User login
MMRV vaccine cut chickenpox hospitalizations in young Brazilian children
(VZV) in children aged 1-4 years, said Marcelo Comerlato Scotta and associates at Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil.
Of the 69,791 admissions caused by VZV in Brazilian patients younger than 20 years, the rate of such hospitalizations for children aged 1-4 years significantly decreased from 27 cases per 100,000 children per year to 14 cases per 100,000 children per year after the vaccine was introduced, a reduction of 48% (P < .001). Changes in other age groups were not significant. That decrease in the rate of VZV admissions remained statistically significant in the vaccinated group after adjusting for seasonality (P < .001).
Direct costs of VZV-related admissions dropped 38% after introducing the MMRV vaccine, the researchers said. “Further studies are needed to evaluate long-term direct and indirect impact on the epidemiology of VZV infections.”
Read more in Vaccine (2017 Dec 1. doi: 10.1016/j.vaccine.2017.11.057.)
(VZV) in children aged 1-4 years, said Marcelo Comerlato Scotta and associates at Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil.
Of the 69,791 admissions caused by VZV in Brazilian patients younger than 20 years, the rate of such hospitalizations for children aged 1-4 years significantly decreased from 27 cases per 100,000 children per year to 14 cases per 100,000 children per year after the vaccine was introduced, a reduction of 48% (P < .001). Changes in other age groups were not significant. That decrease in the rate of VZV admissions remained statistically significant in the vaccinated group after adjusting for seasonality (P < .001).
Direct costs of VZV-related admissions dropped 38% after introducing the MMRV vaccine, the researchers said. “Further studies are needed to evaluate long-term direct and indirect impact on the epidemiology of VZV infections.”
Read more in Vaccine (2017 Dec 1. doi: 10.1016/j.vaccine.2017.11.057.)
(VZV) in children aged 1-4 years, said Marcelo Comerlato Scotta and associates at Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil.
Of the 69,791 admissions caused by VZV in Brazilian patients younger than 20 years, the rate of such hospitalizations for children aged 1-4 years significantly decreased from 27 cases per 100,000 children per year to 14 cases per 100,000 children per year after the vaccine was introduced, a reduction of 48% (P < .001). Changes in other age groups were not significant. That decrease in the rate of VZV admissions remained statistically significant in the vaccinated group after adjusting for seasonality (P < .001).
Direct costs of VZV-related admissions dropped 38% after introducing the MMRV vaccine, the researchers said. “Further studies are needed to evaluate long-term direct and indirect impact on the epidemiology of VZV infections.”
Read more in Vaccine (2017 Dec 1. doi: 10.1016/j.vaccine.2017.11.057.)
FROM VACCINE
FDA approves Eskata for treatment of raised SKs
, according to Aclaris Therapeutics.
Approval for Eskata is based on results from two phase III clinical trials in which patients with raised SKs received either Eskata or a placebo for two doses, one at baseline and one after 2 weeks. Patients who received Eskata were more likely to have their SKs clear completely, compared with the placebo group.
Eskata is approved only for use in the office of a health care provider and is not for home usage.
The most common adverse events associated with Eskata are itching, stinging, crusting, swelling, redness, and scaling at the application site. Serious skin reactions are possible, and if the medication accidentally enters a patient’s eyes, the patient should flush his or her eyes with water for 15-30 minutes.
“This achievement delivers on Aclaris’ commitment to bringing innovative therapies to market that address significant unmet needs in dermatology. For the first time, with the approval of Eskata, patients will have access to an FDA-approved topical, non-invasive treatment for raised SKs,” Dr. Neal Walker, president and chief executive officer of Aclaris, said in written statement.
, according to Aclaris Therapeutics.
Approval for Eskata is based on results from two phase III clinical trials in which patients with raised SKs received either Eskata or a placebo for two doses, one at baseline and one after 2 weeks. Patients who received Eskata were more likely to have their SKs clear completely, compared with the placebo group.
Eskata is approved only for use in the office of a health care provider and is not for home usage.
The most common adverse events associated with Eskata are itching, stinging, crusting, swelling, redness, and scaling at the application site. Serious skin reactions are possible, and if the medication accidentally enters a patient’s eyes, the patient should flush his or her eyes with water for 15-30 minutes.
“This achievement delivers on Aclaris’ commitment to bringing innovative therapies to market that address significant unmet needs in dermatology. For the first time, with the approval of Eskata, patients will have access to an FDA-approved topical, non-invasive treatment for raised SKs,” Dr. Neal Walker, president and chief executive officer of Aclaris, said in written statement.
, according to Aclaris Therapeutics.
Approval for Eskata is based on results from two phase III clinical trials in which patients with raised SKs received either Eskata or a placebo for two doses, one at baseline and one after 2 weeks. Patients who received Eskata were more likely to have their SKs clear completely, compared with the placebo group.
Eskata is approved only for use in the office of a health care provider and is not for home usage.
The most common adverse events associated with Eskata are itching, stinging, crusting, swelling, redness, and scaling at the application site. Serious skin reactions are possible, and if the medication accidentally enters a patient’s eyes, the patient should flush his or her eyes with water for 15-30 minutes.
“This achievement delivers on Aclaris’ commitment to bringing innovative therapies to market that address significant unmet needs in dermatology. For the first time, with the approval of Eskata, patients will have access to an FDA-approved topical, non-invasive treatment for raised SKs,” Dr. Neal Walker, president and chief executive officer of Aclaris, said in written statement.
Zika virus testing shows low incidence in donor blood outside of high-infection areas
SAN DIEGO – The Zika virus is primarily transmitted via the Aedes mosquitoes, most commonly by A. aegypti, but recent outbreaks have revealed that nonvector transmission routes may also spread the infection. Some data suggest that blood transfusion can be a source of transmission.
While the number of contaminated blood donations remains very small, three studies presented at the American Association of Blood Banks annual meeting confirmed the ability of new investigational assays to detect Zika virus in donated blood.
There have been no confirmed transfusion-transmission cases of Zika virus in the United States, but as cases have now been documented in Brazil, the Food and Drug Administration issued revised guidance in August 2016 recommending that blood centers in all states and U.S. territories screen individual units of donated whole blood and blood components.
In the first report (C7-A01C), Paula Saá, PhD, and her colleagues at the American Red Cross initially investigated the use of mini-pool (MP)- nucleic acid testing (NAT) using the Procleix Zika Virus Assay (TMA). Testing was initially implemented on blood collections from Florida, Georgia, South Carolina, Mississippi, and Alabama – five states that were presumed to be at high risk of Zika virus infection. After the FDA revised its guidance, the protocol changed and testing was extended to all blood donations. The use of the MP-NAT was also converted to individual donation (ID)-NAT, and questions concerning travel history was also eventually discontinued.
However, even with the use of ID-NAT, the rate of confirmed positive donations was quite small but the associated cost was quite high, the researchers pointed out. “In the first year of testing at the American Red Cross, we identified nine confirmed positive donations,” said Dr. Saá.
The rate of confirmed positive donations was 1:354, 602 during the study period, but if the period up until September 2017 is taken into account (no additional cases were identified), the rate increases to 1:514,266. “This is a very low rate,” Dr. Saá said. “If there are no changes to the current guidelines, we have estimated that the yearly cost for the American Red Cross of testing will exceed $48 million.”
These figures extrapolate to approximately $6 million per confirmed case, according to the results of this study sponsored by the American Red Cross.
Confirmatory testing included repeat TMA; in addition, RT-PCR, serology and red blood cell count (RBC) TMA were performed. Estimates of viral loads were performed by endpoint TMA on plasma and RBCs.
A total of 2,288,855 blood donations had been tested as of April 2017, including 393,713 (17%) in 24,611 MPs, which did not detect any reactive donations.
Of the confirmed positive blood donors, three lived in Florida and two of those were from local transmission. Six individuals had traveled to a region highly active for Zika virus, and returned to the United States between 2 and 73 days before donating blood. Clinical symptoms were reported in two individuals with a travel risk; the other donors with a confirmed positive test (75%) remained asymptomatic. The longest period for detection in RBCs was 91 days thus far, but in the same person, detection in plasma was only 17 days.
“The data that we are showing here recommends a testing strategy with mini pool testing in areas at low risk of Zika transmission,” said Dr. Saá.
A second related study (C9-A01C), described the detection of ZIKV RNA in blood donations collected in U.S. states between April 3, 2016, and September 23, 2017, using the cobas Zika test, to be used on the cobas 6800/8800 Systems.
Although the test was investigational during the study period, it has just been approved by the FDA, said study author Lisa Pate, MD, who is with Roche Molecular Systems, the manufacturer of the cobas Zika test and cobas 6800/8800 systems. “This is now the first licensed test for screening blood donations for Zika virus.”
Overall, testing showed that Zika contamination in the U.S. blood supply was quite low. Only 0.001% of screened blood donations in United States were confirmed as true positives.
The development of this test came about after the first cases of Zika virus in the United States were detected in Puerto Rico in December 2015, explained Dr. Pate. Shortly after that, the FDA issued guidance prohibiting the use of blood collected in Zika active areas, unless the donations were screened.
“The impact was significant in Puerto Rico, as blood donations were halted, which then forced Puerto Rico to rely on imported blood,” she said.
About that time the FDA reached out to Roche and competitors to see if a test could be developed to screen for Zika.
The cobas Zika test was approved under an investigational new drug application on March 30, 2016, and although initially used to test blood samples in Puerto Rico, testing was expanded to include donor blood from all over the country.
Screening was conducted by individual donation testing, with all initial reactive results repeated in duplicate. Supplemental testing was also done, and included an alternative NAT (AltNAT) assay which was considered to be less sensitive than cobas Zika and serology testing for anti-Zika IgM and IgG. A donor confirmed Zika confirmed positive if at least one replicate of the repeat testing by cobas Zika was reactive on index donation or follow-up, reactive by AltNAT on the index donation, or positive for anti-Zika IgM on index or follow-up.
Screening was conducted at 12 testing labs in the United States, and more than 4 million donations were screened and 27 positive donations were confirmed. Overall, that amounted to less than 1 in 100,000.
“For donors in the U.S. with confirmed positive results, and for whom follow-up information is available, 84% of them report recent travel to Zika active areas,” noted Dr. Pate.
For Puerto Rico, 111,842 blood donations were screened and there were 356 confirmed positive results. The incidence is much higher than in United States, and was 1.27% during peak incidence in July 2016.
A third paper (C12-A01C), also reported on testing the blood supply in Singapore, which had reported its first locally transmitted Zika case last August, using the investigational Procleix ZIKV nucleic acid technology (NAT) assay.
The presence of Zika virus in screened blood was also quite low, with an incidence of only 0.0032%. The Procleix ZIKV assay was found to suitable for screening for Zika infection in an asymptomatic population, as it showed good analytical sensitivity and clinical performance.
The Zika virus came to Singapore in May 2016, imported by an individual who had recently traveled to Brazil, said Sally Lam, laboratory director, Blood Services Group, Health Sciences Authority, Singapore.
“Then in August we had 41 confirmed local Zika virus cases,” she said.
In 2016, there were 458 clinical Zika cases reported, with 8 clusters identified. This year, 63 cases have been reported to date, she said.
Mandatory Zika virus screening in donor blood with ID-NAT began after the onset of local outbreaks, and was implemented in January 2017. A total of 126,906 blood donations were screened.
Researchers in Singapore assessed the performance of the Procleix ZIKV NAT assay for universal blood donation screening. They screened all blood that was donated, beginning Oct. 1, 2016, a confirmed case was defined as having Zika RNA by PCR and/or Zika antibodies. Analytical sensitivity was assessed by use of 300 blinded frozen samples containing Zika virus and 25 negative controls. The performance of the Procleix ZIKV assay was also evaluated by use of samples from the local patient population.
Of four confirmed positive cases, only one was available for follow-up. “In the index donation, the viral load was quite high in the plasma but at 10 days, it was reduced to about 400 copies/mL in the plasma,” said Ms. Lam. “The donor did not develop any symptoms.”
The analytical sensitivity for the Procleix ZIKV assay was determined to be 2.1 copies/mL at 50% LOD and 10.0 copies/mL at 95% LOD, and it detected RNA in six out of nine patient samples for an 85.7% agreement with reference material, according to the researchers.
SAN DIEGO – The Zika virus is primarily transmitted via the Aedes mosquitoes, most commonly by A. aegypti, but recent outbreaks have revealed that nonvector transmission routes may also spread the infection. Some data suggest that blood transfusion can be a source of transmission.
While the number of contaminated blood donations remains very small, three studies presented at the American Association of Blood Banks annual meeting confirmed the ability of new investigational assays to detect Zika virus in donated blood.
There have been no confirmed transfusion-transmission cases of Zika virus in the United States, but as cases have now been documented in Brazil, the Food and Drug Administration issued revised guidance in August 2016 recommending that blood centers in all states and U.S. territories screen individual units of donated whole blood and blood components.
In the first report (C7-A01C), Paula Saá, PhD, and her colleagues at the American Red Cross initially investigated the use of mini-pool (MP)- nucleic acid testing (NAT) using the Procleix Zika Virus Assay (TMA). Testing was initially implemented on blood collections from Florida, Georgia, South Carolina, Mississippi, and Alabama – five states that were presumed to be at high risk of Zika virus infection. After the FDA revised its guidance, the protocol changed and testing was extended to all blood donations. The use of the MP-NAT was also converted to individual donation (ID)-NAT, and questions concerning travel history was also eventually discontinued.
However, even with the use of ID-NAT, the rate of confirmed positive donations was quite small but the associated cost was quite high, the researchers pointed out. “In the first year of testing at the American Red Cross, we identified nine confirmed positive donations,” said Dr. Saá.
The rate of confirmed positive donations was 1:354, 602 during the study period, but if the period up until September 2017 is taken into account (no additional cases were identified), the rate increases to 1:514,266. “This is a very low rate,” Dr. Saá said. “If there are no changes to the current guidelines, we have estimated that the yearly cost for the American Red Cross of testing will exceed $48 million.”
These figures extrapolate to approximately $6 million per confirmed case, according to the results of this study sponsored by the American Red Cross.
Confirmatory testing included repeat TMA; in addition, RT-PCR, serology and red blood cell count (RBC) TMA were performed. Estimates of viral loads were performed by endpoint TMA on plasma and RBCs.
A total of 2,288,855 blood donations had been tested as of April 2017, including 393,713 (17%) in 24,611 MPs, which did not detect any reactive donations.
Of the confirmed positive blood donors, three lived in Florida and two of those were from local transmission. Six individuals had traveled to a region highly active for Zika virus, and returned to the United States between 2 and 73 days before donating blood. Clinical symptoms were reported in two individuals with a travel risk; the other donors with a confirmed positive test (75%) remained asymptomatic. The longest period for detection in RBCs was 91 days thus far, but in the same person, detection in plasma was only 17 days.
“The data that we are showing here recommends a testing strategy with mini pool testing in areas at low risk of Zika transmission,” said Dr. Saá.
A second related study (C9-A01C), described the detection of ZIKV RNA in blood donations collected in U.S. states between April 3, 2016, and September 23, 2017, using the cobas Zika test, to be used on the cobas 6800/8800 Systems.
Although the test was investigational during the study period, it has just been approved by the FDA, said study author Lisa Pate, MD, who is with Roche Molecular Systems, the manufacturer of the cobas Zika test and cobas 6800/8800 systems. “This is now the first licensed test for screening blood donations for Zika virus.”
Overall, testing showed that Zika contamination in the U.S. blood supply was quite low. Only 0.001% of screened blood donations in United States were confirmed as true positives.
The development of this test came about after the first cases of Zika virus in the United States were detected in Puerto Rico in December 2015, explained Dr. Pate. Shortly after that, the FDA issued guidance prohibiting the use of blood collected in Zika active areas, unless the donations were screened.
“The impact was significant in Puerto Rico, as blood donations were halted, which then forced Puerto Rico to rely on imported blood,” she said.
About that time the FDA reached out to Roche and competitors to see if a test could be developed to screen for Zika.
The cobas Zika test was approved under an investigational new drug application on March 30, 2016, and although initially used to test blood samples in Puerto Rico, testing was expanded to include donor blood from all over the country.
Screening was conducted by individual donation testing, with all initial reactive results repeated in duplicate. Supplemental testing was also done, and included an alternative NAT (AltNAT) assay which was considered to be less sensitive than cobas Zika and serology testing for anti-Zika IgM and IgG. A donor confirmed Zika confirmed positive if at least one replicate of the repeat testing by cobas Zika was reactive on index donation or follow-up, reactive by AltNAT on the index donation, or positive for anti-Zika IgM on index or follow-up.
Screening was conducted at 12 testing labs in the United States, and more than 4 million donations were screened and 27 positive donations were confirmed. Overall, that amounted to less than 1 in 100,000.
“For donors in the U.S. with confirmed positive results, and for whom follow-up information is available, 84% of them report recent travel to Zika active areas,” noted Dr. Pate.
For Puerto Rico, 111,842 blood donations were screened and there were 356 confirmed positive results. The incidence is much higher than in United States, and was 1.27% during peak incidence in July 2016.
A third paper (C12-A01C), also reported on testing the blood supply in Singapore, which had reported its first locally transmitted Zika case last August, using the investigational Procleix ZIKV nucleic acid technology (NAT) assay.
The presence of Zika virus in screened blood was also quite low, with an incidence of only 0.0032%. The Procleix ZIKV assay was found to suitable for screening for Zika infection in an asymptomatic population, as it showed good analytical sensitivity and clinical performance.
The Zika virus came to Singapore in May 2016, imported by an individual who had recently traveled to Brazil, said Sally Lam, laboratory director, Blood Services Group, Health Sciences Authority, Singapore.
“Then in August we had 41 confirmed local Zika virus cases,” she said.
In 2016, there were 458 clinical Zika cases reported, with 8 clusters identified. This year, 63 cases have been reported to date, she said.
Mandatory Zika virus screening in donor blood with ID-NAT began after the onset of local outbreaks, and was implemented in January 2017. A total of 126,906 blood donations were screened.
Researchers in Singapore assessed the performance of the Procleix ZIKV NAT assay for universal blood donation screening. They screened all blood that was donated, beginning Oct. 1, 2016, a confirmed case was defined as having Zika RNA by PCR and/or Zika antibodies. Analytical sensitivity was assessed by use of 300 blinded frozen samples containing Zika virus and 25 negative controls. The performance of the Procleix ZIKV assay was also evaluated by use of samples from the local patient population.
Of four confirmed positive cases, only one was available for follow-up. “In the index donation, the viral load was quite high in the plasma but at 10 days, it was reduced to about 400 copies/mL in the plasma,” said Ms. Lam. “The donor did not develop any symptoms.”
The analytical sensitivity for the Procleix ZIKV assay was determined to be 2.1 copies/mL at 50% LOD and 10.0 copies/mL at 95% LOD, and it detected RNA in six out of nine patient samples for an 85.7% agreement with reference material, according to the researchers.
SAN DIEGO – The Zika virus is primarily transmitted via the Aedes mosquitoes, most commonly by A. aegypti, but recent outbreaks have revealed that nonvector transmission routes may also spread the infection. Some data suggest that blood transfusion can be a source of transmission.
While the number of contaminated blood donations remains very small, three studies presented at the American Association of Blood Banks annual meeting confirmed the ability of new investigational assays to detect Zika virus in donated blood.
There have been no confirmed transfusion-transmission cases of Zika virus in the United States, but as cases have now been documented in Brazil, the Food and Drug Administration issued revised guidance in August 2016 recommending that blood centers in all states and U.S. territories screen individual units of donated whole blood and blood components.
In the first report (C7-A01C), Paula Saá, PhD, and her colleagues at the American Red Cross initially investigated the use of mini-pool (MP)- nucleic acid testing (NAT) using the Procleix Zika Virus Assay (TMA). Testing was initially implemented on blood collections from Florida, Georgia, South Carolina, Mississippi, and Alabama – five states that were presumed to be at high risk of Zika virus infection. After the FDA revised its guidance, the protocol changed and testing was extended to all blood donations. The use of the MP-NAT was also converted to individual donation (ID)-NAT, and questions concerning travel history was also eventually discontinued.
However, even with the use of ID-NAT, the rate of confirmed positive donations was quite small but the associated cost was quite high, the researchers pointed out. “In the first year of testing at the American Red Cross, we identified nine confirmed positive donations,” said Dr. Saá.
The rate of confirmed positive donations was 1:354, 602 during the study period, but if the period up until September 2017 is taken into account (no additional cases were identified), the rate increases to 1:514,266. “This is a very low rate,” Dr. Saá said. “If there are no changes to the current guidelines, we have estimated that the yearly cost for the American Red Cross of testing will exceed $48 million.”
These figures extrapolate to approximately $6 million per confirmed case, according to the results of this study sponsored by the American Red Cross.
Confirmatory testing included repeat TMA; in addition, RT-PCR, serology and red blood cell count (RBC) TMA were performed. Estimates of viral loads were performed by endpoint TMA on plasma and RBCs.
A total of 2,288,855 blood donations had been tested as of April 2017, including 393,713 (17%) in 24,611 MPs, which did not detect any reactive donations.
Of the confirmed positive blood donors, three lived in Florida and two of those were from local transmission. Six individuals had traveled to a region highly active for Zika virus, and returned to the United States between 2 and 73 days before donating blood. Clinical symptoms were reported in two individuals with a travel risk; the other donors with a confirmed positive test (75%) remained asymptomatic. The longest period for detection in RBCs was 91 days thus far, but in the same person, detection in plasma was only 17 days.
“The data that we are showing here recommends a testing strategy with mini pool testing in areas at low risk of Zika transmission,” said Dr. Saá.
A second related study (C9-A01C), described the detection of ZIKV RNA in blood donations collected in U.S. states between April 3, 2016, and September 23, 2017, using the cobas Zika test, to be used on the cobas 6800/8800 Systems.
Although the test was investigational during the study period, it has just been approved by the FDA, said study author Lisa Pate, MD, who is with Roche Molecular Systems, the manufacturer of the cobas Zika test and cobas 6800/8800 systems. “This is now the first licensed test for screening blood donations for Zika virus.”
Overall, testing showed that Zika contamination in the U.S. blood supply was quite low. Only 0.001% of screened blood donations in United States were confirmed as true positives.
The development of this test came about after the first cases of Zika virus in the United States were detected in Puerto Rico in December 2015, explained Dr. Pate. Shortly after that, the FDA issued guidance prohibiting the use of blood collected in Zika active areas, unless the donations were screened.
“The impact was significant in Puerto Rico, as blood donations were halted, which then forced Puerto Rico to rely on imported blood,” she said.
About that time the FDA reached out to Roche and competitors to see if a test could be developed to screen for Zika.
The cobas Zika test was approved under an investigational new drug application on March 30, 2016, and although initially used to test blood samples in Puerto Rico, testing was expanded to include donor blood from all over the country.
Screening was conducted by individual donation testing, with all initial reactive results repeated in duplicate. Supplemental testing was also done, and included an alternative NAT (AltNAT) assay which was considered to be less sensitive than cobas Zika and serology testing for anti-Zika IgM and IgG. A donor confirmed Zika confirmed positive if at least one replicate of the repeat testing by cobas Zika was reactive on index donation or follow-up, reactive by AltNAT on the index donation, or positive for anti-Zika IgM on index or follow-up.
Screening was conducted at 12 testing labs in the United States, and more than 4 million donations were screened and 27 positive donations were confirmed. Overall, that amounted to less than 1 in 100,000.
“For donors in the U.S. with confirmed positive results, and for whom follow-up information is available, 84% of them report recent travel to Zika active areas,” noted Dr. Pate.
For Puerto Rico, 111,842 blood donations were screened and there were 356 confirmed positive results. The incidence is much higher than in United States, and was 1.27% during peak incidence in July 2016.
A third paper (C12-A01C), also reported on testing the blood supply in Singapore, which had reported its first locally transmitted Zika case last August, using the investigational Procleix ZIKV nucleic acid technology (NAT) assay.
The presence of Zika virus in screened blood was also quite low, with an incidence of only 0.0032%. The Procleix ZIKV assay was found to suitable for screening for Zika infection in an asymptomatic population, as it showed good analytical sensitivity and clinical performance.
The Zika virus came to Singapore in May 2016, imported by an individual who had recently traveled to Brazil, said Sally Lam, laboratory director, Blood Services Group, Health Sciences Authority, Singapore.
“Then in August we had 41 confirmed local Zika virus cases,” she said.
In 2016, there were 458 clinical Zika cases reported, with 8 clusters identified. This year, 63 cases have been reported to date, she said.
Mandatory Zika virus screening in donor blood with ID-NAT began after the onset of local outbreaks, and was implemented in January 2017. A total of 126,906 blood donations were screened.
Researchers in Singapore assessed the performance of the Procleix ZIKV NAT assay for universal blood donation screening. They screened all blood that was donated, beginning Oct. 1, 2016, a confirmed case was defined as having Zika RNA by PCR and/or Zika antibodies. Analytical sensitivity was assessed by use of 300 blinded frozen samples containing Zika virus and 25 negative controls. The performance of the Procleix ZIKV assay was also evaluated by use of samples from the local patient population.
Of four confirmed positive cases, only one was available for follow-up. “In the index donation, the viral load was quite high in the plasma but at 10 days, it was reduced to about 400 copies/mL in the plasma,” said Ms. Lam. “The donor did not develop any symptoms.”
The analytical sensitivity for the Procleix ZIKV assay was determined to be 2.1 copies/mL at 50% LOD and 10.0 copies/mL at 95% LOD, and it detected RNA in six out of nine patient samples for an 85.7% agreement with reference material, according to the researchers.
AT AABB17
FDA approves tofacitinib for psoriatic arthritis
(PsA) who have had an inadequate response or intolerance to methotrexate or other disease-modifying antirheumatic drugs (DMARDs), according to a Dec. 14 announcement from its manufacturer, Pfizer.
The approvals of tofacitinib (Xeljanz) at 5 mg twice daily and extended-release tofacitinib (Xeljanz XR) at 11 mg once daily are based on data from the phase 3 Oral Psoriatic Arthritis Trial (OPAL) clinical development program, which consisted of two pivotal studies, OPAL Broaden and OPAL Beyond.
In the OPAL Broaden study, 50% of patients who received tofacitinib 5 mg twice daily in combination with a nonbiologic DMARD achieved an ACR20 response at 3 months, compared with 33% of those treated with placebo (P equal to or less than .05). In the OPAL Beyond study, 50% of patients achieved an ACR20 response with tofacitinib 5 mg twice daily at 3 months, when compared with 24% of patients taking placebo (P equal to or less than .05).
Tofacitinib is the first and only Janus kinase inhibitor approved by the FDA to treat both moderate to severe rheumatoid arthritis and active PsA, Pfizer said in its announcement. It is noted that the recommended dose of tofacitinib is in combination with nonbiologic DMARDs, and use in combination with biologic DMARDs or with potent immunosuppressants such as azathioprine and cyclosporine is not recommended.
The safety of tofacitinib in these trials was consistent with the safety profile observed in rheumatoid arthritis patients. The most common adverse events that occurred in greater than 3% of patients on tofacitinib 5 mg twice daily were nasopharyngitis, upper respiratory tract infection, headache, and diarrhea.
(PsA) who have had an inadequate response or intolerance to methotrexate or other disease-modifying antirheumatic drugs (DMARDs), according to a Dec. 14 announcement from its manufacturer, Pfizer.
The approvals of tofacitinib (Xeljanz) at 5 mg twice daily and extended-release tofacitinib (Xeljanz XR) at 11 mg once daily are based on data from the phase 3 Oral Psoriatic Arthritis Trial (OPAL) clinical development program, which consisted of two pivotal studies, OPAL Broaden and OPAL Beyond.
In the OPAL Broaden study, 50% of patients who received tofacitinib 5 mg twice daily in combination with a nonbiologic DMARD achieved an ACR20 response at 3 months, compared with 33% of those treated with placebo (P equal to or less than .05). In the OPAL Beyond study, 50% of patients achieved an ACR20 response with tofacitinib 5 mg twice daily at 3 months, when compared with 24% of patients taking placebo (P equal to or less than .05).
Tofacitinib is the first and only Janus kinase inhibitor approved by the FDA to treat both moderate to severe rheumatoid arthritis and active PsA, Pfizer said in its announcement. It is noted that the recommended dose of tofacitinib is in combination with nonbiologic DMARDs, and use in combination with biologic DMARDs or with potent immunosuppressants such as azathioprine and cyclosporine is not recommended.
The safety of tofacitinib in these trials was consistent with the safety profile observed in rheumatoid arthritis patients. The most common adverse events that occurred in greater than 3% of patients on tofacitinib 5 mg twice daily were nasopharyngitis, upper respiratory tract infection, headache, and diarrhea.
(PsA) who have had an inadequate response or intolerance to methotrexate or other disease-modifying antirheumatic drugs (DMARDs), according to a Dec. 14 announcement from its manufacturer, Pfizer.
The approvals of tofacitinib (Xeljanz) at 5 mg twice daily and extended-release tofacitinib (Xeljanz XR) at 11 mg once daily are based on data from the phase 3 Oral Psoriatic Arthritis Trial (OPAL) clinical development program, which consisted of two pivotal studies, OPAL Broaden and OPAL Beyond.
In the OPAL Broaden study, 50% of patients who received tofacitinib 5 mg twice daily in combination with a nonbiologic DMARD achieved an ACR20 response at 3 months, compared with 33% of those treated with placebo (P equal to or less than .05). In the OPAL Beyond study, 50% of patients achieved an ACR20 response with tofacitinib 5 mg twice daily at 3 months, when compared with 24% of patients taking placebo (P equal to or less than .05).
Tofacitinib is the first and only Janus kinase inhibitor approved by the FDA to treat both moderate to severe rheumatoid arthritis and active PsA, Pfizer said in its announcement. It is noted that the recommended dose of tofacitinib is in combination with nonbiologic DMARDs, and use in combination with biologic DMARDs or with potent immunosuppressants such as azathioprine and cyclosporine is not recommended.
The safety of tofacitinib in these trials was consistent with the safety profile observed in rheumatoid arthritis patients. The most common adverse events that occurred in greater than 3% of patients on tofacitinib 5 mg twice daily were nasopharyngitis, upper respiratory tract infection, headache, and diarrhea.
Adolescents’ use of opioids, cigarettes is down; pot use is up
The positive trends in drug use among adolescents appear to be continuing – overall, according to the 2017 Monitoring the Future survey.
“This year, we have very good news, because ... the pattern of drug use among teenagers in the United States is continuing to go down, and it’s most notable in the case of opioid drugs,” Nora D. Volkow, MD, director of the National Institute on Drug Abuse said in a video interview posted by NIDA. “We are observing some of the lowest rates of opioid use that we have been monitoring through the survey.”
The national survey results of 8th, 10th, and 12th graders, released Dec. 14, found that lifetime, past-year, and past-month misuse of prescription pain medications are at lows that NIDA called historic. Among high school seniors, the survey showed, Vicodin use was at its lowest point since 2002 at 2% – a decrease from its high of 10.5% in 2003. Similarly, the reported use of OxyContin among high school seniors dropped from 5.5% in 2005 to 2.7% this year.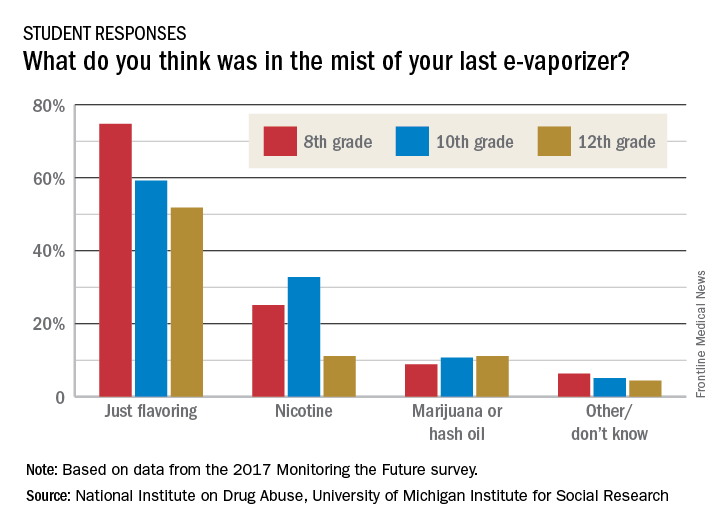
“We’re also seeing continuing decreases in cigarette smoking that are at the lowest levels that we’ve ever seen,” Dr. Volkow said in the interview. Daily cigarette use was 0.6% among 8th graders, 2.2% among 10th graders, and 4.2% among 12th graders in 2017. Those numbers were down from 10.4% and 18.3% among 8th and 10th graders in 1996, and a peak of 24.6 % of 12th graders in 1997.
Twelfth graders also reported lower past-year use of hookah: Use of that substance declined to 10% this year from 13% a year earlier.
As the use of traditional and smokeless tobacco has decreased, however, overall vaping levels held steady in 2017. For the first time, Monitoring the Future reported national, standard estimates of four categories of vaping: nicotine, marijuana, flavoring-only, and any vaping. The reported past-year rates of use under the “any vaping” category in the 2017 survey was 13.3% for 8th graders, 23.9% for 10th graders, and 27.8% for 12th graders. Vaping for flavoring only was the most popular option, and the highest rates were reported by 12th graders – who reported past-month use of 9.7%.
Dr. Volkow said a key concern is that it is unclear that adolescents know what substance they are vaping. Furthermore, adolescents who start vaping any substance are more likely to start to vape nicotine than are those who do not vape. “And we know that when an individual is exposed to nicotine and then gets exposed to another drug, that drug will be more rewarding,” Dr. Volkow said in a teleconference announcing the results.
Richard A. Miech, PhD, MPH, principal investigator of the survey, echoed Dr. Volkow’s concerns.
“These findings emphasize that vaping has progressed well beyond a cigarette alternative,” Dr. Miech said in a statement. “.”
Meanwhile, the past-year use of marijuana among adolescents climbed significantly by 1.3% in 2017, to 24% for all three grades levels combined.
“Historically marijuana use has gone up as adolescents see less risk of harm in using it,” Dr. Miech, of the University of Michigan, Ann Arbor, said in another statement. “We’ve found that the risk adolescents see in marijuana use has been steadily going down for years to the point that it is now at the lowest level we’ve seen in 4 decades.”
Reported overall illicit use of substances such as cocaine, anabolic steroids, and “synthetic marijuana” was relatively low.
“We should take encouragement from this year’s MTF results,” Dr. Volkow wrote on her blog. “Most substance use by middle and high school students is the lowest it has ever been, which suggests that prevention interventions and policies continue to have the desired effect. ...“There is also need to expand on prevention strategies to further reduce drug use – such as use of marijuana.”
The Monitoring the Future survey has been conducted since 1975. The 2017 survey, taken at the beginning of the year, is based on reports from almost 45,000 students in about 380 public and private secondary schools across the country. The survey is designed and conducted by research scientists at the University of Michigan, and is funded by NIDA.
SOURCE: Miech RA et al. 2017 Monitoring the Future
The positive trends in drug use among adolescents appear to be continuing – overall, according to the 2017 Monitoring the Future survey.
“This year, we have very good news, because ... the pattern of drug use among teenagers in the United States is continuing to go down, and it’s most notable in the case of opioid drugs,” Nora D. Volkow, MD, director of the National Institute on Drug Abuse said in a video interview posted by NIDA. “We are observing some of the lowest rates of opioid use that we have been monitoring through the survey.”
The national survey results of 8th, 10th, and 12th graders, released Dec. 14, found that lifetime, past-year, and past-month misuse of prescription pain medications are at lows that NIDA called historic. Among high school seniors, the survey showed, Vicodin use was at its lowest point since 2002 at 2% – a decrease from its high of 10.5% in 2003. Similarly, the reported use of OxyContin among high school seniors dropped from 5.5% in 2005 to 2.7% this year.
“We’re also seeing continuing decreases in cigarette smoking that are at the lowest levels that we’ve ever seen,” Dr. Volkow said in the interview. Daily cigarette use was 0.6% among 8th graders, 2.2% among 10th graders, and 4.2% among 12th graders in 2017. Those numbers were down from 10.4% and 18.3% among 8th and 10th graders in 1996, and a peak of 24.6 % of 12th graders in 1997.
Twelfth graders also reported lower past-year use of hookah: Use of that substance declined to 10% this year from 13% a year earlier.
As the use of traditional and smokeless tobacco has decreased, however, overall vaping levels held steady in 2017. For the first time, Monitoring the Future reported national, standard estimates of four categories of vaping: nicotine, marijuana, flavoring-only, and any vaping. The reported past-year rates of use under the “any vaping” category in the 2017 survey was 13.3% for 8th graders, 23.9% for 10th graders, and 27.8% for 12th graders. Vaping for flavoring only was the most popular option, and the highest rates were reported by 12th graders – who reported past-month use of 9.7%.
Dr. Volkow said a key concern is that it is unclear that adolescents know what substance they are vaping. Furthermore, adolescents who start vaping any substance are more likely to start to vape nicotine than are those who do not vape. “And we know that when an individual is exposed to nicotine and then gets exposed to another drug, that drug will be more rewarding,” Dr. Volkow said in a teleconference announcing the results.
Richard A. Miech, PhD, MPH, principal investigator of the survey, echoed Dr. Volkow’s concerns.
“These findings emphasize that vaping has progressed well beyond a cigarette alternative,” Dr. Miech said in a statement. “.”
Meanwhile, the past-year use of marijuana among adolescents climbed significantly by 1.3% in 2017, to 24% for all three grades levels combined.
“Historically marijuana use has gone up as adolescents see less risk of harm in using it,” Dr. Miech, of the University of Michigan, Ann Arbor, said in another statement. “We’ve found that the risk adolescents see in marijuana use has been steadily going down for years to the point that it is now at the lowest level we’ve seen in 4 decades.”
Reported overall illicit use of substances such as cocaine, anabolic steroids, and “synthetic marijuana” was relatively low.
“We should take encouragement from this year’s MTF results,” Dr. Volkow wrote on her blog. “Most substance use by middle and high school students is the lowest it has ever been, which suggests that prevention interventions and policies continue to have the desired effect. ...“There is also need to expand on prevention strategies to further reduce drug use – such as use of marijuana.”
The Monitoring the Future survey has been conducted since 1975. The 2017 survey, taken at the beginning of the year, is based on reports from almost 45,000 students in about 380 public and private secondary schools across the country. The survey is designed and conducted by research scientists at the University of Michigan, and is funded by NIDA.
SOURCE: Miech RA et al. 2017 Monitoring the Future
The positive trends in drug use among adolescents appear to be continuing – overall, according to the 2017 Monitoring the Future survey.
“This year, we have very good news, because ... the pattern of drug use among teenagers in the United States is continuing to go down, and it’s most notable in the case of opioid drugs,” Nora D. Volkow, MD, director of the National Institute on Drug Abuse said in a video interview posted by NIDA. “We are observing some of the lowest rates of opioid use that we have been monitoring through the survey.”
The national survey results of 8th, 10th, and 12th graders, released Dec. 14, found that lifetime, past-year, and past-month misuse of prescription pain medications are at lows that NIDA called historic. Among high school seniors, the survey showed, Vicodin use was at its lowest point since 2002 at 2% – a decrease from its high of 10.5% in 2003. Similarly, the reported use of OxyContin among high school seniors dropped from 5.5% in 2005 to 2.7% this year.
“We’re also seeing continuing decreases in cigarette smoking that are at the lowest levels that we’ve ever seen,” Dr. Volkow said in the interview. Daily cigarette use was 0.6% among 8th graders, 2.2% among 10th graders, and 4.2% among 12th graders in 2017. Those numbers were down from 10.4% and 18.3% among 8th and 10th graders in 1996, and a peak of 24.6 % of 12th graders in 1997.
Twelfth graders also reported lower past-year use of hookah: Use of that substance declined to 10% this year from 13% a year earlier.
As the use of traditional and smokeless tobacco has decreased, however, overall vaping levels held steady in 2017. For the first time, Monitoring the Future reported national, standard estimates of four categories of vaping: nicotine, marijuana, flavoring-only, and any vaping. The reported past-year rates of use under the “any vaping” category in the 2017 survey was 13.3% for 8th graders, 23.9% for 10th graders, and 27.8% for 12th graders. Vaping for flavoring only was the most popular option, and the highest rates were reported by 12th graders – who reported past-month use of 9.7%.
Dr. Volkow said a key concern is that it is unclear that adolescents know what substance they are vaping. Furthermore, adolescents who start vaping any substance are more likely to start to vape nicotine than are those who do not vape. “And we know that when an individual is exposed to nicotine and then gets exposed to another drug, that drug will be more rewarding,” Dr. Volkow said in a teleconference announcing the results.
Richard A. Miech, PhD, MPH, principal investigator of the survey, echoed Dr. Volkow’s concerns.
“These findings emphasize that vaping has progressed well beyond a cigarette alternative,” Dr. Miech said in a statement. “.”
Meanwhile, the past-year use of marijuana among adolescents climbed significantly by 1.3% in 2017, to 24% for all three grades levels combined.
“Historically marijuana use has gone up as adolescents see less risk of harm in using it,” Dr. Miech, of the University of Michigan, Ann Arbor, said in another statement. “We’ve found that the risk adolescents see in marijuana use has been steadily going down for years to the point that it is now at the lowest level we’ve seen in 4 decades.”
Reported overall illicit use of substances such as cocaine, anabolic steroids, and “synthetic marijuana” was relatively low.
“We should take encouragement from this year’s MTF results,” Dr. Volkow wrote on her blog. “Most substance use by middle and high school students is the lowest it has ever been, which suggests that prevention interventions and policies continue to have the desired effect. ...“There is also need to expand on prevention strategies to further reduce drug use – such as use of marijuana.”
The Monitoring the Future survey has been conducted since 1975. The 2017 survey, taken at the beginning of the year, is based on reports from almost 45,000 students in about 380 public and private secondary schools across the country. The survey is designed and conducted by research scientists at the University of Michigan, and is funded by NIDA.
SOURCE: Miech RA et al. 2017 Monitoring the Future
Key clinical point: Eighth, 10th, and 12th grade students are reporting higher rates of marijuana use.
Major finding: Teen misuse of Vicodin stood at 2% in 2017, a decline from a 10.5% peak in 2003.
Study details: Survey of 43,703 students from 360 public and private schools in the United States.
Disclosures: This survey is sponsored by a grant from the National Institute on Drug Abuse.
Source: Miech RA et al. 2017 Monitoring the Future.
Survey: Safety, rather than ethics, tops concerns about uterine transplantation
Uterine transplantation is generally seen as ethical, with acceptable risk levels for both donors and recipients, according to two parallel surveys given to women’s health physicians and to the general public.
The results, presented at the annual meeting of the American Society for Reproductive Medicine (ASRM), showed that “the majority of gynecologists surveyed find uterine transplantation to be an acceptable and ethical option for patients with uterine factor infertility,” wrote Pietro Bortoletto, MD, and his coauthors.
Similarly, about two-thirds of the general public found uterine transplantation permissible and ethical, according to responses from an age- and gender-balanced nationally representative survey.
The web-based surveys were designed to assess the personal beliefs of both the public and of physicians about the permissibility of uterine transplantation, and to evaluate respondents’ concerns about perceived risks associated with the procedure. Respondents in each survey were also asked to identify any ethical concerns they might have; recipients of both surveys received background information about uterine transplantation.
Dr. Bortoletto and his colleagues sent the survey by email to physicians who were members of ASRM and AAGL. Of the 4,216 physicians who were invited to take the survey, 447 (28.4%) completed it, though results were tallied just for the 414 respondents who were United States–based physicians.
Physician respondents, when asked whether women should be allowed to donate or receive a transplanted uterus, responded mostly in the affirmative: 20% strongly agreed and 36% agreed, while 23% were neutral. The remainder disagreed or strongly disagreed.
The possibility of complications for the recipient was identified as the top concern by about 50% of physician respondents. Next most concerning was fetal outcomes, of primary concern for about 28%, followed by complications to the donor and cost, each of which was of primary concern to 10% or fewer of the physician respondents.
The risk to donors of uterine transplantation was seen as acceptable by 73.7% of AAGL members and 71.7% of ASRM members; just over half of each group saw the risk as acceptable for the recipients and the infants, however.
Though over half of physician respondents (57.9% of AAGL members and 59.5% of ASRM members) felt that uterine transplantation should be a potential treatment option for women with absolute uterine factor infertility, fewer felt it should be covered by health insurance – 35.4% of AAGL members and 40.5% of ASRM members held this opinion.
Among the general public, over three quarters (78%) felt that women should be allowed to undergo uterine transplantation. Slightly fewer (67%) respondents to the public survey felt that uterine transplantation is ethical; those who agreed had slightly higher incomes and education levels (relative risk, 1.11 and 1.09, respectively). A similar number (66%) felt that uterine transplantation was an acceptable alternative to using a gestational carrier.
As was the case for physicians, fewer members of the general public (45%) agreed that health insurance should cover the procedure; here, women and Hispanics were more likely to agree (relative risk, 1.11 and 1.18, respectively).
The results of the survey of the general public were presented by first author Eduardo Hariton, MD, a coauthor of Dr. Bortoletto’s in the study of physician survey results. Both Dr. Hariton and Dr. Bortoletto are resident physicians in the department of obstetrics, gynecology, and reproductive biology at Brigham and Women’s Hospital, Boston.
The public survey was sent to a nationally representative sample, balanced by gender and age. Of 1,444 individuals who were recruited to receive the survey, 1,337 completed it. Ninety respondents reported that they found in vitro fertilization unacceptable; these responses were excluded, Dr. Hariton said in an interview. “We wanted to get at uterine transplantation per se,” rather than assisted reproductive technology in general.
Placing the ethics of uterine transplantation in a broader context, Dr. Bortoletto said in an interview that the United States is one of just a few countries that permit gestational surrogacy, with regulations varying by state. To his knowledge, he said, Ukraine and Russia are the only other two nations that permit compensation for surrogacy. Greece and the United Kingdom permit altruistic surrogacy, while gestational surrogacy of any sort is forbidden in the European Union. Thus, in those nations, uterine transplantation will be the only option for women who wish to bear their biological children.
“I think the main takeaway is that for people who had hesitation about [uterine transplantation], it was mainly around safety,” and not ethical concerns, said Dr. Hariton. “I was a bit surprised, but also encouraged, by the degree of support.”
Neither Dr. Hariton nor Dr. Bortoletto reported any conflicts of interest. The public opinion study was funded by an Expanding the Boundaries grant.
Uterine transplantation is generally seen as ethical, with acceptable risk levels for both donors and recipients, according to two parallel surveys given to women’s health physicians and to the general public.
The results, presented at the annual meeting of the American Society for Reproductive Medicine (ASRM), showed that “the majority of gynecologists surveyed find uterine transplantation to be an acceptable and ethical option for patients with uterine factor infertility,” wrote Pietro Bortoletto, MD, and his coauthors.
Similarly, about two-thirds of the general public found uterine transplantation permissible and ethical, according to responses from an age- and gender-balanced nationally representative survey.
The web-based surveys were designed to assess the personal beliefs of both the public and of physicians about the permissibility of uterine transplantation, and to evaluate respondents’ concerns about perceived risks associated with the procedure. Respondents in each survey were also asked to identify any ethical concerns they might have; recipients of both surveys received background information about uterine transplantation.
Dr. Bortoletto and his colleagues sent the survey by email to physicians who were members of ASRM and AAGL. Of the 4,216 physicians who were invited to take the survey, 447 (28.4%) completed it, though results were tallied just for the 414 respondents who were United States–based physicians.
Physician respondents, when asked whether women should be allowed to donate or receive a transplanted uterus, responded mostly in the affirmative: 20% strongly agreed and 36% agreed, while 23% were neutral. The remainder disagreed or strongly disagreed.
The possibility of complications for the recipient was identified as the top concern by about 50% of physician respondents. Next most concerning was fetal outcomes, of primary concern for about 28%, followed by complications to the donor and cost, each of which was of primary concern to 10% or fewer of the physician respondents.
The risk to donors of uterine transplantation was seen as acceptable by 73.7% of AAGL members and 71.7% of ASRM members; just over half of each group saw the risk as acceptable for the recipients and the infants, however.
Though over half of physician respondents (57.9% of AAGL members and 59.5% of ASRM members) felt that uterine transplantation should be a potential treatment option for women with absolute uterine factor infertility, fewer felt it should be covered by health insurance – 35.4% of AAGL members and 40.5% of ASRM members held this opinion.
Among the general public, over three quarters (78%) felt that women should be allowed to undergo uterine transplantation. Slightly fewer (67%) respondents to the public survey felt that uterine transplantation is ethical; those who agreed had slightly higher incomes and education levels (relative risk, 1.11 and 1.09, respectively). A similar number (66%) felt that uterine transplantation was an acceptable alternative to using a gestational carrier.
As was the case for physicians, fewer members of the general public (45%) agreed that health insurance should cover the procedure; here, women and Hispanics were more likely to agree (relative risk, 1.11 and 1.18, respectively).
The results of the survey of the general public were presented by first author Eduardo Hariton, MD, a coauthor of Dr. Bortoletto’s in the study of physician survey results. Both Dr. Hariton and Dr. Bortoletto are resident physicians in the department of obstetrics, gynecology, and reproductive biology at Brigham and Women’s Hospital, Boston.
The public survey was sent to a nationally representative sample, balanced by gender and age. Of 1,444 individuals who were recruited to receive the survey, 1,337 completed it. Ninety respondents reported that they found in vitro fertilization unacceptable; these responses were excluded, Dr. Hariton said in an interview. “We wanted to get at uterine transplantation per se,” rather than assisted reproductive technology in general.
Placing the ethics of uterine transplantation in a broader context, Dr. Bortoletto said in an interview that the United States is one of just a few countries that permit gestational surrogacy, with regulations varying by state. To his knowledge, he said, Ukraine and Russia are the only other two nations that permit compensation for surrogacy. Greece and the United Kingdom permit altruistic surrogacy, while gestational surrogacy of any sort is forbidden in the European Union. Thus, in those nations, uterine transplantation will be the only option for women who wish to bear their biological children.
“I think the main takeaway is that for people who had hesitation about [uterine transplantation], it was mainly around safety,” and not ethical concerns, said Dr. Hariton. “I was a bit surprised, but also encouraged, by the degree of support.”
Neither Dr. Hariton nor Dr. Bortoletto reported any conflicts of interest. The public opinion study was funded by an Expanding the Boundaries grant.
Uterine transplantation is generally seen as ethical, with acceptable risk levels for both donors and recipients, according to two parallel surveys given to women’s health physicians and to the general public.
The results, presented at the annual meeting of the American Society for Reproductive Medicine (ASRM), showed that “the majority of gynecologists surveyed find uterine transplantation to be an acceptable and ethical option for patients with uterine factor infertility,” wrote Pietro Bortoletto, MD, and his coauthors.
Similarly, about two-thirds of the general public found uterine transplantation permissible and ethical, according to responses from an age- and gender-balanced nationally representative survey.
The web-based surveys were designed to assess the personal beliefs of both the public and of physicians about the permissibility of uterine transplantation, and to evaluate respondents’ concerns about perceived risks associated with the procedure. Respondents in each survey were also asked to identify any ethical concerns they might have; recipients of both surveys received background information about uterine transplantation.
Dr. Bortoletto and his colleagues sent the survey by email to physicians who were members of ASRM and AAGL. Of the 4,216 physicians who were invited to take the survey, 447 (28.4%) completed it, though results were tallied just for the 414 respondents who were United States–based physicians.
Physician respondents, when asked whether women should be allowed to donate or receive a transplanted uterus, responded mostly in the affirmative: 20% strongly agreed and 36% agreed, while 23% were neutral. The remainder disagreed or strongly disagreed.
The possibility of complications for the recipient was identified as the top concern by about 50% of physician respondents. Next most concerning was fetal outcomes, of primary concern for about 28%, followed by complications to the donor and cost, each of which was of primary concern to 10% or fewer of the physician respondents.
The risk to donors of uterine transplantation was seen as acceptable by 73.7% of AAGL members and 71.7% of ASRM members; just over half of each group saw the risk as acceptable for the recipients and the infants, however.
Though over half of physician respondents (57.9% of AAGL members and 59.5% of ASRM members) felt that uterine transplantation should be a potential treatment option for women with absolute uterine factor infertility, fewer felt it should be covered by health insurance – 35.4% of AAGL members and 40.5% of ASRM members held this opinion.
Among the general public, over three quarters (78%) felt that women should be allowed to undergo uterine transplantation. Slightly fewer (67%) respondents to the public survey felt that uterine transplantation is ethical; those who agreed had slightly higher incomes and education levels (relative risk, 1.11 and 1.09, respectively). A similar number (66%) felt that uterine transplantation was an acceptable alternative to using a gestational carrier.
As was the case for physicians, fewer members of the general public (45%) agreed that health insurance should cover the procedure; here, women and Hispanics were more likely to agree (relative risk, 1.11 and 1.18, respectively).
The results of the survey of the general public were presented by first author Eduardo Hariton, MD, a coauthor of Dr. Bortoletto’s in the study of physician survey results. Both Dr. Hariton and Dr. Bortoletto are resident physicians in the department of obstetrics, gynecology, and reproductive biology at Brigham and Women’s Hospital, Boston.
The public survey was sent to a nationally representative sample, balanced by gender and age. Of 1,444 individuals who were recruited to receive the survey, 1,337 completed it. Ninety respondents reported that they found in vitro fertilization unacceptable; these responses were excluded, Dr. Hariton said in an interview. “We wanted to get at uterine transplantation per se,” rather than assisted reproductive technology in general.
Placing the ethics of uterine transplantation in a broader context, Dr. Bortoletto said in an interview that the United States is one of just a few countries that permit gestational surrogacy, with regulations varying by state. To his knowledge, he said, Ukraine and Russia are the only other two nations that permit compensation for surrogacy. Greece and the United Kingdom permit altruistic surrogacy, while gestational surrogacy of any sort is forbidden in the European Union. Thus, in those nations, uterine transplantation will be the only option for women who wish to bear their biological children.
“I think the main takeaway is that for people who had hesitation about [uterine transplantation], it was mainly around safety,” and not ethical concerns, said Dr. Hariton. “I was a bit surprised, but also encouraged, by the degree of support.”
Neither Dr. Hariton nor Dr. Bortoletto reported any conflicts of interest. The public opinion study was funded by an Expanding the Boundaries grant.
FDA approves premixed, low-volume colon-cleansing solution
in adults preparing to undergo colonoscopy, according to Ferring Pharmaceuticals.
The sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution is a relatively low-volume, premixed, cranberry-flavored solution, making it easier to use and more palatable for patients.
Sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution is approved with two dosing options: split dose, one dose the evening prior and one dose the morning of the procedure, or the day before dose, which involves taking both doses the day prior to the procedure. Day before dosing is an alternative and should be used when split dosing is not appropriate. After each dose of sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution, clear liquids should be consumed based on the dosing recommendation. The American College of Gastroenterology recommends the split-dose regimen because of its improved cleansing quality of the colon and better tolerability of the liquid volume by patients.
Patients with impaired renal function should exercise caution if using sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution as it may effect renal function. A more comprehensive list of safety information is available at www.clenpiq.com.
To help your patients understand and prepare for a colonoscopy, use AGA’s patient education materials at http://www.gastro.org/patient-care/procedures/colonoscopy.
in adults preparing to undergo colonoscopy, according to Ferring Pharmaceuticals.
The sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution is a relatively low-volume, premixed, cranberry-flavored solution, making it easier to use and more palatable for patients.
Sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution is approved with two dosing options: split dose, one dose the evening prior and one dose the morning of the procedure, or the day before dose, which involves taking both doses the day prior to the procedure. Day before dosing is an alternative and should be used when split dosing is not appropriate. After each dose of sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution, clear liquids should be consumed based on the dosing recommendation. The American College of Gastroenterology recommends the split-dose regimen because of its improved cleansing quality of the colon and better tolerability of the liquid volume by patients.
Patients with impaired renal function should exercise caution if using sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution as it may effect renal function. A more comprehensive list of safety information is available at www.clenpiq.com.
To help your patients understand and prepare for a colonoscopy, use AGA’s patient education materials at http://www.gastro.org/patient-care/procedures/colonoscopy.
in adults preparing to undergo colonoscopy, according to Ferring Pharmaceuticals.
The sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution is a relatively low-volume, premixed, cranberry-flavored solution, making it easier to use and more palatable for patients.
Sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution is approved with two dosing options: split dose, one dose the evening prior and one dose the morning of the procedure, or the day before dose, which involves taking both doses the day prior to the procedure. Day before dosing is an alternative and should be used when split dosing is not appropriate. After each dose of sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution, clear liquids should be consumed based on the dosing recommendation. The American College of Gastroenterology recommends the split-dose regimen because of its improved cleansing quality of the colon and better tolerability of the liquid volume by patients.
Patients with impaired renal function should exercise caution if using sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution as it may effect renal function. A more comprehensive list of safety information is available at www.clenpiq.com.
To help your patients understand and prepare for a colonoscopy, use AGA’s patient education materials at http://www.gastro.org/patient-care/procedures/colonoscopy.
Translunate, Transradial, Transtriquetral, Transtrapezoid Perilunate Dislocation With Multiple Metacarpal Neck Fractures
Take-Home Points
- Emergency physicians should be aware of radiological markers to avoid missing perilunate injuries.
- They should have a low threshold to refer a suspected perilunate injury for urgent specialist assessment.
- Although majority of the injuries demonstrate the classical pattern, one should be aware of atypical injuries.
- The principles of early anatomic reduction and stable fixation remain the same.
- Salvage procedures are only indicated in extensive irreparable injuries.
Perilunate fracture-dislocations, rare injuries representing <10% of wrist injuries,1 are part of a wide spectrum of high-energy trauma injuries. The typical mechanism of injury is a fall on a dorsiflexed and ulnar-deviated wrist with forces progressively traversing the scapholunate, lunocapitate, and lunotriquetral ligaments.2
In this article, we report a very unusual case of translunate, transradial, transtriquetral, transtrapezoid perilunate dislocation with multiple metacarpal neck fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
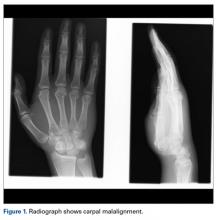
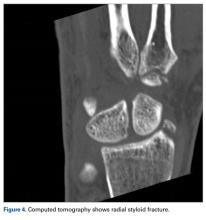
A fit and healthy 30-year-old male software professional fell down stairs, landed on his nondominant right hand, and sustained a high-energy wrist injury. The patient also sustained a concussion, without focal neurologic deficit, and was unable to recall the exact mechanism of the wrist injury (there were no other witnesses). Radiographs of the right wrist in the emergency department showed only a nondisplaced fracture of the neck of the second, third, fourth, and fifth metacarpals and a nondisplaced fracture of the radial styloid.
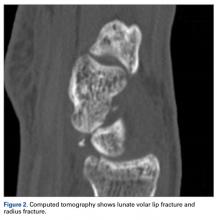
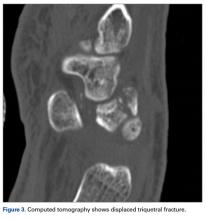
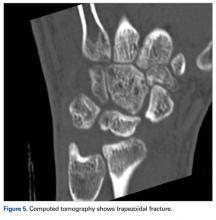
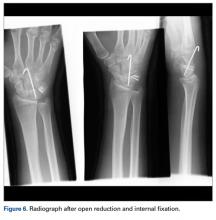
The next day, with the patient under general anesthesia, an attempt to reduce the perilunate dislocation by manipulation was unsuccessful. Open reduction and internal fixation (ORIF) were performed through a dorsal approach; the perilunate dislocation was reduced and stabilized with lunocapitate 1.2-mm Kirschner wire (K-wire). The scapholunate and lunotriquetral ligaments were found to be intact, and the significantly displaced triquetral fracture was treated with internal fixation involving 2 minifragment screws (Figure 6).
Discussion
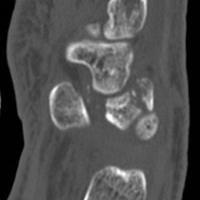
Perilunate injuries are classified as lesser arc injuries (purely ligamentous) or greater arc injuries (osseoligamentous). Greater arc injuries involve fracture of one or more carpal bones with associated ligamentous injuries.3 The greater or lesser arc injuries described by Mayfield and colleagues2 imply a specific pattern of force transmission with axial loading in a dorsiflexed and ulnar-deviated wrist with intercarpal supination. Graham4 introduced a concept of inferior arc injury with the forces passing through the radiocarpal joint with fracture of the radial styloid or juxta-articular margin. Similarly, lunate fracture in perilunate dislocations was explained by Bain and colleagues5 in the translunate arc concept in which forces pass through the lunate bone. A study involving a literature review of translunate perilunate dislocations noted associated transradial, trans-scaphoid, transcapitate, and transtriquetral fractures in order of decreasing frequency.6 To our knowledge, no case of translunate perilunate dislocation with multiple carpal and metacarpal fractures with radial styloid fracture has been reported in the literature.
Our patient’s associated multiple metacarpal neck fractures can be explained by the peculiar double-impact injury with initial axial loading across the hyperextended metacarpophalangeal joint, followed by axial loading across the hyperextended and ulnar-deviated wrist, causing greater arc perilunate fracture-dislocation. The mechanism of lunate injury in this case seems to be longitudinal impaction of the capitate shearing against the volar lunate in the axial plane causing a volar lip fracture (Teisen type I), and this may be accentuated by tension in the volar radiolunate ligament.6,7 Associated triquetral fracture in perilunate dislocation is well described in the literature.6 However, the trapezoid fracture in our case implies a very atypical pattern of force transmission with the arc probably passing more distally through the trapezoid laterally and the triquetrum medially.
This case, which represents a very rare fracture pattern associated with perilunate dislocation, may have been caused by the variable position of the wrist and the pattern of load transmission at time of impact. Although the majority of cases demonstrate the classical pattern described in the literature, it may not be unusual to find atypical fracture patterns, especially those associated with high-energy trauma.
Perilunate injuries have been missed in busy emergency departments and orthopedic practices. An estimated 25% of such injuries can be missed on initial presentation.8 In the present case, fracture of the radial styloid provided a clue to possible more complex carpal injuries involving the scaphoid, lunate, or scapholunate ligament, as Graham4 suggested with the concept of the “transverse pattern” of force transmission. In this case as well, the injury was initially missed, and its extent became evident only with CT. Therefore, emergency teams should have a very low threshold for suspecting and evaluating high-energy wrist injuries.
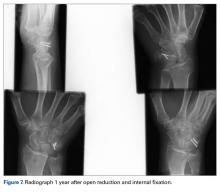
The goal in the treatment of perilunate dislocation with multiple carpal fractures is anatomical reduction and restoration of carpal alignment—which frequently require ORIF, though acute salvage procedures like proximal row carpectomy may be considered in irreparable fractures with extensive ligament injuries.9 For open reduction, the approach can be dorsal, volar, or a combination. The approach in our patient’s case was dorsal. His triquetral fracture, his only displaced fracture, was treated with internal fixation. All other fractures were nondisplaced, stable, and did not warrant internal fixation.
A high index of suspicion and urgent specialist consultation are essential in suspected perilunate injuries. The injury and fracture pattern may be atypical, but the principles of early anatomical reduction and stable fixation remain the same.
1. Youssef B, Deshmukh SC. Volar perilunate dislocation: a case report and review of the literature. Open Orthop J. 2008;2:57-58.
2. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
3. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
4. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
5. Bain GI, McLean JM, Turner PC, Sood A, Pourgiezis N. Translunate fracture with associated perilunate injury: 3 case reports with introduction of the translunate arc concept. J Hand Surg Am. 2008;33(10):1770-1776.
6. Bain GI, Pallapati S, Eng K. Translunate perilunate injuries—a spectrum of this uncommon injury. J Wrist Surg. 2013;2(1):63-68.
7. Teisen H, Hjarbaek J. Classification of fresh fractures of the lunate. J Hand Surg Br. 1988;13(4):458-462.
8. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
9. Huish EG Jr, Vitale MA, Shin AY. Acute proximal row carpectomy to treat a transscaphoid, transtriquetral perilunate fracture dislocation: case report and review of the literature. Hand
(N Y). 2013;8(1):105-109.
Take-Home Points
- Emergency physicians should be aware of radiological markers to avoid missing perilunate injuries.
- They should have a low threshold to refer a suspected perilunate injury for urgent specialist assessment.
- Although majority of the injuries demonstrate the classical pattern, one should be aware of atypical injuries.
- The principles of early anatomic reduction and stable fixation remain the same.
- Salvage procedures are only indicated in extensive irreparable injuries.
Perilunate fracture-dislocations, rare injuries representing <10% of wrist injuries,1 are part of a wide spectrum of high-energy trauma injuries. The typical mechanism of injury is a fall on a dorsiflexed and ulnar-deviated wrist with forces progressively traversing the scapholunate, lunocapitate, and lunotriquetral ligaments.2
In this article, we report a very unusual case of translunate, transradial, transtriquetral, transtrapezoid perilunate dislocation with multiple metacarpal neck fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A fit and healthy 30-year-old male software professional fell down stairs, landed on his nondominant right hand, and sustained a high-energy wrist injury. The patient also sustained a concussion, without focal neurologic deficit, and was unable to recall the exact mechanism of the wrist injury (there were no other witnesses). Radiographs of the right wrist in the emergency department showed only a nondisplaced fracture of the neck of the second, third, fourth, and fifth metacarpals and a nondisplaced fracture of the radial styloid.
The next day, with the patient under general anesthesia, an attempt to reduce the perilunate dislocation by manipulation was unsuccessful. Open reduction and internal fixation (ORIF) were performed through a dorsal approach; the perilunate dislocation was reduced and stabilized with lunocapitate 1.2-mm Kirschner wire (K-wire). The scapholunate and lunotriquetral ligaments were found to be intact, and the significantly displaced triquetral fracture was treated with internal fixation involving 2 minifragment screws (Figure 6).
Discussion
Perilunate injuries are classified as lesser arc injuries (purely ligamentous) or greater arc injuries (osseoligamentous). Greater arc injuries involve fracture of one or more carpal bones with associated ligamentous injuries.3 The greater or lesser arc injuries described by Mayfield and colleagues2 imply a specific pattern of force transmission with axial loading in a dorsiflexed and ulnar-deviated wrist with intercarpal supination. Graham4 introduced a concept of inferior arc injury with the forces passing through the radiocarpal joint with fracture of the radial styloid or juxta-articular margin. Similarly, lunate fracture in perilunate dislocations was explained by Bain and colleagues5 in the translunate arc concept in which forces pass through the lunate bone. A study involving a literature review of translunate perilunate dislocations noted associated transradial, trans-scaphoid, transcapitate, and transtriquetral fractures in order of decreasing frequency.6 To our knowledge, no case of translunate perilunate dislocation with multiple carpal and metacarpal fractures with radial styloid fracture has been reported in the literature.
Our patient’s associated multiple metacarpal neck fractures can be explained by the peculiar double-impact injury with initial axial loading across the hyperextended metacarpophalangeal joint, followed by axial loading across the hyperextended and ulnar-deviated wrist, causing greater arc perilunate fracture-dislocation. The mechanism of lunate injury in this case seems to be longitudinal impaction of the capitate shearing against the volar lunate in the axial plane causing a volar lip fracture (Teisen type I), and this may be accentuated by tension in the volar radiolunate ligament.6,7 Associated triquetral fracture in perilunate dislocation is well described in the literature.6 However, the trapezoid fracture in our case implies a very atypical pattern of force transmission with the arc probably passing more distally through the trapezoid laterally and the triquetrum medially.
This case, which represents a very rare fracture pattern associated with perilunate dislocation, may have been caused by the variable position of the wrist and the pattern of load transmission at time of impact. Although the majority of cases demonstrate the classical pattern described in the literature, it may not be unusual to find atypical fracture patterns, especially those associated with high-energy trauma.
Perilunate injuries have been missed in busy emergency departments and orthopedic practices. An estimated 25% of such injuries can be missed on initial presentation.8 In the present case, fracture of the radial styloid provided a clue to possible more complex carpal injuries involving the scaphoid, lunate, or scapholunate ligament, as Graham4 suggested with the concept of the “transverse pattern” of force transmission. In this case as well, the injury was initially missed, and its extent became evident only with CT. Therefore, emergency teams should have a very low threshold for suspecting and evaluating high-energy wrist injuries.
The goal in the treatment of perilunate dislocation with multiple carpal fractures is anatomical reduction and restoration of carpal alignment—which frequently require ORIF, though acute salvage procedures like proximal row carpectomy may be considered in irreparable fractures with extensive ligament injuries.9 For open reduction, the approach can be dorsal, volar, or a combination. The approach in our patient’s case was dorsal. His triquetral fracture, his only displaced fracture, was treated with internal fixation. All other fractures were nondisplaced, stable, and did not warrant internal fixation.
A high index of suspicion and urgent specialist consultation are essential in suspected perilunate injuries. The injury and fracture pattern may be atypical, but the principles of early anatomical reduction and stable fixation remain the same.
Take-Home Points
- Emergency physicians should be aware of radiological markers to avoid missing perilunate injuries.
- They should have a low threshold to refer a suspected perilunate injury for urgent specialist assessment.
- Although majority of the injuries demonstrate the classical pattern, one should be aware of atypical injuries.
- The principles of early anatomic reduction and stable fixation remain the same.
- Salvage procedures are only indicated in extensive irreparable injuries.
Perilunate fracture-dislocations, rare injuries representing <10% of wrist injuries,1 are part of a wide spectrum of high-energy trauma injuries. The typical mechanism of injury is a fall on a dorsiflexed and ulnar-deviated wrist with forces progressively traversing the scapholunate, lunocapitate, and lunotriquetral ligaments.2
In this article, we report a very unusual case of translunate, transradial, transtriquetral, transtrapezoid perilunate dislocation with multiple metacarpal neck fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A fit and healthy 30-year-old male software professional fell down stairs, landed on his nondominant right hand, and sustained a high-energy wrist injury. The patient also sustained a concussion, without focal neurologic deficit, and was unable to recall the exact mechanism of the wrist injury (there were no other witnesses). Radiographs of the right wrist in the emergency department showed only a nondisplaced fracture of the neck of the second, third, fourth, and fifth metacarpals and a nondisplaced fracture of the radial styloid.
The next day, with the patient under general anesthesia, an attempt to reduce the perilunate dislocation by manipulation was unsuccessful. Open reduction and internal fixation (ORIF) were performed through a dorsal approach; the perilunate dislocation was reduced and stabilized with lunocapitate 1.2-mm Kirschner wire (K-wire). The scapholunate and lunotriquetral ligaments were found to be intact, and the significantly displaced triquetral fracture was treated with internal fixation involving 2 minifragment screws (Figure 6).
Discussion
Perilunate injuries are classified as lesser arc injuries (purely ligamentous) or greater arc injuries (osseoligamentous). Greater arc injuries involve fracture of one or more carpal bones with associated ligamentous injuries.3 The greater or lesser arc injuries described by Mayfield and colleagues2 imply a specific pattern of force transmission with axial loading in a dorsiflexed and ulnar-deviated wrist with intercarpal supination. Graham4 introduced a concept of inferior arc injury with the forces passing through the radiocarpal joint with fracture of the radial styloid or juxta-articular margin. Similarly, lunate fracture in perilunate dislocations was explained by Bain and colleagues5 in the translunate arc concept in which forces pass through the lunate bone. A study involving a literature review of translunate perilunate dislocations noted associated transradial, trans-scaphoid, transcapitate, and transtriquetral fractures in order of decreasing frequency.6 To our knowledge, no case of translunate perilunate dislocation with multiple carpal and metacarpal fractures with radial styloid fracture has been reported in the literature.
Our patient’s associated multiple metacarpal neck fractures can be explained by the peculiar double-impact injury with initial axial loading across the hyperextended metacarpophalangeal joint, followed by axial loading across the hyperextended and ulnar-deviated wrist, causing greater arc perilunate fracture-dislocation. The mechanism of lunate injury in this case seems to be longitudinal impaction of the capitate shearing against the volar lunate in the axial plane causing a volar lip fracture (Teisen type I), and this may be accentuated by tension in the volar radiolunate ligament.6,7 Associated triquetral fracture in perilunate dislocation is well described in the literature.6 However, the trapezoid fracture in our case implies a very atypical pattern of force transmission with the arc probably passing more distally through the trapezoid laterally and the triquetrum medially.
This case, which represents a very rare fracture pattern associated with perilunate dislocation, may have been caused by the variable position of the wrist and the pattern of load transmission at time of impact. Although the majority of cases demonstrate the classical pattern described in the literature, it may not be unusual to find atypical fracture patterns, especially those associated with high-energy trauma.
Perilunate injuries have been missed in busy emergency departments and orthopedic practices. An estimated 25% of such injuries can be missed on initial presentation.8 In the present case, fracture of the radial styloid provided a clue to possible more complex carpal injuries involving the scaphoid, lunate, or scapholunate ligament, as Graham4 suggested with the concept of the “transverse pattern” of force transmission. In this case as well, the injury was initially missed, and its extent became evident only with CT. Therefore, emergency teams should have a very low threshold for suspecting and evaluating high-energy wrist injuries.
The goal in the treatment of perilunate dislocation with multiple carpal fractures is anatomical reduction and restoration of carpal alignment—which frequently require ORIF, though acute salvage procedures like proximal row carpectomy may be considered in irreparable fractures with extensive ligament injuries.9 For open reduction, the approach can be dorsal, volar, or a combination. The approach in our patient’s case was dorsal. His triquetral fracture, his only displaced fracture, was treated with internal fixation. All other fractures were nondisplaced, stable, and did not warrant internal fixation.
A high index of suspicion and urgent specialist consultation are essential in suspected perilunate injuries. The injury and fracture pattern may be atypical, but the principles of early anatomical reduction and stable fixation remain the same.
1. Youssef B, Deshmukh SC. Volar perilunate dislocation: a case report and review of the literature. Open Orthop J. 2008;2:57-58.
2. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
3. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
4. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
5. Bain GI, McLean JM, Turner PC, Sood A, Pourgiezis N. Translunate fracture with associated perilunate injury: 3 case reports with introduction of the translunate arc concept. J Hand Surg Am. 2008;33(10):1770-1776.
6. Bain GI, Pallapati S, Eng K. Translunate perilunate injuries—a spectrum of this uncommon injury. J Wrist Surg. 2013;2(1):63-68.
7. Teisen H, Hjarbaek J. Classification of fresh fractures of the lunate. J Hand Surg Br. 1988;13(4):458-462.
8. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
9. Huish EG Jr, Vitale MA, Shin AY. Acute proximal row carpectomy to treat a transscaphoid, transtriquetral perilunate fracture dislocation: case report and review of the literature. Hand
(N Y). 2013;8(1):105-109.
1. Youssef B, Deshmukh SC. Volar perilunate dislocation: a case report and review of the literature. Open Orthop J. 2008;2:57-58.
2. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
3. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
4. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
5. Bain GI, McLean JM, Turner PC, Sood A, Pourgiezis N. Translunate fracture with associated perilunate injury: 3 case reports with introduction of the translunate arc concept. J Hand Surg Am. 2008;33(10):1770-1776.
6. Bain GI, Pallapati S, Eng K. Translunate perilunate injuries—a spectrum of this uncommon injury. J Wrist Surg. 2013;2(1):63-68.
7. Teisen H, Hjarbaek J. Classification of fresh fractures of the lunate. J Hand Surg Br. 1988;13(4):458-462.
8. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
9. Huish EG Jr, Vitale MA, Shin AY. Acute proximal row carpectomy to treat a transscaphoid, transtriquetral perilunate fracture dislocation: case report and review of the literature. Hand
(N Y). 2013;8(1):105-109.
Complex atypical hyperplasia: When is it appropriate to refer?
Complex atypical hyperplasia (CAH) of the endometrium is considered the precursor for endometrioid endometrial cancer, the most common gynecologic cancer in the United States. This disease is most frequently diagnosed by gynecologists who are evaluating symptoms of abnormal uterine bleeding in premenopausal women or in postmenopausal women who experience new bleeding. Medical therapies, typically progestin-based treatments, can be employed, particularly when fertility preservation is desired or among patients who are poor surgical candidates. However, the most definitive therapy remains surgery with total hysterectomy for two reasons: CAH is associated with a 28% risk for the development of invasive cancer, and occult invasive cancer frequently coexists with CAH.1,2 This raises a question for gynecologists: Given the risk for occult endometrial cancer, should patients be referred to a gynecologic oncologist for their surgery?
What is the risk for cancer?
What is the significance of occult malignancy with CAH?
If surgeons are aware of endometrial cancer preoperatively or intraoperatively, decisions can be made about staging, particularly the need for lymphadenectomy. The virtues of staging in endometrial cancer is a controversial and frequently debated topic. No survival (therapeutic) benefit from lymphadenectomy has been observed in prospective trials when the information from staging results is not used to guide adjuvant therapy.4 However, the administration of adjuvant chemotherapy is associated with improved survival for patients with lymph node metastases.5 Therefore, if there is a benefit to staging with lymphadenectomy, it is its ability to identify patients who most need this life-saving systemic therapy.
Not all patients with endometrial cancer are at equal risk for harboring lymph node metastases and the majority may not benefit from lymphadenectomy. Patients with tumors that are deeply invasive, moderate or high grade, larger than 2 cm, or that have lymphovascular space invasion are at higher risk for lymph node metastases. Women with low grade, minimally invasive tumors that are smaller than 2 cm have extremely low risk for metastases.6 These criteria are commonly employed to stratify women at lowest risk and minimize unnecessary lymphadenectomy procedures. It should be noted that all three of these low risk features must be present to convey that negligible risk profile. The finding of a grade 1 invasive tumor alone is not enough to exclude potential lymph node metastases, particularly in the case of large or deeply invasive cancers.
How can the diagnosis be made preoperatively or intraoperatively?
The gold standard for discriminating between CAH and endometrial cancer is definitive surgical pathology. However, if surgeons wait until these results are available, they have lost the opportunity to stage the patient without subjecting them to a second surgery. The preoperative discovery of cancer may be increased by performing diagnostic curettage rather than relying on office endometrial biopsy sampling.7 This is likely due to the increased volume of tissue removed with dilation and curettage, and a reduction in the risk for sampling error. The addition of hysteroscopy to curettage does not improve upon the detection of cancer. Preoperative MRI to evaluate for depth of myometrial invasion has been described in cases of known endometrial cancer; however, its role in discriminating between CAH and invasive cancer is not well studied.
Intraoperative frozen section is commonly employed to evaluate the hysterectomy specimen for cancer in order to triage patients to staging during that same surgery. However, the accuracy of frozen section with definitive pathology is only approximately 50%.8 This means that at least half of women with CAH will have a false negative frozen section result and will have lost the opportunity for staging at the same procedure. The inaccuracy of frozen section is often overlooked by surgeons who may feel that it is a very straightforward diagnostic procedure. In reality, the characterization of CAH and invasive cancer is technically challenging and relies on multiple sectioning and significant experience in gynecologic pathology.9
Should all patients with CAH be referred and staged?
An alternative to relying on the frozen section process and its inherent inaccuracies would be to routinely stage all women with CAH, knowing that approximately 40% of them have occult cancer, and more than a third of those will have high risk features for lymph node metastases. However, due to the risks associated with lymphadenectomy, particularly lymphedema, most gynecologic oncologists do not routinely stage patients with preoperative CAH with complete lymphadenectomy.
An alternative to the all (complete lymphadenectomy) or none (hysterectomy alone) approach is to perform sentinel lymph node (SLN) biopsy for patients with CAH. SLN biopsy involves removing scant, but high yield lymphatic tissue, and has been shown to be extremely sensitive in detecting metastatic disease.10 This approach is commonly employed by surgeons in the treatment of ductal carcinoma in situ of the breast which, like CAH, is a stage 0 cancer that can be associated with invasive carcinoma on final pathology. In the case of ductal carcinoma in situ, the risk for upstaging is actually substantially lower (25%) than what is observed in CAH.11 Therefore, it would seem even more compelling to apply this approach for endometrial pathologies. The ability to apply the SLN technique is lost after hysterectomy is performed, as there is no longer the target organ into which tracer can be injected; therefore, if SLN biopsy is to be offered to these patients, it needs to be performed using only the preoperative diagnosis of CAH. In this approach, there will be overtreatment of approximately two-thirds of patients, albeit with a less radical and morbid staging procedure.
Making the decision to refer
Ultimately, decisions to refer or not are guided by comprehensive discussions between patient and provider that outline the potential risks and benefits of various approaches. Patients frequently have strong relationships with confidence in their gynecologists who may have cared for them for many years, and may be motivated to have them perform their surgery. For others, the uncertainty and possibility of an unstaged cancer and the potential of a second surgery drives their decision to seek an oncology consultation. Clinicians should discuss the inherent uncertainties in the diagnosis of CAH and the potential for underlying cancer and lymph node metastases, and help patients determine the balance of their underlying competing concerns regarding the risk for inadequate surgery versus the risk of unnecessary surgical procedures.
Summary of recommendations
Invasive endometrial cancer will be identified in the hysterectomy specimens of approximately 40% of women with a preoperative diagnosis of complex endometrial hyperplasia. Preoperative dilation and curettage may reduce the potential for missed occult cancer. Frozen section is an option for determining which patients might benefit from staging but is associated with significant inaccuracies. Failure to diagnose malignancy pre- or intraoperatively handicaps postoperative decision making regarding the necessity of adjuvant chemotherapy, and prevents the ability to offer patients potentially less morbid staging techniques such as SLN biopsy. When gynecologists without oncology training perform these hysterectomies, they should discuss these scenarios to patients and consider referral to gynecologic oncology for patients who desire the potential for comprehensive staging if necessary.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. She reports no relevant financial disclosures.
References
1. J Clin Oncol. 2010 Feb;28:788-92.
2. Cancer. 2006 Feb;106:812-9.
3. Int J Gynecol Cancer. 2005 Jan-Feb;15:127-31.
4. Lancet. 2009 Jan;373(9658):125-36.
5. J Clin Oncol. 2006 Jan;24:36-44.
6. Gynecol Oncol 2008 Apr;109:11-8.
7. Am J Obstet Gynecol. 2010 Oct;203(4):349. e1-6.
8. Am J Obstet Gynecol. 2007 May;196(5):e40-2.
9. Obstet Gynecol. 2012 Nov;120(5):1160-75.
10. Lancet Oncol. 2017 Mar;18(3):384-92.
11. Radiology. 2011 Jul;260:119-28.
Complex atypical hyperplasia (CAH) of the endometrium is considered the precursor for endometrioid endometrial cancer, the most common gynecologic cancer in the United States. This disease is most frequently diagnosed by gynecologists who are evaluating symptoms of abnormal uterine bleeding in premenopausal women or in postmenopausal women who experience new bleeding. Medical therapies, typically progestin-based treatments, can be employed, particularly when fertility preservation is desired or among patients who are poor surgical candidates. However, the most definitive therapy remains surgery with total hysterectomy for two reasons: CAH is associated with a 28% risk for the development of invasive cancer, and occult invasive cancer frequently coexists with CAH.1,2 This raises a question for gynecologists: Given the risk for occult endometrial cancer, should patients be referred to a gynecologic oncologist for their surgery?
What is the risk for cancer?
What is the significance of occult malignancy with CAH?
If surgeons are aware of endometrial cancer preoperatively or intraoperatively, decisions can be made about staging, particularly the need for lymphadenectomy. The virtues of staging in endometrial cancer is a controversial and frequently debated topic. No survival (therapeutic) benefit from lymphadenectomy has been observed in prospective trials when the information from staging results is not used to guide adjuvant therapy.4 However, the administration of adjuvant chemotherapy is associated with improved survival for patients with lymph node metastases.5 Therefore, if there is a benefit to staging with lymphadenectomy, it is its ability to identify patients who most need this life-saving systemic therapy.
Not all patients with endometrial cancer are at equal risk for harboring lymph node metastases and the majority may not benefit from lymphadenectomy. Patients with tumors that are deeply invasive, moderate or high grade, larger than 2 cm, or that have lymphovascular space invasion are at higher risk for lymph node metastases. Women with low grade, minimally invasive tumors that are smaller than 2 cm have extremely low risk for metastases.6 These criteria are commonly employed to stratify women at lowest risk and minimize unnecessary lymphadenectomy procedures. It should be noted that all three of these low risk features must be present to convey that negligible risk profile. The finding of a grade 1 invasive tumor alone is not enough to exclude potential lymph node metastases, particularly in the case of large or deeply invasive cancers.
How can the diagnosis be made preoperatively or intraoperatively?
The gold standard for discriminating between CAH and endometrial cancer is definitive surgical pathology. However, if surgeons wait until these results are available, they have lost the opportunity to stage the patient without subjecting them to a second surgery. The preoperative discovery of cancer may be increased by performing diagnostic curettage rather than relying on office endometrial biopsy sampling.7 This is likely due to the increased volume of tissue removed with dilation and curettage, and a reduction in the risk for sampling error. The addition of hysteroscopy to curettage does not improve upon the detection of cancer. Preoperative MRI to evaluate for depth of myometrial invasion has been described in cases of known endometrial cancer; however, its role in discriminating between CAH and invasive cancer is not well studied.
Intraoperative frozen section is commonly employed to evaluate the hysterectomy specimen for cancer in order to triage patients to staging during that same surgery. However, the accuracy of frozen section with definitive pathology is only approximately 50%.8 This means that at least half of women with CAH will have a false negative frozen section result and will have lost the opportunity for staging at the same procedure. The inaccuracy of frozen section is often overlooked by surgeons who may feel that it is a very straightforward diagnostic procedure. In reality, the characterization of CAH and invasive cancer is technically challenging and relies on multiple sectioning and significant experience in gynecologic pathology.9
Should all patients with CAH be referred and staged?
An alternative to relying on the frozen section process and its inherent inaccuracies would be to routinely stage all women with CAH, knowing that approximately 40% of them have occult cancer, and more than a third of those will have high risk features for lymph node metastases. However, due to the risks associated with lymphadenectomy, particularly lymphedema, most gynecologic oncologists do not routinely stage patients with preoperative CAH with complete lymphadenectomy.
An alternative to the all (complete lymphadenectomy) or none (hysterectomy alone) approach is to perform sentinel lymph node (SLN) biopsy for patients with CAH. SLN biopsy involves removing scant, but high yield lymphatic tissue, and has been shown to be extremely sensitive in detecting metastatic disease.10 This approach is commonly employed by surgeons in the treatment of ductal carcinoma in situ of the breast which, like CAH, is a stage 0 cancer that can be associated with invasive carcinoma on final pathology. In the case of ductal carcinoma in situ, the risk for upstaging is actually substantially lower (25%) than what is observed in CAH.11 Therefore, it would seem even more compelling to apply this approach for endometrial pathologies. The ability to apply the SLN technique is lost after hysterectomy is performed, as there is no longer the target organ into which tracer can be injected; therefore, if SLN biopsy is to be offered to these patients, it needs to be performed using only the preoperative diagnosis of CAH. In this approach, there will be overtreatment of approximately two-thirds of patients, albeit with a less radical and morbid staging procedure.
Making the decision to refer
Ultimately, decisions to refer or not are guided by comprehensive discussions between patient and provider that outline the potential risks and benefits of various approaches. Patients frequently have strong relationships with confidence in their gynecologists who may have cared for them for many years, and may be motivated to have them perform their surgery. For others, the uncertainty and possibility of an unstaged cancer and the potential of a second surgery drives their decision to seek an oncology consultation. Clinicians should discuss the inherent uncertainties in the diagnosis of CAH and the potential for underlying cancer and lymph node metastases, and help patients determine the balance of their underlying competing concerns regarding the risk for inadequate surgery versus the risk of unnecessary surgical procedures.
Summary of recommendations
Invasive endometrial cancer will be identified in the hysterectomy specimens of approximately 40% of women with a preoperative diagnosis of complex endometrial hyperplasia. Preoperative dilation and curettage may reduce the potential for missed occult cancer. Frozen section is an option for determining which patients might benefit from staging but is associated with significant inaccuracies. Failure to diagnose malignancy pre- or intraoperatively handicaps postoperative decision making regarding the necessity of adjuvant chemotherapy, and prevents the ability to offer patients potentially less morbid staging techniques such as SLN biopsy. When gynecologists without oncology training perform these hysterectomies, they should discuss these scenarios to patients and consider referral to gynecologic oncology for patients who desire the potential for comprehensive staging if necessary.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. She reports no relevant financial disclosures.
References
1. J Clin Oncol. 2010 Feb;28:788-92.
2. Cancer. 2006 Feb;106:812-9.
3. Int J Gynecol Cancer. 2005 Jan-Feb;15:127-31.
4. Lancet. 2009 Jan;373(9658):125-36.
5. J Clin Oncol. 2006 Jan;24:36-44.
6. Gynecol Oncol 2008 Apr;109:11-8.
7. Am J Obstet Gynecol. 2010 Oct;203(4):349. e1-6.
8. Am J Obstet Gynecol. 2007 May;196(5):e40-2.
9. Obstet Gynecol. 2012 Nov;120(5):1160-75.
10. Lancet Oncol. 2017 Mar;18(3):384-92.
11. Radiology. 2011 Jul;260:119-28.
Complex atypical hyperplasia (CAH) of the endometrium is considered the precursor for endometrioid endometrial cancer, the most common gynecologic cancer in the United States. This disease is most frequently diagnosed by gynecologists who are evaluating symptoms of abnormal uterine bleeding in premenopausal women or in postmenopausal women who experience new bleeding. Medical therapies, typically progestin-based treatments, can be employed, particularly when fertility preservation is desired or among patients who are poor surgical candidates. However, the most definitive therapy remains surgery with total hysterectomy for two reasons: CAH is associated with a 28% risk for the development of invasive cancer, and occult invasive cancer frequently coexists with CAH.1,2 This raises a question for gynecologists: Given the risk for occult endometrial cancer, should patients be referred to a gynecologic oncologist for their surgery?
What is the risk for cancer?
What is the significance of occult malignancy with CAH?
If surgeons are aware of endometrial cancer preoperatively or intraoperatively, decisions can be made about staging, particularly the need for lymphadenectomy. The virtues of staging in endometrial cancer is a controversial and frequently debated topic. No survival (therapeutic) benefit from lymphadenectomy has been observed in prospective trials when the information from staging results is not used to guide adjuvant therapy.4 However, the administration of adjuvant chemotherapy is associated with improved survival for patients with lymph node metastases.5 Therefore, if there is a benefit to staging with lymphadenectomy, it is its ability to identify patients who most need this life-saving systemic therapy.
Not all patients with endometrial cancer are at equal risk for harboring lymph node metastases and the majority may not benefit from lymphadenectomy. Patients with tumors that are deeply invasive, moderate or high grade, larger than 2 cm, or that have lymphovascular space invasion are at higher risk for lymph node metastases. Women with low grade, minimally invasive tumors that are smaller than 2 cm have extremely low risk for metastases.6 These criteria are commonly employed to stratify women at lowest risk and minimize unnecessary lymphadenectomy procedures. It should be noted that all three of these low risk features must be present to convey that negligible risk profile. The finding of a grade 1 invasive tumor alone is not enough to exclude potential lymph node metastases, particularly in the case of large or deeply invasive cancers.
How can the diagnosis be made preoperatively or intraoperatively?
The gold standard for discriminating between CAH and endometrial cancer is definitive surgical pathology. However, if surgeons wait until these results are available, they have lost the opportunity to stage the patient without subjecting them to a second surgery. The preoperative discovery of cancer may be increased by performing diagnostic curettage rather than relying on office endometrial biopsy sampling.7 This is likely due to the increased volume of tissue removed with dilation and curettage, and a reduction in the risk for sampling error. The addition of hysteroscopy to curettage does not improve upon the detection of cancer. Preoperative MRI to evaluate for depth of myometrial invasion has been described in cases of known endometrial cancer; however, its role in discriminating between CAH and invasive cancer is not well studied.
Intraoperative frozen section is commonly employed to evaluate the hysterectomy specimen for cancer in order to triage patients to staging during that same surgery. However, the accuracy of frozen section with definitive pathology is only approximately 50%.8 This means that at least half of women with CAH will have a false negative frozen section result and will have lost the opportunity for staging at the same procedure. The inaccuracy of frozen section is often overlooked by surgeons who may feel that it is a very straightforward diagnostic procedure. In reality, the characterization of CAH and invasive cancer is technically challenging and relies on multiple sectioning and significant experience in gynecologic pathology.9
Should all patients with CAH be referred and staged?
An alternative to relying on the frozen section process and its inherent inaccuracies would be to routinely stage all women with CAH, knowing that approximately 40% of them have occult cancer, and more than a third of those will have high risk features for lymph node metastases. However, due to the risks associated with lymphadenectomy, particularly lymphedema, most gynecologic oncologists do not routinely stage patients with preoperative CAH with complete lymphadenectomy.
An alternative to the all (complete lymphadenectomy) or none (hysterectomy alone) approach is to perform sentinel lymph node (SLN) biopsy for patients with CAH. SLN biopsy involves removing scant, but high yield lymphatic tissue, and has been shown to be extremely sensitive in detecting metastatic disease.10 This approach is commonly employed by surgeons in the treatment of ductal carcinoma in situ of the breast which, like CAH, is a stage 0 cancer that can be associated with invasive carcinoma on final pathology. In the case of ductal carcinoma in situ, the risk for upstaging is actually substantially lower (25%) than what is observed in CAH.11 Therefore, it would seem even more compelling to apply this approach for endometrial pathologies. The ability to apply the SLN technique is lost after hysterectomy is performed, as there is no longer the target organ into which tracer can be injected; therefore, if SLN biopsy is to be offered to these patients, it needs to be performed using only the preoperative diagnosis of CAH. In this approach, there will be overtreatment of approximately two-thirds of patients, albeit with a less radical and morbid staging procedure.
Making the decision to refer
Ultimately, decisions to refer or not are guided by comprehensive discussions between patient and provider that outline the potential risks and benefits of various approaches. Patients frequently have strong relationships with confidence in their gynecologists who may have cared for them for many years, and may be motivated to have them perform their surgery. For others, the uncertainty and possibility of an unstaged cancer and the potential of a second surgery drives their decision to seek an oncology consultation. Clinicians should discuss the inherent uncertainties in the diagnosis of CAH and the potential for underlying cancer and lymph node metastases, and help patients determine the balance of their underlying competing concerns regarding the risk for inadequate surgery versus the risk of unnecessary surgical procedures.
Summary of recommendations
Invasive endometrial cancer will be identified in the hysterectomy specimens of approximately 40% of women with a preoperative diagnosis of complex endometrial hyperplasia. Preoperative dilation and curettage may reduce the potential for missed occult cancer. Frozen section is an option for determining which patients might benefit from staging but is associated with significant inaccuracies. Failure to diagnose malignancy pre- or intraoperatively handicaps postoperative decision making regarding the necessity of adjuvant chemotherapy, and prevents the ability to offer patients potentially less morbid staging techniques such as SLN biopsy. When gynecologists without oncology training perform these hysterectomies, they should discuss these scenarios to patients and consider referral to gynecologic oncology for patients who desire the potential for comprehensive staging if necessary.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. She reports no relevant financial disclosures.
References
1. J Clin Oncol. 2010 Feb;28:788-92.
2. Cancer. 2006 Feb;106:812-9.
3. Int J Gynecol Cancer. 2005 Jan-Feb;15:127-31.
4. Lancet. 2009 Jan;373(9658):125-36.
5. J Clin Oncol. 2006 Jan;24:36-44.
6. Gynecol Oncol 2008 Apr;109:11-8.
7. Am J Obstet Gynecol. 2010 Oct;203(4):349. e1-6.
8. Am J Obstet Gynecol. 2007 May;196(5):e40-2.
9. Obstet Gynecol. 2012 Nov;120(5):1160-75.
10. Lancet Oncol. 2017 Mar;18(3):384-92.
11. Radiology. 2011 Jul;260:119-28.
‘We have met the enemy and he is us’
In a recent article, the New York Times publicly exposed behavior that can only be described as appalling: Some dermatologists have been using unsupervised physician assistants (PAs) and nurse practitioners (NPs) to perform dermatology services on patients, including frail nursing home patients, many with dementia or other similar cognitive impairments, to increase their profits.
The dermatologic organizations responding to the article included the American Academy of Dermatology, which, in a letter to the editor, stated that a board-certified dermatologist should “provide direct supervision of any nonphysician (PA/ARNP) for optimum dermatologic care,” a statement that was affirmed by the American College of Mohs Surgery. The American Society for Dermatologic Surgery and the Women’s Dermatologic Society sent similar, even more strongly worded letters.
Well, talk is cheap, and it is time for societies to step up and enforce their bylaws or amend them in order to remove members who practice in such an unethical fashion.
Also in November, an important paper by Adewole S. Adamson, MD, and his colleagues was published in JAMA Dermatology concerning the geographic distribution of physician extenders who billed Medicare independently for common dermatologic procedures in 2014 (doi: 10.1001/jamadermatol.2017.5039). The study found that they are geographically distributed in the same areas – suburbs and cities – where dermatologists already are located. In fact, 92% of those who were independently billing were employed by a dermatologist. The majority (71%) were in counties with high dermatologist density, while only 3% were in counties without dermatologists.
The argument that paraprofessionals provide care for the neglected in underserved areas of the country is unfounded. Physician extenders practice mostly in the same areas in which physicians practice, predominantly in the suburbs. This has been previously demonstrated by the American Medical Association and other surveys (N Engl J Med. 2013;368:1935-41).
In addition, as noted in the past, there are midlevel professionals who bill independently (that is, without direct supervision) for destruction of premalignant lesions, biopsies of skin lesions, excisions of skin cancer, surgical repairs, and flaps/grafts (JAMA Dermatol. 2014 Nov;150[11]:1153-9). But – based on a new finding in the Adamson study, and perhaps the most concerning – this list now includes the interpretation of pathology.
It must be noted that NPs and PAs are trained in primary care, even if up to a master’s degree or PhD level. This does not qualify them to practice specialty medicine independently. Neither does working for, or shadowing, a dermatologist, even if it is a near equivalent to completing a dermatology residency after medical school. PAs and NPs are qualified to practice primary care (PAs with a physician, NPs sometimes without) but not specialty medicine. They can work in a specialty medicine setting if they are directly supervised by a specialty physician.
In summary, “we have met the enemy and he is us,” to quote the comic strip character Pogo. As pointed out in the New York Times article, a few dermatologists are enabling and financially benefiting from paraprofessionals who practice dermatology, without any formal training.
The malpractice risk is huge. The insurance industry will surely realize that this costs them more, not less, because of additional biopsies, pathology interpretations, and missed diagnoses. Now the lay press has caught on to this abuse, and the exposure will not stop here. They will flip over every dermatologist using midlevel professionals in this fashion, starting with the biggest and working their way down
Fair warning has been given to those who use this practice model for their personal gain by the New York Times exposure and the hard lines drawn by the dermatology specialty societies. This technique of boosting profits is unsustainable. Dermatologists must supervise midlevel professionals or face public embarrassment, ethics probes from professional societies, audits, and possibly worse.
Our specialty should take this opportunity to distance itself from these profiteers. The patients hopefully will learn that they are being shortchanged, and demand to see a “real” dermatologist instead of a dermatology “provider.”
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
In a recent article, the New York Times publicly exposed behavior that can only be described as appalling: Some dermatologists have been using unsupervised physician assistants (PAs) and nurse practitioners (NPs) to perform dermatology services on patients, including frail nursing home patients, many with dementia or other similar cognitive impairments, to increase their profits.
The dermatologic organizations responding to the article included the American Academy of Dermatology, which, in a letter to the editor, stated that a board-certified dermatologist should “provide direct supervision of any nonphysician (PA/ARNP) for optimum dermatologic care,” a statement that was affirmed by the American College of Mohs Surgery. The American Society for Dermatologic Surgery and the Women’s Dermatologic Society sent similar, even more strongly worded letters.
Well, talk is cheap, and it is time for societies to step up and enforce their bylaws or amend them in order to remove members who practice in such an unethical fashion.
Also in November, an important paper by Adewole S. Adamson, MD, and his colleagues was published in JAMA Dermatology concerning the geographic distribution of physician extenders who billed Medicare independently for common dermatologic procedures in 2014 (doi: 10.1001/jamadermatol.2017.5039). The study found that they are geographically distributed in the same areas – suburbs and cities – where dermatologists already are located. In fact, 92% of those who were independently billing were employed by a dermatologist. The majority (71%) were in counties with high dermatologist density, while only 3% were in counties without dermatologists.
The argument that paraprofessionals provide care for the neglected in underserved areas of the country is unfounded. Physician extenders practice mostly in the same areas in which physicians practice, predominantly in the suburbs. This has been previously demonstrated by the American Medical Association and other surveys (N Engl J Med. 2013;368:1935-41).
In addition, as noted in the past, there are midlevel professionals who bill independently (that is, without direct supervision) for destruction of premalignant lesions, biopsies of skin lesions, excisions of skin cancer, surgical repairs, and flaps/grafts (JAMA Dermatol. 2014 Nov;150[11]:1153-9). But – based on a new finding in the Adamson study, and perhaps the most concerning – this list now includes the interpretation of pathology.
It must be noted that NPs and PAs are trained in primary care, even if up to a master’s degree or PhD level. This does not qualify them to practice specialty medicine independently. Neither does working for, or shadowing, a dermatologist, even if it is a near equivalent to completing a dermatology residency after medical school. PAs and NPs are qualified to practice primary care (PAs with a physician, NPs sometimes without) but not specialty medicine. They can work in a specialty medicine setting if they are directly supervised by a specialty physician.
In summary, “we have met the enemy and he is us,” to quote the comic strip character Pogo. As pointed out in the New York Times article, a few dermatologists are enabling and financially benefiting from paraprofessionals who practice dermatology, without any formal training.
The malpractice risk is huge. The insurance industry will surely realize that this costs them more, not less, because of additional biopsies, pathology interpretations, and missed diagnoses. Now the lay press has caught on to this abuse, and the exposure will not stop here. They will flip over every dermatologist using midlevel professionals in this fashion, starting with the biggest and working their way down
Fair warning has been given to those who use this practice model for their personal gain by the New York Times exposure and the hard lines drawn by the dermatology specialty societies. This technique of boosting profits is unsustainable. Dermatologists must supervise midlevel professionals or face public embarrassment, ethics probes from professional societies, audits, and possibly worse.
Our specialty should take this opportunity to distance itself from these profiteers. The patients hopefully will learn that they are being shortchanged, and demand to see a “real” dermatologist instead of a dermatology “provider.”
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
In a recent article, the New York Times publicly exposed behavior that can only be described as appalling: Some dermatologists have been using unsupervised physician assistants (PAs) and nurse practitioners (NPs) to perform dermatology services on patients, including frail nursing home patients, many with dementia or other similar cognitive impairments, to increase their profits.
The dermatologic organizations responding to the article included the American Academy of Dermatology, which, in a letter to the editor, stated that a board-certified dermatologist should “provide direct supervision of any nonphysician (PA/ARNP) for optimum dermatologic care,” a statement that was affirmed by the American College of Mohs Surgery. The American Society for Dermatologic Surgery and the Women’s Dermatologic Society sent similar, even more strongly worded letters.
Well, talk is cheap, and it is time for societies to step up and enforce their bylaws or amend them in order to remove members who practice in such an unethical fashion.
Also in November, an important paper by Adewole S. Adamson, MD, and his colleagues was published in JAMA Dermatology concerning the geographic distribution of physician extenders who billed Medicare independently for common dermatologic procedures in 2014 (doi: 10.1001/jamadermatol.2017.5039). The study found that they are geographically distributed in the same areas – suburbs and cities – where dermatologists already are located. In fact, 92% of those who were independently billing were employed by a dermatologist. The majority (71%) were in counties with high dermatologist density, while only 3% were in counties without dermatologists.
The argument that paraprofessionals provide care for the neglected in underserved areas of the country is unfounded. Physician extenders practice mostly in the same areas in which physicians practice, predominantly in the suburbs. This has been previously demonstrated by the American Medical Association and other surveys (N Engl J Med. 2013;368:1935-41).
In addition, as noted in the past, there are midlevel professionals who bill independently (that is, without direct supervision) for destruction of premalignant lesions, biopsies of skin lesions, excisions of skin cancer, surgical repairs, and flaps/grafts (JAMA Dermatol. 2014 Nov;150[11]:1153-9). But – based on a new finding in the Adamson study, and perhaps the most concerning – this list now includes the interpretation of pathology.
It must be noted that NPs and PAs are trained in primary care, even if up to a master’s degree or PhD level. This does not qualify them to practice specialty medicine independently. Neither does working for, or shadowing, a dermatologist, even if it is a near equivalent to completing a dermatology residency after medical school. PAs and NPs are qualified to practice primary care (PAs with a physician, NPs sometimes without) but not specialty medicine. They can work in a specialty medicine setting if they are directly supervised by a specialty physician.
In summary, “we have met the enemy and he is us,” to quote the comic strip character Pogo. As pointed out in the New York Times article, a few dermatologists are enabling and financially benefiting from paraprofessionals who practice dermatology, without any formal training.
The malpractice risk is huge. The insurance industry will surely realize that this costs them more, not less, because of additional biopsies, pathology interpretations, and missed diagnoses. Now the lay press has caught on to this abuse, and the exposure will not stop here. They will flip over every dermatologist using midlevel professionals in this fashion, starting with the biggest and working their way down
Fair warning has been given to those who use this practice model for their personal gain by the New York Times exposure and the hard lines drawn by the dermatology specialty societies. This technique of boosting profits is unsustainable. Dermatologists must supervise midlevel professionals or face public embarrassment, ethics probes from professional societies, audits, and possibly worse.
Our specialty should take this opportunity to distance itself from these profiteers. The patients hopefully will learn that they are being shortchanged, and demand to see a “real” dermatologist instead of a dermatology “provider.”
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].