National Conference of State Legislatures. State medical marijuana laws. http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx. Updated September 14, 2017. Accessed November 16, 2017.
13. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163(7):1344-1364.
14. Alger BE. Getting high on the endocannabinoid system. Cerebrum. 2013:14. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3997295. Accessed December 5, 2017.
15. Galal AM, Slade D, Gul W, et al. Naturally occurring and related synthetic cannabinoids and their potential therapeutic applications. Recent Pat CNS Drug Discov. 2009;4(2):112-136.
16. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804.
17. Cesamet [package insert]. Somerset, NJ: Meda Pharmaceuticals; 2013.
18. Marinol [package insert]. Chicago, IL: AbbVie Inc.; 2017.
19. Sativex [package insert]. Mississauga, Ontario: Bayer Inc.; 2015.
20. Torrance N, Ferguson JA, Afolabi E, et al. Neuropathic pain in the community: more under-treated than refractory? Pain. 2013;154(5):690-699.
21. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
22. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473.
23. Wilsey B, Marcotte, T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9(6):506-521.
24. Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ. 2010;182(14):E694-E701.
25. Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14(2):136-148.
26. National Cancer Institute. Treatment-related nausea and vomiting (PDQ®)-health professional version. https://www.cancer.gov/about-cancer/treatment/side-effects/nausea/nausea-hp-pdq. Updated May 10, 2017. Accessed November 7, 2017.
27. Meiri E, Jhangiani H, Vrendenburgh JJ, et al. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin. 2007;23(3):533-543.
28. Tramèr MR, Carroll D, Campbell FA, et al. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001;323(7303):16-21.
29. Smith LA, Azariah F, Lavender VT, et al. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev. 2015;(11):CD009464.
30. Maa E, Figi P. The case for medical marijuana in epilepsy. Epilepsia. 2014;55(6):783-786.
31. dos Santos RG, Hallak JE, Leite JP, et al. Phytocannabinoids and epilepsy. J Clin Pharm Ther. 2015;40(2):135-143.
32. Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;(3):CD009270.
33. Yadav V, Bever C Jr, Bowen J, et al. Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis: report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2014;82(12):1083-1092.
34. Corey-Bloom J, Wolfson T, Gamst A, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ. 2012;184(10):1143-1150.
35. Zajicek J, Ball S, Wright D, et al; CUPID investigator group. Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): a randomised, placebo-controlled trial. Lancet Neurol. 2013;12(9):857-865.
36. Novotna A, Mares J, Ratcliffe S, et al; Sativex Spasticity Study Group. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex(®)), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. 2011;18(9):1122-1131.
37. Merritt JC, Crawford WJ, Alexander PC, et al. Effect of marihuana on intraocular and blood pressure in glaucoma. Ophthalmology. 1980;87(3):222-228.
38. American Academy of Ophthalmology. American Academy of Ophthalmology reiterates position that marijuana is not a proven treatment for glaucoma. https://www.aao.org/newsroom/news-releases/detail/american-academy-of-ophthalmology-reiterates-posit. Published June 27, 2014. Accessed May 29, 2017.
39. Naftali T, Bar-Lev Schleider L, Dotan I, et al. Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol. 2013;11(10):1276.e1-1280.e1.
40. Koppel BS Brust JC, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in certain neurological disorders. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556-1563.
41. Chagas MH, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol. 2014;28(11):1088-1098.
42. Carroll CB, Bain PG, Teare L, et al. Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology. 2004;63(7):1245-1250.
43. Weber M, Goldman B, Truniger S. Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatry. 2010;81(10):1135-1140.
44. Walther S, Mahlberg R, Eichmann U, et al. Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology (Berl). 2006;185(4):524-528.
45. Woodward MR, Harper DG, Stolyar A, et al. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am J Geriatr Psychiatry. 2014;22(4):415-419.
46. van den Elsen GA, Ahmed A, Verkes RJ, et al. Tetrahydrocannabinol for neuropsychiatric symptoms in dementia: a randomized controlled trial. Neurology. 2015;84(23):2338-2346.
47. Ahmed AI, van den Elsen GA, Colbers A, et al. Safety, pharmacodynamics, and pharmacokinetics of multiple oral doses of delta-9-tetrahydrocannabinol in older persons with dementia. Psychopharmacology (Berl). 2015;232(14):25872595.
48. Krishnan S, Cairns R, Howard R. Cannabinoids for the treatment of dementia. Cochrane Database Syst Rev. 2009;(2):CD007204.
49. de Bitencourt RM, Pamplona FA, Takahashi RN. A current overview of cannabinoids and glucocorticoids in facilitating extinction of aversive memories: potential extinction enhancers. Neuropharmacology. 2013;64:389-395.
50. Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther. 2009;15(1):84-88.
51. Greer GR, Grob CS, Halberstadt AL. PTSD symptom reports of patients evaluated for the New Mexico Medical Cannabis Program. J Psychoactive Drugs. 2014;46(1):73-77.
52. Cameron C, Watson D, Robinson J. Use of a synthetic cannabinoid in a correctional population for posttraumatic stress disorder-related insomnia and nightmares, chronic pain, harm reduction, and other indications: a retrospective evaluation. J Clin Psychopharmacol. 2014;34(5):559-564.
53. Jetly R, Heber A, Fraser G, et al. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51:585-588.
54. Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear, memory extinction, and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol. 2008;18(12):849-859.
55. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199-215.
56. Thomas A, Baillie GL, Phillips AM, et al. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150(5):613-623.
57. Das RK, Kamboj SK, Ramadas M, et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology (Berl). 2013;226(4):781-792.
58. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016;79(7):613-619.
59. Volkow ND, Swanson JM, Evins AE, et al. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry. 2016;73(3):292297.
There is a need for additional treatment options to improve symptoms, enhance the quality of life (QOL), and reduce suffering among patients who have chronic medical illness. Medical marijuana (MM) has the potential to help patients who have certain medical conditions in states where it is legal for prescription by a licensed medical provider.
Cannabis has a long history of medicinal use (Box 11-12). Two derivatives of the Cannabis plant—cannabinoid delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD)—are responsible for most of its effects. Some of these effects, including analgesia, decreased muscle spasticity, and reduced eye pressure, have been harnessed for their potential therapeutic effects (Box 213-19). As of November 2017, 29 states had legalized Cannabis for medical use, and several had legalized its recreational use.12
With the increasing availability of MM, psychiatrists are likely to encounter patients who are using it or who will ask them about it. This article reviews evidence related to using MM to treat patients with neuropathic pain; chemotherapyinduced nausea and vomiting (CINV); epilepsy; multiple sclerosis (MS); glaucoma; Crohn’s disease; Parkinson’s disease; amyotrophic lateral sclerosis; dementia-related behavioral disturbances; posttraumatic stress disorder (PTSD); and anxiety.
Box 1
Cannabis: A history of medicinal use
Cannabis has been cultivated since ancient times, beginning in China and India. The earliest reference of its use for healing purposes may have been in the Chinese Pharmacopeia, circa 1500 BC.1 In 1839, Dr. William Brooke O’Shaughnessy introduced Cannabis Indica, or “Indian hemp,” to the western world after a professorship in Calcutta, India.2 In the early 1840s, an English physician, Dr. John Clendinning, prescribed Cannabis for migraine headache.3 In the 19th and early 20th centuries, several prominent physicians advocated using Cannabis for migraines; Sir William Osler did so in his textbook, The principles and practice of medicine.4 It was listed in the U.S. Pharmacopeia in 1850 but removed in 1942.5,6
Until 1937, Cannabis was used in the United States for medicinal purposes, such as for treating inflamed skin, incontinence, and sexually transmitted diseases.7 In 1937, the Marihuana Tax Act, which prohibited the production, importation, possession, use, and dispersal of Cannabis, was passed.8 Cannabis became a Schedule I drug under the Controlled Substance Act of 1970.9
In 1999, based on available evidence, the Institute of Medicine (IOM) concluded Cannabis had less likelihood of dependence than benzodiazepines, opiates, cocaine, or nicotine. The IOM also concluded that the symptoms of withdrawal were mild in comparison with benzodiazepines or opiates. Finally, the IOM stated that Cannabis was not a “gateway” drug.10
In 1996, California was the first state to reimplement medicinal use of Cannabis under the Compassionate Use Act, also known as Proposition 215.11 This act allowed individuals to retain or produce Cannabis for personal consumption with a physician’s approval. Many states eventually followed California’s lead. As of November 2017, 29 states, the District of Columbia, Guam, and Puerto Rico had regulated Cannabis use for medical purposes,12 and recreational use had been approved in 7 states and the District of Columbia.
Medical illnesses
Neuropathic pain. Chronic neuropathic pain affects an estimated 7% to 8% of adults.20 Patients with neuropathic pain are often treated with anticonvulsants, antidepressants, opioids, and local anesthetics21; however, these medications may not provide substantial relief. Research has revealed that THC and CBD can improve central and peripheral neuropathic pain, as well as pain associated with rheumatoid arthritis and fibromyalgia.22
Wilsey et al23 evaluated the analgesic effects of smoked MM for neuropathic pain in a small (N = 38) double-blind, randomized controlled trial (RCT). Patients in this study had a preexisting diagnosis of complex regional pain syndrome, spinal cord injury, peripheral neuropathy, or nerve injury. To prevent any unforeseen adverse outcomes related to Cannabis use, participants were required to have previous exposure to Cannabis. Patients were excluded if they had major mental illness, substance abuse, or other major medical ailments.
Participants smoked high-dose Cannabis cigarettes (7% THC), low-dose Cannabis cigarettes (3.5% THC), or placebo cigarettes. Pain was measured on a visual analog scale (VAS) that ranged from 0 (no pain) to 100 (worst possible pain). Compared with the placebo group, significant analgesia was achieved in both Cannabis groups (P = .016). The high-dose group had greater neurocognitive impairment.
Ware et al24 conducted a crossover RCT (N = 23) to determine the efficacy of smoked MM for neuropathic pain. Participants had neuropathic pain for at least 3 months that was caused by trauma or surgery, with an average weekly pain intensity score >4 on scale of 0 to 10. Patients with pain due to cancer, nociceptive causes, unstable medical conditions, current substance abuse, history of a psychotic disorder, or suicidal ideation were excluded. Participants were assigned to a 9.4% THC group or a 0% THC group. Pain intensity was evaluated daily via telephone. Participants in the 9.4% THC group had statistically lower pain intensity compared with the 0% THC group (P = .023). Common adverse effects reported by those in the 9.4% group included headache, dry eyes, burning sensation, dizziness, numbness, and cough.
Box 2
The effects of Cannabis
Marijuana is harvested from the plant Cannabis sativa and composed of 400 lipophilic chemical compounds, including phytocannabinoids, terpenoids, and flavonoids.13 The plant contains compounds termed “cannabinoids.” Two of these derivatives in particular are responsible for most of the effects of marijuana: cannabinoid delta-9- tetrahydrocannabinol (THC) and cannabidiol (CBD). THC has a comparable structure and binding mechanism to anandamide, a naturally occurring fatty acid neurotransmitter present within the human brain.14-16 The endogenous endocannabinoid system and its receptors are found throughout the entire body (brain, organs, glands, immune cells, and connective tissues).
THC binds to cannabinoid receptors CB1 and CB2. CB1 is found predominantly in the CNS. CB2 is found predominantly outside the CNS and is associated with the immune system.14-16 The effects of THC include euphoria, relaxation, appetite stimulation, improvement of nausea and vomiting, analgesia, decreased muscle spasticity, and reduced eye pressure.14,15 CBD may have anxiolytic, antipsychotic, anticonvulsive, and analgesic effects.
The rate of absorption of THC and CBD depends both on the potency of the cannabinoid as well as the mechanism of consumption. Cannabis can be administered by multiple routes, including via smoking, oral ingestion, or IV.16 When Cannabis is smoked (the route for the most rapid delivery), THC is transported from the lungs to the bloodstream and reaches peak concentrations in 3 to 10 minutes. Oral ingestion (capsules, tinctures, sprays, and edibles) has a more flexible onset of action, usually occurring in 30 to 120 minutes, with effects lasting 5 to 6 hours. IV administration has rapid effects; the onset can occur within seconds to minutes, and effects can last 2 to 3 hours. The IV form allows 90% of THC to be distributed in plasma and can rapidly penetrate highly vascularized tissues, such as the liver, heart, fat, lungs, and muscles.
Pharmaceutical manufacturers have used cannabinoid derivatives to produce Cannabis-based medications for treating medical conditions. Nabilone, a potent agonist of the CB1 receptor, became available as a Schedule II medication in 1981 and was approved for patients with chemotherapy-induced nausea and vomiting (CINV).17 In 1985, dronabinol was introduced as an antiemetic for CINV as well as an appetite stimulant for patients with conditions associated with excessive weight loss.18 Another option, nabiximols, is an oral mucosal spray that consists of THC and CBD in a 1:1 ratio.19 Nabiximols is approved in Canada for pain relief in end-stage cancer patients and pain associated with multiple sclerosis.19
In an RCT of vaporized Cannabis, 39 patients with a diagnosis of complex regional pain syndrome, thalamic pain, spinal cord injury, peripheral neuropathy, radiculopathy, or nerve injury were assigned to a medium-dose (3.53% THC), low-dose (1.29% THC), or placebo group.25 Serious mental illness, substance abuse, and medical conditions were cause for exclusion. Participants received vaporized marijuana (average 8 to 12 puffs per visit) over 3 sessions. A 30% pain reduction was achieved by 26% of those in the placebo group, 57% of those in the low-dose group, and 61% of individuals in the high-dose group; the difference between placebo and each Cannabis group was statistically significant.
Chemotherapy-induced nausea and vomiting. Up to 80% of patients who receive chemotherapy experience CINV, which occurs from 24 hours to 7 days after receiving such therapy.26 CINV negatively influences a patient’s QOL and may impact the decision to continue with chemotherapy. Use of MM can help to diminish vomiting by binding to central CB1 receptors and averting the proemetic effects of dopamine and serotonin.27 Two synthetically derived cannabinoids, dronabinol and nabilone, are FDA-approved for treating CINV.
In a small (N = 64) parallel-group RCT, Meiri et al27 compared dronabinol with the commonly used antiemetic ondansetron and with a combination of dronabinol and ondansetron for treating CINV in adults. The primary outcome was prevention of delayed-onset CINV. Patients were eligible for this study if they had a malignancy that did not involve bone marrow, were receiving treatment with a moderately to highly emetogenic regimen, were not pregnant, and had an estimated life expectancy of at least 6 weeks after chemotherapy. The patients were randomized to 1 of 4 treatment groups: dronabinol alone, ondansetron alone, dronabinol plus ondansetron, or placebo. Overall, 47% to 58% of the active treatment groups improved, compared with 20% of the placebo group. Combination therapy did not provide any benefit beyond any single agent alone. All active treatments reduced nausea compared with placebo; there was no difference between active treatment groups. This study was limited by low enrollment.
Tramèr et al28 conducted a systematic review of 30 randomized comparisons of MM with placebo or antiemetics. The reviewed studies were completed between 1975 to 1997 and analyzed a total of 1,366 patients. Nabilone was evaluated in 16 trials; dronabinol was utilized in 13 trials; and IM levonantradol, a synthetic cannabinoid analog of dronabinol, was used in 1 trial. These agents were found to be more effective as an antiemetic compared with prochlorperazine, metoclopramide, chlorpromazine, thiethylperazine, haloperidol, domperidone, or alizapride. In addition, 38% to 90% of patients in these studies preferred MM over the traditional antiemetics.
A Cochrane review29 suggested that MM may be a viable option for treatment-resistant CINV; however, further studies are needed because current studies have methodological limitations.
Epilepsy. Maa and Figi30 reported a case of a 5-year-old girl who had Dravet syndrome, which resulted in 50 generalized tonic-clonic seizures daily; multiple anticonvulsants did not alleviate these seizures. Because of her recurring seizures, the patient had multiple cognitive and motor delays and needed a feeding tube. In addition to her existing antiepileptic drug regimen, she was started on adjunctive therapy with a sublingual Cannabis extract containing a high concentration of CBD. Her seizures decreased from 50 per day to 2 to 3 nocturnal convulsions per month. The treatment enabled her to stop using a feeding tube, resume walking and talking, and sleep soundly.
dos Santos et al31 reviewed studies of MM for treating epilepsy. One was a double-blind, placebo-controlled trial that included 15 patients ages 14 to 49 who had secondary generalized epilepsy with a temporal lobe focus. Eight patients received 200 to 300 mg/d of oral CBD for 8 to 18 weeks, and 7 received placebo. Seven patients had fewer seizures and 4 had no seizures. Only 1 patient in the placebo group demonstrated any improvement. Another study in this review included 19 children with treatment-resistant epilepsy: Dravet syndrome (n = 13), Doose syndrome (n = 4), Lennox-Gastaut syndrome (n = 1), or idiopathic epilepsy (n = 1). These patients experienced various types of seizures with a frequency ranging from 2 per week to 250 per day. Overall, 84% of children treated with CBD had fewer seizures: 11% were seizure-free, 42% had a >80% reduction in seizures, and 32% had a 25% to 60% reduction in seizures. Parents also noted additional benefits, including increased attention, improved mood, and improved sleep. CBD was well tolerated in most patients in both studies.
Despite these results, a Cochrane review32 found that no reliable conclusions can be drawn regarding the efficacy of MM for treating epilepsy.
Multiple sclerosis. According to American Academy of Neurology guidelines, physicians may provide MM as an alternative treatment for patients with MS-related spasticity.33 Multiple studies have tested MM and MM-related extracts for treating spasticity related to MS.34,35 In a placebo-controlled crossover study, Corey-Bloom et al34 reported a significant reduction in spasticity, measured using the modified Ashworth scale, in MS patients receiving Cannabis cigarettes vs placebo cigarettes (P < .0001). However, compared with the placebo group, patients who received MM had significant adverse effects, primarily cognitive impairment (P = .003).
In a multicenter RCT (N = 572 patients with refractory MS spasticity), Novotna et al36 evaluated nabiximols, an oral mucosal spray of a formulated extract of Cannabis that contains THC and CBD in a 1:1 ratio. They assessed spasticity using the Numerical Spasticity Rating Scale (NRS). Results were confirmed by measuring the number of daily spasms, self-report of sleep quality, and activities of daily living. After 4 weeks of single-blind treatment, patients who responded to nabiximols (≥20% improvement in spasticity) were randomized to a placebo group or nabiximols group for 12 additional weeks. After 12 weeks, compared with those who received placebo, those in the nabiximols group experienced a statistically significant reduction in spasticity based on NRS score (P = .0002).
For a summary of evidence on MM for treating glaucoma, Crohn’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis, see Box 3.37-43
Box 3
Cannabis for treating glaucoma, Crohn’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis
Glaucoma. In a placebo-controlled study, oromucosal administration of medical marijuana (MM) reduced intraocular pressure from 28 mm Hg to 22 mm Hg, with a duration of action of 3.5 hours.37However, the American Academy of Ophthalmologists does not recommend treating glaucoma with MM because the effect is short-lasting, and MM causes significant cognitive impairment compared with other standardized treatments.38 MM also leads to decreased blood pressure, which lowers blood flow to the optic nerve, thus increasing the risk of blindness.
Crohn’s disease. A randomized controlled trial (RCT) of MM for Crohn’s disease was conducted using the Crohn’s Disease Activity Index (CDAI) to assess for remission. In this 8-week study,21 individuals with Crohn’s disease were administered smoked MM (115 mg of delta-9-tetrahydrocannabinol [THC]) or placebo.39 Eligible patients were at least 20 years old, had active Crohn’s disease (CDAI >200), and had not responded to medical treatment for the illness. Compared with those who received placebo, patients who received MM experienced a statistically significant reduction in CDAI scores (P < .05). However, at follow-up 2 weeks after the study, when MM was no longer administered, there was no difference in mean CDAI scores between the 2 groups. Five of the 11 patients in the MM group achieved clinical remission, compared with 1 of 10 in the placebo group, but this difference was not statistically significant.
Parkinson’s disease (PD). According to the American Academy of Neurology, oral Cannabis extracts are “probably ineffective” for levodopa-induced dyskinesia in patients with PD.40 Reported benefits have come mainly from self-report studies. A 2014 survey (22 patients) found a significant reduction in PD symptoms—mainly relief from drug-induced tremor and pain—when measured using the Unified Parkinson’s Disease Rating Scale (UPDRS). Patients also reported better sleep and reduced pain (measured with a visual analog scale [VAS]). An exploratory double-blind placebo trial (N = 119) found no difference in mean UPDRS and no difference in any neuroprotective measures.41 However, the experimental group had a significantly higher quality of life (QOL; P = .05). A similar double-blind crossover study that included 19 patients found no significant difference in dyskinesia, as measured with the UPDRS, in the group receiving oral Cannabis extract compared with the placebo group.42
Amyotrophic lateral sclerosis (ALS). A randomized double-blind crossover trial of 27 ALS patients found that an oral THC extract (dronabinol, 5 mg, twice daily) had no significant effects on spasticity, as measured with the VAS.43 There was also no significant difference between the experimental and placebo groups on number of spasms (also measured with a VAS), quality of sleep (measured with the Sleep Disorders Questionnaire), or QOL (measured with the Amyotrophic Lateral Sclerosis Assessment questionnaire).
Psychiatric illnesses
Dementia-related behavioral disturbances. A few clinical trials with small sample sizes have found evidence supporting the use of MM compounds for alleviating neuropsychiatric symptoms of patients with dementia. An open-label pilot study of 6 individuals with late-stage dementia who received dronabinol, 2.5 mg/d, for 2 weeks, found a significant reduction (compared with baseline) in nighttime motor activity as measured with an actometer (P < .0028).44 The secondary Neuropsychiatric Inventory (NPI) assessment found reductions in aberrant motor behavior (P = .042), agitation (P = .042), and nighttime behaviors (P = .42).
A 2014 retrospective analysis of 40 inpatients with dementia-related agitation and appetite loss who were treated with dronabinol (mean dosage: 7.03 mg/d) found reductions in all aspects of agitation, including aberrant vocalization, motor agitation, aggressiveness, and treatment resistance, as measured with the Pittsburgh Agitation Scale (P < .0001).45 The study found no significant improvements in appetite, Global Assessment of Functioning mean score, or number of times patients awoke during the night. Adverse effects included sedation and delirium.
A RCT of 50 dementia patients with clinically relevant neuropsychiatric symptoms found no significant difference in mean NPI scores between patients given placebo and those who received nabiximols, 1.5 mg, 3 times daily.46 There were no significant differences found in agitation, QOL, life activities, or caregiver-scored Caregiver Global Impression of Change scale.
In a small RCT, THC was safe and well tolerated in 10 older patients with dementia.47 A 2009 Cochrane review48 concluded that there was no evidence for the efficacy of MM in treating the neuropsychiatric symptoms related to dementia.
PTSD. Preclinical evidence shows that the endocannabinoid system is involved in regulating emotional memory. Evidence also suggests that cannabinoids may facilitate the extinction of aversive memories.49,50
In 2009, New Mexico became the first state to authorize the use of MM for patients with PTSD. In a study of patients applying for the New Mexico Medical Cannabis Program, researchers used the Clinician Administered Posttraumatic Scale (CAPS) to assess PTSD symptoms.51 A retrospective chart review of the first 80 patients evaluated found significant (P < .0001) reductions of several PTSD symptoms, including intrusive memories, distressing dreams, flashbacks, numbing and avoidance, and hyperarousal, in the group using MM vs those not using MM. There also was a significant difference in CAPS total score (P < .0001). Patients reported a 75% reduction in PTSD symptoms while using MM. This study has several limitations: It was a retrospective review, not an RCT, and patients were prescreened and knew before the study began that MM helped their PTSD symptoms.
In another retrospective study, researchers evaluated treatment with nabilone, 0.5 to 6 mg/d, in 104 incarcerated men with various major mental illnesses; most (91%) met criteria for Cannabis dependence.52 They found significant improvements in sleep and PTSD symptoms.
A double-blind RCT evaluated MM in 10 Canadian male soldiers with PTSD who experienced nightmares despite standard medication treatment. Adjunctive nabilone (maximum dose: 3 mg/d) resulted in a reduction in nightmares as measured by the CAPS recurrent distressing dream of the event item score.53
Currently, there are no adequately powered RCTs of MM in a diverse group of PTSD patients. Most studies are open-label, enriched design, and included white male veterans. No well-conducted trials have evaluated patients with noncombat-related PTSD. Most of the relevant literature consists of case reports of Cannabis use by patients with PTSD.
Anxiety disorders.Patients frequently indicate that smoking Cannabis helps relieve their anxiety, although there is no replicated evidence based on double-blind RCTs to support this. However, in rat models CBD has been shown to facilitate extinction of conditioned fear via the endocannabinoid system.54-56 The mechanism of action is not completely understood. CBD has been shown to have antagonistic action at CB1 and CB2 receptors. It may have similar effects on memory extinction and may be an adjunct to exposure therapies for anxiety disorders.
Das et al57 studied the effects of CBD (32 mg) on extinction and consolidation of memory related to contextual fear in 48 individuals. They found that CBD can enhance extinction learning, and suggested it may have potential as an adjunct to extinction-based therapies for anxiety disorders.
Caveats: Adverse effects, lack of RCTs
Cannabis use causes impairment of learning, memory, attention, and working memory. Adolescents are particularly vulnerable to the effects of Cannabis on brain development at a time when synaptic pruning and increased myelination occur. Normal brain development could be disrupted. Some studies have linked Cannabis use to abnormalities in the amygdala, hippocampus, frontal lobe, and cerebellum. From 1995 to 2014, the potency of Cannabis (THC concentration) increased from 4% to 12%.58 This has substantial implications for increased abuse among adolescents and the deleterious effects of Cannabis on the brain.
Heavy Cannabis use impairs motivation and could precipitate psychosis in vulnerable individuals. Cannabis use may be linked to the development of schizophrenia.59
There are no well-conducted RCTs on the efficacy of MM, and adequate safety data are lacking. There is also lack of consensus among qualified experts. There is soft evidence that MM may be helpful in some medical conditions, including but not limited to CINV, neuropathic pain, epilepsy, and MS-related spasticity. Currently, the benefits of using MM do not appear to outweigh the risks.
Bottom Line
Limited evidence suggests medical marijuana (MM) may be beneficial for treating a few medical conditions, including neuropathic pain and chemotherapy-induced nausea and vomiting. There is no clear and convincing evidence MM is beneficial for psychiatric disorders, and Cannabis can impair cognition and attention and may precipitate psychosis. The risk of deleterious effects are greater in adolescents.
Related Resources
- Nguyen DH, Thant TM. Caring for medical marijuana patients who request controlled prescriptions. Current Psychiatry. 2017;16(8):50-51.
- National Institute on Drug Abuse. Marijuana as medicine. https://www.drugabuse.gov/publications/drugfacts/ marijuana-medicine.
Drug Brand Names
Alizapride • Litican, Superan
Chlorpromazine • Thorazine
Domperidone • Motilium
Dronabinol • Marinol, Syndros
Haloperidol • Haldol
Metoclopramide • Reglan
Nabilone • Cesamet
Nabiximols • Sativex
Ondansetron • Zofran, Zuplenz
Prochlorperazine • Compazine
Thiethylperazine • Torecan
There is a need for additional treatment options to improve symptoms, enhance the quality of life (QOL), and reduce suffering among patients who have chronic medical illness. Medical marijuana (MM) has the potential to help patients who have certain medical conditions in states where it is legal for prescription by a licensed medical provider.
Cannabis has a long history of medicinal use (Box 11-12). Two derivatives of the Cannabis plant—cannabinoid delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD)—are responsible for most of its effects. Some of these effects, including analgesia, decreased muscle spasticity, and reduced eye pressure, have been harnessed for their potential therapeutic effects (Box 213-19). As of November 2017, 29 states had legalized Cannabis for medical use, and several had legalized its recreational use.12
With the increasing availability of MM, psychiatrists are likely to encounter patients who are using it or who will ask them about it. This article reviews evidence related to using MM to treat patients with neuropathic pain; chemotherapyinduced nausea and vomiting (CINV); epilepsy; multiple sclerosis (MS); glaucoma; Crohn’s disease; Parkinson’s disease; amyotrophic lateral sclerosis; dementia-related behavioral disturbances; posttraumatic stress disorder (PTSD); and anxiety.
Box 1
Cannabis: A history of medicinal use
Cannabis has been cultivated since ancient times, beginning in China and India. The earliest reference of its use for healing purposes may have been in the Chinese Pharmacopeia, circa 1500 BC.1 In 1839, Dr. William Brooke O’Shaughnessy introduced Cannabis Indica, or “Indian hemp,” to the western world after a professorship in Calcutta, India.2 In the early 1840s, an English physician, Dr. John Clendinning, prescribed Cannabis for migraine headache.3 In the 19th and early 20th centuries, several prominent physicians advocated using Cannabis for migraines; Sir William Osler did so in his textbook, The principles and practice of medicine.4 It was listed in the U.S. Pharmacopeia in 1850 but removed in 1942.5,6
Until 1937, Cannabis was used in the United States for medicinal purposes, such as for treating inflamed skin, incontinence, and sexually transmitted diseases.7 In 1937, the Marihuana Tax Act, which prohibited the production, importation, possession, use, and dispersal of Cannabis, was passed.8 Cannabis became a Schedule I drug under the Controlled Substance Act of 1970.9
In 1999, based on available evidence, the Institute of Medicine (IOM) concluded Cannabis had less likelihood of dependence than benzodiazepines, opiates, cocaine, or nicotine. The IOM also concluded that the symptoms of withdrawal were mild in comparison with benzodiazepines or opiates. Finally, the IOM stated that Cannabis was not a “gateway” drug.10
In 1996, California was the first state to reimplement medicinal use of Cannabis under the Compassionate Use Act, also known as Proposition 215.11 This act allowed individuals to retain or produce Cannabis for personal consumption with a physician’s approval. Many states eventually followed California’s lead. As of November 2017, 29 states, the District of Columbia, Guam, and Puerto Rico had regulated Cannabis use for medical purposes,12 and recreational use had been approved in 7 states and the District of Columbia.
Medical illnesses
Neuropathic pain. Chronic neuropathic pain affects an estimated 7% to 8% of adults.20 Patients with neuropathic pain are often treated with anticonvulsants, antidepressants, opioids, and local anesthetics21; however, these medications may not provide substantial relief. Research has revealed that THC and CBD can improve central and peripheral neuropathic pain, as well as pain associated with rheumatoid arthritis and fibromyalgia.22
Wilsey et al23 evaluated the analgesic effects of smoked MM for neuropathic pain in a small (N = 38) double-blind, randomized controlled trial (RCT). Patients in this study had a preexisting diagnosis of complex regional pain syndrome, spinal cord injury, peripheral neuropathy, or nerve injury. To prevent any unforeseen adverse outcomes related to Cannabis use, participants were required to have previous exposure to Cannabis. Patients were excluded if they had major mental illness, substance abuse, or other major medical ailments.
Participants smoked high-dose Cannabis cigarettes (7% THC), low-dose Cannabis cigarettes (3.5% THC), or placebo cigarettes. Pain was measured on a visual analog scale (VAS) that ranged from 0 (no pain) to 100 (worst possible pain). Compared with the placebo group, significant analgesia was achieved in both Cannabis groups (P = .016). The high-dose group had greater neurocognitive impairment.
Ware et al24 conducted a crossover RCT (N = 23) to determine the efficacy of smoked MM for neuropathic pain. Participants had neuropathic pain for at least 3 months that was caused by trauma or surgery, with an average weekly pain intensity score >4 on scale of 0 to 10. Patients with pain due to cancer, nociceptive causes, unstable medical conditions, current substance abuse, history of a psychotic disorder, or suicidal ideation were excluded. Participants were assigned to a 9.4% THC group or a 0% THC group. Pain intensity was evaluated daily via telephone. Participants in the 9.4% THC group had statistically lower pain intensity compared with the 0% THC group (P = .023). Common adverse effects reported by those in the 9.4% group included headache, dry eyes, burning sensation, dizziness, numbness, and cough.
Box 2
The effects of Cannabis
Marijuana is harvested from the plant Cannabis sativa and composed of 400 lipophilic chemical compounds, including phytocannabinoids, terpenoids, and flavonoids.13 The plant contains compounds termed “cannabinoids.” Two of these derivatives in particular are responsible for most of the effects of marijuana: cannabinoid delta-9- tetrahydrocannabinol (THC) and cannabidiol (CBD). THC has a comparable structure and binding mechanism to anandamide, a naturally occurring fatty acid neurotransmitter present within the human brain.14-16 The endogenous endocannabinoid system and its receptors are found throughout the entire body (brain, organs, glands, immune cells, and connective tissues).
THC binds to cannabinoid receptors CB1 and CB2. CB1 is found predominantly in the CNS. CB2 is found predominantly outside the CNS and is associated with the immune system.14-16 The effects of THC include euphoria, relaxation, appetite stimulation, improvement of nausea and vomiting, analgesia, decreased muscle spasticity, and reduced eye pressure.14,15 CBD may have anxiolytic, antipsychotic, anticonvulsive, and analgesic effects.
The rate of absorption of THC and CBD depends both on the potency of the cannabinoid as well as the mechanism of consumption. Cannabis can be administered by multiple routes, including via smoking, oral ingestion, or IV.16 When Cannabis is smoked (the route for the most rapid delivery), THC is transported from the lungs to the bloodstream and reaches peak concentrations in 3 to 10 minutes. Oral ingestion (capsules, tinctures, sprays, and edibles) has a more flexible onset of action, usually occurring in 30 to 120 minutes, with effects lasting 5 to 6 hours. IV administration has rapid effects; the onset can occur within seconds to minutes, and effects can last 2 to 3 hours. The IV form allows 90% of THC to be distributed in plasma and can rapidly penetrate highly vascularized tissues, such as the liver, heart, fat, lungs, and muscles.
Pharmaceutical manufacturers have used cannabinoid derivatives to produce Cannabis-based medications for treating medical conditions. Nabilone, a potent agonist of the CB1 receptor, became available as a Schedule II medication in 1981 and was approved for patients with chemotherapy-induced nausea and vomiting (CINV).17 In 1985, dronabinol was introduced as an antiemetic for CINV as well as an appetite stimulant for patients with conditions associated with excessive weight loss.18 Another option, nabiximols, is an oral mucosal spray that consists of THC and CBD in a 1:1 ratio.19 Nabiximols is approved in Canada for pain relief in end-stage cancer patients and pain associated with multiple sclerosis.19
In an RCT of vaporized Cannabis, 39 patients with a diagnosis of complex regional pain syndrome, thalamic pain, spinal cord injury, peripheral neuropathy, radiculopathy, or nerve injury were assigned to a medium-dose (3.53% THC), low-dose (1.29% THC), or placebo group.25 Serious mental illness, substance abuse, and medical conditions were cause for exclusion. Participants received vaporized marijuana (average 8 to 12 puffs per visit) over 3 sessions. A 30% pain reduction was achieved by 26% of those in the placebo group, 57% of those in the low-dose group, and 61% of individuals in the high-dose group; the difference between placebo and each Cannabis group was statistically significant.
Chemotherapy-induced nausea and vomiting. Up to 80% of patients who receive chemotherapy experience CINV, which occurs from 24 hours to 7 days after receiving such therapy.26 CINV negatively influences a patient’s QOL and may impact the decision to continue with chemotherapy. Use of MM can help to diminish vomiting by binding to central CB1 receptors and averting the proemetic effects of dopamine and serotonin.27 Two synthetically derived cannabinoids, dronabinol and nabilone, are FDA-approved for treating CINV.
In a small (N = 64) parallel-group RCT, Meiri et al27 compared dronabinol with the commonly used antiemetic ondansetron and with a combination of dronabinol and ondansetron for treating CINV in adults. The primary outcome was prevention of delayed-onset CINV. Patients were eligible for this study if they had a malignancy that did not involve bone marrow, were receiving treatment with a moderately to highly emetogenic regimen, were not pregnant, and had an estimated life expectancy of at least 6 weeks after chemotherapy. The patients were randomized to 1 of 4 treatment groups: dronabinol alone, ondansetron alone, dronabinol plus ondansetron, or placebo. Overall, 47% to 58% of the active treatment groups improved, compared with 20% of the placebo group. Combination therapy did not provide any benefit beyond any single agent alone. All active treatments reduced nausea compared with placebo; there was no difference between active treatment groups. This study was limited by low enrollment.
Tramèr et al28 conducted a systematic review of 30 randomized comparisons of MM with placebo or antiemetics. The reviewed studies were completed between 1975 to 1997 and analyzed a total of 1,366 patients. Nabilone was evaluated in 16 trials; dronabinol was utilized in 13 trials; and IM levonantradol, a synthetic cannabinoid analog of dronabinol, was used in 1 trial. These agents were found to be more effective as an antiemetic compared with prochlorperazine, metoclopramide, chlorpromazine, thiethylperazine, haloperidol, domperidone, or alizapride. In addition, 38% to 90% of patients in these studies preferred MM over the traditional antiemetics.
A Cochrane review29 suggested that MM may be a viable option for treatment-resistant CINV; however, further studies are needed because current studies have methodological limitations.
Epilepsy. Maa and Figi30 reported a case of a 5-year-old girl who had Dravet syndrome, which resulted in 50 generalized tonic-clonic seizures daily; multiple anticonvulsants did not alleviate these seizures. Because of her recurring seizures, the patient had multiple cognitive and motor delays and needed a feeding tube. In addition to her existing antiepileptic drug regimen, she was started on adjunctive therapy with a sublingual Cannabis extract containing a high concentration of CBD. Her seizures decreased from 50 per day to 2 to 3 nocturnal convulsions per month. The treatment enabled her to stop using a feeding tube, resume walking and talking, and sleep soundly.
dos Santos et al31 reviewed studies of MM for treating epilepsy. One was a double-blind, placebo-controlled trial that included 15 patients ages 14 to 49 who had secondary generalized epilepsy with a temporal lobe focus. Eight patients received 200 to 300 mg/d of oral CBD for 8 to 18 weeks, and 7 received placebo. Seven patients had fewer seizures and 4 had no seizures. Only 1 patient in the placebo group demonstrated any improvement. Another study in this review included 19 children with treatment-resistant epilepsy: Dravet syndrome (n = 13), Doose syndrome (n = 4), Lennox-Gastaut syndrome (n = 1), or idiopathic epilepsy (n = 1). These patients experienced various types of seizures with a frequency ranging from 2 per week to 250 per day. Overall, 84% of children treated with CBD had fewer seizures: 11% were seizure-free, 42% had a >80% reduction in seizures, and 32% had a 25% to 60% reduction in seizures. Parents also noted additional benefits, including increased attention, improved mood, and improved sleep. CBD was well tolerated in most patients in both studies.
Despite these results, a Cochrane review32 found that no reliable conclusions can be drawn regarding the efficacy of MM for treating epilepsy.
Multiple sclerosis. According to American Academy of Neurology guidelines, physicians may provide MM as an alternative treatment for patients with MS-related spasticity.33 Multiple studies have tested MM and MM-related extracts for treating spasticity related to MS.34,35 In a placebo-controlled crossover study, Corey-Bloom et al34 reported a significant reduction in spasticity, measured using the modified Ashworth scale, in MS patients receiving Cannabis cigarettes vs placebo cigarettes (P < .0001). However, compared with the placebo group, patients who received MM had significant adverse effects, primarily cognitive impairment (P = .003).
In a multicenter RCT (N = 572 patients with refractory MS spasticity), Novotna et al36 evaluated nabiximols, an oral mucosal spray of a formulated extract of Cannabis that contains THC and CBD in a 1:1 ratio. They assessed spasticity using the Numerical Spasticity Rating Scale (NRS). Results were confirmed by measuring the number of daily spasms, self-report of sleep quality, and activities of daily living. After 4 weeks of single-blind treatment, patients who responded to nabiximols (≥20% improvement in spasticity) were randomized to a placebo group or nabiximols group for 12 additional weeks. After 12 weeks, compared with those who received placebo, those in the nabiximols group experienced a statistically significant reduction in spasticity based on NRS score (P = .0002).
For a summary of evidence on MM for treating glaucoma, Crohn’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis, see Box 3.37-43
Box 3
Cannabis for treating glaucoma, Crohn’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis
Glaucoma. In a placebo-controlled study, oromucosal administration of medical marijuana (MM) reduced intraocular pressure from 28 mm Hg to 22 mm Hg, with a duration of action of 3.5 hours.37However, the American Academy of Ophthalmologists does not recommend treating glaucoma with MM because the effect is short-lasting, and MM causes significant cognitive impairment compared with other standardized treatments.38 MM also leads to decreased blood pressure, which lowers blood flow to the optic nerve, thus increasing the risk of blindness.
Crohn’s disease. A randomized controlled trial (RCT) of MM for Crohn’s disease was conducted using the Crohn’s Disease Activity Index (CDAI) to assess for remission. In this 8-week study,21 individuals with Crohn’s disease were administered smoked MM (115 mg of delta-9-tetrahydrocannabinol [THC]) or placebo.39 Eligible patients were at least 20 years old, had active Crohn’s disease (CDAI >200), and had not responded to medical treatment for the illness. Compared with those who received placebo, patients who received MM experienced a statistically significant reduction in CDAI scores (P < .05). However, at follow-up 2 weeks after the study, when MM was no longer administered, there was no difference in mean CDAI scores between the 2 groups. Five of the 11 patients in the MM group achieved clinical remission, compared with 1 of 10 in the placebo group, but this difference was not statistically significant.
Parkinson’s disease (PD). According to the American Academy of Neurology, oral Cannabis extracts are “probably ineffective” for levodopa-induced dyskinesia in patients with PD.40 Reported benefits have come mainly from self-report studies. A 2014 survey (22 patients) found a significant reduction in PD symptoms—mainly relief from drug-induced tremor and pain—when measured using the Unified Parkinson’s Disease Rating Scale (UPDRS). Patients also reported better sleep and reduced pain (measured with a visual analog scale [VAS]). An exploratory double-blind placebo trial (N = 119) found no difference in mean UPDRS and no difference in any neuroprotective measures.41 However, the experimental group had a significantly higher quality of life (QOL; P = .05). A similar double-blind crossover study that included 19 patients found no significant difference in dyskinesia, as measured with the UPDRS, in the group receiving oral Cannabis extract compared with the placebo group.42
Amyotrophic lateral sclerosis (ALS). A randomized double-blind crossover trial of 27 ALS patients found that an oral THC extract (dronabinol, 5 mg, twice daily) had no significant effects on spasticity, as measured with the VAS.43 There was also no significant difference between the experimental and placebo groups on number of spasms (also measured with a VAS), quality of sleep (measured with the Sleep Disorders Questionnaire), or QOL (measured with the Amyotrophic Lateral Sclerosis Assessment questionnaire).
Psychiatric illnesses
Dementia-related behavioral disturbances. A few clinical trials with small sample sizes have found evidence supporting the use of MM compounds for alleviating neuropsychiatric symptoms of patients with dementia. An open-label pilot study of 6 individuals with late-stage dementia who received dronabinol, 2.5 mg/d, for 2 weeks, found a significant reduction (compared with baseline) in nighttime motor activity as measured with an actometer (P < .0028).44 The secondary Neuropsychiatric Inventory (NPI) assessment found reductions in aberrant motor behavior (P = .042), agitation (P = .042), and nighttime behaviors (P = .42).
A 2014 retrospective analysis of 40 inpatients with dementia-related agitation and appetite loss who were treated with dronabinol (mean dosage: 7.03 mg/d) found reductions in all aspects of agitation, including aberrant vocalization, motor agitation, aggressiveness, and treatment resistance, as measured with the Pittsburgh Agitation Scale (P < .0001).45 The study found no significant improvements in appetite, Global Assessment of Functioning mean score, or number of times patients awoke during the night. Adverse effects included sedation and delirium.
A RCT of 50 dementia patients with clinically relevant neuropsychiatric symptoms found no significant difference in mean NPI scores between patients given placebo and those who received nabiximols, 1.5 mg, 3 times daily.46 There were no significant differences found in agitation, QOL, life activities, or caregiver-scored Caregiver Global Impression of Change scale.
In a small RCT, THC was safe and well tolerated in 10 older patients with dementia.47 A 2009 Cochrane review48 concluded that there was no evidence for the efficacy of MM in treating the neuropsychiatric symptoms related to dementia.
PTSD. Preclinical evidence shows that the endocannabinoid system is involved in regulating emotional memory. Evidence also suggests that cannabinoids may facilitate the extinction of aversive memories.49,50
In 2009, New Mexico became the first state to authorize the use of MM for patients with PTSD. In a study of patients applying for the New Mexico Medical Cannabis Program, researchers used the Clinician Administered Posttraumatic Scale (CAPS) to assess PTSD symptoms.51 A retrospective chart review of the first 80 patients evaluated found significant (P < .0001) reductions of several PTSD symptoms, including intrusive memories, distressing dreams, flashbacks, numbing and avoidance, and hyperarousal, in the group using MM vs those not using MM. There also was a significant difference in CAPS total score (P < .0001). Patients reported a 75% reduction in PTSD symptoms while using MM. This study has several limitations: It was a retrospective review, not an RCT, and patients were prescreened and knew before the study began that MM helped their PTSD symptoms.
In another retrospective study, researchers evaluated treatment with nabilone, 0.5 to 6 mg/d, in 104 incarcerated men with various major mental illnesses; most (91%) met criteria for Cannabis dependence.52 They found significant improvements in sleep and PTSD symptoms.
A double-blind RCT evaluated MM in 10 Canadian male soldiers with PTSD who experienced nightmares despite standard medication treatment. Adjunctive nabilone (maximum dose: 3 mg/d) resulted in a reduction in nightmares as measured by the CAPS recurrent distressing dream of the event item score.53
Currently, there are no adequately powered RCTs of MM in a diverse group of PTSD patients. Most studies are open-label, enriched design, and included white male veterans. No well-conducted trials have evaluated patients with noncombat-related PTSD. Most of the relevant literature consists of case reports of Cannabis use by patients with PTSD.
Anxiety disorders.Patients frequently indicate that smoking Cannabis helps relieve their anxiety, although there is no replicated evidence based on double-blind RCTs to support this. However, in rat models CBD has been shown to facilitate extinction of conditioned fear via the endocannabinoid system.54-56 The mechanism of action is not completely understood. CBD has been shown to have antagonistic action at CB1 and CB2 receptors. It may have similar effects on memory extinction and may be an adjunct to exposure therapies for anxiety disorders.
Das et al57 studied the effects of CBD (32 mg) on extinction and consolidation of memory related to contextual fear in 48 individuals. They found that CBD can enhance extinction learning, and suggested it may have potential as an adjunct to extinction-based therapies for anxiety disorders.
Caveats: Adverse effects, lack of RCTs
Cannabis use causes impairment of learning, memory, attention, and working memory. Adolescents are particularly vulnerable to the effects of Cannabis on brain development at a time when synaptic pruning and increased myelination occur. Normal brain development could be disrupted. Some studies have linked Cannabis use to abnormalities in the amygdala, hippocampus, frontal lobe, and cerebellum. From 1995 to 2014, the potency of Cannabis (THC concentration) increased from 4% to 12%.58 This has substantial implications for increased abuse among adolescents and the deleterious effects of Cannabis on the brain.
Heavy Cannabis use impairs motivation and could precipitate psychosis in vulnerable individuals. Cannabis use may be linked to the development of schizophrenia.59
There are no well-conducted RCTs on the efficacy of MM, and adequate safety data are lacking. There is also lack of consensus among qualified experts. There is soft evidence that MM may be helpful in some medical conditions, including but not limited to CINV, neuropathic pain, epilepsy, and MS-related spasticity. Currently, the benefits of using MM do not appear to outweigh the risks.
Bottom Line
Limited evidence suggests medical marijuana (MM) may be beneficial for treating a few medical conditions, including neuropathic pain and chemotherapy-induced nausea and vomiting. There is no clear and convincing evidence MM is beneficial for psychiatric disorders, and Cannabis can impair cognition and attention and may precipitate psychosis. The risk of deleterious effects are greater in adolescents.
Related Resources
- Nguyen DH, Thant TM. Caring for medical marijuana patients who request controlled prescriptions. Current Psychiatry. 2017;16(8):50-51.
- National Institute on Drug Abuse. Marijuana as medicine. https://www.drugabuse.gov/publications/drugfacts/ marijuana-medicine.
Drug Brand Names
Alizapride • Litican, Superan
Chlorpromazine • Thorazine
Domperidone • Motilium
Dronabinol • Marinol, Syndros
Haloperidol • Haldol
Metoclopramide • Reglan
Nabilone • Cesamet
Nabiximols • Sativex
Ondansetron • Zofran, Zuplenz
Prochlorperazine • Compazine
Thiethylperazine • Torecan
1. National Institute on Drug Abuse (NIDA). Marijuana Research Findings 1976. NIDA research monograph 14. https://archives.drugabuse.gov/sites/default/files/monograph14.pdf. Published July 1977. Accessed November 15, 2017.
2. O’Shaughnessy WB. On the preparations of the Indian hemp, or gunjah- cannabis indica their effects on the animal system in health, and their utility in the treatment of tetanus and other convulsive diseases. Prov Med J Retrosp Med Sci. 1843;5(123):363-369.
3. Clendinning J. Observations on the medical properties of the Cannabis Sativa of India. Med Chir Trans. 1843;26:188-210.
4. Osler W, McCrae T. The principles and practice of medicine. 9th ed. New York, NY: D. Appleton and Company; 1921.
5. The pharmacopoeia of the United States of America. 3rd ed. Philadelphia, PA: Lippincott; 1851.
6. The pharmacopoeia of the United States of America. 12th ed. Easton, PA: Mack Printing Company; 1942.
7. Philipsen N, Butler RD, Simon C, et al. Medical marijuana: a primer on ethics, evidence, and politics. Journal Nurse Pract. 2014;10(9):633-640.
8. Marihuana Tax Act of 1937, Pub L No. 75-238, 75th Cong, 50 Stat 551 (1937).
9. Controlled Substances Act, 21 USC §812.
10. Watson SJ, Benson JA, Joy JE, eds. Marijuana and medicine: assessing the science base. Washington, DC: National Academy Press; 1999.
11. California Proposition 215, the medical marijuana initiative (1996). https://ballotpedia.org/California_Proposition_215,_the_Medical_Marijuana_Initiative_(1996). Accessed November 16, 2017.
12. National Conference of State Legislatures. State medical marijuana laws. http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx. Updated September 14, 2017. Accessed November 16, 2017.
13. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163(7):1344-1364.
14. Alger BE. Getting high on the endocannabinoid system. Cerebrum. 2013:14. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3997295. Accessed December 5, 2017.
15. Galal AM, Slade D, Gul W, et al. Naturally occurring and related synthetic cannabinoids and their potential therapeutic applications. Recent Pat CNS Drug Discov. 2009;4(2):112-136.
16. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804.
17. Cesamet [package insert]. Somerset, NJ: Meda Pharmaceuticals; 2013.
18. Marinol [package insert]. Chicago, IL: AbbVie Inc.; 2017.
19. Sativex [package insert]. Mississauga, Ontario: Bayer Inc.; 2015.
20. Torrance N, Ferguson JA, Afolabi E, et al. Neuropathic pain in the community: more under-treated than refractory? Pain. 2013;154(5):690-699.
21. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
22. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473.
23. Wilsey B, Marcotte, T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9(6):506-521.
24. Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ. 2010;182(14):E694-E701.
25. Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14(2):136-148.
26. National Cancer Institute. Treatment-related nausea and vomiting (PDQ®)-health professional version. https://www.cancer.gov/about-cancer/treatment/side-effects/nausea/nausea-hp-pdq. Updated May 10, 2017. Accessed November 7, 2017.
27. Meiri E, Jhangiani H, Vrendenburgh JJ, et al. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin. 2007;23(3):533-543.
28. Tramèr MR, Carroll D, Campbell FA, et al. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001;323(7303):16-21.
29. Smith LA, Azariah F, Lavender VT, et al. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev. 2015;(11):CD009464.
30. Maa E, Figi P. The case for medical marijuana in epilepsy. Epilepsia. 2014;55(6):783-786.
31. dos Santos RG, Hallak JE, Leite JP, et al. Phytocannabinoids and epilepsy. J Clin Pharm Ther. 2015;40(2):135-143.
32. Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;(3):CD009270.
33. Yadav V, Bever C Jr, Bowen J, et al. Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis: report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2014;82(12):1083-1092.
34. Corey-Bloom J, Wolfson T, Gamst A, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ. 2012;184(10):1143-1150.
35. Zajicek J, Ball S, Wright D, et al; CUPID investigator group. Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): a randomised, placebo-controlled trial. Lancet Neurol. 2013;12(9):857-865.
36. Novotna A, Mares J, Ratcliffe S, et al; Sativex Spasticity Study Group. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex(®)), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. 2011;18(9):1122-1131.
37. Merritt JC, Crawford WJ, Alexander PC, et al. Effect of marihuana on intraocular and blood pressure in glaucoma. Ophthalmology. 1980;87(3):222-228.
38. American Academy of Ophthalmology. American Academy of Ophthalmology reiterates position that marijuana is not a proven treatment for glaucoma. https://www.aao.org/newsroom/news-releases/detail/american-academy-of-ophthalmology-reiterates-posit. Published June 27, 2014. Accessed May 29, 2017.
39. Naftali T, Bar-Lev Schleider L, Dotan I, et al. Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol. 2013;11(10):1276.e1-1280.e1.
40. Koppel BS Brust JC, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in certain neurological disorders. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556-1563.
41. Chagas MH, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol. 2014;28(11):1088-1098.
42. Carroll CB, Bain PG, Teare L, et al. Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology. 2004;63(7):1245-1250.
43. Weber M, Goldman B, Truniger S. Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatry. 2010;81(10):1135-1140.
44. Walther S, Mahlberg R, Eichmann U, et al. Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology (Berl). 2006;185(4):524-528.
45. Woodward MR, Harper DG, Stolyar A, et al. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am J Geriatr Psychiatry. 2014;22(4):415-419.
46. van den Elsen GA, Ahmed A, Verkes RJ, et al. Tetrahydrocannabinol for neuropsychiatric symptoms in dementia: a randomized controlled trial. Neurology. 2015;84(23):2338-2346.
47. Ahmed AI, van den Elsen GA, Colbers A, et al. Safety, pharmacodynamics, and pharmacokinetics of multiple oral doses of delta-9-tetrahydrocannabinol in older persons with dementia. Psychopharmacology (Berl). 2015;232(14):25872595.
48. Krishnan S, Cairns R, Howard R. Cannabinoids for the treatment of dementia. Cochrane Database Syst Rev. 2009;(2):CD007204.
49. de Bitencourt RM, Pamplona FA, Takahashi RN. A current overview of cannabinoids and glucocorticoids in facilitating extinction of aversive memories: potential extinction enhancers. Neuropharmacology. 2013;64:389-395.
50. Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther. 2009;15(1):84-88.
51. Greer GR, Grob CS, Halberstadt AL. PTSD symptom reports of patients evaluated for the New Mexico Medical Cannabis Program. J Psychoactive Drugs. 2014;46(1):73-77.
52. Cameron C, Watson D, Robinson J. Use of a synthetic cannabinoid in a correctional population for posttraumatic stress disorder-related insomnia and nightmares, chronic pain, harm reduction, and other indications: a retrospective evaluation. J Clin Psychopharmacol. 2014;34(5):559-564.
53. Jetly R, Heber A, Fraser G, et al. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51:585-588.
54. Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear, memory extinction, and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol. 2008;18(12):849-859.
55. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199-215.
56. Thomas A, Baillie GL, Phillips AM, et al. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150(5):613-623.
57. Das RK, Kamboj SK, Ramadas M, et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology (Berl). 2013;226(4):781-792.
58. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016;79(7):613-619.
59. Volkow ND, Swanson JM, Evins AE, et al. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry. 2016;73(3):292297.
1. National Institute on Drug Abuse (NIDA). Marijuana Research Findings 1976. NIDA research monograph 14. https://archives.drugabuse.gov/sites/default/files/monograph14.pdf. Published July 1977. Accessed November 15, 2017.
2. O’Shaughnessy WB. On the preparations of the Indian hemp, or gunjah- cannabis indica their effects on the animal system in health, and their utility in the treatment of tetanus and other convulsive diseases. Prov Med J Retrosp Med Sci. 1843;5(123):363-369.
3. Clendinning J. Observations on the medical properties of the Cannabis Sativa of India. Med Chir Trans. 1843;26:188-210.
4. Osler W, McCrae T. The principles and practice of medicine. 9th ed. New York, NY: D. Appleton and Company; 1921.
5. The pharmacopoeia of the United States of America. 3rd ed. Philadelphia, PA: Lippincott; 1851.
6. The pharmacopoeia of the United States of America. 12th ed. Easton, PA: Mack Printing Company; 1942.
7. Philipsen N, Butler RD, Simon C, et al. Medical marijuana: a primer on ethics, evidence, and politics. Journal Nurse Pract. 2014;10(9):633-640.
8. Marihuana Tax Act of 1937, Pub L No. 75-238, 75th Cong, 50 Stat 551 (1937).
9. Controlled Substances Act, 21 USC §812.
10. Watson SJ, Benson JA, Joy JE, eds. Marijuana and medicine: assessing the science base. Washington, DC: National Academy Press; 1999.
11. California Proposition 215, the medical marijuana initiative (1996). https://ballotpedia.org/California_Proposition_215,_the_Medical_Marijuana_Initiative_(1996). Accessed November 16, 2017.
12. National Conference of State Legislatures. State medical marijuana laws. http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx. Updated September 14, 2017. Accessed November 16, 2017.
13. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163(7):1344-1364.
14. Alger BE. Getting high on the endocannabinoid system. Cerebrum. 2013:14. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3997295. Accessed December 5, 2017.
15. Galal AM, Slade D, Gul W, et al. Naturally occurring and related synthetic cannabinoids and their potential therapeutic applications. Recent Pat CNS Drug Discov. 2009;4(2):112-136.
16. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804.
17. Cesamet [package insert]. Somerset, NJ: Meda Pharmaceuticals; 2013.
18. Marinol [package insert]. Chicago, IL: AbbVie Inc.; 2017.
19. Sativex [package insert]. Mississauga, Ontario: Bayer Inc.; 2015.
20. Torrance N, Ferguson JA, Afolabi E, et al. Neuropathic pain in the community: more under-treated than refractory? Pain. 2013;154(5):690-699.
21. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
22. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473.
23. Wilsey B, Marcotte, T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9(6):506-521.
24. Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ. 2010;182(14):E694-E701.
25. Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14(2):136-148.
26. National Cancer Institute. Treatment-related nausea and vomiting (PDQ®)-health professional version. https://www.cancer.gov/about-cancer/treatment/side-effects/nausea/nausea-hp-pdq. Updated May 10, 2017. Accessed November 7, 2017.
27. Meiri E, Jhangiani H, Vrendenburgh JJ, et al. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin. 2007;23(3):533-543.
28. Tramèr MR, Carroll D, Campbell FA, et al. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001;323(7303):16-21.
29. Smith LA, Azariah F, Lavender VT, et al. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev. 2015;(11):CD009464.
30. Maa E, Figi P. The case for medical marijuana in epilepsy. Epilepsia. 2014;55(6):783-786.
31. dos Santos RG, Hallak JE, Leite JP, et al. Phytocannabinoids and epilepsy. J Clin Pharm Ther. 2015;40(2):135-143.
32. Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;(3):CD009270.
33. Yadav V, Bever C Jr, Bowen J, et al. Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis: report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2014;82(12):1083-1092.
34. Corey-Bloom J, Wolfson T, Gamst A, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ. 2012;184(10):1143-1150.
35. Zajicek J, Ball S, Wright D, et al; CUPID investigator group. Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): a randomised, placebo-controlled trial. Lancet Neurol. 2013;12(9):857-865.
36. Novotna A, Mares J, Ratcliffe S, et al; Sativex Spasticity Study Group. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex(®)), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. 2011;18(9):1122-1131.
37. Merritt JC, Crawford WJ, Alexander PC, et al. Effect of marihuana on intraocular and blood pressure in glaucoma. Ophthalmology. 1980;87(3):222-228.
38. American Academy of Ophthalmology. American Academy of Ophthalmology reiterates position that marijuana is not a proven treatment for glaucoma. https://www.aao.org/newsroom/news-releases/detail/american-academy-of-ophthalmology-reiterates-posit. Published June 27, 2014. Accessed May 29, 2017.
39. Naftali T, Bar-Lev Schleider L, Dotan I, et al. Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol. 2013;11(10):1276.e1-1280.e1.
40. Koppel BS Brust JC, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in certain neurological disorders. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556-1563.
41. Chagas MH, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol. 2014;28(11):1088-1098.
42. Carroll CB, Bain PG, Teare L, et al. Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology. 2004;63(7):1245-1250.
43. Weber M, Goldman B, Truniger S. Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatry. 2010;81(10):1135-1140.
44. Walther S, Mahlberg R, Eichmann U, et al. Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology (Berl). 2006;185(4):524-528.
45. Woodward MR, Harper DG, Stolyar A, et al. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am J Geriatr Psychiatry. 2014;22(4):415-419.
46. van den Elsen GA, Ahmed A, Verkes RJ, et al. Tetrahydrocannabinol for neuropsychiatric symptoms in dementia: a randomized controlled trial. Neurology. 2015;84(23):2338-2346.
47. Ahmed AI, van den Elsen GA, Colbers A, et al. Safety, pharmacodynamics, and pharmacokinetics of multiple oral doses of delta-9-tetrahydrocannabinol in older persons with dementia. Psychopharmacology (Berl). 2015;232(14):25872595.
48. Krishnan S, Cairns R, Howard R. Cannabinoids for the treatment of dementia. Cochrane Database Syst Rev. 2009;(2):CD007204.
49. de Bitencourt RM, Pamplona FA, Takahashi RN. A current overview of cannabinoids and glucocorticoids in facilitating extinction of aversive memories: potential extinction enhancers. Neuropharmacology. 2013;64:389-395.
50. Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther. 2009;15(1):84-88.
51. Greer GR, Grob CS, Halberstadt AL. PTSD symptom reports of patients evaluated for the New Mexico Medical Cannabis Program. J Psychoactive Drugs. 2014;46(1):73-77.
52. Cameron C, Watson D, Robinson J. Use of a synthetic cannabinoid in a correctional population for posttraumatic stress disorder-related insomnia and nightmares, chronic pain, harm reduction, and other indications: a retrospective evaluation. J Clin Psychopharmacol. 2014;34(5):559-564.
53. Jetly R, Heber A, Fraser G, et al. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51:585-588.
54. Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear, memory extinction, and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol. 2008;18(12):849-859.
55. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199-215.
56. Thomas A, Baillie GL, Phillips AM, et al. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150(5):613-623.
57. Das RK, Kamboj SK, Ramadas M, et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology (Berl). 2013;226(4):781-792.
58. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016;79(7):613-619.
59. Volkow ND, Swanson JM, Evins AE, et al. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry. 2016;73(3):292297.
The evidence on medical marijuana
A concise guide to monoamine oxidase inhibitors: How to avoid drug interactions
Monoamine oxidase inhibitors (MAOIs) have well-established efficacy for treating depression, panic disorder, and social phobia. However, a lack of familiarity with these agents and misconceptions about the risks associated with their use have led to MAOIs being substantially underutilized. The goal of this 2-part guide to MAOIs is to educate clinicians about this often-overlooked class of medications. Part 1 (“A concise guide to monoamine inhibitors,”
MAOIs and potential drug interactions
One source of concern in patients receiving irreversible nonselective MAOIs is the development of excessive serotonergic neurotransmission resulting in SS. In the 1960s, researchers noted that administering large doses of
- mild symptoms: tremor, akathisia, inducible clonus
- moderate symptoms: spontaneous or sustained clonus, muscular hypertonicity
- severe symptoms: hyperthermia, diaphoresis.2
Although SS can be induced by significant exposure to individual agents that promote excess synaptic serotonin (eg, overdose of selective serotonin reuptake inhibitors [SSRIs]), the majority of fatal cases have occurred among patients taking MAOIs who were coadministered an agent that inhibited serotonin reuptake (Table 13). Animal studies have determined that excessive stimulation of the 5HT2A receptor is primarily responsible for SS,4 and that 5HT2A antagonists, such as mirtazapine, can block the development of SS in a mouse coadministered
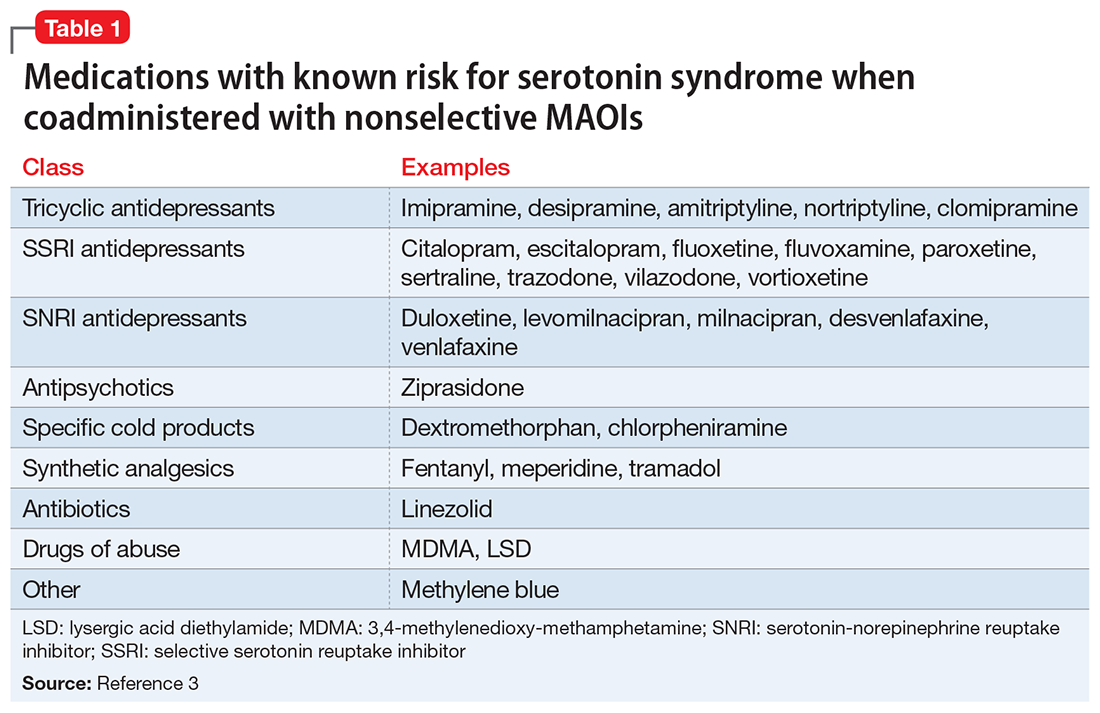
Risk for SS. Most medications that promote serotonergic activity are well known for their use as antidepressants, but other agents that have 5HT reuptake properties (eg, the antihistamine chlorpheniramine) must be avoided. Although older literature suggests that the use of lower doses of certain tricyclic antidepressants concurrently with MAOIs may not be as dangerous as once believed,6 there are sufficient reports of serious outcomes that tricyclics should be avoided in patients taking MAOIs because of the risk of SS, and also because, in general, tricyclics are poorly tolerated.7
Desipramine, a potent norepinephrine transporter (NET) inhibitor, blocks the entry of tyramine into cells by NET, thereby preventing hypertensive events in animal models of tyramine overexposure. However, in some assays, the affinity for the serotonin transporter is not insignificant, so at higher doses desipramine may pose the same theoretical risk for SS as seen with other tricyclics.3
Lastly
Astute clinicians will recognize that antidepressants that lack 5HT reuptake (eg, bupropion, mirtazapine) are not on this list of agents that may increase SS risk when taken with an MAOI. Older papers often list mirtazapine, but as a 5HT2A antagonist, it does not possess a plausible mechanism by which it can induce 5HT toxicity.9,10 Most atypical antipsychotics have significant 5HT2A antagonism and can be combined with MAOIs, but ziprasidone is an exception: as a moderate SNRI, it has been associated with SS when administered with an MAOI.11
Pressor reactions. The only theoretical sources of concern for pressor effects are medications that act as norepinephrine releasers through interactions at the trace amine-associated receptor 1 (TAAR1) (for more information on TAAR1, see
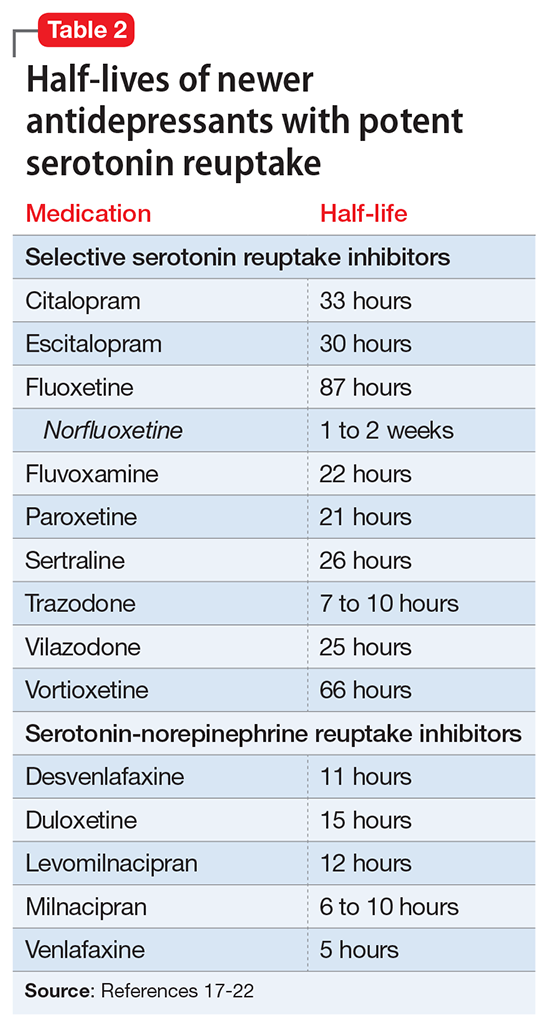
Starting a patient on an MAOI

Contraindicated medications need to be tapered before beginning MAOI treatment. The duration of the washout period depends on the half-life of the medication and any active metabolites. Antidepressants with half-lives of approximately ≤24 hours should be tapered over 7 to 14 days (depending on the dose) to minimize the risk of withdrawal syndromes, while those with long half-lives (eg, fluoxetine,
Initiation of an MAOI is always based on whether the patient can reliably follow the basic dietary advice (see “A concise guide to monoamine inhibitors,”
The orthostasis management strategy is similar to that employed for
Augmentation options for patients taking MAOIs
For depressed patients who do not achieve remission of symptoms from MAOI therapy, augmentation options should be sought, as patients who respond but fail to remit are at increased risk of relapse.26 Lithium augmentation is one of the more common strategies, with abundant data supporting its use.27,28 Case reports dating back >12 years describe the concurrent use o
1. Krishnamoorthy S, Ma Z, Zhang G, et al. Involvement of 5-HT2A receptors in the serotonin (5-HT) syndrome caused by excessive 5-HT efflux in rat brain. Basic Clin Pharmacol Toxicol. 2010;107(4):830-841.
2. Sternbach H. The serotonin syndrome. Am J Psychiatry 1991;148(6):705-713.
3. Gillman PK. Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. PsychoTropical Commentaries. 2016;16(6):1-90.
4. Haberzettl R, Fink H, Bert B. Role of 5-HT(1A)- and 5-HT(2A) receptors for the murine model of the serotonin syndrome. J Pharmacol Toxicol Methods. 2014;70(2):129-133.
5. Shioda K, Nisijima K, Yoshino T, et al. Mirtazapine abolishes hyperthermia in an animal model of serotonin syndrome. Neurosci Lett. 2010;482(3):216-219.
6. White K, Simpson G. Combined MAOI-tricyclic antidepressant treatment: a reevaluation. J Clin Psychopharmacol. 1981;1(5):264-282.
7. Otte W, Birkenhager TK, van den Broek WW. Fatal interaction between tranylcypromine and imipramine. Eur Psychiatry. 2003;18(5):264-265.
8. Panisset M, Chen JJ, Rhyee SH, et al. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250-1258.
9. Gillman PK. Mirtazapine: unable to induce serotonin toxicity? Clin Neuropharmacol. 2003;26(6):288-289; author reply 289-290.
10. Gillman PK. A systematic review of the serotonergic effects of mirtazapine in humans: implications for its dual action status. Hum Psychopharmacol. 2006;21(2):117-125.
11. Meyer JM, Cummings MA, Proctor G. Augmentation of phenelzine with aripiprazole and quetiapine in a treatment resistant patient with psychotic unipolar depression: case report and literature review. CNS Spectr. 2017;22(5):391-396.
12. Feinberg SS. Combining stimulants with monoamine oxidase inhibitors: a review of uses and one possible additional indication. J Clin Psychiatry. 2004;65(11):1520-1524.
13. Israel JA. Combining stimulants and monoamine oxidase inhibitors: a reexamination of the literature and a report of a new treatment combination. Prim Care Companion CNS Disord. 2015;17(6). doi: 10.4088/PCC.15br01836.
14. Simmler LD, Buchy D, Chaboz S, et al. In vitro characterization of psychoactive substances at rat, mouse, and human trace amine-associated receptor 1. J Pharmacol Exp Ther. 2016;357(1):134-144.
15. Froimowitz M, Gu Y, Dakin LA, et al. Slow-onset, long-duration, alkyl analogues of methylphenidate with enhanced selectivity for the dopamine transporter. J Med Chem. 2007;50(2):219-232.
16. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-780.
17. Hiemke C, Härtter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther. 2000;85(1):11-28.
18. Pristiq [package insert]. New York, NY: Pfizer Inc; 2016.
19. Savella [package insert]. Irvine, CA: Allergan USA Inc; 2016.
20. Viibryd [package insert]. Irvine, CA: Allergan USA Inc; 2016.
21. Trintellix [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; 2016.
22. Fetzima [package insert]. Irvine, CA: Allergan USA Inc; 2017.
23. Nardil [package insert]. New York, NY: Pfizer Inc; 2009.
24. Testani M Jr. Clozapine-induced orthostatic hypotension treated with fludrocortisone. J Clin Psychiatry. 1994;55(11):497-498.
25. Emsam [package insert]. Morgantown, WV: Somerset Pharmaceuticals Inc; 2015.
26. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
27. Tariot PN, Murphy DL, Sunderland T, et al. Rapid antidepressant effect of addition of lithium to tranylcypromine. J Clin Psychopharmacol. 1986;6(3):165-167.
28. Kok RM, Vink D, Heeren TJ, et al. Lithium augmentation compared with phenelzine in treatment-resistant depression in the elderly: an open, randomized, controlled trial. J Clin Psychiatry. 2007;68(8):1177-1185.
29. Quante A, Zeugmann S. Tranylcypromine and bupropion combination therapy in treatment-resistant major depression: a report of 2 cases. J Clin Psychopharmacol. 2012;32(4):572-574.
30. Joffe RT. Triiodothyronine potentiation of the antidepressant effect of phenelzine. J Clin Psychiatry. 1988;49(10):409-410.
31. Hullett FJ, Bidder TG. Phenelzine plus triiodothyronine combination in a case of refractory depression. J Nerv Ment Dis. 1983;171(5):318-320.
Monoamine oxidase inhibitors (MAOIs) have well-established efficacy for treating depression, panic disorder, and social phobia. However, a lack of familiarity with these agents and misconceptions about the risks associated with their use have led to MAOIs being substantially underutilized. The goal of this 2-part guide to MAOIs is to educate clinicians about this often-overlooked class of medications. Part 1 (“A concise guide to monoamine inhibitors,”
MAOIs and potential drug interactions
One source of concern in patients receiving irreversible nonselective MAOIs is the development of excessive serotonergic neurotransmission resulting in SS. In the 1960s, researchers noted that administering large doses of
- mild symptoms: tremor, akathisia, inducible clonus
- moderate symptoms: spontaneous or sustained clonus, muscular hypertonicity
- severe symptoms: hyperthermia, diaphoresis.2
Although SS can be induced by significant exposure to individual agents that promote excess synaptic serotonin (eg, overdose of selective serotonin reuptake inhibitors [SSRIs]), the majority of fatal cases have occurred among patients taking MAOIs who were coadministered an agent that inhibited serotonin reuptake (Table 13). Animal studies have determined that excessive stimulation of the 5HT2A receptor is primarily responsible for SS,4 and that 5HT2A antagonists, such as mirtazapine, can block the development of SS in a mouse coadministered

Risk for SS. Most medications that promote serotonergic activity are well known for their use as antidepressants, but other agents that have 5HT reuptake properties (eg, the antihistamine chlorpheniramine) must be avoided. Although older literature suggests that the use of lower doses of certain tricyclic antidepressants concurrently with MAOIs may not be as dangerous as once believed,6 there are sufficient reports of serious outcomes that tricyclics should be avoided in patients taking MAOIs because of the risk of SS, and also because, in general, tricyclics are poorly tolerated.7
Desipramine, a potent norepinephrine transporter (NET) inhibitor, blocks the entry of tyramine into cells by NET, thereby preventing hypertensive events in animal models of tyramine overexposure. However, in some assays, the affinity for the serotonin transporter is not insignificant, so at higher doses desipramine may pose the same theoretical risk for SS as seen with other tricyclics.3
Lastly
Astute clinicians will recognize that antidepressants that lack 5HT reuptake (eg, bupropion, mirtazapine) are not on this list of agents that may increase SS risk when taken with an MAOI. Older papers often list mirtazapine, but as a 5HT2A antagonist, it does not possess a plausible mechanism by which it can induce 5HT toxicity.9,10 Most atypical antipsychotics have significant 5HT2A antagonism and can be combined with MAOIs, but ziprasidone is an exception: as a moderate SNRI, it has been associated with SS when administered with an MAOI.11
Pressor reactions. The only theoretical sources of concern for pressor effects are medications that act as norepinephrine releasers through interactions at the trace amine-associated receptor 1 (TAAR1) (for more information on TAAR1, see

Starting a patient on an MAOI

Contraindicated medications need to be tapered before beginning MAOI treatment. The duration of the washout period depends on the half-life of the medication and any active metabolites. Antidepressants with half-lives of approximately ≤24 hours should be tapered over 7 to 14 days (depending on the dose) to minimize the risk of withdrawal syndromes, while those with long half-lives (eg, fluoxetine,
Initiation of an MAOI is always based on whether the patient can reliably follow the basic dietary advice (see “A concise guide to monoamine inhibitors,”
The orthostasis management strategy is similar to that employed for
Augmentation options for patients taking MAOIs
For depressed patients who do not achieve remission of symptoms from MAOI therapy, augmentation options should be sought, as patients who respond but fail to remit are at increased risk of relapse.26 Lithium augmentation is one of the more common strategies, with abundant data supporting its use.27,28 Case reports dating back >12 years describe the concurrent use o
Monoamine oxidase inhibitors (MAOIs) have well-established efficacy for treating depression, panic disorder, and social phobia. However, a lack of familiarity with these agents and misconceptions about the risks associated with their use have led to MAOIs being substantially underutilized. The goal of this 2-part guide to MAOIs is to educate clinicians about this often-overlooked class of medications. Part 1 (“A concise guide to monoamine inhibitors,”
MAOIs and potential drug interactions
One source of concern in patients receiving irreversible nonselective MAOIs is the development of excessive serotonergic neurotransmission resulting in SS. In the 1960s, researchers noted that administering large doses of
- mild symptoms: tremor, akathisia, inducible clonus
- moderate symptoms: spontaneous or sustained clonus, muscular hypertonicity
- severe symptoms: hyperthermia, diaphoresis.2
Although SS can be induced by significant exposure to individual agents that promote excess synaptic serotonin (eg, overdose of selective serotonin reuptake inhibitors [SSRIs]), the majority of fatal cases have occurred among patients taking MAOIs who were coadministered an agent that inhibited serotonin reuptake (Table 13). Animal studies have determined that excessive stimulation of the 5HT2A receptor is primarily responsible for SS,4 and that 5HT2A antagonists, such as mirtazapine, can block the development of SS in a mouse coadministered

Risk for SS. Most medications that promote serotonergic activity are well known for their use as antidepressants, but other agents that have 5HT reuptake properties (eg, the antihistamine chlorpheniramine) must be avoided. Although older literature suggests that the use of lower doses of certain tricyclic antidepressants concurrently with MAOIs may not be as dangerous as once believed,6 there are sufficient reports of serious outcomes that tricyclics should be avoided in patients taking MAOIs because of the risk of SS, and also because, in general, tricyclics are poorly tolerated.7
Desipramine, a potent norepinephrine transporter (NET) inhibitor, blocks the entry of tyramine into cells by NET, thereby preventing hypertensive events in animal models of tyramine overexposure. However, in some assays, the affinity for the serotonin transporter is not insignificant, so at higher doses desipramine may pose the same theoretical risk for SS as seen with other tricyclics.3
Lastly
Astute clinicians will recognize that antidepressants that lack 5HT reuptake (eg, bupropion, mirtazapine) are not on this list of agents that may increase SS risk when taken with an MAOI. Older papers often list mirtazapine, but as a 5HT2A antagonist, it does not possess a plausible mechanism by which it can induce 5HT toxicity.9,10 Most atypical antipsychotics have significant 5HT2A antagonism and can be combined with MAOIs, but ziprasidone is an exception: as a moderate SNRI, it has been associated with SS when administered with an MAOI.11
Pressor reactions. The only theoretical sources of concern for pressor effects are medications that act as norepinephrine releasers through interactions at the trace amine-associated receptor 1 (TAAR1) (for more information on TAAR1, see

Starting a patient on an MAOI

Contraindicated medications need to be tapered before beginning MAOI treatment. The duration of the washout period depends on the half-life of the medication and any active metabolites. Antidepressants with half-lives of approximately ≤24 hours should be tapered over 7 to 14 days (depending on the dose) to minimize the risk of withdrawal syndromes, while those with long half-lives (eg, fluoxetine,
Initiation of an MAOI is always based on whether the patient can reliably follow the basic dietary advice (see “A concise guide to monoamine inhibitors,”
The orthostasis management strategy is similar to that employed for
Augmentation options for patients taking MAOIs
For depressed patients who do not achieve remission of symptoms from MAOI therapy, augmentation options should be sought, as patients who respond but fail to remit are at increased risk of relapse.26 Lithium augmentation is one of the more common strategies, with abundant data supporting its use.27,28 Case reports dating back >12 years describe the concurrent use o
1. Krishnamoorthy S, Ma Z, Zhang G, et al. Involvement of 5-HT2A receptors in the serotonin (5-HT) syndrome caused by excessive 5-HT efflux in rat brain. Basic Clin Pharmacol Toxicol. 2010;107(4):830-841.
2. Sternbach H. The serotonin syndrome. Am J Psychiatry 1991;148(6):705-713.
3. Gillman PK. Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. PsychoTropical Commentaries. 2016;16(6):1-90.
4. Haberzettl R, Fink H, Bert B. Role of 5-HT(1A)- and 5-HT(2A) receptors for the murine model of the serotonin syndrome. J Pharmacol Toxicol Methods. 2014;70(2):129-133.
5. Shioda K, Nisijima K, Yoshino T, et al. Mirtazapine abolishes hyperthermia in an animal model of serotonin syndrome. Neurosci Lett. 2010;482(3):216-219.
6. White K, Simpson G. Combined MAOI-tricyclic antidepressant treatment: a reevaluation. J Clin Psychopharmacol. 1981;1(5):264-282.
7. Otte W, Birkenhager TK, van den Broek WW. Fatal interaction between tranylcypromine and imipramine. Eur Psychiatry. 2003;18(5):264-265.
8. Panisset M, Chen JJ, Rhyee SH, et al. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250-1258.
9. Gillman PK. Mirtazapine: unable to induce serotonin toxicity? Clin Neuropharmacol. 2003;26(6):288-289; author reply 289-290.
10. Gillman PK. A systematic review of the serotonergic effects of mirtazapine in humans: implications for its dual action status. Hum Psychopharmacol. 2006;21(2):117-125.
11. Meyer JM, Cummings MA, Proctor G. Augmentation of phenelzine with aripiprazole and quetiapine in a treatment resistant patient with psychotic unipolar depression: case report and literature review. CNS Spectr. 2017;22(5):391-396.
12. Feinberg SS. Combining stimulants with monoamine oxidase inhibitors: a review of uses and one possible additional indication. J Clin Psychiatry. 2004;65(11):1520-1524.
13. Israel JA. Combining stimulants and monoamine oxidase inhibitors: a reexamination of the literature and a report of a new treatment combination. Prim Care Companion CNS Disord. 2015;17(6). doi: 10.4088/PCC.15br01836.
14. Simmler LD, Buchy D, Chaboz S, et al. In vitro characterization of psychoactive substances at rat, mouse, and human trace amine-associated receptor 1. J Pharmacol Exp Ther. 2016;357(1):134-144.
15. Froimowitz M, Gu Y, Dakin LA, et al. Slow-onset, long-duration, alkyl analogues of methylphenidate with enhanced selectivity for the dopamine transporter. J Med Chem. 2007;50(2):219-232.
16. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-780.
17. Hiemke C, Härtter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther. 2000;85(1):11-28.
18. Pristiq [package insert]. New York, NY: Pfizer Inc; 2016.
19. Savella [package insert]. Irvine, CA: Allergan USA Inc; 2016.
20. Viibryd [package insert]. Irvine, CA: Allergan USA Inc; 2016.
21. Trintellix [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; 2016.
22. Fetzima [package insert]. Irvine, CA: Allergan USA Inc; 2017.
23. Nardil [package insert]. New York, NY: Pfizer Inc; 2009.
24. Testani M Jr. Clozapine-induced orthostatic hypotension treated with fludrocortisone. J Clin Psychiatry. 1994;55(11):497-498.
25. Emsam [package insert]. Morgantown, WV: Somerset Pharmaceuticals Inc; 2015.
26. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
27. Tariot PN, Murphy DL, Sunderland T, et al. Rapid antidepressant effect of addition of lithium to tranylcypromine. J Clin Psychopharmacol. 1986;6(3):165-167.
28. Kok RM, Vink D, Heeren TJ, et al. Lithium augmentation compared with phenelzine in treatment-resistant depression in the elderly: an open, randomized, controlled trial. J Clin Psychiatry. 2007;68(8):1177-1185.
29. Quante A, Zeugmann S. Tranylcypromine and bupropion combination therapy in treatment-resistant major depression: a report of 2 cases. J Clin Psychopharmacol. 2012;32(4):572-574.
30. Joffe RT. Triiodothyronine potentiation of the antidepressant effect of phenelzine. J Clin Psychiatry. 1988;49(10):409-410.
31. Hullett FJ, Bidder TG. Phenelzine plus triiodothyronine combination in a case of refractory depression. J Nerv Ment Dis. 1983;171(5):318-320.
1. Krishnamoorthy S, Ma Z, Zhang G, et al. Involvement of 5-HT2A receptors in the serotonin (5-HT) syndrome caused by excessive 5-HT efflux in rat brain. Basic Clin Pharmacol Toxicol. 2010;107(4):830-841.
2. Sternbach H. The serotonin syndrome. Am J Psychiatry 1991;148(6):705-713.
3. Gillman PK. Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. PsychoTropical Commentaries. 2016;16(6):1-90.
4. Haberzettl R, Fink H, Bert B. Role of 5-HT(1A)- and 5-HT(2A) receptors for the murine model of the serotonin syndrome. J Pharmacol Toxicol Methods. 2014;70(2):129-133.
5. Shioda K, Nisijima K, Yoshino T, et al. Mirtazapine abolishes hyperthermia in an animal model of serotonin syndrome. Neurosci Lett. 2010;482(3):216-219.
6. White K, Simpson G. Combined MAOI-tricyclic antidepressant treatment: a reevaluation. J Clin Psychopharmacol. 1981;1(5):264-282.
7. Otte W, Birkenhager TK, van den Broek WW. Fatal interaction between tranylcypromine and imipramine. Eur Psychiatry. 2003;18(5):264-265.
8. Panisset M, Chen JJ, Rhyee SH, et al. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250-1258.
9. Gillman PK. Mirtazapine: unable to induce serotonin toxicity? Clin Neuropharmacol. 2003;26(6):288-289; author reply 289-290.
10. Gillman PK. A systematic review of the serotonergic effects of mirtazapine in humans: implications for its dual action status. Hum Psychopharmacol. 2006;21(2):117-125.
11. Meyer JM, Cummings MA, Proctor G. Augmentation of phenelzine with aripiprazole and quetiapine in a treatment resistant patient with psychotic unipolar depression: case report and literature review. CNS Spectr. 2017;22(5):391-396.
12. Feinberg SS. Combining stimulants with monoamine oxidase inhibitors: a review of uses and one possible additional indication. J Clin Psychiatry. 2004;65(11):1520-1524.
13. Israel JA. Combining stimulants and monoamine oxidase inhibitors: a reexamination of the literature and a report of a new treatment combination. Prim Care Companion CNS Disord. 2015;17(6). doi: 10.4088/PCC.15br01836.
14. Simmler LD, Buchy D, Chaboz S, et al. In vitro characterization of psychoactive substances at rat, mouse, and human trace amine-associated receptor 1. J Pharmacol Exp Ther. 2016;357(1):134-144.
15. Froimowitz M, Gu Y, Dakin LA, et al. Slow-onset, long-duration, alkyl analogues of methylphenidate with enhanced selectivity for the dopamine transporter. J Med Chem. 2007;50(2):219-232.
16. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-780.
17. Hiemke C, Härtter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther. 2000;85(1):11-28.
18. Pristiq [package insert]. New York, NY: Pfizer Inc; 2016.
19. Savella [package insert]. Irvine, CA: Allergan USA Inc; 2016.
20. Viibryd [package insert]. Irvine, CA: Allergan USA Inc; 2016.
21. Trintellix [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; 2016.
22. Fetzima [package insert]. Irvine, CA: Allergan USA Inc; 2017.
23. Nardil [package insert]. New York, NY: Pfizer Inc; 2009.
24. Testani M Jr. Clozapine-induced orthostatic hypotension treated with fludrocortisone. J Clin Psychiatry. 1994;55(11):497-498.
25. Emsam [package insert]. Morgantown, WV: Somerset Pharmaceuticals Inc; 2015.
26. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
27. Tariot PN, Murphy DL, Sunderland T, et al. Rapid antidepressant effect of addition of lithium to tranylcypromine. J Clin Psychopharmacol. 1986;6(3):165-167.
28. Kok RM, Vink D, Heeren TJ, et al. Lithium augmentation compared with phenelzine in treatment-resistant depression in the elderly: an open, randomized, controlled trial. J Clin Psychiatry. 2007;68(8):1177-1185.
29. Quante A, Zeugmann S. Tranylcypromine and bupropion combination therapy in treatment-resistant major depression: a report of 2 cases. J Clin Psychopharmacol. 2012;32(4):572-574.
30. Joffe RT. Triiodothyronine potentiation of the antidepressant effect of phenelzine. J Clin Psychiatry. 1988;49(10):409-410.
31. Hullett FJ, Bidder TG. Phenelzine plus triiodothyronine combination in a case of refractory depression. J Nerv Ment Dis. 1983;171(5):318-320.
The puzzling relationship between cholesterol and psychopathology
Cholesterol generally is regarded as a cardiovascular risk factor when elevated. However, numerous studies suggest that cholesterol levels—both high and low—may be associated with various psychiatric brai
The relationship between cholesterol and mental illness is fascinating, complex, and perplexing. Whether elevated or reduced, cholesterol’s effects can be deleterious or salutary, but the literature is riddled with conflicting reports. Physicians should measure their patients’ serum cholesterol levels not only to assess cardiovascular risk, but because cholesterol can be associated with certain neuropsychiatric disorders or may predict the lack of response to psychopharmacotherapy.2
The fact that lowering total cholesterol levels in people with hypercholesterolemia reduces the risk of coronary heart disease is indisputable. Large-scale cardiology clinical trials have shown a significant reduction in mortality from heart disease or stroke with cholesterol-lowering drugs (statins). However, the same trials found an uptick in “unnatural deaths,” mostly suicide or homicide.3 Those findings triggered numerous intriguing reports of the association between cholesterol levels and psychopathology.
Consider the following:
- Low cholesterol levels have been associated with depression, antisocial personality disorder, borderline personality disorder, and dissociative disorder.4
- High cholesterol levels have been associated with schizophrenia, obsessive-compulsive disorder, panic disorder, generalized anxiety disorder, and posttraumatic stress disorder.4
- Some studies suggest that high cholesterol levels are associated with better mental health, mental processing speed, social skills, responsibility, self-control, and self-awareness.5
- In the Clinical Antipsychotic Trials of Intervention Effectiveness schizophrenia study, better cognitive scores were found in patients with higher fasting cholesterol and triglyceride levels (H.A.N., unpublished data, 2017).
The brain is only 2% of body weight, but it contains 25% of the body’s cholesterol.6 Cholesterol is important for brain function and neurotransmission because neuroactive steroids (NASs) are synthesized from cholesterol and they modulate brain processes and interact with γ-aminobutyric acid, N-methyl-
Interestingly, both extremes in cholesterol levels represent a high risk for premature mortality.10 Hypercholesterolemia leads to early death from coronary artery disease. Studies that evaluated statins to lower cholesterol found increased mortality from suicide, accidents, and violence.11 Even without statin treatment, among persons with naturally low cholesterol, there is a significant increase in mortality from non-medical causes.12 However, some studies did not find an association between hypocholesterolemia and suicide.13,14
There also is some evidence that elevated cholesterol may play a role in dementia.15 Reducing cholesterol with statins decreases beta-amyloid in mice, while the opposite occurs with elevated cholesterol.2 Another possible mechanism by which high cholesterol worsens dementia is that neurodegeneration in Alzheimer’s disease (AD) breaks down neuronal cell membranes, which releases the neurotoxic metabolite of cholesterol (24-hydroxycholesterol), which leads to further neurodegeneration.16 Statins may decrease the production of 24-hydroxycholesterol in AD patients and slow down neurodegeneration.16
A large study of 4,444 consecutive patients in Taiwan found that those with low total cholesterol (<160 mg/dL) had higher scores of anxiety, phobia, psychoticism, and aggressive hostility.17 In the same study, women with low high-density lipoprotein cholesterol (<35 mg/dL) had significantly higher scores for depression, phobia, anxiety, interpersonal sensitivity, somatization, and aggressive hostility.17
Not surprisingly, low cholesterol has been proposed as a biomarker for mood dysregulation, depression, and suicidality,18 as well as a predictor of the depression severity and increased suicide risk.19 Clinical recovery in depression may be accompanied by a significant increase of total cholesterol20 but, interestingly, a decrease in cholesterol levels after treatment of mania. High cholesterol was reported to predict poorer response to selective serotonin reuptake inhibitors, and total cholesterol levels >200 mg/dL were associated with lack of response to fluoxetine and nortriptyline.2 Interestingly, clozapine, which elevates lipids, exerts a strong anti-suicide effect in schizophrenia and schizoaffective disorder, but that may not be the main reason for its efficacy in preventing suicide in patients with psychosis.
Cholesterol is an important lipid for brain function. At lower levels, it appears to be associated with depression, suicide, violence, anxiety, schizophrenia, and severe personality disorders (including antisocial personality disorder and borderline personality disorder). However, at high levels, it may improve cognition in schizophrenia and ameliorate the pace of AD and neurodegeneration. Psychiatrists should monitor patients for hypercholesterolemia and hypocholesterolemia, both of which are common among psychiatric patients. High levels may be genetic or the result of weight gain, hypercortisolemia, diabetes, or immune or inflammatory processes. Similarly, low levels may be genetic or secondary to statin therapy.
The bottom line: As psychiatric physicians, we should protect both the hearts and brains of our patients.
1. Hallahan B, Garland MR. Essential fatty acids and mental health. British J Psychiatry. 2005;186(4):275-277.
2. Papakostas GI, Ongür D, Iosifescu DV, et al. Cholesterol in mood and anxiety disorders: review of the literature and new hypotheses. Eur Neuropsychopharmacol. 2004;14(2):135-142.
3. Muldoon MF, Manuck SB, Matthews KA, et al. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301(647):309-314.
4. Jakovljevic
5. Rogers PJ. A healthy body, a healthy mind: long-term impact of diet on mood and cognitive function. Pro Nutr Soc. 2001;60(1):135-143.
6. Björkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med. 2006;260(6):493-508.
7. Tuem KB, Atey TM. Neuroactive steroids: receptor interactions and responses. Front Neurol. 2017;8:442.
8. Borroni MV, Vallés AS, Barrantes FJ. The lipid habitats of neurotransmitter receptors in the brain. Biochim Biophys Acta. 2016;1858(1):2662-2670.
9. Pfrieger FW. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci. 2003;60(6):1158-1171.
10. Graham I, Atar D, Borch-Johnsen K, et al; European Society of Cardiology (ESC); European Association for Cardiovascular Prevention and Rehabilitation (EACPR); Council on Cardiovascular Nursing; European Association for Study of Diabetes (EASD); International Diabetes Federation Europe (IDF-Europe); European Stroke Initiative (EUSI); Society of Behavioural Medicine (ISBM); European Society of Hypertension (ESH); WONCA Europe (European Society of General Practice/Family Medicine); European Heart Network (EHN); European Atherosclerosis Society (EAS). European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of none societies and by invited experts). Eur J Cardiovasc Prev Rehabil. 2007;14(suppl 2):S1-S113.
11. Almeida-Montes LG, Valles-Sanchez V, Moreno-Aguilar J, et al. Relation of serum cholesterol, lipid, serotonin and tryptophan levels to severity of depression and to suicide attempts. J Psychiatry Neurosci. 2000;25(4):371-377.
12. Ryman A. Cholesterol, violent death, and mental disorder. BMJ. 1994;309(69525):421-422.
13. Wardle J. Cholesterol and psychological well-being. J Psychosom Res. 1995;39(5):549-562.
14. Irribarren C, Reed DM, Chen R, et al. Low serum cholesterol and mortality. Which is the cause and which is the effect? Circulation. 1995;92(9):2396-2403.
15. Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: common links. J Intern Med. 2006;260(3):211-223.
16. Raffai RL, Weisgraber KH. Cholesterol: from heart attacks to Alzheimer’s disease. J Lipid Res. 2003;44(8):1423-1430.
17. Chen CC, Lu FH, Wu JS, et al. Correlation between serum lipid concentrations and psychological distress. Psychiatry Res. 2003;102(2):153-162.
18. Mössmer R, Mikova O, Koutsilieri E, et al. Consensus paper of the WFSBP Task Force on Biological Markers: biological markers in depression. World J Biol Psychiatry. 2007;8(3):141-174.
19. Papakostas GI, Petersen T, Sonawalla SB, et al. Serum cholesterol in treatment-resistant depression. Neuropsychobiology. 2003;47(3):146-151.
20. Gabriel A. Changes in plasma cholesterol in mood disorder patients: does treatment make a difference? J Affect Disord. 2007;99(1-3):273-278.
Cholesterol generally is regarded as a cardiovascular risk factor when elevated. However, numerous studies suggest that cholesterol levels—both high and low—may be associated with various psychiatric brai
The relationship between cholesterol and mental illness is fascinating, complex, and perplexing. Whether elevated or reduced, cholesterol’s effects can be deleterious or salutary, but the literature is riddled with conflicting reports. Physicians should measure their patients’ serum cholesterol levels not only to assess cardiovascular risk, but because cholesterol can be associated with certain neuropsychiatric disorders or may predict the lack of response to psychopharmacotherapy.2
The fact that lowering total cholesterol levels in people with hypercholesterolemia reduces the risk of coronary heart disease is indisputable. Large-scale cardiology clinical trials have shown a significant reduction in mortality from heart disease or stroke with cholesterol-lowering drugs (statins). However, the same trials found an uptick in “unnatural deaths,” mostly suicide or homicide.3 Those findings triggered numerous intriguing reports of the association between cholesterol levels and psychopathology.
Consider the following:
- Low cholesterol levels have been associated with depression, antisocial personality disorder, borderline personality disorder, and dissociative disorder.4
- High cholesterol levels have been associated with schizophrenia, obsessive-compulsive disorder, panic disorder, generalized anxiety disorder, and posttraumatic stress disorder.4
- Some studies suggest that high cholesterol levels are associated with better mental health, mental processing speed, social skills, responsibility, self-control, and self-awareness.5
- In the Clinical Antipsychotic Trials of Intervention Effectiveness schizophrenia study, better cognitive scores were found in patients with higher fasting cholesterol and triglyceride levels (H.A.N., unpublished data, 2017).
The brain is only 2% of body weight, but it contains 25% of the body’s cholesterol.6 Cholesterol is important for brain function and neurotransmission because neuroactive steroids (NASs) are synthesized from cholesterol and they modulate brain processes and interact with γ-aminobutyric acid, N-methyl-
Interestingly, both extremes in cholesterol levels represent a high risk for premature mortality.10 Hypercholesterolemia leads to early death from coronary artery disease. Studies that evaluated statins to lower cholesterol found increased mortality from suicide, accidents, and violence.11 Even without statin treatment, among persons with naturally low cholesterol, there is a significant increase in mortality from non-medical causes.12 However, some studies did not find an association between hypocholesterolemia and suicide.13,14
There also is some evidence that elevated cholesterol may play a role in dementia.15 Reducing cholesterol with statins decreases beta-amyloid in mice, while the opposite occurs with elevated cholesterol.2 Another possible mechanism by which high cholesterol worsens dementia is that neurodegeneration in Alzheimer’s disease (AD) breaks down neuronal cell membranes, which releases the neurotoxic metabolite of cholesterol (24-hydroxycholesterol), which leads to further neurodegeneration.16 Statins may decrease the production of 24-hydroxycholesterol in AD patients and slow down neurodegeneration.16
A large study of 4,444 consecutive patients in Taiwan found that those with low total cholesterol (<160 mg/dL) had higher scores of anxiety, phobia, psychoticism, and aggressive hostility.17 In the same study, women with low high-density lipoprotein cholesterol (<35 mg/dL) had significantly higher scores for depression, phobia, anxiety, interpersonal sensitivity, somatization, and aggressive hostility.17
Not surprisingly, low cholesterol has been proposed as a biomarker for mood dysregulation, depression, and suicidality,18 as well as a predictor of the depression severity and increased suicide risk.19 Clinical recovery in depression may be accompanied by a significant increase of total cholesterol20 but, interestingly, a decrease in cholesterol levels after treatment of mania. High cholesterol was reported to predict poorer response to selective serotonin reuptake inhibitors, and total cholesterol levels >200 mg/dL were associated with lack of response to fluoxetine and nortriptyline.2 Interestingly, clozapine, which elevates lipids, exerts a strong anti-suicide effect in schizophrenia and schizoaffective disorder, but that may not be the main reason for its efficacy in preventing suicide in patients with psychosis.
Cholesterol is an important lipid for brain function. At lower levels, it appears to be associated with depression, suicide, violence, anxiety, schizophrenia, and severe personality disorders (including antisocial personality disorder and borderline personality disorder). However, at high levels, it may improve cognition in schizophrenia and ameliorate the pace of AD and neurodegeneration. Psychiatrists should monitor patients for hypercholesterolemia and hypocholesterolemia, both of which are common among psychiatric patients. High levels may be genetic or the result of weight gain, hypercortisolemia, diabetes, or immune or inflammatory processes. Similarly, low levels may be genetic or secondary to statin therapy.
The bottom line: As psychiatric physicians, we should protect both the hearts and brains of our patients.
Cholesterol generally is regarded as a cardiovascular risk factor when elevated. However, numerous studies suggest that cholesterol levels—both high and low—may be associated with various psychiatric brai
The relationship between cholesterol and mental illness is fascinating, complex, and perplexing. Whether elevated or reduced, cholesterol’s effects can be deleterious or salutary, but the literature is riddled with conflicting reports. Physicians should measure their patients’ serum cholesterol levels not only to assess cardiovascular risk, but because cholesterol can be associated with certain neuropsychiatric disorders or may predict the lack of response to psychopharmacotherapy.2
The fact that lowering total cholesterol levels in people with hypercholesterolemia reduces the risk of coronary heart disease is indisputable. Large-scale cardiology clinical trials have shown a significant reduction in mortality from heart disease or stroke with cholesterol-lowering drugs (statins). However, the same trials found an uptick in “unnatural deaths,” mostly suicide or homicide.3 Those findings triggered numerous intriguing reports of the association between cholesterol levels and psychopathology.
Consider the following:
- Low cholesterol levels have been associated with depression, antisocial personality disorder, borderline personality disorder, and dissociative disorder.4
- High cholesterol levels have been associated with schizophrenia, obsessive-compulsive disorder, panic disorder, generalized anxiety disorder, and posttraumatic stress disorder.4
- Some studies suggest that high cholesterol levels are associated with better mental health, mental processing speed, social skills, responsibility, self-control, and self-awareness.5
- In the Clinical Antipsychotic Trials of Intervention Effectiveness schizophrenia study, better cognitive scores were found in patients with higher fasting cholesterol and triglyceride levels (H.A.N., unpublished data, 2017).
The brain is only 2% of body weight, but it contains 25% of the body’s cholesterol.6 Cholesterol is important for brain function and neurotransmission because neuroactive steroids (NASs) are synthesized from cholesterol and they modulate brain processes and interact with γ-aminobutyric acid, N-methyl-
Interestingly, both extremes in cholesterol levels represent a high risk for premature mortality.10 Hypercholesterolemia leads to early death from coronary artery disease. Studies that evaluated statins to lower cholesterol found increased mortality from suicide, accidents, and violence.11 Even without statin treatment, among persons with naturally low cholesterol, there is a significant increase in mortality from non-medical causes.12 However, some studies did not find an association between hypocholesterolemia and suicide.13,14
There also is some evidence that elevated cholesterol may play a role in dementia.15 Reducing cholesterol with statins decreases beta-amyloid in mice, while the opposite occurs with elevated cholesterol.2 Another possible mechanism by which high cholesterol worsens dementia is that neurodegeneration in Alzheimer’s disease (AD) breaks down neuronal cell membranes, which releases the neurotoxic metabolite of cholesterol (24-hydroxycholesterol), which leads to further neurodegeneration.16 Statins may decrease the production of 24-hydroxycholesterol in AD patients and slow down neurodegeneration.16
A large study of 4,444 consecutive patients in Taiwan found that those with low total cholesterol (<160 mg/dL) had higher scores of anxiety, phobia, psychoticism, and aggressive hostility.17 In the same study, women with low high-density lipoprotein cholesterol (<35 mg/dL) had significantly higher scores for depression, phobia, anxiety, interpersonal sensitivity, somatization, and aggressive hostility.17
Not surprisingly, low cholesterol has been proposed as a biomarker for mood dysregulation, depression, and suicidality,18 as well as a predictor of the depression severity and increased suicide risk.19 Clinical recovery in depression may be accompanied by a significant increase of total cholesterol20 but, interestingly, a decrease in cholesterol levels after treatment of mania. High cholesterol was reported to predict poorer response to selective serotonin reuptake inhibitors, and total cholesterol levels >200 mg/dL were associated with lack of response to fluoxetine and nortriptyline.2 Interestingly, clozapine, which elevates lipids, exerts a strong anti-suicide effect in schizophrenia and schizoaffective disorder, but that may not be the main reason for its efficacy in preventing suicide in patients with psychosis.
Cholesterol is an important lipid for brain function. At lower levels, it appears to be associated with depression, suicide, violence, anxiety, schizophrenia, and severe personality disorders (including antisocial personality disorder and borderline personality disorder). However, at high levels, it may improve cognition in schizophrenia and ameliorate the pace of AD and neurodegeneration. Psychiatrists should monitor patients for hypercholesterolemia and hypocholesterolemia, both of which are common among psychiatric patients. High levels may be genetic or the result of weight gain, hypercortisolemia, diabetes, or immune or inflammatory processes. Similarly, low levels may be genetic or secondary to statin therapy.
The bottom line: As psychiatric physicians, we should protect both the hearts and brains of our patients.
1. Hallahan B, Garland MR. Essential fatty acids and mental health. British J Psychiatry. 2005;186(4):275-277.
2. Papakostas GI, Ongür D, Iosifescu DV, et al. Cholesterol in mood and anxiety disorders: review of the literature and new hypotheses. Eur Neuropsychopharmacol. 2004;14(2):135-142.
3. Muldoon MF, Manuck SB, Matthews KA, et al. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301(647):309-314.
4. Jakovljevic
5. Rogers PJ. A healthy body, a healthy mind: long-term impact of diet on mood and cognitive function. Pro Nutr Soc. 2001;60(1):135-143.
6. Björkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med. 2006;260(6):493-508.
7. Tuem KB, Atey TM. Neuroactive steroids: receptor interactions and responses. Front Neurol. 2017;8:442.
8. Borroni MV, Vallés AS, Barrantes FJ. The lipid habitats of neurotransmitter receptors in the brain. Biochim Biophys Acta. 2016;1858(1):2662-2670.
9. Pfrieger FW. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci. 2003;60(6):1158-1171.
10. Graham I, Atar D, Borch-Johnsen K, et al; European Society of Cardiology (ESC); European Association for Cardiovascular Prevention and Rehabilitation (EACPR); Council on Cardiovascular Nursing; European Association for Study of Diabetes (EASD); International Diabetes Federation Europe (IDF-Europe); European Stroke Initiative (EUSI); Society of Behavioural Medicine (ISBM); European Society of Hypertension (ESH); WONCA Europe (European Society of General Practice/Family Medicine); European Heart Network (EHN); European Atherosclerosis Society (EAS). European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of none societies and by invited experts). Eur J Cardiovasc Prev Rehabil. 2007;14(suppl 2):S1-S113.
11. Almeida-Montes LG, Valles-Sanchez V, Moreno-Aguilar J, et al. Relation of serum cholesterol, lipid, serotonin and tryptophan levels to severity of depression and to suicide attempts. J Psychiatry Neurosci. 2000;25(4):371-377.
12. Ryman A. Cholesterol, violent death, and mental disorder. BMJ. 1994;309(69525):421-422.
13. Wardle J. Cholesterol and psychological well-being. J Psychosom Res. 1995;39(5):549-562.
14. Irribarren C, Reed DM, Chen R, et al. Low serum cholesterol and mortality. Which is the cause and which is the effect? Circulation. 1995;92(9):2396-2403.
15. Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: common links. J Intern Med. 2006;260(3):211-223.
16. Raffai RL, Weisgraber KH. Cholesterol: from heart attacks to Alzheimer’s disease. J Lipid Res. 2003;44(8):1423-1430.
17. Chen CC, Lu FH, Wu JS, et al. Correlation between serum lipid concentrations and psychological distress. Psychiatry Res. 2003;102(2):153-162.
18. Mössmer R, Mikova O, Koutsilieri E, et al. Consensus paper of the WFSBP Task Force on Biological Markers: biological markers in depression. World J Biol Psychiatry. 2007;8(3):141-174.
19. Papakostas GI, Petersen T, Sonawalla SB, et al. Serum cholesterol in treatment-resistant depression. Neuropsychobiology. 2003;47(3):146-151.
20. Gabriel A. Changes in plasma cholesterol in mood disorder patients: does treatment make a difference? J Affect Disord. 2007;99(1-3):273-278.
1. Hallahan B, Garland MR. Essential fatty acids and mental health. British J Psychiatry. 2005;186(4):275-277.
2. Papakostas GI, Ongür D, Iosifescu DV, et al. Cholesterol in mood and anxiety disorders: review of the literature and new hypotheses. Eur Neuropsychopharmacol. 2004;14(2):135-142.
3. Muldoon MF, Manuck SB, Matthews KA, et al. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301(647):309-314.
4. Jakovljevic
5. Rogers PJ. A healthy body, a healthy mind: long-term impact of diet on mood and cognitive function. Pro Nutr Soc. 2001;60(1):135-143.
6. Björkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med. 2006;260(6):493-508.
7. Tuem KB, Atey TM. Neuroactive steroids: receptor interactions and responses. Front Neurol. 2017;8:442.
8. Borroni MV, Vallés AS, Barrantes FJ. The lipid habitats of neurotransmitter receptors in the brain. Biochim Biophys Acta. 2016;1858(1):2662-2670.
9. Pfrieger FW. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci. 2003;60(6):1158-1171.
10. Graham I, Atar D, Borch-Johnsen K, et al; European Society of Cardiology (ESC); European Association for Cardiovascular Prevention and Rehabilitation (EACPR); Council on Cardiovascular Nursing; European Association for Study of Diabetes (EASD); International Diabetes Federation Europe (IDF-Europe); European Stroke Initiative (EUSI); Society of Behavioural Medicine (ISBM); European Society of Hypertension (ESH); WONCA Europe (European Society of General Practice/Family Medicine); European Heart Network (EHN); European Atherosclerosis Society (EAS). European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of none societies and by invited experts). Eur J Cardiovasc Prev Rehabil. 2007;14(suppl 2):S1-S113.
11. Almeida-Montes LG, Valles-Sanchez V, Moreno-Aguilar J, et al. Relation of serum cholesterol, lipid, serotonin and tryptophan levels to severity of depression and to suicide attempts. J Psychiatry Neurosci. 2000;25(4):371-377.
12. Ryman A. Cholesterol, violent death, and mental disorder. BMJ. 1994;309(69525):421-422.
13. Wardle J. Cholesterol and psychological well-being. J Psychosom Res. 1995;39(5):549-562.
14. Irribarren C, Reed DM, Chen R, et al. Low serum cholesterol and mortality. Which is the cause and which is the effect? Circulation. 1995;92(9):2396-2403.
15. Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: common links. J Intern Med. 2006;260(3):211-223.
16. Raffai RL, Weisgraber KH. Cholesterol: from heart attacks to Alzheimer’s disease. J Lipid Res. 2003;44(8):1423-1430.
17. Chen CC, Lu FH, Wu JS, et al. Correlation between serum lipid concentrations and psychological distress. Psychiatry Res. 2003;102(2):153-162.
18. Mössmer R, Mikova O, Koutsilieri E, et al. Consensus paper of the WFSBP Task Force on Biological Markers: biological markers in depression. World J Biol Psychiatry. 2007;8(3):141-174.
19. Papakostas GI, Petersen T, Sonawalla SB, et al. Serum cholesterol in treatment-resistant depression. Neuropsychobiology. 2003;47(3):146-151.
20. Gabriel A. Changes in plasma cholesterol in mood disorder patients: does treatment make a difference? J Affect Disord. 2007;99(1-3):273-278.
Using pharmacogenetics guidelines when prescribing: What’s available
Ms. C, age 45, has a history of generalized anxiety disorder, which has been controlled for the past 6 weeks with extended-release (ER) venlafaxine, 37.5 mg/d. Previous medication trials included fluvoxamine, 300 mg/d, for 2 weeks; paroxetine, 20 mg/d, for 1 week; sertraline, 100 mg/d, for 1 week; and citalopram, 20 mg/d, for 2 weeks. For each trial, Ms. C was unable to tolerate standard doses because of substantial adverse effects; she complained that her anxiety would significantly worsen with each course of treatment. Although the adverse effects would eventually subside with continued treatment, they appeared to be the dose-limiting factor for treatment, even when much lower doses were started.
Ms. C’s son recently suggested that she undergo pharmacogenomics testing, and she brings in the results of this testing. The report states that Ms. C has cytochrome P450 (CYP) pharmacogenotypes CYP2D6 *5/*9, CYP2C19 *2/*3, CYP2C9 *2/*2, and CYP1A2 *1A/*1F. Ms. C wants to know if these results explain some of the issues she has had with previous medication trials, and if these results mean that she should be taking a different medication.
The human genome project was a vast, international effort to sequence the entire human genome1 and identify individual differences in drug response, which serves as the basis for pharmacogenomics. Since completion of the human genome project in the early 2000s, the field of pharmacogenomics has advanced, and using pharmacogenomic testing to make therapeutic decisions for medication management is becoming commonplace.2 Although this critical change to how medicine is practiced is exciting, implementation of pharmacogenomics into practice has been varied.2 Therefore, having an understanding of the resources available to guide pharmacogenomics into practice is critical, because the FDA now lists >160 medications that include specific pharmacogenomics information within their package insert.3
CPIC provides guidance for implementing pharmacogenomics
In 2000, the National Institutes of Health established the Pharmacogenomics Knowledge Base (PharmGKB) and the Pharmacogenomics Research Network (PGRN). These 2 resources provide information from cutting-edge research on genomic variation and therapeutic and adverse events, as well as practical implementation of this research.4 As part of their partnership, PharmGKB and PGRN established the Clinical Pharmacogenomics Implementation Consortium (CPIC), which has begun to provide clinical practice guidelines for implementing pharmacogenomic results. Although CPIC does not advocate for pharmacogenomics testing as a standard, it recognizes that this testing is becoming more commonplace, and therefore its guidelines can help clinicians make rational prescribing decisions.4
In a recent partnership among several PGRN members, investigators found that 1 out of 4 pharmacogenomic test results had a potential clinically actionable outcome.2 There are currently >43 gene/drug pairs for which CPIC has provided guidelines; however, >200 other gene/drug pairs are being evaluated.5
Table 15 lists the current CPIC gene/drug combinations with accompanying published guidelines that are pertinent to psychiatry. For each of these guidelines, experts reviewed the available literature to provide graded therapeutic recommendations: A (“preponderance of evidence is high or moderate in favor of changing prescribing”), B (“preponderance of evidence is weak with little conflicting data”), and C and D (“evidence levels can vary”).4 Looking at the specific genotypes for Ms. C, we can use the information within the CPIC to assign a drug metabolism phenotype for her genotype combinations (Table 2).6
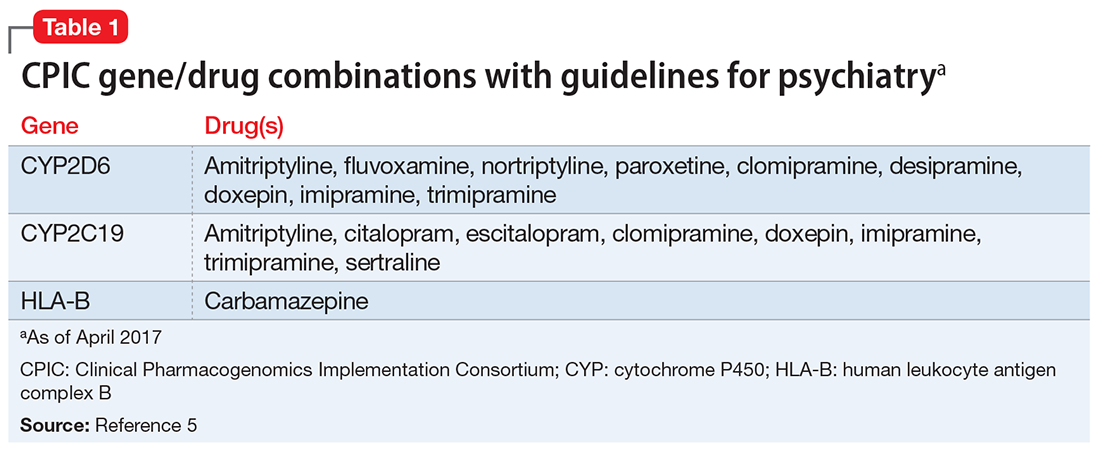
Consider additional resources
In addition to those from the CPIC, guidelines have been developed by other scientific groups, such as the Dutch Pharmacogenetics Working Group and the European Pharmacogenomics Implementation Consortium. Although most of these guidelines are concordant with CPIC, differences exist, which makes it important to be aware of all available resources.
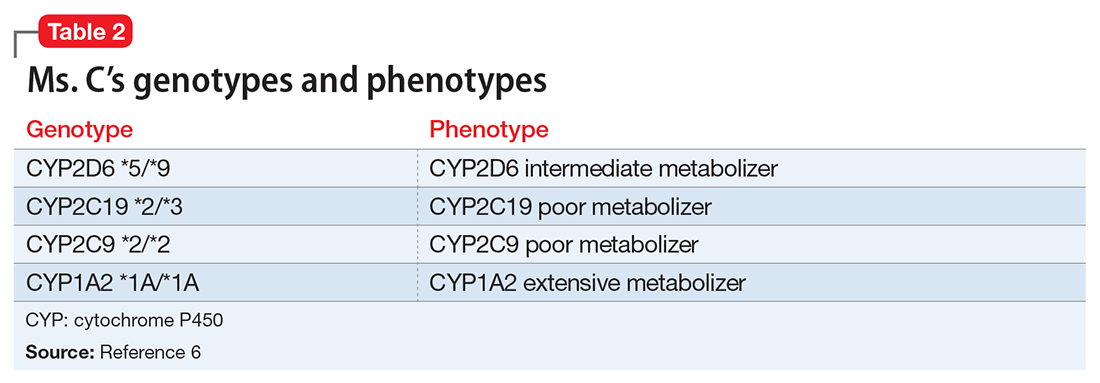
As well as working on the CPIC guidelines, PGRN investigators also provide numerous free online educational resources related to the principles behind pharmacogenomics, including additional resources necessary for systematic implementation. Examples include tables that outline all possible diplotypes (genotypes) for genes in the guidelines and how these are related to the metabolic phenotypes.2,4 Drug metabolizing phenotypes, for example, often are described as poor, intermediate, extensive, and ultra-rapid; in this system, metabolizing ability labeled as poor is less-than-average, and ultra-rapid describes greater-than-average ability. The extensive phenotype is considered average. The data files on the CPIC Web site also can be used as resources to “double check” interpretation results for the diplotype phenotype combinations currently available from various pharmacogenomics companies.7
Based on Ms. C’s presentation, as well as information from the CPIC guidelines, we expect that she might experience substantial adverse effects from most selective serotonin reuptake inhibitors and tricycle antidepressants because of her intermediate metabolizer status for CYP2D6 and poor metabolizer status for CYP2C19. The CPIC’s recommendation for using paroxetine and fluvoxamine in patients with a CYP2D6 intermediate metabolism phenotype is to initiate the recommend starting dose, but acknowledge that reduced metabolic capacity through CYP2D6 may result in higher blood levels and greater probability of adverse drug reactions. For a patient with the CYP2C19 poor metabolizer phenotype, the recommendation is to reduce the starting dose of citalopram or sertraline by 50%, or to prescribe a drug that is not metabolized by CYP2C19.8 Therefore, this pharmacogenomic information may help us understand why Ms. C is unable to tolerate these medications.
Although the CPIC guidelines do not address venlafaxine, the PharmGKB Web site contains literature supporting CYP2D6 as important in venlafaxine metabolism. Current recommendations from the Dutch Pharmacogenetics Working Group Guidelines9 are to either use a non–CYP2D6 metabolized medication or to adjust the dose to clinical response. Because Ms. C has been taking venlafaxine ER for the last 6 weeks and is taking a relatively low but effective dose, our recommendation is to continue current therapy.
It is also important to consider drug interactions when interpreting pharmacogenomic test results. In Ms. C’s case, the impact of a CYP2D6 intermediate metabolism phenotype would be increased if she also was taking a strong CYP2D6 inhibitor such as bupropion. Pharmacogenomics is another clinical tool and discontinuation of an effective treatment that is adequately tolerated should not be done based on pharmacogenomics recommendations alone.
1. Collins FS, Patrinos A, Jordan E, et al. New goals for the U.S. Human Genome Project: 1998-2003. Science. 1998;282(5389):682-689.
2. Luzum JA, Pakyz RE, Elsey AR, et al; Pharmacogenomics Research Network Translational Pharmacogenetics Program. The Pharmacogenomics Research Network Translational Pharmacogenetics Program: outcomes and metrics of pharmacogenetic implementations across diverse healthcare systems. Clin Pharmacol Ther. 2017;102(3):502-510.
3. U.S. Food and Drug Administration. Table of pharmacogenomic biomarkers in drug labeling. https://www.fda.gov/Drugs/ScienceResearch/ucm572698.htm. Updated October 3, 2017. Accessed October 23, 2017.
4. Caudle KE, Gammal RS, Whirl-Carrillo M, et al. Evidence and resources to implement pharmacogenetic knowledge for precision medicine. Am J Health Syst Pharm. 2016;73(23):1977-1985.
5. Clinical Pharmacogenomics Implementation Consortium. Genes-drugs. https://cpicpgx.org/genes-drugs. Updated October 2, 2017. Accessed October 23, 2017.
6. PharmGKB. PGx gene-specific information tables. https://www.pharmgkb.org/page/pgxGeneRef. Accessed October 27, 2017.
7. Whirl-Carrillo M, McDonagh EM, Hebert JM, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414-417.
8. Hicks JK, Bishop JR, Sangkuhl K, et al; Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98(2):127-134.
9
Ms. C, age 45, has a history of generalized anxiety disorder, which has been controlled for the past 6 weeks with extended-release (ER) venlafaxine, 37.5 mg/d. Previous medication trials included fluvoxamine, 300 mg/d, for 2 weeks; paroxetine, 20 mg/d, for 1 week; sertraline, 100 mg/d, for 1 week; and citalopram, 20 mg/d, for 2 weeks. For each trial, Ms. C was unable to tolerate standard doses because of substantial adverse effects; she complained that her anxiety would significantly worsen with each course of treatment. Although the adverse effects would eventually subside with continued treatment, they appeared to be the dose-limiting factor for treatment, even when much lower doses were started.
Ms. C’s son recently suggested that she undergo pharmacogenomics testing, and she brings in the results of this testing. The report states that Ms. C has cytochrome P450 (CYP) pharmacogenotypes CYP2D6 *5/*9, CYP2C19 *2/*3, CYP2C9 *2/*2, and CYP1A2 *1A/*1F. Ms. C wants to know if these results explain some of the issues she has had with previous medication trials, and if these results mean that she should be taking a different medication.
The human genome project was a vast, international effort to sequence the entire human genome1 and identify individual differences in drug response, which serves as the basis for pharmacogenomics. Since completion of the human genome project in the early 2000s, the field of pharmacogenomics has advanced, and using pharmacogenomic testing to make therapeutic decisions for medication management is becoming commonplace.2 Although this critical change to how medicine is practiced is exciting, implementation of pharmacogenomics into practice has been varied.2 Therefore, having an understanding of the resources available to guide pharmacogenomics into practice is critical, because the FDA now lists >160 medications that include specific pharmacogenomics information within their package insert.3
CPIC provides guidance for implementing pharmacogenomics
In 2000, the National Institutes of Health established the Pharmacogenomics Knowledge Base (PharmGKB) and the Pharmacogenomics Research Network (PGRN). These 2 resources provide information from cutting-edge research on genomic variation and therapeutic and adverse events, as well as practical implementation of this research.4 As part of their partnership, PharmGKB and PGRN established the Clinical Pharmacogenomics Implementation Consortium (CPIC), which has begun to provide clinical practice guidelines for implementing pharmacogenomic results. Although CPIC does not advocate for pharmacogenomics testing as a standard, it recognizes that this testing is becoming more commonplace, and therefore its guidelines can help clinicians make rational prescribing decisions.4
In a recent partnership among several PGRN members, investigators found that 1 out of 4 pharmacogenomic test results had a potential clinically actionable outcome.2 There are currently >43 gene/drug pairs for which CPIC has provided guidelines; however, >200 other gene/drug pairs are being evaluated.5
Table 15 lists the current CPIC gene/drug combinations with accompanying published guidelines that are pertinent to psychiatry. For each of these guidelines, experts reviewed the available literature to provide graded therapeutic recommendations: A (“preponderance of evidence is high or moderate in favor of changing prescribing”), B (“preponderance of evidence is weak with little conflicting data”), and C and D (“evidence levels can vary”).4 Looking at the specific genotypes for Ms. C, we can use the information within the CPIC to assign a drug metabolism phenotype for her genotype combinations (Table 2).6

Consider additional resources
In addition to those from the CPIC, guidelines have been developed by other scientific groups, such as the Dutch Pharmacogenetics Working Group and the European Pharmacogenomics Implementation Consortium. Although most of these guidelines are concordant with CPIC, differences exist, which makes it important to be aware of all available resources.

As well as working on the CPIC guidelines, PGRN investigators also provide numerous free online educational resources related to the principles behind pharmacogenomics, including additional resources necessary for systematic implementation. Examples include tables that outline all possible diplotypes (genotypes) for genes in the guidelines and how these are related to the metabolic phenotypes.2,4 Drug metabolizing phenotypes, for example, often are described as poor, intermediate, extensive, and ultra-rapid; in this system, metabolizing ability labeled as poor is less-than-average, and ultra-rapid describes greater-than-average ability. The extensive phenotype is considered average. The data files on the CPIC Web site also can be used as resources to “double check” interpretation results for the diplotype phenotype combinations currently available from various pharmacogenomics companies.7
Based on Ms. C’s presentation, as well as information from the CPIC guidelines, we expect that she might experience substantial adverse effects from most selective serotonin reuptake inhibitors and tricycle antidepressants because of her intermediate metabolizer status for CYP2D6 and poor metabolizer status for CYP2C19. The CPIC’s recommendation for using paroxetine and fluvoxamine in patients with a CYP2D6 intermediate metabolism phenotype is to initiate the recommend starting dose, but acknowledge that reduced metabolic capacity through CYP2D6 may result in higher blood levels and greater probability of adverse drug reactions. For a patient with the CYP2C19 poor metabolizer phenotype, the recommendation is to reduce the starting dose of citalopram or sertraline by 50%, or to prescribe a drug that is not metabolized by CYP2C19.8 Therefore, this pharmacogenomic information may help us understand why Ms. C is unable to tolerate these medications.
Although the CPIC guidelines do not address venlafaxine, the PharmGKB Web site contains literature supporting CYP2D6 as important in venlafaxine metabolism. Current recommendations from the Dutch Pharmacogenetics Working Group Guidelines9 are to either use a non–CYP2D6 metabolized medication or to adjust the dose to clinical response. Because Ms. C has been taking venlafaxine ER for the last 6 weeks and is taking a relatively low but effective dose, our recommendation is to continue current therapy.
It is also important to consider drug interactions when interpreting pharmacogenomic test results. In Ms. C’s case, the impact of a CYP2D6 intermediate metabolism phenotype would be increased if she also was taking a strong CYP2D6 inhibitor such as bupropion. Pharmacogenomics is another clinical tool and discontinuation of an effective treatment that is adequately tolerated should not be done based on pharmacogenomics recommendations alone.
Ms. C, age 45, has a history of generalized anxiety disorder, which has been controlled for the past 6 weeks with extended-release (ER) venlafaxine, 37.5 mg/d. Previous medication trials included fluvoxamine, 300 mg/d, for 2 weeks; paroxetine, 20 mg/d, for 1 week; sertraline, 100 mg/d, for 1 week; and citalopram, 20 mg/d, for 2 weeks. For each trial, Ms. C was unable to tolerate standard doses because of substantial adverse effects; she complained that her anxiety would significantly worsen with each course of treatment. Although the adverse effects would eventually subside with continued treatment, they appeared to be the dose-limiting factor for treatment, even when much lower doses were started.
Ms. C’s son recently suggested that she undergo pharmacogenomics testing, and she brings in the results of this testing. The report states that Ms. C has cytochrome P450 (CYP) pharmacogenotypes CYP2D6 *5/*9, CYP2C19 *2/*3, CYP2C9 *2/*2, and CYP1A2 *1A/*1F. Ms. C wants to know if these results explain some of the issues she has had with previous medication trials, and if these results mean that she should be taking a different medication.
The human genome project was a vast, international effort to sequence the entire human genome1 and identify individual differences in drug response, which serves as the basis for pharmacogenomics. Since completion of the human genome project in the early 2000s, the field of pharmacogenomics has advanced, and using pharmacogenomic testing to make therapeutic decisions for medication management is becoming commonplace.2 Although this critical change to how medicine is practiced is exciting, implementation of pharmacogenomics into practice has been varied.2 Therefore, having an understanding of the resources available to guide pharmacogenomics into practice is critical, because the FDA now lists >160 medications that include specific pharmacogenomics information within their package insert.3
CPIC provides guidance for implementing pharmacogenomics
In 2000, the National Institutes of Health established the Pharmacogenomics Knowledge Base (PharmGKB) and the Pharmacogenomics Research Network (PGRN). These 2 resources provide information from cutting-edge research on genomic variation and therapeutic and adverse events, as well as practical implementation of this research.4 As part of their partnership, PharmGKB and PGRN established the Clinical Pharmacogenomics Implementation Consortium (CPIC), which has begun to provide clinical practice guidelines for implementing pharmacogenomic results. Although CPIC does not advocate for pharmacogenomics testing as a standard, it recognizes that this testing is becoming more commonplace, and therefore its guidelines can help clinicians make rational prescribing decisions.4
In a recent partnership among several PGRN members, investigators found that 1 out of 4 pharmacogenomic test results had a potential clinically actionable outcome.2 There are currently >43 gene/drug pairs for which CPIC has provided guidelines; however, >200 other gene/drug pairs are being evaluated.5
Table 15 lists the current CPIC gene/drug combinations with accompanying published guidelines that are pertinent to psychiatry. For each of these guidelines, experts reviewed the available literature to provide graded therapeutic recommendations: A (“preponderance of evidence is high or moderate in favor of changing prescribing”), B (“preponderance of evidence is weak with little conflicting data”), and C and D (“evidence levels can vary”).4 Looking at the specific genotypes for Ms. C, we can use the information within the CPIC to assign a drug metabolism phenotype for her genotype combinations (Table 2).6

Consider additional resources
In addition to those from the CPIC, guidelines have been developed by other scientific groups, such as the Dutch Pharmacogenetics Working Group and the European Pharmacogenomics Implementation Consortium. Although most of these guidelines are concordant with CPIC, differences exist, which makes it important to be aware of all available resources.

As well as working on the CPIC guidelines, PGRN investigators also provide numerous free online educational resources related to the principles behind pharmacogenomics, including additional resources necessary for systematic implementation. Examples include tables that outline all possible diplotypes (genotypes) for genes in the guidelines and how these are related to the metabolic phenotypes.2,4 Drug metabolizing phenotypes, for example, often are described as poor, intermediate, extensive, and ultra-rapid; in this system, metabolizing ability labeled as poor is less-than-average, and ultra-rapid describes greater-than-average ability. The extensive phenotype is considered average. The data files on the CPIC Web site also can be used as resources to “double check” interpretation results for the diplotype phenotype combinations currently available from various pharmacogenomics companies.7
Based on Ms. C’s presentation, as well as information from the CPIC guidelines, we expect that she might experience substantial adverse effects from most selective serotonin reuptake inhibitors and tricycle antidepressants because of her intermediate metabolizer status for CYP2D6 and poor metabolizer status for CYP2C19. The CPIC’s recommendation for using paroxetine and fluvoxamine in patients with a CYP2D6 intermediate metabolism phenotype is to initiate the recommend starting dose, but acknowledge that reduced metabolic capacity through CYP2D6 may result in higher blood levels and greater probability of adverse drug reactions. For a patient with the CYP2C19 poor metabolizer phenotype, the recommendation is to reduce the starting dose of citalopram or sertraline by 50%, or to prescribe a drug that is not metabolized by CYP2C19.8 Therefore, this pharmacogenomic information may help us understand why Ms. C is unable to tolerate these medications.
Although the CPIC guidelines do not address venlafaxine, the PharmGKB Web site contains literature supporting CYP2D6 as important in venlafaxine metabolism. Current recommendations from the Dutch Pharmacogenetics Working Group Guidelines9 are to either use a non–CYP2D6 metabolized medication or to adjust the dose to clinical response. Because Ms. C has been taking venlafaxine ER for the last 6 weeks and is taking a relatively low but effective dose, our recommendation is to continue current therapy.
It is also important to consider drug interactions when interpreting pharmacogenomic test results. In Ms. C’s case, the impact of a CYP2D6 intermediate metabolism phenotype would be increased if she also was taking a strong CYP2D6 inhibitor such as bupropion. Pharmacogenomics is another clinical tool and discontinuation of an effective treatment that is adequately tolerated should not be done based on pharmacogenomics recommendations alone.
1. Collins FS, Patrinos A, Jordan E, et al. New goals for the U.S. Human Genome Project: 1998-2003. Science. 1998;282(5389):682-689.
2. Luzum JA, Pakyz RE, Elsey AR, et al; Pharmacogenomics Research Network Translational Pharmacogenetics Program. The Pharmacogenomics Research Network Translational Pharmacogenetics Program: outcomes and metrics of pharmacogenetic implementations across diverse healthcare systems. Clin Pharmacol Ther. 2017;102(3):502-510.
3. U.S. Food and Drug Administration. Table of pharmacogenomic biomarkers in drug labeling. https://www.fda.gov/Drugs/ScienceResearch/ucm572698.htm. Updated October 3, 2017. Accessed October 23, 2017.
4. Caudle KE, Gammal RS, Whirl-Carrillo M, et al. Evidence and resources to implement pharmacogenetic knowledge for precision medicine. Am J Health Syst Pharm. 2016;73(23):1977-1985.
5. Clinical Pharmacogenomics Implementation Consortium. Genes-drugs. https://cpicpgx.org/genes-drugs. Updated October 2, 2017. Accessed October 23, 2017.
6. PharmGKB. PGx gene-specific information tables. https://www.pharmgkb.org/page/pgxGeneRef. Accessed October 27, 2017.
7. Whirl-Carrillo M, McDonagh EM, Hebert JM, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414-417.
8. Hicks JK, Bishop JR, Sangkuhl K, et al; Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98(2):127-134.
9
1. Collins FS, Patrinos A, Jordan E, et al. New goals for the U.S. Human Genome Project: 1998-2003. Science. 1998;282(5389):682-689.
2. Luzum JA, Pakyz RE, Elsey AR, et al; Pharmacogenomics Research Network Translational Pharmacogenetics Program. The Pharmacogenomics Research Network Translational Pharmacogenetics Program: outcomes and metrics of pharmacogenetic implementations across diverse healthcare systems. Clin Pharmacol Ther. 2017;102(3):502-510.
3. U.S. Food and Drug Administration. Table of pharmacogenomic biomarkers in drug labeling. https://www.fda.gov/Drugs/ScienceResearch/ucm572698.htm. Updated October 3, 2017. Accessed October 23, 2017.
4. Caudle KE, Gammal RS, Whirl-Carrillo M, et al. Evidence and resources to implement pharmacogenetic knowledge for precision medicine. Am J Health Syst Pharm. 2016;73(23):1977-1985.
5. Clinical Pharmacogenomics Implementation Consortium. Genes-drugs. https://cpicpgx.org/genes-drugs. Updated October 2, 2017. Accessed October 23, 2017.
6. PharmGKB. PGx gene-specific information tables. https://www.pharmgkb.org/page/pgxGeneRef. Accessed October 27, 2017.
7. Whirl-Carrillo M, McDonagh EM, Hebert JM, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414-417.
8. Hicks JK, Bishop JR, Sangkuhl K, et al; Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98(2):127-134.
9
Career Choices: State hospital psychiatry
Editor’s note: Career Choices is a new feature of Residents’ Voices. It features a psychiatry resident/fellow interviewing a psychiatrist about why he (she) has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths. Future installments will feature interviews with psychiatrists who have focused their careers on consultation-liaison psychiatry, academic psychiatry, rural psychiatry, and other career paths.
In this first Career Choices, Cornel Stanciu, MD, talked with Samantha Gnanasegaram, MD, a state hospital psychiatrist at New Hampshire Hospital, where she treats severe and chronic mental illness and testifies in various court proceedings.
Dr. Stanciu: What made you choose to become a state hospital psychiatrist?
Dr. Gnanasegaram: When I started thinking about career options after residency, I knew I wanted to start my career in a facility where I could be challenged, remain up-to-date with the most current evidence-based literature, and have the support and mentorship of seasoned psychiatrists in the field. The opportunity to work under the auspices of a great academic institution with the “bread and butter” of psychiatry reminds me every day why I chose the field in the first place. The often chronic and sometimes refractory cases I encounter daily are extremely thought-provoking, and they motivate me to think and pursue more complex management options. [This setting] also enables me to work closely as [part of] an interdisciplinary team with nursing, social work, and recreational and occupational therapy in ensuring these individuals get the best care and aftercare plans.
We often forget that psychosis often takes weeks to respond [to treatment]. Unfortunately, often in private hospitals, the longer stays that are necessary for patient care are not always possible, leading to premature psychotropic changes and discharge. In this setting, I am able to practice medicine based on what is best for the patient from an evidence-based standpoint. Additionally, being in the state system also allows me to learn first-hand and work closely with the legal system in this state and to testify in various settings to ensure my patients get the best possible care.
Dr. Stanciu: How did your career path prepare you to become a state hospital psychiatrist?
Dr. Gnanasegaram: During my residency, I had exposure to the affiliated state psychiatric hospital and spent some time on various units, each geared toward different patient populations. I also became very familiar with a wide range of psychotropics, ranging from first-line to second- and third-tier medications, as well as off-label. The ECT exposure as well as Crisis Prevention Institute training in how to deal with violent and aggressive individuals certainly added extra layers to my proficiency.
Dr. Stanciu: How would you describe a physician who is well-suited for such a setting?
Dr. Gnanasegaram: This setting is great for someone who likes to be challenged and stay current with literature. Furthermore, this is a great setting for those who are comfortable with the use of medications such as [clozapine] and long-acting injectables, and procedures such as ECT. Additionally, an ideal candidate is someone who understands the chronicity and complexity of mental illness, and has the patience to follow the course and does not rush to make drastic changes or panics at the first sign of a patient taking a step back.
A good candidate also should be comfortable with medical comorbidities, because severe mental illness often leads to poor self-care, diabetes, hypertension, etc., and should be able to work effectively in a team setting and interact with other specialties. State hospital physicians need to be cognizant of outpatient resources available to prevent decompensation in the community and not only focus on acute stabilization. Additionally, this is a great setting for those who enjoy working in an interdisciplinary team and learning from the expertise of different members of a treatment team.
Dr. Stanciu: What challenges and surprises did you encounter when you first began to practice in this setting?
Dr. Gnanasegaram: When I started, the biggest challenge was learning about the differences in practice and legislature in a different state, because all states vary in their involuntary commitment laws, process, and ability to institute forced medications. Learning this as well as how they apply to my practice occurred quicker than I anticipated. As I started practicing, I became more proficient in being able to incorporate the resources I have available.
Dr. Stanciu: What are the disadvantages compared with other branches of psychiatry?
Dr. Gnanasegaram: This is a subjective question. Some physicians may desire a rapid turnaround of patients, which is not always the case in state psychiatric hospitals. Even at discharge, some patients may have low-functioning baselines, requiring guardianship and/or placement in a more supervised setting to ensure they receive the care they need. It is also important to realize these are primarily not voluntary patients, but rather patients committed here involuntarily for treatment due to impaired insight and judgment. At times, the acuity can be high, but the potential for violence is mitigated through comprehensive risk assessments, staff training, and prevention strategies to help ensure patient and staff safety.
Dr. Stanciu: What advice do you have for early career psychiatrists and trainees who are contemplating a state hospital career?
Dr. Gnanasegaram: I would recommend seeking exposure to working in a state psychiatric hospital early in your training so you can see the daily routine and protocol. It would help to obtain mentorship from a state hospital psychiatrist in the state where you intend to work. Ask as many questions as needed and seek their insight into the challenges and benefits of working there. During training, it’s important to familiarize yourself with managing difficult and refractory cases, and don’t shy away from challenging patients. The next step would be to apply for a position of interest to interview and learn more about the facility and the staff that you will be working with.
Dr. Stanciu: How important is the academic affiliation?
Dr. Gnanasegaram: Very important. Especially during the early phase of your career, it is important to have at your fingertips senior mentors and to be involved in the conferences and CME activities offered. This ensures good quality measures in patient care. The academic affiliation helps keep you up-to-date with advancements and maintains an atmosphere that fosters ongoing learning and the best possible care for your patients. Working with trainees at various levels, such as medical students, residents, and fellows, allows you to maintain an evidence-based practice approach as well as share your knowledge and experience with those in training. Being in this academic setting, you also have the opportunity for involvement in research activities and publications.
Editor’s note: Career Choices is a new feature of Residents’ Voices. It features a psychiatry resident/fellow interviewing a psychiatrist about why he (she) has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths. Future installments will feature interviews with psychiatrists who have focused their careers on consultation-liaison psychiatry, academic psychiatry, rural psychiatry, and other career paths.
In this first Career Choices, Cornel Stanciu, MD, talked with Samantha Gnanasegaram, MD, a state hospital psychiatrist at New Hampshire Hospital, where she treats severe and chronic mental illness and testifies in various court proceedings.
Dr. Stanciu: What made you choose to become a state hospital psychiatrist?
Dr. Gnanasegaram: When I started thinking about career options after residency, I knew I wanted to start my career in a facility where I could be challenged, remain up-to-date with the most current evidence-based literature, and have the support and mentorship of seasoned psychiatrists in the field. The opportunity to work under the auspices of a great academic institution with the “bread and butter” of psychiatry reminds me every day why I chose the field in the first place. The often chronic and sometimes refractory cases I encounter daily are extremely thought-provoking, and they motivate me to think and pursue more complex management options. [This setting] also enables me to work closely as [part of] an interdisciplinary team with nursing, social work, and recreational and occupational therapy in ensuring these individuals get the best care and aftercare plans.
We often forget that psychosis often takes weeks to respond [to treatment]. Unfortunately, often in private hospitals, the longer stays that are necessary for patient care are not always possible, leading to premature psychotropic changes and discharge. In this setting, I am able to practice medicine based on what is best for the patient from an evidence-based standpoint. Additionally, being in the state system also allows me to learn first-hand and work closely with the legal system in this state and to testify in various settings to ensure my patients get the best possible care.
Dr. Stanciu: How did your career path prepare you to become a state hospital psychiatrist?
Dr. Gnanasegaram: During my residency, I had exposure to the affiliated state psychiatric hospital and spent some time on various units, each geared toward different patient populations. I also became very familiar with a wide range of psychotropics, ranging from first-line to second- and third-tier medications, as well as off-label. The ECT exposure as well as Crisis Prevention Institute training in how to deal with violent and aggressive individuals certainly added extra layers to my proficiency.
Dr. Stanciu: How would you describe a physician who is well-suited for such a setting?
Dr. Gnanasegaram: This setting is great for someone who likes to be challenged and stay current with literature. Furthermore, this is a great setting for those who are comfortable with the use of medications such as [clozapine] and long-acting injectables, and procedures such as ECT. Additionally, an ideal candidate is someone who understands the chronicity and complexity of mental illness, and has the patience to follow the course and does not rush to make drastic changes or panics at the first sign of a patient taking a step back.
A good candidate also should be comfortable with medical comorbidities, because severe mental illness often leads to poor self-care, diabetes, hypertension, etc., and should be able to work effectively in a team setting and interact with other specialties. State hospital physicians need to be cognizant of outpatient resources available to prevent decompensation in the community and not only focus on acute stabilization. Additionally, this is a great setting for those who enjoy working in an interdisciplinary team and learning from the expertise of different members of a treatment team.
Dr. Stanciu: What challenges and surprises did you encounter when you first began to practice in this setting?
Dr. Gnanasegaram: When I started, the biggest challenge was learning about the differences in practice and legislature in a different state, because all states vary in their involuntary commitment laws, process, and ability to institute forced medications. Learning this as well as how they apply to my practice occurred quicker than I anticipated. As I started practicing, I became more proficient in being able to incorporate the resources I have available.
Dr. Stanciu: What are the disadvantages compared with other branches of psychiatry?
Dr. Gnanasegaram: This is a subjective question. Some physicians may desire a rapid turnaround of patients, which is not always the case in state psychiatric hospitals. Even at discharge, some patients may have low-functioning baselines, requiring guardianship and/or placement in a more supervised setting to ensure they receive the care they need. It is also important to realize these are primarily not voluntary patients, but rather patients committed here involuntarily for treatment due to impaired insight and judgment. At times, the acuity can be high, but the potential for violence is mitigated through comprehensive risk assessments, staff training, and prevention strategies to help ensure patient and staff safety.
Dr. Stanciu: What advice do you have for early career psychiatrists and trainees who are contemplating a state hospital career?
Dr. Gnanasegaram: I would recommend seeking exposure to working in a state psychiatric hospital early in your training so you can see the daily routine and protocol. It would help to obtain mentorship from a state hospital psychiatrist in the state where you intend to work. Ask as many questions as needed and seek their insight into the challenges and benefits of working there. During training, it’s important to familiarize yourself with managing difficult and refractory cases, and don’t shy away from challenging patients. The next step would be to apply for a position of interest to interview and learn more about the facility and the staff that you will be working with.
Dr. Stanciu: How important is the academic affiliation?
Dr. Gnanasegaram: Very important. Especially during the early phase of your career, it is important to have at your fingertips senior mentors and to be involved in the conferences and CME activities offered. This ensures good quality measures in patient care. The academic affiliation helps keep you up-to-date with advancements and maintains an atmosphere that fosters ongoing learning and the best possible care for your patients. Working with trainees at various levels, such as medical students, residents, and fellows, allows you to maintain an evidence-based practice approach as well as share your knowledge and experience with those in training. Being in this academic setting, you also have the opportunity for involvement in research activities and publications.
Editor’s note: Career Choices is a new feature of Residents’ Voices. It features a psychiatry resident/fellow interviewing a psychiatrist about why he (she) has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths. Future installments will feature interviews with psychiatrists who have focused their careers on consultation-liaison psychiatry, academic psychiatry, rural psychiatry, and other career paths.
In this first Career Choices, Cornel Stanciu, MD, talked with Samantha Gnanasegaram, MD, a state hospital psychiatrist at New Hampshire Hospital, where she treats severe and chronic mental illness and testifies in various court proceedings.
Dr. Stanciu: What made you choose to become a state hospital psychiatrist?
Dr. Gnanasegaram: When I started thinking about career options after residency, I knew I wanted to start my career in a facility where I could be challenged, remain up-to-date with the most current evidence-based literature, and have the support and mentorship of seasoned psychiatrists in the field. The opportunity to work under the auspices of a great academic institution with the “bread and butter” of psychiatry reminds me every day why I chose the field in the first place. The often chronic and sometimes refractory cases I encounter daily are extremely thought-provoking, and they motivate me to think and pursue more complex management options. [This setting] also enables me to work closely as [part of] an interdisciplinary team with nursing, social work, and recreational and occupational therapy in ensuring these individuals get the best care and aftercare plans.
We often forget that psychosis often takes weeks to respond [to treatment]. Unfortunately, often in private hospitals, the longer stays that are necessary for patient care are not always possible, leading to premature psychotropic changes and discharge. In this setting, I am able to practice medicine based on what is best for the patient from an evidence-based standpoint. Additionally, being in the state system also allows me to learn first-hand and work closely with the legal system in this state and to testify in various settings to ensure my patients get the best possible care.
Dr. Stanciu: How did your career path prepare you to become a state hospital psychiatrist?
Dr. Gnanasegaram: During my residency, I had exposure to the affiliated state psychiatric hospital and spent some time on various units, each geared toward different patient populations. I also became very familiar with a wide range of psychotropics, ranging from first-line to second- and third-tier medications, as well as off-label. The ECT exposure as well as Crisis Prevention Institute training in how to deal with violent and aggressive individuals certainly added extra layers to my proficiency.
Dr. Stanciu: How would you describe a physician who is well-suited for such a setting?
Dr. Gnanasegaram: This setting is great for someone who likes to be challenged and stay current with literature. Furthermore, this is a great setting for those who are comfortable with the use of medications such as [clozapine] and long-acting injectables, and procedures such as ECT. Additionally, an ideal candidate is someone who understands the chronicity and complexity of mental illness, and has the patience to follow the course and does not rush to make drastic changes or panics at the first sign of a patient taking a step back.
A good candidate also should be comfortable with medical comorbidities, because severe mental illness often leads to poor self-care, diabetes, hypertension, etc., and should be able to work effectively in a team setting and interact with other specialties. State hospital physicians need to be cognizant of outpatient resources available to prevent decompensation in the community and not only focus on acute stabilization. Additionally, this is a great setting for those who enjoy working in an interdisciplinary team and learning from the expertise of different members of a treatment team.
Dr. Stanciu: What challenges and surprises did you encounter when you first began to practice in this setting?
Dr. Gnanasegaram: When I started, the biggest challenge was learning about the differences in practice and legislature in a different state, because all states vary in their involuntary commitment laws, process, and ability to institute forced medications. Learning this as well as how they apply to my practice occurred quicker than I anticipated. As I started practicing, I became more proficient in being able to incorporate the resources I have available.
Dr. Stanciu: What are the disadvantages compared with other branches of psychiatry?
Dr. Gnanasegaram: This is a subjective question. Some physicians may desire a rapid turnaround of patients, which is not always the case in state psychiatric hospitals. Even at discharge, some patients may have low-functioning baselines, requiring guardianship and/or placement in a more supervised setting to ensure they receive the care they need. It is also important to realize these are primarily not voluntary patients, but rather patients committed here involuntarily for treatment due to impaired insight and judgment. At times, the acuity can be high, but the potential for violence is mitigated through comprehensive risk assessments, staff training, and prevention strategies to help ensure patient and staff safety.
Dr. Stanciu: What advice do you have for early career psychiatrists and trainees who are contemplating a state hospital career?
Dr. Gnanasegaram: I would recommend seeking exposure to working in a state psychiatric hospital early in your training so you can see the daily routine and protocol. It would help to obtain mentorship from a state hospital psychiatrist in the state where you intend to work. Ask as many questions as needed and seek their insight into the challenges and benefits of working there. During training, it’s important to familiarize yourself with managing difficult and refractory cases, and don’t shy away from challenging patients. The next step would be to apply for a position of interest to interview and learn more about the facility and the staff that you will be working with.
Dr. Stanciu: How important is the academic affiliation?
Dr. Gnanasegaram: Very important. Especially during the early phase of your career, it is important to have at your fingertips senior mentors and to be involved in the conferences and CME activities offered. This ensures good quality measures in patient care. The academic affiliation helps keep you up-to-date with advancements and maintains an atmosphere that fosters ongoing learning and the best possible care for your patients. Working with trainees at various levels, such as medical students, residents, and fellows, allows you to maintain an evidence-based practice approach as well as share your knowledge and experience with those in training. Being in this academic setting, you also have the opportunity for involvement in research activities and publications.
A 95-year-old man with treatment-resistant depression
CASE Depressed, avoidant
Mr. R, age 95, has a history of recurrent major depressive disorder. He presents to the emergency department with depressive symptoms that began 6 weeks ago. His symptoms include depressed mood, hopelessness, anhedonia, anxiety, and insomnia. Co-occurring anorexia nervosa has resulted in a 20-lb weight loss. He denies suicidal ideation.
A mental status examination reveals profound psychomotor agitation, dysphoric mood, tearfulness, and mood-congruent delusions. Mr. R’s Mini-Mental State Examination (MMSE) score is 14/30; his Hamilton Depression Rating Scale (HAM-D) score is 21, indicating severe depression (19 to 22). However, the examiner feels that these scores may not reflect an accurate assessment because Mr. R gave flippant responses and did not cooperate during the interview. Physical examination is unremarkable. Previous medication trials included buspirone, escitalopram, and risperidone; none of these medications successfully alleviated his depressive symptoms.
On admission, Mr. R is given oral mirtazapine, 15 mg/d, and quetiapine, 25 mg/d, to target depressive mood, insomnia, and weight loss. Urgent intervention is indicated because his depressive symptoms are profoundly causing failure to thrive and are compromising his physical health. Mr. R’s deterioration concerns the physician team. Because of a history of failed pharmacotherapy trials, the team reassesses Mr. R’s treatment options.
[polldaddy:9903171]
The authors’ observations
The physician team recommends that Mr. R undergo ECT to obtain rapid relief from his depressive symptoms. After discussion of the potential risks and benefits, Mr. R agrees to this treatment. Quetiapine is discontinued prior to initiating ECT to avoid unnecessary medications; mirtazapine is continued.
Mr. R’s lack of response to previous antidepressants and significant deterioration were concerning. The physicians wanted to avoid higher-dose medications because of the risk of falls or somnolence. Their clinical experience and the literature supporting ECT for patients of Mr. R’s age lead them to select ECT as the most appropriate therapeutic option.
ECT has no absolute contraindications.1 The rate of ECT use in the United States has fluctuated over time because of factors unrelated to the efficacy and availability of ECT or alternative treatments.2 This form of intervention is also somewhat stigmatized.
Some psychiatrists are reluctant to prescribe ECT for geriatric patients because of concerns of potential neurocognitive or medical complications and risks during anesthesia. However, in the United States, older patients with depression are more likely to be treated with ECT than their younger counterparts.3 ECT usually induces greater immediate efficacy than antidepressants.4
Evidence supports using ECT in older patients
Multiple studies have found that ECT is a rapid, safe, and efficacious intervention for treating older persons with depression. Patients age >60 who receive ECT plus pharmacotherapy have lower HAM-D scores than those receiving pharmacotherapy alone.5 Overall, the rates of remission for depression range from 50% to 70%; yet geriatric patients who receive only ECT have response rates around 90%.6 Older age, presence of psychotic symptoms, and shorter duration of illness can predict a rapidly positive ECT response.7
When treated with ECT, older patients, including those age >85, have fewer subsequent episodes of depression compared with those who receive pharmacotherapy alone.1 Older individuals with physical illness or cognitive impairment respond to and tolerate ECT much like younger patients.6 Older patients receiving ECT may experience less cognitive decline than younger ones.7 Those in their ninth decade of life with treatment-resistant depression, psychotic features, and post-stroke depression often respond robustly with improvement following ECT.8
Remission rates also depend on the technique of administration. Interactions between electrode placement and stimulus parameter dosage affect efficacy and adverse effects.9 Right-sided, unilateral ECT induces less cognitive dysfunction compared with bilateral electrode placement,9 but bilateral ECT is more clinically effective.10 However, the efficacy of right-sided ECT is more dose-sensitive, and some data suggest that suboptimal response is due to insufficient stimulus dosages.11 One double-blind randomized controlled trial documented that when using a high-dose stimulus parameter, unilateral ECT is as effective as bilateral ECT.12 When there is a suboptimal response to unilateral ECT, bilateral ECT might be beneficial.12,13 For preventing relapse in older patients, increasing the interval between ECT treatments is more effective than stopping ECT abruptly.13
[polldaddy:9903172]
Indications of ECT
ECT is indicated for patients with severe depression, mania, and other conditions (Table).14 The most common indication for ECT in older persons is a history of treatment-resistant depression, with melancholia, psychosis, or suicidal ideations.1-6,12 There are also age-related and clinical factors to consider with ECT. This treatment provides a safe, rapid remission for patients age >65, even after adjusting for somatic conditions, duration of illness, medication resistance, or case severity.15 Compared with younger patients, older adults may not tolerate antidepressants as well because of age-related pharmacokinetic alterations, including increased sensitivity to anticholinergic and/or hypotensive effects.1
Factors that favor ECT include a previous good response to it; patient preference; and an indication for rapid intervention, such as suicidality, catatonia, dehydration, malnutrition, or a suboptimal result from pharmacotherapy.3 Mortality among individuals age >85 who receive ECT reportedly is lower than that among their counterparts who receive alternative treatments.16 ECT has been administered safely and effectively in patients with comorbid medical illnesses such as stroke, cerebral aneurysm, cardiovascular disease with ischemia or arrhythmia, dementia, and osteoporosis.17
Neurocognitive effects
Reports on the effects of ECT on neurocognitive functioning have varied. In some studies, performance improved or did not change in severely depressed older patients who received ECT.18,19 In older people who receive ECT, MMSE scores often return to baseline by the end of treatment.20 There often is only mild transient cognitive impairment in patients with late-life depression who receive ECT. Areas of concern include attention span, orientation, and speed of mental processing.20 Physicians should conduct cognitive tests before, during, and after ECT sessions to monitor their patient’s mental status.20
Cognitive stability can be maintained by administering ECT twice a week; applying right-sided, unilateral electrode placement; and using short, ultra-brief stimulus pulse width parameters.21 Cognitive impairment induced by ECT is not associated with age in geriatric patients with depression.22 Older adults who experienced longer postictal reorientation time periods have better outcomes than others who reach orientation faster; their intellectual impairment returned to baseline.20 Falling is another complication associated with ECT. A longitudinal cohort study found the incident of falls among patients receiving ECT was 13%.22 Risk factors for falls during a course of ECT include the number of treatments and the presence of coexisting Parkinson’s disease.23
OUTCOME Improvement
Mr. R receives 8 sessions of right-sided, unilateral ECT with an individualized dosage titration method. Treatments are completed with a stimulus intensity at 6 times seizure threshold, with an ultra-brief pulse width at 0.3 milliseconds. Mr. R’s mood and affect begin to improve after 3 ECT sessions. His MMSE score increases to 28/30 (Figure). His clinical improvement is progressively sustained; he develops an increasingly jovial attitude and experiences less anxiety. Mr. R’s confidence, appetite, and sleep also improve. There are no complications with treatment, and Mr. R has no complaints. After 8 ECT sessions, Mr. R has no affective symptoms and does not experience any cognitive impairment.
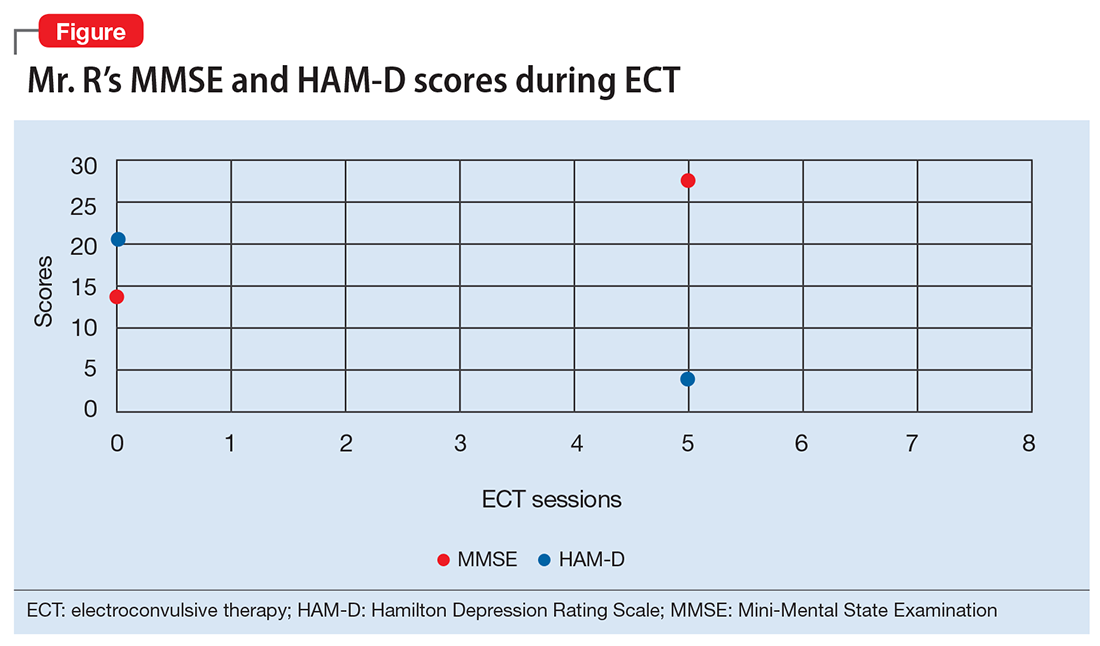
The authors’ observations
Depression among older people is a growing public health concern. It is a leading cause of disability, and often leads to nursing home placement.24 ECT is a safe, effective treatment for late-life depression, but is underutilized in patients age >75 because of concerns for cognitive impairment.6 However, there is evidence that response rates to ECT are higher in patients ages 45 to 85, compared with young individuals ages 18 to 45.25 ECT is a viable intervention for older depressed patients, particularly for those who do not tolerate or fail to respond to pharmacotherapy. Many of these patients are at risk for drug-induced toxicities or interactions or suicide.1
1. Kerner N, Prudic J. Current electroconvulsive therapy practice and research in the geriatric population. Neuropsychiatry (London). 2014;4(1):33-54.
2. Dombrovski AY, Mulsant BH. The evidence for electroconvulsive therapy (ECT) in the treatment of severe late-life depression. ECT: the preferred treatment for severe depression in late life. Int Psychogeriatr. 2007;19(1):10-14,27-35; discussion 24-26.
3. Olfson M, Marcus S, Sackeim HA, et al. Use of ECT for the inpatient treatment of recurrent major depression. Am J Psychiatry. 1998;155(1):22-29.
4. Salzman C, Wong E, Wright BC. Drug and ECT treatment of depression in the elderly, 1996-2001: a literature review. Biol Psychiatry. 2002;52(3):265-284.
5. Kellner CH, Husain MM, Knapp RG, et al; CORE/PRIDE Work Group. A novel strategy for continuation ect in geriatric depression: phase 2 of the PRIDE study. A
6. Tew JD Jr, Mulsant BH, Haskett RF, et al. Acute efficacy of ECT in the treatment of major depression in the old-old. Am J Psychiatry. 1999;156(12):1865-1870.
7. Dombrovski AY, Mulsant BH, Haskett RF, et al. Predictors of remission after electroconvulsive therapy in unipolar major depression. J Clin Psychiatry. 2005;66(8):1043-1049.
8. Charles K. UpToDate. Unipolar major depression in adults: indications for efficacy of electroconvulsive therapy (ECT). https://www.uptodate.com/contents/unipolar-major-depression-in-adults-indications-for-and-efficacy-of-electroconvulsive-therapy-ect. Updated May 16, 2017. Accessed November 26, 2017.
9. Sackeim HA, Prudic J, Devanand DP, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57(5):425-434.
10. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
11. Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357(19):1939-1945.
12. Stoppe A, Louzã M, Rosa M, et al. Fixed high dose electroconvulsive therapy in elderly with depression: a double-blind, randomized comparison of efficacy and tolerability between unilateral and bilateral electrode placement. J ECT. 2006;22(2):92-99.
13. Geduldig ET, Kellner CH. Electroconvulsive therapy in the elderly: new findings in geriatric depression. Curr Psychiatry Rep. 2016;18(4):40.
14. Practice guideline for the treatment of patients with major depressive disorder (revision). American Psychiatric Association. Am J Psychiatry. 2000;157(suppl 4):1-45.
15. Rhebergen D, Huisman A, Bouckaert F, et al. Older age is associated with rapid remission of depression after electroconvulsive therapy: a latent class growth analysis. Am J Geriatr Psychiatry. 2015;23(3):274-282.
16. Philibert RA, Richards L, Lynch CF, et al. Effect of ECT on mortality and clinical outcome in geriatric unipolar depression. J Clin Psychiatry. 1995;56(9):390-394.
17. Tomac TA, Rummans TA, Pileggi TS, et al. Safety and efficacy of electroconvulsive therapy in patients over age 85. Am J Geriatr Psychiatry. 1997;5(2):126-130.
18. Verwijk E, Comijs HC, Kok RM, et al. Short and long-term neurocognitive functioning after electroconvulsive therapy in depressed elderly: a prospective naturalistic study. Int Psychogeriatr. 2014;26(2):315-324.
19. Flint AJ, Gagnon N. Effective use of electroconvulsive therapy in late life depression. Can J Psychiatry. 2002;47(8):734-741.
20. Bjolseth TM, Engedal K, Benth JS, et al. Speed of recovery from disorientation may predict the treatment outcome of electroconvulsive therapy (ECT) in elderly patients with major depression. J Affect Disord. 2016;190:178-186.
21. Sackeim HA, Prudic J, Nobler MS, et al. Ultra-brief pulse ECT and the affective and cognitive consequences of ECT. J ECT. 2001;17(1):77.
22. Bjolseth TM, Engedal K, Benth JS, et al. Baseline cognitive function does not predict the treatment outcome of electroconvulsive therapy (ECT) in late-life depression. J Affect Disord. 2015;185:67-75.
23. de Carle AJ, Kohn R. Electroconvulsive therapy and falls in the elderly. J ECT. 2000;16(3):252-257.
24. Hoover DR, Siegel M, Lucas J, et al. Depression in the first year of stay for elderly long-term nursing home residents in the USA. Int Psychogeriatr. 2010;22:1161.
25. O’Connor MK, Knapp R, Husain M, et al. The influence of age on the response of major depression to electroconvulsive therapy: a C.O.R.E. Report. Am J Geriatr Psychiatry. 2001; 9:382.
CASE Depressed, avoidant
Mr. R, age 95, has a history of recurrent major depressive disorder. He presents to the emergency department with depressive symptoms that began 6 weeks ago. His symptoms include depressed mood, hopelessness, anhedonia, anxiety, and insomnia. Co-occurring anorexia nervosa has resulted in a 20-lb weight loss. He denies suicidal ideation.
A mental status examination reveals profound psychomotor agitation, dysphoric mood, tearfulness, and mood-congruent delusions. Mr. R’s Mini-Mental State Examination (MMSE) score is 14/30; his Hamilton Depression Rating Scale (HAM-D) score is 21, indicating severe depression (19 to 22). However, the examiner feels that these scores may not reflect an accurate assessment because Mr. R gave flippant responses and did not cooperate during the interview. Physical examination is unremarkable. Previous medication trials included buspirone, escitalopram, and risperidone; none of these medications successfully alleviated his depressive symptoms.
On admission, Mr. R is given oral mirtazapine, 15 mg/d, and quetiapine, 25 mg/d, to target depressive mood, insomnia, and weight loss. Urgent intervention is indicated because his depressive symptoms are profoundly causing failure to thrive and are compromising his physical health. Mr. R’s deterioration concerns the physician team. Because of a history of failed pharmacotherapy trials, the team reassesses Mr. R’s treatment options.
[polldaddy:9903171]
The authors’ observations
The physician team recommends that Mr. R undergo ECT to obtain rapid relief from his depressive symptoms. After discussion of the potential risks and benefits, Mr. R agrees to this treatment. Quetiapine is discontinued prior to initiating ECT to avoid unnecessary medications; mirtazapine is continued.
Mr. R’s lack of response to previous antidepressants and significant deterioration were concerning. The physicians wanted to avoid higher-dose medications because of the risk of falls or somnolence. Their clinical experience and the literature supporting ECT for patients of Mr. R’s age lead them to select ECT as the most appropriate therapeutic option.
ECT has no absolute contraindications.1 The rate of ECT use in the United States has fluctuated over time because of factors unrelated to the efficacy and availability of ECT or alternative treatments.2 This form of intervention is also somewhat stigmatized.
Some psychiatrists are reluctant to prescribe ECT for geriatric patients because of concerns of potential neurocognitive or medical complications and risks during anesthesia. However, in the United States, older patients with depression are more likely to be treated with ECT than their younger counterparts.3 ECT usually induces greater immediate efficacy than antidepressants.4
Evidence supports using ECT in older patients
Multiple studies have found that ECT is a rapid, safe, and efficacious intervention for treating older persons with depression. Patients age >60 who receive ECT plus pharmacotherapy have lower HAM-D scores than those receiving pharmacotherapy alone.5 Overall, the rates of remission for depression range from 50% to 70%; yet geriatric patients who receive only ECT have response rates around 90%.6 Older age, presence of psychotic symptoms, and shorter duration of illness can predict a rapidly positive ECT response.7
When treated with ECT, older patients, including those age >85, have fewer subsequent episodes of depression compared with those who receive pharmacotherapy alone.1 Older individuals with physical illness or cognitive impairment respond to and tolerate ECT much like younger patients.6 Older patients receiving ECT may experience less cognitive decline than younger ones.7 Those in their ninth decade of life with treatment-resistant depression, psychotic features, and post-stroke depression often respond robustly with improvement following ECT.8
Remission rates also depend on the technique of administration. Interactions between electrode placement and stimulus parameter dosage affect efficacy and adverse effects.9 Right-sided, unilateral ECT induces less cognitive dysfunction compared with bilateral electrode placement,9 but bilateral ECT is more clinically effective.10 However, the efficacy of right-sided ECT is more dose-sensitive, and some data suggest that suboptimal response is due to insufficient stimulus dosages.11 One double-blind randomized controlled trial documented that when using a high-dose stimulus parameter, unilateral ECT is as effective as bilateral ECT.12 When there is a suboptimal response to unilateral ECT, bilateral ECT might be beneficial.12,13 For preventing relapse in older patients, increasing the interval between ECT treatments is more effective than stopping ECT abruptly.13
[polldaddy:9903172]
Indications of ECT
ECT is indicated for patients with severe depression, mania, and other conditions (Table).14 The most common indication for ECT in older persons is a history of treatment-resistant depression, with melancholia, psychosis, or suicidal ideations.1-6,12 There are also age-related and clinical factors to consider with ECT. This treatment provides a safe, rapid remission for patients age >65, even after adjusting for somatic conditions, duration of illness, medication resistance, or case severity.15 Compared with younger patients, older adults may not tolerate antidepressants as well because of age-related pharmacokinetic alterations, including increased sensitivity to anticholinergic and/or hypotensive effects.1
Factors that favor ECT include a previous good response to it; patient preference; and an indication for rapid intervention, such as suicidality, catatonia, dehydration, malnutrition, or a suboptimal result from pharmacotherapy.3 Mortality among individuals age >85 who receive ECT reportedly is lower than that among their counterparts who receive alternative treatments.16 ECT has been administered safely and effectively in patients with comorbid medical illnesses such as stroke, cerebral aneurysm, cardiovascular disease with ischemia or arrhythmia, dementia, and osteoporosis.17
Neurocognitive effects
Reports on the effects of ECT on neurocognitive functioning have varied. In some studies, performance improved or did not change in severely depressed older patients who received ECT.18,19 In older people who receive ECT, MMSE scores often return to baseline by the end of treatment.20 There often is only mild transient cognitive impairment in patients with late-life depression who receive ECT. Areas of concern include attention span, orientation, and speed of mental processing.20 Physicians should conduct cognitive tests before, during, and after ECT sessions to monitor their patient’s mental status.20
Cognitive stability can be maintained by administering ECT twice a week; applying right-sided, unilateral electrode placement; and using short, ultra-brief stimulus pulse width parameters.21 Cognitive impairment induced by ECT is not associated with age in geriatric patients with depression.22 Older adults who experienced longer postictal reorientation time periods have better outcomes than others who reach orientation faster; their intellectual impairment returned to baseline.20 Falling is another complication associated with ECT. A longitudinal cohort study found the incident of falls among patients receiving ECT was 13%.22 Risk factors for falls during a course of ECT include the number of treatments and the presence of coexisting Parkinson’s disease.23
OUTCOME Improvement
Mr. R receives 8 sessions of right-sided, unilateral ECT with an individualized dosage titration method. Treatments are completed with a stimulus intensity at 6 times seizure threshold, with an ultra-brief pulse width at 0.3 milliseconds. Mr. R’s mood and affect begin to improve after 3 ECT sessions. His MMSE score increases to 28/30 (Figure). His clinical improvement is progressively sustained; he develops an increasingly jovial attitude and experiences less anxiety. Mr. R’s confidence, appetite, and sleep also improve. There are no complications with treatment, and Mr. R has no complaints. After 8 ECT sessions, Mr. R has no affective symptoms and does not experience any cognitive impairment.

The authors’ observations
Depression among older people is a growing public health concern. It is a leading cause of disability, and often leads to nursing home placement.24 ECT is a safe, effective treatment for late-life depression, but is underutilized in patients age >75 because of concerns for cognitive impairment.6 However, there is evidence that response rates to ECT are higher in patients ages 45 to 85, compared with young individuals ages 18 to 45.25 ECT is a viable intervention for older depressed patients, particularly for those who do not tolerate or fail to respond to pharmacotherapy. Many of these patients are at risk for drug-induced toxicities or interactions or suicide.1
CASE Depressed, avoidant
Mr. R, age 95, has a history of recurrent major depressive disorder. He presents to the emergency department with depressive symptoms that began 6 weeks ago. His symptoms include depressed mood, hopelessness, anhedonia, anxiety, and insomnia. Co-occurring anorexia nervosa has resulted in a 20-lb weight loss. He denies suicidal ideation.
A mental status examination reveals profound psychomotor agitation, dysphoric mood, tearfulness, and mood-congruent delusions. Mr. R’s Mini-Mental State Examination (MMSE) score is 14/30; his Hamilton Depression Rating Scale (HAM-D) score is 21, indicating severe depression (19 to 22). However, the examiner feels that these scores may not reflect an accurate assessment because Mr. R gave flippant responses and did not cooperate during the interview. Physical examination is unremarkable. Previous medication trials included buspirone, escitalopram, and risperidone; none of these medications successfully alleviated his depressive symptoms.
On admission, Mr. R is given oral mirtazapine, 15 mg/d, and quetiapine, 25 mg/d, to target depressive mood, insomnia, and weight loss. Urgent intervention is indicated because his depressive symptoms are profoundly causing failure to thrive and are compromising his physical health. Mr. R’s deterioration concerns the physician team. Because of a history of failed pharmacotherapy trials, the team reassesses Mr. R’s treatment options.
[polldaddy:9903171]
The authors’ observations
The physician team recommends that Mr. R undergo ECT to obtain rapid relief from his depressive symptoms. After discussion of the potential risks and benefits, Mr. R agrees to this treatment. Quetiapine is discontinued prior to initiating ECT to avoid unnecessary medications; mirtazapine is continued.
Mr. R’s lack of response to previous antidepressants and significant deterioration were concerning. The physicians wanted to avoid higher-dose medications because of the risk of falls or somnolence. Their clinical experience and the literature supporting ECT for patients of Mr. R’s age lead them to select ECT as the most appropriate therapeutic option.
ECT has no absolute contraindications.1 The rate of ECT use in the United States has fluctuated over time because of factors unrelated to the efficacy and availability of ECT or alternative treatments.2 This form of intervention is also somewhat stigmatized.
Some psychiatrists are reluctant to prescribe ECT for geriatric patients because of concerns of potential neurocognitive or medical complications and risks during anesthesia. However, in the United States, older patients with depression are more likely to be treated with ECT than their younger counterparts.3 ECT usually induces greater immediate efficacy than antidepressants.4
Evidence supports using ECT in older patients
Multiple studies have found that ECT is a rapid, safe, and efficacious intervention for treating older persons with depression. Patients age >60 who receive ECT plus pharmacotherapy have lower HAM-D scores than those receiving pharmacotherapy alone.5 Overall, the rates of remission for depression range from 50% to 70%; yet geriatric patients who receive only ECT have response rates around 90%.6 Older age, presence of psychotic symptoms, and shorter duration of illness can predict a rapidly positive ECT response.7
When treated with ECT, older patients, including those age >85, have fewer subsequent episodes of depression compared with those who receive pharmacotherapy alone.1 Older individuals with physical illness or cognitive impairment respond to and tolerate ECT much like younger patients.6 Older patients receiving ECT may experience less cognitive decline than younger ones.7 Those in their ninth decade of life with treatment-resistant depression, psychotic features, and post-stroke depression often respond robustly with improvement following ECT.8
Remission rates also depend on the technique of administration. Interactions between electrode placement and stimulus parameter dosage affect efficacy and adverse effects.9 Right-sided, unilateral ECT induces less cognitive dysfunction compared with bilateral electrode placement,9 but bilateral ECT is more clinically effective.10 However, the efficacy of right-sided ECT is more dose-sensitive, and some data suggest that suboptimal response is due to insufficient stimulus dosages.11 One double-blind randomized controlled trial documented that when using a high-dose stimulus parameter, unilateral ECT is as effective as bilateral ECT.12 When there is a suboptimal response to unilateral ECT, bilateral ECT might be beneficial.12,13 For preventing relapse in older patients, increasing the interval between ECT treatments is more effective than stopping ECT abruptly.13
[polldaddy:9903172]
Indications of ECT
ECT is indicated for patients with severe depression, mania, and other conditions (Table).14 The most common indication for ECT in older persons is a history of treatment-resistant depression, with melancholia, psychosis, or suicidal ideations.1-6,12 There are also age-related and clinical factors to consider with ECT. This treatment provides a safe, rapid remission for patients age >65, even after adjusting for somatic conditions, duration of illness, medication resistance, or case severity.15 Compared with younger patients, older adults may not tolerate antidepressants as well because of age-related pharmacokinetic alterations, including increased sensitivity to anticholinergic and/or hypotensive effects.1
Factors that favor ECT include a previous good response to it; patient preference; and an indication for rapid intervention, such as suicidality, catatonia, dehydration, malnutrition, or a suboptimal result from pharmacotherapy.3 Mortality among individuals age >85 who receive ECT reportedly is lower than that among their counterparts who receive alternative treatments.16 ECT has been administered safely and effectively in patients with comorbid medical illnesses such as stroke, cerebral aneurysm, cardiovascular disease with ischemia or arrhythmia, dementia, and osteoporosis.17
Neurocognitive effects
Reports on the effects of ECT on neurocognitive functioning have varied. In some studies, performance improved or did not change in severely depressed older patients who received ECT.18,19 In older people who receive ECT, MMSE scores often return to baseline by the end of treatment.20 There often is only mild transient cognitive impairment in patients with late-life depression who receive ECT. Areas of concern include attention span, orientation, and speed of mental processing.20 Physicians should conduct cognitive tests before, during, and after ECT sessions to monitor their patient’s mental status.20
Cognitive stability can be maintained by administering ECT twice a week; applying right-sided, unilateral electrode placement; and using short, ultra-brief stimulus pulse width parameters.21 Cognitive impairment induced by ECT is not associated with age in geriatric patients with depression.22 Older adults who experienced longer postictal reorientation time periods have better outcomes than others who reach orientation faster; their intellectual impairment returned to baseline.20 Falling is another complication associated with ECT. A longitudinal cohort study found the incident of falls among patients receiving ECT was 13%.22 Risk factors for falls during a course of ECT include the number of treatments and the presence of coexisting Parkinson’s disease.23
OUTCOME Improvement
Mr. R receives 8 sessions of right-sided, unilateral ECT with an individualized dosage titration method. Treatments are completed with a stimulus intensity at 6 times seizure threshold, with an ultra-brief pulse width at 0.3 milliseconds. Mr. R’s mood and affect begin to improve after 3 ECT sessions. His MMSE score increases to 28/30 (Figure). His clinical improvement is progressively sustained; he develops an increasingly jovial attitude and experiences less anxiety. Mr. R’s confidence, appetite, and sleep also improve. There are no complications with treatment, and Mr. R has no complaints. After 8 ECT sessions, Mr. R has no affective symptoms and does not experience any cognitive impairment.

The authors’ observations
Depression among older people is a growing public health concern. It is a leading cause of disability, and often leads to nursing home placement.24 ECT is a safe, effective treatment for late-life depression, but is underutilized in patients age >75 because of concerns for cognitive impairment.6 However, there is evidence that response rates to ECT are higher in patients ages 45 to 85, compared with young individuals ages 18 to 45.25 ECT is a viable intervention for older depressed patients, particularly for those who do not tolerate or fail to respond to pharmacotherapy. Many of these patients are at risk for drug-induced toxicities or interactions or suicide.1
1. Kerner N, Prudic J. Current electroconvulsive therapy practice and research in the geriatric population. Neuropsychiatry (London). 2014;4(1):33-54.
2. Dombrovski AY, Mulsant BH. The evidence for electroconvulsive therapy (ECT) in the treatment of severe late-life depression. ECT: the preferred treatment for severe depression in late life. Int Psychogeriatr. 2007;19(1):10-14,27-35; discussion 24-26.
3. Olfson M, Marcus S, Sackeim HA, et al. Use of ECT for the inpatient treatment of recurrent major depression. Am J Psychiatry. 1998;155(1):22-29.
4. Salzman C, Wong E, Wright BC. Drug and ECT treatment of depression in the elderly, 1996-2001: a literature review. Biol Psychiatry. 2002;52(3):265-284.
5. Kellner CH, Husain MM, Knapp RG, et al; CORE/PRIDE Work Group. A novel strategy for continuation ect in geriatric depression: phase 2 of the PRIDE study. A
6. Tew JD Jr, Mulsant BH, Haskett RF, et al. Acute efficacy of ECT in the treatment of major depression in the old-old. Am J Psychiatry. 1999;156(12):1865-1870.
7. Dombrovski AY, Mulsant BH, Haskett RF, et al. Predictors of remission after electroconvulsive therapy in unipolar major depression. J Clin Psychiatry. 2005;66(8):1043-1049.
8. Charles K. UpToDate. Unipolar major depression in adults: indications for efficacy of electroconvulsive therapy (ECT). https://www.uptodate.com/contents/unipolar-major-depression-in-adults-indications-for-and-efficacy-of-electroconvulsive-therapy-ect. Updated May 16, 2017. Accessed November 26, 2017.
9. Sackeim HA, Prudic J, Devanand DP, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57(5):425-434.
10. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
11. Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357(19):1939-1945.
12. Stoppe A, Louzã M, Rosa M, et al. Fixed high dose electroconvulsive therapy in elderly with depression: a double-blind, randomized comparison of efficacy and tolerability between unilateral and bilateral electrode placement. J ECT. 2006;22(2):92-99.
13. Geduldig ET, Kellner CH. Electroconvulsive therapy in the elderly: new findings in geriatric depression. Curr Psychiatry Rep. 2016;18(4):40.
14. Practice guideline for the treatment of patients with major depressive disorder (revision). American Psychiatric Association. Am J Psychiatry. 2000;157(suppl 4):1-45.
15. Rhebergen D, Huisman A, Bouckaert F, et al. Older age is associated with rapid remission of depression after electroconvulsive therapy: a latent class growth analysis. Am J Geriatr Psychiatry. 2015;23(3):274-282.
16. Philibert RA, Richards L, Lynch CF, et al. Effect of ECT on mortality and clinical outcome in geriatric unipolar depression. J Clin Psychiatry. 1995;56(9):390-394.
17. Tomac TA, Rummans TA, Pileggi TS, et al. Safety and efficacy of electroconvulsive therapy in patients over age 85. Am J Geriatr Psychiatry. 1997;5(2):126-130.
18. Verwijk E, Comijs HC, Kok RM, et al. Short and long-term neurocognitive functioning after electroconvulsive therapy in depressed elderly: a prospective naturalistic study. Int Psychogeriatr. 2014;26(2):315-324.
19. Flint AJ, Gagnon N. Effective use of electroconvulsive therapy in late life depression. Can J Psychiatry. 2002;47(8):734-741.
20. Bjolseth TM, Engedal K, Benth JS, et al. Speed of recovery from disorientation may predict the treatment outcome of electroconvulsive therapy (ECT) in elderly patients with major depression. J Affect Disord. 2016;190:178-186.
21. Sackeim HA, Prudic J, Nobler MS, et al. Ultra-brief pulse ECT and the affective and cognitive consequences of ECT. J ECT. 2001;17(1):77.
22. Bjolseth TM, Engedal K, Benth JS, et al. Baseline cognitive function does not predict the treatment outcome of electroconvulsive therapy (ECT) in late-life depression. J Affect Disord. 2015;185:67-75.
23. de Carle AJ, Kohn R. Electroconvulsive therapy and falls in the elderly. J ECT. 2000;16(3):252-257.
24. Hoover DR, Siegel M, Lucas J, et al. Depression in the first year of stay for elderly long-term nursing home residents in the USA. Int Psychogeriatr. 2010;22:1161.
25. O’Connor MK, Knapp R, Husain M, et al. The influence of age on the response of major depression to electroconvulsive therapy: a C.O.R.E. Report. Am J Geriatr Psychiatry. 2001; 9:382.
1. Kerner N, Prudic J. Current electroconvulsive therapy practice and research in the geriatric population. Neuropsychiatry (London). 2014;4(1):33-54.
2. Dombrovski AY, Mulsant BH. The evidence for electroconvulsive therapy (ECT) in the treatment of severe late-life depression. ECT: the preferred treatment for severe depression in late life. Int Psychogeriatr. 2007;19(1):10-14,27-35; discussion 24-26.
3. Olfson M, Marcus S, Sackeim HA, et al. Use of ECT for the inpatient treatment of recurrent major depression. Am J Psychiatry. 1998;155(1):22-29.
4. Salzman C, Wong E, Wright BC. Drug and ECT treatment of depression in the elderly, 1996-2001: a literature review. Biol Psychiatry. 2002;52(3):265-284.
5. Kellner CH, Husain MM, Knapp RG, et al; CORE/PRIDE Work Group. A novel strategy for continuation ect in geriatric depression: phase 2 of the PRIDE study. A
6. Tew JD Jr, Mulsant BH, Haskett RF, et al. Acute efficacy of ECT in the treatment of major depression in the old-old. Am J Psychiatry. 1999;156(12):1865-1870.
7. Dombrovski AY, Mulsant BH, Haskett RF, et al. Predictors of remission after electroconvulsive therapy in unipolar major depression. J Clin Psychiatry. 2005;66(8):1043-1049.
8. Charles K. UpToDate. Unipolar major depression in adults: indications for efficacy of electroconvulsive therapy (ECT). https://www.uptodate.com/contents/unipolar-major-depression-in-adults-indications-for-and-efficacy-of-electroconvulsive-therapy-ect. Updated May 16, 2017. Accessed November 26, 2017.
9. Sackeim HA, Prudic J, Devanand DP, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57(5):425-434.
10. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
11. Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357(19):1939-1945.
12. Stoppe A, Louzã M, Rosa M, et al. Fixed high dose electroconvulsive therapy in elderly with depression: a double-blind, randomized comparison of efficacy and tolerability between unilateral and bilateral electrode placement. J ECT. 2006;22(2):92-99.
13. Geduldig ET, Kellner CH. Electroconvulsive therapy in the elderly: new findings in geriatric depression. Curr Psychiatry Rep. 2016;18(4):40.
14. Practice guideline for the treatment of patients with major depressive disorder (revision). American Psychiatric Association. Am J Psychiatry. 2000;157(suppl 4):1-45.
15. Rhebergen D, Huisman A, Bouckaert F, et al. Older age is associated with rapid remission of depression after electroconvulsive therapy: a latent class growth analysis. Am J Geriatr Psychiatry. 2015;23(3):274-282.
16. Philibert RA, Richards L, Lynch CF, et al. Effect of ECT on mortality and clinical outcome in geriatric unipolar depression. J Clin Psychiatry. 1995;56(9):390-394.
17. Tomac TA, Rummans TA, Pileggi TS, et al. Safety and efficacy of electroconvulsive therapy in patients over age 85. Am J Geriatr Psychiatry. 1997;5(2):126-130.
18. Verwijk E, Comijs HC, Kok RM, et al. Short and long-term neurocognitive functioning after electroconvulsive therapy in depressed elderly: a prospective naturalistic study. Int Psychogeriatr. 2014;26(2):315-324.
19. Flint AJ, Gagnon N. Effective use of electroconvulsive therapy in late life depression. Can J Psychiatry. 2002;47(8):734-741.
20. Bjolseth TM, Engedal K, Benth JS, et al. Speed of recovery from disorientation may predict the treatment outcome of electroconvulsive therapy (ECT) in elderly patients with major depression. J Affect Disord. 2016;190:178-186.
21. Sackeim HA, Prudic J, Nobler MS, et al. Ultra-brief pulse ECT and the affective and cognitive consequences of ECT. J ECT. 2001;17(1):77.
22. Bjolseth TM, Engedal K, Benth JS, et al. Baseline cognitive function does not predict the treatment outcome of electroconvulsive therapy (ECT) in late-life depression. J Affect Disord. 2015;185:67-75.
23. de Carle AJ, Kohn R. Electroconvulsive therapy and falls in the elderly. J ECT. 2000;16(3):252-257.
24. Hoover DR, Siegel M, Lucas J, et al. Depression in the first year of stay for elderly long-term nursing home residents in the USA. Int Psychogeriatr. 2010;22:1161.
25. O’Connor MK, Knapp R, Husain M, et al. The influence of age on the response of major depression to electroconvulsive therapy: a C.O.R.E. Report. Am J Geriatr Psychiatry. 2001; 9:382.
The role of psychiatric APRNs
In Dr. Mary Moller’s Guest Editorial “Advancing the role of advanced practice psychiatric nurses in today’s psychiatric workforce” (
Dr. Moller cited a source from the Federal Trade Commission1 that encourages the autonomous practice of APRNs to increase competition. This again implies the false equivalency between physicians and APRNs. Competition implies that the players are providing the same service. If, as nurse practitioners argue, they practice “nursing,” then they are not practicing “medicine.” Physicians and APRNs do not have the same background. Although both are charged with the care of patients, nursing is not medicine, nor should it be. Both are important and needed, but nursing was never designed to be an autonomous practice. According to the American Association of Colleges of Nursing, “Nursing and medicine are distinct health disciplines that prepare clinicians to assume different roles and meet different practice expectations.”2 In fact, the curriculum and requirements to become an APRN vary depending on the program, and some programs do not even require a BSN.3 There are online programs available for earning an APRN degree. Additionally, APRNs are only required to have 500 to 700 total hours of patient care,4 compared with the >10,000 hours physicians have once they have finished a 3-year residency, which when combined with their education amounts to >20,000 hours.5 This doesn’t account for those who have longer residencies or fellowships to further specialize in their area of training.
Dr. Moller’s main argument is that there is a dire shortage of psychiatrists and that the only way to meet this need for more providers is to make APRNs autonomous. However, no data indicate that autonomous practice of mid-level providers leads to an influx of these providers in rural areas, where the need would be greatest. Although current data on this are quite sparse, some studies indicate that the majority practice in urban areas, even in states with independent practice authority.6,7 Dr. Moller cites a source that only reviewed home zip codes of psychiatric APRNs but did not include zip codes of employment.8 Only 13% of psychiatric APRNs live in rural areas across the United States. Therefore, it is a false assertion to state that these APRNs are found primarily in rural and less populated urban areas. It is also false to imply and assume that these APRNs practice in the rural areas.
In 2017, there were 43,157 registered physician applications, with 35,969 active applications for 31,757 residency positions in the United States, and at least 11,400 medical school graduates were unmatched.9 Imagine how much more we could serve our patients by matching these graduates, whose training far surpasses that of a mid-level provider. The Resident Physician Shortage Reduction Act of 2017 aims to address this problem by increasing Medicare-funded graduate medical education (GME) residency programs in the United States.10 We can make a difference by contacting our members of Congress to encourage them to support this bill. In addition, the AMA is advocating to save funding for GME and provides an easy-to-use Web site (https://savegme.org/take-action) to contact your legislators directly to show your support for GME.
Nurse practitioners have tremendous value when their role is a part of a team; however, they should not practice without supervision, and physicians who supervise them absolutely should be providing adequate supervision. I applaud the APA and the AMA for standing up for the practice of medicine and for our patients. I hope that they continue to do so, and I encourage them to increase their efforts.
Laura Kendall, MD
Assistant Professor of Clinical Psychiatry
Department of Psychiatry and Behavioral Sciences
Keck School of Medicine
University of Southern California
Los Angeles, California
References
1. Koslov T; Office of Policy Planning. The doctor (or nurse practitioner) will see you now: competition and the regulation of advanced practice nurses. Federal Trade Commission. https://www.ftc.gov/news-events/blogs/competition-matters/2014/03/doctor-or-nurse-practitioner-will-see-you-now. Published March 7, 2014. Accessed July 26, 2017.
2. American Association of Colleges of Nursing. DNP talking points. http://www.aacnnursing.org/DNP/about/talking-points. Updated July, 2014. Accessed August 12, 2017.
3. Keyes L. MSN without a BSN? MastersInNursing.com. https://www.mastersinnursing.com/msn-without-a-bsn. Accessed August 12, 2017.
4. Iglehart JK. Expanding the role of advanced nurse practitioners—risks and rewards. New Engl J Med. 2013;368(20):1935-1941.
5. Primary Care Coalition. Issue brief: collaboration between physicians and nurses works. Compare the education gaps between primary care physicians and nurse practitioners. http://www.tafp.org/Media/Default/Downloads/advocacy/scope-education.pdf. Published November 1, 2010. Accessed October 11, 2017.
6. American Medical Association. Issue brief: independent nursing practice. https://www.ama-assn.org/system/files/media-browser/premium/arc/ama-issue-brief-independent-nursing-practice.pdf. Updated 2017.
7. Tabor J, Jennings N, Kohler L, et al. The supply of physician assistants, nurse practitioners, and certified nurse midwives in Arizona. University of Arizona. http://azahec.uahs.arizona.edu/sites/default/files/u9/supply_of_pa_np_cnm.pdf. Accessed October 11, 2017.
8. Hanrahan NP, Hartley D. Employment of advanced-practice psychiatric nurses to stem rural mental health workforce shortages. Psychiatr Serv. 2008;59(1):109-111.
9. 2017 NRMP Main Residency Match the largest match on record [press release]. Washington, DC: National Resident Matching Program; March 17, 2017. http://www.nrmp.org/press-release-2017-nrmp-main-residency-match-the-largest-match-on-record. Accessed October 11, 2017.
10. Resident Physician Shortage Reduction Act of 2017, HR 2267, 115th Cong, 1st session (2017).
The author responds
I would like to thank Dr. Kendall for her passionate letter about my editorial and provide the following response. I neither asserted the equivalency of doctors and nurses or that APRNs can do what MDs do. Rather, APRNs are educated to provide highly qualified, specialty-specific advanced practice nursing, according to the tightly regulated scope of practice defined by individual states. As stated in my editorial, psychiatric mental health (PMH) APRNs engage in the practice of advanced practice PMH nursing. Is there overlap with medicine, social work, and psychology? Of course, but we are not criticized by social workers and psychologists when we engage in various psychotherapeutic approaches; rather, we are collegial and refer to each other. Why are we criticized by physicians when we prescribe from our tightly regulated legend drugs or conduct a psychiatric intake and develop a differential diagnosis and formulation that may save a life in the absence of an available psychiatrist? I would offer that PMH-APRNs are proud of their vast history of collegial relationships with psychiatrists, and that in states where turf is not an issue, there is remarkable respect and mutual referrals based on the ultimate need of finding the most appropriate care for a patient and/or family struggling to live with a psychiatric disorder.
Currently, 26 states have legislated independent practice for APRNs. This legislation was passed after decades of compiling data on the safety and efficacy of patient care outcomes in those states, and then was submitted as testimony to the legislature. State legislature decisions often are influenced by the fact that malpractice claims are decreased in areas where APRNs are independent and increased when APRNs are associated with MDs. A 2009 study1 found that between 1991 and 2007—the first 17 years that the National Practitioner Data Bank was in operation—payments were made on behalf of 37% of physicians but only 3.1% of physician assistants (PAs) and 1.5% of nurse practitioners. The study concluded: “There were no observations or trends to suggest that PAs and APNs increase liability. If anything, they may decrease the rate of reporting malpractice and adverse events.”1
To respond to Dr. Kendall’s comment, “nursing was never designed to be an autonomous practice,” nursing at the entry level of registration was originally conceived by Florence Nightingale as an autonomous profession working side-by-side with physicians, each performing different yet complementary aspects of patient care, each answering to a different hierarchy. Her work in the Crimean War attests to the positive effects of nursing on saving soldiers’ lives, which was heretofore unknown due to all the measures she initiated and meticulously documented. This autonomy, however, was gradually usurped in the private sector. Comparing RNs with MDs is like comparing apples with oranges. We would need to compare all MDs with the 3.4 million registered nurses in the United States, and that is not what my editorial addressed.
For >50 years, master’s prepared advanced practice nurses in psychiatry have been independent in their ability to have private practices, initially focusing on the provision of individual, group, and family psychotherapy. Psychiatrists did not object to this because it opened services they were unable to provide. As psychopharmacologic treatments for psychiatric disorders emerged, APRNs who had the minimum of a master’s degree and substantial psychopharmacology education, which was mandated and regulated by states, were gradually allowed to prescribe starting in the late 1970s. Most typically, these practices were in collaboration with or under supervision of an MD, but as data and outcomes were collected, legislatures began to drop this requirement.
Regarding hours, we could compare the >2,000 classroom and clinical hours and years of clinical experience accumulated by PMH-APRNs in their undergraduate and graduate psychiatric nursing curricula with the 60-hour Psychiatric Medicine course taken in the second semester of the first year of medical school.2 For many physicians, this often is the only psychiatric education they receive when going into primary care. When we consider that 70% of psychiatric care is now provided in a primary care setting, we all should be concerned and be attempting to recruit highly qualified PMH-APRNs to assist in the development and delivery of integrated primary care.
Regarding APRNs working in rural areas, Hanrahan and Hartley3 found that psychiatric APRNs were more likely than psychiatrists to live in rural areas. I contend that the issue is not the zip code of the psychiatric APRN, but rather the need to fix the problem of providers not being drawn to practice in rural and underserved populations due to salary.
Promoting autonomy for PMH-APRNs in all states is not the only way to solve the provider supply shortage, but it is a reasonable way. Unfortunately, there will be a shortage of psychiatric providers no matter what we do. Those of us who are dedicated to providing care to this vulnerable population should be finding ways to maximize our efforts and efficiencies to lessen the critical shortage. Anything less only adds to the problem and sends a negative message to the public. If we psychiatric providers cannot be supportive of each discipline practicing to the full scope and authority of their hard-earned licenses, then we are saying that we are more interested in protecting turf than providing desperately needed care.
Mary D. Moller, DNP, ARNP, PMHCNS-BC, CPRP, FAAN
Associate Professor and Coordinator PMH-DNP ProgramPacific Lutheran University School of Nursing
Director of Psychiatric Services
Northwest Integrated Health
Tacoma, Washington
References
1. Hooker RS, Nicholson JG, Le T. Does the employment of physician assistants and nurse practitioners increase liability? Journal of Medical Licensure and Discipline. 2009;95(2): 6-16.
2. Columbia University Medical Center. Medical student education in psychiatry. https://www.columbiapsychiatry.org/education-and-training/medical-student-education-psychiatry. Accessed November 16, 2017.
3. Hanrahan NP, Hartley D. Employment of advanced-practice psychiatric nurses to stem rural mental health workforce shortages. Psychiatr Serv. 2008;59(1):109-111.
In Dr. Mary Moller’s Guest Editorial “Advancing the role of advanced practice psychiatric nurses in today’s psychiatric workforce” (
Dr. Moller cited a source from the Federal Trade Commission1 that encourages the autonomous practice of APRNs to increase competition. This again implies the false equivalency between physicians and APRNs. Competition implies that the players are providing the same service. If, as nurse practitioners argue, they practice “nursing,” then they are not practicing “medicine.” Physicians and APRNs do not have the same background. Although both are charged with the care of patients, nursing is not medicine, nor should it be. Both are important and needed, but nursing was never designed to be an autonomous practice. According to the American Association of Colleges of Nursing, “Nursing and medicine are distinct health disciplines that prepare clinicians to assume different roles and meet different practice expectations.”2 In fact, the curriculum and requirements to become an APRN vary depending on the program, and some programs do not even require a BSN.3 There are online programs available for earning an APRN degree. Additionally, APRNs are only required to have 500 to 700 total hours of patient care,4 compared with the >10,000 hours physicians have once they have finished a 3-year residency, which when combined with their education amounts to >20,000 hours.5 This doesn’t account for those who have longer residencies or fellowships to further specialize in their area of training.
Dr. Moller’s main argument is that there is a dire shortage of psychiatrists and that the only way to meet this need for more providers is to make APRNs autonomous. However, no data indicate that autonomous practice of mid-level providers leads to an influx of these providers in rural areas, where the need would be greatest. Although current data on this are quite sparse, some studies indicate that the majority practice in urban areas, even in states with independent practice authority.6,7 Dr. Moller cites a source that only reviewed home zip codes of psychiatric APRNs but did not include zip codes of employment.8 Only 13% of psychiatric APRNs live in rural areas across the United States. Therefore, it is a false assertion to state that these APRNs are found primarily in rural and less populated urban areas. It is also false to imply and assume that these APRNs practice in the rural areas.
In 2017, there were 43,157 registered physician applications, with 35,969 active applications for 31,757 residency positions in the United States, and at least 11,400 medical school graduates were unmatched.9 Imagine how much more we could serve our patients by matching these graduates, whose training far surpasses that of a mid-level provider. The Resident Physician Shortage Reduction Act of 2017 aims to address this problem by increasing Medicare-funded graduate medical education (GME) residency programs in the United States.10 We can make a difference by contacting our members of Congress to encourage them to support this bill. In addition, the AMA is advocating to save funding for GME and provides an easy-to-use Web site (https://savegme.org/take-action) to contact your legislators directly to show your support for GME.
Nurse practitioners have tremendous value when their role is a part of a team; however, they should not practice without supervision, and physicians who supervise them absolutely should be providing adequate supervision. I applaud the APA and the AMA for standing up for the practice of medicine and for our patients. I hope that they continue to do so, and I encourage them to increase their efforts.
Laura Kendall, MD
Assistant Professor of Clinical Psychiatry
Department of Psychiatry and Behavioral Sciences
Keck School of Medicine
University of Southern California
Los Angeles, California
References
1. Koslov T; Office of Policy Planning. The doctor (or nurse practitioner) will see you now: competition and the regulation of advanced practice nurses. Federal Trade Commission. https://www.ftc.gov/news-events/blogs/competition-matters/2014/03/doctor-or-nurse-practitioner-will-see-you-now. Published March 7, 2014. Accessed July 26, 2017.
2. American Association of Colleges of Nursing. DNP talking points. http://www.aacnnursing.org/DNP/about/talking-points. Updated July, 2014. Accessed August 12, 2017.
3. Keyes L. MSN without a BSN? MastersInNursing.com. https://www.mastersinnursing.com/msn-without-a-bsn. Accessed August 12, 2017.
4. Iglehart JK. Expanding the role of advanced nurse practitioners—risks and rewards. New Engl J Med. 2013;368(20):1935-1941.
5. Primary Care Coalition. Issue brief: collaboration between physicians and nurses works. Compare the education gaps between primary care physicians and nurse practitioners. http://www.tafp.org/Media/Default/Downloads/advocacy/scope-education.pdf. Published November 1, 2010. Accessed October 11, 2017.
6. American Medical Association. Issue brief: independent nursing practice. https://www.ama-assn.org/system/files/media-browser/premium/arc/ama-issue-brief-independent-nursing-practice.pdf. Updated 2017.
7. Tabor J, Jennings N, Kohler L, et al. The supply of physician assistants, nurse practitioners, and certified nurse midwives in Arizona. University of Arizona. http://azahec.uahs.arizona.edu/sites/default/files/u9/supply_of_pa_np_cnm.pdf. Accessed October 11, 2017.
8. Hanrahan NP, Hartley D. Employment of advanced-practice psychiatric nurses to stem rural mental health workforce shortages. Psychiatr Serv. 2008;59(1):109-111.
9. 2017 NRMP Main Residency Match the largest match on record [press release]. Washington, DC: National Resident Matching Program; March 17, 2017. http://www.nrmp.org/press-release-2017-nrmp-main-residency-match-the-largest-match-on-record. Accessed October 11, 2017.
10. Resident Physician Shortage Reduction Act of 2017, HR 2267, 115th Cong, 1st session (2017).
The author responds
I would like to thank Dr. Kendall for her passionate letter about my editorial and provide the following response. I neither asserted the equivalency of doctors and nurses or that APRNs can do what MDs do. Rather, APRNs are educated to provide highly qualified, specialty-specific advanced practice nursing, according to the tightly regulated scope of practice defined by individual states. As stated in my editorial, psychiatric mental health (PMH) APRNs engage in the practice of advanced practice PMH nursing. Is there overlap with medicine, social work, and psychology? Of course, but we are not criticized by social workers and psychologists when we engage in various psychotherapeutic approaches; rather, we are collegial and refer to each other. Why are we criticized by physicians when we prescribe from our tightly regulated legend drugs or conduct a psychiatric intake and develop a differential diagnosis and formulation that may save a life in the absence of an available psychiatrist? I would offer that PMH-APRNs are proud of their vast history of collegial relationships with psychiatrists, and that in states where turf is not an issue, there is remarkable respect and mutual referrals based on the ultimate need of finding the most appropriate care for a patient and/or family struggling to live with a psychiatric disorder.
Currently, 26 states have legislated independent practice for APRNs. This legislation was passed after decades of compiling data on the safety and efficacy of patient care outcomes in those states, and then was submitted as testimony to the legislature. State legislature decisions often are influenced by the fact that malpractice claims are decreased in areas where APRNs are independent and increased when APRNs are associated with MDs. A 2009 study1 found that between 1991 and 2007—the first 17 years that the National Practitioner Data Bank was in operation—payments were made on behalf of 37% of physicians but only 3.1% of physician assistants (PAs) and 1.5% of nurse practitioners. The study concluded: “There were no observations or trends to suggest that PAs and APNs increase liability. If anything, they may decrease the rate of reporting malpractice and adverse events.”1
To respond to Dr. Kendall’s comment, “nursing was never designed to be an autonomous practice,” nursing at the entry level of registration was originally conceived by Florence Nightingale as an autonomous profession working side-by-side with physicians, each performing different yet complementary aspects of patient care, each answering to a different hierarchy. Her work in the Crimean War attests to the positive effects of nursing on saving soldiers’ lives, which was heretofore unknown due to all the measures she initiated and meticulously documented. This autonomy, however, was gradually usurped in the private sector. Comparing RNs with MDs is like comparing apples with oranges. We would need to compare all MDs with the 3.4 million registered nurses in the United States, and that is not what my editorial addressed.
For >50 years, master’s prepared advanced practice nurses in psychiatry have been independent in their ability to have private practices, initially focusing on the provision of individual, group, and family psychotherapy. Psychiatrists did not object to this because it opened services they were unable to provide. As psychopharmacologic treatments for psychiatric disorders emerged, APRNs who had the minimum of a master’s degree and substantial psychopharmacology education, which was mandated and regulated by states, were gradually allowed to prescribe starting in the late 1970s. Most typically, these practices were in collaboration with or under supervision of an MD, but as data and outcomes were collected, legislatures began to drop this requirement.
Regarding hours, we could compare the >2,000 classroom and clinical hours and years of clinical experience accumulated by PMH-APRNs in their undergraduate and graduate psychiatric nursing curricula with the 60-hour Psychiatric Medicine course taken in the second semester of the first year of medical school.2 For many physicians, this often is the only psychiatric education they receive when going into primary care. When we consider that 70% of psychiatric care is now provided in a primary care setting, we all should be concerned and be attempting to recruit highly qualified PMH-APRNs to assist in the development and delivery of integrated primary care.
Regarding APRNs working in rural areas, Hanrahan and Hartley3 found that psychiatric APRNs were more likely than psychiatrists to live in rural areas. I contend that the issue is not the zip code of the psychiatric APRN, but rather the need to fix the problem of providers not being drawn to practice in rural and underserved populations due to salary.
Promoting autonomy for PMH-APRNs in all states is not the only way to solve the provider supply shortage, but it is a reasonable way. Unfortunately, there will be a shortage of psychiatric providers no matter what we do. Those of us who are dedicated to providing care to this vulnerable population should be finding ways to maximize our efforts and efficiencies to lessen the critical shortage. Anything less only adds to the problem and sends a negative message to the public. If we psychiatric providers cannot be supportive of each discipline practicing to the full scope and authority of their hard-earned licenses, then we are saying that we are more interested in protecting turf than providing desperately needed care.
Mary D. Moller, DNP, ARNP, PMHCNS-BC, CPRP, FAAN
Associate Professor and Coordinator PMH-DNP ProgramPacific Lutheran University School of Nursing
Director of Psychiatric Services
Northwest Integrated Health
Tacoma, Washington
References
1. Hooker RS, Nicholson JG, Le T. Does the employment of physician assistants and nurse practitioners increase liability? Journal of Medical Licensure and Discipline. 2009;95(2): 6-16.
2. Columbia University Medical Center. Medical student education in psychiatry. https://www.columbiapsychiatry.org/education-and-training/medical-student-education-psychiatry. Accessed November 16, 2017.
3. Hanrahan NP, Hartley D. Employment of advanced-practice psychiatric nurses to stem rural mental health workforce shortages. Psychiatr Serv. 2008;59(1):109-111.
In Dr. Mary Moller’s Guest Editorial “Advancing the role of advanced practice psychiatric nurses in today’s psychiatric workforce” (
Dr. Moller cited a source from the Federal Trade Commission1 that encourages the autonomous practice of APRNs to increase competition. This again implies the false equivalency between physicians and APRNs. Competition implies that the players are providing the same service. If, as nurse practitioners argue, they practice “nursing,” then they are not practicing “medicine.” Physicians and APRNs do not have the same background. Although both are charged with the care of patients, nursing is not medicine, nor should it be. Both are important and needed, but nursing was never designed to be an autonomous practice. According to the American Association of Colleges of Nursing, “Nursing and medicine are distinct health disciplines that prepare clinicians to assume different roles and meet different practice expectations.”2 In fact, the curriculum and requirements to become an APRN vary depending on the program, and some programs do not even require a BSN.3 There are online programs available for earning an APRN degree. Additionally, APRNs are only required to have 500 to 700 total hours of patient care,4 compared with the >10,000 hours physicians have once they have finished a 3-year residency, which when combined with their education amounts to >20,000 hours.5 This doesn’t account for those who have longer residencies or fellowships to further specialize in their area of training.
Dr. Moller’s main argument is that there is a dire shortage of psychiatrists and that the only way to meet this need for more providers is to make APRNs autonomous. However, no data indicate that autonomous practice of mid-level providers leads to an influx of these providers in rural areas, where the need would be greatest. Although current data on this are quite sparse, some studies indicate that the majority practice in urban areas, even in states with independent practice authority.6,7 Dr. Moller cites a source that only reviewed home zip codes of psychiatric APRNs but did not include zip codes of employment.8 Only 13% of psychiatric APRNs live in rural areas across the United States. Therefore, it is a false assertion to state that these APRNs are found primarily in rural and less populated urban areas. It is also false to imply and assume that these APRNs practice in the rural areas.
In 2017, there were 43,157 registered physician applications, with 35,969 active applications for 31,757 residency positions in the United States, and at least 11,400 medical school graduates were unmatched.9 Imagine how much more we could serve our patients by matching these graduates, whose training far surpasses that of a mid-level provider. The Resident Physician Shortage Reduction Act of 2017 aims to address this problem by increasing Medicare-funded graduate medical education (GME) residency programs in the United States.10 We can make a difference by contacting our members of Congress to encourage them to support this bill. In addition, the AMA is advocating to save funding for GME and provides an easy-to-use Web site (https://savegme.org/take-action) to contact your legislators directly to show your support for GME.
Nurse practitioners have tremendous value when their role is a part of a team; however, they should not practice without supervision, and physicians who supervise them absolutely should be providing adequate supervision. I applaud the APA and the AMA for standing up for the practice of medicine and for our patients. I hope that they continue to do so, and I encourage them to increase their efforts.
Laura Kendall, MD
Assistant Professor of Clinical Psychiatry
Department of Psychiatry and Behavioral Sciences
Keck School of Medicine
University of Southern California
Los Angeles, California
References
1. Koslov T; Office of Policy Planning. The doctor (or nurse practitioner) will see you now: competition and the regulation of advanced practice nurses. Federal Trade Commission. https://www.ftc.gov/news-events/blogs/competition-matters/2014/03/doctor-or-nurse-practitioner-will-see-you-now. Published March 7, 2014. Accessed July 26, 2017.
2. American Association of Colleges of Nursing. DNP talking points. http://www.aacnnursing.org/DNP/about/talking-points. Updated July, 2014. Accessed August 12, 2017.
3. Keyes L. MSN without a BSN? MastersInNursing.com. https://www.mastersinnursing.com/msn-without-a-bsn. Accessed August 12, 2017.
4. Iglehart JK. Expanding the role of advanced nurse practitioners—risks and rewards. New Engl J Med. 2013;368(20):1935-1941.
5. Primary Care Coalition. Issue brief: collaboration between physicians and nurses works. Compare the education gaps between primary care physicians and nurse practitioners. http://www.tafp.org/Media/Default/Downloads/advocacy/scope-education.pdf. Published November 1, 2010. Accessed October 11, 2017.
6. American Medical Association. Issue brief: independent nursing practice. https://www.ama-assn.org/system/files/media-browser/premium/arc/ama-issue-brief-independent-nursing-practice.pdf. Updated 2017.
7. Tabor J, Jennings N, Kohler L, et al. The supply of physician assistants, nurse practitioners, and certified nurse midwives in Arizona. University of Arizona. http://azahec.uahs.arizona.edu/sites/default/files/u9/supply_of_pa_np_cnm.pdf. Accessed October 11, 2017.
8. Hanrahan NP, Hartley D. Employment of advanced-practice psychiatric nurses to stem rural mental health workforce shortages. Psychiatr Serv. 2008;59(1):109-111.
9. 2017 NRMP Main Residency Match the largest match on record [press release]. Washington, DC: National Resident Matching Program; March 17, 2017. http://www.nrmp.org/press-release-2017-nrmp-main-residency-match-the-largest-match-on-record. Accessed October 11, 2017.
10. Resident Physician Shortage Reduction Act of 2017, HR 2267, 115th Cong, 1st session (2017).
The author responds
I would like to thank Dr. Kendall for her passionate letter about my editorial and provide the following response. I neither asserted the equivalency of doctors and nurses or that APRNs can do what MDs do. Rather, APRNs are educated to provide highly qualified, specialty-specific advanced practice nursing, according to the tightly regulated scope of practice defined by individual states. As stated in my editorial, psychiatric mental health (PMH) APRNs engage in the practice of advanced practice PMH nursing. Is there overlap with medicine, social work, and psychology? Of course, but we are not criticized by social workers and psychologists when we engage in various psychotherapeutic approaches; rather, we are collegial and refer to each other. Why are we criticized by physicians when we prescribe from our tightly regulated legend drugs or conduct a psychiatric intake and develop a differential diagnosis and formulation that may save a life in the absence of an available psychiatrist? I would offer that PMH-APRNs are proud of their vast history of collegial relationships with psychiatrists, and that in states where turf is not an issue, there is remarkable respect and mutual referrals based on the ultimate need of finding the most appropriate care for a patient and/or family struggling to live with a psychiatric disorder.
Currently, 26 states have legislated independent practice for APRNs. This legislation was passed after decades of compiling data on the safety and efficacy of patient care outcomes in those states, and then was submitted as testimony to the legislature. State legislature decisions often are influenced by the fact that malpractice claims are decreased in areas where APRNs are independent and increased when APRNs are associated with MDs. A 2009 study1 found that between 1991 and 2007—the first 17 years that the National Practitioner Data Bank was in operation—payments were made on behalf of 37% of physicians but only 3.1% of physician assistants (PAs) and 1.5% of nurse practitioners. The study concluded: “There were no observations or trends to suggest that PAs and APNs increase liability. If anything, they may decrease the rate of reporting malpractice and adverse events.”1
To respond to Dr. Kendall’s comment, “nursing was never designed to be an autonomous practice,” nursing at the entry level of registration was originally conceived by Florence Nightingale as an autonomous profession working side-by-side with physicians, each performing different yet complementary aspects of patient care, each answering to a different hierarchy. Her work in the Crimean War attests to the positive effects of nursing on saving soldiers’ lives, which was heretofore unknown due to all the measures she initiated and meticulously documented. This autonomy, however, was gradually usurped in the private sector. Comparing RNs with MDs is like comparing apples with oranges. We would need to compare all MDs with the 3.4 million registered nurses in the United States, and that is not what my editorial addressed.
For >50 years, master’s prepared advanced practice nurses in psychiatry have been independent in their ability to have private practices, initially focusing on the provision of individual, group, and family psychotherapy. Psychiatrists did not object to this because it opened services they were unable to provide. As psychopharmacologic treatments for psychiatric disorders emerged, APRNs who had the minimum of a master’s degree and substantial psychopharmacology education, which was mandated and regulated by states, were gradually allowed to prescribe starting in the late 1970s. Most typically, these practices were in collaboration with or under supervision of an MD, but as data and outcomes were collected, legislatures began to drop this requirement.
Regarding hours, we could compare the >2,000 classroom and clinical hours and years of clinical experience accumulated by PMH-APRNs in their undergraduate and graduate psychiatric nursing curricula with the 60-hour Psychiatric Medicine course taken in the second semester of the first year of medical school.2 For many physicians, this often is the only psychiatric education they receive when going into primary care. When we consider that 70% of psychiatric care is now provided in a primary care setting, we all should be concerned and be attempting to recruit highly qualified PMH-APRNs to assist in the development and delivery of integrated primary care.
Regarding APRNs working in rural areas, Hanrahan and Hartley3 found that psychiatric APRNs were more likely than psychiatrists to live in rural areas. I contend that the issue is not the zip code of the psychiatric APRN, but rather the need to fix the problem of providers not being drawn to practice in rural and underserved populations due to salary.
Promoting autonomy for PMH-APRNs in all states is not the only way to solve the provider supply shortage, but it is a reasonable way. Unfortunately, there will be a shortage of psychiatric providers no matter what we do. Those of us who are dedicated to providing care to this vulnerable population should be finding ways to maximize our efforts and efficiencies to lessen the critical shortage. Anything less only adds to the problem and sends a negative message to the public. If we psychiatric providers cannot be supportive of each discipline practicing to the full scope and authority of their hard-earned licenses, then we are saying that we are more interested in protecting turf than providing desperately needed care.
Mary D. Moller, DNP, ARNP, PMHCNS-BC, CPRP, FAAN
Associate Professor and Coordinator PMH-DNP ProgramPacific Lutheran University School of Nursing
Director of Psychiatric Services
Northwest Integrated Health
Tacoma, Washington
References
1. Hooker RS, Nicholson JG, Le T. Does the employment of physician assistants and nurse practitioners increase liability? Journal of Medical Licensure and Discipline. 2009;95(2): 6-16.
2. Columbia University Medical Center. Medical student education in psychiatry. https://www.columbiapsychiatry.org/education-and-training/medical-student-education-psychiatry. Accessed November 16, 2017.
3. Hanrahan NP, Hartley D. Employment of advanced-practice psychiatric nurses to stem rural mental health workforce shortages. Psychiatr Serv. 2008;59(1):109-111.
Yoga for psychiatrists
Being a psychiatrist today often entails long hours immersed in charts or on computers, a lack of fresh air, and eating meals in a hurry. Being on call, facing deadline pressures, and juggling multiple responsibilities can lead to fatigue, frustration, and a lack of adequate socialization. These circumstances can take their toll on us in unpleasant and unhealthy ways, resulting in exhaustion, illness, an
What is yoga?
Yoga is an ancient practice that originated in India thousands of years ago. It was introduced to the West in the 19th century. Yoga is a holistic lifestyle of well-being that includes physical and meditative practices. Today, the most popular forms of yoga typically incorporate a combination of physical postures, controlled breathing, deep relaxation, and/or meditation.2
How to begin yoga practice
Start slow and simple.
- develop balance, endurance, strength, flexibility, and coordination
- release chronic muscular tension
- rejuvenate the body.
Explore different schools. Over time, numerous schools of yoga have evolved. They vary from gentle to strenuous, with an emphasis on postures, breath work, meditation, singing, or a combination of these skills. Choose what feels good and safe based on your personal preference and physical ability.
Be mindful. Focusing solely on the present moment calms the mind and increases awareness. Meditative practice can sharpen clarity and focus. Meditation can involve focusing your attention on sounds, images, or inspirational words or phrases. Each of our movements can invite self-respect and further awareness of the daily toll that modern life places on our minds and bodies. Active breath work is believed to cultivate vitality. Calm breath work and meditative practices help still the mind and decrease physiologic overarousal.
Stay consistent. Regardless of your physical ability or level of mobility, consistent yoga practice is necessary to realize its benefits. Therefore, a weekly class may be a good way to start. Eventually, a good goal is to practice twice a day, at dawn and dusk.
Appreciate the experience. Immerse yourself in each moment of yoga practice. There is no need to rush. Enjoy your journey!
1. Harvard Mental Health Letter. Yoga for anxiety and depression. Harvard Health Publishing. https://www.health.harvard.edu/mind-and-mood/yoga-for-anxiety-and-depression. Updated September 18, 2017. Accessed November 21, 2017.
2. Balasubramaniam M, Telles S, Doraiswamy PM. Yoga on our minds: a systematic review of yoga for neuropsychiatric disorders. Front Psychiatry. 2013;3:117. doi: 10.3389/fpsyt.2012.00117.
Being a psychiatrist today often entails long hours immersed in charts or on computers, a lack of fresh air, and eating meals in a hurry. Being on call, facing deadline pressures, and juggling multiple responsibilities can lead to fatigue, frustration, and a lack of adequate socialization. These circumstances can take their toll on us in unpleasant and unhealthy ways, resulting in exhaustion, illness, an
What is yoga?
Yoga is an ancient practice that originated in India thousands of years ago. It was introduced to the West in the 19th century. Yoga is a holistic lifestyle of well-being that includes physical and meditative practices. Today, the most popular forms of yoga typically incorporate a combination of physical postures, controlled breathing, deep relaxation, and/or meditation.2
How to begin yoga practice
Start slow and simple.
- develop balance, endurance, strength, flexibility, and coordination
- release chronic muscular tension
- rejuvenate the body.
Explore different schools. Over time, numerous schools of yoga have evolved. They vary from gentle to strenuous, with an emphasis on postures, breath work, meditation, singing, or a combination of these skills. Choose what feels good and safe based on your personal preference and physical ability.
Be mindful. Focusing solely on the present moment calms the mind and increases awareness. Meditative practice can sharpen clarity and focus. Meditation can involve focusing your attention on sounds, images, or inspirational words or phrases. Each of our movements can invite self-respect and further awareness of the daily toll that modern life places on our minds and bodies. Active breath work is believed to cultivate vitality. Calm breath work and meditative practices help still the mind and decrease physiologic overarousal.
Stay consistent. Regardless of your physical ability or level of mobility, consistent yoga practice is necessary to realize its benefits. Therefore, a weekly class may be a good way to start. Eventually, a good goal is to practice twice a day, at dawn and dusk.
Appreciate the experience. Immerse yourself in each moment of yoga practice. There is no need to rush. Enjoy your journey!
Being a psychiatrist today often entails long hours immersed in charts or on computers, a lack of fresh air, and eating meals in a hurry. Being on call, facing deadline pressures, and juggling multiple responsibilities can lead to fatigue, frustration, and a lack of adequate socialization. These circumstances can take their toll on us in unpleasant and unhealthy ways, resulting in exhaustion, illness, an
What is yoga?
Yoga is an ancient practice that originated in India thousands of years ago. It was introduced to the West in the 19th century. Yoga is a holistic lifestyle of well-being that includes physical and meditative practices. Today, the most popular forms of yoga typically incorporate a combination of physical postures, controlled breathing, deep relaxation, and/or meditation.2
How to begin yoga practice
Start slow and simple.
- develop balance, endurance, strength, flexibility, and coordination
- release chronic muscular tension
- rejuvenate the body.
Explore different schools. Over time, numerous schools of yoga have evolved. They vary from gentle to strenuous, with an emphasis on postures, breath work, meditation, singing, or a combination of these skills. Choose what feels good and safe based on your personal preference and physical ability.
Be mindful. Focusing solely on the present moment calms the mind and increases awareness. Meditative practice can sharpen clarity and focus. Meditation can involve focusing your attention on sounds, images, or inspirational words or phrases. Each of our movements can invite self-respect and further awareness of the daily toll that modern life places on our minds and bodies. Active breath work is believed to cultivate vitality. Calm breath work and meditative practices help still the mind and decrease physiologic overarousal.
Stay consistent. Regardless of your physical ability or level of mobility, consistent yoga practice is necessary to realize its benefits. Therefore, a weekly class may be a good way to start. Eventually, a good goal is to practice twice a day, at dawn and dusk.
Appreciate the experience. Immerse yourself in each moment of yoga practice. There is no need to rush. Enjoy your journey!
1. Harvard Mental Health Letter. Yoga for anxiety and depression. Harvard Health Publishing. https://www.health.harvard.edu/mind-and-mood/yoga-for-anxiety-and-depression. Updated September 18, 2017. Accessed November 21, 2017.
2. Balasubramaniam M, Telles S, Doraiswamy PM. Yoga on our minds: a systematic review of yoga for neuropsychiatric disorders. Front Psychiatry. 2013;3:117. doi: 10.3389/fpsyt.2012.00117.
1. Harvard Mental Health Letter. Yoga for anxiety and depression. Harvard Health Publishing. https://www.health.harvard.edu/mind-and-mood/yoga-for-anxiety-and-depression. Updated September 18, 2017. Accessed November 21, 2017.
2. Balasubramaniam M, Telles S, Doraiswamy PM. Yoga on our minds: a systematic review of yoga for neuropsychiatric disorders. Front Psychiatry. 2013;3:117. doi: 10.3389/fpsyt.2012.00117.
Nonpharmacologic strategies for helping children with ADHD
Attention-deficit/hyperactivity disorder (ADHD) affects 5% of children and adolescents worldwide.1 Children with ADHD commonly have trouble with attention, hyperactivity, impulsivity, organization, and emotional reactivity, and these difficulties can result in behaviors that frustrate, worry, and overwhelm parents, teachers, and other caregivers.
Extensive evidence supports stimulants as a first-line treatment. However, nonpharmacologic interventions are important, yet often overlooked, adjuncts that can be helpful for children who have a partial response to stimulants or are not prescribed medication. Teaching caregivers to use the following interventions will allow them to help children better navigate situations that require managing their symptoms, such as in a classroom setting.2
Attention. Children with ADHD typically find it challenging to prioritize what to focus on, sustain that focus, and switch between tasks. Shouting instructions often is unproductive. Therefore, encourage parents and teachers to use clear and concise instructions with supplementary visual tools to aid these children. When providing instructions in classrooms, teachers should look directly at the student and call him (her) by name. It also can be helpful to have the student repeat the instructions. Seating students with ADHD near the front of the classroom, close to the teacher and away from other distracting students, can improve their focus and allow the teacher to more easily give nonverbal cues, such as tapping on the student’s desk if his attention is waning.
Hyperactivity. Children with ADHD are prone to excessive talkativeness and continuous motor movement; therefore, sitting still for long periods can be exceptionally difficult. Teachers and caregivers should keep assignments short. For students whose primary manifestation of ADHD is hyperactivity, sitting near the back of the classroom will allow them to stand and stretch without disrupting the class. Occasionally giving these students a time-limited, acceptable outlet for their urge to move may be beneficial.
Impulsivity. Children who exhibit this symptom are more focused on the present and have difficulty weighing the consequences of their actions. Allowing these children to take frequent breaks (eg, more play time) will let their brains rest and recharge so that they can take a step back to evaluate the outcomes of their actions. Instruct parents and teachers to give children with ADHD regular verbal or written feedback to monitor and modify behaviors over time. Consequences for not following the rules should be immediate and consistent.
Organization. School assignments require sequencing, planning, and time management. Therefore, having daily visual reminders of prioritized assignments and schedules is helpful for children with ADHD, both at school and at home. Teachers and parents can help children stay organized by checking and reviewing the child’s agenda with him several times a day; this will allow him more time to think about what he needs to do to complete assignments.Emotional reactivity. Children with ADHD become frustrated easily and often are particularly sensitive to disappointment because of the continuous redirection they receive. Normalizing their mistakes by reinforcing that everyone makes mistakes and teaching them to learn from their mistakes can help reduce their embarrassment.
It also can be helpful to identify triggers for emotional reactivity. Parents and teachers should minimize the amount of talking when a child is unable to control his emotions. Helping children label their emotions, developing strategies for when they become upset, and outlining clear consequences for unacceptable behaviors can help modify their reactions.
1. Faraone SV, Asherson P, Banaschewski T, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015;1:15020. doi: 10.1038/nrdp.2015.20.
2. Barkley RA. Classroom accommodations for children with ADHD. The ADHD Report. 2008;16(4):7-10.
Attention-deficit/hyperactivity disorder (ADHD) affects 5% of children and adolescents worldwide.1 Children with ADHD commonly have trouble with attention, hyperactivity, impulsivity, organization, and emotional reactivity, and these difficulties can result in behaviors that frustrate, worry, and overwhelm parents, teachers, and other caregivers.
Extensive evidence supports stimulants as a first-line treatment. However, nonpharmacologic interventions are important, yet often overlooked, adjuncts that can be helpful for children who have a partial response to stimulants or are not prescribed medication. Teaching caregivers to use the following interventions will allow them to help children better navigate situations that require managing their symptoms, such as in a classroom setting.2
Attention. Children with ADHD typically find it challenging to prioritize what to focus on, sustain that focus, and switch between tasks. Shouting instructions often is unproductive. Therefore, encourage parents and teachers to use clear and concise instructions with supplementary visual tools to aid these children. When providing instructions in classrooms, teachers should look directly at the student and call him (her) by name. It also can be helpful to have the student repeat the instructions. Seating students with ADHD near the front of the classroom, close to the teacher and away from other distracting students, can improve their focus and allow the teacher to more easily give nonverbal cues, such as tapping on the student’s desk if his attention is waning.
Hyperactivity. Children with ADHD are prone to excessive talkativeness and continuous motor movement; therefore, sitting still for long periods can be exceptionally difficult. Teachers and caregivers should keep assignments short. For students whose primary manifestation of ADHD is hyperactivity, sitting near the back of the classroom will allow them to stand and stretch without disrupting the class. Occasionally giving these students a time-limited, acceptable outlet for their urge to move may be beneficial.
Impulsivity. Children who exhibit this symptom are more focused on the present and have difficulty weighing the consequences of their actions. Allowing these children to take frequent breaks (eg, more play time) will let their brains rest and recharge so that they can take a step back to evaluate the outcomes of their actions. Instruct parents and teachers to give children with ADHD regular verbal or written feedback to monitor and modify behaviors over time. Consequences for not following the rules should be immediate and consistent.
Organization. School assignments require sequencing, planning, and time management. Therefore, having daily visual reminders of prioritized assignments and schedules is helpful for children with ADHD, both at school and at home. Teachers and parents can help children stay organized by checking and reviewing the child’s agenda with him several times a day; this will allow him more time to think about what he needs to do to complete assignments.Emotional reactivity. Children with ADHD become frustrated easily and often are particularly sensitive to disappointment because of the continuous redirection they receive. Normalizing their mistakes by reinforcing that everyone makes mistakes and teaching them to learn from their mistakes can help reduce their embarrassment.
It also can be helpful to identify triggers for emotional reactivity. Parents and teachers should minimize the amount of talking when a child is unable to control his emotions. Helping children label their emotions, developing strategies for when they become upset, and outlining clear consequences for unacceptable behaviors can help modify their reactions.
Attention-deficit/hyperactivity disorder (ADHD) affects 5% of children and adolescents worldwide.1 Children with ADHD commonly have trouble with attention, hyperactivity, impulsivity, organization, and emotional reactivity, and these difficulties can result in behaviors that frustrate, worry, and overwhelm parents, teachers, and other caregivers.
Extensive evidence supports stimulants as a first-line treatment. However, nonpharmacologic interventions are important, yet often overlooked, adjuncts that can be helpful for children who have a partial response to stimulants or are not prescribed medication. Teaching caregivers to use the following interventions will allow them to help children better navigate situations that require managing their symptoms, such as in a classroom setting.2
Attention. Children with ADHD typically find it challenging to prioritize what to focus on, sustain that focus, and switch between tasks. Shouting instructions often is unproductive. Therefore, encourage parents and teachers to use clear and concise instructions with supplementary visual tools to aid these children. When providing instructions in classrooms, teachers should look directly at the student and call him (her) by name. It also can be helpful to have the student repeat the instructions. Seating students with ADHD near the front of the classroom, close to the teacher and away from other distracting students, can improve their focus and allow the teacher to more easily give nonverbal cues, such as tapping on the student’s desk if his attention is waning.
Hyperactivity. Children with ADHD are prone to excessive talkativeness and continuous motor movement; therefore, sitting still for long periods can be exceptionally difficult. Teachers and caregivers should keep assignments short. For students whose primary manifestation of ADHD is hyperactivity, sitting near the back of the classroom will allow them to stand and stretch without disrupting the class. Occasionally giving these students a time-limited, acceptable outlet for their urge to move may be beneficial.
Impulsivity. Children who exhibit this symptom are more focused on the present and have difficulty weighing the consequences of their actions. Allowing these children to take frequent breaks (eg, more play time) will let their brains rest and recharge so that they can take a step back to evaluate the outcomes of their actions. Instruct parents and teachers to give children with ADHD regular verbal or written feedback to monitor and modify behaviors over time. Consequences for not following the rules should be immediate and consistent.
Organization. School assignments require sequencing, planning, and time management. Therefore, having daily visual reminders of prioritized assignments and schedules is helpful for children with ADHD, both at school and at home. Teachers and parents can help children stay organized by checking and reviewing the child’s agenda with him several times a day; this will allow him more time to think about what he needs to do to complete assignments.Emotional reactivity. Children with ADHD become frustrated easily and often are particularly sensitive to disappointment because of the continuous redirection they receive. Normalizing their mistakes by reinforcing that everyone makes mistakes and teaching them to learn from their mistakes can help reduce their embarrassment.
It also can be helpful to identify triggers for emotional reactivity. Parents and teachers should minimize the amount of talking when a child is unable to control his emotions. Helping children label their emotions, developing strategies for when they become upset, and outlining clear consequences for unacceptable behaviors can help modify their reactions.
1. Faraone SV, Asherson P, Banaschewski T, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015;1:15020. doi: 10.1038/nrdp.2015.20.
2. Barkley RA. Classroom accommodations for children with ADHD. The ADHD Report. 2008;16(4):7-10.
1. Faraone SV, Asherson P, Banaschewski T, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015;1:15020. doi: 10.1038/nrdp.2015.20.
2. Barkley RA. Classroom accommodations for children with ADHD. The ADHD Report. 2008;16(4):7-10.