User login
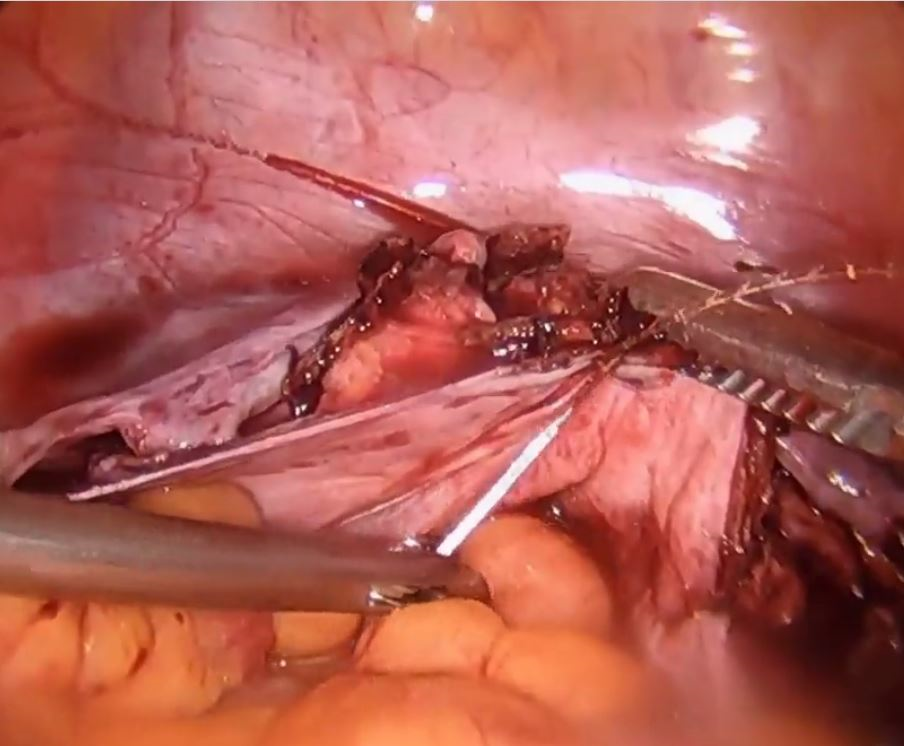
Technique for apical suspension at the time of total laparoscopic hysterectomy

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
This video is brought to you by
Schizophrenia’s silent epidemic: Iatrogenic sexual dysfunction
PARIS – Many psychiatrists have no inkling of how often the antipsychotic agents they prescribe cause hyperprolactinemia-related sexual dysfunction, or the serious negative consequences these common side effects have on quality of life and treatment adherence, Ángel L. Montejo, MD, PhD, declared at the annual congress of the European College of Neuropsychopharmacology.
Psychiatrists are largely uninformed about these matters, because for the most part, they avoid talking with their schizophrenia patients about their sexual activity or inquiring about sexual dysfunction. And they don’t perform the physical examinations that might reveal tell-tale stigmatizing gynecomastia or galactorrhea, according to Dr. Montejo, professor of psychiatry at the University of Salamanca (Spain).
The consensus report includes recommendations for the detection and management of the full range of manifestations of iatrogenic hyperprolactinemia, including osteoporosis and hip fracture, hypogonadism, premature menopause, cardiovascular disease, breast and endometrial cancer, and immunologic disorders.
However, the most common expression of antipsychotic-related hyperprolactinemia is sexual dysfunction, and that’s what Dr. Montejo focused on. This is an issue that directly relates to clinical outcomes. The literature shows that roughly 36% of male and 19% of female schizophrenia patients either stop taking their medication because of its sexual side effects, which include decreased libido, erectile difficulty, vaginal dryness, and anorgasmia, or are thinking about doing so. At least two in three schizophrenia patients engage in some sort of sexual activity. Sixty percent of patients say that having a sexual life is important to them. Yet 73% of psychiatrists in one survey indicated that they don’t interview their patients about their sexual relations.
“If sexual activity is good for you, why isn’t it good for your patients? We’re talking about love, about human relationships. Having a sexual and emotional life can help patients get the best outcomes,” said Dr. Montejo.
“If you don’t ask about sexual dysfunction, your patients won’t tell. Therefore, you may not see it, but your patients will experience it. They know the difference between their sexual life before treatment and during treatment,” he continued.
The incidence of sexual dysfunction in patients on antipsychotic therapy is a function of the agent they are on. Iatrogenic hyperprolactinemia is a consequence of intense blockade of dopamine D2 receptors. The most potent antipsychotic dopamine D2 receptor antagonists are risperidone and paliperidone, followed by haloperidol and most other first-generation antipsychotics.
A normal prolactin level is less than 25 ng/mL in women and less than 20 ng/mL in men. Endocrinologists divide hyperprolactinemia into three categories: mild hyperprolactinemia is a level of 25-50 ng/mL, moderate is 51-100 ng/mL, and severe is anything over 100 ng/mL.
In one study, 44% of patients on oral risperidone at a mean dose of 4.9 mg/day had moderate and 23% had severe hyperprolactinemia; in patients on the injectable long-acting formulation of the drug at a mean dose of 46.2 mg/month, 23% of patients had moderate and 31% had severe hyperprolactinemia. Patients on oral paliperidone at a mean dose of 8.5 mg/day had a 45% prevalence of moderate and an 18% rate of severe hyperprolactinemia, while 40% of those on long-acting injectable paliperidone at a mean of 104 mg/month had moderate and another 40% had severe hyperprolactinemia.
Sixty to 80% of patients on risperidone or paliperidone experience hyperprolactinemia-induced sexual dysfunction. At the other end of the spectrum is the prolactin-sparing antipsychotic aripiprazole, which is associated with sexual dysfunction in about 5% of treated patients. In a meta-analysis, the other prolactin-sparing antipsychotics included ziprasidone, with a 10% rate, quetiapine at 12%, and olanzapine with a 20% rate.
Screening tests for sexual dysfunction
Psychiatrists who are uncomfortable asking patients about sexual dysfunction or don’t want to take the time to do so have other good options. Four easy-to-use, validated instruments are available for free online: the four-question Arizona Sexual Experiences Scale; the Change in Sexual Function Questionnaire, or CSFQ; the Sex Effects Scale, or SexFX; and the Psychotropic-Related Sexual Dysfunction Questionnaire (PRSexDQ-SALSEX).
Managing antipsychotic-induced hyperprolactinemia
The Spanish consensus panel recommended that before psychiatrists prescribe an antipsychotic, they should always explain to patients their risk of antipsychotic-related hyperprolactinemia and make an assessment of personal and family history of risk factors for its possible downstream consequences, including osteoporosis and cancer. And Dr. Montejo stressed that clinicians must “always, always, always” get a baseline serum prolactin measurement, to be repeated after increasing the drug dosage, changing antipsychotics, or upon development of symptoms of hyperprolactinemia.
When a patient’s prolactin level climbs above 50 ng/mL or symptoms of hyperprolactinemia arise, the preferred strategy is to switch to a prolactin-sparing antipsychotic. Most of the alternative options are unsatisfactory. For example, waiting for a sexual side effect to pass is pointless, because it won’t happen spontaneously. A drug holiday undermines compliance. Reducing the antipsychotic dose increases the risk of relapse.
And as for prescribing adjunctive dopamine agonist therapy, well: “Adding a dopamine agonist is not the first step. It should be one of the last steps, because it greatly increases the odds of worsening psychosis,” Dr. Montejo said.
If switching to a different antipsychotic is not possible, the expert panel recommended add-on aripiprazole, accompanied if possible by a downward dose adjustment of the offending antipsychotic. This is an effective way to lower elevated serum prolactin levels.
It’s appropriate to order a lumbar spine and proximal femur bone mineral density measurement in patients on antipsychotic agents if they are older than age 65, have had amenorrhea for at least 6 months, experienced menopause before age 35, have a body mass index below 19 kg/m2, or are experiencing any symptoms of hyperprolactinemia, including sexual dysfunction, according to the Spanish consensus algorithm.
Dr. Montejo reported receiving research grants from and/or serving as a consultant to more than half a dozen pharmaceutical companies.
PARIS – Many psychiatrists have no inkling of how often the antipsychotic agents they prescribe cause hyperprolactinemia-related sexual dysfunction, or the serious negative consequences these common side effects have on quality of life and treatment adherence, Ángel L. Montejo, MD, PhD, declared at the annual congress of the European College of Neuropsychopharmacology.
Psychiatrists are largely uninformed about these matters, because for the most part, they avoid talking with their schizophrenia patients about their sexual activity or inquiring about sexual dysfunction. And they don’t perform the physical examinations that might reveal tell-tale stigmatizing gynecomastia or galactorrhea, according to Dr. Montejo, professor of psychiatry at the University of Salamanca (Spain).
The consensus report includes recommendations for the detection and management of the full range of manifestations of iatrogenic hyperprolactinemia, including osteoporosis and hip fracture, hypogonadism, premature menopause, cardiovascular disease, breast and endometrial cancer, and immunologic disorders.
However, the most common expression of antipsychotic-related hyperprolactinemia is sexual dysfunction, and that’s what Dr. Montejo focused on. This is an issue that directly relates to clinical outcomes. The literature shows that roughly 36% of male and 19% of female schizophrenia patients either stop taking their medication because of its sexual side effects, which include decreased libido, erectile difficulty, vaginal dryness, and anorgasmia, or are thinking about doing so. At least two in three schizophrenia patients engage in some sort of sexual activity. Sixty percent of patients say that having a sexual life is important to them. Yet 73% of psychiatrists in one survey indicated that they don’t interview their patients about their sexual relations.
“If sexual activity is good for you, why isn’t it good for your patients? We’re talking about love, about human relationships. Having a sexual and emotional life can help patients get the best outcomes,” said Dr. Montejo.
“If you don’t ask about sexual dysfunction, your patients won’t tell. Therefore, you may not see it, but your patients will experience it. They know the difference between their sexual life before treatment and during treatment,” he continued.
The incidence of sexual dysfunction in patients on antipsychotic therapy is a function of the agent they are on. Iatrogenic hyperprolactinemia is a consequence of intense blockade of dopamine D2 receptors. The most potent antipsychotic dopamine D2 receptor antagonists are risperidone and paliperidone, followed by haloperidol and most other first-generation antipsychotics.
A normal prolactin level is less than 25 ng/mL in women and less than 20 ng/mL in men. Endocrinologists divide hyperprolactinemia into three categories: mild hyperprolactinemia is a level of 25-50 ng/mL, moderate is 51-100 ng/mL, and severe is anything over 100 ng/mL.
In one study, 44% of patients on oral risperidone at a mean dose of 4.9 mg/day had moderate and 23% had severe hyperprolactinemia; in patients on the injectable long-acting formulation of the drug at a mean dose of 46.2 mg/month, 23% of patients had moderate and 31% had severe hyperprolactinemia. Patients on oral paliperidone at a mean dose of 8.5 mg/day had a 45% prevalence of moderate and an 18% rate of severe hyperprolactinemia, while 40% of those on long-acting injectable paliperidone at a mean of 104 mg/month had moderate and another 40% had severe hyperprolactinemia.
Sixty to 80% of patients on risperidone or paliperidone experience hyperprolactinemia-induced sexual dysfunction. At the other end of the spectrum is the prolactin-sparing antipsychotic aripiprazole, which is associated with sexual dysfunction in about 5% of treated patients. In a meta-analysis, the other prolactin-sparing antipsychotics included ziprasidone, with a 10% rate, quetiapine at 12%, and olanzapine with a 20% rate.
Screening tests for sexual dysfunction
Psychiatrists who are uncomfortable asking patients about sexual dysfunction or don’t want to take the time to do so have other good options. Four easy-to-use, validated instruments are available for free online: the four-question Arizona Sexual Experiences Scale; the Change in Sexual Function Questionnaire, or CSFQ; the Sex Effects Scale, or SexFX; and the Psychotropic-Related Sexual Dysfunction Questionnaire (PRSexDQ-SALSEX).
Managing antipsychotic-induced hyperprolactinemia
The Spanish consensus panel recommended that before psychiatrists prescribe an antipsychotic, they should always explain to patients their risk of antipsychotic-related hyperprolactinemia and make an assessment of personal and family history of risk factors for its possible downstream consequences, including osteoporosis and cancer. And Dr. Montejo stressed that clinicians must “always, always, always” get a baseline serum prolactin measurement, to be repeated after increasing the drug dosage, changing antipsychotics, or upon development of symptoms of hyperprolactinemia.
When a patient’s prolactin level climbs above 50 ng/mL or symptoms of hyperprolactinemia arise, the preferred strategy is to switch to a prolactin-sparing antipsychotic. Most of the alternative options are unsatisfactory. For example, waiting for a sexual side effect to pass is pointless, because it won’t happen spontaneously. A drug holiday undermines compliance. Reducing the antipsychotic dose increases the risk of relapse.
And as for prescribing adjunctive dopamine agonist therapy, well: “Adding a dopamine agonist is not the first step. It should be one of the last steps, because it greatly increases the odds of worsening psychosis,” Dr. Montejo said.
If switching to a different antipsychotic is not possible, the expert panel recommended add-on aripiprazole, accompanied if possible by a downward dose adjustment of the offending antipsychotic. This is an effective way to lower elevated serum prolactin levels.
It’s appropriate to order a lumbar spine and proximal femur bone mineral density measurement in patients on antipsychotic agents if they are older than age 65, have had amenorrhea for at least 6 months, experienced menopause before age 35, have a body mass index below 19 kg/m2, or are experiencing any symptoms of hyperprolactinemia, including sexual dysfunction, according to the Spanish consensus algorithm.
Dr. Montejo reported receiving research grants from and/or serving as a consultant to more than half a dozen pharmaceutical companies.
PARIS – Many psychiatrists have no inkling of how often the antipsychotic agents they prescribe cause hyperprolactinemia-related sexual dysfunction, or the serious negative consequences these common side effects have on quality of life and treatment adherence, Ángel L. Montejo, MD, PhD, declared at the annual congress of the European College of Neuropsychopharmacology.
Psychiatrists are largely uninformed about these matters, because for the most part, they avoid talking with their schizophrenia patients about their sexual activity or inquiring about sexual dysfunction. And they don’t perform the physical examinations that might reveal tell-tale stigmatizing gynecomastia or galactorrhea, according to Dr. Montejo, professor of psychiatry at the University of Salamanca (Spain).
The consensus report includes recommendations for the detection and management of the full range of manifestations of iatrogenic hyperprolactinemia, including osteoporosis and hip fracture, hypogonadism, premature menopause, cardiovascular disease, breast and endometrial cancer, and immunologic disorders.
However, the most common expression of antipsychotic-related hyperprolactinemia is sexual dysfunction, and that’s what Dr. Montejo focused on. This is an issue that directly relates to clinical outcomes. The literature shows that roughly 36% of male and 19% of female schizophrenia patients either stop taking their medication because of its sexual side effects, which include decreased libido, erectile difficulty, vaginal dryness, and anorgasmia, or are thinking about doing so. At least two in three schizophrenia patients engage in some sort of sexual activity. Sixty percent of patients say that having a sexual life is important to them. Yet 73% of psychiatrists in one survey indicated that they don’t interview their patients about their sexual relations.
“If sexual activity is good for you, why isn’t it good for your patients? We’re talking about love, about human relationships. Having a sexual and emotional life can help patients get the best outcomes,” said Dr. Montejo.
“If you don’t ask about sexual dysfunction, your patients won’t tell. Therefore, you may not see it, but your patients will experience it. They know the difference between their sexual life before treatment and during treatment,” he continued.
The incidence of sexual dysfunction in patients on antipsychotic therapy is a function of the agent they are on. Iatrogenic hyperprolactinemia is a consequence of intense blockade of dopamine D2 receptors. The most potent antipsychotic dopamine D2 receptor antagonists are risperidone and paliperidone, followed by haloperidol and most other first-generation antipsychotics.
A normal prolactin level is less than 25 ng/mL in women and less than 20 ng/mL in men. Endocrinologists divide hyperprolactinemia into three categories: mild hyperprolactinemia is a level of 25-50 ng/mL, moderate is 51-100 ng/mL, and severe is anything over 100 ng/mL.
In one study, 44% of patients on oral risperidone at a mean dose of 4.9 mg/day had moderate and 23% had severe hyperprolactinemia; in patients on the injectable long-acting formulation of the drug at a mean dose of 46.2 mg/month, 23% of patients had moderate and 31% had severe hyperprolactinemia. Patients on oral paliperidone at a mean dose of 8.5 mg/day had a 45% prevalence of moderate and an 18% rate of severe hyperprolactinemia, while 40% of those on long-acting injectable paliperidone at a mean of 104 mg/month had moderate and another 40% had severe hyperprolactinemia.
Sixty to 80% of patients on risperidone or paliperidone experience hyperprolactinemia-induced sexual dysfunction. At the other end of the spectrum is the prolactin-sparing antipsychotic aripiprazole, which is associated with sexual dysfunction in about 5% of treated patients. In a meta-analysis, the other prolactin-sparing antipsychotics included ziprasidone, with a 10% rate, quetiapine at 12%, and olanzapine with a 20% rate.
Screening tests for sexual dysfunction
Psychiatrists who are uncomfortable asking patients about sexual dysfunction or don’t want to take the time to do so have other good options. Four easy-to-use, validated instruments are available for free online: the four-question Arizona Sexual Experiences Scale; the Change in Sexual Function Questionnaire, or CSFQ; the Sex Effects Scale, or SexFX; and the Psychotropic-Related Sexual Dysfunction Questionnaire (PRSexDQ-SALSEX).
Managing antipsychotic-induced hyperprolactinemia
The Spanish consensus panel recommended that before psychiatrists prescribe an antipsychotic, they should always explain to patients their risk of antipsychotic-related hyperprolactinemia and make an assessment of personal and family history of risk factors for its possible downstream consequences, including osteoporosis and cancer. And Dr. Montejo stressed that clinicians must “always, always, always” get a baseline serum prolactin measurement, to be repeated after increasing the drug dosage, changing antipsychotics, or upon development of symptoms of hyperprolactinemia.
When a patient’s prolactin level climbs above 50 ng/mL or symptoms of hyperprolactinemia arise, the preferred strategy is to switch to a prolactin-sparing antipsychotic. Most of the alternative options are unsatisfactory. For example, waiting for a sexual side effect to pass is pointless, because it won’t happen spontaneously. A drug holiday undermines compliance. Reducing the antipsychotic dose increases the risk of relapse.
And as for prescribing adjunctive dopamine agonist therapy, well: “Adding a dopamine agonist is not the first step. It should be one of the last steps, because it greatly increases the odds of worsening psychosis,” Dr. Montejo said.
If switching to a different antipsychotic is not possible, the expert panel recommended add-on aripiprazole, accompanied if possible by a downward dose adjustment of the offending antipsychotic. This is an effective way to lower elevated serum prolactin levels.
It’s appropriate to order a lumbar spine and proximal femur bone mineral density measurement in patients on antipsychotic agents if they are older than age 65, have had amenorrhea for at least 6 months, experienced menopause before age 35, have a body mass index below 19 kg/m2, or are experiencing any symptoms of hyperprolactinemia, including sexual dysfunction, according to the Spanish consensus algorithm.
Dr. Montejo reported receiving research grants from and/or serving as a consultant to more than half a dozen pharmaceutical companies.
EXPERT ANALYSIS FROM THE ECNP CONGRESS
VIDEO: Dr. Charles E. Miller’s AAGL highlights
NATIONAL HARBOR, MD. – The biggest theme of the 2017 AAGL Global Congress was the importance of understanding anatomy, Charles E. Miller, MD, a minimally invasive gynecologic surgeon in Naperville, Ill., and past president of the AAGL, said at the meeting.
“It’s about doing surgery in the right place, in the right space,” he said.
One of the advantages of this year’s Congress is a greater emphasis on cadaveric dissections, Dr. Miller said during an interview. “Understanding how the nerves are placed, how the vessels are in place, the muscles and the different spaces, and how that all relates to our most complex dissections.”
In a presentation on neuropelveology, Michael Hibner, MD, and Mario Castellanos, MD, of St. Joseph’s Hospital and Medical Center, Phoenix, performed a live cadaveric dissection showing how to deal with a trapped pudendal nerve, working over the gluteus maximus and dissecting down.
In a video session, surgeons demonstrated a needleless robotic-assisted transabdominal cerclage. The nonneedle procedure used a unique, posterior placement of the cerclage knot, a technique which Dr. Miller said he plans to use in his own practice.
The incorporation of colleagues from around the country, and around the world, was another strength of this year’s Congress, said Dr. Miller, particularly a presentation from a Chinese ob.gyn. association on isthmoceles. “To be able to see that this transcends miles upon miles upon miles, but yet we’re seeing the same type of problems, is quite interesting,” he said.
On Twitter @eaztweets
NATIONAL HARBOR, MD. – The biggest theme of the 2017 AAGL Global Congress was the importance of understanding anatomy, Charles E. Miller, MD, a minimally invasive gynecologic surgeon in Naperville, Ill., and past president of the AAGL, said at the meeting.
“It’s about doing surgery in the right place, in the right space,” he said.
One of the advantages of this year’s Congress is a greater emphasis on cadaveric dissections, Dr. Miller said during an interview. “Understanding how the nerves are placed, how the vessels are in place, the muscles and the different spaces, and how that all relates to our most complex dissections.”
In a presentation on neuropelveology, Michael Hibner, MD, and Mario Castellanos, MD, of St. Joseph’s Hospital and Medical Center, Phoenix, performed a live cadaveric dissection showing how to deal with a trapped pudendal nerve, working over the gluteus maximus and dissecting down.
In a video session, surgeons demonstrated a needleless robotic-assisted transabdominal cerclage. The nonneedle procedure used a unique, posterior placement of the cerclage knot, a technique which Dr. Miller said he plans to use in his own practice.
The incorporation of colleagues from around the country, and around the world, was another strength of this year’s Congress, said Dr. Miller, particularly a presentation from a Chinese ob.gyn. association on isthmoceles. “To be able to see that this transcends miles upon miles upon miles, but yet we’re seeing the same type of problems, is quite interesting,” he said.
On Twitter @eaztweets
NATIONAL HARBOR, MD. – The biggest theme of the 2017 AAGL Global Congress was the importance of understanding anatomy, Charles E. Miller, MD, a minimally invasive gynecologic surgeon in Naperville, Ill., and past president of the AAGL, said at the meeting.
“It’s about doing surgery in the right place, in the right space,” he said.
One of the advantages of this year’s Congress is a greater emphasis on cadaveric dissections, Dr. Miller said during an interview. “Understanding how the nerves are placed, how the vessels are in place, the muscles and the different spaces, and how that all relates to our most complex dissections.”
In a presentation on neuropelveology, Michael Hibner, MD, and Mario Castellanos, MD, of St. Joseph’s Hospital and Medical Center, Phoenix, performed a live cadaveric dissection showing how to deal with a trapped pudendal nerve, working over the gluteus maximus and dissecting down.
In a video session, surgeons demonstrated a needleless robotic-assisted transabdominal cerclage. The nonneedle procedure used a unique, posterior placement of the cerclage knot, a technique which Dr. Miller said he plans to use in his own practice.
The incorporation of colleagues from around the country, and around the world, was another strength of this year’s Congress, said Dr. Miller, particularly a presentation from a Chinese ob.gyn. association on isthmoceles. “To be able to see that this transcends miles upon miles upon miles, but yet we’re seeing the same type of problems, is quite interesting,” he said.
On Twitter @eaztweets
AT AAGL 2017
In bariatric surgery, leak test may backfire
SCOTTSDALE, ARIZ. – A test used to detect anastomotic leaks during bariatric surgery may in fact be a potential cause of leaks, since performance of the test was associated with double the frequency of 30-day postoperative leaks, a study showed.
Based on this finding from an analysis of the 2015 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) database, the researchers suggested that the use of an endoscope could lead to fewer complications.
The test itself is valuable, since surgeons hope to find and repair a leak immediately, but Dr. Nguyen said the wrong method is being used. “The technique of doing the test should be endoscopy rather than the use of an orogastric tube,” he said. The database did not indicate which technique was being used, but Dr. Nguyen said that use of endoscopy is rare. Surgeons “don’t want to break scrub to go around and perform the endoscopy. It’s easier to ask the anesthesiologist to put a tube down,” said Dr. Nguyen, who is chair of surgery at University of California Irvine Medical Center.
The study, which was presented at the annual meeting of the Western Surgical Association, is the first to look at intraoperative and postoperative procedures and risk of leaks during bariatric surgery, and was possible only because of the recent availability of the MBSAQIP database. The study cannot prove causation between performance of the provocative test and heightened leak risk, and one audience member suggested the possibility that the tests were ordered when a surgeon believed the patient was at higher risk. If so, the association wouldn’t be causative.
However, the provocation test was performed 82% of the time, suggesting that the test was being carried out routinely, said Dr. Nguyen.
By contrast, when the surgeon inserted a surgical drain, the risk of leak was nearly four times higher. “It is most likely that the surgeon only decided to place the drain because they were worried about that particular operation, since it was only done a small percentage of the time. Our results suggest that they were right [to be concerned]. We believe it’s a reflection of the knowledge of the surgeon for that particular case,” said Dr. Nguyen.
If indeed there is a risk associated with the provocation test, the use of endoscopy could reduce that risk. Dr. Nguyen also pointed out that endoscopy provides anatomical detail that can help guide revision surgery, and it’s a useful training exercise for residents. “This is an important skill that you need when you graduate from general surgery,” said Dr. Nguyen.
The researchers analyzed data from 133,478 patients who underwent laparoscopic sleeve gastrectomy (LSG) or laparoscopic Roux-en-Y gastric bypass (LRYGB), excluding emergent and revisional cases; 69.3% of patients underwent LSG, while 30.7% underwent LRYGB. The researchers looked at the association between leak frequency and the presence of the provocative test, surgical drain, and swallow study.
The 30-day leak rate was 0.7% overall, and 0.5% in LSG and 1.2% in LRYGB (adjusted odds ratio for LSG, 0.52; 95% confidence interval, 0.44-0.61; P less than .001). The rate was higher in the 81.9% of patients who received the provocative test than in those who did not (0.8% vs. 0.4%; aOR, 1.41; 95% CI, 1.14-1.76; P = .02). The leak risk was also higher in the 24.5% of patients who had a drain placed (1.6% vs. 0.4%; aOR, 3.46; 95% CI, 3.01-3.98; P less than .001).
A total of 41.1% of patients received a swallow study, but their leak rate (0.7%) was identical to that of those who did not have a swallow study.
The study received no outside funding. Dr. Nguyen reported having no relevant financial disclosures.
SCOTTSDALE, ARIZ. – A test used to detect anastomotic leaks during bariatric surgery may in fact be a potential cause of leaks, since performance of the test was associated with double the frequency of 30-day postoperative leaks, a study showed.
Based on this finding from an analysis of the 2015 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) database, the researchers suggested that the use of an endoscope could lead to fewer complications.
The test itself is valuable, since surgeons hope to find and repair a leak immediately, but Dr. Nguyen said the wrong method is being used. “The technique of doing the test should be endoscopy rather than the use of an orogastric tube,” he said. The database did not indicate which technique was being used, but Dr. Nguyen said that use of endoscopy is rare. Surgeons “don’t want to break scrub to go around and perform the endoscopy. It’s easier to ask the anesthesiologist to put a tube down,” said Dr. Nguyen, who is chair of surgery at University of California Irvine Medical Center.
The study, which was presented at the annual meeting of the Western Surgical Association, is the first to look at intraoperative and postoperative procedures and risk of leaks during bariatric surgery, and was possible only because of the recent availability of the MBSAQIP database. The study cannot prove causation between performance of the provocative test and heightened leak risk, and one audience member suggested the possibility that the tests were ordered when a surgeon believed the patient was at higher risk. If so, the association wouldn’t be causative.
However, the provocation test was performed 82% of the time, suggesting that the test was being carried out routinely, said Dr. Nguyen.
By contrast, when the surgeon inserted a surgical drain, the risk of leak was nearly four times higher. “It is most likely that the surgeon only decided to place the drain because they were worried about that particular operation, since it was only done a small percentage of the time. Our results suggest that they were right [to be concerned]. We believe it’s a reflection of the knowledge of the surgeon for that particular case,” said Dr. Nguyen.
If indeed there is a risk associated with the provocation test, the use of endoscopy could reduce that risk. Dr. Nguyen also pointed out that endoscopy provides anatomical detail that can help guide revision surgery, and it’s a useful training exercise for residents. “This is an important skill that you need when you graduate from general surgery,” said Dr. Nguyen.
The researchers analyzed data from 133,478 patients who underwent laparoscopic sleeve gastrectomy (LSG) or laparoscopic Roux-en-Y gastric bypass (LRYGB), excluding emergent and revisional cases; 69.3% of patients underwent LSG, while 30.7% underwent LRYGB. The researchers looked at the association between leak frequency and the presence of the provocative test, surgical drain, and swallow study.
The 30-day leak rate was 0.7% overall, and 0.5% in LSG and 1.2% in LRYGB (adjusted odds ratio for LSG, 0.52; 95% confidence interval, 0.44-0.61; P less than .001). The rate was higher in the 81.9% of patients who received the provocative test than in those who did not (0.8% vs. 0.4%; aOR, 1.41; 95% CI, 1.14-1.76; P = .02). The leak risk was also higher in the 24.5% of patients who had a drain placed (1.6% vs. 0.4%; aOR, 3.46; 95% CI, 3.01-3.98; P less than .001).
A total of 41.1% of patients received a swallow study, but their leak rate (0.7%) was identical to that of those who did not have a swallow study.
The study received no outside funding. Dr. Nguyen reported having no relevant financial disclosures.
SCOTTSDALE, ARIZ. – A test used to detect anastomotic leaks during bariatric surgery may in fact be a potential cause of leaks, since performance of the test was associated with double the frequency of 30-day postoperative leaks, a study showed.
Based on this finding from an analysis of the 2015 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) database, the researchers suggested that the use of an endoscope could lead to fewer complications.
The test itself is valuable, since surgeons hope to find and repair a leak immediately, but Dr. Nguyen said the wrong method is being used. “The technique of doing the test should be endoscopy rather than the use of an orogastric tube,” he said. The database did not indicate which technique was being used, but Dr. Nguyen said that use of endoscopy is rare. Surgeons “don’t want to break scrub to go around and perform the endoscopy. It’s easier to ask the anesthesiologist to put a tube down,” said Dr. Nguyen, who is chair of surgery at University of California Irvine Medical Center.
The study, which was presented at the annual meeting of the Western Surgical Association, is the first to look at intraoperative and postoperative procedures and risk of leaks during bariatric surgery, and was possible only because of the recent availability of the MBSAQIP database. The study cannot prove causation between performance of the provocative test and heightened leak risk, and one audience member suggested the possibility that the tests were ordered when a surgeon believed the patient was at higher risk. If so, the association wouldn’t be causative.
However, the provocation test was performed 82% of the time, suggesting that the test was being carried out routinely, said Dr. Nguyen.
By contrast, when the surgeon inserted a surgical drain, the risk of leak was nearly four times higher. “It is most likely that the surgeon only decided to place the drain because they were worried about that particular operation, since it was only done a small percentage of the time. Our results suggest that they were right [to be concerned]. We believe it’s a reflection of the knowledge of the surgeon for that particular case,” said Dr. Nguyen.
If indeed there is a risk associated with the provocation test, the use of endoscopy could reduce that risk. Dr. Nguyen also pointed out that endoscopy provides anatomical detail that can help guide revision surgery, and it’s a useful training exercise for residents. “This is an important skill that you need when you graduate from general surgery,” said Dr. Nguyen.
The researchers analyzed data from 133,478 patients who underwent laparoscopic sleeve gastrectomy (LSG) or laparoscopic Roux-en-Y gastric bypass (LRYGB), excluding emergent and revisional cases; 69.3% of patients underwent LSG, while 30.7% underwent LRYGB. The researchers looked at the association between leak frequency and the presence of the provocative test, surgical drain, and swallow study.
The 30-day leak rate was 0.7% overall, and 0.5% in LSG and 1.2% in LRYGB (adjusted odds ratio for LSG, 0.52; 95% confidence interval, 0.44-0.61; P less than .001). The rate was higher in the 81.9% of patients who received the provocative test than in those who did not (0.8% vs. 0.4%; aOR, 1.41; 95% CI, 1.14-1.76; P = .02). The leak risk was also higher in the 24.5% of patients who had a drain placed (1.6% vs. 0.4%; aOR, 3.46; 95% CI, 3.01-3.98; P less than .001).
A total of 41.1% of patients received a swallow study, but their leak rate (0.7%) was identical to that of those who did not have a swallow study.
The study received no outside funding. Dr. Nguyen reported having no relevant financial disclosures.
AT WSA 2017
Key clinical point: Use of endoscopy to perform the provocative test may reduce the incidence of anastomotic leaks.
Major finding: The rate of leaks was 0.8% in patients who had the provocative test, compared with 0.4% in patients who didn’t have the test.
Data source: A retrospective analysis of 133,478 procedures.
Disclosures: The study received no outside funding. Dr. Nguyen reported having no relevant financial disclosures.
Dedicated sickle cell center offers roadmap for care
CONCORD, N.C. – A care center for acute sickle cell pain management, which includes a dedicated emergency room and a daytime management unit in the hospital, decreased health system costs and the frequency of acute care visits by sickle cell patients, James Eckman, MD, reported at Sickle Cell Disease Symposium held by Carolinas Health Care System.
“In the first 5 years of the center, acute care visits dropped from 16 per patient per year to 10, and admissions per active patient per year dropped from 2.1 to less than one,” said Dr. Eckman, former medical director of the Georgia Comprehensive Sickle Cell Center at Grady Health System in Atlanta.
By 2011, those numbers had dropped further, falling to less than four acute care visits per patient per year and less than 0.5 admissions per patient per year.
The results Dr. Eckman reported are based on 37 years of his experience at Grady Health System, which included setting up an emergency room dedicated to patients with sickle cell disease (SCD) and the launch of a tertiary care clinic in 1985. The Grady SCD database includes more than 4,500 patients, with about 1,000 adults active at any given time.
It’s , according to Emory University.
“We really developed a model that was very cost effective for the management of this disease,” said Dr. Eckman, professor emeritus in hematology and medical oncology at Emory University. “We actually consistently turned a profit in our budget.”
Previously, SCD patients went to the regular emergency department for their acute pain crises, and they would often wait for hours without treatment. “You need to initiate treatment rapidly in these patients,” Dr. Eckman said. “It’s really unacceptable now what’s happening in our emergency rooms, where they have to wait 3, 4, or more hours to get treated while they’re in intolerable pain.”
In 2014, an expert panel issued guidelines for pain management in SCD calling for the initiation of pain treatment for acute crisis within 30 minutes of the patient’s arrival in the emergency department (JAMA. 2014 Sep 10;312[10]:1033-48). “Our goal is 20 minutes to have a complete assessment, get a laboratory draw, and have them on therapy,” he said. “And we were relatively successful in being able to do that.”
Each patient at the center was enrolled in a care management program consisting of 35 assessment and intervention elements. Assessment includes a complete medical evaluation, along with social and psychological evaluations. Intervention entails developing a detailed problem list – including medical, social, and psychological issues – a detailed management plan, and a social support plan. The initial assessment can take 4-8 hours.
For the first decade, the program tracked acute care visits and admissions in 166 continuing patients and saw dramatic declines in both. “The data only go through 1995, but they actually look exactly the same after 1995 all the way up to 2015,” Dr. Eckman said. “This sustained a really marked decrease in health care utilization.”
The program also identified a small group of patients – fewer than 75 out of a base of 1,000 – who accounted for 90% of visits, he said.
Although the Georgia experience is based on a dedicated care center for SCD, the results can be replicated without that type of dedicated infrastructure, Dr. Eckman said. “It is not the 24-hour acute care center,” he said. “It’s the carefully thought out and implemented comprehensive care plan by a multidisciplinary care team dedicated to care of the individuals with sickle cell disease that makes the difference.”
Dr. Eckman reported having no financial disclosures.
CONCORD, N.C. – A care center for acute sickle cell pain management, which includes a dedicated emergency room and a daytime management unit in the hospital, decreased health system costs and the frequency of acute care visits by sickle cell patients, James Eckman, MD, reported at Sickle Cell Disease Symposium held by Carolinas Health Care System.
“In the first 5 years of the center, acute care visits dropped from 16 per patient per year to 10, and admissions per active patient per year dropped from 2.1 to less than one,” said Dr. Eckman, former medical director of the Georgia Comprehensive Sickle Cell Center at Grady Health System in Atlanta.
By 2011, those numbers had dropped further, falling to less than four acute care visits per patient per year and less than 0.5 admissions per patient per year.
The results Dr. Eckman reported are based on 37 years of his experience at Grady Health System, which included setting up an emergency room dedicated to patients with sickle cell disease (SCD) and the launch of a tertiary care clinic in 1985. The Grady SCD database includes more than 4,500 patients, with about 1,000 adults active at any given time.
It’s , according to Emory University.
“We really developed a model that was very cost effective for the management of this disease,” said Dr. Eckman, professor emeritus in hematology and medical oncology at Emory University. “We actually consistently turned a profit in our budget.”
Previously, SCD patients went to the regular emergency department for their acute pain crises, and they would often wait for hours without treatment. “You need to initiate treatment rapidly in these patients,” Dr. Eckman said. “It’s really unacceptable now what’s happening in our emergency rooms, where they have to wait 3, 4, or more hours to get treated while they’re in intolerable pain.”
In 2014, an expert panel issued guidelines for pain management in SCD calling for the initiation of pain treatment for acute crisis within 30 minutes of the patient’s arrival in the emergency department (JAMA. 2014 Sep 10;312[10]:1033-48). “Our goal is 20 minutes to have a complete assessment, get a laboratory draw, and have them on therapy,” he said. “And we were relatively successful in being able to do that.”
Each patient at the center was enrolled in a care management program consisting of 35 assessment and intervention elements. Assessment includes a complete medical evaluation, along with social and psychological evaluations. Intervention entails developing a detailed problem list – including medical, social, and psychological issues – a detailed management plan, and a social support plan. The initial assessment can take 4-8 hours.
For the first decade, the program tracked acute care visits and admissions in 166 continuing patients and saw dramatic declines in both. “The data only go through 1995, but they actually look exactly the same after 1995 all the way up to 2015,” Dr. Eckman said. “This sustained a really marked decrease in health care utilization.”
The program also identified a small group of patients – fewer than 75 out of a base of 1,000 – who accounted for 90% of visits, he said.
Although the Georgia experience is based on a dedicated care center for SCD, the results can be replicated without that type of dedicated infrastructure, Dr. Eckman said. “It is not the 24-hour acute care center,” he said. “It’s the carefully thought out and implemented comprehensive care plan by a multidisciplinary care team dedicated to care of the individuals with sickle cell disease that makes the difference.”
Dr. Eckman reported having no financial disclosures.
CONCORD, N.C. – A care center for acute sickle cell pain management, which includes a dedicated emergency room and a daytime management unit in the hospital, decreased health system costs and the frequency of acute care visits by sickle cell patients, James Eckman, MD, reported at Sickle Cell Disease Symposium held by Carolinas Health Care System.
“In the first 5 years of the center, acute care visits dropped from 16 per patient per year to 10, and admissions per active patient per year dropped from 2.1 to less than one,” said Dr. Eckman, former medical director of the Georgia Comprehensive Sickle Cell Center at Grady Health System in Atlanta.
By 2011, those numbers had dropped further, falling to less than four acute care visits per patient per year and less than 0.5 admissions per patient per year.
The results Dr. Eckman reported are based on 37 years of his experience at Grady Health System, which included setting up an emergency room dedicated to patients with sickle cell disease (SCD) and the launch of a tertiary care clinic in 1985. The Grady SCD database includes more than 4,500 patients, with about 1,000 adults active at any given time.
It’s , according to Emory University.
“We really developed a model that was very cost effective for the management of this disease,” said Dr. Eckman, professor emeritus in hematology and medical oncology at Emory University. “We actually consistently turned a profit in our budget.”
Previously, SCD patients went to the regular emergency department for their acute pain crises, and they would often wait for hours without treatment. “You need to initiate treatment rapidly in these patients,” Dr. Eckman said. “It’s really unacceptable now what’s happening in our emergency rooms, where they have to wait 3, 4, or more hours to get treated while they’re in intolerable pain.”
In 2014, an expert panel issued guidelines for pain management in SCD calling for the initiation of pain treatment for acute crisis within 30 minutes of the patient’s arrival in the emergency department (JAMA. 2014 Sep 10;312[10]:1033-48). “Our goal is 20 minutes to have a complete assessment, get a laboratory draw, and have them on therapy,” he said. “And we were relatively successful in being able to do that.”
Each patient at the center was enrolled in a care management program consisting of 35 assessment and intervention elements. Assessment includes a complete medical evaluation, along with social and psychological evaluations. Intervention entails developing a detailed problem list – including medical, social, and psychological issues – a detailed management plan, and a social support plan. The initial assessment can take 4-8 hours.
For the first decade, the program tracked acute care visits and admissions in 166 continuing patients and saw dramatic declines in both. “The data only go through 1995, but they actually look exactly the same after 1995 all the way up to 2015,” Dr. Eckman said. “This sustained a really marked decrease in health care utilization.”
The program also identified a small group of patients – fewer than 75 out of a base of 1,000 – who accounted for 90% of visits, he said.
Although the Georgia experience is based on a dedicated care center for SCD, the results can be replicated without that type of dedicated infrastructure, Dr. Eckman said. “It is not the 24-hour acute care center,” he said. “It’s the carefully thought out and implemented comprehensive care plan by a multidisciplinary care team dedicated to care of the individuals with sickle cell disease that makes the difference.”
Dr. Eckman reported having no financial disclosures.
EXPERT ANALYSIS FROM A MEETING ON SICKLE CELL DISEASE
Living liver donation safety supported in single-center study
SCOTTSDALE, ARIZ. – A single-center analysis of complications following living liver donation found a low rate of severe complications, and a high quality of life among donors. The results are similar to what has been seen in a previous multicenter study in the United States, and the authors hope that the results can help inform potential donors and their physicians.
There is a significant shortage of deceased liver donors, leading to the death of about 3,500 liver transplant hopefuls in 2016, compared with about 2,900 who received a transplant. Living donor transplant was developed and first attempted in 1996 as an attempt to counter this shortage, and for a brief period it was popular, peaking at 500 donor surgeries in 2001. But that year the death of a donor occurred in New York and received widespread publicity.
Overall, though, the study showed relatively few complications, and that donors reported good quality of life. “There was a slight dip in health-related quality of life at 5 years and 10 years, but at all times the donors had significantly better quality of life compared to the standard population,” said Dr. Chinnakotla, clinical director of pediatric transplantation at the University of Minnesota, Minneapolis.
The researchers examined long-term complications and quality of life among 176 liver donors who underwent surgery between 1997 and 2016 at the University of Minnesota. At total of 140 donors underwent a right-lobe hepatectomy without middle hepatic vein, 14 underwent right lobe with middle hepatic vein, 4 underwent left lobe, and 18 underwent left lateral segmentectomy.
The researchers then analyzed complications graded by the Clavien scale. They found that 59.1% of right-lobe donors experienced no complications at all; 5.8% had Clavien scale 1 complications, meaning something abnormal occurred but required no intervention; and 27.3% had a Clavien 2 complication, requiring pharmaceutical treatment, a blood transfusion, or parenteral nutrition. Clavien 3a complications, which required an intervention without general anesthesia, occurred in 1.9% of cases, and Clavien 3b complications, which required anesthesia, occurred in 5.8%.
A total of 81.8% of left-lobe donors experienced no complications, 4.5% had a Clavien 1 complication, and 13.6% a Clavien 2. There were no Clavien 3 or 4 complications in left-lobe donors.
Overall, the incidence of Clavien grade 3 or higher complications was 7%, there were no complications involving organ failure, and there were no deaths.
Quality of life, as measured by the 36-item Short Form Health Survey and an internally designed donor-specific survey, was higher among recipients than in the general population at all time points. The primary long-term complaints were incisional discomfort, which ranged from about 23% to 38% in frequency, and intolerance to fatty meals, which had a frequency of 20%-30%, and is likely attributable to accompanying cholecystectomy, according to Dr. Chinnakotla.
“The overall results appear to have been excellent,” said William C. Chapman, MD, who was invited by the meeting organizers to review and comment on the study. Dr. Chapman is surgical director of transplant surgery at Washington University in St. Louis.
Dr. Chapman also noted that some studies in Asia have looked at reducing complications in donors, while avoiding a small-for-size graft, by using two left-lobe grafts from separate living donors (Liver Transpl 2015;21[11]1438-48). “We haven’t been brave enough to do that in the United States, but I think that is a strategy we can look forward to in the future,” said Dr. Chinnakotla.
No funding source was disclosed.
SCOTTSDALE, ARIZ. – A single-center analysis of complications following living liver donation found a low rate of severe complications, and a high quality of life among donors. The results are similar to what has been seen in a previous multicenter study in the United States, and the authors hope that the results can help inform potential donors and their physicians.
There is a significant shortage of deceased liver donors, leading to the death of about 3,500 liver transplant hopefuls in 2016, compared with about 2,900 who received a transplant. Living donor transplant was developed and first attempted in 1996 as an attempt to counter this shortage, and for a brief period it was popular, peaking at 500 donor surgeries in 2001. But that year the death of a donor occurred in New York and received widespread publicity.
Overall, though, the study showed relatively few complications, and that donors reported good quality of life. “There was a slight dip in health-related quality of life at 5 years and 10 years, but at all times the donors had significantly better quality of life compared to the standard population,” said Dr. Chinnakotla, clinical director of pediatric transplantation at the University of Minnesota, Minneapolis.
The researchers examined long-term complications and quality of life among 176 liver donors who underwent surgery between 1997 and 2016 at the University of Minnesota. At total of 140 donors underwent a right-lobe hepatectomy without middle hepatic vein, 14 underwent right lobe with middle hepatic vein, 4 underwent left lobe, and 18 underwent left lateral segmentectomy.
The researchers then analyzed complications graded by the Clavien scale. They found that 59.1% of right-lobe donors experienced no complications at all; 5.8% had Clavien scale 1 complications, meaning something abnormal occurred but required no intervention; and 27.3% had a Clavien 2 complication, requiring pharmaceutical treatment, a blood transfusion, or parenteral nutrition. Clavien 3a complications, which required an intervention without general anesthesia, occurred in 1.9% of cases, and Clavien 3b complications, which required anesthesia, occurred in 5.8%.
A total of 81.8% of left-lobe donors experienced no complications, 4.5% had a Clavien 1 complication, and 13.6% a Clavien 2. There were no Clavien 3 or 4 complications in left-lobe donors.
Overall, the incidence of Clavien grade 3 or higher complications was 7%, there were no complications involving organ failure, and there were no deaths.
Quality of life, as measured by the 36-item Short Form Health Survey and an internally designed donor-specific survey, was higher among recipients than in the general population at all time points. The primary long-term complaints were incisional discomfort, which ranged from about 23% to 38% in frequency, and intolerance to fatty meals, which had a frequency of 20%-30%, and is likely attributable to accompanying cholecystectomy, according to Dr. Chinnakotla.
“The overall results appear to have been excellent,” said William C. Chapman, MD, who was invited by the meeting organizers to review and comment on the study. Dr. Chapman is surgical director of transplant surgery at Washington University in St. Louis.
Dr. Chapman also noted that some studies in Asia have looked at reducing complications in donors, while avoiding a small-for-size graft, by using two left-lobe grafts from separate living donors (Liver Transpl 2015;21[11]1438-48). “We haven’t been brave enough to do that in the United States, but I think that is a strategy we can look forward to in the future,” said Dr. Chinnakotla.
No funding source was disclosed.
SCOTTSDALE, ARIZ. – A single-center analysis of complications following living liver donation found a low rate of severe complications, and a high quality of life among donors. The results are similar to what has been seen in a previous multicenter study in the United States, and the authors hope that the results can help inform potential donors and their physicians.
There is a significant shortage of deceased liver donors, leading to the death of about 3,500 liver transplant hopefuls in 2016, compared with about 2,900 who received a transplant. Living donor transplant was developed and first attempted in 1996 as an attempt to counter this shortage, and for a brief period it was popular, peaking at 500 donor surgeries in 2001. But that year the death of a donor occurred in New York and received widespread publicity.
Overall, though, the study showed relatively few complications, and that donors reported good quality of life. “There was a slight dip in health-related quality of life at 5 years and 10 years, but at all times the donors had significantly better quality of life compared to the standard population,” said Dr. Chinnakotla, clinical director of pediatric transplantation at the University of Minnesota, Minneapolis.
The researchers examined long-term complications and quality of life among 176 liver donors who underwent surgery between 1997 and 2016 at the University of Minnesota. At total of 140 donors underwent a right-lobe hepatectomy without middle hepatic vein, 14 underwent right lobe with middle hepatic vein, 4 underwent left lobe, and 18 underwent left lateral segmentectomy.
The researchers then analyzed complications graded by the Clavien scale. They found that 59.1% of right-lobe donors experienced no complications at all; 5.8% had Clavien scale 1 complications, meaning something abnormal occurred but required no intervention; and 27.3% had a Clavien 2 complication, requiring pharmaceutical treatment, a blood transfusion, or parenteral nutrition. Clavien 3a complications, which required an intervention without general anesthesia, occurred in 1.9% of cases, and Clavien 3b complications, which required anesthesia, occurred in 5.8%.
A total of 81.8% of left-lobe donors experienced no complications, 4.5% had a Clavien 1 complication, and 13.6% a Clavien 2. There were no Clavien 3 or 4 complications in left-lobe donors.
Overall, the incidence of Clavien grade 3 or higher complications was 7%, there were no complications involving organ failure, and there were no deaths.
Quality of life, as measured by the 36-item Short Form Health Survey and an internally designed donor-specific survey, was higher among recipients than in the general population at all time points. The primary long-term complaints were incisional discomfort, which ranged from about 23% to 38% in frequency, and intolerance to fatty meals, which had a frequency of 20%-30%, and is likely attributable to accompanying cholecystectomy, according to Dr. Chinnakotla.
“The overall results appear to have been excellent,” said William C. Chapman, MD, who was invited by the meeting organizers to review and comment on the study. Dr. Chapman is surgical director of transplant surgery at Washington University in St. Louis.
Dr. Chapman also noted that some studies in Asia have looked at reducing complications in donors, while avoiding a small-for-size graft, by using two left-lobe grafts from separate living donors (Liver Transpl 2015;21[11]1438-48). “We haven’t been brave enough to do that in the United States, but I think that is a strategy we can look forward to in the future,” said Dr. Chinnakotla.
No funding source was disclosed.
AT WSA 2017
Interprofessional communication demystified with hands-on EHR training
MONTREAL – A hands-on training program designed to improve communication between physicians and allied health professionals, such as physical therapists, produced gains in comfort and confidence to medical students who completed the training.
After completing the training, which involved both a didactic and a hands-on component, 92.58% of third-year medical students agreed or strongly agreed that they had the skills needed for effective interprofessional communication in the EHR, up from 43.86% before the training.
The idea for the training began when Zaiba Jetpuri, DO, and her physician colleagues at the University of Texas Southwestern Medical Center, Dallas, noticed that medical students and residents often were unsure how best to communicate with nurses, pharmacists, physical therapists, and other members of the healthcare team via the EHR.
Knowing who has what role in an interdisciplinary setting is far from obvious, and knowing how to strike the right tone in EHR communications was a challenge for trainees, Dr. Jetpuri said in an interview during her poster presentation at annual meeting of the North American Primary Care Research Groups.
To address such uncertainties, which can impact both quality and continuity of care, Dr. Jetpuri and her colleagues devised an intervention aimed at third-year medical students who were doing family medicine clerkships. The intervention has been given to about 360 students thus far.
The intervention consisted of two components. The first, a didactic module, delivered information about the importance of professionalism and teamwork and also defined interprofessional roles.
The second phase of the training had students using the “sandbox” of Epic, UT Southwestern’s EHR, to practice responding to hypothetical messages from a patient, a nurse, a pharmacist, and a physical therapist. One message would arrive in the medical student’s EHR inbox each week, and the student would have the week to craft a response.
Dr. Jetpuri said that she and her colleagues worked hard to make the scenarios as realistic as possible: The lengthy patient email included several questions about the safety of taking supplements or herbal remedies. They also tried to make sure that the nuts and bolts of interprofessional communication were covered so that, for example, medical students would end a module knowing what should be included when writing orders for physical therapy and how to place the order correctly.
The responses then went through peer evaluation according to a rubric constructed by the investigators. The training and evaluation were designed to make sure that students acquired a better understanding of the mechanics of EHR interprofessional communication and of the soft skills needed for collegial and professional communication.
Dr. Jetpuri said that she and her colleagues would like to extend this realistic educational tool through the clerkship year for better continuity. They also are working on some technical aspects of the EHR to make communication easier for medical students. Furthermore, they are in the process of validating the peer evaluation process by having instructors use the rubric to duplicate the students’ evaluation. She said, though, that both trainees and faculty see the value in early, realistic experience using the electronic record in a multidisciplinary team.
“Simulated EHR experiences are an important tool to utilize in a medical school curriculum to better train and prepare our students for the postgraduate stage,” wrote Dr. Jetpuri and her colleagues.
Dr. Jetpuri reported that she has no conflicts of interest.
[email protected]
On Twitter @karioakes
MONTREAL – A hands-on training program designed to improve communication between physicians and allied health professionals, such as physical therapists, produced gains in comfort and confidence to medical students who completed the training.
After completing the training, which involved both a didactic and a hands-on component, 92.58% of third-year medical students agreed or strongly agreed that they had the skills needed for effective interprofessional communication in the EHR, up from 43.86% before the training.
The idea for the training began when Zaiba Jetpuri, DO, and her physician colleagues at the University of Texas Southwestern Medical Center, Dallas, noticed that medical students and residents often were unsure how best to communicate with nurses, pharmacists, physical therapists, and other members of the healthcare team via the EHR.
Knowing who has what role in an interdisciplinary setting is far from obvious, and knowing how to strike the right tone in EHR communications was a challenge for trainees, Dr. Jetpuri said in an interview during her poster presentation at annual meeting of the North American Primary Care Research Groups.
To address such uncertainties, which can impact both quality and continuity of care, Dr. Jetpuri and her colleagues devised an intervention aimed at third-year medical students who were doing family medicine clerkships. The intervention has been given to about 360 students thus far.
The intervention consisted of two components. The first, a didactic module, delivered information about the importance of professionalism and teamwork and also defined interprofessional roles.
The second phase of the training had students using the “sandbox” of Epic, UT Southwestern’s EHR, to practice responding to hypothetical messages from a patient, a nurse, a pharmacist, and a physical therapist. One message would arrive in the medical student’s EHR inbox each week, and the student would have the week to craft a response.
Dr. Jetpuri said that she and her colleagues worked hard to make the scenarios as realistic as possible: The lengthy patient email included several questions about the safety of taking supplements or herbal remedies. They also tried to make sure that the nuts and bolts of interprofessional communication were covered so that, for example, medical students would end a module knowing what should be included when writing orders for physical therapy and how to place the order correctly.
The responses then went through peer evaluation according to a rubric constructed by the investigators. The training and evaluation were designed to make sure that students acquired a better understanding of the mechanics of EHR interprofessional communication and of the soft skills needed for collegial and professional communication.
Dr. Jetpuri said that she and her colleagues would like to extend this realistic educational tool through the clerkship year for better continuity. They also are working on some technical aspects of the EHR to make communication easier for medical students. Furthermore, they are in the process of validating the peer evaluation process by having instructors use the rubric to duplicate the students’ evaluation. She said, though, that both trainees and faculty see the value in early, realistic experience using the electronic record in a multidisciplinary team.
“Simulated EHR experiences are an important tool to utilize in a medical school curriculum to better train and prepare our students for the postgraduate stage,” wrote Dr. Jetpuri and her colleagues.
Dr. Jetpuri reported that she has no conflicts of interest.
[email protected]
On Twitter @karioakes
MONTREAL – A hands-on training program designed to improve communication between physicians and allied health professionals, such as physical therapists, produced gains in comfort and confidence to medical students who completed the training.
After completing the training, which involved both a didactic and a hands-on component, 92.58% of third-year medical students agreed or strongly agreed that they had the skills needed for effective interprofessional communication in the EHR, up from 43.86% before the training.
The idea for the training began when Zaiba Jetpuri, DO, and her physician colleagues at the University of Texas Southwestern Medical Center, Dallas, noticed that medical students and residents often were unsure how best to communicate with nurses, pharmacists, physical therapists, and other members of the healthcare team via the EHR.
Knowing who has what role in an interdisciplinary setting is far from obvious, and knowing how to strike the right tone in EHR communications was a challenge for trainees, Dr. Jetpuri said in an interview during her poster presentation at annual meeting of the North American Primary Care Research Groups.
To address such uncertainties, which can impact both quality and continuity of care, Dr. Jetpuri and her colleagues devised an intervention aimed at third-year medical students who were doing family medicine clerkships. The intervention has been given to about 360 students thus far.
The intervention consisted of two components. The first, a didactic module, delivered information about the importance of professionalism and teamwork and also defined interprofessional roles.
The second phase of the training had students using the “sandbox” of Epic, UT Southwestern’s EHR, to practice responding to hypothetical messages from a patient, a nurse, a pharmacist, and a physical therapist. One message would arrive in the medical student’s EHR inbox each week, and the student would have the week to craft a response.
Dr. Jetpuri said that she and her colleagues worked hard to make the scenarios as realistic as possible: The lengthy patient email included several questions about the safety of taking supplements or herbal remedies. They also tried to make sure that the nuts and bolts of interprofessional communication were covered so that, for example, medical students would end a module knowing what should be included when writing orders for physical therapy and how to place the order correctly.
The responses then went through peer evaluation according to a rubric constructed by the investigators. The training and evaluation were designed to make sure that students acquired a better understanding of the mechanics of EHR interprofessional communication and of the soft skills needed for collegial and professional communication.
Dr. Jetpuri said that she and her colleagues would like to extend this realistic educational tool through the clerkship year for better continuity. They also are working on some technical aspects of the EHR to make communication easier for medical students. Furthermore, they are in the process of validating the peer evaluation process by having instructors use the rubric to duplicate the students’ evaluation. She said, though, that both trainees and faculty see the value in early, realistic experience using the electronic record in a multidisciplinary team.
“Simulated EHR experiences are an important tool to utilize in a medical school curriculum to better train and prepare our students for the postgraduate stage,” wrote Dr. Jetpuri and her colleagues.
Dr. Jetpuri reported that she has no conflicts of interest.
[email protected]
On Twitter @karioakes
AT NAPCRG 2017
Key clinical point:
Major finding: After the training, 93% of students felt they had the skills needed for effective interprofessional communication via the EHR.
Data source: Summary data from pilot involving about 360 third-year medical students completing family medicine clerkships.
Disclosures: Dr. Jetpuri reported no outside sources of funding and had no relevant disclosures.
Large database analysis suggests safety of bariatric surgery in seniors
NATIONAL HARBOR, MD. – despite a slight increase in unadjusted mortality rates, according to an analysis of data from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP).
Based on data that was collected in 2015 and submitted to MBSAQIP, “bariatric surgery is safe in the elderly, even in those 70 years old and older,” reported Tallal Zeni, MD, director of the Michigan Bariatric Institute in Livonia.
There were 16,568 patients older than age 60 years entered into the MBSAQIP database in 2015. When those were compared with the 117,443 younger patients, the unadjusted rates of morbidity (6.5% vs. 6.0%) and mortality (0.3% vs. 0.1%) were higher for the older patients, but “they are close,” according to Dr. Zeni. Both rates reached significance by the conventional definition (P < .05), but he suggested that they are lower in this study than those in prior studies of MBSAQIP datasets and that they are acceptable relative to the anticipated health benefits.
Above the age of 60 years, no correlation could be made between increasing age and increasing risk of morbidity, mortality, or rate of reoperations, according to Dr. Zeni.
Why should bariatric surgery be considered in older patients? He cited data from a study that showed the life expectancy in a 70-year-old without functional limitations is 13 years. As a result, he added, “it behooves us to provide them with the best quality of life we can.”
Relative to prior MBSAQIP evaluations of bariatric surgery in the elderly, the proportion of patients undergoing sleeve gastrectomy relative to gastric bypass has been increasing, Dr. Zeni reported. In the analysis, approximately two-thirds of the bariatric procedures were performed with sleeve gastrectomy, which is higher relative to what previous MBSAQIP analyses have shown.
Based on rates of morbidity for those two surgical approaches in the analysis, that trend makes sense. While the higher 30-day mortality for gastric bypass, compared with sleeve gastrectomy, was not significant (0.38% vs. 0.26%; P = .221), all-cause morbidity was almost two times greater for those undergoing gastric bypass than it was for those undergoing sleeve gastrectomy (10.61% vs. 5.81%; P < .001), Dr. Zeni reported.
However, some of that difference may be explained by baseline disparities between the two groups. In the gastric bypass group, there were higher rates of preoperative diabetes (54% vs. 40%; P < .001), sleep apnea (57% vs. 50%; P < .001) and hyperlipidemia (59% vs. 54%; P < .001). Also, gastric bypass patients were more likely to have a history of a previous bariatric procedure (11% vs. 8.5%; P < .001) and to be in the American Society of Anesthesiologists Physical Status score of 3 (84% vs. 80%; P < .001), according to Dr. Zeni.
The specific complications more common in the gastric bypass group than the sleeve gastrectomy group included anastomotic leak (0.56% vs. 0.3%; P = .017), surgical site infection (1.74% vs. 0.61%; P < .001), pneumonia (0.87% vs. 0.32%; P < .001), and bleeding (1.14% vs. 0.5%; P = .024). Although the average operating time was 40 minutes longer in the bypass group, there were no significant differences in thromboembolic complications.
Overall, despite a modest increase in the risk of complications for bariatric surgery in elderly patients, that risk can be considered acceptable in relation to the potential health benefits, according to Dr. Zeni. He suggested that the data might encourage further growth in the rates of bariatric procedures among patients older than 60 years.
Dr. Zeni reports no relevant financial relationships.
NATIONAL HARBOR, MD. – despite a slight increase in unadjusted mortality rates, according to an analysis of data from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP).
Based on data that was collected in 2015 and submitted to MBSAQIP, “bariatric surgery is safe in the elderly, even in those 70 years old and older,” reported Tallal Zeni, MD, director of the Michigan Bariatric Institute in Livonia.
There were 16,568 patients older than age 60 years entered into the MBSAQIP database in 2015. When those were compared with the 117,443 younger patients, the unadjusted rates of morbidity (6.5% vs. 6.0%) and mortality (0.3% vs. 0.1%) were higher for the older patients, but “they are close,” according to Dr. Zeni. Both rates reached significance by the conventional definition (P < .05), but he suggested that they are lower in this study than those in prior studies of MBSAQIP datasets and that they are acceptable relative to the anticipated health benefits.
Above the age of 60 years, no correlation could be made between increasing age and increasing risk of morbidity, mortality, or rate of reoperations, according to Dr. Zeni.
Why should bariatric surgery be considered in older patients? He cited data from a study that showed the life expectancy in a 70-year-old without functional limitations is 13 years. As a result, he added, “it behooves us to provide them with the best quality of life we can.”
Relative to prior MBSAQIP evaluations of bariatric surgery in the elderly, the proportion of patients undergoing sleeve gastrectomy relative to gastric bypass has been increasing, Dr. Zeni reported. In the analysis, approximately two-thirds of the bariatric procedures were performed with sleeve gastrectomy, which is higher relative to what previous MBSAQIP analyses have shown.
Based on rates of morbidity for those two surgical approaches in the analysis, that trend makes sense. While the higher 30-day mortality for gastric bypass, compared with sleeve gastrectomy, was not significant (0.38% vs. 0.26%; P = .221), all-cause morbidity was almost two times greater for those undergoing gastric bypass than it was for those undergoing sleeve gastrectomy (10.61% vs. 5.81%; P < .001), Dr. Zeni reported.
However, some of that difference may be explained by baseline disparities between the two groups. In the gastric bypass group, there were higher rates of preoperative diabetes (54% vs. 40%; P < .001), sleep apnea (57% vs. 50%; P < .001) and hyperlipidemia (59% vs. 54%; P < .001). Also, gastric bypass patients were more likely to have a history of a previous bariatric procedure (11% vs. 8.5%; P < .001) and to be in the American Society of Anesthesiologists Physical Status score of 3 (84% vs. 80%; P < .001), according to Dr. Zeni.
The specific complications more common in the gastric bypass group than the sleeve gastrectomy group included anastomotic leak (0.56% vs. 0.3%; P = .017), surgical site infection (1.74% vs. 0.61%; P < .001), pneumonia (0.87% vs. 0.32%; P < .001), and bleeding (1.14% vs. 0.5%; P = .024). Although the average operating time was 40 minutes longer in the bypass group, there were no significant differences in thromboembolic complications.
Overall, despite a modest increase in the risk of complications for bariatric surgery in elderly patients, that risk can be considered acceptable in relation to the potential health benefits, according to Dr. Zeni. He suggested that the data might encourage further growth in the rates of bariatric procedures among patients older than 60 years.
Dr. Zeni reports no relevant financial relationships.
NATIONAL HARBOR, MD. – despite a slight increase in unadjusted mortality rates, according to an analysis of data from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP).
Based on data that was collected in 2015 and submitted to MBSAQIP, “bariatric surgery is safe in the elderly, even in those 70 years old and older,” reported Tallal Zeni, MD, director of the Michigan Bariatric Institute in Livonia.
There were 16,568 patients older than age 60 years entered into the MBSAQIP database in 2015. When those were compared with the 117,443 younger patients, the unadjusted rates of morbidity (6.5% vs. 6.0%) and mortality (0.3% vs. 0.1%) were higher for the older patients, but “they are close,” according to Dr. Zeni. Both rates reached significance by the conventional definition (P < .05), but he suggested that they are lower in this study than those in prior studies of MBSAQIP datasets and that they are acceptable relative to the anticipated health benefits.
Above the age of 60 years, no correlation could be made between increasing age and increasing risk of morbidity, mortality, or rate of reoperations, according to Dr. Zeni.
Why should bariatric surgery be considered in older patients? He cited data from a study that showed the life expectancy in a 70-year-old without functional limitations is 13 years. As a result, he added, “it behooves us to provide them with the best quality of life we can.”
Relative to prior MBSAQIP evaluations of bariatric surgery in the elderly, the proportion of patients undergoing sleeve gastrectomy relative to gastric bypass has been increasing, Dr. Zeni reported. In the analysis, approximately two-thirds of the bariatric procedures were performed with sleeve gastrectomy, which is higher relative to what previous MBSAQIP analyses have shown.
Based on rates of morbidity for those two surgical approaches in the analysis, that trend makes sense. While the higher 30-day mortality for gastric bypass, compared with sleeve gastrectomy, was not significant (0.38% vs. 0.26%; P = .221), all-cause morbidity was almost two times greater for those undergoing gastric bypass than it was for those undergoing sleeve gastrectomy (10.61% vs. 5.81%; P < .001), Dr. Zeni reported.
However, some of that difference may be explained by baseline disparities between the two groups. In the gastric bypass group, there were higher rates of preoperative diabetes (54% vs. 40%; P < .001), sleep apnea (57% vs. 50%; P < .001) and hyperlipidemia (59% vs. 54%; P < .001). Also, gastric bypass patients were more likely to have a history of a previous bariatric procedure (11% vs. 8.5%; P < .001) and to be in the American Society of Anesthesiologists Physical Status score of 3 (84% vs. 80%; P < .001), according to Dr. Zeni.
The specific complications more common in the gastric bypass group than the sleeve gastrectomy group included anastomotic leak (0.56% vs. 0.3%; P = .017), surgical site infection (1.74% vs. 0.61%; P < .001), pneumonia (0.87% vs. 0.32%; P < .001), and bleeding (1.14% vs. 0.5%; P = .024). Although the average operating time was 40 minutes longer in the bypass group, there were no significant differences in thromboembolic complications.
Overall, despite a modest increase in the risk of complications for bariatric surgery in elderly patients, that risk can be considered acceptable in relation to the potential health benefits, according to Dr. Zeni. He suggested that the data might encourage further growth in the rates of bariatric procedures among patients older than 60 years.
Dr. Zeni reports no relevant financial relationships.
AT OBESITY WEEK 2017
Key clinical point: Based on mortality and morbidity rates, bariatric surgery is acceptably safe in patients older than 60 years of age.
Major finding: Compared with patients younger than 60 years, older patients had only modestly increased rates of morbidity (6.5% vs. 6.0%) and mortality (0.3% vs. 0.1%).
Data source: A retrospective database analysis.
Disclosures: Dr. Zeni reports no relevant financial relationships.
Ending hazing as a rite of manhood on college campuses
Is hazing a necessary rite of passage in Greek life, or a terrible tradition that needs to end once and for all?
There can be no justification for hazing, especially after the recent tragic deaths of fraternity pledges at Florida State University, Texas State University, and Louisiana State University. The horror of hazing has been brought home by the refiling of charges against several Penn State fraternity members in the torturous death last February of Timothy Piazza, which was recorded on videotape. In response to recent deaths and injuries, some colleges have suspended Greek life activities on campus. Unfortunately, hazing deaths are not new to college campuses but have a been a problem for several years, with 40 deaths in the last decade. The majority of these deaths involved the forced consumption of large amounts of alcohol, but some have involved beatings and other forms of abuse.
What exactly is hazing? According to the organization StopHazing (stophazing.org), it is “any activity expected of someone joining or participating in a group that humiliates, degrades, abuses, or endangers them regardless of a person’s willingness to participate.” Activities may involve alcohol consumption, humiliation, sleep deprivation, physical abuse, and sexual abuse. Hazing is not just a problem of fraternities; half of college students joining clubs, teams, and other organizations experience hazing. In fact, half of young adults have been hazed by the time they graduate from high school.
Given its inherent dangers, we have to wonder, why does hazing continue? The National Public Radio show 1A offered one answer to this troubling question on its Nov. 15 show, “How to Stop Hazing.” Two panel members, a filmmaker and a professor, discussed their own hazing experiences in college fraternities that included being forced to drink too much alcohol, eating noxious products, and being subjected to violence. One of the panel members talked about hazing other people. Both men admitted that the hazing process made them feel closer to their fraternity brothers: They formed lifelong bonds and also became stronger in facing adversity. In many ways, hazing was a masculine rite of passage. Neither panel member condoned the behaviors they were subjected to or participated in, and in fact suggested that college men should find new ways to bond and have a sense of belonging.
Even though the panelists were not promoting hazing, I was struck by their almost fond recollection of these experiences. I, in contrast, have no fond memory of an incident that I would consider medical hazing. During my internship when working on an internal medicine unit, I was ordered back to work after 2 days at home with the flu, although I was still febrile and coughing up a storm. That week, I was punished with an extra night of on-call duty. This incident did not leave me embracing the camaraderie and hardiness of my medical colleagues. It left me more determined than ever to treat peers and trainees with care and compassion, and never to abuse my power.
In our own practices as psychiatrists, we can play a role in helping our young adult male patients avoid hazing experiences, which have the potential to lead to depression, anxiety, posttraumatic stress disorder, and suicidal behaviors. We can work with our male patients to develop a sense of belonging and an understanding of who they are as men, without putting their lives or others’ lives at risk. In my work as a college counseling center psychiatrist for over 2 decades, I have often addressed the issues of masculinity, friendship, and peer pressure with my male patients. For those of you who work with young adult men, particularly in the college population, here are some tips:
1. Talk with your male patients about healthy versus harmful relationships. No relationship should involve the intentional infliction of physical or emotional pain. Men will acknowledge that a man hitting a girlfriend is abusive. They need to understand that male fraternity brothers hitting each other or forcing someone to drink a large volume of alcohol is equally abusive. Encourage your patients to know their limits and set boundaries if they are asked to do something dangerous to themselves or others.
2. Role play with your patients how to say no to their peers. I did that with a patient who was drinking too much in general with his fraternity brothers. He was afraid they would reject him if he drank less. He was pleasantly surprised when they did not pressure him to drink more, but instead encouraged him to do what is healthy for him.
3. Encourage your patients to have strong social connections on campus. Well-run fraternities can provide these friendship without inflicting pain. Intramural sports, singing groups, bands, and volunteer organizations all provide great ways to connect and also have a sense of accomplishment. Social connections improve grades, physical health, and emotional health.
4. Encourage your male patients to accept who they are, without embracing one stereotype of what it means to be a man. Social media often promotes unattainable physical images, and some male patients will take supplements or even steroids to build up muscle mass. Promote a healthy lifestyle without extremes in exercise and diet. Explore with your patient what it means to be a man in the 21st century, at a time when typical gender roles are being challenged.
5. Listen for cues about your patients’ relationship with their fathers, which have a large impact on how they view masculinity. Many of the male patients I see discuss how they are trying to be more in touch with and expressive about their feelings, after watching their fathers hold in their emotions or use alcohol to numb emotional pain. Some patients have been able to model and encourage a greater openness with their fathers, while others have been met with silence. As a patient is creating his own life story, his father’s history is always in the background.
Should all fraternities be shut down to end the hazing problem? I don’t believe this is the answer. Each campus has a different fraternity culture, and fraternities on many campuses can be a positive force. I have heard young men describe how fraternities encouraged them academically, discouraged excessive drinking, and promoted ethical behavior. But given that abuses have been prevalent on certain campuses, it is incumbent upon universities to enforce safe behaviors. Fraternity brothers who hurt others should be prosecuted, not protected.
The hazing on campuses needs to stop, and we as psychiatrists should talk about this important issue with our patients and sometimes their parents. We can educate our patients about this insidious form of physical and emotional abuse; we can encourage them not to be bystanders when this happens; and we can promote a culture of respect on our campuses.
Hazing is not just a campus but a national cultural problem, as we are finding from the avalanche of news reports about sexual harassment and assault in the political and entertainment worlds. Victims are exposed to abuses and then deterred from reporting them as a condition of staying in and advancing in the professions they love. Hazing is an abuse of power that we as psychiatrists must continue to fight. We should teach our young adult men the mantra that is now being used by some fraternities, “Real men don’t haze.”
Dr. Morris is an associate professor of psychiatry and associate program director for student health psychiatry at the University of Florida, Gainesville. She is the author of The Campus Cure: A Parent’s Guide to Mental Health and Wellness for College Students, which will be published by Rowman & Littlefield of Lanham, Md., in January 2018.
Is hazing a necessary rite of passage in Greek life, or a terrible tradition that needs to end once and for all?
There can be no justification for hazing, especially after the recent tragic deaths of fraternity pledges at Florida State University, Texas State University, and Louisiana State University. The horror of hazing has been brought home by the refiling of charges against several Penn State fraternity members in the torturous death last February of Timothy Piazza, which was recorded on videotape. In response to recent deaths and injuries, some colleges have suspended Greek life activities on campus. Unfortunately, hazing deaths are not new to college campuses but have a been a problem for several years, with 40 deaths in the last decade. The majority of these deaths involved the forced consumption of large amounts of alcohol, but some have involved beatings and other forms of abuse.
What exactly is hazing? According to the organization StopHazing (stophazing.org), it is “any activity expected of someone joining or participating in a group that humiliates, degrades, abuses, or endangers them regardless of a person’s willingness to participate.” Activities may involve alcohol consumption, humiliation, sleep deprivation, physical abuse, and sexual abuse. Hazing is not just a problem of fraternities; half of college students joining clubs, teams, and other organizations experience hazing. In fact, half of young adults have been hazed by the time they graduate from high school.
Given its inherent dangers, we have to wonder, why does hazing continue? The National Public Radio show 1A offered one answer to this troubling question on its Nov. 15 show, “How to Stop Hazing.” Two panel members, a filmmaker and a professor, discussed their own hazing experiences in college fraternities that included being forced to drink too much alcohol, eating noxious products, and being subjected to violence. One of the panel members talked about hazing other people. Both men admitted that the hazing process made them feel closer to their fraternity brothers: They formed lifelong bonds and also became stronger in facing adversity. In many ways, hazing was a masculine rite of passage. Neither panel member condoned the behaviors they were subjected to or participated in, and in fact suggested that college men should find new ways to bond and have a sense of belonging.
Even though the panelists were not promoting hazing, I was struck by their almost fond recollection of these experiences. I, in contrast, have no fond memory of an incident that I would consider medical hazing. During my internship when working on an internal medicine unit, I was ordered back to work after 2 days at home with the flu, although I was still febrile and coughing up a storm. That week, I was punished with an extra night of on-call duty. This incident did not leave me embracing the camaraderie and hardiness of my medical colleagues. It left me more determined than ever to treat peers and trainees with care and compassion, and never to abuse my power.
In our own practices as psychiatrists, we can play a role in helping our young adult male patients avoid hazing experiences, which have the potential to lead to depression, anxiety, posttraumatic stress disorder, and suicidal behaviors. We can work with our male patients to develop a sense of belonging and an understanding of who they are as men, without putting their lives or others’ lives at risk. In my work as a college counseling center psychiatrist for over 2 decades, I have often addressed the issues of masculinity, friendship, and peer pressure with my male patients. For those of you who work with young adult men, particularly in the college population, here are some tips:
1. Talk with your male patients about healthy versus harmful relationships. No relationship should involve the intentional infliction of physical or emotional pain. Men will acknowledge that a man hitting a girlfriend is abusive. They need to understand that male fraternity brothers hitting each other or forcing someone to drink a large volume of alcohol is equally abusive. Encourage your patients to know their limits and set boundaries if they are asked to do something dangerous to themselves or others.
2. Role play with your patients how to say no to their peers. I did that with a patient who was drinking too much in general with his fraternity brothers. He was afraid they would reject him if he drank less. He was pleasantly surprised when they did not pressure him to drink more, but instead encouraged him to do what is healthy for him.
3. Encourage your patients to have strong social connections on campus. Well-run fraternities can provide these friendship without inflicting pain. Intramural sports, singing groups, bands, and volunteer organizations all provide great ways to connect and also have a sense of accomplishment. Social connections improve grades, physical health, and emotional health.
4. Encourage your male patients to accept who they are, without embracing one stereotype of what it means to be a man. Social media often promotes unattainable physical images, and some male patients will take supplements or even steroids to build up muscle mass. Promote a healthy lifestyle without extremes in exercise and diet. Explore with your patient what it means to be a man in the 21st century, at a time when typical gender roles are being challenged.
5. Listen for cues about your patients’ relationship with their fathers, which have a large impact on how they view masculinity. Many of the male patients I see discuss how they are trying to be more in touch with and expressive about their feelings, after watching their fathers hold in their emotions or use alcohol to numb emotional pain. Some patients have been able to model and encourage a greater openness with their fathers, while others have been met with silence. As a patient is creating his own life story, his father’s history is always in the background.
Should all fraternities be shut down to end the hazing problem? I don’t believe this is the answer. Each campus has a different fraternity culture, and fraternities on many campuses can be a positive force. I have heard young men describe how fraternities encouraged them academically, discouraged excessive drinking, and promoted ethical behavior. But given that abuses have been prevalent on certain campuses, it is incumbent upon universities to enforce safe behaviors. Fraternity brothers who hurt others should be prosecuted, not protected.
The hazing on campuses needs to stop, and we as psychiatrists should talk about this important issue with our patients and sometimes their parents. We can educate our patients about this insidious form of physical and emotional abuse; we can encourage them not to be bystanders when this happens; and we can promote a culture of respect on our campuses.
Hazing is not just a campus but a national cultural problem, as we are finding from the avalanche of news reports about sexual harassment and assault in the political and entertainment worlds. Victims are exposed to abuses and then deterred from reporting them as a condition of staying in and advancing in the professions they love. Hazing is an abuse of power that we as psychiatrists must continue to fight. We should teach our young adult men the mantra that is now being used by some fraternities, “Real men don’t haze.”
Dr. Morris is an associate professor of psychiatry and associate program director for student health psychiatry at the University of Florida, Gainesville. She is the author of The Campus Cure: A Parent’s Guide to Mental Health and Wellness for College Students, which will be published by Rowman & Littlefield of Lanham, Md., in January 2018.
Is hazing a necessary rite of passage in Greek life, or a terrible tradition that needs to end once and for all?
There can be no justification for hazing, especially after the recent tragic deaths of fraternity pledges at Florida State University, Texas State University, and Louisiana State University. The horror of hazing has been brought home by the refiling of charges against several Penn State fraternity members in the torturous death last February of Timothy Piazza, which was recorded on videotape. In response to recent deaths and injuries, some colleges have suspended Greek life activities on campus. Unfortunately, hazing deaths are not new to college campuses but have a been a problem for several years, with 40 deaths in the last decade. The majority of these deaths involved the forced consumption of large amounts of alcohol, but some have involved beatings and other forms of abuse.
What exactly is hazing? According to the organization StopHazing (stophazing.org), it is “any activity expected of someone joining or participating in a group that humiliates, degrades, abuses, or endangers them regardless of a person’s willingness to participate.” Activities may involve alcohol consumption, humiliation, sleep deprivation, physical abuse, and sexual abuse. Hazing is not just a problem of fraternities; half of college students joining clubs, teams, and other organizations experience hazing. In fact, half of young adults have been hazed by the time they graduate from high school.
Given its inherent dangers, we have to wonder, why does hazing continue? The National Public Radio show 1A offered one answer to this troubling question on its Nov. 15 show, “How to Stop Hazing.” Two panel members, a filmmaker and a professor, discussed their own hazing experiences in college fraternities that included being forced to drink too much alcohol, eating noxious products, and being subjected to violence. One of the panel members talked about hazing other people. Both men admitted that the hazing process made them feel closer to their fraternity brothers: They formed lifelong bonds and also became stronger in facing adversity. In many ways, hazing was a masculine rite of passage. Neither panel member condoned the behaviors they were subjected to or participated in, and in fact suggested that college men should find new ways to bond and have a sense of belonging.
Even though the panelists were not promoting hazing, I was struck by their almost fond recollection of these experiences. I, in contrast, have no fond memory of an incident that I would consider medical hazing. During my internship when working on an internal medicine unit, I was ordered back to work after 2 days at home with the flu, although I was still febrile and coughing up a storm. That week, I was punished with an extra night of on-call duty. This incident did not leave me embracing the camaraderie and hardiness of my medical colleagues. It left me more determined than ever to treat peers and trainees with care and compassion, and never to abuse my power.
In our own practices as psychiatrists, we can play a role in helping our young adult male patients avoid hazing experiences, which have the potential to lead to depression, anxiety, posttraumatic stress disorder, and suicidal behaviors. We can work with our male patients to develop a sense of belonging and an understanding of who they are as men, without putting their lives or others’ lives at risk. In my work as a college counseling center psychiatrist for over 2 decades, I have often addressed the issues of masculinity, friendship, and peer pressure with my male patients. For those of you who work with young adult men, particularly in the college population, here are some tips:
1. Talk with your male patients about healthy versus harmful relationships. No relationship should involve the intentional infliction of physical or emotional pain. Men will acknowledge that a man hitting a girlfriend is abusive. They need to understand that male fraternity brothers hitting each other or forcing someone to drink a large volume of alcohol is equally abusive. Encourage your patients to know their limits and set boundaries if they are asked to do something dangerous to themselves or others.
2. Role play with your patients how to say no to their peers. I did that with a patient who was drinking too much in general with his fraternity brothers. He was afraid they would reject him if he drank less. He was pleasantly surprised when they did not pressure him to drink more, but instead encouraged him to do what is healthy for him.
3. Encourage your patients to have strong social connections on campus. Well-run fraternities can provide these friendship without inflicting pain. Intramural sports, singing groups, bands, and volunteer organizations all provide great ways to connect and also have a sense of accomplishment. Social connections improve grades, physical health, and emotional health.
4. Encourage your male patients to accept who they are, without embracing one stereotype of what it means to be a man. Social media often promotes unattainable physical images, and some male patients will take supplements or even steroids to build up muscle mass. Promote a healthy lifestyle without extremes in exercise and diet. Explore with your patient what it means to be a man in the 21st century, at a time when typical gender roles are being challenged.
5. Listen for cues about your patients’ relationship with their fathers, which have a large impact on how they view masculinity. Many of the male patients I see discuss how they are trying to be more in touch with and expressive about their feelings, after watching their fathers hold in their emotions or use alcohol to numb emotional pain. Some patients have been able to model and encourage a greater openness with their fathers, while others have been met with silence. As a patient is creating his own life story, his father’s history is always in the background.
Should all fraternities be shut down to end the hazing problem? I don’t believe this is the answer. Each campus has a different fraternity culture, and fraternities on many campuses can be a positive force. I have heard young men describe how fraternities encouraged them academically, discouraged excessive drinking, and promoted ethical behavior. But given that abuses have been prevalent on certain campuses, it is incumbent upon universities to enforce safe behaviors. Fraternity brothers who hurt others should be prosecuted, not protected.
The hazing on campuses needs to stop, and we as psychiatrists should talk about this important issue with our patients and sometimes their parents. We can educate our patients about this insidious form of physical and emotional abuse; we can encourage them not to be bystanders when this happens; and we can promote a culture of respect on our campuses.
Hazing is not just a campus but a national cultural problem, as we are finding from the avalanche of news reports about sexual harassment and assault in the political and entertainment worlds. Victims are exposed to abuses and then deterred from reporting them as a condition of staying in and advancing in the professions they love. Hazing is an abuse of power that we as psychiatrists must continue to fight. We should teach our young adult men the mantra that is now being used by some fraternities, “Real men don’t haze.”
Dr. Morris is an associate professor of psychiatry and associate program director for student health psychiatry at the University of Florida, Gainesville. She is the author of The Campus Cure: A Parent’s Guide to Mental Health and Wellness for College Students, which will be published by Rowman & Littlefield of Lanham, Md., in January 2018.
FDA approves epinephrine autoinjector for infants, small children
The Food and Drug Administration approved an epinephrine autoinjector constructed specifically to treat life-threatening allergic reactions in infants and small children weighing 16.5-33 pounds.
The Auvi-Q 0.1 mg autoinjector by kaléo was approved after a priority review by the FDA, with features such as “a voice prompt system that guides a user with step-by-step instructions through the delivery process,” according to a written statement from the company. This auto-injector has a shorter needle length and lower dose of epinephrine than other FDA-approved 0.15-mg and 0.3-mg epinephrine autoinjectors.
In a previous study of 51 infants with a mean weight of 24 pounds who were treated with a 0.15-mg epinephrine auto-injector with a standard 12.7-mm needle length, 43% were at risk of having the needle strike the bone. Unintentional injection of epinephrine into the intraosseous space can cause systemic absorption of the epinephrine and possible cardiac complications (Ann Allergy Asthma Immunol. 2017 Jun;118[6]:719-25.e1).
This new autoinjector with a shorter needle length was designed to obviate this problem, according to kaléo’s statement.
The Auvi-Q 0.1 mg autoinjector should be available to patients in the first half of 2018, the company said.
The Food and Drug Administration approved an epinephrine autoinjector constructed specifically to treat life-threatening allergic reactions in infants and small children weighing 16.5-33 pounds.
The Auvi-Q 0.1 mg autoinjector by kaléo was approved after a priority review by the FDA, with features such as “a voice prompt system that guides a user with step-by-step instructions through the delivery process,” according to a written statement from the company. This auto-injector has a shorter needle length and lower dose of epinephrine than other FDA-approved 0.15-mg and 0.3-mg epinephrine autoinjectors.
In a previous study of 51 infants with a mean weight of 24 pounds who were treated with a 0.15-mg epinephrine auto-injector with a standard 12.7-mm needle length, 43% were at risk of having the needle strike the bone. Unintentional injection of epinephrine into the intraosseous space can cause systemic absorption of the epinephrine and possible cardiac complications (Ann Allergy Asthma Immunol. 2017 Jun;118[6]:719-25.e1).
This new autoinjector with a shorter needle length was designed to obviate this problem, according to kaléo’s statement.
The Auvi-Q 0.1 mg autoinjector should be available to patients in the first half of 2018, the company said.
The Food and Drug Administration approved an epinephrine autoinjector constructed specifically to treat life-threatening allergic reactions in infants and small children weighing 16.5-33 pounds.
The Auvi-Q 0.1 mg autoinjector by kaléo was approved after a priority review by the FDA, with features such as “a voice prompt system that guides a user with step-by-step instructions through the delivery process,” according to a written statement from the company. This auto-injector has a shorter needle length and lower dose of epinephrine than other FDA-approved 0.15-mg and 0.3-mg epinephrine autoinjectors.
In a previous study of 51 infants with a mean weight of 24 pounds who were treated with a 0.15-mg epinephrine auto-injector with a standard 12.7-mm needle length, 43% were at risk of having the needle strike the bone. Unintentional injection of epinephrine into the intraosseous space can cause systemic absorption of the epinephrine and possible cardiac complications (Ann Allergy Asthma Immunol. 2017 Jun;118[6]:719-25.e1).
This new autoinjector with a shorter needle length was designed to obviate this problem, according to kaléo’s statement.
The Auvi-Q 0.1 mg autoinjector should be available to patients in the first half of 2018, the company said.