User login
Phytoestrogens may ease late-onset asthma in older women
Phytoestrogens show potential as a treatment for menopausal women with late-onset asthma that may relieve symptoms of both conditions, according to a new review.
and the absence of these hormones during childhood and menopause has been associated with fewer and less severe asthma episodes, wrote Bettina Sommer, PhD, of the Instituto Nacional de Enfermedades Respiratorias, Mexico City, and colleagues.
Late-onset asthma (LOA) has been categorized as a specific asthmatic phenotype that includes menopausal women, and research is needed to explore therapeutic alternatives that might provide relief to older women suffering from LOA, they said.
In a review published in the International Journal of Molecular Sciences, the researchers outlined the potential of phytoestrogens to manage LOA as well as symptoms of menopause.
LOA is often nonatopic and distinguished by a lack of eosinophilic inflammation; it is also associated with obesity and pollutants such as cigarette smoke. LOA is more common in women versus men, and develops between ages 27 and 65 years, the researchers wrote. Very late-onset asthma, which develops in women aged 65 years and older, is related to low levels of total lack of circulating estrogens.
Previous studies have shown that hormone therapy reduces the risk of LOA in menopausal women, but concerns about side effects persist. Phytochemicals offer a low-risk alternative, but phytoestrogen-based hormone therapy and its role in LOA have not been well studied, the researchers wrote.
Estrogen receptors (ERs) have two intracellular isoforms, alpha and beta. “Notably, the literature sustains that ERs expression differs between asthmatics and nonasthmatics,” and mainly the beta ERs are up-regulated in asthma or during inflammations, the researchers said. Phytoestrogens activate ER and benefit postmenopausal women, especially those with asthma, in addition to their anti-inflammatory and antioxidant properties.
Studies using mouse models have shown that E2 phytoestrogen supplementation in mice both increases the expression of antioxidant enzymes and reduces inflammation, according to the researchers. Age-related changes in hormonal statues, immunology, and systemic inflammation may predispose older adults to more infections and asthma exacerbations, but also might drive the development of LOA.
As another example of potential connections between phytoestrogen and asthma, phytoestrogen’s action on an estrogen receptor, notably the beta-ER, was associated with lowered airway hyperresponsiveness in a mouse model, and beta-ER knockout mice showed reduced lung function, compared with wild-type and alpha-ER knockout mice.
More research is needed, but novel therapies using phytoestrogens offer an added advantage to older women with LOA by potentially easing some menopause symptoms with fewer side effects than other options, the researchers wrote. “They may also contribute to more efficient responses to infection and inflammation leading menopausal women to a much better quality of life.”
The study was funded by the Instituto Nacional de Enfermedades Respiratorias, Consejo Nacional de Ciencia y Tecnología, Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica, and the Universidad Nacional Autonoma de Mexico. The researchers had no financial conflicts to disclose.
Phytoestrogens show potential as a treatment for menopausal women with late-onset asthma that may relieve symptoms of both conditions, according to a new review.
and the absence of these hormones during childhood and menopause has been associated with fewer and less severe asthma episodes, wrote Bettina Sommer, PhD, of the Instituto Nacional de Enfermedades Respiratorias, Mexico City, and colleagues.
Late-onset asthma (LOA) has been categorized as a specific asthmatic phenotype that includes menopausal women, and research is needed to explore therapeutic alternatives that might provide relief to older women suffering from LOA, they said.
In a review published in the International Journal of Molecular Sciences, the researchers outlined the potential of phytoestrogens to manage LOA as well as symptoms of menopause.
LOA is often nonatopic and distinguished by a lack of eosinophilic inflammation; it is also associated with obesity and pollutants such as cigarette smoke. LOA is more common in women versus men, and develops between ages 27 and 65 years, the researchers wrote. Very late-onset asthma, which develops in women aged 65 years and older, is related to low levels of total lack of circulating estrogens.
Previous studies have shown that hormone therapy reduces the risk of LOA in menopausal women, but concerns about side effects persist. Phytochemicals offer a low-risk alternative, but phytoestrogen-based hormone therapy and its role in LOA have not been well studied, the researchers wrote.
Estrogen receptors (ERs) have two intracellular isoforms, alpha and beta. “Notably, the literature sustains that ERs expression differs between asthmatics and nonasthmatics,” and mainly the beta ERs are up-regulated in asthma or during inflammations, the researchers said. Phytoestrogens activate ER and benefit postmenopausal women, especially those with asthma, in addition to their anti-inflammatory and antioxidant properties.
Studies using mouse models have shown that E2 phytoestrogen supplementation in mice both increases the expression of antioxidant enzymes and reduces inflammation, according to the researchers. Age-related changes in hormonal statues, immunology, and systemic inflammation may predispose older adults to more infections and asthma exacerbations, but also might drive the development of LOA.
As another example of potential connections between phytoestrogen and asthma, phytoestrogen’s action on an estrogen receptor, notably the beta-ER, was associated with lowered airway hyperresponsiveness in a mouse model, and beta-ER knockout mice showed reduced lung function, compared with wild-type and alpha-ER knockout mice.
More research is needed, but novel therapies using phytoestrogens offer an added advantage to older women with LOA by potentially easing some menopause symptoms with fewer side effects than other options, the researchers wrote. “They may also contribute to more efficient responses to infection and inflammation leading menopausal women to a much better quality of life.”
The study was funded by the Instituto Nacional de Enfermedades Respiratorias, Consejo Nacional de Ciencia y Tecnología, Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica, and the Universidad Nacional Autonoma de Mexico. The researchers had no financial conflicts to disclose.
Phytoestrogens show potential as a treatment for menopausal women with late-onset asthma that may relieve symptoms of both conditions, according to a new review.
and the absence of these hormones during childhood and menopause has been associated with fewer and less severe asthma episodes, wrote Bettina Sommer, PhD, of the Instituto Nacional de Enfermedades Respiratorias, Mexico City, and colleagues.
Late-onset asthma (LOA) has been categorized as a specific asthmatic phenotype that includes menopausal women, and research is needed to explore therapeutic alternatives that might provide relief to older women suffering from LOA, they said.
In a review published in the International Journal of Molecular Sciences, the researchers outlined the potential of phytoestrogens to manage LOA as well as symptoms of menopause.
LOA is often nonatopic and distinguished by a lack of eosinophilic inflammation; it is also associated with obesity and pollutants such as cigarette smoke. LOA is more common in women versus men, and develops between ages 27 and 65 years, the researchers wrote. Very late-onset asthma, which develops in women aged 65 years and older, is related to low levels of total lack of circulating estrogens.
Previous studies have shown that hormone therapy reduces the risk of LOA in menopausal women, but concerns about side effects persist. Phytochemicals offer a low-risk alternative, but phytoestrogen-based hormone therapy and its role in LOA have not been well studied, the researchers wrote.
Estrogen receptors (ERs) have two intracellular isoforms, alpha and beta. “Notably, the literature sustains that ERs expression differs between asthmatics and nonasthmatics,” and mainly the beta ERs are up-regulated in asthma or during inflammations, the researchers said. Phytoestrogens activate ER and benefit postmenopausal women, especially those with asthma, in addition to their anti-inflammatory and antioxidant properties.
Studies using mouse models have shown that E2 phytoestrogen supplementation in mice both increases the expression of antioxidant enzymes and reduces inflammation, according to the researchers. Age-related changes in hormonal statues, immunology, and systemic inflammation may predispose older adults to more infections and asthma exacerbations, but also might drive the development of LOA.
As another example of potential connections between phytoestrogen and asthma, phytoestrogen’s action on an estrogen receptor, notably the beta-ER, was associated with lowered airway hyperresponsiveness in a mouse model, and beta-ER knockout mice showed reduced lung function, compared with wild-type and alpha-ER knockout mice.
More research is needed, but novel therapies using phytoestrogens offer an added advantage to older women with LOA by potentially easing some menopause symptoms with fewer side effects than other options, the researchers wrote. “They may also contribute to more efficient responses to infection and inflammation leading menopausal women to a much better quality of life.”
The study was funded by the Instituto Nacional de Enfermedades Respiratorias, Consejo Nacional de Ciencia y Tecnología, Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica, and the Universidad Nacional Autonoma de Mexico. The researchers had no financial conflicts to disclose.
FROM THE INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES
Nasal ventilation function may factor into children’s OSA
, based on data from more than 200 individuals.
Previous research has shown an increased risk of obstructive sleep apnea syndrome (OSAS) in patients with compromised nasal respiration, but the association between increased nasal resistance (NR) and OSAS in children is controversial and remains unclear, wrote Ying Pang, MD, of Children’s Hospital of Chongqing Medical University, China, and colleagues.
In a study published in the Ear, Nose & Throat Journal, the researchers enrolled 109 children aged 6-12 years with OSAS and 116 healthy control children, with the goal of examining the role of nasal ventilation function on OSAS. Participants underwent acoustic rhinometry (AR) following polysomnography, and measurements of the nasal minimal cross-sectional area (NMCA) were taken in 3 segments, as were nasal cavity volume (NCV) from 0 cm to 5 cm, nasopharyngeal volume (NPV) from 6 cm to 8 cm, and distance of the minimal cross-sectional area to the nostril (DCAN). The children also underwent NR testing in both nostrils while awake and lying in a supine position.
Overall, the NR of children with OSAS were significantly higher than that of controls (P < .05). For AR, children with OSAS had significantly lower measures of NMCA, NCV, and NPV, but DCAN values were between the groups. Both AR and NR measures were similar among children with mild, moderate, or severe OSAS.
A subset of 90 children with mild or moderate OSAS were treated with intranasal corticosteroids (ICS) and oral montelukast for 12 weeks. Of these, 69 completed the study and were divided into three groups: effectively cured (group A), successfully treated (group B), and treatment failure (group C). The researchers compared the size of the tonsil adenoids, the polysomnography, NR, and AR before and after treatment and found significant differences in NR, NMCA, and NCV for the A and B groups but no significant changes in DCAN following treatment.
For group A, treatment was associated with a significant reduction in adenoid size and increase in NPV, but these changes did not occur in group B.
The findings were limited by several factors, including the small sample size and measurement of NR when patients were awake and sitting upright, and larger studies are needed to confirm the results, the researchers noted.
However, the results suggest that NVF plays a role in the pathogenesis of OSAS in children and suggest a need to improve NVF in treating these patients they concluded.
This study was supported by the Medical Project of Chongqing Municipal Science and Health Bureau of China. The researchers had no financial conflicts to disclose.
, based on data from more than 200 individuals.
Previous research has shown an increased risk of obstructive sleep apnea syndrome (OSAS) in patients with compromised nasal respiration, but the association between increased nasal resistance (NR) and OSAS in children is controversial and remains unclear, wrote Ying Pang, MD, of Children’s Hospital of Chongqing Medical University, China, and colleagues.
In a study published in the Ear, Nose & Throat Journal, the researchers enrolled 109 children aged 6-12 years with OSAS and 116 healthy control children, with the goal of examining the role of nasal ventilation function on OSAS. Participants underwent acoustic rhinometry (AR) following polysomnography, and measurements of the nasal minimal cross-sectional area (NMCA) were taken in 3 segments, as were nasal cavity volume (NCV) from 0 cm to 5 cm, nasopharyngeal volume (NPV) from 6 cm to 8 cm, and distance of the minimal cross-sectional area to the nostril (DCAN). The children also underwent NR testing in both nostrils while awake and lying in a supine position.
Overall, the NR of children with OSAS were significantly higher than that of controls (P < .05). For AR, children with OSAS had significantly lower measures of NMCA, NCV, and NPV, but DCAN values were between the groups. Both AR and NR measures were similar among children with mild, moderate, or severe OSAS.
A subset of 90 children with mild or moderate OSAS were treated with intranasal corticosteroids (ICS) and oral montelukast for 12 weeks. Of these, 69 completed the study and were divided into three groups: effectively cured (group A), successfully treated (group B), and treatment failure (group C). The researchers compared the size of the tonsil adenoids, the polysomnography, NR, and AR before and after treatment and found significant differences in NR, NMCA, and NCV for the A and B groups but no significant changes in DCAN following treatment.
For group A, treatment was associated with a significant reduction in adenoid size and increase in NPV, but these changes did not occur in group B.
The findings were limited by several factors, including the small sample size and measurement of NR when patients were awake and sitting upright, and larger studies are needed to confirm the results, the researchers noted.
However, the results suggest that NVF plays a role in the pathogenesis of OSAS in children and suggest a need to improve NVF in treating these patients they concluded.
This study was supported by the Medical Project of Chongqing Municipal Science and Health Bureau of China. The researchers had no financial conflicts to disclose.
, based on data from more than 200 individuals.
Previous research has shown an increased risk of obstructive sleep apnea syndrome (OSAS) in patients with compromised nasal respiration, but the association between increased nasal resistance (NR) and OSAS in children is controversial and remains unclear, wrote Ying Pang, MD, of Children’s Hospital of Chongqing Medical University, China, and colleagues.
In a study published in the Ear, Nose & Throat Journal, the researchers enrolled 109 children aged 6-12 years with OSAS and 116 healthy control children, with the goal of examining the role of nasal ventilation function on OSAS. Participants underwent acoustic rhinometry (AR) following polysomnography, and measurements of the nasal minimal cross-sectional area (NMCA) were taken in 3 segments, as were nasal cavity volume (NCV) from 0 cm to 5 cm, nasopharyngeal volume (NPV) from 6 cm to 8 cm, and distance of the minimal cross-sectional area to the nostril (DCAN). The children also underwent NR testing in both nostrils while awake and lying in a supine position.
Overall, the NR of children with OSAS were significantly higher than that of controls (P < .05). For AR, children with OSAS had significantly lower measures of NMCA, NCV, and NPV, but DCAN values were between the groups. Both AR and NR measures were similar among children with mild, moderate, or severe OSAS.
A subset of 90 children with mild or moderate OSAS were treated with intranasal corticosteroids (ICS) and oral montelukast for 12 weeks. Of these, 69 completed the study and were divided into three groups: effectively cured (group A), successfully treated (group B), and treatment failure (group C). The researchers compared the size of the tonsil adenoids, the polysomnography, NR, and AR before and after treatment and found significant differences in NR, NMCA, and NCV for the A and B groups but no significant changes in DCAN following treatment.
For group A, treatment was associated with a significant reduction in adenoid size and increase in NPV, but these changes did not occur in group B.
The findings were limited by several factors, including the small sample size and measurement of NR when patients were awake and sitting upright, and larger studies are needed to confirm the results, the researchers noted.
However, the results suggest that NVF plays a role in the pathogenesis of OSAS in children and suggest a need to improve NVF in treating these patients they concluded.
This study was supported by the Medical Project of Chongqing Municipal Science and Health Bureau of China. The researchers had no financial conflicts to disclose.
FROM THE EAR, NOSE & THROAT JOURNAL
Keep COVID-19 vaccination on your patients’ radar
The Advisory Committee on Immunization Practices (ACIP) recently issued updated recommendations on the use of vaccines to protect against COVID-19.1 In addition, 3 new COVID-19 vaccine products have been approved for use in the United States since September. Before we discuss both of these items, it’s important to understand why we’re still talking about COVID-19 vaccines.
The impact of vaccination can’t be understated. Vaccines to protect against COVID-19 have been hugely successful in preventing mortality and morbidity from illness caused by SARS-CoV-2. It is estimated that in the first year alone, after vaccines became widely available, they saved more than 14 million lives globally.2 By the end of 2022, they had prevented 18.5 million hospitalizations and 3.2 million deaths in the United States.3 However, waning levels of vaccine-induced immunity and the continuous mutation of the virus have prompted the need for booster doses of vaccine and development of new vaccines.
Enter this year’s vaccines. The new products include updated (2023-2024 formula) COVID-19 mRNA vaccines from Moderna and Pfizer-BioNTech, for use in those ages 6 months and older, and Novavax COVID-19 vaccine for use in those ages 12 years and older. All 3 provide protection against the currently circulating XBB variants, which by September 2023 accounted for > 99% of circulating SARS-CoV-2 strains in the United States.1
Novavax is an option for those who are hesitant to use an mRNA-based vaccine, although the exact recommendations for its use are still pending. Of note, the previously approved bivalent vaccines and the previous Novavax monovalent vaccine are no longer approved for use in the United States.
Current recommendations. For those ages 5 years and older, the recommendation is for a single dose of the 2023-2024 COVID-19 vaccine regardless of previous vaccination history—except for those who were previously unvaccinated and choose Novavax. (Those individuals should receive 2 doses, 3 to 8 weeks apart.) For those ages 6 months through 4 years, the recommended number of doses varies by vaccine and previous vaccination history1; a table can be found at www.cdc.gov/mmwr/volumes/72/wr/mm7242e1.htm.
Those who are moderately to severely immunocompromised should receive a 3-dose series with one of the 2023-2024 COVID-19 vaccines and may receive 1 or more additional updated doses.1 These recommendations are more nuanced, and a full description of them can be found at www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html.
Major changes in this year’s recommendations,4 compared to those previously made on the use of the bivalent vaccines, include:
- Eliminating complex recommendations for 5-year-olds, who are now included in the standard recommendation
- Reducing the number of COVID-19 vaccine products in use by standardizing the dose (25 mcg) for those ages 6 months to 11 years
- Choosing to monitor epidemiology and vaccine effectiveness data to determine whether an additional dose of this year’s vaccine will be needed for those ages 65 years and older, rather than making a recommendation now.
Who’s paying? Another change is how COVID-19 vaccines are paid for. The United States is moving from a system of federal procurement and distribution to the commercial marketplace. This may lead to some disruption and confusion.
All commercial health plans, as well as Medicare and Medicaid, must cover vaccines recommend by the ACIP with no out-of-pocket cost. The Vaccines for Children program provides free vaccine for uninsured and underinsured children up to age 19 years.
However, that leaves no payer for uninsured adults. In response, the CDC has announced the establishment of the Bridge Access Program, which is a private/government partnership to provide the vaccine to this age group. Details about where an adult can obtain a free COVID-19 vaccine through this program can be found by visiting www.cdc.gov/vaccines/programs/bridge/index.html or by calling 800-CDC-INFO.
A dynamic situation. COVID-19 vaccines and associated recommendations are likely to change with time, as we learn how best to formulate them to adjust to virus mutations and determine the optimal intervals to adjust and administer these vaccines. The result may (or may not) eventually resemble the approach recommended for influenza vaccines, which is annual assessment and adjustment of the targeted antigens, when needed, and annual universal vaccination.
1. Regan JJ, Moulia DL, Link-Guelles R, et al. Use of updated COVID-19 vaccines 2023-2024 formula for persons aged > 6 months: recommendations of the Advisory Committee on Immunization Practices—United States, September 2023. MMWR Morb Mortal Wkly Rep. 2023;72:1140-1146. doi: 10.15585/mmwr.mm7242e1
2. Watson OJ, Barnsley G, Toor J, et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22:1293-302. doi: 10.1016/S1473-3099(22)00320-6
3. Fitzpatrick M, Moghadas S, Pandey A, et al. Two years of US COVID-19 vaccines have prevented millions of hospitalizations and deaths. The Commonwealth Fund; 2022. Published December 13, 2022. Accessed November 2, 2023. www.commonwealthfund.org/blog/2022/two-years-covid-vaccines-prevented-millions-deaths-hospitalizations https://doi.org/10.26099/whsf-fp90
4. Wallace M. Evidence to recommendations framework: 2023-2024 (monovalent, XBB containing) COVID-19 vaccine. Presented to the Advisory Committee on Immunization Practices, September 12, 2023. Accessed November 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-12/11-COVID-Wallace-508.pdf
The Advisory Committee on Immunization Practices (ACIP) recently issued updated recommendations on the use of vaccines to protect against COVID-19.1 In addition, 3 new COVID-19 vaccine products have been approved for use in the United States since September. Before we discuss both of these items, it’s important to understand why we’re still talking about COVID-19 vaccines.
The impact of vaccination can’t be understated. Vaccines to protect against COVID-19 have been hugely successful in preventing mortality and morbidity from illness caused by SARS-CoV-2. It is estimated that in the first year alone, after vaccines became widely available, they saved more than 14 million lives globally.2 By the end of 2022, they had prevented 18.5 million hospitalizations and 3.2 million deaths in the United States.3 However, waning levels of vaccine-induced immunity and the continuous mutation of the virus have prompted the need for booster doses of vaccine and development of new vaccines.
Enter this year’s vaccines. The new products include updated (2023-2024 formula) COVID-19 mRNA vaccines from Moderna and Pfizer-BioNTech, for use in those ages 6 months and older, and Novavax COVID-19 vaccine for use in those ages 12 years and older. All 3 provide protection against the currently circulating XBB variants, which by September 2023 accounted for > 99% of circulating SARS-CoV-2 strains in the United States.1
Novavax is an option for those who are hesitant to use an mRNA-based vaccine, although the exact recommendations for its use are still pending. Of note, the previously approved bivalent vaccines and the previous Novavax monovalent vaccine are no longer approved for use in the United States.
Current recommendations. For those ages 5 years and older, the recommendation is for a single dose of the 2023-2024 COVID-19 vaccine regardless of previous vaccination history—except for those who were previously unvaccinated and choose Novavax. (Those individuals should receive 2 doses, 3 to 8 weeks apart.) For those ages 6 months through 4 years, the recommended number of doses varies by vaccine and previous vaccination history1; a table can be found at www.cdc.gov/mmwr/volumes/72/wr/mm7242e1.htm.
Those who are moderately to severely immunocompromised should receive a 3-dose series with one of the 2023-2024 COVID-19 vaccines and may receive 1 or more additional updated doses.1 These recommendations are more nuanced, and a full description of them can be found at www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html.
Major changes in this year’s recommendations,4 compared to those previously made on the use of the bivalent vaccines, include:
- Eliminating complex recommendations for 5-year-olds, who are now included in the standard recommendation
- Reducing the number of COVID-19 vaccine products in use by standardizing the dose (25 mcg) for those ages 6 months to 11 years
- Choosing to monitor epidemiology and vaccine effectiveness data to determine whether an additional dose of this year’s vaccine will be needed for those ages 65 years and older, rather than making a recommendation now.
Who’s paying? Another change is how COVID-19 vaccines are paid for. The United States is moving from a system of federal procurement and distribution to the commercial marketplace. This may lead to some disruption and confusion.
All commercial health plans, as well as Medicare and Medicaid, must cover vaccines recommend by the ACIP with no out-of-pocket cost. The Vaccines for Children program provides free vaccine for uninsured and underinsured children up to age 19 years.
However, that leaves no payer for uninsured adults. In response, the CDC has announced the establishment of the Bridge Access Program, which is a private/government partnership to provide the vaccine to this age group. Details about where an adult can obtain a free COVID-19 vaccine through this program can be found by visiting www.cdc.gov/vaccines/programs/bridge/index.html or by calling 800-CDC-INFO.
A dynamic situation. COVID-19 vaccines and associated recommendations are likely to change with time, as we learn how best to formulate them to adjust to virus mutations and determine the optimal intervals to adjust and administer these vaccines. The result may (or may not) eventually resemble the approach recommended for influenza vaccines, which is annual assessment and adjustment of the targeted antigens, when needed, and annual universal vaccination.
The Advisory Committee on Immunization Practices (ACIP) recently issued updated recommendations on the use of vaccines to protect against COVID-19.1 In addition, 3 new COVID-19 vaccine products have been approved for use in the United States since September. Before we discuss both of these items, it’s important to understand why we’re still talking about COVID-19 vaccines.
The impact of vaccination can’t be understated. Vaccines to protect against COVID-19 have been hugely successful in preventing mortality and morbidity from illness caused by SARS-CoV-2. It is estimated that in the first year alone, after vaccines became widely available, they saved more than 14 million lives globally.2 By the end of 2022, they had prevented 18.5 million hospitalizations and 3.2 million deaths in the United States.3 However, waning levels of vaccine-induced immunity and the continuous mutation of the virus have prompted the need for booster doses of vaccine and development of new vaccines.
Enter this year’s vaccines. The new products include updated (2023-2024 formula) COVID-19 mRNA vaccines from Moderna and Pfizer-BioNTech, for use in those ages 6 months and older, and Novavax COVID-19 vaccine for use in those ages 12 years and older. All 3 provide protection against the currently circulating XBB variants, which by September 2023 accounted for > 99% of circulating SARS-CoV-2 strains in the United States.1
Novavax is an option for those who are hesitant to use an mRNA-based vaccine, although the exact recommendations for its use are still pending. Of note, the previously approved bivalent vaccines and the previous Novavax monovalent vaccine are no longer approved for use in the United States.
Current recommendations. For those ages 5 years and older, the recommendation is for a single dose of the 2023-2024 COVID-19 vaccine regardless of previous vaccination history—except for those who were previously unvaccinated and choose Novavax. (Those individuals should receive 2 doses, 3 to 8 weeks apart.) For those ages 6 months through 4 years, the recommended number of doses varies by vaccine and previous vaccination history1; a table can be found at www.cdc.gov/mmwr/volumes/72/wr/mm7242e1.htm.
Those who are moderately to severely immunocompromised should receive a 3-dose series with one of the 2023-2024 COVID-19 vaccines and may receive 1 or more additional updated doses.1 These recommendations are more nuanced, and a full description of them can be found at www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html.
Major changes in this year’s recommendations,4 compared to those previously made on the use of the bivalent vaccines, include:
- Eliminating complex recommendations for 5-year-olds, who are now included in the standard recommendation
- Reducing the number of COVID-19 vaccine products in use by standardizing the dose (25 mcg) for those ages 6 months to 11 years
- Choosing to monitor epidemiology and vaccine effectiveness data to determine whether an additional dose of this year’s vaccine will be needed for those ages 65 years and older, rather than making a recommendation now.
Who’s paying? Another change is how COVID-19 vaccines are paid for. The United States is moving from a system of federal procurement and distribution to the commercial marketplace. This may lead to some disruption and confusion.
All commercial health plans, as well as Medicare and Medicaid, must cover vaccines recommend by the ACIP with no out-of-pocket cost. The Vaccines for Children program provides free vaccine for uninsured and underinsured children up to age 19 years.
However, that leaves no payer for uninsured adults. In response, the CDC has announced the establishment of the Bridge Access Program, which is a private/government partnership to provide the vaccine to this age group. Details about where an adult can obtain a free COVID-19 vaccine through this program can be found by visiting www.cdc.gov/vaccines/programs/bridge/index.html or by calling 800-CDC-INFO.
A dynamic situation. COVID-19 vaccines and associated recommendations are likely to change with time, as we learn how best to formulate them to adjust to virus mutations and determine the optimal intervals to adjust and administer these vaccines. The result may (or may not) eventually resemble the approach recommended for influenza vaccines, which is annual assessment and adjustment of the targeted antigens, when needed, and annual universal vaccination.
1. Regan JJ, Moulia DL, Link-Guelles R, et al. Use of updated COVID-19 vaccines 2023-2024 formula for persons aged > 6 months: recommendations of the Advisory Committee on Immunization Practices—United States, September 2023. MMWR Morb Mortal Wkly Rep. 2023;72:1140-1146. doi: 10.15585/mmwr.mm7242e1
2. Watson OJ, Barnsley G, Toor J, et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22:1293-302. doi: 10.1016/S1473-3099(22)00320-6
3. Fitzpatrick M, Moghadas S, Pandey A, et al. Two years of US COVID-19 vaccines have prevented millions of hospitalizations and deaths. The Commonwealth Fund; 2022. Published December 13, 2022. Accessed November 2, 2023. www.commonwealthfund.org/blog/2022/two-years-covid-vaccines-prevented-millions-deaths-hospitalizations https://doi.org/10.26099/whsf-fp90
4. Wallace M. Evidence to recommendations framework: 2023-2024 (monovalent, XBB containing) COVID-19 vaccine. Presented to the Advisory Committee on Immunization Practices, September 12, 2023. Accessed November 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-12/11-COVID-Wallace-508.pdf
1. Regan JJ, Moulia DL, Link-Guelles R, et al. Use of updated COVID-19 vaccines 2023-2024 formula for persons aged > 6 months: recommendations of the Advisory Committee on Immunization Practices—United States, September 2023. MMWR Morb Mortal Wkly Rep. 2023;72:1140-1146. doi: 10.15585/mmwr.mm7242e1
2. Watson OJ, Barnsley G, Toor J, et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22:1293-302. doi: 10.1016/S1473-3099(22)00320-6
3. Fitzpatrick M, Moghadas S, Pandey A, et al. Two years of US COVID-19 vaccines have prevented millions of hospitalizations and deaths. The Commonwealth Fund; 2022. Published December 13, 2022. Accessed November 2, 2023. www.commonwealthfund.org/blog/2022/two-years-covid-vaccines-prevented-millions-deaths-hospitalizations https://doi.org/10.26099/whsf-fp90
4. Wallace M. Evidence to recommendations framework: 2023-2024 (monovalent, XBB containing) COVID-19 vaccine. Presented to the Advisory Committee on Immunization Practices, September 12, 2023. Accessed November 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-12/11-COVID-Wallace-508.pdf
Early cryoprecipitate fails to improve trauma hemorrhage outcomes
TOPLINE:
(MHP).
METHODOLOGY:
- CRYOSTAT-2 was an interventional, randomized, open-label, parallel-group controlled, international, multicenter study.
- A total of 1,604 patients were enrolled from 25 major trauma centers in the United Kingdom (n = 1,555) and 1 in the United States (n = 49) between August 2017 and November 2021.
- A total of 805 patients were randomly assigned to receive the standard MHP (standard care), and 799 were randomly assigned to receive an additional three pools of cryoprecipitate.
- The primary outcome was all-cause mortality at 28 days.
TAKEAWAY:
- Addition of early cryoprecipitate versus standard care did not improve all-cause 28-day mortality in the intent-to-treat population (25.3% vs. 26.1%; P = .74).
- In patient subgroup with penetrating trauma, 28-day mortality was significantly higher in the cryoprecipitate group than in the standard care group (16.2% vs. 10.0%; odds ratio, 1.74; P = .006).
- Massive transfusion (RBC ≥ 10 U) was similar between the cryoprecipitate and standard care groups.
IN PRACTICE:
According to the authors, it is possible that certain patients may have benefited from cryoprecipitate, but they did not receive it promptly or in adequate doses to restore functional fibrinogen levels. Despite the study’s goal of early cryoprecipitate administration, the median time to the first transfusion exceeded 1 hour after the patient’s arrival, which highlights the logistical challenges of preparing and delivering a frozen blood component from a distant blood laboratory to the patient.
SOURCE:
The study, with first author Ross Davenport, PhD, of Queen Mary University of London and colleagues, was published in JAMA).
LIMITATIONS:
There was variability of timing of cryoprecipitate administration and an overlap with patients in the standard care group receiving the intervention as part of their usual MHP treatment.
DISCLOSURES:
The study was funded by the U.K. National Institute for Health and Care Research: Health Technology Assessment and Barts Charity, U.K.
A version of this article first appeared on Medscape.com.
TOPLINE:
(MHP).
METHODOLOGY:
- CRYOSTAT-2 was an interventional, randomized, open-label, parallel-group controlled, international, multicenter study.
- A total of 1,604 patients were enrolled from 25 major trauma centers in the United Kingdom (n = 1,555) and 1 in the United States (n = 49) between August 2017 and November 2021.
- A total of 805 patients were randomly assigned to receive the standard MHP (standard care), and 799 were randomly assigned to receive an additional three pools of cryoprecipitate.
- The primary outcome was all-cause mortality at 28 days.
TAKEAWAY:
- Addition of early cryoprecipitate versus standard care did not improve all-cause 28-day mortality in the intent-to-treat population (25.3% vs. 26.1%; P = .74).
- In patient subgroup with penetrating trauma, 28-day mortality was significantly higher in the cryoprecipitate group than in the standard care group (16.2% vs. 10.0%; odds ratio, 1.74; P = .006).
- Massive transfusion (RBC ≥ 10 U) was similar between the cryoprecipitate and standard care groups.
IN PRACTICE:
According to the authors, it is possible that certain patients may have benefited from cryoprecipitate, but they did not receive it promptly or in adequate doses to restore functional fibrinogen levels. Despite the study’s goal of early cryoprecipitate administration, the median time to the first transfusion exceeded 1 hour after the patient’s arrival, which highlights the logistical challenges of preparing and delivering a frozen blood component from a distant blood laboratory to the patient.
SOURCE:
The study, with first author Ross Davenport, PhD, of Queen Mary University of London and colleagues, was published in JAMA).
LIMITATIONS:
There was variability of timing of cryoprecipitate administration and an overlap with patients in the standard care group receiving the intervention as part of their usual MHP treatment.
DISCLOSURES:
The study was funded by the U.K. National Institute for Health and Care Research: Health Technology Assessment and Barts Charity, U.K.
A version of this article first appeared on Medscape.com.
TOPLINE:
(MHP).
METHODOLOGY:
- CRYOSTAT-2 was an interventional, randomized, open-label, parallel-group controlled, international, multicenter study.
- A total of 1,604 patients were enrolled from 25 major trauma centers in the United Kingdom (n = 1,555) and 1 in the United States (n = 49) between August 2017 and November 2021.
- A total of 805 patients were randomly assigned to receive the standard MHP (standard care), and 799 were randomly assigned to receive an additional three pools of cryoprecipitate.
- The primary outcome was all-cause mortality at 28 days.
TAKEAWAY:
- Addition of early cryoprecipitate versus standard care did not improve all-cause 28-day mortality in the intent-to-treat population (25.3% vs. 26.1%; P = .74).
- In patient subgroup with penetrating trauma, 28-day mortality was significantly higher in the cryoprecipitate group than in the standard care group (16.2% vs. 10.0%; odds ratio, 1.74; P = .006).
- Massive transfusion (RBC ≥ 10 U) was similar between the cryoprecipitate and standard care groups.
IN PRACTICE:
According to the authors, it is possible that certain patients may have benefited from cryoprecipitate, but they did not receive it promptly or in adequate doses to restore functional fibrinogen levels. Despite the study’s goal of early cryoprecipitate administration, the median time to the first transfusion exceeded 1 hour after the patient’s arrival, which highlights the logistical challenges of preparing and delivering a frozen blood component from a distant blood laboratory to the patient.
SOURCE:
The study, with first author Ross Davenport, PhD, of Queen Mary University of London and colleagues, was published in JAMA).
LIMITATIONS:
There was variability of timing of cryoprecipitate administration and an overlap with patients in the standard care group receiving the intervention as part of their usual MHP treatment.
DISCLOSURES:
The study was funded by the U.K. National Institute for Health and Care Research: Health Technology Assessment and Barts Charity, U.K.
A version of this article first appeared on Medscape.com.
Drug-coated balloon beats conventional angioplasty for high-risk patients with in-stent restenosis
SAN FRANCISCO – For the treatment of coronary artery in-stent restenosis, angioplasty with a drug-coated balloon (AGENT DCB; Boston Scientific) was superior to conventional balloon angioplasty in preventing target lesion failure at 1 year in a high-risk patient population.
Approximate 50% reductions in the rates of target lesion restenosis and target vessel myocardial infarction (MI) accounted for the superior findings with the AGENT DCB over conventional balloon angioplasty.
Robert Yeh, MD, of Beth Israel Deaconess Medical Center in Boston reported at the annual Transcatheter Cardiovascular Therapeutics congress. “This represented a 38% relative risk reduction as well as a 10% absolute risk reduction in the endpoint. The P value for superiority was 0.0063, highly statistically significant.”
In-stent restenosis is clinically challenging and accounts for about 10% of all percutaneous coronary interventions. “Sometimes these patients have multiple layers, and that could be a third or fourth layer of stent, something that we try to avoid,” he said.
Drug-coated balloons, which are not currently approved in the United States, can deliver drugs that inhibit blockages from reforming, “without leaving additional layers of metal behind,” he added. Such devices are already available in Europe and Japan.
AGENT IDE was a prospective, multicenter, superiority trial that randomly assigned 480 patients 2:1 to the AGENT DCB (n = 321) or to conventional balloon angioplasty (n = 159). Randomization occurred after successful pre-dilation of the target vessel.
The trial included patients with in-stent restenosis previously treated with a bare metal or a drug-eluting stent with lesion lengths < 26 mm (reference vessel diameter: > 2 mm to ≤ 4), and percent diameter stenosis of more than 70% if they were asymptomatic or of more than 50% if they were symptomatic. Patients were excluded if they had a recent ST-elevation MI, bifurcation, saphenous vein or arterial graft, or thrombus in the target vessel.
All received dual antiplatelet therapy for at least 1 month and then antiplatelet monotherapy for the duration of the trial. The primary endpoint was target lesion failure at 1 year, a composite of target lesion restenosis, target vessel-related MI, or cardiac death. More than 93% of patients in each arm were available for evaluation of the primary endpoint.
The two groups were well balanced at baseline: Approximate age was 68 years, 27% were women, and three quarters were White. Approximately 28%-32% had had a prior coronary artery bypass graft, 20%-22% had previous heart failure, and about 22% had a history of left main coronary artery disease. Half had diabetes, and about half had stable angina.
Multiple stent layers were common in 43% of each group. Stenosis diameter was about 65% at baseline for the two groups and was reduced to 22% post procedure.
Outcomes all favored AGENT DCB
In the AGENT DCB group, the technical success rate was 92.9% vs 89.3% for balloon angioplasty. Intravascular imaging was used during the procedure in 72.3% of DCB cases and in 76.7% of balloon cases.
Besides demonstrating a nearly 38% reduction in the primary endpoint of target lesion failure at 1 year for the DCB over conventional balloon angioplasty, DCB nearly halved the rate of target lesion revascularization and target vessel MI and was superior on other measures of clinical outcome.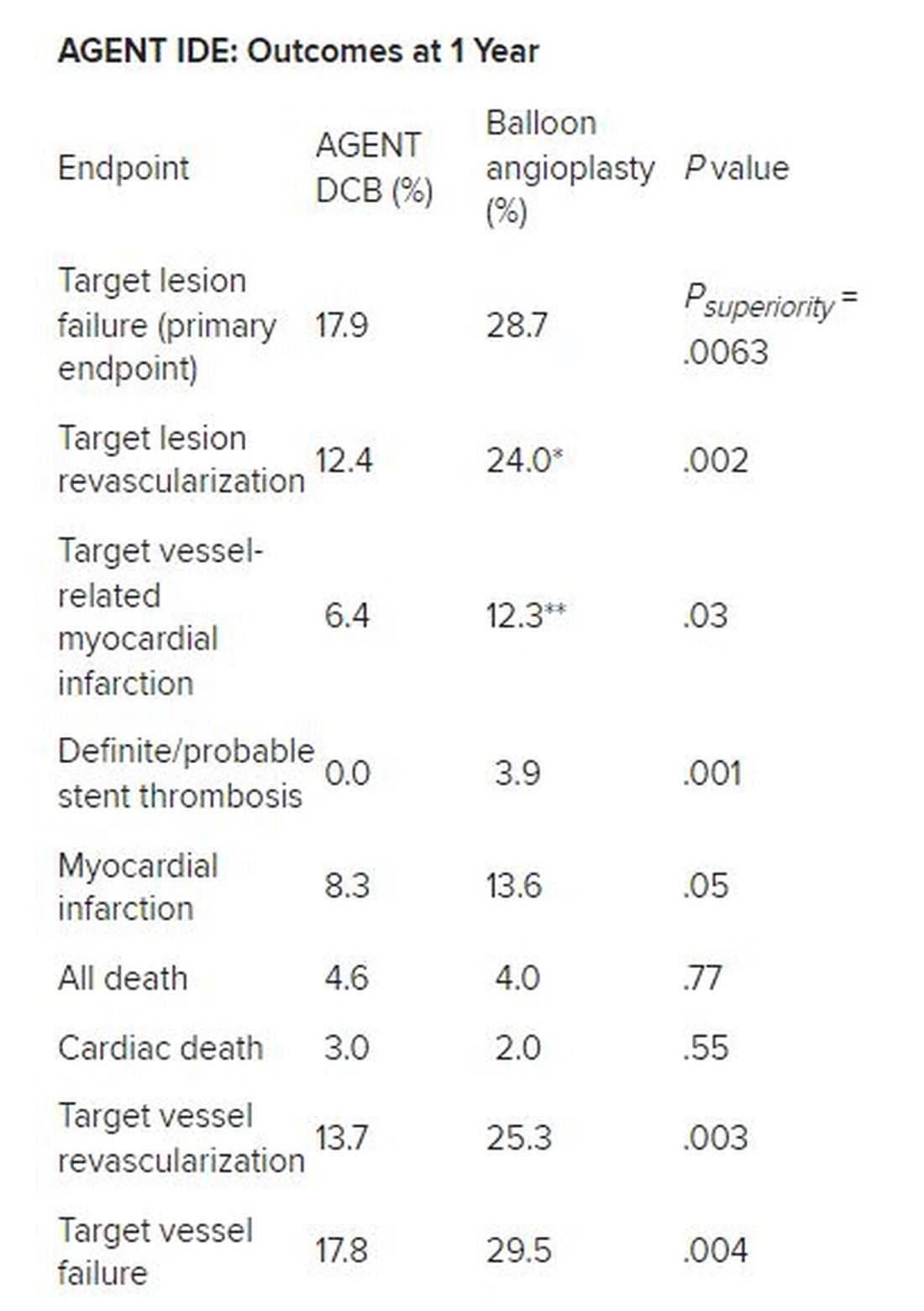
*Hazard ratio, 0.49; 95% CI, 0.31-0.79; ** HR, 0.51; 95% CI, 0.27-0.95
There was no stent rethrombosis with the DCB vs 3.9% with the conventional balloon angioplasty. Of note, there were no differences between the groups in terms of cardiac or noncardiac death.
Subgroup analyses of the primary outcome in terms of sex, age, diabetes, vessel size, or single or multiple stent layers all trended in favor of AGENT DCB but were not statistically significant for interaction.
The study is being expanded to include 600 patients. This device is a US Food and Drug Administration–designated breakthrough device, “and this pivotal trial will be the primary evidence used to support FDA approval,” Dr. Yeh said. “And given the marked superiority over conventional balloon angioplasty, I believe that the AGENT DCB is likely to become an important new treatment option for patients with coronary stenosis in the United States.”
Long overdue
Róisín Colleran, MBBCh, of the Cardiovascular Research Institute Dublin at Mater Private Hospital in Ireland, the designated discussant, first congratulated Dr. Yeh and his coinvestigators on the study’s conduct and findings.
“This study is long overdue,” she said. As Dr. Yeh noted, about 10% of PCI procedures are done for in-stent restenosis, Dr. Colleran said, but in 2023, there is still no coronary drug eluting balloon approved for this indication in the US, despite the class 1 recommendation in the 2014 European guidelines.
She pointed to the trial results, saying they are “clear...a significant reduction in target lesion failure driven by halving in rates of both target lesion revascularization and target vessel MI.”
Strengths of the study are it is the largest of its kind to date, with 480 patients, conducted at 40 US centers, using device-specific endpoints. There was a “very high” intravascular imaging rate of 75% in a cohort with a high risk for in-stent restenosis, consisting of 50% of patients with diabetes and more than 40% with multiple stents.
“The main limitation is the choice of comparator,” Dr. Colleran said. Balloon angioplasty is inferior to both stenting and drug coated balloon therapy for treatment of in-stent restenosis but is the standard of care in the United States, she noted. “I think...for regulatory reasons this was the comparator chosen,” she said.
“I think the implications are clear,” Dr. Colleran added. “This trial should provide a basis for regulatory approval of the drug coated balloon treatment of in-stent restenosis in the U.S. and finally provide this as an available treatment option for such patients.”
Dr. Yeh reported receiving grant/research support from Abbott Vascular, BD Bard, Boston Scientific, Cook Medical, Philips Medical, and Medtronic, and consulting for Abbott Vascular, Boston Scientific, CathWorks, Elixir Medical, Infraredx, Medtronic, Shockwave Medical, and Zol. Dr. Colleran had no disclosures. The trial was supported by Boston Scientific.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – For the treatment of coronary artery in-stent restenosis, angioplasty with a drug-coated balloon (AGENT DCB; Boston Scientific) was superior to conventional balloon angioplasty in preventing target lesion failure at 1 year in a high-risk patient population.
Approximate 50% reductions in the rates of target lesion restenosis and target vessel myocardial infarction (MI) accounted for the superior findings with the AGENT DCB over conventional balloon angioplasty.
Robert Yeh, MD, of Beth Israel Deaconess Medical Center in Boston reported at the annual Transcatheter Cardiovascular Therapeutics congress. “This represented a 38% relative risk reduction as well as a 10% absolute risk reduction in the endpoint. The P value for superiority was 0.0063, highly statistically significant.”
In-stent restenosis is clinically challenging and accounts for about 10% of all percutaneous coronary interventions. “Sometimes these patients have multiple layers, and that could be a third or fourth layer of stent, something that we try to avoid,” he said.
Drug-coated balloons, which are not currently approved in the United States, can deliver drugs that inhibit blockages from reforming, “without leaving additional layers of metal behind,” he added. Such devices are already available in Europe and Japan.
AGENT IDE was a prospective, multicenter, superiority trial that randomly assigned 480 patients 2:1 to the AGENT DCB (n = 321) or to conventional balloon angioplasty (n = 159). Randomization occurred after successful pre-dilation of the target vessel.
The trial included patients with in-stent restenosis previously treated with a bare metal or a drug-eluting stent with lesion lengths < 26 mm (reference vessel diameter: > 2 mm to ≤ 4), and percent diameter stenosis of more than 70% if they were asymptomatic or of more than 50% if they were symptomatic. Patients were excluded if they had a recent ST-elevation MI, bifurcation, saphenous vein or arterial graft, or thrombus in the target vessel.
All received dual antiplatelet therapy for at least 1 month and then antiplatelet monotherapy for the duration of the trial. The primary endpoint was target lesion failure at 1 year, a composite of target lesion restenosis, target vessel-related MI, or cardiac death. More than 93% of patients in each arm were available for evaluation of the primary endpoint.
The two groups were well balanced at baseline: Approximate age was 68 years, 27% were women, and three quarters were White. Approximately 28%-32% had had a prior coronary artery bypass graft, 20%-22% had previous heart failure, and about 22% had a history of left main coronary artery disease. Half had diabetes, and about half had stable angina.
Multiple stent layers were common in 43% of each group. Stenosis diameter was about 65% at baseline for the two groups and was reduced to 22% post procedure.
Outcomes all favored AGENT DCB
In the AGENT DCB group, the technical success rate was 92.9% vs 89.3% for balloon angioplasty. Intravascular imaging was used during the procedure in 72.3% of DCB cases and in 76.7% of balloon cases.
Besides demonstrating a nearly 38% reduction in the primary endpoint of target lesion failure at 1 year for the DCB over conventional balloon angioplasty, DCB nearly halved the rate of target lesion revascularization and target vessel MI and was superior on other measures of clinical outcome.
*Hazard ratio, 0.49; 95% CI, 0.31-0.79; ** HR, 0.51; 95% CI, 0.27-0.95
There was no stent rethrombosis with the DCB vs 3.9% with the conventional balloon angioplasty. Of note, there were no differences between the groups in terms of cardiac or noncardiac death.
Subgroup analyses of the primary outcome in terms of sex, age, diabetes, vessel size, or single or multiple stent layers all trended in favor of AGENT DCB but were not statistically significant for interaction.
The study is being expanded to include 600 patients. This device is a US Food and Drug Administration–designated breakthrough device, “and this pivotal trial will be the primary evidence used to support FDA approval,” Dr. Yeh said. “And given the marked superiority over conventional balloon angioplasty, I believe that the AGENT DCB is likely to become an important new treatment option for patients with coronary stenosis in the United States.”
Long overdue
Róisín Colleran, MBBCh, of the Cardiovascular Research Institute Dublin at Mater Private Hospital in Ireland, the designated discussant, first congratulated Dr. Yeh and his coinvestigators on the study’s conduct and findings.
“This study is long overdue,” she said. As Dr. Yeh noted, about 10% of PCI procedures are done for in-stent restenosis, Dr. Colleran said, but in 2023, there is still no coronary drug eluting balloon approved for this indication in the US, despite the class 1 recommendation in the 2014 European guidelines.
She pointed to the trial results, saying they are “clear...a significant reduction in target lesion failure driven by halving in rates of both target lesion revascularization and target vessel MI.”
Strengths of the study are it is the largest of its kind to date, with 480 patients, conducted at 40 US centers, using device-specific endpoints. There was a “very high” intravascular imaging rate of 75% in a cohort with a high risk for in-stent restenosis, consisting of 50% of patients with diabetes and more than 40% with multiple stents.
“The main limitation is the choice of comparator,” Dr. Colleran said. Balloon angioplasty is inferior to both stenting and drug coated balloon therapy for treatment of in-stent restenosis but is the standard of care in the United States, she noted. “I think...for regulatory reasons this was the comparator chosen,” she said.
“I think the implications are clear,” Dr. Colleran added. “This trial should provide a basis for regulatory approval of the drug coated balloon treatment of in-stent restenosis in the U.S. and finally provide this as an available treatment option for such patients.”
Dr. Yeh reported receiving grant/research support from Abbott Vascular, BD Bard, Boston Scientific, Cook Medical, Philips Medical, and Medtronic, and consulting for Abbott Vascular, Boston Scientific, CathWorks, Elixir Medical, Infraredx, Medtronic, Shockwave Medical, and Zol. Dr. Colleran had no disclosures. The trial was supported by Boston Scientific.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – For the treatment of coronary artery in-stent restenosis, angioplasty with a drug-coated balloon (AGENT DCB; Boston Scientific) was superior to conventional balloon angioplasty in preventing target lesion failure at 1 year in a high-risk patient population.
Approximate 50% reductions in the rates of target lesion restenosis and target vessel myocardial infarction (MI) accounted for the superior findings with the AGENT DCB over conventional balloon angioplasty.
Robert Yeh, MD, of Beth Israel Deaconess Medical Center in Boston reported at the annual Transcatheter Cardiovascular Therapeutics congress. “This represented a 38% relative risk reduction as well as a 10% absolute risk reduction in the endpoint. The P value for superiority was 0.0063, highly statistically significant.”
In-stent restenosis is clinically challenging and accounts for about 10% of all percutaneous coronary interventions. “Sometimes these patients have multiple layers, and that could be a third or fourth layer of stent, something that we try to avoid,” he said.
Drug-coated balloons, which are not currently approved in the United States, can deliver drugs that inhibit blockages from reforming, “without leaving additional layers of metal behind,” he added. Such devices are already available in Europe and Japan.
AGENT IDE was a prospective, multicenter, superiority trial that randomly assigned 480 patients 2:1 to the AGENT DCB (n = 321) or to conventional balloon angioplasty (n = 159). Randomization occurred after successful pre-dilation of the target vessel.
The trial included patients with in-stent restenosis previously treated with a bare metal or a drug-eluting stent with lesion lengths < 26 mm (reference vessel diameter: > 2 mm to ≤ 4), and percent diameter stenosis of more than 70% if they were asymptomatic or of more than 50% if they were symptomatic. Patients were excluded if they had a recent ST-elevation MI, bifurcation, saphenous vein or arterial graft, or thrombus in the target vessel.
All received dual antiplatelet therapy for at least 1 month and then antiplatelet monotherapy for the duration of the trial. The primary endpoint was target lesion failure at 1 year, a composite of target lesion restenosis, target vessel-related MI, or cardiac death. More than 93% of patients in each arm were available for evaluation of the primary endpoint.
The two groups were well balanced at baseline: Approximate age was 68 years, 27% were women, and three quarters were White. Approximately 28%-32% had had a prior coronary artery bypass graft, 20%-22% had previous heart failure, and about 22% had a history of left main coronary artery disease. Half had diabetes, and about half had stable angina.
Multiple stent layers were common in 43% of each group. Stenosis diameter was about 65% at baseline for the two groups and was reduced to 22% post procedure.
Outcomes all favored AGENT DCB
In the AGENT DCB group, the technical success rate was 92.9% vs 89.3% for balloon angioplasty. Intravascular imaging was used during the procedure in 72.3% of DCB cases and in 76.7% of balloon cases.
Besides demonstrating a nearly 38% reduction in the primary endpoint of target lesion failure at 1 year for the DCB over conventional balloon angioplasty, DCB nearly halved the rate of target lesion revascularization and target vessel MI and was superior on other measures of clinical outcome.
*Hazard ratio, 0.49; 95% CI, 0.31-0.79; ** HR, 0.51; 95% CI, 0.27-0.95
There was no stent rethrombosis with the DCB vs 3.9% with the conventional balloon angioplasty. Of note, there were no differences between the groups in terms of cardiac or noncardiac death.
Subgroup analyses of the primary outcome in terms of sex, age, diabetes, vessel size, or single or multiple stent layers all trended in favor of AGENT DCB but were not statistically significant for interaction.
The study is being expanded to include 600 patients. This device is a US Food and Drug Administration–designated breakthrough device, “and this pivotal trial will be the primary evidence used to support FDA approval,” Dr. Yeh said. “And given the marked superiority over conventional balloon angioplasty, I believe that the AGENT DCB is likely to become an important new treatment option for patients with coronary stenosis in the United States.”
Long overdue
Róisín Colleran, MBBCh, of the Cardiovascular Research Institute Dublin at Mater Private Hospital in Ireland, the designated discussant, first congratulated Dr. Yeh and his coinvestigators on the study’s conduct and findings.
“This study is long overdue,” she said. As Dr. Yeh noted, about 10% of PCI procedures are done for in-stent restenosis, Dr. Colleran said, but in 2023, there is still no coronary drug eluting balloon approved for this indication in the US, despite the class 1 recommendation in the 2014 European guidelines.
She pointed to the trial results, saying they are “clear...a significant reduction in target lesion failure driven by halving in rates of both target lesion revascularization and target vessel MI.”
Strengths of the study are it is the largest of its kind to date, with 480 patients, conducted at 40 US centers, using device-specific endpoints. There was a “very high” intravascular imaging rate of 75% in a cohort with a high risk for in-stent restenosis, consisting of 50% of patients with diabetes and more than 40% with multiple stents.
“The main limitation is the choice of comparator,” Dr. Colleran said. Balloon angioplasty is inferior to both stenting and drug coated balloon therapy for treatment of in-stent restenosis but is the standard of care in the United States, she noted. “I think...for regulatory reasons this was the comparator chosen,” she said.
“I think the implications are clear,” Dr. Colleran added. “This trial should provide a basis for regulatory approval of the drug coated balloon treatment of in-stent restenosis in the U.S. and finally provide this as an available treatment option for such patients.”
Dr. Yeh reported receiving grant/research support from Abbott Vascular, BD Bard, Boston Scientific, Cook Medical, Philips Medical, and Medtronic, and consulting for Abbott Vascular, Boston Scientific, CathWorks, Elixir Medical, Infraredx, Medtronic, Shockwave Medical, and Zol. Dr. Colleran had no disclosures. The trial was supported by Boston Scientific.
A version of this article first appeared on Medscape.com.
AT TCT 2023
Laissez-faire
I read a few articles recently that raised my concern about a laissez faire attitude regarding treatment and prevention of infectious disease and lack of a broader understanding of why we treat our patients.
Strep throat
Let’s start with group A streptococcal pharyngitis – strep throat. There are at least five reasons to treat strep throat with antibiotics.
Lest we forget, there is the prevention of acute rheumatic fever! Of course, acute rheumatic fever is rare in high-income countries like the United States, but we have had outbreaks in the past and we will have outbreaks in the future. All it takes is circulation of rheumatogenic strains and susceptible hosts.
Also, antibiotic treatment may prevent acute post-streptococcal glomerulonephritis, although that benefit is somewhat controversial.
Antibiotic treatment may prevent development of another controversial, nonsuppurative streptococcal complication, namely, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS).
Second, group A strep causes suppurative complications such as acute otitis media, peritonsillar abscess, mastoiditis, and sepsis, among others, and antibiotic treatment reduces those risks. Group A strep can cause impetigo, cellulitis, necrotizing fasciitis (flesh-eating disease), and toxic shock syndrome; antibiotics reduce those risks.
Third, while strep throat is a self-limited infection in terms of symptoms, it has been clearly shown that antibiotics cause symptoms to resolve more quickly. I must confess that it galls me when pundits suggest that reducing symptoms of any infectious disease by a day or 2 doesn’t matter for children, when adults with even mild symptoms rush to a clinician with hopes of treatment to shorten illness by a day.
Fourth, antibiotics shorten contagion. In fact, treatment in the morning of an office visit can allow a child to return to school the next day.1
Lastly on this topic, if a clinician had a positive strep culture or rapid test on a patient and did not treat with antibiotics, which is not the standard of care, and that patient went on to a nonsuppurative or suppurative complication, then what?
I am not advocating wholesale antibiotic treatment of all sore throats because antibiotics carry risks from use. Most sore throats are not strep throats. The first step is the examination to decide if a strep test is warranted. There are clinical scoring systems available. But the essence of the clinical criteria relies on age of child (strep is mostly seen in 5- to 15-year-olds), season (not summer), known exposure to strep, absence of rhinorrhea, absence of cough, presence of rapid onset of symptoms, usually with fever, and moderate to severe redness, often with exudates. Gratefully, in the United States, we have rapid strep tests that are covered by insurance. This is not the case even in many other high-income countries and certainly, generally, not available at all in moderate to low income countries. With a rapid test, a point-of-care microbiologic diagnosis can be made with reasonable accuracy. Antibiotic treatment should be reserved for patients with positive laboratory confirmation of Group A streptococci, either by rapid test or culture.
Ear infections
Next, let’s address treatment of acute otitis media – ear infections. There are at least six reasons to treat ear infections with antibiotics. Worldwide, the No. 1 cause of acquired deafness in children today is ear infections. This is rarely seen in the United States because we rarely have patients with chronic suppurative otitis media since antibiotics are typically prescribed.
Second, ear infections have suppurative complications such as mastoiditis, labyrinthitis, malignant otitis, brain abscess, sepsis, and meningitis. The World Health Organization attributes 20,000 deaths per year to complications from ear infections.
Third, ear infections can lead to eardrum rupture and subsequent chronic middle ear drainage.
Fourth, untreated otitis more often progresses to a nonsuppurative complication – a cholesteatoma.
Fifth, while earache is a self-limited illness, antibiotics shorten the acute symptoms by a day or 2 and lessen the duration of middle ear effusion after infection that can cause temporary hearing loss. Once again, as a child advocate, I would point out that pain from an ear infection is often severe and the lingering effects of a middle ear effusion are annoying to say the least.
Lastly on this topic, if a clinician makes the diagnosis of an ear infection in a patient and does not treat with antibiotics, the decision should be within the guidelines of the standard of care as described by the American Academy of Pediatrics2 with decision-making based on patient age and severity of symptoms.
I am not advocating wholesale antibiotic treatment of all ear pain or presumed ear pain. With this clinical condition we currently do not have a diagnostic test, and therein lies the conundrum. Most acute otitis media occurs among children age 6-24 months old, and this leads most clinicians to overdiagnose the infection. A child in that age group is nonverbal and in the context of a viral upper respiratory illness the symptoms of acute otitis media overlap completely with those of a viral URI. Therefore, an adequate examination is necessary. Confronted with an irritable child who is uncooperative with a challenging otoscopic examination, an ear canal with wax blocking an adequate view of the tympanic membrane, and a parent in a hurry to get back to work or home, the inclination is to observe a “little bit of redness” and prescribe unnecessary antibiotics. Even though redness is not a good diagnostic indicator, whereas a full or bulging eardrum is for the diagnosis of acute otitis media, I shudder at how often I see in a medical record a description of redness of the eardrum and no comment on the fullness that occurs when an authentic infection is most likely.
I could extend this column discussing acute sinusitis and cough illnesses as they are two other conditions associated with infection where antibiotics have their important place and where antibiotics are also overused. Instead, I will end by summarizing my viewpoint that judicious antibiotic use is of high importance for prevention of antibiotic resistance at the individual patient level and the community level. However, we should not become complacent about the risks to untreated children experiencing common respiratory infections because there are many justifiable reasons to treat children as discussed here.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Schwartz RH et al. A reappraisal of the minimum duration of antibiotic treatment before approval of return to school for children with streptococcal pharyngitis. Pediatr Infect Dis J. 2015 Dec. doi: 10.1097/INF.0000000000000883.
2. Lieberthal AS et al. The diagnosis and management of acute otitis media. Pediatrics. 2013 Mar. doi: 10.1542/peds.2012-3488.
I read a few articles recently that raised my concern about a laissez faire attitude regarding treatment and prevention of infectious disease and lack of a broader understanding of why we treat our patients.
Strep throat
Let’s start with group A streptococcal pharyngitis – strep throat. There are at least five reasons to treat strep throat with antibiotics.
Lest we forget, there is the prevention of acute rheumatic fever! Of course, acute rheumatic fever is rare in high-income countries like the United States, but we have had outbreaks in the past and we will have outbreaks in the future. All it takes is circulation of rheumatogenic strains and susceptible hosts.
Also, antibiotic treatment may prevent acute post-streptococcal glomerulonephritis, although that benefit is somewhat controversial.
Antibiotic treatment may prevent development of another controversial, nonsuppurative streptococcal complication, namely, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS).
Second, group A strep causes suppurative complications such as acute otitis media, peritonsillar abscess, mastoiditis, and sepsis, among others, and antibiotic treatment reduces those risks. Group A strep can cause impetigo, cellulitis, necrotizing fasciitis (flesh-eating disease), and toxic shock syndrome; antibiotics reduce those risks.
Third, while strep throat is a self-limited infection in terms of symptoms, it has been clearly shown that antibiotics cause symptoms to resolve more quickly. I must confess that it galls me when pundits suggest that reducing symptoms of any infectious disease by a day or 2 doesn’t matter for children, when adults with even mild symptoms rush to a clinician with hopes of treatment to shorten illness by a day.
Fourth, antibiotics shorten contagion. In fact, treatment in the morning of an office visit can allow a child to return to school the next day.1
Lastly on this topic, if a clinician had a positive strep culture or rapid test on a patient and did not treat with antibiotics, which is not the standard of care, and that patient went on to a nonsuppurative or suppurative complication, then what?
I am not advocating wholesale antibiotic treatment of all sore throats because antibiotics carry risks from use. Most sore throats are not strep throats. The first step is the examination to decide if a strep test is warranted. There are clinical scoring systems available. But the essence of the clinical criteria relies on age of child (strep is mostly seen in 5- to 15-year-olds), season (not summer), known exposure to strep, absence of rhinorrhea, absence of cough, presence of rapid onset of symptoms, usually with fever, and moderate to severe redness, often with exudates. Gratefully, in the United States, we have rapid strep tests that are covered by insurance. This is not the case even in many other high-income countries and certainly, generally, not available at all in moderate to low income countries. With a rapid test, a point-of-care microbiologic diagnosis can be made with reasonable accuracy. Antibiotic treatment should be reserved for patients with positive laboratory confirmation of Group A streptococci, either by rapid test or culture.
Ear infections
Next, let’s address treatment of acute otitis media – ear infections. There are at least six reasons to treat ear infections with antibiotics. Worldwide, the No. 1 cause of acquired deafness in children today is ear infections. This is rarely seen in the United States because we rarely have patients with chronic suppurative otitis media since antibiotics are typically prescribed.
Second, ear infections have suppurative complications such as mastoiditis, labyrinthitis, malignant otitis, brain abscess, sepsis, and meningitis. The World Health Organization attributes 20,000 deaths per year to complications from ear infections.
Third, ear infections can lead to eardrum rupture and subsequent chronic middle ear drainage.
Fourth, untreated otitis more often progresses to a nonsuppurative complication – a cholesteatoma.
Fifth, while earache is a self-limited illness, antibiotics shorten the acute symptoms by a day or 2 and lessen the duration of middle ear effusion after infection that can cause temporary hearing loss. Once again, as a child advocate, I would point out that pain from an ear infection is often severe and the lingering effects of a middle ear effusion are annoying to say the least.
Lastly on this topic, if a clinician makes the diagnosis of an ear infection in a patient and does not treat with antibiotics, the decision should be within the guidelines of the standard of care as described by the American Academy of Pediatrics2 with decision-making based on patient age and severity of symptoms.
I am not advocating wholesale antibiotic treatment of all ear pain or presumed ear pain. With this clinical condition we currently do not have a diagnostic test, and therein lies the conundrum. Most acute otitis media occurs among children age 6-24 months old, and this leads most clinicians to overdiagnose the infection. A child in that age group is nonverbal and in the context of a viral upper respiratory illness the symptoms of acute otitis media overlap completely with those of a viral URI. Therefore, an adequate examination is necessary. Confronted with an irritable child who is uncooperative with a challenging otoscopic examination, an ear canal with wax blocking an adequate view of the tympanic membrane, and a parent in a hurry to get back to work or home, the inclination is to observe a “little bit of redness” and prescribe unnecessary antibiotics. Even though redness is not a good diagnostic indicator, whereas a full or bulging eardrum is for the diagnosis of acute otitis media, I shudder at how often I see in a medical record a description of redness of the eardrum and no comment on the fullness that occurs when an authentic infection is most likely.
I could extend this column discussing acute sinusitis and cough illnesses as they are two other conditions associated with infection where antibiotics have their important place and where antibiotics are also overused. Instead, I will end by summarizing my viewpoint that judicious antibiotic use is of high importance for prevention of antibiotic resistance at the individual patient level and the community level. However, we should not become complacent about the risks to untreated children experiencing common respiratory infections because there are many justifiable reasons to treat children as discussed here.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Schwartz RH et al. A reappraisal of the minimum duration of antibiotic treatment before approval of return to school for children with streptococcal pharyngitis. Pediatr Infect Dis J. 2015 Dec. doi: 10.1097/INF.0000000000000883.
2. Lieberthal AS et al. The diagnosis and management of acute otitis media. Pediatrics. 2013 Mar. doi: 10.1542/peds.2012-3488.
I read a few articles recently that raised my concern about a laissez faire attitude regarding treatment and prevention of infectious disease and lack of a broader understanding of why we treat our patients.
Strep throat
Let’s start with group A streptococcal pharyngitis – strep throat. There are at least five reasons to treat strep throat with antibiotics.
Lest we forget, there is the prevention of acute rheumatic fever! Of course, acute rheumatic fever is rare in high-income countries like the United States, but we have had outbreaks in the past and we will have outbreaks in the future. All it takes is circulation of rheumatogenic strains and susceptible hosts.
Also, antibiotic treatment may prevent acute post-streptococcal glomerulonephritis, although that benefit is somewhat controversial.
Antibiotic treatment may prevent development of another controversial, nonsuppurative streptococcal complication, namely, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS).
Second, group A strep causes suppurative complications such as acute otitis media, peritonsillar abscess, mastoiditis, and sepsis, among others, and antibiotic treatment reduces those risks. Group A strep can cause impetigo, cellulitis, necrotizing fasciitis (flesh-eating disease), and toxic shock syndrome; antibiotics reduce those risks.
Third, while strep throat is a self-limited infection in terms of symptoms, it has been clearly shown that antibiotics cause symptoms to resolve more quickly. I must confess that it galls me when pundits suggest that reducing symptoms of any infectious disease by a day or 2 doesn’t matter for children, when adults with even mild symptoms rush to a clinician with hopes of treatment to shorten illness by a day.
Fourth, antibiotics shorten contagion. In fact, treatment in the morning of an office visit can allow a child to return to school the next day.1
Lastly on this topic, if a clinician had a positive strep culture or rapid test on a patient and did not treat with antibiotics, which is not the standard of care, and that patient went on to a nonsuppurative or suppurative complication, then what?
I am not advocating wholesale antibiotic treatment of all sore throats because antibiotics carry risks from use. Most sore throats are not strep throats. The first step is the examination to decide if a strep test is warranted. There are clinical scoring systems available. But the essence of the clinical criteria relies on age of child (strep is mostly seen in 5- to 15-year-olds), season (not summer), known exposure to strep, absence of rhinorrhea, absence of cough, presence of rapid onset of symptoms, usually with fever, and moderate to severe redness, often with exudates. Gratefully, in the United States, we have rapid strep tests that are covered by insurance. This is not the case even in many other high-income countries and certainly, generally, not available at all in moderate to low income countries. With a rapid test, a point-of-care microbiologic diagnosis can be made with reasonable accuracy. Antibiotic treatment should be reserved for patients with positive laboratory confirmation of Group A streptococci, either by rapid test or culture.
Ear infections
Next, let’s address treatment of acute otitis media – ear infections. There are at least six reasons to treat ear infections with antibiotics. Worldwide, the No. 1 cause of acquired deafness in children today is ear infections. This is rarely seen in the United States because we rarely have patients with chronic suppurative otitis media since antibiotics are typically prescribed.
Second, ear infections have suppurative complications such as mastoiditis, labyrinthitis, malignant otitis, brain abscess, sepsis, and meningitis. The World Health Organization attributes 20,000 deaths per year to complications from ear infections.
Third, ear infections can lead to eardrum rupture and subsequent chronic middle ear drainage.
Fourth, untreated otitis more often progresses to a nonsuppurative complication – a cholesteatoma.
Fifth, while earache is a self-limited illness, antibiotics shorten the acute symptoms by a day or 2 and lessen the duration of middle ear effusion after infection that can cause temporary hearing loss. Once again, as a child advocate, I would point out that pain from an ear infection is often severe and the lingering effects of a middle ear effusion are annoying to say the least.
Lastly on this topic, if a clinician makes the diagnosis of an ear infection in a patient and does not treat with antibiotics, the decision should be within the guidelines of the standard of care as described by the American Academy of Pediatrics2 with decision-making based on patient age and severity of symptoms.
I am not advocating wholesale antibiotic treatment of all ear pain or presumed ear pain. With this clinical condition we currently do not have a diagnostic test, and therein lies the conundrum. Most acute otitis media occurs among children age 6-24 months old, and this leads most clinicians to overdiagnose the infection. A child in that age group is nonverbal and in the context of a viral upper respiratory illness the symptoms of acute otitis media overlap completely with those of a viral URI. Therefore, an adequate examination is necessary. Confronted with an irritable child who is uncooperative with a challenging otoscopic examination, an ear canal with wax blocking an adequate view of the tympanic membrane, and a parent in a hurry to get back to work or home, the inclination is to observe a “little bit of redness” and prescribe unnecessary antibiotics. Even though redness is not a good diagnostic indicator, whereas a full or bulging eardrum is for the diagnosis of acute otitis media, I shudder at how often I see in a medical record a description of redness of the eardrum and no comment on the fullness that occurs when an authentic infection is most likely.
I could extend this column discussing acute sinusitis and cough illnesses as they are two other conditions associated with infection where antibiotics have their important place and where antibiotics are also overused. Instead, I will end by summarizing my viewpoint that judicious antibiotic use is of high importance for prevention of antibiotic resistance at the individual patient level and the community level. However, we should not become complacent about the risks to untreated children experiencing common respiratory infections because there are many justifiable reasons to treat children as discussed here.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Schwartz RH et al. A reappraisal of the minimum duration of antibiotic treatment before approval of return to school for children with streptococcal pharyngitis. Pediatr Infect Dis J. 2015 Dec. doi: 10.1097/INF.0000000000000883.
2. Lieberthal AS et al. The diagnosis and management of acute otitis media. Pediatrics. 2013 Mar. doi: 10.1542/peds.2012-3488.
Genetic therapies bring change to neurology clinics
PHOENIX – New therapies are on the horizon for genetic neuromuscular diseases, and this will raise both hopes for patients and challenges for neurologists. , according to Nicolas Madigan, MBBCh, PhD, who spoke at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
“I think we will very soon be in a position to tell these patients that they might actually have a better treatment outcome with a genetic treatment than if they had a sporadic or inflammatory disorder,” said Dr. Madigan, who is an assistant professor of clinical research at Mayo Clinic, Rochester, N.Y.
To illustrate how genetic therapies are changing neurology practice, Dr. Madigan focused his talk on CMT neuropathy, which is the most common hereditary neuropathy and, as a result, has become a prime focus of gene therapy development. “In a city of about a million people, there will be 100-800 patients with one of these disorders,” said Dr. Madigan.
Case report illustrates a change in approach
There are more than 100 known genes that can contribute to CMT, but about 90% of patients harbor alterations in one of four genes: PMP22, GJB1, MFN2, and MPZ.
The trick is determining which patients are candidates for genetic testing, according to Dr. Madigan. He presented a case report of a 39-year-old woman who had experienced sensory symptoms for years, with a sudden exacerbation along with allodynia following COVID-19 vaccination. Her cerebrospinal fluid protein was high and outside electromyography indicated mild demyelinating neuropathy, consistent with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). After her insurance denied IVIG treatment, she received solumedrol, but her symptoms worsened and she was referred to Dr. Madigan.
After 6 months of methotrexate treatment, her sensory symptoms had not improved, and she was referred for genetic testing, which revealed a truncating mutation of the MPZ gene. “What I learned from this case really was that, in a young patient with conduction slowing, you might be considering CIDP. It might actually be better to do genetic testing first as opposed to starting inflammatory neuropathy type treatments with respect to cost – the genetic tests costs $300 versus tens of thousands of dollars for IVIG – and for [patient] welfare as well,” said Dr. Madigan.
Specifically, when clinical signs point to inherited neuropathy and there is conduction slowing, “the biggest bang for your buck might to be to go straight to PMP22 deletion or duplication testing and see if you can get a diagnosis. If that is negative or the clinical features are not as you might suspect, then, if you have other supportive features such as a very young age or there’s predominance of motor or sensory symptoms, you could test more broadly with a panel. If both of these are negative, then you could consider exome sequencing if the clinical phenotype really is consistent with that,” said Dr. Madigan.
The treatment landscape
With a diagnosis in hand, it’s possible to turn to treatment options, and the CMT landscape is promising. Dr. Madigan’s group recently reviewed 286 CMT clinical trials published between 1999 and 2022, 86% of which were interventions. Most were procedures based on carpal or cubital tunnel release, extracorporeal shockwave therapy, or nerve hydrodissection.
The small-molecule drug combination PXT3003 (Pharnext) – comprising baclofen, naltrexone, and sorbitol – downregulated PMP22 mRNA expression and led to improved myelination in animal models. It is currently being studied in a phase 2 clinical trial. Other approaches include supplements, stem cells, anesthetics, and various devices.
Genetic therapy is in the preclinical stage, including gene replacement using adeno-associated virus (AAV) vectors, gene silencing using antisense oligonucleotides or RNA interference, and gene editing using CRISPR-Cas 9 approaches.
Gene replacement strategies include delivering a normal copy of the gene, a supportive gene, or a gene that delays or reduces axon degeneration. Gene silencing targets PMP22, while CRISPR-Cas9 gene editing aims for PMP22 or neurofilament light polypeptide (NEFL) gene knockout.
The most clinically advanced AAV program delivers neurotrophin-3 via the viral vector to the target muscle, which has been demonstrated to improve symptoms in a mouse model using a muscle-specific promoter. A phase 1/2a trial will test the approach in three patients.
In the antisense space, chemical advances have improved the profile of the RNA, including modifications that influence inflammatory properties, stability, and targeting of specific tissues through conjugation to specific lipids, proteins, or antibodies. A 2018 study sponsored by DTxPharma showed that the formulation could improve outcomes and histologic myelination in a mouse model. In the wake of Novartis’s acquisition of the technology, Dr. Madigan anticipates that clinical trials will likely begin in 2024.
Finally, CRISPR-Cas9 targeting of a promoter region that leads to PMP22 transcription improved remyelination and electrophysiological parameters after injection into the sciatic nerve of mice.
A need for genetic counseling
Advances in testing and therapies represent exciting developments, but they also create a need for genetic counselors, according to Dr. Madigan. His clinic has two certified genetic counselors who meet with patients and discuss testing options, including risks and benefits to family members. The counselors also provide psychological support and assist in shared decision-making. They also handle testing paperwork, which eases the burden on physicians.
If the tests are negative, the genetic counselor informs the patient and lets them know of any additional testing required. In case of a positive test, the genetic counselor informs the patient, but the physician also makes contact to discuss clinical implications of the result. “I think it’s working extremely well, and I would encourage all practices to begin to explore those options moving forward,” said Dr. Madigan.
During the Q&A session after the talk, an audience member noted that genetic counselors are not covered by insurance, which places a financial burden on providers to hire them. He noted that his facility has a large clinical genomics department that was able to fund the two counselors, though they are both part-time. “It wasn’t easy. I think there was at least a year of trying to work out how to do it in terms of finding positions and negotiating, but I think once it’s accomplished it’s incredibly cost effective in terms of getting patients what they need from that perspective, and helping with the testing,” said Dr. Madigan.
Dr. Madigan reported no relevant financial disclosures.
PHOENIX – New therapies are on the horizon for genetic neuromuscular diseases, and this will raise both hopes for patients and challenges for neurologists. , according to Nicolas Madigan, MBBCh, PhD, who spoke at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
“I think we will very soon be in a position to tell these patients that they might actually have a better treatment outcome with a genetic treatment than if they had a sporadic or inflammatory disorder,” said Dr. Madigan, who is an assistant professor of clinical research at Mayo Clinic, Rochester, N.Y.
To illustrate how genetic therapies are changing neurology practice, Dr. Madigan focused his talk on CMT neuropathy, which is the most common hereditary neuropathy and, as a result, has become a prime focus of gene therapy development. “In a city of about a million people, there will be 100-800 patients with one of these disorders,” said Dr. Madigan.
Case report illustrates a change in approach
There are more than 100 known genes that can contribute to CMT, but about 90% of patients harbor alterations in one of four genes: PMP22, GJB1, MFN2, and MPZ.
The trick is determining which patients are candidates for genetic testing, according to Dr. Madigan. He presented a case report of a 39-year-old woman who had experienced sensory symptoms for years, with a sudden exacerbation along with allodynia following COVID-19 vaccination. Her cerebrospinal fluid protein was high and outside electromyography indicated mild demyelinating neuropathy, consistent with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). After her insurance denied IVIG treatment, she received solumedrol, but her symptoms worsened and she was referred to Dr. Madigan.
After 6 months of methotrexate treatment, her sensory symptoms had not improved, and she was referred for genetic testing, which revealed a truncating mutation of the MPZ gene. “What I learned from this case really was that, in a young patient with conduction slowing, you might be considering CIDP. It might actually be better to do genetic testing first as opposed to starting inflammatory neuropathy type treatments with respect to cost – the genetic tests costs $300 versus tens of thousands of dollars for IVIG – and for [patient] welfare as well,” said Dr. Madigan.
Specifically, when clinical signs point to inherited neuropathy and there is conduction slowing, “the biggest bang for your buck might to be to go straight to PMP22 deletion or duplication testing and see if you can get a diagnosis. If that is negative or the clinical features are not as you might suspect, then, if you have other supportive features such as a very young age or there’s predominance of motor or sensory symptoms, you could test more broadly with a panel. If both of these are negative, then you could consider exome sequencing if the clinical phenotype really is consistent with that,” said Dr. Madigan.
The treatment landscape
With a diagnosis in hand, it’s possible to turn to treatment options, and the CMT landscape is promising. Dr. Madigan’s group recently reviewed 286 CMT clinical trials published between 1999 and 2022, 86% of which were interventions. Most were procedures based on carpal or cubital tunnel release, extracorporeal shockwave therapy, or nerve hydrodissection.
The small-molecule drug combination PXT3003 (Pharnext) – comprising baclofen, naltrexone, and sorbitol – downregulated PMP22 mRNA expression and led to improved myelination in animal models. It is currently being studied in a phase 2 clinical trial. Other approaches include supplements, stem cells, anesthetics, and various devices.
Genetic therapy is in the preclinical stage, including gene replacement using adeno-associated virus (AAV) vectors, gene silencing using antisense oligonucleotides or RNA interference, and gene editing using CRISPR-Cas 9 approaches.
Gene replacement strategies include delivering a normal copy of the gene, a supportive gene, or a gene that delays or reduces axon degeneration. Gene silencing targets PMP22, while CRISPR-Cas9 gene editing aims for PMP22 or neurofilament light polypeptide (NEFL) gene knockout.
The most clinically advanced AAV program delivers neurotrophin-3 via the viral vector to the target muscle, which has been demonstrated to improve symptoms in a mouse model using a muscle-specific promoter. A phase 1/2a trial will test the approach in three patients.
In the antisense space, chemical advances have improved the profile of the RNA, including modifications that influence inflammatory properties, stability, and targeting of specific tissues through conjugation to specific lipids, proteins, or antibodies. A 2018 study sponsored by DTxPharma showed that the formulation could improve outcomes and histologic myelination in a mouse model. In the wake of Novartis’s acquisition of the technology, Dr. Madigan anticipates that clinical trials will likely begin in 2024.
Finally, CRISPR-Cas9 targeting of a promoter region that leads to PMP22 transcription improved remyelination and electrophysiological parameters after injection into the sciatic nerve of mice.
A need for genetic counseling
Advances in testing and therapies represent exciting developments, but they also create a need for genetic counselors, according to Dr. Madigan. His clinic has two certified genetic counselors who meet with patients and discuss testing options, including risks and benefits to family members. The counselors also provide psychological support and assist in shared decision-making. They also handle testing paperwork, which eases the burden on physicians.
If the tests are negative, the genetic counselor informs the patient and lets them know of any additional testing required. In case of a positive test, the genetic counselor informs the patient, but the physician also makes contact to discuss clinical implications of the result. “I think it’s working extremely well, and I would encourage all practices to begin to explore those options moving forward,” said Dr. Madigan.
During the Q&A session after the talk, an audience member noted that genetic counselors are not covered by insurance, which places a financial burden on providers to hire them. He noted that his facility has a large clinical genomics department that was able to fund the two counselors, though they are both part-time. “It wasn’t easy. I think there was at least a year of trying to work out how to do it in terms of finding positions and negotiating, but I think once it’s accomplished it’s incredibly cost effective in terms of getting patients what they need from that perspective, and helping with the testing,” said Dr. Madigan.
Dr. Madigan reported no relevant financial disclosures.
PHOENIX – New therapies are on the horizon for genetic neuromuscular diseases, and this will raise both hopes for patients and challenges for neurologists. , according to Nicolas Madigan, MBBCh, PhD, who spoke at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
“I think we will very soon be in a position to tell these patients that they might actually have a better treatment outcome with a genetic treatment than if they had a sporadic or inflammatory disorder,” said Dr. Madigan, who is an assistant professor of clinical research at Mayo Clinic, Rochester, N.Y.
To illustrate how genetic therapies are changing neurology practice, Dr. Madigan focused his talk on CMT neuropathy, which is the most common hereditary neuropathy and, as a result, has become a prime focus of gene therapy development. “In a city of about a million people, there will be 100-800 patients with one of these disorders,” said Dr. Madigan.
Case report illustrates a change in approach
There are more than 100 known genes that can contribute to CMT, but about 90% of patients harbor alterations in one of four genes: PMP22, GJB1, MFN2, and MPZ.
The trick is determining which patients are candidates for genetic testing, according to Dr. Madigan. He presented a case report of a 39-year-old woman who had experienced sensory symptoms for years, with a sudden exacerbation along with allodynia following COVID-19 vaccination. Her cerebrospinal fluid protein was high and outside electromyography indicated mild demyelinating neuropathy, consistent with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). After her insurance denied IVIG treatment, she received solumedrol, but her symptoms worsened and she was referred to Dr. Madigan.
After 6 months of methotrexate treatment, her sensory symptoms had not improved, and she was referred for genetic testing, which revealed a truncating mutation of the MPZ gene. “What I learned from this case really was that, in a young patient with conduction slowing, you might be considering CIDP. It might actually be better to do genetic testing first as opposed to starting inflammatory neuropathy type treatments with respect to cost – the genetic tests costs $300 versus tens of thousands of dollars for IVIG – and for [patient] welfare as well,” said Dr. Madigan.
Specifically, when clinical signs point to inherited neuropathy and there is conduction slowing, “the biggest bang for your buck might to be to go straight to PMP22 deletion or duplication testing and see if you can get a diagnosis. If that is negative or the clinical features are not as you might suspect, then, if you have other supportive features such as a very young age or there’s predominance of motor or sensory symptoms, you could test more broadly with a panel. If both of these are negative, then you could consider exome sequencing if the clinical phenotype really is consistent with that,” said Dr. Madigan.
The treatment landscape
With a diagnosis in hand, it’s possible to turn to treatment options, and the CMT landscape is promising. Dr. Madigan’s group recently reviewed 286 CMT clinical trials published between 1999 and 2022, 86% of which were interventions. Most were procedures based on carpal or cubital tunnel release, extracorporeal shockwave therapy, or nerve hydrodissection.
The small-molecule drug combination PXT3003 (Pharnext) – comprising baclofen, naltrexone, and sorbitol – downregulated PMP22 mRNA expression and led to improved myelination in animal models. It is currently being studied in a phase 2 clinical trial. Other approaches include supplements, stem cells, anesthetics, and various devices.
Genetic therapy is in the preclinical stage, including gene replacement using adeno-associated virus (AAV) vectors, gene silencing using antisense oligonucleotides or RNA interference, and gene editing using CRISPR-Cas 9 approaches.
Gene replacement strategies include delivering a normal copy of the gene, a supportive gene, or a gene that delays or reduces axon degeneration. Gene silencing targets PMP22, while CRISPR-Cas9 gene editing aims for PMP22 or neurofilament light polypeptide (NEFL) gene knockout.
The most clinically advanced AAV program delivers neurotrophin-3 via the viral vector to the target muscle, which has been demonstrated to improve symptoms in a mouse model using a muscle-specific promoter. A phase 1/2a trial will test the approach in three patients.
In the antisense space, chemical advances have improved the profile of the RNA, including modifications that influence inflammatory properties, stability, and targeting of specific tissues through conjugation to specific lipids, proteins, or antibodies. A 2018 study sponsored by DTxPharma showed that the formulation could improve outcomes and histologic myelination in a mouse model. In the wake of Novartis’s acquisition of the technology, Dr. Madigan anticipates that clinical trials will likely begin in 2024.
Finally, CRISPR-Cas9 targeting of a promoter region that leads to PMP22 transcription improved remyelination and electrophysiological parameters after injection into the sciatic nerve of mice.
A need for genetic counseling
Advances in testing and therapies represent exciting developments, but they also create a need for genetic counselors, according to Dr. Madigan. His clinic has two certified genetic counselors who meet with patients and discuss testing options, including risks and benefits to family members. The counselors also provide psychological support and assist in shared decision-making. They also handle testing paperwork, which eases the burden on physicians.
If the tests are negative, the genetic counselor informs the patient and lets them know of any additional testing required. In case of a positive test, the genetic counselor informs the patient, but the physician also makes contact to discuss clinical implications of the result. “I think it’s working extremely well, and I would encourage all practices to begin to explore those options moving forward,” said Dr. Madigan.
During the Q&A session after the talk, an audience member noted that genetic counselors are not covered by insurance, which places a financial burden on providers to hire them. He noted that his facility has a large clinical genomics department that was able to fund the two counselors, though they are both part-time. “It wasn’t easy. I think there was at least a year of trying to work out how to do it in terms of finding positions and negotiating, but I think once it’s accomplished it’s incredibly cost effective in terms of getting patients what they need from that perspective, and helping with the testing,” said Dr. Madigan.
Dr. Madigan reported no relevant financial disclosures.
At AANEM 2023
Test all perinatally exposed infants for HCV: CDC
In utero–exposed infants should be tested at 2-6 months of life, much earlier than the current strategy of testing at 18 months.
HCV infection, which can lead to liver fibrosis and cirrhosis, liver failure, hepatic cancer, and transplant, will develop in 6%-7% of all perinatally exposed infants and children. Curative therapy with direct-acting antivirals can be administered starting at age 3, the CDC noted in Morbidity and Mortality Week Report (MMWR).
About 70% of children 18 months and older are not being tested with the current strategy of anti-HCV testing.
This current MMWR report supplements the 2020 CDC recommendations for adult HCV screening, which includes universal screening among pregnant persons during each pregnancy.
The new recommendations
- Perinatally exposed infants should receive a nucleic acid amplification test for HCV RNA at 2-6 months of age to identify those who might develop chronic HCV infection if not treated.
- Those with detectable HCV RNA should be managed in consultation with an expert in pediatric HCV.
- Infants with undetectable HCV RNA do not require further follow-up unless clinically warranted.
“Testing perinatally exposed infants beginning at age 2 months with a NAT for HCV RNA is cost-effective and allows for earlier linkage to care, appropriate evaluation, and the opportunity to provide curative, life-saving therapy,” the MMWR report said.
A growing problem
The CDC noted that rates of HCV infections during pregnancy are on the rise, corresponding with the ongoing opioid crisis and intravenous drug use.
Yet most perinatally exposed children are not tested for HCV infection and are not referred for hepatitis C care. Reasons might include lack of awareness of perinatal exposure by pediatric providers, lack of regular pediatric care among exposed children, and switching of health care providers before the former recommended testing age of 18 months.
The CDC’s testing recommendation is welcome news to Dawnette A. Lewis, MD, a maternal fetal medicine specialist at Northwell Health in New Hyde Park, N.Y. “As opposed to data for hep B and HIV, we have traditionally had little information and experience regarding the transmission and impact of hep C in pregnant women and their babies. We’ve been having that conversation about the lack of information for some time, and now there’s an opportunity to get evolving data on hep C and how it affects the baby, ” she said.
In her view, mothers will likely be quite accepting of testing for their infants. “It could be integrated into the routine newborn screening panel, so there should not be barriers to accessibility if they’re getting prenatal and neonatal care.”
Commenting on HCV testing for babies in an interview at his institution, Ravi R. Jhaveri, MD, division head of pediatric infectious diseases at Northwestern Medicine’s Ann & Robert H. Lurie Children’s Hospital of Chicago, said, “This is a terrific way to capitalize on the fact that infants already come to the doctor for many visits during the first months of life for their vaccines and their well-child check. And so this should be an easy way to streamline our testing strategy and hopefully lose many fewer patients.”
Northwestern Medicine is an innovative clinic offering HCV testing and treatment outside of clinical trials for pregnant women and their infants with the goal of preventing transmission from mother to child.
Northwestern is launching a clinical trial of treatment for HCV-positive pregnant patients during regular prenatal care. “With very simple treatments similar to taking a prenatal vitamin, it would be easy and seamless to fit into the existing schedule,” said Lyn Yee, MD, a Northwestern maternal-fetal medicine specialist.
Dr. Yee stressed that eliminating hepatitis C will likely be one of the most significant health advancements of the decade.
Dr. Lewis, Dr. Jhaveri, and Dr. Yee had no relevant conflicts of interest to declare with regard to their comments.
In utero–exposed infants should be tested at 2-6 months of life, much earlier than the current strategy of testing at 18 months.
HCV infection, which can lead to liver fibrosis and cirrhosis, liver failure, hepatic cancer, and transplant, will develop in 6%-7% of all perinatally exposed infants and children. Curative therapy with direct-acting antivirals can be administered starting at age 3, the CDC noted in Morbidity and Mortality Week Report (MMWR).
About 70% of children 18 months and older are not being tested with the current strategy of anti-HCV testing.
This current MMWR report supplements the 2020 CDC recommendations for adult HCV screening, which includes universal screening among pregnant persons during each pregnancy.
The new recommendations
- Perinatally exposed infants should receive a nucleic acid amplification test for HCV RNA at 2-6 months of age to identify those who might develop chronic HCV infection if not treated.
- Those with detectable HCV RNA should be managed in consultation with an expert in pediatric HCV.
- Infants with undetectable HCV RNA do not require further follow-up unless clinically warranted.
“Testing perinatally exposed infants beginning at age 2 months with a NAT for HCV RNA is cost-effective and allows for earlier linkage to care, appropriate evaluation, and the opportunity to provide curative, life-saving therapy,” the MMWR report said.
A growing problem
The CDC noted that rates of HCV infections during pregnancy are on the rise, corresponding with the ongoing opioid crisis and intravenous drug use.
Yet most perinatally exposed children are not tested for HCV infection and are not referred for hepatitis C care. Reasons might include lack of awareness of perinatal exposure by pediatric providers, lack of regular pediatric care among exposed children, and switching of health care providers before the former recommended testing age of 18 months.
The CDC’s testing recommendation is welcome news to Dawnette A. Lewis, MD, a maternal fetal medicine specialist at Northwell Health in New Hyde Park, N.Y. “As opposed to data for hep B and HIV, we have traditionally had little information and experience regarding the transmission and impact of hep C in pregnant women and their babies. We’ve been having that conversation about the lack of information for some time, and now there’s an opportunity to get evolving data on hep C and how it affects the baby, ” she said.
In her view, mothers will likely be quite accepting of testing for their infants. “It could be integrated into the routine newborn screening panel, so there should not be barriers to accessibility if they’re getting prenatal and neonatal care.”
Commenting on HCV testing for babies in an interview at his institution, Ravi R. Jhaveri, MD, division head of pediatric infectious diseases at Northwestern Medicine’s Ann & Robert H. Lurie Children’s Hospital of Chicago, said, “This is a terrific way to capitalize on the fact that infants already come to the doctor for many visits during the first months of life for their vaccines and their well-child check. And so this should be an easy way to streamline our testing strategy and hopefully lose many fewer patients.”
Northwestern Medicine is an innovative clinic offering HCV testing and treatment outside of clinical trials for pregnant women and their infants with the goal of preventing transmission from mother to child.
Northwestern is launching a clinical trial of treatment for HCV-positive pregnant patients during regular prenatal care. “With very simple treatments similar to taking a prenatal vitamin, it would be easy and seamless to fit into the existing schedule,” said Lyn Yee, MD, a Northwestern maternal-fetal medicine specialist.
Dr. Yee stressed that eliminating hepatitis C will likely be one of the most significant health advancements of the decade.
Dr. Lewis, Dr. Jhaveri, and Dr. Yee had no relevant conflicts of interest to declare with regard to their comments.
In utero–exposed infants should be tested at 2-6 months of life, much earlier than the current strategy of testing at 18 months.
HCV infection, which can lead to liver fibrosis and cirrhosis, liver failure, hepatic cancer, and transplant, will develop in 6%-7% of all perinatally exposed infants and children. Curative therapy with direct-acting antivirals can be administered starting at age 3, the CDC noted in Morbidity and Mortality Week Report (MMWR).
About 70% of children 18 months and older are not being tested with the current strategy of anti-HCV testing.
This current MMWR report supplements the 2020 CDC recommendations for adult HCV screening, which includes universal screening among pregnant persons during each pregnancy.
The new recommendations
- Perinatally exposed infants should receive a nucleic acid amplification test for HCV RNA at 2-6 months of age to identify those who might develop chronic HCV infection if not treated.
- Those with detectable HCV RNA should be managed in consultation with an expert in pediatric HCV.
- Infants with undetectable HCV RNA do not require further follow-up unless clinically warranted.
“Testing perinatally exposed infants beginning at age 2 months with a NAT for HCV RNA is cost-effective and allows for earlier linkage to care, appropriate evaluation, and the opportunity to provide curative, life-saving therapy,” the MMWR report said.
A growing problem
The CDC noted that rates of HCV infections during pregnancy are on the rise, corresponding with the ongoing opioid crisis and intravenous drug use.
Yet most perinatally exposed children are not tested for HCV infection and are not referred for hepatitis C care. Reasons might include lack of awareness of perinatal exposure by pediatric providers, lack of regular pediatric care among exposed children, and switching of health care providers before the former recommended testing age of 18 months.
The CDC’s testing recommendation is welcome news to Dawnette A. Lewis, MD, a maternal fetal medicine specialist at Northwell Health in New Hyde Park, N.Y. “As opposed to data for hep B and HIV, we have traditionally had little information and experience regarding the transmission and impact of hep C in pregnant women and their babies. We’ve been having that conversation about the lack of information for some time, and now there’s an opportunity to get evolving data on hep C and how it affects the baby, ” she said.
In her view, mothers will likely be quite accepting of testing for their infants. “It could be integrated into the routine newborn screening panel, so there should not be barriers to accessibility if they’re getting prenatal and neonatal care.”
Commenting on HCV testing for babies in an interview at his institution, Ravi R. Jhaveri, MD, division head of pediatric infectious diseases at Northwestern Medicine’s Ann & Robert H. Lurie Children’s Hospital of Chicago, said, “This is a terrific way to capitalize on the fact that infants already come to the doctor for many visits during the first months of life for their vaccines and their well-child check. And so this should be an easy way to streamline our testing strategy and hopefully lose many fewer patients.”
Northwestern Medicine is an innovative clinic offering HCV testing and treatment outside of clinical trials for pregnant women and their infants with the goal of preventing transmission from mother to child.
Northwestern is launching a clinical trial of treatment for HCV-positive pregnant patients during regular prenatal care. “With very simple treatments similar to taking a prenatal vitamin, it would be easy and seamless to fit into the existing schedule,” said Lyn Yee, MD, a Northwestern maternal-fetal medicine specialist.
Dr. Yee stressed that eliminating hepatitis C will likely be one of the most significant health advancements of the decade.
Dr. Lewis, Dr. Jhaveri, and Dr. Yee had no relevant conflicts of interest to declare with regard to their comments.
Adverse events related to embryo transfer catheters may be underreported to the FDA
, according to a new study presented at the American Society for Reproductive Medicine’s 2023 meeting.
ETCs are medical devices used routinely in assisted reproduction. The findings highlight the need for increased vigilance in tracking and reporting adverse events associated with these devices, according to the investigators.
“With hundreds of thousands of embryo transfers being performed per year, surveillance of the safety, performance, and quality of embryo transfer catheter devices is critical and should not be taken for granted,” said Anita Madison, MD, MPH, from the division of reproductive endocrinology and infertility at Johns Hopkins School of Medicine, Baltimore, who led the study. “There are a variety of transfer catheters with different indications, with little data on the superiority and safety of the brands compared to one another.”
Although the number of reported adverse events associated with ETCs is relatively small, the problems can significantly affect patient care, the researchers said.
Dr. Madison and her colleagues used the Manufacturer and User Facility Device Experience (MAUDE) database to identify adverse events associated with ETC devices. The MAUDE database is a voluntary reporting system that holds hundreds of thousands of medical device reports of suspected device-associated deaths, injuries, and malfunctions reported to the FDA annually.
For each adverse event in the database linked to an ECT, the researchers collected information related to the brand of the device, the nature of the event, and the nature of the reporter. The researchers omitted the device and manufacturer names from the presentation of the study findings, delineating them only as “Brand 1,” “Brand 2,” “Brand 3,” “Brand 4,” or “Other.”
Problems with devices included contamination, packaging problems, malfunction, mechanical flaws, and material separation. Patient-level adverse events included retaining of foreign body, trauma, malfunction, or failed embryo transfer.
Between 2014 and 2023, Dr. Madison and her colleagues identified 101 adverse events associated with ECTs in the database. About 25% of these occurred in 2018, with 27 cases reported. Contamination was the most prevalent problem, found in 68 reports; oil was the most common contaminant.
The distribution of types of adverse events varied, depending on ETC brand. A breakdown of occurrences revealed high numbers for Brand 2, with 52 adverse events. Although Brand 3 accounted for only 16 adverse events, the majority of these were related to device separation.
“That finding stood out,” Dr. Madison said.
Nearly 1 in 4 (22%) of all reported incidents led to overt patient harm. Retention of a foreign body was the prime type of injury, occurring in 12 cases. Malfunction and injury were found in four cases each, with two failed embryo transfers reported, Dr. Madison said.
Because the majority of these adverse event reports were submitted by manufacturers (87%) and were rarely submitted by end users (for example, physicians, lab staff), the researchers said their findings likely underestimate such problems.
“I’m surprised the [number of reported adverse events] is as low as it is,” said Kimball Pomeroy, PhD, scientific director at the World Egg and Sperm Bank, Scottsdale, Ariz., who was not part of the study team. “Laboratories are required to report failed devices; they have to have a plan for that.”
“It just comes down to underreporting,” added Valerie L. Baker, MD, director in the Division of Reproductive Endocrinology and Infertility at Johns Hopkins Medicine, Lutherville, Md., who was not affiliated with the study.
“In two of these reports, they failed to transfer the embryo; they actually lost the embryo,” Dr. Pomeroy added. “That’s drastic for those patients; it’s a serious problem that needs to be addressed.”
Citing these findings, the authors underscored the need for heightened surveillance of ETC devices and recommend further studies to assess the sensitivity of these procedures for attempting pregnancy. They urge physicians and lab staff involved in these procedures to exercise continued vigilance and to improve the reporting of problems with ETC devices.
Dr. Madison, Dr. Baker, and Dr. Pomeroy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new study presented at the American Society for Reproductive Medicine’s 2023 meeting.
ETCs are medical devices used routinely in assisted reproduction. The findings highlight the need for increased vigilance in tracking and reporting adverse events associated with these devices, according to the investigators.
“With hundreds of thousands of embryo transfers being performed per year, surveillance of the safety, performance, and quality of embryo transfer catheter devices is critical and should not be taken for granted,” said Anita Madison, MD, MPH, from the division of reproductive endocrinology and infertility at Johns Hopkins School of Medicine, Baltimore, who led the study. “There are a variety of transfer catheters with different indications, with little data on the superiority and safety of the brands compared to one another.”
Although the number of reported adverse events associated with ETCs is relatively small, the problems can significantly affect patient care, the researchers said.
Dr. Madison and her colleagues used the Manufacturer and User Facility Device Experience (MAUDE) database to identify adverse events associated with ETC devices. The MAUDE database is a voluntary reporting system that holds hundreds of thousands of medical device reports of suspected device-associated deaths, injuries, and malfunctions reported to the FDA annually.
For each adverse event in the database linked to an ECT, the researchers collected information related to the brand of the device, the nature of the event, and the nature of the reporter. The researchers omitted the device and manufacturer names from the presentation of the study findings, delineating them only as “Brand 1,” “Brand 2,” “Brand 3,” “Brand 4,” or “Other.”
Problems with devices included contamination, packaging problems, malfunction, mechanical flaws, and material separation. Patient-level adverse events included retaining of foreign body, trauma, malfunction, or failed embryo transfer.
Between 2014 and 2023, Dr. Madison and her colleagues identified 101 adverse events associated with ECTs in the database. About 25% of these occurred in 2018, with 27 cases reported. Contamination was the most prevalent problem, found in 68 reports; oil was the most common contaminant.
The distribution of types of adverse events varied, depending on ETC brand. A breakdown of occurrences revealed high numbers for Brand 2, with 52 adverse events. Although Brand 3 accounted for only 16 adverse events, the majority of these were related to device separation.
“That finding stood out,” Dr. Madison said.
Nearly 1 in 4 (22%) of all reported incidents led to overt patient harm. Retention of a foreign body was the prime type of injury, occurring in 12 cases. Malfunction and injury were found in four cases each, with two failed embryo transfers reported, Dr. Madison said.
Because the majority of these adverse event reports were submitted by manufacturers (87%) and were rarely submitted by end users (for example, physicians, lab staff), the researchers said their findings likely underestimate such problems.
“I’m surprised the [number of reported adverse events] is as low as it is,” said Kimball Pomeroy, PhD, scientific director at the World Egg and Sperm Bank, Scottsdale, Ariz., who was not part of the study team. “Laboratories are required to report failed devices; they have to have a plan for that.”
“It just comes down to underreporting,” added Valerie L. Baker, MD, director in the Division of Reproductive Endocrinology and Infertility at Johns Hopkins Medicine, Lutherville, Md., who was not affiliated with the study.
“In two of these reports, they failed to transfer the embryo; they actually lost the embryo,” Dr. Pomeroy added. “That’s drastic for those patients; it’s a serious problem that needs to be addressed.”
Citing these findings, the authors underscored the need for heightened surveillance of ETC devices and recommend further studies to assess the sensitivity of these procedures for attempting pregnancy. They urge physicians and lab staff involved in these procedures to exercise continued vigilance and to improve the reporting of problems with ETC devices.
Dr. Madison, Dr. Baker, and Dr. Pomeroy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new study presented at the American Society for Reproductive Medicine’s 2023 meeting.
ETCs are medical devices used routinely in assisted reproduction. The findings highlight the need for increased vigilance in tracking and reporting adverse events associated with these devices, according to the investigators.
“With hundreds of thousands of embryo transfers being performed per year, surveillance of the safety, performance, and quality of embryo transfer catheter devices is critical and should not be taken for granted,” said Anita Madison, MD, MPH, from the division of reproductive endocrinology and infertility at Johns Hopkins School of Medicine, Baltimore, who led the study. “There are a variety of transfer catheters with different indications, with little data on the superiority and safety of the brands compared to one another.”
Although the number of reported adverse events associated with ETCs is relatively small, the problems can significantly affect patient care, the researchers said.
Dr. Madison and her colleagues used the Manufacturer and User Facility Device Experience (MAUDE) database to identify adverse events associated with ETC devices. The MAUDE database is a voluntary reporting system that holds hundreds of thousands of medical device reports of suspected device-associated deaths, injuries, and malfunctions reported to the FDA annually.
For each adverse event in the database linked to an ECT, the researchers collected information related to the brand of the device, the nature of the event, and the nature of the reporter. The researchers omitted the device and manufacturer names from the presentation of the study findings, delineating them only as “Brand 1,” “Brand 2,” “Brand 3,” “Brand 4,” or “Other.”
Problems with devices included contamination, packaging problems, malfunction, mechanical flaws, and material separation. Patient-level adverse events included retaining of foreign body, trauma, malfunction, or failed embryo transfer.
Between 2014 and 2023, Dr. Madison and her colleagues identified 101 adverse events associated with ECTs in the database. About 25% of these occurred in 2018, with 27 cases reported. Contamination was the most prevalent problem, found in 68 reports; oil was the most common contaminant.
The distribution of types of adverse events varied, depending on ETC brand. A breakdown of occurrences revealed high numbers for Brand 2, with 52 adverse events. Although Brand 3 accounted for only 16 adverse events, the majority of these were related to device separation.
“That finding stood out,” Dr. Madison said.
Nearly 1 in 4 (22%) of all reported incidents led to overt patient harm. Retention of a foreign body was the prime type of injury, occurring in 12 cases. Malfunction and injury were found in four cases each, with two failed embryo transfers reported, Dr. Madison said.
Because the majority of these adverse event reports were submitted by manufacturers (87%) and were rarely submitted by end users (for example, physicians, lab staff), the researchers said their findings likely underestimate such problems.
“I’m surprised the [number of reported adverse events] is as low as it is,” said Kimball Pomeroy, PhD, scientific director at the World Egg and Sperm Bank, Scottsdale, Ariz., who was not part of the study team. “Laboratories are required to report failed devices; they have to have a plan for that.”
“It just comes down to underreporting,” added Valerie L. Baker, MD, director in the Division of Reproductive Endocrinology and Infertility at Johns Hopkins Medicine, Lutherville, Md., who was not affiliated with the study.
“In two of these reports, they failed to transfer the embryo; they actually lost the embryo,” Dr. Pomeroy added. “That’s drastic for those patients; it’s a serious problem that needs to be addressed.”
Citing these findings, the authors underscored the need for heightened surveillance of ETC devices and recommend further studies to assess the sensitivity of these procedures for attempting pregnancy. They urge physicians and lab staff involved in these procedures to exercise continued vigilance and to improve the reporting of problems with ETC devices.
Dr. Madison, Dr. Baker, and Dr. Pomeroy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASRM 2023
Digital tool clarifies menopause symptoms
GLASGOW – An interactive digital decision tool that individualizes menopause care received praise from primary care clinicians in the United Kingdom, who said it could improve patient care and streamline office visits.
“Access to hormone replacement therapy [HRT], as well as decision-making around treatment for menopausal symptoms, is often complicated by concerns around its safety, and there is still a knowledge and a confidence gap among health care professionals causing reluctance to prescribe HRT,” said Aini Kamal, MSc, from University College London. Ms. Kamal presented results of a survey about the tool at the annual meeting of the Royal College of General Practitioners.
For the study, Ms. Kamal, Daniel Reisel, MBBS, PhD, a gynecologist at UCL, and colleagues evaluated Wellspring with doctors, nurses, and pharmacists.
“Ensuring that women receive education around symptoms, so that they are empowered, is a key part of optimizing their care and sharing decision-making,” Dr. Reisel said in an interview. He added that U.K. primary care had seen an increase in cases of women presenting with symptoms associated with the perimenopause and menopause at a time when U.K. Members of Parliament are debating whether to make it mandatory for all women to have menopause check-up in their early 40s.
The online survey was completed by 280 participants, and respondents were primarily GPs with several years of relevant prescribing practice. Of those, 93% found information from national guidelines to be accurately presented in the tool, and 97% said they would recommend this decision aid to other health care professionals, Ms. Kamal reported.
Nearly all participants said they could see themselves using the tool with patients in the clinic or as an adjunct to virtual sessions. “This [finding] was particularly important because it demonstrates the clinical potential this tool has,” she said.
One consult, too many problems
Louise Newson, MBChB, who runs the U.K.’s largest menopause clinic, said primary care appointments are often time-pressured and follow a “’one problem-one consultation’” policy. As such, women are often thinking ‘Do I go with my joint pains, or my palpitations, tinnitus, or what?’ If a patient presents with tinnitus, a doctor might focus on the potential of an inner ear problem rather than a hormone deficiency, but I do know that if the woman is perimenopausal or menopausal, we often look to replace the missing hormones, and then if the tinnitus doesn’t improve we can revisit the ear problem.”
Dr. Newson noted that 17% of women in her clinics have had more than six GP visits in the year before she sees them, but in the year following, this figure drops to 1%. Acknowledging that a menopause consultation for a GP is time-consuming, Dr. Newson pointed out that taking time initially with the patient “means it will reduce the number of future consultations quickly, but more importantly, we also know that taking HRT reduces long-term risk of serious diseases, including heart disease and osteoporosis.”
The digital tool can be used by both doctors and patients to help women work through their symptoms and equip them with knowledge so their GP visits are more productive.
“When we see women who are empowered with knowledge [about menopause symptoms], then the consultations are quicker and essentially place the patient central to the discussion,” Dr. Newson said.
Ed Russell-Smith, MBChB, a GP in Scotland who moderated the session, said the tool “lays out a nicely structured approach and provides modern treatment options and resources for patients.”
However, he added “we also need to remember there are potential harms to be done from HRT too. It’s vitally important that while patients might see HRT as a panacea, doctors need to balance this with the risks involved for each individual. As a tool, I think Wellspring can help us in this respect to apply general principles to that patient and individualize treatment.”
Dr. Reisel, Dr. Newson, Ms. Kamal, and Dr. Russell-Smith disclosed no relevant financial relationships. The Wellspring Decision Aid was supported by UCL’s Institute for Women’s Health. The Newson Health clinic is fully private, but research is done via the nonprofit arm, which is supported by the clinic. There is no pharma involvement.
A version of this article first appeared on Medscape.com.
GLASGOW – An interactive digital decision tool that individualizes menopause care received praise from primary care clinicians in the United Kingdom, who said it could improve patient care and streamline office visits.
“Access to hormone replacement therapy [HRT], as well as decision-making around treatment for menopausal symptoms, is often complicated by concerns around its safety, and there is still a knowledge and a confidence gap among health care professionals causing reluctance to prescribe HRT,” said Aini Kamal, MSc, from University College London. Ms. Kamal presented results of a survey about the tool at the annual meeting of the Royal College of General Practitioners.
For the study, Ms. Kamal, Daniel Reisel, MBBS, PhD, a gynecologist at UCL, and colleagues evaluated Wellspring with doctors, nurses, and pharmacists.
“Ensuring that women receive education around symptoms, so that they are empowered, is a key part of optimizing their care and sharing decision-making,” Dr. Reisel said in an interview. He added that U.K. primary care had seen an increase in cases of women presenting with symptoms associated with the perimenopause and menopause at a time when U.K. Members of Parliament are debating whether to make it mandatory for all women to have menopause check-up in their early 40s.
The online survey was completed by 280 participants, and respondents were primarily GPs with several years of relevant prescribing practice. Of those, 93% found information from national guidelines to be accurately presented in the tool, and 97% said they would recommend this decision aid to other health care professionals, Ms. Kamal reported.
Nearly all participants said they could see themselves using the tool with patients in the clinic or as an adjunct to virtual sessions. “This [finding] was particularly important because it demonstrates the clinical potential this tool has,” she said.
One consult, too many problems
Louise Newson, MBChB, who runs the U.K.’s largest menopause clinic, said primary care appointments are often time-pressured and follow a “’one problem-one consultation’” policy. As such, women are often thinking ‘Do I go with my joint pains, or my palpitations, tinnitus, or what?’ If a patient presents with tinnitus, a doctor might focus on the potential of an inner ear problem rather than a hormone deficiency, but I do know that if the woman is perimenopausal or menopausal, we often look to replace the missing hormones, and then if the tinnitus doesn’t improve we can revisit the ear problem.”
Dr. Newson noted that 17% of women in her clinics have had more than six GP visits in the year before she sees them, but in the year following, this figure drops to 1%. Acknowledging that a menopause consultation for a GP is time-consuming, Dr. Newson pointed out that taking time initially with the patient “means it will reduce the number of future consultations quickly, but more importantly, we also know that taking HRT reduces long-term risk of serious diseases, including heart disease and osteoporosis.”
The digital tool can be used by both doctors and patients to help women work through their symptoms and equip them with knowledge so their GP visits are more productive.
“When we see women who are empowered with knowledge [about menopause symptoms], then the consultations are quicker and essentially place the patient central to the discussion,” Dr. Newson said.
Ed Russell-Smith, MBChB, a GP in Scotland who moderated the session, said the tool “lays out a nicely structured approach and provides modern treatment options and resources for patients.”
However, he added “we also need to remember there are potential harms to be done from HRT too. It’s vitally important that while patients might see HRT as a panacea, doctors need to balance this with the risks involved for each individual. As a tool, I think Wellspring can help us in this respect to apply general principles to that patient and individualize treatment.”
Dr. Reisel, Dr. Newson, Ms. Kamal, and Dr. Russell-Smith disclosed no relevant financial relationships. The Wellspring Decision Aid was supported by UCL’s Institute for Women’s Health. The Newson Health clinic is fully private, but research is done via the nonprofit arm, which is supported by the clinic. There is no pharma involvement.
A version of this article first appeared on Medscape.com.
GLASGOW – An interactive digital decision tool that individualizes menopause care received praise from primary care clinicians in the United Kingdom, who said it could improve patient care and streamline office visits.
“Access to hormone replacement therapy [HRT], as well as decision-making around treatment for menopausal symptoms, is often complicated by concerns around its safety, and there is still a knowledge and a confidence gap among health care professionals causing reluctance to prescribe HRT,” said Aini Kamal, MSc, from University College London. Ms. Kamal presented results of a survey about the tool at the annual meeting of the Royal College of General Practitioners.
For the study, Ms. Kamal, Daniel Reisel, MBBS, PhD, a gynecologist at UCL, and colleagues evaluated Wellspring with doctors, nurses, and pharmacists.
“Ensuring that women receive education around symptoms, so that they are empowered, is a key part of optimizing their care and sharing decision-making,” Dr. Reisel said in an interview. He added that U.K. primary care had seen an increase in cases of women presenting with symptoms associated with the perimenopause and menopause at a time when U.K. Members of Parliament are debating whether to make it mandatory for all women to have menopause check-up in their early 40s.
The online survey was completed by 280 participants, and respondents were primarily GPs with several years of relevant prescribing practice. Of those, 93% found information from national guidelines to be accurately presented in the tool, and 97% said they would recommend this decision aid to other health care professionals, Ms. Kamal reported.
Nearly all participants said they could see themselves using the tool with patients in the clinic or as an adjunct to virtual sessions. “This [finding] was particularly important because it demonstrates the clinical potential this tool has,” she said.
One consult, too many problems
Louise Newson, MBChB, who runs the U.K.’s largest menopause clinic, said primary care appointments are often time-pressured and follow a “’one problem-one consultation’” policy. As such, women are often thinking ‘Do I go with my joint pains, or my palpitations, tinnitus, or what?’ If a patient presents with tinnitus, a doctor might focus on the potential of an inner ear problem rather than a hormone deficiency, but I do know that if the woman is perimenopausal or menopausal, we often look to replace the missing hormones, and then if the tinnitus doesn’t improve we can revisit the ear problem.”
Dr. Newson noted that 17% of women in her clinics have had more than six GP visits in the year before she sees them, but in the year following, this figure drops to 1%. Acknowledging that a menopause consultation for a GP is time-consuming, Dr. Newson pointed out that taking time initially with the patient “means it will reduce the number of future consultations quickly, but more importantly, we also know that taking HRT reduces long-term risk of serious diseases, including heart disease and osteoporosis.”
The digital tool can be used by both doctors and patients to help women work through their symptoms and equip them with knowledge so their GP visits are more productive.
“When we see women who are empowered with knowledge [about menopause symptoms], then the consultations are quicker and essentially place the patient central to the discussion,” Dr. Newson said.
Ed Russell-Smith, MBChB, a GP in Scotland who moderated the session, said the tool “lays out a nicely structured approach and provides modern treatment options and resources for patients.”
However, he added “we also need to remember there are potential harms to be done from HRT too. It’s vitally important that while patients might see HRT as a panacea, doctors need to balance this with the risks involved for each individual. As a tool, I think Wellspring can help us in this respect to apply general principles to that patient and individualize treatment.”
Dr. Reisel, Dr. Newson, Ms. Kamal, and Dr. Russell-Smith disclosed no relevant financial relationships. The Wellspring Decision Aid was supported by UCL’s Institute for Women’s Health. The Newson Health clinic is fully private, but research is done via the nonprofit arm, which is supported by the clinic. There is no pharma involvement.
A version of this article first appeared on Medscape.com.
AT RCGP 2023








