User login
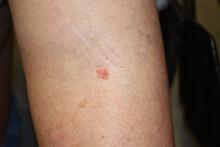
Squamous cell carcinoma linked to 25% increase in all-cause mortality
FROM JAAD
Squamous cell carcinomas (SCC), but not basal cell carcinomas (BCC), were associated with a risk of death from any cause that was 25% higher than that seen in the general population, based on a systematic literature review and meta-analysis published in the Journal of American Academy of Dermatology (2017. doi: 10.1016/j.jaad.2017.11.026).
“Because these tumors often occur in the same patients and are both often caused by exposure to ultraviolet radiation, patients with BCC and SCC are often grouped together,” Mackenzie R. Wehner, MD, of the University of Pennsylvania, Philadelphia, and co-authors wrote. “Our data contributes to the argument that the carcinogenesis of these tumors and long-term outcomes for patients with these tumors may be distinct.”
Patients with SCC “may need additional education and age-appropriate screening to prevent deaths from major diseases,” the authors concluded.
Dr. Wehner and colleagues systematically searched the medical literature and found four studies encompassing a total of 175,849 patients with SCC and 464,230 patients with BCC.
Relative to the general population, mortality for those with an SCC was 1.25 (95% CI, 1.17-1.32). At 0.92 (95% CI 0.83-1.02), there was no significant difference in mortality for patients with a BCC.
Collectively and individually, the studies found a statistically significant increased relative mortality for having SCC.
There are clear distinctions between BCC and SCC with regard to histology, pathophysiology, survival, and other parameters, the study authors said. “While many patients get both BCC and SCC, future research should take into account that these cancers may have different long-term risks and outcomes.”
FROM JAAD
Squamous cell carcinomas (SCC), but not basal cell carcinomas (BCC), were associated with a risk of death from any cause that was 25% higher than that seen in the general population, based on a systematic literature review and meta-analysis published in the Journal of American Academy of Dermatology (2017. doi: 10.1016/j.jaad.2017.11.026).
“Because these tumors often occur in the same patients and are both often caused by exposure to ultraviolet radiation, patients with BCC and SCC are often grouped together,” Mackenzie R. Wehner, MD, of the University of Pennsylvania, Philadelphia, and co-authors wrote. “Our data contributes to the argument that the carcinogenesis of these tumors and long-term outcomes for patients with these tumors may be distinct.”
Patients with SCC “may need additional education and age-appropriate screening to prevent deaths from major diseases,” the authors concluded.
Dr. Wehner and colleagues systematically searched the medical literature and found four studies encompassing a total of 175,849 patients with SCC and 464,230 patients with BCC.
Relative to the general population, mortality for those with an SCC was 1.25 (95% CI, 1.17-1.32). At 0.92 (95% CI 0.83-1.02), there was no significant difference in mortality for patients with a BCC.
Collectively and individually, the studies found a statistically significant increased relative mortality for having SCC.
There are clear distinctions between BCC and SCC with regard to histology, pathophysiology, survival, and other parameters, the study authors said. “While many patients get both BCC and SCC, future research should take into account that these cancers may have different long-term risks and outcomes.”
FROM JAAD
Squamous cell carcinomas (SCC), but not basal cell carcinomas (BCC), were associated with a risk of death from any cause that was 25% higher than that seen in the general population, based on a systematic literature review and meta-analysis published in the Journal of American Academy of Dermatology (2017. doi: 10.1016/j.jaad.2017.11.026).
“Because these tumors often occur in the same patients and are both often caused by exposure to ultraviolet radiation, patients with BCC and SCC are often grouped together,” Mackenzie R. Wehner, MD, of the University of Pennsylvania, Philadelphia, and co-authors wrote. “Our data contributes to the argument that the carcinogenesis of these tumors and long-term outcomes for patients with these tumors may be distinct.”
Patients with SCC “may need additional education and age-appropriate screening to prevent deaths from major diseases,” the authors concluded.
Dr. Wehner and colleagues systematically searched the medical literature and found four studies encompassing a total of 175,849 patients with SCC and 464,230 patients with BCC.
Relative to the general population, mortality for those with an SCC was 1.25 (95% CI, 1.17-1.32). At 0.92 (95% CI 0.83-1.02), there was no significant difference in mortality for patients with a BCC.
Collectively and individually, the studies found a statistically significant increased relative mortality for having SCC.
There are clear distinctions between BCC and SCC with regard to histology, pathophysiology, survival, and other parameters, the study authors said. “While many patients get both BCC and SCC, future research should take into account that these cancers may have different long-term risks and outcomes.”
Studies need to address best follow-on therapy to ibrutinib in CLL
Reporting AT LYMPHOMA & MYELOMA 2017
NEW YORK – Clinical trials are needed to determine the best follow-on therapies when patients discontinue the ibrutinib due to adverse events or disease progression, according to a leading expert on chronic lymphocytic leukemia (CLL).
Anthony Mato, MD, MSCE, from the Abramson Cancer Center at the University of Pennsylvania in Philadelphia, discussed how real-world experience with the use of ibrutinib (Imbruvica) can fill the gaps in knowledge left by clinical trials and point to the need for further study.
“Regulatory bodies around the world are more and more interested in what’s going on in the clinic, and there is a question about whether or not the experiences for patients that we take care of might actually answer some important questions that aren’t easily answered in the context of clinical research,” he said at the annual Lymphoma & Myeloma International Congress on Hematologic Malignancies here.
“Are the experiences in practice with novel agents similar to experiences from clinical trials? I think that’s very important,” he added.
Other important questions that real-world experience may help to answer include whether it’s possible to refine adverse event profiles and reasons for ibrutinib discontinuation, what therapies should be prescribed after ibrutinib, and what is the optimal sequencing of therapies for CLL.
For example, in the RESONATE-2 trial, an open-label, international phase 3 study comparing ibrutinib with chlorambucil in previously untreated patients 65 and older, ibrutinib was found to be superior to chlorambucil in terms of progression-free survival (PFS), overall survival (OS), response rate, and improvements in hematologic variables.
However, this trial excluded patients with the deleterious chromosome 17p deletion (del17p) and included only patients 65 and older, a population that does not necessarily reflect clinical experience.
To get a better sense of how ibrutinib is used to treat CLL in the front-line setting Dr. Mato and colleagues conducted a retrospective cohort study of 391 patients treated in 19 US and international academic and community centers.
The median age of the sample was 68 years, but 41% of the patients were younger than 65. In all, 62% were male, and 80% had Rai stage 2 or greater disease. Genetic analyses showed that 30% of the patients were positive for del17p, and 17% had both del17p and the 11q deletion (del11q). Mutations in TP53 were seen in 20% of patients, 23% had a complex karyotype, and 67% had an unmutated immuglobulin heavy chain variable region (IGHV). Only 57 patients (14.5%) were classified as genetically low risk.
Additionally, only 79 of the 391 patients had complete data for CLL International Prognostic Index (CLL-IPI) scoring, “which goes, I think, to show how often this is actually being tested and utilized in clinical practice,” Dr. Mato said.
Off-label use of ibrutinib in combination therapy was given to 16% of patients, most commonly with an anti-CD20 inhibitor such as rituximab.
In all, 17% of patients required permanent dose reductions; and 42% had a dose interruption, with a median hiatus of 12 days.
Grade 3 or 4 adverse events were uncommon, but more than 20% of patients experienced arthralgias or myalgias of any kind, about 19% reported fatigue, 18% had dermatologic toxicities, 18% reported bruising, 17% had diarrhea or colitis and 15% had infections.
The toxicities seen in RESONATE-2 were somewhat similar, but generally occurred in higher frequencies in the trial than in real-world practice.
Dr. Mato and colleagues found that at a median of 12 months of follow-up, 24% of patients had discontinued ibrutinib. In contrast, in RESONATE-2, after 18 months of follow-up, 13% of patients had discontinued the drug.
The most common reasons for discontinuation in clinical practice were for toxicities (59.5% of 94 discontinuations) including atrial fibrillation in 20% of the patients who discontinued, arthralgias/myalgias and skin toxicities in 14.5% each, and bleeding in 9.1%.
Other reasons for discontinuation included Richter’s transformation in 9.6%, doctor or patient preference in 7.4%, and deaths that were not secondary to CLL progression in 3.2%.
“We also tried to get a sense of whether or not cost was a factor for patients, and in this series and the relapsed refractory setting, 1% or less of patients discontinued due to financial issues,” Dr. Mato said.
Outcomes in the real word were quite good, he noted, with an overall response rate (ORR) of 81.7%, which included 17.4% complete responses (CR), Neither median PFS nor OS have been reached and the respective PFS and OS at 12 months were 92% and 95%. The respective PFS and OS rates for patients with del17p were 87% and 89%. An analysis of predictors of survival showed that only the presence of del17p was associated with inferior PFS (odds ratio 1.91, P = .035)
Dr. Mato noted that there was no clear standard treatment approach for patients who discontinued ibrutinib or for whom ibrutinib did not work. The top three second-line approaches used included an anti-CD20 agent combined with chlorambucil, venetoclax (Venclexta), or a different kinase inhibitor. Chemoimmunotherapy with either fludarabine, cyclophosphamide, and rituximab or bendamustine and rituximab was given to only 5 patients as a second line therapy.
Dr. Mato disclosed serving as a consultant for AbbVie, AstraZeneca, Janssen/Pharmacyclics, and TG Therapeutics.
Reporting AT LYMPHOMA & MYELOMA 2017
NEW YORK – Clinical trials are needed to determine the best follow-on therapies when patients discontinue the ibrutinib due to adverse events or disease progression, according to a leading expert on chronic lymphocytic leukemia (CLL).
Anthony Mato, MD, MSCE, from the Abramson Cancer Center at the University of Pennsylvania in Philadelphia, discussed how real-world experience with the use of ibrutinib (Imbruvica) can fill the gaps in knowledge left by clinical trials and point to the need for further study.
“Regulatory bodies around the world are more and more interested in what’s going on in the clinic, and there is a question about whether or not the experiences for patients that we take care of might actually answer some important questions that aren’t easily answered in the context of clinical research,” he said at the annual Lymphoma & Myeloma International Congress on Hematologic Malignancies here.
“Are the experiences in practice with novel agents similar to experiences from clinical trials? I think that’s very important,” he added.
Other important questions that real-world experience may help to answer include whether it’s possible to refine adverse event profiles and reasons for ibrutinib discontinuation, what therapies should be prescribed after ibrutinib, and what is the optimal sequencing of therapies for CLL.
For example, in the RESONATE-2 trial, an open-label, international phase 3 study comparing ibrutinib with chlorambucil in previously untreated patients 65 and older, ibrutinib was found to be superior to chlorambucil in terms of progression-free survival (PFS), overall survival (OS), response rate, and improvements in hematologic variables.
However, this trial excluded patients with the deleterious chromosome 17p deletion (del17p) and included only patients 65 and older, a population that does not necessarily reflect clinical experience.
To get a better sense of how ibrutinib is used to treat CLL in the front-line setting Dr. Mato and colleagues conducted a retrospective cohort study of 391 patients treated in 19 US and international academic and community centers.
The median age of the sample was 68 years, but 41% of the patients were younger than 65. In all, 62% were male, and 80% had Rai stage 2 or greater disease. Genetic analyses showed that 30% of the patients were positive for del17p, and 17% had both del17p and the 11q deletion (del11q). Mutations in TP53 were seen in 20% of patients, 23% had a complex karyotype, and 67% had an unmutated immuglobulin heavy chain variable region (IGHV). Only 57 patients (14.5%) were classified as genetically low risk.
Additionally, only 79 of the 391 patients had complete data for CLL International Prognostic Index (CLL-IPI) scoring, “which goes, I think, to show how often this is actually being tested and utilized in clinical practice,” Dr. Mato said.
Off-label use of ibrutinib in combination therapy was given to 16% of patients, most commonly with an anti-CD20 inhibitor such as rituximab.
In all, 17% of patients required permanent dose reductions; and 42% had a dose interruption, with a median hiatus of 12 days.
Grade 3 or 4 adverse events were uncommon, but more than 20% of patients experienced arthralgias or myalgias of any kind, about 19% reported fatigue, 18% had dermatologic toxicities, 18% reported bruising, 17% had diarrhea or colitis and 15% had infections.
The toxicities seen in RESONATE-2 were somewhat similar, but generally occurred in higher frequencies in the trial than in real-world practice.
Dr. Mato and colleagues found that at a median of 12 months of follow-up, 24% of patients had discontinued ibrutinib. In contrast, in RESONATE-2, after 18 months of follow-up, 13% of patients had discontinued the drug.
The most common reasons for discontinuation in clinical practice were for toxicities (59.5% of 94 discontinuations) including atrial fibrillation in 20% of the patients who discontinued, arthralgias/myalgias and skin toxicities in 14.5% each, and bleeding in 9.1%.
Other reasons for discontinuation included Richter’s transformation in 9.6%, doctor or patient preference in 7.4%, and deaths that were not secondary to CLL progression in 3.2%.
“We also tried to get a sense of whether or not cost was a factor for patients, and in this series and the relapsed refractory setting, 1% or less of patients discontinued due to financial issues,” Dr. Mato said.
Outcomes in the real word were quite good, he noted, with an overall response rate (ORR) of 81.7%, which included 17.4% complete responses (CR), Neither median PFS nor OS have been reached and the respective PFS and OS at 12 months were 92% and 95%. The respective PFS and OS rates for patients with del17p were 87% and 89%. An analysis of predictors of survival showed that only the presence of del17p was associated with inferior PFS (odds ratio 1.91, P = .035)
Dr. Mato noted that there was no clear standard treatment approach for patients who discontinued ibrutinib or for whom ibrutinib did not work. The top three second-line approaches used included an anti-CD20 agent combined with chlorambucil, venetoclax (Venclexta), or a different kinase inhibitor. Chemoimmunotherapy with either fludarabine, cyclophosphamide, and rituximab or bendamustine and rituximab was given to only 5 patients as a second line therapy.
Dr. Mato disclosed serving as a consultant for AbbVie, AstraZeneca, Janssen/Pharmacyclics, and TG Therapeutics.
Reporting AT LYMPHOMA & MYELOMA 2017
NEW YORK – Clinical trials are needed to determine the best follow-on therapies when patients discontinue the ibrutinib due to adverse events or disease progression, according to a leading expert on chronic lymphocytic leukemia (CLL).
Anthony Mato, MD, MSCE, from the Abramson Cancer Center at the University of Pennsylvania in Philadelphia, discussed how real-world experience with the use of ibrutinib (Imbruvica) can fill the gaps in knowledge left by clinical trials and point to the need for further study.
“Regulatory bodies around the world are more and more interested in what’s going on in the clinic, and there is a question about whether or not the experiences for patients that we take care of might actually answer some important questions that aren’t easily answered in the context of clinical research,” he said at the annual Lymphoma & Myeloma International Congress on Hematologic Malignancies here.
“Are the experiences in practice with novel agents similar to experiences from clinical trials? I think that’s very important,” he added.
Other important questions that real-world experience may help to answer include whether it’s possible to refine adverse event profiles and reasons for ibrutinib discontinuation, what therapies should be prescribed after ibrutinib, and what is the optimal sequencing of therapies for CLL.
For example, in the RESONATE-2 trial, an open-label, international phase 3 study comparing ibrutinib with chlorambucil in previously untreated patients 65 and older, ibrutinib was found to be superior to chlorambucil in terms of progression-free survival (PFS), overall survival (OS), response rate, and improvements in hematologic variables.
However, this trial excluded patients with the deleterious chromosome 17p deletion (del17p) and included only patients 65 and older, a population that does not necessarily reflect clinical experience.
To get a better sense of how ibrutinib is used to treat CLL in the front-line setting Dr. Mato and colleagues conducted a retrospective cohort study of 391 patients treated in 19 US and international academic and community centers.
The median age of the sample was 68 years, but 41% of the patients were younger than 65. In all, 62% were male, and 80% had Rai stage 2 or greater disease. Genetic analyses showed that 30% of the patients were positive for del17p, and 17% had both del17p and the 11q deletion (del11q). Mutations in TP53 were seen in 20% of patients, 23% had a complex karyotype, and 67% had an unmutated immuglobulin heavy chain variable region (IGHV). Only 57 patients (14.5%) were classified as genetically low risk.
Additionally, only 79 of the 391 patients had complete data for CLL International Prognostic Index (CLL-IPI) scoring, “which goes, I think, to show how often this is actually being tested and utilized in clinical practice,” Dr. Mato said.
Off-label use of ibrutinib in combination therapy was given to 16% of patients, most commonly with an anti-CD20 inhibitor such as rituximab.
In all, 17% of patients required permanent dose reductions; and 42% had a dose interruption, with a median hiatus of 12 days.
Grade 3 or 4 adverse events were uncommon, but more than 20% of patients experienced arthralgias or myalgias of any kind, about 19% reported fatigue, 18% had dermatologic toxicities, 18% reported bruising, 17% had diarrhea or colitis and 15% had infections.
The toxicities seen in RESONATE-2 were somewhat similar, but generally occurred in higher frequencies in the trial than in real-world practice.
Dr. Mato and colleagues found that at a median of 12 months of follow-up, 24% of patients had discontinued ibrutinib. In contrast, in RESONATE-2, after 18 months of follow-up, 13% of patients had discontinued the drug.
The most common reasons for discontinuation in clinical practice were for toxicities (59.5% of 94 discontinuations) including atrial fibrillation in 20% of the patients who discontinued, arthralgias/myalgias and skin toxicities in 14.5% each, and bleeding in 9.1%.
Other reasons for discontinuation included Richter’s transformation in 9.6%, doctor or patient preference in 7.4%, and deaths that were not secondary to CLL progression in 3.2%.
“We also tried to get a sense of whether or not cost was a factor for patients, and in this series and the relapsed refractory setting, 1% or less of patients discontinued due to financial issues,” Dr. Mato said.
Outcomes in the real word were quite good, he noted, with an overall response rate (ORR) of 81.7%, which included 17.4% complete responses (CR), Neither median PFS nor OS have been reached and the respective PFS and OS at 12 months were 92% and 95%. The respective PFS and OS rates for patients with del17p were 87% and 89%. An analysis of predictors of survival showed that only the presence of del17p was associated with inferior PFS (odds ratio 1.91, P = .035)
Dr. Mato noted that there was no clear standard treatment approach for patients who discontinued ibrutinib or for whom ibrutinib did not work. The top three second-line approaches used included an anti-CD20 agent combined with chlorambucil, venetoclax (Venclexta), or a different kinase inhibitor. Chemoimmunotherapy with either fludarabine, cyclophosphamide, and rituximab or bendamustine and rituximab was given to only 5 patients as a second line therapy.
Dr. Mato disclosed serving as a consultant for AbbVie, AstraZeneca, Janssen/Pharmacyclics, and TG Therapeutics.
Heart failure readmission penalties linked with rise in deaths
ANAHEIM, CALIF. – Evidence continues to mount that Medicare’s penalization of hospitals with excess heart failure readmissions has cut readmissions but at the apparent price of more deaths.
During the penalty phase of the Hospital Readmission Reduction Program (HRRP), which started in Oct. 2012, 30-day all-cause mortality following a heart failure hospitalization was 18% higher compared with the adjusted rate during 2006-2010, based on Medicare data from 2006-2014 that underwent “extensive” risk adjustment using prospectively-collected clinical data, Gregg C. Fonarow, MD, and his associates reported in a poster at the American Heart Association scientific sessions. During the same 2012-2014 period with imposed penalties, 30-day all-cause readmissions following an index heart failure hospitalization fell by a risk-adjusted 9% compared to the era just before the HRRP. Both the drop in readmissions and rise in deaths were statistically significant.
A similar pattern existed for the risk-adjusted readmissions and mortality rates during the year following the index hospitalization: readmissions fell by 8% compared with the time before the program but deaths rose by a relative 10%, also statistically significant differences.
“This is urgent and alarming. The Centers for Medicare & Medicaid Services needs to revamp the program to exclude heart failure patients and take steps to mitigate the damage,” Dr. Fonarow said in an interview. He estimated that the uptick in mortality following heart failure hospitalizations is causing 5,000-10,000 excess annual deaths among U.S. heart failure patients that are directly attributable to the HRRP. Similar effects have not been seen for patients with an index hospitalization of pneumonia or acute MI, two other targets of the HRRP, he noted.
The HRRP “currently has penalties for readmissions that are 15-fold higher than for mortality. They need to penalize equally, and they need to get at the gaming that hospitals are doing” to shift outcomes away from readmissions even if it means more patients will die. Heart failure patients “who need hospitalization are being denied admission by hospitals out of fear of the readmissions penalty,” said Dr. Fonarow, professor and co-chief of cardiology at the University of California, Los Angeles. “Seeing increased mortality linked with implementation of the penalty is completely unacceptable.”
Although a prior report used similar Medicare data from 2008-2014 to initially find this inverse association, that analysis relied entirely on administrative data collected in Medicare records to perform risk adjustments (JAMA. 2017 July 17;318[3]:270-8). The new analysis reported by Dr. Fonarow and his associates combined the Medicare data with detailed clinical records for the same patients collected by the Get With the Guidelines--Heart Failure program. The extensive clinical data that the researchers used for risk-adjustment allowed for a more reliable attribution to the HRRP of readmission and mortality differences between the two time periods. Despite the extensive risk adjustment “we see exactly the same result” as initially reported, Dr. Fonarow said.
The findings “remind us that it is very important to look at the unintended consequences” of interventions that might initially seem reasonable, commented Lynne Warner Stevenson, MD, professor and director of cardiomyopathy at Vanderbilt University in Nashville, Tenn.
Concurrent with the presentation at the meeting the results also appeared in an article published online (JAMA Cardiol. 2017 Nov 12;doi:10.1001/jamacardio.2017.4265).
A separate analysis of data collected in the Get With the Guidelines--Heart Failure during 2005-2009 showed that within the past decade the 5-year survival of U.S. hospitalized heart failure patients has remained dismally low, and similar regardless of whether patients had heart failure with reduced ejection fraction (HFrEF, 46% of all heart failure patients in the analysis), heart failure with preserved ejection fraction (HFpEF, also 46% of patients), or the in-between patients who had heart failure with borderline ejection fraction (HFbEF, an ejection fraction of 41%-49%, in 8% of patients).
The results, from 39,982 patients, showed a 75% mortality rate during 5-years of follow-up, with similar mortality rates regardless of the patient’s ejection-fraction level, reported Dr. Fonarow and his associates in a separate poster. In every age group examined, patients with heart failure had dramatically reduced life expectancies compared with the general population. For example, among heart failure patients aged 65-69 years in the study, median survival was less than 4 years compared with a 19-year expected median survival for people in the general U.S. population in the same age range.
These very low survival rates of heart failure patients initially hospitalized for heart failure during the relatively recent era of 2005-2009 “is a call to action to prevent heart failure,” said Dr. Fonarow.
The poor prognosis most heart failure patients face should also spur aggressive treatment of HFrEF patients with all proven treatments, Dr. Fonarow said. It should also spur more effort to find effective treatments for HFpEF, which currently has no clearly-proven effective treatment.
These results also appeared in a report simultaneously published online (J Amer Coll Cardiol. 2017 Nov 12;doi: 10.1016/j.jacc.2017.08.074).
[email protected]
On Twitter @mitchelzoler
ANAHEIM, CALIF. – Evidence continues to mount that Medicare’s penalization of hospitals with excess heart failure readmissions has cut readmissions but at the apparent price of more deaths.
During the penalty phase of the Hospital Readmission Reduction Program (HRRP), which started in Oct. 2012, 30-day all-cause mortality following a heart failure hospitalization was 18% higher compared with the adjusted rate during 2006-2010, based on Medicare data from 2006-2014 that underwent “extensive” risk adjustment using prospectively-collected clinical data, Gregg C. Fonarow, MD, and his associates reported in a poster at the American Heart Association scientific sessions. During the same 2012-2014 period with imposed penalties, 30-day all-cause readmissions following an index heart failure hospitalization fell by a risk-adjusted 9% compared to the era just before the HRRP. Both the drop in readmissions and rise in deaths were statistically significant.
A similar pattern existed for the risk-adjusted readmissions and mortality rates during the year following the index hospitalization: readmissions fell by 8% compared with the time before the program but deaths rose by a relative 10%, also statistically significant differences.
“This is urgent and alarming. The Centers for Medicare & Medicaid Services needs to revamp the program to exclude heart failure patients and take steps to mitigate the damage,” Dr. Fonarow said in an interview. He estimated that the uptick in mortality following heart failure hospitalizations is causing 5,000-10,000 excess annual deaths among U.S. heart failure patients that are directly attributable to the HRRP. Similar effects have not been seen for patients with an index hospitalization of pneumonia or acute MI, two other targets of the HRRP, he noted.
The HRRP “currently has penalties for readmissions that are 15-fold higher than for mortality. They need to penalize equally, and they need to get at the gaming that hospitals are doing” to shift outcomes away from readmissions even if it means more patients will die. Heart failure patients “who need hospitalization are being denied admission by hospitals out of fear of the readmissions penalty,” said Dr. Fonarow, professor and co-chief of cardiology at the University of California, Los Angeles. “Seeing increased mortality linked with implementation of the penalty is completely unacceptable.”
Although a prior report used similar Medicare data from 2008-2014 to initially find this inverse association, that analysis relied entirely on administrative data collected in Medicare records to perform risk adjustments (JAMA. 2017 July 17;318[3]:270-8). The new analysis reported by Dr. Fonarow and his associates combined the Medicare data with detailed clinical records for the same patients collected by the Get With the Guidelines--Heart Failure program. The extensive clinical data that the researchers used for risk-adjustment allowed for a more reliable attribution to the HRRP of readmission and mortality differences between the two time periods. Despite the extensive risk adjustment “we see exactly the same result” as initially reported, Dr. Fonarow said.
The findings “remind us that it is very important to look at the unintended consequences” of interventions that might initially seem reasonable, commented Lynne Warner Stevenson, MD, professor and director of cardiomyopathy at Vanderbilt University in Nashville, Tenn.
Concurrent with the presentation at the meeting the results also appeared in an article published online (JAMA Cardiol. 2017 Nov 12;doi:10.1001/jamacardio.2017.4265).
A separate analysis of data collected in the Get With the Guidelines--Heart Failure during 2005-2009 showed that within the past decade the 5-year survival of U.S. hospitalized heart failure patients has remained dismally low, and similar regardless of whether patients had heart failure with reduced ejection fraction (HFrEF, 46% of all heart failure patients in the analysis), heart failure with preserved ejection fraction (HFpEF, also 46% of patients), or the in-between patients who had heart failure with borderline ejection fraction (HFbEF, an ejection fraction of 41%-49%, in 8% of patients).
The results, from 39,982 patients, showed a 75% mortality rate during 5-years of follow-up, with similar mortality rates regardless of the patient’s ejection-fraction level, reported Dr. Fonarow and his associates in a separate poster. In every age group examined, patients with heart failure had dramatically reduced life expectancies compared with the general population. For example, among heart failure patients aged 65-69 years in the study, median survival was less than 4 years compared with a 19-year expected median survival for people in the general U.S. population in the same age range.
These very low survival rates of heart failure patients initially hospitalized for heart failure during the relatively recent era of 2005-2009 “is a call to action to prevent heart failure,” said Dr. Fonarow.
The poor prognosis most heart failure patients face should also spur aggressive treatment of HFrEF patients with all proven treatments, Dr. Fonarow said. It should also spur more effort to find effective treatments for HFpEF, which currently has no clearly-proven effective treatment.
These results also appeared in a report simultaneously published online (J Amer Coll Cardiol. 2017 Nov 12;doi: 10.1016/j.jacc.2017.08.074).
[email protected]
On Twitter @mitchelzoler
ANAHEIM, CALIF. – Evidence continues to mount that Medicare’s penalization of hospitals with excess heart failure readmissions has cut readmissions but at the apparent price of more deaths.
During the penalty phase of the Hospital Readmission Reduction Program (HRRP), which started in Oct. 2012, 30-day all-cause mortality following a heart failure hospitalization was 18% higher compared with the adjusted rate during 2006-2010, based on Medicare data from 2006-2014 that underwent “extensive” risk adjustment using prospectively-collected clinical data, Gregg C. Fonarow, MD, and his associates reported in a poster at the American Heart Association scientific sessions. During the same 2012-2014 period with imposed penalties, 30-day all-cause readmissions following an index heart failure hospitalization fell by a risk-adjusted 9% compared to the era just before the HRRP. Both the drop in readmissions and rise in deaths were statistically significant.
A similar pattern existed for the risk-adjusted readmissions and mortality rates during the year following the index hospitalization: readmissions fell by 8% compared with the time before the program but deaths rose by a relative 10%, also statistically significant differences.
“This is urgent and alarming. The Centers for Medicare & Medicaid Services needs to revamp the program to exclude heart failure patients and take steps to mitigate the damage,” Dr. Fonarow said in an interview. He estimated that the uptick in mortality following heart failure hospitalizations is causing 5,000-10,000 excess annual deaths among U.S. heart failure patients that are directly attributable to the HRRP. Similar effects have not been seen for patients with an index hospitalization of pneumonia or acute MI, two other targets of the HRRP, he noted.
The HRRP “currently has penalties for readmissions that are 15-fold higher than for mortality. They need to penalize equally, and they need to get at the gaming that hospitals are doing” to shift outcomes away from readmissions even if it means more patients will die. Heart failure patients “who need hospitalization are being denied admission by hospitals out of fear of the readmissions penalty,” said Dr. Fonarow, professor and co-chief of cardiology at the University of California, Los Angeles. “Seeing increased mortality linked with implementation of the penalty is completely unacceptable.”
Although a prior report used similar Medicare data from 2008-2014 to initially find this inverse association, that analysis relied entirely on administrative data collected in Medicare records to perform risk adjustments (JAMA. 2017 July 17;318[3]:270-8). The new analysis reported by Dr. Fonarow and his associates combined the Medicare data with detailed clinical records for the same patients collected by the Get With the Guidelines--Heart Failure program. The extensive clinical data that the researchers used for risk-adjustment allowed for a more reliable attribution to the HRRP of readmission and mortality differences between the two time periods. Despite the extensive risk adjustment “we see exactly the same result” as initially reported, Dr. Fonarow said.
The findings “remind us that it is very important to look at the unintended consequences” of interventions that might initially seem reasonable, commented Lynne Warner Stevenson, MD, professor and director of cardiomyopathy at Vanderbilt University in Nashville, Tenn.
Concurrent with the presentation at the meeting the results also appeared in an article published online (JAMA Cardiol. 2017 Nov 12;doi:10.1001/jamacardio.2017.4265).
A separate analysis of data collected in the Get With the Guidelines--Heart Failure during 2005-2009 showed that within the past decade the 5-year survival of U.S. hospitalized heart failure patients has remained dismally low, and similar regardless of whether patients had heart failure with reduced ejection fraction (HFrEF, 46% of all heart failure patients in the analysis), heart failure with preserved ejection fraction (HFpEF, also 46% of patients), or the in-between patients who had heart failure with borderline ejection fraction (HFbEF, an ejection fraction of 41%-49%, in 8% of patients).
The results, from 39,982 patients, showed a 75% mortality rate during 5-years of follow-up, with similar mortality rates regardless of the patient’s ejection-fraction level, reported Dr. Fonarow and his associates in a separate poster. In every age group examined, patients with heart failure had dramatically reduced life expectancies compared with the general population. For example, among heart failure patients aged 65-69 years in the study, median survival was less than 4 years compared with a 19-year expected median survival for people in the general U.S. population in the same age range.
These very low survival rates of heart failure patients initially hospitalized for heart failure during the relatively recent era of 2005-2009 “is a call to action to prevent heart failure,” said Dr. Fonarow.
The poor prognosis most heart failure patients face should also spur aggressive treatment of HFrEF patients with all proven treatments, Dr. Fonarow said. It should also spur more effort to find effective treatments for HFpEF, which currently has no clearly-proven effective treatment.
These results also appeared in a report simultaneously published online (J Amer Coll Cardiol. 2017 Nov 12;doi: 10.1016/j.jacc.2017.08.074).
[email protected]
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Risk-adjusted 30-day readmissions fell by a relative 9% during the reduction program but relative mortality rose by 18%.
Data source: Review of 115,245 Medicare beneficiaries with heart failure treated at hospitals in the Get With the Guidelines--Heart Failure program.
Disclosures: Dr. Fonarow has been a consultant to Amgen, Janssen, Medtronic, Novartis, and St. Jude. Dr. Stevenson has been a consultant to Abbott, has received travel support from St. Jude, and has received research funding and food from Novartis.
FDA approves first-in-class drug for hemophilia A with Factor VIII
The Food and Drug Administration has approved emicizumab-kxwh (Hemlibra) for the prevention or reduction of bleeding episodes for adult and pediatric patients with hemophilia A with Factor VIII inhibitors.
The drug received the FDA’s Priority Review, Breakthrough Therapy, and Orphan Drug designations.
It was shown to be safe and effective in two clinical trials, one of boys and men aged 12 years and older and the other of boys younger than 12 years. In the first trial, patients taking emicizumab-kxwh had an 87% reduction in the rate of treated bleeding episodes per year, compared with patients not receiving prophylactic treatment (2.9 vs. 23.3; P less than .0001).
In the second trial, 87% of the children receiving emicizumab-kxwh did not experience a bleeding episode that required treatment.
The most common adverse events were injection site reactions, headache, and arthralgia. The drug labeling includes a boxed warning about the possibility of thrombotic microangiopathy and thromboembolism in patients given an activated prothrombin complex concentrate rescue treatment for 24 hours or more while taking emicizumab-kxwh.
The Food and Drug Administration has approved emicizumab-kxwh (Hemlibra) for the prevention or reduction of bleeding episodes for adult and pediatric patients with hemophilia A with Factor VIII inhibitors.
The drug received the FDA’s Priority Review, Breakthrough Therapy, and Orphan Drug designations.
It was shown to be safe and effective in two clinical trials, one of boys and men aged 12 years and older and the other of boys younger than 12 years. In the first trial, patients taking emicizumab-kxwh had an 87% reduction in the rate of treated bleeding episodes per year, compared with patients not receiving prophylactic treatment (2.9 vs. 23.3; P less than .0001).
In the second trial, 87% of the children receiving emicizumab-kxwh did not experience a bleeding episode that required treatment.
The most common adverse events were injection site reactions, headache, and arthralgia. The drug labeling includes a boxed warning about the possibility of thrombotic microangiopathy and thromboembolism in patients given an activated prothrombin complex concentrate rescue treatment for 24 hours or more while taking emicizumab-kxwh.
The Food and Drug Administration has approved emicizumab-kxwh (Hemlibra) for the prevention or reduction of bleeding episodes for adult and pediatric patients with hemophilia A with Factor VIII inhibitors.
The drug received the FDA’s Priority Review, Breakthrough Therapy, and Orphan Drug designations.
It was shown to be safe and effective in two clinical trials, one of boys and men aged 12 years and older and the other of boys younger than 12 years. In the first trial, patients taking emicizumab-kxwh had an 87% reduction in the rate of treated bleeding episodes per year, compared with patients not receiving prophylactic treatment (2.9 vs. 23.3; P less than .0001).
In the second trial, 87% of the children receiving emicizumab-kxwh did not experience a bleeding episode that required treatment.
The most common adverse events were injection site reactions, headache, and arthralgia. The drug labeling includes a boxed warning about the possibility of thrombotic microangiopathy and thromboembolism in patients given an activated prothrombin complex concentrate rescue treatment for 24 hours or more while taking emicizumab-kxwh.
Scandinavian registries answer key questions about ADHD
PARIS – Huge longitudinal Scandinavian population registries constitute a unique data source that, in recent years, has provided new insights into attention-deficit/hyperactivity disorder and its associated risks of suicidal behavior, accidents, and early mortality, Henrik Larsson, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“Randomized, controlled trials have provided little information about real-world effectiveness of ADHD medications, such as their potential effects on adverse health outcomes,” said Dr. Larsson of the Karolinska Institute in Stockholm.
Suicidality
Dr. Larsson was senior author of a Swedish national registry study that identified 51,707 patients with ADHD matched by sex and birth year to 258,535 controls. The ADHD patients had significantly higher rates of both attempted and completed suicide.
After adjustment for socioeconomic status, these individuals were at 8.46-fold increased risk for attempted suicide. However, after further adjustment for comorbid psychiatric disorders, this dropped substantially to a 3.62-fold increased risk.
The same pattern pertained to completed suicide: The risk after adjustment for socioeconomic status was increased 12.3-fold in individuals with ADHD, compared with controls, but the risk dropped to 5.91-fold after further adjustment for comorbid psychiatric disorders (JAMA Psychiatry. 2014 Aug;71[8]:958-64).
The clinical take home point: “Detection and treatment of comorbid conditions probably will help reduce suicidal behavior in ADHD,” Dr. Larsson said.
This study also showed that increased familial risk also is a key factor in the increased risk of suicidal behavior in the ADHD population. Parents of individuals with ADHD were at 2.42-fold increased risk of attempted suicide, compared with controls, and full siblings were at 2.28-fold increased risk. In contrast, the risk in half-siblings, while significantly greater than in controls, was lower than in the genetically closer first-degree relatives: Maternal half-siblings were at 1.57-fold increased risk, and paternal half-siblings were at 1.57-fold greater risk. Cousins were at 1.39-fold increased risk.
The same held true for completed suicide risk. And the familial associations remained significant even after excluding relatives with ADHD.
“Regarding the shared familial factors, I’m tempted to hypothesize that this might involve pleiotropic effects reflecting genetic variants associated with impulsivity,” said Dr. Larsson. To further understand the biological mechanisms underlying ADHD and associated adverse health outcomes requires multiple disciplines to work together. For such work, Dr. Larsson collaborates with international colleagues in several consortia.
ADHD medications and suicidality
Concerns regarding this question were raised by a meta-analysis based on clinical trials data that suggested patients’ increased suicidality might be caused by the effects of ADHD medications. But the meta-analysis was seriously flawed by what epidemiologists call confounding by indication, which is the potential for bias to be introduced when a group of patients on medication is compared with another group off medication. The confounding results from the fact that ADHD patients on medication are different from those who aren’t: They are likely to be more symptomatic and have more comorbidities.
To bypass the confounding issue, Dr. Larsson and his coinvestigators turned to the Swedish registries and identified 37,936 patients with ADHD with a total of 7,019 suicide-related events during nearly 151,000 person-years of follow-up. When they compared patients on drug treatment with those who were not, they found – as in the other investigators’ meta-analysis – that drug treatment was associated with a statistically significant 1.31-fold increased risk of suicide-related events. However, when they performed a more appropriate between-individual analysis, Dr. Larsson and his colleagues found that, when patients with ADHD were using stimulant medications, they had a significant 19% lower risk of suicide-related events than when they were off medication. While on nonstimulant ADHD medications, their suicidality risk was no different from when off medication (BMJ. 2014 Jun 18;348:g3769. doi: 10.1136/bmj.g3769).
ADHD and early mortality risk
Prior studies have established that ADHD is associated with a proclivity to engage in risk-taking behaviors, including substance abuse, criminality, risky sexual behavior, and accidents, which are themselves associated with early mortality.
Sure enough, when Danish investigators turned to their national registries, identified 32,061 individuals with ADHD born during 1981-2011, and followed them through 2013, they found that, during nearly 25 million person-years of follow-up, the mortality rate was 5.85 deaths per 10,000 person-years in individuals with ADHD, compared with 2.21 deaths per 10,000 person-years in controls, resulting in a fully adjusted mortality rate ratio of 2.07. The rate ratio was 1.86 for ADHD patients under age 6 years, 1.58 in those aged 6-17 years, and 4.25 for patients aged 18 years and older (Lancet. 2015 May 30;385[9983]:2190-6).
Accidents were the most common cause of death. Could ADHD medications modify this risk of fatal accidents?
Serious motor vehicle accidents
Dr. Larsson and his coinvestigators used registry data to follow 17,408 Swedish adults with ADHD for serious transport accidents involving a trip to the emergency room or death during 2006-2009. The risk was increased by an adjusted 1.47-fold in men with ADHD and by 1.45-fold in women with the disorder. However, in the within-individual analysis, men were 58% less likely to have a serious transport accident when they were on ADHD medication than when off medication. There was no statistically significant effect of ADHD medications on the risk in women with ADHD.
The investigators estimated that 41%-49% of transport accidents in men with ADHD could have been avoided had they been on drug therapy continuously throughout the follow-up period (JAMA Psychiatry. 2014 Mar;71[3]:319-25).
Similar results – that is, data showing that being on ADHD medication reduces the elevated risk of serious accidents – have been reported in four other independent studies conducted in Denmark, Germany, Hong Kong, and most recently in a U.S. analysis by Dr. Larsson and coinvestigators of more than 2.3 million patients with ADHD in a U.S. commercial health insurance claims database (JAMA Psychiatry. 2017 Jun 1;74[6]:597-603).
These findings collectively highlight the public health importance of diagnosing and treating ADHD.
But Dr. Larsson wanted his audience to take home another key lesson: “ADHD is a disorder that can be associated with serious outcomes, including suicide and accidents. It’s nevertheless important to remember that the absolute risks here are very low for any of these outcomes, so the majority of individuals with ADHD will never suffer from any of these outcomes. It’s important to keep that in mind.”
Dr. Larsson’s research is funded by the Swedish Research Council, the National Institute of Mental Health, FORTE, Horizon 2020, and Shire.
[email protected]
PARIS – Huge longitudinal Scandinavian population registries constitute a unique data source that, in recent years, has provided new insights into attention-deficit/hyperactivity disorder and its associated risks of suicidal behavior, accidents, and early mortality, Henrik Larsson, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“Randomized, controlled trials have provided little information about real-world effectiveness of ADHD medications, such as their potential effects on adverse health outcomes,” said Dr. Larsson of the Karolinska Institute in Stockholm.
Suicidality
Dr. Larsson was senior author of a Swedish national registry study that identified 51,707 patients with ADHD matched by sex and birth year to 258,535 controls. The ADHD patients had significantly higher rates of both attempted and completed suicide.
After adjustment for socioeconomic status, these individuals were at 8.46-fold increased risk for attempted suicide. However, after further adjustment for comorbid psychiatric disorders, this dropped substantially to a 3.62-fold increased risk.
The same pattern pertained to completed suicide: The risk after adjustment for socioeconomic status was increased 12.3-fold in individuals with ADHD, compared with controls, but the risk dropped to 5.91-fold after further adjustment for comorbid psychiatric disorders (JAMA Psychiatry. 2014 Aug;71[8]:958-64).
The clinical take home point: “Detection and treatment of comorbid conditions probably will help reduce suicidal behavior in ADHD,” Dr. Larsson said.
This study also showed that increased familial risk also is a key factor in the increased risk of suicidal behavior in the ADHD population. Parents of individuals with ADHD were at 2.42-fold increased risk of attempted suicide, compared with controls, and full siblings were at 2.28-fold increased risk. In contrast, the risk in half-siblings, while significantly greater than in controls, was lower than in the genetically closer first-degree relatives: Maternal half-siblings were at 1.57-fold increased risk, and paternal half-siblings were at 1.57-fold greater risk. Cousins were at 1.39-fold increased risk.
The same held true for completed suicide risk. And the familial associations remained significant even after excluding relatives with ADHD.
“Regarding the shared familial factors, I’m tempted to hypothesize that this might involve pleiotropic effects reflecting genetic variants associated with impulsivity,” said Dr. Larsson. To further understand the biological mechanisms underlying ADHD and associated adverse health outcomes requires multiple disciplines to work together. For such work, Dr. Larsson collaborates with international colleagues in several consortia.
ADHD medications and suicidality
Concerns regarding this question were raised by a meta-analysis based on clinical trials data that suggested patients’ increased suicidality might be caused by the effects of ADHD medications. But the meta-analysis was seriously flawed by what epidemiologists call confounding by indication, which is the potential for bias to be introduced when a group of patients on medication is compared with another group off medication. The confounding results from the fact that ADHD patients on medication are different from those who aren’t: They are likely to be more symptomatic and have more comorbidities.
To bypass the confounding issue, Dr. Larsson and his coinvestigators turned to the Swedish registries and identified 37,936 patients with ADHD with a total of 7,019 suicide-related events during nearly 151,000 person-years of follow-up. When they compared patients on drug treatment with those who were not, they found – as in the other investigators’ meta-analysis – that drug treatment was associated with a statistically significant 1.31-fold increased risk of suicide-related events. However, when they performed a more appropriate between-individual analysis, Dr. Larsson and his colleagues found that, when patients with ADHD were using stimulant medications, they had a significant 19% lower risk of suicide-related events than when they were off medication. While on nonstimulant ADHD medications, their suicidality risk was no different from when off medication (BMJ. 2014 Jun 18;348:g3769. doi: 10.1136/bmj.g3769).
ADHD and early mortality risk
Prior studies have established that ADHD is associated with a proclivity to engage in risk-taking behaviors, including substance abuse, criminality, risky sexual behavior, and accidents, which are themselves associated with early mortality.
Sure enough, when Danish investigators turned to their national registries, identified 32,061 individuals with ADHD born during 1981-2011, and followed them through 2013, they found that, during nearly 25 million person-years of follow-up, the mortality rate was 5.85 deaths per 10,000 person-years in individuals with ADHD, compared with 2.21 deaths per 10,000 person-years in controls, resulting in a fully adjusted mortality rate ratio of 2.07. The rate ratio was 1.86 for ADHD patients under age 6 years, 1.58 in those aged 6-17 years, and 4.25 for patients aged 18 years and older (Lancet. 2015 May 30;385[9983]:2190-6).
Accidents were the most common cause of death. Could ADHD medications modify this risk of fatal accidents?
Serious motor vehicle accidents
Dr. Larsson and his coinvestigators used registry data to follow 17,408 Swedish adults with ADHD for serious transport accidents involving a trip to the emergency room or death during 2006-2009. The risk was increased by an adjusted 1.47-fold in men with ADHD and by 1.45-fold in women with the disorder. However, in the within-individual analysis, men were 58% less likely to have a serious transport accident when they were on ADHD medication than when off medication. There was no statistically significant effect of ADHD medications on the risk in women with ADHD.
The investigators estimated that 41%-49% of transport accidents in men with ADHD could have been avoided had they been on drug therapy continuously throughout the follow-up period (JAMA Psychiatry. 2014 Mar;71[3]:319-25).
Similar results – that is, data showing that being on ADHD medication reduces the elevated risk of serious accidents – have been reported in four other independent studies conducted in Denmark, Germany, Hong Kong, and most recently in a U.S. analysis by Dr. Larsson and coinvestigators of more than 2.3 million patients with ADHD in a U.S. commercial health insurance claims database (JAMA Psychiatry. 2017 Jun 1;74[6]:597-603).
These findings collectively highlight the public health importance of diagnosing and treating ADHD.
But Dr. Larsson wanted his audience to take home another key lesson: “ADHD is a disorder that can be associated with serious outcomes, including suicide and accidents. It’s nevertheless important to remember that the absolute risks here are very low for any of these outcomes, so the majority of individuals with ADHD will never suffer from any of these outcomes. It’s important to keep that in mind.”
Dr. Larsson’s research is funded by the Swedish Research Council, the National Institute of Mental Health, FORTE, Horizon 2020, and Shire.
[email protected]
PARIS – Huge longitudinal Scandinavian population registries constitute a unique data source that, in recent years, has provided new insights into attention-deficit/hyperactivity disorder and its associated risks of suicidal behavior, accidents, and early mortality, Henrik Larsson, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“Randomized, controlled trials have provided little information about real-world effectiveness of ADHD medications, such as their potential effects on adverse health outcomes,” said Dr. Larsson of the Karolinska Institute in Stockholm.
Suicidality
Dr. Larsson was senior author of a Swedish national registry study that identified 51,707 patients with ADHD matched by sex and birth year to 258,535 controls. The ADHD patients had significantly higher rates of both attempted and completed suicide.
After adjustment for socioeconomic status, these individuals were at 8.46-fold increased risk for attempted suicide. However, after further adjustment for comorbid psychiatric disorders, this dropped substantially to a 3.62-fold increased risk.
The same pattern pertained to completed suicide: The risk after adjustment for socioeconomic status was increased 12.3-fold in individuals with ADHD, compared with controls, but the risk dropped to 5.91-fold after further adjustment for comorbid psychiatric disorders (JAMA Psychiatry. 2014 Aug;71[8]:958-64).
The clinical take home point: “Detection and treatment of comorbid conditions probably will help reduce suicidal behavior in ADHD,” Dr. Larsson said.
This study also showed that increased familial risk also is a key factor in the increased risk of suicidal behavior in the ADHD population. Parents of individuals with ADHD were at 2.42-fold increased risk of attempted suicide, compared with controls, and full siblings were at 2.28-fold increased risk. In contrast, the risk in half-siblings, while significantly greater than in controls, was lower than in the genetically closer first-degree relatives: Maternal half-siblings were at 1.57-fold increased risk, and paternal half-siblings were at 1.57-fold greater risk. Cousins were at 1.39-fold increased risk.
The same held true for completed suicide risk. And the familial associations remained significant even after excluding relatives with ADHD.
“Regarding the shared familial factors, I’m tempted to hypothesize that this might involve pleiotropic effects reflecting genetic variants associated with impulsivity,” said Dr. Larsson. To further understand the biological mechanisms underlying ADHD and associated adverse health outcomes requires multiple disciplines to work together. For such work, Dr. Larsson collaborates with international colleagues in several consortia.
ADHD medications and suicidality
Concerns regarding this question were raised by a meta-analysis based on clinical trials data that suggested patients’ increased suicidality might be caused by the effects of ADHD medications. But the meta-analysis was seriously flawed by what epidemiologists call confounding by indication, which is the potential for bias to be introduced when a group of patients on medication is compared with another group off medication. The confounding results from the fact that ADHD patients on medication are different from those who aren’t: They are likely to be more symptomatic and have more comorbidities.
To bypass the confounding issue, Dr. Larsson and his coinvestigators turned to the Swedish registries and identified 37,936 patients with ADHD with a total of 7,019 suicide-related events during nearly 151,000 person-years of follow-up. When they compared patients on drug treatment with those who were not, they found – as in the other investigators’ meta-analysis – that drug treatment was associated with a statistically significant 1.31-fold increased risk of suicide-related events. However, when they performed a more appropriate between-individual analysis, Dr. Larsson and his colleagues found that, when patients with ADHD were using stimulant medications, they had a significant 19% lower risk of suicide-related events than when they were off medication. While on nonstimulant ADHD medications, their suicidality risk was no different from when off medication (BMJ. 2014 Jun 18;348:g3769. doi: 10.1136/bmj.g3769).
ADHD and early mortality risk
Prior studies have established that ADHD is associated with a proclivity to engage in risk-taking behaviors, including substance abuse, criminality, risky sexual behavior, and accidents, which are themselves associated with early mortality.
Sure enough, when Danish investigators turned to their national registries, identified 32,061 individuals with ADHD born during 1981-2011, and followed them through 2013, they found that, during nearly 25 million person-years of follow-up, the mortality rate was 5.85 deaths per 10,000 person-years in individuals with ADHD, compared with 2.21 deaths per 10,000 person-years in controls, resulting in a fully adjusted mortality rate ratio of 2.07. The rate ratio was 1.86 for ADHD patients under age 6 years, 1.58 in those aged 6-17 years, and 4.25 for patients aged 18 years and older (Lancet. 2015 May 30;385[9983]:2190-6).
Accidents were the most common cause of death. Could ADHD medications modify this risk of fatal accidents?
Serious motor vehicle accidents
Dr. Larsson and his coinvestigators used registry data to follow 17,408 Swedish adults with ADHD for serious transport accidents involving a trip to the emergency room or death during 2006-2009. The risk was increased by an adjusted 1.47-fold in men with ADHD and by 1.45-fold in women with the disorder. However, in the within-individual analysis, men were 58% less likely to have a serious transport accident when they were on ADHD medication than when off medication. There was no statistically significant effect of ADHD medications on the risk in women with ADHD.
The investigators estimated that 41%-49% of transport accidents in men with ADHD could have been avoided had they been on drug therapy continuously throughout the follow-up period (JAMA Psychiatry. 2014 Mar;71[3]:319-25).
Similar results – that is, data showing that being on ADHD medication reduces the elevated risk of serious accidents – have been reported in four other independent studies conducted in Denmark, Germany, Hong Kong, and most recently in a U.S. analysis by Dr. Larsson and coinvestigators of more than 2.3 million patients with ADHD in a U.S. commercial health insurance claims database (JAMA Psychiatry. 2017 Jun 1;74[6]:597-603).
These findings collectively highlight the public health importance of diagnosing and treating ADHD.
But Dr. Larsson wanted his audience to take home another key lesson: “ADHD is a disorder that can be associated with serious outcomes, including suicide and accidents. It’s nevertheless important to remember that the absolute risks here are very low for any of these outcomes, so the majority of individuals with ADHD will never suffer from any of these outcomes. It’s important to keep that in mind.”
Dr. Larsson’s research is funded by the Swedish Research Council, the National Institute of Mental Health, FORTE, Horizon 2020, and Shire.
[email protected]
expert analysis from THE ECNP CONGRESS
A program to increase flu vaccine compliance
. It won’t hurt your bottom line either and actually will help it. A flu shot program potentially can be run by a licensed practical nurse, registered nurse, physician’s assistant, or pediatric nurse practitioner, depending on your state’s law regarding vaccine administration by other than a physician, thus freeing up the physician to see well-child and sick-call patients.
It’s easy to set up a flu shot program and run it. Start preparing in June, preceding the upcoming flu season. Designate several Saturdays and or Sundays in September, October, November, December, and January as flu shot Saturdays and/or Sundays. And if Columbus day falls on a weekday, consider adding Columbus Day to your program dates as the kids often are off from school that day (check the local school calendar).
Next, prepare a postcard to be mailed to all patients on the lists your EMR produced for you. Keep the postcard simple. Announce the program, and state the dates the flu shot program is running. Ask parents to call to make an appointment for a flu vaccine only by appointment “with the program.” In addition to mailing a postcard, announce the flu shot program by sending out automated telephone calls and emails to all three lists the EMR has produced for you. The postcard mailing is your first contact, essentially announcing the program with dates and times. An automated phone call may be used to announce a specific date for which you are “now booking.” A good option when using automated phone calls is to allow the caller to press “zero” to be connected to the office to schedule a “flu shot only” appointment! Finally, emails announcing the dates of the program simply will reinforce information about the program.
Mr. Berman has been providing practice management services to physicians and other medical providers since 1983. He is the CEO of a pediatrics practice with locations in Staten Island and Brooklyn, N.Y. He holds a faculty appointment at State University of New York, Brooklyn, as a lecturer for the department of family medicine’s residency training program. He has no disclosures to report. Email him at [email protected].
. It won’t hurt your bottom line either and actually will help it. A flu shot program potentially can be run by a licensed practical nurse, registered nurse, physician’s assistant, or pediatric nurse practitioner, depending on your state’s law regarding vaccine administration by other than a physician, thus freeing up the physician to see well-child and sick-call patients.
It’s easy to set up a flu shot program and run it. Start preparing in June, preceding the upcoming flu season. Designate several Saturdays and or Sundays in September, October, November, December, and January as flu shot Saturdays and/or Sundays. And if Columbus day falls on a weekday, consider adding Columbus Day to your program dates as the kids often are off from school that day (check the local school calendar).
Next, prepare a postcard to be mailed to all patients on the lists your EMR produced for you. Keep the postcard simple. Announce the program, and state the dates the flu shot program is running. Ask parents to call to make an appointment for a flu vaccine only by appointment “with the program.” In addition to mailing a postcard, announce the flu shot program by sending out automated telephone calls and emails to all three lists the EMR has produced for you. The postcard mailing is your first contact, essentially announcing the program with dates and times. An automated phone call may be used to announce a specific date for which you are “now booking.” A good option when using automated phone calls is to allow the caller to press “zero” to be connected to the office to schedule a “flu shot only” appointment! Finally, emails announcing the dates of the program simply will reinforce information about the program.
Mr. Berman has been providing practice management services to physicians and other medical providers since 1983. He is the CEO of a pediatrics practice with locations in Staten Island and Brooklyn, N.Y. He holds a faculty appointment at State University of New York, Brooklyn, as a lecturer for the department of family medicine’s residency training program. He has no disclosures to report. Email him at [email protected].
. It won’t hurt your bottom line either and actually will help it. A flu shot program potentially can be run by a licensed practical nurse, registered nurse, physician’s assistant, or pediatric nurse practitioner, depending on your state’s law regarding vaccine administration by other than a physician, thus freeing up the physician to see well-child and sick-call patients.
It’s easy to set up a flu shot program and run it. Start preparing in June, preceding the upcoming flu season. Designate several Saturdays and or Sundays in September, October, November, December, and January as flu shot Saturdays and/or Sundays. And if Columbus day falls on a weekday, consider adding Columbus Day to your program dates as the kids often are off from school that day (check the local school calendar).
Next, prepare a postcard to be mailed to all patients on the lists your EMR produced for you. Keep the postcard simple. Announce the program, and state the dates the flu shot program is running. Ask parents to call to make an appointment for a flu vaccine only by appointment “with the program.” In addition to mailing a postcard, announce the flu shot program by sending out automated telephone calls and emails to all three lists the EMR has produced for you. The postcard mailing is your first contact, essentially announcing the program with dates and times. An automated phone call may be used to announce a specific date for which you are “now booking.” A good option when using automated phone calls is to allow the caller to press “zero” to be connected to the office to schedule a “flu shot only” appointment! Finally, emails announcing the dates of the program simply will reinforce information about the program.
Mr. Berman has been providing practice management services to physicians and other medical providers since 1983. He is the CEO of a pediatrics practice with locations in Staten Island and Brooklyn, N.Y. He holds a faculty appointment at State University of New York, Brooklyn, as a lecturer for the department of family medicine’s residency training program. He has no disclosures to report. Email him at [email protected].
Team discovers mechanism of resistance in AML
Researchers say they have uncovered a target to overcome drug resistance in acute myeloid leukemia (AML).
The team discovered how a linkage between 2 proteins enables AML cells to resist chemotherapy and showed that disrupting the linkage could render the cells vulnerable to treatment.
The researchers believe their discovery could lead to drugs to enhance chemotherapy in patients with AML and other cancers.
John Schuetz, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues described this research in Nature Communications.
The team launched their experiments based on previous findings that a protein called ABCC4 was greatly elevated in aggressive cases of AML.
Dr Schuetz and his colleagues searched for other proteins that might interact with ABCC4 and enable its function. The team’s screening of candidate proteins yielded one, MPP1, which was also greatly increased in AML.
The researchers found the 2 proteins are connected, and the connection enables cells to assume the characteristics of highly proliferative leukemia cells.
These experiments involved genetically altering hematopoietic progenitor cells to have high MPP1 and ABCC4 levels. The cells were grown in culture and then replated to see if they would continue to grow, as such self-renewal is a hallmark of leukemia cells.
The researchers found that serial regrowth depended on the cells having high levels of both ABCC4 and MPP1.
“Typically, if you take normal progenitors and you replate, you could do that one time, maybe twice,” Dr Schuetz said. “But our big surprise was that overexpressing MPP1—analogous to what you would see in leukemia—allows those progenitors to self-renew, to be replated over and over, to form new colonies.”
The experiments also revealed that MPP1 and ABCC4 functioned at the cell membrane, where they could play a role in the machinery that would rid the leukemia cells of chemotherapy drugs.
“When we disrupted their interaction, ABCC4 moved off the membrane and the cells became more sensitive to drugs used in AML—drugs that are pumped out of the cell by ABCC4,” Dr Schuetz said.
By screening thousands of compounds, the researchers identified some that could disrupt the ABCC4-MPP1 connection. One, called Antimycin-A, reversed drug resistance in AML cell lines and in cells from AML patients.
Antimycin-A is too toxic to be used in chemotherapy, but the researchers believe identification of the compound should aid the search for other, less-toxic drugs to disrupt the ABCC4-MPP1 interaction.
The team’s findings could also enable clinicians to identify AML patients with high levels of ABCC4 and MPP1. In such patients, drugs that disrupt ABCC4-MPP1 might enhance the effectiveness of standard chemotherapy, Dr Schuetz said.
He also noted that other cancers, including breast and colon cancer and medulloblastoma, show high levels of both ABCC4 and MPP1. Chemotherapy for those cancers might also be enhanced by drugs that disrupt ABCC4-MPP1. ![]()
Researchers say they have uncovered a target to overcome drug resistance in acute myeloid leukemia (AML).
The team discovered how a linkage between 2 proteins enables AML cells to resist chemotherapy and showed that disrupting the linkage could render the cells vulnerable to treatment.
The researchers believe their discovery could lead to drugs to enhance chemotherapy in patients with AML and other cancers.
John Schuetz, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues described this research in Nature Communications.
The team launched their experiments based on previous findings that a protein called ABCC4 was greatly elevated in aggressive cases of AML.
Dr Schuetz and his colleagues searched for other proteins that might interact with ABCC4 and enable its function. The team’s screening of candidate proteins yielded one, MPP1, which was also greatly increased in AML.
The researchers found the 2 proteins are connected, and the connection enables cells to assume the characteristics of highly proliferative leukemia cells.
These experiments involved genetically altering hematopoietic progenitor cells to have high MPP1 and ABCC4 levels. The cells were grown in culture and then replated to see if they would continue to grow, as such self-renewal is a hallmark of leukemia cells.
The researchers found that serial regrowth depended on the cells having high levels of both ABCC4 and MPP1.
“Typically, if you take normal progenitors and you replate, you could do that one time, maybe twice,” Dr Schuetz said. “But our big surprise was that overexpressing MPP1—analogous to what you would see in leukemia—allows those progenitors to self-renew, to be replated over and over, to form new colonies.”
The experiments also revealed that MPP1 and ABCC4 functioned at the cell membrane, where they could play a role in the machinery that would rid the leukemia cells of chemotherapy drugs.
“When we disrupted their interaction, ABCC4 moved off the membrane and the cells became more sensitive to drugs used in AML—drugs that are pumped out of the cell by ABCC4,” Dr Schuetz said.
By screening thousands of compounds, the researchers identified some that could disrupt the ABCC4-MPP1 connection. One, called Antimycin-A, reversed drug resistance in AML cell lines and in cells from AML patients.
Antimycin-A is too toxic to be used in chemotherapy, but the researchers believe identification of the compound should aid the search for other, less-toxic drugs to disrupt the ABCC4-MPP1 interaction.
The team’s findings could also enable clinicians to identify AML patients with high levels of ABCC4 and MPP1. In such patients, drugs that disrupt ABCC4-MPP1 might enhance the effectiveness of standard chemotherapy, Dr Schuetz said.
He also noted that other cancers, including breast and colon cancer and medulloblastoma, show high levels of both ABCC4 and MPP1. Chemotherapy for those cancers might also be enhanced by drugs that disrupt ABCC4-MPP1. ![]()
Researchers say they have uncovered a target to overcome drug resistance in acute myeloid leukemia (AML).
The team discovered how a linkage between 2 proteins enables AML cells to resist chemotherapy and showed that disrupting the linkage could render the cells vulnerable to treatment.
The researchers believe their discovery could lead to drugs to enhance chemotherapy in patients with AML and other cancers.
John Schuetz, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues described this research in Nature Communications.
The team launched their experiments based on previous findings that a protein called ABCC4 was greatly elevated in aggressive cases of AML.
Dr Schuetz and his colleagues searched for other proteins that might interact with ABCC4 and enable its function. The team’s screening of candidate proteins yielded one, MPP1, which was also greatly increased in AML.
The researchers found the 2 proteins are connected, and the connection enables cells to assume the characteristics of highly proliferative leukemia cells.
These experiments involved genetically altering hematopoietic progenitor cells to have high MPP1 and ABCC4 levels. The cells were grown in culture and then replated to see if they would continue to grow, as such self-renewal is a hallmark of leukemia cells.
The researchers found that serial regrowth depended on the cells having high levels of both ABCC4 and MPP1.
“Typically, if you take normal progenitors and you replate, you could do that one time, maybe twice,” Dr Schuetz said. “But our big surprise was that overexpressing MPP1—analogous to what you would see in leukemia—allows those progenitors to self-renew, to be replated over and over, to form new colonies.”
The experiments also revealed that MPP1 and ABCC4 functioned at the cell membrane, where they could play a role in the machinery that would rid the leukemia cells of chemotherapy drugs.
“When we disrupted their interaction, ABCC4 moved off the membrane and the cells became more sensitive to drugs used in AML—drugs that are pumped out of the cell by ABCC4,” Dr Schuetz said.
By screening thousands of compounds, the researchers identified some that could disrupt the ABCC4-MPP1 connection. One, called Antimycin-A, reversed drug resistance in AML cell lines and in cells from AML patients.
Antimycin-A is too toxic to be used in chemotherapy, but the researchers believe identification of the compound should aid the search for other, less-toxic drugs to disrupt the ABCC4-MPP1 interaction.
The team’s findings could also enable clinicians to identify AML patients with high levels of ABCC4 and MPP1. In such patients, drugs that disrupt ABCC4-MPP1 might enhance the effectiveness of standard chemotherapy, Dr Schuetz said.
He also noted that other cancers, including breast and colon cancer and medulloblastoma, show high levels of both ABCC4 and MPP1. Chemotherapy for those cancers might also be enhanced by drugs that disrupt ABCC4-MPP1. ![]()
Price transparency of laboratory testing does not change provider ordering habits
Clinical question: Does price transparency of laboratory tests at the point of order entry affect provider ordering behavior?
Background: Up to 30% of laboratory testing may be unnecessary, and health systems are seeking ways to effectively influence provider ordering of tests to reduce costs and improve value to patients. Price transparency and cost displaying is one strategy that has had mixed results in influencing provider ordering and reducing the amount of unnecessary laboratory testing.
Setting: Three urban academic hospitals in Philadelphia.
Synopsis: Sixty inpatient laboratory tests were randomized to either display Medicare fees at the point of order entry or not. Changes in outcomes were followed for 1 year preintervention and 1 year post intervention. The population included 98,529 patients comprising 142,921 hospital admissions. Tests ordered per patient-day and Medicare-associated fees did not significantly change in the intervention group or the control group in the year after the intervention, compared to the year preintervention.
Bottom line: Displaying laboratory testing fees at the point of order entry did not lead to a significant change in provider ordering behavior or reduction in costs.
Citation: Sedrak MS, Myers JS, Small DS, et al. Effect of a price transparency intervention in the electronic health record on clinician ordering of inpatient laboratory tests: The PRICE randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):939-45.
Dr. Chung is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
Clinical question: Does price transparency of laboratory tests at the point of order entry affect provider ordering behavior?
Background: Up to 30% of laboratory testing may be unnecessary, and health systems are seeking ways to effectively influence provider ordering of tests to reduce costs and improve value to patients. Price transparency and cost displaying is one strategy that has had mixed results in influencing provider ordering and reducing the amount of unnecessary laboratory testing.
Setting: Three urban academic hospitals in Philadelphia.
Synopsis: Sixty inpatient laboratory tests were randomized to either display Medicare fees at the point of order entry or not. Changes in outcomes were followed for 1 year preintervention and 1 year post intervention. The population included 98,529 patients comprising 142,921 hospital admissions. Tests ordered per patient-day and Medicare-associated fees did not significantly change in the intervention group or the control group in the year after the intervention, compared to the year preintervention.
Bottom line: Displaying laboratory testing fees at the point of order entry did not lead to a significant change in provider ordering behavior or reduction in costs.
Citation: Sedrak MS, Myers JS, Small DS, et al. Effect of a price transparency intervention in the electronic health record on clinician ordering of inpatient laboratory tests: The PRICE randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):939-45.
Dr. Chung is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
Clinical question: Does price transparency of laboratory tests at the point of order entry affect provider ordering behavior?
Background: Up to 30% of laboratory testing may be unnecessary, and health systems are seeking ways to effectively influence provider ordering of tests to reduce costs and improve value to patients. Price transparency and cost displaying is one strategy that has had mixed results in influencing provider ordering and reducing the amount of unnecessary laboratory testing.
Setting: Three urban academic hospitals in Philadelphia.
Synopsis: Sixty inpatient laboratory tests were randomized to either display Medicare fees at the point of order entry or not. Changes in outcomes were followed for 1 year preintervention and 1 year post intervention. The population included 98,529 patients comprising 142,921 hospital admissions. Tests ordered per patient-day and Medicare-associated fees did not significantly change in the intervention group or the control group in the year after the intervention, compared to the year preintervention.
Bottom line: Displaying laboratory testing fees at the point of order entry did not lead to a significant change in provider ordering behavior or reduction in costs.
Citation: Sedrak MS, Myers JS, Small DS, et al. Effect of a price transparency intervention in the electronic health record on clinician ordering of inpatient laboratory tests: The PRICE randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):939-45.
Dr. Chung is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
FDA: Febuxostat may have increased heart-related death risk
The urate-lowering therapy febuxostat may have a higher risk of heart-related death than does another urate-lowering drug, allopurinol, according to a Safety Alert from the Food and Drug Administration.
The safety trial was commissioned after febuxostat was approved by the FDA in 2009. Clinical trials conducted pre-approval showed an increased risk of heart-related problems, compared with allopurinol, and the drug label already carries a warning about cardiovascular events.
“Once the final results from the manufacturer are received, the FDA will conduct a comprehensive review and will update the public with any new information,” the agency said in the Safety Alert.
[email protected]
The urate-lowering therapy febuxostat may have a higher risk of heart-related death than does another urate-lowering drug, allopurinol, according to a Safety Alert from the Food and Drug Administration.
The safety trial was commissioned after febuxostat was approved by the FDA in 2009. Clinical trials conducted pre-approval showed an increased risk of heart-related problems, compared with allopurinol, and the drug label already carries a warning about cardiovascular events.
“Once the final results from the manufacturer are received, the FDA will conduct a comprehensive review and will update the public with any new information,” the agency said in the Safety Alert.
[email protected]
The urate-lowering therapy febuxostat may have a higher risk of heart-related death than does another urate-lowering drug, allopurinol, according to a Safety Alert from the Food and Drug Administration.
The safety trial was commissioned after febuxostat was approved by the FDA in 2009. Clinical trials conducted pre-approval showed an increased risk of heart-related problems, compared with allopurinol, and the drug label already carries a warning about cardiovascular events.
“Once the final results from the manufacturer are received, the FDA will conduct a comprehensive review and will update the public with any new information,” the agency said in the Safety Alert.
[email protected]
Researchers say there is room for improvement in ObGyn opioid prescribing practices
Drug overdose is the leading cause of death for Americans under age 50. And the number of men and women dying from drug overdose shows no abating, with a sharp 17% increase in 2016 over 2015.1 The rate of fatal overdoses rose to nearly 20 people per 100,000 in 2016, according to the Centers for Disease Control and Prevention. The highest death rates are reported in West Virginia, New Hampshire, Kentucky, Ohio, and Rhode Island.2
Dr. Andrew Kolodny, director of opioid policy research at Brandeis University, told The New York Times that there are “roughly two groups of Americans that are getting addicted. We have an older group that is overdosing on pain medicine, and we have a younger group that is overdosing on black market opioids.”1
Are ObGyns contributing to the over-prescription of opioid pain medications? Investigators from Florida Hospital, the University of Florida, and Orlando VA Medical Center are researching the question, and preliminary findings indicate that, yes, in fact “gynecologists are overprescribing postoperative prescription opioids in all levels of gynecologic surgery,” including after laparotomies and major and minor minimally invasive surgeries. Their work thus far, a prospective cohort study involving 113 patients enrolled to date, was presented November 15, 2017, in Washington, DC, as part of the 46th AAGL Global Congress on MIGS. They found that, on average, patients were prescribed 29.6 (SD, 9.3) opioid tablets, and they had 19.1 (SD, 12.6) tablets left over after surgery. Not surprisingly, patients undergoing major minimally invasive surgery and laparotomy were prescribed larger amounts of opioids than those undergoing minor minimally invasive surgery, but the amount of pain medication left over was similar regardless of surgery type.
The researchers also asked patients if they were told how to dispose of their leftover opioids; only 3 patients reported being told what to do if they had leftover medication.
Too many pills prescribed
In separate research presented at the meeting, investigators from Tufts Medical Center and Lahey Hospital and Medical Center in Boston, Massachusetts, sought to determine opioid prescription practices and patient opioid use after benign hysterectomy. Using retrospective online physician and telephone patient surveys, they found that 51 gynecologists prescribed a median of 30 tabs of oxycodone or hydromorphone after abdominal hysterectomy and a median of 20 tabs after laparoscopic or vaginal hysterectomy. Nearly 65% (36/56) of women used less than half of the opioids they were prescribed, and 16.1% (9/56) used zero tabs. Opioid use was not found to be significantly different for women undergoing abdominal versus minimally invasive hysterectomy.
Managing pain expectations
It takes only 3 days of opioid use for a patient to be at risk for continued use (1 to 3 years) of opioids, said Georgine Lamvu, MD, MPH, CPE, in the educational session “Perioperative Management of the Chronic Pain Patient” at the AAGL meeting. And long-term opioid use is associated with addiction, misuse, and mortality.3 It is therefore crucial to understand how to prescribe pain medications and how to educate women on expectations of pain relief.
Dr. Lamvu and fellow presenters described a 4-step process to pain management: 1) assess—including taking a history and physical exam and providing a risk assessment; 2) check—for other medications that a patient may be taking and possible interactions, as well make sure that the patient is not obtaining opioids or benzodiazepines from other providers; 3) discuss—what pain expectations you have for the patient following surgery; and 4) observe—for clinical improvement, overuse, and misuse, and go slow with dose increases and consult support pain management teams when needed.
Overall, they recommended that surgeons perform a risk assessment (determine the risks and benefits of available therapies); educate themselves and their patients on the risks and benefits; and document the risk assessment, the final recommendations to the patient, and the education provided to the patient.
“We need to do a lot better job at educating ourselves and our patients about pain medications and pain management strategies,” said Dr. Lamvu. The presenters provided these key points for patient and family education:
- Opioids are not first-line or routine therapy for chronic pain, and for acute pain they are used only for severe pain and in short-duration amounts.
- Analgesia will not make you pain free. It only helps to alleviate some pain, and most pain medications take 1 to 2 hours to take effect.
- A 30% improvement in pain and function can be expected for most therapies.
- Recovery from surgical or acute traumatic injury is not immediate; it is expected to take 2 to 4 weeks.
- Do not take extra medication doses beyond what is prescribed, and tell all of your providers what medications you are taking.
- Do not stop taking opioids suddenly, instead taper the dose slowly as instructed by your provider.
- Dispose of excess drugs appropriately—by taking unused pills back to your provider or crushing the pills, placing them in a small amount of liquid, and putting them in the trash.
“The reality is that the majority of patients are not using as many pills as we give them,” said Dr. Lamvu. “Adequate pain control does not supersede patient safety or the responsibility that we have as providers to our society.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kaplan S. CDC reports a record jump in drug overdose deaths last year. The New York Times. November 3, 2017. https://www.nytimes.com/2017/11/03/health/deaths-drug-overdose-cdc.html. Accessed November 15, 2017.
- Drug overdose death data. Centers for Disease Control and Prevention website. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Accessed November 15, 2017.
- Wapner J. CDC study finds opioid dependency begins within a few days of initial use. Newsweek. March 22, 2017. http://www.newsweek.com/cdc-opiate-addiction-572498. Accessed November 17, 2017.
Drug overdose is the leading cause of death for Americans under age 50. And the number of men and women dying from drug overdose shows no abating, with a sharp 17% increase in 2016 over 2015.1 The rate of fatal overdoses rose to nearly 20 people per 100,000 in 2016, according to the Centers for Disease Control and Prevention. The highest death rates are reported in West Virginia, New Hampshire, Kentucky, Ohio, and Rhode Island.2
Dr. Andrew Kolodny, director of opioid policy research at Brandeis University, told The New York Times that there are “roughly two groups of Americans that are getting addicted. We have an older group that is overdosing on pain medicine, and we have a younger group that is overdosing on black market opioids.”1
Are ObGyns contributing to the over-prescription of opioid pain medications? Investigators from Florida Hospital, the University of Florida, and Orlando VA Medical Center are researching the question, and preliminary findings indicate that, yes, in fact “gynecologists are overprescribing postoperative prescription opioids in all levels of gynecologic surgery,” including after laparotomies and major and minor minimally invasive surgeries. Their work thus far, a prospective cohort study involving 113 patients enrolled to date, was presented November 15, 2017, in Washington, DC, as part of the 46th AAGL Global Congress on MIGS. They found that, on average, patients were prescribed 29.6 (SD, 9.3) opioid tablets, and they had 19.1 (SD, 12.6) tablets left over after surgery. Not surprisingly, patients undergoing major minimally invasive surgery and laparotomy were prescribed larger amounts of opioids than those undergoing minor minimally invasive surgery, but the amount of pain medication left over was similar regardless of surgery type.
The researchers also asked patients if they were told how to dispose of their leftover opioids; only 3 patients reported being told what to do if they had leftover medication.
Too many pills prescribed
In separate research presented at the meeting, investigators from Tufts Medical Center and Lahey Hospital and Medical Center in Boston, Massachusetts, sought to determine opioid prescription practices and patient opioid use after benign hysterectomy. Using retrospective online physician and telephone patient surveys, they found that 51 gynecologists prescribed a median of 30 tabs of oxycodone or hydromorphone after abdominal hysterectomy and a median of 20 tabs after laparoscopic or vaginal hysterectomy. Nearly 65% (36/56) of women used less than half of the opioids they were prescribed, and 16.1% (9/56) used zero tabs. Opioid use was not found to be significantly different for women undergoing abdominal versus minimally invasive hysterectomy.
Managing pain expectations
It takes only 3 days of opioid use for a patient to be at risk for continued use (1 to 3 years) of opioids, said Georgine Lamvu, MD, MPH, CPE, in the educational session “Perioperative Management of the Chronic Pain Patient” at the AAGL meeting. And long-term opioid use is associated with addiction, misuse, and mortality.3 It is therefore crucial to understand how to prescribe pain medications and how to educate women on expectations of pain relief.
Dr. Lamvu and fellow presenters described a 4-step process to pain management: 1) assess—including taking a history and physical exam and providing a risk assessment; 2) check—for other medications that a patient may be taking and possible interactions, as well make sure that the patient is not obtaining opioids or benzodiazepines from other providers; 3) discuss—what pain expectations you have for the patient following surgery; and 4) observe—for clinical improvement, overuse, and misuse, and go slow with dose increases and consult support pain management teams when needed.
Overall, they recommended that surgeons perform a risk assessment (determine the risks and benefits of available therapies); educate themselves and their patients on the risks and benefits; and document the risk assessment, the final recommendations to the patient, and the education provided to the patient.
“We need to do a lot better job at educating ourselves and our patients about pain medications and pain management strategies,” said Dr. Lamvu. The presenters provided these key points for patient and family education:
- Opioids are not first-line or routine therapy for chronic pain, and for acute pain they are used only for severe pain and in short-duration amounts.
- Analgesia will not make you pain free. It only helps to alleviate some pain, and most pain medications take 1 to 2 hours to take effect.
- A 30% improvement in pain and function can be expected for most therapies.
- Recovery from surgical or acute traumatic injury is not immediate; it is expected to take 2 to 4 weeks.
- Do not take extra medication doses beyond what is prescribed, and tell all of your providers what medications you are taking.
- Do not stop taking opioids suddenly, instead taper the dose slowly as instructed by your provider.
- Dispose of excess drugs appropriately—by taking unused pills back to your provider or crushing the pills, placing them in a small amount of liquid, and putting them in the trash.
“The reality is that the majority of patients are not using as many pills as we give them,” said Dr. Lamvu. “Adequate pain control does not supersede patient safety or the responsibility that we have as providers to our society.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Drug overdose is the leading cause of death for Americans under age 50. And the number of men and women dying from drug overdose shows no abating, with a sharp 17% increase in 2016 over 2015.1 The rate of fatal overdoses rose to nearly 20 people per 100,000 in 2016, according to the Centers for Disease Control and Prevention. The highest death rates are reported in West Virginia, New Hampshire, Kentucky, Ohio, and Rhode Island.2
Dr. Andrew Kolodny, director of opioid policy research at Brandeis University, told The New York Times that there are “roughly two groups of Americans that are getting addicted. We have an older group that is overdosing on pain medicine, and we have a younger group that is overdosing on black market opioids.”1
Are ObGyns contributing to the over-prescription of opioid pain medications? Investigators from Florida Hospital, the University of Florida, and Orlando VA Medical Center are researching the question, and preliminary findings indicate that, yes, in fact “gynecologists are overprescribing postoperative prescription opioids in all levels of gynecologic surgery,” including after laparotomies and major and minor minimally invasive surgeries. Their work thus far, a prospective cohort study involving 113 patients enrolled to date, was presented November 15, 2017, in Washington, DC, as part of the 46th AAGL Global Congress on MIGS. They found that, on average, patients were prescribed 29.6 (SD, 9.3) opioid tablets, and they had 19.1 (SD, 12.6) tablets left over after surgery. Not surprisingly, patients undergoing major minimally invasive surgery and laparotomy were prescribed larger amounts of opioids than those undergoing minor minimally invasive surgery, but the amount of pain medication left over was similar regardless of surgery type.
The researchers also asked patients if they were told how to dispose of their leftover opioids; only 3 patients reported being told what to do if they had leftover medication.
Too many pills prescribed
In separate research presented at the meeting, investigators from Tufts Medical Center and Lahey Hospital and Medical Center in Boston, Massachusetts, sought to determine opioid prescription practices and patient opioid use after benign hysterectomy. Using retrospective online physician and telephone patient surveys, they found that 51 gynecologists prescribed a median of 30 tabs of oxycodone or hydromorphone after abdominal hysterectomy and a median of 20 tabs after laparoscopic or vaginal hysterectomy. Nearly 65% (36/56) of women used less than half of the opioids they were prescribed, and 16.1% (9/56) used zero tabs. Opioid use was not found to be significantly different for women undergoing abdominal versus minimally invasive hysterectomy.
Managing pain expectations
It takes only 3 days of opioid use for a patient to be at risk for continued use (1 to 3 years) of opioids, said Georgine Lamvu, MD, MPH, CPE, in the educational session “Perioperative Management of the Chronic Pain Patient” at the AAGL meeting. And long-term opioid use is associated with addiction, misuse, and mortality.3 It is therefore crucial to understand how to prescribe pain medications and how to educate women on expectations of pain relief.
Dr. Lamvu and fellow presenters described a 4-step process to pain management: 1) assess—including taking a history and physical exam and providing a risk assessment; 2) check—for other medications that a patient may be taking and possible interactions, as well make sure that the patient is not obtaining opioids or benzodiazepines from other providers; 3) discuss—what pain expectations you have for the patient following surgery; and 4) observe—for clinical improvement, overuse, and misuse, and go slow with dose increases and consult support pain management teams when needed.
Overall, they recommended that surgeons perform a risk assessment (determine the risks and benefits of available therapies); educate themselves and their patients on the risks and benefits; and document the risk assessment, the final recommendations to the patient, and the education provided to the patient.
“We need to do a lot better job at educating ourselves and our patients about pain medications and pain management strategies,” said Dr. Lamvu. The presenters provided these key points for patient and family education:
- Opioids are not first-line or routine therapy for chronic pain, and for acute pain they are used only for severe pain and in short-duration amounts.
- Analgesia will not make you pain free. It only helps to alleviate some pain, and most pain medications take 1 to 2 hours to take effect.
- A 30% improvement in pain and function can be expected for most therapies.
- Recovery from surgical or acute traumatic injury is not immediate; it is expected to take 2 to 4 weeks.
- Do not take extra medication doses beyond what is prescribed, and tell all of your providers what medications you are taking.
- Do not stop taking opioids suddenly, instead taper the dose slowly as instructed by your provider.
- Dispose of excess drugs appropriately—by taking unused pills back to your provider or crushing the pills, placing them in a small amount of liquid, and putting them in the trash.
“The reality is that the majority of patients are not using as many pills as we give them,” said Dr. Lamvu. “Adequate pain control does not supersede patient safety or the responsibility that we have as providers to our society.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kaplan S. CDC reports a record jump in drug overdose deaths last year. The New York Times. November 3, 2017. https://www.nytimes.com/2017/11/03/health/deaths-drug-overdose-cdc.html. Accessed November 15, 2017.
- Drug overdose death data. Centers for Disease Control and Prevention website. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Accessed November 15, 2017.
- Wapner J. CDC study finds opioid dependency begins within a few days of initial use. Newsweek. March 22, 2017. http://www.newsweek.com/cdc-opiate-addiction-572498. Accessed November 17, 2017.
- Kaplan S. CDC reports a record jump in drug overdose deaths last year. The New York Times. November 3, 2017. https://www.nytimes.com/2017/11/03/health/deaths-drug-overdose-cdc.html. Accessed November 15, 2017.
- Drug overdose death data. Centers for Disease Control and Prevention website. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Accessed November 15, 2017.
- Wapner J. CDC study finds opioid dependency begins within a few days of initial use. Newsweek. March 22, 2017. http://www.newsweek.com/cdc-opiate-addiction-572498. Accessed November 17, 2017.