User login
Systematic Review of Novel Synovial Fluid Markers and Polymerase Chain Reaction in the Diagnosis of Prosthetic Joint Infection
Take-Home Points
- Novel synovial markers and PCR have the potential to improve the detection of PJIs.
- 10Difficult-to-detect infections of prosthetic joints pose a diagnostic problem to surgeons and can lead to suboptimal outcomes.
- AD is a highly sensitive and specific synovial fluid marker for detecting PJIs.
- AD has shown promising results in detecting low virulence organisms.
- Studies are needed to determine how to best incorporate novel synovial markers and PCR to current diagnostic criteria in order to improve diagnostic accuracy.
Approximately 7 million Americans are living with a hip or knee replacement.1 According to projections, primary hip arthroplasties will increase by 174% and knee arthroplasties by 673% by 2030. Revision arthroplasties are projected to increase by 137% for hips and 601% for knees during the same time period.2 Infection and aseptic loosening are the most common causes of implant failure.3 The literature shows that infection is the most common cause of failure within 2 years after surgery and that aseptic loosening is the most common cause for late revision.3
Recent studies suggest that prosthetic joint infection (PJI) may be underreported because of difficulty making a diagnosis and that cases of aseptic loosening may in fact be attributable to infections with low-virulence organisms.2,3 These findings have led to new efforts to develop uniform criteria for diagnosing PJIs. In 2011, the Musculoskeletal Infection Society (MSIS) offered a new definition for PJI diagnosis, based on clinical and laboratory criteria, to increase the accuracy of PJI diagnosis.4 The MSIS committee acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-virulence organisms, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. Reports of PJI cases misdiagnosed as aseptic loosening suggest that current screening and diagnostic tools are not sensitive enough to detect all infections and that PJI is likely underdiagnosed.
According to MSIS criteria, the diagnosis of PJI can be made when there is a sinus tract communicating with the prosthesis, when a pathogen is isolated by culture from 2 or more separate tissue or fluid samples obtained from the affected prosthetic joint, or when 4 of 6 criteria are met. The 6 criteria are (1) elevated serum erythrocyte sedimentation rate (ESR) (>30 mm/hour) and elevated C-reactive protein (CRP) level (>10 mg/L); (2) elevated synovial white blood cell (WBC) count (1100-4000 cells/μL); (3) elevated synovial polymorphonuclear leukocytes (>64%); (4) purulence in affected joint; (5) isolation of a microorganism in a culture of periprosthetic tissue or fluid; and (6) more than 5 neutrophils per high-power field in 5 high-power fields observed.
In this review article, we discuss recently developed novel synovial biomarkers and polymerase chain reaction (PCR) technologies that may help increase the sensitivity and specificity of diagnostic guidelines for PJI.
Methods
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), we performed a systematic review of specific synovial fluid markers and PCR used in PJI diagnosis. In May 2016, we searched the PubMed database for these criteria: ((((((PCR[Text Word]) OR IL-6[Text Word]) OR leukocyte esterase[Text Word]) OR alpha defensin[Text Word]) AND ((“infection/diagnosis”[MeSH Terms] OR “infection/surgery”[MeSH Terms])))) AND (prosthetic joint infection[MeSH Terms] OR periprosthetic joint infection[MeSH Terms]).
We included patients who had undergone total hip, knee, or shoulder arthroplasty (THA, TKA, TSA). Index tests were PCR and the synovial fluid markers α-defensin (AD), interleukin 6 (IL-6), and leukocyte esterase (LE). Reference tests included joint fluid/serum analysis or tissue analysis (ESR/CRP level, cell count, culture, frozen section), which defined the MSIS criteria for PJI. Primary outcomes of interest were sensitivity and specificity, and secondary outcomes of interest included positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (–LR). Randomized controlled trials and controlled cohort studies in humans published within the past 10 years were included.
Results
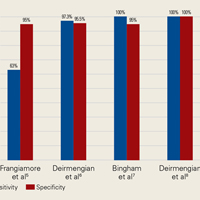
Our full-text review yielded 15 papers that met our study inclusion criteria (Figure 1).
α-Defensin
One of the novel synovial biomarkers that has shown significant promise in diagnosing PJIs, even with difficult-to-detect organisms, is AD.
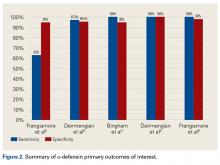
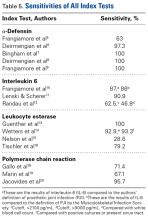
AD has shown even more impressive results as a biomarker for PJI in the hip and knee, where infection with low virulence organism is less common. In 2014, Deirmengian and colleagues6 conducted a prospective clinical study of 149 patients who underwent revision THA or TKA for aseptic loosening (n = 112) or PJI (n = 37) as defined by MSIS criteria. Aseptic loosening was diagnosed when there was no identifiable reason for pain, and MSIS criteria were not met. Synovial fluid aspirates were collected before or during surgery. AD correctly identified 143 of the 149 patients with confirmed infection with sensitivity of 97.3% (95% confidence interval [CI], 85.8%-99.6%) and specificity of 95.5% (95% CI, 89.9%-98.5%). Similarly, Bingham and colleagues7 conducted a retrospective clinical study of 61 assays done on 57 patients who underwent revision arthroplasty for PJI as defined by MSIS criteria. Synovial fluid aspirates were collected before or during surgery. AD correctly identified all 19 PJIs with sensitivity of 100% (95% CI, 79%-100%) and specificity of 95% (95% CI, 83%-99%). Sensitivity and specificity of the AD assay more accurately predicted infection than synovial cell count or serum ESR/CRP level did.
These results are supported by another prospective study by Deirmengian and colleagues8 differentiating aseptic failures and PJIs in THA or TKA. The sensitivity and specificity of AD in diagnosing PJI were 100% (95% CI, 85.05%-100%).
In a prospective study of 102 patients who underwent revision THA or TKA secondary to aseptic loosening or PJI, Frangiamore and colleagues9 also demonstrated the value of AD as a diagnostic for PJI in primary and revision hip and knee arthroplasty.
Table 1 and Figure 2 provide a concise review of the findings of each study.
Interleukin 6
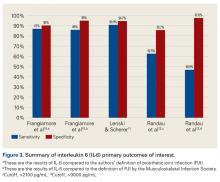
Another synovial fluid biomarker that has shown promise in PJI diagnosis is IL-6. In 2015, Frangiamore and colleagues10 conducted a prospective clinical study of 32 patients who underwent revision TSA. Synovial fluid aspiration was obtained before or during surgery. MSIS criteria were used to establish the diagnosis of PJI. IL-6 had sensitivity of 87% and specificity of 90%, with +LR of 8.45 and –LR of 0.15 in predicting PJI. Synovial fluid IL-6 had strong associations with frozen section histology and growth of P acnes. Frangiamore and colleagues10 recommended an ideal IL-6 cutoff of 359.1 pg/mL and reported that, though not as accurate as AD, synovial fluid IL-6 levels can help predict positive cultures in patients who undergo revision TSA.
Lenski and Scherer11 conducted another retrospective clinical study of the diagnostic value of IL-6 in PJI.
Randau and colleagues12 conducted a prospective clinical study of 120 patients who presented with painful THA or TKA and underwent revision for PJI, aseptic failure, or aseptic revision without signs of infection or loosening. Synovial fluid aspirate was collected before or during surgery.
Table 2 and Figure 3 provide a concise review of the findings of each study.
Leukocyte Esterase
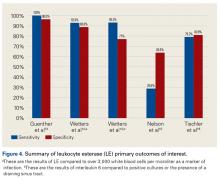
LE strips are an inexpensive screening tool for PJI, according to some studies. In a prospective clinical study of 364 endoprosthetic joint (hip, knee, shoulder) interventions, Guenther and colleagues13 collected synovial fluid before surgery. Samples were tested with graded LE strips using PJI criteria set by the authors. Results were correlated with preoperative synovial fluid aspirations, serum CRP level, serum WBC count, and intraoperative histopathologic and microbiological findings. Whereas 293 (93.31%) of the 314 aseptic cases had negative test strip readings, 100% of the 50 infected cases were positive. LE had sensitivity of 100%, specificity of 96.5%, PPV of 82%, and NPV of 100%.
Wetters et al14 performed a prospective clinical study on 223 patients who underwent TKAs and THAs for suspected PJI based on having criteria defined by the authors of the study. Synovial fluid samples were collected either preoperatively or intraoperatively.
Other authors have reported different findings that LE is an unreliable marker in PJI diagnosis. In one prospective clinical study of 85 patients who underwent primary or revision TSA, synovial fluid was collected during surgery.15 According to MSIS criteria, only 5 positive LE results predicted PJI among 21 primary and revision patients with positive cultures. Of the 7 revision patients who met the MSIS criteria for PJI, only 2 had a positive LE test. LE had sensitivity of 28.6%, specificity of 63.6%, PPV of 28.6%, and NPV of 87.5%. Six of the 7 revision patients grew P acnes. These results showed that LE was unreliable in detecting shoulder PJI.15
In another prospective clinical study, Tischler and colleagues16 enrolled 189 patients who underwent revision TKA or THA for aseptic failure or PJI as defined by the MSIS criteria. Synovial fluid was collected intraoperatively.
Table 3 and Figure 4 provide a concise review of the findings of each study.
Polymerase Chain Reaction
Studies have found that PCR analysis of synovial fluid is effective in detecting bacteria on the surface of implants removed during revision arthroplasties. Comparison of the 16S rRNA gene sequences of bacterial genomes showed a diverse range of bacterial species within biofilms on the surface of clinical and subclinical infections.17 These findings, along with those of other studies, suggest that PCR analysis of synovial fluid is useful in diagnosing PJI and identifying organisms and their sensitivities to antibiotics.
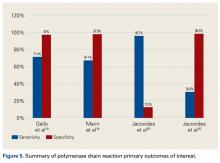
Gallo and colleagues18 performed a prospective clinical study on 115 patients who underwent revision TKAs or THAs. Synovial fluid was collected intraoperatively. PCR assays targeting the 16S rDNA were carried out on 101 patients. PJIs were classified based on criteria of the authors of this study, of which there were 42. The sensitivity, specificity, PPV, NPV, +LR, and -LR for PCR were 71.4% (95% CI, 61.5%-75.5%), 97% (95% CI, 91.7%-99.1%), 92.6% (95% CI, 79.8%-97.9%), 86.5% (95% CI, 81.8%-88.4%), 23.6 (95% CI, 5.9%-93.8%), and 0.29 (95% CI, 0.17%-0.49%), respectively. Of note the most common organism detected in 42 PJIs was coagulase-negative Staphylococcus.
Marin and colleagues19 conducted a prospective study of 122 patients who underwent arthroplasty for suspected infection or aseptic loosening as defined by the authors’ clinicohistopathologic criteria. Synovial fluid and biopsy specimens were collected during surgery, and 40 patients met the infection criteria. The authors concluded that 16S PCR is more specific and has better PPV than culture does as one positive 16S PCR resulted in a specificity and PPV of PJI of 96.3% and 91.7%, respectively. However, they noted that culture was more sensitive in diagnosing PJI.
Jacovides and colleagues20 conducted a prospective study on 82 patients undergoing primary TKA, revision TKA, and revision THA.
The low PCR sensitivities reported in the literature were explained in a review by Hartley and Harris.21 They wrote that BR 16S rDNA and sequencing of PJI samples inherently have low sensitivity because of the contamination that can occur from the PCR reagents themselves or from sample mishandling. Techniques that address contaminant (extraneous DNA) removal, such as ultraviolet irradiation and DNase treatment, reduce Taq DNA polymerase activity, which reduces PCR sensitivity.
Table 4 and Figure 5 provide a concise review of the findings of each study.
Discussion
Although there is no gold standard for the diagnosis of PJIs, several clinical and laboratory criteria guidelines are currently used to help clinicians diagnose infections of prosthetic joints. However, despite standardization of diagnostic criteria, PJI continue to be a diagnostic challenge.
AD is a highly sensitive and specific synovial fluid biomarker in detecting common PJIs.
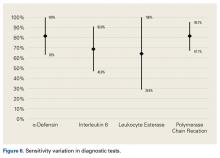
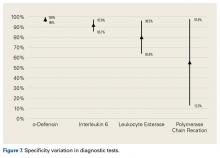
In summary, 5 AD studies5-9 had sensitivity ranging from 63% to 100% and specificity ranging from 95% to 100%; 3 IL-6 studies10-12 had sensitivity ranging from 46.8% to 90.9% and specificity ranging from 85.7% to 97.6%; 4 LE studies13-16 had sensitivity ranging from 28.6% to 100% and specificity ranging from 63.6% to 96.5%; and 3 PCR studies18-20 had sensitivity ranging from 67.1% to 95.7% and specificity ranging from 12.3% to 97.8%. Sensitivity and specificity were consistently higher for AD than for IL-6, LE, and PCR, though there was significant overlap, heterogeneity, and variation across all the included studies.
Although the overall incidence of PJI is low, infected revisions remain a substantial financial burden to hospitals, as annual costs of infected revisions is estimated to exceed $1.62 billion by 2020.25 The usefulness of novel biomarkers and PCR in diagnosing PJI can be found in their ability to diagnose infections and facilitate appropriate early treatment. Several of these tests are readily available commercially and have the potential to be cost-effective diagnostic tools. The price to perform an AD test from Synovasure TM (Zimmer Biomet) ranges from $93 to $143. LE also provides an economic option for diagnosing PJI, as LE strips are commercially available for the cost of about 25 cents. PCR has also become an economic option, as costs can average $15.50 per sample extraction or PCR assay and $42.50 per amplicon sequence as reported in a study by Vandercam and colleagues.26 Future studies are needed to determine a diagnostic algorithm which incorporates these novel synovial markers to improve diagnostic accuracy of PJI in the most cost effective manner.
The current literature supports that AD can potentially be used to screen for PJI. Our findings suggest novel synovial fluid biomarkers may become of significant diagnostic use when combined with current laboratory and clinical diagnostic criteria. We recommend use of AD in cases in which pain, stiffness, and poor TJA outcome cannot be explained by errors in surgical technique, and infection is suspected despite MSIS criteria not being met.
The studies reviewed in this manuscript were limited in that none presented level I evidence (12 had level II evidence, and 3 had level III evidence), and there was significant heterogeneity (some studies used their own diagnostic standard, and others used the MSIS criteria). Larger scale prospective studies comparing serum ESR/CRP level and synovial fluid analysis to novel synovial markers are needed.
Am J Orthop. 2017;46(4):190-198. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014;29(9):1774-1778.
4. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
5. Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015;24(7):1021-1027.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
7. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
8. Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473(1):198-203.
9. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
10. Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97(1):63-70.
11. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
12. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
13. Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38(11):2385-2390.
14. Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 suppl):8-11.
15. Nelson GN, Paxton ES, Narzikul A, Williams G, Lazarus MD, Abboud JA. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg. 2015;24(9):1421-1426.
16. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
17. Dempsey KE, Riggio MP, Lennon A, et al. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther. 2007;9(3):R46.
18. Gallo J, Kolar M, Dendis M, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97-104.
19. Marin M, Garcia-Lechuz JM, Alonso P, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50(3):583-589.
20. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
21. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
22. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
23. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
24. Hunt RW, Bond MJ, Pater GD. Psychological responses to cancer: a case for cancer support groups. Community Health Stud. 1990;14(1):35-38.
25. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
26. Vandercam B, Jeumont S, Cornu O, et al. Amplification-based DNA analysis in the diagnosis of prosthetic joint infection. J Mol Diagn. 2008;10(6):537-543.
Take-Home Points
- Novel synovial markers and PCR have the potential to improve the detection of PJIs.
- 10Difficult-to-detect infections of prosthetic joints pose a diagnostic problem to surgeons and can lead to suboptimal outcomes.
- AD is a highly sensitive and specific synovial fluid marker for detecting PJIs.
- AD has shown promising results in detecting low virulence organisms.
- Studies are needed to determine how to best incorporate novel synovial markers and PCR to current diagnostic criteria in order to improve diagnostic accuracy.
Approximately 7 million Americans are living with a hip or knee replacement.1 According to projections, primary hip arthroplasties will increase by 174% and knee arthroplasties by 673% by 2030. Revision arthroplasties are projected to increase by 137% for hips and 601% for knees during the same time period.2 Infection and aseptic loosening are the most common causes of implant failure.3 The literature shows that infection is the most common cause of failure within 2 years after surgery and that aseptic loosening is the most common cause for late revision.3
Recent studies suggest that prosthetic joint infection (PJI) may be underreported because of difficulty making a diagnosis and that cases of aseptic loosening may in fact be attributable to infections with low-virulence organisms.2,3 These findings have led to new efforts to develop uniform criteria for diagnosing PJIs. In 2011, the Musculoskeletal Infection Society (MSIS) offered a new definition for PJI diagnosis, based on clinical and laboratory criteria, to increase the accuracy of PJI diagnosis.4 The MSIS committee acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-virulence organisms, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. Reports of PJI cases misdiagnosed as aseptic loosening suggest that current screening and diagnostic tools are not sensitive enough to detect all infections and that PJI is likely underdiagnosed.
According to MSIS criteria, the diagnosis of PJI can be made when there is a sinus tract communicating with the prosthesis, when a pathogen is isolated by culture from 2 or more separate tissue or fluid samples obtained from the affected prosthetic joint, or when 4 of 6 criteria are met. The 6 criteria are (1) elevated serum erythrocyte sedimentation rate (ESR) (>30 mm/hour) and elevated C-reactive protein (CRP) level (>10 mg/L); (2) elevated synovial white blood cell (WBC) count (1100-4000 cells/μL); (3) elevated synovial polymorphonuclear leukocytes (>64%); (4) purulence in affected joint; (5) isolation of a microorganism in a culture of periprosthetic tissue or fluid; and (6) more than 5 neutrophils per high-power field in 5 high-power fields observed.
In this review article, we discuss recently developed novel synovial biomarkers and polymerase chain reaction (PCR) technologies that may help increase the sensitivity and specificity of diagnostic guidelines for PJI.
Methods
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), we performed a systematic review of specific synovial fluid markers and PCR used in PJI diagnosis. In May 2016, we searched the PubMed database for these criteria: ((((((PCR[Text Word]) OR IL-6[Text Word]) OR leukocyte esterase[Text Word]) OR alpha defensin[Text Word]) AND ((“infection/diagnosis”[MeSH Terms] OR “infection/surgery”[MeSH Terms])))) AND (prosthetic joint infection[MeSH Terms] OR periprosthetic joint infection[MeSH Terms]).
We included patients who had undergone total hip, knee, or shoulder arthroplasty (THA, TKA, TSA). Index tests were PCR and the synovial fluid markers α-defensin (AD), interleukin 6 (IL-6), and leukocyte esterase (LE). Reference tests included joint fluid/serum analysis or tissue analysis (ESR/CRP level, cell count, culture, frozen section), which defined the MSIS criteria for PJI. Primary outcomes of interest were sensitivity and specificity, and secondary outcomes of interest included positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (–LR). Randomized controlled trials and controlled cohort studies in humans published within the past 10 years were included.
Results
Our full-text review yielded 15 papers that met our study inclusion criteria (Figure 1).
α-Defensin
One of the novel synovial biomarkers that has shown significant promise in diagnosing PJIs, even with difficult-to-detect organisms, is AD.
AD has shown even more impressive results as a biomarker for PJI in the hip and knee, where infection with low virulence organism is less common. In 2014, Deirmengian and colleagues6 conducted a prospective clinical study of 149 patients who underwent revision THA or TKA for aseptic loosening (n = 112) or PJI (n = 37) as defined by MSIS criteria. Aseptic loosening was diagnosed when there was no identifiable reason for pain, and MSIS criteria were not met. Synovial fluid aspirates were collected before or during surgery. AD correctly identified 143 of the 149 patients with confirmed infection with sensitivity of 97.3% (95% confidence interval [CI], 85.8%-99.6%) and specificity of 95.5% (95% CI, 89.9%-98.5%). Similarly, Bingham and colleagues7 conducted a retrospective clinical study of 61 assays done on 57 patients who underwent revision arthroplasty for PJI as defined by MSIS criteria. Synovial fluid aspirates were collected before or during surgery. AD correctly identified all 19 PJIs with sensitivity of 100% (95% CI, 79%-100%) and specificity of 95% (95% CI, 83%-99%). Sensitivity and specificity of the AD assay more accurately predicted infection than synovial cell count or serum ESR/CRP level did.
These results are supported by another prospective study by Deirmengian and colleagues8 differentiating aseptic failures and PJIs in THA or TKA. The sensitivity and specificity of AD in diagnosing PJI were 100% (95% CI, 85.05%-100%).
In a prospective study of 102 patients who underwent revision THA or TKA secondary to aseptic loosening or PJI, Frangiamore and colleagues9 also demonstrated the value of AD as a diagnostic for PJI in primary and revision hip and knee arthroplasty.
Table 1 and Figure 2 provide a concise review of the findings of each study.
Interleukin 6
Another synovial fluid biomarker that has shown promise in PJI diagnosis is IL-6. In 2015, Frangiamore and colleagues10 conducted a prospective clinical study of 32 patients who underwent revision TSA. Synovial fluid aspiration was obtained before or during surgery. MSIS criteria were used to establish the diagnosis of PJI. IL-6 had sensitivity of 87% and specificity of 90%, with +LR of 8.45 and –LR of 0.15 in predicting PJI. Synovial fluid IL-6 had strong associations with frozen section histology and growth of P acnes. Frangiamore and colleagues10 recommended an ideal IL-6 cutoff of 359.1 pg/mL and reported that, though not as accurate as AD, synovial fluid IL-6 levels can help predict positive cultures in patients who undergo revision TSA.
Lenski and Scherer11 conducted another retrospective clinical study of the diagnostic value of IL-6 in PJI.
Randau and colleagues12 conducted a prospective clinical study of 120 patients who presented with painful THA or TKA and underwent revision for PJI, aseptic failure, or aseptic revision without signs of infection or loosening. Synovial fluid aspirate was collected before or during surgery.
Table 2 and Figure 3 provide a concise review of the findings of each study.
Leukocyte Esterase
LE strips are an inexpensive screening tool for PJI, according to some studies. In a prospective clinical study of 364 endoprosthetic joint (hip, knee, shoulder) interventions, Guenther and colleagues13 collected synovial fluid before surgery. Samples were tested with graded LE strips using PJI criteria set by the authors. Results were correlated with preoperative synovial fluid aspirations, serum CRP level, serum WBC count, and intraoperative histopathologic and microbiological findings. Whereas 293 (93.31%) of the 314 aseptic cases had negative test strip readings, 100% of the 50 infected cases were positive. LE had sensitivity of 100%, specificity of 96.5%, PPV of 82%, and NPV of 100%.
Wetters et al14 performed a prospective clinical study on 223 patients who underwent TKAs and THAs for suspected PJI based on having criteria defined by the authors of the study. Synovial fluid samples were collected either preoperatively or intraoperatively.
Other authors have reported different findings that LE is an unreliable marker in PJI diagnosis. In one prospective clinical study of 85 patients who underwent primary or revision TSA, synovial fluid was collected during surgery.15 According to MSIS criteria, only 5 positive LE results predicted PJI among 21 primary and revision patients with positive cultures. Of the 7 revision patients who met the MSIS criteria for PJI, only 2 had a positive LE test. LE had sensitivity of 28.6%, specificity of 63.6%, PPV of 28.6%, and NPV of 87.5%. Six of the 7 revision patients grew P acnes. These results showed that LE was unreliable in detecting shoulder PJI.15
In another prospective clinical study, Tischler and colleagues16 enrolled 189 patients who underwent revision TKA or THA for aseptic failure or PJI as defined by the MSIS criteria. Synovial fluid was collected intraoperatively.
Table 3 and Figure 4 provide a concise review of the findings of each study.
Polymerase Chain Reaction
Studies have found that PCR analysis of synovial fluid is effective in detecting bacteria on the surface of implants removed during revision arthroplasties. Comparison of the 16S rRNA gene sequences of bacterial genomes showed a diverse range of bacterial species within biofilms on the surface of clinical and subclinical infections.17 These findings, along with those of other studies, suggest that PCR analysis of synovial fluid is useful in diagnosing PJI and identifying organisms and their sensitivities to antibiotics.
Gallo and colleagues18 performed a prospective clinical study on 115 patients who underwent revision TKAs or THAs. Synovial fluid was collected intraoperatively. PCR assays targeting the 16S rDNA were carried out on 101 patients. PJIs were classified based on criteria of the authors of this study, of which there were 42. The sensitivity, specificity, PPV, NPV, +LR, and -LR for PCR were 71.4% (95% CI, 61.5%-75.5%), 97% (95% CI, 91.7%-99.1%), 92.6% (95% CI, 79.8%-97.9%), 86.5% (95% CI, 81.8%-88.4%), 23.6 (95% CI, 5.9%-93.8%), and 0.29 (95% CI, 0.17%-0.49%), respectively. Of note the most common organism detected in 42 PJIs was coagulase-negative Staphylococcus.
Marin and colleagues19 conducted a prospective study of 122 patients who underwent arthroplasty for suspected infection or aseptic loosening as defined by the authors’ clinicohistopathologic criteria. Synovial fluid and biopsy specimens were collected during surgery, and 40 patients met the infection criteria. The authors concluded that 16S PCR is more specific and has better PPV than culture does as one positive 16S PCR resulted in a specificity and PPV of PJI of 96.3% and 91.7%, respectively. However, they noted that culture was more sensitive in diagnosing PJI.
Jacovides and colleagues20 conducted a prospective study on 82 patients undergoing primary TKA, revision TKA, and revision THA.
The low PCR sensitivities reported in the literature were explained in a review by Hartley and Harris.21 They wrote that BR 16S rDNA and sequencing of PJI samples inherently have low sensitivity because of the contamination that can occur from the PCR reagents themselves or from sample mishandling. Techniques that address contaminant (extraneous DNA) removal, such as ultraviolet irradiation and DNase treatment, reduce Taq DNA polymerase activity, which reduces PCR sensitivity.
Table 4 and Figure 5 provide a concise review of the findings of each study.
Discussion
Although there is no gold standard for the diagnosis of PJIs, several clinical and laboratory criteria guidelines are currently used to help clinicians diagnose infections of prosthetic joints. However, despite standardization of diagnostic criteria, PJI continue to be a diagnostic challenge.
AD is a highly sensitive and specific synovial fluid biomarker in detecting common PJIs.
In summary, 5 AD studies5-9 had sensitivity ranging from 63% to 100% and specificity ranging from 95% to 100%; 3 IL-6 studies10-12 had sensitivity ranging from 46.8% to 90.9% and specificity ranging from 85.7% to 97.6%; 4 LE studies13-16 had sensitivity ranging from 28.6% to 100% and specificity ranging from 63.6% to 96.5%; and 3 PCR studies18-20 had sensitivity ranging from 67.1% to 95.7% and specificity ranging from 12.3% to 97.8%. Sensitivity and specificity were consistently higher for AD than for IL-6, LE, and PCR, though there was significant overlap, heterogeneity, and variation across all the included studies.
Although the overall incidence of PJI is low, infected revisions remain a substantial financial burden to hospitals, as annual costs of infected revisions is estimated to exceed $1.62 billion by 2020.25 The usefulness of novel biomarkers and PCR in diagnosing PJI can be found in their ability to diagnose infections and facilitate appropriate early treatment. Several of these tests are readily available commercially and have the potential to be cost-effective diagnostic tools. The price to perform an AD test from Synovasure TM (Zimmer Biomet) ranges from $93 to $143. LE also provides an economic option for diagnosing PJI, as LE strips are commercially available for the cost of about 25 cents. PCR has also become an economic option, as costs can average $15.50 per sample extraction or PCR assay and $42.50 per amplicon sequence as reported in a study by Vandercam and colleagues.26 Future studies are needed to determine a diagnostic algorithm which incorporates these novel synovial markers to improve diagnostic accuracy of PJI in the most cost effective manner.
The current literature supports that AD can potentially be used to screen for PJI. Our findings suggest novel synovial fluid biomarkers may become of significant diagnostic use when combined with current laboratory and clinical diagnostic criteria. We recommend use of AD in cases in which pain, stiffness, and poor TJA outcome cannot be explained by errors in surgical technique, and infection is suspected despite MSIS criteria not being met.
The studies reviewed in this manuscript were limited in that none presented level I evidence (12 had level II evidence, and 3 had level III evidence), and there was significant heterogeneity (some studies used their own diagnostic standard, and others used the MSIS criteria). Larger scale prospective studies comparing serum ESR/CRP level and synovial fluid analysis to novel synovial markers are needed.
Am J Orthop. 2017;46(4):190-198. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Novel synovial markers and PCR have the potential to improve the detection of PJIs.
- 10Difficult-to-detect infections of prosthetic joints pose a diagnostic problem to surgeons and can lead to suboptimal outcomes.
- AD is a highly sensitive and specific synovial fluid marker for detecting PJIs.
- AD has shown promising results in detecting low virulence organisms.
- Studies are needed to determine how to best incorporate novel synovial markers and PCR to current diagnostic criteria in order to improve diagnostic accuracy.
Approximately 7 million Americans are living with a hip or knee replacement.1 According to projections, primary hip arthroplasties will increase by 174% and knee arthroplasties by 673% by 2030. Revision arthroplasties are projected to increase by 137% for hips and 601% for knees during the same time period.2 Infection and aseptic loosening are the most common causes of implant failure.3 The literature shows that infection is the most common cause of failure within 2 years after surgery and that aseptic loosening is the most common cause for late revision.3
Recent studies suggest that prosthetic joint infection (PJI) may be underreported because of difficulty making a diagnosis and that cases of aseptic loosening may in fact be attributable to infections with low-virulence organisms.2,3 These findings have led to new efforts to develop uniform criteria for diagnosing PJIs. In 2011, the Musculoskeletal Infection Society (MSIS) offered a new definition for PJI diagnosis, based on clinical and laboratory criteria, to increase the accuracy of PJI diagnosis.4 The MSIS committee acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-virulence organisms, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. Reports of PJI cases misdiagnosed as aseptic loosening suggest that current screening and diagnostic tools are not sensitive enough to detect all infections and that PJI is likely underdiagnosed.
According to MSIS criteria, the diagnosis of PJI can be made when there is a sinus tract communicating with the prosthesis, when a pathogen is isolated by culture from 2 or more separate tissue or fluid samples obtained from the affected prosthetic joint, or when 4 of 6 criteria are met. The 6 criteria are (1) elevated serum erythrocyte sedimentation rate (ESR) (>30 mm/hour) and elevated C-reactive protein (CRP) level (>10 mg/L); (2) elevated synovial white blood cell (WBC) count (1100-4000 cells/μL); (3) elevated synovial polymorphonuclear leukocytes (>64%); (4) purulence in affected joint; (5) isolation of a microorganism in a culture of periprosthetic tissue or fluid; and (6) more than 5 neutrophils per high-power field in 5 high-power fields observed.
In this review article, we discuss recently developed novel synovial biomarkers and polymerase chain reaction (PCR) technologies that may help increase the sensitivity and specificity of diagnostic guidelines for PJI.
Methods
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), we performed a systematic review of specific synovial fluid markers and PCR used in PJI diagnosis. In May 2016, we searched the PubMed database for these criteria: ((((((PCR[Text Word]) OR IL-6[Text Word]) OR leukocyte esterase[Text Word]) OR alpha defensin[Text Word]) AND ((“infection/diagnosis”[MeSH Terms] OR “infection/surgery”[MeSH Terms])))) AND (prosthetic joint infection[MeSH Terms] OR periprosthetic joint infection[MeSH Terms]).
We included patients who had undergone total hip, knee, or shoulder arthroplasty (THA, TKA, TSA). Index tests were PCR and the synovial fluid markers α-defensin (AD), interleukin 6 (IL-6), and leukocyte esterase (LE). Reference tests included joint fluid/serum analysis or tissue analysis (ESR/CRP level, cell count, culture, frozen section), which defined the MSIS criteria for PJI. Primary outcomes of interest were sensitivity and specificity, and secondary outcomes of interest included positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (–LR). Randomized controlled trials and controlled cohort studies in humans published within the past 10 years were included.
Results
Our full-text review yielded 15 papers that met our study inclusion criteria (Figure 1).
α-Defensin
One of the novel synovial biomarkers that has shown significant promise in diagnosing PJIs, even with difficult-to-detect organisms, is AD.
AD has shown even more impressive results as a biomarker for PJI in the hip and knee, where infection with low virulence organism is less common. In 2014, Deirmengian and colleagues6 conducted a prospective clinical study of 149 patients who underwent revision THA or TKA for aseptic loosening (n = 112) or PJI (n = 37) as defined by MSIS criteria. Aseptic loosening was diagnosed when there was no identifiable reason for pain, and MSIS criteria were not met. Synovial fluid aspirates were collected before or during surgery. AD correctly identified 143 of the 149 patients with confirmed infection with sensitivity of 97.3% (95% confidence interval [CI], 85.8%-99.6%) and specificity of 95.5% (95% CI, 89.9%-98.5%). Similarly, Bingham and colleagues7 conducted a retrospective clinical study of 61 assays done on 57 patients who underwent revision arthroplasty for PJI as defined by MSIS criteria. Synovial fluid aspirates were collected before or during surgery. AD correctly identified all 19 PJIs with sensitivity of 100% (95% CI, 79%-100%) and specificity of 95% (95% CI, 83%-99%). Sensitivity and specificity of the AD assay more accurately predicted infection than synovial cell count or serum ESR/CRP level did.
These results are supported by another prospective study by Deirmengian and colleagues8 differentiating aseptic failures and PJIs in THA or TKA. The sensitivity and specificity of AD in diagnosing PJI were 100% (95% CI, 85.05%-100%).
In a prospective study of 102 patients who underwent revision THA or TKA secondary to aseptic loosening or PJI, Frangiamore and colleagues9 also demonstrated the value of AD as a diagnostic for PJI in primary and revision hip and knee arthroplasty.
Table 1 and Figure 2 provide a concise review of the findings of each study.
Interleukin 6
Another synovial fluid biomarker that has shown promise in PJI diagnosis is IL-6. In 2015, Frangiamore and colleagues10 conducted a prospective clinical study of 32 patients who underwent revision TSA. Synovial fluid aspiration was obtained before or during surgery. MSIS criteria were used to establish the diagnosis of PJI. IL-6 had sensitivity of 87% and specificity of 90%, with +LR of 8.45 and –LR of 0.15 in predicting PJI. Synovial fluid IL-6 had strong associations with frozen section histology and growth of P acnes. Frangiamore and colleagues10 recommended an ideal IL-6 cutoff of 359.1 pg/mL and reported that, though not as accurate as AD, synovial fluid IL-6 levels can help predict positive cultures in patients who undergo revision TSA.
Lenski and Scherer11 conducted another retrospective clinical study of the diagnostic value of IL-6 in PJI.
Randau and colleagues12 conducted a prospective clinical study of 120 patients who presented with painful THA or TKA and underwent revision for PJI, aseptic failure, or aseptic revision without signs of infection or loosening. Synovial fluid aspirate was collected before or during surgery.
Table 2 and Figure 3 provide a concise review of the findings of each study.
Leukocyte Esterase
LE strips are an inexpensive screening tool for PJI, according to some studies. In a prospective clinical study of 364 endoprosthetic joint (hip, knee, shoulder) interventions, Guenther and colleagues13 collected synovial fluid before surgery. Samples were tested with graded LE strips using PJI criteria set by the authors. Results were correlated with preoperative synovial fluid aspirations, serum CRP level, serum WBC count, and intraoperative histopathologic and microbiological findings. Whereas 293 (93.31%) of the 314 aseptic cases had negative test strip readings, 100% of the 50 infected cases were positive. LE had sensitivity of 100%, specificity of 96.5%, PPV of 82%, and NPV of 100%.
Wetters et al14 performed a prospective clinical study on 223 patients who underwent TKAs and THAs for suspected PJI based on having criteria defined by the authors of the study. Synovial fluid samples were collected either preoperatively or intraoperatively.
Other authors have reported different findings that LE is an unreliable marker in PJI diagnosis. In one prospective clinical study of 85 patients who underwent primary or revision TSA, synovial fluid was collected during surgery.15 According to MSIS criteria, only 5 positive LE results predicted PJI among 21 primary and revision patients with positive cultures. Of the 7 revision patients who met the MSIS criteria for PJI, only 2 had a positive LE test. LE had sensitivity of 28.6%, specificity of 63.6%, PPV of 28.6%, and NPV of 87.5%. Six of the 7 revision patients grew P acnes. These results showed that LE was unreliable in detecting shoulder PJI.15
In another prospective clinical study, Tischler and colleagues16 enrolled 189 patients who underwent revision TKA or THA for aseptic failure or PJI as defined by the MSIS criteria. Synovial fluid was collected intraoperatively.
Table 3 and Figure 4 provide a concise review of the findings of each study.
Polymerase Chain Reaction
Studies have found that PCR analysis of synovial fluid is effective in detecting bacteria on the surface of implants removed during revision arthroplasties. Comparison of the 16S rRNA gene sequences of bacterial genomes showed a diverse range of bacterial species within biofilms on the surface of clinical and subclinical infections.17 These findings, along with those of other studies, suggest that PCR analysis of synovial fluid is useful in diagnosing PJI and identifying organisms and their sensitivities to antibiotics.
Gallo and colleagues18 performed a prospective clinical study on 115 patients who underwent revision TKAs or THAs. Synovial fluid was collected intraoperatively. PCR assays targeting the 16S rDNA were carried out on 101 patients. PJIs were classified based on criteria of the authors of this study, of which there were 42. The sensitivity, specificity, PPV, NPV, +LR, and -LR for PCR were 71.4% (95% CI, 61.5%-75.5%), 97% (95% CI, 91.7%-99.1%), 92.6% (95% CI, 79.8%-97.9%), 86.5% (95% CI, 81.8%-88.4%), 23.6 (95% CI, 5.9%-93.8%), and 0.29 (95% CI, 0.17%-0.49%), respectively. Of note the most common organism detected in 42 PJIs was coagulase-negative Staphylococcus.
Marin and colleagues19 conducted a prospective study of 122 patients who underwent arthroplasty for suspected infection or aseptic loosening as defined by the authors’ clinicohistopathologic criteria. Synovial fluid and biopsy specimens were collected during surgery, and 40 patients met the infection criteria. The authors concluded that 16S PCR is more specific and has better PPV than culture does as one positive 16S PCR resulted in a specificity and PPV of PJI of 96.3% and 91.7%, respectively. However, they noted that culture was more sensitive in diagnosing PJI.
Jacovides and colleagues20 conducted a prospective study on 82 patients undergoing primary TKA, revision TKA, and revision THA.
The low PCR sensitivities reported in the literature were explained in a review by Hartley and Harris.21 They wrote that BR 16S rDNA and sequencing of PJI samples inherently have low sensitivity because of the contamination that can occur from the PCR reagents themselves or from sample mishandling. Techniques that address contaminant (extraneous DNA) removal, such as ultraviolet irradiation and DNase treatment, reduce Taq DNA polymerase activity, which reduces PCR sensitivity.
Table 4 and Figure 5 provide a concise review of the findings of each study.
Discussion
Although there is no gold standard for the diagnosis of PJIs, several clinical and laboratory criteria guidelines are currently used to help clinicians diagnose infections of prosthetic joints. However, despite standardization of diagnostic criteria, PJI continue to be a diagnostic challenge.
AD is a highly sensitive and specific synovial fluid biomarker in detecting common PJIs.
In summary, 5 AD studies5-9 had sensitivity ranging from 63% to 100% and specificity ranging from 95% to 100%; 3 IL-6 studies10-12 had sensitivity ranging from 46.8% to 90.9% and specificity ranging from 85.7% to 97.6%; 4 LE studies13-16 had sensitivity ranging from 28.6% to 100% and specificity ranging from 63.6% to 96.5%; and 3 PCR studies18-20 had sensitivity ranging from 67.1% to 95.7% and specificity ranging from 12.3% to 97.8%. Sensitivity and specificity were consistently higher for AD than for IL-6, LE, and PCR, though there was significant overlap, heterogeneity, and variation across all the included studies.
Although the overall incidence of PJI is low, infected revisions remain a substantial financial burden to hospitals, as annual costs of infected revisions is estimated to exceed $1.62 billion by 2020.25 The usefulness of novel biomarkers and PCR in diagnosing PJI can be found in their ability to diagnose infections and facilitate appropriate early treatment. Several of these tests are readily available commercially and have the potential to be cost-effective diagnostic tools. The price to perform an AD test from Synovasure TM (Zimmer Biomet) ranges from $93 to $143. LE also provides an economic option for diagnosing PJI, as LE strips are commercially available for the cost of about 25 cents. PCR has also become an economic option, as costs can average $15.50 per sample extraction or PCR assay and $42.50 per amplicon sequence as reported in a study by Vandercam and colleagues.26 Future studies are needed to determine a diagnostic algorithm which incorporates these novel synovial markers to improve diagnostic accuracy of PJI in the most cost effective manner.
The current literature supports that AD can potentially be used to screen for PJI. Our findings suggest novel synovial fluid biomarkers may become of significant diagnostic use when combined with current laboratory and clinical diagnostic criteria. We recommend use of AD in cases in which pain, stiffness, and poor TJA outcome cannot be explained by errors in surgical technique, and infection is suspected despite MSIS criteria not being met.
The studies reviewed in this manuscript were limited in that none presented level I evidence (12 had level II evidence, and 3 had level III evidence), and there was significant heterogeneity (some studies used their own diagnostic standard, and others used the MSIS criteria). Larger scale prospective studies comparing serum ESR/CRP level and synovial fluid analysis to novel synovial markers are needed.
Am J Orthop. 2017;46(4):190-198. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014;29(9):1774-1778.
4. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
5. Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015;24(7):1021-1027.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
7. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
8. Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473(1):198-203.
9. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
10. Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97(1):63-70.
11. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
12. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
13. Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38(11):2385-2390.
14. Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 suppl):8-11.
15. Nelson GN, Paxton ES, Narzikul A, Williams G, Lazarus MD, Abboud JA. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg. 2015;24(9):1421-1426.
16. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
17. Dempsey KE, Riggio MP, Lennon A, et al. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther. 2007;9(3):R46.
18. Gallo J, Kolar M, Dendis M, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97-104.
19. Marin M, Garcia-Lechuz JM, Alonso P, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50(3):583-589.
20. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
21. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
22. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
23. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
24. Hunt RW, Bond MJ, Pater GD. Psychological responses to cancer: a case for cancer support groups. Community Health Stud. 1990;14(1):35-38.
25. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
26. Vandercam B, Jeumont S, Cornu O, et al. Amplification-based DNA analysis in the diagnosis of prosthetic joint infection. J Mol Diagn. 2008;10(6):537-543.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014;29(9):1774-1778.
4. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
5. Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015;24(7):1021-1027.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
7. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
8. Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473(1):198-203.
9. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
10. Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97(1):63-70.
11. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
12. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
13. Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38(11):2385-2390.
14. Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 suppl):8-11.
15. Nelson GN, Paxton ES, Narzikul A, Williams G, Lazarus MD, Abboud JA. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg. 2015;24(9):1421-1426.
16. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
17. Dempsey KE, Riggio MP, Lennon A, et al. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther. 2007;9(3):R46.
18. Gallo J, Kolar M, Dendis M, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97-104.
19. Marin M, Garcia-Lechuz JM, Alonso P, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50(3):583-589.
20. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
21. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
22. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
23. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
24. Hunt RW, Bond MJ, Pater GD. Psychological responses to cancer: a case for cancer support groups. Community Health Stud. 1990;14(1):35-38.
25. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
26. Vandercam B, Jeumont S, Cornu O, et al. Amplification-based DNA analysis in the diagnosis of prosthetic joint infection. J Mol Diagn. 2008;10(6):537-543.
Traumatic Anterior Shoulder Instability: The US Military Experience
Take-Home Points
- Arthroscopic stabilization performed early results in better outcomes in patients with Bankart lesions.
- A subcritical level of bone loss of 13.5% has been shown to have a significant effect on outcomes, in addition to the established “critical amount”.
- Bone loss is a bipolar issue. Both sides must be considered in order to properly address shoulder instability.
- Off-track measurement has been shown to be even more positively predictive of outcomes than glenoid bone loss assessment.
- There are several bone loss management options including, the most common coracoid transfer, as well as distal tibial allograft and distal clavicular autograft.
Given its relatively young age, high activity level, and centralized medical care system, the US military population is ideal for studying traumatic anterior shoulder instability. There is a long history of military surgeons who have made significant contributions that have advanced our understanding of this pathology and its treatment and results. In this article, we describe the scope, treatment, and results of this pathology in the US military population.
Incidence and Pathology
At the United States Military Academy (USMA), Owens and colleagues1 studied the incidence of shoulder instability, including dislocation and subluxation, and found anterior instability events were far more common than in civilian populations. The incidence of shoulder instability was 0.08 per 1000 person-years in the general US population vs 1.69 per 1000 person-years in US military personnel. The factors associated with increased risk of shoulder instability injury in the military population were male sex, white race, junior enlisted rank, and age under 30 years. Owens and colleagues2 noted that subluxation accounted for almost 85% of the total anterior instability events. Owens and colleagues3 found the pathology in subluxation events was similar to that in full dislocations, with a soft-tissue anterior Bankart lesion and a Hill-Sachs lesion detected on magnetic resonance imaging in more than 90% of patients. In another study at the USMA, DeBerardino and colleagues4 noted that 97% of arthroscopically assessed shoulders in first-time dislocators involved complete detachment of the capsuloligamentous complex from the anterior glenoid rim and neck—a so-called Bankart lesion. Thus, in a military population, anterior instability resulting from subluxation or dislocation is a common finding that is often represented by a soft-tissue Bankart lesion and a Hill-Sachs defect.
Natural History of Traumatic Anterior Shoulder Instability in the Military
Several studies have evaluated the outcomes of nonoperative and operative treatment of shoulder instability. Although most have found better outcomes with operative intervention, Aronen and Regan5 reported good results (25% recurrence at nearly 3-year follow-up) with nonoperative treatment and adherence to a strict rehabilitation program. Most other comparative studies in this population have published contrary results. Wheeler and colleagues6 studied the natural history of anterior shoulder dislocations in a USMA cadet cohort and found recurrent instability after shoulder dislocation in 92% of cadets who had nonoperative treatment. Similarly, DeBerardino and colleagues4 found that, in the USMA, 90% of first-time traumatic anterior shoulder dislocations managed nonoperatively experienced recurrent instability. In a series of Army soldiers with shoulder instability, Bottoni and colleagues7 reported that 75% of nonoperatively managed patients had recurrent instability, and, of these, 67% progressed to surgical intervention. Nonoperative treatment for a first-time dislocation is still reasonable if a cadet or soldier needs to quickly return to functional duties. Athletes who develop shoulder instability during their playing season have been studied in a military population as well. In a multicenter study of service academy athletes with anterior instability, Dickens and colleagues8 found that, with conservative management and accelerated rehabilitation of in-season shoulder instability, 73% of athletes returned to sport by a mean of 5 days. However, the durability of this treatment should be questioned, as 64% later experienced recurrence.
Arthroscopic Stabilization of Acute Anterior Shoulder Dislocations
In an early series of cases of traumatic anterior shoulder instability in USMA cadets, Wheeler and colleagues6 found that, at 14 months, 78% of arthroscopically stabilized cases and 92% of nonoperatively treated cases were successful. Then, in the 1990s, DeBerardino and colleagues4 studied a series of young, active patients in the USMA and noted significantly better results with arthroscopic treatment, vs nonoperative treatment, at 2- to 5-year follow-up. Of the arthroscopically treated shoulders, 88% remained stable during the study and returned to preinjury activity levels, and 12% experienced recurrent instability (risk factors included 2+ sulcus sign, poor capsular labral tissue, and history of bilateral shoulder instability). In a long-term follow-up (mean, 11.7 years; range, 9.1-13.9 years) of the same cohort, Owens and colleagues9 found that 14% of patients available for follow-up had undergone revision stabilization surgery, and, of these, 21% reported experiencing subluxation events. The authors concluded that, in first-time dislocators in this active military population, acute arthroscopic Bankart repair resulted in excellent return to athletics and subjective function, and had acceptable recurrence and reoperation rates. Bottoni and colleagues,7 in a prospective, randomized evaluation of arthroscopic stabilization of acute, traumatic, first-time shoulder dislocations in the Army, noted an 89% success rate for arthroscopic treatment at an average follow-up of 36 months, with no recurrent instability. DeBerardino and colleagues10 compared West Point patients treated nonoperatively with those arthroscopically treated with staples, transglenoid sutures, or bioabsorbable anchors. Recurrence rates were 85% for nonoperative treatment, 22% for staples, 14% for transglenoid sutures, and 10% for bioabsorbable anchors.
Arthroscopic Versus Open Stabilization of Anterior Shoulder Instability
In a prospective, randomized clinical trial comparing open and arthroscopic shoulder stabilization for recurrent anterior instability in active-duty Army personnel, Bottoni and colleagues11 found comparable clinical outcomes. Stabilization surgery failed clinically in only 3 cases, 2 open and 1 arthroscopic. The authors concluded that arthroscopic stabilization can be safely performed for recurrent shoulder instability and that arthroscopic outcomes are similar to open outcomes. In a series of anterior shoulder subluxations in young athletes with Bankart lesions, Owens and colleagues12 found that open and arthroscopic stabilization performed early resulted in better outcomes, regardless of technique used. Recurrent subluxation occurred at a mean of 17 months in 3 of the 10 patients in the open group and 3 of the 9 patients in the arthroscopic group, for an overall recurrence rate of 31%. The authors concluded that, in this patient population with Bankart lesions caused by anterior subluxation events, surgery should be performed early.
Bone Lesions
Burkhart and De Beer13 first noted that bone loss has emerged as one of the most important considerations in the setting of shoulder instability in active patients. Other authors have found this to be true in military populations.14,15
The diagnosis of bone loss may include historical findings, such as increased number and ease of dislocations, as well as dislocation in lower positions of abduction. Physical examination findings may include apprehension in the midrange of motion. Advanced imaging, such as magnetic resonance arthrography, has since been validated as equivalent to 3-dimensional computed tomography (3-D CT) in determining glenoid bone loss.16 In 2007, Mologne and colleagues15 studied the amount of glenoid bone loss and the presence of fragmented bone or attritional bone loss and its effect on outcomes. They evaluated 21 patients who had arthroscopic treatment for anterior instability with anteroinferior glenoid bone loss between 20% and 30%. Average follow-up was 34 months. All patients received 3 or 4 anterior anchors. No patient with a bone fragment incorporated into the repair experienced recurrence or subluxation, whereas 30% of patients with attritional bone loss had recurrent instability.15
Classifying Bone Loss and Recognizing Its Effects
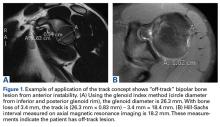
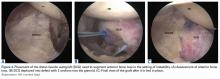
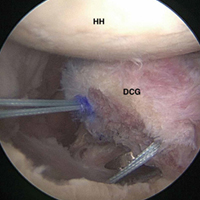
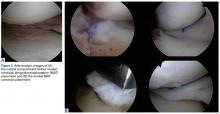
Burkhart and De Beer13 helped define the role and significance of bone loss in the setting of shoulder instability. They defined significant bone loss as an engaging Hill-Sachs lesion of the humerus in an abducted and externally rotated position or an “inverted pear” lesion of the glenoid. Overall analysis revealed recurrence in 4% of cases without significant bone loss and 65% of cases with significant bone loss. In a subanalysis of contact-sport athletes in the setting of bone loss, the failure rate increased to 89%, from 6.5%. Aiding in the quantitative assessment of glenoid bone loss, Itoi and colleagues17 showed that 21% glenoid bone loss resulted in instability that would not be corrected by a soft-tissue procedure alone. Bone loss of 20% to 25% has since been considered a “critical amount,” above which an arthroscopic Bankart has been questioned. More recently, several authors have shown that even less bone loss can have a significant effect on outcomes. Shaha and colleagues18 established that a subcritical level of bone loss (13.5%) on the anteroinferior glenoid resulted in clinical failure (as determined with the Western Ontario Shoulder Instability Index) even in cases in which frank recurrence or subluxation was avoided. It is thought that, in recurrent instability, glenoid bone loss incident rate is as high as 90%, and the corresponding percentage of patients with Hill-Sachs lesions is almost 100%.19,20 Thus, it is increasingly understood that bone loss is a bipolar issue and that both sides must be considered in order to properly address shoulder instability in this setting. In 2007, Yamamoto and colleagues21 introduced the glenoid track, a method for predicting whether a Hill-Sachs lesion will engage. Di Giacomo and colleagues22 refined the track concept to quantitatively determine which lesions will engage in the setting of both glenoid and humeral bone loss. Metzger and colleagues,23 confirming the track concept arthroscopically, found that manipulation with anesthesia and arthroscopic visualization was well predicted by preoperative track measurements, and thus these measurements can be a good guide for surgical management (Figures 1A, 1B).
Strategies for Addressing Bone Loss in Anterior Shoulder Instability
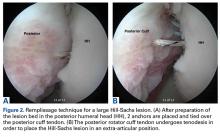
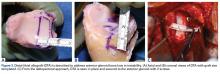
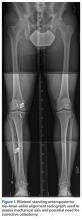
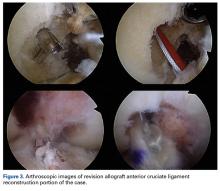
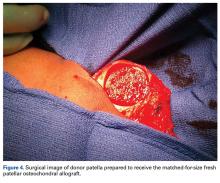
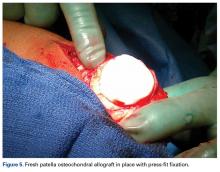
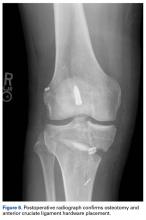
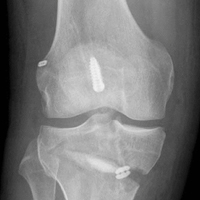
Several approaches for managing bone loss in shoulder instability have been described—the most common being coracoid transfer (Latarjet procedure). Waterman and colleagues25 recently studied the effects of coracoid transfer, distal tibial allograft, and iliac crest augmentation on anterior shoulder instability in US military patients treated between 2006 and 2012. Of 64 patients who underwent a bone block procedure, 16 (25%) had a complication during short-term follow-up. Complications included neurologic injury, pain, infection, hardware failure, and recurrent instability.
Conclusion
Traumatic anterior shoulder instability is a common pathology that continues to significantly challenge the readiness of the US military. Military surgeon-researchers have a long history of investigating approaches to the treatment of this pathology—applying good science to a large controlled population, using a single medical record, and demonstrating a commitment to return service members to the ready defense of the nation.
Am J Orthop. 2017;46(4):184-189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Owens BD, Dawson L, Burks R, Cameron KL. Incidence of shoulder dislocation in the United States military: demographic considerations from a high-risk population. J Bone Joint Surg Am. 2009;91(4):791-796.
2. Owens BD, Duffey ML, Nelson BJ, DeBerardino TM, Taylor DC, Mountcastle SB. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
3. Owens BD, Nelson BJ, Duffey ML, et al. Pathoanatomy of first-time, traumatic, anterior glenohumeral subluxation events. J Bone Joint Surg Am. 2010;92(7):1605-1611.
4. DeBerardino TM, Arciero RA, Taylor DC, Uhorchak JM. Prospective evaluation of arthroscopic stabilization of acute, initial anterior shoulder dislocations in young athletes. Two- to five-year follow-up. Am J Sports Med. 2001;29(5):586-592.
5. Aronen JG, Regan K. Decreasing the incidence of recurrence of first time anterior shoulder dislocations with rehabilitation. Am J Sports Med. 1984;12(4):283-291.
6. Wheeler JH, Ryan JB, Arciero RA, Molinari RN. Arthroscopic versus nonoperative treatment of acute shoulder dislocations in young athletes. Arthroscopy. 1989;5(3):213-217.
7. Bottoni CR, Wilckens JH, DeBerardino TM, et al. A prospective, randomized evaluation of arthroscopic stabilization versus nonoperative treatment in patients with acute, traumatic, first-time shoulder dislocations. Am J Sports Med. 2002;30(4):576-580.
8. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42(12):2842-2850.
9. Owens BD, DeBerardino TM, Nelson BJ, et al. Long-term follow-up of acute arthroscopic Bankart repair for initial anterior shoulder dislocations in young athletes. Am J Sports Med. 2009;37(4):669-673.
10. DeBerardino TM, Arciero RA, Taylor DC. Arthroscopic stabilization of acute initial anterior shoulder dislocation: the West Point experience. J South Orthop Assoc. 1996;5(4):263-271.
11. Bottoni CR, Smith EL, Berkowitz MJ, Towle RB, Moore JH. Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized clinical trial. Am J Sports Med. 2006;34(11):1730-1737.
12. Owens BD, Cameron KL, Peck KY, et al. Arthroscopic versus open stabilization for anterior shoulder subluxations. Orthop J Sports Med. 2015;3(1):2325967115571084.
13. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.14. Shaha JS, Cook JB, Rowles DJ, Bottoni CR, Shaha SH, Tokish JM. Clinical validation of the glenoid track concept in anterior glenohumeral instability. J Bone Joint Surg Am. 2016;98(22):1918-1923.
15. Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35(8):1276-1283.
16. Markenstein JE, Jaspars KC, van der Hulst VP, Willems WJ. The quantification of glenoid bone loss in anterior shoulder instability; MR-arthro compared to 3D-CT. Skeletal Radiol. 2014;43(4):475-483.
17. Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
18. Shaha JS, Cook JB, Song DJ, et al. Redefining “critical” bone loss in shoulder instability: functional outcomes worsen with “subcritical” bone loss. Am J Sports Med. 2015;43(7):1719-1725.
19. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
20. Provencher MT, Frank RM, Leclere LE, et al. The Hill-Sachs lesion: diagnosis, classification, and management. J Am Acad Orthop Surg. 2012;20(4):242-252.
21. Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16(5):649-656.
22. Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from “engaging/non-engaging” lesion to “on-track/off-track” lesion. Arthroscopy. 2014;30(1):90-98.
23. Metzger PD, Barlow B, Leonardelli D, Peace W, Solomon DJ, Provencher MT. Clinical application of the “glenoid track” concept for defining humeral head engagement in anterior shoulder instability: a preliminary report. Orthop J Sports Med. 2013;1(2):2325967113496213.
24. Arciero RA, Parrino A, Bernhardson AS, et al. The effect of a combined glenoid and Hill-Sachs defect on glenohumeral stability: a biomechanical cadaveric study using 3-dimensional modeling of 142 patients. Am J Sports Med. 2015;43(6):1422-1429.
25. Waterman BR, Chandler PJ, Teague E, Provencher MT, Tokish JM, Pallis MP. Short-term outcomes of glenoid bone block augmentation for complex anterior shoulder instability in a high-risk population. Arthroscopy. 2016;32(9):1784-1790.
26. Schroder DT, Provencher MT, Mologne TS, Muldoon MP, Cox JS. The modified Bristow procedure for anterior shoulder instability: 26-year outcomes in Naval Academy midshipmen. Am J Sports Med. 2006;34(5):778-786.
27. Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891-897.
28. Tokish JM, Fitzpatrick K, Cook JB, Mallon WJ. Arthroscopic distal clavicular autograft for treating shoulder instability with glenoid bone loss. Arthrosc Tech. 2014;3(4):e475-e481.
Take-Home Points
- Arthroscopic stabilization performed early results in better outcomes in patients with Bankart lesions.
- A subcritical level of bone loss of 13.5% has been shown to have a significant effect on outcomes, in addition to the established “critical amount”.
- Bone loss is a bipolar issue. Both sides must be considered in order to properly address shoulder instability.
- Off-track measurement has been shown to be even more positively predictive of outcomes than glenoid bone loss assessment.
- There are several bone loss management options including, the most common coracoid transfer, as well as distal tibial allograft and distal clavicular autograft.
Given its relatively young age, high activity level, and centralized medical care system, the US military population is ideal for studying traumatic anterior shoulder instability. There is a long history of military surgeons who have made significant contributions that have advanced our understanding of this pathology and its treatment and results. In this article, we describe the scope, treatment, and results of this pathology in the US military population.
Incidence and Pathology
At the United States Military Academy (USMA), Owens and colleagues1 studied the incidence of shoulder instability, including dislocation and subluxation, and found anterior instability events were far more common than in civilian populations. The incidence of shoulder instability was 0.08 per 1000 person-years in the general US population vs 1.69 per 1000 person-years in US military personnel. The factors associated with increased risk of shoulder instability injury in the military population were male sex, white race, junior enlisted rank, and age under 30 years. Owens and colleagues2 noted that subluxation accounted for almost 85% of the total anterior instability events. Owens and colleagues3 found the pathology in subluxation events was similar to that in full dislocations, with a soft-tissue anterior Bankart lesion and a Hill-Sachs lesion detected on magnetic resonance imaging in more than 90% of patients. In another study at the USMA, DeBerardino and colleagues4 noted that 97% of arthroscopically assessed shoulders in first-time dislocators involved complete detachment of the capsuloligamentous complex from the anterior glenoid rim and neck—a so-called Bankart lesion. Thus, in a military population, anterior instability resulting from subluxation or dislocation is a common finding that is often represented by a soft-tissue Bankart lesion and a Hill-Sachs defect.
Natural History of Traumatic Anterior Shoulder Instability in the Military
Several studies have evaluated the outcomes of nonoperative and operative treatment of shoulder instability. Although most have found better outcomes with operative intervention, Aronen and Regan5 reported good results (25% recurrence at nearly 3-year follow-up) with nonoperative treatment and adherence to a strict rehabilitation program. Most other comparative studies in this population have published contrary results. Wheeler and colleagues6 studied the natural history of anterior shoulder dislocations in a USMA cadet cohort and found recurrent instability after shoulder dislocation in 92% of cadets who had nonoperative treatment. Similarly, DeBerardino and colleagues4 found that, in the USMA, 90% of first-time traumatic anterior shoulder dislocations managed nonoperatively experienced recurrent instability. In a series of Army soldiers with shoulder instability, Bottoni and colleagues7 reported that 75% of nonoperatively managed patients had recurrent instability, and, of these, 67% progressed to surgical intervention. Nonoperative treatment for a first-time dislocation is still reasonable if a cadet or soldier needs to quickly return to functional duties. Athletes who develop shoulder instability during their playing season have been studied in a military population as well. In a multicenter study of service academy athletes with anterior instability, Dickens and colleagues8 found that, with conservative management and accelerated rehabilitation of in-season shoulder instability, 73% of athletes returned to sport by a mean of 5 days. However, the durability of this treatment should be questioned, as 64% later experienced recurrence.
Arthroscopic Stabilization of Acute Anterior Shoulder Dislocations
In an early series of cases of traumatic anterior shoulder instability in USMA cadets, Wheeler and colleagues6 found that, at 14 months, 78% of arthroscopically stabilized cases and 92% of nonoperatively treated cases were successful. Then, in the 1990s, DeBerardino and colleagues4 studied a series of young, active patients in the USMA and noted significantly better results with arthroscopic treatment, vs nonoperative treatment, at 2- to 5-year follow-up. Of the arthroscopically treated shoulders, 88% remained stable during the study and returned to preinjury activity levels, and 12% experienced recurrent instability (risk factors included 2+ sulcus sign, poor capsular labral tissue, and history of bilateral shoulder instability). In a long-term follow-up (mean, 11.7 years; range, 9.1-13.9 years) of the same cohort, Owens and colleagues9 found that 14% of patients available for follow-up had undergone revision stabilization surgery, and, of these, 21% reported experiencing subluxation events. The authors concluded that, in first-time dislocators in this active military population, acute arthroscopic Bankart repair resulted in excellent return to athletics and subjective function, and had acceptable recurrence and reoperation rates. Bottoni and colleagues,7 in a prospective, randomized evaluation of arthroscopic stabilization of acute, traumatic, first-time shoulder dislocations in the Army, noted an 89% success rate for arthroscopic treatment at an average follow-up of 36 months, with no recurrent instability. DeBerardino and colleagues10 compared West Point patients treated nonoperatively with those arthroscopically treated with staples, transglenoid sutures, or bioabsorbable anchors. Recurrence rates were 85% for nonoperative treatment, 22% for staples, 14% for transglenoid sutures, and 10% for bioabsorbable anchors.
Arthroscopic Versus Open Stabilization of Anterior Shoulder Instability
In a prospective, randomized clinical trial comparing open and arthroscopic shoulder stabilization for recurrent anterior instability in active-duty Army personnel, Bottoni and colleagues11 found comparable clinical outcomes. Stabilization surgery failed clinically in only 3 cases, 2 open and 1 arthroscopic. The authors concluded that arthroscopic stabilization can be safely performed for recurrent shoulder instability and that arthroscopic outcomes are similar to open outcomes. In a series of anterior shoulder subluxations in young athletes with Bankart lesions, Owens and colleagues12 found that open and arthroscopic stabilization performed early resulted in better outcomes, regardless of technique used. Recurrent subluxation occurred at a mean of 17 months in 3 of the 10 patients in the open group and 3 of the 9 patients in the arthroscopic group, for an overall recurrence rate of 31%. The authors concluded that, in this patient population with Bankart lesions caused by anterior subluxation events, surgery should be performed early.
Bone Lesions
Burkhart and De Beer13 first noted that bone loss has emerged as one of the most important considerations in the setting of shoulder instability in active patients. Other authors have found this to be true in military populations.14,15
The diagnosis of bone loss may include historical findings, such as increased number and ease of dislocations, as well as dislocation in lower positions of abduction. Physical examination findings may include apprehension in the midrange of motion. Advanced imaging, such as magnetic resonance arthrography, has since been validated as equivalent to 3-dimensional computed tomography (3-D CT) in determining glenoid bone loss.16 In 2007, Mologne and colleagues15 studied the amount of glenoid bone loss and the presence of fragmented bone or attritional bone loss and its effect on outcomes. They evaluated 21 patients who had arthroscopic treatment for anterior instability with anteroinferior glenoid bone loss between 20% and 30%. Average follow-up was 34 months. All patients received 3 or 4 anterior anchors. No patient with a bone fragment incorporated into the repair experienced recurrence or subluxation, whereas 30% of patients with attritional bone loss had recurrent instability.15
Classifying Bone Loss and Recognizing Its Effects
Burkhart and De Beer13 helped define the role and significance of bone loss in the setting of shoulder instability. They defined significant bone loss as an engaging Hill-Sachs lesion of the humerus in an abducted and externally rotated position or an “inverted pear” lesion of the glenoid. Overall analysis revealed recurrence in 4% of cases without significant bone loss and 65% of cases with significant bone loss. In a subanalysis of contact-sport athletes in the setting of bone loss, the failure rate increased to 89%, from 6.5%. Aiding in the quantitative assessment of glenoid bone loss, Itoi and colleagues17 showed that 21% glenoid bone loss resulted in instability that would not be corrected by a soft-tissue procedure alone. Bone loss of 20% to 25% has since been considered a “critical amount,” above which an arthroscopic Bankart has been questioned. More recently, several authors have shown that even less bone loss can have a significant effect on outcomes. Shaha and colleagues18 established that a subcritical level of bone loss (13.5%) on the anteroinferior glenoid resulted in clinical failure (as determined with the Western Ontario Shoulder Instability Index) even in cases in which frank recurrence or subluxation was avoided. It is thought that, in recurrent instability, glenoid bone loss incident rate is as high as 90%, and the corresponding percentage of patients with Hill-Sachs lesions is almost 100%.19,20 Thus, it is increasingly understood that bone loss is a bipolar issue and that both sides must be considered in order to properly address shoulder instability in this setting. In 2007, Yamamoto and colleagues21 introduced the glenoid track, a method for predicting whether a Hill-Sachs lesion will engage. Di Giacomo and colleagues22 refined the track concept to quantitatively determine which lesions will engage in the setting of both glenoid and humeral bone loss. Metzger and colleagues,23 confirming the track concept arthroscopically, found that manipulation with anesthesia and arthroscopic visualization was well predicted by preoperative track measurements, and thus these measurements can be a good guide for surgical management (Figures 1A, 1B).
Strategies for Addressing Bone Loss in Anterior Shoulder Instability
Several approaches for managing bone loss in shoulder instability have been described—the most common being coracoid transfer (Latarjet procedure). Waterman and colleagues25 recently studied the effects of coracoid transfer, distal tibial allograft, and iliac crest augmentation on anterior shoulder instability in US military patients treated between 2006 and 2012. Of 64 patients who underwent a bone block procedure, 16 (25%) had a complication during short-term follow-up. Complications included neurologic injury, pain, infection, hardware failure, and recurrent instability.
Conclusion
Traumatic anterior shoulder instability is a common pathology that continues to significantly challenge the readiness of the US military. Military surgeon-researchers have a long history of investigating approaches to the treatment of this pathology—applying good science to a large controlled population, using a single medical record, and demonstrating a commitment to return service members to the ready defense of the nation.
Am J Orthop. 2017;46(4):184-189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Arthroscopic stabilization performed early results in better outcomes in patients with Bankart lesions.
- A subcritical level of bone loss of 13.5% has been shown to have a significant effect on outcomes, in addition to the established “critical amount”.
- Bone loss is a bipolar issue. Both sides must be considered in order to properly address shoulder instability.
- Off-track measurement has been shown to be even more positively predictive of outcomes than glenoid bone loss assessment.
- There are several bone loss management options including, the most common coracoid transfer, as well as distal tibial allograft and distal clavicular autograft.
Given its relatively young age, high activity level, and centralized medical care system, the US military population is ideal for studying traumatic anterior shoulder instability. There is a long history of military surgeons who have made significant contributions that have advanced our understanding of this pathology and its treatment and results. In this article, we describe the scope, treatment, and results of this pathology in the US military population.
Incidence and Pathology
At the United States Military Academy (USMA), Owens and colleagues1 studied the incidence of shoulder instability, including dislocation and subluxation, and found anterior instability events were far more common than in civilian populations. The incidence of shoulder instability was 0.08 per 1000 person-years in the general US population vs 1.69 per 1000 person-years in US military personnel. The factors associated with increased risk of shoulder instability injury in the military population were male sex, white race, junior enlisted rank, and age under 30 years. Owens and colleagues2 noted that subluxation accounted for almost 85% of the total anterior instability events. Owens and colleagues3 found the pathology in subluxation events was similar to that in full dislocations, with a soft-tissue anterior Bankart lesion and a Hill-Sachs lesion detected on magnetic resonance imaging in more than 90% of patients. In another study at the USMA, DeBerardino and colleagues4 noted that 97% of arthroscopically assessed shoulders in first-time dislocators involved complete detachment of the capsuloligamentous complex from the anterior glenoid rim and neck—a so-called Bankart lesion. Thus, in a military population, anterior instability resulting from subluxation or dislocation is a common finding that is often represented by a soft-tissue Bankart lesion and a Hill-Sachs defect.
Natural History of Traumatic Anterior Shoulder Instability in the Military
Several studies have evaluated the outcomes of nonoperative and operative treatment of shoulder instability. Although most have found better outcomes with operative intervention, Aronen and Regan5 reported good results (25% recurrence at nearly 3-year follow-up) with nonoperative treatment and adherence to a strict rehabilitation program. Most other comparative studies in this population have published contrary results. Wheeler and colleagues6 studied the natural history of anterior shoulder dislocations in a USMA cadet cohort and found recurrent instability after shoulder dislocation in 92% of cadets who had nonoperative treatment. Similarly, DeBerardino and colleagues4 found that, in the USMA, 90% of first-time traumatic anterior shoulder dislocations managed nonoperatively experienced recurrent instability. In a series of Army soldiers with shoulder instability, Bottoni and colleagues7 reported that 75% of nonoperatively managed patients had recurrent instability, and, of these, 67% progressed to surgical intervention. Nonoperative treatment for a first-time dislocation is still reasonable if a cadet or soldier needs to quickly return to functional duties. Athletes who develop shoulder instability during their playing season have been studied in a military population as well. In a multicenter study of service academy athletes with anterior instability, Dickens and colleagues8 found that, with conservative management and accelerated rehabilitation of in-season shoulder instability, 73% of athletes returned to sport by a mean of 5 days. However, the durability of this treatment should be questioned, as 64% later experienced recurrence.
Arthroscopic Stabilization of Acute Anterior Shoulder Dislocations
In an early series of cases of traumatic anterior shoulder instability in USMA cadets, Wheeler and colleagues6 found that, at 14 months, 78% of arthroscopically stabilized cases and 92% of nonoperatively treated cases were successful. Then, in the 1990s, DeBerardino and colleagues4 studied a series of young, active patients in the USMA and noted significantly better results with arthroscopic treatment, vs nonoperative treatment, at 2- to 5-year follow-up. Of the arthroscopically treated shoulders, 88% remained stable during the study and returned to preinjury activity levels, and 12% experienced recurrent instability (risk factors included 2+ sulcus sign, poor capsular labral tissue, and history of bilateral shoulder instability). In a long-term follow-up (mean, 11.7 years; range, 9.1-13.9 years) of the same cohort, Owens and colleagues9 found that 14% of patients available for follow-up had undergone revision stabilization surgery, and, of these, 21% reported experiencing subluxation events. The authors concluded that, in first-time dislocators in this active military population, acute arthroscopic Bankart repair resulted in excellent return to athletics and subjective function, and had acceptable recurrence and reoperation rates. Bottoni and colleagues,7 in a prospective, randomized evaluation of arthroscopic stabilization of acute, traumatic, first-time shoulder dislocations in the Army, noted an 89% success rate for arthroscopic treatment at an average follow-up of 36 months, with no recurrent instability. DeBerardino and colleagues10 compared West Point patients treated nonoperatively with those arthroscopically treated with staples, transglenoid sutures, or bioabsorbable anchors. Recurrence rates were 85% for nonoperative treatment, 22% for staples, 14% for transglenoid sutures, and 10% for bioabsorbable anchors.
Arthroscopic Versus Open Stabilization of Anterior Shoulder Instability
In a prospective, randomized clinical trial comparing open and arthroscopic shoulder stabilization for recurrent anterior instability in active-duty Army personnel, Bottoni and colleagues11 found comparable clinical outcomes. Stabilization surgery failed clinically in only 3 cases, 2 open and 1 arthroscopic. The authors concluded that arthroscopic stabilization can be safely performed for recurrent shoulder instability and that arthroscopic outcomes are similar to open outcomes. In a series of anterior shoulder subluxations in young athletes with Bankart lesions, Owens and colleagues12 found that open and arthroscopic stabilization performed early resulted in better outcomes, regardless of technique used. Recurrent subluxation occurred at a mean of 17 months in 3 of the 10 patients in the open group and 3 of the 9 patients in the arthroscopic group, for an overall recurrence rate of 31%. The authors concluded that, in this patient population with Bankart lesions caused by anterior subluxation events, surgery should be performed early.
Bone Lesions
Burkhart and De Beer13 first noted that bone loss has emerged as one of the most important considerations in the setting of shoulder instability in active patients. Other authors have found this to be true in military populations.14,15
The diagnosis of bone loss may include historical findings, such as increased number and ease of dislocations, as well as dislocation in lower positions of abduction. Physical examination findings may include apprehension in the midrange of motion. Advanced imaging, such as magnetic resonance arthrography, has since been validated as equivalent to 3-dimensional computed tomography (3-D CT) in determining glenoid bone loss.16 In 2007, Mologne and colleagues15 studied the amount of glenoid bone loss and the presence of fragmented bone or attritional bone loss and its effect on outcomes. They evaluated 21 patients who had arthroscopic treatment for anterior instability with anteroinferior glenoid bone loss between 20% and 30%. Average follow-up was 34 months. All patients received 3 or 4 anterior anchors. No patient with a bone fragment incorporated into the repair experienced recurrence or subluxation, whereas 30% of patients with attritional bone loss had recurrent instability.15
Classifying Bone Loss and Recognizing Its Effects
Burkhart and De Beer13 helped define the role and significance of bone loss in the setting of shoulder instability. They defined significant bone loss as an engaging Hill-Sachs lesion of the humerus in an abducted and externally rotated position or an “inverted pear” lesion of the glenoid. Overall analysis revealed recurrence in 4% of cases without significant bone loss and 65% of cases with significant bone loss. In a subanalysis of contact-sport athletes in the setting of bone loss, the failure rate increased to 89%, from 6.5%. Aiding in the quantitative assessment of glenoid bone loss, Itoi and colleagues17 showed that 21% glenoid bone loss resulted in instability that would not be corrected by a soft-tissue procedure alone. Bone loss of 20% to 25% has since been considered a “critical amount,” above which an arthroscopic Bankart has been questioned. More recently, several authors have shown that even less bone loss can have a significant effect on outcomes. Shaha and colleagues18 established that a subcritical level of bone loss (13.5%) on the anteroinferior glenoid resulted in clinical failure (as determined with the Western Ontario Shoulder Instability Index) even in cases in which frank recurrence or subluxation was avoided. It is thought that, in recurrent instability, glenoid bone loss incident rate is as high as 90%, and the corresponding percentage of patients with Hill-Sachs lesions is almost 100%.19,20 Thus, it is increasingly understood that bone loss is a bipolar issue and that both sides must be considered in order to properly address shoulder instability in this setting. In 2007, Yamamoto and colleagues21 introduced the glenoid track, a method for predicting whether a Hill-Sachs lesion will engage. Di Giacomo and colleagues22 refined the track concept to quantitatively determine which lesions will engage in the setting of both glenoid and humeral bone loss. Metzger and colleagues,23 confirming the track concept arthroscopically, found that manipulation with anesthesia and arthroscopic visualization was well predicted by preoperative track measurements, and thus these measurements can be a good guide for surgical management (Figures 1A, 1B).
Strategies for Addressing Bone Loss in Anterior Shoulder Instability
Several approaches for managing bone loss in shoulder instability have been described—the most common being coracoid transfer (Latarjet procedure). Waterman and colleagues25 recently studied the effects of coracoid transfer, distal tibial allograft, and iliac crest augmentation on anterior shoulder instability in US military patients treated between 2006 and 2012. Of 64 patients who underwent a bone block procedure, 16 (25%) had a complication during short-term follow-up. Complications included neurologic injury, pain, infection, hardware failure, and recurrent instability.
Conclusion
Traumatic anterior shoulder instability is a common pathology that continues to significantly challenge the readiness of the US military. Military surgeon-researchers have a long history of investigating approaches to the treatment of this pathology—applying good science to a large controlled population, using a single medical record, and demonstrating a commitment to return service members to the ready defense of the nation.
Am J Orthop. 2017;46(4):184-189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Owens BD, Dawson L, Burks R, Cameron KL. Incidence of shoulder dislocation in the United States military: demographic considerations from a high-risk population. J Bone Joint Surg Am. 2009;91(4):791-796.
2. Owens BD, Duffey ML, Nelson BJ, DeBerardino TM, Taylor DC, Mountcastle SB. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
3. Owens BD, Nelson BJ, Duffey ML, et al. Pathoanatomy of first-time, traumatic, anterior glenohumeral subluxation events. J Bone Joint Surg Am. 2010;92(7):1605-1611.
4. DeBerardino TM, Arciero RA, Taylor DC, Uhorchak JM. Prospective evaluation of arthroscopic stabilization of acute, initial anterior shoulder dislocations in young athletes. Two- to five-year follow-up. Am J Sports Med. 2001;29(5):586-592.
5. Aronen JG, Regan K. Decreasing the incidence of recurrence of first time anterior shoulder dislocations with rehabilitation. Am J Sports Med. 1984;12(4):283-291.
6. Wheeler JH, Ryan JB, Arciero RA, Molinari RN. Arthroscopic versus nonoperative treatment of acute shoulder dislocations in young athletes. Arthroscopy. 1989;5(3):213-217.
7. Bottoni CR, Wilckens JH, DeBerardino TM, et al. A prospective, randomized evaluation of arthroscopic stabilization versus nonoperative treatment in patients with acute, traumatic, first-time shoulder dislocations. Am J Sports Med. 2002;30(4):576-580.
8. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42(12):2842-2850.
9. Owens BD, DeBerardino TM, Nelson BJ, et al. Long-term follow-up of acute arthroscopic Bankart repair for initial anterior shoulder dislocations in young athletes. Am J Sports Med. 2009;37(4):669-673.
10. DeBerardino TM, Arciero RA, Taylor DC. Arthroscopic stabilization of acute initial anterior shoulder dislocation: the West Point experience. J South Orthop Assoc. 1996;5(4):263-271.
11. Bottoni CR, Smith EL, Berkowitz MJ, Towle RB, Moore JH. Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized clinical trial. Am J Sports Med. 2006;34(11):1730-1737.
12. Owens BD, Cameron KL, Peck KY, et al. Arthroscopic versus open stabilization for anterior shoulder subluxations. Orthop J Sports Med. 2015;3(1):2325967115571084.
13. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.14. Shaha JS, Cook JB, Rowles DJ, Bottoni CR, Shaha SH, Tokish JM. Clinical validation of the glenoid track concept in anterior glenohumeral instability. J Bone Joint Surg Am. 2016;98(22):1918-1923.
15. Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35(8):1276-1283.
16. Markenstein JE, Jaspars KC, van der Hulst VP, Willems WJ. The quantification of glenoid bone loss in anterior shoulder instability; MR-arthro compared to 3D-CT. Skeletal Radiol. 2014;43(4):475-483.
17. Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
18. Shaha JS, Cook JB, Song DJ, et al. Redefining “critical” bone loss in shoulder instability: functional outcomes worsen with “subcritical” bone loss. Am J Sports Med. 2015;43(7):1719-1725.
19. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
20. Provencher MT, Frank RM, Leclere LE, et al. The Hill-Sachs lesion: diagnosis, classification, and management. J Am Acad Orthop Surg. 2012;20(4):242-252.
21. Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16(5):649-656.
22. Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from “engaging/non-engaging” lesion to “on-track/off-track” lesion. Arthroscopy. 2014;30(1):90-98.
23. Metzger PD, Barlow B, Leonardelli D, Peace W, Solomon DJ, Provencher MT. Clinical application of the “glenoid track” concept for defining humeral head engagement in anterior shoulder instability: a preliminary report. Orthop J Sports Med. 2013;1(2):2325967113496213.
24. Arciero RA, Parrino A, Bernhardson AS, et al. The effect of a combined glenoid and Hill-Sachs defect on glenohumeral stability: a biomechanical cadaveric study using 3-dimensional modeling of 142 patients. Am J Sports Med. 2015;43(6):1422-1429.
25. Waterman BR, Chandler PJ, Teague E, Provencher MT, Tokish JM, Pallis MP. Short-term outcomes of glenoid bone block augmentation for complex anterior shoulder instability in a high-risk population. Arthroscopy. 2016;32(9):1784-1790.
26. Schroder DT, Provencher MT, Mologne TS, Muldoon MP, Cox JS. The modified Bristow procedure for anterior shoulder instability: 26-year outcomes in Naval Academy midshipmen. Am J Sports Med. 2006;34(5):778-786.
27. Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891-897.
28. Tokish JM, Fitzpatrick K, Cook JB, Mallon WJ. Arthroscopic distal clavicular autograft for treating shoulder instability with glenoid bone loss. Arthrosc Tech. 2014;3(4):e475-e481.
1. Owens BD, Dawson L, Burks R, Cameron KL. Incidence of shoulder dislocation in the United States military: demographic considerations from a high-risk population. J Bone Joint Surg Am. 2009;91(4):791-796.
2. Owens BD, Duffey ML, Nelson BJ, DeBerardino TM, Taylor DC, Mountcastle SB. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
3. Owens BD, Nelson BJ, Duffey ML, et al. Pathoanatomy of first-time, traumatic, anterior glenohumeral subluxation events. J Bone Joint Surg Am. 2010;92(7):1605-1611.
4. DeBerardino TM, Arciero RA, Taylor DC, Uhorchak JM. Prospective evaluation of arthroscopic stabilization of acute, initial anterior shoulder dislocations in young athletes. Two- to five-year follow-up. Am J Sports Med. 2001;29(5):586-592.
5. Aronen JG, Regan K. Decreasing the incidence of recurrence of first time anterior shoulder dislocations with rehabilitation. Am J Sports Med. 1984;12(4):283-291.
6. Wheeler JH, Ryan JB, Arciero RA, Molinari RN. Arthroscopic versus nonoperative treatment of acute shoulder dislocations in young athletes. Arthroscopy. 1989;5(3):213-217.
7. Bottoni CR, Wilckens JH, DeBerardino TM, et al. A prospective, randomized evaluation of arthroscopic stabilization versus nonoperative treatment in patients with acute, traumatic, first-time shoulder dislocations. Am J Sports Med. 2002;30(4):576-580.
8. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42(12):2842-2850.
9. Owens BD, DeBerardino TM, Nelson BJ, et al. Long-term follow-up of acute arthroscopic Bankart repair for initial anterior shoulder dislocations in young athletes. Am J Sports Med. 2009;37(4):669-673.
10. DeBerardino TM, Arciero RA, Taylor DC. Arthroscopic stabilization of acute initial anterior shoulder dislocation: the West Point experience. J South Orthop Assoc. 1996;5(4):263-271.
11. Bottoni CR, Smith EL, Berkowitz MJ, Towle RB, Moore JH. Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized clinical trial. Am J Sports Med. 2006;34(11):1730-1737.
12. Owens BD, Cameron KL, Peck KY, et al. Arthroscopic versus open stabilization for anterior shoulder subluxations. Orthop J Sports Med. 2015;3(1):2325967115571084.
13. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.14. Shaha JS, Cook JB, Rowles DJ, Bottoni CR, Shaha SH, Tokish JM. Clinical validation of the glenoid track concept in anterior glenohumeral instability. J Bone Joint Surg Am. 2016;98(22):1918-1923.
15. Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35(8):1276-1283.
16. Markenstein JE, Jaspars KC, van der Hulst VP, Willems WJ. The quantification of glenoid bone loss in anterior shoulder instability; MR-arthro compared to 3D-CT. Skeletal Radiol. 2014;43(4):475-483.
17. Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
18. Shaha JS, Cook JB, Song DJ, et al. Redefining “critical” bone loss in shoulder instability: functional outcomes worsen with “subcritical” bone loss. Am J Sports Med. 2015;43(7):1719-1725.
19. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
20. Provencher MT, Frank RM, Leclere LE, et al. The Hill-Sachs lesion: diagnosis, classification, and management. J Am Acad Orthop Surg. 2012;20(4):242-252.
21. Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16(5):649-656.
22. Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from “engaging/non-engaging” lesion to “on-track/off-track” lesion. Arthroscopy. 2014;30(1):90-98.
23. Metzger PD, Barlow B, Leonardelli D, Peace W, Solomon DJ, Provencher MT. Clinical application of the “glenoid track” concept for defining humeral head engagement in anterior shoulder instability: a preliminary report. Orthop J Sports Med. 2013;1(2):2325967113496213.
24. Arciero RA, Parrino A, Bernhardson AS, et al. The effect of a combined glenoid and Hill-Sachs defect on glenohumeral stability: a biomechanical cadaveric study using 3-dimensional modeling of 142 patients. Am J Sports Med. 2015;43(6):1422-1429.
25. Waterman BR, Chandler PJ, Teague E, Provencher MT, Tokish JM, Pallis MP. Short-term outcomes of glenoid bone block augmentation for complex anterior shoulder instability in a high-risk population. Arthroscopy. 2016;32(9):1784-1790.
26. Schroder DT, Provencher MT, Mologne TS, Muldoon MP, Cox JS. The modified Bristow procedure for anterior shoulder instability: 26-year outcomes in Naval Academy midshipmen. Am J Sports Med. 2006;34(5):778-786.
27. Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891-897.
28. Tokish JM, Fitzpatrick K, Cook JB, Mallon WJ. Arthroscopic distal clavicular autograft for treating shoulder instability with glenoid bone loss. Arthrosc Tech. 2014;3(4):e475-e481.
Bone Stress Injuries in the Military: Diagnosis, Management, and Prevention
Take-Home Points
- Stress injuries, specifically of the lower extremity, are very common in new military trainees.
- Stress injury can range from benign periosteal reaction to displaced fracture.
- Stress injury should be treated on a case-by-case basis, depending on the severity of injury, the location of the injury, and the likelihood of healing with nonoperative management.
- Modifiable risk factors such as nutritional status, training regiment, and even footwear should be investigated to determine potential causes of injury.
- Prevention is a crucial part of the treatment of these injuries, and early intervention such as careful pre-enrollment physicals and vitamin supplementation can be essential in lowering injury rates.
Bone stress injuries, which are common in military recruits, present in weight-bearing (WB) areas as indolent pain caused by repetitive stress and microtrauma. They were first reported in the metatarsals of Prussian soldiers in 1855.1 Today, stress injuries are increasingly common. One study estimated they account for 10% of patients seen by sports medicine practitioners.2 This injury most commonly affects military members, endurance athletes, and dancers.3-5 Specifically, the incidence of stress fractures in military members has been reported to range from 0.8% to 6.9% for men and from 3.4% to 21.0% for women.4 Because of repetitive vigorous lower extremity loading, stress fractures typically occur in the pelvis, femoral neck, tibial shaft, and metatarsals. Delayed diagnosis and the subsequent duration of treatment required for adequate healing can result in significant morbidity. In a 2009 to 2012 study of US military members, Waterman and colleagues6 found an incidence rate of 5.69 stress fractures per 1000 person-years. Fractures most frequently involved the tibia/fibula (2.26/1000), followed by the metatarsals (0.92/1000) and the femoral neck (0.49/1000).6 In addition, these injuries were most commonly encountered in new recruits, who were less accustomed to the high-volume, high-intensity training required during basic training.4,7 Enlisted junior service members have been reported to account for 77.5% of all stress fractures.6 Age under 20 years or over 40 years and white race have also been found to be risk factors for stress injury.6
The pathogenesis of stress injury is controversial. Stanitski and colleagues8 theorized that multiple submaximal mechanical insults create cumulative stress greater than bone capacity, eventually leading to fracture. Johnson9 conducted a biopsy study and postulated that an accelerated remodeling phase was responsible, whereas Friedenberg10 argued that stress injuries are a form of reduced healing, not an attempt to increase healing, caused by the absence of callous formation in the disease process.
Various other nonmodifiable and modifiable risk factors predispose military service members to stress injury. Nonmodifiable risk factors include sex, bone geometry, limb alignment, race, age, and anatomy. Lower extremity movement biomechanics resulting from dynamic limb alignment during activity may be important. Cameron and colleagues11 examined 1843 patients and found that those with knees in >5° of valgus or >5° of external rotation had higher injury rates. Although variables such as sex and limb alignment cannot be changed, proper identification of modifiable risk factors can assist with injury prevention, and nonmodifiable risk factors can help clinicians and researchers target injury prevention interventions to patients at highest risk.
Metabolic, hormonal, and nutritional status is crucial to overall bone health. Multiple studies have found that low body mass index (BMI) is a significant risk factor for stress fracture.7,12,13 Although low BMI is a concern, patients with abnormally high BMI may also be at increased risk for bone stress injury. In a recently released consensus statement on relative energy deficiency in sport (RED-S), the International Olympic Committee addressed the complex interplay of impairments in physiologic function—including metabolic rate, menstrual function, bone health, immunity, protein synthesis, and cardiovascular health—caused by relative energy deficiency.14 The committee stated that the cause of this syndrome is energy deficiency relative to the balance between dietary energy intake and energy expenditure required for health and activities of daily living, growth, and sporting activities. This finding reveals that conditions such as stress injury often may represent a much broader systemic deficit that may be influenced by a patient’s overall physiologic imbalance.
Diagnosis
History and Physical Examination
The onset of stress reaction typically is insidious, with the classic presentation being a new military recruit who is experiencing a sudden increase in pain during physical activity.15 Pain typically is initially present only during activity, and is relieved with rest, but with disease progression this evolves to pain at rest. It is crucial that the physician elicit the patient’s history of training and physical activity. Hsu and colleagues7 reported increased prevalence of overweight civilian recruits, indicating an increase in the number of new recruits having limited experience with the repetitive physical activity encountered in basic training. Stress injury should be suspected in the setting of worsening, indolent lower extremity pain that has been present for several days, especially in the higher-risk patient populations mentioned. Diet should be assessed, with specific attention given to the intake of fruits, vegetables, and foods high in vitamin D and calcium and, most important, the energy balance between intake and output.16 Special attention should also be given to female patients, who may experience the female athlete triad, a spectrum of low energy availability, menstrual dysfunction, and impaired bone turnover (high amount of resorption relative to formation). A key part of the RED-S consensus statement14 alerted healthcare providers that metabolic derangements do not solely affect female patients. These types of patients sustain a major insult to the homeostatic balance of the hormones that sustain adequate bone health. Beck and colleagues17 found that women with disrupted menstrual cycles are 2 to 4 times more likely to sustain a stress fracture than women without disrupted menstrual cycles, making this abnormality an important part of the history.
Examination should begin with careful evaluation of limb alignment and specific attention given to varus or valgus alignment of the knees.11 The feet should also be inspected, as pes planus or cavus foot may increase the risk of stress fracture.18 Identification of the area of maximal tenderness is important. The area in question may also be erythematous or warm secondary to the inflammatory response associated with attempted fracture healing. In chronic fractures in superficial areas such as the metatarsals, callus may be palpable. Although there are few specific tests for stress injury, pain may be reproducible with deep palpation and WB.
Laboratory Testing
When a pathology is thought to have a nutritional or metabolic cause, particularly in a low-weight or underweight patient, a laboratory workup should be obtained. Specific laboratory tests that all patients should undergo are 25-hydroxyvitamin D3, complete blood cell count, and basic chemistry panel, including calcium and thyroid-stimulating hormone levels. Although not necessary for diagnosis, phosphate, parathyroid hormone, albumin, and prealbumin should also be considered. Females should undergo testing of follicle stimulating hormone, luteinizing hormone, estradiol, and testosterone and have a urine pregnancy test. In patients with signs of excessive cortisone, a dexamethasone suppression test can be administered.21 In males, low testosterone is a documented risk factor for stress injury.22
Imaging
Given their low cost and availability, plain radiographs typically are used for initial examination of a suspected stress injury. However, they often lack sensitivity, particularly in the early stages of stress fracture development (Figure 2).
Management
Management of bone stress injury depends on many factors, including symptom duration, fracture location and severity, and risk of progression or nonunion (Table).13
Pelvis
Pelvic stress fractures are rare and represent only 1.6% to 7.1% of all stress fractures.13,27,28 Given the low frequency, physicians must have a high index of suspicion to make the correct diagnosis. These fractures typically occur in marathon runners and other patients who present with persistent pain and a history of high levels of activity. As pelvic stress fractures typically involve the superior or inferior pubic rami, or sacrum, and are at low risk for nonunion,13 most are managed with nonoperative treatment and activity modification for 8 to 12 weeks.27
Femur
Femoral stress fractures are also relatively uncommon, accounting for about 10% of all stress fractures. Depending on their location, these fractures can be at high risk for progression, nonunion, and significant morbidity.29 Especially concerning are femoral neck stress fractures, which can involve either the tension side (lateral cortex) or the compression side (medial cortex) of the bone. Suspicion of a femoral neck stress fracture should prompt immediate NWB.5 Early recognition of these injuries is crucial because once displacement occurs, their complication and morbidity rates become high.13 Patients with compression-side fractures should undergo NWB treatment for 4 to 6 weeks and then slow progression to WB activity. Most return to light-impact activity by 3 to 4 months. By contrast, tension-side fractures are less likely to heal without operative intervention.11 All tension-side fractures (and any compression-side fractures >50% of the width of the femoral neck) should be treated with percutaneous placement of cannulated screws (Figure 3).
Stress fractures of the femoral shaft are less common than those of the femoral neck and represent as little as 3% of all stress fractures.32 However, femoral shaft stress fractures are more common in military populations. In French military recruits, Niva and colleagues33 found an 18% incidence. Similar to femoral neck fractures, femoral shaft fractures typically are diagnosed with advanced imaging, though the fulcrum test and pain on WB can aid in the diagnosis.19 These injuries are often managed nonoperatively with NWB for a period. Weishaar and colleagues34 described US military cadets treated with progressive rehabilitation who returned to full activity within 12 weeks. Displaced femoral shaft fractures associated with bone stress injury are even less common, and should be managed operatively. Salminen and colleagues35 found an incidence of 1.5 fractures per 100,000 years of military service. Over a 20-year period, they surgically treated 10 of these fractures. Average time from intramedullary nailing to union was 3.5 months.
Tibia
The tibia is one of the more common locations for stress injury and fracture. In a prospective study with members of the military, Giladi and colleagues36 found that 71% of stress fractures were tibia fractures. In addition, a large study of 320 athletes with stress fractures found 49.1% in the tibia.37 Fractures typically are diaphyseal and transverse, usually occurring along the posteromedial cortex, where the bone experiences maximal compressive forces (Figure 4).5,13
Compression-side fractures often heal with nonoperative management, though healing may take several months. Swenson and colleagues40 studied the effects of pneumatic bracing on conservative management and return to play in athletes with tibial stress fractures. Patients with bracing returned to light activity within 7 days and full activity within 21 days, whereas those without bracing returned to light activity within 21 days and full activity within 77 days. Pulsed electromagnetic therapy is of controversial benefit in the management of these injuries. Rettig
Metatarsals
Stress fractures were first discovered by Briethaupt1 in the painful swollen feet of Prussian army members in 1855 and were initially named march fractures. Waterman and colleagues6 reported that metatarsal stress fractures accounted for 16% of all stress fractures in the US military between 2009 and 2012. The second metatarsal neck is the most common location for stress fractures, followed by the third and fourth metatarsals, with the fifth metatarsal being the least common.5 The second metatarsal is thought to sustain these injuries more often than the other metatarsals because of its relative lack of immobility. Donahue and Sharkey43 found that the dorsal aspect of the second metatarsal experiences twice the amount of strain experienced by the fifth metatarsal during gait, and that peak strain in the second metatarsal was further increased by simulated muscle fatigue. The risk of stress fracture can be additionally increased with use of minimalist footwear, as shown by Giuliani and colleagues,44 particularly in the absence of a progressive transition in gait and training volume with a change toward minimalist footwear. In patients with a suspected or confirmed fracture of the second, third, or fourth metatarsal, treatment typically is NWB and immobilization for at least 4 weeks.5 Fifth metatarsal stress injuries (Figure 2) typically are treated differently because of their higher risk of nonunion. Patients with a fifth metatarsal stress fracture complain of lateral midfoot pain with running and jumping. For those who present with this fracture early, acceptable treatment consists of 6 weeks of casting and NWB.5 In cases of failed nonoperative therapy, or presentation with radiographic evidence of nonunion, treatment should be intramedullary screw fixation, with bone graft supplementation based on surgeon preference. DeLee and colleagues45 reported on the results of 10 athletes with fifth metatarsal stress fractures treated with intramedullary screw fixation without bone grafting. All 10 experienced fracture union, at a mean of 7.5 weeks, and returned to sport within 8.5 weeks. One complication with this procedure is pain at the screw insertion site, but this can be successfully managed with footwear modification.45
Prevention
Proper identification of patients at high risk for stress injuries has the potential of reducing the incidence of these injuries. Lappe and colleagues46 prospectively examined female army recruits before and after 8 weeks of basic training and found that those who developed a stress fracture were more likely to have a smoking history, to drink more than 10 alcoholic beverages a week, to have a history of corticosteroid or depot medroxyprogesterone use, and to have lower body weight. In addition, the authors found that a history of prolonged exercise before enrollment was protective against fracture. This finding identifies the importance of having new recruits undergo risk factor screening, which could result in adjusting training regimens to try to reduce injury. The RED-S consensus statement14 offers a comprehensive description of the physiologic factors that can contribute to such injury. Similar to proper risk factor identification, implementation of proper exercise progression programs is a simple, modifiable method of limiting stress injuries. For new recruits or athletes who are resuming activity, injury can be effectively prevented by adjusting the frequency, duration, and intensity of training and the training loads used.47
Vitamin D and calcium supplementation is a simple intervention that can be helpful in injury prevention, and its use has very little downside. A double-blind study found a 20% lower incidence of stress fracture in female navy recruits who took 2000 mg of calcium and 800 IU of vitamin D as daily supplemention.48 Of importance, a meta-analysis of more than 65,000 patients found vitamin D supplementation was effective in reducing fracture risk only when combined with calcium, irrespective of age, sex, or prior fracture.49 In female patients with the female athlete triad, psychological counseling and nutritional consultation are essential in bone health maintenance and long-term prevention.50 Other therapies have been evaluated as well. Use of bisphosphonates is controversial for both treatment and prevention of stress fractures. In a randomized, double-blind study of the potential prophylactic effects of risedronate in 324 new infantry recruits, Milgrom and colleagues51 found no statistically significant differences in tibial, femoral, metatarsal, or total stress fracture incidence between the treatment and placebo groups. Therefore, bisphosphonates are seldom recommended as prevention or in primary management of stress fracture.
In addition to nutritional and pharmacologic therapy, activity modification may have a role in injury prevention. Gait retraining has been identified as a potential intervention for reducing stress fractures in patients with poor biomechanics.47 Crowell and Davis52 investigated the effect of gait retraining on the forces operating in the tibia in runners. After 1 month of gait retraining, tibial acceleration while running decreased by 50%, vertical force loading rate by 30%, and peak vertical force impact by 20%. Such studies indicate the importance of proper mechanics during repetitive activity, especially in patients not as accustomed to the rigorous training methods used with new military recruits. However, whether these reduced loads translate into reduced risk of stress fracture remains unclear. In addition, biomechanical shoe orthoses may lower the stress fracture risk in military recruits by reducing peak tibial strain.53 Warden and colleagues54 found a mechanical loading program was effective in enchaining the structural properties of bone in rats, leading the authors to hypothesize that a similar program aimed at modifying bone structure in humans could help prevent stress fracture. Although there have been no studies of such a strategy in humans, pretraining may be an area for future research, especially for military recruits.
Conclusion
Compared with the general population, members of the military (new recruits in particular) are at increased risk for bone stress injuries. Most of these injuries occur during basic training, when recruits significantly increase their repetitive physical activity. Although the exact pathophysiology of stress injury is debated, nutritional and metabolic abnormalities are contributors. The indolent nature of these injuries, and their high rate of false-negative plain radiographs, may result in a significant delay in diagnosis in the absence of advanced imaging studies. Although a majority of injuries heal with nonoperative management and NWB, several patterns, especially those on the tension side of the bone, are at high risk for progression to fracture and nonunion. These include lateral femoral cortex stress injuries and anterior tibial cortex fractures. There should be a low threshold for operative management in the setting of delayed union or failed nonoperative therapy. Of equal importance to orthopedic management of these injuries is the management of underlying systemic deficits, which may have subjected the patient to injury in the first place. Supplementation with vitamin D and calcium can be an important prophylaxis against stress injury. In addition, military recruits and athletes with underlying metabolic or hormonal deficiencies should receive proper attention with a focus on balancing energy intake and energy expenditure. Stress injury leading to fracture—increasingly common in military populations—often requires a multimodal approach for treatment and subsequent prevention.
Am J Orthop. 2017;46(4):176-183. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Briethaupt MD. Zur Pathologie des menschlichen Fusses [To the pathology of the human foot]. Med Zeitung. 1855;24:169-177.
2. Berger FH, de Jonge MC, Maas M. Stress fractures in the lower extremity. Eur J Radiol. 2007;62(1):16-26.
3. Almeida SA, Williams KM, Shaffer RA, Brodine SK. Epidemiological patterns of musculoskeletal injuries and physical training. Med Sci Sports Exerc. 1999;31(8):1176-1182.
4. Jones BH, Thacker SB, Gilchrist J, Kimsey CD, Sosin DM. Prevention of lower extremity stress fractures in athletes and soldiers: a systematic review. Epidemiol Rev. 2002;24(2):228-247.
5. Jacobs JM, Cameron KL, Bojescul JA. Lower extremity stress fractures in the military. Clin Sports Med. 2014;33(4):591-613.
6. Waterman BR, Gun B, Bader JO, Orr JD, Belmont PJ. Epidemiology of lower extremity stress fractures in the United States military. Mil Med. 2016;181(10):1308-1313.
7. Hsu LL, Nevin RL, Tobler SK, Rubertone MV. Trends in overweight and obesity among 18-year-old applicants to the United States military, 1993–2006. J Adolesc Health. 2007;41(6):610-612.
8. Stanitski CL, McMaster JH, Scranton PE. On the nature of stress fractures. Am J Sports Med. 1978;6(6):391-396.
9. Johnson LC. Histogenesis of stress fractures [annual lecture]. Washington, DC: Armed Forces Institute of Pathology; 1963.
10. Friedenberg ZB. Fatigue fractures of the tibia. Clin Orthop Relat Res. 1971;(76):111-115.
11. Cameron KL, Peck KY, Owens BD, et al. Biomechanical risk factors for lower extremity stress fracture. Orthop J Sports Med. 2013;1(4 suppl).
12. Knapik J, Montain S, McGraw S, Grier T, Ely M, Jones B. Stress fracture risk factors in basic combat training. Int J Sports Med. 2012;33(11):940-946.
13. Behrens SB, Deren ME, Matson A, Fadale PD, Monchik KO. Stress fractures of the pelvis and legs in athletes. Sports Health. 2013;5(2):165-174.
14. Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med. 2014;48(7):491-497.
15. Maitra RS, Johnson DL. Stress fractures. Clinical history and physical examination. Clin Sports Med. 1997;16(2):259-274.
16. Nieves JW, Melsop K, Curtis M, et al. Nutritional factors that influence change in bone density and stress fracture risk among young female cross-country runners. PM R. 2010;2(8):740-750.
17. Beck BR, Matheson GO, Bergman G, et al. Do capacitively coupled electric fields accelerate tibial stress fracture healing? Am J Sports Med. 2008;36(3):545-553.
18. Simkin A, Leichter I, Giladi M, Stein M, Milgrom C. Combined effect of foot arch structure and an orthotic device on stress fractures. Foot Ankle. 1989;10(1):25-29.
19. Johnson AW, Weiss CB, Wheeler DL. Stress fractures of the femoral shaft in athletes—more common than expected: a new clinical test. Am J Sports Med. 1994;22(2):248-256.
20. Clement D, Ammann W, Taunton J, et al. Exercise-induced stress injuries to the femur. Int J Sports Med. 1993;14(6):347-352.
21. Wood PJ, Barth JH, Freedman DB, Perry L, Sheridan B. Evidence for the low dose dexamethasone suppression test to screen for Cushing’s syndrome—recommendations for a protocol for biochemistry laboratories. Ann Clin Biochem. 1997;34(pt 3):222-229.
22. Bennell K, Matheson G, Meeuwisse W, Brukner P. Risk factors for stress fractures. Sports Med. 1999;28(2):91-122.
23. Prather JL, Nusynowitz ML, Snowdy HA, Hughes AD, McCartney WH, Bagg RJ. Scintigraphic findings in stress fractures. J Bone Joint Surg Am. 1977;59(7):869-874.
24. Arendt EA, Griffiths HJ. The use of MR imaging in the assessment and clinical management of stress reactions of bone in high-performance athletes. Clin Sports Med. 1997;16(2):291-306.
25. Boden BP, Osbahr DC. High-risk stress fractures: evaluation and treatment. J Am Acad Orthop Surg. 2000;8(6):344-353.
26. Gaeta M, Minutoli F, Scribano E, et al. CT and MR imaging findings in athletes with early tibial stress injuries: comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235(2):553-561.
27. Matheson GO, Clement DB, Mckenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. Am J Sports Med. 1987;15(1):46-58.
28. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
29. Noakes TD, Smith JA, Lindenberg G, Wills CE. Pelvic stress fractures in long distance runners. Am J Sports Med. 1985;13(2):120-123.
30. Neubauer T, Brand J, Lidder S, Krawany M. Stress fractures of the femoral neck in runners: a review. Res Sports Med. 2016;24(3):283-297.
31. Evans JT, Guyver PM, Kassam AM, Hubble MJW. Displaced femoral neck stress fractures in Royal Marine recruits—management and results of operative treatment. J R Nav Med Serv. 2012;98(2):3-5.
32. Orava S. Stress fractures. Br J Sports Med. 1980;14(1):40-44.
33. Niva MH, Kiuru MJ, Haataja R, Pihlajamäki HK. Fatigue injuries of the femur. J Bone Joint Surg Br. 2005;87(10):1385-1390.
34. Weishaar MD, McMillian DJ, Moore JH. Identification and management of 2 femoral shaft stress injuries. J Orthop Sports Phys Ther. 2005;35(10):665-673.
35. Salminen ST, Pihlajamäki HK, Visuri TI, Böstman OM. Displaced fatigue fractures of the femoral shaft. Clin Orthop Relat Res. 2003;(409):250-259.
36. Giladi M, Ahronson Z, Stein M, Danon YL, Milgrom C. Unusual distribution and onset of stress fractures in soldiers. Clin Orthop Relat Res. 1985;(192):142-146.
37. Matheson GO, Clement DB, Mckenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. Am J Sports Med. 1987;15(1):46-58.
38. Green NE, Rogers RA, Lipscomb AB. Nonunions of stress fractures of the tibia. Am J Sports Med. 1985;13(3):171-176.
39. Orava S, Hulkko A. Stress fracture of the mid-tibial shaft. Acta Orthop Scand. 1984;55(1):35-37.
40. Swenson EJ Jr, DeHaven KE, Sebastianelli WJ, Hanks G, Kalenak A, Lynch JM. The effect of a pneumatic leg brace on return to play in athletes with tibial stress fractures. Am J Sports Med. 1997;25(3):322-328.
41. Rettig AC, Shelbourne KD, McCarroll JR, Bisesi M, Watts J. The natural history and treatment of delayed union stress fractures of the anterior cortex of the tibia. Am J Sports Med. 1988;16(3):250-255.
42. Varner KE, Younas SA, Lintner DM, Marymont JV. Chronic anterior midtibial stress fractures in athletes treated with reamed intramedullary nailing. Am J Sports Med. 2005;33(7):1071-1076.
43. Donahue SW, Sharkey NA. Strains in the metatarsals during the stance phase of gait: implications for stress fractures. J Bone Joint Surg Am. 1999;81(9):1236-1244.
44. Giuliani J, Masini B, Alitz C, Owens BD. Barefoot-simulating footwear associated with metatarsal stress injury in 2 runners. Orthopedics. 2011;34(7):e320-e323.
45. DeLee JC, Evans JP, Julian J. Stress fracture of the fifth metatarsal. Am J Sports Med. 1983;11(5):349-353.
46. Lappe JM, Stegman MR, Recker RR. The impact of lifestyle factors on stress fractures in female army recruits. Osteoporos Int. 2001;12(1):35-42.
47. Friedl KE, Evans RK, Moran DS. Stress fracture and military medical readiness: bridging basic and applied research. Med Sci Sports Exerc. 2008;40(11 suppl):S609-S622.
48. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23(5):741-749.
49. DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group. Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ. 2010;340:b5463.
50. Duckham RL, Peirce N, Meyer C, Summers GD, Cameron N, Brooke-Wavell K. Risk factors for stress fracture in female endurance athletes: a cross-sectional study. BMJ Open. 2012;2(6).
51. Milgrom C, Finestone A, Novack V, et al. The effect of prophylactic treatment with risedronate on stress fracture incidence among infantry recruits. Bone. 2004;35(2):418-424.
52. Crowell HP, Davis IS. Gait retraining to reduce lower extremity loading in runners. Clin Biomech. 2011;26(1):78-83.
53. Ekenman I, Milgrom C, Finestone A, et al. The role of biomechanical shoe orthoses in tibial stress fracture prevention. Am J Sports Med. 2002;30(6):866-870.
54. Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res. 2005;20(5):809-816.
Take-Home Points
- Stress injuries, specifically of the lower extremity, are very common in new military trainees.
- Stress injury can range from benign periosteal reaction to displaced fracture.
- Stress injury should be treated on a case-by-case basis, depending on the severity of injury, the location of the injury, and the likelihood of healing with nonoperative management.
- Modifiable risk factors such as nutritional status, training regiment, and even footwear should be investigated to determine potential causes of injury.
- Prevention is a crucial part of the treatment of these injuries, and early intervention such as careful pre-enrollment physicals and vitamin supplementation can be essential in lowering injury rates.
Bone stress injuries, which are common in military recruits, present in weight-bearing (WB) areas as indolent pain caused by repetitive stress and microtrauma. They were first reported in the metatarsals of Prussian soldiers in 1855.1 Today, stress injuries are increasingly common. One study estimated they account for 10% of patients seen by sports medicine practitioners.2 This injury most commonly affects military members, endurance athletes, and dancers.3-5 Specifically, the incidence of stress fractures in military members has been reported to range from 0.8% to 6.9% for men and from 3.4% to 21.0% for women.4 Because of repetitive vigorous lower extremity loading, stress fractures typically occur in the pelvis, femoral neck, tibial shaft, and metatarsals. Delayed diagnosis and the subsequent duration of treatment required for adequate healing can result in significant morbidity. In a 2009 to 2012 study of US military members, Waterman and colleagues6 found an incidence rate of 5.69 stress fractures per 1000 person-years. Fractures most frequently involved the tibia/fibula (2.26/1000), followed by the metatarsals (0.92/1000) and the femoral neck (0.49/1000).6 In addition, these injuries were most commonly encountered in new recruits, who were less accustomed to the high-volume, high-intensity training required during basic training.4,7 Enlisted junior service members have been reported to account for 77.5% of all stress fractures.6 Age under 20 years or over 40 years and white race have also been found to be risk factors for stress injury.6
The pathogenesis of stress injury is controversial. Stanitski and colleagues8 theorized that multiple submaximal mechanical insults create cumulative stress greater than bone capacity, eventually leading to fracture. Johnson9 conducted a biopsy study and postulated that an accelerated remodeling phase was responsible, whereas Friedenberg10 argued that stress injuries are a form of reduced healing, not an attempt to increase healing, caused by the absence of callous formation in the disease process.
Various other nonmodifiable and modifiable risk factors predispose military service members to stress injury. Nonmodifiable risk factors include sex, bone geometry, limb alignment, race, age, and anatomy. Lower extremity movement biomechanics resulting from dynamic limb alignment during activity may be important. Cameron and colleagues11 examined 1843 patients and found that those with knees in >5° of valgus or >5° of external rotation had higher injury rates. Although variables such as sex and limb alignment cannot be changed, proper identification of modifiable risk factors can assist with injury prevention, and nonmodifiable risk factors can help clinicians and researchers target injury prevention interventions to patients at highest risk.
Metabolic, hormonal, and nutritional status is crucial to overall bone health. Multiple studies have found that low body mass index (BMI) is a significant risk factor for stress fracture.7,12,13 Although low BMI is a concern, patients with abnormally high BMI may also be at increased risk for bone stress injury. In a recently released consensus statement on relative energy deficiency in sport (RED-S), the International Olympic Committee addressed the complex interplay of impairments in physiologic function—including metabolic rate, menstrual function, bone health, immunity, protein synthesis, and cardiovascular health—caused by relative energy deficiency.14 The committee stated that the cause of this syndrome is energy deficiency relative to the balance between dietary energy intake and energy expenditure required for health and activities of daily living, growth, and sporting activities. This finding reveals that conditions such as stress injury often may represent a much broader systemic deficit that may be influenced by a patient’s overall physiologic imbalance.
Diagnosis
History and Physical Examination
The onset of stress reaction typically is insidious, with the classic presentation being a new military recruit who is experiencing a sudden increase in pain during physical activity.15 Pain typically is initially present only during activity, and is relieved with rest, but with disease progression this evolves to pain at rest. It is crucial that the physician elicit the patient’s history of training and physical activity. Hsu and colleagues7 reported increased prevalence of overweight civilian recruits, indicating an increase in the number of new recruits having limited experience with the repetitive physical activity encountered in basic training. Stress injury should be suspected in the setting of worsening, indolent lower extremity pain that has been present for several days, especially in the higher-risk patient populations mentioned. Diet should be assessed, with specific attention given to the intake of fruits, vegetables, and foods high in vitamin D and calcium and, most important, the energy balance between intake and output.16 Special attention should also be given to female patients, who may experience the female athlete triad, a spectrum of low energy availability, menstrual dysfunction, and impaired bone turnover (high amount of resorption relative to formation). A key part of the RED-S consensus statement14 alerted healthcare providers that metabolic derangements do not solely affect female patients. These types of patients sustain a major insult to the homeostatic balance of the hormones that sustain adequate bone health. Beck and colleagues17 found that women with disrupted menstrual cycles are 2 to 4 times more likely to sustain a stress fracture than women without disrupted menstrual cycles, making this abnormality an important part of the history.
Examination should begin with careful evaluation of limb alignment and specific attention given to varus or valgus alignment of the knees.11 The feet should also be inspected, as pes planus or cavus foot may increase the risk of stress fracture.18 Identification of the area of maximal tenderness is important. The area in question may also be erythematous or warm secondary to the inflammatory response associated with attempted fracture healing. In chronic fractures in superficial areas such as the metatarsals, callus may be palpable. Although there are few specific tests for stress injury, pain may be reproducible with deep palpation and WB.
Laboratory Testing
When a pathology is thought to have a nutritional or metabolic cause, particularly in a low-weight or underweight patient, a laboratory workup should be obtained. Specific laboratory tests that all patients should undergo are 25-hydroxyvitamin D3, complete blood cell count, and basic chemistry panel, including calcium and thyroid-stimulating hormone levels. Although not necessary for diagnosis, phosphate, parathyroid hormone, albumin, and prealbumin should also be considered. Females should undergo testing of follicle stimulating hormone, luteinizing hormone, estradiol, and testosterone and have a urine pregnancy test. In patients with signs of excessive cortisone, a dexamethasone suppression test can be administered.21 In males, low testosterone is a documented risk factor for stress injury.22
Imaging
Given their low cost and availability, plain radiographs typically are used for initial examination of a suspected stress injury. However, they often lack sensitivity, particularly in the early stages of stress fracture development (Figure 2).
Management
Management of bone stress injury depends on many factors, including symptom duration, fracture location and severity, and risk of progression or nonunion (Table).13
Pelvis
Pelvic stress fractures are rare and represent only 1.6% to 7.1% of all stress fractures.13,27,28 Given the low frequency, physicians must have a high index of suspicion to make the correct diagnosis. These fractures typically occur in marathon runners and other patients who present with persistent pain and a history of high levels of activity. As pelvic stress fractures typically involve the superior or inferior pubic rami, or sacrum, and are at low risk for nonunion,13 most are managed with nonoperative treatment and activity modification for 8 to 12 weeks.27
Femur
Femoral stress fractures are also relatively uncommon, accounting for about 10% of all stress fractures. Depending on their location, these fractures can be at high risk for progression, nonunion, and significant morbidity.29 Especially concerning are femoral neck stress fractures, which can involve either the tension side (lateral cortex) or the compression side (medial cortex) of the bone. Suspicion of a femoral neck stress fracture should prompt immediate NWB.5 Early recognition of these injuries is crucial because once displacement occurs, their complication and morbidity rates become high.13 Patients with compression-side fractures should undergo NWB treatment for 4 to 6 weeks and then slow progression to WB activity. Most return to light-impact activity by 3 to 4 months. By contrast, tension-side fractures are less likely to heal without operative intervention.11 All tension-side fractures (and any compression-side fractures >50% of the width of the femoral neck) should be treated with percutaneous placement of cannulated screws (Figure 3).
Stress fractures of the femoral shaft are less common than those of the femoral neck and represent as little as 3% of all stress fractures.32 However, femoral shaft stress fractures are more common in military populations. In French military recruits, Niva and colleagues33 found an 18% incidence. Similar to femoral neck fractures, femoral shaft fractures typically are diagnosed with advanced imaging, though the fulcrum test and pain on WB can aid in the diagnosis.19 These injuries are often managed nonoperatively with NWB for a period. Weishaar and colleagues34 described US military cadets treated with progressive rehabilitation who returned to full activity within 12 weeks. Displaced femoral shaft fractures associated with bone stress injury are even less common, and should be managed operatively. Salminen and colleagues35 found an incidence of 1.5 fractures per 100,000 years of military service. Over a 20-year period, they surgically treated 10 of these fractures. Average time from intramedullary nailing to union was 3.5 months.
Tibia
The tibia is one of the more common locations for stress injury and fracture. In a prospective study with members of the military, Giladi and colleagues36 found that 71% of stress fractures were tibia fractures. In addition, a large study of 320 athletes with stress fractures found 49.1% in the tibia.37 Fractures typically are diaphyseal and transverse, usually occurring along the posteromedial cortex, where the bone experiences maximal compressive forces (Figure 4).5,13
Compression-side fractures often heal with nonoperative management, though healing may take several months. Swenson and colleagues40 studied the effects of pneumatic bracing on conservative management and return to play in athletes with tibial stress fractures. Patients with bracing returned to light activity within 7 days and full activity within 21 days, whereas those without bracing returned to light activity within 21 days and full activity within 77 days. Pulsed electromagnetic therapy is of controversial benefit in the management of these injuries. Rettig
Metatarsals
Stress fractures were first discovered by Briethaupt1 in the painful swollen feet of Prussian army members in 1855 and were initially named march fractures. Waterman and colleagues6 reported that metatarsal stress fractures accounted for 16% of all stress fractures in the US military between 2009 and 2012. The second metatarsal neck is the most common location for stress fractures, followed by the third and fourth metatarsals, with the fifth metatarsal being the least common.5 The second metatarsal is thought to sustain these injuries more often than the other metatarsals because of its relative lack of immobility. Donahue and Sharkey43 found that the dorsal aspect of the second metatarsal experiences twice the amount of strain experienced by the fifth metatarsal during gait, and that peak strain in the second metatarsal was further increased by simulated muscle fatigue. The risk of stress fracture can be additionally increased with use of minimalist footwear, as shown by Giuliani and colleagues,44 particularly in the absence of a progressive transition in gait and training volume with a change toward minimalist footwear. In patients with a suspected or confirmed fracture of the second, third, or fourth metatarsal, treatment typically is NWB and immobilization for at least 4 weeks.5 Fifth metatarsal stress injuries (Figure 2) typically are treated differently because of their higher risk of nonunion. Patients with a fifth metatarsal stress fracture complain of lateral midfoot pain with running and jumping. For those who present with this fracture early, acceptable treatment consists of 6 weeks of casting and NWB.5 In cases of failed nonoperative therapy, or presentation with radiographic evidence of nonunion, treatment should be intramedullary screw fixation, with bone graft supplementation based on surgeon preference. DeLee and colleagues45 reported on the results of 10 athletes with fifth metatarsal stress fractures treated with intramedullary screw fixation without bone grafting. All 10 experienced fracture union, at a mean of 7.5 weeks, and returned to sport within 8.5 weeks. One complication with this procedure is pain at the screw insertion site, but this can be successfully managed with footwear modification.45
Prevention
Proper identification of patients at high risk for stress injuries has the potential of reducing the incidence of these injuries. Lappe and colleagues46 prospectively examined female army recruits before and after 8 weeks of basic training and found that those who developed a stress fracture were more likely to have a smoking history, to drink more than 10 alcoholic beverages a week, to have a history of corticosteroid or depot medroxyprogesterone use, and to have lower body weight. In addition, the authors found that a history of prolonged exercise before enrollment was protective against fracture. This finding identifies the importance of having new recruits undergo risk factor screening, which could result in adjusting training regimens to try to reduce injury. The RED-S consensus statement14 offers a comprehensive description of the physiologic factors that can contribute to such injury. Similar to proper risk factor identification, implementation of proper exercise progression programs is a simple, modifiable method of limiting stress injuries. For new recruits or athletes who are resuming activity, injury can be effectively prevented by adjusting the frequency, duration, and intensity of training and the training loads used.47
Vitamin D and calcium supplementation is a simple intervention that can be helpful in injury prevention, and its use has very little downside. A double-blind study found a 20% lower incidence of stress fracture in female navy recruits who took 2000 mg of calcium and 800 IU of vitamin D as daily supplemention.48 Of importance, a meta-analysis of more than 65,000 patients found vitamin D supplementation was effective in reducing fracture risk only when combined with calcium, irrespective of age, sex, or prior fracture.49 In female patients with the female athlete triad, psychological counseling and nutritional consultation are essential in bone health maintenance and long-term prevention.50 Other therapies have been evaluated as well. Use of bisphosphonates is controversial for both treatment and prevention of stress fractures. In a randomized, double-blind study of the potential prophylactic effects of risedronate in 324 new infantry recruits, Milgrom and colleagues51 found no statistically significant differences in tibial, femoral, metatarsal, or total stress fracture incidence between the treatment and placebo groups. Therefore, bisphosphonates are seldom recommended as prevention or in primary management of stress fracture.
In addition to nutritional and pharmacologic therapy, activity modification may have a role in injury prevention. Gait retraining has been identified as a potential intervention for reducing stress fractures in patients with poor biomechanics.47 Crowell and Davis52 investigated the effect of gait retraining on the forces operating in the tibia in runners. After 1 month of gait retraining, tibial acceleration while running decreased by 50%, vertical force loading rate by 30%, and peak vertical force impact by 20%. Such studies indicate the importance of proper mechanics during repetitive activity, especially in patients not as accustomed to the rigorous training methods used with new military recruits. However, whether these reduced loads translate into reduced risk of stress fracture remains unclear. In addition, biomechanical shoe orthoses may lower the stress fracture risk in military recruits by reducing peak tibial strain.53 Warden and colleagues54 found a mechanical loading program was effective in enchaining the structural properties of bone in rats, leading the authors to hypothesize that a similar program aimed at modifying bone structure in humans could help prevent stress fracture. Although there have been no studies of such a strategy in humans, pretraining may be an area for future research, especially for military recruits.
Conclusion
Compared with the general population, members of the military (new recruits in particular) are at increased risk for bone stress injuries. Most of these injuries occur during basic training, when recruits significantly increase their repetitive physical activity. Although the exact pathophysiology of stress injury is debated, nutritional and metabolic abnormalities are contributors. The indolent nature of these injuries, and their high rate of false-negative plain radiographs, may result in a significant delay in diagnosis in the absence of advanced imaging studies. Although a majority of injuries heal with nonoperative management and NWB, several patterns, especially those on the tension side of the bone, are at high risk for progression to fracture and nonunion. These include lateral femoral cortex stress injuries and anterior tibial cortex fractures. There should be a low threshold for operative management in the setting of delayed union or failed nonoperative therapy. Of equal importance to orthopedic management of these injuries is the management of underlying systemic deficits, which may have subjected the patient to injury in the first place. Supplementation with vitamin D and calcium can be an important prophylaxis against stress injury. In addition, military recruits and athletes with underlying metabolic or hormonal deficiencies should receive proper attention with a focus on balancing energy intake and energy expenditure. Stress injury leading to fracture—increasingly common in military populations—often requires a multimodal approach for treatment and subsequent prevention.
Am J Orthop. 2017;46(4):176-183. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Stress injuries, specifically of the lower extremity, are very common in new military trainees.
- Stress injury can range from benign periosteal reaction to displaced fracture.
- Stress injury should be treated on a case-by-case basis, depending on the severity of injury, the location of the injury, and the likelihood of healing with nonoperative management.
- Modifiable risk factors such as nutritional status, training regiment, and even footwear should be investigated to determine potential causes of injury.
- Prevention is a crucial part of the treatment of these injuries, and early intervention such as careful pre-enrollment physicals and vitamin supplementation can be essential in lowering injury rates.
Bone stress injuries, which are common in military recruits, present in weight-bearing (WB) areas as indolent pain caused by repetitive stress and microtrauma. They were first reported in the metatarsals of Prussian soldiers in 1855.1 Today, stress injuries are increasingly common. One study estimated they account for 10% of patients seen by sports medicine practitioners.2 This injury most commonly affects military members, endurance athletes, and dancers.3-5 Specifically, the incidence of stress fractures in military members has been reported to range from 0.8% to 6.9% for men and from 3.4% to 21.0% for women.4 Because of repetitive vigorous lower extremity loading, stress fractures typically occur in the pelvis, femoral neck, tibial shaft, and metatarsals. Delayed diagnosis and the subsequent duration of treatment required for adequate healing can result in significant morbidity. In a 2009 to 2012 study of US military members, Waterman and colleagues6 found an incidence rate of 5.69 stress fractures per 1000 person-years. Fractures most frequently involved the tibia/fibula (2.26/1000), followed by the metatarsals (0.92/1000) and the femoral neck (0.49/1000).6 In addition, these injuries were most commonly encountered in new recruits, who were less accustomed to the high-volume, high-intensity training required during basic training.4,7 Enlisted junior service members have been reported to account for 77.5% of all stress fractures.6 Age under 20 years or over 40 years and white race have also been found to be risk factors for stress injury.6
The pathogenesis of stress injury is controversial. Stanitski and colleagues8 theorized that multiple submaximal mechanical insults create cumulative stress greater than bone capacity, eventually leading to fracture. Johnson9 conducted a biopsy study and postulated that an accelerated remodeling phase was responsible, whereas Friedenberg10 argued that stress injuries are a form of reduced healing, not an attempt to increase healing, caused by the absence of callous formation in the disease process.
Various other nonmodifiable and modifiable risk factors predispose military service members to stress injury. Nonmodifiable risk factors include sex, bone geometry, limb alignment, race, age, and anatomy. Lower extremity movement biomechanics resulting from dynamic limb alignment during activity may be important. Cameron and colleagues11 examined 1843 patients and found that those with knees in >5° of valgus or >5° of external rotation had higher injury rates. Although variables such as sex and limb alignment cannot be changed, proper identification of modifiable risk factors can assist with injury prevention, and nonmodifiable risk factors can help clinicians and researchers target injury prevention interventions to patients at highest risk.
Metabolic, hormonal, and nutritional status is crucial to overall bone health. Multiple studies have found that low body mass index (BMI) is a significant risk factor for stress fracture.7,12,13 Although low BMI is a concern, patients with abnormally high BMI may also be at increased risk for bone stress injury. In a recently released consensus statement on relative energy deficiency in sport (RED-S), the International Olympic Committee addressed the complex interplay of impairments in physiologic function—including metabolic rate, menstrual function, bone health, immunity, protein synthesis, and cardiovascular health—caused by relative energy deficiency.14 The committee stated that the cause of this syndrome is energy deficiency relative to the balance between dietary energy intake and energy expenditure required for health and activities of daily living, growth, and sporting activities. This finding reveals that conditions such as stress injury often may represent a much broader systemic deficit that may be influenced by a patient’s overall physiologic imbalance.
Diagnosis
History and Physical Examination
The onset of stress reaction typically is insidious, with the classic presentation being a new military recruit who is experiencing a sudden increase in pain during physical activity.15 Pain typically is initially present only during activity, and is relieved with rest, but with disease progression this evolves to pain at rest. It is crucial that the physician elicit the patient’s history of training and physical activity. Hsu and colleagues7 reported increased prevalence of overweight civilian recruits, indicating an increase in the number of new recruits having limited experience with the repetitive physical activity encountered in basic training. Stress injury should be suspected in the setting of worsening, indolent lower extremity pain that has been present for several days, especially in the higher-risk patient populations mentioned. Diet should be assessed, with specific attention given to the intake of fruits, vegetables, and foods high in vitamin D and calcium and, most important, the energy balance between intake and output.16 Special attention should also be given to female patients, who may experience the female athlete triad, a spectrum of low energy availability, menstrual dysfunction, and impaired bone turnover (high amount of resorption relative to formation). A key part of the RED-S consensus statement14 alerted healthcare providers that metabolic derangements do not solely affect female patients. These types of patients sustain a major insult to the homeostatic balance of the hormones that sustain adequate bone health. Beck and colleagues17 found that women with disrupted menstrual cycles are 2 to 4 times more likely to sustain a stress fracture than women without disrupted menstrual cycles, making this abnormality an important part of the history.
Examination should begin with careful evaluation of limb alignment and specific attention given to varus or valgus alignment of the knees.11 The feet should also be inspected, as pes planus or cavus foot may increase the risk of stress fracture.18 Identification of the area of maximal tenderness is important. The area in question may also be erythematous or warm secondary to the inflammatory response associated with attempted fracture healing. In chronic fractures in superficial areas such as the metatarsals, callus may be palpable. Although there are few specific tests for stress injury, pain may be reproducible with deep palpation and WB.
Laboratory Testing
When a pathology is thought to have a nutritional or metabolic cause, particularly in a low-weight or underweight patient, a laboratory workup should be obtained. Specific laboratory tests that all patients should undergo are 25-hydroxyvitamin D3, complete blood cell count, and basic chemistry panel, including calcium and thyroid-stimulating hormone levels. Although not necessary for diagnosis, phosphate, parathyroid hormone, albumin, and prealbumin should also be considered. Females should undergo testing of follicle stimulating hormone, luteinizing hormone, estradiol, and testosterone and have a urine pregnancy test. In patients with signs of excessive cortisone, a dexamethasone suppression test can be administered.21 In males, low testosterone is a documented risk factor for stress injury.22
Imaging
Given their low cost and availability, plain radiographs typically are used for initial examination of a suspected stress injury. However, they often lack sensitivity, particularly in the early stages of stress fracture development (Figure 2).
Management
Management of bone stress injury depends on many factors, including symptom duration, fracture location and severity, and risk of progression or nonunion (Table).13
Pelvis
Pelvic stress fractures are rare and represent only 1.6% to 7.1% of all stress fractures.13,27,28 Given the low frequency, physicians must have a high index of suspicion to make the correct diagnosis. These fractures typically occur in marathon runners and other patients who present with persistent pain and a history of high levels of activity. As pelvic stress fractures typically involve the superior or inferior pubic rami, or sacrum, and are at low risk for nonunion,13 most are managed with nonoperative treatment and activity modification for 8 to 12 weeks.27
Femur
Femoral stress fractures are also relatively uncommon, accounting for about 10% of all stress fractures. Depending on their location, these fractures can be at high risk for progression, nonunion, and significant morbidity.29 Especially concerning are femoral neck stress fractures, which can involve either the tension side (lateral cortex) or the compression side (medial cortex) of the bone. Suspicion of a femoral neck stress fracture should prompt immediate NWB.5 Early recognition of these injuries is crucial because once displacement occurs, their complication and morbidity rates become high.13 Patients with compression-side fractures should undergo NWB treatment for 4 to 6 weeks and then slow progression to WB activity. Most return to light-impact activity by 3 to 4 months. By contrast, tension-side fractures are less likely to heal without operative intervention.11 All tension-side fractures (and any compression-side fractures >50% of the width of the femoral neck) should be treated with percutaneous placement of cannulated screws (Figure 3).
Stress fractures of the femoral shaft are less common than those of the femoral neck and represent as little as 3% of all stress fractures.32 However, femoral shaft stress fractures are more common in military populations. In French military recruits, Niva and colleagues33 found an 18% incidence. Similar to femoral neck fractures, femoral shaft fractures typically are diagnosed with advanced imaging, though the fulcrum test and pain on WB can aid in the diagnosis.19 These injuries are often managed nonoperatively with NWB for a period. Weishaar and colleagues34 described US military cadets treated with progressive rehabilitation who returned to full activity within 12 weeks. Displaced femoral shaft fractures associated with bone stress injury are even less common, and should be managed operatively. Salminen and colleagues35 found an incidence of 1.5 fractures per 100,000 years of military service. Over a 20-year period, they surgically treated 10 of these fractures. Average time from intramedullary nailing to union was 3.5 months.
Tibia
The tibia is one of the more common locations for stress injury and fracture. In a prospective study with members of the military, Giladi and colleagues36 found that 71% of stress fractures were tibia fractures. In addition, a large study of 320 athletes with stress fractures found 49.1% in the tibia.37 Fractures typically are diaphyseal and transverse, usually occurring along the posteromedial cortex, where the bone experiences maximal compressive forces (Figure 4).5,13
Compression-side fractures often heal with nonoperative management, though healing may take several months. Swenson and colleagues40 studied the effects of pneumatic bracing on conservative management and return to play in athletes with tibial stress fractures. Patients with bracing returned to light activity within 7 days and full activity within 21 days, whereas those without bracing returned to light activity within 21 days and full activity within 77 days. Pulsed electromagnetic therapy is of controversial benefit in the management of these injuries. Rettig
Metatarsals
Stress fractures were first discovered by Briethaupt1 in the painful swollen feet of Prussian army members in 1855 and were initially named march fractures. Waterman and colleagues6 reported that metatarsal stress fractures accounted for 16% of all stress fractures in the US military between 2009 and 2012. The second metatarsal neck is the most common location for stress fractures, followed by the third and fourth metatarsals, with the fifth metatarsal being the least common.5 The second metatarsal is thought to sustain these injuries more often than the other metatarsals because of its relative lack of immobility. Donahue and Sharkey43 found that the dorsal aspect of the second metatarsal experiences twice the amount of strain experienced by the fifth metatarsal during gait, and that peak strain in the second metatarsal was further increased by simulated muscle fatigue. The risk of stress fracture can be additionally increased with use of minimalist footwear, as shown by Giuliani and colleagues,44 particularly in the absence of a progressive transition in gait and training volume with a change toward minimalist footwear. In patients with a suspected or confirmed fracture of the second, third, or fourth metatarsal, treatment typically is NWB and immobilization for at least 4 weeks.5 Fifth metatarsal stress injuries (Figure 2) typically are treated differently because of their higher risk of nonunion. Patients with a fifth metatarsal stress fracture complain of lateral midfoot pain with running and jumping. For those who present with this fracture early, acceptable treatment consists of 6 weeks of casting and NWB.5 In cases of failed nonoperative therapy, or presentation with radiographic evidence of nonunion, treatment should be intramedullary screw fixation, with bone graft supplementation based on surgeon preference. DeLee and colleagues45 reported on the results of 10 athletes with fifth metatarsal stress fractures treated with intramedullary screw fixation without bone grafting. All 10 experienced fracture union, at a mean of 7.5 weeks, and returned to sport within 8.5 weeks. One complication with this procedure is pain at the screw insertion site, but this can be successfully managed with footwear modification.45
Prevention
Proper identification of patients at high risk for stress injuries has the potential of reducing the incidence of these injuries. Lappe and colleagues46 prospectively examined female army recruits before and after 8 weeks of basic training and found that those who developed a stress fracture were more likely to have a smoking history, to drink more than 10 alcoholic beverages a week, to have a history of corticosteroid or depot medroxyprogesterone use, and to have lower body weight. In addition, the authors found that a history of prolonged exercise before enrollment was protective against fracture. This finding identifies the importance of having new recruits undergo risk factor screening, which could result in adjusting training regimens to try to reduce injury. The RED-S consensus statement14 offers a comprehensive description of the physiologic factors that can contribute to such injury. Similar to proper risk factor identification, implementation of proper exercise progression programs is a simple, modifiable method of limiting stress injuries. For new recruits or athletes who are resuming activity, injury can be effectively prevented by adjusting the frequency, duration, and intensity of training and the training loads used.47
Vitamin D and calcium supplementation is a simple intervention that can be helpful in injury prevention, and its use has very little downside. A double-blind study found a 20% lower incidence of stress fracture in female navy recruits who took 2000 mg of calcium and 800 IU of vitamin D as daily supplemention.48 Of importance, a meta-analysis of more than 65,000 patients found vitamin D supplementation was effective in reducing fracture risk only when combined with calcium, irrespective of age, sex, or prior fracture.49 In female patients with the female athlete triad, psychological counseling and nutritional consultation are essential in bone health maintenance and long-term prevention.50 Other therapies have been evaluated as well. Use of bisphosphonates is controversial for both treatment and prevention of stress fractures. In a randomized, double-blind study of the potential prophylactic effects of risedronate in 324 new infantry recruits, Milgrom and colleagues51 found no statistically significant differences in tibial, femoral, metatarsal, or total stress fracture incidence between the treatment and placebo groups. Therefore, bisphosphonates are seldom recommended as prevention or in primary management of stress fracture.
In addition to nutritional and pharmacologic therapy, activity modification may have a role in injury prevention. Gait retraining has been identified as a potential intervention for reducing stress fractures in patients with poor biomechanics.47 Crowell and Davis52 investigated the effect of gait retraining on the forces operating in the tibia in runners. After 1 month of gait retraining, tibial acceleration while running decreased by 50%, vertical force loading rate by 30%, and peak vertical force impact by 20%. Such studies indicate the importance of proper mechanics during repetitive activity, especially in patients not as accustomed to the rigorous training methods used with new military recruits. However, whether these reduced loads translate into reduced risk of stress fracture remains unclear. In addition, biomechanical shoe orthoses may lower the stress fracture risk in military recruits by reducing peak tibial strain.53 Warden and colleagues54 found a mechanical loading program was effective in enchaining the structural properties of bone in rats, leading the authors to hypothesize that a similar program aimed at modifying bone structure in humans could help prevent stress fracture. Although there have been no studies of such a strategy in humans, pretraining may be an area for future research, especially for military recruits.
Conclusion
Compared with the general population, members of the military (new recruits in particular) are at increased risk for bone stress injuries. Most of these injuries occur during basic training, when recruits significantly increase their repetitive physical activity. Although the exact pathophysiology of stress injury is debated, nutritional and metabolic abnormalities are contributors. The indolent nature of these injuries, and their high rate of false-negative plain radiographs, may result in a significant delay in diagnosis in the absence of advanced imaging studies. Although a majority of injuries heal with nonoperative management and NWB, several patterns, especially those on the tension side of the bone, are at high risk for progression to fracture and nonunion. These include lateral femoral cortex stress injuries and anterior tibial cortex fractures. There should be a low threshold for operative management in the setting of delayed union or failed nonoperative therapy. Of equal importance to orthopedic management of these injuries is the management of underlying systemic deficits, which may have subjected the patient to injury in the first place. Supplementation with vitamin D and calcium can be an important prophylaxis against stress injury. In addition, military recruits and athletes with underlying metabolic or hormonal deficiencies should receive proper attention with a focus on balancing energy intake and energy expenditure. Stress injury leading to fracture—increasingly common in military populations—often requires a multimodal approach for treatment and subsequent prevention.
Am J Orthop. 2017;46(4):176-183. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Briethaupt MD. Zur Pathologie des menschlichen Fusses [To the pathology of the human foot]. Med Zeitung. 1855;24:169-177.
2. Berger FH, de Jonge MC, Maas M. Stress fractures in the lower extremity. Eur J Radiol. 2007;62(1):16-26.
3. Almeida SA, Williams KM, Shaffer RA, Brodine SK. Epidemiological patterns of musculoskeletal injuries and physical training. Med Sci Sports Exerc. 1999;31(8):1176-1182.
4. Jones BH, Thacker SB, Gilchrist J, Kimsey CD, Sosin DM. Prevention of lower extremity stress fractures in athletes and soldiers: a systematic review. Epidemiol Rev. 2002;24(2):228-247.
5. Jacobs JM, Cameron KL, Bojescul JA. Lower extremity stress fractures in the military. Clin Sports Med. 2014;33(4):591-613.
6. Waterman BR, Gun B, Bader JO, Orr JD, Belmont PJ. Epidemiology of lower extremity stress fractures in the United States military. Mil Med. 2016;181(10):1308-1313.
7. Hsu LL, Nevin RL, Tobler SK, Rubertone MV. Trends in overweight and obesity among 18-year-old applicants to the United States military, 1993–2006. J Adolesc Health. 2007;41(6):610-612.
8. Stanitski CL, McMaster JH, Scranton PE. On the nature of stress fractures. Am J Sports Med. 1978;6(6):391-396.
9. Johnson LC. Histogenesis of stress fractures [annual lecture]. Washington, DC: Armed Forces Institute of Pathology; 1963.
10. Friedenberg ZB. Fatigue fractures of the tibia. Clin Orthop Relat Res. 1971;(76):111-115.
11. Cameron KL, Peck KY, Owens BD, et al. Biomechanical risk factors for lower extremity stress fracture. Orthop J Sports Med. 2013;1(4 suppl).
12. Knapik J, Montain S, McGraw S, Grier T, Ely M, Jones B. Stress fracture risk factors in basic combat training. Int J Sports Med. 2012;33(11):940-946.
13. Behrens SB, Deren ME, Matson A, Fadale PD, Monchik KO. Stress fractures of the pelvis and legs in athletes. Sports Health. 2013;5(2):165-174.
14. Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med. 2014;48(7):491-497.
15. Maitra RS, Johnson DL. Stress fractures. Clinical history and physical examination. Clin Sports Med. 1997;16(2):259-274.
16. Nieves JW, Melsop K, Curtis M, et al. Nutritional factors that influence change in bone density and stress fracture risk among young female cross-country runners. PM R. 2010;2(8):740-750.
17. Beck BR, Matheson GO, Bergman G, et al. Do capacitively coupled electric fields accelerate tibial stress fracture healing? Am J Sports Med. 2008;36(3):545-553.
18. Simkin A, Leichter I, Giladi M, Stein M, Milgrom C. Combined effect of foot arch structure and an orthotic device on stress fractures. Foot Ankle. 1989;10(1):25-29.
19. Johnson AW, Weiss CB, Wheeler DL. Stress fractures of the femoral shaft in athletes—more common than expected: a new clinical test. Am J Sports Med. 1994;22(2):248-256.
20. Clement D, Ammann W, Taunton J, et al. Exercise-induced stress injuries to the femur. Int J Sports Med. 1993;14(6):347-352.
21. Wood PJ, Barth JH, Freedman DB, Perry L, Sheridan B. Evidence for the low dose dexamethasone suppression test to screen for Cushing’s syndrome—recommendations for a protocol for biochemistry laboratories. Ann Clin Biochem. 1997;34(pt 3):222-229.
22. Bennell K, Matheson G, Meeuwisse W, Brukner P. Risk factors for stress fractures. Sports Med. 1999;28(2):91-122.
23. Prather JL, Nusynowitz ML, Snowdy HA, Hughes AD, McCartney WH, Bagg RJ. Scintigraphic findings in stress fractures. J Bone Joint Surg Am. 1977;59(7):869-874.
24. Arendt EA, Griffiths HJ. The use of MR imaging in the assessment and clinical management of stress reactions of bone in high-performance athletes. Clin Sports Med. 1997;16(2):291-306.
25. Boden BP, Osbahr DC. High-risk stress fractures: evaluation and treatment. J Am Acad Orthop Surg. 2000;8(6):344-353.
26. Gaeta M, Minutoli F, Scribano E, et al. CT and MR imaging findings in athletes with early tibial stress injuries: comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235(2):553-561.
27. Matheson GO, Clement DB, Mckenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. Am J Sports Med. 1987;15(1):46-58.
28. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
29. Noakes TD, Smith JA, Lindenberg G, Wills CE. Pelvic stress fractures in long distance runners. Am J Sports Med. 1985;13(2):120-123.
30. Neubauer T, Brand J, Lidder S, Krawany M. Stress fractures of the femoral neck in runners: a review. Res Sports Med. 2016;24(3):283-297.
31. Evans JT, Guyver PM, Kassam AM, Hubble MJW. Displaced femoral neck stress fractures in Royal Marine recruits—management and results of operative treatment. J R Nav Med Serv. 2012;98(2):3-5.
32. Orava S. Stress fractures. Br J Sports Med. 1980;14(1):40-44.
33. Niva MH, Kiuru MJ, Haataja R, Pihlajamäki HK. Fatigue injuries of the femur. J Bone Joint Surg Br. 2005;87(10):1385-1390.
34. Weishaar MD, McMillian DJ, Moore JH. Identification and management of 2 femoral shaft stress injuries. J Orthop Sports Phys Ther. 2005;35(10):665-673.
35. Salminen ST, Pihlajamäki HK, Visuri TI, Böstman OM. Displaced fatigue fractures of the femoral shaft. Clin Orthop Relat Res. 2003;(409):250-259.
36. Giladi M, Ahronson Z, Stein M, Danon YL, Milgrom C. Unusual distribution and onset of stress fractures in soldiers. Clin Orthop Relat Res. 1985;(192):142-146.
37. Matheson GO, Clement DB, Mckenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. Am J Sports Med. 1987;15(1):46-58.
38. Green NE, Rogers RA, Lipscomb AB. Nonunions of stress fractures of the tibia. Am J Sports Med. 1985;13(3):171-176.
39. Orava S, Hulkko A. Stress fracture of the mid-tibial shaft. Acta Orthop Scand. 1984;55(1):35-37.
40. Swenson EJ Jr, DeHaven KE, Sebastianelli WJ, Hanks G, Kalenak A, Lynch JM. The effect of a pneumatic leg brace on return to play in athletes with tibial stress fractures. Am J Sports Med. 1997;25(3):322-328.
41. Rettig AC, Shelbourne KD, McCarroll JR, Bisesi M, Watts J. The natural history and treatment of delayed union stress fractures of the anterior cortex of the tibia. Am J Sports Med. 1988;16(3):250-255.
42. Varner KE, Younas SA, Lintner DM, Marymont JV. Chronic anterior midtibial stress fractures in athletes treated with reamed intramedullary nailing. Am J Sports Med. 2005;33(7):1071-1076.
43. Donahue SW, Sharkey NA. Strains in the metatarsals during the stance phase of gait: implications for stress fractures. J Bone Joint Surg Am. 1999;81(9):1236-1244.
44. Giuliani J, Masini B, Alitz C, Owens BD. Barefoot-simulating footwear associated with metatarsal stress injury in 2 runners. Orthopedics. 2011;34(7):e320-e323.
45. DeLee JC, Evans JP, Julian J. Stress fracture of the fifth metatarsal. Am J Sports Med. 1983;11(5):349-353.
46. Lappe JM, Stegman MR, Recker RR. The impact of lifestyle factors on stress fractures in female army recruits. Osteoporos Int. 2001;12(1):35-42.
47. Friedl KE, Evans RK, Moran DS. Stress fracture and military medical readiness: bridging basic and applied research. Med Sci Sports Exerc. 2008;40(11 suppl):S609-S622.
48. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23(5):741-749.
49. DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group. Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ. 2010;340:b5463.
50. Duckham RL, Peirce N, Meyer C, Summers GD, Cameron N, Brooke-Wavell K. Risk factors for stress fracture in female endurance athletes: a cross-sectional study. BMJ Open. 2012;2(6).
51. Milgrom C, Finestone A, Novack V, et al. The effect of prophylactic treatment with risedronate on stress fracture incidence among infantry recruits. Bone. 2004;35(2):418-424.
52. Crowell HP, Davis IS. Gait retraining to reduce lower extremity loading in runners. Clin Biomech. 2011;26(1):78-83.
53. Ekenman I, Milgrom C, Finestone A, et al. The role of biomechanical shoe orthoses in tibial stress fracture prevention. Am J Sports Med. 2002;30(6):866-870.
54. Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res. 2005;20(5):809-816.
1. Briethaupt MD. Zur Pathologie des menschlichen Fusses [To the pathology of the human foot]. Med Zeitung. 1855;24:169-177.
2. Berger FH, de Jonge MC, Maas M. Stress fractures in the lower extremity. Eur J Radiol. 2007;62(1):16-26.
3. Almeida SA, Williams KM, Shaffer RA, Brodine SK. Epidemiological patterns of musculoskeletal injuries and physical training. Med Sci Sports Exerc. 1999;31(8):1176-1182.
4. Jones BH, Thacker SB, Gilchrist J, Kimsey CD, Sosin DM. Prevention of lower extremity stress fractures in athletes and soldiers: a systematic review. Epidemiol Rev. 2002;24(2):228-247.
5. Jacobs JM, Cameron KL, Bojescul JA. Lower extremity stress fractures in the military. Clin Sports Med. 2014;33(4):591-613.
6. Waterman BR, Gun B, Bader JO, Orr JD, Belmont PJ. Epidemiology of lower extremity stress fractures in the United States military. Mil Med. 2016;181(10):1308-1313.
7. Hsu LL, Nevin RL, Tobler SK, Rubertone MV. Trends in overweight and obesity among 18-year-old applicants to the United States military, 1993–2006. J Adolesc Health. 2007;41(6):610-612.
8. Stanitski CL, McMaster JH, Scranton PE. On the nature of stress fractures. Am J Sports Med. 1978;6(6):391-396.
9. Johnson LC. Histogenesis of stress fractures [annual lecture]. Washington, DC: Armed Forces Institute of Pathology; 1963.
10. Friedenberg ZB. Fatigue fractures of the tibia. Clin Orthop Relat Res. 1971;(76):111-115.
11. Cameron KL, Peck KY, Owens BD, et al. Biomechanical risk factors for lower extremity stress fracture. Orthop J Sports Med. 2013;1(4 suppl).
12. Knapik J, Montain S, McGraw S, Grier T, Ely M, Jones B. Stress fracture risk factors in basic combat training. Int J Sports Med. 2012;33(11):940-946.
13. Behrens SB, Deren ME, Matson A, Fadale PD, Monchik KO. Stress fractures of the pelvis and legs in athletes. Sports Health. 2013;5(2):165-174.
14. Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med. 2014;48(7):491-497.
15. Maitra RS, Johnson DL. Stress fractures. Clinical history and physical examination. Clin Sports Med. 1997;16(2):259-274.
16. Nieves JW, Melsop K, Curtis M, et al. Nutritional factors that influence change in bone density and stress fracture risk among young female cross-country runners. PM R. 2010;2(8):740-750.
17. Beck BR, Matheson GO, Bergman G, et al. Do capacitively coupled electric fields accelerate tibial stress fracture healing? Am J Sports Med. 2008;36(3):545-553.
18. Simkin A, Leichter I, Giladi M, Stein M, Milgrom C. Combined effect of foot arch structure and an orthotic device on stress fractures. Foot Ankle. 1989;10(1):25-29.
19. Johnson AW, Weiss CB, Wheeler DL. Stress fractures of the femoral shaft in athletes—more common than expected: a new clinical test. Am J Sports Med. 1994;22(2):248-256.
20. Clement D, Ammann W, Taunton J, et al. Exercise-induced stress injuries to the femur. Int J Sports Med. 1993;14(6):347-352.
21. Wood PJ, Barth JH, Freedman DB, Perry L, Sheridan B. Evidence for the low dose dexamethasone suppression test to screen for Cushing’s syndrome—recommendations for a protocol for biochemistry laboratories. Ann Clin Biochem. 1997;34(pt 3):222-229.
22. Bennell K, Matheson G, Meeuwisse W, Brukner P. Risk factors for stress fractures. Sports Med. 1999;28(2):91-122.
23. Prather JL, Nusynowitz ML, Snowdy HA, Hughes AD, McCartney WH, Bagg RJ. Scintigraphic findings in stress fractures. J Bone Joint Surg Am. 1977;59(7):869-874.
24. Arendt EA, Griffiths HJ. The use of MR imaging in the assessment and clinical management of stress reactions of bone in high-performance athletes. Clin Sports Med. 1997;16(2):291-306.
25. Boden BP, Osbahr DC. High-risk stress fractures: evaluation and treatment. J Am Acad Orthop Surg. 2000;8(6):344-353.
26. Gaeta M, Minutoli F, Scribano E, et al. CT and MR imaging findings in athletes with early tibial stress injuries: comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235(2):553-561.
27. Matheson GO, Clement DB, Mckenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. Am J Sports Med. 1987;15(1):46-58.
28. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
29. Noakes TD, Smith JA, Lindenberg G, Wills CE. Pelvic stress fractures in long distance runners. Am J Sports Med. 1985;13(2):120-123.
30. Neubauer T, Brand J, Lidder S, Krawany M. Stress fractures of the femoral neck in runners: a review. Res Sports Med. 2016;24(3):283-297.
31. Evans JT, Guyver PM, Kassam AM, Hubble MJW. Displaced femoral neck stress fractures in Royal Marine recruits—management and results of operative treatment. J R Nav Med Serv. 2012;98(2):3-5.
32. Orava S. Stress fractures. Br J Sports Med. 1980;14(1):40-44.
33. Niva MH, Kiuru MJ, Haataja R, Pihlajamäki HK. Fatigue injuries of the femur. J Bone Joint Surg Br. 2005;87(10):1385-1390.
34. Weishaar MD, McMillian DJ, Moore JH. Identification and management of 2 femoral shaft stress injuries. J Orthop Sports Phys Ther. 2005;35(10):665-673.
35. Salminen ST, Pihlajamäki HK, Visuri TI, Böstman OM. Displaced fatigue fractures of the femoral shaft. Clin Orthop Relat Res. 2003;(409):250-259.
36. Giladi M, Ahronson Z, Stein M, Danon YL, Milgrom C. Unusual distribution and onset of stress fractures in soldiers. Clin Orthop Relat Res. 1985;(192):142-146.
37. Matheson GO, Clement DB, Mckenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. Am J Sports Med. 1987;15(1):46-58.
38. Green NE, Rogers RA, Lipscomb AB. Nonunions of stress fractures of the tibia. Am J Sports Med. 1985;13(3):171-176.
39. Orava S, Hulkko A. Stress fracture of the mid-tibial shaft. Acta Orthop Scand. 1984;55(1):35-37.
40. Swenson EJ Jr, DeHaven KE, Sebastianelli WJ, Hanks G, Kalenak A, Lynch JM. The effect of a pneumatic leg brace on return to play in athletes with tibial stress fractures. Am J Sports Med. 1997;25(3):322-328.
41. Rettig AC, Shelbourne KD, McCarroll JR, Bisesi M, Watts J. The natural history and treatment of delayed union stress fractures of the anterior cortex of the tibia. Am J Sports Med. 1988;16(3):250-255.
42. Varner KE, Younas SA, Lintner DM, Marymont JV. Chronic anterior midtibial stress fractures in athletes treated with reamed intramedullary nailing. Am J Sports Med. 2005;33(7):1071-1076.
43. Donahue SW, Sharkey NA. Strains in the metatarsals during the stance phase of gait: implications for stress fractures. J Bone Joint Surg Am. 1999;81(9):1236-1244.
44. Giuliani J, Masini B, Alitz C, Owens BD. Barefoot-simulating footwear associated with metatarsal stress injury in 2 runners. Orthopedics. 2011;34(7):e320-e323.
45. DeLee JC, Evans JP, Julian J. Stress fracture of the fifth metatarsal. Am J Sports Med. 1983;11(5):349-353.
46. Lappe JM, Stegman MR, Recker RR. The impact of lifestyle factors on stress fractures in female army recruits. Osteoporos Int. 2001;12(1):35-42.
47. Friedl KE, Evans RK, Moran DS. Stress fracture and military medical readiness: bridging basic and applied research. Med Sci Sports Exerc. 2008;40(11 suppl):S609-S622.
48. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23(5):741-749.
49. DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group. Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ. 2010;340:b5463.
50. Duckham RL, Peirce N, Meyer C, Summers GD, Cameron N, Brooke-Wavell K. Risk factors for stress fracture in female endurance athletes: a cross-sectional study. BMJ Open. 2012;2(6).
51. Milgrom C, Finestone A, Novack V, et al. The effect of prophylactic treatment with risedronate on stress fracture incidence among infantry recruits. Bone. 2004;35(2):418-424.
52. Crowell HP, Davis IS. Gait retraining to reduce lower extremity loading in runners. Clin Biomech. 2011;26(1):78-83.
53. Ekenman I, Milgrom C, Finestone A, et al. The role of biomechanical shoe orthoses in tibial stress fracture prevention. Am J Sports Med. 2002;30(6):866-870.
54. Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res. 2005;20(5):809-816.
In Memoriam
Warren R. Kadrmas, MD, COL, MC, USAF
November 6, 1969-May 8, 2014
Matthew T. Provencher, MD, CAPT, MC, USNR, and John M. Tokish, MD
A Selfless Leader and Former Head of Air Force Orthopedics
In 2014, we tragically lost a true friend, outstanding clinician, great family man, and incredible human being. As one of the preeminent sports orthopedists in the military, Dr. Kadrmas was beloved by all and heralded for his many selfless contributions to military musculoskeletal medicine and injury prevention. He was known for his humble nature and steadfast integrity, and served as an exemplary role model whom we all aspired to emulate. We all remember our time with Warren fondly, and he left us all with lasting memories to cherish and countless stories sure to regale.
Warren Kadrmas was born in 1969 in Vermillion, South Dakota and grew up in Sheridan, Wyoming. Dr. Kadrmas graduated with distinction from both the US Air Force Academy in 1992 and Duke University School of Medicine in 1996. He then went on to complete his residency in 2003 at the Hospital for Special Surgery (HSS) in New York City and was recognized with the Jean C. McDaniel Outstanding Resident Award. He began his Air Force orthopedic career at Wilford Hall Ambulatory Surgical Center on the grounds of Lackland Air Force Base in San Antonio, Texas as part of the 59th Medical Wing. Warren was deployed and served as 1 of 5 people on the mobile-field surgical team assigned to the 379th Expeditionary Medical Group. Subsequently, he returned to HSS, where he excelled in sports medicine and shoulder service subspecialty training.
After his fellowship, Warren returned to San Antonio to continue his work as a top military sports surgeon, serving as a mentor, educator, and leader for all of Air Force orthopedics. During this time he served several tours overseas, becoming an invaluable member of the 332nd Expeditionary Medical Group operating out of the Air Force Theater Hospital at Balad Air Base, Iraq. Warren served as the Program Director of the Orthopedic Residency Program at Wilford Hall Ambulatory Surgical Center. He held the position of Head of Orthopedics for the Air Force as Orthopedic Surgery Consultant to the Air Force Surgeon General for 5 years, a role that entailed coordinating all orthopedic assets for the Global War on Terror for the Air Force. Selfless to a fault, he would never ask anything of anyone that he had not done himself. He completed 6 deployments away from family, loved ones, and work in San Antonio.
A true innovator and visionary, Warren was a pioneer in the integration of high-caliber hip arthroscopy, as well as cutting-edge shoulder and knee care for our active-duty military personnel. He was a prominent member of the American Orthopaedic Society for Sports Medicine (AOSSM) and Arthroscopy Association of North America, and was in line to be the incoming President of the Society of Military Orthopaedic Surgeons, after having previously served as the society’s 2nd Vice President. He was selected for and was scheduled to participate in the AOSSM Traveling Fellowship touring Asia just prior to his untimely accident.
One of Warren’s favorite quotes was on the topic of leading from behind. Nelson Mandela said, “It is better to lead from behind and to put others in front, especially when you celebrate victory when nice things occur. You take the front line when there is danger. Then people will appreciate your leadership.” Warren was the embodiment of this quote. He led from the front, and by example, in times of danger to inspire those he led. But he also honed the skill of leading from behind, with quiet self-sacrifice, to celebrate the success of those he led. His tireless dedication was prominent in all the facets of his life, whether as a father, son, brother, surgeon, educator, mentor, or friend. We miss him dearly, and try to embody his spirit by living our lives through what he taught us all.
Brian Allgood, MD, COL
1960-2007
Dean Taylor, MD
An Exemplary Selfless Leader in Orthopedics and Medicine
When people ask me what effective, ethical healthcare leadership looks like, I think of Brian Allgood. Brian was the epitome of leadership. He led quietly, by example and selflessly–always putting the interests of patients and those on his team ahead of his own.
Brian was a 1982 graduate of the United States Military Academy at West Point, and received a Doctor of Medicine degree from the University of Oklahoma. He completed his orthopedic training at Brooke Army Medical Center in San Antonio. I first met Brian in 1994 when he was practicing as an orthopedic surgeon at Womack Army Medical Center at Ft. Bragg, North Carolina, where he also served at the Division Surgeon for the 82nd Airborne Division. At the time, I was extremely impressed with Brian’s outstanding orthopedic skills, and his unwavering commitment to leadership in orthopedics, military medicine, and medicine.
Brian’s role as the 82nd Airborne Division Surgeon was on the leadership track in Army medicine, a track that many of us who enjoyed and were good at patient care shunned because it was structured to limit the amount of time an administrative leader could spend in patient care. Brian was certainly a skilled orthopedic surgeon who loved caring for patients; however, he was courageous enough to put his responsibility to military medicine and the medical profession ahead of his own clinical interests. He realized that he could provide exceptional leadership that would benefit many instead of only those in his sphere of care. And what an exceptional leader he was!
From 2002 to 2004, I saw firsthand Brian’s extraordinary leadership when he served as the hospital commander of Keller Army Community Hospital at West Point. He was the best hospital commander I worked with during my 11 years at West Point. I saw the sacrifices he made for the rest of us. He gave up something he loved–orthopedic surgery–so that he could effectively lead our hospital. While we operated, he occasionally would look longingly through the operating room (OR) windows. When we saw him, we would invite him to scrub in, much to his delight. He would also show up in other services’ ORs and the hospital’s clinics, staying connected to patients and patient care. This patient-centeredness contributed significantly to the beloved leader he was.
Brian’s final assignment was in 2006 as the Command Surgeon of Multi-National Forces, the highest-ranking medical officer in Iraq. On January 20, 2007, Brian Allgood—on the verge of promotion to brigadier general and on the fast track to Surgeon General of the Army—was killed along with 11 other American service members when their helicopter was shot down.
In his life, Brian was an exemplary leader. After his death, he lives on in our memories as an example to which we should all aspire–an ethical, selfless leader who cared for all patients, always striving to do the right thing.
LCpl Benjamin Whetstone Schmidt
1987-2011
David R. Schmidt, MD
A Fallen Hero’s Legacy
On September 11, 2011, LCpl Benjamin Whetstone Schmidt posted on his Facebook page, “I guess you can use today as a reason for us to be here in Afghanistan. Just know I am fighting for myself, but most of all for my friends and family who read this. Everyone, it’s an honor to be your ambassador.”
Benjamin was a Marine Scout Sniper on his second tour to Afghanistan, this time voluntarily. Not one member of his platoon had combat experience. He felt called to lead, to be with his boys. During his first deployment to Afghanistan he was awarded the Navy/USMC Achievement Medal with Valor for his action in combat.
Less than a month later, on October 6, 2011, he was killed while on patrol in Helmand Province. Even now, 6 years after his death, his comrades continue to hail his virtues as a leader, a friend, a patriot, and an inspiration. He was also a fine athlete and a courageous, energetic young man with bold plans for his future.
Other than his family, few knew what Benjamin would inspire in his death. He left $200,000 of his life insurance to establish a scholarship in the History Department at his beloved Texas Christian University (TCU). With a matching gift from his father, orthopedic surgeon David R. Schmidt, MD, and stepmom Teresa, the scholarship provides annual funding for a graduate student. Asked why he chose to support graduate students, Benjamin replied with his signature humor and wisdom, “I wouldn’t invest in a freshman like myself.” Benjamin had spent 2 years at TCU prior to enlisting in the Marine Corps, and intended to return to TCU to complete his undergraduate and graduate degrees.
Certainly not many young men at age 24 years, prior to going to war, have the foresight to envision and implement a legacy bigger than themselves, with the promise of influencing generations into the future. For his actions, Benjamin was a finalist for a Congressional Medal of Honor Society “Citizen Service Before Self” award.
David and Teresa Schmidt subsequently raised $1 million dollars to endow the LCpl Benjamin W. Schmidt Professor of War, Conflict and Society. It is truly inspirational to know that a young man’s selfless vision and his friends’ and family’s support could produce such a lasting legacy.
Warren R. Kadrmas, MD, COL, MC, USAF
November 6, 1969-May 8, 2014
Matthew T. Provencher, MD, CAPT, MC, USNR, and John M. Tokish, MD
A Selfless Leader and Former Head of Air Force Orthopedics
In 2014, we tragically lost a true friend, outstanding clinician, great family man, and incredible human being. As one of the preeminent sports orthopedists in the military, Dr. Kadrmas was beloved by all and heralded for his many selfless contributions to military musculoskeletal medicine and injury prevention. He was known for his humble nature and steadfast integrity, and served as an exemplary role model whom we all aspired to emulate. We all remember our time with Warren fondly, and he left us all with lasting memories to cherish and countless stories sure to regale.
Warren Kadrmas was born in 1969 in Vermillion, South Dakota and grew up in Sheridan, Wyoming. Dr. Kadrmas graduated with distinction from both the US Air Force Academy in 1992 and Duke University School of Medicine in 1996. He then went on to complete his residency in 2003 at the Hospital for Special Surgery (HSS) in New York City and was recognized with the Jean C. McDaniel Outstanding Resident Award. He began his Air Force orthopedic career at Wilford Hall Ambulatory Surgical Center on the grounds of Lackland Air Force Base in San Antonio, Texas as part of the 59th Medical Wing. Warren was deployed and served as 1 of 5 people on the mobile-field surgical team assigned to the 379th Expeditionary Medical Group. Subsequently, he returned to HSS, where he excelled in sports medicine and shoulder service subspecialty training.
After his fellowship, Warren returned to San Antonio to continue his work as a top military sports surgeon, serving as a mentor, educator, and leader for all of Air Force orthopedics. During this time he served several tours overseas, becoming an invaluable member of the 332nd Expeditionary Medical Group operating out of the Air Force Theater Hospital at Balad Air Base, Iraq. Warren served as the Program Director of the Orthopedic Residency Program at Wilford Hall Ambulatory Surgical Center. He held the position of Head of Orthopedics for the Air Force as Orthopedic Surgery Consultant to the Air Force Surgeon General for 5 years, a role that entailed coordinating all orthopedic assets for the Global War on Terror for the Air Force. Selfless to a fault, he would never ask anything of anyone that he had not done himself. He completed 6 deployments away from family, loved ones, and work in San Antonio.
A true innovator and visionary, Warren was a pioneer in the integration of high-caliber hip arthroscopy, as well as cutting-edge shoulder and knee care for our active-duty military personnel. He was a prominent member of the American Orthopaedic Society for Sports Medicine (AOSSM) and Arthroscopy Association of North America, and was in line to be the incoming President of the Society of Military Orthopaedic Surgeons, after having previously served as the society’s 2nd Vice President. He was selected for and was scheduled to participate in the AOSSM Traveling Fellowship touring Asia just prior to his untimely accident.
One of Warren’s favorite quotes was on the topic of leading from behind. Nelson Mandela said, “It is better to lead from behind and to put others in front, especially when you celebrate victory when nice things occur. You take the front line when there is danger. Then people will appreciate your leadership.” Warren was the embodiment of this quote. He led from the front, and by example, in times of danger to inspire those he led. But he also honed the skill of leading from behind, with quiet self-sacrifice, to celebrate the success of those he led. His tireless dedication was prominent in all the facets of his life, whether as a father, son, brother, surgeon, educator, mentor, or friend. We miss him dearly, and try to embody his spirit by living our lives through what he taught us all.
Brian Allgood, MD, COL
1960-2007
Dean Taylor, MD
An Exemplary Selfless Leader in Orthopedics and Medicine
When people ask me what effective, ethical healthcare leadership looks like, I think of Brian Allgood. Brian was the epitome of leadership. He led quietly, by example and selflessly–always putting the interests of patients and those on his team ahead of his own.
Brian was a 1982 graduate of the United States Military Academy at West Point, and received a Doctor of Medicine degree from the University of Oklahoma. He completed his orthopedic training at Brooke Army Medical Center in San Antonio. I first met Brian in 1994 when he was practicing as an orthopedic surgeon at Womack Army Medical Center at Ft. Bragg, North Carolina, where he also served at the Division Surgeon for the 82nd Airborne Division. At the time, I was extremely impressed with Brian’s outstanding orthopedic skills, and his unwavering commitment to leadership in orthopedics, military medicine, and medicine.
Brian’s role as the 82nd Airborne Division Surgeon was on the leadership track in Army medicine, a track that many of us who enjoyed and were good at patient care shunned because it was structured to limit the amount of time an administrative leader could spend in patient care. Brian was certainly a skilled orthopedic surgeon who loved caring for patients; however, he was courageous enough to put his responsibility to military medicine and the medical profession ahead of his own clinical interests. He realized that he could provide exceptional leadership that would benefit many instead of only those in his sphere of care. And what an exceptional leader he was!
From 2002 to 2004, I saw firsthand Brian’s extraordinary leadership when he served as the hospital commander of Keller Army Community Hospital at West Point. He was the best hospital commander I worked with during my 11 years at West Point. I saw the sacrifices he made for the rest of us. He gave up something he loved–orthopedic surgery–so that he could effectively lead our hospital. While we operated, he occasionally would look longingly through the operating room (OR) windows. When we saw him, we would invite him to scrub in, much to his delight. He would also show up in other services’ ORs and the hospital’s clinics, staying connected to patients and patient care. This patient-centeredness contributed significantly to the beloved leader he was.
Brian’s final assignment was in 2006 as the Command Surgeon of Multi-National Forces, the highest-ranking medical officer in Iraq. On January 20, 2007, Brian Allgood—on the verge of promotion to brigadier general and on the fast track to Surgeon General of the Army—was killed along with 11 other American service members when their helicopter was shot down.
In his life, Brian was an exemplary leader. After his death, he lives on in our memories as an example to which we should all aspire–an ethical, selfless leader who cared for all patients, always striving to do the right thing.
LCpl Benjamin Whetstone Schmidt
1987-2011
David R. Schmidt, MD
A Fallen Hero’s Legacy
On September 11, 2011, LCpl Benjamin Whetstone Schmidt posted on his Facebook page, “I guess you can use today as a reason for us to be here in Afghanistan. Just know I am fighting for myself, but most of all for my friends and family who read this. Everyone, it’s an honor to be your ambassador.”
Benjamin was a Marine Scout Sniper on his second tour to Afghanistan, this time voluntarily. Not one member of his platoon had combat experience. He felt called to lead, to be with his boys. During his first deployment to Afghanistan he was awarded the Navy/USMC Achievement Medal with Valor for his action in combat.
Less than a month later, on October 6, 2011, he was killed while on patrol in Helmand Province. Even now, 6 years after his death, his comrades continue to hail his virtues as a leader, a friend, a patriot, and an inspiration. He was also a fine athlete and a courageous, energetic young man with bold plans for his future.
Other than his family, few knew what Benjamin would inspire in his death. He left $200,000 of his life insurance to establish a scholarship in the History Department at his beloved Texas Christian University (TCU). With a matching gift from his father, orthopedic surgeon David R. Schmidt, MD, and stepmom Teresa, the scholarship provides annual funding for a graduate student. Asked why he chose to support graduate students, Benjamin replied with his signature humor and wisdom, “I wouldn’t invest in a freshman like myself.” Benjamin had spent 2 years at TCU prior to enlisting in the Marine Corps, and intended to return to TCU to complete his undergraduate and graduate degrees.
Certainly not many young men at age 24 years, prior to going to war, have the foresight to envision and implement a legacy bigger than themselves, with the promise of influencing generations into the future. For his actions, Benjamin was a finalist for a Congressional Medal of Honor Society “Citizen Service Before Self” award.
David and Teresa Schmidt subsequently raised $1 million dollars to endow the LCpl Benjamin W. Schmidt Professor of War, Conflict and Society. It is truly inspirational to know that a young man’s selfless vision and his friends’ and family’s support could produce such a lasting legacy.
Warren R. Kadrmas, MD, COL, MC, USAF
November 6, 1969-May 8, 2014
Matthew T. Provencher, MD, CAPT, MC, USNR, and John M. Tokish, MD
A Selfless Leader and Former Head of Air Force Orthopedics
In 2014, we tragically lost a true friend, outstanding clinician, great family man, and incredible human being. As one of the preeminent sports orthopedists in the military, Dr. Kadrmas was beloved by all and heralded for his many selfless contributions to military musculoskeletal medicine and injury prevention. He was known for his humble nature and steadfast integrity, and served as an exemplary role model whom we all aspired to emulate. We all remember our time with Warren fondly, and he left us all with lasting memories to cherish and countless stories sure to regale.
Warren Kadrmas was born in 1969 in Vermillion, South Dakota and grew up in Sheridan, Wyoming. Dr. Kadrmas graduated with distinction from both the US Air Force Academy in 1992 and Duke University School of Medicine in 1996. He then went on to complete his residency in 2003 at the Hospital for Special Surgery (HSS) in New York City and was recognized with the Jean C. McDaniel Outstanding Resident Award. He began his Air Force orthopedic career at Wilford Hall Ambulatory Surgical Center on the grounds of Lackland Air Force Base in San Antonio, Texas as part of the 59th Medical Wing. Warren was deployed and served as 1 of 5 people on the mobile-field surgical team assigned to the 379th Expeditionary Medical Group. Subsequently, he returned to HSS, where he excelled in sports medicine and shoulder service subspecialty training.
After his fellowship, Warren returned to San Antonio to continue his work as a top military sports surgeon, serving as a mentor, educator, and leader for all of Air Force orthopedics. During this time he served several tours overseas, becoming an invaluable member of the 332nd Expeditionary Medical Group operating out of the Air Force Theater Hospital at Balad Air Base, Iraq. Warren served as the Program Director of the Orthopedic Residency Program at Wilford Hall Ambulatory Surgical Center. He held the position of Head of Orthopedics for the Air Force as Orthopedic Surgery Consultant to the Air Force Surgeon General for 5 years, a role that entailed coordinating all orthopedic assets for the Global War on Terror for the Air Force. Selfless to a fault, he would never ask anything of anyone that he had not done himself. He completed 6 deployments away from family, loved ones, and work in San Antonio.
A true innovator and visionary, Warren was a pioneer in the integration of high-caliber hip arthroscopy, as well as cutting-edge shoulder and knee care for our active-duty military personnel. He was a prominent member of the American Orthopaedic Society for Sports Medicine (AOSSM) and Arthroscopy Association of North America, and was in line to be the incoming President of the Society of Military Orthopaedic Surgeons, after having previously served as the society’s 2nd Vice President. He was selected for and was scheduled to participate in the AOSSM Traveling Fellowship touring Asia just prior to his untimely accident.
One of Warren’s favorite quotes was on the topic of leading from behind. Nelson Mandela said, “It is better to lead from behind and to put others in front, especially when you celebrate victory when nice things occur. You take the front line when there is danger. Then people will appreciate your leadership.” Warren was the embodiment of this quote. He led from the front, and by example, in times of danger to inspire those he led. But he also honed the skill of leading from behind, with quiet self-sacrifice, to celebrate the success of those he led. His tireless dedication was prominent in all the facets of his life, whether as a father, son, brother, surgeon, educator, mentor, or friend. We miss him dearly, and try to embody his spirit by living our lives through what he taught us all.
Brian Allgood, MD, COL
1960-2007
Dean Taylor, MD
An Exemplary Selfless Leader in Orthopedics and Medicine
When people ask me what effective, ethical healthcare leadership looks like, I think of Brian Allgood. Brian was the epitome of leadership. He led quietly, by example and selflessly–always putting the interests of patients and those on his team ahead of his own.
Brian was a 1982 graduate of the United States Military Academy at West Point, and received a Doctor of Medicine degree from the University of Oklahoma. He completed his orthopedic training at Brooke Army Medical Center in San Antonio. I first met Brian in 1994 when he was practicing as an orthopedic surgeon at Womack Army Medical Center at Ft. Bragg, North Carolina, where he also served at the Division Surgeon for the 82nd Airborne Division. At the time, I was extremely impressed with Brian’s outstanding orthopedic skills, and his unwavering commitment to leadership in orthopedics, military medicine, and medicine.
Brian’s role as the 82nd Airborne Division Surgeon was on the leadership track in Army medicine, a track that many of us who enjoyed and were good at patient care shunned because it was structured to limit the amount of time an administrative leader could spend in patient care. Brian was certainly a skilled orthopedic surgeon who loved caring for patients; however, he was courageous enough to put his responsibility to military medicine and the medical profession ahead of his own clinical interests. He realized that he could provide exceptional leadership that would benefit many instead of only those in his sphere of care. And what an exceptional leader he was!
From 2002 to 2004, I saw firsthand Brian’s extraordinary leadership when he served as the hospital commander of Keller Army Community Hospital at West Point. He was the best hospital commander I worked with during my 11 years at West Point. I saw the sacrifices he made for the rest of us. He gave up something he loved–orthopedic surgery–so that he could effectively lead our hospital. While we operated, he occasionally would look longingly through the operating room (OR) windows. When we saw him, we would invite him to scrub in, much to his delight. He would also show up in other services’ ORs and the hospital’s clinics, staying connected to patients and patient care. This patient-centeredness contributed significantly to the beloved leader he was.
Brian’s final assignment was in 2006 as the Command Surgeon of Multi-National Forces, the highest-ranking medical officer in Iraq. On January 20, 2007, Brian Allgood—on the verge of promotion to brigadier general and on the fast track to Surgeon General of the Army—was killed along with 11 other American service members when their helicopter was shot down.
In his life, Brian was an exemplary leader. After his death, he lives on in our memories as an example to which we should all aspire–an ethical, selfless leader who cared for all patients, always striving to do the right thing.
LCpl Benjamin Whetstone Schmidt
1987-2011
David R. Schmidt, MD
A Fallen Hero’s Legacy
On September 11, 2011, LCpl Benjamin Whetstone Schmidt posted on his Facebook page, “I guess you can use today as a reason for us to be here in Afghanistan. Just know I am fighting for myself, but most of all for my friends and family who read this. Everyone, it’s an honor to be your ambassador.”
Benjamin was a Marine Scout Sniper on his second tour to Afghanistan, this time voluntarily. Not one member of his platoon had combat experience. He felt called to lead, to be with his boys. During his first deployment to Afghanistan he was awarded the Navy/USMC Achievement Medal with Valor for his action in combat.
Less than a month later, on October 6, 2011, he was killed while on patrol in Helmand Province. Even now, 6 years after his death, his comrades continue to hail his virtues as a leader, a friend, a patriot, and an inspiration. He was also a fine athlete and a courageous, energetic young man with bold plans for his future.
Other than his family, few knew what Benjamin would inspire in his death. He left $200,000 of his life insurance to establish a scholarship in the History Department at his beloved Texas Christian University (TCU). With a matching gift from his father, orthopedic surgeon David R. Schmidt, MD, and stepmom Teresa, the scholarship provides annual funding for a graduate student. Asked why he chose to support graduate students, Benjamin replied with his signature humor and wisdom, “I wouldn’t invest in a freshman like myself.” Benjamin had spent 2 years at TCU prior to enlisting in the Marine Corps, and intended to return to TCU to complete his undergraduate and graduate degrees.
Certainly not many young men at age 24 years, prior to going to war, have the foresight to envision and implement a legacy bigger than themselves, with the promise of influencing generations into the future. For his actions, Benjamin was a finalist for a Congressional Medal of Honor Society “Citizen Service Before Self” award.
David and Teresa Schmidt subsequently raised $1 million dollars to endow the LCpl Benjamin W. Schmidt Professor of War, Conflict and Society. It is truly inspirational to know that a young man’s selfless vision and his friends’ and family’s support could produce such a lasting legacy.
Applying Military Strategy to Complex Knee Reconstruction: Tips for Planning and Executing Advanced Surgery
Take-Home Points
- Thorough preoperative planning is imperative and inclusive of history, physical examination, radiographs, and MRI and potentially CT scan.
- Plan carefully for needed graft sources (autografts and allografts).
- Rehabilitation starts preoperatively and a detailed individualized plan is often warranted.
- Indicated ligamentous repair or augmented repair with reconstruction is more likely to succeed when performed within 2 weeks of injury.
- Complex combined knee restoration surgery can be safely performed in an outpatient setting.
Complex combined knee restoration surgery can be safely performed in an outpatient setting. The term complex knee restoration is used to describe management of knee injuries that are more involved—that is, there is damage to the menisci, cartilage, ligaments, and bones. Management entails not only determining the best treatment options but navigating the more complex logistics of making sure all necessary grafts (fresh and frozen allografts and autografts), implants, and instrumentation are readily available as these cases come to fruition.
The military healthcare paradigm often involves the added logistics of transporting the service member to the correct military treatment facility at the correct time and ensuring the patient’s work-up is complete before he or she arrives for the complex knee restoration. Such cases require significant rehabilitation and time away from family and work, so anything that reduces the morbidity of the surgical undertaking and the overall “morbidity footprint” of time away and that helps the patient return to normal function are value-added and worthy of our attention and diligence in developing an efficient system for managing complex cases.
The globally integrated military healthcare system that is in place has matured over the past decades to allow for the significant majority of the necessary preoperative work-up to be performed at a soldier’s current duty station, wherever in the world that may be, under the guidance of local healthcare providers with specific inputs from the knee restoration surgeon who eventually receives the patient for the planned surgical intervention.
Algorithm for Knee Restoration Planning
Alignment Issues
The first task is to confirm the realignment indication. Realignment may be performed with a proximal opening-wedge medial tibial osteotomy (OWMTO), a distal opening-wedge lateral femoral osteotomy (OWLFO), or a tibial tubercle osteotomy (TTO).1 Given the reproducible clinical improvement achieved and the robust nature of the fixation, these osteotomies are often the first surgical step in complex knee restorations.2 The final determination, made by the surgeon in consultation with the patient, is whether to perform the indicated osteotomy alone or in combination with the rest of the planned restoration surgery. In the vast majority of cases I have managed over the past 2 decades, I have performed the entire knee restoration in a single operation.3 Within the past 5 years, combining the procedures has become even more feasible with the important progress made in multimodal pain management and with the close collaboration of anesthesiologists.4
Meniscus and Cartilage Status
The integration status of meniscus and cartilage within the medial and lateral tibiofemoral compartments is crucial to the comprehensive restoration plan. In fact, the success of the restoration can be said to be dependent on the functional status and health of meniscus and cartilage—which either succeed together or fail apart.
Important covariables are age, prior surgical interventions, activity level expected or allowed after surgery, and size, location, and depth of cartilage injury.5 Whether a cartilage injury is monopolar or bipolar is determined with advanced imaging (magnetic resonance imaging [MRI], computed tomography [CT], weight-bearing radiography) along with analysis of a thorough history (including a review of prior operative reports and arthroscopic images) and a knee examination. Bipolar injuries that involve the condyle and juxtaposed plateau often bode poorly for good clinical outcomes—compared with unipolar lesions, which usually involve the condylar surfaces in isolation. The same thinking regarding the patellofemoral compartment is appropriate. Cartilage lesions that involve the juxtaposed surfaces of the patellar and trochlear groove do poorer than isolated lesions, which are more amenable to cartilage restoration options. The literature on potential cartilage restoration options for the patella and trochlea is expanding. I use the 3-dimensional cartilage restoration option of a fresh patellar osteochondral allograft (OCA) for high-grade cartilage lesions thought to be clinically significant. Other options, such as microfracture, cell-based cartilage restoration, and Osteochondral Autograft Transfer System (Arthrex) procedures (from the thinner condylar cartilage), have varied in their outcomes for patellar lesions. According to more recent literature and a review of my clinical results, fresh patellar OCAs are a good option for patellar lesions.6 Similarly, trochlear lesions can be managed with microfracture, cell-based therapies, or fresh OCAs, depending on surgeon preference.
Functional total or subtotal meniscectomies are often best managed with meniscal allograft transplantation (MAT). An intact or replaced medial or lateral meniscus works synergistically with any planned anterior cruciate ligament (ACL) reconstruction. Again, the adage that meniscus and cartilage succeed together or fail apart is appropriate when planning complex knee restoration. Signs of extrusion or joint-space narrowing and root avulsion or significant loss of meniscal tissue, visualized on MRI or on prior surgical images, often help substantiate a MAT plan. MAT has had the best long-term results when performed in compartments with cartilage damage limited to grade I and grade II changes, in stable knees, and in knees that can be concurrently stabilized.5 Technological advances have increased the value of MAT by limiting the morbidity of the operation and thus allowing for other surgery to be performed concomitantly and safely as part of comprehensive knee restoration. Over the past 20 years, I have arthroscopically performed MAT with bone plugs for medial and lateral procedures, and my results with active-duty soldiers have been promising, paralleling the clinic success reported in the literature.5 Alignment must be considered when performing MAT or cartilage restoration. If the addition of meniscal transplantation or cartilage restoration leaves the knee with residual malalignment of 6° or more, corrective osteotomy is performed.
My view and practice have been to plan for an unloading chondroprotective osteotomy. The goal is a balanced mechanical axis, whether achieved with mere joint-space restoration or with an osteotomy added.
Ligament Status
A comprehensive plan for establishing ligamentous stability is paramount to the overall clinical success of complex knee restorations. Meniscus and cartilage restoration efforts are wasted if clinically significant ligamentous laxity is not concomitantly treated with reconstruction surgery. Revision ACL surgery is by far the most commonly performed surgery in complex knee cases. Diligence in interpreting advanced MRI and physical examination findings is required to make sure there are no concomitant patholaxities in the medial, lateral, posterior, posteromedial, and posterolateral ligamentous complexes. Appropriate ligamentous reconstruction is warranted to maximize clinical results in complex knee restorations. Such cases more commonly require allograft tissue, as the availability of autograft tissue is the limiting issue with 2 or more ligament reconstructions. Military treatment facilities, in which comprehensive knee restorations are performed, have soft-tissue allografts on hand at all times. Having tissue readily available makes it less imperative to determine the most appropriate combined ligamentous reconstruction surgery before the patient arrives—a process that is often difficult. This situation is in contradistinction to the need for specific matched-for-size allograft frozen meniscus and fresh cartilage tissues, both of which require tissue-form procurement in advance of planned restoration surgery.
Rehabilitation Plan
The rehabilitation plan is driven by the part of the complex knee restoration that demands the most caution with respect to weight-bearing and range of motion (ROM) during the first 6 weeks after surgery. The most limiting restorative surgeries involve meniscus and cartilage. Recent clinical trial results support weight-bearing soon after tibial osteotomy performed in the absence of meniscus and cartilage restoration that would otherwise limit weight-bearing for 6 weeks.7 Therefore, most of these complex knee restorations are appropriately managed with a hinged brace locked in extension for toe-touch weight-bearing ambulation, with ROM usually limited to 0° to 90° during the first 6 weeks. Quadriceps rehabilitation with straight-leg raises and isometric contractions is prescribed with a focus on maintaining full extension as the default resting knee position until normalized resting quadriceps tone returns. Full weight-bearing and advancement to full flexion are routinely allowed by 6 weeks.
Case Report
A 41-year-old male service member who was overseas was referred to my clinic for high tibial osteotomy consideration and possible revision ACL reconstruction. His symptoms were medial pain, recurrent instability, and patellofemoral crepitance. Three years earlier, he underwent autograft transtibial ACL reconstruction with significant débridement of the medial meniscus. Before his trip to the United States, I asked that new MRI scans, full-length standing hip–knee–ankle bilateral alignment radiographs, and a 4-view weight-bearing knee series (including a posteroanterior Rosenberg view) be obtained and sent for my review (Figure 1).
Review of the patient’s detailed preoperative imaging work-up and electronic medical record (available through the military’s healthcare system) made it clear that far more surgical intervention was needed than originally assumed. A significant full-thickness chondral lesion of the patella and a subtotal medial meniscectomy would necessitate patellar cartilage restoration and medial MAT in addition to the high tibial osteotomy and revision ACL reconstruction.
Had this patient been sent through the military medical evacuation system, he would have had to make 2 overseas trips—one trip for preoperative evaluation and advanced imaging, whereby he would have been placed on a match list and had to wait for a requested meniscal allograft and an appropriate graft for his patella, and the other trip for his complex surgery. Fortunately, the military’s integrated healthcare network with true 2-way communication and the collaborative use of integrated electronic medical records proved extremely valuable in making management of this complex knee restoration as efficient as possible. From the perspective of the soldier and his military unit, only 1 big overseas trip was needed; from the perspective of the military healthcare system, responsible use of healthcare personnel and monetary resources and well-planned complex knee restoration surgery saved a knee and allowed a soldier-athlete to rejoin the fields of friendly strife.
This patient had undergone functional complete medial meniscectomy and had significant medial compartment pain, varus alignment, and minimal medial joint-space narrowing (assumed grossly intact cartilage about plateau and condyle), plus patellofemoral pain and crepitance with a large high-grade posttraumatic patellar chondral lesion with normal patellofemoral alignment. He also had an isolated failed ACL graft from prior ACL reconstruction. The previous hardware placement was analyzed, and it was determined that the femoral interference screw could be left in place and that the tibial interference screw most likely would be removed. The mechanical axis determined from the bilateral long-leg standing images dictated a need for proximal OWMTO for correction up to 8° to allow the axis to cross the center of the knee. The 8° correction is the measured correction needed to move the axis from its pass through the medial compartment to a more balanced position across the middle of the knee.
The overall plan encompassed major concomitant corrective and restorative surgery: tibial osteotomy, medial MAT, revision ACL reconstruction, and fresh mega-patellar OCA. Once the frozen meniscus and eventually the fresh patella (both matched for size) were obtained, arrangements for the patient’s trip for the complex surgery were finalized.
Surgery was started with brief arthroscopic evaluation to confirm the overall appropriateness of the planned procedure and to determine if any other minor deficiencies would warrant operative intervention. Once confirmed, the restoration proceeded as planned. The OWMTO was performed with a PEEK (polyetheretherketone) wedge implant (iBalance; Arthrex) followed by arthroscopic preparation for medial MAT with removal of any meniscal remnants and placement of passing sutures (Figure 2A).
When the arthroscopic portion of the surgery was finished, a medial parapatellar arthrotomy was made to allow the patella to be inverted and complete fresh mega-patellar OCA placement (Figure 4).
The knee was placed in a ROM brace locked in full extension. The patient was able to do straight-leg raises and calf pumps in the recovery room and was discharged home with a saphenous nerve block and an iPACK (Interspace between the Popliteal Artery and the Capsule of the posterior Knee) nerve block in place. Home-based therapy was started immediately. After the patient’s first postoperative visit, formal therapy (discussed earlier) was initiated (Figure 6).
Discussion
All-inside GraftLink ACL reconstruction with cortical suspensory fixation appears well suited to combined medial and lateral MAT and/or cartilage restoration—whether it be large fresh OCA combined with medial MAT (as in this patient’s case) or another form of cartilage restoration. Arthroscopic MAT with anatomically fashioned and placed bone plugs minimizes the morbidity within the notch footprints and allows for discrete revision socket formation for both femoral and tibial ACL graft placement. In this case, preparation for the medial MAT and ACL sockets was followed by MAT/ACL construct implantation and secure fixation. The arthrotomy was thereby minimized and placed to allow for efficient mega-patellar OCA graft placement.
Over the past decade, I have performed similar concomitant procedures using the same surgical principles that allow for efficient and reproducible complex knee restoration (Figure 7).
Although use of an algorithm for the management of complex knee restorations is not universally feasible, I offer guidelines for complex knee injuries:
- At each decision point, determine whether the knee and the patient can withstand the planned surgical intervention.
- After deciding to proceed with knee restoration, list the meniscus, cartilage, and ligament injuries that must be addressed.
- Determine which repairs (meniscus, cartilage, ligament) are warranted. Repairs generally are best performed within a period of 7 to 14 days.
- Determine which ligament injuries warrant reconstruction. Allograft tissue typically is used for multiligament reconstruction.
- Rank-order the ligament reconstruction requirements. It is fine to proceed with all of the reconstructions if the case is moving smoothly, if there are no developing tourniquet-time issues, and if the soft-tissue envelope is responding as expected.
- Consider autograft and/or allograft tissue needs for concomitant or staged meniscus and cartilage restoration options/requirements.
Am J Orthop. 2017;46(4):170-175, 202. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Uquillas C, Rossy W, Nathasingh CK, Strauss E, Jazrawi L, Gonzalez-Lomas G. Osteotomies about the knee: AAOS exhibit selection. J Bone Joint Surg Am. 2014;96(24):e199.
2. Mehl J, Paul J, Feucht MJ, et al. ACL deficiency and varus osteoarthritis: high tibial osteotomy alone or combined with ACL reconstruction? Arch Orthop Trauma Surg. 2017;137(2):233-240.
3. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
4. Ferrari D, Lopes TJ, França PF, Azevedo FM, Pappas E. Outpatient versus inpatient anterior cruciate ligament reconstruction: a systematic review with meta-analysis. Knee. 2017;24(2):197-206.
5. Weber AE, Gitelis ME, McCarthy MA, Yanke AB, Cole BJ. Malalignment: a requirement for cartilage and organ restoration. Sports Med Arthrosc. 2016;24(2):e14-e22.
6. Prince MR, King AH, Stuart MJ, Dahm DL, Krych AJ. Treatment of patellofemoral cartilage lesions in the young, active patient. J Knee Surg. 2015;28(4):285-295.
7. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
Take-Home Points
- Thorough preoperative planning is imperative and inclusive of history, physical examination, radiographs, and MRI and potentially CT scan.
- Plan carefully for needed graft sources (autografts and allografts).
- Rehabilitation starts preoperatively and a detailed individualized plan is often warranted.
- Indicated ligamentous repair or augmented repair with reconstruction is more likely to succeed when performed within 2 weeks of injury.
- Complex combined knee restoration surgery can be safely performed in an outpatient setting.
Complex combined knee restoration surgery can be safely performed in an outpatient setting. The term complex knee restoration is used to describe management of knee injuries that are more involved—that is, there is damage to the menisci, cartilage, ligaments, and bones. Management entails not only determining the best treatment options but navigating the more complex logistics of making sure all necessary grafts (fresh and frozen allografts and autografts), implants, and instrumentation are readily available as these cases come to fruition.
The military healthcare paradigm often involves the added logistics of transporting the service member to the correct military treatment facility at the correct time and ensuring the patient’s work-up is complete before he or she arrives for the complex knee restoration. Such cases require significant rehabilitation and time away from family and work, so anything that reduces the morbidity of the surgical undertaking and the overall “morbidity footprint” of time away and that helps the patient return to normal function are value-added and worthy of our attention and diligence in developing an efficient system for managing complex cases.
The globally integrated military healthcare system that is in place has matured over the past decades to allow for the significant majority of the necessary preoperative work-up to be performed at a soldier’s current duty station, wherever in the world that may be, under the guidance of local healthcare providers with specific inputs from the knee restoration surgeon who eventually receives the patient for the planned surgical intervention.
Algorithm for Knee Restoration Planning
Alignment Issues
The first task is to confirm the realignment indication. Realignment may be performed with a proximal opening-wedge medial tibial osteotomy (OWMTO), a distal opening-wedge lateral femoral osteotomy (OWLFO), or a tibial tubercle osteotomy (TTO).1 Given the reproducible clinical improvement achieved and the robust nature of the fixation, these osteotomies are often the first surgical step in complex knee restorations.2 The final determination, made by the surgeon in consultation with the patient, is whether to perform the indicated osteotomy alone or in combination with the rest of the planned restoration surgery. In the vast majority of cases I have managed over the past 2 decades, I have performed the entire knee restoration in a single operation.3 Within the past 5 years, combining the procedures has become even more feasible with the important progress made in multimodal pain management and with the close collaboration of anesthesiologists.4
Meniscus and Cartilage Status
The integration status of meniscus and cartilage within the medial and lateral tibiofemoral compartments is crucial to the comprehensive restoration plan. In fact, the success of the restoration can be said to be dependent on the functional status and health of meniscus and cartilage—which either succeed together or fail apart.
Important covariables are age, prior surgical interventions, activity level expected or allowed after surgery, and size, location, and depth of cartilage injury.5 Whether a cartilage injury is monopolar or bipolar is determined with advanced imaging (magnetic resonance imaging [MRI], computed tomography [CT], weight-bearing radiography) along with analysis of a thorough history (including a review of prior operative reports and arthroscopic images) and a knee examination. Bipolar injuries that involve the condyle and juxtaposed plateau often bode poorly for good clinical outcomes—compared with unipolar lesions, which usually involve the condylar surfaces in isolation. The same thinking regarding the patellofemoral compartment is appropriate. Cartilage lesions that involve the juxtaposed surfaces of the patellar and trochlear groove do poorer than isolated lesions, which are more amenable to cartilage restoration options. The literature on potential cartilage restoration options for the patella and trochlea is expanding. I use the 3-dimensional cartilage restoration option of a fresh patellar osteochondral allograft (OCA) for high-grade cartilage lesions thought to be clinically significant. Other options, such as microfracture, cell-based cartilage restoration, and Osteochondral Autograft Transfer System (Arthrex) procedures (from the thinner condylar cartilage), have varied in their outcomes for patellar lesions. According to more recent literature and a review of my clinical results, fresh patellar OCAs are a good option for patellar lesions.6 Similarly, trochlear lesions can be managed with microfracture, cell-based therapies, or fresh OCAs, depending on surgeon preference.
Functional total or subtotal meniscectomies are often best managed with meniscal allograft transplantation (MAT). An intact or replaced medial or lateral meniscus works synergistically with any planned anterior cruciate ligament (ACL) reconstruction. Again, the adage that meniscus and cartilage succeed together or fail apart is appropriate when planning complex knee restoration. Signs of extrusion or joint-space narrowing and root avulsion or significant loss of meniscal tissue, visualized on MRI or on prior surgical images, often help substantiate a MAT plan. MAT has had the best long-term results when performed in compartments with cartilage damage limited to grade I and grade II changes, in stable knees, and in knees that can be concurrently stabilized.5 Technological advances have increased the value of MAT by limiting the morbidity of the operation and thus allowing for other surgery to be performed concomitantly and safely as part of comprehensive knee restoration. Over the past 20 years, I have arthroscopically performed MAT with bone plugs for medial and lateral procedures, and my results with active-duty soldiers have been promising, paralleling the clinic success reported in the literature.5 Alignment must be considered when performing MAT or cartilage restoration. If the addition of meniscal transplantation or cartilage restoration leaves the knee with residual malalignment of 6° or more, corrective osteotomy is performed.
My view and practice have been to plan for an unloading chondroprotective osteotomy. The goal is a balanced mechanical axis, whether achieved with mere joint-space restoration or with an osteotomy added.
Ligament Status
A comprehensive plan for establishing ligamentous stability is paramount to the overall clinical success of complex knee restorations. Meniscus and cartilage restoration efforts are wasted if clinically significant ligamentous laxity is not concomitantly treated with reconstruction surgery. Revision ACL surgery is by far the most commonly performed surgery in complex knee cases. Diligence in interpreting advanced MRI and physical examination findings is required to make sure there are no concomitant patholaxities in the medial, lateral, posterior, posteromedial, and posterolateral ligamentous complexes. Appropriate ligamentous reconstruction is warranted to maximize clinical results in complex knee restorations. Such cases more commonly require allograft tissue, as the availability of autograft tissue is the limiting issue with 2 or more ligament reconstructions. Military treatment facilities, in which comprehensive knee restorations are performed, have soft-tissue allografts on hand at all times. Having tissue readily available makes it less imperative to determine the most appropriate combined ligamentous reconstruction surgery before the patient arrives—a process that is often difficult. This situation is in contradistinction to the need for specific matched-for-size allograft frozen meniscus and fresh cartilage tissues, both of which require tissue-form procurement in advance of planned restoration surgery.
Rehabilitation Plan
The rehabilitation plan is driven by the part of the complex knee restoration that demands the most caution with respect to weight-bearing and range of motion (ROM) during the first 6 weeks after surgery. The most limiting restorative surgeries involve meniscus and cartilage. Recent clinical trial results support weight-bearing soon after tibial osteotomy performed in the absence of meniscus and cartilage restoration that would otherwise limit weight-bearing for 6 weeks.7 Therefore, most of these complex knee restorations are appropriately managed with a hinged brace locked in extension for toe-touch weight-bearing ambulation, with ROM usually limited to 0° to 90° during the first 6 weeks. Quadriceps rehabilitation with straight-leg raises and isometric contractions is prescribed with a focus on maintaining full extension as the default resting knee position until normalized resting quadriceps tone returns. Full weight-bearing and advancement to full flexion are routinely allowed by 6 weeks.
Case Report
A 41-year-old male service member who was overseas was referred to my clinic for high tibial osteotomy consideration and possible revision ACL reconstruction. His symptoms were medial pain, recurrent instability, and patellofemoral crepitance. Three years earlier, he underwent autograft transtibial ACL reconstruction with significant débridement of the medial meniscus. Before his trip to the United States, I asked that new MRI scans, full-length standing hip–knee–ankle bilateral alignment radiographs, and a 4-view weight-bearing knee series (including a posteroanterior Rosenberg view) be obtained and sent for my review (Figure 1).
Review of the patient’s detailed preoperative imaging work-up and electronic medical record (available through the military’s healthcare system) made it clear that far more surgical intervention was needed than originally assumed. A significant full-thickness chondral lesion of the patella and a subtotal medial meniscectomy would necessitate patellar cartilage restoration and medial MAT in addition to the high tibial osteotomy and revision ACL reconstruction.
Had this patient been sent through the military medical evacuation system, he would have had to make 2 overseas trips—one trip for preoperative evaluation and advanced imaging, whereby he would have been placed on a match list and had to wait for a requested meniscal allograft and an appropriate graft for his patella, and the other trip for his complex surgery. Fortunately, the military’s integrated healthcare network with true 2-way communication and the collaborative use of integrated electronic medical records proved extremely valuable in making management of this complex knee restoration as efficient as possible. From the perspective of the soldier and his military unit, only 1 big overseas trip was needed; from the perspective of the military healthcare system, responsible use of healthcare personnel and monetary resources and well-planned complex knee restoration surgery saved a knee and allowed a soldier-athlete to rejoin the fields of friendly strife.
This patient had undergone functional complete medial meniscectomy and had significant medial compartment pain, varus alignment, and minimal medial joint-space narrowing (assumed grossly intact cartilage about plateau and condyle), plus patellofemoral pain and crepitance with a large high-grade posttraumatic patellar chondral lesion with normal patellofemoral alignment. He also had an isolated failed ACL graft from prior ACL reconstruction. The previous hardware placement was analyzed, and it was determined that the femoral interference screw could be left in place and that the tibial interference screw most likely would be removed. The mechanical axis determined from the bilateral long-leg standing images dictated a need for proximal OWMTO for correction up to 8° to allow the axis to cross the center of the knee. The 8° correction is the measured correction needed to move the axis from its pass through the medial compartment to a more balanced position across the middle of the knee.
The overall plan encompassed major concomitant corrective and restorative surgery: tibial osteotomy, medial MAT, revision ACL reconstruction, and fresh mega-patellar OCA. Once the frozen meniscus and eventually the fresh patella (both matched for size) were obtained, arrangements for the patient’s trip for the complex surgery were finalized.
Surgery was started with brief arthroscopic evaluation to confirm the overall appropriateness of the planned procedure and to determine if any other minor deficiencies would warrant operative intervention. Once confirmed, the restoration proceeded as planned. The OWMTO was performed with a PEEK (polyetheretherketone) wedge implant (iBalance; Arthrex) followed by arthroscopic preparation for medial MAT with removal of any meniscal remnants and placement of passing sutures (Figure 2A).
When the arthroscopic portion of the surgery was finished, a medial parapatellar arthrotomy was made to allow the patella to be inverted and complete fresh mega-patellar OCA placement (Figure 4).
The knee was placed in a ROM brace locked in full extension. The patient was able to do straight-leg raises and calf pumps in the recovery room and was discharged home with a saphenous nerve block and an iPACK (Interspace between the Popliteal Artery and the Capsule of the posterior Knee) nerve block in place. Home-based therapy was started immediately. After the patient’s first postoperative visit, formal therapy (discussed earlier) was initiated (Figure 6).
Discussion
All-inside GraftLink ACL reconstruction with cortical suspensory fixation appears well suited to combined medial and lateral MAT and/or cartilage restoration—whether it be large fresh OCA combined with medial MAT (as in this patient’s case) or another form of cartilage restoration. Arthroscopic MAT with anatomically fashioned and placed bone plugs minimizes the morbidity within the notch footprints and allows for discrete revision socket formation for both femoral and tibial ACL graft placement. In this case, preparation for the medial MAT and ACL sockets was followed by MAT/ACL construct implantation and secure fixation. The arthrotomy was thereby minimized and placed to allow for efficient mega-patellar OCA graft placement.
Over the past decade, I have performed similar concomitant procedures using the same surgical principles that allow for efficient and reproducible complex knee restoration (Figure 7).
Although use of an algorithm for the management of complex knee restorations is not universally feasible, I offer guidelines for complex knee injuries:
- At each decision point, determine whether the knee and the patient can withstand the planned surgical intervention.
- After deciding to proceed with knee restoration, list the meniscus, cartilage, and ligament injuries that must be addressed.
- Determine which repairs (meniscus, cartilage, ligament) are warranted. Repairs generally are best performed within a period of 7 to 14 days.
- Determine which ligament injuries warrant reconstruction. Allograft tissue typically is used for multiligament reconstruction.
- Rank-order the ligament reconstruction requirements. It is fine to proceed with all of the reconstructions if the case is moving smoothly, if there are no developing tourniquet-time issues, and if the soft-tissue envelope is responding as expected.
- Consider autograft and/or allograft tissue needs for concomitant or staged meniscus and cartilage restoration options/requirements.
Am J Orthop. 2017;46(4):170-175, 202. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Thorough preoperative planning is imperative and inclusive of history, physical examination, radiographs, and MRI and potentially CT scan.
- Plan carefully for needed graft sources (autografts and allografts).
- Rehabilitation starts preoperatively and a detailed individualized plan is often warranted.
- Indicated ligamentous repair or augmented repair with reconstruction is more likely to succeed when performed within 2 weeks of injury.
- Complex combined knee restoration surgery can be safely performed in an outpatient setting.
Complex combined knee restoration surgery can be safely performed in an outpatient setting. The term complex knee restoration is used to describe management of knee injuries that are more involved—that is, there is damage to the menisci, cartilage, ligaments, and bones. Management entails not only determining the best treatment options but navigating the more complex logistics of making sure all necessary grafts (fresh and frozen allografts and autografts), implants, and instrumentation are readily available as these cases come to fruition.
The military healthcare paradigm often involves the added logistics of transporting the service member to the correct military treatment facility at the correct time and ensuring the patient’s work-up is complete before he or she arrives for the complex knee restoration. Such cases require significant rehabilitation and time away from family and work, so anything that reduces the morbidity of the surgical undertaking and the overall “morbidity footprint” of time away and that helps the patient return to normal function are value-added and worthy of our attention and diligence in developing an efficient system for managing complex cases.
The globally integrated military healthcare system that is in place has matured over the past decades to allow for the significant majority of the necessary preoperative work-up to be performed at a soldier’s current duty station, wherever in the world that may be, under the guidance of local healthcare providers with specific inputs from the knee restoration surgeon who eventually receives the patient for the planned surgical intervention.
Algorithm for Knee Restoration Planning
Alignment Issues
The first task is to confirm the realignment indication. Realignment may be performed with a proximal opening-wedge medial tibial osteotomy (OWMTO), a distal opening-wedge lateral femoral osteotomy (OWLFO), or a tibial tubercle osteotomy (TTO).1 Given the reproducible clinical improvement achieved and the robust nature of the fixation, these osteotomies are often the first surgical step in complex knee restorations.2 The final determination, made by the surgeon in consultation with the patient, is whether to perform the indicated osteotomy alone or in combination with the rest of the planned restoration surgery. In the vast majority of cases I have managed over the past 2 decades, I have performed the entire knee restoration in a single operation.3 Within the past 5 years, combining the procedures has become even more feasible with the important progress made in multimodal pain management and with the close collaboration of anesthesiologists.4
Meniscus and Cartilage Status
The integration status of meniscus and cartilage within the medial and lateral tibiofemoral compartments is crucial to the comprehensive restoration plan. In fact, the success of the restoration can be said to be dependent on the functional status and health of meniscus and cartilage—which either succeed together or fail apart.
Important covariables are age, prior surgical interventions, activity level expected or allowed after surgery, and size, location, and depth of cartilage injury.5 Whether a cartilage injury is monopolar or bipolar is determined with advanced imaging (magnetic resonance imaging [MRI], computed tomography [CT], weight-bearing radiography) along with analysis of a thorough history (including a review of prior operative reports and arthroscopic images) and a knee examination. Bipolar injuries that involve the condyle and juxtaposed plateau often bode poorly for good clinical outcomes—compared with unipolar lesions, which usually involve the condylar surfaces in isolation. The same thinking regarding the patellofemoral compartment is appropriate. Cartilage lesions that involve the juxtaposed surfaces of the patellar and trochlear groove do poorer than isolated lesions, which are more amenable to cartilage restoration options. The literature on potential cartilage restoration options for the patella and trochlea is expanding. I use the 3-dimensional cartilage restoration option of a fresh patellar osteochondral allograft (OCA) for high-grade cartilage lesions thought to be clinically significant. Other options, such as microfracture, cell-based cartilage restoration, and Osteochondral Autograft Transfer System (Arthrex) procedures (from the thinner condylar cartilage), have varied in their outcomes for patellar lesions. According to more recent literature and a review of my clinical results, fresh patellar OCAs are a good option for patellar lesions.6 Similarly, trochlear lesions can be managed with microfracture, cell-based therapies, or fresh OCAs, depending on surgeon preference.
Functional total or subtotal meniscectomies are often best managed with meniscal allograft transplantation (MAT). An intact or replaced medial or lateral meniscus works synergistically with any planned anterior cruciate ligament (ACL) reconstruction. Again, the adage that meniscus and cartilage succeed together or fail apart is appropriate when planning complex knee restoration. Signs of extrusion or joint-space narrowing and root avulsion or significant loss of meniscal tissue, visualized on MRI or on prior surgical images, often help substantiate a MAT plan. MAT has had the best long-term results when performed in compartments with cartilage damage limited to grade I and grade II changes, in stable knees, and in knees that can be concurrently stabilized.5 Technological advances have increased the value of MAT by limiting the morbidity of the operation and thus allowing for other surgery to be performed concomitantly and safely as part of comprehensive knee restoration. Over the past 20 years, I have arthroscopically performed MAT with bone plugs for medial and lateral procedures, and my results with active-duty soldiers have been promising, paralleling the clinic success reported in the literature.5 Alignment must be considered when performing MAT or cartilage restoration. If the addition of meniscal transplantation or cartilage restoration leaves the knee with residual malalignment of 6° or more, corrective osteotomy is performed.
My view and practice have been to plan for an unloading chondroprotective osteotomy. The goal is a balanced mechanical axis, whether achieved with mere joint-space restoration or with an osteotomy added.
Ligament Status
A comprehensive plan for establishing ligamentous stability is paramount to the overall clinical success of complex knee restorations. Meniscus and cartilage restoration efforts are wasted if clinically significant ligamentous laxity is not concomitantly treated with reconstruction surgery. Revision ACL surgery is by far the most commonly performed surgery in complex knee cases. Diligence in interpreting advanced MRI and physical examination findings is required to make sure there are no concomitant patholaxities in the medial, lateral, posterior, posteromedial, and posterolateral ligamentous complexes. Appropriate ligamentous reconstruction is warranted to maximize clinical results in complex knee restorations. Such cases more commonly require allograft tissue, as the availability of autograft tissue is the limiting issue with 2 or more ligament reconstructions. Military treatment facilities, in which comprehensive knee restorations are performed, have soft-tissue allografts on hand at all times. Having tissue readily available makes it less imperative to determine the most appropriate combined ligamentous reconstruction surgery before the patient arrives—a process that is often difficult. This situation is in contradistinction to the need for specific matched-for-size allograft frozen meniscus and fresh cartilage tissues, both of which require tissue-form procurement in advance of planned restoration surgery.
Rehabilitation Plan
The rehabilitation plan is driven by the part of the complex knee restoration that demands the most caution with respect to weight-bearing and range of motion (ROM) during the first 6 weeks after surgery. The most limiting restorative surgeries involve meniscus and cartilage. Recent clinical trial results support weight-bearing soon after tibial osteotomy performed in the absence of meniscus and cartilage restoration that would otherwise limit weight-bearing for 6 weeks.7 Therefore, most of these complex knee restorations are appropriately managed with a hinged brace locked in extension for toe-touch weight-bearing ambulation, with ROM usually limited to 0° to 90° during the first 6 weeks. Quadriceps rehabilitation with straight-leg raises and isometric contractions is prescribed with a focus on maintaining full extension as the default resting knee position until normalized resting quadriceps tone returns. Full weight-bearing and advancement to full flexion are routinely allowed by 6 weeks.
Case Report
A 41-year-old male service member who was overseas was referred to my clinic for high tibial osteotomy consideration and possible revision ACL reconstruction. His symptoms were medial pain, recurrent instability, and patellofemoral crepitance. Three years earlier, he underwent autograft transtibial ACL reconstruction with significant débridement of the medial meniscus. Before his trip to the United States, I asked that new MRI scans, full-length standing hip–knee–ankle bilateral alignment radiographs, and a 4-view weight-bearing knee series (including a posteroanterior Rosenberg view) be obtained and sent for my review (Figure 1).
Review of the patient’s detailed preoperative imaging work-up and electronic medical record (available through the military’s healthcare system) made it clear that far more surgical intervention was needed than originally assumed. A significant full-thickness chondral lesion of the patella and a subtotal medial meniscectomy would necessitate patellar cartilage restoration and medial MAT in addition to the high tibial osteotomy and revision ACL reconstruction.
Had this patient been sent through the military medical evacuation system, he would have had to make 2 overseas trips—one trip for preoperative evaluation and advanced imaging, whereby he would have been placed on a match list and had to wait for a requested meniscal allograft and an appropriate graft for his patella, and the other trip for his complex surgery. Fortunately, the military’s integrated healthcare network with true 2-way communication and the collaborative use of integrated electronic medical records proved extremely valuable in making management of this complex knee restoration as efficient as possible. From the perspective of the soldier and his military unit, only 1 big overseas trip was needed; from the perspective of the military healthcare system, responsible use of healthcare personnel and monetary resources and well-planned complex knee restoration surgery saved a knee and allowed a soldier-athlete to rejoin the fields of friendly strife.
This patient had undergone functional complete medial meniscectomy and had significant medial compartment pain, varus alignment, and minimal medial joint-space narrowing (assumed grossly intact cartilage about plateau and condyle), plus patellofemoral pain and crepitance with a large high-grade posttraumatic patellar chondral lesion with normal patellofemoral alignment. He also had an isolated failed ACL graft from prior ACL reconstruction. The previous hardware placement was analyzed, and it was determined that the femoral interference screw could be left in place and that the tibial interference screw most likely would be removed. The mechanical axis determined from the bilateral long-leg standing images dictated a need for proximal OWMTO for correction up to 8° to allow the axis to cross the center of the knee. The 8° correction is the measured correction needed to move the axis from its pass through the medial compartment to a more balanced position across the middle of the knee.
The overall plan encompassed major concomitant corrective and restorative surgery: tibial osteotomy, medial MAT, revision ACL reconstruction, and fresh mega-patellar OCA. Once the frozen meniscus and eventually the fresh patella (both matched for size) were obtained, arrangements for the patient’s trip for the complex surgery were finalized.
Surgery was started with brief arthroscopic evaluation to confirm the overall appropriateness of the planned procedure and to determine if any other minor deficiencies would warrant operative intervention. Once confirmed, the restoration proceeded as planned. The OWMTO was performed with a PEEK (polyetheretherketone) wedge implant (iBalance; Arthrex) followed by arthroscopic preparation for medial MAT with removal of any meniscal remnants and placement of passing sutures (Figure 2A).
When the arthroscopic portion of the surgery was finished, a medial parapatellar arthrotomy was made to allow the patella to be inverted and complete fresh mega-patellar OCA placement (Figure 4).
The knee was placed in a ROM brace locked in full extension. The patient was able to do straight-leg raises and calf pumps in the recovery room and was discharged home with a saphenous nerve block and an iPACK (Interspace between the Popliteal Artery and the Capsule of the posterior Knee) nerve block in place. Home-based therapy was started immediately. After the patient’s first postoperative visit, formal therapy (discussed earlier) was initiated (Figure 6).
Discussion
All-inside GraftLink ACL reconstruction with cortical suspensory fixation appears well suited to combined medial and lateral MAT and/or cartilage restoration—whether it be large fresh OCA combined with medial MAT (as in this patient’s case) or another form of cartilage restoration. Arthroscopic MAT with anatomically fashioned and placed bone plugs minimizes the morbidity within the notch footprints and allows for discrete revision socket formation for both femoral and tibial ACL graft placement. In this case, preparation for the medial MAT and ACL sockets was followed by MAT/ACL construct implantation and secure fixation. The arthrotomy was thereby minimized and placed to allow for efficient mega-patellar OCA graft placement.
Over the past decade, I have performed similar concomitant procedures using the same surgical principles that allow for efficient and reproducible complex knee restoration (Figure 7).
Although use of an algorithm for the management of complex knee restorations is not universally feasible, I offer guidelines for complex knee injuries:
- At each decision point, determine whether the knee and the patient can withstand the planned surgical intervention.
- After deciding to proceed with knee restoration, list the meniscus, cartilage, and ligament injuries that must be addressed.
- Determine which repairs (meniscus, cartilage, ligament) are warranted. Repairs generally are best performed within a period of 7 to 14 days.
- Determine which ligament injuries warrant reconstruction. Allograft tissue typically is used for multiligament reconstruction.
- Rank-order the ligament reconstruction requirements. It is fine to proceed with all of the reconstructions if the case is moving smoothly, if there are no developing tourniquet-time issues, and if the soft-tissue envelope is responding as expected.
- Consider autograft and/or allograft tissue needs for concomitant or staged meniscus and cartilage restoration options/requirements.
Am J Orthop. 2017;46(4):170-175, 202. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Uquillas C, Rossy W, Nathasingh CK, Strauss E, Jazrawi L, Gonzalez-Lomas G. Osteotomies about the knee: AAOS exhibit selection. J Bone Joint Surg Am. 2014;96(24):e199.
2. Mehl J, Paul J, Feucht MJ, et al. ACL deficiency and varus osteoarthritis: high tibial osteotomy alone or combined with ACL reconstruction? Arch Orthop Trauma Surg. 2017;137(2):233-240.
3. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
4. Ferrari D, Lopes TJ, França PF, Azevedo FM, Pappas E. Outpatient versus inpatient anterior cruciate ligament reconstruction: a systematic review with meta-analysis. Knee. 2017;24(2):197-206.
5. Weber AE, Gitelis ME, McCarthy MA, Yanke AB, Cole BJ. Malalignment: a requirement for cartilage and organ restoration. Sports Med Arthrosc. 2016;24(2):e14-e22.
6. Prince MR, King AH, Stuart MJ, Dahm DL, Krych AJ. Treatment of patellofemoral cartilage lesions in the young, active patient. J Knee Surg. 2015;28(4):285-295.
7. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
1. Uquillas C, Rossy W, Nathasingh CK, Strauss E, Jazrawi L, Gonzalez-Lomas G. Osteotomies about the knee: AAOS exhibit selection. J Bone Joint Surg Am. 2014;96(24):e199.
2. Mehl J, Paul J, Feucht MJ, et al. ACL deficiency and varus osteoarthritis: high tibial osteotomy alone or combined with ACL reconstruction? Arch Orthop Trauma Surg. 2017;137(2):233-240.
3. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
4. Ferrari D, Lopes TJ, França PF, Azevedo FM, Pappas E. Outpatient versus inpatient anterior cruciate ligament reconstruction: a systematic review with meta-analysis. Knee. 2017;24(2):197-206.
5. Weber AE, Gitelis ME, McCarthy MA, Yanke AB, Cole BJ. Malalignment: a requirement for cartilage and organ restoration. Sports Med Arthrosc. 2016;24(2):e14-e22.
6. Prince MR, King AH, Stuart MJ, Dahm DL, Krych AJ. Treatment of patellofemoral cartilage lesions in the young, active patient. J Knee Surg. 2015;28(4):285-295.
7. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
Home of the Brave
This Memorial Day, with all that is taking place in the world, it was hard not to think about the brave men and women who have sacrificed so much to preserve our freedom. They are away from their families, sometimes for years at a time, and they operate in the most dangerous places in the world. A safe return is not guaranteed. I am thankful for these intrepid men and women whose sacrifices and commitment to their country allow me to live comfortably at home with my family and practice orthopedic surgery.
A few years ago, my wife and I traveled to Normandy and visited the American cemetery. It was a moving experience that I will never forget. We then toured Pointe du Hoc, the elevated peninsula separating Omaha and Utah beach and the location of German gun emplacements covering both beaches. The bunkers, and even the craters from the bombs, are still there. Army Rangers were tasked with launching an amphibious assault on the beach and then scaling the 100-foot cliffs using grappling hooks, ropes, and ladders. Once at the top, they faced a heavily fortified German force that was dug in. Looking down at the beach and out over the ocean from above, I thought of the troops who landed there and the impossible task they faced. Despite the overwhelming odds stacked against them, the Rangers took Pointe du Hoc in 25 minutes and then repelled multiple counterattacks with their backs against the cliff. In my opinion, it’s one of the greatest testaments to the incredible determination and ability of our military personnel.
Speaking of incredible ability, AJO would like to recognize our military orthopedists. They are often deployed in combat zones and provide the best of care for our soldiers while working in the most stressful of conditions, and doing it all on a government salary. In their spare time, they’ve contributed so much to the orthopedic literature, authoring numerous landmark articles.
In this issue, AJO looks at classic military injuries: shoulder instability, stress fractures, and multi-ligamentous knee injuries. Provencher and colleagues authored a comprehensive review of instability with current guidelines for determining surgical approach. DeBerardino shows our readers how to take a military approach to multi-ligament and complex knee injuries, and Owens and colleagues provide a guide to the diagnosis and treatment of stress injuries to bone.
We also take a moment to recognize 3 members of our military orthopedic family whose lives were tragically cut short. Warren R. Kadrmas, Brian Allgood, and Benjamin Whetstone Schmidt’s memorials are included on the following pages. Benjamin Whetstone Schmidt, son of orthopedist David R. Schmidt from San Antonio, was a Marine Sniper killed in action in Afghanistan after volunteering for a second tour. After his death, the LCpl Benjamin Whetstone Schmidt Endowed Professorship in History was created at the Texas Christian University. Contributions can be made in his memory at www.heartofpurple.com.
As Independence Day is celebrated, AJO is pleased to present “Military Orthopedics” to honor our troops and the military doctors who support them. As you read this issue, take a moment to reflect on the freedoms you enjoy because America is truly the Home of the Brave.
Am J Orthop. 2017;46(4):166. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
This Memorial Day, with all that is taking place in the world, it was hard not to think about the brave men and women who have sacrificed so much to preserve our freedom. They are away from their families, sometimes for years at a time, and they operate in the most dangerous places in the world. A safe return is not guaranteed. I am thankful for these intrepid men and women whose sacrifices and commitment to their country allow me to live comfortably at home with my family and practice orthopedic surgery.
A few years ago, my wife and I traveled to Normandy and visited the American cemetery. It was a moving experience that I will never forget. We then toured Pointe du Hoc, the elevated peninsula separating Omaha and Utah beach and the location of German gun emplacements covering both beaches. The bunkers, and even the craters from the bombs, are still there. Army Rangers were tasked with launching an amphibious assault on the beach and then scaling the 100-foot cliffs using grappling hooks, ropes, and ladders. Once at the top, they faced a heavily fortified German force that was dug in. Looking down at the beach and out over the ocean from above, I thought of the troops who landed there and the impossible task they faced. Despite the overwhelming odds stacked against them, the Rangers took Pointe du Hoc in 25 minutes and then repelled multiple counterattacks with their backs against the cliff. In my opinion, it’s one of the greatest testaments to the incredible determination and ability of our military personnel.
Speaking of incredible ability, AJO would like to recognize our military orthopedists. They are often deployed in combat zones and provide the best of care for our soldiers while working in the most stressful of conditions, and doing it all on a government salary. In their spare time, they’ve contributed so much to the orthopedic literature, authoring numerous landmark articles.
In this issue, AJO looks at classic military injuries: shoulder instability, stress fractures, and multi-ligamentous knee injuries. Provencher and colleagues authored a comprehensive review of instability with current guidelines for determining surgical approach. DeBerardino shows our readers how to take a military approach to multi-ligament and complex knee injuries, and Owens and colleagues provide a guide to the diagnosis and treatment of stress injuries to bone.
We also take a moment to recognize 3 members of our military orthopedic family whose lives were tragically cut short. Warren R. Kadrmas, Brian Allgood, and Benjamin Whetstone Schmidt’s memorials are included on the following pages. Benjamin Whetstone Schmidt, son of orthopedist David R. Schmidt from San Antonio, was a Marine Sniper killed in action in Afghanistan after volunteering for a second tour. After his death, the LCpl Benjamin Whetstone Schmidt Endowed Professorship in History was created at the Texas Christian University. Contributions can be made in his memory at www.heartofpurple.com.
As Independence Day is celebrated, AJO is pleased to present “Military Orthopedics” to honor our troops and the military doctors who support them. As you read this issue, take a moment to reflect on the freedoms you enjoy because America is truly the Home of the Brave.
Am J Orthop. 2017;46(4):166. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
This Memorial Day, with all that is taking place in the world, it was hard not to think about the brave men and women who have sacrificed so much to preserve our freedom. They are away from their families, sometimes for years at a time, and they operate in the most dangerous places in the world. A safe return is not guaranteed. I am thankful for these intrepid men and women whose sacrifices and commitment to their country allow me to live comfortably at home with my family and practice orthopedic surgery.
A few years ago, my wife and I traveled to Normandy and visited the American cemetery. It was a moving experience that I will never forget. We then toured Pointe du Hoc, the elevated peninsula separating Omaha and Utah beach and the location of German gun emplacements covering both beaches. The bunkers, and even the craters from the bombs, are still there. Army Rangers were tasked with launching an amphibious assault on the beach and then scaling the 100-foot cliffs using grappling hooks, ropes, and ladders. Once at the top, they faced a heavily fortified German force that was dug in. Looking down at the beach and out over the ocean from above, I thought of the troops who landed there and the impossible task they faced. Despite the overwhelming odds stacked against them, the Rangers took Pointe du Hoc in 25 minutes and then repelled multiple counterattacks with their backs against the cliff. In my opinion, it’s one of the greatest testaments to the incredible determination and ability of our military personnel.
Speaking of incredible ability, AJO would like to recognize our military orthopedists. They are often deployed in combat zones and provide the best of care for our soldiers while working in the most stressful of conditions, and doing it all on a government salary. In their spare time, they’ve contributed so much to the orthopedic literature, authoring numerous landmark articles.
In this issue, AJO looks at classic military injuries: shoulder instability, stress fractures, and multi-ligamentous knee injuries. Provencher and colleagues authored a comprehensive review of instability with current guidelines for determining surgical approach. DeBerardino shows our readers how to take a military approach to multi-ligament and complex knee injuries, and Owens and colleagues provide a guide to the diagnosis and treatment of stress injuries to bone.
We also take a moment to recognize 3 members of our military orthopedic family whose lives were tragically cut short. Warren R. Kadrmas, Brian Allgood, and Benjamin Whetstone Schmidt’s memorials are included on the following pages. Benjamin Whetstone Schmidt, son of orthopedist David R. Schmidt from San Antonio, was a Marine Sniper killed in action in Afghanistan after volunteering for a second tour. After his death, the LCpl Benjamin Whetstone Schmidt Endowed Professorship in History was created at the Texas Christian University. Contributions can be made in his memory at www.heartofpurple.com.
As Independence Day is celebrated, AJO is pleased to present “Military Orthopedics” to honor our troops and the military doctors who support them. As you read this issue, take a moment to reflect on the freedoms you enjoy because America is truly the Home of the Brave.
Am J Orthop. 2017;46(4):166. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Segregated Neighborhoods Can Raise Blood Pressure
Living in a racially segregated neighborhood can be bad for the blood pressure (BP) if you are a black adult, according to Northwestern University researchers. They found that simply moving away from that neighborhood is enough to reduce systolic blood pressure 1 to 5 points.
In the study, which was partly funded by the NIH, the researchers examined BP readings for 2,280 black adults who participated in the Coronary Artery Risk Development in Young Adults (CARDIA) study. The participants were initially screened in 1985 and 1986 then reexamined several times over the next 25 years. The Northwestern study is the first to look at the longitudinal effects of segregation on BP compare the effect within the same individuals. Previous research had looked at single points in time.
The researchers categorized neighborhood segregation as high, medium, or low, using a scale that compareed the percentage of black residents in a neighborhood to the surrounding area. When neighborhoods were more segregated, the participants had small but statistically significant increases in systolic BP. Less segregation equaled a “notable” drop in BP.
Participants who lived in a highly segregated neighborhood and moved to a less segregated one saw the most significant improvements. Those who moved temporarily saw a 1 mm Hg drop. A permanent move equaled 3 to 5 mm Hg. That’s a “powerful effect,” said lead author Kiarri Kershaw, assistant professor of preventive medicine at Northwestern. Just 1 mm Hg lower at the population level, she notes, could mean “meaningful” reductions in heart attacks, strokes, and heart failure. The associations persisted even after the researchers accounted for marital status, body mass index, smoking history, physical activity, and socioeconomic status of the community.
The changes in blood pressure were not related to poverty or household income. Kershaw says less stress, achieved by reducing exposure to violence and improving opportunities for socioeconomic mobility, is “likely a key factor.” Other factors that could help include improving access to health-promoting resources, such as full-service grocery stores, recreation centers, and health care clinics.
Living in a racially segregated neighborhood can be bad for the blood pressure (BP) if you are a black adult, according to Northwestern University researchers. They found that simply moving away from that neighborhood is enough to reduce systolic blood pressure 1 to 5 points.
In the study, which was partly funded by the NIH, the researchers examined BP readings for 2,280 black adults who participated in the Coronary Artery Risk Development in Young Adults (CARDIA) study. The participants were initially screened in 1985 and 1986 then reexamined several times over the next 25 years. The Northwestern study is the first to look at the longitudinal effects of segregation on BP compare the effect within the same individuals. Previous research had looked at single points in time.
The researchers categorized neighborhood segregation as high, medium, or low, using a scale that compareed the percentage of black residents in a neighborhood to the surrounding area. When neighborhoods were more segregated, the participants had small but statistically significant increases in systolic BP. Less segregation equaled a “notable” drop in BP.
Participants who lived in a highly segregated neighborhood and moved to a less segregated one saw the most significant improvements. Those who moved temporarily saw a 1 mm Hg drop. A permanent move equaled 3 to 5 mm Hg. That’s a “powerful effect,” said lead author Kiarri Kershaw, assistant professor of preventive medicine at Northwestern. Just 1 mm Hg lower at the population level, she notes, could mean “meaningful” reductions in heart attacks, strokes, and heart failure. The associations persisted even after the researchers accounted for marital status, body mass index, smoking history, physical activity, and socioeconomic status of the community.
The changes in blood pressure were not related to poverty or household income. Kershaw says less stress, achieved by reducing exposure to violence and improving opportunities for socioeconomic mobility, is “likely a key factor.” Other factors that could help include improving access to health-promoting resources, such as full-service grocery stores, recreation centers, and health care clinics.
Living in a racially segregated neighborhood can be bad for the blood pressure (BP) if you are a black adult, according to Northwestern University researchers. They found that simply moving away from that neighborhood is enough to reduce systolic blood pressure 1 to 5 points.
In the study, which was partly funded by the NIH, the researchers examined BP readings for 2,280 black adults who participated in the Coronary Artery Risk Development in Young Adults (CARDIA) study. The participants were initially screened in 1985 and 1986 then reexamined several times over the next 25 years. The Northwestern study is the first to look at the longitudinal effects of segregation on BP compare the effect within the same individuals. Previous research had looked at single points in time.
The researchers categorized neighborhood segregation as high, medium, or low, using a scale that compareed the percentage of black residents in a neighborhood to the surrounding area. When neighborhoods were more segregated, the participants had small but statistically significant increases in systolic BP. Less segregation equaled a “notable” drop in BP.
Participants who lived in a highly segregated neighborhood and moved to a less segregated one saw the most significant improvements. Those who moved temporarily saw a 1 mm Hg drop. A permanent move equaled 3 to 5 mm Hg. That’s a “powerful effect,” said lead author Kiarri Kershaw, assistant professor of preventive medicine at Northwestern. Just 1 mm Hg lower at the population level, she notes, could mean “meaningful” reductions in heart attacks, strokes, and heart failure. The associations persisted even after the researchers accounted for marital status, body mass index, smoking history, physical activity, and socioeconomic status of the community.
The changes in blood pressure were not related to poverty or household income. Kershaw says less stress, achieved by reducing exposure to violence and improving opportunities for socioeconomic mobility, is “likely a key factor.” Other factors that could help include improving access to health-promoting resources, such as full-service grocery stores, recreation centers, and health care clinics.
Zollinger-Ellison Syndrome: Not Your Average Peptic Ulcer Disease
IN THIS ARTICLE
- Diagnostic criteria
- Pharmacologic management
- Patient education
A more severe variant of peptic ulcer disease, Zollinger-Ellison syndrome (ZES) is a rare, chronic, and potentially life-threatening ulcerative disorder. Because the syndrome can be easily misdiagnosed based on clinical presentation alone, primary care clinicians need to be aware of its diagnostic features and know when referral to a gastroenterologist is necessary. Clinicians should suspect ZES in patients with peptic ulcer disease that is refractory to traditional medications.
Caused by a gastrin-secreting neuroendocrine tumor of the pancreas or duodenum called a gastrinoma, ZES can be benign or malignant. It typically manifests in white men ages 30 to 50.1 Due to the significant number of patients treated for a benign cause of peptic ulcer disease (eg, Helicobacter pylori or NSAID-induced ulcers) who are never tested for ZES, the exact incidence is difficult to determine.2 However, it is estimated that approximately 0.1 to 3 people per million develop the disease annually.3
PATHOPHYSIOLOGY
Approximately 80% of gastrinomas occur in the “gastrinoma triangle,” outlined by the hepatic portal vein, neck and body of the pancreas, and latter two-thirds of the duodenum (see Figure).1,4,5 Most gastrinomas involved in ZES occur sporadically, but there is a hereditary component associated with multiple endocrine neoplasia type 1 (MEN1), an autosomal dominant disorder.4
The overproduction and secretion of gastrin by the gastrinoma stimulates hypersecretion of hydrochloric acid.4 This is distinguished from high gastrin levels in the setting of fasting hypochlorhydria or achlorhydria, which may be caused by chronic atrophic gastritis, proton pump inhibitor (PPI) use, or pernicious anemia.5 The chronic hypersecretion of acid causes ulcerations to form. Most commonly, a single ulcer forms in the first portion of the duodenum.3
CLINICAL PRESENTATION
Patients with ZES often report vague abdominal pain that may mimic peptic ulcer disease on initial presentation. The widespread use of PPIs can mask symptoms, and one-fourth of patients present with no abdominal pain at all.6 Patients may also present with
The physical exam may be within normal limits, and no physical finding is considered pathognomonic for ZES. Findings may include epigastric tenderness; pallor, due to an ulcer-related anemia or GI bleed; jaundice, if there is liver involvement; and esophageal or dental erosions, due to excessive acid.8
DIAGNOSIS
Patients with symptoms refractory to medical management should be referred to a specialist for further testing. Once a patient is referred, a gastroenterologist will perform lab tests and imaging studies. In order to be diagnosed with ZES, the patient must exhibit an acidic environment with a pH less than 2 and an inappropriate release of gastrin with a basal acid output greater than 15 mEq/h (or > 5 mEq/h in a patient with prior acid reduction surgery).5,6
Fasting serum gastrin (FSG) is the initial study of choice, followed by a secretin-stimulating test when necessary.9 Diagnosis is established by an FSG level greater than 100 pg/mL; if more than 10-fold the normal level, no further testing is needed. However, results often range from 100 to 1,000 pg/mL.6,10 At these values, further testing with secretin stimulation is warranted.9 The test is performed with an IV injection of secretin, and blood samples are obtained to measure serum gastrin levels.10 An increase greater than 100 pg/mL is considered positive; one greater than 200 pg/mL is diagnostic.3
Once lab tests have been performed, a series of imaging studies are indicated. Endoscopy is used to identify active ulcers and erosions due to long-term acid secretion.3 CT, MRI, and somatostatin receptor scintigraphy (a specialized form of imaging that is the study of choice for localizing gastrinomas) are performed to localize primary tumors and identify any metastatic disease that may be present.10 Finally, after lab tests and imaging studies have been completed, genetic screening for MEN1 is used to determine if the patient has a sporadic or hereditary gastrinoma.3
MANAGEMENT
Once ZES has been diagnosed, the specialist will refer the patient for surgical opinion. The main objectives of surgery are to determine whether the tumor is malignant via biopsy, and to resect the tumor to suppress the acid hypersecretion, if indicated in the absence of liver metastasis and large pancreatic tumor size. Medical management should begin immediately to prevent any further damage from prolonged gastric hypersecretion.1
Pharmacologic options include PPIs, H2-receptor antagonists, and somatostatin analogues; PPIs are considered firstline therapy. Many patients with ZES require a higher dosage than is needed with typical GERD (60-100 mg/d vs 20-40 mg/d). Somatostatin analogues can be used in conjunction with PPIs and have been shown to inhibit tumor growth in patients with malignant ZES.1
Once a ZES diagnosis has been made, the tumor(s) resected (if appropriate), and vagotomy considered or performed, patients will need routine follow-up with their gastroenterologist and their primary care provider, who can manage medications and recommend any lifestyle changes.5
PROGNOSIS
The most important prognostic factor of patients with ZES is whether the gastrinoma is benign or malignant. There are two patterns: aggressive disease (25%) and nonaggressive disease (75%).5 At diagnosis, 40% to 70% of patients with sporadic ZES present with lymph node metastases, and 20% to 40% present with liver metastases. Patients with liver metastases have a 10-year survival rate of 30%, compared to a 15-year survival rate of 83% in patients without liver metastases.11,12
Along with the tumor itself, another prognostic factor to consider is the FSG level at diagnosis. Patients with higher FSG levels have decreased five- and 10-year survival rates compared to patients with lower FSG values. The 10-year survival rate for patients with a lower FSG value (0-499 pg/mL) is 86%, while the 10-year survival rate for those with a greater FSG value (> 1,000 pg/mL) is 73%.11,12 Overall, the prognosis is good for patients with ZES. The 10-year survival rate is high, and management is possible with medications and surgical resection of the gastrinoma.
PATIENT EDUCATION
Once patients are diagnosed, treatment with PPIs is typically lifelong unless they are considered cured by surgical resection. Patients need to understand that compliance is necessary to properly manage symptoms; certain foods, alcohol, and tobacco can affect the condition, and lifestyle modifications should be made, as they would with typical GERD or peptic ulcer disease.
CONCLUSION
ZES is frequently overlooked, and patients often continue to experience unresolved symptoms related to hypergastrinemia. Due to its complexity and ability to mimic other disorders—as well as the implications of duodenal versus pancreatic location, and other disorders of the kidney or endocrine system suggestive of MEN1—ZES should be ruled out in any patient with unexplained persistent GERD, peptic ulcer disease, elevated FSG, chronic diarrhea, and/or abdominal pain.5
The gastrinoma itself is a well-differentiated and slow-growing tumor in the majority of cases, making the prognosis for ZES favorable for long-term survival. Proper pharmacologic management is instrumental for controlling symptoms and decreasing acid production. Surgical resection offers patients the best chance for a complete cure. Clinicians and patients should be well educated about ZES in order to successfully manage the disorder.
1. Tomassetti P, Campana D, Piscitelli L, et al. Treatment of Zollinger-Ellison syndrome. World J Gastroenterol. 2005; 11(35):5423-5432.
2. Metz DC. Diagnosis of the Zollinger-Ellison syndrome. Clin Gastroenterol Hepatol. 2016;10(2):126-130.
3. Epelboym I, Mazeh H. Zollinger-Ellison syndrome: classical considerations and current controversies. Oncologist. 2014; 19(1):44-50.
4. Papadakis M, McPhee S, Rabow M. Current Medical Diagnosis and Treatment 2014. New York, NY: McGraw-Hill Education; 2014:600-601.
5. Feldman M, Friedman LS, Lawrence BJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Philadelphia, PA: Saunders/Elsevier; 2016:511-515.
6. Ito T, Cadiot G, Jensen RT. Diagnosis of Zollinger-Ellison syndrome: increasingly difficult. World J Gastroenterol. 2012; 18(39):5495-5503.
7. Blonski WC, Katzka DA, Lichtenstein GR, Metz DC. Idiopathic gastric acid hypersecretion presenting as a diarrheal disorder and mimicking both Zollinger-Ellison syndrome and Crohn’s disease. Eur J Gastroenterol Hepatol. 2005;17(4):441-444.
8. Roy PK. Zollinger-Ellison syndrome clinical presentation. http://emedicine.medscape.com/article/183555-clinical#b4. Accessed June 14, 2017.
9. Berna MJ, Hoffmann KM, Serrano J, et al. Serum gastrin in Zollinger-Ellison syndrome: I. prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore). 2006;85(6):295-330.
10. Moore AR, Varro A, Pritchard M. Zollinger-Ellison syndrome. Gastrointestinal Nursing. 2012;10(5):44-49.
11. Weber HC, Venzon DJ, Lin JT, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108(6):1637-1649.
12. Berger AC, Gibril F, Venzon DJ, et al. Prognostic value of initial fasting serum gastrin levels in patients with Zollinger-Ellison syndrome. J Clin Oncol. 2001;19(12):3051-3057.
IN THIS ARTICLE
- Diagnostic criteria
- Pharmacologic management
- Patient education
A more severe variant of peptic ulcer disease, Zollinger-Ellison syndrome (ZES) is a rare, chronic, and potentially life-threatening ulcerative disorder. Because the syndrome can be easily misdiagnosed based on clinical presentation alone, primary care clinicians need to be aware of its diagnostic features and know when referral to a gastroenterologist is necessary. Clinicians should suspect ZES in patients with peptic ulcer disease that is refractory to traditional medications.
Caused by a gastrin-secreting neuroendocrine tumor of the pancreas or duodenum called a gastrinoma, ZES can be benign or malignant. It typically manifests in white men ages 30 to 50.1 Due to the significant number of patients treated for a benign cause of peptic ulcer disease (eg, Helicobacter pylori or NSAID-induced ulcers) who are never tested for ZES, the exact incidence is difficult to determine.2 However, it is estimated that approximately 0.1 to 3 people per million develop the disease annually.3
PATHOPHYSIOLOGY
Approximately 80% of gastrinomas occur in the “gastrinoma triangle,” outlined by the hepatic portal vein, neck and body of the pancreas, and latter two-thirds of the duodenum (see Figure).1,4,5 Most gastrinomas involved in ZES occur sporadically, but there is a hereditary component associated with multiple endocrine neoplasia type 1 (MEN1), an autosomal dominant disorder.4

The overproduction and secretion of gastrin by the gastrinoma stimulates hypersecretion of hydrochloric acid.4 This is distinguished from high gastrin levels in the setting of fasting hypochlorhydria or achlorhydria, which may be caused by chronic atrophic gastritis, proton pump inhibitor (PPI) use, or pernicious anemia.5 The chronic hypersecretion of acid causes ulcerations to form. Most commonly, a single ulcer forms in the first portion of the duodenum.3
CLINICAL PRESENTATION
Patients with ZES often report vague abdominal pain that may mimic peptic ulcer disease on initial presentation. The widespread use of PPIs can mask symptoms, and one-fourth of patients present with no abdominal pain at all.6 Patients may also present with
The physical exam may be within normal limits, and no physical finding is considered pathognomonic for ZES. Findings may include epigastric tenderness; pallor, due to an ulcer-related anemia or GI bleed; jaundice, if there is liver involvement; and esophageal or dental erosions, due to excessive acid.8

DIAGNOSIS
Patients with symptoms refractory to medical management should be referred to a specialist for further testing. Once a patient is referred, a gastroenterologist will perform lab tests and imaging studies. In order to be diagnosed with ZES, the patient must exhibit an acidic environment with a pH less than 2 and an inappropriate release of gastrin with a basal acid output greater than 15 mEq/h (or > 5 mEq/h in a patient with prior acid reduction surgery).5,6
Fasting serum gastrin (FSG) is the initial study of choice, followed by a secretin-stimulating test when necessary.9 Diagnosis is established by an FSG level greater than 100 pg/mL; if more than 10-fold the normal level, no further testing is needed. However, results often range from 100 to 1,000 pg/mL.6,10 At these values, further testing with secretin stimulation is warranted.9 The test is performed with an IV injection of secretin, and blood samples are obtained to measure serum gastrin levels.10 An increase greater than 100 pg/mL is considered positive; one greater than 200 pg/mL is diagnostic.3
Once lab tests have been performed, a series of imaging studies are indicated. Endoscopy is used to identify active ulcers and erosions due to long-term acid secretion.3 CT, MRI, and somatostatin receptor scintigraphy (a specialized form of imaging that is the study of choice for localizing gastrinomas) are performed to localize primary tumors and identify any metastatic disease that may be present.10 Finally, after lab tests and imaging studies have been completed, genetic screening for MEN1 is used to determine if the patient has a sporadic or hereditary gastrinoma.3
MANAGEMENT
Once ZES has been diagnosed, the specialist will refer the patient for surgical opinion. The main objectives of surgery are to determine whether the tumor is malignant via biopsy, and to resect the tumor to suppress the acid hypersecretion, if indicated in the absence of liver metastasis and large pancreatic tumor size. Medical management should begin immediately to prevent any further damage from prolonged gastric hypersecretion.1
Pharmacologic options include PPIs, H2-receptor antagonists, and somatostatin analogues; PPIs are considered firstline therapy. Many patients with ZES require a higher dosage than is needed with typical GERD (60-100 mg/d vs 20-40 mg/d). Somatostatin analogues can be used in conjunction with PPIs and have been shown to inhibit tumor growth in patients with malignant ZES.1
Once a ZES diagnosis has been made, the tumor(s) resected (if appropriate), and vagotomy considered or performed, patients will need routine follow-up with their gastroenterologist and their primary care provider, who can manage medications and recommend any lifestyle changes.5
PROGNOSIS
The most important prognostic factor of patients with ZES is whether the gastrinoma is benign or malignant. There are two patterns: aggressive disease (25%) and nonaggressive disease (75%).5 At diagnosis, 40% to 70% of patients with sporadic ZES present with lymph node metastases, and 20% to 40% present with liver metastases. Patients with liver metastases have a 10-year survival rate of 30%, compared to a 15-year survival rate of 83% in patients without liver metastases.11,12
Along with the tumor itself, another prognostic factor to consider is the FSG level at diagnosis. Patients with higher FSG levels have decreased five- and 10-year survival rates compared to patients with lower FSG values. The 10-year survival rate for patients with a lower FSG value (0-499 pg/mL) is 86%, while the 10-year survival rate for those with a greater FSG value (> 1,000 pg/mL) is 73%.11,12 Overall, the prognosis is good for patients with ZES. The 10-year survival rate is high, and management is possible with medications and surgical resection of the gastrinoma.
PATIENT EDUCATION
Once patients are diagnosed, treatment with PPIs is typically lifelong unless they are considered cured by surgical resection. Patients need to understand that compliance is necessary to properly manage symptoms; certain foods, alcohol, and tobacco can affect the condition, and lifestyle modifications should be made, as they would with typical GERD or peptic ulcer disease.
CONCLUSION
ZES is frequently overlooked, and patients often continue to experience unresolved symptoms related to hypergastrinemia. Due to its complexity and ability to mimic other disorders—as well as the implications of duodenal versus pancreatic location, and other disorders of the kidney or endocrine system suggestive of MEN1—ZES should be ruled out in any patient with unexplained persistent GERD, peptic ulcer disease, elevated FSG, chronic diarrhea, and/or abdominal pain.5
The gastrinoma itself is a well-differentiated and slow-growing tumor in the majority of cases, making the prognosis for ZES favorable for long-term survival. Proper pharmacologic management is instrumental for controlling symptoms and decreasing acid production. Surgical resection offers patients the best chance for a complete cure. Clinicians and patients should be well educated about ZES in order to successfully manage the disorder.
IN THIS ARTICLE
- Diagnostic criteria
- Pharmacologic management
- Patient education
A more severe variant of peptic ulcer disease, Zollinger-Ellison syndrome (ZES) is a rare, chronic, and potentially life-threatening ulcerative disorder. Because the syndrome can be easily misdiagnosed based on clinical presentation alone, primary care clinicians need to be aware of its diagnostic features and know when referral to a gastroenterologist is necessary. Clinicians should suspect ZES in patients with peptic ulcer disease that is refractory to traditional medications.
Caused by a gastrin-secreting neuroendocrine tumor of the pancreas or duodenum called a gastrinoma, ZES can be benign or malignant. It typically manifests in white men ages 30 to 50.1 Due to the significant number of patients treated for a benign cause of peptic ulcer disease (eg, Helicobacter pylori or NSAID-induced ulcers) who are never tested for ZES, the exact incidence is difficult to determine.2 However, it is estimated that approximately 0.1 to 3 people per million develop the disease annually.3
PATHOPHYSIOLOGY
Approximately 80% of gastrinomas occur in the “gastrinoma triangle,” outlined by the hepatic portal vein, neck and body of the pancreas, and latter two-thirds of the duodenum (see Figure).1,4,5 Most gastrinomas involved in ZES occur sporadically, but there is a hereditary component associated with multiple endocrine neoplasia type 1 (MEN1), an autosomal dominant disorder.4

The overproduction and secretion of gastrin by the gastrinoma stimulates hypersecretion of hydrochloric acid.4 This is distinguished from high gastrin levels in the setting of fasting hypochlorhydria or achlorhydria, which may be caused by chronic atrophic gastritis, proton pump inhibitor (PPI) use, or pernicious anemia.5 The chronic hypersecretion of acid causes ulcerations to form. Most commonly, a single ulcer forms in the first portion of the duodenum.3
CLINICAL PRESENTATION
Patients with ZES often report vague abdominal pain that may mimic peptic ulcer disease on initial presentation. The widespread use of PPIs can mask symptoms, and one-fourth of patients present with no abdominal pain at all.6 Patients may also present with
The physical exam may be within normal limits, and no physical finding is considered pathognomonic for ZES. Findings may include epigastric tenderness; pallor, due to an ulcer-related anemia or GI bleed; jaundice, if there is liver involvement; and esophageal or dental erosions, due to excessive acid.8

DIAGNOSIS
Patients with symptoms refractory to medical management should be referred to a specialist for further testing. Once a patient is referred, a gastroenterologist will perform lab tests and imaging studies. In order to be diagnosed with ZES, the patient must exhibit an acidic environment with a pH less than 2 and an inappropriate release of gastrin with a basal acid output greater than 15 mEq/h (or > 5 mEq/h in a patient with prior acid reduction surgery).5,6
Fasting serum gastrin (FSG) is the initial study of choice, followed by a secretin-stimulating test when necessary.9 Diagnosis is established by an FSG level greater than 100 pg/mL; if more than 10-fold the normal level, no further testing is needed. However, results often range from 100 to 1,000 pg/mL.6,10 At these values, further testing with secretin stimulation is warranted.9 The test is performed with an IV injection of secretin, and blood samples are obtained to measure serum gastrin levels.10 An increase greater than 100 pg/mL is considered positive; one greater than 200 pg/mL is diagnostic.3
Once lab tests have been performed, a series of imaging studies are indicated. Endoscopy is used to identify active ulcers and erosions due to long-term acid secretion.3 CT, MRI, and somatostatin receptor scintigraphy (a specialized form of imaging that is the study of choice for localizing gastrinomas) are performed to localize primary tumors and identify any metastatic disease that may be present.10 Finally, after lab tests and imaging studies have been completed, genetic screening for MEN1 is used to determine if the patient has a sporadic or hereditary gastrinoma.3
MANAGEMENT
Once ZES has been diagnosed, the specialist will refer the patient for surgical opinion. The main objectives of surgery are to determine whether the tumor is malignant via biopsy, and to resect the tumor to suppress the acid hypersecretion, if indicated in the absence of liver metastasis and large pancreatic tumor size. Medical management should begin immediately to prevent any further damage from prolonged gastric hypersecretion.1
Pharmacologic options include PPIs, H2-receptor antagonists, and somatostatin analogues; PPIs are considered firstline therapy. Many patients with ZES require a higher dosage than is needed with typical GERD (60-100 mg/d vs 20-40 mg/d). Somatostatin analogues can be used in conjunction with PPIs and have been shown to inhibit tumor growth in patients with malignant ZES.1
Once a ZES diagnosis has been made, the tumor(s) resected (if appropriate), and vagotomy considered or performed, patients will need routine follow-up with their gastroenterologist and their primary care provider, who can manage medications and recommend any lifestyle changes.5
PROGNOSIS
The most important prognostic factor of patients with ZES is whether the gastrinoma is benign or malignant. There are two patterns: aggressive disease (25%) and nonaggressive disease (75%).5 At diagnosis, 40% to 70% of patients with sporadic ZES present with lymph node metastases, and 20% to 40% present with liver metastases. Patients with liver metastases have a 10-year survival rate of 30%, compared to a 15-year survival rate of 83% in patients without liver metastases.11,12
Along with the tumor itself, another prognostic factor to consider is the FSG level at diagnosis. Patients with higher FSG levels have decreased five- and 10-year survival rates compared to patients with lower FSG values. The 10-year survival rate for patients with a lower FSG value (0-499 pg/mL) is 86%, while the 10-year survival rate for those with a greater FSG value (> 1,000 pg/mL) is 73%.11,12 Overall, the prognosis is good for patients with ZES. The 10-year survival rate is high, and management is possible with medications and surgical resection of the gastrinoma.
PATIENT EDUCATION
Once patients are diagnosed, treatment with PPIs is typically lifelong unless they are considered cured by surgical resection. Patients need to understand that compliance is necessary to properly manage symptoms; certain foods, alcohol, and tobacco can affect the condition, and lifestyle modifications should be made, as they would with typical GERD or peptic ulcer disease.
CONCLUSION
ZES is frequently overlooked, and patients often continue to experience unresolved symptoms related to hypergastrinemia. Due to its complexity and ability to mimic other disorders—as well as the implications of duodenal versus pancreatic location, and other disorders of the kidney or endocrine system suggestive of MEN1—ZES should be ruled out in any patient with unexplained persistent GERD, peptic ulcer disease, elevated FSG, chronic diarrhea, and/or abdominal pain.5
The gastrinoma itself is a well-differentiated and slow-growing tumor in the majority of cases, making the prognosis for ZES favorable for long-term survival. Proper pharmacologic management is instrumental for controlling symptoms and decreasing acid production. Surgical resection offers patients the best chance for a complete cure. Clinicians and patients should be well educated about ZES in order to successfully manage the disorder.
1. Tomassetti P, Campana D, Piscitelli L, et al. Treatment of Zollinger-Ellison syndrome. World J Gastroenterol. 2005; 11(35):5423-5432.
2. Metz DC. Diagnosis of the Zollinger-Ellison syndrome. Clin Gastroenterol Hepatol. 2016;10(2):126-130.
3. Epelboym I, Mazeh H. Zollinger-Ellison syndrome: classical considerations and current controversies. Oncologist. 2014; 19(1):44-50.
4. Papadakis M, McPhee S, Rabow M. Current Medical Diagnosis and Treatment 2014. New York, NY: McGraw-Hill Education; 2014:600-601.
5. Feldman M, Friedman LS, Lawrence BJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Philadelphia, PA: Saunders/Elsevier; 2016:511-515.
6. Ito T, Cadiot G, Jensen RT. Diagnosis of Zollinger-Ellison syndrome: increasingly difficult. World J Gastroenterol. 2012; 18(39):5495-5503.
7. Blonski WC, Katzka DA, Lichtenstein GR, Metz DC. Idiopathic gastric acid hypersecretion presenting as a diarrheal disorder and mimicking both Zollinger-Ellison syndrome and Crohn’s disease. Eur J Gastroenterol Hepatol. 2005;17(4):441-444.
8. Roy PK. Zollinger-Ellison syndrome clinical presentation. http://emedicine.medscape.com/article/183555-clinical#b4. Accessed June 14, 2017.
9. Berna MJ, Hoffmann KM, Serrano J, et al. Serum gastrin in Zollinger-Ellison syndrome: I. prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore). 2006;85(6):295-330.
10. Moore AR, Varro A, Pritchard M. Zollinger-Ellison syndrome. Gastrointestinal Nursing. 2012;10(5):44-49.
11. Weber HC, Venzon DJ, Lin JT, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108(6):1637-1649.
12. Berger AC, Gibril F, Venzon DJ, et al. Prognostic value of initial fasting serum gastrin levels in patients with Zollinger-Ellison syndrome. J Clin Oncol. 2001;19(12):3051-3057.
1. Tomassetti P, Campana D, Piscitelli L, et al. Treatment of Zollinger-Ellison syndrome. World J Gastroenterol. 2005; 11(35):5423-5432.
2. Metz DC. Diagnosis of the Zollinger-Ellison syndrome. Clin Gastroenterol Hepatol. 2016;10(2):126-130.
3. Epelboym I, Mazeh H. Zollinger-Ellison syndrome: classical considerations and current controversies. Oncologist. 2014; 19(1):44-50.
4. Papadakis M, McPhee S, Rabow M. Current Medical Diagnosis and Treatment 2014. New York, NY: McGraw-Hill Education; 2014:600-601.
5. Feldman M, Friedman LS, Lawrence BJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Philadelphia, PA: Saunders/Elsevier; 2016:511-515.
6. Ito T, Cadiot G, Jensen RT. Diagnosis of Zollinger-Ellison syndrome: increasingly difficult. World J Gastroenterol. 2012; 18(39):5495-5503.
7. Blonski WC, Katzka DA, Lichtenstein GR, Metz DC. Idiopathic gastric acid hypersecretion presenting as a diarrheal disorder and mimicking both Zollinger-Ellison syndrome and Crohn’s disease. Eur J Gastroenterol Hepatol. 2005;17(4):441-444.
8. Roy PK. Zollinger-Ellison syndrome clinical presentation. http://emedicine.medscape.com/article/183555-clinical#b4. Accessed June 14, 2017.
9. Berna MJ, Hoffmann KM, Serrano J, et al. Serum gastrin in Zollinger-Ellison syndrome: I. prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore). 2006;85(6):295-330.
10. Moore AR, Varro A, Pritchard M. Zollinger-Ellison syndrome. Gastrointestinal Nursing. 2012;10(5):44-49.
11. Weber HC, Venzon DJ, Lin JT, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108(6):1637-1649.
12. Berger AC, Gibril F, Venzon DJ, et al. Prognostic value of initial fasting serum gastrin levels in patients with Zollinger-Ellison syndrome. J Clin Oncol. 2001;19(12):3051-3057.
Deprescribing: A simple method for reducing polypharmacy
CASE An 82-year-old woman with a history of hypertension, diabetes, hyperlipidemia, stage 3 chronic kidney disease, anxiety, urge urinary incontinence, constipation, and bilateral knee osteoarthritis presents to her primary care physician’s office after a fall. She reports that she visited the emergency department (ED) a week ago after falling in the middle of the night on her way to the bathroom. This is the third fall she’s had this year. On chart review, she had a blood pressure (BP) of 112/60 mm Hg and a blood glucose level of 65 mg/dL in the ED. All other testing (head imaging, chest x-ray, urinalysis) was normal. The ED physician recommended that she stop taking her lisinopril-hydrochlorothiazide (HCTZ) and glipizide extended release (XL) until her follow-up appointment. Today, she asks about the need to restart these medications.
Polypharmacy is common among older adults due to a high prevalence of chronic conditions that often require multiple medications for optimal management. Cut points of 5 or 9 medications are frequently used to define polypharmacy. However, some define polypharmacy as taking a medication that lacks an indication, is ineffective, or is duplicating treatment provided by another medication.
Either way, polypharmacy is associated with multiple negative consequences, including an increased risk for adverse drug events (ADEs),1-4 drug-drug and drug-disease interactions (TABLE 15,6),7 reduced functional capacity,8 multiple geriatric syndromes (TABLE 25,9-12), medication non-adherence,13 and increased mortality.14 Polypharmacy also contributes to increased health care costs for both the patient and the health care system.15

Taking a step back. Polypharmacy often results from prescribing cascades, which occur when an adverse drug effect is misinterpreted as a new medical problem, leading to the prescribing of more medication to treat the initial drug-induced symptom. Potentially inappropriate medications (PIMs), which are medications that should be avoided in older adults and in those with certain conditions, are also more likely to be prescribed in the setting of polypharmacy.16
Deprescribing is the process of identifying and discontinuing medications that are unnecessary, ineffective, and/or inappropriate in order to reduce polypharmacy and improve health outcomes. Deprescribing is a collaborative process that involves weighing the benefits and harms of medications in the context of a patient’s care goals, current level of functioning, life expectancy, values, and preferences. This article reviews polypharmacy and discusses safe and effective deprescribing strategies for older adults in the primary care setting.
[polldaddy:9781245]
How many people on how many meds?
According to a 2016 study, 36% of community-dwelling older adults (ages 62-85 years) were taking 5 or more prescription medications in 2010 to 2011—up from 31% in 2005 to 2006.17 When one narrows the population to older adults in the United States who are hospitalized, almost half (46%) take 7 or more medications.18 Among frail, older US veterans at hospital discharge, 40% were prescribed 9 or more medications, with 44% of these patients receiving at least one unnecessary drug.19
The challenges of multimorbidity
In the United States, 80% of those 65 and older have 2 or more chronic conditions, or multimorbidity.20 Clinical practice guidelines making recommendations for the management of single conditions, such as heart failure, hypertension, or diabetes, often suggest the use of 2 or more medications to achieve optimal management and fail to provide guidance in the setting of multimorbidity. Following treatment recommendations for multiple conditions predictably leads to polypharmacy, with complicated, costly, and burdensome regimens.
Further, the research contributing to the development of clinical practice guidelines frequently excludes older adults and those with multimorbidity, reducing applicability in this population. As a result, many treatment recommendations have uncertain benefit and may be harmful in the multimorbid older patient.21
CASE In addition to the patient’s multimorbidity, she had a stroke at age 73 and has some mild residual left-sided weakness. Functionally, she is independent and able to perform her activities of daily living and her instrumental activities of daily living. She lives alone, quit smoking at age 65, and has an occasional glass of wine during family parties. The patient’s daughter and granddaughter live 2 blocks away.
Her current medications include glipizide XL 10 mg/d and lisinopril-HCTZ 20-25 mg/d, which she has temporarily discontinued at the ED doctor’s recommendation, as well as: amlodipine 10 mg/d, metformin 1000 mg BID, senna 8.6 mg/d, docusate 100 mg BID, furosemide 40 mg/d, and ibuprofen 600 mg/d (for knee pain). She reports taking omeprazole 20 mg/d “for almost 20 years,” even though she has not had any reflux symptoms in recent memory. After her stroke, she began taking atorvastatin 10 mg/d, aspirin 81 mg/d, and clopidogrel 75 mg/d, which she continues to take today. About a year ago, she started oxybutynin 5 mg/d for urinary incontinence, but she has not noticed significant relief. Additionally, she takes lorazepam 1 mg for insomnia most nights of the week.

A review of systems reveals issues with chronic constipation and intermittent dizziness, but is otherwise negative. The physical examination reveals a well-appearing woman with a body mass index of 26. Her temperature is 98.5° F, her heart rate is 78 beats/min and regular, her respirations are 14 breaths/min, and her BP is 117/65 mm Hg. Orthostatic testing is negative. Her heart, lung, and abdominal exams are within normal limits. Her timed up and go test is 14 seconds. Her blood glucose level today in the office after eating breakfast 2 hours ago is 135 mg/dL (normal: <140 mg/dL). Laboratory tests performed at the time of the ED visit show a creatinine level of 1.2 mg/dL (normal range: 0.6 to 1.1 mg/dL), a glomerular filtration rate (GFR) of 44 units (normal range: >60 units), a hemoglobin level of 9.8 g/dL (normal range: 12-15.5 g/dL), and a thyroid stimulating hormone level of 1.4 mIU/L (normal range: 0.5-8.9 mIU/L). A recent hemoglobin A1C is 6.8% (normal: <5.7%), low-density lipoprotein (LDL) level is 103 mg/dL (optimal <100 mg/dL), and high-density lipoprotein (HDL) level is 65 mg/dL (optimal >60 mg/dL). An echocardiogram performed a year ago showed mild aortic stenosis with normal systolic and diastolic function.
Starting the deprescribing process: Several approaches to choose from
The goal of deprescribing is to reduce polypharmacy and improve health outcomes. It is a process defined as, “reviewing all current medications; identifying medications to be ceased, substituted, or reduced; planning a deprescribing regimen in partnership with the patient; and frequently reviewing and supporting the patient.”22 A medication review should include prescription, over-the-counter (OTC), and complementary/alternative medicine (CAM) agents.
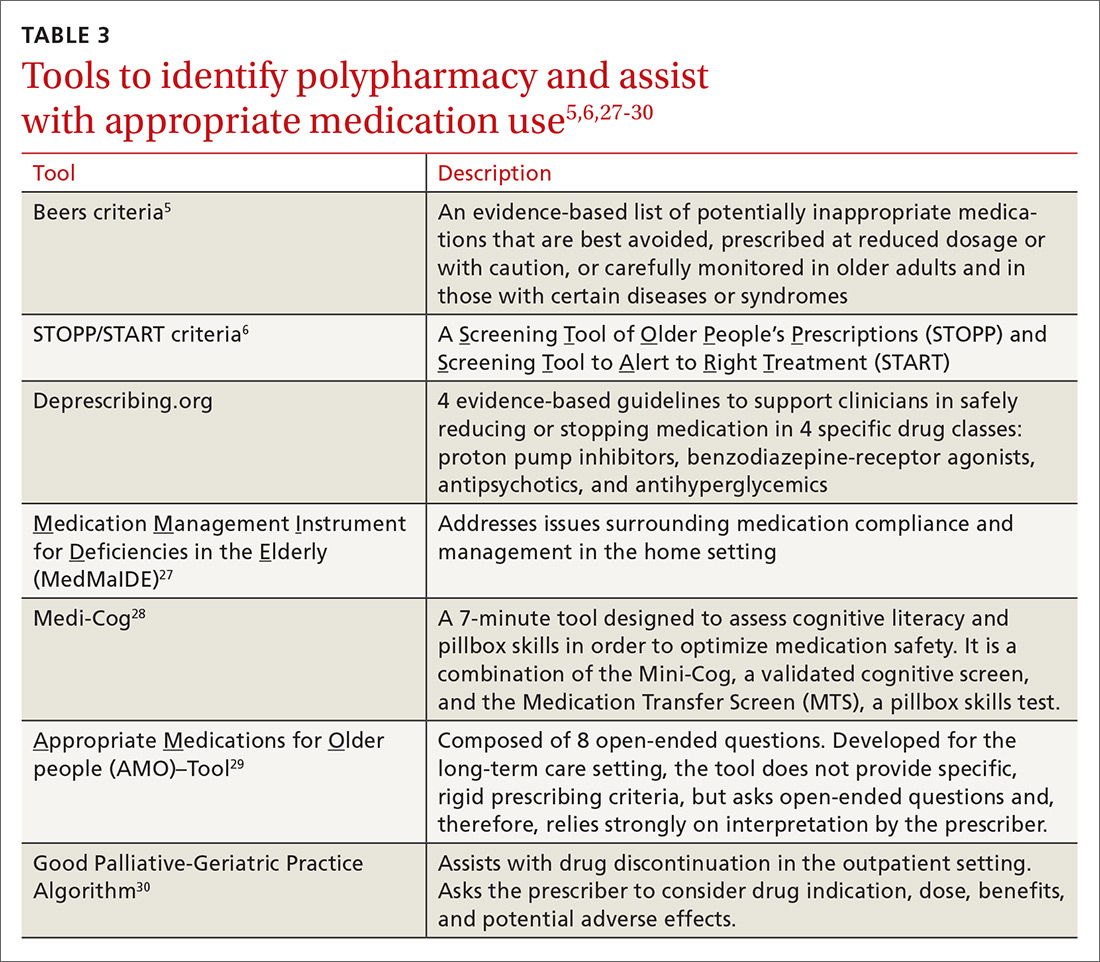
Until recently, studies evaluating the process of deprescribing across drug classes and disease conditions were limited, but new research is beginning to show its potential impact. After deprescribing, patients experience fewer falls and show improvements in cognition.23 While there have not yet been large randomized trials to evaluate deprescribing, a recent systematic review and meta-analysis showed that use of patient-specific deprescribing interventions is associated with improved survival.24 Importantly, there have been no reported adverse drug withdrawal events or deaths associated with deprescribing.23

Smaller studies have reported additional benefits including decreases in health care costs, reductions in drug-drug interactions and PIMs, improvements in medication adherence, and increases in patient satisfaction.25 In addition, the removal of unnecessary medications may allow for increased consideration of prescribing appropriate medications with known benefit.25
Practically speaking, every encounter between a patient and health care provider is an opportunity to reduce unnecessary medications. Electronic alert systems at pharmacies and those embedded within electronic health record (EHR) systems can also prompt a medication review and an effort to deprescribe.26 Evidence-based tools to identify polypharmacy and guide appropriate medication use are listed in TABLE 3.5,6,27-30 In addition, suggested approaches to beginning the deprescribing process are included in TABLE 4.5,31-33 And a medication class-based approach to deprescribing is provided in TABLE 5.5,34-45
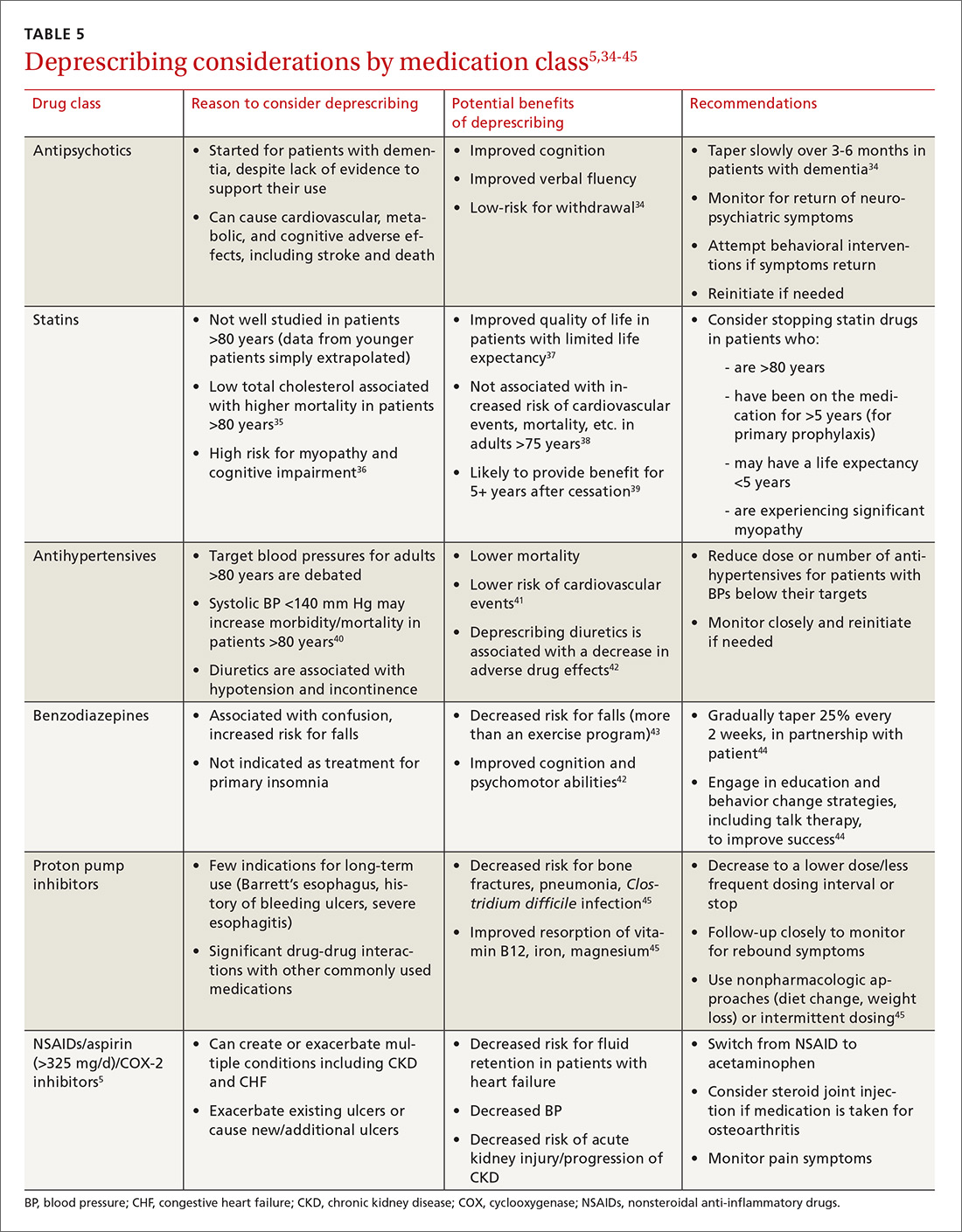
Although no gold standard process exists for deprescribing, experts suggest that any deprescribing protocol should include the following steps:32,46
1. Start with a “brown bag” review of the patient’s medications.
Have the patient bring all of his/her medications in a bag to the visit; review them together or have the medication history taken by a pharmacist. Determine and discuss the indication for each medication and its effectiveness for that indication. Consider the potential benefits and harms of each medication in the context of the patient’s care goals and preferences. Assess whether the patient is taking all of the medications that have been prescribed, and identify any reasons for missed pills (eg, adverse effects, dosing regimens, understanding, cognitive issues).
2. Talk to the patient about the deprescribing process.
Talk with the patient about the risks and benefits of deprescribing, and prioritize which medications to address in the process. Prioritize the medications by balancing patient preferences with available pharmacologic evidence. If there is a lack of evidence supporting the benefits for a particular medication, consider known or suspected adverse effects, the ease or burden of the dosing regimen, the patient’s preferences and goals of care, remaining life expectancy, the time until drug benefit is appreciated, and the length of drug benefit after discontinuation.
3. Deprescribe medications.
If you are going to taper a medication, develop a schedule in partnership with the patient. Stop one medication at a time so that you can monitor for withdrawal symptoms or for the return of a condition.
Acknowledging potential barriers to deprescribing may help structure conversations and provide anticipatory guidance to patients and their families. Working to overcome these barriers will help maximize the benefits of deprescribing and help to build trust with patients.
Patient-driven barriers include fear of a condition worsening or returning, lack of a suitable alternative, lack of ongoing support to manage a particular condition, a previous bad experience with medication cessation, and influence from other care providers (eg, family, home caregivers, nurses, specialists, friends). Patients and family members sometimes cling to the hope of future effectiveness of a treatment, especially in the case of medications like donepezil for dementia.47 Utilizing a team-based and stepwise patient approach to deprescribing aims to provide hesitant patients with appropriate amounts of education and support to begin to reduce unnecessary medicines.
Provider-driven barriers include feeling uneasy about contradicting a specialist’s recommendations for initiation/continuation of specific medications, fear of causing withdrawal symptoms or disease relapse, and lack of specific data to adequately understand and assess benefits and harms in the older adult population. Primary care physicians have also acknowledged worry about discussing life expectancy and that patients will feel their care is being reduced or “downgraded.”48 Finally, there is limited time in which these complex shared decision-making conversations can take place. Thus, if medications are not causing a noticeable problem, it is often easier to just continue them.
One way to overcome some of these concerns is to consider working with a clinical pharmacist. By gaining information regarding medication-specific factors, such as half-life and expected withdrawal patterns, you can feel more confident deprescribing or continuing medications.
Additionally, communicating closely with specialists, ideally with the help of an integrated EHR, can allow you to discuss indications for particular medications or concerns about adverse effects, limited benefits, or difficulty with compliance, so that you can develop a collaborative, cohesive, and patient-centered plan. This, in turn, may improve patient understanding and compliance.
4. Create a follow-up plan.
At the time of deprescribing a medication, develop a plan with the patient for monitoring and assessment. Ensure that the patient understands which symptoms may occur in the event of drug withdrawal and which symptoms may suggest the return of a condition. Make sure that other supports are in place if needed (eg, cognitive behavioral therapy, physical therapy, social support or assistance) to help ensure that medication cessation is successful.
CASE During the office visit, you advise the patient that her BP looks normal, her blood sugar is within an appropriate range, and she is lucky to have not sustained any injuries after her most recent fall. In addition to discussing the benefits of some outpatient physical therapy to help with her balance, you ask if she would like to discuss reducing her medications. She is agreeable and asks for your recommendations.
You are aware of several resources that can help you with your recommendations, among them the STOPP/START6 and Beers criteria,5 as well as the Good Geriatric-Palliative Algorithm.30
If you were to use the STOPP/START and Beers criteria, you might consider stopping:
- lorazepam, which increases the risk of falls and confusion.
- ibuprofen, since this patient has only mild osteoarthritis pain, and ibuprofen has the potential for renal, cardiac, and gastrointestinal toxicities.
- oxybutynin, because it could be contributing to the patient’s constipation and cause confusion and falls.
- furosemide, since the patient has no clinical heart failure.
- omeprazole, since the indication is unknown and the patient has no history of ulceration, esophagitis, or symptomatic gastroesophageal reflux disease.
After reviewing the Good Geriatric-Palliative Algorithm,30 you might consider stopping:
- clopidogrel, as there is no clear indication for this medication in combination with aspirin in this patient.
- glipizide XL, as this patient’s A1c is below goal and this medication puts her at risk of hypoglycemia and its associated morbidities.
- metformin, as it increases her risk of lactic acidosis because her GFR is <45 units.
- docusate, as the evidence to show clear benefit in improving chronic constipation in older adults is lacking.
You tell your patient that there are multiple medications to consider stopping. In order to monitor any symptoms of withdrawal or return of a condition, it would be best to stop one at a time and follow-up closely. Since she has done well for the past week without the glipizide and lisinopril-HCTZ combination, she can remain off the glipizide and the HCTZ. Lisinopril, however, may provide renal protection in the setting of diabetes and will be continued at this time.
You ask her about adverse effects from her other medications. She indicates that the furosemide makes her run to the bathroom all the time, so she would like to try stopping it. You agree and make a plan for her to monitor her weight, watch for edema, and return in 4 weeks for a follow-up visit.
On follow-up, she is feeling well, has no edema on exam, and is happy to report her urinary incontinence has resolved. You therefore suggest her next deprescribing trial be discontinuation of her oxybutynin. She thanks you for your recommendations about her medications and heads off to her physical therapy appointment.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Division of Geriatric Medicine and Palliative Care, Thomas Jefferson University, 2422 S Broad St, 2nd Floor, Philadelphia, PA 19145; [email protected].
1. Bourgeois FT, Shannon MW, Valim C, et al. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010;19:901-910.
2. Nair NP, Chalmers L, Peterson GM, et al. Hospitalization in older patients due to adverse drug reactions–the need for a prediction tool. Clin Interv Aging. 2016;11:497-506.
3. Nguyen JK, Fouts MM, Kotabe SE, et al. Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. Am J Geriatr Pharmacother. 2006; 4:36-41.
4. Hohl CM, Dankoff J, Colacone A, et al. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med. 2001;38:666-671.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213-218.
7. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28:173-186.
8. Magaziner J, Cadigan DA, Fedder DO, et al. Medication use and functional decline among community-dwelling older women. J Aging Health. 1989;1:470-484.
9. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
10. Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174:588-595.
11. Weiss BD. Diagnostic evaluation of urinary incontinence in geriatric patients. Am Fam Physician. 1998;57:2675-2694.
12. Syed Q, Hendler KT, Koncilja K. The impact of aging and medical status on dysgeusia. Am J Med. 2016;129:753, E1-E6.
13. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
14. Espino DV, Bazaldua OV, Palmer RF, et al. Suboptimal medication use and mortality in an older adult community-based cohort: results from the Hispanic EPESE Study. J Gerontol A Biol Sci Med Sci. 2006;61:170-175.
15. Akazawa M, Imai H, Igarashi A, et al. Potentially inappropriate medication use in elderly Japanese patients. Am J Geriatr Pharmacother. 2010; 8:146-160.
16. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516-1523.
17. Qato DM, Wilder J, Schumm LP, et al. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176:473-482.
18. Flaherty JH, Perry HM 3rd, Lynchard GS, et al. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci. 2000;55:554-559.
19. Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518-1523.
20. Gerteis J, Izrael D, Deitz D, et al. Multiple chronic conditions chartbook. Rockville, MD: Agency for Healthcare Research and Quality. 2014.
21. American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. J Am Geriatr Soc. 2012;60:E1-E25.
22. Woodward M. Deprescribing: achieving better health outcomes for older people through reducing medications. J Pharm Pract Res. 2003;33:323-328.
23. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
24. Page AT, Clifford RM, Potter K, et al. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2016;82:583-623.
25. Reeve E, Shakib S, Hendrix I, et al. The benefits and harms of deprescribing. Med J Aust. 2014;201:386-389.
26. Walsh K, Kwan D, Marr P, et al. Deprescribing in a family health team: a study of chronic proton pump inhibitor use. J Prim Health Care. 2016;8:164-171.
27. Orwig D, Brandt N, Gruber-Baldini AL. Medication management assessment for older adults in the community. Gerontologist. 2006;46:661-668.
28. Anderson K, Jue SG, Madaras-Kelly KJ. Identifying patients at risk for medication mismanagement: using cognitive screens to predict a patient’s accuracy in filling a pillbox. Consult Pharm. 2008;23:459-472.
29. Lenaerts E, De Knijf F, Schoenmakers B. Appropriate prescribing for older people: a new tool for the general practitioner. J Frailty & Aging. 2013;2:8-14.
30. Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. IMAJ. 2007;9:430-434.
31. Holmes HM, Todd A. Evidence-based deprescribing of statins in patients with advanced illness. JAMA Intern Med. 2015;175:701-702.
32. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
33. Guirguis-Blake JM, Evans CV,Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
34. Declercq T, Petrovic M, Azermai M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;3:CD007726.
35. Petersen LK, Christensen K, Kragstrup J. Lipid-lowering treatment to the end? A review of observational studies and RCTs on cholesterol and mortality in 80+-year olds. Age Ageing. 2010;39:674-680.
36. Banach M, Serban MC. Discussion around statin discontinuation in older adults and patients with wasting diseases. J Cachexia Sarcopenia Muscle. 2016;7:396-399.
37. Goldstein MR, Mascitelli L, Pezzetta F. Statin therapy in the elderly: misconceptions. J Am Geriatr Soc. 2008;56:1365.
38. Han BH, Sutin D, Williamson JD, et al, for the ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT Randomized Clinical Trial. JAMA Intern Med. Published online May 22, 2017.
39. Sever PS, Chang CL, Gupta AK, et al. The Anglo-Scandinavian Cardiac Outcomes Trial: 11-year mortality follow-up of the lipid-lowering arm in the U.K. Eur Heart J. 2011;32:2525-2532.
40. Denardo SJ, Gong Y, Nichols WW, et al. Blood pressure and outcomes in very old hypertensive coronary artery disease patients: an INVEST substudy. Am J Med. 2010;123:719-726.
41. Ekbom T, Lindholm LH, Oden A, et al. A 5‐year prospective, observational study of the withdrawal of antihypertensive treatment in elderly people. J Intern Med. 1994;235:581-588.
42. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older. Drugs Aging. 2008;25:1021-1031.
43. Campbell AJ, Robertson MC, Gardner MM, et al. Psychotropic medication withdrawal and a home‐based exercise program to prevent falls: a randomized, controlled trial. J Am Geriatr Soc. 1999;47:850-853.
44. Pollmann AS, Murphy AL, Bergman JC, et al. Deprescribing benzodiazepines and Z-drugs in community-dwelling adults: a scoping review. BMC Pharmacol Toxicol. 2015;16:19.
45. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors. Can Fam Phys. 2017; 63:354-364.
46. Duncan P, Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24:37-42.
47. Schuling J, Gebben H, Veehof LJ, et al. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract. 2012;13:56.
48. Scott I, Anderson K, Freeman CR, et al. First do no harm: a real need to deprescribe in older patients. Med J Aust. 2014;201:390-392.
CASE An 82-year-old woman with a history of hypertension, diabetes, hyperlipidemia, stage 3 chronic kidney disease, anxiety, urge urinary incontinence, constipation, and bilateral knee osteoarthritis presents to her primary care physician’s office after a fall. She reports that she visited the emergency department (ED) a week ago after falling in the middle of the night on her way to the bathroom. This is the third fall she’s had this year. On chart review, she had a blood pressure (BP) of 112/60 mm Hg and a blood glucose level of 65 mg/dL in the ED. All other testing (head imaging, chest x-ray, urinalysis) was normal. The ED physician recommended that she stop taking her lisinopril-hydrochlorothiazide (HCTZ) and glipizide extended release (XL) until her follow-up appointment. Today, she asks about the need to restart these medications.
Polypharmacy is common among older adults due to a high prevalence of chronic conditions that often require multiple medications for optimal management. Cut points of 5 or 9 medications are frequently used to define polypharmacy. However, some define polypharmacy as taking a medication that lacks an indication, is ineffective, or is duplicating treatment provided by another medication.

Either way, polypharmacy is associated with multiple negative consequences, including an increased risk for adverse drug events (ADEs),1-4 drug-drug and drug-disease interactions (TABLE 15,6),7 reduced functional capacity,8 multiple geriatric syndromes (TABLE 25,9-12), medication non-adherence,13 and increased mortality.14 Polypharmacy also contributes to increased health care costs for both the patient and the health care system.15

Taking a step back. Polypharmacy often results from prescribing cascades, which occur when an adverse drug effect is misinterpreted as a new medical problem, leading to the prescribing of more medication to treat the initial drug-induced symptom. Potentially inappropriate medications (PIMs), which are medications that should be avoided in older adults and in those with certain conditions, are also more likely to be prescribed in the setting of polypharmacy.16
Deprescribing is the process of identifying and discontinuing medications that are unnecessary, ineffective, and/or inappropriate in order to reduce polypharmacy and improve health outcomes. Deprescribing is a collaborative process that involves weighing the benefits and harms of medications in the context of a patient’s care goals, current level of functioning, life expectancy, values, and preferences. This article reviews polypharmacy and discusses safe and effective deprescribing strategies for older adults in the primary care setting.
[polldaddy:9781245]
How many people on how many meds?
According to a 2016 study, 36% of community-dwelling older adults (ages 62-85 years) were taking 5 or more prescription medications in 2010 to 2011—up from 31% in 2005 to 2006.17 When one narrows the population to older adults in the United States who are hospitalized, almost half (46%) take 7 or more medications.18 Among frail, older US veterans at hospital discharge, 40% were prescribed 9 or more medications, with 44% of these patients receiving at least one unnecessary drug.19
The challenges of multimorbidity
In the United States, 80% of those 65 and older have 2 or more chronic conditions, or multimorbidity.20 Clinical practice guidelines making recommendations for the management of single conditions, such as heart failure, hypertension, or diabetes, often suggest the use of 2 or more medications to achieve optimal management and fail to provide guidance in the setting of multimorbidity. Following treatment recommendations for multiple conditions predictably leads to polypharmacy, with complicated, costly, and burdensome regimens.
Further, the research contributing to the development of clinical practice guidelines frequently excludes older adults and those with multimorbidity, reducing applicability in this population. As a result, many treatment recommendations have uncertain benefit and may be harmful in the multimorbid older patient.21
CASE In addition to the patient’s multimorbidity, she had a stroke at age 73 and has some mild residual left-sided weakness. Functionally, she is independent and able to perform her activities of daily living and her instrumental activities of daily living. She lives alone, quit smoking at age 65, and has an occasional glass of wine during family parties. The patient’s daughter and granddaughter live 2 blocks away.
Her current medications include glipizide XL 10 mg/d and lisinopril-HCTZ 20-25 mg/d, which she has temporarily discontinued at the ED doctor’s recommendation, as well as: amlodipine 10 mg/d, metformin 1000 mg BID, senna 8.6 mg/d, docusate 100 mg BID, furosemide 40 mg/d, and ibuprofen 600 mg/d (for knee pain). She reports taking omeprazole 20 mg/d “for almost 20 years,” even though she has not had any reflux symptoms in recent memory. After her stroke, she began taking atorvastatin 10 mg/d, aspirin 81 mg/d, and clopidogrel 75 mg/d, which she continues to take today. About a year ago, she started oxybutynin 5 mg/d for urinary incontinence, but she has not noticed significant relief. Additionally, she takes lorazepam 1 mg for insomnia most nights of the week.

A review of systems reveals issues with chronic constipation and intermittent dizziness, but is otherwise negative. The physical examination reveals a well-appearing woman with a body mass index of 26. Her temperature is 98.5° F, her heart rate is 78 beats/min and regular, her respirations are 14 breaths/min, and her BP is 117/65 mm Hg. Orthostatic testing is negative. Her heart, lung, and abdominal exams are within normal limits. Her timed up and go test is 14 seconds. Her blood glucose level today in the office after eating breakfast 2 hours ago is 135 mg/dL (normal: <140 mg/dL). Laboratory tests performed at the time of the ED visit show a creatinine level of 1.2 mg/dL (normal range: 0.6 to 1.1 mg/dL), a glomerular filtration rate (GFR) of 44 units (normal range: >60 units), a hemoglobin level of 9.8 g/dL (normal range: 12-15.5 g/dL), and a thyroid stimulating hormone level of 1.4 mIU/L (normal range: 0.5-8.9 mIU/L). A recent hemoglobin A1C is 6.8% (normal: <5.7%), low-density lipoprotein (LDL) level is 103 mg/dL (optimal <100 mg/dL), and high-density lipoprotein (HDL) level is 65 mg/dL (optimal >60 mg/dL). An echocardiogram performed a year ago showed mild aortic stenosis with normal systolic and diastolic function.
Starting the deprescribing process: Several approaches to choose from
The goal of deprescribing is to reduce polypharmacy and improve health outcomes. It is a process defined as, “reviewing all current medications; identifying medications to be ceased, substituted, or reduced; planning a deprescribing regimen in partnership with the patient; and frequently reviewing and supporting the patient.”22 A medication review should include prescription, over-the-counter (OTC), and complementary/alternative medicine (CAM) agents.

Until recently, studies evaluating the process of deprescribing across drug classes and disease conditions were limited, but new research is beginning to show its potential impact. After deprescribing, patients experience fewer falls and show improvements in cognition.23 While there have not yet been large randomized trials to evaluate deprescribing, a recent systematic review and meta-analysis showed that use of patient-specific deprescribing interventions is associated with improved survival.24 Importantly, there have been no reported adverse drug withdrawal events or deaths associated with deprescribing.23

Smaller studies have reported additional benefits including decreases in health care costs, reductions in drug-drug interactions and PIMs, improvements in medication adherence, and increases in patient satisfaction.25 In addition, the removal of unnecessary medications may allow for increased consideration of prescribing appropriate medications with known benefit.25
Practically speaking, every encounter between a patient and health care provider is an opportunity to reduce unnecessary medications. Electronic alert systems at pharmacies and those embedded within electronic health record (EHR) systems can also prompt a medication review and an effort to deprescribe.26 Evidence-based tools to identify polypharmacy and guide appropriate medication use are listed in TABLE 3.5,6,27-30 In addition, suggested approaches to beginning the deprescribing process are included in TABLE 4.5,31-33 And a medication class-based approach to deprescribing is provided in TABLE 5.5,34-45

Although no gold standard process exists for deprescribing, experts suggest that any deprescribing protocol should include the following steps:32,46
1. Start with a “brown bag” review of the patient’s medications.
Have the patient bring all of his/her medications in a bag to the visit; review them together or have the medication history taken by a pharmacist. Determine and discuss the indication for each medication and its effectiveness for that indication. Consider the potential benefits and harms of each medication in the context of the patient’s care goals and preferences. Assess whether the patient is taking all of the medications that have been prescribed, and identify any reasons for missed pills (eg, adverse effects, dosing regimens, understanding, cognitive issues).
2. Talk to the patient about the deprescribing process.
Talk with the patient about the risks and benefits of deprescribing, and prioritize which medications to address in the process. Prioritize the medications by balancing patient preferences with available pharmacologic evidence. If there is a lack of evidence supporting the benefits for a particular medication, consider known or suspected adverse effects, the ease or burden of the dosing regimen, the patient’s preferences and goals of care, remaining life expectancy, the time until drug benefit is appreciated, and the length of drug benefit after discontinuation.
3. Deprescribe medications.
If you are going to taper a medication, develop a schedule in partnership with the patient. Stop one medication at a time so that you can monitor for withdrawal symptoms or for the return of a condition.
Acknowledging potential barriers to deprescribing may help structure conversations and provide anticipatory guidance to patients and their families. Working to overcome these barriers will help maximize the benefits of deprescribing and help to build trust with patients.
Patient-driven barriers include fear of a condition worsening or returning, lack of a suitable alternative, lack of ongoing support to manage a particular condition, a previous bad experience with medication cessation, and influence from other care providers (eg, family, home caregivers, nurses, specialists, friends). Patients and family members sometimes cling to the hope of future effectiveness of a treatment, especially in the case of medications like donepezil for dementia.47 Utilizing a team-based and stepwise patient approach to deprescribing aims to provide hesitant patients with appropriate amounts of education and support to begin to reduce unnecessary medicines.
Provider-driven barriers include feeling uneasy about contradicting a specialist’s recommendations for initiation/continuation of specific medications, fear of causing withdrawal symptoms or disease relapse, and lack of specific data to adequately understand and assess benefits and harms in the older adult population. Primary care physicians have also acknowledged worry about discussing life expectancy and that patients will feel their care is being reduced or “downgraded.”48 Finally, there is limited time in which these complex shared decision-making conversations can take place. Thus, if medications are not causing a noticeable problem, it is often easier to just continue them.
One way to overcome some of these concerns is to consider working with a clinical pharmacist. By gaining information regarding medication-specific factors, such as half-life and expected withdrawal patterns, you can feel more confident deprescribing or continuing medications.
Additionally, communicating closely with specialists, ideally with the help of an integrated EHR, can allow you to discuss indications for particular medications or concerns about adverse effects, limited benefits, or difficulty with compliance, so that you can develop a collaborative, cohesive, and patient-centered plan. This, in turn, may improve patient understanding and compliance.
4. Create a follow-up plan.
At the time of deprescribing a medication, develop a plan with the patient for monitoring and assessment. Ensure that the patient understands which symptoms may occur in the event of drug withdrawal and which symptoms may suggest the return of a condition. Make sure that other supports are in place if needed (eg, cognitive behavioral therapy, physical therapy, social support or assistance) to help ensure that medication cessation is successful.
CASE During the office visit, you advise the patient that her BP looks normal, her blood sugar is within an appropriate range, and she is lucky to have not sustained any injuries after her most recent fall. In addition to discussing the benefits of some outpatient physical therapy to help with her balance, you ask if she would like to discuss reducing her medications. She is agreeable and asks for your recommendations.
You are aware of several resources that can help you with your recommendations, among them the STOPP/START6 and Beers criteria,5 as well as the Good Geriatric-Palliative Algorithm.30
If you were to use the STOPP/START and Beers criteria, you might consider stopping:
- lorazepam, which increases the risk of falls and confusion.
- ibuprofen, since this patient has only mild osteoarthritis pain, and ibuprofen has the potential for renal, cardiac, and gastrointestinal toxicities.
- oxybutynin, because it could be contributing to the patient’s constipation and cause confusion and falls.
- furosemide, since the patient has no clinical heart failure.
- omeprazole, since the indication is unknown and the patient has no history of ulceration, esophagitis, or symptomatic gastroesophageal reflux disease.
After reviewing the Good Geriatric-Palliative Algorithm,30 you might consider stopping:
- clopidogrel, as there is no clear indication for this medication in combination with aspirin in this patient.
- glipizide XL, as this patient’s A1c is below goal and this medication puts her at risk of hypoglycemia and its associated morbidities.
- metformin, as it increases her risk of lactic acidosis because her GFR is <45 units.
- docusate, as the evidence to show clear benefit in improving chronic constipation in older adults is lacking.
You tell your patient that there are multiple medications to consider stopping. In order to monitor any symptoms of withdrawal or return of a condition, it would be best to stop one at a time and follow-up closely. Since she has done well for the past week without the glipizide and lisinopril-HCTZ combination, she can remain off the glipizide and the HCTZ. Lisinopril, however, may provide renal protection in the setting of diabetes and will be continued at this time.
You ask her about adverse effects from her other medications. She indicates that the furosemide makes her run to the bathroom all the time, so she would like to try stopping it. You agree and make a plan for her to monitor her weight, watch for edema, and return in 4 weeks for a follow-up visit.
On follow-up, she is feeling well, has no edema on exam, and is happy to report her urinary incontinence has resolved. You therefore suggest her next deprescribing trial be discontinuation of her oxybutynin. She thanks you for your recommendations about her medications and heads off to her physical therapy appointment.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Division of Geriatric Medicine and Palliative Care, Thomas Jefferson University, 2422 S Broad St, 2nd Floor, Philadelphia, PA 19145; [email protected].
CASE An 82-year-old woman with a history of hypertension, diabetes, hyperlipidemia, stage 3 chronic kidney disease, anxiety, urge urinary incontinence, constipation, and bilateral knee osteoarthritis presents to her primary care physician’s office after a fall. She reports that she visited the emergency department (ED) a week ago after falling in the middle of the night on her way to the bathroom. This is the third fall she’s had this year. On chart review, she had a blood pressure (BP) of 112/60 mm Hg and a blood glucose level of 65 mg/dL in the ED. All other testing (head imaging, chest x-ray, urinalysis) was normal. The ED physician recommended that she stop taking her lisinopril-hydrochlorothiazide (HCTZ) and glipizide extended release (XL) until her follow-up appointment. Today, she asks about the need to restart these medications.
Polypharmacy is common among older adults due to a high prevalence of chronic conditions that often require multiple medications for optimal management. Cut points of 5 or 9 medications are frequently used to define polypharmacy. However, some define polypharmacy as taking a medication that lacks an indication, is ineffective, or is duplicating treatment provided by another medication.

Either way, polypharmacy is associated with multiple negative consequences, including an increased risk for adverse drug events (ADEs),1-4 drug-drug and drug-disease interactions (TABLE 15,6),7 reduced functional capacity,8 multiple geriatric syndromes (TABLE 25,9-12), medication non-adherence,13 and increased mortality.14 Polypharmacy also contributes to increased health care costs for both the patient and the health care system.15

Taking a step back. Polypharmacy often results from prescribing cascades, which occur when an adverse drug effect is misinterpreted as a new medical problem, leading to the prescribing of more medication to treat the initial drug-induced symptom. Potentially inappropriate medications (PIMs), which are medications that should be avoided in older adults and in those with certain conditions, are also more likely to be prescribed in the setting of polypharmacy.16
Deprescribing is the process of identifying and discontinuing medications that are unnecessary, ineffective, and/or inappropriate in order to reduce polypharmacy and improve health outcomes. Deprescribing is a collaborative process that involves weighing the benefits and harms of medications in the context of a patient’s care goals, current level of functioning, life expectancy, values, and preferences. This article reviews polypharmacy and discusses safe and effective deprescribing strategies for older adults in the primary care setting.
[polldaddy:9781245]
How many people on how many meds?
According to a 2016 study, 36% of community-dwelling older adults (ages 62-85 years) were taking 5 or more prescription medications in 2010 to 2011—up from 31% in 2005 to 2006.17 When one narrows the population to older adults in the United States who are hospitalized, almost half (46%) take 7 or more medications.18 Among frail, older US veterans at hospital discharge, 40% were prescribed 9 or more medications, with 44% of these patients receiving at least one unnecessary drug.19
The challenges of multimorbidity
In the United States, 80% of those 65 and older have 2 or more chronic conditions, or multimorbidity.20 Clinical practice guidelines making recommendations for the management of single conditions, such as heart failure, hypertension, or diabetes, often suggest the use of 2 or more medications to achieve optimal management and fail to provide guidance in the setting of multimorbidity. Following treatment recommendations for multiple conditions predictably leads to polypharmacy, with complicated, costly, and burdensome regimens.
Further, the research contributing to the development of clinical practice guidelines frequently excludes older adults and those with multimorbidity, reducing applicability in this population. As a result, many treatment recommendations have uncertain benefit and may be harmful in the multimorbid older patient.21
CASE In addition to the patient’s multimorbidity, she had a stroke at age 73 and has some mild residual left-sided weakness. Functionally, she is independent and able to perform her activities of daily living and her instrumental activities of daily living. She lives alone, quit smoking at age 65, and has an occasional glass of wine during family parties. The patient’s daughter and granddaughter live 2 blocks away.
Her current medications include glipizide XL 10 mg/d and lisinopril-HCTZ 20-25 mg/d, which she has temporarily discontinued at the ED doctor’s recommendation, as well as: amlodipine 10 mg/d, metformin 1000 mg BID, senna 8.6 mg/d, docusate 100 mg BID, furosemide 40 mg/d, and ibuprofen 600 mg/d (for knee pain). She reports taking omeprazole 20 mg/d “for almost 20 years,” even though she has not had any reflux symptoms in recent memory. After her stroke, she began taking atorvastatin 10 mg/d, aspirin 81 mg/d, and clopidogrel 75 mg/d, which she continues to take today. About a year ago, she started oxybutynin 5 mg/d for urinary incontinence, but she has not noticed significant relief. Additionally, she takes lorazepam 1 mg for insomnia most nights of the week.

A review of systems reveals issues with chronic constipation and intermittent dizziness, but is otherwise negative. The physical examination reveals a well-appearing woman with a body mass index of 26. Her temperature is 98.5° F, her heart rate is 78 beats/min and regular, her respirations are 14 breaths/min, and her BP is 117/65 mm Hg. Orthostatic testing is negative. Her heart, lung, and abdominal exams are within normal limits. Her timed up and go test is 14 seconds. Her blood glucose level today in the office after eating breakfast 2 hours ago is 135 mg/dL (normal: <140 mg/dL). Laboratory tests performed at the time of the ED visit show a creatinine level of 1.2 mg/dL (normal range: 0.6 to 1.1 mg/dL), a glomerular filtration rate (GFR) of 44 units (normal range: >60 units), a hemoglobin level of 9.8 g/dL (normal range: 12-15.5 g/dL), and a thyroid stimulating hormone level of 1.4 mIU/L (normal range: 0.5-8.9 mIU/L). A recent hemoglobin A1C is 6.8% (normal: <5.7%), low-density lipoprotein (LDL) level is 103 mg/dL (optimal <100 mg/dL), and high-density lipoprotein (HDL) level is 65 mg/dL (optimal >60 mg/dL). An echocardiogram performed a year ago showed mild aortic stenosis with normal systolic and diastolic function.
Starting the deprescribing process: Several approaches to choose from
The goal of deprescribing is to reduce polypharmacy and improve health outcomes. It is a process defined as, “reviewing all current medications; identifying medications to be ceased, substituted, or reduced; planning a deprescribing regimen in partnership with the patient; and frequently reviewing and supporting the patient.”22 A medication review should include prescription, over-the-counter (OTC), and complementary/alternative medicine (CAM) agents.

Until recently, studies evaluating the process of deprescribing across drug classes and disease conditions were limited, but new research is beginning to show its potential impact. After deprescribing, patients experience fewer falls and show improvements in cognition.23 While there have not yet been large randomized trials to evaluate deprescribing, a recent systematic review and meta-analysis showed that use of patient-specific deprescribing interventions is associated with improved survival.24 Importantly, there have been no reported adverse drug withdrawal events or deaths associated with deprescribing.23

Smaller studies have reported additional benefits including decreases in health care costs, reductions in drug-drug interactions and PIMs, improvements in medication adherence, and increases in patient satisfaction.25 In addition, the removal of unnecessary medications may allow for increased consideration of prescribing appropriate medications with known benefit.25
Practically speaking, every encounter between a patient and health care provider is an opportunity to reduce unnecessary medications. Electronic alert systems at pharmacies and those embedded within electronic health record (EHR) systems can also prompt a medication review and an effort to deprescribe.26 Evidence-based tools to identify polypharmacy and guide appropriate medication use are listed in TABLE 3.5,6,27-30 In addition, suggested approaches to beginning the deprescribing process are included in TABLE 4.5,31-33 And a medication class-based approach to deprescribing is provided in TABLE 5.5,34-45

Although no gold standard process exists for deprescribing, experts suggest that any deprescribing protocol should include the following steps:32,46
1. Start with a “brown bag” review of the patient’s medications.
Have the patient bring all of his/her medications in a bag to the visit; review them together or have the medication history taken by a pharmacist. Determine and discuss the indication for each medication and its effectiveness for that indication. Consider the potential benefits and harms of each medication in the context of the patient’s care goals and preferences. Assess whether the patient is taking all of the medications that have been prescribed, and identify any reasons for missed pills (eg, adverse effects, dosing regimens, understanding, cognitive issues).
2. Talk to the patient about the deprescribing process.
Talk with the patient about the risks and benefits of deprescribing, and prioritize which medications to address in the process. Prioritize the medications by balancing patient preferences with available pharmacologic evidence. If there is a lack of evidence supporting the benefits for a particular medication, consider known or suspected adverse effects, the ease or burden of the dosing regimen, the patient’s preferences and goals of care, remaining life expectancy, the time until drug benefit is appreciated, and the length of drug benefit after discontinuation.
3. Deprescribe medications.
If you are going to taper a medication, develop a schedule in partnership with the patient. Stop one medication at a time so that you can monitor for withdrawal symptoms or for the return of a condition.
Acknowledging potential barriers to deprescribing may help structure conversations and provide anticipatory guidance to patients and their families. Working to overcome these barriers will help maximize the benefits of deprescribing and help to build trust with patients.
Patient-driven barriers include fear of a condition worsening or returning, lack of a suitable alternative, lack of ongoing support to manage a particular condition, a previous bad experience with medication cessation, and influence from other care providers (eg, family, home caregivers, nurses, specialists, friends). Patients and family members sometimes cling to the hope of future effectiveness of a treatment, especially in the case of medications like donepezil for dementia.47 Utilizing a team-based and stepwise patient approach to deprescribing aims to provide hesitant patients with appropriate amounts of education and support to begin to reduce unnecessary medicines.
Provider-driven barriers include feeling uneasy about contradicting a specialist’s recommendations for initiation/continuation of specific medications, fear of causing withdrawal symptoms or disease relapse, and lack of specific data to adequately understand and assess benefits and harms in the older adult population. Primary care physicians have also acknowledged worry about discussing life expectancy and that patients will feel their care is being reduced or “downgraded.”48 Finally, there is limited time in which these complex shared decision-making conversations can take place. Thus, if medications are not causing a noticeable problem, it is often easier to just continue them.
One way to overcome some of these concerns is to consider working with a clinical pharmacist. By gaining information regarding medication-specific factors, such as half-life and expected withdrawal patterns, you can feel more confident deprescribing or continuing medications.
Additionally, communicating closely with specialists, ideally with the help of an integrated EHR, can allow you to discuss indications for particular medications or concerns about adverse effects, limited benefits, or difficulty with compliance, so that you can develop a collaborative, cohesive, and patient-centered plan. This, in turn, may improve patient understanding and compliance.
4. Create a follow-up plan.
At the time of deprescribing a medication, develop a plan with the patient for monitoring and assessment. Ensure that the patient understands which symptoms may occur in the event of drug withdrawal and which symptoms may suggest the return of a condition. Make sure that other supports are in place if needed (eg, cognitive behavioral therapy, physical therapy, social support or assistance) to help ensure that medication cessation is successful.
CASE During the office visit, you advise the patient that her BP looks normal, her blood sugar is within an appropriate range, and she is lucky to have not sustained any injuries after her most recent fall. In addition to discussing the benefits of some outpatient physical therapy to help with her balance, you ask if she would like to discuss reducing her medications. She is agreeable and asks for your recommendations.
You are aware of several resources that can help you with your recommendations, among them the STOPP/START6 and Beers criteria,5 as well as the Good Geriatric-Palliative Algorithm.30
If you were to use the STOPP/START and Beers criteria, you might consider stopping:
- lorazepam, which increases the risk of falls and confusion.
- ibuprofen, since this patient has only mild osteoarthritis pain, and ibuprofen has the potential for renal, cardiac, and gastrointestinal toxicities.
- oxybutynin, because it could be contributing to the patient’s constipation and cause confusion and falls.
- furosemide, since the patient has no clinical heart failure.
- omeprazole, since the indication is unknown and the patient has no history of ulceration, esophagitis, or symptomatic gastroesophageal reflux disease.
After reviewing the Good Geriatric-Palliative Algorithm,30 you might consider stopping:
- clopidogrel, as there is no clear indication for this medication in combination with aspirin in this patient.
- glipizide XL, as this patient’s A1c is below goal and this medication puts her at risk of hypoglycemia and its associated morbidities.
- metformin, as it increases her risk of lactic acidosis because her GFR is <45 units.
- docusate, as the evidence to show clear benefit in improving chronic constipation in older adults is lacking.
You tell your patient that there are multiple medications to consider stopping. In order to monitor any symptoms of withdrawal or return of a condition, it would be best to stop one at a time and follow-up closely. Since she has done well for the past week without the glipizide and lisinopril-HCTZ combination, she can remain off the glipizide and the HCTZ. Lisinopril, however, may provide renal protection in the setting of diabetes and will be continued at this time.
You ask her about adverse effects from her other medications. She indicates that the furosemide makes her run to the bathroom all the time, so she would like to try stopping it. You agree and make a plan for her to monitor her weight, watch for edema, and return in 4 weeks for a follow-up visit.
On follow-up, she is feeling well, has no edema on exam, and is happy to report her urinary incontinence has resolved. You therefore suggest her next deprescribing trial be discontinuation of her oxybutynin. She thanks you for your recommendations about her medications and heads off to her physical therapy appointment.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Division of Geriatric Medicine and Palliative Care, Thomas Jefferson University, 2422 S Broad St, 2nd Floor, Philadelphia, PA 19145; [email protected].
1. Bourgeois FT, Shannon MW, Valim C, et al. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010;19:901-910.
2. Nair NP, Chalmers L, Peterson GM, et al. Hospitalization in older patients due to adverse drug reactions–the need for a prediction tool. Clin Interv Aging. 2016;11:497-506.
3. Nguyen JK, Fouts MM, Kotabe SE, et al. Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. Am J Geriatr Pharmacother. 2006; 4:36-41.
4. Hohl CM, Dankoff J, Colacone A, et al. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med. 2001;38:666-671.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213-218.
7. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28:173-186.
8. Magaziner J, Cadigan DA, Fedder DO, et al. Medication use and functional decline among community-dwelling older women. J Aging Health. 1989;1:470-484.
9. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
10. Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174:588-595.
11. Weiss BD. Diagnostic evaluation of urinary incontinence in geriatric patients. Am Fam Physician. 1998;57:2675-2694.
12. Syed Q, Hendler KT, Koncilja K. The impact of aging and medical status on dysgeusia. Am J Med. 2016;129:753, E1-E6.
13. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
14. Espino DV, Bazaldua OV, Palmer RF, et al. Suboptimal medication use and mortality in an older adult community-based cohort: results from the Hispanic EPESE Study. J Gerontol A Biol Sci Med Sci. 2006;61:170-175.
15. Akazawa M, Imai H, Igarashi A, et al. Potentially inappropriate medication use in elderly Japanese patients. Am J Geriatr Pharmacother. 2010; 8:146-160.
16. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516-1523.
17. Qato DM, Wilder J, Schumm LP, et al. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176:473-482.
18. Flaherty JH, Perry HM 3rd, Lynchard GS, et al. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci. 2000;55:554-559.
19. Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518-1523.
20. Gerteis J, Izrael D, Deitz D, et al. Multiple chronic conditions chartbook. Rockville, MD: Agency for Healthcare Research and Quality. 2014.
21. American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. J Am Geriatr Soc. 2012;60:E1-E25.
22. Woodward M. Deprescribing: achieving better health outcomes for older people through reducing medications. J Pharm Pract Res. 2003;33:323-328.
23. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
24. Page AT, Clifford RM, Potter K, et al. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2016;82:583-623.
25. Reeve E, Shakib S, Hendrix I, et al. The benefits and harms of deprescribing. Med J Aust. 2014;201:386-389.
26. Walsh K, Kwan D, Marr P, et al. Deprescribing in a family health team: a study of chronic proton pump inhibitor use. J Prim Health Care. 2016;8:164-171.
27. Orwig D, Brandt N, Gruber-Baldini AL. Medication management assessment for older adults in the community. Gerontologist. 2006;46:661-668.
28. Anderson K, Jue SG, Madaras-Kelly KJ. Identifying patients at risk for medication mismanagement: using cognitive screens to predict a patient’s accuracy in filling a pillbox. Consult Pharm. 2008;23:459-472.
29. Lenaerts E, De Knijf F, Schoenmakers B. Appropriate prescribing for older people: a new tool for the general practitioner. J Frailty & Aging. 2013;2:8-14.
30. Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. IMAJ. 2007;9:430-434.
31. Holmes HM, Todd A. Evidence-based deprescribing of statins in patients with advanced illness. JAMA Intern Med. 2015;175:701-702.
32. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
33. Guirguis-Blake JM, Evans CV,Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
34. Declercq T, Petrovic M, Azermai M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;3:CD007726.
35. Petersen LK, Christensen K, Kragstrup J. Lipid-lowering treatment to the end? A review of observational studies and RCTs on cholesterol and mortality in 80+-year olds. Age Ageing. 2010;39:674-680.
36. Banach M, Serban MC. Discussion around statin discontinuation in older adults and patients with wasting diseases. J Cachexia Sarcopenia Muscle. 2016;7:396-399.
37. Goldstein MR, Mascitelli L, Pezzetta F. Statin therapy in the elderly: misconceptions. J Am Geriatr Soc. 2008;56:1365.
38. Han BH, Sutin D, Williamson JD, et al, for the ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT Randomized Clinical Trial. JAMA Intern Med. Published online May 22, 2017.
39. Sever PS, Chang CL, Gupta AK, et al. The Anglo-Scandinavian Cardiac Outcomes Trial: 11-year mortality follow-up of the lipid-lowering arm in the U.K. Eur Heart J. 2011;32:2525-2532.
40. Denardo SJ, Gong Y, Nichols WW, et al. Blood pressure and outcomes in very old hypertensive coronary artery disease patients: an INVEST substudy. Am J Med. 2010;123:719-726.
41. Ekbom T, Lindholm LH, Oden A, et al. A 5‐year prospective, observational study of the withdrawal of antihypertensive treatment in elderly people. J Intern Med. 1994;235:581-588.
42. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older. Drugs Aging. 2008;25:1021-1031.
43. Campbell AJ, Robertson MC, Gardner MM, et al. Psychotropic medication withdrawal and a home‐based exercise program to prevent falls: a randomized, controlled trial. J Am Geriatr Soc. 1999;47:850-853.
44. Pollmann AS, Murphy AL, Bergman JC, et al. Deprescribing benzodiazepines and Z-drugs in community-dwelling adults: a scoping review. BMC Pharmacol Toxicol. 2015;16:19.
45. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors. Can Fam Phys. 2017; 63:354-364.
46. Duncan P, Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24:37-42.
47. Schuling J, Gebben H, Veehof LJ, et al. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract. 2012;13:56.
48. Scott I, Anderson K, Freeman CR, et al. First do no harm: a real need to deprescribe in older patients. Med J Aust. 2014;201:390-392.
1. Bourgeois FT, Shannon MW, Valim C, et al. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010;19:901-910.
2. Nair NP, Chalmers L, Peterson GM, et al. Hospitalization in older patients due to adverse drug reactions–the need for a prediction tool. Clin Interv Aging. 2016;11:497-506.
3. Nguyen JK, Fouts MM, Kotabe SE, et al. Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. Am J Geriatr Pharmacother. 2006; 4:36-41.
4. Hohl CM, Dankoff J, Colacone A, et al. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med. 2001;38:666-671.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213-218.
7. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28:173-186.
8. Magaziner J, Cadigan DA, Fedder DO, et al. Medication use and functional decline among community-dwelling older women. J Aging Health. 1989;1:470-484.
9. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
10. Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174:588-595.
11. Weiss BD. Diagnostic evaluation of urinary incontinence in geriatric patients. Am Fam Physician. 1998;57:2675-2694.
12. Syed Q, Hendler KT, Koncilja K. The impact of aging and medical status on dysgeusia. Am J Med. 2016;129:753, E1-E6.
13. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
14. Espino DV, Bazaldua OV, Palmer RF, et al. Suboptimal medication use and mortality in an older adult community-based cohort: results from the Hispanic EPESE Study. J Gerontol A Biol Sci Med Sci. 2006;61:170-175.
15. Akazawa M, Imai H, Igarashi A, et al. Potentially inappropriate medication use in elderly Japanese patients. Am J Geriatr Pharmacother. 2010; 8:146-160.
16. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516-1523.
17. Qato DM, Wilder J, Schumm LP, et al. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176:473-482.
18. Flaherty JH, Perry HM 3rd, Lynchard GS, et al. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci. 2000;55:554-559.
19. Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518-1523.
20. Gerteis J, Izrael D, Deitz D, et al. Multiple chronic conditions chartbook. Rockville, MD: Agency for Healthcare Research and Quality. 2014.
21. American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. J Am Geriatr Soc. 2012;60:E1-E25.
22. Woodward M. Deprescribing: achieving better health outcomes for older people through reducing medications. J Pharm Pract Res. 2003;33:323-328.
23. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
24. Page AT, Clifford RM, Potter K, et al. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2016;82:583-623.
25. Reeve E, Shakib S, Hendrix I, et al. The benefits and harms of deprescribing. Med J Aust. 2014;201:386-389.
26. Walsh K, Kwan D, Marr P, et al. Deprescribing in a family health team: a study of chronic proton pump inhibitor use. J Prim Health Care. 2016;8:164-171.
27. Orwig D, Brandt N, Gruber-Baldini AL. Medication management assessment for older adults in the community. Gerontologist. 2006;46:661-668.
28. Anderson K, Jue SG, Madaras-Kelly KJ. Identifying patients at risk for medication mismanagement: using cognitive screens to predict a patient’s accuracy in filling a pillbox. Consult Pharm. 2008;23:459-472.
29. Lenaerts E, De Knijf F, Schoenmakers B. Appropriate prescribing for older people: a new tool for the general practitioner. J Frailty & Aging. 2013;2:8-14.
30. Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. IMAJ. 2007;9:430-434.
31. Holmes HM, Todd A. Evidence-based deprescribing of statins in patients with advanced illness. JAMA Intern Med. 2015;175:701-702.
32. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
33. Guirguis-Blake JM, Evans CV,Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
34. Declercq T, Petrovic M, Azermai M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;3:CD007726.
35. Petersen LK, Christensen K, Kragstrup J. Lipid-lowering treatment to the end? A review of observational studies and RCTs on cholesterol and mortality in 80+-year olds. Age Ageing. 2010;39:674-680.
36. Banach M, Serban MC. Discussion around statin discontinuation in older adults and patients with wasting diseases. J Cachexia Sarcopenia Muscle. 2016;7:396-399.
37. Goldstein MR, Mascitelli L, Pezzetta F. Statin therapy in the elderly: misconceptions. J Am Geriatr Soc. 2008;56:1365.
38. Han BH, Sutin D, Williamson JD, et al, for the ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT Randomized Clinical Trial. JAMA Intern Med. Published online May 22, 2017.
39. Sever PS, Chang CL, Gupta AK, et al. The Anglo-Scandinavian Cardiac Outcomes Trial: 11-year mortality follow-up of the lipid-lowering arm in the U.K. Eur Heart J. 2011;32:2525-2532.
40. Denardo SJ, Gong Y, Nichols WW, et al. Blood pressure and outcomes in very old hypertensive coronary artery disease patients: an INVEST substudy. Am J Med. 2010;123:719-726.
41. Ekbom T, Lindholm LH, Oden A, et al. A 5‐year prospective, observational study of the withdrawal of antihypertensive treatment in elderly people. J Intern Med. 1994;235:581-588.
42. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older. Drugs Aging. 2008;25:1021-1031.
43. Campbell AJ, Robertson MC, Gardner MM, et al. Psychotropic medication withdrawal and a home‐based exercise program to prevent falls: a randomized, controlled trial. J Am Geriatr Soc. 1999;47:850-853.
44. Pollmann AS, Murphy AL, Bergman JC, et al. Deprescribing benzodiazepines and Z-drugs in community-dwelling adults: a scoping review. BMC Pharmacol Toxicol. 2015;16:19.
45. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors. Can Fam Phys. 2017; 63:354-364.
46. Duncan P, Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24:37-42.
47. Schuling J, Gebben H, Veehof LJ, et al. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract. 2012;13:56.
48. Scott I, Anderson K, Freeman CR, et al. First do no harm: a real need to deprescribe in older patients. Med J Aust. 2014;201:390-392.
From The Journal of Family Practice | 2017;66(7):436-445.
PRACTICE RECOMMENDATIONS
› Avoid medications that are inappropriate for older adults because of adverse effects, lack of efficacy, and/or potential for interactions. A
› Discontinue medications when the harms outweigh the benefits in the context of the patient’s care goals, life expectancy, and/or preferences. C
› Utilize resources such as the STOPP/START and Beers criteria to help you decide where to begin the deprescribing process. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Consider this probiotic for functional abdominal pain
In the article, “When can infants and children benefit from probiotics?” (J Fam Pract. 2016;65:789-794), Dassow et al recommended probiotics as a therapeutic tool for reducing abdominal pain associated with pediatric irritable bowel syndrome (IBS). There are several types of functional disorders in childhood with related abdominal pain, the most common of which are IBS and functional abdominal pain (FAP).1,2
Several recent randomized placebo-controlled trials—one of which I led—have shown that Lactobacillus reuteri DSM 17938 is a beneficial treatment for FAP in children.3-5 When compared with placebo, this probiotic agent significantly reduced the frequency and intensity of FAP in children.
Family physicians should consider this probiotic microorganism as a potential therapeutic tool for IBS, as well as childhood FAP.
Zvi Weizman, MD
Beer-Sheva, Israel
1. Childhood functional GI disorders: child/adolescent. In: Drossman DA CE, Delvaux M, Spiller RC, et al, eds. Rome III: the functional gastrointestinal disorders. 3rd ed. McLean, VA: Degnon Associates, Inc; 2006:895-897.
2. Brown LK, Beattie RM, Tighe MP. Practical management of functional abdominal pain in children. Arch Dis Child. 2016;101:677-683.
3. Romano C, Ferrau’ V, Cavataio F, et al. Lactobacillus reuteri in children with functional abdominal pain (FAP). J Paediatr Child Health. 2014;50:E68-E71.
4. Weizman Z, Abu-Abed J, Binsztok M. Lactobacillus reuteri DSM 17938 for the management of functional abdominal pain in childhood: A randomized, double-blind, placebo-controlled trial. J Pediatr. 2016;174:160-164.e1.
5. Jadrešin O, Hojsak I, Mišak Z, et al. Lactobacillus reuteri DSM 17938 in the treatment of functional abdominal pain in children - RCT study. J Pediatr Gastroenterol Nutr. 2017;64:925-929.
In the article, “When can infants and children benefit from probiotics?” (J Fam Pract. 2016;65:789-794), Dassow et al recommended probiotics as a therapeutic tool for reducing abdominal pain associated with pediatric irritable bowel syndrome (IBS). There are several types of functional disorders in childhood with related abdominal pain, the most common of which are IBS and functional abdominal pain (FAP).1,2
Several recent randomized placebo-controlled trials—one of which I led—have shown that Lactobacillus reuteri DSM 17938 is a beneficial treatment for FAP in children.3-5 When compared with placebo, this probiotic agent significantly reduced the frequency and intensity of FAP in children.
Family physicians should consider this probiotic microorganism as a potential therapeutic tool for IBS, as well as childhood FAP.
Zvi Weizman, MD
Beer-Sheva, Israel
In the article, “When can infants and children benefit from probiotics?” (J Fam Pract. 2016;65:789-794), Dassow et al recommended probiotics as a therapeutic tool for reducing abdominal pain associated with pediatric irritable bowel syndrome (IBS). There are several types of functional disorders in childhood with related abdominal pain, the most common of which are IBS and functional abdominal pain (FAP).1,2
Several recent randomized placebo-controlled trials—one of which I led—have shown that Lactobacillus reuteri DSM 17938 is a beneficial treatment for FAP in children.3-5 When compared with placebo, this probiotic agent significantly reduced the frequency and intensity of FAP in children.
Family physicians should consider this probiotic microorganism as a potential therapeutic tool for IBS, as well as childhood FAP.
Zvi Weizman, MD
Beer-Sheva, Israel
1. Childhood functional GI disorders: child/adolescent. In: Drossman DA CE, Delvaux M, Spiller RC, et al, eds. Rome III: the functional gastrointestinal disorders. 3rd ed. McLean, VA: Degnon Associates, Inc; 2006:895-897.
2. Brown LK, Beattie RM, Tighe MP. Practical management of functional abdominal pain in children. Arch Dis Child. 2016;101:677-683.
3. Romano C, Ferrau’ V, Cavataio F, et al. Lactobacillus reuteri in children with functional abdominal pain (FAP). J Paediatr Child Health. 2014;50:E68-E71.
4. Weizman Z, Abu-Abed J, Binsztok M. Lactobacillus reuteri DSM 17938 for the management of functional abdominal pain in childhood: A randomized, double-blind, placebo-controlled trial. J Pediatr. 2016;174:160-164.e1.
5. Jadrešin O, Hojsak I, Mišak Z, et al. Lactobacillus reuteri DSM 17938 in the treatment of functional abdominal pain in children - RCT study. J Pediatr Gastroenterol Nutr. 2017;64:925-929.
1. Childhood functional GI disorders: child/adolescent. In: Drossman DA CE, Delvaux M, Spiller RC, et al, eds. Rome III: the functional gastrointestinal disorders. 3rd ed. McLean, VA: Degnon Associates, Inc; 2006:895-897.
2. Brown LK, Beattie RM, Tighe MP. Practical management of functional abdominal pain in children. Arch Dis Child. 2016;101:677-683.
3. Romano C, Ferrau’ V, Cavataio F, et al. Lactobacillus reuteri in children with functional abdominal pain (FAP). J Paediatr Child Health. 2014;50:E68-E71.
4. Weizman Z, Abu-Abed J, Binsztok M. Lactobacillus reuteri DSM 17938 for the management of functional abdominal pain in childhood: A randomized, double-blind, placebo-controlled trial. J Pediatr. 2016;174:160-164.e1.
5. Jadrešin O, Hojsak I, Mišak Z, et al. Lactobacillus reuteri DSM 17938 in the treatment of functional abdominal pain in children - RCT study. J Pediatr Gastroenterol Nutr. 2017;64:925-929.