User login
13th Annual AVAHO Meeting Set to Begin
This year’s annual Assocaition of VA Hematology and Oncology (AVAHO) conference offers nearly 50 sessions over the course of 3 days that focus on providing evidence-based hematology and oncology practice. According to AVAHO President Mary Thomas, MS, “I am hopeful that people come away from this meeting with a different conception of evidence-based practice and how they might apply it.”
John P.A. Ioannidis, MD, DSc, the C. F. Rehnborg Professor in Disease Prevention in the School of Medicine and Professor of Health Research and Policy (Epidemiology) at Stanford University will offer the opening keynote address on integrating evidence into patient care. According to Ms. Thomas, Dr. Ioannidis is a “provocative speaker” who may challenge conceptions and execution of evidence-based practice. Other keynote addresses will be presented by Steve Harvey, vice president of IBM Watson Health, to discuss the use of artificial intelligence in health care and specifically cancer care and playwright Maggie Edson, winner of a Pulitzer Prize for the play “Wit.” Ms. Edson’s presentation should provide a unique look at the patient experience and a new consideration of patients’ perspectives and values.
The full agenda can be found here: http://www.avaho.org/2017-annual-meeting.html
This year’s annual Assocaition of VA Hematology and Oncology (AVAHO) conference offers nearly 50 sessions over the course of 3 days that focus on providing evidence-based hematology and oncology practice. According to AVAHO President Mary Thomas, MS, “I am hopeful that people come away from this meeting with a different conception of evidence-based practice and how they might apply it.”
John P.A. Ioannidis, MD, DSc, the C. F. Rehnborg Professor in Disease Prevention in the School of Medicine and Professor of Health Research and Policy (Epidemiology) at Stanford University will offer the opening keynote address on integrating evidence into patient care. According to Ms. Thomas, Dr. Ioannidis is a “provocative speaker” who may challenge conceptions and execution of evidence-based practice. Other keynote addresses will be presented by Steve Harvey, vice president of IBM Watson Health, to discuss the use of artificial intelligence in health care and specifically cancer care and playwright Maggie Edson, winner of a Pulitzer Prize for the play “Wit.” Ms. Edson’s presentation should provide a unique look at the patient experience and a new consideration of patients’ perspectives and values.
The full agenda can be found here: http://www.avaho.org/2017-annual-meeting.html
This year’s annual Assocaition of VA Hematology and Oncology (AVAHO) conference offers nearly 50 sessions over the course of 3 days that focus on providing evidence-based hematology and oncology practice. According to AVAHO President Mary Thomas, MS, “I am hopeful that people come away from this meeting with a different conception of evidence-based practice and how they might apply it.”
John P.A. Ioannidis, MD, DSc, the C. F. Rehnborg Professor in Disease Prevention in the School of Medicine and Professor of Health Research and Policy (Epidemiology) at Stanford University will offer the opening keynote address on integrating evidence into patient care. According to Ms. Thomas, Dr. Ioannidis is a “provocative speaker” who may challenge conceptions and execution of evidence-based practice. Other keynote addresses will be presented by Steve Harvey, vice president of IBM Watson Health, to discuss the use of artificial intelligence in health care and specifically cancer care and playwright Maggie Edson, winner of a Pulitzer Prize for the play “Wit.” Ms. Edson’s presentation should provide a unique look at the patient experience and a new consideration of patients’ perspectives and values.
The full agenda can be found here: http://www.avaho.org/2017-annual-meeting.html
How to Have a Good Time in Denver
From Friday September 15 through Sunday September 17, the Association of VA Hematology and Oncology (AVAHO) will be in Denver, Colorado for their 13th Annual Meeting. If you are looking for something to do in the Mile High City outside of the convention center, here are 4 ways to get the most of a little time to spare.
1. Know the best way to get around
Transportation in a new city can be a stressor, especially when you’re on a compressed schedule. In Denver, there are multiple modes of public transportation with inexpensive price tags. The Regional Transportation District (RTD) has bus and light rail options that take you around Denver and the surrounding areas. For only $5.20, you can get a 1-day pass that allows you to hop on and off as you please. Activity duty U.S. military members ride for free on all RTD services. If you want a more open air option to see the city, try Denver B-cycle. The city-wide bike rental allows you to rent a bike and drop it off at any of the racks around town. Just buy an Access Pass for $9 and you’re good to go!
For more info on RTD, visit http://www.rtd-denver.com/index.shtml
For info in Denver B-cycle, visit https://www.denverbcycle.com/
2. Immerse yourself in culture
Denver has a rich culture and history worth exploring. All around the city there are public art exhibitions for viewing, from the 40-foot tall Blue Bear at the Colorado Convention Center to vibrant murals like the “Love This City” series in 3 different art districts. Information on walking tours is available here. Every 2 years, a week long arts and culture festival called The Biennial of the Americas is held in Denver. Fortunately, the biennial coincides with the AVAHO meeting and offers the opportunity to experience symposiums and concerts by artists, leaders, innovators, and experts from North, South, and Central America and the Caribbean.
For more about Denver Arts and culture visit https://www.denver.org/things-to-do/denver-arts-culture/
3. Eat well
Sometimes the best way to enjoy a city is by eating your way around it. In Denver you can get a taste of the old west when trying Rocky Mountain oysters (bull, sheep, or pig testicles) and locally brewed beers or experience a “smooth as silk, tough as nails” master sommelier like Bobby Stuckey. There are 4 thriving foodie neighborhoods; RiNo where the hipsters get sushi and live jazz, LoHi that continues to be a haven for group outings, Uptown for a mix of old and new school Americana eats, and LoDo as a drinking and dining hub perfect for lunch or dinner. All are easy to access from the meeting via public transportation, taxi, or a short walk.
For more about eating around Denver visit https://denver.eater.com/2017/8/29/16180854/best-food-denver-restaurants-city-guide or https://www.thrillist.com/eat/denver
4. Enjoy the scenery
If nature and fresh air is where you find solace, Denver has no shortage of places for you to experience and enjoy. The Denver Parks and Recreation was founded in 1868 and has about 20,00 acres of mountain parkland and urban parks to visit and more than 80 miles of trails within the city. Just steps from the meeting there are paths for walking or jogging that wind along the South Platte River and Cherry Creek. The architecture in Denver is also a great way take in the scenery and a little bit of history too. There’s a healthy mix of old styles like Beaux Arts at Union Station and French Gothic at Cathedral Basilica of the Immaculate Conception, with new modern designs in the Denver Art Museum: Frederic C. Hamilton Building and the Boettcher Memorial Tropical Conservatory. Whether marveling at nature or buildings, there’s plenty to see.
For more on Denver Parks and Recreation, visit https://www.denvergov.org/content/denvergov/en/denver-parks-and-recreation.html
From Friday September 15 through Sunday September 17, the Association of VA Hematology and Oncology (AVAHO) will be in Denver, Colorado for their 13th Annual Meeting. If you are looking for something to do in the Mile High City outside of the convention center, here are 4 ways to get the most of a little time to spare.
1. Know the best way to get around
Transportation in a new city can be a stressor, especially when you’re on a compressed schedule. In Denver, there are multiple modes of public transportation with inexpensive price tags. The Regional Transportation District (RTD) has bus and light rail options that take you around Denver and the surrounding areas. For only $5.20, you can get a 1-day pass that allows you to hop on and off as you please. Activity duty U.S. military members ride for free on all RTD services. If you want a more open air option to see the city, try Denver B-cycle. The city-wide bike rental allows you to rent a bike and drop it off at any of the racks around town. Just buy an Access Pass for $9 and you’re good to go!
For more info on RTD, visit http://www.rtd-denver.com/index.shtml
For info in Denver B-cycle, visit https://www.denverbcycle.com/
2. Immerse yourself in culture
Denver has a rich culture and history worth exploring. All around the city there are public art exhibitions for viewing, from the 40-foot tall Blue Bear at the Colorado Convention Center to vibrant murals like the “Love This City” series in 3 different art districts. Information on walking tours is available here. Every 2 years, a week long arts and culture festival called The Biennial of the Americas is held in Denver. Fortunately, the biennial coincides with the AVAHO meeting and offers the opportunity to experience symposiums and concerts by artists, leaders, innovators, and experts from North, South, and Central America and the Caribbean.
For more about Denver Arts and culture visit https://www.denver.org/things-to-do/denver-arts-culture/
3. Eat well
Sometimes the best way to enjoy a city is by eating your way around it. In Denver you can get a taste of the old west when trying Rocky Mountain oysters (bull, sheep, or pig testicles) and locally brewed beers or experience a “smooth as silk, tough as nails” master sommelier like Bobby Stuckey. There are 4 thriving foodie neighborhoods; RiNo where the hipsters get sushi and live jazz, LoHi that continues to be a haven for group outings, Uptown for a mix of old and new school Americana eats, and LoDo as a drinking and dining hub perfect for lunch or dinner. All are easy to access from the meeting via public transportation, taxi, or a short walk.
For more about eating around Denver visit https://denver.eater.com/2017/8/29/16180854/best-food-denver-restaurants-city-guide or https://www.thrillist.com/eat/denver
4. Enjoy the scenery
If nature and fresh air is where you find solace, Denver has no shortage of places for you to experience and enjoy. The Denver Parks and Recreation was founded in 1868 and has about 20,00 acres of mountain parkland and urban parks to visit and more than 80 miles of trails within the city. Just steps from the meeting there are paths for walking or jogging that wind along the South Platte River and Cherry Creek. The architecture in Denver is also a great way take in the scenery and a little bit of history too. There’s a healthy mix of old styles like Beaux Arts at Union Station and French Gothic at Cathedral Basilica of the Immaculate Conception, with new modern designs in the Denver Art Museum: Frederic C. Hamilton Building and the Boettcher Memorial Tropical Conservatory. Whether marveling at nature or buildings, there’s plenty to see.
For more on Denver Parks and Recreation, visit https://www.denvergov.org/content/denvergov/en/denver-parks-and-recreation.html
From Friday September 15 through Sunday September 17, the Association of VA Hematology and Oncology (AVAHO) will be in Denver, Colorado for their 13th Annual Meeting. If you are looking for something to do in the Mile High City outside of the convention center, here are 4 ways to get the most of a little time to spare.
1. Know the best way to get around
Transportation in a new city can be a stressor, especially when you’re on a compressed schedule. In Denver, there are multiple modes of public transportation with inexpensive price tags. The Regional Transportation District (RTD) has bus and light rail options that take you around Denver and the surrounding areas. For only $5.20, you can get a 1-day pass that allows you to hop on and off as you please. Activity duty U.S. military members ride for free on all RTD services. If you want a more open air option to see the city, try Denver B-cycle. The city-wide bike rental allows you to rent a bike and drop it off at any of the racks around town. Just buy an Access Pass for $9 and you’re good to go!
For more info on RTD, visit http://www.rtd-denver.com/index.shtml
For info in Denver B-cycle, visit https://www.denverbcycle.com/
2. Immerse yourself in culture
Denver has a rich culture and history worth exploring. All around the city there are public art exhibitions for viewing, from the 40-foot tall Blue Bear at the Colorado Convention Center to vibrant murals like the “Love This City” series in 3 different art districts. Information on walking tours is available here. Every 2 years, a week long arts and culture festival called The Biennial of the Americas is held in Denver. Fortunately, the biennial coincides with the AVAHO meeting and offers the opportunity to experience symposiums and concerts by artists, leaders, innovators, and experts from North, South, and Central America and the Caribbean.
For more about Denver Arts and culture visit https://www.denver.org/things-to-do/denver-arts-culture/
3. Eat well
Sometimes the best way to enjoy a city is by eating your way around it. In Denver you can get a taste of the old west when trying Rocky Mountain oysters (bull, sheep, or pig testicles) and locally brewed beers or experience a “smooth as silk, tough as nails” master sommelier like Bobby Stuckey. There are 4 thriving foodie neighborhoods; RiNo where the hipsters get sushi and live jazz, LoHi that continues to be a haven for group outings, Uptown for a mix of old and new school Americana eats, and LoDo as a drinking and dining hub perfect for lunch or dinner. All are easy to access from the meeting via public transportation, taxi, or a short walk.
For more about eating around Denver visit https://denver.eater.com/2017/8/29/16180854/best-food-denver-restaurants-city-guide or https://www.thrillist.com/eat/denver
4. Enjoy the scenery
If nature and fresh air is where you find solace, Denver has no shortage of places for you to experience and enjoy. The Denver Parks and Recreation was founded in 1868 and has about 20,00 acres of mountain parkland and urban parks to visit and more than 80 miles of trails within the city. Just steps from the meeting there are paths for walking or jogging that wind along the South Platte River and Cherry Creek. The architecture in Denver is also a great way take in the scenery and a little bit of history too. There’s a healthy mix of old styles like Beaux Arts at Union Station and French Gothic at Cathedral Basilica of the Immaculate Conception, with new modern designs in the Denver Art Museum: Frederic C. Hamilton Building and the Boettcher Memorial Tropical Conservatory. Whether marveling at nature or buildings, there’s plenty to see.
For more on Denver Parks and Recreation, visit https://www.denvergov.org/content/denvergov/en/denver-parks-and-recreation.html
Report details progress, obstacles in cancer research and care
Deaths from cancer are on the decline in the US, but new cases of cancer are on the rise, according to the 7th annual American Association for Cancer Research (AACR) Cancer Progress Report.
The data suggest the cancer death rate declined by 35% from 1991 to 2014 for children and by 25% for adults, a reduction that translates to 2.1 million cancer deaths avoided.
However, 600,920 people in the US are projected to die from cancer in 2017.
And the number of new cancer cases is predicted to rise from 1.7 million in 2017 to 2.3 million in 2030.
The report also estimates there will be 62,130 new cases of leukemia in 2017 and 24,500 leukemia deaths this year.
This includes:
- 5970 cases of acute lymphocytic leukemia and 1440 deaths
- 20,110 cases of chronic lymphocytic leukemia and 4660 deaths
- 21,380 cases of acute myeloid leukemia (AML) and 10,590 deaths
- 8950 cases of chronic myeloid leukemia and 1080 deaths.
The estimate for lymphomas is 80,500 new cases and 21,210 deaths.
This includes:
- 8260 cases of Hodgkin lymphoma (HL) and 1070 deaths
- 72,240 cases of non-Hodgkin lymphoma and 20,140 deaths.
The estimate for myeloma is 30,280 new cases and 12,590 deaths.
The report says the estimated new cases of cancer are based on cancer incidence rates from 49 states and the District of Columbia from 1995 through 2013, as reported by the North American Association of Central Cancer Registries. This represents about 98% of the US population.
The estimated deaths are based on US mortality data from 1997 through 2013, taken from the National Center for Health Statistics of the Centers for Disease Control and Prevention.
Drug approvals
The AACR report notes that, between August 1, 2016, and July 31, 2017, the US Food and Drug Administration (FDA) approved new uses for 15 anticancer agents, 9 of which had no previous FDA approval.
Five of the agents are immunotherapies, which the report dubs “revolutionary treatments that are increasing survival and improving quality of life for patients.”
Among the recently approved therapies are 3 used for hematology indications:
- Ibrutinib (Imbruvica), approved to treat patients with relapsed/refractory marginal zone lymphoma who require systemic therapy and have received at least 1 prior anti-CD20-based therapy
- Midostaurin (Rydapt), approved as monotherapy for adults with advanced systemic mastocytosis and for use in combination with standard cytarabine and daunorubicin induction, followed by cytarabine consolidation, in adults with newly diagnosed AML who are FLT3 mutation-positive, as detected by an FDA-approved test.
- Pembrolizumab (Keytruda), approved to treat adult and pediatric patients with refractory classical HL or those with classical HL who have relapsed after 3 or more prior lines of therapy.
Disparities and costs
The AACR report points out that advances against cancer have not benefited everyone equally, and cancer health disparities are some of the most pressing challenges.
Among the disparities listed is the fact that adolescents and young adults (ages 15 to 39) with AML have a 5-year relative survival rate that is 22% lower than that of children (ages 1 to 14) with AML.
And Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
Another concern mentioned in the report is the cost of cancer care. The direct medical costs of cancer care in 2014 were estimated to be nearly $87.6 billion. This number does not include the indirect costs of lost productivity due to cancer-related morbidity and mortality.
With this in mind, the AACR is calling for a $2 billion increase in funding for the National Institutes of Health in fiscal year 2018, for a total funding level of $36.2 billion.
The AACR also recommends an $80 million increase in the FDA budget, bringing it to $2.8 billion for fiscal year 2018. ![]()
Deaths from cancer are on the decline in the US, but new cases of cancer are on the rise, according to the 7th annual American Association for Cancer Research (AACR) Cancer Progress Report.
The data suggest the cancer death rate declined by 35% from 1991 to 2014 for children and by 25% for adults, a reduction that translates to 2.1 million cancer deaths avoided.
However, 600,920 people in the US are projected to die from cancer in 2017.
And the number of new cancer cases is predicted to rise from 1.7 million in 2017 to 2.3 million in 2030.
The report also estimates there will be 62,130 new cases of leukemia in 2017 and 24,500 leukemia deaths this year.
This includes:
- 5970 cases of acute lymphocytic leukemia and 1440 deaths
- 20,110 cases of chronic lymphocytic leukemia and 4660 deaths
- 21,380 cases of acute myeloid leukemia (AML) and 10,590 deaths
- 8950 cases of chronic myeloid leukemia and 1080 deaths.
The estimate for lymphomas is 80,500 new cases and 21,210 deaths.
This includes:
- 8260 cases of Hodgkin lymphoma (HL) and 1070 deaths
- 72,240 cases of non-Hodgkin lymphoma and 20,140 deaths.
The estimate for myeloma is 30,280 new cases and 12,590 deaths.
The report says the estimated new cases of cancer are based on cancer incidence rates from 49 states and the District of Columbia from 1995 through 2013, as reported by the North American Association of Central Cancer Registries. This represents about 98% of the US population.
The estimated deaths are based on US mortality data from 1997 through 2013, taken from the National Center for Health Statistics of the Centers for Disease Control and Prevention.
Drug approvals
The AACR report notes that, between August 1, 2016, and July 31, 2017, the US Food and Drug Administration (FDA) approved new uses for 15 anticancer agents, 9 of which had no previous FDA approval.
Five of the agents are immunotherapies, which the report dubs “revolutionary treatments that are increasing survival and improving quality of life for patients.”
Among the recently approved therapies are 3 used for hematology indications:
- Ibrutinib (Imbruvica), approved to treat patients with relapsed/refractory marginal zone lymphoma who require systemic therapy and have received at least 1 prior anti-CD20-based therapy
- Midostaurin (Rydapt), approved as monotherapy for adults with advanced systemic mastocytosis and for use in combination with standard cytarabine and daunorubicin induction, followed by cytarabine consolidation, in adults with newly diagnosed AML who are FLT3 mutation-positive, as detected by an FDA-approved test.
- Pembrolizumab (Keytruda), approved to treat adult and pediatric patients with refractory classical HL or those with classical HL who have relapsed after 3 or more prior lines of therapy.
Disparities and costs
The AACR report points out that advances against cancer have not benefited everyone equally, and cancer health disparities are some of the most pressing challenges.
Among the disparities listed is the fact that adolescents and young adults (ages 15 to 39) with AML have a 5-year relative survival rate that is 22% lower than that of children (ages 1 to 14) with AML.
And Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
Another concern mentioned in the report is the cost of cancer care. The direct medical costs of cancer care in 2014 were estimated to be nearly $87.6 billion. This number does not include the indirect costs of lost productivity due to cancer-related morbidity and mortality.
With this in mind, the AACR is calling for a $2 billion increase in funding for the National Institutes of Health in fiscal year 2018, for a total funding level of $36.2 billion.
The AACR also recommends an $80 million increase in the FDA budget, bringing it to $2.8 billion for fiscal year 2018. ![]()
Deaths from cancer are on the decline in the US, but new cases of cancer are on the rise, according to the 7th annual American Association for Cancer Research (AACR) Cancer Progress Report.
The data suggest the cancer death rate declined by 35% from 1991 to 2014 for children and by 25% for adults, a reduction that translates to 2.1 million cancer deaths avoided.
However, 600,920 people in the US are projected to die from cancer in 2017.
And the number of new cancer cases is predicted to rise from 1.7 million in 2017 to 2.3 million in 2030.
The report also estimates there will be 62,130 new cases of leukemia in 2017 and 24,500 leukemia deaths this year.
This includes:
- 5970 cases of acute lymphocytic leukemia and 1440 deaths
- 20,110 cases of chronic lymphocytic leukemia and 4660 deaths
- 21,380 cases of acute myeloid leukemia (AML) and 10,590 deaths
- 8950 cases of chronic myeloid leukemia and 1080 deaths.
The estimate for lymphomas is 80,500 new cases and 21,210 deaths.
This includes:
- 8260 cases of Hodgkin lymphoma (HL) and 1070 deaths
- 72,240 cases of non-Hodgkin lymphoma and 20,140 deaths.
The estimate for myeloma is 30,280 new cases and 12,590 deaths.
The report says the estimated new cases of cancer are based on cancer incidence rates from 49 states and the District of Columbia from 1995 through 2013, as reported by the North American Association of Central Cancer Registries. This represents about 98% of the US population.
The estimated deaths are based on US mortality data from 1997 through 2013, taken from the National Center for Health Statistics of the Centers for Disease Control and Prevention.
Drug approvals
The AACR report notes that, between August 1, 2016, and July 31, 2017, the US Food and Drug Administration (FDA) approved new uses for 15 anticancer agents, 9 of which had no previous FDA approval.
Five of the agents are immunotherapies, which the report dubs “revolutionary treatments that are increasing survival and improving quality of life for patients.”
Among the recently approved therapies are 3 used for hematology indications:
- Ibrutinib (Imbruvica), approved to treat patients with relapsed/refractory marginal zone lymphoma who require systemic therapy and have received at least 1 prior anti-CD20-based therapy
- Midostaurin (Rydapt), approved as monotherapy for adults with advanced systemic mastocytosis and for use in combination with standard cytarabine and daunorubicin induction, followed by cytarabine consolidation, in adults with newly diagnosed AML who are FLT3 mutation-positive, as detected by an FDA-approved test.
- Pembrolizumab (Keytruda), approved to treat adult and pediatric patients with refractory classical HL or those with classical HL who have relapsed after 3 or more prior lines of therapy.
Disparities and costs
The AACR report points out that advances against cancer have not benefited everyone equally, and cancer health disparities are some of the most pressing challenges.
Among the disparities listed is the fact that adolescents and young adults (ages 15 to 39) with AML have a 5-year relative survival rate that is 22% lower than that of children (ages 1 to 14) with AML.
And Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
Another concern mentioned in the report is the cost of cancer care. The direct medical costs of cancer care in 2014 were estimated to be nearly $87.6 billion. This number does not include the indirect costs of lost productivity due to cancer-related morbidity and mortality.
With this in mind, the AACR is calling for a $2 billion increase in funding for the National Institutes of Health in fiscal year 2018, for a total funding level of $36.2 billion.
The AACR also recommends an $80 million increase in the FDA budget, bringing it to $2.8 billion for fiscal year 2018. ![]()
Study raises questions about anemia in astronauts
Previous research has suggested that astronauts develop anemia during space flight, but a new study indicates this is not the case for astronauts on long space missions.
“There is an idea of ‘space anemia’ that is associated with space flight,” said Richard Simpson, PhD, of the University of Houston in Texas.
“However, this is based on blood samples from astronauts collected after flight, which may be influenced by various factors—for example, the stress of landing and re-adaptation to conditions on Earth.”
“For this study . . ., living, whole blood samples were collected during space flight and returned to Earth for analysis. This unique sample allowed us to track hematological parameters—such as concentrations of red blood cells, hemoglobin, or hematocrit—in astronauts on board the International Space Station during flight.”
Dr Simpson and his colleagues reported their findings in BMC Hematology.
The researchers found that, during space flight, concentrations of red blood cells (RBCs), platelets, and hemoglobin were higher compared to pre-flight levels. Hematocrit also increased significantly during space flight.
While previous studies had shown this to be the case during the first few days of flight, this is the first study to show that RBC concentrations and hematocrit remain at higher levels even after astronauts’ bodies have adapted to microgravity.
To find out how the blood of astronauts may change if they spend a long time in space, the researchers collected blood samples from 31 astronauts who spent up to 6 months on the International Space Station (ISS). There were 6 female and 25 male subjects, and their mean age was 52 (range, 38–61).
Samples were collected at 180 days and 45 days before the astronauts flew to the ISS. Blood was also collected while they were in space—during the first 2 weeks (early time point), between 2 and 4 months (mid time point), and about 6 months into the mission (late time point).
Samples were returned to Earth for analysis either in Houston or at Star City, Russia, within 48 hours of collection. Post-flight samples were collected 3 to 8 hours after landing and 30 days after the mission had ended.
Results
The researchers said some of the changes observed in the in-flight blood samples were to be expected due to the 48-hour processing delay between sample collection and analysis.
However, the team found that RBC concentration was significantly elevated at all 3 in-flight time points, when compared to the 180-day pre-mission time point (baseline). And RBC counts returned to baseline levels upon Earth landing.
The mean RBC concentrations (x 106 cells/μL) were:
- 4.4 ± 0.4 (range, 3.5–5.1) at baseline
- 4.8 ± 0.5 (range, 3.9–5.7) at the early time point (P<0.05)
- 4.7 ± 0.4 (range, 3.9–5.4) at the mid time point (P<0.05)
- 4.7 ± 0.4 (range, 4.1–5.6) at the late time point (P<0.05).
Hemoglobin was elevated early in flight but returned to pre-flight levels during the mission and fell below baseline levels on landing day. The mean hemoglobin (g/dL) levels were:
- 14.1 ± 1.4 (range, 11.0–17.8) at baseline
- 15.0 ± 1.9 (range, 10.7–17.5) at the early time point (P<0.05)
- 13.5 ± 1.4 (range, 10.1–15.9) on landing day (P<0.05).
Mean corpuscular hemoglobin (MCH) decreased during the mission and was significantly lower than baseline at the late time point. However, MCH returned to pre-flight values upon landing. The mean MCH (pg) was:
- 31.7 ± 1.6 (range, 28.8–36.4) at baseline
- 31.3 ± 1.9 (range 26.3–34.0) at the late time point (P<0.05).
The researchers said they observed significant increases in mean corpuscular volume (MCV) during space flight. However, these reflect the changes observed following the 48-hour processing delay. So there were no variations in MCV attributable to space flight.
The changes observed in hematocrit during space flight were “striking,” according to the researchers. The mean hematocrit levels were:
- 40.9 ± 3.9 (range, 33.1–48.0) at baseline
- 45.9 ± 4.7 (range 38.2–52.1) at the early time point (P<0.05)
- 45.9 ± 5.5 (range 38.9–58.3) at the mid time point (P<0.05)
- 45.0 ± 2.5 (range 38.9–49.9) at the late time point (P<0.05).
Compared to pre-flight levels, hematocrit increased by 12.2% at early, 12.2% at mid, and 10.0% at late time points. In comparison, there was a 4.7% increase in hematocrit in reference samples from non-astronauts after the 48-hour processing delay.
Finally, the researchers found that platelet concentrations were elevated in flight, at the early and mid time points. However, platelets were not significantly elevated at the late time point, and they were stable upon landing.
For all of the astronauts, all blood parameters returned to pre-flight levels within 30 days of landing on Earth.
The researchers said these results are susceptible to the possible influence of dehydration or plasma volume alterations. However, the findings do suggest the increases observed in the ISS samples are partly due to a true in-flight increase in RBC count.
“Although the data does not indicate that significant anemia is present, it must be interpreted in the context of crew plasma volume during flight,” Dr Simpson said. “Overall plasma volume has been shown to be reduced during space flight, but this has not been assessed during long-duration missions.”
“In order to fully interpret the changes to RBC, hematocrit, and other parameters observed in this study, further research into plasma volume during long space missions is needed. This will be addressed in a separate, ongoing NASA investigation.” ![]()
Previous research has suggested that astronauts develop anemia during space flight, but a new study indicates this is not the case for astronauts on long space missions.
“There is an idea of ‘space anemia’ that is associated with space flight,” said Richard Simpson, PhD, of the University of Houston in Texas.
“However, this is based on blood samples from astronauts collected after flight, which may be influenced by various factors—for example, the stress of landing and re-adaptation to conditions on Earth.”
“For this study . . ., living, whole blood samples were collected during space flight and returned to Earth for analysis. This unique sample allowed us to track hematological parameters—such as concentrations of red blood cells, hemoglobin, or hematocrit—in astronauts on board the International Space Station during flight.”
Dr Simpson and his colleagues reported their findings in BMC Hematology.
The researchers found that, during space flight, concentrations of red blood cells (RBCs), platelets, and hemoglobin were higher compared to pre-flight levels. Hematocrit also increased significantly during space flight.
While previous studies had shown this to be the case during the first few days of flight, this is the first study to show that RBC concentrations and hematocrit remain at higher levels even after astronauts’ bodies have adapted to microgravity.
To find out how the blood of astronauts may change if they spend a long time in space, the researchers collected blood samples from 31 astronauts who spent up to 6 months on the International Space Station (ISS). There were 6 female and 25 male subjects, and their mean age was 52 (range, 38–61).
Samples were collected at 180 days and 45 days before the astronauts flew to the ISS. Blood was also collected while they were in space—during the first 2 weeks (early time point), between 2 and 4 months (mid time point), and about 6 months into the mission (late time point).
Samples were returned to Earth for analysis either in Houston or at Star City, Russia, within 48 hours of collection. Post-flight samples were collected 3 to 8 hours after landing and 30 days after the mission had ended.
Results
The researchers said some of the changes observed in the in-flight blood samples were to be expected due to the 48-hour processing delay between sample collection and analysis.
However, the team found that RBC concentration was significantly elevated at all 3 in-flight time points, when compared to the 180-day pre-mission time point (baseline). And RBC counts returned to baseline levels upon Earth landing.
The mean RBC concentrations (x 106 cells/μL) were:
- 4.4 ± 0.4 (range, 3.5–5.1) at baseline
- 4.8 ± 0.5 (range, 3.9–5.7) at the early time point (P<0.05)
- 4.7 ± 0.4 (range, 3.9–5.4) at the mid time point (P<0.05)
- 4.7 ± 0.4 (range, 4.1–5.6) at the late time point (P<0.05).
Hemoglobin was elevated early in flight but returned to pre-flight levels during the mission and fell below baseline levels on landing day. The mean hemoglobin (g/dL) levels were:
- 14.1 ± 1.4 (range, 11.0–17.8) at baseline
- 15.0 ± 1.9 (range, 10.7–17.5) at the early time point (P<0.05)
- 13.5 ± 1.4 (range, 10.1–15.9) on landing day (P<0.05).
Mean corpuscular hemoglobin (MCH) decreased during the mission and was significantly lower than baseline at the late time point. However, MCH returned to pre-flight values upon landing. The mean MCH (pg) was:
- 31.7 ± 1.6 (range, 28.8–36.4) at baseline
- 31.3 ± 1.9 (range 26.3–34.0) at the late time point (P<0.05).
The researchers said they observed significant increases in mean corpuscular volume (MCV) during space flight. However, these reflect the changes observed following the 48-hour processing delay. So there were no variations in MCV attributable to space flight.
The changes observed in hematocrit during space flight were “striking,” according to the researchers. The mean hematocrit levels were:
- 40.9 ± 3.9 (range, 33.1–48.0) at baseline
- 45.9 ± 4.7 (range 38.2–52.1) at the early time point (P<0.05)
- 45.9 ± 5.5 (range 38.9–58.3) at the mid time point (P<0.05)
- 45.0 ± 2.5 (range 38.9–49.9) at the late time point (P<0.05).
Compared to pre-flight levels, hematocrit increased by 12.2% at early, 12.2% at mid, and 10.0% at late time points. In comparison, there was a 4.7% increase in hematocrit in reference samples from non-astronauts after the 48-hour processing delay.
Finally, the researchers found that platelet concentrations were elevated in flight, at the early and mid time points. However, platelets were not significantly elevated at the late time point, and they were stable upon landing.
For all of the astronauts, all blood parameters returned to pre-flight levels within 30 days of landing on Earth.
The researchers said these results are susceptible to the possible influence of dehydration or plasma volume alterations. However, the findings do suggest the increases observed in the ISS samples are partly due to a true in-flight increase in RBC count.
“Although the data does not indicate that significant anemia is present, it must be interpreted in the context of crew plasma volume during flight,” Dr Simpson said. “Overall plasma volume has been shown to be reduced during space flight, but this has not been assessed during long-duration missions.”
“In order to fully interpret the changes to RBC, hematocrit, and other parameters observed in this study, further research into plasma volume during long space missions is needed. This will be addressed in a separate, ongoing NASA investigation.” ![]()
Previous research has suggested that astronauts develop anemia during space flight, but a new study indicates this is not the case for astronauts on long space missions.
“There is an idea of ‘space anemia’ that is associated with space flight,” said Richard Simpson, PhD, of the University of Houston in Texas.
“However, this is based on blood samples from astronauts collected after flight, which may be influenced by various factors—for example, the stress of landing and re-adaptation to conditions on Earth.”
“For this study . . ., living, whole blood samples were collected during space flight and returned to Earth for analysis. This unique sample allowed us to track hematological parameters—such as concentrations of red blood cells, hemoglobin, or hematocrit—in astronauts on board the International Space Station during flight.”
Dr Simpson and his colleagues reported their findings in BMC Hematology.
The researchers found that, during space flight, concentrations of red blood cells (RBCs), platelets, and hemoglobin were higher compared to pre-flight levels. Hematocrit also increased significantly during space flight.
While previous studies had shown this to be the case during the first few days of flight, this is the first study to show that RBC concentrations and hematocrit remain at higher levels even after astronauts’ bodies have adapted to microgravity.
To find out how the blood of astronauts may change if they spend a long time in space, the researchers collected blood samples from 31 astronauts who spent up to 6 months on the International Space Station (ISS). There were 6 female and 25 male subjects, and their mean age was 52 (range, 38–61).
Samples were collected at 180 days and 45 days before the astronauts flew to the ISS. Blood was also collected while they were in space—during the first 2 weeks (early time point), between 2 and 4 months (mid time point), and about 6 months into the mission (late time point).
Samples were returned to Earth for analysis either in Houston or at Star City, Russia, within 48 hours of collection. Post-flight samples were collected 3 to 8 hours after landing and 30 days after the mission had ended.
Results
The researchers said some of the changes observed in the in-flight blood samples were to be expected due to the 48-hour processing delay between sample collection and analysis.
However, the team found that RBC concentration was significantly elevated at all 3 in-flight time points, when compared to the 180-day pre-mission time point (baseline). And RBC counts returned to baseline levels upon Earth landing.
The mean RBC concentrations (x 106 cells/μL) were:
- 4.4 ± 0.4 (range, 3.5–5.1) at baseline
- 4.8 ± 0.5 (range, 3.9–5.7) at the early time point (P<0.05)
- 4.7 ± 0.4 (range, 3.9–5.4) at the mid time point (P<0.05)
- 4.7 ± 0.4 (range, 4.1–5.6) at the late time point (P<0.05).
Hemoglobin was elevated early in flight but returned to pre-flight levels during the mission and fell below baseline levels on landing day. The mean hemoglobin (g/dL) levels were:
- 14.1 ± 1.4 (range, 11.0–17.8) at baseline
- 15.0 ± 1.9 (range, 10.7–17.5) at the early time point (P<0.05)
- 13.5 ± 1.4 (range, 10.1–15.9) on landing day (P<0.05).
Mean corpuscular hemoglobin (MCH) decreased during the mission and was significantly lower than baseline at the late time point. However, MCH returned to pre-flight values upon landing. The mean MCH (pg) was:
- 31.7 ± 1.6 (range, 28.8–36.4) at baseline
- 31.3 ± 1.9 (range 26.3–34.0) at the late time point (P<0.05).
The researchers said they observed significant increases in mean corpuscular volume (MCV) during space flight. However, these reflect the changes observed following the 48-hour processing delay. So there were no variations in MCV attributable to space flight.
The changes observed in hematocrit during space flight were “striking,” according to the researchers. The mean hematocrit levels were:
- 40.9 ± 3.9 (range, 33.1–48.0) at baseline
- 45.9 ± 4.7 (range 38.2–52.1) at the early time point (P<0.05)
- 45.9 ± 5.5 (range 38.9–58.3) at the mid time point (P<0.05)
- 45.0 ± 2.5 (range 38.9–49.9) at the late time point (P<0.05).
Compared to pre-flight levels, hematocrit increased by 12.2% at early, 12.2% at mid, and 10.0% at late time points. In comparison, there was a 4.7% increase in hematocrit in reference samples from non-astronauts after the 48-hour processing delay.
Finally, the researchers found that platelet concentrations were elevated in flight, at the early and mid time points. However, platelets were not significantly elevated at the late time point, and they were stable upon landing.
For all of the astronauts, all blood parameters returned to pre-flight levels within 30 days of landing on Earth.
The researchers said these results are susceptible to the possible influence of dehydration or plasma volume alterations. However, the findings do suggest the increases observed in the ISS samples are partly due to a true in-flight increase in RBC count.
“Although the data does not indicate that significant anemia is present, it must be interpreted in the context of crew plasma volume during flight,” Dr Simpson said. “Overall plasma volume has been shown to be reduced during space flight, but this has not been assessed during long-duration missions.”
“In order to fully interpret the changes to RBC, hematocrit, and other parameters observed in this study, further research into plasma volume during long space missions is needed. This will be addressed in a separate, ongoing NASA investigation.” ![]()
FDA grants priority review for drug to treat APL
The US Food and Drug Administration (FDA) has accepted for priority review a supplemental new drug application (sNDA) for arsenic trioxide (TRISENOX®) injection.
With this sNDA, Teva Pharmaceutical Industries Ltd. is seeking approval for arsenic trioxide to be used in combination with all-trans retinoic acid (ATRA) for induction of remission and consolidation in patients with newly diagnosed, low- or intermediate-risk acute promyelocytic leukemia (APL) characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
Currently, arsenic trioxide is FDA-approved as monotherapy for induction of remission and consolidation in patients with APL who are refractory to, or have relapsed from, retinoid and anthracycline chemotherapy, and whose APL is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
For more details, see the full prescribing information.
The FDA has accepted the arsenic trioxide sNDA for priority review and expects to make a decision on the application in the first quarter of 2018.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
Phase 3 study results
The sNDA for arsenic trioxide is supported by results from the APL0406 study. Updated results from this phase 3 study were published in the Journal of Clinical Oncology in February.
The study included 276 adults (ages 18 to 71) with newly diagnosed, low- or intermediate-risk APL. Patients were randomized to receive ATRA plus arsenic trioxide or ATRA plus chemotherapy.
A total of 263 patients were evaluable for response to induction. One hundred percent of patients in the arsenic trioxide arm (127/127) achieved a complete response (CR), as did 97% (132/136) of patients in the chemotherapy arm (P=0.12).
After a median follow-up of 40.6 months, the event-free survival was 97.3% in the arsenic trioxide arm and 80% in the chemotherapy arm (P<0.001). The cumulative incidence of relapse was 1.9% and 13.9%, respectively (P=0.0013).
At 50 months, the overall survival was 99.2% in the arsenic trioxide arm and 92.6% in the chemotherapy arm (P=0.0073).
After induction, there were 2 relapses and 1 death in CR in the arsenic trioxide arm.
In the chemotherapy arm, there were 2 instances of molecular resistance after third consolidation, 15 relapses, 5 deaths in CR, and 2 patients who developed a therapy-related myeloid neoplasm. ![]()
The US Food and Drug Administration (FDA) has accepted for priority review a supplemental new drug application (sNDA) for arsenic trioxide (TRISENOX®) injection.
With this sNDA, Teva Pharmaceutical Industries Ltd. is seeking approval for arsenic trioxide to be used in combination with all-trans retinoic acid (ATRA) for induction of remission and consolidation in patients with newly diagnosed, low- or intermediate-risk acute promyelocytic leukemia (APL) characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
Currently, arsenic trioxide is FDA-approved as monotherapy for induction of remission and consolidation in patients with APL who are refractory to, or have relapsed from, retinoid and anthracycline chemotherapy, and whose APL is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
For more details, see the full prescribing information.
The FDA has accepted the arsenic trioxide sNDA for priority review and expects to make a decision on the application in the first quarter of 2018.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
Phase 3 study results
The sNDA for arsenic trioxide is supported by results from the APL0406 study. Updated results from this phase 3 study were published in the Journal of Clinical Oncology in February.
The study included 276 adults (ages 18 to 71) with newly diagnosed, low- or intermediate-risk APL. Patients were randomized to receive ATRA plus arsenic trioxide or ATRA plus chemotherapy.
A total of 263 patients were evaluable for response to induction. One hundred percent of patients in the arsenic trioxide arm (127/127) achieved a complete response (CR), as did 97% (132/136) of patients in the chemotherapy arm (P=0.12).
After a median follow-up of 40.6 months, the event-free survival was 97.3% in the arsenic trioxide arm and 80% in the chemotherapy arm (P<0.001). The cumulative incidence of relapse was 1.9% and 13.9%, respectively (P=0.0013).
At 50 months, the overall survival was 99.2% in the arsenic trioxide arm and 92.6% in the chemotherapy arm (P=0.0073).
After induction, there were 2 relapses and 1 death in CR in the arsenic trioxide arm.
In the chemotherapy arm, there were 2 instances of molecular resistance after third consolidation, 15 relapses, 5 deaths in CR, and 2 patients who developed a therapy-related myeloid neoplasm. ![]()
The US Food and Drug Administration (FDA) has accepted for priority review a supplemental new drug application (sNDA) for arsenic trioxide (TRISENOX®) injection.
With this sNDA, Teva Pharmaceutical Industries Ltd. is seeking approval for arsenic trioxide to be used in combination with all-trans retinoic acid (ATRA) for induction of remission and consolidation in patients with newly diagnosed, low- or intermediate-risk acute promyelocytic leukemia (APL) characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
Currently, arsenic trioxide is FDA-approved as monotherapy for induction of remission and consolidation in patients with APL who are refractory to, or have relapsed from, retinoid and anthracycline chemotherapy, and whose APL is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
For more details, see the full prescribing information.
The FDA has accepted the arsenic trioxide sNDA for priority review and expects to make a decision on the application in the first quarter of 2018.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
Phase 3 study results
The sNDA for arsenic trioxide is supported by results from the APL0406 study. Updated results from this phase 3 study were published in the Journal of Clinical Oncology in February.
The study included 276 adults (ages 18 to 71) with newly diagnosed, low- or intermediate-risk APL. Patients were randomized to receive ATRA plus arsenic trioxide or ATRA plus chemotherapy.
A total of 263 patients were evaluable for response to induction. One hundred percent of patients in the arsenic trioxide arm (127/127) achieved a complete response (CR), as did 97% (132/136) of patients in the chemotherapy arm (P=0.12).
After a median follow-up of 40.6 months, the event-free survival was 97.3% in the arsenic trioxide arm and 80% in the chemotherapy arm (P<0.001). The cumulative incidence of relapse was 1.9% and 13.9%, respectively (P=0.0013).
At 50 months, the overall survival was 99.2% in the arsenic trioxide arm and 92.6% in the chemotherapy arm (P=0.0073).
After induction, there were 2 relapses and 1 death in CR in the arsenic trioxide arm.
In the chemotherapy arm, there were 2 instances of molecular resistance after third consolidation, 15 relapses, 5 deaths in CR, and 2 patients who developed a therapy-related myeloid neoplasm. ![]()
Rash on trunk and upper arms
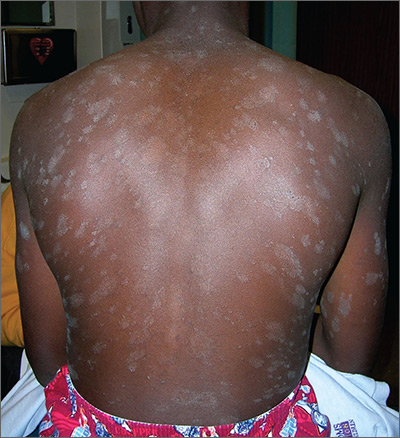
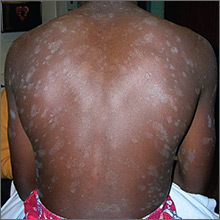
The FP recognized a “Christmas tree” pattern on the patient’s back, which led to a diagnosis of pityriasis rosea. Because there are no lab tests to confirm pityriasis rosea, it is usually a clinical diagnosis.
The Christmas tree pattern that signals pityriasis rosea is caused by the long axis of the plaques following the skin lines of the back. While it is a helpful indicator of pityriasis rosea when present, most cases do not show this pattern. In this case, a collarette scale pattern was also visible. This is seen when scaling is visibly raised on the edge of the plaques, like the collar of a shirt.
Pityriasis rosea can be difficult to distinguish from secondary syphilis, which can also present as a papulosquamous eruption. Therefore, taking a sexual history is important when a diagnosis of pityriasis rosea is being considered. In patients with a history of sexually transmitted diseases or high-risk sexual practices, a rapid plasma reagin test should be ordered.
The FP assured the patient and his mother that the pityriasis rosea rash was not contagious, would not cause scarring, and would resolve in one to 2 months without any treatment. The patient declined any medication for the pruritus. At a subsequent visit for a sports physical, the skin was found to be completely clear with no evidence of scarring.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D, Usatine R. Pityriasis rosea. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 896-900.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP recognized a “Christmas tree” pattern on the patient’s back, which led to a diagnosis of pityriasis rosea. Because there are no lab tests to confirm pityriasis rosea, it is usually a clinical diagnosis.
The Christmas tree pattern that signals pityriasis rosea is caused by the long axis of the plaques following the skin lines of the back. While it is a helpful indicator of pityriasis rosea when present, most cases do not show this pattern. In this case, a collarette scale pattern was also visible. This is seen when scaling is visibly raised on the edge of the plaques, like the collar of a shirt.
Pityriasis rosea can be difficult to distinguish from secondary syphilis, which can also present as a papulosquamous eruption. Therefore, taking a sexual history is important when a diagnosis of pityriasis rosea is being considered. In patients with a history of sexually transmitted diseases or high-risk sexual practices, a rapid plasma reagin test should be ordered.
The FP assured the patient and his mother that the pityriasis rosea rash was not contagious, would not cause scarring, and would resolve in one to 2 months without any treatment. The patient declined any medication for the pruritus. At a subsequent visit for a sports physical, the skin was found to be completely clear with no evidence of scarring.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D, Usatine R. Pityriasis rosea. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 896-900.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP recognized a “Christmas tree” pattern on the patient’s back, which led to a diagnosis of pityriasis rosea. Because there are no lab tests to confirm pityriasis rosea, it is usually a clinical diagnosis.
The Christmas tree pattern that signals pityriasis rosea is caused by the long axis of the plaques following the skin lines of the back. While it is a helpful indicator of pityriasis rosea when present, most cases do not show this pattern. In this case, a collarette scale pattern was also visible. This is seen when scaling is visibly raised on the edge of the plaques, like the collar of a shirt.
Pityriasis rosea can be difficult to distinguish from secondary syphilis, which can also present as a papulosquamous eruption. Therefore, taking a sexual history is important when a diagnosis of pityriasis rosea is being considered. In patients with a history of sexually transmitted diseases or high-risk sexual practices, a rapid plasma reagin test should be ordered.
The FP assured the patient and his mother that the pityriasis rosea rash was not contagious, would not cause scarring, and would resolve in one to 2 months without any treatment. The patient declined any medication for the pruritus. At a subsequent visit for a sports physical, the skin was found to be completely clear with no evidence of scarring.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D, Usatine R. Pityriasis rosea. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 896-900.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
There’s More Where That Came From
ANSWER
The correct answer is pityriasis rosea (choice “d”).
Virtually all patients with this condition believe that they have a terrible case of “ringworm” (ie, fungal infection; choice “a”)—an opinion all too often corroborated by the medical provider unacquainted with pityriasis rosea. The two can be difficult to distinguish, but the “herald” patch, oval shape, odd color, and fine scale all serve to confirm the diagnosis. When necessary, a KOH prep or biopsy can be done.
Psoriasis (choice “b”) can manifest acutely, but it is distinctly salmon-pink under coarse, white scale that affects the palms, nails (with pits), and scalp. Psoriasis lesions are round (rather than oval), with coarser scale and without a herald patch.
The lack of antecedent sores and denial of sexual contact ruled out secondary syphilis (choice “c”). Patients with secondary syphilis often present with low-grade fever, malaise, and palmar scaly papules—all of which were missing in this case. Syphilis serology (rapid plasma reagin) can be easily obtained if doubt persists.
DISCUSSION
Pityriasis rosea (PR) is a papulosquamous eruption that is common in younger populations. About 40% of affected patients present with a large scaly lesion, which is followed by the appearance of multiple smaller oval lesions within days. In most cases, PR is fairly easy to diagnose: the lesion’s oval shape, pinkish-brown color, centripetal fine scale, herald patch, and adherence to skin tension lines are all characteristic findings.
Though the exact organism has not been identified, PR is almost certainly viral in origin. Like many viral exanthems, occurrence tends to peak in the spring and fall. There are also indications that the body builds immunity to the infection, since recurrence outside the acute phase is rare.
Treatment options are unsatisfactory, though exposure to UV sources appears to help. Patients must be informed that their condition will, unfortunately, last for at least six to nine weeks, during which crops of lesions will come and go.
ANSWER
The correct answer is pityriasis rosea (choice “d”).
Virtually all patients with this condition believe that they have a terrible case of “ringworm” (ie, fungal infection; choice “a”)—an opinion all too often corroborated by the medical provider unacquainted with pityriasis rosea. The two can be difficult to distinguish, but the “herald” patch, oval shape, odd color, and fine scale all serve to confirm the diagnosis. When necessary, a KOH prep or biopsy can be done.
Psoriasis (choice “b”) can manifest acutely, but it is distinctly salmon-pink under coarse, white scale that affects the palms, nails (with pits), and scalp. Psoriasis lesions are round (rather than oval), with coarser scale and without a herald patch.
The lack of antecedent sores and denial of sexual contact ruled out secondary syphilis (choice “c”). Patients with secondary syphilis often present with low-grade fever, malaise, and palmar scaly papules—all of which were missing in this case. Syphilis serology (rapid plasma reagin) can be easily obtained if doubt persists.
DISCUSSION
Pityriasis rosea (PR) is a papulosquamous eruption that is common in younger populations. About 40% of affected patients present with a large scaly lesion, which is followed by the appearance of multiple smaller oval lesions within days. In most cases, PR is fairly easy to diagnose: the lesion’s oval shape, pinkish-brown color, centripetal fine scale, herald patch, and adherence to skin tension lines are all characteristic findings.
Though the exact organism has not been identified, PR is almost certainly viral in origin. Like many viral exanthems, occurrence tends to peak in the spring and fall. There are also indications that the body builds immunity to the infection, since recurrence outside the acute phase is rare.
Treatment options are unsatisfactory, though exposure to UV sources appears to help. Patients must be informed that their condition will, unfortunately, last for at least six to nine weeks, during which crops of lesions will come and go.
ANSWER
The correct answer is pityriasis rosea (choice “d”).
Virtually all patients with this condition believe that they have a terrible case of “ringworm” (ie, fungal infection; choice “a”)—an opinion all too often corroborated by the medical provider unacquainted with pityriasis rosea. The two can be difficult to distinguish, but the “herald” patch, oval shape, odd color, and fine scale all serve to confirm the diagnosis. When necessary, a KOH prep or biopsy can be done.
Psoriasis (choice “b”) can manifest acutely, but it is distinctly salmon-pink under coarse, white scale that affects the palms, nails (with pits), and scalp. Psoriasis lesions are round (rather than oval), with coarser scale and without a herald patch.
The lack of antecedent sores and denial of sexual contact ruled out secondary syphilis (choice “c”). Patients with secondary syphilis often present with low-grade fever, malaise, and palmar scaly papules—all of which were missing in this case. Syphilis serology (rapid plasma reagin) can be easily obtained if doubt persists.
DISCUSSION
Pityriasis rosea (PR) is a papulosquamous eruption that is common in younger populations. About 40% of affected patients present with a large scaly lesion, which is followed by the appearance of multiple smaller oval lesions within days. In most cases, PR is fairly easy to diagnose: the lesion’s oval shape, pinkish-brown color, centripetal fine scale, herald patch, and adherence to skin tension lines are all characteristic findings.
Though the exact organism has not been identified, PR is almost certainly viral in origin. Like many viral exanthems, occurrence tends to peak in the spring and fall. There are also indications that the body builds immunity to the infection, since recurrence outside the acute phase is rare.
Treatment options are unsatisfactory, though exposure to UV sources appears to help. Patients must be informed that their condition will, unfortunately, last for at least six to nine weeks, during which crops of lesions will come and go.

About 10 days ago, an asymptomatic, scaly lesion arose overnight on this 21-year-old man’s upper left chest, near the anterior deltoid area. Within the past week, multiple scaly lesions—much smaller than the original—have appeared on his trunk and arms.
The patient claims to be in good health otherwise, denying fever, malaise, or myalgia. He denies sexual contact of any kind in the past two months. There is no personal or family history of skin disease or atopy.
On exam, the patient looks his stated age, is afebrile, and is in no acute distress. On his chest is an oval, pinkish brown, papulosquamous lesion with very fine, sparse scaling in the center. The long axis of the lesion parallels local skin tension lines.
About 20 additional lesions are observed elsewhere, primarily on the truncal skin, sparing the upper neck, face, and palms. All are oval, with the same odd color and scale, and follow skin tension lines. The patient’s elbows, knees, scalp, and nails are unaffected, as are the areas around and below the waist.
Obtaining cystatin-C levels useful in chronic kidney disease
SAN FRANCISCO – Routine lab tests for estimating the average glomerular filtration rate (GFR) are too imprecise, according to Michael G. Shlipak, MD.
“If you study 100 patients, the average GFR based on creatinine is going to be pretty close to estimated GFR,” Dr. Shlipak said at the UCSF Annual Advances in Internal Medicine meeting. “But with individual patients, the average GFR is going to be plus or minus 30%, which is a lot. If the estimated GFR is 70 mL/min per 1.73 m2, the real GFR could be between 50 and 90 mL/min per 1.73 m2; so that’s a wide range.”
“The main appeal of the Cockcroft-Gault equation is that you can almost do it in your head, so that’s a real positive,” said Dr. Shlipak, who is also scientific director of the Kidney Health Research Collaborative at the University of California, San Francisco, and professor of internal medicine, epidemiology, and biostatistics at UCSF Medical Center. “The main disadvantage is that it’s really a terrible equation. The Cockcroft-Gault equation is clearly inadequate, as it is standardized to creatinine clearance and very inaccurate, so it should no longer be used. The MDRD and the CKD-EPI are newer equations that are at least validated to real GFR and not creatinine clearance. In our system, the pharmacy uses the Cockcroft-Gault equation, and the lab gives us the MDRD GFR equation, so it’s quite confusing.”
Dr. Shlipak described using estimated GFR in clinical decision making as “better than using just creatinine, because it integrates demographic characteristics, which are determinants in part of the creatinine production, which is what determines how much creatinine is in the blood before it gets filtered. The equations make us think of GFR and kidney function instead of just the lab value.”
The downsides of using GFRs, he added, include the fact that they are mostly validated in younger patients with kidney disease, they rely on the assumption that demographic characteristics alone can define muscle mass, they were only developed in whites and blacks, and estimated GFR can be interpreted only as “suggested GFR.”
A blood test of kidney function known as cystatin C has been shown to be an alternative, better marker of creatinine, compared with GFR, and is supported by the Kidney Disease: Improving Global Outcomes CKD work group’s 2012 clinical practice guidelines for the evaluation and management of CKD. “Because cystatin C is not related to muscle mass, age, sex, and race, it has major advantages over creatinine,” Dr. Shlipak said. “It is a reliable, standardized, and automated measure that is available for clinical use.”
He and his associates conducted a meta-analysis comparing cystatin C and creatinine in determining prognosis for patients with baseline kidney disease. They included 16 studies involving 90,750 patients and compared associations of estimated GFR (eGFR) as measured with creatinine, cystatin C, and both creatinine and cystatin C with mortality risk; they also determined proportions reclassified by cystatin C in each subgroup of eGFR using creatinine and by the effect on risk associations (N Engl J Med. 2013 Sep 5; 369[10]:932-43).
In the general-population cohorts, the prevalence of an eGFR of less than 60 mL/min per 1.73 m2 of body surface area was higher with the cystatin C–based eGFR than with the creatinine-based eGFR (13.7% vs. 9.7%). Across all eGFR categories, the reclassification of the eGFR to a higher value with the measurement of cystatin C, compared with creatinine, was associated with a reduced risk of all three study outcomes. Reclassification to a lower eGFR was associated with an increased risk.
“When we looked at the threshold for where risk begins, traditionally we’ve said it starts when the GFR declines below 60 mL/min per 1.73 m2,” Dr. Shlipak explained. “That’s exactly what we found with creatinine. But with cystatin C, the threshold of risk was at 88 mL/min per 1.73 m2.
“So, from 88 mL/min per 1.73 m2 downward, every incremental reduction in GFR is associated with higher mortality and cardiovascular risk,” he added. “So cystatin C opens this new window of between 60 and 90 mL/min per 1.73 m2 to start measuring relative declines in kidney function. If you combine creatinine and cystatin C together in an equation, you get a similar estimate. Many advocate that the combined CKD-EPI creatinine-cystatin C equation is the best way to measure GFR.”
Dr. Shlipak reported that he is on the scientific advisory boards of TAI Diagnostics and Cricket Health.
SAN FRANCISCO – Routine lab tests for estimating the average glomerular filtration rate (GFR) are too imprecise, according to Michael G. Shlipak, MD.
“If you study 100 patients, the average GFR based on creatinine is going to be pretty close to estimated GFR,” Dr. Shlipak said at the UCSF Annual Advances in Internal Medicine meeting. “But with individual patients, the average GFR is going to be plus or minus 30%, which is a lot. If the estimated GFR is 70 mL/min per 1.73 m2, the real GFR could be between 50 and 90 mL/min per 1.73 m2; so that’s a wide range.”
“The main appeal of the Cockcroft-Gault equation is that you can almost do it in your head, so that’s a real positive,” said Dr. Shlipak, who is also scientific director of the Kidney Health Research Collaborative at the University of California, San Francisco, and professor of internal medicine, epidemiology, and biostatistics at UCSF Medical Center. “The main disadvantage is that it’s really a terrible equation. The Cockcroft-Gault equation is clearly inadequate, as it is standardized to creatinine clearance and very inaccurate, so it should no longer be used. The MDRD and the CKD-EPI are newer equations that are at least validated to real GFR and not creatinine clearance. In our system, the pharmacy uses the Cockcroft-Gault equation, and the lab gives us the MDRD GFR equation, so it’s quite confusing.”
Dr. Shlipak described using estimated GFR in clinical decision making as “better than using just creatinine, because it integrates demographic characteristics, which are determinants in part of the creatinine production, which is what determines how much creatinine is in the blood before it gets filtered. The equations make us think of GFR and kidney function instead of just the lab value.”
The downsides of using GFRs, he added, include the fact that they are mostly validated in younger patients with kidney disease, they rely on the assumption that demographic characteristics alone can define muscle mass, they were only developed in whites and blacks, and estimated GFR can be interpreted only as “suggested GFR.”
A blood test of kidney function known as cystatin C has been shown to be an alternative, better marker of creatinine, compared with GFR, and is supported by the Kidney Disease: Improving Global Outcomes CKD work group’s 2012 clinical practice guidelines for the evaluation and management of CKD. “Because cystatin C is not related to muscle mass, age, sex, and race, it has major advantages over creatinine,” Dr. Shlipak said. “It is a reliable, standardized, and automated measure that is available for clinical use.”
He and his associates conducted a meta-analysis comparing cystatin C and creatinine in determining prognosis for patients with baseline kidney disease. They included 16 studies involving 90,750 patients and compared associations of estimated GFR (eGFR) as measured with creatinine, cystatin C, and both creatinine and cystatin C with mortality risk; they also determined proportions reclassified by cystatin C in each subgroup of eGFR using creatinine and by the effect on risk associations (N Engl J Med. 2013 Sep 5; 369[10]:932-43).
In the general-population cohorts, the prevalence of an eGFR of less than 60 mL/min per 1.73 m2 of body surface area was higher with the cystatin C–based eGFR than with the creatinine-based eGFR (13.7% vs. 9.7%). Across all eGFR categories, the reclassification of the eGFR to a higher value with the measurement of cystatin C, compared with creatinine, was associated with a reduced risk of all three study outcomes. Reclassification to a lower eGFR was associated with an increased risk.
“When we looked at the threshold for where risk begins, traditionally we’ve said it starts when the GFR declines below 60 mL/min per 1.73 m2,” Dr. Shlipak explained. “That’s exactly what we found with creatinine. But with cystatin C, the threshold of risk was at 88 mL/min per 1.73 m2.
“So, from 88 mL/min per 1.73 m2 downward, every incremental reduction in GFR is associated with higher mortality and cardiovascular risk,” he added. “So cystatin C opens this new window of between 60 and 90 mL/min per 1.73 m2 to start measuring relative declines in kidney function. If you combine creatinine and cystatin C together in an equation, you get a similar estimate. Many advocate that the combined CKD-EPI creatinine-cystatin C equation is the best way to measure GFR.”
Dr. Shlipak reported that he is on the scientific advisory boards of TAI Diagnostics and Cricket Health.
SAN FRANCISCO – Routine lab tests for estimating the average glomerular filtration rate (GFR) are too imprecise, according to Michael G. Shlipak, MD.
“If you study 100 patients, the average GFR based on creatinine is going to be pretty close to estimated GFR,” Dr. Shlipak said at the UCSF Annual Advances in Internal Medicine meeting. “But with individual patients, the average GFR is going to be plus or minus 30%, which is a lot. If the estimated GFR is 70 mL/min per 1.73 m2, the real GFR could be between 50 and 90 mL/min per 1.73 m2; so that’s a wide range.”
“The main appeal of the Cockcroft-Gault equation is that you can almost do it in your head, so that’s a real positive,” said Dr. Shlipak, who is also scientific director of the Kidney Health Research Collaborative at the University of California, San Francisco, and professor of internal medicine, epidemiology, and biostatistics at UCSF Medical Center. “The main disadvantage is that it’s really a terrible equation. The Cockcroft-Gault equation is clearly inadequate, as it is standardized to creatinine clearance and very inaccurate, so it should no longer be used. The MDRD and the CKD-EPI are newer equations that are at least validated to real GFR and not creatinine clearance. In our system, the pharmacy uses the Cockcroft-Gault equation, and the lab gives us the MDRD GFR equation, so it’s quite confusing.”
Dr. Shlipak described using estimated GFR in clinical decision making as “better than using just creatinine, because it integrates demographic characteristics, which are determinants in part of the creatinine production, which is what determines how much creatinine is in the blood before it gets filtered. The equations make us think of GFR and kidney function instead of just the lab value.”
The downsides of using GFRs, he added, include the fact that they are mostly validated in younger patients with kidney disease, they rely on the assumption that demographic characteristics alone can define muscle mass, they were only developed in whites and blacks, and estimated GFR can be interpreted only as “suggested GFR.”
A blood test of kidney function known as cystatin C has been shown to be an alternative, better marker of creatinine, compared with GFR, and is supported by the Kidney Disease: Improving Global Outcomes CKD work group’s 2012 clinical practice guidelines for the evaluation and management of CKD. “Because cystatin C is not related to muscle mass, age, sex, and race, it has major advantages over creatinine,” Dr. Shlipak said. “It is a reliable, standardized, and automated measure that is available for clinical use.”
He and his associates conducted a meta-analysis comparing cystatin C and creatinine in determining prognosis for patients with baseline kidney disease. They included 16 studies involving 90,750 patients and compared associations of estimated GFR (eGFR) as measured with creatinine, cystatin C, and both creatinine and cystatin C with mortality risk; they also determined proportions reclassified by cystatin C in each subgroup of eGFR using creatinine and by the effect on risk associations (N Engl J Med. 2013 Sep 5; 369[10]:932-43).
In the general-population cohorts, the prevalence of an eGFR of less than 60 mL/min per 1.73 m2 of body surface area was higher with the cystatin C–based eGFR than with the creatinine-based eGFR (13.7% vs. 9.7%). Across all eGFR categories, the reclassification of the eGFR to a higher value with the measurement of cystatin C, compared with creatinine, was associated with a reduced risk of all three study outcomes. Reclassification to a lower eGFR was associated with an increased risk.
“When we looked at the threshold for where risk begins, traditionally we’ve said it starts when the GFR declines below 60 mL/min per 1.73 m2,” Dr. Shlipak explained. “That’s exactly what we found with creatinine. But with cystatin C, the threshold of risk was at 88 mL/min per 1.73 m2.
“So, from 88 mL/min per 1.73 m2 downward, every incremental reduction in GFR is associated with higher mortality and cardiovascular risk,” he added. “So cystatin C opens this new window of between 60 and 90 mL/min per 1.73 m2 to start measuring relative declines in kidney function. If you combine creatinine and cystatin C together in an equation, you get a similar estimate. Many advocate that the combined CKD-EPI creatinine-cystatin C equation is the best way to measure GFR.”
Dr. Shlipak reported that he is on the scientific advisory boards of TAI Diagnostics and Cricket Health.
AT THE ANNUAL ADVANCES IN INTERNAL MEDICINE
Chronic Epilepsy Linked to Cognitive Deficits
Patients in their 50s who have chronic epilepsy seem to have cognitive deficits that may be linked to metabolic, inflammatory, and vascular abnormalities, according to a clinical study that included 40 patients with chronic localized epilepsy and 152 controls.
- To reach that conclusion, researchers conducted neuropsychological assessment, clinical examination, and blood testing to evaluate patients’ vascular status.
- The evaluation included systolic and diastolic blood pressure, body mass index (BMI), high density lipoprotein, homocysteine levels, and inflammatory markers including high-sensitivity C-reactive protein (hs-CRP), and interleukin-6.
- The metabolic assessment included measurement of insulin resistance by means of glucose levels and homeostatic model assessment of insulin resistance (HOMA-IR).
- Patients with epilepsy had impaired cognitive factor scores across the board.
- These patients also had abnormal BMI, hs-CRP, fasting glucose, and HOMA-IR.
- There was also a link between higher HOMA-IR and poor immediate memory and visuospatial ability.
- Elevated hs-CRP was associated with poorer visuospatial and verbal abilities.
Hermann BP, Sager MA, Koscik RL, Young K, Nakamura K. Vascular, inflammatory, and metabolic factors associated with cognition in aging persons with chronic epilepsy. [Published online ahead of print Sept 4, 2017]. Epilepsia.
Patients in their 50s who have chronic epilepsy seem to have cognitive deficits that may be linked to metabolic, inflammatory, and vascular abnormalities, according to a clinical study that included 40 patients with chronic localized epilepsy and 152 controls.
- To reach that conclusion, researchers conducted neuropsychological assessment, clinical examination, and blood testing to evaluate patients’ vascular status.
- The evaluation included systolic and diastolic blood pressure, body mass index (BMI), high density lipoprotein, homocysteine levels, and inflammatory markers including high-sensitivity C-reactive protein (hs-CRP), and interleukin-6.
- The metabolic assessment included measurement of insulin resistance by means of glucose levels and homeostatic model assessment of insulin resistance (HOMA-IR).
- Patients with epilepsy had impaired cognitive factor scores across the board.
- These patients also had abnormal BMI, hs-CRP, fasting glucose, and HOMA-IR.
- There was also a link between higher HOMA-IR and poor immediate memory and visuospatial ability.
- Elevated hs-CRP was associated with poorer visuospatial and verbal abilities.
Hermann BP, Sager MA, Koscik RL, Young K, Nakamura K. Vascular, inflammatory, and metabolic factors associated with cognition in aging persons with chronic epilepsy. [Published online ahead of print Sept 4, 2017]. Epilepsia.
Patients in their 50s who have chronic epilepsy seem to have cognitive deficits that may be linked to metabolic, inflammatory, and vascular abnormalities, according to a clinical study that included 40 patients with chronic localized epilepsy and 152 controls.
- To reach that conclusion, researchers conducted neuropsychological assessment, clinical examination, and blood testing to evaluate patients’ vascular status.
- The evaluation included systolic and diastolic blood pressure, body mass index (BMI), high density lipoprotein, homocysteine levels, and inflammatory markers including high-sensitivity C-reactive protein (hs-CRP), and interleukin-6.
- The metabolic assessment included measurement of insulin resistance by means of glucose levels and homeostatic model assessment of insulin resistance (HOMA-IR).
- Patients with epilepsy had impaired cognitive factor scores across the board.
- These patients also had abnormal BMI, hs-CRP, fasting glucose, and HOMA-IR.
- There was also a link between higher HOMA-IR and poor immediate memory and visuospatial ability.
- Elevated hs-CRP was associated with poorer visuospatial and verbal abilities.
Hermann BP, Sager MA, Koscik RL, Young K, Nakamura K. Vascular, inflammatory, and metabolic factors associated with cognition in aging persons with chronic epilepsy. [Published online ahead of print Sept 4, 2017]. Epilepsia.
SUDEP More Likely to Happen During Sleep
Patients who have seizures at night are more likely to die suddenly from epilepsy, according to a recent study that compared seizures occurring while patients were awake to those who experienced them during sleep.
- Researchers point out that patients with epilepsy are 20 times more likely to experience sudden death, compared with the general public.
- The most frequent risk factor for these deaths is uncontrolled seizures.
- Researchers tracked respiratory parameters, comparing adults with epilepsy during wake and sleep periods.
- Sleep-related seizures were linked to lower oxygen saturation when compared with wakeful seizures, and this association remained significant during the ictal and postictal periods.
- Sleep-related seizures were also associated with greater drops in saturation.
- Postseizure EEG suppression occurred more often after sleep-related seizures as well.
- Researchers concluded that sudden unexpected death in epilepsy (SUDEP) is more likely to happen during sleep and that it is linked to more severe and longer occurrences of hypoxemia.
Latreille V, Abdennadher M, Dworetzky BA, et al. Nocturnal seizures are associated with more severe hypoxemia and increased risk of postictal generalized EEG suppression. Epilepsia. 2017;58(9):e127-e131.
Patients who have seizures at night are more likely to die suddenly from epilepsy, according to a recent study that compared seizures occurring while patients were awake to those who experienced them during sleep.
- Researchers point out that patients with epilepsy are 20 times more likely to experience sudden death, compared with the general public.
- The most frequent risk factor for these deaths is uncontrolled seizures.
- Researchers tracked respiratory parameters, comparing adults with epilepsy during wake and sleep periods.
- Sleep-related seizures were linked to lower oxygen saturation when compared with wakeful seizures, and this association remained significant during the ictal and postictal periods.
- Sleep-related seizures were also associated with greater drops in saturation.
- Postseizure EEG suppression occurred more often after sleep-related seizures as well.
- Researchers concluded that sudden unexpected death in epilepsy (SUDEP) is more likely to happen during sleep and that it is linked to more severe and longer occurrences of hypoxemia.
Latreille V, Abdennadher M, Dworetzky BA, et al. Nocturnal seizures are associated with more severe hypoxemia and increased risk of postictal generalized EEG suppression. Epilepsia. 2017;58(9):e127-e131.
Patients who have seizures at night are more likely to die suddenly from epilepsy, according to a recent study that compared seizures occurring while patients were awake to those who experienced them during sleep.
- Researchers point out that patients with epilepsy are 20 times more likely to experience sudden death, compared with the general public.
- The most frequent risk factor for these deaths is uncontrolled seizures.
- Researchers tracked respiratory parameters, comparing adults with epilepsy during wake and sleep periods.
- Sleep-related seizures were linked to lower oxygen saturation when compared with wakeful seizures, and this association remained significant during the ictal and postictal periods.
- Sleep-related seizures were also associated with greater drops in saturation.
- Postseizure EEG suppression occurred more often after sleep-related seizures as well.
- Researchers concluded that sudden unexpected death in epilepsy (SUDEP) is more likely to happen during sleep and that it is linked to more severe and longer occurrences of hypoxemia.
Latreille V, Abdennadher M, Dworetzky BA, et al. Nocturnal seizures are associated with more severe hypoxemia and increased risk of postictal generalized EEG suppression. Epilepsia. 2017;58(9):e127-e131.