User login
Shoulder Pain—Is It From the Shoulder, Neck, or Both?
Neck and shoulder pain are common presenting symptoms in the general adult population with a 41.7% and 50.9% lifetime incidence in males and females, respectively.1 Generally, a single diagnosis is sought to explain a patient’s signs and symptoms, but occasionally 2 or more different causes are responsible. Only by conducting a thorough history and physical examination with proper follow-up will all contributing diseases be discovered. The following case illustrates 2 distinct etiologies responsible for the patient’s pain, one with an extremely unusual presentation.
Case Presentation
A 23-year-old male presented with a 3-month history of pain, spasm, and tightness of his right upper extremity along his posterior neck, shoulder, and triceps area. The patient reported no history of trauma, but he revealed increasing the amount of weight lifting, and his symptoms were especially worse when bench pressing or performing overhead exercises. No paresthesias were reported.
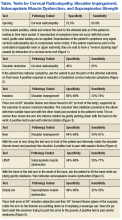
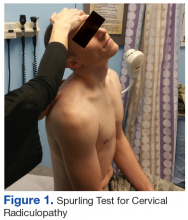
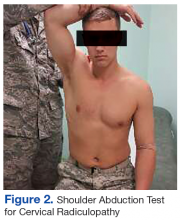
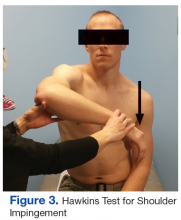
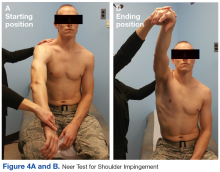
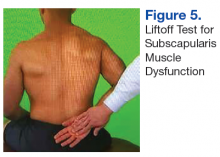
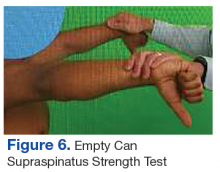
The initial examination revealed a well-developed muscular male with no visible atrophy or tenderness to palpation. He had full range of motion (ROM) of his neck, a normal motor and sensory examination of the C5-T1 nerve roots, and a negative Spurling maneuver. The patient had full ROM of both shoulders but had pain with right shoulder abduction starting at about 120° to 140°. He had pain with resisted supraspinatus muscle testing as well as pain with the liftoff test. The results of the patient’s Hawkins and Neer tests were negative (Table, Figures 1 to 6).2-4 A point-of-care shoulder ultrasound examination revealed no abnormalities.
The working diagnosis was external shoulder impingement with a differential diagnosis of internal impingement and/or cervical radiculopathy. A diagnostic/therapeutic injection of 40 mg of triamcinolone and 4 mL of 1% xylocaine without epinephrine was administered into the right subacromial bursa. The patient experienced immediate and complete relief of pain with repeat shoulder abduction, supraspinatus muscle testing, and the liftoff test. Although this procedure temporarily relieved the pain with movement, a sensation of tightness, pain, and spasm in the posterior shoulder and right posterior arm was still present. The patient was asked to perform therapeutic rotator cuff and scapular strengthening exercises, annotated on a patient information handout, for 15 minutes a day, every other day and to follow-up in 4 weeks.
At follow-up the external impingement symptoms (pain with shoulder abduction, resisted supraspinatus testing and the liftoff test) were fully resolved, but the patient reported persistent pain, spasm, and sensation of tightness in his right posterior shoulder and arm with intermittent extension into forearm and hand. A review of the history reminded the patient of a wrestling episode that caused neck pain months earlier. The patient reported that his current symptoms began after the wrestling episode.
Physical examination at this time revealed pain in the right posterior arm with left lateral neck movement but no neck pain with right lateral neck movement or flexion and extension. There was again a normal motor and sensory examination in the C5-T1 nerve distribution. Of note, there was full painless abduction in the right shoulder, which had improved from the previous examination, and there was no pain with resisted supraspinatus testing or the liftoff test, both of which had been abnormal at the initial encounter.
Due to the patient’s persistent posterior shoulder pain and exacerbation of symptoms with neck movement and the now revealed antecedent event of neck trauma, a higher concern for cervical disc pathology was entertained. A cervical magnetic resonance imaging examination (MRI) was ordered. A moderately sized left paracentral herniation of the disk at C5-C6 was found. The disk herniation was compressing the left ventral hemi-cord with narrowing of the left neuronal foramina. Additionally, there was a mild posterior disc osteophyte complex that caused mild left foraminal narrowing at C6-C7.
Neurosurgical consultation was obtained. Extensive discussion of nonsurgical vs surgical options were conducted, and a trial of nonsurgical therapy was agreed on. Physical therapy with cervical traction was prescribed with 2 sessions a week for 4 weeks. The patient also continued his therapeutic rotator cuff and scapular stabilizing exercises and decreased the amount and intensity of his weight lifting.
At the next 4-week follow-up, his symptoms were greatly reduced. He was discharged from supervised physical therapy and continued his at-home neck and shoulder strengthening regimen. At the 1-year follow-up, the patient reported that the radiating pain had essentially resolved—only occasionally being present with heavy upper-extremity weight lifting or grappling activities. He continues to be symptom free of his external impingement symptoms as well.
The final diagnosis was cervical radiculopathy of C5/6 nerve root due to left paracentral disc herniation with concomitant cord compression as well as external impingement (rotator cuff dysfunction) of the right shoulder. It is unclear whether the disc herniation contributed to the external shoulder impingement due to alterations in biomechanics or whether the 2 diseases were unrelated.
Discussion
The patient’s cervical disc herniation most likely was due to his earlier grappling episode when he had acute trauma to the neck or an exacerbation of an older asymptomatic herniation. His external shoulder impingement likely was due to overuse with heavy weight lifting, which also caused enough mechanical strain to exacerbate the patient’s cervical disc herniation symptoms. What is most unusual about this case is the right-sided cervical radicular symptoms due to a left-sided cervical disc herniation.
With an annual incidence of 107.3 in men and 63.5 in women per 100,000 patients, cervical radiculopathy is caused by compression or irritation of the cervical nerve roots as they exit the spine. The most common cause of cervical radiculopathy is spondylosis followed by disc herniation, but both can be present in the same patient. Spondylosis refers to degeneration of the discs and facet joints but generally without frank disc herniation.5
External shoulder impingement and cervical radiculopathy can have nearly identical symptoms of shoulder and upper arm pain as in this illustrated case. Patients with cervical radiculopathy generally present with neck, shoulder, and arm pain or neurologic deficits. These symptoms alone are very broad and present a wide differential diagnosis. One must determine whether the pain is from the neck or shoulder region.1 The Table and Figures 1 to 6 describe the physical examination maneuvers used to differentiate the etiology.
The decision to pursue imaging should be based on injury severity and patient treatment goals. Although plain radiographic imaging may reveal spondylotic changes, such as degenerative joint changes at the vertebral facets and uncovertebral joints as well as decreased disc space, MRI is the imaging modality of choice for viewing disc herniations.6Nonoperative management of cervical radiculopathy focuses on restoration of full pain-free neck ROM, cervical muscle strengthening, and consideration for cervical traction. The use of either topical or oral medications can be considered if needed to aid in sleep and/or participation in active rehabilitation. Complimentary methods, such as acupuncture, yoga, or therapeutic massage also should be considered. Additionally, corticosteroid epidural injections can be considered, but these have increased risk compared with lumbar epidural injections.7 Surgical indications include persistent symptoms after 6 to 12 weeks of conservative therapy with no improvement of symptoms or progressively worsening motor/neurologic deficits.8
Conclusion
This case illustrates how 2 different conditions can present similarly and lead to diagnostic uncertainty. In this case, both the shoulder impingement and cervical radiculopathy manifested as shoulder and upper arm pain and could be separated only once the impingement had been treated. In addition the left-sided disc herniation causing right-sided symptoms was very unusual. To the best of the authors’ knowledge, this is only the second report of cervical disc herniation causing contralateral symptoms. In the only other available case report on cervical disc herniation with contralateral symptoms, the symptoms occurred in both the contralateral arm and leg.9
1. Briggs AM, Straker LM, Bear NL, Smith AJ. Neck/shoulder pain in adolescents is not related to the level or nature of self-reported physical activity or type of sedentary activity in an Australian pregnancy cohort. BMC Musculoskelet Disord. 2009;10(1):87.
2. Hermans J, Luime JJ, Meuffels DE, Reijman M, Simel DL, Bierma-Zeinstra SM. Does this patient with shoulder pain have rotator cuff disease? JAMA. 2013;310(8):837-847.
3. Rubinstein SM, Pool JJ, van Tulder MW, Riphagen I, Riphagen II, de Vet HC. A systematic review of the diagnostic accuracy of provocative tests of the neck for diagnosing cervical radiculopathy. Eur Spine J. 2007;16(3):307-319.
4. Ghasemi M, Golabchi K, Mousavi SA, et al. The value of provocative tests in diagnosis of cervical radiculopathy. J Res Med Sci. 2013;18(suppl 1):S35-S38.
5. Woods BI, Hilibrand AS. Cervical radiculopathy: epidemiology, etiology, diagnosis, and treatment. J Spinal Disord Tech. 2015;28(5):E251-E259.
6. Green C, Butler J, Eustace S, Poynton A, O’Byrne JM. Imaging modalities for cervical spondylotic stenosis and myelopathy. Adv Orthop. 2012;2012:908324. [Epub July 20, 2011].
7. Childress MA, Beckers BA. Nonoperative management of cervical radiculopathy. Am Fam Physician. 2016;93(9):746-754.
8. Carette S, Fehlings MG. Clinical practice. Cervical radiculopathy. N Engl J Med. 2005;353(4):392-399.
9. Yeung JT, Johnson JI, Karim AS. Cervical disc herniation presenting with neck pain and contralateral symptoms: a case report. J Med Case Rep. 2012;6:166.
Neck and shoulder pain are common presenting symptoms in the general adult population with a 41.7% and 50.9% lifetime incidence in males and females, respectively.1 Generally, a single diagnosis is sought to explain a patient’s signs and symptoms, but occasionally 2 or more different causes are responsible. Only by conducting a thorough history and physical examination with proper follow-up will all contributing diseases be discovered. The following case illustrates 2 distinct etiologies responsible for the patient’s pain, one with an extremely unusual presentation.
Case Presentation
A 23-year-old male presented with a 3-month history of pain, spasm, and tightness of his right upper extremity along his posterior neck, shoulder, and triceps area. The patient reported no history of trauma, but he revealed increasing the amount of weight lifting, and his symptoms were especially worse when bench pressing or performing overhead exercises. No paresthesias were reported.
The initial examination revealed a well-developed muscular male with no visible atrophy or tenderness to palpation. He had full range of motion (ROM) of his neck, a normal motor and sensory examination of the C5-T1 nerve roots, and a negative Spurling maneuver. The patient had full ROM of both shoulders but had pain with right shoulder abduction starting at about 120° to 140°. He had pain with resisted supraspinatus muscle testing as well as pain with the liftoff test. The results of the patient’s Hawkins and Neer tests were negative (Table, Figures 1 to 6).2-4 A point-of-care shoulder ultrasound examination revealed no abnormalities.
The working diagnosis was external shoulder impingement with a differential diagnosis of internal impingement and/or cervical radiculopathy. A diagnostic/therapeutic injection of 40 mg of triamcinolone and 4 mL of 1% xylocaine without epinephrine was administered into the right subacromial bursa. The patient experienced immediate and complete relief of pain with repeat shoulder abduction, supraspinatus muscle testing, and the liftoff test. Although this procedure temporarily relieved the pain with movement, a sensation of tightness, pain, and spasm in the posterior shoulder and right posterior arm was still present. The patient was asked to perform therapeutic rotator cuff and scapular strengthening exercises, annotated on a patient information handout, for 15 minutes a day, every other day and to follow-up in 4 weeks.
At follow-up the external impingement symptoms (pain with shoulder abduction, resisted supraspinatus testing and the liftoff test) were fully resolved, but the patient reported persistent pain, spasm, and sensation of tightness in his right posterior shoulder and arm with intermittent extension into forearm and hand. A review of the history reminded the patient of a wrestling episode that caused neck pain months earlier. The patient reported that his current symptoms began after the wrestling episode.
Physical examination at this time revealed pain in the right posterior arm with left lateral neck movement but no neck pain with right lateral neck movement or flexion and extension. There was again a normal motor and sensory examination in the C5-T1 nerve distribution. Of note, there was full painless abduction in the right shoulder, which had improved from the previous examination, and there was no pain with resisted supraspinatus testing or the liftoff test, both of which had been abnormal at the initial encounter.
Due to the patient’s persistent posterior shoulder pain and exacerbation of symptoms with neck movement and the now revealed antecedent event of neck trauma, a higher concern for cervical disc pathology was entertained. A cervical magnetic resonance imaging examination (MRI) was ordered. A moderately sized left paracentral herniation of the disk at C5-C6 was found. The disk herniation was compressing the left ventral hemi-cord with narrowing of the left neuronal foramina. Additionally, there was a mild posterior disc osteophyte complex that caused mild left foraminal narrowing at C6-C7.
Neurosurgical consultation was obtained. Extensive discussion of nonsurgical vs surgical options were conducted, and a trial of nonsurgical therapy was agreed on. Physical therapy with cervical traction was prescribed with 2 sessions a week for 4 weeks. The patient also continued his therapeutic rotator cuff and scapular stabilizing exercises and decreased the amount and intensity of his weight lifting.
At the next 4-week follow-up, his symptoms were greatly reduced. He was discharged from supervised physical therapy and continued his at-home neck and shoulder strengthening regimen. At the 1-year follow-up, the patient reported that the radiating pain had essentially resolved—only occasionally being present with heavy upper-extremity weight lifting or grappling activities. He continues to be symptom free of his external impingement symptoms as well.
The final diagnosis was cervical radiculopathy of C5/6 nerve root due to left paracentral disc herniation with concomitant cord compression as well as external impingement (rotator cuff dysfunction) of the right shoulder. It is unclear whether the disc herniation contributed to the external shoulder impingement due to alterations in biomechanics or whether the 2 diseases were unrelated.
Discussion
The patient’s cervical disc herniation most likely was due to his earlier grappling episode when he had acute trauma to the neck or an exacerbation of an older asymptomatic herniation. His external shoulder impingement likely was due to overuse with heavy weight lifting, which also caused enough mechanical strain to exacerbate the patient’s cervical disc herniation symptoms. What is most unusual about this case is the right-sided cervical radicular symptoms due to a left-sided cervical disc herniation.
With an annual incidence of 107.3 in men and 63.5 in women per 100,000 patients, cervical radiculopathy is caused by compression or irritation of the cervical nerve roots as they exit the spine. The most common cause of cervical radiculopathy is spondylosis followed by disc herniation, but both can be present in the same patient. Spondylosis refers to degeneration of the discs and facet joints but generally without frank disc herniation.5
External shoulder impingement and cervical radiculopathy can have nearly identical symptoms of shoulder and upper arm pain as in this illustrated case. Patients with cervical radiculopathy generally present with neck, shoulder, and arm pain or neurologic deficits. These symptoms alone are very broad and present a wide differential diagnosis. One must determine whether the pain is from the neck or shoulder region.1 The Table and Figures 1 to 6 describe the physical examination maneuvers used to differentiate the etiology.
The decision to pursue imaging should be based on injury severity and patient treatment goals. Although plain radiographic imaging may reveal spondylotic changes, such as degenerative joint changes at the vertebral facets and uncovertebral joints as well as decreased disc space, MRI is the imaging modality of choice for viewing disc herniations.6Nonoperative management of cervical radiculopathy focuses on restoration of full pain-free neck ROM, cervical muscle strengthening, and consideration for cervical traction. The use of either topical or oral medications can be considered if needed to aid in sleep and/or participation in active rehabilitation. Complimentary methods, such as acupuncture, yoga, or therapeutic massage also should be considered. Additionally, corticosteroid epidural injections can be considered, but these have increased risk compared with lumbar epidural injections.7 Surgical indications include persistent symptoms after 6 to 12 weeks of conservative therapy with no improvement of symptoms or progressively worsening motor/neurologic deficits.8
Conclusion
This case illustrates how 2 different conditions can present similarly and lead to diagnostic uncertainty. In this case, both the shoulder impingement and cervical radiculopathy manifested as shoulder and upper arm pain and could be separated only once the impingement had been treated. In addition the left-sided disc herniation causing right-sided symptoms was very unusual. To the best of the authors’ knowledge, this is only the second report of cervical disc herniation causing contralateral symptoms. In the only other available case report on cervical disc herniation with contralateral symptoms, the symptoms occurred in both the contralateral arm and leg.9
Neck and shoulder pain are common presenting symptoms in the general adult population with a 41.7% and 50.9% lifetime incidence in males and females, respectively.1 Generally, a single diagnosis is sought to explain a patient’s signs and symptoms, but occasionally 2 or more different causes are responsible. Only by conducting a thorough history and physical examination with proper follow-up will all contributing diseases be discovered. The following case illustrates 2 distinct etiologies responsible for the patient’s pain, one with an extremely unusual presentation.
Case Presentation
A 23-year-old male presented with a 3-month history of pain, spasm, and tightness of his right upper extremity along his posterior neck, shoulder, and triceps area. The patient reported no history of trauma, but he revealed increasing the amount of weight lifting, and his symptoms were especially worse when bench pressing or performing overhead exercises. No paresthesias were reported.
The initial examination revealed a well-developed muscular male with no visible atrophy or tenderness to palpation. He had full range of motion (ROM) of his neck, a normal motor and sensory examination of the C5-T1 nerve roots, and a negative Spurling maneuver. The patient had full ROM of both shoulders but had pain with right shoulder abduction starting at about 120° to 140°. He had pain with resisted supraspinatus muscle testing as well as pain with the liftoff test. The results of the patient’s Hawkins and Neer tests were negative (Table, Figures 1 to 6).2-4 A point-of-care shoulder ultrasound examination revealed no abnormalities.
The working diagnosis was external shoulder impingement with a differential diagnosis of internal impingement and/or cervical radiculopathy. A diagnostic/therapeutic injection of 40 mg of triamcinolone and 4 mL of 1% xylocaine without epinephrine was administered into the right subacromial bursa. The patient experienced immediate and complete relief of pain with repeat shoulder abduction, supraspinatus muscle testing, and the liftoff test. Although this procedure temporarily relieved the pain with movement, a sensation of tightness, pain, and spasm in the posterior shoulder and right posterior arm was still present. The patient was asked to perform therapeutic rotator cuff and scapular strengthening exercises, annotated on a patient information handout, for 15 minutes a day, every other day and to follow-up in 4 weeks.
At follow-up the external impingement symptoms (pain with shoulder abduction, resisted supraspinatus testing and the liftoff test) were fully resolved, but the patient reported persistent pain, spasm, and sensation of tightness in his right posterior shoulder and arm with intermittent extension into forearm and hand. A review of the history reminded the patient of a wrestling episode that caused neck pain months earlier. The patient reported that his current symptoms began after the wrestling episode.
Physical examination at this time revealed pain in the right posterior arm with left lateral neck movement but no neck pain with right lateral neck movement or flexion and extension. There was again a normal motor and sensory examination in the C5-T1 nerve distribution. Of note, there was full painless abduction in the right shoulder, which had improved from the previous examination, and there was no pain with resisted supraspinatus testing or the liftoff test, both of which had been abnormal at the initial encounter.
Due to the patient’s persistent posterior shoulder pain and exacerbation of symptoms with neck movement and the now revealed antecedent event of neck trauma, a higher concern for cervical disc pathology was entertained. A cervical magnetic resonance imaging examination (MRI) was ordered. A moderately sized left paracentral herniation of the disk at C5-C6 was found. The disk herniation was compressing the left ventral hemi-cord with narrowing of the left neuronal foramina. Additionally, there was a mild posterior disc osteophyte complex that caused mild left foraminal narrowing at C6-C7.
Neurosurgical consultation was obtained. Extensive discussion of nonsurgical vs surgical options were conducted, and a trial of nonsurgical therapy was agreed on. Physical therapy with cervical traction was prescribed with 2 sessions a week for 4 weeks. The patient also continued his therapeutic rotator cuff and scapular stabilizing exercises and decreased the amount and intensity of his weight lifting.
At the next 4-week follow-up, his symptoms were greatly reduced. He was discharged from supervised physical therapy and continued his at-home neck and shoulder strengthening regimen. At the 1-year follow-up, the patient reported that the radiating pain had essentially resolved—only occasionally being present with heavy upper-extremity weight lifting or grappling activities. He continues to be symptom free of his external impingement symptoms as well.
The final diagnosis was cervical radiculopathy of C5/6 nerve root due to left paracentral disc herniation with concomitant cord compression as well as external impingement (rotator cuff dysfunction) of the right shoulder. It is unclear whether the disc herniation contributed to the external shoulder impingement due to alterations in biomechanics or whether the 2 diseases were unrelated.
Discussion
The patient’s cervical disc herniation most likely was due to his earlier grappling episode when he had acute trauma to the neck or an exacerbation of an older asymptomatic herniation. His external shoulder impingement likely was due to overuse with heavy weight lifting, which also caused enough mechanical strain to exacerbate the patient’s cervical disc herniation symptoms. What is most unusual about this case is the right-sided cervical radicular symptoms due to a left-sided cervical disc herniation.
With an annual incidence of 107.3 in men and 63.5 in women per 100,000 patients, cervical radiculopathy is caused by compression or irritation of the cervical nerve roots as they exit the spine. The most common cause of cervical radiculopathy is spondylosis followed by disc herniation, but both can be present in the same patient. Spondylosis refers to degeneration of the discs and facet joints but generally without frank disc herniation.5
External shoulder impingement and cervical radiculopathy can have nearly identical symptoms of shoulder and upper arm pain as in this illustrated case. Patients with cervical radiculopathy generally present with neck, shoulder, and arm pain or neurologic deficits. These symptoms alone are very broad and present a wide differential diagnosis. One must determine whether the pain is from the neck or shoulder region.1 The Table and Figures 1 to 6 describe the physical examination maneuvers used to differentiate the etiology.
The decision to pursue imaging should be based on injury severity and patient treatment goals. Although plain radiographic imaging may reveal spondylotic changes, such as degenerative joint changes at the vertebral facets and uncovertebral joints as well as decreased disc space, MRI is the imaging modality of choice for viewing disc herniations.6Nonoperative management of cervical radiculopathy focuses on restoration of full pain-free neck ROM, cervical muscle strengthening, and consideration for cervical traction. The use of either topical or oral medications can be considered if needed to aid in sleep and/or participation in active rehabilitation. Complimentary methods, such as acupuncture, yoga, or therapeutic massage also should be considered. Additionally, corticosteroid epidural injections can be considered, but these have increased risk compared with lumbar epidural injections.7 Surgical indications include persistent symptoms after 6 to 12 weeks of conservative therapy with no improvement of symptoms or progressively worsening motor/neurologic deficits.8
Conclusion
This case illustrates how 2 different conditions can present similarly and lead to diagnostic uncertainty. In this case, both the shoulder impingement and cervical radiculopathy manifested as shoulder and upper arm pain and could be separated only once the impingement had been treated. In addition the left-sided disc herniation causing right-sided symptoms was very unusual. To the best of the authors’ knowledge, this is only the second report of cervical disc herniation causing contralateral symptoms. In the only other available case report on cervical disc herniation with contralateral symptoms, the symptoms occurred in both the contralateral arm and leg.9
1. Briggs AM, Straker LM, Bear NL, Smith AJ. Neck/shoulder pain in adolescents is not related to the level or nature of self-reported physical activity or type of sedentary activity in an Australian pregnancy cohort. BMC Musculoskelet Disord. 2009;10(1):87.
2. Hermans J, Luime JJ, Meuffels DE, Reijman M, Simel DL, Bierma-Zeinstra SM. Does this patient with shoulder pain have rotator cuff disease? JAMA. 2013;310(8):837-847.
3. Rubinstein SM, Pool JJ, van Tulder MW, Riphagen I, Riphagen II, de Vet HC. A systematic review of the diagnostic accuracy of provocative tests of the neck for diagnosing cervical radiculopathy. Eur Spine J. 2007;16(3):307-319.
4. Ghasemi M, Golabchi K, Mousavi SA, et al. The value of provocative tests in diagnosis of cervical radiculopathy. J Res Med Sci. 2013;18(suppl 1):S35-S38.
5. Woods BI, Hilibrand AS. Cervical radiculopathy: epidemiology, etiology, diagnosis, and treatment. J Spinal Disord Tech. 2015;28(5):E251-E259.
6. Green C, Butler J, Eustace S, Poynton A, O’Byrne JM. Imaging modalities for cervical spondylotic stenosis and myelopathy. Adv Orthop. 2012;2012:908324. [Epub July 20, 2011].
7. Childress MA, Beckers BA. Nonoperative management of cervical radiculopathy. Am Fam Physician. 2016;93(9):746-754.
8. Carette S, Fehlings MG. Clinical practice. Cervical radiculopathy. N Engl J Med. 2005;353(4):392-399.
9. Yeung JT, Johnson JI, Karim AS. Cervical disc herniation presenting with neck pain and contralateral symptoms: a case report. J Med Case Rep. 2012;6:166.
1. Briggs AM, Straker LM, Bear NL, Smith AJ. Neck/shoulder pain in adolescents is not related to the level or nature of self-reported physical activity or type of sedentary activity in an Australian pregnancy cohort. BMC Musculoskelet Disord. 2009;10(1):87.
2. Hermans J, Luime JJ, Meuffels DE, Reijman M, Simel DL, Bierma-Zeinstra SM. Does this patient with shoulder pain have rotator cuff disease? JAMA. 2013;310(8):837-847.
3. Rubinstein SM, Pool JJ, van Tulder MW, Riphagen I, Riphagen II, de Vet HC. A systematic review of the diagnostic accuracy of provocative tests of the neck for diagnosing cervical radiculopathy. Eur Spine J. 2007;16(3):307-319.
4. Ghasemi M, Golabchi K, Mousavi SA, et al. The value of provocative tests in diagnosis of cervical radiculopathy. J Res Med Sci. 2013;18(suppl 1):S35-S38.
5. Woods BI, Hilibrand AS. Cervical radiculopathy: epidemiology, etiology, diagnosis, and treatment. J Spinal Disord Tech. 2015;28(5):E251-E259.
6. Green C, Butler J, Eustace S, Poynton A, O’Byrne JM. Imaging modalities for cervical spondylotic stenosis and myelopathy. Adv Orthop. 2012;2012:908324. [Epub July 20, 2011].
7. Childress MA, Beckers BA. Nonoperative management of cervical radiculopathy. Am Fam Physician. 2016;93(9):746-754.
8. Carette S, Fehlings MG. Clinical practice. Cervical radiculopathy. N Engl J Med. 2005;353(4):392-399.
9. Yeung JT, Johnson JI, Karim AS. Cervical disc herniation presenting with neck pain and contralateral symptoms: a case report. J Med Case Rep. 2012;6:166.
Product approved to treat patients with hemophilia A and inhibitors
The US Food and Drug Administration (FDA) has approved use of emicizumab-kxwh (Hemlibra®), a bispecific factor IXa- and factor X-directed antibody.
Emicizumab is approved as routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children who have hemophilia A and factor VIII (FVIII) inhibitors.
Emicizumab can be self-administered once-weekly via subcutaneous injection.
The labeling for emicizumab contains a boxed warning noting that patients who received emicizumab in conjunction with activated prothrombin complex concentrate developed thrombotic microangiopathy (TMA) and thromboembolic events (TEs).
Therefore, patients should discontinue prophylactic use of bypassing agents (BPAs) the day before starting prophylaxis with emicizumab.
The FDA granted the approval of emicizumab to Genentech, Inc. The agency granted the application for emicizumab priority review, and the product received breakthrough therapy and orphan drug designations.
Access to emicizumab
According to Genentech, emicizumab will be available shortly.
The company said it will be offering comprehensive services to help minimize barriers to access and reimbursement. Patients can call 866-436-5427 (866-HEMLIBRA) for more information.
For people who qualify, Genentech plans to offer patient assistance programs through Genentech Access Solutions. More information is available at 866-422-2377 (866-4ACCESS) or http://www.Genentech-Access.com.
Emicizumab trials
The biologics license application for emicizumab was supported by results from a pair of phase 3 studies—HAVEN 1 and HAVEN 2.
Results from HAVEN 1 were published in NEJM and presented at the 26th ISTH Congress in July. Interim results from HAVEN 2 were presented at ISTH as well.
HAVEN 1
The study enrolled 109 patients (age 12 and older) with hemophilia A and FVIII inhibitors who were previously treated with BPAs on-demand or as prophylaxis.
The patients were randomized to receive emicizumab prophylaxis or no prophylaxis. On-demand treatment of breakthrough bleeds with BPAs was allowed.
There was a significant reduction in treated bleeds of 87% with emicizumab prophylaxis compared to no prophylaxis (95% CI: 72.3; 94.3, P<0.0001). And there was an 80% reduction in all bleeds with emicizumab (95% CI: 62.5; 89.8, P<0.0001).
Adverse events occurring in at least 5% of patients treated with emicizumab were local injection site reactions, headache, fatigue, upper respiratory tract infection, and arthralgia.
Two patients experienced TEs, and 3 had TMA while receiving emicizumab prophylaxis and more than 100 u/kg/day of activated prothrombin complex concentrate, on average, for 24 hours or more before the event. Two of these patients had also received recombinant factor VIIa.
Neither TE required anticoagulation therapy, and 1 patient restarted emicizumab. The cases of TMA observed were transient, and 1 patient restarted emicizumab.
HAVEN 2
In this single-arm trial, researchers evaluated emicizumab prophylaxis in children younger than 12 years of age who had hemophilia A with FVIII inhibitors.
The interim efficacy analysis, after at least 12 weeks of treatment, included 23 children.
After a median observation time of 38.1 weeks, 87% (95% CI: 66.4; 97.2) of children who received emicizumab experienced 0 treated bleeds. The percentage with 0 treated or non-treated bleeds was lower, at 34.8% (95% CI: 16.4; 57.3).
The most common adverse events (observed in at least 10% of patients) were mild injection site reactions and nasopharyngitis. No TEs or TMAs were observed.
HAVEN 3 and 4
Emicizumab is now being studied in 2 additional phase 3 trials.
In HAVEN 3, researchers are evaluating emicizumab prophylaxis dosed once weekly or once every other week in patients age 12 and older with hemophilia A without FVIII inhibitors.
In HAVEN 4, researchers are evaluating emicizumab prophylaxis dosed every 4 weeks in patients age 12 and older with hemophilia A, with or without inhibitors. ![]()
The US Food and Drug Administration (FDA) has approved use of emicizumab-kxwh (Hemlibra®), a bispecific factor IXa- and factor X-directed antibody.
Emicizumab is approved as routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children who have hemophilia A and factor VIII (FVIII) inhibitors.
Emicizumab can be self-administered once-weekly via subcutaneous injection.
The labeling for emicizumab contains a boxed warning noting that patients who received emicizumab in conjunction with activated prothrombin complex concentrate developed thrombotic microangiopathy (TMA) and thromboembolic events (TEs).
Therefore, patients should discontinue prophylactic use of bypassing agents (BPAs) the day before starting prophylaxis with emicizumab.
The FDA granted the approval of emicizumab to Genentech, Inc. The agency granted the application for emicizumab priority review, and the product received breakthrough therapy and orphan drug designations.
Access to emicizumab
According to Genentech, emicizumab will be available shortly.
The company said it will be offering comprehensive services to help minimize barriers to access and reimbursement. Patients can call 866-436-5427 (866-HEMLIBRA) for more information.
For people who qualify, Genentech plans to offer patient assistance programs through Genentech Access Solutions. More information is available at 866-422-2377 (866-4ACCESS) or http://www.Genentech-Access.com.
Emicizumab trials
The biologics license application for emicizumab was supported by results from a pair of phase 3 studies—HAVEN 1 and HAVEN 2.
Results from HAVEN 1 were published in NEJM and presented at the 26th ISTH Congress in July. Interim results from HAVEN 2 were presented at ISTH as well.
HAVEN 1
The study enrolled 109 patients (age 12 and older) with hemophilia A and FVIII inhibitors who were previously treated with BPAs on-demand or as prophylaxis.
The patients were randomized to receive emicizumab prophylaxis or no prophylaxis. On-demand treatment of breakthrough bleeds with BPAs was allowed.
There was a significant reduction in treated bleeds of 87% with emicizumab prophylaxis compared to no prophylaxis (95% CI: 72.3; 94.3, P<0.0001). And there was an 80% reduction in all bleeds with emicizumab (95% CI: 62.5; 89.8, P<0.0001).
Adverse events occurring in at least 5% of patients treated with emicizumab were local injection site reactions, headache, fatigue, upper respiratory tract infection, and arthralgia.
Two patients experienced TEs, and 3 had TMA while receiving emicizumab prophylaxis and more than 100 u/kg/day of activated prothrombin complex concentrate, on average, for 24 hours or more before the event. Two of these patients had also received recombinant factor VIIa.
Neither TE required anticoagulation therapy, and 1 patient restarted emicizumab. The cases of TMA observed were transient, and 1 patient restarted emicizumab.
HAVEN 2
In this single-arm trial, researchers evaluated emicizumab prophylaxis in children younger than 12 years of age who had hemophilia A with FVIII inhibitors.
The interim efficacy analysis, after at least 12 weeks of treatment, included 23 children.
After a median observation time of 38.1 weeks, 87% (95% CI: 66.4; 97.2) of children who received emicizumab experienced 0 treated bleeds. The percentage with 0 treated or non-treated bleeds was lower, at 34.8% (95% CI: 16.4; 57.3).
The most common adverse events (observed in at least 10% of patients) were mild injection site reactions and nasopharyngitis. No TEs or TMAs were observed.
HAVEN 3 and 4
Emicizumab is now being studied in 2 additional phase 3 trials.
In HAVEN 3, researchers are evaluating emicizumab prophylaxis dosed once weekly or once every other week in patients age 12 and older with hemophilia A without FVIII inhibitors.
In HAVEN 4, researchers are evaluating emicizumab prophylaxis dosed every 4 weeks in patients age 12 and older with hemophilia A, with or without inhibitors. ![]()
The US Food and Drug Administration (FDA) has approved use of emicizumab-kxwh (Hemlibra®), a bispecific factor IXa- and factor X-directed antibody.
Emicizumab is approved as routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children who have hemophilia A and factor VIII (FVIII) inhibitors.
Emicizumab can be self-administered once-weekly via subcutaneous injection.
The labeling for emicizumab contains a boxed warning noting that patients who received emicizumab in conjunction with activated prothrombin complex concentrate developed thrombotic microangiopathy (TMA) and thromboembolic events (TEs).
Therefore, patients should discontinue prophylactic use of bypassing agents (BPAs) the day before starting prophylaxis with emicizumab.
The FDA granted the approval of emicizumab to Genentech, Inc. The agency granted the application for emicizumab priority review, and the product received breakthrough therapy and orphan drug designations.
Access to emicizumab
According to Genentech, emicizumab will be available shortly.
The company said it will be offering comprehensive services to help minimize barriers to access and reimbursement. Patients can call 866-436-5427 (866-HEMLIBRA) for more information.
For people who qualify, Genentech plans to offer patient assistance programs through Genentech Access Solutions. More information is available at 866-422-2377 (866-4ACCESS) or http://www.Genentech-Access.com.
Emicizumab trials
The biologics license application for emicizumab was supported by results from a pair of phase 3 studies—HAVEN 1 and HAVEN 2.
Results from HAVEN 1 were published in NEJM and presented at the 26th ISTH Congress in July. Interim results from HAVEN 2 were presented at ISTH as well.
HAVEN 1
The study enrolled 109 patients (age 12 and older) with hemophilia A and FVIII inhibitors who were previously treated with BPAs on-demand or as prophylaxis.
The patients were randomized to receive emicizumab prophylaxis or no prophylaxis. On-demand treatment of breakthrough bleeds with BPAs was allowed.
There was a significant reduction in treated bleeds of 87% with emicizumab prophylaxis compared to no prophylaxis (95% CI: 72.3; 94.3, P<0.0001). And there was an 80% reduction in all bleeds with emicizumab (95% CI: 62.5; 89.8, P<0.0001).
Adverse events occurring in at least 5% of patients treated with emicizumab were local injection site reactions, headache, fatigue, upper respiratory tract infection, and arthralgia.
Two patients experienced TEs, and 3 had TMA while receiving emicizumab prophylaxis and more than 100 u/kg/day of activated prothrombin complex concentrate, on average, for 24 hours or more before the event. Two of these patients had also received recombinant factor VIIa.
Neither TE required anticoagulation therapy, and 1 patient restarted emicizumab. The cases of TMA observed were transient, and 1 patient restarted emicizumab.
HAVEN 2
In this single-arm trial, researchers evaluated emicizumab prophylaxis in children younger than 12 years of age who had hemophilia A with FVIII inhibitors.
The interim efficacy analysis, after at least 12 weeks of treatment, included 23 children.
After a median observation time of 38.1 weeks, 87% (95% CI: 66.4; 97.2) of children who received emicizumab experienced 0 treated bleeds. The percentage with 0 treated or non-treated bleeds was lower, at 34.8% (95% CI: 16.4; 57.3).
The most common adverse events (observed in at least 10% of patients) were mild injection site reactions and nasopharyngitis. No TEs or TMAs were observed.
HAVEN 3 and 4
Emicizumab is now being studied in 2 additional phase 3 trials.
In HAVEN 3, researchers are evaluating emicizumab prophylaxis dosed once weekly or once every other week in patients age 12 and older with hemophilia A without FVIII inhibitors.
In HAVEN 4, researchers are evaluating emicizumab prophylaxis dosed every 4 weeks in patients age 12 and older with hemophilia A, with or without inhibitors. ![]()
FDA expands approval of obinutuzumab
The US Food and Drug Administration (FDA) has expanded the approved use of obinutuzumab (Gazyva®).
The drug is now approved for use in combination with chemotherapy to treat patients with previously untreated follicular lymphoma (FL) that is advanced (stage II bulky, stage III, or stage IV) .
In patients who respond to this treatment, obinutuzumab monotherapy can be given as maintenance.
The FDA granted this new approval of obinutuzumab to Genentech, Inc. The application for obinutuzumab in this indication received priority review.
The latest FDA approval means obinutuzumab is available in the US for the following indications:
- In combination with chlorambucil to treat chronic lymphocytic leukemia (CLL) in adults who have not had previous CLL treatment
- In combination with bendamustine, followed by obinutuzumab alone, to treat FL in adults who did not respond to a rituximab-containing regimen or whose FL returned after such treatment
- In combination with chemotherapy, followed by obinutuzumab alone in responders, to treat stage II bulky, stage III, or stage IV FL in adults who have not had previous FL treatment.
Phase 3 results
The latest approval of obinutuzumab is based on results from the phase 3 GALLIUM study, which were presented at the 2016 ASH Annual Meeting and published in NEJM in October.
The following are updated data from the obinutuzumab prescribing information.
GALLIUM included 1385 patients with previously untreated non-Hodgkin lymphoma, and 1202 of these patients had advanced FL.
Half of the FL patients (n=601) were randomized to receive obinutuzumab plus chemotherapy (followed by obinutuzumab maintenance for up to 2 years), and half were randomized to rituximab plus chemotherapy (followed by rituximab maintenance for up to 2 years).
The different chemotherapies used were CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone), CVP (cyclophosphamide, vincristine, and prednisolone), and bendamustine.
At a median observation time of 38 months, the overall response rate was 91% in the obinutuzumab arm and 88% in the rituximab arm. The complete response rates were 28% and 27%, respectively.
The median progression-free survival was not reached in either arm. The hazard ratio, for obinutuzumab compared to rituximab, was 0.72 (95% CI, 0.56-0.93, P=0.0118).
Safety was evaluated based on all 1385 patients in the study, 86% of whom had previously untreated FL and 14% of whom had marginal zone lymphoma.
Serious adverse events (AEs) occurred in 50% of patients in the obinutuzumab arm and 43% in the rituximab arm. Fatal AEs occurred in 5% and 4%, respectively. Infections and second malignancies were the leading causes of these deaths.
The most common AEs (incidence ≥ 20%) observed at least 2% more patients in the obinutuzumab arm were infusion-related reactions, neutropenia, upper respiratory tract infection, cough, constipation, and diarrhea.
The most common grade 3 to 5 AEs (incidence ≥ 5%) observed more frequently in the obinutuzumab arm were neutropenia, infusion-related reactions, febrile neutropenia, and thrombocytopenia. ![]()
The US Food and Drug Administration (FDA) has expanded the approved use of obinutuzumab (Gazyva®).
The drug is now approved for use in combination with chemotherapy to treat patients with previously untreated follicular lymphoma (FL) that is advanced (stage II bulky, stage III, or stage IV) .
In patients who respond to this treatment, obinutuzumab monotherapy can be given as maintenance.
The FDA granted this new approval of obinutuzumab to Genentech, Inc. The application for obinutuzumab in this indication received priority review.
The latest FDA approval means obinutuzumab is available in the US for the following indications:
- In combination with chlorambucil to treat chronic lymphocytic leukemia (CLL) in adults who have not had previous CLL treatment
- In combination with bendamustine, followed by obinutuzumab alone, to treat FL in adults who did not respond to a rituximab-containing regimen or whose FL returned after such treatment
- In combination with chemotherapy, followed by obinutuzumab alone in responders, to treat stage II bulky, stage III, or stage IV FL in adults who have not had previous FL treatment.
Phase 3 results
The latest approval of obinutuzumab is based on results from the phase 3 GALLIUM study, which were presented at the 2016 ASH Annual Meeting and published in NEJM in October.
The following are updated data from the obinutuzumab prescribing information.
GALLIUM included 1385 patients with previously untreated non-Hodgkin lymphoma, and 1202 of these patients had advanced FL.
Half of the FL patients (n=601) were randomized to receive obinutuzumab plus chemotherapy (followed by obinutuzumab maintenance for up to 2 years), and half were randomized to rituximab plus chemotherapy (followed by rituximab maintenance for up to 2 years).
The different chemotherapies used were CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone), CVP (cyclophosphamide, vincristine, and prednisolone), and bendamustine.
At a median observation time of 38 months, the overall response rate was 91% in the obinutuzumab arm and 88% in the rituximab arm. The complete response rates were 28% and 27%, respectively.
The median progression-free survival was not reached in either arm. The hazard ratio, for obinutuzumab compared to rituximab, was 0.72 (95% CI, 0.56-0.93, P=0.0118).
Safety was evaluated based on all 1385 patients in the study, 86% of whom had previously untreated FL and 14% of whom had marginal zone lymphoma.
Serious adverse events (AEs) occurred in 50% of patients in the obinutuzumab arm and 43% in the rituximab arm. Fatal AEs occurred in 5% and 4%, respectively. Infections and second malignancies were the leading causes of these deaths.
The most common AEs (incidence ≥ 20%) observed at least 2% more patients in the obinutuzumab arm were infusion-related reactions, neutropenia, upper respiratory tract infection, cough, constipation, and diarrhea.
The most common grade 3 to 5 AEs (incidence ≥ 5%) observed more frequently in the obinutuzumab arm were neutropenia, infusion-related reactions, febrile neutropenia, and thrombocytopenia. ![]()
The US Food and Drug Administration (FDA) has expanded the approved use of obinutuzumab (Gazyva®).
The drug is now approved for use in combination with chemotherapy to treat patients with previously untreated follicular lymphoma (FL) that is advanced (stage II bulky, stage III, or stage IV) .
In patients who respond to this treatment, obinutuzumab monotherapy can be given as maintenance.
The FDA granted this new approval of obinutuzumab to Genentech, Inc. The application for obinutuzumab in this indication received priority review.
The latest FDA approval means obinutuzumab is available in the US for the following indications:
- In combination with chlorambucil to treat chronic lymphocytic leukemia (CLL) in adults who have not had previous CLL treatment
- In combination with bendamustine, followed by obinutuzumab alone, to treat FL in adults who did not respond to a rituximab-containing regimen or whose FL returned after such treatment
- In combination with chemotherapy, followed by obinutuzumab alone in responders, to treat stage II bulky, stage III, or stage IV FL in adults who have not had previous FL treatment.
Phase 3 results
The latest approval of obinutuzumab is based on results from the phase 3 GALLIUM study, which were presented at the 2016 ASH Annual Meeting and published in NEJM in October.
The following are updated data from the obinutuzumab prescribing information.
GALLIUM included 1385 patients with previously untreated non-Hodgkin lymphoma, and 1202 of these patients had advanced FL.
Half of the FL patients (n=601) were randomized to receive obinutuzumab plus chemotherapy (followed by obinutuzumab maintenance for up to 2 years), and half were randomized to rituximab plus chemotherapy (followed by rituximab maintenance for up to 2 years).
The different chemotherapies used were CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone), CVP (cyclophosphamide, vincristine, and prednisolone), and bendamustine.
At a median observation time of 38 months, the overall response rate was 91% in the obinutuzumab arm and 88% in the rituximab arm. The complete response rates were 28% and 27%, respectively.
The median progression-free survival was not reached in either arm. The hazard ratio, for obinutuzumab compared to rituximab, was 0.72 (95% CI, 0.56-0.93, P=0.0118).
Safety was evaluated based on all 1385 patients in the study, 86% of whom had previously untreated FL and 14% of whom had marginal zone lymphoma.
Serious adverse events (AEs) occurred in 50% of patients in the obinutuzumab arm and 43% in the rituximab arm. Fatal AEs occurred in 5% and 4%, respectively. Infections and second malignancies were the leading causes of these deaths.
The most common AEs (incidence ≥ 20%) observed at least 2% more patients in the obinutuzumab arm were infusion-related reactions, neutropenia, upper respiratory tract infection, cough, constipation, and diarrhea.
The most common grade 3 to 5 AEs (incidence ≥ 5%) observed more frequently in the obinutuzumab arm were neutropenia, infusion-related reactions, febrile neutropenia, and thrombocytopenia. ![]()
CAR T-cell therapy on fast track with FDA, EMA
A chimeric antigen receptor (CAR) T-cell therapy, bb2121, has received breakthrough therapy designation from the US Food and Drug Administration (FDA) and was granted access to the European Medicines Agency’s (EMA’s) PRIority MEdicines (PRIME) program.
The CAR T-cell therapy is designed to target B-cell maturation antigen in previously treated patients with multiple myeloma.
bb2121 is being developed by bluebird bio, Inc., and Celgene Corporation.
The EMA’s and FDA’s decisions on bb2121 were based on preliminary data from an ongoing phase 1 study, CRB-401 (NCT02658929).
Results from this study were presented at the 2017 ASCO Annual Meeting (abstract 3010).
The study enrolled patients with relapsed and/or refractory multiple myeloma. As of the May 4, 2017 data cut-off, 21 patients had been enrolled.
Patients had a median of 7 prior lines of therapy (range, 3-14). Their previous treatments included lenalidomide and bortezomib (100%), pomalidomide and carfilzomib (91%), daratumumab (71%), and autologous stem cell transplant (100% at least once).
Twenty-nine percent of patients were refractory to bortezomib, lenalidomide, carfilzomib, pomalidomide, and daratumumab.
Patients received a conditioning regimen of cyclophosphamide and fludarabine, followed by an infusion of bb2121 at 1 of 4 doses: 50 x 106, 150 x 106, 450 x 106 and 800 x 106 CAR+ T cells.
All 21 patients were evaluable for safety.
The most common treatment-emergent grade 3-4 adverse events were cytopenias commonly associated with the lymphodepletion regimen, as well as grade 3 events of hyponatremia (n=4), upper respiratory infection (n=2), syncope (n=2), and cytokine release syndrome (CRS, n=2).
In all, 71% of patients (15/21) had CRS, mostly grade 1 and 2. For the 2 patients with grade 3 CRS, it resolved within 24 hours. To manage CRS, 4 patients received tocilizumab, and 1 (with grade 2) received steroids as well.
Eighteen patients were evaluable for efficacy, and the overall response rate was 89% (16/18).
There were 4 complete responses—2 in the 150 x 106 cohort, 1 in the 450 x 106 cohort, and 1 in the 800 x 106 cohort. The complete responder in the 450 x 106 cohort ultimately died of cardiopulmonary arrest that was deemed unrelated to treatment.
Five patients had a partial response—2 in the 450 x 106 cohort and 1 in each of the other cohorts. Seven patients had a very good partial response—5 in the 450 x 106 cohort and 1 each in the 150 x 106 cohort and 800 x 106 cohort.
Updated data from this study are scheduled to be presented at the 2017 ASH Annual Meeting (abstract 740).
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
The designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
About PRIME
The EMA launched its PRIME program to enhance support for the development of medicines that target an unmet medical need.
The program involves enhanced interaction and early dialogue with developers of promising medicines to optimize development plans and speed up evaluation so these medicines can reach patients earlier.
PRIME focuses on medicines that may offer a major therapeutic advantage over existing treatments or benefit patients without treatment options. To be accepted for PRIME, a medicine must have demonstrated the potential to benefit patients with unmet medical needs based on early clinical data. ![]()
A chimeric antigen receptor (CAR) T-cell therapy, bb2121, has received breakthrough therapy designation from the US Food and Drug Administration (FDA) and was granted access to the European Medicines Agency’s (EMA’s) PRIority MEdicines (PRIME) program.
The CAR T-cell therapy is designed to target B-cell maturation antigen in previously treated patients with multiple myeloma.
bb2121 is being developed by bluebird bio, Inc., and Celgene Corporation.
The EMA’s and FDA’s decisions on bb2121 were based on preliminary data from an ongoing phase 1 study, CRB-401 (NCT02658929).
Results from this study were presented at the 2017 ASCO Annual Meeting (abstract 3010).
The study enrolled patients with relapsed and/or refractory multiple myeloma. As of the May 4, 2017 data cut-off, 21 patients had been enrolled.
Patients had a median of 7 prior lines of therapy (range, 3-14). Their previous treatments included lenalidomide and bortezomib (100%), pomalidomide and carfilzomib (91%), daratumumab (71%), and autologous stem cell transplant (100% at least once).
Twenty-nine percent of patients were refractory to bortezomib, lenalidomide, carfilzomib, pomalidomide, and daratumumab.
Patients received a conditioning regimen of cyclophosphamide and fludarabine, followed by an infusion of bb2121 at 1 of 4 doses: 50 x 106, 150 x 106, 450 x 106 and 800 x 106 CAR+ T cells.
All 21 patients were evaluable for safety.
The most common treatment-emergent grade 3-4 adverse events were cytopenias commonly associated with the lymphodepletion regimen, as well as grade 3 events of hyponatremia (n=4), upper respiratory infection (n=2), syncope (n=2), and cytokine release syndrome (CRS, n=2).
In all, 71% of patients (15/21) had CRS, mostly grade 1 and 2. For the 2 patients with grade 3 CRS, it resolved within 24 hours. To manage CRS, 4 patients received tocilizumab, and 1 (with grade 2) received steroids as well.
Eighteen patients were evaluable for efficacy, and the overall response rate was 89% (16/18).
There were 4 complete responses—2 in the 150 x 106 cohort, 1 in the 450 x 106 cohort, and 1 in the 800 x 106 cohort. The complete responder in the 450 x 106 cohort ultimately died of cardiopulmonary arrest that was deemed unrelated to treatment.
Five patients had a partial response—2 in the 450 x 106 cohort and 1 in each of the other cohorts. Seven patients had a very good partial response—5 in the 450 x 106 cohort and 1 each in the 150 x 106 cohort and 800 x 106 cohort.
Updated data from this study are scheduled to be presented at the 2017 ASH Annual Meeting (abstract 740).
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
The designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
About PRIME
The EMA launched its PRIME program to enhance support for the development of medicines that target an unmet medical need.
The program involves enhanced interaction and early dialogue with developers of promising medicines to optimize development plans and speed up evaluation so these medicines can reach patients earlier.
PRIME focuses on medicines that may offer a major therapeutic advantage over existing treatments or benefit patients without treatment options. To be accepted for PRIME, a medicine must have demonstrated the potential to benefit patients with unmet medical needs based on early clinical data. ![]()
A chimeric antigen receptor (CAR) T-cell therapy, bb2121, has received breakthrough therapy designation from the US Food and Drug Administration (FDA) and was granted access to the European Medicines Agency’s (EMA’s) PRIority MEdicines (PRIME) program.
The CAR T-cell therapy is designed to target B-cell maturation antigen in previously treated patients with multiple myeloma.
bb2121 is being developed by bluebird bio, Inc., and Celgene Corporation.
The EMA’s and FDA’s decisions on bb2121 were based on preliminary data from an ongoing phase 1 study, CRB-401 (NCT02658929).
Results from this study were presented at the 2017 ASCO Annual Meeting (abstract 3010).
The study enrolled patients with relapsed and/or refractory multiple myeloma. As of the May 4, 2017 data cut-off, 21 patients had been enrolled.
Patients had a median of 7 prior lines of therapy (range, 3-14). Their previous treatments included lenalidomide and bortezomib (100%), pomalidomide and carfilzomib (91%), daratumumab (71%), and autologous stem cell transplant (100% at least once).
Twenty-nine percent of patients were refractory to bortezomib, lenalidomide, carfilzomib, pomalidomide, and daratumumab.
Patients received a conditioning regimen of cyclophosphamide and fludarabine, followed by an infusion of bb2121 at 1 of 4 doses: 50 x 106, 150 x 106, 450 x 106 and 800 x 106 CAR+ T cells.
All 21 patients were evaluable for safety.
The most common treatment-emergent grade 3-4 adverse events were cytopenias commonly associated with the lymphodepletion regimen, as well as grade 3 events of hyponatremia (n=4), upper respiratory infection (n=2), syncope (n=2), and cytokine release syndrome (CRS, n=2).
In all, 71% of patients (15/21) had CRS, mostly grade 1 and 2. For the 2 patients with grade 3 CRS, it resolved within 24 hours. To manage CRS, 4 patients received tocilizumab, and 1 (with grade 2) received steroids as well.
Eighteen patients were evaluable for efficacy, and the overall response rate was 89% (16/18).
There were 4 complete responses—2 in the 150 x 106 cohort, 1 in the 450 x 106 cohort, and 1 in the 800 x 106 cohort. The complete responder in the 450 x 106 cohort ultimately died of cardiopulmonary arrest that was deemed unrelated to treatment.
Five patients had a partial response—2 in the 450 x 106 cohort and 1 in each of the other cohorts. Seven patients had a very good partial response—5 in the 450 x 106 cohort and 1 each in the 150 x 106 cohort and 800 x 106 cohort.
Updated data from this study are scheduled to be presented at the 2017 ASH Annual Meeting (abstract 740).
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
The designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
About PRIME
The EMA launched its PRIME program to enhance support for the development of medicines that target an unmet medical need.
The program involves enhanced interaction and early dialogue with developers of promising medicines to optimize development plans and speed up evaluation so these medicines can reach patients earlier.
PRIME focuses on medicines that may offer a major therapeutic advantage over existing treatments or benefit patients without treatment options. To be accepted for PRIME, a medicine must have demonstrated the potential to benefit patients with unmet medical needs based on early clinical data. ![]()
Antibiotics after incision and drainage for simple abscesses improves outcomes
Clinical question: Does antibiotic administration after incision and drainage of simple abscesses improve cure rates?
Background: Abscesses are the most common skin and soft-tissue infection, and most patients seek outpatient treatment. Limited data have shown that antibiotic treatment directed at Staphylococcus aureus after incision and drainage is effective, though the efficacy of adjunctive antibiotic therapy versus incision and drainage alone is unclear.
Setting: Multicenter outpatient facilities across the United States.
Synopsis: After successful incision and drainage of a simple abscess, 786 outpatients (505 adults, 281 children) were randomized to receive either clindamycin, trimethoprim-sulfamethoxazole (TMP-SMX), or placebo for 10 days. The rate of clinical cure was 83.1% in the clindamycin group, 81.7% in the TMP-SMX group, and 68.9% in the placebo group. The cure rate was significantly lower in the placebo group, compared with either of the antibiotic groups. The difference in cure rate was not significant between the clindamycin and TMP-SMX groups. Cure rates in culture-positive S. aureus patients were significantly higher in both antibiotic groups, compared with placebo. Rates of adverse events were higher in the clindamycin group (21.9%) than the TMP-SMX group (11.1%) or the placebo group (12.5%), though all adverse events resolved without sequelae.
Bottom line: Antibiotic therapy after incision and drainage for simple abscesses is associated with improved cure rate and decreased recurrence.
Citation: Daum RS, Miller LG, Immergluck L, et al. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017 Jun 29;376(26):2545-55.
Dr. Chung is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
Clinical question: Does antibiotic administration after incision and drainage of simple abscesses improve cure rates?
Background: Abscesses are the most common skin and soft-tissue infection, and most patients seek outpatient treatment. Limited data have shown that antibiotic treatment directed at Staphylococcus aureus after incision and drainage is effective, though the efficacy of adjunctive antibiotic therapy versus incision and drainage alone is unclear.
Setting: Multicenter outpatient facilities across the United States.
Synopsis: After successful incision and drainage of a simple abscess, 786 outpatients (505 adults, 281 children) were randomized to receive either clindamycin, trimethoprim-sulfamethoxazole (TMP-SMX), or placebo for 10 days. The rate of clinical cure was 83.1% in the clindamycin group, 81.7% in the TMP-SMX group, and 68.9% in the placebo group. The cure rate was significantly lower in the placebo group, compared with either of the antibiotic groups. The difference in cure rate was not significant between the clindamycin and TMP-SMX groups. Cure rates in culture-positive S. aureus patients were significantly higher in both antibiotic groups, compared with placebo. Rates of adverse events were higher in the clindamycin group (21.9%) than the TMP-SMX group (11.1%) or the placebo group (12.5%), though all adverse events resolved without sequelae.
Bottom line: Antibiotic therapy after incision and drainage for simple abscesses is associated with improved cure rate and decreased recurrence.
Citation: Daum RS, Miller LG, Immergluck L, et al. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017 Jun 29;376(26):2545-55.
Dr. Chung is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
Clinical question: Does antibiotic administration after incision and drainage of simple abscesses improve cure rates?
Background: Abscesses are the most common skin and soft-tissue infection, and most patients seek outpatient treatment. Limited data have shown that antibiotic treatment directed at Staphylococcus aureus after incision and drainage is effective, though the efficacy of adjunctive antibiotic therapy versus incision and drainage alone is unclear.
Setting: Multicenter outpatient facilities across the United States.
Synopsis: After successful incision and drainage of a simple abscess, 786 outpatients (505 adults, 281 children) were randomized to receive either clindamycin, trimethoprim-sulfamethoxazole (TMP-SMX), or placebo for 10 days. The rate of clinical cure was 83.1% in the clindamycin group, 81.7% in the TMP-SMX group, and 68.9% in the placebo group. The cure rate was significantly lower in the placebo group, compared with either of the antibiotic groups. The difference in cure rate was not significant between the clindamycin and TMP-SMX groups. Cure rates in culture-positive S. aureus patients were significantly higher in both antibiotic groups, compared with placebo. Rates of adverse events were higher in the clindamycin group (21.9%) than the TMP-SMX group (11.1%) or the placebo group (12.5%), though all adverse events resolved without sequelae.
Bottom line: Antibiotic therapy after incision and drainage for simple abscesses is associated with improved cure rate and decreased recurrence.
Citation: Daum RS, Miller LG, Immergluck L, et al. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017 Jun 29;376(26):2545-55.
Dr. Chung is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
From the Editors: How about that!
For all those who say that the surgeon in the trenches doesn’t have a voice in the world of organized surgery, I have a story or two for you.
About 10 or more years ago, I was at a lovely old hotel in Cooperstown, N.Y., listening to a group of elder rural surgery colleagues hold forth about feeling ignored by the American College of Surgeons and how we should all band together to form a new society of rural surgeons. Like most crowds, they were getting pretty worked up. They were pretty sure such a revolution would solve our problems and we would quit being the Rodney Dangerfields of surgery (not getting any respect, as the great comic used to say).
It took a while – a few years – for the rural surgeons and ACS leadership to get to know each other better. We did, however, get there. An Advisory Council was created with the help of some very heavy hitters not often regarded as rural champions, most of whom had roots in the rural world that helped them understand what this group of surgeons wanted and needed.
Not too much removed from this event, many surgeons were feeling isolated in general due to the many challenges of rural practice familiar to so many of us. ACS leadership sensed that surgeons needed to be connected and with considerable effort they formed listservs and found that they were successful to some degree. So, under Dr. David Hoyt’s guidance a better technology was found, resources were committed, and the ACS Communities was launched. A new era in surgeon-to-surgeon and surgeon-to-leadership communication was born. It was as if we had developed talk radio for surgeons. Some stations were loud, others came in rather softly, but all were on the air. The pulse of the Fellows became audible.
Almost simultaneously, dissatisfaction with Maintenance of Certification grew as surgeons in practice began reaching their second and third recertification and the practice of surgery became ever more specialized. Many surgeons believed that nothing would ever change, that the front-line surgeon didn’t have a chance to affect change, and that the big dogs wouldn’t listen. But the College leadership heard the voices of concern and took steps to support a new approach to certification.
So here we are in 2017, coming off a very successful Clinical Congress. The formerly obscure rural surgeon contingent had several panels on the program. Surgeons practicing in towns as small as 3,000 moderated sessions. Some of those sessions were standing room only. The College assisted (and had for a few years) those rural surgeons in organizing a Rural Surgery Dinner, which filled a restaurant full of surgeons delighted to meet in person surgeons from small communities all over the country. They shared their common experiences and planned for a better tomorrow. Later in the week, the 2nd Vice President-Elect was announced. He is a surgeon from Keokuk, IA, and one of the leaders of the rural surgeon movement within the College, Philip Caropreso, MD, FACS. A new Regent was announced: Gary Timmerman, MD, FACS. Dr. Timmerman started his career in Watertown, S.D., and now runs a rural-based surgery training program.
The Clinical Congress program included many topics and issues that Fellows from every branch of surgery had posted about on the ACS Communities. The American Board of Surgery announced sweeping coming changes to help surgeons move on from an MOC system no one was really happy with to one that has great hope of making ongoing certification more than a hoop to jump through, but instead, a real value to the Diplomate. Sitting on the main committee for the American Board of Surgery’s sprint team on certification is a surgeon from Crockett, Texas, Pat Walker, MD, FACS, who practiced surgery “on the ground” in a small community setting for three decades.
Most of us would agree that rural surgeons are facing truly daunting professional headwinds, despite their critical work in serving rural patients. Yet I have been gladdened at the response and respect that the College leadership has given to rural surgeons in recent years. The outreach by the College to support rural surgeons is part of a broader effort to hear every Fellow and make the College more relevant in the life of hard-working surgeons of every type. Changes in how we retain and improve our certification process in some significant ways came out of College efforts to listen and respond to the concerns of Fellows in the College. These and other initiatives by the College leadership show a degree of farsightedness and caring that should be gratifying to all Fellows.
When Lindsay Fox, MD, FACS, a young rural surgeon in private practice with an 8-week-old baby, receives an award for excellence, moderates her panel at the Clinical Congress to a standing-room-only crowd, and then shares her experience through the Communities, I’d say the American College of Surgeons has done an amazing job in a short period of time to make our organization reflect all of us. As Mel Allen, the voice of the Yankees in their heyday, would say, “How about that!”
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
For all those who say that the surgeon in the trenches doesn’t have a voice in the world of organized surgery, I have a story or two for you.
About 10 or more years ago, I was at a lovely old hotel in Cooperstown, N.Y., listening to a group of elder rural surgery colleagues hold forth about feeling ignored by the American College of Surgeons and how we should all band together to form a new society of rural surgeons. Like most crowds, they were getting pretty worked up. They were pretty sure such a revolution would solve our problems and we would quit being the Rodney Dangerfields of surgery (not getting any respect, as the great comic used to say).
It took a while – a few years – for the rural surgeons and ACS leadership to get to know each other better. We did, however, get there. An Advisory Council was created with the help of some very heavy hitters not often regarded as rural champions, most of whom had roots in the rural world that helped them understand what this group of surgeons wanted and needed.
Not too much removed from this event, many surgeons were feeling isolated in general due to the many challenges of rural practice familiar to so many of us. ACS leadership sensed that surgeons needed to be connected and with considerable effort they formed listservs and found that they were successful to some degree. So, under Dr. David Hoyt’s guidance a better technology was found, resources were committed, and the ACS Communities was launched. A new era in surgeon-to-surgeon and surgeon-to-leadership communication was born. It was as if we had developed talk radio for surgeons. Some stations were loud, others came in rather softly, but all were on the air. The pulse of the Fellows became audible.
Almost simultaneously, dissatisfaction with Maintenance of Certification grew as surgeons in practice began reaching their second and third recertification and the practice of surgery became ever more specialized. Many surgeons believed that nothing would ever change, that the front-line surgeon didn’t have a chance to affect change, and that the big dogs wouldn’t listen. But the College leadership heard the voices of concern and took steps to support a new approach to certification.
So here we are in 2017, coming off a very successful Clinical Congress. The formerly obscure rural surgeon contingent had several panels on the program. Surgeons practicing in towns as small as 3,000 moderated sessions. Some of those sessions were standing room only. The College assisted (and had for a few years) those rural surgeons in organizing a Rural Surgery Dinner, which filled a restaurant full of surgeons delighted to meet in person surgeons from small communities all over the country. They shared their common experiences and planned for a better tomorrow. Later in the week, the 2nd Vice President-Elect was announced. He is a surgeon from Keokuk, IA, and one of the leaders of the rural surgeon movement within the College, Philip Caropreso, MD, FACS. A new Regent was announced: Gary Timmerman, MD, FACS. Dr. Timmerman started his career in Watertown, S.D., and now runs a rural-based surgery training program.
The Clinical Congress program included many topics and issues that Fellows from every branch of surgery had posted about on the ACS Communities. The American Board of Surgery announced sweeping coming changes to help surgeons move on from an MOC system no one was really happy with to one that has great hope of making ongoing certification more than a hoop to jump through, but instead, a real value to the Diplomate. Sitting on the main committee for the American Board of Surgery’s sprint team on certification is a surgeon from Crockett, Texas, Pat Walker, MD, FACS, who practiced surgery “on the ground” in a small community setting for three decades.
Most of us would agree that rural surgeons are facing truly daunting professional headwinds, despite their critical work in serving rural patients. Yet I have been gladdened at the response and respect that the College leadership has given to rural surgeons in recent years. The outreach by the College to support rural surgeons is part of a broader effort to hear every Fellow and make the College more relevant in the life of hard-working surgeons of every type. Changes in how we retain and improve our certification process in some significant ways came out of College efforts to listen and respond to the concerns of Fellows in the College. These and other initiatives by the College leadership show a degree of farsightedness and caring that should be gratifying to all Fellows.
When Lindsay Fox, MD, FACS, a young rural surgeon in private practice with an 8-week-old baby, receives an award for excellence, moderates her panel at the Clinical Congress to a standing-room-only crowd, and then shares her experience through the Communities, I’d say the American College of Surgeons has done an amazing job in a short period of time to make our organization reflect all of us. As Mel Allen, the voice of the Yankees in their heyday, would say, “How about that!”
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
For all those who say that the surgeon in the trenches doesn’t have a voice in the world of organized surgery, I have a story or two for you.
About 10 or more years ago, I was at a lovely old hotel in Cooperstown, N.Y., listening to a group of elder rural surgery colleagues hold forth about feeling ignored by the American College of Surgeons and how we should all band together to form a new society of rural surgeons. Like most crowds, they were getting pretty worked up. They were pretty sure such a revolution would solve our problems and we would quit being the Rodney Dangerfields of surgery (not getting any respect, as the great comic used to say).
It took a while – a few years – for the rural surgeons and ACS leadership to get to know each other better. We did, however, get there. An Advisory Council was created with the help of some very heavy hitters not often regarded as rural champions, most of whom had roots in the rural world that helped them understand what this group of surgeons wanted and needed.
Not too much removed from this event, many surgeons were feeling isolated in general due to the many challenges of rural practice familiar to so many of us. ACS leadership sensed that surgeons needed to be connected and with considerable effort they formed listservs and found that they were successful to some degree. So, under Dr. David Hoyt’s guidance a better technology was found, resources were committed, and the ACS Communities was launched. A new era in surgeon-to-surgeon and surgeon-to-leadership communication was born. It was as if we had developed talk radio for surgeons. Some stations were loud, others came in rather softly, but all were on the air. The pulse of the Fellows became audible.
Almost simultaneously, dissatisfaction with Maintenance of Certification grew as surgeons in practice began reaching their second and third recertification and the practice of surgery became ever more specialized. Many surgeons believed that nothing would ever change, that the front-line surgeon didn’t have a chance to affect change, and that the big dogs wouldn’t listen. But the College leadership heard the voices of concern and took steps to support a new approach to certification.
So here we are in 2017, coming off a very successful Clinical Congress. The formerly obscure rural surgeon contingent had several panels on the program. Surgeons practicing in towns as small as 3,000 moderated sessions. Some of those sessions were standing room only. The College assisted (and had for a few years) those rural surgeons in organizing a Rural Surgery Dinner, which filled a restaurant full of surgeons delighted to meet in person surgeons from small communities all over the country. They shared their common experiences and planned for a better tomorrow. Later in the week, the 2nd Vice President-Elect was announced. He is a surgeon from Keokuk, IA, and one of the leaders of the rural surgeon movement within the College, Philip Caropreso, MD, FACS. A new Regent was announced: Gary Timmerman, MD, FACS. Dr. Timmerman started his career in Watertown, S.D., and now runs a rural-based surgery training program.
The Clinical Congress program included many topics and issues that Fellows from every branch of surgery had posted about on the ACS Communities. The American Board of Surgery announced sweeping coming changes to help surgeons move on from an MOC system no one was really happy with to one that has great hope of making ongoing certification more than a hoop to jump through, but instead, a real value to the Diplomate. Sitting on the main committee for the American Board of Surgery’s sprint team on certification is a surgeon from Crockett, Texas, Pat Walker, MD, FACS, who practiced surgery “on the ground” in a small community setting for three decades.
Most of us would agree that rural surgeons are facing truly daunting professional headwinds, despite their critical work in serving rural patients. Yet I have been gladdened at the response and respect that the College leadership has given to rural surgeons in recent years. The outreach by the College to support rural surgeons is part of a broader effort to hear every Fellow and make the College more relevant in the life of hard-working surgeons of every type. Changes in how we retain and improve our certification process in some significant ways came out of College efforts to listen and respond to the concerns of Fellows in the College. These and other initiatives by the College leadership show a degree of farsightedness and caring that should be gratifying to all Fellows.
When Lindsay Fox, MD, FACS, a young rural surgeon in private practice with an 8-week-old baby, receives an award for excellence, moderates her panel at the Clinical Congress to a standing-room-only crowd, and then shares her experience through the Communities, I’d say the American College of Surgeons has done an amazing job in a short period of time to make our organization reflect all of us. As Mel Allen, the voice of the Yankees in their heyday, would say, “How about that!”
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
Benralizumab approved for eosinophilic asthma
This is the only respiratory biologic to provide fast and “near-complete depletion of eosinophils within 24 hours,” according to the statement from AstraZeneca.
Similar adverse events were seen in patients who took benralizumab and the placebo. AstraZeneca will market benralizumab under the trade name Fasenra.
“This is an important day for severe, eosinophilic asthma patients who have had limited treatment options for far too long,” said Eugene Bleecker, MD, professor and codirector of genetics, genomics, and precision medicine at the University of Arizona in Tucson, in the statement.
This is the only respiratory biologic to provide fast and “near-complete depletion of eosinophils within 24 hours,” according to the statement from AstraZeneca.
Similar adverse events were seen in patients who took benralizumab and the placebo. AstraZeneca will market benralizumab under the trade name Fasenra.
“This is an important day for severe, eosinophilic asthma patients who have had limited treatment options for far too long,” said Eugene Bleecker, MD, professor and codirector of genetics, genomics, and precision medicine at the University of Arizona in Tucson, in the statement.
This is the only respiratory biologic to provide fast and “near-complete depletion of eosinophils within 24 hours,” according to the statement from AstraZeneca.
Similar adverse events were seen in patients who took benralizumab and the placebo. AstraZeneca will market benralizumab under the trade name Fasenra.
“This is an important day for severe, eosinophilic asthma patients who have had limited treatment options for far too long,” said Eugene Bleecker, MD, professor and codirector of genetics, genomics, and precision medicine at the University of Arizona in Tucson, in the statement.
FDA addresses cell-based regenerative medicine in comprehensive new policy
The Food and Drug Administration has announced a new policy that addresses the rapid growth and development of regenerative medicine products, which include novel cellular therapies, with the aim of ensuring their safety and effectiveness.
“The framework – outlined in a suite of four guidance documents – builds upon the FDA’s existing risk-based regulatory approach to more clearly describe what products are regulated as drugs, devices, and/or biological products,” the FDA announced in a statement released on Nov. 16.
He added: “This is no longer the stuff of science fiction. This is the practical promise of modern applications of regenerative medicine.” But, while advances have benefited many patients, he referred to a small number of “unscrupulous actors” that have provided treatments that have harmed patients, which is why stricter FDA enforcement is needed.
Clarification of the existing regulations will “promote responsible and flexible regulation that leverages science to advance public health,” Dr. Gottlieb said during a media briefing held by the FDA to discuss the new framework.
During the briefing, in response to a question concerning adipose tissue injections and their associated risks, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, explained that the guidance documents will clearly delineate when adipose tissue will be classified as a structural tissue and a stem cell product. If a provider is purveying dangerous products, the FDA can “when necessary, undertake seizures, ask for injunctions, and in some cases when it has been determined that certain violations have occurred even criminal actions can be taken,” he said.
Earlier this year, a report of three patients who had severe visual loss after treatment with intravitreal injections of autologous adipose tissue for age-related macular degeneration was published (N Engl J Med. 2017;376:1047-53).
The new framework is composed of two final guidance documents and two draft guidance documents. The first final guidance document provides details about regulations concerning cell and tissue-based products and when those products are subject to regulation in surgical procedures. The second final guidance document elaborates on the definition of “minimal manipulation” and “homologous use” with the hopes of clarifying what products are subject to regulation. These documents will also explain how the FDA will provide a framework for premarket authorization for cell-based regenerative products.
One draft guidance outlines the FDA’s plan to simplify and expedite the application of the regulatory requirements for devices used in relation to regenerative medicine advanced therapies; the second draft guidance outlines the expedited programs that may be available to sponsors of regenerative therapies.
The Food and Drug Administration has announced a new policy that addresses the rapid growth and development of regenerative medicine products, which include novel cellular therapies, with the aim of ensuring their safety and effectiveness.
“The framework – outlined in a suite of four guidance documents – builds upon the FDA’s existing risk-based regulatory approach to more clearly describe what products are regulated as drugs, devices, and/or biological products,” the FDA announced in a statement released on Nov. 16.
He added: “This is no longer the stuff of science fiction. This is the practical promise of modern applications of regenerative medicine.” But, while advances have benefited many patients, he referred to a small number of “unscrupulous actors” that have provided treatments that have harmed patients, which is why stricter FDA enforcement is needed.
Clarification of the existing regulations will “promote responsible and flexible regulation that leverages science to advance public health,” Dr. Gottlieb said during a media briefing held by the FDA to discuss the new framework.
During the briefing, in response to a question concerning adipose tissue injections and their associated risks, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, explained that the guidance documents will clearly delineate when adipose tissue will be classified as a structural tissue and a stem cell product. If a provider is purveying dangerous products, the FDA can “when necessary, undertake seizures, ask for injunctions, and in some cases when it has been determined that certain violations have occurred even criminal actions can be taken,” he said.
Earlier this year, a report of three patients who had severe visual loss after treatment with intravitreal injections of autologous adipose tissue for age-related macular degeneration was published (N Engl J Med. 2017;376:1047-53).
The new framework is composed of two final guidance documents and two draft guidance documents. The first final guidance document provides details about regulations concerning cell and tissue-based products and when those products are subject to regulation in surgical procedures. The second final guidance document elaborates on the definition of “minimal manipulation” and “homologous use” with the hopes of clarifying what products are subject to regulation. These documents will also explain how the FDA will provide a framework for premarket authorization for cell-based regenerative products.
One draft guidance outlines the FDA’s plan to simplify and expedite the application of the regulatory requirements for devices used in relation to regenerative medicine advanced therapies; the second draft guidance outlines the expedited programs that may be available to sponsors of regenerative therapies.
The Food and Drug Administration has announced a new policy that addresses the rapid growth and development of regenerative medicine products, which include novel cellular therapies, with the aim of ensuring their safety and effectiveness.
“The framework – outlined in a suite of four guidance documents – builds upon the FDA’s existing risk-based regulatory approach to more clearly describe what products are regulated as drugs, devices, and/or biological products,” the FDA announced in a statement released on Nov. 16.
He added: “This is no longer the stuff of science fiction. This is the practical promise of modern applications of regenerative medicine.” But, while advances have benefited many patients, he referred to a small number of “unscrupulous actors” that have provided treatments that have harmed patients, which is why stricter FDA enforcement is needed.
Clarification of the existing regulations will “promote responsible and flexible regulation that leverages science to advance public health,” Dr. Gottlieb said during a media briefing held by the FDA to discuss the new framework.
During the briefing, in response to a question concerning adipose tissue injections and their associated risks, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, explained that the guidance documents will clearly delineate when adipose tissue will be classified as a structural tissue and a stem cell product. If a provider is purveying dangerous products, the FDA can “when necessary, undertake seizures, ask for injunctions, and in some cases when it has been determined that certain violations have occurred even criminal actions can be taken,” he said.
Earlier this year, a report of three patients who had severe visual loss after treatment with intravitreal injections of autologous adipose tissue for age-related macular degeneration was published (N Engl J Med. 2017;376:1047-53).
The new framework is composed of two final guidance documents and two draft guidance documents. The first final guidance document provides details about regulations concerning cell and tissue-based products and when those products are subject to regulation in surgical procedures. The second final guidance document elaborates on the definition of “minimal manipulation” and “homologous use” with the hopes of clarifying what products are subject to regulation. These documents will also explain how the FDA will provide a framework for premarket authorization for cell-based regenerative products.
One draft guidance outlines the FDA’s plan to simplify and expedite the application of the regulatory requirements for devices used in relation to regenerative medicine advanced therapies; the second draft guidance outlines the expedited programs that may be available to sponsors of regenerative therapies.
FROM an FDA MEDIA BRIEFING
Career (of your dreams) advice for young MIGS surgeons
From the 46th AAGL Global Congress on MIGS
From the 46th AAGL Global Congress on MIGS
From the 46th AAGL Global Congress on MIGS
AGA launches new registry to track patient outcomes
The AGA Center for GI Innovation and Technology is excited to announce a new clinical research registry to track and evaluate patient outcomes after trans-oral endoscopic suturing procedures.
The Prospective Registry for Trans-Oral Suturing Applications (“Endoscopic Suturing Registry”) will collect real-world data related to the safety and effectiveness of procedures done with Apollo Endosurgery’s OverStitch™ Endoscopic Suturing System. Jennifer Maranki, MD, director of endoscopy, Penn State Milton S. Hershey School of Medicine, and Brian Dunkin, MD, head of endoscopic surgery and medical director, Houston Methodist Institute for Technology, Innovation and Education, will serve as principal investigators for the Endoscopic Suturing Registry. The Registry will begin collecting patient data in early 2018.
We asked Michael Kochman, MD, AGAF, past chair of the AGA Center for GI Innovation and Technology and director of the Center for Endoscopic Innovation, Research and Training at the University of Pennsylvania, to weigh in on the value of this new registry.
“Flexible endoscopic suturing is an important tool for the treatment of a number of GI disorders. As these procedures become more routine in GI and surgery practices across the country, the real-world data AGA will collect through the Endoscopic Suturing Registry will guide all stakeholders in making informed decisions around the continued adoption of these procedures in clinical practice.”
Learn more about AGA’s registry initiative at www.gastro.org/patient-care/registries-studies.
The AGA Center for GI Innovation and Technology is excited to announce a new clinical research registry to track and evaluate patient outcomes after trans-oral endoscopic suturing procedures.
The Prospective Registry for Trans-Oral Suturing Applications (“Endoscopic Suturing Registry”) will collect real-world data related to the safety and effectiveness of procedures done with Apollo Endosurgery’s OverStitch™ Endoscopic Suturing System. Jennifer Maranki, MD, director of endoscopy, Penn State Milton S. Hershey School of Medicine, and Brian Dunkin, MD, head of endoscopic surgery and medical director, Houston Methodist Institute for Technology, Innovation and Education, will serve as principal investigators for the Endoscopic Suturing Registry. The Registry will begin collecting patient data in early 2018.
We asked Michael Kochman, MD, AGAF, past chair of the AGA Center for GI Innovation and Technology and director of the Center for Endoscopic Innovation, Research and Training at the University of Pennsylvania, to weigh in on the value of this new registry.
“Flexible endoscopic suturing is an important tool for the treatment of a number of GI disorders. As these procedures become more routine in GI and surgery practices across the country, the real-world data AGA will collect through the Endoscopic Suturing Registry will guide all stakeholders in making informed decisions around the continued adoption of these procedures in clinical practice.”
Learn more about AGA’s registry initiative at www.gastro.org/patient-care/registries-studies.
The AGA Center for GI Innovation and Technology is excited to announce a new clinical research registry to track and evaluate patient outcomes after trans-oral endoscopic suturing procedures.
The Prospective Registry for Trans-Oral Suturing Applications (“Endoscopic Suturing Registry”) will collect real-world data related to the safety and effectiveness of procedures done with Apollo Endosurgery’s OverStitch™ Endoscopic Suturing System. Jennifer Maranki, MD, director of endoscopy, Penn State Milton S. Hershey School of Medicine, and Brian Dunkin, MD, head of endoscopic surgery and medical director, Houston Methodist Institute for Technology, Innovation and Education, will serve as principal investigators for the Endoscopic Suturing Registry. The Registry will begin collecting patient data in early 2018.
We asked Michael Kochman, MD, AGAF, past chair of the AGA Center for GI Innovation and Technology and director of the Center for Endoscopic Innovation, Research and Training at the University of Pennsylvania, to weigh in on the value of this new registry.
“Flexible endoscopic suturing is an important tool for the treatment of a number of GI disorders. As these procedures become more routine in GI and surgery practices across the country, the real-world data AGA will collect through the Endoscopic Suturing Registry will guide all stakeholders in making informed decisions around the continued adoption of these procedures in clinical practice.”
Learn more about AGA’s registry initiative at www.gastro.org/patient-care/registries-studies.