User login
Simplified HOSPITAL score predicts 30-day readmissions
Clinical Question: Will a simplified HOSPITAL score accurately predict 30-day readmissions?
Background: Hospital readmissions stress patients and health care systems. Interventions to prevent avoidable readmissions are complex and expensive. The HOSPITAL score predicts 30-day readmissions which may help direct resources toward high risk patients.
Setting: Nine hospitals in four countries.
Synopsis: The HOSPITAL score was simplified by removing the procedure variable, expanding the oncology criteria to include a diagnosis of cancer, and dividing patients into high and low risk groups. The simplified HOSPITAL score was used to predict avoidable readmissions of 117,065 patients from nine hospitals. Readmission rates predicted by the simplified HOSPITAL score matched observed outcomes with a sensitivity of 94% and specificity of 73%. Its discriminatory power was comparable with the original HOSPITAL score.
This was a robust study of medical patients but may not be generalizable to surgical patients. The score does not include patients’ socioeconomic status or support systems. It also cannot indicate what type of intervention may prevent readmissions.
Bottom Line: The simplified HOSPITAL score accurately predicts avoidable 30-day readmission rates.
Citation: Aubert CE, Schnipper JL, Williams MV, et al. Simplification of the HOSPITAL score for predicting 30-day readmissions. BMJ Qual Saf. Published online first. 17 Apr 2017. doi: 10.1136/bmjqs-2016-006239.
Dr. Helfrich is an assistant professor in the University of Kentucky division of hospital medicine.
Clinical Question: Will a simplified HOSPITAL score accurately predict 30-day readmissions?
Background: Hospital readmissions stress patients and health care systems. Interventions to prevent avoidable readmissions are complex and expensive. The HOSPITAL score predicts 30-day readmissions which may help direct resources toward high risk patients.
Setting: Nine hospitals in four countries.
Synopsis: The HOSPITAL score was simplified by removing the procedure variable, expanding the oncology criteria to include a diagnosis of cancer, and dividing patients into high and low risk groups. The simplified HOSPITAL score was used to predict avoidable readmissions of 117,065 patients from nine hospitals. Readmission rates predicted by the simplified HOSPITAL score matched observed outcomes with a sensitivity of 94% and specificity of 73%. Its discriminatory power was comparable with the original HOSPITAL score.
This was a robust study of medical patients but may not be generalizable to surgical patients. The score does not include patients’ socioeconomic status or support systems. It also cannot indicate what type of intervention may prevent readmissions.
Bottom Line: The simplified HOSPITAL score accurately predicts avoidable 30-day readmission rates.
Citation: Aubert CE, Schnipper JL, Williams MV, et al. Simplification of the HOSPITAL score for predicting 30-day readmissions. BMJ Qual Saf. Published online first. 17 Apr 2017. doi: 10.1136/bmjqs-2016-006239.
Dr. Helfrich is an assistant professor in the University of Kentucky division of hospital medicine.
Clinical Question: Will a simplified HOSPITAL score accurately predict 30-day readmissions?
Background: Hospital readmissions stress patients and health care systems. Interventions to prevent avoidable readmissions are complex and expensive. The HOSPITAL score predicts 30-day readmissions which may help direct resources toward high risk patients.
Setting: Nine hospitals in four countries.
Synopsis: The HOSPITAL score was simplified by removing the procedure variable, expanding the oncology criteria to include a diagnosis of cancer, and dividing patients into high and low risk groups. The simplified HOSPITAL score was used to predict avoidable readmissions of 117,065 patients from nine hospitals. Readmission rates predicted by the simplified HOSPITAL score matched observed outcomes with a sensitivity of 94% and specificity of 73%. Its discriminatory power was comparable with the original HOSPITAL score.
This was a robust study of medical patients but may not be generalizable to surgical patients. The score does not include patients’ socioeconomic status or support systems. It also cannot indicate what type of intervention may prevent readmissions.
Bottom Line: The simplified HOSPITAL score accurately predicts avoidable 30-day readmission rates.
Citation: Aubert CE, Schnipper JL, Williams MV, et al. Simplification of the HOSPITAL score for predicting 30-day readmissions. BMJ Qual Saf. Published online first. 17 Apr 2017. doi: 10.1136/bmjqs-2016-006239.
Dr. Helfrich is an assistant professor in the University of Kentucky division of hospital medicine.
Patient Preference Before and After Arthroscopic Rotator Cuff Repair: Which Is More Important, Pain Relief or Strength Return?
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
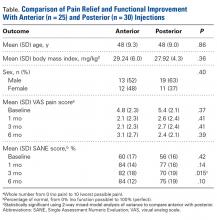
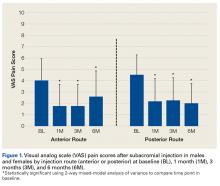
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.
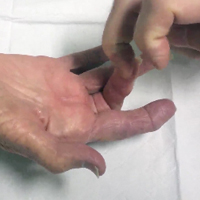
Percutaneous Trigger Finger Release
PD3 G1 SNA found to correlate with pentavalent rotavirus vaccine efficacy
(RV5), reported G. Frank Liu, PhD, and his associates at Merck in Kenilworth, N.J.
The researchers discovered this by analyzing data from five clinical trials of RV5 at both the individual and aggregated population level. Also, by looking at immunogenicity measures and case status of individuals from phase 2 and 3 trials of RV5, they found that “higher PD3 G1 SNA titers are associated with lower odds of contracting any [rotavirus gastroenteritis].”
Read more at (Hum Vaccin Immunother. 2017. doi: 10.1080/21645515.2017.1356522).
[email protected]
(RV5), reported G. Frank Liu, PhD, and his associates at Merck in Kenilworth, N.J.
The researchers discovered this by analyzing data from five clinical trials of RV5 at both the individual and aggregated population level. Also, by looking at immunogenicity measures and case status of individuals from phase 2 and 3 trials of RV5, they found that “higher PD3 G1 SNA titers are associated with lower odds of contracting any [rotavirus gastroenteritis].”
Read more at (Hum Vaccin Immunother. 2017. doi: 10.1080/21645515.2017.1356522).
[email protected]
(RV5), reported G. Frank Liu, PhD, and his associates at Merck in Kenilworth, N.J.
The researchers discovered this by analyzing data from five clinical trials of RV5 at both the individual and aggregated population level. Also, by looking at immunogenicity measures and case status of individuals from phase 2 and 3 trials of RV5, they found that “higher PD3 G1 SNA titers are associated with lower odds of contracting any [rotavirus gastroenteritis].”
Read more at (Hum Vaccin Immunother. 2017. doi: 10.1080/21645515.2017.1356522).
[email protected]
FROM HUMAN VACCINES & IMMUNOTHERAPEUTICS
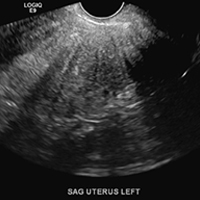
Adenomyosis in the spotlight, but which sign is featured?
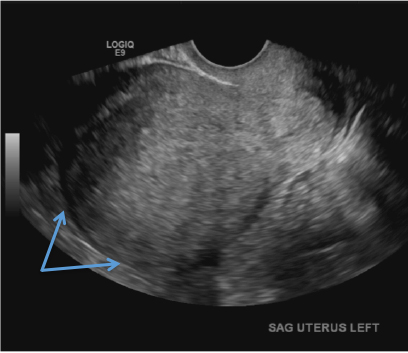
A) Globular enlarged uterus INCORRECT
A homogeneously enlarged uterus in the absence of fibroids is characteristic of adenomyosis.1
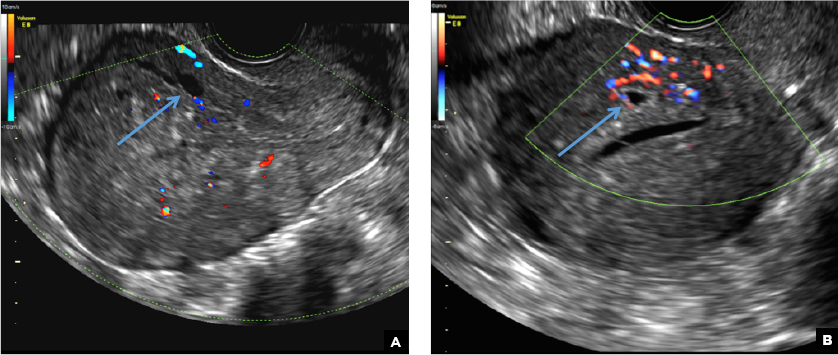
B) Cystic myometrial spaces INCORRECT
Myometrial cysts are dilated cystic glands or foci of hemorrhage within heterotopic endometrial tissue.2 They are often less than 5 mm in size but can be extensive and of variable sizes and can be differentiated from arcuate veins seen in the outer myometrium by the use of color Doppler.1,2
C) Asymmetric myometrial thickening INCORRECT
The finding of asymmetric uterine wall thickening in adenomyosis is usually seen when there is focal disease and presents with wall thickening that demonstrates anteroposterior asymmetry.1
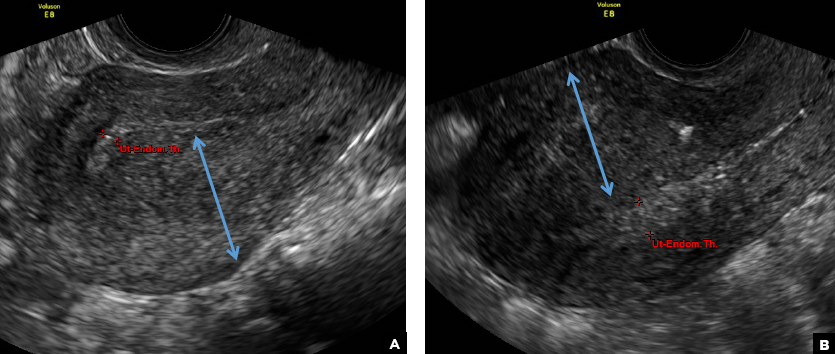
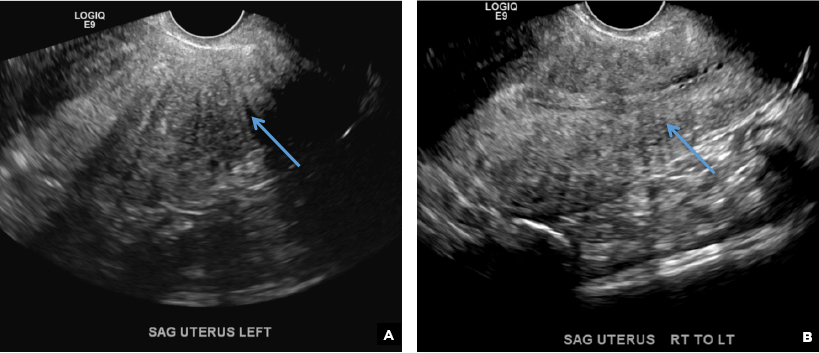
D) Indistinct endomyometrial interface INCORRECT
The heterotopic endometrial tissue invading the myometrium obscures the normal endometrial myometrial border with pseudo-widening of the endometrial echo resulting in poor definition of the endomyometrial junction.1,2

E) Myometrial linear striations CORRECT
A hyperplastic reaction to the infiltration of the heterotrophic endometrial glands into the myometrium results in radiating linear echogenic striations (sometimes referred to as the "venetian blinds" sign).1,2
- Sakhel A, Abuhamad A. Sonography of adenomyosis. J Ultrasound Med. 2012;31(5):805-808.
- Reinhold C, Tafazoli F, Mehio A, et al. Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. Radiographics. 1999;19:S147-S160.
A) Globular enlarged uterus INCORRECT
A homogeneously enlarged uterus in the absence of fibroids is characteristic of adenomyosis.1

B) Cystic myometrial spaces INCORRECT
Myometrial cysts are dilated cystic glands or foci of hemorrhage within heterotopic endometrial tissue.2 They are often less than 5 mm in size but can be extensive and of variable sizes and can be differentiated from arcuate veins seen in the outer myometrium by the use of color Doppler.1,2

C) Asymmetric myometrial thickening INCORRECT
The finding of asymmetric uterine wall thickening in adenomyosis is usually seen when there is focal disease and presents with wall thickening that demonstrates anteroposterior asymmetry.1

D) Indistinct endomyometrial interface INCORRECT
The heterotopic endometrial tissue invading the myometrium obscures the normal endometrial myometrial border with pseudo-widening of the endometrial echo resulting in poor definition of the endomyometrial junction.1,2

E) Myometrial linear striations CORRECT
A hyperplastic reaction to the infiltration of the heterotrophic endometrial glands into the myometrium results in radiating linear echogenic striations (sometimes referred to as the "venetian blinds" sign).1,2

A) Globular enlarged uterus INCORRECT
A homogeneously enlarged uterus in the absence of fibroids is characteristic of adenomyosis.1

B) Cystic myometrial spaces INCORRECT
Myometrial cysts are dilated cystic glands or foci of hemorrhage within heterotopic endometrial tissue.2 They are often less than 5 mm in size but can be extensive and of variable sizes and can be differentiated from arcuate veins seen in the outer myometrium by the use of color Doppler.1,2

C) Asymmetric myometrial thickening INCORRECT
The finding of asymmetric uterine wall thickening in adenomyosis is usually seen when there is focal disease and presents with wall thickening that demonstrates anteroposterior asymmetry.1

D) Indistinct endomyometrial interface INCORRECT
The heterotopic endometrial tissue invading the myometrium obscures the normal endometrial myometrial border with pseudo-widening of the endometrial echo resulting in poor definition of the endomyometrial junction.1,2

E) Myometrial linear striations CORRECT
A hyperplastic reaction to the infiltration of the heterotrophic endometrial glands into the myometrium results in radiating linear echogenic striations (sometimes referred to as the "venetian blinds" sign).1,2

- Sakhel A, Abuhamad A. Sonography of adenomyosis. J Ultrasound Med. 2012;31(5):805-808.
- Reinhold C, Tafazoli F, Mehio A, et al. Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. Radiographics. 1999;19:S147-S160.
- Sakhel A, Abuhamad A. Sonography of adenomyosis. J Ultrasound Med. 2012;31(5):805-808.
- Reinhold C, Tafazoli F, Mehio A, et al. Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. Radiographics. 1999;19:S147-S160.
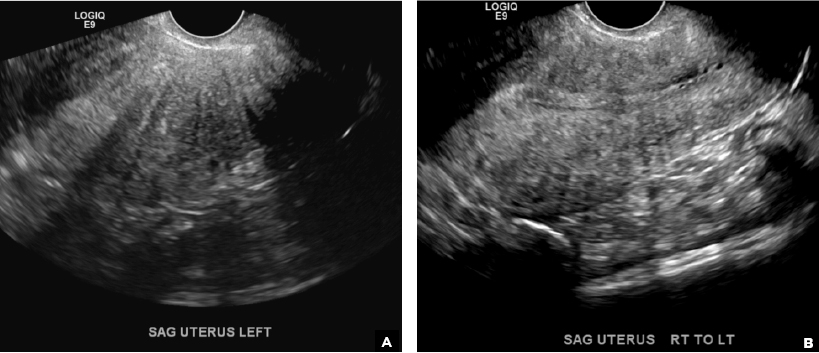
A 37-year-old woman with heavy menstrual bleeding and dysmenorrhea presents to her gynecologist. Physical exam suggests an enlarged uterus; the gynecologist orders pelvic ultrasonography.
Real-world adverse events reported after bivalent MenB vaccination in college students
, said Theresa M. Fiorito, MD, of Brown University, Providence, R.I., and her associates.
In February 2015, two unlinked, culture-confirmed cases of Neisseria meningitidis serogroup B (MenB) disease occurred at a local college in Rhode Island, and a bivalent MenB vaccine subsequently was administered. After the first-dose vaccination clinic, a 94% coverage rate was achieved, and there were no other cases of MenB disease. Injection site pain occurred in 78% of 1,736 students after dose one, in 64% of 1,395 after dose two, and in 71% of 609 students after dose three.
Serious adverse events, such as any allergic reaction; difficulty breathing; hives, welts, or a severe rash; and swelling of the face, mouth, or throat occurred in fewer than 5% of students after any of the three doses, the investigators reported.
“We found an overall lower rate of headache within our sample relative to the rates in reported clinical trials. The rates of injection site pain, fatigue, thermometer-confirmed fevers (100.4° F or higher), and chills were approximately similar in our study as in clinical trials. The rate of myalgia was higher for our sample than in clinical trials following the first and second doses of the vaccine, but similar after the third dose of vaccine,” Dr. Fiorito and her associates wrote.
Read more at (Pediatr Infect Dis J. 2017. doi: 10.1097/INF.0000000000001742).
, said Theresa M. Fiorito, MD, of Brown University, Providence, R.I., and her associates.
In February 2015, two unlinked, culture-confirmed cases of Neisseria meningitidis serogroup B (MenB) disease occurred at a local college in Rhode Island, and a bivalent MenB vaccine subsequently was administered. After the first-dose vaccination clinic, a 94% coverage rate was achieved, and there were no other cases of MenB disease. Injection site pain occurred in 78% of 1,736 students after dose one, in 64% of 1,395 after dose two, and in 71% of 609 students after dose three.
Serious adverse events, such as any allergic reaction; difficulty breathing; hives, welts, or a severe rash; and swelling of the face, mouth, or throat occurred in fewer than 5% of students after any of the three doses, the investigators reported.
“We found an overall lower rate of headache within our sample relative to the rates in reported clinical trials. The rates of injection site pain, fatigue, thermometer-confirmed fevers (100.4° F or higher), and chills were approximately similar in our study as in clinical trials. The rate of myalgia was higher for our sample than in clinical trials following the first and second doses of the vaccine, but similar after the third dose of vaccine,” Dr. Fiorito and her associates wrote.
Read more at (Pediatr Infect Dis J. 2017. doi: 10.1097/INF.0000000000001742).
, said Theresa M. Fiorito, MD, of Brown University, Providence, R.I., and her associates.
In February 2015, two unlinked, culture-confirmed cases of Neisseria meningitidis serogroup B (MenB) disease occurred at a local college in Rhode Island, and a bivalent MenB vaccine subsequently was administered. After the first-dose vaccination clinic, a 94% coverage rate was achieved, and there were no other cases of MenB disease. Injection site pain occurred in 78% of 1,736 students after dose one, in 64% of 1,395 after dose two, and in 71% of 609 students after dose three.
Serious adverse events, such as any allergic reaction; difficulty breathing; hives, welts, or a severe rash; and swelling of the face, mouth, or throat occurred in fewer than 5% of students after any of the three doses, the investigators reported.
“We found an overall lower rate of headache within our sample relative to the rates in reported clinical trials. The rates of injection site pain, fatigue, thermometer-confirmed fevers (100.4° F or higher), and chills were approximately similar in our study as in clinical trials. The rate of myalgia was higher for our sample than in clinical trials following the first and second doses of the vaccine, but similar after the third dose of vaccine,” Dr. Fiorito and her associates wrote.
Read more at (Pediatr Infect Dis J. 2017. doi: 10.1097/INF.0000000000001742).
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
Dr. Britt awarded NIH grant to develop strategies to address health care disparities
L. D. Britt, MD, MPH, DSc(Hon), FACS, FCCM, FRCSEng(Hon), FRCSEd(Hon), FWACS(Hon), FRCSI(Hon), FCS(SA)(Hon), FRCSGlasg(Hon), Henry Ford Professor and Edward Brickhouse Chairman, department of surgery, Eastern Virginia Medical School, Norfolk, and a Past-President of the American College of Surgeons (ACS), was recently awarded a multimillion-dollar National Institutes of Health (NIH) research grant. The grant will be used to develop strategies to address health care disparities in the various surgical specialties. Specifically, the emphasis of this research is “to determine the specific measures of health care disparities in the various surgical specialties in order to develop targeted interventions to mitigate such disparities,” said Dr. Britt, principal investigator of the research project.
The NIH R01 grants are among the most competitive awards in scientific research. Dr. Britt’s research team comprises experts in the field who work in medical organizations and academic institutions, such as the ACS; Harvard Medical School, Boston, MA; and the University of California, Los Angeles.
Dr. Britt has dedicated his career to patient care and addressing the multifaceted disparities in health care, and he believes that this research grant is a pivotal step toward countering one of the greatest challenges facing this country. He is particularly thankful for the unwavering support of David B. Hoyt, MD, FACS, ACS Executive Director; the Board of Regents; and the ACS Committee on Health Care Disparities, which he chairs. Adil Haider, MD, MPH, FACS, professor and director of the Center for Surgery and Public Health, Harvard Medical School, serves as Vice-Chair of the committee.
“This is a big step for the American College of Surgeons,” Dr. Britt said. “With its 100-plus year history of using data to address quality of care in surgery, if the College, in collaboration with the NIH, can’t solve this problem, no one can.”
Dr. Britt added that he anticipates that the College’s efforts to address disparities in health care with the help of the NIH will serve as a template for other professional organizations so that all patients have access to the services they need, from primary care to obstetrics-gynecology, and from cardiology to psychiatry. “Dr. Hoyt and I hope this is the start of movement to address health care disparities in all specialties, but it starts with the College.”
L. D. Britt, MD, MPH, DSc(Hon), FACS, FCCM, FRCSEng(Hon), FRCSEd(Hon), FWACS(Hon), FRCSI(Hon), FCS(SA)(Hon), FRCSGlasg(Hon), Henry Ford Professor and Edward Brickhouse Chairman, department of surgery, Eastern Virginia Medical School, Norfolk, and a Past-President of the American College of Surgeons (ACS), was recently awarded a multimillion-dollar National Institutes of Health (NIH) research grant. The grant will be used to develop strategies to address health care disparities in the various surgical specialties. Specifically, the emphasis of this research is “to determine the specific measures of health care disparities in the various surgical specialties in order to develop targeted interventions to mitigate such disparities,” said Dr. Britt, principal investigator of the research project.
The NIH R01 grants are among the most competitive awards in scientific research. Dr. Britt’s research team comprises experts in the field who work in medical organizations and academic institutions, such as the ACS; Harvard Medical School, Boston, MA; and the University of California, Los Angeles.
Dr. Britt has dedicated his career to patient care and addressing the multifaceted disparities in health care, and he believes that this research grant is a pivotal step toward countering one of the greatest challenges facing this country. He is particularly thankful for the unwavering support of David B. Hoyt, MD, FACS, ACS Executive Director; the Board of Regents; and the ACS Committee on Health Care Disparities, which he chairs. Adil Haider, MD, MPH, FACS, professor and director of the Center for Surgery and Public Health, Harvard Medical School, serves as Vice-Chair of the committee.
“This is a big step for the American College of Surgeons,” Dr. Britt said. “With its 100-plus year history of using data to address quality of care in surgery, if the College, in collaboration with the NIH, can’t solve this problem, no one can.”
Dr. Britt added that he anticipates that the College’s efforts to address disparities in health care with the help of the NIH will serve as a template for other professional organizations so that all patients have access to the services they need, from primary care to obstetrics-gynecology, and from cardiology to psychiatry. “Dr. Hoyt and I hope this is the start of movement to address health care disparities in all specialties, but it starts with the College.”
L. D. Britt, MD, MPH, DSc(Hon), FACS, FCCM, FRCSEng(Hon), FRCSEd(Hon), FWACS(Hon), FRCSI(Hon), FCS(SA)(Hon), FRCSGlasg(Hon), Henry Ford Professor and Edward Brickhouse Chairman, department of surgery, Eastern Virginia Medical School, Norfolk, and a Past-President of the American College of Surgeons (ACS), was recently awarded a multimillion-dollar National Institutes of Health (NIH) research grant. The grant will be used to develop strategies to address health care disparities in the various surgical specialties. Specifically, the emphasis of this research is “to determine the specific measures of health care disparities in the various surgical specialties in order to develop targeted interventions to mitigate such disparities,” said Dr. Britt, principal investigator of the research project.
The NIH R01 grants are among the most competitive awards in scientific research. Dr. Britt’s research team comprises experts in the field who work in medical organizations and academic institutions, such as the ACS; Harvard Medical School, Boston, MA; and the University of California, Los Angeles.
Dr. Britt has dedicated his career to patient care and addressing the multifaceted disparities in health care, and he believes that this research grant is a pivotal step toward countering one of the greatest challenges facing this country. He is particularly thankful for the unwavering support of David B. Hoyt, MD, FACS, ACS Executive Director; the Board of Regents; and the ACS Committee on Health Care Disparities, which he chairs. Adil Haider, MD, MPH, FACS, professor and director of the Center for Surgery and Public Health, Harvard Medical School, serves as Vice-Chair of the committee.
“This is a big step for the American College of Surgeons,” Dr. Britt said. “With its 100-plus year history of using data to address quality of care in surgery, if the College, in collaboration with the NIH, can’t solve this problem, no one can.”
Dr. Britt added that he anticipates that the College’s efforts to address disparities in health care with the help of the NIH will serve as a template for other professional organizations so that all patients have access to the services they need, from primary care to obstetrics-gynecology, and from cardiology to psychiatry. “Dr. Hoyt and I hope this is the start of movement to address health care disparities in all specialties, but it starts with the College.”
FDA approves second adalimumab biosimilar for multiple conditions
The Food and Drug Administration has approved Cyltezo (adalimumab-adbm) for multiple conditions.
Cyltezo is an injectable tumor necrosis factor blocker, and is a biosimilar to adalimumab (Humira). The drug is indicated to treat moderate to severe active rheumatoid arthritis, active psoriatic arthritis, active ankylosing spondylitis, moderate to severe active Crohn’s disease, moderate to severe active ulcerative colitis, moderately to severely active polyarticular juvenile idiopathic arthritis in patients 4 years of age and older, and moderate to severe plaque psoriasis.
Find the Cyltezo labeling information here.
The Food and Drug Administration has approved Cyltezo (adalimumab-adbm) for multiple conditions.
Cyltezo is an injectable tumor necrosis factor blocker, and is a biosimilar to adalimumab (Humira). The drug is indicated to treat moderate to severe active rheumatoid arthritis, active psoriatic arthritis, active ankylosing spondylitis, moderate to severe active Crohn’s disease, moderate to severe active ulcerative colitis, moderately to severely active polyarticular juvenile idiopathic arthritis in patients 4 years of age and older, and moderate to severe plaque psoriasis.
Find the Cyltezo labeling information here.
The Food and Drug Administration has approved Cyltezo (adalimumab-adbm) for multiple conditions.
Cyltezo is an injectable tumor necrosis factor blocker, and is a biosimilar to adalimumab (Humira). The drug is indicated to treat moderate to severe active rheumatoid arthritis, active psoriatic arthritis, active ankylosing spondylitis, moderate to severe active Crohn’s disease, moderate to severe active ulcerative colitis, moderately to severely active polyarticular juvenile idiopathic arthritis in patients 4 years of age and older, and moderate to severe plaque psoriasis.
Find the Cyltezo labeling information here.
Pembrolizumab showed ‘promising’ antitumor activity in small-cell lung cancer
One patient died of treatment-related mesenteric ischemia, reported Patrick A. Ott, MD, of Dana-Farber Cancer Institute in Boston, and his associates. Nonetheless, with an objective response rate of 33% and a median duration of response of 19 months, the checkpoint inhibitor “demonstrated a favorable safety profile and promising durable clinical activity,” they concluded.
Study participants received pembrolizumab (10 mg/kg) every 2 weeks for 24 months or until disease progression or intolerable toxicity occurred. After a median follow-up of 9.8 months (range, 0.5-24 months), one patient (4%) had a complete response, and seven (29%) had partial responses. The median onset of response was 2 months, and responses lasted from 3.6 to 20 months, Dr. Ott and his associates reported (J Clin Oncol. 2017 Aug 16. doi: 10.1200/JCO.2017.72.5069). Two-thirds of patients developed treatment-related adverse events, most often arthralgia, asthenia, rash, diarrhea, and fatigue. Two patients developed grade 3 or worse treatment-related adverse events, including a 65-year-old man with small cell lung cancer and liver metastasis who developed grade 3 bilirubin elevation, and a 58-year-old woman with a history of sleeve gastrectomy who developed grade 3 asthenia, grade 5 colitis, and intestinal ischemia.
The patient who died had received 10 cycles of pembrolizumab, was hospitalized with abdominal pain, nausea, and vomiting, and was discharged home with a diagnosis of food intolerance, the researchers reported. She received an 11th cycle of pembrolizumab and was readmitted with abdominal pain. A rectal biopsy showed chronic colitis. She received systemic corticosteroids and was discharged, was admitted to a different hospital several weeks later with diffuse abdominal pain and septic shock, and subsequently died. “Mesenteric ischemia resulted in death,” the researchers wrote. “The cause of the colitis and intestinal ischemia was reported as probably related to pembrolizumab.”
Pembrolizumab (Keytruda) is a programmed death receptor–1 blocking antibody approved for treating non–small cell lung cancer, head and neck squamous cell cancer, classical Hodgkin lymphoma, urothelial carcinoma, and microsatellite instability–high cancer or mismatch repair deficient solid tumors. Treatment with the checkpoint inhibitor led to grade 5 treatment-related adverse events in other trials. Most recently, in July 2017, the Food and Drug Administration placed clinical holds on phase 1 and phase 3 studies of pembrolizumab for treating multiple myeloma after more patients died in the pembrolizumab arms than did in the comparison arms. Pembrolizumab also recently came up short in a phase 3 trial of patients with head and neck cancer, although it has kept its FDA label for this indication. Multiple trials of pembrolizumab for small cell lung cancer are recruiting or ongoing.
Merck funded the study. Dr. Ott disclosed research funding from Merck and several other pharmaceutical companies, and advisory or consulting relationships with several companies, excluding Merck.
One patient died of treatment-related mesenteric ischemia, reported Patrick A. Ott, MD, of Dana-Farber Cancer Institute in Boston, and his associates. Nonetheless, with an objective response rate of 33% and a median duration of response of 19 months, the checkpoint inhibitor “demonstrated a favorable safety profile and promising durable clinical activity,” they concluded.
Study participants received pembrolizumab (10 mg/kg) every 2 weeks for 24 months or until disease progression or intolerable toxicity occurred. After a median follow-up of 9.8 months (range, 0.5-24 months), one patient (4%) had a complete response, and seven (29%) had partial responses. The median onset of response was 2 months, and responses lasted from 3.6 to 20 months, Dr. Ott and his associates reported (J Clin Oncol. 2017 Aug 16. doi: 10.1200/JCO.2017.72.5069). Two-thirds of patients developed treatment-related adverse events, most often arthralgia, asthenia, rash, diarrhea, and fatigue. Two patients developed grade 3 or worse treatment-related adverse events, including a 65-year-old man with small cell lung cancer and liver metastasis who developed grade 3 bilirubin elevation, and a 58-year-old woman with a history of sleeve gastrectomy who developed grade 3 asthenia, grade 5 colitis, and intestinal ischemia.
The patient who died had received 10 cycles of pembrolizumab, was hospitalized with abdominal pain, nausea, and vomiting, and was discharged home with a diagnosis of food intolerance, the researchers reported. She received an 11th cycle of pembrolizumab and was readmitted with abdominal pain. A rectal biopsy showed chronic colitis. She received systemic corticosteroids and was discharged, was admitted to a different hospital several weeks later with diffuse abdominal pain and septic shock, and subsequently died. “Mesenteric ischemia resulted in death,” the researchers wrote. “The cause of the colitis and intestinal ischemia was reported as probably related to pembrolizumab.”
Pembrolizumab (Keytruda) is a programmed death receptor–1 blocking antibody approved for treating non–small cell lung cancer, head and neck squamous cell cancer, classical Hodgkin lymphoma, urothelial carcinoma, and microsatellite instability–high cancer or mismatch repair deficient solid tumors. Treatment with the checkpoint inhibitor led to grade 5 treatment-related adverse events in other trials. Most recently, in July 2017, the Food and Drug Administration placed clinical holds on phase 1 and phase 3 studies of pembrolizumab for treating multiple myeloma after more patients died in the pembrolizumab arms than did in the comparison arms. Pembrolizumab also recently came up short in a phase 3 trial of patients with head and neck cancer, although it has kept its FDA label for this indication. Multiple trials of pembrolizumab for small cell lung cancer are recruiting or ongoing.
Merck funded the study. Dr. Ott disclosed research funding from Merck and several other pharmaceutical companies, and advisory or consulting relationships with several companies, excluding Merck.
One patient died of treatment-related mesenteric ischemia, reported Patrick A. Ott, MD, of Dana-Farber Cancer Institute in Boston, and his associates. Nonetheless, with an objective response rate of 33% and a median duration of response of 19 months, the checkpoint inhibitor “demonstrated a favorable safety profile and promising durable clinical activity,” they concluded.
Study participants received pembrolizumab (10 mg/kg) every 2 weeks for 24 months or until disease progression or intolerable toxicity occurred. After a median follow-up of 9.8 months (range, 0.5-24 months), one patient (4%) had a complete response, and seven (29%) had partial responses. The median onset of response was 2 months, and responses lasted from 3.6 to 20 months, Dr. Ott and his associates reported (J Clin Oncol. 2017 Aug 16. doi: 10.1200/JCO.2017.72.5069). Two-thirds of patients developed treatment-related adverse events, most often arthralgia, asthenia, rash, diarrhea, and fatigue. Two patients developed grade 3 or worse treatment-related adverse events, including a 65-year-old man with small cell lung cancer and liver metastasis who developed grade 3 bilirubin elevation, and a 58-year-old woman with a history of sleeve gastrectomy who developed grade 3 asthenia, grade 5 colitis, and intestinal ischemia.
The patient who died had received 10 cycles of pembrolizumab, was hospitalized with abdominal pain, nausea, and vomiting, and was discharged home with a diagnosis of food intolerance, the researchers reported. She received an 11th cycle of pembrolizumab and was readmitted with abdominal pain. A rectal biopsy showed chronic colitis. She received systemic corticosteroids and was discharged, was admitted to a different hospital several weeks later with diffuse abdominal pain and septic shock, and subsequently died. “Mesenteric ischemia resulted in death,” the researchers wrote. “The cause of the colitis and intestinal ischemia was reported as probably related to pembrolizumab.”
Pembrolizumab (Keytruda) is a programmed death receptor–1 blocking antibody approved for treating non–small cell lung cancer, head and neck squamous cell cancer, classical Hodgkin lymphoma, urothelial carcinoma, and microsatellite instability–high cancer or mismatch repair deficient solid tumors. Treatment with the checkpoint inhibitor led to grade 5 treatment-related adverse events in other trials. Most recently, in July 2017, the Food and Drug Administration placed clinical holds on phase 1 and phase 3 studies of pembrolizumab for treating multiple myeloma after more patients died in the pembrolizumab arms than did in the comparison arms. Pembrolizumab also recently came up short in a phase 3 trial of patients with head and neck cancer, although it has kept its FDA label for this indication. Multiple trials of pembrolizumab for small cell lung cancer are recruiting or ongoing.
Merck funded the study. Dr. Ott disclosed research funding from Merck and several other pharmaceutical companies, and advisory or consulting relationships with several companies, excluding Merck.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Pembrolizumab showed antitumor activity and was usually safe for treating extensive-stage small cell lung cancer.
Major finding: The objective response rate was 33%. Two patients developed grade 3 or worse treatment-related adverse events, which included fatal mesenteric ischemia and colitis.
Data source: A phase 1b open-label trial of 24 patients with PD-L1–positive extensive-stage small cell lung cancer.
Disclosures: Merck funded the study. Dr. Ott disclosed research funding from Merck and several other pharmaceutical companies, and advisory or consulting relationships with several companies, excluding Merck.
Comparison of Anterior and Posterior Corticosteroid Injections for Pain Relief and Functional Improvement in Shoulder Impingement Syndrome
Take-Home Points
When conservative treatments for SIS do not resolve symptoms, inflammation and pain can be reduced with use of subacromial CSI.
Both anterior CSI and posterior CSI significantly improved pain and function for up to 6 months
CSI combined with structured PT produced significant improvement in pain and function in patients with SIS, regardless of injection route used.
Clinical response to CSI may not depend on injection accuracy.
Clinicians should rely on their clinical acumen when selecting injection routes, as anterior and posterior are both beneficial.
Shoulder pain, a common clinical problem, occurs in 7% to 34% of the general population and in 21% of people older than 70 years.1 Subacromial impingement refers to shoulder pain resulting from mechanical impingement of the rotator cuff underneath the coracoacromial arch between the acromion and the humeral head.2,3 Subacromial impingement syndrome (SIS) is the most common cause of shoulder pain, resulting in significant functional deficits and disability.3
Treatment options for SIS include conservative modalities such as use of nonsteroidal anti-inflammatory drugs, physical therapy (PT), and subacromial corticosteroid injections (CSIs). Studies have found short- and long-term improvement in pain, function, and range of motion after CSI.4-8 Subacromial CSI can be administered through an anterior or a posterior route.4,9 There have been several studies of the accuracy of anterior and posterior CSIs,10-12 with 2 studies finding similar accuracy for these routes.10,11 However, there may be a sex difference: In women, a posterior route may be less accurate than an anterior or a lateral route.12
Although the accuracy of anterior and posterior routes has been studied, their effect on clinical outcomes has not. We conducted a study to understand the effects of anterior and posterior CSIs on SIS. As one of the accuracy studies suggested anterior CSI is more accurate—the anterior route was theorized to provide easier access to the subacromial space12—we hypothesized patients treated with anterior CSI would have superior clinical outcomes 6 months after injection.12,13
Materials and Methods
Study Participants and Randomization
After this study received Institutional Review Board approval, patients with shoulder pain of more than 3 months’ duration and consistent with SIS were screened for inclusion. Eligible patients had pain in the anterior biceps and over the top of the shoulder with overhead activities as well as one or more clinical findings on physical examination: Hawkins-Kennedy sign, Neer sign, painful arc, and infraspinatus pain (pain with external rotation).
Patients were excluded if their history included prior subacromial CSI, adhesive capsulitis (inability to passively abduct shoulder to 90° with scapular stabilization), calcific tendonitis, radiographic evidence of os acromiale, cervical radiculopathy, Spurling sign, neck pain, radiating arm pain or numbness, sensory deficits, or neck and upper extremity motor dysfunction. Also excluded were patients with full-thickness rotator cuff tear, weakness on arm elevation, positive "drop arm sign," or high-riding humerus on standing shoulder radiograph. Patients who had work-related injuries or who were involved in worker compensation were excluded as well.
Enrolled patients were randomly assigned (with use of a computer-based random number generator) to receive either anterior CSI or posterior CSI.
Injection Procedures
All patients were administered 5 mL of lidocaine 1% (without epinephrine) and 2 mL (80 mg) of triamcinolone by 2 board-certified orthopedic surgeons using a 22-gauge 1½-inch needle. For patients who received their subacromial CSI by the anterior route, the arm was held in 0° of abduction and 20° of external rotation. The needle was inserted medial to the humeral head, lateral to the coracoid process, beginning 1 cm inferior to the clavicle with the needle directed posteriorly and laterally toward the acromion.10 For patients who received their CSI by the posterior route, the arm was held in 0° of abduction, the posterolateral corner of the acromion was identified by palpation, and the needle was inserted 1 cm inferior and medial to this point with the needle directed anteriorly and laterally toward the acromion.10,12 In both groups, the subacromial space was identified when a drop in pressure was felt during needle insertion. Accuracy was assessed post hoc by asking patients to grade their response to the injection on a visual analog scale (VAS); VAS score was used as a surrogate for improvement. All patients had a positive Neer test: Pain decreased with impingement maneuvers immediately after injection.
All patients were referred for PT provided according to an evidence-based rehabilitation protocol.14 This program emphasized range of motion with shoulder shrugs, scapular retraction, and pendulum exercises; flexibility with stretching exercises targeting the anterior and posterior aspects of the shoulder and cane stretching for forward elevation and external rotation; and strength with strengthening exercises involving the rotator cuff and scapular stabilizers.
Outcome Measures
Pain was measured with VAS scores and function with Single Assessment Numeric Evaluation (SANE) scores. The VAS is a validated outcome measure of pain intensity. A numeric version of the VAS was used: Patients selected the whole number, from 0 (no pain) to 10 (worst possible pain), that best reflected their pain intensity. On SANE, another validated outcome measure, patients rated their shoulder function as a percentage of normal, from 0% (no function possible) to 100% (perfect).15 Before injection, all patients were administered the VAS and SANE questionnaires to establish their baseline pain level and opinion of shoulder function. These measures were repeated 1, 3, and 6 months after injection. Telephone interviews were conducted at 1 month and 6 months. Patients were asked to return to clinic 3 months after injection as part of the standard of care. At 3 months, 47 (86%) of the 55 patients returned for follow-up and were administered the VAS and SANE questionnaires; the other 8 completed the questionnaires by telephone. At each time point, patients were asked to report on their participation in PT and/or adherence to their home exercise program.
Statistical Analysis
Power analysis performed with Student t test and a 2-sided level of P = .05 compared VAS pain scores between the anterior and posterior injection routes and found a mean (SD) difference of 1.4 (1.7).16 Power calculations made with nQuery Advisor Version 7.0 (Statistical Solutions) indicated a total sample size of 60 patients (30/group) would provide 80% power for detecting a significant difference assuming a 20% dropout rate.
Two-way mixed-model analysis of variance (ANOVA) was used to compare the anterior and posterior routes for statistical differences in both VAS pain scores and SANE function scores at baseline and 1, 3, and 6 months after injection. Likewise, time at baseline (just before injection)was compared with follow-up (1, 3, 6 months) with 2-way mixed-model ANOVA adjusting for anterior or posterior route. Multivariate analysis was performed to evaluate differences between baseline and 6-month follow-up with respect to anterior and posterior injection routes, controlling for age, sex, and body mass index (BMI) for VAS and SANE scores. Parametric testing methods were used for statistical analysis, which was performed with IBM SPSS Statistics Version 21.0 (IBM Corp). Significance was set at P < .05.
Results
Patient Characteristics
Of the 55 patients enrolled, 25 (46%) received anterior subacromial CSI and 30 (54%) received posterior CSI. All enrolled patients had a positive Neer impingement test immediately after injection. Mean (SD) age was 48 (9.3) years for anterior group patients and 48 (9.0) years for posterior group patients. There was no significant difference in age or BMI between the 2 groups (Table).
Five patients (9%) were excluded from the study after randomization and CSI: 2 for a full-thickness rotator cuff tear, 1 for a Bankart lesion, 1 for adhesive capsulitis, and 1 for a worker compensation claim.
One month after injection, 41 patients (75%) reported having engaged in PT as prescribed. Of the 47 patients (86%) who returned for the 3-month follow-up, 25 (46%) reported having engaged in PT between 1 month and 3 months after injection. Fourteen patients (26%) reported attending PT between 3 and 6 months post-injection.
Outcome Measures
Two-way repeated-measures ANOVA with age, sex, and BMI included as covariates revealed no significant differences in VAS scores between the anterior and posterior groups at any time point (P = .45). Both groups had highly significant pain reductions (anterior, F = 9.71, P < .001; posterior, F = 13.46, P < .001). Figure 1 shows mean VAS scores and significant reductions in pain 1, 3, and 6 months after injection (see asterisks for anterior and posterior groups; P < .001 for all). The groups had parallel rates of pain reduction over time, as indicated by a nonsignificant (P = .50) difference in slopes. These pain score reductions were significant for both injection routes and were independent of age, sex, and BMI (P > .05 for all).
Two-way repeated-measures ANOVA with age, sex, and BMI included as covariates revealed no significant differences in SANE scores between the anterior and posterior groups, except for a higher mean score in the anterior group at 3 months
(P = .02). There were no other group differences (P > .10 for all). Both groups had highly significant improvements in function (anterior, F = 17.34,
P < .001; posterior, F = 13.57, P < .001). Figure 2 shows mean SANE scores and significant improvement at 1, 3, and 6 months (see asterisks for anterior and posterior groups; P < .001 for all). The groups had parallel rates of improved function over time, as indicated by a nonsignificant (P = .51) difference in slopes. These function score improvements were significant for both injection routes and were independent of age, sex, and BMI (P > .05 for all).
From the results of this prospective randomized study, we concluded subacromial CSI significantly reduces pain and improves function regardless of route used. In addition, age, sex, and BMI do not significantly affect the efficacy of either anterior CSI or posterior CSI.
Discussion
In patients with SIS, anterior CSI and posterior CSI provided significant improvements in pain and function 1, 3, and 6 months after injection. These effects were independent of age, sex, BMI, and PT participation. There were no significant differences in outcomes between injection routes.
When conservative treatments for SIS do not resolve symptoms, inflammation and pain can be reduced with use of subacromial CSI.4-8 Although clinical outcomes are inconsistent, CSI can be used to address SIS symptoms in appropriate patients. Specifically, Blair and colleagues6 found that, CSI consisting of 4 mL of lidocaine 1% (without epinephrine) and 2 mL (80 mg) of triamcinolone was effective in alleviating shoulder pain and improving shoulder range of motion. Other authors have similarly reported improved outcomes after subacromial injection and short-term follow-up with PT.4,7,8 Our findings are consistent with these reports: CSI coupled with a structured rehabilitation program is effective in alleviating symptoms associated with acute or subacute SIS.
Numerous studies have found improved clinical outcomes after anterior CSI and after posterior CSI,6-8 but no study has directly compared the clinical impact of anterior CSI with that of posterior CSI—which suggests injection route may not affect ultimate clinical outcomes.
CSI accuracy has been studied extensively.10-12,17-20 Although 2 studies found similar accuracy for anterior and posterior routes,10,11 there may be a sex difference: In women, a posterior route may be less accurate than an anterior or a lateral route.12 Collectively, these studies expose the inherent difficulty in treating shoulder pain with localized subacromial injection. Therapy may fail because of errant needle positioning. Two prospective studies found improved clinical outcomes with successful delivery of medication into the subacromial space.17,18 Poor clinical outcomes may result from inaccurate CSI.
In contrast to other clinical studies, our study found that injection route was not associated with differences in clinical response. In a prospective randomized clinical trial in which 75 patients received a subacromial injection, Marder and colleagues12 found anterior routes 84% accurate and posterior routes 56% accurate; they concluded acromion anatomy and subacromial bursa anatomy make posterior injections more difficult. As theorized by Gruson and colleagues,13 with use of an anterior route, the needle enters inferior to the concavity of the acromion and provides easier access to the subacromial space. This idea is in line with Marder and colleagues’12 conclusion that subacromial bursa anatomy provides a favorable environment for accurate CSI.
If accuracy is positively correlated with clinical improvement and anterior routes are more accurate, there should be a difference in response to posterior injections. Our results provide evidence that clinical response to CSI may not depend on injection accuracy. Perhaps merely placing the corticosteroid near the bursa is adequate for improving symptoms or perhaps some of the clinical improvement is due to the systemic effect of corticosteroids. These possibilities require further analysis.
Establishing the efficacy of CSI in SIS is difficult. The literature includes various study designs, different CSI indications and medication formulations, and varying emphasis on the role of organized PT. Rehabilitation has been found to alleviate joint pain by reducing inflammation,14 but data do not universally support this finding.21,22 Nevertheless, use of PT might explain the divergence in clinical outcomes reported by Marder and colleagues,12 who found anterior CSI more accurate than posterior CSI. In our practice, PT is recommended for all SIS patients, not only those who have CSI. Thus, our findings are framed within the context of successful CSI but may include patients who improved with PT alone. This issue raises the question of whether subacromial CSI should be guided by ultrasound. Ultrasound guidance can improve CSI accuracy and clinical outcomes,23-25 though the value of this benefit is debated.26
This study had several limitations. First, pain relief was patient reported. Second, the treatment plan involved CSI with PT but did not control for CSI used alone. PT, which is part of the standard of care for patients with SIS, added another degree of complexity to the study. Third, there may have been some variability in SIS severity (stage 1, 2, or 3). Fourth, although the study design controlled for various shoulder pathologies, advanced imaging, which could have provided diagnosis confirmation, was not available for all patients. Therefore, concurrent conditions may have confounded results. However, randomization was used to try to minimize this effect. Fifth, although injection routes were randomly assigned, the trial was not blinded. Sixth, the study was underpowered by 1 patient, as there was an estimated 20% dropout rate over 3 and 6 months of follow-up. However, we do not think our results were significantly affected.
Although more research is needed to fully describe the role of subacromial CSI in SIS, our study findings suggested that CSI using either an anterior or a posterior route creates a window of symptomatic relief in which patients may be able to engage in PT.
Conclusion
Both anterior CSI and posterior CSI significantly improved pain and function for up to 6 months. No differences were found between anterior and posterior CSIs. In the context of this study, CSI combined with structured PT produced significant improvement in pain and function in patients with SIS, regardless of injection route used. Clinicians should rely on their clinical acumen when selecting injection routes, as anterior and posterior are both beneficial.
1. Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database Syst Rev. 2003;(1):CD004016.
2. Bell AD, Conaway D. Corticosteroid injections for painful shoulders. Int J Clin Pract. 2005;59(10):1178-1186.
3. Michener LA, McClure PW, Karduna AR. Anatomical and biomechanical mechanisms of subacromial impingement syndrome. Clin Biomech. 2003;18(5):369-379.
4. Akgün K, Birtane M, Akarirmak U. Is local subacromial corticosteroid injection beneficial in subacromial impingement syndrome? Clin Rheumatol. 2004;23(6):496-500.
5. Bhagra A, Syed H, Reed DA, et al. Efficacy of musculoskeletal injections by primary care providers in the office: a retrospective cohort study. Int J Gen Med. 2013;6:237-243.
6. Blair B, Rokito AS, Cuomo F, Jarolem K, Zuckerman JD. Efficacy of injections of corticosteroids for subacromial impingement syndrome. J Bone Joint Surg Am. 1996;78(11):1685-1689.
7. Cummins CA, Sasso LM, Nicholson D. Impingement syndrome: temporal outcomes of nonoperative treatment.
J Shoulder Elbow Surg. 2009;18(2):172-177.
8. Yu C, Chen CH, Liu HT, Dai MH, Wang IC, Wang KC. Subacromial injections of corticosteroids and Xylocaine for painful subacromial impingement syndrome. Chang Gung Med J. 2006;29(5):474-478.
9. Codsi MJ. The painful shoulder: when to inject and when to refer. Cleve Clin J Med. 2007;74(7):473-474, 477-478, 480-482 passim.
10. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
11. Kang MN, Rizio L, Prybicien M, Middlemas DA, Blacksin MF. The accuracy of subacromial corticosteroid injections: a comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(15):61S-66S.
12. Marder RA, Kim SH, Labson JD, Hunter JC. Injection of the subacromial bursa in patients with rotator cuff syndrome: a prospective, randomized study comparing the effectiveness of different routes. J Bone Joint Surg Am. 2012;94(16):
1442-1447.
13. Gruson, KI, Ruchelsman DE, Zuckerman JD. Subacromial corticosteroid injections. J Shoulder Elbow Surg. 2008;17(1 suppl):118S-130S.
14. Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18(1):138-160.
15. Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214-221.
16. Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease.
J Shoulder Elbow Surg. 2009;88(6):927-932.
17. Eustace JA, Brophy DP, Gibney RP, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
18. Esenyel CZ, Esenyel M, Yeiltepe R, et al. The correlation between the accuracy of steroid injections and subsequent shoulder pain and function in subacromial impingement
syndrome [in Turkish]. Acta Orthop Traumatol Turc. 2003;37(1):
41-45.
19. Powell SE, Davis SM, Lee EH, et al. Accuracy of palpation-directed intra-articular glenohumeral injection confirmed by magnetic resonance arthrography. Arthroscopy. 2015;31(2):205-208.
20. Rutten MJ, Maresch BJ, Jager GJ, de Waal Malefijt MC. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
21. Desmeules F, Côté CH, Frémont P. Therapeutic exercise and orthopedic manual therapy for impingement syndrome: a systematic review. Clin J Sport Med. 2003;13(3):176-182.
22. Winters JC, Sobel JS, Groenier KH, Arendzen HJ, Meyboom-de Jong B. Comparison of physiotherapy, manipulation, and corticosteroid injection for treating shoulder complaints in general practice: randomised, single blind study. BMJ. 1997;314(7090):1320-1325.
23. Chen MJ, Lew HL, Hsu TC, et al. Ultrasound-guided shoulder injections in the treatment of subacromial bursitis. Am J Phys Med Rehabil. 2006;85(1):31-35.
24. Hsieh LF, Hsu WC, Lin YJ, Wu SH, Chang KC, Chang HL. Is ultrasound-guided injection more effective in chronic subacromial bursitis? Med Sci Sports Exerc. 2013;45(12):
2205-2213.
25. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
26. Hall S, Buchbinder R. Do imaging methods that guide needle placement improve outcome? Ann Rheum Dis. 2004;63(9):1007-1008.
Take-Home Points
When conservative treatments for SIS do not resolve symptoms, inflammation and pain can be reduced with use of subacromial CSI.
Both anterior CSI and posterior CSI significantly improved pain and function for up to 6 months
CSI combined with structured PT produced significant improvement in pain and function in patients with SIS, regardless of injection route used.
Clinical response to CSI may not depend on injection accuracy.
Clinicians should rely on their clinical acumen when selecting injection routes, as anterior and posterior are both beneficial.
Shoulder pain, a common clinical problem, occurs in 7% to 34% of the general population and in 21% of people older than 70 years.1 Subacromial impingement refers to shoulder pain resulting from mechanical impingement of the rotator cuff underneath the coracoacromial arch between the acromion and the humeral head.2,3 Subacromial impingement syndrome (SIS) is the most common cause of shoulder pain, resulting in significant functional deficits and disability.3
Treatment options for SIS include conservative modalities such as use of nonsteroidal anti-inflammatory drugs, physical therapy (PT), and subacromial corticosteroid injections (CSIs). Studies have found short- and long-term improvement in pain, function, and range of motion after CSI.4-8 Subacromial CSI can be administered through an anterior or a posterior route.4,9 There have been several studies of the accuracy of anterior and posterior CSIs,10-12 with 2 studies finding similar accuracy for these routes.10,11 However, there may be a sex difference: In women, a posterior route may be less accurate than an anterior or a lateral route.12
Although the accuracy of anterior and posterior routes has been studied, their effect on clinical outcomes has not. We conducted a study to understand the effects of anterior and posterior CSIs on SIS. As one of the accuracy studies suggested anterior CSI is more accurate—the anterior route was theorized to provide easier access to the subacromial space12—we hypothesized patients treated with anterior CSI would have superior clinical outcomes 6 months after injection.12,13
Materials and Methods
Study Participants and Randomization
After this study received Institutional Review Board approval, patients with shoulder pain of more than 3 months’ duration and consistent with SIS were screened for inclusion. Eligible patients had pain in the anterior biceps and over the top of the shoulder with overhead activities as well as one or more clinical findings on physical examination: Hawkins-Kennedy sign, Neer sign, painful arc, and infraspinatus pain (pain with external rotation).
Patients were excluded if their history included prior subacromial CSI, adhesive capsulitis (inability to passively abduct shoulder to 90° with scapular stabilization), calcific tendonitis, radiographic evidence of os acromiale, cervical radiculopathy, Spurling sign, neck pain, radiating arm pain or numbness, sensory deficits, or neck and upper extremity motor dysfunction. Also excluded were patients with full-thickness rotator cuff tear, weakness on arm elevation, positive "drop arm sign," or high-riding humerus on standing shoulder radiograph. Patients who had work-related injuries or who were involved in worker compensation were excluded as well.
Enrolled patients were randomly assigned (with use of a computer-based random number generator) to receive either anterior CSI or posterior CSI.
Injection Procedures
All patients were administered 5 mL of lidocaine 1% (without epinephrine) and 2 mL (80 mg) of triamcinolone by 2 board-certified orthopedic surgeons using a 22-gauge 1½-inch needle. For patients who received their subacromial CSI by the anterior route, the arm was held in 0° of abduction and 20° of external rotation. The needle was inserted medial to the humeral head, lateral to the coracoid process, beginning 1 cm inferior to the clavicle with the needle directed posteriorly and laterally toward the acromion.10 For patients who received their CSI by the posterior route, the arm was held in 0° of abduction, the posterolateral corner of the acromion was identified by palpation, and the needle was inserted 1 cm inferior and medial to this point with the needle directed anteriorly and laterally toward the acromion.10,12 In both groups, the subacromial space was identified when a drop in pressure was felt during needle insertion. Accuracy was assessed post hoc by asking patients to grade their response to the injection on a visual analog scale (VAS); VAS score was used as a surrogate for improvement. All patients had a positive Neer test: Pain decreased with impingement maneuvers immediately after injection.
All patients were referred for PT provided according to an evidence-based rehabilitation protocol.14 This program emphasized range of motion with shoulder shrugs, scapular retraction, and pendulum exercises; flexibility with stretching exercises targeting the anterior and posterior aspects of the shoulder and cane stretching for forward elevation and external rotation; and strength with strengthening exercises involving the rotator cuff and scapular stabilizers.
Outcome Measures
Pain was measured with VAS scores and function with Single Assessment Numeric Evaluation (SANE) scores. The VAS is a validated outcome measure of pain intensity. A numeric version of the VAS was used: Patients selected the whole number, from 0 (no pain) to 10 (worst possible pain), that best reflected their pain intensity. On SANE, another validated outcome measure, patients rated their shoulder function as a percentage of normal, from 0% (no function possible) to 100% (perfect).15 Before injection, all patients were administered the VAS and SANE questionnaires to establish their baseline pain level and opinion of shoulder function. These measures were repeated 1, 3, and 6 months after injection. Telephone interviews were conducted at 1 month and 6 months. Patients were asked to return to clinic 3 months after injection as part of the standard of care. At 3 months, 47 (86%) of the 55 patients returned for follow-up and were administered the VAS and SANE questionnaires; the other 8 completed the questionnaires by telephone. At each time point, patients were asked to report on their participation in PT and/or adherence to their home exercise program.
Statistical Analysis
Power analysis performed with Student t test and a 2-sided level of P = .05 compared VAS pain scores between the anterior and posterior injection routes and found a mean (SD) difference of 1.4 (1.7).16 Power calculations made with nQuery Advisor Version 7.0 (Statistical Solutions) indicated a total sample size of 60 patients (30/group) would provide 80% power for detecting a significant difference assuming a 20% dropout rate.
Two-way mixed-model analysis of variance (ANOVA) was used to compare the anterior and posterior routes for statistical differences in both VAS pain scores and SANE function scores at baseline and 1, 3, and 6 months after injection. Likewise, time at baseline (just before injection)was compared with follow-up (1, 3, 6 months) with 2-way mixed-model ANOVA adjusting for anterior or posterior route. Multivariate analysis was performed to evaluate differences between baseline and 6-month follow-up with respect to anterior and posterior injection routes, controlling for age, sex, and body mass index (BMI) for VAS and SANE scores. Parametric testing methods were used for statistical analysis, which was performed with IBM SPSS Statistics Version 21.0 (IBM Corp). Significance was set at P < .05.
Results
Patient Characteristics
Of the 55 patients enrolled, 25 (46%) received anterior subacromial CSI and 30 (54%) received posterior CSI. All enrolled patients had a positive Neer impingement test immediately after injection. Mean (SD) age was 48 (9.3) years for anterior group patients and 48 (9.0) years for posterior group patients. There was no significant difference in age or BMI between the 2 groups (Table).
Five patients (9%) were excluded from the study after randomization and CSI: 2 for a full-thickness rotator cuff tear, 1 for a Bankart lesion, 1 for adhesive capsulitis, and 1 for a worker compensation claim.
One month after injection, 41 patients (75%) reported having engaged in PT as prescribed. Of the 47 patients (86%) who returned for the 3-month follow-up, 25 (46%) reported having engaged in PT between 1 month and 3 months after injection. Fourteen patients (26%) reported attending PT between 3 and 6 months post-injection.
Outcome Measures
Two-way repeated-measures ANOVA with age, sex, and BMI included as covariates revealed no significant differences in VAS scores between the anterior and posterior groups at any time point (P = .45). Both groups had highly significant pain reductions (anterior, F = 9.71, P < .001; posterior, F = 13.46, P < .001). Figure 1 shows mean VAS scores and significant reductions in pain 1, 3, and 6 months after injection (see asterisks for anterior and posterior groups; P < .001 for all). The groups had parallel rates of pain reduction over time, as indicated by a nonsignificant (P = .50) difference in slopes. These pain score reductions were significant for both injection routes and were independent of age, sex, and BMI (P > .05 for all).
Two-way repeated-measures ANOVA with age, sex, and BMI included as covariates revealed no significant differences in SANE scores between the anterior and posterior groups, except for a higher mean score in the anterior group at 3 months
(P = .02). There were no other group differences (P > .10 for all). Both groups had highly significant improvements in function (anterior, F = 17.34,
P < .001; posterior, F = 13.57, P < .001). Figure 2 shows mean SANE scores and significant improvement at 1, 3, and 6 months (see asterisks for anterior and posterior groups; P < .001 for all). The groups had parallel rates of improved function over time, as indicated by a nonsignificant (P = .51) difference in slopes. These function score improvements were significant for both injection routes and were independent of age, sex, and BMI (P > .05 for all).
From the results of this prospective randomized study, we concluded subacromial CSI significantly reduces pain and improves function regardless of route used. In addition, age, sex, and BMI do not significantly affect the efficacy of either anterior CSI or posterior CSI.
Discussion
In patients with SIS, anterior CSI and posterior CSI provided significant improvements in pain and function 1, 3, and 6 months after injection. These effects were independent of age, sex, BMI, and PT participation. There were no significant differences in outcomes between injection routes.
When conservative treatments for SIS do not resolve symptoms, inflammation and pain can be reduced with use of subacromial CSI.4-8 Although clinical outcomes are inconsistent, CSI can be used to address SIS symptoms in appropriate patients. Specifically, Blair and colleagues6 found that, CSI consisting of 4 mL of lidocaine 1% (without epinephrine) and 2 mL (80 mg) of triamcinolone was effective in alleviating shoulder pain and improving shoulder range of motion. Other authors have similarly reported improved outcomes after subacromial injection and short-term follow-up with PT.4,7,8 Our findings are consistent with these reports: CSI coupled with a structured rehabilitation program is effective in alleviating symptoms associated with acute or subacute SIS.
Numerous studies have found improved clinical outcomes after anterior CSI and after posterior CSI,6-8 but no study has directly compared the clinical impact of anterior CSI with that of posterior CSI—which suggests injection route may not affect ultimate clinical outcomes.
CSI accuracy has been studied extensively.10-12,17-20 Although 2 studies found similar accuracy for anterior and posterior routes,10,11 there may be a sex difference: In women, a posterior route may be less accurate than an anterior or a lateral route.12 Collectively, these studies expose the inherent difficulty in treating shoulder pain with localized subacromial injection. Therapy may fail because of errant needle positioning. Two prospective studies found improved clinical outcomes with successful delivery of medication into the subacromial space.17,18 Poor clinical outcomes may result from inaccurate CSI.
In contrast to other clinical studies, our study found that injection route was not associated with differences in clinical response. In a prospective randomized clinical trial in which 75 patients received a subacromial injection, Marder and colleagues12 found anterior routes 84% accurate and posterior routes 56% accurate; they concluded acromion anatomy and subacromial bursa anatomy make posterior injections more difficult. As theorized by Gruson and colleagues,13 with use of an anterior route, the needle enters inferior to the concavity of the acromion and provides easier access to the subacromial space. This idea is in line with Marder and colleagues’12 conclusion that subacromial bursa anatomy provides a favorable environment for accurate CSI.
If accuracy is positively correlated with clinical improvement and anterior routes are more accurate, there should be a difference in response to posterior injections. Our results provide evidence that clinical response to CSI may not depend on injection accuracy. Perhaps merely placing the corticosteroid near the bursa is adequate for improving symptoms or perhaps some of the clinical improvement is due to the systemic effect of corticosteroids. These possibilities require further analysis.
Establishing the efficacy of CSI in SIS is difficult. The literature includes various study designs, different CSI indications and medication formulations, and varying emphasis on the role of organized PT. Rehabilitation has been found to alleviate joint pain by reducing inflammation,14 but data do not universally support this finding.21,22 Nevertheless, use of PT might explain the divergence in clinical outcomes reported by Marder and colleagues,12 who found anterior CSI more accurate than posterior CSI. In our practice, PT is recommended for all SIS patients, not only those who have CSI. Thus, our findings are framed within the context of successful CSI but may include patients who improved with PT alone. This issue raises the question of whether subacromial CSI should be guided by ultrasound. Ultrasound guidance can improve CSI accuracy and clinical outcomes,23-25 though the value of this benefit is debated.26
This study had several limitations. First, pain relief was patient reported. Second, the treatment plan involved CSI with PT but did not control for CSI used alone. PT, which is part of the standard of care for patients with SIS, added another degree of complexity to the study. Third, there may have been some variability in SIS severity (stage 1, 2, or 3). Fourth, although the study design controlled for various shoulder pathologies, advanced imaging, which could have provided diagnosis confirmation, was not available for all patients. Therefore, concurrent conditions may have confounded results. However, randomization was used to try to minimize this effect. Fifth, although injection routes were randomly assigned, the trial was not blinded. Sixth, the study was underpowered by 1 patient, as there was an estimated 20% dropout rate over 3 and 6 months of follow-up. However, we do not think our results were significantly affected.
Although more research is needed to fully describe the role of subacromial CSI in SIS, our study findings suggested that CSI using either an anterior or a posterior route creates a window of symptomatic relief in which patients may be able to engage in PT.
Conclusion
Both anterior CSI and posterior CSI significantly improved pain and function for up to 6 months. No differences were found between anterior and posterior CSIs. In the context of this study, CSI combined with structured PT produced significant improvement in pain and function in patients with SIS, regardless of injection route used. Clinicians should rely on their clinical acumen when selecting injection routes, as anterior and posterior are both beneficial.
Take-Home Points
When conservative treatments for SIS do not resolve symptoms, inflammation and pain can be reduced with use of subacromial CSI.
Both anterior CSI and posterior CSI significantly improved pain and function for up to 6 months
CSI combined with structured PT produced significant improvement in pain and function in patients with SIS, regardless of injection route used.
Clinical response to CSI may not depend on injection accuracy.
Clinicians should rely on their clinical acumen when selecting injection routes, as anterior and posterior are both beneficial.
Shoulder pain, a common clinical problem, occurs in 7% to 34% of the general population and in 21% of people older than 70 years.1 Subacromial impingement refers to shoulder pain resulting from mechanical impingement of the rotator cuff underneath the coracoacromial arch between the acromion and the humeral head.2,3 Subacromial impingement syndrome (SIS) is the most common cause of shoulder pain, resulting in significant functional deficits and disability.3
Treatment options for SIS include conservative modalities such as use of nonsteroidal anti-inflammatory drugs, physical therapy (PT), and subacromial corticosteroid injections (CSIs). Studies have found short- and long-term improvement in pain, function, and range of motion after CSI.4-8 Subacromial CSI can be administered through an anterior or a posterior route.4,9 There have been several studies of the accuracy of anterior and posterior CSIs,10-12 with 2 studies finding similar accuracy for these routes.10,11 However, there may be a sex difference: In women, a posterior route may be less accurate than an anterior or a lateral route.12
Although the accuracy of anterior and posterior routes has been studied, their effect on clinical outcomes has not. We conducted a study to understand the effects of anterior and posterior CSIs on SIS. As one of the accuracy studies suggested anterior CSI is more accurate—the anterior route was theorized to provide easier access to the subacromial space12—we hypothesized patients treated with anterior CSI would have superior clinical outcomes 6 months after injection.12,13
Materials and Methods
Study Participants and Randomization
After this study received Institutional Review Board approval, patients with shoulder pain of more than 3 months’ duration and consistent with SIS were screened for inclusion. Eligible patients had pain in the anterior biceps and over the top of the shoulder with overhead activities as well as one or more clinical findings on physical examination: Hawkins-Kennedy sign, Neer sign, painful arc, and infraspinatus pain (pain with external rotation).
Patients were excluded if their history included prior subacromial CSI, adhesive capsulitis (inability to passively abduct shoulder to 90° with scapular stabilization), calcific tendonitis, radiographic evidence of os acromiale, cervical radiculopathy, Spurling sign, neck pain, radiating arm pain or numbness, sensory deficits, or neck and upper extremity motor dysfunction. Also excluded were patients with full-thickness rotator cuff tear, weakness on arm elevation, positive "drop arm sign," or high-riding humerus on standing shoulder radiograph. Patients who had work-related injuries or who were involved in worker compensation were excluded as well.
Enrolled patients were randomly assigned (with use of a computer-based random number generator) to receive either anterior CSI or posterior CSI.
Injection Procedures
All patients were administered 5 mL of lidocaine 1% (without epinephrine) and 2 mL (80 mg) of triamcinolone by 2 board-certified orthopedic surgeons using a 22-gauge 1½-inch needle. For patients who received their subacromial CSI by the anterior route, the arm was held in 0° of abduction and 20° of external rotation. The needle was inserted medial to the humeral head, lateral to the coracoid process, beginning 1 cm inferior to the clavicle with the needle directed posteriorly and laterally toward the acromion.10 For patients who received their CSI by the posterior route, the arm was held in 0° of abduction, the posterolateral corner of the acromion was identified by palpation, and the needle was inserted 1 cm inferior and medial to this point with the needle directed anteriorly and laterally toward the acromion.10,12 In both groups, the subacromial space was identified when a drop in pressure was felt during needle insertion. Accuracy was assessed post hoc by asking patients to grade their response to the injection on a visual analog scale (VAS); VAS score was used as a surrogate for improvement. All patients had a positive Neer test: Pain decreased with impingement maneuvers immediately after injection.
All patients were referred for PT provided according to an evidence-based rehabilitation protocol.14 This program emphasized range of motion with shoulder shrugs, scapular retraction, and pendulum exercises; flexibility with stretching exercises targeting the anterior and posterior aspects of the shoulder and cane stretching for forward elevation and external rotation; and strength with strengthening exercises involving the rotator cuff and scapular stabilizers.
Outcome Measures
Pain was measured with VAS scores and function with Single Assessment Numeric Evaluation (SANE) scores. The VAS is a validated outcome measure of pain intensity. A numeric version of the VAS was used: Patients selected the whole number, from 0 (no pain) to 10 (worst possible pain), that best reflected their pain intensity. On SANE, another validated outcome measure, patients rated their shoulder function as a percentage of normal, from 0% (no function possible) to 100% (perfect).15 Before injection, all patients were administered the VAS and SANE questionnaires to establish their baseline pain level and opinion of shoulder function. These measures were repeated 1, 3, and 6 months after injection. Telephone interviews were conducted at 1 month and 6 months. Patients were asked to return to clinic 3 months after injection as part of the standard of care. At 3 months, 47 (86%) of the 55 patients returned for follow-up and were administered the VAS and SANE questionnaires; the other 8 completed the questionnaires by telephone. At each time point, patients were asked to report on their participation in PT and/or adherence to their home exercise program.
Statistical Analysis
Power analysis performed with Student t test and a 2-sided level of P = .05 compared VAS pain scores between the anterior and posterior injection routes and found a mean (SD) difference of 1.4 (1.7).16 Power calculations made with nQuery Advisor Version 7.0 (Statistical Solutions) indicated a total sample size of 60 patients (30/group) would provide 80% power for detecting a significant difference assuming a 20% dropout rate.
Two-way mixed-model analysis of variance (ANOVA) was used to compare the anterior and posterior routes for statistical differences in both VAS pain scores and SANE function scores at baseline and 1, 3, and 6 months after injection. Likewise, time at baseline (just before injection)was compared with follow-up (1, 3, 6 months) with 2-way mixed-model ANOVA adjusting for anterior or posterior route. Multivariate analysis was performed to evaluate differences between baseline and 6-month follow-up with respect to anterior and posterior injection routes, controlling for age, sex, and body mass index (BMI) for VAS and SANE scores. Parametric testing methods were used for statistical analysis, which was performed with IBM SPSS Statistics Version 21.0 (IBM Corp). Significance was set at P < .05.
Results
Patient Characteristics
Of the 55 patients enrolled, 25 (46%) received anterior subacromial CSI and 30 (54%) received posterior CSI. All enrolled patients had a positive Neer impingement test immediately after injection. Mean (SD) age was 48 (9.3) years for anterior group patients and 48 (9.0) years for posterior group patients. There was no significant difference in age or BMI between the 2 groups (Table).
Five patients (9%) were excluded from the study after randomization and CSI: 2 for a full-thickness rotator cuff tear, 1 for a Bankart lesion, 1 for adhesive capsulitis, and 1 for a worker compensation claim.
One month after injection, 41 patients (75%) reported having engaged in PT as prescribed. Of the 47 patients (86%) who returned for the 3-month follow-up, 25 (46%) reported having engaged in PT between 1 month and 3 months after injection. Fourteen patients (26%) reported attending PT between 3 and 6 months post-injection.
Outcome Measures
Two-way repeated-measures ANOVA with age, sex, and BMI included as covariates revealed no significant differences in VAS scores between the anterior and posterior groups at any time point (P = .45). Both groups had highly significant pain reductions (anterior, F = 9.71, P < .001; posterior, F = 13.46, P < .001). Figure 1 shows mean VAS scores and significant reductions in pain 1, 3, and 6 months after injection (see asterisks for anterior and posterior groups; P < .001 for all). The groups had parallel rates of pain reduction over time, as indicated by a nonsignificant (P = .50) difference in slopes. These pain score reductions were significant for both injection routes and were independent of age, sex, and BMI (P > .05 for all).
Two-way repeated-measures ANOVA with age, sex, and BMI included as covariates revealed no significant differences in SANE scores between the anterior and posterior groups, except for a higher mean score in the anterior group at 3 months
(P = .02). There were no other group differences (P > .10 for all). Both groups had highly significant improvements in function (anterior, F = 17.34,
P < .001; posterior, F = 13.57, P < .001). Figure 2 shows mean SANE scores and significant improvement at 1, 3, and 6 months (see asterisks for anterior and posterior groups; P < .001 for all). The groups had parallel rates of improved function over time, as indicated by a nonsignificant (P = .51) difference in slopes. These function score improvements were significant for both injection routes and were independent of age, sex, and BMI (P > .05 for all).
From the results of this prospective randomized study, we concluded subacromial CSI significantly reduces pain and improves function regardless of route used. In addition, age, sex, and BMI do not significantly affect the efficacy of either anterior CSI or posterior CSI.
Discussion
In patients with SIS, anterior CSI and posterior CSI provided significant improvements in pain and function 1, 3, and 6 months after injection. These effects were independent of age, sex, BMI, and PT participation. There were no significant differences in outcomes between injection routes.
When conservative treatments for SIS do not resolve symptoms, inflammation and pain can be reduced with use of subacromial CSI.4-8 Although clinical outcomes are inconsistent, CSI can be used to address SIS symptoms in appropriate patients. Specifically, Blair and colleagues6 found that, CSI consisting of 4 mL of lidocaine 1% (without epinephrine) and 2 mL (80 mg) of triamcinolone was effective in alleviating shoulder pain and improving shoulder range of motion. Other authors have similarly reported improved outcomes after subacromial injection and short-term follow-up with PT.4,7,8 Our findings are consistent with these reports: CSI coupled with a structured rehabilitation program is effective in alleviating symptoms associated with acute or subacute SIS.
Numerous studies have found improved clinical outcomes after anterior CSI and after posterior CSI,6-8 but no study has directly compared the clinical impact of anterior CSI with that of posterior CSI—which suggests injection route may not affect ultimate clinical outcomes.
CSI accuracy has been studied extensively.10-12,17-20 Although 2 studies found similar accuracy for anterior and posterior routes,10,11 there may be a sex difference: In women, a posterior route may be less accurate than an anterior or a lateral route.12 Collectively, these studies expose the inherent difficulty in treating shoulder pain with localized subacromial injection. Therapy may fail because of errant needle positioning. Two prospective studies found improved clinical outcomes with successful delivery of medication into the subacromial space.17,18 Poor clinical outcomes may result from inaccurate CSI.
In contrast to other clinical studies, our study found that injection route was not associated with differences in clinical response. In a prospective randomized clinical trial in which 75 patients received a subacromial injection, Marder and colleagues12 found anterior routes 84% accurate and posterior routes 56% accurate; they concluded acromion anatomy and subacromial bursa anatomy make posterior injections more difficult. As theorized by Gruson and colleagues,13 with use of an anterior route, the needle enters inferior to the concavity of the acromion and provides easier access to the subacromial space. This idea is in line with Marder and colleagues’12 conclusion that subacromial bursa anatomy provides a favorable environment for accurate CSI.
If accuracy is positively correlated with clinical improvement and anterior routes are more accurate, there should be a difference in response to posterior injections. Our results provide evidence that clinical response to CSI may not depend on injection accuracy. Perhaps merely placing the corticosteroid near the bursa is adequate for improving symptoms or perhaps some of the clinical improvement is due to the systemic effect of corticosteroids. These possibilities require further analysis.
Establishing the efficacy of CSI in SIS is difficult. The literature includes various study designs, different CSI indications and medication formulations, and varying emphasis on the role of organized PT. Rehabilitation has been found to alleviate joint pain by reducing inflammation,14 but data do not universally support this finding.21,22 Nevertheless, use of PT might explain the divergence in clinical outcomes reported by Marder and colleagues,12 who found anterior CSI more accurate than posterior CSI. In our practice, PT is recommended for all SIS patients, not only those who have CSI. Thus, our findings are framed within the context of successful CSI but may include patients who improved with PT alone. This issue raises the question of whether subacromial CSI should be guided by ultrasound. Ultrasound guidance can improve CSI accuracy and clinical outcomes,23-25 though the value of this benefit is debated.26
This study had several limitations. First, pain relief was patient reported. Second, the treatment plan involved CSI with PT but did not control for CSI used alone. PT, which is part of the standard of care for patients with SIS, added another degree of complexity to the study. Third, there may have been some variability in SIS severity (stage 1, 2, or 3). Fourth, although the study design controlled for various shoulder pathologies, advanced imaging, which could have provided diagnosis confirmation, was not available for all patients. Therefore, concurrent conditions may have confounded results. However, randomization was used to try to minimize this effect. Fifth, although injection routes were randomly assigned, the trial was not blinded. Sixth, the study was underpowered by 1 patient, as there was an estimated 20% dropout rate over 3 and 6 months of follow-up. However, we do not think our results were significantly affected.
Although more research is needed to fully describe the role of subacromial CSI in SIS, our study findings suggested that CSI using either an anterior or a posterior route creates a window of symptomatic relief in which patients may be able to engage in PT.
Conclusion
Both anterior CSI and posterior CSI significantly improved pain and function for up to 6 months. No differences were found between anterior and posterior CSIs. In the context of this study, CSI combined with structured PT produced significant improvement in pain and function in patients with SIS, regardless of injection route used. Clinicians should rely on their clinical acumen when selecting injection routes, as anterior and posterior are both beneficial.
1. Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database Syst Rev. 2003;(1):CD004016.
2. Bell AD, Conaway D. Corticosteroid injections for painful shoulders. Int J Clin Pract. 2005;59(10):1178-1186.
3. Michener LA, McClure PW, Karduna AR. Anatomical and biomechanical mechanisms of subacromial impingement syndrome. Clin Biomech. 2003;18(5):369-379.
4. Akgün K, Birtane M, Akarirmak U. Is local subacromial corticosteroid injection beneficial in subacromial impingement syndrome? Clin Rheumatol. 2004;23(6):496-500.
5. Bhagra A, Syed H, Reed DA, et al. Efficacy of musculoskeletal injections by primary care providers in the office: a retrospective cohort study. Int J Gen Med. 2013;6:237-243.
6. Blair B, Rokito AS, Cuomo F, Jarolem K, Zuckerman JD. Efficacy of injections of corticosteroids for subacromial impingement syndrome. J Bone Joint Surg Am. 1996;78(11):1685-1689.
7. Cummins CA, Sasso LM, Nicholson D. Impingement syndrome: temporal outcomes of nonoperative treatment.
J Shoulder Elbow Surg. 2009;18(2):172-177.
8. Yu C, Chen CH, Liu HT, Dai MH, Wang IC, Wang KC. Subacromial injections of corticosteroids and Xylocaine for painful subacromial impingement syndrome. Chang Gung Med J. 2006;29(5):474-478.
9. Codsi MJ. The painful shoulder: when to inject and when to refer. Cleve Clin J Med. 2007;74(7):473-474, 477-478, 480-482 passim.
10. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
11. Kang MN, Rizio L, Prybicien M, Middlemas DA, Blacksin MF. The accuracy of subacromial corticosteroid injections: a comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(15):61S-66S.
12. Marder RA, Kim SH, Labson JD, Hunter JC. Injection of the subacromial bursa in patients with rotator cuff syndrome: a prospective, randomized study comparing the effectiveness of different routes. J Bone Joint Surg Am. 2012;94(16):
1442-1447.
13. Gruson, KI, Ruchelsman DE, Zuckerman JD. Subacromial corticosteroid injections. J Shoulder Elbow Surg. 2008;17(1 suppl):118S-130S.
14. Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18(1):138-160.
15. Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214-221.
16. Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease.
J Shoulder Elbow Surg. 2009;88(6):927-932.
17. Eustace JA, Brophy DP, Gibney RP, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
18. Esenyel CZ, Esenyel M, Yeiltepe R, et al. The correlation between the accuracy of steroid injections and subsequent shoulder pain and function in subacromial impingement
syndrome [in Turkish]. Acta Orthop Traumatol Turc. 2003;37(1):
41-45.
19. Powell SE, Davis SM, Lee EH, et al. Accuracy of palpation-directed intra-articular glenohumeral injection confirmed by magnetic resonance arthrography. Arthroscopy. 2015;31(2):205-208.
20. Rutten MJ, Maresch BJ, Jager GJ, de Waal Malefijt MC. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
21. Desmeules F, Côté CH, Frémont P. Therapeutic exercise and orthopedic manual therapy for impingement syndrome: a systematic review. Clin J Sport Med. 2003;13(3):176-182.
22. Winters JC, Sobel JS, Groenier KH, Arendzen HJ, Meyboom-de Jong B. Comparison of physiotherapy, manipulation, and corticosteroid injection for treating shoulder complaints in general practice: randomised, single blind study. BMJ. 1997;314(7090):1320-1325.
23. Chen MJ, Lew HL, Hsu TC, et al. Ultrasound-guided shoulder injections in the treatment of subacromial bursitis. Am J Phys Med Rehabil. 2006;85(1):31-35.
24. Hsieh LF, Hsu WC, Lin YJ, Wu SH, Chang KC, Chang HL. Is ultrasound-guided injection more effective in chronic subacromial bursitis? Med Sci Sports Exerc. 2013;45(12):
2205-2213.
25. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
26. Hall S, Buchbinder R. Do imaging methods that guide needle placement improve outcome? Ann Rheum Dis. 2004;63(9):1007-1008.
1. Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database Syst Rev. 2003;(1):CD004016.
2. Bell AD, Conaway D. Corticosteroid injections for painful shoulders. Int J Clin Pract. 2005;59(10):1178-1186.
3. Michener LA, McClure PW, Karduna AR. Anatomical and biomechanical mechanisms of subacromial impingement syndrome. Clin Biomech. 2003;18(5):369-379.
4. Akgün K, Birtane M, Akarirmak U. Is local subacromial corticosteroid injection beneficial in subacromial impingement syndrome? Clin Rheumatol. 2004;23(6):496-500.
5. Bhagra A, Syed H, Reed DA, et al. Efficacy of musculoskeletal injections by primary care providers in the office: a retrospective cohort study. Int J Gen Med. 2013;6:237-243.
6. Blair B, Rokito AS, Cuomo F, Jarolem K, Zuckerman JD. Efficacy of injections of corticosteroids for subacromial impingement syndrome. J Bone Joint Surg Am. 1996;78(11):1685-1689.
7. Cummins CA, Sasso LM, Nicholson D. Impingement syndrome: temporal outcomes of nonoperative treatment.
J Shoulder Elbow Surg. 2009;18(2):172-177.
8. Yu C, Chen CH, Liu HT, Dai MH, Wang IC, Wang KC. Subacromial injections of corticosteroids and Xylocaine for painful subacromial impingement syndrome. Chang Gung Med J. 2006;29(5):474-478.
9. Codsi MJ. The painful shoulder: when to inject and when to refer. Cleve Clin J Med. 2007;74(7):473-474, 477-478, 480-482 passim.
10. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
11. Kang MN, Rizio L, Prybicien M, Middlemas DA, Blacksin MF. The accuracy of subacromial corticosteroid injections: a comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(15):61S-66S.
12. Marder RA, Kim SH, Labson JD, Hunter JC. Injection of the subacromial bursa in patients with rotator cuff syndrome: a prospective, randomized study comparing the effectiveness of different routes. J Bone Joint Surg Am. 2012;94(16):
1442-1447.
13. Gruson, KI, Ruchelsman DE, Zuckerman JD. Subacromial corticosteroid injections. J Shoulder Elbow Surg. 2008;17(1 suppl):118S-130S.
14. Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18(1):138-160.
15. Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214-221.
16. Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease.
J Shoulder Elbow Surg. 2009;88(6):927-932.
17. Eustace JA, Brophy DP, Gibney RP, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
18. Esenyel CZ, Esenyel M, Yeiltepe R, et al. The correlation between the accuracy of steroid injections and subsequent shoulder pain and function in subacromial impingement
syndrome [in Turkish]. Acta Orthop Traumatol Turc. 2003;37(1):
41-45.
19. Powell SE, Davis SM, Lee EH, et al. Accuracy of palpation-directed intra-articular glenohumeral injection confirmed by magnetic resonance arthrography. Arthroscopy. 2015;31(2):205-208.
20. Rutten MJ, Maresch BJ, Jager GJ, de Waal Malefijt MC. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
21. Desmeules F, Côté CH, Frémont P. Therapeutic exercise and orthopedic manual therapy for impingement syndrome: a systematic review. Clin J Sport Med. 2003;13(3):176-182.
22. Winters JC, Sobel JS, Groenier KH, Arendzen HJ, Meyboom-de Jong B. Comparison of physiotherapy, manipulation, and corticosteroid injection for treating shoulder complaints in general practice: randomised, single blind study. BMJ. 1997;314(7090):1320-1325.
23. Chen MJ, Lew HL, Hsu TC, et al. Ultrasound-guided shoulder injections in the treatment of subacromial bursitis. Am J Phys Med Rehabil. 2006;85(1):31-35.
24. Hsieh LF, Hsu WC, Lin YJ, Wu SH, Chang KC, Chang HL. Is ultrasound-guided injection more effective in chronic subacromial bursitis? Med Sci Sports Exerc. 2013;45(12):
2205-2213.
25. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
26. Hall S, Buchbinder R. Do imaging methods that guide needle placement improve outcome? Ann Rheum Dis. 2004;63(9):1007-1008.